User login
Health sector has spent $464 million on lobbying in 2020
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
CDC panel takes on COVID vaccine rollout, risks, and side effects
Federal advisers who will help determine which Americans get the first COVID vaccines took an in-depth look Oct. 30 at the challenges they face in selecting priority groups.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) will face two key decisions once a COVID vaccine wins clearance from the US Food and Drug Administration (FDA).
ACIP will need to decide whether to recommend its use in adults (the age group in which vaccines are currently being tested). The group will also need to offer direction on which groups should get priority in vaccine allocation, inasmuch as early supplies will not be sufficient to vaccinate everyone.
At the Oct. 30 meeting, CDC’s Kathleen Dooling, MD, MPH, suggested that ACIP plan on tackling these issues as two separate questions when it comes time to weigh in on an approved vaccine. Although there was no formal vote among ACIP members at the meeting, Dooling’s proposal for tackling a future recommendation in a two-part fashion drew positive feedback.
ACIP member Katherine A. Poehling, MD, MPH, suggested that the panel and CDC be ready to reexamine the situation frequently regarding COVID vaccination. “Perhaps we could think about reviewing data on a monthly basis and updating the recommendation, so that we can account for the concerns and balance both the benefits and the [potential] harm,” Poehling said.
Dooling agreed. “Both the vaccine recommendation and allocation will be revisited in what is a very dynamic situation,” Dooling replied to Poehling. “So all new evidence will be brought to ACIP, and certainly the allocation as vaccine distribution proceeds will need to be adjusted accordingly.”
Ethics and limited evidence
During the meeting, ACIP members repeatedly expressed discomfort with the prospect of having to weigh in on widespread use of COVID vaccines on the basis of limited evidence.
Within months, FDA may opt for a special clearance, known as an emergency use authorization (EUA), for one or more of the experimental COVID vaccines now in advanced testing. Many of FDA’s past EUA clearances were granted for test kits. For those EUA approvals, the agency considered risks of false results but not longer-term, direct harm to patients from these products.
With a COVID vaccine, there will be strong pressure to distribute doses as quickly as possible with the hope of curbing the pandemic, which has already led to more than 229,000 deaths in the United States alone and has disrupted lives and economies around the world. But questions will persist about the possibility of serious complications from these vaccines, ACIP members noted.
“My personal struggle is the ethical side and how to balance these two,” said ACIP member Robert L. Atmar, MD, of Baylor College of Medicine, Houston, Texas, who noted that he expects his fellow panelists to share this concern.
Currently, four experimental COVID vaccines likely to be used in the United States have advanced to phase 3 testing. Pfizer Inc and BioNtech have enrolled more than 42,000 participants in a test of their candidate, BNT162b2 vaccine, and rival Moderna has enrolled about 30,000 participants in a test of its mRNA-1273 vaccine, CDC staff said.
The other two advanced COVID vaccine candidates have overcome recent hurdles. AstraZeneca Plc on Oct. 23 announced that FDA had removed a hold on the testing of its AZD1222 vaccine candidate; the trial will enroll approximately 30,000 people. Johnson & Johnson’s Janssen unit also announced that day the lifting of a safety pause for its Ad26.COV2.S vaccine; the phase 3 trial for that vaccine will enroll approximately 60,000 volunteers. Federal agencies, states, and territories have developed plans for future distribution of COVID vaccines, CDC staff said in briefing materials for today’s ACIP meeting.
Several ACIP members raised many of the same concerns that members of an FDA advisory committee raised at a meeting earlier in October. ACIP and FDA advisers honed in on the FDA’s decision to set a median follow-up duration of 2 months in phase 3 trials in connection with expected EUA applications for COVID-19 vaccines.
“I struggle with following people for 2 months after their second vaccination as a time point to start making final decisions about safety,” said ACIP member Sharon E. Frey, MD, a professor at St. Louis University School of Medicine, St. Louis, Missouri. “I just want to put that out there.”
Medical front line, then who?
There is consensus that healthcare workers be in the first stage ― Phase 1 ― of distribution. That recommendation was made in a report from the National Academies of Sciences, Engineering, and Medicine (NASEM). Phase 1A would include first responders; Phase 1B might include people of all ages who have two or more comorbidities that put them at significantly higher risk for COVID-19 or death, as well as older adults living in congregate or overcrowded settings, the NASEM report said.
A presentation from the CDC’s Matthew Biggerstaff, ScD, MPH, underscored challenges in distributing what are expected to be limited initial supplies of COVID vaccines.
Biggerstaff showed several scenarios the CDC’s Data, Analytics, and Modeling Task Force had studied. The initial allocation of vaccines would be for healthcare workers, followed by what the CDC called Phase 1B.
Choices for a rollout may include next giving COVID vaccines to people at high risk, such as persons who have one or more chronic medical conditions, including heart disease, diabetes, kidney disease, or obesity. Other options for the rollout could be to vaccinate people aged 65 years and older or essential workers whose employment puts them in contact with the public, thus raising the risk of contracting the virus.
The CDC’s research found that the greatest impact in preventing death was to initially vaccinate adults aged 65 and older in Phase 1B. The agency staff described this approach as likely to result in an about “1 to 11% increase in averted deaths across the scenarios.”
Initially vaccinating essential workers or high-risk adults in Phase 1B would avert the most infections. The agency staff described this approach as yielding about “1 to 5% increase in averted infections across the scenarios,” Biggerstaff said during his presentation.
The following are other findings of the CDC staff:
The earlier the vaccine rollout relative to increasing transmission, the greater the averted percentage and differences between the strategies.
Differences were not substantial in some scenarios.
The need to continue efforts to slow the spread of COVID-19 should be emphasized.
Adverse effects
ACIP members also heard about strategies for tracking potential side effects of future vaccines. A presentation by Tom Shimabukuro, MD, MPH, MBA, from the CDC’s COVID-19 Vaccine Task Force/Vaccine Safety Team, included details about a new smartphone-based active surveillance program for COVID-19 vaccine safety.
Known as v-safe, this system would use Web-based survey monitoring and incorporate text messaging. It would conduct electronic health checks on vaccine recipients, which would occur daily during the first week post vaccination and weekly thereafter for 6 weeks from the time of vaccination.
Clinicians “can play an important role in helping CDC enroll patients in v-safe at the time of vaccination,” Shimabukuro noted in his presentation. This would add another task, though, for clinicians, the CDC staff noted.
Pregnancy and breastfeeding are special concerns
Of special concern with the rollout of a COVID vaccine are recommendations regarding pregnancy and breastfeeding. Women constitute about 75% of the healthcare workforce, CDC staff noted.
At the time the initial ACIP COVID vaccination recommendations are made, there could be approximately 330,000 healthcare personnel who are pregnant or who have recently given birth. Available data indicate potentially increased risks for severe maternal illness and preterm birth associated with SARS-CoV-2 infection, said CDC’s Megan Wallace, DrPH, MPH, in a presentation for the Friday meeting.
In an Oct. 27 letter to ACIP, Chair Jose Romero, the American College of Obstetricians and Gynecologists (ACOG), urged the panel to ensure that pregnant women and new mothers in the healthcare workforce have priority access to a COVID vaccine. Pregnant and lactating women were “noticeably and alarmingly absent from the NASEM vaccine allocation plan for COVID-19,” wrote Christopher M. Zahn, MD, vice president for practice activities at ACOG, in the letter to Romero.
“ACOG urges ACIP to incorporate pregnant and lactating women clearly and explicitly into its COVID-19 vaccine allocation and prioritization framework,” Zahn wrote. “Should an Emergency Use Authorization be executed for one or more COVID-19 vaccines and provide a permissive recommendation for pregnant and lactating women, pregnant health care workers, pregnant first responders, and pregnant individuals with underlying conditions should be prioritized for vaccination alongside their non-pregnant peers.”
This article first appeared on Medscape.com.
Federal advisers who will help determine which Americans get the first COVID vaccines took an in-depth look Oct. 30 at the challenges they face in selecting priority groups.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) will face two key decisions once a COVID vaccine wins clearance from the US Food and Drug Administration (FDA).
ACIP will need to decide whether to recommend its use in adults (the age group in which vaccines are currently being tested). The group will also need to offer direction on which groups should get priority in vaccine allocation, inasmuch as early supplies will not be sufficient to vaccinate everyone.
At the Oct. 30 meeting, CDC’s Kathleen Dooling, MD, MPH, suggested that ACIP plan on tackling these issues as two separate questions when it comes time to weigh in on an approved vaccine. Although there was no formal vote among ACIP members at the meeting, Dooling’s proposal for tackling a future recommendation in a two-part fashion drew positive feedback.
ACIP member Katherine A. Poehling, MD, MPH, suggested that the panel and CDC be ready to reexamine the situation frequently regarding COVID vaccination. “Perhaps we could think about reviewing data on a monthly basis and updating the recommendation, so that we can account for the concerns and balance both the benefits and the [potential] harm,” Poehling said.
Dooling agreed. “Both the vaccine recommendation and allocation will be revisited in what is a very dynamic situation,” Dooling replied to Poehling. “So all new evidence will be brought to ACIP, and certainly the allocation as vaccine distribution proceeds will need to be adjusted accordingly.”
Ethics and limited evidence
During the meeting, ACIP members repeatedly expressed discomfort with the prospect of having to weigh in on widespread use of COVID vaccines on the basis of limited evidence.
Within months, FDA may opt for a special clearance, known as an emergency use authorization (EUA), for one or more of the experimental COVID vaccines now in advanced testing. Many of FDA’s past EUA clearances were granted for test kits. For those EUA approvals, the agency considered risks of false results but not longer-term, direct harm to patients from these products.
With a COVID vaccine, there will be strong pressure to distribute doses as quickly as possible with the hope of curbing the pandemic, which has already led to more than 229,000 deaths in the United States alone and has disrupted lives and economies around the world. But questions will persist about the possibility of serious complications from these vaccines, ACIP members noted.
“My personal struggle is the ethical side and how to balance these two,” said ACIP member Robert L. Atmar, MD, of Baylor College of Medicine, Houston, Texas, who noted that he expects his fellow panelists to share this concern.
Currently, four experimental COVID vaccines likely to be used in the United States have advanced to phase 3 testing. Pfizer Inc and BioNtech have enrolled more than 42,000 participants in a test of their candidate, BNT162b2 vaccine, and rival Moderna has enrolled about 30,000 participants in a test of its mRNA-1273 vaccine, CDC staff said.
The other two advanced COVID vaccine candidates have overcome recent hurdles. AstraZeneca Plc on Oct. 23 announced that FDA had removed a hold on the testing of its AZD1222 vaccine candidate; the trial will enroll approximately 30,000 people. Johnson & Johnson’s Janssen unit also announced that day the lifting of a safety pause for its Ad26.COV2.S vaccine; the phase 3 trial for that vaccine will enroll approximately 60,000 volunteers. Federal agencies, states, and territories have developed plans for future distribution of COVID vaccines, CDC staff said in briefing materials for today’s ACIP meeting.
Several ACIP members raised many of the same concerns that members of an FDA advisory committee raised at a meeting earlier in October. ACIP and FDA advisers honed in on the FDA’s decision to set a median follow-up duration of 2 months in phase 3 trials in connection with expected EUA applications for COVID-19 vaccines.
“I struggle with following people for 2 months after their second vaccination as a time point to start making final decisions about safety,” said ACIP member Sharon E. Frey, MD, a professor at St. Louis University School of Medicine, St. Louis, Missouri. “I just want to put that out there.”
Medical front line, then who?
There is consensus that healthcare workers be in the first stage ― Phase 1 ― of distribution. That recommendation was made in a report from the National Academies of Sciences, Engineering, and Medicine (NASEM). Phase 1A would include first responders; Phase 1B might include people of all ages who have two or more comorbidities that put them at significantly higher risk for COVID-19 or death, as well as older adults living in congregate or overcrowded settings, the NASEM report said.
A presentation from the CDC’s Matthew Biggerstaff, ScD, MPH, underscored challenges in distributing what are expected to be limited initial supplies of COVID vaccines.
Biggerstaff showed several scenarios the CDC’s Data, Analytics, and Modeling Task Force had studied. The initial allocation of vaccines would be for healthcare workers, followed by what the CDC called Phase 1B.
Choices for a rollout may include next giving COVID vaccines to people at high risk, such as persons who have one or more chronic medical conditions, including heart disease, diabetes, kidney disease, or obesity. Other options for the rollout could be to vaccinate people aged 65 years and older or essential workers whose employment puts them in contact with the public, thus raising the risk of contracting the virus.
The CDC’s research found that the greatest impact in preventing death was to initially vaccinate adults aged 65 and older in Phase 1B. The agency staff described this approach as likely to result in an about “1 to 11% increase in averted deaths across the scenarios.”
Initially vaccinating essential workers or high-risk adults in Phase 1B would avert the most infections. The agency staff described this approach as yielding about “1 to 5% increase in averted infections across the scenarios,” Biggerstaff said during his presentation.
The following are other findings of the CDC staff:
The earlier the vaccine rollout relative to increasing transmission, the greater the averted percentage and differences between the strategies.
Differences were not substantial in some scenarios.
The need to continue efforts to slow the spread of COVID-19 should be emphasized.
Adverse effects
ACIP members also heard about strategies for tracking potential side effects of future vaccines. A presentation by Tom Shimabukuro, MD, MPH, MBA, from the CDC’s COVID-19 Vaccine Task Force/Vaccine Safety Team, included details about a new smartphone-based active surveillance program for COVID-19 vaccine safety.
Known as v-safe, this system would use Web-based survey monitoring and incorporate text messaging. It would conduct electronic health checks on vaccine recipients, which would occur daily during the first week post vaccination and weekly thereafter for 6 weeks from the time of vaccination.
Clinicians “can play an important role in helping CDC enroll patients in v-safe at the time of vaccination,” Shimabukuro noted in his presentation. This would add another task, though, for clinicians, the CDC staff noted.
Pregnancy and breastfeeding are special concerns
Of special concern with the rollout of a COVID vaccine are recommendations regarding pregnancy and breastfeeding. Women constitute about 75% of the healthcare workforce, CDC staff noted.
At the time the initial ACIP COVID vaccination recommendations are made, there could be approximately 330,000 healthcare personnel who are pregnant or who have recently given birth. Available data indicate potentially increased risks for severe maternal illness and preterm birth associated with SARS-CoV-2 infection, said CDC’s Megan Wallace, DrPH, MPH, in a presentation for the Friday meeting.
In an Oct. 27 letter to ACIP, Chair Jose Romero, the American College of Obstetricians and Gynecologists (ACOG), urged the panel to ensure that pregnant women and new mothers in the healthcare workforce have priority access to a COVID vaccine. Pregnant and lactating women were “noticeably and alarmingly absent from the NASEM vaccine allocation plan for COVID-19,” wrote Christopher M. Zahn, MD, vice president for practice activities at ACOG, in the letter to Romero.
“ACOG urges ACIP to incorporate pregnant and lactating women clearly and explicitly into its COVID-19 vaccine allocation and prioritization framework,” Zahn wrote. “Should an Emergency Use Authorization be executed for one or more COVID-19 vaccines and provide a permissive recommendation for pregnant and lactating women, pregnant health care workers, pregnant first responders, and pregnant individuals with underlying conditions should be prioritized for vaccination alongside their non-pregnant peers.”
This article first appeared on Medscape.com.
Federal advisers who will help determine which Americans get the first COVID vaccines took an in-depth look Oct. 30 at the challenges they face in selecting priority groups.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) will face two key decisions once a COVID vaccine wins clearance from the US Food and Drug Administration (FDA).
ACIP will need to decide whether to recommend its use in adults (the age group in which vaccines are currently being tested). The group will also need to offer direction on which groups should get priority in vaccine allocation, inasmuch as early supplies will not be sufficient to vaccinate everyone.
At the Oct. 30 meeting, CDC’s Kathleen Dooling, MD, MPH, suggested that ACIP plan on tackling these issues as two separate questions when it comes time to weigh in on an approved vaccine. Although there was no formal vote among ACIP members at the meeting, Dooling’s proposal for tackling a future recommendation in a two-part fashion drew positive feedback.
ACIP member Katherine A. Poehling, MD, MPH, suggested that the panel and CDC be ready to reexamine the situation frequently regarding COVID vaccination. “Perhaps we could think about reviewing data on a monthly basis and updating the recommendation, so that we can account for the concerns and balance both the benefits and the [potential] harm,” Poehling said.
Dooling agreed. “Both the vaccine recommendation and allocation will be revisited in what is a very dynamic situation,” Dooling replied to Poehling. “So all new evidence will be brought to ACIP, and certainly the allocation as vaccine distribution proceeds will need to be adjusted accordingly.”
Ethics and limited evidence
During the meeting, ACIP members repeatedly expressed discomfort with the prospect of having to weigh in on widespread use of COVID vaccines on the basis of limited evidence.
Within months, FDA may opt for a special clearance, known as an emergency use authorization (EUA), for one or more of the experimental COVID vaccines now in advanced testing. Many of FDA’s past EUA clearances were granted for test kits. For those EUA approvals, the agency considered risks of false results but not longer-term, direct harm to patients from these products.
With a COVID vaccine, there will be strong pressure to distribute doses as quickly as possible with the hope of curbing the pandemic, which has already led to more than 229,000 deaths in the United States alone and has disrupted lives and economies around the world. But questions will persist about the possibility of serious complications from these vaccines, ACIP members noted.
“My personal struggle is the ethical side and how to balance these two,” said ACIP member Robert L. Atmar, MD, of Baylor College of Medicine, Houston, Texas, who noted that he expects his fellow panelists to share this concern.
Currently, four experimental COVID vaccines likely to be used in the United States have advanced to phase 3 testing. Pfizer Inc and BioNtech have enrolled more than 42,000 participants in a test of their candidate, BNT162b2 vaccine, and rival Moderna has enrolled about 30,000 participants in a test of its mRNA-1273 vaccine, CDC staff said.
The other two advanced COVID vaccine candidates have overcome recent hurdles. AstraZeneca Plc on Oct. 23 announced that FDA had removed a hold on the testing of its AZD1222 vaccine candidate; the trial will enroll approximately 30,000 people. Johnson & Johnson’s Janssen unit also announced that day the lifting of a safety pause for its Ad26.COV2.S vaccine; the phase 3 trial for that vaccine will enroll approximately 60,000 volunteers. Federal agencies, states, and territories have developed plans for future distribution of COVID vaccines, CDC staff said in briefing materials for today’s ACIP meeting.
Several ACIP members raised many of the same concerns that members of an FDA advisory committee raised at a meeting earlier in October. ACIP and FDA advisers honed in on the FDA’s decision to set a median follow-up duration of 2 months in phase 3 trials in connection with expected EUA applications for COVID-19 vaccines.
“I struggle with following people for 2 months after their second vaccination as a time point to start making final decisions about safety,” said ACIP member Sharon E. Frey, MD, a professor at St. Louis University School of Medicine, St. Louis, Missouri. “I just want to put that out there.”
Medical front line, then who?
There is consensus that healthcare workers be in the first stage ― Phase 1 ― of distribution. That recommendation was made in a report from the National Academies of Sciences, Engineering, and Medicine (NASEM). Phase 1A would include first responders; Phase 1B might include people of all ages who have two or more comorbidities that put them at significantly higher risk for COVID-19 or death, as well as older adults living in congregate or overcrowded settings, the NASEM report said.
A presentation from the CDC’s Matthew Biggerstaff, ScD, MPH, underscored challenges in distributing what are expected to be limited initial supplies of COVID vaccines.
Biggerstaff showed several scenarios the CDC’s Data, Analytics, and Modeling Task Force had studied. The initial allocation of vaccines would be for healthcare workers, followed by what the CDC called Phase 1B.
Choices for a rollout may include next giving COVID vaccines to people at high risk, such as persons who have one or more chronic medical conditions, including heart disease, diabetes, kidney disease, or obesity. Other options for the rollout could be to vaccinate people aged 65 years and older or essential workers whose employment puts them in contact with the public, thus raising the risk of contracting the virus.
The CDC’s research found that the greatest impact in preventing death was to initially vaccinate adults aged 65 and older in Phase 1B. The agency staff described this approach as likely to result in an about “1 to 11% increase in averted deaths across the scenarios.”
Initially vaccinating essential workers or high-risk adults in Phase 1B would avert the most infections. The agency staff described this approach as yielding about “1 to 5% increase in averted infections across the scenarios,” Biggerstaff said during his presentation.
The following are other findings of the CDC staff:
The earlier the vaccine rollout relative to increasing transmission, the greater the averted percentage and differences between the strategies.
Differences were not substantial in some scenarios.
The need to continue efforts to slow the spread of COVID-19 should be emphasized.
Adverse effects
ACIP members also heard about strategies for tracking potential side effects of future vaccines. A presentation by Tom Shimabukuro, MD, MPH, MBA, from the CDC’s COVID-19 Vaccine Task Force/Vaccine Safety Team, included details about a new smartphone-based active surveillance program for COVID-19 vaccine safety.
Known as v-safe, this system would use Web-based survey monitoring and incorporate text messaging. It would conduct electronic health checks on vaccine recipients, which would occur daily during the first week post vaccination and weekly thereafter for 6 weeks from the time of vaccination.
Clinicians “can play an important role in helping CDC enroll patients in v-safe at the time of vaccination,” Shimabukuro noted in his presentation. This would add another task, though, for clinicians, the CDC staff noted.
Pregnancy and breastfeeding are special concerns
Of special concern with the rollout of a COVID vaccine are recommendations regarding pregnancy and breastfeeding. Women constitute about 75% of the healthcare workforce, CDC staff noted.
At the time the initial ACIP COVID vaccination recommendations are made, there could be approximately 330,000 healthcare personnel who are pregnant or who have recently given birth. Available data indicate potentially increased risks for severe maternal illness and preterm birth associated with SARS-CoV-2 infection, said CDC’s Megan Wallace, DrPH, MPH, in a presentation for the Friday meeting.
In an Oct. 27 letter to ACIP, Chair Jose Romero, the American College of Obstetricians and Gynecologists (ACOG), urged the panel to ensure that pregnant women and new mothers in the healthcare workforce have priority access to a COVID vaccine. Pregnant and lactating women were “noticeably and alarmingly absent from the NASEM vaccine allocation plan for COVID-19,” wrote Christopher M. Zahn, MD, vice president for practice activities at ACOG, in the letter to Romero.
“ACOG urges ACIP to incorporate pregnant and lactating women clearly and explicitly into its COVID-19 vaccine allocation and prioritization framework,” Zahn wrote. “Should an Emergency Use Authorization be executed for one or more COVID-19 vaccines and provide a permissive recommendation for pregnant and lactating women, pregnant health care workers, pregnant first responders, and pregnant individuals with underlying conditions should be prioritized for vaccination alongside their non-pregnant peers.”
This article first appeared on Medscape.com.
HHS extends deadline for patient access to your clinical notes
The Department of Health & Human Services on Oct. 29 extended the deadline for health care groups to provide patients with immediate electronic access to their doctors’ clinical notes as well as test results and reports from pathology and imaging.
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, and will now go into effect April 5.
The announcement comes just 4 days before the previously established Nov. 2 deadline and gives the pandemic as the reason for the delay.
“We are hearing that, while there is strong support for advancing patient access … stakeholders also must manage the needs being experienced during the current pandemic,” Don Rucker, MD, national coordinator for health information technology at HHS, said in a press statement.
“To be clear, the Office of the National Coordinator is not removing the requirements advancing patient access to their health information,” he added.
‘What you make of it’
Scott MacDonald, MD, electronic health record medical director at the University of California, Davis, said his organization is proceeding anyway. “UC Davis is going to start releasing notes and test results on Nov. 12,” he said in an interview.
Other organizations and practices now have more time, he said, but the law stays the same. “There’s no change to the what or why – only to the when,” Dr. MacDonald pointed out.
Vanderbilt University Medical Center in Nashville, Tenn., will take advantage of the extra time, Trent Rosenbloom, MD, MPH, director of patient portals, said in an interview.
“Given the super-short time frame we had to work under as this emerged out from dealing with COVID, we feel that we have not addressed all the potential legal-edge cases such as dealing with adolescent medicine and child abuse,” he said.
On Oct. 21, this news organization reported on the then-imminent start of the new law, which irked many readers. They cited, among other things, the likelihood of patient confusion with fast patient access to all clinical notes.
“To me, the biggest issue is that we speak a foreign language that most outside of medicine don’t speak. Our job is to explain it to the patient at a level they can understand. What will 100% happen now is that a patient will not be able to reconcile what is in the note to what they’ve been told,” Andrew White, MD, wrote in a reader comment.
But benefits of open notes outweigh the risks, say proponents, who claim that doctor-patient communication and trust actually improve with information access and that research indicates other benefits such as improved medication adherence.
Open notes are “what you make of it,” said Marlene Millen, MD, an internist at UC San Diego Health, which has had a pilot open-notes program for 3 years.
“I actually end all of my appointments with: ‘Don’t forget to read your note later,’ ” she said in an interview.
Dr. Millen feared open notes initially but, within the first 3 months of usage, about 15 patients gave her direct feedback on how much they appreciated her notes. “It seemed to really reassure them that they were getting good care.”
Dr. MacDonald and Dr. Millen disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Department of Health & Human Services on Oct. 29 extended the deadline for health care groups to provide patients with immediate electronic access to their doctors’ clinical notes as well as test results and reports from pathology and imaging.
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, and will now go into effect April 5.
The announcement comes just 4 days before the previously established Nov. 2 deadline and gives the pandemic as the reason for the delay.
“We are hearing that, while there is strong support for advancing patient access … stakeholders also must manage the needs being experienced during the current pandemic,” Don Rucker, MD, national coordinator for health information technology at HHS, said in a press statement.
“To be clear, the Office of the National Coordinator is not removing the requirements advancing patient access to their health information,” he added.
‘What you make of it’
Scott MacDonald, MD, electronic health record medical director at the University of California, Davis, said his organization is proceeding anyway. “UC Davis is going to start releasing notes and test results on Nov. 12,” he said in an interview.
Other organizations and practices now have more time, he said, but the law stays the same. “There’s no change to the what or why – only to the when,” Dr. MacDonald pointed out.
Vanderbilt University Medical Center in Nashville, Tenn., will take advantage of the extra time, Trent Rosenbloom, MD, MPH, director of patient portals, said in an interview.
“Given the super-short time frame we had to work under as this emerged out from dealing with COVID, we feel that we have not addressed all the potential legal-edge cases such as dealing with adolescent medicine and child abuse,” he said.
On Oct. 21, this news organization reported on the then-imminent start of the new law, which irked many readers. They cited, among other things, the likelihood of patient confusion with fast patient access to all clinical notes.
“To me, the biggest issue is that we speak a foreign language that most outside of medicine don’t speak. Our job is to explain it to the patient at a level they can understand. What will 100% happen now is that a patient will not be able to reconcile what is in the note to what they’ve been told,” Andrew White, MD, wrote in a reader comment.
But benefits of open notes outweigh the risks, say proponents, who claim that doctor-patient communication and trust actually improve with information access and that research indicates other benefits such as improved medication adherence.
Open notes are “what you make of it,” said Marlene Millen, MD, an internist at UC San Diego Health, which has had a pilot open-notes program for 3 years.
“I actually end all of my appointments with: ‘Don’t forget to read your note later,’ ” she said in an interview.
Dr. Millen feared open notes initially but, within the first 3 months of usage, about 15 patients gave her direct feedback on how much they appreciated her notes. “It seemed to really reassure them that they were getting good care.”
Dr. MacDonald and Dr. Millen disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Department of Health & Human Services on Oct. 29 extended the deadline for health care groups to provide patients with immediate electronic access to their doctors’ clinical notes as well as test results and reports from pathology and imaging.
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, and will now go into effect April 5.
The announcement comes just 4 days before the previously established Nov. 2 deadline and gives the pandemic as the reason for the delay.
“We are hearing that, while there is strong support for advancing patient access … stakeholders also must manage the needs being experienced during the current pandemic,” Don Rucker, MD, national coordinator for health information technology at HHS, said in a press statement.
“To be clear, the Office of the National Coordinator is not removing the requirements advancing patient access to their health information,” he added.
‘What you make of it’
Scott MacDonald, MD, electronic health record medical director at the University of California, Davis, said his organization is proceeding anyway. “UC Davis is going to start releasing notes and test results on Nov. 12,” he said in an interview.
Other organizations and practices now have more time, he said, but the law stays the same. “There’s no change to the what or why – only to the when,” Dr. MacDonald pointed out.
Vanderbilt University Medical Center in Nashville, Tenn., will take advantage of the extra time, Trent Rosenbloom, MD, MPH, director of patient portals, said in an interview.
“Given the super-short time frame we had to work under as this emerged out from dealing with COVID, we feel that we have not addressed all the potential legal-edge cases such as dealing with adolescent medicine and child abuse,” he said.
On Oct. 21, this news organization reported on the then-imminent start of the new law, which irked many readers. They cited, among other things, the likelihood of patient confusion with fast patient access to all clinical notes.
“To me, the biggest issue is that we speak a foreign language that most outside of medicine don’t speak. Our job is to explain it to the patient at a level they can understand. What will 100% happen now is that a patient will not be able to reconcile what is in the note to what they’ve been told,” Andrew White, MD, wrote in a reader comment.
But benefits of open notes outweigh the risks, say proponents, who claim that doctor-patient communication and trust actually improve with information access and that research indicates other benefits such as improved medication adherence.
Open notes are “what you make of it,” said Marlene Millen, MD, an internist at UC San Diego Health, which has had a pilot open-notes program for 3 years.
“I actually end all of my appointments with: ‘Don’t forget to read your note later,’ ” she said in an interview.
Dr. Millen feared open notes initially but, within the first 3 months of usage, about 15 patients gave her direct feedback on how much they appreciated her notes. “It seemed to really reassure them that they were getting good care.”
Dr. MacDonald and Dr. Millen disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Florida will investigate all COVID-19 deaths
The Florida Department of Health will investigate the state’s 16,000 coronavirus deaths due to questions about the integrity of the data, according to an announcement issued Wednesday.
State health department officials said the “fatality data reported to the state consistently presents confusion and warrants a rigorous review.” The review is meant to “ensure data integrity.”
“During a pandemic, the public must be able to rely on accurate public health data to make informed decisions,” Scott Rivkees, the surgeon general for Florida, said in the statement.
Among the 95 deaths reported Wednesday for instance, 16 had more than a 2-month separation between the time of testing positive for COVID-19 and passing away, and 5 cases had a 3-month gap. In addition, 11 of the deaths occurred more than a month ago.
The health department then listed data for all 95 cases, including the age, gender, county and the dates of test positivity and death. Palm Beach County had 50 of the COVID-19 deaths.
“To ensure the accuracy of COVID-19 related deaths, the department will be performing additional reviews of all deaths,” Rivkees said. “Timely and accurate data remains a top priority of the Department of Health.”
Last week, Jose Oliva, speaker of the Florida House of Representatives, said medical examiner reports were “often lacking in rigor.” House Democrats then said Republicans were trying to “downplay the death toll,” according to the South Florida Sun Sentinel .
Fred Piccolo Jr., a spokesman for Florida Gov. Ron DeSantis, told the newspaper Wednesday that officials have struggled to obtain timely data. Labs sometimes report test results from weeks before, he added.
“It’s really one of those things that you gotta know if someone is dying of COVID or if they’re not,” Piccolo said. “Then you can legitimately say, here are the numbers.”
Sources
Florida Department of Health, “Florida Surgeon General Implements Additional Review Process for Fatalities Attributed to COVID-19 to Ensure Data Integrity.”
South Florida Sun Sentinel, “Florida to investigate all COVID-19 deaths after questions about ‘integrity’ of data.”
WebMD Health News © 2020
This article first appeared on Medscape.com.
The Florida Department of Health will investigate the state’s 16,000 coronavirus deaths due to questions about the integrity of the data, according to an announcement issued Wednesday.
State health department officials said the “fatality data reported to the state consistently presents confusion and warrants a rigorous review.” The review is meant to “ensure data integrity.”
“During a pandemic, the public must be able to rely on accurate public health data to make informed decisions,” Scott Rivkees, the surgeon general for Florida, said in the statement.
Among the 95 deaths reported Wednesday for instance, 16 had more than a 2-month separation between the time of testing positive for COVID-19 and passing away, and 5 cases had a 3-month gap. In addition, 11 of the deaths occurred more than a month ago.
The health department then listed data for all 95 cases, including the age, gender, county and the dates of test positivity and death. Palm Beach County had 50 of the COVID-19 deaths.
“To ensure the accuracy of COVID-19 related deaths, the department will be performing additional reviews of all deaths,” Rivkees said. “Timely and accurate data remains a top priority of the Department of Health.”
Last week, Jose Oliva, speaker of the Florida House of Representatives, said medical examiner reports were “often lacking in rigor.” House Democrats then said Republicans were trying to “downplay the death toll,” according to the South Florida Sun Sentinel .
Fred Piccolo Jr., a spokesman for Florida Gov. Ron DeSantis, told the newspaper Wednesday that officials have struggled to obtain timely data. Labs sometimes report test results from weeks before, he added.
“It’s really one of those things that you gotta know if someone is dying of COVID or if they’re not,” Piccolo said. “Then you can legitimately say, here are the numbers.”
Sources
Florida Department of Health, “Florida Surgeon General Implements Additional Review Process for Fatalities Attributed to COVID-19 to Ensure Data Integrity.”
South Florida Sun Sentinel, “Florida to investigate all COVID-19 deaths after questions about ‘integrity’ of data.”
WebMD Health News © 2020
This article first appeared on Medscape.com.
The Florida Department of Health will investigate the state’s 16,000 coronavirus deaths due to questions about the integrity of the data, according to an announcement issued Wednesday.
State health department officials said the “fatality data reported to the state consistently presents confusion and warrants a rigorous review.” The review is meant to “ensure data integrity.”
“During a pandemic, the public must be able to rely on accurate public health data to make informed decisions,” Scott Rivkees, the surgeon general for Florida, said in the statement.
Among the 95 deaths reported Wednesday for instance, 16 had more than a 2-month separation between the time of testing positive for COVID-19 and passing away, and 5 cases had a 3-month gap. In addition, 11 of the deaths occurred more than a month ago.
The health department then listed data for all 95 cases, including the age, gender, county and the dates of test positivity and death. Palm Beach County had 50 of the COVID-19 deaths.
“To ensure the accuracy of COVID-19 related deaths, the department will be performing additional reviews of all deaths,” Rivkees said. “Timely and accurate data remains a top priority of the Department of Health.”
Last week, Jose Oliva, speaker of the Florida House of Representatives, said medical examiner reports were “often lacking in rigor.” House Democrats then said Republicans were trying to “downplay the death toll,” according to the South Florida Sun Sentinel .
Fred Piccolo Jr., a spokesman for Florida Gov. Ron DeSantis, told the newspaper Wednesday that officials have struggled to obtain timely data. Labs sometimes report test results from weeks before, he added.
“It’s really one of those things that you gotta know if someone is dying of COVID or if they’re not,” Piccolo said. “Then you can legitimately say, here are the numbers.”
Sources
Florida Department of Health, “Florida Surgeon General Implements Additional Review Process for Fatalities Attributed to COVID-19 to Ensure Data Integrity.”
South Florida Sun Sentinel, “Florida to investigate all COVID-19 deaths after questions about ‘integrity’ of data.”
WebMD Health News © 2020
This article first appeared on Medscape.com.
COVID-19 vaccine standards questioned at FDA advisory meeting
The FDA’s Vaccines and Related Biological Products Advisory Committee met for a wide-ranging discussion beginning around 10 am. The FDA did not ask the panel to weigh in on any particular vaccine. Instead, the FDA asked for the panel’s feedback on a series of questions, including considerations for continuing phase 3 trials if a product were to get an interim clearance known as an emergency use authorization (EUA).
Speakers at the hearing made a variety of requests, including asking for data showing COVID-19 vaccines can prevent serious illness and urging transparency about the agency’s deliberations for each product to be considered.
FDA staff are closely tracking the crop of experimental vaccines that have made it into advanced stages of testing, including products from Pfizer Inc, AstraZeneca, Johnson & Johnson, and Moderna.
‘Time for a reset’
Among the speakers at the public hearing was Peter Lurie, MD, who served as an FDA associate commissioner from 2014 to 2017. Now the president of the Center for Science in the Public Interest, Lurie was among the speakers who asked the agency to make its independence clear.
President Donald Trump has for months been making predictions about COVID-19 vaccine approvals that have been overly optimistic. In one example, the president, who is seeking re-election on November 3, last month spoke about being able to begin distributing a vaccine in October.
“Until now the process of developing candidate vaccines has been inappropriately politicized with an eye on the election calendar, rather than the deliberate timeframe science requires,” Lurie told the FDA advisory panel. “Now is the time for a reset. This committee has a unique opportunity to set a new tone for vaccine deliberations going forward.”
Lurie asked the panel to press the FDA to commit to hold an advisory committee meeting on requests by drugmakers for EUAs. He also asked the panel to demand that informed consent forms and minutes from institutional review board (IRB) discussions of COVID-19 vaccines trials be made public.
Also among the speakers at the public hearing was Peter Doshi, PhD, an associate professor at the University of Maryland School of Pharmacy, who argued that the current trials won’t answer the right questions about the COVID-19 vaccines.
“We could end up with approved vaccines that reduce the risk of mild infection, but do not decrease the risk of hospitalization, ICU use, or death — either at all or by a clinically relevant amount,” Doshi told the panel.
In his presentation, he reiterated points he had made previously, including in an October 21 article in the BMJ, for which he is an associate editor. Doshi also raised these concerns in a September opinion article in The New York Times, co-authored with Eric Topol, MD, director of the Scripps Research Translational Institute and editor-in-chief of Medscape.
Risks of a ‘rushed vaccine’
Other complaints about the FDA’s approach included criticism of a 2-month follow-up time after vaccination, which was seen as too short. ECRI, a nonprofit organization that seeks to improve the safety, quality, and cost-effectiveness of medicines, has argued that approving a weak COVID-19 vaccine might worsen the pandemic.
In an October 21 statement, ECRI noted the risk of a partially effective vaccine, which could be welcomed as a means of slowing transmission of the virus. But public response and attitudes over the past 9 months in the United States suggest that people would relax their precautions as soon as a vaccine is available.
“Resulting infections may offset the vaccine’s impact and end up increasing the mortality and morbidity burden,” ECRI said in the brief.
“The risks and consequences of a rushed vaccine could be very severe if the review is anything shy of thorough,” ECRI Chief Executive Officer Marcus Schabacker, MD, PhD, said in a statement prepared for the hearing.
This article first appeared on Medscape.com.
The FDA’s Vaccines and Related Biological Products Advisory Committee met for a wide-ranging discussion beginning around 10 am. The FDA did not ask the panel to weigh in on any particular vaccine. Instead, the FDA asked for the panel’s feedback on a series of questions, including considerations for continuing phase 3 trials if a product were to get an interim clearance known as an emergency use authorization (EUA).
Speakers at the hearing made a variety of requests, including asking for data showing COVID-19 vaccines can prevent serious illness and urging transparency about the agency’s deliberations for each product to be considered.
FDA staff are closely tracking the crop of experimental vaccines that have made it into advanced stages of testing, including products from Pfizer Inc, AstraZeneca, Johnson & Johnson, and Moderna.
‘Time for a reset’
Among the speakers at the public hearing was Peter Lurie, MD, who served as an FDA associate commissioner from 2014 to 2017. Now the president of the Center for Science in the Public Interest, Lurie was among the speakers who asked the agency to make its independence clear.
President Donald Trump has for months been making predictions about COVID-19 vaccine approvals that have been overly optimistic. In one example, the president, who is seeking re-election on November 3, last month spoke about being able to begin distributing a vaccine in October.
“Until now the process of developing candidate vaccines has been inappropriately politicized with an eye on the election calendar, rather than the deliberate timeframe science requires,” Lurie told the FDA advisory panel. “Now is the time for a reset. This committee has a unique opportunity to set a new tone for vaccine deliberations going forward.”
Lurie asked the panel to press the FDA to commit to hold an advisory committee meeting on requests by drugmakers for EUAs. He also asked the panel to demand that informed consent forms and minutes from institutional review board (IRB) discussions of COVID-19 vaccines trials be made public.
Also among the speakers at the public hearing was Peter Doshi, PhD, an associate professor at the University of Maryland School of Pharmacy, who argued that the current trials won’t answer the right questions about the COVID-19 vaccines.
“We could end up with approved vaccines that reduce the risk of mild infection, but do not decrease the risk of hospitalization, ICU use, or death — either at all or by a clinically relevant amount,” Doshi told the panel.
In his presentation, he reiterated points he had made previously, including in an October 21 article in the BMJ, for which he is an associate editor. Doshi also raised these concerns in a September opinion article in The New York Times, co-authored with Eric Topol, MD, director of the Scripps Research Translational Institute and editor-in-chief of Medscape.
Risks of a ‘rushed vaccine’
Other complaints about the FDA’s approach included criticism of a 2-month follow-up time after vaccination, which was seen as too short. ECRI, a nonprofit organization that seeks to improve the safety, quality, and cost-effectiveness of medicines, has argued that approving a weak COVID-19 vaccine might worsen the pandemic.
In an October 21 statement, ECRI noted the risk of a partially effective vaccine, which could be welcomed as a means of slowing transmission of the virus. But public response and attitudes over the past 9 months in the United States suggest that people would relax their precautions as soon as a vaccine is available.
“Resulting infections may offset the vaccine’s impact and end up increasing the mortality and morbidity burden,” ECRI said in the brief.
“The risks and consequences of a rushed vaccine could be very severe if the review is anything shy of thorough,” ECRI Chief Executive Officer Marcus Schabacker, MD, PhD, said in a statement prepared for the hearing.
This article first appeared on Medscape.com.
The FDA’s Vaccines and Related Biological Products Advisory Committee met for a wide-ranging discussion beginning around 10 am. The FDA did not ask the panel to weigh in on any particular vaccine. Instead, the FDA asked for the panel’s feedback on a series of questions, including considerations for continuing phase 3 trials if a product were to get an interim clearance known as an emergency use authorization (EUA).
Speakers at the hearing made a variety of requests, including asking for data showing COVID-19 vaccines can prevent serious illness and urging transparency about the agency’s deliberations for each product to be considered.
FDA staff are closely tracking the crop of experimental vaccines that have made it into advanced stages of testing, including products from Pfizer Inc, AstraZeneca, Johnson & Johnson, and Moderna.
‘Time for a reset’
Among the speakers at the public hearing was Peter Lurie, MD, who served as an FDA associate commissioner from 2014 to 2017. Now the president of the Center for Science in the Public Interest, Lurie was among the speakers who asked the agency to make its independence clear.
President Donald Trump has for months been making predictions about COVID-19 vaccine approvals that have been overly optimistic. In one example, the president, who is seeking re-election on November 3, last month spoke about being able to begin distributing a vaccine in October.
“Until now the process of developing candidate vaccines has been inappropriately politicized with an eye on the election calendar, rather than the deliberate timeframe science requires,” Lurie told the FDA advisory panel. “Now is the time for a reset. This committee has a unique opportunity to set a new tone for vaccine deliberations going forward.”
Lurie asked the panel to press the FDA to commit to hold an advisory committee meeting on requests by drugmakers for EUAs. He also asked the panel to demand that informed consent forms and minutes from institutional review board (IRB) discussions of COVID-19 vaccines trials be made public.
Also among the speakers at the public hearing was Peter Doshi, PhD, an associate professor at the University of Maryland School of Pharmacy, who argued that the current trials won’t answer the right questions about the COVID-19 vaccines.
“We could end up with approved vaccines that reduce the risk of mild infection, but do not decrease the risk of hospitalization, ICU use, or death — either at all or by a clinically relevant amount,” Doshi told the panel.
In his presentation, he reiterated points he had made previously, including in an October 21 article in the BMJ, for which he is an associate editor. Doshi also raised these concerns in a September opinion article in The New York Times, co-authored with Eric Topol, MD, director of the Scripps Research Translational Institute and editor-in-chief of Medscape.
Risks of a ‘rushed vaccine’
Other complaints about the FDA’s approach included criticism of a 2-month follow-up time after vaccination, which was seen as too short. ECRI, a nonprofit organization that seeks to improve the safety, quality, and cost-effectiveness of medicines, has argued that approving a weak COVID-19 vaccine might worsen the pandemic.
In an October 21 statement, ECRI noted the risk of a partially effective vaccine, which could be welcomed as a means of slowing transmission of the virus. But public response and attitudes over the past 9 months in the United States suggest that people would relax their precautions as soon as a vaccine is available.
“Resulting infections may offset the vaccine’s impact and end up increasing the mortality and morbidity burden,” ECRI said in the brief.
“The risks and consequences of a rushed vaccine could be very severe if the review is anything shy of thorough,” ECRI Chief Executive Officer Marcus Schabacker, MD, PhD, said in a statement prepared for the hearing.
This article first appeared on Medscape.com.
Human Papillomavirus Vaccination in LGBTQ Patients: The Need for Dermatologists on the Front Lines
Human papillomavirus (HPV) is one of the most common sexually transmitted infections in the United States. It is the causative agent of genital warts, as well as cervical, anal, penile, vulvar, vaginal, and some head and neck cancers.1 Development of the HPV vaccine and its introduction into the scheduled vaccine series recommended by the Centers for Disease Control and Prevention (CDC) represented a major public health milestone. The CDC recommends the HPV vaccine for all children beginning at 11 or 12 years of age, even as early as 9 years, regardless of gender identity or sexuality. As of late 2016, the 9-valent formulation (Gardasil 9 [Merck]) is the only HPV vaccine distributed in the United States, and the vaccination schedule depends specifically on age. The Advisory Committee on Immunization Practices (ACIP) of the CDC revised its recommendations in 2019 to include “shared clinical decision-making regarding HPV vaccination . . . for some adults aged 27 through 45 years.”2 This change in policy has notable implications for sexual and gender minority populations, such as lesbian, gay, bisexual, transgender, and queer or questioning (LGBTQ) patients, especially in the context of dermatologic care. Herein, we discuss HPV-related conditions for LGBTQ patients, barriers to vaccine administration, and the role of dermatologists in promoting an increased vaccination rate in the LGBTQ community.
HPV-Related Conditions
A 2019 review of dermatologic care for LGBTQ patients identified many specific health disparities of HPV.3 Specifically, men who have sex with men (MSM) are more likely than heterosexual men to have oral, anal, and penile HPV infections, including high-risk HPV types.3 From 2011 to 2014, 18% and 13% of MSM had oral HPV infection and high-risk oral HPV infection, respectively, compared to only 11% and 7%, respectively, of men who reported never having had a same-sex sexual partner.4
Similarly, despite the CDC’s position that patients with perianal warts might benefit from digital anal examination or referral for standard or high-resolution anoscopy to detect intra-anal warts, improvements in morbidity have not yet been realized. In 2017, anal cancer incidence was 45.9 cases for every 100,000 person-years among human immunodeficiency (HIV)–positive MSM and 5.1 cases for every 100,000 person-years among HIV-negative MSM vs only 1.5 cases for every 100,000 person-years among men in the United States overall.3 Yet the CDC states that there is insufficient evidence to recommend routine anal cancer screening among MSM, even when a patient is HIV positive. Therefore, current screening practices and treatments are insufficient as MSM continue to have a disproportionately higher rate of HPV-associated disease compared to other populations.
Barriers to HPV Vaccine Administration
The HPV vaccination rate among MSM in adolescent populations varies across reports.5-7 Interestingly, a 2016 survey study found that MSM had approximately 2-times greater odds of initiating the HPV vaccine than heterosexual men.8 However, a study specifically sampling young gay and bisexual men (N=428) found that only 13% had received any doses of the HPV vaccine.6
Regardless, HPV vaccination is much less common among all males than it is among all females, and the low rate of vaccination among sexual minority men has a disproportionate impact, given their higher risk for HPV infection.4 Although the HPV vaccination rate increased from 2014 to 2017, the HPV vaccination rate in MSM overall is less than half of the Healthy People 2020 goal of 80%.9 A 2018 review determined that HPV vaccination is a cost-effective strategy for preventing anal cancer in MSM10; yet male patients might still view the HPV vaccine as a “women’s issue” and are less likely to be vaccinated if they are not prompted by health care providers. Additionally, HPV vaccination is remarkably less likely in MSM when patients are older, uninsured, of lower socioeconomic status, or have not disclosed their sexual identity to their health care provider.9 Dermatologists should be mindful of these barriers to promote HPV vaccination in MSM before, or soon after, sexual debut.
Other members of the LGBTQ community, such as women who have sex with women, face notable HPV-related health disparities and would benefit from increased vaccination efforts by dermatologists. Adolescent and young adult women who have sex with women are less likely than heterosexual adolescent and young adult women to receive routine Papanicolaou tests and initiate HPV vaccination, despite having a higher number of lifetime sexual partners and a higher risk for HPV exposure.11 A 2015 survey study (N=3253) found that after adjusting for covariates, only 8.5% of lesbians and 33.2% of bisexual women and girls who had heard of the HPV vaccine had initiated vaccination compared to 28.4% of their heterosexual counterparts.11 The HPV vaccine is an effective public health tool for the prevention of cervical cancer in these populations. A study of women aged 15 to 19 years in the HPV vaccination era (2007-2014) found significant (P<.05) observed population-level decreases in cervical intraepithelial neoplasia incidence across all grades.12
Transgender women also face a high rate of HPV infection, HIV infection, and other structural and financial disparities, such as low insurance coverage, that can limit their access to vaccination. Transgender men have a higher rate of HPV infection than cisgender men, and those with female internal reproductive organs are less likely to receive routine Papanicolaou tests. A 2018 survey study found that approximately one-third of transgender men and women reported initiating the HPV vaccination series,13 but further investigation is required to make balanced comparisons to cisgender patients.
The Role of the Dermatologist
Collectively, these disparities emphasize the need for increased involvement by dermatologists in HPV vaccination efforts for all LGBTQ patients. Adult patients may have concerns about ties of the HPV vaccine to drug manufacturers and the general safety of vaccination. For pediatric patients, parents/guardians also may be concerned about an assumed but not evidence-based increase in sexual promiscuity following HPV vaccination.14 These topics can be challenging to discuss, but dermatologists have the duty to be proactive and initiate conversation about HPV vaccination, as opposed to waiting for patients to express interest. Dermatologists should stress the safety of the vaccine as well as its potential to protect against multiple, even life-threatening diseases. Providers also can explain that the ACIP recommends catch-up vaccination for all individuals through 26 years of age, regardless of sexual orientation or gender identity.
With the ACIP having recently expanded the appropriate age range for HPV vaccination, we encourage dermatologists to engage in education and shared decision-making to ensure that adult patients with specific risk factors receive the HPV vaccine. Because the expanded ACIP recommendations are aimed at vaccination before HPV exposure, vaccination might not be appropriate for all LGBTQ patients. However, eliciting a sexual history with routine patient intake forms or during the clinical encounter ensures equal access to the HPV vaccine.
Greater awareness of HPV-related disparities and barriers to vaccination in LGBTQ populations has the potential to notably decrease HPV-associated mortality and morbidity. Increased involvement by dermatologists contributes to the efforts of other specialties in universal HPV vaccination, regardless of sexual orientation or gender identity—ideally in younger age groups, such that patients receive the vaccine prior to coitarche.
There are many ways that dermatologists can advocate for HPV vaccination. Those in a multispecialty or academic practice can readily refer patients to an associated internist, primary care physician, or vaccination clinic in the same building or institution. Dermatologists in private practice might be able to administer the HPV vaccine themselves or can advocate for patients to receive the vaccine at a local facility of the Department of Health or at a nonprofit organization, such as a Planned Parenthood center. Although pediatricians and family physicians remain front-line providers of these services, dermatologists represent an additional member of a patient’s care team, capable of advocating for this important intervention.
- Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40:80-85.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Sonawane K, Suk R, Chiao EY, et al. Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med. 2017;167:714-724.
- Kosche C, Mansh M, Luskus M, et al. Dermatologic care of sexual and gender minority/LGBTQIA youth, part 2: recognition and management of the unique dermatologic needs of SGM adolescents. Pediatr Dermatol. 2019;35:587-593.
- Reiter PL, McRee A-L, Katz ML, et al. Human papillomavirus vaccination among young adult gay and bisexual men in the United States. Am J Public Health. 2015;105:96-102.
- Charlton BM, Reisner SL, Ag
énor M, et al. Sexual orientation disparities in human papillomavirus vaccination in a longitudinal cohort of U.S. males and females. LGBT Health. 2017;4:202-209. - Agénor M, Peitzmeier SM, Gordon AR, et al. Sexual orientation identity disparities in human papillomavirus vaccination initiation and completion among young adult US women and men. Cancer Causes Control. 2016;27:1187-1196.
- Loretan C, Chamberlain AT, Sanchez T, et al. Trends and characteristics associated with human papillomavirus vaccination uptake among men who have sex with men in the United States, 2014-2017. Sex Transm Dis. 2019;46:465-473.
- Setiawan D, Wondimu A, Ong K, et al. Cost effectiveness of human papillomavirus vaccination for men who have sex with men; reviewing the available evidence. Pharmacoeconomics. 2018;36:929-939.
- Agénor M, Peitzmeier S, Gordon AR, et al. Sexual orientation identity disparities in awareness and initiation of the human papillomavirus vaccine among U.S. women and girls: a national survey. Ann Intern Med. 2015;163:99-106.
- Benard VB, Castle PE, Jenison SA, et al. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3:833-837.
- McRee A-L, Gower AL, Reiter PL. Preventive healthcare services use among transgender young adults. Int J Transgend. 2018;19:417-423.
- Trinidad J. Policy focus: promoting human papilloma virus vaccine to prevent genital warts and cancer. Boston, MA: The Fenway Institute; 2012. https://fenwayhealth.org/documents/the-fenway-institute/policy-briefs/PolicyFocus_HPV_v4_10.09.12.pdf. Accessed September 15, 2020.
Human papillomavirus (HPV) is one of the most common sexually transmitted infections in the United States. It is the causative agent of genital warts, as well as cervical, anal, penile, vulvar, vaginal, and some head and neck cancers.1 Development of the HPV vaccine and its introduction into the scheduled vaccine series recommended by the Centers for Disease Control and Prevention (CDC) represented a major public health milestone. The CDC recommends the HPV vaccine for all children beginning at 11 or 12 years of age, even as early as 9 years, regardless of gender identity or sexuality. As of late 2016, the 9-valent formulation (Gardasil 9 [Merck]) is the only HPV vaccine distributed in the United States, and the vaccination schedule depends specifically on age. The Advisory Committee on Immunization Practices (ACIP) of the CDC revised its recommendations in 2019 to include “shared clinical decision-making regarding HPV vaccination . . . for some adults aged 27 through 45 years.”2 This change in policy has notable implications for sexual and gender minority populations, such as lesbian, gay, bisexual, transgender, and queer or questioning (LGBTQ) patients, especially in the context of dermatologic care. Herein, we discuss HPV-related conditions for LGBTQ patients, barriers to vaccine administration, and the role of dermatologists in promoting an increased vaccination rate in the LGBTQ community.
HPV-Related Conditions
A 2019 review of dermatologic care for LGBTQ patients identified many specific health disparities of HPV.3 Specifically, men who have sex with men (MSM) are more likely than heterosexual men to have oral, anal, and penile HPV infections, including high-risk HPV types.3 From 2011 to 2014, 18% and 13% of MSM had oral HPV infection and high-risk oral HPV infection, respectively, compared to only 11% and 7%, respectively, of men who reported never having had a same-sex sexual partner.4
Similarly, despite the CDC’s position that patients with perianal warts might benefit from digital anal examination or referral for standard or high-resolution anoscopy to detect intra-anal warts, improvements in morbidity have not yet been realized. In 2017, anal cancer incidence was 45.9 cases for every 100,000 person-years among human immunodeficiency (HIV)–positive MSM and 5.1 cases for every 100,000 person-years among HIV-negative MSM vs only 1.5 cases for every 100,000 person-years among men in the United States overall.3 Yet the CDC states that there is insufficient evidence to recommend routine anal cancer screening among MSM, even when a patient is HIV positive. Therefore, current screening practices and treatments are insufficient as MSM continue to have a disproportionately higher rate of HPV-associated disease compared to other populations.
Barriers to HPV Vaccine Administration
The HPV vaccination rate among MSM in adolescent populations varies across reports.5-7 Interestingly, a 2016 survey study found that MSM had approximately 2-times greater odds of initiating the HPV vaccine than heterosexual men.8 However, a study specifically sampling young gay and bisexual men (N=428) found that only 13% had received any doses of the HPV vaccine.6
Regardless, HPV vaccination is much less common among all males than it is among all females, and the low rate of vaccination among sexual minority men has a disproportionate impact, given their higher risk for HPV infection.4 Although the HPV vaccination rate increased from 2014 to 2017, the HPV vaccination rate in MSM overall is less than half of the Healthy People 2020 goal of 80%.9 A 2018 review determined that HPV vaccination is a cost-effective strategy for preventing anal cancer in MSM10; yet male patients might still view the HPV vaccine as a “women’s issue” and are less likely to be vaccinated if they are not prompted by health care providers. Additionally, HPV vaccination is remarkably less likely in MSM when patients are older, uninsured, of lower socioeconomic status, or have not disclosed their sexual identity to their health care provider.9 Dermatologists should be mindful of these barriers to promote HPV vaccination in MSM before, or soon after, sexual debut.
Other members of the LGBTQ community, such as women who have sex with women, face notable HPV-related health disparities and would benefit from increased vaccination efforts by dermatologists. Adolescent and young adult women who have sex with women are less likely than heterosexual adolescent and young adult women to receive routine Papanicolaou tests and initiate HPV vaccination, despite having a higher number of lifetime sexual partners and a higher risk for HPV exposure.11 A 2015 survey study (N=3253) found that after adjusting for covariates, only 8.5% of lesbians and 33.2% of bisexual women and girls who had heard of the HPV vaccine had initiated vaccination compared to 28.4% of their heterosexual counterparts.11 The HPV vaccine is an effective public health tool for the prevention of cervical cancer in these populations. A study of women aged 15 to 19 years in the HPV vaccination era (2007-2014) found significant (P<.05) observed population-level decreases in cervical intraepithelial neoplasia incidence across all grades.12
Transgender women also face a high rate of HPV infection, HIV infection, and other structural and financial disparities, such as low insurance coverage, that can limit their access to vaccination. Transgender men have a higher rate of HPV infection than cisgender men, and those with female internal reproductive organs are less likely to receive routine Papanicolaou tests. A 2018 survey study found that approximately one-third of transgender men and women reported initiating the HPV vaccination series,13 but further investigation is required to make balanced comparisons to cisgender patients.
The Role of the Dermatologist
Collectively, these disparities emphasize the need for increased involvement by dermatologists in HPV vaccination efforts for all LGBTQ patients. Adult patients may have concerns about ties of the HPV vaccine to drug manufacturers and the general safety of vaccination. For pediatric patients, parents/guardians also may be concerned about an assumed but not evidence-based increase in sexual promiscuity following HPV vaccination.14 These topics can be challenging to discuss, but dermatologists have the duty to be proactive and initiate conversation about HPV vaccination, as opposed to waiting for patients to express interest. Dermatologists should stress the safety of the vaccine as well as its potential to protect against multiple, even life-threatening diseases. Providers also can explain that the ACIP recommends catch-up vaccination for all individuals through 26 years of age, regardless of sexual orientation or gender identity.
With the ACIP having recently expanded the appropriate age range for HPV vaccination, we encourage dermatologists to engage in education and shared decision-making to ensure that adult patients with specific risk factors receive the HPV vaccine. Because the expanded ACIP recommendations are aimed at vaccination before HPV exposure, vaccination might not be appropriate for all LGBTQ patients. However, eliciting a sexual history with routine patient intake forms or during the clinical encounter ensures equal access to the HPV vaccine.
Greater awareness of HPV-related disparities and barriers to vaccination in LGBTQ populations has the potential to notably decrease HPV-associated mortality and morbidity. Increased involvement by dermatologists contributes to the efforts of other specialties in universal HPV vaccination, regardless of sexual orientation or gender identity—ideally in younger age groups, such that patients receive the vaccine prior to coitarche.
There are many ways that dermatologists can advocate for HPV vaccination. Those in a multispecialty or academic practice can readily refer patients to an associated internist, primary care physician, or vaccination clinic in the same building or institution. Dermatologists in private practice might be able to administer the HPV vaccine themselves or can advocate for patients to receive the vaccine at a local facility of the Department of Health or at a nonprofit organization, such as a Planned Parenthood center. Although pediatricians and family physicians remain front-line providers of these services, dermatologists represent an additional member of a patient’s care team, capable of advocating for this important intervention.
Human papillomavirus (HPV) is one of the most common sexually transmitted infections in the United States. It is the causative agent of genital warts, as well as cervical, anal, penile, vulvar, vaginal, and some head and neck cancers.1 Development of the HPV vaccine and its introduction into the scheduled vaccine series recommended by the Centers for Disease Control and Prevention (CDC) represented a major public health milestone. The CDC recommends the HPV vaccine for all children beginning at 11 or 12 years of age, even as early as 9 years, regardless of gender identity or sexuality. As of late 2016, the 9-valent formulation (Gardasil 9 [Merck]) is the only HPV vaccine distributed in the United States, and the vaccination schedule depends specifically on age. The Advisory Committee on Immunization Practices (ACIP) of the CDC revised its recommendations in 2019 to include “shared clinical decision-making regarding HPV vaccination . . . for some adults aged 27 through 45 years.”2 This change in policy has notable implications for sexual and gender minority populations, such as lesbian, gay, bisexual, transgender, and queer or questioning (LGBTQ) patients, especially in the context of dermatologic care. Herein, we discuss HPV-related conditions for LGBTQ patients, barriers to vaccine administration, and the role of dermatologists in promoting an increased vaccination rate in the LGBTQ community.
HPV-Related Conditions
A 2019 review of dermatologic care for LGBTQ patients identified many specific health disparities of HPV.3 Specifically, men who have sex with men (MSM) are more likely than heterosexual men to have oral, anal, and penile HPV infections, including high-risk HPV types.3 From 2011 to 2014, 18% and 13% of MSM had oral HPV infection and high-risk oral HPV infection, respectively, compared to only 11% and 7%, respectively, of men who reported never having had a same-sex sexual partner.4
Similarly, despite the CDC’s position that patients with perianal warts might benefit from digital anal examination or referral for standard or high-resolution anoscopy to detect intra-anal warts, improvements in morbidity have not yet been realized. In 2017, anal cancer incidence was 45.9 cases for every 100,000 person-years among human immunodeficiency (HIV)–positive MSM and 5.1 cases for every 100,000 person-years among HIV-negative MSM vs only 1.5 cases for every 100,000 person-years among men in the United States overall.3 Yet the CDC states that there is insufficient evidence to recommend routine anal cancer screening among MSM, even when a patient is HIV positive. Therefore, current screening practices and treatments are insufficient as MSM continue to have a disproportionately higher rate of HPV-associated disease compared to other populations.
Barriers to HPV Vaccine Administration
The HPV vaccination rate among MSM in adolescent populations varies across reports.5-7 Interestingly, a 2016 survey study found that MSM had approximately 2-times greater odds of initiating the HPV vaccine than heterosexual men.8 However, a study specifically sampling young gay and bisexual men (N=428) found that only 13% had received any doses of the HPV vaccine.6
Regardless, HPV vaccination is much less common among all males than it is among all females, and the low rate of vaccination among sexual minority men has a disproportionate impact, given their higher risk for HPV infection.4 Although the HPV vaccination rate increased from 2014 to 2017, the HPV vaccination rate in MSM overall is less than half of the Healthy People 2020 goal of 80%.9 A 2018 review determined that HPV vaccination is a cost-effective strategy for preventing anal cancer in MSM10; yet male patients might still view the HPV vaccine as a “women’s issue” and are less likely to be vaccinated if they are not prompted by health care providers. Additionally, HPV vaccination is remarkably less likely in MSM when patients are older, uninsured, of lower socioeconomic status, or have not disclosed their sexual identity to their health care provider.9 Dermatologists should be mindful of these barriers to promote HPV vaccination in MSM before, or soon after, sexual debut.
Other members of the LGBTQ community, such as women who have sex with women, face notable HPV-related health disparities and would benefit from increased vaccination efforts by dermatologists. Adolescent and young adult women who have sex with women are less likely than heterosexual adolescent and young adult women to receive routine Papanicolaou tests and initiate HPV vaccination, despite having a higher number of lifetime sexual partners and a higher risk for HPV exposure.11 A 2015 survey study (N=3253) found that after adjusting for covariates, only 8.5% of lesbians and 33.2% of bisexual women and girls who had heard of the HPV vaccine had initiated vaccination compared to 28.4% of their heterosexual counterparts.11 The HPV vaccine is an effective public health tool for the prevention of cervical cancer in these populations. A study of women aged 15 to 19 years in the HPV vaccination era (2007-2014) found significant (P<.05) observed population-level decreases in cervical intraepithelial neoplasia incidence across all grades.12
Transgender women also face a high rate of HPV infection, HIV infection, and other structural and financial disparities, such as low insurance coverage, that can limit their access to vaccination. Transgender men have a higher rate of HPV infection than cisgender men, and those with female internal reproductive organs are less likely to receive routine Papanicolaou tests. A 2018 survey study found that approximately one-third of transgender men and women reported initiating the HPV vaccination series,13 but further investigation is required to make balanced comparisons to cisgender patients.
The Role of the Dermatologist
Collectively, these disparities emphasize the need for increased involvement by dermatologists in HPV vaccination efforts for all LGBTQ patients. Adult patients may have concerns about ties of the HPV vaccine to drug manufacturers and the general safety of vaccination. For pediatric patients, parents/guardians also may be concerned about an assumed but not evidence-based increase in sexual promiscuity following HPV vaccination.14 These topics can be challenging to discuss, but dermatologists have the duty to be proactive and initiate conversation about HPV vaccination, as opposed to waiting for patients to express interest. Dermatologists should stress the safety of the vaccine as well as its potential to protect against multiple, even life-threatening diseases. Providers also can explain that the ACIP recommends catch-up vaccination for all individuals through 26 years of age, regardless of sexual orientation or gender identity.
With the ACIP having recently expanded the appropriate age range for HPV vaccination, we encourage dermatologists to engage in education and shared decision-making to ensure that adult patients with specific risk factors receive the HPV vaccine. Because the expanded ACIP recommendations are aimed at vaccination before HPV exposure, vaccination might not be appropriate for all LGBTQ patients. However, eliciting a sexual history with routine patient intake forms or during the clinical encounter ensures equal access to the HPV vaccine.
Greater awareness of HPV-related disparities and barriers to vaccination in LGBTQ populations has the potential to notably decrease HPV-associated mortality and morbidity. Increased involvement by dermatologists contributes to the efforts of other specialties in universal HPV vaccination, regardless of sexual orientation or gender identity—ideally in younger age groups, such that patients receive the vaccine prior to coitarche.
There are many ways that dermatologists can advocate for HPV vaccination. Those in a multispecialty or academic practice can readily refer patients to an associated internist, primary care physician, or vaccination clinic in the same building or institution. Dermatologists in private practice might be able to administer the HPV vaccine themselves or can advocate for patients to receive the vaccine at a local facility of the Department of Health or at a nonprofit organization, such as a Planned Parenthood center. Although pediatricians and family physicians remain front-line providers of these services, dermatologists represent an additional member of a patient’s care team, capable of advocating for this important intervention.
- Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40:80-85.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Sonawane K, Suk R, Chiao EY, et al. Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med. 2017;167:714-724.
- Kosche C, Mansh M, Luskus M, et al. Dermatologic care of sexual and gender minority/LGBTQIA youth, part 2: recognition and management of the unique dermatologic needs of SGM adolescents. Pediatr Dermatol. 2019;35:587-593.
- Reiter PL, McRee A-L, Katz ML, et al. Human papillomavirus vaccination among young adult gay and bisexual men in the United States. Am J Public Health. 2015;105:96-102.
- Charlton BM, Reisner SL, Ag
énor M, et al. Sexual orientation disparities in human papillomavirus vaccination in a longitudinal cohort of U.S. males and females. LGBT Health. 2017;4:202-209. - Agénor M, Peitzmeier SM, Gordon AR, et al. Sexual orientation identity disparities in human papillomavirus vaccination initiation and completion among young adult US women and men. Cancer Causes Control. 2016;27:1187-1196.
- Loretan C, Chamberlain AT, Sanchez T, et al. Trends and characteristics associated with human papillomavirus vaccination uptake among men who have sex with men in the United States, 2014-2017. Sex Transm Dis. 2019;46:465-473.
- Setiawan D, Wondimu A, Ong K, et al. Cost effectiveness of human papillomavirus vaccination for men who have sex with men; reviewing the available evidence. Pharmacoeconomics. 2018;36:929-939.
- Agénor M, Peitzmeier S, Gordon AR, et al. Sexual orientation identity disparities in awareness and initiation of the human papillomavirus vaccine among U.S. women and girls: a national survey. Ann Intern Med. 2015;163:99-106.
- Benard VB, Castle PE, Jenison SA, et al. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3:833-837.
- McRee A-L, Gower AL, Reiter PL. Preventive healthcare services use among transgender young adults. Int J Transgend. 2018;19:417-423.
- Trinidad J. Policy focus: promoting human papilloma virus vaccine to prevent genital warts and cancer. Boston, MA: The Fenway Institute; 2012. https://fenwayhealth.org/documents/the-fenway-institute/policy-briefs/PolicyFocus_HPV_v4_10.09.12.pdf. Accessed September 15, 2020.
- Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40:80-85.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Sonawane K, Suk R, Chiao EY, et al. Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med. 2017;167:714-724.
- Kosche C, Mansh M, Luskus M, et al. Dermatologic care of sexual and gender minority/LGBTQIA youth, part 2: recognition and management of the unique dermatologic needs of SGM adolescents. Pediatr Dermatol. 2019;35:587-593.
- Reiter PL, McRee A-L, Katz ML, et al. Human papillomavirus vaccination among young adult gay and bisexual men in the United States. Am J Public Health. 2015;105:96-102.
- Charlton BM, Reisner SL, Ag
énor M, et al. Sexual orientation disparities in human papillomavirus vaccination in a longitudinal cohort of U.S. males and females. LGBT Health. 2017;4:202-209. - Agénor M, Peitzmeier SM, Gordon AR, et al. Sexual orientation identity disparities in human papillomavirus vaccination initiation and completion among young adult US women and men. Cancer Causes Control. 2016;27:1187-1196.
- Loretan C, Chamberlain AT, Sanchez T, et al. Trends and characteristics associated with human papillomavirus vaccination uptake among men who have sex with men in the United States, 2014-2017. Sex Transm Dis. 2019;46:465-473.
- Setiawan D, Wondimu A, Ong K, et al. Cost effectiveness of human papillomavirus vaccination for men who have sex with men; reviewing the available evidence. Pharmacoeconomics. 2018;36:929-939.
- Agénor M, Peitzmeier S, Gordon AR, et al. Sexual orientation identity disparities in awareness and initiation of the human papillomavirus vaccine among U.S. women and girls: a national survey. Ann Intern Med. 2015;163:99-106.
- Benard VB, Castle PE, Jenison SA, et al. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3:833-837.
- McRee A-L, Gower AL, Reiter PL. Preventive healthcare services use among transgender young adults. Int J Transgend. 2018;19:417-423.
- Trinidad J. Policy focus: promoting human papilloma virus vaccine to prevent genital warts and cancer. Boston, MA: The Fenway Institute; 2012. https://fenwayhealth.org/documents/the-fenway-institute/policy-briefs/PolicyFocus_HPV_v4_10.09.12.pdf. Accessed September 15, 2020.
CDER chief reflects on advances in rare diseases
, from helping to usher the approval of the first treatments for cystic fibrosis and multiple sclerosis during her tenure as director of the Office of Therapeutics Research and Review, to introducing the concept of risk management in the agency’s analysis of drug safety during her role as acting director of the Center for Drug Evaluation and Research (CDER).
During an online event on Oct. 9, Dr. Woodcock, who became CDER’s director in 2008, will receive a lifetime achievement award from the National Organization for Rare Disorders*. In this interview, she reflects on the CDER’s accomplishments in the field of rare diseases, from which she draws inspiration, and what it’s like to be overseeing the therapeutics component of Operation Warp Speed amid the COVID-19 pandemic.
Q: What does this lifetime achievement award from the National Organization for Rare Disorders mean to you at this stage in your career?
Dr. Woodcock: According to NORD, there are more than 7,000 rare diseases that affect an estimated 25 million Americans. More than half of those affected are children. Many of these diseases are very serious, so there is a great deal of suffering that goes on, sometimes for a lifetime. I’ve always felt that people suffering like this don’t really have a voice. I’ve always tried to push the regulatory science, the science behind evaluation, and all of the efforts we can make to help those who are trying to develop products for people suffering from these rare diseases. The science is really picking up. We’re seeing more drug approvals every year for rare disorders. Hopefully, the lives of people with rare disorders will improve and we will continue to see a trajectory of better outcomes for people.
Q: Who inspired you most early in your career as a physician? What was it about that person (or persons) that made a difference to you?
Dr. Woodcock: During my training I had the privilege to be exposed to a wide range of stellar diagnosticians and people who were good clinicians who cared about their patients. That experience modeled for me what I would like to be as a doctor.
Q: In 2017, the National Consumers League described you as “a passionate advocate for American patients and consumers, an ally to patient advocacy groups, and a fearless leader at the FDA.” In your own words, how do you describe your leadership style?
Dr. Woodcock: People always call me fearless, but I feel like I just state the facts. I care about getting technical input from everyone, but I’m not terribly concerned about people’s disapproval of my actions. I’m a leader who tries to do the right thing, the thing that will benefit patients. I try to keep them at the center of what we’re doing, who we’re regulating for. We work for the American public. As far as CDER, it’s the people who take medicine, people who administer medicine, and people who need treatments.
Q: Since joining CDER as director in 2008, what are some accomplishments you are most proud of as it relates to treatments for patients with rare diseases?
Dr. Woodcock: I undertook a transformation and modernization of the New Drugs Regulatory Program, which created offices that align interrelated disease areas, and divisions with clearer and more focused areas of expertise. These changes will bring efficiency and effectiveness. We also set up an Office of Translational Sciences. All of these actions are important. In developing drugs for rare disorders, we need more flexibility. We have a lot of critics who say, “Rare disease trials are too small.” If you look at a cardiovascular trial of 25,000 people, for example, the investigators might only have .1% of the affected population enrolled. On the other hand, a rare disease trial of 100 people might represent half of the entire population with that disease. We often get criticism because it’s more difficult to define endpoints. The diseases aren’t that well understood, and you’re going to have smaller trials because there aren’t that many people with the disease. We need to figure out how to appropriately exercise that flexibility in regulation and make sure people have access, but have a high probability of getting products that work and have been adequately tested for safely. We also started a Rare Disease Cures Accelerator, which is enrolling people online in natural history studies to see what happens to them so we can better plan studies. We have Patient-Focused Drug Development meetings as a way to gather patients’ perspectives on their conditions and available therapies to treat those conditions. That is eye-opening, because what the doctor thinks about the disease may not be what the patient thinks about the disease. The patients are the ones taking the medicine, so we need to collect their opinions. Such approaches make it easier to study rare diseases and get new treatments.
Q: How do the challenges of drug research and development in the field of rare diseases differ from those associated with more prevalent diseases?
Dr. Woodcock: There is one advantage today for people with rare diseases. That is, when there is a known genetic mutation causing a disease, RNA interference and other gene therapy approaches can be used. There are challenges, though. Patients with rare disorders often don’t have a uniform disease course. They often have a multisystem impact, so they might have things wrong with their GI tract and/or skin, so it’s difficult to know what to measure. We’re trying to remedy this by gathering better natural history information on what happens to people. That is empowering for patients as well.
Q: In what practical ways can physicians become advocates for patients and their families who are navigating life with a rare disease?
Dr. Woodcock: I advise people to get involved in the association or advocacy group for their rare disease. It’s empowering. They can share stories and information with others who have been suffering from the disease. Also, they would get information about what trials might be available. As for physicians themselves, they have a bewildering variety of jobs they’re supposed to do, so it’s hard to be good in any one of them. People with rare disease often suffer terribly because they don’t get diagnosed for 10 years even though they have classic symptoms of a particular disorder. If physicians have never seen it or never heard of it, they may not know how to treat it. It’s a huge problem.
Q: Who inspires you most in your work today?
Dr. Woodcock: The dedication of the staff at the FDA is unbelievable. When you look at responses to the Federal Employee Viewpoint Survey administered by the Office of Personnel Management, FDA workers consistently express a strong sense of mission and dedication. It’s out of the park, really. They have worked night and day during this pandemic. I’m inspired by everyone who works at the FDA and their incredible dedication to their work.
Q: In what ways do you cope with the pressure that comes with your line of work? Do you have a favorite hobby or that activity that helps keep you grounded?
Dr. Woodcock: I’m an avid gardener, so I have a garden with vegetables, fruits, and flowers, including a large orchid collection. I’m also a hiker and a physical fitness buff, so I feel like there isn’t enough time in the day for all of my hobbies. Formal hiking trails near me are very crowded now, so I’ve been hiking around my neighborhood, taking long walks and going up and down hills quickly. Last November, I went hiking in New Zealand with my daughter. We hiked the Milford Track, which is about 33 miles long. It goes from an inland lake, over a mountain pass, and to the Pacific Ocean. It was fun, with unbelievable scenery.
Q: What novel treatment developments in rare disorders are you most excited about in the next 5 years?
Dr. Woodcock: I think gene therapy will come into its own. I think that could be a game-changer for people with genetic mutations causing rare diseases, and even cancer. We’ll see. It takes the technology a long time to mature. There are also gene-directed therapies such as RNA inhibition. We’ve already approved a couple of products like that for rare diseases, including treatments for the cardiomyopathy and neuropathy associated with ATTR amyloidosis. As our knowledge of biology continues to grow, I think more of these diseases will be amenable to interventions.
Q: In May of 2020 you were asked to temporarily step aside from your post as director of CDER to work on Operation Warp Speed. Please describe what your role is in this effort to accelerate COVID-19 treatments.
Dr. Woodcock: I’m the lead on therapeutics. Operation Warp Speed is mainly focused on developing vaccines for COVID-19. In the meantime, people who don’t respond to vaccines are going to need therapeutics, such as the elderly, or those who refuse to take vaccines, or those who are immunosuppressed and can’t mount a response to a vaccine. If we can develop those therapeutics now, that would be good to get that populous vaccinated. The team identified what we thought were the five highest priority agents to work on, and we’re testing them. We have identified many more in a priority list. We have five master protocols running for different times in the disease, such as when you’re an outpatient, when you’re an inpatient, or when you’re in the ICU. The work is stressful, because we need these treatments as soon as possible, but we have a great team working on this. I feel like I’m making a contribution in this role, because I know people in industry and in the National Institutes of Health. I try to bring everyone together and get things done.
*Correction, 10/22/20: An earlier version of this article misstated the name of the National Organization for Rare Disorders.
, from helping to usher the approval of the first treatments for cystic fibrosis and multiple sclerosis during her tenure as director of the Office of Therapeutics Research and Review, to introducing the concept of risk management in the agency’s analysis of drug safety during her role as acting director of the Center for Drug Evaluation and Research (CDER).
During an online event on Oct. 9, Dr. Woodcock, who became CDER’s director in 2008, will receive a lifetime achievement award from the National Organization for Rare Disorders*. In this interview, she reflects on the CDER’s accomplishments in the field of rare diseases, from which she draws inspiration, and what it’s like to be overseeing the therapeutics component of Operation Warp Speed amid the COVID-19 pandemic.
Q: What does this lifetime achievement award from the National Organization for Rare Disorders mean to you at this stage in your career?
Dr. Woodcock: According to NORD, there are more than 7,000 rare diseases that affect an estimated 25 million Americans. More than half of those affected are children. Many of these diseases are very serious, so there is a great deal of suffering that goes on, sometimes for a lifetime. I’ve always felt that people suffering like this don’t really have a voice. I’ve always tried to push the regulatory science, the science behind evaluation, and all of the efforts we can make to help those who are trying to develop products for people suffering from these rare diseases. The science is really picking up. We’re seeing more drug approvals every year for rare disorders. Hopefully, the lives of people with rare disorders will improve and we will continue to see a trajectory of better outcomes for people.
Q: Who inspired you most early in your career as a physician? What was it about that person (or persons) that made a difference to you?
Dr. Woodcock: During my training I had the privilege to be exposed to a wide range of stellar diagnosticians and people who were good clinicians who cared about their patients. That experience modeled for me what I would like to be as a doctor.
Q: In 2017, the National Consumers League described you as “a passionate advocate for American patients and consumers, an ally to patient advocacy groups, and a fearless leader at the FDA.” In your own words, how do you describe your leadership style?
Dr. Woodcock: People always call me fearless, but I feel like I just state the facts. I care about getting technical input from everyone, but I’m not terribly concerned about people’s disapproval of my actions. I’m a leader who tries to do the right thing, the thing that will benefit patients. I try to keep them at the center of what we’re doing, who we’re regulating for. We work for the American public. As far as CDER, it’s the people who take medicine, people who administer medicine, and people who need treatments.
Q: Since joining CDER as director in 2008, what are some accomplishments you are most proud of as it relates to treatments for patients with rare diseases?
Dr. Woodcock: I undertook a transformation and modernization of the New Drugs Regulatory Program, which created offices that align interrelated disease areas, and divisions with clearer and more focused areas of expertise. These changes will bring efficiency and effectiveness. We also set up an Office of Translational Sciences. All of these actions are important. In developing drugs for rare disorders, we need more flexibility. We have a lot of critics who say, “Rare disease trials are too small.” If you look at a cardiovascular trial of 25,000 people, for example, the investigators might only have .1% of the affected population enrolled. On the other hand, a rare disease trial of 100 people might represent half of the entire population with that disease. We often get criticism because it’s more difficult to define endpoints. The diseases aren’t that well understood, and you’re going to have smaller trials because there aren’t that many people with the disease. We need to figure out how to appropriately exercise that flexibility in regulation and make sure people have access, but have a high probability of getting products that work and have been adequately tested for safely. We also started a Rare Disease Cures Accelerator, which is enrolling people online in natural history studies to see what happens to them so we can better plan studies. We have Patient-Focused Drug Development meetings as a way to gather patients’ perspectives on their conditions and available therapies to treat those conditions. That is eye-opening, because what the doctor thinks about the disease may not be what the patient thinks about the disease. The patients are the ones taking the medicine, so we need to collect their opinions. Such approaches make it easier to study rare diseases and get new treatments.
Q: How do the challenges of drug research and development in the field of rare diseases differ from those associated with more prevalent diseases?
Dr. Woodcock: There is one advantage today for people with rare diseases. That is, when there is a known genetic mutation causing a disease, RNA interference and other gene therapy approaches can be used. There are challenges, though. Patients with rare disorders often don’t have a uniform disease course. They often have a multisystem impact, so they might have things wrong with their GI tract and/or skin, so it’s difficult to know what to measure. We’re trying to remedy this by gathering better natural history information on what happens to people. That is empowering for patients as well.
Q: In what practical ways can physicians become advocates for patients and their families who are navigating life with a rare disease?
Dr. Woodcock: I advise people to get involved in the association or advocacy group for their rare disease. It’s empowering. They can share stories and information with others who have been suffering from the disease. Also, they would get information about what trials might be available. As for physicians themselves, they have a bewildering variety of jobs they’re supposed to do, so it’s hard to be good in any one of them. People with rare disease often suffer terribly because they don’t get diagnosed for 10 years even though they have classic symptoms of a particular disorder. If physicians have never seen it or never heard of it, they may not know how to treat it. It’s a huge problem.
Q: Who inspires you most in your work today?
Dr. Woodcock: The dedication of the staff at the FDA is unbelievable. When you look at responses to the Federal Employee Viewpoint Survey administered by the Office of Personnel Management, FDA workers consistently express a strong sense of mission and dedication. It’s out of the park, really. They have worked night and day during this pandemic. I’m inspired by everyone who works at the FDA and their incredible dedication to their work.
Q: In what ways do you cope with the pressure that comes with your line of work? Do you have a favorite hobby or that activity that helps keep you grounded?
Dr. Woodcock: I’m an avid gardener, so I have a garden with vegetables, fruits, and flowers, including a large orchid collection. I’m also a hiker and a physical fitness buff, so I feel like there isn’t enough time in the day for all of my hobbies. Formal hiking trails near me are very crowded now, so I’ve been hiking around my neighborhood, taking long walks and going up and down hills quickly. Last November, I went hiking in New Zealand with my daughter. We hiked the Milford Track, which is about 33 miles long. It goes from an inland lake, over a mountain pass, and to the Pacific Ocean. It was fun, with unbelievable scenery.
Q: What novel treatment developments in rare disorders are you most excited about in the next 5 years?
Dr. Woodcock: I think gene therapy will come into its own. I think that could be a game-changer for people with genetic mutations causing rare diseases, and even cancer. We’ll see. It takes the technology a long time to mature. There are also gene-directed therapies such as RNA inhibition. We’ve already approved a couple of products like that for rare diseases, including treatments for the cardiomyopathy and neuropathy associated with ATTR amyloidosis. As our knowledge of biology continues to grow, I think more of these diseases will be amenable to interventions.
Q: In May of 2020 you were asked to temporarily step aside from your post as director of CDER to work on Operation Warp Speed. Please describe what your role is in this effort to accelerate COVID-19 treatments.
Dr. Woodcock: I’m the lead on therapeutics. Operation Warp Speed is mainly focused on developing vaccines for COVID-19. In the meantime, people who don’t respond to vaccines are going to need therapeutics, such as the elderly, or those who refuse to take vaccines, or those who are immunosuppressed and can’t mount a response to a vaccine. If we can develop those therapeutics now, that would be good to get that populous vaccinated. The team identified what we thought were the five highest priority agents to work on, and we’re testing them. We have identified many more in a priority list. We have five master protocols running for different times in the disease, such as when you’re an outpatient, when you’re an inpatient, or when you’re in the ICU. The work is stressful, because we need these treatments as soon as possible, but we have a great team working on this. I feel like I’m making a contribution in this role, because I know people in industry and in the National Institutes of Health. I try to bring everyone together and get things done.
*Correction, 10/22/20: An earlier version of this article misstated the name of the National Organization for Rare Disorders.
, from helping to usher the approval of the first treatments for cystic fibrosis and multiple sclerosis during her tenure as director of the Office of Therapeutics Research and Review, to introducing the concept of risk management in the agency’s analysis of drug safety during her role as acting director of the Center for Drug Evaluation and Research (CDER).
During an online event on Oct. 9, Dr. Woodcock, who became CDER’s director in 2008, will receive a lifetime achievement award from the National Organization for Rare Disorders*. In this interview, she reflects on the CDER’s accomplishments in the field of rare diseases, from which she draws inspiration, and what it’s like to be overseeing the therapeutics component of Operation Warp Speed amid the COVID-19 pandemic.
Q: What does this lifetime achievement award from the National Organization for Rare Disorders mean to you at this stage in your career?
Dr. Woodcock: According to NORD, there are more than 7,000 rare diseases that affect an estimated 25 million Americans. More than half of those affected are children. Many of these diseases are very serious, so there is a great deal of suffering that goes on, sometimes for a lifetime. I’ve always felt that people suffering like this don’t really have a voice. I’ve always tried to push the regulatory science, the science behind evaluation, and all of the efforts we can make to help those who are trying to develop products for people suffering from these rare diseases. The science is really picking up. We’re seeing more drug approvals every year for rare disorders. Hopefully, the lives of people with rare disorders will improve and we will continue to see a trajectory of better outcomes for people.
Q: Who inspired you most early in your career as a physician? What was it about that person (or persons) that made a difference to you?
Dr. Woodcock: During my training I had the privilege to be exposed to a wide range of stellar diagnosticians and people who were good clinicians who cared about their patients. That experience modeled for me what I would like to be as a doctor.
Q: In 2017, the National Consumers League described you as “a passionate advocate for American patients and consumers, an ally to patient advocacy groups, and a fearless leader at the FDA.” In your own words, how do you describe your leadership style?
Dr. Woodcock: People always call me fearless, but I feel like I just state the facts. I care about getting technical input from everyone, but I’m not terribly concerned about people’s disapproval of my actions. I’m a leader who tries to do the right thing, the thing that will benefit patients. I try to keep them at the center of what we’re doing, who we’re regulating for. We work for the American public. As far as CDER, it’s the people who take medicine, people who administer medicine, and people who need treatments.
Q: Since joining CDER as director in 2008, what are some accomplishments you are most proud of as it relates to treatments for patients with rare diseases?
Dr. Woodcock: I undertook a transformation and modernization of the New Drugs Regulatory Program, which created offices that align interrelated disease areas, and divisions with clearer and more focused areas of expertise. These changes will bring efficiency and effectiveness. We also set up an Office of Translational Sciences. All of these actions are important. In developing drugs for rare disorders, we need more flexibility. We have a lot of critics who say, “Rare disease trials are too small.” If you look at a cardiovascular trial of 25,000 people, for example, the investigators might only have .1% of the affected population enrolled. On the other hand, a rare disease trial of 100 people might represent half of the entire population with that disease. We often get criticism because it’s more difficult to define endpoints. The diseases aren’t that well understood, and you’re going to have smaller trials because there aren’t that many people with the disease. We need to figure out how to appropriately exercise that flexibility in regulation and make sure people have access, but have a high probability of getting products that work and have been adequately tested for safely. We also started a Rare Disease Cures Accelerator, which is enrolling people online in natural history studies to see what happens to them so we can better plan studies. We have Patient-Focused Drug Development meetings as a way to gather patients’ perspectives on their conditions and available therapies to treat those conditions. That is eye-opening, because what the doctor thinks about the disease may not be what the patient thinks about the disease. The patients are the ones taking the medicine, so we need to collect their opinions. Such approaches make it easier to study rare diseases and get new treatments.
Q: How do the challenges of drug research and development in the field of rare diseases differ from those associated with more prevalent diseases?
Dr. Woodcock: There is one advantage today for people with rare diseases. That is, when there is a known genetic mutation causing a disease, RNA interference and other gene therapy approaches can be used. There are challenges, though. Patients with rare disorders often don’t have a uniform disease course. They often have a multisystem impact, so they might have things wrong with their GI tract and/or skin, so it’s difficult to know what to measure. We’re trying to remedy this by gathering better natural history information on what happens to people. That is empowering for patients as well.
Q: In what practical ways can physicians become advocates for patients and their families who are navigating life with a rare disease?
Dr. Woodcock: I advise people to get involved in the association or advocacy group for their rare disease. It’s empowering. They can share stories and information with others who have been suffering from the disease. Also, they would get information about what trials might be available. As for physicians themselves, they have a bewildering variety of jobs they’re supposed to do, so it’s hard to be good in any one of them. People with rare disease often suffer terribly because they don’t get diagnosed for 10 years even though they have classic symptoms of a particular disorder. If physicians have never seen it or never heard of it, they may not know how to treat it. It’s a huge problem.
Q: Who inspires you most in your work today?
Dr. Woodcock: The dedication of the staff at the FDA is unbelievable. When you look at responses to the Federal Employee Viewpoint Survey administered by the Office of Personnel Management, FDA workers consistently express a strong sense of mission and dedication. It’s out of the park, really. They have worked night and day during this pandemic. I’m inspired by everyone who works at the FDA and their incredible dedication to their work.
Q: In what ways do you cope with the pressure that comes with your line of work? Do you have a favorite hobby or that activity that helps keep you grounded?
Dr. Woodcock: I’m an avid gardener, so I have a garden with vegetables, fruits, and flowers, including a large orchid collection. I’m also a hiker and a physical fitness buff, so I feel like there isn’t enough time in the day for all of my hobbies. Formal hiking trails near me are very crowded now, so I’ve been hiking around my neighborhood, taking long walks and going up and down hills quickly. Last November, I went hiking in New Zealand with my daughter. We hiked the Milford Track, which is about 33 miles long. It goes from an inland lake, over a mountain pass, and to the Pacific Ocean. It was fun, with unbelievable scenery.
Q: What novel treatment developments in rare disorders are you most excited about in the next 5 years?
Dr. Woodcock: I think gene therapy will come into its own. I think that could be a game-changer for people with genetic mutations causing rare diseases, and even cancer. We’ll see. It takes the technology a long time to mature. There are also gene-directed therapies such as RNA inhibition. We’ve already approved a couple of products like that for rare diseases, including treatments for the cardiomyopathy and neuropathy associated with ATTR amyloidosis. As our knowledge of biology continues to grow, I think more of these diseases will be amenable to interventions.
Q: In May of 2020 you were asked to temporarily step aside from your post as director of CDER to work on Operation Warp Speed. Please describe what your role is in this effort to accelerate COVID-19 treatments.
Dr. Woodcock: I’m the lead on therapeutics. Operation Warp Speed is mainly focused on developing vaccines for COVID-19. In the meantime, people who don’t respond to vaccines are going to need therapeutics, such as the elderly, or those who refuse to take vaccines, or those who are immunosuppressed and can’t mount a response to a vaccine. If we can develop those therapeutics now, that would be good to get that populous vaccinated. The team identified what we thought were the five highest priority agents to work on, and we’re testing them. We have identified many more in a priority list. We have five master protocols running for different times in the disease, such as when you’re an outpatient, when you’re an inpatient, or when you’re in the ICU. The work is stressful, because we need these treatments as soon as possible, but we have a great team working on this. I feel like I’m making a contribution in this role, because I know people in industry and in the National Institutes of Health. I try to bring everyone together and get things done.
*Correction, 10/22/20: An earlier version of this article misstated the name of the National Organization for Rare Disorders.
Surgeon general pushes for improved hypertension control
Roughly half of American adults have hypertension, and about 71% of these cases are uncontrolled, according to data from the American Heart Association.
If left uncontrolled, hypertension can increase risk for conditions including heart disease, stroke, kidney disease, pregnancy complications, and cognitive decline, surgeon general Vice Adm. Jerome M. Adams, MD, said in a teleconference on Oct. 7. Hispanic and Black individuals are disproportionately affected, he added.
“We cannot wait to deal with this epidemic of uncontrolled high blood pressure,” even in the midst of the ongoing COVID-19 pandemic, said Dr. Adams. “We know what works” to help control hypertension, he added, citing his own use of a blood pressure monitoring device at home.
The Department of Health & Human Services has issued a Call to Action to Control Hypertension based on the latest science and research.
Dr. Adams outlined three goals to improve hypertension control, starting with making it a national priority. The Call to Action supports increasing awareness of the health risks associated with hypertension, recognizing the economic impact, overcoming barriers to controlling hypertension, and promoting health equity.
“In 2020, disparities in the burden of disease – especially among minority populations – have been recognized during the COVID-19 pandemic. A growing body of evidence has shown that people with underlying health conditions, including cardiovascular disease, are at increased risk of worse outcomes related to COVID-19 infection,” according to the Call to Action.
A second goal is to build and sustain communities that support individuals in taking responsibility for their health and blood pressure control, Dr. Adams said. He cited the need to create places for safe physical activity, access to healthy food, and opportunities to connect to resources to support lifestyle changes.
Finally, clinicians should continue to use standardized treatment approaches and promote team-based care to maximize outcomes for patients, Dr. Adams said.
Success starts with making hypertension control a priority across the leadership team, regardless of the size, location, or demographic population at a health care setting, he said. Dr. Adams cited the Million Hearts 2022 program, an ongoing initiative to prevent 1 million heart attacks in the United States over 5 years, as a way that HHS is recognizing and rewarding success stories in hypertension control from across the country.
Empowering patients and equipping them to take charge of their hypertension essential to reducing the epidemic of high blood pressure, especially during the ongoing pandemic, Dr. Adams said. His message to clinicians to extend to patients is that it is safe to visit their doctors. Hospitals have worked to create a safe environment, however, patients can and should monitor their blood pressure regularly at home, using a self-measured blood pressure monitoring (SMBP) device, which may be covered by some insurers.
“I would encourage people to know their numbers,” and that 130/80 mm Hg is considered high and a risk factor for poor health outcomes, Dr. Adams said. Clinicians also should continue to support patients in lifestyle changes such as healthy eating and exercising regularly to help control high blood pressure.
The AHA expressed support for the surgeon general’s Call to Action. “Today’s call to action references updated hypertension guidelines the AHA and the American College of Cardiology issued in 2017 that apply the latest science to help clinicians work with patients to control their blood pressure,” the AHA said in a statement. The AHA also called on the Centers for Medicare & Medicaid Services and other insurance providers “to include coverage of SMBP devices for treatment and management of hypertension.”
The Call to Action was accompanied by a Viewpoint from Dr. Adams and Janet S. Wright, MD, also of the HHS, published in JAMA. Dr. Adams and Dr. Wright emphasized that the timing of the Call to Action recognizes that many of the same social factors that support or impede successful high blood pressure control are factors in worse outcomes from COVID-19 infections as well.
“When coupled with widespread implementation of best practices in clinical settings and empowering individuals to actively manage their blood pressure, acknowledging and addressing a community’s social conditions may generate sustained improvements in control of both hypertension and COVID-19,” they said.
Read and download the full Call to Action here, and read the Executive Summary at hhs.gov.
Roughly half of American adults have hypertension, and about 71% of these cases are uncontrolled, according to data from the American Heart Association.
If left uncontrolled, hypertension can increase risk for conditions including heart disease, stroke, kidney disease, pregnancy complications, and cognitive decline, surgeon general Vice Adm. Jerome M. Adams, MD, said in a teleconference on Oct. 7. Hispanic and Black individuals are disproportionately affected, he added.
“We cannot wait to deal with this epidemic of uncontrolled high blood pressure,” even in the midst of the ongoing COVID-19 pandemic, said Dr. Adams. “We know what works” to help control hypertension, he added, citing his own use of a blood pressure monitoring device at home.
The Department of Health & Human Services has issued a Call to Action to Control Hypertension based on the latest science and research.
Dr. Adams outlined three goals to improve hypertension control, starting with making it a national priority. The Call to Action supports increasing awareness of the health risks associated with hypertension, recognizing the economic impact, overcoming barriers to controlling hypertension, and promoting health equity.
“In 2020, disparities in the burden of disease – especially among minority populations – have been recognized during the COVID-19 pandemic. A growing body of evidence has shown that people with underlying health conditions, including cardiovascular disease, are at increased risk of worse outcomes related to COVID-19 infection,” according to the Call to Action.
A second goal is to build and sustain communities that support individuals in taking responsibility for their health and blood pressure control, Dr. Adams said. He cited the need to create places for safe physical activity, access to healthy food, and opportunities to connect to resources to support lifestyle changes.
Finally, clinicians should continue to use standardized treatment approaches and promote team-based care to maximize outcomes for patients, Dr. Adams said.
Success starts with making hypertension control a priority across the leadership team, regardless of the size, location, or demographic population at a health care setting, he said. Dr. Adams cited the Million Hearts 2022 program, an ongoing initiative to prevent 1 million heart attacks in the United States over 5 years, as a way that HHS is recognizing and rewarding success stories in hypertension control from across the country.
Empowering patients and equipping them to take charge of their hypertension essential to reducing the epidemic of high blood pressure, especially during the ongoing pandemic, Dr. Adams said. His message to clinicians to extend to patients is that it is safe to visit their doctors. Hospitals have worked to create a safe environment, however, patients can and should monitor their blood pressure regularly at home, using a self-measured blood pressure monitoring (SMBP) device, which may be covered by some insurers.
“I would encourage people to know their numbers,” and that 130/80 mm Hg is considered high and a risk factor for poor health outcomes, Dr. Adams said. Clinicians also should continue to support patients in lifestyle changes such as healthy eating and exercising regularly to help control high blood pressure.
The AHA expressed support for the surgeon general’s Call to Action. “Today’s call to action references updated hypertension guidelines the AHA and the American College of Cardiology issued in 2017 that apply the latest science to help clinicians work with patients to control their blood pressure,” the AHA said in a statement. The AHA also called on the Centers for Medicare & Medicaid Services and other insurance providers “to include coverage of SMBP devices for treatment and management of hypertension.”
The Call to Action was accompanied by a Viewpoint from Dr. Adams and Janet S. Wright, MD, also of the HHS, published in JAMA. Dr. Adams and Dr. Wright emphasized that the timing of the Call to Action recognizes that many of the same social factors that support or impede successful high blood pressure control are factors in worse outcomes from COVID-19 infections as well.
“When coupled with widespread implementation of best practices in clinical settings and empowering individuals to actively manage their blood pressure, acknowledging and addressing a community’s social conditions may generate sustained improvements in control of both hypertension and COVID-19,” they said.
Read and download the full Call to Action here, and read the Executive Summary at hhs.gov.
Roughly half of American adults have hypertension, and about 71% of these cases are uncontrolled, according to data from the American Heart Association.
If left uncontrolled, hypertension can increase risk for conditions including heart disease, stroke, kidney disease, pregnancy complications, and cognitive decline, surgeon general Vice Adm. Jerome M. Adams, MD, said in a teleconference on Oct. 7. Hispanic and Black individuals are disproportionately affected, he added.
“We cannot wait to deal with this epidemic of uncontrolled high blood pressure,” even in the midst of the ongoing COVID-19 pandemic, said Dr. Adams. “We know what works” to help control hypertension, he added, citing his own use of a blood pressure monitoring device at home.
The Department of Health & Human Services has issued a Call to Action to Control Hypertension based on the latest science and research.
Dr. Adams outlined three goals to improve hypertension control, starting with making it a national priority. The Call to Action supports increasing awareness of the health risks associated with hypertension, recognizing the economic impact, overcoming barriers to controlling hypertension, and promoting health equity.
“In 2020, disparities in the burden of disease – especially among minority populations – have been recognized during the COVID-19 pandemic. A growing body of evidence has shown that people with underlying health conditions, including cardiovascular disease, are at increased risk of worse outcomes related to COVID-19 infection,” according to the Call to Action.
A second goal is to build and sustain communities that support individuals in taking responsibility for their health and blood pressure control, Dr. Adams said. He cited the need to create places for safe physical activity, access to healthy food, and opportunities to connect to resources to support lifestyle changes.
Finally, clinicians should continue to use standardized treatment approaches and promote team-based care to maximize outcomes for patients, Dr. Adams said.
Success starts with making hypertension control a priority across the leadership team, regardless of the size, location, or demographic population at a health care setting, he said. Dr. Adams cited the Million Hearts 2022 program, an ongoing initiative to prevent 1 million heart attacks in the United States over 5 years, as a way that HHS is recognizing and rewarding success stories in hypertension control from across the country.
Empowering patients and equipping them to take charge of their hypertension essential to reducing the epidemic of high blood pressure, especially during the ongoing pandemic, Dr. Adams said. His message to clinicians to extend to patients is that it is safe to visit their doctors. Hospitals have worked to create a safe environment, however, patients can and should monitor their blood pressure regularly at home, using a self-measured blood pressure monitoring (SMBP) device, which may be covered by some insurers.
“I would encourage people to know their numbers,” and that 130/80 mm Hg is considered high and a risk factor for poor health outcomes, Dr. Adams said. Clinicians also should continue to support patients in lifestyle changes such as healthy eating and exercising regularly to help control high blood pressure.
The AHA expressed support for the surgeon general’s Call to Action. “Today’s call to action references updated hypertension guidelines the AHA and the American College of Cardiology issued in 2017 that apply the latest science to help clinicians work with patients to control their blood pressure,” the AHA said in a statement. The AHA also called on the Centers for Medicare & Medicaid Services and other insurance providers “to include coverage of SMBP devices for treatment and management of hypertension.”
The Call to Action was accompanied by a Viewpoint from Dr. Adams and Janet S. Wright, MD, also of the HHS, published in JAMA. Dr. Adams and Dr. Wright emphasized that the timing of the Call to Action recognizes that many of the same social factors that support or impede successful high blood pressure control are factors in worse outcomes from COVID-19 infections as well.
“When coupled with widespread implementation of best practices in clinical settings and empowering individuals to actively manage their blood pressure, acknowledging and addressing a community’s social conditions may generate sustained improvements in control of both hypertension and COVID-19,” they said.
Read and download the full Call to Action here, and read the Executive Summary at hhs.gov.
How ObGyns can best work with radiologists to optimize screening for patients with dense breasts
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.
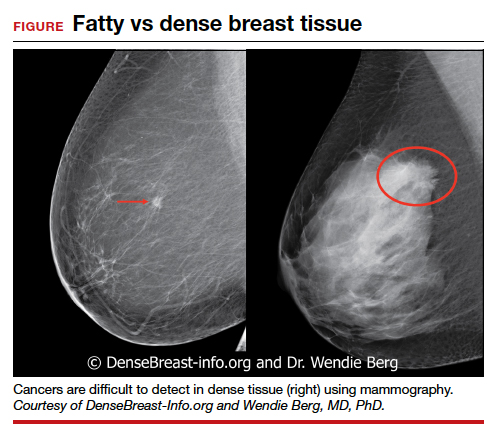
MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.

MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.

MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
Please stop using the adjective “elective” to describe the important health services ObGyns provide
During the April 2020 peak of patient admissions to our hospital caused by coronavirus disease 2019 (COVID-19), we severely limited the number of surgical procedures performed to conserve health system resources. During this stressful time, some administrators and physicians began categorizing operations for cancer as "elective" procedures that could be postponed for months. Personally, I think the use of elective to describe cancer surgery is not optimal, even during a pandemic. In reality, the surgeries for patients with cancer were being postponed to ensure that services were available for patients with severe and critical COVID-19 disease, not because the surgeries were "elective." The health system leaders were making the rational decision to prioritize the needs of patients with COVID-19 infections over the needs of patients with cancer. However, they were using an inappropriate description of the rationale for postponing the surgery for patients with cancer—an intellectual short-cut.
This experience prompted me to explore all the medical interventions commonly described as elective. Surprisingly, among medical specialists, obstetricians excel in using the adjective elective to describe our important work. For example, in the medical record we commonly use terms such as “elective induction of labor,” “elective cesarean delivery” (CD) and “elective termination of pregnancy.” I believe it would advance our field if obstetricians stopped using the term elective to describe the important health services we provide.
Stop using the term “elective induction of labor”
Ghartey and Macones recently advocated for all obstetricians to stop using the term elective when describing induction of labor.1 The ARRIVE trial (A Randomized Trial of Induction vs Expectant Management)2 demonstrated that, among nulliparous women at 39 weeks’ gestation, induction of labor resulted in a lower CD rate than expectant management (18.6% vs 22.2%, respectively; relative risk, 0.84; 95% confidence interval [CI], 0.76-0.93). These findings indicate that induction of labor is not elective because it provides a clear health benefit over the alternative of expectant management. Given current expert guidance, induction of labor prior to 39 weeks’ gestation must be based on an accepted medical indication and provide a health benefit; hence, these inductions are medically indicated. Similarly, since induction of labor at 39 weeks’ gestation also provides a clear health benefit it is also medically indicated and not “elective.” Ghartey and Macones conclude1:
"The words we choose to
describe medical interventions
matter. They send a message
to patients, physicians, nurses,
and hospital administrators.
When the term 'elective' is applied to a medical intervention,
it implies that it is not really
necessary. That is certainly not
the case when it comes to 39-
week nulliparous induction. The
ARRIVE trial provides grade A
(good and consistent) evidence
that labor induction provided
benefit with no harm to women
and their infants. These inductions are not 'elective'."
An alternative descriptor is “medically indicated” induction.
Continue to: Stop using the term “elective cesarean delivery”...
Stop using the term “elective cesarean delivery”
I recently searched PubMed for publications using the key words, “elective cesarean delivery,” and more than 7,000 publications were identified by the National Library of Medicine. “Elective cesarean delivery” is clearly an important term used by obstetrical authorities. What do we mean by elective CD?
At 39 weeks’ gestation, a low-risk nulliparous pregnant woman has a limited number of options:
- induction of labor
- expectant management awaiting the onset of labor
- scheduled CD before the onset of labor.
For a low-risk pregnant woman at 39 weeks’ gestation, the American College of Obstetricians and Gynecologists recommends vaginal delivery because it best balances the risks and benefits for the woman and newborn.3 When a low-risk nulliparous pregnant woman asks a clinician about a scheduled CD, we are trained to thoroughly explore the reasons for the woman’s request, including her intellectual, fact-based, concerns about labor and vaginal birth and her emotional reaction to the thought of a vaginal or cesarean birth. In this situation the clinician will provide information about the risks and benefits of vaginal versus CD. In the vast majority of situations, the pregnant woman will agree to attempting vaginal delivery. In one study of 458,767 births, only 0.2% of women choose a “maternal request cesarean delivery.”4
After thorough counseling, if a woman and her clinician jointly agree to schedule a primary CD it will be the result of hours of intensive discussion, not an imprudent and hasty decision. In this case, the delivery is best characterized as a “maternal request cesarean delivery,” not an “elective” CD.
Stop using the terms “elective termination of pregnancy” and “elective abortion”
Janiak and Goldberg have advocated for the elimination of the phrase elective abortion.5 They write5:
"Support for abortion varies
depending on the reason for
the abortion—whether it is
'elective' or 'indicated.' In the
case of abortion, these terms
generally differentiate between
women seeking abortion for
reasons of maternal or fetal
health (an 'indicated abortion')
defined in contrast to women
seeking abortion for other
reasons (an 'elective abortion').
We argue that such a distinction is impossible to operationalize in a just manner. The use
of the phrase 'elective abortion'
promotes the institutionalization of a false hierarchy of need
among abortion patients."
My experience is that pregnant women never seek an abortion based on whimsy. Most pregnant women who consider an abortion struggle greatly with the choice, using reason and judgment to arrive at their final decision. The choice to seek an abortion is always a difficult one, influenced by a constellation of hard facts that impact the woman’s life. Using the term elective to describe an abortion implies a moral judgment and stigmatizes the choice to have an abortion. Janiak and Goldberg conclude by recommending the elimination of the phrase 'elective abortion' in favor of the phrase “induced abortion.”5
Continue to: Time for change...
Time for change
Shockingly, in searching the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD10), the word elective is most commonly used in the context of health services provided to pregnant women, including: elective induction of labor (Z34.90), elective cesarean delivery (O82), elective termination of pregnancy (Z33.2), and elective fetal reduction (Z031.30X0). In ICD10, other specialties do not describe the scope of their health services with the adjective elective.
There are many definitions and interpretations of elective. The most benign use of the word in the context of surgery is to contrast procedures that can be scheduled in the future with those that need to be performed urgently. In this context elective only refers to the timing, not the medical necessity, of the procedure. By contrast, describing a procedure as elective may signal that it is not medically necessary and is being performed based on the capricious preference of the patient or physician. Given the confusion and misunderstanding that may be caused by describing our important health services as “elective,” I hope that we can permanently sunset use of the term. ●
- Ghartey J, Macones GA. 39-week nulliparous inductions are not elective. Am J Obstet Gynecol. 2020;222:519-520.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- ACOG Committee Opinion No 761: cesarean delivery on maternal request. Obstet Gynecol. 2019;133.e73-e77.
- Gossman GL, Joesch JM, Tanfer K. Trends in maternal request cesarean delivery from 1991 to 2004. Obstet Gynecol. 2006;108:1506-1516.
- Janiak E, Goldberg AB. Eliminating the phrase “elective abortion”: why language matters. Contraception. 2016;93:89-92.
During the April 2020 peak of patient admissions to our hospital caused by coronavirus disease 2019 (COVID-19), we severely limited the number of surgical procedures performed to conserve health system resources. During this stressful time, some administrators and physicians began categorizing operations for cancer as "elective" procedures that could be postponed for months. Personally, I think the use of elective to describe cancer surgery is not optimal, even during a pandemic. In reality, the surgeries for patients with cancer were being postponed to ensure that services were available for patients with severe and critical COVID-19 disease, not because the surgeries were "elective." The health system leaders were making the rational decision to prioritize the needs of patients with COVID-19 infections over the needs of patients with cancer. However, they were using an inappropriate description of the rationale for postponing the surgery for patients with cancer—an intellectual short-cut.
This experience prompted me to explore all the medical interventions commonly described as elective. Surprisingly, among medical specialists, obstetricians excel in using the adjective elective to describe our important work. For example, in the medical record we commonly use terms such as “elective induction of labor,” “elective cesarean delivery” (CD) and “elective termination of pregnancy.” I believe it would advance our field if obstetricians stopped using the term elective to describe the important health services we provide.
Stop using the term “elective induction of labor”
Ghartey and Macones recently advocated for all obstetricians to stop using the term elective when describing induction of labor.1 The ARRIVE trial (A Randomized Trial of Induction vs Expectant Management)2 demonstrated that, among nulliparous women at 39 weeks’ gestation, induction of labor resulted in a lower CD rate than expectant management (18.6% vs 22.2%, respectively; relative risk, 0.84; 95% confidence interval [CI], 0.76-0.93). These findings indicate that induction of labor is not elective because it provides a clear health benefit over the alternative of expectant management. Given current expert guidance, induction of labor prior to 39 weeks’ gestation must be based on an accepted medical indication and provide a health benefit; hence, these inductions are medically indicated. Similarly, since induction of labor at 39 weeks’ gestation also provides a clear health benefit it is also medically indicated and not “elective.” Ghartey and Macones conclude1:
"The words we choose to
describe medical interventions
matter. They send a message
to patients, physicians, nurses,
and hospital administrators.
When the term 'elective' is applied to a medical intervention,
it implies that it is not really
necessary. That is certainly not
the case when it comes to 39-
week nulliparous induction. The
ARRIVE trial provides grade A
(good and consistent) evidence
that labor induction provided
benefit with no harm to women
and their infants. These inductions are not 'elective'."
An alternative descriptor is “medically indicated” induction.
Continue to: Stop using the term “elective cesarean delivery”...
Stop using the term “elective cesarean delivery”
I recently searched PubMed for publications using the key words, “elective cesarean delivery,” and more than 7,000 publications were identified by the National Library of Medicine. “Elective cesarean delivery” is clearly an important term used by obstetrical authorities. What do we mean by elective CD?
At 39 weeks’ gestation, a low-risk nulliparous pregnant woman has a limited number of options:
- induction of labor
- expectant management awaiting the onset of labor
- scheduled CD before the onset of labor.
For a low-risk pregnant woman at 39 weeks’ gestation, the American College of Obstetricians and Gynecologists recommends vaginal delivery because it best balances the risks and benefits for the woman and newborn.3 When a low-risk nulliparous pregnant woman asks a clinician about a scheduled CD, we are trained to thoroughly explore the reasons for the woman’s request, including her intellectual, fact-based, concerns about labor and vaginal birth and her emotional reaction to the thought of a vaginal or cesarean birth. In this situation the clinician will provide information about the risks and benefits of vaginal versus CD. In the vast majority of situations, the pregnant woman will agree to attempting vaginal delivery. In one study of 458,767 births, only 0.2% of women choose a “maternal request cesarean delivery.”4
After thorough counseling, if a woman and her clinician jointly agree to schedule a primary CD it will be the result of hours of intensive discussion, not an imprudent and hasty decision. In this case, the delivery is best characterized as a “maternal request cesarean delivery,” not an “elective” CD.
Stop using the terms “elective termination of pregnancy” and “elective abortion”
Janiak and Goldberg have advocated for the elimination of the phrase elective abortion.5 They write5:
"Support for abortion varies
depending on the reason for
the abortion—whether it is
'elective' or 'indicated.' In the
case of abortion, these terms
generally differentiate between
women seeking abortion for
reasons of maternal or fetal
health (an 'indicated abortion')
defined in contrast to women
seeking abortion for other
reasons (an 'elective abortion').
We argue that such a distinction is impossible to operationalize in a just manner. The use
of the phrase 'elective abortion'
promotes the institutionalization of a false hierarchy of need
among abortion patients."
My experience is that pregnant women never seek an abortion based on whimsy. Most pregnant women who consider an abortion struggle greatly with the choice, using reason and judgment to arrive at their final decision. The choice to seek an abortion is always a difficult one, influenced by a constellation of hard facts that impact the woman’s life. Using the term elective to describe an abortion implies a moral judgment and stigmatizes the choice to have an abortion. Janiak and Goldberg conclude by recommending the elimination of the phrase 'elective abortion' in favor of the phrase “induced abortion.”5
Continue to: Time for change...
Time for change
Shockingly, in searching the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD10), the word elective is most commonly used in the context of health services provided to pregnant women, including: elective induction of labor (Z34.90), elective cesarean delivery (O82), elective termination of pregnancy (Z33.2), and elective fetal reduction (Z031.30X0). In ICD10, other specialties do not describe the scope of their health services with the adjective elective.
There are many definitions and interpretations of elective. The most benign use of the word in the context of surgery is to contrast procedures that can be scheduled in the future with those that need to be performed urgently. In this context elective only refers to the timing, not the medical necessity, of the procedure. By contrast, describing a procedure as elective may signal that it is not medically necessary and is being performed based on the capricious preference of the patient or physician. Given the confusion and misunderstanding that may be caused by describing our important health services as “elective,” I hope that we can permanently sunset use of the term. ●
During the April 2020 peak of patient admissions to our hospital caused by coronavirus disease 2019 (COVID-19), we severely limited the number of surgical procedures performed to conserve health system resources. During this stressful time, some administrators and physicians began categorizing operations for cancer as "elective" procedures that could be postponed for months. Personally, I think the use of elective to describe cancer surgery is not optimal, even during a pandemic. In reality, the surgeries for patients with cancer were being postponed to ensure that services were available for patients with severe and critical COVID-19 disease, not because the surgeries were "elective." The health system leaders were making the rational decision to prioritize the needs of patients with COVID-19 infections over the needs of patients with cancer. However, they were using an inappropriate description of the rationale for postponing the surgery for patients with cancer—an intellectual short-cut.
This experience prompted me to explore all the medical interventions commonly described as elective. Surprisingly, among medical specialists, obstetricians excel in using the adjective elective to describe our important work. For example, in the medical record we commonly use terms such as “elective induction of labor,” “elective cesarean delivery” (CD) and “elective termination of pregnancy.” I believe it would advance our field if obstetricians stopped using the term elective to describe the important health services we provide.
Stop using the term “elective induction of labor”
Ghartey and Macones recently advocated for all obstetricians to stop using the term elective when describing induction of labor.1 The ARRIVE trial (A Randomized Trial of Induction vs Expectant Management)2 demonstrated that, among nulliparous women at 39 weeks’ gestation, induction of labor resulted in a lower CD rate than expectant management (18.6% vs 22.2%, respectively; relative risk, 0.84; 95% confidence interval [CI], 0.76-0.93). These findings indicate that induction of labor is not elective because it provides a clear health benefit over the alternative of expectant management. Given current expert guidance, induction of labor prior to 39 weeks’ gestation must be based on an accepted medical indication and provide a health benefit; hence, these inductions are medically indicated. Similarly, since induction of labor at 39 weeks’ gestation also provides a clear health benefit it is also medically indicated and not “elective.” Ghartey and Macones conclude1:
"The words we choose to
describe medical interventions
matter. They send a message
to patients, physicians, nurses,
and hospital administrators.
When the term 'elective' is applied to a medical intervention,
it implies that it is not really
necessary. That is certainly not
the case when it comes to 39-
week nulliparous induction. The
ARRIVE trial provides grade A
(good and consistent) evidence
that labor induction provided
benefit with no harm to women
and their infants. These inductions are not 'elective'."
An alternative descriptor is “medically indicated” induction.
Continue to: Stop using the term “elective cesarean delivery”...
Stop using the term “elective cesarean delivery”
I recently searched PubMed for publications using the key words, “elective cesarean delivery,” and more than 7,000 publications were identified by the National Library of Medicine. “Elective cesarean delivery” is clearly an important term used by obstetrical authorities. What do we mean by elective CD?
At 39 weeks’ gestation, a low-risk nulliparous pregnant woman has a limited number of options:
- induction of labor
- expectant management awaiting the onset of labor
- scheduled CD before the onset of labor.
For a low-risk pregnant woman at 39 weeks’ gestation, the American College of Obstetricians and Gynecologists recommends vaginal delivery because it best balances the risks and benefits for the woman and newborn.3 When a low-risk nulliparous pregnant woman asks a clinician about a scheduled CD, we are trained to thoroughly explore the reasons for the woman’s request, including her intellectual, fact-based, concerns about labor and vaginal birth and her emotional reaction to the thought of a vaginal or cesarean birth. In this situation the clinician will provide information about the risks and benefits of vaginal versus CD. In the vast majority of situations, the pregnant woman will agree to attempting vaginal delivery. In one study of 458,767 births, only 0.2% of women choose a “maternal request cesarean delivery.”4
After thorough counseling, if a woman and her clinician jointly agree to schedule a primary CD it will be the result of hours of intensive discussion, not an imprudent and hasty decision. In this case, the delivery is best characterized as a “maternal request cesarean delivery,” not an “elective” CD.
Stop using the terms “elective termination of pregnancy” and “elective abortion”
Janiak and Goldberg have advocated for the elimination of the phrase elective abortion.5 They write5:
"Support for abortion varies
depending on the reason for
the abortion—whether it is
'elective' or 'indicated.' In the
case of abortion, these terms
generally differentiate between
women seeking abortion for
reasons of maternal or fetal
health (an 'indicated abortion')
defined in contrast to women
seeking abortion for other
reasons (an 'elective abortion').
We argue that such a distinction is impossible to operationalize in a just manner. The use
of the phrase 'elective abortion'
promotes the institutionalization of a false hierarchy of need
among abortion patients."
My experience is that pregnant women never seek an abortion based on whimsy. Most pregnant women who consider an abortion struggle greatly with the choice, using reason and judgment to arrive at their final decision. The choice to seek an abortion is always a difficult one, influenced by a constellation of hard facts that impact the woman’s life. Using the term elective to describe an abortion implies a moral judgment and stigmatizes the choice to have an abortion. Janiak and Goldberg conclude by recommending the elimination of the phrase 'elective abortion' in favor of the phrase “induced abortion.”5
Continue to: Time for change...
Time for change
Shockingly, in searching the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD10), the word elective is most commonly used in the context of health services provided to pregnant women, including: elective induction of labor (Z34.90), elective cesarean delivery (O82), elective termination of pregnancy (Z33.2), and elective fetal reduction (Z031.30X0). In ICD10, other specialties do not describe the scope of their health services with the adjective elective.
There are many definitions and interpretations of elective. The most benign use of the word in the context of surgery is to contrast procedures that can be scheduled in the future with those that need to be performed urgently. In this context elective only refers to the timing, not the medical necessity, of the procedure. By contrast, describing a procedure as elective may signal that it is not medically necessary and is being performed based on the capricious preference of the patient or physician. Given the confusion and misunderstanding that may be caused by describing our important health services as “elective,” I hope that we can permanently sunset use of the term. ●
- Ghartey J, Macones GA. 39-week nulliparous inductions are not elective. Am J Obstet Gynecol. 2020;222:519-520.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- ACOG Committee Opinion No 761: cesarean delivery on maternal request. Obstet Gynecol. 2019;133.e73-e77.
- Gossman GL, Joesch JM, Tanfer K. Trends in maternal request cesarean delivery from 1991 to 2004. Obstet Gynecol. 2006;108:1506-1516.
- Janiak E, Goldberg AB. Eliminating the phrase “elective abortion”: why language matters. Contraception. 2016;93:89-92.
- Ghartey J, Macones GA. 39-week nulliparous inductions are not elective. Am J Obstet Gynecol. 2020;222:519-520.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- ACOG Committee Opinion No 761: cesarean delivery on maternal request. Obstet Gynecol. 2019;133.e73-e77.
- Gossman GL, Joesch JM, Tanfer K. Trends in maternal request cesarean delivery from 1991 to 2004. Obstet Gynecol. 2006;108:1506-1516.
- Janiak E, Goldberg AB. Eliminating the phrase “elective abortion”: why language matters. Contraception. 2016;93:89-92.