User login
ICYMI: Prednisone lowers IRIS risk in patients with HIV
In patients with HIV, treatment with prednisone for 4 weeks after antiretroviral therapy initiation significantly reduced the risk of tuberculosis-associated immune reconstitution inflammatory syndrome (IRIS). The results from the randomized, double-blind, placebo-controlled trial were published in the New England Journal of Medicine (2018 Nov 14. doi: 10.1056/NEJMoa1800762).
A total of 240 patients were enrolled in the study, with a median age of 36 years; 60% were men, and 73% had microbiologically confirmed tuberculosis. The median CD4 count of the patients was 49 cells/mcL and the median HIV type 1 RNA viral load was 5.5 log10 copies/mL. A total of 120 patients were assigned to each group, with 18 patients lost to follow-up or withdrawn. Tuberculosis-associated IRIS was diagnosed in 39 patients (32.5%) in the prednisone group and in 56 (46.7%) in the placebo group, yielding a relative IRIS risk of 0.70 (95% confidence interval, 0.51-0.96; P = .03), according to the researchers for the PredART (Preventing TB-IRIS in High-Risk Patients: a Randomized Placebo-Controlled Trial of Prednisone) study team.
We covered this story before it was published in the journal. Find our coverage from the Conference on Retroviruses & Opportunistic Infections at the link below.
In patients with HIV, treatment with prednisone for 4 weeks after antiretroviral therapy initiation significantly reduced the risk of tuberculosis-associated immune reconstitution inflammatory syndrome (IRIS). The results from the randomized, double-blind, placebo-controlled trial were published in the New England Journal of Medicine (2018 Nov 14. doi: 10.1056/NEJMoa1800762).
A total of 240 patients were enrolled in the study, with a median age of 36 years; 60% were men, and 73% had microbiologically confirmed tuberculosis. The median CD4 count of the patients was 49 cells/mcL and the median HIV type 1 RNA viral load was 5.5 log10 copies/mL. A total of 120 patients were assigned to each group, with 18 patients lost to follow-up or withdrawn. Tuberculosis-associated IRIS was diagnosed in 39 patients (32.5%) in the prednisone group and in 56 (46.7%) in the placebo group, yielding a relative IRIS risk of 0.70 (95% confidence interval, 0.51-0.96; P = .03), according to the researchers for the PredART (Preventing TB-IRIS in High-Risk Patients: a Randomized Placebo-Controlled Trial of Prednisone) study team.
We covered this story before it was published in the journal. Find our coverage from the Conference on Retroviruses & Opportunistic Infections at the link below.
In patients with HIV, treatment with prednisone for 4 weeks after antiretroviral therapy initiation significantly reduced the risk of tuberculosis-associated immune reconstitution inflammatory syndrome (IRIS). The results from the randomized, double-blind, placebo-controlled trial were published in the New England Journal of Medicine (2018 Nov 14. doi: 10.1056/NEJMoa1800762).
A total of 240 patients were enrolled in the study, with a median age of 36 years; 60% were men, and 73% had microbiologically confirmed tuberculosis. The median CD4 count of the patients was 49 cells/mcL and the median HIV type 1 RNA viral load was 5.5 log10 copies/mL. A total of 120 patients were assigned to each group, with 18 patients lost to follow-up or withdrawn. Tuberculosis-associated IRIS was diagnosed in 39 patients (32.5%) in the prednisone group and in 56 (46.7%) in the placebo group, yielding a relative IRIS risk of 0.70 (95% confidence interval, 0.51-0.96; P = .03), according to the researchers for the PredART (Preventing TB-IRIS in High-Risk Patients: a Randomized Placebo-Controlled Trial of Prednisone) study team.
We covered this story before it was published in the journal. Find our coverage from the Conference on Retroviruses & Opportunistic Infections at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Ganglion stimulation boosts cerebral blood flow, improves stroke outcomes
MONTREAL – Stimulation of the sphenopalatine ganglion (SPG) using a small, implanted electrode for 5 days in patients who had just had an acute ischemic stroke led to statistically significant and clinically meaningful improvements in the subset of patients with confirmed cortical involvement in a pivotal, sham-controlled trial.
SPG stimulation started within 24 hours of an acute ischemic stroke “reduced poststroke disability over the entire outcome range and increased the proportion of patients who were alive and independent 3 months after their stroke” in the subgroup with a confirmed cortical infarction (CCI), Jeffrey L. Saver, MD, said at the World Stroke Congress. Five days of SPG stimulation, done once daily starting within 24 hours of stroke onset, “enhances ipsilateral collateral blood flow” and may also stabilize the blood brain barrier, explained Dr. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles. The study included a prespecified primary endpoint analysis that focused exclusively on the CCI subgroup, 52% of the total enrolled population.
If the reported data result in Food and Drug Administration marketing approval for the system, Dr. Saver said that he anticipated “substantial uptake” of the strategy, which he tested in patients who had not undergone thrombectomy or thrombolysis treatment. In current U.S. practice, there is “a large group of patients with a missed opportunity for recanalization” who would be candidates for treatment with SPG stimulation, a treatment that appeared to provide benefits beyond current standard care, he said in an interview.
Ongoing studies are also testing whether SPG stimulation can benefit acute ischemic stroke patients who have already undergone treatment with thrombectomy or thrombolysis, he added. The same SPG stimulation device is additionally undergoing U.S. testing as a treatment for headache and has regulatory approval in the European Union for treating headache and migraine.
The ImpACT-24B (Implant for Augmentation of Cerebral Blood Flow Trial, Effectiveness and Safety in a 24-Hour Window) trial involved 1,000 patients at 73 centers in 18 countries, including the United States. The investigators enrolled acute ischemic stroke patients 8-24 hours after stroke onset who had a National Institutes of Health Stroke Scale (NIHSS) score of 7-18.
Each patient received an implant of a short, thin metal electrode placed through the soft palate at the rear roof of the mouth, near the SPG. Neurologists primarily performed the implants in a procedure that had a “skin to skin” time of less than 5 minutes. Patients received either electrical stimulation or a sham stimulation through the electrode immediately after placement and then daily for the next 4 days. The investigators titrated the strength of the treatment stimulation in each patient to maximize its strength while maintaining patient comfort. Subsequent analysis of the results showed that the stronger the tolerated stimulation, the bigger the treatment effect in a clear dose-response pattern, Dr. Saver reported.
The study’s primary endpoint was improvement in the modified Rankin scale (mRS) score at 90 days after the index stroke when measured against historical expectations. By this measure, the overall study cohort showed a small, statistically insignificant improvement in actively treated patients, compared with sham-treated patients. However, in the prespecified, coprimary endpoint cohort of patients with a CCI, active treatment resulted in 50% of patients having a better-than-expected 90-day outcome, compared with 40% of controls, a 48% relative improvement in this measure that met the prespecified definition of statistical significance. The results also showed about a 50% relative improvement in each of three secondary outcomes in the CCI cohort: the percentage of patients with a mRS score of 0-2 after 90 days, the percentage with a mRS score of 0-3 after 90 days, and average stroke-related quality of life at 90 days.
Dr. Saver also reported results of a meta-analysis that combined the results he reported from 520 patients with CCI with results from 87 CCI patients enrolled in the preceding pilot study of this treatment strategy, ImpACT-1. The pilot findings were completely consistent and when combined with the current results strengthened the statistical significance of the primary and secondary endpoints.
“There is a compelling story” of efficacy based on the study results, the dose-response relationship, and the meta-analysis results, Dr. Saver said. “I think it’s a very strong case.”
He also reported “no safety concerns” raised in the new study, with no serious adverse effects seen in or experienced by the patients on active treatment.
“The data are compelling” for safety and efficacy, for this novel approach for treating acute ischemic stroke, commented Pooja Khatri, MD, professor of neurology and director of the acute stroke program at the University of Cincinnati.
The study was sponsored by BrainsGate, the company developing the tested device. Dr. Saver has been a consultant to BrainsGate. Dr. Khatri has been a consultant to Biogen, Greenwich, Lumosa, and PTC Therapeutics.
SOURCE: Saver J et al. Int J. Stroke. 2018 Oct;13(2S):28, Abstract 104.
MONTREAL – Stimulation of the sphenopalatine ganglion (SPG) using a small, implanted electrode for 5 days in patients who had just had an acute ischemic stroke led to statistically significant and clinically meaningful improvements in the subset of patients with confirmed cortical involvement in a pivotal, sham-controlled trial.
SPG stimulation started within 24 hours of an acute ischemic stroke “reduced poststroke disability over the entire outcome range and increased the proportion of patients who were alive and independent 3 months after their stroke” in the subgroup with a confirmed cortical infarction (CCI), Jeffrey L. Saver, MD, said at the World Stroke Congress. Five days of SPG stimulation, done once daily starting within 24 hours of stroke onset, “enhances ipsilateral collateral blood flow” and may also stabilize the blood brain barrier, explained Dr. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles. The study included a prespecified primary endpoint analysis that focused exclusively on the CCI subgroup, 52% of the total enrolled population.
If the reported data result in Food and Drug Administration marketing approval for the system, Dr. Saver said that he anticipated “substantial uptake” of the strategy, which he tested in patients who had not undergone thrombectomy or thrombolysis treatment. In current U.S. practice, there is “a large group of patients with a missed opportunity for recanalization” who would be candidates for treatment with SPG stimulation, a treatment that appeared to provide benefits beyond current standard care, he said in an interview.
Ongoing studies are also testing whether SPG stimulation can benefit acute ischemic stroke patients who have already undergone treatment with thrombectomy or thrombolysis, he added. The same SPG stimulation device is additionally undergoing U.S. testing as a treatment for headache and has regulatory approval in the European Union for treating headache and migraine.
The ImpACT-24B (Implant for Augmentation of Cerebral Blood Flow Trial, Effectiveness and Safety in a 24-Hour Window) trial involved 1,000 patients at 73 centers in 18 countries, including the United States. The investigators enrolled acute ischemic stroke patients 8-24 hours after stroke onset who had a National Institutes of Health Stroke Scale (NIHSS) score of 7-18.
Each patient received an implant of a short, thin metal electrode placed through the soft palate at the rear roof of the mouth, near the SPG. Neurologists primarily performed the implants in a procedure that had a “skin to skin” time of less than 5 minutes. Patients received either electrical stimulation or a sham stimulation through the electrode immediately after placement and then daily for the next 4 days. The investigators titrated the strength of the treatment stimulation in each patient to maximize its strength while maintaining patient comfort. Subsequent analysis of the results showed that the stronger the tolerated stimulation, the bigger the treatment effect in a clear dose-response pattern, Dr. Saver reported.
The study’s primary endpoint was improvement in the modified Rankin scale (mRS) score at 90 days after the index stroke when measured against historical expectations. By this measure, the overall study cohort showed a small, statistically insignificant improvement in actively treated patients, compared with sham-treated patients. However, in the prespecified, coprimary endpoint cohort of patients with a CCI, active treatment resulted in 50% of patients having a better-than-expected 90-day outcome, compared with 40% of controls, a 48% relative improvement in this measure that met the prespecified definition of statistical significance. The results also showed about a 50% relative improvement in each of three secondary outcomes in the CCI cohort: the percentage of patients with a mRS score of 0-2 after 90 days, the percentage with a mRS score of 0-3 after 90 days, and average stroke-related quality of life at 90 days.
Dr. Saver also reported results of a meta-analysis that combined the results he reported from 520 patients with CCI with results from 87 CCI patients enrolled in the preceding pilot study of this treatment strategy, ImpACT-1. The pilot findings were completely consistent and when combined with the current results strengthened the statistical significance of the primary and secondary endpoints.
“There is a compelling story” of efficacy based on the study results, the dose-response relationship, and the meta-analysis results, Dr. Saver said. “I think it’s a very strong case.”
He also reported “no safety concerns” raised in the new study, with no serious adverse effects seen in or experienced by the patients on active treatment.
“The data are compelling” for safety and efficacy, for this novel approach for treating acute ischemic stroke, commented Pooja Khatri, MD, professor of neurology and director of the acute stroke program at the University of Cincinnati.
The study was sponsored by BrainsGate, the company developing the tested device. Dr. Saver has been a consultant to BrainsGate. Dr. Khatri has been a consultant to Biogen, Greenwich, Lumosa, and PTC Therapeutics.
SOURCE: Saver J et al. Int J. Stroke. 2018 Oct;13(2S):28, Abstract 104.
MONTREAL – Stimulation of the sphenopalatine ganglion (SPG) using a small, implanted electrode for 5 days in patients who had just had an acute ischemic stroke led to statistically significant and clinically meaningful improvements in the subset of patients with confirmed cortical involvement in a pivotal, sham-controlled trial.
SPG stimulation started within 24 hours of an acute ischemic stroke “reduced poststroke disability over the entire outcome range and increased the proportion of patients who were alive and independent 3 months after their stroke” in the subgroup with a confirmed cortical infarction (CCI), Jeffrey L. Saver, MD, said at the World Stroke Congress. Five days of SPG stimulation, done once daily starting within 24 hours of stroke onset, “enhances ipsilateral collateral blood flow” and may also stabilize the blood brain barrier, explained Dr. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles. The study included a prespecified primary endpoint analysis that focused exclusively on the CCI subgroup, 52% of the total enrolled population.
If the reported data result in Food and Drug Administration marketing approval for the system, Dr. Saver said that he anticipated “substantial uptake” of the strategy, which he tested in patients who had not undergone thrombectomy or thrombolysis treatment. In current U.S. practice, there is “a large group of patients with a missed opportunity for recanalization” who would be candidates for treatment with SPG stimulation, a treatment that appeared to provide benefits beyond current standard care, he said in an interview.
Ongoing studies are also testing whether SPG stimulation can benefit acute ischemic stroke patients who have already undergone treatment with thrombectomy or thrombolysis, he added. The same SPG stimulation device is additionally undergoing U.S. testing as a treatment for headache and has regulatory approval in the European Union for treating headache and migraine.
The ImpACT-24B (Implant for Augmentation of Cerebral Blood Flow Trial, Effectiveness and Safety in a 24-Hour Window) trial involved 1,000 patients at 73 centers in 18 countries, including the United States. The investigators enrolled acute ischemic stroke patients 8-24 hours after stroke onset who had a National Institutes of Health Stroke Scale (NIHSS) score of 7-18.
Each patient received an implant of a short, thin metal electrode placed through the soft palate at the rear roof of the mouth, near the SPG. Neurologists primarily performed the implants in a procedure that had a “skin to skin” time of less than 5 minutes. Patients received either electrical stimulation or a sham stimulation through the electrode immediately after placement and then daily for the next 4 days. The investigators titrated the strength of the treatment stimulation in each patient to maximize its strength while maintaining patient comfort. Subsequent analysis of the results showed that the stronger the tolerated stimulation, the bigger the treatment effect in a clear dose-response pattern, Dr. Saver reported.
The study’s primary endpoint was improvement in the modified Rankin scale (mRS) score at 90 days after the index stroke when measured against historical expectations. By this measure, the overall study cohort showed a small, statistically insignificant improvement in actively treated patients, compared with sham-treated patients. However, in the prespecified, coprimary endpoint cohort of patients with a CCI, active treatment resulted in 50% of patients having a better-than-expected 90-day outcome, compared with 40% of controls, a 48% relative improvement in this measure that met the prespecified definition of statistical significance. The results also showed about a 50% relative improvement in each of three secondary outcomes in the CCI cohort: the percentage of patients with a mRS score of 0-2 after 90 days, the percentage with a mRS score of 0-3 after 90 days, and average stroke-related quality of life at 90 days.
Dr. Saver also reported results of a meta-analysis that combined the results he reported from 520 patients with CCI with results from 87 CCI patients enrolled in the preceding pilot study of this treatment strategy, ImpACT-1. The pilot findings were completely consistent and when combined with the current results strengthened the statistical significance of the primary and secondary endpoints.
“There is a compelling story” of efficacy based on the study results, the dose-response relationship, and the meta-analysis results, Dr. Saver said. “I think it’s a very strong case.”
He also reported “no safety concerns” raised in the new study, with no serious adverse effects seen in or experienced by the patients on active treatment.
“The data are compelling” for safety and efficacy, for this novel approach for treating acute ischemic stroke, commented Pooja Khatri, MD, professor of neurology and director of the acute stroke program at the University of Cincinnati.
The study was sponsored by BrainsGate, the company developing the tested device. Dr. Saver has been a consultant to BrainsGate. Dr. Khatri has been a consultant to Biogen, Greenwich, Lumosa, and PTC Therapeutics.
SOURCE: Saver J et al. Int J. Stroke. 2018 Oct;13(2S):28, Abstract 104.
REPORTING FROM THE WORLD STROKE CONGRESS
Key clinical point: Sphenopalatine ganglion stimulation of acute ischemic stroke patients boosted cerebral blood flow and improved 90-day outcomes in patients with confirmed cortical infarctions.
Major finding: For confirmed cortical infarctions ganglion stimulation led to a 48% higher rate of better-than-expected outcomes, compared with controls.
Study details: ImpACT-24B, a multicenter pivotal trial with 1,000 acute ischemic stroke patients.
Disclosures: The study was sponsored by BrainsGate, the company developing the tested device. Dr. Saver has been a consultant to BrainsGate. Dr. Khatri has been a consultant to Biogen, Greenwich, Lumosa, and PTC Therapeutics.
Source: Saver J et al. Int J. Stroke. 2018 Oct;13(2S):28, Abstract 104.
Tofacitinib impresses in first trial for dermatomyositis
CHICAGO – Oral tofacitinib demonstrated strong clinical efficacy and good safety in the first-ever formal study of a Janus kinase inhibitor in patients with active, treatment-resistant dermatomyositis, Julie J. Paik, MD, reported at the annual meeting of the American College of Rheumatology.
Based upon the encouraging results of this small, open-label, proof-of-concept study, a larger randomized controlled trial is being planned, according to Dr. Paik, a rheumatologist at Johns Hopkins University in Baltimore.
The study included 10 dermatomyositis patients enrolled at the Johns Hopkins Myositis Clinic. All had previously failed at least two steroid-sparing drugs and/or high-dose steroids. After first going off all steroid-sparing agents and being limited to a maximum of 20 mg/day of prednisone, the participants were placed on 11 mg of open-label, extended-release tofacitinib (Xeljanz XR) once daily. Dr. Paik only reported results in 9 patients because the 10th hadn’t yet reached the 12-week mark.
The primary outcome was the rate of achievement of significant improvement at week 12 as defined using the validated International Myositis Assessment and Clinical Studies (IMACS) criteria, which require at least a 20% improvement in three of six core set measures coupled with no more than two measures worsening by 25% or more. By this standard, all nine patients met the primary endpoint. Five were rated as showing moderate improvement, and the other four demonstrated minimal improvement, based on the Total Improvement Score of the Myositis Response criteria. The median Total Improvement Score was 40, indicative of at least moderate improvement.
The mean CDASI (Cutaneous Dermatomyositis Disease Area and Severity Index) activity score – a key secondary endpoint – improved from 28 at baseline to 9.5 at week 12, which translates to a shift from moderate or severe disease to mild disease.
Levels of the alpha-chemokines CXCL9 and CXCL10, which are expressed in muscle affected by idiopathic inflammatory myopathies such as dermatomyositis, declined strongly over the course of 12 weeks of treatment to an extent that was just shy of statistical significance.
Myositis antibody titers didn’t change in response to tofacitinib therapy. Of note, six patients were positive for antitranscriptional intermediary factor 1-gamma, and five of the six were moderate responders to JAK inhibitor therapy.
Four patients were on 20 mg/day of prednisone at baseline; three of the four were able to go off steroids altogether over the course of 12 weeks.
The treatment was well tolerated, with no serious adverse events.
Asked if she thought 11 mg/day of tofacitinib was the right dose for this population of refractory dermatomyositis patients, Dr. Paik replied, “We’re not sure we have the right dose. We had one patient who didn’t have as robust a response, and I really wonder, if we gave her a higher dose, it would have made a difference.”
Extended-release tofacitinib at 11 mg/day is the approved dose for rheumatoid arthritis and psoriatic arthritis. However, 20 mg/day is approved for patients with ulcerative colitis, and Dr. Paik is lobbying for inclusion of a higher-dose arm in the randomized, controlled trial.
Prior to this formal proof-of-concept study, which was funded by Pfizer, the experience with tofacitinib in refractory dermatomyositis was limited to anecdotal case reports.
Dr. Paik reported having no financial conflicts.
SOURCE: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02.
CHICAGO – Oral tofacitinib demonstrated strong clinical efficacy and good safety in the first-ever formal study of a Janus kinase inhibitor in patients with active, treatment-resistant dermatomyositis, Julie J. Paik, MD, reported at the annual meeting of the American College of Rheumatology.
Based upon the encouraging results of this small, open-label, proof-of-concept study, a larger randomized controlled trial is being planned, according to Dr. Paik, a rheumatologist at Johns Hopkins University in Baltimore.
The study included 10 dermatomyositis patients enrolled at the Johns Hopkins Myositis Clinic. All had previously failed at least two steroid-sparing drugs and/or high-dose steroids. After first going off all steroid-sparing agents and being limited to a maximum of 20 mg/day of prednisone, the participants were placed on 11 mg of open-label, extended-release tofacitinib (Xeljanz XR) once daily. Dr. Paik only reported results in 9 patients because the 10th hadn’t yet reached the 12-week mark.
The primary outcome was the rate of achievement of significant improvement at week 12 as defined using the validated International Myositis Assessment and Clinical Studies (IMACS) criteria, which require at least a 20% improvement in three of six core set measures coupled with no more than two measures worsening by 25% or more. By this standard, all nine patients met the primary endpoint. Five were rated as showing moderate improvement, and the other four demonstrated minimal improvement, based on the Total Improvement Score of the Myositis Response criteria. The median Total Improvement Score was 40, indicative of at least moderate improvement.
The mean CDASI (Cutaneous Dermatomyositis Disease Area and Severity Index) activity score – a key secondary endpoint – improved from 28 at baseline to 9.5 at week 12, which translates to a shift from moderate or severe disease to mild disease.
Levels of the alpha-chemokines CXCL9 and CXCL10, which are expressed in muscle affected by idiopathic inflammatory myopathies such as dermatomyositis, declined strongly over the course of 12 weeks of treatment to an extent that was just shy of statistical significance.
Myositis antibody titers didn’t change in response to tofacitinib therapy. Of note, six patients were positive for antitranscriptional intermediary factor 1-gamma, and five of the six were moderate responders to JAK inhibitor therapy.
Four patients were on 20 mg/day of prednisone at baseline; three of the four were able to go off steroids altogether over the course of 12 weeks.
The treatment was well tolerated, with no serious adverse events.
Asked if she thought 11 mg/day of tofacitinib was the right dose for this population of refractory dermatomyositis patients, Dr. Paik replied, “We’re not sure we have the right dose. We had one patient who didn’t have as robust a response, and I really wonder, if we gave her a higher dose, it would have made a difference.”
Extended-release tofacitinib at 11 mg/day is the approved dose for rheumatoid arthritis and psoriatic arthritis. However, 20 mg/day is approved for patients with ulcerative colitis, and Dr. Paik is lobbying for inclusion of a higher-dose arm in the randomized, controlled trial.
Prior to this formal proof-of-concept study, which was funded by Pfizer, the experience with tofacitinib in refractory dermatomyositis was limited to anecdotal case reports.
Dr. Paik reported having no financial conflicts.
SOURCE: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02.
CHICAGO – Oral tofacitinib demonstrated strong clinical efficacy and good safety in the first-ever formal study of a Janus kinase inhibitor in patients with active, treatment-resistant dermatomyositis, Julie J. Paik, MD, reported at the annual meeting of the American College of Rheumatology.
Based upon the encouraging results of this small, open-label, proof-of-concept study, a larger randomized controlled trial is being planned, according to Dr. Paik, a rheumatologist at Johns Hopkins University in Baltimore.
The study included 10 dermatomyositis patients enrolled at the Johns Hopkins Myositis Clinic. All had previously failed at least two steroid-sparing drugs and/or high-dose steroids. After first going off all steroid-sparing agents and being limited to a maximum of 20 mg/day of prednisone, the participants were placed on 11 mg of open-label, extended-release tofacitinib (Xeljanz XR) once daily. Dr. Paik only reported results in 9 patients because the 10th hadn’t yet reached the 12-week mark.
The primary outcome was the rate of achievement of significant improvement at week 12 as defined using the validated International Myositis Assessment and Clinical Studies (IMACS) criteria, which require at least a 20% improvement in three of six core set measures coupled with no more than two measures worsening by 25% or more. By this standard, all nine patients met the primary endpoint. Five were rated as showing moderate improvement, and the other four demonstrated minimal improvement, based on the Total Improvement Score of the Myositis Response criteria. The median Total Improvement Score was 40, indicative of at least moderate improvement.
The mean CDASI (Cutaneous Dermatomyositis Disease Area and Severity Index) activity score – a key secondary endpoint – improved from 28 at baseline to 9.5 at week 12, which translates to a shift from moderate or severe disease to mild disease.
Levels of the alpha-chemokines CXCL9 and CXCL10, which are expressed in muscle affected by idiopathic inflammatory myopathies such as dermatomyositis, declined strongly over the course of 12 weeks of treatment to an extent that was just shy of statistical significance.
Myositis antibody titers didn’t change in response to tofacitinib therapy. Of note, six patients were positive for antitranscriptional intermediary factor 1-gamma, and five of the six were moderate responders to JAK inhibitor therapy.
Four patients were on 20 mg/day of prednisone at baseline; three of the four were able to go off steroids altogether over the course of 12 weeks.
The treatment was well tolerated, with no serious adverse events.
Asked if she thought 11 mg/day of tofacitinib was the right dose for this population of refractory dermatomyositis patients, Dr. Paik replied, “We’re not sure we have the right dose. We had one patient who didn’t have as robust a response, and I really wonder, if we gave her a higher dose, it would have made a difference.”
Extended-release tofacitinib at 11 mg/day is the approved dose for rheumatoid arthritis and psoriatic arthritis. However, 20 mg/day is approved for patients with ulcerative colitis, and Dr. Paik is lobbying for inclusion of a higher-dose arm in the randomized, controlled trial.
Prior to this formal proof-of-concept study, which was funded by Pfizer, the experience with tofacitinib in refractory dermatomyositis was limited to anecdotal case reports.
Dr. Paik reported having no financial conflicts.
SOURCE: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Among patients with refractory dermatomyositis, the mean Cutaneous Dermatomyositis Disease Area and Severity Index activity score improved from 28 at baseline to 9.5 after 12 weeks on oral tofacitinib.
Study details: This first-in-class, open-label, 12-week study included 10 patients with refractory dermatomyositis placed on extended-release tofacitinib at 11 mg once daily.
Disclosures: The presenter reported having no financial conflicts regarding the study, sponsored by Pfizer.
Source: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02
Assess ADHD at developmental age, rather than chronological
ORLANDO – said David O. Childers, MD, chief of the division of developmental pediatrics at the University of Florida in Jacksonville.
“That is a huge consideration, and the vast majority of pediatricians don’t understand that,” Dr. Childers said at the annual meeting of the American Academy of Pediatrics.
A diagnosis of ADHD in children should be reserved for situations in which the symptoms clearly interfere with how they function in social, academic, and occupational settings. ADHD should not be diagnosed in children who exhibit hostility, defiance, a failure to understand, or who have oppositional defiant disorder (ODD). In addition, ADHD in children often overlaps with other conditions, such as learning disabilities, ODD, anxiety, depression, poor self-esteem, conduct disorder, motor coordination problems, and encopresis and enuresis.
One mistake you can make is starting the medication too young in patients who exhibit symptoms of ADHD. Receptive language relative to developmental age should be taken into consideration when deciding whether to treat very young patients. “If the voice in your head is at a 3-year-old level, that’s going to be your behavior picture regardless of your chronological age,” Dr. Childers said.
You also can misdiagnose ADHD in young patients because you don’t recognize behavioral phenotypes and diagnoses that are similar to ADHD. Extreme prematurity, global developmental delay, fetal alcohol syndrome, spina bifida, genetic syndrome, intellectual and learning disabilities, closed head injuries, and depression all can mimic ADHD symptoms. “Everything that wheezes is not asthma, and everything that is ‘hyper’ or ‘drifty’ is not ADHD,” he noted.
Another mistake is considering mixed amphetamine salts (MAS) the same as methylphenidate (MPH), Dr. Childers said. MAS has 5 mg of active isomer versus 2.5 mg in MPH doses, and patients could see side effects from the increased potency in MAS in the form of appetite suppression and tics. You should start with a low dose of the medication and titrate up until patients get the most out of the medication to avoid situations in which patients report a change in personality or that the treatment is not effective.
“The medicine should never change a kid’s personality,” Dr. Childers said. “We are not here to treat the kid to the point of hyperactivity management; we’re here to treat for attention.”
In cases where patients ask for the short-acting medication rather than long-acting stimulant, remember that short-acting medication for ADHD has a higher potential for abuse. “Medication diversion is real. It does occur,” he said.
Establishing a basal sleep history and whether the child has insomnia is important before prescribing ADHD medication. Dr. Childers noted he uses a short-acting stimulant as an off-label treatment to treat some of his patients with insomnia, but he said to be aware of side effects like headache, abdominal pain, anorexia, and tics, as well as cardiac issues.
You also should rethink your diagnosis if the medication is not having an effect after two trials of a stimulant, Dr. Childers noted, and when a child has conditions like anxiety and depression that can mimic ADHD symptoms, a stimulant will increase irritability and have a paradoxical response to the medication. Inattentive ADHD can be missed in adolescent patients who struggle academically until they fall behind their peers, which can result in developing depression that complicates treatment, he said.
A learning disorder also can be mistaken for ADHD in children and presents in different ways depending on what grade the child is in. As a child ages and learns how to read, he or she will begin to fall behind as learning through reading becomes more complex. Learning disorders should be ruled out before treating for ADHD because, if the child improves after taking a stimulant, it could be more difficult to find a diagnosis of learning disorder later, Dr. Childers noted.
Bipolar disorder can be overdiagnosed in children; in these patients, the most likely diagnoses are ADHD and ODD, he said. According to a 2004 National Alliance of Mental Illness fact sheet, approximately 7% of patients in caseloads for federally funded studies in psychiatric facilities met the criteria for bipolar disorder, but the lifelong prevalence of the disease is between 1% and 1.6%.
“Bipolar disorder is frequently oppositional defiant disorder, and the word ‘no’ can be a trigger,” Dr. Childers said.
Dr. Childers reported no relevant conflicts of interest.
ORLANDO – said David O. Childers, MD, chief of the division of developmental pediatrics at the University of Florida in Jacksonville.
“That is a huge consideration, and the vast majority of pediatricians don’t understand that,” Dr. Childers said at the annual meeting of the American Academy of Pediatrics.
A diagnosis of ADHD in children should be reserved for situations in which the symptoms clearly interfere with how they function in social, academic, and occupational settings. ADHD should not be diagnosed in children who exhibit hostility, defiance, a failure to understand, or who have oppositional defiant disorder (ODD). In addition, ADHD in children often overlaps with other conditions, such as learning disabilities, ODD, anxiety, depression, poor self-esteem, conduct disorder, motor coordination problems, and encopresis and enuresis.
One mistake you can make is starting the medication too young in patients who exhibit symptoms of ADHD. Receptive language relative to developmental age should be taken into consideration when deciding whether to treat very young patients. “If the voice in your head is at a 3-year-old level, that’s going to be your behavior picture regardless of your chronological age,” Dr. Childers said.
You also can misdiagnose ADHD in young patients because you don’t recognize behavioral phenotypes and diagnoses that are similar to ADHD. Extreme prematurity, global developmental delay, fetal alcohol syndrome, spina bifida, genetic syndrome, intellectual and learning disabilities, closed head injuries, and depression all can mimic ADHD symptoms. “Everything that wheezes is not asthma, and everything that is ‘hyper’ or ‘drifty’ is not ADHD,” he noted.
Another mistake is considering mixed amphetamine salts (MAS) the same as methylphenidate (MPH), Dr. Childers said. MAS has 5 mg of active isomer versus 2.5 mg in MPH doses, and patients could see side effects from the increased potency in MAS in the form of appetite suppression and tics. You should start with a low dose of the medication and titrate up until patients get the most out of the medication to avoid situations in which patients report a change in personality or that the treatment is not effective.
“The medicine should never change a kid’s personality,” Dr. Childers said. “We are not here to treat the kid to the point of hyperactivity management; we’re here to treat for attention.”
In cases where patients ask for the short-acting medication rather than long-acting stimulant, remember that short-acting medication for ADHD has a higher potential for abuse. “Medication diversion is real. It does occur,” he said.
Establishing a basal sleep history and whether the child has insomnia is important before prescribing ADHD medication. Dr. Childers noted he uses a short-acting stimulant as an off-label treatment to treat some of his patients with insomnia, but he said to be aware of side effects like headache, abdominal pain, anorexia, and tics, as well as cardiac issues.
You also should rethink your diagnosis if the medication is not having an effect after two trials of a stimulant, Dr. Childers noted, and when a child has conditions like anxiety and depression that can mimic ADHD symptoms, a stimulant will increase irritability and have a paradoxical response to the medication. Inattentive ADHD can be missed in adolescent patients who struggle academically until they fall behind their peers, which can result in developing depression that complicates treatment, he said.
A learning disorder also can be mistaken for ADHD in children and presents in different ways depending on what grade the child is in. As a child ages and learns how to read, he or she will begin to fall behind as learning through reading becomes more complex. Learning disorders should be ruled out before treating for ADHD because, if the child improves after taking a stimulant, it could be more difficult to find a diagnosis of learning disorder later, Dr. Childers noted.
Bipolar disorder can be overdiagnosed in children; in these patients, the most likely diagnoses are ADHD and ODD, he said. According to a 2004 National Alliance of Mental Illness fact sheet, approximately 7% of patients in caseloads for federally funded studies in psychiatric facilities met the criteria for bipolar disorder, but the lifelong prevalence of the disease is between 1% and 1.6%.
“Bipolar disorder is frequently oppositional defiant disorder, and the word ‘no’ can be a trigger,” Dr. Childers said.
Dr. Childers reported no relevant conflicts of interest.
ORLANDO – said David O. Childers, MD, chief of the division of developmental pediatrics at the University of Florida in Jacksonville.
“That is a huge consideration, and the vast majority of pediatricians don’t understand that,” Dr. Childers said at the annual meeting of the American Academy of Pediatrics.
A diagnosis of ADHD in children should be reserved for situations in which the symptoms clearly interfere with how they function in social, academic, and occupational settings. ADHD should not be diagnosed in children who exhibit hostility, defiance, a failure to understand, or who have oppositional defiant disorder (ODD). In addition, ADHD in children often overlaps with other conditions, such as learning disabilities, ODD, anxiety, depression, poor self-esteem, conduct disorder, motor coordination problems, and encopresis and enuresis.
One mistake you can make is starting the medication too young in patients who exhibit symptoms of ADHD. Receptive language relative to developmental age should be taken into consideration when deciding whether to treat very young patients. “If the voice in your head is at a 3-year-old level, that’s going to be your behavior picture regardless of your chronological age,” Dr. Childers said.
You also can misdiagnose ADHD in young patients because you don’t recognize behavioral phenotypes and diagnoses that are similar to ADHD. Extreme prematurity, global developmental delay, fetal alcohol syndrome, spina bifida, genetic syndrome, intellectual and learning disabilities, closed head injuries, and depression all can mimic ADHD symptoms. “Everything that wheezes is not asthma, and everything that is ‘hyper’ or ‘drifty’ is not ADHD,” he noted.
Another mistake is considering mixed amphetamine salts (MAS) the same as methylphenidate (MPH), Dr. Childers said. MAS has 5 mg of active isomer versus 2.5 mg in MPH doses, and patients could see side effects from the increased potency in MAS in the form of appetite suppression and tics. You should start with a low dose of the medication and titrate up until patients get the most out of the medication to avoid situations in which patients report a change in personality or that the treatment is not effective.
“The medicine should never change a kid’s personality,” Dr. Childers said. “We are not here to treat the kid to the point of hyperactivity management; we’re here to treat for attention.”
In cases where patients ask for the short-acting medication rather than long-acting stimulant, remember that short-acting medication for ADHD has a higher potential for abuse. “Medication diversion is real. It does occur,” he said.
Establishing a basal sleep history and whether the child has insomnia is important before prescribing ADHD medication. Dr. Childers noted he uses a short-acting stimulant as an off-label treatment to treat some of his patients with insomnia, but he said to be aware of side effects like headache, abdominal pain, anorexia, and tics, as well as cardiac issues.
You also should rethink your diagnosis if the medication is not having an effect after two trials of a stimulant, Dr. Childers noted, and when a child has conditions like anxiety and depression that can mimic ADHD symptoms, a stimulant will increase irritability and have a paradoxical response to the medication. Inattentive ADHD can be missed in adolescent patients who struggle academically until they fall behind their peers, which can result in developing depression that complicates treatment, he said.
A learning disorder also can be mistaken for ADHD in children and presents in different ways depending on what grade the child is in. As a child ages and learns how to read, he or she will begin to fall behind as learning through reading becomes more complex. Learning disorders should be ruled out before treating for ADHD because, if the child improves after taking a stimulant, it could be more difficult to find a diagnosis of learning disorder later, Dr. Childers noted.
Bipolar disorder can be overdiagnosed in children; in these patients, the most likely diagnoses are ADHD and ODD, he said. According to a 2004 National Alliance of Mental Illness fact sheet, approximately 7% of patients in caseloads for federally funded studies in psychiatric facilities met the criteria for bipolar disorder, but the lifelong prevalence of the disease is between 1% and 1.6%.
“Bipolar disorder is frequently oppositional defiant disorder, and the word ‘no’ can be a trigger,” Dr. Childers said.
Dr. Childers reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM AAP 18
Necrotizing lunchitis, pneumonia throwdown, global gamete warming
Global gamete warming
Apparently, increasing deadly wildfires, hurricanes, and global famine aren’t enough. Turns out, climate change has found yet another way to harm its arch nemeses, a.k.a. every single species on the planet. A study originally published in Nature Communications found that rising temperatures also have a significant effect on male (but not female) fertility. Men: so fragile.
Testing fertility in flour beetles, researchers concluded that successive heat waves of 5-7° C above normal for 5 days reduced sperm competitiveness and practically sterilized the males. Inseminated sperm inside females were also not spared the devastating effects of the heat-wave conditions. And, as the icing on the cake, reduced fertility persisted amongst later generations.
Unless we can figure out how robot sperm can deliver DNA, we’re in trouble.
Pneumonia throwdown
Previously, we pitted Clostridium difficile against cockroaches in a battle of toughness. In this week’s edition of Bacteria vs. the World, bacterial pneumonia goes up against another worthy adversary, viral pneumonia.
“We’ve always known pneumonia was a risk factor for a major adverse cardiac event,” said J. Brent Muhlestein, MD, of Intermountain Medical Center in Salt Lake City. “What we didn’t know was which type of pneumonia was more dangerous.”
To find out, he and his associates followed almost 4,800 patients hospitalized with pneumonia and tracked nonfatal heart attacks, stroke, heart failure, or death. Data they presented at the American Heart Association scientific sessions in Chicago show that 34% of patients with bacterial pneumonia had a major cardiovascular event within 90 days, compared with 26% of those diagnosed with viral pneumonia. It is likely “that bacterial pneumonia causes greater inflammation of the arteries compared to viral pneumonia,” Dr. Muhlestein said.
So the bacteria stay undefeated, and somewhere Chuck Norris, who will never have a heart attack – even a heart isn’t foolish enough to attack Chuck Norris – is smiling.
Ch-ch-check it out
Drop the beat! Researchers at the University of California were interested in examining beatboxing processes to explore how the human mind works.
These crazy scientists threw some beatboxers into an MRI for an exclusive performance and studied the movements of their mouth and tongue. Researchers hypothesized that beatboxers base their sounds on already-known speech. But they discovered that these talented virtuosos are creating a whole new language.
“They’re coming up with ways to create these really complex acrobatic sounds by taking approaches drawn from different parts of the mouth that they don’t use in any language, and nobody uses for any language,” according to the lead researcher.
Does that mean beatboxing will be taught in schools as a foreign language? Perhaps. It might be more useful than learning Latin.
Keloid castration
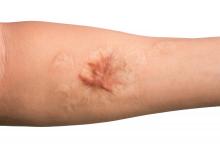
Keloids – those pesky overgrowths of scar tissue – can be mighty hard to treat.
“Virtually every patient says, ‘I want this cut off – I want it gone,’ ” dermatologist Hilary E. Baldwin, MD, said in a presentation at the recent Las Vegas Dermatology Seminar. She responds to patients with reality checks about what’s actually possible in keloid treatment.
But sometimes, they just want to adjust the appearance of their keloids. Like the man who complained that “my keloid looks like my junk.” Dr. Baldwin took a look and had to agree – the keloid on his deltoid was the spitting image of male genitalia. She treated the keloid with the equivalent of castration (removing its “testicles” via surgery) and circumcision of sorts (flattening its “glans penis” via corticosteroids).
“It didn’t look pretty,” she said, as at least one male member of the audience squirmed, “but it no longer looked offensive to him.”
‘Necrotizing lunchitis’
Here at the Bureau of Livin’ on the MDedge, we pride ourselves on having the best words. And being University of Michigan graduates. So, the pain is Likert-scale 10 when Big Ten rivals have better words – and worse office fridges.
Exhibit A: the operative report surgical-taped to a Penn State University call room refrigerator, which general surgery resident and American hero Dr. Cassie Sonntag shared on Twitter. The 18-cubic-foot communal Kenmore’s diagnosis? “Necrotizing lunchitis.”
The grave condition called for immediate intervention by surgeon “Whitt,” with assistance from circulating nurse “Liu.” The surgical team performed “debridement of the upper, middle, and lower compartments of the call room refrigerator with extension into the fridge door, disarticulation and washout of the lower chamber, explantation of necrotic lunches of varying ages.” Complications? “Multiple never-before-seen species of mold casually exterminated.” The patient’s postprocedure condition is “guarded.” The complete report is well worth your review. Even if the Sears appliance’s specimens were “refused by path.”

Global gamete warming
Apparently, increasing deadly wildfires, hurricanes, and global famine aren’t enough. Turns out, climate change has found yet another way to harm its arch nemeses, a.k.a. every single species on the planet. A study originally published in Nature Communications found that rising temperatures also have a significant effect on male (but not female) fertility. Men: so fragile.
Testing fertility in flour beetles, researchers concluded that successive heat waves of 5-7° C above normal for 5 days reduced sperm competitiveness and practically sterilized the males. Inseminated sperm inside females were also not spared the devastating effects of the heat-wave conditions. And, as the icing on the cake, reduced fertility persisted amongst later generations.
Unless we can figure out how robot sperm can deliver DNA, we’re in trouble.
Pneumonia throwdown
Previously, we pitted Clostridium difficile against cockroaches in a battle of toughness. In this week’s edition of Bacteria vs. the World, bacterial pneumonia goes up against another worthy adversary, viral pneumonia.
“We’ve always known pneumonia was a risk factor for a major adverse cardiac event,” said J. Brent Muhlestein, MD, of Intermountain Medical Center in Salt Lake City. “What we didn’t know was which type of pneumonia was more dangerous.”
To find out, he and his associates followed almost 4,800 patients hospitalized with pneumonia and tracked nonfatal heart attacks, stroke, heart failure, or death. Data they presented at the American Heart Association scientific sessions in Chicago show that 34% of patients with bacterial pneumonia had a major cardiovascular event within 90 days, compared with 26% of those diagnosed with viral pneumonia. It is likely “that bacterial pneumonia causes greater inflammation of the arteries compared to viral pneumonia,” Dr. Muhlestein said.
So the bacteria stay undefeated, and somewhere Chuck Norris, who will never have a heart attack – even a heart isn’t foolish enough to attack Chuck Norris – is smiling.
Ch-ch-check it out
Drop the beat! Researchers at the University of California were interested in examining beatboxing processes to explore how the human mind works.
These crazy scientists threw some beatboxers into an MRI for an exclusive performance and studied the movements of their mouth and tongue. Researchers hypothesized that beatboxers base their sounds on already-known speech. But they discovered that these talented virtuosos are creating a whole new language.
“They’re coming up with ways to create these really complex acrobatic sounds by taking approaches drawn from different parts of the mouth that they don’t use in any language, and nobody uses for any language,” according to the lead researcher.
Does that mean beatboxing will be taught in schools as a foreign language? Perhaps. It might be more useful than learning Latin.
Keloid castration
Keloids – those pesky overgrowths of scar tissue – can be mighty hard to treat.
“Virtually every patient says, ‘I want this cut off – I want it gone,’ ” dermatologist Hilary E. Baldwin, MD, said in a presentation at the recent Las Vegas Dermatology Seminar. She responds to patients with reality checks about what’s actually possible in keloid treatment.
But sometimes, they just want to adjust the appearance of their keloids. Like the man who complained that “my keloid looks like my junk.” Dr. Baldwin took a look and had to agree – the keloid on his deltoid was the spitting image of male genitalia. She treated the keloid with the equivalent of castration (removing its “testicles” via surgery) and circumcision of sorts (flattening its “glans penis” via corticosteroids).
“It didn’t look pretty,” she said, as at least one male member of the audience squirmed, “but it no longer looked offensive to him.”
‘Necrotizing lunchitis’
Here at the Bureau of Livin’ on the MDedge, we pride ourselves on having the best words. And being University of Michigan graduates. So, the pain is Likert-scale 10 when Big Ten rivals have better words – and worse office fridges.
Exhibit A: the operative report surgical-taped to a Penn State University call room refrigerator, which general surgery resident and American hero Dr. Cassie Sonntag shared on Twitter. The 18-cubic-foot communal Kenmore’s diagnosis? “Necrotizing lunchitis.”
The grave condition called for immediate intervention by surgeon “Whitt,” with assistance from circulating nurse “Liu.” The surgical team performed “debridement of the upper, middle, and lower compartments of the call room refrigerator with extension into the fridge door, disarticulation and washout of the lower chamber, explantation of necrotic lunches of varying ages.” Complications? “Multiple never-before-seen species of mold casually exterminated.” The patient’s postprocedure condition is “guarded.” The complete report is well worth your review. Even if the Sears appliance’s specimens were “refused by path.”

Global gamete warming
Apparently, increasing deadly wildfires, hurricanes, and global famine aren’t enough. Turns out, climate change has found yet another way to harm its arch nemeses, a.k.a. every single species on the planet. A study originally published in Nature Communications found that rising temperatures also have a significant effect on male (but not female) fertility. Men: so fragile.
Testing fertility in flour beetles, researchers concluded that successive heat waves of 5-7° C above normal for 5 days reduced sperm competitiveness and practically sterilized the males. Inseminated sperm inside females were also not spared the devastating effects of the heat-wave conditions. And, as the icing on the cake, reduced fertility persisted amongst later generations.
Unless we can figure out how robot sperm can deliver DNA, we’re in trouble.
Pneumonia throwdown
Previously, we pitted Clostridium difficile against cockroaches in a battle of toughness. In this week’s edition of Bacteria vs. the World, bacterial pneumonia goes up against another worthy adversary, viral pneumonia.
“We’ve always known pneumonia was a risk factor for a major adverse cardiac event,” said J. Brent Muhlestein, MD, of Intermountain Medical Center in Salt Lake City. “What we didn’t know was which type of pneumonia was more dangerous.”
To find out, he and his associates followed almost 4,800 patients hospitalized with pneumonia and tracked nonfatal heart attacks, stroke, heart failure, or death. Data they presented at the American Heart Association scientific sessions in Chicago show that 34% of patients with bacterial pneumonia had a major cardiovascular event within 90 days, compared with 26% of those diagnosed with viral pneumonia. It is likely “that bacterial pneumonia causes greater inflammation of the arteries compared to viral pneumonia,” Dr. Muhlestein said.
So the bacteria stay undefeated, and somewhere Chuck Norris, who will never have a heart attack – even a heart isn’t foolish enough to attack Chuck Norris – is smiling.
Ch-ch-check it out
Drop the beat! Researchers at the University of California were interested in examining beatboxing processes to explore how the human mind works.
These crazy scientists threw some beatboxers into an MRI for an exclusive performance and studied the movements of their mouth and tongue. Researchers hypothesized that beatboxers base their sounds on already-known speech. But they discovered that these talented virtuosos are creating a whole new language.
“They’re coming up with ways to create these really complex acrobatic sounds by taking approaches drawn from different parts of the mouth that they don’t use in any language, and nobody uses for any language,” according to the lead researcher.
Does that mean beatboxing will be taught in schools as a foreign language? Perhaps. It might be more useful than learning Latin.
Keloid castration
Keloids – those pesky overgrowths of scar tissue – can be mighty hard to treat.
“Virtually every patient says, ‘I want this cut off – I want it gone,’ ” dermatologist Hilary E. Baldwin, MD, said in a presentation at the recent Las Vegas Dermatology Seminar. She responds to patients with reality checks about what’s actually possible in keloid treatment.
But sometimes, they just want to adjust the appearance of their keloids. Like the man who complained that “my keloid looks like my junk.” Dr. Baldwin took a look and had to agree – the keloid on his deltoid was the spitting image of male genitalia. She treated the keloid with the equivalent of castration (removing its “testicles” via surgery) and circumcision of sorts (flattening its “glans penis” via corticosteroids).
“It didn’t look pretty,” she said, as at least one male member of the audience squirmed, “but it no longer looked offensive to him.”
‘Necrotizing lunchitis’
Here at the Bureau of Livin’ on the MDedge, we pride ourselves on having the best words. And being University of Michigan graduates. So, the pain is Likert-scale 10 when Big Ten rivals have better words – and worse office fridges.
Exhibit A: the operative report surgical-taped to a Penn State University call room refrigerator, which general surgery resident and American hero Dr. Cassie Sonntag shared on Twitter. The 18-cubic-foot communal Kenmore’s diagnosis? “Necrotizing lunchitis.”
The grave condition called for immediate intervention by surgeon “Whitt,” with assistance from circulating nurse “Liu.” The surgical team performed “debridement of the upper, middle, and lower compartments of the call room refrigerator with extension into the fridge door, disarticulation and washout of the lower chamber, explantation of necrotic lunches of varying ages.” Complications? “Multiple never-before-seen species of mold casually exterminated.” The patient’s postprocedure condition is “guarded.” The complete report is well worth your review. Even if the Sears appliance’s specimens were “refused by path.”

Increased risk of atrial fibrillation with migraine aura
The presence of visual aura during migraine is associated with an increased risk of atrial fibrillation, a study in Neurology has found.
Researchers reported an analysis of data from the longitudinal, community-based Atherosclerosis Risk in Communities (ARIC) Study, which included 11,939 individuals with no history of atrial fibrillation or stroke. Of these, 426 experienced migraines with visual aura, 1,090 experienced migraines without aura, 1,018 experienced nonmigraine headache, and 9,405 experienced no headache.
After adjustment for age and sex, individuals who had migraine with visual aura showed a significant 46% increase in the risk of incident atrial fibrillation when compared with those who experienced migraine without aura and a 39% increased risk when compared with individuals who did not experience headache (P = .004). After adjustment for risk factors such as hypertension, smoking, coronary artery disease, and congestive heart failure, the hazard ratio of incident atrial fibrillation was 1.30 for migraineurs with aura, compared with people without headache. In addition, the hazard ratio of incident atrial fibrillation was 1.39 for migraineurs with aura, compared with migraineurs without aura.
In contrast, individuals who experienced migraines without aura did not show a significantly increased risk of atrial fibrillation.
“This finding has important clinical implications and may help us better understand the atrial fibrillation mediation of the migraine-stroke link,” wrote Souvik Sen, MD, MPH, a professor in the department of neurology at the University of South Carolina, Columbia, and his coauthors. “A randomized clinical trial may help ascertain whether patients with migraine with visual aura may benefit from atrial fibrillation detection and subsequent anticoagulation or antiplatelet therapy as a primary stroke prevention strategy.”
The study also showed a significant interaction with age and sex. While men who experienced migraine with aura had an 89% higher risk of atrial fibrillation, women with aura showed no increase in risk, compared with individuals who experienced no headache. Similarly, only individuals aged 60 years or older who experienced migraine with aura showed an increased risk of atrial fibrillation, while those younger than 60 years did not.
The authors noted that previous case reports have recorded the incidence of atrial fibrillation during a migraine attack. Autonomic dysfunction influences the pathophysiology of atrial fibrillation and migraine.
“Cardiac arrhythmia recordings have been shown to be present in ECGs of patients while experiencing migraine headaches as compared with migraine-free phases,” they wrote. “This hypothesis is further supported by atrial fibrillation ablation procedures that have shown tendencies to reduce migraine symptoms and frequencies.”
In regard to the role that migraine aura played in this, they speculated as to whether migraine aura could be the result of cardioembolic stroke that might have occurred because of the atrial fibrillation.
Overall, 167 patients had incident cardioembolic strokes, and researchers suggested strokes in 87% of these cases could be attributed to the atrial fibrillation that came before the stroke.
The stroke incidence rate also was around twice as high in individuals who experienced migraine with aura, compared with those who experienced migraine without aura (4.1 per 1,000 person-years vs. 2.07 per 1,000 person-years).
The study authors acknowledged that patent foramen ovale, which was not assessed in ARIC, is a possible confounder. Previous studies have showed that patent foramen ovale is more common in younger individuals with migraine and particularly in patients who experience migraine with aura.
However, they also noted that trials of patent foramen ovale closures as a treatment for migraine have not shown success in reducing migraine frequency and, therefore, argued against patent foramen ovale as being a major confounder.
The study was supported by the National Heart, Lung, and Blood Institute and the American Heart Association. One author declared grants from the National Institutes of health, one declared research support from Tian Medical, and one author is an associate editor for Neurology. No other conflicts of interest were declared.
SOURCE: Sen S et al. Neurology. 2018;91:1-9.
This article was updated 12/12/18.
The presence of visual aura during migraine is associated with an increased risk of atrial fibrillation, a study in Neurology has found.
Researchers reported an analysis of data from the longitudinal, community-based Atherosclerosis Risk in Communities (ARIC) Study, which included 11,939 individuals with no history of atrial fibrillation or stroke. Of these, 426 experienced migraines with visual aura, 1,090 experienced migraines without aura, 1,018 experienced nonmigraine headache, and 9,405 experienced no headache.
After adjustment for age and sex, individuals who had migraine with visual aura showed a significant 46% increase in the risk of incident atrial fibrillation when compared with those who experienced migraine without aura and a 39% increased risk when compared with individuals who did not experience headache (P = .004). After adjustment for risk factors such as hypertension, smoking, coronary artery disease, and congestive heart failure, the hazard ratio of incident atrial fibrillation was 1.30 for migraineurs with aura, compared with people without headache. In addition, the hazard ratio of incident atrial fibrillation was 1.39 for migraineurs with aura, compared with migraineurs without aura.
In contrast, individuals who experienced migraines without aura did not show a significantly increased risk of atrial fibrillation.
“This finding has important clinical implications and may help us better understand the atrial fibrillation mediation of the migraine-stroke link,” wrote Souvik Sen, MD, MPH, a professor in the department of neurology at the University of South Carolina, Columbia, and his coauthors. “A randomized clinical trial may help ascertain whether patients with migraine with visual aura may benefit from atrial fibrillation detection and subsequent anticoagulation or antiplatelet therapy as a primary stroke prevention strategy.”
The study also showed a significant interaction with age and sex. While men who experienced migraine with aura had an 89% higher risk of atrial fibrillation, women with aura showed no increase in risk, compared with individuals who experienced no headache. Similarly, only individuals aged 60 years or older who experienced migraine with aura showed an increased risk of atrial fibrillation, while those younger than 60 years did not.
The authors noted that previous case reports have recorded the incidence of atrial fibrillation during a migraine attack. Autonomic dysfunction influences the pathophysiology of atrial fibrillation and migraine.
“Cardiac arrhythmia recordings have been shown to be present in ECGs of patients while experiencing migraine headaches as compared with migraine-free phases,” they wrote. “This hypothesis is further supported by atrial fibrillation ablation procedures that have shown tendencies to reduce migraine symptoms and frequencies.”
In regard to the role that migraine aura played in this, they speculated as to whether migraine aura could be the result of cardioembolic stroke that might have occurred because of the atrial fibrillation.
Overall, 167 patients had incident cardioembolic strokes, and researchers suggested strokes in 87% of these cases could be attributed to the atrial fibrillation that came before the stroke.
The stroke incidence rate also was around twice as high in individuals who experienced migraine with aura, compared with those who experienced migraine without aura (4.1 per 1,000 person-years vs. 2.07 per 1,000 person-years).
The study authors acknowledged that patent foramen ovale, which was not assessed in ARIC, is a possible confounder. Previous studies have showed that patent foramen ovale is more common in younger individuals with migraine and particularly in patients who experience migraine with aura.
However, they also noted that trials of patent foramen ovale closures as a treatment for migraine have not shown success in reducing migraine frequency and, therefore, argued against patent foramen ovale as being a major confounder.
The study was supported by the National Heart, Lung, and Blood Institute and the American Heart Association. One author declared grants from the National Institutes of health, one declared research support from Tian Medical, and one author is an associate editor for Neurology. No other conflicts of interest were declared.
SOURCE: Sen S et al. Neurology. 2018;91:1-9.
This article was updated 12/12/18.
The presence of visual aura during migraine is associated with an increased risk of atrial fibrillation, a study in Neurology has found.
Researchers reported an analysis of data from the longitudinal, community-based Atherosclerosis Risk in Communities (ARIC) Study, which included 11,939 individuals with no history of atrial fibrillation or stroke. Of these, 426 experienced migraines with visual aura, 1,090 experienced migraines without aura, 1,018 experienced nonmigraine headache, and 9,405 experienced no headache.
After adjustment for age and sex, individuals who had migraine with visual aura showed a significant 46% increase in the risk of incident atrial fibrillation when compared with those who experienced migraine without aura and a 39% increased risk when compared with individuals who did not experience headache (P = .004). After adjustment for risk factors such as hypertension, smoking, coronary artery disease, and congestive heart failure, the hazard ratio of incident atrial fibrillation was 1.30 for migraineurs with aura, compared with people without headache. In addition, the hazard ratio of incident atrial fibrillation was 1.39 for migraineurs with aura, compared with migraineurs without aura.
In contrast, individuals who experienced migraines without aura did not show a significantly increased risk of atrial fibrillation.
“This finding has important clinical implications and may help us better understand the atrial fibrillation mediation of the migraine-stroke link,” wrote Souvik Sen, MD, MPH, a professor in the department of neurology at the University of South Carolina, Columbia, and his coauthors. “A randomized clinical trial may help ascertain whether patients with migraine with visual aura may benefit from atrial fibrillation detection and subsequent anticoagulation or antiplatelet therapy as a primary stroke prevention strategy.”
The study also showed a significant interaction with age and sex. While men who experienced migraine with aura had an 89% higher risk of atrial fibrillation, women with aura showed no increase in risk, compared with individuals who experienced no headache. Similarly, only individuals aged 60 years or older who experienced migraine with aura showed an increased risk of atrial fibrillation, while those younger than 60 years did not.
The authors noted that previous case reports have recorded the incidence of atrial fibrillation during a migraine attack. Autonomic dysfunction influences the pathophysiology of atrial fibrillation and migraine.
“Cardiac arrhythmia recordings have been shown to be present in ECGs of patients while experiencing migraine headaches as compared with migraine-free phases,” they wrote. “This hypothesis is further supported by atrial fibrillation ablation procedures that have shown tendencies to reduce migraine symptoms and frequencies.”
In regard to the role that migraine aura played in this, they speculated as to whether migraine aura could be the result of cardioembolic stroke that might have occurred because of the atrial fibrillation.
Overall, 167 patients had incident cardioembolic strokes, and researchers suggested strokes in 87% of these cases could be attributed to the atrial fibrillation that came before the stroke.
The stroke incidence rate also was around twice as high in individuals who experienced migraine with aura, compared with those who experienced migraine without aura (4.1 per 1,000 person-years vs. 2.07 per 1,000 person-years).
The study authors acknowledged that patent foramen ovale, which was not assessed in ARIC, is a possible confounder. Previous studies have showed that patent foramen ovale is more common in younger individuals with migraine and particularly in patients who experience migraine with aura.
However, they also noted that trials of patent foramen ovale closures as a treatment for migraine have not shown success in reducing migraine frequency and, therefore, argued against patent foramen ovale as being a major confounder.
The study was supported by the National Heart, Lung, and Blood Institute and the American Heart Association. One author declared grants from the National Institutes of health, one declared research support from Tian Medical, and one author is an associate editor for Neurology. No other conflicts of interest were declared.
SOURCE: Sen S et al. Neurology. 2018;91:1-9.
This article was updated 12/12/18.
FROM NEUROLOGY
Key clinical point: Aura in migraine is associated with an increased risk of atrial fibrillation.
Major finding: Individuals who experience migraine with aura have a 39% higher risk of atrial fibrillation than do those without aura or without migraine.
Study details: The longitudinal, community-based Atherosclerosis Risk in Communities Study in 11,939 individuals.
Disclosures: The study was supported by the National Heart, Lung, and Blood Institute and the American Heart Association. One author declared grants from the National Institutes of health, one declared research support from Tian Medical, and one author is an associate editor for Neurology. No other conflicts of interest were declared.
Source: Sen S et al. Neurology. 2018;91:1-9.
New pediatric therapies show promise for influenza, multidrug-resistant pathogens
ORLANDO – John S. Bradley, MD, said at the annual meeting of the American Academy of Pediatrics.
Dr. Bradley, director of the division of infectious diseases at Rady Children’s Hospital–San Diego, discussed a therapy for influenza, baloxavir, which was recently approved as a fast-acting single-dose medication and currently is under study in children. Also, a recent double-blind, phase 3 trial in the New England Journal of Medicine recruited patients as young as 12 years old. In the study, patients in the intervention group resolved their fever in median 25 hours, compared with 42 hours in the placebo group. Baloxavir better reduced viral load at day 2, compared with oseltamivir and placebo, but there was a similar alleviation of symptoms between both groups. There was a greater incidence of nausea and vomiting among the oseltamivir group, while the baloxavir group had a higher rate of diarrhea (N Engl J Med 2018;379:913-23).
However, Dr. Bradley noted baloxavir is much more expensive than oseltamivir, which may not justify the better tolerance of the drug for influenza treatment.
You don’t get better with it faster, so I’m not going to be recommending you all run to baloxavir this flu season for kids 12 years of age and older,” Dr. Bradley said. “I think oseltamivir is still fine, unless we end up with oseltamivir resistance.”
Solithromycin, an intravenous and oral fluoroketolide, has shown promising results against gram-positive and gram-negative pathogens for community-acquired pneumonia and other infections. During the drug’s study period, Cempra sold solithromycin to Melinta. However, one trial showed elevated liver functions in a higher number of patients than expected, and the Food and Drug Administration asked Melinta to conduct additional studies. Investigations on solithromycin have currently stopped until Melinta secures funding. “Until they get better resources, this particular drug is on hold, but you’ll see it again, I’m sure,” said Dr. Bradley, who also is professor and chief of the division of infectious diseases at the University of California, San Diego.
Dr. Bradley also discussed the efficacy of tedizolid, a protein synthesis inhibitor similar to linezolid approved in adults for the treatment of skin infections. He noted tedizolid is more active than linezolid, but the treatment course is a shorter dose for a shorter amount of time. Compared with linezolid, which can cause thrombocytopenia or neutropenia if taken for more than 10 days to 14 days, there also are fewer side effects.
“The tedizolid is much, much safer,” Dr. Bradley said, who added that trials for efficacy of tedizolid are currently underway in pediatric patients. “We’re hoping that will end up being the pediatric oxazolidinone.”
Other investigative therapies approved for adults and under study for use in children include ceftazidime/avibactam for treatment of urinary tract and complicated intra-abdominal infections, which is effective against meropenem-resistant Enterobacteriaceae and resistant Escherichia coli with extended-spectrum beta-lactamases (ESBL); ceftolozane/tazobactam has also been approved for adults, is pending approval in pediatric patients, and is active against ESBLs such as Pseudomonas; and meropenem/vaborbactam, which is active against Klebsiella pneumoniae carbapenemase (KPC)–producing isolates. Plazomicin, an aminoglycoside similar to gentamicin used to treat KPC-producing isolates, is stable against enzymes that degrade gentamicin and tobramycin.
Therapies currently under study for adults and being considered for children include imipenem/relebactam for treatment against E. coli, Enterobacter species, and KPC-producing isolates, and cefiderocol, a siderophore cephalosporin antibiotic – commonly described as a “Trojan horse” antibiotic because it binds to iron and is actively transported into the organism – is effective against Pseudomonas and has finished phase 2 trials in adults, with researchers looking to do single-dose trials in children, Dr. Bradley noted.
More experimentally, phage therapy for multidrug-resistant Acinetobacter baumannii proved effective in a 68-year-old patient with necrotizing pancreatitis who continued to deteriorate over a 4-month period despite multiple courses of antibiotics and attempted drainage of a pancreatic pseudocyst. Researchers selected a phage-specific bacterium with specificity for A. baumannii and cured him. “This is like science fiction,” Dr. Bradley said.
Dr. Bradley reported no relevant conflicts of interest.
ORLANDO – John S. Bradley, MD, said at the annual meeting of the American Academy of Pediatrics.
Dr. Bradley, director of the division of infectious diseases at Rady Children’s Hospital–San Diego, discussed a therapy for influenza, baloxavir, which was recently approved as a fast-acting single-dose medication and currently is under study in children. Also, a recent double-blind, phase 3 trial in the New England Journal of Medicine recruited patients as young as 12 years old. In the study, patients in the intervention group resolved their fever in median 25 hours, compared with 42 hours in the placebo group. Baloxavir better reduced viral load at day 2, compared with oseltamivir and placebo, but there was a similar alleviation of symptoms between both groups. There was a greater incidence of nausea and vomiting among the oseltamivir group, while the baloxavir group had a higher rate of diarrhea (N Engl J Med 2018;379:913-23).
However, Dr. Bradley noted baloxavir is much more expensive than oseltamivir, which may not justify the better tolerance of the drug for influenza treatment.
You don’t get better with it faster, so I’m not going to be recommending you all run to baloxavir this flu season for kids 12 years of age and older,” Dr. Bradley said. “I think oseltamivir is still fine, unless we end up with oseltamivir resistance.”
Solithromycin, an intravenous and oral fluoroketolide, has shown promising results against gram-positive and gram-negative pathogens for community-acquired pneumonia and other infections. During the drug’s study period, Cempra sold solithromycin to Melinta. However, one trial showed elevated liver functions in a higher number of patients than expected, and the Food and Drug Administration asked Melinta to conduct additional studies. Investigations on solithromycin have currently stopped until Melinta secures funding. “Until they get better resources, this particular drug is on hold, but you’ll see it again, I’m sure,” said Dr. Bradley, who also is professor and chief of the division of infectious diseases at the University of California, San Diego.
Dr. Bradley also discussed the efficacy of tedizolid, a protein synthesis inhibitor similar to linezolid approved in adults for the treatment of skin infections. He noted tedizolid is more active than linezolid, but the treatment course is a shorter dose for a shorter amount of time. Compared with linezolid, which can cause thrombocytopenia or neutropenia if taken for more than 10 days to 14 days, there also are fewer side effects.
“The tedizolid is much, much safer,” Dr. Bradley said, who added that trials for efficacy of tedizolid are currently underway in pediatric patients. “We’re hoping that will end up being the pediatric oxazolidinone.”
Other investigative therapies approved for adults and under study for use in children include ceftazidime/avibactam for treatment of urinary tract and complicated intra-abdominal infections, which is effective against meropenem-resistant Enterobacteriaceae and resistant Escherichia coli with extended-spectrum beta-lactamases (ESBL); ceftolozane/tazobactam has also been approved for adults, is pending approval in pediatric patients, and is active against ESBLs such as Pseudomonas; and meropenem/vaborbactam, which is active against Klebsiella pneumoniae carbapenemase (KPC)–producing isolates. Plazomicin, an aminoglycoside similar to gentamicin used to treat KPC-producing isolates, is stable against enzymes that degrade gentamicin and tobramycin.
Therapies currently under study for adults and being considered for children include imipenem/relebactam for treatment against E. coli, Enterobacter species, and KPC-producing isolates, and cefiderocol, a siderophore cephalosporin antibiotic – commonly described as a “Trojan horse” antibiotic because it binds to iron and is actively transported into the organism – is effective against Pseudomonas and has finished phase 2 trials in adults, with researchers looking to do single-dose trials in children, Dr. Bradley noted.
More experimentally, phage therapy for multidrug-resistant Acinetobacter baumannii proved effective in a 68-year-old patient with necrotizing pancreatitis who continued to deteriorate over a 4-month period despite multiple courses of antibiotics and attempted drainage of a pancreatic pseudocyst. Researchers selected a phage-specific bacterium with specificity for A. baumannii and cured him. “This is like science fiction,” Dr. Bradley said.
Dr. Bradley reported no relevant conflicts of interest.
ORLANDO – John S. Bradley, MD, said at the annual meeting of the American Academy of Pediatrics.
Dr. Bradley, director of the division of infectious diseases at Rady Children’s Hospital–San Diego, discussed a therapy for influenza, baloxavir, which was recently approved as a fast-acting single-dose medication and currently is under study in children. Also, a recent double-blind, phase 3 trial in the New England Journal of Medicine recruited patients as young as 12 years old. In the study, patients in the intervention group resolved their fever in median 25 hours, compared with 42 hours in the placebo group. Baloxavir better reduced viral load at day 2, compared with oseltamivir and placebo, but there was a similar alleviation of symptoms between both groups. There was a greater incidence of nausea and vomiting among the oseltamivir group, while the baloxavir group had a higher rate of diarrhea (N Engl J Med 2018;379:913-23).
However, Dr. Bradley noted baloxavir is much more expensive than oseltamivir, which may not justify the better tolerance of the drug for influenza treatment.
You don’t get better with it faster, so I’m not going to be recommending you all run to baloxavir this flu season for kids 12 years of age and older,” Dr. Bradley said. “I think oseltamivir is still fine, unless we end up with oseltamivir resistance.”
Solithromycin, an intravenous and oral fluoroketolide, has shown promising results against gram-positive and gram-negative pathogens for community-acquired pneumonia and other infections. During the drug’s study period, Cempra sold solithromycin to Melinta. However, one trial showed elevated liver functions in a higher number of patients than expected, and the Food and Drug Administration asked Melinta to conduct additional studies. Investigations on solithromycin have currently stopped until Melinta secures funding. “Until they get better resources, this particular drug is on hold, but you’ll see it again, I’m sure,” said Dr. Bradley, who also is professor and chief of the division of infectious diseases at the University of California, San Diego.
Dr. Bradley also discussed the efficacy of tedizolid, a protein synthesis inhibitor similar to linezolid approved in adults for the treatment of skin infections. He noted tedizolid is more active than linezolid, but the treatment course is a shorter dose for a shorter amount of time. Compared with linezolid, which can cause thrombocytopenia or neutropenia if taken for more than 10 days to 14 days, there also are fewer side effects.
“The tedizolid is much, much safer,” Dr. Bradley said, who added that trials for efficacy of tedizolid are currently underway in pediatric patients. “We’re hoping that will end up being the pediatric oxazolidinone.”
Other investigative therapies approved for adults and under study for use in children include ceftazidime/avibactam for treatment of urinary tract and complicated intra-abdominal infections, which is effective against meropenem-resistant Enterobacteriaceae and resistant Escherichia coli with extended-spectrum beta-lactamases (ESBL); ceftolozane/tazobactam has also been approved for adults, is pending approval in pediatric patients, and is active against ESBLs such as Pseudomonas; and meropenem/vaborbactam, which is active against Klebsiella pneumoniae carbapenemase (KPC)–producing isolates. Plazomicin, an aminoglycoside similar to gentamicin used to treat KPC-producing isolates, is stable against enzymes that degrade gentamicin and tobramycin.
Therapies currently under study for adults and being considered for children include imipenem/relebactam for treatment against E. coli, Enterobacter species, and KPC-producing isolates, and cefiderocol, a siderophore cephalosporin antibiotic – commonly described as a “Trojan horse” antibiotic because it binds to iron and is actively transported into the organism – is effective against Pseudomonas and has finished phase 2 trials in adults, with researchers looking to do single-dose trials in children, Dr. Bradley noted.
More experimentally, phage therapy for multidrug-resistant Acinetobacter baumannii proved effective in a 68-year-old patient with necrotizing pancreatitis who continued to deteriorate over a 4-month period despite multiple courses of antibiotics and attempted drainage of a pancreatic pseudocyst. Researchers selected a phage-specific bacterium with specificity for A. baumannii and cured him. “This is like science fiction,” Dr. Bradley said.
Dr. Bradley reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM AAP 18
Team approach needed to tackle complex care for fostered, adopted children
ORLANDO – Children in foster care or who are adopted are at a higher risk of developing medical conditions, and a team approach is needed when caring for these children with a focus on medical, developmental, and mental health, Judith K. Eckerle, MD, said at the annual meeting of the American Academy of Pediatrics.
“One of the things that I’m advocating more these days is [that] we don’t operate as physicians or medical people in a vacuum. We certainly have to operate within a team approach, and that really is the best approach for these kids,” said Dr. Eckerle, a pediatrician who specializes in neurobehavioral development and is director of the Adoption Medicine Clinic at the University of Minnesota, Minneapolis.
Recognizing how early adversity is associated with conditions such as growth, brain development, motor skills, cognition, mental health, attachment, stress sensitivity, sensory processing, language, and chromosome structure can help in the medical assessment of these patients. Dr. Eckerle noted that these issues can even present in children with no early adversity who are adopted or placed into healthy foster environments.
“What we’ve learned is, we’ve just seen dozens if not hundreds of kids now in the exact same scenario who are having exactly the same types of bonding, or attachment or stress issues, as kids who did spend those few years in a really neglectful or orphanage scenarios,” Dr. Eckerle said.
“The majority of adopted and foster care kids do great in the end,” she emphasized. “As a group, they can do beautifully, but we just have to be cognizant of the things that we should be looking for and not just gloss over them so we’re not missing things along the way.”
At her institution, Dr. Eckerle said a medical assessment for new foster care or adoption patients begins with explaining the exam process with the parent and child before the child is screened by an occupational therapist. During the screening, the occupational therapist will screen the child for signs of developmental delay such as delay in gross or fine motor skills, speech, cognition, musculoskeletal, psychosocial, and sensory processing, as well as in school-based, self-care, and functional skills.
While the child is out of the room, Dr. Eckerle will ask whether the parent has any concerns about the child. After that, she will check the child’s eyes, heart, and ears to make sure they are healthy; during the end of the exam, she may order lab tests based on any concerns about prenatal or foster care trauma. Adolescents and children in whom sexual abuse is suspected should be screened for hepatitis B, hepatitis C, syphilis, and HIV.
International adoptees should undergo ova and parasite screening and the Giardia antigen test. International adoptees as well as those who had contact with the homeless or prison systems should undergo QuantiFERON-TB Gold testing for tuberculosis, and children aged under 5 years should receive targeted tuberculin skin testing. She said her center also screens children for intellectual disability, fetal alcohol spectrum disorder, short stature, and precocious puberty.
Lastly, the child is seen by a pediatric psychologist for additional screening. Dr. Eckerle noted the most common diagnoses seen at her center are reactive attachment disorder, oppositional defiant disorder, conduct disorder, pervasive development delay, autism, learning disabilities, emotional or behavioral problems, and ADHD. With regard to mental health, doctors should help a caregiver understand a child with a mental health conditions and consider pairing with a pediatric psychologist to offer evidence-based interventions for children aged under 5 years, such as child-parent psychotherapy, circle of security, and attachment biobehavioral catch-up, and offer trauma-focused cognitive behavioral therapy in children aged between 4 and 5 years.
Bad behavior often is misinterpreted as dishonesty and willful misconduct on the part of the child, but instead could be a neurodevelopmental or mental health problem, Dr. Eckerle noted. For example, a parent might interpret a child who takes a sparkling watch off of a teacher’s desk as stealing, but the child may not yet understand the concept of ownership. “Reframing these things will lead to the goal of helping that parent understand the child.”
Above all, Dr. Eckerle said she emphasizes what will happen after the exam with children she sees to establish a routine, as many children she sees have gone through multiple foster environments. It also is important to let parents know the exam is just a first step and they likely will not receive a diagnosis that day, particularly with out-of-state or international families. “I want them to have appropriate expectations that we’re not going to cure or solve or fix everything the second that they leave that room, but that we are starting the process and we are on the right track.”
Dr. Eckerle reported no conflicts of interest.
ORLANDO – Children in foster care or who are adopted are at a higher risk of developing medical conditions, and a team approach is needed when caring for these children with a focus on medical, developmental, and mental health, Judith K. Eckerle, MD, said at the annual meeting of the American Academy of Pediatrics.
“One of the things that I’m advocating more these days is [that] we don’t operate as physicians or medical people in a vacuum. We certainly have to operate within a team approach, and that really is the best approach for these kids,” said Dr. Eckerle, a pediatrician who specializes in neurobehavioral development and is director of the Adoption Medicine Clinic at the University of Minnesota, Minneapolis.
Recognizing how early adversity is associated with conditions such as growth, brain development, motor skills, cognition, mental health, attachment, stress sensitivity, sensory processing, language, and chromosome structure can help in the medical assessment of these patients. Dr. Eckerle noted that these issues can even present in children with no early adversity who are adopted or placed into healthy foster environments.
“What we’ve learned is, we’ve just seen dozens if not hundreds of kids now in the exact same scenario who are having exactly the same types of bonding, or attachment or stress issues, as kids who did spend those few years in a really neglectful or orphanage scenarios,” Dr. Eckerle said.
“The majority of adopted and foster care kids do great in the end,” she emphasized. “As a group, they can do beautifully, but we just have to be cognizant of the things that we should be looking for and not just gloss over them so we’re not missing things along the way.”
At her institution, Dr. Eckerle said a medical assessment for new foster care or adoption patients begins with explaining the exam process with the parent and child before the child is screened by an occupational therapist. During the screening, the occupational therapist will screen the child for signs of developmental delay such as delay in gross or fine motor skills, speech, cognition, musculoskeletal, psychosocial, and sensory processing, as well as in school-based, self-care, and functional skills.
While the child is out of the room, Dr. Eckerle will ask whether the parent has any concerns about the child. After that, she will check the child’s eyes, heart, and ears to make sure they are healthy; during the end of the exam, she may order lab tests based on any concerns about prenatal or foster care trauma. Adolescents and children in whom sexual abuse is suspected should be screened for hepatitis B, hepatitis C, syphilis, and HIV.
International adoptees should undergo ova and parasite screening and the Giardia antigen test. International adoptees as well as those who had contact with the homeless or prison systems should undergo QuantiFERON-TB Gold testing for tuberculosis, and children aged under 5 years should receive targeted tuberculin skin testing. She said her center also screens children for intellectual disability, fetal alcohol spectrum disorder, short stature, and precocious puberty.
Lastly, the child is seen by a pediatric psychologist for additional screening. Dr. Eckerle noted the most common diagnoses seen at her center are reactive attachment disorder, oppositional defiant disorder, conduct disorder, pervasive development delay, autism, learning disabilities, emotional or behavioral problems, and ADHD. With regard to mental health, doctors should help a caregiver understand a child with a mental health conditions and consider pairing with a pediatric psychologist to offer evidence-based interventions for children aged under 5 years, such as child-parent psychotherapy, circle of security, and attachment biobehavioral catch-up, and offer trauma-focused cognitive behavioral therapy in children aged between 4 and 5 years.
Bad behavior often is misinterpreted as dishonesty and willful misconduct on the part of the child, but instead could be a neurodevelopmental or mental health problem, Dr. Eckerle noted. For example, a parent might interpret a child who takes a sparkling watch off of a teacher’s desk as stealing, but the child may not yet understand the concept of ownership. “Reframing these things will lead to the goal of helping that parent understand the child.”
Above all, Dr. Eckerle said she emphasizes what will happen after the exam with children she sees to establish a routine, as many children she sees have gone through multiple foster environments. It also is important to let parents know the exam is just a first step and they likely will not receive a diagnosis that day, particularly with out-of-state or international families. “I want them to have appropriate expectations that we’re not going to cure or solve or fix everything the second that they leave that room, but that we are starting the process and we are on the right track.”
Dr. Eckerle reported no conflicts of interest.
ORLANDO – Children in foster care or who are adopted are at a higher risk of developing medical conditions, and a team approach is needed when caring for these children with a focus on medical, developmental, and mental health, Judith K. Eckerle, MD, said at the annual meeting of the American Academy of Pediatrics.
“One of the things that I’m advocating more these days is [that] we don’t operate as physicians or medical people in a vacuum. We certainly have to operate within a team approach, and that really is the best approach for these kids,” said Dr. Eckerle, a pediatrician who specializes in neurobehavioral development and is director of the Adoption Medicine Clinic at the University of Minnesota, Minneapolis.
Recognizing how early adversity is associated with conditions such as growth, brain development, motor skills, cognition, mental health, attachment, stress sensitivity, sensory processing, language, and chromosome structure can help in the medical assessment of these patients. Dr. Eckerle noted that these issues can even present in children with no early adversity who are adopted or placed into healthy foster environments.
“What we’ve learned is, we’ve just seen dozens if not hundreds of kids now in the exact same scenario who are having exactly the same types of bonding, or attachment or stress issues, as kids who did spend those few years in a really neglectful or orphanage scenarios,” Dr. Eckerle said.
“The majority of adopted and foster care kids do great in the end,” she emphasized. “As a group, they can do beautifully, but we just have to be cognizant of the things that we should be looking for and not just gloss over them so we’re not missing things along the way.”
At her institution, Dr. Eckerle said a medical assessment for new foster care or adoption patients begins with explaining the exam process with the parent and child before the child is screened by an occupational therapist. During the screening, the occupational therapist will screen the child for signs of developmental delay such as delay in gross or fine motor skills, speech, cognition, musculoskeletal, psychosocial, and sensory processing, as well as in school-based, self-care, and functional skills.
While the child is out of the room, Dr. Eckerle will ask whether the parent has any concerns about the child. After that, she will check the child’s eyes, heart, and ears to make sure they are healthy; during the end of the exam, she may order lab tests based on any concerns about prenatal or foster care trauma. Adolescents and children in whom sexual abuse is suspected should be screened for hepatitis B, hepatitis C, syphilis, and HIV.
International adoptees should undergo ova and parasite screening and the Giardia antigen test. International adoptees as well as those who had contact with the homeless or prison systems should undergo QuantiFERON-TB Gold testing for tuberculosis, and children aged under 5 years should receive targeted tuberculin skin testing. She said her center also screens children for intellectual disability, fetal alcohol spectrum disorder, short stature, and precocious puberty.
Lastly, the child is seen by a pediatric psychologist for additional screening. Dr. Eckerle noted the most common diagnoses seen at her center are reactive attachment disorder, oppositional defiant disorder, conduct disorder, pervasive development delay, autism, learning disabilities, emotional or behavioral problems, and ADHD. With regard to mental health, doctors should help a caregiver understand a child with a mental health conditions and consider pairing with a pediatric psychologist to offer evidence-based interventions for children aged under 5 years, such as child-parent psychotherapy, circle of security, and attachment biobehavioral catch-up, and offer trauma-focused cognitive behavioral therapy in children aged between 4 and 5 years.
Bad behavior often is misinterpreted as dishonesty and willful misconduct on the part of the child, but instead could be a neurodevelopmental or mental health problem, Dr. Eckerle noted. For example, a parent might interpret a child who takes a sparkling watch off of a teacher’s desk as stealing, but the child may not yet understand the concept of ownership. “Reframing these things will lead to the goal of helping that parent understand the child.”
Above all, Dr. Eckerle said she emphasizes what will happen after the exam with children she sees to establish a routine, as many children she sees have gone through multiple foster environments. It also is important to let parents know the exam is just a first step and they likely will not receive a diagnosis that day, particularly with out-of-state or international families. “I want them to have appropriate expectations that we’re not going to cure or solve or fix everything the second that they leave that room, but that we are starting the process and we are on the right track.”
Dr. Eckerle reported no conflicts of interest.
EXPERT ANALYSIS FROM AAP 18
Children with headache disorders may benefit from anti-CGRP mAb treatment
Use of anti–calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) may benefit children with more than 8 headache days each month, a high Pediatric Migraine Disability Assessment (PedMIDAS) score, and failure of other treatments; however, researchers cautioned that long-term safety outcomes for the treatment are not yet known, according to a recent set of recommendations published in the journal Headache.
Christina L. Szperka, MD, MSCE, of the division of neurology at the Children’s Hospital of Philadelphia and members of the Pediatric and Adolescent Headache special interest group of the American Headache Society discussed the topic of anti-CGRP mAbs at the 2018 Annual Scientific Meeting of the American Headache Society. They noted clinical outcomes for anti-CGRP mAbs in pediatric patients will likely not be available for several years and created a set of recommendations based on expert opinion of anti-CGRP mAb use in children and adolescents.
Their recommendations support using anti-CGRP mAbs for children with migraine if patients meet the following criteria: headache frequency exceeding 8 headache days per month; a PedMIDAS score of 30 or greater; failure of two or more therapies, such as pharmacologic, nonpharmacologic, or nutraceutical ones; and in patients who are past puberty.
The special interest group recommended against use of anti-CGRP mAbs in children and adolescents with recent meningitis, recent peripheral nerve injury, neurosurgery, or a central nervous system injury caused by a potentially compromised blood-brain barrier. Children and adolescents with immunodeficiency, receiving immunosuppressive medications, with structural heart defects, with pulmonary hypertension, with coronary artery disease, with cardiomyopathy, or at risk for stroke should also avoid use of anti-CGRP mAbs. Anti-CGRP mAbs are also potentially teratogenic and should not be used by adolescents or women who are pregnant, breastfeeding, or have a pregnancy wish.
Pediatric patients with significant osteoporosis or bone disease should be monitored when prescribed anti-CGRP mAbs, and the recommendations specified monitoring height and linear growth or waiting until after puberty to prescribe anti-CGRP mAbs. Although there is currently no evidence that use of anti-CGRP mAb requires pituitary hormone monitoring, the recommendations noted that weight and body mass index should also be observed.
“Pediatric and adolescent trials of anti-CGRP mAbs should be designed to maximize the chances of determining efficacy in these age groups and should focus on those who have not been successful with current multidisciplinary care,” Dr. Szperka and her colleagues wrote in the recommendations. “In the interim, the use of anti-CGRP mAbs for the treatment of headache disorders in children and adolescents may be considered in appropriate cases but should be done with close follow-up and attention to patient characteristics such as age, pubertal state, and medical comorbidities.”
Dr. Szperka receives grant support from Pfizer and Amgen and research funding from NIH. Other authors have reported grants, consulting fees, speaking fees, royalties, advisory board memberships, speaker’s bureau memberships and travel funds from Alder, Allergan, American Academy of Neurology, Amgen, Aralez, Avanir, Autonomic Technologies Inc., Biohaven, Cambridge University Press, Curelator, Depomed, Dr. Reddy’s Laboratories, Electrocore, eNeura, Genentech, Healint, Impax, JAMA Neurology, Journal Watch, Lilly, Massachusetts Medical Society, MedDay, MedicoLegal, Merck, NIH, Novartis, Oxford University Press, Quest Diagnostics, Scion, Supernus, Teva, Trigemina Inc., Upsher-Smith, UpToDate, Wolters Kluwer and Zosano.
SOURCE: Szperka CL et al. Headache. 2018. doi: 10.1111/head.13414.
Use of anti–calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) may benefit children with more than 8 headache days each month, a high Pediatric Migraine Disability Assessment (PedMIDAS) score, and failure of other treatments; however, researchers cautioned that long-term safety outcomes for the treatment are not yet known, according to a recent set of recommendations published in the journal Headache.
Christina L. Szperka, MD, MSCE, of the division of neurology at the Children’s Hospital of Philadelphia and members of the Pediatric and Adolescent Headache special interest group of the American Headache Society discussed the topic of anti-CGRP mAbs at the 2018 Annual Scientific Meeting of the American Headache Society. They noted clinical outcomes for anti-CGRP mAbs in pediatric patients will likely not be available for several years and created a set of recommendations based on expert opinion of anti-CGRP mAb use in children and adolescents.
Their recommendations support using anti-CGRP mAbs for children with migraine if patients meet the following criteria: headache frequency exceeding 8 headache days per month; a PedMIDAS score of 30 or greater; failure of two or more therapies, such as pharmacologic, nonpharmacologic, or nutraceutical ones; and in patients who are past puberty.
The special interest group recommended against use of anti-CGRP mAbs in children and adolescents with recent meningitis, recent peripheral nerve injury, neurosurgery, or a central nervous system injury caused by a potentially compromised blood-brain barrier. Children and adolescents with immunodeficiency, receiving immunosuppressive medications, with structural heart defects, with pulmonary hypertension, with coronary artery disease, with cardiomyopathy, or at risk for stroke should also avoid use of anti-CGRP mAbs. Anti-CGRP mAbs are also potentially teratogenic and should not be used by adolescents or women who are pregnant, breastfeeding, or have a pregnancy wish.
Pediatric patients with significant osteoporosis or bone disease should be monitored when prescribed anti-CGRP mAbs, and the recommendations specified monitoring height and linear growth or waiting until after puberty to prescribe anti-CGRP mAbs. Although there is currently no evidence that use of anti-CGRP mAb requires pituitary hormone monitoring, the recommendations noted that weight and body mass index should also be observed.
“Pediatric and adolescent trials of anti-CGRP mAbs should be designed to maximize the chances of determining efficacy in these age groups and should focus on those who have not been successful with current multidisciplinary care,” Dr. Szperka and her colleagues wrote in the recommendations. “In the interim, the use of anti-CGRP mAbs for the treatment of headache disorders in children and adolescents may be considered in appropriate cases but should be done with close follow-up and attention to patient characteristics such as age, pubertal state, and medical comorbidities.”
Dr. Szperka receives grant support from Pfizer and Amgen and research funding from NIH. Other authors have reported grants, consulting fees, speaking fees, royalties, advisory board memberships, speaker’s bureau memberships and travel funds from Alder, Allergan, American Academy of Neurology, Amgen, Aralez, Avanir, Autonomic Technologies Inc., Biohaven, Cambridge University Press, Curelator, Depomed, Dr. Reddy’s Laboratories, Electrocore, eNeura, Genentech, Healint, Impax, JAMA Neurology, Journal Watch, Lilly, Massachusetts Medical Society, MedDay, MedicoLegal, Merck, NIH, Novartis, Oxford University Press, Quest Diagnostics, Scion, Supernus, Teva, Trigemina Inc., Upsher-Smith, UpToDate, Wolters Kluwer and Zosano.
SOURCE: Szperka CL et al. Headache. 2018. doi: 10.1111/head.13414.
Use of anti–calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) may benefit children with more than 8 headache days each month, a high Pediatric Migraine Disability Assessment (PedMIDAS) score, and failure of other treatments; however, researchers cautioned that long-term safety outcomes for the treatment are not yet known, according to a recent set of recommendations published in the journal Headache.
Christina L. Szperka, MD, MSCE, of the division of neurology at the Children’s Hospital of Philadelphia and members of the Pediatric and Adolescent Headache special interest group of the American Headache Society discussed the topic of anti-CGRP mAbs at the 2018 Annual Scientific Meeting of the American Headache Society. They noted clinical outcomes for anti-CGRP mAbs in pediatric patients will likely not be available for several years and created a set of recommendations based on expert opinion of anti-CGRP mAb use in children and adolescents.
Their recommendations support using anti-CGRP mAbs for children with migraine if patients meet the following criteria: headache frequency exceeding 8 headache days per month; a PedMIDAS score of 30 or greater; failure of two or more therapies, such as pharmacologic, nonpharmacologic, or nutraceutical ones; and in patients who are past puberty.
The special interest group recommended against use of anti-CGRP mAbs in children and adolescents with recent meningitis, recent peripheral nerve injury, neurosurgery, or a central nervous system injury caused by a potentially compromised blood-brain barrier. Children and adolescents with immunodeficiency, receiving immunosuppressive medications, with structural heart defects, with pulmonary hypertension, with coronary artery disease, with cardiomyopathy, or at risk for stroke should also avoid use of anti-CGRP mAbs. Anti-CGRP mAbs are also potentially teratogenic and should not be used by adolescents or women who are pregnant, breastfeeding, or have a pregnancy wish.
Pediatric patients with significant osteoporosis or bone disease should be monitored when prescribed anti-CGRP mAbs, and the recommendations specified monitoring height and linear growth or waiting until after puberty to prescribe anti-CGRP mAbs. Although there is currently no evidence that use of anti-CGRP mAb requires pituitary hormone monitoring, the recommendations noted that weight and body mass index should also be observed.
“Pediatric and adolescent trials of anti-CGRP mAbs should be designed to maximize the chances of determining efficacy in these age groups and should focus on those who have not been successful with current multidisciplinary care,” Dr. Szperka and her colleagues wrote in the recommendations. “In the interim, the use of anti-CGRP mAbs for the treatment of headache disorders in children and adolescents may be considered in appropriate cases but should be done with close follow-up and attention to patient characteristics such as age, pubertal state, and medical comorbidities.”
Dr. Szperka receives grant support from Pfizer and Amgen and research funding from NIH. Other authors have reported grants, consulting fees, speaking fees, royalties, advisory board memberships, speaker’s bureau memberships and travel funds from Alder, Allergan, American Academy of Neurology, Amgen, Aralez, Avanir, Autonomic Technologies Inc., Biohaven, Cambridge University Press, Curelator, Depomed, Dr. Reddy’s Laboratories, Electrocore, eNeura, Genentech, Healint, Impax, JAMA Neurology, Journal Watch, Lilly, Massachusetts Medical Society, MedDay, MedicoLegal, Merck, NIH, Novartis, Oxford University Press, Quest Diagnostics, Scion, Supernus, Teva, Trigemina Inc., Upsher-Smith, UpToDate, Wolters Kluwer and Zosano.
SOURCE: Szperka CL et al. Headache. 2018. doi: 10.1111/head.13414.
FROM HEADACHE
Key clinical point: Treatment of headache disorders with anti–calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) in children and adolescents may be indicated in some cases.
Major finding: Pediatric patients with more than eight headache days per month, PedMIDAS score of 30 or higher, and failure of other pharmacological, nonpharmacological, and nutraceutical treatments may benefit from anti-CGRP mAb treatment.
Study details: Expert opinion from the members of the Pediatric and Adolescent Headache special interest group based on recommendations made at the 2018 Annual Scientific Meeting of the American Headache Society.
Disclosures: Dr. Szperka receives grant support from Pfizer and Amgen and research funding from NIH. Other authors have reported grants, consulting fees, speaking fees, royalties, advisory board memberships, speaker’s bureau memberships, and travel funds from Alder, Allergan, American Academy of Neurology, Amgen, Aralez, Avanir, Autonomic Technologies, Biohaven, Cambridge University Press, Curelator, Depomed, Dr. Reddy’s Laboratories, Electrocore, eNeura, Genentech, Healint, Impax, JAMA Neurology, Journal Watch, Lilly, Massachusetts Medical Society, MedDay, MedicoLegal, Merck, NIH, Novartis, Oxford University Press, Quest Diagnostics, Scion, Supernus, Teva, Trigemina, Upsher-Smith, UpToDate, Wolters Kluwer, and Zosano.
Source: Szperka CL et al. Headache. 2018. doi: 10.1111/head.13414.
Risk of cancer in NAFLD is 91% higher than in control subjects
SAN FRANCISCO –
Those are key findings from a community cohort study with up to 21 years of follow-up, which one of the authors, Alina M. Allen, MD, discussed during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD is the most common chronic liver disease in the Western world,” said Dr. Allen, a gastroenterologist at the Mayo Clinic, Rochester, Minn. “It affects one in four adults in the U.S. It is related to fat accumulation in the liver in people who are overweight or obese and can lead to cirrhosis and liver-related mortality. However, the most common cause of death in this population is not liver disease but malignancy and cardiovascular disease. There is a paucity of epidemiologic studies of extrahepatic cancer in NAFLD. It is not clear what types of cancers and how much higher their cancer risk is in reference to the general population.”
In an effort to determine the incidence of cancer diagnoses in NALFD, compared with controls, in a U.S. community, Dr. Allen and her colleagues drew from the Rochester Epidemiology Project to evaluate 4,791 adults diagnosed with NAFLD and 14,432 age- and sex-matched control subjects in Olmstead County, Minn., during 1997-2017. The researchers obtained corresponding Surveillance, Epidemiology, and End Results Program (SEER) rates as a quality check and used a regression model to assess the malignancy risk in NAFLD overall and by cancer type, age, sex, and body mass index. They recorded all new diagnoses of cancers that developed in both groups until 2018, for a total possible follow-up of 21 years, and they reported results in incidence rate ratios, a risk estimate similar to hazard ratios.
The mean age of the study population was 53 years, 53% were female, and the mean follow-up was 8 years with a range from 1 to 21 years. New cancers were identified in 16% of subjects with NALFD and in 12% of control subjects. The overall risk of malignancy was 91% higher in NAFLD subjects, compared with control subjects; there were higher rates in the NAFLD subjects for most types of cancers, but the largest increases were in GI cancers. The greatest malignancy risk was for cancer of the liver (RR, 3.24), followed by cancer of the uterus (RR, 2.39), stomach (RR, 2.34), pancreas (RR, 2.09), and colon (RR, 1.75). “Interestingly, the risk of colon cancer increased only in men but not in women,” Dr. Allen said. “These data provide an important hierarchical overview of the top most important malignancy risks associated with NAFLD that the medical community should be aware of.”
When the researchers looked for differences in age at cancer diagnosis between NAFLD and controls, they found that pancreas cancer occurred at a younger age among subjects with NAFLD. They also observed that colon cancer occurred at a younger age in men with NAFLD, but not in women with the disease. “What was most interesting to us was the assessment of cancer risk in NAFLD versus obesity alone,” Dr. Allen said. “Previous studies from the general population have linked obesity to a higher risk of cancer. Whether the presence of fatty liver disease would impact that risk has not been assessed. We showed that obesity is associated with a higher risk of cancer only in those with NAFLD, not in those without. If validated in independent cohorts, these findings could change our understanding of the relationship between obesity and cancer and the importance of screening for NAFLD – not only to risk-stratify liver disease but also for the risk of extrahepatic malignancy.”
Dr. Allen concluded her presentation by noting that findings from large population-based studies such as the Rochester Epidemiology Project “can offer important epidemiologic data regarding the biggest threats to the health of a community,” she said. “Such data increase awareness, enable appropriate counseling, and could inform screening policies. There is a signal in the fact that the GI cancers are increased [in NAFLD]. It’s an interesting signal that needs to be studied further.”
Dr. Allen reported having no financial conflicts.
Source: Allen AM. Hepatol. 2018;68[S1]: Abstract 31.
SAN FRANCISCO –
Those are key findings from a community cohort study with up to 21 years of follow-up, which one of the authors, Alina M. Allen, MD, discussed during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD is the most common chronic liver disease in the Western world,” said Dr. Allen, a gastroenterologist at the Mayo Clinic, Rochester, Minn. “It affects one in four adults in the U.S. It is related to fat accumulation in the liver in people who are overweight or obese and can lead to cirrhosis and liver-related mortality. However, the most common cause of death in this population is not liver disease but malignancy and cardiovascular disease. There is a paucity of epidemiologic studies of extrahepatic cancer in NAFLD. It is not clear what types of cancers and how much higher their cancer risk is in reference to the general population.”
In an effort to determine the incidence of cancer diagnoses in NALFD, compared with controls, in a U.S. community, Dr. Allen and her colleagues drew from the Rochester Epidemiology Project to evaluate 4,791 adults diagnosed with NAFLD and 14,432 age- and sex-matched control subjects in Olmstead County, Minn., during 1997-2017. The researchers obtained corresponding Surveillance, Epidemiology, and End Results Program (SEER) rates as a quality check and used a regression model to assess the malignancy risk in NAFLD overall and by cancer type, age, sex, and body mass index. They recorded all new diagnoses of cancers that developed in both groups until 2018, for a total possible follow-up of 21 years, and they reported results in incidence rate ratios, a risk estimate similar to hazard ratios.
The mean age of the study population was 53 years, 53% were female, and the mean follow-up was 8 years with a range from 1 to 21 years. New cancers were identified in 16% of subjects with NALFD and in 12% of control subjects. The overall risk of malignancy was 91% higher in NAFLD subjects, compared with control subjects; there were higher rates in the NAFLD subjects for most types of cancers, but the largest increases were in GI cancers. The greatest malignancy risk was for cancer of the liver (RR, 3.24), followed by cancer of the uterus (RR, 2.39), stomach (RR, 2.34), pancreas (RR, 2.09), and colon (RR, 1.75). “Interestingly, the risk of colon cancer increased only in men but not in women,” Dr. Allen said. “These data provide an important hierarchical overview of the top most important malignancy risks associated with NAFLD that the medical community should be aware of.”
When the researchers looked for differences in age at cancer diagnosis between NAFLD and controls, they found that pancreas cancer occurred at a younger age among subjects with NAFLD. They also observed that colon cancer occurred at a younger age in men with NAFLD, but not in women with the disease. “What was most interesting to us was the assessment of cancer risk in NAFLD versus obesity alone,” Dr. Allen said. “Previous studies from the general population have linked obesity to a higher risk of cancer. Whether the presence of fatty liver disease would impact that risk has not been assessed. We showed that obesity is associated with a higher risk of cancer only in those with NAFLD, not in those without. If validated in independent cohorts, these findings could change our understanding of the relationship between obesity and cancer and the importance of screening for NAFLD – not only to risk-stratify liver disease but also for the risk of extrahepatic malignancy.”
Dr. Allen concluded her presentation by noting that findings from large population-based studies such as the Rochester Epidemiology Project “can offer important epidemiologic data regarding the biggest threats to the health of a community,” she said. “Such data increase awareness, enable appropriate counseling, and could inform screening policies. There is a signal in the fact that the GI cancers are increased [in NAFLD]. It’s an interesting signal that needs to be studied further.”
Dr. Allen reported having no financial conflicts.
Source: Allen AM. Hepatol. 2018;68[S1]: Abstract 31.
SAN FRANCISCO –
Those are key findings from a community cohort study with up to 21 years of follow-up, which one of the authors, Alina M. Allen, MD, discussed during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD is the most common chronic liver disease in the Western world,” said Dr. Allen, a gastroenterologist at the Mayo Clinic, Rochester, Minn. “It affects one in four adults in the U.S. It is related to fat accumulation in the liver in people who are overweight or obese and can lead to cirrhosis and liver-related mortality. However, the most common cause of death in this population is not liver disease but malignancy and cardiovascular disease. There is a paucity of epidemiologic studies of extrahepatic cancer in NAFLD. It is not clear what types of cancers and how much higher their cancer risk is in reference to the general population.”
In an effort to determine the incidence of cancer diagnoses in NALFD, compared with controls, in a U.S. community, Dr. Allen and her colleagues drew from the Rochester Epidemiology Project to evaluate 4,791 adults diagnosed with NAFLD and 14,432 age- and sex-matched control subjects in Olmstead County, Minn., during 1997-2017. The researchers obtained corresponding Surveillance, Epidemiology, and End Results Program (SEER) rates as a quality check and used a regression model to assess the malignancy risk in NAFLD overall and by cancer type, age, sex, and body mass index. They recorded all new diagnoses of cancers that developed in both groups until 2018, for a total possible follow-up of 21 years, and they reported results in incidence rate ratios, a risk estimate similar to hazard ratios.
The mean age of the study population was 53 years, 53% were female, and the mean follow-up was 8 years with a range from 1 to 21 years. New cancers were identified in 16% of subjects with NALFD and in 12% of control subjects. The overall risk of malignancy was 91% higher in NAFLD subjects, compared with control subjects; there were higher rates in the NAFLD subjects for most types of cancers, but the largest increases were in GI cancers. The greatest malignancy risk was for cancer of the liver (RR, 3.24), followed by cancer of the uterus (RR, 2.39), stomach (RR, 2.34), pancreas (RR, 2.09), and colon (RR, 1.75). “Interestingly, the risk of colon cancer increased only in men but not in women,” Dr. Allen said. “These data provide an important hierarchical overview of the top most important malignancy risks associated with NAFLD that the medical community should be aware of.”
When the researchers looked for differences in age at cancer diagnosis between NAFLD and controls, they found that pancreas cancer occurred at a younger age among subjects with NAFLD. They also observed that colon cancer occurred at a younger age in men with NAFLD, but not in women with the disease. “What was most interesting to us was the assessment of cancer risk in NAFLD versus obesity alone,” Dr. Allen said. “Previous studies from the general population have linked obesity to a higher risk of cancer. Whether the presence of fatty liver disease would impact that risk has not been assessed. We showed that obesity is associated with a higher risk of cancer only in those with NAFLD, not in those without. If validated in independent cohorts, these findings could change our understanding of the relationship between obesity and cancer and the importance of screening for NAFLD – not only to risk-stratify liver disease but also for the risk of extrahepatic malignancy.”
Dr. Allen concluded her presentation by noting that findings from large population-based studies such as the Rochester Epidemiology Project “can offer important epidemiologic data regarding the biggest threats to the health of a community,” she said. “Such data increase awareness, enable appropriate counseling, and could inform screening policies. There is a signal in the fact that the GI cancers are increased [in NAFLD]. It’s an interesting signal that needs to be studied further.”
Dr. Allen reported having no financial conflicts.
Source: Allen AM. Hepatol. 2018;68[S1]: Abstract 31.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: The risk of cancer in patients with nonalcoholic fatty liver disease is 91% higher than in control subjects.
Major finding: Among subjects with NAFLD, the greatest malignancy risk was for cancer of the liver (risk ratio, 3.24).
Study details: A cohort study of 4,791 adults diagnosed with NAFLD and 14,432 age- and sex-matched control subjects from the Rochester Epidemiology Project.
Disclosures: Dr. Allen reported having no financial conflicts.
Source: Allen AM. Hepatol. 2018;68[S1]:Abstract 31