User login
Infectious Arthritis of Native and Prosthetic Joints
Introduction
Acute bacterial arthritis is a potentially serious and rapidly progressive infection that may involve native or prosthetic joints. The epidemiology, pathophysiology, repertoire of potential infecting pathogens, clinical presentation and treatment differ for these two forms of infectious arthritis, but both are associated with significant morbidity and mortality. Infectious arthritis of native and prosthetic joints may be caused by viruses, or fungi, but the most common cause is bacteria.
Acute Bacterial Arthritis
Epidemiology
The burden of septic arthritis in the general population is considerable. The incidence of native joint septic arthritis is approximately 5 cases per 100,000 persons per year and is much higher in patients with rheumatoid arthritis (1,2). Between 1% and 5% of joints with indwelling prostheses become infected and the total number of infections per year is increasing due to a rise in the number of patients who have had prosthetic replacement surgery (3). The mortality from joint infection is difficult to estimate due to differing comorbidity in afflicted patients, but is likely between 15% and 30% (4-6). There is substantial morbidity from these infections because of decreased joint function and mobility, and in cases involving joint prostheses from the excisional or exchange arthroplastic surgery that is often required for treatment.
The most common route of infection for native joint infection is hematogenous (1), but may also be a result of direct inoculation of bacteria through trauma or joint surgery (including arthrocentesis, corticosteroid injection, or arthroscopy) (7), or via contiguous spread from adjacent infected soft tissue or bone (1,8). While hematogenous infection of prosthetic joints occurs, the majority of these infections are the result of joint contamination in the course of implantation surgery or post-surgical wound infection (3). Host factors that increase the risk of septic arthritis include pre-existing joint disease (especially rheumatoid arthritis), immunosuppression, diabetes mellitus, malignancy, chronic renal failure, intravenous drug use, severe skin diseases, and advanced age (1,2,4,6). The extent of joint injury resulting from infection depends on the virulence of the infecting pathogen and degree of host immune response (9).
Microbiology
Native Joint
The most common causes of bacterial septic arthritis are outlined in Table 1. In adults, the most frequent etiology is S. aureus (37–65% of cases) (1,4,6,8,12,15,16) followed by Streptococcus sp. (12,15). An increasing percentage of S. aureus isolated from septic joints are resistant to antistaphylococcal penicillins and cephalosporins (methicillin-resistant S. aureus, MRSA). In adults with diabetes, malignancy, and genitourinary structural abnormalities, group B Streptococcus is a frequently isolated pathogen (5,6,17). Gram-negative bacilli are commonly found in neonates, intravenous drug users, and immunocompromised hosts (18). N. gonorrhoeae is a significant cause of bacterial arthritis in sexually active adults and adolescents (19) and Kingella kingae and Haemophilus influenzae are likely pediatric isolates (20,21). Joint infections that follow bite trauma usually are seen in the small joints of the hand and involve Pasteurella multocida in the case of animal bites, and Eikenella corrodens in the case of humans bites (22-24). Polymicrobial floras are found in up to 8% of cases of septic arthritis.
Prosthetic Joint
The bacteria that cause prosthetic joint arthritis vary depending on the stage of infection as defined by the elapsed time after implantation surgery (Table 1 on page 31). The coagulase negative staphylococci are the most common (30–43% of cases) (3,10), followed by S. aureus (12–23%) (25).
Nonbacterial Pathogens
Nonbacterial pathogens that may cause septic arthritis include viruses, fungi, and mycobacteria. Viral arthritis is often associated with a systemic febrile illness and other manifestations of infection such as rash. Parvovirus B19 is the most common viral arthritide, presenting as a symmetric polyarticular arthritis involving the joints of the hand as well as larger joints (26). The classic red “slapped cheeks” associated with this viral infection in children is usually not present in adults, although a faint lacy reticular rash may be seen.
Fungi and mycobacteria usually cause a subacute or chronic mono- or oligoarticular arthritis (27). Candida species are an increasing cause of both native and prosthetic joint septic arthritis. Risk factors for this infection include loss of skin integrity, diabetes, malignancy, intravenous drug use, and immunosuppressive therapy including glucocorticoids (28). Patients are often chronically ill and have exposure to broad-spectrum antimicrobials, hyperalimentation fluid, and/or indwelling central intravenous catheters. Other fungi, including Cryptococcus, Blastomyces, Histoplasma, Coccidioides, and Sporothrix are rare causes of septic arthritis (29,30). Mycobacterium tuberculosis is the most common cause of mycobacterial arthritis worldwide and should be considered in a patient presenting with chronic arthritis with risk factors for tuberculosis, including being foreign-born (31).
Clinical Features
The clinical manifestations, severity, treatment, and prognosis of septic arthritis are dependent on the identity and virulence of the bacterium, source of joint infection, and underlying host factors. Nongonococcal septic arthritis is monoarticular in 80% to 90% of cases. The knee is usually affected (50% of cases) (27) followed by the hip, wrists, and ankles (2). In adults, the majority of hip infections involve prosthetic or osteosynthetic material (1). Arthritis of the small joints of the foot is most often seen in diabetic patients and is usually secondary to contiguous skin and soft tissue ulcerations or adjacent osteomyelitis.
Gonococcal arthritis may present as febrile monoarticular arthritis, usually of the knees, wrists, and ankles (27), or as one of the manifestations of disseminated gonococcal infection. The latter is characterized by fever, dermatitis, tenosynovitis, and migratory polyarthralgia or polyarthritis (19). Skin lesions are often pustular and occur simultaneously with tenosynovitis, predominately affecting the fingers, hands, wrists, or feet. Concomitant mucosal infection of the urethra or cervix is often present but usually asymptomatic. Urethral and cervical cultures or a nucleic amplification test will frequently yield N. gonorrhoeae (19,32).
Symptoms of acute septic arthritis include pain and loss of joint function. Fever and chills are often present. The acutely infected native joint is usually red, warm, and swollen with an obvious effusion. Range of motion is limited and extremely painful. For deep and axial joint, pain is often the only focal symptom. More subtle symptoms and signs may result in a delay of diagnosis and are particularly seen in patients receiving systemic or intra-articular steroids, and in those with immunocompromised status, advanced comorbidities (including rheumatoid arthritis), and extreme age (33). A thorough physical examination may reveal a distant source of joint infection in up to 50% of patients (27).
Prosthetic joint infection may present acutely as above, particularly in early stage infection, or more indolently with progressive joint pain, minimal swelling or effusion, and absence of fever (34). In late infection a cutaneous draining sinus tract may be present. Rarely, the involved prosthesis may be visible beneath an ulceration or focus of soft-tissue breakdown.
Diseases that can mimic septic arthritis are crystalinduced arthritis, rheumatoid arthritis, systemic lupus erythematosus, spondyloarthropathy, Still’s disease, rheumatic fever, and Kawasaki syndrome.
Diagnostic Approach
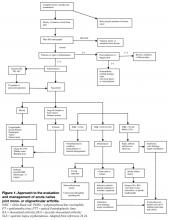
A diagnostic approach to acute native joint arthritis is outlined in Figure 1 on page 32 (35,36). Important aspects include exclusion of other causes of arthritis including trauma, rheumatic diseases, and crystalline arthritis. The most important diagnostic test upon which management hinges is diagnostic arthrocentesis. Fluoroscopic or CT-guided arthrocentesis is indicated for axial and deep joints (e.g., sacroiliac or pubic symphysis) or in the event of a “dry tap” of a peripheral joint. Synovial fluid analysis will often suggest whether an acutely painful joint is due to noninflammatory, sterile inflammatory, or septic causes (Table 2 on page 33). In addition, it will provide fluid for culture and gram stain, a rapid test that can guide early empiric antibiotic therapy. Bacterial, fungal, and mycobacterial cultures should always be performed in order to direct pathogen-specific antimicrobial therapy, which is often given as a prolonged course. Antimicrobial therapy should be delayed until arthrocentesis and other appropriate diagnostic cultures are obtained unless the patient shows signs of sepsis.
For prosthetic joint infections the diagnostic approach is essentially the same although early radiographic imaging is more important than in native joint infection as it may show signs of prosthesis failure or loosening (seen in many late prosthesis infections). Additionally, the synovial fluid white blood cell (WBC) is often lower than in nativejoint infection, with a diagnostic cutoff suggested as greater than 1,700 cells/mm3 or >65% neutrophils (37).
Nonspecific blood tests such as a white blood count, erythrocyte sedimentation rate, or C-reactive protein argue against joint infection if they are normal, but do not specifically suggest septic arthritis if elevated. Other important diagnostic tests include blood cultures (positive in 50–70% of acute bacterial arthritides) (27), but in only 30% or less of gonococcal arthritis cases) (38), wound cultures (although these often correlate poorly with synovial fluid culture results, except when the pathogen is S. aureus), and serologic testing for B. burgdorferi in selected cases with clinical features of Lyme arthritis in endemic areas. If gonococcal arthritis is suspected urethral and cervical specimens should be sent for N. gonorrhoeae culture and nucleic acid amplification tests. Radiographic and scintillographic imaging may yield additional information that will assist in identifying preexisting joint disease or for confirming a diagnosis of native or prosthetic joint infection or its complications (Table 3 on page 33).
Treatment
Native joint
Prompt joint drainage and antimicrobial therapy are the mainstays of treatment in bacterial, fungal, or mycobacterial joint infection. Drainage can be through closed needle aspiration performed daily, or arthroscopy. The former modality allows direct visual inspection of the joint with concomitant irrigation, lysis of adhesions, and removal of necrotic tissue and purulent material (42). Open surgical drainage is recommended for septic arthritis of the hip and when less invasive methods fail to control infection.
Initial antimicrobial therapy should be withheld until synovial fluid has been obtained and should be based on synovial fluid gram staining (Table 4). In the case of a nondiagnostic gram stain, empiric antimicrobial coverage of likely infecting pathogens is indicated. Therapy should be narrowed based on identification and antimicrobial susceptibility testing of bacteria cultured from synovial fluid, blood, or in some cases from ancillary cultures. For patients with MRSA-related infection who are allergic to or intolerant of vancomycin, linezolid or daptomycin are potential alternatives, although not approved by the U.S. Food and Drug Administration for this indication. Linezolid is a potentially attractive option for treatment as it is available as an oral tablet, but for bone and joint infection treatment experience is limited. For septic arthritis related to animal or human bites ampicillin-sulbactam or amoxicillin-clavulanate (clindamycin plus ciprofloxacin in penicillin-allergic patients) provides activity against Pasteurella multocida and other oral bacteria. Gonococcal arthritis is best treated initially with ceftriaxone or cefotaxime; oral ciprofloxacin or levofloxacin may be substituted in regions without fluoroquinolone resistance as the patient improves (Table 4). Septic arthritis due to Candida sp. should be treated initially with an amphotericin B preparation followed by a prolonged course of fluconazole if susceptibility testing confirms activity against the cultured yeast isolate (43).
Duration of intravenous antimicrobials for bacterial joint infections is usually 2 to 4 weeks, while for gonococcal arthritis 2 weeks is sufficient. Antimicrobial therapy that continues for 2 weeks or longer should have weekly followup and laboratory monitoring for hematologic, renal, and liver toxicity.
Prosthetic Joint
Treatment of prosthetic joint septic arthritis is complex, and early consultation with an orthopedic surgeon and infectious diseases physician is recommended. Extensive surgical debridement of the afflicted joint and effective, prolonged antimicrobial therapy is necessary in almost all cases. In order to achieve an optimal synovial fluid and tissue culture yield, antimicrobial therapy should be delayed until the time of debridement surgery unless the patient is septic or exhibiting serious systemic complications of infection. Suggestions for early empiric therapy while awaiting culture results are given in Table 4. Final antimicrobial choices should be based on culture results with assistance from an infectious diseases consultant.
Carefully selected cases of prosthetic joint infection may be treated with simple surgical debridement of the joint with prosthesis retention and at least 3 months of antimicrobial therapy that includes rifampin if the organism is gram positive (44). Patients who present with a short duration of symptoms within 1 month of joint implantation, or those with acute hematogenous infection, are the best candidates for such a treatment strategy. Unfortunately, relapse is common in these cases, particularly if the infection is due to S. aureus, gram-negative bacilli, or drug-resistant pathogens. Thus, the optimal treatment protocols involve surgical excision of the infected prosthesis and prolonged antimicrobial therapy.
Surgical prosthesis extraction and reimplantation can be performed in either a one- or two-stage approach. The two-stage procedure is the more successful strategy and involves removal of the prosthesis and cement followed by a 6-week course of bactericidal antimicrobial therapy. Subsequently a new prosthesis is reimplanted. Using this approach, a 90% to 96% success rate in total hip replacement infections and a 97% success rate in total knee infections has been realized (45-47). An alternative tactic is a one-stage surgical procedure that excises the infected prosthesis with immediate reimplantation of a new joint using antibiotic-impregnated methacrylate cement. This method is effective in 77% to 83% of cases (48-50). Higher failure rates are observed for S. aureus and gram-negative bacillary infections (51). One-stage procedures are often used for elderly or infirm patients who might not tolerate protracted bed rest and a second major operation (52). A recent review article by Zimmerli et al. provides an excellent overview of antimicrobial and surgical treatment options for prosthetic joint infections (34).
Suppressive Antibiotic Therapy
Lifelong oral antimicrobial therapy plays a limited role for definitive therapy but is useful when a surgical approach is not possible because of medical or surgical contraindications. The goal of suppressive therapy is to control the infection and retain prosthesis function. It is important that patients and their families understand that the intention of such treatment is not to cure but to suppress the infection. Generally, oral suppressive therapy is initiated after a course of intravenous therapy. Goulet et al. (53) demonstrated a 63% success rate in maintaining function of hip arthroplasty in patients who met 5 criteria: 1) prostheses removal is not possible, 2) the pathogen is avirulent, 3) the pathogen is sensitive to oral antibiotics, 4) the patient is adherent to and tolerates antibiotics, and 5) the prosthesis is not loose. Patients being treated with lifelong suppressive therapy are at risk for the development of antibiotic resistance (in either the joint infecting pathogen or other commensal organism), local or systemic progression of infection, and adverse effects from chronic antibiotic usage.
Antimicrobial Prophylaxis to Prevent Joint Prosthesis Infection
Patients undergoing elective total joint replacement surgery should be evaluated for symptoms or signs of local infection that predispose to occult or overt bacteremia (particularly odontogenic, urologic, and dermatologic). Surgery should be delayed until such infections and coexisting medical conditions have been treated. Perioperative antibiotic prophylaxis has been shown to reduce deep wound infection and prosthetic joint infection in joint reimplant surgery but should not be continued for more than 24 hours after the preoperative dose (54,55). In order to decrease the risk of hematogenous seeding of established implants, early recognition and treatment of overt infection is crucial. The use of prophylactic antibiotics for patients with joint implants prior to or after dental or other procedures such as colonoscopy or cystoscopy is controversial. The American Academy of Orthopedic Surgeons recommends that a single dose of prophylactic antibiotic be given to certain patients undergoing urologic instrumentation or dental procedures that are accompanied by significant bleeding (56,57). Patients who are candidates for such prophylaxis include those with rheumatoid arthritis or other inflammatory arthropathy, immunosuppression, diabetes, malnutrition, hemophilia, or who have had a previous joint infection.
Dr. Ohl can be contacted at cohl@wfubmc.edu.
References
- Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56:470-5.
- Ohl C. Infectious arthritis of native joints. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier, 2005:1311-1322.
- Brause B. Infections with prostheses in bones and joints. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practices of Infectious Diseases. 6th ed. Philadelphia: Elsevier, 2005:1332-7.
- Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology (Oxford). 2001;40:24-30.
- Nolla JM, Gomez-Vaquero C, Fiter J, et al. Pyarthrosis in patients with rheumatoid arthritis: a detailed analysis of 10 cases and literature review. Semin Arthritis Rheum 2000;30: 121-6.
- Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis 1999;58:214-219.
- Kuzmanova SI, Atanassov AN, Andreev SA, Solakov PT. Minor and major complications of arthroscopic synovectomy of the knee joint performed by rheumatologist. Folia Med (Plovdiv). 2003;45:55-9.
- Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect 1996;117:423-8.
- Mader JT, Shirtliff M, Calhoun JH. The host and the skeletal infection: classification and pathogenesis of acute bacterial bone and joint sepsis. Baillieres Best Pract Res Clin Rheumatol 1999;13:1-20.
- Berendt A. Infections of prosthetic joints and related problems. In: Cohen J, Powderly W, eds. Infectious Diseases. Edinburgh: Mosby, 2005: 583-589.
- Raymond NJ, Henry J, Workowski KA. Enterococcal arthritis: case report and review. Clin Infect Dis. 1995;21: 516-522.
- Ross JJ, Saltzman CL, Carling P, Shapiro DS. Pneumococcal septic arthritis: review of 190 cases. Clin Infect Dis. 2003;36:319-27.
- Kortekangas P, Aro HT, Tuominen J, Toivanen A. Synovial fluid leukocytosis in bacterial arthritis vs. reactive arthritis and rheumatoid arthritis in the adult knee. Scand J Rheumatol. 1992;21:283-8.
- Sack K. Monarthritis: differential diagnosis. Am J Med. 1997; 102(1A):30S-34S.
- Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61:267-9.
- Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36:370-3.
- Nolla JM, Gomez-Vaquero C, Corbella X, et al. Group B streptococcus (Streptococcus agalactiae) pyogenic arthritis in nonpregnant adults. Medicine (Baltimore). 2003;82: 119-28.
- Shirtliff ME, Mader JT. Acute septic arthritis. Clin Microbiol Rev. 2002;15:527-44.
- Bardin T. Gonococcal arthritis. Best Pract Res Clin Rheumatol. 2003;17:201-8.
- Yagupsky P, Dagan R. Kingella kingae: an emerging cause of invasive infections in young children. Clin Infect Dis. 1997;24:860-66.
- Bowerman SG, Green NE, Mencio GA. Decline of bone and joint infections attributable to haemophilus influenzae type b. Clin Orthop. 1997;(341):128-33.
- Ewing R, Fainstein V, Musher DM, Lidsky M, Clarridge J. Articular and skeletal infections caused by Pasteurella multocida. South Med J. 1980;73:1349-52.
- Murray PM. Septic arthritis of the hand and wrist. Hand Clin. 1998;14:579-87, viii.
- Resnick D, Pineda CJ, Weisman MH, Kerr R. Osteomyelitis and septic arthritis of the hand following human bites. Skeletal Radiol. 1985;14:263-6.
- Murdoch DR, Roberts SA, Fowler JV Jr, et al. Infection of orthopedic prostheses after Staphylococcus aureus bacteremia. Clin Infect Dis. 2001;32:647-9.
- Woolf AD, Campion GV, Chishick A, et al. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med. 1989;149:1153-6.
- Goldenberg DL. Septic arthritis. Lancet 1998; 351:197-202.
- Silveira LH, Cuellar ML, Citera G, Cabrera GE, Scopelitis E, Espinoza LR. Candida arthritis. Rheum Dis Clin North Am. 1993;19:427-37.
- Cuellar ML, Silveira LH, Espinoza LR. Fungal arthritis. Ann Rheum Dis. 1992;51:690-7.
- Cuellar ML, Silveira LH, Citera G, Cabrera GE, Valle R. Other fungal arthritides. Rheum Dis Clin North Am. 1993;19:439-55.
- Malaviya AN, Kotwal PP. Arthritis associated with tuberculosis. Best Pract Res Clin Rheumatol. 2003;17:319-43.
- Van Der PB, Ferrero DV, Buck-Barrington L, et al. Multicenter evaluation of the BDProbeTec ET System for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J Clin Microbiol. 2001;39:1008-16.
- Kaandorp CJ, van Schaardenburg D, Krijnen P, Habbema JD, van de Laar MA. Risk factors for septic arthritis in patients with joint disease: a prospective study. Arthritis Rheum. 1995;38:1819-25.
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-54.
- Guidelines for the initial evaluation of the adult patient with acute musculoskeletal symptoms. American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Arthritis Rheum. 1996;39:1-8.
- Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68:83-90.
- Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004; 117:556-62.
- Cucurull E, Espinoza LR. Gonococcal arthritis. Rheum Dis Clin North Am. 1998; 24:305-22.
- Chhem RK, Kaplan PA, Dussault RG. Ultrasonography of the musculoskeletal system. Radiol Clin North Am. 1994;32:275-289.
- Learch TJ, Farooki S. Magnetic resonance imaging of septic arthritis. Clin Imaging. 2000;24:236-42.
- Mohana-Borges AV, Chung CB, Resnick D. Monoarticular arthritis. Radiol Clin North Am. 2004;42:135-49.
- Donatto KC. Orthopedic management of septic arthritis. Rheum Dis Clin North Am. 1998;24:275-86.
- Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161-89.
- Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537-41.
- Garvin KL, Salvati EA, Brause BD. Role of gentamicin-impregnated cement in total joint arthroplasty. Orthop Clin North Am. 1988;19:605-10.
- Lieberman JR, Callaway GH, Salvati EA, Pellicci PM, Brause BD. Treatment of the infected total hip arthroplasty with a two-stage reimplantation protocol. Clin Orthop Relat Res. 1994;205-12.
- Windsor RE, Insall JN, Urs WK, Miller DV, Brause BD. Twostage reimplantation for the salvage of total knee arthroplasty complicated by infection. Further follow-up and refinement of indications. J Bone Joint Surg Am. 1990;72:272-8.
- Buchholz HW, Elson RA, Engelbrecht E, Lodenkamper H, Rottger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981; 63-B(3):342-53.
- Carlsson AS, Josefsson G, Lindberg L. Revision with gentamicin-impregnated cement for deep infections in total hip arthroplasties. J Bone Joint Surg Am. 1978;60:1059-64.
- Jackson WO, Schmalzried TP. Limited role of direct exchange arthroplasty in the treatment of infected total hip replacements. Clin Orthop Relat Res. 2000;(381):101-5.
- Fitzgerald RH Jr, Jones DR. Hip implant infection. Treatment with resection arthroplasty and late total hip arthroplasty. Am J Med. 1985; 78(6B):225-8.
- Garvin KL, Hanssen AD. Infection after total hip arthroplasty. Past, present, and future. J Bone Joint Surg Am. 1995;77:1576-88.
- Goulet JA, Pellicci PM, Brause BD, Salvati EM. Prolonged suppression of infection in total hip arthroplasty. J Arthroplasty. 1988; 3:109-16.
- Engesaeter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003; 74:644-51.
- Norden CW. A critical review of antibiotic prophylaxis in orthopedic surgery. Rev Infect Dis 1983;5:928-32.
- Antibiotic prophylaxis for urological patients with total joint replacements. J Urol. 2003; 169:1796-7.
- Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc. 2003; 134:895-9.
Introduction
Acute bacterial arthritis is a potentially serious and rapidly progressive infection that may involve native or prosthetic joints. The epidemiology, pathophysiology, repertoire of potential infecting pathogens, clinical presentation and treatment differ for these two forms of infectious arthritis, but both are associated with significant morbidity and mortality. Infectious arthritis of native and prosthetic joints may be caused by viruses, or fungi, but the most common cause is bacteria.
Acute Bacterial Arthritis
Epidemiology
The burden of septic arthritis in the general population is considerable. The incidence of native joint septic arthritis is approximately 5 cases per 100,000 persons per year and is much higher in patients with rheumatoid arthritis (1,2). Between 1% and 5% of joints with indwelling prostheses become infected and the total number of infections per year is increasing due to a rise in the number of patients who have had prosthetic replacement surgery (3). The mortality from joint infection is difficult to estimate due to differing comorbidity in afflicted patients, but is likely between 15% and 30% (4-6). There is substantial morbidity from these infections because of decreased joint function and mobility, and in cases involving joint prostheses from the excisional or exchange arthroplastic surgery that is often required for treatment.
The most common route of infection for native joint infection is hematogenous (1), but may also be a result of direct inoculation of bacteria through trauma or joint surgery (including arthrocentesis, corticosteroid injection, or arthroscopy) (7), or via contiguous spread from adjacent infected soft tissue or bone (1,8). While hematogenous infection of prosthetic joints occurs, the majority of these infections are the result of joint contamination in the course of implantation surgery or post-surgical wound infection (3). Host factors that increase the risk of septic arthritis include pre-existing joint disease (especially rheumatoid arthritis), immunosuppression, diabetes mellitus, malignancy, chronic renal failure, intravenous drug use, severe skin diseases, and advanced age (1,2,4,6). The extent of joint injury resulting from infection depends on the virulence of the infecting pathogen and degree of host immune response (9).
Microbiology
Native Joint
The most common causes of bacterial septic arthritis are outlined in Table 1. In adults, the most frequent etiology is S. aureus (37–65% of cases) (1,4,6,8,12,15,16) followed by Streptococcus sp. (12,15). An increasing percentage of S. aureus isolated from septic joints are resistant to antistaphylococcal penicillins and cephalosporins (methicillin-resistant S. aureus, MRSA). In adults with diabetes, malignancy, and genitourinary structural abnormalities, group B Streptococcus is a frequently isolated pathogen (5,6,17). Gram-negative bacilli are commonly found in neonates, intravenous drug users, and immunocompromised hosts (18). N. gonorrhoeae is a significant cause of bacterial arthritis in sexually active adults and adolescents (19) and Kingella kingae and Haemophilus influenzae are likely pediatric isolates (20,21). Joint infections that follow bite trauma usually are seen in the small joints of the hand and involve Pasteurella multocida in the case of animal bites, and Eikenella corrodens in the case of humans bites (22-24). Polymicrobial floras are found in up to 8% of cases of septic arthritis.
Prosthetic Joint
The bacteria that cause prosthetic joint arthritis vary depending on the stage of infection as defined by the elapsed time after implantation surgery (Table 1 on page 31). The coagulase negative staphylococci are the most common (30–43% of cases) (3,10), followed by S. aureus (12–23%) (25).
Nonbacterial Pathogens
Nonbacterial pathogens that may cause septic arthritis include viruses, fungi, and mycobacteria. Viral arthritis is often associated with a systemic febrile illness and other manifestations of infection such as rash. Parvovirus B19 is the most common viral arthritide, presenting as a symmetric polyarticular arthritis involving the joints of the hand as well as larger joints (26). The classic red “slapped cheeks” associated with this viral infection in children is usually not present in adults, although a faint lacy reticular rash may be seen.
Fungi and mycobacteria usually cause a subacute or chronic mono- or oligoarticular arthritis (27). Candida species are an increasing cause of both native and prosthetic joint septic arthritis. Risk factors for this infection include loss of skin integrity, diabetes, malignancy, intravenous drug use, and immunosuppressive therapy including glucocorticoids (28). Patients are often chronically ill and have exposure to broad-spectrum antimicrobials, hyperalimentation fluid, and/or indwelling central intravenous catheters. Other fungi, including Cryptococcus, Blastomyces, Histoplasma, Coccidioides, and Sporothrix are rare causes of septic arthritis (29,30). Mycobacterium tuberculosis is the most common cause of mycobacterial arthritis worldwide and should be considered in a patient presenting with chronic arthritis with risk factors for tuberculosis, including being foreign-born (31).
Clinical Features
The clinical manifestations, severity, treatment, and prognosis of septic arthritis are dependent on the identity and virulence of the bacterium, source of joint infection, and underlying host factors. Nongonococcal septic arthritis is monoarticular in 80% to 90% of cases. The knee is usually affected (50% of cases) (27) followed by the hip, wrists, and ankles (2). In adults, the majority of hip infections involve prosthetic or osteosynthetic material (1). Arthritis of the small joints of the foot is most often seen in diabetic patients and is usually secondary to contiguous skin and soft tissue ulcerations or adjacent osteomyelitis.
Gonococcal arthritis may present as febrile monoarticular arthritis, usually of the knees, wrists, and ankles (27), or as one of the manifestations of disseminated gonococcal infection. The latter is characterized by fever, dermatitis, tenosynovitis, and migratory polyarthralgia or polyarthritis (19). Skin lesions are often pustular and occur simultaneously with tenosynovitis, predominately affecting the fingers, hands, wrists, or feet. Concomitant mucosal infection of the urethra or cervix is often present but usually asymptomatic. Urethral and cervical cultures or a nucleic amplification test will frequently yield N. gonorrhoeae (19,32).
Symptoms of acute septic arthritis include pain and loss of joint function. Fever and chills are often present. The acutely infected native joint is usually red, warm, and swollen with an obvious effusion. Range of motion is limited and extremely painful. For deep and axial joint, pain is often the only focal symptom. More subtle symptoms and signs may result in a delay of diagnosis and are particularly seen in patients receiving systemic or intra-articular steroids, and in those with immunocompromised status, advanced comorbidities (including rheumatoid arthritis), and extreme age (33). A thorough physical examination may reveal a distant source of joint infection in up to 50% of patients (27).
Prosthetic joint infection may present acutely as above, particularly in early stage infection, or more indolently with progressive joint pain, minimal swelling or effusion, and absence of fever (34). In late infection a cutaneous draining sinus tract may be present. Rarely, the involved prosthesis may be visible beneath an ulceration or focus of soft-tissue breakdown.
Diseases that can mimic septic arthritis are crystalinduced arthritis, rheumatoid arthritis, systemic lupus erythematosus, spondyloarthropathy, Still’s disease, rheumatic fever, and Kawasaki syndrome.
Diagnostic Approach
A diagnostic approach to acute native joint arthritis is outlined in Figure 1 on page 32 (35,36). Important aspects include exclusion of other causes of arthritis including trauma, rheumatic diseases, and crystalline arthritis. The most important diagnostic test upon which management hinges is diagnostic arthrocentesis. Fluoroscopic or CT-guided arthrocentesis is indicated for axial and deep joints (e.g., sacroiliac or pubic symphysis) or in the event of a “dry tap” of a peripheral joint. Synovial fluid analysis will often suggest whether an acutely painful joint is due to noninflammatory, sterile inflammatory, or septic causes (Table 2 on page 33). In addition, it will provide fluid for culture and gram stain, a rapid test that can guide early empiric antibiotic therapy. Bacterial, fungal, and mycobacterial cultures should always be performed in order to direct pathogen-specific antimicrobial therapy, which is often given as a prolonged course. Antimicrobial therapy should be delayed until arthrocentesis and other appropriate diagnostic cultures are obtained unless the patient shows signs of sepsis.
For prosthetic joint infections the diagnostic approach is essentially the same although early radiographic imaging is more important than in native joint infection as it may show signs of prosthesis failure or loosening (seen in many late prosthesis infections). Additionally, the synovial fluid white blood cell (WBC) is often lower than in nativejoint infection, with a diagnostic cutoff suggested as greater than 1,700 cells/mm3 or >65% neutrophils (37).
Nonspecific blood tests such as a white blood count, erythrocyte sedimentation rate, or C-reactive protein argue against joint infection if they are normal, but do not specifically suggest septic arthritis if elevated. Other important diagnostic tests include blood cultures (positive in 50–70% of acute bacterial arthritides) (27), but in only 30% or less of gonococcal arthritis cases) (38), wound cultures (although these often correlate poorly with synovial fluid culture results, except when the pathogen is S. aureus), and serologic testing for B. burgdorferi in selected cases with clinical features of Lyme arthritis in endemic areas. If gonococcal arthritis is suspected urethral and cervical specimens should be sent for N. gonorrhoeae culture and nucleic acid amplification tests. Radiographic and scintillographic imaging may yield additional information that will assist in identifying preexisting joint disease or for confirming a diagnosis of native or prosthetic joint infection or its complications (Table 3 on page 33).
Treatment
Native joint
Prompt joint drainage and antimicrobial therapy are the mainstays of treatment in bacterial, fungal, or mycobacterial joint infection. Drainage can be through closed needle aspiration performed daily, or arthroscopy. The former modality allows direct visual inspection of the joint with concomitant irrigation, lysis of adhesions, and removal of necrotic tissue and purulent material (42). Open surgical drainage is recommended for septic arthritis of the hip and when less invasive methods fail to control infection.
Initial antimicrobial therapy should be withheld until synovial fluid has been obtained and should be based on synovial fluid gram staining (Table 4). In the case of a nondiagnostic gram stain, empiric antimicrobial coverage of likely infecting pathogens is indicated. Therapy should be narrowed based on identification and antimicrobial susceptibility testing of bacteria cultured from synovial fluid, blood, or in some cases from ancillary cultures. For patients with MRSA-related infection who are allergic to or intolerant of vancomycin, linezolid or daptomycin are potential alternatives, although not approved by the U.S. Food and Drug Administration for this indication. Linezolid is a potentially attractive option for treatment as it is available as an oral tablet, but for bone and joint infection treatment experience is limited. For septic arthritis related to animal or human bites ampicillin-sulbactam or amoxicillin-clavulanate (clindamycin plus ciprofloxacin in penicillin-allergic patients) provides activity against Pasteurella multocida and other oral bacteria. Gonococcal arthritis is best treated initially with ceftriaxone or cefotaxime; oral ciprofloxacin or levofloxacin may be substituted in regions without fluoroquinolone resistance as the patient improves (Table 4). Septic arthritis due to Candida sp. should be treated initially with an amphotericin B preparation followed by a prolonged course of fluconazole if susceptibility testing confirms activity against the cultured yeast isolate (43).
Duration of intravenous antimicrobials for bacterial joint infections is usually 2 to 4 weeks, while for gonococcal arthritis 2 weeks is sufficient. Antimicrobial therapy that continues for 2 weeks or longer should have weekly followup and laboratory monitoring for hematologic, renal, and liver toxicity.
Prosthetic Joint
Treatment of prosthetic joint septic arthritis is complex, and early consultation with an orthopedic surgeon and infectious diseases physician is recommended. Extensive surgical debridement of the afflicted joint and effective, prolonged antimicrobial therapy is necessary in almost all cases. In order to achieve an optimal synovial fluid and tissue culture yield, antimicrobial therapy should be delayed until the time of debridement surgery unless the patient is septic or exhibiting serious systemic complications of infection. Suggestions for early empiric therapy while awaiting culture results are given in Table 4. Final antimicrobial choices should be based on culture results with assistance from an infectious diseases consultant.
Carefully selected cases of prosthetic joint infection may be treated with simple surgical debridement of the joint with prosthesis retention and at least 3 months of antimicrobial therapy that includes rifampin if the organism is gram positive (44). Patients who present with a short duration of symptoms within 1 month of joint implantation, or those with acute hematogenous infection, are the best candidates for such a treatment strategy. Unfortunately, relapse is common in these cases, particularly if the infection is due to S. aureus, gram-negative bacilli, or drug-resistant pathogens. Thus, the optimal treatment protocols involve surgical excision of the infected prosthesis and prolonged antimicrobial therapy.
Surgical prosthesis extraction and reimplantation can be performed in either a one- or two-stage approach. The two-stage procedure is the more successful strategy and involves removal of the prosthesis and cement followed by a 6-week course of bactericidal antimicrobial therapy. Subsequently a new prosthesis is reimplanted. Using this approach, a 90% to 96% success rate in total hip replacement infections and a 97% success rate in total knee infections has been realized (45-47). An alternative tactic is a one-stage surgical procedure that excises the infected prosthesis with immediate reimplantation of a new joint using antibiotic-impregnated methacrylate cement. This method is effective in 77% to 83% of cases (48-50). Higher failure rates are observed for S. aureus and gram-negative bacillary infections (51). One-stage procedures are often used for elderly or infirm patients who might not tolerate protracted bed rest and a second major operation (52). A recent review article by Zimmerli et al. provides an excellent overview of antimicrobial and surgical treatment options for prosthetic joint infections (34).
Suppressive Antibiotic Therapy
Lifelong oral antimicrobial therapy plays a limited role for definitive therapy but is useful when a surgical approach is not possible because of medical or surgical contraindications. The goal of suppressive therapy is to control the infection and retain prosthesis function. It is important that patients and their families understand that the intention of such treatment is not to cure but to suppress the infection. Generally, oral suppressive therapy is initiated after a course of intravenous therapy. Goulet et al. (53) demonstrated a 63% success rate in maintaining function of hip arthroplasty in patients who met 5 criteria: 1) prostheses removal is not possible, 2) the pathogen is avirulent, 3) the pathogen is sensitive to oral antibiotics, 4) the patient is adherent to and tolerates antibiotics, and 5) the prosthesis is not loose. Patients being treated with lifelong suppressive therapy are at risk for the development of antibiotic resistance (in either the joint infecting pathogen or other commensal organism), local or systemic progression of infection, and adverse effects from chronic antibiotic usage.
Antimicrobial Prophylaxis to Prevent Joint Prosthesis Infection
Patients undergoing elective total joint replacement surgery should be evaluated for symptoms or signs of local infection that predispose to occult or overt bacteremia (particularly odontogenic, urologic, and dermatologic). Surgery should be delayed until such infections and coexisting medical conditions have been treated. Perioperative antibiotic prophylaxis has been shown to reduce deep wound infection and prosthetic joint infection in joint reimplant surgery but should not be continued for more than 24 hours after the preoperative dose (54,55). In order to decrease the risk of hematogenous seeding of established implants, early recognition and treatment of overt infection is crucial. The use of prophylactic antibiotics for patients with joint implants prior to or after dental or other procedures such as colonoscopy or cystoscopy is controversial. The American Academy of Orthopedic Surgeons recommends that a single dose of prophylactic antibiotic be given to certain patients undergoing urologic instrumentation or dental procedures that are accompanied by significant bleeding (56,57). Patients who are candidates for such prophylaxis include those with rheumatoid arthritis or other inflammatory arthropathy, immunosuppression, diabetes, malnutrition, hemophilia, or who have had a previous joint infection.
Dr. Ohl can be contacted at cohl@wfubmc.edu.
References
- Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56:470-5.
- Ohl C. Infectious arthritis of native joints. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier, 2005:1311-1322.
- Brause B. Infections with prostheses in bones and joints. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practices of Infectious Diseases. 6th ed. Philadelphia: Elsevier, 2005:1332-7.
- Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology (Oxford). 2001;40:24-30.
- Nolla JM, Gomez-Vaquero C, Fiter J, et al. Pyarthrosis in patients with rheumatoid arthritis: a detailed analysis of 10 cases and literature review. Semin Arthritis Rheum 2000;30: 121-6.
- Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis 1999;58:214-219.
- Kuzmanova SI, Atanassov AN, Andreev SA, Solakov PT. Minor and major complications of arthroscopic synovectomy of the knee joint performed by rheumatologist. Folia Med (Plovdiv). 2003;45:55-9.
- Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect 1996;117:423-8.
- Mader JT, Shirtliff M, Calhoun JH. The host and the skeletal infection: classification and pathogenesis of acute bacterial bone and joint sepsis. Baillieres Best Pract Res Clin Rheumatol 1999;13:1-20.
- Berendt A. Infections of prosthetic joints and related problems. In: Cohen J, Powderly W, eds. Infectious Diseases. Edinburgh: Mosby, 2005: 583-589.
- Raymond NJ, Henry J, Workowski KA. Enterococcal arthritis: case report and review. Clin Infect Dis. 1995;21: 516-522.
- Ross JJ, Saltzman CL, Carling P, Shapiro DS. Pneumococcal septic arthritis: review of 190 cases. Clin Infect Dis. 2003;36:319-27.
- Kortekangas P, Aro HT, Tuominen J, Toivanen A. Synovial fluid leukocytosis in bacterial arthritis vs. reactive arthritis and rheumatoid arthritis in the adult knee. Scand J Rheumatol. 1992;21:283-8.
- Sack K. Monarthritis: differential diagnosis. Am J Med. 1997; 102(1A):30S-34S.
- Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61:267-9.
- Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36:370-3.
- Nolla JM, Gomez-Vaquero C, Corbella X, et al. Group B streptococcus (Streptococcus agalactiae) pyogenic arthritis in nonpregnant adults. Medicine (Baltimore). 2003;82: 119-28.
- Shirtliff ME, Mader JT. Acute septic arthritis. Clin Microbiol Rev. 2002;15:527-44.
- Bardin T. Gonococcal arthritis. Best Pract Res Clin Rheumatol. 2003;17:201-8.
- Yagupsky P, Dagan R. Kingella kingae: an emerging cause of invasive infections in young children. Clin Infect Dis. 1997;24:860-66.
- Bowerman SG, Green NE, Mencio GA. Decline of bone and joint infections attributable to haemophilus influenzae type b. Clin Orthop. 1997;(341):128-33.
- Ewing R, Fainstein V, Musher DM, Lidsky M, Clarridge J. Articular and skeletal infections caused by Pasteurella multocida. South Med J. 1980;73:1349-52.
- Murray PM. Septic arthritis of the hand and wrist. Hand Clin. 1998;14:579-87, viii.
- Resnick D, Pineda CJ, Weisman MH, Kerr R. Osteomyelitis and septic arthritis of the hand following human bites. Skeletal Radiol. 1985;14:263-6.
- Murdoch DR, Roberts SA, Fowler JV Jr, et al. Infection of orthopedic prostheses after Staphylococcus aureus bacteremia. Clin Infect Dis. 2001;32:647-9.
- Woolf AD, Campion GV, Chishick A, et al. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med. 1989;149:1153-6.
- Goldenberg DL. Septic arthritis. Lancet 1998; 351:197-202.
- Silveira LH, Cuellar ML, Citera G, Cabrera GE, Scopelitis E, Espinoza LR. Candida arthritis. Rheum Dis Clin North Am. 1993;19:427-37.
- Cuellar ML, Silveira LH, Espinoza LR. Fungal arthritis. Ann Rheum Dis. 1992;51:690-7.
- Cuellar ML, Silveira LH, Citera G, Cabrera GE, Valle R. Other fungal arthritides. Rheum Dis Clin North Am. 1993;19:439-55.
- Malaviya AN, Kotwal PP. Arthritis associated with tuberculosis. Best Pract Res Clin Rheumatol. 2003;17:319-43.
- Van Der PB, Ferrero DV, Buck-Barrington L, et al. Multicenter evaluation of the BDProbeTec ET System for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J Clin Microbiol. 2001;39:1008-16.
- Kaandorp CJ, van Schaardenburg D, Krijnen P, Habbema JD, van de Laar MA. Risk factors for septic arthritis in patients with joint disease: a prospective study. Arthritis Rheum. 1995;38:1819-25.
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-54.
- Guidelines for the initial evaluation of the adult patient with acute musculoskeletal symptoms. American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Arthritis Rheum. 1996;39:1-8.
- Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68:83-90.
- Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004; 117:556-62.
- Cucurull E, Espinoza LR. Gonococcal arthritis. Rheum Dis Clin North Am. 1998; 24:305-22.
- Chhem RK, Kaplan PA, Dussault RG. Ultrasonography of the musculoskeletal system. Radiol Clin North Am. 1994;32:275-289.
- Learch TJ, Farooki S. Magnetic resonance imaging of septic arthritis. Clin Imaging. 2000;24:236-42.
- Mohana-Borges AV, Chung CB, Resnick D. Monoarticular arthritis. Radiol Clin North Am. 2004;42:135-49.
- Donatto KC. Orthopedic management of septic arthritis. Rheum Dis Clin North Am. 1998;24:275-86.
- Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161-89.
- Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537-41.
- Garvin KL, Salvati EA, Brause BD. Role of gentamicin-impregnated cement in total joint arthroplasty. Orthop Clin North Am. 1988;19:605-10.
- Lieberman JR, Callaway GH, Salvati EA, Pellicci PM, Brause BD. Treatment of the infected total hip arthroplasty with a two-stage reimplantation protocol. Clin Orthop Relat Res. 1994;205-12.
- Windsor RE, Insall JN, Urs WK, Miller DV, Brause BD. Twostage reimplantation for the salvage of total knee arthroplasty complicated by infection. Further follow-up and refinement of indications. J Bone Joint Surg Am. 1990;72:272-8.
- Buchholz HW, Elson RA, Engelbrecht E, Lodenkamper H, Rottger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981; 63-B(3):342-53.
- Carlsson AS, Josefsson G, Lindberg L. Revision with gentamicin-impregnated cement for deep infections in total hip arthroplasties. J Bone Joint Surg Am. 1978;60:1059-64.
- Jackson WO, Schmalzried TP. Limited role of direct exchange arthroplasty in the treatment of infected total hip replacements. Clin Orthop Relat Res. 2000;(381):101-5.
- Fitzgerald RH Jr, Jones DR. Hip implant infection. Treatment with resection arthroplasty and late total hip arthroplasty. Am J Med. 1985; 78(6B):225-8.
- Garvin KL, Hanssen AD. Infection after total hip arthroplasty. Past, present, and future. J Bone Joint Surg Am. 1995;77:1576-88.
- Goulet JA, Pellicci PM, Brause BD, Salvati EM. Prolonged suppression of infection in total hip arthroplasty. J Arthroplasty. 1988; 3:109-16.
- Engesaeter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003; 74:644-51.
- Norden CW. A critical review of antibiotic prophylaxis in orthopedic surgery. Rev Infect Dis 1983;5:928-32.
- Antibiotic prophylaxis for urological patients with total joint replacements. J Urol. 2003; 169:1796-7.
- Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc. 2003; 134:895-9.
Introduction
Acute bacterial arthritis is a potentially serious and rapidly progressive infection that may involve native or prosthetic joints. The epidemiology, pathophysiology, repertoire of potential infecting pathogens, clinical presentation and treatment differ for these two forms of infectious arthritis, but both are associated with significant morbidity and mortality. Infectious arthritis of native and prosthetic joints may be caused by viruses, or fungi, but the most common cause is bacteria.
Acute Bacterial Arthritis
Epidemiology
The burden of septic arthritis in the general population is considerable. The incidence of native joint septic arthritis is approximately 5 cases per 100,000 persons per year and is much higher in patients with rheumatoid arthritis (1,2). Between 1% and 5% of joints with indwelling prostheses become infected and the total number of infections per year is increasing due to a rise in the number of patients who have had prosthetic replacement surgery (3). The mortality from joint infection is difficult to estimate due to differing comorbidity in afflicted patients, but is likely between 15% and 30% (4-6). There is substantial morbidity from these infections because of decreased joint function and mobility, and in cases involving joint prostheses from the excisional or exchange arthroplastic surgery that is often required for treatment.
The most common route of infection for native joint infection is hematogenous (1), but may also be a result of direct inoculation of bacteria through trauma or joint surgery (including arthrocentesis, corticosteroid injection, or arthroscopy) (7), or via contiguous spread from adjacent infected soft tissue or bone (1,8). While hematogenous infection of prosthetic joints occurs, the majority of these infections are the result of joint contamination in the course of implantation surgery or post-surgical wound infection (3). Host factors that increase the risk of septic arthritis include pre-existing joint disease (especially rheumatoid arthritis), immunosuppression, diabetes mellitus, malignancy, chronic renal failure, intravenous drug use, severe skin diseases, and advanced age (1,2,4,6). The extent of joint injury resulting from infection depends on the virulence of the infecting pathogen and degree of host immune response (9).
Microbiology
Native Joint
The most common causes of bacterial septic arthritis are outlined in Table 1. In adults, the most frequent etiology is S. aureus (37–65% of cases) (1,4,6,8,12,15,16) followed by Streptococcus sp. (12,15). An increasing percentage of S. aureus isolated from septic joints are resistant to antistaphylococcal penicillins and cephalosporins (methicillin-resistant S. aureus, MRSA). In adults with diabetes, malignancy, and genitourinary structural abnormalities, group B Streptococcus is a frequently isolated pathogen (5,6,17). Gram-negative bacilli are commonly found in neonates, intravenous drug users, and immunocompromised hosts (18). N. gonorrhoeae is a significant cause of bacterial arthritis in sexually active adults and adolescents (19) and Kingella kingae and Haemophilus influenzae are likely pediatric isolates (20,21). Joint infections that follow bite trauma usually are seen in the small joints of the hand and involve Pasteurella multocida in the case of animal bites, and Eikenella corrodens in the case of humans bites (22-24). Polymicrobial floras are found in up to 8% of cases of septic arthritis.
Prosthetic Joint
The bacteria that cause prosthetic joint arthritis vary depending on the stage of infection as defined by the elapsed time after implantation surgery (Table 1 on page 31). The coagulase negative staphylococci are the most common (30–43% of cases) (3,10), followed by S. aureus (12–23%) (25).
Nonbacterial Pathogens
Nonbacterial pathogens that may cause septic arthritis include viruses, fungi, and mycobacteria. Viral arthritis is often associated with a systemic febrile illness and other manifestations of infection such as rash. Parvovirus B19 is the most common viral arthritide, presenting as a symmetric polyarticular arthritis involving the joints of the hand as well as larger joints (26). The classic red “slapped cheeks” associated with this viral infection in children is usually not present in adults, although a faint lacy reticular rash may be seen.
Fungi and mycobacteria usually cause a subacute or chronic mono- or oligoarticular arthritis (27). Candida species are an increasing cause of both native and prosthetic joint septic arthritis. Risk factors for this infection include loss of skin integrity, diabetes, malignancy, intravenous drug use, and immunosuppressive therapy including glucocorticoids (28). Patients are often chronically ill and have exposure to broad-spectrum antimicrobials, hyperalimentation fluid, and/or indwelling central intravenous catheters. Other fungi, including Cryptococcus, Blastomyces, Histoplasma, Coccidioides, and Sporothrix are rare causes of septic arthritis (29,30). Mycobacterium tuberculosis is the most common cause of mycobacterial arthritis worldwide and should be considered in a patient presenting with chronic arthritis with risk factors for tuberculosis, including being foreign-born (31).
Clinical Features
The clinical manifestations, severity, treatment, and prognosis of septic arthritis are dependent on the identity and virulence of the bacterium, source of joint infection, and underlying host factors. Nongonococcal septic arthritis is monoarticular in 80% to 90% of cases. The knee is usually affected (50% of cases) (27) followed by the hip, wrists, and ankles (2). In adults, the majority of hip infections involve prosthetic or osteosynthetic material (1). Arthritis of the small joints of the foot is most often seen in diabetic patients and is usually secondary to contiguous skin and soft tissue ulcerations or adjacent osteomyelitis.
Gonococcal arthritis may present as febrile monoarticular arthritis, usually of the knees, wrists, and ankles (27), or as one of the manifestations of disseminated gonococcal infection. The latter is characterized by fever, dermatitis, tenosynovitis, and migratory polyarthralgia or polyarthritis (19). Skin lesions are often pustular and occur simultaneously with tenosynovitis, predominately affecting the fingers, hands, wrists, or feet. Concomitant mucosal infection of the urethra or cervix is often present but usually asymptomatic. Urethral and cervical cultures or a nucleic amplification test will frequently yield N. gonorrhoeae (19,32).
Symptoms of acute septic arthritis include pain and loss of joint function. Fever and chills are often present. The acutely infected native joint is usually red, warm, and swollen with an obvious effusion. Range of motion is limited and extremely painful. For deep and axial joint, pain is often the only focal symptom. More subtle symptoms and signs may result in a delay of diagnosis and are particularly seen in patients receiving systemic or intra-articular steroids, and in those with immunocompromised status, advanced comorbidities (including rheumatoid arthritis), and extreme age (33). A thorough physical examination may reveal a distant source of joint infection in up to 50% of patients (27).
Prosthetic joint infection may present acutely as above, particularly in early stage infection, or more indolently with progressive joint pain, minimal swelling or effusion, and absence of fever (34). In late infection a cutaneous draining sinus tract may be present. Rarely, the involved prosthesis may be visible beneath an ulceration or focus of soft-tissue breakdown.
Diseases that can mimic septic arthritis are crystalinduced arthritis, rheumatoid arthritis, systemic lupus erythematosus, spondyloarthropathy, Still’s disease, rheumatic fever, and Kawasaki syndrome.
Diagnostic Approach
A diagnostic approach to acute native joint arthritis is outlined in Figure 1 on page 32 (35,36). Important aspects include exclusion of other causes of arthritis including trauma, rheumatic diseases, and crystalline arthritis. The most important diagnostic test upon which management hinges is diagnostic arthrocentesis. Fluoroscopic or CT-guided arthrocentesis is indicated for axial and deep joints (e.g., sacroiliac or pubic symphysis) or in the event of a “dry tap” of a peripheral joint. Synovial fluid analysis will often suggest whether an acutely painful joint is due to noninflammatory, sterile inflammatory, or septic causes (Table 2 on page 33). In addition, it will provide fluid for culture and gram stain, a rapid test that can guide early empiric antibiotic therapy. Bacterial, fungal, and mycobacterial cultures should always be performed in order to direct pathogen-specific antimicrobial therapy, which is often given as a prolonged course. Antimicrobial therapy should be delayed until arthrocentesis and other appropriate diagnostic cultures are obtained unless the patient shows signs of sepsis.
For prosthetic joint infections the diagnostic approach is essentially the same although early radiographic imaging is more important than in native joint infection as it may show signs of prosthesis failure or loosening (seen in many late prosthesis infections). Additionally, the synovial fluid white blood cell (WBC) is often lower than in nativejoint infection, with a diagnostic cutoff suggested as greater than 1,700 cells/mm3 or >65% neutrophils (37).
Nonspecific blood tests such as a white blood count, erythrocyte sedimentation rate, or C-reactive protein argue against joint infection if they are normal, but do not specifically suggest septic arthritis if elevated. Other important diagnostic tests include blood cultures (positive in 50–70% of acute bacterial arthritides) (27), but in only 30% or less of gonococcal arthritis cases) (38), wound cultures (although these often correlate poorly with synovial fluid culture results, except when the pathogen is S. aureus), and serologic testing for B. burgdorferi in selected cases with clinical features of Lyme arthritis in endemic areas. If gonococcal arthritis is suspected urethral and cervical specimens should be sent for N. gonorrhoeae culture and nucleic acid amplification tests. Radiographic and scintillographic imaging may yield additional information that will assist in identifying preexisting joint disease or for confirming a diagnosis of native or prosthetic joint infection or its complications (Table 3 on page 33).
Treatment
Native joint
Prompt joint drainage and antimicrobial therapy are the mainstays of treatment in bacterial, fungal, or mycobacterial joint infection. Drainage can be through closed needle aspiration performed daily, or arthroscopy. The former modality allows direct visual inspection of the joint with concomitant irrigation, lysis of adhesions, and removal of necrotic tissue and purulent material (42). Open surgical drainage is recommended for septic arthritis of the hip and when less invasive methods fail to control infection.
Initial antimicrobial therapy should be withheld until synovial fluid has been obtained and should be based on synovial fluid gram staining (Table 4). In the case of a nondiagnostic gram stain, empiric antimicrobial coverage of likely infecting pathogens is indicated. Therapy should be narrowed based on identification and antimicrobial susceptibility testing of bacteria cultured from synovial fluid, blood, or in some cases from ancillary cultures. For patients with MRSA-related infection who are allergic to or intolerant of vancomycin, linezolid or daptomycin are potential alternatives, although not approved by the U.S. Food and Drug Administration for this indication. Linezolid is a potentially attractive option for treatment as it is available as an oral tablet, but for bone and joint infection treatment experience is limited. For septic arthritis related to animal or human bites ampicillin-sulbactam or amoxicillin-clavulanate (clindamycin plus ciprofloxacin in penicillin-allergic patients) provides activity against Pasteurella multocida and other oral bacteria. Gonococcal arthritis is best treated initially with ceftriaxone or cefotaxime; oral ciprofloxacin or levofloxacin may be substituted in regions without fluoroquinolone resistance as the patient improves (Table 4). Septic arthritis due to Candida sp. should be treated initially with an amphotericin B preparation followed by a prolonged course of fluconazole if susceptibility testing confirms activity against the cultured yeast isolate (43).
Duration of intravenous antimicrobials for bacterial joint infections is usually 2 to 4 weeks, while for gonococcal arthritis 2 weeks is sufficient. Antimicrobial therapy that continues for 2 weeks or longer should have weekly followup and laboratory monitoring for hematologic, renal, and liver toxicity.
Prosthetic Joint
Treatment of prosthetic joint septic arthritis is complex, and early consultation with an orthopedic surgeon and infectious diseases physician is recommended. Extensive surgical debridement of the afflicted joint and effective, prolonged antimicrobial therapy is necessary in almost all cases. In order to achieve an optimal synovial fluid and tissue culture yield, antimicrobial therapy should be delayed until the time of debridement surgery unless the patient is septic or exhibiting serious systemic complications of infection. Suggestions for early empiric therapy while awaiting culture results are given in Table 4. Final antimicrobial choices should be based on culture results with assistance from an infectious diseases consultant.
Carefully selected cases of prosthetic joint infection may be treated with simple surgical debridement of the joint with prosthesis retention and at least 3 months of antimicrobial therapy that includes rifampin if the organism is gram positive (44). Patients who present with a short duration of symptoms within 1 month of joint implantation, or those with acute hematogenous infection, are the best candidates for such a treatment strategy. Unfortunately, relapse is common in these cases, particularly if the infection is due to S. aureus, gram-negative bacilli, or drug-resistant pathogens. Thus, the optimal treatment protocols involve surgical excision of the infected prosthesis and prolonged antimicrobial therapy.
Surgical prosthesis extraction and reimplantation can be performed in either a one- or two-stage approach. The two-stage procedure is the more successful strategy and involves removal of the prosthesis and cement followed by a 6-week course of bactericidal antimicrobial therapy. Subsequently a new prosthesis is reimplanted. Using this approach, a 90% to 96% success rate in total hip replacement infections and a 97% success rate in total knee infections has been realized (45-47). An alternative tactic is a one-stage surgical procedure that excises the infected prosthesis with immediate reimplantation of a new joint using antibiotic-impregnated methacrylate cement. This method is effective in 77% to 83% of cases (48-50). Higher failure rates are observed for S. aureus and gram-negative bacillary infections (51). One-stage procedures are often used for elderly or infirm patients who might not tolerate protracted bed rest and a second major operation (52). A recent review article by Zimmerli et al. provides an excellent overview of antimicrobial and surgical treatment options for prosthetic joint infections (34).
Suppressive Antibiotic Therapy
Lifelong oral antimicrobial therapy plays a limited role for definitive therapy but is useful when a surgical approach is not possible because of medical or surgical contraindications. The goal of suppressive therapy is to control the infection and retain prosthesis function. It is important that patients and their families understand that the intention of such treatment is not to cure but to suppress the infection. Generally, oral suppressive therapy is initiated after a course of intravenous therapy. Goulet et al. (53) demonstrated a 63% success rate in maintaining function of hip arthroplasty in patients who met 5 criteria: 1) prostheses removal is not possible, 2) the pathogen is avirulent, 3) the pathogen is sensitive to oral antibiotics, 4) the patient is adherent to and tolerates antibiotics, and 5) the prosthesis is not loose. Patients being treated with lifelong suppressive therapy are at risk for the development of antibiotic resistance (in either the joint infecting pathogen or other commensal organism), local or systemic progression of infection, and adverse effects from chronic antibiotic usage.
Antimicrobial Prophylaxis to Prevent Joint Prosthesis Infection
Patients undergoing elective total joint replacement surgery should be evaluated for symptoms or signs of local infection that predispose to occult or overt bacteremia (particularly odontogenic, urologic, and dermatologic). Surgery should be delayed until such infections and coexisting medical conditions have been treated. Perioperative antibiotic prophylaxis has been shown to reduce deep wound infection and prosthetic joint infection in joint reimplant surgery but should not be continued for more than 24 hours after the preoperative dose (54,55). In order to decrease the risk of hematogenous seeding of established implants, early recognition and treatment of overt infection is crucial. The use of prophylactic antibiotics for patients with joint implants prior to or after dental or other procedures such as colonoscopy or cystoscopy is controversial. The American Academy of Orthopedic Surgeons recommends that a single dose of prophylactic antibiotic be given to certain patients undergoing urologic instrumentation or dental procedures that are accompanied by significant bleeding (56,57). Patients who are candidates for such prophylaxis include those with rheumatoid arthritis or other inflammatory arthropathy, immunosuppression, diabetes, malnutrition, hemophilia, or who have had a previous joint infection.
Dr. Ohl can be contacted at cohl@wfubmc.edu.
References
- Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56:470-5.
- Ohl C. Infectious arthritis of native joints. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier, 2005:1311-1322.
- Brause B. Infections with prostheses in bones and joints. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practices of Infectious Diseases. 6th ed. Philadelphia: Elsevier, 2005:1332-7.
- Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology (Oxford). 2001;40:24-30.
- Nolla JM, Gomez-Vaquero C, Fiter J, et al. Pyarthrosis in patients with rheumatoid arthritis: a detailed analysis of 10 cases and literature review. Semin Arthritis Rheum 2000;30: 121-6.
- Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis 1999;58:214-219.
- Kuzmanova SI, Atanassov AN, Andreev SA, Solakov PT. Minor and major complications of arthroscopic synovectomy of the knee joint performed by rheumatologist. Folia Med (Plovdiv). 2003;45:55-9.
- Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect 1996;117:423-8.
- Mader JT, Shirtliff M, Calhoun JH. The host and the skeletal infection: classification and pathogenesis of acute bacterial bone and joint sepsis. Baillieres Best Pract Res Clin Rheumatol 1999;13:1-20.
- Berendt A. Infections of prosthetic joints and related problems. In: Cohen J, Powderly W, eds. Infectious Diseases. Edinburgh: Mosby, 2005: 583-589.
- Raymond NJ, Henry J, Workowski KA. Enterococcal arthritis: case report and review. Clin Infect Dis. 1995;21: 516-522.
- Ross JJ, Saltzman CL, Carling P, Shapiro DS. Pneumococcal septic arthritis: review of 190 cases. Clin Infect Dis. 2003;36:319-27.
- Kortekangas P, Aro HT, Tuominen J, Toivanen A. Synovial fluid leukocytosis in bacterial arthritis vs. reactive arthritis and rheumatoid arthritis in the adult knee. Scand J Rheumatol. 1992;21:283-8.
- Sack K. Monarthritis: differential diagnosis. Am J Med. 1997; 102(1A):30S-34S.
- Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61:267-9.
- Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36:370-3.
- Nolla JM, Gomez-Vaquero C, Corbella X, et al. Group B streptococcus (Streptococcus agalactiae) pyogenic arthritis in nonpregnant adults. Medicine (Baltimore). 2003;82: 119-28.
- Shirtliff ME, Mader JT. Acute septic arthritis. Clin Microbiol Rev. 2002;15:527-44.
- Bardin T. Gonococcal arthritis. Best Pract Res Clin Rheumatol. 2003;17:201-8.
- Yagupsky P, Dagan R. Kingella kingae: an emerging cause of invasive infections in young children. Clin Infect Dis. 1997;24:860-66.
- Bowerman SG, Green NE, Mencio GA. Decline of bone and joint infections attributable to haemophilus influenzae type b. Clin Orthop. 1997;(341):128-33.
- Ewing R, Fainstein V, Musher DM, Lidsky M, Clarridge J. Articular and skeletal infections caused by Pasteurella multocida. South Med J. 1980;73:1349-52.
- Murray PM. Septic arthritis of the hand and wrist. Hand Clin. 1998;14:579-87, viii.
- Resnick D, Pineda CJ, Weisman MH, Kerr R. Osteomyelitis and septic arthritis of the hand following human bites. Skeletal Radiol. 1985;14:263-6.
- Murdoch DR, Roberts SA, Fowler JV Jr, et al. Infection of orthopedic prostheses after Staphylococcus aureus bacteremia. Clin Infect Dis. 2001;32:647-9.
- Woolf AD, Campion GV, Chishick A, et al. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med. 1989;149:1153-6.
- Goldenberg DL. Septic arthritis. Lancet 1998; 351:197-202.
- Silveira LH, Cuellar ML, Citera G, Cabrera GE, Scopelitis E, Espinoza LR. Candida arthritis. Rheum Dis Clin North Am. 1993;19:427-37.
- Cuellar ML, Silveira LH, Espinoza LR. Fungal arthritis. Ann Rheum Dis. 1992;51:690-7.
- Cuellar ML, Silveira LH, Citera G, Cabrera GE, Valle R. Other fungal arthritides. Rheum Dis Clin North Am. 1993;19:439-55.
- Malaviya AN, Kotwal PP. Arthritis associated with tuberculosis. Best Pract Res Clin Rheumatol. 2003;17:319-43.
- Van Der PB, Ferrero DV, Buck-Barrington L, et al. Multicenter evaluation of the BDProbeTec ET System for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J Clin Microbiol. 2001;39:1008-16.
- Kaandorp CJ, van Schaardenburg D, Krijnen P, Habbema JD, van de Laar MA. Risk factors for septic arthritis in patients with joint disease: a prospective study. Arthritis Rheum. 1995;38:1819-25.
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-54.
- Guidelines for the initial evaluation of the adult patient with acute musculoskeletal symptoms. American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Arthritis Rheum. 1996;39:1-8.
- Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68:83-90.
- Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004; 117:556-62.
- Cucurull E, Espinoza LR. Gonococcal arthritis. Rheum Dis Clin North Am. 1998; 24:305-22.
- Chhem RK, Kaplan PA, Dussault RG. Ultrasonography of the musculoskeletal system. Radiol Clin North Am. 1994;32:275-289.
- Learch TJ, Farooki S. Magnetic resonance imaging of septic arthritis. Clin Imaging. 2000;24:236-42.
- Mohana-Borges AV, Chung CB, Resnick D. Monoarticular arthritis. Radiol Clin North Am. 2004;42:135-49.
- Donatto KC. Orthopedic management of septic arthritis. Rheum Dis Clin North Am. 1998;24:275-86.
- Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161-89.
- Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537-41.
- Garvin KL, Salvati EA, Brause BD. Role of gentamicin-impregnated cement in total joint arthroplasty. Orthop Clin North Am. 1988;19:605-10.
- Lieberman JR, Callaway GH, Salvati EA, Pellicci PM, Brause BD. Treatment of the infected total hip arthroplasty with a two-stage reimplantation protocol. Clin Orthop Relat Res. 1994;205-12.
- Windsor RE, Insall JN, Urs WK, Miller DV, Brause BD. Twostage reimplantation for the salvage of total knee arthroplasty complicated by infection. Further follow-up and refinement of indications. J Bone Joint Surg Am. 1990;72:272-8.
- Buchholz HW, Elson RA, Engelbrecht E, Lodenkamper H, Rottger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981; 63-B(3):342-53.
- Carlsson AS, Josefsson G, Lindberg L. Revision with gentamicin-impregnated cement for deep infections in total hip arthroplasties. J Bone Joint Surg Am. 1978;60:1059-64.
- Jackson WO, Schmalzried TP. Limited role of direct exchange arthroplasty in the treatment of infected total hip replacements. Clin Orthop Relat Res. 2000;(381):101-5.
- Fitzgerald RH Jr, Jones DR. Hip implant infection. Treatment with resection arthroplasty and late total hip arthroplasty. Am J Med. 1985; 78(6B):225-8.
- Garvin KL, Hanssen AD. Infection after total hip arthroplasty. Past, present, and future. J Bone Joint Surg Am. 1995;77:1576-88.
- Goulet JA, Pellicci PM, Brause BD, Salvati EM. Prolonged suppression of infection in total hip arthroplasty. J Arthroplasty. 1988; 3:109-16.
- Engesaeter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003; 74:644-51.
- Norden CW. A critical review of antibiotic prophylaxis in orthopedic surgery. Rev Infect Dis 1983;5:928-32.
- Antibiotic prophylaxis for urological patients with total joint replacements. J Urol. 2003; 169:1796-7.
- Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc. 2003; 134:895-9.
Reducing Antimicrobial Resistance and Hospital-Associated Infections: The Role of the Hospitalist
While infections that develop during hospitalization may appear to be an uncommon but recognized risk of hospital care today, the incidence of these infections has been increasing dramatically during the last 2 to 3 decades, and the risk of acquiring an organism that is resistant to 1 or more antibiotics is becoming increasingly common. Recent studies estimate that approximately 2 million patients contract healthcare-associated infections each year (1). These infections are the most common type of serious adverse event in health care, affecting up to 5–10% of hospitalized patients, leading to approximately 90,000 deaths annually, and adding approximately $5 billion to annual healthcare costs (1-3). Increasingly, healthcare-associated infection risk is viewed as a patient safety issue, as many of these infections may be avoidable or preventable by following evidence-based best practices in infection control and patient care while patients are hospitalized. This article will summarize some of the overlap between patient safety and infection control, explain some of the pressures that have led to development and cultivation of antimicrobial resistance, and describe the Centers for Disease Control and Prevention (CDC) campaign for prevention of healthcare-associated infections and antimicrobial resistance, as well as the role of hospitalists in such prevention.
Patient Safety
The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) specifically identifies in its 2005 National Patient Safety Goals that hospitals and clinicians reduce the risk of healthcare-associated infections. The goals encourage clinicians to comply with current CDC hand hygiene guidelines and that hospitals and clinicians manage as sentinel events all identified cases of unanticipated death or permanent loss of function associated with a heathcare-associated infection. A sentinel event is defined by JCAHO as an unexpected occurrence involving death or serious physical or psychological injury. Such an event signals the need for immediate investigation and response by the institution. By including healthcare-associated infections in this category of high-risk event, with potential morbidity and mortality, JCAHO highlights the frequency and importance of infections acquired in our healthcare system today.
Further, the Agency for Healthcare Research and Quality (AHRQ) recently published an evidence-based report, developed and written primarily by hospitalists, delineating 79 patient safety practices, of which 22 (28%) involved infection control (4). At least 5 of these 22 infection control practices were considered valuable enough, and with sufficiently strong supporting evidence, to mandate widespread implementation. Additionally, the Institute for Healthcare Improvement (IHI; www.ihi.org) recently launched its 100,000 Lives Campaign, enlisting hundreds of hospitals around the United States in a commitment to implement changes that have been proven to prevent avoidable deaths. Three of their first 6 interventions involve the reduction of healthcare-associated infections, including central-line infections, surgical-site infections, and hospital-acquired pneumonia.
Increasingly, hospital-onset infections have become a patient safety issue, and they will remain under public and institutional scrutiny while hospitals take efforts to reduce their incidence and improve care quality. Hospitalists have evolved to serve a unique role as advocate of both patients and hospitals. They should therefore foster quality improvement in the hospital, as well as lead and support initiatives that reduce hospital-acquired infections and resistance.
Healthcare-Associated Infections and Development of Resistance
Bacteria have developed multiple microbiologic and genetic mechanisms to elude antimicrobial agents. Certain practices in medical care, whether intentional or not, can promote persistence or spread of resistant microbes that can cause infections. Such practices may include:
- Inattention to basic infection control measures (e.g., hand washing)
- Unrecognized colonization (e.g., treating colonized urinary or vascular catheters, without evidence of infection)
- Unrecognized reservoirs (e.g., environmental)
- Selective pressure from overuse or inappropriate use of antibiotics
- Movement of patients and staff within a single institution and between institutions
Inappropriate use or overuse of antibiotics can actually remove or “select” the sensitive microbes and promote overgrowth of resistant organisms when present. Each of these practices may serve as a focus for quality improvement interventions to reduce resistance.
Most healthcare-associated infections (more than 80%) originate from 4 specific patient sites: urinary tract, surgical-site (wound), bloodstream, and lung (pneumonia) (5). It is not coincidental that these infection sites are frequently associated with invasive procedures, and many times with indwelling invasive devices that may be used during the course of inpatient care. For example, urinary tract infections, the most common hospital-acquired infections, are usually associated with urinary catheter use. Similarly, bloodstream infections are usually associated with intravascular catheters, and hospital-acquired pneumonia is usually associated with ventilator use.
Because many of the invasive devices that are utilized during the course of inpatient care carry significant risk, including infection risk, it is incumbent upon hospitalists to be aware of these risks, to explain these risks to their patients, and to take all steps at their disposal to help reduce such risk in their patients. Dr. Julie Gerberding, Director of the CDC, has emphasized that the 2 greatest predictors of infection risk in the hospital are length of stay and use of invasive devices (6). While excellent evidence already demonstrates that hospitalists reduce length of stay (7), they should also spearhead the efforts to minimize the use of invasive devices whenever possible, and lead evidence-based efforts to minimize infection in hospitalized patients when invasive devices must be used.
Prevention of Resistance: Best Practices
CDC/SHM Collaboration
In September 2003, the Society of Hospital Medicine (SHM) and the CDC entered into a collaborative agreement to educate hospitalists about the reduction of hospital-acquired infections and the prevention of antimicrobial resistance. The long-term goals of this agreement include developing quality-improvement initiatives and research in the area of antimicrobial resistance reduction. The short-term goals include development of educational materials and resources for hospitalists aimed at reducing hospital-acquired infections and resistance. SHM has provided instruction in the reduction of hospital-acquired infections and antimicrobial resistance, in workshop format, to its membership at national, regional, and local chapter meetings. SHM has also developed an Internet-based educational tool for antimicrobial resistance on its Web site, which will soon be transformed into a new Web-based Resource Room to educate membership on antimicrobial resistance and reduction of hospital-acquired infections.
CDC Campaign
(www.cdc.gov/drugresistance/healthcare/)
The CDC, in collaboration with the National Institutes of Health (NIH) and the Food and Drug Administration (FDA), as well as professional societies, healthcare organizations, public health agencies, and corporate partners, has developed its Campaign to Prevent Antimicrobial Resistance to facilitate the implementation of educational and behavioral interventions that will assist clinicians in appropriate antimicrobial prescribing. The goals of these intervention programs are to improve clinician practices and prevent antimicrobial resistance. The campaign focuses on 4 main strategies: prevent infection, diagnose and treat infection, use antimicrobials wisely, and prevent transmission. Multiple 12-step programs have been developed (or are in the process of development), targeting specific patient populations, including hospitalized adults, dialysis patients, surgical patients, hospitalized children, and long-term-care patients. Each of these patient populations is relevant to the practicing hospitalist, who may access the educational materials and resources cost-free on the Internet. The CDC provides on-line resources (Web site listed above), including a downloadable slide-set, a 12-step fact sheet, and tips for patients. The program translates existing scientific evidence and national guidelines into action steps that can be taken now to prevent antimicrobial resistance.
The 12 Steps to Prevent Antimicrobial Resistance in Hospitalized Adults was the first intervention program to be initiated, because hospital patients are at especially high risk for serious antimicrobial-resistant infections. The rate of multiple drug-resistant organisms causing infection within our hospitals is increasing at a rapid rate. Currently, national data demonstrate that more than 50% of Staphylococcus aureus isolates causing infections in intensive care units (ICUs) are resistant to methicillin (MRSA), while more than 40% are resistant in other non-ICU hospital units (9). Similarly, gram-negative organisms have developed resistance, with more than 25% of Pseudomonas aeruginosa ICU isolates now resistant to fluoroquinolones (9), with a much higher percentage resistant at some institutions. This rapidly growing problem has led the CDC to develop the following 12 Steps to Prevent Antimicrobial Resistance in Hospitalized Adults:
- Vaccinate
- Get the catheters out
- Target the pathogen
- Access the experts
- Practice antimicrobial control
- Use local data
- Treat infection, not contamination
- Treat infection, not colonization
- Know when to say “no” to vanco
- Stop treatment when infection is cured or unlikely
- Isolate the pathogen
- Break the chain of contagion
Prevent Infection
Diagnose and Treat Infection Effectively
Use Antimicrobials Wisely
Prevent Transmission
These steps are designed to optimize patient safety and the outcome of infectious disease management, and hospitalists have the ability to utilize these recommendations to improve the care of their patients.
Hospitalists must employ efforts to prevent infections that may occur during hospitalization as well as those that may bring patients back to the hospital. Such efforts include predischarge influenza and pneumococcal vaccination when indicated, to reduce the more than 100,000 hospitalizations and 20,000 deaths due to influenza and the more than 12,000 deaths due to Streptococcus pneumoniae (10). Clinicians should get annual influenza vaccines as well, to reduce transmission to patients and to other healthcare workers.
Because catheters and other invasive devices are the No. 1 cause of hospital-acquired infections, evidence-based efforts must be utilized to reduce the likelihood of such infections. An estimated 250,000 catheter-related bloodstream infections (CR-BSI) occur each year, with an attributable cost of at least $25,000 per infection and an attributable mortality of 12–25% (11). Because of this, the CDC has recommended adherence to performance indicators for reducing bloodstream infections (8,12). Such performance indicators are based on strong evidence (13-15) and include the following:
- Appropriate site selection for catheter placement (i.e., subclavian over femoral or internal jugular) (14)
- Appropriate hand hygiene and aseptic technique (including use of maximal sterile barriers) during catheter placement
- Adequate skin asepsis (using chlorhexidine preferentially over iodine or alcohol based solutions) (15)
- Catheter discontinuation when no longer essential
- Antibiotic-impregnated catheters in high-risk patients
Recent studies have demonstrated that CR-BSI can be significantly reduced or even virtually eliminated with educational efforts combined with strict adherence to evidence based guidelines for prevention, as well as efforts to remove catheters early (16).
To diagnose and treat infections effectively, hospitalists must obtain appropriate cultures, target empiric therapy to the likely pathogens and local antibiogram data, and target final therapy to the known pathogens and antimicrobial susceptibility test results. The correct regimen, timing, dosage, route, and duration of antibiotic can impact morbidity and mortality in patients presenting with infectious diseases. Therefore, careful selection becomes crucial, and accessing infectious disease expertise in complex or critically ill patients with infectious diseases can be lifesaving.
Wise or appropriate use of antimicrobials can be facilitated by multiple efforts within hospitals. First, practicing antimicrobial control at the institutional level may involve use of standardized antimicrobial order forms, formulary restrictions, prior approval to start or continue specific antimicrobials, pharmacy substitution or switch, multidisciplinary drug utilization evaluation, provider performance feedback, or computerized decision support ordering systems. Many of these efforts can reduce costs while improving outcomes. Second, because the prevalence of resistance can vary by location, patient population, hospital unit, and length of stay, knowledge of the inpatient population that one treats (e.g., community vs. tertiary care, immunocompetent vs. immunosuppressed, or ICU vs. non-ICU) as well as the local antibiogram can help clinicians make decisions regarding initial antimicrobial selections.
Third, curbing antimicrobial overuse can be fostered by avoiding treatment of contamination or colonization. Contaminated cultures may be reduced by using and advocating proper antisepsis for blood cultures and other culture specimens. Recognition of organisms unlikely to represent true bacteremia (e.g., Corynebacterium), as well as those very likely to represent true bacteremia (e.g., Staphylococcus aureus or Entero-bacteriaceae), and interpreting culture results within clinical context help clinicians effectively treat positive cultures when indicated and avoid treating contaminants. Additionally, recognizing when cultures from urinary catheters, intravascular catheters, and endotracheal tubes represent colonization rather than infection and taking active steps to obtain accurate (rather than colonized) cultures can further curb nonindicated antibiotic use. For example, routinely sending catheter tips for culture is not indicated. Also, urinalysis should always accompany urine cultures sent from urinary catheters. Fourth, stopping antimicrobial therapy when infections are cured, cultures are negative and infection unlikely, or when infection is not diagnosed also limits antimicrobial overuse.
Finally, prevention of infection transmission from patient to patient or from healthcare worker to patient can be accomplished by use of standard infection control precautions, use of appropriate isolation precautions and handling of bodily fluids, and accessing infection control experts when questions arise. Frequent and effective hand hygiene as well as empowering all hospital staff to take part in and enforce infection control measures will help reduce transmission of infection by healthcare personnel.
In summary, antimicrobial resistance and hospital-acquired infections represent an enormous issue for patients, providers, hospitals, and the public. Hospitalists are positioned to take a large role in improving patient safety by supporting, following, and advocating the recommended guidelines and evidence-based measures to reduce the incidence of hospital-acquired infections at the local and national levels. Great investment of time, resources, and efforts in quality-improvement initiatives are necessary to reduce resistance, reduce infection, and improve overall outcomes for our patients.
References
- Burke JP. Infection control—a problem for patient safety. N Engl J Med. 2003; 348:651-6.
- Jarvis WR. Infection control and changing health-care delivery systems. Emerg Infect Dis. 2001;7:170-3.
- Stone PW, Larson E, Kawar LN. A systematic audit of economic evidence linking nosocomial infections and infection control interventions: 1990–2000. Am J Infect Control. 2002;30:145-52.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess. 2001;43: i-x, 1-668. Review. Full report available at www.ahrq.gov.
- National Nosocomial Infections Surveillance (NNIS) system report, data summary from October 1986- April 1996, issued May 1996. A report from the National Nosocomial Infections Surveillance (NNIS) system. Am J Infect Control. 1996;24:380-8.
- Gerberding JL. Hospital-onset infections: a patient safety issue. Ann Intern Med. 2002;137:665-70.
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-94.
- O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51:1-29.
- National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32: 470-85.
- Influenza and Pneumococcal Vaccination Levels Among Persons Aged ≥65 Years--United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:532-7.
- Kluger DM, Maki DG. The relative risk of intravascular device related bloodstream infections in adults. Abstracts of the 39th Interscience Conference on Antimicrob Agents Chemother. 1999:514.
- Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132:391-402.
- McGee DC, Gould MK. Preventing Complications of Central Venous Catheterization. N Engl J Med. 2003;348:1123-33.
- Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286:700-7.
- Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136:792-801.
- Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014-20.
While infections that develop during hospitalization may appear to be an uncommon but recognized risk of hospital care today, the incidence of these infections has been increasing dramatically during the last 2 to 3 decades, and the risk of acquiring an organism that is resistant to 1 or more antibiotics is becoming increasingly common. Recent studies estimate that approximately 2 million patients contract healthcare-associated infections each year (1). These infections are the most common type of serious adverse event in health care, affecting up to 5–10% of hospitalized patients, leading to approximately 90,000 deaths annually, and adding approximately $5 billion to annual healthcare costs (1-3). Increasingly, healthcare-associated infection risk is viewed as a patient safety issue, as many of these infections may be avoidable or preventable by following evidence-based best practices in infection control and patient care while patients are hospitalized. This article will summarize some of the overlap between patient safety and infection control, explain some of the pressures that have led to development and cultivation of antimicrobial resistance, and describe the Centers for Disease Control and Prevention (CDC) campaign for prevention of healthcare-associated infections and antimicrobial resistance, as well as the role of hospitalists in such prevention.
Patient Safety
The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) specifically identifies in its 2005 National Patient Safety Goals that hospitals and clinicians reduce the risk of healthcare-associated infections. The goals encourage clinicians to comply with current CDC hand hygiene guidelines and that hospitals and clinicians manage as sentinel events all identified cases of unanticipated death or permanent loss of function associated with a heathcare-associated infection. A sentinel event is defined by JCAHO as an unexpected occurrence involving death or serious physical or psychological injury. Such an event signals the need for immediate investigation and response by the institution. By including healthcare-associated infections in this category of high-risk event, with potential morbidity and mortality, JCAHO highlights the frequency and importance of infections acquired in our healthcare system today.
Further, the Agency for Healthcare Research and Quality (AHRQ) recently published an evidence-based report, developed and written primarily by hospitalists, delineating 79 patient safety practices, of which 22 (28%) involved infection control (4). At least 5 of these 22 infection control practices were considered valuable enough, and with sufficiently strong supporting evidence, to mandate widespread implementation. Additionally, the Institute for Healthcare Improvement (IHI; www.ihi.org) recently launched its 100,000 Lives Campaign, enlisting hundreds of hospitals around the United States in a commitment to implement changes that have been proven to prevent avoidable deaths. Three of their first 6 interventions involve the reduction of healthcare-associated infections, including central-line infections, surgical-site infections, and hospital-acquired pneumonia.
Increasingly, hospital-onset infections have become a patient safety issue, and they will remain under public and institutional scrutiny while hospitals take efforts to reduce their incidence and improve care quality. Hospitalists have evolved to serve a unique role as advocate of both patients and hospitals. They should therefore foster quality improvement in the hospital, as well as lead and support initiatives that reduce hospital-acquired infections and resistance.
Healthcare-Associated Infections and Development of Resistance
Bacteria have developed multiple microbiologic and genetic mechanisms to elude antimicrobial agents. Certain practices in medical care, whether intentional or not, can promote persistence or spread of resistant microbes that can cause infections. Such practices may include:
- Inattention to basic infection control measures (e.g., hand washing)
- Unrecognized colonization (e.g., treating colonized urinary or vascular catheters, without evidence of infection)
- Unrecognized reservoirs (e.g., environmental)
- Selective pressure from overuse or inappropriate use of antibiotics
- Movement of patients and staff within a single institution and between institutions
Inappropriate use or overuse of antibiotics can actually remove or “select” the sensitive microbes and promote overgrowth of resistant organisms when present. Each of these practices may serve as a focus for quality improvement interventions to reduce resistance.
Most healthcare-associated infections (more than 80%) originate from 4 specific patient sites: urinary tract, surgical-site (wound), bloodstream, and lung (pneumonia) (5). It is not coincidental that these infection sites are frequently associated with invasive procedures, and many times with indwelling invasive devices that may be used during the course of inpatient care. For example, urinary tract infections, the most common hospital-acquired infections, are usually associated with urinary catheter use. Similarly, bloodstream infections are usually associated with intravascular catheters, and hospital-acquired pneumonia is usually associated with ventilator use.
Because many of the invasive devices that are utilized during the course of inpatient care carry significant risk, including infection risk, it is incumbent upon hospitalists to be aware of these risks, to explain these risks to their patients, and to take all steps at their disposal to help reduce such risk in their patients. Dr. Julie Gerberding, Director of the CDC, has emphasized that the 2 greatest predictors of infection risk in the hospital are length of stay and use of invasive devices (6). While excellent evidence already demonstrates that hospitalists reduce length of stay (7), they should also spearhead the efforts to minimize the use of invasive devices whenever possible, and lead evidence-based efforts to minimize infection in hospitalized patients when invasive devices must be used.
Prevention of Resistance: Best Practices
CDC/SHM Collaboration
In September 2003, the Society of Hospital Medicine (SHM) and the CDC entered into a collaborative agreement to educate hospitalists about the reduction of hospital-acquired infections and the prevention of antimicrobial resistance. The long-term goals of this agreement include developing quality-improvement initiatives and research in the area of antimicrobial resistance reduction. The short-term goals include development of educational materials and resources for hospitalists aimed at reducing hospital-acquired infections and resistance. SHM has provided instruction in the reduction of hospital-acquired infections and antimicrobial resistance, in workshop format, to its membership at national, regional, and local chapter meetings. SHM has also developed an Internet-based educational tool for antimicrobial resistance on its Web site, which will soon be transformed into a new Web-based Resource Room to educate membership on antimicrobial resistance and reduction of hospital-acquired infections.
CDC Campaign
(www.cdc.gov/drugresistance/healthcare/)
The CDC, in collaboration with the National Institutes of Health (NIH) and the Food and Drug Administration (FDA), as well as professional societies, healthcare organizations, public health agencies, and corporate partners, has developed its Campaign to Prevent Antimicrobial Resistance to facilitate the implementation of educational and behavioral interventions that will assist clinicians in appropriate antimicrobial prescribing. The goals of these intervention programs are to improve clinician practices and prevent antimicrobial resistance. The campaign focuses on 4 main strategies: prevent infection, diagnose and treat infection, use antimicrobials wisely, and prevent transmission. Multiple 12-step programs have been developed (or are in the process of development), targeting specific patient populations, including hospitalized adults, dialysis patients, surgical patients, hospitalized children, and long-term-care patients. Each of these patient populations is relevant to the practicing hospitalist, who may access the educational materials and resources cost-free on the Internet. The CDC provides on-line resources (Web site listed above), including a downloadable slide-set, a 12-step fact sheet, and tips for patients. The program translates existing scientific evidence and national guidelines into action steps that can be taken now to prevent antimicrobial resistance.
The 12 Steps to Prevent Antimicrobial Resistance in Hospitalized Adults was the first intervention program to be initiated, because hospital patients are at especially high risk for serious antimicrobial-resistant infections. The rate of multiple drug-resistant organisms causing infection within our hospitals is increasing at a rapid rate. Currently, national data demonstrate that more than 50% of Staphylococcus aureus isolates causing infections in intensive care units (ICUs) are resistant to methicillin (MRSA), while more than 40% are resistant in other non-ICU hospital units (9). Similarly, gram-negative organisms have developed resistance, with more than 25% of Pseudomonas aeruginosa ICU isolates now resistant to fluoroquinolones (9), with a much higher percentage resistant at some institutions. This rapidly growing problem has led the CDC to develop the following 12 Steps to Prevent Antimicrobial Resistance in Hospitalized Adults:
- Vaccinate
- Get the catheters out
- Target the pathogen
- Access the experts
- Practice antimicrobial control
- Use local data
- Treat infection, not contamination
- Treat infection, not colonization
- Know when to say “no” to vanco
- Stop treatment when infection is cured or unlikely
- Isolate the pathogen
- Break the chain of contagion
Prevent Infection
Diagnose and Treat Infection Effectively
Use Antimicrobials Wisely
Prevent Transmission
These steps are designed to optimize patient safety and the outcome of infectious disease management, and hospitalists have the ability to utilize these recommendations to improve the care of their patients.
Hospitalists must employ efforts to prevent infections that may occur during hospitalization as well as those that may bring patients back to the hospital. Such efforts include predischarge influenza and pneumococcal vaccination when indicated, to reduce the more than 100,000 hospitalizations and 20,000 deaths due to influenza and the more than 12,000 deaths due to Streptococcus pneumoniae (10). Clinicians should get annual influenza vaccines as well, to reduce transmission to patients and to other healthcare workers.
Because catheters and other invasive devices are the No. 1 cause of hospital-acquired infections, evidence-based efforts must be utilized to reduce the likelihood of such infections. An estimated 250,000 catheter-related bloodstream infections (CR-BSI) occur each year, with an attributable cost of at least $25,000 per infection and an attributable mortality of 12–25% (11). Because of this, the CDC has recommended adherence to performance indicators for reducing bloodstream infections (8,12). Such performance indicators are based on strong evidence (13-15) and include the following:
- Appropriate site selection for catheter placement (i.e., subclavian over femoral or internal jugular) (14)
- Appropriate hand hygiene and aseptic technique (including use of maximal sterile barriers) during catheter placement
- Adequate skin asepsis (using chlorhexidine preferentially over iodine or alcohol based solutions) (15)
- Catheter discontinuation when no longer essential
- Antibiotic-impregnated catheters in high-risk patients
Recent studies have demonstrated that CR-BSI can be significantly reduced or even virtually eliminated with educational efforts combined with strict adherence to evidence based guidelines for prevention, as well as efforts to remove catheters early (16).
To diagnose and treat infections effectively, hospitalists must obtain appropriate cultures, target empiric therapy to the likely pathogens and local antibiogram data, and target final therapy to the known pathogens and antimicrobial susceptibility test results. The correct regimen, timing, dosage, route, and duration of antibiotic can impact morbidity and mortality in patients presenting with infectious diseases. Therefore, careful selection becomes crucial, and accessing infectious disease expertise in complex or critically ill patients with infectious diseases can be lifesaving.
Wise or appropriate use of antimicrobials can be facilitated by multiple efforts within hospitals. First, practicing antimicrobial control at the institutional level may involve use of standardized antimicrobial order forms, formulary restrictions, prior approval to start or continue specific antimicrobials, pharmacy substitution or switch, multidisciplinary drug utilization evaluation, provider performance feedback, or computerized decision support ordering systems. Many of these efforts can reduce costs while improving outcomes. Second, because the prevalence of resistance can vary by location, patient population, hospital unit, and length of stay, knowledge of the inpatient population that one treats (e.g., community vs. tertiary care, immunocompetent vs. immunosuppressed, or ICU vs. non-ICU) as well as the local antibiogram can help clinicians make decisions regarding initial antimicrobial selections.
Third, curbing antimicrobial overuse can be fostered by avoiding treatment of contamination or colonization. Contaminated cultures may be reduced by using and advocating proper antisepsis for blood cultures and other culture specimens. Recognition of organisms unlikely to represent true bacteremia (e.g., Corynebacterium), as well as those very likely to represent true bacteremia (e.g., Staphylococcus aureus or Entero-bacteriaceae), and interpreting culture results within clinical context help clinicians effectively treat positive cultures when indicated and avoid treating contaminants. Additionally, recognizing when cultures from urinary catheters, intravascular catheters, and endotracheal tubes represent colonization rather than infection and taking active steps to obtain accurate (rather than colonized) cultures can further curb nonindicated antibiotic use. For example, routinely sending catheter tips for culture is not indicated. Also, urinalysis should always accompany urine cultures sent from urinary catheters. Fourth, stopping antimicrobial therapy when infections are cured, cultures are negative and infection unlikely, or when infection is not diagnosed also limits antimicrobial overuse.
Finally, prevention of infection transmission from patient to patient or from healthcare worker to patient can be accomplished by use of standard infection control precautions, use of appropriate isolation precautions and handling of bodily fluids, and accessing infection control experts when questions arise. Frequent and effective hand hygiene as well as empowering all hospital staff to take part in and enforce infection control measures will help reduce transmission of infection by healthcare personnel.
In summary, antimicrobial resistance and hospital-acquired infections represent an enormous issue for patients, providers, hospitals, and the public. Hospitalists are positioned to take a large role in improving patient safety by supporting, following, and advocating the recommended guidelines and evidence-based measures to reduce the incidence of hospital-acquired infections at the local and national levels. Great investment of time, resources, and efforts in quality-improvement initiatives are necessary to reduce resistance, reduce infection, and improve overall outcomes for our patients.
References
- Burke JP. Infection control—a problem for patient safety. N Engl J Med. 2003; 348:651-6.
- Jarvis WR. Infection control and changing health-care delivery systems. Emerg Infect Dis. 2001;7:170-3.
- Stone PW, Larson E, Kawar LN. A systematic audit of economic evidence linking nosocomial infections and infection control interventions: 1990–2000. Am J Infect Control. 2002;30:145-52.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess. 2001;43: i-x, 1-668. Review. Full report available at www.ahrq.gov.
- National Nosocomial Infections Surveillance (NNIS) system report, data summary from October 1986- April 1996, issued May 1996. A report from the National Nosocomial Infections Surveillance (NNIS) system. Am J Infect Control. 1996;24:380-8.
- Gerberding JL. Hospital-onset infections: a patient safety issue. Ann Intern Med. 2002;137:665-70.
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-94.
- O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51:1-29.
- National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32: 470-85.
- Influenza and Pneumococcal Vaccination Levels Among Persons Aged ≥65 Years--United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:532-7.
- Kluger DM, Maki DG. The relative risk of intravascular device related bloodstream infections in adults. Abstracts of the 39th Interscience Conference on Antimicrob Agents Chemother. 1999:514.
- Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132:391-402.
- McGee DC, Gould MK. Preventing Complications of Central Venous Catheterization. N Engl J Med. 2003;348:1123-33.
- Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286:700-7.
- Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136:792-801.
- Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014-20.
While infections that develop during hospitalization may appear to be an uncommon but recognized risk of hospital care today, the incidence of these infections has been increasing dramatically during the last 2 to 3 decades, and the risk of acquiring an organism that is resistant to 1 or more antibiotics is becoming increasingly common. Recent studies estimate that approximately 2 million patients contract healthcare-associated infections each year (1). These infections are the most common type of serious adverse event in health care, affecting up to 5–10% of hospitalized patients, leading to approximately 90,000 deaths annually, and adding approximately $5 billion to annual healthcare costs (1-3). Increasingly, healthcare-associated infection risk is viewed as a patient safety issue, as many of these infections may be avoidable or preventable by following evidence-based best practices in infection control and patient care while patients are hospitalized. This article will summarize some of the overlap between patient safety and infection control, explain some of the pressures that have led to development and cultivation of antimicrobial resistance, and describe the Centers for Disease Control and Prevention (CDC) campaign for prevention of healthcare-associated infections and antimicrobial resistance, as well as the role of hospitalists in such prevention.
Patient Safety
The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) specifically identifies in its 2005 National Patient Safety Goals that hospitals and clinicians reduce the risk of healthcare-associated infections. The goals encourage clinicians to comply with current CDC hand hygiene guidelines and that hospitals and clinicians manage as sentinel events all identified cases of unanticipated death or permanent loss of function associated with a heathcare-associated infection. A sentinel event is defined by JCAHO as an unexpected occurrence involving death or serious physical or psychological injury. Such an event signals the need for immediate investigation and response by the institution. By including healthcare-associated infections in this category of high-risk event, with potential morbidity and mortality, JCAHO highlights the frequency and importance of infections acquired in our healthcare system today.
Further, the Agency for Healthcare Research and Quality (AHRQ) recently published an evidence-based report, developed and written primarily by hospitalists, delineating 79 patient safety practices, of which 22 (28%) involved infection control (4). At least 5 of these 22 infection control practices were considered valuable enough, and with sufficiently strong supporting evidence, to mandate widespread implementation. Additionally, the Institute for Healthcare Improvement (IHI; www.ihi.org) recently launched its 100,000 Lives Campaign, enlisting hundreds of hospitals around the United States in a commitment to implement changes that have been proven to prevent avoidable deaths. Three of their first 6 interventions involve the reduction of healthcare-associated infections, including central-line infections, surgical-site infections, and hospital-acquired pneumonia.
Increasingly, hospital-onset infections have become a patient safety issue, and they will remain under public and institutional scrutiny while hospitals take efforts to reduce their incidence and improve care quality. Hospitalists have evolved to serve a unique role as advocate of both patients and hospitals. They should therefore foster quality improvement in the hospital, as well as lead and support initiatives that reduce hospital-acquired infections and resistance.
Healthcare-Associated Infections and Development of Resistance
Bacteria have developed multiple microbiologic and genetic mechanisms to elude antimicrobial agents. Certain practices in medical care, whether intentional or not, can promote persistence or spread of resistant microbes that can cause infections. Such practices may include:
- Inattention to basic infection control measures (e.g., hand washing)
- Unrecognized colonization (e.g., treating colonized urinary or vascular catheters, without evidence of infection)
- Unrecognized reservoirs (e.g., environmental)
- Selective pressure from overuse or inappropriate use of antibiotics
- Movement of patients and staff within a single institution and between institutions
Inappropriate use or overuse of antibiotics can actually remove or “select” the sensitive microbes and promote overgrowth of resistant organisms when present. Each of these practices may serve as a focus for quality improvement interventions to reduce resistance.
Most healthcare-associated infections (more than 80%) originate from 4 specific patient sites: urinary tract, surgical-site (wound), bloodstream, and lung (pneumonia) (5). It is not coincidental that these infection sites are frequently associated with invasive procedures, and many times with indwelling invasive devices that may be used during the course of inpatient care. For example, urinary tract infections, the most common hospital-acquired infections, are usually associated with urinary catheter use. Similarly, bloodstream infections are usually associated with intravascular catheters, and hospital-acquired pneumonia is usually associated with ventilator use.
Because many of the invasive devices that are utilized during the course of inpatient care carry significant risk, including infection risk, it is incumbent upon hospitalists to be aware of these risks, to explain these risks to their patients, and to take all steps at their disposal to help reduce such risk in their patients. Dr. Julie Gerberding, Director of the CDC, has emphasized that the 2 greatest predictors of infection risk in the hospital are length of stay and use of invasive devices (6). While excellent evidence already demonstrates that hospitalists reduce length of stay (7), they should also spearhead the efforts to minimize the use of invasive devices whenever possible, and lead evidence-based efforts to minimize infection in hospitalized patients when invasive devices must be used.
Prevention of Resistance: Best Practices
CDC/SHM Collaboration
In September 2003, the Society of Hospital Medicine (SHM) and the CDC entered into a collaborative agreement to educate hospitalists about the reduction of hospital-acquired infections and the prevention of antimicrobial resistance. The long-term goals of this agreement include developing quality-improvement initiatives and research in the area of antimicrobial resistance reduction. The short-term goals include development of educational materials and resources for hospitalists aimed at reducing hospital-acquired infections and resistance. SHM has provided instruction in the reduction of hospital-acquired infections and antimicrobial resistance, in workshop format, to its membership at national, regional, and local chapter meetings. SHM has also developed an Internet-based educational tool for antimicrobial resistance on its Web site, which will soon be transformed into a new Web-based Resource Room to educate membership on antimicrobial resistance and reduction of hospital-acquired infections.
CDC Campaign
(www.cdc.gov/drugresistance/healthcare/)
The CDC, in collaboration with the National Institutes of Health (NIH) and the Food and Drug Administration (FDA), as well as professional societies, healthcare organizations, public health agencies, and corporate partners, has developed its Campaign to Prevent Antimicrobial Resistance to facilitate the implementation of educational and behavioral interventions that will assist clinicians in appropriate antimicrobial prescribing. The goals of these intervention programs are to improve clinician practices and prevent antimicrobial resistance. The campaign focuses on 4 main strategies: prevent infection, diagnose and treat infection, use antimicrobials wisely, and prevent transmission. Multiple 12-step programs have been developed (or are in the process of development), targeting specific patient populations, including hospitalized adults, dialysis patients, surgical patients, hospitalized children, and long-term-care patients. Each of these patient populations is relevant to the practicing hospitalist, who may access the educational materials and resources cost-free on the Internet. The CDC provides on-line resources (Web site listed above), including a downloadable slide-set, a 12-step fact sheet, and tips for patients. The program translates existing scientific evidence and national guidelines into action steps that can be taken now to prevent antimicrobial resistance.
The 12 Steps to Prevent Antimicrobial Resistance in Hospitalized Adults was the first intervention program to be initiated, because hospital patients are at especially high risk for serious antimicrobial-resistant infections. The rate of multiple drug-resistant organisms causing infection within our hospitals is increasing at a rapid rate. Currently, national data demonstrate that more than 50% of Staphylococcus aureus isolates causing infections in intensive care units (ICUs) are resistant to methicillin (MRSA), while more than 40% are resistant in other non-ICU hospital units (9). Similarly, gram-negative organisms have developed resistance, with more than 25% of Pseudomonas aeruginosa ICU isolates now resistant to fluoroquinolones (9), with a much higher percentage resistant at some institutions. This rapidly growing problem has led the CDC to develop the following 12 Steps to Prevent Antimicrobial Resistance in Hospitalized Adults:
- Vaccinate
- Get the catheters out
- Target the pathogen
- Access the experts
- Practice antimicrobial control
- Use local data
- Treat infection, not contamination
- Treat infection, not colonization
- Know when to say “no” to vanco
- Stop treatment when infection is cured or unlikely
- Isolate the pathogen
- Break the chain of contagion
Prevent Infection
Diagnose and Treat Infection Effectively
Use Antimicrobials Wisely
Prevent Transmission
These steps are designed to optimize patient safety and the outcome of infectious disease management, and hospitalists have the ability to utilize these recommendations to improve the care of their patients.
Hospitalists must employ efforts to prevent infections that may occur during hospitalization as well as those that may bring patients back to the hospital. Such efforts include predischarge influenza and pneumococcal vaccination when indicated, to reduce the more than 100,000 hospitalizations and 20,000 deaths due to influenza and the more than 12,000 deaths due to Streptococcus pneumoniae (10). Clinicians should get annual influenza vaccines as well, to reduce transmission to patients and to other healthcare workers.
Because catheters and other invasive devices are the No. 1 cause of hospital-acquired infections, evidence-based efforts must be utilized to reduce the likelihood of such infections. An estimated 250,000 catheter-related bloodstream infections (CR-BSI) occur each year, with an attributable cost of at least $25,000 per infection and an attributable mortality of 12–25% (11). Because of this, the CDC has recommended adherence to performance indicators for reducing bloodstream infections (8,12). Such performance indicators are based on strong evidence (13-15) and include the following:
- Appropriate site selection for catheter placement (i.e., subclavian over femoral or internal jugular) (14)
- Appropriate hand hygiene and aseptic technique (including use of maximal sterile barriers) during catheter placement
- Adequate skin asepsis (using chlorhexidine preferentially over iodine or alcohol based solutions) (15)
- Catheter discontinuation when no longer essential
- Antibiotic-impregnated catheters in high-risk patients
Recent studies have demonstrated that CR-BSI can be significantly reduced or even virtually eliminated with educational efforts combined with strict adherence to evidence based guidelines for prevention, as well as efforts to remove catheters early (16).
To diagnose and treat infections effectively, hospitalists must obtain appropriate cultures, target empiric therapy to the likely pathogens and local antibiogram data, and target final therapy to the known pathogens and antimicrobial susceptibility test results. The correct regimen, timing, dosage, route, and duration of antibiotic can impact morbidity and mortality in patients presenting with infectious diseases. Therefore, careful selection becomes crucial, and accessing infectious disease expertise in complex or critically ill patients with infectious diseases can be lifesaving.
Wise or appropriate use of antimicrobials can be facilitated by multiple efforts within hospitals. First, practicing antimicrobial control at the institutional level may involve use of standardized antimicrobial order forms, formulary restrictions, prior approval to start or continue specific antimicrobials, pharmacy substitution or switch, multidisciplinary drug utilization evaluation, provider performance feedback, or computerized decision support ordering systems. Many of these efforts can reduce costs while improving outcomes. Second, because the prevalence of resistance can vary by location, patient population, hospital unit, and length of stay, knowledge of the inpatient population that one treats (e.g., community vs. tertiary care, immunocompetent vs. immunosuppressed, or ICU vs. non-ICU) as well as the local antibiogram can help clinicians make decisions regarding initial antimicrobial selections.
Third, curbing antimicrobial overuse can be fostered by avoiding treatment of contamination or colonization. Contaminated cultures may be reduced by using and advocating proper antisepsis for blood cultures and other culture specimens. Recognition of organisms unlikely to represent true bacteremia (e.g., Corynebacterium), as well as those very likely to represent true bacteremia (e.g., Staphylococcus aureus or Entero-bacteriaceae), and interpreting culture results within clinical context help clinicians effectively treat positive cultures when indicated and avoid treating contaminants. Additionally, recognizing when cultures from urinary catheters, intravascular catheters, and endotracheal tubes represent colonization rather than infection and taking active steps to obtain accurate (rather than colonized) cultures can further curb nonindicated antibiotic use. For example, routinely sending catheter tips for culture is not indicated. Also, urinalysis should always accompany urine cultures sent from urinary catheters. Fourth, stopping antimicrobial therapy when infections are cured, cultures are negative and infection unlikely, or when infection is not diagnosed also limits antimicrobial overuse.
Finally, prevention of infection transmission from patient to patient or from healthcare worker to patient can be accomplished by use of standard infection control precautions, use of appropriate isolation precautions and handling of bodily fluids, and accessing infection control experts when questions arise. Frequent and effective hand hygiene as well as empowering all hospital staff to take part in and enforce infection control measures will help reduce transmission of infection by healthcare personnel.
In summary, antimicrobial resistance and hospital-acquired infections represent an enormous issue for patients, providers, hospitals, and the public. Hospitalists are positioned to take a large role in improving patient safety by supporting, following, and advocating the recommended guidelines and evidence-based measures to reduce the incidence of hospital-acquired infections at the local and national levels. Great investment of time, resources, and efforts in quality-improvement initiatives are necessary to reduce resistance, reduce infection, and improve overall outcomes for our patients.
References
- Burke JP. Infection control—a problem for patient safety. N Engl J Med. 2003; 348:651-6.
- Jarvis WR. Infection control and changing health-care delivery systems. Emerg Infect Dis. 2001;7:170-3.
- Stone PW, Larson E, Kawar LN. A systematic audit of economic evidence linking nosocomial infections and infection control interventions: 1990–2000. Am J Infect Control. 2002;30:145-52.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess. 2001;43: i-x, 1-668. Review. Full report available at www.ahrq.gov.
- National Nosocomial Infections Surveillance (NNIS) system report, data summary from October 1986- April 1996, issued May 1996. A report from the National Nosocomial Infections Surveillance (NNIS) system. Am J Infect Control. 1996;24:380-8.
- Gerberding JL. Hospital-onset infections: a patient safety issue. Ann Intern Med. 2002;137:665-70.
- Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-94.
- O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51:1-29.
- National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32: 470-85.
- Influenza and Pneumococcal Vaccination Levels Among Persons Aged ≥65 Years--United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:532-7.
- Kluger DM, Maki DG. The relative risk of intravascular device related bloodstream infections in adults. Abstracts of the 39th Interscience Conference on Antimicrob Agents Chemother. 1999:514.
- Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132:391-402.
- McGee DC, Gould MK. Preventing Complications of Central Venous Catheterization. N Engl J Med. 2003;348:1123-33.
- Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286:700-7.
- Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136:792-801.
- Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014-20.
The Inpatient with AIDS: What the Hospitalist Needs to Know
The opinions and assertions contained herein are those of the authors and are not to be construed as official or as reflecting the views of the Department of Defense, the Department of the Navy, or the naval services at large.
Introduction
An estimated 850,000 to 950,000 persons in the United States are living with human immunodeficiency virus (HIV), 280,000 of whom are unaware of their infection and another 43,000 of whom meet the definition of acquired immunodeficiency syndrome (AIDS) (www.cdc.gov). The use of highly active antiretroviral therapy (HAART) has produced significant declines in morbidity and mortality from AIDS. Compared with the first 2 decades of the HIV pandemic, the number of HIV-related hospital admissions has declined. However, recently, this rate of decline has markedly slowed (1-3). The reasons for this plateau are many including a steady number of admissions for complications related to HAART, treatment failures, and the overall increased prevalence of HIV infection. Not only will HIV-infected patients still frequently require admission to the hospital, but the complexity of their inpatient care will continue to increase with the advancements in multiple drug regimens, aging of the HIV-infected population, and the interaction of HIV infection with medical comorbidities, many of which are attributable to HAART.
The hospitalist caring for the inpatient with AIDS is presented with several challenges including not only the diagnosis and management of opportunistic infections, but also the complications of HAART. In this article we review the guidelines for the initiation and continuation of HAART in the hospital, review important clinical complications of antiretroviral therapy, and review conditions that may result in the hospitalization of AIDS patients.
Initiation of HAART in the Hospital
In those who do not have access to health care, the initial diagnosis of HIV infection frequently occurs during a hospitalization for an AIDS-defining illness. Initiation of antiretrovirals is contingent on several issues, including CD4 count, viral load, clinical status, likelihood of continued adherence, and the concurrent treatment of opportunistic infections (OIs). All patients with HIV infection and a CD4 count <200 cells/mm3 or an AIDS-defining illness should receive antiretroviral therapy. Controversy exists as to whether a patient admitted for the treatment of an opportunistic infection should begin antiretroviral therapy immediately, or whether this therapy should be deferred until after acute treatment of the OI. The potential detrimental effects of drug-drug interactions, the need for treatment interruptions, and drug-related toxicity between antiretrovirals and OI-specific therapy may support initiating HAART after control of an OI is achieved. Conversely, for some opportunistic infections, such as cryptosporidiosis, the use of HAART is essential for successful treatment of the infection.
An ongoing randomized controlled trial initiated within the Adult AIDS Clinical Trials Group (ACTG) comparing outcomes between patients who start HAART immediately after presentation with an acute OI and patients who start HAART at least 4 weeks after the OI has resolved should help identify the factors supporting early or delayed initiation of antiretrovirals (4).
Generally speaking, HAART can be administered by combining either a protease inhibitor (PI) or a nonnucleoside reverse transcriptase inhibitor (NNRTI) with 2 nucleoside reverse transcriptase inhibitors (NRTIs). There are currently more than 20 FDA-approved antiretrovirals. Frequent updates on the guidelines for the use of antiretroviral agents in HIV-infected adults are available at www.AIDSinfo.nih.gov, and a discussion of this topic is beyond the scope of this review.
Continuation of HAART in the Hospital
In most cases, every effort should be made to minimize interruption of HAART during a hospitalization. Although some investigators are examining the virologic and immunologic safety of interrupting HAART as a treatment strategy, there are few data on viral replication, CD4 cell count decline, and rate of acquisition of new mutations in hospitalized patients who have unexpected treatment interruptions (5). The long half-life of some antiretrovirals promotes the emergence of resistance once HAART is stopped. For example, once NNRTIs are stopped, subtherapeutic levels remain in the plasma and cells for several days. HIV then replicates in a milieu that may select for resistance mutations.
Because zidovudine is the only antiretroviral available in a parenteral preparation, it is often difficult to continue HAART when a patient cannot take medications by mouth. Drugs given by the enteral route in a hospitalized patient may also be poorly absorbed, and few data exist on the absorption of antiretrovirals administered through a gastrostomy or jejunostomy tube (6).
Prescribing HAART in the Hospital
Antiretroviral prescribing errors occur frequently in the hospitalized AIDS patient. The most common errors include overdosing or underdosing, missing components of multidrug regimens, or missing critical drug-drug interactions (7). Underdosing may lead to resistance, and overdosing contributes to increased toxicity. In one report, prescribing errors occurred in 12% of admissions in the post-HAART era (1998) compared with 2% of admissions in the pre-HAART era (1996) (7). The NRTIs, including didanosine, emtricitabine, lamivudine, stavudine, and zidovudine, require decreased dosing in renal insufficiency. Tenofovir is not recommended for use if the creatine clearance is less than 60 mL/minute. Dosage adjustments in hepatic disease are recommended for amprenavir, fosamprenavir, delavirdine, efavirenz, and nevirapine.
Immune Reconstitution Syndrome
The widespread use of HAART has produced sustained suppression of HIV replication and recovery of CD4 cell counts. It also became evident that HAART resulted in not only a numerical increase in CD4 cells, but also in a functional immune recovery (8-10). This improved T-cell response to antigens results in adequate protection against specific opportunistic infections, allowing for discontinuation of primary and secondary prophylaxis in HIV-infected patients. Immune reconstitution syndrome (IRS), an inflammatory syndrome, is recognized as a potential complication that can occur days to months after starting HAART. The onset of IRS is characterized by a paradoxical worsening of clinical or laboratory parameters despite a favorable response in CD4 cell counts and the suppression of viral replication (9,11,12). IRS has been reported to occur in 10–25% of patients who receive HAART and more commonly in those whose CD4 cell counts are <50 cells/mm3 at the start of HAART (9,11). It is postulated that the inflammatory response is triggered by the recognition of antigens associated with ongoing infection or recognition of persisting antigens associated with past (nonreplicating) infections. Mycobacterial antigens, frequently implicated in IRS, are responsible for about one third of cases. Other antigens associated with IRS include cytomegalovirus and hepatitis B and C (11). In most circumstances, with the management of IRS, HAART should be continued, while specific antimicrobial therapy and steroids should be considered (10).
Medical Conditions that Should Prompt HIV Screening
There are several medical conditions that should prompt screening for HIV infection. Generally, anyone presenting with a fever of unknown etiology who is sexually active or had a blood transfusion prior to 1985 should be screened for HIV infection. Symptoms consistent with acute retroviral syndrome (fever, sore throat, malaise, and skin rash) may be more commonly recognized by clinicians now than previously, and this remains a “golden opportunity” to intervene. Frequently, acute retroviral syndrome will be attributed to Epstein-Barr virus; however, caution should be used in the diagnosis of mononucleosis in those other than teenage populations. It is recommended that all persons presenting with any sexually transmitted disease, unexplained generalized lymphadenopathy, oral candidiasis, or tuberculosis should also be tested. Other conditions where HIV infection should be considered include enigmatic pneumonia, acute hepatitis B infection, herpes zoster infection (particularly in younger, seemingly immunocompetent individuals), idiopathic thrombocytopenic purpura, and nephropathy of unknown cause.
Drug Interactions
Drug interactions are an important consideration in the treatment of HIV infection. Interactions between HAART and other drugs used for the treatment or prophylaxis of opportunistic infections along with those used for the treatment of drug-induced endocrinopathies (hyperlipidemia, diabetes mellitus) are virtually unavoidable. Drug interactions occur either because of drug metabolism or absorption. The multiple metabolic pathways of some drugs make it difficult to predict the outcome of drug interactions. All protease inhibitors and non-nucleoside reverse transcriptase inhibitors are metabolized by the cytochrome P-450 enzyme system and each of these drugs may alter the metabolism of other antiretrovirals and concomitantly administered drugs (13,14). A decrease in trough plasma concentrations of the protease inhibitors to a level below the in vitro concentration required to inhibit replication of 50% of viral strains (IC50) may lead to development of resistance. Because nucleoside analogue reverse transcriptase inhibitors are primarily eliminated by the kidney, they do not interact with other drugs through the cytochrome P-450 system.
One noteworthy interaction that the clinician caring for HIV-infected patients should be aware of is the interaction of ribavirin with zidovudine. Ribavirin decreases the phosphorylation of zidovudine and stavudine in vitro, resulting in decreased concentrations of the active compound. HIV-infected patients who are coinfected with hepatitis C may be treated with regimens that include ribavirin, which may reduce the efficacy of zidovudine (15). Another important interaction is the effect of nevirapine or efavirenz on plasma methadone concentrations. Both drugs can decrease methadone plasma levels by 50%, and patients receiving chronic therapy may need increased methadone doses to prevent withdrawal symptoms (16).
Protease inhibitors are associated with numerous interactions including certain antiarrhythmics, sedatives, hypnotics, ergot derivatives, and several lipid-lowering agents (statins). Not only do protease inhibitors affect the metabolism of certain drugs, but also their own metabolism is altered by other inducers or inhibitors of cytochrome activity that can cause clinically important decreases in serum levels of protease inhibitors. One widely recognized interaction is that of rifampin, which may decrease levels of some protease inhibitors by 80%. The resulting low plasma concentrations may promote viral resistance and result in treatment failure. Patients being treated for tuberculosis, who are also receiving protease inhibitors should be treated with a four-drug regimen that includes rifabutin (at half dose) instead of rifampin. Updated guidelines for the use of rifabutin or rifampin in HIV-infected patients receiving antiretroviral agents have been reviewed recently (17).
Other potent inducers such as phenytoin, phenobarbital, and carbamazepine can cause similar reductions in serum levels of protease inhibitors. Azole antifungal drugs and macrolides also have important interactions that complicate both the treatment and prophylaxis of opportunistic infections.
Interactions that interfere with absorption can also affect plasma drug concentrations. For example, the absorption of fluconazole is unaffected by variations in gastric pH, while itraconazole and ketoconazole require an acidic environment for optimal absorption. The protease inhibitor, atazanavir, also requires a low pH for absorption and thus is contraindicated with the use of proton pump inhibitors; taking atazanavir with acidic beverages is not sufficient to overcome this (18).
New information about drug interactions becomes known on almost a daily basis in patients with HIV infection. The number of documented and theoretical interactions can become overwhelming to the clinician. Clinicians should suspect potential drug interactions in a patient who is failing therapy but who is adherent to HAART. Fortunately, there are extensive tables on Web sites (www.hivatis.org) and product information to aid in the recognition and management of drug interactions.
Complications of HAART
Diabetes mellitus, hyperlipidemias, lipodystrophy, and insulin resistance are among the many complex metabolic abnormalities attributable to the use of HAART. For the most part, these complications are managed conservatively and usually do not mandate the discontinuation of HAART. Pancreatitis, hepatic steatosis, and lactic acidosis are wellrecognized complications of NRTIs. These are usually more acute and may result in hospitalization and necessitate the discontinuation of medications. Cessation of the offending agent (didanosine [ddI], stavudine [d4T], and zalcitabine [ddC) usually results in resolution of pancreatitis, but the episode may limit use of these agents in the future. Hepatic steatosis and lactic acidosis are rare but life-threatening adverse effects associated with the mitochondrial toxicity seen with the NRTIs. Symptoms usually develop insidiously with nausea, vomiting, abdominal pain, weight loss, or dyspnea and can progress rapidly to fatal lactic acidosis. Hepatomegaly, ascites, elevated liver associated enzymes, and an increased anion gap with lactic acidemia are usually present (19,20). Discontinuation of antiretroivirals is imperative.
There is an accumulating body of evidence that suggests that HIV-infected patients receiving HAART may be at risk for accelerated coronary disease (21). In addition, some cohort studies have reported an increased incidence of myocardial infarction (MI) following the introduction of HAART and the risk for MI rose progressively with the number of years on antiretroviral therapy (22,23). However, it is important to note that many traditional risk factors for coronary artery disease contribute more substantially to the risk for a cardiovascular event than does HAART. Therefore, aggressive modification of primary cardiac risk factors is warranted.
Hypersensitivity Drug Reactions
Drug hypersensitivity reactions are life-threatening reactions that result in a systemic illness that usually includes fever and maculopapular rash accompanied by constitutional symptoms (fatigue, myalgias, and arthralgias), visceral involvement (lymphadenopathy, mucositis, pneumonitis, myocarditis, hepatitis, and interstitial nephritis), and hematologic abnormalities (eosinophilia) (24).
Abacavir, an NRTI, is a relatively new antiretroviral agent used in many HAART regimens. Abacavir is associated with a hypersensitivity reaction, which can be fatal if abacavir use is continued despite the reaction, or if re-challenge with the drug takes place after the reaction (25). The overall incidence of this reaction appears to be around 4% (25). Prior antiretroviral experience and being of African descent are associated with a nearly 40% reduction in the risk of this hypersensitivity reaction, while patients of white race are at a significantly greater risk. CD4 cell counts do not appear to be significantly related to abacavir hypersensitivity (26). The exact metabolite that is likely responsible for abacavir hypersensitivity is unknown.
The most common symptoms of abacavir hypersensitivity reaction are fever, rash, nausea, vomiting, and abdominal pain. Occasionally, respiratory symptoms will be present and can mimic influenza. However, gastrointestinal symptoms are the most prominent complaints after fever and rash and help to distinguish between influenza and abacavir hypersensitivity. More than 90% of hypersensitivity reactions occur during the first 6 weeks of treatment, with a median time to development of 8 days. A fever that develops within a few weeks after the initiation of therapy with abacavir may be due to causes other than hypersensitivity. One of the most common situations is the simultaneous initiation of treatment with other drugs, such as trimethoprimsulfamethxazole, efavirenz, or nevirapine, all of which are associated with a higher incidence of hypersensitivity than abacavir (27,28). Symptoms may be sudden and worsen over a few days if abacavir is continued. Symptoms tend to improve in 48 hours after abacavir is discontinued. Supportive therapy includes intravenous hydration and withdrawal of abacavir as well as all other antiretrovirals. Early in the use of this medication, 20% of patients who were re-challenged with the drug experienced unanticipated life-threatening events manifesting as an anaphylactic or immediate type hypersensitivity reaction. Hypotension, renal insufficiency, and bronchospasm have resulted in death. Rechallenge symptoms have been seen with the first dose (29). A discussion of the potential for this hypersensitivity is warranted when prescribing this agent. In the United States, a patient information card warning of this hypersensitivity reaction is distributed to the patient with each bottle of abacavir. Prednisone does not prevent the development of hypersensitivity reaction.
Symptoms of toxicity from TMP-SMX are more likely to occur in the HIV infected than in patients without HIV infection. Fever and rash can occur in up to 50% of HIV-infected patients. The rash can be treatment limiting or severe in up to 20% of HIV-infected patients who receive it. Life-threatening reactions may occur, including fatal Stevens Johnson-type exfoliative skin reactions. Most toxicity in HIV-infected patients appears to be related to metabolites of the sulfamethoxazole component and decreased levels of glutathione. There have been reports of severe systemic reactions that resemble anaphylaxis or septic shock occurring in HIV-infected patients who are re-challenged with TMP-SMX after experiencing toxicity within the previous 6–8 weeks (30).
The NNRTIf nevirapine and efavirenz can cause a delayed hypersensitivity reaction similar to that seen with abacavir. Cutaneous involvement is a prominent component of both nevirapine and efavirenz hypersensitivity reaction, with rash more likely to occur with the use of nevirapine. In addition, female patients have a higher propensity of developing Stevens-Johnson syndrome and symptomatic hepatic events from nevirapine (28,31).
Laboratory Abnormalities Related to Drugs
Hyperbilirubinemia and Atazanavir and Indinavir
Atazanavir, a protease inhibitor, is metabolized by the liver via CYP3A and also inhibits both CTP3A and UGT1A1. UGT1A1 is required for conjugation of bilirubin and inhibition of this enzyme results in elevated levels of unconjugated bilirubin. This effect is similar to what is observed in Gilbert’s syndrome. Asymptomatic indirect hyperbilirubemia may be seen in up to 60% of patients receiving atazanavir. Total bilirubin levels may rise to greater than 5 mg/dL, and more than 17% of patients may experience jaundice (18). Concurrent elevations in hepatic serum transaminases should not be attributed to atazanavir and alternative etiologies for these elevations should be sought. This hyperbilirubinemia is reversible upon discontinuation of the atazanavir.
Similarly, but to a lesser degree, asymptomatic unconjugated hyperbilirubinemia (>2 mg/dL) has been reported in up to 14% of patients treated with indinavir. Elevated serum transaminases were seen in less than 1%.
Renal Abnormalities and Indinavir
Several renal syndromes have been associated with indinavir use, ranging from obstructive uropathy and acute renal failure to asymptomatic pyuria. The range of clinical syndromes is a consequence of indinavir crystals aggregating within or irritating the urinary tract (32). Symptomatic nephrolithiasis (indinavir crystallization) has been reported to affect up to 12% (range, 5–35 %) of patients who receive indinavir, while up to 67% of patients will have asymptomatic crystalluria. The cumulative frequency of nephrolithiasis events increases with increasing exposure to indinavir. Therapy may be continued or interrupted for a few days. Adequate hydration is necessary with the administration of indinavir. Indinavir associated pyuria is frequently associated with interstitial nephritis or urothelial inflammation. Discontinuation of indinavir will lead to resolution of urine abnormalities.
Elevated Mean Corpuscular Volume (MCV) and NRTIs
Elevation of MCV or macrocytosis occurs in more than 90% of patients treated with zidovudine, but is not correlated with the development of anemia. Macrocytosis (MCV values exceeding 110/fl) develops within 2 weeks following the initiation of zidovudine therapy, and its presence can be used as a marker for medical adherence. When anemia does occur it is associated with a dose related bone marrow toxicity manifested as a macrocytic anemia. Serum B12 and folate levels are normal. Stavudine use is also associated with macrocytosis, in non-zidovudine-containing regimens (33).
Drug Screens and Efavirenz
The use of efavirenz, a potent non-nucleoside reverse transcriptase inhibitor, can cause a false-positive urine drug screen for cannabinoid. Efavirenz does not bind to cannabinoid receptors. The false-positive test results are specific to the assay kit used (34).
Evaluation of the AIDS Patient with Fever
Fever is a common symptom in HIV-infected patients, the etiology of which can be identified in more than 80% of cases (35,36). The AIDS patient with fever poses a considerable challenge given that the expanded differential may include a wide range of OIs. The CD4 cell count remains a valuable predictor of risk for infection. Patients with CD4 cell counts greater than 500 cells/mm3 should be evaluated as if immunocompetent. Patients with CD4 cell counts between 200 and 500 cells/mm3 are at increased risk for upper and lower bacterial respiratory infections, tuberculosis, and sinusitis, but overall their risk for opportunistic infection is not increased. In patients with CD4 counts less than 200 cells/mm3, Pneumocystis jiroveci pneumonia, formerly known as Pneumocystis carinii pneumonia, is the most common cause of fever in those not receiving primary prophylaxis. As the CD4 cell count decreases below 100 cells/mm3, the risk for disseminated MAC, toxoplasmosis, CMV, disseminated fungal infections, and lymphoma should be considered possible causes of fever. HIV itself is usually not the cause of fever in patients with advanced immunosuppression (37).
In patients with CD4 counts >200 cells/mm3, the clinician can usually construct a laboratory and radiographic evaluation guided by symptoms, while in patients with severe immunosuppression a broader evaluation is required. A serum cryptococcal antigen should be obtained, as it has high sensitivity and specificity for both systemic disease as well as meningitis (38). Bacterial, mycobacterial, and fungal isolator blood cultures should be performed, as well as a urine culture, despite lack of symptoms. Urine AFB cultures can be added if there is a suspicion for tuberculosis. Sputum should be evaluated with gram stain, AFB smear, and culture, as well as PCP direct fluorescent antibody. If diarrhea is present, stool studies should include bacterial culture, ova and parasites evaluation, an assay for C. difficile, and Cryptosporidia and Giardia stool antigen assays. A serum LDH may be elevated in PCP, disseminated histoplasmosis, or lymphoma. A serum CMV antigen may be useful in the patient with fever and diarrhea, hepatitis, or retinitis.
A chest radiograph should be performed in all febrile AIDS patients. Chest films may be normal in 5–10% of HIV-infected patients with tuberculosis (TB). The typical radiographic appearance of Pneumocystis jiroveci pneumonia is a bilateral interstitial pattern characterized by reticular or ground-glass opacities. However, normal chest radiographs may be seen in one third of AIDS patients with active PCP. High-resolution computed tomography (HRCT) of the chest should be obtained if there is still a clinical suspicion for Pneumocystis. HRCT has been shown in several reports to have a 100% negative predictive value in the evaluation for PCP (39,40).
A lumbar puncture should be performed if the patient is symptomatic or if the serum cryptococcal antigen is reactive. A bone marrow biopsy and culture is also useful particularly in the evaluation of the patient with cytopenias. Bacterial, fungal, and AFB cultures may yield disseminated mycobacterial or fungal disease. Histopathologic evaluation may reveal granulomas with organisms or lymphoma. Bronchoscopy may be pursued in cases of high suspicion for TB or PCP.
Guidelines for the management of opportunistic infections associated with human immunodeficiency virus are available at www.AIDSinfo.nih.gov.
Evaluation of the AIDS Patient with Focal Neurological Disease
Toxoplasma encephalitis (TE) may be distinguished from primary CNS lymphoma without a brain biopsy. TE is caused by reactivation of latent infection by the protozoan Toxoplasma gondii. Almost 90% of patients have CD4 counts less than 200 cells/mm3 and 75% have CD4 counts less than 100 cells/mm3. Serum anti-Toxoplasma IgG antibodies are detected in more than 90% of patients with TE. Lesions on CT or MRI (a more sensitive modality) are typically multiple ring-enhancing lesions with a predilection for the basal ganglia. An emipiric trial of therapy is recommended, and a response confirms a diagnosis in the patient who has a positive Toxoplasma antibody and is not receiving trimethoprim-sulfamethoxazole prophylaxis. A lumbar puncture in this setting is not necessary and maybe ill advised if cerebral edema is present (Figure 1, page 24).
Primary CNS lymphoma (PCNSL) has a similar radiographic appearance as TE. Solitary lesions are more frequent in PCNSL. Positron emission tomography (PET) and single photon emission CT (SPECT) are useful adjunctive imaging modalities as they are positive in PCNSL due to the increased metabolic activity of the tumor. Cytologic analysis of the CSF may show lymphomatous cells. Epstein-Barr virus (EBV) DNA is uniformly detected in PCNSL in AIDS patients and detection of EBV DNA by PCR on the CSF has a sensitivity of 90–100% and a specificity of 87–98% for the diagnosis of PCNSL (41,42). Combining SPECT imaging and EBV PCR provides 100% sensitivity and 100% negative predictive value in the evaluation of AIDS-related primary CNS lymphoma (43), obviating the need for brain biopsy.
Progressive multifocal leukoencephalopathy (PML) is characterized radiographically by multiple and bilateral hypodense lesions of the white matter without mass effect or enhancement on CT. MRI demonstrates areas of hypointensity on T1-weighted images and increased intensity on T2-weighted images. JC virus DNA can be detected by PCR of CSF or brain tissue with sensitivity of approximately 80% and specificity of 95%. Because of the high positive predictive value of a positive PCR, a patient with AIDS who also has a compatible MRI can be diagnosed with PML (44,45).
Other focal neurologic disease seen in AIDS patients includes cryptococcomas, tuberculomas, CMV encephalitis, neurosyphilis, Nocardia and Aspergillus infection, and bacterial brain abscesses (46).
Conclusion
As survival of the HIV-infected population improves, more patients may require hospitalization for HAART treatment failures or complications attributed to antiretroviral therapy. The hospitalist should be familiar with the complications of antiretroviral agents, the interactions between HAART and medications used to treat opportunistic infections, and medical conditions induced by HAART. Evaluation of the HIV-infected patient presenting with fever can be based on the CD4 cell count, which predicts risk for opportunistic infections. Finally, using combined diagnostic approaches along with modern imaging and laboratory assays may preclude the need for more invasive procedures in the HIV-infected hospitalized patient.
Dr. Decker may be reached at cfdecker@bethesda.med.navy.mil.
References
- Torres RA, Barr M. Impact of combination therapy for HIV infection on inpatient census. N Engl J Med. 1997;336:1531-2.
- Fleishman JA, Hellinger FJ. Trends in HIV-related inpatient admissions from 1993 to 1997: a seven-state study. J Acquir Immune Defic Syndr. 2001;28:73-80.
- Fleishman JA, Hellinger FH. Recent trends in HIV-related inpatient admissions 1996-2000: a 7-state study. J Acquir Immune Defic Syndr. 2003;34:102-10.
- Powderly WG. Should patients with an acute opportunistic infection receive antiretroviral therapy immediately? Advanced Studies in Medicine. 2005;5:S111-S116.
- Fagard C, Oxenius A, Gunthard H, et al. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch Intern Med. 2003;163:1220-6.
- Soni N, Pozniak A. Continuing HIV therapy in the ICU. Crit Care. 2001;5:247-8.
- Purdy BD, Raymond AM, Lesar TS. Antiretroviral prescribing errors in hospitalized patients. Ann Pharmacother. 2000;34:833-8.
- Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112-6.
- Cooney EL. Clinical indicators of immune restoration following highly active antiretroviral therapy. Clin Infect Dis. 2002;34:224-33.
- Hirsch HH, Kaufmann G, Sendi P, BaĴegay M. Immune reconstitution in HIV-infected patients. Clin Infect Dis. 2004;38:1159-66.
- Cheng VC, Yuen KY, Chan WM, Wong SS, Ma ES, Chan RM. Immunorestitution disease involving the innate and adaptive response. Clin Infect Dis. 2000;30:882-92.
- Fishman JE, Saraf-Lavi E, Narita M, Hollender ES, Ramsinghani R, Ashkin D. Pulmonary tuberculosis in AIDS patients: transient chest radiographic worsening after initiation of antiretroviral therapy. AJR Am J Roentgenol. 2000;174:43-9.
- Piscitelli SC, Gallicano KD. Interactions among drugs for HIV and opportunistic infections. N Engl J Med. 2001;344:984-96.
- Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36:289-304.
- Sim SM, Hoggard PG, Sales SD, Phiboonbanakit D, Hart CA, Back DJ. Effect of ribavirin on zidovudine efficacy and toxicity in vitro: a concentration-dependent interaction. AIDS Res Hum Retroviruses. 1998;14:1661-7.
- Clarke SM, Mulcahy FM, Tjia J, et al. The pharmacokinetics of methadone in HIV-positive patients receiving the non-nucleoside reverse transcriptase inhibitor efavirenz. Br J Clin Pharmacol. 2001;51:213-7.
- Centers for Disease Control and Prevention. Updated guidelines for the use of rifabutin or rifampin for the treatment and prevention of tuberculosis among HIV-infected patients taking protease inhibitors or nonnucleoside reverse transcriptase inhibitors. Morb Mortal Wkly Rep. 2000;49:185-9.
- Havlir DV, O’Marro SD. Atazanavir: new option for treatment of HIV infection. Clin Infect Dis. 2004;38:1599-604.
- Sheng WH, Hsieh SM, Lee SC, et al. Fatal lactic acidosis associated with highly active antiretroviral therapy in patients with advanced human immunodeficiency virus infection in Taiwan. Int J STD AIDS. 2004;15:249-53.
- Herman JS, Easterbrook PJ. The metabolic toxicities of antiretroviral therapy. Int J STD AIDS. 2001;12:555-62;quiz 563-4.
- Behrens G, Schmidt H, Meyer D, Stoll M, Schmidt RE. Vascular complications associated with use of HIV protease inhibitors. Lancet. 1998;351:1958.
- Klein D, Hurley LB, Sidney S. Cardiovascular Disease and HIV Infection. N Engl J Med. 2003;349:1869-70; author reply 1869-70.
- Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993-2003.
- Anderson JA, Adkinson NF Jr. Allergic reactions to drugs and biologic agents. JAMA. 1987;258:2891-9.
- Hewitt RG. Abacavir hypersensitivity reaction. Clin Infect Dis. 2002;34:1137-42.
- Hetherington S, Hughes AR, Mosteller M, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121-2.
- Bossi P, Colin D, Bricaire F, Caumes E. Hypersensitivity syndrome associated with efavirenz therapy. Clin Infect Dis. 2000;30:227-8.
- Bourezane Y, Salard D, Hoen B, Vandel S, Drobacheff C, Laurent R. DRESS (drug rash with eosinophilia and systemic symptoms) syndrome associated with nevirapine therapy. Clin Infect Dis. 1998;27:1321-2.
- Walensky RP, Goldberg JH, Daily JP. Anaphylaxis after rechallenge with abacavir. AIDS. 1999;13:999-1000.
- Martin GJ, Paparello SF, Decker CF. A severe systemic reaction to trimethoprim-sulfamethoxazole in a patient infected with the human immunodeficiency virus. Clin Infect Dis. 1993;16:175-6.
- Bersoff-Matcha SJ, Miller WC, Aberg JA, et al. Sex differences in nevirapine rash. Clin Infect Dis. 2001;32:124-9.
- Kopp JB, Falloon J, Filie A, et al. Indinavir-associated interstitial nephritis and urothelial inflammation: clinical and cytologic findings. Clin Infect Dis. 2002;34:1122-8.
- Geene D, Sudre P, Anwar D, Goehring C, Saaidia A, Hirschel B. Causes of macrocytosis in HIV-infected patients not treated with zidovudine. Swiss HIV Cohort Study. J Infect. 2000;40:160-3.
- Gottesman L. Sustiva may cause false positive on marijuana test. WORLD. 1999:7.
- Sepkowitz KA, Telzak EE, Carrow M, Armstrong D. Fever among outpatients with advanced human immunodeficiency virus infection. Arch Intern Med. 1993;153: 1909-12.
- Barat LM, Gunn JE, Steger KA, Perkins CJ, Craven DE. Causes of fever in patients infected with human immunodeficiency virus who were admitted to Boston City Hospital. Clin Infect Dis. 1996;23:320-8.
- Sepkowitz KA. FUO and AIDS. Curr Clin Top Infect Dis. 1999;19:1-15.
- Asawavichienjinda T, Sitthi-Amorn C, Tanyanont V. Serum cyrptococcal antigen: diagnostic value in the diagnosis of AIDS-related cryptococcal meningitis. J Med Assoc Thai. 1999;82:65-71.
- Richards PJ, Riddell L, Reznek RH, Armstrong P, Pinching AJ, Parkin JM. High resolution computed tomography in HIV patients with suspected Pneumocystis carinii pneumonia and a normal chest radiograph. Clin Radiol. 1996;51:689-93.
- Gruden JF, Huang L, Turner J, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR Am J Roentgenol. 1997;169:967-75.
- MacMahon EM, Glass JD, Hayward SD, et al. Association of Epstein-Barr virus with primary central nervous system lymphoma in AIDS. AIDS Res Hum Retroviruses. 1992;8:740-2.
- Rao CR, Jain K, Bhatia K, Laksmaiah KC, Shankar SK. Association of primary central nervous system lymphomas with the Epstein-Barr virus. Neurol India. 2003;51:237-40.
- Antinori A, De Rossi G, Ammassari A, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol. 1999;17:554-60.
- Post MJ, Yiannoutsos C, Simpson D, et al. Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? AIDS Clinical Trials Group, 243 Team. AJNR Am J Neuroradiol. 1999;20:1896-906.
- Weber T. Cerebrospinal fluid analysis for the diagnosis of human immunodeficiency virus-related neurologic diseases. Semin Neurol. 1999;19:223-33.
- Skiest DJ. Focal neurological disease in patients with acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34:103-15.
The opinions and assertions contained herein are those of the authors and are not to be construed as official or as reflecting the views of the Department of Defense, the Department of the Navy, or the naval services at large.
Introduction
An estimated 850,000 to 950,000 persons in the United States are living with human immunodeficiency virus (HIV), 280,000 of whom are unaware of their infection and another 43,000 of whom meet the definition of acquired immunodeficiency syndrome (AIDS) (www.cdc.gov). The use of highly active antiretroviral therapy (HAART) has produced significant declines in morbidity and mortality from AIDS. Compared with the first 2 decades of the HIV pandemic, the number of HIV-related hospital admissions has declined. However, recently, this rate of decline has markedly slowed (1-3). The reasons for this plateau are many including a steady number of admissions for complications related to HAART, treatment failures, and the overall increased prevalence of HIV infection. Not only will HIV-infected patients still frequently require admission to the hospital, but the complexity of their inpatient care will continue to increase with the advancements in multiple drug regimens, aging of the HIV-infected population, and the interaction of HIV infection with medical comorbidities, many of which are attributable to HAART.
The hospitalist caring for the inpatient with AIDS is presented with several challenges including not only the diagnosis and management of opportunistic infections, but also the complications of HAART. In this article we review the guidelines for the initiation and continuation of HAART in the hospital, review important clinical complications of antiretroviral therapy, and review conditions that may result in the hospitalization of AIDS patients.
Initiation of HAART in the Hospital
In those who do not have access to health care, the initial diagnosis of HIV infection frequently occurs during a hospitalization for an AIDS-defining illness. Initiation of antiretrovirals is contingent on several issues, including CD4 count, viral load, clinical status, likelihood of continued adherence, and the concurrent treatment of opportunistic infections (OIs). All patients with HIV infection and a CD4 count <200 cells/mm3 or an AIDS-defining illness should receive antiretroviral therapy. Controversy exists as to whether a patient admitted for the treatment of an opportunistic infection should begin antiretroviral therapy immediately, or whether this therapy should be deferred until after acute treatment of the OI. The potential detrimental effects of drug-drug interactions, the need for treatment interruptions, and drug-related toxicity between antiretrovirals and OI-specific therapy may support initiating HAART after control of an OI is achieved. Conversely, for some opportunistic infections, such as cryptosporidiosis, the use of HAART is essential for successful treatment of the infection.
An ongoing randomized controlled trial initiated within the Adult AIDS Clinical Trials Group (ACTG) comparing outcomes between patients who start HAART immediately after presentation with an acute OI and patients who start HAART at least 4 weeks after the OI has resolved should help identify the factors supporting early or delayed initiation of antiretrovirals (4).
Generally speaking, HAART can be administered by combining either a protease inhibitor (PI) or a nonnucleoside reverse transcriptase inhibitor (NNRTI) with 2 nucleoside reverse transcriptase inhibitors (NRTIs). There are currently more than 20 FDA-approved antiretrovirals. Frequent updates on the guidelines for the use of antiretroviral agents in HIV-infected adults are available at www.AIDSinfo.nih.gov, and a discussion of this topic is beyond the scope of this review.
Continuation of HAART in the Hospital
In most cases, every effort should be made to minimize interruption of HAART during a hospitalization. Although some investigators are examining the virologic and immunologic safety of interrupting HAART as a treatment strategy, there are few data on viral replication, CD4 cell count decline, and rate of acquisition of new mutations in hospitalized patients who have unexpected treatment interruptions (5). The long half-life of some antiretrovirals promotes the emergence of resistance once HAART is stopped. For example, once NNRTIs are stopped, subtherapeutic levels remain in the plasma and cells for several days. HIV then replicates in a milieu that may select for resistance mutations.
Because zidovudine is the only antiretroviral available in a parenteral preparation, it is often difficult to continue HAART when a patient cannot take medications by mouth. Drugs given by the enteral route in a hospitalized patient may also be poorly absorbed, and few data exist on the absorption of antiretrovirals administered through a gastrostomy or jejunostomy tube (6).
Prescribing HAART in the Hospital
Antiretroviral prescribing errors occur frequently in the hospitalized AIDS patient. The most common errors include overdosing or underdosing, missing components of multidrug regimens, or missing critical drug-drug interactions (7). Underdosing may lead to resistance, and overdosing contributes to increased toxicity. In one report, prescribing errors occurred in 12% of admissions in the post-HAART era (1998) compared with 2% of admissions in the pre-HAART era (1996) (7). The NRTIs, including didanosine, emtricitabine, lamivudine, stavudine, and zidovudine, require decreased dosing in renal insufficiency. Tenofovir is not recommended for use if the creatine clearance is less than 60 mL/minute. Dosage adjustments in hepatic disease are recommended for amprenavir, fosamprenavir, delavirdine, efavirenz, and nevirapine.
Immune Reconstitution Syndrome
The widespread use of HAART has produced sustained suppression of HIV replication and recovery of CD4 cell counts. It also became evident that HAART resulted in not only a numerical increase in CD4 cells, but also in a functional immune recovery (8-10). This improved T-cell response to antigens results in adequate protection against specific opportunistic infections, allowing for discontinuation of primary and secondary prophylaxis in HIV-infected patients. Immune reconstitution syndrome (IRS), an inflammatory syndrome, is recognized as a potential complication that can occur days to months after starting HAART. The onset of IRS is characterized by a paradoxical worsening of clinical or laboratory parameters despite a favorable response in CD4 cell counts and the suppression of viral replication (9,11,12). IRS has been reported to occur in 10–25% of patients who receive HAART and more commonly in those whose CD4 cell counts are <50 cells/mm3 at the start of HAART (9,11). It is postulated that the inflammatory response is triggered by the recognition of antigens associated with ongoing infection or recognition of persisting antigens associated with past (nonreplicating) infections. Mycobacterial antigens, frequently implicated in IRS, are responsible for about one third of cases. Other antigens associated with IRS include cytomegalovirus and hepatitis B and C (11). In most circumstances, with the management of IRS, HAART should be continued, while specific antimicrobial therapy and steroids should be considered (10).
Medical Conditions that Should Prompt HIV Screening
There are several medical conditions that should prompt screening for HIV infection. Generally, anyone presenting with a fever of unknown etiology who is sexually active or had a blood transfusion prior to 1985 should be screened for HIV infection. Symptoms consistent with acute retroviral syndrome (fever, sore throat, malaise, and skin rash) may be more commonly recognized by clinicians now than previously, and this remains a “golden opportunity” to intervene. Frequently, acute retroviral syndrome will be attributed to Epstein-Barr virus; however, caution should be used in the diagnosis of mononucleosis in those other than teenage populations. It is recommended that all persons presenting with any sexually transmitted disease, unexplained generalized lymphadenopathy, oral candidiasis, or tuberculosis should also be tested. Other conditions where HIV infection should be considered include enigmatic pneumonia, acute hepatitis B infection, herpes zoster infection (particularly in younger, seemingly immunocompetent individuals), idiopathic thrombocytopenic purpura, and nephropathy of unknown cause.
Drug Interactions
Drug interactions are an important consideration in the treatment of HIV infection. Interactions between HAART and other drugs used for the treatment or prophylaxis of opportunistic infections along with those used for the treatment of drug-induced endocrinopathies (hyperlipidemia, diabetes mellitus) are virtually unavoidable. Drug interactions occur either because of drug metabolism or absorption. The multiple metabolic pathways of some drugs make it difficult to predict the outcome of drug interactions. All protease inhibitors and non-nucleoside reverse transcriptase inhibitors are metabolized by the cytochrome P-450 enzyme system and each of these drugs may alter the metabolism of other antiretrovirals and concomitantly administered drugs (13,14). A decrease in trough plasma concentrations of the protease inhibitors to a level below the in vitro concentration required to inhibit replication of 50% of viral strains (IC50) may lead to development of resistance. Because nucleoside analogue reverse transcriptase inhibitors are primarily eliminated by the kidney, they do not interact with other drugs through the cytochrome P-450 system.
One noteworthy interaction that the clinician caring for HIV-infected patients should be aware of is the interaction of ribavirin with zidovudine. Ribavirin decreases the phosphorylation of zidovudine and stavudine in vitro, resulting in decreased concentrations of the active compound. HIV-infected patients who are coinfected with hepatitis C may be treated with regimens that include ribavirin, which may reduce the efficacy of zidovudine (15). Another important interaction is the effect of nevirapine or efavirenz on plasma methadone concentrations. Both drugs can decrease methadone plasma levels by 50%, and patients receiving chronic therapy may need increased methadone doses to prevent withdrawal symptoms (16).
Protease inhibitors are associated with numerous interactions including certain antiarrhythmics, sedatives, hypnotics, ergot derivatives, and several lipid-lowering agents (statins). Not only do protease inhibitors affect the metabolism of certain drugs, but also their own metabolism is altered by other inducers or inhibitors of cytochrome activity that can cause clinically important decreases in serum levels of protease inhibitors. One widely recognized interaction is that of rifampin, which may decrease levels of some protease inhibitors by 80%. The resulting low plasma concentrations may promote viral resistance and result in treatment failure. Patients being treated for tuberculosis, who are also receiving protease inhibitors should be treated with a four-drug regimen that includes rifabutin (at half dose) instead of rifampin. Updated guidelines for the use of rifabutin or rifampin in HIV-infected patients receiving antiretroviral agents have been reviewed recently (17).
Other potent inducers such as phenytoin, phenobarbital, and carbamazepine can cause similar reductions in serum levels of protease inhibitors. Azole antifungal drugs and macrolides also have important interactions that complicate both the treatment and prophylaxis of opportunistic infections.
Interactions that interfere with absorption can also affect plasma drug concentrations. For example, the absorption of fluconazole is unaffected by variations in gastric pH, while itraconazole and ketoconazole require an acidic environment for optimal absorption. The protease inhibitor, atazanavir, also requires a low pH for absorption and thus is contraindicated with the use of proton pump inhibitors; taking atazanavir with acidic beverages is not sufficient to overcome this (18).
New information about drug interactions becomes known on almost a daily basis in patients with HIV infection. The number of documented and theoretical interactions can become overwhelming to the clinician. Clinicians should suspect potential drug interactions in a patient who is failing therapy but who is adherent to HAART. Fortunately, there are extensive tables on Web sites (www.hivatis.org) and product information to aid in the recognition and management of drug interactions.
Complications of HAART
Diabetes mellitus, hyperlipidemias, lipodystrophy, and insulin resistance are among the many complex metabolic abnormalities attributable to the use of HAART. For the most part, these complications are managed conservatively and usually do not mandate the discontinuation of HAART. Pancreatitis, hepatic steatosis, and lactic acidosis are wellrecognized complications of NRTIs. These are usually more acute and may result in hospitalization and necessitate the discontinuation of medications. Cessation of the offending agent (didanosine [ddI], stavudine [d4T], and zalcitabine [ddC) usually results in resolution of pancreatitis, but the episode may limit use of these agents in the future. Hepatic steatosis and lactic acidosis are rare but life-threatening adverse effects associated with the mitochondrial toxicity seen with the NRTIs. Symptoms usually develop insidiously with nausea, vomiting, abdominal pain, weight loss, or dyspnea and can progress rapidly to fatal lactic acidosis. Hepatomegaly, ascites, elevated liver associated enzymes, and an increased anion gap with lactic acidemia are usually present (19,20). Discontinuation of antiretroivirals is imperative.
There is an accumulating body of evidence that suggests that HIV-infected patients receiving HAART may be at risk for accelerated coronary disease (21). In addition, some cohort studies have reported an increased incidence of myocardial infarction (MI) following the introduction of HAART and the risk for MI rose progressively with the number of years on antiretroviral therapy (22,23). However, it is important to note that many traditional risk factors for coronary artery disease contribute more substantially to the risk for a cardiovascular event than does HAART. Therefore, aggressive modification of primary cardiac risk factors is warranted.
Hypersensitivity Drug Reactions
Drug hypersensitivity reactions are life-threatening reactions that result in a systemic illness that usually includes fever and maculopapular rash accompanied by constitutional symptoms (fatigue, myalgias, and arthralgias), visceral involvement (lymphadenopathy, mucositis, pneumonitis, myocarditis, hepatitis, and interstitial nephritis), and hematologic abnormalities (eosinophilia) (24).
Abacavir, an NRTI, is a relatively new antiretroviral agent used in many HAART regimens. Abacavir is associated with a hypersensitivity reaction, which can be fatal if abacavir use is continued despite the reaction, or if re-challenge with the drug takes place after the reaction (25). The overall incidence of this reaction appears to be around 4% (25). Prior antiretroviral experience and being of African descent are associated with a nearly 40% reduction in the risk of this hypersensitivity reaction, while patients of white race are at a significantly greater risk. CD4 cell counts do not appear to be significantly related to abacavir hypersensitivity (26). The exact metabolite that is likely responsible for abacavir hypersensitivity is unknown.
The most common symptoms of abacavir hypersensitivity reaction are fever, rash, nausea, vomiting, and abdominal pain. Occasionally, respiratory symptoms will be present and can mimic influenza. However, gastrointestinal symptoms are the most prominent complaints after fever and rash and help to distinguish between influenza and abacavir hypersensitivity. More than 90% of hypersensitivity reactions occur during the first 6 weeks of treatment, with a median time to development of 8 days. A fever that develops within a few weeks after the initiation of therapy with abacavir may be due to causes other than hypersensitivity. One of the most common situations is the simultaneous initiation of treatment with other drugs, such as trimethoprimsulfamethxazole, efavirenz, or nevirapine, all of which are associated with a higher incidence of hypersensitivity than abacavir (27,28). Symptoms may be sudden and worsen over a few days if abacavir is continued. Symptoms tend to improve in 48 hours after abacavir is discontinued. Supportive therapy includes intravenous hydration and withdrawal of abacavir as well as all other antiretrovirals. Early in the use of this medication, 20% of patients who were re-challenged with the drug experienced unanticipated life-threatening events manifesting as an anaphylactic or immediate type hypersensitivity reaction. Hypotension, renal insufficiency, and bronchospasm have resulted in death. Rechallenge symptoms have been seen with the first dose (29). A discussion of the potential for this hypersensitivity is warranted when prescribing this agent. In the United States, a patient information card warning of this hypersensitivity reaction is distributed to the patient with each bottle of abacavir. Prednisone does not prevent the development of hypersensitivity reaction.
Symptoms of toxicity from TMP-SMX are more likely to occur in the HIV infected than in patients without HIV infection. Fever and rash can occur in up to 50% of HIV-infected patients. The rash can be treatment limiting or severe in up to 20% of HIV-infected patients who receive it. Life-threatening reactions may occur, including fatal Stevens Johnson-type exfoliative skin reactions. Most toxicity in HIV-infected patients appears to be related to metabolites of the sulfamethoxazole component and decreased levels of glutathione. There have been reports of severe systemic reactions that resemble anaphylaxis or septic shock occurring in HIV-infected patients who are re-challenged with TMP-SMX after experiencing toxicity within the previous 6–8 weeks (30).
The NNRTIf nevirapine and efavirenz can cause a delayed hypersensitivity reaction similar to that seen with abacavir. Cutaneous involvement is a prominent component of both nevirapine and efavirenz hypersensitivity reaction, with rash more likely to occur with the use of nevirapine. In addition, female patients have a higher propensity of developing Stevens-Johnson syndrome and symptomatic hepatic events from nevirapine (28,31).
Laboratory Abnormalities Related to Drugs
Hyperbilirubinemia and Atazanavir and Indinavir
Atazanavir, a protease inhibitor, is metabolized by the liver via CYP3A and also inhibits both CTP3A and UGT1A1. UGT1A1 is required for conjugation of bilirubin and inhibition of this enzyme results in elevated levels of unconjugated bilirubin. This effect is similar to what is observed in Gilbert’s syndrome. Asymptomatic indirect hyperbilirubemia may be seen in up to 60% of patients receiving atazanavir. Total bilirubin levels may rise to greater than 5 mg/dL, and more than 17% of patients may experience jaundice (18). Concurrent elevations in hepatic serum transaminases should not be attributed to atazanavir and alternative etiologies for these elevations should be sought. This hyperbilirubinemia is reversible upon discontinuation of the atazanavir.
Similarly, but to a lesser degree, asymptomatic unconjugated hyperbilirubinemia (>2 mg/dL) has been reported in up to 14% of patients treated with indinavir. Elevated serum transaminases were seen in less than 1%.
Renal Abnormalities and Indinavir
Several renal syndromes have been associated with indinavir use, ranging from obstructive uropathy and acute renal failure to asymptomatic pyuria. The range of clinical syndromes is a consequence of indinavir crystals aggregating within or irritating the urinary tract (32). Symptomatic nephrolithiasis (indinavir crystallization) has been reported to affect up to 12% (range, 5–35 %) of patients who receive indinavir, while up to 67% of patients will have asymptomatic crystalluria. The cumulative frequency of nephrolithiasis events increases with increasing exposure to indinavir. Therapy may be continued or interrupted for a few days. Adequate hydration is necessary with the administration of indinavir. Indinavir associated pyuria is frequently associated with interstitial nephritis or urothelial inflammation. Discontinuation of indinavir will lead to resolution of urine abnormalities.
Elevated Mean Corpuscular Volume (MCV) and NRTIs
Elevation of MCV or macrocytosis occurs in more than 90% of patients treated with zidovudine, but is not correlated with the development of anemia. Macrocytosis (MCV values exceeding 110/fl) develops within 2 weeks following the initiation of zidovudine therapy, and its presence can be used as a marker for medical adherence. When anemia does occur it is associated with a dose related bone marrow toxicity manifested as a macrocytic anemia. Serum B12 and folate levels are normal. Stavudine use is also associated with macrocytosis, in non-zidovudine-containing regimens (33).
Drug Screens and Efavirenz
The use of efavirenz, a potent non-nucleoside reverse transcriptase inhibitor, can cause a false-positive urine drug screen for cannabinoid. Efavirenz does not bind to cannabinoid receptors. The false-positive test results are specific to the assay kit used (34).
Evaluation of the AIDS Patient with Fever
Fever is a common symptom in HIV-infected patients, the etiology of which can be identified in more than 80% of cases (35,36). The AIDS patient with fever poses a considerable challenge given that the expanded differential may include a wide range of OIs. The CD4 cell count remains a valuable predictor of risk for infection. Patients with CD4 cell counts greater than 500 cells/mm3 should be evaluated as if immunocompetent. Patients with CD4 cell counts between 200 and 500 cells/mm3 are at increased risk for upper and lower bacterial respiratory infections, tuberculosis, and sinusitis, but overall their risk for opportunistic infection is not increased. In patients with CD4 counts less than 200 cells/mm3, Pneumocystis jiroveci pneumonia, formerly known as Pneumocystis carinii pneumonia, is the most common cause of fever in those not receiving primary prophylaxis. As the CD4 cell count decreases below 100 cells/mm3, the risk for disseminated MAC, toxoplasmosis, CMV, disseminated fungal infections, and lymphoma should be considered possible causes of fever. HIV itself is usually not the cause of fever in patients with advanced immunosuppression (37).
In patients with CD4 counts >200 cells/mm3, the clinician can usually construct a laboratory and radiographic evaluation guided by symptoms, while in patients with severe immunosuppression a broader evaluation is required. A serum cryptococcal antigen should be obtained, as it has high sensitivity and specificity for both systemic disease as well as meningitis (38). Bacterial, mycobacterial, and fungal isolator blood cultures should be performed, as well as a urine culture, despite lack of symptoms. Urine AFB cultures can be added if there is a suspicion for tuberculosis. Sputum should be evaluated with gram stain, AFB smear, and culture, as well as PCP direct fluorescent antibody. If diarrhea is present, stool studies should include bacterial culture, ova and parasites evaluation, an assay for C. difficile, and Cryptosporidia and Giardia stool antigen assays. A serum LDH may be elevated in PCP, disseminated histoplasmosis, or lymphoma. A serum CMV antigen may be useful in the patient with fever and diarrhea, hepatitis, or retinitis.
A chest radiograph should be performed in all febrile AIDS patients. Chest films may be normal in 5–10% of HIV-infected patients with tuberculosis (TB). The typical radiographic appearance of Pneumocystis jiroveci pneumonia is a bilateral interstitial pattern characterized by reticular or ground-glass opacities. However, normal chest radiographs may be seen in one third of AIDS patients with active PCP. High-resolution computed tomography (HRCT) of the chest should be obtained if there is still a clinical suspicion for Pneumocystis. HRCT has been shown in several reports to have a 100% negative predictive value in the evaluation for PCP (39,40).
A lumbar puncture should be performed if the patient is symptomatic or if the serum cryptococcal antigen is reactive. A bone marrow biopsy and culture is also useful particularly in the evaluation of the patient with cytopenias. Bacterial, fungal, and AFB cultures may yield disseminated mycobacterial or fungal disease. Histopathologic evaluation may reveal granulomas with organisms or lymphoma. Bronchoscopy may be pursued in cases of high suspicion for TB or PCP.
Guidelines for the management of opportunistic infections associated with human immunodeficiency virus are available at www.AIDSinfo.nih.gov.
Evaluation of the AIDS Patient with Focal Neurological Disease
Toxoplasma encephalitis (TE) may be distinguished from primary CNS lymphoma without a brain biopsy. TE is caused by reactivation of latent infection by the protozoan Toxoplasma gondii. Almost 90% of patients have CD4 counts less than 200 cells/mm3 and 75% have CD4 counts less than 100 cells/mm3. Serum anti-Toxoplasma IgG antibodies are detected in more than 90% of patients with TE. Lesions on CT or MRI (a more sensitive modality) are typically multiple ring-enhancing lesions with a predilection for the basal ganglia. An emipiric trial of therapy is recommended, and a response confirms a diagnosis in the patient who has a positive Toxoplasma antibody and is not receiving trimethoprim-sulfamethoxazole prophylaxis. A lumbar puncture in this setting is not necessary and maybe ill advised if cerebral edema is present (Figure 1, page 24).
Primary CNS lymphoma (PCNSL) has a similar radiographic appearance as TE. Solitary lesions are more frequent in PCNSL. Positron emission tomography (PET) and single photon emission CT (SPECT) are useful adjunctive imaging modalities as they are positive in PCNSL due to the increased metabolic activity of the tumor. Cytologic analysis of the CSF may show lymphomatous cells. Epstein-Barr virus (EBV) DNA is uniformly detected in PCNSL in AIDS patients and detection of EBV DNA by PCR on the CSF has a sensitivity of 90–100% and a specificity of 87–98% for the diagnosis of PCNSL (41,42). Combining SPECT imaging and EBV PCR provides 100% sensitivity and 100% negative predictive value in the evaluation of AIDS-related primary CNS lymphoma (43), obviating the need for brain biopsy.
Progressive multifocal leukoencephalopathy (PML) is characterized radiographically by multiple and bilateral hypodense lesions of the white matter without mass effect or enhancement on CT. MRI demonstrates areas of hypointensity on T1-weighted images and increased intensity on T2-weighted images. JC virus DNA can be detected by PCR of CSF or brain tissue with sensitivity of approximately 80% and specificity of 95%. Because of the high positive predictive value of a positive PCR, a patient with AIDS who also has a compatible MRI can be diagnosed with PML (44,45).
Other focal neurologic disease seen in AIDS patients includes cryptococcomas, tuberculomas, CMV encephalitis, neurosyphilis, Nocardia and Aspergillus infection, and bacterial brain abscesses (46).
Conclusion
As survival of the HIV-infected population improves, more patients may require hospitalization for HAART treatment failures or complications attributed to antiretroviral therapy. The hospitalist should be familiar with the complications of antiretroviral agents, the interactions between HAART and medications used to treat opportunistic infections, and medical conditions induced by HAART. Evaluation of the HIV-infected patient presenting with fever can be based on the CD4 cell count, which predicts risk for opportunistic infections. Finally, using combined diagnostic approaches along with modern imaging and laboratory assays may preclude the need for more invasive procedures in the HIV-infected hospitalized patient.
Dr. Decker may be reached at cfdecker@bethesda.med.navy.mil.
References
- Torres RA, Barr M. Impact of combination therapy for HIV infection on inpatient census. N Engl J Med. 1997;336:1531-2.
- Fleishman JA, Hellinger FJ. Trends in HIV-related inpatient admissions from 1993 to 1997: a seven-state study. J Acquir Immune Defic Syndr. 2001;28:73-80.
- Fleishman JA, Hellinger FH. Recent trends in HIV-related inpatient admissions 1996-2000: a 7-state study. J Acquir Immune Defic Syndr. 2003;34:102-10.
- Powderly WG. Should patients with an acute opportunistic infection receive antiretroviral therapy immediately? Advanced Studies in Medicine. 2005;5:S111-S116.
- Fagard C, Oxenius A, Gunthard H, et al. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch Intern Med. 2003;163:1220-6.
- Soni N, Pozniak A. Continuing HIV therapy in the ICU. Crit Care. 2001;5:247-8.
- Purdy BD, Raymond AM, Lesar TS. Antiretroviral prescribing errors in hospitalized patients. Ann Pharmacother. 2000;34:833-8.
- Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112-6.
- Cooney EL. Clinical indicators of immune restoration following highly active antiretroviral therapy. Clin Infect Dis. 2002;34:224-33.
- Hirsch HH, Kaufmann G, Sendi P, BaĴegay M. Immune reconstitution in HIV-infected patients. Clin Infect Dis. 2004;38:1159-66.
- Cheng VC, Yuen KY, Chan WM, Wong SS, Ma ES, Chan RM. Immunorestitution disease involving the innate and adaptive response. Clin Infect Dis. 2000;30:882-92.
- Fishman JE, Saraf-Lavi E, Narita M, Hollender ES, Ramsinghani R, Ashkin D. Pulmonary tuberculosis in AIDS patients: transient chest radiographic worsening after initiation of antiretroviral therapy. AJR Am J Roentgenol. 2000;174:43-9.
- Piscitelli SC, Gallicano KD. Interactions among drugs for HIV and opportunistic infections. N Engl J Med. 2001;344:984-96.
- Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36:289-304.
- Sim SM, Hoggard PG, Sales SD, Phiboonbanakit D, Hart CA, Back DJ. Effect of ribavirin on zidovudine efficacy and toxicity in vitro: a concentration-dependent interaction. AIDS Res Hum Retroviruses. 1998;14:1661-7.
- Clarke SM, Mulcahy FM, Tjia J, et al. The pharmacokinetics of methadone in HIV-positive patients receiving the non-nucleoside reverse transcriptase inhibitor efavirenz. Br J Clin Pharmacol. 2001;51:213-7.
- Centers for Disease Control and Prevention. Updated guidelines for the use of rifabutin or rifampin for the treatment and prevention of tuberculosis among HIV-infected patients taking protease inhibitors or nonnucleoside reverse transcriptase inhibitors. Morb Mortal Wkly Rep. 2000;49:185-9.
- Havlir DV, O’Marro SD. Atazanavir: new option for treatment of HIV infection. Clin Infect Dis. 2004;38:1599-604.
- Sheng WH, Hsieh SM, Lee SC, et al. Fatal lactic acidosis associated with highly active antiretroviral therapy in patients with advanced human immunodeficiency virus infection in Taiwan. Int J STD AIDS. 2004;15:249-53.
- Herman JS, Easterbrook PJ. The metabolic toxicities of antiretroviral therapy. Int J STD AIDS. 2001;12:555-62;quiz 563-4.
- Behrens G, Schmidt H, Meyer D, Stoll M, Schmidt RE. Vascular complications associated with use of HIV protease inhibitors. Lancet. 1998;351:1958.
- Klein D, Hurley LB, Sidney S. Cardiovascular Disease and HIV Infection. N Engl J Med. 2003;349:1869-70; author reply 1869-70.
- Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993-2003.
- Anderson JA, Adkinson NF Jr. Allergic reactions to drugs and biologic agents. JAMA. 1987;258:2891-9.
- Hewitt RG. Abacavir hypersensitivity reaction. Clin Infect Dis. 2002;34:1137-42.
- Hetherington S, Hughes AR, Mosteller M, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121-2.
- Bossi P, Colin D, Bricaire F, Caumes E. Hypersensitivity syndrome associated with efavirenz therapy. Clin Infect Dis. 2000;30:227-8.
- Bourezane Y, Salard D, Hoen B, Vandel S, Drobacheff C, Laurent R. DRESS (drug rash with eosinophilia and systemic symptoms) syndrome associated with nevirapine therapy. Clin Infect Dis. 1998;27:1321-2.
- Walensky RP, Goldberg JH, Daily JP. Anaphylaxis after rechallenge with abacavir. AIDS. 1999;13:999-1000.
- Martin GJ, Paparello SF, Decker CF. A severe systemic reaction to trimethoprim-sulfamethoxazole in a patient infected with the human immunodeficiency virus. Clin Infect Dis. 1993;16:175-6.
- Bersoff-Matcha SJ, Miller WC, Aberg JA, et al. Sex differences in nevirapine rash. Clin Infect Dis. 2001;32:124-9.
- Kopp JB, Falloon J, Filie A, et al. Indinavir-associated interstitial nephritis and urothelial inflammation: clinical and cytologic findings. Clin Infect Dis. 2002;34:1122-8.
- Geene D, Sudre P, Anwar D, Goehring C, Saaidia A, Hirschel B. Causes of macrocytosis in HIV-infected patients not treated with zidovudine. Swiss HIV Cohort Study. J Infect. 2000;40:160-3.
- Gottesman L. Sustiva may cause false positive on marijuana test. WORLD. 1999:7.
- Sepkowitz KA, Telzak EE, Carrow M, Armstrong D. Fever among outpatients with advanced human immunodeficiency virus infection. Arch Intern Med. 1993;153: 1909-12.
- Barat LM, Gunn JE, Steger KA, Perkins CJ, Craven DE. Causes of fever in patients infected with human immunodeficiency virus who were admitted to Boston City Hospital. Clin Infect Dis. 1996;23:320-8.
- Sepkowitz KA. FUO and AIDS. Curr Clin Top Infect Dis. 1999;19:1-15.
- Asawavichienjinda T, Sitthi-Amorn C, Tanyanont V. Serum cyrptococcal antigen: diagnostic value in the diagnosis of AIDS-related cryptococcal meningitis. J Med Assoc Thai. 1999;82:65-71.
- Richards PJ, Riddell L, Reznek RH, Armstrong P, Pinching AJ, Parkin JM. High resolution computed tomography in HIV patients with suspected Pneumocystis carinii pneumonia and a normal chest radiograph. Clin Radiol. 1996;51:689-93.
- Gruden JF, Huang L, Turner J, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR Am J Roentgenol. 1997;169:967-75.
- MacMahon EM, Glass JD, Hayward SD, et al. Association of Epstein-Barr virus with primary central nervous system lymphoma in AIDS. AIDS Res Hum Retroviruses. 1992;8:740-2.
- Rao CR, Jain K, Bhatia K, Laksmaiah KC, Shankar SK. Association of primary central nervous system lymphomas with the Epstein-Barr virus. Neurol India. 2003;51:237-40.
- Antinori A, De Rossi G, Ammassari A, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol. 1999;17:554-60.
- Post MJ, Yiannoutsos C, Simpson D, et al. Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? AIDS Clinical Trials Group, 243 Team. AJNR Am J Neuroradiol. 1999;20:1896-906.
- Weber T. Cerebrospinal fluid analysis for the diagnosis of human immunodeficiency virus-related neurologic diseases. Semin Neurol. 1999;19:223-33.
- Skiest DJ. Focal neurological disease in patients with acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34:103-15.
The opinions and assertions contained herein are those of the authors and are not to be construed as official or as reflecting the views of the Department of Defense, the Department of the Navy, or the naval services at large.
Introduction
An estimated 850,000 to 950,000 persons in the United States are living with human immunodeficiency virus (HIV), 280,000 of whom are unaware of their infection and another 43,000 of whom meet the definition of acquired immunodeficiency syndrome (AIDS) (www.cdc.gov). The use of highly active antiretroviral therapy (HAART) has produced significant declines in morbidity and mortality from AIDS. Compared with the first 2 decades of the HIV pandemic, the number of HIV-related hospital admissions has declined. However, recently, this rate of decline has markedly slowed (1-3). The reasons for this plateau are many including a steady number of admissions for complications related to HAART, treatment failures, and the overall increased prevalence of HIV infection. Not only will HIV-infected patients still frequently require admission to the hospital, but the complexity of their inpatient care will continue to increase with the advancements in multiple drug regimens, aging of the HIV-infected population, and the interaction of HIV infection with medical comorbidities, many of which are attributable to HAART.
The hospitalist caring for the inpatient with AIDS is presented with several challenges including not only the diagnosis and management of opportunistic infections, but also the complications of HAART. In this article we review the guidelines for the initiation and continuation of HAART in the hospital, review important clinical complications of antiretroviral therapy, and review conditions that may result in the hospitalization of AIDS patients.
Initiation of HAART in the Hospital
In those who do not have access to health care, the initial diagnosis of HIV infection frequently occurs during a hospitalization for an AIDS-defining illness. Initiation of antiretrovirals is contingent on several issues, including CD4 count, viral load, clinical status, likelihood of continued adherence, and the concurrent treatment of opportunistic infections (OIs). All patients with HIV infection and a CD4 count <200 cells/mm3 or an AIDS-defining illness should receive antiretroviral therapy. Controversy exists as to whether a patient admitted for the treatment of an opportunistic infection should begin antiretroviral therapy immediately, or whether this therapy should be deferred until after acute treatment of the OI. The potential detrimental effects of drug-drug interactions, the need for treatment interruptions, and drug-related toxicity between antiretrovirals and OI-specific therapy may support initiating HAART after control of an OI is achieved. Conversely, for some opportunistic infections, such as cryptosporidiosis, the use of HAART is essential for successful treatment of the infection.
An ongoing randomized controlled trial initiated within the Adult AIDS Clinical Trials Group (ACTG) comparing outcomes between patients who start HAART immediately after presentation with an acute OI and patients who start HAART at least 4 weeks after the OI has resolved should help identify the factors supporting early or delayed initiation of antiretrovirals (4).
Generally speaking, HAART can be administered by combining either a protease inhibitor (PI) or a nonnucleoside reverse transcriptase inhibitor (NNRTI) with 2 nucleoside reverse transcriptase inhibitors (NRTIs). There are currently more than 20 FDA-approved antiretrovirals. Frequent updates on the guidelines for the use of antiretroviral agents in HIV-infected adults are available at www.AIDSinfo.nih.gov, and a discussion of this topic is beyond the scope of this review.
Continuation of HAART in the Hospital
In most cases, every effort should be made to minimize interruption of HAART during a hospitalization. Although some investigators are examining the virologic and immunologic safety of interrupting HAART as a treatment strategy, there are few data on viral replication, CD4 cell count decline, and rate of acquisition of new mutations in hospitalized patients who have unexpected treatment interruptions (5). The long half-life of some antiretrovirals promotes the emergence of resistance once HAART is stopped. For example, once NNRTIs are stopped, subtherapeutic levels remain in the plasma and cells for several days. HIV then replicates in a milieu that may select for resistance mutations.
Because zidovudine is the only antiretroviral available in a parenteral preparation, it is often difficult to continue HAART when a patient cannot take medications by mouth. Drugs given by the enteral route in a hospitalized patient may also be poorly absorbed, and few data exist on the absorption of antiretrovirals administered through a gastrostomy or jejunostomy tube (6).
Prescribing HAART in the Hospital
Antiretroviral prescribing errors occur frequently in the hospitalized AIDS patient. The most common errors include overdosing or underdosing, missing components of multidrug regimens, or missing critical drug-drug interactions (7). Underdosing may lead to resistance, and overdosing contributes to increased toxicity. In one report, prescribing errors occurred in 12% of admissions in the post-HAART era (1998) compared with 2% of admissions in the pre-HAART era (1996) (7). The NRTIs, including didanosine, emtricitabine, lamivudine, stavudine, and zidovudine, require decreased dosing in renal insufficiency. Tenofovir is not recommended for use if the creatine clearance is less than 60 mL/minute. Dosage adjustments in hepatic disease are recommended for amprenavir, fosamprenavir, delavirdine, efavirenz, and nevirapine.
Immune Reconstitution Syndrome
The widespread use of HAART has produced sustained suppression of HIV replication and recovery of CD4 cell counts. It also became evident that HAART resulted in not only a numerical increase in CD4 cells, but also in a functional immune recovery (8-10). This improved T-cell response to antigens results in adequate protection against specific opportunistic infections, allowing for discontinuation of primary and secondary prophylaxis in HIV-infected patients. Immune reconstitution syndrome (IRS), an inflammatory syndrome, is recognized as a potential complication that can occur days to months after starting HAART. The onset of IRS is characterized by a paradoxical worsening of clinical or laboratory parameters despite a favorable response in CD4 cell counts and the suppression of viral replication (9,11,12). IRS has been reported to occur in 10–25% of patients who receive HAART and more commonly in those whose CD4 cell counts are <50 cells/mm3 at the start of HAART (9,11). It is postulated that the inflammatory response is triggered by the recognition of antigens associated with ongoing infection or recognition of persisting antigens associated with past (nonreplicating) infections. Mycobacterial antigens, frequently implicated in IRS, are responsible for about one third of cases. Other antigens associated with IRS include cytomegalovirus and hepatitis B and C (11). In most circumstances, with the management of IRS, HAART should be continued, while specific antimicrobial therapy and steroids should be considered (10).
Medical Conditions that Should Prompt HIV Screening
There are several medical conditions that should prompt screening for HIV infection. Generally, anyone presenting with a fever of unknown etiology who is sexually active or had a blood transfusion prior to 1985 should be screened for HIV infection. Symptoms consistent with acute retroviral syndrome (fever, sore throat, malaise, and skin rash) may be more commonly recognized by clinicians now than previously, and this remains a “golden opportunity” to intervene. Frequently, acute retroviral syndrome will be attributed to Epstein-Barr virus; however, caution should be used in the diagnosis of mononucleosis in those other than teenage populations. It is recommended that all persons presenting with any sexually transmitted disease, unexplained generalized lymphadenopathy, oral candidiasis, or tuberculosis should also be tested. Other conditions where HIV infection should be considered include enigmatic pneumonia, acute hepatitis B infection, herpes zoster infection (particularly in younger, seemingly immunocompetent individuals), idiopathic thrombocytopenic purpura, and nephropathy of unknown cause.
Drug Interactions
Drug interactions are an important consideration in the treatment of HIV infection. Interactions between HAART and other drugs used for the treatment or prophylaxis of opportunistic infections along with those used for the treatment of drug-induced endocrinopathies (hyperlipidemia, diabetes mellitus) are virtually unavoidable. Drug interactions occur either because of drug metabolism or absorption. The multiple metabolic pathways of some drugs make it difficult to predict the outcome of drug interactions. All protease inhibitors and non-nucleoside reverse transcriptase inhibitors are metabolized by the cytochrome P-450 enzyme system and each of these drugs may alter the metabolism of other antiretrovirals and concomitantly administered drugs (13,14). A decrease in trough plasma concentrations of the protease inhibitors to a level below the in vitro concentration required to inhibit replication of 50% of viral strains (IC50) may lead to development of resistance. Because nucleoside analogue reverse transcriptase inhibitors are primarily eliminated by the kidney, they do not interact with other drugs through the cytochrome P-450 system.
One noteworthy interaction that the clinician caring for HIV-infected patients should be aware of is the interaction of ribavirin with zidovudine. Ribavirin decreases the phosphorylation of zidovudine and stavudine in vitro, resulting in decreased concentrations of the active compound. HIV-infected patients who are coinfected with hepatitis C may be treated with regimens that include ribavirin, which may reduce the efficacy of zidovudine (15). Another important interaction is the effect of nevirapine or efavirenz on plasma methadone concentrations. Both drugs can decrease methadone plasma levels by 50%, and patients receiving chronic therapy may need increased methadone doses to prevent withdrawal symptoms (16).
Protease inhibitors are associated with numerous interactions including certain antiarrhythmics, sedatives, hypnotics, ergot derivatives, and several lipid-lowering agents (statins). Not only do protease inhibitors affect the metabolism of certain drugs, but also their own metabolism is altered by other inducers or inhibitors of cytochrome activity that can cause clinically important decreases in serum levels of protease inhibitors. One widely recognized interaction is that of rifampin, which may decrease levels of some protease inhibitors by 80%. The resulting low plasma concentrations may promote viral resistance and result in treatment failure. Patients being treated for tuberculosis, who are also receiving protease inhibitors should be treated with a four-drug regimen that includes rifabutin (at half dose) instead of rifampin. Updated guidelines for the use of rifabutin or rifampin in HIV-infected patients receiving antiretroviral agents have been reviewed recently (17).
Other potent inducers such as phenytoin, phenobarbital, and carbamazepine can cause similar reductions in serum levels of protease inhibitors. Azole antifungal drugs and macrolides also have important interactions that complicate both the treatment and prophylaxis of opportunistic infections.
Interactions that interfere with absorption can also affect plasma drug concentrations. For example, the absorption of fluconazole is unaffected by variations in gastric pH, while itraconazole and ketoconazole require an acidic environment for optimal absorption. The protease inhibitor, atazanavir, also requires a low pH for absorption and thus is contraindicated with the use of proton pump inhibitors; taking atazanavir with acidic beverages is not sufficient to overcome this (18).
New information about drug interactions becomes known on almost a daily basis in patients with HIV infection. The number of documented and theoretical interactions can become overwhelming to the clinician. Clinicians should suspect potential drug interactions in a patient who is failing therapy but who is adherent to HAART. Fortunately, there are extensive tables on Web sites (www.hivatis.org) and product information to aid in the recognition and management of drug interactions.
Complications of HAART
Diabetes mellitus, hyperlipidemias, lipodystrophy, and insulin resistance are among the many complex metabolic abnormalities attributable to the use of HAART. For the most part, these complications are managed conservatively and usually do not mandate the discontinuation of HAART. Pancreatitis, hepatic steatosis, and lactic acidosis are wellrecognized complications of NRTIs. These are usually more acute and may result in hospitalization and necessitate the discontinuation of medications. Cessation of the offending agent (didanosine [ddI], stavudine [d4T], and zalcitabine [ddC) usually results in resolution of pancreatitis, but the episode may limit use of these agents in the future. Hepatic steatosis and lactic acidosis are rare but life-threatening adverse effects associated with the mitochondrial toxicity seen with the NRTIs. Symptoms usually develop insidiously with nausea, vomiting, abdominal pain, weight loss, or dyspnea and can progress rapidly to fatal lactic acidosis. Hepatomegaly, ascites, elevated liver associated enzymes, and an increased anion gap with lactic acidemia are usually present (19,20). Discontinuation of antiretroivirals is imperative.
There is an accumulating body of evidence that suggests that HIV-infected patients receiving HAART may be at risk for accelerated coronary disease (21). In addition, some cohort studies have reported an increased incidence of myocardial infarction (MI) following the introduction of HAART and the risk for MI rose progressively with the number of years on antiretroviral therapy (22,23). However, it is important to note that many traditional risk factors for coronary artery disease contribute more substantially to the risk for a cardiovascular event than does HAART. Therefore, aggressive modification of primary cardiac risk factors is warranted.
Hypersensitivity Drug Reactions
Drug hypersensitivity reactions are life-threatening reactions that result in a systemic illness that usually includes fever and maculopapular rash accompanied by constitutional symptoms (fatigue, myalgias, and arthralgias), visceral involvement (lymphadenopathy, mucositis, pneumonitis, myocarditis, hepatitis, and interstitial nephritis), and hematologic abnormalities (eosinophilia) (24).
Abacavir, an NRTI, is a relatively new antiretroviral agent used in many HAART regimens. Abacavir is associated with a hypersensitivity reaction, which can be fatal if abacavir use is continued despite the reaction, or if re-challenge with the drug takes place after the reaction (25). The overall incidence of this reaction appears to be around 4% (25). Prior antiretroviral experience and being of African descent are associated with a nearly 40% reduction in the risk of this hypersensitivity reaction, while patients of white race are at a significantly greater risk. CD4 cell counts do not appear to be significantly related to abacavir hypersensitivity (26). The exact metabolite that is likely responsible for abacavir hypersensitivity is unknown.
The most common symptoms of abacavir hypersensitivity reaction are fever, rash, nausea, vomiting, and abdominal pain. Occasionally, respiratory symptoms will be present and can mimic influenza. However, gastrointestinal symptoms are the most prominent complaints after fever and rash and help to distinguish between influenza and abacavir hypersensitivity. More than 90% of hypersensitivity reactions occur during the first 6 weeks of treatment, with a median time to development of 8 days. A fever that develops within a few weeks after the initiation of therapy with abacavir may be due to causes other than hypersensitivity. One of the most common situations is the simultaneous initiation of treatment with other drugs, such as trimethoprimsulfamethxazole, efavirenz, or nevirapine, all of which are associated with a higher incidence of hypersensitivity than abacavir (27,28). Symptoms may be sudden and worsen over a few days if abacavir is continued. Symptoms tend to improve in 48 hours after abacavir is discontinued. Supportive therapy includes intravenous hydration and withdrawal of abacavir as well as all other antiretrovirals. Early in the use of this medication, 20% of patients who were re-challenged with the drug experienced unanticipated life-threatening events manifesting as an anaphylactic or immediate type hypersensitivity reaction. Hypotension, renal insufficiency, and bronchospasm have resulted in death. Rechallenge symptoms have been seen with the first dose (29). A discussion of the potential for this hypersensitivity is warranted when prescribing this agent. In the United States, a patient information card warning of this hypersensitivity reaction is distributed to the patient with each bottle of abacavir. Prednisone does not prevent the development of hypersensitivity reaction.
Symptoms of toxicity from TMP-SMX are more likely to occur in the HIV infected than in patients without HIV infection. Fever and rash can occur in up to 50% of HIV-infected patients. The rash can be treatment limiting or severe in up to 20% of HIV-infected patients who receive it. Life-threatening reactions may occur, including fatal Stevens Johnson-type exfoliative skin reactions. Most toxicity in HIV-infected patients appears to be related to metabolites of the sulfamethoxazole component and decreased levels of glutathione. There have been reports of severe systemic reactions that resemble anaphylaxis or septic shock occurring in HIV-infected patients who are re-challenged with TMP-SMX after experiencing toxicity within the previous 6–8 weeks (30).
The NNRTIf nevirapine and efavirenz can cause a delayed hypersensitivity reaction similar to that seen with abacavir. Cutaneous involvement is a prominent component of both nevirapine and efavirenz hypersensitivity reaction, with rash more likely to occur with the use of nevirapine. In addition, female patients have a higher propensity of developing Stevens-Johnson syndrome and symptomatic hepatic events from nevirapine (28,31).
Laboratory Abnormalities Related to Drugs
Hyperbilirubinemia and Atazanavir and Indinavir
Atazanavir, a protease inhibitor, is metabolized by the liver via CYP3A and also inhibits both CTP3A and UGT1A1. UGT1A1 is required for conjugation of bilirubin and inhibition of this enzyme results in elevated levels of unconjugated bilirubin. This effect is similar to what is observed in Gilbert’s syndrome. Asymptomatic indirect hyperbilirubemia may be seen in up to 60% of patients receiving atazanavir. Total bilirubin levels may rise to greater than 5 mg/dL, and more than 17% of patients may experience jaundice (18). Concurrent elevations in hepatic serum transaminases should not be attributed to atazanavir and alternative etiologies for these elevations should be sought. This hyperbilirubinemia is reversible upon discontinuation of the atazanavir.
Similarly, but to a lesser degree, asymptomatic unconjugated hyperbilirubinemia (>2 mg/dL) has been reported in up to 14% of patients treated with indinavir. Elevated serum transaminases were seen in less than 1%.
Renal Abnormalities and Indinavir
Several renal syndromes have been associated with indinavir use, ranging from obstructive uropathy and acute renal failure to asymptomatic pyuria. The range of clinical syndromes is a consequence of indinavir crystals aggregating within or irritating the urinary tract (32). Symptomatic nephrolithiasis (indinavir crystallization) has been reported to affect up to 12% (range, 5–35 %) of patients who receive indinavir, while up to 67% of patients will have asymptomatic crystalluria. The cumulative frequency of nephrolithiasis events increases with increasing exposure to indinavir. Therapy may be continued or interrupted for a few days. Adequate hydration is necessary with the administration of indinavir. Indinavir associated pyuria is frequently associated with interstitial nephritis or urothelial inflammation. Discontinuation of indinavir will lead to resolution of urine abnormalities.
Elevated Mean Corpuscular Volume (MCV) and NRTIs
Elevation of MCV or macrocytosis occurs in more than 90% of patients treated with zidovudine, but is not correlated with the development of anemia. Macrocytosis (MCV values exceeding 110/fl) develops within 2 weeks following the initiation of zidovudine therapy, and its presence can be used as a marker for medical adherence. When anemia does occur it is associated with a dose related bone marrow toxicity manifested as a macrocytic anemia. Serum B12 and folate levels are normal. Stavudine use is also associated with macrocytosis, in non-zidovudine-containing regimens (33).
Drug Screens and Efavirenz
The use of efavirenz, a potent non-nucleoside reverse transcriptase inhibitor, can cause a false-positive urine drug screen for cannabinoid. Efavirenz does not bind to cannabinoid receptors. The false-positive test results are specific to the assay kit used (34).
Evaluation of the AIDS Patient with Fever
Fever is a common symptom in HIV-infected patients, the etiology of which can be identified in more than 80% of cases (35,36). The AIDS patient with fever poses a considerable challenge given that the expanded differential may include a wide range of OIs. The CD4 cell count remains a valuable predictor of risk for infection. Patients with CD4 cell counts greater than 500 cells/mm3 should be evaluated as if immunocompetent. Patients with CD4 cell counts between 200 and 500 cells/mm3 are at increased risk for upper and lower bacterial respiratory infections, tuberculosis, and sinusitis, but overall their risk for opportunistic infection is not increased. In patients with CD4 counts less than 200 cells/mm3, Pneumocystis jiroveci pneumonia, formerly known as Pneumocystis carinii pneumonia, is the most common cause of fever in those not receiving primary prophylaxis. As the CD4 cell count decreases below 100 cells/mm3, the risk for disseminated MAC, toxoplasmosis, CMV, disseminated fungal infections, and lymphoma should be considered possible causes of fever. HIV itself is usually not the cause of fever in patients with advanced immunosuppression (37).
In patients with CD4 counts >200 cells/mm3, the clinician can usually construct a laboratory and radiographic evaluation guided by symptoms, while in patients with severe immunosuppression a broader evaluation is required. A serum cryptococcal antigen should be obtained, as it has high sensitivity and specificity for both systemic disease as well as meningitis (38). Bacterial, mycobacterial, and fungal isolator blood cultures should be performed, as well as a urine culture, despite lack of symptoms. Urine AFB cultures can be added if there is a suspicion for tuberculosis. Sputum should be evaluated with gram stain, AFB smear, and culture, as well as PCP direct fluorescent antibody. If diarrhea is present, stool studies should include bacterial culture, ova and parasites evaluation, an assay for C. difficile, and Cryptosporidia and Giardia stool antigen assays. A serum LDH may be elevated in PCP, disseminated histoplasmosis, or lymphoma. A serum CMV antigen may be useful in the patient with fever and diarrhea, hepatitis, or retinitis.
A chest radiograph should be performed in all febrile AIDS patients. Chest films may be normal in 5–10% of HIV-infected patients with tuberculosis (TB). The typical radiographic appearance of Pneumocystis jiroveci pneumonia is a bilateral interstitial pattern characterized by reticular or ground-glass opacities. However, normal chest radiographs may be seen in one third of AIDS patients with active PCP. High-resolution computed tomography (HRCT) of the chest should be obtained if there is still a clinical suspicion for Pneumocystis. HRCT has been shown in several reports to have a 100% negative predictive value in the evaluation for PCP (39,40).
A lumbar puncture should be performed if the patient is symptomatic or if the serum cryptococcal antigen is reactive. A bone marrow biopsy and culture is also useful particularly in the evaluation of the patient with cytopenias. Bacterial, fungal, and AFB cultures may yield disseminated mycobacterial or fungal disease. Histopathologic evaluation may reveal granulomas with organisms or lymphoma. Bronchoscopy may be pursued in cases of high suspicion for TB or PCP.
Guidelines for the management of opportunistic infections associated with human immunodeficiency virus are available at www.AIDSinfo.nih.gov.
Evaluation of the AIDS Patient with Focal Neurological Disease
Toxoplasma encephalitis (TE) may be distinguished from primary CNS lymphoma without a brain biopsy. TE is caused by reactivation of latent infection by the protozoan Toxoplasma gondii. Almost 90% of patients have CD4 counts less than 200 cells/mm3 and 75% have CD4 counts less than 100 cells/mm3. Serum anti-Toxoplasma IgG antibodies are detected in more than 90% of patients with TE. Lesions on CT or MRI (a more sensitive modality) are typically multiple ring-enhancing lesions with a predilection for the basal ganglia. An emipiric trial of therapy is recommended, and a response confirms a diagnosis in the patient who has a positive Toxoplasma antibody and is not receiving trimethoprim-sulfamethoxazole prophylaxis. A lumbar puncture in this setting is not necessary and maybe ill advised if cerebral edema is present (Figure 1, page 24).
Primary CNS lymphoma (PCNSL) has a similar radiographic appearance as TE. Solitary lesions are more frequent in PCNSL. Positron emission tomography (PET) and single photon emission CT (SPECT) are useful adjunctive imaging modalities as they are positive in PCNSL due to the increased metabolic activity of the tumor. Cytologic analysis of the CSF may show lymphomatous cells. Epstein-Barr virus (EBV) DNA is uniformly detected in PCNSL in AIDS patients and detection of EBV DNA by PCR on the CSF has a sensitivity of 90–100% and a specificity of 87–98% for the diagnosis of PCNSL (41,42). Combining SPECT imaging and EBV PCR provides 100% sensitivity and 100% negative predictive value in the evaluation of AIDS-related primary CNS lymphoma (43), obviating the need for brain biopsy.
Progressive multifocal leukoencephalopathy (PML) is characterized radiographically by multiple and bilateral hypodense lesions of the white matter without mass effect or enhancement on CT. MRI demonstrates areas of hypointensity on T1-weighted images and increased intensity on T2-weighted images. JC virus DNA can be detected by PCR of CSF or brain tissue with sensitivity of approximately 80% and specificity of 95%. Because of the high positive predictive value of a positive PCR, a patient with AIDS who also has a compatible MRI can be diagnosed with PML (44,45).
Other focal neurologic disease seen in AIDS patients includes cryptococcomas, tuberculomas, CMV encephalitis, neurosyphilis, Nocardia and Aspergillus infection, and bacterial brain abscesses (46).
Conclusion
As survival of the HIV-infected population improves, more patients may require hospitalization for HAART treatment failures or complications attributed to antiretroviral therapy. The hospitalist should be familiar with the complications of antiretroviral agents, the interactions between HAART and medications used to treat opportunistic infections, and medical conditions induced by HAART. Evaluation of the HIV-infected patient presenting with fever can be based on the CD4 cell count, which predicts risk for opportunistic infections. Finally, using combined diagnostic approaches along with modern imaging and laboratory assays may preclude the need for more invasive procedures in the HIV-infected hospitalized patient.
Dr. Decker may be reached at cfdecker@bethesda.med.navy.mil.
References
- Torres RA, Barr M. Impact of combination therapy for HIV infection on inpatient census. N Engl J Med. 1997;336:1531-2.
- Fleishman JA, Hellinger FJ. Trends in HIV-related inpatient admissions from 1993 to 1997: a seven-state study. J Acquir Immune Defic Syndr. 2001;28:73-80.
- Fleishman JA, Hellinger FH. Recent trends in HIV-related inpatient admissions 1996-2000: a 7-state study. J Acquir Immune Defic Syndr. 2003;34:102-10.
- Powderly WG. Should patients with an acute opportunistic infection receive antiretroviral therapy immediately? Advanced Studies in Medicine. 2005;5:S111-S116.
- Fagard C, Oxenius A, Gunthard H, et al. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch Intern Med. 2003;163:1220-6.
- Soni N, Pozniak A. Continuing HIV therapy in the ICU. Crit Care. 2001;5:247-8.
- Purdy BD, Raymond AM, Lesar TS. Antiretroviral prescribing errors in hospitalized patients. Ann Pharmacother. 2000;34:833-8.
- Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112-6.
- Cooney EL. Clinical indicators of immune restoration following highly active antiretroviral therapy. Clin Infect Dis. 2002;34:224-33.
- Hirsch HH, Kaufmann G, Sendi P, BaĴegay M. Immune reconstitution in HIV-infected patients. Clin Infect Dis. 2004;38:1159-66.
- Cheng VC, Yuen KY, Chan WM, Wong SS, Ma ES, Chan RM. Immunorestitution disease involving the innate and adaptive response. Clin Infect Dis. 2000;30:882-92.
- Fishman JE, Saraf-Lavi E, Narita M, Hollender ES, Ramsinghani R, Ashkin D. Pulmonary tuberculosis in AIDS patients: transient chest radiographic worsening after initiation of antiretroviral therapy. AJR Am J Roentgenol. 2000;174:43-9.
- Piscitelli SC, Gallicano KD. Interactions among drugs for HIV and opportunistic infections. N Engl J Med. 2001;344:984-96.
- Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36:289-304.
- Sim SM, Hoggard PG, Sales SD, Phiboonbanakit D, Hart CA, Back DJ. Effect of ribavirin on zidovudine efficacy and toxicity in vitro: a concentration-dependent interaction. AIDS Res Hum Retroviruses. 1998;14:1661-7.
- Clarke SM, Mulcahy FM, Tjia J, et al. The pharmacokinetics of methadone in HIV-positive patients receiving the non-nucleoside reverse transcriptase inhibitor efavirenz. Br J Clin Pharmacol. 2001;51:213-7.
- Centers for Disease Control and Prevention. Updated guidelines for the use of rifabutin or rifampin for the treatment and prevention of tuberculosis among HIV-infected patients taking protease inhibitors or nonnucleoside reverse transcriptase inhibitors. Morb Mortal Wkly Rep. 2000;49:185-9.
- Havlir DV, O’Marro SD. Atazanavir: new option for treatment of HIV infection. Clin Infect Dis. 2004;38:1599-604.
- Sheng WH, Hsieh SM, Lee SC, et al. Fatal lactic acidosis associated with highly active antiretroviral therapy in patients with advanced human immunodeficiency virus infection in Taiwan. Int J STD AIDS. 2004;15:249-53.
- Herman JS, Easterbrook PJ. The metabolic toxicities of antiretroviral therapy. Int J STD AIDS. 2001;12:555-62;quiz 563-4.
- Behrens G, Schmidt H, Meyer D, Stoll M, Schmidt RE. Vascular complications associated with use of HIV protease inhibitors. Lancet. 1998;351:1958.
- Klein D, Hurley LB, Sidney S. Cardiovascular Disease and HIV Infection. N Engl J Med. 2003;349:1869-70; author reply 1869-70.
- Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993-2003.
- Anderson JA, Adkinson NF Jr. Allergic reactions to drugs and biologic agents. JAMA. 1987;258:2891-9.
- Hewitt RG. Abacavir hypersensitivity reaction. Clin Infect Dis. 2002;34:1137-42.
- Hetherington S, Hughes AR, Mosteller M, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121-2.
- Bossi P, Colin D, Bricaire F, Caumes E. Hypersensitivity syndrome associated with efavirenz therapy. Clin Infect Dis. 2000;30:227-8.
- Bourezane Y, Salard D, Hoen B, Vandel S, Drobacheff C, Laurent R. DRESS (drug rash with eosinophilia and systemic symptoms) syndrome associated with nevirapine therapy. Clin Infect Dis. 1998;27:1321-2.
- Walensky RP, Goldberg JH, Daily JP. Anaphylaxis after rechallenge with abacavir. AIDS. 1999;13:999-1000.
- Martin GJ, Paparello SF, Decker CF. A severe systemic reaction to trimethoprim-sulfamethoxazole in a patient infected with the human immunodeficiency virus. Clin Infect Dis. 1993;16:175-6.
- Bersoff-Matcha SJ, Miller WC, Aberg JA, et al. Sex differences in nevirapine rash. Clin Infect Dis. 2001;32:124-9.
- Kopp JB, Falloon J, Filie A, et al. Indinavir-associated interstitial nephritis and urothelial inflammation: clinical and cytologic findings. Clin Infect Dis. 2002;34:1122-8.
- Geene D, Sudre P, Anwar D, Goehring C, Saaidia A, Hirschel B. Causes of macrocytosis in HIV-infected patients not treated with zidovudine. Swiss HIV Cohort Study. J Infect. 2000;40:160-3.
- Gottesman L. Sustiva may cause false positive on marijuana test. WORLD. 1999:7.
- Sepkowitz KA, Telzak EE, Carrow M, Armstrong D. Fever among outpatients with advanced human immunodeficiency virus infection. Arch Intern Med. 1993;153: 1909-12.
- Barat LM, Gunn JE, Steger KA, Perkins CJ, Craven DE. Causes of fever in patients infected with human immunodeficiency virus who were admitted to Boston City Hospital. Clin Infect Dis. 1996;23:320-8.
- Sepkowitz KA. FUO and AIDS. Curr Clin Top Infect Dis. 1999;19:1-15.
- Asawavichienjinda T, Sitthi-Amorn C, Tanyanont V. Serum cyrptococcal antigen: diagnostic value in the diagnosis of AIDS-related cryptococcal meningitis. J Med Assoc Thai. 1999;82:65-71.
- Richards PJ, Riddell L, Reznek RH, Armstrong P, Pinching AJ, Parkin JM. High resolution computed tomography in HIV patients with suspected Pneumocystis carinii pneumonia and a normal chest radiograph. Clin Radiol. 1996;51:689-93.
- Gruden JF, Huang L, Turner J, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR Am J Roentgenol. 1997;169:967-75.
- MacMahon EM, Glass JD, Hayward SD, et al. Association of Epstein-Barr virus with primary central nervous system lymphoma in AIDS. AIDS Res Hum Retroviruses. 1992;8:740-2.
- Rao CR, Jain K, Bhatia K, Laksmaiah KC, Shankar SK. Association of primary central nervous system lymphomas with the Epstein-Barr virus. Neurol India. 2003;51:237-40.
- Antinori A, De Rossi G, Ammassari A, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol. 1999;17:554-60.
- Post MJ, Yiannoutsos C, Simpson D, et al. Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? AIDS Clinical Trials Group, 243 Team. AJNR Am J Neuroradiol. 1999;20:1896-906.
- Weber T. Cerebrospinal fluid analysis for the diagnosis of human immunodeficiency virus-related neurologic diseases. Semin Neurol. 1999;19:223-33.
- Skiest DJ. Focal neurological disease in patients with acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34:103-15.
Nosocomial Pneumonia
(This chapter has been reprinted with permission from Williams MV, Hayward R: Comprehensive Hospital Medicine, 1st edition. Philadelphia, WB Saunders, in press.)
Background
Nosocomial pneumonia (NP) is the leading cause of mortality among patients who die from hospital-acquired infections. Defined as pneumonia occurring 48 hours or more after hospital admission, NP also includes the subset of ventilator-associated pneumonia (VAP), defined as pneumonia developing 48 to 72 hours after initiation of mechanical ventilation. The incidence of NP is between 5 and 15 cases per 1000 hospital admissions. Healthcare-associated pneumonia (HCAP), part of the continuum of NP, describes an increasingly common proportion of pneumonia developing outside the hospital (Table I) (1). Typically afflicting people in a nursing home or assisted living setting, these patients are at risk for antibiotic-resistant-organisms and should be approached similarly to cases of nosocomial pneumonia rather than community-acquired pneumonia. Most of the data informing our diagnostic and treatment decisions about NP come from studies performed in mechanically ventilated patients and are extrapolated to make recommendations for non-ventilated patients.
Mortality attributable to NP is debated, but may be as high as 30%. The presence of nosocomial pneumonia increases hospital length of stay an average of 7–10 days, and in the case of VAP, is estimated to cost between $10,000 and $40,000 per case (2).
Assessment
Clinical Presentation
Signs and Symptoms
Nosocomial pneumonia is usually diagnosed based on clinical grounds. Typical symptoms and signs consist of fever, cough with sputum, and shortness of breath in the setting of hypoxia and a new infiltrate on chest radiograph (CXR). In the elderly, signs may be more subtle and delirium, fever, or leukocytosis in the absence of cough should trigger its consideration. The likelihood of NP increases among patients with risk factors for microaspiration, oropharyngeal colonization, or overgrowth of resistant organisms (Table II) (3).
Differential Diagnosis
Prior to settling on a diagnosis of NP, alternative causes of fever, hypoxia, and pulmonary infiltrates should be considered. Most commonly, these include pulmonary embolus, pulmonary edema, or atelectasis. Alternative infectious sources, such as urinary tract, skin and soft-tissue infections, and device-related infections (i.e., central venous catheters) are common in hospitalized patients and should be ruled out before diagnosing nosocomial pneumonia.
Diagnosis
Diagnostic strategies for NP seek to confirm the diagnosis and identify an etiologic pathogen, thus allowing timely, effective, and streamlined antibiotic therapy. Unfortunately, no consensus exists on the best approach to diagnosing nosocomial pneumonia. After obtaining a complete blood count and blood cultures, you can choose between a clinical or microbiologic diagnostic approach to diagnosis. A clinical diagnosis relies on a new or progressive radiographic infiltrate along with signs of infection such as fever, leukocytosis, or purulent sputum. Clinical diagnosis is sensitive, but is likely to lead to antibiotic overuse. The microbiologic approach requires sampling of secretions from the respiratory tract and may reduce inappropriate antibiotic use, but takes longer and may not be available in all hospitals.
Preferred Studies
The microbiologic approach to diagnosis relies on the use of quantitative or semi-quantitative cultures to create thresholds for antibiotic treatment. Bacterial cultures that demonstrate a level of growth above the thresholds described below warrant treatment, while those below it should trigger withholding or discontinuation of antibiotics.
Bronchoscopic Approaches: Bronchoalveolar lavage (BAL) with a cutoff of 10 (4) organisms/mL or protected specimen brush (PSB) with a cutoff of 10 (3) organisms/mL are felt to be the most specific diagnostic tests when performed prior to initiating antibiotics, or prior to changing antibiotics if a patient is already receiving them. In clinically stable patients, antibiotics can be safely discontinued if bacterial growth falls below the thresholds. If cultures are positive, antibiotic therapy should be tailored to target the organism identified. The bronchoscopic approach is favored in patients who are mechanically ventilated, develop their pneumonia late in the hospital stay (>5–7 days), are at risk for unusual pathogens, are failing therapy or suspected of having an alternative diagnosis.
Non-Bronchoscopic Approaches: Qualitative endotracheal aspirates (ETA) have been shown to be quite sensitive in ventilated patients, regularly identify organisms that may be subsequently found by BAL or PSB, and if negative, should result in withholding antibiotics. Quantitative endotracheal aspirates with a cutoff of 10 (6) organisms/mL are often encouraged to reduce antibiotic overuse, but results should be interpreted cautiously as they only have a sensitivity and specificity of about 75% (1). Consideration should be given to withholding antibiotics in a clinically stable patient with a negative quantitative ETA if antibiotics have not been changed in the preceding 72 hours. Many ICUs have begun to perform blinded sampling of lower respiratory tract secretions with suction catheters (blind PSB, blind mini-BAL). These techniques can be performed at all hours by trained respiratory therapists or nurses, provide culture data similar to that of bronchoscopy, and may be safer and less costly than bronchoscopy. In general, non-bronchoscopic techniques are preferred in patients who are not mechanically ventilated. Sputum sampling, while easy to obtain, has not been well studied in NP. However, in patients in whom bronchoscopic or other non-bronchoscopic techniques are not feasible, sputum sampling may be performed to identify potentially resistant organisms and help tailor therapy.
Alternative Options
Clinical Pulmonary Infection Score—Combining Clinical and Microbiologic Approaches
The clinical diagnosis of nosocomial pneumonia (new infiltrate + fever, leukocytosis, or purulent sputum) likely leads to antibiotic overuse, yet pursuing a bronchoscopic diagnosis is invasive, costly, and requires technical expertise. The quantitative ETA, blind PSB, and blind BAL discussed above are examples of some compromises that avoid the need for bronchoscopy, yet add microbiologic data in an attempt to prevent excess antibiotic therapy. Formally combining diagnostic approaches (clinical + microbiologic) may also be useful. One such option is the use of the clinical pulmonary infection score (CPIS), which combines clinical, radiographic, physiological, and microbiologic data into a numerical result. Scores >6 have been shown to correlate well with quantitative BAL (4). More recent studies, however, have suggested a lower specificity which could still result in antibiotic overuse, but this approach remains more accurate than a general clinical approach. Using the CPIS serially at the time NP is suspected and again at 72 hours may be more useful. Patients with an initial low clinical suspicion for pneumonia (CPIS of 6 or less) could have antibiotics safely discontinued at 72 hours if the CPIS remains low (5). Such a strategy may be useful in settings where more sophisticated diagnostic modalities are not available.
Multiple studies of biological markers of infection have attempted to find a non-invasive, rapid, accurate means of determining who needs antibiotics for presumed NP. Unfortunately, the results have largely been disappointing. More recently, measurement of a soluble triggering receptor expressed on myeloid cells (sTREM-1) that is upregulated in the setting of infection has been shown to improve our ability to diagnose NP accurately. Measurement of sTREM-1 was 98% sensitive and 90% specific for the diagnosis of pneumonia in mechanically ventilated patients (6). While promising, more data is needed before this test can be recommended for routine use.
Management
Initial Treatment
Early initiation of adequate empiric antibiotic therapy (i.e., the antibiotics administered are shown to be active against all organisms isolated) is associated with improved survival compared with initial inadequate therapy (1,7). Antibiotics should be started immediately after obtaining blood and sputum samples for culture and should not be withheld in the event of delay in diagnostic testing. The need to choose antibiotics quickly and expeditiously drives the use of broad spectrum antibiotics. In an effort to avoid unnecessary overuse of broad spectrum antibiotics, therapy should be based on risk for multidrug-resistant (MDR) pathogens. Identifying patients at low risk for MDR pathogens by clinical criteria allows for more narrow, but effective, antibiotic therapy. Low risk patients include those who develop their pneumonia early in the hospitalization (<5–7 days), are not immunocompromised, have not had prior broad spectrum antibiotics, and do not have risk factors for HCAP (Table I) (1,7). In these patients antibiotics should target common community-acquired organisms (Table III–low risk pathogens). Appropriate initial antibiotic therapy could include a third generation cephalosporin or a beta-lactam/beta-lactamase inhibitor. In some communities or hospital wards the incidence of methicillin-resistance among Staphylococcus aureus isolates (MRSA) may be high enough to warrant initial empiric therapy with vancomycin or linezolid.
Unfortunately, today’s increasingly complex hospitalized patients are unlikely to be “low risk,” especially in intensive care units.
Patients not meeting low risk criteria are considered to be at high risk for MDR pathogens (Table III–high risk pathogens). Initial empiric therapy needs to be broad and should include one antipseudomonal agent (cefepime or imipenem or beta-lactam/beta-lactamase inhibitor) plus a fluoroquinolone or aminoglycoside plus vancomycin or linezolid. The specific initial empiric therapy should be dictated by local resistance patterns, cost, and availability of preferred agents. When such broad spectrum therapy is initiated, it becomes imperative that antibiotics are “de-escalated” to limit antibiotic overuse. De-escalation therapy focuses on narrowing the antibiotic spectrum based on culture results, and limiting the overall duration of therapy. Hospitalists should aim to accomplish such de-escalation within 48–72 hours of initiating broad-spectrum antibiotics.
Subsequent Treatment
Patients started on initial empiric antibiotic therapy for presumed nosocomial pneumonia should be reassessed at 48–72 hours. Specifically, cultures should be checked and the clinical response to treatment evaluated. Figure I describes an algorithm for guiding treatment (1). In patients who are clinically stable and have negative lower respiratory tract cultures, antibiotics can be stopped. Patients with positive cultures should have antibiotics tailored, or “de-escalated” based on the organisms identified. In general, the most narrow spectrum antibiotic that is active against the bacteria isolated should be used. The use of combination therapy for gram negative organisms (two or more antibiotics active against a bacterial isolate) is widely practiced to achieve synergy, or prevent the development of resistance. However, in the absence of neutropenia, combination therapy has not been shown to be superior to monotherapyy (8), and monotherapy is preferred. The isolation of MRSA from a respiratory sample should also result in use of monotherapy. While some studies have suggested that linezolid may be superior to vancomycin for MRSA pneumonia, this finding needs validation in prospective studies.
A second component of de-escalation is shortening the total duration of therapy. The CPIS may be used to shorten the duration of therapy in patients at low risk for pneumonia. Investigators at a Veterans Affairs medical center randomized patients suspected of having NP, but who had a CPIS score < 6, to either treatment for 10–21 days, or short course therapy. Patients receiving short course therapy were reassessed at day 3, and if their CPIS score remained < 6, antibiotics were stopped (5). The short course therapy group had no difference in mortality when compared to the standard treatment group, but had less antibiotic use, shorter ICU stays, and was less likely to develop a superinfection or infection with a resistant organism. If the CPIS is not used, or if patients are felt to be at higher risk or convincingly demonstrated to have NP, a shorter course of therapy may still be preferred. A large randomized trial showed that 8 days of antibiotic therapy for patients with VAP resulted in similar clinical outcomes when compared to 15 days of therapy. Additionally, shorter duration antibiotic therapy was associated with lower likelihood of developing subsequent infections with multi-resistant pathogens. A subset of patients in the 8 day treatment group infected with non-fermenting gram negative bacilli (e.g., Pseudomonas aeruginosa) did have a higher pulmonary infection recurrence rate, but due to aggressive surveillance, this did not translate into a higher mortality risk in this subset of patients (9).
In summary, treatment of patients with suspected NP starts with immediate initiation of antibiotics and collection of respiratory secretions. While low risk patients can receive narrower spectrum therapy, most patients will require broad initial empiric therapy. The antibiotic regimen, however, should be narrowed at 48–72 hours based on microbiological results if the patient is improving. Overall treatment duration of 1 week is safe and effective with less chance of promoting growth of resistant organisms. In the subset of patients with pseudomonal infections, treatment of 1 week duration should be followed by active surveillance for recurrence, or alternatively, treatment can be extended to two weeks.
Prognosis
Once treatment for NP is initiated, clinical improvement is usually seen by 48–72 hours. There is little support for following either microbiologic response (clearance of positive cultures) or the response by chest radiography. The chest radiograph often lags behind the clinical response, however, a markedly worsening CXR (>50% increase in infiltrate) within the first 48 hours may indicate treatment failure. Clinical resolution as measured by temperature, white blood cell count, and oxygenation usually occurs by 6–7 days (10). Failure of oxygenation to improve by 72 hours has been shown to be predictive of treatment failure.
The overall mortality in patients with NP is as high as 30–70%, largely due to severe comorbid disease in the at risk population. Higher mortality rates are seen in patients with VAP and resistant organisms. The mortality attributable to the episode of NP is about 30%, and can be reduced to <15% with appropriate antibiotic therapy (1).
Prevention
Preventive strategies are either directed at reducing the overall incidence of infectious complications in hospitalized patients, or they are specifically targeted at reducing the incidence of nosocomial pneumonia (3). The majority of the data supporting preventive strategies is limited to patients in the ICU, and in particular, patients receiving mechanical ventilation. However, many of the preventive principles can be extrapolated to the non-ICU population. The preventive strategies are highlighted in Table IV (page 18).
General Preventive Strategies
General preventive strategies aim to avoid contamination of patients with antimicrobial resistant organisms that exist in hospitals, or mitigating the emergence of antimicrobial resistant organisms in the first place. Preventing iatrogenic spread of resistant organisms depends on careful hand hygiene. Hand washing before and after patient contact reduces the incidence of nosocomial infection. Alcohol-based hand rinses placed at the bedside may actually be superior to soap and water, and in addition, improve compliance with hand hygiene.
Minimizing the use of indwelling devices (central lines, urinary catheters) also reduces the emergence of resistant organisms. When these devices are necessary, focusing on their timely removal is critical. The control of antibiotic use has been central to many preventive strategies. Prolonged or unnecessary use of broad-spectrum antibiotics is strongly associated with development and colonization of resistant organisms. Strategies that focus on aggressive antibiotic de-escalation (described above) are a key preventive tool. Some institutions have had success with antibiotic restriction or rotation, but long term data on the effectiveness of these techniques are lacking.
Targeted Preventive Strategies
Preventive strategies to lower the incidence of NP focus on reducing risk factors for oropharyngeal or gastric colonization and subsequent aspiration of contaminated oropharyngeal or gastric secretions (1,3,7,11).
Endotracheal intubation is one of the most important risk factors for NP in patients requiring ventilatory support. The use of non-invasive ventilation (NIV) or positive pressure mask ventilation in selected groups of patients has been effective in preventing nosocomial pneumonia. Non-invasive ventilation has been most successful in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) and pulmonary edema secondary to congestive heart failure (CHF) and should be considered in appropriately selected patients. When intubation is required the use of nasotracheal intubation should be avoided due higher rates of NP when compared to orotracheal intubation.
Supine positioning may contribute to the development of NP, likely due to an increased risk of gastric reflux and subsequent aspiration. Studies of semi-recumbent positioning (elevation of the head of the bed >45 degrees) have shown less reflux, less aspiration, and in one recent randomized control trial, a significant reduction in the rate of VAP (12). Elevation of the head of the bed is clearly indicated in mechanically ventilated patients and is also likely to benefit all patients at risk for aspiration and subsequent NP, although this technique has not been well studied in non-ventilated patients.
Subglottic secretion drainage (SSD) involves the removal of pooled secretions above the cuff of a specialized endotracheal tube that might otherwise leak into the lung. A meta-analysis of five studies evaluating this new technology showed significant reductions in the incidence of VAP. The use of SSD should be considered for use in patients requiring more than 3 days of mechanical ventilation (13).
Medications used for stress ulcer prophylaxis that increase gastric pH-such as H2 antagonists and antacids-allow for colonization of the upper gastrointestinal tract by potentially pathogenic organisms and therefore increase the risk for NP. The use of sucralfate instead of H2 antagonists is felt to lead to less alkalinization of the stomach and less bacterial overgrowth. The ability of sucralfate to prevent nosocomial pneumonia, however, has not been well demonstrated and its routine use is not recommended (14). Instead, efforts should be targeted at limiting use of stress ulcer prophylaxis to populations at high risk for clinically significant bleeding, namely patients with coagulopathy and prolonged ventilatory failure. Most patients who are not in the ICU should not receive stress ulcer prophylaxis. The risk of NP related to use of proton pump inhibitors has not been well studied.
Selective digestive decontamination (SDD) involves sterilization of the oropharynx and gastrointestinal tract in mechanically ventilated patients in order to prevent aspiration of large numbers of potentially pathogenic organisms and subsequent VAP. Most evaluations of SDD have involved oral (and sometimes gastric) application of topical polymixin, aminoglycoside, and amphotericin. In many cases, short courses of IV antibiotics have been added. At least 10 meta-analyses have shown a reduction in the risk of VAP with the use of SDD. The addition of IV antibiotics may also provide a mortality benefit. However, the long-term risk for emergence of resistant organisms, and insufficient data on the cost-effectiveness of SDD prevent its recommendation for routine use (14).
There are several preventive strategies targeted at reducing aspiration of contaminants in ventilator circuits, filters, and tubing. Recommended strategies, listed in Table III, page 16, include avoidance of routine ventilator circuit changes (change the tubing only when visibly contaminated or for a new patient), use of heat and moisture exchangers rather than heated humidifiers, and reduction in the frequency of changes of the heat and moisture exchangers (1,11,14).
Discharge/Follow-up Plans
Patients should be followed in the hospital until it is clear they are responding to therapy and clinically improving. There has been limited evaluation of strategies to rapidly transition patients to oral therapy. However, if patients are improving, are tolerating oral therapy, have a functional GI tract, and have an organism isolated that is sensitive to available oral antibiotics, the switch to oral therapy can be made. If no organism is isolated, but a patient definitely was felt to have NP, the oral antibiotics selected should have the same spectrum of activity as the previously administered IV antibiotics. In many cases, patients will have an infection with an organism that is only susceptible to IV antibiotics. These patients are likely to be ill enough to complete a full one week IV course in the hospital, but if they have no active co-morbid illness and have improved, they can have a PICC line placed (or other long-term IV access) and receive the remainder of their therapy at home or in another lower acuity setting.
In all patients who develop NP, reversible causes of aspiration should be sought, and in cases where multidrug-resistant organisms are isolated, this should be reported to any facility to which a patient is being transferred or to the primary care physician or home nurse who will assume care after discharge.
References
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
- Warren DK, Shukla SJ, Olsen MA, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31:1312-7.
- Flanders SA, Collard HR, Saint S. Preventing Nosocomial Pneumonia. In: Lautenbach E, Woeltje K, eds. The Society for Healthcare Epidemiology of America: Practical Handbook for Healthcare Epidemiologists. Thorofare, NJ: Slack, 2004:69-78.
- Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121-9.
- Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505-11.
- Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451-8.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867-903.
- Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and metaanalysis of randomised trials. BMJ. 2004;328:668.
- Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588-98.
- Dennesen PJ, van der Ven AJ, Kessels AG, Ramsay G, Bonten MJ. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am J Respir Crit Care Med. 2001;163:1371-5.
- Collard HR, Saint S, Matthay MA. Prevention of ventilator-associated pneumonia: an evidence-based systematic review. Ann Intern Med. 2003;138:494-501.
- Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851-8.
- Dezfulian C, Shojania K, Collard HR, Kim HM, Matthay MA, Saint S. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a metaanalysis. Am J Med. 2005;118:11-8.
- Dodek P, Keenan S, Cook D, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141:305-13.
(This chapter has been reprinted with permission from Williams MV, Hayward R: Comprehensive Hospital Medicine, 1st edition. Philadelphia, WB Saunders, in press.)
Background
Nosocomial pneumonia (NP) is the leading cause of mortality among patients who die from hospital-acquired infections. Defined as pneumonia occurring 48 hours or more after hospital admission, NP also includes the subset of ventilator-associated pneumonia (VAP), defined as pneumonia developing 48 to 72 hours after initiation of mechanical ventilation. The incidence of NP is between 5 and 15 cases per 1000 hospital admissions. Healthcare-associated pneumonia (HCAP), part of the continuum of NP, describes an increasingly common proportion of pneumonia developing outside the hospital (Table I) (1). Typically afflicting people in a nursing home or assisted living setting, these patients are at risk for antibiotic-resistant-organisms and should be approached similarly to cases of nosocomial pneumonia rather than community-acquired pneumonia. Most of the data informing our diagnostic and treatment decisions about NP come from studies performed in mechanically ventilated patients and are extrapolated to make recommendations for non-ventilated patients.
Mortality attributable to NP is debated, but may be as high as 30%. The presence of nosocomial pneumonia increases hospital length of stay an average of 7–10 days, and in the case of VAP, is estimated to cost between $10,000 and $40,000 per case (2).
Assessment
Clinical Presentation
Signs and Symptoms
Nosocomial pneumonia is usually diagnosed based on clinical grounds. Typical symptoms and signs consist of fever, cough with sputum, and shortness of breath in the setting of hypoxia and a new infiltrate on chest radiograph (CXR). In the elderly, signs may be more subtle and delirium, fever, or leukocytosis in the absence of cough should trigger its consideration. The likelihood of NP increases among patients with risk factors for microaspiration, oropharyngeal colonization, or overgrowth of resistant organisms (Table II) (3).
Differential Diagnosis
Prior to settling on a diagnosis of NP, alternative causes of fever, hypoxia, and pulmonary infiltrates should be considered. Most commonly, these include pulmonary embolus, pulmonary edema, or atelectasis. Alternative infectious sources, such as urinary tract, skin and soft-tissue infections, and device-related infections (i.e., central venous catheters) are common in hospitalized patients and should be ruled out before diagnosing nosocomial pneumonia.
Diagnosis
Diagnostic strategies for NP seek to confirm the diagnosis and identify an etiologic pathogen, thus allowing timely, effective, and streamlined antibiotic therapy. Unfortunately, no consensus exists on the best approach to diagnosing nosocomial pneumonia. After obtaining a complete blood count and blood cultures, you can choose between a clinical or microbiologic diagnostic approach to diagnosis. A clinical diagnosis relies on a new or progressive radiographic infiltrate along with signs of infection such as fever, leukocytosis, or purulent sputum. Clinical diagnosis is sensitive, but is likely to lead to antibiotic overuse. The microbiologic approach requires sampling of secretions from the respiratory tract and may reduce inappropriate antibiotic use, but takes longer and may not be available in all hospitals.
Preferred Studies
The microbiologic approach to diagnosis relies on the use of quantitative or semi-quantitative cultures to create thresholds for antibiotic treatment. Bacterial cultures that demonstrate a level of growth above the thresholds described below warrant treatment, while those below it should trigger withholding or discontinuation of antibiotics.
Bronchoscopic Approaches: Bronchoalveolar lavage (BAL) with a cutoff of 10 (4) organisms/mL or protected specimen brush (PSB) with a cutoff of 10 (3) organisms/mL are felt to be the most specific diagnostic tests when performed prior to initiating antibiotics, or prior to changing antibiotics if a patient is already receiving them. In clinically stable patients, antibiotics can be safely discontinued if bacterial growth falls below the thresholds. If cultures are positive, antibiotic therapy should be tailored to target the organism identified. The bronchoscopic approach is favored in patients who are mechanically ventilated, develop their pneumonia late in the hospital stay (>5–7 days), are at risk for unusual pathogens, are failing therapy or suspected of having an alternative diagnosis.
Non-Bronchoscopic Approaches: Qualitative endotracheal aspirates (ETA) have been shown to be quite sensitive in ventilated patients, regularly identify organisms that may be subsequently found by BAL or PSB, and if negative, should result in withholding antibiotics. Quantitative endotracheal aspirates with a cutoff of 10 (6) organisms/mL are often encouraged to reduce antibiotic overuse, but results should be interpreted cautiously as they only have a sensitivity and specificity of about 75% (1). Consideration should be given to withholding antibiotics in a clinically stable patient with a negative quantitative ETA if antibiotics have not been changed in the preceding 72 hours. Many ICUs have begun to perform blinded sampling of lower respiratory tract secretions with suction catheters (blind PSB, blind mini-BAL). These techniques can be performed at all hours by trained respiratory therapists or nurses, provide culture data similar to that of bronchoscopy, and may be safer and less costly than bronchoscopy. In general, non-bronchoscopic techniques are preferred in patients who are not mechanically ventilated. Sputum sampling, while easy to obtain, has not been well studied in NP. However, in patients in whom bronchoscopic or other non-bronchoscopic techniques are not feasible, sputum sampling may be performed to identify potentially resistant organisms and help tailor therapy.
Alternative Options
Clinical Pulmonary Infection Score—Combining Clinical and Microbiologic Approaches
The clinical diagnosis of nosocomial pneumonia (new infiltrate + fever, leukocytosis, or purulent sputum) likely leads to antibiotic overuse, yet pursuing a bronchoscopic diagnosis is invasive, costly, and requires technical expertise. The quantitative ETA, blind PSB, and blind BAL discussed above are examples of some compromises that avoid the need for bronchoscopy, yet add microbiologic data in an attempt to prevent excess antibiotic therapy. Formally combining diagnostic approaches (clinical + microbiologic) may also be useful. One such option is the use of the clinical pulmonary infection score (CPIS), which combines clinical, radiographic, physiological, and microbiologic data into a numerical result. Scores >6 have been shown to correlate well with quantitative BAL (4). More recent studies, however, have suggested a lower specificity which could still result in antibiotic overuse, but this approach remains more accurate than a general clinical approach. Using the CPIS serially at the time NP is suspected and again at 72 hours may be more useful. Patients with an initial low clinical suspicion for pneumonia (CPIS of 6 or less) could have antibiotics safely discontinued at 72 hours if the CPIS remains low (5). Such a strategy may be useful in settings where more sophisticated diagnostic modalities are not available.
Multiple studies of biological markers of infection have attempted to find a non-invasive, rapid, accurate means of determining who needs antibiotics for presumed NP. Unfortunately, the results have largely been disappointing. More recently, measurement of a soluble triggering receptor expressed on myeloid cells (sTREM-1) that is upregulated in the setting of infection has been shown to improve our ability to diagnose NP accurately. Measurement of sTREM-1 was 98% sensitive and 90% specific for the diagnosis of pneumonia in mechanically ventilated patients (6). While promising, more data is needed before this test can be recommended for routine use.
Management
Initial Treatment
Early initiation of adequate empiric antibiotic therapy (i.e., the antibiotics administered are shown to be active against all organisms isolated) is associated with improved survival compared with initial inadequate therapy (1,7). Antibiotics should be started immediately after obtaining blood and sputum samples for culture and should not be withheld in the event of delay in diagnostic testing. The need to choose antibiotics quickly and expeditiously drives the use of broad spectrum antibiotics. In an effort to avoid unnecessary overuse of broad spectrum antibiotics, therapy should be based on risk for multidrug-resistant (MDR) pathogens. Identifying patients at low risk for MDR pathogens by clinical criteria allows for more narrow, but effective, antibiotic therapy. Low risk patients include those who develop their pneumonia early in the hospitalization (<5–7 days), are not immunocompromised, have not had prior broad spectrum antibiotics, and do not have risk factors for HCAP (Table I) (1,7). In these patients antibiotics should target common community-acquired organisms (Table III–low risk pathogens). Appropriate initial antibiotic therapy could include a third generation cephalosporin or a beta-lactam/beta-lactamase inhibitor. In some communities or hospital wards the incidence of methicillin-resistance among Staphylococcus aureus isolates (MRSA) may be high enough to warrant initial empiric therapy with vancomycin or linezolid.
Unfortunately, today’s increasingly complex hospitalized patients are unlikely to be “low risk,” especially in intensive care units.
Patients not meeting low risk criteria are considered to be at high risk for MDR pathogens (Table III–high risk pathogens). Initial empiric therapy needs to be broad and should include one antipseudomonal agent (cefepime or imipenem or beta-lactam/beta-lactamase inhibitor) plus a fluoroquinolone or aminoglycoside plus vancomycin or linezolid. The specific initial empiric therapy should be dictated by local resistance patterns, cost, and availability of preferred agents. When such broad spectrum therapy is initiated, it becomes imperative that antibiotics are “de-escalated” to limit antibiotic overuse. De-escalation therapy focuses on narrowing the antibiotic spectrum based on culture results, and limiting the overall duration of therapy. Hospitalists should aim to accomplish such de-escalation within 48–72 hours of initiating broad-spectrum antibiotics.
Subsequent Treatment
Patients started on initial empiric antibiotic therapy for presumed nosocomial pneumonia should be reassessed at 48–72 hours. Specifically, cultures should be checked and the clinical response to treatment evaluated. Figure I describes an algorithm for guiding treatment (1). In patients who are clinically stable and have negative lower respiratory tract cultures, antibiotics can be stopped. Patients with positive cultures should have antibiotics tailored, or “de-escalated” based on the organisms identified. In general, the most narrow spectrum antibiotic that is active against the bacteria isolated should be used. The use of combination therapy for gram negative organisms (two or more antibiotics active against a bacterial isolate) is widely practiced to achieve synergy, or prevent the development of resistance. However, in the absence of neutropenia, combination therapy has not been shown to be superior to monotherapyy (8), and monotherapy is preferred. The isolation of MRSA from a respiratory sample should also result in use of monotherapy. While some studies have suggested that linezolid may be superior to vancomycin for MRSA pneumonia, this finding needs validation in prospective studies.
A second component of de-escalation is shortening the total duration of therapy. The CPIS may be used to shorten the duration of therapy in patients at low risk for pneumonia. Investigators at a Veterans Affairs medical center randomized patients suspected of having NP, but who had a CPIS score < 6, to either treatment for 10–21 days, or short course therapy. Patients receiving short course therapy were reassessed at day 3, and if their CPIS score remained < 6, antibiotics were stopped (5). The short course therapy group had no difference in mortality when compared to the standard treatment group, but had less antibiotic use, shorter ICU stays, and was less likely to develop a superinfection or infection with a resistant organism. If the CPIS is not used, or if patients are felt to be at higher risk or convincingly demonstrated to have NP, a shorter course of therapy may still be preferred. A large randomized trial showed that 8 days of antibiotic therapy for patients with VAP resulted in similar clinical outcomes when compared to 15 days of therapy. Additionally, shorter duration antibiotic therapy was associated with lower likelihood of developing subsequent infections with multi-resistant pathogens. A subset of patients in the 8 day treatment group infected with non-fermenting gram negative bacilli (e.g., Pseudomonas aeruginosa) did have a higher pulmonary infection recurrence rate, but due to aggressive surveillance, this did not translate into a higher mortality risk in this subset of patients (9).
In summary, treatment of patients with suspected NP starts with immediate initiation of antibiotics and collection of respiratory secretions. While low risk patients can receive narrower spectrum therapy, most patients will require broad initial empiric therapy. The antibiotic regimen, however, should be narrowed at 48–72 hours based on microbiological results if the patient is improving. Overall treatment duration of 1 week is safe and effective with less chance of promoting growth of resistant organisms. In the subset of patients with pseudomonal infections, treatment of 1 week duration should be followed by active surveillance for recurrence, or alternatively, treatment can be extended to two weeks.
Prognosis
Once treatment for NP is initiated, clinical improvement is usually seen by 48–72 hours. There is little support for following either microbiologic response (clearance of positive cultures) or the response by chest radiography. The chest radiograph often lags behind the clinical response, however, a markedly worsening CXR (>50% increase in infiltrate) within the first 48 hours may indicate treatment failure. Clinical resolution as measured by temperature, white blood cell count, and oxygenation usually occurs by 6–7 days (10). Failure of oxygenation to improve by 72 hours has been shown to be predictive of treatment failure.
The overall mortality in patients with NP is as high as 30–70%, largely due to severe comorbid disease in the at risk population. Higher mortality rates are seen in patients with VAP and resistant organisms. The mortality attributable to the episode of NP is about 30%, and can be reduced to <15% with appropriate antibiotic therapy (1).
Prevention
Preventive strategies are either directed at reducing the overall incidence of infectious complications in hospitalized patients, or they are specifically targeted at reducing the incidence of nosocomial pneumonia (3). The majority of the data supporting preventive strategies is limited to patients in the ICU, and in particular, patients receiving mechanical ventilation. However, many of the preventive principles can be extrapolated to the non-ICU population. The preventive strategies are highlighted in Table IV (page 18).
General Preventive Strategies
General preventive strategies aim to avoid contamination of patients with antimicrobial resistant organisms that exist in hospitals, or mitigating the emergence of antimicrobial resistant organisms in the first place. Preventing iatrogenic spread of resistant organisms depends on careful hand hygiene. Hand washing before and after patient contact reduces the incidence of nosocomial infection. Alcohol-based hand rinses placed at the bedside may actually be superior to soap and water, and in addition, improve compliance with hand hygiene.
Minimizing the use of indwelling devices (central lines, urinary catheters) also reduces the emergence of resistant organisms. When these devices are necessary, focusing on their timely removal is critical. The control of antibiotic use has been central to many preventive strategies. Prolonged or unnecessary use of broad-spectrum antibiotics is strongly associated with development and colonization of resistant organisms. Strategies that focus on aggressive antibiotic de-escalation (described above) are a key preventive tool. Some institutions have had success with antibiotic restriction or rotation, but long term data on the effectiveness of these techniques are lacking.
Targeted Preventive Strategies
Preventive strategies to lower the incidence of NP focus on reducing risk factors for oropharyngeal or gastric colonization and subsequent aspiration of contaminated oropharyngeal or gastric secretions (1,3,7,11).
Endotracheal intubation is one of the most important risk factors for NP in patients requiring ventilatory support. The use of non-invasive ventilation (NIV) or positive pressure mask ventilation in selected groups of patients has been effective in preventing nosocomial pneumonia. Non-invasive ventilation has been most successful in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) and pulmonary edema secondary to congestive heart failure (CHF) and should be considered in appropriately selected patients. When intubation is required the use of nasotracheal intubation should be avoided due higher rates of NP when compared to orotracheal intubation.
Supine positioning may contribute to the development of NP, likely due to an increased risk of gastric reflux and subsequent aspiration. Studies of semi-recumbent positioning (elevation of the head of the bed >45 degrees) have shown less reflux, less aspiration, and in one recent randomized control trial, a significant reduction in the rate of VAP (12). Elevation of the head of the bed is clearly indicated in mechanically ventilated patients and is also likely to benefit all patients at risk for aspiration and subsequent NP, although this technique has not been well studied in non-ventilated patients.
Subglottic secretion drainage (SSD) involves the removal of pooled secretions above the cuff of a specialized endotracheal tube that might otherwise leak into the lung. A meta-analysis of five studies evaluating this new technology showed significant reductions in the incidence of VAP. The use of SSD should be considered for use in patients requiring more than 3 days of mechanical ventilation (13).
Medications used for stress ulcer prophylaxis that increase gastric pH-such as H2 antagonists and antacids-allow for colonization of the upper gastrointestinal tract by potentially pathogenic organisms and therefore increase the risk for NP. The use of sucralfate instead of H2 antagonists is felt to lead to less alkalinization of the stomach and less bacterial overgrowth. The ability of sucralfate to prevent nosocomial pneumonia, however, has not been well demonstrated and its routine use is not recommended (14). Instead, efforts should be targeted at limiting use of stress ulcer prophylaxis to populations at high risk for clinically significant bleeding, namely patients with coagulopathy and prolonged ventilatory failure. Most patients who are not in the ICU should not receive stress ulcer prophylaxis. The risk of NP related to use of proton pump inhibitors has not been well studied.
Selective digestive decontamination (SDD) involves sterilization of the oropharynx and gastrointestinal tract in mechanically ventilated patients in order to prevent aspiration of large numbers of potentially pathogenic organisms and subsequent VAP. Most evaluations of SDD have involved oral (and sometimes gastric) application of topical polymixin, aminoglycoside, and amphotericin. In many cases, short courses of IV antibiotics have been added. At least 10 meta-analyses have shown a reduction in the risk of VAP with the use of SDD. The addition of IV antibiotics may also provide a mortality benefit. However, the long-term risk for emergence of resistant organisms, and insufficient data on the cost-effectiveness of SDD prevent its recommendation for routine use (14).
There are several preventive strategies targeted at reducing aspiration of contaminants in ventilator circuits, filters, and tubing. Recommended strategies, listed in Table III, page 16, include avoidance of routine ventilator circuit changes (change the tubing only when visibly contaminated or for a new patient), use of heat and moisture exchangers rather than heated humidifiers, and reduction in the frequency of changes of the heat and moisture exchangers (1,11,14).
Discharge/Follow-up Plans
Patients should be followed in the hospital until it is clear they are responding to therapy and clinically improving. There has been limited evaluation of strategies to rapidly transition patients to oral therapy. However, if patients are improving, are tolerating oral therapy, have a functional GI tract, and have an organism isolated that is sensitive to available oral antibiotics, the switch to oral therapy can be made. If no organism is isolated, but a patient definitely was felt to have NP, the oral antibiotics selected should have the same spectrum of activity as the previously administered IV antibiotics. In many cases, patients will have an infection with an organism that is only susceptible to IV antibiotics. These patients are likely to be ill enough to complete a full one week IV course in the hospital, but if they have no active co-morbid illness and have improved, they can have a PICC line placed (or other long-term IV access) and receive the remainder of their therapy at home or in another lower acuity setting.
In all patients who develop NP, reversible causes of aspiration should be sought, and in cases where multidrug-resistant organisms are isolated, this should be reported to any facility to which a patient is being transferred or to the primary care physician or home nurse who will assume care after discharge.
References
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
- Warren DK, Shukla SJ, Olsen MA, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31:1312-7.
- Flanders SA, Collard HR, Saint S. Preventing Nosocomial Pneumonia. In: Lautenbach E, Woeltje K, eds. The Society for Healthcare Epidemiology of America: Practical Handbook for Healthcare Epidemiologists. Thorofare, NJ: Slack, 2004:69-78.
- Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121-9.
- Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505-11.
- Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451-8.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867-903.
- Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and metaanalysis of randomised trials. BMJ. 2004;328:668.
- Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588-98.
- Dennesen PJ, van der Ven AJ, Kessels AG, Ramsay G, Bonten MJ. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am J Respir Crit Care Med. 2001;163:1371-5.
- Collard HR, Saint S, Matthay MA. Prevention of ventilator-associated pneumonia: an evidence-based systematic review. Ann Intern Med. 2003;138:494-501.
- Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851-8.
- Dezfulian C, Shojania K, Collard HR, Kim HM, Matthay MA, Saint S. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a metaanalysis. Am J Med. 2005;118:11-8.
- Dodek P, Keenan S, Cook D, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141:305-13.
(This chapter has been reprinted with permission from Williams MV, Hayward R: Comprehensive Hospital Medicine, 1st edition. Philadelphia, WB Saunders, in press.)
Background
Nosocomial pneumonia (NP) is the leading cause of mortality among patients who die from hospital-acquired infections. Defined as pneumonia occurring 48 hours or more after hospital admission, NP also includes the subset of ventilator-associated pneumonia (VAP), defined as pneumonia developing 48 to 72 hours after initiation of mechanical ventilation. The incidence of NP is between 5 and 15 cases per 1000 hospital admissions. Healthcare-associated pneumonia (HCAP), part of the continuum of NP, describes an increasingly common proportion of pneumonia developing outside the hospital (Table I) (1). Typically afflicting people in a nursing home or assisted living setting, these patients are at risk for antibiotic-resistant-organisms and should be approached similarly to cases of nosocomial pneumonia rather than community-acquired pneumonia. Most of the data informing our diagnostic and treatment decisions about NP come from studies performed in mechanically ventilated patients and are extrapolated to make recommendations for non-ventilated patients.
Mortality attributable to NP is debated, but may be as high as 30%. The presence of nosocomial pneumonia increases hospital length of stay an average of 7–10 days, and in the case of VAP, is estimated to cost between $10,000 and $40,000 per case (2).
Assessment
Clinical Presentation
Signs and Symptoms
Nosocomial pneumonia is usually diagnosed based on clinical grounds. Typical symptoms and signs consist of fever, cough with sputum, and shortness of breath in the setting of hypoxia and a new infiltrate on chest radiograph (CXR). In the elderly, signs may be more subtle and delirium, fever, or leukocytosis in the absence of cough should trigger its consideration. The likelihood of NP increases among patients with risk factors for microaspiration, oropharyngeal colonization, or overgrowth of resistant organisms (Table II) (3).
Differential Diagnosis
Prior to settling on a diagnosis of NP, alternative causes of fever, hypoxia, and pulmonary infiltrates should be considered. Most commonly, these include pulmonary embolus, pulmonary edema, or atelectasis. Alternative infectious sources, such as urinary tract, skin and soft-tissue infections, and device-related infections (i.e., central venous catheters) are common in hospitalized patients and should be ruled out before diagnosing nosocomial pneumonia.
Diagnosis
Diagnostic strategies for NP seek to confirm the diagnosis and identify an etiologic pathogen, thus allowing timely, effective, and streamlined antibiotic therapy. Unfortunately, no consensus exists on the best approach to diagnosing nosocomial pneumonia. After obtaining a complete blood count and blood cultures, you can choose between a clinical or microbiologic diagnostic approach to diagnosis. A clinical diagnosis relies on a new or progressive radiographic infiltrate along with signs of infection such as fever, leukocytosis, or purulent sputum. Clinical diagnosis is sensitive, but is likely to lead to antibiotic overuse. The microbiologic approach requires sampling of secretions from the respiratory tract and may reduce inappropriate antibiotic use, but takes longer and may not be available in all hospitals.
Preferred Studies
The microbiologic approach to diagnosis relies on the use of quantitative or semi-quantitative cultures to create thresholds for antibiotic treatment. Bacterial cultures that demonstrate a level of growth above the thresholds described below warrant treatment, while those below it should trigger withholding or discontinuation of antibiotics.
Bronchoscopic Approaches: Bronchoalveolar lavage (BAL) with a cutoff of 10 (4) organisms/mL or protected specimen brush (PSB) with a cutoff of 10 (3) organisms/mL are felt to be the most specific diagnostic tests when performed prior to initiating antibiotics, or prior to changing antibiotics if a patient is already receiving them. In clinically stable patients, antibiotics can be safely discontinued if bacterial growth falls below the thresholds. If cultures are positive, antibiotic therapy should be tailored to target the organism identified. The bronchoscopic approach is favored in patients who are mechanically ventilated, develop their pneumonia late in the hospital stay (>5–7 days), are at risk for unusual pathogens, are failing therapy or suspected of having an alternative diagnosis.
Non-Bronchoscopic Approaches: Qualitative endotracheal aspirates (ETA) have been shown to be quite sensitive in ventilated patients, regularly identify organisms that may be subsequently found by BAL or PSB, and if negative, should result in withholding antibiotics. Quantitative endotracheal aspirates with a cutoff of 10 (6) organisms/mL are often encouraged to reduce antibiotic overuse, but results should be interpreted cautiously as they only have a sensitivity and specificity of about 75% (1). Consideration should be given to withholding antibiotics in a clinically stable patient with a negative quantitative ETA if antibiotics have not been changed in the preceding 72 hours. Many ICUs have begun to perform blinded sampling of lower respiratory tract secretions with suction catheters (blind PSB, blind mini-BAL). These techniques can be performed at all hours by trained respiratory therapists or nurses, provide culture data similar to that of bronchoscopy, and may be safer and less costly than bronchoscopy. In general, non-bronchoscopic techniques are preferred in patients who are not mechanically ventilated. Sputum sampling, while easy to obtain, has not been well studied in NP. However, in patients in whom bronchoscopic or other non-bronchoscopic techniques are not feasible, sputum sampling may be performed to identify potentially resistant organisms and help tailor therapy.
Alternative Options
Clinical Pulmonary Infection Score—Combining Clinical and Microbiologic Approaches
The clinical diagnosis of nosocomial pneumonia (new infiltrate + fever, leukocytosis, or purulent sputum) likely leads to antibiotic overuse, yet pursuing a bronchoscopic diagnosis is invasive, costly, and requires technical expertise. The quantitative ETA, blind PSB, and blind BAL discussed above are examples of some compromises that avoid the need for bronchoscopy, yet add microbiologic data in an attempt to prevent excess antibiotic therapy. Formally combining diagnostic approaches (clinical + microbiologic) may also be useful. One such option is the use of the clinical pulmonary infection score (CPIS), which combines clinical, radiographic, physiological, and microbiologic data into a numerical result. Scores >6 have been shown to correlate well with quantitative BAL (4). More recent studies, however, have suggested a lower specificity which could still result in antibiotic overuse, but this approach remains more accurate than a general clinical approach. Using the CPIS serially at the time NP is suspected and again at 72 hours may be more useful. Patients with an initial low clinical suspicion for pneumonia (CPIS of 6 or less) could have antibiotics safely discontinued at 72 hours if the CPIS remains low (5). Such a strategy may be useful in settings where more sophisticated diagnostic modalities are not available.
Multiple studies of biological markers of infection have attempted to find a non-invasive, rapid, accurate means of determining who needs antibiotics for presumed NP. Unfortunately, the results have largely been disappointing. More recently, measurement of a soluble triggering receptor expressed on myeloid cells (sTREM-1) that is upregulated in the setting of infection has been shown to improve our ability to diagnose NP accurately. Measurement of sTREM-1 was 98% sensitive and 90% specific for the diagnosis of pneumonia in mechanically ventilated patients (6). While promising, more data is needed before this test can be recommended for routine use.
Management
Initial Treatment
Early initiation of adequate empiric antibiotic therapy (i.e., the antibiotics administered are shown to be active against all organisms isolated) is associated with improved survival compared with initial inadequate therapy (1,7). Antibiotics should be started immediately after obtaining blood and sputum samples for culture and should not be withheld in the event of delay in diagnostic testing. The need to choose antibiotics quickly and expeditiously drives the use of broad spectrum antibiotics. In an effort to avoid unnecessary overuse of broad spectrum antibiotics, therapy should be based on risk for multidrug-resistant (MDR) pathogens. Identifying patients at low risk for MDR pathogens by clinical criteria allows for more narrow, but effective, antibiotic therapy. Low risk patients include those who develop their pneumonia early in the hospitalization (<5–7 days), are not immunocompromised, have not had prior broad spectrum antibiotics, and do not have risk factors for HCAP (Table I) (1,7). In these patients antibiotics should target common community-acquired organisms (Table III–low risk pathogens). Appropriate initial antibiotic therapy could include a third generation cephalosporin or a beta-lactam/beta-lactamase inhibitor. In some communities or hospital wards the incidence of methicillin-resistance among Staphylococcus aureus isolates (MRSA) may be high enough to warrant initial empiric therapy with vancomycin or linezolid.
Unfortunately, today’s increasingly complex hospitalized patients are unlikely to be “low risk,” especially in intensive care units.
Patients not meeting low risk criteria are considered to be at high risk for MDR pathogens (Table III–high risk pathogens). Initial empiric therapy needs to be broad and should include one antipseudomonal agent (cefepime or imipenem or beta-lactam/beta-lactamase inhibitor) plus a fluoroquinolone or aminoglycoside plus vancomycin or linezolid. The specific initial empiric therapy should be dictated by local resistance patterns, cost, and availability of preferred agents. When such broad spectrum therapy is initiated, it becomes imperative that antibiotics are “de-escalated” to limit antibiotic overuse. De-escalation therapy focuses on narrowing the antibiotic spectrum based on culture results, and limiting the overall duration of therapy. Hospitalists should aim to accomplish such de-escalation within 48–72 hours of initiating broad-spectrum antibiotics.
Subsequent Treatment
Patients started on initial empiric antibiotic therapy for presumed nosocomial pneumonia should be reassessed at 48–72 hours. Specifically, cultures should be checked and the clinical response to treatment evaluated. Figure I describes an algorithm for guiding treatment (1). In patients who are clinically stable and have negative lower respiratory tract cultures, antibiotics can be stopped. Patients with positive cultures should have antibiotics tailored, or “de-escalated” based on the organisms identified. In general, the most narrow spectrum antibiotic that is active against the bacteria isolated should be used. The use of combination therapy for gram negative organisms (two or more antibiotics active against a bacterial isolate) is widely practiced to achieve synergy, or prevent the development of resistance. However, in the absence of neutropenia, combination therapy has not been shown to be superior to monotherapyy (8), and monotherapy is preferred. The isolation of MRSA from a respiratory sample should also result in use of monotherapy. While some studies have suggested that linezolid may be superior to vancomycin for MRSA pneumonia, this finding needs validation in prospective studies.
A second component of de-escalation is shortening the total duration of therapy. The CPIS may be used to shorten the duration of therapy in patients at low risk for pneumonia. Investigators at a Veterans Affairs medical center randomized patients suspected of having NP, but who had a CPIS score < 6, to either treatment for 10–21 days, or short course therapy. Patients receiving short course therapy were reassessed at day 3, and if their CPIS score remained < 6, antibiotics were stopped (5). The short course therapy group had no difference in mortality when compared to the standard treatment group, but had less antibiotic use, shorter ICU stays, and was less likely to develop a superinfection or infection with a resistant organism. If the CPIS is not used, or if patients are felt to be at higher risk or convincingly demonstrated to have NP, a shorter course of therapy may still be preferred. A large randomized trial showed that 8 days of antibiotic therapy for patients with VAP resulted in similar clinical outcomes when compared to 15 days of therapy. Additionally, shorter duration antibiotic therapy was associated with lower likelihood of developing subsequent infections with multi-resistant pathogens. A subset of patients in the 8 day treatment group infected with non-fermenting gram negative bacilli (e.g., Pseudomonas aeruginosa) did have a higher pulmonary infection recurrence rate, but due to aggressive surveillance, this did not translate into a higher mortality risk in this subset of patients (9).
In summary, treatment of patients with suspected NP starts with immediate initiation of antibiotics and collection of respiratory secretions. While low risk patients can receive narrower spectrum therapy, most patients will require broad initial empiric therapy. The antibiotic regimen, however, should be narrowed at 48–72 hours based on microbiological results if the patient is improving. Overall treatment duration of 1 week is safe and effective with less chance of promoting growth of resistant organisms. In the subset of patients with pseudomonal infections, treatment of 1 week duration should be followed by active surveillance for recurrence, or alternatively, treatment can be extended to two weeks.
Prognosis
Once treatment for NP is initiated, clinical improvement is usually seen by 48–72 hours. There is little support for following either microbiologic response (clearance of positive cultures) or the response by chest radiography. The chest radiograph often lags behind the clinical response, however, a markedly worsening CXR (>50% increase in infiltrate) within the first 48 hours may indicate treatment failure. Clinical resolution as measured by temperature, white blood cell count, and oxygenation usually occurs by 6–7 days (10). Failure of oxygenation to improve by 72 hours has been shown to be predictive of treatment failure.
The overall mortality in patients with NP is as high as 30–70%, largely due to severe comorbid disease in the at risk population. Higher mortality rates are seen in patients with VAP and resistant organisms. The mortality attributable to the episode of NP is about 30%, and can be reduced to <15% with appropriate antibiotic therapy (1).
Prevention
Preventive strategies are either directed at reducing the overall incidence of infectious complications in hospitalized patients, or they are specifically targeted at reducing the incidence of nosocomial pneumonia (3). The majority of the data supporting preventive strategies is limited to patients in the ICU, and in particular, patients receiving mechanical ventilation. However, many of the preventive principles can be extrapolated to the non-ICU population. The preventive strategies are highlighted in Table IV (page 18).
General Preventive Strategies
General preventive strategies aim to avoid contamination of patients with antimicrobial resistant organisms that exist in hospitals, or mitigating the emergence of antimicrobial resistant organisms in the first place. Preventing iatrogenic spread of resistant organisms depends on careful hand hygiene. Hand washing before and after patient contact reduces the incidence of nosocomial infection. Alcohol-based hand rinses placed at the bedside may actually be superior to soap and water, and in addition, improve compliance with hand hygiene.
Minimizing the use of indwelling devices (central lines, urinary catheters) also reduces the emergence of resistant organisms. When these devices are necessary, focusing on their timely removal is critical. The control of antibiotic use has been central to many preventive strategies. Prolonged or unnecessary use of broad-spectrum antibiotics is strongly associated with development and colonization of resistant organisms. Strategies that focus on aggressive antibiotic de-escalation (described above) are a key preventive tool. Some institutions have had success with antibiotic restriction or rotation, but long term data on the effectiveness of these techniques are lacking.
Targeted Preventive Strategies
Preventive strategies to lower the incidence of NP focus on reducing risk factors for oropharyngeal or gastric colonization and subsequent aspiration of contaminated oropharyngeal or gastric secretions (1,3,7,11).
Endotracheal intubation is one of the most important risk factors for NP in patients requiring ventilatory support. The use of non-invasive ventilation (NIV) or positive pressure mask ventilation in selected groups of patients has been effective in preventing nosocomial pneumonia. Non-invasive ventilation has been most successful in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) and pulmonary edema secondary to congestive heart failure (CHF) and should be considered in appropriately selected patients. When intubation is required the use of nasotracheal intubation should be avoided due higher rates of NP when compared to orotracheal intubation.
Supine positioning may contribute to the development of NP, likely due to an increased risk of gastric reflux and subsequent aspiration. Studies of semi-recumbent positioning (elevation of the head of the bed >45 degrees) have shown less reflux, less aspiration, and in one recent randomized control trial, a significant reduction in the rate of VAP (12). Elevation of the head of the bed is clearly indicated in mechanically ventilated patients and is also likely to benefit all patients at risk for aspiration and subsequent NP, although this technique has not been well studied in non-ventilated patients.
Subglottic secretion drainage (SSD) involves the removal of pooled secretions above the cuff of a specialized endotracheal tube that might otherwise leak into the lung. A meta-analysis of five studies evaluating this new technology showed significant reductions in the incidence of VAP. The use of SSD should be considered for use in patients requiring more than 3 days of mechanical ventilation (13).
Medications used for stress ulcer prophylaxis that increase gastric pH-such as H2 antagonists and antacids-allow for colonization of the upper gastrointestinal tract by potentially pathogenic organisms and therefore increase the risk for NP. The use of sucralfate instead of H2 antagonists is felt to lead to less alkalinization of the stomach and less bacterial overgrowth. The ability of sucralfate to prevent nosocomial pneumonia, however, has not been well demonstrated and its routine use is not recommended (14). Instead, efforts should be targeted at limiting use of stress ulcer prophylaxis to populations at high risk for clinically significant bleeding, namely patients with coagulopathy and prolonged ventilatory failure. Most patients who are not in the ICU should not receive stress ulcer prophylaxis. The risk of NP related to use of proton pump inhibitors has not been well studied.
Selective digestive decontamination (SDD) involves sterilization of the oropharynx and gastrointestinal tract in mechanically ventilated patients in order to prevent aspiration of large numbers of potentially pathogenic organisms and subsequent VAP. Most evaluations of SDD have involved oral (and sometimes gastric) application of topical polymixin, aminoglycoside, and amphotericin. In many cases, short courses of IV antibiotics have been added. At least 10 meta-analyses have shown a reduction in the risk of VAP with the use of SDD. The addition of IV antibiotics may also provide a mortality benefit. However, the long-term risk for emergence of resistant organisms, and insufficient data on the cost-effectiveness of SDD prevent its recommendation for routine use (14).
There are several preventive strategies targeted at reducing aspiration of contaminants in ventilator circuits, filters, and tubing. Recommended strategies, listed in Table III, page 16, include avoidance of routine ventilator circuit changes (change the tubing only when visibly contaminated or for a new patient), use of heat and moisture exchangers rather than heated humidifiers, and reduction in the frequency of changes of the heat and moisture exchangers (1,11,14).
Discharge/Follow-up Plans
Patients should be followed in the hospital until it is clear they are responding to therapy and clinically improving. There has been limited evaluation of strategies to rapidly transition patients to oral therapy. However, if patients are improving, are tolerating oral therapy, have a functional GI tract, and have an organism isolated that is sensitive to available oral antibiotics, the switch to oral therapy can be made. If no organism is isolated, but a patient definitely was felt to have NP, the oral antibiotics selected should have the same spectrum of activity as the previously administered IV antibiotics. In many cases, patients will have an infection with an organism that is only susceptible to IV antibiotics. These patients are likely to be ill enough to complete a full one week IV course in the hospital, but if they have no active co-morbid illness and have improved, they can have a PICC line placed (or other long-term IV access) and receive the remainder of their therapy at home or in another lower acuity setting.
In all patients who develop NP, reversible causes of aspiration should be sought, and in cases where multidrug-resistant organisms are isolated, this should be reported to any facility to which a patient is being transferred or to the primary care physician or home nurse who will assume care after discharge.
References
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
- Warren DK, Shukla SJ, Olsen MA, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31:1312-7.
- Flanders SA, Collard HR, Saint S. Preventing Nosocomial Pneumonia. In: Lautenbach E, Woeltje K, eds. The Society for Healthcare Epidemiology of America: Practical Handbook for Healthcare Epidemiologists. Thorofare, NJ: Slack, 2004:69-78.
- Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121-9.
- Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505-11.
- Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451-8.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867-903.
- Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and metaanalysis of randomised trials. BMJ. 2004;328:668.
- Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588-98.
- Dennesen PJ, van der Ven AJ, Kessels AG, Ramsay G, Bonten MJ. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am J Respir Crit Care Med. 2001;163:1371-5.
- Collard HR, Saint S, Matthay MA. Prevention of ventilator-associated pneumonia: an evidence-based systematic review. Ann Intern Med. 2003;138:494-501.
- Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851-8.
- Dezfulian C, Shojania K, Collard HR, Kim HM, Matthay MA, Saint S. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a metaanalysis. Am J Med. 2005;118:11-8.
- Dodek P, Keenan S, Cook D, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141:305-13.
The Epidemiology and Clinical Manifestations of Community-Acquired Methicillin-Resistant Staphylococcus aureus
A 65-year-old male with no significant past medical history, recently returned from a trip to the Democratic Republic of the Congo, presented with pain, swelling, and ulceration of his right lower leg. The symptoms had progressed despite oral amoxicillin/clavulanate. Evaluation at the time of admission revealed a large fluid collection in the anterior calf with extensive subcutaneous edema. Blood cultures were positive for methicillin–resistant S. aureus susceptible to clindamycin, erythromycin, tetracycline, trimethoprim-sulfamethoxazole, gentamicin, and tetracycline. His infection was successfully treated with surgical debridement, wound care, and vancomycin.
In 1941, Skinner and colleagues described the seriousness of S. aureus bloodstream infections in their series of 122 consecutive patients. The mortality rate was greater than 80% (1). Despite early success with penicillin the subsequent decades have shown this organism to be capable of elaborating resistance mechanisms that make therapy increasingly difficult (2). Methicillin resistance, which first appeared in the 1960s, has come to characterize many of the S. aureus isolates that are identified in the hospital. Recently, distinct strains of methicillin-resistant S. aureus (MRSA) are more commonly being identified in patients presenting for care from the community. This review will discuss recent developments in the clinical presentation and epidemiology of community-acquired MRSA in adults.
Definitions and Epidemiology
For infection control and epidemiological purposes, infections have been traditionally termed nosocomial if they 1) were not incubating at the time of presentation, 2) developed more than 72 hours after hospital admission, or 3) occurred in patients who were recently discharged from the hospital or who reside in a long-term care or skilled nursing facility. Beyond epidemiology, these definitions have been useful in helping the practicing clinician to employ effective empirical antibiotic therapy. The delivery of health care has evolved, however, and the distinction between outpatients and inpatients has been blurred. A broader term that has been suggested for infectious maladies in experienced patients who have moved in and out of the hospital is “healthcare associated” infections (3).
The evolving understanding of the origin of an infection has influenced efforts to define community MRSA. The term “community onset” or “community associated” MRSA can be used to describe a methicillin-resistant S. aureus infection that began incubating outside the hospital. If a patient has historical ties to a traditional treatment setting, the infection is most likely healthcare associated. Notable risk factors include hospitalization or stay in a nursing facility within the past year, use of broad-spectrum antibiotics, surgery, dialysis, intravenous drug use, or the presence of an indwelling vascular catheter. A MRSA infection in a patient presenting from home without any healthcare risk factors can be deemed “community acquired” MRSA (CaMRSA) (4).
A further understanding of CaMRSA can be gleaned from molecular studies of the organism. Methicillin resistance is mediated by a genetic element called staphylococcal cassette chromosome mecA (SCCmecA). MecA codes for a novel penicillin binding protein, PBP 2a, which is not inhibited by beta-lactam antibiotics (2). There are at least 5 types of SCCmecA. Types I through III are typically present in nosocomial MRSA strains. CaMRSA is distinguishable by the presence of SCCmecA IV (4-6).
Another distinctive feature of CaMRSA is the presence of the Panton-Valentine leukocidin (PVL). Previous work has shown that only 2–3% of strains of S. aureus produce this toxin (7). However this virulence factor, encoded by the genes lukS-PV and lukF-PV, appears to be expressed much more commonly in CaMRSA.
The difficulties with defining CaMRSA have influenced attempts to understand its prevalence. The key question in reviewing the available studies is how rigorous an attempt was made to exclude those patients who had significant healthcare contact. Salgado and colleagues performed a meta-analysis to try to determine the prevalence of true CaMRSA. They found that a significant number of subjects included in prevalence studies had identifiable healthcare risk factors, and that when this was accounted for, the overall prevalence of CaMRSA was less than 0.24% (8). The burden of CaMRSA infection will vary however based on location, and certain areas of the United States have demonstrated a higher prevalence. Researchers from the Emerging Infections Program Network examined CaMRSA in Atlanta, Baltimore, and Minnesota and found the prevalence to range from 8% to 20% (9). Of note, only 41% of suspected cases of CaMRSA were confirmed through interviews.
So what is CaMRSA? An acceptable working definition is a methicillin-resistant S. aureus infection occurring in a patient without a history of healthcare risk factors due to an isolate carrying SCCmecA type IV. The isolate is also likely to express the PVL virulence factor. This definition combines what is known about both the clinical and molecular epidemiology of these strains. Further research and time is likely to result in modifications to our understanding of this emerging phenomenon.
Antibiotic Susceptibility Patterns
CaMRSA strains have unique susceptibility patterns compared with traditional MRSA strains. As noted above, SCCmecA codes for methicillin resistance in S. aureus. SCCmecA types II and III are large genetic elements that usually code for resistance to multiple antibiotics. In contrast, type IV is smaller and results in decreased susceptibility to betalactams alone. CaMRSA strains are identifiable as being susceptible to clindamycin, trimethoprim-sulfamethoxazole, and the aminoglycosides (4). Susceptibility to clindamycin must be interpreted cautiously in strains that are erythromycin resistant. If erythromycin resistance is due to an inactivating enzyme (a ribosomal methylase) resistance to clindamycin can be induced. This macrolide-lincosamide-streptogramin–inducible phenotype can be identified in the microbiology lab by performing an erythromycin induction test (D-test). Clinical failures have been described when clindamycin has been used in the presence of this inducible phenotype (10).
Outbreaks
As with many infectious diseases, outbreaks first brought the problem of CaMRSA to wider attention. The first well-described outbreak occurred in the early 1980s among intravenous drug users in Detroit (11). Reports in the early 1990s focused on MRSA infections in young children without risk factors for resistant infection (12). Overwhelming, fatal sepsis due to MRSA was described in 4 pediatric patients in Minnesota and North Dakota. A fulminant, necrotizing pneumonia characterized 3 of the cases (13). Subsequently numerous outbreaks have been described among prison inmates, sexual partners, and competitive sports participants (14-16).
Two well-documented outbreaks have been described in football players. Begier and colleagues identified an outbreak that involved 10 players on the same college football team. Molecular typing demonstrated all recovered isolates to be of the same strain and to carry SCCmecA and the PVL gene. The case-control analysis showed an association between infection and playing wide receiver or cornerback, turf burns, and body shaving (17). An investigation of 8 MRSA infections among professional football players similarly showed all recovered strains to be clonal and to harbor SCCmecA IV and the PVL locus. In contrast to the college outbreak, these investigators found an association between being a lineman or a linebacker and disease. Turf burns were again a significant risk factor (18).
Both of these outbreaks, although geographically separate, were found to be due to the same strain of MRSA, clone USA300-0114. This clone has also been demonstrated as the predominant cause of CaMRSA in other communities (15,19). This would seem to indicate greater fitness of this particular strain that has allowed it to spread widely (20).
Clinical Manifestations
In general, CaMRSA has been reported to cause a similar spectrum of disease as methicillin-susceptible S. aureus (MSSA). As mentioned above, it appears to be seen mostly in otherwise healthy, young individuals. In the population based surveillance project of Fridkin et al., 77% of patients with community MRSA had skin and soft tissue infections (9). Invasive disease was observed in 6%. Similarly, Naimi and colleagues found skin and soft tissue infections in 75% of the subjects in their study of community-associated MRSA in Minnesota (21).
There is concern that CaMRSA may be associated with a greater likelihood of disease compared with other S. aureus strains. Ellis et al. prospectively evaluated active-duty soldiers found to be colonized with CaMRSA. Of the 24 colonized, 38% or 9 individuals developed soft-tissue infections as compared with 3% of those colonized with MSSA. Eight of nine affected patients had abscesses. All 9 of the available clinical isolates were positive for the PVL gene and the presence of this virulence factor was associated with an increased risk of invasive disease (22). Other authors have found an association between PVL-carrying strains of S. aureus and disease and it is perhaps this characteristic, not methicillin resistance, that assists the organism in causing disease in otherwise healthy individuals (23,24). The observed high prevalence of the PVL virulence factor among CaMRSA has been described as the “convergence of resistance and virulence” (25).
Severe disease has also been described due to strains of CaMRSA. Francis described 4 patients with necrotizing pneumonia due to CaMRSA similar to the pediatric cases referred to above. The isolates from all 4 patients carried PVL and SCCmecA IV genes and were of the USA300 strain group (26). Of note, all 4 patients initially had influenza-like illnesses, demonstrating again the association between influenza and staphylococcal pneumonia. This also signifies these presentations were potentially vaccine preventable. Recently, necrotizing fasciitis caused by CaMRSA strains, all again characterized as having PVL genes, has been described (27). This new phenomenon expands the differential diagnosis of causes of this life-threatening soft-tissue syndrome and influences empirical antibiotic selection.
A 41-year-old with Crohn’s disease treated with infliximab undergoes ventral hernia repair. She has a past surgical history of multiple abdominal surgeries. Three weeks postoperatively she is readmitted with a superinfected hematoma requiring operative drainage. Cultures reveal MRSA, susceptible to erythromycin, clindamycin, vancomycin, gentamicin, tetracycline, and trimethoprim-sulfamethoxazole.
The work to date on this new aspect of resistance in S. aureus intimates a trend similar to that previously experienced with penicillin resistance. Penicillinase-producing strains, first recognized in 1944, became increasingly common among hospital isolates after the second World War (28,29). By the 1970s, penicillin-resistant staphylococci had become widespread in the Community as well. Currently, identification of a penicillin-susceptible S. aureus isolate is uncommon.
The potential for further increases in the prevalence of methicillin resistance among staphylococci lies with the SCCmecA complex. Acquisition of this determinant from another resistant clone of either S. aureus or a coagulase-negative staphylococcus is the necessary first step in the process of becoming methicillin resistant. Types I through III are large, and this has been an obstacle to frequent transfers to MSSA strains. The result of this dynamic is that hospital-acquired MRSA to this point has descended from a relatively small number of clones as compared with the wide heterogeneity seen in susceptible S. aureus (30). As mentioned above, SCCmecA IV is smaller and can therefore more easily insert into many different MSSA strains without a loss of fitness. In fact type IV strains have been shown in vitro to replicate faster than hospital MRSA strains (20). This may allow MRSA to begin to displace MSSA as the predominant community phenotype in a manner similar to that in which penicillin-susceptible S. aureus was replaced.
A similar phenomenon may occur in hospitals wherein a typical CaMRSA strain may become the predominant hospital clone. This has been described already in 1 hospital where SCCmecA IV became the major determinant of methicillin resistance in the hospital (31). The trend was identifiable by a “more susceptible” antibiogram of MRSA strains. Future epidemiological surveillance will be necessary as the potential exists for resistant strains to continue to cross the increasingly more permeable barrier between traditional healthcare and the community.
Management
Increasing resistance to S. aureus has several implications for clinicians. Fundamental principles in the management of infectious syndromes become even more important, particularly source control of suppurative foci through debridement and drainage. An added benefit of such procedures is that they facilitate the establishment of a microbiological diagnosis. Clinicians and microbiologists will need to continue to work together closely so as to be aware of resistance trends in their community. In situations where the pathogen is not identified and treatment is prescribed empirically, follow-up is crucial.
Obviously, the emergence of CaMRSA has limited antibiotic choices. Clindamycin, trimethoprim-sulfamethoxazole, and doxycycline remain therapeutic options in the appropriate clinical situation. The severe clinical manifestations described above require consideration of empirical vancomycin in the treatment of patients presenting seriously ill with infectious syndromes that could be potentially due to S. aureus while awaiting culture results. The most extensive experience for inpatient use is with this agent. Linezolid, daptomycin, and quinupristin-dalfopristin are newer agents with activity against MRSA that have been reviewed elsewhere (32,33). Growing experience with these agents has provided options in situations where vancomycin cannot be used. It has also emphasized some of their limitations. Daptomycin and quinupristin-dalfopristin are only given parenterally, while linezolid can be given both orally and intravenously. Expense impacts the use of all three especially outside the hospital. Treatment-limiting cytopenias and peripheral and optic neuropathy have been described with linezolid when it is has been employed for extended courses of therapy. Daptomycin is inhibited by surfactant and therefore should not be used for suspected pulmonary infections. Quinupristin-dalfopristin’s use can be limited by disabling myalgias and the need for central venous access. More data about the use of these newer agents for invasive infections are needed before they can be considered superior to vancomycin.
Dr. Fraser may be reached at frasert@ccf.org.
Dr. Fraser is a member of the Wyeth Emerging Pathogens speakers’ bureau and has participated in a local advisory panel for GlaxoSmithKline. There is no conflict of interest to disclose for this work.
References
- Skinner D, Keefer CS. Significance of bacteremia caused by Staphylococcus aureus. Arch Intern Med. 1941;68:851-75.
- Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111:1265-73.
- Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791-7.
- Said-Salim B, Mathema B, Kreiswirth BN. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging pathogen. Infect Control Hosp Epidemiol. 2003;24:451-5.
- Carleton HA, Diep BA, Charlebois ED, Sensabaugh GF, Perdreau-Remington F. Community-adapted methicillin-resistant Staphylococcus aureus (MRSA): population dynamics of an expanding community reservoir of MRSA. J Infect Dis. 2004;190:1730-8.
- Daum RS, Ito T, Hiramatsu K, et al. A novel methicillin-resistance cassette in community-acquired methicillin resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis. 2002:186;1344-7.
- Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16-34.
- Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a metaanalysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131-9.
- Fridkin SK, Hageman JC, Morrison M, et al. Methicillinresistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-44.
- Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis. 2003;37:1257-60.
- Saravolatz LD, Markowitz N, Arking L, Pohlod D, Fisher E. Methicillin-resistant Staphylococcus aureus. Epidemiologic observations during a community-acquired outbreak. Ann Intern Med. 1982;96:11-6.
- Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593-598.
- Centers for Disease Control and Prevention. Four pediatric deaths from community acquired methicillin resistant Staphylococcus aureus—Minnesota and North Dakota, 1997-1999. MMWR Morb Mortal Wkly Rep. 1999;48:707-10.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus infections in correctional facilities—Georgia, California, and Texas, 2001-2003. MMWR Morb Mortal Wkly Rep. 2003;52:992-6.
- Centers for Disease Control and Prevention. Public Health Dispatch: outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002-2003. MMWR Morb Mortal Wkly Rep. 2003;52:88.
- Lindenmayer JD, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Int Med. 1998;158:895-9.
- Begier EM, Frenette K, Barrett NL, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39:1446-53.
- Kazakova SV, Hagerman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352: 468-75.
- McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113-20.
- Deresinski S. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic, and therapeutic odyssey. Clin Infect Dis. 2005;40:562-73.
- Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976-84.
- Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39:971-9.
- Yamasaki O, Kaneko J, Morizane S, et al. The association between Staphylococcus aureus strains carrying panton-valentine leukocidin genes and the development of deep-seated follicular infection. Clin Infect Dis. 2005;40:381-5.
- Hsu LY, Koh TH, Kurup A, Low J, Chlebicki MP, Tan BH. High incidence of Panton-Valentine leukocidin-producing Staphylococcus aureus in a tertiary care public hospital in Singapore. Clin Infect Dis. 2005;40:486-9.
- Chambers HF. Community-associated MRSA–resistance and virulence converge. N Engl J Med. 2005;352:1485-7.
- Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100-7.
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445-53.
- Kirby WMM. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944;99:452-3.
- Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178-82.
- Kreiswirth B, Kornblum J, Arbeit RD, et al. Evidence for a clonal origin of methicillin resistance in Staphylococcus aureus. Science. 1993;259:227-30.
- Donnio PY, Preney L, Gautier-Lerestif AL, Avril JL, Lafforgue N. Changes in staphylococcal chromosome type and antibiotic resistance profile in methicillin-resistant Staphylococcus aureus isolates from a French hospital over an 11 year period. J Antimicrob Chemother. 2004;53:808-13.
- Eliopoulos GM. Quinupristin-dalfopristin and linezolid: evidence and opinion. Clin Infect Dis. 2003;36: 473-81.
- Carpenter CF, Chambers HF. Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin Infect Dis. 2004;38: 994-1000.
A 65-year-old male with no significant past medical history, recently returned from a trip to the Democratic Republic of the Congo, presented with pain, swelling, and ulceration of his right lower leg. The symptoms had progressed despite oral amoxicillin/clavulanate. Evaluation at the time of admission revealed a large fluid collection in the anterior calf with extensive subcutaneous edema. Blood cultures were positive for methicillin–resistant S. aureus susceptible to clindamycin, erythromycin, tetracycline, trimethoprim-sulfamethoxazole, gentamicin, and tetracycline. His infection was successfully treated with surgical debridement, wound care, and vancomycin.
In 1941, Skinner and colleagues described the seriousness of S. aureus bloodstream infections in their series of 122 consecutive patients. The mortality rate was greater than 80% (1). Despite early success with penicillin the subsequent decades have shown this organism to be capable of elaborating resistance mechanisms that make therapy increasingly difficult (2). Methicillin resistance, which first appeared in the 1960s, has come to characterize many of the S. aureus isolates that are identified in the hospital. Recently, distinct strains of methicillin-resistant S. aureus (MRSA) are more commonly being identified in patients presenting for care from the community. This review will discuss recent developments in the clinical presentation and epidemiology of community-acquired MRSA in adults.
Definitions and Epidemiology
For infection control and epidemiological purposes, infections have been traditionally termed nosocomial if they 1) were not incubating at the time of presentation, 2) developed more than 72 hours after hospital admission, or 3) occurred in patients who were recently discharged from the hospital or who reside in a long-term care or skilled nursing facility. Beyond epidemiology, these definitions have been useful in helping the practicing clinician to employ effective empirical antibiotic therapy. The delivery of health care has evolved, however, and the distinction between outpatients and inpatients has been blurred. A broader term that has been suggested for infectious maladies in experienced patients who have moved in and out of the hospital is “healthcare associated” infections (3).
The evolving understanding of the origin of an infection has influenced efforts to define community MRSA. The term “community onset” or “community associated” MRSA can be used to describe a methicillin-resistant S. aureus infection that began incubating outside the hospital. If a patient has historical ties to a traditional treatment setting, the infection is most likely healthcare associated. Notable risk factors include hospitalization or stay in a nursing facility within the past year, use of broad-spectrum antibiotics, surgery, dialysis, intravenous drug use, or the presence of an indwelling vascular catheter. A MRSA infection in a patient presenting from home without any healthcare risk factors can be deemed “community acquired” MRSA (CaMRSA) (4).
A further understanding of CaMRSA can be gleaned from molecular studies of the organism. Methicillin resistance is mediated by a genetic element called staphylococcal cassette chromosome mecA (SCCmecA). MecA codes for a novel penicillin binding protein, PBP 2a, which is not inhibited by beta-lactam antibiotics (2). There are at least 5 types of SCCmecA. Types I through III are typically present in nosocomial MRSA strains. CaMRSA is distinguishable by the presence of SCCmecA IV (4-6).
Another distinctive feature of CaMRSA is the presence of the Panton-Valentine leukocidin (PVL). Previous work has shown that only 2–3% of strains of S. aureus produce this toxin (7). However this virulence factor, encoded by the genes lukS-PV and lukF-PV, appears to be expressed much more commonly in CaMRSA.
The difficulties with defining CaMRSA have influenced attempts to understand its prevalence. The key question in reviewing the available studies is how rigorous an attempt was made to exclude those patients who had significant healthcare contact. Salgado and colleagues performed a meta-analysis to try to determine the prevalence of true CaMRSA. They found that a significant number of subjects included in prevalence studies had identifiable healthcare risk factors, and that when this was accounted for, the overall prevalence of CaMRSA was less than 0.24% (8). The burden of CaMRSA infection will vary however based on location, and certain areas of the United States have demonstrated a higher prevalence. Researchers from the Emerging Infections Program Network examined CaMRSA in Atlanta, Baltimore, and Minnesota and found the prevalence to range from 8% to 20% (9). Of note, only 41% of suspected cases of CaMRSA were confirmed through interviews.
So what is CaMRSA? An acceptable working definition is a methicillin-resistant S. aureus infection occurring in a patient without a history of healthcare risk factors due to an isolate carrying SCCmecA type IV. The isolate is also likely to express the PVL virulence factor. This definition combines what is known about both the clinical and molecular epidemiology of these strains. Further research and time is likely to result in modifications to our understanding of this emerging phenomenon.
Antibiotic Susceptibility Patterns
CaMRSA strains have unique susceptibility patterns compared with traditional MRSA strains. As noted above, SCCmecA codes for methicillin resistance in S. aureus. SCCmecA types II and III are large genetic elements that usually code for resistance to multiple antibiotics. In contrast, type IV is smaller and results in decreased susceptibility to betalactams alone. CaMRSA strains are identifiable as being susceptible to clindamycin, trimethoprim-sulfamethoxazole, and the aminoglycosides (4). Susceptibility to clindamycin must be interpreted cautiously in strains that are erythromycin resistant. If erythromycin resistance is due to an inactivating enzyme (a ribosomal methylase) resistance to clindamycin can be induced. This macrolide-lincosamide-streptogramin–inducible phenotype can be identified in the microbiology lab by performing an erythromycin induction test (D-test). Clinical failures have been described when clindamycin has been used in the presence of this inducible phenotype (10).
Outbreaks
As with many infectious diseases, outbreaks first brought the problem of CaMRSA to wider attention. The first well-described outbreak occurred in the early 1980s among intravenous drug users in Detroit (11). Reports in the early 1990s focused on MRSA infections in young children without risk factors for resistant infection (12). Overwhelming, fatal sepsis due to MRSA was described in 4 pediatric patients in Minnesota and North Dakota. A fulminant, necrotizing pneumonia characterized 3 of the cases (13). Subsequently numerous outbreaks have been described among prison inmates, sexual partners, and competitive sports participants (14-16).
Two well-documented outbreaks have been described in football players. Begier and colleagues identified an outbreak that involved 10 players on the same college football team. Molecular typing demonstrated all recovered isolates to be of the same strain and to carry SCCmecA and the PVL gene. The case-control analysis showed an association between infection and playing wide receiver or cornerback, turf burns, and body shaving (17). An investigation of 8 MRSA infections among professional football players similarly showed all recovered strains to be clonal and to harbor SCCmecA IV and the PVL locus. In contrast to the college outbreak, these investigators found an association between being a lineman or a linebacker and disease. Turf burns were again a significant risk factor (18).
Both of these outbreaks, although geographically separate, were found to be due to the same strain of MRSA, clone USA300-0114. This clone has also been demonstrated as the predominant cause of CaMRSA in other communities (15,19). This would seem to indicate greater fitness of this particular strain that has allowed it to spread widely (20).
Clinical Manifestations
In general, CaMRSA has been reported to cause a similar spectrum of disease as methicillin-susceptible S. aureus (MSSA). As mentioned above, it appears to be seen mostly in otherwise healthy, young individuals. In the population based surveillance project of Fridkin et al., 77% of patients with community MRSA had skin and soft tissue infections (9). Invasive disease was observed in 6%. Similarly, Naimi and colleagues found skin and soft tissue infections in 75% of the subjects in their study of community-associated MRSA in Minnesota (21).
There is concern that CaMRSA may be associated with a greater likelihood of disease compared with other S. aureus strains. Ellis et al. prospectively evaluated active-duty soldiers found to be colonized with CaMRSA. Of the 24 colonized, 38% or 9 individuals developed soft-tissue infections as compared with 3% of those colonized with MSSA. Eight of nine affected patients had abscesses. All 9 of the available clinical isolates were positive for the PVL gene and the presence of this virulence factor was associated with an increased risk of invasive disease (22). Other authors have found an association between PVL-carrying strains of S. aureus and disease and it is perhaps this characteristic, not methicillin resistance, that assists the organism in causing disease in otherwise healthy individuals (23,24). The observed high prevalence of the PVL virulence factor among CaMRSA has been described as the “convergence of resistance and virulence” (25).
Severe disease has also been described due to strains of CaMRSA. Francis described 4 patients with necrotizing pneumonia due to CaMRSA similar to the pediatric cases referred to above. The isolates from all 4 patients carried PVL and SCCmecA IV genes and were of the USA300 strain group (26). Of note, all 4 patients initially had influenza-like illnesses, demonstrating again the association between influenza and staphylococcal pneumonia. This also signifies these presentations were potentially vaccine preventable. Recently, necrotizing fasciitis caused by CaMRSA strains, all again characterized as having PVL genes, has been described (27). This new phenomenon expands the differential diagnosis of causes of this life-threatening soft-tissue syndrome and influences empirical antibiotic selection.
A 41-year-old with Crohn’s disease treated with infliximab undergoes ventral hernia repair. She has a past surgical history of multiple abdominal surgeries. Three weeks postoperatively she is readmitted with a superinfected hematoma requiring operative drainage. Cultures reveal MRSA, susceptible to erythromycin, clindamycin, vancomycin, gentamicin, tetracycline, and trimethoprim-sulfamethoxazole.
The work to date on this new aspect of resistance in S. aureus intimates a trend similar to that previously experienced with penicillin resistance. Penicillinase-producing strains, first recognized in 1944, became increasingly common among hospital isolates after the second World War (28,29). By the 1970s, penicillin-resistant staphylococci had become widespread in the Community as well. Currently, identification of a penicillin-susceptible S. aureus isolate is uncommon.
The potential for further increases in the prevalence of methicillin resistance among staphylococci lies with the SCCmecA complex. Acquisition of this determinant from another resistant clone of either S. aureus or a coagulase-negative staphylococcus is the necessary first step in the process of becoming methicillin resistant. Types I through III are large, and this has been an obstacle to frequent transfers to MSSA strains. The result of this dynamic is that hospital-acquired MRSA to this point has descended from a relatively small number of clones as compared with the wide heterogeneity seen in susceptible S. aureus (30). As mentioned above, SCCmecA IV is smaller and can therefore more easily insert into many different MSSA strains without a loss of fitness. In fact type IV strains have been shown in vitro to replicate faster than hospital MRSA strains (20). This may allow MRSA to begin to displace MSSA as the predominant community phenotype in a manner similar to that in which penicillin-susceptible S. aureus was replaced.
A similar phenomenon may occur in hospitals wherein a typical CaMRSA strain may become the predominant hospital clone. This has been described already in 1 hospital where SCCmecA IV became the major determinant of methicillin resistance in the hospital (31). The trend was identifiable by a “more susceptible” antibiogram of MRSA strains. Future epidemiological surveillance will be necessary as the potential exists for resistant strains to continue to cross the increasingly more permeable barrier between traditional healthcare and the community.
Management
Increasing resistance to S. aureus has several implications for clinicians. Fundamental principles in the management of infectious syndromes become even more important, particularly source control of suppurative foci through debridement and drainage. An added benefit of such procedures is that they facilitate the establishment of a microbiological diagnosis. Clinicians and microbiologists will need to continue to work together closely so as to be aware of resistance trends in their community. In situations where the pathogen is not identified and treatment is prescribed empirically, follow-up is crucial.
Obviously, the emergence of CaMRSA has limited antibiotic choices. Clindamycin, trimethoprim-sulfamethoxazole, and doxycycline remain therapeutic options in the appropriate clinical situation. The severe clinical manifestations described above require consideration of empirical vancomycin in the treatment of patients presenting seriously ill with infectious syndromes that could be potentially due to S. aureus while awaiting culture results. The most extensive experience for inpatient use is with this agent. Linezolid, daptomycin, and quinupristin-dalfopristin are newer agents with activity against MRSA that have been reviewed elsewhere (32,33). Growing experience with these agents has provided options in situations where vancomycin cannot be used. It has also emphasized some of their limitations. Daptomycin and quinupristin-dalfopristin are only given parenterally, while linezolid can be given both orally and intravenously. Expense impacts the use of all three especially outside the hospital. Treatment-limiting cytopenias and peripheral and optic neuropathy have been described with linezolid when it is has been employed for extended courses of therapy. Daptomycin is inhibited by surfactant and therefore should not be used for suspected pulmonary infections. Quinupristin-dalfopristin’s use can be limited by disabling myalgias and the need for central venous access. More data about the use of these newer agents for invasive infections are needed before they can be considered superior to vancomycin.
Dr. Fraser may be reached at frasert@ccf.org.
Dr. Fraser is a member of the Wyeth Emerging Pathogens speakers’ bureau and has participated in a local advisory panel for GlaxoSmithKline. There is no conflict of interest to disclose for this work.
References
- Skinner D, Keefer CS. Significance of bacteremia caused by Staphylococcus aureus. Arch Intern Med. 1941;68:851-75.
- Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111:1265-73.
- Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791-7.
- Said-Salim B, Mathema B, Kreiswirth BN. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging pathogen. Infect Control Hosp Epidemiol. 2003;24:451-5.
- Carleton HA, Diep BA, Charlebois ED, Sensabaugh GF, Perdreau-Remington F. Community-adapted methicillin-resistant Staphylococcus aureus (MRSA): population dynamics of an expanding community reservoir of MRSA. J Infect Dis. 2004;190:1730-8.
- Daum RS, Ito T, Hiramatsu K, et al. A novel methicillin-resistance cassette in community-acquired methicillin resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis. 2002:186;1344-7.
- Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16-34.
- Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a metaanalysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131-9.
- Fridkin SK, Hageman JC, Morrison M, et al. Methicillinresistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-44.
- Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis. 2003;37:1257-60.
- Saravolatz LD, Markowitz N, Arking L, Pohlod D, Fisher E. Methicillin-resistant Staphylococcus aureus. Epidemiologic observations during a community-acquired outbreak. Ann Intern Med. 1982;96:11-6.
- Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593-598.
- Centers for Disease Control and Prevention. Four pediatric deaths from community acquired methicillin resistant Staphylococcus aureus—Minnesota and North Dakota, 1997-1999. MMWR Morb Mortal Wkly Rep. 1999;48:707-10.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus infections in correctional facilities—Georgia, California, and Texas, 2001-2003. MMWR Morb Mortal Wkly Rep. 2003;52:992-6.
- Centers for Disease Control and Prevention. Public Health Dispatch: outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002-2003. MMWR Morb Mortal Wkly Rep. 2003;52:88.
- Lindenmayer JD, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Int Med. 1998;158:895-9.
- Begier EM, Frenette K, Barrett NL, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39:1446-53.
- Kazakova SV, Hagerman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352: 468-75.
- McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113-20.
- Deresinski S. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic, and therapeutic odyssey. Clin Infect Dis. 2005;40:562-73.
- Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976-84.
- Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39:971-9.
- Yamasaki O, Kaneko J, Morizane S, et al. The association between Staphylococcus aureus strains carrying panton-valentine leukocidin genes and the development of deep-seated follicular infection. Clin Infect Dis. 2005;40:381-5.
- Hsu LY, Koh TH, Kurup A, Low J, Chlebicki MP, Tan BH. High incidence of Panton-Valentine leukocidin-producing Staphylococcus aureus in a tertiary care public hospital in Singapore. Clin Infect Dis. 2005;40:486-9.
- Chambers HF. Community-associated MRSA–resistance and virulence converge. N Engl J Med. 2005;352:1485-7.
- Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100-7.
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445-53.
- Kirby WMM. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944;99:452-3.
- Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178-82.
- Kreiswirth B, Kornblum J, Arbeit RD, et al. Evidence for a clonal origin of methicillin resistance in Staphylococcus aureus. Science. 1993;259:227-30.
- Donnio PY, Preney L, Gautier-Lerestif AL, Avril JL, Lafforgue N. Changes in staphylococcal chromosome type and antibiotic resistance profile in methicillin-resistant Staphylococcus aureus isolates from a French hospital over an 11 year period. J Antimicrob Chemother. 2004;53:808-13.
- Eliopoulos GM. Quinupristin-dalfopristin and linezolid: evidence and opinion. Clin Infect Dis. 2003;36: 473-81.
- Carpenter CF, Chambers HF. Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin Infect Dis. 2004;38: 994-1000.
A 65-year-old male with no significant past medical history, recently returned from a trip to the Democratic Republic of the Congo, presented with pain, swelling, and ulceration of his right lower leg. The symptoms had progressed despite oral amoxicillin/clavulanate. Evaluation at the time of admission revealed a large fluid collection in the anterior calf with extensive subcutaneous edema. Blood cultures were positive for methicillin–resistant S. aureus susceptible to clindamycin, erythromycin, tetracycline, trimethoprim-sulfamethoxazole, gentamicin, and tetracycline. His infection was successfully treated with surgical debridement, wound care, and vancomycin.
In 1941, Skinner and colleagues described the seriousness of S. aureus bloodstream infections in their series of 122 consecutive patients. The mortality rate was greater than 80% (1). Despite early success with penicillin the subsequent decades have shown this organism to be capable of elaborating resistance mechanisms that make therapy increasingly difficult (2). Methicillin resistance, which first appeared in the 1960s, has come to characterize many of the S. aureus isolates that are identified in the hospital. Recently, distinct strains of methicillin-resistant S. aureus (MRSA) are more commonly being identified in patients presenting for care from the community. This review will discuss recent developments in the clinical presentation and epidemiology of community-acquired MRSA in adults.
Definitions and Epidemiology
For infection control and epidemiological purposes, infections have been traditionally termed nosocomial if they 1) were not incubating at the time of presentation, 2) developed more than 72 hours after hospital admission, or 3) occurred in patients who were recently discharged from the hospital or who reside in a long-term care or skilled nursing facility. Beyond epidemiology, these definitions have been useful in helping the practicing clinician to employ effective empirical antibiotic therapy. The delivery of health care has evolved, however, and the distinction between outpatients and inpatients has been blurred. A broader term that has been suggested for infectious maladies in experienced patients who have moved in and out of the hospital is “healthcare associated” infections (3).
The evolving understanding of the origin of an infection has influenced efforts to define community MRSA. The term “community onset” or “community associated” MRSA can be used to describe a methicillin-resistant S. aureus infection that began incubating outside the hospital. If a patient has historical ties to a traditional treatment setting, the infection is most likely healthcare associated. Notable risk factors include hospitalization or stay in a nursing facility within the past year, use of broad-spectrum antibiotics, surgery, dialysis, intravenous drug use, or the presence of an indwelling vascular catheter. A MRSA infection in a patient presenting from home without any healthcare risk factors can be deemed “community acquired” MRSA (CaMRSA) (4).
A further understanding of CaMRSA can be gleaned from molecular studies of the organism. Methicillin resistance is mediated by a genetic element called staphylococcal cassette chromosome mecA (SCCmecA). MecA codes for a novel penicillin binding protein, PBP 2a, which is not inhibited by beta-lactam antibiotics (2). There are at least 5 types of SCCmecA. Types I through III are typically present in nosocomial MRSA strains. CaMRSA is distinguishable by the presence of SCCmecA IV (4-6).
Another distinctive feature of CaMRSA is the presence of the Panton-Valentine leukocidin (PVL). Previous work has shown that only 2–3% of strains of S. aureus produce this toxin (7). However this virulence factor, encoded by the genes lukS-PV and lukF-PV, appears to be expressed much more commonly in CaMRSA.
The difficulties with defining CaMRSA have influenced attempts to understand its prevalence. The key question in reviewing the available studies is how rigorous an attempt was made to exclude those patients who had significant healthcare contact. Salgado and colleagues performed a meta-analysis to try to determine the prevalence of true CaMRSA. They found that a significant number of subjects included in prevalence studies had identifiable healthcare risk factors, and that when this was accounted for, the overall prevalence of CaMRSA was less than 0.24% (8). The burden of CaMRSA infection will vary however based on location, and certain areas of the United States have demonstrated a higher prevalence. Researchers from the Emerging Infections Program Network examined CaMRSA in Atlanta, Baltimore, and Minnesota and found the prevalence to range from 8% to 20% (9). Of note, only 41% of suspected cases of CaMRSA were confirmed through interviews.
So what is CaMRSA? An acceptable working definition is a methicillin-resistant S. aureus infection occurring in a patient without a history of healthcare risk factors due to an isolate carrying SCCmecA type IV. The isolate is also likely to express the PVL virulence factor. This definition combines what is known about both the clinical and molecular epidemiology of these strains. Further research and time is likely to result in modifications to our understanding of this emerging phenomenon.
Antibiotic Susceptibility Patterns
CaMRSA strains have unique susceptibility patterns compared with traditional MRSA strains. As noted above, SCCmecA codes for methicillin resistance in S. aureus. SCCmecA types II and III are large genetic elements that usually code for resistance to multiple antibiotics. In contrast, type IV is smaller and results in decreased susceptibility to betalactams alone. CaMRSA strains are identifiable as being susceptible to clindamycin, trimethoprim-sulfamethoxazole, and the aminoglycosides (4). Susceptibility to clindamycin must be interpreted cautiously in strains that are erythromycin resistant. If erythromycin resistance is due to an inactivating enzyme (a ribosomal methylase) resistance to clindamycin can be induced. This macrolide-lincosamide-streptogramin–inducible phenotype can be identified in the microbiology lab by performing an erythromycin induction test (D-test). Clinical failures have been described when clindamycin has been used in the presence of this inducible phenotype (10).
Outbreaks
As with many infectious diseases, outbreaks first brought the problem of CaMRSA to wider attention. The first well-described outbreak occurred in the early 1980s among intravenous drug users in Detroit (11). Reports in the early 1990s focused on MRSA infections in young children without risk factors for resistant infection (12). Overwhelming, fatal sepsis due to MRSA was described in 4 pediatric patients in Minnesota and North Dakota. A fulminant, necrotizing pneumonia characterized 3 of the cases (13). Subsequently numerous outbreaks have been described among prison inmates, sexual partners, and competitive sports participants (14-16).
Two well-documented outbreaks have been described in football players. Begier and colleagues identified an outbreak that involved 10 players on the same college football team. Molecular typing demonstrated all recovered isolates to be of the same strain and to carry SCCmecA and the PVL gene. The case-control analysis showed an association between infection and playing wide receiver or cornerback, turf burns, and body shaving (17). An investigation of 8 MRSA infections among professional football players similarly showed all recovered strains to be clonal and to harbor SCCmecA IV and the PVL locus. In contrast to the college outbreak, these investigators found an association between being a lineman or a linebacker and disease. Turf burns were again a significant risk factor (18).
Both of these outbreaks, although geographically separate, were found to be due to the same strain of MRSA, clone USA300-0114. This clone has also been demonstrated as the predominant cause of CaMRSA in other communities (15,19). This would seem to indicate greater fitness of this particular strain that has allowed it to spread widely (20).
Clinical Manifestations
In general, CaMRSA has been reported to cause a similar spectrum of disease as methicillin-susceptible S. aureus (MSSA). As mentioned above, it appears to be seen mostly in otherwise healthy, young individuals. In the population based surveillance project of Fridkin et al., 77% of patients with community MRSA had skin and soft tissue infections (9). Invasive disease was observed in 6%. Similarly, Naimi and colleagues found skin and soft tissue infections in 75% of the subjects in their study of community-associated MRSA in Minnesota (21).
There is concern that CaMRSA may be associated with a greater likelihood of disease compared with other S. aureus strains. Ellis et al. prospectively evaluated active-duty soldiers found to be colonized with CaMRSA. Of the 24 colonized, 38% or 9 individuals developed soft-tissue infections as compared with 3% of those colonized with MSSA. Eight of nine affected patients had abscesses. All 9 of the available clinical isolates were positive for the PVL gene and the presence of this virulence factor was associated with an increased risk of invasive disease (22). Other authors have found an association between PVL-carrying strains of S. aureus and disease and it is perhaps this characteristic, not methicillin resistance, that assists the organism in causing disease in otherwise healthy individuals (23,24). The observed high prevalence of the PVL virulence factor among CaMRSA has been described as the “convergence of resistance and virulence” (25).
Severe disease has also been described due to strains of CaMRSA. Francis described 4 patients with necrotizing pneumonia due to CaMRSA similar to the pediatric cases referred to above. The isolates from all 4 patients carried PVL and SCCmecA IV genes and were of the USA300 strain group (26). Of note, all 4 patients initially had influenza-like illnesses, demonstrating again the association between influenza and staphylococcal pneumonia. This also signifies these presentations were potentially vaccine preventable. Recently, necrotizing fasciitis caused by CaMRSA strains, all again characterized as having PVL genes, has been described (27). This new phenomenon expands the differential diagnosis of causes of this life-threatening soft-tissue syndrome and influences empirical antibiotic selection.
A 41-year-old with Crohn’s disease treated with infliximab undergoes ventral hernia repair. She has a past surgical history of multiple abdominal surgeries. Three weeks postoperatively she is readmitted with a superinfected hematoma requiring operative drainage. Cultures reveal MRSA, susceptible to erythromycin, clindamycin, vancomycin, gentamicin, tetracycline, and trimethoprim-sulfamethoxazole.
The work to date on this new aspect of resistance in S. aureus intimates a trend similar to that previously experienced with penicillin resistance. Penicillinase-producing strains, first recognized in 1944, became increasingly common among hospital isolates after the second World War (28,29). By the 1970s, penicillin-resistant staphylococci had become widespread in the Community as well. Currently, identification of a penicillin-susceptible S. aureus isolate is uncommon.
The potential for further increases in the prevalence of methicillin resistance among staphylococci lies with the SCCmecA complex. Acquisition of this determinant from another resistant clone of either S. aureus or a coagulase-negative staphylococcus is the necessary first step in the process of becoming methicillin resistant. Types I through III are large, and this has been an obstacle to frequent transfers to MSSA strains. The result of this dynamic is that hospital-acquired MRSA to this point has descended from a relatively small number of clones as compared with the wide heterogeneity seen in susceptible S. aureus (30). As mentioned above, SCCmecA IV is smaller and can therefore more easily insert into many different MSSA strains without a loss of fitness. In fact type IV strains have been shown in vitro to replicate faster than hospital MRSA strains (20). This may allow MRSA to begin to displace MSSA as the predominant community phenotype in a manner similar to that in which penicillin-susceptible S. aureus was replaced.
A similar phenomenon may occur in hospitals wherein a typical CaMRSA strain may become the predominant hospital clone. This has been described already in 1 hospital where SCCmecA IV became the major determinant of methicillin resistance in the hospital (31). The trend was identifiable by a “more susceptible” antibiogram of MRSA strains. Future epidemiological surveillance will be necessary as the potential exists for resistant strains to continue to cross the increasingly more permeable barrier between traditional healthcare and the community.
Management
Increasing resistance to S. aureus has several implications for clinicians. Fundamental principles in the management of infectious syndromes become even more important, particularly source control of suppurative foci through debridement and drainage. An added benefit of such procedures is that they facilitate the establishment of a microbiological diagnosis. Clinicians and microbiologists will need to continue to work together closely so as to be aware of resistance trends in their community. In situations where the pathogen is not identified and treatment is prescribed empirically, follow-up is crucial.
Obviously, the emergence of CaMRSA has limited antibiotic choices. Clindamycin, trimethoprim-sulfamethoxazole, and doxycycline remain therapeutic options in the appropriate clinical situation. The severe clinical manifestations described above require consideration of empirical vancomycin in the treatment of patients presenting seriously ill with infectious syndromes that could be potentially due to S. aureus while awaiting culture results. The most extensive experience for inpatient use is with this agent. Linezolid, daptomycin, and quinupristin-dalfopristin are newer agents with activity against MRSA that have been reviewed elsewhere (32,33). Growing experience with these agents has provided options in situations where vancomycin cannot be used. It has also emphasized some of their limitations. Daptomycin and quinupristin-dalfopristin are only given parenterally, while linezolid can be given both orally and intravenously. Expense impacts the use of all three especially outside the hospital. Treatment-limiting cytopenias and peripheral and optic neuropathy have been described with linezolid when it is has been employed for extended courses of therapy. Daptomycin is inhibited by surfactant and therefore should not be used for suspected pulmonary infections. Quinupristin-dalfopristin’s use can be limited by disabling myalgias and the need for central venous access. More data about the use of these newer agents for invasive infections are needed before they can be considered superior to vancomycin.
Dr. Fraser may be reached at frasert@ccf.org.
Dr. Fraser is a member of the Wyeth Emerging Pathogens speakers’ bureau and has participated in a local advisory panel for GlaxoSmithKline. There is no conflict of interest to disclose for this work.
References
- Skinner D, Keefer CS. Significance of bacteremia caused by Staphylococcus aureus. Arch Intern Med. 1941;68:851-75.
- Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111:1265-73.
- Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791-7.
- Said-Salim B, Mathema B, Kreiswirth BN. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging pathogen. Infect Control Hosp Epidemiol. 2003;24:451-5.
- Carleton HA, Diep BA, Charlebois ED, Sensabaugh GF, Perdreau-Remington F. Community-adapted methicillin-resistant Staphylococcus aureus (MRSA): population dynamics of an expanding community reservoir of MRSA. J Infect Dis. 2004;190:1730-8.
- Daum RS, Ito T, Hiramatsu K, et al. A novel methicillin-resistance cassette in community-acquired methicillin resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis. 2002:186;1344-7.
- Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16-34.
- Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a metaanalysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131-9.
- Fridkin SK, Hageman JC, Morrison M, et al. Methicillinresistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-44.
- Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis. 2003;37:1257-60.
- Saravolatz LD, Markowitz N, Arking L, Pohlod D, Fisher E. Methicillin-resistant Staphylococcus aureus. Epidemiologic observations during a community-acquired outbreak. Ann Intern Med. 1982;96:11-6.
- Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593-598.
- Centers for Disease Control and Prevention. Four pediatric deaths from community acquired methicillin resistant Staphylococcus aureus—Minnesota and North Dakota, 1997-1999. MMWR Morb Mortal Wkly Rep. 1999;48:707-10.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus infections in correctional facilities—Georgia, California, and Texas, 2001-2003. MMWR Morb Mortal Wkly Rep. 2003;52:992-6.
- Centers for Disease Control and Prevention. Public Health Dispatch: outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002-2003. MMWR Morb Mortal Wkly Rep. 2003;52:88.
- Lindenmayer JD, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Int Med. 1998;158:895-9.
- Begier EM, Frenette K, Barrett NL, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39:1446-53.
- Kazakova SV, Hagerman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352: 468-75.
- McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113-20.
- Deresinski S. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic, and therapeutic odyssey. Clin Infect Dis. 2005;40:562-73.
- Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976-84.
- Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39:971-9.
- Yamasaki O, Kaneko J, Morizane S, et al. The association between Staphylococcus aureus strains carrying panton-valentine leukocidin genes and the development of deep-seated follicular infection. Clin Infect Dis. 2005;40:381-5.
- Hsu LY, Koh TH, Kurup A, Low J, Chlebicki MP, Tan BH. High incidence of Panton-Valentine leukocidin-producing Staphylococcus aureus in a tertiary care public hospital in Singapore. Clin Infect Dis. 2005;40:486-9.
- Chambers HF. Community-associated MRSA–resistance and virulence converge. N Engl J Med. 2005;352:1485-7.
- Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100-7.
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445-53.
- Kirby WMM. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944;99:452-3.
- Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178-82.
- Kreiswirth B, Kornblum J, Arbeit RD, et al. Evidence for a clonal origin of methicillin resistance in Staphylococcus aureus. Science. 1993;259:227-30.
- Donnio PY, Preney L, Gautier-Lerestif AL, Avril JL, Lafforgue N. Changes in staphylococcal chromosome type and antibiotic resistance profile in methicillin-resistant Staphylococcus aureus isolates from a French hospital over an 11 year period. J Antimicrob Chemother. 2004;53:808-13.
- Eliopoulos GM. Quinupristin-dalfopristin and linezolid: evidence and opinion. Clin Infect Dis. 2003;36: 473-81.
- Carpenter CF, Chambers HF. Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin Infect Dis. 2004;38: 994-1000.
The Top 10 Things ID Specialists Wish Every Hospitalist Knew
In my experience, hospitalists usually have a greater knowledge of antibiotics and treatment of infections than other non-infectious disease (ID) practitioners who manage hospital patients. But that doesn’t stop ID physicians from wanting to make suggestions. The following list is not meant to be all-inclusive, but it does reflect an informal poll of my colleagues at a tertiary care medical center. Any opinions are of course my own, and naturally are evidenced based. There is an old joke that if you ask two ID doctors a question you get three answers. Having said that, I believe that there is a good consensus on these issues.
1. Beta-lactam/beta-lactamase inhibitors have excellent anaerobic coverage.
Beta-lactam/beta-lactamase inhibitors such as ampicillin/sulbactam and piperacillin/tazobactam have excellent anaerobic coverage. When treating suspected or proven anaerobic infections with these drugs, addition of other agents such as metronidazole and clindamycin to cover anaerobic infections is not necessary (1). Quite often we see patients treated with ampicillin/sulbactam and metronidazole or piperacillin/tazobactam and metronidazole, which is not necessary and potentially exposes the patient to additional drug toxicities. “Unasyn and Flagyl” for suspected intra-abdominal infections provides unnecessary double coverage for anaerobes, while providing suboptimal coverage for gram-negative rods due to increasing resistance to ampicillin/sulbactam among gram-negative aerobes.
2. Staphylococcus aureus bacteremia “always” gets at least 2 weeks of IV antibiotics.
Clinicians managing patients who have blood cultures positive for Staphylococcus aureus should always think about whether the patient has a deep-seated source such as cardiac or bone, and treat accordingly. But even patients with a self-limited bacteremia related to an intravenous catheter or other easily removable source of infection should get at least 2 weeks of antibiotics (2). One of the goals of treating S. aureus bacteremia is to prevent metastatic infection. Patients with line-related infections may have rapid clinical improvement and resolve the bacteremia quickly, but they are at high risk for relapse with bone, joint, or cardiac infection if the initial antimicrobial course is inadequate. At least once every year or 2 at our teaching hospitals we see a patient who is given a very short course of antibiotics for S. aureus bacteremia related to an intravenous catheter who returns a month or two later with relapse in the spine or in other equally serious sites of infection. It is believed that more aggressive initial antimicrobial therapy can prevent metastatic infections. A frequently employed strategy in evaluating patients with S. aureus bacteremia is to complete a transesophageal echocardiogram to rule out cardiac involvement. If there is no cardiac involvement and other deep seated source such as bone or joint are not suspected, 2 weeks of intravenous antibiotics are generally adequate. In the setting of cardiac involvement or deep-seated involvement in bone or joints, 4–6 weeks of antibiotics are required. Patients with staphylococcal bacteremia who do not have a known source of infection should almost always be treated for 4–6 weeks. And, of course, there is the usual caveat: Oral antibiotics with excellent oral bioavailability such as linezolid can be used as switch therapy to complete a 2-week course in some cases. But the default approach would be to treat all S. aureus bacteremias with at least 2 weeks of intravenous antibiotics.
3. Staphylococcus aureus in the urine should almost always prompt a search for another site of infection.
Patients who have Foley catheters or are status post-genitourinary procedures may develop primary S. aureus urinary tract infection, but it is unusual for patients without history of genitourinary manipulation to present with S. aureus as a cause of urinary tract infection. In patients with no predisposing factors for S. aureus urinary infection, isolation of the organism in the urine should always prompt an evaluation for another site of infection such as bone, joint, or endovascular. Patients with known or suspected S. aureus urinary tract infection should have blood cultures drawn prior to the initiation of antibiotics to detect occult bacteremias. It is not unusual on our Infectious Disease Consult Service to see a patient who is suspected of having a S. aureus UTI that is later shown after consultation and investigation to have S. aureus endocarditis without other obvious manifestations, or another deep-seated infection such as spinal osteomyelitis or epidural abscess.
4. Stool assays for Clostridium difficile lack a high degree of sensitivity.
North America is experiencing an explosion in illness related to Clostridium difficile (primarily C. difficile associated diarrhea, or CDAD) (3). C. difficile is most often a nosocomial infection, and CDAD has become a very common disease in patients hospitalized for any length of time who are given broad-spectrum antibiotics. Hospitalization is the “perfect storm” for CDAD. C. difficile spores exist in the hospital environment and are ingested by patients on broad-spectrum antibiotics that inhibit the normal flora, creating the perfect environment for C. difficile to flourish. The assays to detect C. difficile toxins in the stool are often not highly sensitive; under the best of circumstances the techniques used by most hospital labs will produce a false-negative result 10–20% of the time (4). Not all labs detect all toxin types, and not all kits are highly efficient for detecting toxins. Patients in whom there is a strong suspicion for CDAD should be treated even in the face of negative toxin assays unless there is another likely source of diarrhea.
5. Hospitalized patients who develop diarrhea after admission almost never have enteric infections other than CDAD.
Patients who develop diarrhea after being in the hospital 1 or 2 days almost never have infection with Salmonella, Campylobacter, Entamoeba histolytica, or Giardia sp. It is not uncommon to see hospitalized patients who develop diarrhea after several days in the hospital with shotgun orders for “SSYC and O&P.” Unless there is a history of immunosupression or risk factors for enteric infection such as international travel, these tests are almost always unnecessary (5). 6. The most common cause of leukocytosis of unknown etiology in hospitalized patients is CDAD. There is something about the pathogenesis of CDAD that produces a leukemoid reaction much more often than other infections do. It is not unusual to see white blood cell counts of 30,000 with CDAD, and counts of 50,000 and higher in patients with CDAD are not rare. Patients who develop leukocytosis in the hospital while on antibiotics, or who present from long-term care facilities with marked leukocytosis and recent antibiotic exposure, have a high pretest probability of having CDAD (6). In this setting, the higher the white count, the more likely the patient has CDAD.
6. Blood cultures should always be obtained before parenteral antibiotics are given for a febrile illness.
Patients who are given broad-spectrum antibiotics have 1 opportunity to have interpretable blood cultures obtained: before antibiotics are administered. Once patients are given broad-spectrum antibiotics, blood cultures have a very limited value in diagnosing infections that might not be initially suspected on admission. A common example in our hospital is a patient presenting with pneumonia. About a third of the patients who come through the emergency room with a diagnosis of community-acquired pneumonia end up having another diagnosis. Often the alternative diagnosis is suspected based on blood cultures obtained prior to the patient receiving broad-spectrum antibiotics in the emergency room. In the last 3 months we have seen patients with liver abscesses, endocarditis, and osteomyelitis initially felt to have community-acquired pneumonia whose blood cultures initiated prior to antibiotic therapy revealed a pathogen that caused a search for an alternative source of infection. The vast majority of patients only need 2 blood cultures from 2 sites 20 minutes apart before initiation of antibiotic therapy. Patients in whom common skin contaminants may easily be interpreted as pathogens (such as patients with prosthetic heart valves) should have 3 sets of blood cultures to aid in the interpretation of cultures that are positive for skin contaminants such as coagulase negative staph.
7. In diabetics without foot ulcers, cellulitis is most often due to Streptococcus and occasionally to Staphylococcus species.
Diabetic patients who have infections related to foot ulcers or ischemic lesions require broad-spectrum antimicrobial therapy active against anaerobes, gram-positives, and gram-negatives. However, diabetic patients who are not critically ill who are admitted with a clinical picture typical for cellulitis tend to be infected with the same pathogens as non-diabetic patients. We frequently encounter diabetic patients who present with a clinical picture of an uncomplicated cellulitis without ulcers or other lower-extremity lesions and are treated with broader-spectrum antimicrobial therapy than is needed for cellulitis. Broader therapy is often more expensive, and it puts patients at risk for more adverse effects such as CDAD. The great majority of patients with cellulitis have infection with group A strep and other streptococci, and less often S. aureus. Cellulitis due to anaerobes and gram-negative organisms in the absence of foot ulcers or similar lesions is distinctly unusual.
Another “pearl” about cellulitis: Group A strep cellulitis is often initially slow to respond to therapy. The local findings may take 3 or 4 days to show improvement and there actually may be slight worsening despite 1 or 2 days of appropriate antibiotics. This is believed to be related to toxins produced by group A strep and other local tissue factors. Even if an antimicrobial is successful in eradicating strep, there are still toxins in the tissues that produce aggressive local findings. We often get consulted about patients with cellulitis who after 2 days of antimicrobial therapy may have some improvement in their fever curve and white blood cell count but have worsening of the local findings. These patients almost never need a change in antimicrobial therapy, but need more time—and elevation. I was taught by one of my mentors of the importance of elevating an extremity when treating cellulitis. My clinical experience has borne out this wisdom. In addition, patients with lower-extremity edema or venous insufficiency or venous stasis who present with cellulitis must have edema and stasis aggressively treated for the cellulitis to respond to antimicrobial therapy.
8. Quinolones are no longer highly reliable as empiric therapy against gram-negative infections.
Five years ago in Ohio, if a patient presented with pyelonephritis or a complicated UTI as a community-acquired infection, it was unusual for the causative pathogen to be quinolone resistant. Quinolones such as ciprofloxacin could be used as empiric therapy for serious gram-negative infections with a great deal of confidence that the causative agent would be sensitive. In the last 5 years we have seen a steady, progressive increase in resistance to quinolones in both community acquired and nosocomial infections (7,8). Approximately 5–10% of E. coli are now quinolone resistant, and in some hospitals more than half of Pseudomonas aeruginosa are now quinolone resistant. Seriously ill patients with infections that are likely due to gram-negative rods should not be treated empirically with quinolone monotherapy in most settings. Oral quinolones, due to their excellent oral bioavailability, continue to have in important role in treating gram-negative infections, but their use should be based on the results of a culture with antimicrobial susceptibility.
9. VRE in the stool does not need to be treated.
The great majority of patients who test positive for VRE in a stool specimen never acquire an infection with VRE. Patients who are colonized with VRE in the stool will clear colonization over several weeks or months if there is no antimicrobial pressure to select for VRE. Infectious disease clinicians spend a lot of time trying to allay the fear of patients and families who become extremely nervous due to isolation procedures for VRE. My usual approach is to tell the patients that the only reason they are in isolation is to prevent VRE from spreading to the very, very small group of patients who actually are susceptible to infection with VRE, such as liver transplant patients. I tell the family there is almost no chance that healthy family members will develop a VRE infection and that the VRE bacteria is normally found as a natural part of the human intestinal flora. VRE is simply 1 strain that has particular resistance to antibiotics, making it difficult to treat when infection occurs, but it is not more pathogenic. Infection with VRE is relatively rare and with the possible exception of cystitis (or bladder colonization) there is an extremely low risk of any actual infection despite VRE colonization. Uncomplicated cystitis due to VRE can usually be treated with nitrofurantoin.
10. Community-acquired MRSA is on the rise.
In the last 5 years in the United States, there has been a steady increase in MRSA infections in patients without traditional risk factors (9,10). Historically, clinicians have been concerned about MRSA in nursing home patients, patients in other long-term care facilities, injection drug users, and hospitalized patients. In the last 5 years there have been increasing numbers of patients with MRSA with none of these risk factors. Often these patients present with a serious life-threatening S. aureus infection. It is now appropriate to give vancomycin empirically for patients who have serious illnesses due to suspected S. aureus even if they don’t have traditional risk factors for MRSA. As ID practitioners, we do not want to encourage overuse of vancomycin, and clinicians should quickly switch to other agents if the patient proves not to be infected with MRSA. While vancomycin is a useful drug, it is considered inferior to the beta-lactams for many infections, such as bone or joint infections, and should only be used in patients with documented or suspected MRSA, or patients intolerant of beta-lactams. Several new drugs provide alternatives to vancomycin for MRSA, including linezolid and daptomycin. Both of these agents are more expensive and have not proven in clinical trials to be superior (with the possible exception of linezolid for MRSA pneumonia). Linezolid offers the advantage of having excellent oral availability; however, oral linezolid use is complicated by its high cost. Oral linezolid costs approximately $100 a day, and in almost all cases the use of this drug must be preapproved before an insurance company will pay for it. Insurance companies will almost always approve oral linezolid if the only alternative is continued hospitalization, skilled nursing home placement, or home IV antibiotic therapy. Trimethoprim/sulfa is a much less expensive alternative to oral linezolid for MRSA, and is very useful for less serious MRSA infections such as UTIs. About 85% of MRSA strains are sensitive to trimethoprim/sulfa. Many community-acquired MRSA strains are clindamycin susceptible, and minocycline and doxycycline have activity against many MRSA strains.
So these are 10 things ID physicians wish all hospitalists knew. The 11th is that we enjoy working with our hospitalist colleagues, so please call when you think you need us.
Dr. Armitage may be reached at kba@case.edu.
References
- Young M. Plosker GL. Piperacillin/tazobactam: a pharmacoeconomic review of its use in moderate to severe bacterial infections. Pharmacoeconomics. 2001;19:1135-75.
- Fowler VG Jr., Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clinical Infect Dis. 1998;27:478-86.
- Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004:171:466-72.
- Bartlett JG. Clinical practice. Antibiotic-associated diarrhea [see comment]. N Engl J Med. 2002;346:334-9.
- Chitkara YK, McCasland KA, Kenefic L. Development and implementation of cost-effective guidelines in the laboratory investigation of diarrhea in a community hospital. Arch Intern Med. 1996;156:1445-8.
- Bulusu M, Narayan S, Shetler K, Triadafilopoulos G. Leukocytosis as a harbinger and surrogate marker of Clostridium difficile infection in hospitalized patients with diarrhea. Am J Gastroenterol. 2000;95:3137-41.
- Neuhauser MM, Weinstein RA, Rydman R; Danziger LH, Karam G, Quinn JP. Antibiotic resistance among gramnegative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA. 2003;289:885-8.
- Cizman M. The use and resistance to antibiotics in the community. Int J Antimicrob Agents. 2003;21:297-307.
- Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J. Comparison of community- and health care–associated methicillin-resistant Staphylococcus aureus infection JAMA. 2003;290:2976-84.
- Chambers HF. Community-associated MRSA—resistance and virulence converge. N Engl J Med. 2005;352:1485-7.
In my experience, hospitalists usually have a greater knowledge of antibiotics and treatment of infections than other non-infectious disease (ID) practitioners who manage hospital patients. But that doesn’t stop ID physicians from wanting to make suggestions. The following list is not meant to be all-inclusive, but it does reflect an informal poll of my colleagues at a tertiary care medical center. Any opinions are of course my own, and naturally are evidenced based. There is an old joke that if you ask two ID doctors a question you get three answers. Having said that, I believe that there is a good consensus on these issues.
1. Beta-lactam/beta-lactamase inhibitors have excellent anaerobic coverage.
Beta-lactam/beta-lactamase inhibitors such as ampicillin/sulbactam and piperacillin/tazobactam have excellent anaerobic coverage. When treating suspected or proven anaerobic infections with these drugs, addition of other agents such as metronidazole and clindamycin to cover anaerobic infections is not necessary (1). Quite often we see patients treated with ampicillin/sulbactam and metronidazole or piperacillin/tazobactam and metronidazole, which is not necessary and potentially exposes the patient to additional drug toxicities. “Unasyn and Flagyl” for suspected intra-abdominal infections provides unnecessary double coverage for anaerobes, while providing suboptimal coverage for gram-negative rods due to increasing resistance to ampicillin/sulbactam among gram-negative aerobes.
2. Staphylococcus aureus bacteremia “always” gets at least 2 weeks of IV antibiotics.
Clinicians managing patients who have blood cultures positive for Staphylococcus aureus should always think about whether the patient has a deep-seated source such as cardiac or bone, and treat accordingly. But even patients with a self-limited bacteremia related to an intravenous catheter or other easily removable source of infection should get at least 2 weeks of antibiotics (2). One of the goals of treating S. aureus bacteremia is to prevent metastatic infection. Patients with line-related infections may have rapid clinical improvement and resolve the bacteremia quickly, but they are at high risk for relapse with bone, joint, or cardiac infection if the initial antimicrobial course is inadequate. At least once every year or 2 at our teaching hospitals we see a patient who is given a very short course of antibiotics for S. aureus bacteremia related to an intravenous catheter who returns a month or two later with relapse in the spine or in other equally serious sites of infection. It is believed that more aggressive initial antimicrobial therapy can prevent metastatic infections. A frequently employed strategy in evaluating patients with S. aureus bacteremia is to complete a transesophageal echocardiogram to rule out cardiac involvement. If there is no cardiac involvement and other deep seated source such as bone or joint are not suspected, 2 weeks of intravenous antibiotics are generally adequate. In the setting of cardiac involvement or deep-seated involvement in bone or joints, 4–6 weeks of antibiotics are required. Patients with staphylococcal bacteremia who do not have a known source of infection should almost always be treated for 4–6 weeks. And, of course, there is the usual caveat: Oral antibiotics with excellent oral bioavailability such as linezolid can be used as switch therapy to complete a 2-week course in some cases. But the default approach would be to treat all S. aureus bacteremias with at least 2 weeks of intravenous antibiotics.
3. Staphylococcus aureus in the urine should almost always prompt a search for another site of infection.
Patients who have Foley catheters or are status post-genitourinary procedures may develop primary S. aureus urinary tract infection, but it is unusual for patients without history of genitourinary manipulation to present with S. aureus as a cause of urinary tract infection. In patients with no predisposing factors for S. aureus urinary infection, isolation of the organism in the urine should always prompt an evaluation for another site of infection such as bone, joint, or endovascular. Patients with known or suspected S. aureus urinary tract infection should have blood cultures drawn prior to the initiation of antibiotics to detect occult bacteremias. It is not unusual on our Infectious Disease Consult Service to see a patient who is suspected of having a S. aureus UTI that is later shown after consultation and investigation to have S. aureus endocarditis without other obvious manifestations, or another deep-seated infection such as spinal osteomyelitis or epidural abscess.
4. Stool assays for Clostridium difficile lack a high degree of sensitivity.
North America is experiencing an explosion in illness related to Clostridium difficile (primarily C. difficile associated diarrhea, or CDAD) (3). C. difficile is most often a nosocomial infection, and CDAD has become a very common disease in patients hospitalized for any length of time who are given broad-spectrum antibiotics. Hospitalization is the “perfect storm” for CDAD. C. difficile spores exist in the hospital environment and are ingested by patients on broad-spectrum antibiotics that inhibit the normal flora, creating the perfect environment for C. difficile to flourish. The assays to detect C. difficile toxins in the stool are often not highly sensitive; under the best of circumstances the techniques used by most hospital labs will produce a false-negative result 10–20% of the time (4). Not all labs detect all toxin types, and not all kits are highly efficient for detecting toxins. Patients in whom there is a strong suspicion for CDAD should be treated even in the face of negative toxin assays unless there is another likely source of diarrhea.
5. Hospitalized patients who develop diarrhea after admission almost never have enteric infections other than CDAD.
Patients who develop diarrhea after being in the hospital 1 or 2 days almost never have infection with Salmonella, Campylobacter, Entamoeba histolytica, or Giardia sp. It is not uncommon to see hospitalized patients who develop diarrhea after several days in the hospital with shotgun orders for “SSYC and O&P.” Unless there is a history of immunosupression or risk factors for enteric infection such as international travel, these tests are almost always unnecessary (5). 6. The most common cause of leukocytosis of unknown etiology in hospitalized patients is CDAD. There is something about the pathogenesis of CDAD that produces a leukemoid reaction much more often than other infections do. It is not unusual to see white blood cell counts of 30,000 with CDAD, and counts of 50,000 and higher in patients with CDAD are not rare. Patients who develop leukocytosis in the hospital while on antibiotics, or who present from long-term care facilities with marked leukocytosis and recent antibiotic exposure, have a high pretest probability of having CDAD (6). In this setting, the higher the white count, the more likely the patient has CDAD.
6. Blood cultures should always be obtained before parenteral antibiotics are given for a febrile illness.
Patients who are given broad-spectrum antibiotics have 1 opportunity to have interpretable blood cultures obtained: before antibiotics are administered. Once patients are given broad-spectrum antibiotics, blood cultures have a very limited value in diagnosing infections that might not be initially suspected on admission. A common example in our hospital is a patient presenting with pneumonia. About a third of the patients who come through the emergency room with a diagnosis of community-acquired pneumonia end up having another diagnosis. Often the alternative diagnosis is suspected based on blood cultures obtained prior to the patient receiving broad-spectrum antibiotics in the emergency room. In the last 3 months we have seen patients with liver abscesses, endocarditis, and osteomyelitis initially felt to have community-acquired pneumonia whose blood cultures initiated prior to antibiotic therapy revealed a pathogen that caused a search for an alternative source of infection. The vast majority of patients only need 2 blood cultures from 2 sites 20 minutes apart before initiation of antibiotic therapy. Patients in whom common skin contaminants may easily be interpreted as pathogens (such as patients with prosthetic heart valves) should have 3 sets of blood cultures to aid in the interpretation of cultures that are positive for skin contaminants such as coagulase negative staph.
7. In diabetics without foot ulcers, cellulitis is most often due to Streptococcus and occasionally to Staphylococcus species.
Diabetic patients who have infections related to foot ulcers or ischemic lesions require broad-spectrum antimicrobial therapy active against anaerobes, gram-positives, and gram-negatives. However, diabetic patients who are not critically ill who are admitted with a clinical picture typical for cellulitis tend to be infected with the same pathogens as non-diabetic patients. We frequently encounter diabetic patients who present with a clinical picture of an uncomplicated cellulitis without ulcers or other lower-extremity lesions and are treated with broader-spectrum antimicrobial therapy than is needed for cellulitis. Broader therapy is often more expensive, and it puts patients at risk for more adverse effects such as CDAD. The great majority of patients with cellulitis have infection with group A strep and other streptococci, and less often S. aureus. Cellulitis due to anaerobes and gram-negative organisms in the absence of foot ulcers or similar lesions is distinctly unusual.
Another “pearl” about cellulitis: Group A strep cellulitis is often initially slow to respond to therapy. The local findings may take 3 or 4 days to show improvement and there actually may be slight worsening despite 1 or 2 days of appropriate antibiotics. This is believed to be related to toxins produced by group A strep and other local tissue factors. Even if an antimicrobial is successful in eradicating strep, there are still toxins in the tissues that produce aggressive local findings. We often get consulted about patients with cellulitis who after 2 days of antimicrobial therapy may have some improvement in their fever curve and white blood cell count but have worsening of the local findings. These patients almost never need a change in antimicrobial therapy, but need more time—and elevation. I was taught by one of my mentors of the importance of elevating an extremity when treating cellulitis. My clinical experience has borne out this wisdom. In addition, patients with lower-extremity edema or venous insufficiency or venous stasis who present with cellulitis must have edema and stasis aggressively treated for the cellulitis to respond to antimicrobial therapy.
8. Quinolones are no longer highly reliable as empiric therapy against gram-negative infections.
Five years ago in Ohio, if a patient presented with pyelonephritis or a complicated UTI as a community-acquired infection, it was unusual for the causative pathogen to be quinolone resistant. Quinolones such as ciprofloxacin could be used as empiric therapy for serious gram-negative infections with a great deal of confidence that the causative agent would be sensitive. In the last 5 years we have seen a steady, progressive increase in resistance to quinolones in both community acquired and nosocomial infections (7,8). Approximately 5–10% of E. coli are now quinolone resistant, and in some hospitals more than half of Pseudomonas aeruginosa are now quinolone resistant. Seriously ill patients with infections that are likely due to gram-negative rods should not be treated empirically with quinolone monotherapy in most settings. Oral quinolones, due to their excellent oral bioavailability, continue to have in important role in treating gram-negative infections, but their use should be based on the results of a culture with antimicrobial susceptibility.
9. VRE in the stool does not need to be treated.
The great majority of patients who test positive for VRE in a stool specimen never acquire an infection with VRE. Patients who are colonized with VRE in the stool will clear colonization over several weeks or months if there is no antimicrobial pressure to select for VRE. Infectious disease clinicians spend a lot of time trying to allay the fear of patients and families who become extremely nervous due to isolation procedures for VRE. My usual approach is to tell the patients that the only reason they are in isolation is to prevent VRE from spreading to the very, very small group of patients who actually are susceptible to infection with VRE, such as liver transplant patients. I tell the family there is almost no chance that healthy family members will develop a VRE infection and that the VRE bacteria is normally found as a natural part of the human intestinal flora. VRE is simply 1 strain that has particular resistance to antibiotics, making it difficult to treat when infection occurs, but it is not more pathogenic. Infection with VRE is relatively rare and with the possible exception of cystitis (or bladder colonization) there is an extremely low risk of any actual infection despite VRE colonization. Uncomplicated cystitis due to VRE can usually be treated with nitrofurantoin.
10. Community-acquired MRSA is on the rise.
In the last 5 years in the United States, there has been a steady increase in MRSA infections in patients without traditional risk factors (9,10). Historically, clinicians have been concerned about MRSA in nursing home patients, patients in other long-term care facilities, injection drug users, and hospitalized patients. In the last 5 years there have been increasing numbers of patients with MRSA with none of these risk factors. Often these patients present with a serious life-threatening S. aureus infection. It is now appropriate to give vancomycin empirically for patients who have serious illnesses due to suspected S. aureus even if they don’t have traditional risk factors for MRSA. As ID practitioners, we do not want to encourage overuse of vancomycin, and clinicians should quickly switch to other agents if the patient proves not to be infected with MRSA. While vancomycin is a useful drug, it is considered inferior to the beta-lactams for many infections, such as bone or joint infections, and should only be used in patients with documented or suspected MRSA, or patients intolerant of beta-lactams. Several new drugs provide alternatives to vancomycin for MRSA, including linezolid and daptomycin. Both of these agents are more expensive and have not proven in clinical trials to be superior (with the possible exception of linezolid for MRSA pneumonia). Linezolid offers the advantage of having excellent oral availability; however, oral linezolid use is complicated by its high cost. Oral linezolid costs approximately $100 a day, and in almost all cases the use of this drug must be preapproved before an insurance company will pay for it. Insurance companies will almost always approve oral linezolid if the only alternative is continued hospitalization, skilled nursing home placement, or home IV antibiotic therapy. Trimethoprim/sulfa is a much less expensive alternative to oral linezolid for MRSA, and is very useful for less serious MRSA infections such as UTIs. About 85% of MRSA strains are sensitive to trimethoprim/sulfa. Many community-acquired MRSA strains are clindamycin susceptible, and minocycline and doxycycline have activity against many MRSA strains.
So these are 10 things ID physicians wish all hospitalists knew. The 11th is that we enjoy working with our hospitalist colleagues, so please call when you think you need us.
Dr. Armitage may be reached at kba@case.edu.
References
- Young M. Plosker GL. Piperacillin/tazobactam: a pharmacoeconomic review of its use in moderate to severe bacterial infections. Pharmacoeconomics. 2001;19:1135-75.
- Fowler VG Jr., Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clinical Infect Dis. 1998;27:478-86.
- Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004:171:466-72.
- Bartlett JG. Clinical practice. Antibiotic-associated diarrhea [see comment]. N Engl J Med. 2002;346:334-9.
- Chitkara YK, McCasland KA, Kenefic L. Development and implementation of cost-effective guidelines in the laboratory investigation of diarrhea in a community hospital. Arch Intern Med. 1996;156:1445-8.
- Bulusu M, Narayan S, Shetler K, Triadafilopoulos G. Leukocytosis as a harbinger and surrogate marker of Clostridium difficile infection in hospitalized patients with diarrhea. Am J Gastroenterol. 2000;95:3137-41.
- Neuhauser MM, Weinstein RA, Rydman R; Danziger LH, Karam G, Quinn JP. Antibiotic resistance among gramnegative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA. 2003;289:885-8.
- Cizman M. The use and resistance to antibiotics in the community. Int J Antimicrob Agents. 2003;21:297-307.
- Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J. Comparison of community- and health care–associated methicillin-resistant Staphylococcus aureus infection JAMA. 2003;290:2976-84.
- Chambers HF. Community-associated MRSA—resistance and virulence converge. N Engl J Med. 2005;352:1485-7.
In my experience, hospitalists usually have a greater knowledge of antibiotics and treatment of infections than other non-infectious disease (ID) practitioners who manage hospital patients. But that doesn’t stop ID physicians from wanting to make suggestions. The following list is not meant to be all-inclusive, but it does reflect an informal poll of my colleagues at a tertiary care medical center. Any opinions are of course my own, and naturally are evidenced based. There is an old joke that if you ask two ID doctors a question you get three answers. Having said that, I believe that there is a good consensus on these issues.
1. Beta-lactam/beta-lactamase inhibitors have excellent anaerobic coverage.
Beta-lactam/beta-lactamase inhibitors such as ampicillin/sulbactam and piperacillin/tazobactam have excellent anaerobic coverage. When treating suspected or proven anaerobic infections with these drugs, addition of other agents such as metronidazole and clindamycin to cover anaerobic infections is not necessary (1). Quite often we see patients treated with ampicillin/sulbactam and metronidazole or piperacillin/tazobactam and metronidazole, which is not necessary and potentially exposes the patient to additional drug toxicities. “Unasyn and Flagyl” for suspected intra-abdominal infections provides unnecessary double coverage for anaerobes, while providing suboptimal coverage for gram-negative rods due to increasing resistance to ampicillin/sulbactam among gram-negative aerobes.
2. Staphylococcus aureus bacteremia “always” gets at least 2 weeks of IV antibiotics.
Clinicians managing patients who have blood cultures positive for Staphylococcus aureus should always think about whether the patient has a deep-seated source such as cardiac or bone, and treat accordingly. But even patients with a self-limited bacteremia related to an intravenous catheter or other easily removable source of infection should get at least 2 weeks of antibiotics (2). One of the goals of treating S. aureus bacteremia is to prevent metastatic infection. Patients with line-related infections may have rapid clinical improvement and resolve the bacteremia quickly, but they are at high risk for relapse with bone, joint, or cardiac infection if the initial antimicrobial course is inadequate. At least once every year or 2 at our teaching hospitals we see a patient who is given a very short course of antibiotics for S. aureus bacteremia related to an intravenous catheter who returns a month or two later with relapse in the spine or in other equally serious sites of infection. It is believed that more aggressive initial antimicrobial therapy can prevent metastatic infections. A frequently employed strategy in evaluating patients with S. aureus bacteremia is to complete a transesophageal echocardiogram to rule out cardiac involvement. If there is no cardiac involvement and other deep seated source such as bone or joint are not suspected, 2 weeks of intravenous antibiotics are generally adequate. In the setting of cardiac involvement or deep-seated involvement in bone or joints, 4–6 weeks of antibiotics are required. Patients with staphylococcal bacteremia who do not have a known source of infection should almost always be treated for 4–6 weeks. And, of course, there is the usual caveat: Oral antibiotics with excellent oral bioavailability such as linezolid can be used as switch therapy to complete a 2-week course in some cases. But the default approach would be to treat all S. aureus bacteremias with at least 2 weeks of intravenous antibiotics.
3. Staphylococcus aureus in the urine should almost always prompt a search for another site of infection.
Patients who have Foley catheters or are status post-genitourinary procedures may develop primary S. aureus urinary tract infection, but it is unusual for patients without history of genitourinary manipulation to present with S. aureus as a cause of urinary tract infection. In patients with no predisposing factors for S. aureus urinary infection, isolation of the organism in the urine should always prompt an evaluation for another site of infection such as bone, joint, or endovascular. Patients with known or suspected S. aureus urinary tract infection should have blood cultures drawn prior to the initiation of antibiotics to detect occult bacteremias. It is not unusual on our Infectious Disease Consult Service to see a patient who is suspected of having a S. aureus UTI that is later shown after consultation and investigation to have S. aureus endocarditis without other obvious manifestations, or another deep-seated infection such as spinal osteomyelitis or epidural abscess.
4. Stool assays for Clostridium difficile lack a high degree of sensitivity.
North America is experiencing an explosion in illness related to Clostridium difficile (primarily C. difficile associated diarrhea, or CDAD) (3). C. difficile is most often a nosocomial infection, and CDAD has become a very common disease in patients hospitalized for any length of time who are given broad-spectrum antibiotics. Hospitalization is the “perfect storm” for CDAD. C. difficile spores exist in the hospital environment and are ingested by patients on broad-spectrum antibiotics that inhibit the normal flora, creating the perfect environment for C. difficile to flourish. The assays to detect C. difficile toxins in the stool are often not highly sensitive; under the best of circumstances the techniques used by most hospital labs will produce a false-negative result 10–20% of the time (4). Not all labs detect all toxin types, and not all kits are highly efficient for detecting toxins. Patients in whom there is a strong suspicion for CDAD should be treated even in the face of negative toxin assays unless there is another likely source of diarrhea.
5. Hospitalized patients who develop diarrhea after admission almost never have enteric infections other than CDAD.
Patients who develop diarrhea after being in the hospital 1 or 2 days almost never have infection with Salmonella, Campylobacter, Entamoeba histolytica, or Giardia sp. It is not uncommon to see hospitalized patients who develop diarrhea after several days in the hospital with shotgun orders for “SSYC and O&P.” Unless there is a history of immunosupression or risk factors for enteric infection such as international travel, these tests are almost always unnecessary (5). 6. The most common cause of leukocytosis of unknown etiology in hospitalized patients is CDAD. There is something about the pathogenesis of CDAD that produces a leukemoid reaction much more often than other infections do. It is not unusual to see white blood cell counts of 30,000 with CDAD, and counts of 50,000 and higher in patients with CDAD are not rare. Patients who develop leukocytosis in the hospital while on antibiotics, or who present from long-term care facilities with marked leukocytosis and recent antibiotic exposure, have a high pretest probability of having CDAD (6). In this setting, the higher the white count, the more likely the patient has CDAD.
6. Blood cultures should always be obtained before parenteral antibiotics are given for a febrile illness.
Patients who are given broad-spectrum antibiotics have 1 opportunity to have interpretable blood cultures obtained: before antibiotics are administered. Once patients are given broad-spectrum antibiotics, blood cultures have a very limited value in diagnosing infections that might not be initially suspected on admission. A common example in our hospital is a patient presenting with pneumonia. About a third of the patients who come through the emergency room with a diagnosis of community-acquired pneumonia end up having another diagnosis. Often the alternative diagnosis is suspected based on blood cultures obtained prior to the patient receiving broad-spectrum antibiotics in the emergency room. In the last 3 months we have seen patients with liver abscesses, endocarditis, and osteomyelitis initially felt to have community-acquired pneumonia whose blood cultures initiated prior to antibiotic therapy revealed a pathogen that caused a search for an alternative source of infection. The vast majority of patients only need 2 blood cultures from 2 sites 20 minutes apart before initiation of antibiotic therapy. Patients in whom common skin contaminants may easily be interpreted as pathogens (such as patients with prosthetic heart valves) should have 3 sets of blood cultures to aid in the interpretation of cultures that are positive for skin contaminants such as coagulase negative staph.
7. In diabetics without foot ulcers, cellulitis is most often due to Streptococcus and occasionally to Staphylococcus species.
Diabetic patients who have infections related to foot ulcers or ischemic lesions require broad-spectrum antimicrobial therapy active against anaerobes, gram-positives, and gram-negatives. However, diabetic patients who are not critically ill who are admitted with a clinical picture typical for cellulitis tend to be infected with the same pathogens as non-diabetic patients. We frequently encounter diabetic patients who present with a clinical picture of an uncomplicated cellulitis without ulcers or other lower-extremity lesions and are treated with broader-spectrum antimicrobial therapy than is needed for cellulitis. Broader therapy is often more expensive, and it puts patients at risk for more adverse effects such as CDAD. The great majority of patients with cellulitis have infection with group A strep and other streptococci, and less often S. aureus. Cellulitis due to anaerobes and gram-negative organisms in the absence of foot ulcers or similar lesions is distinctly unusual.
Another “pearl” about cellulitis: Group A strep cellulitis is often initially slow to respond to therapy. The local findings may take 3 or 4 days to show improvement and there actually may be slight worsening despite 1 or 2 days of appropriate antibiotics. This is believed to be related to toxins produced by group A strep and other local tissue factors. Even if an antimicrobial is successful in eradicating strep, there are still toxins in the tissues that produce aggressive local findings. We often get consulted about patients with cellulitis who after 2 days of antimicrobial therapy may have some improvement in their fever curve and white blood cell count but have worsening of the local findings. These patients almost never need a change in antimicrobial therapy, but need more time—and elevation. I was taught by one of my mentors of the importance of elevating an extremity when treating cellulitis. My clinical experience has borne out this wisdom. In addition, patients with lower-extremity edema or venous insufficiency or venous stasis who present with cellulitis must have edema and stasis aggressively treated for the cellulitis to respond to antimicrobial therapy.
8. Quinolones are no longer highly reliable as empiric therapy against gram-negative infections.
Five years ago in Ohio, if a patient presented with pyelonephritis or a complicated UTI as a community-acquired infection, it was unusual for the causative pathogen to be quinolone resistant. Quinolones such as ciprofloxacin could be used as empiric therapy for serious gram-negative infections with a great deal of confidence that the causative agent would be sensitive. In the last 5 years we have seen a steady, progressive increase in resistance to quinolones in both community acquired and nosocomial infections (7,8). Approximately 5–10% of E. coli are now quinolone resistant, and in some hospitals more than half of Pseudomonas aeruginosa are now quinolone resistant. Seriously ill patients with infections that are likely due to gram-negative rods should not be treated empirically with quinolone monotherapy in most settings. Oral quinolones, due to their excellent oral bioavailability, continue to have in important role in treating gram-negative infections, but their use should be based on the results of a culture with antimicrobial susceptibility.
9. VRE in the stool does not need to be treated.
The great majority of patients who test positive for VRE in a stool specimen never acquire an infection with VRE. Patients who are colonized with VRE in the stool will clear colonization over several weeks or months if there is no antimicrobial pressure to select for VRE. Infectious disease clinicians spend a lot of time trying to allay the fear of patients and families who become extremely nervous due to isolation procedures for VRE. My usual approach is to tell the patients that the only reason they are in isolation is to prevent VRE from spreading to the very, very small group of patients who actually are susceptible to infection with VRE, such as liver transplant patients. I tell the family there is almost no chance that healthy family members will develop a VRE infection and that the VRE bacteria is normally found as a natural part of the human intestinal flora. VRE is simply 1 strain that has particular resistance to antibiotics, making it difficult to treat when infection occurs, but it is not more pathogenic. Infection with VRE is relatively rare and with the possible exception of cystitis (or bladder colonization) there is an extremely low risk of any actual infection despite VRE colonization. Uncomplicated cystitis due to VRE can usually be treated with nitrofurantoin.
10. Community-acquired MRSA is on the rise.
In the last 5 years in the United States, there has been a steady increase in MRSA infections in patients without traditional risk factors (9,10). Historically, clinicians have been concerned about MRSA in nursing home patients, patients in other long-term care facilities, injection drug users, and hospitalized patients. In the last 5 years there have been increasing numbers of patients with MRSA with none of these risk factors. Often these patients present with a serious life-threatening S. aureus infection. It is now appropriate to give vancomycin empirically for patients who have serious illnesses due to suspected S. aureus even if they don’t have traditional risk factors for MRSA. As ID practitioners, we do not want to encourage overuse of vancomycin, and clinicians should quickly switch to other agents if the patient proves not to be infected with MRSA. While vancomycin is a useful drug, it is considered inferior to the beta-lactams for many infections, such as bone or joint infections, and should only be used in patients with documented or suspected MRSA, or patients intolerant of beta-lactams. Several new drugs provide alternatives to vancomycin for MRSA, including linezolid and daptomycin. Both of these agents are more expensive and have not proven in clinical trials to be superior (with the possible exception of linezolid for MRSA pneumonia). Linezolid offers the advantage of having excellent oral availability; however, oral linezolid use is complicated by its high cost. Oral linezolid costs approximately $100 a day, and in almost all cases the use of this drug must be preapproved before an insurance company will pay for it. Insurance companies will almost always approve oral linezolid if the only alternative is continued hospitalization, skilled nursing home placement, or home IV antibiotic therapy. Trimethoprim/sulfa is a much less expensive alternative to oral linezolid for MRSA, and is very useful for less serious MRSA infections such as UTIs. About 85% of MRSA strains are sensitive to trimethoprim/sulfa. Many community-acquired MRSA strains are clindamycin susceptible, and minocycline and doxycycline have activity against many MRSA strains.
So these are 10 things ID physicians wish all hospitalists knew. The 11th is that we enjoy working with our hospitalist colleagues, so please call when you think you need us.
Dr. Armitage may be reached at kba@case.edu.
References
- Young M. Plosker GL. Piperacillin/tazobactam: a pharmacoeconomic review of its use in moderate to severe bacterial infections. Pharmacoeconomics. 2001;19:1135-75.
- Fowler VG Jr., Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clinical Infect Dis. 1998;27:478-86.
- Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004:171:466-72.
- Bartlett JG. Clinical practice. Antibiotic-associated diarrhea [see comment]. N Engl J Med. 2002;346:334-9.
- Chitkara YK, McCasland KA, Kenefic L. Development and implementation of cost-effective guidelines in the laboratory investigation of diarrhea in a community hospital. Arch Intern Med. 1996;156:1445-8.
- Bulusu M, Narayan S, Shetler K, Triadafilopoulos G. Leukocytosis as a harbinger and surrogate marker of Clostridium difficile infection in hospitalized patients with diarrhea. Am J Gastroenterol. 2000;95:3137-41.
- Neuhauser MM, Weinstein RA, Rydman R; Danziger LH, Karam G, Quinn JP. Antibiotic resistance among gramnegative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA. 2003;289:885-8.
- Cizman M. The use and resistance to antibiotics in the community. Int J Antimicrob Agents. 2003;21:297-307.
- Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J. Comparison of community- and health care–associated methicillin-resistant Staphylococcus aureus infection JAMA. 2003;290:2976-84.
- Chambers HF. Community-associated MRSA—resistance and virulence converge. N Engl J Med. 2005;352:1485-7.
Editorial
Despite the widespread availability of potent anti-infective agents, infectious diseases remain formidable and common problems in hospitalized patients. In this issue of The Hospitalist, hospitalists and infectious disease specialists provide state-of-the-art reviews of common infectious disease syndromes encountered in hospital medicine. This should be required reading for hospitalists, given the severity of illness of many patients with these syndromes, and the rapidly evolving developments in optimal diagnosis and management in many of these areas.
Hospitalists and infectious disease practitioners share similar challenges and should be natural allies in improving patient care. To name a few, these challenges include the prompt recognition and management of new and emerging infectious diseases; the rising incidence of drug-resistant pathogens; the prevention, prompt recognition, and effective management of nosocomial and opportunistic infections in hospitalized patients, including outbreaks; the implementation of clinical practice guidelines for common infectious disease problems; and the vaccination of high-risk hospitalized patients.
New and emerging infectious diseases have been recognized with increasing frequency, and hospitalists are among those physicians most likely to encounter them. In the past two years alone, these have included SARS (1), monkeypox (2), tanapox (3), and American Boutonneuse fever (4). Drug-resistant pathogens have become increasingly common. Methicillin-resistant Staphylococcus aureus (MRSA) now accounts for more than 50% of Staphylococcus aureus isolates in many hospitals. Until recently, MRSA has afflicted mainly hospitalized patients or those with significant underlying comorbidities. Recent reports, however, have described MRSA in previously healthy patients admitted from the community (5,6) Even more ominously, isolates of Staphylococcus aureus with intermediate or high-level resistance to vancomycin have been reported (7,8).
Drug-resistant Streptococcus pneumoniae is common in many parts of the United States; many isolates are resistant to multiple common antibiotics, and fluoroquinolone resistance, though still uncommon, has been reported (9,10). Vancomycin-resistant enterococci have emerged over the past decade and now account for up to 15% of enterococcal isolates in many centers. Macrolide-resistance in Treponema pallidum was recently described (11). The emergence of drug-resistant pathogens has important implications for hospitalists, who are typically at the front line in choosing empiric or pathogen-specific antimicrobial therapy for hospitalized patients. Knowledge about local trends in antimicrobial resistance is essential for making informed antibiotic selections, and prevention of spread of these organisms within hospitals is crucial.
How have hospitalists partnered with infectious disease specialists in tackling these problems? Surprisingly, very little has been written. In our review of the literature we were able to identify only 3 studies addressing the role of hospitalists and infectious disease physicians in the management of infectious diseases (12-14). Reddy et al. compared the impact of a clinical practice guideline introduced at the University of California San Francisco Moffitt-Long Hospital in 1996 with that of a hospitalist-based reorganization of their medical service in the management of patients with community-acquired pneumonia (CAP) (12). Following implementation of the guideline, average cost per case and length of stay declined similarly among patients cared for by hospitalists versus those cared for by attending physicians on the traditional medical service. Mortality remained unchanged despite shorter length of stay, and readmission rates fell. However, hospitalists achieved statistically greater reductions in cost per case and length of stay for all other diagnoses compared with their traditional attending counterparts. This study therefore concluded that the implementation of a clinical practice guideline was the key driver in improving resource utilization in hospitalized patients with CAP, rather than physician model of care.
In a similar study, Rifkin et al. compared outcomes and resource utilization in hospitalized patients with CAP at Long Island Jewish Medical Center cared for by hospitalists (185 patients) versus primary care physicians (270 patients) (13). No local clinical practice guideline was in place, although appropriateness of therapy was evaluated based upon guidelines disseminated by the American Thoracic Society and the Infectious Disease Society of America at the time. Compared with hospitalists, primary care physicians obtained more subspecialty consultations and administered antibiotics in a more timely fashion. Nevertheless, hospitalist care was associated with shorter length of stay, lower cost per case, more rapid transition from parenteral to oral antibiotic therapy, and improved survival. Hospitalist patients were more likely to be discharged with an unstable vital sign, but 15- and 30-day readmission rates were similar to patients cared for by primary care physicians. This study implies that, absent an enforced clinical practice guideline, hospitalist care of patients with CAP is associated with decreased resource utilization and better outcome.
Finally, in a case-control study, Eron and Passos examined the performance of hospitalists versus infectious disease consultants in the care of hospitalized patients with CAP, cellulitis, or pyelonephritis (14). One hundred eleven patients cared for by infectious disease consultants were compared with 112 historical controls cared for by hospitalists. Patients receiving care from infectious disease specialists had higher patient satisfaction, shorter length of stay, no readmissions, and more rapid return to activities of daily living. The benefits of specialty care were attributable to more frequent use of early outpatient parenteral antibiotic therapy (OPAT) in patients with cellulitis and more rapid switching from parenteral to oral therapy in patients with pyelonephritis and CAP by infectious disease specialists compared with hospitalists. This study has several implications. First, selected inpatients may benefit from early infectious disease consultation. And second, hospitalists may be able to learn from their subspecialty colleagues about the safety and efficacy of early switch therapy and OPAT in selected patient populations.
These limited data suggest that patients with selected infectious diseases may benefit from care provided by hospitalists and infectious disease specialists and that such care is associated with better outcomes and decreased resource utilization. No studies have examined what we believe to be the true potential for improved patient care through the partnership of these 2 disciplines working in collaboration. In the absence of data, we offer several suggestions for areas of fruitful collaboration and further study. First, with the increasing incidence of drug-resistant pathogens and the increasing severity of illness of hospitalized patients with infectious diseases, hospitalists and infectious disease specialists can work together to ensure optimal use of empiric and pathogen-specific antimicrobial therapy and the development and implementation of evidence-based practice guidelines. Second, early infectious disease consultation should be strongly considered in critically ill patients, in those with suspected or confirmed drug-resistant pathogens, and in those with unusual or complicated infectious disease problems. Third, hospitalists, in collaboration with infectious disease specialists, can and should help to improve basic infection control practices, such as hand hygiene and the appropriate use of indwelling devices such as Foley catheters and vascular access devices, which predispose to nosocomial infection. Fourth, hospitalists can facilitate the appropriate immunization of at-risk hospitalized patients against influenza and S. pneumoniae. Finally, hospitalists can partner with their infectious disease colleagues to optimize the use of OPAT and early switch therapy in patients with selected infectious diseases so as to maximize outcome while at the same time reducing hospital length of stay and resource utilization.
In a demonstration project, the Center for Medicare and Medicaid Services has now linked quality measures to reimbursement in several areas of infectious diseases. These include timeliness of antibiotic administration in patients with CAP, blood culture collection prior to antibiotic therapy in patients with CAP, and screening and administering influenza and pneumococcal immunizations to at-risk patients. This trend of linking reimbursement to performance in infectious disease management and prevention is likely to continue.
There are therefore many reasons to encourage partnership and collaboration between these disciplines. These include better quality of care, improved resource utilization, and, hopefully, better reimbursement for hospitals. Through leadership, teamwork, and a multidisciplinary approach, hospitalists and infectious disease physicians should together drive change to realize these goals.
References
- Pieris JSM, Lai ST, Poon LLM, et al. Coronavirus as a cause of severe acute respiratory syndrome. Lancet. 2003;361:1319-25.
- Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the western hemisphere. N Engl J Med. 2004;350:342-50.
- Dhar AD, Werchniak AE, Li Y, et al. Tanapox infection in a college student. N Engl J Med. 2004;350;361-6.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38: 805-11.
- Fridkin SK., Hageman JC, Morrison M, et al. Methicillin resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-44.
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445-53.
- Cosgrove SE, Carroll KC, Perl TM. Staphylococcus aureus with reduced susceptibility to vancomycin. Clin Infect Dis. 2004;39:539-45.
- Whitener CJ, Park SY, Browne FA, et al. Vancomycin resistant Staphylococcus aureus in the absence of vancomycin exposure. Clin Infect Dis. 2004;38:1049-55.
- Whitney CG, Farley MM, Hadler J, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000;343: 1917-24.
- Davidson R, Cavalcanti R, Brunton JL, et al. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N Engl J Med. 2002;346:747-50.
- Lukehart SA, Godornes C, Molini BJ, et al. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004;351:154-8.
- Reddy JC, Katz PP, Goldman L, Wachter RM. A pneumonia practice guideline and a hospitalist-based reorganization lead to equivalent efficiency gains. Am J Manag Care. 2001;7:1142-8.
- Rifkin WD, Connor D, Silver A, Eichorn A. Comparison of processes and outcomes of pneumonia care between hospitalists and community-based primary care physicians. Mayo Clin Proc. 2002;77:1053-8.
- Eron LJ, Passos S: Early discharge of infected patients through appropriate antibiotic use. Arch Intern Med. 2001;161:61-5.
Despite the widespread availability of potent anti-infective agents, infectious diseases remain formidable and common problems in hospitalized patients. In this issue of The Hospitalist, hospitalists and infectious disease specialists provide state-of-the-art reviews of common infectious disease syndromes encountered in hospital medicine. This should be required reading for hospitalists, given the severity of illness of many patients with these syndromes, and the rapidly evolving developments in optimal diagnosis and management in many of these areas.
Hospitalists and infectious disease practitioners share similar challenges and should be natural allies in improving patient care. To name a few, these challenges include the prompt recognition and management of new and emerging infectious diseases; the rising incidence of drug-resistant pathogens; the prevention, prompt recognition, and effective management of nosocomial and opportunistic infections in hospitalized patients, including outbreaks; the implementation of clinical practice guidelines for common infectious disease problems; and the vaccination of high-risk hospitalized patients.
New and emerging infectious diseases have been recognized with increasing frequency, and hospitalists are among those physicians most likely to encounter them. In the past two years alone, these have included SARS (1), monkeypox (2), tanapox (3), and American Boutonneuse fever (4). Drug-resistant pathogens have become increasingly common. Methicillin-resistant Staphylococcus aureus (MRSA) now accounts for more than 50% of Staphylococcus aureus isolates in many hospitals. Until recently, MRSA has afflicted mainly hospitalized patients or those with significant underlying comorbidities. Recent reports, however, have described MRSA in previously healthy patients admitted from the community (5,6) Even more ominously, isolates of Staphylococcus aureus with intermediate or high-level resistance to vancomycin have been reported (7,8).
Drug-resistant Streptococcus pneumoniae is common in many parts of the United States; many isolates are resistant to multiple common antibiotics, and fluoroquinolone resistance, though still uncommon, has been reported (9,10). Vancomycin-resistant enterococci have emerged over the past decade and now account for up to 15% of enterococcal isolates in many centers. Macrolide-resistance in Treponema pallidum was recently described (11). The emergence of drug-resistant pathogens has important implications for hospitalists, who are typically at the front line in choosing empiric or pathogen-specific antimicrobial therapy for hospitalized patients. Knowledge about local trends in antimicrobial resistance is essential for making informed antibiotic selections, and prevention of spread of these organisms within hospitals is crucial.
How have hospitalists partnered with infectious disease specialists in tackling these problems? Surprisingly, very little has been written. In our review of the literature we were able to identify only 3 studies addressing the role of hospitalists and infectious disease physicians in the management of infectious diseases (12-14). Reddy et al. compared the impact of a clinical practice guideline introduced at the University of California San Francisco Moffitt-Long Hospital in 1996 with that of a hospitalist-based reorganization of their medical service in the management of patients with community-acquired pneumonia (CAP) (12). Following implementation of the guideline, average cost per case and length of stay declined similarly among patients cared for by hospitalists versus those cared for by attending physicians on the traditional medical service. Mortality remained unchanged despite shorter length of stay, and readmission rates fell. However, hospitalists achieved statistically greater reductions in cost per case and length of stay for all other diagnoses compared with their traditional attending counterparts. This study therefore concluded that the implementation of a clinical practice guideline was the key driver in improving resource utilization in hospitalized patients with CAP, rather than physician model of care.
In a similar study, Rifkin et al. compared outcomes and resource utilization in hospitalized patients with CAP at Long Island Jewish Medical Center cared for by hospitalists (185 patients) versus primary care physicians (270 patients) (13). No local clinical practice guideline was in place, although appropriateness of therapy was evaluated based upon guidelines disseminated by the American Thoracic Society and the Infectious Disease Society of America at the time. Compared with hospitalists, primary care physicians obtained more subspecialty consultations and administered antibiotics in a more timely fashion. Nevertheless, hospitalist care was associated with shorter length of stay, lower cost per case, more rapid transition from parenteral to oral antibiotic therapy, and improved survival. Hospitalist patients were more likely to be discharged with an unstable vital sign, but 15- and 30-day readmission rates were similar to patients cared for by primary care physicians. This study implies that, absent an enforced clinical practice guideline, hospitalist care of patients with CAP is associated with decreased resource utilization and better outcome.
Finally, in a case-control study, Eron and Passos examined the performance of hospitalists versus infectious disease consultants in the care of hospitalized patients with CAP, cellulitis, or pyelonephritis (14). One hundred eleven patients cared for by infectious disease consultants were compared with 112 historical controls cared for by hospitalists. Patients receiving care from infectious disease specialists had higher patient satisfaction, shorter length of stay, no readmissions, and more rapid return to activities of daily living. The benefits of specialty care were attributable to more frequent use of early outpatient parenteral antibiotic therapy (OPAT) in patients with cellulitis and more rapid switching from parenteral to oral therapy in patients with pyelonephritis and CAP by infectious disease specialists compared with hospitalists. This study has several implications. First, selected inpatients may benefit from early infectious disease consultation. And second, hospitalists may be able to learn from their subspecialty colleagues about the safety and efficacy of early switch therapy and OPAT in selected patient populations.
These limited data suggest that patients with selected infectious diseases may benefit from care provided by hospitalists and infectious disease specialists and that such care is associated with better outcomes and decreased resource utilization. No studies have examined what we believe to be the true potential for improved patient care through the partnership of these 2 disciplines working in collaboration. In the absence of data, we offer several suggestions for areas of fruitful collaboration and further study. First, with the increasing incidence of drug-resistant pathogens and the increasing severity of illness of hospitalized patients with infectious diseases, hospitalists and infectious disease specialists can work together to ensure optimal use of empiric and pathogen-specific antimicrobial therapy and the development and implementation of evidence-based practice guidelines. Second, early infectious disease consultation should be strongly considered in critically ill patients, in those with suspected or confirmed drug-resistant pathogens, and in those with unusual or complicated infectious disease problems. Third, hospitalists, in collaboration with infectious disease specialists, can and should help to improve basic infection control practices, such as hand hygiene and the appropriate use of indwelling devices such as Foley catheters and vascular access devices, which predispose to nosocomial infection. Fourth, hospitalists can facilitate the appropriate immunization of at-risk hospitalized patients against influenza and S. pneumoniae. Finally, hospitalists can partner with their infectious disease colleagues to optimize the use of OPAT and early switch therapy in patients with selected infectious diseases so as to maximize outcome while at the same time reducing hospital length of stay and resource utilization.
In a demonstration project, the Center for Medicare and Medicaid Services has now linked quality measures to reimbursement in several areas of infectious diseases. These include timeliness of antibiotic administration in patients with CAP, blood culture collection prior to antibiotic therapy in patients with CAP, and screening and administering influenza and pneumococcal immunizations to at-risk patients. This trend of linking reimbursement to performance in infectious disease management and prevention is likely to continue.
There are therefore many reasons to encourage partnership and collaboration between these disciplines. These include better quality of care, improved resource utilization, and, hopefully, better reimbursement for hospitals. Through leadership, teamwork, and a multidisciplinary approach, hospitalists and infectious disease physicians should together drive change to realize these goals.
References
- Pieris JSM, Lai ST, Poon LLM, et al. Coronavirus as a cause of severe acute respiratory syndrome. Lancet. 2003;361:1319-25.
- Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the western hemisphere. N Engl J Med. 2004;350:342-50.
- Dhar AD, Werchniak AE, Li Y, et al. Tanapox infection in a college student. N Engl J Med. 2004;350;361-6.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38: 805-11.
- Fridkin SK., Hageman JC, Morrison M, et al. Methicillin resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-44.
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445-53.
- Cosgrove SE, Carroll KC, Perl TM. Staphylococcus aureus with reduced susceptibility to vancomycin. Clin Infect Dis. 2004;39:539-45.
- Whitener CJ, Park SY, Browne FA, et al. Vancomycin resistant Staphylococcus aureus in the absence of vancomycin exposure. Clin Infect Dis. 2004;38:1049-55.
- Whitney CG, Farley MM, Hadler J, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000;343: 1917-24.
- Davidson R, Cavalcanti R, Brunton JL, et al. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N Engl J Med. 2002;346:747-50.
- Lukehart SA, Godornes C, Molini BJ, et al. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004;351:154-8.
- Reddy JC, Katz PP, Goldman L, Wachter RM. A pneumonia practice guideline and a hospitalist-based reorganization lead to equivalent efficiency gains. Am J Manag Care. 2001;7:1142-8.
- Rifkin WD, Connor D, Silver A, Eichorn A. Comparison of processes and outcomes of pneumonia care between hospitalists and community-based primary care physicians. Mayo Clin Proc. 2002;77:1053-8.
- Eron LJ, Passos S: Early discharge of infected patients through appropriate antibiotic use. Arch Intern Med. 2001;161:61-5.
Despite the widespread availability of potent anti-infective agents, infectious diseases remain formidable and common problems in hospitalized patients. In this issue of The Hospitalist, hospitalists and infectious disease specialists provide state-of-the-art reviews of common infectious disease syndromes encountered in hospital medicine. This should be required reading for hospitalists, given the severity of illness of many patients with these syndromes, and the rapidly evolving developments in optimal diagnosis and management in many of these areas.
Hospitalists and infectious disease practitioners share similar challenges and should be natural allies in improving patient care. To name a few, these challenges include the prompt recognition and management of new and emerging infectious diseases; the rising incidence of drug-resistant pathogens; the prevention, prompt recognition, and effective management of nosocomial and opportunistic infections in hospitalized patients, including outbreaks; the implementation of clinical practice guidelines for common infectious disease problems; and the vaccination of high-risk hospitalized patients.
New and emerging infectious diseases have been recognized with increasing frequency, and hospitalists are among those physicians most likely to encounter them. In the past two years alone, these have included SARS (1), monkeypox (2), tanapox (3), and American Boutonneuse fever (4). Drug-resistant pathogens have become increasingly common. Methicillin-resistant Staphylococcus aureus (MRSA) now accounts for more than 50% of Staphylococcus aureus isolates in many hospitals. Until recently, MRSA has afflicted mainly hospitalized patients or those with significant underlying comorbidities. Recent reports, however, have described MRSA in previously healthy patients admitted from the community (5,6) Even more ominously, isolates of Staphylococcus aureus with intermediate or high-level resistance to vancomycin have been reported (7,8).
Drug-resistant Streptococcus pneumoniae is common in many parts of the United States; many isolates are resistant to multiple common antibiotics, and fluoroquinolone resistance, though still uncommon, has been reported (9,10). Vancomycin-resistant enterococci have emerged over the past decade and now account for up to 15% of enterococcal isolates in many centers. Macrolide-resistance in Treponema pallidum was recently described (11). The emergence of drug-resistant pathogens has important implications for hospitalists, who are typically at the front line in choosing empiric or pathogen-specific antimicrobial therapy for hospitalized patients. Knowledge about local trends in antimicrobial resistance is essential for making informed antibiotic selections, and prevention of spread of these organisms within hospitals is crucial.
How have hospitalists partnered with infectious disease specialists in tackling these problems? Surprisingly, very little has been written. In our review of the literature we were able to identify only 3 studies addressing the role of hospitalists and infectious disease physicians in the management of infectious diseases (12-14). Reddy et al. compared the impact of a clinical practice guideline introduced at the University of California San Francisco Moffitt-Long Hospital in 1996 with that of a hospitalist-based reorganization of their medical service in the management of patients with community-acquired pneumonia (CAP) (12). Following implementation of the guideline, average cost per case and length of stay declined similarly among patients cared for by hospitalists versus those cared for by attending physicians on the traditional medical service. Mortality remained unchanged despite shorter length of stay, and readmission rates fell. However, hospitalists achieved statistically greater reductions in cost per case and length of stay for all other diagnoses compared with their traditional attending counterparts. This study therefore concluded that the implementation of a clinical practice guideline was the key driver in improving resource utilization in hospitalized patients with CAP, rather than physician model of care.
In a similar study, Rifkin et al. compared outcomes and resource utilization in hospitalized patients with CAP at Long Island Jewish Medical Center cared for by hospitalists (185 patients) versus primary care physicians (270 patients) (13). No local clinical practice guideline was in place, although appropriateness of therapy was evaluated based upon guidelines disseminated by the American Thoracic Society and the Infectious Disease Society of America at the time. Compared with hospitalists, primary care physicians obtained more subspecialty consultations and administered antibiotics in a more timely fashion. Nevertheless, hospitalist care was associated with shorter length of stay, lower cost per case, more rapid transition from parenteral to oral antibiotic therapy, and improved survival. Hospitalist patients were more likely to be discharged with an unstable vital sign, but 15- and 30-day readmission rates were similar to patients cared for by primary care physicians. This study implies that, absent an enforced clinical practice guideline, hospitalist care of patients with CAP is associated with decreased resource utilization and better outcome.
Finally, in a case-control study, Eron and Passos examined the performance of hospitalists versus infectious disease consultants in the care of hospitalized patients with CAP, cellulitis, or pyelonephritis (14). One hundred eleven patients cared for by infectious disease consultants were compared with 112 historical controls cared for by hospitalists. Patients receiving care from infectious disease specialists had higher patient satisfaction, shorter length of stay, no readmissions, and more rapid return to activities of daily living. The benefits of specialty care were attributable to more frequent use of early outpatient parenteral antibiotic therapy (OPAT) in patients with cellulitis and more rapid switching from parenteral to oral therapy in patients with pyelonephritis and CAP by infectious disease specialists compared with hospitalists. This study has several implications. First, selected inpatients may benefit from early infectious disease consultation. And second, hospitalists may be able to learn from their subspecialty colleagues about the safety and efficacy of early switch therapy and OPAT in selected patient populations.
These limited data suggest that patients with selected infectious diseases may benefit from care provided by hospitalists and infectious disease specialists and that such care is associated with better outcomes and decreased resource utilization. No studies have examined what we believe to be the true potential for improved patient care through the partnership of these 2 disciplines working in collaboration. In the absence of data, we offer several suggestions for areas of fruitful collaboration and further study. First, with the increasing incidence of drug-resistant pathogens and the increasing severity of illness of hospitalized patients with infectious diseases, hospitalists and infectious disease specialists can work together to ensure optimal use of empiric and pathogen-specific antimicrobial therapy and the development and implementation of evidence-based practice guidelines. Second, early infectious disease consultation should be strongly considered in critically ill patients, in those with suspected or confirmed drug-resistant pathogens, and in those with unusual or complicated infectious disease problems. Third, hospitalists, in collaboration with infectious disease specialists, can and should help to improve basic infection control practices, such as hand hygiene and the appropriate use of indwelling devices such as Foley catheters and vascular access devices, which predispose to nosocomial infection. Fourth, hospitalists can facilitate the appropriate immunization of at-risk hospitalized patients against influenza and S. pneumoniae. Finally, hospitalists can partner with their infectious disease colleagues to optimize the use of OPAT and early switch therapy in patients with selected infectious diseases so as to maximize outcome while at the same time reducing hospital length of stay and resource utilization.
In a demonstration project, the Center for Medicare and Medicaid Services has now linked quality measures to reimbursement in several areas of infectious diseases. These include timeliness of antibiotic administration in patients with CAP, blood culture collection prior to antibiotic therapy in patients with CAP, and screening and administering influenza and pneumococcal immunizations to at-risk patients. This trend of linking reimbursement to performance in infectious disease management and prevention is likely to continue.
There are therefore many reasons to encourage partnership and collaboration between these disciplines. These include better quality of care, improved resource utilization, and, hopefully, better reimbursement for hospitals. Through leadership, teamwork, and a multidisciplinary approach, hospitalists and infectious disease physicians should together drive change to realize these goals.
References
- Pieris JSM, Lai ST, Poon LLM, et al. Coronavirus as a cause of severe acute respiratory syndrome. Lancet. 2003;361:1319-25.
- Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the western hemisphere. N Engl J Med. 2004;350:342-50.
- Dhar AD, Werchniak AE, Li Y, et al. Tanapox infection in a college student. N Engl J Med. 2004;350;361-6.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38: 805-11.
- Fridkin SK., Hageman JC, Morrison M, et al. Methicillin resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-44.
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445-53.
- Cosgrove SE, Carroll KC, Perl TM. Staphylococcus aureus with reduced susceptibility to vancomycin. Clin Infect Dis. 2004;39:539-45.
- Whitener CJ, Park SY, Browne FA, et al. Vancomycin resistant Staphylococcus aureus in the absence of vancomycin exposure. Clin Infect Dis. 2004;38:1049-55.
- Whitney CG, Farley MM, Hadler J, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000;343: 1917-24.
- Davidson R, Cavalcanti R, Brunton JL, et al. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N Engl J Med. 2002;346:747-50.
- Lukehart SA, Godornes C, Molini BJ, et al. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004;351:154-8.
- Reddy JC, Katz PP, Goldman L, Wachter RM. A pneumonia practice guideline and a hospitalist-based reorganization lead to equivalent efficiency gains. Am J Manag Care. 2001;7:1142-8.
- Rifkin WD, Connor D, Silver A, Eichorn A. Comparison of processes and outcomes of pneumonia care between hospitalists and community-based primary care physicians. Mayo Clin Proc. 2002;77:1053-8.
- Eron LJ, Passos S: Early discharge of infected patients through appropriate antibiotic use. Arch Intern Med. 2001;161:61-5.
Improving Patient Safety and Quality of Care
Patient safety and improved quality of care have become priority issues in the American healthcare system. The potential for medical errors was highlighted in 1999 when the Quality of Health Care in America Committee of the Institute of Medicine (IOM) published its first report, To Err is Human: Building a Safer Health System. The committee estimated that between 44,000 and 98,000 people die annually from inpatient medical errors. The eighth leading cause of death in this country, preventable medical errors, cost the U.S. approximately $17 billion annually in direct and indirect costs (IOM). These alarming statistics in the IOM report ignited the patient safety movement (I).
The IOM report made a series of recommendations that included the creation of a center for patient safety, the development of a national public reporting system, the establishment of oversight agencies, and the incorporation of safety principles into monitoring systems. Public and private agencies have responded with a series of initiatives that address these recommendations (See Table 1).
One healthcare expert describes three reasons as to why the potential for medical errors has increased. First, technology has created a sophisticated array of test, x-rays, laboratory procedures, and diagnostic tools. Second, pharmaceutical research has introduced thousands of new medications to the marketplace. Finally, specialization has led to experts, both physician and non-physician, in a wide range of body systems, diseases, settings, procedures, and therapies. Hospital medicine represents a new type of medical specialty that has the potential to address this increased complexity and sophistication and to improve patient care in the hospital inpatient environment (2).
Hospitalists as Team Coordinators
To achieve maximum positive outcomes in the complex inpatient environment, a qualified coordinator must educate others and facilitate activity revolving around patient care. Hospitalists as inpatient experts possess the necessary qualifications to integrate hospital systems and maximize efforts to enhance patient safety by monitoring medication distribution, chairing pharmaceuticals and therapeutics (P&T) committees, overseeing computerized physician order entry (CPOE), directing quality/performance improvement projects, and collaborating with discharge planning and case management.
Lakshmi Halasyamani, MD, is vice chair of the department of Internal Medicine at St. Joseph Mercy Hospital in Michigan and chairperson of the Society of Hospital Medicine (SHM) Hospital Quality and Patient Safety Committee. She says, Hospitalists have a ‘lens of understanding the systems under which they care for patients.’ They take care of patients in the hospital every single day so they can examine the processes with which they work. Hospitalists have an ideal perspective from which to reform ineffective systems.”
In spite of all the guidelines established by federal agencies and expert groups, Dr. Halasyamani points out that implementation barriers exist that prevent well-intentioned protocols and best practices from being carried out. Part of the challenge is the performance of a critical piece of the infrastructure—the multidisciplinary team. The very nature of healthcare demands an inherent need to coordinate and communicate. “Treating the patient is not the responsibility of one single individual,” says Halasyamani. “This is a team effort. The hospitalist recognizes that he is part of that team.” By elevating the ideals of teamwork, hospitalists can deliver to the patients the essential care that patients need, both while in the hospital and after they are discharged. In managing hospital inpatients, physicians must cope with high intensity of illness, pressures to reduce length of stay (LOS), and the coordination of handoffs among many specialists. According to Halasyamani, this can be a “recipe for disaster.”
Halasyamani acknowledges the vital role of protocols in reducing medical errors and improving quality of care. The training, education, and experience a hospitalist has acquired enables him to optimize communication and implement protocols, thus facilitating the practice of delivering safe and consistent care to all patients. In fact, with this smaller core group of inpatient physicians, the development and implementation of protocols can potentially be more effective because it targets a smaller group of physicians than the traditional inpatient model (8).
Kaveh C. Shojania, MD, is assistant professor of medicine at the University of Ottawa and co-author of Internal Bleeding: The Terrifying Truth Behind America's Epidemic Medical Mistakes. He points out that the current inpatient medical landscape involves a significant number of clinicians who practice at the hospital but not all their activity is centered there. “From a clinical perspective, no one has ownership,” he says. “On the other hand, hospitalists are based in a single hospital and have a vested interest in that particular hospital.” Typically generalists, hospitalists tend to interact with all specialists and therefore have a good sense of all interests.
Medical errors occur most often during transition times, from the ICU to the floor or from inpatient to outpatient status. There is the potential for a loss of clinical information during these transfers. According to Shojania, a significant portion of the hospitalist’s time is spent managing these transitions and overseeing patients as they are relocated from floor to floor and discharge to home, rehabilitation facility, or nursing home. He notes that the regulatory agencies have begun to acknowledge the importance of hospitalists. “The JCAHO (Joint Commission for the Accreditation of Healthcare Organizations) recognizes hospitalists as a resource because they are always in the hospital and have a vested interest,” he says (9).
Stakeholder Analysis
Patients stand to gain the most benefit from hospitalists insofar as safety and quality of care is concerned. Through the efforts and oversight of hospitalists, patients may experience reduced medical errors and lower mortality rates. For primary care physicians and hospitals, this lower rate of medical error means fewer medical malpractice cases, the potential for lower insurance premiums and, as a result, enhanced reputations. When hospitals are run more efficiently and provide a greater sense of trust and efficient management practices, society in general becomes the benefactor.
Clinical Trials
To date, few research studies measuring the impact of hospitalists on patient safety and quality of care have been conducted. Quality of care has been assessed largely through the surrogate markers of mortality and readmission rates. One study showed decreased in-hospital and 1-year mortality rates for hospitalist patients (10), and another indicated a decrease in 30-day readmission rates (11).
In addition, data from individual programs demonstrate positive findings. For example, Stacy Goldsholl, MD, medical director of the Covenant Healthcare hospital medicine program in Michigan, reports a 17% decrease in the expected mortality rate in the first year of the hospital medicine program. The information was drawn from the Michigan Hospital Association (MHA) databank and matched for severity and diagnosis (See Table 2). “This was significant when compared to the internal medicine comparison group with similar case mix index (CMI),” says Goldsholl. “In the first half of our second year, we have demonstrated a 46% decrease in expected mortality, while internal medicine had a 4% increase” (12).
Additionally, Goldsholl reports that Covenant initiated a Code Blue and emergency consult service to improve patient outcome and experienced a marked increase in efficiency. Table 3 represents elementary data collected during the first 6 months pre- and post-initiation of the hospital medicine program at Covenant (12).
Conclusion
Patient safety and quality of care in the hospital require a team of dedicated people to effect change. Orchestrating the team effectively is the responsibility of an attending physician. With the numerous “handoffs” that take place during hospitalization, the potential for medical errors increases exponentially. Federal mandates requiring the conversion to electronic medical records, which includes basic health information as well as critical data regarding medications, procedures, and surgeries, further complicates efficient and safe patient management. According to Robert Wachter, “Those doctors with the best outcomes were those who tended to treat similar patients with similar problems using similar techniques.” By definition, the hospitalist is a “physician who focuses his practice on the care, coordination, and safety of hospitalized patients.” Who better to stand at the center of the issue of reduced medical errors, improved patient care, and enhanced quality of care than hospitalists (13)?
Dr. Pak can be contacted at mhp@medicine.wisc.edu.
References
- To Err is Human: Building a Safer Health System, Institute of Medicine, November 1999.
- Wachter R. The end of the beginning: patient safety five years after ‘To Err Is Human.’ Health Affairs. November 30, 2004.
- Mission Statement: Center for Quality Improvement and Patient Safety. February 2004. Agency for Healthcare Research and Quality, Rockville, MD. www.ahrq.gov/about/cquips/cquipsmiss.htm.
- Safe Practices for Better Healthcare: a Consensus. The National Quality Forum, 2003.
- Joint Commission for Accreditation of Healthcare Organizations (JCAHO), www.jcaho.org.
- Leapfrog Group, www.leapfroggroup.org.
- Accreditation Council for Graduate Medical Education (ACGME), www.acgme.org.
- Halasyamani L. Telephone interview. February 7, 2005.
- Shojania KG. Assistant professor of medicine, University of Ottawa. Telephone interview. January 31, 2005.
- Auerbach AD, Wachter RM, Katz P. et al. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137:859-65.
- Kulaga ME, Charney P, O’Mahoney SP, et al. The positive impact of initiation of hospitalist clinician educators. J Gen Intern Med. 2004;19:293-301.
- Goldsholl S. Medical director. Covenant Healthcare hospital medicine program, Saginaw, Michigan, email interview. January 31, 2005.
- Wachter R, Shojania K. Internal bleeding: the truth behind America’s terrifying epidemic of medical mistakes. Rugged Land, LLC, 2004.
Patient safety and improved quality of care have become priority issues in the American healthcare system. The potential for medical errors was highlighted in 1999 when the Quality of Health Care in America Committee of the Institute of Medicine (IOM) published its first report, To Err is Human: Building a Safer Health System. The committee estimated that between 44,000 and 98,000 people die annually from inpatient medical errors. The eighth leading cause of death in this country, preventable medical errors, cost the U.S. approximately $17 billion annually in direct and indirect costs (IOM). These alarming statistics in the IOM report ignited the patient safety movement (I).
The IOM report made a series of recommendations that included the creation of a center for patient safety, the development of a national public reporting system, the establishment of oversight agencies, and the incorporation of safety principles into monitoring systems. Public and private agencies have responded with a series of initiatives that address these recommendations (See Table 1).
One healthcare expert describes three reasons as to why the potential for medical errors has increased. First, technology has created a sophisticated array of test, x-rays, laboratory procedures, and diagnostic tools. Second, pharmaceutical research has introduced thousands of new medications to the marketplace. Finally, specialization has led to experts, both physician and non-physician, in a wide range of body systems, diseases, settings, procedures, and therapies. Hospital medicine represents a new type of medical specialty that has the potential to address this increased complexity and sophistication and to improve patient care in the hospital inpatient environment (2).
Hospitalists as Team Coordinators
To achieve maximum positive outcomes in the complex inpatient environment, a qualified coordinator must educate others and facilitate activity revolving around patient care. Hospitalists as inpatient experts possess the necessary qualifications to integrate hospital systems and maximize efforts to enhance patient safety by monitoring medication distribution, chairing pharmaceuticals and therapeutics (P&T) committees, overseeing computerized physician order entry (CPOE), directing quality/performance improvement projects, and collaborating with discharge planning and case management.
Lakshmi Halasyamani, MD, is vice chair of the department of Internal Medicine at St. Joseph Mercy Hospital in Michigan and chairperson of the Society of Hospital Medicine (SHM) Hospital Quality and Patient Safety Committee. She says, Hospitalists have a ‘lens of understanding the systems under which they care for patients.’ They take care of patients in the hospital every single day so they can examine the processes with which they work. Hospitalists have an ideal perspective from which to reform ineffective systems.”
In spite of all the guidelines established by federal agencies and expert groups, Dr. Halasyamani points out that implementation barriers exist that prevent well-intentioned protocols and best practices from being carried out. Part of the challenge is the performance of a critical piece of the infrastructure—the multidisciplinary team. The very nature of healthcare demands an inherent need to coordinate and communicate. “Treating the patient is not the responsibility of one single individual,” says Halasyamani. “This is a team effort. The hospitalist recognizes that he is part of that team.” By elevating the ideals of teamwork, hospitalists can deliver to the patients the essential care that patients need, both while in the hospital and after they are discharged. In managing hospital inpatients, physicians must cope with high intensity of illness, pressures to reduce length of stay (LOS), and the coordination of handoffs among many specialists. According to Halasyamani, this can be a “recipe for disaster.”
Halasyamani acknowledges the vital role of protocols in reducing medical errors and improving quality of care. The training, education, and experience a hospitalist has acquired enables him to optimize communication and implement protocols, thus facilitating the practice of delivering safe and consistent care to all patients. In fact, with this smaller core group of inpatient physicians, the development and implementation of protocols can potentially be more effective because it targets a smaller group of physicians than the traditional inpatient model (8).
Kaveh C. Shojania, MD, is assistant professor of medicine at the University of Ottawa and co-author of Internal Bleeding: The Terrifying Truth Behind America's Epidemic Medical Mistakes. He points out that the current inpatient medical landscape involves a significant number of clinicians who practice at the hospital but not all their activity is centered there. “From a clinical perspective, no one has ownership,” he says. “On the other hand, hospitalists are based in a single hospital and have a vested interest in that particular hospital.” Typically generalists, hospitalists tend to interact with all specialists and therefore have a good sense of all interests.
Medical errors occur most often during transition times, from the ICU to the floor or from inpatient to outpatient status. There is the potential for a loss of clinical information during these transfers. According to Shojania, a significant portion of the hospitalist’s time is spent managing these transitions and overseeing patients as they are relocated from floor to floor and discharge to home, rehabilitation facility, or nursing home. He notes that the regulatory agencies have begun to acknowledge the importance of hospitalists. “The JCAHO (Joint Commission for the Accreditation of Healthcare Organizations) recognizes hospitalists as a resource because they are always in the hospital and have a vested interest,” he says (9).
Stakeholder Analysis
Patients stand to gain the most benefit from hospitalists insofar as safety and quality of care is concerned. Through the efforts and oversight of hospitalists, patients may experience reduced medical errors and lower mortality rates. For primary care physicians and hospitals, this lower rate of medical error means fewer medical malpractice cases, the potential for lower insurance premiums and, as a result, enhanced reputations. When hospitals are run more efficiently and provide a greater sense of trust and efficient management practices, society in general becomes the benefactor.
Clinical Trials
To date, few research studies measuring the impact of hospitalists on patient safety and quality of care have been conducted. Quality of care has been assessed largely through the surrogate markers of mortality and readmission rates. One study showed decreased in-hospital and 1-year mortality rates for hospitalist patients (10), and another indicated a decrease in 30-day readmission rates (11).
In addition, data from individual programs demonstrate positive findings. For example, Stacy Goldsholl, MD, medical director of the Covenant Healthcare hospital medicine program in Michigan, reports a 17% decrease in the expected mortality rate in the first year of the hospital medicine program. The information was drawn from the Michigan Hospital Association (MHA) databank and matched for severity and diagnosis (See Table 2). “This was significant when compared to the internal medicine comparison group with similar case mix index (CMI),” says Goldsholl. “In the first half of our second year, we have demonstrated a 46% decrease in expected mortality, while internal medicine had a 4% increase” (12).
Additionally, Goldsholl reports that Covenant initiated a Code Blue and emergency consult service to improve patient outcome and experienced a marked increase in efficiency. Table 3 represents elementary data collected during the first 6 months pre- and post-initiation of the hospital medicine program at Covenant (12).
Conclusion
Patient safety and quality of care in the hospital require a team of dedicated people to effect change. Orchestrating the team effectively is the responsibility of an attending physician. With the numerous “handoffs” that take place during hospitalization, the potential for medical errors increases exponentially. Federal mandates requiring the conversion to electronic medical records, which includes basic health information as well as critical data regarding medications, procedures, and surgeries, further complicates efficient and safe patient management. According to Robert Wachter, “Those doctors with the best outcomes were those who tended to treat similar patients with similar problems using similar techniques.” By definition, the hospitalist is a “physician who focuses his practice on the care, coordination, and safety of hospitalized patients.” Who better to stand at the center of the issue of reduced medical errors, improved patient care, and enhanced quality of care than hospitalists (13)?
Dr. Pak can be contacted at mhp@medicine.wisc.edu.
References
- To Err is Human: Building a Safer Health System, Institute of Medicine, November 1999.
- Wachter R. The end of the beginning: patient safety five years after ‘To Err Is Human.’ Health Affairs. November 30, 2004.
- Mission Statement: Center for Quality Improvement and Patient Safety. February 2004. Agency for Healthcare Research and Quality, Rockville, MD. www.ahrq.gov/about/cquips/cquipsmiss.htm.
- Safe Practices for Better Healthcare: a Consensus. The National Quality Forum, 2003.
- Joint Commission for Accreditation of Healthcare Organizations (JCAHO), www.jcaho.org.
- Leapfrog Group, www.leapfroggroup.org.
- Accreditation Council for Graduate Medical Education (ACGME), www.acgme.org.
- Halasyamani L. Telephone interview. February 7, 2005.
- Shojania KG. Assistant professor of medicine, University of Ottawa. Telephone interview. January 31, 2005.
- Auerbach AD, Wachter RM, Katz P. et al. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137:859-65.
- Kulaga ME, Charney P, O’Mahoney SP, et al. The positive impact of initiation of hospitalist clinician educators. J Gen Intern Med. 2004;19:293-301.
- Goldsholl S. Medical director. Covenant Healthcare hospital medicine program, Saginaw, Michigan, email interview. January 31, 2005.
- Wachter R, Shojania K. Internal bleeding: the truth behind America’s terrifying epidemic of medical mistakes. Rugged Land, LLC, 2004.
Patient safety and improved quality of care have become priority issues in the American healthcare system. The potential for medical errors was highlighted in 1999 when the Quality of Health Care in America Committee of the Institute of Medicine (IOM) published its first report, To Err is Human: Building a Safer Health System. The committee estimated that between 44,000 and 98,000 people die annually from inpatient medical errors. The eighth leading cause of death in this country, preventable medical errors, cost the U.S. approximately $17 billion annually in direct and indirect costs (IOM). These alarming statistics in the IOM report ignited the patient safety movement (I).
The IOM report made a series of recommendations that included the creation of a center for patient safety, the development of a national public reporting system, the establishment of oversight agencies, and the incorporation of safety principles into monitoring systems. Public and private agencies have responded with a series of initiatives that address these recommendations (See Table 1).
One healthcare expert describes three reasons as to why the potential for medical errors has increased. First, technology has created a sophisticated array of test, x-rays, laboratory procedures, and diagnostic tools. Second, pharmaceutical research has introduced thousands of new medications to the marketplace. Finally, specialization has led to experts, both physician and non-physician, in a wide range of body systems, diseases, settings, procedures, and therapies. Hospital medicine represents a new type of medical specialty that has the potential to address this increased complexity and sophistication and to improve patient care in the hospital inpatient environment (2).
Hospitalists as Team Coordinators
To achieve maximum positive outcomes in the complex inpatient environment, a qualified coordinator must educate others and facilitate activity revolving around patient care. Hospitalists as inpatient experts possess the necessary qualifications to integrate hospital systems and maximize efforts to enhance patient safety by monitoring medication distribution, chairing pharmaceuticals and therapeutics (P&T) committees, overseeing computerized physician order entry (CPOE), directing quality/performance improvement projects, and collaborating with discharge planning and case management.
Lakshmi Halasyamani, MD, is vice chair of the department of Internal Medicine at St. Joseph Mercy Hospital in Michigan and chairperson of the Society of Hospital Medicine (SHM) Hospital Quality and Patient Safety Committee. She says, Hospitalists have a ‘lens of understanding the systems under which they care for patients.’ They take care of patients in the hospital every single day so they can examine the processes with which they work. Hospitalists have an ideal perspective from which to reform ineffective systems.”
In spite of all the guidelines established by federal agencies and expert groups, Dr. Halasyamani points out that implementation barriers exist that prevent well-intentioned protocols and best practices from being carried out. Part of the challenge is the performance of a critical piece of the infrastructure—the multidisciplinary team. The very nature of healthcare demands an inherent need to coordinate and communicate. “Treating the patient is not the responsibility of one single individual,” says Halasyamani. “This is a team effort. The hospitalist recognizes that he is part of that team.” By elevating the ideals of teamwork, hospitalists can deliver to the patients the essential care that patients need, both while in the hospital and after they are discharged. In managing hospital inpatients, physicians must cope with high intensity of illness, pressures to reduce length of stay (LOS), and the coordination of handoffs among many specialists. According to Halasyamani, this can be a “recipe for disaster.”
Halasyamani acknowledges the vital role of protocols in reducing medical errors and improving quality of care. The training, education, and experience a hospitalist has acquired enables him to optimize communication and implement protocols, thus facilitating the practice of delivering safe and consistent care to all patients. In fact, with this smaller core group of inpatient physicians, the development and implementation of protocols can potentially be more effective because it targets a smaller group of physicians than the traditional inpatient model (8).
Kaveh C. Shojania, MD, is assistant professor of medicine at the University of Ottawa and co-author of Internal Bleeding: The Terrifying Truth Behind America's Epidemic Medical Mistakes. He points out that the current inpatient medical landscape involves a significant number of clinicians who practice at the hospital but not all their activity is centered there. “From a clinical perspective, no one has ownership,” he says. “On the other hand, hospitalists are based in a single hospital and have a vested interest in that particular hospital.” Typically generalists, hospitalists tend to interact with all specialists and therefore have a good sense of all interests.
Medical errors occur most often during transition times, from the ICU to the floor or from inpatient to outpatient status. There is the potential for a loss of clinical information during these transfers. According to Shojania, a significant portion of the hospitalist’s time is spent managing these transitions and overseeing patients as they are relocated from floor to floor and discharge to home, rehabilitation facility, or nursing home. He notes that the regulatory agencies have begun to acknowledge the importance of hospitalists. “The JCAHO (Joint Commission for the Accreditation of Healthcare Organizations) recognizes hospitalists as a resource because they are always in the hospital and have a vested interest,” he says (9).
Stakeholder Analysis
Patients stand to gain the most benefit from hospitalists insofar as safety and quality of care is concerned. Through the efforts and oversight of hospitalists, patients may experience reduced medical errors and lower mortality rates. For primary care physicians and hospitals, this lower rate of medical error means fewer medical malpractice cases, the potential for lower insurance premiums and, as a result, enhanced reputations. When hospitals are run more efficiently and provide a greater sense of trust and efficient management practices, society in general becomes the benefactor.
Clinical Trials
To date, few research studies measuring the impact of hospitalists on patient safety and quality of care have been conducted. Quality of care has been assessed largely through the surrogate markers of mortality and readmission rates. One study showed decreased in-hospital and 1-year mortality rates for hospitalist patients (10), and another indicated a decrease in 30-day readmission rates (11).
In addition, data from individual programs demonstrate positive findings. For example, Stacy Goldsholl, MD, medical director of the Covenant Healthcare hospital medicine program in Michigan, reports a 17% decrease in the expected mortality rate in the first year of the hospital medicine program. The information was drawn from the Michigan Hospital Association (MHA) databank and matched for severity and diagnosis (See Table 2). “This was significant when compared to the internal medicine comparison group with similar case mix index (CMI),” says Goldsholl. “In the first half of our second year, we have demonstrated a 46% decrease in expected mortality, while internal medicine had a 4% increase” (12).
Additionally, Goldsholl reports that Covenant initiated a Code Blue and emergency consult service to improve patient outcome and experienced a marked increase in efficiency. Table 3 represents elementary data collected during the first 6 months pre- and post-initiation of the hospital medicine program at Covenant (12).
Conclusion
Patient safety and quality of care in the hospital require a team of dedicated people to effect change. Orchestrating the team effectively is the responsibility of an attending physician. With the numerous “handoffs” that take place during hospitalization, the potential for medical errors increases exponentially. Federal mandates requiring the conversion to electronic medical records, which includes basic health information as well as critical data regarding medications, procedures, and surgeries, further complicates efficient and safe patient management. According to Robert Wachter, “Those doctors with the best outcomes were those who tended to treat similar patients with similar problems using similar techniques.” By definition, the hospitalist is a “physician who focuses his practice on the care, coordination, and safety of hospitalized patients.” Who better to stand at the center of the issue of reduced medical errors, improved patient care, and enhanced quality of care than hospitalists (13)?
Dr. Pak can be contacted at mhp@medicine.wisc.edu.
References
- To Err is Human: Building a Safer Health System, Institute of Medicine, November 1999.
- Wachter R. The end of the beginning: patient safety five years after ‘To Err Is Human.’ Health Affairs. November 30, 2004.
- Mission Statement: Center for Quality Improvement and Patient Safety. February 2004. Agency for Healthcare Research and Quality, Rockville, MD. www.ahrq.gov/about/cquips/cquipsmiss.htm.
- Safe Practices for Better Healthcare: a Consensus. The National Quality Forum, 2003.
- Joint Commission for Accreditation of Healthcare Organizations (JCAHO), www.jcaho.org.
- Leapfrog Group, www.leapfroggroup.org.
- Accreditation Council for Graduate Medical Education (ACGME), www.acgme.org.
- Halasyamani L. Telephone interview. February 7, 2005.
- Shojania KG. Assistant professor of medicine, University of Ottawa. Telephone interview. January 31, 2005.
- Auerbach AD, Wachter RM, Katz P. et al. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137:859-65.
- Kulaga ME, Charney P, O’Mahoney SP, et al. The positive impact of initiation of hospitalist clinician educators. J Gen Intern Med. 2004;19:293-301.
- Goldsholl S. Medical director. Covenant Healthcare hospital medicine program, Saginaw, Michigan, email interview. January 31, 2005.
- Wachter R, Shojania K. Internal bleeding: the truth behind America’s terrifying epidemic of medical mistakes. Rugged Land, LLC, 2004.
Maximizing Throughput and Improving Patient Flow
According to data from the American Hospital Association (1), in 1985, the United States had 5732 operational community hospitals; by 2002, the latest year for which figures are available, the number had decreased to 4927, a loss of approximately 14% (1). In that same timeframe, these hospitals lost approximately 18% of their beds, dropping from just over 1 million to 820,653 beds. This reduction in bed capacity has been accompanied by hospital cost-cutting efforts, staff downsizing, and elimination of services. Many explanations for these trends have been suggested, including changes in Medicare reimbursement and the growth of managed care organizations (MCOs).
However, as the current baby boom generation ages, rising inpatient demands are presenting hospitals with significant challenges. According to a 2001 report from Solucient (2), who maintains the nation’s largest health care database, the senior population—individuals age 65 and older—are projected to experience an 85% growth rate over the next two decades. Since this age group utilizes inpatient services 4.5 times more than younger populations, the number of admissions and beds to accommodate those cases will soar. By the year 2027, hospitals can anticipate a 46% rise in demand for acute inpatient beds as admissions escalate by approximately 13 million cases. Currently, the nation’s healthcare facilities admit 31 million cases; this number will jump to more than 44 million, representing a 41% growth from present admissions figures. For hospitals that maintain an 80% census rate, an additional 238,000 beds will be needed to meet demands (1).
Adding to this increase in demand and pressure on bed capacity, hospitals must address the requirements of the Emergency Medical Treatment and Active Labor Act (EMTALA) passed by the US Congress in 1986 as part of the Consolidated Omnibus Reconciliation Act (COBRA). The law’s initial intent was to ensure patient access to emergency medical care and to prevent the practice of patient dumping, in which uninsured patients were transferred, solely for financial reasons, from private to public hospitals without consideration of their medical condition or stability for the transfer (3). EMTALA mandates that hospitals rank the severity of patients. Thus, tertiary referral centers are required to admit the sickest patients first. This directive presents a significant challenge to many healthcare facilities. High census rates prohibit the admission of elective surgical cases, which, although profitable, are considered second tier. Routine medical cases or complicated emergency surgical cases have the potential to adversely affect the institution’s financial performance.
In addition to the challenge of increased bed demands and EMTALA, hospitals also cite an increasingly smaller number of on-site community physicians. Longstanding trends from inpatient to outpatient care have prompted many community physicians to concentrate their efforts on serving the needs of office-based patients, limiting their accessibility to hospital cases.
To address these pressures, hospitals must execute innovative strategies that deliver efficient throughput and enhance revenue, while still preserving high-quality services. Since 1996, hospital medicine programs have demonstrated a positive impact on the healthcare facility’s ability to increase overall productivity and profitability and still maintain high quality Patients today present to the doctor sicker than in the past and require more careful and frequent outpatient care. Since hospitalists operate solely on an inpatient basis, their availability to efficiently admit and manage hospitalized patients enables delivery of quality care that expedites appropriate treatment and shortens length of stay.
Two Roles of the Hospitalist
According to the Society of Hospital Medicine (SHM), “Hospitalists are physicians whose primary professional focus is the general medical care of hospitalized patients. Their activities include patient care, teaching, research, and leadership related to hospital medicine.” Coined by Drs. Robert Wachter and Lee Goldman in 1996 (4), the term implies an additional point of emphasis. Part of a new paradigm in clinical care, the hospitalist enhances the processes of care surrounding patients and adopts an attitude of accountability for that care. In practice, hospitalists play two key roles.
Primarily, the hospitalist is a practicing clinician — managing throughput on a case-by-case, patient-by-patient basis. In addition, a hospitalist performs a non-clinical role as an “inpatient expert,” taking the lead in creating system changes and communicating those changes to other hospital personnel as well as to community physicians. As an inpatient expert, hospitalists are often asked to lead organization-wide throughput initiatives to identify and implement strategies to facilitate patient flow and efficiency. As dedicated members of multi-disciplinary in-house teams, the hospitalist is in a prime position to foster change and improve systems.
Throughput as Continuum of Care
As suggested by Heffner (5), the process of admission, hospitalization, and discharge resembles a “bell-shaped curve.” To achieve effective throughput, hospitals must expedite patient care and also maintain careful oversight throughout a patient’s entire hospital stay. The hospitalist, as an integral part of a multidisciplinary team, coordinates care to promote a positive outcome and shorten length of stay. Drawing on strong leadership qualities, as well as on intimate knowledge of hospital procedures, layout design and infrastructure, and available community resources, the hospitalist plays a pivotal role in creating efficient throughput from admission to discharge.
Emergency Department
At the front end of the bell-shaped curve, the hospitalist may be engaged by emergency department (ED) physicians to assist in ensuring smooth patient flow and, more important, identifies the “intensity of service” needed. Through the use of clinical criteria, such as lnterQual, the hospitalist, together with the ED physician, may be asked to quantitatively rate the patient’s illness for degree of severity.
Timely patient evaluation helps prevent a backlog of ED cases and enables more patients to be seen. Immediate attention to and initiation of appropriate therapy guarantees a better outcome while minimizing the potential risk for complications, which could possibly lead to longer inpatient stays.
Inpatient Unit
Once a patient has been admitted to an inpatient unit, the hospitalist, together with a multidisciplinary team, facilitates care and determines the inpatient services that will optimize patient recovery through strong interdepartmental communications. Working together with admissions, medical records, nursing, laboratory and diagnostic services, information technology and other pertinent departments, the hospitalist maintains a pulse on all activity surrounding the patient and his care.
Judicious inpatient consultations and treatment decisions result in timely changes in therapy, potentially reducing the length of stay. The frequency with which the hospitalist sees the patient allows him to monitor any changes in condition and reduce possible decompensation, a practice known as vertical continuity (6). Such careful attention may reduce inpatient length of stay significantly. When aggressive management is mandated, the presence of the hospitalist enables initiation of effective therapy and results in quicker discharge and a reduction in potential readmission (7).
Surgery
The surgeon and hospitalist are ideally suited to work together in managing a surgical patient. The hospitalist focuses on the peri-operative management of medical issues and risk reduction, which allows the surgeon to concentrate more on surgical indications and the surgery itself. The hospitalist’s role in the management of a surgical patient enables vertical continuity when the surgeon may be occupied in the operating room with another patient as documented by Huddleston’s Hospitalist Orthopedic Team (HOT) approach (8).
Intensive Care Unit (ICU)
In many hospitals, particularly those that do not have intensivists, hospitalists are able to provide quality care to patients. Even in hospitals where intensivists manage ICU patients, hospitalists work together with the intensivist to ensure smoother transition into and out of the unit.
Discharge
Timing is a critical issue with regard to discharge. Since the hospitalist operates solely in-house and in collaboration with a multidisciplinary team, he is able to round early in the day to discharge patients by mid- or late-morning, freeing a bed for a new patient. In some cases, the hospitalist, in anticipation of early discharge, may begin pre-planning the day prior to discharge, which further expedites the process. Early discharge applies to the ICU, step-down areas and general inpatient care areas, as well as to full discharge from the healthcare facility. Moving a patient from one of these areas enables other patients to fill those empty beds thus optimizing throughput.
Having managed the patient throughout his hospital stay, the hospitalist — again working together with a multidisciplinary team —can facilitate arrangements to send the patient home or to a rehabilitation or skilled nursing facility or alternative housing situation upon discharge, as well as coordinating post-discharge care, whether it be arranging for a visiting nursing or social services or communicating with the primary care physician regarding follow-up appointments. If additional outpatient care is prescribed, the hospitalist will work with the discharge planning staff to contact various community agencies to arrange services best suited to the patient’s needs. Efficient discharge makes possible the admission of other, more critically ill patients, potentially enhancing the hospital’s revenue stream.
Stakeholder Analysis
Five specific stakeholders need to be examined to document the value-added by hospitalists. Anecdotal evidence, as well as documented studies, has demonstrated numerous returns—physical, social, psychological and financial—to stakeholders involved in the hospital process. With regard to throughput, the hospitalist provides benefits to each of the stakeholders listed in Table 1.
Study Results
A dozen studies have been conducted that document the impact of hospital medicine programs on cost and clinical outcomes. Of these trials, nine found a significant decrease in the average length of stay (15%) as well as reductions in cost (9). Two other studies, one from an academic medical center and the other from a community teaching hospital, demonstrate similar reductions during a 2-year follow-up period. At the Western Penn Hospital, a 54% reduction in readmissions was reported with a 12% decrease in hospital costs, while the average LOS was 17% shorter. Additionally, an unpublished study from the University of California, San Francisco Medical Center revealed a consistent 10-15% decline in cost and length of stay between hospitalists and non-hospitalist teaching faculty. More important, those differences remained stable through 6 years of follow-up. In general, hospitals with hospitalist programs realized a 5-39% decrease in costs and a shortened average LOS of 7-25% (6).
According to Robert M. Wachter, author of the 2002 study, “If the average U.S. hospitalist cares for 600 inpatients each year and generates a 10% savings over the average medical inpatient cost of $8,000, the nation’s 4500 hospitalists save approximately $2.2 billion per year while potentially improving quality” (6).
In a study conducted by Douglas Gregory, Walter Baigelman, and Ira B. Wilson, hospitalists at Tufts-New England Medical Center in Boston, MA were found to substantially improve throughput with high baseline occupancy levels. Compared with a control group, the hospitalist group reduced LOS from 3.45 days to 2.19 days (p<.001). Additionally, the total cost of hospital admission decreased from $2,332 to $1,775 (p<.001) when hospitalists were involved. According to the study authors, improved throughput generated an incremental 266 patients per year with a related incremental hospital profitability of $1.3 million with the use of hospitalists (7).
Conclusion
As hospital administrators attempt to address the issue of expeditiously admitting, treating and discharging patients in these days of restricted budgets and increased demand, hospitalist programs are poised as an invaluable factor in the throughput process.
Dr. Cawley can be contacted at pcawley@ushosp.com.
References
- Hospital Statistics: the comprehensive reference source for analysis and comparison of hospital trends. Published annually by Health Forum, an affiliate of the American Hospital Association.
- National and local impact of long-term demographic change on inpatient acute care. 2001. Solucient, LLC.
- Zibulewsky J. The Emergency Medical Treatment and Active Labor Act (EMTALA): what it is and what it means for physicians. Baylor University Medical Center (BUMC) Proceedings. 2001;14:339-46.
- Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Eng J Med. 1996;335:514-7.
- Heffner JE. Executive medical director, Medical University of South Carolina (MUSC). Personal interview. June 24, 2004.
- Whitcomb WF. Director, Mercy Inpatient Medicine Service, Mercy Medical Center, Springfield, MA.
- Gregory D, Baigelman W, Wilson IB. Hospital economics of the hospitalist. Health Serv Res. 2003;38:905-18.
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical co-management after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141:28-38.
- Wachter RM. The evolution of the hospitalist model in the United States. Med Clin North Am. 2002;86:687-706.
According to data from the American Hospital Association (1), in 1985, the United States had 5732 operational community hospitals; by 2002, the latest year for which figures are available, the number had decreased to 4927, a loss of approximately 14% (1). In that same timeframe, these hospitals lost approximately 18% of their beds, dropping from just over 1 million to 820,653 beds. This reduction in bed capacity has been accompanied by hospital cost-cutting efforts, staff downsizing, and elimination of services. Many explanations for these trends have been suggested, including changes in Medicare reimbursement and the growth of managed care organizations (MCOs).
However, as the current baby boom generation ages, rising inpatient demands are presenting hospitals with significant challenges. According to a 2001 report from Solucient (2), who maintains the nation’s largest health care database, the senior population—individuals age 65 and older—are projected to experience an 85% growth rate over the next two decades. Since this age group utilizes inpatient services 4.5 times more than younger populations, the number of admissions and beds to accommodate those cases will soar. By the year 2027, hospitals can anticipate a 46% rise in demand for acute inpatient beds as admissions escalate by approximately 13 million cases. Currently, the nation’s healthcare facilities admit 31 million cases; this number will jump to more than 44 million, representing a 41% growth from present admissions figures. For hospitals that maintain an 80% census rate, an additional 238,000 beds will be needed to meet demands (1).
Adding to this increase in demand and pressure on bed capacity, hospitals must address the requirements of the Emergency Medical Treatment and Active Labor Act (EMTALA) passed by the US Congress in 1986 as part of the Consolidated Omnibus Reconciliation Act (COBRA). The law’s initial intent was to ensure patient access to emergency medical care and to prevent the practice of patient dumping, in which uninsured patients were transferred, solely for financial reasons, from private to public hospitals without consideration of their medical condition or stability for the transfer (3). EMTALA mandates that hospitals rank the severity of patients. Thus, tertiary referral centers are required to admit the sickest patients first. This directive presents a significant challenge to many healthcare facilities. High census rates prohibit the admission of elective surgical cases, which, although profitable, are considered second tier. Routine medical cases or complicated emergency surgical cases have the potential to adversely affect the institution’s financial performance.
In addition to the challenge of increased bed demands and EMTALA, hospitals also cite an increasingly smaller number of on-site community physicians. Longstanding trends from inpatient to outpatient care have prompted many community physicians to concentrate their efforts on serving the needs of office-based patients, limiting their accessibility to hospital cases.
To address these pressures, hospitals must execute innovative strategies that deliver efficient throughput and enhance revenue, while still preserving high-quality services. Since 1996, hospital medicine programs have demonstrated a positive impact on the healthcare facility’s ability to increase overall productivity and profitability and still maintain high quality Patients today present to the doctor sicker than in the past and require more careful and frequent outpatient care. Since hospitalists operate solely on an inpatient basis, their availability to efficiently admit and manage hospitalized patients enables delivery of quality care that expedites appropriate treatment and shortens length of stay.
Two Roles of the Hospitalist
According to the Society of Hospital Medicine (SHM), “Hospitalists are physicians whose primary professional focus is the general medical care of hospitalized patients. Their activities include patient care, teaching, research, and leadership related to hospital medicine.” Coined by Drs. Robert Wachter and Lee Goldman in 1996 (4), the term implies an additional point of emphasis. Part of a new paradigm in clinical care, the hospitalist enhances the processes of care surrounding patients and adopts an attitude of accountability for that care. In practice, hospitalists play two key roles.
Primarily, the hospitalist is a practicing clinician — managing throughput on a case-by-case, patient-by-patient basis. In addition, a hospitalist performs a non-clinical role as an “inpatient expert,” taking the lead in creating system changes and communicating those changes to other hospital personnel as well as to community physicians. As an inpatient expert, hospitalists are often asked to lead organization-wide throughput initiatives to identify and implement strategies to facilitate patient flow and efficiency. As dedicated members of multi-disciplinary in-house teams, the hospitalist is in a prime position to foster change and improve systems.
Throughput as Continuum of Care
As suggested by Heffner (5), the process of admission, hospitalization, and discharge resembles a “bell-shaped curve.” To achieve effective throughput, hospitals must expedite patient care and also maintain careful oversight throughout a patient’s entire hospital stay. The hospitalist, as an integral part of a multidisciplinary team, coordinates care to promote a positive outcome and shorten length of stay. Drawing on strong leadership qualities, as well as on intimate knowledge of hospital procedures, layout design and infrastructure, and available community resources, the hospitalist plays a pivotal role in creating efficient throughput from admission to discharge.
Emergency Department
At the front end of the bell-shaped curve, the hospitalist may be engaged by emergency department (ED) physicians to assist in ensuring smooth patient flow and, more important, identifies the “intensity of service” needed. Through the use of clinical criteria, such as lnterQual, the hospitalist, together with the ED physician, may be asked to quantitatively rate the patient’s illness for degree of severity.
Timely patient evaluation helps prevent a backlog of ED cases and enables more patients to be seen. Immediate attention to and initiation of appropriate therapy guarantees a better outcome while minimizing the potential risk for complications, which could possibly lead to longer inpatient stays.
Inpatient Unit
Once a patient has been admitted to an inpatient unit, the hospitalist, together with a multidisciplinary team, facilitates care and determines the inpatient services that will optimize patient recovery through strong interdepartmental communications. Working together with admissions, medical records, nursing, laboratory and diagnostic services, information technology and other pertinent departments, the hospitalist maintains a pulse on all activity surrounding the patient and his care.
Judicious inpatient consultations and treatment decisions result in timely changes in therapy, potentially reducing the length of stay. The frequency with which the hospitalist sees the patient allows him to monitor any changes in condition and reduce possible decompensation, a practice known as vertical continuity (6). Such careful attention may reduce inpatient length of stay significantly. When aggressive management is mandated, the presence of the hospitalist enables initiation of effective therapy and results in quicker discharge and a reduction in potential readmission (7).
Surgery
The surgeon and hospitalist are ideally suited to work together in managing a surgical patient. The hospitalist focuses on the peri-operative management of medical issues and risk reduction, which allows the surgeon to concentrate more on surgical indications and the surgery itself. The hospitalist’s role in the management of a surgical patient enables vertical continuity when the surgeon may be occupied in the operating room with another patient as documented by Huddleston’s Hospitalist Orthopedic Team (HOT) approach (8).
Intensive Care Unit (ICU)
In many hospitals, particularly those that do not have intensivists, hospitalists are able to provide quality care to patients. Even in hospitals where intensivists manage ICU patients, hospitalists work together with the intensivist to ensure smoother transition into and out of the unit.
Discharge
Timing is a critical issue with regard to discharge. Since the hospitalist operates solely in-house and in collaboration with a multidisciplinary team, he is able to round early in the day to discharge patients by mid- or late-morning, freeing a bed for a new patient. In some cases, the hospitalist, in anticipation of early discharge, may begin pre-planning the day prior to discharge, which further expedites the process. Early discharge applies to the ICU, step-down areas and general inpatient care areas, as well as to full discharge from the healthcare facility. Moving a patient from one of these areas enables other patients to fill those empty beds thus optimizing throughput.
Having managed the patient throughout his hospital stay, the hospitalist — again working together with a multidisciplinary team —can facilitate arrangements to send the patient home or to a rehabilitation or skilled nursing facility or alternative housing situation upon discharge, as well as coordinating post-discharge care, whether it be arranging for a visiting nursing or social services or communicating with the primary care physician regarding follow-up appointments. If additional outpatient care is prescribed, the hospitalist will work with the discharge planning staff to contact various community agencies to arrange services best suited to the patient’s needs. Efficient discharge makes possible the admission of other, more critically ill patients, potentially enhancing the hospital’s revenue stream.
Stakeholder Analysis
Five specific stakeholders need to be examined to document the value-added by hospitalists. Anecdotal evidence, as well as documented studies, has demonstrated numerous returns—physical, social, psychological and financial—to stakeholders involved in the hospital process. With regard to throughput, the hospitalist provides benefits to each of the stakeholders listed in Table 1.
Study Results
A dozen studies have been conducted that document the impact of hospital medicine programs on cost and clinical outcomes. Of these trials, nine found a significant decrease in the average length of stay (15%) as well as reductions in cost (9). Two other studies, one from an academic medical center and the other from a community teaching hospital, demonstrate similar reductions during a 2-year follow-up period. At the Western Penn Hospital, a 54% reduction in readmissions was reported with a 12% decrease in hospital costs, while the average LOS was 17% shorter. Additionally, an unpublished study from the University of California, San Francisco Medical Center revealed a consistent 10-15% decline in cost and length of stay between hospitalists and non-hospitalist teaching faculty. More important, those differences remained stable through 6 years of follow-up. In general, hospitals with hospitalist programs realized a 5-39% decrease in costs and a shortened average LOS of 7-25% (6).
According to Robert M. Wachter, author of the 2002 study, “If the average U.S. hospitalist cares for 600 inpatients each year and generates a 10% savings over the average medical inpatient cost of $8,000, the nation’s 4500 hospitalists save approximately $2.2 billion per year while potentially improving quality” (6).
In a study conducted by Douglas Gregory, Walter Baigelman, and Ira B. Wilson, hospitalists at Tufts-New England Medical Center in Boston, MA were found to substantially improve throughput with high baseline occupancy levels. Compared with a control group, the hospitalist group reduced LOS from 3.45 days to 2.19 days (p<.001). Additionally, the total cost of hospital admission decreased from $2,332 to $1,775 (p<.001) when hospitalists were involved. According to the study authors, improved throughput generated an incremental 266 patients per year with a related incremental hospital profitability of $1.3 million with the use of hospitalists (7).
Conclusion
As hospital administrators attempt to address the issue of expeditiously admitting, treating and discharging patients in these days of restricted budgets and increased demand, hospitalist programs are poised as an invaluable factor in the throughput process.
Dr. Cawley can be contacted at pcawley@ushosp.com.
References
- Hospital Statistics: the comprehensive reference source for analysis and comparison of hospital trends. Published annually by Health Forum, an affiliate of the American Hospital Association.
- National and local impact of long-term demographic change on inpatient acute care. 2001. Solucient, LLC.
- Zibulewsky J. The Emergency Medical Treatment and Active Labor Act (EMTALA): what it is and what it means for physicians. Baylor University Medical Center (BUMC) Proceedings. 2001;14:339-46.
- Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Eng J Med. 1996;335:514-7.
- Heffner JE. Executive medical director, Medical University of South Carolina (MUSC). Personal interview. June 24, 2004.
- Whitcomb WF. Director, Mercy Inpatient Medicine Service, Mercy Medical Center, Springfield, MA.
- Gregory D, Baigelman W, Wilson IB. Hospital economics of the hospitalist. Health Serv Res. 2003;38:905-18.
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical co-management after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141:28-38.
- Wachter RM. The evolution of the hospitalist model in the United States. Med Clin North Am. 2002;86:687-706.
According to data from the American Hospital Association (1), in 1985, the United States had 5732 operational community hospitals; by 2002, the latest year for which figures are available, the number had decreased to 4927, a loss of approximately 14% (1). In that same timeframe, these hospitals lost approximately 18% of their beds, dropping from just over 1 million to 820,653 beds. This reduction in bed capacity has been accompanied by hospital cost-cutting efforts, staff downsizing, and elimination of services. Many explanations for these trends have been suggested, including changes in Medicare reimbursement and the growth of managed care organizations (MCOs).
However, as the current baby boom generation ages, rising inpatient demands are presenting hospitals with significant challenges. According to a 2001 report from Solucient (2), who maintains the nation’s largest health care database, the senior population—individuals age 65 and older—are projected to experience an 85% growth rate over the next two decades. Since this age group utilizes inpatient services 4.5 times more than younger populations, the number of admissions and beds to accommodate those cases will soar. By the year 2027, hospitals can anticipate a 46% rise in demand for acute inpatient beds as admissions escalate by approximately 13 million cases. Currently, the nation’s healthcare facilities admit 31 million cases; this number will jump to more than 44 million, representing a 41% growth from present admissions figures. For hospitals that maintain an 80% census rate, an additional 238,000 beds will be needed to meet demands (1).
Adding to this increase in demand and pressure on bed capacity, hospitals must address the requirements of the Emergency Medical Treatment and Active Labor Act (EMTALA) passed by the US Congress in 1986 as part of the Consolidated Omnibus Reconciliation Act (COBRA). The law’s initial intent was to ensure patient access to emergency medical care and to prevent the practice of patient dumping, in which uninsured patients were transferred, solely for financial reasons, from private to public hospitals without consideration of their medical condition or stability for the transfer (3). EMTALA mandates that hospitals rank the severity of patients. Thus, tertiary referral centers are required to admit the sickest patients first. This directive presents a significant challenge to many healthcare facilities. High census rates prohibit the admission of elective surgical cases, which, although profitable, are considered second tier. Routine medical cases or complicated emergency surgical cases have the potential to adversely affect the institution’s financial performance.
In addition to the challenge of increased bed demands and EMTALA, hospitals also cite an increasingly smaller number of on-site community physicians. Longstanding trends from inpatient to outpatient care have prompted many community physicians to concentrate their efforts on serving the needs of office-based patients, limiting their accessibility to hospital cases.
To address these pressures, hospitals must execute innovative strategies that deliver efficient throughput and enhance revenue, while still preserving high-quality services. Since 1996, hospital medicine programs have demonstrated a positive impact on the healthcare facility’s ability to increase overall productivity and profitability and still maintain high quality Patients today present to the doctor sicker than in the past and require more careful and frequent outpatient care. Since hospitalists operate solely on an inpatient basis, their availability to efficiently admit and manage hospitalized patients enables delivery of quality care that expedites appropriate treatment and shortens length of stay.
Two Roles of the Hospitalist
According to the Society of Hospital Medicine (SHM), “Hospitalists are physicians whose primary professional focus is the general medical care of hospitalized patients. Their activities include patient care, teaching, research, and leadership related to hospital medicine.” Coined by Drs. Robert Wachter and Lee Goldman in 1996 (4), the term implies an additional point of emphasis. Part of a new paradigm in clinical care, the hospitalist enhances the processes of care surrounding patients and adopts an attitude of accountability for that care. In practice, hospitalists play two key roles.
Primarily, the hospitalist is a practicing clinician — managing throughput on a case-by-case, patient-by-patient basis. In addition, a hospitalist performs a non-clinical role as an “inpatient expert,” taking the lead in creating system changes and communicating those changes to other hospital personnel as well as to community physicians. As an inpatient expert, hospitalists are often asked to lead organization-wide throughput initiatives to identify and implement strategies to facilitate patient flow and efficiency. As dedicated members of multi-disciplinary in-house teams, the hospitalist is in a prime position to foster change and improve systems.
Throughput as Continuum of Care
As suggested by Heffner (5), the process of admission, hospitalization, and discharge resembles a “bell-shaped curve.” To achieve effective throughput, hospitals must expedite patient care and also maintain careful oversight throughout a patient’s entire hospital stay. The hospitalist, as an integral part of a multidisciplinary team, coordinates care to promote a positive outcome and shorten length of stay. Drawing on strong leadership qualities, as well as on intimate knowledge of hospital procedures, layout design and infrastructure, and available community resources, the hospitalist plays a pivotal role in creating efficient throughput from admission to discharge.
Emergency Department
At the front end of the bell-shaped curve, the hospitalist may be engaged by emergency department (ED) physicians to assist in ensuring smooth patient flow and, more important, identifies the “intensity of service” needed. Through the use of clinical criteria, such as lnterQual, the hospitalist, together with the ED physician, may be asked to quantitatively rate the patient’s illness for degree of severity.
Timely patient evaluation helps prevent a backlog of ED cases and enables more patients to be seen. Immediate attention to and initiation of appropriate therapy guarantees a better outcome while minimizing the potential risk for complications, which could possibly lead to longer inpatient stays.
Inpatient Unit
Once a patient has been admitted to an inpatient unit, the hospitalist, together with a multidisciplinary team, facilitates care and determines the inpatient services that will optimize patient recovery through strong interdepartmental communications. Working together with admissions, medical records, nursing, laboratory and diagnostic services, information technology and other pertinent departments, the hospitalist maintains a pulse on all activity surrounding the patient and his care.
Judicious inpatient consultations and treatment decisions result in timely changes in therapy, potentially reducing the length of stay. The frequency with which the hospitalist sees the patient allows him to monitor any changes in condition and reduce possible decompensation, a practice known as vertical continuity (6). Such careful attention may reduce inpatient length of stay significantly. When aggressive management is mandated, the presence of the hospitalist enables initiation of effective therapy and results in quicker discharge and a reduction in potential readmission (7).
Surgery
The surgeon and hospitalist are ideally suited to work together in managing a surgical patient. The hospitalist focuses on the peri-operative management of medical issues and risk reduction, which allows the surgeon to concentrate more on surgical indications and the surgery itself. The hospitalist’s role in the management of a surgical patient enables vertical continuity when the surgeon may be occupied in the operating room with another patient as documented by Huddleston’s Hospitalist Orthopedic Team (HOT) approach (8).
Intensive Care Unit (ICU)
In many hospitals, particularly those that do not have intensivists, hospitalists are able to provide quality care to patients. Even in hospitals where intensivists manage ICU patients, hospitalists work together with the intensivist to ensure smoother transition into and out of the unit.
Discharge
Timing is a critical issue with regard to discharge. Since the hospitalist operates solely in-house and in collaboration with a multidisciplinary team, he is able to round early in the day to discharge patients by mid- or late-morning, freeing a bed for a new patient. In some cases, the hospitalist, in anticipation of early discharge, may begin pre-planning the day prior to discharge, which further expedites the process. Early discharge applies to the ICU, step-down areas and general inpatient care areas, as well as to full discharge from the healthcare facility. Moving a patient from one of these areas enables other patients to fill those empty beds thus optimizing throughput.
Having managed the patient throughout his hospital stay, the hospitalist — again working together with a multidisciplinary team —can facilitate arrangements to send the patient home or to a rehabilitation or skilled nursing facility or alternative housing situation upon discharge, as well as coordinating post-discharge care, whether it be arranging for a visiting nursing or social services or communicating with the primary care physician regarding follow-up appointments. If additional outpatient care is prescribed, the hospitalist will work with the discharge planning staff to contact various community agencies to arrange services best suited to the patient’s needs. Efficient discharge makes possible the admission of other, more critically ill patients, potentially enhancing the hospital’s revenue stream.
Stakeholder Analysis
Five specific stakeholders need to be examined to document the value-added by hospitalists. Anecdotal evidence, as well as documented studies, has demonstrated numerous returns—physical, social, psychological and financial—to stakeholders involved in the hospital process. With regard to throughput, the hospitalist provides benefits to each of the stakeholders listed in Table 1.
Study Results
A dozen studies have been conducted that document the impact of hospital medicine programs on cost and clinical outcomes. Of these trials, nine found a significant decrease in the average length of stay (15%) as well as reductions in cost (9). Two other studies, one from an academic medical center and the other from a community teaching hospital, demonstrate similar reductions during a 2-year follow-up period. At the Western Penn Hospital, a 54% reduction in readmissions was reported with a 12% decrease in hospital costs, while the average LOS was 17% shorter. Additionally, an unpublished study from the University of California, San Francisco Medical Center revealed a consistent 10-15% decline in cost and length of stay between hospitalists and non-hospitalist teaching faculty. More important, those differences remained stable through 6 years of follow-up. In general, hospitals with hospitalist programs realized a 5-39% decrease in costs and a shortened average LOS of 7-25% (6).
According to Robert M. Wachter, author of the 2002 study, “If the average U.S. hospitalist cares for 600 inpatients each year and generates a 10% savings over the average medical inpatient cost of $8,000, the nation’s 4500 hospitalists save approximately $2.2 billion per year while potentially improving quality” (6).
In a study conducted by Douglas Gregory, Walter Baigelman, and Ira B. Wilson, hospitalists at Tufts-New England Medical Center in Boston, MA were found to substantially improve throughput with high baseline occupancy levels. Compared with a control group, the hospitalist group reduced LOS from 3.45 days to 2.19 days (p<.001). Additionally, the total cost of hospital admission decreased from $2,332 to $1,775 (p<.001) when hospitalists were involved. According to the study authors, improved throughput generated an incremental 266 patients per year with a related incremental hospital profitability of $1.3 million with the use of hospitalists (7).
Conclusion
As hospital administrators attempt to address the issue of expeditiously admitting, treating and discharging patients in these days of restricted budgets and increased demand, hospitalist programs are poised as an invaluable factor in the throughput process.
Dr. Cawley can be contacted at pcawley@ushosp.com.
References
- Hospital Statistics: the comprehensive reference source for analysis and comparison of hospital trends. Published annually by Health Forum, an affiliate of the American Hospital Association.
- National and local impact of long-term demographic change on inpatient acute care. 2001. Solucient, LLC.
- Zibulewsky J. The Emergency Medical Treatment and Active Labor Act (EMTALA): what it is and what it means for physicians. Baylor University Medical Center (BUMC) Proceedings. 2001;14:339-46.
- Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Eng J Med. 1996;335:514-7.
- Heffner JE. Executive medical director, Medical University of South Carolina (MUSC). Personal interview. June 24, 2004.
- Whitcomb WF. Director, Mercy Inpatient Medicine Service, Mercy Medical Center, Springfield, MA.
- Gregory D, Baigelman W, Wilson IB. Hospital economics of the hospitalist. Health Serv Res. 2003;38:905-18.
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical co-management after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141:28-38.
- Wachter RM. The evolution of the hospitalist model in the United States. Med Clin North Am. 2002;86:687-706.
Improving Resource Utilization
Today’s hospitals must address a variety of challenges stemming from the expectation to provide more services and better quality with fewer financial, material, and human resources. According to the annual survey conducted by the American Hospital Association (AHA) in 2003, total expenses for all U.S. community hospitals were more than $450 billion. In managing these expenditures, hospitals face the following pressures:
- Cost increases in medical supplies and pharmaceuticals.
- Record shortages of nurses, pharmacists, and technicians.
- A growing uncompensated patient pool.
- Annual potential reductions in Medicare and Medicaid reimbursements.
- Rising bad debt resulting from greater patient responsibility for the cost of care.
- The diversion of more profitable cases to specialty and freestanding ambulatory care facilities and surgery centers.
- Soaring costs associated with adequately serving high-risk conditions, such as cancer, heart disease, and HIV/AIDS.
- Discounted reimbursement rates with insurers.
- Increasing pressure to commit financial resources to clinical information technology.
- The need to fund infrastructure improvements and physical plant renovations as well as expansions to address increasing demand (1).
To overcome these challenges, hospitals must find innovative ways to balance revenues and expenses, fund necessary capital investments, and satisfy the public’s demand for quality, safety, and accessibility.
Hospitalist Programs: A Good Investment
One solution to the above-mentioned situations is a hospitalist program, which, in its short history, has already had a profound impact on inpatient care. Robert M. Wachter, MD, associate chair in the department of medicine at the University of California, San Francisco (UCSF) and medical service chief at Moffitt-Long Hospitals, coined the term Hospitalist in an article in the New England Journal of Medicine in 1996 (2). At the 2002 annual meeting of the Society of Hospital Medicine (SHM), Wachter presented findings from a study conducted at his institution. The results demonstrate a significant return on investment (ROI) of 5.8:1 when a hospitalist program is utilized (See Table 1 for details) (3).
How do hospitalists reduce length of stay (LOS) and cost per stay? William David Rifkin, MD, associate director of the Yale Primary Care Residency Program, offers three basic reasons why hospitalist programs contribute to effective and efficient use of resources. Since hospitalists are physically onsite, they are better able to react to condition changes and requests for consultations in a timely manner, he asserts. Also, being familiar with the hospital’s systems of care, the hospitalist knows who to call and how to utilize the services of social workers and other contingency staff when arranging for post-discharge care. Third, Rifkin indicates that inpatients today are sicker than they were in past years, a fact well known and understood by hospitalists. “There is an increased level of acuity,” he says. “Hospitalists are used to seeing these kinds of patients. They are more comfortable taking care of these patients and will see more of them with any given diagnosis” (4).
In one of his studies, Rifkin noted a reduction in LOS for inpatients with a pneumonia diagnosis. “The hospitalist had switched the patient from IV (intravenous) to oral antibiotics,” he says. Reacting quickly to indications that the patient was ready for a change in treatment modality facilitated an earlier discharge (5).
L. Craig Miller, MD, senior vice president of medical affairs at Baptist Health Care, reports that his hospital saved $2.56 million in 2 years as a direct result of its inpatient management program (6). Although attention to technical and clinical details is important, Miller emphasizes the critical role the human factor plays, specifically the impact of teamwork, on achieving resource utilization savings.
“Hospitalists work as a team, collaborating with physicians and ED doctors,” he says. This cooperative spirit enables the efficient use of manpower in patient care. Miller adds that at Baptist, as is the case at most hospitals, the medical complexity of patients dictates a need for cooperation in order to successfully treat illness. The presence of hospitalists facilitates the team effort, causing a positive trickle down effect regarding LOS, readmission and mortality rates, he affirms. “The hospitalist provides focused leadership to utilization resource management,” says Miller (7).
In the role of inpatient leader, the hospitalist also facilitates ED throughput, which results in another area of cost savings for the hospital. Paola Coppola, MD, ED director at Brookhaven Memorial Hospital Medical Center, says, “From an ER perspective, a call to the hospitalist replaces multiple calls to specialists. In general, hospitalists feel much more comfortable treating a wide array of conditions including infectious disease, pneumonias, strokes, and chest pain without the intervention of specialists in that field. Hence, hospital consumption of resources decreases, which in turn lowers length of stay.” He echoes Rifkin’s thoughts on quick response time. “Hospitalists provide an immediately available service, thus saving ER physicians valuable time. This ensures faster turnover, better throughput, makes more ER beds available, and services more patients, eventually helping the hospital’s bottom line,” says Coppola (8).
In addition to teamwork, 24/7 availability is vital to the wise utilization of resources, according to Anthony Shallash, MD, vice president of medical affairs at Brookhaven. “The fact of 24/7 presence allows rapid responses to patient condition and problems. Continuous and close monitoring of patients allows them to be upgraded or downgraded as needed,” he says. “As such, LOS is decreased and quite favorable as compared to peer practitioners for similar disease severity. Resources consumed and tests ordered also show a favorable trend” (9).
A recently published study (10) by researchers at Dartmouth Medical School documents the variation in the volume and cost of services that academic medical centers use in treating patients. Hospitals were categorized as low- and high-intensity, with significant differences in cost per case. For example, the high-intensity hospitals spent up to 47% more on care for acute myocardial infarction. In an interview in Today’s Hospitalist (11), the lead author, Elliott S. Fisher, MD, professor of medicine and community and family medicine at Dartmouth Medical School, described the importance of coordination in achieving efficient care. Fisher says, “I think there’s a real opportunity for hospitalists to improve the care of patients in both high- and low- intensity hospitals. Having ten doctors involved in a given patient’s care may not be a good thing, unless someone [i.e., the hospitalist] is doing a really good job of coordinating that care.”
Hospitalists focus only on inpatient medicine. They are familiar with managing the most common medical diagnoses, such as community acquired pneumonia, diabetes. and congestive heart failure. Hospitalist programs often develop uniform and consistent ways of treating these patients. Cogent Healthcare, a national hospitalist management company has implemented the “Cogent Care Guides,” best practice guidelines for high-volume hospital diagnoses. Ron Greeno, MD, FCCP and Cogent’s chief medical officer, says “The Cogent Care Guides ensure best practices are implemented at critical points in the patient’s care… decreasing the variability of care that results in inefficiencies.” Greeno added, “The care guidelines [also] support the timely notification of the primary care physician of nine critical landmark events related to patient status that can affect outcomes” (12).
Stacy Goldsholl, Director of the Covenant HealthCare Hospital Medicine Program in Saginaw, MI, suggests other ways that hospitalists can generate utilization savings for their hospitals. “Hospitalists often eliminate unnecessary admissions and shift work-ups to the ambulatory setting. For example, I recently arranged an outpatient colonoscopy for a pneumonia patient with a stable hemoglobin and heme positive stool. Because of my experience treating patients with pneumonia, I was able to determine that the circumstances did not require an inpatient stay.” In addition, Dr. Goldsholl has found that the hospitalists in her program are quite effective in classifying “observation” patients, eliminating reimbursement conflicts with Medicare, Medicaid, and other insurers.
Finally, because they are always in the hospital rather than sharing time between the office and hospital, hospitalists can improve inpatient continuity of care, resulting in lower costs and better outcomes. Adrienne Bennett, MD, chief of the hospital medicine service at Newton-Wellesley Hospital near Boston, examined cases managed by hospitalists and non-hospitalist community physicians, comparing the number of “handoffs” of responsibility that occur among attending physicians. Community physicians share inpatient responsibility in their practices and sometimes their partners round on their patients. Every time another physician assumes responsibility for a patient, there is the potential for a loss of information and a discontinuity of care. At Newton-Wellesley Hospital, the hospitalists work a schedule of 14 days on, followed by 7 days off. “We found that hospitalists averaged less than half the number of handoffs as the community physicians,” says Bennett. “This may be one of the reasons that hospitalists have better case mix adjusted utilization performance.”
Stakeholder Analysis
Anecdotal evidence, as well as documented studies, has demonstrated that hospitalists provide value to a wide range of stakeholders involved in the inpatient care process. With regard to resource utilization savings, the hospitalist provides benefits to each of the listed stakeholders (Table 2).
Published Research Results
Dozens of studies demonstrate the positive effects hospitalist programs have on resource utilization. Observational, retrospective, and prospective data analyses have been conducted at community-based hospitals as well as at academic medical institutions. Findings consistently indicate that hospitalist programs result in resource savings for patients, physicians. and hospital medicine. A range of studies shown in Table 3 represent the most recent efforts at tracking hospitalist programs and their effects on resource utilization.
Conclusion
According to the AHA’s 2003 survey of healthcare trends, the fiscal health of the nation’s hospitals will most likely remain fragile and variable in the coming years. The survey cites declining operating margins, a continued decrease in reimbursement, labor shortages, and rising insurance and pharmaceutical costs, as well as the need to invest in technology and facility maintenance and upkeep as key factors. However, hospitalists have proven time and again in clinical studies that they can bring value to the operation of a healthcare facility. With reduced lengths of stay, decreased overall hospital costs, and equivalent — if not superior — quality, hospitalists can contribute significantly to a hospital’s healthy bottom line.
Dr. Syed can be contacted at syed.saeed@CogentHealthcare.com.
References
- ACP Research Center, Environmental Assessment: Trends in hospital financing. 2003. www.aha.org.
- Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335:514-7
- Wachter RM. Presentation, Society of Hospital Medicine (SHM) annual meeting 2002. 4. Rifkin WD. Telephone interview December 15, 2004.
- Rifkin WD, Conner D, Silver A, Eichom A. Comparison of processes and outcomes of pneumonia care between hospitalists and community-based primary care physicians. Mayo Clin Proc. 2002;77:1053-8.
- “Hospitalists save $2.5 million and decrease LOS.” Healthcare Benchmarks and Quality Improvement, May 2004.
- Miller LC. Telephone intewiew, November 16, 2004.
- Coppola P. Email interview, December 15,2004.
- Shallash A. Email interview, December17, 2004.
- Healthaffairs.org, “Use of Medicare claims data to monitor provider-specific performance among patients with severe chronic illness.” 10.1377/hlthaff.var.5. Posting date: October 7, 2004.
- Why less really can be more when it comes to teaching hospitals. Today’s Hospitalist. 2004 December.
- Greeno R. Chief medical officer, Cogent Healthcare, Irvine, California. Telephone interview. December 16, 2004.
- Everett GD, Anton MP Jackson BK, Swigert C, Uddin N. Comparison of hospital costs and length of stay associated with general internists and hospitalist physicians at a community hospital. Am J Manag Care. 2004;10:626-30.
- Kaboli PJ, Barnett MJ, Rosenthal GE. Associations with reduced length of stay and costs on an academic hospitalist service. Am J Manag Care. 2004;10:561-8.
- Gregory D, Baigelman W, Wilson IB. Hospital economics of the hospitalist. Health Services Research. 2003:38(3):905-18; discussion 919-22.
- Wachter RM, Goldman L The hospitalist movement 5 years later. JAMA. 2002;287:487-94.
- Palmer HC, Armistead NS, Elnicki DM, et al. The effect of a hospitalist service with nurse discharge planner on patient care in an academic teaching hospital. Am J Med. 2001;111:627-32.
Today’s hospitals must address a variety of challenges stemming from the expectation to provide more services and better quality with fewer financial, material, and human resources. According to the annual survey conducted by the American Hospital Association (AHA) in 2003, total expenses for all U.S. community hospitals were more than $450 billion. In managing these expenditures, hospitals face the following pressures:
- Cost increases in medical supplies and pharmaceuticals.
- Record shortages of nurses, pharmacists, and technicians.
- A growing uncompensated patient pool.
- Annual potential reductions in Medicare and Medicaid reimbursements.
- Rising bad debt resulting from greater patient responsibility for the cost of care.
- The diversion of more profitable cases to specialty and freestanding ambulatory care facilities and surgery centers.
- Soaring costs associated with adequately serving high-risk conditions, such as cancer, heart disease, and HIV/AIDS.
- Discounted reimbursement rates with insurers.
- Increasing pressure to commit financial resources to clinical information technology.
- The need to fund infrastructure improvements and physical plant renovations as well as expansions to address increasing demand (1).
To overcome these challenges, hospitals must find innovative ways to balance revenues and expenses, fund necessary capital investments, and satisfy the public’s demand for quality, safety, and accessibility.
Hospitalist Programs: A Good Investment
One solution to the above-mentioned situations is a hospitalist program, which, in its short history, has already had a profound impact on inpatient care. Robert M. Wachter, MD, associate chair in the department of medicine at the University of California, San Francisco (UCSF) and medical service chief at Moffitt-Long Hospitals, coined the term Hospitalist in an article in the New England Journal of Medicine in 1996 (2). At the 2002 annual meeting of the Society of Hospital Medicine (SHM), Wachter presented findings from a study conducted at his institution. The results demonstrate a significant return on investment (ROI) of 5.8:1 when a hospitalist program is utilized (See Table 1 for details) (3).
How do hospitalists reduce length of stay (LOS) and cost per stay? William David Rifkin, MD, associate director of the Yale Primary Care Residency Program, offers three basic reasons why hospitalist programs contribute to effective and efficient use of resources. Since hospitalists are physically onsite, they are better able to react to condition changes and requests for consultations in a timely manner, he asserts. Also, being familiar with the hospital’s systems of care, the hospitalist knows who to call and how to utilize the services of social workers and other contingency staff when arranging for post-discharge care. Third, Rifkin indicates that inpatients today are sicker than they were in past years, a fact well known and understood by hospitalists. “There is an increased level of acuity,” he says. “Hospitalists are used to seeing these kinds of patients. They are more comfortable taking care of these patients and will see more of them with any given diagnosis” (4).
In one of his studies, Rifkin noted a reduction in LOS for inpatients with a pneumonia diagnosis. “The hospitalist had switched the patient from IV (intravenous) to oral antibiotics,” he says. Reacting quickly to indications that the patient was ready for a change in treatment modality facilitated an earlier discharge (5).
L. Craig Miller, MD, senior vice president of medical affairs at Baptist Health Care, reports that his hospital saved $2.56 million in 2 years as a direct result of its inpatient management program (6). Although attention to technical and clinical details is important, Miller emphasizes the critical role the human factor plays, specifically the impact of teamwork, on achieving resource utilization savings.
“Hospitalists work as a team, collaborating with physicians and ED doctors,” he says. This cooperative spirit enables the efficient use of manpower in patient care. Miller adds that at Baptist, as is the case at most hospitals, the medical complexity of patients dictates a need for cooperation in order to successfully treat illness. The presence of hospitalists facilitates the team effort, causing a positive trickle down effect regarding LOS, readmission and mortality rates, he affirms. “The hospitalist provides focused leadership to utilization resource management,” says Miller (7).
In the role of inpatient leader, the hospitalist also facilitates ED throughput, which results in another area of cost savings for the hospital. Paola Coppola, MD, ED director at Brookhaven Memorial Hospital Medical Center, says, “From an ER perspective, a call to the hospitalist replaces multiple calls to specialists. In general, hospitalists feel much more comfortable treating a wide array of conditions including infectious disease, pneumonias, strokes, and chest pain without the intervention of specialists in that field. Hence, hospital consumption of resources decreases, which in turn lowers length of stay.” He echoes Rifkin’s thoughts on quick response time. “Hospitalists provide an immediately available service, thus saving ER physicians valuable time. This ensures faster turnover, better throughput, makes more ER beds available, and services more patients, eventually helping the hospital’s bottom line,” says Coppola (8).
In addition to teamwork, 24/7 availability is vital to the wise utilization of resources, according to Anthony Shallash, MD, vice president of medical affairs at Brookhaven. “The fact of 24/7 presence allows rapid responses to patient condition and problems. Continuous and close monitoring of patients allows them to be upgraded or downgraded as needed,” he says. “As such, LOS is decreased and quite favorable as compared to peer practitioners for similar disease severity. Resources consumed and tests ordered also show a favorable trend” (9).
A recently published study (10) by researchers at Dartmouth Medical School documents the variation in the volume and cost of services that academic medical centers use in treating patients. Hospitals were categorized as low- and high-intensity, with significant differences in cost per case. For example, the high-intensity hospitals spent up to 47% more on care for acute myocardial infarction. In an interview in Today’s Hospitalist (11), the lead author, Elliott S. Fisher, MD, professor of medicine and community and family medicine at Dartmouth Medical School, described the importance of coordination in achieving efficient care. Fisher says, “I think there’s a real opportunity for hospitalists to improve the care of patients in both high- and low- intensity hospitals. Having ten doctors involved in a given patient’s care may not be a good thing, unless someone [i.e., the hospitalist] is doing a really good job of coordinating that care.”
Hospitalists focus only on inpatient medicine. They are familiar with managing the most common medical diagnoses, such as community acquired pneumonia, diabetes. and congestive heart failure. Hospitalist programs often develop uniform and consistent ways of treating these patients. Cogent Healthcare, a national hospitalist management company has implemented the “Cogent Care Guides,” best practice guidelines for high-volume hospital diagnoses. Ron Greeno, MD, FCCP and Cogent’s chief medical officer, says “The Cogent Care Guides ensure best practices are implemented at critical points in the patient’s care… decreasing the variability of care that results in inefficiencies.” Greeno added, “The care guidelines [also] support the timely notification of the primary care physician of nine critical landmark events related to patient status that can affect outcomes” (12).
Stacy Goldsholl, Director of the Covenant HealthCare Hospital Medicine Program in Saginaw, MI, suggests other ways that hospitalists can generate utilization savings for their hospitals. “Hospitalists often eliminate unnecessary admissions and shift work-ups to the ambulatory setting. For example, I recently arranged an outpatient colonoscopy for a pneumonia patient with a stable hemoglobin and heme positive stool. Because of my experience treating patients with pneumonia, I was able to determine that the circumstances did not require an inpatient stay.” In addition, Dr. Goldsholl has found that the hospitalists in her program are quite effective in classifying “observation” patients, eliminating reimbursement conflicts with Medicare, Medicaid, and other insurers.
Finally, because they are always in the hospital rather than sharing time between the office and hospital, hospitalists can improve inpatient continuity of care, resulting in lower costs and better outcomes. Adrienne Bennett, MD, chief of the hospital medicine service at Newton-Wellesley Hospital near Boston, examined cases managed by hospitalists and non-hospitalist community physicians, comparing the number of “handoffs” of responsibility that occur among attending physicians. Community physicians share inpatient responsibility in their practices and sometimes their partners round on their patients. Every time another physician assumes responsibility for a patient, there is the potential for a loss of information and a discontinuity of care. At Newton-Wellesley Hospital, the hospitalists work a schedule of 14 days on, followed by 7 days off. “We found that hospitalists averaged less than half the number of handoffs as the community physicians,” says Bennett. “This may be one of the reasons that hospitalists have better case mix adjusted utilization performance.”
Stakeholder Analysis
Anecdotal evidence, as well as documented studies, has demonstrated that hospitalists provide value to a wide range of stakeholders involved in the inpatient care process. With regard to resource utilization savings, the hospitalist provides benefits to each of the listed stakeholders (Table 2).
Published Research Results
Dozens of studies demonstrate the positive effects hospitalist programs have on resource utilization. Observational, retrospective, and prospective data analyses have been conducted at community-based hospitals as well as at academic medical institutions. Findings consistently indicate that hospitalist programs result in resource savings for patients, physicians. and hospital medicine. A range of studies shown in Table 3 represent the most recent efforts at tracking hospitalist programs and their effects on resource utilization.
Conclusion
According to the AHA’s 2003 survey of healthcare trends, the fiscal health of the nation’s hospitals will most likely remain fragile and variable in the coming years. The survey cites declining operating margins, a continued decrease in reimbursement, labor shortages, and rising insurance and pharmaceutical costs, as well as the need to invest in technology and facility maintenance and upkeep as key factors. However, hospitalists have proven time and again in clinical studies that they can bring value to the operation of a healthcare facility. With reduced lengths of stay, decreased overall hospital costs, and equivalent — if not superior — quality, hospitalists can contribute significantly to a hospital’s healthy bottom line.
Dr. Syed can be contacted at syed.saeed@CogentHealthcare.com.
References
- ACP Research Center, Environmental Assessment: Trends in hospital financing. 2003. www.aha.org.
- Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335:514-7
- Wachter RM. Presentation, Society of Hospital Medicine (SHM) annual meeting 2002. 4. Rifkin WD. Telephone interview December 15, 2004.
- Rifkin WD, Conner D, Silver A, Eichom A. Comparison of processes and outcomes of pneumonia care between hospitalists and community-based primary care physicians. Mayo Clin Proc. 2002;77:1053-8.
- “Hospitalists save $2.5 million and decrease LOS.” Healthcare Benchmarks and Quality Improvement, May 2004.
- Miller LC. Telephone intewiew, November 16, 2004.
- Coppola P. Email interview, December 15,2004.
- Shallash A. Email interview, December17, 2004.
- Healthaffairs.org, “Use of Medicare claims data to monitor provider-specific performance among patients with severe chronic illness.” 10.1377/hlthaff.var.5. Posting date: October 7, 2004.
- Why less really can be more when it comes to teaching hospitals. Today’s Hospitalist. 2004 December.
- Greeno R. Chief medical officer, Cogent Healthcare, Irvine, California. Telephone interview. December 16, 2004.
- Everett GD, Anton MP Jackson BK, Swigert C, Uddin N. Comparison of hospital costs and length of stay associated with general internists and hospitalist physicians at a community hospital. Am J Manag Care. 2004;10:626-30.
- Kaboli PJ, Barnett MJ, Rosenthal GE. Associations with reduced length of stay and costs on an academic hospitalist service. Am J Manag Care. 2004;10:561-8.
- Gregory D, Baigelman W, Wilson IB. Hospital economics of the hospitalist. Health Services Research. 2003:38(3):905-18; discussion 919-22.
- Wachter RM, Goldman L The hospitalist movement 5 years later. JAMA. 2002;287:487-94.
- Palmer HC, Armistead NS, Elnicki DM, et al. The effect of a hospitalist service with nurse discharge planner on patient care in an academic teaching hospital. Am J Med. 2001;111:627-32.
Today’s hospitals must address a variety of challenges stemming from the expectation to provide more services and better quality with fewer financial, material, and human resources. According to the annual survey conducted by the American Hospital Association (AHA) in 2003, total expenses for all U.S. community hospitals were more than $450 billion. In managing these expenditures, hospitals face the following pressures:
- Cost increases in medical supplies and pharmaceuticals.
- Record shortages of nurses, pharmacists, and technicians.
- A growing uncompensated patient pool.
- Annual potential reductions in Medicare and Medicaid reimbursements.
- Rising bad debt resulting from greater patient responsibility for the cost of care.
- The diversion of more profitable cases to specialty and freestanding ambulatory care facilities and surgery centers.
- Soaring costs associated with adequately serving high-risk conditions, such as cancer, heart disease, and HIV/AIDS.
- Discounted reimbursement rates with insurers.
- Increasing pressure to commit financial resources to clinical information technology.
- The need to fund infrastructure improvements and physical plant renovations as well as expansions to address increasing demand (1).
To overcome these challenges, hospitals must find innovative ways to balance revenues and expenses, fund necessary capital investments, and satisfy the public’s demand for quality, safety, and accessibility.
Hospitalist Programs: A Good Investment
One solution to the above-mentioned situations is a hospitalist program, which, in its short history, has already had a profound impact on inpatient care. Robert M. Wachter, MD, associate chair in the department of medicine at the University of California, San Francisco (UCSF) and medical service chief at Moffitt-Long Hospitals, coined the term Hospitalist in an article in the New England Journal of Medicine in 1996 (2). At the 2002 annual meeting of the Society of Hospital Medicine (SHM), Wachter presented findings from a study conducted at his institution. The results demonstrate a significant return on investment (ROI) of 5.8:1 when a hospitalist program is utilized (See Table 1 for details) (3).
How do hospitalists reduce length of stay (LOS) and cost per stay? William David Rifkin, MD, associate director of the Yale Primary Care Residency Program, offers three basic reasons why hospitalist programs contribute to effective and efficient use of resources. Since hospitalists are physically onsite, they are better able to react to condition changes and requests for consultations in a timely manner, he asserts. Also, being familiar with the hospital’s systems of care, the hospitalist knows who to call and how to utilize the services of social workers and other contingency staff when arranging for post-discharge care. Third, Rifkin indicates that inpatients today are sicker than they were in past years, a fact well known and understood by hospitalists. “There is an increased level of acuity,” he says. “Hospitalists are used to seeing these kinds of patients. They are more comfortable taking care of these patients and will see more of them with any given diagnosis” (4).
In one of his studies, Rifkin noted a reduction in LOS for inpatients with a pneumonia diagnosis. “The hospitalist had switched the patient from IV (intravenous) to oral antibiotics,” he says. Reacting quickly to indications that the patient was ready for a change in treatment modality facilitated an earlier discharge (5).
L. Craig Miller, MD, senior vice president of medical affairs at Baptist Health Care, reports that his hospital saved $2.56 million in 2 years as a direct result of its inpatient management program (6). Although attention to technical and clinical details is important, Miller emphasizes the critical role the human factor plays, specifically the impact of teamwork, on achieving resource utilization savings.
“Hospitalists work as a team, collaborating with physicians and ED doctors,” he says. This cooperative spirit enables the efficient use of manpower in patient care. Miller adds that at Baptist, as is the case at most hospitals, the medical complexity of patients dictates a need for cooperation in order to successfully treat illness. The presence of hospitalists facilitates the team effort, causing a positive trickle down effect regarding LOS, readmission and mortality rates, he affirms. “The hospitalist provides focused leadership to utilization resource management,” says Miller (7).
In the role of inpatient leader, the hospitalist also facilitates ED throughput, which results in another area of cost savings for the hospital. Paola Coppola, MD, ED director at Brookhaven Memorial Hospital Medical Center, says, “From an ER perspective, a call to the hospitalist replaces multiple calls to specialists. In general, hospitalists feel much more comfortable treating a wide array of conditions including infectious disease, pneumonias, strokes, and chest pain without the intervention of specialists in that field. Hence, hospital consumption of resources decreases, which in turn lowers length of stay.” He echoes Rifkin’s thoughts on quick response time. “Hospitalists provide an immediately available service, thus saving ER physicians valuable time. This ensures faster turnover, better throughput, makes more ER beds available, and services more patients, eventually helping the hospital’s bottom line,” says Coppola (8).
In addition to teamwork, 24/7 availability is vital to the wise utilization of resources, according to Anthony Shallash, MD, vice president of medical affairs at Brookhaven. “The fact of 24/7 presence allows rapid responses to patient condition and problems. Continuous and close monitoring of patients allows them to be upgraded or downgraded as needed,” he says. “As such, LOS is decreased and quite favorable as compared to peer practitioners for similar disease severity. Resources consumed and tests ordered also show a favorable trend” (9).
A recently published study (10) by researchers at Dartmouth Medical School documents the variation in the volume and cost of services that academic medical centers use in treating patients. Hospitals were categorized as low- and high-intensity, with significant differences in cost per case. For example, the high-intensity hospitals spent up to 47% more on care for acute myocardial infarction. In an interview in Today’s Hospitalist (11), the lead author, Elliott S. Fisher, MD, professor of medicine and community and family medicine at Dartmouth Medical School, described the importance of coordination in achieving efficient care. Fisher says, “I think there’s a real opportunity for hospitalists to improve the care of patients in both high- and low- intensity hospitals. Having ten doctors involved in a given patient’s care may not be a good thing, unless someone [i.e., the hospitalist] is doing a really good job of coordinating that care.”
Hospitalists focus only on inpatient medicine. They are familiar with managing the most common medical diagnoses, such as community acquired pneumonia, diabetes. and congestive heart failure. Hospitalist programs often develop uniform and consistent ways of treating these patients. Cogent Healthcare, a national hospitalist management company has implemented the “Cogent Care Guides,” best practice guidelines for high-volume hospital diagnoses. Ron Greeno, MD, FCCP and Cogent’s chief medical officer, says “The Cogent Care Guides ensure best practices are implemented at critical points in the patient’s care… decreasing the variability of care that results in inefficiencies.” Greeno added, “The care guidelines [also] support the timely notification of the primary care physician of nine critical landmark events related to patient status that can affect outcomes” (12).
Stacy Goldsholl, Director of the Covenant HealthCare Hospital Medicine Program in Saginaw, MI, suggests other ways that hospitalists can generate utilization savings for their hospitals. “Hospitalists often eliminate unnecessary admissions and shift work-ups to the ambulatory setting. For example, I recently arranged an outpatient colonoscopy for a pneumonia patient with a stable hemoglobin and heme positive stool. Because of my experience treating patients with pneumonia, I was able to determine that the circumstances did not require an inpatient stay.” In addition, Dr. Goldsholl has found that the hospitalists in her program are quite effective in classifying “observation” patients, eliminating reimbursement conflicts with Medicare, Medicaid, and other insurers.
Finally, because they are always in the hospital rather than sharing time between the office and hospital, hospitalists can improve inpatient continuity of care, resulting in lower costs and better outcomes. Adrienne Bennett, MD, chief of the hospital medicine service at Newton-Wellesley Hospital near Boston, examined cases managed by hospitalists and non-hospitalist community physicians, comparing the number of “handoffs” of responsibility that occur among attending physicians. Community physicians share inpatient responsibility in their practices and sometimes their partners round on their patients. Every time another physician assumes responsibility for a patient, there is the potential for a loss of information and a discontinuity of care. At Newton-Wellesley Hospital, the hospitalists work a schedule of 14 days on, followed by 7 days off. “We found that hospitalists averaged less than half the number of handoffs as the community physicians,” says Bennett. “This may be one of the reasons that hospitalists have better case mix adjusted utilization performance.”
Stakeholder Analysis
Anecdotal evidence, as well as documented studies, has demonstrated that hospitalists provide value to a wide range of stakeholders involved in the inpatient care process. With regard to resource utilization savings, the hospitalist provides benefits to each of the listed stakeholders (Table 2).
Published Research Results
Dozens of studies demonstrate the positive effects hospitalist programs have on resource utilization. Observational, retrospective, and prospective data analyses have been conducted at community-based hospitals as well as at academic medical institutions. Findings consistently indicate that hospitalist programs result in resource savings for patients, physicians. and hospital medicine. A range of studies shown in Table 3 represent the most recent efforts at tracking hospitalist programs and their effects on resource utilization.
Conclusion
According to the AHA’s 2003 survey of healthcare trends, the fiscal health of the nation’s hospitals will most likely remain fragile and variable in the coming years. The survey cites declining operating margins, a continued decrease in reimbursement, labor shortages, and rising insurance and pharmaceutical costs, as well as the need to invest in technology and facility maintenance and upkeep as key factors. However, hospitalists have proven time and again in clinical studies that they can bring value to the operation of a healthcare facility. With reduced lengths of stay, decreased overall hospital costs, and equivalent — if not superior — quality, hospitalists can contribute significantly to a hospital’s healthy bottom line.
Dr. Syed can be contacted at syed.saeed@CogentHealthcare.com.
References
- ACP Research Center, Environmental Assessment: Trends in hospital financing. 2003. www.aha.org.
- Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335:514-7
- Wachter RM. Presentation, Society of Hospital Medicine (SHM) annual meeting 2002. 4. Rifkin WD. Telephone interview December 15, 2004.
- Rifkin WD, Conner D, Silver A, Eichom A. Comparison of processes and outcomes of pneumonia care between hospitalists and community-based primary care physicians. Mayo Clin Proc. 2002;77:1053-8.
- “Hospitalists save $2.5 million and decrease LOS.” Healthcare Benchmarks and Quality Improvement, May 2004.
- Miller LC. Telephone intewiew, November 16, 2004.
- Coppola P. Email interview, December 15,2004.
- Shallash A. Email interview, December17, 2004.
- Healthaffairs.org, “Use of Medicare claims data to monitor provider-specific performance among patients with severe chronic illness.” 10.1377/hlthaff.var.5. Posting date: October 7, 2004.
- Why less really can be more when it comes to teaching hospitals. Today’s Hospitalist. 2004 December.
- Greeno R. Chief medical officer, Cogent Healthcare, Irvine, California. Telephone interview. December 16, 2004.
- Everett GD, Anton MP Jackson BK, Swigert C, Uddin N. Comparison of hospital costs and length of stay associated with general internists and hospitalist physicians at a community hospital. Am J Manag Care. 2004;10:626-30.
- Kaboli PJ, Barnett MJ, Rosenthal GE. Associations with reduced length of stay and costs on an academic hospitalist service. Am J Manag Care. 2004;10:561-8.
- Gregory D, Baigelman W, Wilson IB. Hospital economics of the hospitalist. Health Services Research. 2003:38(3):905-18; discussion 919-22.
- Wachter RM, Goldman L The hospitalist movement 5 years later. JAMA. 2002;287:487-94.
- Palmer HC, Armistead NS, Elnicki DM, et al. The effect of a hospitalist service with nurse discharge planner on patient care in an academic teaching hospital. Am J Med. 2001;111:627-32.