User login
Paradoxical Eczema Risk Low With Biologic Psoriasis Treatments
examined in a large observational analysis.
Using data from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) database, Ali Al-Janabi, MA, from the University of Manchester (England) and associates found that 273 (1%) of approximately 25,000 drug exposures in 13,699 biologic-treated patients with psoriasis were associated with paradoxical eczema.
The incidence of paradoxical eczema was found to vary by class. The highest rate was seen for IL-17 inhibitors, at 1.22 per 100,000 person-years, and the lowest rate was seen with IL-23 inhibitors, at 0.56 per 100,000 person-years. The respective incidence rates for tumor necrosis factor (TNF) inhibitors and IL-12/IL-23 inhibitors were a respective 0.94 and 0.80 per 100,000 person-years.
“Compared with TNF inhibitors, IL-23 inhibitor exposure was associated with significantly lower risk of paradoxical eczema,” the BADBIR Study Group reported in JAMA Dermatology. Indeed, patients treated with IL-23 inhibitors were 61% less likely than were those taking TNF-inhibitors to experience a paradoxical eczema event.
“These findings remained when restricting the analysis to first-line biologic exposures and were specific to this eczema phenotype” the group said.
Cautious Interpretation
As the corresponding author for the work, Mr. Al-Janabi observed in an email that the research needs to be replicated, and the findings need to be interpreted with caution.
“As well as usual clinical variables influencing biologic selection, clinicians could consider IL-23 inhibitors in patients with previous atopic dermatitis, hay fever, or paradoxical eczema episodes, as this class was associated with the lowest risk of paradoxical eczema,” he suggested.
A prior history of atopic dermatitis (AD) and hay fever appears to be particularly relevant, as both substantially upped the chances that paradoxical eczema would occur, with hazard ratios of 12.40 and 3.78, respectively. Increasing age also increased the risk, albeit slightly (hazard ratio [HR], 1.02 per year), and there was an apparent lower risk (HR, 0.60) comparing men and women.
The BADBIR Study Group authors believe that, to the best of their knowledge, this is the first study to compare paradoxical eczema risk by biologic class. “Based on clinical experience and prevalence of eczematous reactions reported in some IL-17 inhibitor clinical trials, we suspected an association between IL-17 inhibitor exposure and paradoxical eczema,” they wrote.
“While the incidence of paradoxical eczema was numerically highest among IL-17 inhibitor exposures, it was not significantly different from the incidence among TNF inhibitor exposures.” The low overall incidence of paradoxical eczema “may be reassuring for patients and clinicians,” they added, “but it is possible that the incidence was underestimated due to underreporting or exclusion of adverse events with insufficient detail.”
Details of the Analysis, Other Findings
To explore the risk of paradoxical eczema by biologic class and identify possible risk factors, the BADBIR Study Group performed a prospective cohort study using data held within the BADBIR database between September 2007 and December 2022.
Adults over the age of 18 year or older with plaque psoriasis and who had been treated with at least one of the following biologics were eligible for inclusion: the TNF inhibitors adalimumab, certolizumab pegol, etanercept, and infliximab; the IL-17 inhibitors bimekizumab, brodalumab, ixekizumab, and secukinumab; the IL-12/23 inhibitor ustekinumab; and the IL-23 inhibitors guselkumab, risankizumab, and tildrakizumab.
Patient records and adverse event data were reviewed to determine the incidence of paradoxical eczema events, using terms such as eczema, eczematized, eczematous, atopy, atopic, and dermatitis.
Of 24,952 drug exposures analyzed, the majority (11,819) were for TNF inhibitors, followed by IL-17 inhibitors (4,776), IL-12/23 inhibitors (6,423), and finally, IL-23 inhibitors (1,934).
Mr. Al-Janabi and coauthors reported that the median time to onset of paradoxical eczema events was 294 days — approximately 9.8 months. The earliest that these events were recorded was at 120 days (4 months), and the latest at 699 days (almost 2 years).
The face and neck were the most common sites affected (26% of exposures), with other sites including the limbs (23%), the trunk (13%), and hands or feet (12%). Itching (18%), redness (7%), and dryness (4%) were the most commonly reported symptoms.
The researchers noted that 21 patients had skin biopsies taken and “all showed spongiosis or a feature of eczema, with 1 having overlapping features of psoriasis.”
In the majority (92 %) of cases, patients experienced only one eczema event. Of the 20 patients who had more than one event, just over one-fifth of repeat events occurred after receiving the same biologic as for the index event. A quarter of events occurred after a different biologic of the same class had been used, and just over half of events occurred after a different class of biologic had been given.
Strengths and Limitations
The “large sample size and inclusion of multiple lines of exposure per participant” are strengths of the study, said the researchers. “We included data for all currently available biologics, originating from more than 160 dermatology centers in the UK and Ireland.”
They added, however, that the “main limitation is the small numbers of observations within certain subgroups, such as specific biologic exposures or participants in ethnic minority groups, restricting generalizability of our findings and the interpretation of some subgroup analyses.”
Moreover, the small number of paradoxical eczema events seen may have resulted in imprecise effect estimates, they observe, noting that the number of exposures to IL-23 inhibitors was low compared with other classes.
“Future studies with more exposures and paradoxical eczema events would enable a more robust analysis of individual drugs and patient subgroups,” the authors concluded.
The study was funded by the Medical Research Council. BADBIR is coordinated by The University of Manchester, and funded by the British Association of Dermatologists (BAD). The BAD receives income from AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Samsung Bioepis, Sandoz Hexal AG, and UCB Pharma for providing pharmacovigilance services. This income finances a separate contract between the BAD and The University of Manchester, which coordinates BADBIR. Mr. Al-Janabi reported receiving grants from the Medical Research Council during the conduct of the study; nonfinancial support from UCB, Almirall, and Janssen; and personal fees from UCB outside the submitted work.
examined in a large observational analysis.
Using data from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) database, Ali Al-Janabi, MA, from the University of Manchester (England) and associates found that 273 (1%) of approximately 25,000 drug exposures in 13,699 biologic-treated patients with psoriasis were associated with paradoxical eczema.
The incidence of paradoxical eczema was found to vary by class. The highest rate was seen for IL-17 inhibitors, at 1.22 per 100,000 person-years, and the lowest rate was seen with IL-23 inhibitors, at 0.56 per 100,000 person-years. The respective incidence rates for tumor necrosis factor (TNF) inhibitors and IL-12/IL-23 inhibitors were a respective 0.94 and 0.80 per 100,000 person-years.
“Compared with TNF inhibitors, IL-23 inhibitor exposure was associated with significantly lower risk of paradoxical eczema,” the BADBIR Study Group reported in JAMA Dermatology. Indeed, patients treated with IL-23 inhibitors were 61% less likely than were those taking TNF-inhibitors to experience a paradoxical eczema event.
“These findings remained when restricting the analysis to first-line biologic exposures and were specific to this eczema phenotype” the group said.
Cautious Interpretation
As the corresponding author for the work, Mr. Al-Janabi observed in an email that the research needs to be replicated, and the findings need to be interpreted with caution.
“As well as usual clinical variables influencing biologic selection, clinicians could consider IL-23 inhibitors in patients with previous atopic dermatitis, hay fever, or paradoxical eczema episodes, as this class was associated with the lowest risk of paradoxical eczema,” he suggested.
A prior history of atopic dermatitis (AD) and hay fever appears to be particularly relevant, as both substantially upped the chances that paradoxical eczema would occur, with hazard ratios of 12.40 and 3.78, respectively. Increasing age also increased the risk, albeit slightly (hazard ratio [HR], 1.02 per year), and there was an apparent lower risk (HR, 0.60) comparing men and women.
The BADBIR Study Group authors believe that, to the best of their knowledge, this is the first study to compare paradoxical eczema risk by biologic class. “Based on clinical experience and prevalence of eczematous reactions reported in some IL-17 inhibitor clinical trials, we suspected an association between IL-17 inhibitor exposure and paradoxical eczema,” they wrote.
“While the incidence of paradoxical eczema was numerically highest among IL-17 inhibitor exposures, it was not significantly different from the incidence among TNF inhibitor exposures.” The low overall incidence of paradoxical eczema “may be reassuring for patients and clinicians,” they added, “but it is possible that the incidence was underestimated due to underreporting or exclusion of adverse events with insufficient detail.”
Details of the Analysis, Other Findings
To explore the risk of paradoxical eczema by biologic class and identify possible risk factors, the BADBIR Study Group performed a prospective cohort study using data held within the BADBIR database between September 2007 and December 2022.
Adults over the age of 18 year or older with plaque psoriasis and who had been treated with at least one of the following biologics were eligible for inclusion: the TNF inhibitors adalimumab, certolizumab pegol, etanercept, and infliximab; the IL-17 inhibitors bimekizumab, brodalumab, ixekizumab, and secukinumab; the IL-12/23 inhibitor ustekinumab; and the IL-23 inhibitors guselkumab, risankizumab, and tildrakizumab.
Patient records and adverse event data were reviewed to determine the incidence of paradoxical eczema events, using terms such as eczema, eczematized, eczematous, atopy, atopic, and dermatitis.
Of 24,952 drug exposures analyzed, the majority (11,819) were for TNF inhibitors, followed by IL-17 inhibitors (4,776), IL-12/23 inhibitors (6,423), and finally, IL-23 inhibitors (1,934).
Mr. Al-Janabi and coauthors reported that the median time to onset of paradoxical eczema events was 294 days — approximately 9.8 months. The earliest that these events were recorded was at 120 days (4 months), and the latest at 699 days (almost 2 years).
The face and neck were the most common sites affected (26% of exposures), with other sites including the limbs (23%), the trunk (13%), and hands or feet (12%). Itching (18%), redness (7%), and dryness (4%) were the most commonly reported symptoms.
The researchers noted that 21 patients had skin biopsies taken and “all showed spongiosis or a feature of eczema, with 1 having overlapping features of psoriasis.”
In the majority (92 %) of cases, patients experienced only one eczema event. Of the 20 patients who had more than one event, just over one-fifth of repeat events occurred after receiving the same biologic as for the index event. A quarter of events occurred after a different biologic of the same class had been used, and just over half of events occurred after a different class of biologic had been given.
Strengths and Limitations
The “large sample size and inclusion of multiple lines of exposure per participant” are strengths of the study, said the researchers. “We included data for all currently available biologics, originating from more than 160 dermatology centers in the UK and Ireland.”
They added, however, that the “main limitation is the small numbers of observations within certain subgroups, such as specific biologic exposures or participants in ethnic minority groups, restricting generalizability of our findings and the interpretation of some subgroup analyses.”
Moreover, the small number of paradoxical eczema events seen may have resulted in imprecise effect estimates, they observe, noting that the number of exposures to IL-23 inhibitors was low compared with other classes.
“Future studies with more exposures and paradoxical eczema events would enable a more robust analysis of individual drugs and patient subgroups,” the authors concluded.
The study was funded by the Medical Research Council. BADBIR is coordinated by The University of Manchester, and funded by the British Association of Dermatologists (BAD). The BAD receives income from AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Samsung Bioepis, Sandoz Hexal AG, and UCB Pharma for providing pharmacovigilance services. This income finances a separate contract between the BAD and The University of Manchester, which coordinates BADBIR. Mr. Al-Janabi reported receiving grants from the Medical Research Council during the conduct of the study; nonfinancial support from UCB, Almirall, and Janssen; and personal fees from UCB outside the submitted work.
examined in a large observational analysis.
Using data from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) database, Ali Al-Janabi, MA, from the University of Manchester (England) and associates found that 273 (1%) of approximately 25,000 drug exposures in 13,699 biologic-treated patients with psoriasis were associated with paradoxical eczema.
The incidence of paradoxical eczema was found to vary by class. The highest rate was seen for IL-17 inhibitors, at 1.22 per 100,000 person-years, and the lowest rate was seen with IL-23 inhibitors, at 0.56 per 100,000 person-years. The respective incidence rates for tumor necrosis factor (TNF) inhibitors and IL-12/IL-23 inhibitors were a respective 0.94 and 0.80 per 100,000 person-years.
“Compared with TNF inhibitors, IL-23 inhibitor exposure was associated with significantly lower risk of paradoxical eczema,” the BADBIR Study Group reported in JAMA Dermatology. Indeed, patients treated with IL-23 inhibitors were 61% less likely than were those taking TNF-inhibitors to experience a paradoxical eczema event.
“These findings remained when restricting the analysis to first-line biologic exposures and were specific to this eczema phenotype” the group said.
Cautious Interpretation
As the corresponding author for the work, Mr. Al-Janabi observed in an email that the research needs to be replicated, and the findings need to be interpreted with caution.
“As well as usual clinical variables influencing biologic selection, clinicians could consider IL-23 inhibitors in patients with previous atopic dermatitis, hay fever, or paradoxical eczema episodes, as this class was associated with the lowest risk of paradoxical eczema,” he suggested.
A prior history of atopic dermatitis (AD) and hay fever appears to be particularly relevant, as both substantially upped the chances that paradoxical eczema would occur, with hazard ratios of 12.40 and 3.78, respectively. Increasing age also increased the risk, albeit slightly (hazard ratio [HR], 1.02 per year), and there was an apparent lower risk (HR, 0.60) comparing men and women.
The BADBIR Study Group authors believe that, to the best of their knowledge, this is the first study to compare paradoxical eczema risk by biologic class. “Based on clinical experience and prevalence of eczematous reactions reported in some IL-17 inhibitor clinical trials, we suspected an association between IL-17 inhibitor exposure and paradoxical eczema,” they wrote.
“While the incidence of paradoxical eczema was numerically highest among IL-17 inhibitor exposures, it was not significantly different from the incidence among TNF inhibitor exposures.” The low overall incidence of paradoxical eczema “may be reassuring for patients and clinicians,” they added, “but it is possible that the incidence was underestimated due to underreporting or exclusion of adverse events with insufficient detail.”
Details of the Analysis, Other Findings
To explore the risk of paradoxical eczema by biologic class and identify possible risk factors, the BADBIR Study Group performed a prospective cohort study using data held within the BADBIR database between September 2007 and December 2022.
Adults over the age of 18 year or older with plaque psoriasis and who had been treated with at least one of the following biologics were eligible for inclusion: the TNF inhibitors adalimumab, certolizumab pegol, etanercept, and infliximab; the IL-17 inhibitors bimekizumab, brodalumab, ixekizumab, and secukinumab; the IL-12/23 inhibitor ustekinumab; and the IL-23 inhibitors guselkumab, risankizumab, and tildrakizumab.
Patient records and adverse event data were reviewed to determine the incidence of paradoxical eczema events, using terms such as eczema, eczematized, eczematous, atopy, atopic, and dermatitis.
Of 24,952 drug exposures analyzed, the majority (11,819) were for TNF inhibitors, followed by IL-17 inhibitors (4,776), IL-12/23 inhibitors (6,423), and finally, IL-23 inhibitors (1,934).
Mr. Al-Janabi and coauthors reported that the median time to onset of paradoxical eczema events was 294 days — approximately 9.8 months. The earliest that these events were recorded was at 120 days (4 months), and the latest at 699 days (almost 2 years).
The face and neck were the most common sites affected (26% of exposures), with other sites including the limbs (23%), the trunk (13%), and hands or feet (12%). Itching (18%), redness (7%), and dryness (4%) were the most commonly reported symptoms.
The researchers noted that 21 patients had skin biopsies taken and “all showed spongiosis or a feature of eczema, with 1 having overlapping features of psoriasis.”
In the majority (92 %) of cases, patients experienced only one eczema event. Of the 20 patients who had more than one event, just over one-fifth of repeat events occurred after receiving the same biologic as for the index event. A quarter of events occurred after a different biologic of the same class had been used, and just over half of events occurred after a different class of biologic had been given.
Strengths and Limitations
The “large sample size and inclusion of multiple lines of exposure per participant” are strengths of the study, said the researchers. “We included data for all currently available biologics, originating from more than 160 dermatology centers in the UK and Ireland.”
They added, however, that the “main limitation is the small numbers of observations within certain subgroups, such as specific biologic exposures or participants in ethnic minority groups, restricting generalizability of our findings and the interpretation of some subgroup analyses.”
Moreover, the small number of paradoxical eczema events seen may have resulted in imprecise effect estimates, they observe, noting that the number of exposures to IL-23 inhibitors was low compared with other classes.
“Future studies with more exposures and paradoxical eczema events would enable a more robust analysis of individual drugs and patient subgroups,” the authors concluded.
The study was funded by the Medical Research Council. BADBIR is coordinated by The University of Manchester, and funded by the British Association of Dermatologists (BAD). The BAD receives income from AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Samsung Bioepis, Sandoz Hexal AG, and UCB Pharma for providing pharmacovigilance services. This income finances a separate contract between the BAD and The University of Manchester, which coordinates BADBIR. Mr. Al-Janabi reported receiving grants from the Medical Research Council during the conduct of the study; nonfinancial support from UCB, Almirall, and Janssen; and personal fees from UCB outside the submitted work.
FROM JAMA DERMATOLOGY
How does lebrikizumab perform across different racial and ethnic subgroups?
.
The finding comes from an analysis of the 16-week induction periods of the phase 3 ADvocate1 and ADvocate2 trials, which Raj Chovatiya, MD, PhD, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. The efficacy of lebrikizumab monotherapy to treat moderate-to-severe AD has been established in phase 3 studies, “but disease characteristic and efficacy outcomes may vary among racial and ethnic subgroups,” said Dr. Chovatiya, assistant professor in the department of dermatology at Northwestern University, Chicago. “The goal of the current study is to report the week 16 efficacy of lebrikizumab-treated patients in racial and ethnic subgroups from ADvocate1 and ADvocate2.”
Key eligibility criteria for both trials included adults or adolescents with a diagnosis of AD as defined by the American Academy of Dermatology Consensus Criteria, for at least 1 year prior to screening. They had moderate-to-severe AD, were candidates for systemic therapy, and were dupilumab- and tralokinumab-naive. Outcomes of interest were the Investigator’s Global Assessment 0 or 1, with at least a 2-point improvement; and the proportions of patients who achieved Eczema Area and Severity Index (EASI75) and EASI90 responses, and an improvement of 4 points or more on the Pruritus Numeric Rating Scale (NRS).
For statistical analysis, the researchers pooled data from Advocate1 and Advocate2 and applied imputation methodology to the 16-week induction period. Subsequent data from patients who received topical or systemic rescue medication or discontinued treatment due to lack of efficacy were imputed as nonresponders. Subsequent data from patients who discontinued treatment for other reasons were set to missing, and the researchers handled missing data with multiple imputation. They used logistic regression to test the interaction between the treatment and subgroup and the Cochran-Mantel-Haenszel method to evaluate the treatment effect within each subgroup after adjusting for stratification factors.
Dr. Chovatiya reported findings from the 851 study participants in the combined studies. Of these, 542 were White, 192 were Asian, 84 were Black, and 33 were from other racial subgroups. By ethnic subgroup, 748 were not Hispanic or Latino, 91 were Hispanic or Latino, and ethnicity was unknown or not reported for 12 subjects. At baseline, the mean body mass index was slightly higher among Blacks (30.4 kg/m2) and Hispanics (29.4 kg/m2) compared with other racial and ethnic groups, “which reflects general epidemiologic data among these groups in the United States,” Dr. Chovatiya said. “You can also see a difference in the balance of IGA scores — they were a little bit more severe in the Black or African American and Hispanic groups as well.” The researchers also observed differences in the baseline EASI score across some of these groups, particularly in the Asian individuals, who had higher EASI scores. Prior use of systemic therapy was lower in the Black and “other” subgroups, compared with other racial subgroups.
At week 16, key efficacy endpoints were generally similar between the different racial subgroups. Specifically, 25.1% of Asians in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 4.1% of those in the placebo group (P < .001), while 33.2% of Blacks in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 13.2% of those in the placebo group (no P value was established because this subgroup represented less than 10% of the entire study population). In addition, 43.3% of Whites in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 14.1% of those in the placebo group (P < .001).
In other findings, 45.5% of Asians in the lebrikizumab treatment group achieved an EASI75, compared with 8.5% of those in the placebo group (P < .001), while 51.7% of Blacks in the lebrikizumab treatment group achieved an EASI75, compared with 18.8% of those in the placebo group. Among whites in the lebrikizumab treatment group, 59.7% of achieved an EASI75, compared with 20.4% of those in the placebo group (P < .001).
Dr. Chovatiya said that 26.5% of Asians in the lebrikizumab treatment group achieved an EASI90, compared with 4.3% of those in the placebo group (P < .001), while 26.9% of Blacks in the lebrikizumab treatment group achieved an EASI90, compared with 13.2% of those in the placebo group. In addition, 38.3% of Whites in the lebrikizumab treatment group achieved an EASI90, compared with 10.9% of those in the placebo group (P < .001).
Finally, 36.4% of Asians in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 5.7% of those in the placebo group (P <. 001), while 41.7% of Blacks in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 17.4% of those in the placebo group. In addition, 45.9% of Whites in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 14.8% of those in the placebo group (P < .001). Statistical analyses of efficacy endpoints conducted by ethnic group yielded similar results.
Dr. Chovatiya acknowledged certain limitations of the study, including the fact that differences in baseline demographics and disease characteristics limit direct comparison across racial and ethnic subgroups. “Due to the relatively small sample size of some racial and ethnic subgroups and the post hoc nature of this analysis, additional studies are needed to verify these results,” he concluded. But for now, he said, the data available indicate that “lebrikizumab is effective across racial and ethnic subgroups for the treatment of moderate-to-severe AD after 16 weeks of monotherapy treatment.”
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly and Company. Dr. Chovatiya disclosed that he is speaker for and/or a consult and advisory board member to many pharmaceutical companies, including Eli Lilly.
.
The finding comes from an analysis of the 16-week induction periods of the phase 3 ADvocate1 and ADvocate2 trials, which Raj Chovatiya, MD, PhD, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. The efficacy of lebrikizumab monotherapy to treat moderate-to-severe AD has been established in phase 3 studies, “but disease characteristic and efficacy outcomes may vary among racial and ethnic subgroups,” said Dr. Chovatiya, assistant professor in the department of dermatology at Northwestern University, Chicago. “The goal of the current study is to report the week 16 efficacy of lebrikizumab-treated patients in racial and ethnic subgroups from ADvocate1 and ADvocate2.”
Key eligibility criteria for both trials included adults or adolescents with a diagnosis of AD as defined by the American Academy of Dermatology Consensus Criteria, for at least 1 year prior to screening. They had moderate-to-severe AD, were candidates for systemic therapy, and were dupilumab- and tralokinumab-naive. Outcomes of interest were the Investigator’s Global Assessment 0 or 1, with at least a 2-point improvement; and the proportions of patients who achieved Eczema Area and Severity Index (EASI75) and EASI90 responses, and an improvement of 4 points or more on the Pruritus Numeric Rating Scale (NRS).
For statistical analysis, the researchers pooled data from Advocate1 and Advocate2 and applied imputation methodology to the 16-week induction period. Subsequent data from patients who received topical or systemic rescue medication or discontinued treatment due to lack of efficacy were imputed as nonresponders. Subsequent data from patients who discontinued treatment for other reasons were set to missing, and the researchers handled missing data with multiple imputation. They used logistic regression to test the interaction between the treatment and subgroup and the Cochran-Mantel-Haenszel method to evaluate the treatment effect within each subgroup after adjusting for stratification factors.
Dr. Chovatiya reported findings from the 851 study participants in the combined studies. Of these, 542 were White, 192 were Asian, 84 were Black, and 33 were from other racial subgroups. By ethnic subgroup, 748 were not Hispanic or Latino, 91 were Hispanic or Latino, and ethnicity was unknown or not reported for 12 subjects. At baseline, the mean body mass index was slightly higher among Blacks (30.4 kg/m2) and Hispanics (29.4 kg/m2) compared with other racial and ethnic groups, “which reflects general epidemiologic data among these groups in the United States,” Dr. Chovatiya said. “You can also see a difference in the balance of IGA scores — they were a little bit more severe in the Black or African American and Hispanic groups as well.” The researchers also observed differences in the baseline EASI score across some of these groups, particularly in the Asian individuals, who had higher EASI scores. Prior use of systemic therapy was lower in the Black and “other” subgroups, compared with other racial subgroups.
At week 16, key efficacy endpoints were generally similar between the different racial subgroups. Specifically, 25.1% of Asians in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 4.1% of those in the placebo group (P < .001), while 33.2% of Blacks in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 13.2% of those in the placebo group (no P value was established because this subgroup represented less than 10% of the entire study population). In addition, 43.3% of Whites in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 14.1% of those in the placebo group (P < .001).
In other findings, 45.5% of Asians in the lebrikizumab treatment group achieved an EASI75, compared with 8.5% of those in the placebo group (P < .001), while 51.7% of Blacks in the lebrikizumab treatment group achieved an EASI75, compared with 18.8% of those in the placebo group. Among whites in the lebrikizumab treatment group, 59.7% of achieved an EASI75, compared with 20.4% of those in the placebo group (P < .001).
Dr. Chovatiya said that 26.5% of Asians in the lebrikizumab treatment group achieved an EASI90, compared with 4.3% of those in the placebo group (P < .001), while 26.9% of Blacks in the lebrikizumab treatment group achieved an EASI90, compared with 13.2% of those in the placebo group. In addition, 38.3% of Whites in the lebrikizumab treatment group achieved an EASI90, compared with 10.9% of those in the placebo group (P < .001).
Finally, 36.4% of Asians in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 5.7% of those in the placebo group (P <. 001), while 41.7% of Blacks in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 17.4% of those in the placebo group. In addition, 45.9% of Whites in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 14.8% of those in the placebo group (P < .001). Statistical analyses of efficacy endpoints conducted by ethnic group yielded similar results.
Dr. Chovatiya acknowledged certain limitations of the study, including the fact that differences in baseline demographics and disease characteristics limit direct comparison across racial and ethnic subgroups. “Due to the relatively small sample size of some racial and ethnic subgroups and the post hoc nature of this analysis, additional studies are needed to verify these results,” he concluded. But for now, he said, the data available indicate that “lebrikizumab is effective across racial and ethnic subgroups for the treatment of moderate-to-severe AD after 16 weeks of monotherapy treatment.”
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly and Company. Dr. Chovatiya disclosed that he is speaker for and/or a consult and advisory board member to many pharmaceutical companies, including Eli Lilly.
.
The finding comes from an analysis of the 16-week induction periods of the phase 3 ADvocate1 and ADvocate2 trials, which Raj Chovatiya, MD, PhD, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. The efficacy of lebrikizumab monotherapy to treat moderate-to-severe AD has been established in phase 3 studies, “but disease characteristic and efficacy outcomes may vary among racial and ethnic subgroups,” said Dr. Chovatiya, assistant professor in the department of dermatology at Northwestern University, Chicago. “The goal of the current study is to report the week 16 efficacy of lebrikizumab-treated patients in racial and ethnic subgroups from ADvocate1 and ADvocate2.”
Key eligibility criteria for both trials included adults or adolescents with a diagnosis of AD as defined by the American Academy of Dermatology Consensus Criteria, for at least 1 year prior to screening. They had moderate-to-severe AD, were candidates for systemic therapy, and were dupilumab- and tralokinumab-naive. Outcomes of interest were the Investigator’s Global Assessment 0 or 1, with at least a 2-point improvement; and the proportions of patients who achieved Eczema Area and Severity Index (EASI75) and EASI90 responses, and an improvement of 4 points or more on the Pruritus Numeric Rating Scale (NRS).
For statistical analysis, the researchers pooled data from Advocate1 and Advocate2 and applied imputation methodology to the 16-week induction period. Subsequent data from patients who received topical or systemic rescue medication or discontinued treatment due to lack of efficacy were imputed as nonresponders. Subsequent data from patients who discontinued treatment for other reasons were set to missing, and the researchers handled missing data with multiple imputation. They used logistic regression to test the interaction between the treatment and subgroup and the Cochran-Mantel-Haenszel method to evaluate the treatment effect within each subgroup after adjusting for stratification factors.
Dr. Chovatiya reported findings from the 851 study participants in the combined studies. Of these, 542 were White, 192 were Asian, 84 were Black, and 33 were from other racial subgroups. By ethnic subgroup, 748 were not Hispanic or Latino, 91 were Hispanic or Latino, and ethnicity was unknown or not reported for 12 subjects. At baseline, the mean body mass index was slightly higher among Blacks (30.4 kg/m2) and Hispanics (29.4 kg/m2) compared with other racial and ethnic groups, “which reflects general epidemiologic data among these groups in the United States,” Dr. Chovatiya said. “You can also see a difference in the balance of IGA scores — they were a little bit more severe in the Black or African American and Hispanic groups as well.” The researchers also observed differences in the baseline EASI score across some of these groups, particularly in the Asian individuals, who had higher EASI scores. Prior use of systemic therapy was lower in the Black and “other” subgroups, compared with other racial subgroups.
At week 16, key efficacy endpoints were generally similar between the different racial subgroups. Specifically, 25.1% of Asians in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 4.1% of those in the placebo group (P < .001), while 33.2% of Blacks in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 13.2% of those in the placebo group (no P value was established because this subgroup represented less than 10% of the entire study population). In addition, 43.3% of Whites in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 14.1% of those in the placebo group (P < .001).
In other findings, 45.5% of Asians in the lebrikizumab treatment group achieved an EASI75, compared with 8.5% of those in the placebo group (P < .001), while 51.7% of Blacks in the lebrikizumab treatment group achieved an EASI75, compared with 18.8% of those in the placebo group. Among whites in the lebrikizumab treatment group, 59.7% of achieved an EASI75, compared with 20.4% of those in the placebo group (P < .001).
Dr. Chovatiya said that 26.5% of Asians in the lebrikizumab treatment group achieved an EASI90, compared with 4.3% of those in the placebo group (P < .001), while 26.9% of Blacks in the lebrikizumab treatment group achieved an EASI90, compared with 13.2% of those in the placebo group. In addition, 38.3% of Whites in the lebrikizumab treatment group achieved an EASI90, compared with 10.9% of those in the placebo group (P < .001).
Finally, 36.4% of Asians in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 5.7% of those in the placebo group (P <. 001), while 41.7% of Blacks in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 17.4% of those in the placebo group. In addition, 45.9% of Whites in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 14.8% of those in the placebo group (P < .001). Statistical analyses of efficacy endpoints conducted by ethnic group yielded similar results.
Dr. Chovatiya acknowledged certain limitations of the study, including the fact that differences in baseline demographics and disease characteristics limit direct comparison across racial and ethnic subgroups. “Due to the relatively small sample size of some racial and ethnic subgroups and the post hoc nature of this analysis, additional studies are needed to verify these results,” he concluded. But for now, he said, the data available indicate that “lebrikizumab is effective across racial and ethnic subgroups for the treatment of moderate-to-severe AD after 16 weeks of monotherapy treatment.”
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly and Company. Dr. Chovatiya disclosed that he is speaker for and/or a consult and advisory board member to many pharmaceutical companies, including Eli Lilly.
FROM RAD 2023
Adequate disease control elusive for many patients on systemic AD therapies, study finds
,” the study’s lead author, Jonathan I. Silverberg, MD, PhD, MPH, reported.
The findings come from an analysis of real-world outcomes from the TARGET-DERM AD registry, which Dr. Silverberg, professor and director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. He characterized the findings as “patients just getting stuck with a therapy and not advancing when they need to.”
TARGET-DERM AD is a longitudinal observational study of people with AD at 39 academic centers in the United States and Canada. Dr. Silverberg and his coinvestigators evaluated the proportion of patients who were experiencing an inadequate response after receiving systemic therapy and continuing on the same treatment for 3-12 months. “These are patients who are receiving their first or advanced systemic therapy, and the question is, how long did they stay on it, even if they’re not doing so well?” Dr. Silverberg said.
The researchers identified and compared the proportions of patients not achieving moderate or optimal clinician-reported outcome targets on patients with AD treated with their first systemic therapy. Advanced systemic therapy (AST) included abrocitinib, dupilumab, tralokinumab, or upadacitinib, while conventional systemic therapy (CST) included methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, and systemic corticosteroids.
Patients in TARGET-DERM AD were treated and maintained on their first systemic therapy (either advanced or conventional) for up to 12 months. They had a validated Investigator’s Global Assessment of AD (vIGA-AD) score of 3 or 4 less than 45 days prior to initiation of systemic therapy or up to 14 days afterward. They also had at least one vIGA-AD assessment 3-12 months after initiating treatment. Outcome measures included IGA (defined as a score of 2 or less, with an optional target of 0 or 1), BSA (defined as a 50% BSA improvement, with an optimal target BSA of 2% or less), and the Worst-Itch Numeric Rating Scale (defined as at least a 4-point reduction, with an optimal target of 1 or less).
The analysis included 445 patients with a mean age of 31 years at enrollment. More than half (62%) were female and 45% were non-Hispanic Whites. Most patients (92%) were on an AST, mainly dupilumab, with smaller proportions treated with either tralokinumab, upadacitinib, or abrocitinib. Fewer than 10% of patients in the registry were being treated with CSTs.
At 6 months, 37% and approximately 67% of the AST-treated patients had inadequate responses in terms of skin clearance and itch outcomes, respectively. At 12 months, these figures were about 30% and 66%, respectively. CST-treated patients showed a similar trend. For patients starting an AST on or after Sept. 21, 2021, when three additional AST options were available (tralokinumab, upadacitinib, and abrocitinib), the proportion of patients demonstrating an adequate response over 12 months was generally similar to the overall cohort of those on ASTs.
“These findings suggest a need for alternative therapies and management strategies in AD treatment,” concluded Dr. Silverberg, who chaired the RAD symposium.
Dr. Silverberg reported being a consultant and/or an adviser for many pharmaceutical companies, and has received grant or research support from Galderma and Pfizer. The TARGET-DERM study is sponsored by Target PharmaSolutions.
,” the study’s lead author, Jonathan I. Silverberg, MD, PhD, MPH, reported.
The findings come from an analysis of real-world outcomes from the TARGET-DERM AD registry, which Dr. Silverberg, professor and director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. He characterized the findings as “patients just getting stuck with a therapy and not advancing when they need to.”
TARGET-DERM AD is a longitudinal observational study of people with AD at 39 academic centers in the United States and Canada. Dr. Silverberg and his coinvestigators evaluated the proportion of patients who were experiencing an inadequate response after receiving systemic therapy and continuing on the same treatment for 3-12 months. “These are patients who are receiving their first or advanced systemic therapy, and the question is, how long did they stay on it, even if they’re not doing so well?” Dr. Silverberg said.
The researchers identified and compared the proportions of patients not achieving moderate or optimal clinician-reported outcome targets on patients with AD treated with their first systemic therapy. Advanced systemic therapy (AST) included abrocitinib, dupilumab, tralokinumab, or upadacitinib, while conventional systemic therapy (CST) included methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, and systemic corticosteroids.
Patients in TARGET-DERM AD were treated and maintained on their first systemic therapy (either advanced or conventional) for up to 12 months. They had a validated Investigator’s Global Assessment of AD (vIGA-AD) score of 3 or 4 less than 45 days prior to initiation of systemic therapy or up to 14 days afterward. They also had at least one vIGA-AD assessment 3-12 months after initiating treatment. Outcome measures included IGA (defined as a score of 2 or less, with an optional target of 0 or 1), BSA (defined as a 50% BSA improvement, with an optimal target BSA of 2% or less), and the Worst-Itch Numeric Rating Scale (defined as at least a 4-point reduction, with an optimal target of 1 or less).
The analysis included 445 patients with a mean age of 31 years at enrollment. More than half (62%) were female and 45% were non-Hispanic Whites. Most patients (92%) were on an AST, mainly dupilumab, with smaller proportions treated with either tralokinumab, upadacitinib, or abrocitinib. Fewer than 10% of patients in the registry were being treated with CSTs.
At 6 months, 37% and approximately 67% of the AST-treated patients had inadequate responses in terms of skin clearance and itch outcomes, respectively. At 12 months, these figures were about 30% and 66%, respectively. CST-treated patients showed a similar trend. For patients starting an AST on or after Sept. 21, 2021, when three additional AST options were available (tralokinumab, upadacitinib, and abrocitinib), the proportion of patients demonstrating an adequate response over 12 months was generally similar to the overall cohort of those on ASTs.
“These findings suggest a need for alternative therapies and management strategies in AD treatment,” concluded Dr. Silverberg, who chaired the RAD symposium.
Dr. Silverberg reported being a consultant and/or an adviser for many pharmaceutical companies, and has received grant or research support from Galderma and Pfizer. The TARGET-DERM study is sponsored by Target PharmaSolutions.
,” the study’s lead author, Jonathan I. Silverberg, MD, PhD, MPH, reported.
The findings come from an analysis of real-world outcomes from the TARGET-DERM AD registry, which Dr. Silverberg, professor and director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. He characterized the findings as “patients just getting stuck with a therapy and not advancing when they need to.”
TARGET-DERM AD is a longitudinal observational study of people with AD at 39 academic centers in the United States and Canada. Dr. Silverberg and his coinvestigators evaluated the proportion of patients who were experiencing an inadequate response after receiving systemic therapy and continuing on the same treatment for 3-12 months. “These are patients who are receiving their first or advanced systemic therapy, and the question is, how long did they stay on it, even if they’re not doing so well?” Dr. Silverberg said.
The researchers identified and compared the proportions of patients not achieving moderate or optimal clinician-reported outcome targets on patients with AD treated with their first systemic therapy. Advanced systemic therapy (AST) included abrocitinib, dupilumab, tralokinumab, or upadacitinib, while conventional systemic therapy (CST) included methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, and systemic corticosteroids.
Patients in TARGET-DERM AD were treated and maintained on their first systemic therapy (either advanced or conventional) for up to 12 months. They had a validated Investigator’s Global Assessment of AD (vIGA-AD) score of 3 or 4 less than 45 days prior to initiation of systemic therapy or up to 14 days afterward. They also had at least one vIGA-AD assessment 3-12 months after initiating treatment. Outcome measures included IGA (defined as a score of 2 or less, with an optional target of 0 or 1), BSA (defined as a 50% BSA improvement, with an optimal target BSA of 2% or less), and the Worst-Itch Numeric Rating Scale (defined as at least a 4-point reduction, with an optimal target of 1 or less).
The analysis included 445 patients with a mean age of 31 years at enrollment. More than half (62%) were female and 45% were non-Hispanic Whites. Most patients (92%) were on an AST, mainly dupilumab, with smaller proportions treated with either tralokinumab, upadacitinib, or abrocitinib. Fewer than 10% of patients in the registry were being treated with CSTs.
At 6 months, 37% and approximately 67% of the AST-treated patients had inadequate responses in terms of skin clearance and itch outcomes, respectively. At 12 months, these figures were about 30% and 66%, respectively. CST-treated patients showed a similar trend. For patients starting an AST on or after Sept. 21, 2021, when three additional AST options were available (tralokinumab, upadacitinib, and abrocitinib), the proportion of patients demonstrating an adequate response over 12 months was generally similar to the overall cohort of those on ASTs.
“These findings suggest a need for alternative therapies and management strategies in AD treatment,” concluded Dr. Silverberg, who chaired the RAD symposium.
Dr. Silverberg reported being a consultant and/or an adviser for many pharmaceutical companies, and has received grant or research support from Galderma and Pfizer. The TARGET-DERM study is sponsored by Target PharmaSolutions.
FROM RAD 2023
Tape strips detect hidradenitis suppurativa biomarkers, novel study shows
, results from a novel study showed.
“Tape strips can provide important clues to when and which drugs to use in HS in patients with both early and late disease, which can change clinical practice,” corresponding study author Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai in New York City, said in an interview. “It is noninvasive and nonscarring,” she added.
Tape stripping has been validated in atopic dermatitis, psoriasis, and other dermatologic conditions in recent years. For the current study, which was published online in the Journal of the American Academy of Dermatology, and is believed to be the first of its kind, Dr. Guttman-Yassky and colleagues performed RNA sequencing from large D-Squame tape strips collected from lesional and nonlesional skin of 22 patients with HS and from 21 age- and sex-matched healthy controls. They correlated the expression of skin biomarkers between tape strips and a previously published gene-signature of HS biopsies. The mean age of patients with HS was 43 years, while the mean age of healthy controls was 35. The average International Hidradenitis Suppurativa Severity Score System (IHS4) score of the HS cohort was 36.
Consistent with published studies, the researchers found that tape strips identified an overall higher inflammatory burden in HS. Specifically, they observed an upregulation of known cytokines within the following pathways: Th1 (such as IFNG, CXCL9/10/11, and CCR5); Th17 (such as interleukin [IL]-17A/F, IL12B, IL23A, CAMP, and CCL20); Th2 (such as IL4R, IL13/IL31/IL10, CCR4, CCL7/CCL13/CCL24, TNFSF4/OX40L, and TNFRSF4/OX40); and Th22 (such as IL22 and IL32).
The researchers also found that the expression of Th17 and tumor necrosis factor (TNF)–alpha pathways were highly correlated between tape strips and biopsies and that HS clinical severity was significantly associated with expression of biomarkers, such as TNF-alpha, IL17A/F, OX40, JAK1-3, and IL4R in HS lesional and/or nonlesional skin.
“It was quite unexpected that we are able to identify, using a minimally invasive approach that samples only the upper layers of the epidermis, products and processes that are considered to be deeper-situated, such as IL-17, and other immune markers,” Dr. Guttman-Yassky said in the interview. “We were also surprised to see how well the tape-stripped–derived skin molecular profile correlated with that of biopsies, as well as how well it correlated with the clinical disease severity of HS.”
Also surprising, she added, was that the biomarkers in nonlesional tape-stripped skin, such as IL-17 and TNF alpha, “show high correlations with disease severity and provide clues to early disease.”
If using tape strips in HS is validated in larger cohort studies, the potential cost implications of using this approach in practice remain unclear, Dr. Guttman-Yassky said. “It is currently not cheap, but we are hoping that one day, we can provide a means to diagnose the disease and treat it early, and appropriately, utilizing this approach,” she commented. “We are excited about the applicability of this study to the early treatment and longitudinal follow up of HS with drugs that are targeting specific immune molecules and pathways,” she said, adding that it will also be useful for helping determine which drug should be used for which patient.
She and her co-authors acknowledged certain limitations of the study, including its small sample size and the fact that tape stripping is limited to the epidermis.
Asked to comment on the study, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, said the findings “have important potential implications for our ability to one day personalize treatments for a patient with early HS in a minimally invasive way.”
As the study authors point out, she added, “tape strips only allow sampling of the epidermis, which is limiting in a disease like HS where much of the disruption is in the dermis with deep nodules and dermal tunnels. However, our overall goal should be to catch patients in the early stages of their disease before the occurrence of irreversible tissue damage such as dermal tunnels. Thus, the ongoing campaign for early diagnosis and early intervention by various stakeholders in the field of HS can help mitigate the impact of this inherent limitation of tape strips. It will be exciting to see larger studies that investigate tape strip results in relation to clinical phenotypes, disease progression, and therapeutic responses.”
The study was funded by an International Dermatology Outcome Measures Hidradenitis Suppurativa Grant. Dr. Guttman-Yassky disclosed that she has been a consultant to, an adviser for, and has received research grants from many pharmaceutical companies. Of the remaining authors, 2 also had multiple disclosures and 11 had no disclosures. Dr. Hsiao disclosed that she is a member of the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
, results from a novel study showed.
“Tape strips can provide important clues to when and which drugs to use in HS in patients with both early and late disease, which can change clinical practice,” corresponding study author Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai in New York City, said in an interview. “It is noninvasive and nonscarring,” she added.
Tape stripping has been validated in atopic dermatitis, psoriasis, and other dermatologic conditions in recent years. For the current study, which was published online in the Journal of the American Academy of Dermatology, and is believed to be the first of its kind, Dr. Guttman-Yassky and colleagues performed RNA sequencing from large D-Squame tape strips collected from lesional and nonlesional skin of 22 patients with HS and from 21 age- and sex-matched healthy controls. They correlated the expression of skin biomarkers between tape strips and a previously published gene-signature of HS biopsies. The mean age of patients with HS was 43 years, while the mean age of healthy controls was 35. The average International Hidradenitis Suppurativa Severity Score System (IHS4) score of the HS cohort was 36.
Consistent with published studies, the researchers found that tape strips identified an overall higher inflammatory burden in HS. Specifically, they observed an upregulation of known cytokines within the following pathways: Th1 (such as IFNG, CXCL9/10/11, and CCR5); Th17 (such as interleukin [IL]-17A/F, IL12B, IL23A, CAMP, and CCL20); Th2 (such as IL4R, IL13/IL31/IL10, CCR4, CCL7/CCL13/CCL24, TNFSF4/OX40L, and TNFRSF4/OX40); and Th22 (such as IL22 and IL32).
The researchers also found that the expression of Th17 and tumor necrosis factor (TNF)–alpha pathways were highly correlated between tape strips and biopsies and that HS clinical severity was significantly associated with expression of biomarkers, such as TNF-alpha, IL17A/F, OX40, JAK1-3, and IL4R in HS lesional and/or nonlesional skin.
“It was quite unexpected that we are able to identify, using a minimally invasive approach that samples only the upper layers of the epidermis, products and processes that are considered to be deeper-situated, such as IL-17, and other immune markers,” Dr. Guttman-Yassky said in the interview. “We were also surprised to see how well the tape-stripped–derived skin molecular profile correlated with that of biopsies, as well as how well it correlated with the clinical disease severity of HS.”
Also surprising, she added, was that the biomarkers in nonlesional tape-stripped skin, such as IL-17 and TNF alpha, “show high correlations with disease severity and provide clues to early disease.”
If using tape strips in HS is validated in larger cohort studies, the potential cost implications of using this approach in practice remain unclear, Dr. Guttman-Yassky said. “It is currently not cheap, but we are hoping that one day, we can provide a means to diagnose the disease and treat it early, and appropriately, utilizing this approach,” she commented. “We are excited about the applicability of this study to the early treatment and longitudinal follow up of HS with drugs that are targeting specific immune molecules and pathways,” she said, adding that it will also be useful for helping determine which drug should be used for which patient.
She and her co-authors acknowledged certain limitations of the study, including its small sample size and the fact that tape stripping is limited to the epidermis.
Asked to comment on the study, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, said the findings “have important potential implications for our ability to one day personalize treatments for a patient with early HS in a minimally invasive way.”
As the study authors point out, she added, “tape strips only allow sampling of the epidermis, which is limiting in a disease like HS where much of the disruption is in the dermis with deep nodules and dermal tunnels. However, our overall goal should be to catch patients in the early stages of their disease before the occurrence of irreversible tissue damage such as dermal tunnels. Thus, the ongoing campaign for early diagnosis and early intervention by various stakeholders in the field of HS can help mitigate the impact of this inherent limitation of tape strips. It will be exciting to see larger studies that investigate tape strip results in relation to clinical phenotypes, disease progression, and therapeutic responses.”
The study was funded by an International Dermatology Outcome Measures Hidradenitis Suppurativa Grant. Dr. Guttman-Yassky disclosed that she has been a consultant to, an adviser for, and has received research grants from many pharmaceutical companies. Of the remaining authors, 2 also had multiple disclosures and 11 had no disclosures. Dr. Hsiao disclosed that she is a member of the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
, results from a novel study showed.
“Tape strips can provide important clues to when and which drugs to use in HS in patients with both early and late disease, which can change clinical practice,” corresponding study author Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai in New York City, said in an interview. “It is noninvasive and nonscarring,” she added.
Tape stripping has been validated in atopic dermatitis, psoriasis, and other dermatologic conditions in recent years. For the current study, which was published online in the Journal of the American Academy of Dermatology, and is believed to be the first of its kind, Dr. Guttman-Yassky and colleagues performed RNA sequencing from large D-Squame tape strips collected from lesional and nonlesional skin of 22 patients with HS and from 21 age- and sex-matched healthy controls. They correlated the expression of skin biomarkers between tape strips and a previously published gene-signature of HS biopsies. The mean age of patients with HS was 43 years, while the mean age of healthy controls was 35. The average International Hidradenitis Suppurativa Severity Score System (IHS4) score of the HS cohort was 36.
Consistent with published studies, the researchers found that tape strips identified an overall higher inflammatory burden in HS. Specifically, they observed an upregulation of known cytokines within the following pathways: Th1 (such as IFNG, CXCL9/10/11, and CCR5); Th17 (such as interleukin [IL]-17A/F, IL12B, IL23A, CAMP, and CCL20); Th2 (such as IL4R, IL13/IL31/IL10, CCR4, CCL7/CCL13/CCL24, TNFSF4/OX40L, and TNFRSF4/OX40); and Th22 (such as IL22 and IL32).
The researchers also found that the expression of Th17 and tumor necrosis factor (TNF)–alpha pathways were highly correlated between tape strips and biopsies and that HS clinical severity was significantly associated with expression of biomarkers, such as TNF-alpha, IL17A/F, OX40, JAK1-3, and IL4R in HS lesional and/or nonlesional skin.
“It was quite unexpected that we are able to identify, using a minimally invasive approach that samples only the upper layers of the epidermis, products and processes that are considered to be deeper-situated, such as IL-17, and other immune markers,” Dr. Guttman-Yassky said in the interview. “We were also surprised to see how well the tape-stripped–derived skin molecular profile correlated with that of biopsies, as well as how well it correlated with the clinical disease severity of HS.”
Also surprising, she added, was that the biomarkers in nonlesional tape-stripped skin, such as IL-17 and TNF alpha, “show high correlations with disease severity and provide clues to early disease.”
If using tape strips in HS is validated in larger cohort studies, the potential cost implications of using this approach in practice remain unclear, Dr. Guttman-Yassky said. “It is currently not cheap, but we are hoping that one day, we can provide a means to diagnose the disease and treat it early, and appropriately, utilizing this approach,” she commented. “We are excited about the applicability of this study to the early treatment and longitudinal follow up of HS with drugs that are targeting specific immune molecules and pathways,” she said, adding that it will also be useful for helping determine which drug should be used for which patient.
She and her co-authors acknowledged certain limitations of the study, including its small sample size and the fact that tape stripping is limited to the epidermis.
Asked to comment on the study, Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, said the findings “have important potential implications for our ability to one day personalize treatments for a patient with early HS in a minimally invasive way.”
As the study authors point out, she added, “tape strips only allow sampling of the epidermis, which is limiting in a disease like HS where much of the disruption is in the dermis with deep nodules and dermal tunnels. However, our overall goal should be to catch patients in the early stages of their disease before the occurrence of irreversible tissue damage such as dermal tunnels. Thus, the ongoing campaign for early diagnosis and early intervention by various stakeholders in the field of HS can help mitigate the impact of this inherent limitation of tape strips. It will be exciting to see larger studies that investigate tape strip results in relation to clinical phenotypes, disease progression, and therapeutic responses.”
The study was funded by an International Dermatology Outcome Measures Hidradenitis Suppurativa Grant. Dr. Guttman-Yassky disclosed that she has been a consultant to, an adviser for, and has received research grants from many pharmaceutical companies. Of the remaining authors, 2 also had multiple disclosures and 11 had no disclosures. Dr. Hsiao disclosed that she is a member of the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Association Between Atopic Dermatitis and Chronic Obstructive Pulmonary Disease Among US Adults in the 1999-2006 NHANES Survey
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).
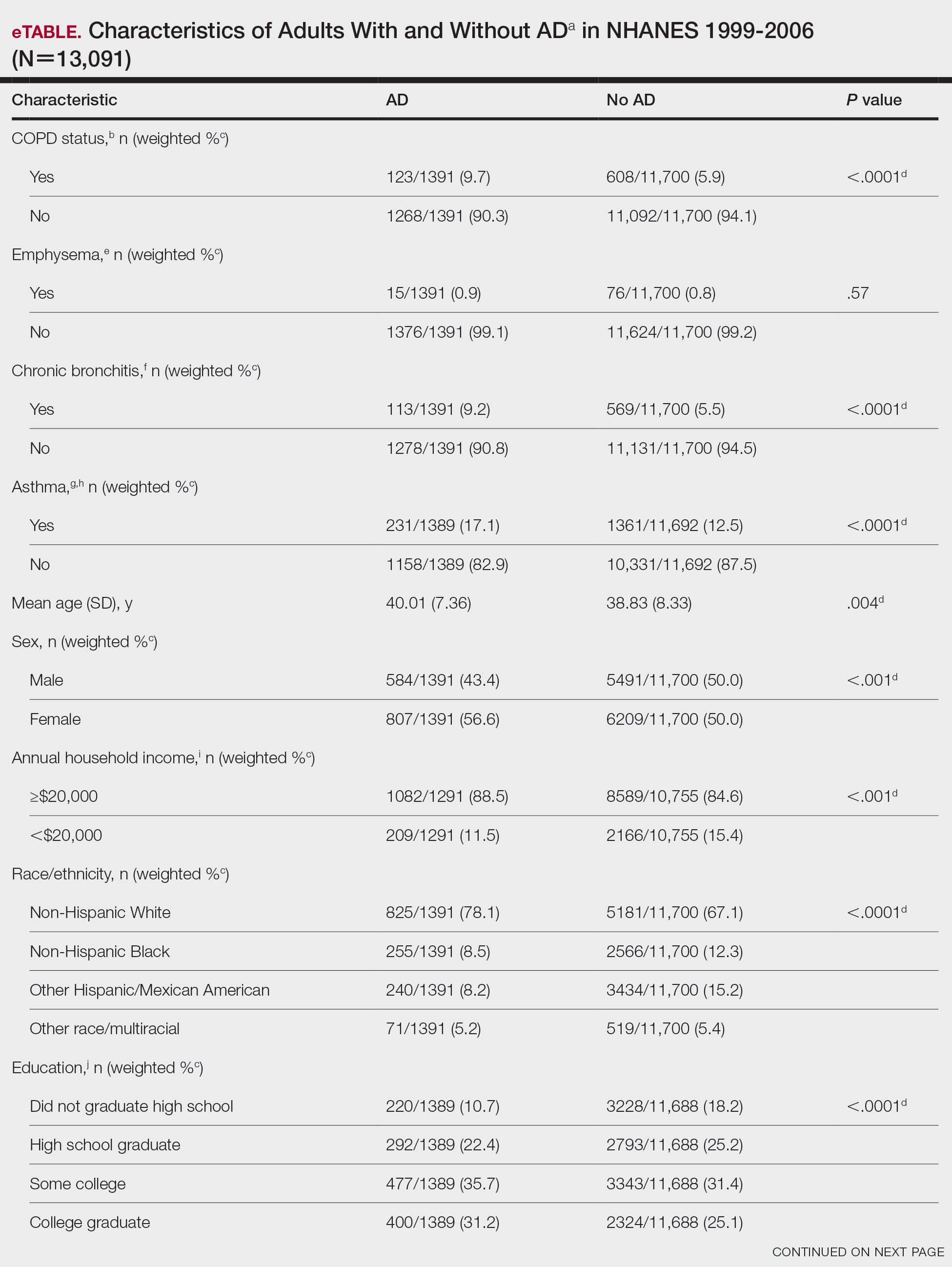
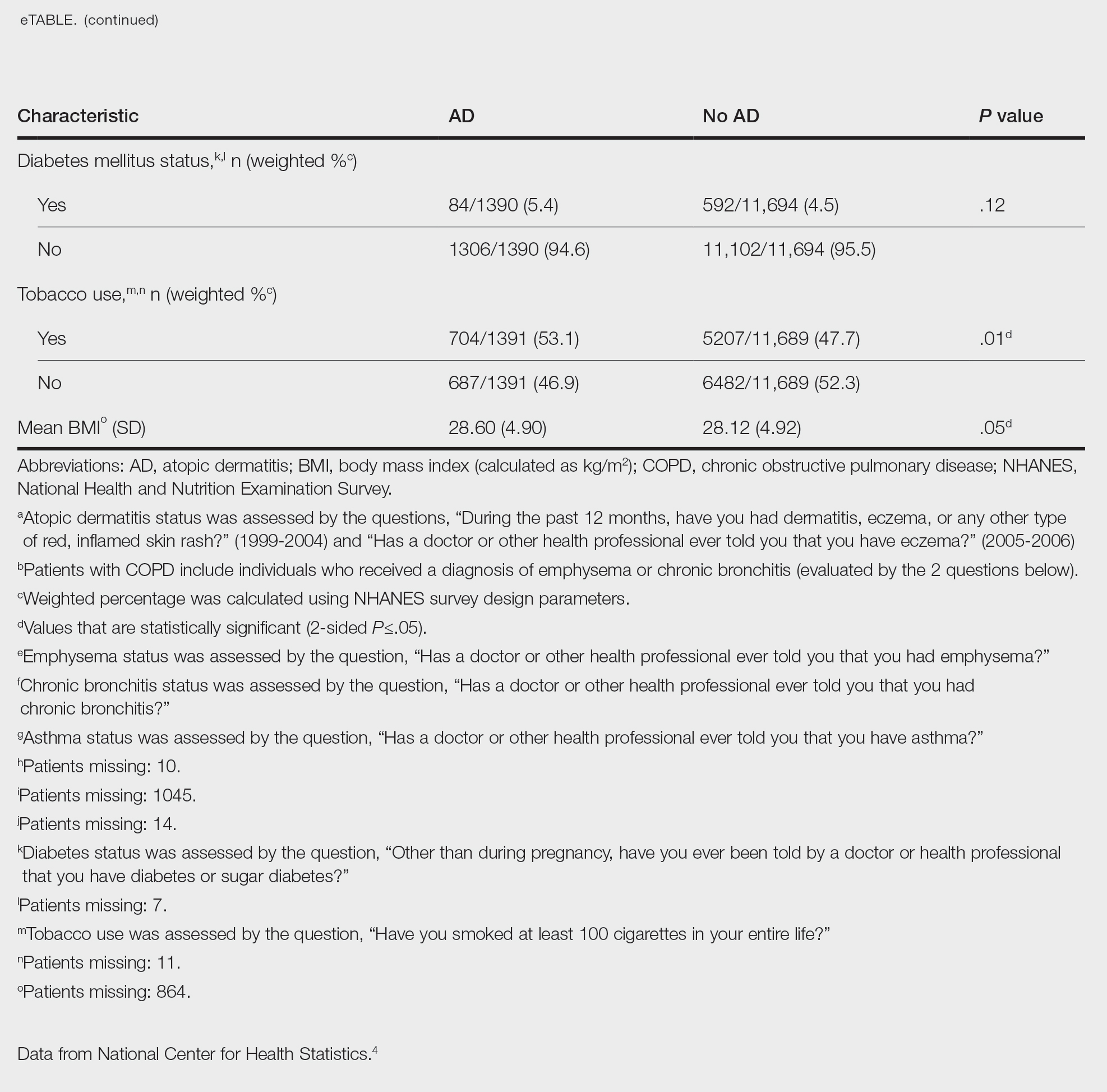
Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).
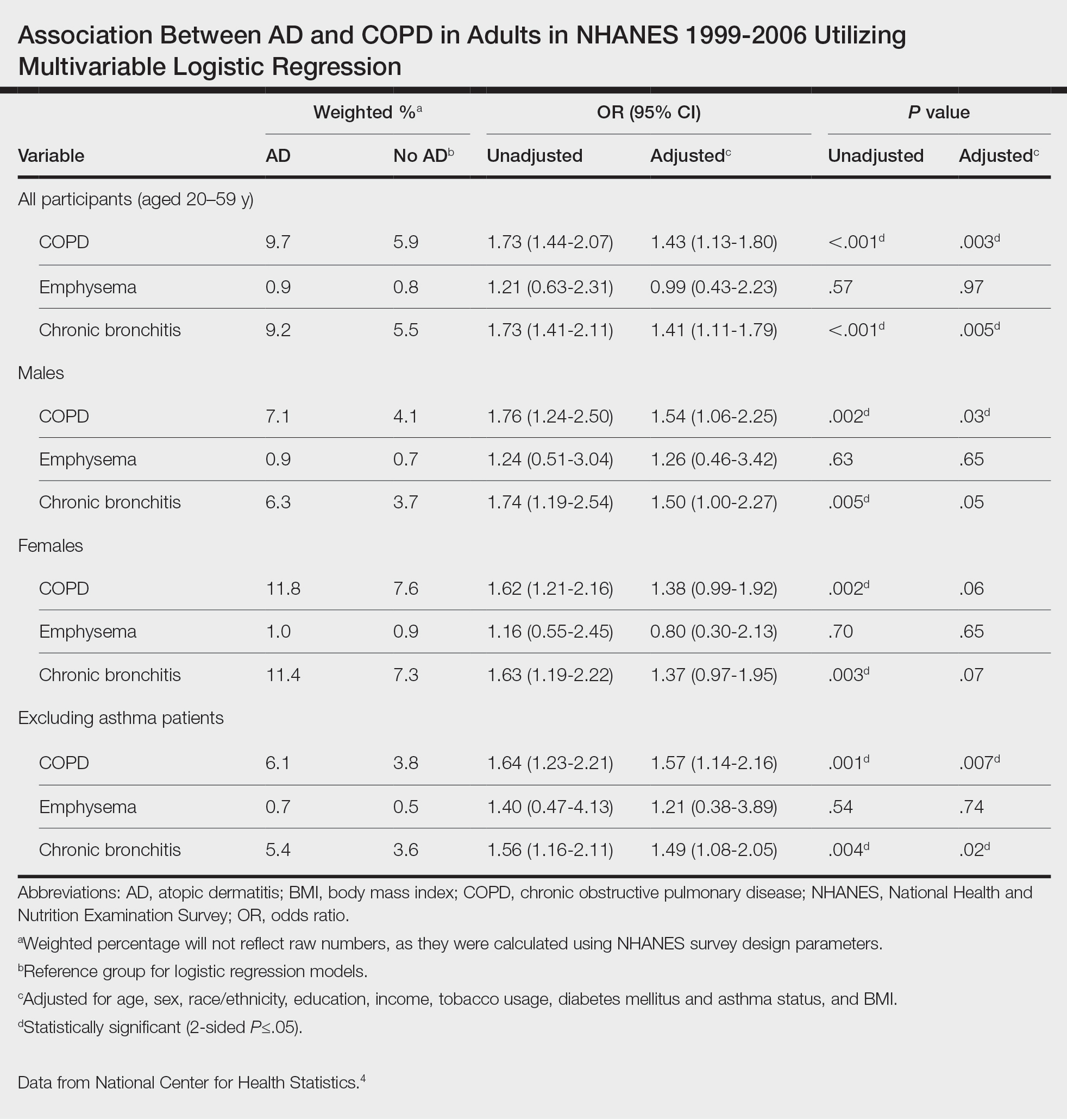
Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).


Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).

Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).


Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).

Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
Practice Points
- Various comorbidities are associated with atopic dermatitis (AD). Currently, research exploring the association between AD and chronic obstructive pulmonary disease is limited.
- Understanding the systemic diseases associated with inflammatory skin diseases can assist with adequate patient management.
Low-dose methotrexate carries higher risk for older patients with CKD
TOPLINE:
The use of low-dose methotrexate among older adults with chronic kidney disease (CKD) was associated with a significantly increased risk at 90 days for serious adverse events requiring a hospital visit, compared with starting treatment with hydroxychloroquine.
METHODOLOGY:
- In a retrospective, population-based cohort study conducted in Ontario, researchers used linked administrative healthcare data to identify adults aged 66 years and older with CKD who were not undergoing dialysis and were new to medication; CKD was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2.
- The study population included 2,309 individuals who began treatment with low-dose methotrexate (5-35 mg/week); they were matched with 2,309 individuals who began treatment with hydroxychloroquine (200-400 mg/day). The median age was 76 years, 69% were women, and rheumatoid arthritis was the most common diagnosis (56%).
- The primary outcome was the risk of a hospital visit at 90 days for a composite of serious adverse events that included myelosuppression, sepsis, pneumotoxic effects, or hepatoxic effects.
TAKEAWAY:
- Overall, 3.55% of methotrexate patients and 1.73% of hydroxychloroquine patients met the primary outcome (risk ratio, 2.05); these events occurred at a median of 49 days and 43 days after starting the medications for the two groups, respectively.
- In an analysis by eGFR category, the risk of serious adverse events at 90 days increased among patients with eGFR levels less than 45 mL/min per 1.73 m2 (RR, 2.79).
- In a secondary comparison, the 90-day risk of serious adverse events was higher among methotrexate patients who began treatment with doses of 15-35 mg/week in comparison with those whose initial doses were 5 to less than 15 mg/week.
IN PRACTICE:
“Patients with CKD starting low-dose methotrexate should have active surveillance, including blood tests and chest radiographs performed regularly to monitor for signs of myelosuppression, infection, hepatotoxic effects, and pneumotoxic effects,” the researchers wrote.
SOURCE:
The lead author on the study was Flory T. Muanda, MD, of Western University, London, Ont. The study was published online in JAMA Network Open.
LIMITATIONS:
The observational design and lack of data on patients’ adherence to medications were among the limiting factors, as were the focus on older adults with CKD and the lack of assessment of the risk-benefit ratio of low-dose methotrexate.
DISCLOSURES:
The study was supported by the Institute for Clinical Evaluative Sciences. Dr. Muanda had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
The use of low-dose methotrexate among older adults with chronic kidney disease (CKD) was associated with a significantly increased risk at 90 days for serious adverse events requiring a hospital visit, compared with starting treatment with hydroxychloroquine.
METHODOLOGY:
- In a retrospective, population-based cohort study conducted in Ontario, researchers used linked administrative healthcare data to identify adults aged 66 years and older with CKD who were not undergoing dialysis and were new to medication; CKD was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2.
- The study population included 2,309 individuals who began treatment with low-dose methotrexate (5-35 mg/week); they were matched with 2,309 individuals who began treatment with hydroxychloroquine (200-400 mg/day). The median age was 76 years, 69% were women, and rheumatoid arthritis was the most common diagnosis (56%).
- The primary outcome was the risk of a hospital visit at 90 days for a composite of serious adverse events that included myelosuppression, sepsis, pneumotoxic effects, or hepatoxic effects.
TAKEAWAY:
- Overall, 3.55% of methotrexate patients and 1.73% of hydroxychloroquine patients met the primary outcome (risk ratio, 2.05); these events occurred at a median of 49 days and 43 days after starting the medications for the two groups, respectively.
- In an analysis by eGFR category, the risk of serious adverse events at 90 days increased among patients with eGFR levels less than 45 mL/min per 1.73 m2 (RR, 2.79).
- In a secondary comparison, the 90-day risk of serious adverse events was higher among methotrexate patients who began treatment with doses of 15-35 mg/week in comparison with those whose initial doses were 5 to less than 15 mg/week.
IN PRACTICE:
“Patients with CKD starting low-dose methotrexate should have active surveillance, including blood tests and chest radiographs performed regularly to monitor for signs of myelosuppression, infection, hepatotoxic effects, and pneumotoxic effects,” the researchers wrote.
SOURCE:
The lead author on the study was Flory T. Muanda, MD, of Western University, London, Ont. The study was published online in JAMA Network Open.
LIMITATIONS:
The observational design and lack of data on patients’ adherence to medications were among the limiting factors, as were the focus on older adults with CKD and the lack of assessment of the risk-benefit ratio of low-dose methotrexate.
DISCLOSURES:
The study was supported by the Institute for Clinical Evaluative Sciences. Dr. Muanda had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
The use of low-dose methotrexate among older adults with chronic kidney disease (CKD) was associated with a significantly increased risk at 90 days for serious adverse events requiring a hospital visit, compared with starting treatment with hydroxychloroquine.
METHODOLOGY:
- In a retrospective, population-based cohort study conducted in Ontario, researchers used linked administrative healthcare data to identify adults aged 66 years and older with CKD who were not undergoing dialysis and were new to medication; CKD was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2.
- The study population included 2,309 individuals who began treatment with low-dose methotrexate (5-35 mg/week); they were matched with 2,309 individuals who began treatment with hydroxychloroquine (200-400 mg/day). The median age was 76 years, 69% were women, and rheumatoid arthritis was the most common diagnosis (56%).
- The primary outcome was the risk of a hospital visit at 90 days for a composite of serious adverse events that included myelosuppression, sepsis, pneumotoxic effects, or hepatoxic effects.
TAKEAWAY:
- Overall, 3.55% of methotrexate patients and 1.73% of hydroxychloroquine patients met the primary outcome (risk ratio, 2.05); these events occurred at a median of 49 days and 43 days after starting the medications for the two groups, respectively.
- In an analysis by eGFR category, the risk of serious adverse events at 90 days increased among patients with eGFR levels less than 45 mL/min per 1.73 m2 (RR, 2.79).
- In a secondary comparison, the 90-day risk of serious adverse events was higher among methotrexate patients who began treatment with doses of 15-35 mg/week in comparison with those whose initial doses were 5 to less than 15 mg/week.
IN PRACTICE:
“Patients with CKD starting low-dose methotrexate should have active surveillance, including blood tests and chest radiographs performed regularly to monitor for signs of myelosuppression, infection, hepatotoxic effects, and pneumotoxic effects,” the researchers wrote.
SOURCE:
The lead author on the study was Flory T. Muanda, MD, of Western University, London, Ont. The study was published online in JAMA Network Open.
LIMITATIONS:
The observational design and lack of data on patients’ adherence to medications were among the limiting factors, as were the focus on older adults with CKD and the lack of assessment of the risk-benefit ratio of low-dose methotrexate.
DISCLOSURES:
The study was supported by the Institute for Clinical Evaluative Sciences. Dr. Muanda had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Tapinarof effective for AD in patients as young as 2 years
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
AT THE EADV CONGRESS
Parent concerns a factor when treating eczema in children with darker skin types
NEW YORK –
Skin diseases pose a greater risk of both hyper- and hypopigmentation in patients with darker skin types, but the fear and concern that this raises for permanent disfigurement is not limited to Blacks, Dr. Heath, assistant professor of pediatric dermatology at Temple University, Philadelphia, said at the Skin of Color Update 2023.
“Culturally, pigmentation changes can be huge. For people of Indian descent, for example, pigmentary changes like light spots on the skin might be an obstacle to marriage, so it can really be life changing,” she added.
In patients with darker skin tones presenting with an inflammatory skin disease, such as AD or psoriasis, Dr. Heath advised asking specifically about change in skin tone even if it is not readily apparent. In pediatric patients, it is also appropriate to include parents in this conversation.
Consider the parent’s perspective
“When you are taking care of a child or adolescent, the patient is likely to be concerned about changes in pigmentation, but it is important to remember that the adult in the room might have had their own journey with brown skin and has dealt with the burden of pigment changes,” Dr. Heath said.
For the parent, the pigmentation changes, rather than the inflammation, might be the governing issue and the reason that he or she brought the child to the clinician. Dr. Heath suggested that it is important for caregivers to explicitly recognize their concern, explain that addressing the pigmentary changes is part of the treatment plan, and to create realistic expectations about how long pigmentary changes will take to resolve.
As an example, Dr. Heath recounted a difficult case of a Black infant with disseminated hyperpigmentation and features that did not preclude pathology other than AD. Dr. Heath created a multifaceted treatment plan to address the inflammation in distinct areas of the body that included low-strength topical steroids for the face, stronger steroids for the body, and advice on scalp and skin care.
“I thought this was a great treatment plan out of the gate – I was covering all of the things on my differential list – I thought that the mom would be thinking, this doctor is amazing,” Dr. Heath said.
Pigmentary changes are a priority
However, that was not what the patient’s mother was thinking. Having failed to explicitly recognize her concern about the pigmentation changes and how the treatment would address this issue, the mother was disappointed.
“She had one question: Will my baby ever be one color? That was her main concern,” said Dr. Heath, indicating that other clinicians seeing inflammatory diseases in children with darker skin types can learn from her experience.
“Really, you have to acknowledge that the condition you are treating is causing the pigmentation change, and we do see that and that we have a treatment plan in place,” she said.
Because of differences in how inflammatory skin diseases present in darker skin types, there is plenty of room for a delayed diagnosis for clinicians who do not see many of these patients, according to Dr. Heath. Follicular eczema, which is common in skin of color, often presents with pruritus but differences in the appearance of the underlying disease can threaten a delay in diagnosis.
In cases of follicular eczema with itch in darker skin, the bumps look and feel like goose bumps, which “means that the eczema is really active and inflamed,” Dr. Heath said. When the skin becomes smooth and the itch dissipates, “you know that they are under great control.”
Psoriasis is often missed in children with darker skin types based on the misperception that it is rare. Although it is true that it is less common in Blacks than Whites, it is not rare, according to Dr. Heath. In inspecting the telltale erythematous plaque–like lesions, clinicians might start to consider alternative diagnoses when they do not detect the same erythematous appearance, but the reddish tone is often concealed in darker skin.
She said that predominant involvement in the head and neck and diaper area is often more common in children of color and that nail or scalp involvement, when present, is often a clue that psoriasis is the diagnosis.
Again, because many clinicians do not think immediately of psoriasis in darker skin children with lesions in the scalp, Dr. Heath advised this is another reason to include psoriasis in the differential diagnosis.
“If you have a child that has failed multiple courses of treatment for tinea capitis and they have well-demarcated plaques, it’s time to really start to think about pediatric psoriasis,” she said.
Restoring skin tone can be the priority
Asked to comment on Dr. Heath’s advice about the importance of acknowledging pigmentary changes associated with inflammatory skin diseases in patients of color, Jenna Lester, MD, the founding director of the Skin of Color Clinic at the University of California, San Francisco, called it an “often unspoken concern of patients.”
“Pigmentary changes that occur secondary to an inflammatory condition should be addressed and treated alongside the inciting condition,” she agreed.
Even if changes in skin color or skin tone are not a specific complaint of the patients, Dr. Lester also urged clinicians to raise the topic. If change in skin pigmentation is part of the clinical picture, this should be targeted in the treatment plan.
“In acne, for example, often times I find that patients are as worried about postinflammatory hyperpigmentation as they are about their acne,” she said, reiterating the advice provided by Dr. Heath.
Dr. Heath has financial relationships with Arcutis, Janssen, Johnson & Johnson, Lilly, and Regeneron. Dr. Lester reported no potential conflicts of interest.
NEW YORK –
Skin diseases pose a greater risk of both hyper- and hypopigmentation in patients with darker skin types, but the fear and concern that this raises for permanent disfigurement is not limited to Blacks, Dr. Heath, assistant professor of pediatric dermatology at Temple University, Philadelphia, said at the Skin of Color Update 2023.
“Culturally, pigmentation changes can be huge. For people of Indian descent, for example, pigmentary changes like light spots on the skin might be an obstacle to marriage, so it can really be life changing,” she added.
In patients with darker skin tones presenting with an inflammatory skin disease, such as AD or psoriasis, Dr. Heath advised asking specifically about change in skin tone even if it is not readily apparent. In pediatric patients, it is also appropriate to include parents in this conversation.
Consider the parent’s perspective
“When you are taking care of a child or adolescent, the patient is likely to be concerned about changes in pigmentation, but it is important to remember that the adult in the room might have had their own journey with brown skin and has dealt with the burden of pigment changes,” Dr. Heath said.
For the parent, the pigmentation changes, rather than the inflammation, might be the governing issue and the reason that he or she brought the child to the clinician. Dr. Heath suggested that it is important for caregivers to explicitly recognize their concern, explain that addressing the pigmentary changes is part of the treatment plan, and to create realistic expectations about how long pigmentary changes will take to resolve.
As an example, Dr. Heath recounted a difficult case of a Black infant with disseminated hyperpigmentation and features that did not preclude pathology other than AD. Dr. Heath created a multifaceted treatment plan to address the inflammation in distinct areas of the body that included low-strength topical steroids for the face, stronger steroids for the body, and advice on scalp and skin care.
“I thought this was a great treatment plan out of the gate – I was covering all of the things on my differential list – I thought that the mom would be thinking, this doctor is amazing,” Dr. Heath said.
Pigmentary changes are a priority
However, that was not what the patient’s mother was thinking. Having failed to explicitly recognize her concern about the pigmentation changes and how the treatment would address this issue, the mother was disappointed.
“She had one question: Will my baby ever be one color? That was her main concern,” said Dr. Heath, indicating that other clinicians seeing inflammatory diseases in children with darker skin types can learn from her experience.
“Really, you have to acknowledge that the condition you are treating is causing the pigmentation change, and we do see that and that we have a treatment plan in place,” she said.
Because of differences in how inflammatory skin diseases present in darker skin types, there is plenty of room for a delayed diagnosis for clinicians who do not see many of these patients, according to Dr. Heath. Follicular eczema, which is common in skin of color, often presents with pruritus but differences in the appearance of the underlying disease can threaten a delay in diagnosis.
In cases of follicular eczema with itch in darker skin, the bumps look and feel like goose bumps, which “means that the eczema is really active and inflamed,” Dr. Heath said. When the skin becomes smooth and the itch dissipates, “you know that they are under great control.”
Psoriasis is often missed in children with darker skin types based on the misperception that it is rare. Although it is true that it is less common in Blacks than Whites, it is not rare, according to Dr. Heath. In inspecting the telltale erythematous plaque–like lesions, clinicians might start to consider alternative diagnoses when they do not detect the same erythematous appearance, but the reddish tone is often concealed in darker skin.
She said that predominant involvement in the head and neck and diaper area is often more common in children of color and that nail or scalp involvement, when present, is often a clue that psoriasis is the diagnosis.
Again, because many clinicians do not think immediately of psoriasis in darker skin children with lesions in the scalp, Dr. Heath advised this is another reason to include psoriasis in the differential diagnosis.
“If you have a child that has failed multiple courses of treatment for tinea capitis and they have well-demarcated plaques, it’s time to really start to think about pediatric psoriasis,” she said.
Restoring skin tone can be the priority
Asked to comment on Dr. Heath’s advice about the importance of acknowledging pigmentary changes associated with inflammatory skin diseases in patients of color, Jenna Lester, MD, the founding director of the Skin of Color Clinic at the University of California, San Francisco, called it an “often unspoken concern of patients.”
“Pigmentary changes that occur secondary to an inflammatory condition should be addressed and treated alongside the inciting condition,” she agreed.
Even if changes in skin color or skin tone are not a specific complaint of the patients, Dr. Lester also urged clinicians to raise the topic. If change in skin pigmentation is part of the clinical picture, this should be targeted in the treatment plan.
“In acne, for example, often times I find that patients are as worried about postinflammatory hyperpigmentation as they are about their acne,” she said, reiterating the advice provided by Dr. Heath.
Dr. Heath has financial relationships with Arcutis, Janssen, Johnson & Johnson, Lilly, and Regeneron. Dr. Lester reported no potential conflicts of interest.
NEW YORK –
Skin diseases pose a greater risk of both hyper- and hypopigmentation in patients with darker skin types, but the fear and concern that this raises for permanent disfigurement is not limited to Blacks, Dr. Heath, assistant professor of pediatric dermatology at Temple University, Philadelphia, said at the Skin of Color Update 2023.
“Culturally, pigmentation changes can be huge. For people of Indian descent, for example, pigmentary changes like light spots on the skin might be an obstacle to marriage, so it can really be life changing,” she added.
In patients with darker skin tones presenting with an inflammatory skin disease, such as AD or psoriasis, Dr. Heath advised asking specifically about change in skin tone even if it is not readily apparent. In pediatric patients, it is also appropriate to include parents in this conversation.
Consider the parent’s perspective
“When you are taking care of a child or adolescent, the patient is likely to be concerned about changes in pigmentation, but it is important to remember that the adult in the room might have had their own journey with brown skin and has dealt with the burden of pigment changes,” Dr. Heath said.
For the parent, the pigmentation changes, rather than the inflammation, might be the governing issue and the reason that he or she brought the child to the clinician. Dr. Heath suggested that it is important for caregivers to explicitly recognize their concern, explain that addressing the pigmentary changes is part of the treatment plan, and to create realistic expectations about how long pigmentary changes will take to resolve.
As an example, Dr. Heath recounted a difficult case of a Black infant with disseminated hyperpigmentation and features that did not preclude pathology other than AD. Dr. Heath created a multifaceted treatment plan to address the inflammation in distinct areas of the body that included low-strength topical steroids for the face, stronger steroids for the body, and advice on scalp and skin care.
“I thought this was a great treatment plan out of the gate – I was covering all of the things on my differential list – I thought that the mom would be thinking, this doctor is amazing,” Dr. Heath said.
Pigmentary changes are a priority
However, that was not what the patient’s mother was thinking. Having failed to explicitly recognize her concern about the pigmentation changes and how the treatment would address this issue, the mother was disappointed.
“She had one question: Will my baby ever be one color? That was her main concern,” said Dr. Heath, indicating that other clinicians seeing inflammatory diseases in children with darker skin types can learn from her experience.
“Really, you have to acknowledge that the condition you are treating is causing the pigmentation change, and we do see that and that we have a treatment plan in place,” she said.
Because of differences in how inflammatory skin diseases present in darker skin types, there is plenty of room for a delayed diagnosis for clinicians who do not see many of these patients, according to Dr. Heath. Follicular eczema, which is common in skin of color, often presents with pruritus but differences in the appearance of the underlying disease can threaten a delay in diagnosis.
In cases of follicular eczema with itch in darker skin, the bumps look and feel like goose bumps, which “means that the eczema is really active and inflamed,” Dr. Heath said. When the skin becomes smooth and the itch dissipates, “you know that they are under great control.”
Psoriasis is often missed in children with darker skin types based on the misperception that it is rare. Although it is true that it is less common in Blacks than Whites, it is not rare, according to Dr. Heath. In inspecting the telltale erythematous plaque–like lesions, clinicians might start to consider alternative diagnoses when they do not detect the same erythematous appearance, but the reddish tone is often concealed in darker skin.
She said that predominant involvement in the head and neck and diaper area is often more common in children of color and that nail or scalp involvement, when present, is often a clue that psoriasis is the diagnosis.
Again, because many clinicians do not think immediately of psoriasis in darker skin children with lesions in the scalp, Dr. Heath advised this is another reason to include psoriasis in the differential diagnosis.
“If you have a child that has failed multiple courses of treatment for tinea capitis and they have well-demarcated plaques, it’s time to really start to think about pediatric psoriasis,” she said.
Restoring skin tone can be the priority
Asked to comment on Dr. Heath’s advice about the importance of acknowledging pigmentary changes associated with inflammatory skin diseases in patients of color, Jenna Lester, MD, the founding director of the Skin of Color Clinic at the University of California, San Francisco, called it an “often unspoken concern of patients.”
“Pigmentary changes that occur secondary to an inflammatory condition should be addressed and treated alongside the inciting condition,” she agreed.
Even if changes in skin color or skin tone are not a specific complaint of the patients, Dr. Lester also urged clinicians to raise the topic. If change in skin pigmentation is part of the clinical picture, this should be targeted in the treatment plan.
“In acne, for example, often times I find that patients are as worried about postinflammatory hyperpigmentation as they are about their acne,” she said, reiterating the advice provided by Dr. Heath.
Dr. Heath has financial relationships with Arcutis, Janssen, Johnson & Johnson, Lilly, and Regeneron. Dr. Lester reported no potential conflicts of interest.
AT SOC 2023
Commentary: JAK Inhibitors and Comorbidities in AD, December 2023
Schlösser and colleagues provide a real-world report of 48 patients treated with upadacitinib for atopic dermatitis, many of whom had previously been treated with cyclosporine and dupilumab. The upbeat authors concluded, "Overall, adverse events were mostly well tolerated." Being a cynical, glass-is-half-empty kind of person, I wondered what that meant. Most patients (56%) reported adverse events, the most common being acne (25% of patients treated), nausea (13%), respiratory tract infections (10%), and herpes virus (8%). The herpes virus signal is not just a bit of a concern for me, but it also makes it hard for me to convince patients to take a Janus kinase (JAK) inhibitor, as when I even mention herpes, patients reply, often rather emphatically, "I don't want herpes!" I'll be encouraging patients to get vaccinated for shingles when starting them on JAK inhibitors.
Dupilumab seems to work great in real-life use. In Martinez-Cabriales and colleagues' study of 62 children age < 12 with atopic dermatitis, only four discontinued the treatment. One of these was a nonresponder who took only one injection and had flushing, and one of the other three discontinued because their skin had completely cleared.
When I saw the title of Rand and colleagues' article, "Matching-Adjusted Indirect Comparison of the Long-Term Efficacy Maintenance and Adverse Event Rates of Lebrikizumab Versus Dupilumab in Moderate-to-Severe Atopic Dermatitis," I thought, Oh, this is great — a head-to-head, long-term trial comparing lebrikizumab and dupilumab. I was disappointed to find that this was simply a retrospective analysis of data reported from different studies. The study found little difference in efficacy or safety of the two drugs. Both seem to be excellent medications for atopic dermatitis.
Here's another study (Zhou et al) that reports possible increased risk for a comorbidity (cognitive dysfunction) associated with atopic dermatitis. This study reports that there is an elevated hazard ratio that is statistically significant; the article fails to report what the increased absolute risk is for cognitive dysfunction associated with atopic dermatitis. My guess is that it is small and probably clinically unimportant. The hazard ratio for developing dementia was 1.16. It's hard to know how that translates into absolute risk, but my brilliant friend and former partner, Dr Alan Fleischer, once told me that the odds ratio for smoking and lung cancer is something like 100; the hazard ratio is in the range of 20. On the basis of a hazard ratio of 1.16, I don't think patients with atopic dermatitis need to be any more worried about dementia than those without. (Though, to be honest, I think we can all be worried about developing dementia.)
In this tour de force analysis of 83 trials with over 20,000 participants, Drucker and colleagues determined that high doses of abrocitinib and upadacitinib are more effective than even dupilumab for atopic dermatitis. The standard doses of these JAK inhibitors were similar in efficacy to dupilumab. I think it's safe to say that JAK inhibitors are, at least at their high doses, more effective than dupilumab, but safety remains a critical factor in treatment decision-making. I think JAK inhibitors are a great option for patients who need the most effective treatment or who fail to respond to dupilumab.
The title of the article by Oh and colleagues, "Increased Risk of Renal Malignancy in Patients With Moderate to Severe Atopic Dermatitis," seems like it could terrify patients. The study involved an analysis of an enormous number of people, including tens of thousands with atopic dermatitis and millions of controls. The investigators did find statistically significant differences in the rate of malignancy. The rate of renal cancer was about 1.6 per 10,000 person-years for people without atopic dermatitis or people with mild atopic dermatitis; the rate was about 2.5 per 10,000 people for patients with moderate to severe atopic dermatitis. While the rate of renal cancer was statistically significantly higher in patients with moderate to severe atopic dermatitis (ie, the higher rate was unlikely to be occurring due to chance alone), these patients have very little risk for renal malignancy. The authors' conclusion that regular checkups for renal malignancy are recommended for patients with severe atopic dermatitis seems unnecessary to me.
Schlösser and colleagues provide a real-world report of 48 patients treated with upadacitinib for atopic dermatitis, many of whom had previously been treated with cyclosporine and dupilumab. The upbeat authors concluded, "Overall, adverse events were mostly well tolerated." Being a cynical, glass-is-half-empty kind of person, I wondered what that meant. Most patients (56%) reported adverse events, the most common being acne (25% of patients treated), nausea (13%), respiratory tract infections (10%), and herpes virus (8%). The herpes virus signal is not just a bit of a concern for me, but it also makes it hard for me to convince patients to take a Janus kinase (JAK) inhibitor, as when I even mention herpes, patients reply, often rather emphatically, "I don't want herpes!" I'll be encouraging patients to get vaccinated for shingles when starting them on JAK inhibitors.
Dupilumab seems to work great in real-life use. In Martinez-Cabriales and colleagues' study of 62 children age < 12 with atopic dermatitis, only four discontinued the treatment. One of these was a nonresponder who took only one injection and had flushing, and one of the other three discontinued because their skin had completely cleared.
When I saw the title of Rand and colleagues' article, "Matching-Adjusted Indirect Comparison of the Long-Term Efficacy Maintenance and Adverse Event Rates of Lebrikizumab Versus Dupilumab in Moderate-to-Severe Atopic Dermatitis," I thought, Oh, this is great — a head-to-head, long-term trial comparing lebrikizumab and dupilumab. I was disappointed to find that this was simply a retrospective analysis of data reported from different studies. The study found little difference in efficacy or safety of the two drugs. Both seem to be excellent medications for atopic dermatitis.
Here's another study (Zhou et al) that reports possible increased risk for a comorbidity (cognitive dysfunction) associated with atopic dermatitis. This study reports that there is an elevated hazard ratio that is statistically significant; the article fails to report what the increased absolute risk is for cognitive dysfunction associated with atopic dermatitis. My guess is that it is small and probably clinically unimportant. The hazard ratio for developing dementia was 1.16. It's hard to know how that translates into absolute risk, but my brilliant friend and former partner, Dr Alan Fleischer, once told me that the odds ratio for smoking and lung cancer is something like 100; the hazard ratio is in the range of 20. On the basis of a hazard ratio of 1.16, I don't think patients with atopic dermatitis need to be any more worried about dementia than those without. (Though, to be honest, I think we can all be worried about developing dementia.)
In this tour de force analysis of 83 trials with over 20,000 participants, Drucker and colleagues determined that high doses of abrocitinib and upadacitinib are more effective than even dupilumab for atopic dermatitis. The standard doses of these JAK inhibitors were similar in efficacy to dupilumab. I think it's safe to say that JAK inhibitors are, at least at their high doses, more effective than dupilumab, but safety remains a critical factor in treatment decision-making. I think JAK inhibitors are a great option for patients who need the most effective treatment or who fail to respond to dupilumab.
The title of the article by Oh and colleagues, "Increased Risk of Renal Malignancy in Patients With Moderate to Severe Atopic Dermatitis," seems like it could terrify patients. The study involved an analysis of an enormous number of people, including tens of thousands with atopic dermatitis and millions of controls. The investigators did find statistically significant differences in the rate of malignancy. The rate of renal cancer was about 1.6 per 10,000 person-years for people without atopic dermatitis or people with mild atopic dermatitis; the rate was about 2.5 per 10,000 people for patients with moderate to severe atopic dermatitis. While the rate of renal cancer was statistically significantly higher in patients with moderate to severe atopic dermatitis (ie, the higher rate was unlikely to be occurring due to chance alone), these patients have very little risk for renal malignancy. The authors' conclusion that regular checkups for renal malignancy are recommended for patients with severe atopic dermatitis seems unnecessary to me.
Schlösser and colleagues provide a real-world report of 48 patients treated with upadacitinib for atopic dermatitis, many of whom had previously been treated with cyclosporine and dupilumab. The upbeat authors concluded, "Overall, adverse events were mostly well tolerated." Being a cynical, glass-is-half-empty kind of person, I wondered what that meant. Most patients (56%) reported adverse events, the most common being acne (25% of patients treated), nausea (13%), respiratory tract infections (10%), and herpes virus (8%). The herpes virus signal is not just a bit of a concern for me, but it also makes it hard for me to convince patients to take a Janus kinase (JAK) inhibitor, as when I even mention herpes, patients reply, often rather emphatically, "I don't want herpes!" I'll be encouraging patients to get vaccinated for shingles when starting them on JAK inhibitors.
Dupilumab seems to work great in real-life use. In Martinez-Cabriales and colleagues' study of 62 children age < 12 with atopic dermatitis, only four discontinued the treatment. One of these was a nonresponder who took only one injection and had flushing, and one of the other three discontinued because their skin had completely cleared.
When I saw the title of Rand and colleagues' article, "Matching-Adjusted Indirect Comparison of the Long-Term Efficacy Maintenance and Adverse Event Rates of Lebrikizumab Versus Dupilumab in Moderate-to-Severe Atopic Dermatitis," I thought, Oh, this is great — a head-to-head, long-term trial comparing lebrikizumab and dupilumab. I was disappointed to find that this was simply a retrospective analysis of data reported from different studies. The study found little difference in efficacy or safety of the two drugs. Both seem to be excellent medications for atopic dermatitis.
Here's another study (Zhou et al) that reports possible increased risk for a comorbidity (cognitive dysfunction) associated with atopic dermatitis. This study reports that there is an elevated hazard ratio that is statistically significant; the article fails to report what the increased absolute risk is for cognitive dysfunction associated with atopic dermatitis. My guess is that it is small and probably clinically unimportant. The hazard ratio for developing dementia was 1.16. It's hard to know how that translates into absolute risk, but my brilliant friend and former partner, Dr Alan Fleischer, once told me that the odds ratio for smoking and lung cancer is something like 100; the hazard ratio is in the range of 20. On the basis of a hazard ratio of 1.16, I don't think patients with atopic dermatitis need to be any more worried about dementia than those without. (Though, to be honest, I think we can all be worried about developing dementia.)
In this tour de force analysis of 83 trials with over 20,000 participants, Drucker and colleagues determined that high doses of abrocitinib and upadacitinib are more effective than even dupilumab for atopic dermatitis. The standard doses of these JAK inhibitors were similar in efficacy to dupilumab. I think it's safe to say that JAK inhibitors are, at least at their high doses, more effective than dupilumab, but safety remains a critical factor in treatment decision-making. I think JAK inhibitors are a great option for patients who need the most effective treatment or who fail to respond to dupilumab.
The title of the article by Oh and colleagues, "Increased Risk of Renal Malignancy in Patients With Moderate to Severe Atopic Dermatitis," seems like it could terrify patients. The study involved an analysis of an enormous number of people, including tens of thousands with atopic dermatitis and millions of controls. The investigators did find statistically significant differences in the rate of malignancy. The rate of renal cancer was about 1.6 per 10,000 person-years for people without atopic dermatitis or people with mild atopic dermatitis; the rate was about 2.5 per 10,000 people for patients with moderate to severe atopic dermatitis. While the rate of renal cancer was statistically significantly higher in patients with moderate to severe atopic dermatitis (ie, the higher rate was unlikely to be occurring due to chance alone), these patients have very little risk for renal malignancy. The authors' conclusion that regular checkups for renal malignancy are recommended for patients with severe atopic dermatitis seems unnecessary to me.
Lebrikizumab gets European nod for treating moderate-to-severe atopic dermatitis
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.









