User login
Scholarly Productivity and Rank in Academic Hospital Medicine
Hospital medicine has grown rapidly, with more than 50,000 hospitalists practicing nationally in 2016.1 Despite the remarkable increase in academic hospital medicine faculty (AHMF), scholarly productivity remains underdeveloped. Prior evidence suggests peer-reviewed publications remain an important aspect of promotion in academic hospital medicine.2 However, there are multiple barriers to robust scholarly productivity among AHMF, including inadequate mentorship,3 lack of protected scholarship time,4 and greater participation in nonclinical activities outside of peer-reviewed clinical research.5 Though research barriers have been described previously, the current state of scholarly productivity among AHMF has not been characterized. In this cross-sectional study, we describe the distribution of academic rank and scholarly output of a national sample of AHMF.
METHODS
Study Design and Data Source
We performed a cross-sectional study of AHMF at the top 25 internal medicine residency programs as determined by Doximity.com as of February 1, 2020 (Appendix Table 1). Between March and August 2020, two authors (NS, MT) visited each residency program’s website, identified all faculty listed as members of the hospital medicine program, and extracted demographic data, including degrees, sex, residency, medical school, year of residency graduation, completion of chief residency, completion of fellowship, and rank. We categorized all academic titles into full professor, associate professor, assistant professor, and instructor/lecturer. Missing information was supplemented by searching state licensing websites and Doximity.com. Sex was validated using Genderize.io. We queried the Scopus database for each AHMF’s name and affiliated institution to extract publications, citations, and H-index (metric of productivity and impact, derived from the number of publications and their associated citations).6 We categorized medical schools by rank (top 25, top 50, or unranked), as defined by the 2020 US News Best Medical Schools, sorted by research7 and by location (United States, international Caribbean, and international non-Caribbean). We excluded programs without hospital medicine section/division webpages and AHMF with nonpromotion titles such as “adjunct professor” or “acting professor” or those with missing data that could not be identified using these methods.
Analysis
Summary statistics were generated using means with standard deviations and medians with interquartile ranges. We evaluated postresidency years 6 to 10 and 14 to 18 as conservative time frames for promotion to associate and full professor, respectively. These windows account for time spent for additional degrees, instructor years, and alternative career pathways. Demographic differences between academic ranks were determined using chi-square and Kruskal-Wallis analyses.
Because promotion occurs sequentially, a proportional odds logistic regression model was used to evaluate the association of academic rank and H-index, number of years post residency, completion of chief residency, graduation from a top 25 medical school, and sex. Since not all programs have the instructor/lecturer rank, only assistant, associate, and full professors were included in this model. Significance was assessed with the likelihood ratio test. The proportional odds assumption was assessed using the score test. All adjusted odds ratios and their associated 95% confidence intervals were recorded. A two-tailed P value < .05 was considered significant for this study, and SAS version 9.4 (SAS Institute Inc) was used to conduct all analyses. This study was approved by the UT Southwestern Institutional Review Board.
RESULTS
Cohort Demographics
Of the top 25 internal medicine programs, 3 were excluded because they did not have websites that listed AHMF. Of the remaining 22 programs, we identified 1,829 AHMF. We excluded 166 AHMF because we could not identify title or year of residency graduation and 109 for having nonpromotion titles, leaving 1,554 AHMF (Appendix Figure). The cohort characteristics are described in Table 1. 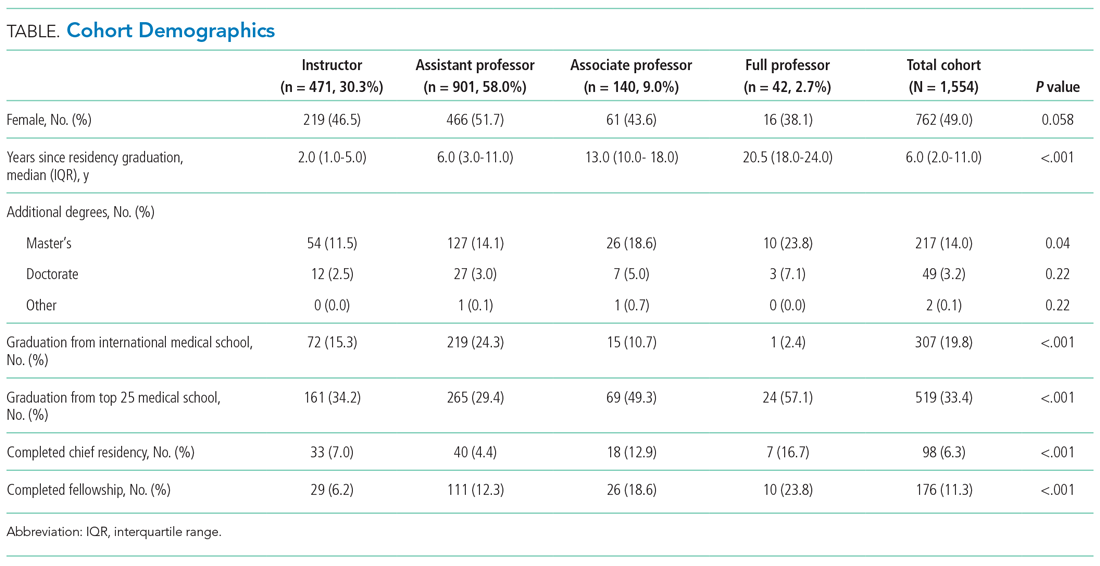
Research Productivity
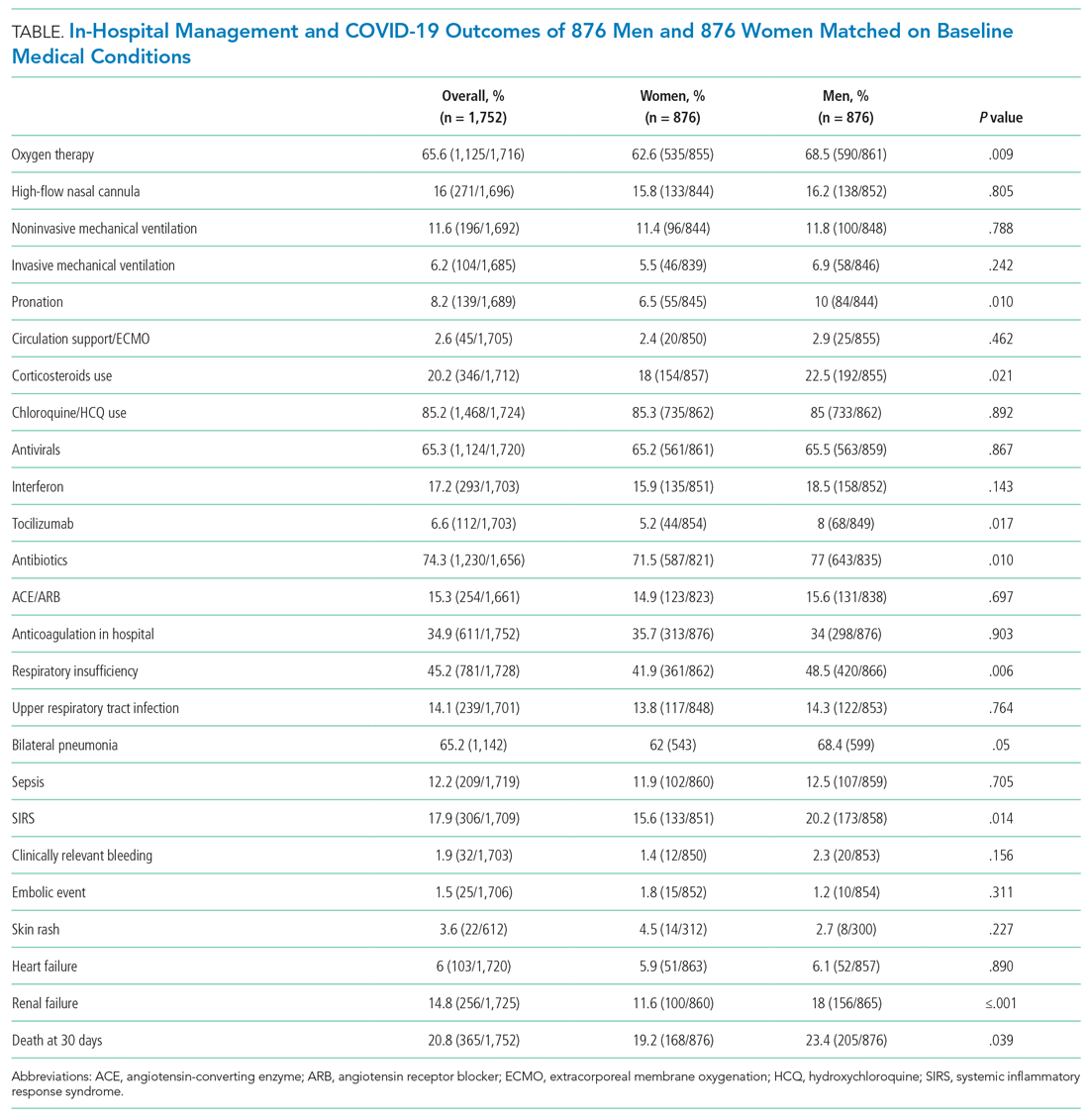
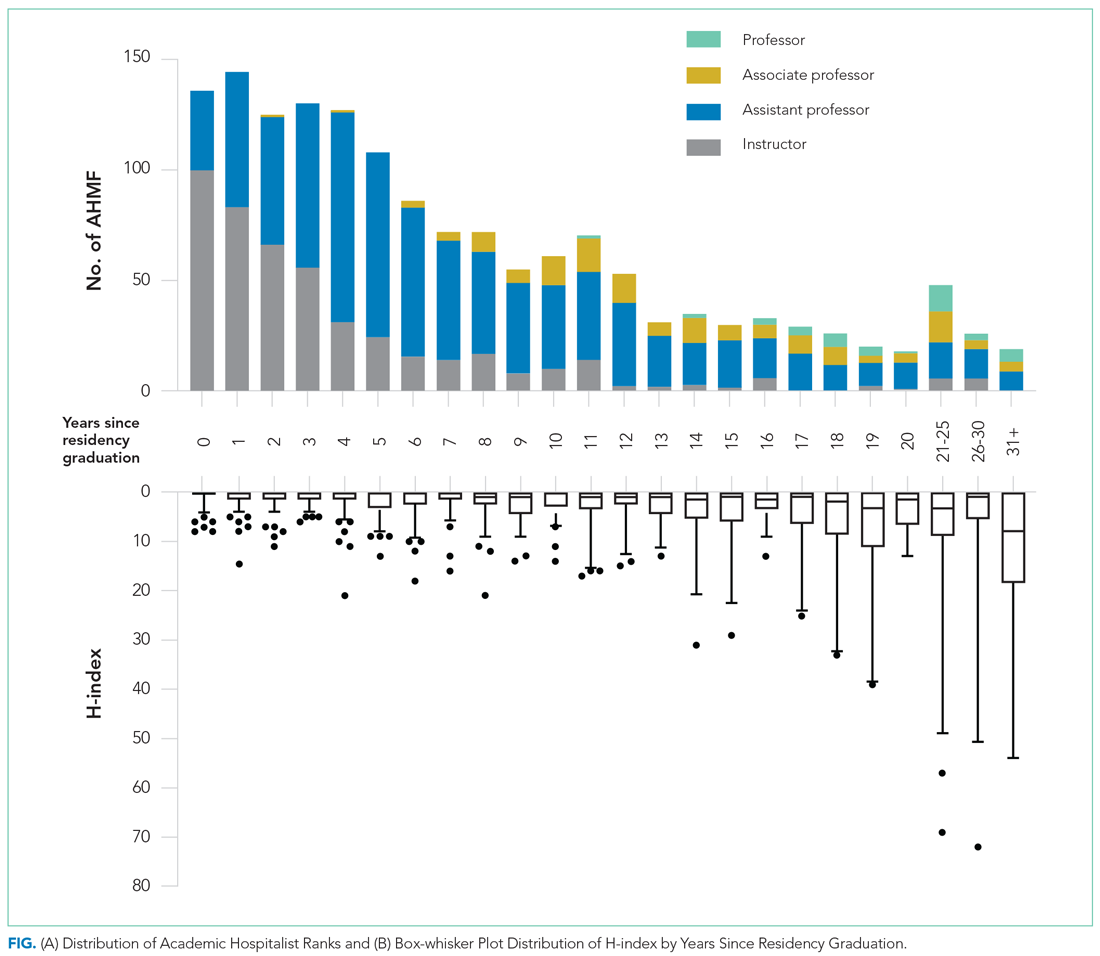
A total of 9,809 documents had been published by this cohort of academic hospitalists (Appendix Table 2). Overall mean (SD) and median (IQR) publications were 6.3 (24.3) and 0.0 (0.0-4.0), respectively. A total of 799 (51.4%) AHMF had no publications, 347 (22.3%) had one to three publications, 209 (13.4%) had 10 or more, and 39 (2.5%) had 50 or more. The median number of publications stratified by academic rank were 0.0 (IQR, 0.0-1.0) for instructors, 0.0 (IQR, 0.0-3.0) for assistant professors, 8.0 (IQR, 2.0-23.0) for associate professors, and 38.0 (IQR, 6.0-99.0) for full professors. Among men, 54.3% had published at least one manuscript, compared to 42.7% of women (P < .0001). The distribution of H-indices by years since residency graduation is shown in the Figure. The median number of documents published by faculty 6 to 10 years post residency was 1.0 (IQR, 0.0-4.0), with 46.8% of these faculty without a publication. For faculty 14 to 18 years post residency, the median number of documents was 3.0 (IQR, 0.0-11.0), with 30.1% of these faculty without a publication. Years post residency and academic rank were correlated with higher H-indices as well as more publications and citations (P < .0001).
Factors Associated With Academic Rank
Factors associated with rank are described in Appendix Table 3. In our multivariable ordinal regression model, H-index (adjusted odds ratio [aOR], 1.16 per single H-index point; 95% CI, 1.12-1.20), years post residency graduation (aOR, 1.14; 95% CI, 1.11-1.17), completion of chief residency (aOR, 2.46; 95% CI, 1.34-4.51), and graduation from a top 25 medical school (aOR, 2.10; 95% CI, 1.44-3.06) were associated with promotion.
DISCUSSION
In this cross-sectional analysis of more than 1,500 AHMF at the top 25 internal medicine residencies in the United States, 88.3% were instructors or assistant professors, while only 11.7% were associate or full professors. Furthermore, 51.4% were without a publication, and only 26.3% had published more than three manuscripts. Last, H-index, completion of a chief residency, years post residency, and graduation from a top 25 medical school were associated with higher academic rank.
Only 2.7% of the cohort were full professors, and 9.0% were associate professors. In comparison, academic cardiology faculty are 28.2% full professors and 22.9% associate professors.8 While the field of hospital medicine is relatively new, many faculty members had practiced for the expected duration of time for promotion consideration, with assistant professors or instructors constituting 89.9% of faculty at 6 to 10 years and 63.6% of faculty at 14 to 18 years post residency. We additionally observed a gender gap in publication history in hospital medicine, consistent with prior studies in hospital medicine that suggested gender disparities in scholarship.9,10 Increased focus will be needed in the future to ensure opportunities for scholarship are equitable for all faculty in hospital medicine.
Our findings suggest that scholarly productivity in academic hospital medicine remains a challenge. Prior studies have reported that less than half of academic hospitalists have ever published, and fewer than one in eight have received research funding.11,12 It is encouraging, however, that publications increase with time after residency. These data are consistent with the literature demonstrating a modest increase in hospitalists who had ever published, increasing from 43.0% in 2012 to 48.6% in 2020.12 Despite these trends, however, some early-career academic hospitalists report ambivalence toward academic productivity and promotion.13 Whether this ambivalence is the source of low scholarship output or the outcome of insufficient mentorship and limited research success is uncertain. But these factors, combined with the pressures of clinical productivity, the existing lack of mentorship, and inadequate protected research time represent barriers to successful scholarship in academic hospital medicine.3,14
Our study has several limitations. First, our inclusion criteria for the top 25 internal medicine residencies may have excluded hospital medicine divisions with substantial scholarly productivity. However, with 21 of the 25 programs listed on Doximity.com in the top 25 for internal medicine research funding, it is likely that our results overestimate scholarly productivity if compared to a complete, national cohort of AHMF.15 Second, our findings may not be generalizable to hospitalists who practice in nonacademic settings. Third, we were unable to account for differences in promotion criteria/tracks or scholarly output expectations between institutions. This limitation has been seen similarly in prior studies linking promotion and H-index.2 Furthermore, our study does not capture promotion via other pathways that may not depend on scholarly output, such as hospital leadership roles. Last, as data were abstracted from academic center websites, it is possible that not all information was accurate or updated. However, we randomly reevaluated 25% of hospital division webpages 6 months after our initial data collection and noted that all had been updated with new faculty and academic ranks, suggesting our data were accurate.
These data highlight that research productivity and academic promotion remain challenges in academic hospital medicine. Future studies may examine topics that include understanding pathways and milestones to promotion, reducing disparities in scholarship, and improving mentorship, protected time, and research funding in academic hospital medicine.
1. Wachter RM, Goldman L. Zero to 50,000—the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Leykum LK, Parekh VI, Sharpe B, Boonyasai RT, Centor RM. Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411-415. https://doi.org/10.1002/jhm.894
3. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
4. Cumbler E, Rendón P, Yirdaw E, et al. Keys to career success: resources and barriers identified by early career academic hospitalists. J Gen Intern Med. 2018;33(5):588-589. https://doi.org/10.1007/s11606-018-4336-7
5. Flanders SA, Centor B, Weber V, McGinn T, DeSalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240-246. https://doi.org/10.1002/jhm.497
6. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572. https://doi.org/10.1073/pnas.0507655102
7. 2021 Best Medical Schools: Research. U.S. News & World Report. Accessed April 23, 2021. https://www.usnews.com/best-graduate-schools/top-medical-schools/research-rankings
8. Blumenthal DM, Olenski AR, Yeh RW, et al. Sex differences in faculty rank among academic cardiologists in the United States. Circulation. 2017;135(6):506-517. https://doi.org/10.1161/CIRCULATIONAHA.116.023520
9. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
10. Adler E, Hobbs A, Dhaliwal G, Babik JM. Gender differences in authorship of clinical problem-solving articles. J Hosp Med. 2020;15(8):475-478. https://doi.org/10.12788/jhm.3465
11. Chopra V, Burden M, Jones CD, et al. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
12. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
13. Cumbler E, Yirdaw E, Kneeland P, et al. What is career success for academic hospitalists? A qualitative analysis of early-career faculty perspectives. J Hosp Med. 2018;13(6):372-377. https://doi.org/10.12788/jhm.2924
14. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
15. Roskoski R Jr, Parslow TG. Ranking tables of NIH funding to US medical schools in 2019. Accessed April 23, 2021. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
Hospital medicine has grown rapidly, with more than 50,000 hospitalists practicing nationally in 2016.1 Despite the remarkable increase in academic hospital medicine faculty (AHMF), scholarly productivity remains underdeveloped. Prior evidence suggests peer-reviewed publications remain an important aspect of promotion in academic hospital medicine.2 However, there are multiple barriers to robust scholarly productivity among AHMF, including inadequate mentorship,3 lack of protected scholarship time,4 and greater participation in nonclinical activities outside of peer-reviewed clinical research.5 Though research barriers have been described previously, the current state of scholarly productivity among AHMF has not been characterized. In this cross-sectional study, we describe the distribution of academic rank and scholarly output of a national sample of AHMF.
METHODS
Study Design and Data Source
We performed a cross-sectional study of AHMF at the top 25 internal medicine residency programs as determined by Doximity.com as of February 1, 2020 (Appendix Table 1). Between March and August 2020, two authors (NS, MT) visited each residency program’s website, identified all faculty listed as members of the hospital medicine program, and extracted demographic data, including degrees, sex, residency, medical school, year of residency graduation, completion of chief residency, completion of fellowship, and rank. We categorized all academic titles into full professor, associate professor, assistant professor, and instructor/lecturer. Missing information was supplemented by searching state licensing websites and Doximity.com. Sex was validated using Genderize.io. We queried the Scopus database for each AHMF’s name and affiliated institution to extract publications, citations, and H-index (metric of productivity and impact, derived from the number of publications and their associated citations).6 We categorized medical schools by rank (top 25, top 50, or unranked), as defined by the 2020 US News Best Medical Schools, sorted by research7 and by location (United States, international Caribbean, and international non-Caribbean). We excluded programs without hospital medicine section/division webpages and AHMF with nonpromotion titles such as “adjunct professor” or “acting professor” or those with missing data that could not be identified using these methods.
Analysis
Summary statistics were generated using means with standard deviations and medians with interquartile ranges. We evaluated postresidency years 6 to 10 and 14 to 18 as conservative time frames for promotion to associate and full professor, respectively. These windows account for time spent for additional degrees, instructor years, and alternative career pathways. Demographic differences between academic ranks were determined using chi-square and Kruskal-Wallis analyses.
Because promotion occurs sequentially, a proportional odds logistic regression model was used to evaluate the association of academic rank and H-index, number of years post residency, completion of chief residency, graduation from a top 25 medical school, and sex. Since not all programs have the instructor/lecturer rank, only assistant, associate, and full professors were included in this model. Significance was assessed with the likelihood ratio test. The proportional odds assumption was assessed using the score test. All adjusted odds ratios and their associated 95% confidence intervals were recorded. A two-tailed P value < .05 was considered significant for this study, and SAS version 9.4 (SAS Institute Inc) was used to conduct all analyses. This study was approved by the UT Southwestern Institutional Review Board.
RESULTS
Cohort Demographics
Of the top 25 internal medicine programs, 3 were excluded because they did not have websites that listed AHMF. Of the remaining 22 programs, we identified 1,829 AHMF. We excluded 166 AHMF because we could not identify title or year of residency graduation and 109 for having nonpromotion titles, leaving 1,554 AHMF (Appendix Figure). The cohort characteristics are described in Table 1. 

Research Productivity
A total of 9,809 documents had been published by this cohort of academic hospitalists (Appendix Table 2). Overall mean (SD) and median (IQR) publications were 6.3 (24.3) and 0.0 (0.0-4.0), respectively. A total of 799 (51.4%) AHMF had no publications, 347 (22.3%) had one to three publications, 209 (13.4%) had 10 or more, and 39 (2.5%) had 50 or more. The median number of publications stratified by academic rank were 0.0 (IQR, 0.0-1.0) for instructors, 0.0 (IQR, 0.0-3.0) for assistant professors, 8.0 (IQR, 2.0-23.0) for associate professors, and 38.0 (IQR, 6.0-99.0) for full professors. Among men, 54.3% had published at least one manuscript, compared to 42.7% of women (P < .0001). The distribution of H-indices by years since residency graduation is shown in the Figure. The median number of documents published by faculty 6 to 10 years post residency was 1.0 (IQR, 0.0-4.0), with 46.8% of these faculty without a publication. For faculty 14 to 18 years post residency, the median number of documents was 3.0 (IQR, 0.0-11.0), with 30.1% of these faculty without a publication. Years post residency and academic rank were correlated with higher H-indices as well as more publications and citations (P < .0001).
Factors Associated With Academic Rank
Factors associated with rank are described in Appendix Table 3. In our multivariable ordinal regression model, H-index (adjusted odds ratio [aOR], 1.16 per single H-index point; 95% CI, 1.12-1.20), years post residency graduation (aOR, 1.14; 95% CI, 1.11-1.17), completion of chief residency (aOR, 2.46; 95% CI, 1.34-4.51), and graduation from a top 25 medical school (aOR, 2.10; 95% CI, 1.44-3.06) were associated with promotion.
DISCUSSION
In this cross-sectional analysis of more than 1,500 AHMF at the top 25 internal medicine residencies in the United States, 88.3% were instructors or assistant professors, while only 11.7% were associate or full professors. Furthermore, 51.4% were without a publication, and only 26.3% had published more than three manuscripts. Last, H-index, completion of a chief residency, years post residency, and graduation from a top 25 medical school were associated with higher academic rank.
Only 2.7% of the cohort were full professors, and 9.0% were associate professors. In comparison, academic cardiology faculty are 28.2% full professors and 22.9% associate professors.8 While the field of hospital medicine is relatively new, many faculty members had practiced for the expected duration of time for promotion consideration, with assistant professors or instructors constituting 89.9% of faculty at 6 to 10 years and 63.6% of faculty at 14 to 18 years post residency. We additionally observed a gender gap in publication history in hospital medicine, consistent with prior studies in hospital medicine that suggested gender disparities in scholarship.9,10 Increased focus will be needed in the future to ensure opportunities for scholarship are equitable for all faculty in hospital medicine.
Our findings suggest that scholarly productivity in academic hospital medicine remains a challenge. Prior studies have reported that less than half of academic hospitalists have ever published, and fewer than one in eight have received research funding.11,12 It is encouraging, however, that publications increase with time after residency. These data are consistent with the literature demonstrating a modest increase in hospitalists who had ever published, increasing from 43.0% in 2012 to 48.6% in 2020.12 Despite these trends, however, some early-career academic hospitalists report ambivalence toward academic productivity and promotion.13 Whether this ambivalence is the source of low scholarship output or the outcome of insufficient mentorship and limited research success is uncertain. But these factors, combined with the pressures of clinical productivity, the existing lack of mentorship, and inadequate protected research time represent barriers to successful scholarship in academic hospital medicine.3,14
Our study has several limitations. First, our inclusion criteria for the top 25 internal medicine residencies may have excluded hospital medicine divisions with substantial scholarly productivity. However, with 21 of the 25 programs listed on Doximity.com in the top 25 for internal medicine research funding, it is likely that our results overestimate scholarly productivity if compared to a complete, national cohort of AHMF.15 Second, our findings may not be generalizable to hospitalists who practice in nonacademic settings. Third, we were unable to account for differences in promotion criteria/tracks or scholarly output expectations between institutions. This limitation has been seen similarly in prior studies linking promotion and H-index.2 Furthermore, our study does not capture promotion via other pathways that may not depend on scholarly output, such as hospital leadership roles. Last, as data were abstracted from academic center websites, it is possible that not all information was accurate or updated. However, we randomly reevaluated 25% of hospital division webpages 6 months after our initial data collection and noted that all had been updated with new faculty and academic ranks, suggesting our data were accurate.
These data highlight that research productivity and academic promotion remain challenges in academic hospital medicine. Future studies may examine topics that include understanding pathways and milestones to promotion, reducing disparities in scholarship, and improving mentorship, protected time, and research funding in academic hospital medicine.
Hospital medicine has grown rapidly, with more than 50,000 hospitalists practicing nationally in 2016.1 Despite the remarkable increase in academic hospital medicine faculty (AHMF), scholarly productivity remains underdeveloped. Prior evidence suggests peer-reviewed publications remain an important aspect of promotion in academic hospital medicine.2 However, there are multiple barriers to robust scholarly productivity among AHMF, including inadequate mentorship,3 lack of protected scholarship time,4 and greater participation in nonclinical activities outside of peer-reviewed clinical research.5 Though research barriers have been described previously, the current state of scholarly productivity among AHMF has not been characterized. In this cross-sectional study, we describe the distribution of academic rank and scholarly output of a national sample of AHMF.
METHODS
Study Design and Data Source
We performed a cross-sectional study of AHMF at the top 25 internal medicine residency programs as determined by Doximity.com as of February 1, 2020 (Appendix Table 1). Between March and August 2020, two authors (NS, MT) visited each residency program’s website, identified all faculty listed as members of the hospital medicine program, and extracted demographic data, including degrees, sex, residency, medical school, year of residency graduation, completion of chief residency, completion of fellowship, and rank. We categorized all academic titles into full professor, associate professor, assistant professor, and instructor/lecturer. Missing information was supplemented by searching state licensing websites and Doximity.com. Sex was validated using Genderize.io. We queried the Scopus database for each AHMF’s name and affiliated institution to extract publications, citations, and H-index (metric of productivity and impact, derived from the number of publications and their associated citations).6 We categorized medical schools by rank (top 25, top 50, or unranked), as defined by the 2020 US News Best Medical Schools, sorted by research7 and by location (United States, international Caribbean, and international non-Caribbean). We excluded programs without hospital medicine section/division webpages and AHMF with nonpromotion titles such as “adjunct professor” or “acting professor” or those with missing data that could not be identified using these methods.
Analysis
Summary statistics were generated using means with standard deviations and medians with interquartile ranges. We evaluated postresidency years 6 to 10 and 14 to 18 as conservative time frames for promotion to associate and full professor, respectively. These windows account for time spent for additional degrees, instructor years, and alternative career pathways. Demographic differences between academic ranks were determined using chi-square and Kruskal-Wallis analyses.
Because promotion occurs sequentially, a proportional odds logistic regression model was used to evaluate the association of academic rank and H-index, number of years post residency, completion of chief residency, graduation from a top 25 medical school, and sex. Since not all programs have the instructor/lecturer rank, only assistant, associate, and full professors were included in this model. Significance was assessed with the likelihood ratio test. The proportional odds assumption was assessed using the score test. All adjusted odds ratios and their associated 95% confidence intervals were recorded. A two-tailed P value < .05 was considered significant for this study, and SAS version 9.4 (SAS Institute Inc) was used to conduct all analyses. This study was approved by the UT Southwestern Institutional Review Board.
RESULTS
Cohort Demographics
Of the top 25 internal medicine programs, 3 were excluded because they did not have websites that listed AHMF. Of the remaining 22 programs, we identified 1,829 AHMF. We excluded 166 AHMF because we could not identify title or year of residency graduation and 109 for having nonpromotion titles, leaving 1,554 AHMF (Appendix Figure). The cohort characteristics are described in Table 1. 

Research Productivity
A total of 9,809 documents had been published by this cohort of academic hospitalists (Appendix Table 2). Overall mean (SD) and median (IQR) publications were 6.3 (24.3) and 0.0 (0.0-4.0), respectively. A total of 799 (51.4%) AHMF had no publications, 347 (22.3%) had one to three publications, 209 (13.4%) had 10 or more, and 39 (2.5%) had 50 or more. The median number of publications stratified by academic rank were 0.0 (IQR, 0.0-1.0) for instructors, 0.0 (IQR, 0.0-3.0) for assistant professors, 8.0 (IQR, 2.0-23.0) for associate professors, and 38.0 (IQR, 6.0-99.0) for full professors. Among men, 54.3% had published at least one manuscript, compared to 42.7% of women (P < .0001). The distribution of H-indices by years since residency graduation is shown in the Figure. The median number of documents published by faculty 6 to 10 years post residency was 1.0 (IQR, 0.0-4.0), with 46.8% of these faculty without a publication. For faculty 14 to 18 years post residency, the median number of documents was 3.0 (IQR, 0.0-11.0), with 30.1% of these faculty without a publication. Years post residency and academic rank were correlated with higher H-indices as well as more publications and citations (P < .0001).
Factors Associated With Academic Rank
Factors associated with rank are described in Appendix Table 3. In our multivariable ordinal regression model, H-index (adjusted odds ratio [aOR], 1.16 per single H-index point; 95% CI, 1.12-1.20), years post residency graduation (aOR, 1.14; 95% CI, 1.11-1.17), completion of chief residency (aOR, 2.46; 95% CI, 1.34-4.51), and graduation from a top 25 medical school (aOR, 2.10; 95% CI, 1.44-3.06) were associated with promotion.
DISCUSSION
In this cross-sectional analysis of more than 1,500 AHMF at the top 25 internal medicine residencies in the United States, 88.3% were instructors or assistant professors, while only 11.7% were associate or full professors. Furthermore, 51.4% were without a publication, and only 26.3% had published more than three manuscripts. Last, H-index, completion of a chief residency, years post residency, and graduation from a top 25 medical school were associated with higher academic rank.
Only 2.7% of the cohort were full professors, and 9.0% were associate professors. In comparison, academic cardiology faculty are 28.2% full professors and 22.9% associate professors.8 While the field of hospital medicine is relatively new, many faculty members had practiced for the expected duration of time for promotion consideration, with assistant professors or instructors constituting 89.9% of faculty at 6 to 10 years and 63.6% of faculty at 14 to 18 years post residency. We additionally observed a gender gap in publication history in hospital medicine, consistent with prior studies in hospital medicine that suggested gender disparities in scholarship.9,10 Increased focus will be needed in the future to ensure opportunities for scholarship are equitable for all faculty in hospital medicine.
Our findings suggest that scholarly productivity in academic hospital medicine remains a challenge. Prior studies have reported that less than half of academic hospitalists have ever published, and fewer than one in eight have received research funding.11,12 It is encouraging, however, that publications increase with time after residency. These data are consistent with the literature demonstrating a modest increase in hospitalists who had ever published, increasing from 43.0% in 2012 to 48.6% in 2020.12 Despite these trends, however, some early-career academic hospitalists report ambivalence toward academic productivity and promotion.13 Whether this ambivalence is the source of low scholarship output or the outcome of insufficient mentorship and limited research success is uncertain. But these factors, combined with the pressures of clinical productivity, the existing lack of mentorship, and inadequate protected research time represent barriers to successful scholarship in academic hospital medicine.3,14
Our study has several limitations. First, our inclusion criteria for the top 25 internal medicine residencies may have excluded hospital medicine divisions with substantial scholarly productivity. However, with 21 of the 25 programs listed on Doximity.com in the top 25 for internal medicine research funding, it is likely that our results overestimate scholarly productivity if compared to a complete, national cohort of AHMF.15 Second, our findings may not be generalizable to hospitalists who practice in nonacademic settings. Third, we were unable to account for differences in promotion criteria/tracks or scholarly output expectations between institutions. This limitation has been seen similarly in prior studies linking promotion and H-index.2 Furthermore, our study does not capture promotion via other pathways that may not depend on scholarly output, such as hospital leadership roles. Last, as data were abstracted from academic center websites, it is possible that not all information was accurate or updated. However, we randomly reevaluated 25% of hospital division webpages 6 months after our initial data collection and noted that all had been updated with new faculty and academic ranks, suggesting our data were accurate.
These data highlight that research productivity and academic promotion remain challenges in academic hospital medicine. Future studies may examine topics that include understanding pathways and milestones to promotion, reducing disparities in scholarship, and improving mentorship, protected time, and research funding in academic hospital medicine.
1. Wachter RM, Goldman L. Zero to 50,000—the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Leykum LK, Parekh VI, Sharpe B, Boonyasai RT, Centor RM. Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411-415. https://doi.org/10.1002/jhm.894
3. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
4. Cumbler E, Rendón P, Yirdaw E, et al. Keys to career success: resources and barriers identified by early career academic hospitalists. J Gen Intern Med. 2018;33(5):588-589. https://doi.org/10.1007/s11606-018-4336-7
5. Flanders SA, Centor B, Weber V, McGinn T, DeSalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240-246. https://doi.org/10.1002/jhm.497
6. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572. https://doi.org/10.1073/pnas.0507655102
7. 2021 Best Medical Schools: Research. U.S. News & World Report. Accessed April 23, 2021. https://www.usnews.com/best-graduate-schools/top-medical-schools/research-rankings
8. Blumenthal DM, Olenski AR, Yeh RW, et al. Sex differences in faculty rank among academic cardiologists in the United States. Circulation. 2017;135(6):506-517. https://doi.org/10.1161/CIRCULATIONAHA.116.023520
9. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
10. Adler E, Hobbs A, Dhaliwal G, Babik JM. Gender differences in authorship of clinical problem-solving articles. J Hosp Med. 2020;15(8):475-478. https://doi.org/10.12788/jhm.3465
11. Chopra V, Burden M, Jones CD, et al. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
12. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
13. Cumbler E, Yirdaw E, Kneeland P, et al. What is career success for academic hospitalists? A qualitative analysis of early-career faculty perspectives. J Hosp Med. 2018;13(6):372-377. https://doi.org/10.12788/jhm.2924
14. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
15. Roskoski R Jr, Parslow TG. Ranking tables of NIH funding to US medical schools in 2019. Accessed April 23, 2021. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
1. Wachter RM, Goldman L. Zero to 50,000—the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Leykum LK, Parekh VI, Sharpe B, Boonyasai RT, Centor RM. Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411-415. https://doi.org/10.1002/jhm.894
3. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
4. Cumbler E, Rendón P, Yirdaw E, et al. Keys to career success: resources and barriers identified by early career academic hospitalists. J Gen Intern Med. 2018;33(5):588-589. https://doi.org/10.1007/s11606-018-4336-7
5. Flanders SA, Centor B, Weber V, McGinn T, DeSalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240-246. https://doi.org/10.1002/jhm.497
6. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572. https://doi.org/10.1073/pnas.0507655102
7. 2021 Best Medical Schools: Research. U.S. News & World Report. Accessed April 23, 2021. https://www.usnews.com/best-graduate-schools/top-medical-schools/research-rankings
8. Blumenthal DM, Olenski AR, Yeh RW, et al. Sex differences in faculty rank among academic cardiologists in the United States. Circulation. 2017;135(6):506-517. https://doi.org/10.1161/CIRCULATIONAHA.116.023520
9. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
10. Adler E, Hobbs A, Dhaliwal G, Babik JM. Gender differences in authorship of clinical problem-solving articles. J Hosp Med. 2020;15(8):475-478. https://doi.org/10.12788/jhm.3465
11. Chopra V, Burden M, Jones CD, et al. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
12. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
13. Cumbler E, Yirdaw E, Kneeland P, et al. What is career success for academic hospitalists? A qualitative analysis of early-career faculty perspectives. J Hosp Med. 2018;13(6):372-377. https://doi.org/10.12788/jhm.2924
14. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
15. Roskoski R Jr, Parslow TG. Ranking tables of NIH funding to US medical schools in 2019. Accessed April 23, 2021. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
© 2021 Society of Hospital Medicine
Hospital Buprenorphine Program for Opioid Use Disorder Is Associated With Increased Inpatient and Outpatient Addiction Treatment
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
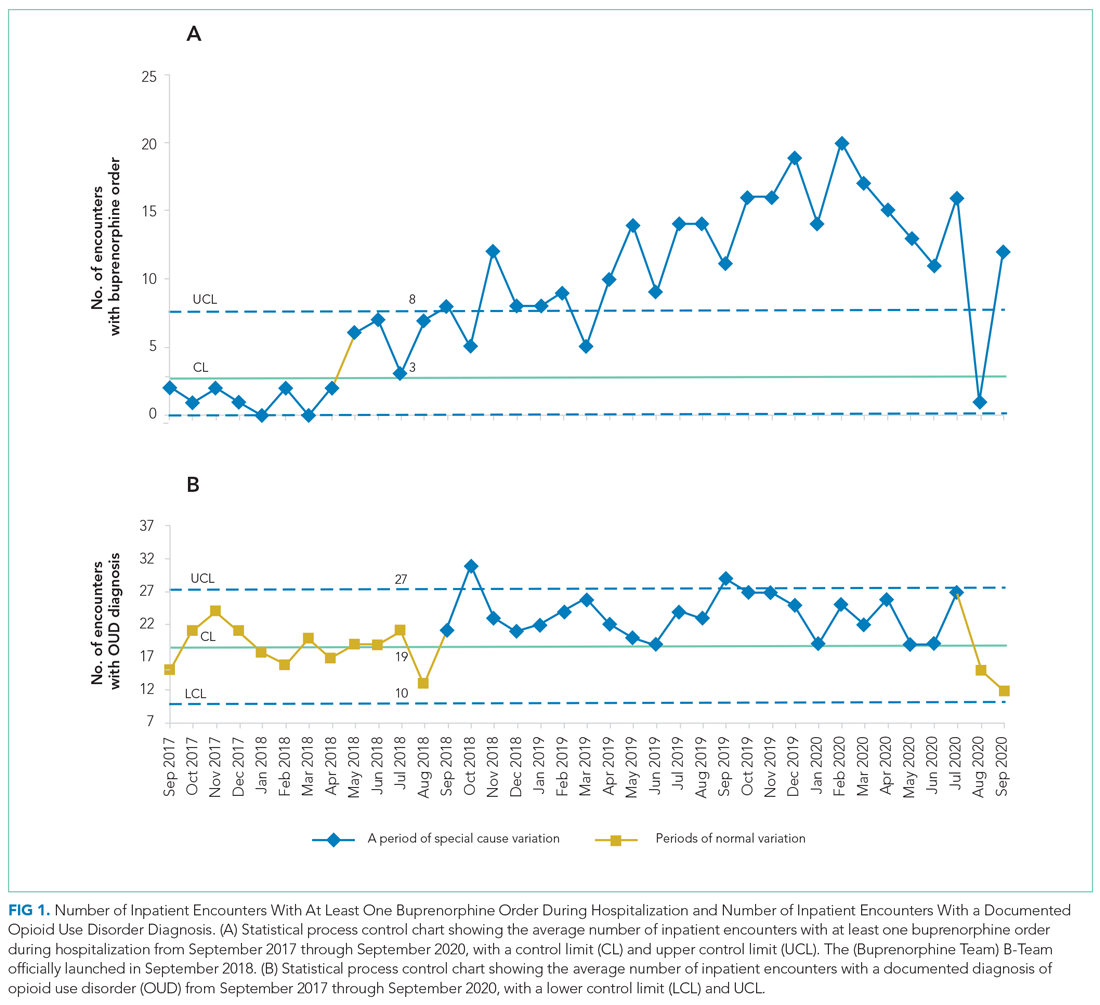
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased
The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).
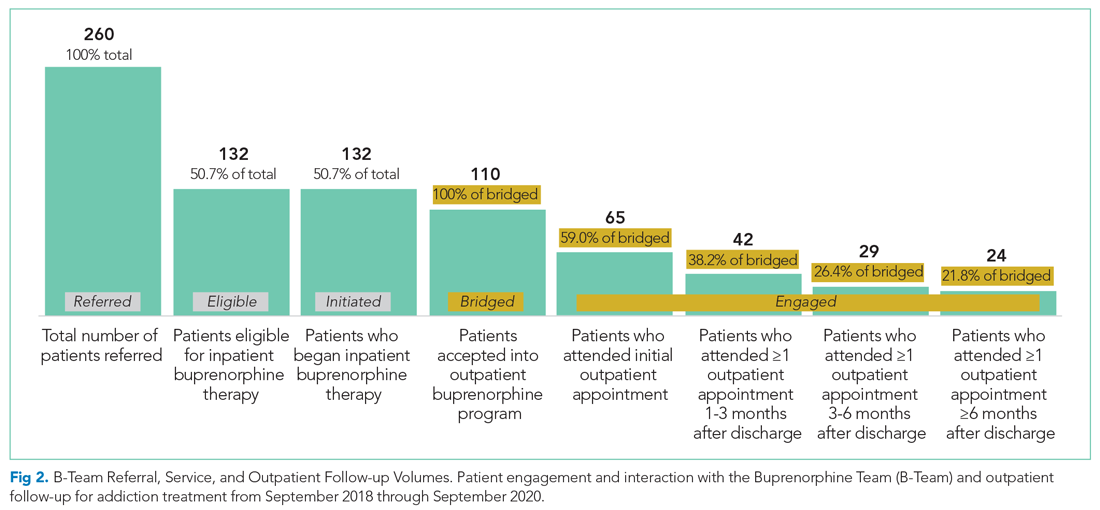
Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased

The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).

Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
Hospitalizations related to opioid use disorder (OUD) have increased and now account for up to 6% of hospital admissions in certain areas of the United States.1 Patients with OUD who are started on buprenorphine during hospitalization are more likely to enter outpatient treatment, stay in treatment longer, and have more drug-free days compared with patients who only receive a referral for outpatient treatment.2,3 Therefore, a crucial comprehensive strategy for OUD care should include hospital-based programs that support initiation of treatment in the inpatient setting and strong bridges to outpatient care. One of the common barriers to initiating treatment in the inpatient setting, however, is a lack of access to addiction medicine specialists.4-6
In 2017, we created a hospitalist-led interprofessional team called the B-Team (Buprenorphine Team) to help primary care teams identify patients with OUD, initiate and maintain buprenorphine therapy during hospitalization, provide warm handoffs to outpatient treatment programs, and reduce institutional stigma related to people with substance use disorders.
METHODS
Program Description
The B-Team is led by a hospital medicine physician assistant and includes physicians from internal medicine, consult-liaison psychiatry, and palliative care; advanced practice and bedside nurses; a social worker; a pharmacist; a chaplain; a peer-recovery specialist; and medical trainees. The B-Team is notified of potential candidates for buprenorphine through a secure texting platform, one that is accessible to any healthcare provider at the hospital. Patients who are referred to the B-Team either self-identify or are identified by their primary team as having an underlying OUD. One of the B-Team providers assesses the patient to determine if they are eligible to receive inpatient therapy. Patients are considered eligible for the program if they meet Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for OUD, have a desire to cease opioid use, and receive medical clearance to take buprenorphine.
For eligible patients, the B-Team provider orders a nurse-driven protocol to initiate buprenorphine for OUD. The chaplain offers psychospiritual counseling, and the social worker provides counseling and coordination of care. The B-Team partners with a nonhospital-affiliated, publicly-funded, office-based opioid treatment (OBOT) program that combines primary care with behavioral health programming. A follow-up outpatient appointment is secured prior to hospital discharge, and a member of the B-Team who has Drug Addiction Treatment Act of 2000 (DATA 2000) X-waiver certification prescribes buprenorphine as a bridge until the follow-up appointment. The medication is dispensed from the hospital’s retail pharmacy, and the patient leaves the hospital with the medication in-hand.
Patients who are not eligible for buprenorphine therapy are offered a harm-reduction intervention or referral to the psychiatry consult liaison service to assess for alternative diagnoses or treatment. These patients are also offered psychospiritual counseling and a prescription for naloxone.
Prior to the creation of the B-Team at our hospital, there was no structure in place to facilitate initiation of buprenorphine therapy during hospitalization and no linkage to outpatient treatment after discharge; furthermore, none of the hospitalists or other providers (including consulting psychiatrists) had an X-waiver to prescribe buprenorphine for OUD.
Program Evaluation
Study data were collected using Research Electronic Data Capture software. Inpatient and outpatient data were entered by a B-Team provider or a researcher via chart review. Patients were considered to be engaged in care if they attended at least one outpatient appointment for buprenorphine therapy during each of the following time periods: (1) 0 to 27 days (initial follow-up), 28-89 days (1- to 3-month follow-up), 90-179 days (3- to 6-month follow-up), and 180 days or more (>6-month follow-up). Only visits specifically for buprenorphine maintenance therapy were counted. If multiple encounters occurred within one time frame, the encounter closest to 0, 30, 90, or 180 days from discharge was used. If a patient did not attend any encounters during a specified time frame, they were considered to no longer be engaged in care and were no longer tracked for purposes of the evaluation. Data for the percentage of patients engaged in outpatient care are presented as the number of patients who attended at least one appointment during each of the follow-up periods (1 to 3 months, 3 to 6 months, or after 6 months, as noted above) divided by the number of patients who had been discharged with coordinated follow-up.
The number of patients admitted per month for whom there was an order to initiate inpatient buprenorphine therapy was analyzed using a statistical process control chart,
This program and study were considered quality improvement by The University of Texas Institutional Review Board and did not meet criteria for human subjects research.
RESULTS
During the first 2 years of the program (September 2018-September 2020), the B-Team received 260 patient referrals. Most of the patients were White (72%), male (62%), and between ages 25 and 44 years (53%) (Appendix Table). The team initiated buprenorphine therapy in 132 hospitalized patients. In the year prior to the creation of the B-Team program, the average number of hospitalized patients receiving buprenorphine for OUD per month was three; after the launch of the B-Team program, this number increased

The B-Team saw a total of 132 eligible patients; members of the team provided counseling, support, and resources regarding buprenorphine therapy. In addition, the B-Team’s chaplain provided emotional support and spiritual connection (if desired) to 40 of these patients (30%). In the study, no cases of precipitated withdrawal were identified. Of the 132 patients seen, 110 (83%) were accepted to an outpatient OUD program upon discharge from the hospital; 98 (89%) of these patients were accepted at our partner OBOT clinic. The remaining patients were not interested in continuing OUD treatment (13%) or were denied acceptance to an outpatient program based on administrative and/or financial eligibility guidelines (4%). Patients who would not be attending an outpatient program were discontinued on buprenorphine therapy prior to discharge, counseled about naloxone, and provided printed resources.
Outpatient appointment attendance was used to measure ongoing treatment engagement of the 110 patients who were discharged with coordinated follow-up care. A total of 65 patients (59%) attended their first outpatient appointment; the average time between discharge and the first outpatient appointment was 5.9 days. Forty-two patients (38%) attended at least one appointment between 1 and 3 months; 29 (26%) between 3 and 6 months; and 24 (22%) after 6 months (Figure 2).

Of the 128 patients who were not administered buprenorphine therapy, 64 (50%) were not interested in starting treatment and/or were not ready to engage in treatment; 36 (28%) did not meet criteria for OUD treatment; 28 (22%) were already receiving treatment or preferred another type of OUD treatment; and 13 (10%) had severe comorbid addiction and/or illness requiring treatment that contraindicates the use of buprenorphine.
DISCUSSION
A volunteer hospitalist-led interprofessional team providing evidence-based care for hospitalized patients with OUD was associated with a substantial increase in patients receiving buprenorphine therapy—both during hospitalization and after discharge. In the program, 59% of patients attended initial follow-up appointments, and 22% of patients were still engaged at 6 months. These outpatient follow-up rates appear to be similar to, or higher than, other programs described in the literature. For example, a buprenorphine OUD-treatment initiative led by the psychiatry consult service at a Boston academic medical center resulted in less than half of patients receiving buprenorphine treatment within 2 months of discharge.7 In another study wherein an addiction medicine consult service administered buprenorphine to patients with OUD during hospitalization, 39%, 27%, and 18% of patients were retained in outpatient treatment at 30, 90, and 180 days, respectively.8
The B-Team model is likely generalizable to other hospital medicine groups that may not otherwise have access to inpatient care for substance use disorder. The B-Team is not an addiction medicine consultation service; rather, it is a hospitalist-led quality improvement initiative seeking to improve the standard of care for hospitalized patients with OUD.
A significant barrier is ensuring ongoing support for patients with OUD after discharge. In the B-Team program, a parallel OBOT program was created by a local nonaffiliated federally qualified health center. Although 89% of patients received treatment at this OBOT clinic, the inpatient team also has relationships with other local treatment centers, including programs that provide methadone. Another important barrier to high-quality outpatient care for OUD is the requirement of an X-waiver. To help overcome this barrier, our inpatient program partnered with a regional medical society to offer periodic X-waiver training to outpatient providers. In less than a year, more than 100 regional prescribers participated in this program.
Our study has several limitations. There was likely some degree of selection bias among the hospitalized patients who received initial buprenorphine treatment. To our knowledge, there is no specific validated screening tool for OUD in the inpatient acute care setting; moreover, we have been unable to implement standardized screening for OUD into the electronic health record. As such, we rely on the totality of the clinical circumstances approach to identify patients with OUD.
Furthermore, we had neither a comparison group nor a prospective plan to follow patients who did not remain engaged in care after discharge. In addition, our analysis of OUD admissions included F11 ICD-10 codes, which are limited by clinical documentation.9,10 Our program focuses exclusively on buprenorphine initiation due to insufficient immediate outpatient capacity for methadone initiated during hospitalization and lack of coverage for extended-release naltrexone. Limitations to outpatient data-sharing prevented the reporting of outpatient appointments external to the identified partner program; since these appointments were included in the analysis as “lost to follow-up,” actual engagement rates may be higher than those reported.
Moving forward, the B-Team is continuing to serve as a role model for appropriate, patient-centered, evidence-based care for hospitalized patients with OUD. Attending physicians and residents with an X-waiver are now encouraged to initiate buprenorphine treatment on their own. In June 2020, we added peer-recovery support services to the program, which has improved care for patients and increased adoption of hospital-initiated substance use disorder interventions.11 Lessons learned from inpatient implementation are being applied to our hospital’s emergency department and to an inpatient obstetrics unit at a partner hospital; they are also being employed to further empower hospitalists to diagnose and treat other substance use disorders, such as alcohol use disorder.
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
1. Owens PL, Weiss AJ, Barrett ML. Hospital Burden of Opioid-Related Inpatient Stays: Metropolitan and Rural Hospitals, 2016. HCUP Statistical Brief #258. Agency for Healthcare Research and Quality. May 2020. Accessed May 24, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559382/pdf/Bookshelf_NBK559382.pdf
2. Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/jamainternmed.2014.2556
3. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/adm.0000000000000499
4. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736
5. Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. https://doi.org/10.1056/nejmp1606157
6. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024
7. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict. 2015;24(1):10-14. https://doi.org/10.1111/ajad.12161
8. Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1-5. https://doi.org/10.1016/j.jsat.2017.05.007
9. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/adm.0000000000000454
10. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918-923. https://doi.org/10.1097/mlr.0000000000000805
11. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. 2019. https://doi.org/10.1007/s11606-019-05311-0
© 2021 Society of Hospital Medicine
Gender Differences in the Presentation and Outcomes of Hospitalized Patients With COVID-19
There is growing evidence that gender may be associated with COVID-19 infection, presentation, and prognosis
METHODS
Study methods and definitions are available in Appendix 1 and Appendix 2, respectively, and detailed in a previous paper5 and online on the web page of the study.6
Enrolled patients were divided into two groups according to their gender, then propensity score matching (PSM) analysis was performed (1:1 nearest neighbor matching, caliper = 0.01, without replacement and maximizing execution performance). Our
Statistical analysis methods are outlined in Appendix 1.
RESULTS
Of the 2,798 patients consecutively enrolled in the HOPE registry, 1,111 were women (39.7%) and 1,687 were men (60.3%). Of the 2,375 (84.9%) patients who had a nasopharyngeal swab positive for COVID-19, 962 were women and 1,413 were men.
Baseline Characteristics and Clinical Presentation
The baseline characteristics and clinical presentation of the overall population included in the study are summarized in Appendix Table 1. In the raw population, men had a significantly higher prevalence of conventional cardiovascular risk factors, such as diabetes, dyslipidemia, and smoking history, as well as a history of lung and cardiovascular diseases. On presentation, the most common symptoms for all patients were fever, cough, and dyspnea. Fever was more common in men, whereas vomiting, diarrhea, and upper airway symptoms (eg, sore throat, hyposmia/anosmia, dysgeusia) were more common in women.
Most patients had increased values of acute phase reactants. C-reactive protein (CRP) was elevated in 90.2% and D-dimer in 64.2% of patients, both significantly more often in men. Lymphocytopenia was present in 75.4% of patients, more commonly among men. Bilateral pneumonia occurred in 69.2% of the population, more frequently in men.
After PSM analysis (Appendix Table 2), a higher prevalence of hyposmia/anosmia and gastrointestinal symptoms in women was confirmed, as well as a higher prevalence of fever in men. Laboratory tests in men still presented alterations consistent with a more severe COVID-19 infection (significantly higher CRP, troponin, transaminases, lymphocytopenia, thrombocytopenia, and ferritin). There was no significant difference in the time between onset of symptoms and hospital admission by gender (6.2 ± 7.1 days in women vs 5.9 ± 7.6 days in men; P = .472).
The main findings after PSM analysis are summarized in Appendix Figure 1 and Appendix Figure 2.
In-Hospital Management and Outcomes
The supportive and pharmacologic treatments of study patients and their outcomes are summarized in Appendix Table 3. During the in-hospital stay, men required oxygen supplementation more frequently than women. Noninvasive mechanical ventilation, invasive mechanical ventilation, and pronation were more commonly used in men. Chloroquine/hydroxychloroquine, antivirals, and antibiotics were the medications most widely used in our population (84.5%, 65.8%, and 74.4% of patients, respectively), without significant differences between male and female patients, with the exception of antibiotics, which were used more often in men (76.6% vs 71.1%). Immunomodulators (corticosteroids, tocilizumab, and interferon) were used more often in male patients.
After PSM (Table), men more frequently received immunomodulators (corticosteroids and tocilizumab), antibiotics, and pronation. No differences in invasive and noninvasive mechanical ventilation were observed.
Thirty-day outcome data were available for all patients included in the analysis. During the in-hospital stay, 48% of patients developed respiratory insufficiency, 18.8%
The PSM analysis continued to show a higher 30-day mortality rate among men (Figure), as well as greater need for oxygen, pronation, and use of immunomodulators and antibiotics (Table).
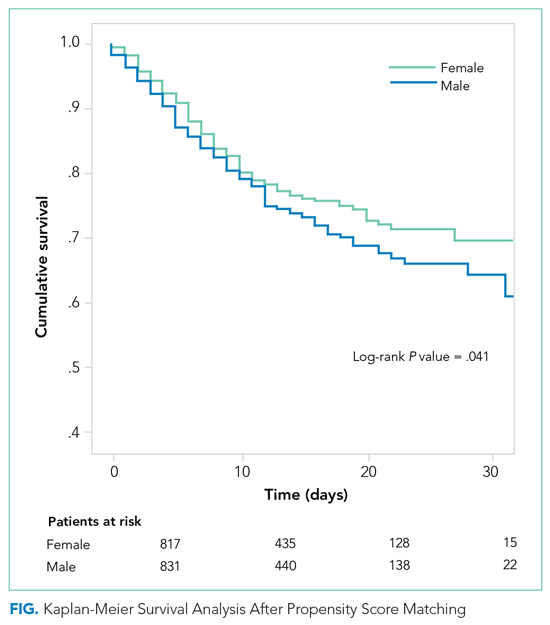
DISCUSSION
The results of our study confirm that among patients with COVID-19, men have a poorer prognosis than women. Because of the design of the study, it is not possible to determine if men are more prone to SARS-CoV-2 infection in our population; however, given the prevalence of men in our unselected, all-comers population, we can assume that men are either infected more often and/or more frequently symptomatic.
After PSM analysis, the 30-day all-cause mortality remained higher among men than women. The poorer prognosis of male patients is attributable not only to a higher burden of cardiovascular risk factors, but may also be related to unmodifiable biological factors, such as sex differences in angiotensin-converting enzyme 2 expression.7,8 The worse prognosis observed in our study confirms the higher incidence of death in male patients that was observed in previous studies.9 Liu et al questioned the role of gender as an independent prognostic factor in COVID-1910; however, that study included fewer patients, who were also younger and had less severe disease.
The clinical presentation of COVID-19 also differed by gender in our study. Gastrointestinal symptoms and hyposmia/anosmia were more common in women, whereas fever was more common in men. The prevalence of olfactory and gustatory dysfunction in women has already been described,11,12 and these symptoms have been linked with milder disease.13 It is possible that women presenting to the hospital had milder forms of COVID-19, or that there were systematic differences in how men and women sought medical care. The results of our study emphasize the need for a high level of suspicion for COVID-19 infection in women, even in the presence of mild mucosal or gastrointestinal symptoms and/or relatively minor laboratory abnormalities.
Laboratory values indicative of more severe COVID-19 infection in men could suggest a higher inflammatory response to the infection. Men also received more immunomodulators and antibiotics in this study. A recent paper from Scully et al14 pointed out the different immune response to viruses observed in men that could partially explain the higher level of inflammation markers and the more severe disease observed in men.
Limitations
Our study has several limitations. As an observational study of hospitalized patients, it may represent patients with more severe COVID-19. Men and women may have sought hospital care differently. Diagnosis, testing, and treatment were not standardized and may have been influenced by patient gender. Although we attempted to match patients on baseline medical conditions, we may not have completely controlled for differences in preexisting health. Finally, gender data were collected as binary and so did not capture other gender categories.
CONCLUSION
In our multicenter cohort of hospitalized COVID-19 patients, men had a higher burden of risk factors; different clinical presentations, with more fever and less olfactory and gastrointestinal symptoms; and a significantly poorer prognosis than women did at 30 days.
Acknowledgments
The authors thank Cardiovascular Excellence SL for their essential support regarding the database and HOPE web page as well as all HOPE researchers. The authors also thank Michael Andrews for his valuable contribution to the English revision.
1. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613-1620. https://doi.org/10.1016/j.mayocp.2020.05.014
2. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. https://doi.org/10.1186/s13293-020-00304-9
3. Gausman J, Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt). 2020;29(4):465-466. https://doi.org/10.1089/jwh.2020.8472
4. Walter LA, McGregor AJ. Sex- and gender-specific observations and implications for COVID-19. West J Emerg Med. 2020;21(3):507-509. https://doi.org/10.5811/westjem.2020.4.47536
5. Núñez-Gil IJ, Estrada V, Fernández-Pérez C, et al. Health outcome predictive evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemp Clin Trials Commun. 2020;20:100654. https://doi.org/10.1016/j.conctc.2020.100654
6. International COVID-19 Clinical Evaluation Registry: HOPE-COVID 19. Accessed February 6, 2021. https://hopeprojectmd.com/en/
7. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. https://doi.org/10.1038/s41420-020-0276-1
8. Ciaglia E, Vecchione C, Puca AA. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. https://doi.org/10.3389/fped.2020.00206
9. Peckham H, de Gruijter N, Raine C, et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. Research Square. April 20, 2020. https://doi.org/10.21203/rs.3.rs-23651/v2
10. Liu J, Zhang L, Chen Y, et al. Association of sex with clinical outcomes in COVID-19 patients: a retrospective analysis of 1190 cases. Respir Med. 2020;173:106159. https://doi.org/10.1016/j.rmed.2020.106159
11. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms—a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722-728. https://doi.org/10.1177/0194599820934380
12. Costa KVTD, Carnaúba ATL, Rocha KW, Andrade KCLD, Ferreira SMS, Menezes PTL. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86(6):781-792. https://doi.org/10.1016/j.bjorl.2020.05.008
13. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. https://doi.org/10.1007/s00405-020-05965-1
14. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-447. https://doi.org/10.1038/s41577-020-0348-8
There is growing evidence that gender may be associated with COVID-19 infection, presentation, and prognosis
METHODS
Study methods and definitions are available in Appendix 1 and Appendix 2, respectively, and detailed in a previous paper5 and online on the web page of the study.6
Enrolled patients were divided into two groups according to their gender, then propensity score matching (PSM) analysis was performed (1:1 nearest neighbor matching, caliper = 0.01, without replacement and maximizing execution performance). Our
Statistical analysis methods are outlined in Appendix 1.
RESULTS
Of the 2,798 patients consecutively enrolled in the HOPE registry, 1,111 were women (39.7%) and 1,687 were men (60.3%). Of the 2,375 (84.9%) patients who had a nasopharyngeal swab positive for COVID-19, 962 were women and 1,413 were men.
Baseline Characteristics and Clinical Presentation
The baseline characteristics and clinical presentation of the overall population included in the study are summarized in Appendix Table 1. In the raw population, men had a significantly higher prevalence of conventional cardiovascular risk factors, such as diabetes, dyslipidemia, and smoking history, as well as a history of lung and cardiovascular diseases. On presentation, the most common symptoms for all patients were fever, cough, and dyspnea. Fever was more common in men, whereas vomiting, diarrhea, and upper airway symptoms (eg, sore throat, hyposmia/anosmia, dysgeusia) were more common in women.
Most patients had increased values of acute phase reactants. C-reactive protein (CRP) was elevated in 90.2% and D-dimer in 64.2% of patients, both significantly more often in men. Lymphocytopenia was present in 75.4% of patients, more commonly among men. Bilateral pneumonia occurred in 69.2% of the population, more frequently in men.
After PSM analysis (Appendix Table 2), a higher prevalence of hyposmia/anosmia and gastrointestinal symptoms in women was confirmed, as well as a higher prevalence of fever in men. Laboratory tests in men still presented alterations consistent with a more severe COVID-19 infection (significantly higher CRP, troponin, transaminases, lymphocytopenia, thrombocytopenia, and ferritin). There was no significant difference in the time between onset of symptoms and hospital admission by gender (6.2 ± 7.1 days in women vs 5.9 ± 7.6 days in men; P = .472).
The main findings after PSM analysis are summarized in Appendix Figure 1 and Appendix Figure 2.
In-Hospital Management and Outcomes
The supportive and pharmacologic treatments of study patients and their outcomes are summarized in Appendix Table 3. During the in-hospital stay, men required oxygen supplementation more frequently than women. Noninvasive mechanical ventilation, invasive mechanical ventilation, and pronation were more commonly used in men. Chloroquine/hydroxychloroquine, antivirals, and antibiotics were the medications most widely used in our population (84.5%, 65.8%, and 74.4% of patients, respectively), without significant differences between male and female patients, with the exception of antibiotics, which were used more often in men (76.6% vs 71.1%). Immunomodulators (corticosteroids, tocilizumab, and interferon) were used more often in male patients.
After PSM (Table), men more frequently received immunomodulators (corticosteroids and tocilizumab), antibiotics, and pronation. No differences in invasive and noninvasive mechanical ventilation were observed.
Thirty-day outcome data were available for all patients included in the analysis. During the in-hospital stay, 48% of patients developed respiratory insufficiency, 18.8%
The PSM analysis continued to show a higher 30-day mortality rate among men (Figure), as well as greater need for oxygen, pronation, and use of immunomodulators and antibiotics (Table).

DISCUSSION
The results of our study confirm that among patients with COVID-19, men have a poorer prognosis than women. Because of the design of the study, it is not possible to determine if men are more prone to SARS-CoV-2 infection in our population; however, given the prevalence of men in our unselected, all-comers population, we can assume that men are either infected more often and/or more frequently symptomatic.
After PSM analysis, the 30-day all-cause mortality remained higher among men than women. The poorer prognosis of male patients is attributable not only to a higher burden of cardiovascular risk factors, but may also be related to unmodifiable biological factors, such as sex differences in angiotensin-converting enzyme 2 expression.7,8 The worse prognosis observed in our study confirms the higher incidence of death in male patients that was observed in previous studies.9 Liu et al questioned the role of gender as an independent prognostic factor in COVID-1910; however, that study included fewer patients, who were also younger and had less severe disease.
The clinical presentation of COVID-19 also differed by gender in our study. Gastrointestinal symptoms and hyposmia/anosmia were more common in women, whereas fever was more common in men. The prevalence of olfactory and gustatory dysfunction in women has already been described,11,12 and these symptoms have been linked with milder disease.13 It is possible that women presenting to the hospital had milder forms of COVID-19, or that there were systematic differences in how men and women sought medical care. The results of our study emphasize the need for a high level of suspicion for COVID-19 infection in women, even in the presence of mild mucosal or gastrointestinal symptoms and/or relatively minor laboratory abnormalities.
Laboratory values indicative of more severe COVID-19 infection in men could suggest a higher inflammatory response to the infection. Men also received more immunomodulators and antibiotics in this study. A recent paper from Scully et al14 pointed out the different immune response to viruses observed in men that could partially explain the higher level of inflammation markers and the more severe disease observed in men.
Limitations
Our study has several limitations. As an observational study of hospitalized patients, it may represent patients with more severe COVID-19. Men and women may have sought hospital care differently. Diagnosis, testing, and treatment were not standardized and may have been influenced by patient gender. Although we attempted to match patients on baseline medical conditions, we may not have completely controlled for differences in preexisting health. Finally, gender data were collected as binary and so did not capture other gender categories.
CONCLUSION
In our multicenter cohort of hospitalized COVID-19 patients, men had a higher burden of risk factors; different clinical presentations, with more fever and less olfactory and gastrointestinal symptoms; and a significantly poorer prognosis than women did at 30 days.
Acknowledgments
The authors thank Cardiovascular Excellence SL for their essential support regarding the database and HOPE web page as well as all HOPE researchers. The authors also thank Michael Andrews for his valuable contribution to the English revision.
There is growing evidence that gender may be associated with COVID-19 infection, presentation, and prognosis
METHODS
Study methods and definitions are available in Appendix 1 and Appendix 2, respectively, and detailed in a previous paper5 and online on the web page of the study.6
Enrolled patients were divided into two groups according to their gender, then propensity score matching (PSM) analysis was performed (1:1 nearest neighbor matching, caliper = 0.01, without replacement and maximizing execution performance). Our
Statistical analysis methods are outlined in Appendix 1.
RESULTS
Of the 2,798 patients consecutively enrolled in the HOPE registry, 1,111 were women (39.7%) and 1,687 were men (60.3%). Of the 2,375 (84.9%) patients who had a nasopharyngeal swab positive for COVID-19, 962 were women and 1,413 were men.
Baseline Characteristics and Clinical Presentation
The baseline characteristics and clinical presentation of the overall population included in the study are summarized in Appendix Table 1. In the raw population, men had a significantly higher prevalence of conventional cardiovascular risk factors, such as diabetes, dyslipidemia, and smoking history, as well as a history of lung and cardiovascular diseases. On presentation, the most common symptoms for all patients were fever, cough, and dyspnea. Fever was more common in men, whereas vomiting, diarrhea, and upper airway symptoms (eg, sore throat, hyposmia/anosmia, dysgeusia) were more common in women.
Most patients had increased values of acute phase reactants. C-reactive protein (CRP) was elevated in 90.2% and D-dimer in 64.2% of patients, both significantly more often in men. Lymphocytopenia was present in 75.4% of patients, more commonly among men. Bilateral pneumonia occurred in 69.2% of the population, more frequently in men.
After PSM analysis (Appendix Table 2), a higher prevalence of hyposmia/anosmia and gastrointestinal symptoms in women was confirmed, as well as a higher prevalence of fever in men. Laboratory tests in men still presented alterations consistent with a more severe COVID-19 infection (significantly higher CRP, troponin, transaminases, lymphocytopenia, thrombocytopenia, and ferritin). There was no significant difference in the time between onset of symptoms and hospital admission by gender (6.2 ± 7.1 days in women vs 5.9 ± 7.6 days in men; P = .472).
The main findings after PSM analysis are summarized in Appendix Figure 1 and Appendix Figure 2.
In-Hospital Management and Outcomes
The supportive and pharmacologic treatments of study patients and their outcomes are summarized in Appendix Table 3. During the in-hospital stay, men required oxygen supplementation more frequently than women. Noninvasive mechanical ventilation, invasive mechanical ventilation, and pronation were more commonly used in men. Chloroquine/hydroxychloroquine, antivirals, and antibiotics were the medications most widely used in our population (84.5%, 65.8%, and 74.4% of patients, respectively), without significant differences between male and female patients, with the exception of antibiotics, which were used more often in men (76.6% vs 71.1%). Immunomodulators (corticosteroids, tocilizumab, and interferon) were used more often in male patients.
After PSM (Table), men more frequently received immunomodulators (corticosteroids and tocilizumab), antibiotics, and pronation. No differences in invasive and noninvasive mechanical ventilation were observed.
Thirty-day outcome data were available for all patients included in the analysis. During the in-hospital stay, 48% of patients developed respiratory insufficiency, 18.8%
The PSM analysis continued to show a higher 30-day mortality rate among men (Figure), as well as greater need for oxygen, pronation, and use of immunomodulators and antibiotics (Table).

DISCUSSION
The results of our study confirm that among patients with COVID-19, men have a poorer prognosis than women. Because of the design of the study, it is not possible to determine if men are more prone to SARS-CoV-2 infection in our population; however, given the prevalence of men in our unselected, all-comers population, we can assume that men are either infected more often and/or more frequently symptomatic.
After PSM analysis, the 30-day all-cause mortality remained higher among men than women. The poorer prognosis of male patients is attributable not only to a higher burden of cardiovascular risk factors, but may also be related to unmodifiable biological factors, such as sex differences in angiotensin-converting enzyme 2 expression.7,8 The worse prognosis observed in our study confirms the higher incidence of death in male patients that was observed in previous studies.9 Liu et al questioned the role of gender as an independent prognostic factor in COVID-1910; however, that study included fewer patients, who were also younger and had less severe disease.
The clinical presentation of COVID-19 also differed by gender in our study. Gastrointestinal symptoms and hyposmia/anosmia were more common in women, whereas fever was more common in men. The prevalence of olfactory and gustatory dysfunction in women has already been described,11,12 and these symptoms have been linked with milder disease.13 It is possible that women presenting to the hospital had milder forms of COVID-19, or that there were systematic differences in how men and women sought medical care. The results of our study emphasize the need for a high level of suspicion for COVID-19 infection in women, even in the presence of mild mucosal or gastrointestinal symptoms and/or relatively minor laboratory abnormalities.
Laboratory values indicative of more severe COVID-19 infection in men could suggest a higher inflammatory response to the infection. Men also received more immunomodulators and antibiotics in this study. A recent paper from Scully et al14 pointed out the different immune response to viruses observed in men that could partially explain the higher level of inflammation markers and the more severe disease observed in men.
Limitations
Our study has several limitations. As an observational study of hospitalized patients, it may represent patients with more severe COVID-19. Men and women may have sought hospital care differently. Diagnosis, testing, and treatment were not standardized and may have been influenced by patient gender. Although we attempted to match patients on baseline medical conditions, we may not have completely controlled for differences in preexisting health. Finally, gender data were collected as binary and so did not capture other gender categories.
CONCLUSION
In our multicenter cohort of hospitalized COVID-19 patients, men had a higher burden of risk factors; different clinical presentations, with more fever and less olfactory and gastrointestinal symptoms; and a significantly poorer prognosis than women did at 30 days.
Acknowledgments
The authors thank Cardiovascular Excellence SL for their essential support regarding the database and HOPE web page as well as all HOPE researchers. The authors also thank Michael Andrews for his valuable contribution to the English revision.
1. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613-1620. https://doi.org/10.1016/j.mayocp.2020.05.014
2. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. https://doi.org/10.1186/s13293-020-00304-9
3. Gausman J, Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt). 2020;29(4):465-466. https://doi.org/10.1089/jwh.2020.8472
4. Walter LA, McGregor AJ. Sex- and gender-specific observations and implications for COVID-19. West J Emerg Med. 2020;21(3):507-509. https://doi.org/10.5811/westjem.2020.4.47536
5. Núñez-Gil IJ, Estrada V, Fernández-Pérez C, et al. Health outcome predictive evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemp Clin Trials Commun. 2020;20:100654. https://doi.org/10.1016/j.conctc.2020.100654
6. International COVID-19 Clinical Evaluation Registry: HOPE-COVID 19. Accessed February 6, 2021. https://hopeprojectmd.com/en/
7. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. https://doi.org/10.1038/s41420-020-0276-1
8. Ciaglia E, Vecchione C, Puca AA. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. https://doi.org/10.3389/fped.2020.00206
9. Peckham H, de Gruijter N, Raine C, et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. Research Square. April 20, 2020. https://doi.org/10.21203/rs.3.rs-23651/v2
10. Liu J, Zhang L, Chen Y, et al. Association of sex with clinical outcomes in COVID-19 patients: a retrospective analysis of 1190 cases. Respir Med. 2020;173:106159. https://doi.org/10.1016/j.rmed.2020.106159
11. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms—a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722-728. https://doi.org/10.1177/0194599820934380
12. Costa KVTD, Carnaúba ATL, Rocha KW, Andrade KCLD, Ferreira SMS, Menezes PTL. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86(6):781-792. https://doi.org/10.1016/j.bjorl.2020.05.008
13. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. https://doi.org/10.1007/s00405-020-05965-1
14. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-447. https://doi.org/10.1038/s41577-020-0348-8
1. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613-1620. https://doi.org/10.1016/j.mayocp.2020.05.014
2. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. https://doi.org/10.1186/s13293-020-00304-9
3. Gausman J, Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt). 2020;29(4):465-466. https://doi.org/10.1089/jwh.2020.8472
4. Walter LA, McGregor AJ. Sex- and gender-specific observations and implications for COVID-19. West J Emerg Med. 2020;21(3):507-509. https://doi.org/10.5811/westjem.2020.4.47536
5. Núñez-Gil IJ, Estrada V, Fernández-Pérez C, et al. Health outcome predictive evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemp Clin Trials Commun. 2020;20:100654. https://doi.org/10.1016/j.conctc.2020.100654
6. International COVID-19 Clinical Evaluation Registry: HOPE-COVID 19. Accessed February 6, 2021. https://hopeprojectmd.com/en/
7. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. https://doi.org/10.1038/s41420-020-0276-1
8. Ciaglia E, Vecchione C, Puca AA. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. https://doi.org/10.3389/fped.2020.00206
9. Peckham H, de Gruijter N, Raine C, et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. Research Square. April 20, 2020. https://doi.org/10.21203/rs.3.rs-23651/v2
10. Liu J, Zhang L, Chen Y, et al. Association of sex with clinical outcomes in COVID-19 patients: a retrospective analysis of 1190 cases. Respir Med. 2020;173:106159. https://doi.org/10.1016/j.rmed.2020.106159
11. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms—a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722-728. https://doi.org/10.1177/0194599820934380
12. Costa KVTD, Carnaúba ATL, Rocha KW, Andrade KCLD, Ferreira SMS, Menezes PTL. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86(6):781-792. https://doi.org/10.1016/j.bjorl.2020.05.008
13. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. https://doi.org/10.1007/s00405-020-05965-1
14. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-447. https://doi.org/10.1038/s41577-020-0348-8
© 2021 Society of Hospital Medicine
Mapping the Clinical Experience of a New York City Residency Program During the COVID-19 Pandemic
The COVID-19 pandemic has disrupted the educational experience of medical trainees around the world, and this has been especially true for those in New York City (NYC), the early epicenter of the global outbreak.1 The pandemic’s surge required redeployment of trainees away from scheduled rotations, focused didactics around emerging COVID-19 data, and seemingly narrowed trainees’ clinical exposure to a single respiratory infection.
While there is a small body of literature describing the programmatic responses2,3 and educational adaptations4-7 that have come about as a result of the pandemic’s disruptive force, a characterization of exactly how trainees’ clinical experiences have been affected is lacking. A detailed understanding of how trainees’ inpatient care activities evolved during the pandemic could provide valuable practice habits feedback, allow for comparisons across training sites, focus content selection for didactic learning and self-study, and potentially help forecast similar clinical changes in the event of a subsequent wave. Perhaps most important, as internal medicine (IM) trainees require broad exposure to diverse clinical conditions to mature toward independent practice, a characterization of exactly how the pandemic has narrowed the diversity of clinical exposure could inform changes in how trainees attain clinical competence.
Profiling IM residents’ clinical experiences in a meaningful way is particularly challenging given the extraordinary breadth of the field. We recently developed a strategy by which resident-attributed International Classification of Diseases, Tenth Revision (ICD-10) principal diagnosis codes are mapped to an educational taxonomy of medical content categories, yielding clinical exposure profiles.8 Here, we apply this mapping strategy to all four training hospitals of a large NYC IM residency program to catalogue the evolution of clinical diversity experienced by residents during the COVID-19 pandemic.
METHODS
Study Population
The NYU IM Residency Program comprises 225 resident physicians rotating at four inpatient training sites: NYU Langone Hospital–Brooklyn (NYU-BK), NYU Langone Hospitals–Manhattan (NYU-MN), Bellevue Hospital (BH), and VA–New York Harbor Healthcare (VA). The study period was defined as February 1, 2020, to May 31, 2020, to capture clinical exposure during baseline, surge, and immediate post-surge periods. The NYU IM residency program declared pandemic emergency status on March 23, 2020, after which all residents were assigned to inpatient acute care and intensive care rotations to augment the inpatient workforce.
Data Source
Clinical data at each training hospital are collected and stored, allowing for asynchronous querying. Given differences in data reporting, strategies for collecting principal ICD-10 codes of patients discharged during the study period differed slightly across sites. Principal ICD-10 codes from patients discharged from NYU-BK and NYU-MN were filtered by nursing unit, allowing selection for resident-staffed units. Principal ICD-10 codes from BH were curated by care team, allowing selection for resident-staffed teams. Principal ICD-10 codes from VA were filtered by both hospital unit and provider service to attribute to resident providers. Dates of each discharge were included, and mortalities were included as discharges. All methods yielded a dataset of principal ICD-10 discharge diagnosis codes attributed primarily to IM residents. Given the rapid changes in hospital staffing to care for increasing patient volumes, in rare circumstances residents and other providers (such as advanced practice providers) shared hospital units. While ICD-10 codes mined from each hospital are attributed primarily to residents, this attribution is not entirely exclusive. Data were analyzed both by training site and in aggregate across the four training sites. No individually identifiable data were analyzed, the primary goal of the project was to improve education, and the data were collected as part of a required aspect of training; as a result, this project met criteria for certification as a quality improvement, and not a human subject, research project.
The Crosswalk Tool
We previously developed a crosswalk tool containing 4,854 ICD-10 diagnoses uniquely mapped to 16 broad medical content areas as defined by the American Board of Internal Medicine (ABIM).8 Custom programs (MATLAB, MathWorks, Inc) captured and subsequently mapped resident-attributed ICD-10 discharge codes to content areas if the syntax of the ICD-10 code in question exactly matched or was nested within an ICD-10 code in the crosswalk. This tool allowed us to measure the daily discharge frequency of each content area across the sites.
Analysis
The sensitivity of the crosswalk tool was calculated as the number of ICD-10 codes captured divided by the total number of patients. Codes missed by the tool were excluded. The total number, as well as the 7-day running average of discharges per content area, across the sites during the study period were measured. To evaluate for differences in the distribution of content before and after pandemic emergency status, 2 × 16 χ2 contingency tables were constructed. To evaluate for changes in the mean relative proportions (%) of each content area, paired t tests were conducted. Confidence intervals were estimated from t distributions.
RESULTS
There were 6,613 patients discharged from all sites (NYU-BK, 2,062; NYU-MN, 2,188; BH, 1,711; VA, 652; Appendix Table). The crosswalk tool captured 6,384 principal discharge ICD-10 codes (96.5%). The five most common content areas during the study period were infectious diseases (ID; n = 2,892), cardiovascular disease (CVD; n = 1,199), gastroenterology (n = 406), pulmonary disease (n = 372), and nephrology and urology (n = 252). These were also the content areas most frequently encountered by residents at baseline (Figure and Table). The distribution of content prior to declaration of pandemic emergency status was significantly different than that after declaration (χ2 = 709; df, 15; P <.001). ID diagnoses, driven by COVID-19, rose steeply in the period following declaration, peaked in mid-April, and slowly waned in May (Figure). The mean relative percentage of ID discharges across the sites rose from 26.0% (16.5%-35.4%) at baseline to 58.3% (41.3%-75.3%) in the period after pandemic emergency status was declared (P = .005).
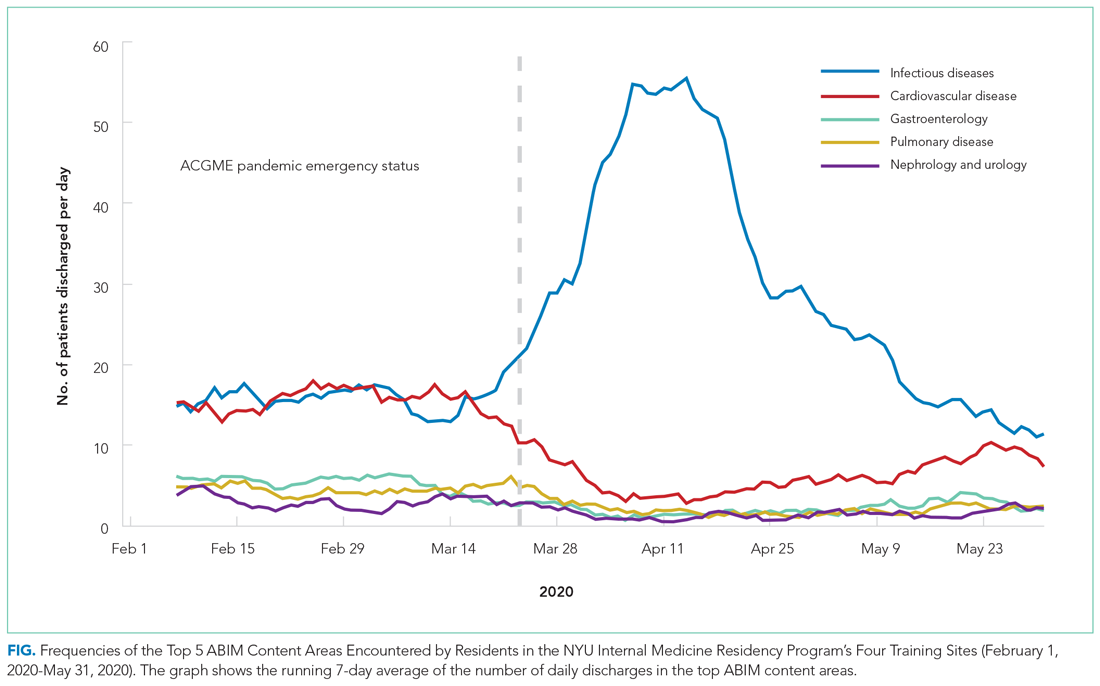
Frequencies of diagnoses mapping to other content areas decreased significantly, reflecting a marked tapering of clinical diversity (Figure and Table). Specifically, decreases were seen in CVD (27.6% [95% CI, 17.9%-37.2%] to 13.9% [95% CI, 5.5%-22.3%]; P = .013); gastroenterology (8.3% [95% CI, 6.2%-10.2%] to 4.6% [95% CI, 2.1%-6.9%]; P = .038); pulmonary disease (8.0% [95% CI, 5.6%-10.2%] to 4.6% [95% CI, 1.6%-7.4%]; P = .040); and nephrology and urology (4.8% [95% CI, 2.6%-6.9%] to 3.1% [95% CI, 1.9%-4.2%]; P = .047) (Table). In late April, diagnoses mapping to these content areas began to repopulate residents’ clinical experiences and by the end of the study period had nearly returned to baseline frequencies. These patterns were similar when discharge diagnoses from each training site were plotted individually (Appendix Figure).
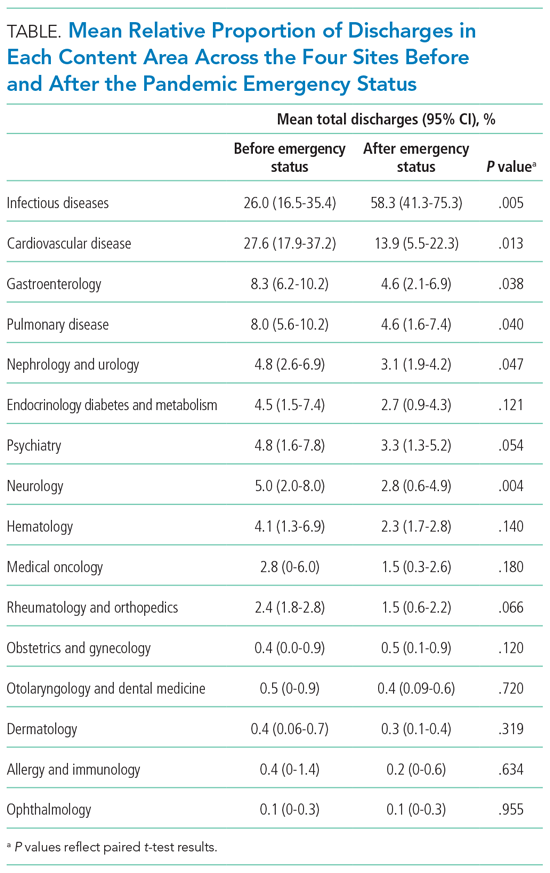
DISCUSSION
Here, we demonstrate how the clinical educational landscape changed for our residents during the COVID-19 pandemic. We uncover a dramatic deviation in the content to which residents were exposed through patient care activities that disproportionately favored ID at the expense of all other content. We demonstrate that this reduction in clinical diversity persisted for nearly 2 months and was similar at each of our training hospitals, and also provide a trajectory on which other content repopulated residents’ clinical experiences.
These data have served several valuable purposes and support ongoing efforts to map residents’ experiential curriculum at our program and others. Sharing this data with residents, as occurred routinely in town hall forums and noon conferences, has provided them with real-time practice feedback during a time of crisis. This has provided scope for their herculean efforts during the pandemic, served as a blueprint for underrepresented content most ripe for self-study, and offered reassurance of a return to normalcy given the trajectory of clinical content curves. As practice habits feedback is an Accreditation Council for Graduate Medical Education requirement, this strategy has also served as a robust and reproducible means of complying.
Our training program used this characterization of clinical content to help guide teaching in the pandemic era. For example, we preferentially structured case conferences and other didactics around reemerging content areas to capitalize on just-in-time education and harness residents’ eagerness for a respite from COVID-specific education. Residents required to quarantine at home were provided with learning plans centered on content underrepresented in clinical practice.
Given the critical importance of experiential learning in IM residents’ training, our findings quantifying significant changes in clinical exposure could form the basis for predicting poor outcomes in competency-based assessments for residents training in the COVID era, which continues to affect our trainees. For example, our characterization of clinical exposure may predict poor in-training exam or even ABIM certification exam performance in the content areas most drastically affected. Knowledge of this association of clinical exposure and clinical competence could allow training programs like ours to preempt poor performance in competency-based assessments by more aggressively shifting lectures, simulations, and other didactic programs toward content areas underrepresented in the pandemic’s wake.
Limitations of this study include the fact that availability of testing and ICD-10 coding for COVID-19 differed slightly across training sites, potentially contributing to site differences in mapping. Additionally, given our 1:1 mapping of ICD-10 codes to content categories, our strategy attributes COVID-19 to ID alone, and does not capture additional areas germane to this diagnosis, such as pulmonary disease.
CONCLUSION
We provide a detailed characterization of the evolution of a single IM program’s patient care experiences across four training hospitals during the COVID-19 pandemic. Such characterization can be leveraged to provide effective practice habits feedback and guide teaching efforts, and could form the basis to predict competency-based outcomes for trainees in the COVID era.
1. Accreditation Council for Graduate Medical Education. ACGME response to pandemic crisis. Accessed April 14, 2021. https://acgme.org/covid-19
2. Manson DK, Shen S, Lavelle MP, et al. Reorganizing a medicine residency program in response to the COVID-19 pandemic in New York. Acad Med. 2020;95(11):1670-1673. https://doi.org/10.1097/ACM.0000000000003548
3. Kee A, Archuleta S, Dan YY. Internal medicine residency training in the COVID-19 era—reflections from Singapore. J Grad Med Educ. 2020;12(4):406-408. https://doi.org/10.4300/JGME-D-20-00315.1
4. Kochis M, Goessling W. Learning during and from a crisis: the student-led development of a COVID-19 curriculum. Acad Med. 2021;96(3):399-401. https://doi.org/10.1097/ACM.0000000000003755
5 . Redinger JW, Cornia PB, Albert TJ. Teaching during a pandemic. J Grad Med Educ. 2020;12(4):403-405. https://doi.org/10.4300/JGME-D-20-00241.1
6. Liang ZC, Ooi SBS, Wang W. Pandemics and their impact on medical training: lessons from Singapore. Acad Med. 2020;95(9):1359-1361. https://doi.org/10.1097/ACM.0000000000003441
7. Tisdale R, Filsoof AR, Singhal S. Novel graduate medical education in the era of a novel virus. J Grad Med Educ. 2020;12(4):409-411. https://doi.org/10.4300/JGME-D-20-00225.1
8. Rhee DW, Chun JW, Stern DT, Sartori DJ. Experience and education in residency training: capturing the resident experience by mapping clinical data. Acad Med. Published online May 11, 2021. https://doi.org/10.1097/ACM.0000000000004162
The COVID-19 pandemic has disrupted the educational experience of medical trainees around the world, and this has been especially true for those in New York City (NYC), the early epicenter of the global outbreak.1 The pandemic’s surge required redeployment of trainees away from scheduled rotations, focused didactics around emerging COVID-19 data, and seemingly narrowed trainees’ clinical exposure to a single respiratory infection.
While there is a small body of literature describing the programmatic responses2,3 and educational adaptations4-7 that have come about as a result of the pandemic’s disruptive force, a characterization of exactly how trainees’ clinical experiences have been affected is lacking. A detailed understanding of how trainees’ inpatient care activities evolved during the pandemic could provide valuable practice habits feedback, allow for comparisons across training sites, focus content selection for didactic learning and self-study, and potentially help forecast similar clinical changes in the event of a subsequent wave. Perhaps most important, as internal medicine (IM) trainees require broad exposure to diverse clinical conditions to mature toward independent practice, a characterization of exactly how the pandemic has narrowed the diversity of clinical exposure could inform changes in how trainees attain clinical competence.
Profiling IM residents’ clinical experiences in a meaningful way is particularly challenging given the extraordinary breadth of the field. We recently developed a strategy by which resident-attributed International Classification of Diseases, Tenth Revision (ICD-10) principal diagnosis codes are mapped to an educational taxonomy of medical content categories, yielding clinical exposure profiles.8 Here, we apply this mapping strategy to all four training hospitals of a large NYC IM residency program to catalogue the evolution of clinical diversity experienced by residents during the COVID-19 pandemic.
METHODS
Study Population
The NYU IM Residency Program comprises 225 resident physicians rotating at four inpatient training sites: NYU Langone Hospital–Brooklyn (NYU-BK), NYU Langone Hospitals–Manhattan (NYU-MN), Bellevue Hospital (BH), and VA–New York Harbor Healthcare (VA). The study period was defined as February 1, 2020, to May 31, 2020, to capture clinical exposure during baseline, surge, and immediate post-surge periods. The NYU IM residency program declared pandemic emergency status on March 23, 2020, after which all residents were assigned to inpatient acute care and intensive care rotations to augment the inpatient workforce.
Data Source
Clinical data at each training hospital are collected and stored, allowing for asynchronous querying. Given differences in data reporting, strategies for collecting principal ICD-10 codes of patients discharged during the study period differed slightly across sites. Principal ICD-10 codes from patients discharged from NYU-BK and NYU-MN were filtered by nursing unit, allowing selection for resident-staffed units. Principal ICD-10 codes from BH were curated by care team, allowing selection for resident-staffed teams. Principal ICD-10 codes from VA were filtered by both hospital unit and provider service to attribute to resident providers. Dates of each discharge were included, and mortalities were included as discharges. All methods yielded a dataset of principal ICD-10 discharge diagnosis codes attributed primarily to IM residents. Given the rapid changes in hospital staffing to care for increasing patient volumes, in rare circumstances residents and other providers (such as advanced practice providers) shared hospital units. While ICD-10 codes mined from each hospital are attributed primarily to residents, this attribution is not entirely exclusive. Data were analyzed both by training site and in aggregate across the four training sites. No individually identifiable data were analyzed, the primary goal of the project was to improve education, and the data were collected as part of a required aspect of training; as a result, this project met criteria for certification as a quality improvement, and not a human subject, research project.
The Crosswalk Tool
We previously developed a crosswalk tool containing 4,854 ICD-10 diagnoses uniquely mapped to 16 broad medical content areas as defined by the American Board of Internal Medicine (ABIM).8 Custom programs (MATLAB, MathWorks, Inc) captured and subsequently mapped resident-attributed ICD-10 discharge codes to content areas if the syntax of the ICD-10 code in question exactly matched or was nested within an ICD-10 code in the crosswalk. This tool allowed us to measure the daily discharge frequency of each content area across the sites.
Analysis
The sensitivity of the crosswalk tool was calculated as the number of ICD-10 codes captured divided by the total number of patients. Codes missed by the tool were excluded. The total number, as well as the 7-day running average of discharges per content area, across the sites during the study period were measured. To evaluate for differences in the distribution of content before and after pandemic emergency status, 2 × 16 χ2 contingency tables were constructed. To evaluate for changes in the mean relative proportions (%) of each content area, paired t tests were conducted. Confidence intervals were estimated from t distributions.
RESULTS
There were 6,613 patients discharged from all sites (NYU-BK, 2,062; NYU-MN, 2,188; BH, 1,711; VA, 652; Appendix Table). The crosswalk tool captured 6,384 principal discharge ICD-10 codes (96.5%). The five most common content areas during the study period were infectious diseases (ID; n = 2,892), cardiovascular disease (CVD; n = 1,199), gastroenterology (n = 406), pulmonary disease (n = 372), and nephrology and urology (n = 252). These were also the content areas most frequently encountered by residents at baseline (Figure and Table). The distribution of content prior to declaration of pandemic emergency status was significantly different than that after declaration (χ2 = 709; df, 15; P <.001). ID diagnoses, driven by COVID-19, rose steeply in the period following declaration, peaked in mid-April, and slowly waned in May (Figure). The mean relative percentage of ID discharges across the sites rose from 26.0% (16.5%-35.4%) at baseline to 58.3% (41.3%-75.3%) in the period after pandemic emergency status was declared (P = .005).

Frequencies of diagnoses mapping to other content areas decreased significantly, reflecting a marked tapering of clinical diversity (Figure and Table). Specifically, decreases were seen in CVD (27.6% [95% CI, 17.9%-37.2%] to 13.9% [95% CI, 5.5%-22.3%]; P = .013); gastroenterology (8.3% [95% CI, 6.2%-10.2%] to 4.6% [95% CI, 2.1%-6.9%]; P = .038); pulmonary disease (8.0% [95% CI, 5.6%-10.2%] to 4.6% [95% CI, 1.6%-7.4%]; P = .040); and nephrology and urology (4.8% [95% CI, 2.6%-6.9%] to 3.1% [95% CI, 1.9%-4.2%]; P = .047) (Table). In late April, diagnoses mapping to these content areas began to repopulate residents’ clinical experiences and by the end of the study period had nearly returned to baseline frequencies. These patterns were similar when discharge diagnoses from each training site were plotted individually (Appendix Figure).

DISCUSSION
Here, we demonstrate how the clinical educational landscape changed for our residents during the COVID-19 pandemic. We uncover a dramatic deviation in the content to which residents were exposed through patient care activities that disproportionately favored ID at the expense of all other content. We demonstrate that this reduction in clinical diversity persisted for nearly 2 months and was similar at each of our training hospitals, and also provide a trajectory on which other content repopulated residents’ clinical experiences.
These data have served several valuable purposes and support ongoing efforts to map residents’ experiential curriculum at our program and others. Sharing this data with residents, as occurred routinely in town hall forums and noon conferences, has provided them with real-time practice feedback during a time of crisis. This has provided scope for their herculean efforts during the pandemic, served as a blueprint for underrepresented content most ripe for self-study, and offered reassurance of a return to normalcy given the trajectory of clinical content curves. As practice habits feedback is an Accreditation Council for Graduate Medical Education requirement, this strategy has also served as a robust and reproducible means of complying.
Our training program used this characterization of clinical content to help guide teaching in the pandemic era. For example, we preferentially structured case conferences and other didactics around reemerging content areas to capitalize on just-in-time education and harness residents’ eagerness for a respite from COVID-specific education. Residents required to quarantine at home were provided with learning plans centered on content underrepresented in clinical practice.
Given the critical importance of experiential learning in IM residents’ training, our findings quantifying significant changes in clinical exposure could form the basis for predicting poor outcomes in competency-based assessments for residents training in the COVID era, which continues to affect our trainees. For example, our characterization of clinical exposure may predict poor in-training exam or even ABIM certification exam performance in the content areas most drastically affected. Knowledge of this association of clinical exposure and clinical competence could allow training programs like ours to preempt poor performance in competency-based assessments by more aggressively shifting lectures, simulations, and other didactic programs toward content areas underrepresented in the pandemic’s wake.
Limitations of this study include the fact that availability of testing and ICD-10 coding for COVID-19 differed slightly across training sites, potentially contributing to site differences in mapping. Additionally, given our 1:1 mapping of ICD-10 codes to content categories, our strategy attributes COVID-19 to ID alone, and does not capture additional areas germane to this diagnosis, such as pulmonary disease.
CONCLUSION
We provide a detailed characterization of the evolution of a single IM program’s patient care experiences across four training hospitals during the COVID-19 pandemic. Such characterization can be leveraged to provide effective practice habits feedback and guide teaching efforts, and could form the basis to predict competency-based outcomes for trainees in the COVID era.
The COVID-19 pandemic has disrupted the educational experience of medical trainees around the world, and this has been especially true for those in New York City (NYC), the early epicenter of the global outbreak.1 The pandemic’s surge required redeployment of trainees away from scheduled rotations, focused didactics around emerging COVID-19 data, and seemingly narrowed trainees’ clinical exposure to a single respiratory infection.
While there is a small body of literature describing the programmatic responses2,3 and educational adaptations4-7 that have come about as a result of the pandemic’s disruptive force, a characterization of exactly how trainees’ clinical experiences have been affected is lacking. A detailed understanding of how trainees’ inpatient care activities evolved during the pandemic could provide valuable practice habits feedback, allow for comparisons across training sites, focus content selection for didactic learning and self-study, and potentially help forecast similar clinical changes in the event of a subsequent wave. Perhaps most important, as internal medicine (IM) trainees require broad exposure to diverse clinical conditions to mature toward independent practice, a characterization of exactly how the pandemic has narrowed the diversity of clinical exposure could inform changes in how trainees attain clinical competence.
Profiling IM residents’ clinical experiences in a meaningful way is particularly challenging given the extraordinary breadth of the field. We recently developed a strategy by which resident-attributed International Classification of Diseases, Tenth Revision (ICD-10) principal diagnosis codes are mapped to an educational taxonomy of medical content categories, yielding clinical exposure profiles.8 Here, we apply this mapping strategy to all four training hospitals of a large NYC IM residency program to catalogue the evolution of clinical diversity experienced by residents during the COVID-19 pandemic.
METHODS
Study Population
The NYU IM Residency Program comprises 225 resident physicians rotating at four inpatient training sites: NYU Langone Hospital–Brooklyn (NYU-BK), NYU Langone Hospitals–Manhattan (NYU-MN), Bellevue Hospital (BH), and VA–New York Harbor Healthcare (VA). The study period was defined as February 1, 2020, to May 31, 2020, to capture clinical exposure during baseline, surge, and immediate post-surge periods. The NYU IM residency program declared pandemic emergency status on March 23, 2020, after which all residents were assigned to inpatient acute care and intensive care rotations to augment the inpatient workforce.
Data Source
Clinical data at each training hospital are collected and stored, allowing for asynchronous querying. Given differences in data reporting, strategies for collecting principal ICD-10 codes of patients discharged during the study period differed slightly across sites. Principal ICD-10 codes from patients discharged from NYU-BK and NYU-MN were filtered by nursing unit, allowing selection for resident-staffed units. Principal ICD-10 codes from BH were curated by care team, allowing selection for resident-staffed teams. Principal ICD-10 codes from VA were filtered by both hospital unit and provider service to attribute to resident providers. Dates of each discharge were included, and mortalities were included as discharges. All methods yielded a dataset of principal ICD-10 discharge diagnosis codes attributed primarily to IM residents. Given the rapid changes in hospital staffing to care for increasing patient volumes, in rare circumstances residents and other providers (such as advanced practice providers) shared hospital units. While ICD-10 codes mined from each hospital are attributed primarily to residents, this attribution is not entirely exclusive. Data were analyzed both by training site and in aggregate across the four training sites. No individually identifiable data were analyzed, the primary goal of the project was to improve education, and the data were collected as part of a required aspect of training; as a result, this project met criteria for certification as a quality improvement, and not a human subject, research project.
The Crosswalk Tool
We previously developed a crosswalk tool containing 4,854 ICD-10 diagnoses uniquely mapped to 16 broad medical content areas as defined by the American Board of Internal Medicine (ABIM).8 Custom programs (MATLAB, MathWorks, Inc) captured and subsequently mapped resident-attributed ICD-10 discharge codes to content areas if the syntax of the ICD-10 code in question exactly matched or was nested within an ICD-10 code in the crosswalk. This tool allowed us to measure the daily discharge frequency of each content area across the sites.
Analysis
The sensitivity of the crosswalk tool was calculated as the number of ICD-10 codes captured divided by the total number of patients. Codes missed by the tool were excluded. The total number, as well as the 7-day running average of discharges per content area, across the sites during the study period were measured. To evaluate for differences in the distribution of content before and after pandemic emergency status, 2 × 16 χ2 contingency tables were constructed. To evaluate for changes in the mean relative proportions (%) of each content area, paired t tests were conducted. Confidence intervals were estimated from t distributions.
RESULTS
There were 6,613 patients discharged from all sites (NYU-BK, 2,062; NYU-MN, 2,188; BH, 1,711; VA, 652; Appendix Table). The crosswalk tool captured 6,384 principal discharge ICD-10 codes (96.5%). The five most common content areas during the study period were infectious diseases (ID; n = 2,892), cardiovascular disease (CVD; n = 1,199), gastroenterology (n = 406), pulmonary disease (n = 372), and nephrology and urology (n = 252). These were also the content areas most frequently encountered by residents at baseline (Figure and Table). The distribution of content prior to declaration of pandemic emergency status was significantly different than that after declaration (χ2 = 709; df, 15; P <.001). ID diagnoses, driven by COVID-19, rose steeply in the period following declaration, peaked in mid-April, and slowly waned in May (Figure). The mean relative percentage of ID discharges across the sites rose from 26.0% (16.5%-35.4%) at baseline to 58.3% (41.3%-75.3%) in the period after pandemic emergency status was declared (P = .005).

Frequencies of diagnoses mapping to other content areas decreased significantly, reflecting a marked tapering of clinical diversity (Figure and Table). Specifically, decreases were seen in CVD (27.6% [95% CI, 17.9%-37.2%] to 13.9% [95% CI, 5.5%-22.3%]; P = .013); gastroenterology (8.3% [95% CI, 6.2%-10.2%] to 4.6% [95% CI, 2.1%-6.9%]; P = .038); pulmonary disease (8.0% [95% CI, 5.6%-10.2%] to 4.6% [95% CI, 1.6%-7.4%]; P = .040); and nephrology and urology (4.8% [95% CI, 2.6%-6.9%] to 3.1% [95% CI, 1.9%-4.2%]; P = .047) (Table). In late April, diagnoses mapping to these content areas began to repopulate residents’ clinical experiences and by the end of the study period had nearly returned to baseline frequencies. These patterns were similar when discharge diagnoses from each training site were plotted individually (Appendix Figure).

DISCUSSION
Here, we demonstrate how the clinical educational landscape changed for our residents during the COVID-19 pandemic. We uncover a dramatic deviation in the content to which residents were exposed through patient care activities that disproportionately favored ID at the expense of all other content. We demonstrate that this reduction in clinical diversity persisted for nearly 2 months and was similar at each of our training hospitals, and also provide a trajectory on which other content repopulated residents’ clinical experiences.
These data have served several valuable purposes and support ongoing efforts to map residents’ experiential curriculum at our program and others. Sharing this data with residents, as occurred routinely in town hall forums and noon conferences, has provided them with real-time practice feedback during a time of crisis. This has provided scope for their herculean efforts during the pandemic, served as a blueprint for underrepresented content most ripe for self-study, and offered reassurance of a return to normalcy given the trajectory of clinical content curves. As practice habits feedback is an Accreditation Council for Graduate Medical Education requirement, this strategy has also served as a robust and reproducible means of complying.
Our training program used this characterization of clinical content to help guide teaching in the pandemic era. For example, we preferentially structured case conferences and other didactics around reemerging content areas to capitalize on just-in-time education and harness residents’ eagerness for a respite from COVID-specific education. Residents required to quarantine at home were provided with learning plans centered on content underrepresented in clinical practice.
Given the critical importance of experiential learning in IM residents’ training, our findings quantifying significant changes in clinical exposure could form the basis for predicting poor outcomes in competency-based assessments for residents training in the COVID era, which continues to affect our trainees. For example, our characterization of clinical exposure may predict poor in-training exam or even ABIM certification exam performance in the content areas most drastically affected. Knowledge of this association of clinical exposure and clinical competence could allow training programs like ours to preempt poor performance in competency-based assessments by more aggressively shifting lectures, simulations, and other didactic programs toward content areas underrepresented in the pandemic’s wake.
Limitations of this study include the fact that availability of testing and ICD-10 coding for COVID-19 differed slightly across training sites, potentially contributing to site differences in mapping. Additionally, given our 1:1 mapping of ICD-10 codes to content categories, our strategy attributes COVID-19 to ID alone, and does not capture additional areas germane to this diagnosis, such as pulmonary disease.
CONCLUSION
We provide a detailed characterization of the evolution of a single IM program’s patient care experiences across four training hospitals during the COVID-19 pandemic. Such characterization can be leveraged to provide effective practice habits feedback and guide teaching efforts, and could form the basis to predict competency-based outcomes for trainees in the COVID era.
1. Accreditation Council for Graduate Medical Education. ACGME response to pandemic crisis. Accessed April 14, 2021. https://acgme.org/covid-19
2. Manson DK, Shen S, Lavelle MP, et al. Reorganizing a medicine residency program in response to the COVID-19 pandemic in New York. Acad Med. 2020;95(11):1670-1673. https://doi.org/10.1097/ACM.0000000000003548
3. Kee A, Archuleta S, Dan YY. Internal medicine residency training in the COVID-19 era—reflections from Singapore. J Grad Med Educ. 2020;12(4):406-408. https://doi.org/10.4300/JGME-D-20-00315.1
4. Kochis M, Goessling W. Learning during and from a crisis: the student-led development of a COVID-19 curriculum. Acad Med. 2021;96(3):399-401. https://doi.org/10.1097/ACM.0000000000003755
5 . Redinger JW, Cornia PB, Albert TJ. Teaching during a pandemic. J Grad Med Educ. 2020;12(4):403-405. https://doi.org/10.4300/JGME-D-20-00241.1
6. Liang ZC, Ooi SBS, Wang W. Pandemics and their impact on medical training: lessons from Singapore. Acad Med. 2020;95(9):1359-1361. https://doi.org/10.1097/ACM.0000000000003441
7. Tisdale R, Filsoof AR, Singhal S. Novel graduate medical education in the era of a novel virus. J Grad Med Educ. 2020;12(4):409-411. https://doi.org/10.4300/JGME-D-20-00225.1
8. Rhee DW, Chun JW, Stern DT, Sartori DJ. Experience and education in residency training: capturing the resident experience by mapping clinical data. Acad Med. Published online May 11, 2021. https://doi.org/10.1097/ACM.0000000000004162
1. Accreditation Council for Graduate Medical Education. ACGME response to pandemic crisis. Accessed April 14, 2021. https://acgme.org/covid-19
2. Manson DK, Shen S, Lavelle MP, et al. Reorganizing a medicine residency program in response to the COVID-19 pandemic in New York. Acad Med. 2020;95(11):1670-1673. https://doi.org/10.1097/ACM.0000000000003548
3. Kee A, Archuleta S, Dan YY. Internal medicine residency training in the COVID-19 era—reflections from Singapore. J Grad Med Educ. 2020;12(4):406-408. https://doi.org/10.4300/JGME-D-20-00315.1
4. Kochis M, Goessling W. Learning during and from a crisis: the student-led development of a COVID-19 curriculum. Acad Med. 2021;96(3):399-401. https://doi.org/10.1097/ACM.0000000000003755
5 . Redinger JW, Cornia PB, Albert TJ. Teaching during a pandemic. J Grad Med Educ. 2020;12(4):403-405. https://doi.org/10.4300/JGME-D-20-00241.1
6. Liang ZC, Ooi SBS, Wang W. Pandemics and their impact on medical training: lessons from Singapore. Acad Med. 2020;95(9):1359-1361. https://doi.org/10.1097/ACM.0000000000003441
7. Tisdale R, Filsoof AR, Singhal S. Novel graduate medical education in the era of a novel virus. J Grad Med Educ. 2020;12(4):409-411. https://doi.org/10.4300/JGME-D-20-00225.1
8. Rhee DW, Chun JW, Stern DT, Sartori DJ. Experience and education in residency training: capturing the resident experience by mapping clinical data. Acad Med. Published online May 11, 2021. https://doi.org/10.1097/ACM.0000000000004162
© 2021 Society of Hospital Medicine
Impact of a Hospitalist-Run Procedure Service on Time to Paracentesis and Length of Stay
Peritoneal fluid examination is often recommended for hospitalized patients with ascites.1
Internal medicine residency programs are establishing procedure services to address concerns about resident training in procedures and patient safety.
METHODS
An inpatient hospitalist-run procedure service was introduced on July 1, 2016. The service was staffed by a hospitalist and second-year internal medicine residents. The service is available 7:00
Data on age, gender, race, ethnicity, date and time of hospital admission, and discharge date and time were retrieved. We also retrieved data on the absolute number of polymorphonuclear leukocytes (PMN) in the peritoneal fluid sample; a patient with a count higher than 250/uL was considered to have SBP. The timestamp for the peritoneal fluid results was used to approximate the A2P time. Paracenteses performed by or under direct supervision of procedure service hospitalists were identified through a procedure log maintained by procedure service hospitalists. We generated a binary variable to differentiate patients who were admitted during the day from those admitted during the night, when the procedure service was not available. For all patients, we calculated the model for end-stage liver disease and sodium (MELD-Na) score.13 Groups performing paracenteses were categorized into procedure service, residents, and radiology. Primary clinical services were categorized into general medicine, gastroenterology, surgery, and others.
Data were summarized as mean (SD) or median (interquartile range) for continuous variables and as percentages for categorical variables. Patients who had paracenteses by radiology or residents during the study period were considered controls. We used concurrent controls to address secular time trends (eg, measures to decrease LOS or changes in ordering tests in the electronic health record) in outcome measures. Patient characteristics were compared using the Wilcoxon rank-sum test or the χ2 test, as appropriate.
Two outcome variables were examined: LOS, and A2P time. Because both outcome variables were right skewed, we used generalized linear models with gamma distribution and log link. The advantage of a generalized linear model approach is that the transformed coefficients are better interpretable than when using the log transformation of the response variable.14 To account for time trends, we included time in months in the model. Models were adjusted for age, gender, race, whether the admission was during day or night, PMN in peritoneal fluid, MELD-Na score, platelet count on the day of procedure, presence or absence of cirrhosis, diagnosis-related groups weight, primary clinical service, and the group performing paracentesis. To address heterogeneity among patients included in our study and the fact that some patients had multiple paracenteses, we conducted sensitivity analyses by excluding all noncirrhotic patients and including only the first paracentesis. A P value less than .05 was considered significant. All statistical analyses were performed using Stata MP 16.0 for Windows (StataCorp LLC).
RESULTS
Of the 1,321 paracenteses included in our study, 509 (38.5%) were performed by the procedure service, 723 (54.7%) by residents, and 89 (6.7%) by radiology.
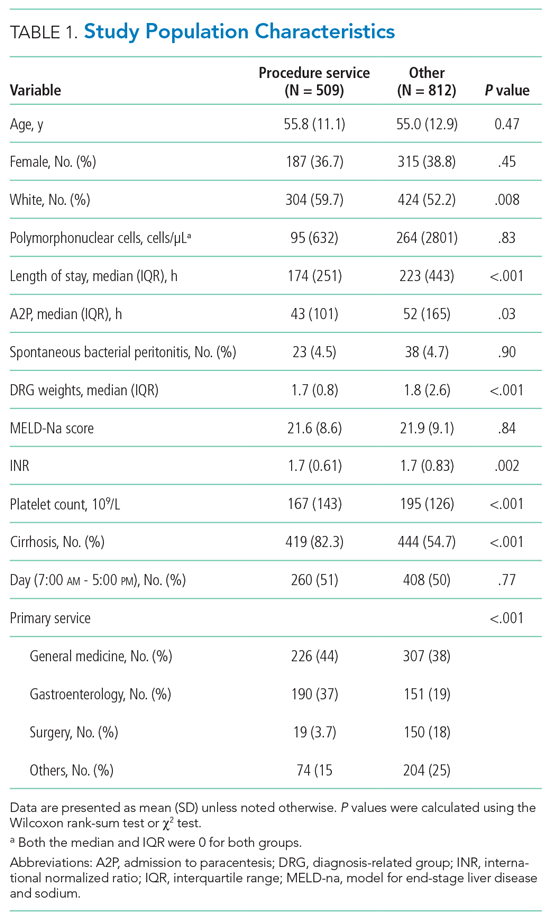
In unadjusted models but accounting for secular time trends, patients who had paracenteses performed by residents or by radiology had a 50% (95% CI, 22%-83%; P = .002) and 127% (95% CI, 65%-211%; P < .001) longer LOS, respectively, than when paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in LOS between radiology and the procedure service remained significant; patients who had a paracentesis performed by radiology had a 27% (95% CI, 2%-58%; P = .03) longer LOS than patients who had the procedure performed by the procedure service. This relative LOS translates into 88 (95% CI, 1-174 hours) additional hours in absolute LOS. There was no difference in LOS between the procedure service and residents in the adjusted analysis (Table 2).

Similarly, in unadjusted models for A2P time and accounting for secular time trends, patients who had a paracentesis performed by residents or by radiology had a 52% (95% CI, 23%-88%; P < .001) and 173% (95% CI, 109%-280%; P < .001) longer A2P time, respectively, than patients whose paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in A2P time between radiology and the procedure service remained significant. Patients who had paracentesis performed by radiology had a 40% (95% CI, 5%-87%; P = .02) longer A2P time than patients who had paracentesis performed by the procedure service. This relative increase translates into 52 (95% CI, 3.3-101 hours) additional hours in absolute A2P time. On the other hand, residents had a significantly shorter A2P time (–19%, 95% CI, –33% to 0.2%; P = .05) (Table 2).
In the sensitivity analysis, excluding noncirrhotic patients and including only the first paracentesis for patients who had multiple procedures performed during admission, the results remained unchanged. In adjusted analysis, patients who had paracentesis performed by radiology had a 47% (95% CI, 3.7%-108%; P = .03) longer LOS and 91% (95% CI, 19%-107%; P = .008) longer A2P time than when paracentesis was performed by the procedure service. There were no differences in LOS or A2P time between the procedure service and residents (Table 2).
DISCUSSION
In this study, we report that a hospitalist-run procedure service, when compared with a radiology service, is associated with decreased LOS and A2P time independent of studied potential confounders and secular time trends. We also showed that, compared with radiology, the A2P time for nonemergent procedures (those performed 6 hours after admission) was not adversely affected by the procedure service. Residents performing paracenteses independently had shorter A2P time than the procedure service.
Although several institutions have bedside procedure services, data are lacking on benefits. Previously, paracenteses performed by residents have been associated with decreased LOS and need for transfusions when compared with radiology.7 Our study extends these findings to show a shortened A2P time. Delays may occur when a patient is referred to radiology because of volume, triaging of higher-acuity procedures, and transportation. Procedure services provide consistent attending supervision, more procedures by upper-level residents, and a lower rate of unsuccessful procedures.12,15 Current study findings support the importance of continuing bedside procedure training for at least those residents who are interested in hospital medicine.7
Our study has several strengths and some potential limitations. The study examined outcomes that are important to patients as well as hospital administrators; it also had a large sample size, spanning 3 years.
CONCLUSION
We found that a hospitalist-run teaching procedure service is associated with shorter LOS and A2P time. Further research is needed to determine if the benefits of a procedure service extend to lowering morbidity and/or mortality, as well as to determine the cost-effectiveness of a procedure service and whether the significant investment by the institution in establishing a procedure service is mitigated by the gains from better patient outcomes and reduced LOS.
1. Runyon BA. AASLD guidelines. Management of adult patients with ascites due to cirrhosis: update 2012. April 2013. https://www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4.pdf
2. Rimola A, García-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32(1):142-153. https://doi.org/10.1016/S0168-8278(00)80201-9
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
4. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. https://doi.org/10.1111/jgh.13255
5. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
6. Chinnock B, Afarian H, Minnigan H, Butler J, Hendey GW. Physician clinical impression does not rule out spontaneous bacterial peritonitis in patients undergoing emergency department paracentesis. Ann Emerg Med. 2008;52(3):268-273. https://doi.org/10.1016/j.annemergmed.2008.02.016
7. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
8. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71.e17-24. https://doi.org/10.1016/j.amjmed.2005.08.007
9. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
10. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors—procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z
11. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018-1026. https://doi.org/10.1056/NEJMoa0801209
14. Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med. 1998;17(1):59-68. https://doi.org/10.1002/(sici)1097-0258(19980115)17:1<59::aid-sim733>3.0.co;2-7
15. Miller R, Garber A, Smith H, Malik M, Kimberly C, Qayyum R. Volume and supervision of resident procedures logged after implementation of a procedure medicine curriculum. J Gen Intern Med. Published online March 17, 2020. https://doi.org/10.1007/s11606-020-05763-9
Peritoneal fluid examination is often recommended for hospitalized patients with ascites.1
Internal medicine residency programs are establishing procedure services to address concerns about resident training in procedures and patient safety.
METHODS
An inpatient hospitalist-run procedure service was introduced on July 1, 2016. The service was staffed by a hospitalist and second-year internal medicine residents. The service is available 7:00
Data on age, gender, race, ethnicity, date and time of hospital admission, and discharge date and time were retrieved. We also retrieved data on the absolute number of polymorphonuclear leukocytes (PMN) in the peritoneal fluid sample; a patient with a count higher than 250/uL was considered to have SBP. The timestamp for the peritoneal fluid results was used to approximate the A2P time. Paracenteses performed by or under direct supervision of procedure service hospitalists were identified through a procedure log maintained by procedure service hospitalists. We generated a binary variable to differentiate patients who were admitted during the day from those admitted during the night, when the procedure service was not available. For all patients, we calculated the model for end-stage liver disease and sodium (MELD-Na) score.13 Groups performing paracenteses were categorized into procedure service, residents, and radiology. Primary clinical services were categorized into general medicine, gastroenterology, surgery, and others.
Data were summarized as mean (SD) or median (interquartile range) for continuous variables and as percentages for categorical variables. Patients who had paracenteses by radiology or residents during the study period were considered controls. We used concurrent controls to address secular time trends (eg, measures to decrease LOS or changes in ordering tests in the electronic health record) in outcome measures. Patient characteristics were compared using the Wilcoxon rank-sum test or the χ2 test, as appropriate.
Two outcome variables were examined: LOS, and A2P time. Because both outcome variables were right skewed, we used generalized linear models with gamma distribution and log link. The advantage of a generalized linear model approach is that the transformed coefficients are better interpretable than when using the log transformation of the response variable.14 To account for time trends, we included time in months in the model. Models were adjusted for age, gender, race, whether the admission was during day or night, PMN in peritoneal fluid, MELD-Na score, platelet count on the day of procedure, presence or absence of cirrhosis, diagnosis-related groups weight, primary clinical service, and the group performing paracentesis. To address heterogeneity among patients included in our study and the fact that some patients had multiple paracenteses, we conducted sensitivity analyses by excluding all noncirrhotic patients and including only the first paracentesis. A P value less than .05 was considered significant. All statistical analyses were performed using Stata MP 16.0 for Windows (StataCorp LLC).
RESULTS
Of the 1,321 paracenteses included in our study, 509 (38.5%) were performed by the procedure service, 723 (54.7%) by residents, and 89 (6.7%) by radiology.

In unadjusted models but accounting for secular time trends, patients who had paracenteses performed by residents or by radiology had a 50% (95% CI, 22%-83%; P = .002) and 127% (95% CI, 65%-211%; P < .001) longer LOS, respectively, than when paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in LOS between radiology and the procedure service remained significant; patients who had a paracentesis performed by radiology had a 27% (95% CI, 2%-58%; P = .03) longer LOS than patients who had the procedure performed by the procedure service. This relative LOS translates into 88 (95% CI, 1-174 hours) additional hours in absolute LOS. There was no difference in LOS between the procedure service and residents in the adjusted analysis (Table 2).

Similarly, in unadjusted models for A2P time and accounting for secular time trends, patients who had a paracentesis performed by residents or by radiology had a 52% (95% CI, 23%-88%; P < .001) and 173% (95% CI, 109%-280%; P < .001) longer A2P time, respectively, than patients whose paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in A2P time between radiology and the procedure service remained significant. Patients who had paracentesis performed by radiology had a 40% (95% CI, 5%-87%; P = .02) longer A2P time than patients who had paracentesis performed by the procedure service. This relative increase translates into 52 (95% CI, 3.3-101 hours) additional hours in absolute A2P time. On the other hand, residents had a significantly shorter A2P time (–19%, 95% CI, –33% to 0.2%; P = .05) (Table 2).
In the sensitivity analysis, excluding noncirrhotic patients and including only the first paracentesis for patients who had multiple procedures performed during admission, the results remained unchanged. In adjusted analysis, patients who had paracentesis performed by radiology had a 47% (95% CI, 3.7%-108%; P = .03) longer LOS and 91% (95% CI, 19%-107%; P = .008) longer A2P time than when paracentesis was performed by the procedure service. There were no differences in LOS or A2P time between the procedure service and residents (Table 2).
DISCUSSION
In this study, we report that a hospitalist-run procedure service, when compared with a radiology service, is associated with decreased LOS and A2P time independent of studied potential confounders and secular time trends. We also showed that, compared with radiology, the A2P time for nonemergent procedures (those performed 6 hours after admission) was not adversely affected by the procedure service. Residents performing paracenteses independently had shorter A2P time than the procedure service.
Although several institutions have bedside procedure services, data are lacking on benefits. Previously, paracenteses performed by residents have been associated with decreased LOS and need for transfusions when compared with radiology.7 Our study extends these findings to show a shortened A2P time. Delays may occur when a patient is referred to radiology because of volume, triaging of higher-acuity procedures, and transportation. Procedure services provide consistent attending supervision, more procedures by upper-level residents, and a lower rate of unsuccessful procedures.12,15 Current study findings support the importance of continuing bedside procedure training for at least those residents who are interested in hospital medicine.7
Our study has several strengths and some potential limitations. The study examined outcomes that are important to patients as well as hospital administrators; it also had a large sample size, spanning 3 years.
CONCLUSION
We found that a hospitalist-run teaching procedure service is associated with shorter LOS and A2P time. Further research is needed to determine if the benefits of a procedure service extend to lowering morbidity and/or mortality, as well as to determine the cost-effectiveness of a procedure service and whether the significant investment by the institution in establishing a procedure service is mitigated by the gains from better patient outcomes and reduced LOS.
Peritoneal fluid examination is often recommended for hospitalized patients with ascites.1
Internal medicine residency programs are establishing procedure services to address concerns about resident training in procedures and patient safety.
METHODS
An inpatient hospitalist-run procedure service was introduced on July 1, 2016. The service was staffed by a hospitalist and second-year internal medicine residents. The service is available 7:00
Data on age, gender, race, ethnicity, date and time of hospital admission, and discharge date and time were retrieved. We also retrieved data on the absolute number of polymorphonuclear leukocytes (PMN) in the peritoneal fluid sample; a patient with a count higher than 250/uL was considered to have SBP. The timestamp for the peritoneal fluid results was used to approximate the A2P time. Paracenteses performed by or under direct supervision of procedure service hospitalists were identified through a procedure log maintained by procedure service hospitalists. We generated a binary variable to differentiate patients who were admitted during the day from those admitted during the night, when the procedure service was not available. For all patients, we calculated the model for end-stage liver disease and sodium (MELD-Na) score.13 Groups performing paracenteses were categorized into procedure service, residents, and radiology. Primary clinical services were categorized into general medicine, gastroenterology, surgery, and others.
Data were summarized as mean (SD) or median (interquartile range) for continuous variables and as percentages for categorical variables. Patients who had paracenteses by radiology or residents during the study period were considered controls. We used concurrent controls to address secular time trends (eg, measures to decrease LOS or changes in ordering tests in the electronic health record) in outcome measures. Patient characteristics were compared using the Wilcoxon rank-sum test or the χ2 test, as appropriate.
Two outcome variables were examined: LOS, and A2P time. Because both outcome variables were right skewed, we used generalized linear models with gamma distribution and log link. The advantage of a generalized linear model approach is that the transformed coefficients are better interpretable than when using the log transformation of the response variable.14 To account for time trends, we included time in months in the model. Models were adjusted for age, gender, race, whether the admission was during day or night, PMN in peritoneal fluid, MELD-Na score, platelet count on the day of procedure, presence or absence of cirrhosis, diagnosis-related groups weight, primary clinical service, and the group performing paracentesis. To address heterogeneity among patients included in our study and the fact that some patients had multiple paracenteses, we conducted sensitivity analyses by excluding all noncirrhotic patients and including only the first paracentesis. A P value less than .05 was considered significant. All statistical analyses were performed using Stata MP 16.0 for Windows (StataCorp LLC).
RESULTS
Of the 1,321 paracenteses included in our study, 509 (38.5%) were performed by the procedure service, 723 (54.7%) by residents, and 89 (6.7%) by radiology.

In unadjusted models but accounting for secular time trends, patients who had paracenteses performed by residents or by radiology had a 50% (95% CI, 22%-83%; P = .002) and 127% (95% CI, 65%-211%; P < .001) longer LOS, respectively, than when paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in LOS between radiology and the procedure service remained significant; patients who had a paracentesis performed by radiology had a 27% (95% CI, 2%-58%; P = .03) longer LOS than patients who had the procedure performed by the procedure service. This relative LOS translates into 88 (95% CI, 1-174 hours) additional hours in absolute LOS. There was no difference in LOS between the procedure service and residents in the adjusted analysis (Table 2).

Similarly, in unadjusted models for A2P time and accounting for secular time trends, patients who had a paracentesis performed by residents or by radiology had a 52% (95% CI, 23%-88%; P < .001) and 173% (95% CI, 109%-280%; P < .001) longer A2P time, respectively, than patients whose paracentesis was performed by the procedure service. After adjusting for potential confounders, the difference in A2P time between radiology and the procedure service remained significant. Patients who had paracentesis performed by radiology had a 40% (95% CI, 5%-87%; P = .02) longer A2P time than patients who had paracentesis performed by the procedure service. This relative increase translates into 52 (95% CI, 3.3-101 hours) additional hours in absolute A2P time. On the other hand, residents had a significantly shorter A2P time (–19%, 95% CI, –33% to 0.2%; P = .05) (Table 2).
In the sensitivity analysis, excluding noncirrhotic patients and including only the first paracentesis for patients who had multiple procedures performed during admission, the results remained unchanged. In adjusted analysis, patients who had paracentesis performed by radiology had a 47% (95% CI, 3.7%-108%; P = .03) longer LOS and 91% (95% CI, 19%-107%; P = .008) longer A2P time than when paracentesis was performed by the procedure service. There were no differences in LOS or A2P time between the procedure service and residents (Table 2).
DISCUSSION
In this study, we report that a hospitalist-run procedure service, when compared with a radiology service, is associated with decreased LOS and A2P time independent of studied potential confounders and secular time trends. We also showed that, compared with radiology, the A2P time for nonemergent procedures (those performed 6 hours after admission) was not adversely affected by the procedure service. Residents performing paracenteses independently had shorter A2P time than the procedure service.
Although several institutions have bedside procedure services, data are lacking on benefits. Previously, paracenteses performed by residents have been associated with decreased LOS and need for transfusions when compared with radiology.7 Our study extends these findings to show a shortened A2P time. Delays may occur when a patient is referred to radiology because of volume, triaging of higher-acuity procedures, and transportation. Procedure services provide consistent attending supervision, more procedures by upper-level residents, and a lower rate of unsuccessful procedures.12,15 Current study findings support the importance of continuing bedside procedure training for at least those residents who are interested in hospital medicine.7
Our study has several strengths and some potential limitations. The study examined outcomes that are important to patients as well as hospital administrators; it also had a large sample size, spanning 3 years.
CONCLUSION
We found that a hospitalist-run teaching procedure service is associated with shorter LOS and A2P time. Further research is needed to determine if the benefits of a procedure service extend to lowering morbidity and/or mortality, as well as to determine the cost-effectiveness of a procedure service and whether the significant investment by the institution in establishing a procedure service is mitigated by the gains from better patient outcomes and reduced LOS.
1. Runyon BA. AASLD guidelines. Management of adult patients with ascites due to cirrhosis: update 2012. April 2013. https://www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4.pdf
2. Rimola A, García-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32(1):142-153. https://doi.org/10.1016/S0168-8278(00)80201-9
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
4. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. https://doi.org/10.1111/jgh.13255
5. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
6. Chinnock B, Afarian H, Minnigan H, Butler J, Hendey GW. Physician clinical impression does not rule out spontaneous bacterial peritonitis in patients undergoing emergency department paracentesis. Ann Emerg Med. 2008;52(3):268-273. https://doi.org/10.1016/j.annemergmed.2008.02.016
7. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
8. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71.e17-24. https://doi.org/10.1016/j.amjmed.2005.08.007
9. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
10. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors—procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z
11. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018-1026. https://doi.org/10.1056/NEJMoa0801209
14. Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med. 1998;17(1):59-68. https://doi.org/10.1002/(sici)1097-0258(19980115)17:1<59::aid-sim733>3.0.co;2-7
15. Miller R, Garber A, Smith H, Malik M, Kimberly C, Qayyum R. Volume and supervision of resident procedures logged after implementation of a procedure medicine curriculum. J Gen Intern Med. Published online March 17, 2020. https://doi.org/10.1007/s11606-020-05763-9
1. Runyon BA. AASLD guidelines. Management of adult patients with ascites due to cirrhosis: update 2012. April 2013. https://www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4.pdf
2. Rimola A, García-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32(1):142-153. https://doi.org/10.1016/S0168-8278(00)80201-9
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
4. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. https://doi.org/10.1111/jgh.13255
5. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
6. Chinnock B, Afarian H, Minnigan H, Butler J, Hendey GW. Physician clinical impression does not rule out spontaneous bacterial peritonitis in patients undergoing emergency department paracentesis. Ann Emerg Med. 2008;52(3):268-273. https://doi.org/10.1016/j.annemergmed.2008.02.016
7. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
8. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71.e17-24. https://doi.org/10.1016/j.amjmed.2005.08.007
9. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
10. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors—procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z
11. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018-1026. https://doi.org/10.1056/NEJMoa0801209
14. Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med. 1998;17(1):59-68. https://doi.org/10.1002/(sici)1097-0258(19980115)17:1<59::aid-sim733>3.0.co;2-7
15. Miller R, Garber A, Smith H, Malik M, Kimberly C, Qayyum R. Volume and supervision of resident procedures logged after implementation of a procedure medicine curriculum. J Gen Intern Med. Published online March 17, 2020. https://doi.org/10.1007/s11606-020-05763-9
© Society of Hospital Medicine
Healthcare Encounter and Financial Impact of COVID-19 on Children’s Hospitals
To benefit patients and the public health of their communities, children’s hospitals across the United States prepared for and responded to COVID-19 by conserving personal protective equipment, suspending noncritical in-person healthcare encounters (including outpatient visits and elective surgeries), and implementing socially distanced essential care.1,2 These measures were promptly instituted during a time of both substantial uncertainty about the pandemic’s behavior in children—including its severity and duration—and extreme variation in local and state governments’ responses to the pandemic.
Congruent with other healthcare institutions, children’s hospitals calibrated their clinical operations to the evolving nature of the pandemic, prioritizing the safety of patients and staff while striving to maintain financial viability in the setting of increased costs and decreased revenue. In some cases, children’s hospitals aided adult hospitals and health systems by admitting young and middle-aged adult patients and by centralizing all pediatric patients requiring intensive care within a region. These efforts occurred while many children’s hospitals remained the sole source of specialized pediatric care, including care for rare complex health problems.
As the COVID-19 pandemic continues, there is a critical need to assess how the initial phase of the pandemic affected healthcare encounters and related finances in children’s hospitals. Understanding these trends will position children’s hospitals to project and prepare for subsequent COVID-19 surges, as well as future related public health crises that necessitate widespread social distancing. Therefore, we compared year-over-year trends in healthcare encounters and hospital charges across US children’s hospitals before and during the COVID-19 pandemic, focusing on the beginning of COVID-19 in the United States, which was defined as February through June 2020.
METHODS
This is a retrospective analysis of 26 children’s hospitals (22 freestanding, 4 nonfreestanding) from all US regions (12 South, 7 Midwest, 5 West, 2 Northeast) contributing encounter and financial data to the PROSPECT database (Children’s Hospital Association, Lenexa, Kansas) from February 1 to June 30 in both 2019 (before COVID-19) and 2020 (during COVID-19). In response to COVID-19, hospitals participating in PROSPECT increased the efficiency of data centralization and reporting in 2020 during the period February 1 to June 30 to expedite analysis and dissemination of findings.
The main outcome measures were the percentage of change in weekly encounters (inpatient bed-days, emergency department [ED] visits, and surgeries) and inflation-adjusted charges (categorized as inpatient care and outpatient care, such as ambulatory surgery, clinics, and ED visits) before vs during COVID-19.
RESULTS
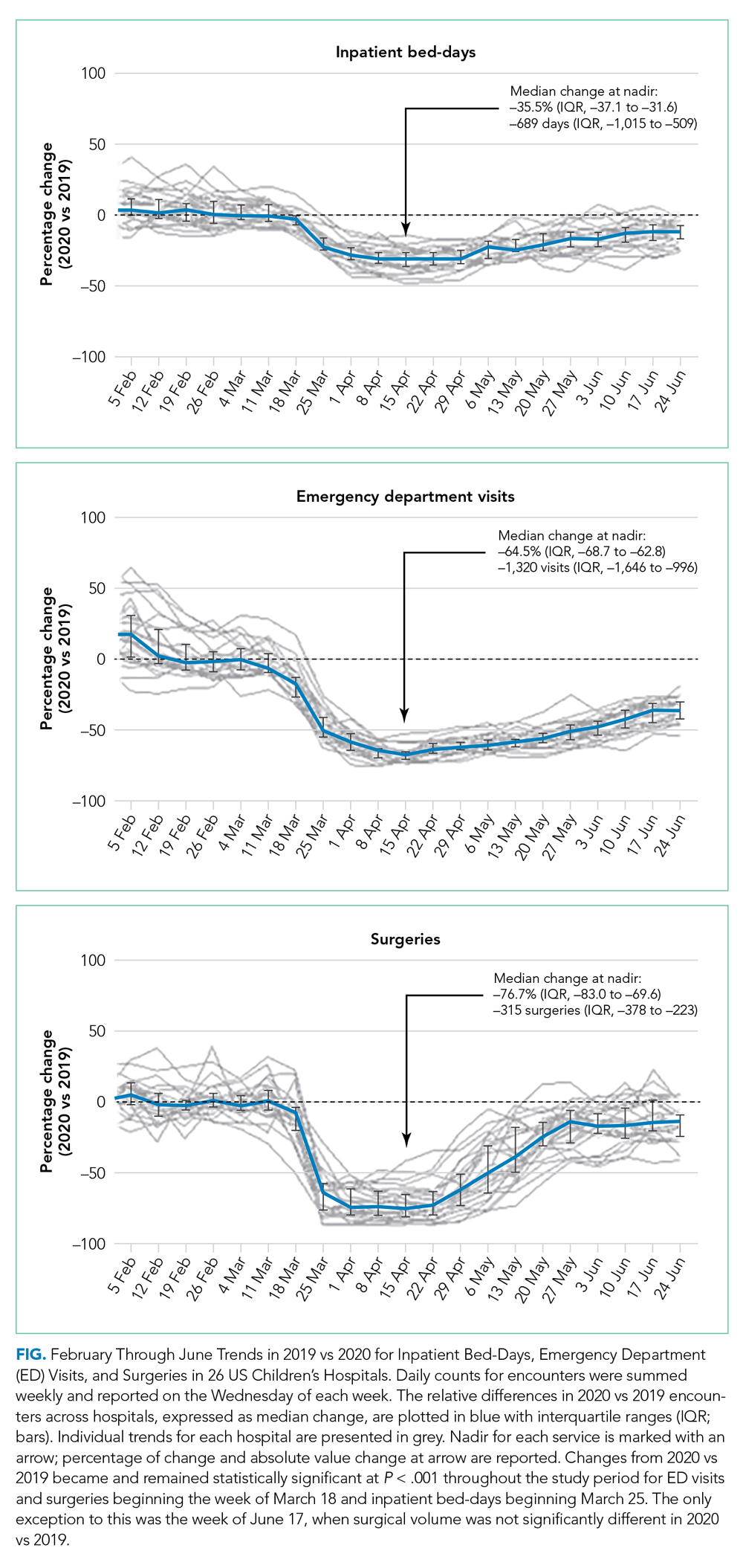
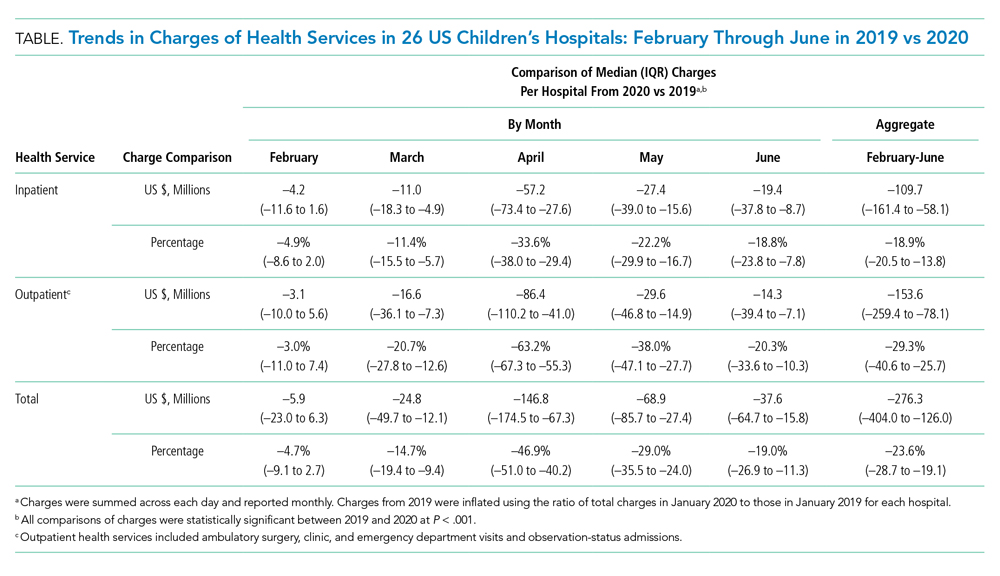
Charges that accrued from February 1 to June 30 were lower in 2020 by a median 23.6% (IQR, –28.7% to –19.1%) per children’s hospital than they were in 2019, corresponding to a median decrease of $276.3 million (IQR, $404.0-$126.0 million) in charges per hospital (Table). Forty percent of this decrease was attributable to decreased charges resulting from fewer inpatient healthcare encounters.
DISCUSSION
These findings beg the question of how well children’s hospitals are positioned to weather a recurrent surge in COVID-19. Because the severity of illness of COVID-19 has been lower to date in the pediatric vs adult populations, an increase in COVID-19-related visits to EDs and admissions to offset the decreased resource use of other pediatric healthcare problems is not anticipated. Existing hospital financial reserves as well as federal aid from the Coronavirus Aid, Relief, and Economic Security Act that helped mitigate the initial encounter and financial losses during the beginning of COVID-19 may not be readily available over time.4,5 Certainly, the findings from the current study support continued lobbying for additional state and federal funds allocated through future relief packages to children’s hospitals.
Additional approaches to financial solvency in children’s hospitals during the sustained COVID-19 pandemic include addressing surgical backlogs and sharing best practices for safe and sustained reopening of clinical operations and financial practices across institutions. Although the PROSPECT database does not contain information on the types of surgeries present within this backlog, our experiences suggest that both same-day and inpatient elective surgeries have been affected, especially lengthy procedures (eg, spinal fusion for neuromuscular scoliosis). Spread and scale of feasible and efficient solutions to reengineer and expand patient capacities and throughput for operating rooms, postanesthesia recovery areas, and intensive care and floor units are needed. Enhanced analytics that accurately predict postoperative length of hospital stay, coupled with early recovery after surgery clinical protocols, could help optimize hospital bed management. Effective ways to convert hospital rooms from single to double occupancy, to manage family visitation, and to proactively test asymptomatic patients, family, and hospital staff will mitigate continued COVID-19 penetration through children’s hospitals.
One important limitation of the current study is the measurement of hospitals’ charges. The charge data were not positioned to comprehensively measure each hospital’s financial state during the COVID-19 pandemic. However, the decrease in hospital charges reported by the children’s hospitals in the current study is comparable with the financial losses reported for many adult hospitals during the pandemic.6,7
CONCLUSION
Children’s hospitals’ ability to serve the nation’s pediatric patients depends on the success of the hospitals’ plans to manage current and future COVID-19 surges and to reopen and recover from the surges that have passed. Additional investigation is needed to identify best operational and financial practices among children’s hospitals that have enabled them to endure the COVID-19 pandemic.
1. COVID-19: ways to prepare your children’s hospital now. Children’s Hospital Association. March 12, 2020. Accessed June 30, 2020. https://www.childrenshospitals.org/Newsroom/Childrens-Hospitals-Today/Articles/2020/03/COVID-19-11-Ways-to-Prepare-Your-Hospital-Now
2. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
3. Oseran AS, Nash D, Kim C, et al. Changes in hospital admissions for urgent conditions during COVID-19 pandemic. Am J Manag Care. 2020;26(8):327-328. https://doi.org/10.37765/ajmc.2020.43837
4. Coronavirus Aid, Relief, and Economic Security Act or the CARES Act. 15 USC Chapter 116 (2020). Pub L No. 116-36, 134 Stat 281. https://www.congress.gov/bill/116th-congress/house-bill/748
5. The Coronavirus Aid, Relief, and Economic Security (CARES) Act Provider Relief Fund: general information. US Department of Health & Human Services. June 25, 2020. Accessed June 30, 2020. https://www.hhs.gov/coronavirus/cares-act-provider-relief-fund/general-information/index.html
6. Hospitals and health systems face unprecedented financial pressures due to COVID-19. American Hospital Association. May 2020. Accessed July 13, 2020. https://www.aha.org/system/files/media/file/2020/05/aha-covid19-financial-impact-0520-FINAL.pdf
7. Birkmeyer J, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
To benefit patients and the public health of their communities, children’s hospitals across the United States prepared for and responded to COVID-19 by conserving personal protective equipment, suspending noncritical in-person healthcare encounters (including outpatient visits and elective surgeries), and implementing socially distanced essential care.1,2 These measures were promptly instituted during a time of both substantial uncertainty about the pandemic’s behavior in children—including its severity and duration—and extreme variation in local and state governments’ responses to the pandemic.
Congruent with other healthcare institutions, children’s hospitals calibrated their clinical operations to the evolving nature of the pandemic, prioritizing the safety of patients and staff while striving to maintain financial viability in the setting of increased costs and decreased revenue. In some cases, children’s hospitals aided adult hospitals and health systems by admitting young and middle-aged adult patients and by centralizing all pediatric patients requiring intensive care within a region. These efforts occurred while many children’s hospitals remained the sole source of specialized pediatric care, including care for rare complex health problems.
As the COVID-19 pandemic continues, there is a critical need to assess how the initial phase of the pandemic affected healthcare encounters and related finances in children’s hospitals. Understanding these trends will position children’s hospitals to project and prepare for subsequent COVID-19 surges, as well as future related public health crises that necessitate widespread social distancing. Therefore, we compared year-over-year trends in healthcare encounters and hospital charges across US children’s hospitals before and during the COVID-19 pandemic, focusing on the beginning of COVID-19 in the United States, which was defined as February through June 2020.
METHODS
This is a retrospective analysis of 26 children’s hospitals (22 freestanding, 4 nonfreestanding) from all US regions (12 South, 7 Midwest, 5 West, 2 Northeast) contributing encounter and financial data to the PROSPECT database (Children’s Hospital Association, Lenexa, Kansas) from February 1 to June 30 in both 2019 (before COVID-19) and 2020 (during COVID-19). In response to COVID-19, hospitals participating in PROSPECT increased the efficiency of data centralization and reporting in 2020 during the period February 1 to June 30 to expedite analysis and dissemination of findings.
The main outcome measures were the percentage of change in weekly encounters (inpatient bed-days, emergency department [ED] visits, and surgeries) and inflation-adjusted charges (categorized as inpatient care and outpatient care, such as ambulatory surgery, clinics, and ED visits) before vs during COVID-19.
RESULTS


Charges that accrued from February 1 to June 30 were lower in 2020 by a median 23.6% (IQR, –28.7% to –19.1%) per children’s hospital than they were in 2019, corresponding to a median decrease of $276.3 million (IQR, $404.0-$126.0 million) in charges per hospital (Table). Forty percent of this decrease was attributable to decreased charges resulting from fewer inpatient healthcare encounters.
DISCUSSION
These findings beg the question of how well children’s hospitals are positioned to weather a recurrent surge in COVID-19. Because the severity of illness of COVID-19 has been lower to date in the pediatric vs adult populations, an increase in COVID-19-related visits to EDs and admissions to offset the decreased resource use of other pediatric healthcare problems is not anticipated. Existing hospital financial reserves as well as federal aid from the Coronavirus Aid, Relief, and Economic Security Act that helped mitigate the initial encounter and financial losses during the beginning of COVID-19 may not be readily available over time.4,5 Certainly, the findings from the current study support continued lobbying for additional state and federal funds allocated through future relief packages to children’s hospitals.
Additional approaches to financial solvency in children’s hospitals during the sustained COVID-19 pandemic include addressing surgical backlogs and sharing best practices for safe and sustained reopening of clinical operations and financial practices across institutions. Although the PROSPECT database does not contain information on the types of surgeries present within this backlog, our experiences suggest that both same-day and inpatient elective surgeries have been affected, especially lengthy procedures (eg, spinal fusion for neuromuscular scoliosis). Spread and scale of feasible and efficient solutions to reengineer and expand patient capacities and throughput for operating rooms, postanesthesia recovery areas, and intensive care and floor units are needed. Enhanced analytics that accurately predict postoperative length of hospital stay, coupled with early recovery after surgery clinical protocols, could help optimize hospital bed management. Effective ways to convert hospital rooms from single to double occupancy, to manage family visitation, and to proactively test asymptomatic patients, family, and hospital staff will mitigate continued COVID-19 penetration through children’s hospitals.
One important limitation of the current study is the measurement of hospitals’ charges. The charge data were not positioned to comprehensively measure each hospital’s financial state during the COVID-19 pandemic. However, the decrease in hospital charges reported by the children’s hospitals in the current study is comparable with the financial losses reported for many adult hospitals during the pandemic.6,7
CONCLUSION
Children’s hospitals’ ability to serve the nation’s pediatric patients depends on the success of the hospitals’ plans to manage current and future COVID-19 surges and to reopen and recover from the surges that have passed. Additional investigation is needed to identify best operational and financial practices among children’s hospitals that have enabled them to endure the COVID-19 pandemic.
To benefit patients and the public health of their communities, children’s hospitals across the United States prepared for and responded to COVID-19 by conserving personal protective equipment, suspending noncritical in-person healthcare encounters (including outpatient visits and elective surgeries), and implementing socially distanced essential care.1,2 These measures were promptly instituted during a time of both substantial uncertainty about the pandemic’s behavior in children—including its severity and duration—and extreme variation in local and state governments’ responses to the pandemic.
Congruent with other healthcare institutions, children’s hospitals calibrated their clinical operations to the evolving nature of the pandemic, prioritizing the safety of patients and staff while striving to maintain financial viability in the setting of increased costs and decreased revenue. In some cases, children’s hospitals aided adult hospitals and health systems by admitting young and middle-aged adult patients and by centralizing all pediatric patients requiring intensive care within a region. These efforts occurred while many children’s hospitals remained the sole source of specialized pediatric care, including care for rare complex health problems.
As the COVID-19 pandemic continues, there is a critical need to assess how the initial phase of the pandemic affected healthcare encounters and related finances in children’s hospitals. Understanding these trends will position children’s hospitals to project and prepare for subsequent COVID-19 surges, as well as future related public health crises that necessitate widespread social distancing. Therefore, we compared year-over-year trends in healthcare encounters and hospital charges across US children’s hospitals before and during the COVID-19 pandemic, focusing on the beginning of COVID-19 in the United States, which was defined as February through June 2020.
METHODS
This is a retrospective analysis of 26 children’s hospitals (22 freestanding, 4 nonfreestanding) from all US regions (12 South, 7 Midwest, 5 West, 2 Northeast) contributing encounter and financial data to the PROSPECT database (Children’s Hospital Association, Lenexa, Kansas) from February 1 to June 30 in both 2019 (before COVID-19) and 2020 (during COVID-19). In response to COVID-19, hospitals participating in PROSPECT increased the efficiency of data centralization and reporting in 2020 during the period February 1 to June 30 to expedite analysis and dissemination of findings.
The main outcome measures were the percentage of change in weekly encounters (inpatient bed-days, emergency department [ED] visits, and surgeries) and inflation-adjusted charges (categorized as inpatient care and outpatient care, such as ambulatory surgery, clinics, and ED visits) before vs during COVID-19.
RESULTS


Charges that accrued from February 1 to June 30 were lower in 2020 by a median 23.6% (IQR, –28.7% to –19.1%) per children’s hospital than they were in 2019, corresponding to a median decrease of $276.3 million (IQR, $404.0-$126.0 million) in charges per hospital (Table). Forty percent of this decrease was attributable to decreased charges resulting from fewer inpatient healthcare encounters.
DISCUSSION
These findings beg the question of how well children’s hospitals are positioned to weather a recurrent surge in COVID-19. Because the severity of illness of COVID-19 has been lower to date in the pediatric vs adult populations, an increase in COVID-19-related visits to EDs and admissions to offset the decreased resource use of other pediatric healthcare problems is not anticipated. Existing hospital financial reserves as well as federal aid from the Coronavirus Aid, Relief, and Economic Security Act that helped mitigate the initial encounter and financial losses during the beginning of COVID-19 may not be readily available over time.4,5 Certainly, the findings from the current study support continued lobbying for additional state and federal funds allocated through future relief packages to children’s hospitals.
Additional approaches to financial solvency in children’s hospitals during the sustained COVID-19 pandemic include addressing surgical backlogs and sharing best practices for safe and sustained reopening of clinical operations and financial practices across institutions. Although the PROSPECT database does not contain information on the types of surgeries present within this backlog, our experiences suggest that both same-day and inpatient elective surgeries have been affected, especially lengthy procedures (eg, spinal fusion for neuromuscular scoliosis). Spread and scale of feasible and efficient solutions to reengineer and expand patient capacities and throughput for operating rooms, postanesthesia recovery areas, and intensive care and floor units are needed. Enhanced analytics that accurately predict postoperative length of hospital stay, coupled with early recovery after surgery clinical protocols, could help optimize hospital bed management. Effective ways to convert hospital rooms from single to double occupancy, to manage family visitation, and to proactively test asymptomatic patients, family, and hospital staff will mitigate continued COVID-19 penetration through children’s hospitals.
One important limitation of the current study is the measurement of hospitals’ charges. The charge data were not positioned to comprehensively measure each hospital’s financial state during the COVID-19 pandemic. However, the decrease in hospital charges reported by the children’s hospitals in the current study is comparable with the financial losses reported for many adult hospitals during the pandemic.6,7
CONCLUSION
Children’s hospitals’ ability to serve the nation’s pediatric patients depends on the success of the hospitals’ plans to manage current and future COVID-19 surges and to reopen and recover from the surges that have passed. Additional investigation is needed to identify best operational and financial practices among children’s hospitals that have enabled them to endure the COVID-19 pandemic.
1. COVID-19: ways to prepare your children’s hospital now. Children’s Hospital Association. March 12, 2020. Accessed June 30, 2020. https://www.childrenshospitals.org/Newsroom/Childrens-Hospitals-Today/Articles/2020/03/COVID-19-11-Ways-to-Prepare-Your-Hospital-Now
2. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
3. Oseran AS, Nash D, Kim C, et al. Changes in hospital admissions for urgent conditions during COVID-19 pandemic. Am J Manag Care. 2020;26(8):327-328. https://doi.org/10.37765/ajmc.2020.43837
4. Coronavirus Aid, Relief, and Economic Security Act or the CARES Act. 15 USC Chapter 116 (2020). Pub L No. 116-36, 134 Stat 281. https://www.congress.gov/bill/116th-congress/house-bill/748
5. The Coronavirus Aid, Relief, and Economic Security (CARES) Act Provider Relief Fund: general information. US Department of Health & Human Services. June 25, 2020. Accessed June 30, 2020. https://www.hhs.gov/coronavirus/cares-act-provider-relief-fund/general-information/index.html
6. Hospitals and health systems face unprecedented financial pressures due to COVID-19. American Hospital Association. May 2020. Accessed July 13, 2020. https://www.aha.org/system/files/media/file/2020/05/aha-covid19-financial-impact-0520-FINAL.pdf
7. Birkmeyer J, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
1. COVID-19: ways to prepare your children’s hospital now. Children’s Hospital Association. March 12, 2020. Accessed June 30, 2020. https://www.childrenshospitals.org/Newsroom/Childrens-Hospitals-Today/Articles/2020/03/COVID-19-11-Ways-to-Prepare-Your-Hospital-Now
2. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172(9):621-622. https://doi.org/10.7326/m20-0907
3. Oseran AS, Nash D, Kim C, et al. Changes in hospital admissions for urgent conditions during COVID-19 pandemic. Am J Manag Care. 2020;26(8):327-328. https://doi.org/10.37765/ajmc.2020.43837
4. Coronavirus Aid, Relief, and Economic Security Act or the CARES Act. 15 USC Chapter 116 (2020). Pub L No. 116-36, 134 Stat 281. https://www.congress.gov/bill/116th-congress/house-bill/748
5. The Coronavirus Aid, Relief, and Economic Security (CARES) Act Provider Relief Fund: general information. US Department of Health & Human Services. June 25, 2020. Accessed June 30, 2020. https://www.hhs.gov/coronavirus/cares-act-provider-relief-fund/general-information/index.html
6. Hospitals and health systems face unprecedented financial pressures due to COVID-19. American Hospital Association. May 2020. Accessed July 13, 2020. https://www.aha.org/system/files/media/file/2020/05/aha-covid19-financial-impact-0520-FINAL.pdf
7. Birkmeyer J, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
© 2021 Society of Hospital Medicine