User login
A Practical Overview of Pediatric Atopic Dermatitis, Part 1: Epidemiology and Pathogenesis
Atopic dermatitis (AD), or eczema, is the leading dermatologic diagnosis worldwide and is vexing to patients due to the itchiness of the rash. It is the leading cause of skin disease burden worldwide with a prevalence of 229,761,000 reported cases in 2010, presenting largely in preadolescence but also persisting through adulthood.1 Using the children’s life quality index, it has been demonstrated that AD has a greater impact on health-related quality of life than renal disease and cystic fibrosis.2 The overall burden of AD includes stress on the patient and his/her family as well as financial burdens that have been estimated to be similar to that of type 1 diabetes mellitus.3
Epidemiology of AD
The worldwide prevalence of AD varies by country and age group surveyed, with a higher prevalence in wealthy developed nations (eg, the United States) compared to poorer developing nations.4 Efforts to identify prevalence data for AD in the United States have been approached through a variety of strategies. A group in Oregon estimated the prevalence of AD in children aged 5 to 9 years to be 17.2% via a survey of parents (N=1465) and 11.8% with doctor-diagnosed eczema. In the same study, the question “Has a doctor ever said that your child has eczema?” was found to have a 91.3% predictive correlation.5 Analysis of the 2003 National Survey of Children’s Health demonstrated the overall US prevalence of pediatric AD to be 10.7% in 102,353 children 17 years or younger, with a range of 8.7% to 18.1% by region.6
In its evaluation of the worldwide prevalence of AD, the International Study of Asthma and Allergies in Childhood ranked the United States 17th.7,8 The prevalence of AD in developed countries such as the United States is fluid and is expected to increase if the trends from the last 20 years remain true. In an assessment of the National Health Interview Survey data from 1997 to 2011 based on responses to the question, “During the past 12 months, has your child had eczema or any kind of skin allergy?”, the Centers for Disease Control and Prevention identified an increase in the prevalence of AD in patients aged 0 to 17 years from 7.4% in 1997-1999 to 12.5% in 2009-2011.9 Rising prevalence seems to be paired with rising incidence in the total number of severe intractable cases, reduced clearance at the approach of grade school, or cases persisting into adulthood.
Racial Disparity in AD
Racial disparity worldwide and migration are thought to contribute to the prevalence of and therapeutic need for AD. For example, in the United Kingdom, the prevalence of AD in London-born Afro-Caribbean children versus white children (total cross-section, N=693 [junior school children]) was 16.3% and 8.7%, respectively.10 In the United States, black children were more likely to have AD than white children (odds ratio, 1.7).6 Asian and black children also were more likely to present to a physician for treatment of AD than white children.6,10-13
Definition and Diagnostic Considerations
According to Hanifin,14 “Eczema represents a family of inflammatory skin conditions characterized by pruritic, papulovesicular, sometimes weeping dermatitis. All demonstrate the histological hallmark of spongiosis, which helps to distinguish the eczemas from papulosquamous diseases such as psoriasis.”14 Atopic dermatitis is a variant of eczema; however, most laymen identify eczema and AD as being one and the same.
The Hanifin and Rajka15 criteria are the major diagnostic criteria for AD but are difficult to use in clinical practice. Three of the following 4 major criteria are needed for diagnosis: (1) pruritus, which is present universally; (2) typical morphology and distribution; (3) chronic or chronically relapsing dermatitis; and (4) personal and/or family history of atopy. Additionally, 3 of the following 23 minor criteria are needed for diagnosis: xerosis; ichthyosis vulgaris, palmar hyperlinearity, or keratosis pilaris; positive skin prick test; elevated serum IgE level; early age of onset; tendency toward cutaneous infections or impaired cell-mediated immunity; tendency toward nonspecific hand or foot dermatitis; nipple eczema; cheilitis; recurrent conjunctivitis; Dennie-Morgan fold (infraorbital fold); keratoconus; anterior subcapsular cataracts; orbital darkening; facial pallor or facial erythema; pityriasis alba; anterior neck folds; itching when sweating; intolerance to wool and lipid solvents; perifollicular accentuation; skin reactions from ingested foods or by food contact; environmental or emotional factors; and lesional/nonlesional white dermographism or delayed blanch.15-17
More pragmatic streamlined diagnostic criteria were established by Eichenfield et al.18 According to these guidelines, essential features for AD include pruritus and eczema. Important features seen in most cases and adding support to the diagnosis include early age of onset, atopy, and xerosis.18 In clinical practice, diagnosis is often made based on a pruritic relapsing condition in typical locations including the face, neck, and extensor surfaces in infants and children.
Age Considerations
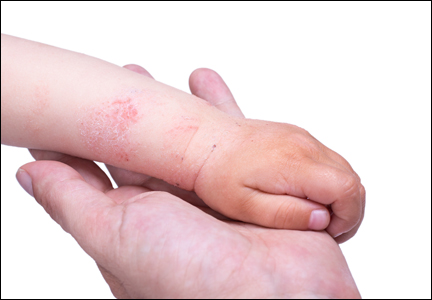
Diagnosis of AD is made by 5 years of age in 85% to 90% of children who will develop the disease and by age 1 year in 60% to 65%.6,19,20 Atopic dermatitis will persist into adulthood in up to one-third of children.21,22 Infantile AD is characterized by erythematous, oozing, excoriated plaques on the cheeks (sparing the nose), scalp, trunk, and extensor surfaces. Pruritus is always seen in AD and can be a source of morbidity.16-18 Seborrheic dermatitis may complicate or overlap with AD in infancy.22
By 2 years of age, most children who are going to develop AD begin to show disease signs of childhood AD characterized by flexural lesions and lesions on the neck and in the postauricular area with sparing of the diaper area.23 Adult AD often presents as eczema of the hands and/or feet. Hand eczema in adulthood is correlated with a prior history of childhood hand eczema and/or childhood AD as well as wet work and caring for small children.24 Children with skin of color may manifest with follicular eczema as their primary disease phenotype. Facial and eyelid dermatitis are more common in Asian females, infants, and teenagers.12,25 Other disease phenotypes that are common in patients with skin of color include lichenoid AD and postinflammatory hypopigmentation.12
Pathogenesis of AD
There are 2 theories on the pathogenesis of AD known as the inside-out and outside-in hypotheses.26 The inside-out hypothesis suggests that allergic triggering leads to a weakened skin barrier that furthers allergen introduction and presentation, while the outside-in hypothesis suggests that the skin barrier is weakened in AD and allows for the presentation of allergens. Both theories have validity and biologic basis, and both may in fact be true in certain individuals.26
The Skin Barrier: An Overview
The skin barrier is a complex set of factors present and functional at birth that seal the keratinocytes and the interkeratinocyte space so that the skin can perform key processes and functions including retention of fluid, exclusion of allergens, protection from UV light and solvents, and prevention of pathogen entry (eg, infections).27-29 The superficial stratum corneum or the cornified envelope consists of keratinocytes with intercellular stripes of hydrophobic and hydrophilic substances formed by various intercellular lipids, largely ceramides, cholesterol, and free fatty acids.30,31 Keratinocytes are the first responders to a variety of environmental insults with the production of IL-18, RANTES (regulated on activation, normal T-expressed, and presumably secreted), granulocyte-macrophage colony-stimulating factor, and thymic stromal lymphopoietin. These inflammatory substances produce acute and chronic inflammation, mast cell reactivity, and T-cell activation.14 Corneodesmosins link the keratinocytes. Peptidases released will cleave the corneodesmosins and allow normal desquamation or shedding of surface skin, which is replaced by division of stem cells in the basal layer.29
The stratum granulosum is the layer beneath the stratum corneum that co-contributes to barrier activity. The stratum granulosum is absent or reduced histologically in ichthyosis vulgaris,32 a form of skin dryness linked to filaggrin mutations and AD. Filaggrin breakdown creates natural moisturizing factor, a series of hygroscopic compounds that attract water into the skin.33 Histidine, a filaggrin breakdown product, is used by urocanic acid to process UV light insults.34 Filaggrin also contributes to other barrier functions including pH and stratum corneum cohesion as well as paracellular permeability of the stratum corneum. Tight junctions in the stratum granulosum include claudin-1 and claudin-6 and provide another barrier feature.29
The skin barrier is composed of lipids and keratinocytes. Ceramides, which represent one type of lipids, are reduced in AD, causing alteration in the lamellar pattern35 and increased transepidermal water loss. Furthermore, the stratum corneum is thickened in AD, possibly in response to trauma, and hydration is reduced.36 Filaggrin (chromosome arm 1q21.3) is formed from the 400-kDa+ precursor profilaggrin through dephosphorylation and cleavage, and it performs an essential function in the skin barrier through its differential cleavage and breakdown as well as release of natural moisturizing factor and other compounds.37 Filaggrin mutations are linked to AD and ichthyosis vulgaris; however, barrier defects as evidenced by transepidermal water loss in the absence of filaggrin mutation are sufficient to allow for sensitization to allergens through the skin.29 Filaggrin mutations have been associated with AD development and vary in prevalence worldwide. In the United Kingdom, a prevalence study of filaggrin mutations in patients aged 7 to 9 years (N=792) demonstrated an 18.4% carrier rate in AD patients versus 12.9% in controls.34 A similar study in Sweden (N=3301) showed carrier rates of 13.5% versus 6.5%, respectively.38 Although filaggrin mutations are lower in black patients,39 ceramide content may be reduced in this population, demonstrating that a variety of skin barrier defects can result in AD. Carriers of filaggrin mutations are more likely to have eczema on skin exposed to environmental factors (eg, face, hands).40
Barrier Defects Contributing to AD
The breakdown of the stratum corneum allows for antigen presentation to Langerhans cells, the dendritic antigen-presenting cells of the skin. Breaks in the stratum corneum may occur from scratching. These macroscopic breaks are large, whereas the breaks that otherwise occur due to barrier breakdown may be more microscopic in nature. Scratching causes aggravation of the helper T cell (TH2) response.29 For example, it allows the dendritic ends of Langerhans cells to be exposed to antigens. The dendritic ends capture allergens through IgE (may be elevated in AD29), which is bound to the high-affinity FCER1 receptors on Langerhans cells. Rather than causing a type I hypersensitivity reaction, these Langerhans cells are activated and move to the lymph nodes where they present antigen and initiate a cascade of proinflammatory activity. This TH2 cascade includes release of cytokines such as IL-2, IL-4, IL-8, IL-10, tumor necrosis factor α, and IFN-γ.26,29
Transepidermal water loss and barrier dysfunction contribute to disease activity and facilitate food/environmental allergen sensitization by allowing increased penetration of allergens through the skin to be presented by Langerhans cells to TH1 cells (sensitization phase). The Langerhans cells can reach their dendritic ends through tight junctions and into the stratum corneum, allowing them to reach surface allergens when the barrier is impaired. Ultimate expansion to systemic allergy (effector phase) occurs when dendritic cells move to draining lymph nodes, causing antigen presentation to CD4 and/or CD8 cells. Langerhans cells and dendritic cell sensitization through the weakened skin is believed to be the basis or role of barrier disruption as a trigger of atopic diseases, including AD and food and environmental allergies.
Many different forms of barrier disruption can cause a TH2 response in AD. The TH2 response triggers a constellation of proinflammatory activities including release of IL-4, associated with eosinophilia and elevated IgE levels, the latter being minor criterion in the diagnosis of AD.15 One mechanism by which the TH2 response is elicited may be the release of molecules such as danger-associated molecule patterns that may elicit recruitment of other inflammatory cells. Helper T cell (TH2) activity also can worsen barrier defects through IL-4 and IL-13 release, which can reduce filaggrin expression,29,41 and can aggravate barrier dysfunction in AD.
Inflammatory activation in AD also may involve inflammatory dendritic epidermal cells (IDECs). The IDECs can be tolerogenic or immunogenic mature phenotypes. The IDECs activate helper T cells (TH1), which may contribute to long-term AD activity.
Conclusion
Atopic dermatitis is a common skin condition worldwide and is characterized by the hallmark of pruritus and features that include a typical pattern, history of atopy (personal or family), and usually xerosis and early disease onset. Barrier dysfunction and immune dysregulation are prominent in AD, both of which aggravate the other and may encourage increased development of allergies and other forms of atopy over time.
1. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
2. Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
3. Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
4. Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
5. Laughter D, Istvan JA, Tofte SJ, et al. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649-655.
6. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
7. Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-1258.
8. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225-1232.
9. Hansen TE, Evjenth B, Holt J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: three surveys during the period 1985-2008. Acta Paediatr. 2013;102:47-52.
10. Williams HC, Pembroke AC, Forsdyke H, et al. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol. 1995;32:212-217.
11. Horii KA, Simon SD, Liu DY, et al. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120:e527-e534.
12. Silverberg NB. Eczematous diseases. In: Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity. New York, NY: Springer; 2012:69-88.
13. Gupta J, Grube E, Ericksen MB, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725-730.
14. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
15. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980;92:44-47.
16. Queille-Roussel C, Raynaud F, Saurat JH. A prospective computerized study of 500 cases of atopic dermatitis in childhood. I. Initial analysis of 250 parameters. Acta Derm Venereol Suppl (Stockh). 1985;114:87-92.
17. Böhme M, Svensson A, Kull I, et al. Hanifin’s and Rajka’s minor criteria for atopic dermatitis: which do 2-year-olds exhibit? J Am Acad Dermatol. 2000;43:785-792.
18. Eichenfield LF, Hanifin JM, Luger TA, et al. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol. 2003;49:1088-1095.
19. Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
20. Perkin MR, Strachan DP, Williams HC, et al. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol. 2004;15:221-229.
21. Ellis CN, Mancini AJ, Paller AS, et al. Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg. 2012;31(suppl 2):S18-S22.
22. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
23. Meding B, Wrangsjö K, Järvholm B. Hand eczema extent and morphology—association and influence on long-term prognosis. J Invest Dermatol. 2007;127:2147-2151.
24. Mortz CG, Bindslev-Jensen C, Andersen KE. Hand eczema in The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS): prevalence, incidence and risk factors from adolescence to adulthood [published online August 7, 2014]. Br J Dermatol. 2014;171:313-323.
25. Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
26. Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
27. Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care [published online December 8, 2014]. Clin Dermatol. 2015;33:271-280.
28. Miyagaki T, Sugaya M. Recent advances in atopic dermatitis and psoriasis: genetic background, barrier function, and therapeutic targets. J Dermatol Sci. 2015;78:89-94.
29. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
30. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
31. Janssens M, van Smeden J, Gooris GS, et al. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131:2136-2138.
32. Fitch N, Segool R, Ferenczy A, et al. Dominant ichthyosis vulgaris with an ultrastructurally normal granular layer. Clin Genet. 1976;9:71-76.
33. Chandar P, Nole G, Johnson AW. Understanding natural moisturizing mechanisms: implications for moisturizer technology. Cutis. 2009;84(suppl 1):2-15.
34. Brown SJ, Relton CL, Liao H, et al. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol. 2008;121:940-946.
35. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
36. Nemoto-Hasebe I, Akiyama M, Nomura T, et al. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009;129:682-689.
37. Hoste E, Kemperman P, Devos M, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233-2241.
38. Ballardini N, Kull I, Söderhäll C, et al. Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol. 2013;168:588-594.
39. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
40. Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
41. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
Atopic dermatitis (AD), or eczema, is the leading dermatologic diagnosis worldwide and is vexing to patients due to the itchiness of the rash. It is the leading cause of skin disease burden worldwide with a prevalence of 229,761,000 reported cases in 2010, presenting largely in preadolescence but also persisting through adulthood.1 Using the children’s life quality index, it has been demonstrated that AD has a greater impact on health-related quality of life than renal disease and cystic fibrosis.2 The overall burden of AD includes stress on the patient and his/her family as well as financial burdens that have been estimated to be similar to that of type 1 diabetes mellitus.3
Epidemiology of AD
The worldwide prevalence of AD varies by country and age group surveyed, with a higher prevalence in wealthy developed nations (eg, the United States) compared to poorer developing nations.4 Efforts to identify prevalence data for AD in the United States have been approached through a variety of strategies. A group in Oregon estimated the prevalence of AD in children aged 5 to 9 years to be 17.2% via a survey of parents (N=1465) and 11.8% with doctor-diagnosed eczema. In the same study, the question “Has a doctor ever said that your child has eczema?” was found to have a 91.3% predictive correlation.5 Analysis of the 2003 National Survey of Children’s Health demonstrated the overall US prevalence of pediatric AD to be 10.7% in 102,353 children 17 years or younger, with a range of 8.7% to 18.1% by region.6
In its evaluation of the worldwide prevalence of AD, the International Study of Asthma and Allergies in Childhood ranked the United States 17th.7,8 The prevalence of AD in developed countries such as the United States is fluid and is expected to increase if the trends from the last 20 years remain true. In an assessment of the National Health Interview Survey data from 1997 to 2011 based on responses to the question, “During the past 12 months, has your child had eczema or any kind of skin allergy?”, the Centers for Disease Control and Prevention identified an increase in the prevalence of AD in patients aged 0 to 17 years from 7.4% in 1997-1999 to 12.5% in 2009-2011.9 Rising prevalence seems to be paired with rising incidence in the total number of severe intractable cases, reduced clearance at the approach of grade school, or cases persisting into adulthood.
Racial Disparity in AD
Racial disparity worldwide and migration are thought to contribute to the prevalence of and therapeutic need for AD. For example, in the United Kingdom, the prevalence of AD in London-born Afro-Caribbean children versus white children (total cross-section, N=693 [junior school children]) was 16.3% and 8.7%, respectively.10 In the United States, black children were more likely to have AD than white children (odds ratio, 1.7).6 Asian and black children also were more likely to present to a physician for treatment of AD than white children.6,10-13
Definition and Diagnostic Considerations
According to Hanifin,14 “Eczema represents a family of inflammatory skin conditions characterized by pruritic, papulovesicular, sometimes weeping dermatitis. All demonstrate the histological hallmark of spongiosis, which helps to distinguish the eczemas from papulosquamous diseases such as psoriasis.”14 Atopic dermatitis is a variant of eczema; however, most laymen identify eczema and AD as being one and the same.
The Hanifin and Rajka15 criteria are the major diagnostic criteria for AD but are difficult to use in clinical practice. Three of the following 4 major criteria are needed for diagnosis: (1) pruritus, which is present universally; (2) typical morphology and distribution; (3) chronic or chronically relapsing dermatitis; and (4) personal and/or family history of atopy. Additionally, 3 of the following 23 minor criteria are needed for diagnosis: xerosis; ichthyosis vulgaris, palmar hyperlinearity, or keratosis pilaris; positive skin prick test; elevated serum IgE level; early age of onset; tendency toward cutaneous infections or impaired cell-mediated immunity; tendency toward nonspecific hand or foot dermatitis; nipple eczema; cheilitis; recurrent conjunctivitis; Dennie-Morgan fold (infraorbital fold); keratoconus; anterior subcapsular cataracts; orbital darkening; facial pallor or facial erythema; pityriasis alba; anterior neck folds; itching when sweating; intolerance to wool and lipid solvents; perifollicular accentuation; skin reactions from ingested foods or by food contact; environmental or emotional factors; and lesional/nonlesional white dermographism or delayed blanch.15-17
More pragmatic streamlined diagnostic criteria were established by Eichenfield et al.18 According to these guidelines, essential features for AD include pruritus and eczema. Important features seen in most cases and adding support to the diagnosis include early age of onset, atopy, and xerosis.18 In clinical practice, diagnosis is often made based on a pruritic relapsing condition in typical locations including the face, neck, and extensor surfaces in infants and children.
Age Considerations
Diagnosis of AD is made by 5 years of age in 85% to 90% of children who will develop the disease and by age 1 year in 60% to 65%.6,19,20 Atopic dermatitis will persist into adulthood in up to one-third of children.21,22 Infantile AD is characterized by erythematous, oozing, excoriated plaques on the cheeks (sparing the nose), scalp, trunk, and extensor surfaces. Pruritus is always seen in AD and can be a source of morbidity.16-18 Seborrheic dermatitis may complicate or overlap with AD in infancy.22
By 2 years of age, most children who are going to develop AD begin to show disease signs of childhood AD characterized by flexural lesions and lesions on the neck and in the postauricular area with sparing of the diaper area.23 Adult AD often presents as eczema of the hands and/or feet. Hand eczema in adulthood is correlated with a prior history of childhood hand eczema and/or childhood AD as well as wet work and caring for small children.24 Children with skin of color may manifest with follicular eczema as their primary disease phenotype. Facial and eyelid dermatitis are more common in Asian females, infants, and teenagers.12,25 Other disease phenotypes that are common in patients with skin of color include lichenoid AD and postinflammatory hypopigmentation.12
Pathogenesis of AD
There are 2 theories on the pathogenesis of AD known as the inside-out and outside-in hypotheses.26 The inside-out hypothesis suggests that allergic triggering leads to a weakened skin barrier that furthers allergen introduction and presentation, while the outside-in hypothesis suggests that the skin barrier is weakened in AD and allows for the presentation of allergens. Both theories have validity and biologic basis, and both may in fact be true in certain individuals.26
The Skin Barrier: An Overview
The skin barrier is a complex set of factors present and functional at birth that seal the keratinocytes and the interkeratinocyte space so that the skin can perform key processes and functions including retention of fluid, exclusion of allergens, protection from UV light and solvents, and prevention of pathogen entry (eg, infections).27-29 The superficial stratum corneum or the cornified envelope consists of keratinocytes with intercellular stripes of hydrophobic and hydrophilic substances formed by various intercellular lipids, largely ceramides, cholesterol, and free fatty acids.30,31 Keratinocytes are the first responders to a variety of environmental insults with the production of IL-18, RANTES (regulated on activation, normal T-expressed, and presumably secreted), granulocyte-macrophage colony-stimulating factor, and thymic stromal lymphopoietin. These inflammatory substances produce acute and chronic inflammation, mast cell reactivity, and T-cell activation.14 Corneodesmosins link the keratinocytes. Peptidases released will cleave the corneodesmosins and allow normal desquamation or shedding of surface skin, which is replaced by division of stem cells in the basal layer.29
The stratum granulosum is the layer beneath the stratum corneum that co-contributes to barrier activity. The stratum granulosum is absent or reduced histologically in ichthyosis vulgaris,32 a form of skin dryness linked to filaggrin mutations and AD. Filaggrin breakdown creates natural moisturizing factor, a series of hygroscopic compounds that attract water into the skin.33 Histidine, a filaggrin breakdown product, is used by urocanic acid to process UV light insults.34 Filaggrin also contributes to other barrier functions including pH and stratum corneum cohesion as well as paracellular permeability of the stratum corneum. Tight junctions in the stratum granulosum include claudin-1 and claudin-6 and provide another barrier feature.29
The skin barrier is composed of lipids and keratinocytes. Ceramides, which represent one type of lipids, are reduced in AD, causing alteration in the lamellar pattern35 and increased transepidermal water loss. Furthermore, the stratum corneum is thickened in AD, possibly in response to trauma, and hydration is reduced.36 Filaggrin (chromosome arm 1q21.3) is formed from the 400-kDa+ precursor profilaggrin through dephosphorylation and cleavage, and it performs an essential function in the skin barrier through its differential cleavage and breakdown as well as release of natural moisturizing factor and other compounds.37 Filaggrin mutations are linked to AD and ichthyosis vulgaris; however, barrier defects as evidenced by transepidermal water loss in the absence of filaggrin mutation are sufficient to allow for sensitization to allergens through the skin.29 Filaggrin mutations have been associated with AD development and vary in prevalence worldwide. In the United Kingdom, a prevalence study of filaggrin mutations in patients aged 7 to 9 years (N=792) demonstrated an 18.4% carrier rate in AD patients versus 12.9% in controls.34 A similar study in Sweden (N=3301) showed carrier rates of 13.5% versus 6.5%, respectively.38 Although filaggrin mutations are lower in black patients,39 ceramide content may be reduced in this population, demonstrating that a variety of skin barrier defects can result in AD. Carriers of filaggrin mutations are more likely to have eczema on skin exposed to environmental factors (eg, face, hands).40
Barrier Defects Contributing to AD
The breakdown of the stratum corneum allows for antigen presentation to Langerhans cells, the dendritic antigen-presenting cells of the skin. Breaks in the stratum corneum may occur from scratching. These macroscopic breaks are large, whereas the breaks that otherwise occur due to barrier breakdown may be more microscopic in nature. Scratching causes aggravation of the helper T cell (TH2) response.29 For example, it allows the dendritic ends of Langerhans cells to be exposed to antigens. The dendritic ends capture allergens through IgE (may be elevated in AD29), which is bound to the high-affinity FCER1 receptors on Langerhans cells. Rather than causing a type I hypersensitivity reaction, these Langerhans cells are activated and move to the lymph nodes where they present antigen and initiate a cascade of proinflammatory activity. This TH2 cascade includes release of cytokines such as IL-2, IL-4, IL-8, IL-10, tumor necrosis factor α, and IFN-γ.26,29
Transepidermal water loss and barrier dysfunction contribute to disease activity and facilitate food/environmental allergen sensitization by allowing increased penetration of allergens through the skin to be presented by Langerhans cells to TH1 cells (sensitization phase). The Langerhans cells can reach their dendritic ends through tight junctions and into the stratum corneum, allowing them to reach surface allergens when the barrier is impaired. Ultimate expansion to systemic allergy (effector phase) occurs when dendritic cells move to draining lymph nodes, causing antigen presentation to CD4 and/or CD8 cells. Langerhans cells and dendritic cell sensitization through the weakened skin is believed to be the basis or role of barrier disruption as a trigger of atopic diseases, including AD and food and environmental allergies.
Many different forms of barrier disruption can cause a TH2 response in AD. The TH2 response triggers a constellation of proinflammatory activities including release of IL-4, associated with eosinophilia and elevated IgE levels, the latter being minor criterion in the diagnosis of AD.15 One mechanism by which the TH2 response is elicited may be the release of molecules such as danger-associated molecule patterns that may elicit recruitment of other inflammatory cells. Helper T cell (TH2) activity also can worsen barrier defects through IL-4 and IL-13 release, which can reduce filaggrin expression,29,41 and can aggravate barrier dysfunction in AD.
Inflammatory activation in AD also may involve inflammatory dendritic epidermal cells (IDECs). The IDECs can be tolerogenic or immunogenic mature phenotypes. The IDECs activate helper T cells (TH1), which may contribute to long-term AD activity.
Conclusion
Atopic dermatitis is a common skin condition worldwide and is characterized by the hallmark of pruritus and features that include a typical pattern, history of atopy (personal or family), and usually xerosis and early disease onset. Barrier dysfunction and immune dysregulation are prominent in AD, both of which aggravate the other and may encourage increased development of allergies and other forms of atopy over time.
Atopic dermatitis (AD), or eczema, is the leading dermatologic diagnosis worldwide and is vexing to patients due to the itchiness of the rash. It is the leading cause of skin disease burden worldwide with a prevalence of 229,761,000 reported cases in 2010, presenting largely in preadolescence but also persisting through adulthood.1 Using the children’s life quality index, it has been demonstrated that AD has a greater impact on health-related quality of life than renal disease and cystic fibrosis.2 The overall burden of AD includes stress on the patient and his/her family as well as financial burdens that have been estimated to be similar to that of type 1 diabetes mellitus.3
Epidemiology of AD
The worldwide prevalence of AD varies by country and age group surveyed, with a higher prevalence in wealthy developed nations (eg, the United States) compared to poorer developing nations.4 Efforts to identify prevalence data for AD in the United States have been approached through a variety of strategies. A group in Oregon estimated the prevalence of AD in children aged 5 to 9 years to be 17.2% via a survey of parents (N=1465) and 11.8% with doctor-diagnosed eczema. In the same study, the question “Has a doctor ever said that your child has eczema?” was found to have a 91.3% predictive correlation.5 Analysis of the 2003 National Survey of Children’s Health demonstrated the overall US prevalence of pediatric AD to be 10.7% in 102,353 children 17 years or younger, with a range of 8.7% to 18.1% by region.6
In its evaluation of the worldwide prevalence of AD, the International Study of Asthma and Allergies in Childhood ranked the United States 17th.7,8 The prevalence of AD in developed countries such as the United States is fluid and is expected to increase if the trends from the last 20 years remain true. In an assessment of the National Health Interview Survey data from 1997 to 2011 based on responses to the question, “During the past 12 months, has your child had eczema or any kind of skin allergy?”, the Centers for Disease Control and Prevention identified an increase in the prevalence of AD in patients aged 0 to 17 years from 7.4% in 1997-1999 to 12.5% in 2009-2011.9 Rising prevalence seems to be paired with rising incidence in the total number of severe intractable cases, reduced clearance at the approach of grade school, or cases persisting into adulthood.
Racial Disparity in AD
Racial disparity worldwide and migration are thought to contribute to the prevalence of and therapeutic need for AD. For example, in the United Kingdom, the prevalence of AD in London-born Afro-Caribbean children versus white children (total cross-section, N=693 [junior school children]) was 16.3% and 8.7%, respectively.10 In the United States, black children were more likely to have AD than white children (odds ratio, 1.7).6 Asian and black children also were more likely to present to a physician for treatment of AD than white children.6,10-13
Definition and Diagnostic Considerations
According to Hanifin,14 “Eczema represents a family of inflammatory skin conditions characterized by pruritic, papulovesicular, sometimes weeping dermatitis. All demonstrate the histological hallmark of spongiosis, which helps to distinguish the eczemas from papulosquamous diseases such as psoriasis.”14 Atopic dermatitis is a variant of eczema; however, most laymen identify eczema and AD as being one and the same.
The Hanifin and Rajka15 criteria are the major diagnostic criteria for AD but are difficult to use in clinical practice. Three of the following 4 major criteria are needed for diagnosis: (1) pruritus, which is present universally; (2) typical morphology and distribution; (3) chronic or chronically relapsing dermatitis; and (4) personal and/or family history of atopy. Additionally, 3 of the following 23 minor criteria are needed for diagnosis: xerosis; ichthyosis vulgaris, palmar hyperlinearity, or keratosis pilaris; positive skin prick test; elevated serum IgE level; early age of onset; tendency toward cutaneous infections or impaired cell-mediated immunity; tendency toward nonspecific hand or foot dermatitis; nipple eczema; cheilitis; recurrent conjunctivitis; Dennie-Morgan fold (infraorbital fold); keratoconus; anterior subcapsular cataracts; orbital darkening; facial pallor or facial erythema; pityriasis alba; anterior neck folds; itching when sweating; intolerance to wool and lipid solvents; perifollicular accentuation; skin reactions from ingested foods or by food contact; environmental or emotional factors; and lesional/nonlesional white dermographism or delayed blanch.15-17
More pragmatic streamlined diagnostic criteria were established by Eichenfield et al.18 According to these guidelines, essential features for AD include pruritus and eczema. Important features seen in most cases and adding support to the diagnosis include early age of onset, atopy, and xerosis.18 In clinical practice, diagnosis is often made based on a pruritic relapsing condition in typical locations including the face, neck, and extensor surfaces in infants and children.
Age Considerations
Diagnosis of AD is made by 5 years of age in 85% to 90% of children who will develop the disease and by age 1 year in 60% to 65%.6,19,20 Atopic dermatitis will persist into adulthood in up to one-third of children.21,22 Infantile AD is characterized by erythematous, oozing, excoriated plaques on the cheeks (sparing the nose), scalp, trunk, and extensor surfaces. Pruritus is always seen in AD and can be a source of morbidity.16-18 Seborrheic dermatitis may complicate or overlap with AD in infancy.22
By 2 years of age, most children who are going to develop AD begin to show disease signs of childhood AD characterized by flexural lesions and lesions on the neck and in the postauricular area with sparing of the diaper area.23 Adult AD often presents as eczema of the hands and/or feet. Hand eczema in adulthood is correlated with a prior history of childhood hand eczema and/or childhood AD as well as wet work and caring for small children.24 Children with skin of color may manifest with follicular eczema as their primary disease phenotype. Facial and eyelid dermatitis are more common in Asian females, infants, and teenagers.12,25 Other disease phenotypes that are common in patients with skin of color include lichenoid AD and postinflammatory hypopigmentation.12
Pathogenesis of AD
There are 2 theories on the pathogenesis of AD known as the inside-out and outside-in hypotheses.26 The inside-out hypothesis suggests that allergic triggering leads to a weakened skin barrier that furthers allergen introduction and presentation, while the outside-in hypothesis suggests that the skin barrier is weakened in AD and allows for the presentation of allergens. Both theories have validity and biologic basis, and both may in fact be true in certain individuals.26
The Skin Barrier: An Overview
The skin barrier is a complex set of factors present and functional at birth that seal the keratinocytes and the interkeratinocyte space so that the skin can perform key processes and functions including retention of fluid, exclusion of allergens, protection from UV light and solvents, and prevention of pathogen entry (eg, infections).27-29 The superficial stratum corneum or the cornified envelope consists of keratinocytes with intercellular stripes of hydrophobic and hydrophilic substances formed by various intercellular lipids, largely ceramides, cholesterol, and free fatty acids.30,31 Keratinocytes are the first responders to a variety of environmental insults with the production of IL-18, RANTES (regulated on activation, normal T-expressed, and presumably secreted), granulocyte-macrophage colony-stimulating factor, and thymic stromal lymphopoietin. These inflammatory substances produce acute and chronic inflammation, mast cell reactivity, and T-cell activation.14 Corneodesmosins link the keratinocytes. Peptidases released will cleave the corneodesmosins and allow normal desquamation or shedding of surface skin, which is replaced by division of stem cells in the basal layer.29
The stratum granulosum is the layer beneath the stratum corneum that co-contributes to barrier activity. The stratum granulosum is absent or reduced histologically in ichthyosis vulgaris,32 a form of skin dryness linked to filaggrin mutations and AD. Filaggrin breakdown creates natural moisturizing factor, a series of hygroscopic compounds that attract water into the skin.33 Histidine, a filaggrin breakdown product, is used by urocanic acid to process UV light insults.34 Filaggrin also contributes to other barrier functions including pH and stratum corneum cohesion as well as paracellular permeability of the stratum corneum. Tight junctions in the stratum granulosum include claudin-1 and claudin-6 and provide another barrier feature.29
The skin barrier is composed of lipids and keratinocytes. Ceramides, which represent one type of lipids, are reduced in AD, causing alteration in the lamellar pattern35 and increased transepidermal water loss. Furthermore, the stratum corneum is thickened in AD, possibly in response to trauma, and hydration is reduced.36 Filaggrin (chromosome arm 1q21.3) is formed from the 400-kDa+ precursor profilaggrin through dephosphorylation and cleavage, and it performs an essential function in the skin barrier through its differential cleavage and breakdown as well as release of natural moisturizing factor and other compounds.37 Filaggrin mutations are linked to AD and ichthyosis vulgaris; however, barrier defects as evidenced by transepidermal water loss in the absence of filaggrin mutation are sufficient to allow for sensitization to allergens through the skin.29 Filaggrin mutations have been associated with AD development and vary in prevalence worldwide. In the United Kingdom, a prevalence study of filaggrin mutations in patients aged 7 to 9 years (N=792) demonstrated an 18.4% carrier rate in AD patients versus 12.9% in controls.34 A similar study in Sweden (N=3301) showed carrier rates of 13.5% versus 6.5%, respectively.38 Although filaggrin mutations are lower in black patients,39 ceramide content may be reduced in this population, demonstrating that a variety of skin barrier defects can result in AD. Carriers of filaggrin mutations are more likely to have eczema on skin exposed to environmental factors (eg, face, hands).40
Barrier Defects Contributing to AD
The breakdown of the stratum corneum allows for antigen presentation to Langerhans cells, the dendritic antigen-presenting cells of the skin. Breaks in the stratum corneum may occur from scratching. These macroscopic breaks are large, whereas the breaks that otherwise occur due to barrier breakdown may be more microscopic in nature. Scratching causes aggravation of the helper T cell (TH2) response.29 For example, it allows the dendritic ends of Langerhans cells to be exposed to antigens. The dendritic ends capture allergens through IgE (may be elevated in AD29), which is bound to the high-affinity FCER1 receptors on Langerhans cells. Rather than causing a type I hypersensitivity reaction, these Langerhans cells are activated and move to the lymph nodes where they present antigen and initiate a cascade of proinflammatory activity. This TH2 cascade includes release of cytokines such as IL-2, IL-4, IL-8, IL-10, tumor necrosis factor α, and IFN-γ.26,29
Transepidermal water loss and barrier dysfunction contribute to disease activity and facilitate food/environmental allergen sensitization by allowing increased penetration of allergens through the skin to be presented by Langerhans cells to TH1 cells (sensitization phase). The Langerhans cells can reach their dendritic ends through tight junctions and into the stratum corneum, allowing them to reach surface allergens when the barrier is impaired. Ultimate expansion to systemic allergy (effector phase) occurs when dendritic cells move to draining lymph nodes, causing antigen presentation to CD4 and/or CD8 cells. Langerhans cells and dendritic cell sensitization through the weakened skin is believed to be the basis or role of barrier disruption as a trigger of atopic diseases, including AD and food and environmental allergies.
Many different forms of barrier disruption can cause a TH2 response in AD. The TH2 response triggers a constellation of proinflammatory activities including release of IL-4, associated with eosinophilia and elevated IgE levels, the latter being minor criterion in the diagnosis of AD.15 One mechanism by which the TH2 response is elicited may be the release of molecules such as danger-associated molecule patterns that may elicit recruitment of other inflammatory cells. Helper T cell (TH2) activity also can worsen barrier defects through IL-4 and IL-13 release, which can reduce filaggrin expression,29,41 and can aggravate barrier dysfunction in AD.
Inflammatory activation in AD also may involve inflammatory dendritic epidermal cells (IDECs). The IDECs can be tolerogenic or immunogenic mature phenotypes. The IDECs activate helper T cells (TH1), which may contribute to long-term AD activity.
Conclusion
Atopic dermatitis is a common skin condition worldwide and is characterized by the hallmark of pruritus and features that include a typical pattern, history of atopy (personal or family), and usually xerosis and early disease onset. Barrier dysfunction and immune dysregulation are prominent in AD, both of which aggravate the other and may encourage increased development of allergies and other forms of atopy over time.
1. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
2. Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
3. Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
4. Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
5. Laughter D, Istvan JA, Tofte SJ, et al. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649-655.
6. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
7. Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-1258.
8. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225-1232.
9. Hansen TE, Evjenth B, Holt J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: three surveys during the period 1985-2008. Acta Paediatr. 2013;102:47-52.
10. Williams HC, Pembroke AC, Forsdyke H, et al. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol. 1995;32:212-217.
11. Horii KA, Simon SD, Liu DY, et al. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120:e527-e534.
12. Silverberg NB. Eczematous diseases. In: Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity. New York, NY: Springer; 2012:69-88.
13. Gupta J, Grube E, Ericksen MB, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725-730.
14. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
15. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980;92:44-47.
16. Queille-Roussel C, Raynaud F, Saurat JH. A prospective computerized study of 500 cases of atopic dermatitis in childhood. I. Initial analysis of 250 parameters. Acta Derm Venereol Suppl (Stockh). 1985;114:87-92.
17. Böhme M, Svensson A, Kull I, et al. Hanifin’s and Rajka’s minor criteria for atopic dermatitis: which do 2-year-olds exhibit? J Am Acad Dermatol. 2000;43:785-792.
18. Eichenfield LF, Hanifin JM, Luger TA, et al. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol. 2003;49:1088-1095.
19. Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
20. Perkin MR, Strachan DP, Williams HC, et al. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol. 2004;15:221-229.
21. Ellis CN, Mancini AJ, Paller AS, et al. Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg. 2012;31(suppl 2):S18-S22.
22. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
23. Meding B, Wrangsjö K, Järvholm B. Hand eczema extent and morphology—association and influence on long-term prognosis. J Invest Dermatol. 2007;127:2147-2151.
24. Mortz CG, Bindslev-Jensen C, Andersen KE. Hand eczema in The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS): prevalence, incidence and risk factors from adolescence to adulthood [published online August 7, 2014]. Br J Dermatol. 2014;171:313-323.
25. Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
26. Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
27. Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care [published online December 8, 2014]. Clin Dermatol. 2015;33:271-280.
28. Miyagaki T, Sugaya M. Recent advances in atopic dermatitis and psoriasis: genetic background, barrier function, and therapeutic targets. J Dermatol Sci. 2015;78:89-94.
29. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
30. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
31. Janssens M, van Smeden J, Gooris GS, et al. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131:2136-2138.
32. Fitch N, Segool R, Ferenczy A, et al. Dominant ichthyosis vulgaris with an ultrastructurally normal granular layer. Clin Genet. 1976;9:71-76.
33. Chandar P, Nole G, Johnson AW. Understanding natural moisturizing mechanisms: implications for moisturizer technology. Cutis. 2009;84(suppl 1):2-15.
34. Brown SJ, Relton CL, Liao H, et al. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol. 2008;121:940-946.
35. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
36. Nemoto-Hasebe I, Akiyama M, Nomura T, et al. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009;129:682-689.
37. Hoste E, Kemperman P, Devos M, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233-2241.
38. Ballardini N, Kull I, Söderhäll C, et al. Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol. 2013;168:588-594.
39. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
40. Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
41. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
1. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
2. Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
3. Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
4. Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
5. Laughter D, Istvan JA, Tofte SJ, et al. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649-655.
6. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
7. Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-1258.
8. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225-1232.
9. Hansen TE, Evjenth B, Holt J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: three surveys during the period 1985-2008. Acta Paediatr. 2013;102:47-52.
10. Williams HC, Pembroke AC, Forsdyke H, et al. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol. 1995;32:212-217.
11. Horii KA, Simon SD, Liu DY, et al. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120:e527-e534.
12. Silverberg NB. Eczematous diseases. In: Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity. New York, NY: Springer; 2012:69-88.
13. Gupta J, Grube E, Ericksen MB, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725-730.
14. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
15. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980;92:44-47.
16. Queille-Roussel C, Raynaud F, Saurat JH. A prospective computerized study of 500 cases of atopic dermatitis in childhood. I. Initial analysis of 250 parameters. Acta Derm Venereol Suppl (Stockh). 1985;114:87-92.
17. Böhme M, Svensson A, Kull I, et al. Hanifin’s and Rajka’s minor criteria for atopic dermatitis: which do 2-year-olds exhibit? J Am Acad Dermatol. 2000;43:785-792.
18. Eichenfield LF, Hanifin JM, Luger TA, et al. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol. 2003;49:1088-1095.
19. Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
20. Perkin MR, Strachan DP, Williams HC, et al. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol. 2004;15:221-229.
21. Ellis CN, Mancini AJ, Paller AS, et al. Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg. 2012;31(suppl 2):S18-S22.
22. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
23. Meding B, Wrangsjö K, Järvholm B. Hand eczema extent and morphology—association and influence on long-term prognosis. J Invest Dermatol. 2007;127:2147-2151.
24. Mortz CG, Bindslev-Jensen C, Andersen KE. Hand eczema in The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS): prevalence, incidence and risk factors from adolescence to adulthood [published online August 7, 2014]. Br J Dermatol. 2014;171:313-323.
25. Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
26. Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
27. Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care [published online December 8, 2014]. Clin Dermatol. 2015;33:271-280.
28. Miyagaki T, Sugaya M. Recent advances in atopic dermatitis and psoriasis: genetic background, barrier function, and therapeutic targets. J Dermatol Sci. 2015;78:89-94.
29. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
30. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
31. Janssens M, van Smeden J, Gooris GS, et al. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131:2136-2138.
32. Fitch N, Segool R, Ferenczy A, et al. Dominant ichthyosis vulgaris with an ultrastructurally normal granular layer. Clin Genet. 1976;9:71-76.
33. Chandar P, Nole G, Johnson AW. Understanding natural moisturizing mechanisms: implications for moisturizer technology. Cutis. 2009;84(suppl 1):2-15.
34. Brown SJ, Relton CL, Liao H, et al. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol. 2008;121:940-946.
35. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
36. Nemoto-Hasebe I, Akiyama M, Nomura T, et al. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009;129:682-689.
37. Hoste E, Kemperman P, Devos M, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233-2241.
38. Ballardini N, Kull I, Söderhäll C, et al. Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol. 2013;168:588-594.
39. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
40. Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
41. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
Practice Points
- The impact of atopic dermatitis (AD) on health-related quality of life mimics that of chronic childhood illnesses such as cystic fibrosis.
- The prevalence of pediatric AD in the United States is estimated at more than 10% of children, with a 1.7 increased odds ratio in black children.
- Diagnosis generally is made based on the presence of a pruritic eczematous eruption with typical morphology and a personal and/or family history of atopy.
- Atopic dermatitis is caused by a complex interplay of skin barrier dysfunction and immune tendency toward allergy development.
Piebaldism in Children
Case Report
A 14-year-old adolescent girl presented with multiple asymptomatic light-colored patches on the forehead, bilateral arms, and legs that had been present since birth. The patient reported that the size of the patches had increased in proportion to her overall growth and that “brown spots” had gradually started to form within and around the patches. She noted that her father and paternal grandfather also had similar clinical findings. A review of systems was negative for hearing impairment, ocular abnormalities, and recurrent infections.
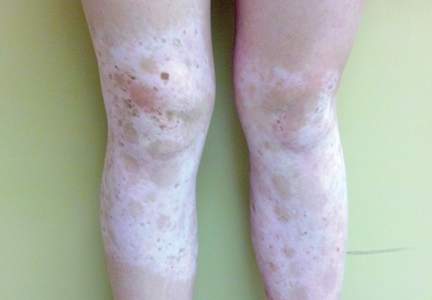
Physical examination revealed an otherwise healthy adolescent girl with Fitzpatrick skin type I and homogeneous blue eyes. Large symmetric depigmented patches were noted on the extensor surfaces of the mid legs and mid forearms (Figure). Macules of baseline pigment and hyperpigmentation were irregularly scattered within and at the periphery of the patches. A triangular hypopigmented patch at the hairline on the mid frontal scalp hairline was accompanied by depigmentation of terminal hairs in this region.
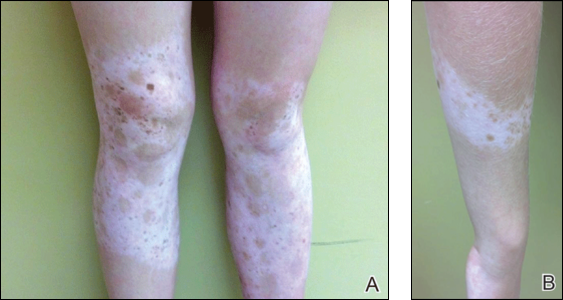
A clinical diagnosis of piebaldism was made and was discussed at length with the patient. Due to the benign nature of the condition and patient preference, no therapeutic intervention was pursued. It was recommended that she apply sunscreen daily for protection of the depigmented areas.
Comment
Piebaldism is a rare hereditary disorder of melanocyte development characterized clinically by the presence of congenital poliosis and leukoderma.1 The exact prevalence of piebaldism is unknown, but it has been estimated that less than 1 in 20,000 children are born with this condition.2 Poliosis circumscripta, traditionally known as white forelock, may be the only manifestation in 80% to 90% of cases and is present at birth.3 The white forelock typically appears in a triangular shape and the underlying skin of the scalp also is amelanotic. The eyebrows and eyelashes also may be involved.3
Characteristically, lesions of leukoderma are well-circumscribed, irregular, white patches that are often accompanied by hyperpigmented macules noted on both depigmented and unaffected adjacent skin.1 The lesions are classically distributed on the central forehead and anterior trunk, with extension to the flanks, anterior mid arms, and mid legs. Sparing of the dorsal midline, hands, feet, and periorificial area is characteristic.1
Depigmented patches typically are nonprogressive and persist into adulthood. Additional hyperpigmented macules may develop at or within the margins of the white patches. Partial or complete repigmentation may occur spontaneously or after trauma in some patients.2 Some children may develop café au lait lesions and may be misdiagnosed as concurrently having neurofibromatosis type I and piebaldism. If neurofibromatosis type I is suspected, patients should be thoroughly evaluated for other diagnostic criteria of this syndrome, as there may be cases of coexistence and overlap with piebaldism.4
Piebaldism is an autosomal-dominant inherited disorder and most commonly develops as a consequence of a mutation in the c-kit proto-oncogene (located on chromosome arm 14q12), which affects melanoblast migration, proliferation, differentiation, and survival.2 In piebaldism, the site of mutation within the gene correlates with the severity of the phenotype.5 Melanocytes are histologically and ultrastructurally absent or considerably reduced in depigmented patches but are normal in number in the hyperpigmented areas.2
Rare cases of piebaldism have been reported in association with other diseases, including congenital megacolon, congenital dyserythropoietic anemia type II, Diamond-Blackfan anemia, Grover disease (transient acantholytic dermatosis), and glycogen-storage disease type 1a.1,6 Poliosis alone may be the initial presentation of certain genetic syndromes, including Waardenburg syndrome (WS) and tuberous sclerosis; it also may be acquired in the setting of several conditions, including vitiligo, Vogt-Koyanagi-Harada syndrome, Alezzandrini syndrome, alopecia areata, and sarcoidosis.3
Notably, the diagnosis of piebaldism should alert the clinician to the possibility of WS, an autosomal-dominant disease characterized by a congenital white forelock, leukoderma in a piebaldlike distribution, lateral displacement of the medial canthi, a hypertrophic nasal root, heterochromia iridis, and progressive sensorineural hearing loss.7 Four clinical subtypes of WS have been described, with various gene mutations implicated: type 1 is the classic form, type 2 lacks dystopia canthorum and has a stonger association with deafness, type 3 is associated with limb abnormalities, and type 4 is associated with congenital megacolon. A case of WS type 1 has been described in association with facial nerve palsy and lingua plicata, 2 main features of Melkerson-Rosenthal syndrome.8 Depigmentation in WS is caused by the absence of melanocytes in the affected areas as well as failed migration of melanocytes to the ears and eyes.3 Waardenburg syndrome may be distinguished from piebaldism by characteristic facial features of the disease and should prompt a thorough ocular and auditory examination in affected patients.9
Although not a diagnostic criterion, poliosis rarely has been reported as one of the earliest associated findings of tuberous sclerosis.3,10 Major cutaneous features of this disease include facial angiofibromas, hypomelanotic macules, shagreen patches (connective tissue nevi), periungual fibromas, molluscum pendulum, and café au lait macules.
Vitiligo also may be considered in the differential diagnosis of piebaldism and can be distinguished by the presence of depigmented patches in a typical acral and periorificial distribution, lack of congential presentation, and relatively progressive course. Vitiligo is characterized by an acquired loss of epidermal melanocytes, leading to depigmented macules and patches.1,3
Vitiligo, poliosis, and alopecia areata usually are late clinical manifestations of Vogt-Koyanagi-Harada syndrome, a rare condition characterized by an autoimmune response to melanocyte-associated antigens. This condition initially presents with neurologic and ocular manifestations including headache, muscle weakness, tinnitus, uveitis, and choroiditis prior to dermatologic manifestations.11
Alezzandrini syndrome, a rare and closely related disorder, is distinctly characterized by whitening of scalp hair, eyebrows, and eyelashes, along with unilateral depigmentation of facial skin. This presentation is associated with ipsilateral visual changes and hearing abnormalities.12
The absence of abnormal ocular, auditory, and neurologic examinations, along with lack of characteristic cutaneous features indicating any of the aforementioned disorders, highly suggests a diagnosis of piebaldism.
Piebaldism is considered a relatively benign disorder but can be highly socially disabling, which presents a therapeutic challenge in affected children. Depigmented skin in piebaldism generally is considered unresponsive to medical or light therapy.1 Topical treatments with makeup or artificial pigmenting agents (eg, dihydroxyacetone [an ingredient used in sunless tanning products]) are useful but temporary. Sunscreen should be used judiciously to avoid sunburn and reduce carcinogenic potential.13
Several surgical techniques have been reported for treatment of leukoderma but with variable success. Of those reported, micropunch transplantation (minigrafting) using epidermal donor sites of 1 to 1.25 mm is a relatively inexpensive and effective method but is limited by scarring at the donor site.14 Autologous cultured epidermal cellular grafting with a controlled number of melanocytes is reported to achieve greater than 75% repigmentation. It requires fewer donor sites and, therefore, results in less scarring.15 Additionally, use of the erbium-doped:YAG laser aids in deepithelialization of the recipient site, allowing for treatment of large piebald lesions during a single operation.16 Despite these advances, additional studies are needed to improve quality of life in those affected.
- Janjua SA, Khachemoune A, Guldbakke KK. Piebaldism: a case report and a concise review of the literature. Cutis. 2007;80:411-414.
- Agarwal S, Ojha A. Piebaldism: a brief report and review of the literature. Indian Dermatol Online J. 2012;3:144-147.
- Sleiman R, Kurban M, Succaria F, et al. Poliosis circumscripta: overview and underlying causes. J Am Acad Dermatol. 2013;69:625-633.
- Oiso N, Fukai K, Kawada A, et al. Piebaldism. J Dermatol. 2013;40:330-355.
- López V, Jordá E. Piebaldism in a 2-year-old girl. Dermatol Online J. 2011;17:13.
- Ghoshal B, Sarkar N, Bhattacharjee M, et al. Glycogen storage disease 1a with piebaldism. Indian Pediatr. 2012;49:235-236.
- Salvatore S, Carnevale C, Infussi R, et al. Waardenburg syndrome: a review of literature and case reports. Clin Ter. 2012;163:e85-e94.
- Dourmishev AL, Dourmishev LA, Schwartz RA, et al. Waardenburg syndrome. Int J Dermatol. 1999;38:656-663.
- Fistarol SK, Itin PH. Disorders of pigmentation. J Dtsch Dermatol Ges. 2010;8:187-201.
- McWilliam RC, Stephenson JB. Depigmented hair. the earliest sign of tuberous sclerosis. Arch Dis Child. 1978;53:961-963.
- Chan EW, Sanjay S, Chang BC. Headache, red eyes, blurred vision and hearing loss. diagnosis: Vogt-Koyanagi-Harada syndrome. CMAJ. 2010;182:1205-1209.
- Andrade A, Pithon M. Alezzandrini syndrome: report of a sixth clinical case. Dermatology (Basel). 2011;222:8-9.
- Suga Y, Ikejima A, Matsuba S, et al. Medical pearl: DHA application for camouflaging segmental vitiligo and piebald lesions. J Am Acad Dermatol. 2002;47:436-438.
- Neves DR, Régis Júnior JR, Oliveira PJ, et al. Melanocyte transplant in piebaldism: case report. An Bras Dermatol. 2010;85:384-388.
- Van geel N, Wallaeys E, Goh BK, et al. Long-term results of noncultured epidermal cellular grafting in vitiligo, halo naevi, piebaldism and naevus depigmentosus. Br J Dermatol. 2010;163:1186-1193.
- Guerra L, Primavera G, Raskovic D, et al. Permanent repigmentation of piebaldism by erbium:YAG laser and autologous cultured epidermis. Br J Dermatol. 2004;150:715-721.
Case Report
A 14-year-old adolescent girl presented with multiple asymptomatic light-colored patches on the forehead, bilateral arms, and legs that had been present since birth. The patient reported that the size of the patches had increased in proportion to her overall growth and that “brown spots” had gradually started to form within and around the patches. She noted that her father and paternal grandfather also had similar clinical findings. A review of systems was negative for hearing impairment, ocular abnormalities, and recurrent infections.
Physical examination revealed an otherwise healthy adolescent girl with Fitzpatrick skin type I and homogeneous blue eyes. Large symmetric depigmented patches were noted on the extensor surfaces of the mid legs and mid forearms (Figure). Macules of baseline pigment and hyperpigmentation were irregularly scattered within and at the periphery of the patches. A triangular hypopigmented patch at the hairline on the mid frontal scalp hairline was accompanied by depigmentation of terminal hairs in this region.

A clinical diagnosis of piebaldism was made and was discussed at length with the patient. Due to the benign nature of the condition and patient preference, no therapeutic intervention was pursued. It was recommended that she apply sunscreen daily for protection of the depigmented areas.
Comment
Piebaldism is a rare hereditary disorder of melanocyte development characterized clinically by the presence of congenital poliosis and leukoderma.1 The exact prevalence of piebaldism is unknown, but it has been estimated that less than 1 in 20,000 children are born with this condition.2 Poliosis circumscripta, traditionally known as white forelock, may be the only manifestation in 80% to 90% of cases and is present at birth.3 The white forelock typically appears in a triangular shape and the underlying skin of the scalp also is amelanotic. The eyebrows and eyelashes also may be involved.3
Characteristically, lesions of leukoderma are well-circumscribed, irregular, white patches that are often accompanied by hyperpigmented macules noted on both depigmented and unaffected adjacent skin.1 The lesions are classically distributed on the central forehead and anterior trunk, with extension to the flanks, anterior mid arms, and mid legs. Sparing of the dorsal midline, hands, feet, and periorificial area is characteristic.1
Depigmented patches typically are nonprogressive and persist into adulthood. Additional hyperpigmented macules may develop at or within the margins of the white patches. Partial or complete repigmentation may occur spontaneously or after trauma in some patients.2 Some children may develop café au lait lesions and may be misdiagnosed as concurrently having neurofibromatosis type I and piebaldism. If neurofibromatosis type I is suspected, patients should be thoroughly evaluated for other diagnostic criteria of this syndrome, as there may be cases of coexistence and overlap with piebaldism.4
Piebaldism is an autosomal-dominant inherited disorder and most commonly develops as a consequence of a mutation in the c-kit proto-oncogene (located on chromosome arm 14q12), which affects melanoblast migration, proliferation, differentiation, and survival.2 In piebaldism, the site of mutation within the gene correlates with the severity of the phenotype.5 Melanocytes are histologically and ultrastructurally absent or considerably reduced in depigmented patches but are normal in number in the hyperpigmented areas.2
Rare cases of piebaldism have been reported in association with other diseases, including congenital megacolon, congenital dyserythropoietic anemia type II, Diamond-Blackfan anemia, Grover disease (transient acantholytic dermatosis), and glycogen-storage disease type 1a.1,6 Poliosis alone may be the initial presentation of certain genetic syndromes, including Waardenburg syndrome (WS) and tuberous sclerosis; it also may be acquired in the setting of several conditions, including vitiligo, Vogt-Koyanagi-Harada syndrome, Alezzandrini syndrome, alopecia areata, and sarcoidosis.3
Notably, the diagnosis of piebaldism should alert the clinician to the possibility of WS, an autosomal-dominant disease characterized by a congenital white forelock, leukoderma in a piebaldlike distribution, lateral displacement of the medial canthi, a hypertrophic nasal root, heterochromia iridis, and progressive sensorineural hearing loss.7 Four clinical subtypes of WS have been described, with various gene mutations implicated: type 1 is the classic form, type 2 lacks dystopia canthorum and has a stonger association with deafness, type 3 is associated with limb abnormalities, and type 4 is associated with congenital megacolon. A case of WS type 1 has been described in association with facial nerve palsy and lingua plicata, 2 main features of Melkerson-Rosenthal syndrome.8 Depigmentation in WS is caused by the absence of melanocytes in the affected areas as well as failed migration of melanocytes to the ears and eyes.3 Waardenburg syndrome may be distinguished from piebaldism by characteristic facial features of the disease and should prompt a thorough ocular and auditory examination in affected patients.9
Although not a diagnostic criterion, poliosis rarely has been reported as one of the earliest associated findings of tuberous sclerosis.3,10 Major cutaneous features of this disease include facial angiofibromas, hypomelanotic macules, shagreen patches (connective tissue nevi), periungual fibromas, molluscum pendulum, and café au lait macules.
Vitiligo also may be considered in the differential diagnosis of piebaldism and can be distinguished by the presence of depigmented patches in a typical acral and periorificial distribution, lack of congential presentation, and relatively progressive course. Vitiligo is characterized by an acquired loss of epidermal melanocytes, leading to depigmented macules and patches.1,3
Vitiligo, poliosis, and alopecia areata usually are late clinical manifestations of Vogt-Koyanagi-Harada syndrome, a rare condition characterized by an autoimmune response to melanocyte-associated antigens. This condition initially presents with neurologic and ocular manifestations including headache, muscle weakness, tinnitus, uveitis, and choroiditis prior to dermatologic manifestations.11
Alezzandrini syndrome, a rare and closely related disorder, is distinctly characterized by whitening of scalp hair, eyebrows, and eyelashes, along with unilateral depigmentation of facial skin. This presentation is associated with ipsilateral visual changes and hearing abnormalities.12
The absence of abnormal ocular, auditory, and neurologic examinations, along with lack of characteristic cutaneous features indicating any of the aforementioned disorders, highly suggests a diagnosis of piebaldism.
Piebaldism is considered a relatively benign disorder but can be highly socially disabling, which presents a therapeutic challenge in affected children. Depigmented skin in piebaldism generally is considered unresponsive to medical or light therapy.1 Topical treatments with makeup or artificial pigmenting agents (eg, dihydroxyacetone [an ingredient used in sunless tanning products]) are useful but temporary. Sunscreen should be used judiciously to avoid sunburn and reduce carcinogenic potential.13
Several surgical techniques have been reported for treatment of leukoderma but with variable success. Of those reported, micropunch transplantation (minigrafting) using epidermal donor sites of 1 to 1.25 mm is a relatively inexpensive and effective method but is limited by scarring at the donor site.14 Autologous cultured epidermal cellular grafting with a controlled number of melanocytes is reported to achieve greater than 75% repigmentation. It requires fewer donor sites and, therefore, results in less scarring.15 Additionally, use of the erbium-doped:YAG laser aids in deepithelialization of the recipient site, allowing for treatment of large piebald lesions during a single operation.16 Despite these advances, additional studies are needed to improve quality of life in those affected.
Case Report
A 14-year-old adolescent girl presented with multiple asymptomatic light-colored patches on the forehead, bilateral arms, and legs that had been present since birth. The patient reported that the size of the patches had increased in proportion to her overall growth and that “brown spots” had gradually started to form within and around the patches. She noted that her father and paternal grandfather also had similar clinical findings. A review of systems was negative for hearing impairment, ocular abnormalities, and recurrent infections.
Physical examination revealed an otherwise healthy adolescent girl with Fitzpatrick skin type I and homogeneous blue eyes. Large symmetric depigmented patches were noted on the extensor surfaces of the mid legs and mid forearms (Figure). Macules of baseline pigment and hyperpigmentation were irregularly scattered within and at the periphery of the patches. A triangular hypopigmented patch at the hairline on the mid frontal scalp hairline was accompanied by depigmentation of terminal hairs in this region.

A clinical diagnosis of piebaldism was made and was discussed at length with the patient. Due to the benign nature of the condition and patient preference, no therapeutic intervention was pursued. It was recommended that she apply sunscreen daily for protection of the depigmented areas.
Comment
Piebaldism is a rare hereditary disorder of melanocyte development characterized clinically by the presence of congenital poliosis and leukoderma.1 The exact prevalence of piebaldism is unknown, but it has been estimated that less than 1 in 20,000 children are born with this condition.2 Poliosis circumscripta, traditionally known as white forelock, may be the only manifestation in 80% to 90% of cases and is present at birth.3 The white forelock typically appears in a triangular shape and the underlying skin of the scalp also is amelanotic. The eyebrows and eyelashes also may be involved.3
Characteristically, lesions of leukoderma are well-circumscribed, irregular, white patches that are often accompanied by hyperpigmented macules noted on both depigmented and unaffected adjacent skin.1 The lesions are classically distributed on the central forehead and anterior trunk, with extension to the flanks, anterior mid arms, and mid legs. Sparing of the dorsal midline, hands, feet, and periorificial area is characteristic.1
Depigmented patches typically are nonprogressive and persist into adulthood. Additional hyperpigmented macules may develop at or within the margins of the white patches. Partial or complete repigmentation may occur spontaneously or after trauma in some patients.2 Some children may develop café au lait lesions and may be misdiagnosed as concurrently having neurofibromatosis type I and piebaldism. If neurofibromatosis type I is suspected, patients should be thoroughly evaluated for other diagnostic criteria of this syndrome, as there may be cases of coexistence and overlap with piebaldism.4
Piebaldism is an autosomal-dominant inherited disorder and most commonly develops as a consequence of a mutation in the c-kit proto-oncogene (located on chromosome arm 14q12), which affects melanoblast migration, proliferation, differentiation, and survival.2 In piebaldism, the site of mutation within the gene correlates with the severity of the phenotype.5 Melanocytes are histologically and ultrastructurally absent or considerably reduced in depigmented patches but are normal in number in the hyperpigmented areas.2
Rare cases of piebaldism have been reported in association with other diseases, including congenital megacolon, congenital dyserythropoietic anemia type II, Diamond-Blackfan anemia, Grover disease (transient acantholytic dermatosis), and glycogen-storage disease type 1a.1,6 Poliosis alone may be the initial presentation of certain genetic syndromes, including Waardenburg syndrome (WS) and tuberous sclerosis; it also may be acquired in the setting of several conditions, including vitiligo, Vogt-Koyanagi-Harada syndrome, Alezzandrini syndrome, alopecia areata, and sarcoidosis.3
Notably, the diagnosis of piebaldism should alert the clinician to the possibility of WS, an autosomal-dominant disease characterized by a congenital white forelock, leukoderma in a piebaldlike distribution, lateral displacement of the medial canthi, a hypertrophic nasal root, heterochromia iridis, and progressive sensorineural hearing loss.7 Four clinical subtypes of WS have been described, with various gene mutations implicated: type 1 is the classic form, type 2 lacks dystopia canthorum and has a stonger association with deafness, type 3 is associated with limb abnormalities, and type 4 is associated with congenital megacolon. A case of WS type 1 has been described in association with facial nerve palsy and lingua plicata, 2 main features of Melkerson-Rosenthal syndrome.8 Depigmentation in WS is caused by the absence of melanocytes in the affected areas as well as failed migration of melanocytes to the ears and eyes.3 Waardenburg syndrome may be distinguished from piebaldism by characteristic facial features of the disease and should prompt a thorough ocular and auditory examination in affected patients.9
Although not a diagnostic criterion, poliosis rarely has been reported as one of the earliest associated findings of tuberous sclerosis.3,10 Major cutaneous features of this disease include facial angiofibromas, hypomelanotic macules, shagreen patches (connective tissue nevi), periungual fibromas, molluscum pendulum, and café au lait macules.
Vitiligo also may be considered in the differential diagnosis of piebaldism and can be distinguished by the presence of depigmented patches in a typical acral and periorificial distribution, lack of congential presentation, and relatively progressive course. Vitiligo is characterized by an acquired loss of epidermal melanocytes, leading to depigmented macules and patches.1,3
Vitiligo, poliosis, and alopecia areata usually are late clinical manifestations of Vogt-Koyanagi-Harada syndrome, a rare condition characterized by an autoimmune response to melanocyte-associated antigens. This condition initially presents with neurologic and ocular manifestations including headache, muscle weakness, tinnitus, uveitis, and choroiditis prior to dermatologic manifestations.11
Alezzandrini syndrome, a rare and closely related disorder, is distinctly characterized by whitening of scalp hair, eyebrows, and eyelashes, along with unilateral depigmentation of facial skin. This presentation is associated with ipsilateral visual changes and hearing abnormalities.12
The absence of abnormal ocular, auditory, and neurologic examinations, along with lack of characteristic cutaneous features indicating any of the aforementioned disorders, highly suggests a diagnosis of piebaldism.
Piebaldism is considered a relatively benign disorder but can be highly socially disabling, which presents a therapeutic challenge in affected children. Depigmented skin in piebaldism generally is considered unresponsive to medical or light therapy.1 Topical treatments with makeup or artificial pigmenting agents (eg, dihydroxyacetone [an ingredient used in sunless tanning products]) are useful but temporary. Sunscreen should be used judiciously to avoid sunburn and reduce carcinogenic potential.13
Several surgical techniques have been reported for treatment of leukoderma but with variable success. Of those reported, micropunch transplantation (minigrafting) using epidermal donor sites of 1 to 1.25 mm is a relatively inexpensive and effective method but is limited by scarring at the donor site.14 Autologous cultured epidermal cellular grafting with a controlled number of melanocytes is reported to achieve greater than 75% repigmentation. It requires fewer donor sites and, therefore, results in less scarring.15 Additionally, use of the erbium-doped:YAG laser aids in deepithelialization of the recipient site, allowing for treatment of large piebald lesions during a single operation.16 Despite these advances, additional studies are needed to improve quality of life in those affected.
- Janjua SA, Khachemoune A, Guldbakke KK. Piebaldism: a case report and a concise review of the literature. Cutis. 2007;80:411-414.
- Agarwal S, Ojha A. Piebaldism: a brief report and review of the literature. Indian Dermatol Online J. 2012;3:144-147.
- Sleiman R, Kurban M, Succaria F, et al. Poliosis circumscripta: overview and underlying causes. J Am Acad Dermatol. 2013;69:625-633.
- Oiso N, Fukai K, Kawada A, et al. Piebaldism. J Dermatol. 2013;40:330-355.
- López V, Jordá E. Piebaldism in a 2-year-old girl. Dermatol Online J. 2011;17:13.
- Ghoshal B, Sarkar N, Bhattacharjee M, et al. Glycogen storage disease 1a with piebaldism. Indian Pediatr. 2012;49:235-236.
- Salvatore S, Carnevale C, Infussi R, et al. Waardenburg syndrome: a review of literature and case reports. Clin Ter. 2012;163:e85-e94.
- Dourmishev AL, Dourmishev LA, Schwartz RA, et al. Waardenburg syndrome. Int J Dermatol. 1999;38:656-663.
- Fistarol SK, Itin PH. Disorders of pigmentation. J Dtsch Dermatol Ges. 2010;8:187-201.
- McWilliam RC, Stephenson JB. Depigmented hair. the earliest sign of tuberous sclerosis. Arch Dis Child. 1978;53:961-963.
- Chan EW, Sanjay S, Chang BC. Headache, red eyes, blurred vision and hearing loss. diagnosis: Vogt-Koyanagi-Harada syndrome. CMAJ. 2010;182:1205-1209.
- Andrade A, Pithon M. Alezzandrini syndrome: report of a sixth clinical case. Dermatology (Basel). 2011;222:8-9.
- Suga Y, Ikejima A, Matsuba S, et al. Medical pearl: DHA application for camouflaging segmental vitiligo and piebald lesions. J Am Acad Dermatol. 2002;47:436-438.
- Neves DR, Régis Júnior JR, Oliveira PJ, et al. Melanocyte transplant in piebaldism: case report. An Bras Dermatol. 2010;85:384-388.
- Van geel N, Wallaeys E, Goh BK, et al. Long-term results of noncultured epidermal cellular grafting in vitiligo, halo naevi, piebaldism and naevus depigmentosus. Br J Dermatol. 2010;163:1186-1193.
- Guerra L, Primavera G, Raskovic D, et al. Permanent repigmentation of piebaldism by erbium:YAG laser and autologous cultured epidermis. Br J Dermatol. 2004;150:715-721.
- Janjua SA, Khachemoune A, Guldbakke KK. Piebaldism: a case report and a concise review of the literature. Cutis. 2007;80:411-414.
- Agarwal S, Ojha A. Piebaldism: a brief report and review of the literature. Indian Dermatol Online J. 2012;3:144-147.
- Sleiman R, Kurban M, Succaria F, et al. Poliosis circumscripta: overview and underlying causes. J Am Acad Dermatol. 2013;69:625-633.
- Oiso N, Fukai K, Kawada A, et al. Piebaldism. J Dermatol. 2013;40:330-355.
- López V, Jordá E. Piebaldism in a 2-year-old girl. Dermatol Online J. 2011;17:13.
- Ghoshal B, Sarkar N, Bhattacharjee M, et al. Glycogen storage disease 1a with piebaldism. Indian Pediatr. 2012;49:235-236.
- Salvatore S, Carnevale C, Infussi R, et al. Waardenburg syndrome: a review of literature and case reports. Clin Ter. 2012;163:e85-e94.
- Dourmishev AL, Dourmishev LA, Schwartz RA, et al. Waardenburg syndrome. Int J Dermatol. 1999;38:656-663.
- Fistarol SK, Itin PH. Disorders of pigmentation. J Dtsch Dermatol Ges. 2010;8:187-201.
- McWilliam RC, Stephenson JB. Depigmented hair. the earliest sign of tuberous sclerosis. Arch Dis Child. 1978;53:961-963.
- Chan EW, Sanjay S, Chang BC. Headache, red eyes, blurred vision and hearing loss. diagnosis: Vogt-Koyanagi-Harada syndrome. CMAJ. 2010;182:1205-1209.
- Andrade A, Pithon M. Alezzandrini syndrome: report of a sixth clinical case. Dermatology (Basel). 2011;222:8-9.
- Suga Y, Ikejima A, Matsuba S, et al. Medical pearl: DHA application for camouflaging segmental vitiligo and piebald lesions. J Am Acad Dermatol. 2002;47:436-438.
- Neves DR, Régis Júnior JR, Oliveira PJ, et al. Melanocyte transplant in piebaldism: case report. An Bras Dermatol. 2010;85:384-388.
- Van geel N, Wallaeys E, Goh BK, et al. Long-term results of noncultured epidermal cellular grafting in vitiligo, halo naevi, piebaldism and naevus depigmentosus. Br J Dermatol. 2010;163:1186-1193.
- Guerra L, Primavera G, Raskovic D, et al. Permanent repigmentation of piebaldism by erbium:YAG laser and autologous cultured epidermis. Br J Dermatol. 2004;150:715-721.
Practice Points
- Poliosis circumscripta (or white forelock) is commonly the only manifestation of piebaldism in children.
- Affected areas of leukoderma in piebaldism are classically distributed on the central forehead, anterior trunk, and mid extremities.
- The presence of congenital leukoderma should prompt a thorough skin examination and review of the patient’s medical history for evidence of ocular, auditory, and/or neurologic abnormalities.
Subcorneal Hematomas in Excessive Video Game Play
Case Report
A 19-year-old man was admitted to our hospital to begin treatment for acute myeloid leukemia that had been diagnosed 2 days prior. Three days after completing a 10-day regimen of induction chemotherapy, he developed bilateral, well-demarcated erythematous patches on the palmar surfaces of the proximal phalanges of the third, fourth, and fifth fingers (Figure 1) and 2 patches on the right palm. The patient was referred to dermatology for evaluation. He recalled no trauma to these sites although he reported pushing his intravenous pole with the right hand when walking. Of note, he had become neutropenic and thrombocytopenic following chemotherapy

On physical examination, the patches measured 1- to 1.5-cm in diameter and were mildly tender to palpation. The 2 patches on the right palm were much smaller than those on the fingers but were otherwise similar in appearance.
A punch biopsy of the erythematous lesion on the left third digit was performed. Histologic examination revealed extensive epidermal denudation associated with vascular proliferation and congestion as well as hemorrhage and a sparse lymphocytic infiltrate (Figures 2–4). There was no evidence of a leukemic infiltrate, and stains for fungal elements and bacteria were negative. Eccrine ducts appeared normal with no evidence of necrosis or metaplasia. These findings were suggestive of a frictional etiology.
Due to the distribution of the skin lesions on the hands, it was suspected that the source of friction was a video game controller. Although the patient denied playing video games since his admission to the hospital, he reported heavy video game use during the weeks prior to admission. We postulated that the thrombocytopenia the patient developed following chemotherapy along with prior friction injury sustained from heavy video game play led to traumatic subcorneal hemorrhage on the hands at the points of contact with the video game controller (Figure 5). The subcorneal hematomas resolved completely over the next 2 months during which the patient abstained from video game play.

This case demonstrates the importance of obtaining a detailed patient history, as our patient’s history of video game play prior to hospitalization proved to be of major diagnostic importance. Although the location, distribution, and well-demarcated nature of the patient’s lesions suggested an external source of trauma and biopsy definitively ruled out leukemia cutis, Sweet syndrome, and eccrine hidradenitis,1 the final diagnosis of traumatic subcorneal hematomas was only possible with specific knowledge of the patient’s video game controller use.
Comment
History of video game play has been key to the diagnosis of a variety of cutaneous lesions documented in the medical literature. Robertson et al2 attributed a similar case of traumatic subcorneal hematomas of the hands in an otherwise healthy 16-year-old boy to excessive use of a video game controller. Similarly, Kasraee et al3 attributed a case of idiopathic eccrine hidradenitis in an otherwise healthy 12-year-old girl to excessive video game use. In both of these reported cases, bilateral skin lesions on the palms of the hands appeared acutely in a pattern consistent with the points of contact of a video game controller. Excessive video game play has also been associated with unilateral dermatologic lesions on the hands, such as knuckle pads,4 onycholysis,5 friction blisters,6 pressure ulcers,7 and hemorrhagic lesions.5,6,8
Video game–related pathologies are not limited to the skin and have been implicated in a variety of clinical presentations. In 1987, Osterman et al9 published an early account of repetitive strain injury (RSI) related to video game use in which the investigators reported 2 cases of video game–related volar flexor tenosynovitis (or trigger finger), which they termed “joystick digit.” Since that time, video game play has greatly evolved along with the types and nature of RSI cases reported in the medical literature. In 1990, Brasington10 described acute tendinopathy of the extensor pollicis longus tendon caused by excessive video game play, which was termed “Nintendinitis.” This term has since been used in reference to any video game–related RSI and reports have increased over time, likely due to the proliferation of an increasing array of video game systems.5,11-16 In recent years, a number of traumatic injuries including fractures, joint dislocations, head injuries, hemothorax, and lacerations have been attributed to interactive gaming systems.6,11,17-20 In rare cases, video game play also has been associated with enuresis,21 encopresis,22 and epilepsy.23
According to a 2011 report from the Entertainment Software Association, women over the age of 18 years now represent a greater proportion of the video game–playing population than boys aged 17 years and younger.24 This same report also noted that the average video game player is 35 years old; 44% of all players are female; and 27% of Americans over the age of 50 years play video games. This shifting demographic data, including the fact that 80% of American households reportedly play video games, reveals the expanding depth and breadth of the market.24 However, the pediatric population is still a high-volume player demographic. Average time per session peaks between 10 to 12 years of age and then falls through the teenage and adults years.24 Hence, the pediatric population is at high risk for clinical pathology because of the increased repetitive movements associated with video game play. Overall, cognizance of the popularity of video games and related pathologies can be an asset for dermatologists who evaluate pediatric patients.
1. Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Elsevier Health Sciences UK; 2007.
2. Robertson SJ, Leonard J, Chamberlain AJ. PlayStation purpura. Australas J Dermatol. 2010;51:220-222.
3. Kasraee B, Masouyé I, Piguet V. PlayStation palmar hidradenitis. Br J Dermatol. 2009;160:892-894.
4. Rushing ME, Sheehan DJ, Davis LS. Video game induced knuckle pad. Pediatr Dermatol. 2006;23:455-457.
5. Bakos RM, Bakos L. Use of dermoscopy to visualize punctate hemorrhages and onycholysis in “playstation thumb.” Arch Dermatol. 2006;142:1664-1665.
6. Wood DJ. The “How!” sign—a central palmar blister induced by overplaying on a Nintendo console. Arch Dis Child. 2001;84:288.
7. Koh TH. Ulcerative “nintendinitis”: a new kind of repetitive strain injury. Med J Aust. 2000;173:671.
8. Bernabeu-Wittel J, Domínguez-Cruz J, Zulueta T, et al. Hemorrhagic parallel-ridge pattern on dermoscopy in “Playstation fingertip.” J Am Acad Dermatol. 2011;65:238-239.
9. Osterman AL, Weinberg P, Miller G. Joystick digit. JAMA. 1987;257:782.
10. Brasington R. Nintendinitis. N Engl J Med. 1990;322:1473-1474.
11. Sparks DA, Coughlin LM, Chase DM. Did too much Wii cause your patient’s injury? J Fam Pract. 2011;60:404-409.
12. Bright DA, Bringhurst DC. Nintendo elbow. West J Med. 1992;156:667-668.
13. Vaidya HJ. Playstation thumb. Lancet. 2004;363:1080.
14. Bonis J. Acute Wiiitis. N Engl J Med. 2007;356:2431-2432.
15. Boehm KM, Pugh A. A new variant of Wiiitis [published online ahead of print June 13, 2008]. J Emerg Med. 2009;36:80.
16. Beddy P, Dunne R, de Blacam C. Achilles wiiitis. AJR Am J Roentgenol. 2009;192:W79.
17. Eley KA. A Wii fracture. N Engl J Med. 2010;362:473-474.
18. Wells JJ. An 8-year-old girl presented to the ER after accidentally being hit by a Wii remote control swung by her brother. J Trauma. 2008;65:1203.
19. Fysh T, Thompson JF. A Wii problem. J R Soc Med. 2009;102:502.
20. George AJ. Musculo-ske Wii tal medicine. Injury. 2012;43:390-391.
21. Schink JC. Nintendo enuresis. Am J Dis Child. 1991;145:1094.
22. Corkery JC. Nintendo power. Am J Dis Child. 1990;144:959.
23. Hart EJ. Nintendo epilepsy. N Engl J Med. 1990;322:1473.
24. Entertainment Software Association. 2015 sales, demographic, and usage data. essential facts about the computer and video game industry. Entertainment Software Association Web site. http://www.theesa.com/wp-content/uploads/2015/04/ESA-Essential-Facts-2015.pdf. Accessed October 16, 2015.
Case Report
A 19-year-old man was admitted to our hospital to begin treatment for acute myeloid leukemia that had been diagnosed 2 days prior. Three days after completing a 10-day regimen of induction chemotherapy, he developed bilateral, well-demarcated erythematous patches on the palmar surfaces of the proximal phalanges of the third, fourth, and fifth fingers (Figure 1) and 2 patches on the right palm. The patient was referred to dermatology for evaluation. He recalled no trauma to these sites although he reported pushing his intravenous pole with the right hand when walking. Of note, he had become neutropenic and thrombocytopenic following chemotherapy

On physical examination, the patches measured 1- to 1.5-cm in diameter and were mildly tender to palpation. The 2 patches on the right palm were much smaller than those on the fingers but were otherwise similar in appearance.
A punch biopsy of the erythematous lesion on the left third digit was performed. Histologic examination revealed extensive epidermal denudation associated with vascular proliferation and congestion as well as hemorrhage and a sparse lymphocytic infiltrate (Figures 2–4). There was no evidence of a leukemic infiltrate, and stains for fungal elements and bacteria were negative. Eccrine ducts appeared normal with no evidence of necrosis or metaplasia. These findings were suggestive of a frictional etiology.



Due to the distribution of the skin lesions on the hands, it was suspected that the source of friction was a video game controller. Although the patient denied playing video games since his admission to the hospital, he reported heavy video game use during the weeks prior to admission. We postulated that the thrombocytopenia the patient developed following chemotherapy along with prior friction injury sustained from heavy video game play led to traumatic subcorneal hemorrhage on the hands at the points of contact with the video game controller (Figure 5). The subcorneal hematomas resolved completely over the next 2 months during which the patient abstained from video game play.

This case demonstrates the importance of obtaining a detailed patient history, as our patient’s history of video game play prior to hospitalization proved to be of major diagnostic importance. Although the location, distribution, and well-demarcated nature of the patient’s lesions suggested an external source of trauma and biopsy definitively ruled out leukemia cutis, Sweet syndrome, and eccrine hidradenitis,1 the final diagnosis of traumatic subcorneal hematomas was only possible with specific knowledge of the patient’s video game controller use.
Comment
History of video game play has been key to the diagnosis of a variety of cutaneous lesions documented in the medical literature. Robertson et al2 attributed a similar case of traumatic subcorneal hematomas of the hands in an otherwise healthy 16-year-old boy to excessive use of a video game controller. Similarly, Kasraee et al3 attributed a case of idiopathic eccrine hidradenitis in an otherwise healthy 12-year-old girl to excessive video game use. In both of these reported cases, bilateral skin lesions on the palms of the hands appeared acutely in a pattern consistent with the points of contact of a video game controller. Excessive video game play has also been associated with unilateral dermatologic lesions on the hands, such as knuckle pads,4 onycholysis,5 friction blisters,6 pressure ulcers,7 and hemorrhagic lesions.5,6,8
Video game–related pathologies are not limited to the skin and have been implicated in a variety of clinical presentations. In 1987, Osterman et al9 published an early account of repetitive strain injury (RSI) related to video game use in which the investigators reported 2 cases of video game–related volar flexor tenosynovitis (or trigger finger), which they termed “joystick digit.” Since that time, video game play has greatly evolved along with the types and nature of RSI cases reported in the medical literature. In 1990, Brasington10 described acute tendinopathy of the extensor pollicis longus tendon caused by excessive video game play, which was termed “Nintendinitis.” This term has since been used in reference to any video game–related RSI and reports have increased over time, likely due to the proliferation of an increasing array of video game systems.5,11-16 In recent years, a number of traumatic injuries including fractures, joint dislocations, head injuries, hemothorax, and lacerations have been attributed to interactive gaming systems.6,11,17-20 In rare cases, video game play also has been associated with enuresis,21 encopresis,22 and epilepsy.23
According to a 2011 report from the Entertainment Software Association, women over the age of 18 years now represent a greater proportion of the video game–playing population than boys aged 17 years and younger.24 This same report also noted that the average video game player is 35 years old; 44% of all players are female; and 27% of Americans over the age of 50 years play video games. This shifting demographic data, including the fact that 80% of American households reportedly play video games, reveals the expanding depth and breadth of the market.24 However, the pediatric population is still a high-volume player demographic. Average time per session peaks between 10 to 12 years of age and then falls through the teenage and adults years.24 Hence, the pediatric population is at high risk for clinical pathology because of the increased repetitive movements associated with video game play. Overall, cognizance of the popularity of video games and related pathologies can be an asset for dermatologists who evaluate pediatric patients.
Case Report
A 19-year-old man was admitted to our hospital to begin treatment for acute myeloid leukemia that had been diagnosed 2 days prior. Three days after completing a 10-day regimen of induction chemotherapy, he developed bilateral, well-demarcated erythematous patches on the palmar surfaces of the proximal phalanges of the third, fourth, and fifth fingers (Figure 1) and 2 patches on the right palm. The patient was referred to dermatology for evaluation. He recalled no trauma to these sites although he reported pushing his intravenous pole with the right hand when walking. Of note, he had become neutropenic and thrombocytopenic following chemotherapy

On physical examination, the patches measured 1- to 1.5-cm in diameter and were mildly tender to palpation. The 2 patches on the right palm were much smaller than those on the fingers but were otherwise similar in appearance.
A punch biopsy of the erythematous lesion on the left third digit was performed. Histologic examination revealed extensive epidermal denudation associated with vascular proliferation and congestion as well as hemorrhage and a sparse lymphocytic infiltrate (Figures 2–4). There was no evidence of a leukemic infiltrate, and stains for fungal elements and bacteria were negative. Eccrine ducts appeared normal with no evidence of necrosis or metaplasia. These findings were suggestive of a frictional etiology.



Due to the distribution of the skin lesions on the hands, it was suspected that the source of friction was a video game controller. Although the patient denied playing video games since his admission to the hospital, he reported heavy video game use during the weeks prior to admission. We postulated that the thrombocytopenia the patient developed following chemotherapy along with prior friction injury sustained from heavy video game play led to traumatic subcorneal hemorrhage on the hands at the points of contact with the video game controller (Figure 5). The subcorneal hematomas resolved completely over the next 2 months during which the patient abstained from video game play.

This case demonstrates the importance of obtaining a detailed patient history, as our patient’s history of video game play prior to hospitalization proved to be of major diagnostic importance. Although the location, distribution, and well-demarcated nature of the patient’s lesions suggested an external source of trauma and biopsy definitively ruled out leukemia cutis, Sweet syndrome, and eccrine hidradenitis,1 the final diagnosis of traumatic subcorneal hematomas was only possible with specific knowledge of the patient’s video game controller use.
Comment
History of video game play has been key to the diagnosis of a variety of cutaneous lesions documented in the medical literature. Robertson et al2 attributed a similar case of traumatic subcorneal hematomas of the hands in an otherwise healthy 16-year-old boy to excessive use of a video game controller. Similarly, Kasraee et al3 attributed a case of idiopathic eccrine hidradenitis in an otherwise healthy 12-year-old girl to excessive video game use. In both of these reported cases, bilateral skin lesions on the palms of the hands appeared acutely in a pattern consistent with the points of contact of a video game controller. Excessive video game play has also been associated with unilateral dermatologic lesions on the hands, such as knuckle pads,4 onycholysis,5 friction blisters,6 pressure ulcers,7 and hemorrhagic lesions.5,6,8
Video game–related pathologies are not limited to the skin and have been implicated in a variety of clinical presentations. In 1987, Osterman et al9 published an early account of repetitive strain injury (RSI) related to video game use in which the investigators reported 2 cases of video game–related volar flexor tenosynovitis (or trigger finger), which they termed “joystick digit.” Since that time, video game play has greatly evolved along with the types and nature of RSI cases reported in the medical literature. In 1990, Brasington10 described acute tendinopathy of the extensor pollicis longus tendon caused by excessive video game play, which was termed “Nintendinitis.” This term has since been used in reference to any video game–related RSI and reports have increased over time, likely due to the proliferation of an increasing array of video game systems.5,11-16 In recent years, a number of traumatic injuries including fractures, joint dislocations, head injuries, hemothorax, and lacerations have been attributed to interactive gaming systems.6,11,17-20 In rare cases, video game play also has been associated with enuresis,21 encopresis,22 and epilepsy.23
According to a 2011 report from the Entertainment Software Association, women over the age of 18 years now represent a greater proportion of the video game–playing population than boys aged 17 years and younger.24 This same report also noted that the average video game player is 35 years old; 44% of all players are female; and 27% of Americans over the age of 50 years play video games. This shifting demographic data, including the fact that 80% of American households reportedly play video games, reveals the expanding depth and breadth of the market.24 However, the pediatric population is still a high-volume player demographic. Average time per session peaks between 10 to 12 years of age and then falls through the teenage and adults years.24 Hence, the pediatric population is at high risk for clinical pathology because of the increased repetitive movements associated with video game play. Overall, cognizance of the popularity of video games and related pathologies can be an asset for dermatologists who evaluate pediatric patients.
1. Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Elsevier Health Sciences UK; 2007.
2. Robertson SJ, Leonard J, Chamberlain AJ. PlayStation purpura. Australas J Dermatol. 2010;51:220-222.
3. Kasraee B, Masouyé I, Piguet V. PlayStation palmar hidradenitis. Br J Dermatol. 2009;160:892-894.
4. Rushing ME, Sheehan DJ, Davis LS. Video game induced knuckle pad. Pediatr Dermatol. 2006;23:455-457.
5. Bakos RM, Bakos L. Use of dermoscopy to visualize punctate hemorrhages and onycholysis in “playstation thumb.” Arch Dermatol. 2006;142:1664-1665.
6. Wood DJ. The “How!” sign—a central palmar blister induced by overplaying on a Nintendo console. Arch Dis Child. 2001;84:288.
7. Koh TH. Ulcerative “nintendinitis”: a new kind of repetitive strain injury. Med J Aust. 2000;173:671.
8. Bernabeu-Wittel J, Domínguez-Cruz J, Zulueta T, et al. Hemorrhagic parallel-ridge pattern on dermoscopy in “Playstation fingertip.” J Am Acad Dermatol. 2011;65:238-239.
9. Osterman AL, Weinberg P, Miller G. Joystick digit. JAMA. 1987;257:782.
10. Brasington R. Nintendinitis. N Engl J Med. 1990;322:1473-1474.
11. Sparks DA, Coughlin LM, Chase DM. Did too much Wii cause your patient’s injury? J Fam Pract. 2011;60:404-409.
12. Bright DA, Bringhurst DC. Nintendo elbow. West J Med. 1992;156:667-668.
13. Vaidya HJ. Playstation thumb. Lancet. 2004;363:1080.
14. Bonis J. Acute Wiiitis. N Engl J Med. 2007;356:2431-2432.
15. Boehm KM, Pugh A. A new variant of Wiiitis [published online ahead of print June 13, 2008]. J Emerg Med. 2009;36:80.
16. Beddy P, Dunne R, de Blacam C. Achilles wiiitis. AJR Am J Roentgenol. 2009;192:W79.
17. Eley KA. A Wii fracture. N Engl J Med. 2010;362:473-474.
18. Wells JJ. An 8-year-old girl presented to the ER after accidentally being hit by a Wii remote control swung by her brother. J Trauma. 2008;65:1203.
19. Fysh T, Thompson JF. A Wii problem. J R Soc Med. 2009;102:502.
20. George AJ. Musculo-ske Wii tal medicine. Injury. 2012;43:390-391.
21. Schink JC. Nintendo enuresis. Am J Dis Child. 1991;145:1094.
22. Corkery JC. Nintendo power. Am J Dis Child. 1990;144:959.
23. Hart EJ. Nintendo epilepsy. N Engl J Med. 1990;322:1473.
24. Entertainment Software Association. 2015 sales, demographic, and usage data. essential facts about the computer and video game industry. Entertainment Software Association Web site. http://www.theesa.com/wp-content/uploads/2015/04/ESA-Essential-Facts-2015.pdf. Accessed October 16, 2015.
1. Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Elsevier Health Sciences UK; 2007.
2. Robertson SJ, Leonard J, Chamberlain AJ. PlayStation purpura. Australas J Dermatol. 2010;51:220-222.
3. Kasraee B, Masouyé I, Piguet V. PlayStation palmar hidradenitis. Br J Dermatol. 2009;160:892-894.
4. Rushing ME, Sheehan DJ, Davis LS. Video game induced knuckle pad. Pediatr Dermatol. 2006;23:455-457.
5. Bakos RM, Bakos L. Use of dermoscopy to visualize punctate hemorrhages and onycholysis in “playstation thumb.” Arch Dermatol. 2006;142:1664-1665.
6. Wood DJ. The “How!” sign—a central palmar blister induced by overplaying on a Nintendo console. Arch Dis Child. 2001;84:288.
7. Koh TH. Ulcerative “nintendinitis”: a new kind of repetitive strain injury. Med J Aust. 2000;173:671.
8. Bernabeu-Wittel J, Domínguez-Cruz J, Zulueta T, et al. Hemorrhagic parallel-ridge pattern on dermoscopy in “Playstation fingertip.” J Am Acad Dermatol. 2011;65:238-239.
9. Osterman AL, Weinberg P, Miller G. Joystick digit. JAMA. 1987;257:782.
10. Brasington R. Nintendinitis. N Engl J Med. 1990;322:1473-1474.
11. Sparks DA, Coughlin LM, Chase DM. Did too much Wii cause your patient’s injury? J Fam Pract. 2011;60:404-409.
12. Bright DA, Bringhurst DC. Nintendo elbow. West J Med. 1992;156:667-668.
13. Vaidya HJ. Playstation thumb. Lancet. 2004;363:1080.
14. Bonis J. Acute Wiiitis. N Engl J Med. 2007;356:2431-2432.
15. Boehm KM, Pugh A. A new variant of Wiiitis [published online ahead of print June 13, 2008]. J Emerg Med. 2009;36:80.
16. Beddy P, Dunne R, de Blacam C. Achilles wiiitis. AJR Am J Roentgenol. 2009;192:W79.
17. Eley KA. A Wii fracture. N Engl J Med. 2010;362:473-474.
18. Wells JJ. An 8-year-old girl presented to the ER after accidentally being hit by a Wii remote control swung by her brother. J Trauma. 2008;65:1203.
19. Fysh T, Thompson JF. A Wii problem. J R Soc Med. 2009;102:502.
20. George AJ. Musculo-ske Wii tal medicine. Injury. 2012;43:390-391.
21. Schink JC. Nintendo enuresis. Am J Dis Child. 1991;145:1094.
22. Corkery JC. Nintendo power. Am J Dis Child. 1990;144:959.
23. Hart EJ. Nintendo epilepsy. N Engl J Med. 1990;322:1473.
24. Entertainment Software Association. 2015 sales, demographic, and usage data. essential facts about the computer and video game industry. Entertainment Software Association Web site. http://www.theesa.com/wp-content/uploads/2015/04/ESA-Essential-Facts-2015.pdf. Accessed October 16, 2015.
Practice Points
- Video game play has been reported as an etiologic factor in multiple musculoskeletal and dermatologic conditions.
- More than two-thirds of US children aged 2 to 18 years live in a home with a video game system.
- Cognizance of the popularity of video games and related pathologies can be an asset for dermatologists who evaluate pediatric patients.
Bullous Henoch-Schönlein Purpura in Children
Henoch-Schönlein purpura (HSP) is a systemic, small vessel vasculitis affecting the skin, joints, gastrointestinal tract, and kidneys. It usually is self-limited, but relapses can be seen in one-third of cases.1 The classic cutaneous presentation includes palpable purpura localized to the legs and buttocks. Painful hemorrhagic bullae are uncommonly observed in childhood HSP and often could lead to a diagnostic dilemma. We report the case of a patient who presented with atypical features of painful hemorrhagic bullae and provide a review of the literature.
Case Report
An otherwise healthy 14-year-old adolescent girl presented to the hospital with painful ulcerative lesions covering the arms, legs, lower abdomen, and buttocks of 3 weeks’ duration. The rash first appeared on the ankles and spread in an ascending fashion, starting with bullous formation that was accompanied by joint pain, especially in the ankles and elbows. No abdominal pain was reported. The patient attributed the lesions to prolonged cold exposure followed by a hot bath. She had tried naproxen without any improvement of pain. She was afebrile with normal blood pressure.
On physical examination, numerous petechiae, palpable purpura, hemorrhagic bullae, and ulcers with surrounding erythematous to violaceous induration as well as central necrosis were noted on the arms, legs (Figure 1), abdomen, and buttocks. The palms, soles, trunk, and face were spared.
Laboratory values on admission revealed leukocytosis (17,500/μL [reference range, 4500–11,000/μL]), elevated erythrocyte sedimentation rate (42 mm/h [reference range, 0–20 mm/h]), elevated C-reactive protein (15.59 mg/L [reference range, 0.08–3.1 mg/L]), elevated C3 (174 mg/dL [reference range, 75–135 mg/dL]), normal C4 (32 mg/dL [reference range, 3–75 mg/dL]), normal blood urea nitrogen (13 mg/dL [reference range, 8–23 mg/dL]), and normal creatinine (0.72 mg/dL [reference range, 0.6–1.2 mg/dL]). Urinalysis showed microscopic hematuria and trace proteinuria. Platelet count was normal.
Diagnostic considerations included HSP, drug-induced leukocytoclastic vasculitis, and bullous pyoderma gangrenosum. The patient was started on oral prednisone 80 mg once daily. Additionally, oral doxycycline 100 mg twice daily was added for prevention of secondary bacterial infections and for anti-inflammatory effects. All nonsteroidal anti-inflammatory drugs were avoided. A commercial ointment containing 8-hydroxyquinoline sulfate 0.3% and triamcinolone acetonide ointment 0.1% were used to minimize skin irritation. Morphine, oxycodone-acetaminophen, and pregabalin followed by gabapentin were used for pain control. Hydrotherapy also was used for the treatment of skin lesions.
Two skin punch biopsies were performed at different stages. Biopsy of an early palpable purpuric lesion showed small vessel leukocytoclastic vasculitis with perivascular IgA on direct immunofluorescence. A second biopsy from a more hemorrhagic lesion performed 96 hours after admission to the hospital showed subepidermal vesicles with partial epidermal necrosis, confluent neutrophilic infiltrate in the papillary dermis, and small vessel vasculitis (Figures 2 and 3). Gram, periodic acid–Schiff, and acid-fast bacilli staining and cultures were negative. With continued treatment for 7 days, the clinical appearance of the lesions improved. On the tenth day of hospitalization, oral dapsone 25 mg once daily was initiated with the goal of weaning the patient off the prednisone as tolerated. She was discharged on prednisone (60 mg once daily) after 14 days of hospitalization. Dapsone also was continued.
 |  | |
Figure 2. Biopsy of a subepidermal bulla revealed neutrophilic inflammation within bullous space and evidence of dermal hemorrhage (H&E, original magnification ×100). | Figure 3. Leukocytoclastic vasculitis on biopsy (H&E, original magnification ×400). |
At 4-week follow-up, the lesions showed healing with mild residual pigmentation. The patient’s blood pressure and serum urea and creatinine levels were normal but the proteinuria was persistent, so the patient was started on oral lisinopril 5 mg once daily. Tapering of steroids over several months was initiated and the dose of dapsone was increased to 50 mg daily. Follow-up with a nephrologist was arranged to monitor renal function. She continued on lisinopril 5 mg once daily for treatment of nonnephrotic-range proteinuria, which was detected at 6 months following discharge.
Comment
The presence of atypical symptoms such as bullae and painful lesions in patients with suspected HSP can complicate the diagnosis. Initially, one of the top diagnostic considerations in our patient was bullous pyoderma gangrenosum, a neutrophilic dermatosis that typically presents with painful ulcerative lesions and inflammatory bullae. Other causes of bullae in children include erythema multiforme, toxic epidermal necrolysis, epidermolysis bullosa, bullous mastocytosis, pemphigus, bullous pemphigoid, dermatitis herpetiformis, linear IgA dermatosis, bullous impetigo, gangrenous cellulitis, and Vibrio vulnificus infection. However, the clinical symptoms of joint pain and hematuria/proteinuria in our patient as well as the punch biopsy findings pointed toward HSP as the most likely diagnosis.
Although bullous lesions are relatively common in adult-onset HSP (16%–60% of patients), they are very rare in pediatric patients (2% of patients).2-4 We performed a PubMed search of articles indexed for MEDLINE for bullous Henoch-Schönlein purpura in childhood using the search term Henoch-Schönlein purpura and bullous. The Table provides a summary of our search results from the English-language literature.5-22
Bullae often develop on several parts of the body but are more commonly observed on the legs.17 Pathergy and edema have been implicated in the pathogenesis, as these findings have been observed in sites such as malleoli and legs, respectively.12 Matrix metalloproteinases secreted in polymorphonuclear neutrophils have been found to be elevated in blister fluid and can cause bullae formation via degrading collagen in the basement membrane.9 Corticosteroids, by virtue of their inhibition of proinflammatory transcription factors (eg, nuclear factor κβ, intranuclear activator protein 1) and decreasing metalloproteinase levels, may be efficacious in bullous HSP. Although there is no consensus, corticosteroid therapy seems to be efficacious in treating the bullae, according to several reports.17-22
The use of glucocorticoids in bullous HSP in childhood remains controversial. Studies report shortening of the duration of abdominal pain, reducing risk of intussusception, decreasing recurrence risk, and reducing the risk of renal involvement with use of steroids in HSP.23-25 The use of systemic steroids has been described in children with bullous HSP to reduce the severity of HSP-related bullae and its associated painful ulcers and necrosis.16,21,25,26 The duration of steroid use ranged from a short burst to a prolonged course of weaning over weeks. Azathioprine also has been used in conjunction with methylprednisolone, prednisone, and dexamethasone.17,22 Because of its anti-IgA antioxidant antineutrophil effects, dapsone has been shown to be effective in the treatment of cutaneous HSP.27 In our patient, we used dapsone to help in weaning the patient off the prednisone. Based on our review of the literature, few cases of bullous HSP in children have reported remission without drug therapy. IgA was not found in all the reported cases in which a skin biopsy was done. As shown by the comparison of the 2 biopsies in our patient, biopsying an early lesion within 48 hours of appearance is essential to make a diagnosis because the biopsy of the older lesion could not rule out bullous pyoderma gangrenosum. Immunoreactants (IgA, C3) are destroyed within 48 hours and might lead to false-negative results on immunofluorescence in old and necrotic lesions.28,29 Most reported cases of bullous HSP showed resolution, but few resulted in scarring and/or pigmentation.10,17,18 Henoch-Schönlein purpura usually is self-limited but relapses can be seen in one-third of cases.1 One of the reported cases of bullous HSP showed recurrence of lesions.15 One of the cases showed persistent hematuria.8 Our patient also was started on lisinopril for persistent proteinuria.
1. Saulsbury FT. Henoch-Schönlein purpura in children. report of 100 patients and the review of literature. Medicine. 1999;78:395-409.
2. Cream JJ, Gumpel JM, Peachey RD. Schönlein-Henoch purpura in the adult. a study of 77 adults with anaphylactoid or Schönlein-Henoch purpura. Q J Med. 1970;39:461-484.
3. Tancrede-Bohin E, Ochonisky S, Vignon-Pennamen MD, et al. Schönlein-Henoch purpura in adult patients. predictive factors for IgA glomerulonephritis in a retrospective study of 57 cases. Arch Dermatol. 1997;133:438-442.
4. Abdel-Al YK, Hejazi Z, Majeed HA. Henoch Schönlein purpura in Arab children. analysis of 52 cases. Trop Geogr Med. 1990;42:52-57.
5. Garland JS, Chusid MJ. Henoch-Schöenlein purpura: association with unusual vesicular lesions. Wis Med J. 1985;84:21-23.
6. Crosby DL, Feldman SD. A pruritic vesicular eruption. Henoch-Schönlein purpura. Arch Dermatol. 1990;126:1497-1498.
7. Wananukul S, Pongprasit P, Korkij W. Henoch-Schönlein purpura presenting as hemorrhagic vesicles and bullae: case report and literature review. Pediatr Dermatol. 1995;12:314-317.
8. Saulsbury FT. Hemorrhagic bullous lesions in Henoch-Schönlein purpura. Pediatr Dermatol. 1998;15:357-359.
9. Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiological role of gelatinase (MMP-9). Dermatology. 1998;197:62-64.
10. Liu PM, Bong CN, Chen HH, et al. Henoch-Schönlein purpura with hemorrhagic bullae in children: report of two cases. J Microbiol Immunol Infect. 2004;37:375-378.
11. Ishii Y, Takizawa T, Arakawa H, et al. Hemorrhagic bullous lesions in Henoch-Schönlein purpura. Pediatr Int. 2005;47:694-697.
12. Leung AK, Robson WL. Hemorrhagic bullous lesions in a child with Henoch-Schönlein purpura. Pediatr Dermatol. 2006;23:139-141.
13. Chan K, Han N, Tang W, et al. Lesions in Henoch-Schönlein purpura. Pediatr Dermatol. 2007;24: 325-326.
14. Kausar S, Yalamanchili A. Management of haemorrhagic bullous lesions in Henoch-Schonlein purpura: is there any consensus? J Dermatolog Treat. 2009;20:88-90.
15. Maguiness S, Balma-Mena A, Pope E, et al. Bullous Henoch-Schönlein purpura in children: a report of 6 cases and review of the literature. Clin Pediatr. 2010;49: 1033-1037.
16. den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch-Schönlein purpura as indication to start systemic prednisone. Acta Paediatr. 2010;99:781-783.
17. Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature. Rheumatol Int. 2010;30:1355-1359.
18. Park SE, Lee JH. Haemorrhagic bullous lesions in a 3-year-old girl with Henoch-Schönlein purpura. Acta Paediatr. 2011;100:e283-e284.
19. Parikh K. 14-year-old boy with bullous lesions. Pediatr Ann. 2012;41:275-277.
20. Raymond M, Spinks J. Bullous Henoch Schönlein purpura. Arch Dis Child. 2012;97:617.
21. Kocaoglu C, Ozturk R, Unlu Y, et al. Successful treatment of hemorrhagic bullous Henoch-Schönlein purpura with oral corticosteroid: a case report [published online ahead of print April 16, 2013]. Case Rep Pediatr. 2013;2013:680208.
22. Mehra S, Suri D, Dogra S, et al. Hemorrhagic bullous lesions in a girl with Henoch Schönlein purpura. Indian J Pediatr. 2014;81:210-211.
23. Ronkainen J, Koskimies O, Ala-Houhala M, et al. Early prednisone therapy in Henoch-Schönlein purpura: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2006;149:241-247.
24. Weiss PF, Klink AJ, Localio R, et al. Corticosteroids may improve clinical outcomes during hospitalization for Henoch-Schönlein purpura. Pediatrics. 2010;126:674-681.
25. Rosato L, Chehade H, Cachat F. Re: steroids in haemorrhagic bullous Henoch-Schönlein purpura. Acta Paediatr. 2011;100:319-320.
26. Park SJ, Kim JH, Ha TS, et al. The role of corticosteroid in hemorrhagic bullous Henoch Schönlein purpura. Acta Paediatr. 2011;100:e3-e4.
27. Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
28. Davin JC, Weening JJ. Diagnosis of Henoch-Schönlein purpura: renal or skin biopsy? Pediatr Nephrol. 2003;18:1201-1203.
29. González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48: 1157-1165.
Henoch-Schönlein purpura (HSP) is a systemic, small vessel vasculitis affecting the skin, joints, gastrointestinal tract, and kidneys. It usually is self-limited, but relapses can be seen in one-third of cases.1 The classic cutaneous presentation includes palpable purpura localized to the legs and buttocks. Painful hemorrhagic bullae are uncommonly observed in childhood HSP and often could lead to a diagnostic dilemma. We report the case of a patient who presented with atypical features of painful hemorrhagic bullae and provide a review of the literature.
Case Report
An otherwise healthy 14-year-old adolescent girl presented to the hospital with painful ulcerative lesions covering the arms, legs, lower abdomen, and buttocks of 3 weeks’ duration. The rash first appeared on the ankles and spread in an ascending fashion, starting with bullous formation that was accompanied by joint pain, especially in the ankles and elbows. No abdominal pain was reported. The patient attributed the lesions to prolonged cold exposure followed by a hot bath. She had tried naproxen without any improvement of pain. She was afebrile with normal blood pressure.
On physical examination, numerous petechiae, palpable purpura, hemorrhagic bullae, and ulcers with surrounding erythematous to violaceous induration as well as central necrosis were noted on the arms, legs (Figure 1), abdomen, and buttocks. The palms, soles, trunk, and face were spared.
Laboratory values on admission revealed leukocytosis (17,500/μL [reference range, 4500–11,000/μL]), elevated erythrocyte sedimentation rate (42 mm/h [reference range, 0–20 mm/h]), elevated C-reactive protein (15.59 mg/L [reference range, 0.08–3.1 mg/L]), elevated C3 (174 mg/dL [reference range, 75–135 mg/dL]), normal C4 (32 mg/dL [reference range, 3–75 mg/dL]), normal blood urea nitrogen (13 mg/dL [reference range, 8–23 mg/dL]), and normal creatinine (0.72 mg/dL [reference range, 0.6–1.2 mg/dL]). Urinalysis showed microscopic hematuria and trace proteinuria. Platelet count was normal.
Diagnostic considerations included HSP, drug-induced leukocytoclastic vasculitis, and bullous pyoderma gangrenosum. The patient was started on oral prednisone 80 mg once daily. Additionally, oral doxycycline 100 mg twice daily was added for prevention of secondary bacterial infections and for anti-inflammatory effects. All nonsteroidal anti-inflammatory drugs were avoided. A commercial ointment containing 8-hydroxyquinoline sulfate 0.3% and triamcinolone acetonide ointment 0.1% were used to minimize skin irritation. Morphine, oxycodone-acetaminophen, and pregabalin followed by gabapentin were used for pain control. Hydrotherapy also was used for the treatment of skin lesions.
Two skin punch biopsies were performed at different stages. Biopsy of an early palpable purpuric lesion showed small vessel leukocytoclastic vasculitis with perivascular IgA on direct immunofluorescence. A second biopsy from a more hemorrhagic lesion performed 96 hours after admission to the hospital showed subepidermal vesicles with partial epidermal necrosis, confluent neutrophilic infiltrate in the papillary dermis, and small vessel vasculitis (Figures 2 and 3). Gram, periodic acid–Schiff, and acid-fast bacilli staining and cultures were negative. With continued treatment for 7 days, the clinical appearance of the lesions improved. On the tenth day of hospitalization, oral dapsone 25 mg once daily was initiated with the goal of weaning the patient off the prednisone as tolerated. She was discharged on prednisone (60 mg once daily) after 14 days of hospitalization. Dapsone also was continued.
 |  | |
Figure 2. Biopsy of a subepidermal bulla revealed neutrophilic inflammation within bullous space and evidence of dermal hemorrhage (H&E, original magnification ×100). | Figure 3. Leukocytoclastic vasculitis on biopsy (H&E, original magnification ×400). |
At 4-week follow-up, the lesions showed healing with mild residual pigmentation. The patient’s blood pressure and serum urea and creatinine levels were normal but the proteinuria was persistent, so the patient was started on oral lisinopril 5 mg once daily. Tapering of steroids over several months was initiated and the dose of dapsone was increased to 50 mg daily. Follow-up with a nephrologist was arranged to monitor renal function. She continued on lisinopril 5 mg once daily for treatment of nonnephrotic-range proteinuria, which was detected at 6 months following discharge.
Comment
The presence of atypical symptoms such as bullae and painful lesions in patients with suspected HSP can complicate the diagnosis. Initially, one of the top diagnostic considerations in our patient was bullous pyoderma gangrenosum, a neutrophilic dermatosis that typically presents with painful ulcerative lesions and inflammatory bullae. Other causes of bullae in children include erythema multiforme, toxic epidermal necrolysis, epidermolysis bullosa, bullous mastocytosis, pemphigus, bullous pemphigoid, dermatitis herpetiformis, linear IgA dermatosis, bullous impetigo, gangrenous cellulitis, and Vibrio vulnificus infection. However, the clinical symptoms of joint pain and hematuria/proteinuria in our patient as well as the punch biopsy findings pointed toward HSP as the most likely diagnosis.
Although bullous lesions are relatively common in adult-onset HSP (16%–60% of patients), they are very rare in pediatric patients (2% of patients).2-4 We performed a PubMed search of articles indexed for MEDLINE for bullous Henoch-Schönlein purpura in childhood using the search term Henoch-Schönlein purpura and bullous. The Table provides a summary of our search results from the English-language literature.5-22
Bullae often develop on several parts of the body but are more commonly observed on the legs.17 Pathergy and edema have been implicated in the pathogenesis, as these findings have been observed in sites such as malleoli and legs, respectively.12 Matrix metalloproteinases secreted in polymorphonuclear neutrophils have been found to be elevated in blister fluid and can cause bullae formation via degrading collagen in the basement membrane.9 Corticosteroids, by virtue of their inhibition of proinflammatory transcription factors (eg, nuclear factor κβ, intranuclear activator protein 1) and decreasing metalloproteinase levels, may be efficacious in bullous HSP. Although there is no consensus, corticosteroid therapy seems to be efficacious in treating the bullae, according to several reports.17-22
The use of glucocorticoids in bullous HSP in childhood remains controversial. Studies report shortening of the duration of abdominal pain, reducing risk of intussusception, decreasing recurrence risk, and reducing the risk of renal involvement with use of steroids in HSP.23-25 The use of systemic steroids has been described in children with bullous HSP to reduce the severity of HSP-related bullae and its associated painful ulcers and necrosis.16,21,25,26 The duration of steroid use ranged from a short burst to a prolonged course of weaning over weeks. Azathioprine also has been used in conjunction with methylprednisolone, prednisone, and dexamethasone.17,22 Because of its anti-IgA antioxidant antineutrophil effects, dapsone has been shown to be effective in the treatment of cutaneous HSP.27 In our patient, we used dapsone to help in weaning the patient off the prednisone. Based on our review of the literature, few cases of bullous HSP in children have reported remission without drug therapy. IgA was not found in all the reported cases in which a skin biopsy was done. As shown by the comparison of the 2 biopsies in our patient, biopsying an early lesion within 48 hours of appearance is essential to make a diagnosis because the biopsy of the older lesion could not rule out bullous pyoderma gangrenosum. Immunoreactants (IgA, C3) are destroyed within 48 hours and might lead to false-negative results on immunofluorescence in old and necrotic lesions.28,29 Most reported cases of bullous HSP showed resolution, but few resulted in scarring and/or pigmentation.10,17,18 Henoch-Schönlein purpura usually is self-limited but relapses can be seen in one-third of cases.1 One of the reported cases of bullous HSP showed recurrence of lesions.15 One of the cases showed persistent hematuria.8 Our patient also was started on lisinopril for persistent proteinuria.
Henoch-Schönlein purpura (HSP) is a systemic, small vessel vasculitis affecting the skin, joints, gastrointestinal tract, and kidneys. It usually is self-limited, but relapses can be seen in one-third of cases.1 The classic cutaneous presentation includes palpable purpura localized to the legs and buttocks. Painful hemorrhagic bullae are uncommonly observed in childhood HSP and often could lead to a diagnostic dilemma. We report the case of a patient who presented with atypical features of painful hemorrhagic bullae and provide a review of the literature.
Case Report
An otherwise healthy 14-year-old adolescent girl presented to the hospital with painful ulcerative lesions covering the arms, legs, lower abdomen, and buttocks of 3 weeks’ duration. The rash first appeared on the ankles and spread in an ascending fashion, starting with bullous formation that was accompanied by joint pain, especially in the ankles and elbows. No abdominal pain was reported. The patient attributed the lesions to prolonged cold exposure followed by a hot bath. She had tried naproxen without any improvement of pain. She was afebrile with normal blood pressure.
On physical examination, numerous petechiae, palpable purpura, hemorrhagic bullae, and ulcers with surrounding erythematous to violaceous induration as well as central necrosis were noted on the arms, legs (Figure 1), abdomen, and buttocks. The palms, soles, trunk, and face were spared.
Laboratory values on admission revealed leukocytosis (17,500/μL [reference range, 4500–11,000/μL]), elevated erythrocyte sedimentation rate (42 mm/h [reference range, 0–20 mm/h]), elevated C-reactive protein (15.59 mg/L [reference range, 0.08–3.1 mg/L]), elevated C3 (174 mg/dL [reference range, 75–135 mg/dL]), normal C4 (32 mg/dL [reference range, 3–75 mg/dL]), normal blood urea nitrogen (13 mg/dL [reference range, 8–23 mg/dL]), and normal creatinine (0.72 mg/dL [reference range, 0.6–1.2 mg/dL]). Urinalysis showed microscopic hematuria and trace proteinuria. Platelet count was normal.
Diagnostic considerations included HSP, drug-induced leukocytoclastic vasculitis, and bullous pyoderma gangrenosum. The patient was started on oral prednisone 80 mg once daily. Additionally, oral doxycycline 100 mg twice daily was added for prevention of secondary bacterial infections and for anti-inflammatory effects. All nonsteroidal anti-inflammatory drugs were avoided. A commercial ointment containing 8-hydroxyquinoline sulfate 0.3% and triamcinolone acetonide ointment 0.1% were used to minimize skin irritation. Morphine, oxycodone-acetaminophen, and pregabalin followed by gabapentin were used for pain control. Hydrotherapy also was used for the treatment of skin lesions.
Two skin punch biopsies were performed at different stages. Biopsy of an early palpable purpuric lesion showed small vessel leukocytoclastic vasculitis with perivascular IgA on direct immunofluorescence. A second biopsy from a more hemorrhagic lesion performed 96 hours after admission to the hospital showed subepidermal vesicles with partial epidermal necrosis, confluent neutrophilic infiltrate in the papillary dermis, and small vessel vasculitis (Figures 2 and 3). Gram, periodic acid–Schiff, and acid-fast bacilli staining and cultures were negative. With continued treatment for 7 days, the clinical appearance of the lesions improved. On the tenth day of hospitalization, oral dapsone 25 mg once daily was initiated with the goal of weaning the patient off the prednisone as tolerated. She was discharged on prednisone (60 mg once daily) after 14 days of hospitalization. Dapsone also was continued.
 |  | |
Figure 2. Biopsy of a subepidermal bulla revealed neutrophilic inflammation within bullous space and evidence of dermal hemorrhage (H&E, original magnification ×100). | Figure 3. Leukocytoclastic vasculitis on biopsy (H&E, original magnification ×400). |
At 4-week follow-up, the lesions showed healing with mild residual pigmentation. The patient’s blood pressure and serum urea and creatinine levels were normal but the proteinuria was persistent, so the patient was started on oral lisinopril 5 mg once daily. Tapering of steroids over several months was initiated and the dose of dapsone was increased to 50 mg daily. Follow-up with a nephrologist was arranged to monitor renal function. She continued on lisinopril 5 mg once daily for treatment of nonnephrotic-range proteinuria, which was detected at 6 months following discharge.
Comment
The presence of atypical symptoms such as bullae and painful lesions in patients with suspected HSP can complicate the diagnosis. Initially, one of the top diagnostic considerations in our patient was bullous pyoderma gangrenosum, a neutrophilic dermatosis that typically presents with painful ulcerative lesions and inflammatory bullae. Other causes of bullae in children include erythema multiforme, toxic epidermal necrolysis, epidermolysis bullosa, bullous mastocytosis, pemphigus, bullous pemphigoid, dermatitis herpetiformis, linear IgA dermatosis, bullous impetigo, gangrenous cellulitis, and Vibrio vulnificus infection. However, the clinical symptoms of joint pain and hematuria/proteinuria in our patient as well as the punch biopsy findings pointed toward HSP as the most likely diagnosis.
Although bullous lesions are relatively common in adult-onset HSP (16%–60% of patients), they are very rare in pediatric patients (2% of patients).2-4 We performed a PubMed search of articles indexed for MEDLINE for bullous Henoch-Schönlein purpura in childhood using the search term Henoch-Schönlein purpura and bullous. The Table provides a summary of our search results from the English-language literature.5-22
Bullae often develop on several parts of the body but are more commonly observed on the legs.17 Pathergy and edema have been implicated in the pathogenesis, as these findings have been observed in sites such as malleoli and legs, respectively.12 Matrix metalloproteinases secreted in polymorphonuclear neutrophils have been found to be elevated in blister fluid and can cause bullae formation via degrading collagen in the basement membrane.9 Corticosteroids, by virtue of their inhibition of proinflammatory transcription factors (eg, nuclear factor κβ, intranuclear activator protein 1) and decreasing metalloproteinase levels, may be efficacious in bullous HSP. Although there is no consensus, corticosteroid therapy seems to be efficacious in treating the bullae, according to several reports.17-22
The use of glucocorticoids in bullous HSP in childhood remains controversial. Studies report shortening of the duration of abdominal pain, reducing risk of intussusception, decreasing recurrence risk, and reducing the risk of renal involvement with use of steroids in HSP.23-25 The use of systemic steroids has been described in children with bullous HSP to reduce the severity of HSP-related bullae and its associated painful ulcers and necrosis.16,21,25,26 The duration of steroid use ranged from a short burst to a prolonged course of weaning over weeks. Azathioprine also has been used in conjunction with methylprednisolone, prednisone, and dexamethasone.17,22 Because of its anti-IgA antioxidant antineutrophil effects, dapsone has been shown to be effective in the treatment of cutaneous HSP.27 In our patient, we used dapsone to help in weaning the patient off the prednisone. Based on our review of the literature, few cases of bullous HSP in children have reported remission without drug therapy. IgA was not found in all the reported cases in which a skin biopsy was done. As shown by the comparison of the 2 biopsies in our patient, biopsying an early lesion within 48 hours of appearance is essential to make a diagnosis because the biopsy of the older lesion could not rule out bullous pyoderma gangrenosum. Immunoreactants (IgA, C3) are destroyed within 48 hours and might lead to false-negative results on immunofluorescence in old and necrotic lesions.28,29 Most reported cases of bullous HSP showed resolution, but few resulted in scarring and/or pigmentation.10,17,18 Henoch-Schönlein purpura usually is self-limited but relapses can be seen in one-third of cases.1 One of the reported cases of bullous HSP showed recurrence of lesions.15 One of the cases showed persistent hematuria.8 Our patient also was started on lisinopril for persistent proteinuria.
1. Saulsbury FT. Henoch-Schönlein purpura in children. report of 100 patients and the review of literature. Medicine. 1999;78:395-409.
2. Cream JJ, Gumpel JM, Peachey RD. Schönlein-Henoch purpura in the adult. a study of 77 adults with anaphylactoid or Schönlein-Henoch purpura. Q J Med. 1970;39:461-484.
3. Tancrede-Bohin E, Ochonisky S, Vignon-Pennamen MD, et al. Schönlein-Henoch purpura in adult patients. predictive factors for IgA glomerulonephritis in a retrospective study of 57 cases. Arch Dermatol. 1997;133:438-442.
4. Abdel-Al YK, Hejazi Z, Majeed HA. Henoch Schönlein purpura in Arab children. analysis of 52 cases. Trop Geogr Med. 1990;42:52-57.
5. Garland JS, Chusid MJ. Henoch-Schöenlein purpura: association with unusual vesicular lesions. Wis Med J. 1985;84:21-23.
6. Crosby DL, Feldman SD. A pruritic vesicular eruption. Henoch-Schönlein purpura. Arch Dermatol. 1990;126:1497-1498.
7. Wananukul S, Pongprasit P, Korkij W. Henoch-Schönlein purpura presenting as hemorrhagic vesicles and bullae: case report and literature review. Pediatr Dermatol. 1995;12:314-317.
8. Saulsbury FT. Hemorrhagic bullous lesions in Henoch-Schönlein purpura. Pediatr Dermatol. 1998;15:357-359.
9. Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiological role of gelatinase (MMP-9). Dermatology. 1998;197:62-64.
10. Liu PM, Bong CN, Chen HH, et al. Henoch-Schönlein purpura with hemorrhagic bullae in children: report of two cases. J Microbiol Immunol Infect. 2004;37:375-378.
11. Ishii Y, Takizawa T, Arakawa H, et al. Hemorrhagic bullous lesions in Henoch-Schönlein purpura. Pediatr Int. 2005;47:694-697.
12. Leung AK, Robson WL. Hemorrhagic bullous lesions in a child with Henoch-Schönlein purpura. Pediatr Dermatol. 2006;23:139-141.
13. Chan K, Han N, Tang W, et al. Lesions in Henoch-Schönlein purpura. Pediatr Dermatol. 2007;24: 325-326.
14. Kausar S, Yalamanchili A. Management of haemorrhagic bullous lesions in Henoch-Schonlein purpura: is there any consensus? J Dermatolog Treat. 2009;20:88-90.
15. Maguiness S, Balma-Mena A, Pope E, et al. Bullous Henoch-Schönlein purpura in children: a report of 6 cases and review of the literature. Clin Pediatr. 2010;49: 1033-1037.
16. den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch-Schönlein purpura as indication to start systemic prednisone. Acta Paediatr. 2010;99:781-783.
17. Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature. Rheumatol Int. 2010;30:1355-1359.
18. Park SE, Lee JH. Haemorrhagic bullous lesions in a 3-year-old girl with Henoch-Schönlein purpura. Acta Paediatr. 2011;100:e283-e284.
19. Parikh K. 14-year-old boy with bullous lesions. Pediatr Ann. 2012;41:275-277.
20. Raymond M, Spinks J. Bullous Henoch Schönlein purpura. Arch Dis Child. 2012;97:617.
21. Kocaoglu C, Ozturk R, Unlu Y, et al. Successful treatment of hemorrhagic bullous Henoch-Schönlein purpura with oral corticosteroid: a case report [published online ahead of print April 16, 2013]. Case Rep Pediatr. 2013;2013:680208.
22. Mehra S, Suri D, Dogra S, et al. Hemorrhagic bullous lesions in a girl with Henoch Schönlein purpura. Indian J Pediatr. 2014;81:210-211.
23. Ronkainen J, Koskimies O, Ala-Houhala M, et al. Early prednisone therapy in Henoch-Schönlein purpura: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2006;149:241-247.
24. Weiss PF, Klink AJ, Localio R, et al. Corticosteroids may improve clinical outcomes during hospitalization for Henoch-Schönlein purpura. Pediatrics. 2010;126:674-681.
25. Rosato L, Chehade H, Cachat F. Re: steroids in haemorrhagic bullous Henoch-Schönlein purpura. Acta Paediatr. 2011;100:319-320.
26. Park SJ, Kim JH, Ha TS, et al. The role of corticosteroid in hemorrhagic bullous Henoch Schönlein purpura. Acta Paediatr. 2011;100:e3-e4.
27. Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
28. Davin JC, Weening JJ. Diagnosis of Henoch-Schönlein purpura: renal or skin biopsy? Pediatr Nephrol. 2003;18:1201-1203.
29. González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48: 1157-1165.
1. Saulsbury FT. Henoch-Schönlein purpura in children. report of 100 patients and the review of literature. Medicine. 1999;78:395-409.
2. Cream JJ, Gumpel JM, Peachey RD. Schönlein-Henoch purpura in the adult. a study of 77 adults with anaphylactoid or Schönlein-Henoch purpura. Q J Med. 1970;39:461-484.
3. Tancrede-Bohin E, Ochonisky S, Vignon-Pennamen MD, et al. Schönlein-Henoch purpura in adult patients. predictive factors for IgA glomerulonephritis in a retrospective study of 57 cases. Arch Dermatol. 1997;133:438-442.
4. Abdel-Al YK, Hejazi Z, Majeed HA. Henoch Schönlein purpura in Arab children. analysis of 52 cases. Trop Geogr Med. 1990;42:52-57.
5. Garland JS, Chusid MJ. Henoch-Schöenlein purpura: association with unusual vesicular lesions. Wis Med J. 1985;84:21-23.
6. Crosby DL, Feldman SD. A pruritic vesicular eruption. Henoch-Schönlein purpura. Arch Dermatol. 1990;126:1497-1498.
7. Wananukul S, Pongprasit P, Korkij W. Henoch-Schönlein purpura presenting as hemorrhagic vesicles and bullae: case report and literature review. Pediatr Dermatol. 1995;12:314-317.
8. Saulsbury FT. Hemorrhagic bullous lesions in Henoch-Schönlein purpura. Pediatr Dermatol. 1998;15:357-359.
9. Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiological role of gelatinase (MMP-9). Dermatology. 1998;197:62-64.
10. Liu PM, Bong CN, Chen HH, et al. Henoch-Schönlein purpura with hemorrhagic bullae in children: report of two cases. J Microbiol Immunol Infect. 2004;37:375-378.
11. Ishii Y, Takizawa T, Arakawa H, et al. Hemorrhagic bullous lesions in Henoch-Schönlein purpura. Pediatr Int. 2005;47:694-697.
12. Leung AK, Robson WL. Hemorrhagic bullous lesions in a child with Henoch-Schönlein purpura. Pediatr Dermatol. 2006;23:139-141.
13. Chan K, Han N, Tang W, et al. Lesions in Henoch-Schönlein purpura. Pediatr Dermatol. 2007;24: 325-326.
14. Kausar S, Yalamanchili A. Management of haemorrhagic bullous lesions in Henoch-Schonlein purpura: is there any consensus? J Dermatolog Treat. 2009;20:88-90.
15. Maguiness S, Balma-Mena A, Pope E, et al. Bullous Henoch-Schönlein purpura in children: a report of 6 cases and review of the literature. Clin Pediatr. 2010;49: 1033-1037.
16. den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch-Schönlein purpura as indication to start systemic prednisone. Acta Paediatr. 2010;99:781-783.
17. Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature. Rheumatol Int. 2010;30:1355-1359.
18. Park SE, Lee JH. Haemorrhagic bullous lesions in a 3-year-old girl with Henoch-Schönlein purpura. Acta Paediatr. 2011;100:e283-e284.
19. Parikh K. 14-year-old boy with bullous lesions. Pediatr Ann. 2012;41:275-277.
20. Raymond M, Spinks J. Bullous Henoch Schönlein purpura. Arch Dis Child. 2012;97:617.
21. Kocaoglu C, Ozturk R, Unlu Y, et al. Successful treatment of hemorrhagic bullous Henoch-Schönlein purpura with oral corticosteroid: a case report [published online ahead of print April 16, 2013]. Case Rep Pediatr. 2013;2013:680208.
22. Mehra S, Suri D, Dogra S, et al. Hemorrhagic bullous lesions in a girl with Henoch Schönlein purpura. Indian J Pediatr. 2014;81:210-211.
23. Ronkainen J, Koskimies O, Ala-Houhala M, et al. Early prednisone therapy in Henoch-Schönlein purpura: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2006;149:241-247.
24. Weiss PF, Klink AJ, Localio R, et al. Corticosteroids may improve clinical outcomes during hospitalization for Henoch-Schönlein purpura. Pediatrics. 2010;126:674-681.
25. Rosato L, Chehade H, Cachat F. Re: steroids in haemorrhagic bullous Henoch-Schönlein purpura. Acta Paediatr. 2011;100:319-320.
26. Park SJ, Kim JH, Ha TS, et al. The role of corticosteroid in hemorrhagic bullous Henoch Schönlein purpura. Acta Paediatr. 2011;100:e3-e4.
27. Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
28. Davin JC, Weening JJ. Diagnosis of Henoch-Schönlein purpura: renal or skin biopsy? Pediatr Nephrol. 2003;18:1201-1203.
29. González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48: 1157-1165.
Practice Points
- The presence of painful hemorrhagic bullae is an uncommon presentation in pediatric patients with Henoch-Schönlein purpura (HSP) and can be a diagnostic challenge.
- Presence of joint pain, abdominal pain, or nephritis could corroborate the diagnosis.
- Early biopsy of the lesion within 48 hours of appearance is important for diagnosis. Presence of IgA deposits on immunofluorescence may aid in diagnosis.
- This finding of bullae in HSP does not seem to have any prognostic significance. Because of the rarity of incidence, there is no consensus on management. Supportive therapy and/or corticosteroids might be effective.
Scalp Hyperkeratosis in Children With Skin of Color: Diagnostic and Therapeutic Considerations
Scalp hyperkeratosis (scaling or flaking) is a common symptom in childhood and is typified by fine to thick hyperkeratosis of the scalp with or without underlying erythema. The causes of scalp hyperkeratosis in childhood vary based on the demographics of the population. In a population where approximately half of the pediatric patients were white, scaling of the scalp was more common in patients with seborrheic dermatitis and/or atopic dermatitis (AD) who were aged 0 to 2 years, and tinea capitis was only noted in children who were black.1 In children with skin of color, scalp hyperkeratosis has been noted as a marker of tinea capitis, especially in patients aged 3 to 11 years,2,3 and the level of suspicion should consistently remain high for this age group. In another study of an all-black population of schoolchildren aged 5 to 13 years (N=224), 3% demonstrated signs and symptoms of tinea capitis and 14% were found to be asymptomatic carriers.4 Although generally benign in nature, scalp hyperkeratosis can be associated with systemic illnesses such as juvenile dermatomyositis and Langerhans cell histiocytosis.5 This article addresses the diagnosis and treatment of scalp hyperkeratosis in children with skin of color, focusing on differences in exposure to contagious cases, hairstyling practices, and biological factors that may impact the disease process.
CAUSES OF SCALP HYPERKERATOSIS IN CHILDHOOD
Scalp hyperkeratosis in childhood usually is caused by common benign conditions, but some level of suspicion should be maintained for more severe etiologic conditions such as Langerhans cell histiocytosis and collagen vascular diseases (eg, juvenile dermatomyositis).6 Langerhans cell histiocytosis of the scalp might be obscured by background pigmentation in black children.
Scalp scaling can be a minor criterion in the diagnosis of AD. Atopic dermatitis should be suspected in Asian children with scalp scaling. Although one study in Bangladesh revealed scalp involvement in only 5.2% of pediatric patients with AD,7 a study in China reported an incidence rate as high as 49.7% (with a similarly high incidence of eyelid dermatitis).8 Children with AD also may have dry hair.9 Atopic dermatitis of the scalp is typified by itching, fine hyperkeratosis, and notably eczematous scalp lesions ranging from excoriated or oozing erythematous plaques to lichenification with hair miniaturization, primarily from scratch-induced breakage.10 The latter finding often is noted in black adolescent girls with long-term moderate to severe AD (personal observation).
Seborrheic dermatitis is a hypersensitivity response to yeast colonization of the scalp with Malassezia species. The infantile form is extremely common (also known as cradle cap). Characteristically, greasy yellow hyperkeratosis in fine to thick sheets is noted on the scalp in children younger than 2 years, especially infants, often with involvement of skin folds. One study noted that seborrheic dermatitis occurs in 6% of school-aged children as opposed to 19% of children younger than 2 years.1 Severe seborrheic dermatitis in infancy may be a prelude to AD, with the incidence being 3 times higher in children with prior seborrheic dermatitis.11 In teenagers, seborrheic dermatitis often accompanies acne onset in the early pubertal years.12
Psoriasis is an autoimmune inflammatory dermatosis that most commonly affects white children. In childhood, pityriasis amiantacea, psoriasiform scalp hyperkeratosis, is more common than in adulthood, with thick, stuck-on scales bound to the hairs. This variant is uncommon in Hispanic and Asian children and is almost never seen in black children but has been reported in cohorts of Turkish children.13 In a series of 85 Egyptian children with pityriasis amiantacea, diagnosis of scalp psoriasis was made in 35.3%, eczematous dermatitis in 34.2%, and tinea capitis in 12.9%.14 Consequently, a high degree of suspicion for tinea capitis should be held if pityriasis amiantacea is found in children with skin of color.15,16
Tinea capitis is a dermatophyte infection of the scalp, hair, and surrounding skin. The presence of tinea capitis on the scalp is associated with environmental exposure to dermatophytes (eg, school, household).4,17 The infection is largely caused by Trichophyton tonsurans in the United States, which causes a seborrheic appearance and less commonly alopecia (black dot or thinning), plaques with scale, or kerion. The presence of cervical lymph nodes and/or alopecia increases the chances of tinea being the diagnosis. Potassium hydroxide preparation and fungal culture can be performed to corroborate the diagnosis.1-3 Other etiologies of scalp hyperkeratosis such as juvenile pityriasis rubra pilaris and lice are extremely uncommon in black children, but lice may be seen in Hispanic and Asian girls with long straight hair who attend school. Discoid lupus is more common in children with skin of color but is rare overall. When noted, accompanying mottled dyspigmentation and scarring alopecia are noted in addition to a high risk for developing systemic lupus erythematosus. Biopsy and screening for systemic lupus are necessary, as the risk for progression from discoid lupus to systemic disease is 26% over 3 years.18
THE BIOLOGY OF HAIR IN CHILDREN WITH SKIN OF COLOR
To some extent, the biology of hair impacts the occurrence, appearance, and treatment of scalp hyperkeratosis in children with skin of color. First, it is important to remember that follicular density is lower in black patients as compared to Asian patients with a consequently lower hair count overall, which results in the easy appearance of hair loss, particularly at the margins of the scalp.19,20 Second, the shape of the hair follicle differs among races and ethnicities. Asian patients have round hair shafts coming from straight follicles, which allows for greater natural hair hydration, resulting in somewhat less aggressive scalp disease. Hispanic patients may have similarly straight hair or may have elliptical or curled shafts, the latter being noted in black patients. Furthermore, a curled hair shaft results in poor flow of sebum across the hair, resulting in greater scalp xerosis, more susceptibility to traction alopecia, and ultimately a greater risk for infections.20-23 Finally, the scalp is continuous with the face and neck, and Asian patients have greater sensitivity to skin care products in these areas, resulting in difficulty of treatment in this patient population and the need for use of gentle products.
HAIR CARE PRACTICES IN CHILDREN WITH SKIN OF COLOR
Hair care in patients with skin of color can be costly, difficult, and potentially damaging, with 99% of black girls reporting pomade or oil usage. Costly and complex hair care practices begin in childhood for patients with skin of color. In a series of 201 surveyed black girls with a mean age of 9.8 years, 80% had used hot combs and 42% used relaxers.24 Traction styles were common with 81% using ponytails, 67% braids, and 49% cornrows in the last 12 months. These styles are thought to affect hair health, particularly through induction of traction-related damage, folliculitis, and alopecia. Furthermore, chemical relaxers, hot combs, blowouts, and hair setting may be introduced during childhood.24 These practices appear to disturb the integrity of the hair follicle, leaving it more susceptible to irritation and infection.
Hair care in the pediatric population often is complicated by the fact that multiple children are being styled in tandem, either at home or in a salon, resulting in shared equipment and fomite spread. Even just proximity to a case of tinea capitis in the household will increase risk for tinea capitis. Furthermore, it is quite commonplace for black patients to use pomades and shampoos that contain antifungals, especially selenium sulfide, which makes it difficult to obtain accurate culture results. In India, use of mustard oil also has been linked to increased risk for tinea capitis.25
Other issues related to hair care include frequent dry scalp in patients with skin of color due to poor sebum distribution along the hair shaft. As a result, frequent washing may exacerbate scalp xerosis and further irritate seborrheic dermatitis and/or AD.
DIAGNOSTIC CONSIDERATIONS FOR SCALP HYPERKERATOSIS IN CHILDHOOD
Dermatologists should have a greater level of suspicion for tinea capitis in black and Hispanic children compared to white children. The index of suspicion should be high given that antifungal shampoos and pomades may minimize the clinical appearance. Although trends in overall incidence in the United States suggest tinea capitis is becoming less common, there still is a stronger representation of the disease in black patients.26 A study of positive fungal cultures from one clinic in Mississippi (N=1220) showed that two-thirds of patients were children younger than 13 years; 87% of patients with positive cultures for dermatophytes were black.27 The endothrix type of tinea capitis caused by T tonsurans often presents with a seborrheic appearance, and fungal culture is warranted in all pediatric patients with skin of color who have scalp hyperkeratosis. Asian children can be regarded with a lower level of suspicion for tinea capitis, similar to white patients in the United States. Variation in incidence of tinea capitis does exist worldwide and the practitioner may need to address these issues in patients who travel or are recent immigrants.
When identifying tinea capitis infections in children with skin of color, physicians should consider the patient’s personal and family history, comorbid skin disorders, dermoscopy, microscopy and fungal staining, and fungal culture (Figure).
A paradigm for the diagnosis of scalp hyperkeratosis in children with skin of color.
Personal and Family History
The first diagnostic consideration is the patient’s personal and family history. A history of AD, asthma, or allergies will support but not confirm the diagnosis of AD. Prior tinea capitis infections and household contacts with tinea infections support the presence of tinea capitis.17 Recent implementation of anti–tumor necrosis factor a inhibitor therapy in a psoriatic child can flare scalp disease, mimicking tinea capitis.28 The patient’s guardians should be queried about potential infectious contacts, whether they themselves have signs of scalp disease or tinea corporis (ringworm) or whether they have a pet with problematic fur. Physicians also should query patients and their guardians about recent use of topical antifungal shampoos, pomades, creams (both over-the-counter [OTC] and prescription), and/or oral antifungals. When these agents are used, there is a possibility that fungal examinations may be negative in the presence of true infection with tinea capitis. Traction alopecia, often preceded by fine scale, is more likely to present in patients who wear their hair in cornrows, while seborrheic dermatitis may be associated with hair extensions, reduced frequency of washing (61% of black girls surveyed wash every 2 weeks), and/or reduced usage of hair oils in black girls.24 Knowledge of the patient’s personal hair care history, such as use of pomades; frequency and method of washing/drying hair; types of hair care products used daily to wash and style hair; use of chemical relaxers; or recent hairstyling with cornrows, braids, or hair extensions, also is essential to the diagnosis of tinea capitis. Usage of traction-related styling practices in patients with chemically relaxed hair can enhance the risk for traction alopecia.29
Comorbid Skin Disorders
The patient also should be examined for comorbid skin disorders, including tinea corporis, alopecia (particularly in the areas of hyperkeratosis), and the presence of nuchal lymphadenopathy. For each extra clinical finding, the chances of a final diagnosis of tinea capitis rises, allowing for empiric diagnosis to be made that can be confirmed by a variety of tests.1-3
Dermoscopy
Next, the patient should undergo dermoscopic evaluation. On dermoscopy, tinea capitis typically presents with broken hairs, black dots on the scalp, comma-shaped hairs, and short corkscrew hairs, all of which should clear with therapy.30-33 Dermoscopic findings of AD would reveal underlying xerosis and prominent vasculature due to inflammation, and alopecia areata would present with yellow dots at the orifices of the hair follicles, exclamation point hairs, and vellus hairs.34,35 Traction alopecia may be noted by retained hairs along the hairline, which is known as the fringe sign.36
Microscopy and Fungal Staining
Microscopic preparations can be performed to identify tinea capitis using fungal stains of slide-based specimens. Breakage of short hairs onto the slide and/or cotton swab is a soft sign corroborating endothrix infection of the hairs. Potassium hydroxide can enhance visualization of the hyperkeratotic scalp, but for most black patients, use of antifungal agents reduces fungal hyphae and spores in the areas of hyperkeratosis and may limit the utility of examining the skin microscopically. Assessment of the broken hairs obtained by gentle friction with one glass slide and catching the scales onto another glass slide may yield the best results in the evaluation of tinea capitis (a technique taught to me by Robin Hornung, MD, Everett, Washington). Hairs obtained in this manner often are fragile and break due to endothrix infection replacing and weakening the shaft of the hairs. In the United States, fungal samples usually are obtained with cotton swabs, but a recent study suggested that brushing is superior to scraping to obtain samples; the combination of sampling techniques may improve the yield of a culture.37 Because topical agents are unable to enter the hair cortex, the hair shaft is the most likely to show fungal spores under the microscope when antifungal shampoos or pomades are used. Other testing methods such as Swartz-Lamkins or calcofluor white staining can be used on similar scrapings. Biopsy and periodic acid–Schiff staining of thick scales or crust can help differentiate tinea capitis from pityriasis amiantacea when the crust is too thick to be softened via potassium hydroxide preparation.38
Fungal Culture
Fungal culture onto media that contains nutrients for dermatophyte growth can be used for 4 purposes in tinea capitis: (1) to confirm infection, (2) to identify species of infection, (3) to confirm mycological cure when difficulty in clearance of disease has been noted, and (4) to obtain a specimen for sensitivity screening regarding antifungals when necessary, an uncommon but occasionally useful test in individuals with disease that has failed treatment with 1 or more antifungals.27
THERAPY FOR SCALP HYPERKERATOSIS IN CHILDREN WITH SKIN OF COLOR
In patients with scalp hyperkeratosis, it is important to address the specific cause of the disease. Therapy for scalp hyperkeratosis in children with skin of color includes altered hair care practices, use of OTC and prescription agents, and containment of fomites in the case of infections. Biopsy of atypical scalp hyperkeratosis cases is needed to diagnose rare etiologies such as discoid lupus or Langerhans cell histiocytosis. For individuals with systemic disease including Langerhans cell histiocytosis, which is generally accompanied by nodes and plaques in the inguinal region or other intertriginous sites, immediate hematology and oncology workup is required.39 For collagen vascular diseases such as lupus or dermatomyositis, appropriate referral to rheumatology and systemic therapy is warranted.
Altered Hair Care Practices
The use of prophylactic ketoconazole 1% shampoo may not reduce the risk for recurrence of tinea capitis over standard good hygiene, removal of fomites, and adherence to prescribed therapy.40 Use of selenium sulfide has been shown to effectively reduce contagion risk.41
Fragrance- and dye-free shampoos can be helpful in providing gentle cleansing of the scalp, which is especially important in Asian patients who have greater facial and eyelid sensitivity. Free-and-clear shampoos can be used alternatively with shampoos containing selenium sulfide or sulfur to eliminate comorbid seborrhea. Black patients should be advised to shampoo and condition their hair once weekly, and Asian and Hispanic patients should shampoo and condition 2 to 3 times weekly to remove scale and potentially reduce risk for tinea acquisition.42 Children with straight hair should shampoo with increased frequency in the summer to manually remove sweat-induced macerated hyperkeratosis. Conditioners also should be used consistently after shampooing to enhance hair health.
Use of OTC and Prescription Agents
Atopic Dermatitis
Topical corticosteroid agents can be used in increasing strengths to treat AD of the scalp in children with skin of color, from OTC scalp products containing hydrocortisone 1% to prescription-based agents. Hydration of the hair also is needed to counteract reduced water content.43 Due to the innate xerosis of the scalp in black patients and atopic patients, the use of oil-based or lotion products may provide the most hydration for patients with scalp disease.44 Alcohol-based agents, either drops or foams, may enhance xerosis and should be used sparingly.
Seborrheic Dermatitis
Alternating treatment with medicated shampoos containing selenium sulfide and ketoconazole can aid in the removal of seborrhea. Pomades including borage seed oil–based agents can be massaged into the scalp,45 particularly for treatment of infantile seborrhea, and should not necessarily be washed off daily in dark-skinned patients. Additional focused application of topical corticosteroids to the scalp also is helpful. Due to innate scalp xerosis in black children, therapy should be similar to AD.
Psoriasis
In the setting of pityriasis amiantacea, albeit rare in children with skin of color, oil-based agents can soften hyperkeratosis for removal. Sterile mineral oil or commercially available scalp preparations of peanut oil with fluocinolone or mineral oil with glycerin can aid in the removal of scales without harming the hair, but usage must be age appropriate. The addition of focused application of age-appropriate topical corticosteroids for areas of severe hyperkeratosis can aid in clearance of the lesions.44 Recently, a stable combination of calcipo-triene 0.005%–betamethasone dipropionate 0.064% has been approved in the United States for the therapy of scalp psoriasis in adolescents.46
Tinea Capitis
Antifungal shampoos including selenium sulfide will reduce contagion risk when used by both the patient and his/her family members. Frequency of shampooing is similar to that described for AD. Between shampooing, pomades with selenium sulfide can be applied to the scalp to enhance overall clearance.
Oral antifungals are the basis of treatment and use of griseofulvin is the gold standard. Terbinafine has been approved by the US Food and Drug Administration for treatment of tinea capitis; for children weighing less than 25 kg the dosage is 125 mg daily, for 25 to 35 kg the dosage is 187.5 mg daily, and for more than 35 kg the dosage is 250 mg daily. Shorter therapeutic courses may be required, making it a good second-line agent. Laboratory screening in children prior to therapy is not always performed but should be done in cases where fatty liver might be suspected.47 Monitoring liver function tests is best when exceeding 3 months of usage or shifting from one antifungal to another.3
Containment of Fomites
There are several procedures that should be followed to contain scalp infection in children with skin of color. First, all objects that come into contact with the scalp (eg, hats, hoods, brushes, pillowcases) should be washed with hot water or replaced weekly. Sharing these objects with friends or family should be strongly discouraged. Patients and their family members also should be instructed to use medicated (eg, selenium sulfide) shampoos and conditioners. Finally, patients are advised to avoid use of shared classroom garments or mats for sleeping.
LONG-TERM SEQUELAE OF SCALP HYPERKERATOSIS
Long-term sequelae of scalp hyperkeratosis often are discounted in children, but the disease can have lasting and damaging effects on the scalp. Sequelae include discomfort from chronicity and psychological distress. In particular, years of scalp pruritus can promote lichenification of the scalp and miniaturization of the hair follicles. Furthermore, itching due to sweating can limit participation in sports. Finally, tinea capitis is thought to be a risk factor for central centrifugal cicatricial alopecia (or can occur comorbidly with central centrifugal cicatricial alopecia causing severe pruritus), a chronic scarring hair loss that is seen primarily in black adult females.48 Erythema nodosum also has been reported as an associated finding in the case of kerion.49 One study reported associated findings that included thyroid cancer in individuals irradiated for tinea capitis in the 1950s.50
Conclusion
Scalp hyperkeratosis in children with skin of color, especially black patients, is more likely to be associated with tinea capitis and is more challenging to treat due to innate scalp xerosis in black patients and increased sensitivity of facial skin in Asian children. Ultimately, institution of therapy when needed and good scalp and hair care may prevent long-term sequelae.
1. Williams JV, Eichenfield LF, Burke BL, et al. Prevalence of scalp scaling in prepubertal children. Pediatrics. 2005;115:e1-e6.
2. Coley MK, Bhanusali DG, Silverberg JI, et al. Scalp hyperkeratosis and alopecia in children of color. J Drugs Dermatol. 2011;10:511-516.
3. Bhanusali D, Coley M, Silverberg JI, et al. Treatment outcomes for tinea capitis in a skin of color population. J Drugs Dermatol. 2012;11:852-856.
4. Williams JV, Honig PJ, McGinley KJ, et al. Semiquantitative study of tinea capitis and the asymptomatic carrier state in inner-city school children. Pediatrics. 1995;96:265-267.
5. McDonald LL, Smith ML. Diagnostic dilemmas in pediatric/adolescent dermatology: scaly scalp. J Pediatr Health Care. 1998;12:80-84.
6. Peloro TM, Miller OF 3rd, Hahn TF, et al. Juvenile dermatomyositis: a retrospective review of a 30-year experience. J Am Acad Dermatol. 2001;45:28-34.
7. Wahab MA, Rahman MH, Khondker L, et al. Minor criteria for atopic dermatitis in children. Mymensingh Med J. 2011;20:419-424.
8. Shi M, Zhang H, Chen X, et al. Clinical features of atopic dermatitis in a hospital-based setting in China. J Eur Acad Dermatol Venereol [published online ahead of print January 9, 2011]. 2011;25:1206-1212.
9. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair. Microsc Res Tech. 2012;75:620-625.
10. Sabin BR, Peters N, Peters AT. Chapter 20: atopic dermatitis. Allergy Asthma Proc. 2012;33:S67-S69.
11. Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis [published online ahead of print November 13, 2013]. Pediatr Dermatol. 2014;31:125-130.
12. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
13. Sarifakioglu E, Yilmaz AE, Gorpelioglu C, et al. Prevalence of scalp disorders and hair loss in children. Cutis. 2012;90:225-229.
14. Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
15. Oostveen AM, Jong EM, Evers AW, et al. Reliability, responsiveness and validity of Scalpdex in children with scalp psoriasis: the Dutch study. Acta Derm Venereol. 2014;94:198-202.
16. Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity: Comparative Dermatologic Atlas of Pediatric Skin of All Colors. New York, NY: Springer; 2012.
17. Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
18. Moises-Alfaro C, Berrón-Pérez R, Carrasco-Daza D, et al. Discoid lupus erythematosus in children: clinical, histopathologic, and follow-up features in 27 cases. Pediatr Dermatol. 2003;20:103-107.
19. Ramos-e-Silva M. Ethnic hair and skin: what is the state of the science? Chicago, Illinois—September 29-30, 2001. Clin Dermatol. 2002;20:321-324.
20. Heath CR, McMichael AJ. Biology of hair follicle. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. New York, NY: McGraw Hill; 2009:105-109.
21. Khumalo NP. African hair morphology: macrostructure to ultrastructure. Int J Dermatol. 2005;44(suppl 1):10-12.
22. Thibaut S, Bernard BA. The biology of hair shape. Int J Dermatol. 2005;44(suppl 1):2-3.
23. Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
24. Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
25. Kumar V, Sharma RC, Chander R. Clinicomycological study of tinea capitis. Indian J Dermatol Venereol Leprol. 1996;62:207-209.
26. Mirmirani P, Tucker LY. Epidemiologic trends in pediatric tinea capitis: a population-based study from Kaiser Permanente Northern California [published online ahead of print October 2, 2013]. J Am Acad Dermatol. 2013;69:916-921.
27. Chapman JC, Daniel CR 3rd, Daniel JG, et al. Tinea capitis caused by dermatophytes: a 15-year retrospective study from a Mississippi Dermatology Clinic. Cutis. 2011;88:230-233.
28. Perman MJ, Lovell DJ, Denson LA, et al. Five cases of anti-tumor necrosis factor alpha-induced psoriasis presenting with severe scalp involvement in children. Pediatr Dermatol. 2012;29:454-459.
29. Khumalo NP, Jessop S, Gumedze F, et al. Determinants of marginal traction alopecia in African girls and women. J Am Acad Dermatol. 2008;59:432-438.
30. Vazquez-Lopez F, Palacios-Garcia L, Argenziano G. Dermoscopic corkscrew hairs dissolve after successful therapy of Trichophyton violaceum tinea capitis: a case report. Australas J Dermatol. 2012;53:118-119.
31. Pinheiro AM, Lobato LA, Varella TC. Dermoscopy findings in tinea capitis: case report and literature review. An Bras Dermatol. 2012;87:313-314.
32. Mapelli ET, Gualandri L, Cerri A, et al. Comma hairs in tinea capitis: a useful dermatoscopic sign for diagnosis of tinea capitis. Pediatr Dermatol. 2012;29:223-224.
33. Hughes R, Chiaverini C, Bahadoran P, et al. Corkscrew hair: a new dermoscopic sign for diagnosis of tinea capitis in black children. Arch Dermatol. 2011;147:355-356.
34. Ekiz O, Sen BB, Rifaiog˘lu EN, et al. Trichoscopy in paediatric patients with tinea capitis: a useful method to differentiate from alopecia areata [published online ahead of print August 24, 2013]. J Eur Acad Dermatol Venereol. 2014;28:1255-1258.
35. Lencastre A, Tosti A. Role of trichoscopy in children’s scalp and hair disorders [published online ahead of print Aug 13, 2013]. Pediatr Dermatol. 2013;30:674-682.
36. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
37. Nasir S, Ralph N, O’Neill C, et al. Trends in tinea capitis in an Irish pediatric population and a comparison of scalp brushings versus scalp scrapings as methods of investigation [published online ahead of print February 22, 2013]. Pediatr Dermatol. 2014;31:622-623.
38. Alvarez MS, Silverberg NB. Tinea capitis. Cutis. 2006;78:189-196.
39. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996.
40. Bookstaver PB, Watson HJ, Winters SD, et al. Prophylactic ketoconazole shampoo for tinea capitis in a high-risk pediatric population. J Pediatr Pharmacol Ther. 2011;16:199-203.
41. Allen HB, Honig PJ, Leyden JJ, et al. Selenium sulfide: adjunctive therapy for tinea capitis. Pediatrics. 1982;69:81-83.
42. Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
43. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair [published online ahead of print November 7, 2011]. Microsc Res Tech. 2012;75:620-625.
44. Kapila S, Hong E, Fischer G. A comparative study of childhood psoriasis and atopic dermatitis and greater understanding of the overlapping condition, psoriasis-dermatitis. Australas J Dermatol. 2012;53:98-105.
45. Tollesson A, Frithz A. Borage oil, an effective new treatment for infantile seborrhoeic dermatitis. Br J Dermatol. 1993;129:95.
46. Gooderham M, Debarre JM, Keddy-Grant J, et al. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12-17 years of age [published online ahead of print October 22, 2014]. Br J Dermatol. 2014;171:1470-1477.
47. Singer C, Stancu P, Coşoveanu S, et al. Non-alcoholic fatty liver disease in children. Curr Health Sci J. 2014;40:170-176.
48. Chiang C, Price V, Mirmirani P. Central centrifugal cicatricial alopecia: superimposed tinea capitis as the etiology of chronic scalp pruritus. Dermatol Online J. 2008;14:3.
49. Morrone A, Calcaterra R, Valenzano M, et al. Erythema nodosum induced by kerion celsi of the scalp in a woman. Mycoses. 2011;54:e237-e239.
50. Boaventura P, Pereira D, Celestino R, et al. Genetic alterations in thyroid tumors from patients irradiated in childhood for tinea capitis treatment. Eur J Endocrinol. 2013;169:673-679.
Scalp hyperkeratosis (scaling or flaking) is a common symptom in childhood and is typified by fine to thick hyperkeratosis of the scalp with or without underlying erythema. The causes of scalp hyperkeratosis in childhood vary based on the demographics of the population. In a population where approximately half of the pediatric patients were white, scaling of the scalp was more common in patients with seborrheic dermatitis and/or atopic dermatitis (AD) who were aged 0 to 2 years, and tinea capitis was only noted in children who were black.1 In children with skin of color, scalp hyperkeratosis has been noted as a marker of tinea capitis, especially in patients aged 3 to 11 years,2,3 and the level of suspicion should consistently remain high for this age group. In another study of an all-black population of schoolchildren aged 5 to 13 years (N=224), 3% demonstrated signs and symptoms of tinea capitis and 14% were found to be asymptomatic carriers.4 Although generally benign in nature, scalp hyperkeratosis can be associated with systemic illnesses such as juvenile dermatomyositis and Langerhans cell histiocytosis.5 This article addresses the diagnosis and treatment of scalp hyperkeratosis in children with skin of color, focusing on differences in exposure to contagious cases, hairstyling practices, and biological factors that may impact the disease process.
CAUSES OF SCALP HYPERKERATOSIS IN CHILDHOOD
Scalp hyperkeratosis in childhood usually is caused by common benign conditions, but some level of suspicion should be maintained for more severe etiologic conditions such as Langerhans cell histiocytosis and collagen vascular diseases (eg, juvenile dermatomyositis).6 Langerhans cell histiocytosis of the scalp might be obscured by background pigmentation in black children.
Scalp scaling can be a minor criterion in the diagnosis of AD. Atopic dermatitis should be suspected in Asian children with scalp scaling. Although one study in Bangladesh revealed scalp involvement in only 5.2% of pediatric patients with AD,7 a study in China reported an incidence rate as high as 49.7% (with a similarly high incidence of eyelid dermatitis).8 Children with AD also may have dry hair.9 Atopic dermatitis of the scalp is typified by itching, fine hyperkeratosis, and notably eczematous scalp lesions ranging from excoriated or oozing erythematous plaques to lichenification with hair miniaturization, primarily from scratch-induced breakage.10 The latter finding often is noted in black adolescent girls with long-term moderate to severe AD (personal observation).
Seborrheic dermatitis is a hypersensitivity response to yeast colonization of the scalp with Malassezia species. The infantile form is extremely common (also known as cradle cap). Characteristically, greasy yellow hyperkeratosis in fine to thick sheets is noted on the scalp in children younger than 2 years, especially infants, often with involvement of skin folds. One study noted that seborrheic dermatitis occurs in 6% of school-aged children as opposed to 19% of children younger than 2 years.1 Severe seborrheic dermatitis in infancy may be a prelude to AD, with the incidence being 3 times higher in children with prior seborrheic dermatitis.11 In teenagers, seborrheic dermatitis often accompanies acne onset in the early pubertal years.12
Psoriasis is an autoimmune inflammatory dermatosis that most commonly affects white children. In childhood, pityriasis amiantacea, psoriasiform scalp hyperkeratosis, is more common than in adulthood, with thick, stuck-on scales bound to the hairs. This variant is uncommon in Hispanic and Asian children and is almost never seen in black children but has been reported in cohorts of Turkish children.13 In a series of 85 Egyptian children with pityriasis amiantacea, diagnosis of scalp psoriasis was made in 35.3%, eczematous dermatitis in 34.2%, and tinea capitis in 12.9%.14 Consequently, a high degree of suspicion for tinea capitis should be held if pityriasis amiantacea is found in children with skin of color.15,16
Tinea capitis is a dermatophyte infection of the scalp, hair, and surrounding skin. The presence of tinea capitis on the scalp is associated with environmental exposure to dermatophytes (eg, school, household).4,17 The infection is largely caused by Trichophyton tonsurans in the United States, which causes a seborrheic appearance and less commonly alopecia (black dot or thinning), plaques with scale, or kerion. The presence of cervical lymph nodes and/or alopecia increases the chances of tinea being the diagnosis. Potassium hydroxide preparation and fungal culture can be performed to corroborate the diagnosis.1-3 Other etiologies of scalp hyperkeratosis such as juvenile pityriasis rubra pilaris and lice are extremely uncommon in black children, but lice may be seen in Hispanic and Asian girls with long straight hair who attend school. Discoid lupus is more common in children with skin of color but is rare overall. When noted, accompanying mottled dyspigmentation and scarring alopecia are noted in addition to a high risk for developing systemic lupus erythematosus. Biopsy and screening for systemic lupus are necessary, as the risk for progression from discoid lupus to systemic disease is 26% over 3 years.18
THE BIOLOGY OF HAIR IN CHILDREN WITH SKIN OF COLOR
To some extent, the biology of hair impacts the occurrence, appearance, and treatment of scalp hyperkeratosis in children with skin of color. First, it is important to remember that follicular density is lower in black patients as compared to Asian patients with a consequently lower hair count overall, which results in the easy appearance of hair loss, particularly at the margins of the scalp.19,20 Second, the shape of the hair follicle differs among races and ethnicities. Asian patients have round hair shafts coming from straight follicles, which allows for greater natural hair hydration, resulting in somewhat less aggressive scalp disease. Hispanic patients may have similarly straight hair or may have elliptical or curled shafts, the latter being noted in black patients. Furthermore, a curled hair shaft results in poor flow of sebum across the hair, resulting in greater scalp xerosis, more susceptibility to traction alopecia, and ultimately a greater risk for infections.20-23 Finally, the scalp is continuous with the face and neck, and Asian patients have greater sensitivity to skin care products in these areas, resulting in difficulty of treatment in this patient population and the need for use of gentle products.
HAIR CARE PRACTICES IN CHILDREN WITH SKIN OF COLOR
Hair care in patients with skin of color can be costly, difficult, and potentially damaging, with 99% of black girls reporting pomade or oil usage. Costly and complex hair care practices begin in childhood for patients with skin of color. In a series of 201 surveyed black girls with a mean age of 9.8 years, 80% had used hot combs and 42% used relaxers.24 Traction styles were common with 81% using ponytails, 67% braids, and 49% cornrows in the last 12 months. These styles are thought to affect hair health, particularly through induction of traction-related damage, folliculitis, and alopecia. Furthermore, chemical relaxers, hot combs, blowouts, and hair setting may be introduced during childhood.24 These practices appear to disturb the integrity of the hair follicle, leaving it more susceptible to irritation and infection.
Hair care in the pediatric population often is complicated by the fact that multiple children are being styled in tandem, either at home or in a salon, resulting in shared equipment and fomite spread. Even just proximity to a case of tinea capitis in the household will increase risk for tinea capitis. Furthermore, it is quite commonplace for black patients to use pomades and shampoos that contain antifungals, especially selenium sulfide, which makes it difficult to obtain accurate culture results. In India, use of mustard oil also has been linked to increased risk for tinea capitis.25
Other issues related to hair care include frequent dry scalp in patients with skin of color due to poor sebum distribution along the hair shaft. As a result, frequent washing may exacerbate scalp xerosis and further irritate seborrheic dermatitis and/or AD.
DIAGNOSTIC CONSIDERATIONS FOR SCALP HYPERKERATOSIS IN CHILDHOOD
Dermatologists should have a greater level of suspicion for tinea capitis in black and Hispanic children compared to white children. The index of suspicion should be high given that antifungal shampoos and pomades may minimize the clinical appearance. Although trends in overall incidence in the United States suggest tinea capitis is becoming less common, there still is a stronger representation of the disease in black patients.26 A study of positive fungal cultures from one clinic in Mississippi (N=1220) showed that two-thirds of patients were children younger than 13 years; 87% of patients with positive cultures for dermatophytes were black.27 The endothrix type of tinea capitis caused by T tonsurans often presents with a seborrheic appearance, and fungal culture is warranted in all pediatric patients with skin of color who have scalp hyperkeratosis. Asian children can be regarded with a lower level of suspicion for tinea capitis, similar to white patients in the United States. Variation in incidence of tinea capitis does exist worldwide and the practitioner may need to address these issues in patients who travel or are recent immigrants.
When identifying tinea capitis infections in children with skin of color, physicians should consider the patient’s personal and family history, comorbid skin disorders, dermoscopy, microscopy and fungal staining, and fungal culture (Figure).
A paradigm for the diagnosis of scalp hyperkeratosis in children with skin of color.
Personal and Family History
The first diagnostic consideration is the patient’s personal and family history. A history of AD, asthma, or allergies will support but not confirm the diagnosis of AD. Prior tinea capitis infections and household contacts with tinea infections support the presence of tinea capitis.17 Recent implementation of anti–tumor necrosis factor a inhibitor therapy in a psoriatic child can flare scalp disease, mimicking tinea capitis.28 The patient’s guardians should be queried about potential infectious contacts, whether they themselves have signs of scalp disease or tinea corporis (ringworm) or whether they have a pet with problematic fur. Physicians also should query patients and their guardians about recent use of topical antifungal shampoos, pomades, creams (both over-the-counter [OTC] and prescription), and/or oral antifungals. When these agents are used, there is a possibility that fungal examinations may be negative in the presence of true infection with tinea capitis. Traction alopecia, often preceded by fine scale, is more likely to present in patients who wear their hair in cornrows, while seborrheic dermatitis may be associated with hair extensions, reduced frequency of washing (61% of black girls surveyed wash every 2 weeks), and/or reduced usage of hair oils in black girls.24 Knowledge of the patient’s personal hair care history, such as use of pomades; frequency and method of washing/drying hair; types of hair care products used daily to wash and style hair; use of chemical relaxers; or recent hairstyling with cornrows, braids, or hair extensions, also is essential to the diagnosis of tinea capitis. Usage of traction-related styling practices in patients with chemically relaxed hair can enhance the risk for traction alopecia.29
Comorbid Skin Disorders
The patient also should be examined for comorbid skin disorders, including tinea corporis, alopecia (particularly in the areas of hyperkeratosis), and the presence of nuchal lymphadenopathy. For each extra clinical finding, the chances of a final diagnosis of tinea capitis rises, allowing for empiric diagnosis to be made that can be confirmed by a variety of tests.1-3
Dermoscopy
Next, the patient should undergo dermoscopic evaluation. On dermoscopy, tinea capitis typically presents with broken hairs, black dots on the scalp, comma-shaped hairs, and short corkscrew hairs, all of which should clear with therapy.30-33 Dermoscopic findings of AD would reveal underlying xerosis and prominent vasculature due to inflammation, and alopecia areata would present with yellow dots at the orifices of the hair follicles, exclamation point hairs, and vellus hairs.34,35 Traction alopecia may be noted by retained hairs along the hairline, which is known as the fringe sign.36
Microscopy and Fungal Staining
Microscopic preparations can be performed to identify tinea capitis using fungal stains of slide-based specimens. Breakage of short hairs onto the slide and/or cotton swab is a soft sign corroborating endothrix infection of the hairs. Potassium hydroxide can enhance visualization of the hyperkeratotic scalp, but for most black patients, use of antifungal agents reduces fungal hyphae and spores in the areas of hyperkeratosis and may limit the utility of examining the skin microscopically. Assessment of the broken hairs obtained by gentle friction with one glass slide and catching the scales onto another glass slide may yield the best results in the evaluation of tinea capitis (a technique taught to me by Robin Hornung, MD, Everett, Washington). Hairs obtained in this manner often are fragile and break due to endothrix infection replacing and weakening the shaft of the hairs. In the United States, fungal samples usually are obtained with cotton swabs, but a recent study suggested that brushing is superior to scraping to obtain samples; the combination of sampling techniques may improve the yield of a culture.37 Because topical agents are unable to enter the hair cortex, the hair shaft is the most likely to show fungal spores under the microscope when antifungal shampoos or pomades are used. Other testing methods such as Swartz-Lamkins or calcofluor white staining can be used on similar scrapings. Biopsy and periodic acid–Schiff staining of thick scales or crust can help differentiate tinea capitis from pityriasis amiantacea when the crust is too thick to be softened via potassium hydroxide preparation.38
Fungal Culture
Fungal culture onto media that contains nutrients for dermatophyte growth can be used for 4 purposes in tinea capitis: (1) to confirm infection, (2) to identify species of infection, (3) to confirm mycological cure when difficulty in clearance of disease has been noted, and (4) to obtain a specimen for sensitivity screening regarding antifungals when necessary, an uncommon but occasionally useful test in individuals with disease that has failed treatment with 1 or more antifungals.27
THERAPY FOR SCALP HYPERKERATOSIS IN CHILDREN WITH SKIN OF COLOR
In patients with scalp hyperkeratosis, it is important to address the specific cause of the disease. Therapy for scalp hyperkeratosis in children with skin of color includes altered hair care practices, use of OTC and prescription agents, and containment of fomites in the case of infections. Biopsy of atypical scalp hyperkeratosis cases is needed to diagnose rare etiologies such as discoid lupus or Langerhans cell histiocytosis. For individuals with systemic disease including Langerhans cell histiocytosis, which is generally accompanied by nodes and plaques in the inguinal region or other intertriginous sites, immediate hematology and oncology workup is required.39 For collagen vascular diseases such as lupus or dermatomyositis, appropriate referral to rheumatology and systemic therapy is warranted.
Altered Hair Care Practices
The use of prophylactic ketoconazole 1% shampoo may not reduce the risk for recurrence of tinea capitis over standard good hygiene, removal of fomites, and adherence to prescribed therapy.40 Use of selenium sulfide has been shown to effectively reduce contagion risk.41
Fragrance- and dye-free shampoos can be helpful in providing gentle cleansing of the scalp, which is especially important in Asian patients who have greater facial and eyelid sensitivity. Free-and-clear shampoos can be used alternatively with shampoos containing selenium sulfide or sulfur to eliminate comorbid seborrhea. Black patients should be advised to shampoo and condition their hair once weekly, and Asian and Hispanic patients should shampoo and condition 2 to 3 times weekly to remove scale and potentially reduce risk for tinea acquisition.42 Children with straight hair should shampoo with increased frequency in the summer to manually remove sweat-induced macerated hyperkeratosis. Conditioners also should be used consistently after shampooing to enhance hair health.
Use of OTC and Prescription Agents
Atopic Dermatitis
Topical corticosteroid agents can be used in increasing strengths to treat AD of the scalp in children with skin of color, from OTC scalp products containing hydrocortisone 1% to prescription-based agents. Hydration of the hair also is needed to counteract reduced water content.43 Due to the innate xerosis of the scalp in black patients and atopic patients, the use of oil-based or lotion products may provide the most hydration for patients with scalp disease.44 Alcohol-based agents, either drops or foams, may enhance xerosis and should be used sparingly.
Seborrheic Dermatitis
Alternating treatment with medicated shampoos containing selenium sulfide and ketoconazole can aid in the removal of seborrhea. Pomades including borage seed oil–based agents can be massaged into the scalp,45 particularly for treatment of infantile seborrhea, and should not necessarily be washed off daily in dark-skinned patients. Additional focused application of topical corticosteroids to the scalp also is helpful. Due to innate scalp xerosis in black children, therapy should be similar to AD.
Psoriasis
In the setting of pityriasis amiantacea, albeit rare in children with skin of color, oil-based agents can soften hyperkeratosis for removal. Sterile mineral oil or commercially available scalp preparations of peanut oil with fluocinolone or mineral oil with glycerin can aid in the removal of scales without harming the hair, but usage must be age appropriate. The addition of focused application of age-appropriate topical corticosteroids for areas of severe hyperkeratosis can aid in clearance of the lesions.44 Recently, a stable combination of calcipo-triene 0.005%–betamethasone dipropionate 0.064% has been approved in the United States for the therapy of scalp psoriasis in adolescents.46
Tinea Capitis
Antifungal shampoos including selenium sulfide will reduce contagion risk when used by both the patient and his/her family members. Frequency of shampooing is similar to that described for AD. Between shampooing, pomades with selenium sulfide can be applied to the scalp to enhance overall clearance.
Oral antifungals are the basis of treatment and use of griseofulvin is the gold standard. Terbinafine has been approved by the US Food and Drug Administration for treatment of tinea capitis; for children weighing less than 25 kg the dosage is 125 mg daily, for 25 to 35 kg the dosage is 187.5 mg daily, and for more than 35 kg the dosage is 250 mg daily. Shorter therapeutic courses may be required, making it a good second-line agent. Laboratory screening in children prior to therapy is not always performed but should be done in cases where fatty liver might be suspected.47 Monitoring liver function tests is best when exceeding 3 months of usage or shifting from one antifungal to another.3
Containment of Fomites
There are several procedures that should be followed to contain scalp infection in children with skin of color. First, all objects that come into contact with the scalp (eg, hats, hoods, brushes, pillowcases) should be washed with hot water or replaced weekly. Sharing these objects with friends or family should be strongly discouraged. Patients and their family members also should be instructed to use medicated (eg, selenium sulfide) shampoos and conditioners. Finally, patients are advised to avoid use of shared classroom garments or mats for sleeping.
LONG-TERM SEQUELAE OF SCALP HYPERKERATOSIS
Long-term sequelae of scalp hyperkeratosis often are discounted in children, but the disease can have lasting and damaging effects on the scalp. Sequelae include discomfort from chronicity and psychological distress. In particular, years of scalp pruritus can promote lichenification of the scalp and miniaturization of the hair follicles. Furthermore, itching due to sweating can limit participation in sports. Finally, tinea capitis is thought to be a risk factor for central centrifugal cicatricial alopecia (or can occur comorbidly with central centrifugal cicatricial alopecia causing severe pruritus), a chronic scarring hair loss that is seen primarily in black adult females.48 Erythema nodosum also has been reported as an associated finding in the case of kerion.49 One study reported associated findings that included thyroid cancer in individuals irradiated for tinea capitis in the 1950s.50
Conclusion
Scalp hyperkeratosis in children with skin of color, especially black patients, is more likely to be associated with tinea capitis and is more challenging to treat due to innate scalp xerosis in black patients and increased sensitivity of facial skin in Asian children. Ultimately, institution of therapy when needed and good scalp and hair care may prevent long-term sequelae.
Scalp hyperkeratosis (scaling or flaking) is a common symptom in childhood and is typified by fine to thick hyperkeratosis of the scalp with or without underlying erythema. The causes of scalp hyperkeratosis in childhood vary based on the demographics of the population. In a population where approximately half of the pediatric patients were white, scaling of the scalp was more common in patients with seborrheic dermatitis and/or atopic dermatitis (AD) who were aged 0 to 2 years, and tinea capitis was only noted in children who were black.1 In children with skin of color, scalp hyperkeratosis has been noted as a marker of tinea capitis, especially in patients aged 3 to 11 years,2,3 and the level of suspicion should consistently remain high for this age group. In another study of an all-black population of schoolchildren aged 5 to 13 years (N=224), 3% demonstrated signs and symptoms of tinea capitis and 14% were found to be asymptomatic carriers.4 Although generally benign in nature, scalp hyperkeratosis can be associated with systemic illnesses such as juvenile dermatomyositis and Langerhans cell histiocytosis.5 This article addresses the diagnosis and treatment of scalp hyperkeratosis in children with skin of color, focusing on differences in exposure to contagious cases, hairstyling practices, and biological factors that may impact the disease process.
CAUSES OF SCALP HYPERKERATOSIS IN CHILDHOOD
Scalp hyperkeratosis in childhood usually is caused by common benign conditions, but some level of suspicion should be maintained for more severe etiologic conditions such as Langerhans cell histiocytosis and collagen vascular diseases (eg, juvenile dermatomyositis).6 Langerhans cell histiocytosis of the scalp might be obscured by background pigmentation in black children.
Scalp scaling can be a minor criterion in the diagnosis of AD. Atopic dermatitis should be suspected in Asian children with scalp scaling. Although one study in Bangladesh revealed scalp involvement in only 5.2% of pediatric patients with AD,7 a study in China reported an incidence rate as high as 49.7% (with a similarly high incidence of eyelid dermatitis).8 Children with AD also may have dry hair.9 Atopic dermatitis of the scalp is typified by itching, fine hyperkeratosis, and notably eczematous scalp lesions ranging from excoriated or oozing erythematous plaques to lichenification with hair miniaturization, primarily from scratch-induced breakage.10 The latter finding often is noted in black adolescent girls with long-term moderate to severe AD (personal observation).
Seborrheic dermatitis is a hypersensitivity response to yeast colonization of the scalp with Malassezia species. The infantile form is extremely common (also known as cradle cap). Characteristically, greasy yellow hyperkeratosis in fine to thick sheets is noted on the scalp in children younger than 2 years, especially infants, often with involvement of skin folds. One study noted that seborrheic dermatitis occurs in 6% of school-aged children as opposed to 19% of children younger than 2 years.1 Severe seborrheic dermatitis in infancy may be a prelude to AD, with the incidence being 3 times higher in children with prior seborrheic dermatitis.11 In teenagers, seborrheic dermatitis often accompanies acne onset in the early pubertal years.12
Psoriasis is an autoimmune inflammatory dermatosis that most commonly affects white children. In childhood, pityriasis amiantacea, psoriasiform scalp hyperkeratosis, is more common than in adulthood, with thick, stuck-on scales bound to the hairs. This variant is uncommon in Hispanic and Asian children and is almost never seen in black children but has been reported in cohorts of Turkish children.13 In a series of 85 Egyptian children with pityriasis amiantacea, diagnosis of scalp psoriasis was made in 35.3%, eczematous dermatitis in 34.2%, and tinea capitis in 12.9%.14 Consequently, a high degree of suspicion for tinea capitis should be held if pityriasis amiantacea is found in children with skin of color.15,16
Tinea capitis is a dermatophyte infection of the scalp, hair, and surrounding skin. The presence of tinea capitis on the scalp is associated with environmental exposure to dermatophytes (eg, school, household).4,17 The infection is largely caused by Trichophyton tonsurans in the United States, which causes a seborrheic appearance and less commonly alopecia (black dot or thinning), plaques with scale, or kerion. The presence of cervical lymph nodes and/or alopecia increases the chances of tinea being the diagnosis. Potassium hydroxide preparation and fungal culture can be performed to corroborate the diagnosis.1-3 Other etiologies of scalp hyperkeratosis such as juvenile pityriasis rubra pilaris and lice are extremely uncommon in black children, but lice may be seen in Hispanic and Asian girls with long straight hair who attend school. Discoid lupus is more common in children with skin of color but is rare overall. When noted, accompanying mottled dyspigmentation and scarring alopecia are noted in addition to a high risk for developing systemic lupus erythematosus. Biopsy and screening for systemic lupus are necessary, as the risk for progression from discoid lupus to systemic disease is 26% over 3 years.18
THE BIOLOGY OF HAIR IN CHILDREN WITH SKIN OF COLOR
To some extent, the biology of hair impacts the occurrence, appearance, and treatment of scalp hyperkeratosis in children with skin of color. First, it is important to remember that follicular density is lower in black patients as compared to Asian patients with a consequently lower hair count overall, which results in the easy appearance of hair loss, particularly at the margins of the scalp.19,20 Second, the shape of the hair follicle differs among races and ethnicities. Asian patients have round hair shafts coming from straight follicles, which allows for greater natural hair hydration, resulting in somewhat less aggressive scalp disease. Hispanic patients may have similarly straight hair or may have elliptical or curled shafts, the latter being noted in black patients. Furthermore, a curled hair shaft results in poor flow of sebum across the hair, resulting in greater scalp xerosis, more susceptibility to traction alopecia, and ultimately a greater risk for infections.20-23 Finally, the scalp is continuous with the face and neck, and Asian patients have greater sensitivity to skin care products in these areas, resulting in difficulty of treatment in this patient population and the need for use of gentle products.
HAIR CARE PRACTICES IN CHILDREN WITH SKIN OF COLOR
Hair care in patients with skin of color can be costly, difficult, and potentially damaging, with 99% of black girls reporting pomade or oil usage. Costly and complex hair care practices begin in childhood for patients with skin of color. In a series of 201 surveyed black girls with a mean age of 9.8 years, 80% had used hot combs and 42% used relaxers.24 Traction styles were common with 81% using ponytails, 67% braids, and 49% cornrows in the last 12 months. These styles are thought to affect hair health, particularly through induction of traction-related damage, folliculitis, and alopecia. Furthermore, chemical relaxers, hot combs, blowouts, and hair setting may be introduced during childhood.24 These practices appear to disturb the integrity of the hair follicle, leaving it more susceptible to irritation and infection.
Hair care in the pediatric population often is complicated by the fact that multiple children are being styled in tandem, either at home or in a salon, resulting in shared equipment and fomite spread. Even just proximity to a case of tinea capitis in the household will increase risk for tinea capitis. Furthermore, it is quite commonplace for black patients to use pomades and shampoos that contain antifungals, especially selenium sulfide, which makes it difficult to obtain accurate culture results. In India, use of mustard oil also has been linked to increased risk for tinea capitis.25
Other issues related to hair care include frequent dry scalp in patients with skin of color due to poor sebum distribution along the hair shaft. As a result, frequent washing may exacerbate scalp xerosis and further irritate seborrheic dermatitis and/or AD.
DIAGNOSTIC CONSIDERATIONS FOR SCALP HYPERKERATOSIS IN CHILDHOOD
Dermatologists should have a greater level of suspicion for tinea capitis in black and Hispanic children compared to white children. The index of suspicion should be high given that antifungal shampoos and pomades may minimize the clinical appearance. Although trends in overall incidence in the United States suggest tinea capitis is becoming less common, there still is a stronger representation of the disease in black patients.26 A study of positive fungal cultures from one clinic in Mississippi (N=1220) showed that two-thirds of patients were children younger than 13 years; 87% of patients with positive cultures for dermatophytes were black.27 The endothrix type of tinea capitis caused by T tonsurans often presents with a seborrheic appearance, and fungal culture is warranted in all pediatric patients with skin of color who have scalp hyperkeratosis. Asian children can be regarded with a lower level of suspicion for tinea capitis, similar to white patients in the United States. Variation in incidence of tinea capitis does exist worldwide and the practitioner may need to address these issues in patients who travel or are recent immigrants.
When identifying tinea capitis infections in children with skin of color, physicians should consider the patient’s personal and family history, comorbid skin disorders, dermoscopy, microscopy and fungal staining, and fungal culture (Figure).
A paradigm for the diagnosis of scalp hyperkeratosis in children with skin of color.
Personal and Family History
The first diagnostic consideration is the patient’s personal and family history. A history of AD, asthma, or allergies will support but not confirm the diagnosis of AD. Prior tinea capitis infections and household contacts with tinea infections support the presence of tinea capitis.17 Recent implementation of anti–tumor necrosis factor a inhibitor therapy in a psoriatic child can flare scalp disease, mimicking tinea capitis.28 The patient’s guardians should be queried about potential infectious contacts, whether they themselves have signs of scalp disease or tinea corporis (ringworm) or whether they have a pet with problematic fur. Physicians also should query patients and their guardians about recent use of topical antifungal shampoos, pomades, creams (both over-the-counter [OTC] and prescription), and/or oral antifungals. When these agents are used, there is a possibility that fungal examinations may be negative in the presence of true infection with tinea capitis. Traction alopecia, often preceded by fine scale, is more likely to present in patients who wear their hair in cornrows, while seborrheic dermatitis may be associated with hair extensions, reduced frequency of washing (61% of black girls surveyed wash every 2 weeks), and/or reduced usage of hair oils in black girls.24 Knowledge of the patient’s personal hair care history, such as use of pomades; frequency and method of washing/drying hair; types of hair care products used daily to wash and style hair; use of chemical relaxers; or recent hairstyling with cornrows, braids, or hair extensions, also is essential to the diagnosis of tinea capitis. Usage of traction-related styling practices in patients with chemically relaxed hair can enhance the risk for traction alopecia.29
Comorbid Skin Disorders
The patient also should be examined for comorbid skin disorders, including tinea corporis, alopecia (particularly in the areas of hyperkeratosis), and the presence of nuchal lymphadenopathy. For each extra clinical finding, the chances of a final diagnosis of tinea capitis rises, allowing for empiric diagnosis to be made that can be confirmed by a variety of tests.1-3
Dermoscopy
Next, the patient should undergo dermoscopic evaluation. On dermoscopy, tinea capitis typically presents with broken hairs, black dots on the scalp, comma-shaped hairs, and short corkscrew hairs, all of which should clear with therapy.30-33 Dermoscopic findings of AD would reveal underlying xerosis and prominent vasculature due to inflammation, and alopecia areata would present with yellow dots at the orifices of the hair follicles, exclamation point hairs, and vellus hairs.34,35 Traction alopecia may be noted by retained hairs along the hairline, which is known as the fringe sign.36
Microscopy and Fungal Staining
Microscopic preparations can be performed to identify tinea capitis using fungal stains of slide-based specimens. Breakage of short hairs onto the slide and/or cotton swab is a soft sign corroborating endothrix infection of the hairs. Potassium hydroxide can enhance visualization of the hyperkeratotic scalp, but for most black patients, use of antifungal agents reduces fungal hyphae and spores in the areas of hyperkeratosis and may limit the utility of examining the skin microscopically. Assessment of the broken hairs obtained by gentle friction with one glass slide and catching the scales onto another glass slide may yield the best results in the evaluation of tinea capitis (a technique taught to me by Robin Hornung, MD, Everett, Washington). Hairs obtained in this manner often are fragile and break due to endothrix infection replacing and weakening the shaft of the hairs. In the United States, fungal samples usually are obtained with cotton swabs, but a recent study suggested that brushing is superior to scraping to obtain samples; the combination of sampling techniques may improve the yield of a culture.37 Because topical agents are unable to enter the hair cortex, the hair shaft is the most likely to show fungal spores under the microscope when antifungal shampoos or pomades are used. Other testing methods such as Swartz-Lamkins or calcofluor white staining can be used on similar scrapings. Biopsy and periodic acid–Schiff staining of thick scales or crust can help differentiate tinea capitis from pityriasis amiantacea when the crust is too thick to be softened via potassium hydroxide preparation.38
Fungal Culture
Fungal culture onto media that contains nutrients for dermatophyte growth can be used for 4 purposes in tinea capitis: (1) to confirm infection, (2) to identify species of infection, (3) to confirm mycological cure when difficulty in clearance of disease has been noted, and (4) to obtain a specimen for sensitivity screening regarding antifungals when necessary, an uncommon but occasionally useful test in individuals with disease that has failed treatment with 1 or more antifungals.27
THERAPY FOR SCALP HYPERKERATOSIS IN CHILDREN WITH SKIN OF COLOR
In patients with scalp hyperkeratosis, it is important to address the specific cause of the disease. Therapy for scalp hyperkeratosis in children with skin of color includes altered hair care practices, use of OTC and prescription agents, and containment of fomites in the case of infections. Biopsy of atypical scalp hyperkeratosis cases is needed to diagnose rare etiologies such as discoid lupus or Langerhans cell histiocytosis. For individuals with systemic disease including Langerhans cell histiocytosis, which is generally accompanied by nodes and plaques in the inguinal region or other intertriginous sites, immediate hematology and oncology workup is required.39 For collagen vascular diseases such as lupus or dermatomyositis, appropriate referral to rheumatology and systemic therapy is warranted.
Altered Hair Care Practices
The use of prophylactic ketoconazole 1% shampoo may not reduce the risk for recurrence of tinea capitis over standard good hygiene, removal of fomites, and adherence to prescribed therapy.40 Use of selenium sulfide has been shown to effectively reduce contagion risk.41
Fragrance- and dye-free shampoos can be helpful in providing gentle cleansing of the scalp, which is especially important in Asian patients who have greater facial and eyelid sensitivity. Free-and-clear shampoos can be used alternatively with shampoos containing selenium sulfide or sulfur to eliminate comorbid seborrhea. Black patients should be advised to shampoo and condition their hair once weekly, and Asian and Hispanic patients should shampoo and condition 2 to 3 times weekly to remove scale and potentially reduce risk for tinea acquisition.42 Children with straight hair should shampoo with increased frequency in the summer to manually remove sweat-induced macerated hyperkeratosis. Conditioners also should be used consistently after shampooing to enhance hair health.
Use of OTC and Prescription Agents
Atopic Dermatitis
Topical corticosteroid agents can be used in increasing strengths to treat AD of the scalp in children with skin of color, from OTC scalp products containing hydrocortisone 1% to prescription-based agents. Hydration of the hair also is needed to counteract reduced water content.43 Due to the innate xerosis of the scalp in black patients and atopic patients, the use of oil-based or lotion products may provide the most hydration for patients with scalp disease.44 Alcohol-based agents, either drops or foams, may enhance xerosis and should be used sparingly.
Seborrheic Dermatitis
Alternating treatment with medicated shampoos containing selenium sulfide and ketoconazole can aid in the removal of seborrhea. Pomades including borage seed oil–based agents can be massaged into the scalp,45 particularly for treatment of infantile seborrhea, and should not necessarily be washed off daily in dark-skinned patients. Additional focused application of topical corticosteroids to the scalp also is helpful. Due to innate scalp xerosis in black children, therapy should be similar to AD.
Psoriasis
In the setting of pityriasis amiantacea, albeit rare in children with skin of color, oil-based agents can soften hyperkeratosis for removal. Sterile mineral oil or commercially available scalp preparations of peanut oil with fluocinolone or mineral oil with glycerin can aid in the removal of scales without harming the hair, but usage must be age appropriate. The addition of focused application of age-appropriate topical corticosteroids for areas of severe hyperkeratosis can aid in clearance of the lesions.44 Recently, a stable combination of calcipo-triene 0.005%–betamethasone dipropionate 0.064% has been approved in the United States for the therapy of scalp psoriasis in adolescents.46
Tinea Capitis
Antifungal shampoos including selenium sulfide will reduce contagion risk when used by both the patient and his/her family members. Frequency of shampooing is similar to that described for AD. Between shampooing, pomades with selenium sulfide can be applied to the scalp to enhance overall clearance.
Oral antifungals are the basis of treatment and use of griseofulvin is the gold standard. Terbinafine has been approved by the US Food and Drug Administration for treatment of tinea capitis; for children weighing less than 25 kg the dosage is 125 mg daily, for 25 to 35 kg the dosage is 187.5 mg daily, and for more than 35 kg the dosage is 250 mg daily. Shorter therapeutic courses may be required, making it a good second-line agent. Laboratory screening in children prior to therapy is not always performed but should be done in cases where fatty liver might be suspected.47 Monitoring liver function tests is best when exceeding 3 months of usage or shifting from one antifungal to another.3
Containment of Fomites
There are several procedures that should be followed to contain scalp infection in children with skin of color. First, all objects that come into contact with the scalp (eg, hats, hoods, brushes, pillowcases) should be washed with hot water or replaced weekly. Sharing these objects with friends or family should be strongly discouraged. Patients and their family members also should be instructed to use medicated (eg, selenium sulfide) shampoos and conditioners. Finally, patients are advised to avoid use of shared classroom garments or mats for sleeping.
LONG-TERM SEQUELAE OF SCALP HYPERKERATOSIS
Long-term sequelae of scalp hyperkeratosis often are discounted in children, but the disease can have lasting and damaging effects on the scalp. Sequelae include discomfort from chronicity and psychological distress. In particular, years of scalp pruritus can promote lichenification of the scalp and miniaturization of the hair follicles. Furthermore, itching due to sweating can limit participation in sports. Finally, tinea capitis is thought to be a risk factor for central centrifugal cicatricial alopecia (or can occur comorbidly with central centrifugal cicatricial alopecia causing severe pruritus), a chronic scarring hair loss that is seen primarily in black adult females.48 Erythema nodosum also has been reported as an associated finding in the case of kerion.49 One study reported associated findings that included thyroid cancer in individuals irradiated for tinea capitis in the 1950s.50
Conclusion
Scalp hyperkeratosis in children with skin of color, especially black patients, is more likely to be associated with tinea capitis and is more challenging to treat due to innate scalp xerosis in black patients and increased sensitivity of facial skin in Asian children. Ultimately, institution of therapy when needed and good scalp and hair care may prevent long-term sequelae.
1. Williams JV, Eichenfield LF, Burke BL, et al. Prevalence of scalp scaling in prepubertal children. Pediatrics. 2005;115:e1-e6.
2. Coley MK, Bhanusali DG, Silverberg JI, et al. Scalp hyperkeratosis and alopecia in children of color. J Drugs Dermatol. 2011;10:511-516.
3. Bhanusali D, Coley M, Silverberg JI, et al. Treatment outcomes for tinea capitis in a skin of color population. J Drugs Dermatol. 2012;11:852-856.
4. Williams JV, Honig PJ, McGinley KJ, et al. Semiquantitative study of tinea capitis and the asymptomatic carrier state in inner-city school children. Pediatrics. 1995;96:265-267.
5. McDonald LL, Smith ML. Diagnostic dilemmas in pediatric/adolescent dermatology: scaly scalp. J Pediatr Health Care. 1998;12:80-84.
6. Peloro TM, Miller OF 3rd, Hahn TF, et al. Juvenile dermatomyositis: a retrospective review of a 30-year experience. J Am Acad Dermatol. 2001;45:28-34.
7. Wahab MA, Rahman MH, Khondker L, et al. Minor criteria for atopic dermatitis in children. Mymensingh Med J. 2011;20:419-424.
8. Shi M, Zhang H, Chen X, et al. Clinical features of atopic dermatitis in a hospital-based setting in China. J Eur Acad Dermatol Venereol [published online ahead of print January 9, 2011]. 2011;25:1206-1212.
9. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair. Microsc Res Tech. 2012;75:620-625.
10. Sabin BR, Peters N, Peters AT. Chapter 20: atopic dermatitis. Allergy Asthma Proc. 2012;33:S67-S69.
11. Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis [published online ahead of print November 13, 2013]. Pediatr Dermatol. 2014;31:125-130.
12. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
13. Sarifakioglu E, Yilmaz AE, Gorpelioglu C, et al. Prevalence of scalp disorders and hair loss in children. Cutis. 2012;90:225-229.
14. Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
15. Oostveen AM, Jong EM, Evers AW, et al. Reliability, responsiveness and validity of Scalpdex in children with scalp psoriasis: the Dutch study. Acta Derm Venereol. 2014;94:198-202.
16. Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity: Comparative Dermatologic Atlas of Pediatric Skin of All Colors. New York, NY: Springer; 2012.
17. Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
18. Moises-Alfaro C, Berrón-Pérez R, Carrasco-Daza D, et al. Discoid lupus erythematosus in children: clinical, histopathologic, and follow-up features in 27 cases. Pediatr Dermatol. 2003;20:103-107.
19. Ramos-e-Silva M. Ethnic hair and skin: what is the state of the science? Chicago, Illinois—September 29-30, 2001. Clin Dermatol. 2002;20:321-324.
20. Heath CR, McMichael AJ. Biology of hair follicle. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. New York, NY: McGraw Hill; 2009:105-109.
21. Khumalo NP. African hair morphology: macrostructure to ultrastructure. Int J Dermatol. 2005;44(suppl 1):10-12.
22. Thibaut S, Bernard BA. The biology of hair shape. Int J Dermatol. 2005;44(suppl 1):2-3.
23. Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
24. Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
25. Kumar V, Sharma RC, Chander R. Clinicomycological study of tinea capitis. Indian J Dermatol Venereol Leprol. 1996;62:207-209.
26. Mirmirani P, Tucker LY. Epidemiologic trends in pediatric tinea capitis: a population-based study from Kaiser Permanente Northern California [published online ahead of print October 2, 2013]. J Am Acad Dermatol. 2013;69:916-921.
27. Chapman JC, Daniel CR 3rd, Daniel JG, et al. Tinea capitis caused by dermatophytes: a 15-year retrospective study from a Mississippi Dermatology Clinic. Cutis. 2011;88:230-233.
28. Perman MJ, Lovell DJ, Denson LA, et al. Five cases of anti-tumor necrosis factor alpha-induced psoriasis presenting with severe scalp involvement in children. Pediatr Dermatol. 2012;29:454-459.
29. Khumalo NP, Jessop S, Gumedze F, et al. Determinants of marginal traction alopecia in African girls and women. J Am Acad Dermatol. 2008;59:432-438.
30. Vazquez-Lopez F, Palacios-Garcia L, Argenziano G. Dermoscopic corkscrew hairs dissolve after successful therapy of Trichophyton violaceum tinea capitis: a case report. Australas J Dermatol. 2012;53:118-119.
31. Pinheiro AM, Lobato LA, Varella TC. Dermoscopy findings in tinea capitis: case report and literature review. An Bras Dermatol. 2012;87:313-314.
32. Mapelli ET, Gualandri L, Cerri A, et al. Comma hairs in tinea capitis: a useful dermatoscopic sign for diagnosis of tinea capitis. Pediatr Dermatol. 2012;29:223-224.
33. Hughes R, Chiaverini C, Bahadoran P, et al. Corkscrew hair: a new dermoscopic sign for diagnosis of tinea capitis in black children. Arch Dermatol. 2011;147:355-356.
34. Ekiz O, Sen BB, Rifaiog˘lu EN, et al. Trichoscopy in paediatric patients with tinea capitis: a useful method to differentiate from alopecia areata [published online ahead of print August 24, 2013]. J Eur Acad Dermatol Venereol. 2014;28:1255-1258.
35. Lencastre A, Tosti A. Role of trichoscopy in children’s scalp and hair disorders [published online ahead of print Aug 13, 2013]. Pediatr Dermatol. 2013;30:674-682.
36. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
37. Nasir S, Ralph N, O’Neill C, et al. Trends in tinea capitis in an Irish pediatric population and a comparison of scalp brushings versus scalp scrapings as methods of investigation [published online ahead of print February 22, 2013]. Pediatr Dermatol. 2014;31:622-623.
38. Alvarez MS, Silverberg NB. Tinea capitis. Cutis. 2006;78:189-196.
39. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996.
40. Bookstaver PB, Watson HJ, Winters SD, et al. Prophylactic ketoconazole shampoo for tinea capitis in a high-risk pediatric population. J Pediatr Pharmacol Ther. 2011;16:199-203.
41. Allen HB, Honig PJ, Leyden JJ, et al. Selenium sulfide: adjunctive therapy for tinea capitis. Pediatrics. 1982;69:81-83.
42. Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
43. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair [published online ahead of print November 7, 2011]. Microsc Res Tech. 2012;75:620-625.
44. Kapila S, Hong E, Fischer G. A comparative study of childhood psoriasis and atopic dermatitis and greater understanding of the overlapping condition, psoriasis-dermatitis. Australas J Dermatol. 2012;53:98-105.
45. Tollesson A, Frithz A. Borage oil, an effective new treatment for infantile seborrhoeic dermatitis. Br J Dermatol. 1993;129:95.
46. Gooderham M, Debarre JM, Keddy-Grant J, et al. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12-17 years of age [published online ahead of print October 22, 2014]. Br J Dermatol. 2014;171:1470-1477.
47. Singer C, Stancu P, Coşoveanu S, et al. Non-alcoholic fatty liver disease in children. Curr Health Sci J. 2014;40:170-176.
48. Chiang C, Price V, Mirmirani P. Central centrifugal cicatricial alopecia: superimposed tinea capitis as the etiology of chronic scalp pruritus. Dermatol Online J. 2008;14:3.
49. Morrone A, Calcaterra R, Valenzano M, et al. Erythema nodosum induced by kerion celsi of the scalp in a woman. Mycoses. 2011;54:e237-e239.
50. Boaventura P, Pereira D, Celestino R, et al. Genetic alterations in thyroid tumors from patients irradiated in childhood for tinea capitis treatment. Eur J Endocrinol. 2013;169:673-679.
1. Williams JV, Eichenfield LF, Burke BL, et al. Prevalence of scalp scaling in prepubertal children. Pediatrics. 2005;115:e1-e6.
2. Coley MK, Bhanusali DG, Silverberg JI, et al. Scalp hyperkeratosis and alopecia in children of color. J Drugs Dermatol. 2011;10:511-516.
3. Bhanusali D, Coley M, Silverberg JI, et al. Treatment outcomes for tinea capitis in a skin of color population. J Drugs Dermatol. 2012;11:852-856.
4. Williams JV, Honig PJ, McGinley KJ, et al. Semiquantitative study of tinea capitis and the asymptomatic carrier state in inner-city school children. Pediatrics. 1995;96:265-267.
5. McDonald LL, Smith ML. Diagnostic dilemmas in pediatric/adolescent dermatology: scaly scalp. J Pediatr Health Care. 1998;12:80-84.
6. Peloro TM, Miller OF 3rd, Hahn TF, et al. Juvenile dermatomyositis: a retrospective review of a 30-year experience. J Am Acad Dermatol. 2001;45:28-34.
7. Wahab MA, Rahman MH, Khondker L, et al. Minor criteria for atopic dermatitis in children. Mymensingh Med J. 2011;20:419-424.
8. Shi M, Zhang H, Chen X, et al. Clinical features of atopic dermatitis in a hospital-based setting in China. J Eur Acad Dermatol Venereol [published online ahead of print January 9, 2011]. 2011;25:1206-1212.
9. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair. Microsc Res Tech. 2012;75:620-625.
10. Sabin BR, Peters N, Peters AT. Chapter 20: atopic dermatitis. Allergy Asthma Proc. 2012;33:S67-S69.
11. Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis [published online ahead of print November 13, 2013]. Pediatr Dermatol. 2014;31:125-130.
12. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
13. Sarifakioglu E, Yilmaz AE, Gorpelioglu C, et al. Prevalence of scalp disorders and hair loss in children. Cutis. 2012;90:225-229.
14. Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
15. Oostveen AM, Jong EM, Evers AW, et al. Reliability, responsiveness and validity of Scalpdex in children with scalp psoriasis: the Dutch study. Acta Derm Venereol. 2014;94:198-202.
16. Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity: Comparative Dermatologic Atlas of Pediatric Skin of All Colors. New York, NY: Springer; 2012.
17. Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
18. Moises-Alfaro C, Berrón-Pérez R, Carrasco-Daza D, et al. Discoid lupus erythematosus in children: clinical, histopathologic, and follow-up features in 27 cases. Pediatr Dermatol. 2003;20:103-107.
19. Ramos-e-Silva M. Ethnic hair and skin: what is the state of the science? Chicago, Illinois—September 29-30, 2001. Clin Dermatol. 2002;20:321-324.
20. Heath CR, McMichael AJ. Biology of hair follicle. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. New York, NY: McGraw Hill; 2009:105-109.
21. Khumalo NP. African hair morphology: macrostructure to ultrastructure. Int J Dermatol. 2005;44(suppl 1):10-12.
22. Thibaut S, Bernard BA. The biology of hair shape. Int J Dermatol. 2005;44(suppl 1):2-3.
23. Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
24. Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
25. Kumar V, Sharma RC, Chander R. Clinicomycological study of tinea capitis. Indian J Dermatol Venereol Leprol. 1996;62:207-209.
26. Mirmirani P, Tucker LY. Epidemiologic trends in pediatric tinea capitis: a population-based study from Kaiser Permanente Northern California [published online ahead of print October 2, 2013]. J Am Acad Dermatol. 2013;69:916-921.
27. Chapman JC, Daniel CR 3rd, Daniel JG, et al. Tinea capitis caused by dermatophytes: a 15-year retrospective study from a Mississippi Dermatology Clinic. Cutis. 2011;88:230-233.
28. Perman MJ, Lovell DJ, Denson LA, et al. Five cases of anti-tumor necrosis factor alpha-induced psoriasis presenting with severe scalp involvement in children. Pediatr Dermatol. 2012;29:454-459.
29. Khumalo NP, Jessop S, Gumedze F, et al. Determinants of marginal traction alopecia in African girls and women. J Am Acad Dermatol. 2008;59:432-438.
30. Vazquez-Lopez F, Palacios-Garcia L, Argenziano G. Dermoscopic corkscrew hairs dissolve after successful therapy of Trichophyton violaceum tinea capitis: a case report. Australas J Dermatol. 2012;53:118-119.
31. Pinheiro AM, Lobato LA, Varella TC. Dermoscopy findings in tinea capitis: case report and literature review. An Bras Dermatol. 2012;87:313-314.
32. Mapelli ET, Gualandri L, Cerri A, et al. Comma hairs in tinea capitis: a useful dermatoscopic sign for diagnosis of tinea capitis. Pediatr Dermatol. 2012;29:223-224.
33. Hughes R, Chiaverini C, Bahadoran P, et al. Corkscrew hair: a new dermoscopic sign for diagnosis of tinea capitis in black children. Arch Dermatol. 2011;147:355-356.
34. Ekiz O, Sen BB, Rifaiog˘lu EN, et al. Trichoscopy in paediatric patients with tinea capitis: a useful method to differentiate from alopecia areata [published online ahead of print August 24, 2013]. J Eur Acad Dermatol Venereol. 2014;28:1255-1258.
35. Lencastre A, Tosti A. Role of trichoscopy in children’s scalp and hair disorders [published online ahead of print Aug 13, 2013]. Pediatr Dermatol. 2013;30:674-682.
36. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
37. Nasir S, Ralph N, O’Neill C, et al. Trends in tinea capitis in an Irish pediatric population and a comparison of scalp brushings versus scalp scrapings as methods of investigation [published online ahead of print February 22, 2013]. Pediatr Dermatol. 2014;31:622-623.
38. Alvarez MS, Silverberg NB. Tinea capitis. Cutis. 2006;78:189-196.
39. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996.
40. Bookstaver PB, Watson HJ, Winters SD, et al. Prophylactic ketoconazole shampoo for tinea capitis in a high-risk pediatric population. J Pediatr Pharmacol Ther. 2011;16:199-203.
41. Allen HB, Honig PJ, Leyden JJ, et al. Selenium sulfide: adjunctive therapy for tinea capitis. Pediatrics. 1982;69:81-83.
42. Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
43. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair [published online ahead of print November 7, 2011]. Microsc Res Tech. 2012;75:620-625.
44. Kapila S, Hong E, Fischer G. A comparative study of childhood psoriasis and atopic dermatitis and greater understanding of the overlapping condition, psoriasis-dermatitis. Australas J Dermatol. 2012;53:98-105.
45. Tollesson A, Frithz A. Borage oil, an effective new treatment for infantile seborrhoeic dermatitis. Br J Dermatol. 1993;129:95.
46. Gooderham M, Debarre JM, Keddy-Grant J, et al. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12-17 years of age [published online ahead of print October 22, 2014]. Br J Dermatol. 2014;171:1470-1477.
47. Singer C, Stancu P, Coşoveanu S, et al. Non-alcoholic fatty liver disease in children. Curr Health Sci J. 2014;40:170-176.
48. Chiang C, Price V, Mirmirani P. Central centrifugal cicatricial alopecia: superimposed tinea capitis as the etiology of chronic scalp pruritus. Dermatol Online J. 2008;14:3.
49. Morrone A, Calcaterra R, Valenzano M, et al. Erythema nodosum induced by kerion celsi of the scalp in a woman. Mycoses. 2011;54:e237-e239.
50. Boaventura P, Pereira D, Celestino R, et al. Genetic alterations in thyroid tumors from patients irradiated in childhood for tinea capitis treatment. Eur J Endocrinol. 2013;169:673-679.
Practice Points
- Scalp hyperkeratosis is a common finding in children, especially those with skin of color.
- Fungal culture may be helpful in the diagnosis of scalp hyperkeratosis in children of any age but should be performed in patients aged 3 to 11 years with skin of color.
- Therapy of scalp disease in children with skin of color should be adjusted based on hair type and disease features.
Update on Pediatric Psoriasis
Psoriasis affects 2% to 4% of the US population, with approximately one-third of cases beginning in childhood. The understanding of pediatric psoriasis has developed at a far slower pace than adult disease, with limitations in care including few medications that are approved by the US Food and Drug Administration for pediatric and adolescent use. Recently, a stable fixed-combination dose of calcipo-triene 0.005%–betamethasone dipropionate 0.064% topical suspension was approved for treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years, which hopefully will lead a trend in psoriasis medication approval for children and teenagers.1 Based on a PubMed search of articles indexed for MEDLINE using the search terms pediatric psoriasis, psoriasis, and strep that were published from April 2012 to April 2014, this article reviews newer data to address the issues that surround pediatric psoriasis and to provide an update on prior review articles on pediatric psoriasis.2-5 This article reviews some of the newer literature on clinical presentation and comorbidities in pediatric psoriasis.5 Based on these recent findings, additional screenings including review of obesity parameters are recommended for pediatric patients with psoriasis (Table 1).
Update on Disease Manifestations, Associations, and Comorbidities
Disease Manifestations
A 2013 multicenter study delineated the clinical features of pediatric psoriasis.6 The study was conducted at 8 geographically diverse dermatology clinics in the United States to delineate the clinical manifestations of pediatric psoriasis. In an assessment of 181 participants aged 5 to 17 years, the investigators sought to determine the frequency of disease sites, severity, and guttate disease. Over a period of approximately 2 years, 43.1% of participants were determined to have mild disease and 56.9% had severe disease. Family history of psoriasis was present in 51.4% of participants, with first-degree relatives affected in 59.8% of cases. Scalp involvement at some time was noted in 79.0% of participants, and nail disease was noted in 55% of boys and 29% of girls. Guttate psoriasis was noted in 30% of participants, with more cases in the severe range (35.9%) versus the mild range (21.8%). Additionally, 22.1% of participants had a precipitating streptococcal infection, with the association being more common in pediatric patients with guttate psoriasis than plaque psoriasis.6 This study highlighted that pediatric psoriasis has a genetic basis, is frequently guttate in nature, commonly affects the nails, shows a trend toward being classified as severe, and may be triggered by streptococcal infections.
Streptococcal Infection
Pediatric psoriasis may be triggered or flared by Streptococcus pyogenes (group A β-hemolytic streptococci) infections, specifically β-hemolytic streptococci groups A, C, and G that have streptococcal M protein,2,3,7 and this tendency can be associated with HLA-Cw6 or guttate psoriasis. Newer data have elucidated the role of streptococcal throat infections in psoriasis. Given that streptococcal throat infections are most common in school-aged children, these studies suggest a putative mechanism in pediatric psoriasis for triggering streptococcal infections, which would need to be confirmed in future studies, specifically in pediatric psoriasis patients.
It has been shown that T cells in psoriasis patients recognize common streptococcal M proteins and keratin determinants.7 Ferran et al8 recently demonstrated activation of circulating cutaneous lymphocyte–associated antigen (CLA)+ T cells but not CLA- memory T cells in 27 psoriasis patients (ages not specified) when mixed with streptococcal throat extracts, causing production of IL-17, IP-10, IL-22, and IFN-γ; activation was not found in 6 healthy control patients. Antistreptolysin O levels were correlated with the messenger RNA up- regulation for IL-17, IP-10, IL-22, and IFN-γ, and also correlated with psoriasis area and severity index score in psoriasis patients. In this same study, injection of the activated culture supernatant into mouse skin caused epidermal hyperkeratosis and activation of nonlesional epidermal cells from psoriatic patients. This study thereby delineated some of the potential pathways of the streptococcal induction of psoriasis and psoriatic flares in childhood8; however, confirmation is needed through further study of pediatric psoriatic lymphocyte activity.
Differential Diagnosis
Additions to the extensive differential list have been cited in the recent literature. The differential diagnosis of pediatric psoriasis now includes sodium valproate–induced psoriasiform drug eruption9 and allergic contact dermatitis to methylchloroisothiazolinone and methylisothiazolinone, which are present in many sanitizing hand and diaper wipes and has been reported to cause psoriasiform dermatitis in a periorificial or perineal distribution.10 Clinicians should inquire about the use of these wipes, as caregivers rarely suspect this agent to be causative of the eruption.
Psoriatic Arthritis
Previously, psoriasis and psoriatic arthritis have been linked to autoimmune thyroid disease in adults.11 A study of the Childhood Arthritis & Rheumatology Research Alliance (CARRA) registry showed that family history of psoriasis, autoimmune thyroiditis, Crohn disease, and ankylosing spondylitis in a first-degree relative has been linked to juvenile idiopathic arthritis, highlighting that pediatric psoriasis can be genetically linked or associated with multiple autoimmune conditions and vice versa.12
Obesity, Metabolic Syndrome, and Cardiovascular Risks
Obesity is associated with pediatric psoriasis as highlighted in a growing body of recent literature.13 Excess adiposity as manifested by body mass index in the 85th percentile or greater (37.9% of 155 pediatric psoriasis patients vs 20.5% of 42 controls) and excess central adiposity as manifested by excess waist circumference and increased waist-to-height ratios are more common in pediatric patients with psoriasis than in controls.14
Obesity may be a trigger or associated with increased disease activity in pediatric psoriasis patients. Excess overall adiposity correlates with more severe disease. Obesity parameters may correlate with the onset of psoriasis and with disease severity. In fact, the odds of obesity may be higher in childhood than in adults.14,15 A 2011 report of pediatric psoriasis patients aged 10 to 17 years (n=12) and wart controls (n=6)(mean age, 13.2 and 13.5 years, respectively) demonstrated that 4 of 12 patients with psoriasis and 0 of 6 patients with warts met criteria for metabolic syndrome as defined by 3 of the following: (1) triglycerides greater than or equal to 100 mg/dL; (2) high-density lipoprotein cholesterol less than 50 mg/dL in females and less than 5 mg/dL in males; (3) fasting blood glucose levels greater than or equal to 110 mg/dL, (4) waist circumference greater than the 75th percentile for age and sex; and (5) systolic or diastolic blood pressure greater than the 90th percentile for age, sex, and height.16 These studies highlight that obesity and metabolic syndrome are of concern in pediatric psoriasis patients; however, the best management approach using diet and weight interventions has yet to be identified.
Adiposity may precede the onset of psoriasis. A recent cohort of 27 pediatric psoriasis patients reported that the average age at onset of psoriasis was 8.7 years and the average age at onset of obesity was 4.1 years.15 In this study, 93% (25/27) of patients had adiposity preceding their psoriasis by 2 or more years. It is unclear if this is nature or nurture, as 48% (13/27) of patients had a family history of obesity, 41% (11/27) had a family history of psoriasis, and 48% (13/27) had a family history of hyperlipidemia.15 Therefore, obesity may be cultivated in some psoriatic families. The issue of household influences on diet and obesity needs to be addressed if successful weight management is to be achieved in future studies of pediatric psoriasis.
Cardiovascular risks in the pediatric psoriasis population are the subject of ongoing assessment but will likely mimic studies of adult psoriasis patients when reviewed longitudinally.16 Weight loss and healthy lifestyle interventions likely are beneficial to long-term health, but there is a lack of published data addressing dietary modification as a disease modifier for long-term care of pediatric psoriasis.
Anxiety and Depression
Anxiety and depression have been noted in adults with chronic skin diseases. A recent study assessed 118 patients and caregivers of pediatric patients with atopic dermatitis (n=50), psoriasis (n=25), or vitiligo (n=43) using the Children’s Dermatology Life Quality Index, the Hamilton Anxiety Scale, and the Beck Depression Inventory.17 Anxiety and depression were found in 36% of caregivers of pediatric psoriasis patients and depression was found in 36% of pediatric psoriasis patients, highlighting the need for interventions on a personal and family level to improve quality of life. As a comparator, anxiety was more prevalent in vitiligo caregivers (42%), but depression was only found in 26% of caregivers in the same group. Extent of disease (25%–75% body surface area affected) correlated with both depression and anxiety in the caregivers of pediatric patients with psoriasis as well as with anxiety in caregivers of pediatric patients with increased visible surface area of vitiligo.17 Parental anxiety has been reported at times to be linked to corticosteroid phobia, or corticophobia, which may interfere with disease therapy, as topical corticosteroids are considered the mainstay of therapy in childhood disease.18 Coordinating care with caregivers and addressing their concerns about the safety of medications should be integral to the pediatric psoriasis visit.
Pustular Psoriasis
Pustular psoriasis can be seen in any age group. Researchers recently have attempted to delineate the features and successful management of this severe subset of pediatric psoriasis patients. Twenty-four pediatric pustular psoriasis cases reviewed by Posso-De Los Rios et al19 revealed that 92% (22/24) had generalized and 8% (2/24) had limited acral disease. The mean (standard deviation) age at onset of pediatric pustular psoriasis was 6.3 (4.9) years. Half of the reported cases required more than one intervention. Treatment with acitretin, cyclosporine, and methotrexate was effective, but the investigators identified that there is a true dearth of evidence-based therapeutics in pediatric pustular psoriasis and much rebound with discontinuation.19 Although the subset of pediatric pustular psoriasis is rare, study of evidence-based intervention is needed.
Therapy
Recent reviews of pediatric and adolescent psoriasis highlight the paucity of therapeutic information for these patient populations. Investigators typically focus on topical therapies as the basis of treatment,20 as well as the addition of phototherapy in mild to moderate plaque or guttate psoriasis and biologic or systemic agents in moderate to severe flares of plaque, erythrodermic, or pustular psoriasis.21 Further studies are needed to identify evidence-based therapeutic paradigms for pediatric psoriasis and to pinpoint therapies associated with the best quality of life in patients and their caregivers.
Tumor Necrosis Factor α Inhibitors
Safety and efficacy of etanercept for juvenile idiopathic arthritis including oligoarthritis, enthesitis-related arthritis, and psoriatic arthritis recently was reviewed by Windschall et al22 using data from the German pediatric Biologika in der Kinderrheumatologie registry. Juvenile Arthritis Disease Activity Score 10 improved from baseline for 127 pediatric patients with psoriatic arthritis in 3 to 24 months (mean [standard deviation], 14.7 [6.4], 5.0 [4.6], 5.3 [6.4] at baseline, 3 months, and 24 months, respectively). Overall side effects were relatively higher in the psoriatic arthritis group; the rate of serious (relative risk, 1.39 [0.95-2.03; P=.08]) and nonserious (relative risk, 1.18 [1.02-1.35; P=.03]) adverse events also was elevated. Uveitis risk was greatest in the psoriatic arthritis group and the number of associated cases of inflammatory bowel disease outnumbered those seen in other forms of arthritis. The investigators concluded that monitoring for extra-articular immunopathies should be conducted in pediatric patients with psoriatic arthritis who are undergoing etanercept therapy.22
Tumor necrosis factor α (TNF-α) inhibitors have been associated with triggering psoriasiform dermatitis in pediatric patients treated for inflammatory bowel disease. A Finnish study of infliximab side effects in pediatric patients with inflammatory bowel disease (n=84; Crohn disease: n=64) demonstrated that almost half (47.6% [40/84]) of the participants presented with chronic skin reactions, 23% of which were severe in nature.23 Psoriasiform lesions of the scalp and ears were most common, followed by the periorificial area, genitals, trunk, and extremities. Rare association with HLA-Cw*0602 genotype was noted. Skin manifestations did not correlate with gut inflammation (as determined by fecal calprotectin levels). Discontinuation of therapy rarely was required.23 Other studies also have highlighted this side effect, suggesting an incidence of 2.7% in adults with colitis treated with TNF-α inhibitors24 and 10.5% in pediatric patients with Crohn disease.25 In a study by Sherlock et al,25 pediatric patients with Crohn disease developing psoriasis following infliximab therapy were more likely to be homozygous for specific polymorphisms in the IL-23R gene (rs10489628, rs10789229, and rs1343151).
Methotrexate
For pediatric patients who are being treated with methotrexate, the polyglutamate assay recently has been reported to be helpful in identifying patients needing a dose escalation.26 Higher numbers on the polyglutamate assay are associated with superior response to methotrexate therapy. Doses can be increased after 12 weeks in patients with low assays.26
IL-23
The safety of IL-23 blockade in pediatric psoriasis patients has not yet been established, but data from adult cases have implicated the IL-17 and IL-23 pathways in psoriasis/psoriatic arthritis, including an association with IL-23R polymorphisms27 and increases in soluble IL-20 and IL-22 associated with disease severity and an association of IL-17 levels with activity on the psoriasis area and severity index scores.28 The data are more limited for pediatric cases. Pediatric patients with inflammatory bowel disease who have an IL-23R polymorphism appear to be susceptible to psoriatic flares while on TNF-α inhibitor therapy,25 which suggests that the IL-23 blockade may be of benefit for some pediatric patients with psoriasis or psoriatic arthritis.
Conclusion
Pediatric psoriasis and psoriatic arthritis have now been identified as being part of the autoimmune spectrum and are associated with metabolic syndrome, including obesity and excess central adiposity, similar to their adult variants. An overview of potential unmet needs in pediatric psoriasis is included in Table 2. These unmet needs include further delineation of diet and weight modification in the care and prevention of psoriasis; expansion of therapeutic trials and US Food and Drug Administration–approved medications for children with psoriasis, especially severe variants such as extensive plaque and pustular disease; and development of guidelines for ongoing monitoring of children with psoriasis. The role of therapeutic interventions and weight management on long-term disease course remains to be shown in extended clinical trials. Despite the great advancements in psoriatic care, knowledge gaps remain in pediatric psoriasis that will need to be addressed in the future.
1. Taclonex Expanded Indication. OptumRx Web site. https://www.optumrx.com/vgnpreview/HCP/Assets/RxNews/Clinical%20Updates_Taclonex_2014-1003.pdf. Published August 29, 2014. Accessed January 28, 2015.
2. Silverberg NB. Update on pediatric psoriasis, part 1: clinical features and demographics. Cutis. 2010;86:118-124.
3. Silverberg NB. Update on pediatric psoriasis, part 2: therapeutic management. Cutis. 2010;86:172-176.
4. Cather JC. Psoriasis in children and women: addressing some special needs. Semin Cutan Med Surg. 2014;33(2 suppl 2):S42-S44.
5. Khorsand K, Sidbury R. Recent advances in pediatric dermatology. Arch Dis Child. 2014;99:944-948.
6. Mercy K, Kwasny M, Cordoro KM, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30:424-428.
7. Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, et al. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol. 2003;149:530-534.
8. Ferran M, Galván AB, Rincón C, et al. Streptococcus induces circulating CLA(+) memory T-cell-dependent epidermal cell activation in psoriasis. J Invest Dermatol. 2013;133:999-1007.
9. Gul Mert G, Incecik F, Gunasti S, et al. Psoriasiform drug eruption associated with sodium valproate [published online ahead of print November 13, 2013]. Case Rep Pediatr. 2013;2013:823469.
10. Chang MW, Nakrani R. Six children with allergic contact dermatitis to methylisothiazolinone in wet wipes (baby wipes). Pediatrics. 2014;133:e434-e438.
11. Gul U, Gonul M, Kaya I, et al. Autoimmune thyroid disorders in patients with psoriasis. Eur J Dermatol. 2009;19:221-223.
12. Prahalad S, McCracken C, Ponder L, et al. A120: Familial autoimmunity in the CARRA registry. Arthritis Rheumatol. 2014;66(suppl 11):S157.
13. Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
14. Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149:166-176.
15. Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
16. Volf EM, Levine DE, Michelon MA, et al. Assessor-blinded study of the metabolic syndrome and surrogate markers of increased cardiovascular risk in children with moderate-to-severe psoriasis compared with age-matched population of children with warts. J Drugs Dermatol. 2011;10:900-901.
17. Manzoni AP, Weber MB, Nagatomi AR, et al. Assessing depression and anxiety in the caregivers of pediatric patients with chronic skin disorders. An Bras Dermatol. 2013;88:894-899.
18. Belloni Fortina A, Neri L. Topical steroids and corticophobia. G Ital Dermatol Venereol. 2013;148:651-654.
19. Posso-De Los Rios CJ, Pope E, Lara-Corrales I. A systematic review of systemic medications for pustular psoriasis in pediatrics. Pediatr Dermatol. 2014;31:430-439.
20. Tollefson MM. Diagnosis and management of psoriasis in children. Pediatr Clin North Am. 2014;61:261-277.
21. Fotiadou C, Lazaridou E, Ioannides D. Management of psoriasis in adolescence. Adolesc Health Med Ther. 2014;5:25-34.
22. Windschall D, Müller T, Becker I, et al. Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis-related arthritis and psoriasis arthritis [published online ahead of print July 18, 2014]. Clin Rheumatol. 2015;34:61-69.
23. Mälkönen T, Wikström A, Heiskanen K, et al. Skin reactions during anti-TNFa therapy for pediatric inflammatory bowel disease: a 2-year prospective study. Inflamm Bowel Dis. 2014;20:1309-1315.
24. Afzali A, Wheat CL, Hu JK, et al. The association of psoriasiform rash with anti-tumor necrosis factor (anti-TNF) therapy in inflammatory bowel disease: a single academic center case series. J Crohns Colitis. 2014;8:480-488.
25. Sherlock ME, Walters T, Tabbers MM, et al. Infliximab-induced psoriasis and psoriasiform skin lesions in pediatric Crohn disease and a potential association with IL-23 receptor polymorphisms. J Pediatr Gastroenterol Nutr. 2013;56:512-518.
26. Rahman SI, Siegfried E, Flanagan KH, et al. The methotrexate polyglutamate assay supports the efficacy of methotrexate for severe inflammatory skin disease in children. J Am Acad Dermatol. 2014;70:252-256.
27. Suzuki E, Mellins ED, Gershwin ME, et al. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13:496-502.
28. Michalak-Stoma A, Bartosi´nska J, Kowal M, et al. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers. 2013;35:625-631.
Psoriasis affects 2% to 4% of the US population, with approximately one-third of cases beginning in childhood. The understanding of pediatric psoriasis has developed at a far slower pace than adult disease, with limitations in care including few medications that are approved by the US Food and Drug Administration for pediatric and adolescent use. Recently, a stable fixed-combination dose of calcipo-triene 0.005%–betamethasone dipropionate 0.064% topical suspension was approved for treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years, which hopefully will lead a trend in psoriasis medication approval for children and teenagers.1 Based on a PubMed search of articles indexed for MEDLINE using the search terms pediatric psoriasis, psoriasis, and strep that were published from April 2012 to April 2014, this article reviews newer data to address the issues that surround pediatric psoriasis and to provide an update on prior review articles on pediatric psoriasis.2-5 This article reviews some of the newer literature on clinical presentation and comorbidities in pediatric psoriasis.5 Based on these recent findings, additional screenings including review of obesity parameters are recommended for pediatric patients with psoriasis (Table 1).
Update on Disease Manifestations, Associations, and Comorbidities
Disease Manifestations
A 2013 multicenter study delineated the clinical features of pediatric psoriasis.6 The study was conducted at 8 geographically diverse dermatology clinics in the United States to delineate the clinical manifestations of pediatric psoriasis. In an assessment of 181 participants aged 5 to 17 years, the investigators sought to determine the frequency of disease sites, severity, and guttate disease. Over a period of approximately 2 years, 43.1% of participants were determined to have mild disease and 56.9% had severe disease. Family history of psoriasis was present in 51.4% of participants, with first-degree relatives affected in 59.8% of cases. Scalp involvement at some time was noted in 79.0% of participants, and nail disease was noted in 55% of boys and 29% of girls. Guttate psoriasis was noted in 30% of participants, with more cases in the severe range (35.9%) versus the mild range (21.8%). Additionally, 22.1% of participants had a precipitating streptococcal infection, with the association being more common in pediatric patients with guttate psoriasis than plaque psoriasis.6 This study highlighted that pediatric psoriasis has a genetic basis, is frequently guttate in nature, commonly affects the nails, shows a trend toward being classified as severe, and may be triggered by streptococcal infections.
Streptococcal Infection
Pediatric psoriasis may be triggered or flared by Streptococcus pyogenes (group A β-hemolytic streptococci) infections, specifically β-hemolytic streptococci groups A, C, and G that have streptococcal M protein,2,3,7 and this tendency can be associated with HLA-Cw6 or guttate psoriasis. Newer data have elucidated the role of streptococcal throat infections in psoriasis. Given that streptococcal throat infections are most common in school-aged children, these studies suggest a putative mechanism in pediatric psoriasis for triggering streptococcal infections, which would need to be confirmed in future studies, specifically in pediatric psoriasis patients.
It has been shown that T cells in psoriasis patients recognize common streptococcal M proteins and keratin determinants.7 Ferran et al8 recently demonstrated activation of circulating cutaneous lymphocyte–associated antigen (CLA)+ T cells but not CLA- memory T cells in 27 psoriasis patients (ages not specified) when mixed with streptococcal throat extracts, causing production of IL-17, IP-10, IL-22, and IFN-γ; activation was not found in 6 healthy control patients. Antistreptolysin O levels were correlated with the messenger RNA up- regulation for IL-17, IP-10, IL-22, and IFN-γ, and also correlated with psoriasis area and severity index score in psoriasis patients. In this same study, injection of the activated culture supernatant into mouse skin caused epidermal hyperkeratosis and activation of nonlesional epidermal cells from psoriatic patients. This study thereby delineated some of the potential pathways of the streptococcal induction of psoriasis and psoriatic flares in childhood8; however, confirmation is needed through further study of pediatric psoriatic lymphocyte activity.
Differential Diagnosis
Additions to the extensive differential list have been cited in the recent literature. The differential diagnosis of pediatric psoriasis now includes sodium valproate–induced psoriasiform drug eruption9 and allergic contact dermatitis to methylchloroisothiazolinone and methylisothiazolinone, which are present in many sanitizing hand and diaper wipes and has been reported to cause psoriasiform dermatitis in a periorificial or perineal distribution.10 Clinicians should inquire about the use of these wipes, as caregivers rarely suspect this agent to be causative of the eruption.
Psoriatic Arthritis
Previously, psoriasis and psoriatic arthritis have been linked to autoimmune thyroid disease in adults.11 A study of the Childhood Arthritis & Rheumatology Research Alliance (CARRA) registry showed that family history of psoriasis, autoimmune thyroiditis, Crohn disease, and ankylosing spondylitis in a first-degree relative has been linked to juvenile idiopathic arthritis, highlighting that pediatric psoriasis can be genetically linked or associated with multiple autoimmune conditions and vice versa.12
Obesity, Metabolic Syndrome, and Cardiovascular Risks
Obesity is associated with pediatric psoriasis as highlighted in a growing body of recent literature.13 Excess adiposity as manifested by body mass index in the 85th percentile or greater (37.9% of 155 pediatric psoriasis patients vs 20.5% of 42 controls) and excess central adiposity as manifested by excess waist circumference and increased waist-to-height ratios are more common in pediatric patients with psoriasis than in controls.14
Obesity may be a trigger or associated with increased disease activity in pediatric psoriasis patients. Excess overall adiposity correlates with more severe disease. Obesity parameters may correlate with the onset of psoriasis and with disease severity. In fact, the odds of obesity may be higher in childhood than in adults.14,15 A 2011 report of pediatric psoriasis patients aged 10 to 17 years (n=12) and wart controls (n=6)(mean age, 13.2 and 13.5 years, respectively) demonstrated that 4 of 12 patients with psoriasis and 0 of 6 patients with warts met criteria for metabolic syndrome as defined by 3 of the following: (1) triglycerides greater than or equal to 100 mg/dL; (2) high-density lipoprotein cholesterol less than 50 mg/dL in females and less than 5 mg/dL in males; (3) fasting blood glucose levels greater than or equal to 110 mg/dL, (4) waist circumference greater than the 75th percentile for age and sex; and (5) systolic or diastolic blood pressure greater than the 90th percentile for age, sex, and height.16 These studies highlight that obesity and metabolic syndrome are of concern in pediatric psoriasis patients; however, the best management approach using diet and weight interventions has yet to be identified.
Adiposity may precede the onset of psoriasis. A recent cohort of 27 pediatric psoriasis patients reported that the average age at onset of psoriasis was 8.7 years and the average age at onset of obesity was 4.1 years.15 In this study, 93% (25/27) of patients had adiposity preceding their psoriasis by 2 or more years. It is unclear if this is nature or nurture, as 48% (13/27) of patients had a family history of obesity, 41% (11/27) had a family history of psoriasis, and 48% (13/27) had a family history of hyperlipidemia.15 Therefore, obesity may be cultivated in some psoriatic families. The issue of household influences on diet and obesity needs to be addressed if successful weight management is to be achieved in future studies of pediatric psoriasis.
Cardiovascular risks in the pediatric psoriasis population are the subject of ongoing assessment but will likely mimic studies of adult psoriasis patients when reviewed longitudinally.16 Weight loss and healthy lifestyle interventions likely are beneficial to long-term health, but there is a lack of published data addressing dietary modification as a disease modifier for long-term care of pediatric psoriasis.
Anxiety and Depression
Anxiety and depression have been noted in adults with chronic skin diseases. A recent study assessed 118 patients and caregivers of pediatric patients with atopic dermatitis (n=50), psoriasis (n=25), or vitiligo (n=43) using the Children’s Dermatology Life Quality Index, the Hamilton Anxiety Scale, and the Beck Depression Inventory.17 Anxiety and depression were found in 36% of caregivers of pediatric psoriasis patients and depression was found in 36% of pediatric psoriasis patients, highlighting the need for interventions on a personal and family level to improve quality of life. As a comparator, anxiety was more prevalent in vitiligo caregivers (42%), but depression was only found in 26% of caregivers in the same group. Extent of disease (25%–75% body surface area affected) correlated with both depression and anxiety in the caregivers of pediatric patients with psoriasis as well as with anxiety in caregivers of pediatric patients with increased visible surface area of vitiligo.17 Parental anxiety has been reported at times to be linked to corticosteroid phobia, or corticophobia, which may interfere with disease therapy, as topical corticosteroids are considered the mainstay of therapy in childhood disease.18 Coordinating care with caregivers and addressing their concerns about the safety of medications should be integral to the pediatric psoriasis visit.
Pustular Psoriasis
Pustular psoriasis can be seen in any age group. Researchers recently have attempted to delineate the features and successful management of this severe subset of pediatric psoriasis patients. Twenty-four pediatric pustular psoriasis cases reviewed by Posso-De Los Rios et al19 revealed that 92% (22/24) had generalized and 8% (2/24) had limited acral disease. The mean (standard deviation) age at onset of pediatric pustular psoriasis was 6.3 (4.9) years. Half of the reported cases required more than one intervention. Treatment with acitretin, cyclosporine, and methotrexate was effective, but the investigators identified that there is a true dearth of evidence-based therapeutics in pediatric pustular psoriasis and much rebound with discontinuation.19 Although the subset of pediatric pustular psoriasis is rare, study of evidence-based intervention is needed.
Therapy
Recent reviews of pediatric and adolescent psoriasis highlight the paucity of therapeutic information for these patient populations. Investigators typically focus on topical therapies as the basis of treatment,20 as well as the addition of phototherapy in mild to moderate plaque or guttate psoriasis and biologic or systemic agents in moderate to severe flares of plaque, erythrodermic, or pustular psoriasis.21 Further studies are needed to identify evidence-based therapeutic paradigms for pediatric psoriasis and to pinpoint therapies associated with the best quality of life in patients and their caregivers.
Tumor Necrosis Factor α Inhibitors
Safety and efficacy of etanercept for juvenile idiopathic arthritis including oligoarthritis, enthesitis-related arthritis, and psoriatic arthritis recently was reviewed by Windschall et al22 using data from the German pediatric Biologika in der Kinderrheumatologie registry. Juvenile Arthritis Disease Activity Score 10 improved from baseline for 127 pediatric patients with psoriatic arthritis in 3 to 24 months (mean [standard deviation], 14.7 [6.4], 5.0 [4.6], 5.3 [6.4] at baseline, 3 months, and 24 months, respectively). Overall side effects were relatively higher in the psoriatic arthritis group; the rate of serious (relative risk, 1.39 [0.95-2.03; P=.08]) and nonserious (relative risk, 1.18 [1.02-1.35; P=.03]) adverse events also was elevated. Uveitis risk was greatest in the psoriatic arthritis group and the number of associated cases of inflammatory bowel disease outnumbered those seen in other forms of arthritis. The investigators concluded that monitoring for extra-articular immunopathies should be conducted in pediatric patients with psoriatic arthritis who are undergoing etanercept therapy.22
Tumor necrosis factor α (TNF-α) inhibitors have been associated with triggering psoriasiform dermatitis in pediatric patients treated for inflammatory bowel disease. A Finnish study of infliximab side effects in pediatric patients with inflammatory bowel disease (n=84; Crohn disease: n=64) demonstrated that almost half (47.6% [40/84]) of the participants presented with chronic skin reactions, 23% of which were severe in nature.23 Psoriasiform lesions of the scalp and ears were most common, followed by the periorificial area, genitals, trunk, and extremities. Rare association with HLA-Cw*0602 genotype was noted. Skin manifestations did not correlate with gut inflammation (as determined by fecal calprotectin levels). Discontinuation of therapy rarely was required.23 Other studies also have highlighted this side effect, suggesting an incidence of 2.7% in adults with colitis treated with TNF-α inhibitors24 and 10.5% in pediatric patients with Crohn disease.25 In a study by Sherlock et al,25 pediatric patients with Crohn disease developing psoriasis following infliximab therapy were more likely to be homozygous for specific polymorphisms in the IL-23R gene (rs10489628, rs10789229, and rs1343151).
Methotrexate
For pediatric patients who are being treated with methotrexate, the polyglutamate assay recently has been reported to be helpful in identifying patients needing a dose escalation.26 Higher numbers on the polyglutamate assay are associated with superior response to methotrexate therapy. Doses can be increased after 12 weeks in patients with low assays.26
IL-23
The safety of IL-23 blockade in pediatric psoriasis patients has not yet been established, but data from adult cases have implicated the IL-17 and IL-23 pathways in psoriasis/psoriatic arthritis, including an association with IL-23R polymorphisms27 and increases in soluble IL-20 and IL-22 associated with disease severity and an association of IL-17 levels with activity on the psoriasis area and severity index scores.28 The data are more limited for pediatric cases. Pediatric patients with inflammatory bowel disease who have an IL-23R polymorphism appear to be susceptible to psoriatic flares while on TNF-α inhibitor therapy,25 which suggests that the IL-23 blockade may be of benefit for some pediatric patients with psoriasis or psoriatic arthritis.
Conclusion
Pediatric psoriasis and psoriatic arthritis have now been identified as being part of the autoimmune spectrum and are associated with metabolic syndrome, including obesity and excess central adiposity, similar to their adult variants. An overview of potential unmet needs in pediatric psoriasis is included in Table 2. These unmet needs include further delineation of diet and weight modification in the care and prevention of psoriasis; expansion of therapeutic trials and US Food and Drug Administration–approved medications for children with psoriasis, especially severe variants such as extensive plaque and pustular disease; and development of guidelines for ongoing monitoring of children with psoriasis. The role of therapeutic interventions and weight management on long-term disease course remains to be shown in extended clinical trials. Despite the great advancements in psoriatic care, knowledge gaps remain in pediatric psoriasis that will need to be addressed in the future.
Psoriasis affects 2% to 4% of the US population, with approximately one-third of cases beginning in childhood. The understanding of pediatric psoriasis has developed at a far slower pace than adult disease, with limitations in care including few medications that are approved by the US Food and Drug Administration for pediatric and adolescent use. Recently, a stable fixed-combination dose of calcipo-triene 0.005%–betamethasone dipropionate 0.064% topical suspension was approved for treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years, which hopefully will lead a trend in psoriasis medication approval for children and teenagers.1 Based on a PubMed search of articles indexed for MEDLINE using the search terms pediatric psoriasis, psoriasis, and strep that were published from April 2012 to April 2014, this article reviews newer data to address the issues that surround pediatric psoriasis and to provide an update on prior review articles on pediatric psoriasis.2-5 This article reviews some of the newer literature on clinical presentation and comorbidities in pediatric psoriasis.5 Based on these recent findings, additional screenings including review of obesity parameters are recommended for pediatric patients with psoriasis (Table 1).
Update on Disease Manifestations, Associations, and Comorbidities
Disease Manifestations
A 2013 multicenter study delineated the clinical features of pediatric psoriasis.6 The study was conducted at 8 geographically diverse dermatology clinics in the United States to delineate the clinical manifestations of pediatric psoriasis. In an assessment of 181 participants aged 5 to 17 years, the investigators sought to determine the frequency of disease sites, severity, and guttate disease. Over a period of approximately 2 years, 43.1% of participants were determined to have mild disease and 56.9% had severe disease. Family history of psoriasis was present in 51.4% of participants, with first-degree relatives affected in 59.8% of cases. Scalp involvement at some time was noted in 79.0% of participants, and nail disease was noted in 55% of boys and 29% of girls. Guttate psoriasis was noted in 30% of participants, with more cases in the severe range (35.9%) versus the mild range (21.8%). Additionally, 22.1% of participants had a precipitating streptococcal infection, with the association being more common in pediatric patients with guttate psoriasis than plaque psoriasis.6 This study highlighted that pediatric psoriasis has a genetic basis, is frequently guttate in nature, commonly affects the nails, shows a trend toward being classified as severe, and may be triggered by streptococcal infections.
Streptococcal Infection
Pediatric psoriasis may be triggered or flared by Streptococcus pyogenes (group A β-hemolytic streptococci) infections, specifically β-hemolytic streptococci groups A, C, and G that have streptococcal M protein,2,3,7 and this tendency can be associated with HLA-Cw6 or guttate psoriasis. Newer data have elucidated the role of streptococcal throat infections in psoriasis. Given that streptococcal throat infections are most common in school-aged children, these studies suggest a putative mechanism in pediatric psoriasis for triggering streptococcal infections, which would need to be confirmed in future studies, specifically in pediatric psoriasis patients.
It has been shown that T cells in psoriasis patients recognize common streptococcal M proteins and keratin determinants.7 Ferran et al8 recently demonstrated activation of circulating cutaneous lymphocyte–associated antigen (CLA)+ T cells but not CLA- memory T cells in 27 psoriasis patients (ages not specified) when mixed with streptococcal throat extracts, causing production of IL-17, IP-10, IL-22, and IFN-γ; activation was not found in 6 healthy control patients. Antistreptolysin O levels were correlated with the messenger RNA up- regulation for IL-17, IP-10, IL-22, and IFN-γ, and also correlated with psoriasis area and severity index score in psoriasis patients. In this same study, injection of the activated culture supernatant into mouse skin caused epidermal hyperkeratosis and activation of nonlesional epidermal cells from psoriatic patients. This study thereby delineated some of the potential pathways of the streptococcal induction of psoriasis and psoriatic flares in childhood8; however, confirmation is needed through further study of pediatric psoriatic lymphocyte activity.
Differential Diagnosis
Additions to the extensive differential list have been cited in the recent literature. The differential diagnosis of pediatric psoriasis now includes sodium valproate–induced psoriasiform drug eruption9 and allergic contact dermatitis to methylchloroisothiazolinone and methylisothiazolinone, which are present in many sanitizing hand and diaper wipes and has been reported to cause psoriasiform dermatitis in a periorificial or perineal distribution.10 Clinicians should inquire about the use of these wipes, as caregivers rarely suspect this agent to be causative of the eruption.
Psoriatic Arthritis
Previously, psoriasis and psoriatic arthritis have been linked to autoimmune thyroid disease in adults.11 A study of the Childhood Arthritis & Rheumatology Research Alliance (CARRA) registry showed that family history of psoriasis, autoimmune thyroiditis, Crohn disease, and ankylosing spondylitis in a first-degree relative has been linked to juvenile idiopathic arthritis, highlighting that pediatric psoriasis can be genetically linked or associated with multiple autoimmune conditions and vice versa.12
Obesity, Metabolic Syndrome, and Cardiovascular Risks
Obesity is associated with pediatric psoriasis as highlighted in a growing body of recent literature.13 Excess adiposity as manifested by body mass index in the 85th percentile or greater (37.9% of 155 pediatric psoriasis patients vs 20.5% of 42 controls) and excess central adiposity as manifested by excess waist circumference and increased waist-to-height ratios are more common in pediatric patients with psoriasis than in controls.14
Obesity may be a trigger or associated with increased disease activity in pediatric psoriasis patients. Excess overall adiposity correlates with more severe disease. Obesity parameters may correlate with the onset of psoriasis and with disease severity. In fact, the odds of obesity may be higher in childhood than in adults.14,15 A 2011 report of pediatric psoriasis patients aged 10 to 17 years (n=12) and wart controls (n=6)(mean age, 13.2 and 13.5 years, respectively) demonstrated that 4 of 12 patients with psoriasis and 0 of 6 patients with warts met criteria for metabolic syndrome as defined by 3 of the following: (1) triglycerides greater than or equal to 100 mg/dL; (2) high-density lipoprotein cholesterol less than 50 mg/dL in females and less than 5 mg/dL in males; (3) fasting blood glucose levels greater than or equal to 110 mg/dL, (4) waist circumference greater than the 75th percentile for age and sex; and (5) systolic or diastolic blood pressure greater than the 90th percentile for age, sex, and height.16 These studies highlight that obesity and metabolic syndrome are of concern in pediatric psoriasis patients; however, the best management approach using diet and weight interventions has yet to be identified.
Adiposity may precede the onset of psoriasis. A recent cohort of 27 pediatric psoriasis patients reported that the average age at onset of psoriasis was 8.7 years and the average age at onset of obesity was 4.1 years.15 In this study, 93% (25/27) of patients had adiposity preceding their psoriasis by 2 or more years. It is unclear if this is nature or nurture, as 48% (13/27) of patients had a family history of obesity, 41% (11/27) had a family history of psoriasis, and 48% (13/27) had a family history of hyperlipidemia.15 Therefore, obesity may be cultivated in some psoriatic families. The issue of household influences on diet and obesity needs to be addressed if successful weight management is to be achieved in future studies of pediatric psoriasis.
Cardiovascular risks in the pediatric psoriasis population are the subject of ongoing assessment but will likely mimic studies of adult psoriasis patients when reviewed longitudinally.16 Weight loss and healthy lifestyle interventions likely are beneficial to long-term health, but there is a lack of published data addressing dietary modification as a disease modifier for long-term care of pediatric psoriasis.
Anxiety and Depression
Anxiety and depression have been noted in adults with chronic skin diseases. A recent study assessed 118 patients and caregivers of pediatric patients with atopic dermatitis (n=50), psoriasis (n=25), or vitiligo (n=43) using the Children’s Dermatology Life Quality Index, the Hamilton Anxiety Scale, and the Beck Depression Inventory.17 Anxiety and depression were found in 36% of caregivers of pediatric psoriasis patients and depression was found in 36% of pediatric psoriasis patients, highlighting the need for interventions on a personal and family level to improve quality of life. As a comparator, anxiety was more prevalent in vitiligo caregivers (42%), but depression was only found in 26% of caregivers in the same group. Extent of disease (25%–75% body surface area affected) correlated with both depression and anxiety in the caregivers of pediatric patients with psoriasis as well as with anxiety in caregivers of pediatric patients with increased visible surface area of vitiligo.17 Parental anxiety has been reported at times to be linked to corticosteroid phobia, or corticophobia, which may interfere with disease therapy, as topical corticosteroids are considered the mainstay of therapy in childhood disease.18 Coordinating care with caregivers and addressing their concerns about the safety of medications should be integral to the pediatric psoriasis visit.
Pustular Psoriasis
Pustular psoriasis can be seen in any age group. Researchers recently have attempted to delineate the features and successful management of this severe subset of pediatric psoriasis patients. Twenty-four pediatric pustular psoriasis cases reviewed by Posso-De Los Rios et al19 revealed that 92% (22/24) had generalized and 8% (2/24) had limited acral disease. The mean (standard deviation) age at onset of pediatric pustular psoriasis was 6.3 (4.9) years. Half of the reported cases required more than one intervention. Treatment with acitretin, cyclosporine, and methotrexate was effective, but the investigators identified that there is a true dearth of evidence-based therapeutics in pediatric pustular psoriasis and much rebound with discontinuation.19 Although the subset of pediatric pustular psoriasis is rare, study of evidence-based intervention is needed.
Therapy
Recent reviews of pediatric and adolescent psoriasis highlight the paucity of therapeutic information for these patient populations. Investigators typically focus on topical therapies as the basis of treatment,20 as well as the addition of phototherapy in mild to moderate plaque or guttate psoriasis and biologic or systemic agents in moderate to severe flares of plaque, erythrodermic, or pustular psoriasis.21 Further studies are needed to identify evidence-based therapeutic paradigms for pediatric psoriasis and to pinpoint therapies associated with the best quality of life in patients and their caregivers.
Tumor Necrosis Factor α Inhibitors
Safety and efficacy of etanercept for juvenile idiopathic arthritis including oligoarthritis, enthesitis-related arthritis, and psoriatic arthritis recently was reviewed by Windschall et al22 using data from the German pediatric Biologika in der Kinderrheumatologie registry. Juvenile Arthritis Disease Activity Score 10 improved from baseline for 127 pediatric patients with psoriatic arthritis in 3 to 24 months (mean [standard deviation], 14.7 [6.4], 5.0 [4.6], 5.3 [6.4] at baseline, 3 months, and 24 months, respectively). Overall side effects were relatively higher in the psoriatic arthritis group; the rate of serious (relative risk, 1.39 [0.95-2.03; P=.08]) and nonserious (relative risk, 1.18 [1.02-1.35; P=.03]) adverse events also was elevated. Uveitis risk was greatest in the psoriatic arthritis group and the number of associated cases of inflammatory bowel disease outnumbered those seen in other forms of arthritis. The investigators concluded that monitoring for extra-articular immunopathies should be conducted in pediatric patients with psoriatic arthritis who are undergoing etanercept therapy.22
Tumor necrosis factor α (TNF-α) inhibitors have been associated with triggering psoriasiform dermatitis in pediatric patients treated for inflammatory bowel disease. A Finnish study of infliximab side effects in pediatric patients with inflammatory bowel disease (n=84; Crohn disease: n=64) demonstrated that almost half (47.6% [40/84]) of the participants presented with chronic skin reactions, 23% of which were severe in nature.23 Psoriasiform lesions of the scalp and ears were most common, followed by the periorificial area, genitals, trunk, and extremities. Rare association with HLA-Cw*0602 genotype was noted. Skin manifestations did not correlate with gut inflammation (as determined by fecal calprotectin levels). Discontinuation of therapy rarely was required.23 Other studies also have highlighted this side effect, suggesting an incidence of 2.7% in adults with colitis treated with TNF-α inhibitors24 and 10.5% in pediatric patients with Crohn disease.25 In a study by Sherlock et al,25 pediatric patients with Crohn disease developing psoriasis following infliximab therapy were more likely to be homozygous for specific polymorphisms in the IL-23R gene (rs10489628, rs10789229, and rs1343151).
Methotrexate
For pediatric patients who are being treated with methotrexate, the polyglutamate assay recently has been reported to be helpful in identifying patients needing a dose escalation.26 Higher numbers on the polyglutamate assay are associated with superior response to methotrexate therapy. Doses can be increased after 12 weeks in patients with low assays.26
IL-23
The safety of IL-23 blockade in pediatric psoriasis patients has not yet been established, but data from adult cases have implicated the IL-17 and IL-23 pathways in psoriasis/psoriatic arthritis, including an association with IL-23R polymorphisms27 and increases in soluble IL-20 and IL-22 associated with disease severity and an association of IL-17 levels with activity on the psoriasis area and severity index scores.28 The data are more limited for pediatric cases. Pediatric patients with inflammatory bowel disease who have an IL-23R polymorphism appear to be susceptible to psoriatic flares while on TNF-α inhibitor therapy,25 which suggests that the IL-23 blockade may be of benefit for some pediatric patients with psoriasis or psoriatic arthritis.
Conclusion
Pediatric psoriasis and psoriatic arthritis have now been identified as being part of the autoimmune spectrum and are associated with metabolic syndrome, including obesity and excess central adiposity, similar to their adult variants. An overview of potential unmet needs in pediatric psoriasis is included in Table 2. These unmet needs include further delineation of diet and weight modification in the care and prevention of psoriasis; expansion of therapeutic trials and US Food and Drug Administration–approved medications for children with psoriasis, especially severe variants such as extensive plaque and pustular disease; and development of guidelines for ongoing monitoring of children with psoriasis. The role of therapeutic interventions and weight management on long-term disease course remains to be shown in extended clinical trials. Despite the great advancements in psoriatic care, knowledge gaps remain in pediatric psoriasis that will need to be addressed in the future.
1. Taclonex Expanded Indication. OptumRx Web site. https://www.optumrx.com/vgnpreview/HCP/Assets/RxNews/Clinical%20Updates_Taclonex_2014-1003.pdf. Published August 29, 2014. Accessed January 28, 2015.
2. Silverberg NB. Update on pediatric psoriasis, part 1: clinical features and demographics. Cutis. 2010;86:118-124.
3. Silverberg NB. Update on pediatric psoriasis, part 2: therapeutic management. Cutis. 2010;86:172-176.
4. Cather JC. Psoriasis in children and women: addressing some special needs. Semin Cutan Med Surg. 2014;33(2 suppl 2):S42-S44.
5. Khorsand K, Sidbury R. Recent advances in pediatric dermatology. Arch Dis Child. 2014;99:944-948.
6. Mercy K, Kwasny M, Cordoro KM, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30:424-428.
7. Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, et al. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol. 2003;149:530-534.
8. Ferran M, Galván AB, Rincón C, et al. Streptococcus induces circulating CLA(+) memory T-cell-dependent epidermal cell activation in psoriasis. J Invest Dermatol. 2013;133:999-1007.
9. Gul Mert G, Incecik F, Gunasti S, et al. Psoriasiform drug eruption associated with sodium valproate [published online ahead of print November 13, 2013]. Case Rep Pediatr. 2013;2013:823469.
10. Chang MW, Nakrani R. Six children with allergic contact dermatitis to methylisothiazolinone in wet wipes (baby wipes). Pediatrics. 2014;133:e434-e438.
11. Gul U, Gonul M, Kaya I, et al. Autoimmune thyroid disorders in patients with psoriasis. Eur J Dermatol. 2009;19:221-223.
12. Prahalad S, McCracken C, Ponder L, et al. A120: Familial autoimmunity in the CARRA registry. Arthritis Rheumatol. 2014;66(suppl 11):S157.
13. Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
14. Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149:166-176.
15. Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
16. Volf EM, Levine DE, Michelon MA, et al. Assessor-blinded study of the metabolic syndrome and surrogate markers of increased cardiovascular risk in children with moderate-to-severe psoriasis compared with age-matched population of children with warts. J Drugs Dermatol. 2011;10:900-901.
17. Manzoni AP, Weber MB, Nagatomi AR, et al. Assessing depression and anxiety in the caregivers of pediatric patients with chronic skin disorders. An Bras Dermatol. 2013;88:894-899.
18. Belloni Fortina A, Neri L. Topical steroids and corticophobia. G Ital Dermatol Venereol. 2013;148:651-654.
19. Posso-De Los Rios CJ, Pope E, Lara-Corrales I. A systematic review of systemic medications for pustular psoriasis in pediatrics. Pediatr Dermatol. 2014;31:430-439.
20. Tollefson MM. Diagnosis and management of psoriasis in children. Pediatr Clin North Am. 2014;61:261-277.
21. Fotiadou C, Lazaridou E, Ioannides D. Management of psoriasis in adolescence. Adolesc Health Med Ther. 2014;5:25-34.
22. Windschall D, Müller T, Becker I, et al. Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis-related arthritis and psoriasis arthritis [published online ahead of print July 18, 2014]. Clin Rheumatol. 2015;34:61-69.
23. Mälkönen T, Wikström A, Heiskanen K, et al. Skin reactions during anti-TNFa therapy for pediatric inflammatory bowel disease: a 2-year prospective study. Inflamm Bowel Dis. 2014;20:1309-1315.
24. Afzali A, Wheat CL, Hu JK, et al. The association of psoriasiform rash with anti-tumor necrosis factor (anti-TNF) therapy in inflammatory bowel disease: a single academic center case series. J Crohns Colitis. 2014;8:480-488.
25. Sherlock ME, Walters T, Tabbers MM, et al. Infliximab-induced psoriasis and psoriasiform skin lesions in pediatric Crohn disease and a potential association with IL-23 receptor polymorphisms. J Pediatr Gastroenterol Nutr. 2013;56:512-518.
26. Rahman SI, Siegfried E, Flanagan KH, et al. The methotrexate polyglutamate assay supports the efficacy of methotrexate for severe inflammatory skin disease in children. J Am Acad Dermatol. 2014;70:252-256.
27. Suzuki E, Mellins ED, Gershwin ME, et al. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13:496-502.
28. Michalak-Stoma A, Bartosi´nska J, Kowal M, et al. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers. 2013;35:625-631.
1. Taclonex Expanded Indication. OptumRx Web site. https://www.optumrx.com/vgnpreview/HCP/Assets/RxNews/Clinical%20Updates_Taclonex_2014-1003.pdf. Published August 29, 2014. Accessed January 28, 2015.
2. Silverberg NB. Update on pediatric psoriasis, part 1: clinical features and demographics. Cutis. 2010;86:118-124.
3. Silverberg NB. Update on pediatric psoriasis, part 2: therapeutic management. Cutis. 2010;86:172-176.
4. Cather JC. Psoriasis in children and women: addressing some special needs. Semin Cutan Med Surg. 2014;33(2 suppl 2):S42-S44.
5. Khorsand K, Sidbury R. Recent advances in pediatric dermatology. Arch Dis Child. 2014;99:944-948.
6. Mercy K, Kwasny M, Cordoro KM, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30:424-428.
7. Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, et al. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol. 2003;149:530-534.
8. Ferran M, Galván AB, Rincón C, et al. Streptococcus induces circulating CLA(+) memory T-cell-dependent epidermal cell activation in psoriasis. J Invest Dermatol. 2013;133:999-1007.
9. Gul Mert G, Incecik F, Gunasti S, et al. Psoriasiform drug eruption associated with sodium valproate [published online ahead of print November 13, 2013]. Case Rep Pediatr. 2013;2013:823469.
10. Chang MW, Nakrani R. Six children with allergic contact dermatitis to methylisothiazolinone in wet wipes (baby wipes). Pediatrics. 2014;133:e434-e438.
11. Gul U, Gonul M, Kaya I, et al. Autoimmune thyroid disorders in patients with psoriasis. Eur J Dermatol. 2009;19:221-223.
12. Prahalad S, McCracken C, Ponder L, et al. A120: Familial autoimmunity in the CARRA registry. Arthritis Rheumatol. 2014;66(suppl 11):S157.
13. Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
14. Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149:166-176.
15. Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
16. Volf EM, Levine DE, Michelon MA, et al. Assessor-blinded study of the metabolic syndrome and surrogate markers of increased cardiovascular risk in children with moderate-to-severe psoriasis compared with age-matched population of children with warts. J Drugs Dermatol. 2011;10:900-901.
17. Manzoni AP, Weber MB, Nagatomi AR, et al. Assessing depression and anxiety in the caregivers of pediatric patients with chronic skin disorders. An Bras Dermatol. 2013;88:894-899.
18. Belloni Fortina A, Neri L. Topical steroids and corticophobia. G Ital Dermatol Venereol. 2013;148:651-654.
19. Posso-De Los Rios CJ, Pope E, Lara-Corrales I. A systematic review of systemic medications for pustular psoriasis in pediatrics. Pediatr Dermatol. 2014;31:430-439.
20. Tollefson MM. Diagnosis and management of psoriasis in children. Pediatr Clin North Am. 2014;61:261-277.
21. Fotiadou C, Lazaridou E, Ioannides D. Management of psoriasis in adolescence. Adolesc Health Med Ther. 2014;5:25-34.
22. Windschall D, Müller T, Becker I, et al. Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis-related arthritis and psoriasis arthritis [published online ahead of print July 18, 2014]. Clin Rheumatol. 2015;34:61-69.
23. Mälkönen T, Wikström A, Heiskanen K, et al. Skin reactions during anti-TNFa therapy for pediatric inflammatory bowel disease: a 2-year prospective study. Inflamm Bowel Dis. 2014;20:1309-1315.
24. Afzali A, Wheat CL, Hu JK, et al. The association of psoriasiform rash with anti-tumor necrosis factor (anti-TNF) therapy in inflammatory bowel disease: a single academic center case series. J Crohns Colitis. 2014;8:480-488.
25. Sherlock ME, Walters T, Tabbers MM, et al. Infliximab-induced psoriasis and psoriasiform skin lesions in pediatric Crohn disease and a potential association with IL-23 receptor polymorphisms. J Pediatr Gastroenterol Nutr. 2013;56:512-518.
26. Rahman SI, Siegfried E, Flanagan KH, et al. The methotrexate polyglutamate assay supports the efficacy of methotrexate for severe inflammatory skin disease in children. J Am Acad Dermatol. 2014;70:252-256.
27. Suzuki E, Mellins ED, Gershwin ME, et al. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13:496-502.
28. Michalak-Stoma A, Bartosi´nska J, Kowal M, et al. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers. 2013;35:625-631.
Practice Points
- The majority of children with psoriasis have severe disease, scalp involvement, and a family history.
- Pediatric psoriasis is associated with metabolic syndrome, especially obesity.
- Anxiety and depression may be noted in children with psoriasis as well as their caregivers.
Cutaneous Side Effects of Chemotherapy in Pediatric Oncology Patients
Pediatric oncology patients can present with various skin lesions related to both their primary disease and immunosuppressive treatments. In the majority of cases, cutaneous findings are associated with the use of chemotherapeutic agents. The toxic effects of chemotherapeutic agents, which generally are associated with treatment of solid organ malignancies (eg, liver, kidneys), can be detected by oncologists using clinical signs and laboratory tests.1-3 However, it also is important for dermatologists to recognize and evaluate cutaneous side effects associated with chemotherapeutic agents. Reports in the literature of cutaneous side effects of chemotherapy in pediatric patients generally are limited to case studies. This study aimed to evaluate the characteristics of cutaneous side effects of chemotherapy in pediatric oncology patients.
Materials and Methods
The study was performed through the collaboration of the departments of dermatology and venereology and pediatric oncology in the Faculty of Medicine at Ege University, Izmir, Turkey. Sixty-five pediatric oncology patients who were scheduled to undergo chemotherapy from May 2011 to May 2013 were included in the study. Clinical examination of dermatologic findings was conducted at baseline (prior to beginning chemotherapy) and at months 1, 3, and 6 of treatment. Patients were examined a total of 4 times during the study. Patients with a history of skin disease prior to diagnosis of their malignancy were excluded, as the study aimed to evaluate cutaneous side effects of chemotherapy. Patients who developed cutaneous side effects during the study period were photographed. Skin biopsy was performed to confirm clinical diagnosis. Patients were split into 5 groups according to oncological diagnoses, including hematological malignancies, solid organ tumors, bone and soft tissue tumors, central nervous system tumors, and Langerhans cell histiocytosis. Data regarding age, gender, treatments administered (ie, chemotherapeutics, antibiotics, antifungals, antivirals), and dermatologic signs were recorded. Mucocutaneous findings were classified as infectious (viral, bacterial, fungal) lesions, bullous lesions, inflammatory dermatoses (eg, diaper dermatitis, asteatotic eczema, contact dermatitis, seborrheic dermatitis), xeroderma, petechiae/ecchymoses, nail signs, alopecia, mucositis, cheilitis, oral aphthae, drug reactions confirmed by histopathology, cushingoid signs (eg, striae, acneform eruption, hypertrichosis), and cutaneous hyperpigmentation.
Statistical analysis was performed using SPSS version 15.0 and χ2 test was applied to the analysis.
Results
Of 65 patients, 62 completed the study and were included in the analysis. Three patients were excluded from the results, as 2 patients died during treatment and 1 patient withdrew from the study prior to completion. Twenty-seven (43.5%) patients were female and 35 (56.5%) were male ranging in age from 1 to 17 years (mean age, 8.14 years; median age [standard deviation], 7.25 [5.42] years). There were 31 (50%) patients in the hematological malignancies group, 11 (17.7%) in the solid organ tumors group, 10 (16.1%) in the bone and soft tissue tumors group, and 9 (14.5%) in the central nervous system tumors group; Langerhans cell histiocytosis was diagnosed in 1 (1.6%) patient. Hodgkin lymphoma made up 29.0% (n=9) of hematological malignancies. Other hematological malignancies included acute myeloblastic leukemia (n=7 [22.5%]), acute lymphoblastic leukemia (n=7 [22.5%]), T-cell lymphoma (n=5 [16.1%]), non-Hodgkin lym-phoma (n=1 [3.2%]), anaplastic giant cell lymphoma (n=1 [3.2%]), and diffuse giant cell lymphoma (n=1 [3.2%]).
In addition to chemotherapeutic agents, 7 (11.3%) patients in this study also received antibiotics and 3 (4.8%) received antivirals. The most frequently employed chemotherapeutic agents were vincristine, methotrexate, cytarabine, etoposide, and dexamethasone. Cyclophosphamide, doxorubicin, ifosfamide, asparaginase, carboplatin, procarbazine, daunorubicin, actinomycin D, vinblastine, cisplatin, bleomycin, idarubicin, 6-mercaptopurine, temozolamide, and cyclosporine also were administered. The most commonly encountered dermatological side effects were alopecia, xeroderma, inflammatory skin lesions, infectious lesions, and mucositis, respectively (Table 1). Cutaneous side effects were frequently seen at months 1 and 3 of treatment.
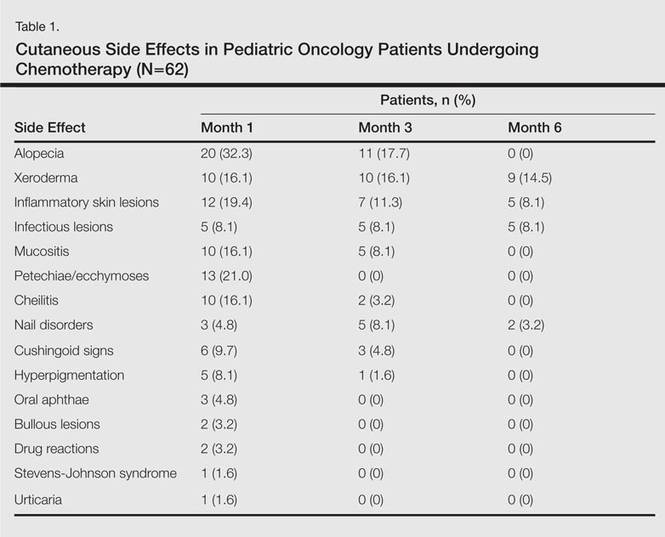
The most commonly encountered dermatologic side effect was alopecia (31/62 [50%]). Anagen effluvium (Figure 1) was detected in half of the cases, while complete scalp hair loss was noted in the rest. Alopecia was encountered more commonly in cases with central nervous system tumors (5/9 [55.6%]) and hematological malignancies (16/31 [51.6%])(Table 2).
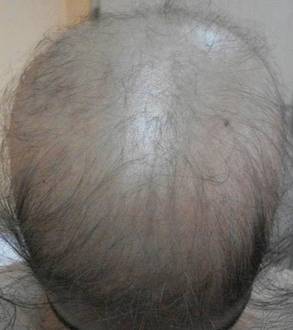
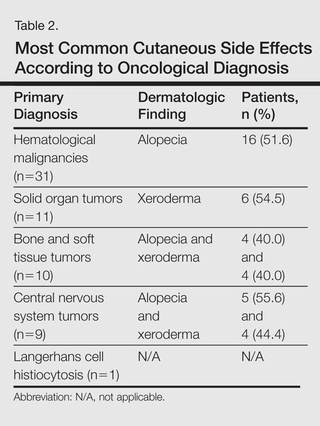
The second most commonly encountered side effect was xeroderma (29/62 [46.8%])(Figure 2). This side effect was most commonly encountered in patients with solid organ tumors (6/11 [54.5%]) and central nervous system tumors (4/9 [44.4%]), and occurred less frequently with bone and soft tissue tumors (4/10 [40.0%]).
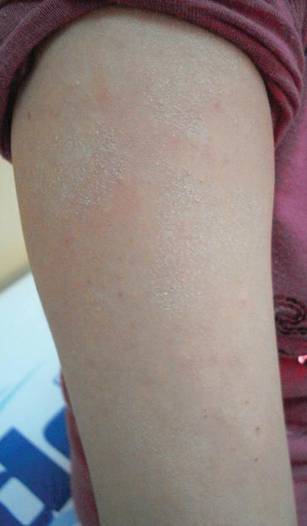
Findings of eczema accounted for the majority of inflammatory lesions, which were the third most commonly encountered side effects. Among 24 cases of inflammatory skin lesions, 8 patients (33.3%) had diaper dermatitis, 7 (29.2%) had asteatotic eczema, 6 (25.0%) had contact dermatitis, and 3 (12.5%) had seborrheic dermatitis. Although inflammatory skin lesions were commonly encountered in patients with hematological malignancies (14/31 [45.2%]), the difference was not statistically significant.
Mucositis and oral aphthous lesions were observed in 15 (24.2%) and 3 (4.8%) patients, respectively. Nail signs were noted in 10 (16.1%) patients; 4 patients had transverse streaks on the nail plates, 3 had linear streaks, 2 had nail plate fragility, and 1 had increased pigmentation at the nail bed and periungual area. Figure 3 shows linear streaks on the nail plate. These side effects were most commonly encountered in patients with solid organ tumors (5/11 [45.5%]); however, the difference was not statistically significant when compared with the other diagnostic groups.
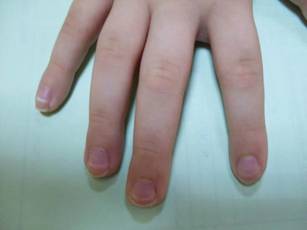
Dermatologic signs with infectious origins were detected in 15 (24.2%) patients; 2 patients had herpes labialis, 2 had verruca vulgaris, 3 had bacterial folliculitis, 1 had acute paronychia, 1 had soft tissue infection, 2 had tinea versicolor, and 4 had mucocutaneous candidiasis. Dermatologic side effects due to infectious causes were more commonly encountered in patients with bone and soft tissue tumors (4/11 [36.4%]), and the difference was statistically significant when compared with the other diagnostic groups (P=.04).
Petechiae and ecchymotic lesions were present in 13 (21.0%) patients. These side effects occurred mainly in the first month of chemotherapy, namely when patients were in the pancytopenic phase.
Comment
Variability among the oncological diagnosis and drugs used in treatment as well as increased numbers of chemotherapeutic agents available have led to many side effects and complications in pediatric oncology patients undergoing chemotherapy.1,2 Comprehensive studies regarding the cutaneous side effects of chemotherapeuticagents in cancer treatment have been conducted in adult patients. Side effects in pediatric patients have only been documented in case reports in the literature. In our study of pediatric oncology patients undergoing treatment with chemotherapy, the most commonly observed dermatologic side effect was alopecia, followed by xeroderma, inflammatory lesions, infectious lesions, mucositis, petechiae/ecchymoses, cheilitis, nail disorders, cushingoid signs, oral aphthae, bullous lesions, and drug reactions confirmed histopathologically (Table 1).
Because the common effects of chemotherapeutic agents used in cancer treatment are greatest in areas of rapidly dividing cells, the skin and skin appendages frequently are affected by these drugs.1-3 Cutaneous signs are frequently observed, especially in regions with increased mitotic activity such as the hair, mucosa, and nails.
Kamil et al1 reported that the incidence of alopecia was 64.3% (74/115) in a study of adult cancer patients who underwent chemotherapy. Chemotherapeutic agents that have commonly caused alopecia are vincristine, daunorubicin, doxorubicin, cyclophosphamide, etoposide, cytarabine, and carboplatin.1,2 In our study, alopecia was noted in 31 (50.0%) patients, especially with the use of vincristine (7/31 [22.6%]), daunorubicin (8/31 [25.8%]), doxorubicin (6/31 [19.4%]), and cyclophosphamide (10/31 [32.3%]).
Darkening of the skin and paleness accompanied the majority of cases of xeroderma in our study. Skin dryness was in an ichthyosiform appearance and was severe in 1 patient who was diagnosed with osteosarcoma. Asteatotic eczema and cheilitis were related to skin dryness. It has been reported that acquired paraneoplastic ichthyosis can develop in hematological malignancies, primarily in patients with Hodgkin lymphoma.4
The incidence of mucositis has been related to the doses of chemotherapeutic agents. Although it is a commonly encountered side effect, there is no standard treatment of mucositis; therefore, preventive care in patients undergoing chemotherapy is important. It has been reported that practicing good oral hygiene before the treatment period can decrease the incidence of mucositis.5-9 The lower incidence of mucositis in our study compared to the literature (55.6%)5 can be attributed to the lower doses of chemotherapy drugs administered to children due to their weights; they also had active oral mucosa care during chemotherapy.
Another common complication observed in our study was nail disorders. Transverse streaks commonly are encountered due to damage in the nail matrix. Other signs are increased linear streaks, longitudinal melanonychia, nail plate fragility, and onycholysis.10
Cancer patients acquire infections more frequently because of immunosuppression from chemotherapy and malignancy.11,12 In our study, cutaneous side effects with infectious causes were noted in 15 patients. Steroids, which are included in the majority of chemotherapeutic protocols, can cause cushingoid changes. Striae from rapid weight gain, acneform eruptions, hypertrichosis, and atrophy of the skin also have been observed among secondary changes to chemotherapy.1,11
Other skin signs observed in the study were acute urticaria in 1 patient (1.6%) following administration of intrathecal methotrexate; Stevens-Johnson syndrome related to voriconazole was noted in 1 (1.6%) patient.
Hyperpigmentation is a common side effect observed in oncology patients.13-15 It can be observed locally in the skin as well as the mucosa, teeth, hair, and nails, and it generally develops secondary to alkylating agents.16 Moreover, hyperpigmentation may develop in regions with occlusions (eg, electrocardiogram pads, adhesion sites of plasters), and commonly is associated with ifosfamide, etoposide, carboplatin, and cyclosporine. Although the development mechanism of hyperpigmentation related to chemotherapy drugs is not clearly known, it is thought to be due to direct toxicity, melanocyte stimulation, or postinflammatory changes.1,6,17 In our study, xeroderma was noted in some patients with hyperpigmentation; all of them had received cyclosporine and systemic steroid treatments. The other chemotherapeutics were defined as etoposide, cytarabine, dacarbazine, and ifosfamide.1 Our patients with hyperpigmentation were not taking these therapies.
Increased skin malignancies have been reported in adult cases with hematological malignancies.18 None of the patients in our study had a secondary skin malignancy, likely because we evaluated a pediatric population and the follow-up period (6 months) was too short for the development of a secondary malignancy.
Conclusion
A wide range of cutaneous side effects can be observed in pediatric oncology patients undergoing chemotherapy based on oncological diagnosis and treatment protocol. Although these side effects are not fatal, they may negatively affect morbidity and can lead to emotional distress. Knowing the possible cutaneous side effects of chemotherapy in pediatric patients and their causes is important for early diagnosis and minimal treatment.
- Kamil N, Kamil S, Ahmed SP, et al. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7-14.
- Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212-216.
- Ozkan A, Apak H, Celkan T, et al. Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside. Pediatr Dermatol. 2001;18:38-40.
- Rizos E, Milionis HJ, Pavlidis N, et al. Acquired ichthyosis: a paraneoplastic skin manifestation of Hodgkin’s disease. Lancet Oncol. 2002;3:727.
- Otmani N, Alami R, Hessissen L, et al. Determinants of severe oral mucositis in pediatric cancer patients: a prospective study. Int J Pediatr Dent. 2011;21:210-216.
- Mateus C, Robert C. New drugs in oncology and skin toxicity [in French]. Rev Med Interne. 2009;30:401-410.
- Manji A, Tomlinson D, Ethier MC, et al. Psychometric properties of the Oral Mucositis Daily Questionnaire for child self-report and importance of mucositis in children treated with chemotherapy. Support Care Cancer. 2012;20:1251-1258.
- Keefe DM. Mucositis management in patients with cancer. Support Cancer Ther. 2006;3:154-157.
- Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452-456.
- Utas S, Kulluk P. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:466-474.
- Ott H, Höger PH. Dermatologic manifestations of infections in pediatric cancer patients [in German]. Klin Padiatr. 2005;217(suppl 1):110-119.
- Ramphal R, Grant RM, Dzolganovski B, et al. Herpes simplex virus in febrile neutropenic children undergoing chemotherapy for cancer: a prospective cohort study. Pediatr Infect Dis J. 2007;26:700-704.
- Yaris N, Cakir M, Kalyoncu M, et al. Bleomycin induced hyperpigmentation with yolk sac tumor. Indian J Pediatr. 2007;74:505-506.
- Kleynberg RL, Sofi AA, Chaudhary RT. Hand-foot hyperpigmentation skin lesions associated with combination gemcitabine-carboplatin (GemCarbo) therapy. Am J Ther. 2011;18:261-263.
- Blaya M, Saba N. Chemotherapy-induced hyperpigmentation of the tongue. N Engl J Med. 2011;365:e20.
- Anandajeya WV, Corrêa ZM, Augsburger JJ. Primary acquired melanosis with atypia treated with mitomycin C. Int Ophthalmol. 2009;29:285-288.
- Torres C, Wong L, Welsh O, et al. Skin manifestations associated with chemotherapy in children with hematologic malignancies. Pediatr Dermatol. 2011;2:123-147.
- Mays SR, Cohen PR. Emerging dermatologic issues in the oncology patient. Semin Cutan Med Surg. 2006;25:179-189.
Pediatric oncology patients can present with various skin lesions related to both their primary disease and immunosuppressive treatments. In the majority of cases, cutaneous findings are associated with the use of chemotherapeutic agents. The toxic effects of chemotherapeutic agents, which generally are associated with treatment of solid organ malignancies (eg, liver, kidneys), can be detected by oncologists using clinical signs and laboratory tests.1-3 However, it also is important for dermatologists to recognize and evaluate cutaneous side effects associated with chemotherapeutic agents. Reports in the literature of cutaneous side effects of chemotherapy in pediatric patients generally are limited to case studies. This study aimed to evaluate the characteristics of cutaneous side effects of chemotherapy in pediatric oncology patients.
Materials and Methods
The study was performed through the collaboration of the departments of dermatology and venereology and pediatric oncology in the Faculty of Medicine at Ege University, Izmir, Turkey. Sixty-five pediatric oncology patients who were scheduled to undergo chemotherapy from May 2011 to May 2013 were included in the study. Clinical examination of dermatologic findings was conducted at baseline (prior to beginning chemotherapy) and at months 1, 3, and 6 of treatment. Patients were examined a total of 4 times during the study. Patients with a history of skin disease prior to diagnosis of their malignancy were excluded, as the study aimed to evaluate cutaneous side effects of chemotherapy. Patients who developed cutaneous side effects during the study period were photographed. Skin biopsy was performed to confirm clinical diagnosis. Patients were split into 5 groups according to oncological diagnoses, including hematological malignancies, solid organ tumors, bone and soft tissue tumors, central nervous system tumors, and Langerhans cell histiocytosis. Data regarding age, gender, treatments administered (ie, chemotherapeutics, antibiotics, antifungals, antivirals), and dermatologic signs were recorded. Mucocutaneous findings were classified as infectious (viral, bacterial, fungal) lesions, bullous lesions, inflammatory dermatoses (eg, diaper dermatitis, asteatotic eczema, contact dermatitis, seborrheic dermatitis), xeroderma, petechiae/ecchymoses, nail signs, alopecia, mucositis, cheilitis, oral aphthae, drug reactions confirmed by histopathology, cushingoid signs (eg, striae, acneform eruption, hypertrichosis), and cutaneous hyperpigmentation.
Statistical analysis was performed using SPSS version 15.0 and χ2 test was applied to the analysis.
Results
Of 65 patients, 62 completed the study and were included in the analysis. Three patients were excluded from the results, as 2 patients died during treatment and 1 patient withdrew from the study prior to completion. Twenty-seven (43.5%) patients were female and 35 (56.5%) were male ranging in age from 1 to 17 years (mean age, 8.14 years; median age [standard deviation], 7.25 [5.42] years). There were 31 (50%) patients in the hematological malignancies group, 11 (17.7%) in the solid organ tumors group, 10 (16.1%) in the bone and soft tissue tumors group, and 9 (14.5%) in the central nervous system tumors group; Langerhans cell histiocytosis was diagnosed in 1 (1.6%) patient. Hodgkin lymphoma made up 29.0% (n=9) of hematological malignancies. Other hematological malignancies included acute myeloblastic leukemia (n=7 [22.5%]), acute lymphoblastic leukemia (n=7 [22.5%]), T-cell lymphoma (n=5 [16.1%]), non-Hodgkin lym-phoma (n=1 [3.2%]), anaplastic giant cell lymphoma (n=1 [3.2%]), and diffuse giant cell lymphoma (n=1 [3.2%]).
In addition to chemotherapeutic agents, 7 (11.3%) patients in this study also received antibiotics and 3 (4.8%) received antivirals. The most frequently employed chemotherapeutic agents were vincristine, methotrexate, cytarabine, etoposide, and dexamethasone. Cyclophosphamide, doxorubicin, ifosfamide, asparaginase, carboplatin, procarbazine, daunorubicin, actinomycin D, vinblastine, cisplatin, bleomycin, idarubicin, 6-mercaptopurine, temozolamide, and cyclosporine also were administered. The most commonly encountered dermatological side effects were alopecia, xeroderma, inflammatory skin lesions, infectious lesions, and mucositis, respectively (Table 1). Cutaneous side effects were frequently seen at months 1 and 3 of treatment.

The most commonly encountered dermatologic side effect was alopecia (31/62 [50%]). Anagen effluvium (Figure 1) was detected in half of the cases, while complete scalp hair loss was noted in the rest. Alopecia was encountered more commonly in cases with central nervous system tumors (5/9 [55.6%]) and hematological malignancies (16/31 [51.6%])(Table 2).


The second most commonly encountered side effect was xeroderma (29/62 [46.8%])(Figure 2). This side effect was most commonly encountered in patients with solid organ tumors (6/11 [54.5%]) and central nervous system tumors (4/9 [44.4%]), and occurred less frequently with bone and soft tissue tumors (4/10 [40.0%]).

Findings of eczema accounted for the majority of inflammatory lesions, which were the third most commonly encountered side effects. Among 24 cases of inflammatory skin lesions, 8 patients (33.3%) had diaper dermatitis, 7 (29.2%) had asteatotic eczema, 6 (25.0%) had contact dermatitis, and 3 (12.5%) had seborrheic dermatitis. Although inflammatory skin lesions were commonly encountered in patients with hematological malignancies (14/31 [45.2%]), the difference was not statistically significant.
Mucositis and oral aphthous lesions were observed in 15 (24.2%) and 3 (4.8%) patients, respectively. Nail signs were noted in 10 (16.1%) patients; 4 patients had transverse streaks on the nail plates, 3 had linear streaks, 2 had nail plate fragility, and 1 had increased pigmentation at the nail bed and periungual area. Figure 3 shows linear streaks on the nail plate. These side effects were most commonly encountered in patients with solid organ tumors (5/11 [45.5%]); however, the difference was not statistically significant when compared with the other diagnostic groups.

Dermatologic signs with infectious origins were detected in 15 (24.2%) patients; 2 patients had herpes labialis, 2 had verruca vulgaris, 3 had bacterial folliculitis, 1 had acute paronychia, 1 had soft tissue infection, 2 had tinea versicolor, and 4 had mucocutaneous candidiasis. Dermatologic side effects due to infectious causes were more commonly encountered in patients with bone and soft tissue tumors (4/11 [36.4%]), and the difference was statistically significant when compared with the other diagnostic groups (P=.04).
Petechiae and ecchymotic lesions were present in 13 (21.0%) patients. These side effects occurred mainly in the first month of chemotherapy, namely when patients were in the pancytopenic phase.
Comment
Variability among the oncological diagnosis and drugs used in treatment as well as increased numbers of chemotherapeutic agents available have led to many side effects and complications in pediatric oncology patients undergoing chemotherapy.1,2 Comprehensive studies regarding the cutaneous side effects of chemotherapeuticagents in cancer treatment have been conducted in adult patients. Side effects in pediatric patients have only been documented in case reports in the literature. In our study of pediatric oncology patients undergoing treatment with chemotherapy, the most commonly observed dermatologic side effect was alopecia, followed by xeroderma, inflammatory lesions, infectious lesions, mucositis, petechiae/ecchymoses, cheilitis, nail disorders, cushingoid signs, oral aphthae, bullous lesions, and drug reactions confirmed histopathologically (Table 1).
Because the common effects of chemotherapeutic agents used in cancer treatment are greatest in areas of rapidly dividing cells, the skin and skin appendages frequently are affected by these drugs.1-3 Cutaneous signs are frequently observed, especially in regions with increased mitotic activity such as the hair, mucosa, and nails.
Kamil et al1 reported that the incidence of alopecia was 64.3% (74/115) in a study of adult cancer patients who underwent chemotherapy. Chemotherapeutic agents that have commonly caused alopecia are vincristine, daunorubicin, doxorubicin, cyclophosphamide, etoposide, cytarabine, and carboplatin.1,2 In our study, alopecia was noted in 31 (50.0%) patients, especially with the use of vincristine (7/31 [22.6%]), daunorubicin (8/31 [25.8%]), doxorubicin (6/31 [19.4%]), and cyclophosphamide (10/31 [32.3%]).
Darkening of the skin and paleness accompanied the majority of cases of xeroderma in our study. Skin dryness was in an ichthyosiform appearance and was severe in 1 patient who was diagnosed with osteosarcoma. Asteatotic eczema and cheilitis were related to skin dryness. It has been reported that acquired paraneoplastic ichthyosis can develop in hematological malignancies, primarily in patients with Hodgkin lymphoma.4
The incidence of mucositis has been related to the doses of chemotherapeutic agents. Although it is a commonly encountered side effect, there is no standard treatment of mucositis; therefore, preventive care in patients undergoing chemotherapy is important. It has been reported that practicing good oral hygiene before the treatment period can decrease the incidence of mucositis.5-9 The lower incidence of mucositis in our study compared to the literature (55.6%)5 can be attributed to the lower doses of chemotherapy drugs administered to children due to their weights; they also had active oral mucosa care during chemotherapy.
Another common complication observed in our study was nail disorders. Transverse streaks commonly are encountered due to damage in the nail matrix. Other signs are increased linear streaks, longitudinal melanonychia, nail plate fragility, and onycholysis.10
Cancer patients acquire infections more frequently because of immunosuppression from chemotherapy and malignancy.11,12 In our study, cutaneous side effects with infectious causes were noted in 15 patients. Steroids, which are included in the majority of chemotherapeutic protocols, can cause cushingoid changes. Striae from rapid weight gain, acneform eruptions, hypertrichosis, and atrophy of the skin also have been observed among secondary changes to chemotherapy.1,11
Other skin signs observed in the study were acute urticaria in 1 patient (1.6%) following administration of intrathecal methotrexate; Stevens-Johnson syndrome related to voriconazole was noted in 1 (1.6%) patient.
Hyperpigmentation is a common side effect observed in oncology patients.13-15 It can be observed locally in the skin as well as the mucosa, teeth, hair, and nails, and it generally develops secondary to alkylating agents.16 Moreover, hyperpigmentation may develop in regions with occlusions (eg, electrocardiogram pads, adhesion sites of plasters), and commonly is associated with ifosfamide, etoposide, carboplatin, and cyclosporine. Although the development mechanism of hyperpigmentation related to chemotherapy drugs is not clearly known, it is thought to be due to direct toxicity, melanocyte stimulation, or postinflammatory changes.1,6,17 In our study, xeroderma was noted in some patients with hyperpigmentation; all of them had received cyclosporine and systemic steroid treatments. The other chemotherapeutics were defined as etoposide, cytarabine, dacarbazine, and ifosfamide.1 Our patients with hyperpigmentation were not taking these therapies.
Increased skin malignancies have been reported in adult cases with hematological malignancies.18 None of the patients in our study had a secondary skin malignancy, likely because we evaluated a pediatric population and the follow-up period (6 months) was too short for the development of a secondary malignancy.
Conclusion
A wide range of cutaneous side effects can be observed in pediatric oncology patients undergoing chemotherapy based on oncological diagnosis and treatment protocol. Although these side effects are not fatal, they may negatively affect morbidity and can lead to emotional distress. Knowing the possible cutaneous side effects of chemotherapy in pediatric patients and their causes is important for early diagnosis and minimal treatment.
Pediatric oncology patients can present with various skin lesions related to both their primary disease and immunosuppressive treatments. In the majority of cases, cutaneous findings are associated with the use of chemotherapeutic agents. The toxic effects of chemotherapeutic agents, which generally are associated with treatment of solid organ malignancies (eg, liver, kidneys), can be detected by oncologists using clinical signs and laboratory tests.1-3 However, it also is important for dermatologists to recognize and evaluate cutaneous side effects associated with chemotherapeutic agents. Reports in the literature of cutaneous side effects of chemotherapy in pediatric patients generally are limited to case studies. This study aimed to evaluate the characteristics of cutaneous side effects of chemotherapy in pediatric oncology patients.
Materials and Methods
The study was performed through the collaboration of the departments of dermatology and venereology and pediatric oncology in the Faculty of Medicine at Ege University, Izmir, Turkey. Sixty-five pediatric oncology patients who were scheduled to undergo chemotherapy from May 2011 to May 2013 were included in the study. Clinical examination of dermatologic findings was conducted at baseline (prior to beginning chemotherapy) and at months 1, 3, and 6 of treatment. Patients were examined a total of 4 times during the study. Patients with a history of skin disease prior to diagnosis of their malignancy were excluded, as the study aimed to evaluate cutaneous side effects of chemotherapy. Patients who developed cutaneous side effects during the study period were photographed. Skin biopsy was performed to confirm clinical diagnosis. Patients were split into 5 groups according to oncological diagnoses, including hematological malignancies, solid organ tumors, bone and soft tissue tumors, central nervous system tumors, and Langerhans cell histiocytosis. Data regarding age, gender, treatments administered (ie, chemotherapeutics, antibiotics, antifungals, antivirals), and dermatologic signs were recorded. Mucocutaneous findings were classified as infectious (viral, bacterial, fungal) lesions, bullous lesions, inflammatory dermatoses (eg, diaper dermatitis, asteatotic eczema, contact dermatitis, seborrheic dermatitis), xeroderma, petechiae/ecchymoses, nail signs, alopecia, mucositis, cheilitis, oral aphthae, drug reactions confirmed by histopathology, cushingoid signs (eg, striae, acneform eruption, hypertrichosis), and cutaneous hyperpigmentation.
Statistical analysis was performed using SPSS version 15.0 and χ2 test was applied to the analysis.
Results
Of 65 patients, 62 completed the study and were included in the analysis. Three patients were excluded from the results, as 2 patients died during treatment and 1 patient withdrew from the study prior to completion. Twenty-seven (43.5%) patients were female and 35 (56.5%) were male ranging in age from 1 to 17 years (mean age, 8.14 years; median age [standard deviation], 7.25 [5.42] years). There were 31 (50%) patients in the hematological malignancies group, 11 (17.7%) in the solid organ tumors group, 10 (16.1%) in the bone and soft tissue tumors group, and 9 (14.5%) in the central nervous system tumors group; Langerhans cell histiocytosis was diagnosed in 1 (1.6%) patient. Hodgkin lymphoma made up 29.0% (n=9) of hematological malignancies. Other hematological malignancies included acute myeloblastic leukemia (n=7 [22.5%]), acute lymphoblastic leukemia (n=7 [22.5%]), T-cell lymphoma (n=5 [16.1%]), non-Hodgkin lym-phoma (n=1 [3.2%]), anaplastic giant cell lymphoma (n=1 [3.2%]), and diffuse giant cell lymphoma (n=1 [3.2%]).
In addition to chemotherapeutic agents, 7 (11.3%) patients in this study also received antibiotics and 3 (4.8%) received antivirals. The most frequently employed chemotherapeutic agents were vincristine, methotrexate, cytarabine, etoposide, and dexamethasone. Cyclophosphamide, doxorubicin, ifosfamide, asparaginase, carboplatin, procarbazine, daunorubicin, actinomycin D, vinblastine, cisplatin, bleomycin, idarubicin, 6-mercaptopurine, temozolamide, and cyclosporine also were administered. The most commonly encountered dermatological side effects were alopecia, xeroderma, inflammatory skin lesions, infectious lesions, and mucositis, respectively (Table 1). Cutaneous side effects were frequently seen at months 1 and 3 of treatment.

The most commonly encountered dermatologic side effect was alopecia (31/62 [50%]). Anagen effluvium (Figure 1) was detected in half of the cases, while complete scalp hair loss was noted in the rest. Alopecia was encountered more commonly in cases with central nervous system tumors (5/9 [55.6%]) and hematological malignancies (16/31 [51.6%])(Table 2).


The second most commonly encountered side effect was xeroderma (29/62 [46.8%])(Figure 2). This side effect was most commonly encountered in patients with solid organ tumors (6/11 [54.5%]) and central nervous system tumors (4/9 [44.4%]), and occurred less frequently with bone and soft tissue tumors (4/10 [40.0%]).

Findings of eczema accounted for the majority of inflammatory lesions, which were the third most commonly encountered side effects. Among 24 cases of inflammatory skin lesions, 8 patients (33.3%) had diaper dermatitis, 7 (29.2%) had asteatotic eczema, 6 (25.0%) had contact dermatitis, and 3 (12.5%) had seborrheic dermatitis. Although inflammatory skin lesions were commonly encountered in patients with hematological malignancies (14/31 [45.2%]), the difference was not statistically significant.
Mucositis and oral aphthous lesions were observed in 15 (24.2%) and 3 (4.8%) patients, respectively. Nail signs were noted in 10 (16.1%) patients; 4 patients had transverse streaks on the nail plates, 3 had linear streaks, 2 had nail plate fragility, and 1 had increased pigmentation at the nail bed and periungual area. Figure 3 shows linear streaks on the nail plate. These side effects were most commonly encountered in patients with solid organ tumors (5/11 [45.5%]); however, the difference was not statistically significant when compared with the other diagnostic groups.

Dermatologic signs with infectious origins were detected in 15 (24.2%) patients; 2 patients had herpes labialis, 2 had verruca vulgaris, 3 had bacterial folliculitis, 1 had acute paronychia, 1 had soft tissue infection, 2 had tinea versicolor, and 4 had mucocutaneous candidiasis. Dermatologic side effects due to infectious causes were more commonly encountered in patients with bone and soft tissue tumors (4/11 [36.4%]), and the difference was statistically significant when compared with the other diagnostic groups (P=.04).
Petechiae and ecchymotic lesions were present in 13 (21.0%) patients. These side effects occurred mainly in the first month of chemotherapy, namely when patients were in the pancytopenic phase.
Comment
Variability among the oncological diagnosis and drugs used in treatment as well as increased numbers of chemotherapeutic agents available have led to many side effects and complications in pediatric oncology patients undergoing chemotherapy.1,2 Comprehensive studies regarding the cutaneous side effects of chemotherapeuticagents in cancer treatment have been conducted in adult patients. Side effects in pediatric patients have only been documented in case reports in the literature. In our study of pediatric oncology patients undergoing treatment with chemotherapy, the most commonly observed dermatologic side effect was alopecia, followed by xeroderma, inflammatory lesions, infectious lesions, mucositis, petechiae/ecchymoses, cheilitis, nail disorders, cushingoid signs, oral aphthae, bullous lesions, and drug reactions confirmed histopathologically (Table 1).
Because the common effects of chemotherapeutic agents used in cancer treatment are greatest in areas of rapidly dividing cells, the skin and skin appendages frequently are affected by these drugs.1-3 Cutaneous signs are frequently observed, especially in regions with increased mitotic activity such as the hair, mucosa, and nails.
Kamil et al1 reported that the incidence of alopecia was 64.3% (74/115) in a study of adult cancer patients who underwent chemotherapy. Chemotherapeutic agents that have commonly caused alopecia are vincristine, daunorubicin, doxorubicin, cyclophosphamide, etoposide, cytarabine, and carboplatin.1,2 In our study, alopecia was noted in 31 (50.0%) patients, especially with the use of vincristine (7/31 [22.6%]), daunorubicin (8/31 [25.8%]), doxorubicin (6/31 [19.4%]), and cyclophosphamide (10/31 [32.3%]).
Darkening of the skin and paleness accompanied the majority of cases of xeroderma in our study. Skin dryness was in an ichthyosiform appearance and was severe in 1 patient who was diagnosed with osteosarcoma. Asteatotic eczema and cheilitis were related to skin dryness. It has been reported that acquired paraneoplastic ichthyosis can develop in hematological malignancies, primarily in patients with Hodgkin lymphoma.4
The incidence of mucositis has been related to the doses of chemotherapeutic agents. Although it is a commonly encountered side effect, there is no standard treatment of mucositis; therefore, preventive care in patients undergoing chemotherapy is important. It has been reported that practicing good oral hygiene before the treatment period can decrease the incidence of mucositis.5-9 The lower incidence of mucositis in our study compared to the literature (55.6%)5 can be attributed to the lower doses of chemotherapy drugs administered to children due to their weights; they also had active oral mucosa care during chemotherapy.
Another common complication observed in our study was nail disorders. Transverse streaks commonly are encountered due to damage in the nail matrix. Other signs are increased linear streaks, longitudinal melanonychia, nail plate fragility, and onycholysis.10
Cancer patients acquire infections more frequently because of immunosuppression from chemotherapy and malignancy.11,12 In our study, cutaneous side effects with infectious causes were noted in 15 patients. Steroids, which are included in the majority of chemotherapeutic protocols, can cause cushingoid changes. Striae from rapid weight gain, acneform eruptions, hypertrichosis, and atrophy of the skin also have been observed among secondary changes to chemotherapy.1,11
Other skin signs observed in the study were acute urticaria in 1 patient (1.6%) following administration of intrathecal methotrexate; Stevens-Johnson syndrome related to voriconazole was noted in 1 (1.6%) patient.
Hyperpigmentation is a common side effect observed in oncology patients.13-15 It can be observed locally in the skin as well as the mucosa, teeth, hair, and nails, and it generally develops secondary to alkylating agents.16 Moreover, hyperpigmentation may develop in regions with occlusions (eg, electrocardiogram pads, adhesion sites of plasters), and commonly is associated with ifosfamide, etoposide, carboplatin, and cyclosporine. Although the development mechanism of hyperpigmentation related to chemotherapy drugs is not clearly known, it is thought to be due to direct toxicity, melanocyte stimulation, or postinflammatory changes.1,6,17 In our study, xeroderma was noted in some patients with hyperpigmentation; all of them had received cyclosporine and systemic steroid treatments. The other chemotherapeutics were defined as etoposide, cytarabine, dacarbazine, and ifosfamide.1 Our patients with hyperpigmentation were not taking these therapies.
Increased skin malignancies have been reported in adult cases with hematological malignancies.18 None of the patients in our study had a secondary skin malignancy, likely because we evaluated a pediatric population and the follow-up period (6 months) was too short for the development of a secondary malignancy.
Conclusion
A wide range of cutaneous side effects can be observed in pediatric oncology patients undergoing chemotherapy based on oncological diagnosis and treatment protocol. Although these side effects are not fatal, they may negatively affect morbidity and can lead to emotional distress. Knowing the possible cutaneous side effects of chemotherapy in pediatric patients and their causes is important for early diagnosis and minimal treatment.
- Kamil N, Kamil S, Ahmed SP, et al. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7-14.
- Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212-216.
- Ozkan A, Apak H, Celkan T, et al. Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside. Pediatr Dermatol. 2001;18:38-40.
- Rizos E, Milionis HJ, Pavlidis N, et al. Acquired ichthyosis: a paraneoplastic skin manifestation of Hodgkin’s disease. Lancet Oncol. 2002;3:727.
- Otmani N, Alami R, Hessissen L, et al. Determinants of severe oral mucositis in pediatric cancer patients: a prospective study. Int J Pediatr Dent. 2011;21:210-216.
- Mateus C, Robert C. New drugs in oncology and skin toxicity [in French]. Rev Med Interne. 2009;30:401-410.
- Manji A, Tomlinson D, Ethier MC, et al. Psychometric properties of the Oral Mucositis Daily Questionnaire for child self-report and importance of mucositis in children treated with chemotherapy. Support Care Cancer. 2012;20:1251-1258.
- Keefe DM. Mucositis management in patients with cancer. Support Cancer Ther. 2006;3:154-157.
- Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452-456.
- Utas S, Kulluk P. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:466-474.
- Ott H, Höger PH. Dermatologic manifestations of infections in pediatric cancer patients [in German]. Klin Padiatr. 2005;217(suppl 1):110-119.
- Ramphal R, Grant RM, Dzolganovski B, et al. Herpes simplex virus in febrile neutropenic children undergoing chemotherapy for cancer: a prospective cohort study. Pediatr Infect Dis J. 2007;26:700-704.
- Yaris N, Cakir M, Kalyoncu M, et al. Bleomycin induced hyperpigmentation with yolk sac tumor. Indian J Pediatr. 2007;74:505-506.
- Kleynberg RL, Sofi AA, Chaudhary RT. Hand-foot hyperpigmentation skin lesions associated with combination gemcitabine-carboplatin (GemCarbo) therapy. Am J Ther. 2011;18:261-263.
- Blaya M, Saba N. Chemotherapy-induced hyperpigmentation of the tongue. N Engl J Med. 2011;365:e20.
- Anandajeya WV, Corrêa ZM, Augsburger JJ. Primary acquired melanosis with atypia treated with mitomycin C. Int Ophthalmol. 2009;29:285-288.
- Torres C, Wong L, Welsh O, et al. Skin manifestations associated with chemotherapy in children with hematologic malignancies. Pediatr Dermatol. 2011;2:123-147.
- Mays SR, Cohen PR. Emerging dermatologic issues in the oncology patient. Semin Cutan Med Surg. 2006;25:179-189.
- Kamil N, Kamil S, Ahmed SP, et al. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7-14.
- Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212-216.
- Ozkan A, Apak H, Celkan T, et al. Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside. Pediatr Dermatol. 2001;18:38-40.
- Rizos E, Milionis HJ, Pavlidis N, et al. Acquired ichthyosis: a paraneoplastic skin manifestation of Hodgkin’s disease. Lancet Oncol. 2002;3:727.
- Otmani N, Alami R, Hessissen L, et al. Determinants of severe oral mucositis in pediatric cancer patients: a prospective study. Int J Pediatr Dent. 2011;21:210-216.
- Mateus C, Robert C. New drugs in oncology and skin toxicity [in French]. Rev Med Interne. 2009;30:401-410.
- Manji A, Tomlinson D, Ethier MC, et al. Psychometric properties of the Oral Mucositis Daily Questionnaire for child self-report and importance of mucositis in children treated with chemotherapy. Support Care Cancer. 2012;20:1251-1258.
- Keefe DM. Mucositis management in patients with cancer. Support Cancer Ther. 2006;3:154-157.
- Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452-456.
- Utas S, Kulluk P. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:466-474.
- Ott H, Höger PH. Dermatologic manifestations of infections in pediatric cancer patients [in German]. Klin Padiatr. 2005;217(suppl 1):110-119.
- Ramphal R, Grant RM, Dzolganovski B, et al. Herpes simplex virus in febrile neutropenic children undergoing chemotherapy for cancer: a prospective cohort study. Pediatr Infect Dis J. 2007;26:700-704.
- Yaris N, Cakir M, Kalyoncu M, et al. Bleomycin induced hyperpigmentation with yolk sac tumor. Indian J Pediatr. 2007;74:505-506.
- Kleynberg RL, Sofi AA, Chaudhary RT. Hand-foot hyperpigmentation skin lesions associated with combination gemcitabine-carboplatin (GemCarbo) therapy. Am J Ther. 2011;18:261-263.
- Blaya M, Saba N. Chemotherapy-induced hyperpigmentation of the tongue. N Engl J Med. 2011;365:e20.
- Anandajeya WV, Corrêa ZM, Augsburger JJ. Primary acquired melanosis with atypia treated with mitomycin C. Int Ophthalmol. 2009;29:285-288.
- Torres C, Wong L, Welsh O, et al. Skin manifestations associated with chemotherapy in children with hematologic malignancies. Pediatr Dermatol. 2011;2:123-147.
- Mays SR, Cohen PR. Emerging dermatologic issues in the oncology patient. Semin Cutan Med Surg. 2006;25:179-189.
Practice Points
- Chemotherapeutic agents can cause a variety of cutaneous side effects.
- Pediatric oncology patients should be examined regularly for cutaneous side effects of chemotherapeutics.
Neonatal and Infantile Acne Vulgaris: An Update
Acne vulgaris typically is associated with adolescence and young adulthood; however, it also can affect neonates, infants, and small children.1 Acne neonatorum occurs in up to 20% of newborns. The clinical importance of neonatal acne lies in its differentiation from infectious diseases, the exclusion of virilization as its underlying cause, and the possible implication of severe acne in adolescence.2 Neonatal acne also must be distinguished from acne that is induced by application of topical oils and ointments (acne venenata) and from acneform eruptions induced by acnegenic maternal medications such as hydantoin (fetal hydantoin syndrome) and lithium.3
Neonatal Acne (Acne Neonatorum)
Clinical Presentation
Neonatal acne (acne neonatorum) typically presents as small closed comedones on the forehead, nose, and cheeks (Figure 1).4 Accompanying sebaceous hyperplasia often is noted.5 Less frequently, open comedones, inflammatory papules, and pustules may develop.6 Neonatal acne may be evident at birth or appear during the first 4 weeks of life7 and is more commonly seen in boys.8
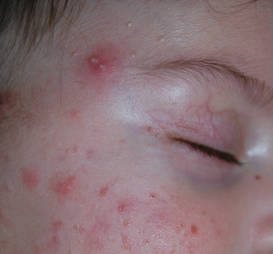
Etiology
Several factors may be pivotal in the etiology of neonatal acne, including increased sebum excretion, stimulation of the sebaceous glands by maternal or neonatal androgens,4 and colonization of sebaceous glands by Malassezia species.2 Increased sebum excretion occurs during the neonatal period due to enlarged sebaceous glands,2 which may result from the substantial production of β-hydroxysteroids from the relatively large adrenal glands.9,10 After 6 months of age, the size of the sebaceous glands and the sebum excretion rate decrease.9,10
Both maternal and neonatal androgens have been implicated in the stimulation of sebaceous glands in neonatal acne.2 The neonatal adrenal gland produces high levels of dehydroepiandrosterone,2 which stimulate sebaceous glands until around 1 year of age when dehydroepiandrosterone levels drop off as a consequence of involution of the neonatal adrenal gland.11 Testicular androgens provide additional stimulation to the sebaceous glands, which may explain why neonatal acne is more common in boys.1 Neonatal acne may be an inflammatory response to Malassezia species; however, Malassezia was not isolated in a series of patients,12 suggesting that neonatal acne is an early presentation of comedonal acne and not a response to Malassezia.2,12
Differential Diagnosis
There are a number of acneform eruptions that should be considered in the differential diagnosis,3 including bacterial folliculitis, secondary syphilis,13 herpes simplex virus and varicella zoster virus,14 and skin colonization by fungi of Malassezia species.15 Other neonatal eruptions such as erythema toxicum neonatorum,16 transient neonatal pustular melanosis, and milia and pustular miliaria, as well as a drug eruption associated with hydantoin, lithium, or halogens should be considered.17 The relationship between neonatal acne and neonatal cephalic pustulosis, which is characterized by papules and pustules without comedones, is controversial; some consider them to be 2 different entities,14 while others do not.18
Treatment
Guardians should be reassured that neonatal acne is mild, self-limited, and generally resolves spontaneously without scarring in approximately 1 to 3 months.1,2 In most cases, no treatment is needed.19 If necessary, comedones may be treated with azelaic acid cream 20% or tretinoin cream 0.025% to 0.05%.1,2 For inflammatory lesions, erythromycin solution 2% and benzoyl peroxide gel 2.5% may be used.1,20 Severe or recalcitrant disease warrants a workup for congenital adrenal hyperplasia, a virilizing tumor, or underlying endocrinopathy.19
Infantile Acne Vulgaris
Clinical Presentation
Infantile acne vulgaris shares similarities with neonatal acne21,22 in that they both affect the face, predominantly the cheeks, and have a male predominance (Figure 2).1,10 However, by definition, onset of infantile acne typically occurs later than acne neonatorum, usually at 3 to 6 months of age.1,4 Lesions are more pleomorphic and inflammatory than in neonatal acne. In addition to closed and open comedones, infantile acne may be first evident with papules, pustules, severe nodules, and cysts with scarring potential (Figure 3).1,2,5 Accordingly, treatment may be required. Most cases of infantile acne resolve by 4 or 5 years of age, but some remain active into puberty.1 Patients with a history of infantile acne have an increased incidence of acne vulgaris during adolescence compared to their peers, with greater severity and enhanced risk for scarring.4,23

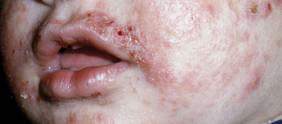
Etiology
The etiology of infantile acne remains unclear.2 Similar to neonatal acne, infantile acne may be a result of elevated androgens produced by the fetal adrenal glands as well as by the testes in males.11 For example, a child with infantile acne had elevated luteinizing hormone, follicle-stimulating hormone, and testosterone levels.24 Therefore, hyperandrogenism should be considered as an etiology. Other causes also have been suggested. Rarely, an adrenocortical tumor may be associated with persistent infantile acne with signs of virilization and rapid development.25Malassezia was implicated in infantile acne in a 6-month-old infant who was successfully treated with ketoconazole cream 2%.26
Differential Diagnosis
Infantile acne often is misdiagnosed because it is rarely considered in the differential diagnosis. When closed comedones predominate, acne venenata induced by topical creams, lotions, or oils may be etiologic. Chloracne also should be considered.14
Treatment
Guardians should be educated about the likely chronicity of infantile acne, which may require long-term treatment, as well as the possibility that acne may recur in severe form during puberty.1 The treatment strategy for infantile acne is similar to treatment of acne at any age, with topical agents including retinoids (eg, tretinoin, benzoyl peroxide) and topical antibacterials (eg, erythromycin). Twice-daily erythromycin 125 to 250 mg is the treatment of choice when oral antibiotics are indicated. Tetracyclines are contraindicated in treatment of neonatal and infantile acne. Intralesional injections with low-concentration triamcinolone acetonide, cryotherapy, or topical corticosteroids for a short period of time can be used to treat deep nodules and cysts.2 Acne that is refractory to treatment with oral antibiotics alone or combined with topical treatments poses a dilemma, given the potential cosmetic sequelae of scarring and quality-of-life concerns. Because reducing or eliminating dairy intake appears beneficial for adolescents with moderate to severe acne,27 this approach may represent a good option for infantile acne.
Conclusion
Neonatal and infantile acne vulgaris may be overlooked or misdiagnosed. It is important to consider and treat. Early childhood acne may represent a virilization syndrome.
- Jansen T, Burgdorf WH, Plewig G. Pathogenesis and treatment of acne in childhood. Pediatr Dermatol. 1997;14:17-21.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Kuflik JH, Schwartz RA. Acneiform eruptions. Cutis. 2000;66:97-100.
- Barbareschi M, Benardon S, Guanziroli E, et al. Classification and grading. In: Schwartz RA, Micali G, eds. Acne. Gurgaon, India: Nature Publishing Group; 2013:67-75.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Nanda S, Reddy BS, Ramji S, et al. Analytical study of pustular eruptions in neonates. Pediatr Dermatol. 2002;19:210-215.
- Yonkosky DM, Pochi PE. Acne vulgaris in childhood. pathogenesis and management. Dermatol Clin. 1986;4:127-136.
- Agache P, Blanc D, Barrand C, et al. Sebum levels during the first year of life. Br J Dermatol. 1980;103:643-649.
- Herane MI, Ando I. Acne in infancy and acne genetics. Dermatology. 2003;206:24-28.
- Lucky AW. A review of infantile and pediatric acne. Dermatology (Basel, Switzerland). 1998;103:643-649.
- Bernier V, Weill FX, Hirigoyen V, et al. Skin colonization by Malassezia species in neonates: a prospective study and relationship with neonatal cephalic pustulosis. Arch Dermatol. 2002;138:215-218.
- Lambert WC, Bagley MP, Khan Y, et al. Pustular acneiform secondary syphilis. Cutis. 1986;37:69-70.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Borton LK, Schwartz RA. Pityrosporum folliculitis: a common acneiform condition of middle age. Ariz Med. 1981;38:598-601.
- Morgan AJ, Steen CJ, Schwartz RA, et al. Erythema toxicum neonatorum revisited. Cutis. 2009;83:13-16.
- Brodkin RH, Schwartz RA. Cutaneous signs of dioxin exposure. Am Fam Physician. 1984;30:189-194.
- Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
- Katsambas AD, Katoulis AC, Stavropoulos P. Acne neonatorum: a study of 22 cases. Int J Dermatol. 1999;38:128-130.
- Van Praag MC, Van Rooij RW, Folkers E, et al. Diagnosis and treatment of pustular disorders in the neonate. Pediatr Dermatol. 1997;14:131-143.
- Barnes CJ, Eichenfield LF, Lee J, et al. A practical approach for the use of oral isotretinoin for infantile acne. Pediatr Dermatol. 2005;22:166-169.
- Janniger CK. Neonatal and infantile acne vulgaris. Cutis. 1993;52:16.
- Chew EW, Bingham A, Burrows D. Incidence of acne vulgaris in patients with infantile acne. Clin Exp Dermatol. 1990;15:376-377.
- Duke EM. Infantile acne associated with transient increases in plasma concentrations of luteinising hormone, follicle-stimulating hormone, and testosterone. Br Med J (Clinical Res Ed). 1981;282:1275-1276.
- Mann MW, Ellis SS, Mallory SB. Infantile acne as the initial sign of an adrenocortical tumor [published online ahead of print September 14, 2006]. J Am Acad Dermatol. 2007;56(suppl 2):S15-S18.
- Kang SK, Jee MS, Choi JH, et al. A case of infantile acne due to Pityrosporum. Pediatr Dermatol. 2003;20:68-70.
- Di Landro A, Cazzaniga S, Parazzini F, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults [published online ahead of print March 3, 2012]. J Am Acad Dermatol. 2012;67:1129-1135.
Acne vulgaris typically is associated with adolescence and young adulthood; however, it also can affect neonates, infants, and small children.1 Acne neonatorum occurs in up to 20% of newborns. The clinical importance of neonatal acne lies in its differentiation from infectious diseases, the exclusion of virilization as its underlying cause, and the possible implication of severe acne in adolescence.2 Neonatal acne also must be distinguished from acne that is induced by application of topical oils and ointments (acne venenata) and from acneform eruptions induced by acnegenic maternal medications such as hydantoin (fetal hydantoin syndrome) and lithium.3
Neonatal Acne (Acne Neonatorum)
Clinical Presentation
Neonatal acne (acne neonatorum) typically presents as small closed comedones on the forehead, nose, and cheeks (Figure 1).4 Accompanying sebaceous hyperplasia often is noted.5 Less frequently, open comedones, inflammatory papules, and pustules may develop.6 Neonatal acne may be evident at birth or appear during the first 4 weeks of life7 and is more commonly seen in boys.8

Etiology
Several factors may be pivotal in the etiology of neonatal acne, including increased sebum excretion, stimulation of the sebaceous glands by maternal or neonatal androgens,4 and colonization of sebaceous glands by Malassezia species.2 Increased sebum excretion occurs during the neonatal period due to enlarged sebaceous glands,2 which may result from the substantial production of β-hydroxysteroids from the relatively large adrenal glands.9,10 After 6 months of age, the size of the sebaceous glands and the sebum excretion rate decrease.9,10
Both maternal and neonatal androgens have been implicated in the stimulation of sebaceous glands in neonatal acne.2 The neonatal adrenal gland produces high levels of dehydroepiandrosterone,2 which stimulate sebaceous glands until around 1 year of age when dehydroepiandrosterone levels drop off as a consequence of involution of the neonatal adrenal gland.11 Testicular androgens provide additional stimulation to the sebaceous glands, which may explain why neonatal acne is more common in boys.1 Neonatal acne may be an inflammatory response to Malassezia species; however, Malassezia was not isolated in a series of patients,12 suggesting that neonatal acne is an early presentation of comedonal acne and not a response to Malassezia.2,12
Differential Diagnosis
There are a number of acneform eruptions that should be considered in the differential diagnosis,3 including bacterial folliculitis, secondary syphilis,13 herpes simplex virus and varicella zoster virus,14 and skin colonization by fungi of Malassezia species.15 Other neonatal eruptions such as erythema toxicum neonatorum,16 transient neonatal pustular melanosis, and milia and pustular miliaria, as well as a drug eruption associated with hydantoin, lithium, or halogens should be considered.17 The relationship between neonatal acne and neonatal cephalic pustulosis, which is characterized by papules and pustules without comedones, is controversial; some consider them to be 2 different entities,14 while others do not.18
Treatment
Guardians should be reassured that neonatal acne is mild, self-limited, and generally resolves spontaneously without scarring in approximately 1 to 3 months.1,2 In most cases, no treatment is needed.19 If necessary, comedones may be treated with azelaic acid cream 20% or tretinoin cream 0.025% to 0.05%.1,2 For inflammatory lesions, erythromycin solution 2% and benzoyl peroxide gel 2.5% may be used.1,20 Severe or recalcitrant disease warrants a workup for congenital adrenal hyperplasia, a virilizing tumor, or underlying endocrinopathy.19
Infantile Acne Vulgaris
Clinical Presentation
Infantile acne vulgaris shares similarities with neonatal acne21,22 in that they both affect the face, predominantly the cheeks, and have a male predominance (Figure 2).1,10 However, by definition, onset of infantile acne typically occurs later than acne neonatorum, usually at 3 to 6 months of age.1,4 Lesions are more pleomorphic and inflammatory than in neonatal acne. In addition to closed and open comedones, infantile acne may be first evident with papules, pustules, severe nodules, and cysts with scarring potential (Figure 3).1,2,5 Accordingly, treatment may be required. Most cases of infantile acne resolve by 4 or 5 years of age, but some remain active into puberty.1 Patients with a history of infantile acne have an increased incidence of acne vulgaris during adolescence compared to their peers, with greater severity and enhanced risk for scarring.4,23


Etiology
The etiology of infantile acne remains unclear.2 Similar to neonatal acne, infantile acne may be a result of elevated androgens produced by the fetal adrenal glands as well as by the testes in males.11 For example, a child with infantile acne had elevated luteinizing hormone, follicle-stimulating hormone, and testosterone levels.24 Therefore, hyperandrogenism should be considered as an etiology. Other causes also have been suggested. Rarely, an adrenocortical tumor may be associated with persistent infantile acne with signs of virilization and rapid development.25Malassezia was implicated in infantile acne in a 6-month-old infant who was successfully treated with ketoconazole cream 2%.26
Differential Diagnosis
Infantile acne often is misdiagnosed because it is rarely considered in the differential diagnosis. When closed comedones predominate, acne venenata induced by topical creams, lotions, or oils may be etiologic. Chloracne also should be considered.14
Treatment
Guardians should be educated about the likely chronicity of infantile acne, which may require long-term treatment, as well as the possibility that acne may recur in severe form during puberty.1 The treatment strategy for infantile acne is similar to treatment of acne at any age, with topical agents including retinoids (eg, tretinoin, benzoyl peroxide) and topical antibacterials (eg, erythromycin). Twice-daily erythromycin 125 to 250 mg is the treatment of choice when oral antibiotics are indicated. Tetracyclines are contraindicated in treatment of neonatal and infantile acne. Intralesional injections with low-concentration triamcinolone acetonide, cryotherapy, or topical corticosteroids for a short period of time can be used to treat deep nodules and cysts.2 Acne that is refractory to treatment with oral antibiotics alone or combined with topical treatments poses a dilemma, given the potential cosmetic sequelae of scarring and quality-of-life concerns. Because reducing or eliminating dairy intake appears beneficial for adolescents with moderate to severe acne,27 this approach may represent a good option for infantile acne.
Conclusion
Neonatal and infantile acne vulgaris may be overlooked or misdiagnosed. It is important to consider and treat. Early childhood acne may represent a virilization syndrome.
Acne vulgaris typically is associated with adolescence and young adulthood; however, it also can affect neonates, infants, and small children.1 Acne neonatorum occurs in up to 20% of newborns. The clinical importance of neonatal acne lies in its differentiation from infectious diseases, the exclusion of virilization as its underlying cause, and the possible implication of severe acne in adolescence.2 Neonatal acne also must be distinguished from acne that is induced by application of topical oils and ointments (acne venenata) and from acneform eruptions induced by acnegenic maternal medications such as hydantoin (fetal hydantoin syndrome) and lithium.3
Neonatal Acne (Acne Neonatorum)
Clinical Presentation
Neonatal acne (acne neonatorum) typically presents as small closed comedones on the forehead, nose, and cheeks (Figure 1).4 Accompanying sebaceous hyperplasia often is noted.5 Less frequently, open comedones, inflammatory papules, and pustules may develop.6 Neonatal acne may be evident at birth or appear during the first 4 weeks of life7 and is more commonly seen in boys.8

Etiology
Several factors may be pivotal in the etiology of neonatal acne, including increased sebum excretion, stimulation of the sebaceous glands by maternal or neonatal androgens,4 and colonization of sebaceous glands by Malassezia species.2 Increased sebum excretion occurs during the neonatal period due to enlarged sebaceous glands,2 which may result from the substantial production of β-hydroxysteroids from the relatively large adrenal glands.9,10 After 6 months of age, the size of the sebaceous glands and the sebum excretion rate decrease.9,10
Both maternal and neonatal androgens have been implicated in the stimulation of sebaceous glands in neonatal acne.2 The neonatal adrenal gland produces high levels of dehydroepiandrosterone,2 which stimulate sebaceous glands until around 1 year of age when dehydroepiandrosterone levels drop off as a consequence of involution of the neonatal adrenal gland.11 Testicular androgens provide additional stimulation to the sebaceous glands, which may explain why neonatal acne is more common in boys.1 Neonatal acne may be an inflammatory response to Malassezia species; however, Malassezia was not isolated in a series of patients,12 suggesting that neonatal acne is an early presentation of comedonal acne and not a response to Malassezia.2,12
Differential Diagnosis
There are a number of acneform eruptions that should be considered in the differential diagnosis,3 including bacterial folliculitis, secondary syphilis,13 herpes simplex virus and varicella zoster virus,14 and skin colonization by fungi of Malassezia species.15 Other neonatal eruptions such as erythema toxicum neonatorum,16 transient neonatal pustular melanosis, and milia and pustular miliaria, as well as a drug eruption associated with hydantoin, lithium, or halogens should be considered.17 The relationship between neonatal acne and neonatal cephalic pustulosis, which is characterized by papules and pustules without comedones, is controversial; some consider them to be 2 different entities,14 while others do not.18
Treatment
Guardians should be reassured that neonatal acne is mild, self-limited, and generally resolves spontaneously without scarring in approximately 1 to 3 months.1,2 In most cases, no treatment is needed.19 If necessary, comedones may be treated with azelaic acid cream 20% or tretinoin cream 0.025% to 0.05%.1,2 For inflammatory lesions, erythromycin solution 2% and benzoyl peroxide gel 2.5% may be used.1,20 Severe or recalcitrant disease warrants a workup for congenital adrenal hyperplasia, a virilizing tumor, or underlying endocrinopathy.19
Infantile Acne Vulgaris
Clinical Presentation
Infantile acne vulgaris shares similarities with neonatal acne21,22 in that they both affect the face, predominantly the cheeks, and have a male predominance (Figure 2).1,10 However, by definition, onset of infantile acne typically occurs later than acne neonatorum, usually at 3 to 6 months of age.1,4 Lesions are more pleomorphic and inflammatory than in neonatal acne. In addition to closed and open comedones, infantile acne may be first evident with papules, pustules, severe nodules, and cysts with scarring potential (Figure 3).1,2,5 Accordingly, treatment may be required. Most cases of infantile acne resolve by 4 or 5 years of age, but some remain active into puberty.1 Patients with a history of infantile acne have an increased incidence of acne vulgaris during adolescence compared to their peers, with greater severity and enhanced risk for scarring.4,23


Etiology
The etiology of infantile acne remains unclear.2 Similar to neonatal acne, infantile acne may be a result of elevated androgens produced by the fetal adrenal glands as well as by the testes in males.11 For example, a child with infantile acne had elevated luteinizing hormone, follicle-stimulating hormone, and testosterone levels.24 Therefore, hyperandrogenism should be considered as an etiology. Other causes also have been suggested. Rarely, an adrenocortical tumor may be associated with persistent infantile acne with signs of virilization and rapid development.25Malassezia was implicated in infantile acne in a 6-month-old infant who was successfully treated with ketoconazole cream 2%.26
Differential Diagnosis
Infantile acne often is misdiagnosed because it is rarely considered in the differential diagnosis. When closed comedones predominate, acne venenata induced by topical creams, lotions, or oils may be etiologic. Chloracne also should be considered.14
Treatment
Guardians should be educated about the likely chronicity of infantile acne, which may require long-term treatment, as well as the possibility that acne may recur in severe form during puberty.1 The treatment strategy for infantile acne is similar to treatment of acne at any age, with topical agents including retinoids (eg, tretinoin, benzoyl peroxide) and topical antibacterials (eg, erythromycin). Twice-daily erythromycin 125 to 250 mg is the treatment of choice when oral antibiotics are indicated. Tetracyclines are contraindicated in treatment of neonatal and infantile acne. Intralesional injections with low-concentration triamcinolone acetonide, cryotherapy, or topical corticosteroids for a short period of time can be used to treat deep nodules and cysts.2 Acne that is refractory to treatment with oral antibiotics alone or combined with topical treatments poses a dilemma, given the potential cosmetic sequelae of scarring and quality-of-life concerns. Because reducing or eliminating dairy intake appears beneficial for adolescents with moderate to severe acne,27 this approach may represent a good option for infantile acne.
Conclusion
Neonatal and infantile acne vulgaris may be overlooked or misdiagnosed. It is important to consider and treat. Early childhood acne may represent a virilization syndrome.
- Jansen T, Burgdorf WH, Plewig G. Pathogenesis and treatment of acne in childhood. Pediatr Dermatol. 1997;14:17-21.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Kuflik JH, Schwartz RA. Acneiform eruptions. Cutis. 2000;66:97-100.
- Barbareschi M, Benardon S, Guanziroli E, et al. Classification and grading. In: Schwartz RA, Micali G, eds. Acne. Gurgaon, India: Nature Publishing Group; 2013:67-75.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Nanda S, Reddy BS, Ramji S, et al. Analytical study of pustular eruptions in neonates. Pediatr Dermatol. 2002;19:210-215.
- Yonkosky DM, Pochi PE. Acne vulgaris in childhood. pathogenesis and management. Dermatol Clin. 1986;4:127-136.
- Agache P, Blanc D, Barrand C, et al. Sebum levels during the first year of life. Br J Dermatol. 1980;103:643-649.
- Herane MI, Ando I. Acne in infancy and acne genetics. Dermatology. 2003;206:24-28.
- Lucky AW. A review of infantile and pediatric acne. Dermatology (Basel, Switzerland). 1998;103:643-649.
- Bernier V, Weill FX, Hirigoyen V, et al. Skin colonization by Malassezia species in neonates: a prospective study and relationship with neonatal cephalic pustulosis. Arch Dermatol. 2002;138:215-218.
- Lambert WC, Bagley MP, Khan Y, et al. Pustular acneiform secondary syphilis. Cutis. 1986;37:69-70.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Borton LK, Schwartz RA. Pityrosporum folliculitis: a common acneiform condition of middle age. Ariz Med. 1981;38:598-601.
- Morgan AJ, Steen CJ, Schwartz RA, et al. Erythema toxicum neonatorum revisited. Cutis. 2009;83:13-16.
- Brodkin RH, Schwartz RA. Cutaneous signs of dioxin exposure. Am Fam Physician. 1984;30:189-194.
- Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
- Katsambas AD, Katoulis AC, Stavropoulos P. Acne neonatorum: a study of 22 cases. Int J Dermatol. 1999;38:128-130.
- Van Praag MC, Van Rooij RW, Folkers E, et al. Diagnosis and treatment of pustular disorders in the neonate. Pediatr Dermatol. 1997;14:131-143.
- Barnes CJ, Eichenfield LF, Lee J, et al. A practical approach for the use of oral isotretinoin for infantile acne. Pediatr Dermatol. 2005;22:166-169.
- Janniger CK. Neonatal and infantile acne vulgaris. Cutis. 1993;52:16.
- Chew EW, Bingham A, Burrows D. Incidence of acne vulgaris in patients with infantile acne. Clin Exp Dermatol. 1990;15:376-377.
- Duke EM. Infantile acne associated with transient increases in plasma concentrations of luteinising hormone, follicle-stimulating hormone, and testosterone. Br Med J (Clinical Res Ed). 1981;282:1275-1276.
- Mann MW, Ellis SS, Mallory SB. Infantile acne as the initial sign of an adrenocortical tumor [published online ahead of print September 14, 2006]. J Am Acad Dermatol. 2007;56(suppl 2):S15-S18.
- Kang SK, Jee MS, Choi JH, et al. A case of infantile acne due to Pityrosporum. Pediatr Dermatol. 2003;20:68-70.
- Di Landro A, Cazzaniga S, Parazzini F, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults [published online ahead of print March 3, 2012]. J Am Acad Dermatol. 2012;67:1129-1135.
- Jansen T, Burgdorf WH, Plewig G. Pathogenesis and treatment of acne in childhood. Pediatr Dermatol. 1997;14:17-21.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Kuflik JH, Schwartz RA. Acneiform eruptions. Cutis. 2000;66:97-100.
- Barbareschi M, Benardon S, Guanziroli E, et al. Classification and grading. In: Schwartz RA, Micali G, eds. Acne. Gurgaon, India: Nature Publishing Group; 2013:67-75.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Nanda S, Reddy BS, Ramji S, et al. Analytical study of pustular eruptions in neonates. Pediatr Dermatol. 2002;19:210-215.
- Yonkosky DM, Pochi PE. Acne vulgaris in childhood. pathogenesis and management. Dermatol Clin. 1986;4:127-136.
- Agache P, Blanc D, Barrand C, et al. Sebum levels during the first year of life. Br J Dermatol. 1980;103:643-649.
- Herane MI, Ando I. Acne in infancy and acne genetics. Dermatology. 2003;206:24-28.
- Lucky AW. A review of infantile and pediatric acne. Dermatology (Basel, Switzerland). 1998;103:643-649.
- Bernier V, Weill FX, Hirigoyen V, et al. Skin colonization by Malassezia species in neonates: a prospective study and relationship with neonatal cephalic pustulosis. Arch Dermatol. 2002;138:215-218.
- Lambert WC, Bagley MP, Khan Y, et al. Pustular acneiform secondary syphilis. Cutis. 1986;37:69-70.
- Antoniou C, Dessinioti C, Stratigos AJ, et al. Clinical and therapeutic approach to childhood acne: an update. Pediatr Dermatol. 2009;26:373-380.
- Borton LK, Schwartz RA. Pityrosporum folliculitis: a common acneiform condition of middle age. Ariz Med. 1981;38:598-601.
- Morgan AJ, Steen CJ, Schwartz RA, et al. Erythema toxicum neonatorum revisited. Cutis. 2009;83:13-16.
- Brodkin RH, Schwartz RA. Cutaneous signs of dioxin exposure. Am Fam Physician. 1984;30:189-194.
- Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
- Katsambas AD, Katoulis AC, Stavropoulos P. Acne neonatorum: a study of 22 cases. Int J Dermatol. 1999;38:128-130.
- Van Praag MC, Van Rooij RW, Folkers E, et al. Diagnosis and treatment of pustular disorders in the neonate. Pediatr Dermatol. 1997;14:131-143.
- Barnes CJ, Eichenfield LF, Lee J, et al. A practical approach for the use of oral isotretinoin for infantile acne. Pediatr Dermatol. 2005;22:166-169.
- Janniger CK. Neonatal and infantile acne vulgaris. Cutis. 1993;52:16.
- Chew EW, Bingham A, Burrows D. Incidence of acne vulgaris in patients with infantile acne. Clin Exp Dermatol. 1990;15:376-377.
- Duke EM. Infantile acne associated with transient increases in plasma concentrations of luteinising hormone, follicle-stimulating hormone, and testosterone. Br Med J (Clinical Res Ed). 1981;282:1275-1276.
- Mann MW, Ellis SS, Mallory SB. Infantile acne as the initial sign of an adrenocortical tumor [published online ahead of print September 14, 2006]. J Am Acad Dermatol. 2007;56(suppl 2):S15-S18.
- Kang SK, Jee MS, Choi JH, et al. A case of infantile acne due to Pityrosporum. Pediatr Dermatol. 2003;20:68-70.
- Di Landro A, Cazzaniga S, Parazzini F, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults [published online ahead of print March 3, 2012]. J Am Acad Dermatol. 2012;67:1129-1135.
Practice Points
- Infantile acne needs to be recognized and treated.
- Acne in early childhood may represent virilization.
Congenital Candidiasis: An Uncommon Skin Eruption Presenting at Birth
Ulerythema Ophryogenes: Updates and Insights
Test your knowledge on ulerythema ophryogenes with MD-IQ: the medical intelligence quiz. Click here to answer 5 questions.