User login
A doctor must go to extremes to save a choking victim
Some time ago I was invited to join a bipartisan congressional task force on valley fever, also known as coccidioidomycosis. A large and diverse crowd attended the task force’s first meeting in Bakersfield, Calif. – a meeting for everyone: the medical profession, the public, it even included veterinarians.
The whole thing was a resounding success. Francis Collins was there, the just-retired director of the NIH. Tom Frieden, then-director of the Centers for Disease Control and Prevention was there, as were several congresspeople and also my college roommate, a retired Navy medical corps captain. I was enjoying it.
Afterward, we had a banquet dinner at a restaurant in downtown Bakersfield. One of the people there was a woman I knew well – her husband was a physician friend. The restaurant served steak and salmon, and this woman made the mistake of ordering the steak.
Not long after the entrees were served, I heard a commotion at the table just behind me. I turned around and saw that woman in distress. A piece of steak had wedged in her trachea and she couldn’t breathe.
Almost immediately, the chef showed up. I don’t know how he got there. The chef at this restaurant was a big guy. I mean, probably 6 feet, 5 inches tall and 275 pounds. He tried the Heimlich maneuver. It didn’t work.
At that point, I jumped up. I thought, “Well, maybe I know how to do this better than him.” Probably not, actually. I tried and couldn’t make it work either. So I knew we were going to have to do something.
Paul Krogstad, my friend and research partner who is a pediatric infectious disease physician, stepped up and tried to put his finger in her throat and dig it out. He couldn’t get it. The patient had lost consciousness.
So, I’m thinking, okay, there’s really only one choice. You have to get an airway surgically.
I said, “We have to put her down on the floor.” And then I said, “Knife!”
I was looking at the steak knives on the table and they weren’t to my liking for doing a procedure. My college roommate – the retired Navy man – whipped out this very good pocketknife.
I had never done this in my life.
While I was making the incision, somebody gave Paul a ballpoint pen and he broke it into pieces to make a tracheostomy tube. Once I’d made the little incision, I put the tube in. She wasn’t breathing, but she still had a pulse.
I leaned forward and blew into the tube and inflated her lungs. I could see her lungs balloon up. It was a nice feeling, because I knew I was clearly in the right place.
I can’t quite explain it, but while I was doing this, I was enormously calm and totally focused. I knew there was a crowd of people around me, all looking at me, but I wasn’t conscious of that.
It was really just the four of us: Paul and Tom and me and our patient. Those were the only people that I was really cognizant of. Paul and Tom were not panic stricken at all. I remember somebody shouting, “We have to start CPR!” and Frieden said, “No. We don’t.”
Moments later, she woke up, sat up, coughed, and shot the piece of steak across the room.
She was breathing on her own, but we still taped that tube into place. Somebody had already summoned an ambulance; they were there not very long after we completed this procedure. I got in the ambulance with her and we rode over to the emergency room at Mercy Truxtun.
She was stable and doing okay. I sat with her until a thoracic surgeon showed up. He checked out the situation and decided we didn’t need that tube and took it out. I didn’t want to take that out until I had a surgeon there who could do a formal tracheostomy.
They kept her in the hospital for 3 or 4 days. Now, this woman had always had difficulties swallowing, so steak may not have been the best choice. She still had trouble swallowing afterward but recovered.
I’ve known her and her husband a long time, so it was certainly rewarding to be able to provide this service. Years later, though, when her husband died, I spoke at his funeral. When she was speaking to the gathering, she said, “And oh, by the way, Royce, thanks for saving my life.”
That surprised me. I didn’t think we were going to go there.
I’d never tried to practice medicine “at the roadside” before. But that’s part of the career.
Royce Johnson, MD, is the chief of the division of infectious disease among other leadership positions at Kern Medical in Bakersfield, Calif., and the medical director of the Valley Fever Institute.
A version of this article first appeared on Medscape.com.
Some time ago I was invited to join a bipartisan congressional task force on valley fever, also known as coccidioidomycosis. A large and diverse crowd attended the task force’s first meeting in Bakersfield, Calif. – a meeting for everyone: the medical profession, the public, it even included veterinarians.
The whole thing was a resounding success. Francis Collins was there, the just-retired director of the NIH. Tom Frieden, then-director of the Centers for Disease Control and Prevention was there, as were several congresspeople and also my college roommate, a retired Navy medical corps captain. I was enjoying it.
Afterward, we had a banquet dinner at a restaurant in downtown Bakersfield. One of the people there was a woman I knew well – her husband was a physician friend. The restaurant served steak and salmon, and this woman made the mistake of ordering the steak.
Not long after the entrees were served, I heard a commotion at the table just behind me. I turned around and saw that woman in distress. A piece of steak had wedged in her trachea and she couldn’t breathe.
Almost immediately, the chef showed up. I don’t know how he got there. The chef at this restaurant was a big guy. I mean, probably 6 feet, 5 inches tall and 275 pounds. He tried the Heimlich maneuver. It didn’t work.
At that point, I jumped up. I thought, “Well, maybe I know how to do this better than him.” Probably not, actually. I tried and couldn’t make it work either. So I knew we were going to have to do something.
Paul Krogstad, my friend and research partner who is a pediatric infectious disease physician, stepped up and tried to put his finger in her throat and dig it out. He couldn’t get it. The patient had lost consciousness.
So, I’m thinking, okay, there’s really only one choice. You have to get an airway surgically.
I said, “We have to put her down on the floor.” And then I said, “Knife!”
I was looking at the steak knives on the table and they weren’t to my liking for doing a procedure. My college roommate – the retired Navy man – whipped out this very good pocketknife.
I had never done this in my life.
While I was making the incision, somebody gave Paul a ballpoint pen and he broke it into pieces to make a tracheostomy tube. Once I’d made the little incision, I put the tube in. She wasn’t breathing, but she still had a pulse.
I leaned forward and blew into the tube and inflated her lungs. I could see her lungs balloon up. It was a nice feeling, because I knew I was clearly in the right place.
I can’t quite explain it, but while I was doing this, I was enormously calm and totally focused. I knew there was a crowd of people around me, all looking at me, but I wasn’t conscious of that.
It was really just the four of us: Paul and Tom and me and our patient. Those were the only people that I was really cognizant of. Paul and Tom were not panic stricken at all. I remember somebody shouting, “We have to start CPR!” and Frieden said, “No. We don’t.”
Moments later, she woke up, sat up, coughed, and shot the piece of steak across the room.
She was breathing on her own, but we still taped that tube into place. Somebody had already summoned an ambulance; they were there not very long after we completed this procedure. I got in the ambulance with her and we rode over to the emergency room at Mercy Truxtun.
She was stable and doing okay. I sat with her until a thoracic surgeon showed up. He checked out the situation and decided we didn’t need that tube and took it out. I didn’t want to take that out until I had a surgeon there who could do a formal tracheostomy.
They kept her in the hospital for 3 or 4 days. Now, this woman had always had difficulties swallowing, so steak may not have been the best choice. She still had trouble swallowing afterward but recovered.
I’ve known her and her husband a long time, so it was certainly rewarding to be able to provide this service. Years later, though, when her husband died, I spoke at his funeral. When she was speaking to the gathering, she said, “And oh, by the way, Royce, thanks for saving my life.”
That surprised me. I didn’t think we were going to go there.
I’d never tried to practice medicine “at the roadside” before. But that’s part of the career.
Royce Johnson, MD, is the chief of the division of infectious disease among other leadership positions at Kern Medical in Bakersfield, Calif., and the medical director of the Valley Fever Institute.
A version of this article first appeared on Medscape.com.
Some time ago I was invited to join a bipartisan congressional task force on valley fever, also known as coccidioidomycosis. A large and diverse crowd attended the task force’s first meeting in Bakersfield, Calif. – a meeting for everyone: the medical profession, the public, it even included veterinarians.
The whole thing was a resounding success. Francis Collins was there, the just-retired director of the NIH. Tom Frieden, then-director of the Centers for Disease Control and Prevention was there, as were several congresspeople and also my college roommate, a retired Navy medical corps captain. I was enjoying it.
Afterward, we had a banquet dinner at a restaurant in downtown Bakersfield. One of the people there was a woman I knew well – her husband was a physician friend. The restaurant served steak and salmon, and this woman made the mistake of ordering the steak.
Not long after the entrees were served, I heard a commotion at the table just behind me. I turned around and saw that woman in distress. A piece of steak had wedged in her trachea and she couldn’t breathe.
Almost immediately, the chef showed up. I don’t know how he got there. The chef at this restaurant was a big guy. I mean, probably 6 feet, 5 inches tall and 275 pounds. He tried the Heimlich maneuver. It didn’t work.
At that point, I jumped up. I thought, “Well, maybe I know how to do this better than him.” Probably not, actually. I tried and couldn’t make it work either. So I knew we were going to have to do something.
Paul Krogstad, my friend and research partner who is a pediatric infectious disease physician, stepped up and tried to put his finger in her throat and dig it out. He couldn’t get it. The patient had lost consciousness.
So, I’m thinking, okay, there’s really only one choice. You have to get an airway surgically.
I said, “We have to put her down on the floor.” And then I said, “Knife!”
I was looking at the steak knives on the table and they weren’t to my liking for doing a procedure. My college roommate – the retired Navy man – whipped out this very good pocketknife.
I had never done this in my life.
While I was making the incision, somebody gave Paul a ballpoint pen and he broke it into pieces to make a tracheostomy tube. Once I’d made the little incision, I put the tube in. She wasn’t breathing, but she still had a pulse.
I leaned forward and blew into the tube and inflated her lungs. I could see her lungs balloon up. It was a nice feeling, because I knew I was clearly in the right place.
I can’t quite explain it, but while I was doing this, I was enormously calm and totally focused. I knew there was a crowd of people around me, all looking at me, but I wasn’t conscious of that.
It was really just the four of us: Paul and Tom and me and our patient. Those were the only people that I was really cognizant of. Paul and Tom were not panic stricken at all. I remember somebody shouting, “We have to start CPR!” and Frieden said, “No. We don’t.”
Moments later, she woke up, sat up, coughed, and shot the piece of steak across the room.
She was breathing on her own, but we still taped that tube into place. Somebody had already summoned an ambulance; they were there not very long after we completed this procedure. I got in the ambulance with her and we rode over to the emergency room at Mercy Truxtun.
She was stable and doing okay. I sat with her until a thoracic surgeon showed up. He checked out the situation and decided we didn’t need that tube and took it out. I didn’t want to take that out until I had a surgeon there who could do a formal tracheostomy.
They kept her in the hospital for 3 or 4 days. Now, this woman had always had difficulties swallowing, so steak may not have been the best choice. She still had trouble swallowing afterward but recovered.
I’ve known her and her husband a long time, so it was certainly rewarding to be able to provide this service. Years later, though, when her husband died, I spoke at his funeral. When she was speaking to the gathering, she said, “And oh, by the way, Royce, thanks for saving my life.”
That surprised me. I didn’t think we were going to go there.
I’d never tried to practice medicine “at the roadside” before. But that’s part of the career.
Royce Johnson, MD, is the chief of the division of infectious disease among other leadership positions at Kern Medical in Bakersfield, Calif., and the medical director of the Valley Fever Institute.
A version of this article first appeared on Medscape.com.
Less invasive NSCLC surgery does not compromise survival
suggest results from the CALGB 140503 trial, although strict patient selection remains key.
These new results contrast with those from a previous study from 1995, which found that local recurrence was three times higher and cancer mortality was twice as high with the less invasive procedure.
Those results from nearly 30 years ago established lobectomy as the standard of surgical care in this patient population, but since then advances in imaging and staging have allowed the detection of smaller and earlier tumors, which has “rekindled interest in sublobar resection,” the authors comment.
Hence, they conducted the new trial, which involved almost 700 U.S. patients with clinical T1aN0 NSCLC and a tumor size up to 2 cm, who were randomly assigned to lobar or sublobar tumor resection, and followed for 7 years.
The rates of both disease-free and overall survival were similar between the two groups, with no significant differences observed. There were also no substantial differences in rates of distant and locoregional recurrence.
In addition, there was a suggestion of less reduction in pulmonary function following the less invasive procedure.
“These findings affirm that sublobar resection ... is an effective management approach for this subgroup of patients with NSCLC,” says lead author Nasser Altorki, MD, Weill Cornell Medicine, NewYork–Presbyterian Hospital, New York.
“It is important that these results are interpreted strictly within the constraints of the eligibility criteria mandated by the trial, he emphasizes. “Specifically, the results are applicable only to a highly selected group of patients ... in whom the absence of metastases to hilar and mediastinal lymph nodes is pathologically confirmed.”
Nevertheless, Dr. Altorki said that “these results will become increasingly relevant as the proportion of patients with early-stage lung cancer increases with expanded implementation of lung cancer screening, and as the number of older persons with early-stage disease in whom sublobar resection may be the preferred surgical option increases.”
The study was published online in the New England Journal of Medicine.
In an accompanying editorial, Valerie W. Rusch, MD, Thoracic Service, Memorial Sloan Kettering Cancer Center, New York, agrees. “As CT screening becomes more widespread, this patient population will increase in clinical practice,” she explains.
However, Dr. Rusch also urges caution around patient selection, underlining that the results do not “provide a license for suboptimal surgical care.”
She says that “safeguards” such as the meticulous and strict patient criteria used in the trial “must be preserved in routine practice.”
“Thoracic surgeons will need to expand their expertise in sublobar resections, especially complex segmentectomies, and will need to collaborate closely with pathologists in assessing margins of resection, adequacy of lymph-node staging, and tumor characteristics that may predict recurrence.”
While emphasizing that lobectomy should still be performed when appropriate, Dr. Rusch nevertheless says: “The era of ‘precision’ surgery for NSCLC has arrived.”
Consistent with Japanese results
The investigators also point out that their findings are “consistent” with those of a recent Japanese study that compared lobectomy with anatomical segmentectomy, which found that the 5-year overall survival was 91.1% for lobectomy and 94.3% for segmentectomy.
The authors suggest that the difference in overall survival rates between the two trials might be due to anatomical segmentectomy being “considered by most surgeons to be more oncologically sound than wedge resection.”
In the current trial, wedge resection was allowed, however, “because it is the most frequently practiced method of sublobar resection in North America and Europe; thus, its inclusion would make the trial more representative of a ‘real world’ setting.”
Another important difference could be that more than 90% of the patients in the Japanese trial had adenocarcinoma, 45% with an associated ground-glass component, which is associated with better survival than a completely solid adenocarcinoma.
Dr. Rusch agrees that there are likely to be various factors related to the survival differences between the two trials, including patient selection, intraoperative management, and tumor characteristics.
“However, these two landmark trials are practice-changing because they establish sublobar resection as the standard of care for a select group of patients with NSCLC,” Dr. Rusch concluded.
Study details
Dr. Altorki and colleagues conducted the multicenter, international, randomized, noninferiority, phase 3 trial in patients with clinically staged T1aN0 NSCLC from 83 academic and community-based institutions in the United States, Canada, and Australia.
Patients were required to have a peripheral lung nodule with a solid component of up to 2 cm on preoperative CT, a tumor center in the outer third of the lung, and a tumor location amenable to sublobar resection, whether wedge or segment, or lobar resection, among other criteria.
In all, 697 patients were randomly assigned to undergo either lobar resection or sublobar resection, of whom 59.1% had wedge resection and 37.9% anatomical segmental resection. The median age was 67.9 years, and 57.4% were female. The vast majority (90%) were White.
After a median follow-up of 7 years, the 5-year disease-free survival was 63.6% with sublobar resection and 64.1% following lobar resection.
The team found that sublobar resection was not inferior to lobectomy for disease-free survival, at a hazard ratio for disease recurrence or death of 1.01 (90% confidence interval, 0.83-1.24), which adjusted to 0.99 after taking into account the site where the patient was treated.
The 5-year overall survival rate was 80.3% after sublobar resection, and 78.9% following lobar resection, at a hazard ratio for death of 0.95 (95% CI, 0.72-1.26).
The results were “generally consistent” when accounting for factors such as age group, sex, tumor location, histologic type, smoking history, tumor size, and ECOG performance status, the team says.
Turning to recurrence, they showed that, among 687 patients eligible for assessment, 30.4% of those in the sublobar resection group and 29.3% of those assigned to lobar resection experienced disease recurrence, with 13.4% and 10%, respectively, having locoregional recurrence.
An exploratory analysis indicated that 5-year recurrence-free survival was similar in the two groups, at 70.2% vs. 71.2% or a hazard ratio for recurrence of 1.05 (95% CI, 0.80-1.39). The cumulative incidence of death was also similar.
It was also notable that reduction in predictive forced expiratory volume in 1 second from baseline was lower with sublobar than lobar resection, at –4.0 vs. –6.0, as was the reduction in predicted forced vital capacity, at –3.0 vs. –5.0.
“Although this difference is arguably not clinically meaningful in this patient population with normal baseline pulmonary functions,” the team writes, “it may be more clinically relevant in patients with compromised pulmonary functions, or in those with lower-lobe disease in whom lobar resection may be associated with greater impairment of pulmonary function.”
Dr. Rusch suggests that “more sensitive or functional assessments” of pulmonary function might include “diffusion capacity and 6-minute walk tests,” although she noted that even short-term differences in pulmonary function “may affect perioperative and functional outcomes, especially for tumors in the lower lobe.”
The study was supported by the National Cancer Institute of the National Institutes of Health, including via grants to the Alliance for Clinical Trials in Oncology and the Canadian Cancer Trials Group, and supported in part by Covidien and Ethicon.
Dr. Altorki reports relationships with AstraZeneca, Genentech, Johnson & Johnson, and Regeneron. Dr. Rusch reports relationships with Cancer Research UK, Genentech, and the National Cancer Institute.
A version of this article first appeared on Medscape.com.
suggest results from the CALGB 140503 trial, although strict patient selection remains key.
These new results contrast with those from a previous study from 1995, which found that local recurrence was three times higher and cancer mortality was twice as high with the less invasive procedure.
Those results from nearly 30 years ago established lobectomy as the standard of surgical care in this patient population, but since then advances in imaging and staging have allowed the detection of smaller and earlier tumors, which has “rekindled interest in sublobar resection,” the authors comment.
Hence, they conducted the new trial, which involved almost 700 U.S. patients with clinical T1aN0 NSCLC and a tumor size up to 2 cm, who were randomly assigned to lobar or sublobar tumor resection, and followed for 7 years.
The rates of both disease-free and overall survival were similar between the two groups, with no significant differences observed. There were also no substantial differences in rates of distant and locoregional recurrence.
In addition, there was a suggestion of less reduction in pulmonary function following the less invasive procedure.
“These findings affirm that sublobar resection ... is an effective management approach for this subgroup of patients with NSCLC,” says lead author Nasser Altorki, MD, Weill Cornell Medicine, NewYork–Presbyterian Hospital, New York.
“It is important that these results are interpreted strictly within the constraints of the eligibility criteria mandated by the trial, he emphasizes. “Specifically, the results are applicable only to a highly selected group of patients ... in whom the absence of metastases to hilar and mediastinal lymph nodes is pathologically confirmed.”
Nevertheless, Dr. Altorki said that “these results will become increasingly relevant as the proportion of patients with early-stage lung cancer increases with expanded implementation of lung cancer screening, and as the number of older persons with early-stage disease in whom sublobar resection may be the preferred surgical option increases.”
The study was published online in the New England Journal of Medicine.
In an accompanying editorial, Valerie W. Rusch, MD, Thoracic Service, Memorial Sloan Kettering Cancer Center, New York, agrees. “As CT screening becomes more widespread, this patient population will increase in clinical practice,” she explains.
However, Dr. Rusch also urges caution around patient selection, underlining that the results do not “provide a license for suboptimal surgical care.”
She says that “safeguards” such as the meticulous and strict patient criteria used in the trial “must be preserved in routine practice.”
“Thoracic surgeons will need to expand their expertise in sublobar resections, especially complex segmentectomies, and will need to collaborate closely with pathologists in assessing margins of resection, adequacy of lymph-node staging, and tumor characteristics that may predict recurrence.”
While emphasizing that lobectomy should still be performed when appropriate, Dr. Rusch nevertheless says: “The era of ‘precision’ surgery for NSCLC has arrived.”
Consistent with Japanese results
The investigators also point out that their findings are “consistent” with those of a recent Japanese study that compared lobectomy with anatomical segmentectomy, which found that the 5-year overall survival was 91.1% for lobectomy and 94.3% for segmentectomy.
The authors suggest that the difference in overall survival rates between the two trials might be due to anatomical segmentectomy being “considered by most surgeons to be more oncologically sound than wedge resection.”
In the current trial, wedge resection was allowed, however, “because it is the most frequently practiced method of sublobar resection in North America and Europe; thus, its inclusion would make the trial more representative of a ‘real world’ setting.”
Another important difference could be that more than 90% of the patients in the Japanese trial had adenocarcinoma, 45% with an associated ground-glass component, which is associated with better survival than a completely solid adenocarcinoma.
Dr. Rusch agrees that there are likely to be various factors related to the survival differences between the two trials, including patient selection, intraoperative management, and tumor characteristics.
“However, these two landmark trials are practice-changing because they establish sublobar resection as the standard of care for a select group of patients with NSCLC,” Dr. Rusch concluded.
Study details
Dr. Altorki and colleagues conducted the multicenter, international, randomized, noninferiority, phase 3 trial in patients with clinically staged T1aN0 NSCLC from 83 academic and community-based institutions in the United States, Canada, and Australia.
Patients were required to have a peripheral lung nodule with a solid component of up to 2 cm on preoperative CT, a tumor center in the outer third of the lung, and a tumor location amenable to sublobar resection, whether wedge or segment, or lobar resection, among other criteria.
In all, 697 patients were randomly assigned to undergo either lobar resection or sublobar resection, of whom 59.1% had wedge resection and 37.9% anatomical segmental resection. The median age was 67.9 years, and 57.4% were female. The vast majority (90%) were White.
After a median follow-up of 7 years, the 5-year disease-free survival was 63.6% with sublobar resection and 64.1% following lobar resection.
The team found that sublobar resection was not inferior to lobectomy for disease-free survival, at a hazard ratio for disease recurrence or death of 1.01 (90% confidence interval, 0.83-1.24), which adjusted to 0.99 after taking into account the site where the patient was treated.
The 5-year overall survival rate was 80.3% after sublobar resection, and 78.9% following lobar resection, at a hazard ratio for death of 0.95 (95% CI, 0.72-1.26).
The results were “generally consistent” when accounting for factors such as age group, sex, tumor location, histologic type, smoking history, tumor size, and ECOG performance status, the team says.
Turning to recurrence, they showed that, among 687 patients eligible for assessment, 30.4% of those in the sublobar resection group and 29.3% of those assigned to lobar resection experienced disease recurrence, with 13.4% and 10%, respectively, having locoregional recurrence.
An exploratory analysis indicated that 5-year recurrence-free survival was similar in the two groups, at 70.2% vs. 71.2% or a hazard ratio for recurrence of 1.05 (95% CI, 0.80-1.39). The cumulative incidence of death was also similar.
It was also notable that reduction in predictive forced expiratory volume in 1 second from baseline was lower with sublobar than lobar resection, at –4.0 vs. –6.0, as was the reduction in predicted forced vital capacity, at –3.0 vs. –5.0.
“Although this difference is arguably not clinically meaningful in this patient population with normal baseline pulmonary functions,” the team writes, “it may be more clinically relevant in patients with compromised pulmonary functions, or in those with lower-lobe disease in whom lobar resection may be associated with greater impairment of pulmonary function.”
Dr. Rusch suggests that “more sensitive or functional assessments” of pulmonary function might include “diffusion capacity and 6-minute walk tests,” although she noted that even short-term differences in pulmonary function “may affect perioperative and functional outcomes, especially for tumors in the lower lobe.”
The study was supported by the National Cancer Institute of the National Institutes of Health, including via grants to the Alliance for Clinical Trials in Oncology and the Canadian Cancer Trials Group, and supported in part by Covidien and Ethicon.
Dr. Altorki reports relationships with AstraZeneca, Genentech, Johnson & Johnson, and Regeneron. Dr. Rusch reports relationships with Cancer Research UK, Genentech, and the National Cancer Institute.
A version of this article first appeared on Medscape.com.
suggest results from the CALGB 140503 trial, although strict patient selection remains key.
These new results contrast with those from a previous study from 1995, which found that local recurrence was three times higher and cancer mortality was twice as high with the less invasive procedure.
Those results from nearly 30 years ago established lobectomy as the standard of surgical care in this patient population, but since then advances in imaging and staging have allowed the detection of smaller and earlier tumors, which has “rekindled interest in sublobar resection,” the authors comment.
Hence, they conducted the new trial, which involved almost 700 U.S. patients with clinical T1aN0 NSCLC and a tumor size up to 2 cm, who were randomly assigned to lobar or sublobar tumor resection, and followed for 7 years.
The rates of both disease-free and overall survival were similar between the two groups, with no significant differences observed. There were also no substantial differences in rates of distant and locoregional recurrence.
In addition, there was a suggestion of less reduction in pulmonary function following the less invasive procedure.
“These findings affirm that sublobar resection ... is an effective management approach for this subgroup of patients with NSCLC,” says lead author Nasser Altorki, MD, Weill Cornell Medicine, NewYork–Presbyterian Hospital, New York.
“It is important that these results are interpreted strictly within the constraints of the eligibility criteria mandated by the trial, he emphasizes. “Specifically, the results are applicable only to a highly selected group of patients ... in whom the absence of metastases to hilar and mediastinal lymph nodes is pathologically confirmed.”
Nevertheless, Dr. Altorki said that “these results will become increasingly relevant as the proportion of patients with early-stage lung cancer increases with expanded implementation of lung cancer screening, and as the number of older persons with early-stage disease in whom sublobar resection may be the preferred surgical option increases.”
The study was published online in the New England Journal of Medicine.
In an accompanying editorial, Valerie W. Rusch, MD, Thoracic Service, Memorial Sloan Kettering Cancer Center, New York, agrees. “As CT screening becomes more widespread, this patient population will increase in clinical practice,” she explains.
However, Dr. Rusch also urges caution around patient selection, underlining that the results do not “provide a license for suboptimal surgical care.”
She says that “safeguards” such as the meticulous and strict patient criteria used in the trial “must be preserved in routine practice.”
“Thoracic surgeons will need to expand their expertise in sublobar resections, especially complex segmentectomies, and will need to collaborate closely with pathologists in assessing margins of resection, adequacy of lymph-node staging, and tumor characteristics that may predict recurrence.”
While emphasizing that lobectomy should still be performed when appropriate, Dr. Rusch nevertheless says: “The era of ‘precision’ surgery for NSCLC has arrived.”
Consistent with Japanese results
The investigators also point out that their findings are “consistent” with those of a recent Japanese study that compared lobectomy with anatomical segmentectomy, which found that the 5-year overall survival was 91.1% for lobectomy and 94.3% for segmentectomy.
The authors suggest that the difference in overall survival rates between the two trials might be due to anatomical segmentectomy being “considered by most surgeons to be more oncologically sound than wedge resection.”
In the current trial, wedge resection was allowed, however, “because it is the most frequently practiced method of sublobar resection in North America and Europe; thus, its inclusion would make the trial more representative of a ‘real world’ setting.”
Another important difference could be that more than 90% of the patients in the Japanese trial had adenocarcinoma, 45% with an associated ground-glass component, which is associated with better survival than a completely solid adenocarcinoma.
Dr. Rusch agrees that there are likely to be various factors related to the survival differences between the two trials, including patient selection, intraoperative management, and tumor characteristics.
“However, these two landmark trials are practice-changing because they establish sublobar resection as the standard of care for a select group of patients with NSCLC,” Dr. Rusch concluded.
Study details
Dr. Altorki and colleagues conducted the multicenter, international, randomized, noninferiority, phase 3 trial in patients with clinically staged T1aN0 NSCLC from 83 academic and community-based institutions in the United States, Canada, and Australia.
Patients were required to have a peripheral lung nodule with a solid component of up to 2 cm on preoperative CT, a tumor center in the outer third of the lung, and a tumor location amenable to sublobar resection, whether wedge or segment, or lobar resection, among other criteria.
In all, 697 patients were randomly assigned to undergo either lobar resection or sublobar resection, of whom 59.1% had wedge resection and 37.9% anatomical segmental resection. The median age was 67.9 years, and 57.4% were female. The vast majority (90%) were White.
After a median follow-up of 7 years, the 5-year disease-free survival was 63.6% with sublobar resection and 64.1% following lobar resection.
The team found that sublobar resection was not inferior to lobectomy for disease-free survival, at a hazard ratio for disease recurrence or death of 1.01 (90% confidence interval, 0.83-1.24), which adjusted to 0.99 after taking into account the site where the patient was treated.
The 5-year overall survival rate was 80.3% after sublobar resection, and 78.9% following lobar resection, at a hazard ratio for death of 0.95 (95% CI, 0.72-1.26).
The results were “generally consistent” when accounting for factors such as age group, sex, tumor location, histologic type, smoking history, tumor size, and ECOG performance status, the team says.
Turning to recurrence, they showed that, among 687 patients eligible for assessment, 30.4% of those in the sublobar resection group and 29.3% of those assigned to lobar resection experienced disease recurrence, with 13.4% and 10%, respectively, having locoregional recurrence.
An exploratory analysis indicated that 5-year recurrence-free survival was similar in the two groups, at 70.2% vs. 71.2% or a hazard ratio for recurrence of 1.05 (95% CI, 0.80-1.39). The cumulative incidence of death was also similar.
It was also notable that reduction in predictive forced expiratory volume in 1 second from baseline was lower with sublobar than lobar resection, at –4.0 vs. –6.0, as was the reduction in predicted forced vital capacity, at –3.0 vs. –5.0.
“Although this difference is arguably not clinically meaningful in this patient population with normal baseline pulmonary functions,” the team writes, “it may be more clinically relevant in patients with compromised pulmonary functions, or in those with lower-lobe disease in whom lobar resection may be associated with greater impairment of pulmonary function.”
Dr. Rusch suggests that “more sensitive or functional assessments” of pulmonary function might include “diffusion capacity and 6-minute walk tests,” although she noted that even short-term differences in pulmonary function “may affect perioperative and functional outcomes, especially for tumors in the lower lobe.”
The study was supported by the National Cancer Institute of the National Institutes of Health, including via grants to the Alliance for Clinical Trials in Oncology and the Canadian Cancer Trials Group, and supported in part by Covidien and Ethicon.
Dr. Altorki reports relationships with AstraZeneca, Genentech, Johnson & Johnson, and Regeneron. Dr. Rusch reports relationships with Cancer Research UK, Genentech, and the National Cancer Institute.
A version of this article first appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Simulation-based training effective for transesophageal echo
Simulation-based teaching of transesophageal echocardiography (TEE) improved cardiology fellows’ knowledge, skills, and comfort with the procedure, compared with traditional training, a new study shows.
“TEE learning may be hampered by the lack of availability of teachers and equipment and by the need for esophageal intubation, which is semi-invasive,” Augustin Coisne, MD, PhD, of the Cardiovascular Research Foundation in New York, said in an interview. “In this setting, simulation emerges as a key educational tool, but we were lacking evidence supporting simulation-based educational programs.”
Fellows in the simulation group achieved higher theoretical test scores and practical test scores after the training than did those in the traditional group.
Furthermore, Dr. Coisne said, “the results of the subgroup analyses were surprising and unexpected. The effect of the simulation-based training was greater among fellows at the beginning of fellowship – i.e., 2 years or less of training – in both theoretical and practical tests and in women [versus men] for the theoretical test.”
Their results, from the randomized SIMULATOR study, were published online in JAMA Cardiology.
More ready, more confident
The researchers randomly assigned 324 cardiology fellows (mean age, 26.4 years; about 30% women) inexperienced in TEE from 42 French university centers to TEE training with or without simulation support. Both groups participated in traditional didactic training using e-learning with an online course that is compulsory for all cardiology fellows in France.
The simulation group also participated in two 2-hour teaching sessions using a TEE simulator.
Each fellow completed a theoretical and a practical test prior to training to assess their baseline TEE level and again 3 months after the end of the training program. A TEE simulator (U/S Mentor Simulator; 3D Systems Simbionix) was used for all tests, and 24 certified echocardiography teachers served as both trainers and raters.
The theoretical tests included 20 online video-based questions to evaluate recognition of standard TEE views, normal anatomy, and some pathological cases. Fellows had 90 seconds to choose the best answer for each question from five multiple-choice options.
For the practical tests, fellows had 3 minutes to familiarize themselves with the handling of the simulator, without specific training and before the probe introduction.
They were asked to show 10 basic views on the simulator and had a maximum of 1 minute for each view.
The coprimary outcomes were the scores in the final theoretical and practical tests. TEE duration and the fellows’ self-assessment of their proficiency were also evaluated.
At baseline, the theoretical and practical test scores were similar between the groups (33.0 for the simulator group vs. 32.5 for the traditional group, and 44.2 vs. 46.1, respectively).
After training, the fellows in the simulation group had higher theoretical and practical test scores than those in the traditional group (47.2% vs. 38.3% and 74.5% vs. 59.0%, respectively).
Score changes were consistently higher when the pretraining scores were lower, an association that was stronger in the simulation group.
Dr. Coisne noted that subgroup analyses showed that the effectiveness of the simulation training was greater when performed at the beginning of the fellowship. On the theoretical test, the point increase was 11.9 for the simulation group versus 4.25 points for the traditional training group; for the practical test, the increases were 24.0 points versus 10.1 points.
After training, it took significantly less time for the simulation group to complete a TEE than it did the traditional group (8.3 vs. 9.4 minutes).
Furthermore, simulation group fellows reported that they felt more ready (mean score, 3.0 vs. 1.7) and more confident (mean score, 3.3 vs. 2.4) about performing a TEE alone after training.
“The simulation approach is definitively scalable to every institution,” Dr. Coisne said. “However, a medico-economic analysis should be interesting because the cost of the simulator and its maintenance might be a limitation to spread simulation-based teaching. The possibility for smaller hospitals to pool their financial input to share a TEE simulator could be considered to increase its cost-effectiveness.”
Real-world outcomes required
Commenting on the study, S. Justin Szawlewicz, MD, chair of cardiovascular medicine at Deborah Heart and Lung Center in Brown Mills, N.J., pointed out that the authors indicated that the number of TEEs performed by the trainees was not collected.
“This would be useful information to determine if those who received simulator training sought out and performed more TEEs, and also to determine if cardiology trainees in France perform a similar number of TEEs as cardiology trainees in the United States.”
In addition, he said, “the 4 hours of simulator training in TEE is extra education and experience that the standard trainees didn’t get. Would 4 extra hours of standard training didactics also improve trainees’ scores?”
Noting that the fellows’ ability to perform TEE in real patients was not assessed, Dr. Szawlewicz said, “a study could be designed that evaluated TEE images from real patients to see if trainees receiving simulator training performed better, more comprehensive and efficient TEEs than standard training.”
Nevertheless, he concluded, “Four hours of simulator training appears to improve TEE knowledge and skills. This is something we would consider at our institution.”
Like Dr. Szawlewicz, Michael Spooner, MD, MBA, of Mercy One North Iowa Heart Center in Mason City, and Kathryn Bertlacher, MD, of the University of Pittsburgh Medical Center, noted in a related editorial, “data are not provided about change in the learner’s behavior or performance on an actual TEE after the course, nor are there data about clinical outcomes such as patient safety or completeness of subsequent TEEs.
“This limitation, which is a limitation of most of the existing TEE simulation literature, is a high bar to cross,” they concluded. “Reaching this bar will require studies such as this to provide foundational understanding.”
Twin-Medical provided the TEE simulators. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
Simulation-based teaching of transesophageal echocardiography (TEE) improved cardiology fellows’ knowledge, skills, and comfort with the procedure, compared with traditional training, a new study shows.
“TEE learning may be hampered by the lack of availability of teachers and equipment and by the need for esophageal intubation, which is semi-invasive,” Augustin Coisne, MD, PhD, of the Cardiovascular Research Foundation in New York, said in an interview. “In this setting, simulation emerges as a key educational tool, but we were lacking evidence supporting simulation-based educational programs.”
Fellows in the simulation group achieved higher theoretical test scores and practical test scores after the training than did those in the traditional group.
Furthermore, Dr. Coisne said, “the results of the subgroup analyses were surprising and unexpected. The effect of the simulation-based training was greater among fellows at the beginning of fellowship – i.e., 2 years or less of training – in both theoretical and practical tests and in women [versus men] for the theoretical test.”
Their results, from the randomized SIMULATOR study, were published online in JAMA Cardiology.
More ready, more confident
The researchers randomly assigned 324 cardiology fellows (mean age, 26.4 years; about 30% women) inexperienced in TEE from 42 French university centers to TEE training with or without simulation support. Both groups participated in traditional didactic training using e-learning with an online course that is compulsory for all cardiology fellows in France.
The simulation group also participated in two 2-hour teaching sessions using a TEE simulator.
Each fellow completed a theoretical and a practical test prior to training to assess their baseline TEE level and again 3 months after the end of the training program. A TEE simulator (U/S Mentor Simulator; 3D Systems Simbionix) was used for all tests, and 24 certified echocardiography teachers served as both trainers and raters.
The theoretical tests included 20 online video-based questions to evaluate recognition of standard TEE views, normal anatomy, and some pathological cases. Fellows had 90 seconds to choose the best answer for each question from five multiple-choice options.
For the practical tests, fellows had 3 minutes to familiarize themselves with the handling of the simulator, without specific training and before the probe introduction.
They were asked to show 10 basic views on the simulator and had a maximum of 1 minute for each view.
The coprimary outcomes were the scores in the final theoretical and practical tests. TEE duration and the fellows’ self-assessment of their proficiency were also evaluated.
At baseline, the theoretical and practical test scores were similar between the groups (33.0 for the simulator group vs. 32.5 for the traditional group, and 44.2 vs. 46.1, respectively).
After training, the fellows in the simulation group had higher theoretical and practical test scores than those in the traditional group (47.2% vs. 38.3% and 74.5% vs. 59.0%, respectively).
Score changes were consistently higher when the pretraining scores were lower, an association that was stronger in the simulation group.
Dr. Coisne noted that subgroup analyses showed that the effectiveness of the simulation training was greater when performed at the beginning of the fellowship. On the theoretical test, the point increase was 11.9 for the simulation group versus 4.25 points for the traditional training group; for the practical test, the increases were 24.0 points versus 10.1 points.
After training, it took significantly less time for the simulation group to complete a TEE than it did the traditional group (8.3 vs. 9.4 minutes).
Furthermore, simulation group fellows reported that they felt more ready (mean score, 3.0 vs. 1.7) and more confident (mean score, 3.3 vs. 2.4) about performing a TEE alone after training.
“The simulation approach is definitively scalable to every institution,” Dr. Coisne said. “However, a medico-economic analysis should be interesting because the cost of the simulator and its maintenance might be a limitation to spread simulation-based teaching. The possibility for smaller hospitals to pool their financial input to share a TEE simulator could be considered to increase its cost-effectiveness.”
Real-world outcomes required
Commenting on the study, S. Justin Szawlewicz, MD, chair of cardiovascular medicine at Deborah Heart and Lung Center in Brown Mills, N.J., pointed out that the authors indicated that the number of TEEs performed by the trainees was not collected.
“This would be useful information to determine if those who received simulator training sought out and performed more TEEs, and also to determine if cardiology trainees in France perform a similar number of TEEs as cardiology trainees in the United States.”
In addition, he said, “the 4 hours of simulator training in TEE is extra education and experience that the standard trainees didn’t get. Would 4 extra hours of standard training didactics also improve trainees’ scores?”
Noting that the fellows’ ability to perform TEE in real patients was not assessed, Dr. Szawlewicz said, “a study could be designed that evaluated TEE images from real patients to see if trainees receiving simulator training performed better, more comprehensive and efficient TEEs than standard training.”
Nevertheless, he concluded, “Four hours of simulator training appears to improve TEE knowledge and skills. This is something we would consider at our institution.”
Like Dr. Szawlewicz, Michael Spooner, MD, MBA, of Mercy One North Iowa Heart Center in Mason City, and Kathryn Bertlacher, MD, of the University of Pittsburgh Medical Center, noted in a related editorial, “data are not provided about change in the learner’s behavior or performance on an actual TEE after the course, nor are there data about clinical outcomes such as patient safety or completeness of subsequent TEEs.
“This limitation, which is a limitation of most of the existing TEE simulation literature, is a high bar to cross,” they concluded. “Reaching this bar will require studies such as this to provide foundational understanding.”
Twin-Medical provided the TEE simulators. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
Simulation-based teaching of transesophageal echocardiography (TEE) improved cardiology fellows’ knowledge, skills, and comfort with the procedure, compared with traditional training, a new study shows.
“TEE learning may be hampered by the lack of availability of teachers and equipment and by the need for esophageal intubation, which is semi-invasive,” Augustin Coisne, MD, PhD, of the Cardiovascular Research Foundation in New York, said in an interview. “In this setting, simulation emerges as a key educational tool, but we were lacking evidence supporting simulation-based educational programs.”
Fellows in the simulation group achieved higher theoretical test scores and practical test scores after the training than did those in the traditional group.
Furthermore, Dr. Coisne said, “the results of the subgroup analyses were surprising and unexpected. The effect of the simulation-based training was greater among fellows at the beginning of fellowship – i.e., 2 years or less of training – in both theoretical and practical tests and in women [versus men] for the theoretical test.”
Their results, from the randomized SIMULATOR study, were published online in JAMA Cardiology.
More ready, more confident
The researchers randomly assigned 324 cardiology fellows (mean age, 26.4 years; about 30% women) inexperienced in TEE from 42 French university centers to TEE training with or without simulation support. Both groups participated in traditional didactic training using e-learning with an online course that is compulsory for all cardiology fellows in France.
The simulation group also participated in two 2-hour teaching sessions using a TEE simulator.
Each fellow completed a theoretical and a practical test prior to training to assess their baseline TEE level and again 3 months after the end of the training program. A TEE simulator (U/S Mentor Simulator; 3D Systems Simbionix) was used for all tests, and 24 certified echocardiography teachers served as both trainers and raters.
The theoretical tests included 20 online video-based questions to evaluate recognition of standard TEE views, normal anatomy, and some pathological cases. Fellows had 90 seconds to choose the best answer for each question from five multiple-choice options.
For the practical tests, fellows had 3 minutes to familiarize themselves with the handling of the simulator, without specific training and before the probe introduction.
They were asked to show 10 basic views on the simulator and had a maximum of 1 minute for each view.
The coprimary outcomes were the scores in the final theoretical and practical tests. TEE duration and the fellows’ self-assessment of their proficiency were also evaluated.
At baseline, the theoretical and practical test scores were similar between the groups (33.0 for the simulator group vs. 32.5 for the traditional group, and 44.2 vs. 46.1, respectively).
After training, the fellows in the simulation group had higher theoretical and practical test scores than those in the traditional group (47.2% vs. 38.3% and 74.5% vs. 59.0%, respectively).
Score changes were consistently higher when the pretraining scores were lower, an association that was stronger in the simulation group.
Dr. Coisne noted that subgroup analyses showed that the effectiveness of the simulation training was greater when performed at the beginning of the fellowship. On the theoretical test, the point increase was 11.9 for the simulation group versus 4.25 points for the traditional training group; for the practical test, the increases were 24.0 points versus 10.1 points.
After training, it took significantly less time for the simulation group to complete a TEE than it did the traditional group (8.3 vs. 9.4 minutes).
Furthermore, simulation group fellows reported that they felt more ready (mean score, 3.0 vs. 1.7) and more confident (mean score, 3.3 vs. 2.4) about performing a TEE alone after training.
“The simulation approach is definitively scalable to every institution,” Dr. Coisne said. “However, a medico-economic analysis should be interesting because the cost of the simulator and its maintenance might be a limitation to spread simulation-based teaching. The possibility for smaller hospitals to pool their financial input to share a TEE simulator could be considered to increase its cost-effectiveness.”
Real-world outcomes required
Commenting on the study, S. Justin Szawlewicz, MD, chair of cardiovascular medicine at Deborah Heart and Lung Center in Brown Mills, N.J., pointed out that the authors indicated that the number of TEEs performed by the trainees was not collected.
“This would be useful information to determine if those who received simulator training sought out and performed more TEEs, and also to determine if cardiology trainees in France perform a similar number of TEEs as cardiology trainees in the United States.”
In addition, he said, “the 4 hours of simulator training in TEE is extra education and experience that the standard trainees didn’t get. Would 4 extra hours of standard training didactics also improve trainees’ scores?”
Noting that the fellows’ ability to perform TEE in real patients was not assessed, Dr. Szawlewicz said, “a study could be designed that evaluated TEE images from real patients to see if trainees receiving simulator training performed better, more comprehensive and efficient TEEs than standard training.”
Nevertheless, he concluded, “Four hours of simulator training appears to improve TEE knowledge and skills. This is something we would consider at our institution.”
Like Dr. Szawlewicz, Michael Spooner, MD, MBA, of Mercy One North Iowa Heart Center in Mason City, and Kathryn Bertlacher, MD, of the University of Pittsburgh Medical Center, noted in a related editorial, “data are not provided about change in the learner’s behavior or performance on an actual TEE after the course, nor are there data about clinical outcomes such as patient safety or completeness of subsequent TEEs.
“This limitation, which is a limitation of most of the existing TEE simulation literature, is a high bar to cross,” they concluded. “Reaching this bar will require studies such as this to provide foundational understanding.”
Twin-Medical provided the TEE simulators. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
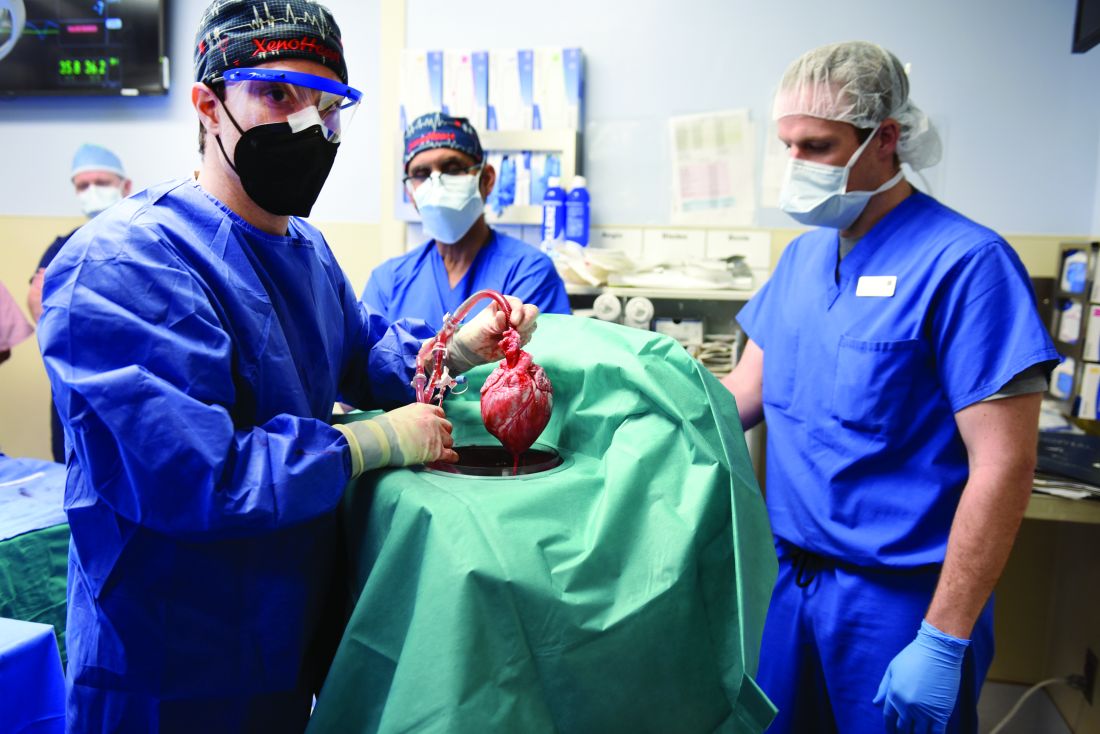
Similar transplant outcomes with hearts donated after circulatory death
Transplantation of hearts donated after circulatory death (DCD) is associated with short-term clinical outcomes similar to those of hearts donated after brain death (DBD), except for transient posttransplant right heart dysfunction, a single-center analysis suggests.
The right-heart dysfunction resolved by 3 weeks post transplant, and recipient mortality was similar for those receiving DCD and DBD, which is considered standard of care (SOC).
Furthermore, the median waiting list time was significantly shorter for DCD recipients than for SOC recipients (17 vs. 70 days).
The authors suggest that use of DCD hearts could expand the donor pool by as much as 30%.
“Now that we and others have demonstrated the safety of this technique, I believe it is our obligation as a transplant community to use these organs and not allow them to be wasted,” David A. D’Alessandro, MD, of Massachusetts General Hospital, Boston, told this news organization.
“I will caution that DCD heart transplantation is labor intensive, and there is a learning curve which can potentially put patients at risk,” he added. “It is vitally important, therefore, that we learn from each other’s experiences to flatten this curve.”
The study was published online in the Journal of the American College of Cardiology.
Similar outcomes
Dr. D’Alessandro and colleagues compared the hemodynamic and clinical profiles of 47 DCD hearts with 166 SOC hearts implanted at Massachusetts General Hospital between 2016 and 2022. DCD hearts were maintained with use of a proprietary warm perfusion circuit organ care system (OCS, TransMedics).
Baseline characteristics were similar between the groups, except the DCD heart recipients were younger (mean age, 55 vs. 59); they were less likely to be an inpatient at the time of transplant (26% vs. 49%); and they had lower pulmonary vascular resistance (1.73 WU vs. 2.26 WU).
The median time from DCD consent to transplant was significantly shorter than for SOC hearts (17 vs. 70 days). However, there was a higher, though not statistically significant, incidence of severe primary graft dysfunction at 24 hours post transplant with DCD (10.6% vs. SOC 3.6%), leading five DCD recipients (10.6%) and nine SOC recipients (5.4%) to receive venoarterial extracorporeal membrane oxygenation.
Right heart function was significantly impaired in DCD vs. SOC recipients 1 week post transplant, with higher median right atrial pressure (10 mm Hg vs. 7 mm Hg); higher right atrial pressure to pulmonary capillary wedge pressure ratio (0.64 vs. 0.57); and lower pulmonary arterial pulsatility index (1.66 vs. 2.52).
However, by 3 weeks post transplant, right heart function was similar between the groups, as was mortality at 30 days (0 vs. 2%) and 1 year (3% vs. 8%).
Furthermore, hospital length of stay following transplant, intensive care unit length of stay, ICU readmissions, and 30-day readmissions were similar between the groups.
“We and others will continue to push the boundaries of this technique to understand if we can safely extend the warm ischemic time, which could make additional organs available,” Dr. D’Alessandro said. “We will also be exploring additional ways to monitor and assess organ health and viability ex situ and potential avenues of treatment which could repair and optimize organ function.
“A successful DCD heart transplant program requires institutional and team commitment,” he added, “and there are clinical nuances which should be appreciated to minimize patient risks associated with the obligate learning curve.”
Ulrich P. Jorde, MD, of Montefiore Medical Center in New York, author of a related editorial, concluded that heart donation after circulatory death “promises significant expansion of the donor pool and will lead to many lives saved” and that “the current investigation is a timely and important contribution to this effort”.
However, he noted, “it must be acknowledged that donation after cardiac death has evoked significant controversy regarding the ethics of this approach,” particularly when using a technique called normothermic regional perfusion (NRP), in which, after declaration of death and ligation of cerebral vessels, the heart is resuscitated in situ using extracorporeal membrane oxygenation, as opposed to the proprietary warm perfusion OCS used in this study.
“Central to this discussion is the definition of death and its irreversibility,” Dr. Jorde noted. “In contrast to DBD, where brain death protocols are well established and accepted by societies across the globe, DCD protocol rules, e.g., standoff times after complete cessation of circulation, continue to vary even within national jurisdictions. Such variability and incomplete standardization of practice is particularly important when the organ is resuscitated in situ using normothermic regional perfusion.
“The International Society of Heart and Lung Transplantation has recently provided a framework within which donation after cardiac death, with or without the use of NRP, can be conducted to comply with ethical and legal norms and regulations, acknowledging that such norms and regulations may differ between societies,” he wrote. “To advance the field, and to ensure ongoing trust in the transplantation system, it is of critical importance that such discussions are held publicly and transparently.”
More ‘dry runs’
“Donor heart allographs are safe for our patients with heart failure if procured and transplanted in an organized and protocolized manner,” Philip J. Spencer, MD, a cardiovascular and transplant surgeon at Mayo Clinic in Rochester, Minn., told this news organization. “As the techniques are adopted globally, our patients will benefit.”
Nevertheless, like Dr. D’Alessandro, he noted that procurement of DCD hearts is more labor intensive. “A program and its patients must be willing to accept a higher number of ‘dry runs,’ which occurs when the team is sent for an organ and the donor does not progress to circulatory death in a time and manner appropriate for safe organ recovery.
“There is no doubt that being open to these organs will increase the patient’s chances of receiving a donor heart in a shorter period of time,” he said. “However, the experience of a dry run, or multiple, can be emotionally and financially stressful for the patient and the program.”
No commercial funding or relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
Transplantation of hearts donated after circulatory death (DCD) is associated with short-term clinical outcomes similar to those of hearts donated after brain death (DBD), except for transient posttransplant right heart dysfunction, a single-center analysis suggests.
The right-heart dysfunction resolved by 3 weeks post transplant, and recipient mortality was similar for those receiving DCD and DBD, which is considered standard of care (SOC).
Furthermore, the median waiting list time was significantly shorter for DCD recipients than for SOC recipients (17 vs. 70 days).
The authors suggest that use of DCD hearts could expand the donor pool by as much as 30%.
“Now that we and others have demonstrated the safety of this technique, I believe it is our obligation as a transplant community to use these organs and not allow them to be wasted,” David A. D’Alessandro, MD, of Massachusetts General Hospital, Boston, told this news organization.
“I will caution that DCD heart transplantation is labor intensive, and there is a learning curve which can potentially put patients at risk,” he added. “It is vitally important, therefore, that we learn from each other’s experiences to flatten this curve.”
The study was published online in the Journal of the American College of Cardiology.
Similar outcomes
Dr. D’Alessandro and colleagues compared the hemodynamic and clinical profiles of 47 DCD hearts with 166 SOC hearts implanted at Massachusetts General Hospital between 2016 and 2022. DCD hearts were maintained with use of a proprietary warm perfusion circuit organ care system (OCS, TransMedics).
Baseline characteristics were similar between the groups, except the DCD heart recipients were younger (mean age, 55 vs. 59); they were less likely to be an inpatient at the time of transplant (26% vs. 49%); and they had lower pulmonary vascular resistance (1.73 WU vs. 2.26 WU).
The median time from DCD consent to transplant was significantly shorter than for SOC hearts (17 vs. 70 days). However, there was a higher, though not statistically significant, incidence of severe primary graft dysfunction at 24 hours post transplant with DCD (10.6% vs. SOC 3.6%), leading five DCD recipients (10.6%) and nine SOC recipients (5.4%) to receive venoarterial extracorporeal membrane oxygenation.
Right heart function was significantly impaired in DCD vs. SOC recipients 1 week post transplant, with higher median right atrial pressure (10 mm Hg vs. 7 mm Hg); higher right atrial pressure to pulmonary capillary wedge pressure ratio (0.64 vs. 0.57); and lower pulmonary arterial pulsatility index (1.66 vs. 2.52).
However, by 3 weeks post transplant, right heart function was similar between the groups, as was mortality at 30 days (0 vs. 2%) and 1 year (3% vs. 8%).
Furthermore, hospital length of stay following transplant, intensive care unit length of stay, ICU readmissions, and 30-day readmissions were similar between the groups.
“We and others will continue to push the boundaries of this technique to understand if we can safely extend the warm ischemic time, which could make additional organs available,” Dr. D’Alessandro said. “We will also be exploring additional ways to monitor and assess organ health and viability ex situ and potential avenues of treatment which could repair and optimize organ function.
“A successful DCD heart transplant program requires institutional and team commitment,” he added, “and there are clinical nuances which should be appreciated to minimize patient risks associated with the obligate learning curve.”
Ulrich P. Jorde, MD, of Montefiore Medical Center in New York, author of a related editorial, concluded that heart donation after circulatory death “promises significant expansion of the donor pool and will lead to many lives saved” and that “the current investigation is a timely and important contribution to this effort”.
However, he noted, “it must be acknowledged that donation after cardiac death has evoked significant controversy regarding the ethics of this approach,” particularly when using a technique called normothermic regional perfusion (NRP), in which, after declaration of death and ligation of cerebral vessels, the heart is resuscitated in situ using extracorporeal membrane oxygenation, as opposed to the proprietary warm perfusion OCS used in this study.
“Central to this discussion is the definition of death and its irreversibility,” Dr. Jorde noted. “In contrast to DBD, where brain death protocols are well established and accepted by societies across the globe, DCD protocol rules, e.g., standoff times after complete cessation of circulation, continue to vary even within national jurisdictions. Such variability and incomplete standardization of practice is particularly important when the organ is resuscitated in situ using normothermic regional perfusion.
“The International Society of Heart and Lung Transplantation has recently provided a framework within which donation after cardiac death, with or without the use of NRP, can be conducted to comply with ethical and legal norms and regulations, acknowledging that such norms and regulations may differ between societies,” he wrote. “To advance the field, and to ensure ongoing trust in the transplantation system, it is of critical importance that such discussions are held publicly and transparently.”
More ‘dry runs’
“Donor heart allographs are safe for our patients with heart failure if procured and transplanted in an organized and protocolized manner,” Philip J. Spencer, MD, a cardiovascular and transplant surgeon at Mayo Clinic in Rochester, Minn., told this news organization. “As the techniques are adopted globally, our patients will benefit.”
Nevertheless, like Dr. D’Alessandro, he noted that procurement of DCD hearts is more labor intensive. “A program and its patients must be willing to accept a higher number of ‘dry runs,’ which occurs when the team is sent for an organ and the donor does not progress to circulatory death in a time and manner appropriate for safe organ recovery.
“There is no doubt that being open to these organs will increase the patient’s chances of receiving a donor heart in a shorter period of time,” he said. “However, the experience of a dry run, or multiple, can be emotionally and financially stressful for the patient and the program.”
No commercial funding or relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
Transplantation of hearts donated after circulatory death (DCD) is associated with short-term clinical outcomes similar to those of hearts donated after brain death (DBD), except for transient posttransplant right heart dysfunction, a single-center analysis suggests.
The right-heart dysfunction resolved by 3 weeks post transplant, and recipient mortality was similar for those receiving DCD and DBD, which is considered standard of care (SOC).
Furthermore, the median waiting list time was significantly shorter for DCD recipients than for SOC recipients (17 vs. 70 days).
The authors suggest that use of DCD hearts could expand the donor pool by as much as 30%.
“Now that we and others have demonstrated the safety of this technique, I believe it is our obligation as a transplant community to use these organs and not allow them to be wasted,” David A. D’Alessandro, MD, of Massachusetts General Hospital, Boston, told this news organization.
“I will caution that DCD heart transplantation is labor intensive, and there is a learning curve which can potentially put patients at risk,” he added. “It is vitally important, therefore, that we learn from each other’s experiences to flatten this curve.”
The study was published online in the Journal of the American College of Cardiology.
Similar outcomes
Dr. D’Alessandro and colleagues compared the hemodynamic and clinical profiles of 47 DCD hearts with 166 SOC hearts implanted at Massachusetts General Hospital between 2016 and 2022. DCD hearts were maintained with use of a proprietary warm perfusion circuit organ care system (OCS, TransMedics).
Baseline characteristics were similar between the groups, except the DCD heart recipients were younger (mean age, 55 vs. 59); they were less likely to be an inpatient at the time of transplant (26% vs. 49%); and they had lower pulmonary vascular resistance (1.73 WU vs. 2.26 WU).
The median time from DCD consent to transplant was significantly shorter than for SOC hearts (17 vs. 70 days). However, there was a higher, though not statistically significant, incidence of severe primary graft dysfunction at 24 hours post transplant with DCD (10.6% vs. SOC 3.6%), leading five DCD recipients (10.6%) and nine SOC recipients (5.4%) to receive venoarterial extracorporeal membrane oxygenation.
Right heart function was significantly impaired in DCD vs. SOC recipients 1 week post transplant, with higher median right atrial pressure (10 mm Hg vs. 7 mm Hg); higher right atrial pressure to pulmonary capillary wedge pressure ratio (0.64 vs. 0.57); and lower pulmonary arterial pulsatility index (1.66 vs. 2.52).
However, by 3 weeks post transplant, right heart function was similar between the groups, as was mortality at 30 days (0 vs. 2%) and 1 year (3% vs. 8%).
Furthermore, hospital length of stay following transplant, intensive care unit length of stay, ICU readmissions, and 30-day readmissions were similar between the groups.
“We and others will continue to push the boundaries of this technique to understand if we can safely extend the warm ischemic time, which could make additional organs available,” Dr. D’Alessandro said. “We will also be exploring additional ways to monitor and assess organ health and viability ex situ and potential avenues of treatment which could repair and optimize organ function.
“A successful DCD heart transplant program requires institutional and team commitment,” he added, “and there are clinical nuances which should be appreciated to minimize patient risks associated with the obligate learning curve.”
Ulrich P. Jorde, MD, of Montefiore Medical Center in New York, author of a related editorial, concluded that heart donation after circulatory death “promises significant expansion of the donor pool and will lead to many lives saved” and that “the current investigation is a timely and important contribution to this effort”.
However, he noted, “it must be acknowledged that donation after cardiac death has evoked significant controversy regarding the ethics of this approach,” particularly when using a technique called normothermic regional perfusion (NRP), in which, after declaration of death and ligation of cerebral vessels, the heart is resuscitated in situ using extracorporeal membrane oxygenation, as opposed to the proprietary warm perfusion OCS used in this study.
“Central to this discussion is the definition of death and its irreversibility,” Dr. Jorde noted. “In contrast to DBD, where brain death protocols are well established and accepted by societies across the globe, DCD protocol rules, e.g., standoff times after complete cessation of circulation, continue to vary even within national jurisdictions. Such variability and incomplete standardization of practice is particularly important when the organ is resuscitated in situ using normothermic regional perfusion.
“The International Society of Heart and Lung Transplantation has recently provided a framework within which donation after cardiac death, with or without the use of NRP, can be conducted to comply with ethical and legal norms and regulations, acknowledging that such norms and regulations may differ between societies,” he wrote. “To advance the field, and to ensure ongoing trust in the transplantation system, it is of critical importance that such discussions are held publicly and transparently.”
More ‘dry runs’
“Donor heart allographs are safe for our patients with heart failure if procured and transplanted in an organized and protocolized manner,” Philip J. Spencer, MD, a cardiovascular and transplant surgeon at Mayo Clinic in Rochester, Minn., told this news organization. “As the techniques are adopted globally, our patients will benefit.”
Nevertheless, like Dr. D’Alessandro, he noted that procurement of DCD hearts is more labor intensive. “A program and its patients must be willing to accept a higher number of ‘dry runs,’ which occurs when the team is sent for an organ and the donor does not progress to circulatory death in a time and manner appropriate for safe organ recovery.
“There is no doubt that being open to these organs will increase the patient’s chances of receiving a donor heart in a shorter period of time,” he said. “However, the experience of a dry run, or multiple, can be emotionally and financially stressful for the patient and the program.”
No commercial funding or relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Pig heart transplants and the ethical challenges that lie ahead
The long-struggling field of cardiac xenotransplantation has had a very good year.
In January, the University of Maryland made history by keeping a 57-year-old man deemed too sick for a human heart transplant alive for 2 months with a genetically engineered pig heart. On July 12, New York University surgeons reported that heart function was “completely normal with excellent contractility” in two brain-dead patients with pig hearts beating in their chests for 72 hours.
The NYU team approached the project with a decedent model in mind and, after discussions with their IRB equivalent, settled on a 72-hour window because that’s the time they typically keep people ventilated when trying to place their organs, explained Robert A. Montgomery, MD, DPhil, director of the NYU Langone Transplant Institute.
“There’s no real ethical argument for that,” he said in an interview. The consideration is what the family is willing to do when trying to balance doing “something very altruistic and good versus having closure.”
Some families have religious beliefs that burial or interment has to occur very rapidly, whereas others, including one of the family donors, were willing to have the research go on much longer, Dr. Montgomery said. Indeed, the next protocol is being written to consider maintaining the bodies for 2-4 weeks.
“People do vary and you have to kind of accommodate that variation,” he said. “For some people, this isn’t going to be what they’re going to want and that’s why you have to go through the consent process.”
Informed authorization
Arthur L. Caplan, PhD, director of medical ethics at the NYU Langone Medical Center, said the Uniform Anatomical Gift Act recognizes an individual’s right to be an organ donor for transplant and research, but it “mentions nothing about maintaining you in a dead state artificially for research purposes.”
“It’s a major shift in what people are thinking about doing when they die or their relatives die,” he said.
Because organ donation is controlled at the state, not federal, level, the possibility of donating organs for xenotransplantation, like medical aid in dying, will vary between states, observed Dr. Caplan. The best way to ensure that patients whose organs are found to be unsuitable for transplantation have the option is to change state laws.
He noted that cases are already springing up where people are requesting postmortem sperm or egg donations without direct consents from the person who died. “So we have this new area opening up of handling the use of the dead body and we need to bring the law into sync with the possibilities that are out there.”
In terms of informed authorization (informed consent is reserved for the living), Dr. Caplan said there should be written evidence the person wanted to be a donor and, while not required by law, all survivors should give their permission and understand what’s going to be done in terms of the experiment, such as the use of animal parts, when the body will be returned, and the possibility of zoonotic viral infection.
“They have to fully accept that the person is dead and we’re just maintaining them artificially,” he said. “There’s no maintaining anyone who’s alive. That’s a source of a lot of confusion.”
Special committees also need to be appointed with voices from people in organ procurement, law, theology, and patient groups to monitor practice to ensure people who have given permission understood the process, that families have their questions answered independent of the research team, and that clear limits are set on how long experiments will last.
As to what those limits should be: “I think in terms of a week or 2,” Dr. Caplan said. “Obviously we could maintain bodies longer and people have. But I think, culturally in our society, going much past that starts to perhaps stress emotionally, psychologically, family and friends about getting closure.”
“I’m not as comfortable when people say things like, ‘How about 2 months?’ ” he said. “That’s a long time to sort of accept the fact that somebody has died but you can’t complete all the things that go along with the death.”
Dr. Caplan is also uncomfortable with the use of one-off emergency authorizations, as used for Maryland resident David Bennett Sr., who was rejected for standard heart transplantation and required mechanical circulatory support to stay alive.
“It’s too premature, I believe, even to try and rescue someone,” he said. “We need to learn more from the deceased models.”
A better model
Dr. Montgomery noted that primates are very imperfect models for predicting what’s going to happen in humans, and that in order to do xenotransplantation in living humans, there are only two pathways – the one-off emergency authorization or a clinical phase 1 trial.
The decedent model, he said, “will make human trials safer because it’s an intermediate step. You don’t have a living human’s life on the line when you’re trying to do iterative changes and improve the procedure.”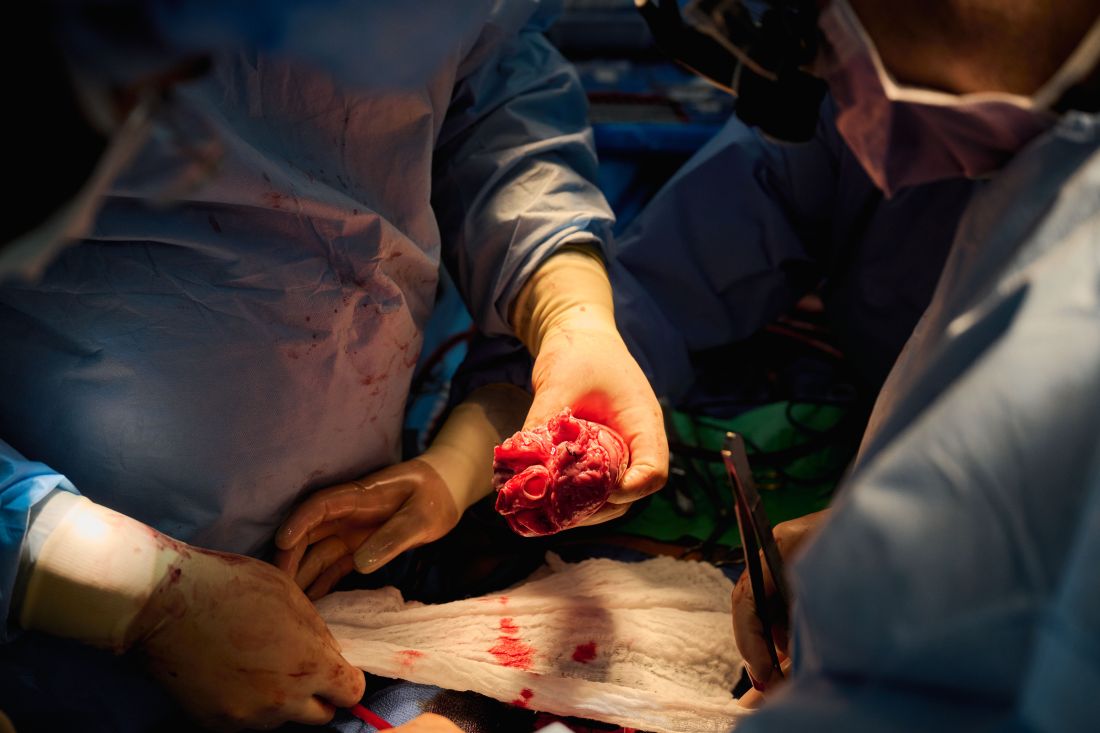
The team, for example, omitted a perfusion pump that was used in the Maryland case and would likely have made its way into phase 1 trials based on baboon data that suggested it was important to have the heart on the pump for hours before it was transplanted, he said. “We didn’t do any of that. We just did it like we would do a regular heart transplant and it started right up, immediately, and started to work.”
The researchers did not release details on the immunosuppression regimen, but noted that, unlike Maryland, they also did not use the experimental anti-CD40 antibody to tamp down the recipients’ immune system.
Although Mr. Bennett’s autopsy did not show any conventional sign of graft rejection, the transplanted pig heart was infected with porcine cytomegalovirus (PCMV) and Mr. Bennett showed traces of DNA from PCMV in his circulation.
Nailing down safety
Dr. Montgomery said he wouldn’t rule out xenotransplantation in a living human, but that the safety issues need to be nailed down. “I think that the tests used on the pig that was the donor for the Bennett case were not sensitive enough for latent virus, and that’s how it slipped through. So there was a bit of going back to the drawing board, really looking at each of the tests, and being sure we had the sensitivity to pick up a latent virus.”
He noted that United Therapeutics, which funded the research and provided the engineered pigs through its subsidiary Revivicor, has created and validated a more sensitive polymerase chain reaction test that covers some 35 different pathogens, microbes, and parasites. NYU has also developed its own platform to repeat the testing and for monitoring after the transplant. “The ones that we’re currently using would have picked up the virus.”
Stuart Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., said “the biggest thing from my perspective is those two amazing families that were willing let this happen. ... If 20 years from now, this is what we’re doing, it’s related to these families being this generous at a really tough time in their lives.”
Dr. Russell said he awaits publication of the data on what the pathology of the heart looks like, but that the experiments “help to give us a lot of reassurance that we don’t need to worry about hyperacute rejection,” which by definition is going to happen in the first 24-48 hours.
That said, longer-term data is essential to potential safety issues. Notably, among the 10 genetic modifications made to the pigs, four were porcine gene knockouts, including a growth hormone receptor knockout to prevent abnormal organ growth inside the recipient’s chest. As a result, the organs seem to be small for the age of the pig and just don’t grow that well, admitted Dr. Montgomery, who said they are currently analyzing this with echocardiography.
Dr. Russell said this may create a sizing issue, but also “if you have a heart that’s more stressed in the pig, from the point of being a donor, maybe it’s not as good a heart as if it was growing normally. But that kind of stuff, I think, is going to take more than two cases and longer-term data to sort out.”
Sharon Hunt, MD, professor emerita, Stanford (Calif.) University Medical Center, and past president of the International Society for Heart Lung Transplantation, said it’s not the technical aspects, but the biology of xenotransplantation that’s really daunting.
“It’s not the physical act of doing it, like they needed a bigger heart or a smaller heart. Those are technical problems but they’ll manage them,” she said. “The big problem is biological – and the bottom line is we don’t really know. We may have overcome hyperacute rejection, which is great, but the rest remains to be seen.”
Dr. Hunt, who worked with heart transplantation pioneer Norman Shumway, MD, and spent decades caring for patients after transplantation, said most families will consent to 24 or 48 hours or even a week of experimentation on a brain-dead loved one, but what the transplant community wants to know is whether this is workable for many months.
“So the fact that the xenotransplant works for 72 hours, yeah, that’s groovy. But, you know, the answer is kind of ‘so what,’ ” she said. “I’d like to see this go for months, like they were trying to do in the human in Maryland.”
For phase 1 trials, even longer-term survival with or without rejection or with rejection that’s treatable is needed, Dr. Hunt suggested.
“We haven’t seen that yet. The Maryland people were very valiant but they lost the cause,” she said. “There’s just so much more to do before we have a viable model to start anything like a phase 1 trial. I’d love it if that happens in my lifetime, but I’m not sure it’s going to.”
Dr. Russell and Dr. Hunt reported no relevant financial relationships. Dr. Caplan reported serving as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
The long-struggling field of cardiac xenotransplantation has had a very good year.
In January, the University of Maryland made history by keeping a 57-year-old man deemed too sick for a human heart transplant alive for 2 months with a genetically engineered pig heart. On July 12, New York University surgeons reported that heart function was “completely normal with excellent contractility” in two brain-dead patients with pig hearts beating in their chests for 72 hours.
The NYU team approached the project with a decedent model in mind and, after discussions with their IRB equivalent, settled on a 72-hour window because that’s the time they typically keep people ventilated when trying to place their organs, explained Robert A. Montgomery, MD, DPhil, director of the NYU Langone Transplant Institute.
“There’s no real ethical argument for that,” he said in an interview. The consideration is what the family is willing to do when trying to balance doing “something very altruistic and good versus having closure.”
Some families have religious beliefs that burial or interment has to occur very rapidly, whereas others, including one of the family donors, were willing to have the research go on much longer, Dr. Montgomery said. Indeed, the next protocol is being written to consider maintaining the bodies for 2-4 weeks.
“People do vary and you have to kind of accommodate that variation,” he said. “For some people, this isn’t going to be what they’re going to want and that’s why you have to go through the consent process.”
Informed authorization
Arthur L. Caplan, PhD, director of medical ethics at the NYU Langone Medical Center, said the Uniform Anatomical Gift Act recognizes an individual’s right to be an organ donor for transplant and research, but it “mentions nothing about maintaining you in a dead state artificially for research purposes.”
“It’s a major shift in what people are thinking about doing when they die or their relatives die,” he said.
Because organ donation is controlled at the state, not federal, level, the possibility of donating organs for xenotransplantation, like medical aid in dying, will vary between states, observed Dr. Caplan. The best way to ensure that patients whose organs are found to be unsuitable for transplantation have the option is to change state laws.
He noted that cases are already springing up where people are requesting postmortem sperm or egg donations without direct consents from the person who died. “So we have this new area opening up of handling the use of the dead body and we need to bring the law into sync with the possibilities that are out there.”
In terms of informed authorization (informed consent is reserved for the living), Dr. Caplan said there should be written evidence the person wanted to be a donor and, while not required by law, all survivors should give their permission and understand what’s going to be done in terms of the experiment, such as the use of animal parts, when the body will be returned, and the possibility of zoonotic viral infection.
“They have to fully accept that the person is dead and we’re just maintaining them artificially,” he said. “There’s no maintaining anyone who’s alive. That’s a source of a lot of confusion.”
Special committees also need to be appointed with voices from people in organ procurement, law, theology, and patient groups to monitor practice to ensure people who have given permission understood the process, that families have their questions answered independent of the research team, and that clear limits are set on how long experiments will last.
As to what those limits should be: “I think in terms of a week or 2,” Dr. Caplan said. “Obviously we could maintain bodies longer and people have. But I think, culturally in our society, going much past that starts to perhaps stress emotionally, psychologically, family and friends about getting closure.”
“I’m not as comfortable when people say things like, ‘How about 2 months?’ ” he said. “That’s a long time to sort of accept the fact that somebody has died but you can’t complete all the things that go along with the death.”
Dr. Caplan is also uncomfortable with the use of one-off emergency authorizations, as used for Maryland resident David Bennett Sr., who was rejected for standard heart transplantation and required mechanical circulatory support to stay alive.
“It’s too premature, I believe, even to try and rescue someone,” he said. “We need to learn more from the deceased models.”
A better model
Dr. Montgomery noted that primates are very imperfect models for predicting what’s going to happen in humans, and that in order to do xenotransplantation in living humans, there are only two pathways – the one-off emergency authorization or a clinical phase 1 trial.
The decedent model, he said, “will make human trials safer because it’s an intermediate step. You don’t have a living human’s life on the line when you’re trying to do iterative changes and improve the procedure.”
The team, for example, omitted a perfusion pump that was used in the Maryland case and would likely have made its way into phase 1 trials based on baboon data that suggested it was important to have the heart on the pump for hours before it was transplanted, he said. “We didn’t do any of that. We just did it like we would do a regular heart transplant and it started right up, immediately, and started to work.”
The researchers did not release details on the immunosuppression regimen, but noted that, unlike Maryland, they also did not use the experimental anti-CD40 antibody to tamp down the recipients’ immune system.
Although Mr. Bennett’s autopsy did not show any conventional sign of graft rejection, the transplanted pig heart was infected with porcine cytomegalovirus (PCMV) and Mr. Bennett showed traces of DNA from PCMV in his circulation.
Nailing down safety
Dr. Montgomery said he wouldn’t rule out xenotransplantation in a living human, but that the safety issues need to be nailed down. “I think that the tests used on the pig that was the donor for the Bennett case were not sensitive enough for latent virus, and that’s how it slipped through. So there was a bit of going back to the drawing board, really looking at each of the tests, and being sure we had the sensitivity to pick up a latent virus.”
He noted that United Therapeutics, which funded the research and provided the engineered pigs through its subsidiary Revivicor, has created and validated a more sensitive polymerase chain reaction test that covers some 35 different pathogens, microbes, and parasites. NYU has also developed its own platform to repeat the testing and for monitoring after the transplant. “The ones that we’re currently using would have picked up the virus.”
Stuart Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., said “the biggest thing from my perspective is those two amazing families that were willing let this happen. ... If 20 years from now, this is what we’re doing, it’s related to these families being this generous at a really tough time in their lives.”
Dr. Russell said he awaits publication of the data on what the pathology of the heart looks like, but that the experiments “help to give us a lot of reassurance that we don’t need to worry about hyperacute rejection,” which by definition is going to happen in the first 24-48 hours.
That said, longer-term data is essential to potential safety issues. Notably, among the 10 genetic modifications made to the pigs, four were porcine gene knockouts, including a growth hormone receptor knockout to prevent abnormal organ growth inside the recipient’s chest. As a result, the organs seem to be small for the age of the pig and just don’t grow that well, admitted Dr. Montgomery, who said they are currently analyzing this with echocardiography.
Dr. Russell said this may create a sizing issue, but also “if you have a heart that’s more stressed in the pig, from the point of being a donor, maybe it’s not as good a heart as if it was growing normally. But that kind of stuff, I think, is going to take more than two cases and longer-term data to sort out.”
Sharon Hunt, MD, professor emerita, Stanford (Calif.) University Medical Center, and past president of the International Society for Heart Lung Transplantation, said it’s not the technical aspects, but the biology of xenotransplantation that’s really daunting.
“It’s not the physical act of doing it, like they needed a bigger heart or a smaller heart. Those are technical problems but they’ll manage them,” she said. “The big problem is biological – and the bottom line is we don’t really know. We may have overcome hyperacute rejection, which is great, but the rest remains to be seen.”
Dr. Hunt, who worked with heart transplantation pioneer Norman Shumway, MD, and spent decades caring for patients after transplantation, said most families will consent to 24 or 48 hours or even a week of experimentation on a brain-dead loved one, but what the transplant community wants to know is whether this is workable for many months.
“So the fact that the xenotransplant works for 72 hours, yeah, that’s groovy. But, you know, the answer is kind of ‘so what,’ ” she said. “I’d like to see this go for months, like they were trying to do in the human in Maryland.”
For phase 1 trials, even longer-term survival with or without rejection or with rejection that’s treatable is needed, Dr. Hunt suggested.
“We haven’t seen that yet. The Maryland people were very valiant but they lost the cause,” she said. “There’s just so much more to do before we have a viable model to start anything like a phase 1 trial. I’d love it if that happens in my lifetime, but I’m not sure it’s going to.”
Dr. Russell and Dr. Hunt reported no relevant financial relationships. Dr. Caplan reported serving as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
The long-struggling field of cardiac xenotransplantation has had a very good year.
In January, the University of Maryland made history by keeping a 57-year-old man deemed too sick for a human heart transplant alive for 2 months with a genetically engineered pig heart. On July 12, New York University surgeons reported that heart function was “completely normal with excellent contractility” in two brain-dead patients with pig hearts beating in their chests for 72 hours.
The NYU team approached the project with a decedent model in mind and, after discussions with their IRB equivalent, settled on a 72-hour window because that’s the time they typically keep people ventilated when trying to place their organs, explained Robert A. Montgomery, MD, DPhil, director of the NYU Langone Transplant Institute.
“There’s no real ethical argument for that,” he said in an interview. The consideration is what the family is willing to do when trying to balance doing “something very altruistic and good versus having closure.”
Some families have religious beliefs that burial or interment has to occur very rapidly, whereas others, including one of the family donors, were willing to have the research go on much longer, Dr. Montgomery said. Indeed, the next protocol is being written to consider maintaining the bodies for 2-4 weeks.
“People do vary and you have to kind of accommodate that variation,” he said. “For some people, this isn’t going to be what they’re going to want and that’s why you have to go through the consent process.”
Informed authorization
Arthur L. Caplan, PhD, director of medical ethics at the NYU Langone Medical Center, said the Uniform Anatomical Gift Act recognizes an individual’s right to be an organ donor for transplant and research, but it “mentions nothing about maintaining you in a dead state artificially for research purposes.”
“It’s a major shift in what people are thinking about doing when they die or their relatives die,” he said.
Because organ donation is controlled at the state, not federal, level, the possibility of donating organs for xenotransplantation, like medical aid in dying, will vary between states, observed Dr. Caplan. The best way to ensure that patients whose organs are found to be unsuitable for transplantation have the option is to change state laws.
He noted that cases are already springing up where people are requesting postmortem sperm or egg donations without direct consents from the person who died. “So we have this new area opening up of handling the use of the dead body and we need to bring the law into sync with the possibilities that are out there.”
In terms of informed authorization (informed consent is reserved for the living), Dr. Caplan said there should be written evidence the person wanted to be a donor and, while not required by law, all survivors should give their permission and understand what’s going to be done in terms of the experiment, such as the use of animal parts, when the body will be returned, and the possibility of zoonotic viral infection.
“They have to fully accept that the person is dead and we’re just maintaining them artificially,” he said. “There’s no maintaining anyone who’s alive. That’s a source of a lot of confusion.”
Special committees also need to be appointed with voices from people in organ procurement, law, theology, and patient groups to monitor practice to ensure people who have given permission understood the process, that families have their questions answered independent of the research team, and that clear limits are set on how long experiments will last.
As to what those limits should be: “I think in terms of a week or 2,” Dr. Caplan said. “Obviously we could maintain bodies longer and people have. But I think, culturally in our society, going much past that starts to perhaps stress emotionally, psychologically, family and friends about getting closure.”
“I’m not as comfortable when people say things like, ‘How about 2 months?’ ” he said. “That’s a long time to sort of accept the fact that somebody has died but you can’t complete all the things that go along with the death.”
Dr. Caplan is also uncomfortable with the use of one-off emergency authorizations, as used for Maryland resident David Bennett Sr., who was rejected for standard heart transplantation and required mechanical circulatory support to stay alive.
“It’s too premature, I believe, even to try and rescue someone,” he said. “We need to learn more from the deceased models.”
A better model
Dr. Montgomery noted that primates are very imperfect models for predicting what’s going to happen in humans, and that in order to do xenotransplantation in living humans, there are only two pathways – the one-off emergency authorization or a clinical phase 1 trial.
The decedent model, he said, “will make human trials safer because it’s an intermediate step. You don’t have a living human’s life on the line when you’re trying to do iterative changes and improve the procedure.”
The team, for example, omitted a perfusion pump that was used in the Maryland case and would likely have made its way into phase 1 trials based on baboon data that suggested it was important to have the heart on the pump for hours before it was transplanted, he said. “We didn’t do any of that. We just did it like we would do a regular heart transplant and it started right up, immediately, and started to work.”
The researchers did not release details on the immunosuppression regimen, but noted that, unlike Maryland, they also did not use the experimental anti-CD40 antibody to tamp down the recipients’ immune system.
Although Mr. Bennett’s autopsy did not show any conventional sign of graft rejection, the transplanted pig heart was infected with porcine cytomegalovirus (PCMV) and Mr. Bennett showed traces of DNA from PCMV in his circulation.
Nailing down safety
Dr. Montgomery said he wouldn’t rule out xenotransplantation in a living human, but that the safety issues need to be nailed down. “I think that the tests used on the pig that was the donor for the Bennett case were not sensitive enough for latent virus, and that’s how it slipped through. So there was a bit of going back to the drawing board, really looking at each of the tests, and being sure we had the sensitivity to pick up a latent virus.”
He noted that United Therapeutics, which funded the research and provided the engineered pigs through its subsidiary Revivicor, has created and validated a more sensitive polymerase chain reaction test that covers some 35 different pathogens, microbes, and parasites. NYU has also developed its own platform to repeat the testing and for monitoring after the transplant. “The ones that we’re currently using would have picked up the virus.”
Stuart Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., said “the biggest thing from my perspective is those two amazing families that were willing let this happen. ... If 20 years from now, this is what we’re doing, it’s related to these families being this generous at a really tough time in their lives.”
Dr. Russell said he awaits publication of the data on what the pathology of the heart looks like, but that the experiments “help to give us a lot of reassurance that we don’t need to worry about hyperacute rejection,” which by definition is going to happen in the first 24-48 hours.
That said, longer-term data is essential to potential safety issues. Notably, among the 10 genetic modifications made to the pigs, four were porcine gene knockouts, including a growth hormone receptor knockout to prevent abnormal organ growth inside the recipient’s chest. As a result, the organs seem to be small for the age of the pig and just don’t grow that well, admitted Dr. Montgomery, who said they are currently analyzing this with echocardiography.
Dr. Russell said this may create a sizing issue, but also “if you have a heart that’s more stressed in the pig, from the point of being a donor, maybe it’s not as good a heart as if it was growing normally. But that kind of stuff, I think, is going to take more than two cases and longer-term data to sort out.”
Sharon Hunt, MD, professor emerita, Stanford (Calif.) University Medical Center, and past president of the International Society for Heart Lung Transplantation, said it’s not the technical aspects, but the biology of xenotransplantation that’s really daunting.
“It’s not the physical act of doing it, like they needed a bigger heart or a smaller heart. Those are technical problems but they’ll manage them,” she said. “The big problem is biological – and the bottom line is we don’t really know. We may have overcome hyperacute rejection, which is great, but the rest remains to be seen.”
Dr. Hunt, who worked with heart transplantation pioneer Norman Shumway, MD, and spent decades caring for patients after transplantation, said most families will consent to 24 or 48 hours or even a week of experimentation on a brain-dead loved one, but what the transplant community wants to know is whether this is workable for many months.
“So the fact that the xenotransplant works for 72 hours, yeah, that’s groovy. But, you know, the answer is kind of ‘so what,’ ” she said. “I’d like to see this go for months, like they were trying to do in the human in Maryland.”
For phase 1 trials, even longer-term survival with or without rejection or with rejection that’s treatable is needed, Dr. Hunt suggested.
“We haven’t seen that yet. The Maryland people were very valiant but they lost the cause,” she said. “There’s just so much more to do before we have a viable model to start anything like a phase 1 trial. I’d love it if that happens in my lifetime, but I’m not sure it’s going to.”
Dr. Russell and Dr. Hunt reported no relevant financial relationships. Dr. Caplan reported serving as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
Transplanted pig hearts functioned normally in deceased persons on ventilator support
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.
Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).
The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.
Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.
Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
Early cardiac rehab as effective as later start after sternotomy
Cardiac rehabilitation (CR) started 2 weeks after sternotomy for a cardiac procedure was noninferior to usual care, in which CR starts 6 weeks after the procedure, with a greater improvement in 6-minute walk test outcomes, a randomized study suggests.
There was no difference in adverse events between groups, although the researchers pointed out that the study was not powered specifically for safety outcomes.
“Cardiac surgical techniques have evolved significantly over the last 60 years, leading to improved survival and shorter hospital stays,” Gordon McGregor, PhD, University of Warwick, Coventry, England, told this news organization. “However, sternal precautions and rehabilitation guidelines have not changed accordingly. There has never been a guideline based on empirical evidence to support rehabilitation professionals working with cardiac surgery patients after median sternotomy.”
“By adopting a progressive individualized approach,” he added, “cardiac surgery sternotomy patients can start cardiac rehabilitation up to 4 weeks earlier than current guidance, and thus potentially complete their recovery sooner.”
Results of the Early Initiation of Poststernotomy Cardiac Rehabilitation Exercise Training study were published online in JAMA Cardiology.
In the study, Dr. McGregor and colleagues randomly assigned 158 patients (mean age, 63 years; 84% men) to 8 weeks of 1-hour, twice-weekly supervised CR exercise training starting 2 weeks (early) or 6 weeks (usual care) after sternotomy.
The primary outcome was change in the 6-minute walk test distance from baseline to 10 or 14 weeks after sternotomy, respectively, and 12 months after randomization.
For usual care, training followed British standards: a warm-up with light cardiovascular and mobility exercises; continuous moderate-intensity cardiovascular exercise; a cooldown; functional exercises using resistance machines and free weights; and upper-body exercises designed to prevent sternal and leg wound pain and complications.
There are no specific outpatient CR exercise guidelines for early CR, so study participants followed an individualized exercise program for the first 2-3 weeks after surgery, starting with light mobility and moderate-intensity cardiovascular training when they could do those exercises with minimal discomfort. They then progressed to current British standards, as per usual care.
Forty patients were lost to follow-up, largely because of the pandemic; about half the participants in each group were included in the primary analysis.
Early CR was not inferior to usual care, the authors wrote. The mean change in 6-minute walk distance from baseline to completion of CR was 28 meters greater in the early group than in the usual-care group, and was achieved 4 weeks earlier in the recovery timeline.
Secondary outcomes (functional fitness and quality of life) improved in both groups and between-group differences were not statistically significant, indicating the noninferiority of early CR, the authors noted.
Safety not proven
There were more adverse events in the early group than in the usual-care group (58 vs. 46) and more serious adverse events (18 vs. 14), but fewer deaths (1 vs. 2).
Although there was no between-group difference in the likelihood of having an adverse or serious adverse event, Dr. McGregor acknowledged that the study was “not powered specifically for safety outcomes.” He added that “there is the potential to run a very large multination definitive superiority [randomized, controlled trial] with safety as the primary outcome; however, a very large sample would be required.”
Meanwhile, he said, “we can say with some degree of certainty that early CR was likely as safe as usual-care CR. In the United Kingdom, we work closely with the British Association for Cardiovascular Prevention and Rehabilitation and the Association of Chartered Physiotherapists in Cardiovascular Rehabilitation, who will incorporate our findings in their guidelines and training courses.”
Questions remain
Asked to comment on the study, John Larry, MD, medical director of cardiology and cardiac rehabilitation at the Ohio State University Wexner Medical Center East Hospital, Columbus, said: “For those under time pressure to return to work, [early CR] could be an advantage to allow more rehab time and improved stamina prior to their return-to-work date.”
That said, he noted, “we typically delay any significant upper-body training activities for 8-10 weeks to avoid impact on healing of the sternum. Thus ... starting sooner would limit the amount of time a patient would have to engage in any upper-body resistance training. Many lose upper body strength after surgery, so this is an important part of the recovery/rehab process.”
Matthew Tomey, MD, director of the cardiac intensive care unit, Mount Sinai Morningside, New York, advised “caution” when interpreting the findings, stating that “there was no evident difference in the primary outcome measure of functional capacity by 14 weeks, and the trial was not designed to directly assess impact on either social functioning or economic productivity.”
“I would be interested to [see] more comprehensive data on safety in a larger, more diverse sample of postoperative patients,” he said, “as well as evidence to indicate clear advantage of an earlier start for patient-centered outcomes specifically after cardiac surgery.
“Perhaps the greatest challenges to full realization of the benefits of CR in practice have been gaps in referral and gaps in enrollment,” he added. “It is incumbent upon us as clinicians to counsel our patients and to provide appropriate referrals.”
The study was supported by the Medical and Life Sciences Research Fund and the Jeremy Pilcher Memorial Fund. No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Cardiac rehabilitation (CR) started 2 weeks after sternotomy for a cardiac procedure was noninferior to usual care, in which CR starts 6 weeks after the procedure, with a greater improvement in 6-minute walk test outcomes, a randomized study suggests.
There was no difference in adverse events between groups, although the researchers pointed out that the study was not powered specifically for safety outcomes.
“Cardiac surgical techniques have evolved significantly over the last 60 years, leading to improved survival and shorter hospital stays,” Gordon McGregor, PhD, University of Warwick, Coventry, England, told this news organization. “However, sternal precautions and rehabilitation guidelines have not changed accordingly. There has never been a guideline based on empirical evidence to support rehabilitation professionals working with cardiac surgery patients after median sternotomy.”
“By adopting a progressive individualized approach,” he added, “cardiac surgery sternotomy patients can start cardiac rehabilitation up to 4 weeks earlier than current guidance, and thus potentially complete their recovery sooner.”
Results of the Early Initiation of Poststernotomy Cardiac Rehabilitation Exercise Training study were published online in JAMA Cardiology.
In the study, Dr. McGregor and colleagues randomly assigned 158 patients (mean age, 63 years; 84% men) to 8 weeks of 1-hour, twice-weekly supervised CR exercise training starting 2 weeks (early) or 6 weeks (usual care) after sternotomy.
The primary outcome was change in the 6-minute walk test distance from baseline to 10 or 14 weeks after sternotomy, respectively, and 12 months after randomization.
For usual care, training followed British standards: a warm-up with light cardiovascular and mobility exercises; continuous moderate-intensity cardiovascular exercise; a cooldown; functional exercises using resistance machines and free weights; and upper-body exercises designed to prevent sternal and leg wound pain and complications.
There are no specific outpatient CR exercise guidelines for early CR, so study participants followed an individualized exercise program for the first 2-3 weeks after surgery, starting with light mobility and moderate-intensity cardiovascular training when they could do those exercises with minimal discomfort. They then progressed to current British standards, as per usual care.
Forty patients were lost to follow-up, largely because of the pandemic; about half the participants in each group were included in the primary analysis.
Early CR was not inferior to usual care, the authors wrote. The mean change in 6-minute walk distance from baseline to completion of CR was 28 meters greater in the early group than in the usual-care group, and was achieved 4 weeks earlier in the recovery timeline.
Secondary outcomes (functional fitness and quality of life) improved in both groups and between-group differences were not statistically significant, indicating the noninferiority of early CR, the authors noted.
Safety not proven
There were more adverse events in the early group than in the usual-care group (58 vs. 46) and more serious adverse events (18 vs. 14), but fewer deaths (1 vs. 2).
Although there was no between-group difference in the likelihood of having an adverse or serious adverse event, Dr. McGregor acknowledged that the study was “not powered specifically for safety outcomes.” He added that “there is the potential to run a very large multination definitive superiority [randomized, controlled trial] with safety as the primary outcome; however, a very large sample would be required.”
Meanwhile, he said, “we can say with some degree of certainty that early CR was likely as safe as usual-care CR. In the United Kingdom, we work closely with the British Association for Cardiovascular Prevention and Rehabilitation and the Association of Chartered Physiotherapists in Cardiovascular Rehabilitation, who will incorporate our findings in their guidelines and training courses.”
Questions remain
Asked to comment on the study, John Larry, MD, medical director of cardiology and cardiac rehabilitation at the Ohio State University Wexner Medical Center East Hospital, Columbus, said: “For those under time pressure to return to work, [early CR] could be an advantage to allow more rehab time and improved stamina prior to their return-to-work date.”
That said, he noted, “we typically delay any significant upper-body training activities for 8-10 weeks to avoid impact on healing of the sternum. Thus ... starting sooner would limit the amount of time a patient would have to engage in any upper-body resistance training. Many lose upper body strength after surgery, so this is an important part of the recovery/rehab process.”
Matthew Tomey, MD, director of the cardiac intensive care unit, Mount Sinai Morningside, New York, advised “caution” when interpreting the findings, stating that “there was no evident difference in the primary outcome measure of functional capacity by 14 weeks, and the trial was not designed to directly assess impact on either social functioning or economic productivity.”
“I would be interested to [see] more comprehensive data on safety in a larger, more diverse sample of postoperative patients,” he said, “as well as evidence to indicate clear advantage of an earlier start for patient-centered outcomes specifically after cardiac surgery.
“Perhaps the greatest challenges to full realization of the benefits of CR in practice have been gaps in referral and gaps in enrollment,” he added. “It is incumbent upon us as clinicians to counsel our patients and to provide appropriate referrals.”
The study was supported by the Medical and Life Sciences Research Fund and the Jeremy Pilcher Memorial Fund. No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
Cardiac rehabilitation (CR) started 2 weeks after sternotomy for a cardiac procedure was noninferior to usual care, in which CR starts 6 weeks after the procedure, with a greater improvement in 6-minute walk test outcomes, a randomized study suggests.
There was no difference in adverse events between groups, although the researchers pointed out that the study was not powered specifically for safety outcomes.
“Cardiac surgical techniques have evolved significantly over the last 60 years, leading to improved survival and shorter hospital stays,” Gordon McGregor, PhD, University of Warwick, Coventry, England, told this news organization. “However, sternal precautions and rehabilitation guidelines have not changed accordingly. There has never been a guideline based on empirical evidence to support rehabilitation professionals working with cardiac surgery patients after median sternotomy.”
“By adopting a progressive individualized approach,” he added, “cardiac surgery sternotomy patients can start cardiac rehabilitation up to 4 weeks earlier than current guidance, and thus potentially complete their recovery sooner.”
Results of the Early Initiation of Poststernotomy Cardiac Rehabilitation Exercise Training study were published online in JAMA Cardiology.
In the study, Dr. McGregor and colleagues randomly assigned 158 patients (mean age, 63 years; 84% men) to 8 weeks of 1-hour, twice-weekly supervised CR exercise training starting 2 weeks (early) or 6 weeks (usual care) after sternotomy.
The primary outcome was change in the 6-minute walk test distance from baseline to 10 or 14 weeks after sternotomy, respectively, and 12 months after randomization.
For usual care, training followed British standards: a warm-up with light cardiovascular and mobility exercises; continuous moderate-intensity cardiovascular exercise; a cooldown; functional exercises using resistance machines and free weights; and upper-body exercises designed to prevent sternal and leg wound pain and complications.
There are no specific outpatient CR exercise guidelines for early CR, so study participants followed an individualized exercise program for the first 2-3 weeks after surgery, starting with light mobility and moderate-intensity cardiovascular training when they could do those exercises with minimal discomfort. They then progressed to current British standards, as per usual care.
Forty patients were lost to follow-up, largely because of the pandemic; about half the participants in each group were included in the primary analysis.
Early CR was not inferior to usual care, the authors wrote. The mean change in 6-minute walk distance from baseline to completion of CR was 28 meters greater in the early group than in the usual-care group, and was achieved 4 weeks earlier in the recovery timeline.
Secondary outcomes (functional fitness and quality of life) improved in both groups and between-group differences were not statistically significant, indicating the noninferiority of early CR, the authors noted.
Safety not proven
There were more adverse events in the early group than in the usual-care group (58 vs. 46) and more serious adverse events (18 vs. 14), but fewer deaths (1 vs. 2).
Although there was no between-group difference in the likelihood of having an adverse or serious adverse event, Dr. McGregor acknowledged that the study was “not powered specifically for safety outcomes.” He added that “there is the potential to run a very large multination definitive superiority [randomized, controlled trial] with safety as the primary outcome; however, a very large sample would be required.”
Meanwhile, he said, “we can say with some degree of certainty that early CR was likely as safe as usual-care CR. In the United Kingdom, we work closely with the British Association for Cardiovascular Prevention and Rehabilitation and the Association of Chartered Physiotherapists in Cardiovascular Rehabilitation, who will incorporate our findings in their guidelines and training courses.”
Questions remain
Asked to comment on the study, John Larry, MD, medical director of cardiology and cardiac rehabilitation at the Ohio State University Wexner Medical Center East Hospital, Columbus, said: “For those under time pressure to return to work, [early CR] could be an advantage to allow more rehab time and improved stamina prior to their return-to-work date.”
That said, he noted, “we typically delay any significant upper-body training activities for 8-10 weeks to avoid impact on healing of the sternum. Thus ... starting sooner would limit the amount of time a patient would have to engage in any upper-body resistance training. Many lose upper body strength after surgery, so this is an important part of the recovery/rehab process.”
Matthew Tomey, MD, director of the cardiac intensive care unit, Mount Sinai Morningside, New York, advised “caution” when interpreting the findings, stating that “there was no evident difference in the primary outcome measure of functional capacity by 14 weeks, and the trial was not designed to directly assess impact on either social functioning or economic productivity.”
“I would be interested to [see] more comprehensive data on safety in a larger, more diverse sample of postoperative patients,” he said, “as well as evidence to indicate clear advantage of an earlier start for patient-centered outcomes specifically after cardiac surgery.
“Perhaps the greatest challenges to full realization of the benefits of CR in practice have been gaps in referral and gaps in enrollment,” he added. “It is incumbent upon us as clinicians to counsel our patients and to provide appropriate referrals.”
The study was supported by the Medical and Life Sciences Research Fund and the Jeremy Pilcher Memorial Fund. No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cause of death in pig heart recipient: New clues
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.