User login
Dr. Mary Edwards Walker Inspiring Women in Surgery Award presented to Dr. Lee
The American College of Surgeons (ACS) presented the Dr. Mary Edwards Walker Inspiring Women in Surgery Award to Yeu-Tsu Margaret Lee, MD, FACS, at the Convocation at Clinical Congress 2018 in Boston, MA. This award was established by the ACS Women in Surgery Committee (WiSC) and is presented annually at the Clinical Congress to recognize an individual’s significant contributions to the advancement of women in surgery.
Dr. Lee is from Honolulu, HI, and was born in Xian, China, in 1936. During her childhood, four of her siblings died from illness, motivating Dr. Lee to become a physician. Her family was forced to flee to Taiwan after the Chinese Civil War, and she immigrated to the U.S. in 1955, graduated from Harvard Medical School, Boston, in 1961, and has worked as a general surgeon and a surgical oncologist for more than 50 years. In the early 1980s, she was a tenured associate professor of surgery, University of Southern California (USC), Los Angeles, and head physician, Los Angeles County-USC Medical Center, but chose to pursue a different path.
In 1983, Dr. Lee moved to Hawaii, worked at Tripler Army Medical Center, Honolulu, as chief, surgical oncology section of general surgical services, and joined the U.S. Army Corps. She was deployed to Iraq during Operation Desert Storm and treated many U.S. soldiers as well as Iraqi prisoners of war. She served on a team of surgeons that performed more than 125 operations in a 400-bed hospital in northern Saudi Arabia. Dr. Lee received several accolades in the military, including an “A” Proficiency Designator from the Army Medical Department and a Certificate of Achievement. After retiring from the Army as a colonel, she became professor of surgery, University of Hawaii at Manoa, Honolulu, where she was the only woman surgeon for most of her career.
Dr. Lee has participated in medical missions to Ghana, Honduras, Cambodia, Laos, the Philippines, and other underserved countries. She has made many international trips to promote friendship and medical exchanges. Notably, in 1995, she was the leader of a Women Surgeons Delegation to Russia and Romania. The trip was a Citizen Ambassador Program sponsored by People to People International, which was established by President Dwight D. Eisenhower. From 2000 to 2017, Dr. Lee taught surgery for a month, four times a year, at the Tzu-Chi University School of Medicine, Hualien, Taiwan.
Dr. Lee was one of 21 women surgeons in attendance at a networking breakfast at the 1981 Clinical Congress—led by ACS Past-President Patricia Numann, MD, FACS—which proved to be the genesis of the Association of Women Surgeons (AWS). She has been a supporter of the association, in time and talent, since its inception, and her presence at the AWS meetings, her academic career at teaching hospitals, and her research publications provide women surgeons and medical students from around the world an example of what women can achieve in the field.
Because her home is in Hawaii, midway between the East and West, she hopes to function as a “bridge,” contributing to global understanding and promoting communication, collaboration, and goodwill, and continues to work in the areas of medical education, international health, and world peace.
Committed to improving the care of the surgical patient, Dr. Lee is an outstanding leader and role model for surgeons everywhere. Her contributions to academic medicine in surgery, in the military, and in surgical volunteerism worldwide have made a lasting impression on the surgical profession. Her passion, endless energy, and dedication to the ACS and to women in surgery are without equal.
The American College of Surgeons (ACS) presented the Dr. Mary Edwards Walker Inspiring Women in Surgery Award to Yeu-Tsu Margaret Lee, MD, FACS, at the Convocation at Clinical Congress 2018 in Boston, MA. This award was established by the ACS Women in Surgery Committee (WiSC) and is presented annually at the Clinical Congress to recognize an individual’s significant contributions to the advancement of women in surgery.
Dr. Lee is from Honolulu, HI, and was born in Xian, China, in 1936. During her childhood, four of her siblings died from illness, motivating Dr. Lee to become a physician. Her family was forced to flee to Taiwan after the Chinese Civil War, and she immigrated to the U.S. in 1955, graduated from Harvard Medical School, Boston, in 1961, and has worked as a general surgeon and a surgical oncologist for more than 50 years. In the early 1980s, she was a tenured associate professor of surgery, University of Southern California (USC), Los Angeles, and head physician, Los Angeles County-USC Medical Center, but chose to pursue a different path.
In 1983, Dr. Lee moved to Hawaii, worked at Tripler Army Medical Center, Honolulu, as chief, surgical oncology section of general surgical services, and joined the U.S. Army Corps. She was deployed to Iraq during Operation Desert Storm and treated many U.S. soldiers as well as Iraqi prisoners of war. She served on a team of surgeons that performed more than 125 operations in a 400-bed hospital in northern Saudi Arabia. Dr. Lee received several accolades in the military, including an “A” Proficiency Designator from the Army Medical Department and a Certificate of Achievement. After retiring from the Army as a colonel, she became professor of surgery, University of Hawaii at Manoa, Honolulu, where she was the only woman surgeon for most of her career.
Dr. Lee has participated in medical missions to Ghana, Honduras, Cambodia, Laos, the Philippines, and other underserved countries. She has made many international trips to promote friendship and medical exchanges. Notably, in 1995, she was the leader of a Women Surgeons Delegation to Russia and Romania. The trip was a Citizen Ambassador Program sponsored by People to People International, which was established by President Dwight D. Eisenhower. From 2000 to 2017, Dr. Lee taught surgery for a month, four times a year, at the Tzu-Chi University School of Medicine, Hualien, Taiwan.
Dr. Lee was one of 21 women surgeons in attendance at a networking breakfast at the 1981 Clinical Congress—led by ACS Past-President Patricia Numann, MD, FACS—which proved to be the genesis of the Association of Women Surgeons (AWS). She has been a supporter of the association, in time and talent, since its inception, and her presence at the AWS meetings, her academic career at teaching hospitals, and her research publications provide women surgeons and medical students from around the world an example of what women can achieve in the field.
Because her home is in Hawaii, midway between the East and West, she hopes to function as a “bridge,” contributing to global understanding and promoting communication, collaboration, and goodwill, and continues to work in the areas of medical education, international health, and world peace.
Committed to improving the care of the surgical patient, Dr. Lee is an outstanding leader and role model for surgeons everywhere. Her contributions to academic medicine in surgery, in the military, and in surgical volunteerism worldwide have made a lasting impression on the surgical profession. Her passion, endless energy, and dedication to the ACS and to women in surgery are without equal.
The American College of Surgeons (ACS) presented the Dr. Mary Edwards Walker Inspiring Women in Surgery Award to Yeu-Tsu Margaret Lee, MD, FACS, at the Convocation at Clinical Congress 2018 in Boston, MA. This award was established by the ACS Women in Surgery Committee (WiSC) and is presented annually at the Clinical Congress to recognize an individual’s significant contributions to the advancement of women in surgery.
Dr. Lee is from Honolulu, HI, and was born in Xian, China, in 1936. During her childhood, four of her siblings died from illness, motivating Dr. Lee to become a physician. Her family was forced to flee to Taiwan after the Chinese Civil War, and she immigrated to the U.S. in 1955, graduated from Harvard Medical School, Boston, in 1961, and has worked as a general surgeon and a surgical oncologist for more than 50 years. In the early 1980s, she was a tenured associate professor of surgery, University of Southern California (USC), Los Angeles, and head physician, Los Angeles County-USC Medical Center, but chose to pursue a different path.
In 1983, Dr. Lee moved to Hawaii, worked at Tripler Army Medical Center, Honolulu, as chief, surgical oncology section of general surgical services, and joined the U.S. Army Corps. She was deployed to Iraq during Operation Desert Storm and treated many U.S. soldiers as well as Iraqi prisoners of war. She served on a team of surgeons that performed more than 125 operations in a 400-bed hospital in northern Saudi Arabia. Dr. Lee received several accolades in the military, including an “A” Proficiency Designator from the Army Medical Department and a Certificate of Achievement. After retiring from the Army as a colonel, she became professor of surgery, University of Hawaii at Manoa, Honolulu, where she was the only woman surgeon for most of her career.
Dr. Lee has participated in medical missions to Ghana, Honduras, Cambodia, Laos, the Philippines, and other underserved countries. She has made many international trips to promote friendship and medical exchanges. Notably, in 1995, she was the leader of a Women Surgeons Delegation to Russia and Romania. The trip was a Citizen Ambassador Program sponsored by People to People International, which was established by President Dwight D. Eisenhower. From 2000 to 2017, Dr. Lee taught surgery for a month, four times a year, at the Tzu-Chi University School of Medicine, Hualien, Taiwan.
Dr. Lee was one of 21 women surgeons in attendance at a networking breakfast at the 1981 Clinical Congress—led by ACS Past-President Patricia Numann, MD, FACS—which proved to be the genesis of the Association of Women Surgeons (AWS). She has been a supporter of the association, in time and talent, since its inception, and her presence at the AWS meetings, her academic career at teaching hospitals, and her research publications provide women surgeons and medical students from around the world an example of what women can achieve in the field.
Because her home is in Hawaii, midway between the East and West, she hopes to function as a “bridge,” contributing to global understanding and promoting communication, collaboration, and goodwill, and continues to work in the areas of medical education, international health, and world peace.
Committed to improving the care of the surgical patient, Dr. Lee is an outstanding leader and role model for surgeons everywhere. Her contributions to academic medicine in surgery, in the military, and in surgical volunteerism worldwide have made a lasting impression on the surgical profession. Her passion, endless energy, and dedication to the ACS and to women in surgery are without equal.
Biography of C. Rollins Hanlon, MD, FACS, Past-Director of the ACS, now available
The American College of Surgeons (ACS) recently published a new biography of C. Rollins Hanlon, MD, FACS, ACS Past-Director, a seminal figure in the history of surgery and the College, titled The Conscience of Surgery: C. Rollins Hanlon, MD, FACS. Written by David L. Nahrwold, MD, FACS, this account examines the life of the erudite, principled cardiothoracic surgeon and innovator, who co-developed the Blalock-Hanlon operation with Alfred P. Blalock, MD, FACS.
The book covers every aspect of Dr. Hanlon’s life—from his boyhood in Baltimore, MD, to his quest to be the best clinician and surgeon-scientist, to his views on the government’s increasing influence on the delivery of surgical care, and to his undying love of the written word. For many surgeons, Dr. Hanlon was the embodiment of what it means to be a Fellow of the ACS.
“I got to know [Dr. Hanlon] as a person and a professional during my stint as the Interim Director of the ACS in 1999 when he was ‘retired’ and serving as Executive Consultant,” Dr. Nahrwold writes in the book’s preface. “He insisted that the mission of the College was to advance the ethical and competent practice of surgery and not to improve the financial well-being of surgeons.”
Throughout his career, Dr. Hanlon’s mentors, colleagues, and students included many eminent surgeons at Johns Hopkins Medical School, Baltimore, MD; Cincinnati General Hospital, OH; and the University of California, San Francisco. He trained under Dean DeWitt Lewis, Walter E. Dandy, Howard C. Naffziger, Warfield “Monty” Firor, and Mont Reid (all MD, FACS). He worked alongside William P. Longmire, MD, FACS; Dr. Blalock; and Mark C. Ravitch, MD, FACS; and his residents and interns at St. Louis University, MO, included Vallee Willman, Theodore Cooper, Theodore Dubuque, and William Stoneman (all MD, FACS), among others.
Dr. Hanlon served in the U.S. Navy in World War II, and followed with a distinguished career at Johns Hopkins and at St. Louis University, where, as chair of surgery, he developed the institution’s cardiac research capabilities, which helped to pioneer early open-heart and heart transplant procedures.
Dr. Hanlon became a Fellow of the College in 1953 and served as the ACS Director for 17 years (1969–1986), making him the longest-serving Director to date. Additionally, he served on the Board of Regents and as the ACS President (1985–1986). After retirement, he stayed on as ACS Executive Consultant, offering his sage advice to his successors, including Paul A. Ebert, MD, FACS; Samuel Wells, MD, FACS; Dr. Nahrwold; Thomas R. Russell, MD, FACS; and David B. Hoyt, MD, FACS. Through these positions, Dr. Hanlon had a profound effect on the direction and philosophy of the College, including in philanthropic endeavors and the establishment of the ACS Archives. He received the first ACS Lifetime Achievement Award in 2010.
“Hanlon’s integrity, faith, hard work, and service to others led him to become a role model for physicians and laypersons alike. These attributes also drove his brilliant career as an innovative surgeon, leadership in academic and organized medicine, and reputation as a humanist and ethicist,” Dr. Narhwold concludes in the preface. “Before he died I knew that I must write his biography to expose his principled life, his goodness, and his devotion to surgery and to surgeons, especially young surgeons, with the hope that they and others will find his life worthy of study and emulation.”
Dr. Nahrwold is Emeritus Professor of Surgery at Northwestern University Feinberg School of Medicine, Chicago, IL, where he was the Loyal and Edith Davis Professor and Chairman, department of surgery, and surgeon-in-chief, Northwestern Memorial Hospital. He is a recipient of the College’s highest honor—the Distinguished Service Award.
Dr. Nahrwold is author of A Mirror Reflecting Surgery, Surgeons, and their College: The Bulletin of the American College of Surgeons, and co-author, with Peter J. Kernahan, MD, PhD, FACS, of A Century of Surgeons and Surgery: The American College of Surgeons 1913–2012.
The Conscience of Surgery: C. Rollins Hanlon, MD, FACS, is available for $15.95 on the ACS E-Store at web4.facs.org/eBusiness/ProductCatalog/Product.aspx?ID=1060 and on amazon.com.
The American College of Surgeons (ACS) recently published a new biography of C. Rollins Hanlon, MD, FACS, ACS Past-Director, a seminal figure in the history of surgery and the College, titled The Conscience of Surgery: C. Rollins Hanlon, MD, FACS. Written by David L. Nahrwold, MD, FACS, this account examines the life of the erudite, principled cardiothoracic surgeon and innovator, who co-developed the Blalock-Hanlon operation with Alfred P. Blalock, MD, FACS.
The book covers every aspect of Dr. Hanlon’s life—from his boyhood in Baltimore, MD, to his quest to be the best clinician and surgeon-scientist, to his views on the government’s increasing influence on the delivery of surgical care, and to his undying love of the written word. For many surgeons, Dr. Hanlon was the embodiment of what it means to be a Fellow of the ACS.
“I got to know [Dr. Hanlon] as a person and a professional during my stint as the Interim Director of the ACS in 1999 when he was ‘retired’ and serving as Executive Consultant,” Dr. Nahrwold writes in the book’s preface. “He insisted that the mission of the College was to advance the ethical and competent practice of surgery and not to improve the financial well-being of surgeons.”
Throughout his career, Dr. Hanlon’s mentors, colleagues, and students included many eminent surgeons at Johns Hopkins Medical School, Baltimore, MD; Cincinnati General Hospital, OH; and the University of California, San Francisco. He trained under Dean DeWitt Lewis, Walter E. Dandy, Howard C. Naffziger, Warfield “Monty” Firor, and Mont Reid (all MD, FACS). He worked alongside William P. Longmire, MD, FACS; Dr. Blalock; and Mark C. Ravitch, MD, FACS; and his residents and interns at St. Louis University, MO, included Vallee Willman, Theodore Cooper, Theodore Dubuque, and William Stoneman (all MD, FACS), among others.
Dr. Hanlon served in the U.S. Navy in World War II, and followed with a distinguished career at Johns Hopkins and at St. Louis University, where, as chair of surgery, he developed the institution’s cardiac research capabilities, which helped to pioneer early open-heart and heart transplant procedures.
Dr. Hanlon became a Fellow of the College in 1953 and served as the ACS Director for 17 years (1969–1986), making him the longest-serving Director to date. Additionally, he served on the Board of Regents and as the ACS President (1985–1986). After retirement, he stayed on as ACS Executive Consultant, offering his sage advice to his successors, including Paul A. Ebert, MD, FACS; Samuel Wells, MD, FACS; Dr. Nahrwold; Thomas R. Russell, MD, FACS; and David B. Hoyt, MD, FACS. Through these positions, Dr. Hanlon had a profound effect on the direction and philosophy of the College, including in philanthropic endeavors and the establishment of the ACS Archives. He received the first ACS Lifetime Achievement Award in 2010.
“Hanlon’s integrity, faith, hard work, and service to others led him to become a role model for physicians and laypersons alike. These attributes also drove his brilliant career as an innovative surgeon, leadership in academic and organized medicine, and reputation as a humanist and ethicist,” Dr. Narhwold concludes in the preface. “Before he died I knew that I must write his biography to expose his principled life, his goodness, and his devotion to surgery and to surgeons, especially young surgeons, with the hope that they and others will find his life worthy of study and emulation.”
Dr. Nahrwold is Emeritus Professor of Surgery at Northwestern University Feinberg School of Medicine, Chicago, IL, where he was the Loyal and Edith Davis Professor and Chairman, department of surgery, and surgeon-in-chief, Northwestern Memorial Hospital. He is a recipient of the College’s highest honor—the Distinguished Service Award.
Dr. Nahrwold is author of A Mirror Reflecting Surgery, Surgeons, and their College: The Bulletin of the American College of Surgeons, and co-author, with Peter J. Kernahan, MD, PhD, FACS, of A Century of Surgeons and Surgery: The American College of Surgeons 1913–2012.
The Conscience of Surgery: C. Rollins Hanlon, MD, FACS, is available for $15.95 on the ACS E-Store at web4.facs.org/eBusiness/ProductCatalog/Product.aspx?ID=1060 and on amazon.com.
The American College of Surgeons (ACS) recently published a new biography of C. Rollins Hanlon, MD, FACS, ACS Past-Director, a seminal figure in the history of surgery and the College, titled The Conscience of Surgery: C. Rollins Hanlon, MD, FACS. Written by David L. Nahrwold, MD, FACS, this account examines the life of the erudite, principled cardiothoracic surgeon and innovator, who co-developed the Blalock-Hanlon operation with Alfred P. Blalock, MD, FACS.
The book covers every aspect of Dr. Hanlon’s life—from his boyhood in Baltimore, MD, to his quest to be the best clinician and surgeon-scientist, to his views on the government’s increasing influence on the delivery of surgical care, and to his undying love of the written word. For many surgeons, Dr. Hanlon was the embodiment of what it means to be a Fellow of the ACS.
“I got to know [Dr. Hanlon] as a person and a professional during my stint as the Interim Director of the ACS in 1999 when he was ‘retired’ and serving as Executive Consultant,” Dr. Nahrwold writes in the book’s preface. “He insisted that the mission of the College was to advance the ethical and competent practice of surgery and not to improve the financial well-being of surgeons.”
Throughout his career, Dr. Hanlon’s mentors, colleagues, and students included many eminent surgeons at Johns Hopkins Medical School, Baltimore, MD; Cincinnati General Hospital, OH; and the University of California, San Francisco. He trained under Dean DeWitt Lewis, Walter E. Dandy, Howard C. Naffziger, Warfield “Monty” Firor, and Mont Reid (all MD, FACS). He worked alongside William P. Longmire, MD, FACS; Dr. Blalock; and Mark C. Ravitch, MD, FACS; and his residents and interns at St. Louis University, MO, included Vallee Willman, Theodore Cooper, Theodore Dubuque, and William Stoneman (all MD, FACS), among others.
Dr. Hanlon served in the U.S. Navy in World War II, and followed with a distinguished career at Johns Hopkins and at St. Louis University, where, as chair of surgery, he developed the institution’s cardiac research capabilities, which helped to pioneer early open-heart and heart transplant procedures.
Dr. Hanlon became a Fellow of the College in 1953 and served as the ACS Director for 17 years (1969–1986), making him the longest-serving Director to date. Additionally, he served on the Board of Regents and as the ACS President (1985–1986). After retirement, he stayed on as ACS Executive Consultant, offering his sage advice to his successors, including Paul A. Ebert, MD, FACS; Samuel Wells, MD, FACS; Dr. Nahrwold; Thomas R. Russell, MD, FACS; and David B. Hoyt, MD, FACS. Through these positions, Dr. Hanlon had a profound effect on the direction and philosophy of the College, including in philanthropic endeavors and the establishment of the ACS Archives. He received the first ACS Lifetime Achievement Award in 2010.
“Hanlon’s integrity, faith, hard work, and service to others led him to become a role model for physicians and laypersons alike. These attributes also drove his brilliant career as an innovative surgeon, leadership in academic and organized medicine, and reputation as a humanist and ethicist,” Dr. Narhwold concludes in the preface. “Before he died I knew that I must write his biography to expose his principled life, his goodness, and his devotion to surgery and to surgeons, especially young surgeons, with the hope that they and others will find his life worthy of study and emulation.”
Dr. Nahrwold is Emeritus Professor of Surgery at Northwestern University Feinberg School of Medicine, Chicago, IL, where he was the Loyal and Edith Davis Professor and Chairman, department of surgery, and surgeon-in-chief, Northwestern Memorial Hospital. He is a recipient of the College’s highest honor—the Distinguished Service Award.
Dr. Nahrwold is author of A Mirror Reflecting Surgery, Surgeons, and their College: The Bulletin of the American College of Surgeons, and co-author, with Peter J. Kernahan, MD, PhD, FACS, of A Century of Surgeons and Surgery: The American College of Surgeons 1913–2012.
The Conscience of Surgery: C. Rollins Hanlon, MD, FACS, is available for $15.95 on the ACS E-Store at web4.facs.org/eBusiness/ProductCatalog/Product.aspx?ID=1060 and on amazon.com.
Heidi Nelson, MD, FACS, named Medical Director of ACS Cancer Programs
The American College of Surgeons (ACS) recently announced that Heidi Nelson, MD, FACS, a colorectal surgeon from Rochester, MN, will be joining the ACS Division of Research and Optimal Patient Care (DROPC) as Medical Director, Cancer Programs, succeeding David P. Winchester, MD, FACS, as he transitions from the position he has served in for more than 30 years. Dr. Nelson comes to the ACS from her position as chair, and vice-chair for research, department of surgery, Mayo Clinic, as well as professor of surgery, Mayo Clinic College of Medicine and Science, Rochester. She has master’s faculty privileges in clinical and translation science at the Mayo Clinic Graduate School of Biomedical Sciences and the Mayo Clinic College of Medicine and Science.
Dr. Nelson received a bachelor’s degree from Western Washington University, Bellingham, and her medical degree from the University of Washington School of Medicine, Seattle. She completed an internship and residency in general surgery at Oregon Health & Science University, Portland, where she also served as an American Cancer Society Fellow. She then went to the Mayo Clinic College of Medicine and Science, where she was a colon and rectal surgery fellow and completed a research fellowship. Dr. Nelson returned to the University of Washington, where she was a Leo Hirsch Traveling Fellow.
Dr. Nelson has received numerous awards and held membership in many professional organizations, including the American Society of Colon and Rectal Surgeons (ASCRS), the Mayo Clinic Board of Governors, the Society of Surgical Oncology, and the Association of Women Surgeons, among others.
Research activities
As the Fred C. Andersen Professor for the Mayo Foundation and a consultant for Mayo Clinic’s division of colon and rectal surgery, Dr. Nelson is internationally renowned for her research in the field of colon and rectal cancer. The goal of her research activities has been to improve the duration and quality of life for these patients. These efforts have helped to reduce the impact of surgery on patients with early-stage disease through the safe introduction of laparoscopic and minimally invasive surgical approaches. Her work also has helped to reduce the cancer burden in patients with locally advanced and recurrent rectal cancer through studies examining the role of complex operations and intraoperative radiation therapy. Dr. Nelson’s work has been funded by the National Institutes of Health, the American Cancer Society, the ASCRS, and many other organizations. In addition to her clinical activities, she has led the Center for Individualized Medicine Microbiome Program at the Mayo Clinic, where she conducts, presents, and publishes research on the human microbiome and its connection to health and disease.
Leadership
Dr. Nelson brings a wealth of experience from leading others and establishing results-oriented teams. She has mentored trainees and investigators and has served as an editor and publisher for high-impact journals. She also has been extensively involved with the ACS throughout her career—Dr. Nelson became an ACS Fellow in 1993 and has served as former Director, ACS Clinical Research Program; former co-chair, ACS Oncology Group; and as a member, Commission on Cancer Executive Committee.
Dr. Nelson started working with the ACS in September on an initial part-time basis, overlapping with Dr. Winchester to ensure a smooth transition and continuity of leadership.
“The American College of Surgeons is excited about Dr. Nelson joining our Executive Leadership Team. Her research acumen and leadership in the cancer care community are well known and widely respected. Her addition to our team will benefit our members, our relationships with cancer care organizations, and the patients whom we serve,” said ACS Executive Director David B. Hoyt, MD, FACS.
The American College of Surgeons (ACS) recently announced that Heidi Nelson, MD, FACS, a colorectal surgeon from Rochester, MN, will be joining the ACS Division of Research and Optimal Patient Care (DROPC) as Medical Director, Cancer Programs, succeeding David P. Winchester, MD, FACS, as he transitions from the position he has served in for more than 30 years. Dr. Nelson comes to the ACS from her position as chair, and vice-chair for research, department of surgery, Mayo Clinic, as well as professor of surgery, Mayo Clinic College of Medicine and Science, Rochester. She has master’s faculty privileges in clinical and translation science at the Mayo Clinic Graduate School of Biomedical Sciences and the Mayo Clinic College of Medicine and Science.
Dr. Nelson received a bachelor’s degree from Western Washington University, Bellingham, and her medical degree from the University of Washington School of Medicine, Seattle. She completed an internship and residency in general surgery at Oregon Health & Science University, Portland, where she also served as an American Cancer Society Fellow. She then went to the Mayo Clinic College of Medicine and Science, where she was a colon and rectal surgery fellow and completed a research fellowship. Dr. Nelson returned to the University of Washington, where she was a Leo Hirsch Traveling Fellow.
Dr. Nelson has received numerous awards and held membership in many professional organizations, including the American Society of Colon and Rectal Surgeons (ASCRS), the Mayo Clinic Board of Governors, the Society of Surgical Oncology, and the Association of Women Surgeons, among others.
Research activities
As the Fred C. Andersen Professor for the Mayo Foundation and a consultant for Mayo Clinic’s division of colon and rectal surgery, Dr. Nelson is internationally renowned for her research in the field of colon and rectal cancer. The goal of her research activities has been to improve the duration and quality of life for these patients. These efforts have helped to reduce the impact of surgery on patients with early-stage disease through the safe introduction of laparoscopic and minimally invasive surgical approaches. Her work also has helped to reduce the cancer burden in patients with locally advanced and recurrent rectal cancer through studies examining the role of complex operations and intraoperative radiation therapy. Dr. Nelson’s work has been funded by the National Institutes of Health, the American Cancer Society, the ASCRS, and many other organizations. In addition to her clinical activities, she has led the Center for Individualized Medicine Microbiome Program at the Mayo Clinic, where she conducts, presents, and publishes research on the human microbiome and its connection to health and disease.
Leadership
Dr. Nelson brings a wealth of experience from leading others and establishing results-oriented teams. She has mentored trainees and investigators and has served as an editor and publisher for high-impact journals. She also has been extensively involved with the ACS throughout her career—Dr. Nelson became an ACS Fellow in 1993 and has served as former Director, ACS Clinical Research Program; former co-chair, ACS Oncology Group; and as a member, Commission on Cancer Executive Committee.
Dr. Nelson started working with the ACS in September on an initial part-time basis, overlapping with Dr. Winchester to ensure a smooth transition and continuity of leadership.
“The American College of Surgeons is excited about Dr. Nelson joining our Executive Leadership Team. Her research acumen and leadership in the cancer care community are well known and widely respected. Her addition to our team will benefit our members, our relationships with cancer care organizations, and the patients whom we serve,” said ACS Executive Director David B. Hoyt, MD, FACS.
The American College of Surgeons (ACS) recently announced that Heidi Nelson, MD, FACS, a colorectal surgeon from Rochester, MN, will be joining the ACS Division of Research and Optimal Patient Care (DROPC) as Medical Director, Cancer Programs, succeeding David P. Winchester, MD, FACS, as he transitions from the position he has served in for more than 30 years. Dr. Nelson comes to the ACS from her position as chair, and vice-chair for research, department of surgery, Mayo Clinic, as well as professor of surgery, Mayo Clinic College of Medicine and Science, Rochester. She has master’s faculty privileges in clinical and translation science at the Mayo Clinic Graduate School of Biomedical Sciences and the Mayo Clinic College of Medicine and Science.
Dr. Nelson received a bachelor’s degree from Western Washington University, Bellingham, and her medical degree from the University of Washington School of Medicine, Seattle. She completed an internship and residency in general surgery at Oregon Health & Science University, Portland, where she also served as an American Cancer Society Fellow. She then went to the Mayo Clinic College of Medicine and Science, where she was a colon and rectal surgery fellow and completed a research fellowship. Dr. Nelson returned to the University of Washington, where she was a Leo Hirsch Traveling Fellow.
Dr. Nelson has received numerous awards and held membership in many professional organizations, including the American Society of Colon and Rectal Surgeons (ASCRS), the Mayo Clinic Board of Governors, the Society of Surgical Oncology, and the Association of Women Surgeons, among others.
Research activities
As the Fred C. Andersen Professor for the Mayo Foundation and a consultant for Mayo Clinic’s division of colon and rectal surgery, Dr. Nelson is internationally renowned for her research in the field of colon and rectal cancer. The goal of her research activities has been to improve the duration and quality of life for these patients. These efforts have helped to reduce the impact of surgery on patients with early-stage disease through the safe introduction of laparoscopic and minimally invasive surgical approaches. Her work also has helped to reduce the cancer burden in patients with locally advanced and recurrent rectal cancer through studies examining the role of complex operations and intraoperative radiation therapy. Dr. Nelson’s work has been funded by the National Institutes of Health, the American Cancer Society, the ASCRS, and many other organizations. In addition to her clinical activities, she has led the Center for Individualized Medicine Microbiome Program at the Mayo Clinic, where she conducts, presents, and publishes research on the human microbiome and its connection to health and disease.
Leadership
Dr. Nelson brings a wealth of experience from leading others and establishing results-oriented teams. She has mentored trainees and investigators and has served as an editor and publisher for high-impact journals. She also has been extensively involved with the ACS throughout her career—Dr. Nelson became an ACS Fellow in 1993 and has served as former Director, ACS Clinical Research Program; former co-chair, ACS Oncology Group; and as a member, Commission on Cancer Executive Committee.
Dr. Nelson started working with the ACS in September on an initial part-time basis, overlapping with Dr. Winchester to ensure a smooth transition and continuity of leadership.
“The American College of Surgeons is excited about Dr. Nelson joining our Executive Leadership Team. Her research acumen and leadership in the cancer care community are well known and widely respected. Her addition to our team will benefit our members, our relationships with cancer care organizations, and the patients whom we serve,” said ACS Executive Director David B. Hoyt, MD, FACS.
Ronald V. Maier, MD, FACS, FRCSEd(Hon), FCSHK(Hon) installed as 2018–2019
Ronald. V. Maier, MD, FACS, FRCSEd(Hon), FCSHK(Hon), the Jane and Donald D. Trunkey Endowed Chair in Trauma Surgery; vice-chairman, department of surgery; and professor of surgery, University of Washington School of Medicine, Seattle, was installed as the 99th President of the American College of Surgeons (ACS) at Convocation, October 21, at Clinical Congress 2018 in Boston, MA.
Dr. Maier is highly esteemed for his contributions to trauma surgery, surgical research, and surgical education. In addition to his positions at the University of Washington, he is director, Northwest Regional Trauma Center; and surgeon-in-chief and co-director, surgical intensive care unit (SICU), Harborview Medical Center, Seattle. He also is associate medical staff, University of Washington Medical Center and Seattle Cancer Care Alliance. A Fellow of the College since 1984, Dr. Maier served as First Vice-President of the ACS (2015−2016) and has played an active role on several key committees, most notably the Committee on Trauma (COT).
Mark C. Weissler, MD, FACS, Past-Chair of the ACS Board of Regents (2014−2015) was installed as the First Vice-President. An otolaryngologist-head and neck surgeon, Dr. Weissler is the Joseph P. Riddle Distinguished Professor, department of otolaryngology–head and neck surgery, and chief, division of head and neck surgery, University of North Carolina (UNC) School of Medicine, Chapel Hill. An ACS Fellow since 1989, Dr. Weissler is a former ACS Regent, serving as Vice-Chair of the Board of Regents for two years (2012–2014) and Chair for one year (2014−2015). He has served on the ACS Board of Governors and in other leadership capacities for the College, including the Committee on Ethics, Central Judiciary Committee, Advisory Council for Otolaryngology−Head and Neck Surgery; and President, North Carolina Chapter of the ACS.
The Second Vice-President is Phillip R. Caropreso, MD, FACS, a general surgeon from Keokuk, IA. A committed rural surgeon, Dr. Caropreso has practiced in Mason City, IA; Keokuk, IA; and Carthage, IL. Academic positions have included serving on the teaching faculty, family practice residency, North Iowa Medical Center, Mason City; adjunct clinical professor of surgery, University of Iowa, Iowa City; and director, general surgery rotation, North Iowa Medical Center. A Fellow of the ACS since 1979, Dr. Caropreso has been active at the local and national level. He was Chair, Iowa State COT; President of the Iowa Chapter; and ACS Governor, serving on the Board of Governors Committee on Surgical Practices.
Read more about Dr. Maier, Dr. Weissler, and Dr. Caropreso in the November Bulletin at www.bulletin.facs.org.
Ronald. V. Maier, MD, FACS, FRCSEd(Hon), FCSHK(Hon), the Jane and Donald D. Trunkey Endowed Chair in Trauma Surgery; vice-chairman, department of surgery; and professor of surgery, University of Washington School of Medicine, Seattle, was installed as the 99th President of the American College of Surgeons (ACS) at Convocation, October 21, at Clinical Congress 2018 in Boston, MA.
Dr. Maier is highly esteemed for his contributions to trauma surgery, surgical research, and surgical education. In addition to his positions at the University of Washington, he is director, Northwest Regional Trauma Center; and surgeon-in-chief and co-director, surgical intensive care unit (SICU), Harborview Medical Center, Seattle. He also is associate medical staff, University of Washington Medical Center and Seattle Cancer Care Alliance. A Fellow of the College since 1984, Dr. Maier served as First Vice-President of the ACS (2015−2016) and has played an active role on several key committees, most notably the Committee on Trauma (COT).
Mark C. Weissler, MD, FACS, Past-Chair of the ACS Board of Regents (2014−2015) was installed as the First Vice-President. An otolaryngologist-head and neck surgeon, Dr. Weissler is the Joseph P. Riddle Distinguished Professor, department of otolaryngology–head and neck surgery, and chief, division of head and neck surgery, University of North Carolina (UNC) School of Medicine, Chapel Hill. An ACS Fellow since 1989, Dr. Weissler is a former ACS Regent, serving as Vice-Chair of the Board of Regents for two years (2012–2014) and Chair for one year (2014−2015). He has served on the ACS Board of Governors and in other leadership capacities for the College, including the Committee on Ethics, Central Judiciary Committee, Advisory Council for Otolaryngology−Head and Neck Surgery; and President, North Carolina Chapter of the ACS.
The Second Vice-President is Phillip R. Caropreso, MD, FACS, a general surgeon from Keokuk, IA. A committed rural surgeon, Dr. Caropreso has practiced in Mason City, IA; Keokuk, IA; and Carthage, IL. Academic positions have included serving on the teaching faculty, family practice residency, North Iowa Medical Center, Mason City; adjunct clinical professor of surgery, University of Iowa, Iowa City; and director, general surgery rotation, North Iowa Medical Center. A Fellow of the ACS since 1979, Dr. Caropreso has been active at the local and national level. He was Chair, Iowa State COT; President of the Iowa Chapter; and ACS Governor, serving on the Board of Governors Committee on Surgical Practices.
Read more about Dr. Maier, Dr. Weissler, and Dr. Caropreso in the November Bulletin at www.bulletin.facs.org.
Ronald. V. Maier, MD, FACS, FRCSEd(Hon), FCSHK(Hon), the Jane and Donald D. Trunkey Endowed Chair in Trauma Surgery; vice-chairman, department of surgery; and professor of surgery, University of Washington School of Medicine, Seattle, was installed as the 99th President of the American College of Surgeons (ACS) at Convocation, October 21, at Clinical Congress 2018 in Boston, MA.
Dr. Maier is highly esteemed for his contributions to trauma surgery, surgical research, and surgical education. In addition to his positions at the University of Washington, he is director, Northwest Regional Trauma Center; and surgeon-in-chief and co-director, surgical intensive care unit (SICU), Harborview Medical Center, Seattle. He also is associate medical staff, University of Washington Medical Center and Seattle Cancer Care Alliance. A Fellow of the College since 1984, Dr. Maier served as First Vice-President of the ACS (2015−2016) and has played an active role on several key committees, most notably the Committee on Trauma (COT).
Mark C. Weissler, MD, FACS, Past-Chair of the ACS Board of Regents (2014−2015) was installed as the First Vice-President. An otolaryngologist-head and neck surgeon, Dr. Weissler is the Joseph P. Riddle Distinguished Professor, department of otolaryngology–head and neck surgery, and chief, division of head and neck surgery, University of North Carolina (UNC) School of Medicine, Chapel Hill. An ACS Fellow since 1989, Dr. Weissler is a former ACS Regent, serving as Vice-Chair of the Board of Regents for two years (2012–2014) and Chair for one year (2014−2015). He has served on the ACS Board of Governors and in other leadership capacities for the College, including the Committee on Ethics, Central Judiciary Committee, Advisory Council for Otolaryngology−Head and Neck Surgery; and President, North Carolina Chapter of the ACS.
The Second Vice-President is Phillip R. Caropreso, MD, FACS, a general surgeon from Keokuk, IA. A committed rural surgeon, Dr. Caropreso has practiced in Mason City, IA; Keokuk, IA; and Carthage, IL. Academic positions have included serving on the teaching faculty, family practice residency, North Iowa Medical Center, Mason City; adjunct clinical professor of surgery, University of Iowa, Iowa City; and director, general surgery rotation, North Iowa Medical Center. A Fellow of the ACS since 1979, Dr. Caropreso has been active at the local and national level. He was Chair, Iowa State COT; President of the Iowa Chapter; and ACS Governor, serving on the Board of Governors Committee on Surgical Practices.
Read more about Dr. Maier, Dr. Weissler, and Dr. Caropreso in the November Bulletin at www.bulletin.facs.org.
Investigation Into New Antimalarial Drug Begins
In 2016, a staggering 216 million people developed malaria, and 445,000 died—primarily children in sub-Saharan Africa. But a new first-in-human study sponsored by the National Institute of Allergy and Infectious Disease may help reduce the numbers of future victims.
Enrollment has begun in a phase I trial to test the safety of DM1157, an investigational modified form of chloroquine. Many strains of Plasmodium falciparum are now resistant to chloroquine. In fact, the parasites can expel the drug before it can affect them. Like chloroquine, DM1157 interferes with the parasite’s metabolism, but it also inhibits the parasite’s ability to expel the drug.
The study will enroll up to 104 healthy volunteers aged 18-45 years. One group will fast overnight and then receive either a single dose of the experimental drug at 1 of 7 dosage levels or a placebo. A second group also will fast and receive 1 dose at 1 of 4 dosage levels and repeat the routine for 2 more consecutive days. A third group will be given either a single 300-mg dose or placebo after eating a high-fat meal.
The study is expected to be completed by June 2019.
In 2016, a staggering 216 million people developed malaria, and 445,000 died—primarily children in sub-Saharan Africa. But a new first-in-human study sponsored by the National Institute of Allergy and Infectious Disease may help reduce the numbers of future victims.
Enrollment has begun in a phase I trial to test the safety of DM1157, an investigational modified form of chloroquine. Many strains of Plasmodium falciparum are now resistant to chloroquine. In fact, the parasites can expel the drug before it can affect them. Like chloroquine, DM1157 interferes with the parasite’s metabolism, but it also inhibits the parasite’s ability to expel the drug.
The study will enroll up to 104 healthy volunteers aged 18-45 years. One group will fast overnight and then receive either a single dose of the experimental drug at 1 of 7 dosage levels or a placebo. A second group also will fast and receive 1 dose at 1 of 4 dosage levels and repeat the routine for 2 more consecutive days. A third group will be given either a single 300-mg dose or placebo after eating a high-fat meal.
The study is expected to be completed by June 2019.
In 2016, a staggering 216 million people developed malaria, and 445,000 died—primarily children in sub-Saharan Africa. But a new first-in-human study sponsored by the National Institute of Allergy and Infectious Disease may help reduce the numbers of future victims.
Enrollment has begun in a phase I trial to test the safety of DM1157, an investigational modified form of chloroquine. Many strains of Plasmodium falciparum are now resistant to chloroquine. In fact, the parasites can expel the drug before it can affect them. Like chloroquine, DM1157 interferes with the parasite’s metabolism, but it also inhibits the parasite’s ability to expel the drug.
The study will enroll up to 104 healthy volunteers aged 18-45 years. One group will fast overnight and then receive either a single dose of the experimental drug at 1 of 7 dosage levels or a placebo. A second group also will fast and receive 1 dose at 1 of 4 dosage levels and repeat the routine for 2 more consecutive days. A third group will be given either a single 300-mg dose or placebo after eating a high-fat meal.
The study is expected to be completed by June 2019.
Robin Cooper: Climate Change
In this edition of the Psychcast, Lorenzo Norris, MD, welcomes Robin Cooper, MD, to discuss the impacts of global climate change on both patients and communities.
Dr. Cooper “has been in private practice with a focus on both psychotherapy and medical management throughout her 35 years of practice.”
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
In this edition of the Psychcast, Lorenzo Norris, MD, welcomes Robin Cooper, MD, to discuss the impacts of global climate change on both patients and communities.
Dr. Cooper “has been in private practice with a focus on both psychotherapy and medical management throughout her 35 years of practice.”
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
In this edition of the Psychcast, Lorenzo Norris, MD, welcomes Robin Cooper, MD, to discuss the impacts of global climate change on both patients and communities.
Dr. Cooper “has been in private practice with a focus on both psychotherapy and medical management throughout her 35 years of practice.”
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Vitamin D, fish out, and primary prevention
Also today, atopic dermatitis can harm both mental health and quality of life, apixaban is the safest effective DOAC for stroke prevention in atrial fibrillation, and a program that is aimed to increase awareness by reduced fetal movement is not effective at preventing stillbirths.
Amazon Alexa
Apple Podcasts
Spotify
Also today, atopic dermatitis can harm both mental health and quality of life, apixaban is the safest effective DOAC for stroke prevention in atrial fibrillation, and a program that is aimed to increase awareness by reduced fetal movement is not effective at preventing stillbirths.
Amazon Alexa
Apple Podcasts
Spotify
Also today, atopic dermatitis can harm both mental health and quality of life, apixaban is the safest effective DOAC for stroke prevention in atrial fibrillation, and a program that is aimed to increase awareness by reduced fetal movement is not effective at preventing stillbirths.
Amazon Alexa
Apple Podcasts
Spotify
Markers associated with efficacy of malaria vaccine
New research has revealed markers associated with efficacy of the RTS,S/AS01E malaria vaccine (Mosquirix™).
The study suggests malaria protection depends on the amount and subclass of antibodies generated upon vaccination and on previous exposure levels to the malaria parasite.
Researchers believe these findings, published in BMC Medicine, could aid the development of more effective vaccines and guide efforts to improve the effectiveness of RTS,S.
The RTS,S vaccine demonstrated partial effectiveness in a phase 3 study—31% in infants ages 6 weeks to 12 weeks and 56% in children ages 5 months to 17 months.
Carlota Dobaño Lázaro, PhD, of ISGlobal in Barcelona, Spain, and her colleagues have been working to understand the reasons for this variability and identify vaccine protection correlates.
The team used a quantitative assay to investigate the levels and types of antibodies induced by RTS,S. In particular, they measured total IgM, IgG, and IgG1–4 subclass antibodies to hepatitis B surface antigen (HBsAg) and three constructs of the Plasmodium falciparum circumsporozoite protein (CSP).
The researchers analyzed serum and plasma from 195 infants and children from Kintampo, Ghana (an area with high malaria transmission) and Manhiça, Mozambique (low malaria transmission), who were vaccinated during the phase 3 trial for RTS,S.
The results confirmed that RTS,S induces significant levels of IgG antibodies against both CSP and HBsAg, which are higher in children than in infants.
The researchers found that higher HBsAg antibody levels post-vaccination were associated with protection from malaria.
However, the same could not be said for all subclasses of CSP antibodies. Higher levels of IgG1 and IgG3 antibodies were associated with protection, while higher levels of IgG2 and IgG4 were associated with a greater risk of developing malaria.
“The balance between the different subclasses seems to be more important than the total IgG levels,” said study author Itzi Ubillos Escriche, of ISGlobal.
“This could be because IgG1 and IgG3 antibodies have the capacity to stick to the parasite and give an ‘eat-me’ signal to cells of the immune system.”
The results also showed that subjects with higher pre-vaccine levels of anti-P falciparum and anti-CSP antibodies were less protected against malaria post-vaccination.
“This means that the vaccine will exert a larger benefit to infants who have not been exposed to the parasite in utero or during the first weeks of life,” Dr. Dobaño Lázaro said.
“This study . . . identifies new correlates of vaccine success and failure in African children and provides a basis for designing more efficacious vaccines.”
This research was funded by the National Institutes of Health-National Institute of Allergy and Infectious Diseases, PATH Malaria Vaccine Initiative, Ministerio de Economía y Competitividad, and EVIMalaR and AGAUR-Catalonia. ISGlobal is a member of the CERCA Program, Generalitat de Catalunya.
The authors said they have no competing interests.
The phase 3 trial of RTS,S was supported by GlaxoSmithKline Biologicals SA and the PATH Malaria Vaccine Initiative.
New research has revealed markers associated with efficacy of the RTS,S/AS01E malaria vaccine (Mosquirix™).
The study suggests malaria protection depends on the amount and subclass of antibodies generated upon vaccination and on previous exposure levels to the malaria parasite.
Researchers believe these findings, published in BMC Medicine, could aid the development of more effective vaccines and guide efforts to improve the effectiveness of RTS,S.
The RTS,S vaccine demonstrated partial effectiveness in a phase 3 study—31% in infants ages 6 weeks to 12 weeks and 56% in children ages 5 months to 17 months.
Carlota Dobaño Lázaro, PhD, of ISGlobal in Barcelona, Spain, and her colleagues have been working to understand the reasons for this variability and identify vaccine protection correlates.
The team used a quantitative assay to investigate the levels and types of antibodies induced by RTS,S. In particular, they measured total IgM, IgG, and IgG1–4 subclass antibodies to hepatitis B surface antigen (HBsAg) and three constructs of the Plasmodium falciparum circumsporozoite protein (CSP).
The researchers analyzed serum and plasma from 195 infants and children from Kintampo, Ghana (an area with high malaria transmission) and Manhiça, Mozambique (low malaria transmission), who were vaccinated during the phase 3 trial for RTS,S.
The results confirmed that RTS,S induces significant levels of IgG antibodies against both CSP and HBsAg, which are higher in children than in infants.
The researchers found that higher HBsAg antibody levels post-vaccination were associated with protection from malaria.
However, the same could not be said for all subclasses of CSP antibodies. Higher levels of IgG1 and IgG3 antibodies were associated with protection, while higher levels of IgG2 and IgG4 were associated with a greater risk of developing malaria.
“The balance between the different subclasses seems to be more important than the total IgG levels,” said study author Itzi Ubillos Escriche, of ISGlobal.
“This could be because IgG1 and IgG3 antibodies have the capacity to stick to the parasite and give an ‘eat-me’ signal to cells of the immune system.”
The results also showed that subjects with higher pre-vaccine levels of anti-P falciparum and anti-CSP antibodies were less protected against malaria post-vaccination.
“This means that the vaccine will exert a larger benefit to infants who have not been exposed to the parasite in utero or during the first weeks of life,” Dr. Dobaño Lázaro said.
“This study . . . identifies new correlates of vaccine success and failure in African children and provides a basis for designing more efficacious vaccines.”
This research was funded by the National Institutes of Health-National Institute of Allergy and Infectious Diseases, PATH Malaria Vaccine Initiative, Ministerio de Economía y Competitividad, and EVIMalaR and AGAUR-Catalonia. ISGlobal is a member of the CERCA Program, Generalitat de Catalunya.
The authors said they have no competing interests.
The phase 3 trial of RTS,S was supported by GlaxoSmithKline Biologicals SA and the PATH Malaria Vaccine Initiative.
New research has revealed markers associated with efficacy of the RTS,S/AS01E malaria vaccine (Mosquirix™).
The study suggests malaria protection depends on the amount and subclass of antibodies generated upon vaccination and on previous exposure levels to the malaria parasite.
Researchers believe these findings, published in BMC Medicine, could aid the development of more effective vaccines and guide efforts to improve the effectiveness of RTS,S.
The RTS,S vaccine demonstrated partial effectiveness in a phase 3 study—31% in infants ages 6 weeks to 12 weeks and 56% in children ages 5 months to 17 months.
Carlota Dobaño Lázaro, PhD, of ISGlobal in Barcelona, Spain, and her colleagues have been working to understand the reasons for this variability and identify vaccine protection correlates.
The team used a quantitative assay to investigate the levels and types of antibodies induced by RTS,S. In particular, they measured total IgM, IgG, and IgG1–4 subclass antibodies to hepatitis B surface antigen (HBsAg) and three constructs of the Plasmodium falciparum circumsporozoite protein (CSP).
The researchers analyzed serum and plasma from 195 infants and children from Kintampo, Ghana (an area with high malaria transmission) and Manhiça, Mozambique (low malaria transmission), who were vaccinated during the phase 3 trial for RTS,S.
The results confirmed that RTS,S induces significant levels of IgG antibodies against both CSP and HBsAg, which are higher in children than in infants.
The researchers found that higher HBsAg antibody levels post-vaccination were associated with protection from malaria.
However, the same could not be said for all subclasses of CSP antibodies. Higher levels of IgG1 and IgG3 antibodies were associated with protection, while higher levels of IgG2 and IgG4 were associated with a greater risk of developing malaria.
“The balance between the different subclasses seems to be more important than the total IgG levels,” said study author Itzi Ubillos Escriche, of ISGlobal.
“This could be because IgG1 and IgG3 antibodies have the capacity to stick to the parasite and give an ‘eat-me’ signal to cells of the immune system.”
The results also showed that subjects with higher pre-vaccine levels of anti-P falciparum and anti-CSP antibodies were less protected against malaria post-vaccination.
“This means that the vaccine will exert a larger benefit to infants who have not been exposed to the parasite in utero or during the first weeks of life,” Dr. Dobaño Lázaro said.
“This study . . . identifies new correlates of vaccine success and failure in African children and provides a basis for designing more efficacious vaccines.”
This research was funded by the National Institutes of Health-National Institute of Allergy and Infectious Diseases, PATH Malaria Vaccine Initiative, Ministerio de Economía y Competitividad, and EVIMalaR and AGAUR-Catalonia. ISGlobal is a member of the CERCA Program, Generalitat de Catalunya.
The authors said they have no competing interests.
The phase 3 trial of RTS,S was supported by GlaxoSmithKline Biologicals SA and the PATH Malaria Vaccine Initiative.
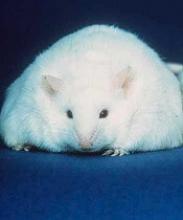
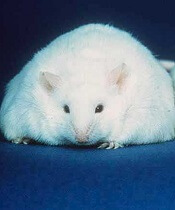
Diet change can improve survival in obese mice with ALL
Switching to a low-fat diet can improve survival in obese mice with acute lymphoblastic leukemia (ALL), according to new research.
Diet-induced obese (DIO) mice with ALL had a survival rate of 17% if they remained on a high-fat diet while treated with vincristine, but survival rose to 92% for mice that were switched to a low-fat diet before treatment.
However, the dietary switch did not impact the survival of DIO mice treated with dexamethasone or L-asparaginase monotherapy.
Researchers reported these findings in Cancer & Metabolism.
“The most exciting thing, to me, about this study is the fact that this shows that a dietary intervention could potentially help us kill leukemia cells in children with acute lymphoblastic leukemia,” said study author Steven Mittelman, MD, PhD, of the University of Southern California, Los Angeles.
“The current treatments for leukemia are very toxic, so finding a way to use a healthy diet, without increasing the toxicity of therapy to treat people with cancer, would be incredible.”
Building on previous research that showed obesity reduced the effectiveness of chemotherapeutic drugs in children with leukemia, the researchers tested whether a dietary intervention could improve ALL outcomes in obese mice.
Methods
The team used NOD/SCID IL2-receptor gamma chain knockout mice raised on either a 60% or 10% fat-calorie diet. Only male mice were studied because female mice do not become as significantly obese on the high-fat diet.
The researchers implanted GFP+ pre-B-cell ALL transgenic mouse cells into DIO and control mice at about 20 weeks old.
Six to seven days after ALL implantation, the researchers randomized the DIO mice to continue their high-fat diet or switch to the control diet (10% calories from fat).
In some experiments, mice received monotherapy with vincristine, and other experiments used L-asparaginase or dexamethasone.
In additional experiments, the researchers implanted DIO and control mice with patient-derived ALL cells with normal karyotype.
After 17 days of engraftment, the researchers switched half the DIO mice to the control diet. The next day, they treated these mice with vincristine, L-asparaginase, or dexamethasone for 4 weeks.
The team monitored the mice daily for food intake, body weight, and onset of progressive leukemia.
Results
Diet had no impact on DIO or control mice that did not receive chemotherapy. Time to progression was the same in these mice.
When vincristine was started on day 7 after ALL implantation, DIO mice switched to the low-fat diet had the best survival, even better than the control mice.
Overall survival was 92% for the DIO mice that switched diets, 42% for the control group, and 17% for the DIO group maintained on the high-fat diet.
Survival experiments performed using L-asparaginase or dexamethasone monotherapy showed no impact of switching diets. The researchers reported there was “no detectable effect on survival in these experiments.”
The team believes this is the first study to test a diet intervention on treatment outcome in hematologic malignancy.
The researchers plan to study dietary intervention further in both obese and non-obese patients.
This study was supported by the National Cancer Institute and funds from the Saban Research Institute, Children’s Hospital of Los Angeles. The researchers had no competing interests to declare.
Switching to a low-fat diet can improve survival in obese mice with acute lymphoblastic leukemia (ALL), according to new research.
Diet-induced obese (DIO) mice with ALL had a survival rate of 17% if they remained on a high-fat diet while treated with vincristine, but survival rose to 92% for mice that were switched to a low-fat diet before treatment.
However, the dietary switch did not impact the survival of DIO mice treated with dexamethasone or L-asparaginase monotherapy.
Researchers reported these findings in Cancer & Metabolism.
“The most exciting thing, to me, about this study is the fact that this shows that a dietary intervention could potentially help us kill leukemia cells in children with acute lymphoblastic leukemia,” said study author Steven Mittelman, MD, PhD, of the University of Southern California, Los Angeles.
“The current treatments for leukemia are very toxic, so finding a way to use a healthy diet, without increasing the toxicity of therapy to treat people with cancer, would be incredible.”
Building on previous research that showed obesity reduced the effectiveness of chemotherapeutic drugs in children with leukemia, the researchers tested whether a dietary intervention could improve ALL outcomes in obese mice.
Methods
The team used NOD/SCID IL2-receptor gamma chain knockout mice raised on either a 60% or 10% fat-calorie diet. Only male mice were studied because female mice do not become as significantly obese on the high-fat diet.
The researchers implanted GFP+ pre-B-cell ALL transgenic mouse cells into DIO and control mice at about 20 weeks old.
Six to seven days after ALL implantation, the researchers randomized the DIO mice to continue their high-fat diet or switch to the control diet (10% calories from fat).
In some experiments, mice received monotherapy with vincristine, and other experiments used L-asparaginase or dexamethasone.
In additional experiments, the researchers implanted DIO and control mice with patient-derived ALL cells with normal karyotype.
After 17 days of engraftment, the researchers switched half the DIO mice to the control diet. The next day, they treated these mice with vincristine, L-asparaginase, or dexamethasone for 4 weeks.
The team monitored the mice daily for food intake, body weight, and onset of progressive leukemia.
Results
Diet had no impact on DIO or control mice that did not receive chemotherapy. Time to progression was the same in these mice.
When vincristine was started on day 7 after ALL implantation, DIO mice switched to the low-fat diet had the best survival, even better than the control mice.
Overall survival was 92% for the DIO mice that switched diets, 42% for the control group, and 17% for the DIO group maintained on the high-fat diet.
Survival experiments performed using L-asparaginase or dexamethasone monotherapy showed no impact of switching diets. The researchers reported there was “no detectable effect on survival in these experiments.”
The team believes this is the first study to test a diet intervention on treatment outcome in hematologic malignancy.
The researchers plan to study dietary intervention further in both obese and non-obese patients.
This study was supported by the National Cancer Institute and funds from the Saban Research Institute, Children’s Hospital of Los Angeles. The researchers had no competing interests to declare.
Switching to a low-fat diet can improve survival in obese mice with acute lymphoblastic leukemia (ALL), according to new research.
Diet-induced obese (DIO) mice with ALL had a survival rate of 17% if they remained on a high-fat diet while treated with vincristine, but survival rose to 92% for mice that were switched to a low-fat diet before treatment.
However, the dietary switch did not impact the survival of DIO mice treated with dexamethasone or L-asparaginase monotherapy.
Researchers reported these findings in Cancer & Metabolism.
“The most exciting thing, to me, about this study is the fact that this shows that a dietary intervention could potentially help us kill leukemia cells in children with acute lymphoblastic leukemia,” said study author Steven Mittelman, MD, PhD, of the University of Southern California, Los Angeles.
“The current treatments for leukemia are very toxic, so finding a way to use a healthy diet, without increasing the toxicity of therapy to treat people with cancer, would be incredible.”
Building on previous research that showed obesity reduced the effectiveness of chemotherapeutic drugs in children with leukemia, the researchers tested whether a dietary intervention could improve ALL outcomes in obese mice.
Methods
The team used NOD/SCID IL2-receptor gamma chain knockout mice raised on either a 60% or 10% fat-calorie diet. Only male mice were studied because female mice do not become as significantly obese on the high-fat diet.
The researchers implanted GFP+ pre-B-cell ALL transgenic mouse cells into DIO and control mice at about 20 weeks old.
Six to seven days after ALL implantation, the researchers randomized the DIO mice to continue their high-fat diet or switch to the control diet (10% calories from fat).
In some experiments, mice received monotherapy with vincristine, and other experiments used L-asparaginase or dexamethasone.
In additional experiments, the researchers implanted DIO and control mice with patient-derived ALL cells with normal karyotype.
After 17 days of engraftment, the researchers switched half the DIO mice to the control diet. The next day, they treated these mice with vincristine, L-asparaginase, or dexamethasone for 4 weeks.
The team monitored the mice daily for food intake, body weight, and onset of progressive leukemia.
Results
Diet had no impact on DIO or control mice that did not receive chemotherapy. Time to progression was the same in these mice.
When vincristine was started on day 7 after ALL implantation, DIO mice switched to the low-fat diet had the best survival, even better than the control mice.
Overall survival was 92% for the DIO mice that switched diets, 42% for the control group, and 17% for the DIO group maintained on the high-fat diet.
Survival experiments performed using L-asparaginase or dexamethasone monotherapy showed no impact of switching diets. The researchers reported there was “no detectable effect on survival in these experiments.”
The team believes this is the first study to test a diet intervention on treatment outcome in hematologic malignancy.
The researchers plan to study dietary intervention further in both obese and non-obese patients.
This study was supported by the National Cancer Institute and funds from the Saban Research Institute, Children’s Hospital of Los Angeles. The researchers had no competing interests to declare.
ADAR1 linked to MM pathogenesis, outcomes
Overly zealous editing of messenger RNA in multiple myeloma (MM) cells contributes to MM pathogenesis and is associated with poor outcomes after certain treatments, investigators contend.
The team found evidence to suggest that overexpression of the RNA editing enzyme ADAR1 leads to hyperediting of the MM transcriptome that appears related to a drug-resistant disease phenotype and worse prognosis.
Phaik Ju Teoh, PhD, of the Cancer Science Institute of Singapore, and colleagues reported these findings in Blood.
The investigators implicate aberrant editing of adenosine (A) to inosine (I) in malignant plasma cells and its effects on NEIL1, a gene that encodes proteins involved in base-excision repair of DNA, as important mechanisms in MM pathogenesis.
A to I editing is the most prevalent form of RNA editing in humans, and aberrant editing mediated by ADAR1 has recently been linked to the development of several cancer types, the investigators noted.
To see whether this process may also be involved in MM, the investigators examined whole blood or bone marrow samples from healthy volunteers and MM patients.
The team found that ADAR1 was overexpressed in MM cells compared to nonmalignant plasma cells.
Furthermore, ADAR1 was expressed at higher levels in patients with newly diagnosed or relapsed MM compared to patients who had smoldering myeloma or monoclonal gammopathy of undetermined significance.
Response to treatment
The investigators also assessed ADAR1 expression in relation to MM patients’ responsiveness to treatment using data from the CoMMpass study.
The team found that patients with poor responses (stable or progressive disease) to bortezomib-based and immunomodulatory-based therapies had high ADAR1 mRNA.
There was no correlation between ADAR1 and responsiveness to carfilzomib-based treatments, but the investigators said this may be because of the relatively lower number of patients who received carfilzomib in this study.
The investigators also found that bortezomib was more effective in inhibiting the growth of MM cells with low ADAR1, and bortezomib-treated cells showed downregulation of ADAR1 expression in a dose- and time-dependent manner.
ADAR1-mediated editing
The investigators determined that ADAR1 directly regulates hyperediting of the MM transcriptome. This was evidenced by a significant increase in A to guanosine (G) editing in newly diagnosed and relapsed MM samples, compared with normal plasma cells.
The team confirmed this finding by observing the effects of ADAR1 levels on editing events across the transcriptome.
The investigators followed this observation with experiments to see whether ADAR1-mediated editing contributes to oncogenesis in MM cells. The MM growth rate slowed when the team silenced ADAR1, and introducing wild-type ADAR1 into cells promoted growth and proliferation.
The investigators also identified NEIL1 as an important ADAR1 editing target in MM. The editing compromised NEIL1’s ability to accurately repair DNA damage.
This study was supported by the National Research Foundation Singapore, the Singapore Ministry of Education, and the National University of Singapore. The investigators reported no competing financial interests.
Overly zealous editing of messenger RNA in multiple myeloma (MM) cells contributes to MM pathogenesis and is associated with poor outcomes after certain treatments, investigators contend.
The team found evidence to suggest that overexpression of the RNA editing enzyme ADAR1 leads to hyperediting of the MM transcriptome that appears related to a drug-resistant disease phenotype and worse prognosis.
Phaik Ju Teoh, PhD, of the Cancer Science Institute of Singapore, and colleagues reported these findings in Blood.
The investigators implicate aberrant editing of adenosine (A) to inosine (I) in malignant plasma cells and its effects on NEIL1, a gene that encodes proteins involved in base-excision repair of DNA, as important mechanisms in MM pathogenesis.
A to I editing is the most prevalent form of RNA editing in humans, and aberrant editing mediated by ADAR1 has recently been linked to the development of several cancer types, the investigators noted.
To see whether this process may also be involved in MM, the investigators examined whole blood or bone marrow samples from healthy volunteers and MM patients.
The team found that ADAR1 was overexpressed in MM cells compared to nonmalignant plasma cells.
Furthermore, ADAR1 was expressed at higher levels in patients with newly diagnosed or relapsed MM compared to patients who had smoldering myeloma or monoclonal gammopathy of undetermined significance.
Response to treatment
The investigators also assessed ADAR1 expression in relation to MM patients’ responsiveness to treatment using data from the CoMMpass study.
The team found that patients with poor responses (stable or progressive disease) to bortezomib-based and immunomodulatory-based therapies had high ADAR1 mRNA.
There was no correlation between ADAR1 and responsiveness to carfilzomib-based treatments, but the investigators said this may be because of the relatively lower number of patients who received carfilzomib in this study.
The investigators also found that bortezomib was more effective in inhibiting the growth of MM cells with low ADAR1, and bortezomib-treated cells showed downregulation of ADAR1 expression in a dose- and time-dependent manner.
ADAR1-mediated editing
The investigators determined that ADAR1 directly regulates hyperediting of the MM transcriptome. This was evidenced by a significant increase in A to guanosine (G) editing in newly diagnosed and relapsed MM samples, compared with normal plasma cells.
The team confirmed this finding by observing the effects of ADAR1 levels on editing events across the transcriptome.
The investigators followed this observation with experiments to see whether ADAR1-mediated editing contributes to oncogenesis in MM cells. The MM growth rate slowed when the team silenced ADAR1, and introducing wild-type ADAR1 into cells promoted growth and proliferation.
The investigators also identified NEIL1 as an important ADAR1 editing target in MM. The editing compromised NEIL1’s ability to accurately repair DNA damage.
This study was supported by the National Research Foundation Singapore, the Singapore Ministry of Education, and the National University of Singapore. The investigators reported no competing financial interests.
Overly zealous editing of messenger RNA in multiple myeloma (MM) cells contributes to MM pathogenesis and is associated with poor outcomes after certain treatments, investigators contend.
The team found evidence to suggest that overexpression of the RNA editing enzyme ADAR1 leads to hyperediting of the MM transcriptome that appears related to a drug-resistant disease phenotype and worse prognosis.
Phaik Ju Teoh, PhD, of the Cancer Science Institute of Singapore, and colleagues reported these findings in Blood.
The investigators implicate aberrant editing of adenosine (A) to inosine (I) in malignant plasma cells and its effects on NEIL1, a gene that encodes proteins involved in base-excision repair of DNA, as important mechanisms in MM pathogenesis.
A to I editing is the most prevalent form of RNA editing in humans, and aberrant editing mediated by ADAR1 has recently been linked to the development of several cancer types, the investigators noted.
To see whether this process may also be involved in MM, the investigators examined whole blood or bone marrow samples from healthy volunteers and MM patients.
The team found that ADAR1 was overexpressed in MM cells compared to nonmalignant plasma cells.
Furthermore, ADAR1 was expressed at higher levels in patients with newly diagnosed or relapsed MM compared to patients who had smoldering myeloma or monoclonal gammopathy of undetermined significance.
Response to treatment
The investigators also assessed ADAR1 expression in relation to MM patients’ responsiveness to treatment using data from the CoMMpass study.
The team found that patients with poor responses (stable or progressive disease) to bortezomib-based and immunomodulatory-based therapies had high ADAR1 mRNA.
There was no correlation between ADAR1 and responsiveness to carfilzomib-based treatments, but the investigators said this may be because of the relatively lower number of patients who received carfilzomib in this study.
The investigators also found that bortezomib was more effective in inhibiting the growth of MM cells with low ADAR1, and bortezomib-treated cells showed downregulation of ADAR1 expression in a dose- and time-dependent manner.
ADAR1-mediated editing
The investigators determined that ADAR1 directly regulates hyperediting of the MM transcriptome. This was evidenced by a significant increase in A to guanosine (G) editing in newly diagnosed and relapsed MM samples, compared with normal plasma cells.
The team confirmed this finding by observing the effects of ADAR1 levels on editing events across the transcriptome.
The investigators followed this observation with experiments to see whether ADAR1-mediated editing contributes to oncogenesis in MM cells. The MM growth rate slowed when the team silenced ADAR1, and introducing wild-type ADAR1 into cells promoted growth and proliferation.
The investigators also identified NEIL1 as an important ADAR1 editing target in MM. The editing compromised NEIL1’s ability to accurately repair DNA damage.
This study was supported by the National Research Foundation Singapore, the Singapore Ministry of Education, and the National University of Singapore. The investigators reported no competing financial interests.