User login
10 tips for overcoming common challenges of intrapartum fetal monitoring
Interpreting continuous fetal heart rate (FHR) monitoring is one of the most common tasks obstetricians perform during the course of intrapartum care. Notably, many providers do not seek ongoing training to optimize their ability to reliably and accurately interpret the FHR. Yet FHR interpretation is one of the most frequent causes of litigation in the modern obstetric practice. Failure to interpret continuous FHR monitoring appropriately is estimated to account for 75% of obstetric-related litigation.1
Continuous FHR monitoring during labor was introduced to identify infants at risk for developing hypoxic-ischemic encephalopathy (HIE). The rate of HIE has not declined, however, despite almost universal adoption of continuous FHR monitoring.2 Numerous reasons account for this failure, including ad hoc interpretation of terminology, lack of standardized protocols for management and intervention, and the oftentimes challenging patterns that must be interpreted.3 The confusion about and dissatisfaction with the current state of FHR monitoring has led to attempts to enhance our ability to identify infants at risk with additional approaches (such as fetal pulse oximetry and fetal ST-segment evaluation), and some have called for a complete overhaul of our approach to interpreting the FHR. Clark and colleagues stated recently, "It is time to start over and establish some common language, standard interpretation, and reasonable management principles and guidelines."3
We must recognize that, as a stand-alone tool, continuous FHR monitoring is ineffective for avoiding preventable adverse outcomes. It is most likely to be effective when used in accordance with published standard guidelines by professionals skilled in interpretation and when timely, appropriate interventions are performed based on that interpretation. Optimal FHR monitoring requires a collaborative perinatal team that performs the monitoring correctly, interprets it appropriately, and communicates the findings effectively, and in a timely fashion, to all members of the care team when a high-risk pattern is detected.
In this article we review some common challenges that clinicians encounter during intrapartum FHR monitoring and we offer 10 simple tips to help overcome these challenges. The clinical scenarios described are derived from published reports in the medical literature, published malpractice claims, and from our personal experience working in a major health care system as part of a team charged with overseeing ongoing certification and training of labor and delivery nurses.
Challenge: Signal ambiguity
CASE 1 Young woman in labor with first pregnancy
A 19-year-old woman presents in spontaneous labor with her first pregnancy, which has been uncomplicated. During the course of her care, it is noted that the FHR changes to a lower baseline than previously recorded. Evaluation reveals that the external monitor is tracking the maternal heart rate and not the FHR (FIGURE 1). After the monitor is adjusted, both the fetal and maternal rates are documented for a short period. Ultimately, continuous monitoring of the maternal heart rate is discontinued. After delivery of the infant several hours later, it is noted that the FHR continues to register on the monitor, and it is determined that for the last few hours the maternal heart rate has been traced.
| FIGURE 1 FHR tracing indicates signal ambiguity | ||
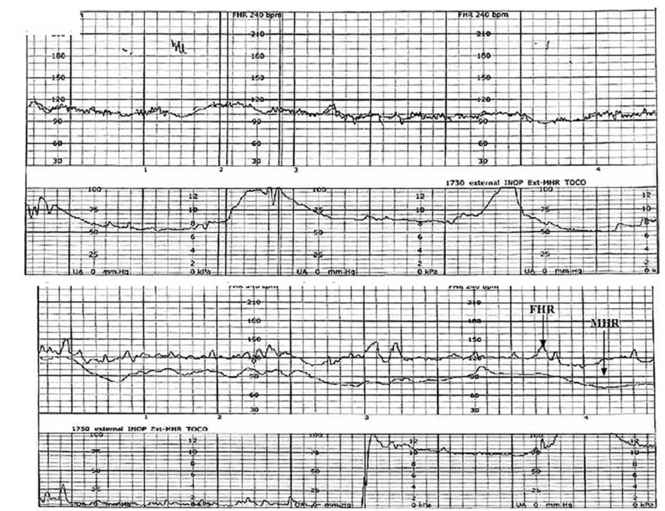 | ||
As described in Case 1, the upper panel of this tracing demonstrates the maternal heart rate confused as the fetal heart rate, while the segment in the lower panel shows a clear distinction between the maternal and fetal heart rates. | ||
TIP #10: Ensure the FHR monitor is tracking the fetal, not the maternal, heart rate
Confusing the maternal and the fetal heart rate with external cardiotocography is common. When the mix-up is noted and corrected expeditiously, it is unlikely to result in an adverse outcome. Signal ambiguity may arise from faulty Doppler equipment or the inability of the cardiotocograph to differentiate between maternal and fetal heart rates. It commonly occurs after repositioning the patient, after fetal movement, or during pushing in the second stage when the maternal heart rate may increase to a baseline that is similar to that of the fetus.
Signal ambiguity should be suspected when the FHR runs in the low-normal range or when FHR accelerations are noted with greater than 50% of contractions (especially when pushing).4 Signal ambiguity also should be ruled out when there is an apparent FHR deceleration to the maternal range that does not recover.
Evaluating for suspected signal ambiguity involves 2 key steps: (1) documentation and verification of the maternal heart rate and (2) definitive documentation of the true FHR. To document the maternal heart rate, manually count the radial pulse for 1 minute or use a pulse oximeter for continuous monitoring. Using a pulse oximeter is a less labor-intensive approach and has the advantage of allowing continuous assessment of the maternal heart rate for comparison. Recording the maternal pulse continuously on the same screen as the FHR enables ongoing differentiation of the mother and fetus in difficult cases, particularly if internal fetal monitoring is not an option (because of maternal infectious disease, low suspicion for an abnormal FHR pattern, or strong maternal preference against internal monitoring, for example).
When clinically appropriate, use of a fetal scalp electrode (FSE) can document the FHR. If intrauterine fetal death has occurred, however, the FSE may transmit the maternal heart rate.5 Using ultrasonography to confirm the FHR prior to placing the FSE is a reliable method of definitive differentiation. If a newly placed FSE shows a clear differentiation of 5 to 10 beats per minute from a continuously assessed maternal pulse rate, then this is also a reliable way to assure that the FHR monitoring represents the fetus, particularly if ultrasonography is not immediately available.
Ultimately, before intervening based on an abnormal FHR tracing, it is paramount to confirm that the data are adequate for interpretation and represent the actual FHR. If signal ambiguity is identified or suspected, correct it by using ultrasonography to locate the FHR and replace the external monitor until a rate that is at least 5 to 10 beats per minute different from the maternal rate is obtained. Alternatively, this is an indication for internal fetal monitoring with an FSE.
Challenge: Inadequate FHR tracing, poor communication, lack of clinical context
CASE 2 Woman with uncomplicated postdates pregnancy presents for induction
A 28-year-old woman (G3P2) at 41 weeks 0 days of gestation presents to labor and delivery for induction of labor for the indication of postdates. There have been no complications with the current pregnancy. The initial cervical exam reveals 1+ cm dilation, 90% effacement, and −3 station, and the patient is started on oxytocin per the hospital protocol. What is your interpretation of the continuous FHR tracing shown in FIGURE 2?
| FIGURE 2 Inadequate, uninterpretable FHR tracing | ||
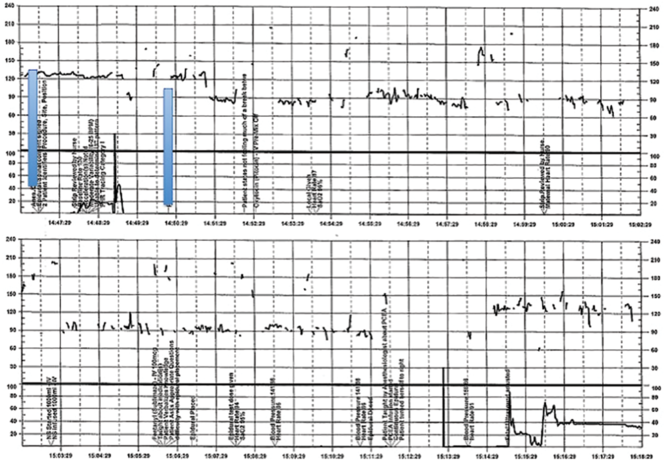 | ||
This FHR tracing, from the patient described in Case 2, is unusable because of the absence of data. | ||
TIP #9: Check that the monitors are providing useful data
The ability to accurately interpret a continuous FHR tracing depends on the quality of data recorded. Unfortunately, the absence of data makes interpretation impossible. This includes both FHR and tocometry data, since both pieces of information are required for appropriate interpretation of a continuous FHR tracing.
Prolonged periods of uninterpretable FHR and uterine activity tracings imply that no one was attending the mother and fetus.6 If it is difficult to obtain an interpretable FHR tracing, document in the medical record that you made ongoing efforts to maintain an adequate tracing, including the amount of time spent holding the external monitor, use of ultrasonography to document the FHR, and plans for potential internal monitoring.
CASE 2 Continued
After several hours, the patient requests an epidural for pain management and one is placed without difficulty. She reports adequate pain relief and is comfortable for the next 1 to 2 hours. Subsequently, the patient reports a sudden onset of increasing pain that does not respond to additional patient-administered doses of anesthesia over a 30-minute period. The labor and delivery nurse becomes concerned about the patient's pain level and contacts the attending physician to discuss her concerns. The physician, who is currently attending to patients in clinic, listens to the nurse and asks her to contact the anesthesia department with her concerns (FIGURE 3).
| FIGURE 3 FHR tracing reveals recurrent variables in a patient with evolving clinical concerns | ||
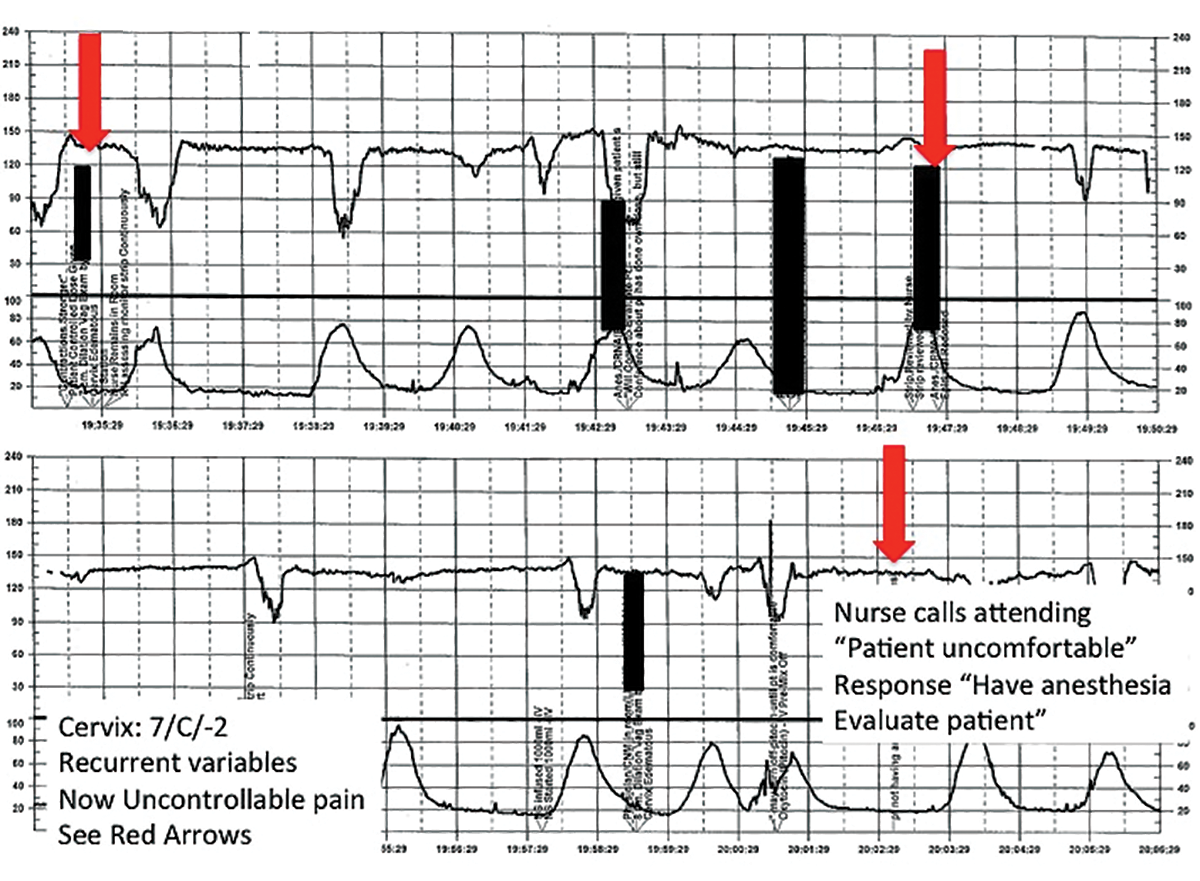 | ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #8: Clearly communicate an urgent situation to the care team
Poor communication underlies many preventable adverse outcomes in medicine.7 Effective communication requires an adequate description of the clinical scenario or problem. A root cause analysis of a series of intrapartum adverse events involving fetal death or injury showed that poor communication about a concerning FHR tracing played a role in 72% of cases.1
In this clinical scenario, the nurse believed that the patient's pain level was unusual or more than anticipated. The person who is communicating his or her concern (the sender) must be sure that the person receiving the message (the responder) clearly understands the sender's level of concern. In this case, it would have been appropriate for the sender to state clearly that she felt the patient's pain was outside of normal expectations and to request that the attending physician come to evaluate the patient.
Clear and effective communication includes (1) an appropriate description of the urgency of the situation and (2) an indication by the sender as to the desired response to this information ("please come evaluate the patient").8 In all cases, both steps are necessary to elicit an appropriate response.
CASE 2 Continued
Over the next 2 hours, recurrent variable decelerations develop, and then sudden, prolonged fetal bradycardia leads to urgent cesarean delivery. At delivery, a uterine rupture is diagnosed and a fetal hand is observed protruding through a lower-uterine segment defect into the maternal abdomen.
TIP #7: Always consider the entire clinical scenario
In this case, the team caring for the patient was not aware that her previous pregnancy had ended with a low transverse cesarean delivery. How does this information change your interpretation of the clinical scenario? The importance of understanding the entire clinical context when interpreting individual characteristics of cardiotocography cannot be overstated. For example, the sudden onset of recurrent, significant variable decelerations is more concerning in the context of a prior cesarean delivery, and late decelerations are more concerning in a patient with placental abruption, fetal growth restriction, or poorly controlled maternal diabetes.
An estimated 70% of fetuses will have an indeterminate FHR pattern (category II) at some time during labor.9 To appropriately interpret the FHR tracing, it is crucial to know the a priori risk for fetal hypoxia and metabolic acidosis (the precursor of fetal injury) due to such identified clinical risk factors as placental insufficiency, medical comorbidities (hypertension, diabetes), or postdates gestational age.
It is well established that cardiotocography has a good negative predictive value for the absence of fetal metabolic acidosis when there is moderate variability and spontaneous or induced accelerations. When attempting to risk stratify the fetus with a category II (indeterminate) FHR tracing, consider these 3 important questions:
- What are the risk factors for this particular patient and her fetus?
- What is the state of the fetus right now, and when was the last time metabolic acidosis could be excluded reasonably (by the presence of moderate variability and accelerations)?
- What is the risk that the fetus will develop acidemia prior to delivery?
The presence of decelerations indicates interruption of oxygen delivery to the fetus, and recurrent decelerations may indicate an evolving process of accumulated oxygen deprivation, hypoxia, and eventually, metabolic acidosis. Most authorities agree that, for the fetus with a previously normal FHR tracing, the onset of significant, recurrent decelerations with slowly cumulative oxygen deficit can lead to fetal acidemia over the course of approximately 1 hour.10 Of course, acidosis also can occur much more quickly with acute events, such as placental abruption or uterine rupture. In deciding whether or not to intervene based on an FHR tracing, the clinician must take into account the clinical context to determine if delivery is likely to occur before significant acidemia develops.
Challenge: Lack of situational awareness, failure to address nursing concerns, reluctance to initiate the chain of command
CASE 3 Spontaneous labor in a second pregnancy
A 28-year-old woman (G2P1) at 40 weeks' gestation presents in spontaneous labor. She has a history of a previous uncomplicated vaginal delivery. After 6 hours she reaches complete dilation with the fetus at −1 station and begins pushing. After 60 minutes, the patient has only progressed to +1 station. She is contracting every 1 to 2 minutes with recurrent variable decelerations (FIGURE 4).
| FIGURE 4 FHR tracing shows time points for initiation and continuation of pushing | ||
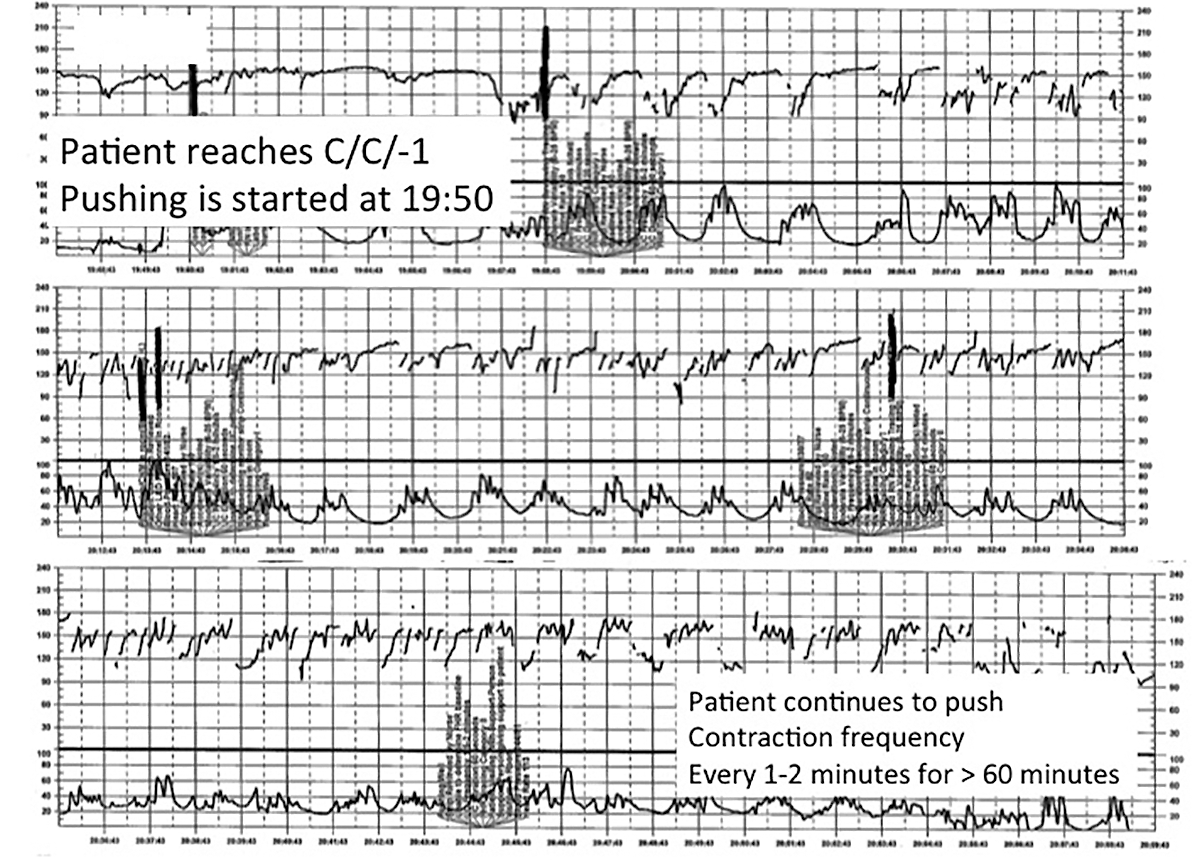 | ||
This tracing, from the patient described in Case 3, documents contraction frequency every 1-2 minutes for more than 60 minutes while the patient continues to push. The fetal heart rate demonstrates repetitive moderate variable decelerations with every push. | ||
TIP #6: Maintain situational awareness
A state of situational awareness exists when caregivers have a clear understanding of all of the factors at play in a clinical situation.11 This can be lost when caregivers focus too intensely on one aspect of care. It often happens when the patient is pushing in the second stage and the provider, focused on the progress of fetal descent, loses track of the amount of time that has passed without reassuring features (such as variability and induced or spontaneous accelerations) in the FHR tracing. The nurse, seeing the physician at the bedside, presumes he or she is aware of the tracing and is thus reluctant to point out the concerning features for fear of appearing insubordinate.
Situational awareness also may be lost at the time of patient hand off between providers wherein critical information, such as a history of previous cesarean delivery, is not communicated to the next care team. When receiving an intrapartum patient hand off, providers must have heightened vigilance to ensure they quickly reach situational awareness and are cognizant of the entire clinical context. Maintaining an environment in which all members of the care team, regardless of their training level, are encouraged to voice their concerns is another way to promote ongoing situational awareness.
CASE 3 Continued
The patient continues pushing for another 20 minutes without delivery, and the nurse raises a concern about the FHR tracing to the physician, who remains in the room but does not respond (FIGURE 5).
| FIGURE 5 FHR tracing reveals ongoing repetitive variable decelerations | ||
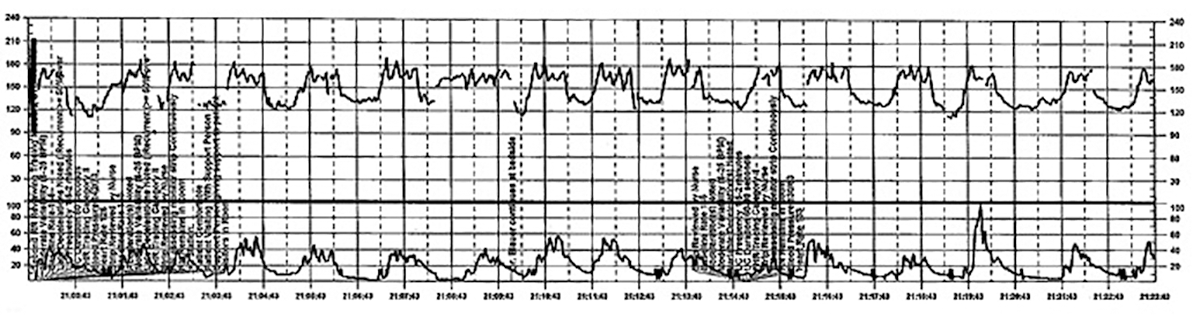 | ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #5: Acknowledge and respond to other caregivers' concerns
A team approach to patient care is essential in all areas of medicine, perhaps none more so than in obstetrics. Each member of the team is engaged in trying to provide optimal patient care and the concerns of every team member--regardless of title or level of training--must be acknowledged and addressed. Good communication requires creating a safe environment wherein each member of the team feels comfortable raising concerns without fear of reprisal. Rather than becoming angry or frustrated when questioned, providers should remain cognizant that these are ongoing efforts to maintain situational awareness and ensure the best possible outcome for mother and baby.
CASE 3 Continued
Pushing continues for another 30 minutes despite the nurse's repeated effort to express concern to the physician about the FHR tracing. After more than 2 hours of pushing, the infant is delivered; Apgar scores are 1, 5, and 7. No cord gas is obtained.
TIP #4: Initiate the chain of command when necessary
Any caregiver, regardless of job title, has a duty to initiate the institution's chain-of-command policy and procedure if he or she has a concern about patient well-being that is not being addressed adequately. It can be uncomfortable for a nurse, midwife, or resident physician to question an attending physician, particularly if that person responds in a dismissive, condescending, or angry manner. If a caregiver has made several attempts to engage the attending physician and feels the concerns are being inadequately addressed, then he or she must respectfully initiate the chain of command to seek additional objective review of the clinical situation.
Failure to follow oxytocin protocols, inadequate surveillance, poor documentation
CASE 4 Induction of an uncomplicated pregnancy due to postdates
A 20-year-old woman (G1P0) at 42 weeks' gestation with an otherwise uncomplicated first pregnancy presents for postdates induction with oxytocin. After 6 hours, she develops uterine tachysystole with recurrent variable decelerations but the oxytocin infusion is continued at the same rate (FIGURE 6).
| FIGURE 6 FHR tracing indicates uterine tachysystole | ||
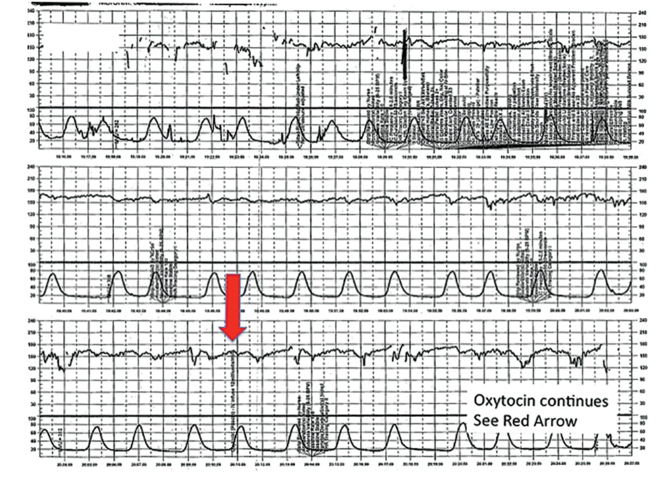 | ||
The patient in Case 4 received oxytocin for induction of postdates pregnancy. The red arrow shown on the FHR tracing points out that oxytocin augmentation continues despite the presence of uterine contractions that are too frequent and initial changes, including subtle late decelerations in the FHR, that suggest early fetal compromise. | ||
TIP #3: Manage oxytocin infusion according to protocol
Inappropriate use of oxytocin is common, including the improper management of oxytocin infusion in the setting of uterine tachysystole (defined as the presence of >5 contractions over a 10-minute period averaged over 30 minutes) and/or an abnormal FHR tracing. The mismanagement of uterine tachysystole is cited in more than two-thirds of obstetric malpractice cases.12
Uterine contractions alter blood flow through the spiral arteries and transiently reduce placental perfusion. Prolonged uterine tachysystole can lead to fetal oxygen debt and early signs of hypoxia, including the loss of spontaneous accelerations, tachycardia, and reduced variability. Continuing or increasing the oxytocin in the setting of such changes is hard to justify. One study found that the use of oxytocin in the setting of tachysystole was significantly associated with signs of fetal asphyxia (odds ratio [OR], 5.6).13 When the FHR pattern suggests significant interruption of fetal oxygen delivery and possible hypoxia, continuing or increasing an oxytocin infusion suggests a lack of understanding of the physiology that is the basis for FHR interpretation.
Appropriate management of tachysystole depends on the accompanying FHR.14 In the setting of a category I (normal) FHR tracing, tachysystole can be treated first with maternal repositioning (left or right lateral) and administration of a 500-cm3 maternal IV fluid bolus. If uterine activity does not return to normal after 10 to 15 minutes, decrease the oxytocin rate by at least half. If it does not return to normal after another 10 to 15 minutes, discontinue oxytocin until the tachysystole has resolved.
In the setting of a concerning category IIFHR tracing, discontinuation of oxytocin should be the first step along with maternal repositioning and administration of a fluid bolus. If these measures do not improve the FHR tracing and tachysystole persists, administration of an acute uterine relaxant, such as terbutaline, should be considered to slow contraction frequency.
If interventions result in normalization of the FHR tracing and resolution of tachysystole for 20 to 30 minutes, then oxytocin may be restarted if necessary for labor progress at no more than half the rate that produced tachysystole.
TIP #2: Recognize an abnormal FHR tracing--and what it means
Misinterpretation of the FHR tracing occurs when there is a failure to recognize characteristics that should raise concern about fetal well-being. Failure to recognize an abnormal FHR tracing occurred in 77% of sentinel cases involving intrapartum birth injury or death.1,12,13 To limit misinterpretation of the FHR tracing, it is critical for nurses and physicians to use standardized terminology for clear, effective communication.
In 2008, the Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) published guidelines standardizing the terminology used to describe cardiotocography and to create consensus around its interpretation.15 Any description of an intrapartum FHR tracing should include a designation of category (I, II, or III). Fetal well-being is reasonably established with a category I FHR tracing. A category III tracing indicates the high likelihood of fetal acidemia and the need for immediate intervention. A category II FHR tracing is considered indeterminate, and further characterization is required to reasonably exclude fetal metabolic acidosis and a risk of fetal injury.
The presence of moderate variability and fetal response to scalp stimulation are considered reassuring findings that reasonably exclude significant metabolic acidosis. In assessing variability, one pitfall is mistaking the appearance of "variability" within a deceleration (including during return to baseline) for baseline FHR variability. In the event of a persistent category II FHR tracing (>30 minutes), nursing staff should request direct physician review of the FHR tracing. In any case in which fetal well-being is uncertain, nursing staff should request direct physician evaluation of the mother in person and also the FHR tracing, with clear documentation of the findings, interpretation, and plan of care.16
TIP #1: Document, document, document
Nursing and physician documentation about the FHR tracing within the patient-specific clinical context is crucial for effective caregiver communication and patient safety. Thoughtful documentation also reduces liability exposure for providers by demonstrating maternal-fetal surveillance, early identification and treatment of an abnormal or indeterminate FHR tracing, and timely intervention on fetal behalf when necessary.
When the medical record aligns with the electronic FHR tracing and includes appropriate descriptions, interpretations, and interventions in line with national guidelines and institutional policy, the record demonstrates that the providers have a thorough understanding of the physiology behind cardiotocography and, more importantly, that they are able to apply that knowledge in clinical practice.6
Minimizing missteps
Several straightforward interventions can help clinicians overcome the most common pitfalls during FHR monitoring. These include accurate and high-quality cardiotocography, a collaborative team-based approach to patient care, and sustained situational awareness among providers. The consistent use of common language for the description and interpretation of FHR monitoring, adherence to hospital oxytocin protocols, and well-defined expectations for fetal surveillance and provider communication are critical to overcoming these challenges. Regularly scheduled nursing and physician education sessions and interdisciplinary case review can promote the adoption and sustained incorporation of these simple techniques into daily practice.3
Some have advocated for an "electronic fetal monitoring bundle," which would serve as a checklist of clinical evaluation steps that should occur every time a given process occurs.17 This approach would ensure that all providers on labor and delivery are qualified to read, accurately interpret, and respond to FHR tracings. It would require a credentialing process to confirm the competency of team members and reinforce the presence of a common language. It would also include an explicit escalation policy for rapid initiation of the chain of command in cases wherein there is a disagreement among team members about the FHR interpretation. Finally, each patient would be required to have, at all times, an identified responsible provider capable of a rapid response.
Although continuous FHR monitoring may not effectively reduce intrapartum fetal asphyxia, it is clearly here to stay. Recognizing--and addressing--the most common challenges encountered during intrapartum FHR monitoring may reduce unnecessary morbidity and potential liability for caregivers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Sentinel event alert issue 30--July 21, 2004. Preventing infant death and injury during delivery. Adv Neonatal Care. 2004;4(4):180–181.
- Shy KK, Luthy DA, Bennett FC, et al. Effects of electronic fetal-heart-rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. N Engl J Med. 1990;322(9):588–593.
- Clark SL, Nageotte MP, Garite TJ, et al. Intrapartum management of category II fetal heart rate tracings: towards standardization of care. Am J Obstet Gynecol. 2013;209(2):89–97.
- Neilson DR Jr, Freeman RK, Mangan S. Signal ambiguity resulting in unexpected outcome with external fetal heart rate monitoring. Am J Obstet Gynecol. 2008;198(6):717–724.
- McWhinney NA, Knowles S, Green HL, Gordon H. Transmission of the maternal electrocardiograph via a fetal scalp electrode in the presence of intrauterine death. Case report. Br J Obstet Gynaecol. 1984;91(10):1046–1048.
- Simpson KR, Knox GE. Risk management and electronic fetal monitoring: decreasing risk of adverse outcomes and liability exposure. J Perinat Neonatal Nurs. 2000;14(3):40–52.
- Gluck PA. Patient safety in women's health care: a framework for progress. Best Pract Res Clin Obstet Gynaecol. 2007;21(4):525–536.
- Lyndon A, Zlatnik MG, Wachter RM. Effective physician-nurse communication: a patient safety essential for labor and delivery. Am J Obstet Gynecol. 2011;205(2):91–96.
- Jackson M, Holmgren CM, Esplin MS, Henry E, Varner MW. Frequency of fetal heart rate categories and short-term neonatal outcome. Obstet Gynecol. 2011;118(4):803–808.
- Parer JT, Ikeda T. A framework for standardized management of intrapartum fetal heart rate patterns. Am J Obstet Gynecol. 2007;197(1):26.e1-e6.
- MacEachin SR, Lopez CM, Powell KJ, Corbett NL. The fetal heart rate collaborative practice project: situational awareness in electronic fetal monitoring--a Kaiser Permanente Perinatal Patient Safety Program Initiative. J Perinat Neonatal Nurs. 2009;23(4):314–323; quiz 24–25.
- Jonsson M, Norden SL, Hanson U. Analysis of malpractice claims with a focus on oxytocin use in labour. Acta Obstet Gynecol Scand. 2007;86(3):315–319.
- Berglund S, Pettersson H, Cnattingius S, Grunewald C. How often is a low Apgar score the result of substandard care during labour? BJOG. 2010;117(8):968–978.
- Doyle J, Kenny TH, Burkett AM, von Gruenigen VE. A performance improvement process to tackle tachysystole. J Obstet Gynecol Neonatal Nurs. 2011;40(5):512–519.
- Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661–666.
- Knox GE, Simpson KR, Garite TJ. High reliability perinatal units: an approach to the prevention of patient injury and medical malpractice claims. J Healthc Risk Manag. 1999;19(2):24–32.
- Minkoff H, Berkowitz R; Greater New York Hospital Association's Perinatal Safety C. Fetal monitoring bundle. Obstet Gynecol. 2009;114(6):1332–1335.
Interpreting continuous fetal heart rate (FHR) monitoring is one of the most common tasks obstetricians perform during the course of intrapartum care. Notably, many providers do not seek ongoing training to optimize their ability to reliably and accurately interpret the FHR. Yet FHR interpretation is one of the most frequent causes of litigation in the modern obstetric practice. Failure to interpret continuous FHR monitoring appropriately is estimated to account for 75% of obstetric-related litigation.1
Continuous FHR monitoring during labor was introduced to identify infants at risk for developing hypoxic-ischemic encephalopathy (HIE). The rate of HIE has not declined, however, despite almost universal adoption of continuous FHR monitoring.2 Numerous reasons account for this failure, including ad hoc interpretation of terminology, lack of standardized protocols for management and intervention, and the oftentimes challenging patterns that must be interpreted.3 The confusion about and dissatisfaction with the current state of FHR monitoring has led to attempts to enhance our ability to identify infants at risk with additional approaches (such as fetal pulse oximetry and fetal ST-segment evaluation), and some have called for a complete overhaul of our approach to interpreting the FHR. Clark and colleagues stated recently, "It is time to start over and establish some common language, standard interpretation, and reasonable management principles and guidelines."3
We must recognize that, as a stand-alone tool, continuous FHR monitoring is ineffective for avoiding preventable adverse outcomes. It is most likely to be effective when used in accordance with published standard guidelines by professionals skilled in interpretation and when timely, appropriate interventions are performed based on that interpretation. Optimal FHR monitoring requires a collaborative perinatal team that performs the monitoring correctly, interprets it appropriately, and communicates the findings effectively, and in a timely fashion, to all members of the care team when a high-risk pattern is detected.
In this article we review some common challenges that clinicians encounter during intrapartum FHR monitoring and we offer 10 simple tips to help overcome these challenges. The clinical scenarios described are derived from published reports in the medical literature, published malpractice claims, and from our personal experience working in a major health care system as part of a team charged with overseeing ongoing certification and training of labor and delivery nurses.
Challenge: Signal ambiguity
CASE 1 Young woman in labor with first pregnancy
A 19-year-old woman presents in spontaneous labor with her first pregnancy, which has been uncomplicated. During the course of her care, it is noted that the FHR changes to a lower baseline than previously recorded. Evaluation reveals that the external monitor is tracking the maternal heart rate and not the FHR (FIGURE 1). After the monitor is adjusted, both the fetal and maternal rates are documented for a short period. Ultimately, continuous monitoring of the maternal heart rate is discontinued. After delivery of the infant several hours later, it is noted that the FHR continues to register on the monitor, and it is determined that for the last few hours the maternal heart rate has been traced.
| FIGURE 1 FHR tracing indicates signal ambiguity | ||
 | ||
As described in Case 1, the upper panel of this tracing demonstrates the maternal heart rate confused as the fetal heart rate, while the segment in the lower panel shows a clear distinction between the maternal and fetal heart rates. | ||
TIP #10: Ensure the FHR monitor is tracking the fetal, not the maternal, heart rate
Confusing the maternal and the fetal heart rate with external cardiotocography is common. When the mix-up is noted and corrected expeditiously, it is unlikely to result in an adverse outcome. Signal ambiguity may arise from faulty Doppler equipment or the inability of the cardiotocograph to differentiate between maternal and fetal heart rates. It commonly occurs after repositioning the patient, after fetal movement, or during pushing in the second stage when the maternal heart rate may increase to a baseline that is similar to that of the fetus.
Signal ambiguity should be suspected when the FHR runs in the low-normal range or when FHR accelerations are noted with greater than 50% of contractions (especially when pushing).4 Signal ambiguity also should be ruled out when there is an apparent FHR deceleration to the maternal range that does not recover.
Evaluating for suspected signal ambiguity involves 2 key steps: (1) documentation and verification of the maternal heart rate and (2) definitive documentation of the true FHR. To document the maternal heart rate, manually count the radial pulse for 1 minute or use a pulse oximeter for continuous monitoring. Using a pulse oximeter is a less labor-intensive approach and has the advantage of allowing continuous assessment of the maternal heart rate for comparison. Recording the maternal pulse continuously on the same screen as the FHR enables ongoing differentiation of the mother and fetus in difficult cases, particularly if internal fetal monitoring is not an option (because of maternal infectious disease, low suspicion for an abnormal FHR pattern, or strong maternal preference against internal monitoring, for example).
When clinically appropriate, use of a fetal scalp electrode (FSE) can document the FHR. If intrauterine fetal death has occurred, however, the FSE may transmit the maternal heart rate.5 Using ultrasonography to confirm the FHR prior to placing the FSE is a reliable method of definitive differentiation. If a newly placed FSE shows a clear differentiation of 5 to 10 beats per minute from a continuously assessed maternal pulse rate, then this is also a reliable way to assure that the FHR monitoring represents the fetus, particularly if ultrasonography is not immediately available.
Ultimately, before intervening based on an abnormal FHR tracing, it is paramount to confirm that the data are adequate for interpretation and represent the actual FHR. If signal ambiguity is identified or suspected, correct it by using ultrasonography to locate the FHR and replace the external monitor until a rate that is at least 5 to 10 beats per minute different from the maternal rate is obtained. Alternatively, this is an indication for internal fetal monitoring with an FSE.
Challenge: Inadequate FHR tracing, poor communication, lack of clinical context
CASE 2 Woman with uncomplicated postdates pregnancy presents for induction
A 28-year-old woman (G3P2) at 41 weeks 0 days of gestation presents to labor and delivery for induction of labor for the indication of postdates. There have been no complications with the current pregnancy. The initial cervical exam reveals 1+ cm dilation, 90% effacement, and −3 station, and the patient is started on oxytocin per the hospital protocol. What is your interpretation of the continuous FHR tracing shown in FIGURE 2?
| FIGURE 2 Inadequate, uninterpretable FHR tracing | ||
 | ||
This FHR tracing, from the patient described in Case 2, is unusable because of the absence of data. | ||
TIP #9: Check that the monitors are providing useful data
The ability to accurately interpret a continuous FHR tracing depends on the quality of data recorded. Unfortunately, the absence of data makes interpretation impossible. This includes both FHR and tocometry data, since both pieces of information are required for appropriate interpretation of a continuous FHR tracing.
Prolonged periods of uninterpretable FHR and uterine activity tracings imply that no one was attending the mother and fetus.6 If it is difficult to obtain an interpretable FHR tracing, document in the medical record that you made ongoing efforts to maintain an adequate tracing, including the amount of time spent holding the external monitor, use of ultrasonography to document the FHR, and plans for potential internal monitoring.
CASE 2 Continued
After several hours, the patient requests an epidural for pain management and one is placed without difficulty. She reports adequate pain relief and is comfortable for the next 1 to 2 hours. Subsequently, the patient reports a sudden onset of increasing pain that does not respond to additional patient-administered doses of anesthesia over a 30-minute period. The labor and delivery nurse becomes concerned about the patient's pain level and contacts the attending physician to discuss her concerns. The physician, who is currently attending to patients in clinic, listens to the nurse and asks her to contact the anesthesia department with her concerns (FIGURE 3).
| FIGURE 3 FHR tracing reveals recurrent variables in a patient with evolving clinical concerns | ||
 | ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #8: Clearly communicate an urgent situation to the care team
Poor communication underlies many preventable adverse outcomes in medicine.7 Effective communication requires an adequate description of the clinical scenario or problem. A root cause analysis of a series of intrapartum adverse events involving fetal death or injury showed that poor communication about a concerning FHR tracing played a role in 72% of cases.1
In this clinical scenario, the nurse believed that the patient's pain level was unusual or more than anticipated. The person who is communicating his or her concern (the sender) must be sure that the person receiving the message (the responder) clearly understands the sender's level of concern. In this case, it would have been appropriate for the sender to state clearly that she felt the patient's pain was outside of normal expectations and to request that the attending physician come to evaluate the patient.
Clear and effective communication includes (1) an appropriate description of the urgency of the situation and (2) an indication by the sender as to the desired response to this information ("please come evaluate the patient").8 In all cases, both steps are necessary to elicit an appropriate response.
CASE 2 Continued
Over the next 2 hours, recurrent variable decelerations develop, and then sudden, prolonged fetal bradycardia leads to urgent cesarean delivery. At delivery, a uterine rupture is diagnosed and a fetal hand is observed protruding through a lower-uterine segment defect into the maternal abdomen.
TIP #7: Always consider the entire clinical scenario
In this case, the team caring for the patient was not aware that her previous pregnancy had ended with a low transverse cesarean delivery. How does this information change your interpretation of the clinical scenario? The importance of understanding the entire clinical context when interpreting individual characteristics of cardiotocography cannot be overstated. For example, the sudden onset of recurrent, significant variable decelerations is more concerning in the context of a prior cesarean delivery, and late decelerations are more concerning in a patient with placental abruption, fetal growth restriction, or poorly controlled maternal diabetes.
An estimated 70% of fetuses will have an indeterminate FHR pattern (category II) at some time during labor.9 To appropriately interpret the FHR tracing, it is crucial to know the a priori risk for fetal hypoxia and metabolic acidosis (the precursor of fetal injury) due to such identified clinical risk factors as placental insufficiency, medical comorbidities (hypertension, diabetes), or postdates gestational age.
It is well established that cardiotocography has a good negative predictive value for the absence of fetal metabolic acidosis when there is moderate variability and spontaneous or induced accelerations. When attempting to risk stratify the fetus with a category II (indeterminate) FHR tracing, consider these 3 important questions:
- What are the risk factors for this particular patient and her fetus?
- What is the state of the fetus right now, and when was the last time metabolic acidosis could be excluded reasonably (by the presence of moderate variability and accelerations)?
- What is the risk that the fetus will develop acidemia prior to delivery?
The presence of decelerations indicates interruption of oxygen delivery to the fetus, and recurrent decelerations may indicate an evolving process of accumulated oxygen deprivation, hypoxia, and eventually, metabolic acidosis. Most authorities agree that, for the fetus with a previously normal FHR tracing, the onset of significant, recurrent decelerations with slowly cumulative oxygen deficit can lead to fetal acidemia over the course of approximately 1 hour.10 Of course, acidosis also can occur much more quickly with acute events, such as placental abruption or uterine rupture. In deciding whether or not to intervene based on an FHR tracing, the clinician must take into account the clinical context to determine if delivery is likely to occur before significant acidemia develops.
Challenge: Lack of situational awareness, failure to address nursing concerns, reluctance to initiate the chain of command
CASE 3 Spontaneous labor in a second pregnancy
A 28-year-old woman (G2P1) at 40 weeks' gestation presents in spontaneous labor. She has a history of a previous uncomplicated vaginal delivery. After 6 hours she reaches complete dilation with the fetus at −1 station and begins pushing. After 60 minutes, the patient has only progressed to +1 station. She is contracting every 1 to 2 minutes with recurrent variable decelerations (FIGURE 4).
| FIGURE 4 FHR tracing shows time points for initiation and continuation of pushing | ||
 | ||
This tracing, from the patient described in Case 3, documents contraction frequency every 1-2 minutes for more than 60 minutes while the patient continues to push. The fetal heart rate demonstrates repetitive moderate variable decelerations with every push. | ||
TIP #6: Maintain situational awareness
A state of situational awareness exists when caregivers have a clear understanding of all of the factors at play in a clinical situation.11 This can be lost when caregivers focus too intensely on one aspect of care. It often happens when the patient is pushing in the second stage and the provider, focused on the progress of fetal descent, loses track of the amount of time that has passed without reassuring features (such as variability and induced or spontaneous accelerations) in the FHR tracing. The nurse, seeing the physician at the bedside, presumes he or she is aware of the tracing and is thus reluctant to point out the concerning features for fear of appearing insubordinate.
Situational awareness also may be lost at the time of patient hand off between providers wherein critical information, such as a history of previous cesarean delivery, is not communicated to the next care team. When receiving an intrapartum patient hand off, providers must have heightened vigilance to ensure they quickly reach situational awareness and are cognizant of the entire clinical context. Maintaining an environment in which all members of the care team, regardless of their training level, are encouraged to voice their concerns is another way to promote ongoing situational awareness.
CASE 3 Continued
The patient continues pushing for another 20 minutes without delivery, and the nurse raises a concern about the FHR tracing to the physician, who remains in the room but does not respond (FIGURE 5).
| FIGURE 5 FHR tracing reveals ongoing repetitive variable decelerations | ||
 | ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #5: Acknowledge and respond to other caregivers' concerns
A team approach to patient care is essential in all areas of medicine, perhaps none more so than in obstetrics. Each member of the team is engaged in trying to provide optimal patient care and the concerns of every team member--regardless of title or level of training--must be acknowledged and addressed. Good communication requires creating a safe environment wherein each member of the team feels comfortable raising concerns without fear of reprisal. Rather than becoming angry or frustrated when questioned, providers should remain cognizant that these are ongoing efforts to maintain situational awareness and ensure the best possible outcome for mother and baby.
CASE 3 Continued
Pushing continues for another 30 minutes despite the nurse's repeated effort to express concern to the physician about the FHR tracing. After more than 2 hours of pushing, the infant is delivered; Apgar scores are 1, 5, and 7. No cord gas is obtained.
TIP #4: Initiate the chain of command when necessary
Any caregiver, regardless of job title, has a duty to initiate the institution's chain-of-command policy and procedure if he or she has a concern about patient well-being that is not being addressed adequately. It can be uncomfortable for a nurse, midwife, or resident physician to question an attending physician, particularly if that person responds in a dismissive, condescending, or angry manner. If a caregiver has made several attempts to engage the attending physician and feels the concerns are being inadequately addressed, then he or she must respectfully initiate the chain of command to seek additional objective review of the clinical situation.
Failure to follow oxytocin protocols, inadequate surveillance, poor documentation
CASE 4 Induction of an uncomplicated pregnancy due to postdates
A 20-year-old woman (G1P0) at 42 weeks' gestation with an otherwise uncomplicated first pregnancy presents for postdates induction with oxytocin. After 6 hours, she develops uterine tachysystole with recurrent variable decelerations but the oxytocin infusion is continued at the same rate (FIGURE 6).
| FIGURE 6 FHR tracing indicates uterine tachysystole | ||
 | ||
The patient in Case 4 received oxytocin for induction of postdates pregnancy. The red arrow shown on the FHR tracing points out that oxytocin augmentation continues despite the presence of uterine contractions that are too frequent and initial changes, including subtle late decelerations in the FHR, that suggest early fetal compromise. | ||
TIP #3: Manage oxytocin infusion according to protocol
Inappropriate use of oxytocin is common, including the improper management of oxytocin infusion in the setting of uterine tachysystole (defined as the presence of >5 contractions over a 10-minute period averaged over 30 minutes) and/or an abnormal FHR tracing. The mismanagement of uterine tachysystole is cited in more than two-thirds of obstetric malpractice cases.12
Uterine contractions alter blood flow through the spiral arteries and transiently reduce placental perfusion. Prolonged uterine tachysystole can lead to fetal oxygen debt and early signs of hypoxia, including the loss of spontaneous accelerations, tachycardia, and reduced variability. Continuing or increasing the oxytocin in the setting of such changes is hard to justify. One study found that the use of oxytocin in the setting of tachysystole was significantly associated with signs of fetal asphyxia (odds ratio [OR], 5.6).13 When the FHR pattern suggests significant interruption of fetal oxygen delivery and possible hypoxia, continuing or increasing an oxytocin infusion suggests a lack of understanding of the physiology that is the basis for FHR interpretation.
Appropriate management of tachysystole depends on the accompanying FHR.14 In the setting of a category I (normal) FHR tracing, tachysystole can be treated first with maternal repositioning (left or right lateral) and administration of a 500-cm3 maternal IV fluid bolus. If uterine activity does not return to normal after 10 to 15 minutes, decrease the oxytocin rate by at least half. If it does not return to normal after another 10 to 15 minutes, discontinue oxytocin until the tachysystole has resolved.
In the setting of a concerning category IIFHR tracing, discontinuation of oxytocin should be the first step along with maternal repositioning and administration of a fluid bolus. If these measures do not improve the FHR tracing and tachysystole persists, administration of an acute uterine relaxant, such as terbutaline, should be considered to slow contraction frequency.
If interventions result in normalization of the FHR tracing and resolution of tachysystole for 20 to 30 minutes, then oxytocin may be restarted if necessary for labor progress at no more than half the rate that produced tachysystole.
TIP #2: Recognize an abnormal FHR tracing--and what it means
Misinterpretation of the FHR tracing occurs when there is a failure to recognize characteristics that should raise concern about fetal well-being. Failure to recognize an abnormal FHR tracing occurred in 77% of sentinel cases involving intrapartum birth injury or death.1,12,13 To limit misinterpretation of the FHR tracing, it is critical for nurses and physicians to use standardized terminology for clear, effective communication.
In 2008, the Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) published guidelines standardizing the terminology used to describe cardiotocography and to create consensus around its interpretation.15 Any description of an intrapartum FHR tracing should include a designation of category (I, II, or III). Fetal well-being is reasonably established with a category I FHR tracing. A category III tracing indicates the high likelihood of fetal acidemia and the need for immediate intervention. A category II FHR tracing is considered indeterminate, and further characterization is required to reasonably exclude fetal metabolic acidosis and a risk of fetal injury.
The presence of moderate variability and fetal response to scalp stimulation are considered reassuring findings that reasonably exclude significant metabolic acidosis. In assessing variability, one pitfall is mistaking the appearance of "variability" within a deceleration (including during return to baseline) for baseline FHR variability. In the event of a persistent category II FHR tracing (>30 minutes), nursing staff should request direct physician review of the FHR tracing. In any case in which fetal well-being is uncertain, nursing staff should request direct physician evaluation of the mother in person and also the FHR tracing, with clear documentation of the findings, interpretation, and plan of care.16
TIP #1: Document, document, document
Nursing and physician documentation about the FHR tracing within the patient-specific clinical context is crucial for effective caregiver communication and patient safety. Thoughtful documentation also reduces liability exposure for providers by demonstrating maternal-fetal surveillance, early identification and treatment of an abnormal or indeterminate FHR tracing, and timely intervention on fetal behalf when necessary.
When the medical record aligns with the electronic FHR tracing and includes appropriate descriptions, interpretations, and interventions in line with national guidelines and institutional policy, the record demonstrates that the providers have a thorough understanding of the physiology behind cardiotocography and, more importantly, that they are able to apply that knowledge in clinical practice.6
Minimizing missteps
Several straightforward interventions can help clinicians overcome the most common pitfalls during FHR monitoring. These include accurate and high-quality cardiotocography, a collaborative team-based approach to patient care, and sustained situational awareness among providers. The consistent use of common language for the description and interpretation of FHR monitoring, adherence to hospital oxytocin protocols, and well-defined expectations for fetal surveillance and provider communication are critical to overcoming these challenges. Regularly scheduled nursing and physician education sessions and interdisciplinary case review can promote the adoption and sustained incorporation of these simple techniques into daily practice.3
Some have advocated for an "electronic fetal monitoring bundle," which would serve as a checklist of clinical evaluation steps that should occur every time a given process occurs.17 This approach would ensure that all providers on labor and delivery are qualified to read, accurately interpret, and respond to FHR tracings. It would require a credentialing process to confirm the competency of team members and reinforce the presence of a common language. It would also include an explicit escalation policy for rapid initiation of the chain of command in cases wherein there is a disagreement among team members about the FHR interpretation. Finally, each patient would be required to have, at all times, an identified responsible provider capable of a rapid response.
Although continuous FHR monitoring may not effectively reduce intrapartum fetal asphyxia, it is clearly here to stay. Recognizing--and addressing--the most common challenges encountered during intrapartum FHR monitoring may reduce unnecessary morbidity and potential liability for caregivers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Interpreting continuous fetal heart rate (FHR) monitoring is one of the most common tasks obstetricians perform during the course of intrapartum care. Notably, many providers do not seek ongoing training to optimize their ability to reliably and accurately interpret the FHR. Yet FHR interpretation is one of the most frequent causes of litigation in the modern obstetric practice. Failure to interpret continuous FHR monitoring appropriately is estimated to account for 75% of obstetric-related litigation.1
Continuous FHR monitoring during labor was introduced to identify infants at risk for developing hypoxic-ischemic encephalopathy (HIE). The rate of HIE has not declined, however, despite almost universal adoption of continuous FHR monitoring.2 Numerous reasons account for this failure, including ad hoc interpretation of terminology, lack of standardized protocols for management and intervention, and the oftentimes challenging patterns that must be interpreted.3 The confusion about and dissatisfaction with the current state of FHR monitoring has led to attempts to enhance our ability to identify infants at risk with additional approaches (such as fetal pulse oximetry and fetal ST-segment evaluation), and some have called for a complete overhaul of our approach to interpreting the FHR. Clark and colleagues stated recently, "It is time to start over and establish some common language, standard interpretation, and reasonable management principles and guidelines."3
We must recognize that, as a stand-alone tool, continuous FHR monitoring is ineffective for avoiding preventable adverse outcomes. It is most likely to be effective when used in accordance with published standard guidelines by professionals skilled in interpretation and when timely, appropriate interventions are performed based on that interpretation. Optimal FHR monitoring requires a collaborative perinatal team that performs the monitoring correctly, interprets it appropriately, and communicates the findings effectively, and in a timely fashion, to all members of the care team when a high-risk pattern is detected.
In this article we review some common challenges that clinicians encounter during intrapartum FHR monitoring and we offer 10 simple tips to help overcome these challenges. The clinical scenarios described are derived from published reports in the medical literature, published malpractice claims, and from our personal experience working in a major health care system as part of a team charged with overseeing ongoing certification and training of labor and delivery nurses.
Challenge: Signal ambiguity
CASE 1 Young woman in labor with first pregnancy
A 19-year-old woman presents in spontaneous labor with her first pregnancy, which has been uncomplicated. During the course of her care, it is noted that the FHR changes to a lower baseline than previously recorded. Evaluation reveals that the external monitor is tracking the maternal heart rate and not the FHR (FIGURE 1). After the monitor is adjusted, both the fetal and maternal rates are documented for a short period. Ultimately, continuous monitoring of the maternal heart rate is discontinued. After delivery of the infant several hours later, it is noted that the FHR continues to register on the monitor, and it is determined that for the last few hours the maternal heart rate has been traced.
| FIGURE 1 FHR tracing indicates signal ambiguity | ||
 | ||
As described in Case 1, the upper panel of this tracing demonstrates the maternal heart rate confused as the fetal heart rate, while the segment in the lower panel shows a clear distinction between the maternal and fetal heart rates. | ||
TIP #10: Ensure the FHR monitor is tracking the fetal, not the maternal, heart rate
Confusing the maternal and the fetal heart rate with external cardiotocography is common. When the mix-up is noted and corrected expeditiously, it is unlikely to result in an adverse outcome. Signal ambiguity may arise from faulty Doppler equipment or the inability of the cardiotocograph to differentiate between maternal and fetal heart rates. It commonly occurs after repositioning the patient, after fetal movement, or during pushing in the second stage when the maternal heart rate may increase to a baseline that is similar to that of the fetus.
Signal ambiguity should be suspected when the FHR runs in the low-normal range or when FHR accelerations are noted with greater than 50% of contractions (especially when pushing).4 Signal ambiguity also should be ruled out when there is an apparent FHR deceleration to the maternal range that does not recover.
Evaluating for suspected signal ambiguity involves 2 key steps: (1) documentation and verification of the maternal heart rate and (2) definitive documentation of the true FHR. To document the maternal heart rate, manually count the radial pulse for 1 minute or use a pulse oximeter for continuous monitoring. Using a pulse oximeter is a less labor-intensive approach and has the advantage of allowing continuous assessment of the maternal heart rate for comparison. Recording the maternal pulse continuously on the same screen as the FHR enables ongoing differentiation of the mother and fetus in difficult cases, particularly if internal fetal monitoring is not an option (because of maternal infectious disease, low suspicion for an abnormal FHR pattern, or strong maternal preference against internal monitoring, for example).
When clinically appropriate, use of a fetal scalp electrode (FSE) can document the FHR. If intrauterine fetal death has occurred, however, the FSE may transmit the maternal heart rate.5 Using ultrasonography to confirm the FHR prior to placing the FSE is a reliable method of definitive differentiation. If a newly placed FSE shows a clear differentiation of 5 to 10 beats per minute from a continuously assessed maternal pulse rate, then this is also a reliable way to assure that the FHR monitoring represents the fetus, particularly if ultrasonography is not immediately available.
Ultimately, before intervening based on an abnormal FHR tracing, it is paramount to confirm that the data are adequate for interpretation and represent the actual FHR. If signal ambiguity is identified or suspected, correct it by using ultrasonography to locate the FHR and replace the external monitor until a rate that is at least 5 to 10 beats per minute different from the maternal rate is obtained. Alternatively, this is an indication for internal fetal monitoring with an FSE.
Challenge: Inadequate FHR tracing, poor communication, lack of clinical context
CASE 2 Woman with uncomplicated postdates pregnancy presents for induction
A 28-year-old woman (G3P2) at 41 weeks 0 days of gestation presents to labor and delivery for induction of labor for the indication of postdates. There have been no complications with the current pregnancy. The initial cervical exam reveals 1+ cm dilation, 90% effacement, and −3 station, and the patient is started on oxytocin per the hospital protocol. What is your interpretation of the continuous FHR tracing shown in FIGURE 2?
| FIGURE 2 Inadequate, uninterpretable FHR tracing | ||
 | ||
This FHR tracing, from the patient described in Case 2, is unusable because of the absence of data. | ||
TIP #9: Check that the monitors are providing useful data
The ability to accurately interpret a continuous FHR tracing depends on the quality of data recorded. Unfortunately, the absence of data makes interpretation impossible. This includes both FHR and tocometry data, since both pieces of information are required for appropriate interpretation of a continuous FHR tracing.
Prolonged periods of uninterpretable FHR and uterine activity tracings imply that no one was attending the mother and fetus.6 If it is difficult to obtain an interpretable FHR tracing, document in the medical record that you made ongoing efforts to maintain an adequate tracing, including the amount of time spent holding the external monitor, use of ultrasonography to document the FHR, and plans for potential internal monitoring.
CASE 2 Continued
After several hours, the patient requests an epidural for pain management and one is placed without difficulty. She reports adequate pain relief and is comfortable for the next 1 to 2 hours. Subsequently, the patient reports a sudden onset of increasing pain that does not respond to additional patient-administered doses of anesthesia over a 30-minute period. The labor and delivery nurse becomes concerned about the patient's pain level and contacts the attending physician to discuss her concerns. The physician, who is currently attending to patients in clinic, listens to the nurse and asks her to contact the anesthesia department with her concerns (FIGURE 3).
| FIGURE 3 FHR tracing reveals recurrent variables in a patient with evolving clinical concerns | ||
 | ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #8: Clearly communicate an urgent situation to the care team
Poor communication underlies many preventable adverse outcomes in medicine.7 Effective communication requires an adequate description of the clinical scenario or problem. A root cause analysis of a series of intrapartum adverse events involving fetal death or injury showed that poor communication about a concerning FHR tracing played a role in 72% of cases.1
In this clinical scenario, the nurse believed that the patient's pain level was unusual or more than anticipated. The person who is communicating his or her concern (the sender) must be sure that the person receiving the message (the responder) clearly understands the sender's level of concern. In this case, it would have been appropriate for the sender to state clearly that she felt the patient's pain was outside of normal expectations and to request that the attending physician come to evaluate the patient.
Clear and effective communication includes (1) an appropriate description of the urgency of the situation and (2) an indication by the sender as to the desired response to this information ("please come evaluate the patient").8 In all cases, both steps are necessary to elicit an appropriate response.
CASE 2 Continued
Over the next 2 hours, recurrent variable decelerations develop, and then sudden, prolonged fetal bradycardia leads to urgent cesarean delivery. At delivery, a uterine rupture is diagnosed and a fetal hand is observed protruding through a lower-uterine segment defect into the maternal abdomen.
TIP #7: Always consider the entire clinical scenario
In this case, the team caring for the patient was not aware that her previous pregnancy had ended with a low transverse cesarean delivery. How does this information change your interpretation of the clinical scenario? The importance of understanding the entire clinical context when interpreting individual characteristics of cardiotocography cannot be overstated. For example, the sudden onset of recurrent, significant variable decelerations is more concerning in the context of a prior cesarean delivery, and late decelerations are more concerning in a patient with placental abruption, fetal growth restriction, or poorly controlled maternal diabetes.
An estimated 70% of fetuses will have an indeterminate FHR pattern (category II) at some time during labor.9 To appropriately interpret the FHR tracing, it is crucial to know the a priori risk for fetal hypoxia and metabolic acidosis (the precursor of fetal injury) due to such identified clinical risk factors as placental insufficiency, medical comorbidities (hypertension, diabetes), or postdates gestational age.
It is well established that cardiotocography has a good negative predictive value for the absence of fetal metabolic acidosis when there is moderate variability and spontaneous or induced accelerations. When attempting to risk stratify the fetus with a category II (indeterminate) FHR tracing, consider these 3 important questions:
- What are the risk factors for this particular patient and her fetus?
- What is the state of the fetus right now, and when was the last time metabolic acidosis could be excluded reasonably (by the presence of moderate variability and accelerations)?
- What is the risk that the fetus will develop acidemia prior to delivery?
The presence of decelerations indicates interruption of oxygen delivery to the fetus, and recurrent decelerations may indicate an evolving process of accumulated oxygen deprivation, hypoxia, and eventually, metabolic acidosis. Most authorities agree that, for the fetus with a previously normal FHR tracing, the onset of significant, recurrent decelerations with slowly cumulative oxygen deficit can lead to fetal acidemia over the course of approximately 1 hour.10 Of course, acidosis also can occur much more quickly with acute events, such as placental abruption or uterine rupture. In deciding whether or not to intervene based on an FHR tracing, the clinician must take into account the clinical context to determine if delivery is likely to occur before significant acidemia develops.
Challenge: Lack of situational awareness, failure to address nursing concerns, reluctance to initiate the chain of command
CASE 3 Spontaneous labor in a second pregnancy
A 28-year-old woman (G2P1) at 40 weeks' gestation presents in spontaneous labor. She has a history of a previous uncomplicated vaginal delivery. After 6 hours she reaches complete dilation with the fetus at −1 station and begins pushing. After 60 minutes, the patient has only progressed to +1 station. She is contracting every 1 to 2 minutes with recurrent variable decelerations (FIGURE 4).
| FIGURE 4 FHR tracing shows time points for initiation and continuation of pushing | ||
 | ||
This tracing, from the patient described in Case 3, documents contraction frequency every 1-2 minutes for more than 60 minutes while the patient continues to push. The fetal heart rate demonstrates repetitive moderate variable decelerations with every push. | ||
TIP #6: Maintain situational awareness
A state of situational awareness exists when caregivers have a clear understanding of all of the factors at play in a clinical situation.11 This can be lost when caregivers focus too intensely on one aspect of care. It often happens when the patient is pushing in the second stage and the provider, focused on the progress of fetal descent, loses track of the amount of time that has passed without reassuring features (such as variability and induced or spontaneous accelerations) in the FHR tracing. The nurse, seeing the physician at the bedside, presumes he or she is aware of the tracing and is thus reluctant to point out the concerning features for fear of appearing insubordinate.
Situational awareness also may be lost at the time of patient hand off between providers wherein critical information, such as a history of previous cesarean delivery, is not communicated to the next care team. When receiving an intrapartum patient hand off, providers must have heightened vigilance to ensure they quickly reach situational awareness and are cognizant of the entire clinical context. Maintaining an environment in which all members of the care team, regardless of their training level, are encouraged to voice their concerns is another way to promote ongoing situational awareness.
CASE 3 Continued
The patient continues pushing for another 20 minutes without delivery, and the nurse raises a concern about the FHR tracing to the physician, who remains in the room but does not respond (FIGURE 5).
| FIGURE 5 FHR tracing reveals ongoing repetitive variable decelerations | ||
 | ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #5: Acknowledge and respond to other caregivers' concerns
A team approach to patient care is essential in all areas of medicine, perhaps none more so than in obstetrics. Each member of the team is engaged in trying to provide optimal patient care and the concerns of every team member--regardless of title or level of training--must be acknowledged and addressed. Good communication requires creating a safe environment wherein each member of the team feels comfortable raising concerns without fear of reprisal. Rather than becoming angry or frustrated when questioned, providers should remain cognizant that these are ongoing efforts to maintain situational awareness and ensure the best possible outcome for mother and baby.
CASE 3 Continued
Pushing continues for another 30 minutes despite the nurse's repeated effort to express concern to the physician about the FHR tracing. After more than 2 hours of pushing, the infant is delivered; Apgar scores are 1, 5, and 7. No cord gas is obtained.
TIP #4: Initiate the chain of command when necessary
Any caregiver, regardless of job title, has a duty to initiate the institution's chain-of-command policy and procedure if he or she has a concern about patient well-being that is not being addressed adequately. It can be uncomfortable for a nurse, midwife, or resident physician to question an attending physician, particularly if that person responds in a dismissive, condescending, or angry manner. If a caregiver has made several attempts to engage the attending physician and feels the concerns are being inadequately addressed, then he or she must respectfully initiate the chain of command to seek additional objective review of the clinical situation.
Failure to follow oxytocin protocols, inadequate surveillance, poor documentation
CASE 4 Induction of an uncomplicated pregnancy due to postdates
A 20-year-old woman (G1P0) at 42 weeks' gestation with an otherwise uncomplicated first pregnancy presents for postdates induction with oxytocin. After 6 hours, she develops uterine tachysystole with recurrent variable decelerations but the oxytocin infusion is continued at the same rate (FIGURE 6).
| FIGURE 6 FHR tracing indicates uterine tachysystole | ||
 | ||
The patient in Case 4 received oxytocin for induction of postdates pregnancy. The red arrow shown on the FHR tracing points out that oxytocin augmentation continues despite the presence of uterine contractions that are too frequent and initial changes, including subtle late decelerations in the FHR, that suggest early fetal compromise. | ||
TIP #3: Manage oxytocin infusion according to protocol
Inappropriate use of oxytocin is common, including the improper management of oxytocin infusion in the setting of uterine tachysystole (defined as the presence of >5 contractions over a 10-minute period averaged over 30 minutes) and/or an abnormal FHR tracing. The mismanagement of uterine tachysystole is cited in more than two-thirds of obstetric malpractice cases.12
Uterine contractions alter blood flow through the spiral arteries and transiently reduce placental perfusion. Prolonged uterine tachysystole can lead to fetal oxygen debt and early signs of hypoxia, including the loss of spontaneous accelerations, tachycardia, and reduced variability. Continuing or increasing the oxytocin in the setting of such changes is hard to justify. One study found that the use of oxytocin in the setting of tachysystole was significantly associated with signs of fetal asphyxia (odds ratio [OR], 5.6).13 When the FHR pattern suggests significant interruption of fetal oxygen delivery and possible hypoxia, continuing or increasing an oxytocin infusion suggests a lack of understanding of the physiology that is the basis for FHR interpretation.
Appropriate management of tachysystole depends on the accompanying FHR.14 In the setting of a category I (normal) FHR tracing, tachysystole can be treated first with maternal repositioning (left or right lateral) and administration of a 500-cm3 maternal IV fluid bolus. If uterine activity does not return to normal after 10 to 15 minutes, decrease the oxytocin rate by at least half. If it does not return to normal after another 10 to 15 minutes, discontinue oxytocin until the tachysystole has resolved.
In the setting of a concerning category IIFHR tracing, discontinuation of oxytocin should be the first step along with maternal repositioning and administration of a fluid bolus. If these measures do not improve the FHR tracing and tachysystole persists, administration of an acute uterine relaxant, such as terbutaline, should be considered to slow contraction frequency.
If interventions result in normalization of the FHR tracing and resolution of tachysystole for 20 to 30 minutes, then oxytocin may be restarted if necessary for labor progress at no more than half the rate that produced tachysystole.
TIP #2: Recognize an abnormal FHR tracing--and what it means
Misinterpretation of the FHR tracing occurs when there is a failure to recognize characteristics that should raise concern about fetal well-being. Failure to recognize an abnormal FHR tracing occurred in 77% of sentinel cases involving intrapartum birth injury or death.1,12,13 To limit misinterpretation of the FHR tracing, it is critical for nurses and physicians to use standardized terminology for clear, effective communication.
In 2008, the Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) published guidelines standardizing the terminology used to describe cardiotocography and to create consensus around its interpretation.15 Any description of an intrapartum FHR tracing should include a designation of category (I, II, or III). Fetal well-being is reasonably established with a category I FHR tracing. A category III tracing indicates the high likelihood of fetal acidemia and the need for immediate intervention. A category II FHR tracing is considered indeterminate, and further characterization is required to reasonably exclude fetal metabolic acidosis and a risk of fetal injury.
The presence of moderate variability and fetal response to scalp stimulation are considered reassuring findings that reasonably exclude significant metabolic acidosis. In assessing variability, one pitfall is mistaking the appearance of "variability" within a deceleration (including during return to baseline) for baseline FHR variability. In the event of a persistent category II FHR tracing (>30 minutes), nursing staff should request direct physician review of the FHR tracing. In any case in which fetal well-being is uncertain, nursing staff should request direct physician evaluation of the mother in person and also the FHR tracing, with clear documentation of the findings, interpretation, and plan of care.16
TIP #1: Document, document, document
Nursing and physician documentation about the FHR tracing within the patient-specific clinical context is crucial for effective caregiver communication and patient safety. Thoughtful documentation also reduces liability exposure for providers by demonstrating maternal-fetal surveillance, early identification and treatment of an abnormal or indeterminate FHR tracing, and timely intervention on fetal behalf when necessary.
When the medical record aligns with the electronic FHR tracing and includes appropriate descriptions, interpretations, and interventions in line with national guidelines and institutional policy, the record demonstrates that the providers have a thorough understanding of the physiology behind cardiotocography and, more importantly, that they are able to apply that knowledge in clinical practice.6
Minimizing missteps
Several straightforward interventions can help clinicians overcome the most common pitfalls during FHR monitoring. These include accurate and high-quality cardiotocography, a collaborative team-based approach to patient care, and sustained situational awareness among providers. The consistent use of common language for the description and interpretation of FHR monitoring, adherence to hospital oxytocin protocols, and well-defined expectations for fetal surveillance and provider communication are critical to overcoming these challenges. Regularly scheduled nursing and physician education sessions and interdisciplinary case review can promote the adoption and sustained incorporation of these simple techniques into daily practice.3
Some have advocated for an "electronic fetal monitoring bundle," which would serve as a checklist of clinical evaluation steps that should occur every time a given process occurs.17 This approach would ensure that all providers on labor and delivery are qualified to read, accurately interpret, and respond to FHR tracings. It would require a credentialing process to confirm the competency of team members and reinforce the presence of a common language. It would also include an explicit escalation policy for rapid initiation of the chain of command in cases wherein there is a disagreement among team members about the FHR interpretation. Finally, each patient would be required to have, at all times, an identified responsible provider capable of a rapid response.
Although continuous FHR monitoring may not effectively reduce intrapartum fetal asphyxia, it is clearly here to stay. Recognizing--and addressing--the most common challenges encountered during intrapartum FHR monitoring may reduce unnecessary morbidity and potential liability for caregivers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Sentinel event alert issue 30--July 21, 2004. Preventing infant death and injury during delivery. Adv Neonatal Care. 2004;4(4):180–181.
- Shy KK, Luthy DA, Bennett FC, et al. Effects of electronic fetal-heart-rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. N Engl J Med. 1990;322(9):588–593.
- Clark SL, Nageotte MP, Garite TJ, et al. Intrapartum management of category II fetal heart rate tracings: towards standardization of care. Am J Obstet Gynecol. 2013;209(2):89–97.
- Neilson DR Jr, Freeman RK, Mangan S. Signal ambiguity resulting in unexpected outcome with external fetal heart rate monitoring. Am J Obstet Gynecol. 2008;198(6):717–724.
- McWhinney NA, Knowles S, Green HL, Gordon H. Transmission of the maternal electrocardiograph via a fetal scalp electrode in the presence of intrauterine death. Case report. Br J Obstet Gynaecol. 1984;91(10):1046–1048.
- Simpson KR, Knox GE. Risk management and electronic fetal monitoring: decreasing risk of adverse outcomes and liability exposure. J Perinat Neonatal Nurs. 2000;14(3):40–52.
- Gluck PA. Patient safety in women's health care: a framework for progress. Best Pract Res Clin Obstet Gynaecol. 2007;21(4):525–536.
- Lyndon A, Zlatnik MG, Wachter RM. Effective physician-nurse communication: a patient safety essential for labor and delivery. Am J Obstet Gynecol. 2011;205(2):91–96.
- Jackson M, Holmgren CM, Esplin MS, Henry E, Varner MW. Frequency of fetal heart rate categories and short-term neonatal outcome. Obstet Gynecol. 2011;118(4):803–808.
- Parer JT, Ikeda T. A framework for standardized management of intrapartum fetal heart rate patterns. Am J Obstet Gynecol. 2007;197(1):26.e1-e6.
- MacEachin SR, Lopez CM, Powell KJ, Corbett NL. The fetal heart rate collaborative practice project: situational awareness in electronic fetal monitoring--a Kaiser Permanente Perinatal Patient Safety Program Initiative. J Perinat Neonatal Nurs. 2009;23(4):314–323; quiz 24–25.
- Jonsson M, Norden SL, Hanson U. Analysis of malpractice claims with a focus on oxytocin use in labour. Acta Obstet Gynecol Scand. 2007;86(3):315–319.
- Berglund S, Pettersson H, Cnattingius S, Grunewald C. How often is a low Apgar score the result of substandard care during labour? BJOG. 2010;117(8):968–978.
- Doyle J, Kenny TH, Burkett AM, von Gruenigen VE. A performance improvement process to tackle tachysystole. J Obstet Gynecol Neonatal Nurs. 2011;40(5):512–519.
- Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661–666.
- Knox GE, Simpson KR, Garite TJ. High reliability perinatal units: an approach to the prevention of patient injury and medical malpractice claims. J Healthc Risk Manag. 1999;19(2):24–32.
- Minkoff H, Berkowitz R; Greater New York Hospital Association's Perinatal Safety C. Fetal monitoring bundle. Obstet Gynecol. 2009;114(6):1332–1335.
- Sentinel event alert issue 30--July 21, 2004. Preventing infant death and injury during delivery. Adv Neonatal Care. 2004;4(4):180–181.
- Shy KK, Luthy DA, Bennett FC, et al. Effects of electronic fetal-heart-rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. N Engl J Med. 1990;322(9):588–593.
- Clark SL, Nageotte MP, Garite TJ, et al. Intrapartum management of category II fetal heart rate tracings: towards standardization of care. Am J Obstet Gynecol. 2013;209(2):89–97.
- Neilson DR Jr, Freeman RK, Mangan S. Signal ambiguity resulting in unexpected outcome with external fetal heart rate monitoring. Am J Obstet Gynecol. 2008;198(6):717–724.
- McWhinney NA, Knowles S, Green HL, Gordon H. Transmission of the maternal electrocardiograph via a fetal scalp electrode in the presence of intrauterine death. Case report. Br J Obstet Gynaecol. 1984;91(10):1046–1048.
- Simpson KR, Knox GE. Risk management and electronic fetal monitoring: decreasing risk of adverse outcomes and liability exposure. J Perinat Neonatal Nurs. 2000;14(3):40–52.
- Gluck PA. Patient safety in women's health care: a framework for progress. Best Pract Res Clin Obstet Gynaecol. 2007;21(4):525–536.
- Lyndon A, Zlatnik MG, Wachter RM. Effective physician-nurse communication: a patient safety essential for labor and delivery. Am J Obstet Gynecol. 2011;205(2):91–96.
- Jackson M, Holmgren CM, Esplin MS, Henry E, Varner MW. Frequency of fetal heart rate categories and short-term neonatal outcome. Obstet Gynecol. 2011;118(4):803–808.
- Parer JT, Ikeda T. A framework for standardized management of intrapartum fetal heart rate patterns. Am J Obstet Gynecol. 2007;197(1):26.e1-e6.
- MacEachin SR, Lopez CM, Powell KJ, Corbett NL. The fetal heart rate collaborative practice project: situational awareness in electronic fetal monitoring--a Kaiser Permanente Perinatal Patient Safety Program Initiative. J Perinat Neonatal Nurs. 2009;23(4):314–323; quiz 24–25.
- Jonsson M, Norden SL, Hanson U. Analysis of malpractice claims with a focus on oxytocin use in labour. Acta Obstet Gynecol Scand. 2007;86(3):315–319.
- Berglund S, Pettersson H, Cnattingius S, Grunewald C. How often is a low Apgar score the result of substandard care during labour? BJOG. 2010;117(8):968–978.
- Doyle J, Kenny TH, Burkett AM, von Gruenigen VE. A performance improvement process to tackle tachysystole. J Obstet Gynecol Neonatal Nurs. 2011;40(5):512–519.
- Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661–666.
- Knox GE, Simpson KR, Garite TJ. High reliability perinatal units: an approach to the prevention of patient injury and medical malpractice claims. J Healthc Risk Manag. 1999;19(2):24–32.
- Minkoff H, Berkowitz R; Greater New York Hospital Association's Perinatal Safety C. Fetal monitoring bundle. Obstet Gynecol. 2009;114(6):1332–1335.
In this article
• Communicate urgency
• Situational awareness
The posterior colpotomy: An alternative approach to tissue extraction
CASE: Patient opts for myomectomy
A 41-year-old woman, G0, with symptomatic myomas wishes to preserve her reproductive organs rather than undergo hysterectomy. She chooses laparoscopic myomectomy.
Preoperative imaging with transvaginal ultrasound reveals a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma. Preoperative videohysteroscopy reveals external compression of the anterior intramural myoma without intracavitary extension. Both tubal ostia appear normal.
During a multipuncture technique with a 5-mm laparoscope and 5-mm accessory ports,1 the abdomen and pelvis are evaluated. The 4-cm pedunculated myoma is visualized posteriorly and to the left of midline. The 5-cm intramural myoma enlarges the contour of the uterine fundus.
How would you proceed?
With intracorporeal electromechanical “power” morcellation under scrutiny due to the potential dissemination of benign and malignant tissue, many surgeons are seeking alternatives that will allow them to continue offering minimally invasive surgical options.2–4
Intracorporeal power morcellation is used during minimally invasive gynecologic procedures, including total hysterectomy, supracervical hysterectomy, and myomectomy. Two current alternatives—laparoscopic-assisted minilaparotomy and tissue extraction through a posterior colpotomy—show promise in minimizing the risks of tissue dissemination.5–7 Regardless of the route selected for tissue extraction, the use of endoscopic specimen bags and surgical retractors may ease tissue removal and limit dissemination.
In this article, we describe contained transvaginal tissue extraction through a posterior colpotomy in the setting of laparoscopic myomectomy, describing an actual case. A video of our technique is available at obgmanagement.com.
Technique, tips, and tricks
Posterior colpotomy allows the removal of fibroids during laparoscopic myomectomy without the need to enlarge the abdominal incisions and without the use of intracorporeal power morcellation. Instead, tissue is extracted transvaginally. The incision is hidden in a natural orifice, the vagina.
Equipment consists of a:
- 5-mm laparoscope and 5-mm accessory ports
- LapSac specimen-retrieval bag (Cook Medical; various sizes available)
- AirSeal Access Port (SurgiQuest), 12 mm in diameter and 150 mm in length (FIGURE 1).
Figure 1: Equipment   The AirSeal Access Port (Top) and LapSac specimen-retrieval bag (Bottom). |
Preparatory steps Place a manipulator in the uterus and elevate it anteriorly. Position the AirSeal Access Port transvaginally, with the sharp tip below the cervix in the posterior fornix. Take care not to injure the rectum.
Confirm proper placement of the Access Port and visualize the posterior cul-de-sac laparoscopically.
Insert the 12-mm Access Port for pneumoperitoneum and the introduction and removal of suture, curved needles, and the specimen-retrieval bag.
The Access Port also provides excellent smoke evacuation and optimal visualization during the myomectomy. It is a new-concept laparoscopic port without any mechanical seal. The technology assists in maintaining pneumoperitoneum at a constant pressure despite the size of the opening.
Amputating the myomas
Choose a specimen-retrieval bag just slightly larger than the largest myoma. In this case, the larger of the two myomas is approximately 5 cm. Therefore, a 5 × 8 cm LapSac is appropriate. We roll up the LapSac and place it through the Access Port using smooth forceps, situating the bag in the abdomen prior to the start of the myomectomy, with the opening toward the uterus, so that the myomas can be collected as they are removed (FIGURE 2).
We then inject dilute vasopressin (one 20-unit ampule in 60 cc normal saline) near the base of the pedunculated myoma stalk and use monopolar electrosurgery to amputate the myoma. We place the myoma in the specimen-retrieval bag (FIGURE 3).
Next, we inject dilute vasopressin into the serosa overlying the intramural myoma and use electrosurgery to incise the serosa and myometrium. We enucleate the second myoma and place it in the bag. We then close the uterine incision using a combination of interrupted Vicryl and running V-Loc sutures on a curved CT-2 needle introduced through the Access Port (FIGURE 4).
Figure 2: Introduce the bag  Introduce the LapSac through the Access Port. Figure 3: Contain the specimen  | Figure 4: Close the uterine incision  In preparation for closure, insert a curved CT-2 needle and suture material through the Access Port. Figure 5: Cinch the sac  |
Tissue extraction
We place a blunt-tipped grasper transvaginally through the 12-mm Access Port to retrieve the blue polypropylene drawstring of the specimen bag (FIGURE 5). We then deactivate the Access Port and AirSeal system.
The bag containing the myomas is too large to fit through the port and the posterior colpotomy, so it is necessary to remove the Access Port from the vagina without losing the drawstrings of the specimen bag (FIGURE 6).
We vaginally exteriorize the opening of the bag (FIGURE 7), reorient the pedunculated myoma, which is oblong in shape, using forceps, and remove it without morcellation.
Manual morcellation will be necessary for the second, larger myoma. We perform that morcellation sharply using a scalpel within the specimen retrieval bag, taking care not to puncture the bag (FIGURE 8). When the myoma pieces are small enough, we remove them, along with the bag, through the posterior colpotomy. We then close the colpotomy laparoscopically using two interrupted 0 Vicryl sutures, and we copiously irrigate the pelvis (FIGURE 9).
Figure 6: Remove the Access Port  Prior to tissue extraction, remove the Access Port from the vagina. Figure 7: Exteriorize the bag  | Figure 8: Contain the morcellation  Manually morcellate the specimen within the bag and remove it transvaginally. Figure 9: Close the colpotomy  |
Benefits of this approach
The greatest benefit of this technique is the safe removal of specimens when performing fertility-sparing surgery. The 5-mm incisions are cosmetically inconspicuous. Moreover, the risk of port-site hernia is lower with 5-mm incisions, as opposed to extended incisions to remove specimens transabdominally.
The posterior colpotomy is associated with reduced pain and does not increase the rate of dyspareunia or infection; it also helps prevent pelvic adhesions.8–11
In 1993, we reported the results of second-look laparoscopy in 22 women who had undergone laparoscopic posterior colpotomy for tissue extraction. None had obliterative adhesions in the posterior cul-de-sac.11 This advantage is especially important in fertility-sparing surgery.
We have used this approach for specimen removal after several different procedures, including laparoscopic cystectomy and appendectomy.12,13 For laparoscopic cystectomy, once the cyst is drained, we enucleate it and place the cyst capsule into a specimen bag that has been inserted transvaginally through a posterior colpotomy.12 Laparoscopic appendectomy can be performed using a 12-mm stapler introduced via the colpotomy. We simply remove the specimen in its entirety through the posterior colpotomy.13
The bottom line: Gynecologic surgeons need to continue performing minimally invasive surgery for the benefit of patients. Moving forward and innovating to develop alternatives to intracorporeal power morcellation, when possible, should be our aim rather than falling back on surgeries through large abdominal incisions.
CASE: Resolved
At her 1-week postoperative visit, the patient’s 5-mm incisions are healing well and she has minimal pain.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. King LP, Nezhat C, Nezhat F, et al. Laparoscopic access. In: Nezhat C, Nezhat F, Nezhat CH, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:41–53.
2. Kho KA, Nezhat CH. Evaluating the risks of electric uterine morcellation. JAMA. 2014;311(9):905–906.
3. Kho KA, Anderson TL, Nezhat CH. Intracorporeal electromechanical tissue morcellation: a critical review and recommendations for clinical practice. Obstet Gynecol. 2014;124(4):787–793.
4. Kho K, Nezhat CH. Parasitic myomas. Obstet Gynecol. 2009;114(3):611–615.
5. Nezhat C, Nezhat F, Bess O, Nezhat CH, Mashiach R. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil. 1994;39(1):39–44.
6. Seidman DS, Nezhat CH, Nezhat F, Nezhat C. The role of laparoscopic-assisted myomectomy (LAM). JSLS. 2001;5(4):299–303.
7. Kho KA, Shin JH, Nezhat C. Vaginal extraction of large uteri with the Alexis retractor. JMIG. 2009;16(5):616–617.
8. Ghezzi F, Cromi A, Uccella S, Bogani G, Serati M, Bolis P. Transumbilical versus transvaginal retrieval of surgical specimens at laparoscopy: a randomized trial. Am J Obstet Gynecol. 2012;207(2):112.e1–e6.
9. Ghezzi F, Raio L, Mueller MD, Gyr T, Buttarelli M, Franchi M. Vaginal extraction of pelvic masses following operative laparoscopy. Surg Endosc. 2002;16(12):1691–1696.
10. Guarner-Argente C, Beltrán M, Martínez-Pallí G, et al. Infection during natural orifice transluminal endoscopic surgery peritoneoscopy: a randomized comparative study in a survival porcine model. J Minim Invasive Gynecol. 2011;18(6):741–746.
11. Nezhat F, Brill AI, Nezhat CH, Nezhat C. Adhesion formation after endoscopic posterior colpotomy. J Reprod Med. 1993;38(7):534–536.
12. Nezhat CH. Laparoscopic large ovarian cystectomy and removal through a natural orifice in a 16-year-old female. Video presented at: 21st Annual Meeting of the Society of Laparoscopic Surgeons; September 5–8, 2012; Boston, Massachusetts.
13. Nezhat CH, Datta MS, DeFazio A, Nezhat F, Nezhat C. Natural orifice-assisted laparoscopic appendectomy. JSLS. 2009;13(1):14–18.
CASE: Patient opts for myomectomy
A 41-year-old woman, G0, with symptomatic myomas wishes to preserve her reproductive organs rather than undergo hysterectomy. She chooses laparoscopic myomectomy.
Preoperative imaging with transvaginal ultrasound reveals a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma. Preoperative videohysteroscopy reveals external compression of the anterior intramural myoma without intracavitary extension. Both tubal ostia appear normal.
During a multipuncture technique with a 5-mm laparoscope and 5-mm accessory ports,1 the abdomen and pelvis are evaluated. The 4-cm pedunculated myoma is visualized posteriorly and to the left of midline. The 5-cm intramural myoma enlarges the contour of the uterine fundus.
How would you proceed?
With intracorporeal electromechanical “power” morcellation under scrutiny due to the potential dissemination of benign and malignant tissue, many surgeons are seeking alternatives that will allow them to continue offering minimally invasive surgical options.2–4
Intracorporeal power morcellation is used during minimally invasive gynecologic procedures, including total hysterectomy, supracervical hysterectomy, and myomectomy. Two current alternatives—laparoscopic-assisted minilaparotomy and tissue extraction through a posterior colpotomy—show promise in minimizing the risks of tissue dissemination.5–7 Regardless of the route selected for tissue extraction, the use of endoscopic specimen bags and surgical retractors may ease tissue removal and limit dissemination.
In this article, we describe contained transvaginal tissue extraction through a posterior colpotomy in the setting of laparoscopic myomectomy, describing an actual case. A video of our technique is available at obgmanagement.com.
Technique, tips, and tricks
Posterior colpotomy allows the removal of fibroids during laparoscopic myomectomy without the need to enlarge the abdominal incisions and without the use of intracorporeal power morcellation. Instead, tissue is extracted transvaginally. The incision is hidden in a natural orifice, the vagina.
Equipment consists of a:
- 5-mm laparoscope and 5-mm accessory ports
- LapSac specimen-retrieval bag (Cook Medical; various sizes available)
- AirSeal Access Port (SurgiQuest), 12 mm in diameter and 150 mm in length (FIGURE 1).
Figure 1: Equipment   The AirSeal Access Port (Top) and LapSac specimen-retrieval bag (Bottom). |
Preparatory steps Place a manipulator in the uterus and elevate it anteriorly. Position the AirSeal Access Port transvaginally, with the sharp tip below the cervix in the posterior fornix. Take care not to injure the rectum.
Confirm proper placement of the Access Port and visualize the posterior cul-de-sac laparoscopically.
Insert the 12-mm Access Port for pneumoperitoneum and the introduction and removal of suture, curved needles, and the specimen-retrieval bag.
The Access Port also provides excellent smoke evacuation and optimal visualization during the myomectomy. It is a new-concept laparoscopic port without any mechanical seal. The technology assists in maintaining pneumoperitoneum at a constant pressure despite the size of the opening.
Amputating the myomas
Choose a specimen-retrieval bag just slightly larger than the largest myoma. In this case, the larger of the two myomas is approximately 5 cm. Therefore, a 5 × 8 cm LapSac is appropriate. We roll up the LapSac and place it through the Access Port using smooth forceps, situating the bag in the abdomen prior to the start of the myomectomy, with the opening toward the uterus, so that the myomas can be collected as they are removed (FIGURE 2).
We then inject dilute vasopressin (one 20-unit ampule in 60 cc normal saline) near the base of the pedunculated myoma stalk and use monopolar electrosurgery to amputate the myoma. We place the myoma in the specimen-retrieval bag (FIGURE 3).
Next, we inject dilute vasopressin into the serosa overlying the intramural myoma and use electrosurgery to incise the serosa and myometrium. We enucleate the second myoma and place it in the bag. We then close the uterine incision using a combination of interrupted Vicryl and running V-Loc sutures on a curved CT-2 needle introduced through the Access Port (FIGURE 4).
Figure 2: Introduce the bag  Introduce the LapSac through the Access Port. Figure 3: Contain the specimen  | Figure 4: Close the uterine incision  In preparation for closure, insert a curved CT-2 needle and suture material through the Access Port. Figure 5: Cinch the sac  |
Tissue extraction
We place a blunt-tipped grasper transvaginally through the 12-mm Access Port to retrieve the blue polypropylene drawstring of the specimen bag (FIGURE 5). We then deactivate the Access Port and AirSeal system.
The bag containing the myomas is too large to fit through the port and the posterior colpotomy, so it is necessary to remove the Access Port from the vagina without losing the drawstrings of the specimen bag (FIGURE 6).
We vaginally exteriorize the opening of the bag (FIGURE 7), reorient the pedunculated myoma, which is oblong in shape, using forceps, and remove it without morcellation.
Manual morcellation will be necessary for the second, larger myoma. We perform that morcellation sharply using a scalpel within the specimen retrieval bag, taking care not to puncture the bag (FIGURE 8). When the myoma pieces are small enough, we remove them, along with the bag, through the posterior colpotomy. We then close the colpotomy laparoscopically using two interrupted 0 Vicryl sutures, and we copiously irrigate the pelvis (FIGURE 9).
Figure 6: Remove the Access Port  Prior to tissue extraction, remove the Access Port from the vagina. Figure 7: Exteriorize the bag  | Figure 8: Contain the morcellation  Manually morcellate the specimen within the bag and remove it transvaginally. Figure 9: Close the colpotomy  |
Benefits of this approach
The greatest benefit of this technique is the safe removal of specimens when performing fertility-sparing surgery. The 5-mm incisions are cosmetically inconspicuous. Moreover, the risk of port-site hernia is lower with 5-mm incisions, as opposed to extended incisions to remove specimens transabdominally.
The posterior colpotomy is associated with reduced pain and does not increase the rate of dyspareunia or infection; it also helps prevent pelvic adhesions.8–11
In 1993, we reported the results of second-look laparoscopy in 22 women who had undergone laparoscopic posterior colpotomy for tissue extraction. None had obliterative adhesions in the posterior cul-de-sac.11 This advantage is especially important in fertility-sparing surgery.
We have used this approach for specimen removal after several different procedures, including laparoscopic cystectomy and appendectomy.12,13 For laparoscopic cystectomy, once the cyst is drained, we enucleate it and place the cyst capsule into a specimen bag that has been inserted transvaginally through a posterior colpotomy.12 Laparoscopic appendectomy can be performed using a 12-mm stapler introduced via the colpotomy. We simply remove the specimen in its entirety through the posterior colpotomy.13
The bottom line: Gynecologic surgeons need to continue performing minimally invasive surgery for the benefit of patients. Moving forward and innovating to develop alternatives to intracorporeal power morcellation, when possible, should be our aim rather than falling back on surgeries through large abdominal incisions.
CASE: Resolved
At her 1-week postoperative visit, the patient’s 5-mm incisions are healing well and she has minimal pain.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE: Patient opts for myomectomy
A 41-year-old woman, G0, with symptomatic myomas wishes to preserve her reproductive organs rather than undergo hysterectomy. She chooses laparoscopic myomectomy.
Preoperative imaging with transvaginal ultrasound reveals a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma. Preoperative videohysteroscopy reveals external compression of the anterior intramural myoma without intracavitary extension. Both tubal ostia appear normal.
During a multipuncture technique with a 5-mm laparoscope and 5-mm accessory ports,1 the abdomen and pelvis are evaluated. The 4-cm pedunculated myoma is visualized posteriorly and to the left of midline. The 5-cm intramural myoma enlarges the contour of the uterine fundus.
How would you proceed?
With intracorporeal electromechanical “power” morcellation under scrutiny due to the potential dissemination of benign and malignant tissue, many surgeons are seeking alternatives that will allow them to continue offering minimally invasive surgical options.2–4
Intracorporeal power morcellation is used during minimally invasive gynecologic procedures, including total hysterectomy, supracervical hysterectomy, and myomectomy. Two current alternatives—laparoscopic-assisted minilaparotomy and tissue extraction through a posterior colpotomy—show promise in minimizing the risks of tissue dissemination.5–7 Regardless of the route selected for tissue extraction, the use of endoscopic specimen bags and surgical retractors may ease tissue removal and limit dissemination.
In this article, we describe contained transvaginal tissue extraction through a posterior colpotomy in the setting of laparoscopic myomectomy, describing an actual case. A video of our technique is available at obgmanagement.com.
Technique, tips, and tricks
Posterior colpotomy allows the removal of fibroids during laparoscopic myomectomy without the need to enlarge the abdominal incisions and without the use of intracorporeal power morcellation. Instead, tissue is extracted transvaginally. The incision is hidden in a natural orifice, the vagina.
Equipment consists of a:
- 5-mm laparoscope and 5-mm accessory ports
- LapSac specimen-retrieval bag (Cook Medical; various sizes available)
- AirSeal Access Port (SurgiQuest), 12 mm in diameter and 150 mm in length (FIGURE 1).
Figure 1: Equipment   The AirSeal Access Port (Top) and LapSac specimen-retrieval bag (Bottom). |
Preparatory steps Place a manipulator in the uterus and elevate it anteriorly. Position the AirSeal Access Port transvaginally, with the sharp tip below the cervix in the posterior fornix. Take care not to injure the rectum.
Confirm proper placement of the Access Port and visualize the posterior cul-de-sac laparoscopically.
Insert the 12-mm Access Port for pneumoperitoneum and the introduction and removal of suture, curved needles, and the specimen-retrieval bag.
The Access Port also provides excellent smoke evacuation and optimal visualization during the myomectomy. It is a new-concept laparoscopic port without any mechanical seal. The technology assists in maintaining pneumoperitoneum at a constant pressure despite the size of the opening.
Amputating the myomas
Choose a specimen-retrieval bag just slightly larger than the largest myoma. In this case, the larger of the two myomas is approximately 5 cm. Therefore, a 5 × 8 cm LapSac is appropriate. We roll up the LapSac and place it through the Access Port using smooth forceps, situating the bag in the abdomen prior to the start of the myomectomy, with the opening toward the uterus, so that the myomas can be collected as they are removed (FIGURE 2).
We then inject dilute vasopressin (one 20-unit ampule in 60 cc normal saline) near the base of the pedunculated myoma stalk and use monopolar electrosurgery to amputate the myoma. We place the myoma in the specimen-retrieval bag (FIGURE 3).
Next, we inject dilute vasopressin into the serosa overlying the intramural myoma and use electrosurgery to incise the serosa and myometrium. We enucleate the second myoma and place it in the bag. We then close the uterine incision using a combination of interrupted Vicryl and running V-Loc sutures on a curved CT-2 needle introduced through the Access Port (FIGURE 4).
Figure 2: Introduce the bag  Introduce the LapSac through the Access Port. Figure 3: Contain the specimen  | Figure 4: Close the uterine incision  In preparation for closure, insert a curved CT-2 needle and suture material through the Access Port. Figure 5: Cinch the sac  |
Tissue extraction
We place a blunt-tipped grasper transvaginally through the 12-mm Access Port to retrieve the blue polypropylene drawstring of the specimen bag (FIGURE 5). We then deactivate the Access Port and AirSeal system.
The bag containing the myomas is too large to fit through the port and the posterior colpotomy, so it is necessary to remove the Access Port from the vagina without losing the drawstrings of the specimen bag (FIGURE 6).
We vaginally exteriorize the opening of the bag (FIGURE 7), reorient the pedunculated myoma, which is oblong in shape, using forceps, and remove it without morcellation.
Manual morcellation will be necessary for the second, larger myoma. We perform that morcellation sharply using a scalpel within the specimen retrieval bag, taking care not to puncture the bag (FIGURE 8). When the myoma pieces are small enough, we remove them, along with the bag, through the posterior colpotomy. We then close the colpotomy laparoscopically using two interrupted 0 Vicryl sutures, and we copiously irrigate the pelvis (FIGURE 9).
Figure 6: Remove the Access Port  Prior to tissue extraction, remove the Access Port from the vagina. Figure 7: Exteriorize the bag  | Figure 8: Contain the morcellation  Manually morcellate the specimen within the bag and remove it transvaginally. Figure 9: Close the colpotomy  |
Benefits of this approach
The greatest benefit of this technique is the safe removal of specimens when performing fertility-sparing surgery. The 5-mm incisions are cosmetically inconspicuous. Moreover, the risk of port-site hernia is lower with 5-mm incisions, as opposed to extended incisions to remove specimens transabdominally.
The posterior colpotomy is associated with reduced pain and does not increase the rate of dyspareunia or infection; it also helps prevent pelvic adhesions.8–11
In 1993, we reported the results of second-look laparoscopy in 22 women who had undergone laparoscopic posterior colpotomy for tissue extraction. None had obliterative adhesions in the posterior cul-de-sac.11 This advantage is especially important in fertility-sparing surgery.
We have used this approach for specimen removal after several different procedures, including laparoscopic cystectomy and appendectomy.12,13 For laparoscopic cystectomy, once the cyst is drained, we enucleate it and place the cyst capsule into a specimen bag that has been inserted transvaginally through a posterior colpotomy.12 Laparoscopic appendectomy can be performed using a 12-mm stapler introduced via the colpotomy. We simply remove the specimen in its entirety through the posterior colpotomy.13
The bottom line: Gynecologic surgeons need to continue performing minimally invasive surgery for the benefit of patients. Moving forward and innovating to develop alternatives to intracorporeal power morcellation, when possible, should be our aim rather than falling back on surgeries through large abdominal incisions.
CASE: Resolved
At her 1-week postoperative visit, the patient’s 5-mm incisions are healing well and she has minimal pain.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. King LP, Nezhat C, Nezhat F, et al. Laparoscopic access. In: Nezhat C, Nezhat F, Nezhat CH, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:41–53.
2. Kho KA, Nezhat CH. Evaluating the risks of electric uterine morcellation. JAMA. 2014;311(9):905–906.
3. Kho KA, Anderson TL, Nezhat CH. Intracorporeal electromechanical tissue morcellation: a critical review and recommendations for clinical practice. Obstet Gynecol. 2014;124(4):787–793.
4. Kho K, Nezhat CH. Parasitic myomas. Obstet Gynecol. 2009;114(3):611–615.
5. Nezhat C, Nezhat F, Bess O, Nezhat CH, Mashiach R. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil. 1994;39(1):39–44.
6. Seidman DS, Nezhat CH, Nezhat F, Nezhat C. The role of laparoscopic-assisted myomectomy (LAM). JSLS. 2001;5(4):299–303.
7. Kho KA, Shin JH, Nezhat C. Vaginal extraction of large uteri with the Alexis retractor. JMIG. 2009;16(5):616–617.
8. Ghezzi F, Cromi A, Uccella S, Bogani G, Serati M, Bolis P. Transumbilical versus transvaginal retrieval of surgical specimens at laparoscopy: a randomized trial. Am J Obstet Gynecol. 2012;207(2):112.e1–e6.
9. Ghezzi F, Raio L, Mueller MD, Gyr T, Buttarelli M, Franchi M. Vaginal extraction of pelvic masses following operative laparoscopy. Surg Endosc. 2002;16(12):1691–1696.
10. Guarner-Argente C, Beltrán M, Martínez-Pallí G, et al. Infection during natural orifice transluminal endoscopic surgery peritoneoscopy: a randomized comparative study in a survival porcine model. J Minim Invasive Gynecol. 2011;18(6):741–746.
11. Nezhat F, Brill AI, Nezhat CH, Nezhat C. Adhesion formation after endoscopic posterior colpotomy. J Reprod Med. 1993;38(7):534–536.
12. Nezhat CH. Laparoscopic large ovarian cystectomy and removal through a natural orifice in a 16-year-old female. Video presented at: 21st Annual Meeting of the Society of Laparoscopic Surgeons; September 5–8, 2012; Boston, Massachusetts.
13. Nezhat CH, Datta MS, DeFazio A, Nezhat F, Nezhat C. Natural orifice-assisted laparoscopic appendectomy. JSLS. 2009;13(1):14–18.
1. King LP, Nezhat C, Nezhat F, et al. Laparoscopic access. In: Nezhat C, Nezhat F, Nezhat CH, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:41–53.
2. Kho KA, Nezhat CH. Evaluating the risks of electric uterine morcellation. JAMA. 2014;311(9):905–906.
3. Kho KA, Anderson TL, Nezhat CH. Intracorporeal electromechanical tissue morcellation: a critical review and recommendations for clinical practice. Obstet Gynecol. 2014;124(4):787–793.
4. Kho K, Nezhat CH. Parasitic myomas. Obstet Gynecol. 2009;114(3):611–615.
5. Nezhat C, Nezhat F, Bess O, Nezhat CH, Mashiach R. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil. 1994;39(1):39–44.
6. Seidman DS, Nezhat CH, Nezhat F, Nezhat C. The role of laparoscopic-assisted myomectomy (LAM). JSLS. 2001;5(4):299–303.
7. Kho KA, Shin JH, Nezhat C. Vaginal extraction of large uteri with the Alexis retractor. JMIG. 2009;16(5):616–617.
8. Ghezzi F, Cromi A, Uccella S, Bogani G, Serati M, Bolis P. Transumbilical versus transvaginal retrieval of surgical specimens at laparoscopy: a randomized trial. Am J Obstet Gynecol. 2012;207(2):112.e1–e6.
9. Ghezzi F, Raio L, Mueller MD, Gyr T, Buttarelli M, Franchi M. Vaginal extraction of pelvic masses following operative laparoscopy. Surg Endosc. 2002;16(12):1691–1696.
10. Guarner-Argente C, Beltrán M, Martínez-Pallí G, et al. Infection during natural orifice transluminal endoscopic surgery peritoneoscopy: a randomized comparative study in a survival porcine model. J Minim Invasive Gynecol. 2011;18(6):741–746.
11. Nezhat F, Brill AI, Nezhat CH, Nezhat C. Adhesion formation after endoscopic posterior colpotomy. J Reprod Med. 1993;38(7):534–536.
12. Nezhat CH. Laparoscopic large ovarian cystectomy and removal through a natural orifice in a 16-year-old female. Video presented at: 21st Annual Meeting of the Society of Laparoscopic Surgeons; September 5–8, 2012; Boston, Massachusetts.
13. Nezhat CH, Datta MS, DeFazio A, Nezhat F, Nezhat C. Natural orifice-assisted laparoscopic appendectomy. JSLS. 2009;13(1):14–18.
Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews
“It takes 20 years to build a reputation and 5 minutes to ruin it. If you think about that, you’ll do things differently.”
—Warren Buffet
CASE: Decline in new patients
A well-respected physician—one of the best in his field—notices that the number of new patients in his practice has fallen off drastically over the past year. Baffled, he hires a consultant, who discovers that the doctor’s online reputation has plummeted, thanks to four negative reviews and no positive ones.
What can the physician do to remedy the situation and restore his reputation?
The problem can be fixed, but it takes time—like major surgery. Rather than wait until negative reviews are posted, we recommend that you become proactive and take steps as soon as possible to secure your online reputation. That way, you won’t get caught by surprise when one or two unhappy patients try to smear your good name. In this article, we step you through a number of remedies and proactive strategies for boosting positive online reviews and combating negative ones.
The Internet: A one-stop source of information
The Internet has become everyone’s go-to source for pretty much any kind of data, including details on products, services, and people. Anyone can access all kinds of information simply by asking.
Today, people research medical conditions on the Web, often using Google. If you have done your search engine optimization, your Web site will come up in the first page of search results, making it possible for prospective patients to click through to your homepage. (For the scoop on search engine optimization, see Part 3 of this series, “Maximizing your online reach through SEO and pay-per-click,” which appeared in the September 2014 issue of OBG Management.)
If visitors like what they see at your site, they may make an appointment. But they are more likely to visit three or four other sites before making a decision. And in all likelihood, they will research each physician to find out what patients have to say about her or him. It’s no different than looking at the reviews of hotels or products you are considering.
You are an open book on the Internet. Only a few short years ago, your peers and patients knew your reputation primarily through word of mouth, which traveled at the speed of molasses. For the most part, that information was favorable. Today your exposure is much greater, and negative comments about you can be viewed by thousands of potential patients. The speed of information has increased, as well. What is posted on the Internet can become readily available to hundreds, thousands, and even millions of Web users in a nanosecond.
The Internet provides a forum for people to say whatever they want about their experiences, both positive and negative. Regrettably, the positive experiences do not find their way online nearly as often as the negative ones!
The bottom line? In today’s Internet-savvy world, you need to pay regular attention to your online reputation. You need to take steps to ensure that your name and practice look their best and to negate any complaints that may appear.
What patients share about their experience with you
Many online review sites provide an opportunity for your patients to describe their experience with you and your practice. To name a few: RateMDs.com, Vitals.com, ZocDoc.com, healthgrades.com, UcompareHealth.com, Citysearch.com, yelp.com, and, of course, Google Plus reviews.
And when patients post comments on the Internet, you likely will be rated on:
- the patient’s wait time
- how your staff treated the patient
- the diagnosis
- your attitude
- the level of trust in your decisions
- treatment and outcome.
The online surfer searching for a reputable physician is likely to believe whatever he or she finds on the leading review sites.
The good news: Most physicians have a very favorable rating, averaging 9.3 out of 10 on a scale of 1 to 10. In fact, 70% of doctors have perfect scores!1
The bad news: Someone who is unhappy with her treatment or outcome will go out of her way to find every online review site possible and proclaim your faults to the cyber-world, using the Internet as a forum, whether her facts are straight or not. Patients who are pleased and satisfied rarely bother to place a positive review.
How you can control your online reputation
It is incumbent upon you to keep an eye on your online reputation at all times. Here are some tips for taking charge:
- If someone posts a negative review, respond to them directly in the review site. Doing so does not violate privacy laws as long as you do not mention the patient’s name or give other identifying details. Explain your side of the story without confirming or denying that the reviewer is or was a patient. Do not mention the specifics of any patient’s condition.
- If you feel that a negative review is completely unjustified, file a dispute with the review site. Many review sites will remove the unfavorable content if you can convince them that the patient is merely ranting.
- To protect your reputation over the long term, use your name or practice name to set up an alert with Google Alerts by visiting the site Google.com/alerts.
- Do a Google search of your name and the name of your practice at least once a month and check out all the review sites that come up. Read the comments!
Develop a proactive system
You have a lot of control when it comes to protecting your online reputation, provided you are willing to take the time to set up a system to regularly request feedback or testimonials from your patients.
Regrettably, this is where most medical practices fall short, by failing to establish a system to solicit positive reviews.
The process need not be complicated. Such a system can be set in motion by scheduling a quick meeting with your staff to announce your plans to solicit testimonials from patients. Often there will be a flurry of activity for a couple of weeks before the task is forgotten. To keep your system from falling through the cracks, make a checklist and decide who on your staff is responsible for each step in the process. Go over the results in your staff meetings on a regular basis—ie, at least monthly.
You want to solicit positive reviews for use in two places:
- your Web site
- the review sites we mentioned earlier.
Posting testimonials on your Web site
Your site is the place prospective patients visit when they are looking for information about you and your services. Here are a few tips on gathering and posting testimonials:
- The best time to solicit feedback from the patient is after the follow-up appointment, when her needs have been met and she has had at least two experiences with your practice. If she is happy with her outcome, she is likely to be receptive to the idea of providing a testimonial while the details are fresh in her mind.
- Post testimonials on your homepage and every other page at your site. They should be visible when each page loads without the need to scroll down. A testimonial is worthless if it can’t be easily seen.
- Post testimonials in italics, with quotation marks around the comments to distinguish them from other elements on the page.
- Give each testimonial a headline in bold italics. Use key words likely to resonate with the reader. For example, if the patient reports: “I had a surgical procedure and it was a game changer. You turned my life around! Thank you!” the headline might be: “You turned my life around.”
- Create a Web page just for testimonials and order the comments and headlines so that they will appeal to a diversity of prospective patients. The visitor may not read every testimonial, but she will at least read and scroll through the headlines.
Gathering feedback: Your options
- One option for automating the gathering of feedback is to include a patient feedback survey on your Web site. It’s a convenient way to ask for comments. When the patient is in the office, you or your staff can simply ask her to visit the survey page on your site and answer the questions. The problem with this approach is that many patients will agree to complete the survey but few will actually follow through.
- A far more effective way to get patients to complete a survey while they are still in your office is to have the receptionist hand the patient an iPad after her appointment and ask her to take a couple of minutes to complete the survey. You can then transcribe her comments and post them on your site.
- Asking patients to post positive comments on review sites such as healthgrades.com is another option—but, again, patients are unlikely to follow through unless you make it as easy and fast as possible. The best way to do this is to provide your patient with a blueprint for how to proceed. We offer a “patient feedback” form that contains four or five questions (FIGURE). The answers to these questions will provide a great testimonial for the doctor and the practice. Providing your patients with the right questions to elicit an emotional response will help them describe their experiences more fully. If you let the patient create a testimonial on her own, you’ll probably just receive comments such as, “I’m very happy with my results” or “She is a great doctor.”
- Also provide patients with a step-by-step process for entering their feedback on the desired review sites. This can be a daunting task for your patient, so your instructions should be clear and simple. Better yet, have someone on your staff sit with the patient at a computer or iPad to help her through the process.
- Another way to control your online reputation is to capture positive comments at the point of service. In our practice, we have a testimonial poster in every exam room as well as the reception area. It contains a quick response (QR) code that can be scanned to allow the patient to submit a testimonial about her experience with the practice. With this system, we are able to collect three to five positive reviews every day.
FIGURE: Patient follow-up satisfaction survey
| It is our intention to provide our patients with the absolute best medical care available to produce optimal results. Your feedback about your procedure and patient care is an important measure of our performance. Please take the time to let us know how you feel about your results:
Your name: _______________________________ Date: ________ Thank you for telling us about the results of your procedure. How you feel about your experience helps us better understand the physical and emotional needs of our patients. We would like to share your experience with others who might be struggling with the same issues. By signing this form, you agree to let us share this information on our Web site and informational material to help other patients understand the benefits of having these types of procedures performed. |
CASE: Resolved
The physician institutes a process in his practice to gather testimonials and positive feedback, and his staff takes time to help willing patients post their reviews online. He also disputes the negative comments that have already been posted online, offering an objective response to the complaints and asking the Web sites to take down the reviews that are merely ranting. In addition, he posts selected testimonials on the homepage of his Web site and adds a page that is just for testimonials.
Within a few weeks, the number of new patients scheduling appointments with him begins to increase until he once again enjoys a bustling practice.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
- Schwartz SK. Online patient feedback: what to do. Physicianspractice.com. http://www.physicianspractice.com/health-it/online-patient-feedback-what-do. Published December 27, 2012. Accessed November 15, 2014.
“It takes 20 years to build a reputation and 5 minutes to ruin it. If you think about that, you’ll do things differently.”
—Warren Buffet
CASE: Decline in new patients
A well-respected physician—one of the best in his field—notices that the number of new patients in his practice has fallen off drastically over the past year. Baffled, he hires a consultant, who discovers that the doctor’s online reputation has plummeted, thanks to four negative reviews and no positive ones.
What can the physician do to remedy the situation and restore his reputation?
The problem can be fixed, but it takes time—like major surgery. Rather than wait until negative reviews are posted, we recommend that you become proactive and take steps as soon as possible to secure your online reputation. That way, you won’t get caught by surprise when one or two unhappy patients try to smear your good name. In this article, we step you through a number of remedies and proactive strategies for boosting positive online reviews and combating negative ones.
The Internet: A one-stop source of information
The Internet has become everyone’s go-to source for pretty much any kind of data, including details on products, services, and people. Anyone can access all kinds of information simply by asking.
Today, people research medical conditions on the Web, often using Google. If you have done your search engine optimization, your Web site will come up in the first page of search results, making it possible for prospective patients to click through to your homepage. (For the scoop on search engine optimization, see Part 3 of this series, “Maximizing your online reach through SEO and pay-per-click,” which appeared in the September 2014 issue of OBG Management.)
If visitors like what they see at your site, they may make an appointment. But they are more likely to visit three or four other sites before making a decision. And in all likelihood, they will research each physician to find out what patients have to say about her or him. It’s no different than looking at the reviews of hotels or products you are considering.
You are an open book on the Internet. Only a few short years ago, your peers and patients knew your reputation primarily through word of mouth, which traveled at the speed of molasses. For the most part, that information was favorable. Today your exposure is much greater, and negative comments about you can be viewed by thousands of potential patients. The speed of information has increased, as well. What is posted on the Internet can become readily available to hundreds, thousands, and even millions of Web users in a nanosecond.
The Internet provides a forum for people to say whatever they want about their experiences, both positive and negative. Regrettably, the positive experiences do not find their way online nearly as often as the negative ones!
The bottom line? In today’s Internet-savvy world, you need to pay regular attention to your online reputation. You need to take steps to ensure that your name and practice look their best and to negate any complaints that may appear.
What patients share about their experience with you
Many online review sites provide an opportunity for your patients to describe their experience with you and your practice. To name a few: RateMDs.com, Vitals.com, ZocDoc.com, healthgrades.com, UcompareHealth.com, Citysearch.com, yelp.com, and, of course, Google Plus reviews.
And when patients post comments on the Internet, you likely will be rated on:
- the patient’s wait time
- how your staff treated the patient
- the diagnosis
- your attitude
- the level of trust in your decisions
- treatment and outcome.
The online surfer searching for a reputable physician is likely to believe whatever he or she finds on the leading review sites.
The good news: Most physicians have a very favorable rating, averaging 9.3 out of 10 on a scale of 1 to 10. In fact, 70% of doctors have perfect scores!1
The bad news: Someone who is unhappy with her treatment or outcome will go out of her way to find every online review site possible and proclaim your faults to the cyber-world, using the Internet as a forum, whether her facts are straight or not. Patients who are pleased and satisfied rarely bother to place a positive review.
How you can control your online reputation
It is incumbent upon you to keep an eye on your online reputation at all times. Here are some tips for taking charge:
- If someone posts a negative review, respond to them directly in the review site. Doing so does not violate privacy laws as long as you do not mention the patient’s name or give other identifying details. Explain your side of the story without confirming or denying that the reviewer is or was a patient. Do not mention the specifics of any patient’s condition.
- If you feel that a negative review is completely unjustified, file a dispute with the review site. Many review sites will remove the unfavorable content if you can convince them that the patient is merely ranting.
- To protect your reputation over the long term, use your name or practice name to set up an alert with Google Alerts by visiting the site Google.com/alerts.
- Do a Google search of your name and the name of your practice at least once a month and check out all the review sites that come up. Read the comments!
Develop a proactive system
You have a lot of control when it comes to protecting your online reputation, provided you are willing to take the time to set up a system to regularly request feedback or testimonials from your patients.
Regrettably, this is where most medical practices fall short, by failing to establish a system to solicit positive reviews.
The process need not be complicated. Such a system can be set in motion by scheduling a quick meeting with your staff to announce your plans to solicit testimonials from patients. Often there will be a flurry of activity for a couple of weeks before the task is forgotten. To keep your system from falling through the cracks, make a checklist and decide who on your staff is responsible for each step in the process. Go over the results in your staff meetings on a regular basis—ie, at least monthly.
You want to solicit positive reviews for use in two places:
- your Web site
- the review sites we mentioned earlier.
Posting testimonials on your Web site
Your site is the place prospective patients visit when they are looking for information about you and your services. Here are a few tips on gathering and posting testimonials:
- The best time to solicit feedback from the patient is after the follow-up appointment, when her needs have been met and she has had at least two experiences with your practice. If she is happy with her outcome, she is likely to be receptive to the idea of providing a testimonial while the details are fresh in her mind.
- Post testimonials on your homepage and every other page at your site. They should be visible when each page loads without the need to scroll down. A testimonial is worthless if it can’t be easily seen.
- Post testimonials in italics, with quotation marks around the comments to distinguish them from other elements on the page.
- Give each testimonial a headline in bold italics. Use key words likely to resonate with the reader. For example, if the patient reports: “I had a surgical procedure and it was a game changer. You turned my life around! Thank you!” the headline might be: “You turned my life around.”
- Create a Web page just for testimonials and order the comments and headlines so that they will appeal to a diversity of prospective patients. The visitor may not read every testimonial, but she will at least read and scroll through the headlines.
Gathering feedback: Your options
- One option for automating the gathering of feedback is to include a patient feedback survey on your Web site. It’s a convenient way to ask for comments. When the patient is in the office, you or your staff can simply ask her to visit the survey page on your site and answer the questions. The problem with this approach is that many patients will agree to complete the survey but few will actually follow through.
- A far more effective way to get patients to complete a survey while they are still in your office is to have the receptionist hand the patient an iPad after her appointment and ask her to take a couple of minutes to complete the survey. You can then transcribe her comments and post them on your site.
- Asking patients to post positive comments on review sites such as healthgrades.com is another option—but, again, patients are unlikely to follow through unless you make it as easy and fast as possible. The best way to do this is to provide your patient with a blueprint for how to proceed. We offer a “patient feedback” form that contains four or five questions (FIGURE). The answers to these questions will provide a great testimonial for the doctor and the practice. Providing your patients with the right questions to elicit an emotional response will help them describe their experiences more fully. If you let the patient create a testimonial on her own, you’ll probably just receive comments such as, “I’m very happy with my results” or “She is a great doctor.”
- Also provide patients with a step-by-step process for entering their feedback on the desired review sites. This can be a daunting task for your patient, so your instructions should be clear and simple. Better yet, have someone on your staff sit with the patient at a computer or iPad to help her through the process.
- Another way to control your online reputation is to capture positive comments at the point of service. In our practice, we have a testimonial poster in every exam room as well as the reception area. It contains a quick response (QR) code that can be scanned to allow the patient to submit a testimonial about her experience with the practice. With this system, we are able to collect three to five positive reviews every day.
FIGURE: Patient follow-up satisfaction survey
| It is our intention to provide our patients with the absolute best medical care available to produce optimal results. Your feedback about your procedure and patient care is an important measure of our performance. Please take the time to let us know how you feel about your results:
Your name: _______________________________ Date: ________ Thank you for telling us about the results of your procedure. How you feel about your experience helps us better understand the physical and emotional needs of our patients. We would like to share your experience with others who might be struggling with the same issues. By signing this form, you agree to let us share this information on our Web site and informational material to help other patients understand the benefits of having these types of procedures performed. |
CASE: Resolved
The physician institutes a process in his practice to gather testimonials and positive feedback, and his staff takes time to help willing patients post their reviews online. He also disputes the negative comments that have already been posted online, offering an objective response to the complaints and asking the Web sites to take down the reviews that are merely ranting. In addition, he posts selected testimonials on the homepage of his Web site and adds a page that is just for testimonials.
Within a few weeks, the number of new patients scheduling appointments with him begins to increase until he once again enjoys a bustling practice.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
“It takes 20 years to build a reputation and 5 minutes to ruin it. If you think about that, you’ll do things differently.”
—Warren Buffet
CASE: Decline in new patients
A well-respected physician—one of the best in his field—notices that the number of new patients in his practice has fallen off drastically over the past year. Baffled, he hires a consultant, who discovers that the doctor’s online reputation has plummeted, thanks to four negative reviews and no positive ones.
What can the physician do to remedy the situation and restore his reputation?
The problem can be fixed, but it takes time—like major surgery. Rather than wait until negative reviews are posted, we recommend that you become proactive and take steps as soon as possible to secure your online reputation. That way, you won’t get caught by surprise when one or two unhappy patients try to smear your good name. In this article, we step you through a number of remedies and proactive strategies for boosting positive online reviews and combating negative ones.
The Internet: A one-stop source of information
The Internet has become everyone’s go-to source for pretty much any kind of data, including details on products, services, and people. Anyone can access all kinds of information simply by asking.
Today, people research medical conditions on the Web, often using Google. If you have done your search engine optimization, your Web site will come up in the first page of search results, making it possible for prospective patients to click through to your homepage. (For the scoop on search engine optimization, see Part 3 of this series, “Maximizing your online reach through SEO and pay-per-click,” which appeared in the September 2014 issue of OBG Management.)
If visitors like what they see at your site, they may make an appointment. But they are more likely to visit three or four other sites before making a decision. And in all likelihood, they will research each physician to find out what patients have to say about her or him. It’s no different than looking at the reviews of hotels or products you are considering.
You are an open book on the Internet. Only a few short years ago, your peers and patients knew your reputation primarily through word of mouth, which traveled at the speed of molasses. For the most part, that information was favorable. Today your exposure is much greater, and negative comments about you can be viewed by thousands of potential patients. The speed of information has increased, as well. What is posted on the Internet can become readily available to hundreds, thousands, and even millions of Web users in a nanosecond.
The Internet provides a forum for people to say whatever they want about their experiences, both positive and negative. Regrettably, the positive experiences do not find their way online nearly as often as the negative ones!
The bottom line? In today’s Internet-savvy world, you need to pay regular attention to your online reputation. You need to take steps to ensure that your name and practice look their best and to negate any complaints that may appear.
What patients share about their experience with you
Many online review sites provide an opportunity for your patients to describe their experience with you and your practice. To name a few: RateMDs.com, Vitals.com, ZocDoc.com, healthgrades.com, UcompareHealth.com, Citysearch.com, yelp.com, and, of course, Google Plus reviews.
And when patients post comments on the Internet, you likely will be rated on:
- the patient’s wait time
- how your staff treated the patient
- the diagnosis
- your attitude
- the level of trust in your decisions
- treatment and outcome.
The online surfer searching for a reputable physician is likely to believe whatever he or she finds on the leading review sites.
The good news: Most physicians have a very favorable rating, averaging 9.3 out of 10 on a scale of 1 to 10. In fact, 70% of doctors have perfect scores!1
The bad news: Someone who is unhappy with her treatment or outcome will go out of her way to find every online review site possible and proclaim your faults to the cyber-world, using the Internet as a forum, whether her facts are straight or not. Patients who are pleased and satisfied rarely bother to place a positive review.
How you can control your online reputation
It is incumbent upon you to keep an eye on your online reputation at all times. Here are some tips for taking charge:
- If someone posts a negative review, respond to them directly in the review site. Doing so does not violate privacy laws as long as you do not mention the patient’s name or give other identifying details. Explain your side of the story without confirming or denying that the reviewer is or was a patient. Do not mention the specifics of any patient’s condition.
- If you feel that a negative review is completely unjustified, file a dispute with the review site. Many review sites will remove the unfavorable content if you can convince them that the patient is merely ranting.
- To protect your reputation over the long term, use your name or practice name to set up an alert with Google Alerts by visiting the site Google.com/alerts.
- Do a Google search of your name and the name of your practice at least once a month and check out all the review sites that come up. Read the comments!
Develop a proactive system
You have a lot of control when it comes to protecting your online reputation, provided you are willing to take the time to set up a system to regularly request feedback or testimonials from your patients.
Regrettably, this is where most medical practices fall short, by failing to establish a system to solicit positive reviews.
The process need not be complicated. Such a system can be set in motion by scheduling a quick meeting with your staff to announce your plans to solicit testimonials from patients. Often there will be a flurry of activity for a couple of weeks before the task is forgotten. To keep your system from falling through the cracks, make a checklist and decide who on your staff is responsible for each step in the process. Go over the results in your staff meetings on a regular basis—ie, at least monthly.
You want to solicit positive reviews for use in two places:
- your Web site
- the review sites we mentioned earlier.
Posting testimonials on your Web site
Your site is the place prospective patients visit when they are looking for information about you and your services. Here are a few tips on gathering and posting testimonials:
- The best time to solicit feedback from the patient is after the follow-up appointment, when her needs have been met and she has had at least two experiences with your practice. If she is happy with her outcome, she is likely to be receptive to the idea of providing a testimonial while the details are fresh in her mind.
- Post testimonials on your homepage and every other page at your site. They should be visible when each page loads without the need to scroll down. A testimonial is worthless if it can’t be easily seen.
- Post testimonials in italics, with quotation marks around the comments to distinguish them from other elements on the page.
- Give each testimonial a headline in bold italics. Use key words likely to resonate with the reader. For example, if the patient reports: “I had a surgical procedure and it was a game changer. You turned my life around! Thank you!” the headline might be: “You turned my life around.”
- Create a Web page just for testimonials and order the comments and headlines so that they will appeal to a diversity of prospective patients. The visitor may not read every testimonial, but she will at least read and scroll through the headlines.
Gathering feedback: Your options
- One option for automating the gathering of feedback is to include a patient feedback survey on your Web site. It’s a convenient way to ask for comments. When the patient is in the office, you or your staff can simply ask her to visit the survey page on your site and answer the questions. The problem with this approach is that many patients will agree to complete the survey but few will actually follow through.
- A far more effective way to get patients to complete a survey while they are still in your office is to have the receptionist hand the patient an iPad after her appointment and ask her to take a couple of minutes to complete the survey. You can then transcribe her comments and post them on your site.
- Asking patients to post positive comments on review sites such as healthgrades.com is another option—but, again, patients are unlikely to follow through unless you make it as easy and fast as possible. The best way to do this is to provide your patient with a blueprint for how to proceed. We offer a “patient feedback” form that contains four or five questions (FIGURE). The answers to these questions will provide a great testimonial for the doctor and the practice. Providing your patients with the right questions to elicit an emotional response will help them describe their experiences more fully. If you let the patient create a testimonial on her own, you’ll probably just receive comments such as, “I’m very happy with my results” or “She is a great doctor.”
- Also provide patients with a step-by-step process for entering their feedback on the desired review sites. This can be a daunting task for your patient, so your instructions should be clear and simple. Better yet, have someone on your staff sit with the patient at a computer or iPad to help her through the process.
- Another way to control your online reputation is to capture positive comments at the point of service. In our practice, we have a testimonial poster in every exam room as well as the reception area. It contains a quick response (QR) code that can be scanned to allow the patient to submit a testimonial about her experience with the practice. With this system, we are able to collect three to five positive reviews every day.
FIGURE: Patient follow-up satisfaction survey
| It is our intention to provide our patients with the absolute best medical care available to produce optimal results. Your feedback about your procedure and patient care is an important measure of our performance. Please take the time to let us know how you feel about your results:
Your name: _______________________________ Date: ________ Thank you for telling us about the results of your procedure. How you feel about your experience helps us better understand the physical and emotional needs of our patients. We would like to share your experience with others who might be struggling with the same issues. By signing this form, you agree to let us share this information on our Web site and informational material to help other patients understand the benefits of having these types of procedures performed. |
CASE: Resolved
The physician institutes a process in his practice to gather testimonials and positive feedback, and his staff takes time to help willing patients post their reviews online. He also disputes the negative comments that have already been posted online, offering an objective response to the complaints and asking the Web sites to take down the reviews that are merely ranting. In addition, he posts selected testimonials on the homepage of his Web site and adds a page that is just for testimonials.
Within a few weeks, the number of new patients scheduling appointments with him begins to increase until he once again enjoys a bustling practice.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Reference
- Schwartz SK. Online patient feedback: what to do. Physicianspractice.com. http://www.physicianspractice.com/health-it/online-patient-feedback-what-do. Published December 27, 2012. Accessed November 15, 2014.
Reference
- Schwartz SK. Online patient feedback: what to do. Physicianspractice.com. http://www.physicianspractice.com/health-it/online-patient-feedback-what-do. Published December 27, 2012. Accessed November 15, 2014.
Dr. Robert L. Barbieri’s Editor’s Picks November 2014
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s November 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the November 2014 issue here.
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s November 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the November 2014 issue here.
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s November 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the November 2014 issue here.
Flight plan for robotic surgery credentialing: New AAGL guidelines
The AAGL, formerly the American Association of Gynecologic Laparoscopists, has approved the first-ever set of privileging and credentialing guidelines for robotic surgery.1
Why has this prestigious minimally invasive surgery organization done that?
Maybe you’ve seen the Internet and TV ads and billboard trucks driving outside of many major medical society meetings recently, advertising “1-800-BAD-Robot.”2 You also are probably aware of recent articles in the headlines of national periodicals like the Wall Street Journal claiming that robotic surgery can be harmful.3
And yet, robotic gynecologic surgery has grown at an unprecedented rate since its approval by the US Food and Drug Administration (FDA) in April 2005. Recent data from the Nationwide Inpatient Sample from the Agency for Healthcare Research and Quality indicate that robot-assisted hysterectomies have increased at a dramatic rate.4 In a recent study of the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database, investigators found that more than 30% of injuries during robotic surgery are related to operator error or robot failure, but the majority of problems are not associated with the technology.5
In this article, I use the aviation industry as an example of a sector that has gotten safety right. By emulating many of its standards, our specialty can make great strides toward patient safety and improved outcomes. I also outline the main points of the new AAGL guidelines and the rationale behind them.1 See, for example, the summary box on page 46.
A “shining example”
The robot clearly is an enabling technology. With its high-definition 3D vision and scaled motion with wristed instruments, surgeons are more comfortable performing many complex gynecologic procedures that previously would have required open surgery to safely accomplish … but the da Vinci Robot does not make a poor surgeon a great surgeon.
Hospitals now are being sued for allowing surgeons to perform robotic surgery on patients without documenting adequate surgeon training or providing consistent oversight.6 This new technology has outpaced the ability of hospital medical staffs to establish practice guidelines and rules to ensure patient safety.
The aviation industry is a shining example of a highly reliable industry. Each day, thousands of commercial aircraft fly all over the world with amazing safety. Most of the time, the pilot and copilot have never flown together. However, each crew member knows his or her role precisely and clearly understands what is expected. Crew members must meet standards that transcend all airlines and all aircraft.7 They all practice communication and undergo standardized training, including simulation, prior to taking off with live passengers on board.
In addition, all pilots must demonstrate their proficiency and competence on a regular basis—by exhibiting actual safe flight performance (over multiple takeoffs and landings) and undergoing check rides with flight examiners and practicing routine and emergency procedures on flight simulators. Airline passengers have come to expect that all pilots are equally proficient and safe. Shouldn’t patients be able to expect the same from their surgeons and hospitals? And yet there is no national or local organization that ensures that all surgeons are equally safe in the operating room. That responsibility is too often left up to the courts.
Three requirements of robotic credentialing
In 2008, the MultiCare Health System in the Pacific Northwest adopted a unique system of robotic credentialing that was based on the aviation model.8 This model has three main components, which are identical to the guidelines imposed on pilots:
- Surgeons selected for training should be likely to be successful in performing robotic surgeries safely and efficiently.
- Practice makes perfect. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills. The aviation world calls this concept “currency.”
- Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Adoption of these tried-and-true safety principles would ensure that hospitals exercise their responsibility to protect patients who undergo robotic surgeries in their systems.
The AAGL’s Robotics Special Interest Group, formed in 2010, is now the largest special interest group in the organization. The group was initially tasked to develop evidence-based guidelines for robotic surgery training and credentialing. Using the aviation industry’s model, the group developed a basic template of robotic surgery credentialing and privileging guidelines that can be used anywhere in the world. This proposal is not meant to be a standard-of-care definition; rather, it is intended simply as a starting point.
Key components of new AAGL robotic surgery credentialing and privileging guidelines1
Initial training
- Train only surgeons who have an adequate case volume to get through the learning curve. Recommended: at least 20 major cases per year.
- Current training pathways include computer-based learning, case observations, pig labs, simulation, and proctored cases. More intense validated simulation training could replace pig labs.
- Surgeons should initially perform only simple, basic procedures with surgeon first-assists until they develop the necessary skills to safely operate the robotic console and start performing more complex cases.
Annual currency
- Surgeons should perform at least 20 major cases per year, with at least one case every 8 weeks.
- If surgeons operate less frequently, proficiency should be verified on a simulator before operation on a live patient.
Annual recertification
- All surgeons should demonstrate competency annually on a simulator, regardless of case volume.
Initial training involves a long learning curve
There is a long learning curve for surgeons to become competent in robotic surgery. In initial studies of experienced advanced laparoscopic surgeons, investigators found that learning curves could involve 50 cases or more.9,10 In a recent study of gynecologic oncologists and urogynecologists at the Mayo Clinic, researchers found that it took 91 cases for experienced surgeons to become proficient on the robot.11
ObGyns in the United States are doing fewer hysterectomies than they used to.12 Many surgeons now perform fewer than 10 hysterectomies per year. These surgeons clearly have worse outcomes than surgeons who operate more frequently.13–15 Therefore, these new guidelines suggest that hospitals should choose to train only surgeons who have a case volume that will allow them to get through their learning curve in a short time and continue to have enough surgeries to maintain their skills. These guidelines recommend that surgeons who are candidates for robotic surgery training already perform a minimum of 20 major gynecologic operations per year.
It is important to learn to walk before you run. New student pilots start out with single-engine propeller planes before graduating to multi-engine props, jets, and commercial aircraft. Similarly, new medical students start out with easy surgical tasks before training for more complex procedures. This approach seems like common sense, although many surgeons may feel that, after orienting on the robot, they can start doing complex cases right away, as the robot enables them to do better and more precise surgery. Nothing could be further from the truth.
It is very important that new robotic surgeons start with easy, basic cases to completely familiarize themselves with the operation of the robot console before attempting more complex and difficult cases.
There is no absolute number of cases that ensures competency with the robot; the number depends on the surgeon’s case load, surgical prowess, and psychomotor skills. A surgeon should be restricted to simple cases initially, and should have an experienced robot-credentialed surgeon operating with him or her during this initial learning period.
Practice makes perfect
Musicians will tell you that the more often you practice, the more skilled you become. This is true for anyone whose job requires special training. It would be naïve to assume that surgeons can maintain optimal skills for robotic surgery by performing only a few cases each year.
Psychomotor skill degradation has been explored in relation to various surgical skills. The more complex the skill, the more likely that skill set will deteriorate without use. In recent studies, investigators have shown that robotic surgery skills begin to decline significantly after only 2 weeks of inactivity, and that skills continue to degrade without use.16,17
Based on this information, the currency requirement for surgeons to maintain privileges was set at 20 cases per year—fewer than two cases per month. Although the members of the Robotics Special Interest Group strongly agree that
maintenance of privileges should not be based entirely on an arbitrary currency number, as Tracy and colleagues also argue in a recent publication,18 it is clear that frequent performance of robotic surgery by high-volume surgeons clearly is more efficient and safer, with lower total operative times and complication rates, than robotic surgery performed by lower-volume surgeons.8
Currency is a well-accepted safety standard in aviation, and pilots know the importance of frequent practice and repetition in the cockpit under real-world conditions.
Ensure annual competency
Although a pilot must accomplish a minimum number of flying hours each year to maintain certification, this does not ensure that passengers will be safe. Pilots also must prove their competence by undergoing periodic check rides and demonstrating their skills on flight simulators.
Surgeons also can use these models to verify competency. Proctors who are independently certified by the FDA or another government agency as examiners could observe and evaluate surgeons performing robotic surgery using standardized checklists and grading forms. If done locally, care must be taken to assure standardization, as local hospital politics could interfere.
The only other methods currently available to verify surgeon competency are to demonstrate proficiency on simulation and to review outcomes data, looking for outliers in important areas such as complications, robotic console times, total operative times, length of stay, etc.
Simulation offers a standardized, independent method to monitor competency.19 A passing test score on a robotic simulator exercise could be a way for a surgeon to prove his or her competency. Basic robotic skills such as camera control and clutching, energy use, and sewing and needle control can be practiced on a robotic simulator.
Virtual cases such as hysterectomy and myomectomy are not yet available on the simulator, nor are cases involving typical complications. These are being developed, however, and will be available shortly.
Several gynecologic resident and fellowship training programs are using simulation to train novice surgeons, and some community hospitals are using simulation as an annual requirement for all practicing surgeons to demonstrate proficiency, similar to pilots.8 Some newer validated training protocols require a surgeon to demonstrate mastery of a particular robotic skill by achieving passing scores at least five times, with at least two consecutive passing scores.20,21
As simulators evolve, they will continue to be incorporated into training, used for surgeon warm-up before surgery, as refreshers for surgeons after a period of robotic inactivity, and for annual recertification.
When robotic surgery leads to legal trouble
A recent medical malpractice case highlights the importance of having guidelines in place to protect patients. In Bremerton, Washington, in 2008,1 a urologist performed his first nonproctored robotic prostatectomy. The challenging and difficult procedure took more than 13 hours; he converted to an open procedure after 7 hours. The patient developed significant postoperative complications and died.1
In the litigation that followed, the surgeon was sued for negligence and for failing to disclose that this was his first solo robot-assisted surgery. The surgeon settled, as did the hospital, which was sued for not supervising the surgeon and failing to ensure that he could use the robot safely. The family also sued Intuitive Surgical, the manufacturer of the da Vinci Robot, for failing to provide adequate training to the surgeon.2
The jury ruled in favor of the manufacturer, stating that the verification of adequate surgeon training was the responsibility of the hospital and specialty medical societies, not the industry.
References
- Estate of Fred Taylor v. Intuitive Surgical Inc., 09-2-03136-5, Superior Court, State of Washington, Kitsap County (Port Orchard).
- Ostrom C. Failed robotic surgery focus of Kitsap trial. Seattle Times. http://seattletimes.com/html/localnews/2020918732_robottrialxml.html Published May 3, 2013. Accessed October 10, 2014.
A word to the wise
If hospital departments really want to ensure that they are doing all that they can to make robotic surgeries safe for their patients, they will utilize the recent guidelines approved by AAGL. In order for these guidelines to work, hospital systems need to commit resources for medical staff oversight, including a robotics peer-review committee with a physician chairman and adequate medical staff support to monitor physicians and manage those who cannot meet these goals.
There clearly will be push-back from surgeons who feel that it is unfair to restrict their ability to perform surgery just because their volumes are low or they can’t master the simulation exercises. However, in the final analysis, would we want the airlines to employ pilots who fly only a couple of times a year or who can’t master the required simulation skills to safely operate a commercial passenger jet?
The important question is, what is our focus? Is it to be “fair” to all surgeons, or is it to provide the best and safest outcomes for our patients? As surgeons, we each need to remember the oath we took when we became physicians to “First, do no harm.” By following these new AAGL robotic surgery guidelines, we will reassure our patients that we, as physicians, do take that oath seriously.
INSTANT POLL
For credentialing and privileging of robotic gynecologic surgery, do you agree that the following points are essential components of the process?
1. Surgeons should be selected for training who are most likely to be successful in performing robotic surgeries safely and efficiently.
2. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills.
3. Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Answer:
a. Yes, I agree.
b. No, I believe this approach is too restrictive.
c. No, I believe this approach is not restrictive enough.
To vote, please visit obgmanagement.com and look for “Quick Poll” on the right side of the homepage.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasive Gynecol. 2014;21(2):157–167.
2. Becnel Law Firm LLC. Bad Robot Surgery. http://badrobotsurgery.com. Accessed October 10, 2014.
3. Burton TM. Report raises concern on robotic surgery device. Wall Street Journal. http://online.wsj.com/news/articles/SB10001424052702304672404579186190568061568 Published November 8, 2013. Accessed October 10, 2014.
4. Rosero E, Kho K, Joshi G, Giesecke M, Schaffer J. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 2013;122(4):778–786.
5. Fuchs Weizman N, Cohen S, Manoucheri E, Wang K, Einarsson J. Surgical errors associated with robotic surgery in gynecology: a review of the FDA MAUDE database. J Minim Invasive Gynecol. 2013;20(6):S171.
6. Lee YL, Kilic G, Phelps J. Medicolegal review of liability risks for gynecologists stemming from lack of training in robotic assisted surgery. J Minim Invasive Gynecol. 2011;18(4):512–515.
7. Federal Aviation Administration. Pilot Regulations. http://www.faa.gov/pilots/regs/. Updated March 20, 2013. Accessed October 10, 2014.
8. Lenihan JP. Navigating credentialing, privileging, and learning curves in robotics with an evidence- and experience-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
9. Lenihan J, Kovanda C, Kreaden U. What is the learning curve for robotic Gyn surgery? J Minim Invasive Gynecol. 2008;15(5):589–594.
10. Payne T, Dauterive F. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invasive Gynecol. 2008;15(3):286–291.
11. Woelk J, Casiano E, Weaver A, Gostout B, Trabuco E, Gebhart A. The learning curve of robotic hysterectomy. Obstet Gynecol. 2013;121(1):87–96.
12. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
13. Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
14. Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volumes on outcomes for laparoscopic hysterectomy for benign conditions. Obstet Gynecol. 2012;119(4):710–716.
15. Doll K, Milad M, Gossett D. Surgeon volume and outcomes in benign hysterectomy. J Minim Invasive Gynecol. 2013;20(5):554–561.
16. Jenison E, Gil K, Lendvay T, Guy M. Robotic surgical skills: acquisition, maintenance and degradation. JSLS. 2012;16(2):218–228.
17. Guseila L, Jenison E. Maintaining robotic surgical skills during periods of robotic inactivity. J Robotic Surg. 2014;8(3):261–268.
18. Tracy E, Zephyrin L, Rosman D, Berkowitz L. Credentialing based on surgical volume. Physician workforce challenges, and patient access. Obstet Gynecol. 2013;122(5):947–951.
19. Brand T. Madigan Protocol – Si Version. Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=17. Accessed October 10, 2014.
20. Culligan P, Salamon C. Validation of a robotic simulator: transferring simulator skills to the operating room. Validation of a robotic surgery simulator protocol—transfer of simulator skills to the operating room. Fem Pelvic Med Recon Surg. 2014;20(1):48–51.
21. Culligan P. Morristown Protocol (Morristown Memorial Hospital). Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=11. Accessed October 10, 2014.
The AAGL, formerly the American Association of Gynecologic Laparoscopists, has approved the first-ever set of privileging and credentialing guidelines for robotic surgery.1
Why has this prestigious minimally invasive surgery organization done that?
Maybe you’ve seen the Internet and TV ads and billboard trucks driving outside of many major medical society meetings recently, advertising “1-800-BAD-Robot.”2 You also are probably aware of recent articles in the headlines of national periodicals like the Wall Street Journal claiming that robotic surgery can be harmful.3
And yet, robotic gynecologic surgery has grown at an unprecedented rate since its approval by the US Food and Drug Administration (FDA) in April 2005. Recent data from the Nationwide Inpatient Sample from the Agency for Healthcare Research and Quality indicate that robot-assisted hysterectomies have increased at a dramatic rate.4 In a recent study of the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database, investigators found that more than 30% of injuries during robotic surgery are related to operator error or robot failure, but the majority of problems are not associated with the technology.5
In this article, I use the aviation industry as an example of a sector that has gotten safety right. By emulating many of its standards, our specialty can make great strides toward patient safety and improved outcomes. I also outline the main points of the new AAGL guidelines and the rationale behind them.1 See, for example, the summary box on page 46.
A “shining example”
The robot clearly is an enabling technology. With its high-definition 3D vision and scaled motion with wristed instruments, surgeons are more comfortable performing many complex gynecologic procedures that previously would have required open surgery to safely accomplish … but the da Vinci Robot does not make a poor surgeon a great surgeon.
Hospitals now are being sued for allowing surgeons to perform robotic surgery on patients without documenting adequate surgeon training or providing consistent oversight.6 This new technology has outpaced the ability of hospital medical staffs to establish practice guidelines and rules to ensure patient safety.
The aviation industry is a shining example of a highly reliable industry. Each day, thousands of commercial aircraft fly all over the world with amazing safety. Most of the time, the pilot and copilot have never flown together. However, each crew member knows his or her role precisely and clearly understands what is expected. Crew members must meet standards that transcend all airlines and all aircraft.7 They all practice communication and undergo standardized training, including simulation, prior to taking off with live passengers on board.
In addition, all pilots must demonstrate their proficiency and competence on a regular basis—by exhibiting actual safe flight performance (over multiple takeoffs and landings) and undergoing check rides with flight examiners and practicing routine and emergency procedures on flight simulators. Airline passengers have come to expect that all pilots are equally proficient and safe. Shouldn’t patients be able to expect the same from their surgeons and hospitals? And yet there is no national or local organization that ensures that all surgeons are equally safe in the operating room. That responsibility is too often left up to the courts.
Three requirements of robotic credentialing
In 2008, the MultiCare Health System in the Pacific Northwest adopted a unique system of robotic credentialing that was based on the aviation model.8 This model has three main components, which are identical to the guidelines imposed on pilots:
- Surgeons selected for training should be likely to be successful in performing robotic surgeries safely and efficiently.
- Practice makes perfect. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills. The aviation world calls this concept “currency.”
- Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Adoption of these tried-and-true safety principles would ensure that hospitals exercise their responsibility to protect patients who undergo robotic surgeries in their systems.
The AAGL’s Robotics Special Interest Group, formed in 2010, is now the largest special interest group in the organization. The group was initially tasked to develop evidence-based guidelines for robotic surgery training and credentialing. Using the aviation industry’s model, the group developed a basic template of robotic surgery credentialing and privileging guidelines that can be used anywhere in the world. This proposal is not meant to be a standard-of-care definition; rather, it is intended simply as a starting point.
Key components of new AAGL robotic surgery credentialing and privileging guidelines1
Initial training
- Train only surgeons who have an adequate case volume to get through the learning curve. Recommended: at least 20 major cases per year.
- Current training pathways include computer-based learning, case observations, pig labs, simulation, and proctored cases. More intense validated simulation training could replace pig labs.
- Surgeons should initially perform only simple, basic procedures with surgeon first-assists until they develop the necessary skills to safely operate the robotic console and start performing more complex cases.
Annual currency
- Surgeons should perform at least 20 major cases per year, with at least one case every 8 weeks.
- If surgeons operate less frequently, proficiency should be verified on a simulator before operation on a live patient.
Annual recertification
- All surgeons should demonstrate competency annually on a simulator, regardless of case volume.
Initial training involves a long learning curve
There is a long learning curve for surgeons to become competent in robotic surgery. In initial studies of experienced advanced laparoscopic surgeons, investigators found that learning curves could involve 50 cases or more.9,10 In a recent study of gynecologic oncologists and urogynecologists at the Mayo Clinic, researchers found that it took 91 cases for experienced surgeons to become proficient on the robot.11
ObGyns in the United States are doing fewer hysterectomies than they used to.12 Many surgeons now perform fewer than 10 hysterectomies per year. These surgeons clearly have worse outcomes than surgeons who operate more frequently.13–15 Therefore, these new guidelines suggest that hospitals should choose to train only surgeons who have a case volume that will allow them to get through their learning curve in a short time and continue to have enough surgeries to maintain their skills. These guidelines recommend that surgeons who are candidates for robotic surgery training already perform a minimum of 20 major gynecologic operations per year.
It is important to learn to walk before you run. New student pilots start out with single-engine propeller planes before graduating to multi-engine props, jets, and commercial aircraft. Similarly, new medical students start out with easy surgical tasks before training for more complex procedures. This approach seems like common sense, although many surgeons may feel that, after orienting on the robot, they can start doing complex cases right away, as the robot enables them to do better and more precise surgery. Nothing could be further from the truth.
It is very important that new robotic surgeons start with easy, basic cases to completely familiarize themselves with the operation of the robot console before attempting more complex and difficult cases.
There is no absolute number of cases that ensures competency with the robot; the number depends on the surgeon’s case load, surgical prowess, and psychomotor skills. A surgeon should be restricted to simple cases initially, and should have an experienced robot-credentialed surgeon operating with him or her during this initial learning period.
Practice makes perfect
Musicians will tell you that the more often you practice, the more skilled you become. This is true for anyone whose job requires special training. It would be naïve to assume that surgeons can maintain optimal skills for robotic surgery by performing only a few cases each year.
Psychomotor skill degradation has been explored in relation to various surgical skills. The more complex the skill, the more likely that skill set will deteriorate without use. In recent studies, investigators have shown that robotic surgery skills begin to decline significantly after only 2 weeks of inactivity, and that skills continue to degrade without use.16,17
Based on this information, the currency requirement for surgeons to maintain privileges was set at 20 cases per year—fewer than two cases per month. Although the members of the Robotics Special Interest Group strongly agree that
maintenance of privileges should not be based entirely on an arbitrary currency number, as Tracy and colleagues also argue in a recent publication,18 it is clear that frequent performance of robotic surgery by high-volume surgeons clearly is more efficient and safer, with lower total operative times and complication rates, than robotic surgery performed by lower-volume surgeons.8
Currency is a well-accepted safety standard in aviation, and pilots know the importance of frequent practice and repetition in the cockpit under real-world conditions.
Ensure annual competency
Although a pilot must accomplish a minimum number of flying hours each year to maintain certification, this does not ensure that passengers will be safe. Pilots also must prove their competence by undergoing periodic check rides and demonstrating their skills on flight simulators.
Surgeons also can use these models to verify competency. Proctors who are independently certified by the FDA or another government agency as examiners could observe and evaluate surgeons performing robotic surgery using standardized checklists and grading forms. If done locally, care must be taken to assure standardization, as local hospital politics could interfere.
The only other methods currently available to verify surgeon competency are to demonstrate proficiency on simulation and to review outcomes data, looking for outliers in important areas such as complications, robotic console times, total operative times, length of stay, etc.
Simulation offers a standardized, independent method to monitor competency.19 A passing test score on a robotic simulator exercise could be a way for a surgeon to prove his or her competency. Basic robotic skills such as camera control and clutching, energy use, and sewing and needle control can be practiced on a robotic simulator.
Virtual cases such as hysterectomy and myomectomy are not yet available on the simulator, nor are cases involving typical complications. These are being developed, however, and will be available shortly.
Several gynecologic resident and fellowship training programs are using simulation to train novice surgeons, and some community hospitals are using simulation as an annual requirement for all practicing surgeons to demonstrate proficiency, similar to pilots.8 Some newer validated training protocols require a surgeon to demonstrate mastery of a particular robotic skill by achieving passing scores at least five times, with at least two consecutive passing scores.20,21
As simulators evolve, they will continue to be incorporated into training, used for surgeon warm-up before surgery, as refreshers for surgeons after a period of robotic inactivity, and for annual recertification.
When robotic surgery leads to legal trouble
A recent medical malpractice case highlights the importance of having guidelines in place to protect patients. In Bremerton, Washington, in 2008,1 a urologist performed his first nonproctored robotic prostatectomy. The challenging and difficult procedure took more than 13 hours; he converted to an open procedure after 7 hours. The patient developed significant postoperative complications and died.1
In the litigation that followed, the surgeon was sued for negligence and for failing to disclose that this was his first solo robot-assisted surgery. The surgeon settled, as did the hospital, which was sued for not supervising the surgeon and failing to ensure that he could use the robot safely. The family also sued Intuitive Surgical, the manufacturer of the da Vinci Robot, for failing to provide adequate training to the surgeon.2
The jury ruled in favor of the manufacturer, stating that the verification of adequate surgeon training was the responsibility of the hospital and specialty medical societies, not the industry.
References
- Estate of Fred Taylor v. Intuitive Surgical Inc., 09-2-03136-5, Superior Court, State of Washington, Kitsap County (Port Orchard).
- Ostrom C. Failed robotic surgery focus of Kitsap trial. Seattle Times. http://seattletimes.com/html/localnews/2020918732_robottrialxml.html Published May 3, 2013. Accessed October 10, 2014.
A word to the wise
If hospital departments really want to ensure that they are doing all that they can to make robotic surgeries safe for their patients, they will utilize the recent guidelines approved by AAGL. In order for these guidelines to work, hospital systems need to commit resources for medical staff oversight, including a robotics peer-review committee with a physician chairman and adequate medical staff support to monitor physicians and manage those who cannot meet these goals.
There clearly will be push-back from surgeons who feel that it is unfair to restrict their ability to perform surgery just because their volumes are low or they can’t master the simulation exercises. However, in the final analysis, would we want the airlines to employ pilots who fly only a couple of times a year or who can’t master the required simulation skills to safely operate a commercial passenger jet?
The important question is, what is our focus? Is it to be “fair” to all surgeons, or is it to provide the best and safest outcomes for our patients? As surgeons, we each need to remember the oath we took when we became physicians to “First, do no harm.” By following these new AAGL robotic surgery guidelines, we will reassure our patients that we, as physicians, do take that oath seriously.
INSTANT POLL
For credentialing and privileging of robotic gynecologic surgery, do you agree that the following points are essential components of the process?
1. Surgeons should be selected for training who are most likely to be successful in performing robotic surgeries safely and efficiently.
2. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills.
3. Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Answer:
a. Yes, I agree.
b. No, I believe this approach is too restrictive.
c. No, I believe this approach is not restrictive enough.
To vote, please visit obgmanagement.com and look for “Quick Poll” on the right side of the homepage.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The AAGL, formerly the American Association of Gynecologic Laparoscopists, has approved the first-ever set of privileging and credentialing guidelines for robotic surgery.1
Why has this prestigious minimally invasive surgery organization done that?
Maybe you’ve seen the Internet and TV ads and billboard trucks driving outside of many major medical society meetings recently, advertising “1-800-BAD-Robot.”2 You also are probably aware of recent articles in the headlines of national periodicals like the Wall Street Journal claiming that robotic surgery can be harmful.3
And yet, robotic gynecologic surgery has grown at an unprecedented rate since its approval by the US Food and Drug Administration (FDA) in April 2005. Recent data from the Nationwide Inpatient Sample from the Agency for Healthcare Research and Quality indicate that robot-assisted hysterectomies have increased at a dramatic rate.4 In a recent study of the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database, investigators found that more than 30% of injuries during robotic surgery are related to operator error or robot failure, but the majority of problems are not associated with the technology.5
In this article, I use the aviation industry as an example of a sector that has gotten safety right. By emulating many of its standards, our specialty can make great strides toward patient safety and improved outcomes. I also outline the main points of the new AAGL guidelines and the rationale behind them.1 See, for example, the summary box on page 46.
A “shining example”
The robot clearly is an enabling technology. With its high-definition 3D vision and scaled motion with wristed instruments, surgeons are more comfortable performing many complex gynecologic procedures that previously would have required open surgery to safely accomplish … but the da Vinci Robot does not make a poor surgeon a great surgeon.
Hospitals now are being sued for allowing surgeons to perform robotic surgery on patients without documenting adequate surgeon training or providing consistent oversight.6 This new technology has outpaced the ability of hospital medical staffs to establish practice guidelines and rules to ensure patient safety.
The aviation industry is a shining example of a highly reliable industry. Each day, thousands of commercial aircraft fly all over the world with amazing safety. Most of the time, the pilot and copilot have never flown together. However, each crew member knows his or her role precisely and clearly understands what is expected. Crew members must meet standards that transcend all airlines and all aircraft.7 They all practice communication and undergo standardized training, including simulation, prior to taking off with live passengers on board.
In addition, all pilots must demonstrate their proficiency and competence on a regular basis—by exhibiting actual safe flight performance (over multiple takeoffs and landings) and undergoing check rides with flight examiners and practicing routine and emergency procedures on flight simulators. Airline passengers have come to expect that all pilots are equally proficient and safe. Shouldn’t patients be able to expect the same from their surgeons and hospitals? And yet there is no national or local organization that ensures that all surgeons are equally safe in the operating room. That responsibility is too often left up to the courts.
Three requirements of robotic credentialing
In 2008, the MultiCare Health System in the Pacific Northwest adopted a unique system of robotic credentialing that was based on the aviation model.8 This model has three main components, which are identical to the guidelines imposed on pilots:
- Surgeons selected for training should be likely to be successful in performing robotic surgeries safely and efficiently.
- Practice makes perfect. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills. The aviation world calls this concept “currency.”
- Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Adoption of these tried-and-true safety principles would ensure that hospitals exercise their responsibility to protect patients who undergo robotic surgeries in their systems.
The AAGL’s Robotics Special Interest Group, formed in 2010, is now the largest special interest group in the organization. The group was initially tasked to develop evidence-based guidelines for robotic surgery training and credentialing. Using the aviation industry’s model, the group developed a basic template of robotic surgery credentialing and privileging guidelines that can be used anywhere in the world. This proposal is not meant to be a standard-of-care definition; rather, it is intended simply as a starting point.
Key components of new AAGL robotic surgery credentialing and privileging guidelines1
Initial training
- Train only surgeons who have an adequate case volume to get through the learning curve. Recommended: at least 20 major cases per year.
- Current training pathways include computer-based learning, case observations, pig labs, simulation, and proctored cases. More intense validated simulation training could replace pig labs.
- Surgeons should initially perform only simple, basic procedures with surgeon first-assists until they develop the necessary skills to safely operate the robotic console and start performing more complex cases.
Annual currency
- Surgeons should perform at least 20 major cases per year, with at least one case every 8 weeks.
- If surgeons operate less frequently, proficiency should be verified on a simulator before operation on a live patient.
Annual recertification
- All surgeons should demonstrate competency annually on a simulator, regardless of case volume.
Initial training involves a long learning curve
There is a long learning curve for surgeons to become competent in robotic surgery. In initial studies of experienced advanced laparoscopic surgeons, investigators found that learning curves could involve 50 cases or more.9,10 In a recent study of gynecologic oncologists and urogynecologists at the Mayo Clinic, researchers found that it took 91 cases for experienced surgeons to become proficient on the robot.11
ObGyns in the United States are doing fewer hysterectomies than they used to.12 Many surgeons now perform fewer than 10 hysterectomies per year. These surgeons clearly have worse outcomes than surgeons who operate more frequently.13–15 Therefore, these new guidelines suggest that hospitals should choose to train only surgeons who have a case volume that will allow them to get through their learning curve in a short time and continue to have enough surgeries to maintain their skills. These guidelines recommend that surgeons who are candidates for robotic surgery training already perform a minimum of 20 major gynecologic operations per year.
It is important to learn to walk before you run. New student pilots start out with single-engine propeller planes before graduating to multi-engine props, jets, and commercial aircraft. Similarly, new medical students start out with easy surgical tasks before training for more complex procedures. This approach seems like common sense, although many surgeons may feel that, after orienting on the robot, they can start doing complex cases right away, as the robot enables them to do better and more precise surgery. Nothing could be further from the truth.
It is very important that new robotic surgeons start with easy, basic cases to completely familiarize themselves with the operation of the robot console before attempting more complex and difficult cases.
There is no absolute number of cases that ensures competency with the robot; the number depends on the surgeon’s case load, surgical prowess, and psychomotor skills. A surgeon should be restricted to simple cases initially, and should have an experienced robot-credentialed surgeon operating with him or her during this initial learning period.
Practice makes perfect
Musicians will tell you that the more often you practice, the more skilled you become. This is true for anyone whose job requires special training. It would be naïve to assume that surgeons can maintain optimal skills for robotic surgery by performing only a few cases each year.
Psychomotor skill degradation has been explored in relation to various surgical skills. The more complex the skill, the more likely that skill set will deteriorate without use. In recent studies, investigators have shown that robotic surgery skills begin to decline significantly after only 2 weeks of inactivity, and that skills continue to degrade without use.16,17
Based on this information, the currency requirement for surgeons to maintain privileges was set at 20 cases per year—fewer than two cases per month. Although the members of the Robotics Special Interest Group strongly agree that
maintenance of privileges should not be based entirely on an arbitrary currency number, as Tracy and colleagues also argue in a recent publication,18 it is clear that frequent performance of robotic surgery by high-volume surgeons clearly is more efficient and safer, with lower total operative times and complication rates, than robotic surgery performed by lower-volume surgeons.8
Currency is a well-accepted safety standard in aviation, and pilots know the importance of frequent practice and repetition in the cockpit under real-world conditions.
Ensure annual competency
Although a pilot must accomplish a minimum number of flying hours each year to maintain certification, this does not ensure that passengers will be safe. Pilots also must prove their competence by undergoing periodic check rides and demonstrating their skills on flight simulators.
Surgeons also can use these models to verify competency. Proctors who are independently certified by the FDA or another government agency as examiners could observe and evaluate surgeons performing robotic surgery using standardized checklists and grading forms. If done locally, care must be taken to assure standardization, as local hospital politics could interfere.
The only other methods currently available to verify surgeon competency are to demonstrate proficiency on simulation and to review outcomes data, looking for outliers in important areas such as complications, robotic console times, total operative times, length of stay, etc.
Simulation offers a standardized, independent method to monitor competency.19 A passing test score on a robotic simulator exercise could be a way for a surgeon to prove his or her competency. Basic robotic skills such as camera control and clutching, energy use, and sewing and needle control can be practiced on a robotic simulator.
Virtual cases such as hysterectomy and myomectomy are not yet available on the simulator, nor are cases involving typical complications. These are being developed, however, and will be available shortly.
Several gynecologic resident and fellowship training programs are using simulation to train novice surgeons, and some community hospitals are using simulation as an annual requirement for all practicing surgeons to demonstrate proficiency, similar to pilots.8 Some newer validated training protocols require a surgeon to demonstrate mastery of a particular robotic skill by achieving passing scores at least five times, with at least two consecutive passing scores.20,21
As simulators evolve, they will continue to be incorporated into training, used for surgeon warm-up before surgery, as refreshers for surgeons after a period of robotic inactivity, and for annual recertification.
When robotic surgery leads to legal trouble
A recent medical malpractice case highlights the importance of having guidelines in place to protect patients. In Bremerton, Washington, in 2008,1 a urologist performed his first nonproctored robotic prostatectomy. The challenging and difficult procedure took more than 13 hours; he converted to an open procedure after 7 hours. The patient developed significant postoperative complications and died.1
In the litigation that followed, the surgeon was sued for negligence and for failing to disclose that this was his first solo robot-assisted surgery. The surgeon settled, as did the hospital, which was sued for not supervising the surgeon and failing to ensure that he could use the robot safely. The family also sued Intuitive Surgical, the manufacturer of the da Vinci Robot, for failing to provide adequate training to the surgeon.2
The jury ruled in favor of the manufacturer, stating that the verification of adequate surgeon training was the responsibility of the hospital and specialty medical societies, not the industry.
References
- Estate of Fred Taylor v. Intuitive Surgical Inc., 09-2-03136-5, Superior Court, State of Washington, Kitsap County (Port Orchard).
- Ostrom C. Failed robotic surgery focus of Kitsap trial. Seattle Times. http://seattletimes.com/html/localnews/2020918732_robottrialxml.html Published May 3, 2013. Accessed October 10, 2014.
A word to the wise
If hospital departments really want to ensure that they are doing all that they can to make robotic surgeries safe for their patients, they will utilize the recent guidelines approved by AAGL. In order for these guidelines to work, hospital systems need to commit resources for medical staff oversight, including a robotics peer-review committee with a physician chairman and adequate medical staff support to monitor physicians and manage those who cannot meet these goals.
There clearly will be push-back from surgeons who feel that it is unfair to restrict their ability to perform surgery just because their volumes are low or they can’t master the simulation exercises. However, in the final analysis, would we want the airlines to employ pilots who fly only a couple of times a year or who can’t master the required simulation skills to safely operate a commercial passenger jet?
The important question is, what is our focus? Is it to be “fair” to all surgeons, or is it to provide the best and safest outcomes for our patients? As surgeons, we each need to remember the oath we took when we became physicians to “First, do no harm.” By following these new AAGL robotic surgery guidelines, we will reassure our patients that we, as physicians, do take that oath seriously.
INSTANT POLL
For credentialing and privileging of robotic gynecologic surgery, do you agree that the following points are essential components of the process?
1. Surgeons should be selected for training who are most likely to be successful in performing robotic surgeries safely and efficiently.
2. There should be a minimum number of procedures performed on a regular basis to ensure that the surgeon maintains his or her psychomotor (hand-eye coordination) skills.
3. Surgeons, like pilots, should be required to demonstrate their competency in operating the robot on a regular basis.
Answer:
a. Yes, I agree.
b. No, I believe this approach is too restrictive.
c. No, I believe this approach is not restrictive enough.
To vote, please visit obgmanagement.com and look for “Quick Poll” on the right side of the homepage.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasive Gynecol. 2014;21(2):157–167.
2. Becnel Law Firm LLC. Bad Robot Surgery. http://badrobotsurgery.com. Accessed October 10, 2014.
3. Burton TM. Report raises concern on robotic surgery device. Wall Street Journal. http://online.wsj.com/news/articles/SB10001424052702304672404579186190568061568 Published November 8, 2013. Accessed October 10, 2014.
4. Rosero E, Kho K, Joshi G, Giesecke M, Schaffer J. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 2013;122(4):778–786.
5. Fuchs Weizman N, Cohen S, Manoucheri E, Wang K, Einarsson J. Surgical errors associated with robotic surgery in gynecology: a review of the FDA MAUDE database. J Minim Invasive Gynecol. 2013;20(6):S171.
6. Lee YL, Kilic G, Phelps J. Medicolegal review of liability risks for gynecologists stemming from lack of training in robotic assisted surgery. J Minim Invasive Gynecol. 2011;18(4):512–515.
7. Federal Aviation Administration. Pilot Regulations. http://www.faa.gov/pilots/regs/. Updated March 20, 2013. Accessed October 10, 2014.
8. Lenihan JP. Navigating credentialing, privileging, and learning curves in robotics with an evidence- and experience-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
9. Lenihan J, Kovanda C, Kreaden U. What is the learning curve for robotic Gyn surgery? J Minim Invasive Gynecol. 2008;15(5):589–594.
10. Payne T, Dauterive F. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invasive Gynecol. 2008;15(3):286–291.
11. Woelk J, Casiano E, Weaver A, Gostout B, Trabuco E, Gebhart A. The learning curve of robotic hysterectomy. Obstet Gynecol. 2013;121(1):87–96.
12. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
13. Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
14. Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volumes on outcomes for laparoscopic hysterectomy for benign conditions. Obstet Gynecol. 2012;119(4):710–716.
15. Doll K, Milad M, Gossett D. Surgeon volume and outcomes in benign hysterectomy. J Minim Invasive Gynecol. 2013;20(5):554–561.
16. Jenison E, Gil K, Lendvay T, Guy M. Robotic surgical skills: acquisition, maintenance and degradation. JSLS. 2012;16(2):218–228.
17. Guseila L, Jenison E. Maintaining robotic surgical skills during periods of robotic inactivity. J Robotic Surg. 2014;8(3):261–268.
18. Tracy E, Zephyrin L, Rosman D, Berkowitz L. Credentialing based on surgical volume. Physician workforce challenges, and patient access. Obstet Gynecol. 2013;122(5):947–951.
19. Brand T. Madigan Protocol – Si Version. Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=17. Accessed October 10, 2014.
20. Culligan P, Salamon C. Validation of a robotic simulator: transferring simulator skills to the operating room. Validation of a robotic surgery simulator protocol—transfer of simulator skills to the operating room. Fem Pelvic Med Recon Surg. 2014;20(1):48–51.
21. Culligan P. Morristown Protocol (Morristown Memorial Hospital). Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=11. Accessed October 10, 2014.
1. Guidelines for privileging for robotic-assisted gynecologic laparoscopy. J Minim Invasive Gynecol. 2014;21(2):157–167.
2. Becnel Law Firm LLC. Bad Robot Surgery. http://badrobotsurgery.com. Accessed October 10, 2014.
3. Burton TM. Report raises concern on robotic surgery device. Wall Street Journal. http://online.wsj.com/news/articles/SB10001424052702304672404579186190568061568 Published November 8, 2013. Accessed October 10, 2014.
4. Rosero E, Kho K, Joshi G, Giesecke M, Schaffer J. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 2013;122(4):778–786.
5. Fuchs Weizman N, Cohen S, Manoucheri E, Wang K, Einarsson J. Surgical errors associated with robotic surgery in gynecology: a review of the FDA MAUDE database. J Minim Invasive Gynecol. 2013;20(6):S171.
6. Lee YL, Kilic G, Phelps J. Medicolegal review of liability risks for gynecologists stemming from lack of training in robotic assisted surgery. J Minim Invasive Gynecol. 2011;18(4):512–515.
7. Federal Aviation Administration. Pilot Regulations. http://www.faa.gov/pilots/regs/. Updated March 20, 2013. Accessed October 10, 2014.
8. Lenihan JP. Navigating credentialing, privileging, and learning curves in robotics with an evidence- and experience-based approach. Clin Obstet Gynecol. 2011;54(3):382–390.
9. Lenihan J, Kovanda C, Kreaden U. What is the learning curve for robotic Gyn surgery? J Minim Invasive Gynecol. 2008;15(5):589–594.
10. Payne T, Dauterive F. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invasive Gynecol. 2008;15(3):286–291.
11. Woelk J, Casiano E, Weaver A, Gostout B, Trabuco E, Gebhart A. The learning curve of robotic hysterectomy. Obstet Gynecol. 2013;121(1):87–96.
12. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
13. Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116(4):909–915.
14. Wallenstein MR, Ananth CV, Kim JH, et al. Effects of surgical volumes on outcomes for laparoscopic hysterectomy for benign conditions. Obstet Gynecol. 2012;119(4):710–716.
15. Doll K, Milad M, Gossett D. Surgeon volume and outcomes in benign hysterectomy. J Minim Invasive Gynecol. 2013;20(5):554–561.
16. Jenison E, Gil K, Lendvay T, Guy M. Robotic surgical skills: acquisition, maintenance and degradation. JSLS. 2012;16(2):218–228.
17. Guseila L, Jenison E. Maintaining robotic surgical skills during periods of robotic inactivity. J Robotic Surg. 2014;8(3):261–268.
18. Tracy E, Zephyrin L, Rosman D, Berkowitz L. Credentialing based on surgical volume. Physician workforce challenges, and patient access. Obstet Gynecol. 2013;122(5):947–951.
19. Brand T. Madigan Protocol – Si Version. Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=17. Accessed October 10, 2014.
20. Culligan P, Salamon C. Validation of a robotic simulator: transferring simulator skills to the operating room. Validation of a robotic surgery simulator protocol—transfer of simulator skills to the operating room. Fem Pelvic Med Recon Surg. 2014;20(1):48–51.
21. Culligan P. Morristown Protocol (Morristown Memorial Hospital). Mimic Technologies Web site. http://www.mimicsimulation.com/training/mshare/curriculum/?id=11. Accessed October 10, 2014.
Optimal obstetric care for women aged 40 and older
CASE: Preterm labor in an older woman
G.S. is a 41-year-old G1P0 with a several-year history of infertility and a medical history of chronic hypertension. She undergoes in vitro fertilization (IVF) using her own oocytes, with transfer of two embryos. Early ultrasonography (US) confirms a diamniotic/dichorionic twin gestation. She undergoes chorionic villus sampling (CVS) during the first trimester, with normal fetal karyotypes noted.
For her chronic hypertension, the patient is treated with oral labetalol 200 mg twice daily, beginning in the first trimester. Results of a baseline comprehensive metabolic profile and complete blood count, and electrocardiogram are normal. Baseline 24-hour urine study results reveal no significant proteinuria and a normal creatinine clearance.
At 18 weeks’ gestation, US results show normal growth and amniotic fluid volume for each fetus, with no anomalies detected. Because of a gradual increase in the patient’s blood pressure, her labetalol dose is increased to 400 mg orally thrice daily. Her urine protein output remains negative on dipstick, and US every 4 weeks until 28 weeks’ gestation continues to show normal fetal growth and amniotic fluid volume.
At 33 weeks’ gestation, the patient presents with regular uterine activity. Nonstress tests for both fetuses are reactive. She is given a 1-L intravenous (IV) fluid bolus of lactated Ringers solution, as well as subcutaneous terbutaline sulfate every 15 minutes for four doses, without resolution of the uterine contractions. Her pulse has increased to 120 bpm.
How do you manage this patient’s care?
Nine times as many women aged 35 and older gave birth to their first child in 2012 than did women of the same age 40 years ago, according to the most recent data from the National Center for Health Statistics.1 The rate of first births for women aged 40 to 44 remained essentially stable during the 1970s and early 1980s but increased more than fourfold from 1985 through 2012—from 0.5 to 2.3 per 1,000 women.1 Clearly, more women are delaying childbearing to a later age by personal choice for reasons such as completion of education and career advancement.2
The path to late motherhood is not without thorns, however. Heightened risks associated with increasing maternal age include:
- fetal aneuploidy
- fetal malformation
- gestational diabetes
- chronic and gestational hypertension
- antepartum hemorrhage
- placenta previa
- prelabor rupture of membranes
- preterm labor.3,4
Women with advanced age at conception also are more likely to have a multifetal gestation because of the need for assisted reproduction and are more likely to require cesarean delivery5 as a result of abnormal placentation, fetal malpresentation, an abnormal pattern of labor, or increased use of oxytocin in labor. In addition, they are more likely to experience rupture of the sphincter, postpartum hemorrhage, and thromboembolism.3 Advanced maternal age also is associated with a higher risk of stillbirth throughout gestation, with the peak risk period reported to occur at 37 to 41 weeks.6
Maternal age-related risks of autosomal trisomies (especially Down syndrome) are well understood and have been quantified for singleton and twin gestations. TABLE 1 shows the risks at term for singleton and twin gestations for at least one chromosomally abnormal fetus by maternal age (40–46 years) and race.7
Preconception considerations
Aging and fertility
These combined result of aging of the ovary and uterus and an escalating risk of underlying medical comorbidities has a detrimental effect on fertility.8 Although assisted reproductive technologies are helpful, they cannot guarantee a live birth or completely compensate for an age-related decline in fertility.9
Many IVF programs refuse infertility treatment to women over age 43 or 44 who want to use their own oocytes. The reason: low pregnancy rates. The use of donor oocytes, however, increases the risks of gestational hypertension and preeclampsia. And if assisted reproductive technologies are needed, the risk for multifetal pregnancy increases.
Women of advanced maternal age are likely to have an older spouse or partner. There is no clearly accepted definition of advanced paternal age, but it is most often defined as an age of 40 years or older at the time of conception. Advanced paternal age has been associated with a higher risk for autism spectrum disorder and schizophrenia, as well as mutations in the FGFR2 and FGFR3 genes that result in skeletal dysplasias and craniosynostosis syndromes.10
Medical conditions are more common
Women of advanced maternal age have an increased rate of such prepregnancy chronic medical complications as diabetes, chronic hypertension, obesity, and renal and cardiac disease. Therefore, it is best to optimize control of these chronic illnesses prior to conception to minimize the risks of miscarriage, fetal anomalies, and gestational hypertension and preeclampsia.
Preeclampsia. Although daily low-dose (60–81 mg) aspirin has been used to reduce the risk of preeclampsia, current recommendations from the American College of Obstetricians and Gynecologists (ACOG) suggest that this therapy be reserved for women with a medical history of early-onset preeclampsia or those who have had preeclampsia in more than one pregnancy.11
Impact of obesity. We recently examined the influence of age and obesity on pregnancy outcomes of nulliparous women aged 40 or older at delivery.12 The study included women aged 20 to 29 years (n = 52,249) and 40 or older (n = 1,231) who delivered singleton infants. Women who reported medical disorders, tobacco use, or conception with assisted reproductive technology were excluded.
In the older age group (≥40 years), obese women had significantly higher rates of cesarean delivery, gestational hypertension, preeclampsia, gestational diabetes, preterm delivery before 37 weeks’ gestation, and preterm delivery before 28 weeks, and their infants had higher rates of admission to the neonatal intensive care unit (NICU), compared with nonobese women (FIGURE).
It would appear, however, that healthy, obese women who delay pregnancy until the age of 40 or later may modify their risk for cesarean delivery, gestational diabetes mellitus, and gestational hypertension and preeclampsia by reducing their body mass index to nonobese levels prior to conception.
In addition to maternal risks for women of advanced maternal age, there are risks to the fetus and neonate, as well as a risk of placental abnormalities. These risks are summarized in TABLE 2.
Placental
- Molar or partial molar pregnancy
- Fetus or twins with a complete mole
- Placenta previa, vasa previa
Fetal/neonatal
- Aneuploidy
- Selective fetal growth restriction in twin gestation
- Twin-twin transfusion syndrome
- Preterm birth
- Perinatal death
Antepartum
- Gestational diabetes
- Insulin-dependent diabetes
- Gestational hypertension and preeclampsia
- Cholestasis of pregnancy
- Acute fatty liver of pregnancy
- Venous thromboembolism
- Preterm labor, preterm premature rupture
of membranes
Intrapartum
- Dysfunctional labor
- Malpresentation
- Cesarean delivery
Postpartum
- Venous thromboembolism
- Postpartum hemorrhage
Folic acid supplementation can reduce some risks
The potential benefit of folic acid supplementation to reduce the risk of fetal open neural tube defects is well documented. More recent data suggest that folic acid also is associated with a reduction in the risks of congenital heart defects, abdominal wall defects, cleft lip and palate, and spontaneous abortion. Supplementation should be initiated at least 3 months prior to conception and continued through the first trimester.
The first trimester
Early pregnancy loss is a risk
Women of advanced maternal age are more likely than younger women to experience early pregnancy loss. This risk is due to higher rates of fetal aneuploidy as well as declining ovarian and uterine function and a higher rate of ectopic pregnancy.
In the First and Second Trimester Evaluation of Risk (FASTER) trial, in which investigators reported pregnancy outcomes by maternal age for 36,056 pregnancies, the rate of spontaneous abortion after 10 weeks of gestation was 0.8% among women younger than 35 years, compared with 2.2% for women aged 40 or older.4
The likelihood of multiple gestation increases
The background risk of multiple births is higher in women of advanced maternal age, compared with younger women. This risk increases further with fertility treatment.
Multiple gestations at any age are associated with increased risks for preterm birth and very-low–birthweight infants. Potential maternal risks are listed in TABLE 3.
- Hypertension (2.5 times the risk of a singleton gestation)
- Abruption (3.0 times the risk)
- Anemia (2.5 times the risk)
- Urinary tract infection (1.5 times the risk)
- Preeclampsia (risk of 26%–75%) (occurs at earlier gestation) — HELLP syndrome (risk of 9%)
- Abruption (20%) (10 times the risk of a singleton gestation)
- Anemia (24%)
- Preterm premature rupture of membranes (24%)
- Gestational diabetes (14%)
- Acute fatty liver (4%) (1 in 10,000 singletons)
- Postpartum hemorrhage (9%)
To reduce the number of multiple gestations with assisted reproduction, consider elective single embryo transfer, especially if the mother has significant underlying medical complications.
Multiple gestations present difficult management issues in older women. Strategies shown to prevent preterm delivery in singleton gestations, including weekly 17-hydroxyprogesterone injections and cervical cerclage, are not effective in multiple gestations. Moreover, many of these therapies—including bed rest—increase the risk of thromboembolic events in multiple gestations, particularly when the mother is of advanced age.
Maternal adaptations in multiple gestations also may be poorly tolerated by older patients, particularly cardiac changes that markedly increase stroke volume, heart rate, cardiac output, and plasma volume.
The range of genetic screening and testing options has broadened
Options include first-trimester CVS, which provides information about the fetal chromosomal complement but not the presence of a fetal open neural tube defect. The procedure-related rate of fetal loss with CVS is quoted as 1%.
Options for genetic testing in the second trimester include transabdominal amniocentesis. A procedure-related fetal loss rate of 1 in 500 to 1 in 1,600 is quoted for midtrimester amniocentesis.
A relatively new screening option is analysis of cell-free fetal DNA in maternal blood, which can be performed after 10 weeks’ gestation in singleton and multiple gestations. This directed analysis measures the relative proportions of chromosomes. The detection rate for fetal Down syndrome using cell-free fetal DNA is greater than 98%, with a false-positive rate of less than 0.5%. However, this screening is unreliable in triplet gestations.
Other screening options include US and biochemical screening to detect fetal aneuploidy and open neural tube defects during the second trimester. These options should be included in counseling of the patient.
Second and third trimesters
Gestational hypertension and preeclampsia are significant risks
Older pregnant women have an incidence of gestational hypertension and preeclampsia 2 to 4 times as high as that of patients younger than 30 years.13 The underlying risk for preeclampsia is further increased if coexisting medical disorders such as diabetes or chronic hypertension are present. Moreover, the risk for preeclampsia increases to 10% to 20% in twin gestations and 25% to 60% in triplet gestations. Le Ray and colleagues reported that, if oocyte donation is used with IVF in women older than age 43, the risk for preeclampsia triples.14
We previously studied 379 women aged 35 and older who had mild gestational hypertension remote from term, comparing them with their younger adult counterparts in a matched cohort design.15 Outpatient management produced similar maternal outcomes in both groups, but older women had a statistically insignificant increase in the rate of stillbirth (5 vs 0; P = .063).15
Gestational diabetes risk doubles
The rates of both diabetes mellitus and gestational diabetes increase with advanced maternal age. Data from the FASTER consortium included an adjusted odds ratio of 2.4 for gestational diabetes in women aged 40 or older, compared with a younger control group.4 This increased risk may be a consequence of greater maternal habitus as well as declining insulin sensitivity.
Diabetes increases the risks of macrosomia, cesarean birth, and gestational hypertension. Among women with pregestational diabetes, the risks of congenital heart disease and fetal neural tube defects increase threefold. Because of this increased risk, perinatal screening is indicated for both anomalies in older women.
Pulmonary complications increase
Another risk facing women of advanced maternal age—particularly those carrying a multiple gestation—is pulmonary edema, owing to the increased cardiac output, heart rate, and blood volume, the decreased systemic vascular resistance, and the physiologic anemia of pregnancy. These risks rise further in women who develop preterm labor that requires therapy and in those who develop gestational hypertension and/or preeclampsia. Judicious use of IV fluids, particularly those with lower sodium concentrations, can reduce the risk of pulmonary complications.
Women who develop pulmonary edema have an increased risk of peripartum cardiomyopathy.16
Preterm delivery is more common
Cleary-Goodman and colleagues noted an increased incidence of preterm delivery in women aged 40 and older, compared with women younger than age 35, but no increase in spontaneous preterm labor.4 Advanced maternal age appears to be associated with an increased risk of preterm birth largely as a consequence of underlying complications of fetal growth restriction and maternal disease, including hypertension. Because preterm birth is an important contributor to perinatal morbidity and mortality, steroids should be administered for fetal lung maturity whenever preterm labor is diagnosed before 34 weeks’ gestation.
Risk of placenta previa is 1.1%
Joseph and colleagues found the risk of placenta previa to be 1.1% in women aged 40 and older, compared with 0.3% in women aged 25 to 29 years.17 This increased risk likely is a consequence not only of maternal age but increased parity and a history of prior uterine surgery. If transabdominal US results are suspicious for placenta previa, transvaginal US is indicated for confirmation. Additional US assessment of the cord insertion site to the placenta also should be performed to rule out vasa previa.
Look for neonatal complications
Ziadeh and colleagues found that, although maternal morbidity was increased in older women, the overall neonatal outcome did not appear to be affected.18 However, we noted a higher rate of neonatal complications in women aged 40 or older, including higher NICU admission rates and more low-birth–weight infants.11
In addition, Odibo and colleagues found advanced maternal age to be an independent risk factor for intrauterine growth restriction (IUGR).19 In that study, the odds ratio for IUGR was 3.2 (95% confidence interval [CI], 1.9–5.4) for a maternal age of 40 years or older, compared with a control group. For that reason, they recommend routine screening for IUGR in all pregnant women of advanced age.
Stillbirth risk peaks at 37 to 41 weeks
In a review of more than 5.4 million singleton pregnancies without reported congenital anomalies, Reddy and colleagues found an association between advanced maternal age and stillbirth, with a higher risk of stillbirth at 37 to 41 weeks’ gestation.6 This effect of maternal age persisted despite adjusting for medical disease, parity, and race/ethnicity.
Many women older than age 40 have independent medical or fetal indications for antenatal testing. Some experts have suggested antepartum surveillance starting at 37 weeks for women of advanced maternal age; they argue that the risk of stillbirth at this gestational age is similar in frequency to other high-risk conditions for which testing is performed routinely. However, the National Institute of Child Health and Human Development (NICHD) workshop on antepartum fetal monitoring found insufficient evidence that antenatal testing for the sole indication of advanced maternal age reduces stillbirth or improves perinatal outcomes.20
If increased antenatal testing is indicated for a high-risk condition or electively chosen given advanced age, it should include electronic fetal monitoring as well as amniotic fluid volume assessment. Because the risk of fetal loss sharply increased at 40 weeks’ gestation in the study by Reddy and colleagues,6 women older than age 40 should be considered for delivery by 40 weeks’ gestation in the presence of good dating criteria.
Some clinicians also would consider delivery by 39 weeks’ gestation with good dating criteria if the Bishop score is favorable.
Risks of labor and delivery
Multiple variables contribute to a higher cesarean delivery rate
The risk of cesarean delivery increases with advancing maternal age.5,11 This increased risk is a consequence of multiple variables, including the rate of previous cesarean delivery, malpresentation, underlying complications such as preeclampsia and diabetes, and a higher prevalence of dysfunctional labor.21 Further, Vaughn and colleagues noted that the cesarean delivery rate increases in direct proportion to age, with a rate of 54.4% in women older than age 40.5
As Cohen pointed out in a commentary accompanying a study of dysfunctional labor in women of advancing age, “the notion of a premium baby (ie, that the fetus of a woman with a reduced likelihood of having another pregnancy is somehow more deserving of being spared the rigours of labour than the fetus of a young woman) may play a role” in the high rate of cesarean delivery.21,22
Postpartum hemorrhage risk may be lower in older women
Advanced maternal age is assumed to be a risk factor for postpartum hemorrhage.23 The increased risk was thought to be related to the increased incidence of multiple underlying factors, such as cesarean delivery, multiple gestation, and hypertensive disorders of pregnancy.
However, in a retrospective cohort study, Lao and colleagues found that advanced maternal age (≥35 years) served only as a surrogate factor for postpartum hemorrhage due to associated risk factors, obstetric complications, and interventions.24 After multivariate analysis, aging was associated with a decreased rate of postpartum hemorrhage, which declined progressively from ages 25 to 40 years and older, compared with women aged 20 to 24.24
Nevertheless, medical interventions should be readily available at the time of delivery for treatment of uterine atony, especially with multiple gestation and grand multiparity.
Case: Resolved
The patient is admitted to the hospital, where she is given IV magnesium sulfate (6-g load followed by an infusion of 3 g/hr) and betamethasone for fetal lung maturity enhancement. She continues to receive IV fluids as well (125 mL/hr lactated Ringers solution). Uterine activity abates.
IV magnesium sulfate is continued for 36 hours, but urine protein output is not monitored. Her heart rate ranges from 105 to 115 bpm, and blood pressure from 130/80 mm Hg to 138/88 mm Hg. Forty-eight hours after admission, she reports a gradual onset of tightness of the chest and breathlessness. She is agitated, with a pulse of 130 bpm, 30 respirations/min, and room air pulse oximetry of 90%. Rales are noted upon auscultation of both lungs. A radiograph of the chest demonstrates bilateral air-space disease consistent with pulmonary edema. IV furosemide and oxygen (by mask) are provided, with some respiratory improvement.
The patient then reports leakage of amniotic fluid, and preterm rupture of membranes is confirmed on examination. Because steroids for fetal lung maturity have been administered, and given improvement in her pulmonary edema and a footling breech presentation for Twin A, cesarean delivery is performed.
The patient’s immediate postoperative course is uncomplicated. On postoperative day 2, however, she develops recurrent pulmonary edema, as confirmed by physical examination and chest radiograph. She also reports headache, and her blood pressure rises to 164/114 mm Hg—findings consistent with postpartum preeclampsia. Magnesium sulfate and antihypertensive therapy are initiated, along with IV furosemide and oxygen, which improves her respiratory status.
An echocardiogram to rule out peripartum dilated cardiomyopathy finds no evidence of a dilated left ventricle, and the calculated left ventricular ejection fraction (55%) is normal.
After diuresis and improvement in her blood pressure, she is discharged home. By the time of her follow-up office visit 7 days later, her blood pressure has normalized on labetalol therapy.
For an overview of evaluation and management of pregnant women aged 40 or older, see TABLE 4.
Preconception
- Identify risk factors (ie, diabetes, obesity, hypertension, cardiac dysfunction, family history
- Review outcome of previous pregnancy, if applicable
- Review risks (multiple gestation, birth defects) associated with assisted reproductive technologies if they were needed to achieve pregnancy
- Optimize maternal health
- Begin folic acid supplementation
- Encourage smoking cessation
- If the patient is ≥45 years old:
– Electrocardiogram
– Glucose screening (fasting plasma glucose or hemoglobin A1c)
– Echocardiogram for patients with chronic hypertension
First trimester
- Ultrasonography for dating and to assess fetal number and chorionicity
- Baseline metabolic profile and complete blood count
- Baseline urinalysis
- Continue folic acid supplementation
- Offer first-trimester genetic testing or other genetic screening
Second trimester
- If first-trimester genetic testing is declined, offer second-trimester testing or screening
- Detailed fetal anomaly evaluation by ultrasound
- Fetal echocardiogram if pregnancy was achieved by in vitro fertilization or if it is a monochorionic twin gestation
- Screen for gestational diabetes
Third trimester
- Increased antenatal testing for routine indications, including hypertension, diabetes, and lupus
- Ultrasonography for growth and later ultrasonographic findings of fetal aneuploidy
- Consider delivery
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Mathews TJ, Hamilton BE. First births to older women continue to rise. National Center for Health Statistics. NCHS Data Brief No. 152. May 2014. http://www.cdc.gov/nchs/data/databriefs/db152.pdf. Accessed October 3, 2014.
2. Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860.
3. Ziadeh SM. Maternal and perinatal outcome in nulliparous women aged 35 and older. Gynecol Obstet Invest. 2002;54(1):6–10.
4. Cleary-Goldman J, Malone FD, Vidaver J, et al; FASTER Consortium. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 pt 1):983–990.
5. Vaughn DA, Cleary BJ, Murphy DJ. Delivery outcomes for nulliparous women at the extremes of maternal age—a cohort study. BJOG. 2014;121(3):261–268.
6. Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth through pregnancy in the United States. Am J Obstet Gynecol. 2006;195(3):764–770.
7. Meyers C, Adam R, Dungan J, Prenger V. Aneuploidy in twin gestations: when is maternal age advanced? Obstet Gynecol. 1997;89(2):248–251.
8. Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update. 2013;19(1):67–83.
9. Johnson JA, Tough S. Delayed child-bearing. J Obstet Gynaecol Can. 2012;34(1):80–93.
10. Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200.
11. Barton JR, Sibai AJ, Istwan NB, Rhea DJ, Desch CN, Sibai BM. Spontaneously conceived pregnancy after 40: influence of age and obesity on outcome. Am J Perinatol. 2014;31(9):795–798.
12. Roberts JM, August PA, Bakris JR, et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
13. Jahromi BN, Husseini Z. Pregnancy outcome at maternal age 40 and older. Taiwan J Obstet Gynecol. 2008;47(3):318–321.
14. Le Ray C, Scherier S, Anselem O, et al. Association between oocyte donation and maternal and perinatal outcomes in women aged 43 years or older. Hum Reprod. 2012;27(3):896–901.
15. Barton JR, Bergauer NK, Jacques DL, Coleman SK, Stanziano GJ, Sibai BM. Does advanced maternal age affect pregnancy outcome in women with mild hypertension remote from term? Am J Obstet Gynecol. 1997;176(6):1236–1243.
16. Habli M, O’Brien T, Nowack E, et al. Peripartum cardiomyopathy: prognostic factors for long-term maternal outcome. Am J Obstet Gynecol. 2008;199(4):415.e1–e5.
17. Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105(6):1410–1418.
18. Ziadeh S, Yahaya A. Pregnancy outcome at age 40 and older. Arch Gynecol Obstet. 2001;265(1):30–33.
19. Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23(5):325–328.
20. Signore C, Freeman RK, Spong CY. Antenatal testing—a reevaluation: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;113(3):687–701.
21. Cohen WR, Newman L, Friedman EA. Risk of labor abnormalities with advancing maternal age. Obstet Gynecol. 1980;55(4):414–416.
22. Cohen WR. Does maternal age affect pregnancy outcome? BJOG. 2014;121(3):252–254.
23. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–1373.
24. Lao TT, Sahota DS, Cheng YK, Law LW, Leung TY. Advanced maternal age and postpartum hemorrhage—risk factor or red herring? J Matern Fetal Neonatal Med. 2014;27(3):243–246.
CASE: Preterm labor in an older woman
G.S. is a 41-year-old G1P0 with a several-year history of infertility and a medical history of chronic hypertension. She undergoes in vitro fertilization (IVF) using her own oocytes, with transfer of two embryos. Early ultrasonography (US) confirms a diamniotic/dichorionic twin gestation. She undergoes chorionic villus sampling (CVS) during the first trimester, with normal fetal karyotypes noted.
For her chronic hypertension, the patient is treated with oral labetalol 200 mg twice daily, beginning in the first trimester. Results of a baseline comprehensive metabolic profile and complete blood count, and electrocardiogram are normal. Baseline 24-hour urine study results reveal no significant proteinuria and a normal creatinine clearance.
At 18 weeks’ gestation, US results show normal growth and amniotic fluid volume for each fetus, with no anomalies detected. Because of a gradual increase in the patient’s blood pressure, her labetalol dose is increased to 400 mg orally thrice daily. Her urine protein output remains negative on dipstick, and US every 4 weeks until 28 weeks’ gestation continues to show normal fetal growth and amniotic fluid volume.
At 33 weeks’ gestation, the patient presents with regular uterine activity. Nonstress tests for both fetuses are reactive. She is given a 1-L intravenous (IV) fluid bolus of lactated Ringers solution, as well as subcutaneous terbutaline sulfate every 15 minutes for four doses, without resolution of the uterine contractions. Her pulse has increased to 120 bpm.
How do you manage this patient’s care?
Nine times as many women aged 35 and older gave birth to their first child in 2012 than did women of the same age 40 years ago, according to the most recent data from the National Center for Health Statistics.1 The rate of first births for women aged 40 to 44 remained essentially stable during the 1970s and early 1980s but increased more than fourfold from 1985 through 2012—from 0.5 to 2.3 per 1,000 women.1 Clearly, more women are delaying childbearing to a later age by personal choice for reasons such as completion of education and career advancement.2
The path to late motherhood is not without thorns, however. Heightened risks associated with increasing maternal age include:
- fetal aneuploidy
- fetal malformation
- gestational diabetes
- chronic and gestational hypertension
- antepartum hemorrhage
- placenta previa
- prelabor rupture of membranes
- preterm labor.3,4
Women with advanced age at conception also are more likely to have a multifetal gestation because of the need for assisted reproduction and are more likely to require cesarean delivery5 as a result of abnormal placentation, fetal malpresentation, an abnormal pattern of labor, or increased use of oxytocin in labor. In addition, they are more likely to experience rupture of the sphincter, postpartum hemorrhage, and thromboembolism.3 Advanced maternal age also is associated with a higher risk of stillbirth throughout gestation, with the peak risk period reported to occur at 37 to 41 weeks.6
Maternal age-related risks of autosomal trisomies (especially Down syndrome) are well understood and have been quantified for singleton and twin gestations. TABLE 1 shows the risks at term for singleton and twin gestations for at least one chromosomally abnormal fetus by maternal age (40–46 years) and race.7
Preconception considerations
Aging and fertility
These combined result of aging of the ovary and uterus and an escalating risk of underlying medical comorbidities has a detrimental effect on fertility.8 Although assisted reproductive technologies are helpful, they cannot guarantee a live birth or completely compensate for an age-related decline in fertility.9
Many IVF programs refuse infertility treatment to women over age 43 or 44 who want to use their own oocytes. The reason: low pregnancy rates. The use of donor oocytes, however, increases the risks of gestational hypertension and preeclampsia. And if assisted reproductive technologies are needed, the risk for multifetal pregnancy increases.
Women of advanced maternal age are likely to have an older spouse or partner. There is no clearly accepted definition of advanced paternal age, but it is most often defined as an age of 40 years or older at the time of conception. Advanced paternal age has been associated with a higher risk for autism spectrum disorder and schizophrenia, as well as mutations in the FGFR2 and FGFR3 genes that result in skeletal dysplasias and craniosynostosis syndromes.10
Medical conditions are more common
Women of advanced maternal age have an increased rate of such prepregnancy chronic medical complications as diabetes, chronic hypertension, obesity, and renal and cardiac disease. Therefore, it is best to optimize control of these chronic illnesses prior to conception to minimize the risks of miscarriage, fetal anomalies, and gestational hypertension and preeclampsia.
Preeclampsia. Although daily low-dose (60–81 mg) aspirin has been used to reduce the risk of preeclampsia, current recommendations from the American College of Obstetricians and Gynecologists (ACOG) suggest that this therapy be reserved for women with a medical history of early-onset preeclampsia or those who have had preeclampsia in more than one pregnancy.11
Impact of obesity. We recently examined the influence of age and obesity on pregnancy outcomes of nulliparous women aged 40 or older at delivery.12 The study included women aged 20 to 29 years (n = 52,249) and 40 or older (n = 1,231) who delivered singleton infants. Women who reported medical disorders, tobacco use, or conception with assisted reproductive technology were excluded.
In the older age group (≥40 years), obese women had significantly higher rates of cesarean delivery, gestational hypertension, preeclampsia, gestational diabetes, preterm delivery before 37 weeks’ gestation, and preterm delivery before 28 weeks, and their infants had higher rates of admission to the neonatal intensive care unit (NICU), compared with nonobese women (FIGURE).
It would appear, however, that healthy, obese women who delay pregnancy until the age of 40 or later may modify their risk for cesarean delivery, gestational diabetes mellitus, and gestational hypertension and preeclampsia by reducing their body mass index to nonobese levels prior to conception.
In addition to maternal risks for women of advanced maternal age, there are risks to the fetus and neonate, as well as a risk of placental abnormalities. These risks are summarized in TABLE 2.
Placental
- Molar or partial molar pregnancy
- Fetus or twins with a complete mole
- Placenta previa, vasa previa
Fetal/neonatal
- Aneuploidy
- Selective fetal growth restriction in twin gestation
- Twin-twin transfusion syndrome
- Preterm birth
- Perinatal death
Antepartum
- Gestational diabetes
- Insulin-dependent diabetes
- Gestational hypertension and preeclampsia
- Cholestasis of pregnancy
- Acute fatty liver of pregnancy
- Venous thromboembolism
- Preterm labor, preterm premature rupture
of membranes
Intrapartum
- Dysfunctional labor
- Malpresentation
- Cesarean delivery
Postpartum
- Venous thromboembolism
- Postpartum hemorrhage
Folic acid supplementation can reduce some risks
The potential benefit of folic acid supplementation to reduce the risk of fetal open neural tube defects is well documented. More recent data suggest that folic acid also is associated with a reduction in the risks of congenital heart defects, abdominal wall defects, cleft lip and palate, and spontaneous abortion. Supplementation should be initiated at least 3 months prior to conception and continued through the first trimester.
The first trimester
Early pregnancy loss is a risk
Women of advanced maternal age are more likely than younger women to experience early pregnancy loss. This risk is due to higher rates of fetal aneuploidy as well as declining ovarian and uterine function and a higher rate of ectopic pregnancy.
In the First and Second Trimester Evaluation of Risk (FASTER) trial, in which investigators reported pregnancy outcomes by maternal age for 36,056 pregnancies, the rate of spontaneous abortion after 10 weeks of gestation was 0.8% among women younger than 35 years, compared with 2.2% for women aged 40 or older.4
The likelihood of multiple gestation increases
The background risk of multiple births is higher in women of advanced maternal age, compared with younger women. This risk increases further with fertility treatment.
Multiple gestations at any age are associated with increased risks for preterm birth and very-low–birthweight infants. Potential maternal risks are listed in TABLE 3.
- Hypertension (2.5 times the risk of a singleton gestation)
- Abruption (3.0 times the risk)
- Anemia (2.5 times the risk)
- Urinary tract infection (1.5 times the risk)
- Preeclampsia (risk of 26%–75%) (occurs at earlier gestation) — HELLP syndrome (risk of 9%)
- Abruption (20%) (10 times the risk of a singleton gestation)
- Anemia (24%)
- Preterm premature rupture of membranes (24%)
- Gestational diabetes (14%)
- Acute fatty liver (4%) (1 in 10,000 singletons)
- Postpartum hemorrhage (9%)
To reduce the number of multiple gestations with assisted reproduction, consider elective single embryo transfer, especially if the mother has significant underlying medical complications.
Multiple gestations present difficult management issues in older women. Strategies shown to prevent preterm delivery in singleton gestations, including weekly 17-hydroxyprogesterone injections and cervical cerclage, are not effective in multiple gestations. Moreover, many of these therapies—including bed rest—increase the risk of thromboembolic events in multiple gestations, particularly when the mother is of advanced age.
Maternal adaptations in multiple gestations also may be poorly tolerated by older patients, particularly cardiac changes that markedly increase stroke volume, heart rate, cardiac output, and plasma volume.
The range of genetic screening and testing options has broadened
Options include first-trimester CVS, which provides information about the fetal chromosomal complement but not the presence of a fetal open neural tube defect. The procedure-related rate of fetal loss with CVS is quoted as 1%.
Options for genetic testing in the second trimester include transabdominal amniocentesis. A procedure-related fetal loss rate of 1 in 500 to 1 in 1,600 is quoted for midtrimester amniocentesis.
A relatively new screening option is analysis of cell-free fetal DNA in maternal blood, which can be performed after 10 weeks’ gestation in singleton and multiple gestations. This directed analysis measures the relative proportions of chromosomes. The detection rate for fetal Down syndrome using cell-free fetal DNA is greater than 98%, with a false-positive rate of less than 0.5%. However, this screening is unreliable in triplet gestations.
Other screening options include US and biochemical screening to detect fetal aneuploidy and open neural tube defects during the second trimester. These options should be included in counseling of the patient.
Second and third trimesters
Gestational hypertension and preeclampsia are significant risks
Older pregnant women have an incidence of gestational hypertension and preeclampsia 2 to 4 times as high as that of patients younger than 30 years.13 The underlying risk for preeclampsia is further increased if coexisting medical disorders such as diabetes or chronic hypertension are present. Moreover, the risk for preeclampsia increases to 10% to 20% in twin gestations and 25% to 60% in triplet gestations. Le Ray and colleagues reported that, if oocyte donation is used with IVF in women older than age 43, the risk for preeclampsia triples.14
We previously studied 379 women aged 35 and older who had mild gestational hypertension remote from term, comparing them with their younger adult counterparts in a matched cohort design.15 Outpatient management produced similar maternal outcomes in both groups, but older women had a statistically insignificant increase in the rate of stillbirth (5 vs 0; P = .063).15
Gestational diabetes risk doubles
The rates of both diabetes mellitus and gestational diabetes increase with advanced maternal age. Data from the FASTER consortium included an adjusted odds ratio of 2.4 for gestational diabetes in women aged 40 or older, compared with a younger control group.4 This increased risk may be a consequence of greater maternal habitus as well as declining insulin sensitivity.
Diabetes increases the risks of macrosomia, cesarean birth, and gestational hypertension. Among women with pregestational diabetes, the risks of congenital heart disease and fetal neural tube defects increase threefold. Because of this increased risk, perinatal screening is indicated for both anomalies in older women.
Pulmonary complications increase
Another risk facing women of advanced maternal age—particularly those carrying a multiple gestation—is pulmonary edema, owing to the increased cardiac output, heart rate, and blood volume, the decreased systemic vascular resistance, and the physiologic anemia of pregnancy. These risks rise further in women who develop preterm labor that requires therapy and in those who develop gestational hypertension and/or preeclampsia. Judicious use of IV fluids, particularly those with lower sodium concentrations, can reduce the risk of pulmonary complications.
Women who develop pulmonary edema have an increased risk of peripartum cardiomyopathy.16
Preterm delivery is more common
Cleary-Goodman and colleagues noted an increased incidence of preterm delivery in women aged 40 and older, compared with women younger than age 35, but no increase in spontaneous preterm labor.4 Advanced maternal age appears to be associated with an increased risk of preterm birth largely as a consequence of underlying complications of fetal growth restriction and maternal disease, including hypertension. Because preterm birth is an important contributor to perinatal morbidity and mortality, steroids should be administered for fetal lung maturity whenever preterm labor is diagnosed before 34 weeks’ gestation.
Risk of placenta previa is 1.1%
Joseph and colleagues found the risk of placenta previa to be 1.1% in women aged 40 and older, compared with 0.3% in women aged 25 to 29 years.17 This increased risk likely is a consequence not only of maternal age but increased parity and a history of prior uterine surgery. If transabdominal US results are suspicious for placenta previa, transvaginal US is indicated for confirmation. Additional US assessment of the cord insertion site to the placenta also should be performed to rule out vasa previa.
Look for neonatal complications
Ziadeh and colleagues found that, although maternal morbidity was increased in older women, the overall neonatal outcome did not appear to be affected.18 However, we noted a higher rate of neonatal complications in women aged 40 or older, including higher NICU admission rates and more low-birth–weight infants.11
In addition, Odibo and colleagues found advanced maternal age to be an independent risk factor for intrauterine growth restriction (IUGR).19 In that study, the odds ratio for IUGR was 3.2 (95% confidence interval [CI], 1.9–5.4) for a maternal age of 40 years or older, compared with a control group. For that reason, they recommend routine screening for IUGR in all pregnant women of advanced age.
Stillbirth risk peaks at 37 to 41 weeks
In a review of more than 5.4 million singleton pregnancies without reported congenital anomalies, Reddy and colleagues found an association between advanced maternal age and stillbirth, with a higher risk of stillbirth at 37 to 41 weeks’ gestation.6 This effect of maternal age persisted despite adjusting for medical disease, parity, and race/ethnicity.
Many women older than age 40 have independent medical or fetal indications for antenatal testing. Some experts have suggested antepartum surveillance starting at 37 weeks for women of advanced maternal age; they argue that the risk of stillbirth at this gestational age is similar in frequency to other high-risk conditions for which testing is performed routinely. However, the National Institute of Child Health and Human Development (NICHD) workshop on antepartum fetal monitoring found insufficient evidence that antenatal testing for the sole indication of advanced maternal age reduces stillbirth or improves perinatal outcomes.20
If increased antenatal testing is indicated for a high-risk condition or electively chosen given advanced age, it should include electronic fetal monitoring as well as amniotic fluid volume assessment. Because the risk of fetal loss sharply increased at 40 weeks’ gestation in the study by Reddy and colleagues,6 women older than age 40 should be considered for delivery by 40 weeks’ gestation in the presence of good dating criteria.
Some clinicians also would consider delivery by 39 weeks’ gestation with good dating criteria if the Bishop score is favorable.
Risks of labor and delivery
Multiple variables contribute to a higher cesarean delivery rate
The risk of cesarean delivery increases with advancing maternal age.5,11 This increased risk is a consequence of multiple variables, including the rate of previous cesarean delivery, malpresentation, underlying complications such as preeclampsia and diabetes, and a higher prevalence of dysfunctional labor.21 Further, Vaughn and colleagues noted that the cesarean delivery rate increases in direct proportion to age, with a rate of 54.4% in women older than age 40.5
As Cohen pointed out in a commentary accompanying a study of dysfunctional labor in women of advancing age, “the notion of a premium baby (ie, that the fetus of a woman with a reduced likelihood of having another pregnancy is somehow more deserving of being spared the rigours of labour than the fetus of a young woman) may play a role” in the high rate of cesarean delivery.21,22
Postpartum hemorrhage risk may be lower in older women
Advanced maternal age is assumed to be a risk factor for postpartum hemorrhage.23 The increased risk was thought to be related to the increased incidence of multiple underlying factors, such as cesarean delivery, multiple gestation, and hypertensive disorders of pregnancy.
However, in a retrospective cohort study, Lao and colleagues found that advanced maternal age (≥35 years) served only as a surrogate factor for postpartum hemorrhage due to associated risk factors, obstetric complications, and interventions.24 After multivariate analysis, aging was associated with a decreased rate of postpartum hemorrhage, which declined progressively from ages 25 to 40 years and older, compared with women aged 20 to 24.24
Nevertheless, medical interventions should be readily available at the time of delivery for treatment of uterine atony, especially with multiple gestation and grand multiparity.
Case: Resolved
The patient is admitted to the hospital, where she is given IV magnesium sulfate (6-g load followed by an infusion of 3 g/hr) and betamethasone for fetal lung maturity enhancement. She continues to receive IV fluids as well (125 mL/hr lactated Ringers solution). Uterine activity abates.
IV magnesium sulfate is continued for 36 hours, but urine protein output is not monitored. Her heart rate ranges from 105 to 115 bpm, and blood pressure from 130/80 mm Hg to 138/88 mm Hg. Forty-eight hours after admission, she reports a gradual onset of tightness of the chest and breathlessness. She is agitated, with a pulse of 130 bpm, 30 respirations/min, and room air pulse oximetry of 90%. Rales are noted upon auscultation of both lungs. A radiograph of the chest demonstrates bilateral air-space disease consistent with pulmonary edema. IV furosemide and oxygen (by mask) are provided, with some respiratory improvement.
The patient then reports leakage of amniotic fluid, and preterm rupture of membranes is confirmed on examination. Because steroids for fetal lung maturity have been administered, and given improvement in her pulmonary edema and a footling breech presentation for Twin A, cesarean delivery is performed.
The patient’s immediate postoperative course is uncomplicated. On postoperative day 2, however, she develops recurrent pulmonary edema, as confirmed by physical examination and chest radiograph. She also reports headache, and her blood pressure rises to 164/114 mm Hg—findings consistent with postpartum preeclampsia. Magnesium sulfate and antihypertensive therapy are initiated, along with IV furosemide and oxygen, which improves her respiratory status.
An echocardiogram to rule out peripartum dilated cardiomyopathy finds no evidence of a dilated left ventricle, and the calculated left ventricular ejection fraction (55%) is normal.
After diuresis and improvement in her blood pressure, she is discharged home. By the time of her follow-up office visit 7 days later, her blood pressure has normalized on labetalol therapy.
For an overview of evaluation and management of pregnant women aged 40 or older, see TABLE 4.
Preconception
- Identify risk factors (ie, diabetes, obesity, hypertension, cardiac dysfunction, family history
- Review outcome of previous pregnancy, if applicable
- Review risks (multiple gestation, birth defects) associated with assisted reproductive technologies if they were needed to achieve pregnancy
- Optimize maternal health
- Begin folic acid supplementation
- Encourage smoking cessation
- If the patient is ≥45 years old:
– Electrocardiogram
– Glucose screening (fasting plasma glucose or hemoglobin A1c)
– Echocardiogram for patients with chronic hypertension
First trimester
- Ultrasonography for dating and to assess fetal number and chorionicity
- Baseline metabolic profile and complete blood count
- Baseline urinalysis
- Continue folic acid supplementation
- Offer first-trimester genetic testing or other genetic screening
Second trimester
- If first-trimester genetic testing is declined, offer second-trimester testing or screening
- Detailed fetal anomaly evaluation by ultrasound
- Fetal echocardiogram if pregnancy was achieved by in vitro fertilization or if it is a monochorionic twin gestation
- Screen for gestational diabetes
Third trimester
- Increased antenatal testing for routine indications, including hypertension, diabetes, and lupus
- Ultrasonography for growth and later ultrasonographic findings of fetal aneuploidy
- Consider delivery
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE: Preterm labor in an older woman
G.S. is a 41-year-old G1P0 with a several-year history of infertility and a medical history of chronic hypertension. She undergoes in vitro fertilization (IVF) using her own oocytes, with transfer of two embryos. Early ultrasonography (US) confirms a diamniotic/dichorionic twin gestation. She undergoes chorionic villus sampling (CVS) during the first trimester, with normal fetal karyotypes noted.
For her chronic hypertension, the patient is treated with oral labetalol 200 mg twice daily, beginning in the first trimester. Results of a baseline comprehensive metabolic profile and complete blood count, and electrocardiogram are normal. Baseline 24-hour urine study results reveal no significant proteinuria and a normal creatinine clearance.
At 18 weeks’ gestation, US results show normal growth and amniotic fluid volume for each fetus, with no anomalies detected. Because of a gradual increase in the patient’s blood pressure, her labetalol dose is increased to 400 mg orally thrice daily. Her urine protein output remains negative on dipstick, and US every 4 weeks until 28 weeks’ gestation continues to show normal fetal growth and amniotic fluid volume.
At 33 weeks’ gestation, the patient presents with regular uterine activity. Nonstress tests for both fetuses are reactive. She is given a 1-L intravenous (IV) fluid bolus of lactated Ringers solution, as well as subcutaneous terbutaline sulfate every 15 minutes for four doses, without resolution of the uterine contractions. Her pulse has increased to 120 bpm.
How do you manage this patient’s care?
Nine times as many women aged 35 and older gave birth to their first child in 2012 than did women of the same age 40 years ago, according to the most recent data from the National Center for Health Statistics.1 The rate of first births for women aged 40 to 44 remained essentially stable during the 1970s and early 1980s but increased more than fourfold from 1985 through 2012—from 0.5 to 2.3 per 1,000 women.1 Clearly, more women are delaying childbearing to a later age by personal choice for reasons such as completion of education and career advancement.2
The path to late motherhood is not without thorns, however. Heightened risks associated with increasing maternal age include:
- fetal aneuploidy
- fetal malformation
- gestational diabetes
- chronic and gestational hypertension
- antepartum hemorrhage
- placenta previa
- prelabor rupture of membranes
- preterm labor.3,4
Women with advanced age at conception also are more likely to have a multifetal gestation because of the need for assisted reproduction and are more likely to require cesarean delivery5 as a result of abnormal placentation, fetal malpresentation, an abnormal pattern of labor, or increased use of oxytocin in labor. In addition, they are more likely to experience rupture of the sphincter, postpartum hemorrhage, and thromboembolism.3 Advanced maternal age also is associated with a higher risk of stillbirth throughout gestation, with the peak risk period reported to occur at 37 to 41 weeks.6
Maternal age-related risks of autosomal trisomies (especially Down syndrome) are well understood and have been quantified for singleton and twin gestations. TABLE 1 shows the risks at term for singleton and twin gestations for at least one chromosomally abnormal fetus by maternal age (40–46 years) and race.7
Preconception considerations
Aging and fertility
These combined result of aging of the ovary and uterus and an escalating risk of underlying medical comorbidities has a detrimental effect on fertility.8 Although assisted reproductive technologies are helpful, they cannot guarantee a live birth or completely compensate for an age-related decline in fertility.9
Many IVF programs refuse infertility treatment to women over age 43 or 44 who want to use their own oocytes. The reason: low pregnancy rates. The use of donor oocytes, however, increases the risks of gestational hypertension and preeclampsia. And if assisted reproductive technologies are needed, the risk for multifetal pregnancy increases.
Women of advanced maternal age are likely to have an older spouse or partner. There is no clearly accepted definition of advanced paternal age, but it is most often defined as an age of 40 years or older at the time of conception. Advanced paternal age has been associated with a higher risk for autism spectrum disorder and schizophrenia, as well as mutations in the FGFR2 and FGFR3 genes that result in skeletal dysplasias and craniosynostosis syndromes.10
Medical conditions are more common
Women of advanced maternal age have an increased rate of such prepregnancy chronic medical complications as diabetes, chronic hypertension, obesity, and renal and cardiac disease. Therefore, it is best to optimize control of these chronic illnesses prior to conception to minimize the risks of miscarriage, fetal anomalies, and gestational hypertension and preeclampsia.
Preeclampsia. Although daily low-dose (60–81 mg) aspirin has been used to reduce the risk of preeclampsia, current recommendations from the American College of Obstetricians and Gynecologists (ACOG) suggest that this therapy be reserved for women with a medical history of early-onset preeclampsia or those who have had preeclampsia in more than one pregnancy.11
Impact of obesity. We recently examined the influence of age and obesity on pregnancy outcomes of nulliparous women aged 40 or older at delivery.12 The study included women aged 20 to 29 years (n = 52,249) and 40 or older (n = 1,231) who delivered singleton infants. Women who reported medical disorders, tobacco use, or conception with assisted reproductive technology were excluded.
In the older age group (≥40 years), obese women had significantly higher rates of cesarean delivery, gestational hypertension, preeclampsia, gestational diabetes, preterm delivery before 37 weeks’ gestation, and preterm delivery before 28 weeks, and their infants had higher rates of admission to the neonatal intensive care unit (NICU), compared with nonobese women (FIGURE).
It would appear, however, that healthy, obese women who delay pregnancy until the age of 40 or later may modify their risk for cesarean delivery, gestational diabetes mellitus, and gestational hypertension and preeclampsia by reducing their body mass index to nonobese levels prior to conception.
In addition to maternal risks for women of advanced maternal age, there are risks to the fetus and neonate, as well as a risk of placental abnormalities. These risks are summarized in TABLE 2.
Placental
- Molar or partial molar pregnancy
- Fetus or twins with a complete mole
- Placenta previa, vasa previa
Fetal/neonatal
- Aneuploidy
- Selective fetal growth restriction in twin gestation
- Twin-twin transfusion syndrome
- Preterm birth
- Perinatal death
Antepartum
- Gestational diabetes
- Insulin-dependent diabetes
- Gestational hypertension and preeclampsia
- Cholestasis of pregnancy
- Acute fatty liver of pregnancy
- Venous thromboembolism
- Preterm labor, preterm premature rupture
of membranes
Intrapartum
- Dysfunctional labor
- Malpresentation
- Cesarean delivery
Postpartum
- Venous thromboembolism
- Postpartum hemorrhage
Folic acid supplementation can reduce some risks
The potential benefit of folic acid supplementation to reduce the risk of fetal open neural tube defects is well documented. More recent data suggest that folic acid also is associated with a reduction in the risks of congenital heart defects, abdominal wall defects, cleft lip and palate, and spontaneous abortion. Supplementation should be initiated at least 3 months prior to conception and continued through the first trimester.
The first trimester
Early pregnancy loss is a risk
Women of advanced maternal age are more likely than younger women to experience early pregnancy loss. This risk is due to higher rates of fetal aneuploidy as well as declining ovarian and uterine function and a higher rate of ectopic pregnancy.
In the First and Second Trimester Evaluation of Risk (FASTER) trial, in which investigators reported pregnancy outcomes by maternal age for 36,056 pregnancies, the rate of spontaneous abortion after 10 weeks of gestation was 0.8% among women younger than 35 years, compared with 2.2% for women aged 40 or older.4
The likelihood of multiple gestation increases
The background risk of multiple births is higher in women of advanced maternal age, compared with younger women. This risk increases further with fertility treatment.
Multiple gestations at any age are associated with increased risks for preterm birth and very-low–birthweight infants. Potential maternal risks are listed in TABLE 3.
- Hypertension (2.5 times the risk of a singleton gestation)
- Abruption (3.0 times the risk)
- Anemia (2.5 times the risk)
- Urinary tract infection (1.5 times the risk)
- Preeclampsia (risk of 26%–75%) (occurs at earlier gestation) — HELLP syndrome (risk of 9%)
- Abruption (20%) (10 times the risk of a singleton gestation)
- Anemia (24%)
- Preterm premature rupture of membranes (24%)
- Gestational diabetes (14%)
- Acute fatty liver (4%) (1 in 10,000 singletons)
- Postpartum hemorrhage (9%)
To reduce the number of multiple gestations with assisted reproduction, consider elective single embryo transfer, especially if the mother has significant underlying medical complications.
Multiple gestations present difficult management issues in older women. Strategies shown to prevent preterm delivery in singleton gestations, including weekly 17-hydroxyprogesterone injections and cervical cerclage, are not effective in multiple gestations. Moreover, many of these therapies—including bed rest—increase the risk of thromboembolic events in multiple gestations, particularly when the mother is of advanced age.
Maternal adaptations in multiple gestations also may be poorly tolerated by older patients, particularly cardiac changes that markedly increase stroke volume, heart rate, cardiac output, and plasma volume.
The range of genetic screening and testing options has broadened
Options include first-trimester CVS, which provides information about the fetal chromosomal complement but not the presence of a fetal open neural tube defect. The procedure-related rate of fetal loss with CVS is quoted as 1%.
Options for genetic testing in the second trimester include transabdominal amniocentesis. A procedure-related fetal loss rate of 1 in 500 to 1 in 1,600 is quoted for midtrimester amniocentesis.
A relatively new screening option is analysis of cell-free fetal DNA in maternal blood, which can be performed after 10 weeks’ gestation in singleton and multiple gestations. This directed analysis measures the relative proportions of chromosomes. The detection rate for fetal Down syndrome using cell-free fetal DNA is greater than 98%, with a false-positive rate of less than 0.5%. However, this screening is unreliable in triplet gestations.
Other screening options include US and biochemical screening to detect fetal aneuploidy and open neural tube defects during the second trimester. These options should be included in counseling of the patient.
Second and third trimesters
Gestational hypertension and preeclampsia are significant risks
Older pregnant women have an incidence of gestational hypertension and preeclampsia 2 to 4 times as high as that of patients younger than 30 years.13 The underlying risk for preeclampsia is further increased if coexisting medical disorders such as diabetes or chronic hypertension are present. Moreover, the risk for preeclampsia increases to 10% to 20% in twin gestations and 25% to 60% in triplet gestations. Le Ray and colleagues reported that, if oocyte donation is used with IVF in women older than age 43, the risk for preeclampsia triples.14
We previously studied 379 women aged 35 and older who had mild gestational hypertension remote from term, comparing them with their younger adult counterparts in a matched cohort design.15 Outpatient management produced similar maternal outcomes in both groups, but older women had a statistically insignificant increase in the rate of stillbirth (5 vs 0; P = .063).15
Gestational diabetes risk doubles
The rates of both diabetes mellitus and gestational diabetes increase with advanced maternal age. Data from the FASTER consortium included an adjusted odds ratio of 2.4 for gestational diabetes in women aged 40 or older, compared with a younger control group.4 This increased risk may be a consequence of greater maternal habitus as well as declining insulin sensitivity.
Diabetes increases the risks of macrosomia, cesarean birth, and gestational hypertension. Among women with pregestational diabetes, the risks of congenital heart disease and fetal neural tube defects increase threefold. Because of this increased risk, perinatal screening is indicated for both anomalies in older women.
Pulmonary complications increase
Another risk facing women of advanced maternal age—particularly those carrying a multiple gestation—is pulmonary edema, owing to the increased cardiac output, heart rate, and blood volume, the decreased systemic vascular resistance, and the physiologic anemia of pregnancy. These risks rise further in women who develop preterm labor that requires therapy and in those who develop gestational hypertension and/or preeclampsia. Judicious use of IV fluids, particularly those with lower sodium concentrations, can reduce the risk of pulmonary complications.
Women who develop pulmonary edema have an increased risk of peripartum cardiomyopathy.16
Preterm delivery is more common
Cleary-Goodman and colleagues noted an increased incidence of preterm delivery in women aged 40 and older, compared with women younger than age 35, but no increase in spontaneous preterm labor.4 Advanced maternal age appears to be associated with an increased risk of preterm birth largely as a consequence of underlying complications of fetal growth restriction and maternal disease, including hypertension. Because preterm birth is an important contributor to perinatal morbidity and mortality, steroids should be administered for fetal lung maturity whenever preterm labor is diagnosed before 34 weeks’ gestation.
Risk of placenta previa is 1.1%
Joseph and colleagues found the risk of placenta previa to be 1.1% in women aged 40 and older, compared with 0.3% in women aged 25 to 29 years.17 This increased risk likely is a consequence not only of maternal age but increased parity and a history of prior uterine surgery. If transabdominal US results are suspicious for placenta previa, transvaginal US is indicated for confirmation. Additional US assessment of the cord insertion site to the placenta also should be performed to rule out vasa previa.
Look for neonatal complications
Ziadeh and colleagues found that, although maternal morbidity was increased in older women, the overall neonatal outcome did not appear to be affected.18 However, we noted a higher rate of neonatal complications in women aged 40 or older, including higher NICU admission rates and more low-birth–weight infants.11
In addition, Odibo and colleagues found advanced maternal age to be an independent risk factor for intrauterine growth restriction (IUGR).19 In that study, the odds ratio for IUGR was 3.2 (95% confidence interval [CI], 1.9–5.4) for a maternal age of 40 years or older, compared with a control group. For that reason, they recommend routine screening for IUGR in all pregnant women of advanced age.
Stillbirth risk peaks at 37 to 41 weeks
In a review of more than 5.4 million singleton pregnancies without reported congenital anomalies, Reddy and colleagues found an association between advanced maternal age and stillbirth, with a higher risk of stillbirth at 37 to 41 weeks’ gestation.6 This effect of maternal age persisted despite adjusting for medical disease, parity, and race/ethnicity.
Many women older than age 40 have independent medical or fetal indications for antenatal testing. Some experts have suggested antepartum surveillance starting at 37 weeks for women of advanced maternal age; they argue that the risk of stillbirth at this gestational age is similar in frequency to other high-risk conditions for which testing is performed routinely. However, the National Institute of Child Health and Human Development (NICHD) workshop on antepartum fetal monitoring found insufficient evidence that antenatal testing for the sole indication of advanced maternal age reduces stillbirth or improves perinatal outcomes.20
If increased antenatal testing is indicated for a high-risk condition or electively chosen given advanced age, it should include electronic fetal monitoring as well as amniotic fluid volume assessment. Because the risk of fetal loss sharply increased at 40 weeks’ gestation in the study by Reddy and colleagues,6 women older than age 40 should be considered for delivery by 40 weeks’ gestation in the presence of good dating criteria.
Some clinicians also would consider delivery by 39 weeks’ gestation with good dating criteria if the Bishop score is favorable.
Risks of labor and delivery
Multiple variables contribute to a higher cesarean delivery rate
The risk of cesarean delivery increases with advancing maternal age.5,11 This increased risk is a consequence of multiple variables, including the rate of previous cesarean delivery, malpresentation, underlying complications such as preeclampsia and diabetes, and a higher prevalence of dysfunctional labor.21 Further, Vaughn and colleagues noted that the cesarean delivery rate increases in direct proportion to age, with a rate of 54.4% in women older than age 40.5
As Cohen pointed out in a commentary accompanying a study of dysfunctional labor in women of advancing age, “the notion of a premium baby (ie, that the fetus of a woman with a reduced likelihood of having another pregnancy is somehow more deserving of being spared the rigours of labour than the fetus of a young woman) may play a role” in the high rate of cesarean delivery.21,22
Postpartum hemorrhage risk may be lower in older women
Advanced maternal age is assumed to be a risk factor for postpartum hemorrhage.23 The increased risk was thought to be related to the increased incidence of multiple underlying factors, such as cesarean delivery, multiple gestation, and hypertensive disorders of pregnancy.
However, in a retrospective cohort study, Lao and colleagues found that advanced maternal age (≥35 years) served only as a surrogate factor for postpartum hemorrhage due to associated risk factors, obstetric complications, and interventions.24 After multivariate analysis, aging was associated with a decreased rate of postpartum hemorrhage, which declined progressively from ages 25 to 40 years and older, compared with women aged 20 to 24.24
Nevertheless, medical interventions should be readily available at the time of delivery for treatment of uterine atony, especially with multiple gestation and grand multiparity.
Case: Resolved
The patient is admitted to the hospital, where she is given IV magnesium sulfate (6-g load followed by an infusion of 3 g/hr) and betamethasone for fetal lung maturity enhancement. She continues to receive IV fluids as well (125 mL/hr lactated Ringers solution). Uterine activity abates.
IV magnesium sulfate is continued for 36 hours, but urine protein output is not monitored. Her heart rate ranges from 105 to 115 bpm, and blood pressure from 130/80 mm Hg to 138/88 mm Hg. Forty-eight hours after admission, she reports a gradual onset of tightness of the chest and breathlessness. She is agitated, with a pulse of 130 bpm, 30 respirations/min, and room air pulse oximetry of 90%. Rales are noted upon auscultation of both lungs. A radiograph of the chest demonstrates bilateral air-space disease consistent with pulmonary edema. IV furosemide and oxygen (by mask) are provided, with some respiratory improvement.
The patient then reports leakage of amniotic fluid, and preterm rupture of membranes is confirmed on examination. Because steroids for fetal lung maturity have been administered, and given improvement in her pulmonary edema and a footling breech presentation for Twin A, cesarean delivery is performed.
The patient’s immediate postoperative course is uncomplicated. On postoperative day 2, however, she develops recurrent pulmonary edema, as confirmed by physical examination and chest radiograph. She also reports headache, and her blood pressure rises to 164/114 mm Hg—findings consistent with postpartum preeclampsia. Magnesium sulfate and antihypertensive therapy are initiated, along with IV furosemide and oxygen, which improves her respiratory status.
An echocardiogram to rule out peripartum dilated cardiomyopathy finds no evidence of a dilated left ventricle, and the calculated left ventricular ejection fraction (55%) is normal.
After diuresis and improvement in her blood pressure, she is discharged home. By the time of her follow-up office visit 7 days later, her blood pressure has normalized on labetalol therapy.
For an overview of evaluation and management of pregnant women aged 40 or older, see TABLE 4.
Preconception
- Identify risk factors (ie, diabetes, obesity, hypertension, cardiac dysfunction, family history
- Review outcome of previous pregnancy, if applicable
- Review risks (multiple gestation, birth defects) associated with assisted reproductive technologies if they were needed to achieve pregnancy
- Optimize maternal health
- Begin folic acid supplementation
- Encourage smoking cessation
- If the patient is ≥45 years old:
– Electrocardiogram
– Glucose screening (fasting plasma glucose or hemoglobin A1c)
– Echocardiogram for patients with chronic hypertension
First trimester
- Ultrasonography for dating and to assess fetal number and chorionicity
- Baseline metabolic profile and complete blood count
- Baseline urinalysis
- Continue folic acid supplementation
- Offer first-trimester genetic testing or other genetic screening
Second trimester
- If first-trimester genetic testing is declined, offer second-trimester testing or screening
- Detailed fetal anomaly evaluation by ultrasound
- Fetal echocardiogram if pregnancy was achieved by in vitro fertilization or if it is a monochorionic twin gestation
- Screen for gestational diabetes
Third trimester
- Increased antenatal testing for routine indications, including hypertension, diabetes, and lupus
- Ultrasonography for growth and later ultrasonographic findings of fetal aneuploidy
- Consider delivery
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Mathews TJ, Hamilton BE. First births to older women continue to rise. National Center for Health Statistics. NCHS Data Brief No. 152. May 2014. http://www.cdc.gov/nchs/data/databriefs/db152.pdf. Accessed October 3, 2014.
2. Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860.
3. Ziadeh SM. Maternal and perinatal outcome in nulliparous women aged 35 and older. Gynecol Obstet Invest. 2002;54(1):6–10.
4. Cleary-Goldman J, Malone FD, Vidaver J, et al; FASTER Consortium. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 pt 1):983–990.
5. Vaughn DA, Cleary BJ, Murphy DJ. Delivery outcomes for nulliparous women at the extremes of maternal age—a cohort study. BJOG. 2014;121(3):261–268.
6. Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth through pregnancy in the United States. Am J Obstet Gynecol. 2006;195(3):764–770.
7. Meyers C, Adam R, Dungan J, Prenger V. Aneuploidy in twin gestations: when is maternal age advanced? Obstet Gynecol. 1997;89(2):248–251.
8. Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update. 2013;19(1):67–83.
9. Johnson JA, Tough S. Delayed child-bearing. J Obstet Gynaecol Can. 2012;34(1):80–93.
10. Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200.
11. Barton JR, Sibai AJ, Istwan NB, Rhea DJ, Desch CN, Sibai BM. Spontaneously conceived pregnancy after 40: influence of age and obesity on outcome. Am J Perinatol. 2014;31(9):795–798.
12. Roberts JM, August PA, Bakris JR, et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
13. Jahromi BN, Husseini Z. Pregnancy outcome at maternal age 40 and older. Taiwan J Obstet Gynecol. 2008;47(3):318–321.
14. Le Ray C, Scherier S, Anselem O, et al. Association between oocyte donation and maternal and perinatal outcomes in women aged 43 years or older. Hum Reprod. 2012;27(3):896–901.
15. Barton JR, Bergauer NK, Jacques DL, Coleman SK, Stanziano GJ, Sibai BM. Does advanced maternal age affect pregnancy outcome in women with mild hypertension remote from term? Am J Obstet Gynecol. 1997;176(6):1236–1243.
16. Habli M, O’Brien T, Nowack E, et al. Peripartum cardiomyopathy: prognostic factors for long-term maternal outcome. Am J Obstet Gynecol. 2008;199(4):415.e1–e5.
17. Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105(6):1410–1418.
18. Ziadeh S, Yahaya A. Pregnancy outcome at age 40 and older. Arch Gynecol Obstet. 2001;265(1):30–33.
19. Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23(5):325–328.
20. Signore C, Freeman RK, Spong CY. Antenatal testing—a reevaluation: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;113(3):687–701.
21. Cohen WR, Newman L, Friedman EA. Risk of labor abnormalities with advancing maternal age. Obstet Gynecol. 1980;55(4):414–416.
22. Cohen WR. Does maternal age affect pregnancy outcome? BJOG. 2014;121(3):252–254.
23. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–1373.
24. Lao TT, Sahota DS, Cheng YK, Law LW, Leung TY. Advanced maternal age and postpartum hemorrhage—risk factor or red herring? J Matern Fetal Neonatal Med. 2014;27(3):243–246.
1. Mathews TJ, Hamilton BE. First births to older women continue to rise. National Center for Health Statistics. NCHS Data Brief No. 152. May 2014. http://www.cdc.gov/nchs/data/databriefs/db152.pdf. Accessed October 3, 2014.
2. Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860.
3. Ziadeh SM. Maternal and perinatal outcome in nulliparous women aged 35 and older. Gynecol Obstet Invest. 2002;54(1):6–10.
4. Cleary-Goldman J, Malone FD, Vidaver J, et al; FASTER Consortium. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 pt 1):983–990.
5. Vaughn DA, Cleary BJ, Murphy DJ. Delivery outcomes for nulliparous women at the extremes of maternal age—a cohort study. BJOG. 2014;121(3):261–268.
6. Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth through pregnancy in the United States. Am J Obstet Gynecol. 2006;195(3):764–770.
7. Meyers C, Adam R, Dungan J, Prenger V. Aneuploidy in twin gestations: when is maternal age advanced? Obstet Gynecol. 1997;89(2):248–251.
8. Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update. 2013;19(1):67–83.
9. Johnson JA, Tough S. Delayed child-bearing. J Obstet Gynaecol Can. 2012;34(1):80–93.
10. Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200.
11. Barton JR, Sibai AJ, Istwan NB, Rhea DJ, Desch CN, Sibai BM. Spontaneously conceived pregnancy after 40: influence of age and obesity on outcome. Am J Perinatol. 2014;31(9):795–798.
12. Roberts JM, August PA, Bakris JR, et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
13. Jahromi BN, Husseini Z. Pregnancy outcome at maternal age 40 and older. Taiwan J Obstet Gynecol. 2008;47(3):318–321.
14. Le Ray C, Scherier S, Anselem O, et al. Association between oocyte donation and maternal and perinatal outcomes in women aged 43 years or older. Hum Reprod. 2012;27(3):896–901.
15. Barton JR, Bergauer NK, Jacques DL, Coleman SK, Stanziano GJ, Sibai BM. Does advanced maternal age affect pregnancy outcome in women with mild hypertension remote from term? Am J Obstet Gynecol. 1997;176(6):1236–1243.
16. Habli M, O’Brien T, Nowack E, et al. Peripartum cardiomyopathy: prognostic factors for long-term maternal outcome. Am J Obstet Gynecol. 2008;199(4):415.e1–e5.
17. Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105(6):1410–1418.
18. Ziadeh S, Yahaya A. Pregnancy outcome at age 40 and older. Arch Gynecol Obstet. 2001;265(1):30–33.
19. Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23(5):325–328.
20. Signore C, Freeman RK, Spong CY. Antenatal testing—a reevaluation: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;113(3):687–701.
21. Cohen WR, Newman L, Friedman EA. Risk of labor abnormalities with advancing maternal age. Obstet Gynecol. 1980;55(4):414–416.
22. Cohen WR. Does maternal age affect pregnancy outcome? BJOG. 2014;121(3):252–254.
23. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–1373.
24. Lao TT, Sahota DS, Cheng YK, Law LW, Leung TY. Advanced maternal age and postpartum hemorrhage—risk factor or red herring? J Matern Fetal Neonatal Med. 2014;27(3):243–246.
A summary of the new ACOG report on neonatal brachial plexus palsy. Part 2: Pathophysiology and causation
Obstetricians are often blamed for causing neonatal brachial plexus palsy (NBPP). For that reason, understanding the true pathophysiology and causation of this birth-related entity is of extreme importance.
In Part 1 of this two-part series, I summarized findings from the new report on NBPP from the American College of Obstetricians and Gynecologists (ACOG), focusing on whether the phenomenon of shoulder dystocia and NBPP can be predicted or prevented.1 Here, in Part 2, I focus on ACOG’s conclusions concerning pathophysiology and causation of NBPP, as well as the College’s recommendations for applying that knowledge to practice.
Some infants are more susceptible than others to the forces of labor and delivery
Babies emerge from the uterus and maternal pelvis by a combination of uterine contractions and maternal pushing (endogenous forces) aided by the traction forces applied by the birth attendant (exogenous forces). Research over the past 2 decades has shown that endogenous forces play a significant—if not dominant—role in the causation of NBPP.
Stretching and potential injury to the brachial plexus occur when the long axis of the fetus is pushed down the birth canal while either the maternal symphysis pubis or sacral promontory catches and holds either the anterior or posterior shoulder of the fetus, respectively. This conjunction of events generates a stretching force on the tissues that connect the fetal trunk and head—the neck—under which lies the brachial plexus. The same anatomic relationships and labor forces also vigorously compress the fetal neck against the maternal symphysis pubis or sacral promontory and may cause compression injury. Any traction applied by the clinician accentuates these stretching and pressure forces acting on the nerves of the brachial plexus.
How the neonate responds to these forces depends on the tensile strength of its tissues, the metabolic condition of the fetus after a potentially long labor (as measured by acid-base status), the degree of protective muscle tone around the fetal shoulder and neck, and other fluctuating conditions. In other words, because of the many variables involved, some fetuses are more or less susceptible to injury than others.
Maternal forces alone can cause NBPP
The ACOG report1 makes an important statement:
Some plaintiff attorneys and their expert witnesses have tried to make the case that, although endogenous forces can cause temporary brachial plexus injuries, they cannot cause permanent brachial plexus injuries. However, as the ACOG report goes on to state:
The report acknowledges that the clinician can increase brachial plexus stretch by applying downward lateral traction to the neonate’s head during delivery efforts. However, contrary to claims often made by the plaintiff bar, in the presence of shoulder dystocia, even properly applied axial traction will necessarily increase the stretching of the brachial plexus. The report also notes that traction applied in the plane of the fetal cervicothoracic spine typically is along a vector estimated to be 25° to 45° below the horizontal plane of a woman in lithotomy position, not in an exact straight line with the maternal trunk. This degree of delivery force below the horizon is defined as normal “axial traction.”
Exogenous forces have yet to be definitively measured
Multiple attempts have been made to quantify the amount of force applied by clinicians in various delivery scenarios. However, in the published studies in which this force has been “measured,” the accuracy of the findings has not been validated. The three studies in which delivery force was directly measured in a clinical setting “provide a limited assessment of exogenous forces” and “do not address the angle at which forces were applied.”3–5 All other studies used artificial models.
As a result, few conclusions from such studies are directly applicable to the clinical arena. Moreover, in other studies using simulated birth scenarios, there was no feedback to participating clinicians as to whether the force they applied would have been sufficient to deliver the “fetus.” It was therefore difficult for participants in such studies to “determine how the situation corresponds with the force they would apply clinically.”1
Cadaver studies have been inadequate to assess the in situ response of the brachial plexus
Many plaintiff claims regarding the cause of brachial plexus injury use cadaver studies as evidence. However, most such studies were conducted between 98 and 140 years ago. In these older studies, quantitative evaluation was rare. And in the few more recent studies, there are several reasons why the data obtained are problematic:
- the nerves being studied were dissected free from supporting tissues
- nerve tissue deteriorates quickly postmortem
- some studies used adult tissues; there may be significant differences between adult and newborn nerve tissue that obscure comparison.
The ACOG report concludes the section on cadaver studies by stating:
Physical models also fall short
The problem with the use of physical models in evaluating NBPP centers on the need to find materials that have the same or similar properties as the tissues of interest. These sorts of bioengineering limitations generally do not allow for findings that have direct clinical applicability.
Of interest, however, is the finding of at least two groups of investigators that less traction is required when simulating delivery of a model infant when rotational maneuvers (Rubin’s) are employed rather than after McRoberts repositioning.
Computer models have yielded data on the relative effects of endogenous and exogenous forces
Sophisticated computer analysis has been used to investigate both endogenous and exogenous delivery forces. Results of such studies have shown that maternal endogenous forces exert twice as much pressure on the base of the fetal neck against the maternal symphysis pubis as do deliverer-induced exogenous forces.
Is there a threshold of force?
Data that include measurement of the force applied to the brachial plexus nerves of a live infant during a real delivery are almost nonexistent. One group—on the basis of a single case of transient NBPP and potentially flawed pressure measurements—has suggested that the threshold for NBPP in the human is 100 Newtons.3 However, other studies have shown that physician-applied forces in routine deliveries commonly exceed this hypothesized cutoff—yet the rate of NBPP remains low. In measuring delivery forces it must be remembered that significant variation exists between individual neonates, both in terms of mechanical properties and anatomy. Because of this variation—and the nonlinear behavior of nerve tissues—the specific force needed to cause a nerve injury or rupture in a given neonate has not been established.
Chapter 3 of the ACOG report closes with a statement:
NBPP and shoulder dystocia
Shoulder dystocia is defined as a delivery that requires additional obstetric maneuvers after gentle downward traction on the fetal head fails to deliver the fetal shoulders. The ACOG report makes the important point that shoulder dystocia is not formally diagnosed until a trial of downward axial traction has been unsuccessful in delivering the anterior shoulder. This point is a refutation of the frequent plaintiff claim that, once a shoulder dystocia is thought to be present, no traction whatsoever should be applied by the clinician at any time during the remainder of the delivery.
Shoulder dystocia incidence is rising
The reported incidence of shoulder dystocia has increased over the past several decades. It is unclear whether this increase is related to maternal obesity, fetal macrosomia, or more widespread reporting. However, paradoxes exist in the relationship among risk factors, shoulder dystocia, and brachial plexus injury:
- although there is an increased incidence of shoulder dystocia with increased birth weight, the mean birth weight of neonates with recognized shoulder dystocia is not significantly higher than the mean birth weight of all term infants
- strategies to reduce NBPP by preventing shoulder dystocia—including early induction of labor and prophylactic use of McRoberts maneuver and suprapubic pressure—have not been effective in reducing the incidence of NBPP.
The ACOG report makes the statement: “Maternal and fetal factors associated with shoulder dystocia do not allow for reliable prediction of persistent NBPP.”1
What is optimal management of shoulder dystocia?
The last obstetric part of the ACOG report takes as its focus the management of shoulder dystocia. It discusses the importance of communication among members of the delivery team and with the mother whose neonate is experiencing a shoulder dystocia. The report states:
This statement contrasts with claims frequently made by plaintiff medical expert witnesses that the woman experiencing a shoulder dystocia should absolutely cease from pushing.
In a section on team training, the report describes the delivery team’s priorities:
- resolving the shoulder dystocia
- avoiding neonatal hypoxic-ischemic central nervous system injury
- minimizing strain on the neonatal brachial plexus.
Studies evaluating process standardization, the use of checklists, teamwork training, crew resource management, and evidence-based medicine have shown that these tools improve neonatal and maternal outcomes.
Simulation training also has been shown to help reduce transient NBPP (see the box below for more on simulation programs for shoulder dystocia). Whether it also can lower the rate of permanent NBPP is unclear.1
Can simulation training reduce the rate of neonatal brachial plexus injury after shoulder dystocia?
In the new ACOG report on neonatal brachial plexus injury, simulation training is discussed as one solution to the dilemma of how clinicians can gain experience in managing obstetric events that occur infrequently.1 Simulation training also has the potential to improve teamwork, communication, and the situational awareness of the health-care team as a whole. Several studies over the past few years have shown that, in some units, the implementation of simulation training actually has decreased the number of cases of neonatal brachial plexus palsy (NBPP), compared with no simulation training.
For example, Draycott and colleagues explored the rate of neonatal injury associated with shoulder dystocia before and after implementation of a mandatory 1-day simulation training program at Southmead Hospital in Bristol, United Kingdom.2 The program consisted of practice on a shoulder dystocia training mannequin and covered risk factors, recognition of shoulder dystocia, maneuvers, and documentation. The training used a stepwise approach, beginning with a call for help and continuing through McRoberts’ positioning, suprapubic pressure, and internal maneuvers such as delivery of the posterior arm (Figure).
There were 15,908 births in the pretraining period and 13,117 in the posttraining period, with shoulder dystocia rates comparable between the two periods. Not only did clinical management of shoulder dystocia improve after training, but there was a significant reduction in neonatal injury at birth after shoulder dystocia (30 injuries of 324 shoulder dystocia cases [9.3%] before training vs six injuries of 262 shoulder dystocia cases [2.3%] afterward).2
In another study of obstetric brachial plexus injury before and after implementation of simulation training for shoulder dystocia, Inglis and colleagues found a decline in the rate of such injury from 30% to 10.67% (P<.01).3 Shoulder dystocia training remained associated with reduced obstetric brachial plexus injury after logistic-regression analysis.3
Shoulder dystocia training is now recommended by the Joint Commission on Accreditation of Healthcare Organizations in the United States. However, in its report, ACOG concludes—despite studies from Draycott and colleagues and others—that, owing to “limited data,” “there remains no evidence that introduction of simulation can reduce the frequency of persistent NBPP.”1
References
- American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
- Draycott TJ, Crofts FJ, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14–20.
- Inglis SR, Feier N, Chetiyaar JB, et al. Effects of shoulder dystocia training on the incidence of brachial plexus palsy. Am J Obstet Gynecol. 2011;204(4):322.e1–e6.
Delivery of the posterior arm
The report reaffirms the previous statement from the ACOG practice bulletin on shoulder dystocia, which asserts that no specific sequence of maneuvers for resolving shoulder dystocia has been shown to be superior to any other.6 It does note, however, that recent studies seem to demonstrate a benefit when delivery of the posterior arm is prioritized over the usual first-line maneuvers of McRoberts positioning and the application of suprapubic pressure. If confirmed, such findings may alter the standard of care for shoulder dystocia resolution and result in a change in ACOG recommendations.
Documentation may be enhanced by use of a checklist
The ACOG report stresses the importance of accurate, contemporaneous documentation of the management of shoulder dystocia, observing that checklists and documentation reminders help ensure the completeness and relevance of notes after shoulder dystocia deliveries and NBPP. ACOG has produced such a checklist, which can be found in the appendix of the report itself.1
How long before central neurologic injury occurs?
Another issue covered in the report is how long a clinician has to resolve a shoulder dystocia before central neurologic damage occurs. Studies have shown that permanent neurologic injury can occur as soon as 2 minutes after shoulder impaction, although the risk of acidosis or severe hypoxic-ischemic encephalopathy remains low until impaction has lasted at least 5 minutes.
Other issues covered in the report
The last chapters of the ACOG report focus on orthopedic aspects of brachial plexus injury, including diagnosis, treatment, and prognosis.
The report concludes with a glossary and three appendices:
- Royal College of Obstetricians and Gynecologists Green Top Guidebook #42 on shoulder dystocia
- ACOG Practice Bulletin #40 on shoulder dystocia
- ACOG Patient Safety Checklist #6 on the documentation of shoulder dystocia.
Why the ACOG report is foundational
The ACOG report on NBPP is an important and much-needed document. It includes a comprehensive review of the literature on brachial plexus injury and shoulder dystocia, written by nationally recognized experts in the field. Most important, it makes definitive statements that counteract false and dubious claims often made by the plaintiff bar in brachial plexus injury cases and provides evidence to back those statements.
The report:
- disproves the claim that “excessive” physician traction is the only etiology of brachial plexus injuries
- demonstrates that no differentiation can be made between the etiology of permanent versus temporary brachial plexus injuries
- describes how brachial plexus injuries can occur in the absence of physician traction or even of shoulder dystocia
- provides a summary of scientific information about brachial plexus injuries that will benefit obstetric clinicians
- provides a wealth of literature documentation that will enable physician defendants to counteract many of the claims plaintiffs and their expert witnesses make in brachial plexus injury cases.
The report is—and will remain—a foundational document in obstetrics for many years to come.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com.
1. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
2. Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198(3):e.7–e.8.
3. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77(3):352–355.
4. Poggi SH, Allen RH, Patel CR, Ghidini A, Pezzullo JC, Spong CY. Randomized trial of McRoberts versus lithotomy positioning to decrease the force that is applied to the fetus during delivery. Am J Obstet Gynecol. 2004;191(3):874–878.
5. Poggi SH, Allen RH, Patel C, et al. Effect of epidural anaesthesia on clinician-applied force during vaginal delivery. Am J Obstet Gynecol. 2004;191(3):903–906.
6. American College of Obstetricians and Gynecologists. Practice bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
Obstetricians are often blamed for causing neonatal brachial plexus palsy (NBPP). For that reason, understanding the true pathophysiology and causation of this birth-related entity is of extreme importance.
In Part 1 of this two-part series, I summarized findings from the new report on NBPP from the American College of Obstetricians and Gynecologists (ACOG), focusing on whether the phenomenon of shoulder dystocia and NBPP can be predicted or prevented.1 Here, in Part 2, I focus on ACOG’s conclusions concerning pathophysiology and causation of NBPP, as well as the College’s recommendations for applying that knowledge to practice.
Some infants are more susceptible than others to the forces of labor and delivery
Babies emerge from the uterus and maternal pelvis by a combination of uterine contractions and maternal pushing (endogenous forces) aided by the traction forces applied by the birth attendant (exogenous forces). Research over the past 2 decades has shown that endogenous forces play a significant—if not dominant—role in the causation of NBPP.
Stretching and potential injury to the brachial plexus occur when the long axis of the fetus is pushed down the birth canal while either the maternal symphysis pubis or sacral promontory catches and holds either the anterior or posterior shoulder of the fetus, respectively. This conjunction of events generates a stretching force on the tissues that connect the fetal trunk and head—the neck—under which lies the brachial plexus. The same anatomic relationships and labor forces also vigorously compress the fetal neck against the maternal symphysis pubis or sacral promontory and may cause compression injury. Any traction applied by the clinician accentuates these stretching and pressure forces acting on the nerves of the brachial plexus.
How the neonate responds to these forces depends on the tensile strength of its tissues, the metabolic condition of the fetus after a potentially long labor (as measured by acid-base status), the degree of protective muscle tone around the fetal shoulder and neck, and other fluctuating conditions. In other words, because of the many variables involved, some fetuses are more or less susceptible to injury than others.
Maternal forces alone can cause NBPP
The ACOG report1 makes an important statement:
Some plaintiff attorneys and their expert witnesses have tried to make the case that, although endogenous forces can cause temporary brachial plexus injuries, they cannot cause permanent brachial plexus injuries. However, as the ACOG report goes on to state:
The report acknowledges that the clinician can increase brachial plexus stretch by applying downward lateral traction to the neonate’s head during delivery efforts. However, contrary to claims often made by the plaintiff bar, in the presence of shoulder dystocia, even properly applied axial traction will necessarily increase the stretching of the brachial plexus. The report also notes that traction applied in the plane of the fetal cervicothoracic spine typically is along a vector estimated to be 25° to 45° below the horizontal plane of a woman in lithotomy position, not in an exact straight line with the maternal trunk. This degree of delivery force below the horizon is defined as normal “axial traction.”
Exogenous forces have yet to be definitively measured
Multiple attempts have been made to quantify the amount of force applied by clinicians in various delivery scenarios. However, in the published studies in which this force has been “measured,” the accuracy of the findings has not been validated. The three studies in which delivery force was directly measured in a clinical setting “provide a limited assessment of exogenous forces” and “do not address the angle at which forces were applied.”3–5 All other studies used artificial models.
As a result, few conclusions from such studies are directly applicable to the clinical arena. Moreover, in other studies using simulated birth scenarios, there was no feedback to participating clinicians as to whether the force they applied would have been sufficient to deliver the “fetus.” It was therefore difficult for participants in such studies to “determine how the situation corresponds with the force they would apply clinically.”1
Cadaver studies have been inadequate to assess the in situ response of the brachial plexus
Many plaintiff claims regarding the cause of brachial plexus injury use cadaver studies as evidence. However, most such studies were conducted between 98 and 140 years ago. In these older studies, quantitative evaluation was rare. And in the few more recent studies, there are several reasons why the data obtained are problematic:
- the nerves being studied were dissected free from supporting tissues
- nerve tissue deteriorates quickly postmortem
- some studies used adult tissues; there may be significant differences between adult and newborn nerve tissue that obscure comparison.
The ACOG report concludes the section on cadaver studies by stating:
Physical models also fall short
The problem with the use of physical models in evaluating NBPP centers on the need to find materials that have the same or similar properties as the tissues of interest. These sorts of bioengineering limitations generally do not allow for findings that have direct clinical applicability.
Of interest, however, is the finding of at least two groups of investigators that less traction is required when simulating delivery of a model infant when rotational maneuvers (Rubin’s) are employed rather than after McRoberts repositioning.
Computer models have yielded data on the relative effects of endogenous and exogenous forces
Sophisticated computer analysis has been used to investigate both endogenous and exogenous delivery forces. Results of such studies have shown that maternal endogenous forces exert twice as much pressure on the base of the fetal neck against the maternal symphysis pubis as do deliverer-induced exogenous forces.
Is there a threshold of force?
Data that include measurement of the force applied to the brachial plexus nerves of a live infant during a real delivery are almost nonexistent. One group—on the basis of a single case of transient NBPP and potentially flawed pressure measurements—has suggested that the threshold for NBPP in the human is 100 Newtons.3 However, other studies have shown that physician-applied forces in routine deliveries commonly exceed this hypothesized cutoff—yet the rate of NBPP remains low. In measuring delivery forces it must be remembered that significant variation exists between individual neonates, both in terms of mechanical properties and anatomy. Because of this variation—and the nonlinear behavior of nerve tissues—the specific force needed to cause a nerve injury or rupture in a given neonate has not been established.
Chapter 3 of the ACOG report closes with a statement:
NBPP and shoulder dystocia
Shoulder dystocia is defined as a delivery that requires additional obstetric maneuvers after gentle downward traction on the fetal head fails to deliver the fetal shoulders. The ACOG report makes the important point that shoulder dystocia is not formally diagnosed until a trial of downward axial traction has been unsuccessful in delivering the anterior shoulder. This point is a refutation of the frequent plaintiff claim that, once a shoulder dystocia is thought to be present, no traction whatsoever should be applied by the clinician at any time during the remainder of the delivery.
Shoulder dystocia incidence is rising
The reported incidence of shoulder dystocia has increased over the past several decades. It is unclear whether this increase is related to maternal obesity, fetal macrosomia, or more widespread reporting. However, paradoxes exist in the relationship among risk factors, shoulder dystocia, and brachial plexus injury:
- although there is an increased incidence of shoulder dystocia with increased birth weight, the mean birth weight of neonates with recognized shoulder dystocia is not significantly higher than the mean birth weight of all term infants
- strategies to reduce NBPP by preventing shoulder dystocia—including early induction of labor and prophylactic use of McRoberts maneuver and suprapubic pressure—have not been effective in reducing the incidence of NBPP.
The ACOG report makes the statement: “Maternal and fetal factors associated with shoulder dystocia do not allow for reliable prediction of persistent NBPP.”1
What is optimal management of shoulder dystocia?
The last obstetric part of the ACOG report takes as its focus the management of shoulder dystocia. It discusses the importance of communication among members of the delivery team and with the mother whose neonate is experiencing a shoulder dystocia. The report states:
This statement contrasts with claims frequently made by plaintiff medical expert witnesses that the woman experiencing a shoulder dystocia should absolutely cease from pushing.
In a section on team training, the report describes the delivery team’s priorities:
- resolving the shoulder dystocia
- avoiding neonatal hypoxic-ischemic central nervous system injury
- minimizing strain on the neonatal brachial plexus.
Studies evaluating process standardization, the use of checklists, teamwork training, crew resource management, and evidence-based medicine have shown that these tools improve neonatal and maternal outcomes.
Simulation training also has been shown to help reduce transient NBPP (see the box below for more on simulation programs for shoulder dystocia). Whether it also can lower the rate of permanent NBPP is unclear.1
Can simulation training reduce the rate of neonatal brachial plexus injury after shoulder dystocia?
In the new ACOG report on neonatal brachial plexus injury, simulation training is discussed as one solution to the dilemma of how clinicians can gain experience in managing obstetric events that occur infrequently.1 Simulation training also has the potential to improve teamwork, communication, and the situational awareness of the health-care team as a whole. Several studies over the past few years have shown that, in some units, the implementation of simulation training actually has decreased the number of cases of neonatal brachial plexus palsy (NBPP), compared with no simulation training.
For example, Draycott and colleagues explored the rate of neonatal injury associated with shoulder dystocia before and after implementation of a mandatory 1-day simulation training program at Southmead Hospital in Bristol, United Kingdom.2 The program consisted of practice on a shoulder dystocia training mannequin and covered risk factors, recognition of shoulder dystocia, maneuvers, and documentation. The training used a stepwise approach, beginning with a call for help and continuing through McRoberts’ positioning, suprapubic pressure, and internal maneuvers such as delivery of the posterior arm (Figure).
There were 15,908 births in the pretraining period and 13,117 in the posttraining period, with shoulder dystocia rates comparable between the two periods. Not only did clinical management of shoulder dystocia improve after training, but there was a significant reduction in neonatal injury at birth after shoulder dystocia (30 injuries of 324 shoulder dystocia cases [9.3%] before training vs six injuries of 262 shoulder dystocia cases [2.3%] afterward).2
In another study of obstetric brachial plexus injury before and after implementation of simulation training for shoulder dystocia, Inglis and colleagues found a decline in the rate of such injury from 30% to 10.67% (P<.01).3 Shoulder dystocia training remained associated with reduced obstetric brachial plexus injury after logistic-regression analysis.3
Shoulder dystocia training is now recommended by the Joint Commission on Accreditation of Healthcare Organizations in the United States. However, in its report, ACOG concludes—despite studies from Draycott and colleagues and others—that, owing to “limited data,” “there remains no evidence that introduction of simulation can reduce the frequency of persistent NBPP.”1
References
- American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
- Draycott TJ, Crofts FJ, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14–20.
- Inglis SR, Feier N, Chetiyaar JB, et al. Effects of shoulder dystocia training on the incidence of brachial plexus palsy. Am J Obstet Gynecol. 2011;204(4):322.e1–e6.
Delivery of the posterior arm
The report reaffirms the previous statement from the ACOG practice bulletin on shoulder dystocia, which asserts that no specific sequence of maneuvers for resolving shoulder dystocia has been shown to be superior to any other.6 It does note, however, that recent studies seem to demonstrate a benefit when delivery of the posterior arm is prioritized over the usual first-line maneuvers of McRoberts positioning and the application of suprapubic pressure. If confirmed, such findings may alter the standard of care for shoulder dystocia resolution and result in a change in ACOG recommendations.
Documentation may be enhanced by use of a checklist
The ACOG report stresses the importance of accurate, contemporaneous documentation of the management of shoulder dystocia, observing that checklists and documentation reminders help ensure the completeness and relevance of notes after shoulder dystocia deliveries and NBPP. ACOG has produced such a checklist, which can be found in the appendix of the report itself.1
How long before central neurologic injury occurs?
Another issue covered in the report is how long a clinician has to resolve a shoulder dystocia before central neurologic damage occurs. Studies have shown that permanent neurologic injury can occur as soon as 2 minutes after shoulder impaction, although the risk of acidosis or severe hypoxic-ischemic encephalopathy remains low until impaction has lasted at least 5 minutes.
Other issues covered in the report
The last chapters of the ACOG report focus on orthopedic aspects of brachial plexus injury, including diagnosis, treatment, and prognosis.
The report concludes with a glossary and three appendices:
- Royal College of Obstetricians and Gynecologists Green Top Guidebook #42 on shoulder dystocia
- ACOG Practice Bulletin #40 on shoulder dystocia
- ACOG Patient Safety Checklist #6 on the documentation of shoulder dystocia.
Why the ACOG report is foundational
The ACOG report on NBPP is an important and much-needed document. It includes a comprehensive review of the literature on brachial plexus injury and shoulder dystocia, written by nationally recognized experts in the field. Most important, it makes definitive statements that counteract false and dubious claims often made by the plaintiff bar in brachial plexus injury cases and provides evidence to back those statements.
The report:
- disproves the claim that “excessive” physician traction is the only etiology of brachial plexus injuries
- demonstrates that no differentiation can be made between the etiology of permanent versus temporary brachial plexus injuries
- describes how brachial plexus injuries can occur in the absence of physician traction or even of shoulder dystocia
- provides a summary of scientific information about brachial plexus injuries that will benefit obstetric clinicians
- provides a wealth of literature documentation that will enable physician defendants to counteract many of the claims plaintiffs and their expert witnesses make in brachial plexus injury cases.
The report is—and will remain—a foundational document in obstetrics for many years to come.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com.
Obstetricians are often blamed for causing neonatal brachial plexus palsy (NBPP). For that reason, understanding the true pathophysiology and causation of this birth-related entity is of extreme importance.
In Part 1 of this two-part series, I summarized findings from the new report on NBPP from the American College of Obstetricians and Gynecologists (ACOG), focusing on whether the phenomenon of shoulder dystocia and NBPP can be predicted or prevented.1 Here, in Part 2, I focus on ACOG’s conclusions concerning pathophysiology and causation of NBPP, as well as the College’s recommendations for applying that knowledge to practice.
Some infants are more susceptible than others to the forces of labor and delivery
Babies emerge from the uterus and maternal pelvis by a combination of uterine contractions and maternal pushing (endogenous forces) aided by the traction forces applied by the birth attendant (exogenous forces). Research over the past 2 decades has shown that endogenous forces play a significant—if not dominant—role in the causation of NBPP.
Stretching and potential injury to the brachial plexus occur when the long axis of the fetus is pushed down the birth canal while either the maternal symphysis pubis or sacral promontory catches and holds either the anterior or posterior shoulder of the fetus, respectively. This conjunction of events generates a stretching force on the tissues that connect the fetal trunk and head—the neck—under which lies the brachial plexus. The same anatomic relationships and labor forces also vigorously compress the fetal neck against the maternal symphysis pubis or sacral promontory and may cause compression injury. Any traction applied by the clinician accentuates these stretching and pressure forces acting on the nerves of the brachial plexus.
How the neonate responds to these forces depends on the tensile strength of its tissues, the metabolic condition of the fetus after a potentially long labor (as measured by acid-base status), the degree of protective muscle tone around the fetal shoulder and neck, and other fluctuating conditions. In other words, because of the many variables involved, some fetuses are more or less susceptible to injury than others.
Maternal forces alone can cause NBPP
The ACOG report1 makes an important statement:
Some plaintiff attorneys and their expert witnesses have tried to make the case that, although endogenous forces can cause temporary brachial plexus injuries, they cannot cause permanent brachial plexus injuries. However, as the ACOG report goes on to state:
The report acknowledges that the clinician can increase brachial plexus stretch by applying downward lateral traction to the neonate’s head during delivery efforts. However, contrary to claims often made by the plaintiff bar, in the presence of shoulder dystocia, even properly applied axial traction will necessarily increase the stretching of the brachial plexus. The report also notes that traction applied in the plane of the fetal cervicothoracic spine typically is along a vector estimated to be 25° to 45° below the horizontal plane of a woman in lithotomy position, not in an exact straight line with the maternal trunk. This degree of delivery force below the horizon is defined as normal “axial traction.”
Exogenous forces have yet to be definitively measured
Multiple attempts have been made to quantify the amount of force applied by clinicians in various delivery scenarios. However, in the published studies in which this force has been “measured,” the accuracy of the findings has not been validated. The three studies in which delivery force was directly measured in a clinical setting “provide a limited assessment of exogenous forces” and “do not address the angle at which forces were applied.”3–5 All other studies used artificial models.
As a result, few conclusions from such studies are directly applicable to the clinical arena. Moreover, in other studies using simulated birth scenarios, there was no feedback to participating clinicians as to whether the force they applied would have been sufficient to deliver the “fetus.” It was therefore difficult for participants in such studies to “determine how the situation corresponds with the force they would apply clinically.”1
Cadaver studies have been inadequate to assess the in situ response of the brachial plexus
Many plaintiff claims regarding the cause of brachial plexus injury use cadaver studies as evidence. However, most such studies were conducted between 98 and 140 years ago. In these older studies, quantitative evaluation was rare. And in the few more recent studies, there are several reasons why the data obtained are problematic:
- the nerves being studied were dissected free from supporting tissues
- nerve tissue deteriorates quickly postmortem
- some studies used adult tissues; there may be significant differences between adult and newborn nerve tissue that obscure comparison.
The ACOG report concludes the section on cadaver studies by stating:
Physical models also fall short
The problem with the use of physical models in evaluating NBPP centers on the need to find materials that have the same or similar properties as the tissues of interest. These sorts of bioengineering limitations generally do not allow for findings that have direct clinical applicability.
Of interest, however, is the finding of at least two groups of investigators that less traction is required when simulating delivery of a model infant when rotational maneuvers (Rubin’s) are employed rather than after McRoberts repositioning.
Computer models have yielded data on the relative effects of endogenous and exogenous forces
Sophisticated computer analysis has been used to investigate both endogenous and exogenous delivery forces. Results of such studies have shown that maternal endogenous forces exert twice as much pressure on the base of the fetal neck against the maternal symphysis pubis as do deliverer-induced exogenous forces.
Is there a threshold of force?
Data that include measurement of the force applied to the brachial plexus nerves of a live infant during a real delivery are almost nonexistent. One group—on the basis of a single case of transient NBPP and potentially flawed pressure measurements—has suggested that the threshold for NBPP in the human is 100 Newtons.3 However, other studies have shown that physician-applied forces in routine deliveries commonly exceed this hypothesized cutoff—yet the rate of NBPP remains low. In measuring delivery forces it must be remembered that significant variation exists between individual neonates, both in terms of mechanical properties and anatomy. Because of this variation—and the nonlinear behavior of nerve tissues—the specific force needed to cause a nerve injury or rupture in a given neonate has not been established.
Chapter 3 of the ACOG report closes with a statement:
NBPP and shoulder dystocia
Shoulder dystocia is defined as a delivery that requires additional obstetric maneuvers after gentle downward traction on the fetal head fails to deliver the fetal shoulders. The ACOG report makes the important point that shoulder dystocia is not formally diagnosed until a trial of downward axial traction has been unsuccessful in delivering the anterior shoulder. This point is a refutation of the frequent plaintiff claim that, once a shoulder dystocia is thought to be present, no traction whatsoever should be applied by the clinician at any time during the remainder of the delivery.
Shoulder dystocia incidence is rising
The reported incidence of shoulder dystocia has increased over the past several decades. It is unclear whether this increase is related to maternal obesity, fetal macrosomia, or more widespread reporting. However, paradoxes exist in the relationship among risk factors, shoulder dystocia, and brachial plexus injury:
- although there is an increased incidence of shoulder dystocia with increased birth weight, the mean birth weight of neonates with recognized shoulder dystocia is not significantly higher than the mean birth weight of all term infants
- strategies to reduce NBPP by preventing shoulder dystocia—including early induction of labor and prophylactic use of McRoberts maneuver and suprapubic pressure—have not been effective in reducing the incidence of NBPP.
The ACOG report makes the statement: “Maternal and fetal factors associated with shoulder dystocia do not allow for reliable prediction of persistent NBPP.”1
What is optimal management of shoulder dystocia?
The last obstetric part of the ACOG report takes as its focus the management of shoulder dystocia. It discusses the importance of communication among members of the delivery team and with the mother whose neonate is experiencing a shoulder dystocia. The report states:
This statement contrasts with claims frequently made by plaintiff medical expert witnesses that the woman experiencing a shoulder dystocia should absolutely cease from pushing.
In a section on team training, the report describes the delivery team’s priorities:
- resolving the shoulder dystocia
- avoiding neonatal hypoxic-ischemic central nervous system injury
- minimizing strain on the neonatal brachial plexus.
Studies evaluating process standardization, the use of checklists, teamwork training, crew resource management, and evidence-based medicine have shown that these tools improve neonatal and maternal outcomes.
Simulation training also has been shown to help reduce transient NBPP (see the box below for more on simulation programs for shoulder dystocia). Whether it also can lower the rate of permanent NBPP is unclear.1
Can simulation training reduce the rate of neonatal brachial plexus injury after shoulder dystocia?
In the new ACOG report on neonatal brachial plexus injury, simulation training is discussed as one solution to the dilemma of how clinicians can gain experience in managing obstetric events that occur infrequently.1 Simulation training also has the potential to improve teamwork, communication, and the situational awareness of the health-care team as a whole. Several studies over the past few years have shown that, in some units, the implementation of simulation training actually has decreased the number of cases of neonatal brachial plexus palsy (NBPP), compared with no simulation training.
For example, Draycott and colleagues explored the rate of neonatal injury associated with shoulder dystocia before and after implementation of a mandatory 1-day simulation training program at Southmead Hospital in Bristol, United Kingdom.2 The program consisted of practice on a shoulder dystocia training mannequin and covered risk factors, recognition of shoulder dystocia, maneuvers, and documentation. The training used a stepwise approach, beginning with a call for help and continuing through McRoberts’ positioning, suprapubic pressure, and internal maneuvers such as delivery of the posterior arm (Figure).
There were 15,908 births in the pretraining period and 13,117 in the posttraining period, with shoulder dystocia rates comparable between the two periods. Not only did clinical management of shoulder dystocia improve after training, but there was a significant reduction in neonatal injury at birth after shoulder dystocia (30 injuries of 324 shoulder dystocia cases [9.3%] before training vs six injuries of 262 shoulder dystocia cases [2.3%] afterward).2
In another study of obstetric brachial plexus injury before and after implementation of simulation training for shoulder dystocia, Inglis and colleagues found a decline in the rate of such injury from 30% to 10.67% (P<.01).3 Shoulder dystocia training remained associated with reduced obstetric brachial plexus injury after logistic-regression analysis.3
Shoulder dystocia training is now recommended by the Joint Commission on Accreditation of Healthcare Organizations in the United States. However, in its report, ACOG concludes—despite studies from Draycott and colleagues and others—that, owing to “limited data,” “there remains no evidence that introduction of simulation can reduce the frequency of persistent NBPP.”1
References
- American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
- Draycott TJ, Crofts FJ, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14–20.
- Inglis SR, Feier N, Chetiyaar JB, et al. Effects of shoulder dystocia training on the incidence of brachial plexus palsy. Am J Obstet Gynecol. 2011;204(4):322.e1–e6.
Delivery of the posterior arm
The report reaffirms the previous statement from the ACOG practice bulletin on shoulder dystocia, which asserts that no specific sequence of maneuvers for resolving shoulder dystocia has been shown to be superior to any other.6 It does note, however, that recent studies seem to demonstrate a benefit when delivery of the posterior arm is prioritized over the usual first-line maneuvers of McRoberts positioning and the application of suprapubic pressure. If confirmed, such findings may alter the standard of care for shoulder dystocia resolution and result in a change in ACOG recommendations.
Documentation may be enhanced by use of a checklist
The ACOG report stresses the importance of accurate, contemporaneous documentation of the management of shoulder dystocia, observing that checklists and documentation reminders help ensure the completeness and relevance of notes after shoulder dystocia deliveries and NBPP. ACOG has produced such a checklist, which can be found in the appendix of the report itself.1
How long before central neurologic injury occurs?
Another issue covered in the report is how long a clinician has to resolve a shoulder dystocia before central neurologic damage occurs. Studies have shown that permanent neurologic injury can occur as soon as 2 minutes after shoulder impaction, although the risk of acidosis or severe hypoxic-ischemic encephalopathy remains low until impaction has lasted at least 5 minutes.
Other issues covered in the report
The last chapters of the ACOG report focus on orthopedic aspects of brachial plexus injury, including diagnosis, treatment, and prognosis.
The report concludes with a glossary and three appendices:
- Royal College of Obstetricians and Gynecologists Green Top Guidebook #42 on shoulder dystocia
- ACOG Practice Bulletin #40 on shoulder dystocia
- ACOG Patient Safety Checklist #6 on the documentation of shoulder dystocia.
Why the ACOG report is foundational
The ACOG report on NBPP is an important and much-needed document. It includes a comprehensive review of the literature on brachial plexus injury and shoulder dystocia, written by nationally recognized experts in the field. Most important, it makes definitive statements that counteract false and dubious claims often made by the plaintiff bar in brachial plexus injury cases and provides evidence to back those statements.
The report:
- disproves the claim that “excessive” physician traction is the only etiology of brachial plexus injuries
- demonstrates that no differentiation can be made between the etiology of permanent versus temporary brachial plexus injuries
- describes how brachial plexus injuries can occur in the absence of physician traction or even of shoulder dystocia
- provides a summary of scientific information about brachial plexus injuries that will benefit obstetric clinicians
- provides a wealth of literature documentation that will enable physician defendants to counteract many of the claims plaintiffs and their expert witnesses make in brachial plexus injury cases.
The report is—and will remain—a foundational document in obstetrics for many years to come.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com.
1. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
2. Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198(3):e.7–e.8.
3. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77(3):352–355.
4. Poggi SH, Allen RH, Patel CR, Ghidini A, Pezzullo JC, Spong CY. Randomized trial of McRoberts versus lithotomy positioning to decrease the force that is applied to the fetus during delivery. Am J Obstet Gynecol. 2004;191(3):874–878.
5. Poggi SH, Allen RH, Patel C, et al. Effect of epidural anaesthesia on clinician-applied force during vaginal delivery. Am J Obstet Gynecol. 2004;191(3):903–906.
6. American College of Obstetricians and Gynecologists. Practice bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
1. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
2. Lerner HM, Salamon E. Permanent brachial plexus injury following vaginal delivery without physician traction or shoulder dystocia. Am J Obstet Gynecol. 2008;198(3):e.7–e.8.
3. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77(3):352–355.
4. Poggi SH, Allen RH, Patel CR, Ghidini A, Pezzullo JC, Spong CY. Randomized trial of McRoberts versus lithotomy positioning to decrease the force that is applied to the fetus during delivery. Am J Obstet Gynecol. 2004;191(3):874–878.
5. Poggi SH, Allen RH, Patel C, et al. Effect of epidural anaesthesia on clinician-applied force during vaginal delivery. Am J Obstet Gynecol. 2004;191(3):903–906.
6. American College of Obstetricians and Gynecologists. Practice bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
Dr. Robert L. Barbieri’s Editor’s Picks September 2014
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s September 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the September 2014 issue here.
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s September 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the September 2014 issue here.
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s September 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the September 2014 issue here.
A summary of the new ACOG report on neonatal brachial plexus palsy. Part 1: Can it be predicted?
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
Laparoscopic dual-port contained power morcellation: An offered solution
Minimally invasive surgery utilizing laparoscopy for hysterectomy and myomectomy has become more common in women with gynecologic pathology. The benefits of this approach compared with laparotomy include decreased hospital stay, shorter recovery and, in experienced hands, significantly decreased morbidity.1–3
Approximately 600,000 hysterectomies are performed annually in the United States—30% of which are performed laparoscopically.4 The primary indication for surgical intervention is uterine leiomyoma. This pathology accounts for 40% of procedures.5 During these surgeries, electromechanical morcellation (EMM), or open “power” morcellation, is commonly used to cut large tissue specimens into small pieces for removal and thereby avoid a larger incision. Concerns have been raised regarding the use of open power morcellation because of the risk of spreading an unrecognized malignancy.
Based on case reports and retrospective studies, the FDA issued a statement in April of this year discouraging the use of EMM for hysterectomy and myomectomy in women with uterine fibroids.6 The concern for inadvertent spread of an occult malignancy was the reasoning for the communication. Since that time, the FDA’s Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee held a public meeting in which the panel heard comments from patients, societies, and industry regarding their positions on the safety of laparoscopic power morcellation. The panel made several recommendations to the FDA but, at the time of this writing, the FDA has yet to issue a final decision.
Reaction to FDA’s action/inaction
The FDA’s “safety” communication was in response to the concern of a few who experienced a bad outcome believed to be secondary to open power morcellation of enlarged uteri or fibroid tumors. In its statement, the FDA estimated the risk of an occult sarcoma to be about 1 in 350 and stated that the risk of disseminating a sarcoma with morcellation is substantial. The FDA discouraged the use of the power morcellator during hysterectomy or myomectomy for uterine fibroids.
Many organizations, including the Society of Gynecologic Oncology, The American Association of Gynecologic Laparoscopists (AAGL), and the American College of Obstetricians and Gynecologists, issued less stringent statements regarding this technology.7–9 These organizations stated generally that there were too few data to make a statement at that time, advocated the collection of more data, and encouraged detailed informed consent to be given to patients undergoing these procedures.
However, the FDA’s statement, and lack of a timely follow-up to clarify the role of the laparoscopic power morcellator in gynecologic surgery, has effectively stopped the use of this technology in its current form. In fact, in response to the statement, Ethicon Endosurgery has discontinued the distribution and sales of its power morcellator and many institutions have severely or completely restricted the use of this technology. The reason for these restrictions is that the medicolegal consequences of an adverse outcome would be very difficult to defend given the current, albeit premature, recommendations of the FDA. This statement makes it difficult to defend any adverse outcome that may occur in association with the use of the laparoscopic power morcellator. Furthermore, this statement by the FDA has largely prevented the medical community at large from collecting additional useful information to allow for a data-driven determination.
Power morcellation is not without risks. In fact, we outline them in this article. However, we believe that minimally invasive surgery should be allowed to continue to advance. In that vein, here we describe a technique of dual-port contained EMM. This surgical approach is performed under direct visualization—which solves the problem of poor visualization that hinders other contained EMM techniques.
Risks of power morcellation
The potential for inadvertent spread of occult malignancy is not the only risk of open EMM. Reports of disseminated leiomyomatosis, adenomyosis, and endometriosis also have been described from inadvertent tissue dispersion during open EMM with resulting ectopic reperitonealization.10–12
The procedure itself is not without risks. A recent systematic review documented 55 major and minor complications from EMM.13 Multiple organ systems were injured including bowel, urinary, vascular, and others, resulting in six deaths from these complications. The investigators concluded that “laparoscopic morcellator–related injuries continue to increase and short- and long-term complications are emerging in both the medical literature and device-related databases. Surgeon inexperience is descriptively identified as one of the most common contributing factors.”
All of the above risks must be weighed against the known benefits of laparoscopic surgery and presented to each patient to assist in deciding which route of surgery should be performed.
Tissue extraction options for large specimens
Large specimen extraction options during gynecologic surgery include:
Vaginal coring. Delivery through the vagina or colpotomy during vaginal or laparoscopic hysterectomy uses the technique of coring, which has long been established in our field.
Manual morcellation through a single incision. Mini-laparotomy or laparoendoscopic single-site surgery (LESS) incisions provide another option of removal with manual morcellation after laparoscopic hysterectomy or myomectomy. One study revealed that specimens up to 22 weeks in size can be placed in a large EndoCatch bag and morcellated extracorporeally by circumferentially coring with a scalpel.14
Contained power morcellation through a single port. Finally, the technique of contained EMM was recently described.15 This technique uses a large containment bag placed through a LESS incision with EMM being performed in an artificially created pneumoperitoneum. This technique isolates the specimen so that it can be morcellated without risk of exposing the patient to any malignant cells that might be unrecognized within the specimen.
Each of these techniques allows many patients to consider a minimally invasive option for their surgery. However, the ability to safely morcellate a very large uterus or myoma may be limited by visualization, and the experience of the surgeon is often critical in the successful performance of these procedures.16
Therefore, at Washington Universitywe have developed a technique using dual ports, with isolation of the uterus or myomas to improve visualization and prevent spillage of malignant tumor or dispersion of other benign tissue.
Dual-port EMM: Technique, tips, and tricks
Our technique of dual-port contained EMM allows the removal of large fibroids or uteri much larger than 20 weeks in size safely under direct visualization through a 15-mm incision. The technique uses:
- Karl Storz Rotocut tissue morcellator with spacers (FIGURE 1)
- 15-mm trocar
- 5-mm balloon trocar
- 20320-inch containment bag (FIGURE 2).
Containment bag placement
Once the specimen is free, we place it to the right or left side of the abdomen. The 15-mm trocar is placed through the umbilicus while visualizing from a lateral trocar site. We then fan-fold the containment bag and introduce it through the 15-mm trocar, keeping the bag oriented with the opening anterior (FIGURE 3). The bag is then grasped at the opening along the drawstring with an atraumatic grasper.
Tip: Care must be taken when introducing the bag in order to avoid tearing or making a small hole in it.
The leading edge is then introduced into the deepest part of the pelvis, and the remainder of the bag (left outside of the abdomen) is then fed cephalad into the abdomen.
Once the bag is completely in the abdomen, we orient the bag with the opening as wide as possible. This allows placement of a very large specimen. Once the specimen is within the containment bag, the drawstring is pulled tight and the mouth of the bag is removed through the 15-mm trocar site at the umbilicus.
The abdominal lateral gas port is opened to allow the intra-abdominal pneumoperitoneum to escape. A 5-mm trocar is placed into the bag through the opening at the umbilicus and the containment bag is insufflated with carbon dioxide and the insufflation pressure is set to 30 mm. The laparoscope placed through this trocar allows the artificial pneumoperitoneum being created to be observed (VIDEO).

Tip: The containment bag covers the entire abdominal cavity and should be fully distended. If it does not distend fully, a hole in the bag may be present and the bag must be replaced.
At this point, we place a balloon trocar at the lateral trocar site and into the bag under direct visualization. The balloon tip is inflated and pulled up tightly against the bag and abdominal wall (FIGURE 4). This allows a tight seal so there is no gas leak or spillage of the morcellated specimen. The laparoscope is placed through this trocar and the insufflation tubing is moved to this port.
Morcellator insertion
The morcellator is introduced through the umbilicus under direct visualization using the short morcellator blade in most instances. Spacers are used to set the length of the morcellator within the containment bag. The tip of the morcellator should be approximately 3 cm to 4 cm within the bag but well away from the retroperitoneum. Remember, any bag will be cut easily by the morcellator and should be thought of as peritoneum only and not a tough barrier. Serious injuries could otherwise develop.
At this point, place the patient flat or out of Trendelenburg position. Morcellation may now proceed.
Tip: Morcellation is best performed with the morcellator perpendicular to the abdomen under direct visualization using a 30° laparoscope to optimize the view. Morcellation in this position uses gravity to facilitate “peeling” of the specimen during morcellation and allows for faster removal.
Before removing the morcellator, inspect the containment bag for any large pieces that may have been dispersed during the morcellation process and remove them. Once there are only small fragments remaining, remove the morcellator, allowing the carbon dioxide to escape. Deflate the balloon tip on the trocar.
Now the containment bag with the remaining specimen may be removed through the umbilicus, while simultaneously removing the balloon-tip trocar from the bag.
A safe minimally invasive approach
This technique has allowed us to safely remove specimens larger than 1,500 g while keeping them in a contained environment with no spill of tissue within the abdomen.
Tracking and adaptation needed
The FDA safety communication has severely limited the practice of morcellation in the minimally invasive gynecologic surgical setting. Many hospitals around the country have reacted by placing significant restrictions on the use of EMM or banned it outright. This action may reverse the national trend of increasing rates of laparoscopic hysterectomy and force many practitioners to return to open surgery.
Currently, it is unclear what the true risk of tissue extraction is whether it is performed via EMM or manually. Large national databases including the BOLD database from the Surgical Review Corporation, as well as AAGL, must be utilized to track these cases and their outcomes to guide therapy. In the meantime, in order to continue to offer a minimally invasive approach to gynecologic surgery, new techniques and instrumentation in the operating room will need to be modified to adapt to these new guidelines. This is vital to maintain or even reduce the rates of open hysterectomy and associated morbidity while diminishing the potential risks of inadvertent benign as well as malignant tissue dispersion with tissue extraction.
Share your thoughts on this article! Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677. doi:10.1002/14651858.CD003677.pub4.
2. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
3. Wiser A, Holcroft CA, Tolandi T, Abenhaim HA. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10(2):117–122.
4. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
5. Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198(1):34.e1–e7.
6. U S Food and Drug Administration. Quantitative assessment of the prevalence of unsuspected uterine sarcoma in women undergoing treatment of uterine fibroids: Summary and key findings. Silver Spring, Maryland: FDA. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM393589.pdf. Published April 17, 2014. Accessed August 19, 2014.
7. Society of Gynecologic Oncology (SGO). SGO Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation. Published December 2013. Accessed March 1, 2014.
8. AAGL. Member Update: Disseminated leiomyosarcoma with power morcellation (Update #2). https://www.aagl.org/aaglnews/member-update-disseminated-leiomyosarcoma-with-power-morcellation-update-2/. Published July 11, 2014. Accessed August 19, 2014.
9. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery. http://www.acog.org/Resources_And_Publications/Task_Force_and_Work_Group_Reports/Power_Morcellation_and_Occult_Malignancy_in_Gynecologic_Surgery. Published May 2014. Accessed August 19, 2014.
10. Sepilian V, Della Badia C. Iatrogenic endometriosis caused by uterine morcellation during a supracervical hysterectomy. Obstet Gynecol. 2003;102(5 Pt 2):1125–1127.
11. Takeda A, Mori M, Sakai K, Mitsui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: report of a case and review of the literature. J Minim Invasive Gynecol. 2007;14(6):770–775.
12. Donnez O, Squifflet J, Leconte I, Jadoul P, Donnez J. Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J Minim Invasive Gynecol. 2007;14(2):156–160.
13. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21(3):486–491.
14. Serur E, Lakhi N. Laparoscopic hysterectomy with manual morcellation of the uterus: an original technique that permits the safe and quick removal of a large uterus. Am J. Obstet Gynecol. 2011;204(6):566.e1–e2.
15. Shibley KA. Feasibilty of intra-abdominal tissue isolation and extraction with an artificially created pneumoperitoneum, at laparoscopy for gynecologic procedures. J Min Invasive Gynecol. 2012;19(6):S75.
Minimally invasive surgery utilizing laparoscopy for hysterectomy and myomectomy has become more common in women with gynecologic pathology. The benefits of this approach compared with laparotomy include decreased hospital stay, shorter recovery and, in experienced hands, significantly decreased morbidity.1–3
Approximately 600,000 hysterectomies are performed annually in the United States—30% of which are performed laparoscopically.4 The primary indication for surgical intervention is uterine leiomyoma. This pathology accounts for 40% of procedures.5 During these surgeries, electromechanical morcellation (EMM), or open “power” morcellation, is commonly used to cut large tissue specimens into small pieces for removal and thereby avoid a larger incision. Concerns have been raised regarding the use of open power morcellation because of the risk of spreading an unrecognized malignancy.
Based on case reports and retrospective studies, the FDA issued a statement in April of this year discouraging the use of EMM for hysterectomy and myomectomy in women with uterine fibroids.6 The concern for inadvertent spread of an occult malignancy was the reasoning for the communication. Since that time, the FDA’s Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee held a public meeting in which the panel heard comments from patients, societies, and industry regarding their positions on the safety of laparoscopic power morcellation. The panel made several recommendations to the FDA but, at the time of this writing, the FDA has yet to issue a final decision.
Reaction to FDA’s action/inaction
The FDA’s “safety” communication was in response to the concern of a few who experienced a bad outcome believed to be secondary to open power morcellation of enlarged uteri or fibroid tumors. In its statement, the FDA estimated the risk of an occult sarcoma to be about 1 in 350 and stated that the risk of disseminating a sarcoma with morcellation is substantial. The FDA discouraged the use of the power morcellator during hysterectomy or myomectomy for uterine fibroids.
Many organizations, including the Society of Gynecologic Oncology, The American Association of Gynecologic Laparoscopists (AAGL), and the American College of Obstetricians and Gynecologists, issued less stringent statements regarding this technology.7–9 These organizations stated generally that there were too few data to make a statement at that time, advocated the collection of more data, and encouraged detailed informed consent to be given to patients undergoing these procedures.
However, the FDA’s statement, and lack of a timely follow-up to clarify the role of the laparoscopic power morcellator in gynecologic surgery, has effectively stopped the use of this technology in its current form. In fact, in response to the statement, Ethicon Endosurgery has discontinued the distribution and sales of its power morcellator and many institutions have severely or completely restricted the use of this technology. The reason for these restrictions is that the medicolegal consequences of an adverse outcome would be very difficult to defend given the current, albeit premature, recommendations of the FDA. This statement makes it difficult to defend any adverse outcome that may occur in association with the use of the laparoscopic power morcellator. Furthermore, this statement by the FDA has largely prevented the medical community at large from collecting additional useful information to allow for a data-driven determination.
Power morcellation is not without risks. In fact, we outline them in this article. However, we believe that minimally invasive surgery should be allowed to continue to advance. In that vein, here we describe a technique of dual-port contained EMM. This surgical approach is performed under direct visualization—which solves the problem of poor visualization that hinders other contained EMM techniques.
Risks of power morcellation
The potential for inadvertent spread of occult malignancy is not the only risk of open EMM. Reports of disseminated leiomyomatosis, adenomyosis, and endometriosis also have been described from inadvertent tissue dispersion during open EMM with resulting ectopic reperitonealization.10–12
The procedure itself is not without risks. A recent systematic review documented 55 major and minor complications from EMM.13 Multiple organ systems were injured including bowel, urinary, vascular, and others, resulting in six deaths from these complications. The investigators concluded that “laparoscopic morcellator–related injuries continue to increase and short- and long-term complications are emerging in both the medical literature and device-related databases. Surgeon inexperience is descriptively identified as one of the most common contributing factors.”
All of the above risks must be weighed against the known benefits of laparoscopic surgery and presented to each patient to assist in deciding which route of surgery should be performed.
Tissue extraction options for large specimens
Large specimen extraction options during gynecologic surgery include:
Vaginal coring. Delivery through the vagina or colpotomy during vaginal or laparoscopic hysterectomy uses the technique of coring, which has long been established in our field.
Manual morcellation through a single incision. Mini-laparotomy or laparoendoscopic single-site surgery (LESS) incisions provide another option of removal with manual morcellation after laparoscopic hysterectomy or myomectomy. One study revealed that specimens up to 22 weeks in size can be placed in a large EndoCatch bag and morcellated extracorporeally by circumferentially coring with a scalpel.14
Contained power morcellation through a single port. Finally, the technique of contained EMM was recently described.15 This technique uses a large containment bag placed through a LESS incision with EMM being performed in an artificially created pneumoperitoneum. This technique isolates the specimen so that it can be morcellated without risk of exposing the patient to any malignant cells that might be unrecognized within the specimen.
Each of these techniques allows many patients to consider a minimally invasive option for their surgery. However, the ability to safely morcellate a very large uterus or myoma may be limited by visualization, and the experience of the surgeon is often critical in the successful performance of these procedures.16
Therefore, at Washington Universitywe have developed a technique using dual ports, with isolation of the uterus or myomas to improve visualization and prevent spillage of malignant tumor or dispersion of other benign tissue.
Dual-port EMM: Technique, tips, and tricks
Our technique of dual-port contained EMM allows the removal of large fibroids or uteri much larger than 20 weeks in size safely under direct visualization through a 15-mm incision. The technique uses:
- Karl Storz Rotocut tissue morcellator with spacers (FIGURE 1)
- 15-mm trocar
- 5-mm balloon trocar
- 20320-inch containment bag (FIGURE 2).
Containment bag placement
Once the specimen is free, we place it to the right or left side of the abdomen. The 15-mm trocar is placed through the umbilicus while visualizing from a lateral trocar site. We then fan-fold the containment bag and introduce it through the 15-mm trocar, keeping the bag oriented with the opening anterior (FIGURE 3). The bag is then grasped at the opening along the drawstring with an atraumatic grasper.
Tip: Care must be taken when introducing the bag in order to avoid tearing or making a small hole in it.
The leading edge is then introduced into the deepest part of the pelvis, and the remainder of the bag (left outside of the abdomen) is then fed cephalad into the abdomen.
Once the bag is completely in the abdomen, we orient the bag with the opening as wide as possible. This allows placement of a very large specimen. Once the specimen is within the containment bag, the drawstring is pulled tight and the mouth of the bag is removed through the 15-mm trocar site at the umbilicus.
The abdominal lateral gas port is opened to allow the intra-abdominal pneumoperitoneum to escape. A 5-mm trocar is placed into the bag through the opening at the umbilicus and the containment bag is insufflated with carbon dioxide and the insufflation pressure is set to 30 mm. The laparoscope placed through this trocar allows the artificial pneumoperitoneum being created to be observed (VIDEO).

Tip: The containment bag covers the entire abdominal cavity and should be fully distended. If it does not distend fully, a hole in the bag may be present and the bag must be replaced.
At this point, we place a balloon trocar at the lateral trocar site and into the bag under direct visualization. The balloon tip is inflated and pulled up tightly against the bag and abdominal wall (FIGURE 4). This allows a tight seal so there is no gas leak or spillage of the morcellated specimen. The laparoscope is placed through this trocar and the insufflation tubing is moved to this port.
Morcellator insertion
The morcellator is introduced through the umbilicus under direct visualization using the short morcellator blade in most instances. Spacers are used to set the length of the morcellator within the containment bag. The tip of the morcellator should be approximately 3 cm to 4 cm within the bag but well away from the retroperitoneum. Remember, any bag will be cut easily by the morcellator and should be thought of as peritoneum only and not a tough barrier. Serious injuries could otherwise develop.
At this point, place the patient flat or out of Trendelenburg position. Morcellation may now proceed.
Tip: Morcellation is best performed with the morcellator perpendicular to the abdomen under direct visualization using a 30° laparoscope to optimize the view. Morcellation in this position uses gravity to facilitate “peeling” of the specimen during morcellation and allows for faster removal.
Before removing the morcellator, inspect the containment bag for any large pieces that may have been dispersed during the morcellation process and remove them. Once there are only small fragments remaining, remove the morcellator, allowing the carbon dioxide to escape. Deflate the balloon tip on the trocar.
Now the containment bag with the remaining specimen may be removed through the umbilicus, while simultaneously removing the balloon-tip trocar from the bag.
A safe minimally invasive approach
This technique has allowed us to safely remove specimens larger than 1,500 g while keeping them in a contained environment with no spill of tissue within the abdomen.
Tracking and adaptation needed
The FDA safety communication has severely limited the practice of morcellation in the minimally invasive gynecologic surgical setting. Many hospitals around the country have reacted by placing significant restrictions on the use of EMM or banned it outright. This action may reverse the national trend of increasing rates of laparoscopic hysterectomy and force many practitioners to return to open surgery.
Currently, it is unclear what the true risk of tissue extraction is whether it is performed via EMM or manually. Large national databases including the BOLD database from the Surgical Review Corporation, as well as AAGL, must be utilized to track these cases and their outcomes to guide therapy. In the meantime, in order to continue to offer a minimally invasive approach to gynecologic surgery, new techniques and instrumentation in the operating room will need to be modified to adapt to these new guidelines. This is vital to maintain or even reduce the rates of open hysterectomy and associated morbidity while diminishing the potential risks of inadvertent benign as well as malignant tissue dispersion with tissue extraction.
Share your thoughts on this article! Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
Minimally invasive surgery utilizing laparoscopy for hysterectomy and myomectomy has become more common in women with gynecologic pathology. The benefits of this approach compared with laparotomy include decreased hospital stay, shorter recovery and, in experienced hands, significantly decreased morbidity.1–3
Approximately 600,000 hysterectomies are performed annually in the United States—30% of which are performed laparoscopically.4 The primary indication for surgical intervention is uterine leiomyoma. This pathology accounts for 40% of procedures.5 During these surgeries, electromechanical morcellation (EMM), or open “power” morcellation, is commonly used to cut large tissue specimens into small pieces for removal and thereby avoid a larger incision. Concerns have been raised regarding the use of open power morcellation because of the risk of spreading an unrecognized malignancy.
Based on case reports and retrospective studies, the FDA issued a statement in April of this year discouraging the use of EMM for hysterectomy and myomectomy in women with uterine fibroids.6 The concern for inadvertent spread of an occult malignancy was the reasoning for the communication. Since that time, the FDA’s Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee held a public meeting in which the panel heard comments from patients, societies, and industry regarding their positions on the safety of laparoscopic power morcellation. The panel made several recommendations to the FDA but, at the time of this writing, the FDA has yet to issue a final decision.
Reaction to FDA’s action/inaction
The FDA’s “safety” communication was in response to the concern of a few who experienced a bad outcome believed to be secondary to open power morcellation of enlarged uteri or fibroid tumors. In its statement, the FDA estimated the risk of an occult sarcoma to be about 1 in 350 and stated that the risk of disseminating a sarcoma with morcellation is substantial. The FDA discouraged the use of the power morcellator during hysterectomy or myomectomy for uterine fibroids.
Many organizations, including the Society of Gynecologic Oncology, The American Association of Gynecologic Laparoscopists (AAGL), and the American College of Obstetricians and Gynecologists, issued less stringent statements regarding this technology.7–9 These organizations stated generally that there were too few data to make a statement at that time, advocated the collection of more data, and encouraged detailed informed consent to be given to patients undergoing these procedures.
However, the FDA’s statement, and lack of a timely follow-up to clarify the role of the laparoscopic power morcellator in gynecologic surgery, has effectively stopped the use of this technology in its current form. In fact, in response to the statement, Ethicon Endosurgery has discontinued the distribution and sales of its power morcellator and many institutions have severely or completely restricted the use of this technology. The reason for these restrictions is that the medicolegal consequences of an adverse outcome would be very difficult to defend given the current, albeit premature, recommendations of the FDA. This statement makes it difficult to defend any adverse outcome that may occur in association with the use of the laparoscopic power morcellator. Furthermore, this statement by the FDA has largely prevented the medical community at large from collecting additional useful information to allow for a data-driven determination.
Power morcellation is not without risks. In fact, we outline them in this article. However, we believe that minimally invasive surgery should be allowed to continue to advance. In that vein, here we describe a technique of dual-port contained EMM. This surgical approach is performed under direct visualization—which solves the problem of poor visualization that hinders other contained EMM techniques.
Risks of power morcellation
The potential for inadvertent spread of occult malignancy is not the only risk of open EMM. Reports of disseminated leiomyomatosis, adenomyosis, and endometriosis also have been described from inadvertent tissue dispersion during open EMM with resulting ectopic reperitonealization.10–12
The procedure itself is not without risks. A recent systematic review documented 55 major and minor complications from EMM.13 Multiple organ systems were injured including bowel, urinary, vascular, and others, resulting in six deaths from these complications. The investigators concluded that “laparoscopic morcellator–related injuries continue to increase and short- and long-term complications are emerging in both the medical literature and device-related databases. Surgeon inexperience is descriptively identified as one of the most common contributing factors.”
All of the above risks must be weighed against the known benefits of laparoscopic surgery and presented to each patient to assist in deciding which route of surgery should be performed.
Tissue extraction options for large specimens
Large specimen extraction options during gynecologic surgery include:
Vaginal coring. Delivery through the vagina or colpotomy during vaginal or laparoscopic hysterectomy uses the technique of coring, which has long been established in our field.
Manual morcellation through a single incision. Mini-laparotomy or laparoendoscopic single-site surgery (LESS) incisions provide another option of removal with manual morcellation after laparoscopic hysterectomy or myomectomy. One study revealed that specimens up to 22 weeks in size can be placed in a large EndoCatch bag and morcellated extracorporeally by circumferentially coring with a scalpel.14
Contained power morcellation through a single port. Finally, the technique of contained EMM was recently described.15 This technique uses a large containment bag placed through a LESS incision with EMM being performed in an artificially created pneumoperitoneum. This technique isolates the specimen so that it can be morcellated without risk of exposing the patient to any malignant cells that might be unrecognized within the specimen.
Each of these techniques allows many patients to consider a minimally invasive option for their surgery. However, the ability to safely morcellate a very large uterus or myoma may be limited by visualization, and the experience of the surgeon is often critical in the successful performance of these procedures.16
Therefore, at Washington Universitywe have developed a technique using dual ports, with isolation of the uterus or myomas to improve visualization and prevent spillage of malignant tumor or dispersion of other benign tissue.
Dual-port EMM: Technique, tips, and tricks
Our technique of dual-port contained EMM allows the removal of large fibroids or uteri much larger than 20 weeks in size safely under direct visualization through a 15-mm incision. The technique uses:
- Karl Storz Rotocut tissue morcellator with spacers (FIGURE 1)
- 15-mm trocar
- 5-mm balloon trocar
- 20320-inch containment bag (FIGURE 2).
Containment bag placement
Once the specimen is free, we place it to the right or left side of the abdomen. The 15-mm trocar is placed through the umbilicus while visualizing from a lateral trocar site. We then fan-fold the containment bag and introduce it through the 15-mm trocar, keeping the bag oriented with the opening anterior (FIGURE 3). The bag is then grasped at the opening along the drawstring with an atraumatic grasper.
Tip: Care must be taken when introducing the bag in order to avoid tearing or making a small hole in it.
The leading edge is then introduced into the deepest part of the pelvis, and the remainder of the bag (left outside of the abdomen) is then fed cephalad into the abdomen.
Once the bag is completely in the abdomen, we orient the bag with the opening as wide as possible. This allows placement of a very large specimen. Once the specimen is within the containment bag, the drawstring is pulled tight and the mouth of the bag is removed through the 15-mm trocar site at the umbilicus.
The abdominal lateral gas port is opened to allow the intra-abdominal pneumoperitoneum to escape. A 5-mm trocar is placed into the bag through the opening at the umbilicus and the containment bag is insufflated with carbon dioxide and the insufflation pressure is set to 30 mm. The laparoscope placed through this trocar allows the artificial pneumoperitoneum being created to be observed (VIDEO).

Tip: The containment bag covers the entire abdominal cavity and should be fully distended. If it does not distend fully, a hole in the bag may be present and the bag must be replaced.
At this point, we place a balloon trocar at the lateral trocar site and into the bag under direct visualization. The balloon tip is inflated and pulled up tightly against the bag and abdominal wall (FIGURE 4). This allows a tight seal so there is no gas leak or spillage of the morcellated specimen. The laparoscope is placed through this trocar and the insufflation tubing is moved to this port.
Morcellator insertion
The morcellator is introduced through the umbilicus under direct visualization using the short morcellator blade in most instances. Spacers are used to set the length of the morcellator within the containment bag. The tip of the morcellator should be approximately 3 cm to 4 cm within the bag but well away from the retroperitoneum. Remember, any bag will be cut easily by the morcellator and should be thought of as peritoneum only and not a tough barrier. Serious injuries could otherwise develop.
At this point, place the patient flat or out of Trendelenburg position. Morcellation may now proceed.
Tip: Morcellation is best performed with the morcellator perpendicular to the abdomen under direct visualization using a 30° laparoscope to optimize the view. Morcellation in this position uses gravity to facilitate “peeling” of the specimen during morcellation and allows for faster removal.
Before removing the morcellator, inspect the containment bag for any large pieces that may have been dispersed during the morcellation process and remove them. Once there are only small fragments remaining, remove the morcellator, allowing the carbon dioxide to escape. Deflate the balloon tip on the trocar.
Now the containment bag with the remaining specimen may be removed through the umbilicus, while simultaneously removing the balloon-tip trocar from the bag.
A safe minimally invasive approach
This technique has allowed us to safely remove specimens larger than 1,500 g while keeping them in a contained environment with no spill of tissue within the abdomen.
Tracking and adaptation needed
The FDA safety communication has severely limited the practice of morcellation in the minimally invasive gynecologic surgical setting. Many hospitals around the country have reacted by placing significant restrictions on the use of EMM or banned it outright. This action may reverse the national trend of increasing rates of laparoscopic hysterectomy and force many practitioners to return to open surgery.
Currently, it is unclear what the true risk of tissue extraction is whether it is performed via EMM or manually. Large national databases including the BOLD database from the Surgical Review Corporation, as well as AAGL, must be utilized to track these cases and their outcomes to guide therapy. In the meantime, in order to continue to offer a minimally invasive approach to gynecologic surgery, new techniques and instrumentation in the operating room will need to be modified to adapt to these new guidelines. This is vital to maintain or even reduce the rates of open hysterectomy and associated morbidity while diminishing the potential risks of inadvertent benign as well as malignant tissue dispersion with tissue extraction.
Share your thoughts on this article! Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677. doi:10.1002/14651858.CD003677.pub4.
2. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
3. Wiser A, Holcroft CA, Tolandi T, Abenhaim HA. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10(2):117–122.
4. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
5. Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198(1):34.e1–e7.
6. U S Food and Drug Administration. Quantitative assessment of the prevalence of unsuspected uterine sarcoma in women undergoing treatment of uterine fibroids: Summary and key findings. Silver Spring, Maryland: FDA. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM393589.pdf. Published April 17, 2014. Accessed August 19, 2014.
7. Society of Gynecologic Oncology (SGO). SGO Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation. Published December 2013. Accessed March 1, 2014.
8. AAGL. Member Update: Disseminated leiomyosarcoma with power morcellation (Update #2). https://www.aagl.org/aaglnews/member-update-disseminated-leiomyosarcoma-with-power-morcellation-update-2/. Published July 11, 2014. Accessed August 19, 2014.
9. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery. http://www.acog.org/Resources_And_Publications/Task_Force_and_Work_Group_Reports/Power_Morcellation_and_Occult_Malignancy_in_Gynecologic_Surgery. Published May 2014. Accessed August 19, 2014.
10. Sepilian V, Della Badia C. Iatrogenic endometriosis caused by uterine morcellation during a supracervical hysterectomy. Obstet Gynecol. 2003;102(5 Pt 2):1125–1127.
11. Takeda A, Mori M, Sakai K, Mitsui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: report of a case and review of the literature. J Minim Invasive Gynecol. 2007;14(6):770–775.
12. Donnez O, Squifflet J, Leconte I, Jadoul P, Donnez J. Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J Minim Invasive Gynecol. 2007;14(2):156–160.
13. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21(3):486–491.
14. Serur E, Lakhi N. Laparoscopic hysterectomy with manual morcellation of the uterus: an original technique that permits the safe and quick removal of a large uterus. Am J. Obstet Gynecol. 2011;204(6):566.e1–e2.
15. Shibley KA. Feasibilty of intra-abdominal tissue isolation and extraction with an artificially created pneumoperitoneum, at laparoscopy for gynecologic procedures. J Min Invasive Gynecol. 2012;19(6):S75.
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677. doi:10.1002/14651858.CD003677.pub4.
2. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
3. Wiser A, Holcroft CA, Tolandi T, Abenhaim HA. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10(2):117–122.
4. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698.
5. Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198(1):34.e1–e7.
6. U S Food and Drug Administration. Quantitative assessment of the prevalence of unsuspected uterine sarcoma in women undergoing treatment of uterine fibroids: Summary and key findings. Silver Spring, Maryland: FDA. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM393589.pdf. Published April 17, 2014. Accessed August 19, 2014.
7. Society of Gynecologic Oncology (SGO). SGO Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation. Published December 2013. Accessed March 1, 2014.
8. AAGL. Member Update: Disseminated leiomyosarcoma with power morcellation (Update #2). https://www.aagl.org/aaglnews/member-update-disseminated-leiomyosarcoma-with-power-morcellation-update-2/. Published July 11, 2014. Accessed August 19, 2014.
9. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery. http://www.acog.org/Resources_And_Publications/Task_Force_and_Work_Group_Reports/Power_Morcellation_and_Occult_Malignancy_in_Gynecologic_Surgery. Published May 2014. Accessed August 19, 2014.
10. Sepilian V, Della Badia C. Iatrogenic endometriosis caused by uterine morcellation during a supracervical hysterectomy. Obstet Gynecol. 2003;102(5 Pt 2):1125–1127.
11. Takeda A, Mori M, Sakai K, Mitsui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: report of a case and review of the literature. J Minim Invasive Gynecol. 2007;14(6):770–775.
12. Donnez O, Squifflet J, Leconte I, Jadoul P, Donnez J. Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J Minim Invasive Gynecol. 2007;14(2):156–160.
13. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21(3):486–491.
14. Serur E, Lakhi N. Laparoscopic hysterectomy with manual morcellation of the uterus: an original technique that permits the safe and quick removal of a large uterus. Am J. Obstet Gynecol. 2011;204(6):566.e1–e2.
15. Shibley KA. Feasibilty of intra-abdominal tissue isolation and extraction with an artificially created pneumoperitoneum, at laparoscopy for gynecologic procedures. J Min Invasive Gynecol. 2012;19(6):S75.