User login
Unrelated Death After Colorectal Cancer Screening: Implications for Improving Colonoscopy Referrals
Colorectal cancer (CRC) ranks among the most common causes of cancer and cancer-related death in the US. The US Multi-Society Task Force (USMSTF) on Colorectal Cancer thus strongly endorsed using several available screening options.1 The published guidelines largely rely on age to define the target population (Table 1). For average-risk individuals, national and Veterans Health Administration (VHA) guidelines currently recommend CRC screening in individuals aged between 50 and 75 years with a life expectancy of > 5 years.1
Although case-control studies also point to a potential benefit in persons aged > 75 years,2,3 the USMSTF cited less convincing evidence and suggested an individualized approach that should consider relative cancer risk and comorbidity burden. Such an approach is supported by modeling studies, which suggest reduced benefit and increased risk of screening with increasing age. The reduced benefit also is significantly affected by comorbidity and relative cancer risk.4 The VHA has successfully implemented CRC screening, capturing the majority of eligible patients based on age criteria. A recent survey showed that more than three-quarters of veterans between age 50 and 75 years had undergone some screening test for CRC as part of routine preventive care. Colonoscopy clearly emerged as the dominant modality chosen for CRC screening and accounted for nearly 84% of these screening tests.5 Consistent with these data, a case-control study confirmed that the widespread implementation of colonoscopy as CRC screening method reduced cancer-related mortality in veterans for cases of left but not right-sided colon cancer.6
With calls to expand the age range of CRC screening beyond aged 75 years, we decided to assess survival rates of a cohort of veterans who underwent a screening or surveillance colonoscopy between 2008 and 2014.7 The goals were to characterize the portion of the cohort that had died, the time between a screening colonoscopy and death, the portion of deaths that were aged ≥ 80 years, and the causes of the deaths. In addition, we focused on a subgroup of the cohort, defined by death within 2 years after the index colonoscopy, to identify predictors of early death that were independent of age.
Methods
We queried the endoscopy reporting system (EndoWorks; Olympus America, Center Valley, PA) for all colonoscopies performed by 2 of 14 physicians at the George Wahlen VA Medical Center (GWVAMC) in Salt Lake City, Utah, who performed endoscopic procedures between January 1, 2008 and December 1, 2014. These physicians had focused their clinical practice exclusively on elective outpatient colonoscopies and accounted for 37.4% of the examinations at GWVAMC during the study period. All colonoscopy requests were triaged and assigned based on availability of open and appropriate procedure time slots without direct physician-specific referral, thus reducing the chance of skewing results. The reports were filtered through a text search to focus on examinations that listed screening or surveillance as indication. The central patient electronic health record was then reviewed to extract basic demographic data, survival status (as of August 1, 2018), and survival time in years after the index or subsequent colonoscopy. For deceased veterans, the age at the time of death, cause of death, and comorbidities were queried.
This study compared cases and control across the study. Cases were persons who clearly died early (defined as > 2 years following the index examination). They were matched with controls who lived for ≥ 5 years after their colonoscopy. These periods were selected because the USMSTF recommended that CRC screening or surveillance colonoscopy should be discontinued in persons with a life expectancy of < 5 years, and most study patients underwent their index procedure ≥ 5 years before August 2018. Cases and controls underwent a colonoscopy in the same year and were matched for age, sex, and presence of underlying inflammatory bowel disease (IBD). For cases and controls, we identified the ordering health care provider specialty, (ie, primary care, gastroenterology, or other).
In addition, we reviewed the encounter linked to the order and abstracted relevant comorbidities listed at that time, noted the use of anticoagulants, opioid analgesics, and benzodiazepines. The comorbidity burden was quantified using the Charlson Comorbidity Index.8 In addition, we denoted the presence of psychiatric problems (eg, anxiety, depression, bipolar disease, psychosis, substance abuse), the diagnosis of atrial fibrillation (AF) or other cardiac arrhythmias, and whether the patient had previously been treated for a malignancy that was in apparent clinical remission. Finally, we searched for routine laboratory tests at the time of this visit or, when not obtained, within 6 months of the encounter, and abstracted serum creatinine, hemoglobin (Hgb), platelet number, serum protein, and albumin. In clinical practice, cutoff values of test results are often more helpful in decision making. We, therefore, dichotomized results for Hgb (cutoff: 10 g/dL), creatinine (cutoff: 2 mg/dL), and albumin (cutoff: 3.2 mg/dL).
Descriptive and analytical statistics were obtained with Stata Version 14.1 (College Station, TX). Unless indicated otherwise, continuous data are shown as mean with 95% CIs. For dichotomous data, we used percentages with their 95% CIs. Analytic statistics were performed with the t test for continuous variables and the 2-tailed test for proportions. A P < .05 was considered a significant difference. To determine independent predictors of early death, we performed a logistic regression analysis with results being expressed as odds ratio with 95% CIs. Survival status was chosen as a dependent variable, and we entered variables that significantly correlated with survival in the bivariate analysis as independent variables.
The study was designed and conducted as a quality improvement project to assess colonoscopy performance and outcomes with the Salt Lake City Specialty Care Center of Innovation (COI), one of 5 regional COIs with an operational mission to improve health care access, utilization, and quality. Our work related to colonoscopy and access within the COI region, including Salt Lake City, has been reviewed and acknowledged by the GWVAMC Institutional Review Board as quality improvement. Andrew Gawron has an operational appointment in the GWVAMC COI, which is part of a US Department of Veterans Affairs (VA) central office initiative established in 2015. The COIs are charged with identifying best practices within the VA and applying those practices throughout the COI region. This local project to identify practice patterns and outcomes locally was sponsored by the GWVAMC COI with a focus to generate information to improve colonoscopy referral quality in patients at Salt Lake City and inform regional and national efforts in this domain.
Results
During the study period, 4,879 veterans (96.9% male) underwent at least 1 colonoscopy for screening or surveillance by 1 of the 2 providers. A total of 306 persons (6.3%) were aged > 80 years. The indication for surveillance colonoscopies included IBD in 78 (1.6%) veterans 2 of whom were women. The mean (SD) follow-up period between the index colonoscopy and study closure or death was 7.4 years (1.7). During the study time, 1,439 persons underwent a repeat examination for surveillance. The percentage of veterans with at least 1 additional colonoscopy after the index test was significantly higher in patients with known IBD compared with those without IBD (78.2% vs 28.7%; P < .01).
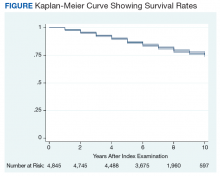
Between the index colonoscopy and August 2018, 974 patients (20.0%) died (Figure). The mean (SD) time between the colonoscopy and recorded year of death was 4.4 years (4.1). The fraction of women in the cohort that died (n = 18) was lower compared with 132 for the group of persons still alive (1.8% vs 3.4%; P < .05). The fraction of veterans with IBD who died by August 2018 did not differ from that of patients with IBD in the cohort of individuals who survived (19.2% vs 20.0%; P = .87). The cohort of veterans who died before study closure included 107 persons who were aged > 80 years at the time of their index colonoscopy, which is significantly more than in the cohort of persons still alive (11.0% vs 5.1%; P < .01).
Cause of Death
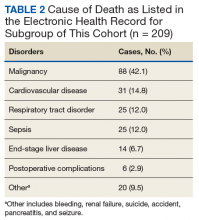
In 209 of the 974 (21.5%) veteran deaths a cause was recorded. Malignancies accounted for 88 of the deaths (42.1%), and CRCs were responsible for 14 (6.7%) deaths (Table 2). In 8 of these patients, the cancer had been identified at an advanced stage, not allowing for curative therapy. One patient had been asked to return for a repeat test as residual fecal matter did not allow proper visualization. He died 1 year later due to complications of sepsis after colonic perforation caused by a proximal colon cancer. Five patients underwent surgery with curative intent but suffered recurrences. In addition to malignancies, advanced diseases, such as cardiovascular, bronchopulmonary illnesses, and infections, were other commonly listed causes of death.
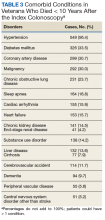
We also abstracted comorbidities that were known at the time of death or the most recent encounter within the VHA system. Hypertension was most commonly listed (549) followed by a current or prior diagnosis of malignancies (355) and diabetes mellitus (DM) (Table 3). Prostate cancer was the most commonly diagnosed malignancy (80), 17 of whom had a second malignancy. CRC accounted for 54 of the malignancies, 1 of which developed in a patient with long-standing ulcerative colitis, 2 were a manifestation of a known hereditary cancer syndrome (Lynch syndrome), and the remaining 51 cases were various cancers without known predisposition. The diagnosis of CRC was made during the study period in 29 veterans. In the remaining 25 patients, the colonoscopy was performed as a surveillance examination after previous surgery for CRC.
Potential Predictors of Early Death
To better define potential predictors of early death, we focused on the 258 persons (5.3%) who died within 2 years after the index procedure and paired them with matched controls. One patient underwent a colonoscopy for surveillance of previously treated cancer and was excluded due to very advanced age, as no matched control could be identified. The mean (SD) age of this male-predominant cohort was 68.2 (9.6) vs 67.9 (9.4) years for cases and controls, respectively. At the time of referral for the test, 29 persons (11.3%) were aged > 80 years, which is significantly more than seen for the overall cohort with 306 (6.3%; P < .001). While primary care providers accounted for most referrals in cases (85.2%) and controls (93.0%), the fraction of veterans referred by gastroenterologists or other specialty care providers was significantly higher in the case group compared with that in the controls (14.8% vs 7.0%; P < .05).
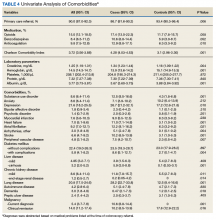
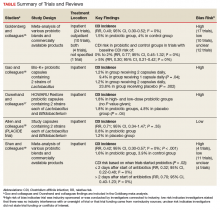
In our age-matched analysis, we examined other potential factors that could influence survival. The burden of comorbid conditions summarized in the Charlson Comorbidity Index significantly correlated with survival status (Table 4). As this composite index does not include psychiatric conditions, we separately examined the impact of anxiety, depression, bipolar disease, psychotic disorders, and substance abuse. The diagnoses of depression and substance use disorders (SUDs) were associated with higher rates of early death. Considering concerns about SUDs, we also assessed the association between prescription for opioids or benzodiazepines and survival status, which showed a marginal correlation. Anticoagulant use, a likely surrogate for cardiovascular disorders, were more commonly listed in the cases than they were in the controls.
Looking at specific comorbid conditions, significant problems affecting key organ systems from heart to lung, liver, kidneys, or brain (dementia) were all predictors of poor outcome. Similarly, DM with secondary complication correlated with early death after the index procedure. In contrast, a history of prior myocardial infarction, prior cancer treatment without evidence of persistent or recurrent disease, or prior peptic ulcer disease did not differ between cases and controls. Focusing on routine blood tests, we noted marginal, but statistically different results for Hgb, serum creatinine, and albumin in cases compared with controls.
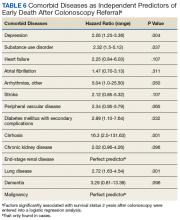
Next we performed a logistic regression to identify independent predictors of survival status. The referring provider specialty, Charlson Comorbidity Index, the diagnosis of a SUD, current benzodiazepine use, and significant anemia or hypoalbuminemia independently predicted death within 2 years of the index examination (Table 5). Considering the composite nature of the Charlson Comorbidity Index, we separately examined the relative importance of different comorbid conditions using a logistic regression analysis. Consistent with the univariate analyses, a known malignancy; severe liver, lung, or kidney disease; and DM with secondary complications were associated with poor outcome. Only arrhythmias other than AF were independent marginal predictors of early death, whereas other variables related to cardiac performance did not reach the level of significance (Table 6). As was true for our analysis examining the composite comorbidity index, the diagnosis of a SUD remained significant as a predictor of death within 2 years of the index colonoscopy.
Discussion
This retrospective analysis followed patients for a mean time of 7 years after a colonoscopy for CRC screening or polyp surveillance. We noted a high rate of all-cause mortality, with 20% of the cohort dying within the period studied. Malignancies, cardiovascular diseases, and advanced lung diseases were most commonly listed causes of death. As expected, CRC was among the 3 most common malignancies and was the cause of death in 6.7% of the group with sufficiently detailed information. While these results fall within the expected range for the mortality related to CRC,9 the results do not allow us to assess the impact of screening, which has been shown to decrease cancer-related mortality in veterans.6 This was limited because the sample size was too small to assess the impact of screening and the cause of death was ascertained for a small percentage of the sample.
Although our findings are limited to a subset of patients seen in a single center, they suggest the importance of appropriate eligibility criteria for screening tests, as also defined in national guidelines.1 As a key anchoring point that describes the target population, age contributed to the rate of relatively early death after the index procedure. Consistent with previously published data, we saw a significant impact of comorbid diseases.10,11 However, our findings go beyond prior reports and show the important impact of psychiatric disease burden, most important the role of SUDs. The predictive value of a summary score, such as the Charlson Comorbidity Index, supports the idea of a cumulative impact, with an increasing disease burden decreasing life expectancy.10-14 It is important to consider the ongoing impact of such coexisting illnesses. Our analysis shows, the mere history of prior problems did not independently predict survival status in our cohort.
Although age is the key anchoring point that defines the target population for CRC screening programs, the benefit of earlier cancer detection or, in the context of colonoscopy with polypectomy, cancer prevention comes with a delay. Thus, cancer risk, procedural risk, and life expectancy should all be weighed when discussing and deciding on the appropriateness of CRC screening. When we disregard inherited cancer syndromes, CRC is clearly a disease of the second half of life with the incidence increasing with age.15 However, other disease burdens rise, which may affects the risk of screening and treatment should cancer be found.
Using our understanding of disease development, researchers have introduced the concept of time to benefit or lag time to help decisions about screening strategies. The period defines the likely time for a precursor or early form of cancer potentially detected by screening to manifest as a clinically relevant lesion. This lag time becomes an especially important consideration in screening of older and/or chronically ill adults with life expectancies that may be close to or even less than the time to benefit.16 Modeling studies suggest that 1,000 flexible sigmoidoscopy screenings are needed to prevent 1 cancer that would manifest about 10 years after the index examination.17,18 The mean life expectancy of a healthy person aged 75 years exceeds 10 years but drops with comorbidity burden. Consistent with these considerations, an analysis of Medicare claims data concluded that individuals with ≥ 3 significant comorbidities do not derive any benefit from screening colonoscopy.14 Looking at the impact of comorbidities, mathematical models concluded that colorectal cancer screening should not be continued in persons with moderate or severe comorbid conditions aged 66 years and 72 years, respectively.19 In contrast, modeling results suggest a benefit of continued screening up to and even above the age of 80 years if persons have an increased cancer risk and if there are no confounding comorbidities.4
Life expectancy and time to benefit describe probabilities. Although such probabilities are relevant in public policy decision, providers and patients may struggle with probabilistic thinking when faced with decisions that involve probabilities of individual health care vs population health care. Both are concerned about the seemingly gloomy or pessimistic undertone of discussing life expectancy and the inherent uncertainty of prognostic tools.20,21 Prior research indicates that this reluctance translates into clinical practice. When faced with vignettes, most clinicians would offer CRC screening to healthy persons aged 80 years with rates falling when the description included a significant comorbid burden; however, more than 40% would still consider screening in octogenarians with poor health.22
Consistent with these responses to theoretical scenarios, CRC screening of veterans dropped with age but was still continued in persons with significant comorbidity.23 Large studies of the veteran population suggest that about 10% of veterans aged > 70 years have chronic medical problems that limit their life expectancy to < 5 years; nonetheless, more than 40% of this cohort underwent colonoscopies for CRC screening.24,25 Interestingly, more illness burden and more clinical encounters translated into more screening examinations in older sick veterans compared with that of the cohort of healthier older persons, suggesting an impact of clinical reminders and the key role of age as the main anchoring variable.23
Ongoing screening despite limited or even no benefit is not unique to CRC. Using validated tools, Pollock and colleagues showed comparable screening rates for breast and prostate cancer when they examined cohorts at either high or low risk of early mortality.26 Similar results have been reported in veterans with about one-third of elderly males with poor life expectancy still undergoing prostate cancer screening.27 Interestingly, inappropriate screening is more common in nonacademic centers and influenced by provider characteristics: nurse practitioner, physician assistants, older attending physicians and male physicians were more likely to order such tests.27,28
Limitations
In this study, we examined a cohort of veterans enrolled in CRC screening within a single institution and obtained survival data for a mean follow-up of > 7 years. We also restricted our study to patients undergoing examinations that explicitly listed screening as indication or polyp surveillance for the test. However, inclusion was based on the indication listed in the report, which may differ from the intent of the ordering provider. Reporting systems often come with default settings, which may skew data. Comorbidities for the entire cohort of veterans who died within the time frame of the study were extracted from the chart without controlling for time-dependent changes, which may more appropriately describe the comorbidity burden at the time of the test. Using a case-control design, we addressed this potential caveat and included only illnesses recorded in the encounter linked to the colonoscopy order. Despite these limitations, our results highlight the importance to more effectively define and target appropriate candidates for CRC screening.
Conclusion
This study shows that age is a simple but not sufficiently accurate criterion to define potential candidates for CRC screening. As automated reminders often prompt discussions about and referral to screening examinations, we should develop algorithms that estimate the individual cancer risk and/or integrate an automatically calculated comorbidity index with these alerts or insert such a tool into order-sets. In addition, providers and patients need to be educated about the rationale and need for a more comprehensive approach to CRC screening that considers anticipated life expectancy. On an individual and health system level, our goal should be to reduce overall mortality rather than only cancer-specific death rates.
1. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323.
2. Kahi CJ, Myers LJ, Slaven JE, et al. Lower endoscopy reduces colorectal cancer incidence in older individuals. Gastroenterology. 2014;146(3):718-725.e3.
3. Wang YR, Cangemi JR, Loftus EV Jr, Picco MF. Decreased risk of colorectal cancer after colonoscopy in patients 76-85 years old in the United States. Digestion. 2016;93(2):132-138.
4. van Hees F, Saini SD, Lansdorp-Vogelaar I, et al. Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology. 2015;149(6):1425-1437.
5. May FP, Yano EM, Provenzale D, Steers NW, Washington DL. The association between primary source of healthcare coverage and colorectal cancer screening among US veterans. Dig Dis Sci. 2017;62(8):1923-1932.
6. Kahi CJ, Pohl H, Myers LJ, Mobarek D, Robertson DJ, Imperiale TF. Colonoscopy and colorectal cancer mortality in the Veterans Affairs Health Care System: a case-control study. Ann Intern Med. 2018;168(7):481-488.
7. Holt PR, Kozuch P, Mewar S. Colon cancer and the elderly: from screening to treatment in management of GI disease in the elderly. Best Pract Res Clin Gastroenterol. 2009;23(6):889-907.
8. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251.
9. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365-1371.
10. Lee TA, Shields AE, Vogeli C, et al. Mortality rate in veterans with multiple chronic conditions. J Gen Intern Med. 2007;22(suppl 3):403-407.
11. Nguyen-Nielsen M, Norgaard M, Jacobsen JB, et al. Comorbidity and survival of Danish prostate cancer patients from 2000-2011: a population-based cohort study. Clin Epidemiol. 2013;5(suppl 1):47-55.
12. Jang SH, Chea JW, Lee KB. Charlson comorbidity index using administrative database in incident PD patients. Clin Nephrol. 2010;73(3):204-209.
13. Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis. 2001;37(2):337-342.
14. Gross CP, Soulos PR, Ross JS, et al. Assessing the impact of screening colonoscopy on mortality in the medicare population. J Gen Intern Med. 2011;26(12):1441-1449.
15. Chouhan V, Mansoor E, Parasa S, Cooper GS. Rates of prevalent colorectal cancer occurrence in persons 75 years of age and older: a population-based national study. Dig Dis Sci. 2018;63(7):1929-1936.
16. Lee SJ, Kim CM. Individualizing prevention for older adults. J Am Geriatr Soc. 2018;66(2):229-234.
17. Tang V, Boscardin WJ, Stijacic-Cenzer I, et al. Time to benefit for colorectal cancer screening: survival meta-analysis of flexible sigmoidoscopy trials. BMJ. 2015;350:h1662.
18. Lee SJ, Boscardin WJ, Stijacic-Cenzer I, et al. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
19. Lansdorp-Vogelaar I, Gulati R, Mariotto AB, et al. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med. 2014;161(2):104-112.
20. Schoenborn NL, Bowman TL II, Cayea D, Pollack CE, Feeser S, Boyd C. Primary care practitioners’ views on incorporating long-term prognosis in the care of older adults. JAMA Intern Med. 2016;176(5):671-678.
21. Schoenborn NL, Lee K, Pollack CE, et al. Older adults’ views and communication preferences about cancer screening cessation. JAMA Intern Med. 2017;177(8):1121-1128.
22. Lewis CL, Esserman D, DeLeon C, Pignone MP, Pathman DE, Golin C. Physician decision making for colorectal cancer screening in the elderly. J Gen Intern Med. 2013;28(9):1202-1217.
23. Saini SD, Vijan S, Schoenfeld P, Powell AA, Moser S, Kerr EA. Role of quality measurement in inappropriate use of screening for colorectal cancer: retrospective cohort study. BMJ. 2014;348:g1247.
24. Walter LC, Lindquist K, Nugent S, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann Intern Med. 2009;150(7):465-473.
25. Powell AA, Saini SD, Breitenstein MK, et al. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015;30(6):732-741.
26. Pollack CE, Blackford AL, Schoenborn NL, Boyd CM, Peairs KS, DuGoff EH. Comparing prognostic tools for cancer screening: considerations for clinical practice and performance assessment. J Am Geriatr Soc. 2016;64(5):1032-1038.
27. So C, Kirby KA, Mehta K, et al. Medical center characteristics associated with PSA screening in elderly veterans with limited life expectancy. J Gen Intern Med. 2012;27(6):653-660.
28. Tang VL, Shi Y, Fung K, et al. Clinician factors associated with prostate-specific antigen screening in older veterans with limited life expectancy. JAMA Intern Med. 2016;176(5):654-661.
Colorectal cancer (CRC) ranks among the most common causes of cancer and cancer-related death in the US. The US Multi-Society Task Force (USMSTF) on Colorectal Cancer thus strongly endorsed using several available screening options.1 The published guidelines largely rely on age to define the target population (Table 1). For average-risk individuals, national and Veterans Health Administration (VHA) guidelines currently recommend CRC screening in individuals aged between 50 and 75 years with a life expectancy of > 5 years.1
Although case-control studies also point to a potential benefit in persons aged > 75 years,2,3 the USMSTF cited less convincing evidence and suggested an individualized approach that should consider relative cancer risk and comorbidity burden. Such an approach is supported by modeling studies, which suggest reduced benefit and increased risk of screening with increasing age. The reduced benefit also is significantly affected by comorbidity and relative cancer risk.4 The VHA has successfully implemented CRC screening, capturing the majority of eligible patients based on age criteria. A recent survey showed that more than three-quarters of veterans between age 50 and 75 years had undergone some screening test for CRC as part of routine preventive care. Colonoscopy clearly emerged as the dominant modality chosen for CRC screening and accounted for nearly 84% of these screening tests.5 Consistent with these data, a case-control study confirmed that the widespread implementation of colonoscopy as CRC screening method reduced cancer-related mortality in veterans for cases of left but not right-sided colon cancer.6
With calls to expand the age range of CRC screening beyond aged 75 years, we decided to assess survival rates of a cohort of veterans who underwent a screening or surveillance colonoscopy between 2008 and 2014.7 The goals were to characterize the portion of the cohort that had died, the time between a screening colonoscopy and death, the portion of deaths that were aged ≥ 80 years, and the causes of the deaths. In addition, we focused on a subgroup of the cohort, defined by death within 2 years after the index colonoscopy, to identify predictors of early death that were independent of age.
Methods
We queried the endoscopy reporting system (EndoWorks; Olympus America, Center Valley, PA) for all colonoscopies performed by 2 of 14 physicians at the George Wahlen VA Medical Center (GWVAMC) in Salt Lake City, Utah, who performed endoscopic procedures between January 1, 2008 and December 1, 2014. These physicians had focused their clinical practice exclusively on elective outpatient colonoscopies and accounted for 37.4% of the examinations at GWVAMC during the study period. All colonoscopy requests were triaged and assigned based on availability of open and appropriate procedure time slots without direct physician-specific referral, thus reducing the chance of skewing results. The reports were filtered through a text search to focus on examinations that listed screening or surveillance as indication. The central patient electronic health record was then reviewed to extract basic demographic data, survival status (as of August 1, 2018), and survival time in years after the index or subsequent colonoscopy. For deceased veterans, the age at the time of death, cause of death, and comorbidities were queried.
This study compared cases and control across the study. Cases were persons who clearly died early (defined as > 2 years following the index examination). They were matched with controls who lived for ≥ 5 years after their colonoscopy. These periods were selected because the USMSTF recommended that CRC screening or surveillance colonoscopy should be discontinued in persons with a life expectancy of < 5 years, and most study patients underwent their index procedure ≥ 5 years before August 2018. Cases and controls underwent a colonoscopy in the same year and were matched for age, sex, and presence of underlying inflammatory bowel disease (IBD). For cases and controls, we identified the ordering health care provider specialty, (ie, primary care, gastroenterology, or other).
In addition, we reviewed the encounter linked to the order and abstracted relevant comorbidities listed at that time, noted the use of anticoagulants, opioid analgesics, and benzodiazepines. The comorbidity burden was quantified using the Charlson Comorbidity Index.8 In addition, we denoted the presence of psychiatric problems (eg, anxiety, depression, bipolar disease, psychosis, substance abuse), the diagnosis of atrial fibrillation (AF) or other cardiac arrhythmias, and whether the patient had previously been treated for a malignancy that was in apparent clinical remission. Finally, we searched for routine laboratory tests at the time of this visit or, when not obtained, within 6 months of the encounter, and abstracted serum creatinine, hemoglobin (Hgb), platelet number, serum protein, and albumin. In clinical practice, cutoff values of test results are often more helpful in decision making. We, therefore, dichotomized results for Hgb (cutoff: 10 g/dL), creatinine (cutoff: 2 mg/dL), and albumin (cutoff: 3.2 mg/dL).
Descriptive and analytical statistics were obtained with Stata Version 14.1 (College Station, TX). Unless indicated otherwise, continuous data are shown as mean with 95% CIs. For dichotomous data, we used percentages with their 95% CIs. Analytic statistics were performed with the t test for continuous variables and the 2-tailed test for proportions. A P < .05 was considered a significant difference. To determine independent predictors of early death, we performed a logistic regression analysis with results being expressed as odds ratio with 95% CIs. Survival status was chosen as a dependent variable, and we entered variables that significantly correlated with survival in the bivariate analysis as independent variables.
The study was designed and conducted as a quality improvement project to assess colonoscopy performance and outcomes with the Salt Lake City Specialty Care Center of Innovation (COI), one of 5 regional COIs with an operational mission to improve health care access, utilization, and quality. Our work related to colonoscopy and access within the COI region, including Salt Lake City, has been reviewed and acknowledged by the GWVAMC Institutional Review Board as quality improvement. Andrew Gawron has an operational appointment in the GWVAMC COI, which is part of a US Department of Veterans Affairs (VA) central office initiative established in 2015. The COIs are charged with identifying best practices within the VA and applying those practices throughout the COI region. This local project to identify practice patterns and outcomes locally was sponsored by the GWVAMC COI with a focus to generate information to improve colonoscopy referral quality in patients at Salt Lake City and inform regional and national efforts in this domain.
Results
During the study period, 4,879 veterans (96.9% male) underwent at least 1 colonoscopy for screening or surveillance by 1 of the 2 providers. A total of 306 persons (6.3%) were aged > 80 years. The indication for surveillance colonoscopies included IBD in 78 (1.6%) veterans 2 of whom were women. The mean (SD) follow-up period between the index colonoscopy and study closure or death was 7.4 years (1.7). During the study time, 1,439 persons underwent a repeat examination for surveillance. The percentage of veterans with at least 1 additional colonoscopy after the index test was significantly higher in patients with known IBD compared with those without IBD (78.2% vs 28.7%; P < .01).
Between the index colonoscopy and August 2018, 974 patients (20.0%) died (Figure). The mean (SD) time between the colonoscopy and recorded year of death was 4.4 years (4.1). The fraction of women in the cohort that died (n = 18) was lower compared with 132 for the group of persons still alive (1.8% vs 3.4%; P < .05). The fraction of veterans with IBD who died by August 2018 did not differ from that of patients with IBD in the cohort of individuals who survived (19.2% vs 20.0%; P = .87). The cohort of veterans who died before study closure included 107 persons who were aged > 80 years at the time of their index colonoscopy, which is significantly more than in the cohort of persons still alive (11.0% vs 5.1%; P < .01).
Cause of Death
In 209 of the 974 (21.5%) veteran deaths a cause was recorded. Malignancies accounted for 88 of the deaths (42.1%), and CRCs were responsible for 14 (6.7%) deaths (Table 2). In 8 of these patients, the cancer had been identified at an advanced stage, not allowing for curative therapy. One patient had been asked to return for a repeat test as residual fecal matter did not allow proper visualization. He died 1 year later due to complications of sepsis after colonic perforation caused by a proximal colon cancer. Five patients underwent surgery with curative intent but suffered recurrences. In addition to malignancies, advanced diseases, such as cardiovascular, bronchopulmonary illnesses, and infections, were other commonly listed causes of death.
We also abstracted comorbidities that were known at the time of death or the most recent encounter within the VHA system. Hypertension was most commonly listed (549) followed by a current or prior diagnosis of malignancies (355) and diabetes mellitus (DM) (Table 3). Prostate cancer was the most commonly diagnosed malignancy (80), 17 of whom had a second malignancy. CRC accounted for 54 of the malignancies, 1 of which developed in a patient with long-standing ulcerative colitis, 2 were a manifestation of a known hereditary cancer syndrome (Lynch syndrome), and the remaining 51 cases were various cancers without known predisposition. The diagnosis of CRC was made during the study period in 29 veterans. In the remaining 25 patients, the colonoscopy was performed as a surveillance examination after previous surgery for CRC.
Potential Predictors of Early Death
To better define potential predictors of early death, we focused on the 258 persons (5.3%) who died within 2 years after the index procedure and paired them with matched controls. One patient underwent a colonoscopy for surveillance of previously treated cancer and was excluded due to very advanced age, as no matched control could be identified. The mean (SD) age of this male-predominant cohort was 68.2 (9.6) vs 67.9 (9.4) years for cases and controls, respectively. At the time of referral for the test, 29 persons (11.3%) were aged > 80 years, which is significantly more than seen for the overall cohort with 306 (6.3%; P < .001). While primary care providers accounted for most referrals in cases (85.2%) and controls (93.0%), the fraction of veterans referred by gastroenterologists or other specialty care providers was significantly higher in the case group compared with that in the controls (14.8% vs 7.0%; P < .05).
In our age-matched analysis, we examined other potential factors that could influence survival. The burden of comorbid conditions summarized in the Charlson Comorbidity Index significantly correlated with survival status (Table 4). As this composite index does not include psychiatric conditions, we separately examined the impact of anxiety, depression, bipolar disease, psychotic disorders, and substance abuse. The diagnoses of depression and substance use disorders (SUDs) were associated with higher rates of early death. Considering concerns about SUDs, we also assessed the association between prescription for opioids or benzodiazepines and survival status, which showed a marginal correlation. Anticoagulant use, a likely surrogate for cardiovascular disorders, were more commonly listed in the cases than they were in the controls.
Looking at specific comorbid conditions, significant problems affecting key organ systems from heart to lung, liver, kidneys, or brain (dementia) were all predictors of poor outcome. Similarly, DM with secondary complication correlated with early death after the index procedure. In contrast, a history of prior myocardial infarction, prior cancer treatment without evidence of persistent or recurrent disease, or prior peptic ulcer disease did not differ between cases and controls. Focusing on routine blood tests, we noted marginal, but statistically different results for Hgb, serum creatinine, and albumin in cases compared with controls.
Next we performed a logistic regression to identify independent predictors of survival status. The referring provider specialty, Charlson Comorbidity Index, the diagnosis of a SUD, current benzodiazepine use, and significant anemia or hypoalbuminemia independently predicted death within 2 years of the index examination (Table 5). Considering the composite nature of the Charlson Comorbidity Index, we separately examined the relative importance of different comorbid conditions using a logistic regression analysis. Consistent with the univariate analyses, a known malignancy; severe liver, lung, or kidney disease; and DM with secondary complications were associated with poor outcome. Only arrhythmias other than AF were independent marginal predictors of early death, whereas other variables related to cardiac performance did not reach the level of significance (Table 6). As was true for our analysis examining the composite comorbidity index, the diagnosis of a SUD remained significant as a predictor of death within 2 years of the index colonoscopy.
Discussion
This retrospective analysis followed patients for a mean time of 7 years after a colonoscopy for CRC screening or polyp surveillance. We noted a high rate of all-cause mortality, with 20% of the cohort dying within the period studied. Malignancies, cardiovascular diseases, and advanced lung diseases were most commonly listed causes of death. As expected, CRC was among the 3 most common malignancies and was the cause of death in 6.7% of the group with sufficiently detailed information. While these results fall within the expected range for the mortality related to CRC,9 the results do not allow us to assess the impact of screening, which has been shown to decrease cancer-related mortality in veterans.6 This was limited because the sample size was too small to assess the impact of screening and the cause of death was ascertained for a small percentage of the sample.
Although our findings are limited to a subset of patients seen in a single center, they suggest the importance of appropriate eligibility criteria for screening tests, as also defined in national guidelines.1 As a key anchoring point that describes the target population, age contributed to the rate of relatively early death after the index procedure. Consistent with previously published data, we saw a significant impact of comorbid diseases.10,11 However, our findings go beyond prior reports and show the important impact of psychiatric disease burden, most important the role of SUDs. The predictive value of a summary score, such as the Charlson Comorbidity Index, supports the idea of a cumulative impact, with an increasing disease burden decreasing life expectancy.10-14 It is important to consider the ongoing impact of such coexisting illnesses. Our analysis shows, the mere history of prior problems did not independently predict survival status in our cohort.
Although age is the key anchoring point that defines the target population for CRC screening programs, the benefit of earlier cancer detection or, in the context of colonoscopy with polypectomy, cancer prevention comes with a delay. Thus, cancer risk, procedural risk, and life expectancy should all be weighed when discussing and deciding on the appropriateness of CRC screening. When we disregard inherited cancer syndromes, CRC is clearly a disease of the second half of life with the incidence increasing with age.15 However, other disease burdens rise, which may affects the risk of screening and treatment should cancer be found.
Using our understanding of disease development, researchers have introduced the concept of time to benefit or lag time to help decisions about screening strategies. The period defines the likely time for a precursor or early form of cancer potentially detected by screening to manifest as a clinically relevant lesion. This lag time becomes an especially important consideration in screening of older and/or chronically ill adults with life expectancies that may be close to or even less than the time to benefit.16 Modeling studies suggest that 1,000 flexible sigmoidoscopy screenings are needed to prevent 1 cancer that would manifest about 10 years after the index examination.17,18 The mean life expectancy of a healthy person aged 75 years exceeds 10 years but drops with comorbidity burden. Consistent with these considerations, an analysis of Medicare claims data concluded that individuals with ≥ 3 significant comorbidities do not derive any benefit from screening colonoscopy.14 Looking at the impact of comorbidities, mathematical models concluded that colorectal cancer screening should not be continued in persons with moderate or severe comorbid conditions aged 66 years and 72 years, respectively.19 In contrast, modeling results suggest a benefit of continued screening up to and even above the age of 80 years if persons have an increased cancer risk and if there are no confounding comorbidities.4
Life expectancy and time to benefit describe probabilities. Although such probabilities are relevant in public policy decision, providers and patients may struggle with probabilistic thinking when faced with decisions that involve probabilities of individual health care vs population health care. Both are concerned about the seemingly gloomy or pessimistic undertone of discussing life expectancy and the inherent uncertainty of prognostic tools.20,21 Prior research indicates that this reluctance translates into clinical practice. When faced with vignettes, most clinicians would offer CRC screening to healthy persons aged 80 years with rates falling when the description included a significant comorbid burden; however, more than 40% would still consider screening in octogenarians with poor health.22
Consistent with these responses to theoretical scenarios, CRC screening of veterans dropped with age but was still continued in persons with significant comorbidity.23 Large studies of the veteran population suggest that about 10% of veterans aged > 70 years have chronic medical problems that limit their life expectancy to < 5 years; nonetheless, more than 40% of this cohort underwent colonoscopies for CRC screening.24,25 Interestingly, more illness burden and more clinical encounters translated into more screening examinations in older sick veterans compared with that of the cohort of healthier older persons, suggesting an impact of clinical reminders and the key role of age as the main anchoring variable.23
Ongoing screening despite limited or even no benefit is not unique to CRC. Using validated tools, Pollock and colleagues showed comparable screening rates for breast and prostate cancer when they examined cohorts at either high or low risk of early mortality.26 Similar results have been reported in veterans with about one-third of elderly males with poor life expectancy still undergoing prostate cancer screening.27 Interestingly, inappropriate screening is more common in nonacademic centers and influenced by provider characteristics: nurse practitioner, physician assistants, older attending physicians and male physicians were more likely to order such tests.27,28
Limitations
In this study, we examined a cohort of veterans enrolled in CRC screening within a single institution and obtained survival data for a mean follow-up of > 7 years. We also restricted our study to patients undergoing examinations that explicitly listed screening as indication or polyp surveillance for the test. However, inclusion was based on the indication listed in the report, which may differ from the intent of the ordering provider. Reporting systems often come with default settings, which may skew data. Comorbidities for the entire cohort of veterans who died within the time frame of the study were extracted from the chart without controlling for time-dependent changes, which may more appropriately describe the comorbidity burden at the time of the test. Using a case-control design, we addressed this potential caveat and included only illnesses recorded in the encounter linked to the colonoscopy order. Despite these limitations, our results highlight the importance to more effectively define and target appropriate candidates for CRC screening.
Conclusion
This study shows that age is a simple but not sufficiently accurate criterion to define potential candidates for CRC screening. As automated reminders often prompt discussions about and referral to screening examinations, we should develop algorithms that estimate the individual cancer risk and/or integrate an automatically calculated comorbidity index with these alerts or insert such a tool into order-sets. In addition, providers and patients need to be educated about the rationale and need for a more comprehensive approach to CRC screening that considers anticipated life expectancy. On an individual and health system level, our goal should be to reduce overall mortality rather than only cancer-specific death rates.
Colorectal cancer (CRC) ranks among the most common causes of cancer and cancer-related death in the US. The US Multi-Society Task Force (USMSTF) on Colorectal Cancer thus strongly endorsed using several available screening options.1 The published guidelines largely rely on age to define the target population (Table 1). For average-risk individuals, national and Veterans Health Administration (VHA) guidelines currently recommend CRC screening in individuals aged between 50 and 75 years with a life expectancy of > 5 years.1
Although case-control studies also point to a potential benefit in persons aged > 75 years,2,3 the USMSTF cited less convincing evidence and suggested an individualized approach that should consider relative cancer risk and comorbidity burden. Such an approach is supported by modeling studies, which suggest reduced benefit and increased risk of screening with increasing age. The reduced benefit also is significantly affected by comorbidity and relative cancer risk.4 The VHA has successfully implemented CRC screening, capturing the majority of eligible patients based on age criteria. A recent survey showed that more than three-quarters of veterans between age 50 and 75 years had undergone some screening test for CRC as part of routine preventive care. Colonoscopy clearly emerged as the dominant modality chosen for CRC screening and accounted for nearly 84% of these screening tests.5 Consistent with these data, a case-control study confirmed that the widespread implementation of colonoscopy as CRC screening method reduced cancer-related mortality in veterans for cases of left but not right-sided colon cancer.6
With calls to expand the age range of CRC screening beyond aged 75 years, we decided to assess survival rates of a cohort of veterans who underwent a screening or surveillance colonoscopy between 2008 and 2014.7 The goals were to characterize the portion of the cohort that had died, the time between a screening colonoscopy and death, the portion of deaths that were aged ≥ 80 years, and the causes of the deaths. In addition, we focused on a subgroup of the cohort, defined by death within 2 years after the index colonoscopy, to identify predictors of early death that were independent of age.
Methods
We queried the endoscopy reporting system (EndoWorks; Olympus America, Center Valley, PA) for all colonoscopies performed by 2 of 14 physicians at the George Wahlen VA Medical Center (GWVAMC) in Salt Lake City, Utah, who performed endoscopic procedures between January 1, 2008 and December 1, 2014. These physicians had focused their clinical practice exclusively on elective outpatient colonoscopies and accounted for 37.4% of the examinations at GWVAMC during the study period. All colonoscopy requests were triaged and assigned based on availability of open and appropriate procedure time slots without direct physician-specific referral, thus reducing the chance of skewing results. The reports were filtered through a text search to focus on examinations that listed screening or surveillance as indication. The central patient electronic health record was then reviewed to extract basic demographic data, survival status (as of August 1, 2018), and survival time in years after the index or subsequent colonoscopy. For deceased veterans, the age at the time of death, cause of death, and comorbidities were queried.
This study compared cases and control across the study. Cases were persons who clearly died early (defined as > 2 years following the index examination). They were matched with controls who lived for ≥ 5 years after their colonoscopy. These periods were selected because the USMSTF recommended that CRC screening or surveillance colonoscopy should be discontinued in persons with a life expectancy of < 5 years, and most study patients underwent their index procedure ≥ 5 years before August 2018. Cases and controls underwent a colonoscopy in the same year and were matched for age, sex, and presence of underlying inflammatory bowel disease (IBD). For cases and controls, we identified the ordering health care provider specialty, (ie, primary care, gastroenterology, or other).
In addition, we reviewed the encounter linked to the order and abstracted relevant comorbidities listed at that time, noted the use of anticoagulants, opioid analgesics, and benzodiazepines. The comorbidity burden was quantified using the Charlson Comorbidity Index.8 In addition, we denoted the presence of psychiatric problems (eg, anxiety, depression, bipolar disease, psychosis, substance abuse), the diagnosis of atrial fibrillation (AF) or other cardiac arrhythmias, and whether the patient had previously been treated for a malignancy that was in apparent clinical remission. Finally, we searched for routine laboratory tests at the time of this visit or, when not obtained, within 6 months of the encounter, and abstracted serum creatinine, hemoglobin (Hgb), platelet number, serum protein, and albumin. In clinical practice, cutoff values of test results are often more helpful in decision making. We, therefore, dichotomized results for Hgb (cutoff: 10 g/dL), creatinine (cutoff: 2 mg/dL), and albumin (cutoff: 3.2 mg/dL).
Descriptive and analytical statistics were obtained with Stata Version 14.1 (College Station, TX). Unless indicated otherwise, continuous data are shown as mean with 95% CIs. For dichotomous data, we used percentages with their 95% CIs. Analytic statistics were performed with the t test for continuous variables and the 2-tailed test for proportions. A P < .05 was considered a significant difference. To determine independent predictors of early death, we performed a logistic regression analysis with results being expressed as odds ratio with 95% CIs. Survival status was chosen as a dependent variable, and we entered variables that significantly correlated with survival in the bivariate analysis as independent variables.
The study was designed and conducted as a quality improvement project to assess colonoscopy performance and outcomes with the Salt Lake City Specialty Care Center of Innovation (COI), one of 5 regional COIs with an operational mission to improve health care access, utilization, and quality. Our work related to colonoscopy and access within the COI region, including Salt Lake City, has been reviewed and acknowledged by the GWVAMC Institutional Review Board as quality improvement. Andrew Gawron has an operational appointment in the GWVAMC COI, which is part of a US Department of Veterans Affairs (VA) central office initiative established in 2015. The COIs are charged with identifying best practices within the VA and applying those practices throughout the COI region. This local project to identify practice patterns and outcomes locally was sponsored by the GWVAMC COI with a focus to generate information to improve colonoscopy referral quality in patients at Salt Lake City and inform regional and national efforts in this domain.
Results
During the study period, 4,879 veterans (96.9% male) underwent at least 1 colonoscopy for screening or surveillance by 1 of the 2 providers. A total of 306 persons (6.3%) were aged > 80 years. The indication for surveillance colonoscopies included IBD in 78 (1.6%) veterans 2 of whom were women. The mean (SD) follow-up period between the index colonoscopy and study closure or death was 7.4 years (1.7). During the study time, 1,439 persons underwent a repeat examination for surveillance. The percentage of veterans with at least 1 additional colonoscopy after the index test was significantly higher in patients with known IBD compared with those without IBD (78.2% vs 28.7%; P < .01).
Between the index colonoscopy and August 2018, 974 patients (20.0%) died (Figure). The mean (SD) time between the colonoscopy and recorded year of death was 4.4 years (4.1). The fraction of women in the cohort that died (n = 18) was lower compared with 132 for the group of persons still alive (1.8% vs 3.4%; P < .05). The fraction of veterans with IBD who died by August 2018 did not differ from that of patients with IBD in the cohort of individuals who survived (19.2% vs 20.0%; P = .87). The cohort of veterans who died before study closure included 107 persons who were aged > 80 years at the time of their index colonoscopy, which is significantly more than in the cohort of persons still alive (11.0% vs 5.1%; P < .01).
Cause of Death
In 209 of the 974 (21.5%) veteran deaths a cause was recorded. Malignancies accounted for 88 of the deaths (42.1%), and CRCs were responsible for 14 (6.7%) deaths (Table 2). In 8 of these patients, the cancer had been identified at an advanced stage, not allowing for curative therapy. One patient had been asked to return for a repeat test as residual fecal matter did not allow proper visualization. He died 1 year later due to complications of sepsis after colonic perforation caused by a proximal colon cancer. Five patients underwent surgery with curative intent but suffered recurrences. In addition to malignancies, advanced diseases, such as cardiovascular, bronchopulmonary illnesses, and infections, were other commonly listed causes of death.
We also abstracted comorbidities that were known at the time of death or the most recent encounter within the VHA system. Hypertension was most commonly listed (549) followed by a current or prior diagnosis of malignancies (355) and diabetes mellitus (DM) (Table 3). Prostate cancer was the most commonly diagnosed malignancy (80), 17 of whom had a second malignancy. CRC accounted for 54 of the malignancies, 1 of which developed in a patient with long-standing ulcerative colitis, 2 were a manifestation of a known hereditary cancer syndrome (Lynch syndrome), and the remaining 51 cases were various cancers without known predisposition. The diagnosis of CRC was made during the study period in 29 veterans. In the remaining 25 patients, the colonoscopy was performed as a surveillance examination after previous surgery for CRC.
Potential Predictors of Early Death
To better define potential predictors of early death, we focused on the 258 persons (5.3%) who died within 2 years after the index procedure and paired them with matched controls. One patient underwent a colonoscopy for surveillance of previously treated cancer and was excluded due to very advanced age, as no matched control could be identified. The mean (SD) age of this male-predominant cohort was 68.2 (9.6) vs 67.9 (9.4) years for cases and controls, respectively. At the time of referral for the test, 29 persons (11.3%) were aged > 80 years, which is significantly more than seen for the overall cohort with 306 (6.3%; P < .001). While primary care providers accounted for most referrals in cases (85.2%) and controls (93.0%), the fraction of veterans referred by gastroenterologists or other specialty care providers was significantly higher in the case group compared with that in the controls (14.8% vs 7.0%; P < .05).
In our age-matched analysis, we examined other potential factors that could influence survival. The burden of comorbid conditions summarized in the Charlson Comorbidity Index significantly correlated with survival status (Table 4). As this composite index does not include psychiatric conditions, we separately examined the impact of anxiety, depression, bipolar disease, psychotic disorders, and substance abuse. The diagnoses of depression and substance use disorders (SUDs) were associated with higher rates of early death. Considering concerns about SUDs, we also assessed the association between prescription for opioids or benzodiazepines and survival status, which showed a marginal correlation. Anticoagulant use, a likely surrogate for cardiovascular disorders, were more commonly listed in the cases than they were in the controls.
Looking at specific comorbid conditions, significant problems affecting key organ systems from heart to lung, liver, kidneys, or brain (dementia) were all predictors of poor outcome. Similarly, DM with secondary complication correlated with early death after the index procedure. In contrast, a history of prior myocardial infarction, prior cancer treatment without evidence of persistent or recurrent disease, or prior peptic ulcer disease did not differ between cases and controls. Focusing on routine blood tests, we noted marginal, but statistically different results for Hgb, serum creatinine, and albumin in cases compared with controls.
Next we performed a logistic regression to identify independent predictors of survival status. The referring provider specialty, Charlson Comorbidity Index, the diagnosis of a SUD, current benzodiazepine use, and significant anemia or hypoalbuminemia independently predicted death within 2 years of the index examination (Table 5). Considering the composite nature of the Charlson Comorbidity Index, we separately examined the relative importance of different comorbid conditions using a logistic regression analysis. Consistent with the univariate analyses, a known malignancy; severe liver, lung, or kidney disease; and DM with secondary complications were associated with poor outcome. Only arrhythmias other than AF were independent marginal predictors of early death, whereas other variables related to cardiac performance did not reach the level of significance (Table 6). As was true for our analysis examining the composite comorbidity index, the diagnosis of a SUD remained significant as a predictor of death within 2 years of the index colonoscopy.
Discussion
This retrospective analysis followed patients for a mean time of 7 years after a colonoscopy for CRC screening or polyp surveillance. We noted a high rate of all-cause mortality, with 20% of the cohort dying within the period studied. Malignancies, cardiovascular diseases, and advanced lung diseases were most commonly listed causes of death. As expected, CRC was among the 3 most common malignancies and was the cause of death in 6.7% of the group with sufficiently detailed information. While these results fall within the expected range for the mortality related to CRC,9 the results do not allow us to assess the impact of screening, which has been shown to decrease cancer-related mortality in veterans.6 This was limited because the sample size was too small to assess the impact of screening and the cause of death was ascertained for a small percentage of the sample.
Although our findings are limited to a subset of patients seen in a single center, they suggest the importance of appropriate eligibility criteria for screening tests, as also defined in national guidelines.1 As a key anchoring point that describes the target population, age contributed to the rate of relatively early death after the index procedure. Consistent with previously published data, we saw a significant impact of comorbid diseases.10,11 However, our findings go beyond prior reports and show the important impact of psychiatric disease burden, most important the role of SUDs. The predictive value of a summary score, such as the Charlson Comorbidity Index, supports the idea of a cumulative impact, with an increasing disease burden decreasing life expectancy.10-14 It is important to consider the ongoing impact of such coexisting illnesses. Our analysis shows, the mere history of prior problems did not independently predict survival status in our cohort.
Although age is the key anchoring point that defines the target population for CRC screening programs, the benefit of earlier cancer detection or, in the context of colonoscopy with polypectomy, cancer prevention comes with a delay. Thus, cancer risk, procedural risk, and life expectancy should all be weighed when discussing and deciding on the appropriateness of CRC screening. When we disregard inherited cancer syndromes, CRC is clearly a disease of the second half of life with the incidence increasing with age.15 However, other disease burdens rise, which may affects the risk of screening and treatment should cancer be found.
Using our understanding of disease development, researchers have introduced the concept of time to benefit or lag time to help decisions about screening strategies. The period defines the likely time for a precursor or early form of cancer potentially detected by screening to manifest as a clinically relevant lesion. This lag time becomes an especially important consideration in screening of older and/or chronically ill adults with life expectancies that may be close to or even less than the time to benefit.16 Modeling studies suggest that 1,000 flexible sigmoidoscopy screenings are needed to prevent 1 cancer that would manifest about 10 years after the index examination.17,18 The mean life expectancy of a healthy person aged 75 years exceeds 10 years but drops with comorbidity burden. Consistent with these considerations, an analysis of Medicare claims data concluded that individuals with ≥ 3 significant comorbidities do not derive any benefit from screening colonoscopy.14 Looking at the impact of comorbidities, mathematical models concluded that colorectal cancer screening should not be continued in persons with moderate or severe comorbid conditions aged 66 years and 72 years, respectively.19 In contrast, modeling results suggest a benefit of continued screening up to and even above the age of 80 years if persons have an increased cancer risk and if there are no confounding comorbidities.4
Life expectancy and time to benefit describe probabilities. Although such probabilities are relevant in public policy decision, providers and patients may struggle with probabilistic thinking when faced with decisions that involve probabilities of individual health care vs population health care. Both are concerned about the seemingly gloomy or pessimistic undertone of discussing life expectancy and the inherent uncertainty of prognostic tools.20,21 Prior research indicates that this reluctance translates into clinical practice. When faced with vignettes, most clinicians would offer CRC screening to healthy persons aged 80 years with rates falling when the description included a significant comorbid burden; however, more than 40% would still consider screening in octogenarians with poor health.22
Consistent with these responses to theoretical scenarios, CRC screening of veterans dropped with age but was still continued in persons with significant comorbidity.23 Large studies of the veteran population suggest that about 10% of veterans aged > 70 years have chronic medical problems that limit their life expectancy to < 5 years; nonetheless, more than 40% of this cohort underwent colonoscopies for CRC screening.24,25 Interestingly, more illness burden and more clinical encounters translated into more screening examinations in older sick veterans compared with that of the cohort of healthier older persons, suggesting an impact of clinical reminders and the key role of age as the main anchoring variable.23
Ongoing screening despite limited or even no benefit is not unique to CRC. Using validated tools, Pollock and colleagues showed comparable screening rates for breast and prostate cancer when they examined cohorts at either high or low risk of early mortality.26 Similar results have been reported in veterans with about one-third of elderly males with poor life expectancy still undergoing prostate cancer screening.27 Interestingly, inappropriate screening is more common in nonacademic centers and influenced by provider characteristics: nurse practitioner, physician assistants, older attending physicians and male physicians were more likely to order such tests.27,28
Limitations
In this study, we examined a cohort of veterans enrolled in CRC screening within a single institution and obtained survival data for a mean follow-up of > 7 years. We also restricted our study to patients undergoing examinations that explicitly listed screening as indication or polyp surveillance for the test. However, inclusion was based on the indication listed in the report, which may differ from the intent of the ordering provider. Reporting systems often come with default settings, which may skew data. Comorbidities for the entire cohort of veterans who died within the time frame of the study were extracted from the chart without controlling for time-dependent changes, which may more appropriately describe the comorbidity burden at the time of the test. Using a case-control design, we addressed this potential caveat and included only illnesses recorded in the encounter linked to the colonoscopy order. Despite these limitations, our results highlight the importance to more effectively define and target appropriate candidates for CRC screening.
Conclusion
This study shows that age is a simple but not sufficiently accurate criterion to define potential candidates for CRC screening. As automated reminders often prompt discussions about and referral to screening examinations, we should develop algorithms that estimate the individual cancer risk and/or integrate an automatically calculated comorbidity index with these alerts or insert such a tool into order-sets. In addition, providers and patients need to be educated about the rationale and need for a more comprehensive approach to CRC screening that considers anticipated life expectancy. On an individual and health system level, our goal should be to reduce overall mortality rather than only cancer-specific death rates.
1. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323.
2. Kahi CJ, Myers LJ, Slaven JE, et al. Lower endoscopy reduces colorectal cancer incidence in older individuals. Gastroenterology. 2014;146(3):718-725.e3.
3. Wang YR, Cangemi JR, Loftus EV Jr, Picco MF. Decreased risk of colorectal cancer after colonoscopy in patients 76-85 years old in the United States. Digestion. 2016;93(2):132-138.
4. van Hees F, Saini SD, Lansdorp-Vogelaar I, et al. Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology. 2015;149(6):1425-1437.
5. May FP, Yano EM, Provenzale D, Steers NW, Washington DL. The association between primary source of healthcare coverage and colorectal cancer screening among US veterans. Dig Dis Sci. 2017;62(8):1923-1932.
6. Kahi CJ, Pohl H, Myers LJ, Mobarek D, Robertson DJ, Imperiale TF. Colonoscopy and colorectal cancer mortality in the Veterans Affairs Health Care System: a case-control study. Ann Intern Med. 2018;168(7):481-488.
7. Holt PR, Kozuch P, Mewar S. Colon cancer and the elderly: from screening to treatment in management of GI disease in the elderly. Best Pract Res Clin Gastroenterol. 2009;23(6):889-907.
8. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251.
9. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365-1371.
10. Lee TA, Shields AE, Vogeli C, et al. Mortality rate in veterans with multiple chronic conditions. J Gen Intern Med. 2007;22(suppl 3):403-407.
11. Nguyen-Nielsen M, Norgaard M, Jacobsen JB, et al. Comorbidity and survival of Danish prostate cancer patients from 2000-2011: a population-based cohort study. Clin Epidemiol. 2013;5(suppl 1):47-55.
12. Jang SH, Chea JW, Lee KB. Charlson comorbidity index using administrative database in incident PD patients. Clin Nephrol. 2010;73(3):204-209.
13. Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis. 2001;37(2):337-342.
14. Gross CP, Soulos PR, Ross JS, et al. Assessing the impact of screening colonoscopy on mortality in the medicare population. J Gen Intern Med. 2011;26(12):1441-1449.
15. Chouhan V, Mansoor E, Parasa S, Cooper GS. Rates of prevalent colorectal cancer occurrence in persons 75 years of age and older: a population-based national study. Dig Dis Sci. 2018;63(7):1929-1936.
16. Lee SJ, Kim CM. Individualizing prevention for older adults. J Am Geriatr Soc. 2018;66(2):229-234.
17. Tang V, Boscardin WJ, Stijacic-Cenzer I, et al. Time to benefit for colorectal cancer screening: survival meta-analysis of flexible sigmoidoscopy trials. BMJ. 2015;350:h1662.
18. Lee SJ, Boscardin WJ, Stijacic-Cenzer I, et al. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
19. Lansdorp-Vogelaar I, Gulati R, Mariotto AB, et al. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med. 2014;161(2):104-112.
20. Schoenborn NL, Bowman TL II, Cayea D, Pollack CE, Feeser S, Boyd C. Primary care practitioners’ views on incorporating long-term prognosis in the care of older adults. JAMA Intern Med. 2016;176(5):671-678.
21. Schoenborn NL, Lee K, Pollack CE, et al. Older adults’ views and communication preferences about cancer screening cessation. JAMA Intern Med. 2017;177(8):1121-1128.
22. Lewis CL, Esserman D, DeLeon C, Pignone MP, Pathman DE, Golin C. Physician decision making for colorectal cancer screening in the elderly. J Gen Intern Med. 2013;28(9):1202-1217.
23. Saini SD, Vijan S, Schoenfeld P, Powell AA, Moser S, Kerr EA. Role of quality measurement in inappropriate use of screening for colorectal cancer: retrospective cohort study. BMJ. 2014;348:g1247.
24. Walter LC, Lindquist K, Nugent S, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann Intern Med. 2009;150(7):465-473.
25. Powell AA, Saini SD, Breitenstein MK, et al. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015;30(6):732-741.
26. Pollack CE, Blackford AL, Schoenborn NL, Boyd CM, Peairs KS, DuGoff EH. Comparing prognostic tools for cancer screening: considerations for clinical practice and performance assessment. J Am Geriatr Soc. 2016;64(5):1032-1038.
27. So C, Kirby KA, Mehta K, et al. Medical center characteristics associated with PSA screening in elderly veterans with limited life expectancy. J Gen Intern Med. 2012;27(6):653-660.
28. Tang VL, Shi Y, Fung K, et al. Clinician factors associated with prostate-specific antigen screening in older veterans with limited life expectancy. JAMA Intern Med. 2016;176(5):654-661.
1. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323.
2. Kahi CJ, Myers LJ, Slaven JE, et al. Lower endoscopy reduces colorectal cancer incidence in older individuals. Gastroenterology. 2014;146(3):718-725.e3.
3. Wang YR, Cangemi JR, Loftus EV Jr, Picco MF. Decreased risk of colorectal cancer after colonoscopy in patients 76-85 years old in the United States. Digestion. 2016;93(2):132-138.
4. van Hees F, Saini SD, Lansdorp-Vogelaar I, et al. Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology. 2015;149(6):1425-1437.
5. May FP, Yano EM, Provenzale D, Steers NW, Washington DL. The association between primary source of healthcare coverage and colorectal cancer screening among US veterans. Dig Dis Sci. 2017;62(8):1923-1932.
6. Kahi CJ, Pohl H, Myers LJ, Mobarek D, Robertson DJ, Imperiale TF. Colonoscopy and colorectal cancer mortality in the Veterans Affairs Health Care System: a case-control study. Ann Intern Med. 2018;168(7):481-488.
7. Holt PR, Kozuch P, Mewar S. Colon cancer and the elderly: from screening to treatment in management of GI disease in the elderly. Best Pract Res Clin Gastroenterol. 2009;23(6):889-907.
8. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251.
9. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365-1371.
10. Lee TA, Shields AE, Vogeli C, et al. Mortality rate in veterans with multiple chronic conditions. J Gen Intern Med. 2007;22(suppl 3):403-407.
11. Nguyen-Nielsen M, Norgaard M, Jacobsen JB, et al. Comorbidity and survival of Danish prostate cancer patients from 2000-2011: a population-based cohort study. Clin Epidemiol. 2013;5(suppl 1):47-55.
12. Jang SH, Chea JW, Lee KB. Charlson comorbidity index using administrative database in incident PD patients. Clin Nephrol. 2010;73(3):204-209.
13. Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis. 2001;37(2):337-342.
14. Gross CP, Soulos PR, Ross JS, et al. Assessing the impact of screening colonoscopy on mortality in the medicare population. J Gen Intern Med. 2011;26(12):1441-1449.
15. Chouhan V, Mansoor E, Parasa S, Cooper GS. Rates of prevalent colorectal cancer occurrence in persons 75 years of age and older: a population-based national study. Dig Dis Sci. 2018;63(7):1929-1936.
16. Lee SJ, Kim CM. Individualizing prevention for older adults. J Am Geriatr Soc. 2018;66(2):229-234.
17. Tang V, Boscardin WJ, Stijacic-Cenzer I, et al. Time to benefit for colorectal cancer screening: survival meta-analysis of flexible sigmoidoscopy trials. BMJ. 2015;350:h1662.
18. Lee SJ, Boscardin WJ, Stijacic-Cenzer I, et al. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
19. Lansdorp-Vogelaar I, Gulati R, Mariotto AB, et al. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med. 2014;161(2):104-112.
20. Schoenborn NL, Bowman TL II, Cayea D, Pollack CE, Feeser S, Boyd C. Primary care practitioners’ views on incorporating long-term prognosis in the care of older adults. JAMA Intern Med. 2016;176(5):671-678.
21. Schoenborn NL, Lee K, Pollack CE, et al. Older adults’ views and communication preferences about cancer screening cessation. JAMA Intern Med. 2017;177(8):1121-1128.
22. Lewis CL, Esserman D, DeLeon C, Pignone MP, Pathman DE, Golin C. Physician decision making for colorectal cancer screening in the elderly. J Gen Intern Med. 2013;28(9):1202-1217.
23. Saini SD, Vijan S, Schoenfeld P, Powell AA, Moser S, Kerr EA. Role of quality measurement in inappropriate use of screening for colorectal cancer: retrospective cohort study. BMJ. 2014;348:g1247.
24. Walter LC, Lindquist K, Nugent S, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann Intern Med. 2009;150(7):465-473.
25. Powell AA, Saini SD, Breitenstein MK, et al. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015;30(6):732-741.
26. Pollack CE, Blackford AL, Schoenborn NL, Boyd CM, Peairs KS, DuGoff EH. Comparing prognostic tools for cancer screening: considerations for clinical practice and performance assessment. J Am Geriatr Soc. 2016;64(5):1032-1038.
27. So C, Kirby KA, Mehta K, et al. Medical center characteristics associated with PSA screening in elderly veterans with limited life expectancy. J Gen Intern Med. 2012;27(6):653-660.
28. Tang VL, Shi Y, Fung K, et al. Clinician factors associated with prostate-specific antigen screening in older veterans with limited life expectancy. JAMA Intern Med. 2016;176(5):654-661.
Probiotic Use for the Prevention of Antibiotic- Associated Clostridium difficile Infection
Clostridium difficile (C difficile) is a gram-positive, toxin-producing bacterium that is of increasing concern among health care providers and patients. Infection with C difficile can have manifestations ranging from mild diarrhea to severe toxic megacolon and can result in prolonged hospitalization with severe cases requiring admission to an intensive care unit.1 In 2014, the US was estimated to have more than 600,000 cases of C difficile infection (CDI), previously known as C difficile–associated diarrhea, and more than 44,000 associated deaths. The annual economic cost of CDI is thought to exceed $5 billion.1 According to studies of health care–associated illness, CDI rates are comparable to or have surpassed rates of methicillin-resistant Staphylococcus aureus infection within the US, including at US Department of Veterans Affairs (VA) acute care centers nationwide.2,3
C difficile has been shown to be the causative agent in 10% to 20% of antibiotic-associated diarrhea episodes.4 Colonization of C difficile is uncommon in healthy adults, but colonization rates are as high as 21% in hospitalized patients, with increasing rates proportional to increasing hospital length of stay.5,6 Although not all colonized patients develop clinically significant CDI, those who do may require multiple treatment courses, over months to years, because of the high risk of disease recurrence. An estimated 25% of patients have a single recurrent episode of CDI within 30 days after treatment completion, and 45% of those patients have additional recurrent infections.7,8 Although probiotics do not have an approved US Food and Drug Administration (FDA) indication, these supplements are often used to try to prevent CDI from developing during concomitant antibiotic use. Probiotics are microorganisms with potential health benefits, but the mechanisms of these benefits are not fully understood. Proposed mechanisms include reduced growth of pathogenic bacteria, modulation of the immune system, and support of the intestinal wall barrier.9 The many probiotic formulations currently marketed include Lactobacillus acidophilus (L acidophilus) capsules and various combinations of L acidophilus, Lactobacillus casei, Bifidobacterium lactis, Bifidobacterium longum, Streptococcus thermophilus, and other bacterial strains.
Dosing and Guidelines
Manufacturers’ suggested dosing for their Lactobacillus capsules, tablets, and packets varies from 1 unit daily to 4 units 4 times daily for dietary supplementation; the products’ labeling does not include any information regarding treatment duration.10-13 In addition, there are no published recommendations or product labeling guiding the dosing of probiotics or their duration of use in the primary prevention of CDI.
In 2017, the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) updated their CDI treatment guidelines.14 As these guidelines indicate that the data on probiotic use in CDI are inadequate, IDSA and SHEA make no recommendation for or against probiotic use in primary prevention of the disease. The guidelines point to several limitations in the literature, including variability in probiotic formulations studied, duration of probiotic administration, definitions of CDI, and duration of study follow-up.
Given the lack of consensus guidelines that clinicians can use when deciding which probiotic dosing and duration are appropriate for a patient for primary prevention of CDI, we evaluated the literature on the topic and summarize their findings here.
Review of Probiotoc Literature
Conflicting data exist about probiotics and their effect on CDI prevention. The literature reviewed was selected based on our assessment of its contribution to the topic and its potential utility to clinicians in determining appropriate probiotic therapies and recommendations. Included in our discussion is a large Cochrane Review of probiotic efficacy, 2 trials of probiotic dosing, the PLACIDE trial, and a systematic review of timely probiotic initiation. All of these studies attempted to determine the effect of probiotics on CDI incidence (Table).
In their 2017 Cochrane Review, Goldenberg and colleagues reviewed 39 trials that investigated the efficacy of probiotics in CDI prevention in 9,955 immunocompetent patients receiving antibiotics.15 The incidence of CDI was significantly lower in patients who received a probiotic than in patients who received placebo or no treatment (1.5% vs 4.0%; relative risk [RR], 0.40; 95% CI, 0.30-0.52; I2 = 0%). It is important to note that trials with a control-group CDI incidence of 0% to 2% (baseline CDI risk) found no statistically significant difference in CDI risk between patients using and not using probiotics (RR, 0.77; 95% CI, 0.45-1.32; I2 = 0%) and that the preceding statistically significant result may have been driven by the inclusion of trials with high baseline CDI risk (> 5%). Trials that enrolled patients who were at this risk level found a statistically significant 70% reduction in CDI risk in those using probiotics (vs no probiotics) while on concomitant antibiotic therapy (RR, 0.30; 95% CI, 0.21-0.42; I2 = 0%).
Probiotic therapy seems to be effective in reducing CDI risk in immunocompetent patients and may be particularly beneficial in patients at higher CDI risk, though Goldenberg and colleagues did not elaborate on what constitutes higher risk and based their conclusion on their control group’s high CDI incidence (> 5%). The most common adverse events (AEs) attributable to probiotics included abdominal cramping, nausea, fever, soft stools, flatulence, and taste disturbance. The review’s findings are limited in that the inclusion of many small trials with high baseline CDI risk likely contributed to a statistically significant result, and 17 of the review’s 39 trials were industry-sponsored or were conducted by investigators with industry associations; another 12 lacked statements about funding or sponsorship.
Two of the trials in the Cochrane Review investigated whether probiotics have a dose effect on CDI prevention. Gao and colleagues randomly assigned 255 hospitalized Asian patients to 3 groups: those receiving placebo, 1 probiotic capsule daily, and 2 probiotic capsules daily.16 Each probiotic capsule contained 50 billion colony-forming units (CFUs) of Lactobacillus. Incidence of CDI was lower in patients taking 2 probiotic capsules daily than in those taking 1 probiotic capsule daily (1.2% vs 9.4%; P = .04) or placebo (1.2% vs 23.8%; P = .002). In the other trial, Ouwehand and colleagues randomly assigned 503 hospitalized Asian patients to 3 groups as well: those receiving placebo, low-dose probiotic (4.17 billion CFUs of Lactobacillus and Bifidobacterium), and high-dose probiotic (17 billion CFUs).17 The incidence of CDI in each probiotic group (low-dose, high-dose) was 1.8%, which was significantly lower than the 4.8% in the placebo group (P = .04).
The Cochrane Review’s largest and most rigorous trial was PLACIDE, a 2013 randomized controlled study of the effect of probiotics on CDI.18 Allen and colleagues randomly assigned 2,981 inpatients (aged ≥ 65 years; exposed to antibiotics within preceding 7 days) to 2 groups: those receiving either 1 probiotic capsule daily, or 1 placebo capsule daily, for 21 days. Results showed no difference in CDI incidence between the probiotic and placebo groups (0.8% vs 1.2%; RR, 0.71; 95% CI, 0.34-1.47; P = .35). Of note, this trial is free of industry sponsorship, is the largest probiotic trial to date, has a control-group baseline CDI rate consistent with the rate in hospital and ambulatory settings in the US, and found a negative result regarding probiotic use in CDI prevention. Findings are limited in that the study allowed for initiating probiotic therapy up to 7 days after the start of antibiotics, and patients were given 1 relatively low-dose capsule daily, which may have contributed to lack of an effect on CDI prevention. No serious AEs were attributed to probiotic use.
In a 2017 systematic meta-analysis of 19 studies, Shen and colleagues investigated whether timely use of probiotics prevented CDI in 6,261 hospitalized patients receiving antibiotics.19 The incidence of CDI was significantly lower in patients receiving vs not receiving probiotics (1.6% vs 3.9%; RR, 0.42; 95% CI, 0.30-0.57; I2 = 0%; P < .001).19 A subgroup analysis was performed to compare studies initiating probiotics within 2 days after the start of antibiotics with studies initiating probiotics more than 2 days after the start. CDI risk was reduced by 68% when probiotics were started within 2 days, vs 30% when started after 2 days (RR, 0.32; 95% CI, 0.22-0.48; I2 = 0% vs RR, 0.70; 95% CI, 0.40-1.23; I2 = 0%; P = .02). Of note, no difference was found in efficacy among the various probiotic formulations, and no significant AEs were noted in any study group.
Trials included in the Cochrane Review used many different probiotic regimens over various durations.15 All these trials continued probiotics for at least the duration of antibiotic therapy. The 2 trials that evaluated the effect of probiotic therapy over an extended period required probiotics be started within 48 hours after initiation of antibiotic therapy; one trial continued probiotics for 5 days after completion of antibiotics, and the other for 7 days after completion.16,20 In both trials, CDI was statistically significantly reduced among adults using probiotics compared with adults receiving placebo.
Probiotic Safety
The FDA has not approved probiotics for the prevention or treatment of any health problems. Most probiotics are FDA-regulated as dietary supplements and do not have to meet stringent drug-approval requirements. The FDA has given many strains of common probiotics the Generally Recognized as Safe designation for use in commercially available products and foods.21-23 Probiotic use has not been associated with significant AEs in clinical trials and generally has been considered safe in immunocompetent and otherwise healthy persons.15-19 However, clinical trials have been inadequate in reporting or investigating AEs; the alternative for evaluating the risks of probiotic therapy is case reports.24,25 Theoretical risks associated with probiotics include sepsis, deleterious effects on normal gut digestion, excessive immune stimulation, and possible transfer of antimicrobial resistance genes among microorganisms.26 Boyle and colleagues further described a handful of case reports of sepsis caused by probiotics in immunocompromised individuals; the other theoretical risks have not been reported outside animal studies.26
CDI Risk Factors
Many factors can increase a patient’s CDI risk. Specific antibiotics (eg, ampicillin, amoxicillin, cephalosporins, clindamycin, fluoroquinolones) confer higher risk.27,28 Other factors include inflammatory bowel disease, organ transplantation, chemotherapy, chronic kidney disease, and immunodeficiency. Advanced age increases CDI risk and can increase the severity of infection. The evidence regarding acid suppression and CDI risk is conflicting, though a recent meta-analysis found that use of proton pump inhibitors is associated with a 2-fold higher risk of developing CDI.29 Patient-specific risk factors should be evaluated when the risk–benefit ratio for probiotic use is being considered.
Conclusion
CDIs are becoming increasingly burdensome to the health care system. More research is needed on the role of probiotics in CDI prevention in patients taking antibiotics. Given the limited risk for AEs when probiotics are used in immunocompetent patients and the relatively low cost of these supplements, the risks likely are outweighed by the postulated benefits, and probiotics may be recommended in select patient populations.
The PLACIDE trial found no benefit of probiotics in preventing CDI in a population similar to that of a typical US hospital or ambulatory setting, but its intervention allowed late initiation of relatively low doses of probiotics. Therefore, probiotics may be recommended for CDI prevention in patients taking antibiotics, especially patients at high risk for developing CDI. When clinicians recommend probiotic use in this setting, the probiotic should be initiated within 2 days after the start of antibiotics and should be continued for the duration of antibiotic therapy and for up to 7 days after that therapy is completed. Optimal probiotic dosing, likely dependent on the product used, remains unclear. PLACIDE trial results suggest that a dosage of at least 1 probiotic capsule 2 times daily may confer additional efficacy.
1. Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis. 2016;16:303.
2. Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387-390.
3. Evans ME, Kralovic SM, Simbartl LA, Jain R, Roselle GA. Effect of a Clostridium difficile infection prevention initiative in Veterans Affairs acute care facilities. Infect Control Hosp Epidemiol. 2016;37(6):720-722.
4. Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346(5):334-339.
5. Johnson S, Clabots CR, Linn FV, Olson MM, Peterson LR, Gerding DN. Nosocomial Clostridium difficile colonisation and disease. Lancet. 1990;336(8707):97-100.
6. McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204-210.
7. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769-1775.
8. Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27.
9. Sartor RB. Probiotics for gastrointestinal diseases. https://www.uptodate.com/contents/probiotics-for-gastrointestinal-diseases. Updated September 4, 2018. Accessed April 4, 2019.
10. VSL#3 (Lactobacillus) [prescribing information]. Covington, LA: Alfasigma USA Inc; July 2017.
11. Culturelle Digestive Health Probiotic Capsules (Lactobacillus) [prescribing information]. Cromwell, CT: I-Health, Inc; 2015.
12. Flora-Q (Lactobacillus) [prescribing information]. Melville, NY: PharmaDerm; May 2012.
13. Lactinex (Lactobacillus) [prescribing information]. Franklin Lakes, NJ: Becton, Dickinson and Company; 2015
14. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose–response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile–associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105(7):1636-1641.
17. Ouwehand AC, DongLian C, Weijian X, et al. Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study. Vaccine. 2014;32(4):458-463.
18. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
19. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.
20. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335(7610):80.
21. Center for Food Safety and Applied Nutrition. GRAS notice inventory. https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm. Updated September 26, 2018. Accessed November 1, 2018.
22. Mattia A, Merker R. Regulation of probiotic substances as ingredients in foods: premarket approval or “generally recognized as safe” notification. Clin Infect Dis. 2008;46(suppl 2):S115-S118.
23. Probiotics: in depth. https://nccih.nih.gov/health/probiotics/introduction.htm. Updated October 2016. Accessed January 15, 2019.
24. Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60(suppl 2):S129-S134.
25. Bafeta A, Koh M, Riveros C, Ravaud P. Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review. Ann Intern Med. 2018;169(4):240-247.
26. Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83(6):1256-1264.
27. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539-1548.
28. Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemoth. 2013;57(5):2326-2332.
29. Oshima T, Wu L, Li M, Fukui H, Watari J, Miwa H. Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: a systematic review and meta-analysis. J Gastroenterol. 2018;53(1):84-94.
Clostridium difficile (C difficile) is a gram-positive, toxin-producing bacterium that is of increasing concern among health care providers and patients. Infection with C difficile can have manifestations ranging from mild diarrhea to severe toxic megacolon and can result in prolonged hospitalization with severe cases requiring admission to an intensive care unit.1 In 2014, the US was estimated to have more than 600,000 cases of C difficile infection (CDI), previously known as C difficile–associated diarrhea, and more than 44,000 associated deaths. The annual economic cost of CDI is thought to exceed $5 billion.1 According to studies of health care–associated illness, CDI rates are comparable to or have surpassed rates of methicillin-resistant Staphylococcus aureus infection within the US, including at US Department of Veterans Affairs (VA) acute care centers nationwide.2,3
C difficile has been shown to be the causative agent in 10% to 20% of antibiotic-associated diarrhea episodes.4 Colonization of C difficile is uncommon in healthy adults, but colonization rates are as high as 21% in hospitalized patients, with increasing rates proportional to increasing hospital length of stay.5,6 Although not all colonized patients develop clinically significant CDI, those who do may require multiple treatment courses, over months to years, because of the high risk of disease recurrence. An estimated 25% of patients have a single recurrent episode of CDI within 30 days after treatment completion, and 45% of those patients have additional recurrent infections.7,8 Although probiotics do not have an approved US Food and Drug Administration (FDA) indication, these supplements are often used to try to prevent CDI from developing during concomitant antibiotic use. Probiotics are microorganisms with potential health benefits, but the mechanisms of these benefits are not fully understood. Proposed mechanisms include reduced growth of pathogenic bacteria, modulation of the immune system, and support of the intestinal wall barrier.9 The many probiotic formulations currently marketed include Lactobacillus acidophilus (L acidophilus) capsules and various combinations of L acidophilus, Lactobacillus casei, Bifidobacterium lactis, Bifidobacterium longum, Streptococcus thermophilus, and other bacterial strains.
Dosing and Guidelines
Manufacturers’ suggested dosing for their Lactobacillus capsules, tablets, and packets varies from 1 unit daily to 4 units 4 times daily for dietary supplementation; the products’ labeling does not include any information regarding treatment duration.10-13 In addition, there are no published recommendations or product labeling guiding the dosing of probiotics or their duration of use in the primary prevention of CDI.
In 2017, the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) updated their CDI treatment guidelines.14 As these guidelines indicate that the data on probiotic use in CDI are inadequate, IDSA and SHEA make no recommendation for or against probiotic use in primary prevention of the disease. The guidelines point to several limitations in the literature, including variability in probiotic formulations studied, duration of probiotic administration, definitions of CDI, and duration of study follow-up.
Given the lack of consensus guidelines that clinicians can use when deciding which probiotic dosing and duration are appropriate for a patient for primary prevention of CDI, we evaluated the literature on the topic and summarize their findings here.
Review of Probiotoc Literature
Conflicting data exist about probiotics and their effect on CDI prevention. The literature reviewed was selected based on our assessment of its contribution to the topic and its potential utility to clinicians in determining appropriate probiotic therapies and recommendations. Included in our discussion is a large Cochrane Review of probiotic efficacy, 2 trials of probiotic dosing, the PLACIDE trial, and a systematic review of timely probiotic initiation. All of these studies attempted to determine the effect of probiotics on CDI incidence (Table).
In their 2017 Cochrane Review, Goldenberg and colleagues reviewed 39 trials that investigated the efficacy of probiotics in CDI prevention in 9,955 immunocompetent patients receiving antibiotics.15 The incidence of CDI was significantly lower in patients who received a probiotic than in patients who received placebo or no treatment (1.5% vs 4.0%; relative risk [RR], 0.40; 95% CI, 0.30-0.52; I2 = 0%). It is important to note that trials with a control-group CDI incidence of 0% to 2% (baseline CDI risk) found no statistically significant difference in CDI risk between patients using and not using probiotics (RR, 0.77; 95% CI, 0.45-1.32; I2 = 0%) and that the preceding statistically significant result may have been driven by the inclusion of trials with high baseline CDI risk (> 5%). Trials that enrolled patients who were at this risk level found a statistically significant 70% reduction in CDI risk in those using probiotics (vs no probiotics) while on concomitant antibiotic therapy (RR, 0.30; 95% CI, 0.21-0.42; I2 = 0%).
Probiotic therapy seems to be effective in reducing CDI risk in immunocompetent patients and may be particularly beneficial in patients at higher CDI risk, though Goldenberg and colleagues did not elaborate on what constitutes higher risk and based their conclusion on their control group’s high CDI incidence (> 5%). The most common adverse events (AEs) attributable to probiotics included abdominal cramping, nausea, fever, soft stools, flatulence, and taste disturbance. The review’s findings are limited in that the inclusion of many small trials with high baseline CDI risk likely contributed to a statistically significant result, and 17 of the review’s 39 trials were industry-sponsored or were conducted by investigators with industry associations; another 12 lacked statements about funding or sponsorship.
Two of the trials in the Cochrane Review investigated whether probiotics have a dose effect on CDI prevention. Gao and colleagues randomly assigned 255 hospitalized Asian patients to 3 groups: those receiving placebo, 1 probiotic capsule daily, and 2 probiotic capsules daily.16 Each probiotic capsule contained 50 billion colony-forming units (CFUs) of Lactobacillus. Incidence of CDI was lower in patients taking 2 probiotic capsules daily than in those taking 1 probiotic capsule daily (1.2% vs 9.4%; P = .04) or placebo (1.2% vs 23.8%; P = .002). In the other trial, Ouwehand and colleagues randomly assigned 503 hospitalized Asian patients to 3 groups as well: those receiving placebo, low-dose probiotic (4.17 billion CFUs of Lactobacillus and Bifidobacterium), and high-dose probiotic (17 billion CFUs).17 The incidence of CDI in each probiotic group (low-dose, high-dose) was 1.8%, which was significantly lower than the 4.8% in the placebo group (P = .04).
The Cochrane Review’s largest and most rigorous trial was PLACIDE, a 2013 randomized controlled study of the effect of probiotics on CDI.18 Allen and colleagues randomly assigned 2,981 inpatients (aged ≥ 65 years; exposed to antibiotics within preceding 7 days) to 2 groups: those receiving either 1 probiotic capsule daily, or 1 placebo capsule daily, for 21 days. Results showed no difference in CDI incidence between the probiotic and placebo groups (0.8% vs 1.2%; RR, 0.71; 95% CI, 0.34-1.47; P = .35). Of note, this trial is free of industry sponsorship, is the largest probiotic trial to date, has a control-group baseline CDI rate consistent with the rate in hospital and ambulatory settings in the US, and found a negative result regarding probiotic use in CDI prevention. Findings are limited in that the study allowed for initiating probiotic therapy up to 7 days after the start of antibiotics, and patients were given 1 relatively low-dose capsule daily, which may have contributed to lack of an effect on CDI prevention. No serious AEs were attributed to probiotic use.
In a 2017 systematic meta-analysis of 19 studies, Shen and colleagues investigated whether timely use of probiotics prevented CDI in 6,261 hospitalized patients receiving antibiotics.19 The incidence of CDI was significantly lower in patients receiving vs not receiving probiotics (1.6% vs 3.9%; RR, 0.42; 95% CI, 0.30-0.57; I2 = 0%; P < .001).19 A subgroup analysis was performed to compare studies initiating probiotics within 2 days after the start of antibiotics with studies initiating probiotics more than 2 days after the start. CDI risk was reduced by 68% when probiotics were started within 2 days, vs 30% when started after 2 days (RR, 0.32; 95% CI, 0.22-0.48; I2 = 0% vs RR, 0.70; 95% CI, 0.40-1.23; I2 = 0%; P = .02). Of note, no difference was found in efficacy among the various probiotic formulations, and no significant AEs were noted in any study group.
Trials included in the Cochrane Review used many different probiotic regimens over various durations.15 All these trials continued probiotics for at least the duration of antibiotic therapy. The 2 trials that evaluated the effect of probiotic therapy over an extended period required probiotics be started within 48 hours after initiation of antibiotic therapy; one trial continued probiotics for 5 days after completion of antibiotics, and the other for 7 days after completion.16,20 In both trials, CDI was statistically significantly reduced among adults using probiotics compared with adults receiving placebo.
Probiotic Safety
The FDA has not approved probiotics for the prevention or treatment of any health problems. Most probiotics are FDA-regulated as dietary supplements and do not have to meet stringent drug-approval requirements. The FDA has given many strains of common probiotics the Generally Recognized as Safe designation for use in commercially available products and foods.21-23 Probiotic use has not been associated with significant AEs in clinical trials and generally has been considered safe in immunocompetent and otherwise healthy persons.15-19 However, clinical trials have been inadequate in reporting or investigating AEs; the alternative for evaluating the risks of probiotic therapy is case reports.24,25 Theoretical risks associated with probiotics include sepsis, deleterious effects on normal gut digestion, excessive immune stimulation, and possible transfer of antimicrobial resistance genes among microorganisms.26 Boyle and colleagues further described a handful of case reports of sepsis caused by probiotics in immunocompromised individuals; the other theoretical risks have not been reported outside animal studies.26
CDI Risk Factors
Many factors can increase a patient’s CDI risk. Specific antibiotics (eg, ampicillin, amoxicillin, cephalosporins, clindamycin, fluoroquinolones) confer higher risk.27,28 Other factors include inflammatory bowel disease, organ transplantation, chemotherapy, chronic kidney disease, and immunodeficiency. Advanced age increases CDI risk and can increase the severity of infection. The evidence regarding acid suppression and CDI risk is conflicting, though a recent meta-analysis found that use of proton pump inhibitors is associated with a 2-fold higher risk of developing CDI.29 Patient-specific risk factors should be evaluated when the risk–benefit ratio for probiotic use is being considered.
Conclusion
CDIs are becoming increasingly burdensome to the health care system. More research is needed on the role of probiotics in CDI prevention in patients taking antibiotics. Given the limited risk for AEs when probiotics are used in immunocompetent patients and the relatively low cost of these supplements, the risks likely are outweighed by the postulated benefits, and probiotics may be recommended in select patient populations.
The PLACIDE trial found no benefit of probiotics in preventing CDI in a population similar to that of a typical US hospital or ambulatory setting, but its intervention allowed late initiation of relatively low doses of probiotics. Therefore, probiotics may be recommended for CDI prevention in patients taking antibiotics, especially patients at high risk for developing CDI. When clinicians recommend probiotic use in this setting, the probiotic should be initiated within 2 days after the start of antibiotics and should be continued for the duration of antibiotic therapy and for up to 7 days after that therapy is completed. Optimal probiotic dosing, likely dependent on the product used, remains unclear. PLACIDE trial results suggest that a dosage of at least 1 probiotic capsule 2 times daily may confer additional efficacy.
Clostridium difficile (C difficile) is a gram-positive, toxin-producing bacterium that is of increasing concern among health care providers and patients. Infection with C difficile can have manifestations ranging from mild diarrhea to severe toxic megacolon and can result in prolonged hospitalization with severe cases requiring admission to an intensive care unit.1 In 2014, the US was estimated to have more than 600,000 cases of C difficile infection (CDI), previously known as C difficile–associated diarrhea, and more than 44,000 associated deaths. The annual economic cost of CDI is thought to exceed $5 billion.1 According to studies of health care–associated illness, CDI rates are comparable to or have surpassed rates of methicillin-resistant Staphylococcus aureus infection within the US, including at US Department of Veterans Affairs (VA) acute care centers nationwide.2,3
C difficile has been shown to be the causative agent in 10% to 20% of antibiotic-associated diarrhea episodes.4 Colonization of C difficile is uncommon in healthy adults, but colonization rates are as high as 21% in hospitalized patients, with increasing rates proportional to increasing hospital length of stay.5,6 Although not all colonized patients develop clinically significant CDI, those who do may require multiple treatment courses, over months to years, because of the high risk of disease recurrence. An estimated 25% of patients have a single recurrent episode of CDI within 30 days after treatment completion, and 45% of those patients have additional recurrent infections.7,8 Although probiotics do not have an approved US Food and Drug Administration (FDA) indication, these supplements are often used to try to prevent CDI from developing during concomitant antibiotic use. Probiotics are microorganisms with potential health benefits, but the mechanisms of these benefits are not fully understood. Proposed mechanisms include reduced growth of pathogenic bacteria, modulation of the immune system, and support of the intestinal wall barrier.9 The many probiotic formulations currently marketed include Lactobacillus acidophilus (L acidophilus) capsules and various combinations of L acidophilus, Lactobacillus casei, Bifidobacterium lactis, Bifidobacterium longum, Streptococcus thermophilus, and other bacterial strains.
Dosing and Guidelines
Manufacturers’ suggested dosing for their Lactobacillus capsules, tablets, and packets varies from 1 unit daily to 4 units 4 times daily for dietary supplementation; the products’ labeling does not include any information regarding treatment duration.10-13 In addition, there are no published recommendations or product labeling guiding the dosing of probiotics or their duration of use in the primary prevention of CDI.
In 2017, the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) updated their CDI treatment guidelines.14 As these guidelines indicate that the data on probiotic use in CDI are inadequate, IDSA and SHEA make no recommendation for or against probiotic use in primary prevention of the disease. The guidelines point to several limitations in the literature, including variability in probiotic formulations studied, duration of probiotic administration, definitions of CDI, and duration of study follow-up.
Given the lack of consensus guidelines that clinicians can use when deciding which probiotic dosing and duration are appropriate for a patient for primary prevention of CDI, we evaluated the literature on the topic and summarize their findings here.
Review of Probiotoc Literature
Conflicting data exist about probiotics and their effect on CDI prevention. The literature reviewed was selected based on our assessment of its contribution to the topic and its potential utility to clinicians in determining appropriate probiotic therapies and recommendations. Included in our discussion is a large Cochrane Review of probiotic efficacy, 2 trials of probiotic dosing, the PLACIDE trial, and a systematic review of timely probiotic initiation. All of these studies attempted to determine the effect of probiotics on CDI incidence (Table).
In their 2017 Cochrane Review, Goldenberg and colleagues reviewed 39 trials that investigated the efficacy of probiotics in CDI prevention in 9,955 immunocompetent patients receiving antibiotics.15 The incidence of CDI was significantly lower in patients who received a probiotic than in patients who received placebo or no treatment (1.5% vs 4.0%; relative risk [RR], 0.40; 95% CI, 0.30-0.52; I2 = 0%). It is important to note that trials with a control-group CDI incidence of 0% to 2% (baseline CDI risk) found no statistically significant difference in CDI risk between patients using and not using probiotics (RR, 0.77; 95% CI, 0.45-1.32; I2 = 0%) and that the preceding statistically significant result may have been driven by the inclusion of trials with high baseline CDI risk (> 5%). Trials that enrolled patients who were at this risk level found a statistically significant 70% reduction in CDI risk in those using probiotics (vs no probiotics) while on concomitant antibiotic therapy (RR, 0.30; 95% CI, 0.21-0.42; I2 = 0%).
Probiotic therapy seems to be effective in reducing CDI risk in immunocompetent patients and may be particularly beneficial in patients at higher CDI risk, though Goldenberg and colleagues did not elaborate on what constitutes higher risk and based their conclusion on their control group’s high CDI incidence (> 5%). The most common adverse events (AEs) attributable to probiotics included abdominal cramping, nausea, fever, soft stools, flatulence, and taste disturbance. The review’s findings are limited in that the inclusion of many small trials with high baseline CDI risk likely contributed to a statistically significant result, and 17 of the review’s 39 trials were industry-sponsored or were conducted by investigators with industry associations; another 12 lacked statements about funding or sponsorship.
Two of the trials in the Cochrane Review investigated whether probiotics have a dose effect on CDI prevention. Gao and colleagues randomly assigned 255 hospitalized Asian patients to 3 groups: those receiving placebo, 1 probiotic capsule daily, and 2 probiotic capsules daily.16 Each probiotic capsule contained 50 billion colony-forming units (CFUs) of Lactobacillus. Incidence of CDI was lower in patients taking 2 probiotic capsules daily than in those taking 1 probiotic capsule daily (1.2% vs 9.4%; P = .04) or placebo (1.2% vs 23.8%; P = .002). In the other trial, Ouwehand and colleagues randomly assigned 503 hospitalized Asian patients to 3 groups as well: those receiving placebo, low-dose probiotic (4.17 billion CFUs of Lactobacillus and Bifidobacterium), and high-dose probiotic (17 billion CFUs).17 The incidence of CDI in each probiotic group (low-dose, high-dose) was 1.8%, which was significantly lower than the 4.8% in the placebo group (P = .04).
The Cochrane Review’s largest and most rigorous trial was PLACIDE, a 2013 randomized controlled study of the effect of probiotics on CDI.18 Allen and colleagues randomly assigned 2,981 inpatients (aged ≥ 65 years; exposed to antibiotics within preceding 7 days) to 2 groups: those receiving either 1 probiotic capsule daily, or 1 placebo capsule daily, for 21 days. Results showed no difference in CDI incidence between the probiotic and placebo groups (0.8% vs 1.2%; RR, 0.71; 95% CI, 0.34-1.47; P = .35). Of note, this trial is free of industry sponsorship, is the largest probiotic trial to date, has a control-group baseline CDI rate consistent with the rate in hospital and ambulatory settings in the US, and found a negative result regarding probiotic use in CDI prevention. Findings are limited in that the study allowed for initiating probiotic therapy up to 7 days after the start of antibiotics, and patients were given 1 relatively low-dose capsule daily, which may have contributed to lack of an effect on CDI prevention. No serious AEs were attributed to probiotic use.
In a 2017 systematic meta-analysis of 19 studies, Shen and colleagues investigated whether timely use of probiotics prevented CDI in 6,261 hospitalized patients receiving antibiotics.19 The incidence of CDI was significantly lower in patients receiving vs not receiving probiotics (1.6% vs 3.9%; RR, 0.42; 95% CI, 0.30-0.57; I2 = 0%; P < .001).19 A subgroup analysis was performed to compare studies initiating probiotics within 2 days after the start of antibiotics with studies initiating probiotics more than 2 days after the start. CDI risk was reduced by 68% when probiotics were started within 2 days, vs 30% when started after 2 days (RR, 0.32; 95% CI, 0.22-0.48; I2 = 0% vs RR, 0.70; 95% CI, 0.40-1.23; I2 = 0%; P = .02). Of note, no difference was found in efficacy among the various probiotic formulations, and no significant AEs were noted in any study group.
Trials included in the Cochrane Review used many different probiotic regimens over various durations.15 All these trials continued probiotics for at least the duration of antibiotic therapy. The 2 trials that evaluated the effect of probiotic therapy over an extended period required probiotics be started within 48 hours after initiation of antibiotic therapy; one trial continued probiotics for 5 days after completion of antibiotics, and the other for 7 days after completion.16,20 In both trials, CDI was statistically significantly reduced among adults using probiotics compared with adults receiving placebo.
Probiotic Safety
The FDA has not approved probiotics for the prevention or treatment of any health problems. Most probiotics are FDA-regulated as dietary supplements and do not have to meet stringent drug-approval requirements. The FDA has given many strains of common probiotics the Generally Recognized as Safe designation for use in commercially available products and foods.21-23 Probiotic use has not been associated with significant AEs in clinical trials and generally has been considered safe in immunocompetent and otherwise healthy persons.15-19 However, clinical trials have been inadequate in reporting or investigating AEs; the alternative for evaluating the risks of probiotic therapy is case reports.24,25 Theoretical risks associated with probiotics include sepsis, deleterious effects on normal gut digestion, excessive immune stimulation, and possible transfer of antimicrobial resistance genes among microorganisms.26 Boyle and colleagues further described a handful of case reports of sepsis caused by probiotics in immunocompromised individuals; the other theoretical risks have not been reported outside animal studies.26
CDI Risk Factors
Many factors can increase a patient’s CDI risk. Specific antibiotics (eg, ampicillin, amoxicillin, cephalosporins, clindamycin, fluoroquinolones) confer higher risk.27,28 Other factors include inflammatory bowel disease, organ transplantation, chemotherapy, chronic kidney disease, and immunodeficiency. Advanced age increases CDI risk and can increase the severity of infection. The evidence regarding acid suppression and CDI risk is conflicting, though a recent meta-analysis found that use of proton pump inhibitors is associated with a 2-fold higher risk of developing CDI.29 Patient-specific risk factors should be evaluated when the risk–benefit ratio for probiotic use is being considered.
Conclusion
CDIs are becoming increasingly burdensome to the health care system. More research is needed on the role of probiotics in CDI prevention in patients taking antibiotics. Given the limited risk for AEs when probiotics are used in immunocompetent patients and the relatively low cost of these supplements, the risks likely are outweighed by the postulated benefits, and probiotics may be recommended in select patient populations.
The PLACIDE trial found no benefit of probiotics in preventing CDI in a population similar to that of a typical US hospital or ambulatory setting, but its intervention allowed late initiation of relatively low doses of probiotics. Therefore, probiotics may be recommended for CDI prevention in patients taking antibiotics, especially patients at high risk for developing CDI. When clinicians recommend probiotic use in this setting, the probiotic should be initiated within 2 days after the start of antibiotics and should be continued for the duration of antibiotic therapy and for up to 7 days after that therapy is completed. Optimal probiotic dosing, likely dependent on the product used, remains unclear. PLACIDE trial results suggest that a dosage of at least 1 probiotic capsule 2 times daily may confer additional efficacy.
1. Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis. 2016;16:303.
2. Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387-390.
3. Evans ME, Kralovic SM, Simbartl LA, Jain R, Roselle GA. Effect of a Clostridium difficile infection prevention initiative in Veterans Affairs acute care facilities. Infect Control Hosp Epidemiol. 2016;37(6):720-722.
4. Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346(5):334-339.
5. Johnson S, Clabots CR, Linn FV, Olson MM, Peterson LR, Gerding DN. Nosocomial Clostridium difficile colonisation and disease. Lancet. 1990;336(8707):97-100.
6. McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204-210.
7. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769-1775.
8. Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27.
9. Sartor RB. Probiotics for gastrointestinal diseases. https://www.uptodate.com/contents/probiotics-for-gastrointestinal-diseases. Updated September 4, 2018. Accessed April 4, 2019.
10. VSL#3 (Lactobacillus) [prescribing information]. Covington, LA: Alfasigma USA Inc; July 2017.
11. Culturelle Digestive Health Probiotic Capsules (Lactobacillus) [prescribing information]. Cromwell, CT: I-Health, Inc; 2015.
12. Flora-Q (Lactobacillus) [prescribing information]. Melville, NY: PharmaDerm; May 2012.
13. Lactinex (Lactobacillus) [prescribing information]. Franklin Lakes, NJ: Becton, Dickinson and Company; 2015
14. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose–response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile–associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105(7):1636-1641.
17. Ouwehand AC, DongLian C, Weijian X, et al. Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study. Vaccine. 2014;32(4):458-463.
18. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
19. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.
20. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335(7610):80.
21. Center for Food Safety and Applied Nutrition. GRAS notice inventory. https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm. Updated September 26, 2018. Accessed November 1, 2018.
22. Mattia A, Merker R. Regulation of probiotic substances as ingredients in foods: premarket approval or “generally recognized as safe” notification. Clin Infect Dis. 2008;46(suppl 2):S115-S118.
23. Probiotics: in depth. https://nccih.nih.gov/health/probiotics/introduction.htm. Updated October 2016. Accessed January 15, 2019.
24. Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60(suppl 2):S129-S134.
25. Bafeta A, Koh M, Riveros C, Ravaud P. Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review. Ann Intern Med. 2018;169(4):240-247.
26. Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83(6):1256-1264.
27. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539-1548.
28. Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemoth. 2013;57(5):2326-2332.
29. Oshima T, Wu L, Li M, Fukui H, Watari J, Miwa H. Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: a systematic review and meta-analysis. J Gastroenterol. 2018;53(1):84-94.
1. Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis. 2016;16:303.
2. Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387-390.
3. Evans ME, Kralovic SM, Simbartl LA, Jain R, Roselle GA. Effect of a Clostridium difficile infection prevention initiative in Veterans Affairs acute care facilities. Infect Control Hosp Epidemiol. 2016;37(6):720-722.
4. Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346(5):334-339.
5. Johnson S, Clabots CR, Linn FV, Olson MM, Peterson LR, Gerding DN. Nosocomial Clostridium difficile colonisation and disease. Lancet. 1990;336(8707):97-100.
6. McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204-210.
7. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769-1775.
8. Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27.
9. Sartor RB. Probiotics for gastrointestinal diseases. https://www.uptodate.com/contents/probiotics-for-gastrointestinal-diseases. Updated September 4, 2018. Accessed April 4, 2019.
10. VSL#3 (Lactobacillus) [prescribing information]. Covington, LA: Alfasigma USA Inc; July 2017.
11. Culturelle Digestive Health Probiotic Capsules (Lactobacillus) [prescribing information]. Cromwell, CT: I-Health, Inc; 2015.
12. Flora-Q (Lactobacillus) [prescribing information]. Melville, NY: PharmaDerm; May 2012.
13. Lactinex (Lactobacillus) [prescribing information]. Franklin Lakes, NJ: Becton, Dickinson and Company; 2015
14. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile–associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose–response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile–associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105(7):1636-1641.
17. Ouwehand AC, DongLian C, Weijian X, et al. Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study. Vaccine. 2014;32(4):458-463.
18. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
19. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.
20. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335(7610):80.
21. Center for Food Safety and Applied Nutrition. GRAS notice inventory. https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm. Updated September 26, 2018. Accessed November 1, 2018.
22. Mattia A, Merker R. Regulation of probiotic substances as ingredients in foods: premarket approval or “generally recognized as safe” notification. Clin Infect Dis. 2008;46(suppl 2):S115-S118.
23. Probiotics: in depth. https://nccih.nih.gov/health/probiotics/introduction.htm. Updated October 2016. Accessed January 15, 2019.
24. Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60(suppl 2):S129-S134.
25. Bafeta A, Koh M, Riveros C, Ravaud P. Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review. Ann Intern Med. 2018;169(4):240-247.
26. Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83(6):1256-1264.
27. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539-1548.
28. Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemoth. 2013;57(5):2326-2332.
29. Oshima T, Wu L, Li M, Fukui H, Watari J, Miwa H. Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: a systematic review and meta-analysis. J Gastroenterol. 2018;53(1):84-94.
Systematic review indicates cutaneous laser therapy may be safe during pregnancy
according to the results of a systematic review of 22 studies.
Among 380 women in all trimesters of pregnancy who were treated with various laser wavelengths, the only clinically significant event was a case of premature rupture of membranes (PROM) “without further morbidity,” wrote Eric C. Wilkerson, MD, of Skin Laser & Surgery Specialists of NY and NJ in New York, and associates. In that case, the cause was not clear, there was no further morbidity, “and it was uncertain whether this was related to the laser procedure.”
However, only 22 studies were identified between 1960 and 2017, all of which were case reports or series, published from 1994 to 2015. “[Thus far,] the best evidence exists for the safety of the carbon dioxide laser, particularly in the treatment of condyloma,” they wrote in Dermatologic Surgery.
Elective laser treatments are usually not recommended during pregnancy, but no evidence supports this, Dr. Wilkerson and coauthors wrote. Therefore, they searched for studies indexed in PubMed, Google Scholar, the Cochrane Library, or the EBSCO CINAHL Plus Database from 1960 to 2017. They also searched LexisNexis for relevant legal cases, but found none.
The women in the 22 case reports and series were aged 14-41 years and received laser therapy for cervical adenocarcinoma, urolithiasis, condyloma acuminata, cervical carcinoma in situ, cutaneous scarring, Buschke-Löwenstein tumor, verrucous carcinoma, and acne vulgaris. Modalities included 504-nm pulsed-dye laser, 532-nm potassium titanyl phosphate, 1,064-nm neodymium:YAG, 2,100-nm holmium:YAG, and 10,600-nm CO2.
Apart from the case of PROM, there were no instances of fetal morbidity or mortality, premature labor or preterm birth, or detectable fetal stress, the authors wrote. The case of PROM occurred at 35 weeks, 4 days after the mother had received CO2 laser therapy for condyloma acuminata. She delivered normally approximately 1 week later. There also were several cases of premature contractions without true labor, all of which responded to tocolytic therapy. (In the same study, there also were two cases of PROM in women 7 and 10 weeks after the same procedure, but were thought to be unrelated.)
The thickness of the pregnant abdomen, uterus, and amniotic fluid makes it “very unlikely” that clinically significant amounts of laser energy would reach the fetus during cutaneous laser therapy, the authors noted. Certain topical anesthetics, such as lidocaine and prilocaine, also appear safe during pregnancy “and may potentially decrease concern for fetal stress secondary to maternal stress or pain during the procedure,” they added. “Appropriate safety measures including eye protection and laser plume management should continue to be used during laser treatment.”
The authors reported no funding sources or conflicts of interest.
SOURCE: Wilkerson EJ et al. Dermatol Surg. 2019 Jun;45(6):818-28.
according to the results of a systematic review of 22 studies.
Among 380 women in all trimesters of pregnancy who were treated with various laser wavelengths, the only clinically significant event was a case of premature rupture of membranes (PROM) “without further morbidity,” wrote Eric C. Wilkerson, MD, of Skin Laser & Surgery Specialists of NY and NJ in New York, and associates. In that case, the cause was not clear, there was no further morbidity, “and it was uncertain whether this was related to the laser procedure.”
However, only 22 studies were identified between 1960 and 2017, all of which were case reports or series, published from 1994 to 2015. “[Thus far,] the best evidence exists for the safety of the carbon dioxide laser, particularly in the treatment of condyloma,” they wrote in Dermatologic Surgery.
Elective laser treatments are usually not recommended during pregnancy, but no evidence supports this, Dr. Wilkerson and coauthors wrote. Therefore, they searched for studies indexed in PubMed, Google Scholar, the Cochrane Library, or the EBSCO CINAHL Plus Database from 1960 to 2017. They also searched LexisNexis for relevant legal cases, but found none.
The women in the 22 case reports and series were aged 14-41 years and received laser therapy for cervical adenocarcinoma, urolithiasis, condyloma acuminata, cervical carcinoma in situ, cutaneous scarring, Buschke-Löwenstein tumor, verrucous carcinoma, and acne vulgaris. Modalities included 504-nm pulsed-dye laser, 532-nm potassium titanyl phosphate, 1,064-nm neodymium:YAG, 2,100-nm holmium:YAG, and 10,600-nm CO2.
Apart from the case of PROM, there were no instances of fetal morbidity or mortality, premature labor or preterm birth, or detectable fetal stress, the authors wrote. The case of PROM occurred at 35 weeks, 4 days after the mother had received CO2 laser therapy for condyloma acuminata. She delivered normally approximately 1 week later. There also were several cases of premature contractions without true labor, all of which responded to tocolytic therapy. (In the same study, there also were two cases of PROM in women 7 and 10 weeks after the same procedure, but were thought to be unrelated.)
The thickness of the pregnant abdomen, uterus, and amniotic fluid makes it “very unlikely” that clinically significant amounts of laser energy would reach the fetus during cutaneous laser therapy, the authors noted. Certain topical anesthetics, such as lidocaine and prilocaine, also appear safe during pregnancy “and may potentially decrease concern for fetal stress secondary to maternal stress or pain during the procedure,” they added. “Appropriate safety measures including eye protection and laser plume management should continue to be used during laser treatment.”
The authors reported no funding sources or conflicts of interest.
SOURCE: Wilkerson EJ et al. Dermatol Surg. 2019 Jun;45(6):818-28.
according to the results of a systematic review of 22 studies.
Among 380 women in all trimesters of pregnancy who were treated with various laser wavelengths, the only clinically significant event was a case of premature rupture of membranes (PROM) “without further morbidity,” wrote Eric C. Wilkerson, MD, of Skin Laser & Surgery Specialists of NY and NJ in New York, and associates. In that case, the cause was not clear, there was no further morbidity, “and it was uncertain whether this was related to the laser procedure.”
However, only 22 studies were identified between 1960 and 2017, all of which were case reports or series, published from 1994 to 2015. “[Thus far,] the best evidence exists for the safety of the carbon dioxide laser, particularly in the treatment of condyloma,” they wrote in Dermatologic Surgery.
Elective laser treatments are usually not recommended during pregnancy, but no evidence supports this, Dr. Wilkerson and coauthors wrote. Therefore, they searched for studies indexed in PubMed, Google Scholar, the Cochrane Library, or the EBSCO CINAHL Plus Database from 1960 to 2017. They also searched LexisNexis for relevant legal cases, but found none.
The women in the 22 case reports and series were aged 14-41 years and received laser therapy for cervical adenocarcinoma, urolithiasis, condyloma acuminata, cervical carcinoma in situ, cutaneous scarring, Buschke-Löwenstein tumor, verrucous carcinoma, and acne vulgaris. Modalities included 504-nm pulsed-dye laser, 532-nm potassium titanyl phosphate, 1,064-nm neodymium:YAG, 2,100-nm holmium:YAG, and 10,600-nm CO2.
Apart from the case of PROM, there were no instances of fetal morbidity or mortality, premature labor or preterm birth, or detectable fetal stress, the authors wrote. The case of PROM occurred at 35 weeks, 4 days after the mother had received CO2 laser therapy for condyloma acuminata. She delivered normally approximately 1 week later. There also were several cases of premature contractions without true labor, all of which responded to tocolytic therapy. (In the same study, there also were two cases of PROM in women 7 and 10 weeks after the same procedure, but were thought to be unrelated.)
The thickness of the pregnant abdomen, uterus, and amniotic fluid makes it “very unlikely” that clinically significant amounts of laser energy would reach the fetus during cutaneous laser therapy, the authors noted. Certain topical anesthetics, such as lidocaine and prilocaine, also appear safe during pregnancy “and may potentially decrease concern for fetal stress secondary to maternal stress or pain during the procedure,” they added. “Appropriate safety measures including eye protection and laser plume management should continue to be used during laser treatment.”
The authors reported no funding sources or conflicts of interest.
SOURCE: Wilkerson EJ et al. Dermatol Surg. 2019 Jun;45(6):818-28.
FROM DERMATOLOGIC SURGERY
Why you should re-credential with Medicare as a hospitalist
CMS needs a better database of hospitalist information
In April 2017, the Centers for Medicare and Medicaid Services implemented the new physician specialty code C6, specifically for hospitalists. There has been a lot of confusion about what this means and some uncertainty about why clinicians should bother to use it.
Some folks thought initially that it was a new CPT code they could use to bill hospitalist services, which might recognize the increased intensity of services hospitalists often provide to their hospitalized patients compared to many traditional internal medicine and family medicine primary care physicians. Others thought it was a code that was added to the HCFA 1500 billing form somewhere to designate that the service was provided by a hospitalist.
Neither is true. The C6 physician specialty code is one of a large number of such codes used by physicians to designate their primary physician specialty when they enroll with Medicare via the PECOS online enrollment system. It describes the unique type of medicine practiced by the enrolling physician and is used by the CMS both for claims processing purposes and for “programmatic” purposes (whatever that means).
It doesn’t change how your claim is processed or how much you get paid. So why bother going through the laborious process of re-credentialing with CMS via PECOS just to change your specialty code? Well, I believe there are several ways in which the C6 specialty code provides value – both to you and to the specialty of hospital medicine.
Reduce concurrent care denials
First, it distinguishes you from a general internal medicine or general family medicine practitioner by recognizing “hospitalist” as a distinct specialty. This can be valuable from a financial perspective because it may reduce the risk that claims for your services might be denied due to “concurrent care” by another provider in the same specialty on the same calendar day.
And it’s not just a general internist or family medicine physician that you might run into concurrent care trouble with. I’ve seen situations where doctors completed critical care or cardiology fellowships but never got around to re-credentialing with Medicare in their new specialty, so their claims still showed up with an “internal medicine” physician specialty code, resulting in denied “concurrent care” claims for either the hospitalist or the specialist.
While Medicare may still see unnecessary overlap between services provided by you and an internal medicine or family physician to the same patient on the same calendar day, you can make a better argument that your services were unique and complementary to (not duplicative of) the services of others if you are credentialed as a hospitalist.
Ensure “apples to apples” comparisons
A second reason to re-credential as a hospitalist is to ensure that when the CMS looks at the services you are providing and the CPT codes you are selecting, it is comparing you to an appropriate peer group for compliance purposes.
The mix of CPT codes reported by hospitalists in the SHM State of Hospital Medicine Survey has historically tilted toward higher-level care than has the mix of CPT codes reported by the CMS for internal medicine or family medicine physicians. But last year when Medicare released the utilization of evaluation and management services by specialty for calendar year 2017, CPT utilization was shown separately for hospitalists for the first time!
The volume of services reported for physicians credentialed as hospitalists was very small relative to the volume of inpatient services provided by internal medicine and family medicine physicians, but the distribution of inpatient admission, subsequent visit, and discharge codes for hospitalists closely mirrored those reported by SHM in its 2018 State of Hospital Medicine Report (see graphic).
If you’re going to be targeted in a RAC audit for the high proportion of 99233s you bill, you want to be sure the CMS is looking at your performance compared to those who are truly your peers, caring for patients of the same type and complexity.
Improve CMS data used for research purposes
Finally, the ability of academic hospitalists and other health services researchers to utilize Medicare claims data to better understand the care provided by hospitalists and its impact on the overall health care system will be significantly enhanced by a more robust presence of physicians who have identified themselves as hospitalists in the PECOS credentialing system.
We care for the majority of patients in most hospitals these days, yet “hospitalists” billed only 2,009,869 inpatient subsequent visits (CPT codes 99231, 99232, and 99233) in 2017 compared to 25,903,829 billed by internal medicine physicians and 4,678,111 billed by family medicine physicians. And regardless of what you think about using claims data as a proxy for health care services and quality, it’s undeniably the best data set we currently have.
So, let’s work together to build a bigger, better database of hospitalist information at the CMS. I urge you to go to your credentialing folks today and find out how you can work with them to get yourself re-credentialed in PECOS using the C6 “hospitalist” physician specialty.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Meeting Committees, and helps to coordinate SHM’s bi-annual State of Hospital Medicine Survey. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
CMS needs a better database of hospitalist information
CMS needs a better database of hospitalist information
In April 2017, the Centers for Medicare and Medicaid Services implemented the new physician specialty code C6, specifically for hospitalists. There has been a lot of confusion about what this means and some uncertainty about why clinicians should bother to use it.
Some folks thought initially that it was a new CPT code they could use to bill hospitalist services, which might recognize the increased intensity of services hospitalists often provide to their hospitalized patients compared to many traditional internal medicine and family medicine primary care physicians. Others thought it was a code that was added to the HCFA 1500 billing form somewhere to designate that the service was provided by a hospitalist.
Neither is true. The C6 physician specialty code is one of a large number of such codes used by physicians to designate their primary physician specialty when they enroll with Medicare via the PECOS online enrollment system. It describes the unique type of medicine practiced by the enrolling physician and is used by the CMS both for claims processing purposes and for “programmatic” purposes (whatever that means).
It doesn’t change how your claim is processed or how much you get paid. So why bother going through the laborious process of re-credentialing with CMS via PECOS just to change your specialty code? Well, I believe there are several ways in which the C6 specialty code provides value – both to you and to the specialty of hospital medicine.
Reduce concurrent care denials
First, it distinguishes you from a general internal medicine or general family medicine practitioner by recognizing “hospitalist” as a distinct specialty. This can be valuable from a financial perspective because it may reduce the risk that claims for your services might be denied due to “concurrent care” by another provider in the same specialty on the same calendar day.
And it’s not just a general internist or family medicine physician that you might run into concurrent care trouble with. I’ve seen situations where doctors completed critical care or cardiology fellowships but never got around to re-credentialing with Medicare in their new specialty, so their claims still showed up with an “internal medicine” physician specialty code, resulting in denied “concurrent care” claims for either the hospitalist or the specialist.
While Medicare may still see unnecessary overlap between services provided by you and an internal medicine or family physician to the same patient on the same calendar day, you can make a better argument that your services were unique and complementary to (not duplicative of) the services of others if you are credentialed as a hospitalist.
Ensure “apples to apples” comparisons
A second reason to re-credential as a hospitalist is to ensure that when the CMS looks at the services you are providing and the CPT codes you are selecting, it is comparing you to an appropriate peer group for compliance purposes.
The mix of CPT codes reported by hospitalists in the SHM State of Hospital Medicine Survey has historically tilted toward higher-level care than has the mix of CPT codes reported by the CMS for internal medicine or family medicine physicians. But last year when Medicare released the utilization of evaluation and management services by specialty for calendar year 2017, CPT utilization was shown separately for hospitalists for the first time!
The volume of services reported for physicians credentialed as hospitalists was very small relative to the volume of inpatient services provided by internal medicine and family medicine physicians, but the distribution of inpatient admission, subsequent visit, and discharge codes for hospitalists closely mirrored those reported by SHM in its 2018 State of Hospital Medicine Report (see graphic).
If you’re going to be targeted in a RAC audit for the high proportion of 99233s you bill, you want to be sure the CMS is looking at your performance compared to those who are truly your peers, caring for patients of the same type and complexity.
Improve CMS data used for research purposes
Finally, the ability of academic hospitalists and other health services researchers to utilize Medicare claims data to better understand the care provided by hospitalists and its impact on the overall health care system will be significantly enhanced by a more robust presence of physicians who have identified themselves as hospitalists in the PECOS credentialing system.
We care for the majority of patients in most hospitals these days, yet “hospitalists” billed only 2,009,869 inpatient subsequent visits (CPT codes 99231, 99232, and 99233) in 2017 compared to 25,903,829 billed by internal medicine physicians and 4,678,111 billed by family medicine physicians. And regardless of what you think about using claims data as a proxy for health care services and quality, it’s undeniably the best data set we currently have.
So, let’s work together to build a bigger, better database of hospitalist information at the CMS. I urge you to go to your credentialing folks today and find out how you can work with them to get yourself re-credentialed in PECOS using the C6 “hospitalist” physician specialty.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Meeting Committees, and helps to coordinate SHM’s bi-annual State of Hospital Medicine Survey. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
In April 2017, the Centers for Medicare and Medicaid Services implemented the new physician specialty code C6, specifically for hospitalists. There has been a lot of confusion about what this means and some uncertainty about why clinicians should bother to use it.
Some folks thought initially that it was a new CPT code they could use to bill hospitalist services, which might recognize the increased intensity of services hospitalists often provide to their hospitalized patients compared to many traditional internal medicine and family medicine primary care physicians. Others thought it was a code that was added to the HCFA 1500 billing form somewhere to designate that the service was provided by a hospitalist.
Neither is true. The C6 physician specialty code is one of a large number of such codes used by physicians to designate their primary physician specialty when they enroll with Medicare via the PECOS online enrollment system. It describes the unique type of medicine practiced by the enrolling physician and is used by the CMS both for claims processing purposes and for “programmatic” purposes (whatever that means).
It doesn’t change how your claim is processed or how much you get paid. So why bother going through the laborious process of re-credentialing with CMS via PECOS just to change your specialty code? Well, I believe there are several ways in which the C6 specialty code provides value – both to you and to the specialty of hospital medicine.
Reduce concurrent care denials
First, it distinguishes you from a general internal medicine or general family medicine practitioner by recognizing “hospitalist” as a distinct specialty. This can be valuable from a financial perspective because it may reduce the risk that claims for your services might be denied due to “concurrent care” by another provider in the same specialty on the same calendar day.
And it’s not just a general internist or family medicine physician that you might run into concurrent care trouble with. I’ve seen situations where doctors completed critical care or cardiology fellowships but never got around to re-credentialing with Medicare in their new specialty, so their claims still showed up with an “internal medicine” physician specialty code, resulting in denied “concurrent care” claims for either the hospitalist or the specialist.
While Medicare may still see unnecessary overlap between services provided by you and an internal medicine or family physician to the same patient on the same calendar day, you can make a better argument that your services were unique and complementary to (not duplicative of) the services of others if you are credentialed as a hospitalist.
Ensure “apples to apples” comparisons
A second reason to re-credential as a hospitalist is to ensure that when the CMS looks at the services you are providing and the CPT codes you are selecting, it is comparing you to an appropriate peer group for compliance purposes.
The mix of CPT codes reported by hospitalists in the SHM State of Hospital Medicine Survey has historically tilted toward higher-level care than has the mix of CPT codes reported by the CMS for internal medicine or family medicine physicians. But last year when Medicare released the utilization of evaluation and management services by specialty for calendar year 2017, CPT utilization was shown separately for hospitalists for the first time!
The volume of services reported for physicians credentialed as hospitalists was very small relative to the volume of inpatient services provided by internal medicine and family medicine physicians, but the distribution of inpatient admission, subsequent visit, and discharge codes for hospitalists closely mirrored those reported by SHM in its 2018 State of Hospital Medicine Report (see graphic).
If you’re going to be targeted in a RAC audit for the high proportion of 99233s you bill, you want to be sure the CMS is looking at your performance compared to those who are truly your peers, caring for patients of the same type and complexity.
Improve CMS data used for research purposes
Finally, the ability of academic hospitalists and other health services researchers to utilize Medicare claims data to better understand the care provided by hospitalists and its impact on the overall health care system will be significantly enhanced by a more robust presence of physicians who have identified themselves as hospitalists in the PECOS credentialing system.
We care for the majority of patients in most hospitals these days, yet “hospitalists” billed only 2,009,869 inpatient subsequent visits (CPT codes 99231, 99232, and 99233) in 2017 compared to 25,903,829 billed by internal medicine physicians and 4,678,111 billed by family medicine physicians. And regardless of what you think about using claims data as a proxy for health care services and quality, it’s undeniably the best data set we currently have.
So, let’s work together to build a bigger, better database of hospitalist information at the CMS. I urge you to go to your credentialing folks today and find out how you can work with them to get yourself re-credentialed in PECOS using the C6 “hospitalist” physician specialty.
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Meeting Committees, and helps to coordinate SHM’s bi-annual State of Hospital Medicine Survey. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
Linear Vulvar Lesions
The Diagnosis: Vestibular Papillomatosis
Vestibular papillomatosis (VP), the female equivalent of pearly penile papules, is characterized by multiple papules in a linear array on the labia minora and is considered a normal anatomic variant. It typically presents as monomorphous, soft, flesh-colored, filiform papules that are distributed in a symmetric fashion. In women, the papules present as linear arrays on the inner aspects of the labia minora, whereas in men, they present in a circumferential array along the sulcus of the glans penis.1 Lesions often are asymptomatic but may cause itching, burning, and dyspareunia.2 Previously believed to be associated with human papillomavirus infection,3 VP is now considered a noninfectious condition. Biopsy reveals parakeratosis and perinuclear vacuolization in the absence of true koilocytes.4,5 Dermoscopy and reflectance confocal microscopy have been used to differentiate VP from clinically similar lesions (eg, condyloma acuminatum).6,7 The prevalence of this condition is not well established; however, one study found VP in 1% of women attending genitourinary medicine clinics.3
Condyloma acuminatum, known colloquially as genital warts, is a human papillomavirus infection. Lesions tend to be painless and firm and are distributed asymmetrically with a cauliflowerlike appearance.1 Condyloma latum, found in secondary syphilis, is characterized by papules that are pale, smooth, flat topped, and moist.8 Molluscum contagiosum is an infection caused by a poxvirus presenting with flesh-colored, dome-shaped papules with central umbilication.9 The lesions of papulosquamous lichen planus are violaceous polygonal papules that affect the clitoral hood and labia minora and may cause pruritus. The cause of lichen planus is unknown; however, clinically similar lesions may occur in a lichenoid drug eruption due to certain medications.
Vestibular papillomatosis typically does not require treatment, except in symptomatic cases. To date, limited studies have reported variable treatment success utilizing destructive techniques such as CO2 laser or topical application of 5-fluorouracil or trichloroacetic acid.10
The lesions on our patient's left medial labia minora were successfully treated with low-voltage (3.0 V) electrodesiccation. Following local anesthesia with 1% lidocaine, each papule was gently electrodesiccated utilizing a standard hyfrecation electrode tip to a light gray discoloration. Postprocedural care involved only twice-daily cleansing with a gentle soap and application of petrolatum. The patient tolerated the procedure well and was satisfied with the cosmetic and functional results. She subsequently underwent treatment of the lesions on the right labia minora with equivalent treatment success.
- Moyal-Barracco M, Leibowitch M, Orth G. Vestibular papillae of the vulva. lack of evidence for human papillomavirus etiology. Arch Dermatol. 1990;126:1594-1598.
- Strand A, Wilander E, Zehbe I, et al. Vulvar papillomatosis, aceto-white lesions, and normal-looking vulvar mucosa evaluated by microscopy and human papillomavirus analysis. Gynecol Obstet Invest. 1995;40:265-270.
- Welch JM, Nayagam M, Parry G, et al. What is vestibular papillomatosis? a study of its prevalence, aetiology and natural history. Br J Obstet Gynaecol. 1993;100:939-942.
- Wilkinson EJ, Guerrero E, Daniel R, et al. Vulvar vestibulitis is rarely associated with human papillomavirus infection types 6, 11, 16, or 18. Int J Gynecol Pathol. 1993;12:344-349.
- Beznos G, Coates V, Focchi J, et al. Biomolecular study of the correlation between papillomatosis of the vulvar vestibule in adolescents and human papillomavirus. ScientificWorldJournal. 2006;6:628-636.
- Kim SH, Seo SH, Ko HC, et al. The use of dermatoscopy to differentiate vestibular papillae, a normal variant of the female external genitalia, from condyloma acuminata. J Am Acad Dermatol. 2009;60:353-355.
- Ozkur E, Falay T, Turgut Erdemir AV, et al. Vestibular papillomatosis: an important differential diagnosis of vulvar papillomas. Dermatol Online J. 2016;22. pii:13030/qt7933q377
- Chang GJ, Welton ML. Human papillomavirus, condylomata acuminata, and anal neoplasia. Clin Colon Rectal Surg. 2004;17:221-230.
- Lynch PJ, Moyal-Barracco M, Bogliatto F, et al. 2006 ISSVD classification of vulvar dermatoses: pathologic subsets and their clinical correlates. J Reprod Med. 2007;52:3-9.
- Bergeron C, Ferenczy A, Richart RM, et al. Micropapillomatosis labialis appears unrelated to human papillomavirus. Obstet Gynecol. 1990;76:281-286.
The Diagnosis: Vestibular Papillomatosis
Vestibular papillomatosis (VP), the female equivalent of pearly penile papules, is characterized by multiple papules in a linear array on the labia minora and is considered a normal anatomic variant. It typically presents as monomorphous, soft, flesh-colored, filiform papules that are distributed in a symmetric fashion. In women, the papules present as linear arrays on the inner aspects of the labia minora, whereas in men, they present in a circumferential array along the sulcus of the glans penis.1 Lesions often are asymptomatic but may cause itching, burning, and dyspareunia.2 Previously believed to be associated with human papillomavirus infection,3 VP is now considered a noninfectious condition. Biopsy reveals parakeratosis and perinuclear vacuolization in the absence of true koilocytes.4,5 Dermoscopy and reflectance confocal microscopy have been used to differentiate VP from clinically similar lesions (eg, condyloma acuminatum).6,7 The prevalence of this condition is not well established; however, one study found VP in 1% of women attending genitourinary medicine clinics.3
Condyloma acuminatum, known colloquially as genital warts, is a human papillomavirus infection. Lesions tend to be painless and firm and are distributed asymmetrically with a cauliflowerlike appearance.1 Condyloma latum, found in secondary syphilis, is characterized by papules that are pale, smooth, flat topped, and moist.8 Molluscum contagiosum is an infection caused by a poxvirus presenting with flesh-colored, dome-shaped papules with central umbilication.9 The lesions of papulosquamous lichen planus are violaceous polygonal papules that affect the clitoral hood and labia minora and may cause pruritus. The cause of lichen planus is unknown; however, clinically similar lesions may occur in a lichenoid drug eruption due to certain medications.
Vestibular papillomatosis typically does not require treatment, except in symptomatic cases. To date, limited studies have reported variable treatment success utilizing destructive techniques such as CO2 laser or topical application of 5-fluorouracil or trichloroacetic acid.10
The lesions on our patient's left medial labia minora were successfully treated with low-voltage (3.0 V) electrodesiccation. Following local anesthesia with 1% lidocaine, each papule was gently electrodesiccated utilizing a standard hyfrecation electrode tip to a light gray discoloration. Postprocedural care involved only twice-daily cleansing with a gentle soap and application of petrolatum. The patient tolerated the procedure well and was satisfied with the cosmetic and functional results. She subsequently underwent treatment of the lesions on the right labia minora with equivalent treatment success.
The Diagnosis: Vestibular Papillomatosis
Vestibular papillomatosis (VP), the female equivalent of pearly penile papules, is characterized by multiple papules in a linear array on the labia minora and is considered a normal anatomic variant. It typically presents as monomorphous, soft, flesh-colored, filiform papules that are distributed in a symmetric fashion. In women, the papules present as linear arrays on the inner aspects of the labia minora, whereas in men, they present in a circumferential array along the sulcus of the glans penis.1 Lesions often are asymptomatic but may cause itching, burning, and dyspareunia.2 Previously believed to be associated with human papillomavirus infection,3 VP is now considered a noninfectious condition. Biopsy reveals parakeratosis and perinuclear vacuolization in the absence of true koilocytes.4,5 Dermoscopy and reflectance confocal microscopy have been used to differentiate VP from clinically similar lesions (eg, condyloma acuminatum).6,7 The prevalence of this condition is not well established; however, one study found VP in 1% of women attending genitourinary medicine clinics.3
Condyloma acuminatum, known colloquially as genital warts, is a human papillomavirus infection. Lesions tend to be painless and firm and are distributed asymmetrically with a cauliflowerlike appearance.1 Condyloma latum, found in secondary syphilis, is characterized by papules that are pale, smooth, flat topped, and moist.8 Molluscum contagiosum is an infection caused by a poxvirus presenting with flesh-colored, dome-shaped papules with central umbilication.9 The lesions of papulosquamous lichen planus are violaceous polygonal papules that affect the clitoral hood and labia minora and may cause pruritus. The cause of lichen planus is unknown; however, clinically similar lesions may occur in a lichenoid drug eruption due to certain medications.
Vestibular papillomatosis typically does not require treatment, except in symptomatic cases. To date, limited studies have reported variable treatment success utilizing destructive techniques such as CO2 laser or topical application of 5-fluorouracil or trichloroacetic acid.10
The lesions on our patient's left medial labia minora were successfully treated with low-voltage (3.0 V) electrodesiccation. Following local anesthesia with 1% lidocaine, each papule was gently electrodesiccated utilizing a standard hyfrecation electrode tip to a light gray discoloration. Postprocedural care involved only twice-daily cleansing with a gentle soap and application of petrolatum. The patient tolerated the procedure well and was satisfied with the cosmetic and functional results. She subsequently underwent treatment of the lesions on the right labia minora with equivalent treatment success.
- Moyal-Barracco M, Leibowitch M, Orth G. Vestibular papillae of the vulva. lack of evidence for human papillomavirus etiology. Arch Dermatol. 1990;126:1594-1598.
- Strand A, Wilander E, Zehbe I, et al. Vulvar papillomatosis, aceto-white lesions, and normal-looking vulvar mucosa evaluated by microscopy and human papillomavirus analysis. Gynecol Obstet Invest. 1995;40:265-270.
- Welch JM, Nayagam M, Parry G, et al. What is vestibular papillomatosis? a study of its prevalence, aetiology and natural history. Br J Obstet Gynaecol. 1993;100:939-942.
- Wilkinson EJ, Guerrero E, Daniel R, et al. Vulvar vestibulitis is rarely associated with human papillomavirus infection types 6, 11, 16, or 18. Int J Gynecol Pathol. 1993;12:344-349.
- Beznos G, Coates V, Focchi J, et al. Biomolecular study of the correlation between papillomatosis of the vulvar vestibule in adolescents and human papillomavirus. ScientificWorldJournal. 2006;6:628-636.
- Kim SH, Seo SH, Ko HC, et al. The use of dermatoscopy to differentiate vestibular papillae, a normal variant of the female external genitalia, from condyloma acuminata. J Am Acad Dermatol. 2009;60:353-355.
- Ozkur E, Falay T, Turgut Erdemir AV, et al. Vestibular papillomatosis: an important differential diagnosis of vulvar papillomas. Dermatol Online J. 2016;22. pii:13030/qt7933q377
- Chang GJ, Welton ML. Human papillomavirus, condylomata acuminata, and anal neoplasia. Clin Colon Rectal Surg. 2004;17:221-230.
- Lynch PJ, Moyal-Barracco M, Bogliatto F, et al. 2006 ISSVD classification of vulvar dermatoses: pathologic subsets and their clinical correlates. J Reprod Med. 2007;52:3-9.
- Bergeron C, Ferenczy A, Richart RM, et al. Micropapillomatosis labialis appears unrelated to human papillomavirus. Obstet Gynecol. 1990;76:281-286.
- Moyal-Barracco M, Leibowitch M, Orth G. Vestibular papillae of the vulva. lack of evidence for human papillomavirus etiology. Arch Dermatol. 1990;126:1594-1598.
- Strand A, Wilander E, Zehbe I, et al. Vulvar papillomatosis, aceto-white lesions, and normal-looking vulvar mucosa evaluated by microscopy and human papillomavirus analysis. Gynecol Obstet Invest. 1995;40:265-270.
- Welch JM, Nayagam M, Parry G, et al. What is vestibular papillomatosis? a study of its prevalence, aetiology and natural history. Br J Obstet Gynaecol. 1993;100:939-942.
- Wilkinson EJ, Guerrero E, Daniel R, et al. Vulvar vestibulitis is rarely associated with human papillomavirus infection types 6, 11, 16, or 18. Int J Gynecol Pathol. 1993;12:344-349.
- Beznos G, Coates V, Focchi J, et al. Biomolecular study of the correlation between papillomatosis of the vulvar vestibule in adolescents and human papillomavirus. ScientificWorldJournal. 2006;6:628-636.
- Kim SH, Seo SH, Ko HC, et al. The use of dermatoscopy to differentiate vestibular papillae, a normal variant of the female external genitalia, from condyloma acuminata. J Am Acad Dermatol. 2009;60:353-355.
- Ozkur E, Falay T, Turgut Erdemir AV, et al. Vestibular papillomatosis: an important differential diagnosis of vulvar papillomas. Dermatol Online J. 2016;22. pii:13030/qt7933q377
- Chang GJ, Welton ML. Human papillomavirus, condylomata acuminata, and anal neoplasia. Clin Colon Rectal Surg. 2004;17:221-230.
- Lynch PJ, Moyal-Barracco M, Bogliatto F, et al. 2006 ISSVD classification of vulvar dermatoses: pathologic subsets and their clinical correlates. J Reprod Med. 2007;52:3-9.
- Bergeron C, Ferenczy A, Richart RM, et al. Micropapillomatosis labialis appears unrelated to human papillomavirus. Obstet Gynecol. 1990;76:281-286.
A 30-year-old woman with congenital absence of the uterus presented to dermatology for a second opinion of vulvar lesions that were first noted during adolescence. The patient reported that the lesions had not changed and were painful during sexual intercourse. The lesions were otherwise asymptomatic, and she had no additional relevant medical history or family history of similar lesions. She denied any history of sexually transmitted infections. Physical examination revealed multiple, soft, flesh-colored, 1- to 2-mm, discrete and coalescing, filiform papules distributed symmetrically in a linear array on the inner aspect of the bilateral medial labia minora. The rest of the mucocutaneous examination was normal.
The lesions on the left medial labia minora were treated with low-voltage (3.0 V) electrodesiccation following local anesthesia with 1% lidocaine (red arrow), while the lesions on the right medial labia minora were left untreated (black arrow). The clinical image shows the left labia minora approximately 1 month after treatment; the papules on the right labia minora were unchanged from the prior examination.
Gone But Not Forgotten: How VA Remembers
Caring for veterans at the end of their lives is a great honor. The US Department of Veterans Affairs (VA) health care professionals (HCPs) find meaning and take pride in providing this care. We are there to support the patient and their family and loved ones around the time of death. When our patients die, we feel the loss and grieve as well. VA health care providers look to our teams to set up rituals that pay tribute to the veteran and to show respect and gratitude for our role in these moments. It is important to recognize the bonds we share and the grief we feel when a veteran dies. The relationships we form, the recognition of loss, and the honoring of the veterans help nourish and maintain us.
Although the number of VA inpatient deaths nationwide has been declining steadily for years, internal reporting by the Palliative and Hospice Care Program Office has shown that the percentage of VA inpatient deaths that occur in hospice settings has steadily grown. Since 2013, more veterans die in VA inpatient hospice beds than in any other hospital setting. Therefore, it is useful to take stock of the way hospice and palliative care providers and staff process and provide support so that they can continue to provide service to veterans.
In the same way that all loss and grief are unique, there are many diverse rituals across VA facilities. This article highlights some of the unique traditions that hospice and palliative care teams have adopted to embrace this remembrance. We hope that by sharing these practices others will be inspired to find ways to reflect on their work and honor the lives of veterans.
The authors reached out to VA palliative care colleagues across the country via the Veterans Health Administration National Hospice and Palliative Care listserve to ask: How does your team practice remembrance? Palliative care providers responded and shared the unique ways they and their teams reflect on these losses.
There are many moments for reflection from the time of death to the weeks and months after, to the entire year of cumulative loss. Some observances start around the time of death. Susan MacDonald, RN, GEC, from Erie VA Medical Center (VAMC) in Pennsylvania reported that following the death of a veteran in the hospice unit, there is a bedside remembrance that includes the chaplain, care team, family, and other loved ones. At the John D. Dingell VAMC in Detroit, Michigan, the clinical chaplain leads a memorial service after a community living center (CLC) resident dies.
Several VAMCs, such as Detroit and Erie, have an Honors Escort or Final Salute. In these ceremonies, family, employees, residents, and other veterans line the hallways to honor the veteran on their departure from the building.1 At the VA Maine Healthcare System, Kate MacFawn, nurse manager, Inpatient Hospice Unit, explained, “We debrief every death the day after it occurs. The doc[tor]s check in with the nursing staff on each shift, and the rest of the multidisciplinary team discusses [it] in our morning report.”
Palliative care providers consider the physical spaces where the veteran has spent those last moments and the void that is left. Karen Pickler, staff chaplain at Northport VAMC Hospice Unit recounts:
At the time of death, we decorate the tray table with the veteran’s picture, a flag, and an angel. In the CLC they will have a memorial service on Friday if a resident has died that week. This is for the unit and staff. In the past, other residents were not informed of the death. This way, the relationships between residents are honored as well as their natural families.
At VA Boston Healthcare System (VABHS) in Massachusetts in the Inpatient Hospice Unit-CLC, after a veteran dies, a flag, a strand of lights, and a rose in a vase are placed outside the veteran’s room to mark the absence. The VABHS remembrance practice has evolved over time based on input from the team. According to Noah Whiddon, LICSW, CLC complex case coordinator, at a weekly interdisciplinary team (IDT) meeting, the names of veterans who have died in the past week are read, and there is a commemorative ribbon cutting. “Any team member may write the last name of the deceased veteran on the ribbon and place it into a vase,” he said. “One of the nurse team members felt that a moment of silence would be appropriate, and we have added that.”
Every 6 months, VABHS holds a flag burning ceremony to appropriately dispose of worn out flags. Veterans and families are invited. The commemorative ribbons are packaged and burned at this ceremony with the following explanation of the ritual:
We’d like to take a moment to reflect on the lives of veterans we’ve lost in the last 6 months. Each week we remember the veterans for whom we have cared who have passed away. As part of this, we cut a ribbon and inscribe their name on it to commemorate their memory. We might have known these veterans for a few days or for a few years, but each of their lives had meaning for us. The courage that our veterans demonstrate at the end of their lives is an extension of the bravery they displayed in their service to our country. Today we will burn their commemorative ribbons with our country’s flag in tribute to and respect for their selflessness to our country. Please join us in a few moments of silence as their ribbons burn together with our flag.
In the VABHS acute care hospital, the palliative care IDT reserves 30 minutes, twice monthly for a chaplain-led remembrance. A large bowl-shaped shell is placed in the center of the table with smaller shells around it. Any team member can read the names of veterans who have died in prior weeks and share a memory of the patient or family, and then place a smaller shell into the larger bowl. This represents the transition from the smallest part of the universe back into the larger part. At the end, a moment of silence is observed or a poem is read. This tradition was brought to the team by the palliative care chaplain, Douglas Falls, MDiv.
Bimonthly bereavement meetings are held at the James A. Haley Veterans’ Hospital-Pasco County branch, and each veteran who has died is remembered. Whoever wants to share is welcome. “We conclude with a poem, usually shared by the physician, but it can be any team member,” explained Linda Falzetta-Gross, ARNP-BC. “This process is led by the team social worker. In the past, we used to ring a bell prior to each name.”
Bells also are used at the Greater West Los Angeles VAMC in California. At the weekly clinic, veterans who have died are remembered, and each team member has an opportunity to share memories. “We ring a Tibetan bell for a moment without words,” explains Geoffrey Tyrell, palliative care chaplain. “It is introduced with a few words to allow new staff members in our clinic to participate, as a moment of mindfulness to let go of our words and to go inside, to see what we might need for our own wellness.” Afterward the chaplain says a few words and wishes for peace for the veterans and their families. The team also has responded well to more participatory group activities, such as placing rocks in a bowl of water, to symbolize letting go of something that has been difficult.
Additionally, there are practices of a larger scope. Many VAMCs have established facility-wide memorial services annually, biannually, or quarterly. At this time, families and staff come together to remember and honor veterans who have died within the VAMC. These memorials might involve a variety of service lines, such as chaplaincy, voluntary services, nursing, and social work and may consist of an honor guard, music, and readings. In accordance with the Health Insurance Portability and Accountability Act (HIPAA) and privacy regulations, only family members of deceased veterans may speak the names at the ceremony unless written consent is given. At the Tennessee Valley Healthcare System in Nashville, family members may stand and give the name of the person they are honoring. Balloons are released, stories are told, and a poem or appropriate passage is read. Families are given a book pinned with a flag, according to Jennifer C. Crenshaw, clinical staff chaplain. Family members are moved knowing that the VA remembers their loved ones even months after they are gone.
Due to the overwhelming positive feedback from veterans’ families who participated in these ceremonies, on January 24, 2018, Carolyn Clancy, MD, VHA Executive-in-Charge, Office of the Under Secretary for Health issued a memorandum requesting that all VAMCs immediately adopt this best practice: to host periodic ceremonies to publicly recognize and honor deceased veterans in the presence of their families, VA care providers, veterans service organizations and community members. Clancy recommended calling the ceremonies “The Last Roll Call Ceremony of Remembrance.”2
These rituals are a small sample of the rich diversity of practice in VAs across the country. What unifies VA palliative care providers is our mission to serve the veterans, honor their deaths, show respect and gratitude, and recognize that we, too, feel the pain of loss. We mark these moments with solemnity and beauty—a bell, a poem, a prayer—to honor our shared experience caring for veterans.
1. Saint S. A VA hospital you may not know: The Final Salute, and how much we doctors care. https://theconversation.com/a-va-hospital-you-may-not-know-the-final-salute-and-how-much-we-doctors-care-94217. Published March 30, 2018. Accessed May 8, 2019.
2. Clancy CM. VAIQ Memorandum 7866347: Implementation of the last roll call ceremony of remembrance at all Veterans Affairs medical centers. Published 2018.
Caring for veterans at the end of their lives is a great honor. The US Department of Veterans Affairs (VA) health care professionals (HCPs) find meaning and take pride in providing this care. We are there to support the patient and their family and loved ones around the time of death. When our patients die, we feel the loss and grieve as well. VA health care providers look to our teams to set up rituals that pay tribute to the veteran and to show respect and gratitude for our role in these moments. It is important to recognize the bonds we share and the grief we feel when a veteran dies. The relationships we form, the recognition of loss, and the honoring of the veterans help nourish and maintain us.
Although the number of VA inpatient deaths nationwide has been declining steadily for years, internal reporting by the Palliative and Hospice Care Program Office has shown that the percentage of VA inpatient deaths that occur in hospice settings has steadily grown. Since 2013, more veterans die in VA inpatient hospice beds than in any other hospital setting. Therefore, it is useful to take stock of the way hospice and palliative care providers and staff process and provide support so that they can continue to provide service to veterans.
In the same way that all loss and grief are unique, there are many diverse rituals across VA facilities. This article highlights some of the unique traditions that hospice and palliative care teams have adopted to embrace this remembrance. We hope that by sharing these practices others will be inspired to find ways to reflect on their work and honor the lives of veterans.
The authors reached out to VA palliative care colleagues across the country via the Veterans Health Administration National Hospice and Palliative Care listserve to ask: How does your team practice remembrance? Palliative care providers responded and shared the unique ways they and their teams reflect on these losses.
There are many moments for reflection from the time of death to the weeks and months after, to the entire year of cumulative loss. Some observances start around the time of death. Susan MacDonald, RN, GEC, from Erie VA Medical Center (VAMC) in Pennsylvania reported that following the death of a veteran in the hospice unit, there is a bedside remembrance that includes the chaplain, care team, family, and other loved ones. At the John D. Dingell VAMC in Detroit, Michigan, the clinical chaplain leads a memorial service after a community living center (CLC) resident dies.
Several VAMCs, such as Detroit and Erie, have an Honors Escort or Final Salute. In these ceremonies, family, employees, residents, and other veterans line the hallways to honor the veteran on their departure from the building.1 At the VA Maine Healthcare System, Kate MacFawn, nurse manager, Inpatient Hospice Unit, explained, “We debrief every death the day after it occurs. The doc[tor]s check in with the nursing staff on each shift, and the rest of the multidisciplinary team discusses [it] in our morning report.”
Palliative care providers consider the physical spaces where the veteran has spent those last moments and the void that is left. Karen Pickler, staff chaplain at Northport VAMC Hospice Unit recounts:
At the time of death, we decorate the tray table with the veteran’s picture, a flag, and an angel. In the CLC they will have a memorial service on Friday if a resident has died that week. This is for the unit and staff. In the past, other residents were not informed of the death. This way, the relationships between residents are honored as well as their natural families.
At VA Boston Healthcare System (VABHS) in Massachusetts in the Inpatient Hospice Unit-CLC, after a veteran dies, a flag, a strand of lights, and a rose in a vase are placed outside the veteran’s room to mark the absence. The VABHS remembrance practice has evolved over time based on input from the team. According to Noah Whiddon, LICSW, CLC complex case coordinator, at a weekly interdisciplinary team (IDT) meeting, the names of veterans who have died in the past week are read, and there is a commemorative ribbon cutting. “Any team member may write the last name of the deceased veteran on the ribbon and place it into a vase,” he said. “One of the nurse team members felt that a moment of silence would be appropriate, and we have added that.”
Every 6 months, VABHS holds a flag burning ceremony to appropriately dispose of worn out flags. Veterans and families are invited. The commemorative ribbons are packaged and burned at this ceremony with the following explanation of the ritual:
We’d like to take a moment to reflect on the lives of veterans we’ve lost in the last 6 months. Each week we remember the veterans for whom we have cared who have passed away. As part of this, we cut a ribbon and inscribe their name on it to commemorate their memory. We might have known these veterans for a few days or for a few years, but each of their lives had meaning for us. The courage that our veterans demonstrate at the end of their lives is an extension of the bravery they displayed in their service to our country. Today we will burn their commemorative ribbons with our country’s flag in tribute to and respect for their selflessness to our country. Please join us in a few moments of silence as their ribbons burn together with our flag.
In the VABHS acute care hospital, the palliative care IDT reserves 30 minutes, twice monthly for a chaplain-led remembrance. A large bowl-shaped shell is placed in the center of the table with smaller shells around it. Any team member can read the names of veterans who have died in prior weeks and share a memory of the patient or family, and then place a smaller shell into the larger bowl. This represents the transition from the smallest part of the universe back into the larger part. At the end, a moment of silence is observed or a poem is read. This tradition was brought to the team by the palliative care chaplain, Douglas Falls, MDiv.
Bimonthly bereavement meetings are held at the James A. Haley Veterans’ Hospital-Pasco County branch, and each veteran who has died is remembered. Whoever wants to share is welcome. “We conclude with a poem, usually shared by the physician, but it can be any team member,” explained Linda Falzetta-Gross, ARNP-BC. “This process is led by the team social worker. In the past, we used to ring a bell prior to each name.”
Bells also are used at the Greater West Los Angeles VAMC in California. At the weekly clinic, veterans who have died are remembered, and each team member has an opportunity to share memories. “We ring a Tibetan bell for a moment without words,” explains Geoffrey Tyrell, palliative care chaplain. “It is introduced with a few words to allow new staff members in our clinic to participate, as a moment of mindfulness to let go of our words and to go inside, to see what we might need for our own wellness.” Afterward the chaplain says a few words and wishes for peace for the veterans and their families. The team also has responded well to more participatory group activities, such as placing rocks in a bowl of water, to symbolize letting go of something that has been difficult.
Additionally, there are practices of a larger scope. Many VAMCs have established facility-wide memorial services annually, biannually, or quarterly. At this time, families and staff come together to remember and honor veterans who have died within the VAMC. These memorials might involve a variety of service lines, such as chaplaincy, voluntary services, nursing, and social work and may consist of an honor guard, music, and readings. In accordance with the Health Insurance Portability and Accountability Act (HIPAA) and privacy regulations, only family members of deceased veterans may speak the names at the ceremony unless written consent is given. At the Tennessee Valley Healthcare System in Nashville, family members may stand and give the name of the person they are honoring. Balloons are released, stories are told, and a poem or appropriate passage is read. Families are given a book pinned with a flag, according to Jennifer C. Crenshaw, clinical staff chaplain. Family members are moved knowing that the VA remembers their loved ones even months after they are gone.
Due to the overwhelming positive feedback from veterans’ families who participated in these ceremonies, on January 24, 2018, Carolyn Clancy, MD, VHA Executive-in-Charge, Office of the Under Secretary for Health issued a memorandum requesting that all VAMCs immediately adopt this best practice: to host periodic ceremonies to publicly recognize and honor deceased veterans in the presence of their families, VA care providers, veterans service organizations and community members. Clancy recommended calling the ceremonies “The Last Roll Call Ceremony of Remembrance.”2
These rituals are a small sample of the rich diversity of practice in VAs across the country. What unifies VA palliative care providers is our mission to serve the veterans, honor their deaths, show respect and gratitude, and recognize that we, too, feel the pain of loss. We mark these moments with solemnity and beauty—a bell, a poem, a prayer—to honor our shared experience caring for veterans.
Caring for veterans at the end of their lives is a great honor. The US Department of Veterans Affairs (VA) health care professionals (HCPs) find meaning and take pride in providing this care. We are there to support the patient and their family and loved ones around the time of death. When our patients die, we feel the loss and grieve as well. VA health care providers look to our teams to set up rituals that pay tribute to the veteran and to show respect and gratitude for our role in these moments. It is important to recognize the bonds we share and the grief we feel when a veteran dies. The relationships we form, the recognition of loss, and the honoring of the veterans help nourish and maintain us.
Although the number of VA inpatient deaths nationwide has been declining steadily for years, internal reporting by the Palliative and Hospice Care Program Office has shown that the percentage of VA inpatient deaths that occur in hospice settings has steadily grown. Since 2013, more veterans die in VA inpatient hospice beds than in any other hospital setting. Therefore, it is useful to take stock of the way hospice and palliative care providers and staff process and provide support so that they can continue to provide service to veterans.
In the same way that all loss and grief are unique, there are many diverse rituals across VA facilities. This article highlights some of the unique traditions that hospice and palliative care teams have adopted to embrace this remembrance. We hope that by sharing these practices others will be inspired to find ways to reflect on their work and honor the lives of veterans.
The authors reached out to VA palliative care colleagues across the country via the Veterans Health Administration National Hospice and Palliative Care listserve to ask: How does your team practice remembrance? Palliative care providers responded and shared the unique ways they and their teams reflect on these losses.
There are many moments for reflection from the time of death to the weeks and months after, to the entire year of cumulative loss. Some observances start around the time of death. Susan MacDonald, RN, GEC, from Erie VA Medical Center (VAMC) in Pennsylvania reported that following the death of a veteran in the hospice unit, there is a bedside remembrance that includes the chaplain, care team, family, and other loved ones. At the John D. Dingell VAMC in Detroit, Michigan, the clinical chaplain leads a memorial service after a community living center (CLC) resident dies.
Several VAMCs, such as Detroit and Erie, have an Honors Escort or Final Salute. In these ceremonies, family, employees, residents, and other veterans line the hallways to honor the veteran on their departure from the building.1 At the VA Maine Healthcare System, Kate MacFawn, nurse manager, Inpatient Hospice Unit, explained, “We debrief every death the day after it occurs. The doc[tor]s check in with the nursing staff on each shift, and the rest of the multidisciplinary team discusses [it] in our morning report.”
Palliative care providers consider the physical spaces where the veteran has spent those last moments and the void that is left. Karen Pickler, staff chaplain at Northport VAMC Hospice Unit recounts:
At the time of death, we decorate the tray table with the veteran’s picture, a flag, and an angel. In the CLC they will have a memorial service on Friday if a resident has died that week. This is for the unit and staff. In the past, other residents were not informed of the death. This way, the relationships between residents are honored as well as their natural families.
At VA Boston Healthcare System (VABHS) in Massachusetts in the Inpatient Hospice Unit-CLC, after a veteran dies, a flag, a strand of lights, and a rose in a vase are placed outside the veteran’s room to mark the absence. The VABHS remembrance practice has evolved over time based on input from the team. According to Noah Whiddon, LICSW, CLC complex case coordinator, at a weekly interdisciplinary team (IDT) meeting, the names of veterans who have died in the past week are read, and there is a commemorative ribbon cutting. “Any team member may write the last name of the deceased veteran on the ribbon and place it into a vase,” he said. “One of the nurse team members felt that a moment of silence would be appropriate, and we have added that.”
Every 6 months, VABHS holds a flag burning ceremony to appropriately dispose of worn out flags. Veterans and families are invited. The commemorative ribbons are packaged and burned at this ceremony with the following explanation of the ritual:
We’d like to take a moment to reflect on the lives of veterans we’ve lost in the last 6 months. Each week we remember the veterans for whom we have cared who have passed away. As part of this, we cut a ribbon and inscribe their name on it to commemorate their memory. We might have known these veterans for a few days or for a few years, but each of their lives had meaning for us. The courage that our veterans demonstrate at the end of their lives is an extension of the bravery they displayed in their service to our country. Today we will burn their commemorative ribbons with our country’s flag in tribute to and respect for their selflessness to our country. Please join us in a few moments of silence as their ribbons burn together with our flag.
In the VABHS acute care hospital, the palliative care IDT reserves 30 minutes, twice monthly for a chaplain-led remembrance. A large bowl-shaped shell is placed in the center of the table with smaller shells around it. Any team member can read the names of veterans who have died in prior weeks and share a memory of the patient or family, and then place a smaller shell into the larger bowl. This represents the transition from the smallest part of the universe back into the larger part. At the end, a moment of silence is observed or a poem is read. This tradition was brought to the team by the palliative care chaplain, Douglas Falls, MDiv.
Bimonthly bereavement meetings are held at the James A. Haley Veterans’ Hospital-Pasco County branch, and each veteran who has died is remembered. Whoever wants to share is welcome. “We conclude with a poem, usually shared by the physician, but it can be any team member,” explained Linda Falzetta-Gross, ARNP-BC. “This process is led by the team social worker. In the past, we used to ring a bell prior to each name.”
Bells also are used at the Greater West Los Angeles VAMC in California. At the weekly clinic, veterans who have died are remembered, and each team member has an opportunity to share memories. “We ring a Tibetan bell for a moment without words,” explains Geoffrey Tyrell, palliative care chaplain. “It is introduced with a few words to allow new staff members in our clinic to participate, as a moment of mindfulness to let go of our words and to go inside, to see what we might need for our own wellness.” Afterward the chaplain says a few words and wishes for peace for the veterans and their families. The team also has responded well to more participatory group activities, such as placing rocks in a bowl of water, to symbolize letting go of something that has been difficult.
Additionally, there are practices of a larger scope. Many VAMCs have established facility-wide memorial services annually, biannually, or quarterly. At this time, families and staff come together to remember and honor veterans who have died within the VAMC. These memorials might involve a variety of service lines, such as chaplaincy, voluntary services, nursing, and social work and may consist of an honor guard, music, and readings. In accordance with the Health Insurance Portability and Accountability Act (HIPAA) and privacy regulations, only family members of deceased veterans may speak the names at the ceremony unless written consent is given. At the Tennessee Valley Healthcare System in Nashville, family members may stand and give the name of the person they are honoring. Balloons are released, stories are told, and a poem or appropriate passage is read. Families are given a book pinned with a flag, according to Jennifer C. Crenshaw, clinical staff chaplain. Family members are moved knowing that the VA remembers their loved ones even months after they are gone.
Due to the overwhelming positive feedback from veterans’ families who participated in these ceremonies, on January 24, 2018, Carolyn Clancy, MD, VHA Executive-in-Charge, Office of the Under Secretary for Health issued a memorandum requesting that all VAMCs immediately adopt this best practice: to host periodic ceremonies to publicly recognize and honor deceased veterans in the presence of their families, VA care providers, veterans service organizations and community members. Clancy recommended calling the ceremonies “The Last Roll Call Ceremony of Remembrance.”2
These rituals are a small sample of the rich diversity of practice in VAs across the country. What unifies VA palliative care providers is our mission to serve the veterans, honor their deaths, show respect and gratitude, and recognize that we, too, feel the pain of loss. We mark these moments with solemnity and beauty—a bell, a poem, a prayer—to honor our shared experience caring for veterans.
1. Saint S. A VA hospital you may not know: The Final Salute, and how much we doctors care. https://theconversation.com/a-va-hospital-you-may-not-know-the-final-salute-and-how-much-we-doctors-care-94217. Published March 30, 2018. Accessed May 8, 2019.
2. Clancy CM. VAIQ Memorandum 7866347: Implementation of the last roll call ceremony of remembrance at all Veterans Affairs medical centers. Published 2018.
1. Saint S. A VA hospital you may not know: The Final Salute, and how much we doctors care. https://theconversation.com/a-va-hospital-you-may-not-know-the-final-salute-and-how-much-we-doctors-care-94217. Published March 30, 2018. Accessed May 8, 2019.
2. Clancy CM. VAIQ Memorandum 7866347: Implementation of the last roll call ceremony of remembrance at all Veterans Affairs medical centers. Published 2018.
Pediatric gastroesophageal reflux
In a 2018 guideline, the writing committee defined GER as reflux of stomach contents to the esophagus. GER is considered pathologic and, therefore, gastroesophageal reflux disease (GERD) when it is associated with troublesome symptoms and/or complications that can include esophagitis and aspiration.
Infants
GERD is difficult to diagnose in infants. The symptoms of GERD, such as crying after feeds, regurgitation, and irritability, occur commonly in all infants and in any individual infant may not be reflective of GERD. Regurgitation is common, frequent and normal in infants up to 6 months of age. A common challenge occurs when families request treatment for infants with irritability, back arching, and/or regurgitation who are otherwise doing well. In this group of infants it is important to recognize that neither testing nor therapy is indicated unless there is difficulty with feeding, growth, acquisition of milestones, or red flag signs.
In infants with recurrent regurgitation history, physical exam is usually sufficient to distinguish uncomplicated GER from GERD and other more worrisome diagnoses. Red flag symptoms raise the possibility of a different diagnosis. Red flag symptoms include weight loss; lethargy; excessive irritability/pain; onset of vomiting for more than 6 months or persisting past 12-18 months of age; rapidly increasing head circumference; persistent forceful, nocturnal, bloody, or bilious vomiting; abdominal distention; rectal bleeding; and chronic diarrhea. GERD that starts after 6 months of age or which persists after 12 months of age warrants further evaluation, often with referral to a pediatric gastroenterologist.
When GERD is suspected, the first therapeutic steps are to institute behavioral changes. Caregivers should avoid overfeeding and modify the feeding pattern to more frequent feedings consisting of less volume at each feed. The addition of thickeners to feeds does reduce regurgitation, although it may not affect other GERD signs and symptoms. Formula can be thickened with rice cereal, which tends to be an affordable choice that doesn’t clog nipples. Enzymes present in breast milk digest cereal thickeners, so breast milk can be thickened with xanthum gum (after 1 year of age) or carob bean–based products (after 42 weeks gestation).
If these modifications do not improve symptoms, the next step is to change the type of feeds. Some infants in whom GERD is suspected actually have cow’s milk protein allergy (CMPA), so a trial of cow’s milk elimination is warranted. A breastfeeding mother can eliminate all dairy from her diet including casein and whey. Caregivers can switch to an extensively hydrolyzed formula or an amino acid–based formula. The guideline do not recommend soy-based formulas because they are not available in Europe and because a significant percentage of infants with CMPA also develop allergy to soy, and they do not recommend rice hydrolysate formula because of a lack of evidence. Dairy can be reintroduced at a later point. While positional changes including elevating the head of the crib or placing the infant in the left lateral position can help decrease GERD, the American Academy of Pediatrics strongly discourages these positions because of safety concerns, so the guidelines do not recommend positional change.
If a 2-4 week trial of nonpharmacologic interventions fails, the next step is referral to a pediatric gastroenterologist. If a pediatric gastroenterologist is not available, a 4-8 week trial of acid suppressive medication may be given. No trial has shown utility of a trial of acid suppression as a diagnostic test for GERD. Medication should only be used in infants with strongly suspected GERD and, per the guidelines, “should not be used for the treatment of visible regurgitation in otherwise healthy infants.” Medications to treat GER do not have evidence of efficacy, and there is evidence of an increased risk of infection with use of acid suppression, including an increased risk of necrotizing enterocolitis, pneumonia, upper respiratory tract infections, sepsis, urinary tract infections, and Clostridium difficile. If used, proton-pump inhibitors are preferred over histamine-2 receptor blockers. Antacids and alginates are not recommended.
Older children
In children with heartburn or regurgitation without red flag symptoms, a trial of lifestyle changes and dietary education may be initiated. If a child is overweight, it is important to inform the patient and parents that excess body weight is associated with GERD. The head of the bed can be elevated along with left lateral positioning. The guidelines do not support any probiotics or herbal medicines.
If bothersome symptoms persist, a trial of acid-suppressing medication for 4-8 weeks is reasonable. A PPI is preferred to a histamine-2 receptor blocker. PPI safety studies are lacking, but case studies suggest an increase in infections in children taking acid-suppressing medications. Therefore, as with infants, if medications are used they should be prescribed at the lowest dose and for the shortest period of time possible. If medications are not helping, or need to be used long term, referral to a pediatric gastroenterologist can be considered. Of note, the guidelines do support a 4-8 week trial of PPIs in older children as a diagnostic test; this differs from the recommendations for infants, in whom a trial for diagnostic purposes is discouraged.
Diagnostic testing
Refer to a gastroenterologist for endoscopy in cases of persistent symptoms despite PPI use or failure to wean off medication. If there are no erosions, pH monitoring with pH-impedance monitoring or pH-metry can help distinguish between nonerosive reflux disease (NERD), reflux hypersensitivity, and functional heartburn. If it is performed when a child is off of PPIs, endoscopy can also diagnose PPI-responsive eosinophilic esophagitis. Barium contrast, abdominal ultrasonography, and manometry may be considered during the course of a search for an alternative diagnosis, but they should not be used to diagnose or confirm GERD.
The bottom line
Most GER is physiologic and does not need treatment. First-line treatment for GERD in infants and children is nonpharmacologic intervention.
Reference
Rosen R et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-554.
Dr. Oh is a third year resident in the Family Medicine Residency at Abington-Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
In a 2018 guideline, the writing committee defined GER as reflux of stomach contents to the esophagus. GER is considered pathologic and, therefore, gastroesophageal reflux disease (GERD) when it is associated with troublesome symptoms and/or complications that can include esophagitis and aspiration.
Infants
GERD is difficult to diagnose in infants. The symptoms of GERD, such as crying after feeds, regurgitation, and irritability, occur commonly in all infants and in any individual infant may not be reflective of GERD. Regurgitation is common, frequent and normal in infants up to 6 months of age. A common challenge occurs when families request treatment for infants with irritability, back arching, and/or regurgitation who are otherwise doing well. In this group of infants it is important to recognize that neither testing nor therapy is indicated unless there is difficulty with feeding, growth, acquisition of milestones, or red flag signs.
In infants with recurrent regurgitation history, physical exam is usually sufficient to distinguish uncomplicated GER from GERD and other more worrisome diagnoses. Red flag symptoms raise the possibility of a different diagnosis. Red flag symptoms include weight loss; lethargy; excessive irritability/pain; onset of vomiting for more than 6 months or persisting past 12-18 months of age; rapidly increasing head circumference; persistent forceful, nocturnal, bloody, or bilious vomiting; abdominal distention; rectal bleeding; and chronic diarrhea. GERD that starts after 6 months of age or which persists after 12 months of age warrants further evaluation, often with referral to a pediatric gastroenterologist.
When GERD is suspected, the first therapeutic steps are to institute behavioral changes. Caregivers should avoid overfeeding and modify the feeding pattern to more frequent feedings consisting of less volume at each feed. The addition of thickeners to feeds does reduce regurgitation, although it may not affect other GERD signs and symptoms. Formula can be thickened with rice cereal, which tends to be an affordable choice that doesn’t clog nipples. Enzymes present in breast milk digest cereal thickeners, so breast milk can be thickened with xanthum gum (after 1 year of age) or carob bean–based products (after 42 weeks gestation).
If these modifications do not improve symptoms, the next step is to change the type of feeds. Some infants in whom GERD is suspected actually have cow’s milk protein allergy (CMPA), so a trial of cow’s milk elimination is warranted. A breastfeeding mother can eliminate all dairy from her diet including casein and whey. Caregivers can switch to an extensively hydrolyzed formula or an amino acid–based formula. The guideline do not recommend soy-based formulas because they are not available in Europe and because a significant percentage of infants with CMPA also develop allergy to soy, and they do not recommend rice hydrolysate formula because of a lack of evidence. Dairy can be reintroduced at a later point. While positional changes including elevating the head of the crib or placing the infant in the left lateral position can help decrease GERD, the American Academy of Pediatrics strongly discourages these positions because of safety concerns, so the guidelines do not recommend positional change.
If a 2-4 week trial of nonpharmacologic interventions fails, the next step is referral to a pediatric gastroenterologist. If a pediatric gastroenterologist is not available, a 4-8 week trial of acid suppressive medication may be given. No trial has shown utility of a trial of acid suppression as a diagnostic test for GERD. Medication should only be used in infants with strongly suspected GERD and, per the guidelines, “should not be used for the treatment of visible regurgitation in otherwise healthy infants.” Medications to treat GER do not have evidence of efficacy, and there is evidence of an increased risk of infection with use of acid suppression, including an increased risk of necrotizing enterocolitis, pneumonia, upper respiratory tract infections, sepsis, urinary tract infections, and Clostridium difficile. If used, proton-pump inhibitors are preferred over histamine-2 receptor blockers. Antacids and alginates are not recommended.
Older children
In children with heartburn or regurgitation without red flag symptoms, a trial of lifestyle changes and dietary education may be initiated. If a child is overweight, it is important to inform the patient and parents that excess body weight is associated with GERD. The head of the bed can be elevated along with left lateral positioning. The guidelines do not support any probiotics or herbal medicines.
If bothersome symptoms persist, a trial of acid-suppressing medication for 4-8 weeks is reasonable. A PPI is preferred to a histamine-2 receptor blocker. PPI safety studies are lacking, but case studies suggest an increase in infections in children taking acid-suppressing medications. Therefore, as with infants, if medications are used they should be prescribed at the lowest dose and for the shortest period of time possible. If medications are not helping, or need to be used long term, referral to a pediatric gastroenterologist can be considered. Of note, the guidelines do support a 4-8 week trial of PPIs in older children as a diagnostic test; this differs from the recommendations for infants, in whom a trial for diagnostic purposes is discouraged.
Diagnostic testing
Refer to a gastroenterologist for endoscopy in cases of persistent symptoms despite PPI use or failure to wean off medication. If there are no erosions, pH monitoring with pH-impedance monitoring or pH-metry can help distinguish between nonerosive reflux disease (NERD), reflux hypersensitivity, and functional heartburn. If it is performed when a child is off of PPIs, endoscopy can also diagnose PPI-responsive eosinophilic esophagitis. Barium contrast, abdominal ultrasonography, and manometry may be considered during the course of a search for an alternative diagnosis, but they should not be used to diagnose or confirm GERD.
The bottom line
Most GER is physiologic and does not need treatment. First-line treatment for GERD in infants and children is nonpharmacologic intervention.
Reference
Rosen R et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-554.
Dr. Oh is a third year resident in the Family Medicine Residency at Abington-Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
In a 2018 guideline, the writing committee defined GER as reflux of stomach contents to the esophagus. GER is considered pathologic and, therefore, gastroesophageal reflux disease (GERD) when it is associated with troublesome symptoms and/or complications that can include esophagitis and aspiration.
Infants
GERD is difficult to diagnose in infants. The symptoms of GERD, such as crying after feeds, regurgitation, and irritability, occur commonly in all infants and in any individual infant may not be reflective of GERD. Regurgitation is common, frequent and normal in infants up to 6 months of age. A common challenge occurs when families request treatment for infants with irritability, back arching, and/or regurgitation who are otherwise doing well. In this group of infants it is important to recognize that neither testing nor therapy is indicated unless there is difficulty with feeding, growth, acquisition of milestones, or red flag signs.
In infants with recurrent regurgitation history, physical exam is usually sufficient to distinguish uncomplicated GER from GERD and other more worrisome diagnoses. Red flag symptoms raise the possibility of a different diagnosis. Red flag symptoms include weight loss; lethargy; excessive irritability/pain; onset of vomiting for more than 6 months or persisting past 12-18 months of age; rapidly increasing head circumference; persistent forceful, nocturnal, bloody, or bilious vomiting; abdominal distention; rectal bleeding; and chronic diarrhea. GERD that starts after 6 months of age or which persists after 12 months of age warrants further evaluation, often with referral to a pediatric gastroenterologist.
When GERD is suspected, the first therapeutic steps are to institute behavioral changes. Caregivers should avoid overfeeding and modify the feeding pattern to more frequent feedings consisting of less volume at each feed. The addition of thickeners to feeds does reduce regurgitation, although it may not affect other GERD signs and symptoms. Formula can be thickened with rice cereal, which tends to be an affordable choice that doesn’t clog nipples. Enzymes present in breast milk digest cereal thickeners, so breast milk can be thickened with xanthum gum (after 1 year of age) or carob bean–based products (after 42 weeks gestation).
If these modifications do not improve symptoms, the next step is to change the type of feeds. Some infants in whom GERD is suspected actually have cow’s milk protein allergy (CMPA), so a trial of cow’s milk elimination is warranted. A breastfeeding mother can eliminate all dairy from her diet including casein and whey. Caregivers can switch to an extensively hydrolyzed formula or an amino acid–based formula. The guideline do not recommend soy-based formulas because they are not available in Europe and because a significant percentage of infants with CMPA also develop allergy to soy, and they do not recommend rice hydrolysate formula because of a lack of evidence. Dairy can be reintroduced at a later point. While positional changes including elevating the head of the crib or placing the infant in the left lateral position can help decrease GERD, the American Academy of Pediatrics strongly discourages these positions because of safety concerns, so the guidelines do not recommend positional change.
If a 2-4 week trial of nonpharmacologic interventions fails, the next step is referral to a pediatric gastroenterologist. If a pediatric gastroenterologist is not available, a 4-8 week trial of acid suppressive medication may be given. No trial has shown utility of a trial of acid suppression as a diagnostic test for GERD. Medication should only be used in infants with strongly suspected GERD and, per the guidelines, “should not be used for the treatment of visible regurgitation in otherwise healthy infants.” Medications to treat GER do not have evidence of efficacy, and there is evidence of an increased risk of infection with use of acid suppression, including an increased risk of necrotizing enterocolitis, pneumonia, upper respiratory tract infections, sepsis, urinary tract infections, and Clostridium difficile. If used, proton-pump inhibitors are preferred over histamine-2 receptor blockers. Antacids and alginates are not recommended.
Older children
In children with heartburn or regurgitation without red flag symptoms, a trial of lifestyle changes and dietary education may be initiated. If a child is overweight, it is important to inform the patient and parents that excess body weight is associated with GERD. The head of the bed can be elevated along with left lateral positioning. The guidelines do not support any probiotics or herbal medicines.
If bothersome symptoms persist, a trial of acid-suppressing medication for 4-8 weeks is reasonable. A PPI is preferred to a histamine-2 receptor blocker. PPI safety studies are lacking, but case studies suggest an increase in infections in children taking acid-suppressing medications. Therefore, as with infants, if medications are used they should be prescribed at the lowest dose and for the shortest period of time possible. If medications are not helping, or need to be used long term, referral to a pediatric gastroenterologist can be considered. Of note, the guidelines do support a 4-8 week trial of PPIs in older children as a diagnostic test; this differs from the recommendations for infants, in whom a trial for diagnostic purposes is discouraged.
Diagnostic testing
Refer to a gastroenterologist for endoscopy in cases of persistent symptoms despite PPI use or failure to wean off medication. If there are no erosions, pH monitoring with pH-impedance monitoring or pH-metry can help distinguish between nonerosive reflux disease (NERD), reflux hypersensitivity, and functional heartburn. If it is performed when a child is off of PPIs, endoscopy can also diagnose PPI-responsive eosinophilic esophagitis. Barium contrast, abdominal ultrasonography, and manometry may be considered during the course of a search for an alternative diagnosis, but they should not be used to diagnose or confirm GERD.
The bottom line
Most GER is physiologic and does not need treatment. First-line treatment for GERD in infants and children is nonpharmacologic intervention.
Reference
Rosen R et al. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018 Mar;66(3):516-554.
Dr. Oh is a third year resident in the Family Medicine Residency at Abington-Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
Teamwork makes the dream work – maximizing the relationship between physicians and advanced practice providers
Advanced practice providers (APPs; physician assistants and nurse practitioners) play a vital role in the success of an academic or private gastroenterology practice. Partnership with APPs in the clinical setting can improve inpatient and outpatient workflow and complex chronic care management, optimizing downstream revenue from endoscopy, radiology, and motility studies and enhancing physician productivity in research or academic affairs. In an informal AGA Community survey of physicians throughout the United States, 86% of respondents worked with advanced practice providers, 61% of whom had done so for over 5 years. While APPs may fill diverse roles in gastroenterology practice, there are common principles that may help optimize the physician-APP relationship. We surveyed both APPs and physicians to gain their perspective and present a tool kit to optimize the relationship among APPs and physicians.
The APP perspective
In qualitative interviews with 12 APPs practicing gastroenterology in a variety of specialties in Massachusetts, we aimed to understand 1) what APPs felt they brought to GI practice and 2) how APPs can be best utilized and integrated into GI practice and flow.
All interviewees independently noted that improving patient access to care and providing continuity of care were key benefits they brought to their practice, resulting in the possible downstream prevention of unnecessary emergency room admissions. Additionally, APPs felt that they brought significant value by having the time to listen to patient concerns to allow the team to prioritize care (83%), and provide patient education on their disease or medications (92%).
Though APPs are often utilized based on the individual needs of the practice, physician understanding of the APP skillset (83%) and a clear job description with set expectations up front (75%) were two critical elements of practice integration and job satisfaction on qualitative APP surveys. Additionally, APPs felt that strong mentorship with opportunities for career growth could enhance career satisfaction and improve the overall retention of the APP (100%).
The physician perspective
Informed by themes identified from the qualitative APP survey, we posted an informal, anonymous online survey to physicians on the AGA Community Forum. Nearly all physicians that worked with an APP felt that they were beneficial to their practice. Ninety-seven percent of respondents found that APPs improved patient access to the clinic, while 47% found that APPs decreased phone calls and 43% found that APPs improved administrative burden. Other less commonly cited benefits of APPs included increased practice revenue, improved efficiency of inpatient care, and assistance with procedures.
In building relationships and developing trust with their APPs, respondents valued communication (94%), observed or measured competency through orientation or standardized training (55%), and increased time comanaging patients (48%). However, 52% of respondents were concerned regarding the time required to train an APP to their standards, 45% were concerned regarding knowledge deficits, and 48% were concerned regarding risk of turnover and burnout. Though patient satisfaction was noted as a possible benefit of a physician/APP team approach, physicians also noted a potential concern that it may compromise the existing physician/patient relationship.
Despite concerns regarding training and knowledge deficits, only 29% of respondents had a standard orientation for APPs, 26% had a clearly defined job description, and 32% had formal teaching in their specialty content area.
Developing a model for success
Based on the results of these surveys and our practice experience, we present seven recommendations to optimize the APP/physician relationship:
1. Create a clear job description that ensures your APP works to the top of their license and training. This key principle can have a great impact on practice revenue and APP job satisfaction.
2. Develop a plan to train the APP to your standards, whether it be through a dedicated content curriculum or a mentored preceptorship. Most APPs finish formal training with very little gastroenterology specialty expertise, and would benefit from content-based learning in the area of gastroenterology in which they will work. The AGA publishes on-demand webcasts in different content areas, geared toward advanced practice providers (https://www.gastro.org/aga-leadership/initiatives-and-programs/nurse-practitioner-and-physician-assistant-resource-center). The AGA also hosts an annual conference to review GI content and prepare APPs to deliver optimal patient care (https://nppa.gastro.org/).
3. Designate objective criteria by which you will measure competency. Share this model with your APP up front to establish transparent expectations, and meet to review competencies and plans for further training at least annually. This structure presents a model for clinical growth and transparent expectations may enhance APP retention.
4. Establish APP mentorship. Just as for physicians, both clinical and career mentorship are an important part of job satisfaction and retention for APPs.
• Meet regularly. We recommend that mentors schedule weekly meetings with their APPs to review cases, questions/concerns, outstanding clinical work, quality-improvement initiatives and/or research. These regular meetings will keep lines of communication open and may enhance APP retention.
• Provide feedback. Both APPs and physicians benefit from constructive feedback. An annual review should not bring any surprises. Keeping feedback honest and constructive will further strengthen the relationship.
5. Introduce the APP as an integral member of the care team during the initial patient encounter. Whether working in a dedicated subspecialty team (inflammatory bowel disease, hepatology, motility, or hepatobiliary) or as part of a general gastroenterology practice, APPs should be introduced during the initial encounter as a key member of the team to establish rapport. The APP’s name should also be listed in the after-visit summary, on business cards, and on stationary to strengthen the team image. Once a patient is established with an APP and a therapeutic relationship is built, patients often report positive outcomes and maintain follow-up with the APP/physician team. We recommend that the physician see the patient at least every other visit (alternating with the APP) to reinforce the team dynamic and dedication of all members of the team to the patient’s health.
6. Provide a sense of community. Depending on the size of your practice, you can connect APPs within your practice, institution, or at a professional organization level. Belonging to a larger group that understands APP practice provides strong support and APP career satisfaction.
7. Create growth opportunities. In addition to clinical growth, APPs can provide value in leading quality-improvement and research initiatives. Establish goals and timelines for achieving goals up front, and be prepared to protect the APP’s time to achieve these goals. Successful APP growth and development may enhance job satisfaction and lead to reduced turnover. In addition, establishment of APP leaders provides candidates to help design and implement an effective APP program as a practice grows.
The authors wish to recognize research coordinators Casey Silvernale and April Mendez, and Dr. Kyle Staller who assisted with the coordination of the surveys that contributed to this work. Dr. Burke is a gastroenterolgist affiliated with Massachusetts General Hospital, Boston; Dr. Thurler is the Ambulatory Director of advanced practice providers and nursing at Massachusetts General Hospital. The authors had no disclosures.
This story was updated on June 26, 2019.
Advanced practice providers (APPs; physician assistants and nurse practitioners) play a vital role in the success of an academic or private gastroenterology practice. Partnership with APPs in the clinical setting can improve inpatient and outpatient workflow and complex chronic care management, optimizing downstream revenue from endoscopy, radiology, and motility studies and enhancing physician productivity in research or academic affairs. In an informal AGA Community survey of physicians throughout the United States, 86% of respondents worked with advanced practice providers, 61% of whom had done so for over 5 years. While APPs may fill diverse roles in gastroenterology practice, there are common principles that may help optimize the physician-APP relationship. We surveyed both APPs and physicians to gain their perspective and present a tool kit to optimize the relationship among APPs and physicians.
The APP perspective
In qualitative interviews with 12 APPs practicing gastroenterology in a variety of specialties in Massachusetts, we aimed to understand 1) what APPs felt they brought to GI practice and 2) how APPs can be best utilized and integrated into GI practice and flow.
All interviewees independently noted that improving patient access to care and providing continuity of care were key benefits they brought to their practice, resulting in the possible downstream prevention of unnecessary emergency room admissions. Additionally, APPs felt that they brought significant value by having the time to listen to patient concerns to allow the team to prioritize care (83%), and provide patient education on their disease or medications (92%).
Though APPs are often utilized based on the individual needs of the practice, physician understanding of the APP skillset (83%) and a clear job description with set expectations up front (75%) were two critical elements of practice integration and job satisfaction on qualitative APP surveys. Additionally, APPs felt that strong mentorship with opportunities for career growth could enhance career satisfaction and improve the overall retention of the APP (100%).
The physician perspective
Informed by themes identified from the qualitative APP survey, we posted an informal, anonymous online survey to physicians on the AGA Community Forum. Nearly all physicians that worked with an APP felt that they were beneficial to their practice. Ninety-seven percent of respondents found that APPs improved patient access to the clinic, while 47% found that APPs decreased phone calls and 43% found that APPs improved administrative burden. Other less commonly cited benefits of APPs included increased practice revenue, improved efficiency of inpatient care, and assistance with procedures.
In building relationships and developing trust with their APPs, respondents valued communication (94%), observed or measured competency through orientation or standardized training (55%), and increased time comanaging patients (48%). However, 52% of respondents were concerned regarding the time required to train an APP to their standards, 45% were concerned regarding knowledge deficits, and 48% were concerned regarding risk of turnover and burnout. Though patient satisfaction was noted as a possible benefit of a physician/APP team approach, physicians also noted a potential concern that it may compromise the existing physician/patient relationship.
Despite concerns regarding training and knowledge deficits, only 29% of respondents had a standard orientation for APPs, 26% had a clearly defined job description, and 32% had formal teaching in their specialty content area.
Developing a model for success
Based on the results of these surveys and our practice experience, we present seven recommendations to optimize the APP/physician relationship:
1. Create a clear job description that ensures your APP works to the top of their license and training. This key principle can have a great impact on practice revenue and APP job satisfaction.
2. Develop a plan to train the APP to your standards, whether it be through a dedicated content curriculum or a mentored preceptorship. Most APPs finish formal training with very little gastroenterology specialty expertise, and would benefit from content-based learning in the area of gastroenterology in which they will work. The AGA publishes on-demand webcasts in different content areas, geared toward advanced practice providers (https://www.gastro.org/aga-leadership/initiatives-and-programs/nurse-practitioner-and-physician-assistant-resource-center). The AGA also hosts an annual conference to review GI content and prepare APPs to deliver optimal patient care (https://nppa.gastro.org/).
3. Designate objective criteria by which you will measure competency. Share this model with your APP up front to establish transparent expectations, and meet to review competencies and plans for further training at least annually. This structure presents a model for clinical growth and transparent expectations may enhance APP retention.
4. Establish APP mentorship. Just as for physicians, both clinical and career mentorship are an important part of job satisfaction and retention for APPs.
• Meet regularly. We recommend that mentors schedule weekly meetings with their APPs to review cases, questions/concerns, outstanding clinical work, quality-improvement initiatives and/or research. These regular meetings will keep lines of communication open and may enhance APP retention.
• Provide feedback. Both APPs and physicians benefit from constructive feedback. An annual review should not bring any surprises. Keeping feedback honest and constructive will further strengthen the relationship.
5. Introduce the APP as an integral member of the care team during the initial patient encounter. Whether working in a dedicated subspecialty team (inflammatory bowel disease, hepatology, motility, or hepatobiliary) or as part of a general gastroenterology practice, APPs should be introduced during the initial encounter as a key member of the team to establish rapport. The APP’s name should also be listed in the after-visit summary, on business cards, and on stationary to strengthen the team image. Once a patient is established with an APP and a therapeutic relationship is built, patients often report positive outcomes and maintain follow-up with the APP/physician team. We recommend that the physician see the patient at least every other visit (alternating with the APP) to reinforce the team dynamic and dedication of all members of the team to the patient’s health.
6. Provide a sense of community. Depending on the size of your practice, you can connect APPs within your practice, institution, or at a professional organization level. Belonging to a larger group that understands APP practice provides strong support and APP career satisfaction.
7. Create growth opportunities. In addition to clinical growth, APPs can provide value in leading quality-improvement and research initiatives. Establish goals and timelines for achieving goals up front, and be prepared to protect the APP’s time to achieve these goals. Successful APP growth and development may enhance job satisfaction and lead to reduced turnover. In addition, establishment of APP leaders provides candidates to help design and implement an effective APP program as a practice grows.
The authors wish to recognize research coordinators Casey Silvernale and April Mendez, and Dr. Kyle Staller who assisted with the coordination of the surveys that contributed to this work. Dr. Burke is a gastroenterolgist affiliated with Massachusetts General Hospital, Boston; Dr. Thurler is the Ambulatory Director of advanced practice providers and nursing at Massachusetts General Hospital. The authors had no disclosures.
This story was updated on June 26, 2019.
Advanced practice providers (APPs; physician assistants and nurse practitioners) play a vital role in the success of an academic or private gastroenterology practice. Partnership with APPs in the clinical setting can improve inpatient and outpatient workflow and complex chronic care management, optimizing downstream revenue from endoscopy, radiology, and motility studies and enhancing physician productivity in research or academic affairs. In an informal AGA Community survey of physicians throughout the United States, 86% of respondents worked with advanced practice providers, 61% of whom had done so for over 5 years. While APPs may fill diverse roles in gastroenterology practice, there are common principles that may help optimize the physician-APP relationship. We surveyed both APPs and physicians to gain their perspective and present a tool kit to optimize the relationship among APPs and physicians.
The APP perspective
In qualitative interviews with 12 APPs practicing gastroenterology in a variety of specialties in Massachusetts, we aimed to understand 1) what APPs felt they brought to GI practice and 2) how APPs can be best utilized and integrated into GI practice and flow.
All interviewees independently noted that improving patient access to care and providing continuity of care were key benefits they brought to their practice, resulting in the possible downstream prevention of unnecessary emergency room admissions. Additionally, APPs felt that they brought significant value by having the time to listen to patient concerns to allow the team to prioritize care (83%), and provide patient education on their disease or medications (92%).
Though APPs are often utilized based on the individual needs of the practice, physician understanding of the APP skillset (83%) and a clear job description with set expectations up front (75%) were two critical elements of practice integration and job satisfaction on qualitative APP surveys. Additionally, APPs felt that strong mentorship with opportunities for career growth could enhance career satisfaction and improve the overall retention of the APP (100%).
The physician perspective
Informed by themes identified from the qualitative APP survey, we posted an informal, anonymous online survey to physicians on the AGA Community Forum. Nearly all physicians that worked with an APP felt that they were beneficial to their practice. Ninety-seven percent of respondents found that APPs improved patient access to the clinic, while 47% found that APPs decreased phone calls and 43% found that APPs improved administrative burden. Other less commonly cited benefits of APPs included increased practice revenue, improved efficiency of inpatient care, and assistance with procedures.
In building relationships and developing trust with their APPs, respondents valued communication (94%), observed or measured competency through orientation or standardized training (55%), and increased time comanaging patients (48%). However, 52% of respondents were concerned regarding the time required to train an APP to their standards, 45% were concerned regarding knowledge deficits, and 48% were concerned regarding risk of turnover and burnout. Though patient satisfaction was noted as a possible benefit of a physician/APP team approach, physicians also noted a potential concern that it may compromise the existing physician/patient relationship.
Despite concerns regarding training and knowledge deficits, only 29% of respondents had a standard orientation for APPs, 26% had a clearly defined job description, and 32% had formal teaching in their specialty content area.
Developing a model for success
Based on the results of these surveys and our practice experience, we present seven recommendations to optimize the APP/physician relationship:
1. Create a clear job description that ensures your APP works to the top of their license and training. This key principle can have a great impact on practice revenue and APP job satisfaction.
2. Develop a plan to train the APP to your standards, whether it be through a dedicated content curriculum or a mentored preceptorship. Most APPs finish formal training with very little gastroenterology specialty expertise, and would benefit from content-based learning in the area of gastroenterology in which they will work. The AGA publishes on-demand webcasts in different content areas, geared toward advanced practice providers (https://www.gastro.org/aga-leadership/initiatives-and-programs/nurse-practitioner-and-physician-assistant-resource-center). The AGA also hosts an annual conference to review GI content and prepare APPs to deliver optimal patient care (https://nppa.gastro.org/).
3. Designate objective criteria by which you will measure competency. Share this model with your APP up front to establish transparent expectations, and meet to review competencies and plans for further training at least annually. This structure presents a model for clinical growth and transparent expectations may enhance APP retention.
4. Establish APP mentorship. Just as for physicians, both clinical and career mentorship are an important part of job satisfaction and retention for APPs.
• Meet regularly. We recommend that mentors schedule weekly meetings with their APPs to review cases, questions/concerns, outstanding clinical work, quality-improvement initiatives and/or research. These regular meetings will keep lines of communication open and may enhance APP retention.
• Provide feedback. Both APPs and physicians benefit from constructive feedback. An annual review should not bring any surprises. Keeping feedback honest and constructive will further strengthen the relationship.
5. Introduce the APP as an integral member of the care team during the initial patient encounter. Whether working in a dedicated subspecialty team (inflammatory bowel disease, hepatology, motility, or hepatobiliary) or as part of a general gastroenterology practice, APPs should be introduced during the initial encounter as a key member of the team to establish rapport. The APP’s name should also be listed in the after-visit summary, on business cards, and on stationary to strengthen the team image. Once a patient is established with an APP and a therapeutic relationship is built, patients often report positive outcomes and maintain follow-up with the APP/physician team. We recommend that the physician see the patient at least every other visit (alternating with the APP) to reinforce the team dynamic and dedication of all members of the team to the patient’s health.
6. Provide a sense of community. Depending on the size of your practice, you can connect APPs within your practice, institution, or at a professional organization level. Belonging to a larger group that understands APP practice provides strong support and APP career satisfaction.
7. Create growth opportunities. In addition to clinical growth, APPs can provide value in leading quality-improvement and research initiatives. Establish goals and timelines for achieving goals up front, and be prepared to protect the APP’s time to achieve these goals. Successful APP growth and development may enhance job satisfaction and lead to reduced turnover. In addition, establishment of APP leaders provides candidates to help design and implement an effective APP program as a practice grows.
The authors wish to recognize research coordinators Casey Silvernale and April Mendez, and Dr. Kyle Staller who assisted with the coordination of the surveys that contributed to this work. Dr. Burke is a gastroenterolgist affiliated with Massachusetts General Hospital, Boston; Dr. Thurler is the Ambulatory Director of advanced practice providers and nursing at Massachusetts General Hospital. The authors had no disclosures.
This story was updated on June 26, 2019.
Peanut desensitization comes at cost of anaphylaxis
based on a meta-analysis from more than 1,000 patients published in the Lancet.
In the Peanut Allergen immunotherapy, Clarifying the Evidence (PACE) systematic review and meta-analysis, Derek K. Chu, MD, of McMaster University, Hamilton, Ont., and colleagues reviewed 12 trials conducted between 2011 and 2018 with a total of 1,041 patients (median age, 9 years).
Overall, the risk of anaphylaxis was significantly higher among children who received oral immunotherapy, compared with no therapy (risk ratio, 3.12) as was anaphylaxis frequency (incidence rate ratio, 2.72) and use of epinephrine (RR, 2.21).
In addition, oral immunotherapy increased serious adverse events, compared with no therapy (RR, 1.92). Nonanaphylactic reactions also went up among oral immunotherapy patients, with increased risk for vomiting (RR, 1.79), angioedema (RR, 2.25), upper respiratory tract reactions (RR, 1.36), and lower respiratory tract infections (RR, 1.55).
Quality of life scores were not significantly different between patients who did and did not receive oral immunotherapy, the researchers noted.
The oral immunotherapy consisted of defatted, lightly roasted peanut flour in 10 studies, and a combination of peanut paste, peanut extract, or ground and defatted peanut in the other studies.
The oral immunotherapy did induce desensitization to peanuts in support of earlier studies including the subcutaneous immunotherapy trial, but “this outcome does not translate into achieving the clinical and patient-desired aim of less allergic reactions and anaphylaxis,” Dr. Chu and associates wrote.
However, “rather than take the view that these data denounce current research in oral immunotherapy as not successful, we instead suggest that this research has reached an important milestone in mechanistic but not clinical efficacy. From a clinical or biological perspective, the apparently paradoxical desensitization versus longitudinal clinical findings show the lability and unreliability of allergen thresholds identified during oral food challenges because patients often unpredictably reacted to previously tolerated doses outside of clinic,” they emphasized.
The findings were limited by several factors including the small sample size, compared with similar studies for asthma or cardiovascular conditions, and by incomplete or inconsistent data reporting, the researchers noted. However, the results are the most comprehensive to date, and support the need for food allergy treatments with better safety profiles, using peanut allergy immunotherapy as a model for other food allergies.
Dr. Chu and two other authors reported being investigators on a federally funded ongoing peanut oral immunotherapy trial. Two authors reported receiving a variety of grants from organizations such as the National Institutes of Health; the American Academy of Allergy, Asthma, & Immunology; or pharmaceutical companies.
SOURCE: Chu DK et al. Lancet. 2019 June 1;393:2222-32.
“The key criticism of this systematic review is inherent in its method because studies with different designs were grouped together,” Graham Roberts, MD, and Elizabeth Angier, MD, wrote in an accompanying editorial. In addition, the studies chosen did not account for the development of long-term peanut tolerance after the therapy was discontinued.
Also, the researchers did not factor in the variation in patterns of anaphylactic events, with patients in the treatment groups having events at home in conjunction with daily peanut doses, while the control patients would have had events mainly away from home.
“Unfortunately, the trials have not provided information about which participants benefited most from the intervention,” they wrote.
“Trading treatment-related side effects at home for allergic reactions to accidental exposures out of the house [i.e., in social situations] might beneficial for some patients,” they added. However, more research is needed to determine which patients would benefit from different treatment options at home and outside the home. The less effective but safer option of epicutaneous immunotherapy might be preferred by some patients. And early introduction of peanut products during infancy may prevent many cases of peanut allergy.
Dr. Roberts and Dr. Angier are at the University of Southampton (England). Both are members of the European Academy of Allergy and Clinical Immunology Allergen Immunotherapy Guidelines Group, which has recently published guidelines on immunotherapy. They wrote an editorial to accompany the article by Chu et al (Lancet. 2019 June 1;393:2180-1). They had no financial conflicts to disclose.
“The key criticism of this systematic review is inherent in its method because studies with different designs were grouped together,” Graham Roberts, MD, and Elizabeth Angier, MD, wrote in an accompanying editorial. In addition, the studies chosen did not account for the development of long-term peanut tolerance after the therapy was discontinued.
Also, the researchers did not factor in the variation in patterns of anaphylactic events, with patients in the treatment groups having events at home in conjunction with daily peanut doses, while the control patients would have had events mainly away from home.
“Unfortunately, the trials have not provided information about which participants benefited most from the intervention,” they wrote.
“Trading treatment-related side effects at home for allergic reactions to accidental exposures out of the house [i.e., in social situations] might beneficial for some patients,” they added. However, more research is needed to determine which patients would benefit from different treatment options at home and outside the home. The less effective but safer option of epicutaneous immunotherapy might be preferred by some patients. And early introduction of peanut products during infancy may prevent many cases of peanut allergy.
Dr. Roberts and Dr. Angier are at the University of Southampton (England). Both are members of the European Academy of Allergy and Clinical Immunology Allergen Immunotherapy Guidelines Group, which has recently published guidelines on immunotherapy. They wrote an editorial to accompany the article by Chu et al (Lancet. 2019 June 1;393:2180-1). They had no financial conflicts to disclose.
“The key criticism of this systematic review is inherent in its method because studies with different designs were grouped together,” Graham Roberts, MD, and Elizabeth Angier, MD, wrote in an accompanying editorial. In addition, the studies chosen did not account for the development of long-term peanut tolerance after the therapy was discontinued.
Also, the researchers did not factor in the variation in patterns of anaphylactic events, with patients in the treatment groups having events at home in conjunction with daily peanut doses, while the control patients would have had events mainly away from home.
“Unfortunately, the trials have not provided information about which participants benefited most from the intervention,” they wrote.
“Trading treatment-related side effects at home for allergic reactions to accidental exposures out of the house [i.e., in social situations] might beneficial for some patients,” they added. However, more research is needed to determine which patients would benefit from different treatment options at home and outside the home. The less effective but safer option of epicutaneous immunotherapy might be preferred by some patients. And early introduction of peanut products during infancy may prevent many cases of peanut allergy.
Dr. Roberts and Dr. Angier are at the University of Southampton (England). Both are members of the European Academy of Allergy and Clinical Immunology Allergen Immunotherapy Guidelines Group, which has recently published guidelines on immunotherapy. They wrote an editorial to accompany the article by Chu et al (Lancet. 2019 June 1;393:2180-1). They had no financial conflicts to disclose.
based on a meta-analysis from more than 1,000 patients published in the Lancet.
In the Peanut Allergen immunotherapy, Clarifying the Evidence (PACE) systematic review and meta-analysis, Derek K. Chu, MD, of McMaster University, Hamilton, Ont., and colleagues reviewed 12 trials conducted between 2011 and 2018 with a total of 1,041 patients (median age, 9 years).
Overall, the risk of anaphylaxis was significantly higher among children who received oral immunotherapy, compared with no therapy (risk ratio, 3.12) as was anaphylaxis frequency (incidence rate ratio, 2.72) and use of epinephrine (RR, 2.21).
In addition, oral immunotherapy increased serious adverse events, compared with no therapy (RR, 1.92). Nonanaphylactic reactions also went up among oral immunotherapy patients, with increased risk for vomiting (RR, 1.79), angioedema (RR, 2.25), upper respiratory tract reactions (RR, 1.36), and lower respiratory tract infections (RR, 1.55).
Quality of life scores were not significantly different between patients who did and did not receive oral immunotherapy, the researchers noted.
The oral immunotherapy consisted of defatted, lightly roasted peanut flour in 10 studies, and a combination of peanut paste, peanut extract, or ground and defatted peanut in the other studies.
The oral immunotherapy did induce desensitization to peanuts in support of earlier studies including the subcutaneous immunotherapy trial, but “this outcome does not translate into achieving the clinical and patient-desired aim of less allergic reactions and anaphylaxis,” Dr. Chu and associates wrote.
However, “rather than take the view that these data denounce current research in oral immunotherapy as not successful, we instead suggest that this research has reached an important milestone in mechanistic but not clinical efficacy. From a clinical or biological perspective, the apparently paradoxical desensitization versus longitudinal clinical findings show the lability and unreliability of allergen thresholds identified during oral food challenges because patients often unpredictably reacted to previously tolerated doses outside of clinic,” they emphasized.
The findings were limited by several factors including the small sample size, compared with similar studies for asthma or cardiovascular conditions, and by incomplete or inconsistent data reporting, the researchers noted. However, the results are the most comprehensive to date, and support the need for food allergy treatments with better safety profiles, using peanut allergy immunotherapy as a model for other food allergies.
Dr. Chu and two other authors reported being investigators on a federally funded ongoing peanut oral immunotherapy trial. Two authors reported receiving a variety of grants from organizations such as the National Institutes of Health; the American Academy of Allergy, Asthma, & Immunology; or pharmaceutical companies.
SOURCE: Chu DK et al. Lancet. 2019 June 1;393:2222-32.
based on a meta-analysis from more than 1,000 patients published in the Lancet.
In the Peanut Allergen immunotherapy, Clarifying the Evidence (PACE) systematic review and meta-analysis, Derek K. Chu, MD, of McMaster University, Hamilton, Ont., and colleagues reviewed 12 trials conducted between 2011 and 2018 with a total of 1,041 patients (median age, 9 years).
Overall, the risk of anaphylaxis was significantly higher among children who received oral immunotherapy, compared with no therapy (risk ratio, 3.12) as was anaphylaxis frequency (incidence rate ratio, 2.72) and use of epinephrine (RR, 2.21).
In addition, oral immunotherapy increased serious adverse events, compared with no therapy (RR, 1.92). Nonanaphylactic reactions also went up among oral immunotherapy patients, with increased risk for vomiting (RR, 1.79), angioedema (RR, 2.25), upper respiratory tract reactions (RR, 1.36), and lower respiratory tract infections (RR, 1.55).
Quality of life scores were not significantly different between patients who did and did not receive oral immunotherapy, the researchers noted.
The oral immunotherapy consisted of defatted, lightly roasted peanut flour in 10 studies, and a combination of peanut paste, peanut extract, or ground and defatted peanut in the other studies.
The oral immunotherapy did induce desensitization to peanuts in support of earlier studies including the subcutaneous immunotherapy trial, but “this outcome does not translate into achieving the clinical and patient-desired aim of less allergic reactions and anaphylaxis,” Dr. Chu and associates wrote.
However, “rather than take the view that these data denounce current research in oral immunotherapy as not successful, we instead suggest that this research has reached an important milestone in mechanistic but not clinical efficacy. From a clinical or biological perspective, the apparently paradoxical desensitization versus longitudinal clinical findings show the lability and unreliability of allergen thresholds identified during oral food challenges because patients often unpredictably reacted to previously tolerated doses outside of clinic,” they emphasized.
The findings were limited by several factors including the small sample size, compared with similar studies for asthma or cardiovascular conditions, and by incomplete or inconsistent data reporting, the researchers noted. However, the results are the most comprehensive to date, and support the need for food allergy treatments with better safety profiles, using peanut allergy immunotherapy as a model for other food allergies.
Dr. Chu and two other authors reported being investigators on a federally funded ongoing peanut oral immunotherapy trial. Two authors reported receiving a variety of grants from organizations such as the National Institutes of Health; the American Academy of Allergy, Asthma, & Immunology; or pharmaceutical companies.
SOURCE: Chu DK et al. Lancet. 2019 June 1;393:2222-32.
FROM THE LANCET
Topical calcineurin inhibitors prove beneficial for patients with vitiligo
Though responses to topical calcineurin inhibitors (TCIs) plus phototherapy were found to be higher than TCI monotherapy, a meta-analysis of studies on TCI therapy found that both should be used in treatment for patients with vitiligo.
“In addition, the proactive use of TCIs to maintain remission of vitiligo could be promising, considering its high recurrence rate,” wrote Ji Hae Lee, MD, PhD, of the Catholic University of Korea, Seoul, and coauthors in JAMA Dermatology.
To assess TCIs as treatment for vitiligo, the researchers undertook a systematic review and analysis of 56 relevant studies. Eleven of the studies were on the TCI mechanism; 36 were on TCI monotherapy; 12 were on TCI plus phototherapy; and 1 was on TCI maintenance therapy. Treatment responses for each study were measured via the degree of repigmentation on a quartile scale: an at least mild response (25% or greater repigmentation), at least moderate response (50% or greater repigmentation), and marked response (75% or greater repigmentation).
In regard to TCI monotherapy, an at least mild response was achieved in 55% (95% confidence interval, 42.2%-67.8%) of 560 patients in 21 studies. An at least moderate response was achieved in 38.5% (95% CI, 28.2%-48.8%) of 619 patients in 23 studies, and there was a marked response in 18.1% (95% CI, 13.2%-23.1%) of 520 patients in 19 studies.
For TCI plus phototherapy, an at least mild response was achieved in 89.5% (95% CI, 81.1%-97.9%) of 433 patients in eight studies. An at least moderate response was achieved in 72.9% (95% CI, 57.6%-88.2%) of 486 patients in 10 studies, and a marked response was achieved in 47.5% (95% CI, 30.6%-64.4%) of 490 patients in 9 studies.
The authors noted several limitations with their review, including a level of heterogeneity in the study designs, characteristics of the patients, and protocols. They also acknowledged that the quartile scale may be somewhat arbitrary in nature, though they added that it has been the “most commonly used measure and would have been one of the best estimates of the treatment response at this time.”
The authors report no conflicts of interest.
SOURCE: Lee JH et al. Jama Dermatol. 2019 May 29. doi: 10.1001/Jamadermatol.2019.0696.
Though responses to topical calcineurin inhibitors (TCIs) plus phototherapy were found to be higher than TCI monotherapy, a meta-analysis of studies on TCI therapy found that both should be used in treatment for patients with vitiligo.
“In addition, the proactive use of TCIs to maintain remission of vitiligo could be promising, considering its high recurrence rate,” wrote Ji Hae Lee, MD, PhD, of the Catholic University of Korea, Seoul, and coauthors in JAMA Dermatology.
To assess TCIs as treatment for vitiligo, the researchers undertook a systematic review and analysis of 56 relevant studies. Eleven of the studies were on the TCI mechanism; 36 were on TCI monotherapy; 12 were on TCI plus phototherapy; and 1 was on TCI maintenance therapy. Treatment responses for each study were measured via the degree of repigmentation on a quartile scale: an at least mild response (25% or greater repigmentation), at least moderate response (50% or greater repigmentation), and marked response (75% or greater repigmentation).
In regard to TCI monotherapy, an at least mild response was achieved in 55% (95% confidence interval, 42.2%-67.8%) of 560 patients in 21 studies. An at least moderate response was achieved in 38.5% (95% CI, 28.2%-48.8%) of 619 patients in 23 studies, and there was a marked response in 18.1% (95% CI, 13.2%-23.1%) of 520 patients in 19 studies.
For TCI plus phototherapy, an at least mild response was achieved in 89.5% (95% CI, 81.1%-97.9%) of 433 patients in eight studies. An at least moderate response was achieved in 72.9% (95% CI, 57.6%-88.2%) of 486 patients in 10 studies, and a marked response was achieved in 47.5% (95% CI, 30.6%-64.4%) of 490 patients in 9 studies.
The authors noted several limitations with their review, including a level of heterogeneity in the study designs, characteristics of the patients, and protocols. They also acknowledged that the quartile scale may be somewhat arbitrary in nature, though they added that it has been the “most commonly used measure and would have been one of the best estimates of the treatment response at this time.”
The authors report no conflicts of interest.
SOURCE: Lee JH et al. Jama Dermatol. 2019 May 29. doi: 10.1001/Jamadermatol.2019.0696.
Though responses to topical calcineurin inhibitors (TCIs) plus phototherapy were found to be higher than TCI monotherapy, a meta-analysis of studies on TCI therapy found that both should be used in treatment for patients with vitiligo.
“In addition, the proactive use of TCIs to maintain remission of vitiligo could be promising, considering its high recurrence rate,” wrote Ji Hae Lee, MD, PhD, of the Catholic University of Korea, Seoul, and coauthors in JAMA Dermatology.
To assess TCIs as treatment for vitiligo, the researchers undertook a systematic review and analysis of 56 relevant studies. Eleven of the studies were on the TCI mechanism; 36 were on TCI monotherapy; 12 were on TCI plus phototherapy; and 1 was on TCI maintenance therapy. Treatment responses for each study were measured via the degree of repigmentation on a quartile scale: an at least mild response (25% or greater repigmentation), at least moderate response (50% or greater repigmentation), and marked response (75% or greater repigmentation).
In regard to TCI monotherapy, an at least mild response was achieved in 55% (95% confidence interval, 42.2%-67.8%) of 560 patients in 21 studies. An at least moderate response was achieved in 38.5% (95% CI, 28.2%-48.8%) of 619 patients in 23 studies, and there was a marked response in 18.1% (95% CI, 13.2%-23.1%) of 520 patients in 19 studies.
For TCI plus phototherapy, an at least mild response was achieved in 89.5% (95% CI, 81.1%-97.9%) of 433 patients in eight studies. An at least moderate response was achieved in 72.9% (95% CI, 57.6%-88.2%) of 486 patients in 10 studies, and a marked response was achieved in 47.5% (95% CI, 30.6%-64.4%) of 490 patients in 9 studies.
The authors noted several limitations with their review, including a level of heterogeneity in the study designs, characteristics of the patients, and protocols. They also acknowledged that the quartile scale may be somewhat arbitrary in nature, though they added that it has been the “most commonly used measure and would have been one of the best estimates of the treatment response at this time.”
The authors report no conflicts of interest.
SOURCE: Lee JH et al. Jama Dermatol. 2019 May 29. doi: 10.1001/Jamadermatol.2019.0696.
FROM JAMA Dermatology