User login
Improving Physicians’ Bowel Documentation on Geriatric Wards
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
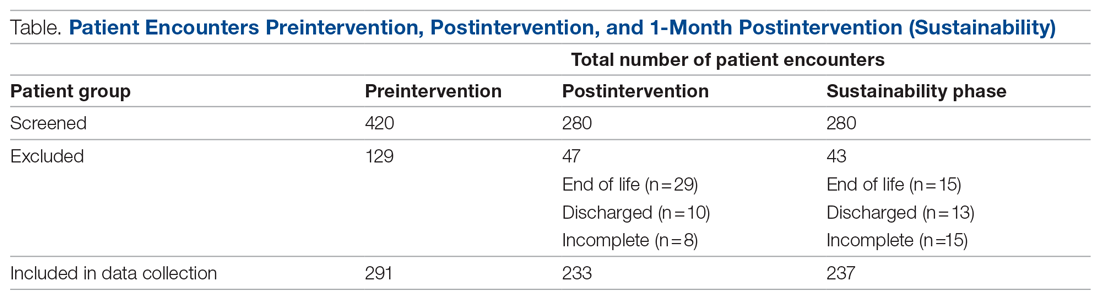
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).
The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
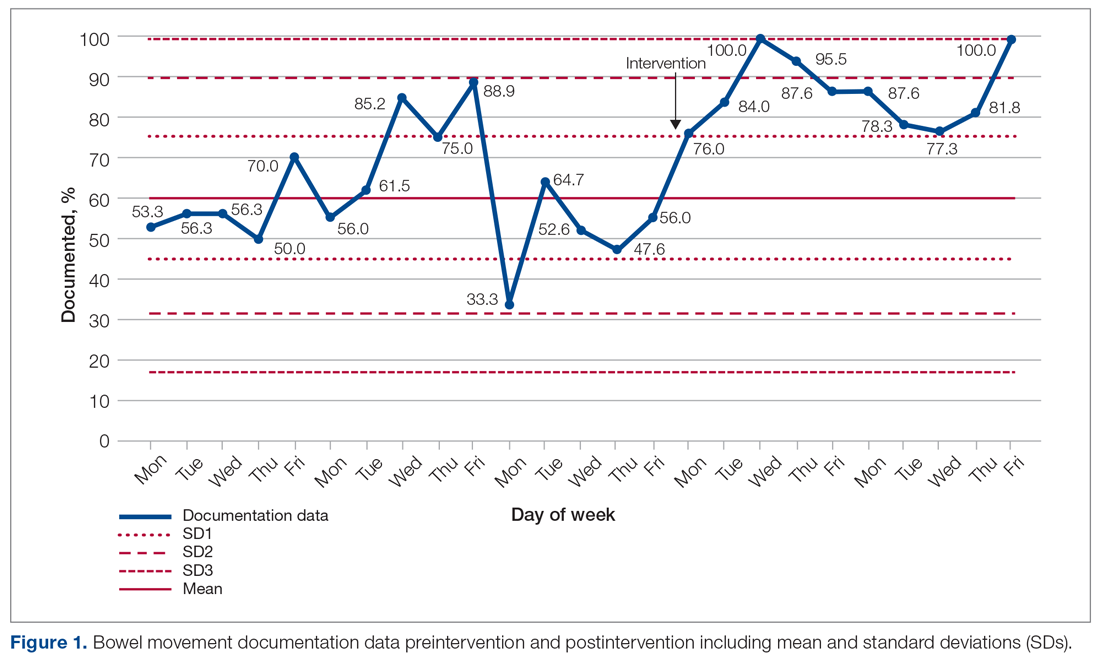
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.
The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
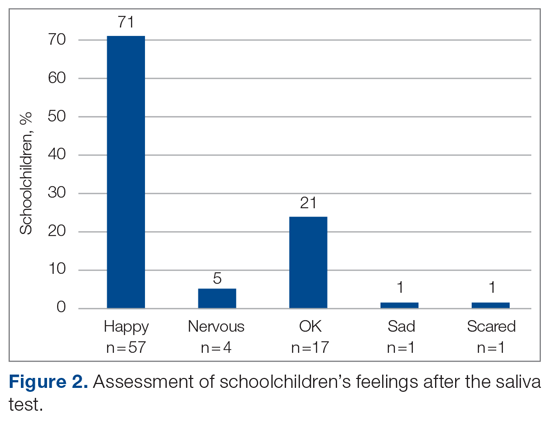
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.
Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; alecnoar@live.co.uk.
Financial disclosures: None.
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).
The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.
The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.
Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; alecnoar@live.co.uk.
Financial disclosures: None.
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).
The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.
The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.
Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; alecnoar@live.co.uk.
Financial disclosures: None.
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002
Feasibility of a Saliva-Based COVID-19 Screening Program in Abu Dhabi Primary Schools
From Health Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates (Dr. Virji and Aisha Al Hamiz), Public Health, Abu Dhabi Public Health Center, Abu Dhabi, United Arab Emirates (Drs. Al Hajeri, Al Shehhi, Al Memari, and Ahlam Al Maskari), College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, United Arab Emirates, Department of Medicine, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates (Dr. Alhajri), Public Health Research Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates, Oxford University Hospitals NHS Foundation Trust, Oxford, England, and the MRC Epidemiology Unit, University of Cambridge, Cambridge, England (Dr. Ali).
Objective: The pandemic has forced closures of primary schools, resulting in loss of learning time on a global scale. In addition to face coverings, social distancing, and hand hygiene, an efficient testing method is important to mitigate the spread of COVID-19 in schools. We evaluated the feasibility of a saliva-based SARS-CoV-2 polymerase chain reaction testing program among 18 primary schools in the Emirate of Abu Dhabi, United Arab Emirates. Qualitative results show that children 4 to 5 years old had difficulty producing an adequate saliva specimen compared to those 6 to 12 years old.
Methods: A short training video on saliva collection beforehand helps demystify the process for students and parents alike. Informed consent was challenging yet should be done beforehand by school health nurses or other medical professionals to reassure parents and maximize participation.
Results: Telephone interviews with school administrators resulted in an 83% response rate. Overall, 93% of school administrators had a positive experience with saliva testing and felt the program improved the safety of their schools. The ongoing use of saliva testing for SARS-CoV-2 was supported by 73% of respondents.
Conclusion: On-campus saliva testing is a feasible option for primary schools to screen for COVID-19 in their student population to help keep their campuses safe and open for learning.
Keywords: COVID-19; saliva testing; mitigation; primary school.
The COVID-19 pandemic is a leading cause of morbidity and mortality worldwide and continues to exhaust health care resources on a large scale.1 Efficient testing is critical to identify cases early and to help mitigate the deleterious effects of the pandemic.2 Saliva polymerase chain reaction (PCR) nucleic acid amplification testing (NAAT) is more comfortable than nasopharyngeal (NP) NAAT and has been validated as a test for SARS-CoV-2.1 Although children are less susceptible to severe disease, primary schools are considered a vector for transmission and community spread.3 Efficient and scalable methods of routine testing are needed globally to help keep schools open. Saliva testing has proven a useful resource for this population.4,5
Abu Dhabi is the largest Emirate in the United Arab Emirates (UAE), with an estimated population of 2.5 million.6 The first case of COVID-19 was discovered in the UAE on January 29, 2020.7 The UAE has been recognized worldwide for its robust pandemic response. Along with the coordinated and swift application of public health measures, the country has one of the highest COVID-19 testing rates per capita and one of the highest vaccination rates worldwide.8,9 The Abu Dhabi Public Health Center (ADPHC) works alongside the Ministry of Education (MOE) to establish testing, quarantine, and general safety guidelines for primary schools. In December 2020, the ADPHC partnered with a local, accredited diagnostic laboratory to test the feasibility of a saliva-based screening program for COVID-19 directly on school campuses for 18 primary schools in the Emirate.
Saliva-based PCR testing for COVID-19 was approved for use in schools in the UAE on January 24, 2021.10 As part of a greater mitigation strategy to reduce both school-based transmission and, hence, community spread, the ADPHC focused its on-site testing program on children aged 4 to 12 years. The program required collaboration among medical professionals, school administrators and teachers, students, and parents. Our study evaluates the feasibility of implementing a saliva-based COVID-19 screening program directly on primary school campuses involving children as young as 4 years of age.
Methods
The ADPHC, in collaboration with G42 Biogenix Labs, conducted a saliva SARS-CoV-2 NAAT testing program in 18 primary schools in the Emirate. Schools were selected based on outbreak prevalence at the time and focused on “hot spot” areas. The school on-site saliva testing program included children aged 4 to 12 years old in a “bubble” attendance model during the school day. This model involved children being assigned to groups or “pods.” This allowed us to limit a potential outbreak to a single pod, as opposed to risk exposing the entire school, should a single student test positive. The well-established SalivaDirect protocol developed at Yale University was used for testing and included an RNA extraction-free, RT-qPCR method for SARS-CoV-2 detection.11
We conducted a qualitative study involving telephone interviews of school administrators to evaluate their experience with the ADPHC testing program at their schools. In addition, we interviewed the G42 Biogenix Lab providers to understand the logistics that supported on-campus collection of saliva specimens for this age group. We also gathered the attitudes of school children before and after testing. This study was reviewed and approved by the Abu Dhabi Health Research and Technology Committee and the Institutional Review Board (IRB), New York University Abu Dhabi (NYUAD).
Sample and recruitment
The original sample collection of saliva specimens was performed by the ADPHC in collaboration with G42 Biogenix Lab providers on school campuses between December 6 and December 10, 2020. During this time, schools operated in a hybrid teaching model, where learning took place both online and in person. Infection control measures were deployed based on ADPHC standards and guidelines. Nurses utilized appropriate patient protective equipment, frequent hand hygiene, and social distancing during the collection process. Inclusion criteria included asymptomatic students aged 4 to 12 years attending in-person classes on campus. Students with respiratory symptoms who were asked to stay home or those not attending in-person classes were excluded.
Data collection
Data with regard to school children’s attitudes before and after testing were compiled through an online survey sent randomly to participants postintervention. Data from school administrators were collected through video and telephone interviews between April 14 and April 29, 2021. We first interviewed G42 Biogenix Lab providers to obtain previously acquired qualitative and quantitative data, which were collected during the intervention itself. After obtaining this information, we designed a questionnaire and proceeded with a structured interview process for school officials.
We interviewed school principals and administrators to collect their overall experiences with the saliva testing program. Before starting each interview, we established the interviewees preferred language, either English or Arabic. We then introduced the meeting attendees and provided study details, aims, and objectives, and described collaborating entities. We obtained verbal informed consent from a script approved by the NYUAD IRB and then proceeded with the interview, which included 4 questions. The first 3 questions were answered on a 5-point Likert scale model that consisted of 5 answer options: 5 being completely agree, 4 agree, 3 somewhat agree, 2 somewhat disagree, and 1 completely disagree. The fourth question invited open-ended feedback and comments on the following statements:
- I believe the COVID-19 saliva testing program improved the safety for my school campus.
- Our community had an overall positive experience with the COVID saliva testing.
- We would like to continue a saliva-based COVID testing program on our school campus.
- Please provide any additional comments you feel important about the program.
During the interview, we transcribed the answers as the interviewee was answering. We then translated those in Arabic into English and collected the data in 1 Excel spreadsheet. School interviewees and school names were de-identified in the collection and storage process.
Results
A total of 2011 saliva samples were collected from 18 different primary school campuses. Samples were sent the same day to G42 Biogenix Labs in Abu Dhabi for COVID PCR testing. A team consisting of 5 doctors providing general oversight, along with 2 to 6 nurses per site, were able to manage the collection process for all 18 school campuses. Samples were collected between 8
Sample stations were set up in either the school auditorium or gymnasium to ensure appropriate crowd control and ventilation. Teachers and other school staff, including public safety, were able to manage lines and the shuttling of students back and forth from classes to testing stations, which allowed medical staff to focus on sample collection.
Informed consent was obtained by prior electronic communication to parents from school staff, asking them to agree to allow their child to participate in the testing program. Informed consent was identified as a challenge: Getting parents to understand that saliva testing was more comfortable than NP testing, and that the results were only being used to help keep the school safe, took time. School staff are used to obtaining consent from parents for field trips, but this was clearly more challenging for them.
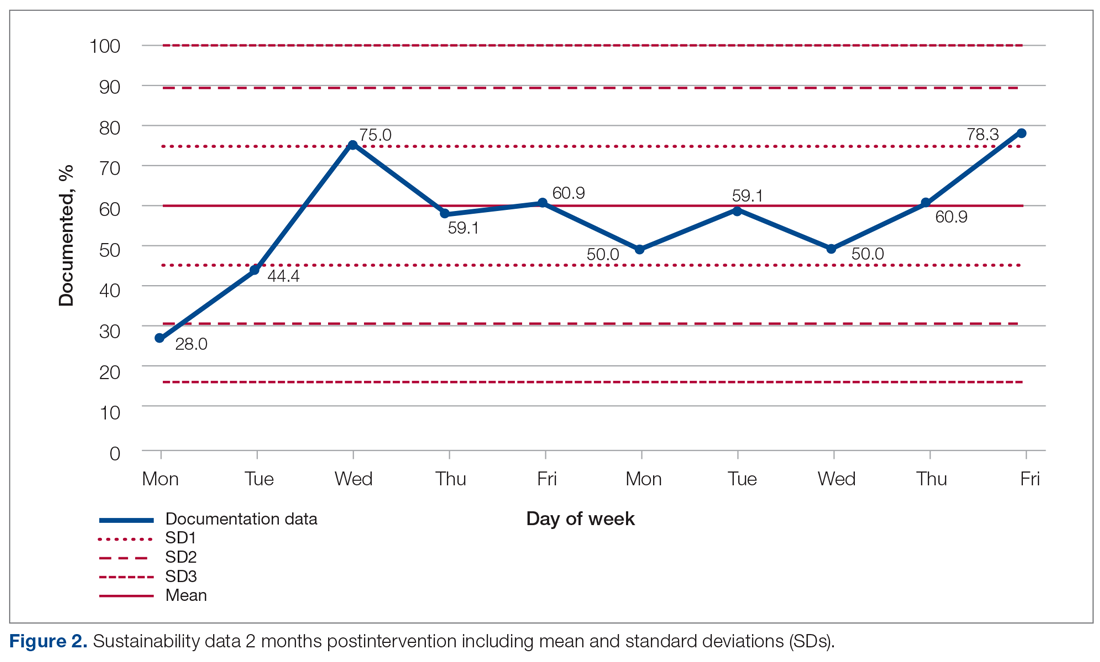
The saliva collection process per child took more time than expected. Children fasted for 45 minutes before saliva collection. We used an active drool technique, which required children to pool saliva in their mouth then express it into a collection tube. Adults can generally do this on command, but we found it took 10 to 12 minutes per child. Saliva production was cued by asking the children to think about food, and by showing them pictures and TV commercials depicting food. Children 4 to 5 years old had more difficulty with the process despite active cueing, while those 6 to 12 years old had an easier time with the process. We collected data on a cohort of 80 children regarding their attitudes pre (Figure 1) and post collection (Figure 2). Children felt happier, less nervous, and less scared after collection than before collection. This trend reassured us that future collections would be easier for students.
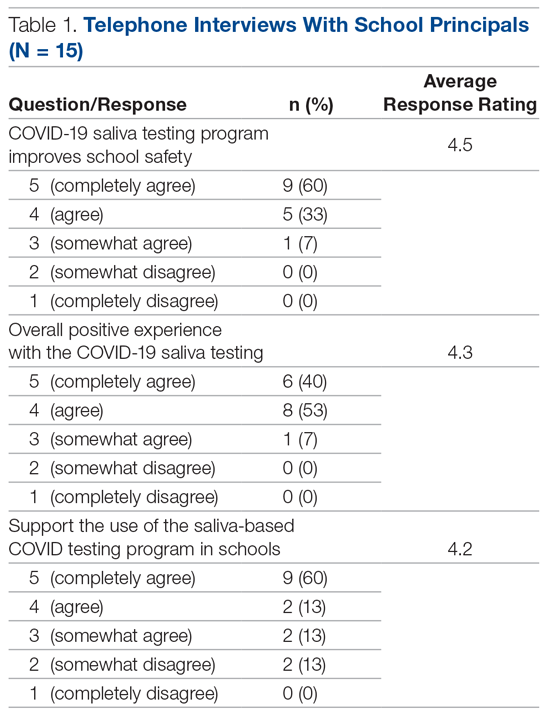
A total of 15 of 18 school principals completed the telephone interview, yielding a response rate of 83%. Overall, 93% of the school principals agreed or completely agreed that the COVID-19 saliva testing program improved school safety; 93% agreed or completely agreed that they had an overall positive experience with the program; and 73% supported the ongoing use of saliva testing in their schools (Table 1). Administrators’ open-ended comments on their experience were positive overall (Table 2).
Discussion
By March 2020, many kindergarten to grade 12 public and private schools suspended in-person classes due to the pandemic and turned to online learning platforms. The negative impact of school closures on academic achievement is projected to be significant.7,12,13 Ensuring schools can stay open and run operations safely will require routine SARS-CoV-2 testing. Our study investigated the feasibility of routine saliva testing on children aged 4 to 12 years on their school campuses. The ADPHC school on-site saliva testing program involved bringing lab providers onto 18 primary school campuses and required cooperation among parents, students, school administrators, and health care professionals.
Children younger than 6 years had difficulty producing an adequate saliva specimen, whereas those 6 to 12 years did so with relative ease when cued by thoughts or pictures of food while waiting in line for collection. Schools considering on-site testing programs should consider the age range of 6 to 12 years as a viable age range for saliva screening. Children should fast for a minimum of 45 minutes prior to saliva collection and should be cued by thoughts of food, food pictures, or food commercials. Setting up a sampling station close to the cafeteria where students can smell meal preparation may also help.14,15 Sampling before breakfast or lunch, when children are potentially at their hungriest, should also be considered.
The greatest challenge was obtaining informed consent from parents who were not yet familiar with the reliability of saliva testing as a tool for SARS-CoV-2 screening or with the saliva collection process as a whole. Informed consent was initially done electronically, lacking direct human interaction to answer parents’ questions. Parents who refused had a follow-up call from the school nurse to further explain the logistics and rationale for saliva screening. Having medical professionals directly answer parents’ questions was helpful. Parents were reassured that the process was painless, confidential, and only to be used for school safety purposes. Despite school administrators being experienced in obtaining consent from parents for field trips, obtaining informed consent for a medical testing procedure is more complicated, and parents aren’t accustomed to providing such consent in a school environment. Schools considering on-site testing should ensure that their school nurse or other health care providers are on the front line obtaining informed consent and allaying parents’ fears.
School staff were able to effectively provide crowd control for testing, and children felt at ease being in a familiar environment. Teachers and public safety officers are well-equipped at managing the shuttling of students to class, to lunch, to physical education, and, finally, to dismissal. They were equally equipped at handling the logistics of students to and from testing, including minimizing crowds and helping students feel at ease during the process. This effective collaboration allowed the lab personnel to focus on sample collection and storage, while school staff managed all other aspects of the children’s safety and care.
Conclusion
Overall, school administrators had a positive experience with the testing program, felt the program improved the safety of their schools, and supported the ongoing use of saliva testing for SARS-CoV-2 on their school campuses. Children aged 6 years and older were able to provide adequate saliva samples, and children felt happier and less nervous after the process, indicating repeatability. Our findings highlight the feasibility of an integrated on-site saliva testing model for primary school campuses. Further research is needed to determine the scalability of such a model and whether the added compliance and safety of on-site testing compensates for the potential loss of learning time that testing during school hours would require.
Corresponding author: Ayaz Virji, MD, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, United Arab Emirates; av102@nyu.edu.
Financial disclosures: None.
1. Kuehn BM. Despite improvements, COVID-19’s health care disruptions persist. JAMA. 2021;325(23):2335. doi:10.1001/jama.2021.9134
2. National Institute on Aging. Why COVID-19 testing is the key to getting back to normal. September 4, 2020. Accessed September 8, 2021. https://www.nia.nih.gov/news/why-covid-19-testing-key-getting-back-normal
3. Centers for Disease Control and Prevention. Science brief: Transmission of SARS-CoV-2 in K-12 schools. Updated July 9, 2021. Accessed September 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html
4. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-360. doi:10.1001/jamainternmed.2020.8876
5. Al Suwaidi H, Senok A, Varghese R, et al. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27(9):1330-1335. doi:10.1016/j.cmi.2021.02.009
6. Abu Dhabi. Accessed September 8, 2021. https://u.ae/en/about-the-uae/the-seven-emirates/abu-dhabi
7. Alsuwaidi AR, Al Hosani FI, Al Memari S, et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol. 2021;50(4):1077-1090. doi:10.1093/ije/dyab077
8. Al Hosany F, Ganesan S, Al Memari S, et al. Response to COVID-19 pandemic in the UAE: a public health perspective. J Glob Health. 2021;11:03050. doi:10.7189/jogh.11.03050
9. Bremmer I. The best global responses to the COVID-19 pandemic, 1 year later. Time Magazine. Updated February 23, 2021. Accessed September 8, 2021. https://time.com/5851633/best-global-responses-covid-19/
10. Department of Health, Abu Dhabi. Laboratory diagnostic test for COVID-19: update regarding saliva-based testing using RT-PCR test. 2021.
11. Vogels C, Brackney DE, Kalinich CC, et al. SalivaDirect: RNA extraction-free SARS-CoV-2 diagnostics. Protocols.io. Accessed September 8, 2021. https://www.protocols.io/view/salivadirect-rna-extraction-free-sars-cov-2-diagno-bh6jj9cn?version_warning=no
12. Education Endowment Foundation. Impact of school closures on the attainment gap: rapid evidence assessment. June 2020. Accessed September 8, 2021. https://www.researchgate.net/publication/342501263_EEF_2020_-_Impact_of_School_Closures_on_the_Attainment_Gap
13. United Nations. Policy brief: Education during COVID-19 and beyond. Accessed September 8, 2021. https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2020/08/sg_policy_brief_covid-19_and_education_august_2020.pdf
14. Schiffman SS, Miletic ID. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J Nutr Health Aging. 1999;3(3):158-164.
15. Lee VM, Linden RW. The effect of odours on stimulated parotid salivary flow in humans. Physiol Behav. 1992;52(6):1121-1125. doi:10.1016/0031-9384(92)90470-m
From Health Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates (Dr. Virji and Aisha Al Hamiz), Public Health, Abu Dhabi Public Health Center, Abu Dhabi, United Arab Emirates (Drs. Al Hajeri, Al Shehhi, Al Memari, and Ahlam Al Maskari), College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, United Arab Emirates, Department of Medicine, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates (Dr. Alhajri), Public Health Research Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates, Oxford University Hospitals NHS Foundation Trust, Oxford, England, and the MRC Epidemiology Unit, University of Cambridge, Cambridge, England (Dr. Ali).
Objective: The pandemic has forced closures of primary schools, resulting in loss of learning time on a global scale. In addition to face coverings, social distancing, and hand hygiene, an efficient testing method is important to mitigate the spread of COVID-19 in schools. We evaluated the feasibility of a saliva-based SARS-CoV-2 polymerase chain reaction testing program among 18 primary schools in the Emirate of Abu Dhabi, United Arab Emirates. Qualitative results show that children 4 to 5 years old had difficulty producing an adequate saliva specimen compared to those 6 to 12 years old.
Methods: A short training video on saliva collection beforehand helps demystify the process for students and parents alike. Informed consent was challenging yet should be done beforehand by school health nurses or other medical professionals to reassure parents and maximize participation.
Results: Telephone interviews with school administrators resulted in an 83% response rate. Overall, 93% of school administrators had a positive experience with saliva testing and felt the program improved the safety of their schools. The ongoing use of saliva testing for SARS-CoV-2 was supported by 73% of respondents.
Conclusion: On-campus saliva testing is a feasible option for primary schools to screen for COVID-19 in their student population to help keep their campuses safe and open for learning.
Keywords: COVID-19; saliva testing; mitigation; primary school.
The COVID-19 pandemic is a leading cause of morbidity and mortality worldwide and continues to exhaust health care resources on a large scale.1 Efficient testing is critical to identify cases early and to help mitigate the deleterious effects of the pandemic.2 Saliva polymerase chain reaction (PCR) nucleic acid amplification testing (NAAT) is more comfortable than nasopharyngeal (NP) NAAT and has been validated as a test for SARS-CoV-2.1 Although children are less susceptible to severe disease, primary schools are considered a vector for transmission and community spread.3 Efficient and scalable methods of routine testing are needed globally to help keep schools open. Saliva testing has proven a useful resource for this population.4,5
Abu Dhabi is the largest Emirate in the United Arab Emirates (UAE), with an estimated population of 2.5 million.6 The first case of COVID-19 was discovered in the UAE on January 29, 2020.7 The UAE has been recognized worldwide for its robust pandemic response. Along with the coordinated and swift application of public health measures, the country has one of the highest COVID-19 testing rates per capita and one of the highest vaccination rates worldwide.8,9 The Abu Dhabi Public Health Center (ADPHC) works alongside the Ministry of Education (MOE) to establish testing, quarantine, and general safety guidelines for primary schools. In December 2020, the ADPHC partnered with a local, accredited diagnostic laboratory to test the feasibility of a saliva-based screening program for COVID-19 directly on school campuses for 18 primary schools in the Emirate.
Saliva-based PCR testing for COVID-19 was approved for use in schools in the UAE on January 24, 2021.10 As part of a greater mitigation strategy to reduce both school-based transmission and, hence, community spread, the ADPHC focused its on-site testing program on children aged 4 to 12 years. The program required collaboration among medical professionals, school administrators and teachers, students, and parents. Our study evaluates the feasibility of implementing a saliva-based COVID-19 screening program directly on primary school campuses involving children as young as 4 years of age.
Methods
The ADPHC, in collaboration with G42 Biogenix Labs, conducted a saliva SARS-CoV-2 NAAT testing program in 18 primary schools in the Emirate. Schools were selected based on outbreak prevalence at the time and focused on “hot spot” areas. The school on-site saliva testing program included children aged 4 to 12 years old in a “bubble” attendance model during the school day. This model involved children being assigned to groups or “pods.” This allowed us to limit a potential outbreak to a single pod, as opposed to risk exposing the entire school, should a single student test positive. The well-established SalivaDirect protocol developed at Yale University was used for testing and included an RNA extraction-free, RT-qPCR method for SARS-CoV-2 detection.11
We conducted a qualitative study involving telephone interviews of school administrators to evaluate their experience with the ADPHC testing program at their schools. In addition, we interviewed the G42 Biogenix Lab providers to understand the logistics that supported on-campus collection of saliva specimens for this age group. We also gathered the attitudes of school children before and after testing. This study was reviewed and approved by the Abu Dhabi Health Research and Technology Committee and the Institutional Review Board (IRB), New York University Abu Dhabi (NYUAD).
Sample and recruitment
The original sample collection of saliva specimens was performed by the ADPHC in collaboration with G42 Biogenix Lab providers on school campuses between December 6 and December 10, 2020. During this time, schools operated in a hybrid teaching model, where learning took place both online and in person. Infection control measures were deployed based on ADPHC standards and guidelines. Nurses utilized appropriate patient protective equipment, frequent hand hygiene, and social distancing during the collection process. Inclusion criteria included asymptomatic students aged 4 to 12 years attending in-person classes on campus. Students with respiratory symptoms who were asked to stay home or those not attending in-person classes were excluded.
Data collection
Data with regard to school children’s attitudes before and after testing were compiled through an online survey sent randomly to participants postintervention. Data from school administrators were collected through video and telephone interviews between April 14 and April 29, 2021. We first interviewed G42 Biogenix Lab providers to obtain previously acquired qualitative and quantitative data, which were collected during the intervention itself. After obtaining this information, we designed a questionnaire and proceeded with a structured interview process for school officials.
We interviewed school principals and administrators to collect their overall experiences with the saliva testing program. Before starting each interview, we established the interviewees preferred language, either English or Arabic. We then introduced the meeting attendees and provided study details, aims, and objectives, and described collaborating entities. We obtained verbal informed consent from a script approved by the NYUAD IRB and then proceeded with the interview, which included 4 questions. The first 3 questions were answered on a 5-point Likert scale model that consisted of 5 answer options: 5 being completely agree, 4 agree, 3 somewhat agree, 2 somewhat disagree, and 1 completely disagree. The fourth question invited open-ended feedback and comments on the following statements:
- I believe the COVID-19 saliva testing program improved the safety for my school campus.
- Our community had an overall positive experience with the COVID saliva testing.
- We would like to continue a saliva-based COVID testing program on our school campus.
- Please provide any additional comments you feel important about the program.
During the interview, we transcribed the answers as the interviewee was answering. We then translated those in Arabic into English and collected the data in 1 Excel spreadsheet. School interviewees and school names were de-identified in the collection and storage process.
Results
A total of 2011 saliva samples were collected from 18 different primary school campuses. Samples were sent the same day to G42 Biogenix Labs in Abu Dhabi for COVID PCR testing. A team consisting of 5 doctors providing general oversight, along with 2 to 6 nurses per site, were able to manage the collection process for all 18 school campuses. Samples were collected between 8
Sample stations were set up in either the school auditorium or gymnasium to ensure appropriate crowd control and ventilation. Teachers and other school staff, including public safety, were able to manage lines and the shuttling of students back and forth from classes to testing stations, which allowed medical staff to focus on sample collection.
Informed consent was obtained by prior electronic communication to parents from school staff, asking them to agree to allow their child to participate in the testing program. Informed consent was identified as a challenge: Getting parents to understand that saliva testing was more comfortable than NP testing, and that the results were only being used to help keep the school safe, took time. School staff are used to obtaining consent from parents for field trips, but this was clearly more challenging for them.
The saliva collection process per child took more time than expected. Children fasted for 45 minutes before saliva collection. We used an active drool technique, which required children to pool saliva in their mouth then express it into a collection tube. Adults can generally do this on command, but we found it took 10 to 12 minutes per child. Saliva production was cued by asking the children to think about food, and by showing them pictures and TV commercials depicting food. Children 4 to 5 years old had more difficulty with the process despite active cueing, while those 6 to 12 years old had an easier time with the process. We collected data on a cohort of 80 children regarding their attitudes pre (Figure 1) and post collection (Figure 2). Children felt happier, less nervous, and less scared after collection than before collection. This trend reassured us that future collections would be easier for students.
A total of 15 of 18 school principals completed the telephone interview, yielding a response rate of 83%. Overall, 93% of the school principals agreed or completely agreed that the COVID-19 saliva testing program improved school safety; 93% agreed or completely agreed that they had an overall positive experience with the program; and 73% supported the ongoing use of saliva testing in their schools (Table 1). Administrators’ open-ended comments on their experience were positive overall (Table 2).
Discussion
By March 2020, many kindergarten to grade 12 public and private schools suspended in-person classes due to the pandemic and turned to online learning platforms. The negative impact of school closures on academic achievement is projected to be significant.7,12,13 Ensuring schools can stay open and run operations safely will require routine SARS-CoV-2 testing. Our study investigated the feasibility of routine saliva testing on children aged 4 to 12 years on their school campuses. The ADPHC school on-site saliva testing program involved bringing lab providers onto 18 primary school campuses and required cooperation among parents, students, school administrators, and health care professionals.
Children younger than 6 years had difficulty producing an adequate saliva specimen, whereas those 6 to 12 years did so with relative ease when cued by thoughts or pictures of food while waiting in line for collection. Schools considering on-site testing programs should consider the age range of 6 to 12 years as a viable age range for saliva screening. Children should fast for a minimum of 45 minutes prior to saliva collection and should be cued by thoughts of food, food pictures, or food commercials. Setting up a sampling station close to the cafeteria where students can smell meal preparation may also help.14,15 Sampling before breakfast or lunch, when children are potentially at their hungriest, should also be considered.
The greatest challenge was obtaining informed consent from parents who were not yet familiar with the reliability of saliva testing as a tool for SARS-CoV-2 screening or with the saliva collection process as a whole. Informed consent was initially done electronically, lacking direct human interaction to answer parents’ questions. Parents who refused had a follow-up call from the school nurse to further explain the logistics and rationale for saliva screening. Having medical professionals directly answer parents’ questions was helpful. Parents were reassured that the process was painless, confidential, and only to be used for school safety purposes. Despite school administrators being experienced in obtaining consent from parents for field trips, obtaining informed consent for a medical testing procedure is more complicated, and parents aren’t accustomed to providing such consent in a school environment. Schools considering on-site testing should ensure that their school nurse or other health care providers are on the front line obtaining informed consent and allaying parents’ fears.
School staff were able to effectively provide crowd control for testing, and children felt at ease being in a familiar environment. Teachers and public safety officers are well-equipped at managing the shuttling of students to class, to lunch, to physical education, and, finally, to dismissal. They were equally equipped at handling the logistics of students to and from testing, including minimizing crowds and helping students feel at ease during the process. This effective collaboration allowed the lab personnel to focus on sample collection and storage, while school staff managed all other aspects of the children’s safety and care.
Conclusion
Overall, school administrators had a positive experience with the testing program, felt the program improved the safety of their schools, and supported the ongoing use of saliva testing for SARS-CoV-2 on their school campuses. Children aged 6 years and older were able to provide adequate saliva samples, and children felt happier and less nervous after the process, indicating repeatability. Our findings highlight the feasibility of an integrated on-site saliva testing model for primary school campuses. Further research is needed to determine the scalability of such a model and whether the added compliance and safety of on-site testing compensates for the potential loss of learning time that testing during school hours would require.
Corresponding author: Ayaz Virji, MD, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, United Arab Emirates; av102@nyu.edu.
Financial disclosures: None.
From Health Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates (Dr. Virji and Aisha Al Hamiz), Public Health, Abu Dhabi Public Health Center, Abu Dhabi, United Arab Emirates (Drs. Al Hajeri, Al Shehhi, Al Memari, and Ahlam Al Maskari), College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, United Arab Emirates, Department of Medicine, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates (Dr. Alhajri), Public Health Research Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates, Oxford University Hospitals NHS Foundation Trust, Oxford, England, and the MRC Epidemiology Unit, University of Cambridge, Cambridge, England (Dr. Ali).
Objective: The pandemic has forced closures of primary schools, resulting in loss of learning time on a global scale. In addition to face coverings, social distancing, and hand hygiene, an efficient testing method is important to mitigate the spread of COVID-19 in schools. We evaluated the feasibility of a saliva-based SARS-CoV-2 polymerase chain reaction testing program among 18 primary schools in the Emirate of Abu Dhabi, United Arab Emirates. Qualitative results show that children 4 to 5 years old had difficulty producing an adequate saliva specimen compared to those 6 to 12 years old.
Methods: A short training video on saliva collection beforehand helps demystify the process for students and parents alike. Informed consent was challenging yet should be done beforehand by school health nurses or other medical professionals to reassure parents and maximize participation.
Results: Telephone interviews with school administrators resulted in an 83% response rate. Overall, 93% of school administrators had a positive experience with saliva testing and felt the program improved the safety of their schools. The ongoing use of saliva testing for SARS-CoV-2 was supported by 73% of respondents.
Conclusion: On-campus saliva testing is a feasible option for primary schools to screen for COVID-19 in their student population to help keep their campuses safe and open for learning.
Keywords: COVID-19; saliva testing; mitigation; primary school.
The COVID-19 pandemic is a leading cause of morbidity and mortality worldwide and continues to exhaust health care resources on a large scale.1 Efficient testing is critical to identify cases early and to help mitigate the deleterious effects of the pandemic.2 Saliva polymerase chain reaction (PCR) nucleic acid amplification testing (NAAT) is more comfortable than nasopharyngeal (NP) NAAT and has been validated as a test for SARS-CoV-2.1 Although children are less susceptible to severe disease, primary schools are considered a vector for transmission and community spread.3 Efficient and scalable methods of routine testing are needed globally to help keep schools open. Saliva testing has proven a useful resource for this population.4,5
Abu Dhabi is the largest Emirate in the United Arab Emirates (UAE), with an estimated population of 2.5 million.6 The first case of COVID-19 was discovered in the UAE on January 29, 2020.7 The UAE has been recognized worldwide for its robust pandemic response. Along with the coordinated and swift application of public health measures, the country has one of the highest COVID-19 testing rates per capita and one of the highest vaccination rates worldwide.8,9 The Abu Dhabi Public Health Center (ADPHC) works alongside the Ministry of Education (MOE) to establish testing, quarantine, and general safety guidelines for primary schools. In December 2020, the ADPHC partnered with a local, accredited diagnostic laboratory to test the feasibility of a saliva-based screening program for COVID-19 directly on school campuses for 18 primary schools in the Emirate.
Saliva-based PCR testing for COVID-19 was approved for use in schools in the UAE on January 24, 2021.10 As part of a greater mitigation strategy to reduce both school-based transmission and, hence, community spread, the ADPHC focused its on-site testing program on children aged 4 to 12 years. The program required collaboration among medical professionals, school administrators and teachers, students, and parents. Our study evaluates the feasibility of implementing a saliva-based COVID-19 screening program directly on primary school campuses involving children as young as 4 years of age.
Methods
The ADPHC, in collaboration with G42 Biogenix Labs, conducted a saliva SARS-CoV-2 NAAT testing program in 18 primary schools in the Emirate. Schools were selected based on outbreak prevalence at the time and focused on “hot spot” areas. The school on-site saliva testing program included children aged 4 to 12 years old in a “bubble” attendance model during the school day. This model involved children being assigned to groups or “pods.” This allowed us to limit a potential outbreak to a single pod, as opposed to risk exposing the entire school, should a single student test positive. The well-established SalivaDirect protocol developed at Yale University was used for testing and included an RNA extraction-free, RT-qPCR method for SARS-CoV-2 detection.11
We conducted a qualitative study involving telephone interviews of school administrators to evaluate their experience with the ADPHC testing program at their schools. In addition, we interviewed the G42 Biogenix Lab providers to understand the logistics that supported on-campus collection of saliva specimens for this age group. We also gathered the attitudes of school children before and after testing. This study was reviewed and approved by the Abu Dhabi Health Research and Technology Committee and the Institutional Review Board (IRB), New York University Abu Dhabi (NYUAD).
Sample and recruitment
The original sample collection of saliva specimens was performed by the ADPHC in collaboration with G42 Biogenix Lab providers on school campuses between December 6 and December 10, 2020. During this time, schools operated in a hybrid teaching model, where learning took place both online and in person. Infection control measures were deployed based on ADPHC standards and guidelines. Nurses utilized appropriate patient protective equipment, frequent hand hygiene, and social distancing during the collection process. Inclusion criteria included asymptomatic students aged 4 to 12 years attending in-person classes on campus. Students with respiratory symptoms who were asked to stay home or those not attending in-person classes were excluded.
Data collection
Data with regard to school children’s attitudes before and after testing were compiled through an online survey sent randomly to participants postintervention. Data from school administrators were collected through video and telephone interviews between April 14 and April 29, 2021. We first interviewed G42 Biogenix Lab providers to obtain previously acquired qualitative and quantitative data, which were collected during the intervention itself. After obtaining this information, we designed a questionnaire and proceeded with a structured interview process for school officials.
We interviewed school principals and administrators to collect their overall experiences with the saliva testing program. Before starting each interview, we established the interviewees preferred language, either English or Arabic. We then introduced the meeting attendees and provided study details, aims, and objectives, and described collaborating entities. We obtained verbal informed consent from a script approved by the NYUAD IRB and then proceeded with the interview, which included 4 questions. The first 3 questions were answered on a 5-point Likert scale model that consisted of 5 answer options: 5 being completely agree, 4 agree, 3 somewhat agree, 2 somewhat disagree, and 1 completely disagree. The fourth question invited open-ended feedback and comments on the following statements:
- I believe the COVID-19 saliva testing program improved the safety for my school campus.
- Our community had an overall positive experience with the COVID saliva testing.
- We would like to continue a saliva-based COVID testing program on our school campus.
- Please provide any additional comments you feel important about the program.
During the interview, we transcribed the answers as the interviewee was answering. We then translated those in Arabic into English and collected the data in 1 Excel spreadsheet. School interviewees and school names were de-identified in the collection and storage process.
Results
A total of 2011 saliva samples were collected from 18 different primary school campuses. Samples were sent the same day to G42 Biogenix Labs in Abu Dhabi for COVID PCR testing. A team consisting of 5 doctors providing general oversight, along with 2 to 6 nurses per site, were able to manage the collection process for all 18 school campuses. Samples were collected between 8
Sample stations were set up in either the school auditorium or gymnasium to ensure appropriate crowd control and ventilation. Teachers and other school staff, including public safety, were able to manage lines and the shuttling of students back and forth from classes to testing stations, which allowed medical staff to focus on sample collection.
Informed consent was obtained by prior electronic communication to parents from school staff, asking them to agree to allow their child to participate in the testing program. Informed consent was identified as a challenge: Getting parents to understand that saliva testing was more comfortable than NP testing, and that the results were only being used to help keep the school safe, took time. School staff are used to obtaining consent from parents for field trips, but this was clearly more challenging for them.
The saliva collection process per child took more time than expected. Children fasted for 45 minutes before saliva collection. We used an active drool technique, which required children to pool saliva in their mouth then express it into a collection tube. Adults can generally do this on command, but we found it took 10 to 12 minutes per child. Saliva production was cued by asking the children to think about food, and by showing them pictures and TV commercials depicting food. Children 4 to 5 years old had more difficulty with the process despite active cueing, while those 6 to 12 years old had an easier time with the process. We collected data on a cohort of 80 children regarding their attitudes pre (Figure 1) and post collection (Figure 2). Children felt happier, less nervous, and less scared after collection than before collection. This trend reassured us that future collections would be easier for students.
A total of 15 of 18 school principals completed the telephone interview, yielding a response rate of 83%. Overall, 93% of the school principals agreed or completely agreed that the COVID-19 saliva testing program improved school safety; 93% agreed or completely agreed that they had an overall positive experience with the program; and 73% supported the ongoing use of saliva testing in their schools (Table 1). Administrators’ open-ended comments on their experience were positive overall (Table 2).
Discussion
By March 2020, many kindergarten to grade 12 public and private schools suspended in-person classes due to the pandemic and turned to online learning platforms. The negative impact of school closures on academic achievement is projected to be significant.7,12,13 Ensuring schools can stay open and run operations safely will require routine SARS-CoV-2 testing. Our study investigated the feasibility of routine saliva testing on children aged 4 to 12 years on their school campuses. The ADPHC school on-site saliva testing program involved bringing lab providers onto 18 primary school campuses and required cooperation among parents, students, school administrators, and health care professionals.
Children younger than 6 years had difficulty producing an adequate saliva specimen, whereas those 6 to 12 years did so with relative ease when cued by thoughts or pictures of food while waiting in line for collection. Schools considering on-site testing programs should consider the age range of 6 to 12 years as a viable age range for saliva screening. Children should fast for a minimum of 45 minutes prior to saliva collection and should be cued by thoughts of food, food pictures, or food commercials. Setting up a sampling station close to the cafeteria where students can smell meal preparation may also help.14,15 Sampling before breakfast or lunch, when children are potentially at their hungriest, should also be considered.
The greatest challenge was obtaining informed consent from parents who were not yet familiar with the reliability of saliva testing as a tool for SARS-CoV-2 screening or with the saliva collection process as a whole. Informed consent was initially done electronically, lacking direct human interaction to answer parents’ questions. Parents who refused had a follow-up call from the school nurse to further explain the logistics and rationale for saliva screening. Having medical professionals directly answer parents’ questions was helpful. Parents were reassured that the process was painless, confidential, and only to be used for school safety purposes. Despite school administrators being experienced in obtaining consent from parents for field trips, obtaining informed consent for a medical testing procedure is more complicated, and parents aren’t accustomed to providing such consent in a school environment. Schools considering on-site testing should ensure that their school nurse or other health care providers are on the front line obtaining informed consent and allaying parents’ fears.
School staff were able to effectively provide crowd control for testing, and children felt at ease being in a familiar environment. Teachers and public safety officers are well-equipped at managing the shuttling of students to class, to lunch, to physical education, and, finally, to dismissal. They were equally equipped at handling the logistics of students to and from testing, including minimizing crowds and helping students feel at ease during the process. This effective collaboration allowed the lab personnel to focus on sample collection and storage, while school staff managed all other aspects of the children’s safety and care.
Conclusion
Overall, school administrators had a positive experience with the testing program, felt the program improved the safety of their schools, and supported the ongoing use of saliva testing for SARS-CoV-2 on their school campuses. Children aged 6 years and older were able to provide adequate saliva samples, and children felt happier and less nervous after the process, indicating repeatability. Our findings highlight the feasibility of an integrated on-site saliva testing model for primary school campuses. Further research is needed to determine the scalability of such a model and whether the added compliance and safety of on-site testing compensates for the potential loss of learning time that testing during school hours would require.
Corresponding author: Ayaz Virji, MD, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, United Arab Emirates; av102@nyu.edu.
Financial disclosures: None.
1. Kuehn BM. Despite improvements, COVID-19’s health care disruptions persist. JAMA. 2021;325(23):2335. doi:10.1001/jama.2021.9134
2. National Institute on Aging. Why COVID-19 testing is the key to getting back to normal. September 4, 2020. Accessed September 8, 2021. https://www.nia.nih.gov/news/why-covid-19-testing-key-getting-back-normal
3. Centers for Disease Control and Prevention. Science brief: Transmission of SARS-CoV-2 in K-12 schools. Updated July 9, 2021. Accessed September 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html
4. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-360. doi:10.1001/jamainternmed.2020.8876
5. Al Suwaidi H, Senok A, Varghese R, et al. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27(9):1330-1335. doi:10.1016/j.cmi.2021.02.009
6. Abu Dhabi. Accessed September 8, 2021. https://u.ae/en/about-the-uae/the-seven-emirates/abu-dhabi
7. Alsuwaidi AR, Al Hosani FI, Al Memari S, et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol. 2021;50(4):1077-1090. doi:10.1093/ije/dyab077
8. Al Hosany F, Ganesan S, Al Memari S, et al. Response to COVID-19 pandemic in the UAE: a public health perspective. J Glob Health. 2021;11:03050. doi:10.7189/jogh.11.03050
9. Bremmer I. The best global responses to the COVID-19 pandemic, 1 year later. Time Magazine. Updated February 23, 2021. Accessed September 8, 2021. https://time.com/5851633/best-global-responses-covid-19/
10. Department of Health, Abu Dhabi. Laboratory diagnostic test for COVID-19: update regarding saliva-based testing using RT-PCR test. 2021.
11. Vogels C, Brackney DE, Kalinich CC, et al. SalivaDirect: RNA extraction-free SARS-CoV-2 diagnostics. Protocols.io. Accessed September 8, 2021. https://www.protocols.io/view/salivadirect-rna-extraction-free-sars-cov-2-diagno-bh6jj9cn?version_warning=no
12. Education Endowment Foundation. Impact of school closures on the attainment gap: rapid evidence assessment. June 2020. Accessed September 8, 2021. https://www.researchgate.net/publication/342501263_EEF_2020_-_Impact_of_School_Closures_on_the_Attainment_Gap
13. United Nations. Policy brief: Education during COVID-19 and beyond. Accessed September 8, 2021. https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2020/08/sg_policy_brief_covid-19_and_education_august_2020.pdf
14. Schiffman SS, Miletic ID. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J Nutr Health Aging. 1999;3(3):158-164.
15. Lee VM, Linden RW. The effect of odours on stimulated parotid salivary flow in humans. Physiol Behav. 1992;52(6):1121-1125. doi:10.1016/0031-9384(92)90470-m
1. Kuehn BM. Despite improvements, COVID-19’s health care disruptions persist. JAMA. 2021;325(23):2335. doi:10.1001/jama.2021.9134
2. National Institute on Aging. Why COVID-19 testing is the key to getting back to normal. September 4, 2020. Accessed September 8, 2021. https://www.nia.nih.gov/news/why-covid-19-testing-key-getting-back-normal
3. Centers for Disease Control and Prevention. Science brief: Transmission of SARS-CoV-2 in K-12 schools. Updated July 9, 2021. Accessed September 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html
4. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-360. doi:10.1001/jamainternmed.2020.8876
5. Al Suwaidi H, Senok A, Varghese R, et al. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27(9):1330-1335. doi:10.1016/j.cmi.2021.02.009
6. Abu Dhabi. Accessed September 8, 2021. https://u.ae/en/about-the-uae/the-seven-emirates/abu-dhabi
7. Alsuwaidi AR, Al Hosani FI, Al Memari S, et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol. 2021;50(4):1077-1090. doi:10.1093/ije/dyab077
8. Al Hosany F, Ganesan S, Al Memari S, et al. Response to COVID-19 pandemic in the UAE: a public health perspective. J Glob Health. 2021;11:03050. doi:10.7189/jogh.11.03050
9. Bremmer I. The best global responses to the COVID-19 pandemic, 1 year later. Time Magazine. Updated February 23, 2021. Accessed September 8, 2021. https://time.com/5851633/best-global-responses-covid-19/
10. Department of Health, Abu Dhabi. Laboratory diagnostic test for COVID-19: update regarding saliva-based testing using RT-PCR test. 2021.
11. Vogels C, Brackney DE, Kalinich CC, et al. SalivaDirect: RNA extraction-free SARS-CoV-2 diagnostics. Protocols.io. Accessed September 8, 2021. https://www.protocols.io/view/salivadirect-rna-extraction-free-sars-cov-2-diagno-bh6jj9cn?version_warning=no
12. Education Endowment Foundation. Impact of school closures on the attainment gap: rapid evidence assessment. June 2020. Accessed September 8, 2021. https://www.researchgate.net/publication/342501263_EEF_2020_-_Impact_of_School_Closures_on_the_Attainment_Gap
13. United Nations. Policy brief: Education during COVID-19 and beyond. Accessed September 8, 2021. https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2020/08/sg_policy_brief_covid-19_and_education_august_2020.pdf
14. Schiffman SS, Miletic ID. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J Nutr Health Aging. 1999;3(3):158-164.
15. Lee VM, Linden RW. The effect of odours on stimulated parotid salivary flow in humans. Physiol Behav. 1992;52(6):1121-1125. doi:10.1016/0031-9384(92)90470-m
Improving the Efficiency of Ordering Next Generation Sequencing During New Patient Triage: A Quality Improvement Project
Objective
To decrease the time to treatment by streamlining ordering of next generation sequencing (NGS) during new patient triage utilizing a centralized document of indications for testing.
Background
Use of NGS in management of patients with cancer is rapidly expanding. In 2017, over 75% of oncologists reported using NGS to guide treatment decisions (1). NGS testing is also now incorporated into 67% of NCCN guidelines (2). However, due to the wide variety and changing indications for NGS, integrating testing into routine clinical care can be challenging.
Results
A total of 118 new patients were seen at the SLC VA Oncology Clinic between 2020-2021 of which 21 met criteria for NGS testing at time of triage consult, 10 before and 11 after the intervention. Median time from triage to treatment initiation was 30 days (30-33) after the incorporation of the document into clinic workflow compared to 63 days (47-66). Median time from biopsy to NGS results was similar between pre- and post-intervention groups, 28 (25-49) vs 26 days (18.5-26.5).
Conclusion
Our centralized summary of NGS indications is easily updated and accessible to staff. To date, shorter times from triage to treatment have been seen after integrating this document into clinic workflow. As our sample size is small, further evaluation of this trend is required. However, our data suggests that additional improvement may be achieved through incorporating this document into the Pathology department’s workflow.
References
(1) Freedman A et al. Use of NGS sequencing tests to guide cancer treatment: results from a nationally representative survey of oncologists in the United States. JCO Precis Oncol. 2018;2:1-13. (2) Conway J et al. NGS and the clinical oncology workflow: data challenges, proposed solutions and a call to action. JCO Precis Oncol. 2019;3:1-10.
Objective
To decrease the time to treatment by streamlining ordering of next generation sequencing (NGS) during new patient triage utilizing a centralized document of indications for testing.
Background
Use of NGS in management of patients with cancer is rapidly expanding. In 2017, over 75% of oncologists reported using NGS to guide treatment decisions (1). NGS testing is also now incorporated into 67% of NCCN guidelines (2). However, due to the wide variety and changing indications for NGS, integrating testing into routine clinical care can be challenging.
Results
A total of 118 new patients were seen at the SLC VA Oncology Clinic between 2020-2021 of which 21 met criteria for NGS testing at time of triage consult, 10 before and 11 after the intervention. Median time from triage to treatment initiation was 30 days (30-33) after the incorporation of the document into clinic workflow compared to 63 days (47-66). Median time from biopsy to NGS results was similar between pre- and post-intervention groups, 28 (25-49) vs 26 days (18.5-26.5).
Conclusion
Our centralized summary of NGS indications is easily updated and accessible to staff. To date, shorter times from triage to treatment have been seen after integrating this document into clinic workflow. As our sample size is small, further evaluation of this trend is required. However, our data suggests that additional improvement may be achieved through incorporating this document into the Pathology department’s workflow.
References
(1) Freedman A et al. Use of NGS sequencing tests to guide cancer treatment: results from a nationally representative survey of oncologists in the United States. JCO Precis Oncol. 2018;2:1-13. (2) Conway J et al. NGS and the clinical oncology workflow: data challenges, proposed solutions and a call to action. JCO Precis Oncol. 2019;3:1-10.
Objective
To decrease the time to treatment by streamlining ordering of next generation sequencing (NGS) during new patient triage utilizing a centralized document of indications for testing.
Background
Use of NGS in management of patients with cancer is rapidly expanding. In 2017, over 75% of oncologists reported using NGS to guide treatment decisions (1). NGS testing is also now incorporated into 67% of NCCN guidelines (2). However, due to the wide variety and changing indications for NGS, integrating testing into routine clinical care can be challenging.
Results
A total of 118 new patients were seen at the SLC VA Oncology Clinic between 2020-2021 of which 21 met criteria for NGS testing at time of triage consult, 10 before and 11 after the intervention. Median time from triage to treatment initiation was 30 days (30-33) after the incorporation of the document into clinic workflow compared to 63 days (47-66). Median time from biopsy to NGS results was similar between pre- and post-intervention groups, 28 (25-49) vs 26 days (18.5-26.5).
Conclusion
Our centralized summary of NGS indications is easily updated and accessible to staff. To date, shorter times from triage to treatment have been seen after integrating this document into clinic workflow. As our sample size is small, further evaluation of this trend is required. However, our data suggests that additional improvement may be achieved through incorporating this document into the Pathology department’s workflow.
References
(1) Freedman A et al. Use of NGS sequencing tests to guide cancer treatment: results from a nationally representative survey of oncologists in the United States. JCO Precis Oncol. 2018;2:1-13. (2) Conway J et al. NGS and the clinical oncology workflow: data challenges, proposed solutions and a call to action. JCO Precis Oncol. 2019;3:1-10.
Want to see what COVID strain you have? The government says no
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.
The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.
The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.
The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Biden vaccine mandate rule could be ready within weeks
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
The Delta Factor
Several weeks ago, I received a call from my brother who, though not a health care professional, wanted me to know he thought the public was being too critical of scientists and physicians who “are giving us the best advice they can about COVID. People think they should have all the answers. But this virus is complicated, and they don’t always know what is going to happen next.” What makes his charitable read of the public health situation remarkable is that he is a COVID-19 survivor of one of the first reported cases of Guillain-Barre syndrome, which several expert neurologists believe is the result of COVID-19. Like so many other COVID-19 long-haul patients, he is left with lingering symptoms and residual deficits.1
I use this personal story as the overture to this piece on why I am changing my opinion regarding a COVID-19 mandate for federal practitioners. In June I raised ethical concerns about compelling vaccination especially for service members of color based on a current and historical climate of mistrust and discrimination in health care that compulsory vaccination could exacerbate.2 Instead, I followed the lead of Secretary of Defense J. Lloyd Austin III and advocated continued education and encouragement for vaccine-hesitant troops.3 So in 2 months what has so radically changed to lead Secretary Austin and US Department of Veterans Affairs (VA) Secretary Denis R. McDonough to mandate vaccination for their workforce?4,5
I am calling the change the Delta Factor. This is not to be confused with the spy-thrillers that ironically involved rescuing a scientist! The Delta Factor is a catch-all phrase to cover the protean public health impacts of the devastating COVID-19 Delta variant now ravaging the country. Depending on the area of the country as of mid-August, the Centers for Disease Control and Prevention (CDC) estimated that 80% to > 90% of new cases were the Delta variant.6 An increasing number of these cases sadly are in children.7
According to the CDC, the Delta variant is more than twice as contagious as index or subsequent strains: making it about as contagious as chicken pox. The unvaccinated are the most susceptible to Delta and may develop more serious illness and risk of death than with other strains. Those who are fully vaccinated can still contract the virus although usually with milder cases. More worrisome is that individuals with these breakthrough infections have the same viral load as those without vaccinations, rendering them vectors of transmission, although for a shorter time than unvaccinated persons.8
The VA first mandated vaccination among its health care employees in July and then expanded it to all staff in August.9 The US Department of Defense (DoD) mandatory vaccination was announced prior to US Food and Drug Administration’s (FDA) full approval of the Pfizer-BioNTech vaccine.10 Secretary Austin asked President Biden to grant a waiver to permit mandatory vaccination even without full FDA approval, and Biden has indicated his support, but the full approval expedited the time line for implementation.11
Both agencies directly referenced Delta as a primary reason for their vaccination mandates. The VA argued that the mandate was necessary to protect the safety of veterans, while the DoD noted that vaccination was essential to ensure the health of the fighting force. In his initial announcement, Secretary McDonough explicitly mentioned the Delta variant as a primary reason for his decision. noting “it’s the best way to keep veterans safe, especially as the Delta variant spreads across the country.”4 Similarly, Secretary Austin declared, “We will also be keeping a close eye on infection rates, which are on the rise now due to the Delta variant and the impact these rates might have on our readiness.”5
VA and DoD leadership emphasized the safety and effectiveness of the vaccine and urged employees to voluntarily obtain the vaccine or obtain a religious or medical exemption. Those without such an exemption must adhere to masking, testing, and other restrictions.5 As anticipated in the earlier editorial, there has been opposition to the mandate from the workforce of the 2 agencies and their political supporters some of whom view vaccine mandates as violations of personal liberty and bodily integrity and for whom rampant disinformation has amplified entrenched distrust of the government.12
The decision to shift from voluntary to mandatory vaccination of federal employees responsible for the health care of veterans and the defense of citizens, which may seem
Finally and most important, for a vaccine or other public health intervention to be ethically mandated it must have a high probability of attaining a serious purpose: here preventing the harms of sickness and death especially in the most vulnerable. In July, the White House COVID-19 Response Team reported that “preliminary data from several states over the last few months suggest that 99.5% of deaths from COVID-19 in the United States were in unvaccinated people” and were preventable.15 Ethically, even as mandates are implemented across the federal workforce, efforts to educate, encourage, and empower vaccination especially among disenfranchised cohorts must continue. But as a recently leaked CDC internal document acknowledged about the Delta Factor, “the war has changed” and so has my opinion about mandating vaccination among those upon whose service depends the life and security of us all.16
1. CBS Good Morning. Christopher Cross on his near-fatal COVID illness. Published October 18, 2020. Accessed August 21, 2021. https://www.cbsnews.com/news/christopher-cross-on-his-near-fatal-covid-illness
2. Geppert CM. Mistrust and mandates: COVID-19 vaccination in the military. Fed Pract. 2021;38(6):254-255. doi:10.12788/fp.0143
3. Garmone J, US Department of Defense. Secretary of defense addresses vaccine hesitancy in the military. Published February 25, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military
4. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA mandates COVID-19 vaccines among its medical employees including VHA facilities staff [press release]. Published July 26, 2021. Accessed August 21, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5696
5. US Department of Defense, Secretary of Defense. Memorandum for all Department of Defense employees. Published August 9, 2021. Accessed August 23, 2021. https://media.defense.gov/2021/Aug/09/2002826254/-1/-1/0/MESSAGE-TO-THE-FORCE-MEMO-VACCINE.PDF
6. Centers for Disease Control and Prevention COVID data tracker. Variant proportions. Updated August 17, 2021. Accessed August 23, 2021. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
7. American Academy of Pediatrics. Children and COVID-19: state data level report. Updated August 23, 2021. Accessed August 23, 2021. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state|-level-data-report
8. Centers for Disease Control and Prevention. Delta variant: what we know about the science. Update August 19, 2021. Accessed August 23, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
9. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands mandate for COVID-19 vaccines among VHA employees [press release]. Published August 12, 2021. Accessed August 23, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5703
10. US Food and Drug Administration. FDA approves first COVID-19 vaccine [press release]. Published August 23, 2021. Accessed August 23, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
11. Garamone J, US Department of Defense. Biden to approve Austin’s request to make COVID-19 vaccine mandatory for service members. Published August 9, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2724982/biden-to-approve-austins-request-to-make-covid-19-vaccine-mandatory-for-service
12. Watson J. Potential military vaccine mandate brings distrust, support. Associated Press. August 5, 2021. Accessed August 23, 2021. https://apnews.com/article/joe-biden-business-health-coronavirus-pandemic-6a0f94e11f5af1e0de740d44d7931d65
13. Giubilini A. Vaccination ethics. Br Med Bull. 2021;137(1):4-12. doi:10.1093/bmb/ldaa036
14. Steinhauer J. Military and V.A. struggle with vaccination rates in their ranks. The New York Times. July 1, 2021. Accessed August 23, 2021. https://www.nytimes.com/2021/07/01/us/politics/military-va-vaccines.html
15. The White House. Press briefing by White House COVID-19 Response Team and public health officials. Published July 8, 2021. Accessed August 23, 2021. https://www.whitehouse.gov/briefing-room/press-briefings/2021/07/08/press-briefing-by-white-house-covid-19-response-team-and-public-health-officials-44
16. Adutaleb Y, Johnson CY, Achenbach J. ‘The war has changed’: Internal CDC document urges new messaging, warns delta infections likely more severe. The Washington Post. July 29, 2021. Accessed August 21, 2021 https://www.washingtonpost.com/health/2021/07/29/cdc-mask-guidance
Several weeks ago, I received a call from my brother who, though not a health care professional, wanted me to know he thought the public was being too critical of scientists and physicians who “are giving us the best advice they can about COVID. People think they should have all the answers. But this virus is complicated, and they don’t always know what is going to happen next.” What makes his charitable read of the public health situation remarkable is that he is a COVID-19 survivor of one of the first reported cases of Guillain-Barre syndrome, which several expert neurologists believe is the result of COVID-19. Like so many other COVID-19 long-haul patients, he is left with lingering symptoms and residual deficits.1
I use this personal story as the overture to this piece on why I am changing my opinion regarding a COVID-19 mandate for federal practitioners. In June I raised ethical concerns about compelling vaccination especially for service members of color based on a current and historical climate of mistrust and discrimination in health care that compulsory vaccination could exacerbate.2 Instead, I followed the lead of Secretary of Defense J. Lloyd Austin III and advocated continued education and encouragement for vaccine-hesitant troops.3 So in 2 months what has so radically changed to lead Secretary Austin and US Department of Veterans Affairs (VA) Secretary Denis R. McDonough to mandate vaccination for their workforce?4,5
I am calling the change the Delta Factor. This is not to be confused with the spy-thrillers that ironically involved rescuing a scientist! The Delta Factor is a catch-all phrase to cover the protean public health impacts of the devastating COVID-19 Delta variant now ravaging the country. Depending on the area of the country as of mid-August, the Centers for Disease Control and Prevention (CDC) estimated that 80% to > 90% of new cases were the Delta variant.6 An increasing number of these cases sadly are in children.7
According to the CDC, the Delta variant is more than twice as contagious as index or subsequent strains: making it about as contagious as chicken pox. The unvaccinated are the most susceptible to Delta and may develop more serious illness and risk of death than with other strains. Those who are fully vaccinated can still contract the virus although usually with milder cases. More worrisome is that individuals with these breakthrough infections have the same viral load as those without vaccinations, rendering them vectors of transmission, although for a shorter time than unvaccinated persons.8
The VA first mandated vaccination among its health care employees in July and then expanded it to all staff in August.9 The US Department of Defense (DoD) mandatory vaccination was announced prior to US Food and Drug Administration’s (FDA) full approval of the Pfizer-BioNTech vaccine.10 Secretary Austin asked President Biden to grant a waiver to permit mandatory vaccination even without full FDA approval, and Biden has indicated his support, but the full approval expedited the time line for implementation.11
Both agencies directly referenced Delta as a primary reason for their vaccination mandates. The VA argued that the mandate was necessary to protect the safety of veterans, while the DoD noted that vaccination was essential to ensure the health of the fighting force. In his initial announcement, Secretary McDonough explicitly mentioned the Delta variant as a primary reason for his decision. noting “it’s the best way to keep veterans safe, especially as the Delta variant spreads across the country.”4 Similarly, Secretary Austin declared, “We will also be keeping a close eye on infection rates, which are on the rise now due to the Delta variant and the impact these rates might have on our readiness.”5
VA and DoD leadership emphasized the safety and effectiveness of the vaccine and urged employees to voluntarily obtain the vaccine or obtain a religious or medical exemption. Those without such an exemption must adhere to masking, testing, and other restrictions.5 As anticipated in the earlier editorial, there has been opposition to the mandate from the workforce of the 2 agencies and their political supporters some of whom view vaccine mandates as violations of personal liberty and bodily integrity and for whom rampant disinformation has amplified entrenched distrust of the government.12
The decision to shift from voluntary to mandatory vaccination of federal employees responsible for the health care of veterans and the defense of citizens, which may seem
Finally and most important, for a vaccine or other public health intervention to be ethically mandated it must have a high probability of attaining a serious purpose: here preventing the harms of sickness and death especially in the most vulnerable. In July, the White House COVID-19 Response Team reported that “preliminary data from several states over the last few months suggest that 99.5% of deaths from COVID-19 in the United States were in unvaccinated people” and were preventable.15 Ethically, even as mandates are implemented across the federal workforce, efforts to educate, encourage, and empower vaccination especially among disenfranchised cohorts must continue. But as a recently leaked CDC internal document acknowledged about the Delta Factor, “the war has changed” and so has my opinion about mandating vaccination among those upon whose service depends the life and security of us all.16
Several weeks ago, I received a call from my brother who, though not a health care professional, wanted me to know he thought the public was being too critical of scientists and physicians who “are giving us the best advice they can about COVID. People think they should have all the answers. But this virus is complicated, and they don’t always know what is going to happen next.” What makes his charitable read of the public health situation remarkable is that he is a COVID-19 survivor of one of the first reported cases of Guillain-Barre syndrome, which several expert neurologists believe is the result of COVID-19. Like so many other COVID-19 long-haul patients, he is left with lingering symptoms and residual deficits.1
I use this personal story as the overture to this piece on why I am changing my opinion regarding a COVID-19 mandate for federal practitioners. In June I raised ethical concerns about compelling vaccination especially for service members of color based on a current and historical climate of mistrust and discrimination in health care that compulsory vaccination could exacerbate.2 Instead, I followed the lead of Secretary of Defense J. Lloyd Austin III and advocated continued education and encouragement for vaccine-hesitant troops.3 So in 2 months what has so radically changed to lead Secretary Austin and US Department of Veterans Affairs (VA) Secretary Denis R. McDonough to mandate vaccination for their workforce?4,5
I am calling the change the Delta Factor. This is not to be confused with the spy-thrillers that ironically involved rescuing a scientist! The Delta Factor is a catch-all phrase to cover the protean public health impacts of the devastating COVID-19 Delta variant now ravaging the country. Depending on the area of the country as of mid-August, the Centers for Disease Control and Prevention (CDC) estimated that 80% to > 90% of new cases were the Delta variant.6 An increasing number of these cases sadly are in children.7
According to the CDC, the Delta variant is more than twice as contagious as index or subsequent strains: making it about as contagious as chicken pox. The unvaccinated are the most susceptible to Delta and may develop more serious illness and risk of death than with other strains. Those who are fully vaccinated can still contract the virus although usually with milder cases. More worrisome is that individuals with these breakthrough infections have the same viral load as those without vaccinations, rendering them vectors of transmission, although for a shorter time than unvaccinated persons.8
The VA first mandated vaccination among its health care employees in July and then expanded it to all staff in August.9 The US Department of Defense (DoD) mandatory vaccination was announced prior to US Food and Drug Administration’s (FDA) full approval of the Pfizer-BioNTech vaccine.10 Secretary Austin asked President Biden to grant a waiver to permit mandatory vaccination even without full FDA approval, and Biden has indicated his support, but the full approval expedited the time line for implementation.11
Both agencies directly referenced Delta as a primary reason for their vaccination mandates. The VA argued that the mandate was necessary to protect the safety of veterans, while the DoD noted that vaccination was essential to ensure the health of the fighting force. In his initial announcement, Secretary McDonough explicitly mentioned the Delta variant as a primary reason for his decision. noting “it’s the best way to keep veterans safe, especially as the Delta variant spreads across the country.”4 Similarly, Secretary Austin declared, “We will also be keeping a close eye on infection rates, which are on the rise now due to the Delta variant and the impact these rates might have on our readiness.”5
VA and DoD leadership emphasized the safety and effectiveness of the vaccine and urged employees to voluntarily obtain the vaccine or obtain a religious or medical exemption. Those without such an exemption must adhere to masking, testing, and other restrictions.5 As anticipated in the earlier editorial, there has been opposition to the mandate from the workforce of the 2 agencies and their political supporters some of whom view vaccine mandates as violations of personal liberty and bodily integrity and for whom rampant disinformation has amplified entrenched distrust of the government.12
The decision to shift from voluntary to mandatory vaccination of federal employees responsible for the health care of veterans and the defense of citizens, which may seem
Finally and most important, for a vaccine or other public health intervention to be ethically mandated it must have a high probability of attaining a serious purpose: here preventing the harms of sickness and death especially in the most vulnerable. In July, the White House COVID-19 Response Team reported that “preliminary data from several states over the last few months suggest that 99.5% of deaths from COVID-19 in the United States were in unvaccinated people” and were preventable.15 Ethically, even as mandates are implemented across the federal workforce, efforts to educate, encourage, and empower vaccination especially among disenfranchised cohorts must continue. But as a recently leaked CDC internal document acknowledged about the Delta Factor, “the war has changed” and so has my opinion about mandating vaccination among those upon whose service depends the life and security of us all.16
1. CBS Good Morning. Christopher Cross on his near-fatal COVID illness. Published October 18, 2020. Accessed August 21, 2021. https://www.cbsnews.com/news/christopher-cross-on-his-near-fatal-covid-illness
2. Geppert CM. Mistrust and mandates: COVID-19 vaccination in the military. Fed Pract. 2021;38(6):254-255. doi:10.12788/fp.0143
3. Garmone J, US Department of Defense. Secretary of defense addresses vaccine hesitancy in the military. Published February 25, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military
4. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA mandates COVID-19 vaccines among its medical employees including VHA facilities staff [press release]. Published July 26, 2021. Accessed August 21, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5696
5. US Department of Defense, Secretary of Defense. Memorandum for all Department of Defense employees. Published August 9, 2021. Accessed August 23, 2021. https://media.defense.gov/2021/Aug/09/2002826254/-1/-1/0/MESSAGE-TO-THE-FORCE-MEMO-VACCINE.PDF
6. Centers for Disease Control and Prevention COVID data tracker. Variant proportions. Updated August 17, 2021. Accessed August 23, 2021. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
7. American Academy of Pediatrics. Children and COVID-19: state data level report. Updated August 23, 2021. Accessed August 23, 2021. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state|-level-data-report
8. Centers for Disease Control and Prevention. Delta variant: what we know about the science. Update August 19, 2021. Accessed August 23, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
9. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands mandate for COVID-19 vaccines among VHA employees [press release]. Published August 12, 2021. Accessed August 23, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5703
10. US Food and Drug Administration. FDA approves first COVID-19 vaccine [press release]. Published August 23, 2021. Accessed August 23, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
11. Garamone J, US Department of Defense. Biden to approve Austin’s request to make COVID-19 vaccine mandatory for service members. Published August 9, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2724982/biden-to-approve-austins-request-to-make-covid-19-vaccine-mandatory-for-service
12. Watson J. Potential military vaccine mandate brings distrust, support. Associated Press. August 5, 2021. Accessed August 23, 2021. https://apnews.com/article/joe-biden-business-health-coronavirus-pandemic-6a0f94e11f5af1e0de740d44d7931d65
13. Giubilini A. Vaccination ethics. Br Med Bull. 2021;137(1):4-12. doi:10.1093/bmb/ldaa036
14. Steinhauer J. Military and V.A. struggle with vaccination rates in their ranks. The New York Times. July 1, 2021. Accessed August 23, 2021. https://www.nytimes.com/2021/07/01/us/politics/military-va-vaccines.html
15. The White House. Press briefing by White House COVID-19 Response Team and public health officials. Published July 8, 2021. Accessed August 23, 2021. https://www.whitehouse.gov/briefing-room/press-briefings/2021/07/08/press-briefing-by-white-house-covid-19-response-team-and-public-health-officials-44
16. Adutaleb Y, Johnson CY, Achenbach J. ‘The war has changed’: Internal CDC document urges new messaging, warns delta infections likely more severe. The Washington Post. July 29, 2021. Accessed August 21, 2021 https://www.washingtonpost.com/health/2021/07/29/cdc-mask-guidance
1. CBS Good Morning. Christopher Cross on his near-fatal COVID illness. Published October 18, 2020. Accessed August 21, 2021. https://www.cbsnews.com/news/christopher-cross-on-his-near-fatal-covid-illness
2. Geppert CM. Mistrust and mandates: COVID-19 vaccination in the military. Fed Pract. 2021;38(6):254-255. doi:10.12788/fp.0143
3. Garmone J, US Department of Defense. Secretary of defense addresses vaccine hesitancy in the military. Published February 25, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military
4. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA mandates COVID-19 vaccines among its medical employees including VHA facilities staff [press release]. Published July 26, 2021. Accessed August 21, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5696
5. US Department of Defense, Secretary of Defense. Memorandum for all Department of Defense employees. Published August 9, 2021. Accessed August 23, 2021. https://media.defense.gov/2021/Aug/09/2002826254/-1/-1/0/MESSAGE-TO-THE-FORCE-MEMO-VACCINE.PDF
6. Centers for Disease Control and Prevention COVID data tracker. Variant proportions. Updated August 17, 2021. Accessed August 23, 2021. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
7. American Academy of Pediatrics. Children and COVID-19: state data level report. Updated August 23, 2021. Accessed August 23, 2021. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state|-level-data-report
8. Centers for Disease Control and Prevention. Delta variant: what we know about the science. Update August 19, 2021. Accessed August 23, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
9. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands mandate for COVID-19 vaccines among VHA employees [press release]. Published August 12, 2021. Accessed August 23, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5703
10. US Food and Drug Administration. FDA approves first COVID-19 vaccine [press release]. Published August 23, 2021. Accessed August 23, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
11. Garamone J, US Department of Defense. Biden to approve Austin’s request to make COVID-19 vaccine mandatory for service members. Published August 9, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2724982/biden-to-approve-austins-request-to-make-covid-19-vaccine-mandatory-for-service
12. Watson J. Potential military vaccine mandate brings distrust, support. Associated Press. August 5, 2021. Accessed August 23, 2021. https://apnews.com/article/joe-biden-business-health-coronavirus-pandemic-6a0f94e11f5af1e0de740d44d7931d65
13. Giubilini A. Vaccination ethics. Br Med Bull. 2021;137(1):4-12. doi:10.1093/bmb/ldaa036
14. Steinhauer J. Military and V.A. struggle with vaccination rates in their ranks. The New York Times. July 1, 2021. Accessed August 23, 2021. https://www.nytimes.com/2021/07/01/us/politics/military-va-vaccines.html
15. The White House. Press briefing by White House COVID-19 Response Team and public health officials. Published July 8, 2021. Accessed August 23, 2021. https://www.whitehouse.gov/briefing-room/press-briefings/2021/07/08/press-briefing-by-white-house-covid-19-response-team-and-public-health-officials-44
16. Adutaleb Y, Johnson CY, Achenbach J. ‘The war has changed’: Internal CDC document urges new messaging, warns delta infections likely more severe. The Washington Post. July 29, 2021. Accessed August 21, 2021 https://www.washingtonpost.com/health/2021/07/29/cdc-mask-guidance
Modifier -25 and the New 2021 E/M Codes: Documentation of Separate and Distinct Just Got Easier
Insurers Target Modifier -25
Modifier -25 allows reporting of both a minor procedure (ie, one with a 0- or 10-day global period) and a separate and distinct evaluation and management (E/M) service on the same date of service.1 Because of the multicomplaint nature of dermatology, the ability to report a same-day procedure and an E/M service is critical for efficient, cost-effective, and patient-centered dermatologic care. However, it is well known that the use of modifier -25 has been under notable insurer scrutiny and is a common reason for medical record audits.2,3 Some insurers have responded to increased utilization of modifier -25 by cutting reimbursement for claims that include both a procedure and an E/M service or by denying one of the services altogether.4-6 The Centers for Medicare and Medicaid Services also have expressed concern about this coding combination with proposed cuts to reimbursement.7 Moreover, the Office of Inspector General has announced a work plan to investigate the frequent utilization of E/M codes and minor procedures by dermatologists.8 Clearly, modifier -25 is a continued target by insurers and regulators; therefore, dermatologists will want to make sure their coding and documentation meet all requirements and are updated for the new E/M codes for 2021.
The American Medical Association’s Current Procedural Terminology indicates that modifier -25 allows reporting of a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of a procedure or other service.”1 Given that dermatology patients typically present with multiple concerns, dermatologists commonly evaluate and treat numerous conditions during one visit. Understanding what constitutes a separately identifiable E/M service is critical to bill accurately and to pass insurer audits.
Global Surgical Package
To appropriately bill both a procedure and an E/M service, the physician must indicate that the patient’s condition required an E/M service above and beyond the usual work of the procedure. The compilation of evaluation and work included in the payment for a procedure is called the global surgical package.9 In general, the global surgical package includes local or topical anesthesia; the surgical service/procedure itself; immediate postoperative care, including dictating the operative note; meeting/discussing the patient’s procedure with family and other physicians; and writing orders for the patient. For minor procedures (ie, those with either 0- or 10-day global periods), the surgical package also includes same-day E/M services associated with the decision to perform surgery. An appropriate history and physical examination as well as a discussion of the differential diagnosis, treatment options, and risk and benefits of treatment are all included in the payment of a minor procedure itself. Therefore, an evaluation to discuss a patient’s condition or change in condition, alternatives to treatment, or next steps after a diagnosis related to a treatment or diagnostic procedure should not be separately reported. Moreover, the fact that the patient is new to the physician is not in itself sufficient to allow reporting of an E/M service with these minor procedures. For major procedures (ie, those with 90-day postoperative periods), the decision for surgery is excluded from the global surgical package.
2021 E/M Codes Simplify Documentation
The biggest coding change of 2021 was the new E/M codes.10 Prior to this year, the descriptors of E/M services recognized 7 components to define the levels of E/M services11: history and nature of the presenting problem; physical examination; medical decision-making (MDM); counseling; coordination of care; and time. Furthermore, history, physical examination, and MDM were all broken down into more granular elements that were summed to determine the level for each component; for example, the history of the presenting problem was defined as a chronological description of the development of the patient’s present illness, including the following elements: location, quality, severity, duration, timing, context, modifying factors, and associated signs and symptoms. Each of these categories would constitute bullet points to be summed to determine the level of history. Physical examination and MDM bullet points also would be summed to determine a proper coding level.11 Understandably, this coding scheme was complicated and burdensome to medical providers.
The redefinition of the E/M codes for 2021 substantially simplified the determination of coding level and documentation.10 The revisions to the E/M office visit code descriptors and documentation standards are now centered around how physicians think and take care of patients and not on mandatory standards and checking boxes. The main changes involve MDM as the prime determinant of the coding level. Elements of MDM affecting coding for an outpatient or office visit now include only 3 components: the number and complexity of problems addressed in the encounter, the amount or complexity of data to be reviewed and analyzed, and the risk of complications or morbidity of patient management. Gone are the requirements from the earlier criteria requiring so many bullet points for the history, physical examination, and MDM.
Dermatologists may ask, “How does the new E/M coding structure affect reporting and documenting an E/M and a procedure on the same day?” The answer is that the determination of separate and distinct is basically unchanged with the new E/M codes; however, the documentation requirements for modifier -25 using the new E/M codes are simplified.
As always, the key to determining whether a separate and distinct E/M service was provided and subsequently documented is to deconstruct the medical note. All evaluation services associated with the procedure—making a clinical diagnosis or differential diagnosis, decision to perform surgery, and discussion of alternative treatments—should be removed from one’s documentation as shown in the example below. If a complete E/M service still exists, then an E/M may be billed in addition to the procedure. Physical examination of the treatment area is included in the surgical package. With the prior E/M criteria, physical examination of the procedural area could not be used again as a bullet point to count for the E/M level. However, with the new 2021 coding requirements, the documentation of a separate MDM will be sufficient to meet criteria because documentation of physical examination is not a requirement.
Modifier -25 Examples
Let’s examine a typical dermatologist medical note. An established patient presents to the dermatologist complaining of an itchy rash on the left wrist after a hiking trip. Treatment with topical hydrocortisone 1% did not help. The patient also complains of a growing tender lesion on the left elbow of 2 months’ duration. Physical examination reveals a linear vesicular eruption on the left wrist and a tender hyperkeratotic papule on the left elbow. No data is evaluated. A diagnosis of acute rhus dermatitis of the left wrist is made, and betamethasone cream is prescribed. The decision is made to perform a tangential biopsy of the lesion on the left elbow because of the suspicion for malignancy. The biopsy is performed the same day.
This case clearly illustrates performance of an E/M service in the treatment of rhus dermatitis, which is separate and distinct from the biopsy procedure; however, in evaluating whether the case meets the documentation requirements for modifier -25, the information in the medical note inclusive to the procedure’s global surgical package, including history associated with establishing the diagnosis, physical examination of the procedure area(s), and discussion of treatment options, is eliminated, leaving the following notes: An established patient presents to the dermatologist complaining of an itchy rash on the left wrist after a hiking trip. Treatment with topical hydrocortisone 1% did not help. No data is evaluated. A diagnosis of acute rhus dermatitis of the left wrist is made, and betamethasone cream is prescribed.
Because the physical examination of the body part (left arm) is included in the procedure’s global surgical package, the examination of the left wrist cannot be used as coding support for the E/M service. This makes a difference for coding level in the prior E/M coding requirements, which required examination bullet points. However, with the 2021 E/M codes, documentation of physical examination bullet points is irrelevant to the coding level. Therefore, qualifying for a modifier -25 claim is more straightforward in this case with the new code set. Because bullet points are not integral to the 2021 E/M codes, qualifying and properly documenting for a higher level of service will likely be more common in dermatology.
Final Thoughts
Frequent use of modifier -25 is a critical part of a high-quality and cost-effective dermatology practice. Same-day performance of minor procedures and E/M services allows for more rapid and efficient diagnosis and treatment of various conditions as well as minimizing unnecessary office visits. The new E/M codes for 2021 actually make the documentation of a separate and distinct E/M service less complicated because the bullet point requirements associated with the old E/M codes have been eliminated. Understanding how the new E/M code descriptors affect modifier -25 reporting and clear documentation of separate, distinct, and medically necessary E/M services will be needed due to increased insurer scrutiny and audits.
- Current Procedural Terminology 2021, Professional Edition. American Medical Association; 2020.
- Rogers HW. Modifier −25 victory, but the battle is not over. Cutis. 2018;101:409-410.
- Rogers HW. One diagnosis and modifier −25: appropriate or audit target? Cutis. 2017;99:165-166.
- Update regarding E/M with modifier −25—professional. Anthem Blue Cross Blue Shield website. Published February 1, 2019. Accessed August 17, 2021. https://providernews.anthem.com/ohio/article/update-regarding-em-with-modifier-25-professional
- Payment policies—surgery. Harvard Pilgrim Health Care website. Updated May 2021. Accessed August 17, 2021. https://www.harvardpilgrim.org/provider/wp-content/uploads/sites/7/2020/07/H-6-Surgery-PM.pdf
- Modifier 25: frequently asked questions. Independence Blue Cross website. Updated September 25, 2017. Accessed August 17, 2021. https://provcomm.ibx.com/ibc/archive/pages/A86603B03881756B8525817E00768006.aspx
- Huang G. CMS 2019 fee schedule takes modifier 25 cuts, runs with them. Doctors Management website. Accessed August 17, 2021. https://www.doctors-management.com/cms-2019-feeschedule-modifier25/
- Dermatologist claims for evaluation and management services on the same day as minor surgical procedures. US Department of Health and Humans Services Office of Inspector General website. Accessed August 17, 2021. https://oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000577.asp
- Global surgery booklet. Centers for Medicare and Medicaid Services website. Updated September 2018. Accessed August 17, 2021. https://www.cms.gov/outreach-and-education/medicare-learning-network-mln/mlnproducts/downloads/globallsurgery-icn907166.pdf
- American Medical Association. CPT® Evaluation and management (E/M)—office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Updated March 9, 2021. Accessed August 17, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
- 1997 documentation guidelines for evaluation and management services. Centers for Medicare and Medicaid Services website. Accessed August 17, 2021. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNEdWebGuide/Downloads/97Docguidelines.pdf
Insurers Target Modifier -25
Modifier -25 allows reporting of both a minor procedure (ie, one with a 0- or 10-day global period) and a separate and distinct evaluation and management (E/M) service on the same date of service.1 Because of the multicomplaint nature of dermatology, the ability to report a same-day procedure and an E/M service is critical for efficient, cost-effective, and patient-centered dermatologic care. However, it is well known that the use of modifier -25 has been under notable insurer scrutiny and is a common reason for medical record audits.2,3 Some insurers have responded to increased utilization of modifier -25 by cutting reimbursement for claims that include both a procedure and an E/M service or by denying one of the services altogether.4-6 The Centers for Medicare and Medicaid Services also have expressed concern about this coding combination with proposed cuts to reimbursement.7 Moreover, the Office of Inspector General has announced a work plan to investigate the frequent utilization of E/M codes and minor procedures by dermatologists.8 Clearly, modifier -25 is a continued target by insurers and regulators; therefore, dermatologists will want to make sure their coding and documentation meet all requirements and are updated for the new E/M codes for 2021.
The American Medical Association’s Current Procedural Terminology indicates that modifier -25 allows reporting of a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of a procedure or other service.”1 Given that dermatology patients typically present with multiple concerns, dermatologists commonly evaluate and treat numerous conditions during one visit. Understanding what constitutes a separately identifiable E/M service is critical to bill accurately and to pass insurer audits.
Global Surgical Package
To appropriately bill both a procedure and an E/M service, the physician must indicate that the patient’s condition required an E/M service above and beyond the usual work of the procedure. The compilation of evaluation and work included in the payment for a procedure is called the global surgical package.9 In general, the global surgical package includes local or topical anesthesia; the surgical service/procedure itself; immediate postoperative care, including dictating the operative note; meeting/discussing the patient’s procedure with family and other physicians; and writing orders for the patient. For minor procedures (ie, those with either 0- or 10-day global periods), the surgical package also includes same-day E/M services associated with the decision to perform surgery. An appropriate history and physical examination as well as a discussion of the differential diagnosis, treatment options, and risk and benefits of treatment are all included in the payment of a minor procedure itself. Therefore, an evaluation to discuss a patient’s condition or change in condition, alternatives to treatment, or next steps after a diagnosis related to a treatment or diagnostic procedure should not be separately reported. Moreover, the fact that the patient is new to the physician is not in itself sufficient to allow reporting of an E/M service with these minor procedures. For major procedures (ie, those with 90-day postoperative periods), the decision for surgery is excluded from the global surgical package.
2021 E/M Codes Simplify Documentation
The biggest coding change of 2021 was the new E/M codes.10 Prior to this year, the descriptors of E/M services recognized 7 components to define the levels of E/M services11: history and nature of the presenting problem; physical examination; medical decision-making (MDM); counseling; coordination of care; and time. Furthermore, history, physical examination, and MDM were all broken down into more granular elements that were summed to determine the level for each component; for example, the history of the presenting problem was defined as a chronological description of the development of the patient’s present illness, including the following elements: location, quality, severity, duration, timing, context, modifying factors, and associated signs and symptoms. Each of these categories would constitute bullet points to be summed to determine the level of history. Physical examination and MDM bullet points also would be summed to determine a proper coding level.11 Understandably, this coding scheme was complicated and burdensome to medical providers.
The redefinition of the E/M codes for 2021 substantially simplified the determination of coding level and documentation.10 The revisions to the E/M office visit code descriptors and documentation standards are now centered around how physicians think and take care of patients and not on mandatory standards and checking boxes. The main changes involve MDM as the prime determinant of the coding level. Elements of MDM affecting coding for an outpatient or office visit now include only 3 components: the number and complexity of problems addressed in the encounter, the amount or complexity of data to be reviewed and analyzed, and the risk of complications or morbidity of patient management. Gone are the requirements from the earlier criteria requiring so many bullet points for the history, physical examination, and MDM.
Dermatologists may ask, “How does the new E/M coding structure affect reporting and documenting an E/M and a procedure on the same day?” The answer is that the determination of separate and distinct is basically unchanged with the new E/M codes; however, the documentation requirements for modifier -25 using the new E/M codes are simplified.
As always, the key to determining whether a separate and distinct E/M service was provided and subsequently documented is to deconstruct the medical note. All evaluation services associated with the procedure—making a clinical diagnosis or differential diagnosis, decision to perform surgery, and discussion of alternative treatments—should be removed from one’s documentation as shown in the example below. If a complete E/M service still exists, then an E/M may be billed in addition to the procedure. Physical examination of the treatment area is included in the surgical package. With the prior E/M criteria, physical examination of the procedural area could not be used again as a bullet point to count for the E/M level. However, with the new 2021 coding requirements, the documentation of a separate MDM will be sufficient to meet criteria because documentation of physical examination is not a requirement.
Modifier -25 Examples
Let’s examine a typical dermatologist medical note. An established patient presents to the dermatologist complaining of an itchy rash on the left wrist after a hiking trip. Treatment with topical hydrocortisone 1% did not help. The patient also complains of a growing tender lesion on the left elbow of 2 months’ duration. Physical examination reveals a linear vesicular eruption on the left wrist and a tender hyperkeratotic papule on the left elbow. No data is evaluated. A diagnosis of acute rhus dermatitis of the left wrist is made, and betamethasone cream is prescribed. The decision is made to perform a tangential biopsy of the lesion on the left elbow because of the suspicion for malignancy. The biopsy is performed the same day.
This case clearly illustrates performance of an E/M service in the treatment of rhus dermatitis, which is separate and distinct from the biopsy procedure; however, in evaluating whether the case meets the documentation requirements for modifier -25, the information in the medical note inclusive to the procedure’s global surgical package, including history associated with establishing the diagnosis, physical examination of the procedure area(s), and discussion of treatment options, is eliminated, leaving the following notes: An established patient presents to the dermatologist complaining of an itchy rash on the left wrist after a hiking trip. Treatment with topical hydrocortisone 1% did not help. No data is evaluated. A diagnosis of acute rhus dermatitis of the left wrist is made, and betamethasone cream is prescribed.
Because the physical examination of the body part (left arm) is included in the procedure’s global surgical package, the examination of the left wrist cannot be used as coding support for the E/M service. This makes a difference for coding level in the prior E/M coding requirements, which required examination bullet points. However, with the 2021 E/M codes, documentation of physical examination bullet points is irrelevant to the coding level. Therefore, qualifying for a modifier -25 claim is more straightforward in this case with the new code set. Because bullet points are not integral to the 2021 E/M codes, qualifying and properly documenting for a higher level of service will likely be more common in dermatology.
Final Thoughts
Frequent use of modifier -25 is a critical part of a high-quality and cost-effective dermatology practice. Same-day performance of minor procedures and E/M services allows for more rapid and efficient diagnosis and treatment of various conditions as well as minimizing unnecessary office visits. The new E/M codes for 2021 actually make the documentation of a separate and distinct E/M service less complicated because the bullet point requirements associated with the old E/M codes have been eliminated. Understanding how the new E/M code descriptors affect modifier -25 reporting and clear documentation of separate, distinct, and medically necessary E/M services will be needed due to increased insurer scrutiny and audits.
Insurers Target Modifier -25
Modifier -25 allows reporting of both a minor procedure (ie, one with a 0- or 10-day global period) and a separate and distinct evaluation and management (E/M) service on the same date of service.1 Because of the multicomplaint nature of dermatology, the ability to report a same-day procedure and an E/M service is critical for efficient, cost-effective, and patient-centered dermatologic care. However, it is well known that the use of modifier -25 has been under notable insurer scrutiny and is a common reason for medical record audits.2,3 Some insurers have responded to increased utilization of modifier -25 by cutting reimbursement for claims that include both a procedure and an E/M service or by denying one of the services altogether.4-6 The Centers for Medicare and Medicaid Services also have expressed concern about this coding combination with proposed cuts to reimbursement.7 Moreover, the Office of Inspector General has announced a work plan to investigate the frequent utilization of E/M codes and minor procedures by dermatologists.8 Clearly, modifier -25 is a continued target by insurers and regulators; therefore, dermatologists will want to make sure their coding and documentation meet all requirements and are updated for the new E/M codes for 2021.
The American Medical Association’s Current Procedural Terminology indicates that modifier -25 allows reporting of a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of a procedure or other service.”1 Given that dermatology patients typically present with multiple concerns, dermatologists commonly evaluate and treat numerous conditions during one visit. Understanding what constitutes a separately identifiable E/M service is critical to bill accurately and to pass insurer audits.
Global Surgical Package
To appropriately bill both a procedure and an E/M service, the physician must indicate that the patient’s condition required an E/M service above and beyond the usual work of the procedure. The compilation of evaluation and work included in the payment for a procedure is called the global surgical package.9 In general, the global surgical package includes local or topical anesthesia; the surgical service/procedure itself; immediate postoperative care, including dictating the operative note; meeting/discussing the patient’s procedure with family and other physicians; and writing orders for the patient. For minor procedures (ie, those with either 0- or 10-day global periods), the surgical package also includes same-day E/M services associated with the decision to perform surgery. An appropriate history and physical examination as well as a discussion of the differential diagnosis, treatment options, and risk and benefits of treatment are all included in the payment of a minor procedure itself. Therefore, an evaluation to discuss a patient’s condition or change in condition, alternatives to treatment, or next steps after a diagnosis related to a treatment or diagnostic procedure should not be separately reported. Moreover, the fact that the patient is new to the physician is not in itself sufficient to allow reporting of an E/M service with these minor procedures. For major procedures (ie, those with 90-day postoperative periods), the decision for surgery is excluded from the global surgical package.
2021 E/M Codes Simplify Documentation
The biggest coding change of 2021 was the new E/M codes.10 Prior to this year, the descriptors of E/M services recognized 7 components to define the levels of E/M services11: history and nature of the presenting problem; physical examination; medical decision-making (MDM); counseling; coordination of care; and time. Furthermore, history, physical examination, and MDM were all broken down into more granular elements that were summed to determine the level for each component; for example, the history of the presenting problem was defined as a chronological description of the development of the patient’s present illness, including the following elements: location, quality, severity, duration, timing, context, modifying factors, and associated signs and symptoms. Each of these categories would constitute bullet points to be summed to determine the level of history. Physical examination and MDM bullet points also would be summed to determine a proper coding level.11 Understandably, this coding scheme was complicated and burdensome to medical providers.
The redefinition of the E/M codes for 2021 substantially simplified the determination of coding level and documentation.10 The revisions to the E/M office visit code descriptors and documentation standards are now centered around how physicians think and take care of patients and not on mandatory standards and checking boxes. The main changes involve MDM as the prime determinant of the coding level. Elements of MDM affecting coding for an outpatient or office visit now include only 3 components: the number and complexity of problems addressed in the encounter, the amount or complexity of data to be reviewed and analyzed, and the risk of complications or morbidity of patient management. Gone are the requirements from the earlier criteria requiring so many bullet points for the history, physical examination, and MDM.
Dermatologists may ask, “How does the new E/M coding structure affect reporting and documenting an E/M and a procedure on the same day?” The answer is that the determination of separate and distinct is basically unchanged with the new E/M codes; however, the documentation requirements for modifier -25 using the new E/M codes are simplified.
As always, the key to determining whether a separate and distinct E/M service was provided and subsequently documented is to deconstruct the medical note. All evaluation services associated with the procedure—making a clinical diagnosis or differential diagnosis, decision to perform surgery, and discussion of alternative treatments—should be removed from one’s documentation as shown in the example below. If a complete E/M service still exists, then an E/M may be billed in addition to the procedure. Physical examination of the treatment area is included in the surgical package. With the prior E/M criteria, physical examination of the procedural area could not be used again as a bullet point to count for the E/M level. However, with the new 2021 coding requirements, the documentation of a separate MDM will be sufficient to meet criteria because documentation of physical examination is not a requirement.
Modifier -25 Examples
Let’s examine a typical dermatologist medical note. An established patient presents to the dermatologist complaining of an itchy rash on the left wrist after a hiking trip. Treatment with topical hydrocortisone 1% did not help. The patient also complains of a growing tender lesion on the left elbow of 2 months’ duration. Physical examination reveals a linear vesicular eruption on the left wrist and a tender hyperkeratotic papule on the left elbow. No data is evaluated. A diagnosis of acute rhus dermatitis of the left wrist is made, and betamethasone cream is prescribed. The decision is made to perform a tangential biopsy of the lesion on the left elbow because of the suspicion for malignancy. The biopsy is performed the same day.
This case clearly illustrates performance of an E/M service in the treatment of rhus dermatitis, which is separate and distinct from the biopsy procedure; however, in evaluating whether the case meets the documentation requirements for modifier -25, the information in the medical note inclusive to the procedure’s global surgical package, including history associated with establishing the diagnosis, physical examination of the procedure area(s), and discussion of treatment options, is eliminated, leaving the following notes: An established patient presents to the dermatologist complaining of an itchy rash on the left wrist after a hiking trip. Treatment with topical hydrocortisone 1% did not help. No data is evaluated. A diagnosis of acute rhus dermatitis of the left wrist is made, and betamethasone cream is prescribed.
Because the physical examination of the body part (left arm) is included in the procedure’s global surgical package, the examination of the left wrist cannot be used as coding support for the E/M service. This makes a difference for coding level in the prior E/M coding requirements, which required examination bullet points. However, with the 2021 E/M codes, documentation of physical examination bullet points is irrelevant to the coding level. Therefore, qualifying for a modifier -25 claim is more straightforward in this case with the new code set. Because bullet points are not integral to the 2021 E/M codes, qualifying and properly documenting for a higher level of service will likely be more common in dermatology.
Final Thoughts
Frequent use of modifier -25 is a critical part of a high-quality and cost-effective dermatology practice. Same-day performance of minor procedures and E/M services allows for more rapid and efficient diagnosis and treatment of various conditions as well as minimizing unnecessary office visits. The new E/M codes for 2021 actually make the documentation of a separate and distinct E/M service less complicated because the bullet point requirements associated with the old E/M codes have been eliminated. Understanding how the new E/M code descriptors affect modifier -25 reporting and clear documentation of separate, distinct, and medically necessary E/M services will be needed due to increased insurer scrutiny and audits.
- Current Procedural Terminology 2021, Professional Edition. American Medical Association; 2020.
- Rogers HW. Modifier −25 victory, but the battle is not over. Cutis. 2018;101:409-410.
- Rogers HW. One diagnosis and modifier −25: appropriate or audit target? Cutis. 2017;99:165-166.
- Update regarding E/M with modifier −25—professional. Anthem Blue Cross Blue Shield website. Published February 1, 2019. Accessed August 17, 2021. https://providernews.anthem.com/ohio/article/update-regarding-em-with-modifier-25-professional
- Payment policies—surgery. Harvard Pilgrim Health Care website. Updated May 2021. Accessed August 17, 2021. https://www.harvardpilgrim.org/provider/wp-content/uploads/sites/7/2020/07/H-6-Surgery-PM.pdf
- Modifier 25: frequently asked questions. Independence Blue Cross website. Updated September 25, 2017. Accessed August 17, 2021. https://provcomm.ibx.com/ibc/archive/pages/A86603B03881756B8525817E00768006.aspx
- Huang G. CMS 2019 fee schedule takes modifier 25 cuts, runs with them. Doctors Management website. Accessed August 17, 2021. https://www.doctors-management.com/cms-2019-feeschedule-modifier25/
- Dermatologist claims for evaluation and management services on the same day as minor surgical procedures. US Department of Health and Humans Services Office of Inspector General website. Accessed August 17, 2021. https://oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000577.asp
- Global surgery booklet. Centers for Medicare and Medicaid Services website. Updated September 2018. Accessed August 17, 2021. https://www.cms.gov/outreach-and-education/medicare-learning-network-mln/mlnproducts/downloads/globallsurgery-icn907166.pdf
- American Medical Association. CPT® Evaluation and management (E/M)—office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Updated March 9, 2021. Accessed August 17, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
- 1997 documentation guidelines for evaluation and management services. Centers for Medicare and Medicaid Services website. Accessed August 17, 2021. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNEdWebGuide/Downloads/97Docguidelines.pdf
- Current Procedural Terminology 2021, Professional Edition. American Medical Association; 2020.
- Rogers HW. Modifier −25 victory, but the battle is not over. Cutis. 2018;101:409-410.
- Rogers HW. One diagnosis and modifier −25: appropriate or audit target? Cutis. 2017;99:165-166.
- Update regarding E/M with modifier −25—professional. Anthem Blue Cross Blue Shield website. Published February 1, 2019. Accessed August 17, 2021. https://providernews.anthem.com/ohio/article/update-regarding-em-with-modifier-25-professional
- Payment policies—surgery. Harvard Pilgrim Health Care website. Updated May 2021. Accessed August 17, 2021. https://www.harvardpilgrim.org/provider/wp-content/uploads/sites/7/2020/07/H-6-Surgery-PM.pdf
- Modifier 25: frequently asked questions. Independence Blue Cross website. Updated September 25, 2017. Accessed August 17, 2021. https://provcomm.ibx.com/ibc/archive/pages/A86603B03881756B8525817E00768006.aspx
- Huang G. CMS 2019 fee schedule takes modifier 25 cuts, runs with them. Doctors Management website. Accessed August 17, 2021. https://www.doctors-management.com/cms-2019-feeschedule-modifier25/
- Dermatologist claims for evaluation and management services on the same day as minor surgical procedures. US Department of Health and Humans Services Office of Inspector General website. Accessed August 17, 2021. https://oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000577.asp
- Global surgery booklet. Centers for Medicare and Medicaid Services website. Updated September 2018. Accessed August 17, 2021. https://www.cms.gov/outreach-and-education/medicare-learning-network-mln/mlnproducts/downloads/globallsurgery-icn907166.pdf
- American Medical Association. CPT® Evaluation and management (E/M)—office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Updated March 9, 2021. Accessed August 17, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
- 1997 documentation guidelines for evaluation and management services. Centers for Medicare and Medicaid Services website. Accessed August 17, 2021. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNEdWebGuide/Downloads/97Docguidelines.pdf
Practice Points
- Insurer scrutiny of same-day evaluation and management (E/M) and procedure services has increased, and dermatologists should be prepared for more frequent medical record reviews and audits.
- The new 2021 E/M codes actually reduce the hurdles for reporting a separate and distinct E/M service by eliminating the history and physical examination bullet points of the previous code set.
Supreme Court Case: Dobbs v Jackson Women’s Health Organization: What you need to know
This fall, the Supreme Court of the United States (SCOTUS) will announce when they will hear oral arguments for Dobbs v Jackson Women’s Health Organization. The court will examine a Mississippi law, known as the “Gestational Age Act,” originally passed in 2018, that sought to “limit abortions to fifteen weeks’ gestation except in a medical emergency or in cases of severe fetal abnormality.”1 This sets the stage for SCOTUS to make a major ruling on abortion, one which could affirm or upend landmark decisions and nearly 50 years of abortion legislative precedent. Additionally, SCOTUS’ recent decision to not intervene on Texas’ Senate Bill 8 (SB8), which essentially bans all abortions after 6 weeks’ gestational age, may foreshadow how this case will be decided. The current abortion restrictions in Texas and the implications of SB8 will be discussed in a forthcoming column.
SCOTUS and abortion rights
The decision to hear this case comes on the heels of another recent decision regarding a Louisiana law in June Medical Services v Russo. This case examined Louisiana Act 620, which would have required physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.2 The law was deemed constitutionally invalid, with the majority noting the law would have drastically burdened a woman’s right to access abortion services. The Court ruled similarly in 2016 in Whole Women’s Health (WWH) v Hellerstedt, in which WWH challenged Texas House Bill 2, a nearly identical law requiring admitting privileges for abortion care providers. In both of these cases, SCOTUS pointed to precedent set by Southeastern Pennsylvania v Casey, which established that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 The precedent to this, Roe v Wade, and 5 decades of abortion legislation set may be upended by a SCOTUS decision this next term.
Dobbs v Jackson
On March 19, 2018, Mississippi enacted the “Gestational Age Act” into law. The newly enacted law would limit abortions to 15 weeks’ gestation except in a medical emergency or in cases of severe fetal anomalies. Jackson Women’s Health Organization, the only licensed abortion provider in the state, challenged the constitutionality of the law with legal support from Center for Reproductive Rights (CRR). The US District Court for the Southern District of Mississippi granted summary judgement in favor of the clinic and placed an injunction on the law’s enforcement. The state appealed to the Fifth Circuit Court of Appeals, which upheld the district court decision in a 3-0 decision in November 2019. Mississippi appealed to the Supreme Court, with their petition focusing on multiple questions from the appeals process. After repeatedly rescheduling the case, and multiple reviews in conference, SCOTUS agreed to hear the case. Most recently, the state has narrowed its argument, changing course, and attacking Roe v Wade directly. In a brief submitted in July 2021, the state argues the court should hold that all pre-viability prohibitions on elective abortions are constitutional.
Interestingly, during this time the Mississippi legislature also passed a law, House Bill 2116, also known as the “fetal heartbeat bill,” banning abortion with gestational ages after detection of a fetal heartbeat. This was also challenged, deemed unconstitutional, and affirmed on appeal by the Fifth US Circuit Court.
While recent challenges have focused on the “undue burden” state laws placed on those trying to access abortion care, this case will bring the issue of “viability” and gestational age limits to the forefront.4,5 In addition to Roe v Wade, the Court will have the opportunity to reexamine other relevant precedent, such as Southeastern Pennsylvania v Casey, in considering the most recent arguments of the state. In this most recent brief, the state argues that the Court should, “reject viability as a barrier to prohibiting elective abortions” and that a “viability rule has no constitutional basis.” The state goes on to argue the “Constitution does not protect a right to abortion or limit States’ authority to restrict it.”6 The language and tone in this brief are more direct and aggressive than the states’ petition submitted last June.
However, the composition of the Court is different than in the past. This case will be argued with Justice Amy Coney Barrett seated in place of Justice Ruth Bader Ginsburg, who was a strong advocate for women’s rights.7 She joins Justices Neil Gorsuch and Brett Kavanaugh, also appointed by President Donald Trump and widely viewed as conservative judges, tipping the scales to a more conservative Supreme Court. This case will also be argued in a polarized political environment.8,9 Given the conservative Supreme Court in the setting of an increasingly politically charged environment, reproductive right advocates are understandably worried that members of the anti-abortion movement view this as an opportunity to weaken or remove federal constitutional protections for abortion.
Continue to: Potential outcome of Dobbs v Jackson...
Potential outcome of Dobbs v Jackson
Should SCOTUS choose to rule in favor of Mississippi, it could severely weaken, or even overturn Roe v Wade. This would leave a legal path for states with pre-Roe abortion bans and currently unenforced post-Roe bans to take effect. These “trigger” laws are bans or severe restrictions on abortion providers and patients intended to take effect if Roe were to be overturned. Alternatively, the Court may overturn Southeastern Pennsylvania v Casey, but maintain Roe v Wade, essentially leaving the regulation of pre-viability abortion care to individual states. Currently 21 states have laws that would restrict the legal status of abortion.10 In addition, state legislatures are aggressively introducing abortion restrictions. As of June 2021, there have been 561 abortion restrictions, including 165 abortion bans, introduced across 47 states, putting 2021 on course to be the most devastating anti-abortion state legislative session in decades.11
The damage caused by such restriction on abortion care would be significant. It would block or push access out of reach for many. The negative effects of such legislative action would most heavily burden those already marginalized by systemic, structural inequalities including those of low socioeconomic status, people of color, young people, those in rural communities, and members of the LGBTQ community. The medical community has long recognized the harm caused by restricting access to abortion care. Restriction of access to safe abortion care paradoxically has been shown not to decrease the incidence of abortion, but rather increases the number of unsafe abortions.12 The American College of Obstetricians and Gynecologists (ACOG) acknowledge “individuals require access to safe, legal abortion” and that this represents “a necessary component for comprehensive health care.”13,14 They joined the American Medical Association and other professional groups in a 2019 amicus brief to SCOTUS opposing restrictions on abortion access.15 In addition, government laws restricting access to abortion care undermine the fundamental relationship between a person and their physician, limiting a physician’s obligation to honor patient autonomy and provide appropriate medical care.
By taking up the question whether all pre-viability bans on elective abortions violate the Constitution, SCOTUS is indicating a possible willingness to revisit the central holding of abortion jurisprudence. Their decision regarding this case will likely be the most significant ruling regarding the legal status of abortion care in decades, and will significantly affect the delivery of abortion care in the future.
Action items
- Reach out to your representatives to support the Women’s Health Protection Act, an initiative introduced to Congress to protect access to abortion care. If you reside in a state where your federal representatives support the Women’s Health Protection Act, reach out to friends and colleagues in states without supportive elected officials and ask them to call their representatives and ask them to support the bill.
- Get involved with local grassroots groups fighting to protect abortion access.
- Continue to speak out against laws and policies designed to limit access to safe abortion care.
- Connect with your local ACOG chapter for more ways to become involved.
- As always, make sure you are registered to vote, and exercise your right whenever you can.
- HB1510 (As Introduced) - 2018 Regular Session. http://billstatus.ls.state.ms.us/documents/2018/html/HB/1500-1599/HB1510IN.htm Accessed August 13, 2021.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed August 13, 2021.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed August 13, 2021.
- 15-274 Whole Woman’s Health v. Hellerstedt (06/27/2016). Published online 2016:107.
- 18-1323 June Medical Services L. L. C. v. Russo (06/29/2020). Published online 2020:138.
- 19-1392 Dobbs v. Jackson Women’s Health Organization (07/22/2021). Published online 2021.
- What Ruth Bader Ginsburg said about abortion and Roe v. Wade. Time. August 2, 2018. https://time.com/5354490/ruth-bader-ginsburg-roe-v-wade/. Accessed August 13, 2021.
- Montanaro D. Poll: majority want to keep abortion legal, but they also want restrictions. NPR. June 7, 2019. https://www.npr.org/2019/06/07/730183531/poll-majority-want-to-keep-abortion-legal-but-they-also-want-restrictions. Accessed August 13, 2021.
- Abortion support remains steady despite growing partisan divide, survey finds. Washington Post. August 13, 2019. https://www.washingtonpost.com/health/2019/08/13/one-largest-ever-abortion-surveys-shows-growing-partisan-divide/. Accessed August 13, 2021.
- Abortion policy in the absence of Roe. Guttmacher Institute. September 1, 2021. https://www.guttmacher.org/state-policy/explore/abortion-policy-absence-roe#. Accessed September 8, 2021.
- 2021 is on track to become the most devastating antiabortion state legislative session in decades. Guttmacher Institute. Published April 30, 2021. Updated June 14, 2021. https://www.guttmacher.org/article/2021/04/2021-track-become-most-devastating-antiabortion-state-legislative-session-decades. Accessed August 13, 2021.
- Facts and consequences: legality, incidence and safety of abortion worldwide. Guttmacher Institute. November 20, 2009. https://www.guttmacher.org/gpr/2009/11/facts-and-consequences-legality-incidence-and-safety-abortion-worldwide. Accessed August 13, 2021.
- Increasing access to abortion. https://www.acog.org/en/clinical/clinical-guidance/committee-opinion/articles/2020/12/increasing-access-to-abortion. Accessed August 13, 2021.
- ACOG statement on Dobbs vs. Jackson Women’s Health. May 17, 2021. https://www.acog.org/en/news/news-releases/2021/05/acog-statement-dobbs-vs-jackson-womens-health. Accessed August 13, 2021.
- Perryman SL, Parker KA, Hickman SA. Brief of amici curiae American College of Obstetricians and Gynecologists, American Medical Associations, American Academy of Family Physicians, American Academy of Nursing, American Academy of Pediatrics, et al. In support of June Medical Services, LLC, et al. https://www.supremecourt.gov/
DocketPDF/18/18-1323/124091/ . Accessed August 13, 2021.20191202145531124_18-1323% 2018-1460%20tsac%20American% 20College%20of% 20Obstetricians%20and% 20Gynecologists%20et%20al.pdf
This fall, the Supreme Court of the United States (SCOTUS) will announce when they will hear oral arguments for Dobbs v Jackson Women’s Health Organization. The court will examine a Mississippi law, known as the “Gestational Age Act,” originally passed in 2018, that sought to “limit abortions to fifteen weeks’ gestation except in a medical emergency or in cases of severe fetal abnormality.”1 This sets the stage for SCOTUS to make a major ruling on abortion, one which could affirm or upend landmark decisions and nearly 50 years of abortion legislative precedent. Additionally, SCOTUS’ recent decision to not intervene on Texas’ Senate Bill 8 (SB8), which essentially bans all abortions after 6 weeks’ gestational age, may foreshadow how this case will be decided. The current abortion restrictions in Texas and the implications of SB8 will be discussed in a forthcoming column.
SCOTUS and abortion rights
The decision to hear this case comes on the heels of another recent decision regarding a Louisiana law in June Medical Services v Russo. This case examined Louisiana Act 620, which would have required physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.2 The law was deemed constitutionally invalid, with the majority noting the law would have drastically burdened a woman’s right to access abortion services. The Court ruled similarly in 2016 in Whole Women’s Health (WWH) v Hellerstedt, in which WWH challenged Texas House Bill 2, a nearly identical law requiring admitting privileges for abortion care providers. In both of these cases, SCOTUS pointed to precedent set by Southeastern Pennsylvania v Casey, which established that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 The precedent to this, Roe v Wade, and 5 decades of abortion legislation set may be upended by a SCOTUS decision this next term.
Dobbs v Jackson
On March 19, 2018, Mississippi enacted the “Gestational Age Act” into law. The newly enacted law would limit abortions to 15 weeks’ gestation except in a medical emergency or in cases of severe fetal anomalies. Jackson Women’s Health Organization, the only licensed abortion provider in the state, challenged the constitutionality of the law with legal support from Center for Reproductive Rights (CRR). The US District Court for the Southern District of Mississippi granted summary judgement in favor of the clinic and placed an injunction on the law’s enforcement. The state appealed to the Fifth Circuit Court of Appeals, which upheld the district court decision in a 3-0 decision in November 2019. Mississippi appealed to the Supreme Court, with their petition focusing on multiple questions from the appeals process. After repeatedly rescheduling the case, and multiple reviews in conference, SCOTUS agreed to hear the case. Most recently, the state has narrowed its argument, changing course, and attacking Roe v Wade directly. In a brief submitted in July 2021, the state argues the court should hold that all pre-viability prohibitions on elective abortions are constitutional.
Interestingly, during this time the Mississippi legislature also passed a law, House Bill 2116, also known as the “fetal heartbeat bill,” banning abortion with gestational ages after detection of a fetal heartbeat. This was also challenged, deemed unconstitutional, and affirmed on appeal by the Fifth US Circuit Court.
While recent challenges have focused on the “undue burden” state laws placed on those trying to access abortion care, this case will bring the issue of “viability” and gestational age limits to the forefront.4,5 In addition to Roe v Wade, the Court will have the opportunity to reexamine other relevant precedent, such as Southeastern Pennsylvania v Casey, in considering the most recent arguments of the state. In this most recent brief, the state argues that the Court should, “reject viability as a barrier to prohibiting elective abortions” and that a “viability rule has no constitutional basis.” The state goes on to argue the “Constitution does not protect a right to abortion or limit States’ authority to restrict it.”6 The language and tone in this brief are more direct and aggressive than the states’ petition submitted last June.
However, the composition of the Court is different than in the past. This case will be argued with Justice Amy Coney Barrett seated in place of Justice Ruth Bader Ginsburg, who was a strong advocate for women’s rights.7 She joins Justices Neil Gorsuch and Brett Kavanaugh, also appointed by President Donald Trump and widely viewed as conservative judges, tipping the scales to a more conservative Supreme Court. This case will also be argued in a polarized political environment.8,9 Given the conservative Supreme Court in the setting of an increasingly politically charged environment, reproductive right advocates are understandably worried that members of the anti-abortion movement view this as an opportunity to weaken or remove federal constitutional protections for abortion.
Continue to: Potential outcome of Dobbs v Jackson...
Potential outcome of Dobbs v Jackson
Should SCOTUS choose to rule in favor of Mississippi, it could severely weaken, or even overturn Roe v Wade. This would leave a legal path for states with pre-Roe abortion bans and currently unenforced post-Roe bans to take effect. These “trigger” laws are bans or severe restrictions on abortion providers and patients intended to take effect if Roe were to be overturned. Alternatively, the Court may overturn Southeastern Pennsylvania v Casey, but maintain Roe v Wade, essentially leaving the regulation of pre-viability abortion care to individual states. Currently 21 states have laws that would restrict the legal status of abortion.10 In addition, state legislatures are aggressively introducing abortion restrictions. As of June 2021, there have been 561 abortion restrictions, including 165 abortion bans, introduced across 47 states, putting 2021 on course to be the most devastating anti-abortion state legislative session in decades.11
The damage caused by such restriction on abortion care would be significant. It would block or push access out of reach for many. The negative effects of such legislative action would most heavily burden those already marginalized by systemic, structural inequalities including those of low socioeconomic status, people of color, young people, those in rural communities, and members of the LGBTQ community. The medical community has long recognized the harm caused by restricting access to abortion care. Restriction of access to safe abortion care paradoxically has been shown not to decrease the incidence of abortion, but rather increases the number of unsafe abortions.12 The American College of Obstetricians and Gynecologists (ACOG) acknowledge “individuals require access to safe, legal abortion” and that this represents “a necessary component for comprehensive health care.”13,14 They joined the American Medical Association and other professional groups in a 2019 amicus brief to SCOTUS opposing restrictions on abortion access.15 In addition, government laws restricting access to abortion care undermine the fundamental relationship between a person and their physician, limiting a physician’s obligation to honor patient autonomy and provide appropriate medical care.
By taking up the question whether all pre-viability bans on elective abortions violate the Constitution, SCOTUS is indicating a possible willingness to revisit the central holding of abortion jurisprudence. Their decision regarding this case will likely be the most significant ruling regarding the legal status of abortion care in decades, and will significantly affect the delivery of abortion care in the future.
Action items
- Reach out to your representatives to support the Women’s Health Protection Act, an initiative introduced to Congress to protect access to abortion care. If you reside in a state where your federal representatives support the Women’s Health Protection Act, reach out to friends and colleagues in states without supportive elected officials and ask them to call their representatives and ask them to support the bill.
- Get involved with local grassroots groups fighting to protect abortion access.
- Continue to speak out against laws and policies designed to limit access to safe abortion care.
- Connect with your local ACOG chapter for more ways to become involved.
- As always, make sure you are registered to vote, and exercise your right whenever you can.
This fall, the Supreme Court of the United States (SCOTUS) will announce when they will hear oral arguments for Dobbs v Jackson Women’s Health Organization. The court will examine a Mississippi law, known as the “Gestational Age Act,” originally passed in 2018, that sought to “limit abortions to fifteen weeks’ gestation except in a medical emergency or in cases of severe fetal abnormality.”1 This sets the stage for SCOTUS to make a major ruling on abortion, one which could affirm or upend landmark decisions and nearly 50 years of abortion legislative precedent. Additionally, SCOTUS’ recent decision to not intervene on Texas’ Senate Bill 8 (SB8), which essentially bans all abortions after 6 weeks’ gestational age, may foreshadow how this case will be decided. The current abortion restrictions in Texas and the implications of SB8 will be discussed in a forthcoming column.
SCOTUS and abortion rights
The decision to hear this case comes on the heels of another recent decision regarding a Louisiana law in June Medical Services v Russo. This case examined Louisiana Act 620, which would have required physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.2 The law was deemed constitutionally invalid, with the majority noting the law would have drastically burdened a woman’s right to access abortion services. The Court ruled similarly in 2016 in Whole Women’s Health (WWH) v Hellerstedt, in which WWH challenged Texas House Bill 2, a nearly identical law requiring admitting privileges for abortion care providers. In both of these cases, SCOTUS pointed to precedent set by Southeastern Pennsylvania v Casey, which established that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 The precedent to this, Roe v Wade, and 5 decades of abortion legislation set may be upended by a SCOTUS decision this next term.
Dobbs v Jackson
On March 19, 2018, Mississippi enacted the “Gestational Age Act” into law. The newly enacted law would limit abortions to 15 weeks’ gestation except in a medical emergency or in cases of severe fetal anomalies. Jackson Women’s Health Organization, the only licensed abortion provider in the state, challenged the constitutionality of the law with legal support from Center for Reproductive Rights (CRR). The US District Court for the Southern District of Mississippi granted summary judgement in favor of the clinic and placed an injunction on the law’s enforcement. The state appealed to the Fifth Circuit Court of Appeals, which upheld the district court decision in a 3-0 decision in November 2019. Mississippi appealed to the Supreme Court, with their petition focusing on multiple questions from the appeals process. After repeatedly rescheduling the case, and multiple reviews in conference, SCOTUS agreed to hear the case. Most recently, the state has narrowed its argument, changing course, and attacking Roe v Wade directly. In a brief submitted in July 2021, the state argues the court should hold that all pre-viability prohibitions on elective abortions are constitutional.
Interestingly, during this time the Mississippi legislature also passed a law, House Bill 2116, also known as the “fetal heartbeat bill,” banning abortion with gestational ages after detection of a fetal heartbeat. This was also challenged, deemed unconstitutional, and affirmed on appeal by the Fifth US Circuit Court.
While recent challenges have focused on the “undue burden” state laws placed on those trying to access abortion care, this case will bring the issue of “viability” and gestational age limits to the forefront.4,5 In addition to Roe v Wade, the Court will have the opportunity to reexamine other relevant precedent, such as Southeastern Pennsylvania v Casey, in considering the most recent arguments of the state. In this most recent brief, the state argues that the Court should, “reject viability as a barrier to prohibiting elective abortions” and that a “viability rule has no constitutional basis.” The state goes on to argue the “Constitution does not protect a right to abortion or limit States’ authority to restrict it.”6 The language and tone in this brief are more direct and aggressive than the states’ petition submitted last June.
However, the composition of the Court is different than in the past. This case will be argued with Justice Amy Coney Barrett seated in place of Justice Ruth Bader Ginsburg, who was a strong advocate for women’s rights.7 She joins Justices Neil Gorsuch and Brett Kavanaugh, also appointed by President Donald Trump and widely viewed as conservative judges, tipping the scales to a more conservative Supreme Court. This case will also be argued in a polarized political environment.8,9 Given the conservative Supreme Court in the setting of an increasingly politically charged environment, reproductive right advocates are understandably worried that members of the anti-abortion movement view this as an opportunity to weaken or remove federal constitutional protections for abortion.
Continue to: Potential outcome of Dobbs v Jackson...
Potential outcome of Dobbs v Jackson
Should SCOTUS choose to rule in favor of Mississippi, it could severely weaken, or even overturn Roe v Wade. This would leave a legal path for states with pre-Roe abortion bans and currently unenforced post-Roe bans to take effect. These “trigger” laws are bans or severe restrictions on abortion providers and patients intended to take effect if Roe were to be overturned. Alternatively, the Court may overturn Southeastern Pennsylvania v Casey, but maintain Roe v Wade, essentially leaving the regulation of pre-viability abortion care to individual states. Currently 21 states have laws that would restrict the legal status of abortion.10 In addition, state legislatures are aggressively introducing abortion restrictions. As of June 2021, there have been 561 abortion restrictions, including 165 abortion bans, introduced across 47 states, putting 2021 on course to be the most devastating anti-abortion state legislative session in decades.11
The damage caused by such restriction on abortion care would be significant. It would block or push access out of reach for many. The negative effects of such legislative action would most heavily burden those already marginalized by systemic, structural inequalities including those of low socioeconomic status, people of color, young people, those in rural communities, and members of the LGBTQ community. The medical community has long recognized the harm caused by restricting access to abortion care. Restriction of access to safe abortion care paradoxically has been shown not to decrease the incidence of abortion, but rather increases the number of unsafe abortions.12 The American College of Obstetricians and Gynecologists (ACOG) acknowledge “individuals require access to safe, legal abortion” and that this represents “a necessary component for comprehensive health care.”13,14 They joined the American Medical Association and other professional groups in a 2019 amicus brief to SCOTUS opposing restrictions on abortion access.15 In addition, government laws restricting access to abortion care undermine the fundamental relationship between a person and their physician, limiting a physician’s obligation to honor patient autonomy and provide appropriate medical care.
By taking up the question whether all pre-viability bans on elective abortions violate the Constitution, SCOTUS is indicating a possible willingness to revisit the central holding of abortion jurisprudence. Their decision regarding this case will likely be the most significant ruling regarding the legal status of abortion care in decades, and will significantly affect the delivery of abortion care in the future.
Action items
- Reach out to your representatives to support the Women’s Health Protection Act, an initiative introduced to Congress to protect access to abortion care. If you reside in a state where your federal representatives support the Women’s Health Protection Act, reach out to friends and colleagues in states without supportive elected officials and ask them to call their representatives and ask them to support the bill.
- Get involved with local grassroots groups fighting to protect abortion access.
- Continue to speak out against laws and policies designed to limit access to safe abortion care.
- Connect with your local ACOG chapter for more ways to become involved.
- As always, make sure you are registered to vote, and exercise your right whenever you can.
- HB1510 (As Introduced) - 2018 Regular Session. http://billstatus.ls.state.ms.us/documents/2018/html/HB/1500-1599/HB1510IN.htm Accessed August 13, 2021.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed August 13, 2021.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed August 13, 2021.
- 15-274 Whole Woman’s Health v. Hellerstedt (06/27/2016). Published online 2016:107.
- 18-1323 June Medical Services L. L. C. v. Russo (06/29/2020). Published online 2020:138.
- 19-1392 Dobbs v. Jackson Women’s Health Organization (07/22/2021). Published online 2021.
- What Ruth Bader Ginsburg said about abortion and Roe v. Wade. Time. August 2, 2018. https://time.com/5354490/ruth-bader-ginsburg-roe-v-wade/. Accessed August 13, 2021.
- Montanaro D. Poll: majority want to keep abortion legal, but they also want restrictions. NPR. June 7, 2019. https://www.npr.org/2019/06/07/730183531/poll-majority-want-to-keep-abortion-legal-but-they-also-want-restrictions. Accessed August 13, 2021.
- Abortion support remains steady despite growing partisan divide, survey finds. Washington Post. August 13, 2019. https://www.washingtonpost.com/health/2019/08/13/one-largest-ever-abortion-surveys-shows-growing-partisan-divide/. Accessed August 13, 2021.
- Abortion policy in the absence of Roe. Guttmacher Institute. September 1, 2021. https://www.guttmacher.org/state-policy/explore/abortion-policy-absence-roe#. Accessed September 8, 2021.
- 2021 is on track to become the most devastating antiabortion state legislative session in decades. Guttmacher Institute. Published April 30, 2021. Updated June 14, 2021. https://www.guttmacher.org/article/2021/04/2021-track-become-most-devastating-antiabortion-state-legislative-session-decades. Accessed August 13, 2021.
- Facts and consequences: legality, incidence and safety of abortion worldwide. Guttmacher Institute. November 20, 2009. https://www.guttmacher.org/gpr/2009/11/facts-and-consequences-legality-incidence-and-safety-abortion-worldwide. Accessed August 13, 2021.
- Increasing access to abortion. https://www.acog.org/en/clinical/clinical-guidance/committee-opinion/articles/2020/12/increasing-access-to-abortion. Accessed August 13, 2021.
- ACOG statement on Dobbs vs. Jackson Women’s Health. May 17, 2021. https://www.acog.org/en/news/news-releases/2021/05/acog-statement-dobbs-vs-jackson-womens-health. Accessed August 13, 2021.
- Perryman SL, Parker KA, Hickman SA. Brief of amici curiae American College of Obstetricians and Gynecologists, American Medical Associations, American Academy of Family Physicians, American Academy of Nursing, American Academy of Pediatrics, et al. In support of June Medical Services, LLC, et al. https://www.supremecourt.gov/
DocketPDF/18/18-1323/124091/ . Accessed August 13, 2021.20191202145531124_18-1323% 2018-1460%20tsac%20American% 20College%20of% 20Obstetricians%20and% 20Gynecologists%20et%20al.pdf
- HB1510 (As Introduced) - 2018 Regular Session. http://billstatus.ls.state.ms.us/documents/2018/html/HB/1500-1599/HB1510IN.htm Accessed August 13, 2021.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed August 13, 2021.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed August 13, 2021.
- 15-274 Whole Woman’s Health v. Hellerstedt (06/27/2016). Published online 2016:107.
- 18-1323 June Medical Services L. L. C. v. Russo (06/29/2020). Published online 2020:138.
- 19-1392 Dobbs v. Jackson Women’s Health Organization (07/22/2021). Published online 2021.
- What Ruth Bader Ginsburg said about abortion and Roe v. Wade. Time. August 2, 2018. https://time.com/5354490/ruth-bader-ginsburg-roe-v-wade/. Accessed August 13, 2021.
- Montanaro D. Poll: majority want to keep abortion legal, but they also want restrictions. NPR. June 7, 2019. https://www.npr.org/2019/06/07/730183531/poll-majority-want-to-keep-abortion-legal-but-they-also-want-restrictions. Accessed August 13, 2021.
- Abortion support remains steady despite growing partisan divide, survey finds. Washington Post. August 13, 2019. https://www.washingtonpost.com/health/2019/08/13/one-largest-ever-abortion-surveys-shows-growing-partisan-divide/. Accessed August 13, 2021.
- Abortion policy in the absence of Roe. Guttmacher Institute. September 1, 2021. https://www.guttmacher.org/state-policy/explore/abortion-policy-absence-roe#. Accessed September 8, 2021.
- 2021 is on track to become the most devastating antiabortion state legislative session in decades. Guttmacher Institute. Published April 30, 2021. Updated June 14, 2021. https://www.guttmacher.org/article/2021/04/2021-track-become-most-devastating-antiabortion-state-legislative-session-decades. Accessed August 13, 2021.
- Facts and consequences: legality, incidence and safety of abortion worldwide. Guttmacher Institute. November 20, 2009. https://www.guttmacher.org/gpr/2009/11/facts-and-consequences-legality-incidence-and-safety-abortion-worldwide. Accessed August 13, 2021.
- Increasing access to abortion. https://www.acog.org/en/clinical/clinical-guidance/committee-opinion/articles/2020/12/increasing-access-to-abortion. Accessed August 13, 2021.
- ACOG statement on Dobbs vs. Jackson Women’s Health. May 17, 2021. https://www.acog.org/en/news/news-releases/2021/05/acog-statement-dobbs-vs-jackson-womens-health. Accessed August 13, 2021.
- Perryman SL, Parker KA, Hickman SA. Brief of amici curiae American College of Obstetricians and Gynecologists, American Medical Associations, American Academy of Family Physicians, American Academy of Nursing, American Academy of Pediatrics, et al. In support of June Medical Services, LLC, et al. https://www.supremecourt.gov/
DocketPDF/18/18-1323/124091/ . Accessed August 13, 2021.20191202145531124_18-1323% 2018-1460%20tsac%20American% 20College%20of% 20Obstetricians%20and% 20Gynecologists%20et%20al.pdf
Business Education in Dermatology Residency: A Survey of Program Directors
Globally, the United States has the highest per-capita cost of health care; total costs are expected to account for approximately 20% of the nation’s gross domestic product by 2025.1 These rising costs have prompted residency programs and medical schools to incorporate business education into their curricula.2-5 Although medical training is demanding—with little room to add curricular components—these business-focused curricula have consistently received positive feedback from residents.5,6
In dermatology, more than 50% of residents opt to join a private practice upon graduation.7 In the United States, there also is an upward trend of practice acquisition and consolidation by private equity firms. Therefore, dermatology trainees are uniquely positioned to benefit from business education to make well-informed decisions about joining or starting a practice.Furthermore, whether in a private or academic setting, knowledge of foundational economics, business strategy, finance, marketing, and health care policy can equip dermatologists to more effectively advocate for local and national policies that benefit their patient population.7
We conducted a survey of dermatology program directors (PDs) to determine the availability of and perceptions regarding business education during residency training.
Materials and Methods
Institutional review board (Vanderbilt University Medical Center, Nashville, Tennessee) approval was obtained. The survey was distributed weekly during a 5-week period from July 2020 to August 2020 through the Research Electronic Data Capture survey application (www.project-redcap.org). Program director email addresses were obtained through the Accreditation Council for Graduate Medical Education (ACGME) program list. A PD was included in the survey if they were employed by an accredited US osteopathic or allopathic program and their email address was provided in the ACGME program list or on their program’s faculty web page; a PD was excluded if an email address was not provided in the ACGME program list or on their program’s faculty web page.
The 8-part questionnaire was designed to assess the following characteristics: details about the respondent’s residency program (institutional affiliation, number of residents), the respondent’s professional background (number of years as a PD, business training experience), resources for business education provided by the program, the respondent’s opinion about business education for residents, and the respondent’s perception of the most important topics to include in a dermatology curriculum’s business education component, which included economics/finance, health care policy/government, management, marketing, negotiation, private equity involvement in health care, business strategy, supply chain/operations, and technology/product development. Responses were kept anonymous. Categorical and continuous variables were analyzed with medians and proportions.
Results
Of the 139 surveys distributed, 35 were completed and returned (response rate, 25.2%). Most programs were university-affiliated (71.4%) or community-affiliated (22.9%). The median number of residents was 12. The respondents had a median of 5 years’ experience in their role. Most respondents (65.7%) had no business training, although 20.0% had completed undergraduate business coursework, and 8.6% had attended formal seminars on business topics; 5.7% were self-taught on business topics.
Business Education Availability
Approximately half (51.4%) of programs offered business training to residents, primarily through seminars or lectures (94.4%) and take-home modules (16.7%). None of the programs offered a formal gap year during which residents could pursue a professional business degree. Most respondents thought business education during residency was important (82.8%) and that programs should implement more training (57.1%). When asked whether residents were competent to handle business aspects of dermatology upon graduation, most respondents disagreed somewhat (22.9%) or were neutral (40.0%).
Topics for Business Education
The most important topics identified for inclusion in a business curriculum were economics or finance (68.6%), management (68.6%), and health care policy or government (57.1%). Other identified topics included negotiation (40.0%), private equity involvement in health care (40.0%), strategy (11.4%), supply chain or operations (11.4%), marketing (2.9%), and technology (2.9%).
Comment
Residency programs and medical schools in the United States have started to integrate formal business training into their curricula; however, the state of business training in dermatology has not been characterized. Overall, this survey revealed largely positive perceptions about business education and identified a demand for more resources.
Whereas most PDs identified business education as important, only one half (51.4%) of the representative programs offered structured training. Notably, most PDs did not agree that graduating residents were competent to handle the business demands of dermatology practice. These responses highlight a gap in the demand and resources available for business training.
Identifying Curricular Resources
During an already demanding residency, additional curricular components need to be beneficial and worthwhile. To avoid significant disruption, business training could take place in the form of online lectures or take-home modules. Most programs represented in the survey responses had an academic affiliation and therefore commonly have access to an affiliated graduate business school and/or hospital administrators who have clinical and business training.
Community dermatologists who own or run their own practice also are uniquely positioned to provide residents with practical, dermatology-specific business education. Programs can utilize their institutional and local colleagues to aid in curricular design and implementation. In addition, a potential long-term solution to obtaining resources for business education is to coordinate with a national dermatology organization to create standardized modules that are available to all residency programs.
Key Curriculum Topics
Our survey identified the most important topics to include in a business curriculum for dermatology residents. Economics and finance, management, and health care policy would be valuable to a trainee regardless of whether they ultimately choose a career in academia or private practice. A thorough understanding of complex health care policy reinforces knowledge about insurance and regional and national regulations, which could ultimately benefit patient care. As an example, the American Academy of Dermatology outlines several advocacy priorities such as Medicare reimbursement policies, access to dermatologic care through public and private insurance, medication access and pricing, and preservation of private practice in the setting of market consolidation. Having a better understanding of health care policy and business could better equip dermatologists to lead these often business-driven advocacy efforts to ultimately improve patient care and advance the specialty.8
Limitations
There were notable limitations to this survey, primarily related to its design. With a 25% response rate, there was the potential for response and selection biases; therefore, these results might not be generalizable to all programs. In addition, views held by PDs might not be consistent with those of other members of the dermatology community; for example, surveying residents, other faculty members, and dermatologists in private practice would have provided a more comprehensive characterization of the topic.
Conclusion
This study assessed residency program directors’ perceptions of business education in dermatology training. There appears to be an imbalance between the perceived importance of such education and the resources that are available to provide it. More attention is needed to address this gap to ensure that dermatologists are prepared to manage a rapidly changing health care environment. Results of this survey should encourage efforts to establish (1) a standardized, dermatology-specific business curriculum and (2) a plan to make that curriculum accessible to trainees and other members of the dermatology community.
- Branning G, Vater M. Healthcare spending: plenty of blame to go around. Am Health Drug Benefits. 2016;9:445-447.
- Bayard M, Peeples CR, Holt J, et al. An interactive approach to teaching practice management to family practice residents. Fam Med. 2003;35:622-624.
- Chan S. Management education during radiology residency: development of an educational practice. Acad Radiol. 2004;11:1308-1317.
- Ninan D, Patel D. Career and leadership education in anesthesia residency training. Cureus. 2018;10:e2546.
- Yu-Chin R. Teaching administration and management within psychiatric residency training. Acad Psychiatry. 2002;26:245-252.
- Winkelman JW, Brugnara C. Management training for pathology residents. II. experience with a focused curriculum. Am J Clin Pathol. 1994;101:564-568.
- Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021.
- Academy advocacy priorities. American Academy of Dermatology website. Accessed August 11, 2021. www.aad.org/member/advocacy/priorities
Globally, the United States has the highest per-capita cost of health care; total costs are expected to account for approximately 20% of the nation’s gross domestic product by 2025.1 These rising costs have prompted residency programs and medical schools to incorporate business education into their curricula.2-5 Although medical training is demanding—with little room to add curricular components—these business-focused curricula have consistently received positive feedback from residents.5,6
In dermatology, more than 50% of residents opt to join a private practice upon graduation.7 In the United States, there also is an upward trend of practice acquisition and consolidation by private equity firms. Therefore, dermatology trainees are uniquely positioned to benefit from business education to make well-informed decisions about joining or starting a practice.Furthermore, whether in a private or academic setting, knowledge of foundational economics, business strategy, finance, marketing, and health care policy can equip dermatologists to more effectively advocate for local and national policies that benefit their patient population.7
We conducted a survey of dermatology program directors (PDs) to determine the availability of and perceptions regarding business education during residency training.
Materials and Methods
Institutional review board (Vanderbilt University Medical Center, Nashville, Tennessee) approval was obtained. The survey was distributed weekly during a 5-week period from July 2020 to August 2020 through the Research Electronic Data Capture survey application (www.project-redcap.org). Program director email addresses were obtained through the Accreditation Council for Graduate Medical Education (ACGME) program list. A PD was included in the survey if they were employed by an accredited US osteopathic or allopathic program and their email address was provided in the ACGME program list or on their program’s faculty web page; a PD was excluded if an email address was not provided in the ACGME program list or on their program’s faculty web page.
The 8-part questionnaire was designed to assess the following characteristics: details about the respondent’s residency program (institutional affiliation, number of residents), the respondent’s professional background (number of years as a PD, business training experience), resources for business education provided by the program, the respondent’s opinion about business education for residents, and the respondent’s perception of the most important topics to include in a dermatology curriculum’s business education component, which included economics/finance, health care policy/government, management, marketing, negotiation, private equity involvement in health care, business strategy, supply chain/operations, and technology/product development. Responses were kept anonymous. Categorical and continuous variables were analyzed with medians and proportions.
Results
Of the 139 surveys distributed, 35 were completed and returned (response rate, 25.2%). Most programs were university-affiliated (71.4%) or community-affiliated (22.9%). The median number of residents was 12. The respondents had a median of 5 years’ experience in their role. Most respondents (65.7%) had no business training, although 20.0% had completed undergraduate business coursework, and 8.6% had attended formal seminars on business topics; 5.7% were self-taught on business topics.
Business Education Availability
Approximately half (51.4%) of programs offered business training to residents, primarily through seminars or lectures (94.4%) and take-home modules (16.7%). None of the programs offered a formal gap year during which residents could pursue a professional business degree. Most respondents thought business education during residency was important (82.8%) and that programs should implement more training (57.1%). When asked whether residents were competent to handle business aspects of dermatology upon graduation, most respondents disagreed somewhat (22.9%) or were neutral (40.0%).
Topics for Business Education
The most important topics identified for inclusion in a business curriculum were economics or finance (68.6%), management (68.6%), and health care policy or government (57.1%). Other identified topics included negotiation (40.0%), private equity involvement in health care (40.0%), strategy (11.4%), supply chain or operations (11.4%), marketing (2.9%), and technology (2.9%).
Comment
Residency programs and medical schools in the United States have started to integrate formal business training into their curricula; however, the state of business training in dermatology has not been characterized. Overall, this survey revealed largely positive perceptions about business education and identified a demand for more resources.
Whereas most PDs identified business education as important, only one half (51.4%) of the representative programs offered structured training. Notably, most PDs did not agree that graduating residents were competent to handle the business demands of dermatology practice. These responses highlight a gap in the demand and resources available for business training.
Identifying Curricular Resources
During an already demanding residency, additional curricular components need to be beneficial and worthwhile. To avoid significant disruption, business training could take place in the form of online lectures or take-home modules. Most programs represented in the survey responses had an academic affiliation and therefore commonly have access to an affiliated graduate business school and/or hospital administrators who have clinical and business training.
Community dermatologists who own or run their own practice also are uniquely positioned to provide residents with practical, dermatology-specific business education. Programs can utilize their institutional and local colleagues to aid in curricular design and implementation. In addition, a potential long-term solution to obtaining resources for business education is to coordinate with a national dermatology organization to create standardized modules that are available to all residency programs.
Key Curriculum Topics
Our survey identified the most important topics to include in a business curriculum for dermatology residents. Economics and finance, management, and health care policy would be valuable to a trainee regardless of whether they ultimately choose a career in academia or private practice. A thorough understanding of complex health care policy reinforces knowledge about insurance and regional and national regulations, which could ultimately benefit patient care. As an example, the American Academy of Dermatology outlines several advocacy priorities such as Medicare reimbursement policies, access to dermatologic care through public and private insurance, medication access and pricing, and preservation of private practice in the setting of market consolidation. Having a better understanding of health care policy and business could better equip dermatologists to lead these often business-driven advocacy efforts to ultimately improve patient care and advance the specialty.8
Limitations
There were notable limitations to this survey, primarily related to its design. With a 25% response rate, there was the potential for response and selection biases; therefore, these results might not be generalizable to all programs. In addition, views held by PDs might not be consistent with those of other members of the dermatology community; for example, surveying residents, other faculty members, and dermatologists in private practice would have provided a more comprehensive characterization of the topic.
Conclusion
This study assessed residency program directors’ perceptions of business education in dermatology training. There appears to be an imbalance between the perceived importance of such education and the resources that are available to provide it. More attention is needed to address this gap to ensure that dermatologists are prepared to manage a rapidly changing health care environment. Results of this survey should encourage efforts to establish (1) a standardized, dermatology-specific business curriculum and (2) a plan to make that curriculum accessible to trainees and other members of the dermatology community.
Globally, the United States has the highest per-capita cost of health care; total costs are expected to account for approximately 20% of the nation’s gross domestic product by 2025.1 These rising costs have prompted residency programs and medical schools to incorporate business education into their curricula.2-5 Although medical training is demanding—with little room to add curricular components—these business-focused curricula have consistently received positive feedback from residents.5,6
In dermatology, more than 50% of residents opt to join a private practice upon graduation.7 In the United States, there also is an upward trend of practice acquisition and consolidation by private equity firms. Therefore, dermatology trainees are uniquely positioned to benefit from business education to make well-informed decisions about joining or starting a practice.Furthermore, whether in a private or academic setting, knowledge of foundational economics, business strategy, finance, marketing, and health care policy can equip dermatologists to more effectively advocate for local and national policies that benefit their patient population.7
We conducted a survey of dermatology program directors (PDs) to determine the availability of and perceptions regarding business education during residency training.
Materials and Methods
Institutional review board (Vanderbilt University Medical Center, Nashville, Tennessee) approval was obtained. The survey was distributed weekly during a 5-week period from July 2020 to August 2020 through the Research Electronic Data Capture survey application (www.project-redcap.org). Program director email addresses were obtained through the Accreditation Council for Graduate Medical Education (ACGME) program list. A PD was included in the survey if they were employed by an accredited US osteopathic or allopathic program and their email address was provided in the ACGME program list or on their program’s faculty web page; a PD was excluded if an email address was not provided in the ACGME program list or on their program’s faculty web page.
The 8-part questionnaire was designed to assess the following characteristics: details about the respondent’s residency program (institutional affiliation, number of residents), the respondent’s professional background (number of years as a PD, business training experience), resources for business education provided by the program, the respondent’s opinion about business education for residents, and the respondent’s perception of the most important topics to include in a dermatology curriculum’s business education component, which included economics/finance, health care policy/government, management, marketing, negotiation, private equity involvement in health care, business strategy, supply chain/operations, and technology/product development. Responses were kept anonymous. Categorical and continuous variables were analyzed with medians and proportions.
Results
Of the 139 surveys distributed, 35 were completed and returned (response rate, 25.2%). Most programs were university-affiliated (71.4%) or community-affiliated (22.9%). The median number of residents was 12. The respondents had a median of 5 years’ experience in their role. Most respondents (65.7%) had no business training, although 20.0% had completed undergraduate business coursework, and 8.6% had attended formal seminars on business topics; 5.7% were self-taught on business topics.
Business Education Availability
Approximately half (51.4%) of programs offered business training to residents, primarily through seminars or lectures (94.4%) and take-home modules (16.7%). None of the programs offered a formal gap year during which residents could pursue a professional business degree. Most respondents thought business education during residency was important (82.8%) and that programs should implement more training (57.1%). When asked whether residents were competent to handle business aspects of dermatology upon graduation, most respondents disagreed somewhat (22.9%) or were neutral (40.0%).
Topics for Business Education
The most important topics identified for inclusion in a business curriculum were economics or finance (68.6%), management (68.6%), and health care policy or government (57.1%). Other identified topics included negotiation (40.0%), private equity involvement in health care (40.0%), strategy (11.4%), supply chain or operations (11.4%), marketing (2.9%), and technology (2.9%).
Comment
Residency programs and medical schools in the United States have started to integrate formal business training into their curricula; however, the state of business training in dermatology has not been characterized. Overall, this survey revealed largely positive perceptions about business education and identified a demand for more resources.
Whereas most PDs identified business education as important, only one half (51.4%) of the representative programs offered structured training. Notably, most PDs did not agree that graduating residents were competent to handle the business demands of dermatology practice. These responses highlight a gap in the demand and resources available for business training.
Identifying Curricular Resources
During an already demanding residency, additional curricular components need to be beneficial and worthwhile. To avoid significant disruption, business training could take place in the form of online lectures or take-home modules. Most programs represented in the survey responses had an academic affiliation and therefore commonly have access to an affiliated graduate business school and/or hospital administrators who have clinical and business training.
Community dermatologists who own or run their own practice also are uniquely positioned to provide residents with practical, dermatology-specific business education. Programs can utilize their institutional and local colleagues to aid in curricular design and implementation. In addition, a potential long-term solution to obtaining resources for business education is to coordinate with a national dermatology organization to create standardized modules that are available to all residency programs.
Key Curriculum Topics
Our survey identified the most important topics to include in a business curriculum for dermatology residents. Economics and finance, management, and health care policy would be valuable to a trainee regardless of whether they ultimately choose a career in academia or private practice. A thorough understanding of complex health care policy reinforces knowledge about insurance and regional and national regulations, which could ultimately benefit patient care. As an example, the American Academy of Dermatology outlines several advocacy priorities such as Medicare reimbursement policies, access to dermatologic care through public and private insurance, medication access and pricing, and preservation of private practice in the setting of market consolidation. Having a better understanding of health care policy and business could better equip dermatologists to lead these often business-driven advocacy efforts to ultimately improve patient care and advance the specialty.8
Limitations
There were notable limitations to this survey, primarily related to its design. With a 25% response rate, there was the potential for response and selection biases; therefore, these results might not be generalizable to all programs. In addition, views held by PDs might not be consistent with those of other members of the dermatology community; for example, surveying residents, other faculty members, and dermatologists in private practice would have provided a more comprehensive characterization of the topic.
Conclusion
This study assessed residency program directors’ perceptions of business education in dermatology training. There appears to be an imbalance between the perceived importance of such education and the resources that are available to provide it. More attention is needed to address this gap to ensure that dermatologists are prepared to manage a rapidly changing health care environment. Results of this survey should encourage efforts to establish (1) a standardized, dermatology-specific business curriculum and (2) a plan to make that curriculum accessible to trainees and other members of the dermatology community.
- Branning G, Vater M. Healthcare spending: plenty of blame to go around. Am Health Drug Benefits. 2016;9:445-447.
- Bayard M, Peeples CR, Holt J, et al. An interactive approach to teaching practice management to family practice residents. Fam Med. 2003;35:622-624.
- Chan S. Management education during radiology residency: development of an educational practice. Acad Radiol. 2004;11:1308-1317.
- Ninan D, Patel D. Career and leadership education in anesthesia residency training. Cureus. 2018;10:e2546.
- Yu-Chin R. Teaching administration and management within psychiatric residency training. Acad Psychiatry. 2002;26:245-252.
- Winkelman JW, Brugnara C. Management training for pathology residents. II. experience with a focused curriculum. Am J Clin Pathol. 1994;101:564-568.
- Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021.
- Academy advocacy priorities. American Academy of Dermatology website. Accessed August 11, 2021. www.aad.org/member/advocacy/priorities
- Branning G, Vater M. Healthcare spending: plenty of blame to go around. Am Health Drug Benefits. 2016;9:445-447.
- Bayard M, Peeples CR, Holt J, et al. An interactive approach to teaching practice management to family practice residents. Fam Med. 2003;35:622-624.
- Chan S. Management education during radiology residency: development of an educational practice. Acad Radiol. 2004;11:1308-1317.
- Ninan D, Patel D. Career and leadership education in anesthesia residency training. Cureus. 2018;10:e2546.
- Yu-Chin R. Teaching administration and management within psychiatric residency training. Acad Psychiatry. 2002;26:245-252.
- Winkelman JW, Brugnara C. Management training for pathology residents. II. experience with a focused curriculum. Am J Clin Pathol. 1994;101:564-568.
- Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021.
- Academy advocacy priorities. American Academy of Dermatology website. Accessed August 11, 2021. www.aad.org/member/advocacy/priorities
Practice Points
- In our survey of dermatology program directors, most felt inclusion of business education in residency training was important.
- Approximately half of the dermatology programs that responded to our survey offer business training to their residents.
- Economics and finance, management, and health care policy were the most important topics identified to include in a business curriculum for dermatology residents
Secretary of Defense Seeks Approval To Make COVID Vaccines Mandatory For DoD Employees
New policy hopes to be in line with full FDA approval expected in September. When the largest employer in the world makes any significant decision, everyone sits up and takes notice.
That’s what happened when Secretary of Defense Lloyd Austin III sent out a memo to all US Department of Defense (DoD) employees saying he was seeking President Biden’s approval to make COVID-19 vaccines mandatory. His decision affects not only the 3.2 million employees on the payroll, but their families, communities, and states. Florida, for instance, where approximately 40% of the population remains unvaccinated has about 55,000 active duty service members and 36,000 reservists.
Vaccination rates in the military have lagged behind other populations, especially among Black and Hispanic service members. An April study published in Medical Surveillance Monthly Report found that “non-Hispanic Blacks, as well as those who were female, younger, of lower rank, with lower education levels, and those serving in the Army were less likely to initiate COVID-19 vaccination after adjusting for other factors.”
The decision had been in the offing for some time but when cases of the Delta variant of the virus began to spike in July, President Biden asked Sec. Austin to consider how and when the COVID vaccine could be added to the list of required vaccines for service members. It’s a long list already: Depending on their location, service members can get as many as 17 vaccines. It also folllows on the heals of the decision by the US Department of Veterans Affairs to require vaccinations for frontline health care workers.
Austin promised to “not let grass grow.” He consulted with Army Gen. Mark Milley, the Joint Chiefs of Staff, service chiefs, service secretaries, and medical professionals. Based on those discussions, he decided to ask for approval to make the vaccines mandatory no later than mid-September or immediately upon FDA licensure, whichever comes first.
However, he added, “[t]o defend this Nation, we need a healthy and ready force. I strongly encourage all DoD military and civilian personnel—as well as contractor personnel—to get vaccinated now and for military Service members to not wait for the mandate.” Currently, 73% of active-duty personnel have had at least one dose of the vaccine.
Leaping upon the news—and based on the wording in the memo—some in the media were reporting that it meant all troops have to be vaccinated by mid-September. “He’ll make the request by mid-September, unless or until FDA licensure occurs before that time, at which point the Secretary has the authority he needs…to make whatever vaccine is then given that license mandatory.” That’s not the case, said Pentagon press secretary John Kirby in a briefing. Some voices also have called on the DoD to do more to dispel vaccine hesitancy among the troops.
In the meantime, Kirby said, “[T]wo things are going to happen. One, the services are going to be tasked to come back to the Secretary with implementation plans for how they’re going to get this moving.” Noting that mid-September isn’t far away, he pointed out that the services have a “fair but limited amount of time” to arrange their implementation plans. “I have every confidence that service leadership and your commanders will implement this new vaccination program with professionalism, skill, and compassion,” Austin wrote in his memo.
The second thing, Kirby said, was that DoD would be developing policies that comply with the President’s direction that the unvaccinated will have to be subjected to “certain requirements and restrictions.” The Delta variant is hitting the unvaccinated hardest. Austin said the DoD will keep a close eye on infection rates “and the impact these rates might have on our readiness. I will not hesitate to act sooner or recommend a different course to the President if I feel the need to do so.”
Kirby said he didn’t have all the details for that yet, but the department is “working hard” on a policy directive that will clarify what those requirements and restrictions might be.
President Biden replied almost immediately to Austin’s message. “I strongly support Secretary Austin’s message to the force today…. Secretary Austin and I share an unshakeable commitment to making sure our troops have every tool they need to do their jobs as safely as possible. These vaccines will save lives. Period.”
“All FDA-authorized COVID-19 vaccines are safe and highly effective,” Austin said in the close to his memo. “They will protect you and your family. They will protect your unit, your ship, and your co-workers. …Get the shot. Stay healthy. Stay ready.”
New policy hopes to be in line with full FDA approval expected in September. When the largest employer in the world makes any significant decision, everyone sits up and takes notice.
That’s what happened when Secretary of Defense Lloyd Austin III sent out a memo to all US Department of Defense (DoD) employees saying he was seeking President Biden’s approval to make COVID-19 vaccines mandatory. His decision affects not only the 3.2 million employees on the payroll, but their families, communities, and states. Florida, for instance, where approximately 40% of the population remains unvaccinated has about 55,000 active duty service members and 36,000 reservists.
Vaccination rates in the military have lagged behind other populations, especially among Black and Hispanic service members. An April study published in Medical Surveillance Monthly Report found that “non-Hispanic Blacks, as well as those who were female, younger, of lower rank, with lower education levels, and those serving in the Army were less likely to initiate COVID-19 vaccination after adjusting for other factors.”
The decision had been in the offing for some time but when cases of the Delta variant of the virus began to spike in July, President Biden asked Sec. Austin to consider how and when the COVID vaccine could be added to the list of required vaccines for service members. It’s a long list already: Depending on their location, service members can get as many as 17 vaccines. It also folllows on the heals of the decision by the US Department of Veterans Affairs to require vaccinations for frontline health care workers.
Austin promised to “not let grass grow.” He consulted with Army Gen. Mark Milley, the Joint Chiefs of Staff, service chiefs, service secretaries, and medical professionals. Based on those discussions, he decided to ask for approval to make the vaccines mandatory no later than mid-September or immediately upon FDA licensure, whichever comes first.
However, he added, “[t]o defend this Nation, we need a healthy and ready force. I strongly encourage all DoD military and civilian personnel—as well as contractor personnel—to get vaccinated now and for military Service members to not wait for the mandate.” Currently, 73% of active-duty personnel have had at least one dose of the vaccine.
Leaping upon the news—and based on the wording in the memo—some in the media were reporting that it meant all troops have to be vaccinated by mid-September. “He’ll make the request by mid-September, unless or until FDA licensure occurs before that time, at which point the Secretary has the authority he needs…to make whatever vaccine is then given that license mandatory.” That’s not the case, said Pentagon press secretary John Kirby in a briefing. Some voices also have called on the DoD to do more to dispel vaccine hesitancy among the troops.
In the meantime, Kirby said, “[T]wo things are going to happen. One, the services are going to be tasked to come back to the Secretary with implementation plans for how they’re going to get this moving.” Noting that mid-September isn’t far away, he pointed out that the services have a “fair but limited amount of time” to arrange their implementation plans. “I have every confidence that service leadership and your commanders will implement this new vaccination program with professionalism, skill, and compassion,” Austin wrote in his memo.
The second thing, Kirby said, was that DoD would be developing policies that comply with the President’s direction that the unvaccinated will have to be subjected to “certain requirements and restrictions.” The Delta variant is hitting the unvaccinated hardest. Austin said the DoD will keep a close eye on infection rates “and the impact these rates might have on our readiness. I will not hesitate to act sooner or recommend a different course to the President if I feel the need to do so.”
Kirby said he didn’t have all the details for that yet, but the department is “working hard” on a policy directive that will clarify what those requirements and restrictions might be.
President Biden replied almost immediately to Austin’s message. “I strongly support Secretary Austin’s message to the force today…. Secretary Austin and I share an unshakeable commitment to making sure our troops have every tool they need to do their jobs as safely as possible. These vaccines will save lives. Period.”
“All FDA-authorized COVID-19 vaccines are safe and highly effective,” Austin said in the close to his memo. “They will protect you and your family. They will protect your unit, your ship, and your co-workers. …Get the shot. Stay healthy. Stay ready.”
New policy hopes to be in line with full FDA approval expected in September. When the largest employer in the world makes any significant decision, everyone sits up and takes notice.
That’s what happened when Secretary of Defense Lloyd Austin III sent out a memo to all US Department of Defense (DoD) employees saying he was seeking President Biden’s approval to make COVID-19 vaccines mandatory. His decision affects not only the 3.2 million employees on the payroll, but their families, communities, and states. Florida, for instance, where approximately 40% of the population remains unvaccinated has about 55,000 active duty service members and 36,000 reservists.
Vaccination rates in the military have lagged behind other populations, especially among Black and Hispanic service members. An April study published in Medical Surveillance Monthly Report found that “non-Hispanic Blacks, as well as those who were female, younger, of lower rank, with lower education levels, and those serving in the Army were less likely to initiate COVID-19 vaccination after adjusting for other factors.”
The decision had been in the offing for some time but when cases of the Delta variant of the virus began to spike in July, President Biden asked Sec. Austin to consider how and when the COVID vaccine could be added to the list of required vaccines for service members. It’s a long list already: Depending on their location, service members can get as many as 17 vaccines. It also folllows on the heals of the decision by the US Department of Veterans Affairs to require vaccinations for frontline health care workers.
Austin promised to “not let grass grow.” He consulted with Army Gen. Mark Milley, the Joint Chiefs of Staff, service chiefs, service secretaries, and medical professionals. Based on those discussions, he decided to ask for approval to make the vaccines mandatory no later than mid-September or immediately upon FDA licensure, whichever comes first.
However, he added, “[t]o defend this Nation, we need a healthy and ready force. I strongly encourage all DoD military and civilian personnel—as well as contractor personnel—to get vaccinated now and for military Service members to not wait for the mandate.” Currently, 73% of active-duty personnel have had at least one dose of the vaccine.
Leaping upon the news—and based on the wording in the memo—some in the media were reporting that it meant all troops have to be vaccinated by mid-September. “He’ll make the request by mid-September, unless or until FDA licensure occurs before that time, at which point the Secretary has the authority he needs…to make whatever vaccine is then given that license mandatory.” That’s not the case, said Pentagon press secretary John Kirby in a briefing. Some voices also have called on the DoD to do more to dispel vaccine hesitancy among the troops.
In the meantime, Kirby said, “[T]wo things are going to happen. One, the services are going to be tasked to come back to the Secretary with implementation plans for how they’re going to get this moving.” Noting that mid-September isn’t far away, he pointed out that the services have a “fair but limited amount of time” to arrange their implementation plans. “I have every confidence that service leadership and your commanders will implement this new vaccination program with professionalism, skill, and compassion,” Austin wrote in his memo.
The second thing, Kirby said, was that DoD would be developing policies that comply with the President’s direction that the unvaccinated will have to be subjected to “certain requirements and restrictions.” The Delta variant is hitting the unvaccinated hardest. Austin said the DoD will keep a close eye on infection rates “and the impact these rates might have on our readiness. I will not hesitate to act sooner or recommend a different course to the President if I feel the need to do so.”
Kirby said he didn’t have all the details for that yet, but the department is “working hard” on a policy directive that will clarify what those requirements and restrictions might be.
President Biden replied almost immediately to Austin’s message. “I strongly support Secretary Austin’s message to the force today…. Secretary Austin and I share an unshakeable commitment to making sure our troops have every tool they need to do their jobs as safely as possible. These vaccines will save lives. Period.”
“All FDA-authorized COVID-19 vaccines are safe and highly effective,” Austin said in the close to his memo. “They will protect you and your family. They will protect your unit, your ship, and your co-workers. …Get the shot. Stay healthy. Stay ready.”