User login
Vascular Trainees: Apply for the VRIC Travel Scholarship
The SVS Foundation is supporting travel scholarships for trainees to attend the annual Vascular Research Initiatives Conference (VRIC), which will be held on May 13, 2019, in Boston. Recipients of the scholarship will receive complimentary registration to both VRIC and the Vascular Discovery Scientific Sessions, as well as a $1,000 award for conference travel. VRIC is considered a key event for connecting with vascular researchers and the theme this year is Hard Science: Calcification and Vascular Solution. Learn more and apply today. VRIC registration is now open; register today.
The SVS Foundation is supporting travel scholarships for trainees to attend the annual Vascular Research Initiatives Conference (VRIC), which will be held on May 13, 2019, in Boston. Recipients of the scholarship will receive complimentary registration to both VRIC and the Vascular Discovery Scientific Sessions, as well as a $1,000 award for conference travel. VRIC is considered a key event for connecting with vascular researchers and the theme this year is Hard Science: Calcification and Vascular Solution. Learn more and apply today. VRIC registration is now open; register today.
The SVS Foundation is supporting travel scholarships for trainees to attend the annual Vascular Research Initiatives Conference (VRIC), which will be held on May 13, 2019, in Boston. Recipients of the scholarship will receive complimentary registration to both VRIC and the Vascular Discovery Scientific Sessions, as well as a $1,000 award for conference travel. VRIC is considered a key event for connecting with vascular researchers and the theme this year is Hard Science: Calcification and Vascular Solution. Learn more and apply today. VRIC registration is now open; register today.
If You Had to Do It Again …
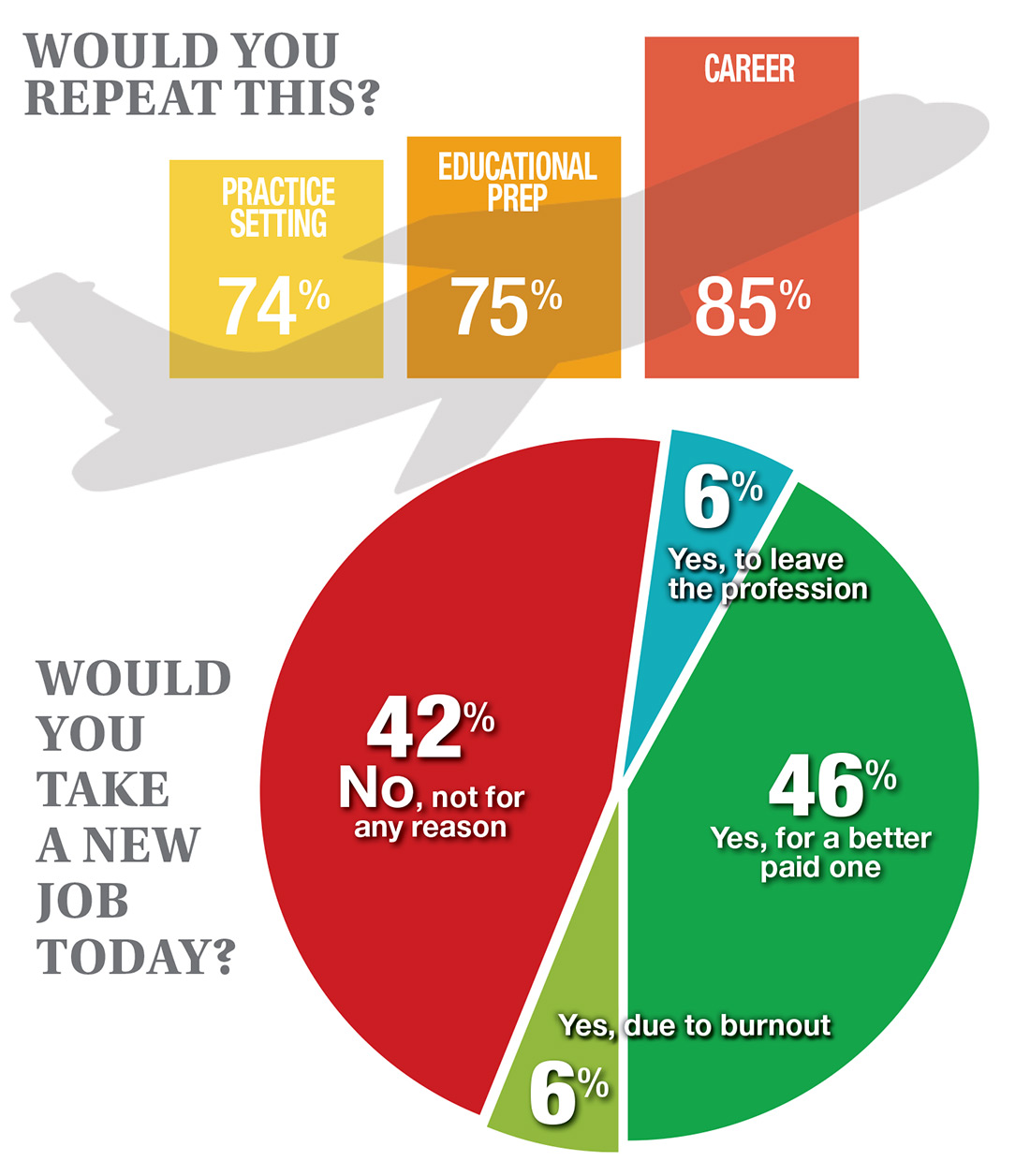
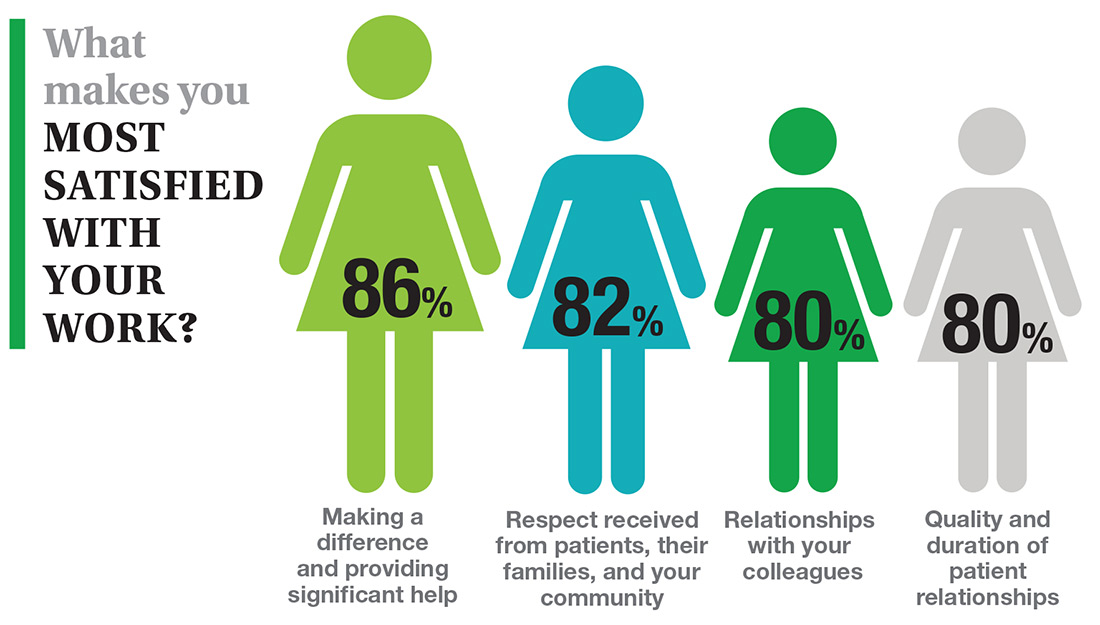
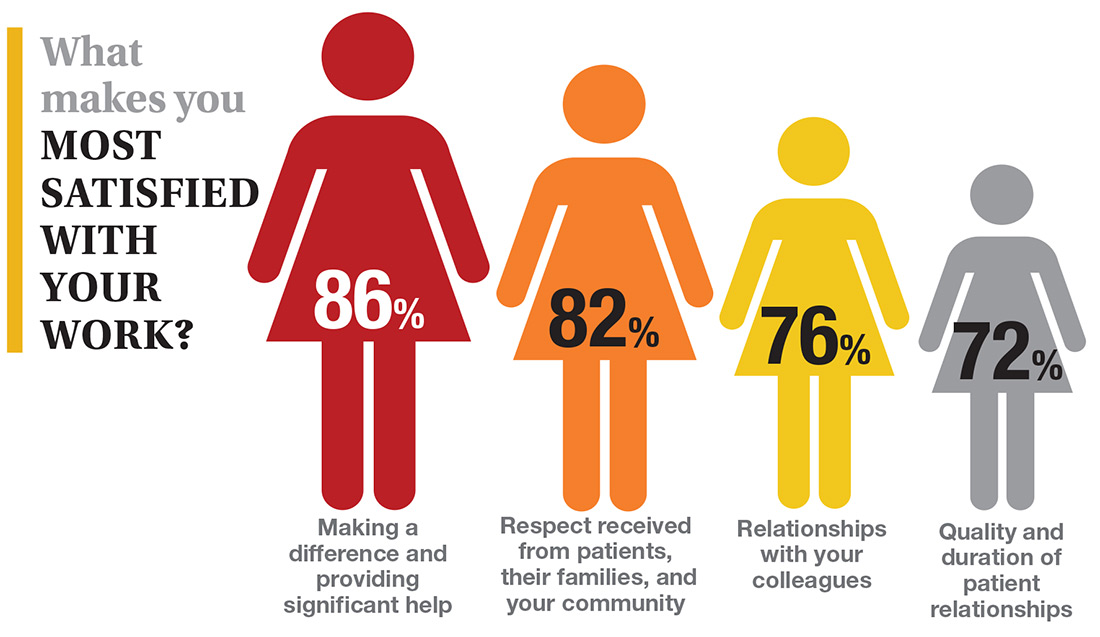
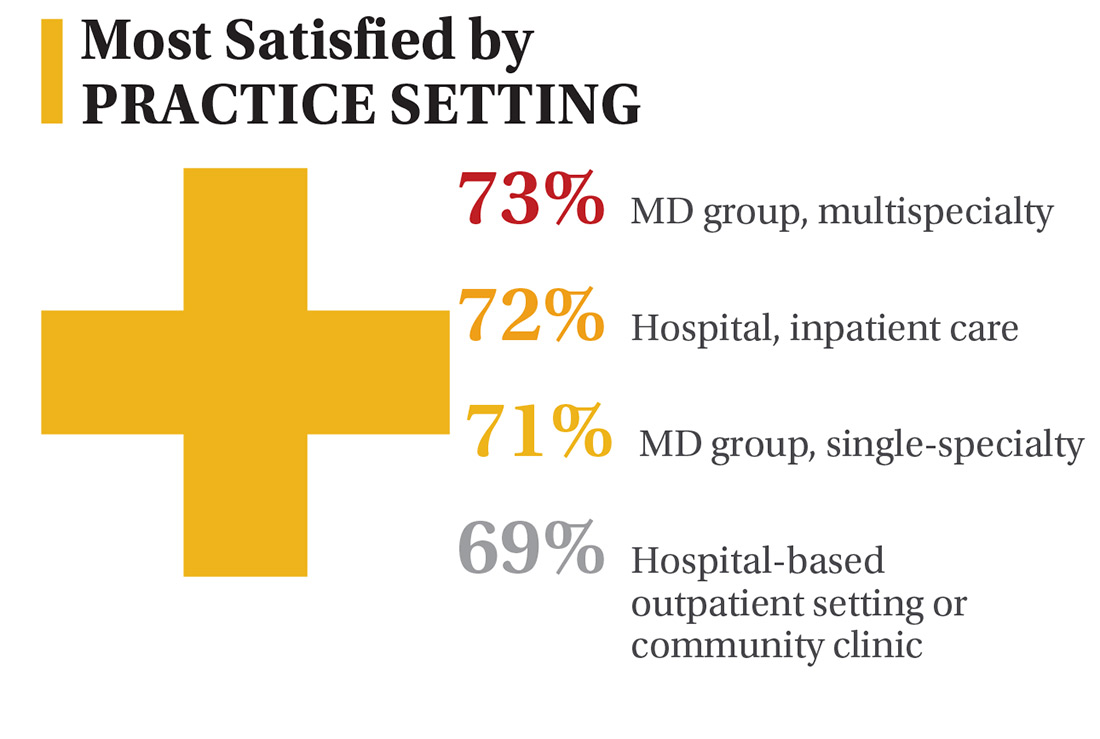
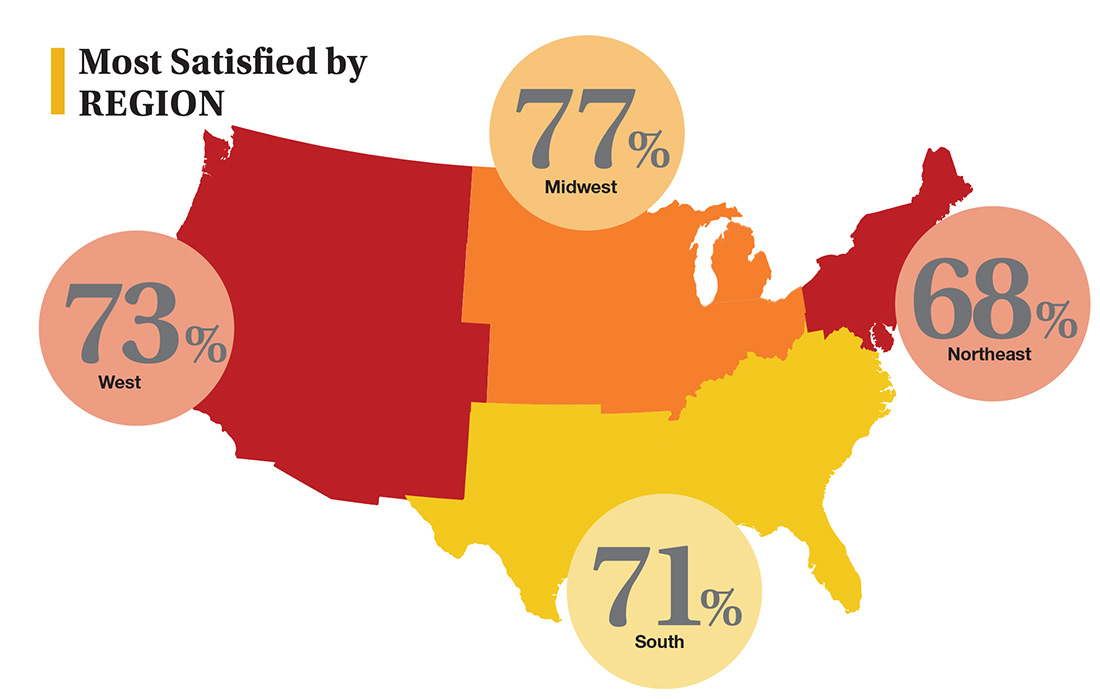
In this era of “burnout”—when a PubMed search of the term yields more than 13,000 results—it’s heartening to discover that 72% of all clinicians are always and almost always satisfied at work. In contrast to physicians, who report a 42% burnout rate, only 6% of NPs and PAs report the same.1 This bodes well for your patients’ satisfaction.2
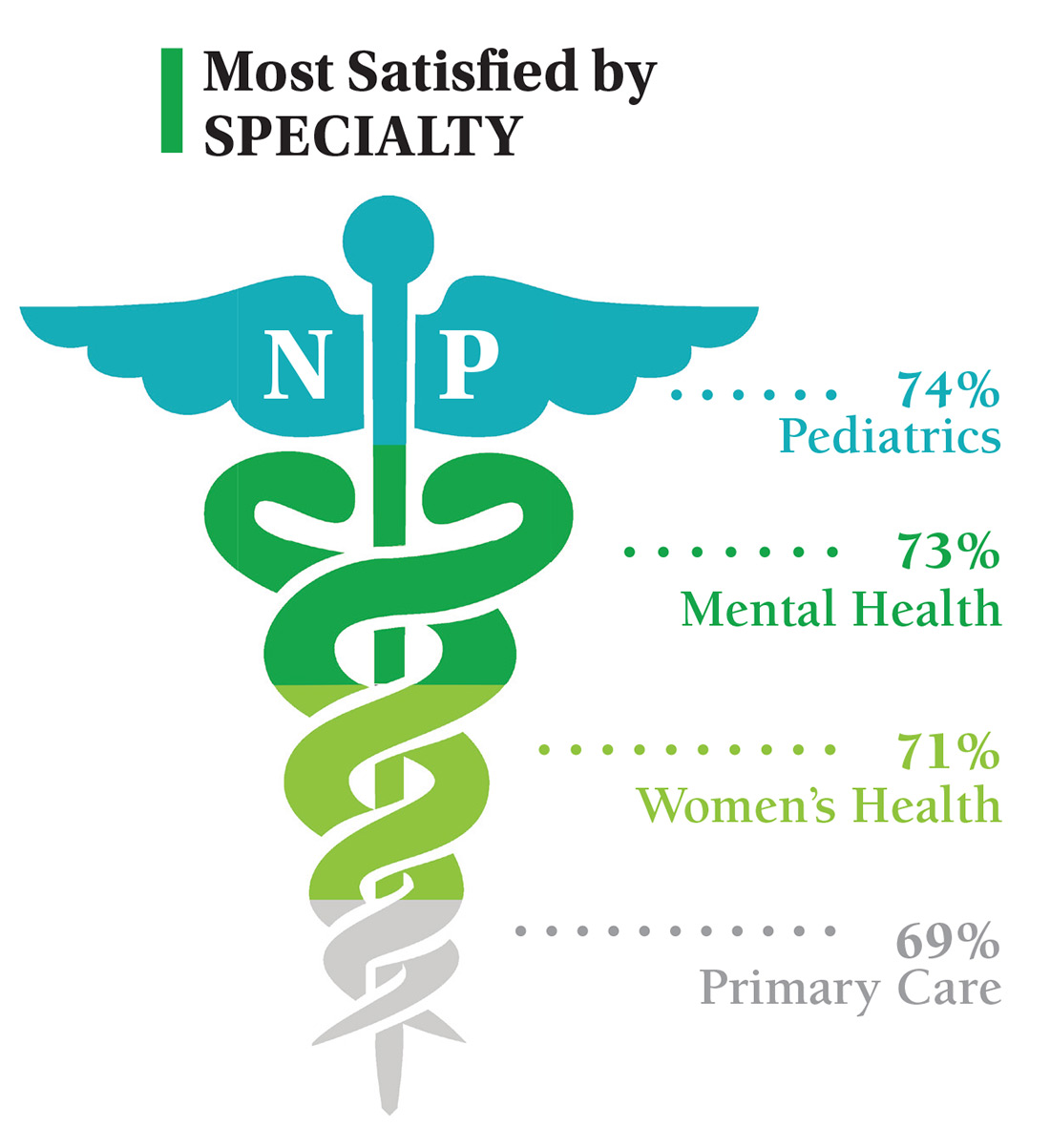
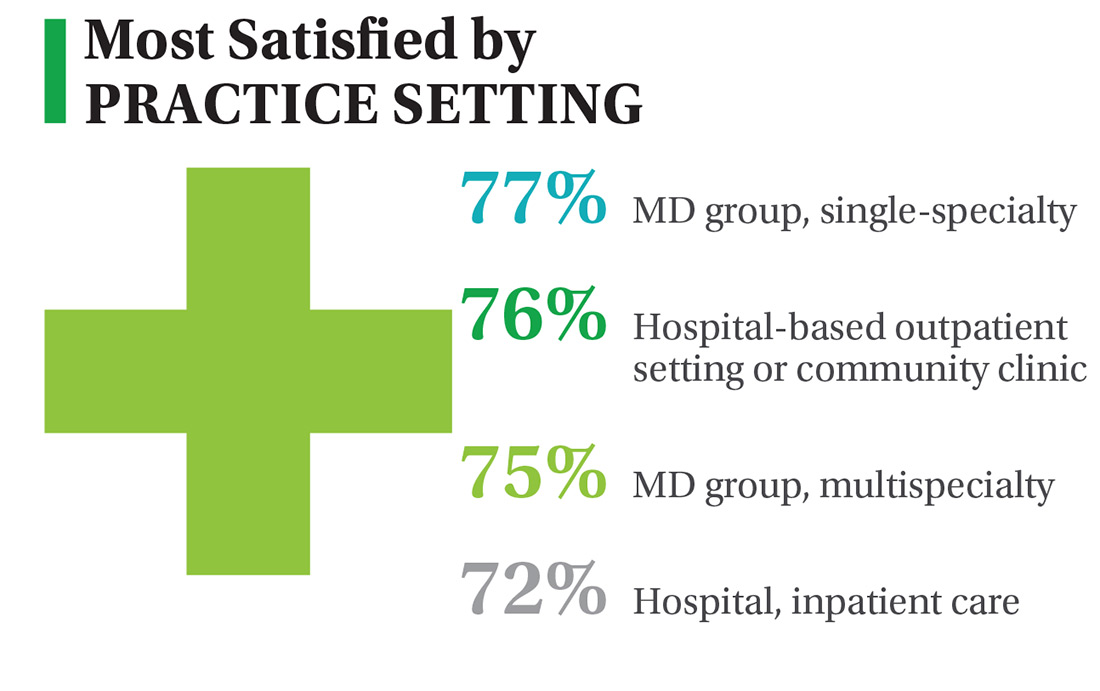
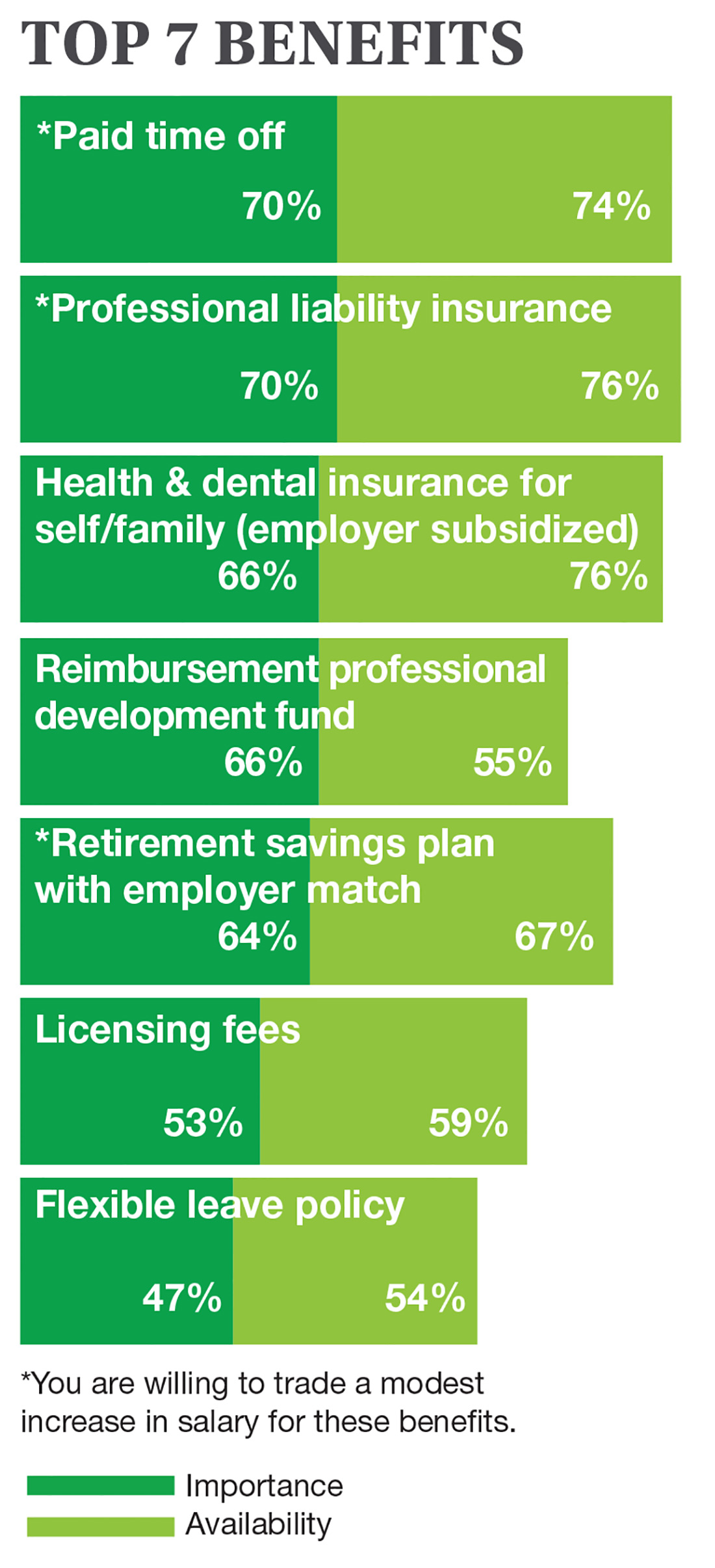
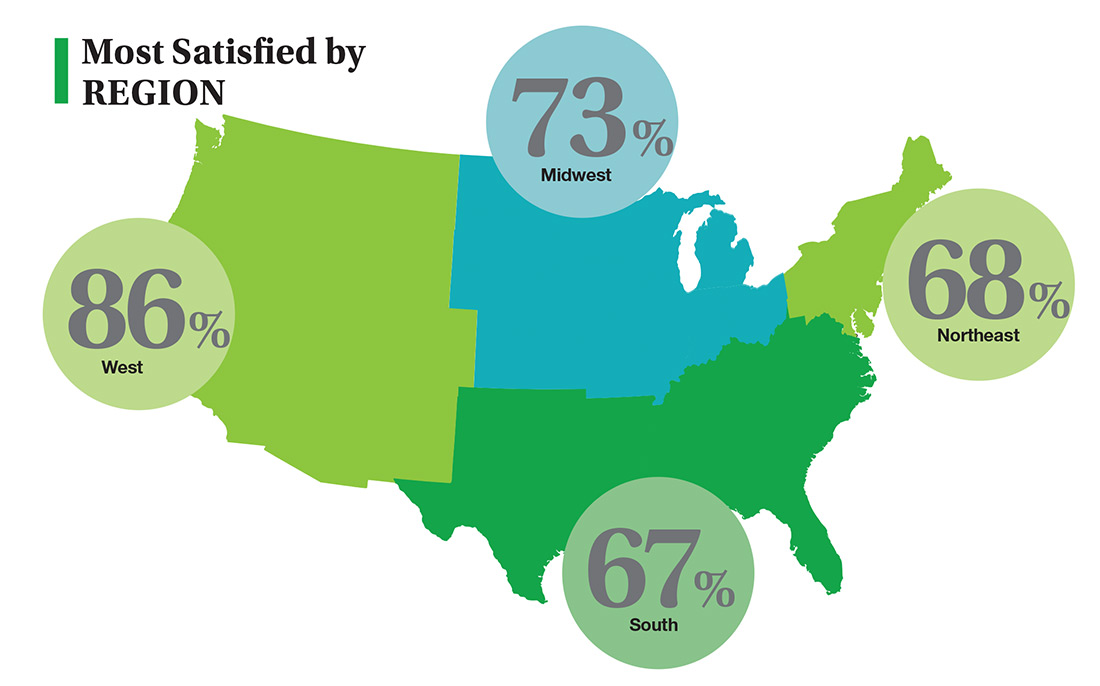
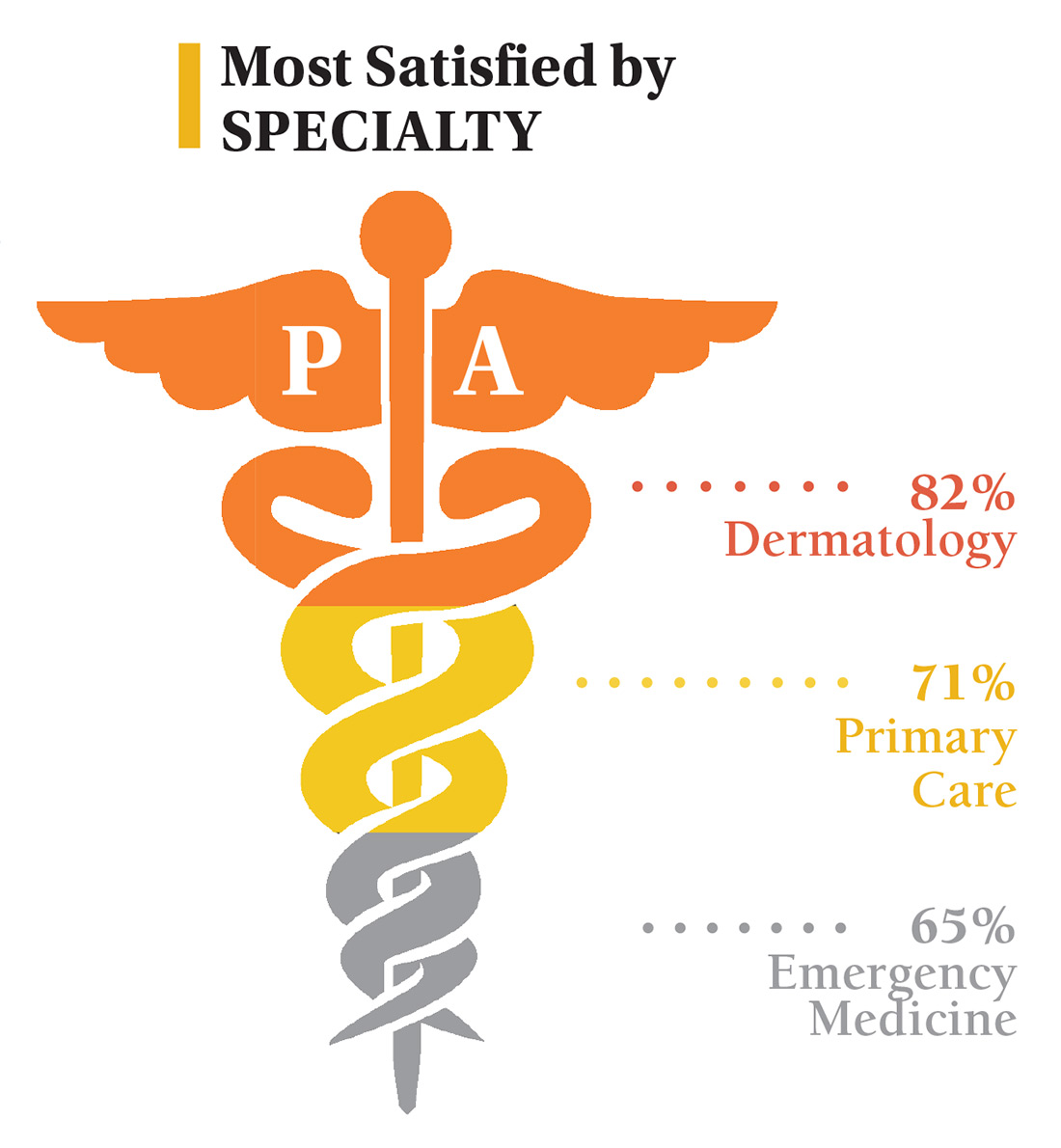
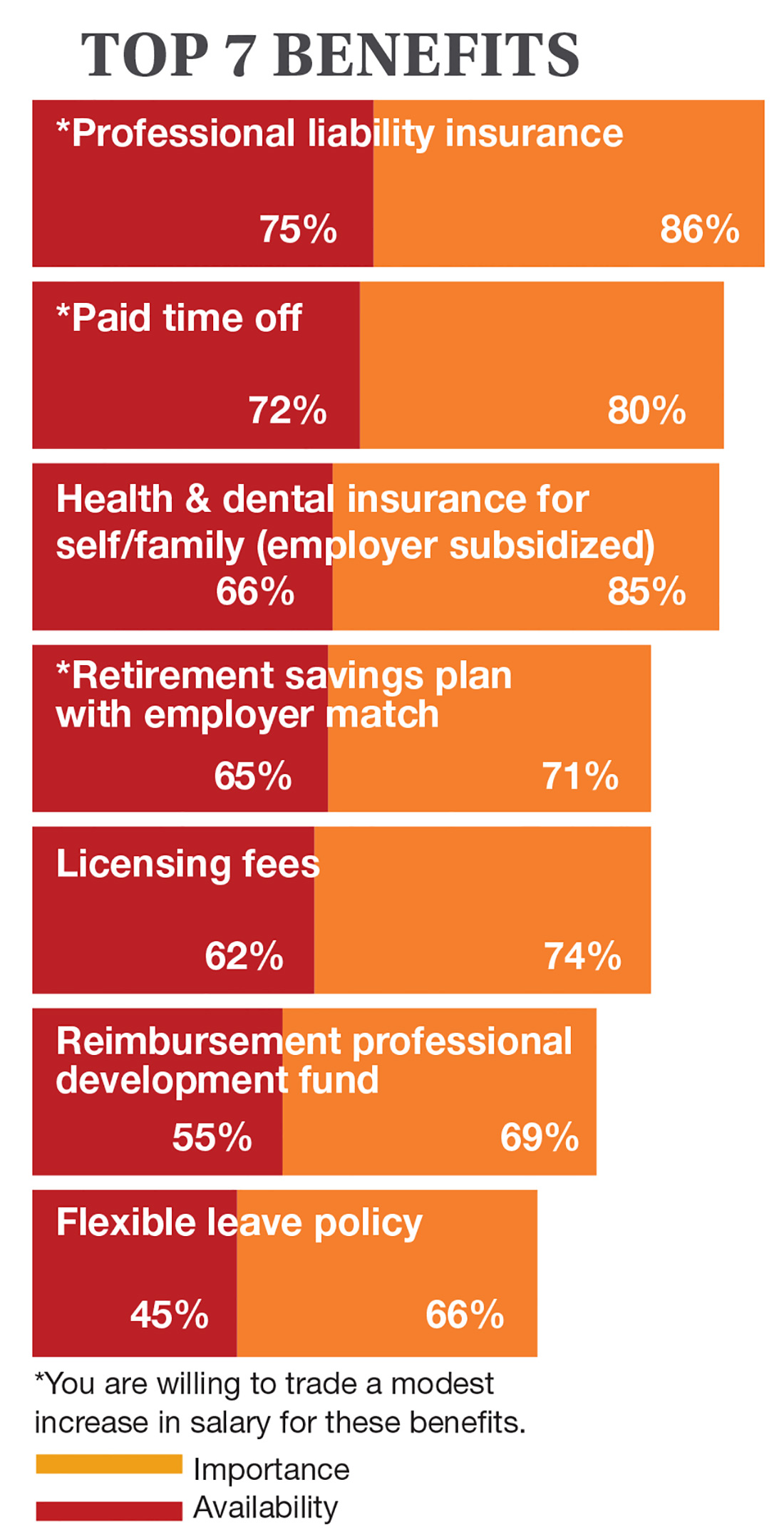
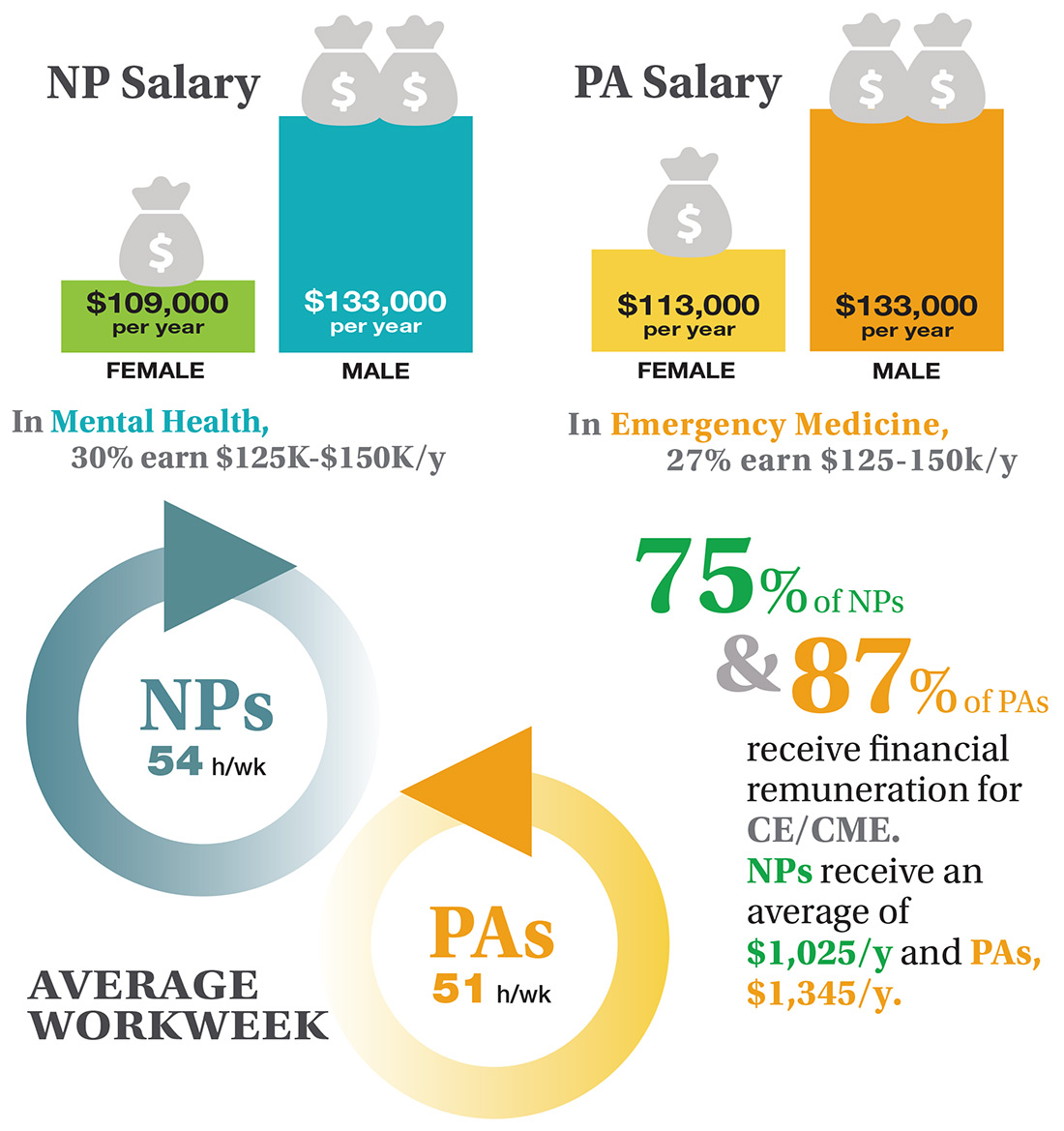
On the following pages, we focus on the details, with breakouts by specialty, region, and practice setting. Be sure to check out your top seven most desirable benefits by availability; CE/CME reimbursements; and salary information by gender and workweek.
1. Medscape National Physician Burnout & Depression Report 2018. www.medscape.com/slideshow/2018-lifestyle-burnoutdepression-6009235#2. Accessed November 17, 2018.
2. The Connection Between Employee Satisfaction and Patient Satisfaction. www.amnhealthcare.com/latest-healthcare-news/459/1033. AMN Healthcare. Accessed November 17, 2018.
Continue to: Nurse Practitioners
NURSE PRACTITIONERS
Continue to: Physician Assistants
PHYSICIAN ASSISTANTS
Continue to: NPs & PAs
NPs & PAs
METHODOLOGY
Fielded electronically under the Clinician Reviews logo, an introductory email letter signed by the Editors-in-Chief invited participation in the online 3rd annual NP/PA Job Satisfaction Survey of 35 questions.
The survey was fielded October 4, 2018, to a random representative sample of 16,000 NPs and 9,000 PAs within the United States, excluding students. The first 150 respondents to complete the survey received a $25 Amazon.com gift certificate.
A total of 1,207 usable responses—a projectable sample size—were received by October 19, 2018, the final cut-off date.
Of the total respondents, 65% are NPs (789) and 35% are PAs (418), which is proportional to the universe of NPs and PAs.1,2 This summary of results is based on only those respondents who designated their profession as NP or PA.
1. American Association of Nurse Practitioners. NP Fact Sheet. www.aanp.org/all-about-nps/np-fact-sheet. Accessed November 20, 2018.
2. NCCPA. 2017 Statistical Profile of Certified Physician Assistants: an Annual Report of the National Commission on Certification of Physician Assistants. http://prodcmsstoragesa.blob.core.windows.net/uploads/files/2017StatisticalProfileofCertifiedPhysicianAssistants6.27.pdf. Accessed November 20, 2018.
In this era of “burnout”—when a PubMed search of the term yields more than 13,000 results—it’s heartening to discover that 72% of all clinicians are always and almost always satisfied at work. In contrast to physicians, who report a 42% burnout rate, only 6% of NPs and PAs report the same.1 This bodes well for your patients’ satisfaction.2
On the following pages, we focus on the details, with breakouts by specialty, region, and practice setting. Be sure to check out your top seven most desirable benefits by availability; CE/CME reimbursements; and salary information by gender and workweek.
1. Medscape National Physician Burnout & Depression Report 2018. www.medscape.com/slideshow/2018-lifestyle-burnoutdepression-6009235#2. Accessed November 17, 2018.
2. The Connection Between Employee Satisfaction and Patient Satisfaction. www.amnhealthcare.com/latest-healthcare-news/459/1033. AMN Healthcare. Accessed November 17, 2018.
Continue to: Nurse Practitioners
NURSE PRACTITIONERS
Continue to: Physician Assistants
PHYSICIAN ASSISTANTS
Continue to: NPs & PAs
NPs & PAs
METHODOLOGY
Fielded electronically under the Clinician Reviews logo, an introductory email letter signed by the Editors-in-Chief invited participation in the online 3rd annual NP/PA Job Satisfaction Survey of 35 questions.
The survey was fielded October 4, 2018, to a random representative sample of 16,000 NPs and 9,000 PAs within the United States, excluding students. The first 150 respondents to complete the survey received a $25 Amazon.com gift certificate.
A total of 1,207 usable responses—a projectable sample size—were received by October 19, 2018, the final cut-off date.
Of the total respondents, 65% are NPs (789) and 35% are PAs (418), which is proportional to the universe of NPs and PAs.1,2 This summary of results is based on only those respondents who designated their profession as NP or PA.
1. American Association of Nurse Practitioners. NP Fact Sheet. www.aanp.org/all-about-nps/np-fact-sheet. Accessed November 20, 2018.
2. NCCPA. 2017 Statistical Profile of Certified Physician Assistants: an Annual Report of the National Commission on Certification of Physician Assistants. http://prodcmsstoragesa.blob.core.windows.net/uploads/files/2017StatisticalProfileofCertifiedPhysicianAssistants6.27.pdf. Accessed November 20, 2018.
In this era of “burnout”—when a PubMed search of the term yields more than 13,000 results—it’s heartening to discover that 72% of all clinicians are always and almost always satisfied at work. In contrast to physicians, who report a 42% burnout rate, only 6% of NPs and PAs report the same.1 This bodes well for your patients’ satisfaction.2
On the following pages, we focus on the details, with breakouts by specialty, region, and practice setting. Be sure to check out your top seven most desirable benefits by availability; CE/CME reimbursements; and salary information by gender and workweek.
1. Medscape National Physician Burnout & Depression Report 2018. www.medscape.com/slideshow/2018-lifestyle-burnoutdepression-6009235#2. Accessed November 17, 2018.
2. The Connection Between Employee Satisfaction and Patient Satisfaction. www.amnhealthcare.com/latest-healthcare-news/459/1033. AMN Healthcare. Accessed November 17, 2018.
Continue to: Nurse Practitioners
NURSE PRACTITIONERS
Continue to: Physician Assistants
PHYSICIAN ASSISTANTS
Continue to: NPs & PAs
NPs & PAs
METHODOLOGY
Fielded electronically under the Clinician Reviews logo, an introductory email letter signed by the Editors-in-Chief invited participation in the online 3rd annual NP/PA Job Satisfaction Survey of 35 questions.
The survey was fielded October 4, 2018, to a random representative sample of 16,000 NPs and 9,000 PAs within the United States, excluding students. The first 150 respondents to complete the survey received a $25 Amazon.com gift certificate.
A total of 1,207 usable responses—a projectable sample size—were received by October 19, 2018, the final cut-off date.
Of the total respondents, 65% are NPs (789) and 35% are PAs (418), which is proportional to the universe of NPs and PAs.1,2 This summary of results is based on only those respondents who designated their profession as NP or PA.
1. American Association of Nurse Practitioners. NP Fact Sheet. www.aanp.org/all-about-nps/np-fact-sheet. Accessed November 20, 2018.
2. NCCPA. 2017 Statistical Profile of Certified Physician Assistants: an Annual Report of the National Commission on Certification of Physician Assistants. http://prodcmsstoragesa.blob.core.windows.net/uploads/files/2017StatisticalProfileofCertifiedPhysicianAssistants6.27.pdf. Accessed November 20, 2018.
Early switch to dasatinib offers clinical benefit to CML patients
SAN DIEGO—Early results of the DASCERN trial indicate that patients with chronic myeloid leukemia (CML) in chronic phase who have a suboptimal response to imatinib as a first-line treatment benefit from switching to dasatinib at 3 months.
Twenty-nine percent of dasatinib-treated patients achieved a major molecular response (MMR) at 12 months, compared to 13% of patients who remained on imatinib (P=0.005).
Dasatinib-treated patients also attained MMR much faster than those on imatinib, at a median of 14 months, compared to 20 months for those treated with imatinib.
DASCERN is the first study, according to investigators, to explore the significance of an early switch for patients who have not achieved an early molecular response (EMR) with imatinib.
“[EMRs] are important because they correlate with the outcome of patients, certainly with progression-free survival and overall survival,” explained Jorge E. Cortes, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“[T]he possibility of changing to dasatinib appears, with these early results, to suggest that there may be a benefit to switching these patients to achieve better long-term outcomes. But also, those patients that have these early molecular responses have a better probability of achieving deep molecular responses that we desire for treatment-free remission.”
Dr. Cortes elaborated on the DASCERN data at the 2018 ASH Annual Meeting (abstract 788*).
Study design
DASCERN (NCT01593254) is a randomized, open-label, international, phase 2b trial in adult patients with chronic-phase CML who had achieved a complete hematologic response but still had more than 10% BCR-ABL1 transcripts at 3 months.
Patients were initially treated with imatinib at 400 mg daily, and 1,126 patients had a molecular assessment at 3 months.
Those who did not achieve an MMR (n=260) were randomized in a 2:1 fashion to 100 mg daily of dasatinib (n=174) or 400 mg daily or twice daily of imatinib (n=86).
If patients subsequently failed imatinib treatment by European LeukemiaNet standards, they could cross over to dasatinib.
“Importantly, there was a window of enrollment up to 8 weeks after the 3-month assessment, allowing for time to get response results and screen and enroll patients onto the study,” Dr. Cortes clarified.
Patients were also stratified according to Sokal risk score and time from molecular assessment to randomization.
Dr. Cortes noted that about 40% of patients were started on treatment within 4 weeks of the 3-month assessment. And another 58% were enrolled between 4 and 8 weeks from the 3-month assessment.
The primary endpoint is the achievement of MMR at 12 months from the first day of imatinib treatment; that is, at about 9 months from the start of the protocol treatment, in both trial arms.
Patient characteristics
Seventy-eight percent of patients were male, and 95% were younger than 65.
“This is a relatively younger patient population,” Dr. Cortes noted. “This has to do with the fact that this was an international study with a significant representation of patients that were from Asia (73%).”
The Asian patients were primarily from China, Dr. Cortes said, “and we know that, in some parts of the world, including Asia, patients seem to be younger.”
“This is also associated with a higher percentage of patients with high-risk Sokal scores, more than 20%,” he added. “That is different than what’s seen, for example, in the U.S. or in Europe.”
The prevalence of male patients, he said, broadly represents the distribution of patients in other parts of the world.
Patient disposition
Patients were followed for a median of 30 months.
Of the randomized patients—the intent-to-treat (ITT) population—143 (84%) in the dasatinib arm and 72 (84%) in the imatinib arm continued on treatment.
Study drug toxicity was the most common reason for discontinuing treatment and occurred in 9 patients (5%) in the dasatinib arm and 3 (4%) in the imatinib arm.
Nearly half the imatinib patients (n=42, 49%) crossed over to dasatinib at a median of 9 months.
The median duration of treatment was 22 months (range, 1 – 44) for patients on imatinib who did not cross over and 15 months (range, <1 – 38) for patients on dasatinib after crossing over from imatinib.
Response
In the ITT population, the rate of MMR at 12 months was 29% in the dasatinib arm and 13% in the imatinib arm (P=0.005).
The median time to MMR was significantly shorter for patients who received dasatinib compared with imatinib—14 months and 20 months, respectively (P=0.053).
“Over 60% of patients on the dasatinib arm achieved a major molecular response,” Dr. Cortes said. “This compares to about 55% of patients on the imatinib arm, even when you consider the crossover of a significant number of these patients.”
A few patients achieved a molecular response of a 4.5-log reduction in BCR-ABL1 transcripts (MR4.5).
“Of course, the follow-up is short, but we had twice as many patients [on dasatinib] at 12 months with MR4.5— 5% with dasatinib versus 2% with imatinib,” Dr. Cortes said.
Survival outcomes
Both overall and progression-free survival “look very good with both treatment approaches,” Dr. Cortes pointed out.
At a median follow-up of 30 months, the overall survival in the ITT population was 98.8% in each treatment arm.
Progression-free survival was 96.9% in the dasatinib arm and 97.6% in the imatinib arm.
“It is important to note that no patient in either treatment arm has transformed to accelerated or blast phase,” Dr. Cortes said.
Safety
Treatment was well tolerated with both agents, Dr. Cortes observed, with very few grade 3 adverse events (AEs) noted to date.
“The one that stands out here, and it’s only 2% of patients, is headache with dasatinib,” he said.
The headache did not lead to treatment discontinuation, and the patients were managed with dose adjustments.
The investigators observed no new safety signals with either drug.
Treatment-emergent AEs of any grade occurring in 15% or fewer dasatinib- or imatinib-treated patients, respectively, in the ITT population included headache (15%, 9%), diarrhea (9%, 8%), nausea (9%, 8%), eyelid edema (1%, 9%), hypocalcemia (1%, 7%), and muscle spasms (1%, 8%).
Patients who crossed over to dasatinib had similar rates of AEs to those documented for imatinib.
“They are typical for what we know of both of these tyrosine kinase inhibitors,” Dr. Cortes stated.
Investigators observed pleural effusion in 9 (5%) patients on dasatinib, and only one grade 3. That patient discontinued therapy due to the AE.
Of the patients who were on imatinib and crossed over to dasatinib, 3 (7%) experienced pleural effusion, most of them grade 1 or 2. One patient with grade 4 discontinued therapy with dasatinib due to the AE.
“Hematologic toxicity has been mild,” Dr. Cortes said, and in keeping with the known toxicities of dasatinib and imatinib.
Grade 3/4 treatment-emergent AEs in the dasatinib and imatinib arms, respectively, in the ITT population included anemia (5%, 4%), neutropenia (11%, 16%), thrombocytopenia (11%, 11%), and leukopenia (1%, 1%).
Patients who crossed over to dasatinib experienced more of these AEs than patients who did not cross over, Dr. Cortes clarified, but they are still within the expected range with these agents.
“We acknowledge the results are early and we need to continue following for both safety and efficacy, as it will be important to see if those rates of MR4.5 continue to increase with the same difference in favor of dasatinib that we are starting to see very early on,” Dr. Cortes said.
He disclosed serving as a consultant for Pfizer, Daiichi Sankyo, Astellas Pharma, Novartis, and Bristol-Myers Squibb, and he received research funding from Pfizer, Daiichi Sankyo, Arog Pharmaceuticals, Astellas Pharma, Novartis, and Bristol-Myers Squibb.
The study was supported by Bristol-Myers Squibb.
*Data in the abstract differ from the presentation.
SAN DIEGO—Early results of the DASCERN trial indicate that patients with chronic myeloid leukemia (CML) in chronic phase who have a suboptimal response to imatinib as a first-line treatment benefit from switching to dasatinib at 3 months.
Twenty-nine percent of dasatinib-treated patients achieved a major molecular response (MMR) at 12 months, compared to 13% of patients who remained on imatinib (P=0.005).
Dasatinib-treated patients also attained MMR much faster than those on imatinib, at a median of 14 months, compared to 20 months for those treated with imatinib.
DASCERN is the first study, according to investigators, to explore the significance of an early switch for patients who have not achieved an early molecular response (EMR) with imatinib.
“[EMRs] are important because they correlate with the outcome of patients, certainly with progression-free survival and overall survival,” explained Jorge E. Cortes, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“[T]he possibility of changing to dasatinib appears, with these early results, to suggest that there may be a benefit to switching these patients to achieve better long-term outcomes. But also, those patients that have these early molecular responses have a better probability of achieving deep molecular responses that we desire for treatment-free remission.”
Dr. Cortes elaborated on the DASCERN data at the 2018 ASH Annual Meeting (abstract 788*).
Study design
DASCERN (NCT01593254) is a randomized, open-label, international, phase 2b trial in adult patients with chronic-phase CML who had achieved a complete hematologic response but still had more than 10% BCR-ABL1 transcripts at 3 months.
Patients were initially treated with imatinib at 400 mg daily, and 1,126 patients had a molecular assessment at 3 months.
Those who did not achieve an MMR (n=260) were randomized in a 2:1 fashion to 100 mg daily of dasatinib (n=174) or 400 mg daily or twice daily of imatinib (n=86).
If patients subsequently failed imatinib treatment by European LeukemiaNet standards, they could cross over to dasatinib.
“Importantly, there was a window of enrollment up to 8 weeks after the 3-month assessment, allowing for time to get response results and screen and enroll patients onto the study,” Dr. Cortes clarified.
Patients were also stratified according to Sokal risk score and time from molecular assessment to randomization.
Dr. Cortes noted that about 40% of patients were started on treatment within 4 weeks of the 3-month assessment. And another 58% were enrolled between 4 and 8 weeks from the 3-month assessment.
The primary endpoint is the achievement of MMR at 12 months from the first day of imatinib treatment; that is, at about 9 months from the start of the protocol treatment, in both trial arms.
Patient characteristics
Seventy-eight percent of patients were male, and 95% were younger than 65.
“This is a relatively younger patient population,” Dr. Cortes noted. “This has to do with the fact that this was an international study with a significant representation of patients that were from Asia (73%).”
The Asian patients were primarily from China, Dr. Cortes said, “and we know that, in some parts of the world, including Asia, patients seem to be younger.”
“This is also associated with a higher percentage of patients with high-risk Sokal scores, more than 20%,” he added. “That is different than what’s seen, for example, in the U.S. or in Europe.”
The prevalence of male patients, he said, broadly represents the distribution of patients in other parts of the world.
Patient disposition
Patients were followed for a median of 30 months.
Of the randomized patients—the intent-to-treat (ITT) population—143 (84%) in the dasatinib arm and 72 (84%) in the imatinib arm continued on treatment.
Study drug toxicity was the most common reason for discontinuing treatment and occurred in 9 patients (5%) in the dasatinib arm and 3 (4%) in the imatinib arm.
Nearly half the imatinib patients (n=42, 49%) crossed over to dasatinib at a median of 9 months.
The median duration of treatment was 22 months (range, 1 – 44) for patients on imatinib who did not cross over and 15 months (range, <1 – 38) for patients on dasatinib after crossing over from imatinib.
Response
In the ITT population, the rate of MMR at 12 months was 29% in the dasatinib arm and 13% in the imatinib arm (P=0.005).
The median time to MMR was significantly shorter for patients who received dasatinib compared with imatinib—14 months and 20 months, respectively (P=0.053).
“Over 60% of patients on the dasatinib arm achieved a major molecular response,” Dr. Cortes said. “This compares to about 55% of patients on the imatinib arm, even when you consider the crossover of a significant number of these patients.”
A few patients achieved a molecular response of a 4.5-log reduction in BCR-ABL1 transcripts (MR4.5).
“Of course, the follow-up is short, but we had twice as many patients [on dasatinib] at 12 months with MR4.5— 5% with dasatinib versus 2% with imatinib,” Dr. Cortes said.
Survival outcomes
Both overall and progression-free survival “look very good with both treatment approaches,” Dr. Cortes pointed out.
At a median follow-up of 30 months, the overall survival in the ITT population was 98.8% in each treatment arm.
Progression-free survival was 96.9% in the dasatinib arm and 97.6% in the imatinib arm.
“It is important to note that no patient in either treatment arm has transformed to accelerated or blast phase,” Dr. Cortes said.
Safety
Treatment was well tolerated with both agents, Dr. Cortes observed, with very few grade 3 adverse events (AEs) noted to date.
“The one that stands out here, and it’s only 2% of patients, is headache with dasatinib,” he said.
The headache did not lead to treatment discontinuation, and the patients were managed with dose adjustments.
The investigators observed no new safety signals with either drug.
Treatment-emergent AEs of any grade occurring in 15% or fewer dasatinib- or imatinib-treated patients, respectively, in the ITT population included headache (15%, 9%), diarrhea (9%, 8%), nausea (9%, 8%), eyelid edema (1%, 9%), hypocalcemia (1%, 7%), and muscle spasms (1%, 8%).
Patients who crossed over to dasatinib had similar rates of AEs to those documented for imatinib.
“They are typical for what we know of both of these tyrosine kinase inhibitors,” Dr. Cortes stated.
Investigators observed pleural effusion in 9 (5%) patients on dasatinib, and only one grade 3. That patient discontinued therapy due to the AE.
Of the patients who were on imatinib and crossed over to dasatinib, 3 (7%) experienced pleural effusion, most of them grade 1 or 2. One patient with grade 4 discontinued therapy with dasatinib due to the AE.
“Hematologic toxicity has been mild,” Dr. Cortes said, and in keeping with the known toxicities of dasatinib and imatinib.
Grade 3/4 treatment-emergent AEs in the dasatinib and imatinib arms, respectively, in the ITT population included anemia (5%, 4%), neutropenia (11%, 16%), thrombocytopenia (11%, 11%), and leukopenia (1%, 1%).
Patients who crossed over to dasatinib experienced more of these AEs than patients who did not cross over, Dr. Cortes clarified, but they are still within the expected range with these agents.
“We acknowledge the results are early and we need to continue following for both safety and efficacy, as it will be important to see if those rates of MR4.5 continue to increase with the same difference in favor of dasatinib that we are starting to see very early on,” Dr. Cortes said.
He disclosed serving as a consultant for Pfizer, Daiichi Sankyo, Astellas Pharma, Novartis, and Bristol-Myers Squibb, and he received research funding from Pfizer, Daiichi Sankyo, Arog Pharmaceuticals, Astellas Pharma, Novartis, and Bristol-Myers Squibb.
The study was supported by Bristol-Myers Squibb.
*Data in the abstract differ from the presentation.
SAN DIEGO—Early results of the DASCERN trial indicate that patients with chronic myeloid leukemia (CML) in chronic phase who have a suboptimal response to imatinib as a first-line treatment benefit from switching to dasatinib at 3 months.
Twenty-nine percent of dasatinib-treated patients achieved a major molecular response (MMR) at 12 months, compared to 13% of patients who remained on imatinib (P=0.005).
Dasatinib-treated patients also attained MMR much faster than those on imatinib, at a median of 14 months, compared to 20 months for those treated with imatinib.
DASCERN is the first study, according to investigators, to explore the significance of an early switch for patients who have not achieved an early molecular response (EMR) with imatinib.
“[EMRs] are important because they correlate with the outcome of patients, certainly with progression-free survival and overall survival,” explained Jorge E. Cortes, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“[T]he possibility of changing to dasatinib appears, with these early results, to suggest that there may be a benefit to switching these patients to achieve better long-term outcomes. But also, those patients that have these early molecular responses have a better probability of achieving deep molecular responses that we desire for treatment-free remission.”
Dr. Cortes elaborated on the DASCERN data at the 2018 ASH Annual Meeting (abstract 788*).
Study design
DASCERN (NCT01593254) is a randomized, open-label, international, phase 2b trial in adult patients with chronic-phase CML who had achieved a complete hematologic response but still had more than 10% BCR-ABL1 transcripts at 3 months.
Patients were initially treated with imatinib at 400 mg daily, and 1,126 patients had a molecular assessment at 3 months.
Those who did not achieve an MMR (n=260) were randomized in a 2:1 fashion to 100 mg daily of dasatinib (n=174) or 400 mg daily or twice daily of imatinib (n=86).
If patients subsequently failed imatinib treatment by European LeukemiaNet standards, they could cross over to dasatinib.
“Importantly, there was a window of enrollment up to 8 weeks after the 3-month assessment, allowing for time to get response results and screen and enroll patients onto the study,” Dr. Cortes clarified.
Patients were also stratified according to Sokal risk score and time from molecular assessment to randomization.
Dr. Cortes noted that about 40% of patients were started on treatment within 4 weeks of the 3-month assessment. And another 58% were enrolled between 4 and 8 weeks from the 3-month assessment.
The primary endpoint is the achievement of MMR at 12 months from the first day of imatinib treatment; that is, at about 9 months from the start of the protocol treatment, in both trial arms.
Patient characteristics
Seventy-eight percent of patients were male, and 95% were younger than 65.
“This is a relatively younger patient population,” Dr. Cortes noted. “This has to do with the fact that this was an international study with a significant representation of patients that were from Asia (73%).”
The Asian patients were primarily from China, Dr. Cortes said, “and we know that, in some parts of the world, including Asia, patients seem to be younger.”
“This is also associated with a higher percentage of patients with high-risk Sokal scores, more than 20%,” he added. “That is different than what’s seen, for example, in the U.S. or in Europe.”
The prevalence of male patients, he said, broadly represents the distribution of patients in other parts of the world.
Patient disposition
Patients were followed for a median of 30 months.
Of the randomized patients—the intent-to-treat (ITT) population—143 (84%) in the dasatinib arm and 72 (84%) in the imatinib arm continued on treatment.
Study drug toxicity was the most common reason for discontinuing treatment and occurred in 9 patients (5%) in the dasatinib arm and 3 (4%) in the imatinib arm.
Nearly half the imatinib patients (n=42, 49%) crossed over to dasatinib at a median of 9 months.
The median duration of treatment was 22 months (range, 1 – 44) for patients on imatinib who did not cross over and 15 months (range, <1 – 38) for patients on dasatinib after crossing over from imatinib.
Response
In the ITT population, the rate of MMR at 12 months was 29% in the dasatinib arm and 13% in the imatinib arm (P=0.005).
The median time to MMR was significantly shorter for patients who received dasatinib compared with imatinib—14 months and 20 months, respectively (P=0.053).
“Over 60% of patients on the dasatinib arm achieved a major molecular response,” Dr. Cortes said. “This compares to about 55% of patients on the imatinib arm, even when you consider the crossover of a significant number of these patients.”
A few patients achieved a molecular response of a 4.5-log reduction in BCR-ABL1 transcripts (MR4.5).
“Of course, the follow-up is short, but we had twice as many patients [on dasatinib] at 12 months with MR4.5— 5% with dasatinib versus 2% with imatinib,” Dr. Cortes said.
Survival outcomes
Both overall and progression-free survival “look very good with both treatment approaches,” Dr. Cortes pointed out.
At a median follow-up of 30 months, the overall survival in the ITT population was 98.8% in each treatment arm.
Progression-free survival was 96.9% in the dasatinib arm and 97.6% in the imatinib arm.
“It is important to note that no patient in either treatment arm has transformed to accelerated or blast phase,” Dr. Cortes said.
Safety
Treatment was well tolerated with both agents, Dr. Cortes observed, with very few grade 3 adverse events (AEs) noted to date.
“The one that stands out here, and it’s only 2% of patients, is headache with dasatinib,” he said.
The headache did not lead to treatment discontinuation, and the patients were managed with dose adjustments.
The investigators observed no new safety signals with either drug.
Treatment-emergent AEs of any grade occurring in 15% or fewer dasatinib- or imatinib-treated patients, respectively, in the ITT population included headache (15%, 9%), diarrhea (9%, 8%), nausea (9%, 8%), eyelid edema (1%, 9%), hypocalcemia (1%, 7%), and muscle spasms (1%, 8%).
Patients who crossed over to dasatinib had similar rates of AEs to those documented for imatinib.
“They are typical for what we know of both of these tyrosine kinase inhibitors,” Dr. Cortes stated.
Investigators observed pleural effusion in 9 (5%) patients on dasatinib, and only one grade 3. That patient discontinued therapy due to the AE.
Of the patients who were on imatinib and crossed over to dasatinib, 3 (7%) experienced pleural effusion, most of them grade 1 or 2. One patient with grade 4 discontinued therapy with dasatinib due to the AE.
“Hematologic toxicity has been mild,” Dr. Cortes said, and in keeping with the known toxicities of dasatinib and imatinib.
Grade 3/4 treatment-emergent AEs in the dasatinib and imatinib arms, respectively, in the ITT population included anemia (5%, 4%), neutropenia (11%, 16%), thrombocytopenia (11%, 11%), and leukopenia (1%, 1%).
Patients who crossed over to dasatinib experienced more of these AEs than patients who did not cross over, Dr. Cortes clarified, but they are still within the expected range with these agents.
“We acknowledge the results are early and we need to continue following for both safety and efficacy, as it will be important to see if those rates of MR4.5 continue to increase with the same difference in favor of dasatinib that we are starting to see very early on,” Dr. Cortes said.
He disclosed serving as a consultant for Pfizer, Daiichi Sankyo, Astellas Pharma, Novartis, and Bristol-Myers Squibb, and he received research funding from Pfizer, Daiichi Sankyo, Arog Pharmaceuticals, Astellas Pharma, Novartis, and Bristol-Myers Squibb.
The study was supported by Bristol-Myers Squibb.
*Data in the abstract differ from the presentation.
Emapalumab found safe, effective in primary HLH
SAN DIEGO—Emapalumab, an interferon gamma-blocking antibody, controls disease activity and has a favorable safety profile in pediatric patients with primary hemophagocytic lymphohistiocytosis (HLH), according to research presented at the 2018 ASH Annual Meeting.
Investigators believe emapalumab, which was recently approved to treat HLH in the United States, should be considered a new therapeutic option for this rare and life-threatening syndrome because of the drug’s targeted mode of action.
Multiple lines of evidence have pointed to interferon gamma as a “rational target” in HLH, and elevated levels of interferon gamma are consistently observed in patients with HLH, said Franco Locatelli, MD, of Ospedale Pediatrico Bambino Gesù in Rome, Italy.
Emapalumab binds to its target with high affinity, recognizing both free and receptor-bound interferon gamma, he added.
Dr. Locatelli described trial results with emapalumab in children with HLH during the late-breaking abstracts session at ASH (abstract LBA-6).
This phase 2/3 study (NCT01818492) included 34 children with primary HLH—7 who were treatment-naïve and 27 who had failed conventional HLH therapy.
The patients received emapalumab intravenously with concomitant dexamethasone for up to 8 weeks or extended to the point of allogeneic hematopoietic stem cell transplant if needed.
The study met its primary endpoint of overall response rate higher than 40%, Dr. Locatelli reported.
The overall response rate was 64.7% for all 34 treated patients (95% confidence interval [CI], 46% to 80%; P=0.0031) and 63% for the 27 patients who had failed prior therapy (95% CI, 42% to 81%; P=0.0134).
Response was rapid, occurring at a median of 8 days after starting emapalumab, and patients were in response for a median of 75% of days during treatment, Dr. Locatelli said.
Ninety-four percent of patients had at least one adverse event (AE), and 63% had serious AEs. Common AEs included infections (56%), hypertension (35%), infusion-related reactions (27%), and pyrexia (24%).
One patient had disseminated histoplasmosis that led to discontinuation of emapalumab but resolved with appropriate treatment.
This study was sponsored by Novimmune. Investigators provided disclosures related to Sobi, Novimmune, Rocket Pharmaceuticals, Inc., AB2Bio, Novartis, Eli Lilly, Sanofi, UCB, Pfizer, and AbbVie. Two investigators reported employment with Novimmune.
SAN DIEGO—Emapalumab, an interferon gamma-blocking antibody, controls disease activity and has a favorable safety profile in pediatric patients with primary hemophagocytic lymphohistiocytosis (HLH), according to research presented at the 2018 ASH Annual Meeting.
Investigators believe emapalumab, which was recently approved to treat HLH in the United States, should be considered a new therapeutic option for this rare and life-threatening syndrome because of the drug’s targeted mode of action.
Multiple lines of evidence have pointed to interferon gamma as a “rational target” in HLH, and elevated levels of interferon gamma are consistently observed in patients with HLH, said Franco Locatelli, MD, of Ospedale Pediatrico Bambino Gesù in Rome, Italy.
Emapalumab binds to its target with high affinity, recognizing both free and receptor-bound interferon gamma, he added.
Dr. Locatelli described trial results with emapalumab in children with HLH during the late-breaking abstracts session at ASH (abstract LBA-6).
This phase 2/3 study (NCT01818492) included 34 children with primary HLH—7 who were treatment-naïve and 27 who had failed conventional HLH therapy.
The patients received emapalumab intravenously with concomitant dexamethasone for up to 8 weeks or extended to the point of allogeneic hematopoietic stem cell transplant if needed.
The study met its primary endpoint of overall response rate higher than 40%, Dr. Locatelli reported.
The overall response rate was 64.7% for all 34 treated patients (95% confidence interval [CI], 46% to 80%; P=0.0031) and 63% for the 27 patients who had failed prior therapy (95% CI, 42% to 81%; P=0.0134).
Response was rapid, occurring at a median of 8 days after starting emapalumab, and patients were in response for a median of 75% of days during treatment, Dr. Locatelli said.
Ninety-four percent of patients had at least one adverse event (AE), and 63% had serious AEs. Common AEs included infections (56%), hypertension (35%), infusion-related reactions (27%), and pyrexia (24%).
One patient had disseminated histoplasmosis that led to discontinuation of emapalumab but resolved with appropriate treatment.
This study was sponsored by Novimmune. Investigators provided disclosures related to Sobi, Novimmune, Rocket Pharmaceuticals, Inc., AB2Bio, Novartis, Eli Lilly, Sanofi, UCB, Pfizer, and AbbVie. Two investigators reported employment with Novimmune.
SAN DIEGO—Emapalumab, an interferon gamma-blocking antibody, controls disease activity and has a favorable safety profile in pediatric patients with primary hemophagocytic lymphohistiocytosis (HLH), according to research presented at the 2018 ASH Annual Meeting.
Investigators believe emapalumab, which was recently approved to treat HLH in the United States, should be considered a new therapeutic option for this rare and life-threatening syndrome because of the drug’s targeted mode of action.
Multiple lines of evidence have pointed to interferon gamma as a “rational target” in HLH, and elevated levels of interferon gamma are consistently observed in patients with HLH, said Franco Locatelli, MD, of Ospedale Pediatrico Bambino Gesù in Rome, Italy.
Emapalumab binds to its target with high affinity, recognizing both free and receptor-bound interferon gamma, he added.
Dr. Locatelli described trial results with emapalumab in children with HLH during the late-breaking abstracts session at ASH (abstract LBA-6).
This phase 2/3 study (NCT01818492) included 34 children with primary HLH—7 who were treatment-naïve and 27 who had failed conventional HLH therapy.
The patients received emapalumab intravenously with concomitant dexamethasone for up to 8 weeks or extended to the point of allogeneic hematopoietic stem cell transplant if needed.
The study met its primary endpoint of overall response rate higher than 40%, Dr. Locatelli reported.
The overall response rate was 64.7% for all 34 treated patients (95% confidence interval [CI], 46% to 80%; P=0.0031) and 63% for the 27 patients who had failed prior therapy (95% CI, 42% to 81%; P=0.0134).
Response was rapid, occurring at a median of 8 days after starting emapalumab, and patients were in response for a median of 75% of days during treatment, Dr. Locatelli said.
Ninety-four percent of patients had at least one adverse event (AE), and 63% had serious AEs. Common AEs included infections (56%), hypertension (35%), infusion-related reactions (27%), and pyrexia (24%).
One patient had disseminated histoplasmosis that led to discontinuation of emapalumab but resolved with appropriate treatment.
This study was sponsored by Novimmune. Investigators provided disclosures related to Sobi, Novimmune, Rocket Pharmaceuticals, Inc., AB2Bio, Novartis, Eli Lilly, Sanofi, UCB, Pfizer, and AbbVie. Two investigators reported employment with Novimmune.
ASH 2018 meeting wrap-up
In this special meetings edition of the MDedge Daily News we hear from Dr. Arok Khorana of the Cleveland Clinic on rivaroxaban for the prevention of VTE in cancer patients. We also hear from Dr. Ify Osunkwo of the Levine Cancer Institute on recent advances in sickle cell disease.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
In this special meetings edition of the MDedge Daily News we hear from Dr. Arok Khorana of the Cleveland Clinic on rivaroxaban for the prevention of VTE in cancer patients. We also hear from Dr. Ify Osunkwo of the Levine Cancer Institute on recent advances in sickle cell disease.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
In this special meetings edition of the MDedge Daily News we hear from Dr. Arok Khorana of the Cleveland Clinic on rivaroxaban for the prevention of VTE in cancer patients. We also hear from Dr. Ify Osunkwo of the Levine Cancer Institute on recent advances in sickle cell disease.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Inhibitor can improve symptoms of systemic mastocytosis
SAN DIEGO—The KIT D816V inhibitor avapritinib can improve symptoms of systemic mastocytosis (SM), according to researchers.
Patients treated with avapritinib in a phase 1 trial had an overall response rate (ORR) of 83%, a 41% mean reduction in mastocytosis symptoms from baseline, and a 58% mean reduction in the most bothersome symptom domain.
Most adverse events (AEs) in this trial were grade 1 or 2. However, 66% of patients did have grade 3 or higher treatment-related AEs that necessitated dose reductions.
Jason R. Gotlib, MD, of Stanford University School of Medicine in California, presented these results, from the EXPLORER trial (NCT02561988), at the 2018 ASH Annual Meeting (abstract 351).
Patients and treatment
The trial enrolled 67 patients—90% with advanced SM and 10% with indolent or smoldering SM. The patients’ median age was 62 (range, 34 to 83), and 49% were female.
Patients had received a median of 3 (range, 0 to 3) prior therapies. Sixty percent of patients had received any prior therapy, and 23% had received midostaurin. Thirty-three percent were on steroid therapy at baseline.
Eighty-four percent of patients had the KIT D816V mutation, and 1% had the KIT D816Y mutation. Forty-five percent of patients had mutations in SRSF2, ASXL1, and RUNX1.
In the dose-escalation portion of the trial, patients received avapritinib at 30 mg to 400 mg once daily in continuous 28-day cycles. In the expansion portion of the trial, patients received avapritinib at 200 mg or 300 mg once daily.
Response
There were 29 patients evaluable for response. The ORR was 83% (n=24). The rate of complete response (CR) was 10% (n=3), the rate of CR with partial hematologic recovery (CRh) was 14% (n=4), and the partial response rate was 48% (n=14).
Ten percent (n=3) of patients had clinical improvement, 17% (n=5) had stable disease, and none of the patients progressed.
Among the 10 patients treated at a dose of 200 mg or lower, the ORR was 90% (n=9). The CR rate was 30% (n=3), the rate of CRh was 20% (n=2), and the partial response rate was 30% (n=3). Ten percent (n=1) of patients each had clinical improvement or stable disease.
Dr. Gotlib noted that responses have been durable and deepened over time.
At a median follow-up of 14 months, the median duration of response had not been reached. The 12-month response rate is 76%.
The median time to initial response is 2 months, and the median time to CR/CRh is 9 months.
Safety
Seventy-eight percent of patients (52/67) were still on treatment at last follow-up. Four percent (4/67) discontinued treatment due to related AEs, and 66% (44/67) had grade 3 or higher AEs that prompted dose reductions.
AEs prompting discontinuation included refractory ascites, encephalopathy, and intracranial bleeding. AEs necessitating dose reductions were largely hematologic events.
The most common AEs were periorbital edema (67%), anemia (52%), fatigue (37%), nausea (36%), diarrhea (34%), peripheral edema (34%), thrombocytopenia (31%) vomiting (28%), cognitive effects (28%), and hair color changes (25%).
The most common grade 3/4 AEs were anemia (26%), thrombocytopenia (17%), and neutropenia (10%). There were no treatment-related deaths.
Symptoms
For symptom assessment, patients completed the Advanced Systemic Mastocytosis Symptom Assessment Form (AdvSM-SAF), a patient-reported outcomes (PRO) tool that included eight symptoms:
- Abdominal pain
- Diarrhea
- Nausea
- Vomiting
- Spots
- Itching
- Flushing
- Fatigue.
Symptoms were scored on a scale of 1 to 10. Results were analyzed as a total symptom score (TSS) combining all eight items, as a gastrointestinal domain (combining nausea, vomiting, diarrhea, and abdominal pain), and as a skin domain (combining itching, flushing, and spots). Analyses were based on 7-day average scores.
Among the 32 evaluable patients, there was a 41% reduction in TSS from baseline (P=0.043). Among the 16 most symptomatic patients, there was a 46% reduction in TSS from baseline (P=0.038).
In all 32 evaluable patients, there was a 58% reduction from baseline in the score for most bothersome symptom domain (gastrointestinal or skin; P=0.0034). In the 16 most symptomatic patients, there was 63% reduction from baseline (P=0.0038).
“This is the first advanced SM-specific PRO to demonstrate significant improvements in total symptom score,” Dr. Gotlib said. “The clinical activity and initial PRO data do support further evaluation of avapritinib in both advanced and indolent disease.”
Dr. Gotlib noted that the PATHFINDER trial (NCT03580655), a study of avapritinib in advanced SM, is now enrolling. And the PIONEER trial (NCT03731260), a study of avapritinib in patients with indolent or smoldering SM, is scheduled to begin at the end of the year.
The EXPLORER trial was sponsored by Blueprint Medicines Corporation. Dr. Gotlib reported relationships with Blueprint Medicines, Celgene, Incyte, Novartis, Deciphera, Gilead, Promedior, and Kartos.
SAN DIEGO—The KIT D816V inhibitor avapritinib can improve symptoms of systemic mastocytosis (SM), according to researchers.
Patients treated with avapritinib in a phase 1 trial had an overall response rate (ORR) of 83%, a 41% mean reduction in mastocytosis symptoms from baseline, and a 58% mean reduction in the most bothersome symptom domain.
Most adverse events (AEs) in this trial were grade 1 or 2. However, 66% of patients did have grade 3 or higher treatment-related AEs that necessitated dose reductions.
Jason R. Gotlib, MD, of Stanford University School of Medicine in California, presented these results, from the EXPLORER trial (NCT02561988), at the 2018 ASH Annual Meeting (abstract 351).
Patients and treatment
The trial enrolled 67 patients—90% with advanced SM and 10% with indolent or smoldering SM. The patients’ median age was 62 (range, 34 to 83), and 49% were female.
Patients had received a median of 3 (range, 0 to 3) prior therapies. Sixty percent of patients had received any prior therapy, and 23% had received midostaurin. Thirty-three percent were on steroid therapy at baseline.
Eighty-four percent of patients had the KIT D816V mutation, and 1% had the KIT D816Y mutation. Forty-five percent of patients had mutations in SRSF2, ASXL1, and RUNX1.
In the dose-escalation portion of the trial, patients received avapritinib at 30 mg to 400 mg once daily in continuous 28-day cycles. In the expansion portion of the trial, patients received avapritinib at 200 mg or 300 mg once daily.
Response
There were 29 patients evaluable for response. The ORR was 83% (n=24). The rate of complete response (CR) was 10% (n=3), the rate of CR with partial hematologic recovery (CRh) was 14% (n=4), and the partial response rate was 48% (n=14).
Ten percent (n=3) of patients had clinical improvement, 17% (n=5) had stable disease, and none of the patients progressed.
Among the 10 patients treated at a dose of 200 mg or lower, the ORR was 90% (n=9). The CR rate was 30% (n=3), the rate of CRh was 20% (n=2), and the partial response rate was 30% (n=3). Ten percent (n=1) of patients each had clinical improvement or stable disease.
Dr. Gotlib noted that responses have been durable and deepened over time.
At a median follow-up of 14 months, the median duration of response had not been reached. The 12-month response rate is 76%.
The median time to initial response is 2 months, and the median time to CR/CRh is 9 months.
Safety
Seventy-eight percent of patients (52/67) were still on treatment at last follow-up. Four percent (4/67) discontinued treatment due to related AEs, and 66% (44/67) had grade 3 or higher AEs that prompted dose reductions.
AEs prompting discontinuation included refractory ascites, encephalopathy, and intracranial bleeding. AEs necessitating dose reductions were largely hematologic events.
The most common AEs were periorbital edema (67%), anemia (52%), fatigue (37%), nausea (36%), diarrhea (34%), peripheral edema (34%), thrombocytopenia (31%) vomiting (28%), cognitive effects (28%), and hair color changes (25%).
The most common grade 3/4 AEs were anemia (26%), thrombocytopenia (17%), and neutropenia (10%). There were no treatment-related deaths.
Symptoms
For symptom assessment, patients completed the Advanced Systemic Mastocytosis Symptom Assessment Form (AdvSM-SAF), a patient-reported outcomes (PRO) tool that included eight symptoms:
- Abdominal pain
- Diarrhea
- Nausea
- Vomiting
- Spots
- Itching
- Flushing
- Fatigue.
Symptoms were scored on a scale of 1 to 10. Results were analyzed as a total symptom score (TSS) combining all eight items, as a gastrointestinal domain (combining nausea, vomiting, diarrhea, and abdominal pain), and as a skin domain (combining itching, flushing, and spots). Analyses were based on 7-day average scores.
Among the 32 evaluable patients, there was a 41% reduction in TSS from baseline (P=0.043). Among the 16 most symptomatic patients, there was a 46% reduction in TSS from baseline (P=0.038).
In all 32 evaluable patients, there was a 58% reduction from baseline in the score for most bothersome symptom domain (gastrointestinal or skin; P=0.0034). In the 16 most symptomatic patients, there was 63% reduction from baseline (P=0.0038).
“This is the first advanced SM-specific PRO to demonstrate significant improvements in total symptom score,” Dr. Gotlib said. “The clinical activity and initial PRO data do support further evaluation of avapritinib in both advanced and indolent disease.”
Dr. Gotlib noted that the PATHFINDER trial (NCT03580655), a study of avapritinib in advanced SM, is now enrolling. And the PIONEER trial (NCT03731260), a study of avapritinib in patients with indolent or smoldering SM, is scheduled to begin at the end of the year.
The EXPLORER trial was sponsored by Blueprint Medicines Corporation. Dr. Gotlib reported relationships with Blueprint Medicines, Celgene, Incyte, Novartis, Deciphera, Gilead, Promedior, and Kartos.
SAN DIEGO—The KIT D816V inhibitor avapritinib can improve symptoms of systemic mastocytosis (SM), according to researchers.
Patients treated with avapritinib in a phase 1 trial had an overall response rate (ORR) of 83%, a 41% mean reduction in mastocytosis symptoms from baseline, and a 58% mean reduction in the most bothersome symptom domain.
Most adverse events (AEs) in this trial were grade 1 or 2. However, 66% of patients did have grade 3 or higher treatment-related AEs that necessitated dose reductions.
Jason R. Gotlib, MD, of Stanford University School of Medicine in California, presented these results, from the EXPLORER trial (NCT02561988), at the 2018 ASH Annual Meeting (abstract 351).
Patients and treatment
The trial enrolled 67 patients—90% with advanced SM and 10% with indolent or smoldering SM. The patients’ median age was 62 (range, 34 to 83), and 49% were female.
Patients had received a median of 3 (range, 0 to 3) prior therapies. Sixty percent of patients had received any prior therapy, and 23% had received midostaurin. Thirty-three percent were on steroid therapy at baseline.
Eighty-four percent of patients had the KIT D816V mutation, and 1% had the KIT D816Y mutation. Forty-five percent of patients had mutations in SRSF2, ASXL1, and RUNX1.
In the dose-escalation portion of the trial, patients received avapritinib at 30 mg to 400 mg once daily in continuous 28-day cycles. In the expansion portion of the trial, patients received avapritinib at 200 mg or 300 mg once daily.
Response
There were 29 patients evaluable for response. The ORR was 83% (n=24). The rate of complete response (CR) was 10% (n=3), the rate of CR with partial hematologic recovery (CRh) was 14% (n=4), and the partial response rate was 48% (n=14).
Ten percent (n=3) of patients had clinical improvement, 17% (n=5) had stable disease, and none of the patients progressed.
Among the 10 patients treated at a dose of 200 mg or lower, the ORR was 90% (n=9). The CR rate was 30% (n=3), the rate of CRh was 20% (n=2), and the partial response rate was 30% (n=3). Ten percent (n=1) of patients each had clinical improvement or stable disease.
Dr. Gotlib noted that responses have been durable and deepened over time.
At a median follow-up of 14 months, the median duration of response had not been reached. The 12-month response rate is 76%.
The median time to initial response is 2 months, and the median time to CR/CRh is 9 months.
Safety
Seventy-eight percent of patients (52/67) were still on treatment at last follow-up. Four percent (4/67) discontinued treatment due to related AEs, and 66% (44/67) had grade 3 or higher AEs that prompted dose reductions.
AEs prompting discontinuation included refractory ascites, encephalopathy, and intracranial bleeding. AEs necessitating dose reductions were largely hematologic events.
The most common AEs were periorbital edema (67%), anemia (52%), fatigue (37%), nausea (36%), diarrhea (34%), peripheral edema (34%), thrombocytopenia (31%) vomiting (28%), cognitive effects (28%), and hair color changes (25%).
The most common grade 3/4 AEs were anemia (26%), thrombocytopenia (17%), and neutropenia (10%). There were no treatment-related deaths.
Symptoms
For symptom assessment, patients completed the Advanced Systemic Mastocytosis Symptom Assessment Form (AdvSM-SAF), a patient-reported outcomes (PRO) tool that included eight symptoms:
- Abdominal pain
- Diarrhea
- Nausea
- Vomiting
- Spots
- Itching
- Flushing
- Fatigue.
Symptoms were scored on a scale of 1 to 10. Results were analyzed as a total symptom score (TSS) combining all eight items, as a gastrointestinal domain (combining nausea, vomiting, diarrhea, and abdominal pain), and as a skin domain (combining itching, flushing, and spots). Analyses were based on 7-day average scores.
Among the 32 evaluable patients, there was a 41% reduction in TSS from baseline (P=0.043). Among the 16 most symptomatic patients, there was a 46% reduction in TSS from baseline (P=0.038).
In all 32 evaluable patients, there was a 58% reduction from baseline in the score for most bothersome symptom domain (gastrointestinal or skin; P=0.0034). In the 16 most symptomatic patients, there was 63% reduction from baseline (P=0.0038).
“This is the first advanced SM-specific PRO to demonstrate significant improvements in total symptom score,” Dr. Gotlib said. “The clinical activity and initial PRO data do support further evaluation of avapritinib in both advanced and indolent disease.”
Dr. Gotlib noted that the PATHFINDER trial (NCT03580655), a study of avapritinib in advanced SM, is now enrolling. And the PIONEER trial (NCT03731260), a study of avapritinib in patients with indolent or smoldering SM, is scheduled to begin at the end of the year.
The EXPLORER trial was sponsored by Blueprint Medicines Corporation. Dr. Gotlib reported relationships with Blueprint Medicines, Celgene, Incyte, Novartis, Deciphera, Gilead, Promedior, and Kartos.
Decreased insulin clearance may be first step on path to insulin resistance
LOS ANGELES – As obese, nondiabetic individuals become more insulin resistant, a decrease in insulin clearance is the first change to occur, according to Sun H. Kim, MD.
“You will often hear about how insulin resistance enhances demand on beta cells to increase insulin secretion, which leads to hyperinsulinemia,” Dr. Kim said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “While well accepted, this model ignores the role of insulin clearance rate in maintaining hyperinsulinemia in insulin resistance states.”
In an effort to understand the physiologic adaptation to insulin resistance prior to the development of type 2 diabetes mellitus, Dr. Kim, an associate professor of endocrinology at Stanford (Calif) University, Stanford, and her colleagues enrolled 91 adults who had a body mass index of at least 30 kg/m2. The study was published in the March 2018 issue of Diabetologia. Each subject underwent a 75-g oral glucose tolerance test as well as the insulin suppression test to measure insulin resistance and the graded glucose infusion test to determine each subject’s insulin secretion rate and insulin clearance rate. For the graded glucose infusion test, the researchers increased the glucose infusion rate every 40 minutes, from 1 mg/kg per minute up to 8 mg/kg per minute. Next, they divided the cohort of obese individuals into tertiles of insulin resistance as quantified by the steady-state plasma glucose (SSPG): less than 9.7 mmol/L (tertile 1), 9.7-12.7 mmol/L (tertile 2), and 12.8 mmol/L or greater (tertile 3).
The mean age of subjects was 54 years. The mean SSPG level was 7.2 mmol/L among subjects in tertile 1, 11.3 mmol/L among those in tertile 2, and 14.3 mmol/L among those in tertile 3. The remainder of the demographics was similar. “Most importantly, body mass index among tertiles was nearly identical,” Dr. Kim said. “The only biomarker that was different was ALT, which increased with increasing tertiles. The individuals who were more insulin resistant likely had more fatty liver. We didn’t do imaging in this particular study.”
When the researchers evaluated oral glucose tolerance test data, they observed that subjects who were most insulin resistant had slightly higher glucose levels, “which we often see,” she said. “The body does try to keep glucose in a narrow range. What was dramatic were the insulin levels. The most insulin-resistant subjects had insulin levels that were double those of the least insulin-resistant subjects in tertile 1 during the oral glucose tolerance test.”
During the intravenous glucose infusion test, glucose levels rose similarly in the three groups, but those in tertile 3 had slightly higher glucose levels (P = .04). The insulin secretion rate, meanwhile, was similar among subjects in tertiles 1 and 2 but was increased significantly among subjects in tertile 3 (P less than .001). In contrast, the researchers observed a stepwise decline in insulin clearance rate from tertiles 1 to 3. Thus the insulin clearance rate was significantly different between subjects in tertile 1 and tertile 2 (P = .04) as well as between subjects in tertile 2 and those in tertile 3 (P less than .001).
“We propose that insulin resistance leads to an increase in intrahepatic fat, which decreases the insulin clearance rate and helps maintain euglycemia,” Dr. Kim concluded. “In the most insulin-resistant tertile, a decrease in insulin clearance rate is not sufficient, and an increase in the insulin secretion rate is also required. If you look at the relationship between insulin resistance and insulin clearance rate, there is a negative correlation, so the more insulin resistant you are, the lower your insulin clearance rate. However, there are insulin-resistant individuals who perhaps have higher insulin clearance rates than we think they should have. Could those individuals be at the highest risk to develop diabetes? That’s the story to which I don’t yet have an ending.” She reported having no financial disclosures.
SOURCE: Jung SH et al. Diabetologia. 2018;61(3):681-7.
LOS ANGELES – As obese, nondiabetic individuals become more insulin resistant, a decrease in insulin clearance is the first change to occur, according to Sun H. Kim, MD.
“You will often hear about how insulin resistance enhances demand on beta cells to increase insulin secretion, which leads to hyperinsulinemia,” Dr. Kim said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “While well accepted, this model ignores the role of insulin clearance rate in maintaining hyperinsulinemia in insulin resistance states.”
In an effort to understand the physiologic adaptation to insulin resistance prior to the development of type 2 diabetes mellitus, Dr. Kim, an associate professor of endocrinology at Stanford (Calif) University, Stanford, and her colleagues enrolled 91 adults who had a body mass index of at least 30 kg/m2. The study was published in the March 2018 issue of Diabetologia. Each subject underwent a 75-g oral glucose tolerance test as well as the insulin suppression test to measure insulin resistance and the graded glucose infusion test to determine each subject’s insulin secretion rate and insulin clearance rate. For the graded glucose infusion test, the researchers increased the glucose infusion rate every 40 minutes, from 1 mg/kg per minute up to 8 mg/kg per minute. Next, they divided the cohort of obese individuals into tertiles of insulin resistance as quantified by the steady-state plasma glucose (SSPG): less than 9.7 mmol/L (tertile 1), 9.7-12.7 mmol/L (tertile 2), and 12.8 mmol/L or greater (tertile 3).
The mean age of subjects was 54 years. The mean SSPG level was 7.2 mmol/L among subjects in tertile 1, 11.3 mmol/L among those in tertile 2, and 14.3 mmol/L among those in tertile 3. The remainder of the demographics was similar. “Most importantly, body mass index among tertiles was nearly identical,” Dr. Kim said. “The only biomarker that was different was ALT, which increased with increasing tertiles. The individuals who were more insulin resistant likely had more fatty liver. We didn’t do imaging in this particular study.”
When the researchers evaluated oral glucose tolerance test data, they observed that subjects who were most insulin resistant had slightly higher glucose levels, “which we often see,” she said. “The body does try to keep glucose in a narrow range. What was dramatic were the insulin levels. The most insulin-resistant subjects had insulin levels that were double those of the least insulin-resistant subjects in tertile 1 during the oral glucose tolerance test.”
During the intravenous glucose infusion test, glucose levels rose similarly in the three groups, but those in tertile 3 had slightly higher glucose levels (P = .04). The insulin secretion rate, meanwhile, was similar among subjects in tertiles 1 and 2 but was increased significantly among subjects in tertile 3 (P less than .001). In contrast, the researchers observed a stepwise decline in insulin clearance rate from tertiles 1 to 3. Thus the insulin clearance rate was significantly different between subjects in tertile 1 and tertile 2 (P = .04) as well as between subjects in tertile 2 and those in tertile 3 (P less than .001).
“We propose that insulin resistance leads to an increase in intrahepatic fat, which decreases the insulin clearance rate and helps maintain euglycemia,” Dr. Kim concluded. “In the most insulin-resistant tertile, a decrease in insulin clearance rate is not sufficient, and an increase in the insulin secretion rate is also required. If you look at the relationship between insulin resistance and insulin clearance rate, there is a negative correlation, so the more insulin resistant you are, the lower your insulin clearance rate. However, there are insulin-resistant individuals who perhaps have higher insulin clearance rates than we think they should have. Could those individuals be at the highest risk to develop diabetes? That’s the story to which I don’t yet have an ending.” She reported having no financial disclosures.
SOURCE: Jung SH et al. Diabetologia. 2018;61(3):681-7.
LOS ANGELES – As obese, nondiabetic individuals become more insulin resistant, a decrease in insulin clearance is the first change to occur, according to Sun H. Kim, MD.
“You will often hear about how insulin resistance enhances demand on beta cells to increase insulin secretion, which leads to hyperinsulinemia,” Dr. Kim said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “While well accepted, this model ignores the role of insulin clearance rate in maintaining hyperinsulinemia in insulin resistance states.”
In an effort to understand the physiologic adaptation to insulin resistance prior to the development of type 2 diabetes mellitus, Dr. Kim, an associate professor of endocrinology at Stanford (Calif) University, Stanford, and her colleagues enrolled 91 adults who had a body mass index of at least 30 kg/m2. The study was published in the March 2018 issue of Diabetologia. Each subject underwent a 75-g oral glucose tolerance test as well as the insulin suppression test to measure insulin resistance and the graded glucose infusion test to determine each subject’s insulin secretion rate and insulin clearance rate. For the graded glucose infusion test, the researchers increased the glucose infusion rate every 40 minutes, from 1 mg/kg per minute up to 8 mg/kg per minute. Next, they divided the cohort of obese individuals into tertiles of insulin resistance as quantified by the steady-state plasma glucose (SSPG): less than 9.7 mmol/L (tertile 1), 9.7-12.7 mmol/L (tertile 2), and 12.8 mmol/L or greater (tertile 3).
The mean age of subjects was 54 years. The mean SSPG level was 7.2 mmol/L among subjects in tertile 1, 11.3 mmol/L among those in tertile 2, and 14.3 mmol/L among those in tertile 3. The remainder of the demographics was similar. “Most importantly, body mass index among tertiles was nearly identical,” Dr. Kim said. “The only biomarker that was different was ALT, which increased with increasing tertiles. The individuals who were more insulin resistant likely had more fatty liver. We didn’t do imaging in this particular study.”
When the researchers evaluated oral glucose tolerance test data, they observed that subjects who were most insulin resistant had slightly higher glucose levels, “which we often see,” she said. “The body does try to keep glucose in a narrow range. What was dramatic were the insulin levels. The most insulin-resistant subjects had insulin levels that were double those of the least insulin-resistant subjects in tertile 1 during the oral glucose tolerance test.”
During the intravenous glucose infusion test, glucose levels rose similarly in the three groups, but those in tertile 3 had slightly higher glucose levels (P = .04). The insulin secretion rate, meanwhile, was similar among subjects in tertiles 1 and 2 but was increased significantly among subjects in tertile 3 (P less than .001). In contrast, the researchers observed a stepwise decline in insulin clearance rate from tertiles 1 to 3. Thus the insulin clearance rate was significantly different between subjects in tertile 1 and tertile 2 (P = .04) as well as between subjects in tertile 2 and those in tertile 3 (P less than .001).
“We propose that insulin resistance leads to an increase in intrahepatic fat, which decreases the insulin clearance rate and helps maintain euglycemia,” Dr. Kim concluded. “In the most insulin-resistant tertile, a decrease in insulin clearance rate is not sufficient, and an increase in the insulin secretion rate is also required. If you look at the relationship between insulin resistance and insulin clearance rate, there is a negative correlation, so the more insulin resistant you are, the lower your insulin clearance rate. However, there are insulin-resistant individuals who perhaps have higher insulin clearance rates than we think they should have. Could those individuals be at the highest risk to develop diabetes? That’s the story to which I don’t yet have an ending.” She reported having no financial disclosures.
SOURCE: Jung SH et al. Diabetologia. 2018;61(3):681-7.
REPORTING FROM WCIRDC 2018
Key clinical point:
Major finding: In the most insulin-resistant subgroup, the insulin secretion rate increases and the insulin clearance rate decreases to compensate for insulin resistance.
Study details: A study of 91 obese adults without diabetes.
Disclosures: Dr. Kim reported having no disclosures.
Source: Jung SH et al. Diabetologia. 2018;61(3):681-7.
Oxybutynin nets dramatic reduction in hot flashes
SAN ANTONIO – Oxybutynin (Ditropan), a drug approved to treat overactive bladder, is highly efficacious and well tolerated when used to alleviate hot flashes, according to results of a randomized, controlled trial of 150 women reported by lead author Roberto A. Leon-Ferre, MD.
The women, about two-thirds of whom were breast cancer survivors taking tamoxifen or aromatase inhibitors, were having at least 28 hot flashes weekly at baseline. Results of the trial showed that the 6-week reduction in a hot flash score capturing both frequency and severity was about 30% with placebo, 65% with oxybutynin 2.5 mg b.i.d., and 80% with oxybutynin 5 mg b.i.d. (P less than .01 across groups).
There also was a significant difference in quality of life in favor of the drug and, in the higher-dose group, significantly better scores for mood and life enjoyment. In a video interview, Dr. Leon-Ferre discussed how oxybutynin compares with other available treatment options, which women are good or poor candidates for this drug, and how the findings have influenced his own practice.
Dr. Leon-Ferre of the Mayo Clinic, Rochester, Minn., disclosed that he had no relevant conflicts of interest. The study was funded by the Breast Cancer Research Foundation.
SAN ANTONIO – Oxybutynin (Ditropan), a drug approved to treat overactive bladder, is highly efficacious and well tolerated when used to alleviate hot flashes, according to results of a randomized, controlled trial of 150 women reported by lead author Roberto A. Leon-Ferre, MD.
The women, about two-thirds of whom were breast cancer survivors taking tamoxifen or aromatase inhibitors, were having at least 28 hot flashes weekly at baseline. Results of the trial showed that the 6-week reduction in a hot flash score capturing both frequency and severity was about 30% with placebo, 65% with oxybutynin 2.5 mg b.i.d., and 80% with oxybutynin 5 mg b.i.d. (P less than .01 across groups).
There also was a significant difference in quality of life in favor of the drug and, in the higher-dose group, significantly better scores for mood and life enjoyment. In a video interview, Dr. Leon-Ferre discussed how oxybutynin compares with other available treatment options, which women are good or poor candidates for this drug, and how the findings have influenced his own practice.
Dr. Leon-Ferre of the Mayo Clinic, Rochester, Minn., disclosed that he had no relevant conflicts of interest. The study was funded by the Breast Cancer Research Foundation.
SAN ANTONIO – Oxybutynin (Ditropan), a drug approved to treat overactive bladder, is highly efficacious and well tolerated when used to alleviate hot flashes, according to results of a randomized, controlled trial of 150 women reported by lead author Roberto A. Leon-Ferre, MD.
The women, about two-thirds of whom were breast cancer survivors taking tamoxifen or aromatase inhibitors, were having at least 28 hot flashes weekly at baseline. Results of the trial showed that the 6-week reduction in a hot flash score capturing both frequency and severity was about 30% with placebo, 65% with oxybutynin 2.5 mg b.i.d., and 80% with oxybutynin 5 mg b.i.d. (P less than .01 across groups).
There also was a significant difference in quality of life in favor of the drug and, in the higher-dose group, significantly better scores for mood and life enjoyment. In a video interview, Dr. Leon-Ferre discussed how oxybutynin compares with other available treatment options, which women are good or poor candidates for this drug, and how the findings have influenced his own practice.
Dr. Leon-Ferre of the Mayo Clinic, Rochester, Minn., disclosed that he had no relevant conflicts of interest. The study was funded by the Breast Cancer Research Foundation.
REPORTING FROM SABCS 2018
In Medicare population, carotid revascularization has declined
NEW YORK – The rates of carotid artery revascularization with either endarterectomy or stenting declined precipitously over a recent 15-year period, at least among Medicare fee-for-service beneficiaries, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
A reduction in carotid endarterectomies (CEA) largely accounted for the decline during 1999-2014 although there was a cumulative decline in all carotid revascularization procedures when rates of CEA and stenting were combined, according to Brajesh K. Lal, MD, professor of surgery, University of Maryland Medical System, Baltimore.
In 1999, when enthusiasm for CEA appears to have peaked, 81,306 patients received this procedure, but a steady decline was observed until 2014, when 36,325 patients were being treated annually in the Medicare database. When calculated as endarterectomies per 100,000 beneficiaries, the rate declined from 298 to 128 (57%; P less than .001) over this 15-year period.
The number of stenting procedures had not reached its peak in 1999, when 10,416 were performed. Rather, the number performed annually nearly doubled to, 22.865 by 2006. However, it then began to decline and reached 10,208 by 2014, which was slightly fewer than in 1999, according to Dr. Lal.
These trends have been observed even though outcomes are getting better, at least for CEA, according to Dr. Lal. From the same pool of data, there was a 31% (1.1% vs. 1.6%) reduction from 1999 to 2014 in mortality at 30 days following CEA. For a composite of ischemic stroke and all-cause mortality, the rate fell 29.5% (3.1% vs. 4.4%). Both reductions were called statistically significant by Dr. Lal.
The improvements in CEA outcomes were observed even though “the treated patients got sicker when looking at comorbidities and risk factors, particularly hypertension, renal insufficiency, and diabetes,” Dr. Lal said.
Outcomes also improved among patients undergoing carotid stenting in general, although the patterns were described as “more complex.” In general, there was steady improvement on outcomes during 1999-2006, but there was no further gain and some lost ground during 2006-2014. For example, ischemic stroke or death fell from 7.0% in 1999 to 4.8% in 2006, but it had climbed back to 7.0% by 2014 with no net change when the first and last year were compared.
However, with risk adjustment, there was a reduction in in-hospital mortality (1.13% vs. 2.78%) over the study period for patients undergoing carotid stenting, according to Dr. Lal, who said this reached statistical significance. Like the CEA group, there was more comorbidity among those treated with stenting at the end, relative to the early part of the study period.
In the stenting group, patients with symptomatic carotid disease rose from 14.4% in 1999 to 25.9% in 2014. This tracks with Medicare policy, which required patients after 2005 to have symptomatic disease for reimbursement, according to Dr. Lal. Prior to 2005, reimbursement was granted for patients participating in clinical trials only.
The rates of carotid revascularization are not evenly distributed geographically in the United States, according to the Medicare data. Endarterectomy in particular has been more common in the south and Midwest than on either coast. This was true in 1999 and remained so in 2014. The distribution was similar for stenting, although it was also relatively common in the southwest in the early part of the study period.
In the beginning of the study, the increased rate of stenting might have contributed to the decline in endarterectomy, but there are several other factors that are implicated in the observed trends, according to Dr. Lal. He suggested that decreasing reimbursement for the performance of these procedures, better clinical management of risk factors, and advances in medical therapy. He cited a physician survey that showed a growing preference for medical management over invasive procedures in patients with high-grade stenosis and indicated that this last factor might be a particularly important driver of the decline in revascularization referrals for asymptomatic carotid disease.
The degree to which these Medicare data are representative of overall trends in the United States is unclear, but Dr. Lal called for further work to understand the forces that these data suggest are driving the changing patterns of carotid revascularization.
NEW YORK – The rates of carotid artery revascularization with either endarterectomy or stenting declined precipitously over a recent 15-year period, at least among Medicare fee-for-service beneficiaries, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
A reduction in carotid endarterectomies (CEA) largely accounted for the decline during 1999-2014 although there was a cumulative decline in all carotid revascularization procedures when rates of CEA and stenting were combined, according to Brajesh K. Lal, MD, professor of surgery, University of Maryland Medical System, Baltimore.
In 1999, when enthusiasm for CEA appears to have peaked, 81,306 patients received this procedure, but a steady decline was observed until 2014, when 36,325 patients were being treated annually in the Medicare database. When calculated as endarterectomies per 100,000 beneficiaries, the rate declined from 298 to 128 (57%; P less than .001) over this 15-year period.
The number of stenting procedures had not reached its peak in 1999, when 10,416 were performed. Rather, the number performed annually nearly doubled to, 22.865 by 2006. However, it then began to decline and reached 10,208 by 2014, which was slightly fewer than in 1999, according to Dr. Lal.
These trends have been observed even though outcomes are getting better, at least for CEA, according to Dr. Lal. From the same pool of data, there was a 31% (1.1% vs. 1.6%) reduction from 1999 to 2014 in mortality at 30 days following CEA. For a composite of ischemic stroke and all-cause mortality, the rate fell 29.5% (3.1% vs. 4.4%). Both reductions were called statistically significant by Dr. Lal.
The improvements in CEA outcomes were observed even though “the treated patients got sicker when looking at comorbidities and risk factors, particularly hypertension, renal insufficiency, and diabetes,” Dr. Lal said.
Outcomes also improved among patients undergoing carotid stenting in general, although the patterns were described as “more complex.” In general, there was steady improvement on outcomes during 1999-2006, but there was no further gain and some lost ground during 2006-2014. For example, ischemic stroke or death fell from 7.0% in 1999 to 4.8% in 2006, but it had climbed back to 7.0% by 2014 with no net change when the first and last year were compared.
However, with risk adjustment, there was a reduction in in-hospital mortality (1.13% vs. 2.78%) over the study period for patients undergoing carotid stenting, according to Dr. Lal, who said this reached statistical significance. Like the CEA group, there was more comorbidity among those treated with stenting at the end, relative to the early part of the study period.
In the stenting group, patients with symptomatic carotid disease rose from 14.4% in 1999 to 25.9% in 2014. This tracks with Medicare policy, which required patients after 2005 to have symptomatic disease for reimbursement, according to Dr. Lal. Prior to 2005, reimbursement was granted for patients participating in clinical trials only.
The rates of carotid revascularization are not evenly distributed geographically in the United States, according to the Medicare data. Endarterectomy in particular has been more common in the south and Midwest than on either coast. This was true in 1999 and remained so in 2014. The distribution was similar for stenting, although it was also relatively common in the southwest in the early part of the study period.
In the beginning of the study, the increased rate of stenting might have contributed to the decline in endarterectomy, but there are several other factors that are implicated in the observed trends, according to Dr. Lal. He suggested that decreasing reimbursement for the performance of these procedures, better clinical management of risk factors, and advances in medical therapy. He cited a physician survey that showed a growing preference for medical management over invasive procedures in patients with high-grade stenosis and indicated that this last factor might be a particularly important driver of the decline in revascularization referrals for asymptomatic carotid disease.
The degree to which these Medicare data are representative of overall trends in the United States is unclear, but Dr. Lal called for further work to understand the forces that these data suggest are driving the changing patterns of carotid revascularization.
NEW YORK – The rates of carotid artery revascularization with either endarterectomy or stenting declined precipitously over a recent 15-year period, at least among Medicare fee-for-service beneficiaries, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
A reduction in carotid endarterectomies (CEA) largely accounted for the decline during 1999-2014 although there was a cumulative decline in all carotid revascularization procedures when rates of CEA and stenting were combined, according to Brajesh K. Lal, MD, professor of surgery, University of Maryland Medical System, Baltimore.
In 1999, when enthusiasm for CEA appears to have peaked, 81,306 patients received this procedure, but a steady decline was observed until 2014, when 36,325 patients were being treated annually in the Medicare database. When calculated as endarterectomies per 100,000 beneficiaries, the rate declined from 298 to 128 (57%; P less than .001) over this 15-year period.
The number of stenting procedures had not reached its peak in 1999, when 10,416 were performed. Rather, the number performed annually nearly doubled to, 22.865 by 2006. However, it then began to decline and reached 10,208 by 2014, which was slightly fewer than in 1999, according to Dr. Lal.
These trends have been observed even though outcomes are getting better, at least for CEA, according to Dr. Lal. From the same pool of data, there was a 31% (1.1% vs. 1.6%) reduction from 1999 to 2014 in mortality at 30 days following CEA. For a composite of ischemic stroke and all-cause mortality, the rate fell 29.5% (3.1% vs. 4.4%). Both reductions were called statistically significant by Dr. Lal.
The improvements in CEA outcomes were observed even though “the treated patients got sicker when looking at comorbidities and risk factors, particularly hypertension, renal insufficiency, and diabetes,” Dr. Lal said.
Outcomes also improved among patients undergoing carotid stenting in general, although the patterns were described as “more complex.” In general, there was steady improvement on outcomes during 1999-2006, but there was no further gain and some lost ground during 2006-2014. For example, ischemic stroke or death fell from 7.0% in 1999 to 4.8% in 2006, but it had climbed back to 7.0% by 2014 with no net change when the first and last year were compared.
However, with risk adjustment, there was a reduction in in-hospital mortality (1.13% vs. 2.78%) over the study period for patients undergoing carotid stenting, according to Dr. Lal, who said this reached statistical significance. Like the CEA group, there was more comorbidity among those treated with stenting at the end, relative to the early part of the study period.
In the stenting group, patients with symptomatic carotid disease rose from 14.4% in 1999 to 25.9% in 2014. This tracks with Medicare policy, which required patients after 2005 to have symptomatic disease for reimbursement, according to Dr. Lal. Prior to 2005, reimbursement was granted for patients participating in clinical trials only.
The rates of carotid revascularization are not evenly distributed geographically in the United States, according to the Medicare data. Endarterectomy in particular has been more common in the south and Midwest than on either coast. This was true in 1999 and remained so in 2014. The distribution was similar for stenting, although it was also relatively common in the southwest in the early part of the study period.
In the beginning of the study, the increased rate of stenting might have contributed to the decline in endarterectomy, but there are several other factors that are implicated in the observed trends, according to Dr. Lal. He suggested that decreasing reimbursement for the performance of these procedures, better clinical management of risk factors, and advances in medical therapy. He cited a physician survey that showed a growing preference for medical management over invasive procedures in patients with high-grade stenosis and indicated that this last factor might be a particularly important driver of the decline in revascularization referrals for asymptomatic carotid disease.
The degree to which these Medicare data are representative of overall trends in the United States is unclear, but Dr. Lal called for further work to understand the forces that these data suggest are driving the changing patterns of carotid revascularization.
REPORTING FROM VEITHSYMPOSIUM
Key clinical point:
Major finding: During 1999-2014, the rate of carotid endarterectomy per 100,000 beneficiaries fell from 291 to 128 (57%; P less than .001).
Study details: Retrospective database review.
Disclosures: Dr. Lal reported having no financial conflicts relevant to the study.
Addressing patients’ complaints
. More than ever, it seems impossible to construct any sort of template for consistent, mutually satisfactory resolutions of such disputes.
But it is possible, and it’s not as complex as it appears, once you realize what the vast majority of complaints have in common: Expectations have not been met. Sometimes it’s your fault, sometimes the patient’s, and often a bit of both, but either way, the result is the same: You have an unhappy patient, and you must deal with it.
Why, you might ask? Is the expenditure of time and effort necessary to resolve complaints really worth it? Absolutely, because the old cliché is true: A satisfied patient will refer five new patients, but a dissatisfied one will chase away twenty or more. Besides, if the complaint is significant, and you decline to resolve it, the patient is likely to find someone who will; and chances are you won’t like the choice, or the venue – or the resolution.
As such, this is not a job you should delegate. Unless the complaint is trivial or purely administrative, you should address it yourself. It’s what you would want if you were the complainant, and it’s often too important to trust to a subordinate.
I have distilled this unpleasant duty down to a three-part strategy:
- Discover which expectations went unmet, and why.
- Agree on a solution.
- Learn from the experience, to prevent similar future complaints.
Of course, the easiest way to deal with complaints is to prevent as many as possible in the first place. Take the time to explain all treatments and procedures, and their most likely outcomes. Nip unrealistic expectations in the bud. Make it clear (preferably in writing) that reputable practitioners cannot guarantee perfect results. And, of course, document everything you have explained. Documentation is like garlic: There is no such thing as too much of it.
Of course, despite your best efforts at prevention, there will always be complaints, and handling them is a skill set worth honing, especially the one most of us do poorly: listening to the complaint.
Before you can resolve a problem you have to know what it is, and this is precisely the wrong time to make assumptions or jump to conclusions. So listen to the entire complaint without interrupting, defending, or justifying. Angry patients don’t care why the problem occurred, and they are not interested in your side of the story. This is not about you, so listen and understand.
As you listen, the unmet expectations will become clear. When the patient is finished, I like to summarize the complaint in that context: “So if I understand you correctly, you expected ‘X’ to happen, but ‘Y’ happened instead.” If I’m wrong, I modify my summary until the patient agrees that I understand the issue.
Once you know the problem, you can talk about a solution. The patient usually has one in mind – additional treatment, a referral elsewhere, a fee adjustment, or sometimes simply an apology. Consider it.
If the patient’s solution is reasonable, by all means, agree to it; if it is unreasonable, try to offer a reasonable alternative. The temptation here is to think more about protecting yourself than making the patient happy, but that often leads to bigger problems. Don’t be defensive. Again, this is not about you.
I am often asked if a refund is a reasonable option. Some patients (and lawyers) will interpret a refund as a tacit admission of guilt, so I generally try to avoid them. However, canceling a small fee or copay for an angry patient can be an expedient solution (particularly if it is still unpaid), and in my opinion, looks exactly like what it is: an honest effort to rectify the situation. But in general, additional materials or services, at reduced or waived fees, are a better alternative than refunding money.
Once you have arrived at a mutually satisfactory solution, again, document everything but consider reserving a “private” chart area for such documentation (unless it is a bona fide clinical issue), so that it won’t go out to referrers and other third parties with copies of your clinical notes. Also, consider having the patient sign off on the documentation, acknowledging that the complaint has been resolved.
Finally, always try to learn something from the experience. Ask yourself what you can do (or avoid doing) next time, and how you might prevent similar unrealistic expectations in a future situation.
Above all, never take complaints personally – even when they are personal. It’s always worth reminding yourself that no matter how hard you try, you will never please everyone.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
. More than ever, it seems impossible to construct any sort of template for consistent, mutually satisfactory resolutions of such disputes.
But it is possible, and it’s not as complex as it appears, once you realize what the vast majority of complaints have in common: Expectations have not been met. Sometimes it’s your fault, sometimes the patient’s, and often a bit of both, but either way, the result is the same: You have an unhappy patient, and you must deal with it.
Why, you might ask? Is the expenditure of time and effort necessary to resolve complaints really worth it? Absolutely, because the old cliché is true: A satisfied patient will refer five new patients, but a dissatisfied one will chase away twenty or more. Besides, if the complaint is significant, and you decline to resolve it, the patient is likely to find someone who will; and chances are you won’t like the choice, or the venue – or the resolution.
As such, this is not a job you should delegate. Unless the complaint is trivial or purely administrative, you should address it yourself. It’s what you would want if you were the complainant, and it’s often too important to trust to a subordinate.
I have distilled this unpleasant duty down to a three-part strategy:
- Discover which expectations went unmet, and why.
- Agree on a solution.
- Learn from the experience, to prevent similar future complaints.
Of course, the easiest way to deal with complaints is to prevent as many as possible in the first place. Take the time to explain all treatments and procedures, and their most likely outcomes. Nip unrealistic expectations in the bud. Make it clear (preferably in writing) that reputable practitioners cannot guarantee perfect results. And, of course, document everything you have explained. Documentation is like garlic: There is no such thing as too much of it.
Of course, despite your best efforts at prevention, there will always be complaints, and handling them is a skill set worth honing, especially the one most of us do poorly: listening to the complaint.
Before you can resolve a problem you have to know what it is, and this is precisely the wrong time to make assumptions or jump to conclusions. So listen to the entire complaint without interrupting, defending, or justifying. Angry patients don’t care why the problem occurred, and they are not interested in your side of the story. This is not about you, so listen and understand.
As you listen, the unmet expectations will become clear. When the patient is finished, I like to summarize the complaint in that context: “So if I understand you correctly, you expected ‘X’ to happen, but ‘Y’ happened instead.” If I’m wrong, I modify my summary until the patient agrees that I understand the issue.
Once you know the problem, you can talk about a solution. The patient usually has one in mind – additional treatment, a referral elsewhere, a fee adjustment, or sometimes simply an apology. Consider it.
If the patient’s solution is reasonable, by all means, agree to it; if it is unreasonable, try to offer a reasonable alternative. The temptation here is to think more about protecting yourself than making the patient happy, but that often leads to bigger problems. Don’t be defensive. Again, this is not about you.
I am often asked if a refund is a reasonable option. Some patients (and lawyers) will interpret a refund as a tacit admission of guilt, so I generally try to avoid them. However, canceling a small fee or copay for an angry patient can be an expedient solution (particularly if it is still unpaid), and in my opinion, looks exactly like what it is: an honest effort to rectify the situation. But in general, additional materials or services, at reduced or waived fees, are a better alternative than refunding money.
Once you have arrived at a mutually satisfactory solution, again, document everything but consider reserving a “private” chart area for such documentation (unless it is a bona fide clinical issue), so that it won’t go out to referrers and other third parties with copies of your clinical notes. Also, consider having the patient sign off on the documentation, acknowledging that the complaint has been resolved.
Finally, always try to learn something from the experience. Ask yourself what you can do (or avoid doing) next time, and how you might prevent similar unrealistic expectations in a future situation.
Above all, never take complaints personally – even when they are personal. It’s always worth reminding yourself that no matter how hard you try, you will never please everyone.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
. More than ever, it seems impossible to construct any sort of template for consistent, mutually satisfactory resolutions of such disputes.
But it is possible, and it’s not as complex as it appears, once you realize what the vast majority of complaints have in common: Expectations have not been met. Sometimes it’s your fault, sometimes the patient’s, and often a bit of both, but either way, the result is the same: You have an unhappy patient, and you must deal with it.
Why, you might ask? Is the expenditure of time and effort necessary to resolve complaints really worth it? Absolutely, because the old cliché is true: A satisfied patient will refer five new patients, but a dissatisfied one will chase away twenty or more. Besides, if the complaint is significant, and you decline to resolve it, the patient is likely to find someone who will; and chances are you won’t like the choice, or the venue – or the resolution.
As such, this is not a job you should delegate. Unless the complaint is trivial or purely administrative, you should address it yourself. It’s what you would want if you were the complainant, and it’s often too important to trust to a subordinate.
I have distilled this unpleasant duty down to a three-part strategy:
- Discover which expectations went unmet, and why.
- Agree on a solution.
- Learn from the experience, to prevent similar future complaints.
Of course, the easiest way to deal with complaints is to prevent as many as possible in the first place. Take the time to explain all treatments and procedures, and their most likely outcomes. Nip unrealistic expectations in the bud. Make it clear (preferably in writing) that reputable practitioners cannot guarantee perfect results. And, of course, document everything you have explained. Documentation is like garlic: There is no such thing as too much of it.
Of course, despite your best efforts at prevention, there will always be complaints, and handling them is a skill set worth honing, especially the one most of us do poorly: listening to the complaint.
Before you can resolve a problem you have to know what it is, and this is precisely the wrong time to make assumptions or jump to conclusions. So listen to the entire complaint without interrupting, defending, or justifying. Angry patients don’t care why the problem occurred, and they are not interested in your side of the story. This is not about you, so listen and understand.
As you listen, the unmet expectations will become clear. When the patient is finished, I like to summarize the complaint in that context: “So if I understand you correctly, you expected ‘X’ to happen, but ‘Y’ happened instead.” If I’m wrong, I modify my summary until the patient agrees that I understand the issue.
Once you know the problem, you can talk about a solution. The patient usually has one in mind – additional treatment, a referral elsewhere, a fee adjustment, or sometimes simply an apology. Consider it.
If the patient’s solution is reasonable, by all means, agree to it; if it is unreasonable, try to offer a reasonable alternative. The temptation here is to think more about protecting yourself than making the patient happy, but that often leads to bigger problems. Don’t be defensive. Again, this is not about you.
I am often asked if a refund is a reasonable option. Some patients (and lawyers) will interpret a refund as a tacit admission of guilt, so I generally try to avoid them. However, canceling a small fee or copay for an angry patient can be an expedient solution (particularly if it is still unpaid), and in my opinion, looks exactly like what it is: an honest effort to rectify the situation. But in general, additional materials or services, at reduced or waived fees, are a better alternative than refunding money.
Once you have arrived at a mutually satisfactory solution, again, document everything but consider reserving a “private” chart area for such documentation (unless it is a bona fide clinical issue), so that it won’t go out to referrers and other third parties with copies of your clinical notes. Also, consider having the patient sign off on the documentation, acknowledging that the complaint has been resolved.
Finally, always try to learn something from the experience. Ask yourself what you can do (or avoid doing) next time, and how you might prevent similar unrealistic expectations in a future situation.
Above all, never take complaints personally – even when they are personal. It’s always worth reminding yourself that no matter how hard you try, you will never please everyone.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.