User login
Myth busting: SARS-CoV-2 vaccine
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
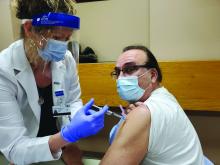
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
The lost year – even for common respiratory viruses
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.
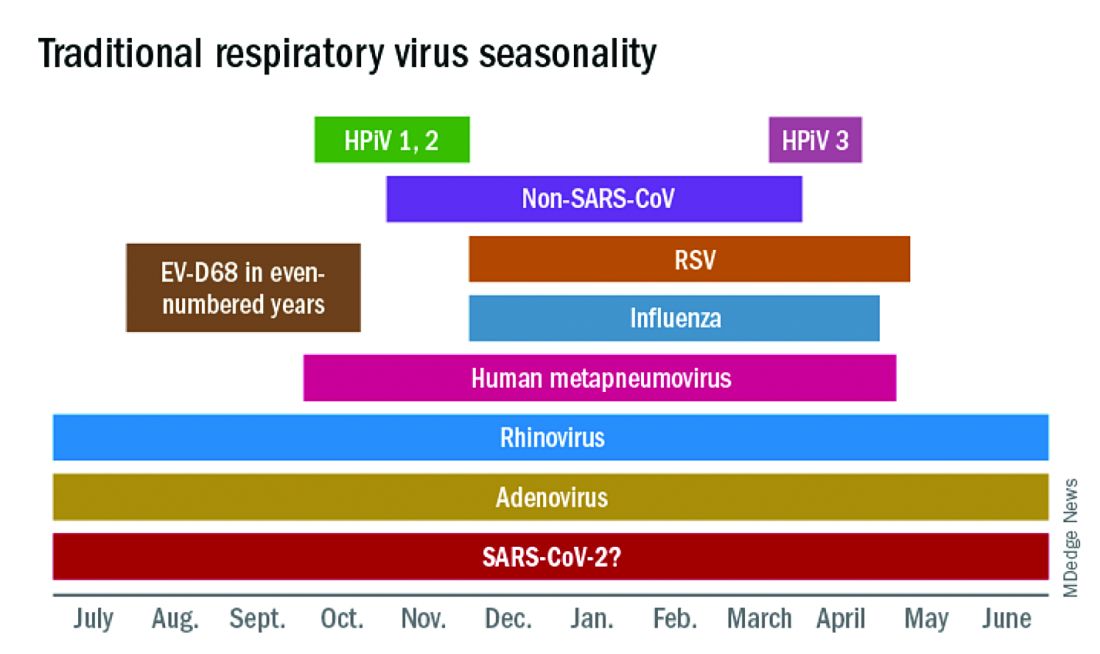
The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).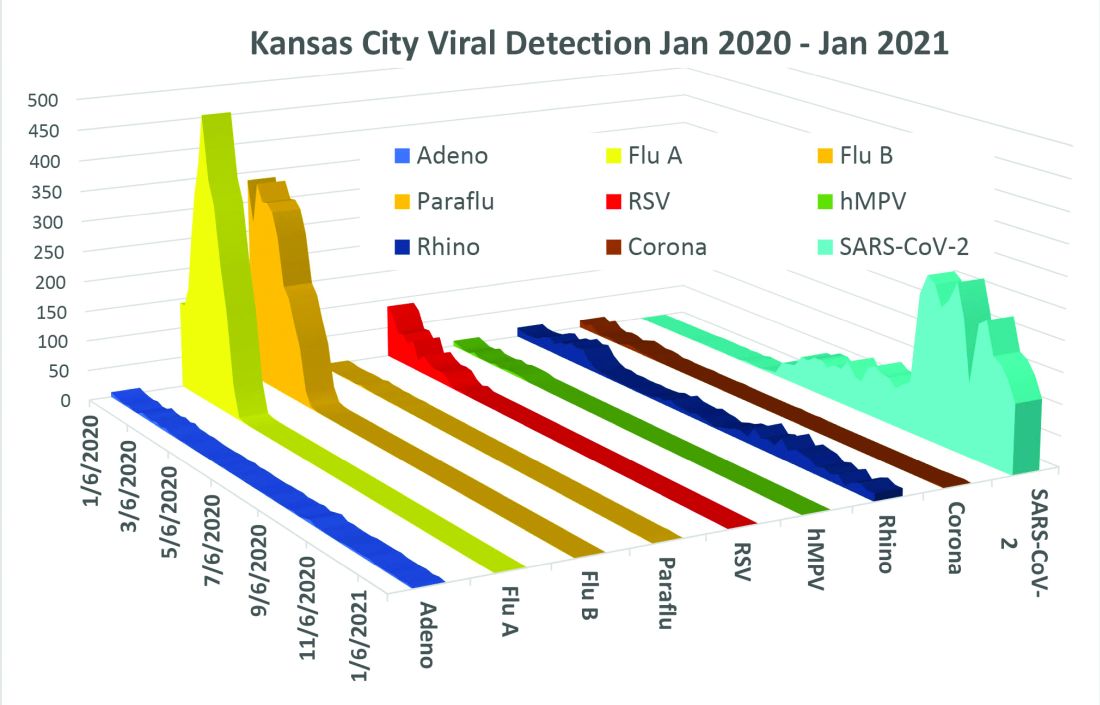
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
Waiting for the COVID 19 vaccine, or not?
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 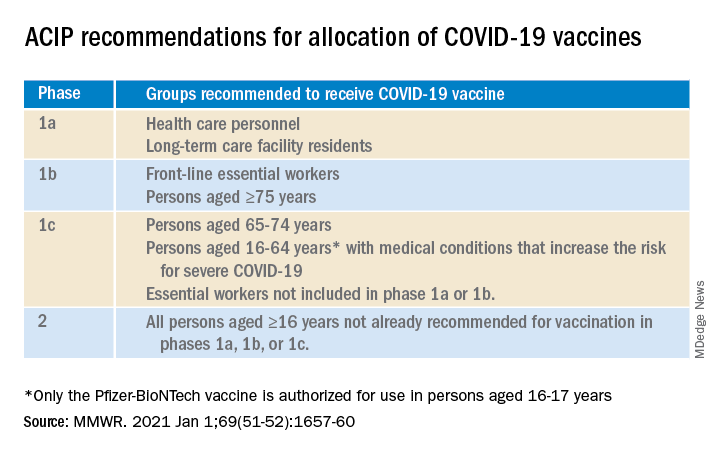
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Understanding messenger RNA and other SARS-CoV-2 vaccines
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at pdnews@mdedge.com.
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at pdnews@mdedge.com.
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at pdnews@mdedge.com.
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
Should our patients really go home for the holidays?
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Direct-acting agents cure hepatitis C in children
Between 23,000 and 46,000 U.S. children live with chronic hepatitis C virus with a prevalence of 0.17% anti–hepatitis C virus (HCV) antibody positivity in those aged 6-11 years and 0.39% among children aged 12-19 years. In the United States, genotype 1 is most frequent, followed by genotypes 2 and 3. About 99% of cases result from vertical transmission; transfusion-related cases have not been observed in recent decades.Only viremic mothers are at risk of transmission as those who have spontaneously cleared HCV viremia or have been treated successfully do not risk transmission. Maternal HCV viral load appears to be a risk factor for HCV transmission, however transmission is reported at all levels of viremia.
In conjunction with the opioid epidemics, the prevalence of HCV infection has increased over the last decade. The Centers for Disease Control and Prevention reported that, between 2009 and 2014, the prevalence of HCV infection increased from 1.8 to 3.4 per 1,000 live births. They identified substantial state-to-state variation with the highest rate in West Virginia (22.6 per 1,000 live births), and the lowest in Hawaii (0.7 per 1,000 live births). The implications are clear that increasing numbers of newborns are exposed to HCV and, if transmission rates are between 1% and 5%, 80-400 U.S. infants each year acquire HCV infection.
HCV in children
HCV in children is almost always associated with persistent transaminitis. Chronic infection is defined as the persistence of HCV RNA for at least 6 months, and clearance of HCV infection is determined by the persistent disappearance of HCV RNA. Regardless of infection status, an infant may have detectable maternal anti-HCV antibody in serum until 18 months of age, resulting from passive transfer. In addition, prolonged infection can lead to cirrhosis, hepatocellular carcinoma, or decompensated liver disease. Potential extrahepatic manifestations including reduced physical and psychosocial health also are linked to chronic HCV. Autoimmune disease also has been reported in children with HCV. As well, the stigma of HCV elicits fear in school and child care settings that is a result of public misunderstanding regarding routes of hepatitis C transmission. No restriction of regular childhood activities is required in the daily life of HCV-infected children.
Taken together, increasing rates of HCV infection in pregnant women, increasing numbers of exposed and infected infants annually, potential for both short- and long-term morbidity, and curative nontoxic treatment,
Screening for HCV
There is considerable discussion about which strategy for screening of at-risk infants is more appropriate. Some groups advocate for HCV-RNA testing within the first year of life. Proponents argue the use of a highly sensitive RNA assay early in life has potential to increase detection of infected infants while a negative result allows the conclusion the infant is not infected. Advocates hypothesize that early identification has potential to improve continued follow-up.
Opponents argue that early testing does not change the need for repeat testing after 18 months to confirm diagnosis. They also argue that HCV RNA is more expensive than an antibody-based testing; and treatment will not begin prior to age 3 as there is still opportunity for viremia to spontaneously clear.
Direct acting agents licensed
Ledipasvir/sofosbuvir (Harvoni) was initially demonstrated as curative for genotype 1, 4, 5, or 6 infection in a phase 2, multicenter, open-label study of 100 adolescents with genotype 1 treated for 12 weeks. Sustained virologic response (SVR) was documented in 98% of participants.The regimen was safe and well tolerated in this population, and the adult dosage formulation resulted in pharmacokinetic characteristics similar to those observed in adults. Two clinical trials supported the efficacy of ledipasvir/sofosbuvir in the pediatric population aged 3-11 years. This regimen also is recommended for interferon-experienced (± ribavirin, with or without an HCV protease inhibitor) children and adolescents aged 3 years or older with genotype 1 or 4. A 12-week course is recommended for patients without cirrhosis; 24 weeks is recommended for those with compensated cirrhosis. The combination of ledipasvir/sofosbuvir is the only treatment option for children aged 3-6 years with genotype 1, 4, 5, or 6 infection.
The efficacy of sofosbuvir/velpatasvir (Epclusa) once daily for 12 weeks was first evaluated in an open-label trial among children aged 6 years and older with genotype 1, 2, 3, 4, or 6 infection, without cirrhosis or with compensated cirrhosis. Subsequently, the “cocktail” was evaluated in children aged 6-12 years, with 76% genotype 1, 3% genotype 2, 15% genotype 3, and 6% genotype 4. SVR12 rates were 93% (50/54) in children with genotype 1, 91% (10/11) in those with genotype 3, and 100% in participants with genotype 2 (2/2) or genotype 4 (4/4). Sofosbuvir/velpatasvir was approved in March 2020 by the Food and Drug Administration for pediatric patients aged 6 years and older. Given its pangenotypic activity, safety, and efficacy, sofosbuvir/velpatasvir is currently recommended as a first choice for HCV treatment in children and adolescents aged at least 6 years.
The daily fixed-dose combination of glecaprevir/pibrentasvir (Mavyret) was approved in April 2019 for adolescents aged 12-17 years, and weighing at least 45 kg.Treatment is for 8 weeks, and includes treatment-naive patients without cirrhosis or those with compensated cirrhosis. SVR12 rates for Mavyret have ranged from 91% to 100 % across clinic trials. FDA approval and HCV guideline treatment recommendations for direct-acting antiviral (DAA)–experienced adolescents are based on clinical trial data from adults. Given its pangenotypic activity, safety, and efficacy record in adult patients, glecaprevir/pibrentasvir is recommended as a first choice for adolescent HCV treatment. Glecaprevir/pibrentasvir once approved for children less than 3 years of age will be safe and efficacious as a pangenotypic treatment option in children with chronic HCV infection.
Current recommendations
Tools for identifying HCV infected infants as early as a few months of age are available, yet studies demonstrate that a minority of at-risk children are tested for HCV using either an HCV polymerase chain reaction strategy early in life or an anti-HCV antibody strategy after 18 months of age.
Therapy with direct-acting agents is now licensed to those aged 3 years and offers the potential for cure, eliminating concern for possible progression after prolonged infection. Such therapy offers the potential to eliminate the stigma faced by many children as well as the hepatic and extrahepatic manifestations observed in children. Medication formulation and the child’s abilities to take the medication needs to be considered when prescribing DAAs. It is important to assess if the child can successfully swallow pills. Currently, Harvoni is the only medication that comes in both pellet and pill formulations. The dose is based on weight. The pellets need to be given in a small amount of nonacidic food; they cannot be chewed.
All children with chronic HCV infection are candidates for treatment. When significant fibrosis and/or cirrhosis is present treatment should not be delayed once the child is age 3 years; when only transaminitis is present, treatment can be delayed. In our experience, parents are eager to complete treatment before starting kindergarten.
Liver biopsy for obtaining liver tissue for histopathologic examination is not routinely indicated in children with chronic HCV infection but should be evaluated case by case. Noninvasive tests of hepatic fibrosis have been used in children, these include serologic markers (i.e., FibroSure) and radiologic tests such as ultrasound-based transient elastography (i.e., Fibroscan). Validation for pediatric patients is variable for the different serologic tests. Studies have shown that Fibroscan using the M probe is feasible for a wide range of ages, but poor patient cooperation may make measurement difficult.
Further details regarding dosing and choice of formulation is available at https://www.hcvguidelines.org/unique-populations/children.
Dr. Sabharwal is assistant professor of pediatrics at Boston University and attending physician in pediatric infectious diseases at Boston Medical Center. Ms. Moloney is an instructor in pediatrics at Boston University and a pediatric nurse practitioner in pediatric infectious diseases at Boston Medicine Center. Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. Boston Medical Center received funding from AbbVie for study of Harvoni in Children 3 years of age and older. Email them at pdnews@mdedge.com.
References
MMWR Morb Mortal Wkly Rep. 2017 May 12;66(18):470-3. Hepatol Commun. 2017 March 23. doi: 10.1002/hep4.1028. Hepatology. 2020 Feb;71(2):422-30. Lancet Gastroenterol Hepatol. 2019 Apr 11. doi: 10.1016/S2468-1253(19)30046-9. Arch Dis Child. 2006 Sep;91(9):781-5. J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):123-31.
Between 23,000 and 46,000 U.S. children live with chronic hepatitis C virus with a prevalence of 0.17% anti–hepatitis C virus (HCV) antibody positivity in those aged 6-11 years and 0.39% among children aged 12-19 years. In the United States, genotype 1 is most frequent, followed by genotypes 2 and 3. About 99% of cases result from vertical transmission; transfusion-related cases have not been observed in recent decades.Only viremic mothers are at risk of transmission as those who have spontaneously cleared HCV viremia or have been treated successfully do not risk transmission. Maternal HCV viral load appears to be a risk factor for HCV transmission, however transmission is reported at all levels of viremia.
In conjunction with the opioid epidemics, the prevalence of HCV infection has increased over the last decade. The Centers for Disease Control and Prevention reported that, between 2009 and 2014, the prevalence of HCV infection increased from 1.8 to 3.4 per 1,000 live births. They identified substantial state-to-state variation with the highest rate in West Virginia (22.6 per 1,000 live births), and the lowest in Hawaii (0.7 per 1,000 live births). The implications are clear that increasing numbers of newborns are exposed to HCV and, if transmission rates are between 1% and 5%, 80-400 U.S. infants each year acquire HCV infection.
HCV in children
HCV in children is almost always associated with persistent transaminitis. Chronic infection is defined as the persistence of HCV RNA for at least 6 months, and clearance of HCV infection is determined by the persistent disappearance of HCV RNA. Regardless of infection status, an infant may have detectable maternal anti-HCV antibody in serum until 18 months of age, resulting from passive transfer. In addition, prolonged infection can lead to cirrhosis, hepatocellular carcinoma, or decompensated liver disease. Potential extrahepatic manifestations including reduced physical and psychosocial health also are linked to chronic HCV. Autoimmune disease also has been reported in children with HCV. As well, the stigma of HCV elicits fear in school and child care settings that is a result of public misunderstanding regarding routes of hepatitis C transmission. No restriction of regular childhood activities is required in the daily life of HCV-infected children.
Taken together, increasing rates of HCV infection in pregnant women, increasing numbers of exposed and infected infants annually, potential for both short- and long-term morbidity, and curative nontoxic treatment,
Screening for HCV
There is considerable discussion about which strategy for screening of at-risk infants is more appropriate. Some groups advocate for HCV-RNA testing within the first year of life. Proponents argue the use of a highly sensitive RNA assay early in life has potential to increase detection of infected infants while a negative result allows the conclusion the infant is not infected. Advocates hypothesize that early identification has potential to improve continued follow-up.
Opponents argue that early testing does not change the need for repeat testing after 18 months to confirm diagnosis. They also argue that HCV RNA is more expensive than an antibody-based testing; and treatment will not begin prior to age 3 as there is still opportunity for viremia to spontaneously clear.
Direct acting agents licensed
Ledipasvir/sofosbuvir (Harvoni) was initially demonstrated as curative for genotype 1, 4, 5, or 6 infection in a phase 2, multicenter, open-label study of 100 adolescents with genotype 1 treated for 12 weeks. Sustained virologic response (SVR) was documented in 98% of participants.The regimen was safe and well tolerated in this population, and the adult dosage formulation resulted in pharmacokinetic characteristics similar to those observed in adults. Two clinical trials supported the efficacy of ledipasvir/sofosbuvir in the pediatric population aged 3-11 years. This regimen also is recommended for interferon-experienced (± ribavirin, with or without an HCV protease inhibitor) children and adolescents aged 3 years or older with genotype 1 or 4. A 12-week course is recommended for patients without cirrhosis; 24 weeks is recommended for those with compensated cirrhosis. The combination of ledipasvir/sofosbuvir is the only treatment option for children aged 3-6 years with genotype 1, 4, 5, or 6 infection.
The efficacy of sofosbuvir/velpatasvir (Epclusa) once daily for 12 weeks was first evaluated in an open-label trial among children aged 6 years and older with genotype 1, 2, 3, 4, or 6 infection, without cirrhosis or with compensated cirrhosis. Subsequently, the “cocktail” was evaluated in children aged 6-12 years, with 76% genotype 1, 3% genotype 2, 15% genotype 3, and 6% genotype 4. SVR12 rates were 93% (50/54) in children with genotype 1, 91% (10/11) in those with genotype 3, and 100% in participants with genotype 2 (2/2) or genotype 4 (4/4). Sofosbuvir/velpatasvir was approved in March 2020 by the Food and Drug Administration for pediatric patients aged 6 years and older. Given its pangenotypic activity, safety, and efficacy, sofosbuvir/velpatasvir is currently recommended as a first choice for HCV treatment in children and adolescents aged at least 6 years.
The daily fixed-dose combination of glecaprevir/pibrentasvir (Mavyret) was approved in April 2019 for adolescents aged 12-17 years, and weighing at least 45 kg.Treatment is for 8 weeks, and includes treatment-naive patients without cirrhosis or those with compensated cirrhosis. SVR12 rates for Mavyret have ranged from 91% to 100 % across clinic trials. FDA approval and HCV guideline treatment recommendations for direct-acting antiviral (DAA)–experienced adolescents are based on clinical trial data from adults. Given its pangenotypic activity, safety, and efficacy record in adult patients, glecaprevir/pibrentasvir is recommended as a first choice for adolescent HCV treatment. Glecaprevir/pibrentasvir once approved for children less than 3 years of age will be safe and efficacious as a pangenotypic treatment option in children with chronic HCV infection.
Current recommendations
Tools for identifying HCV infected infants as early as a few months of age are available, yet studies demonstrate that a minority of at-risk children are tested for HCV using either an HCV polymerase chain reaction strategy early in life or an anti-HCV antibody strategy after 18 months of age.
Therapy with direct-acting agents is now licensed to those aged 3 years and offers the potential for cure, eliminating concern for possible progression after prolonged infection. Such therapy offers the potential to eliminate the stigma faced by many children as well as the hepatic and extrahepatic manifestations observed in children. Medication formulation and the child’s abilities to take the medication needs to be considered when prescribing DAAs. It is important to assess if the child can successfully swallow pills. Currently, Harvoni is the only medication that comes in both pellet and pill formulations. The dose is based on weight. The pellets need to be given in a small amount of nonacidic food; they cannot be chewed.
All children with chronic HCV infection are candidates for treatment. When significant fibrosis and/or cirrhosis is present treatment should not be delayed once the child is age 3 years; when only transaminitis is present, treatment can be delayed. In our experience, parents are eager to complete treatment before starting kindergarten.
Liver biopsy for obtaining liver tissue for histopathologic examination is not routinely indicated in children with chronic HCV infection but should be evaluated case by case. Noninvasive tests of hepatic fibrosis have been used in children, these include serologic markers (i.e., FibroSure) and radiologic tests such as ultrasound-based transient elastography (i.e., Fibroscan). Validation for pediatric patients is variable for the different serologic tests. Studies have shown that Fibroscan using the M probe is feasible for a wide range of ages, but poor patient cooperation may make measurement difficult.
Further details regarding dosing and choice of formulation is available at https://www.hcvguidelines.org/unique-populations/children.
Dr. Sabharwal is assistant professor of pediatrics at Boston University and attending physician in pediatric infectious diseases at Boston Medical Center. Ms. Moloney is an instructor in pediatrics at Boston University and a pediatric nurse practitioner in pediatric infectious diseases at Boston Medicine Center. Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. Boston Medical Center received funding from AbbVie for study of Harvoni in Children 3 years of age and older. Email them at pdnews@mdedge.com.
References
MMWR Morb Mortal Wkly Rep. 2017 May 12;66(18):470-3. Hepatol Commun. 2017 March 23. doi: 10.1002/hep4.1028. Hepatology. 2020 Feb;71(2):422-30. Lancet Gastroenterol Hepatol. 2019 Apr 11. doi: 10.1016/S2468-1253(19)30046-9. Arch Dis Child. 2006 Sep;91(9):781-5. J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):123-31.
Between 23,000 and 46,000 U.S. children live with chronic hepatitis C virus with a prevalence of 0.17% anti–hepatitis C virus (HCV) antibody positivity in those aged 6-11 years and 0.39% among children aged 12-19 years. In the United States, genotype 1 is most frequent, followed by genotypes 2 and 3. About 99% of cases result from vertical transmission; transfusion-related cases have not been observed in recent decades.Only viremic mothers are at risk of transmission as those who have spontaneously cleared HCV viremia or have been treated successfully do not risk transmission. Maternal HCV viral load appears to be a risk factor for HCV transmission, however transmission is reported at all levels of viremia.
In conjunction with the opioid epidemics, the prevalence of HCV infection has increased over the last decade. The Centers for Disease Control and Prevention reported that, between 2009 and 2014, the prevalence of HCV infection increased from 1.8 to 3.4 per 1,000 live births. They identified substantial state-to-state variation with the highest rate in West Virginia (22.6 per 1,000 live births), and the lowest in Hawaii (0.7 per 1,000 live births). The implications are clear that increasing numbers of newborns are exposed to HCV and, if transmission rates are between 1% and 5%, 80-400 U.S. infants each year acquire HCV infection.
HCV in children
HCV in children is almost always associated with persistent transaminitis. Chronic infection is defined as the persistence of HCV RNA for at least 6 months, and clearance of HCV infection is determined by the persistent disappearance of HCV RNA. Regardless of infection status, an infant may have detectable maternal anti-HCV antibody in serum until 18 months of age, resulting from passive transfer. In addition, prolonged infection can lead to cirrhosis, hepatocellular carcinoma, or decompensated liver disease. Potential extrahepatic manifestations including reduced physical and psychosocial health also are linked to chronic HCV. Autoimmune disease also has been reported in children with HCV. As well, the stigma of HCV elicits fear in school and child care settings that is a result of public misunderstanding regarding routes of hepatitis C transmission. No restriction of regular childhood activities is required in the daily life of HCV-infected children.
Taken together, increasing rates of HCV infection in pregnant women, increasing numbers of exposed and infected infants annually, potential for both short- and long-term morbidity, and curative nontoxic treatment,
Screening for HCV
There is considerable discussion about which strategy for screening of at-risk infants is more appropriate. Some groups advocate for HCV-RNA testing within the first year of life. Proponents argue the use of a highly sensitive RNA assay early in life has potential to increase detection of infected infants while a negative result allows the conclusion the infant is not infected. Advocates hypothesize that early identification has potential to improve continued follow-up.
Opponents argue that early testing does not change the need for repeat testing after 18 months to confirm diagnosis. They also argue that HCV RNA is more expensive than an antibody-based testing; and treatment will not begin prior to age 3 as there is still opportunity for viremia to spontaneously clear.
Direct acting agents licensed
Ledipasvir/sofosbuvir (Harvoni) was initially demonstrated as curative for genotype 1, 4, 5, or 6 infection in a phase 2, multicenter, open-label study of 100 adolescents with genotype 1 treated for 12 weeks. Sustained virologic response (SVR) was documented in 98% of participants.The regimen was safe and well tolerated in this population, and the adult dosage formulation resulted in pharmacokinetic characteristics similar to those observed in adults. Two clinical trials supported the efficacy of ledipasvir/sofosbuvir in the pediatric population aged 3-11 years. This regimen also is recommended for interferon-experienced (± ribavirin, with or without an HCV protease inhibitor) children and adolescents aged 3 years or older with genotype 1 or 4. A 12-week course is recommended for patients without cirrhosis; 24 weeks is recommended for those with compensated cirrhosis. The combination of ledipasvir/sofosbuvir is the only treatment option for children aged 3-6 years with genotype 1, 4, 5, or 6 infection.
The efficacy of sofosbuvir/velpatasvir (Epclusa) once daily for 12 weeks was first evaluated in an open-label trial among children aged 6 years and older with genotype 1, 2, 3, 4, or 6 infection, without cirrhosis or with compensated cirrhosis. Subsequently, the “cocktail” was evaluated in children aged 6-12 years, with 76% genotype 1, 3% genotype 2, 15% genotype 3, and 6% genotype 4. SVR12 rates were 93% (50/54) in children with genotype 1, 91% (10/11) in those with genotype 3, and 100% in participants with genotype 2 (2/2) or genotype 4 (4/4). Sofosbuvir/velpatasvir was approved in March 2020 by the Food and Drug Administration for pediatric patients aged 6 years and older. Given its pangenotypic activity, safety, and efficacy, sofosbuvir/velpatasvir is currently recommended as a first choice for HCV treatment in children and adolescents aged at least 6 years.
The daily fixed-dose combination of glecaprevir/pibrentasvir (Mavyret) was approved in April 2019 for adolescents aged 12-17 years, and weighing at least 45 kg.Treatment is for 8 weeks, and includes treatment-naive patients without cirrhosis or those with compensated cirrhosis. SVR12 rates for Mavyret have ranged from 91% to 100 % across clinic trials. FDA approval and HCV guideline treatment recommendations for direct-acting antiviral (DAA)–experienced adolescents are based on clinical trial data from adults. Given its pangenotypic activity, safety, and efficacy record in adult patients, glecaprevir/pibrentasvir is recommended as a first choice for adolescent HCV treatment. Glecaprevir/pibrentasvir once approved for children less than 3 years of age will be safe and efficacious as a pangenotypic treatment option in children with chronic HCV infection.
Current recommendations
Tools for identifying HCV infected infants as early as a few months of age are available, yet studies demonstrate that a minority of at-risk children are tested for HCV using either an HCV polymerase chain reaction strategy early in life or an anti-HCV antibody strategy after 18 months of age.
Therapy with direct-acting agents is now licensed to those aged 3 years and offers the potential for cure, eliminating concern for possible progression after prolonged infection. Such therapy offers the potential to eliminate the stigma faced by many children as well as the hepatic and extrahepatic manifestations observed in children. Medication formulation and the child’s abilities to take the medication needs to be considered when prescribing DAAs. It is important to assess if the child can successfully swallow pills. Currently, Harvoni is the only medication that comes in both pellet and pill formulations. The dose is based on weight. The pellets need to be given in a small amount of nonacidic food; they cannot be chewed.
All children with chronic HCV infection are candidates for treatment. When significant fibrosis and/or cirrhosis is present treatment should not be delayed once the child is age 3 years; when only transaminitis is present, treatment can be delayed. In our experience, parents are eager to complete treatment before starting kindergarten.
Liver biopsy for obtaining liver tissue for histopathologic examination is not routinely indicated in children with chronic HCV infection but should be evaluated case by case. Noninvasive tests of hepatic fibrosis have been used in children, these include serologic markers (i.e., FibroSure) and radiologic tests such as ultrasound-based transient elastography (i.e., Fibroscan). Validation for pediatric patients is variable for the different serologic tests. Studies have shown that Fibroscan using the M probe is feasible for a wide range of ages, but poor patient cooperation may make measurement difficult.
Further details regarding dosing and choice of formulation is available at https://www.hcvguidelines.org/unique-populations/children.
Dr. Sabharwal is assistant professor of pediatrics at Boston University and attending physician in pediatric infectious diseases at Boston Medical Center. Ms. Moloney is an instructor in pediatrics at Boston University and a pediatric nurse practitioner in pediatric infectious diseases at Boston Medicine Center. Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. Boston Medical Center received funding from AbbVie for study of Harvoni in Children 3 years of age and older. Email them at pdnews@mdedge.com.
References
MMWR Morb Mortal Wkly Rep. 2017 May 12;66(18):470-3. Hepatol Commun. 2017 March 23. doi: 10.1002/hep4.1028. Hepatology. 2020 Feb;71(2):422-30. Lancet Gastroenterol Hepatol. 2019 Apr 11. doi: 10.1016/S2468-1253(19)30046-9. Arch Dis Child. 2006 Sep;91(9):781-5. J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):123-31.
2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic
Respiratory virus seasons usually follow a fairly well-known pattern. Enterovirus 68 (EV-D68) is a summer-to-early fall virus with biennial peak years. Rhinovirus (HRv) and adenovirus (Adv) occur nearly year-round but may have small upticks in the first month or so that children return to school. Early in the school year, upper respiratory infections from both HRv and Adv and viral sore throats from Adv are common, with conjunctivitis from Adv outbreaks in some years. October to November is human parainfluenza (HPiV) 1 and 2 season, often presenting as croup. Human metapneumovirus infections span October through April. In late November to December, influenza begins, usually with an A type, later transitioning to a B type in February through April. Also in December, respiratory syncytial virus (RSV) starts, characteristically with bronchiolitis presentations, peaking in February to March and tapering off in May. In late March to April, HPiV 3 also appears for 4-6 weeks.

Will 2020-2021 be different?
Summer was remarkably free of expected enterovirus activity, suggesting that the seasonal parade may differ this year. Remember that the 2019-2020 respiratory season suddenly and nearly completely stopped in March because of social distancing and lockdowns needed to address the SARS-CoV-2 pandemic.
The mild influenza season in the southern hemisphere suggests that our influenza season also could be mild. But perhaps not – most southern hemisphere countries that are surveyed for influenza activities had the most intense SARS-CoV-2 mitigations, making the observed mildness potentially related more to social mitigation than less virulent influenza strains. If so, southern hemisphere influenza data may not apply to the United States, where social distancing and masks are ignored or used inconsistently by almost half the population.
Further, the stop-and-go pattern of in-person school/college attendance adds to uncertainties for the usual orderly virus-specific seasonality. The result may be multiple stop-and-go “pop-up” or “mini” outbreaks for any given virus potentially reflected as exaggerated local or regional differences in circulation of various viruses. The erratic seasonality also would increase coinfections, which could present with more severe or different symptoms.
SARS-CoV-2’s potential interaction
Will the relatively mild presentations for most children with SARS-CoV-2 hold up in the setting of coinfections or sequential respiratory viral infections? Could SARS-CoV-2 cause worse/more prolonged symptoms or more sequelae if paired simultaneously or in tandem with a traditional respiratory virus? To date, data on the frequency and severity of SARS-CoV-2 coinfections are conflicting and sparse, but it appears that non-SARS-CoV-2 viruses can be involved in 15%-50% pediatric acute respiratory infections.1,2
However, it may not be important to know about coinfecting viruses other than influenza (can be treated) or SARS-CoV-2 (needs quarantine and contact tracing), unless symptoms are atypical or more severe than usual. For example, a young child with bronchiolitis is most likely infected with RSV, but HPiV, influenza, metapneumovirus, HRv, and even SARS-CoV-2 can cause bronchiolitis. Even so, testing outpatients for RSV or non-influenza is not routine or even clinically helpful. Supportive treatment and restriction from daycare attendance are sufficient management for outpatient ARIs whether presenting as bronchiolitis or not.
Considerations for SARS-CoV-2 testing: Outpatient bronchiolitis
If a child presents with classic bronchiolitis but has above moderate to severe symptoms, is SARS-CoV-2 a consideration? Perhaps, if SARS-CoV-2 acts similarly to non-SARS-CoV-2s.
A recent report from the 30th Multicenter Airway Research Collaboration (MARC-30) surveillance study (2007-2014) of children hospitalized with clinical bronchiolitis evaluated respiratory viruses, including RSV and the four common non-SARS coronaviruses using molecular testing.3 Among 1,880 subjects, a CoV (alpha CoV: NL63 or 229E, or beta CoV: KKU1 or OC43) was detected in 12%. Yet most had only RSV (n = 1,661); 32 had only CoV (n = 32). But note that 219 had both.
Bronchiolitis subjects with CoV were older – median 3.7 (1.4-5.8) vs. 2.8 (1.9-7.2) years – and more likely male than were RSV subjects (68% vs. 58%). OC43 was most frequent followed by equal numbers of HKU1 and NL63, while 229E was the least frequent. Medical utilization and severity did not differ among the CoVs, or between RSV+CoV vs. RSV alone, unless one considered CoV viral load as a variable. ICU use increased when the polymerase chain reaction cycle threshold result indicated a high CoV viral load.
These data suggest CoVs are not infrequent coinfectors with RSV in bronchiolitis – and that SARS-CoV-2 is the same. Therefore, a bronchiolitis presentation doesn’t necessarily take us off the hook for the need to consider SARS-CoV-2 testing, particularly in the somewhat older bronchiolitis patient with more than mild symptoms.
Considerations for SARS-CoV-2 testing: Outpatient influenza-like illness
In 2020-2021, the Centers for Disease Control and Prevention recommends considering empiric antiviral treatment for ILIs (fever plus either cough or sore throat) based upon our clinical judgement, even in non-high-risk children.4
While pediatric COVID-19 illnesses are predominantly asymptomatic or mild, a febrile ARI is also a SARS-CoV-2 compatible presentation. So, if all we use is our clinical judgment, how do we know if the febrile ARI is due to influenza or SARS-CoV-2 or both? At least one study used a highly sensitive and specific molecular influenza test to show that the accuracy of clinically diagnosing influenza in children is not much better than flipping a coin and would lead to potential antiviral overuse.5
So, it seems ideal to test for influenza when possible. Point-of-care (POC) tests are frequently used for outpatients. Eight POC Clinical Laboratory Improvement Amendments (CLIA)–waived kits, some also detecting RSV, are available but most have modest sensitivity (60%-80%) compared with lab-based molecular tests.6 That said, if supplies and kits for one of the POC tests are available to us during these SARS-CoV-2 stressed times (back orders seem more common this year), a positive influenza test in the first 48 hours of symptoms confirms the option to prescribe an antiviral. Yet how will we have confidence that the febrile ARI is not also partly due to SARS-CoV-2? Currently febrile ARIs usually are considered SARS-CoV-2 and the children are sent for SARS-CoV-2 testing. During influenza season, it seems we will need to continue to send febrile outpatients for SARS-CoV-2 testing, even if POC influenza positive, via whatever mechanisms are available as time goes on.
We expect more rapid pediatric testing modalities for SARS-CoV-2 (maybe even saliva tests) to become available over the next months. Indeed, rapid antigen tests and rapid molecular tests are being evaluated in adults and seem destined for CLIA waivers as POC tests, and even home testing kits. Pediatric approvals hopefully also will occur. So, the pathways for SARS-CoV-2 testing available now will likely change over this winter. But be aware that supplies/kits will be prioritized to locations within high need areas and bulk purchase contracts. So POC kits may remain scarce for practices, meaning a reference laboratory still could be the way to go for SARS-CoV-2 for at least the rest of 2020. Reference labs are becoming creative as well; one combined detection of influenza A, influenza B, RSV, and SARS-CoV-2 into one test, and hopes to get approval for swab collection that can be done by families at home and mailed in.
Summary
Expect variations on the traditional parade of seasonal respiratory viruses, with increased numbers of coinfections. Choosing the outpatient who needs influenza testing is the same as in past years, although we have CDC permissive recommendations to prescribe antivirals for any outpatient ILI within the first 48 hours of symptoms. Still, POC testing for influenza remains potentially valuable in the ILI patient. The choice of whether and how to test for SARS-CoV-2 given its potential to be a primary or coinfecting agent in presentations linked more closely to a traditional virus (e.g. RSV bronchiolitis) will be a test of our clinical judgement until more data and easier testing are available. Further complicating coinfection recognition is the fact that many sick visits occur by telehealth and much testing is done at drive-through SARS-CoV-2 testing facilities with no clinician exam. Unless we are liberal in SARS-CoV-2 testing, detecting SARS-CoV-2 coinfections is easier said than done given its usually mild presentation being overshadowed by any coinfecting virus.
But understanding who has SARS-CoV-2, even as a coinfection, still is essential in controlling the pandemic. We will need to be vigilant for evolving approaches to SARS-CoV-2 testing in the context of symptomatic ARI presentations, knowing this will likely remain a moving target for the foreseeable future.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at pdnews@mdedge.com.
References
1. Pediatrics. 2020;146(1):e20200961.
2. JAMA. 2020 May 26;323(20):2085-6.
3. Pediatrics. 2020. doi: 10.1542/peds.2020-1267.
4. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
5. J. Pediatr. 2020. doi: 10.1016/j.jpeds.2020.08.007.
6. www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html.
Respiratory virus seasons usually follow a fairly well-known pattern. Enterovirus 68 (EV-D68) is a summer-to-early fall virus with biennial peak years. Rhinovirus (HRv) and adenovirus (Adv) occur nearly year-round but may have small upticks in the first month or so that children return to school. Early in the school year, upper respiratory infections from both HRv and Adv and viral sore throats from Adv are common, with conjunctivitis from Adv outbreaks in some years. October to November is human parainfluenza (HPiV) 1 and 2 season, often presenting as croup. Human metapneumovirus infections span October through April. In late November to December, influenza begins, usually with an A type, later transitioning to a B type in February through April. Also in December, respiratory syncytial virus (RSV) starts, characteristically with bronchiolitis presentations, peaking in February to March and tapering off in May. In late March to April, HPiV 3 also appears for 4-6 weeks.

Will 2020-2021 be different?
Summer was remarkably free of expected enterovirus activity, suggesting that the seasonal parade may differ this year. Remember that the 2019-2020 respiratory season suddenly and nearly completely stopped in March because of social distancing and lockdowns needed to address the SARS-CoV-2 pandemic.
The mild influenza season in the southern hemisphere suggests that our influenza season also could be mild. But perhaps not – most southern hemisphere countries that are surveyed for influenza activities had the most intense SARS-CoV-2 mitigations, making the observed mildness potentially related more to social mitigation than less virulent influenza strains. If so, southern hemisphere influenza data may not apply to the United States, where social distancing and masks are ignored or used inconsistently by almost half the population.
Further, the stop-and-go pattern of in-person school/college attendance adds to uncertainties for the usual orderly virus-specific seasonality. The result may be multiple stop-and-go “pop-up” or “mini” outbreaks for any given virus potentially reflected as exaggerated local or regional differences in circulation of various viruses. The erratic seasonality also would increase coinfections, which could present with more severe or different symptoms.
SARS-CoV-2’s potential interaction
Will the relatively mild presentations for most children with SARS-CoV-2 hold up in the setting of coinfections or sequential respiratory viral infections? Could SARS-CoV-2 cause worse/more prolonged symptoms or more sequelae if paired simultaneously or in tandem with a traditional respiratory virus? To date, data on the frequency and severity of SARS-CoV-2 coinfections are conflicting and sparse, but it appears that non-SARS-CoV-2 viruses can be involved in 15%-50% pediatric acute respiratory infections.1,2
However, it may not be important to know about coinfecting viruses other than influenza (can be treated) or SARS-CoV-2 (needs quarantine and contact tracing), unless symptoms are atypical or more severe than usual. For example, a young child with bronchiolitis is most likely infected with RSV, but HPiV, influenza, metapneumovirus, HRv, and even SARS-CoV-2 can cause bronchiolitis. Even so, testing outpatients for RSV or non-influenza is not routine or even clinically helpful. Supportive treatment and restriction from daycare attendance are sufficient management for outpatient ARIs whether presenting as bronchiolitis or not.
Considerations for SARS-CoV-2 testing: Outpatient bronchiolitis
If a child presents with classic bronchiolitis but has above moderate to severe symptoms, is SARS-CoV-2 a consideration? Perhaps, if SARS-CoV-2 acts similarly to non-SARS-CoV-2s.
A recent report from the 30th Multicenter Airway Research Collaboration (MARC-30) surveillance study (2007-2014) of children hospitalized with clinical bronchiolitis evaluated respiratory viruses, including RSV and the four common non-SARS coronaviruses using molecular testing.3 Among 1,880 subjects, a CoV (alpha CoV: NL63 or 229E, or beta CoV: KKU1 or OC43) was detected in 12%. Yet most had only RSV (n = 1,661); 32 had only CoV (n = 32). But note that 219 had both.
Bronchiolitis subjects with CoV were older – median 3.7 (1.4-5.8) vs. 2.8 (1.9-7.2) years – and more likely male than were RSV subjects (68% vs. 58%). OC43 was most frequent followed by equal numbers of HKU1 and NL63, while 229E was the least frequent. Medical utilization and severity did not differ among the CoVs, or between RSV+CoV vs. RSV alone, unless one considered CoV viral load as a variable. ICU use increased when the polymerase chain reaction cycle threshold result indicated a high CoV viral load.
These data suggest CoVs are not infrequent coinfectors with RSV in bronchiolitis – and that SARS-CoV-2 is the same. Therefore, a bronchiolitis presentation doesn’t necessarily take us off the hook for the need to consider SARS-CoV-2 testing, particularly in the somewhat older bronchiolitis patient with more than mild symptoms.
Considerations for SARS-CoV-2 testing: Outpatient influenza-like illness
In 2020-2021, the Centers for Disease Control and Prevention recommends considering empiric antiviral treatment for ILIs (fever plus either cough or sore throat) based upon our clinical judgement, even in non-high-risk children.4
While pediatric COVID-19 illnesses are predominantly asymptomatic or mild, a febrile ARI is also a SARS-CoV-2 compatible presentation. So, if all we use is our clinical judgment, how do we know if the febrile ARI is due to influenza or SARS-CoV-2 or both? At least one study used a highly sensitive and specific molecular influenza test to show that the accuracy of clinically diagnosing influenza in children is not much better than flipping a coin and would lead to potential antiviral overuse.5
So, it seems ideal to test for influenza when possible. Point-of-care (POC) tests are frequently used for outpatients. Eight POC Clinical Laboratory Improvement Amendments (CLIA)–waived kits, some also detecting RSV, are available but most have modest sensitivity (60%-80%) compared with lab-based molecular tests.6 That said, if supplies and kits for one of the POC tests are available to us during these SARS-CoV-2 stressed times (back orders seem more common this year), a positive influenza test in the first 48 hours of symptoms confirms the option to prescribe an antiviral. Yet how will we have confidence that the febrile ARI is not also partly due to SARS-CoV-2? Currently febrile ARIs usually are considered SARS-CoV-2 and the children are sent for SARS-CoV-2 testing. During influenza season, it seems we will need to continue to send febrile outpatients for SARS-CoV-2 testing, even if POC influenza positive, via whatever mechanisms are available as time goes on.
We expect more rapid pediatric testing modalities for SARS-CoV-2 (maybe even saliva tests) to become available over the next months. Indeed, rapid antigen tests and rapid molecular tests are being evaluated in adults and seem destined for CLIA waivers as POC tests, and even home testing kits. Pediatric approvals hopefully also will occur. So, the pathways for SARS-CoV-2 testing available now will likely change over this winter. But be aware that supplies/kits will be prioritized to locations within high need areas and bulk purchase contracts. So POC kits may remain scarce for practices, meaning a reference laboratory still could be the way to go for SARS-CoV-2 for at least the rest of 2020. Reference labs are becoming creative as well; one combined detection of influenza A, influenza B, RSV, and SARS-CoV-2 into one test, and hopes to get approval for swab collection that can be done by families at home and mailed in.
Summary
Expect variations on the traditional parade of seasonal respiratory viruses, with increased numbers of coinfections. Choosing the outpatient who needs influenza testing is the same as in past years, although we have CDC permissive recommendations to prescribe antivirals for any outpatient ILI within the first 48 hours of symptoms. Still, POC testing for influenza remains potentially valuable in the ILI patient. The choice of whether and how to test for SARS-CoV-2 given its potential to be a primary or coinfecting agent in presentations linked more closely to a traditional virus (e.g. RSV bronchiolitis) will be a test of our clinical judgement until more data and easier testing are available. Further complicating coinfection recognition is the fact that many sick visits occur by telehealth and much testing is done at drive-through SARS-CoV-2 testing facilities with no clinician exam. Unless we are liberal in SARS-CoV-2 testing, detecting SARS-CoV-2 coinfections is easier said than done given its usually mild presentation being overshadowed by any coinfecting virus.
But understanding who has SARS-CoV-2, even as a coinfection, still is essential in controlling the pandemic. We will need to be vigilant for evolving approaches to SARS-CoV-2 testing in the context of symptomatic ARI presentations, knowing this will likely remain a moving target for the foreseeable future.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at pdnews@mdedge.com.
References
1. Pediatrics. 2020;146(1):e20200961.
2. JAMA. 2020 May 26;323(20):2085-6.
3. Pediatrics. 2020. doi: 10.1542/peds.2020-1267.
4. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
5. J. Pediatr. 2020. doi: 10.1016/j.jpeds.2020.08.007.
6. www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html.
Respiratory virus seasons usually follow a fairly well-known pattern. Enterovirus 68 (EV-D68) is a summer-to-early fall virus with biennial peak years. Rhinovirus (HRv) and adenovirus (Adv) occur nearly year-round but may have small upticks in the first month or so that children return to school. Early in the school year, upper respiratory infections from both HRv and Adv and viral sore throats from Adv are common, with conjunctivitis from Adv outbreaks in some years. October to November is human parainfluenza (HPiV) 1 and 2 season, often presenting as croup. Human metapneumovirus infections span October through April. In late November to December, influenza begins, usually with an A type, later transitioning to a B type in February through April. Also in December, respiratory syncytial virus (RSV) starts, characteristically with bronchiolitis presentations, peaking in February to March and tapering off in May. In late March to April, HPiV 3 also appears for 4-6 weeks.

Will 2020-2021 be different?
Summer was remarkably free of expected enterovirus activity, suggesting that the seasonal parade may differ this year. Remember that the 2019-2020 respiratory season suddenly and nearly completely stopped in March because of social distancing and lockdowns needed to address the SARS-CoV-2 pandemic.
The mild influenza season in the southern hemisphere suggests that our influenza season also could be mild. But perhaps not – most southern hemisphere countries that are surveyed for influenza activities had the most intense SARS-CoV-2 mitigations, making the observed mildness potentially related more to social mitigation than less virulent influenza strains. If so, southern hemisphere influenza data may not apply to the United States, where social distancing and masks are ignored or used inconsistently by almost half the population.
Further, the stop-and-go pattern of in-person school/college attendance adds to uncertainties for the usual orderly virus-specific seasonality. The result may be multiple stop-and-go “pop-up” or “mini” outbreaks for any given virus potentially reflected as exaggerated local or regional differences in circulation of various viruses. The erratic seasonality also would increase coinfections, which could present with more severe or different symptoms.
SARS-CoV-2’s potential interaction
Will the relatively mild presentations for most children with SARS-CoV-2 hold up in the setting of coinfections or sequential respiratory viral infections? Could SARS-CoV-2 cause worse/more prolonged symptoms or more sequelae if paired simultaneously or in tandem with a traditional respiratory virus? To date, data on the frequency and severity of SARS-CoV-2 coinfections are conflicting and sparse, but it appears that non-SARS-CoV-2 viruses can be involved in 15%-50% pediatric acute respiratory infections.1,2
However, it may not be important to know about coinfecting viruses other than influenza (can be treated) or SARS-CoV-2 (needs quarantine and contact tracing), unless symptoms are atypical or more severe than usual. For example, a young child with bronchiolitis is most likely infected with RSV, but HPiV, influenza, metapneumovirus, HRv, and even SARS-CoV-2 can cause bronchiolitis. Even so, testing outpatients for RSV or non-influenza is not routine or even clinically helpful. Supportive treatment and restriction from daycare attendance are sufficient management for outpatient ARIs whether presenting as bronchiolitis or not.
Considerations for SARS-CoV-2 testing: Outpatient bronchiolitis
If a child presents with classic bronchiolitis but has above moderate to severe symptoms, is SARS-CoV-2 a consideration? Perhaps, if SARS-CoV-2 acts similarly to non-SARS-CoV-2s.
A recent report from the 30th Multicenter Airway Research Collaboration (MARC-30) surveillance study (2007-2014) of children hospitalized with clinical bronchiolitis evaluated respiratory viruses, including RSV and the four common non-SARS coronaviruses using molecular testing.3 Among 1,880 subjects, a CoV (alpha CoV: NL63 or 229E, or beta CoV: KKU1 or OC43) was detected in 12%. Yet most had only RSV (n = 1,661); 32 had only CoV (n = 32). But note that 219 had both.
Bronchiolitis subjects with CoV were older – median 3.7 (1.4-5.8) vs. 2.8 (1.9-7.2) years – and more likely male than were RSV subjects (68% vs. 58%). OC43 was most frequent followed by equal numbers of HKU1 and NL63, while 229E was the least frequent. Medical utilization and severity did not differ among the CoVs, or between RSV+CoV vs. RSV alone, unless one considered CoV viral load as a variable. ICU use increased when the polymerase chain reaction cycle threshold result indicated a high CoV viral load.
These data suggest CoVs are not infrequent coinfectors with RSV in bronchiolitis – and that SARS-CoV-2 is the same. Therefore, a bronchiolitis presentation doesn’t necessarily take us off the hook for the need to consider SARS-CoV-2 testing, particularly in the somewhat older bronchiolitis patient with more than mild symptoms.
Considerations for SARS-CoV-2 testing: Outpatient influenza-like illness
In 2020-2021, the Centers for Disease Control and Prevention recommends considering empiric antiviral treatment for ILIs (fever plus either cough or sore throat) based upon our clinical judgement, even in non-high-risk children.4
While pediatric COVID-19 illnesses are predominantly asymptomatic or mild, a febrile ARI is also a SARS-CoV-2 compatible presentation. So, if all we use is our clinical judgment, how do we know if the febrile ARI is due to influenza or SARS-CoV-2 or both? At least one study used a highly sensitive and specific molecular influenza test to show that the accuracy of clinically diagnosing influenza in children is not much better than flipping a coin and would lead to potential antiviral overuse.5
So, it seems ideal to test for influenza when possible. Point-of-care (POC) tests are frequently used for outpatients. Eight POC Clinical Laboratory Improvement Amendments (CLIA)–waived kits, some also detecting RSV, are available but most have modest sensitivity (60%-80%) compared with lab-based molecular tests.6 That said, if supplies and kits for one of the POC tests are available to us during these SARS-CoV-2 stressed times (back orders seem more common this year), a positive influenza test in the first 48 hours of symptoms confirms the option to prescribe an antiviral. Yet how will we have confidence that the febrile ARI is not also partly due to SARS-CoV-2? Currently febrile ARIs usually are considered SARS-CoV-2 and the children are sent for SARS-CoV-2 testing. During influenza season, it seems we will need to continue to send febrile outpatients for SARS-CoV-2 testing, even if POC influenza positive, via whatever mechanisms are available as time goes on.
We expect more rapid pediatric testing modalities for SARS-CoV-2 (maybe even saliva tests) to become available over the next months. Indeed, rapid antigen tests and rapid molecular tests are being evaluated in adults and seem destined for CLIA waivers as POC tests, and even home testing kits. Pediatric approvals hopefully also will occur. So, the pathways for SARS-CoV-2 testing available now will likely change over this winter. But be aware that supplies/kits will be prioritized to locations within high need areas and bulk purchase contracts. So POC kits may remain scarce for practices, meaning a reference laboratory still could be the way to go for SARS-CoV-2 for at least the rest of 2020. Reference labs are becoming creative as well; one combined detection of influenza A, influenza B, RSV, and SARS-CoV-2 into one test, and hopes to get approval for swab collection that can be done by families at home and mailed in.
Summary
Expect variations on the traditional parade of seasonal respiratory viruses, with increased numbers of coinfections. Choosing the outpatient who needs influenza testing is the same as in past years, although we have CDC permissive recommendations to prescribe antivirals for any outpatient ILI within the first 48 hours of symptoms. Still, POC testing for influenza remains potentially valuable in the ILI patient. The choice of whether and how to test for SARS-CoV-2 given its potential to be a primary or coinfecting agent in presentations linked more closely to a traditional virus (e.g. RSV bronchiolitis) will be a test of our clinical judgement until more data and easier testing are available. Further complicating coinfection recognition is the fact that many sick visits occur by telehealth and much testing is done at drive-through SARS-CoV-2 testing facilities with no clinician exam. Unless we are liberal in SARS-CoV-2 testing, detecting SARS-CoV-2 coinfections is easier said than done given its usually mild presentation being overshadowed by any coinfecting virus.
But understanding who has SARS-CoV-2, even as a coinfection, still is essential in controlling the pandemic. We will need to be vigilant for evolving approaches to SARS-CoV-2 testing in the context of symptomatic ARI presentations, knowing this will likely remain a moving target for the foreseeable future.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at pdnews@mdedge.com.
References
1. Pediatrics. 2020;146(1):e20200961.
2. JAMA. 2020 May 26;323(20):2085-6.
3. Pediatrics. 2020. doi: 10.1542/peds.2020-1267.
4. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
5. J. Pediatr. 2020. doi: 10.1016/j.jpeds.2020.08.007.
6. www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html.
Action and awareness are needed to increase immunization rates
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
COVID-19: A primary care perspective
With the COVID-19 pandemic, we are experiencing a once-in-a-100-year event. Dr. Steven A. Schulz, who is serving children on the front line in upstate New York, and I outline some of the challenges primary care pediatricians have been facing and solutions that have succeeded.
Reduction in direct patient care and its consequences
Because of the unknowns of COVID-19, many parents have not wanted to bring their children to a medical office because of fear of contracting SARS-CoV-2. At the same time, pediatricians have restricted in-person visits to prevent spread of SARS-CoV-2 and to help flatten the curve of infection. Use of pediatric medical professional services, compared with last year, dropped by 52% in March 2020 and by 58% in April, according to FAIR Health, a nonprofit organization that manages a database of 31 million claims. This is resulting in decreased immunization rates, which increases concern for secondary spikes of other preventable illnesses; for example, data from the Centers for Disease Control and Prevention showed that, from mid-March to mid-April 2020, physicians in the Vaccines for Children program ordered 2.5 million fewer doses of vaccines and 250,000 fewer doses of measles-containing vaccines, compared with the same period in 2019. Fewer children are being seen for well visits, which means opportunities are lost for adequate monitoring of growth, development, physical wellness, and social determinants of health.
This is occurring at a time when families have been experiencing increased stress in terms of finances, social isolation, finding adequate child care, and serving as parent, teacher, and breadwinner. An increase in injuries is occurring because of inadequate parental supervision because many parents have been distracted while working from home. An increase in cases of severe abuse is occurring because schools, child care providers, physicians, and other mandated reporters in the community have decreased interaction with children. Children’s Hospital Colorado in Colorado Springs saw a 118% increase in the number of trauma cases in its ED between January and April 2020. Some of these were accidental injuries caused by falls or bicycle accidents, but there was a 200% increase in nonaccidental trauma, which was associated with a steep fall in calls to the state’s child abuse hotline. Academic gains are being lost, and there has been worry for a prolonged “summer slide” risk, especially for children living in poverty and children with developmental disabilities.
The COVID-19 pandemic also is affecting physicians and staff. As frontline personnel, we are at risk to contract the virus, and news media reminds us of severe illness and deaths among health care workers. The pandemic is affecting financial viability; estimated revenue of pediatric offices fell by 45% in March 2020 and 48% in April, compared with the previous year, according to FAIR Health. Nurses and staff have been furloughed. Practices have had to apply for grants and Paycheck Protection Program funds while extending credit lines.
Limited testing capability for SARS-CoV-2
Testing for SARS-CoV-2 has been variably available. There have been problems with false positive and especially false negative results (BMJ. 2020 May 12. doi: 10.1136/bmj.m1808).The best specimen collection method has yet to be determined. Blood testing for antibody has been touted, but it remains unclear if there is clinical benefit because a positive result offers no guarantee of immunity, and immunity may quickly wane. Perhaps widespread primary care office–based testing will be in place by the fall, with hope for future reliable point of care results.
Evolving knowledge regarding SARS-CoV-2 and MIS-C
It initially was thought that children were relatively spared from serious illness caused by COVID-19. Then reports of cases of newly identified multisystem inflammatory syndrome of children occurred. It has been unclear how children contribute to the spread of COVID-19 illness, although emerging evidence indicates it is lower than adult transmission. What will happen when children return to school and daycare in the fall?
The challenges have led to creative solutions for how to deliver care.
Adapting to telehealth to provide care
At least for the short term, HIPAA regulations have been relaxed to allow for video visits using platforms such as FaceTime, Skype, Zoom, Doximity, and Doxy.me. Some of these platforms are HIPAA compliant and will be long-term solutions; however, electronic medical record portals allowing for video visits are the more secure option, according to HIPAA.
It has been a learning experience to see what can be accomplished with a video visit. Taking a history and visual examination of injuries and rashes has been possible. Addressing mental health concerns through the video exchange generally has been effective.
However, video visits change the provider-patient interpersonal dynamic and offer only visual exam capabilities, compared with an in-person visit. We cannot look in ears, palpate a liver and spleen, touch and examine a joint or bone, or feel a rash. Video visits also are dependent on the quality of patient Internet access, sufficient data plans, and mutual capabilities to address the inevitable technological glitches on the provider’s end as well. Expanding information technology infrastructure ability and added licensure costs have occurred. Practices and health systems have been working with insurance companies to ensure telephone and video visits are reimbursed on a comparable level to in-office visits.
A new type of office visit and developing appropriate safety plans
Patients must be universally screened prior to arrival during appointment scheduling for well and illness visits. Patients aged older than 2 years and caregivers must wear masks on entering the facility. In many practices, patients are scheduled during specific sick or well visit time slots throughout the day. Waiting rooms chairs need to be spaced for 6-foot social distancing, and cars in the parking lot often serve as waiting rooms until staff can meet patients at the door and take them to the exam room. Alternate entrances, car-side exams, and drive-by and/or tent testing facilities often have become part of the new normal everyday practice. Creating virtual visit time blocks in provider’s schedules has allowed for decreased office congestion. Patients often are checked out from their room, as opposed to waiting in a line at a check out desk. Nurse triage protocols also have been adapted and enhanced to meet needs and concerns.
With the need for summer physicals and many regions opening up, a gradual return toward baseline has been evolving, although some of the twists of a “new normal” will stay in place. The new normal has been for providers and staff to wear surgical masks and face shields; sometimes N95 masks, gloves, and gowns have been needed. Cleaning rooms and equipment between patient visits has become a major, new time-consuming task. Acquiring and maintaining adequate supplies has been a challenge.
Summary
The American Academy of Pediatrics, CDC, and state and local health departments have been providing informative and regular updates, webinars, and best practices guidelines. Pediatricians, community organizations, schools, and mental health professionals have been collaborating, overcoming hurdles, and working together to help mitigate the effects of the pandemic on children, their families, and our communities. Continued education, cooperation, and adaptation will be needed in the months ahead. If there is a silver lining to this pandemic experience, it may be that families have grown closer together as they sheltered in place (and we have grown closer to our own families as well). One day perhaps a child who lived through this pandemic might be asked what it was like, and their recollection might be that it was a wonderful time because their parents stayed home all the time, took care of them, taught them their school work, and took lots of long family walks.
Dr. Schulz is pediatric medical director, Rochester (N.Y.) Regional Health. Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz and Dr. Pichichero said they have no relevant financial disclosures. Email them at pdnews@mdedge.com.
This article was updated 7/16/2020.
With the COVID-19 pandemic, we are experiencing a once-in-a-100-year event. Dr. Steven A. Schulz, who is serving children on the front line in upstate New York, and I outline some of the challenges primary care pediatricians have been facing and solutions that have succeeded.
Reduction in direct patient care and its consequences
Because of the unknowns of COVID-19, many parents have not wanted to bring their children to a medical office because of fear of contracting SARS-CoV-2. At the same time, pediatricians have restricted in-person visits to prevent spread of SARS-CoV-2 and to help flatten the curve of infection. Use of pediatric medical professional services, compared with last year, dropped by 52% in March 2020 and by 58% in April, according to FAIR Health, a nonprofit organization that manages a database of 31 million claims. This is resulting in decreased immunization rates, which increases concern for secondary spikes of other preventable illnesses; for example, data from the Centers for Disease Control and Prevention showed that, from mid-March to mid-April 2020, physicians in the Vaccines for Children program ordered 2.5 million fewer doses of vaccines and 250,000 fewer doses of measles-containing vaccines, compared with the same period in 2019. Fewer children are being seen for well visits, which means opportunities are lost for adequate monitoring of growth, development, physical wellness, and social determinants of health.
This is occurring at a time when families have been experiencing increased stress in terms of finances, social isolation, finding adequate child care, and serving as parent, teacher, and breadwinner. An increase in injuries is occurring because of inadequate parental supervision because many parents have been distracted while working from home. An increase in cases of severe abuse is occurring because schools, child care providers, physicians, and other mandated reporters in the community have decreased interaction with children. Children’s Hospital Colorado in Colorado Springs saw a 118% increase in the number of trauma cases in its ED between January and April 2020. Some of these were accidental injuries caused by falls or bicycle accidents, but there was a 200% increase in nonaccidental trauma, which was associated with a steep fall in calls to the state’s child abuse hotline. Academic gains are being lost, and there has been worry for a prolonged “summer slide” risk, especially for children living in poverty and children with developmental disabilities.
The COVID-19 pandemic also is affecting physicians and staff. As frontline personnel, we are at risk to contract the virus, and news media reminds us of severe illness and deaths among health care workers. The pandemic is affecting financial viability; estimated revenue of pediatric offices fell by 45% in March 2020 and 48% in April, compared with the previous year, according to FAIR Health. Nurses and staff have been furloughed. Practices have had to apply for grants and Paycheck Protection Program funds while extending credit lines.
Limited testing capability for SARS-CoV-2
Testing for SARS-CoV-2 has been variably available. There have been problems with false positive and especially false negative results (BMJ. 2020 May 12. doi: 10.1136/bmj.m1808).The best specimen collection method has yet to be determined. Blood testing for antibody has been touted, but it remains unclear if there is clinical benefit because a positive result offers no guarantee of immunity, and immunity may quickly wane. Perhaps widespread primary care office–based testing will be in place by the fall, with hope for future reliable point of care results.
Evolving knowledge regarding SARS-CoV-2 and MIS-C
It initially was thought that children were relatively spared from serious illness caused by COVID-19. Then reports of cases of newly identified multisystem inflammatory syndrome of children occurred. It has been unclear how children contribute to the spread of COVID-19 illness, although emerging evidence indicates it is lower than adult transmission. What will happen when children return to school and daycare in the fall?
The challenges have led to creative solutions for how to deliver care.
Adapting to telehealth to provide care
At least for the short term, HIPAA regulations have been relaxed to allow for video visits using platforms such as FaceTime, Skype, Zoom, Doximity, and Doxy.me. Some of these platforms are HIPAA compliant and will be long-term solutions; however, electronic medical record portals allowing for video visits are the more secure option, according to HIPAA.
It has been a learning experience to see what can be accomplished with a video visit. Taking a history and visual examination of injuries and rashes has been possible. Addressing mental health concerns through the video exchange generally has been effective.
However, video visits change the provider-patient interpersonal dynamic and offer only visual exam capabilities, compared with an in-person visit. We cannot look in ears, palpate a liver and spleen, touch and examine a joint or bone, or feel a rash. Video visits also are dependent on the quality of patient Internet access, sufficient data plans, and mutual capabilities to address the inevitable technological glitches on the provider’s end as well. Expanding information technology infrastructure ability and added licensure costs have occurred. Practices and health systems have been working with insurance companies to ensure telephone and video visits are reimbursed on a comparable level to in-office visits.
A new type of office visit and developing appropriate safety plans
Patients must be universally screened prior to arrival during appointment scheduling for well and illness visits. Patients aged older than 2 years and caregivers must wear masks on entering the facility. In many practices, patients are scheduled during specific sick or well visit time slots throughout the day. Waiting rooms chairs need to be spaced for 6-foot social distancing, and cars in the parking lot often serve as waiting rooms until staff can meet patients at the door and take them to the exam room. Alternate entrances, car-side exams, and drive-by and/or tent testing facilities often have become part of the new normal everyday practice. Creating virtual visit time blocks in provider’s schedules has allowed for decreased office congestion. Patients often are checked out from their room, as opposed to waiting in a line at a check out desk. Nurse triage protocols also have been adapted and enhanced to meet needs and concerns.
With the need for summer physicals and many regions opening up, a gradual return toward baseline has been evolving, although some of the twists of a “new normal” will stay in place. The new normal has been for providers and staff to wear surgical masks and face shields; sometimes N95 masks, gloves, and gowns have been needed. Cleaning rooms and equipment between patient visits has become a major, new time-consuming task. Acquiring and maintaining adequate supplies has been a challenge.
Summary
The American Academy of Pediatrics, CDC, and state and local health departments have been providing informative and regular updates, webinars, and best practices guidelines. Pediatricians, community organizations, schools, and mental health professionals have been collaborating, overcoming hurdles, and working together to help mitigate the effects of the pandemic on children, their families, and our communities. Continued education, cooperation, and adaptation will be needed in the months ahead. If there is a silver lining to this pandemic experience, it may be that families have grown closer together as they sheltered in place (and we have grown closer to our own families as well). One day perhaps a child who lived through this pandemic might be asked what it was like, and their recollection might be that it was a wonderful time because their parents stayed home all the time, took care of them, taught them their school work, and took lots of long family walks.
Dr. Schulz is pediatric medical director, Rochester (N.Y.) Regional Health. Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz and Dr. Pichichero said they have no relevant financial disclosures. Email them at pdnews@mdedge.com.
This article was updated 7/16/2020.
With the COVID-19 pandemic, we are experiencing a once-in-a-100-year event. Dr. Steven A. Schulz, who is serving children on the front line in upstate New York, and I outline some of the challenges primary care pediatricians have been facing and solutions that have succeeded.
Reduction in direct patient care and its consequences
Because of the unknowns of COVID-19, many parents have not wanted to bring their children to a medical office because of fear of contracting SARS-CoV-2. At the same time, pediatricians have restricted in-person visits to prevent spread of SARS-CoV-2 and to help flatten the curve of infection. Use of pediatric medical professional services, compared with last year, dropped by 52% in March 2020 and by 58% in April, according to FAIR Health, a nonprofit organization that manages a database of 31 million claims. This is resulting in decreased immunization rates, which increases concern for secondary spikes of other preventable illnesses; for example, data from the Centers for Disease Control and Prevention showed that, from mid-March to mid-April 2020, physicians in the Vaccines for Children program ordered 2.5 million fewer doses of vaccines and 250,000 fewer doses of measles-containing vaccines, compared with the same period in 2019. Fewer children are being seen for well visits, which means opportunities are lost for adequate monitoring of growth, development, physical wellness, and social determinants of health.
This is occurring at a time when families have been experiencing increased stress in terms of finances, social isolation, finding adequate child care, and serving as parent, teacher, and breadwinner. An increase in injuries is occurring because of inadequate parental supervision because many parents have been distracted while working from home. An increase in cases of severe abuse is occurring because schools, child care providers, physicians, and other mandated reporters in the community have decreased interaction with children. Children’s Hospital Colorado in Colorado Springs saw a 118% increase in the number of trauma cases in its ED between January and April 2020. Some of these were accidental injuries caused by falls or bicycle accidents, but there was a 200% increase in nonaccidental trauma, which was associated with a steep fall in calls to the state’s child abuse hotline. Academic gains are being lost, and there has been worry for a prolonged “summer slide” risk, especially for children living in poverty and children with developmental disabilities.
The COVID-19 pandemic also is affecting physicians and staff. As frontline personnel, we are at risk to contract the virus, and news media reminds us of severe illness and deaths among health care workers. The pandemic is affecting financial viability; estimated revenue of pediatric offices fell by 45% in March 2020 and 48% in April, compared with the previous year, according to FAIR Health. Nurses and staff have been furloughed. Practices have had to apply for grants and Paycheck Protection Program funds while extending credit lines.
Limited testing capability for SARS-CoV-2
Testing for SARS-CoV-2 has been variably available. There have been problems with false positive and especially false negative results (BMJ. 2020 May 12. doi: 10.1136/bmj.m1808).The best specimen collection method has yet to be determined. Blood testing for antibody has been touted, but it remains unclear if there is clinical benefit because a positive result offers no guarantee of immunity, and immunity may quickly wane. Perhaps widespread primary care office–based testing will be in place by the fall, with hope for future reliable point of care results.
Evolving knowledge regarding SARS-CoV-2 and MIS-C
It initially was thought that children were relatively spared from serious illness caused by COVID-19. Then reports of cases of newly identified multisystem inflammatory syndrome of children occurred. It has been unclear how children contribute to the spread of COVID-19 illness, although emerging evidence indicates it is lower than adult transmission. What will happen when children return to school and daycare in the fall?
The challenges have led to creative solutions for how to deliver care.
Adapting to telehealth to provide care
At least for the short term, HIPAA regulations have been relaxed to allow for video visits using platforms such as FaceTime, Skype, Zoom, Doximity, and Doxy.me. Some of these platforms are HIPAA compliant and will be long-term solutions; however, electronic medical record portals allowing for video visits are the more secure option, according to HIPAA.
It has been a learning experience to see what can be accomplished with a video visit. Taking a history and visual examination of injuries and rashes has been possible. Addressing mental health concerns through the video exchange generally has been effective.
However, video visits change the provider-patient interpersonal dynamic and offer only visual exam capabilities, compared with an in-person visit. We cannot look in ears, palpate a liver and spleen, touch and examine a joint or bone, or feel a rash. Video visits also are dependent on the quality of patient Internet access, sufficient data plans, and mutual capabilities to address the inevitable technological glitches on the provider’s end as well. Expanding information technology infrastructure ability and added licensure costs have occurred. Practices and health systems have been working with insurance companies to ensure telephone and video visits are reimbursed on a comparable level to in-office visits.
A new type of office visit and developing appropriate safety plans
Patients must be universally screened prior to arrival during appointment scheduling for well and illness visits. Patients aged older than 2 years and caregivers must wear masks on entering the facility. In many practices, patients are scheduled during specific sick or well visit time slots throughout the day. Waiting rooms chairs need to be spaced for 6-foot social distancing, and cars in the parking lot often serve as waiting rooms until staff can meet patients at the door and take them to the exam room. Alternate entrances, car-side exams, and drive-by and/or tent testing facilities often have become part of the new normal everyday practice. Creating virtual visit time blocks in provider’s schedules has allowed for decreased office congestion. Patients often are checked out from their room, as opposed to waiting in a line at a check out desk. Nurse triage protocols also have been adapted and enhanced to meet needs and concerns.
With the need for summer physicals and many regions opening up, a gradual return toward baseline has been evolving, although some of the twists of a “new normal” will stay in place. The new normal has been for providers and staff to wear surgical masks and face shields; sometimes N95 masks, gloves, and gowns have been needed. Cleaning rooms and equipment between patient visits has become a major, new time-consuming task. Acquiring and maintaining adequate supplies has been a challenge.
Summary
The American Academy of Pediatrics, CDC, and state and local health departments have been providing informative and regular updates, webinars, and best practices guidelines. Pediatricians, community organizations, schools, and mental health professionals have been collaborating, overcoming hurdles, and working together to help mitigate the effects of the pandemic on children, their families, and our communities. Continued education, cooperation, and adaptation will be needed in the months ahead. If there is a silver lining to this pandemic experience, it may be that families have grown closer together as they sheltered in place (and we have grown closer to our own families as well). One day perhaps a child who lived through this pandemic might be asked what it was like, and their recollection might be that it was a wonderful time because their parents stayed home all the time, took care of them, taught them their school work, and took lots of long family walks.
Dr. Schulz is pediatric medical director, Rochester (N.Y.) Regional Health. Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz and Dr. Pichichero said they have no relevant financial disclosures. Email them at pdnews@mdedge.com.
This article was updated 7/16/2020.
Perfect storm of SARS-CoV-2 during flu season
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.

Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at pdnews@mdedge.com.
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.

Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at pdnews@mdedge.com.
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.

Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at pdnews@mdedge.com.
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.