User login
AMA takes on vaccine misinformation, physician vaccines, racism
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
Trump could clean house at health agencies
Others may soon depart voluntarily. Politico reported in late October that more than two dozen political appointees had already left the U.S. Department Health and Human Services (HHS) since the start of the COVID-19 pandemic in February and that potentially dozens of the more than 100 in the department would leave if Trump was not reelected.
Trump hasn’t conceded, he is challenging the election results, and he has already fired his Defense Secretary, Mark Esper.
Among those possibly in Trump’s sights: HHS Secretary Alex Azar, US Food and Drug Administration (FDA) Commissioner Stephen Hahn, MD, Centers for Disease Control and Prevention (CDC) Director Robert Redfield, MD, and White House Coronavirus Task Force member Anthony Fauci, MD, who is also the director of the National Institutes of Allergy and Infectious Diseases.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services (CMS), is likely safe. According to Politico, Verma is expected to leave on her own terms.
Azar has had a long run as a Trump appointee. He took office in January 2018 and has been a staunch loyalist. But he’s frequently been the butt of grousing by Trump for not doing enough to help lower drug prices and for his handling of the coronavirus pandemic. Azar was initially in charge of the Trump virus effort but was quickly replaced by Vice President Mike Pence.
It was widely reported in late April that Trump was considering firing Azar, but the president called that “fake news” in a tweet.
Azar has complained about Hahn, who was confirmed in December 2019. According to Politico, Azar was looking into how to remove Hahn as commissioner because of the FDA’s battle with the White House over standards for emergency use authorization of a coronavirus vaccine.
In addition, Trump was infuriated by the agency’s insistence that it stick to the highest bar for an emergency approval. “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd,” Trump tweeted at Hahn.
Fauci on the firing line?
Most of the president’s ire has been directed at Fauci. As far back as April, Trump retweeted a call for Fauci’s firing. Twitter removed the original tweet but kept Trump’s comments on the original tweet.
The president has frequently questioned Fauci’s advice, sidelined him from task force meetings, and infrequently met with him. Trump called Fauci a “disaster” during a call with supporters in October, and then, at a campaign rally in November, intimated that he would fire the scientist after the election, according to The Washington Post.
But such a firing cannot be easily done. Some have speculated that Trump could pressure Fauci’s boss, Francis Collins, MD, PhD — the director of the National Institutes of Health (NIH), who is a political appointee — to get rid of him. But Collins would have to come up with a reason to fire Fauci. Because he is not a political appointee, Fauci is afforded a raft of protections given to civil service employees of the federal government.
To demote or fire Fauci, Collins would have to give him 30 days’ notice unless there’s a belief that he committed a crime. Fauci would have at least a week to offer evidence and affidavits in support of his service.
He’d also be entitled to legal representation, a written decision, and the specific reasons for the action being taken quickly. He could also request a hearing, and he’d be able to appeal any action to the Merit Systems Protection Board. The process could take months, if not years.
In late October, Trump issued an executive order that would reclassify certain federal employees so that they wouldn’t have such protections. But agencies have until mid-January to come up with lists of such workers, according to Government Executive.
Collins has been with NIH since 1993, when he headed the Human Genome Project and the National Human Genome Research Institute. Politico has speculated that Collins, 70, might retire if Trump was reelected. It’s unclear what he’ll do now.
Redfield, who has taken heat for his leadership from many in public health — and was asked in October to stand up to Trump by former CDC Director William H. Foege, MD — has been openly contradicted by the president on more than one occasion, according to The New York Times.
In September, The Hill reported that Trump told reporters that he’d chastised Redfield by phone soon after Redfield had told a Senate committee that a coronavirus vaccine would not be available until mid-2021.
This article first appeared on Medscape.com.
Others may soon depart voluntarily. Politico reported in late October that more than two dozen political appointees had already left the U.S. Department Health and Human Services (HHS) since the start of the COVID-19 pandemic in February and that potentially dozens of the more than 100 in the department would leave if Trump was not reelected.
Trump hasn’t conceded, he is challenging the election results, and he has already fired his Defense Secretary, Mark Esper.
Among those possibly in Trump’s sights: HHS Secretary Alex Azar, US Food and Drug Administration (FDA) Commissioner Stephen Hahn, MD, Centers for Disease Control and Prevention (CDC) Director Robert Redfield, MD, and White House Coronavirus Task Force member Anthony Fauci, MD, who is also the director of the National Institutes of Allergy and Infectious Diseases.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services (CMS), is likely safe. According to Politico, Verma is expected to leave on her own terms.
Azar has had a long run as a Trump appointee. He took office in January 2018 and has been a staunch loyalist. But he’s frequently been the butt of grousing by Trump for not doing enough to help lower drug prices and for his handling of the coronavirus pandemic. Azar was initially in charge of the Trump virus effort but was quickly replaced by Vice President Mike Pence.
It was widely reported in late April that Trump was considering firing Azar, but the president called that “fake news” in a tweet.
Azar has complained about Hahn, who was confirmed in December 2019. According to Politico, Azar was looking into how to remove Hahn as commissioner because of the FDA’s battle with the White House over standards for emergency use authorization of a coronavirus vaccine.
In addition, Trump was infuriated by the agency’s insistence that it stick to the highest bar for an emergency approval. “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd,” Trump tweeted at Hahn.
Fauci on the firing line?
Most of the president’s ire has been directed at Fauci. As far back as April, Trump retweeted a call for Fauci’s firing. Twitter removed the original tweet but kept Trump’s comments on the original tweet.
The president has frequently questioned Fauci’s advice, sidelined him from task force meetings, and infrequently met with him. Trump called Fauci a “disaster” during a call with supporters in October, and then, at a campaign rally in November, intimated that he would fire the scientist after the election, according to The Washington Post.
But such a firing cannot be easily done. Some have speculated that Trump could pressure Fauci’s boss, Francis Collins, MD, PhD — the director of the National Institutes of Health (NIH), who is a political appointee — to get rid of him. But Collins would have to come up with a reason to fire Fauci. Because he is not a political appointee, Fauci is afforded a raft of protections given to civil service employees of the federal government.
To demote or fire Fauci, Collins would have to give him 30 days’ notice unless there’s a belief that he committed a crime. Fauci would have at least a week to offer evidence and affidavits in support of his service.
He’d also be entitled to legal representation, a written decision, and the specific reasons for the action being taken quickly. He could also request a hearing, and he’d be able to appeal any action to the Merit Systems Protection Board. The process could take months, if not years.
In late October, Trump issued an executive order that would reclassify certain federal employees so that they wouldn’t have such protections. But agencies have until mid-January to come up with lists of such workers, according to Government Executive.
Collins has been with NIH since 1993, when he headed the Human Genome Project and the National Human Genome Research Institute. Politico has speculated that Collins, 70, might retire if Trump was reelected. It’s unclear what he’ll do now.
Redfield, who has taken heat for his leadership from many in public health — and was asked in October to stand up to Trump by former CDC Director William H. Foege, MD — has been openly contradicted by the president on more than one occasion, according to The New York Times.
In September, The Hill reported that Trump told reporters that he’d chastised Redfield by phone soon after Redfield had told a Senate committee that a coronavirus vaccine would not be available until mid-2021.
This article first appeared on Medscape.com.
Others may soon depart voluntarily. Politico reported in late October that more than two dozen political appointees had already left the U.S. Department Health and Human Services (HHS) since the start of the COVID-19 pandemic in February and that potentially dozens of the more than 100 in the department would leave if Trump was not reelected.
Trump hasn’t conceded, he is challenging the election results, and he has already fired his Defense Secretary, Mark Esper.
Among those possibly in Trump’s sights: HHS Secretary Alex Azar, US Food and Drug Administration (FDA) Commissioner Stephen Hahn, MD, Centers for Disease Control and Prevention (CDC) Director Robert Redfield, MD, and White House Coronavirus Task Force member Anthony Fauci, MD, who is also the director of the National Institutes of Allergy and Infectious Diseases.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services (CMS), is likely safe. According to Politico, Verma is expected to leave on her own terms.
Azar has had a long run as a Trump appointee. He took office in January 2018 and has been a staunch loyalist. But he’s frequently been the butt of grousing by Trump for not doing enough to help lower drug prices and for his handling of the coronavirus pandemic. Azar was initially in charge of the Trump virus effort but was quickly replaced by Vice President Mike Pence.
It was widely reported in late April that Trump was considering firing Azar, but the president called that “fake news” in a tweet.
Azar has complained about Hahn, who was confirmed in December 2019. According to Politico, Azar was looking into how to remove Hahn as commissioner because of the FDA’s battle with the White House over standards for emergency use authorization of a coronavirus vaccine.
In addition, Trump was infuriated by the agency’s insistence that it stick to the highest bar for an emergency approval. “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd,” Trump tweeted at Hahn.
Fauci on the firing line?
Most of the president’s ire has been directed at Fauci. As far back as April, Trump retweeted a call for Fauci’s firing. Twitter removed the original tweet but kept Trump’s comments on the original tweet.
The president has frequently questioned Fauci’s advice, sidelined him from task force meetings, and infrequently met with him. Trump called Fauci a “disaster” during a call with supporters in October, and then, at a campaign rally in November, intimated that he would fire the scientist after the election, according to The Washington Post.
But such a firing cannot be easily done. Some have speculated that Trump could pressure Fauci’s boss, Francis Collins, MD, PhD — the director of the National Institutes of Health (NIH), who is a political appointee — to get rid of him. But Collins would have to come up with a reason to fire Fauci. Because he is not a political appointee, Fauci is afforded a raft of protections given to civil service employees of the federal government.
To demote or fire Fauci, Collins would have to give him 30 days’ notice unless there’s a belief that he committed a crime. Fauci would have at least a week to offer evidence and affidavits in support of his service.
He’d also be entitled to legal representation, a written decision, and the specific reasons for the action being taken quickly. He could also request a hearing, and he’d be able to appeal any action to the Merit Systems Protection Board. The process could take months, if not years.
In late October, Trump issued an executive order that would reclassify certain federal employees so that they wouldn’t have such protections. But agencies have until mid-January to come up with lists of such workers, according to Government Executive.
Collins has been with NIH since 1993, when he headed the Human Genome Project and the National Human Genome Research Institute. Politico has speculated that Collins, 70, might retire if Trump was reelected. It’s unclear what he’ll do now.
Redfield, who has taken heat for his leadership from many in public health — and was asked in October to stand up to Trump by former CDC Director William H. Foege, MD — has been openly contradicted by the president on more than one occasion, according to The New York Times.
In September, The Hill reported that Trump told reporters that he’d chastised Redfield by phone soon after Redfield had told a Senate committee that a coronavirus vaccine would not be available until mid-2021.
This article first appeared on Medscape.com.
FDA grants emergency use authorization to Lilly’s antibody COVID-19 therapy
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
Template Design and Analysis: Integrating Informatics Solutions to Improve Clinical Documentation
Standardized template design is a useful tool to improve clinical documentation and reliable reporting of health care outcomes when constructed with clear objectives and with collaboration of key stakeholders. A standardized template should not only capture accurate diagnostic information, but also inform quality improvement (QI) measures and best practices.
Kang and colleagues showed that a correlation exists between organizational satisfaction and improved quality outcomes.1 A new initiative should have a well-defined purpose reinforced by collaborative workgroups and engaged employees who understand their clinical care role with electronic health record (EHR) modifications.
Several studies have shown how the usefulness of templates achieve multipurpose goals, such as accurate documentation and improved care. Valluru and colleagues showed a significant increase in vaccination rates for patients with inflammatory bowel disease after implementing a standardized template.2 By using a standardized template, Thaker and colleagues showed improved documentation regarding obesity and increased nutritional and physical activity counseling.3 Furthermore, Grogan and colleagues showed that templates are useful for house staff education on International Classification of Diseases (ICD) terminology and demonstrated improved documentation in the postintervention group.4,5
This article discusses the US Department of Veterans Affairs (VA) North Florida/South Georgia Veterans Health System (NF/SGVHS) integrated informatics solutions within template design in the Veterans Health Administration (VHA) EHR system that was associated with an increase in its case severity index (CSI) through improved clinical documentation capture.
Methods
According to policy activities that constitute research at NF/SGVHS, institutional review board approval was not required as this work met the criteria for operational improvement activities exempt from ethics review.
NF/SGVHS includes 2 hospitals: Malcom Randall VA Medical Center (MRVAMC) in Gainesville, Florida, and Lake City VA Medical Center (LCVAMC) in Lake City, Florida. MRVAMC is a large, 1a, academic VA facility composed of rotating residents and fellows and includes multiple specialty care services. LCVAMC is a smaller, nonteaching facility.
Template Design Impact
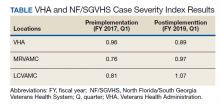
CSI is a risk-adjusted formula developed by the Inpatient Evaluation Center within VHA. CSI is incorporated into the VHA quality metrics reporting system, Strategic Analytics for Improvement and Learning (SAIL). CSI risk-adjusts metrics such as length of stay and mortality before releasing SAIL reports. CSI is calculated separately for acute level of care (LOC) and for the intensive care unit (ICU). In fiscal year (FY) 2017, acute LOC preimplementation data for CSI at NF/SGVHS were 0.76 for MRVAMC and 0.81 for LCVAMC, which was significantly below the national VHA average of 0.96 (Table).
A below-average CSI conveys a less complicated case mix compared with most other VA facilities. Although smaller VA facilities may have a less complicated case mix, it is unusual for large, tertiary care 1a VA facilities to have a low CSI. This low CSI is usually due to inadequate documentation, which affects not only risk-adjusted quality metrics outcomes, but also potential reimbursement.6
An interdisciplinary team composed of attendings, residents, and a clinical document improvement specialist identified the below-average acute LOC CSI for MRVAMC and LCVAMC compared with that of the national VHA average. Further analysis by chart reviews showed inconsistencies with standardized documentation despite prior health care provider education on ICD terminology and specific groups of common comorbidities analyzed in administrative data reviews for risk-adjustment purposes, known as Elixhauser comorbidities.5,7
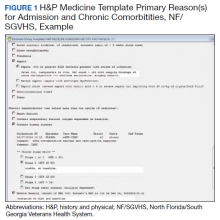
A chart review showed lack of clarity regarding primary reason(s) for admission and chronic comorbidities within NF/SGVHS. Using Pareto chart analysis, the template team designed a standardized history and physical (H&P) medicine template based on NF/SGVHS common medicine admissions (Figure 1). A Pareto chart is a valuable QI tool that assists with identifying majority contributors to a problem(s) being analyzed when evaluating a large set of data points. Subsequently, this tool helps focus direction on QI efforts.8
The template had the usual H&P elements not shown (eg, chief complaint, history of present illness, etc), and highlights the assessment/plan section containing primary reason(s) for admission and chronic comorbidities (Figure 1). The complete assessment and plan section on the template can be found in the Appendix.
To simplify the template interface, only single clicks were required to expand diagnostic and chronic comorbidity checkboxes. Subcategories then appeared to select diagnosis and chronic comorbidities along with free text for additional documentation.
In addition, data objects were created within the template that permitted the ability to retrieve information from the VHA EHR and insert specific data points of interest in the template; for example, body mass index to assess degree of obesity and estimated glomerular filtration rate to determine the stage of chronic kidney disease. This allowed users to easily reference data in one template in lieu of searching for data in multiple places in the EHR.9
Results
The standardized H&P medicine template was implemented at MRVAMC and LCVAMC in June 2018 (the final month of the third quarter of FY 2018). As clinical providers throughout NF/SGVHS used the standardized template, acute LOC postimplementation data for CSI significantly improved. Although the national VHA average slightly decreased from 0.96 in the first quarter of FY 2017 to 0.89, in the first quarter of FY 2019, MRVAMC acute LOC CSI improved from 0.76 to 0.97, and LCVAMC acute LOC CSI improved from 0.81 to 1.07 during the same period.
In addition, compliance also was monitored within MRVAMC and LCVAMC for about 1 year after standardized H&P medicine template implementation. Compliance was determined by how often the standardized H&P medicine template was used for inpatient medicine admissions to the acute care wards vs other H&P notes used (such as personalized templates).
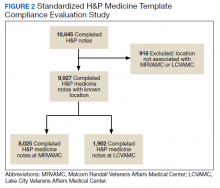
Methodology for compliance analysis included acquisition of completed H&P medicine notes from June 18, 2018 to June 30, 2019, within the VHA Veterans Information Systems and Technology Architecture (VistA) clinical and business information system using the search strings: “H&P admission history and physical” and “history of present illness.”10
A review identified 10,845 completed medicine H&P notes. Nine hundred eighteen notes were excluded as their search function yielded a location not corresponding to MRVAMC or LCVAMC. Of the 9,927 notes remaining, 8,025 of these were completed medicine H&P notes at MRVAMC and 1,902 were completed medicine H&P notes at LCVAMC (Figure 2).
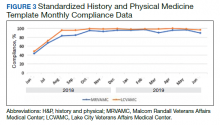
From June 18, 2018 to June 30, 2019 at MRVAMC, compliance was reviewed monthly for the 8,025 completed H&P medicine notes. Of the completed H&P medicine notes, the standardized H&P medicine template was used 43.2% in June 2018. By June 2019, MRVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use at 89.9% (Figure 3). Total average compliance from June 18, 2018 to June 30, 2019, was 88.4%, which doubled compliance from the initial introduction of the standardized H&P medicine template.
Compliance was reviewed monthly for the 1,902 completed H&P medicine notes from June 18, 2018 to June 30, 2019, at LCVAMC. Of the completed H&P medicine notes, the standardized template was used 48.2% of the time in June 2018. By June 2019, LCVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use, which increased to 96.9%. Total average compliance from June 18, 2018 to June 30, 2019, was 93.8%, which was almost double the baseline compliance rate.
Discussion
Template design with clear objectives, strategic collaboration, and integrated informatics solutions has the potential to increase accuracy of documentation. As shown, the NF/SGVHS template design was associated with significant improvement in acute LOC CSI for both MRVAMC and LCVAMC due to more accurate documentation using the standardized H&P medicine template.
Numerous factors contributed to the success of this template design. First, a clear vision for application of the template was communicated with key stakeholders. In addition, the template design team was focused on specific goals rather than a one size fits all approach, which was crucial for sustainable execution. Although interdisciplinary teamwork has the potential to result in innovative practices, large multidisciplinary teams also may have difficulty establishing a shared vision that can result in barriers to achieving project goals.
Balancing standardization and customization was essential for user buy-in. As noted by Gardner and Pearce, inviting clinical providers to participate in template design and allowing for customization has the potential to increase acceptance and use of templates.11 Although the original design for the standardized H&P medicine template started with the medicine service at NF/SGVHS, the design framework is applicable to numerous services where various clinical care elements can be customized.
Explaining the informatics tools built into the template allowed clinicians to see opportunities to improve clinical documentation and the impact it has on reporting health care outcomes. When improvement work involves integrating clinical care delivery and administrative expectations, it is essential that health care systems understand and strategically execute project initiatives at this critical juncture.
Finally, incorporation of a sustainability plan when process improvement strategies are implemented is vital. In addition to collaboration with the clinical providers during design and implementation of the standardized template, leadership buy-in was key. Compliance with standardized H&P medicine template use was monitored monthly and reviewed by the NF/SGVHS Chief of Staff.
As noted, LCVAMC postimplementation acute LOC CSI was higher than that of MRVAMC despite being a smaller facility. This might be due to the MRVAMC designation as a teaching institution. Medicine is the only inpatient service at LCVAMC staffed by hospitalists with limited specialists available for consultation, whereas MRVAMC is a tertiary care teaching facility with numerous inpatient services and subspecialties. As LCVAMC has more continuity, house staff rotating at MRVAMC require continued training/education on new templates and process changes.
Limitations
Although standardized template design was successful at NF/SGVHS, limitations should be noted. Our clinical documentation improvement (CDI) program also was expanded about the same time as the new templates were released. The expansion of the CDI program in addition to new template design likely had a synergistic effect on acute LOC CSI.
CSI is a complex, risk-adjusted model that includes numerous factors, including but not limited to diagnosis and comorbid conditions. Other factors include age, marital status, procedures, source of admission, specific laboratory values, medical or surgical diagnosis-related group, intensive care unit stays, and immunosuppressive status. CSI also includes operative and nonoperative components that average into an overall CSI. As the majority of CSI is composed of nonoperative constituents within NF/SGVHS, we do not believe this had any substantial impact on reporting of CSI improvements.
In addition, template entry into VHA EHR requires a location selection (such as a clinic name or ward name following an inpatient admission). Of the 10,845 completed H&P medicine notes identified in VistA, 918 notes were excluded from analysis as their search function yielded a location not corresponding to MRVAMC or LCVAMC. For the 918 notes excluded, we believe this was likely due to user error where locations not related to MRVAMC or LCVAMC were selected during standardized H&P medicine template entry.
Conclusions
After the NF/SGVHS implementation of a uniquely designed template embedded with informatics solutions within the VHA EHR, the CSI increased due to more accurate documentation.
Next steps include determining the impact of the NF/SGVHS template design on potential reimbursement and expanding template design into the outpatient setting where there are additional opportunities to improve clinical documentation and reliable reporting of health care outcomes.
Acknowledgments
The authors thank the following individuals for their experience and contribution: Beverley White is the Clinical Documentation Improvement Coordinator at North Florida/South Georgia Veterans Health System and provided expertise on documentation requirements. Russell Jacobitz and Susan Rozelle provided technical expertise on electronic health record system enhancements and implemented the template design. Jess Delaune, MD, and Robert Carroll, MD, provided additional physician input during template design. We also acknowledge the Inpatient Evaluation Center (IPEC) within the Veterans Health Administration (VHA). IPEC developed the case severity index, a risk-adjusted formula incorporated into the VHA quality metric reporting system, Strategic Analytics for Improvement and Learning (SAIL).
1. Kang R, Kunkel S, Columbo J, et al. Association of Hospital Employee satisfaction with patient safety and satisfaction within Veterans Affairs Medical Centers. Am J Med. 2019;132(4):530-534.e1. doi: 10.1016/j.amjmed.2018.11.031
2. Valluru, N, Kang L, Gaidos JK. Health maintenance documentation improves for veterans with IBD using a template in the Computerized Patient Record System. Dig Dis Sci. 2018;63(7):1782-1786. doi:10.1007%2Fs10620-018-5093-5
3. Thaker VV, Lee F, Bottino CJ, et al. Impact of an electronic template on documentation of obesity in a primary care clinic. Clin Pediatr. 2016;55(12):1152-1159. doi:10.1177/0009922815621331
4. Grogan EL, Speroff T, Deppen S, et al. Improving documentation of patient acuity level using a progress note template. J Am Coll Surg. 2004;199(3):468-475. doi:10.1016/j.jamcollsurg.2004.05.254
5. Centers for Disease Control and Prevention. Classification of diseases, functioning, and disability. https://www .cdc.gov/nchs/icd/index.htm. Updated June 30, 2020. Accessed October 12, 2020.
6. Marill K A, Gauharou ES, Nelson BK, et al. Prospective, randomized trial of template-assisted versus undirected written recording of physician records in the emergency department. Ann Emerg Med. 1999;33(5):500- 509. doi:10.1016/S0196-0644(99)70336-7
7. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004
8. Hart KA, Steinfeldt BA, Braun RD. Formulation and applications of a probalistic Pareto chart. AIAA. 2015;0804. doi:10.2514/6.2015-0804
9. IBM. IBM knowledge center: overview of data objects. https://www.ibm.com/support/knowledgecenter /en/SSLTBW_2.3.0/com.ibm.zos.v2r3.cbclx01/data _objects.htm. Accessed October 12, 2020.
10. US Department of Veterans Affairs. History of IT at VA. https://www.oit.va.gov/about/history.cfm. Accessed October 18, 2020.
11. Gardner CL, Pearce PF. Customization of electronic medical record templates to improve end-user satisfaction. Comput Inform Nurs. 2013;31(3):115-121. doi:10.1097/NXN.0b013e3182771814
Standardized template design is a useful tool to improve clinical documentation and reliable reporting of health care outcomes when constructed with clear objectives and with collaboration of key stakeholders. A standardized template should not only capture accurate diagnostic information, but also inform quality improvement (QI) measures and best practices.
Kang and colleagues showed that a correlation exists between organizational satisfaction and improved quality outcomes.1 A new initiative should have a well-defined purpose reinforced by collaborative workgroups and engaged employees who understand their clinical care role with electronic health record (EHR) modifications.
Several studies have shown how the usefulness of templates achieve multipurpose goals, such as accurate documentation and improved care. Valluru and colleagues showed a significant increase in vaccination rates for patients with inflammatory bowel disease after implementing a standardized template.2 By using a standardized template, Thaker and colleagues showed improved documentation regarding obesity and increased nutritional and physical activity counseling.3 Furthermore, Grogan and colleagues showed that templates are useful for house staff education on International Classification of Diseases (ICD) terminology and demonstrated improved documentation in the postintervention group.4,5
This article discusses the US Department of Veterans Affairs (VA) North Florida/South Georgia Veterans Health System (NF/SGVHS) integrated informatics solutions within template design in the Veterans Health Administration (VHA) EHR system that was associated with an increase in its case severity index (CSI) through improved clinical documentation capture.
Methods
According to policy activities that constitute research at NF/SGVHS, institutional review board approval was not required as this work met the criteria for operational improvement activities exempt from ethics review.
NF/SGVHS includes 2 hospitals: Malcom Randall VA Medical Center (MRVAMC) in Gainesville, Florida, and Lake City VA Medical Center (LCVAMC) in Lake City, Florida. MRVAMC is a large, 1a, academic VA facility composed of rotating residents and fellows and includes multiple specialty care services. LCVAMC is a smaller, nonteaching facility.
Template Design Impact
CSI is a risk-adjusted formula developed by the Inpatient Evaluation Center within VHA. CSI is incorporated into the VHA quality metrics reporting system, Strategic Analytics for Improvement and Learning (SAIL). CSI risk-adjusts metrics such as length of stay and mortality before releasing SAIL reports. CSI is calculated separately for acute level of care (LOC) and for the intensive care unit (ICU). In fiscal year (FY) 2017, acute LOC preimplementation data for CSI at NF/SGVHS were 0.76 for MRVAMC and 0.81 for LCVAMC, which was significantly below the national VHA average of 0.96 (Table).
A below-average CSI conveys a less complicated case mix compared with most other VA facilities. Although smaller VA facilities may have a less complicated case mix, it is unusual for large, tertiary care 1a VA facilities to have a low CSI. This low CSI is usually due to inadequate documentation, which affects not only risk-adjusted quality metrics outcomes, but also potential reimbursement.6
An interdisciplinary team composed of attendings, residents, and a clinical document improvement specialist identified the below-average acute LOC CSI for MRVAMC and LCVAMC compared with that of the national VHA average. Further analysis by chart reviews showed inconsistencies with standardized documentation despite prior health care provider education on ICD terminology and specific groups of common comorbidities analyzed in administrative data reviews for risk-adjustment purposes, known as Elixhauser comorbidities.5,7
A chart review showed lack of clarity regarding primary reason(s) for admission and chronic comorbidities within NF/SGVHS. Using Pareto chart analysis, the template team designed a standardized history and physical (H&P) medicine template based on NF/SGVHS common medicine admissions (Figure 1). A Pareto chart is a valuable QI tool that assists with identifying majority contributors to a problem(s) being analyzed when evaluating a large set of data points. Subsequently, this tool helps focus direction on QI efforts.8
The template had the usual H&P elements not shown (eg, chief complaint, history of present illness, etc), and highlights the assessment/plan section containing primary reason(s) for admission and chronic comorbidities (Figure 1). The complete assessment and plan section on the template can be found in the Appendix.
To simplify the template interface, only single clicks were required to expand diagnostic and chronic comorbidity checkboxes. Subcategories then appeared to select diagnosis and chronic comorbidities along with free text for additional documentation.
In addition, data objects were created within the template that permitted the ability to retrieve information from the VHA EHR and insert specific data points of interest in the template; for example, body mass index to assess degree of obesity and estimated glomerular filtration rate to determine the stage of chronic kidney disease. This allowed users to easily reference data in one template in lieu of searching for data in multiple places in the EHR.9
Results
The standardized H&P medicine template was implemented at MRVAMC and LCVAMC in June 2018 (the final month of the third quarter of FY 2018). As clinical providers throughout NF/SGVHS used the standardized template, acute LOC postimplementation data for CSI significantly improved. Although the national VHA average slightly decreased from 0.96 in the first quarter of FY 2017 to 0.89, in the first quarter of FY 2019, MRVAMC acute LOC CSI improved from 0.76 to 0.97, and LCVAMC acute LOC CSI improved from 0.81 to 1.07 during the same period.
In addition, compliance also was monitored within MRVAMC and LCVAMC for about 1 year after standardized H&P medicine template implementation. Compliance was determined by how often the standardized H&P medicine template was used for inpatient medicine admissions to the acute care wards vs other H&P notes used (such as personalized templates).
Methodology for compliance analysis included acquisition of completed H&P medicine notes from June 18, 2018 to June 30, 2019, within the VHA Veterans Information Systems and Technology Architecture (VistA) clinical and business information system using the search strings: “H&P admission history and physical” and “history of present illness.”10
A review identified 10,845 completed medicine H&P notes. Nine hundred eighteen notes were excluded as their search function yielded a location not corresponding to MRVAMC or LCVAMC. Of the 9,927 notes remaining, 8,025 of these were completed medicine H&P notes at MRVAMC and 1,902 were completed medicine H&P notes at LCVAMC (Figure 2).
From June 18, 2018 to June 30, 2019 at MRVAMC, compliance was reviewed monthly for the 8,025 completed H&P medicine notes. Of the completed H&P medicine notes, the standardized H&P medicine template was used 43.2% in June 2018. By June 2019, MRVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use at 89.9% (Figure 3). Total average compliance from June 18, 2018 to June 30, 2019, was 88.4%, which doubled compliance from the initial introduction of the standardized H&P medicine template.
Compliance was reviewed monthly for the 1,902 completed H&P medicine notes from June 18, 2018 to June 30, 2019, at LCVAMC. Of the completed H&P medicine notes, the standardized template was used 48.2% of the time in June 2018. By June 2019, LCVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use, which increased to 96.9%. Total average compliance from June 18, 2018 to June 30, 2019, was 93.8%, which was almost double the baseline compliance rate.
Discussion
Template design with clear objectives, strategic collaboration, and integrated informatics solutions has the potential to increase accuracy of documentation. As shown, the NF/SGVHS template design was associated with significant improvement in acute LOC CSI for both MRVAMC and LCVAMC due to more accurate documentation using the standardized H&P medicine template.
Numerous factors contributed to the success of this template design. First, a clear vision for application of the template was communicated with key stakeholders. In addition, the template design team was focused on specific goals rather than a one size fits all approach, which was crucial for sustainable execution. Although interdisciplinary teamwork has the potential to result in innovative practices, large multidisciplinary teams also may have difficulty establishing a shared vision that can result in barriers to achieving project goals.
Balancing standardization and customization was essential for user buy-in. As noted by Gardner and Pearce, inviting clinical providers to participate in template design and allowing for customization has the potential to increase acceptance and use of templates.11 Although the original design for the standardized H&P medicine template started with the medicine service at NF/SGVHS, the design framework is applicable to numerous services where various clinical care elements can be customized.
Explaining the informatics tools built into the template allowed clinicians to see opportunities to improve clinical documentation and the impact it has on reporting health care outcomes. When improvement work involves integrating clinical care delivery and administrative expectations, it is essential that health care systems understand and strategically execute project initiatives at this critical juncture.
Finally, incorporation of a sustainability plan when process improvement strategies are implemented is vital. In addition to collaboration with the clinical providers during design and implementation of the standardized template, leadership buy-in was key. Compliance with standardized H&P medicine template use was monitored monthly and reviewed by the NF/SGVHS Chief of Staff.
As noted, LCVAMC postimplementation acute LOC CSI was higher than that of MRVAMC despite being a smaller facility. This might be due to the MRVAMC designation as a teaching institution. Medicine is the only inpatient service at LCVAMC staffed by hospitalists with limited specialists available for consultation, whereas MRVAMC is a tertiary care teaching facility with numerous inpatient services and subspecialties. As LCVAMC has more continuity, house staff rotating at MRVAMC require continued training/education on new templates and process changes.
Limitations
Although standardized template design was successful at NF/SGVHS, limitations should be noted. Our clinical documentation improvement (CDI) program also was expanded about the same time as the new templates were released. The expansion of the CDI program in addition to new template design likely had a synergistic effect on acute LOC CSI.
CSI is a complex, risk-adjusted model that includes numerous factors, including but not limited to diagnosis and comorbid conditions. Other factors include age, marital status, procedures, source of admission, specific laboratory values, medical or surgical diagnosis-related group, intensive care unit stays, and immunosuppressive status. CSI also includes operative and nonoperative components that average into an overall CSI. As the majority of CSI is composed of nonoperative constituents within NF/SGVHS, we do not believe this had any substantial impact on reporting of CSI improvements.
In addition, template entry into VHA EHR requires a location selection (such as a clinic name or ward name following an inpatient admission). Of the 10,845 completed H&P medicine notes identified in VistA, 918 notes were excluded from analysis as their search function yielded a location not corresponding to MRVAMC or LCVAMC. For the 918 notes excluded, we believe this was likely due to user error where locations not related to MRVAMC or LCVAMC were selected during standardized H&P medicine template entry.
Conclusions
After the NF/SGVHS implementation of a uniquely designed template embedded with informatics solutions within the VHA EHR, the CSI increased due to more accurate documentation.
Next steps include determining the impact of the NF/SGVHS template design on potential reimbursement and expanding template design into the outpatient setting where there are additional opportunities to improve clinical documentation and reliable reporting of health care outcomes.
Acknowledgments
The authors thank the following individuals for their experience and contribution: Beverley White is the Clinical Documentation Improvement Coordinator at North Florida/South Georgia Veterans Health System and provided expertise on documentation requirements. Russell Jacobitz and Susan Rozelle provided technical expertise on electronic health record system enhancements and implemented the template design. Jess Delaune, MD, and Robert Carroll, MD, provided additional physician input during template design. We also acknowledge the Inpatient Evaluation Center (IPEC) within the Veterans Health Administration (VHA). IPEC developed the case severity index, a risk-adjusted formula incorporated into the VHA quality metric reporting system, Strategic Analytics for Improvement and Learning (SAIL).
Standardized template design is a useful tool to improve clinical documentation and reliable reporting of health care outcomes when constructed with clear objectives and with collaboration of key stakeholders. A standardized template should not only capture accurate diagnostic information, but also inform quality improvement (QI) measures and best practices.
Kang and colleagues showed that a correlation exists between organizational satisfaction and improved quality outcomes.1 A new initiative should have a well-defined purpose reinforced by collaborative workgroups and engaged employees who understand their clinical care role with electronic health record (EHR) modifications.
Several studies have shown how the usefulness of templates achieve multipurpose goals, such as accurate documentation and improved care. Valluru and colleagues showed a significant increase in vaccination rates for patients with inflammatory bowel disease after implementing a standardized template.2 By using a standardized template, Thaker and colleagues showed improved documentation regarding obesity and increased nutritional and physical activity counseling.3 Furthermore, Grogan and colleagues showed that templates are useful for house staff education on International Classification of Diseases (ICD) terminology and demonstrated improved documentation in the postintervention group.4,5
This article discusses the US Department of Veterans Affairs (VA) North Florida/South Georgia Veterans Health System (NF/SGVHS) integrated informatics solutions within template design in the Veterans Health Administration (VHA) EHR system that was associated with an increase in its case severity index (CSI) through improved clinical documentation capture.
Methods
According to policy activities that constitute research at NF/SGVHS, institutional review board approval was not required as this work met the criteria for operational improvement activities exempt from ethics review.
NF/SGVHS includes 2 hospitals: Malcom Randall VA Medical Center (MRVAMC) in Gainesville, Florida, and Lake City VA Medical Center (LCVAMC) in Lake City, Florida. MRVAMC is a large, 1a, academic VA facility composed of rotating residents and fellows and includes multiple specialty care services. LCVAMC is a smaller, nonteaching facility.
Template Design Impact
CSI is a risk-adjusted formula developed by the Inpatient Evaluation Center within VHA. CSI is incorporated into the VHA quality metrics reporting system, Strategic Analytics for Improvement and Learning (SAIL). CSI risk-adjusts metrics such as length of stay and mortality before releasing SAIL reports. CSI is calculated separately for acute level of care (LOC) and for the intensive care unit (ICU). In fiscal year (FY) 2017, acute LOC preimplementation data for CSI at NF/SGVHS were 0.76 for MRVAMC and 0.81 for LCVAMC, which was significantly below the national VHA average of 0.96 (Table).
A below-average CSI conveys a less complicated case mix compared with most other VA facilities. Although smaller VA facilities may have a less complicated case mix, it is unusual for large, tertiary care 1a VA facilities to have a low CSI. This low CSI is usually due to inadequate documentation, which affects not only risk-adjusted quality metrics outcomes, but also potential reimbursement.6
An interdisciplinary team composed of attendings, residents, and a clinical document improvement specialist identified the below-average acute LOC CSI for MRVAMC and LCVAMC compared with that of the national VHA average. Further analysis by chart reviews showed inconsistencies with standardized documentation despite prior health care provider education on ICD terminology and specific groups of common comorbidities analyzed in administrative data reviews for risk-adjustment purposes, known as Elixhauser comorbidities.5,7
A chart review showed lack of clarity regarding primary reason(s) for admission and chronic comorbidities within NF/SGVHS. Using Pareto chart analysis, the template team designed a standardized history and physical (H&P) medicine template based on NF/SGVHS common medicine admissions (Figure 1). A Pareto chart is a valuable QI tool that assists with identifying majority contributors to a problem(s) being analyzed when evaluating a large set of data points. Subsequently, this tool helps focus direction on QI efforts.8
The template had the usual H&P elements not shown (eg, chief complaint, history of present illness, etc), and highlights the assessment/plan section containing primary reason(s) for admission and chronic comorbidities (Figure 1). The complete assessment and plan section on the template can be found in the Appendix.
To simplify the template interface, only single clicks were required to expand diagnostic and chronic comorbidity checkboxes. Subcategories then appeared to select diagnosis and chronic comorbidities along with free text for additional documentation.
In addition, data objects were created within the template that permitted the ability to retrieve information from the VHA EHR and insert specific data points of interest in the template; for example, body mass index to assess degree of obesity and estimated glomerular filtration rate to determine the stage of chronic kidney disease. This allowed users to easily reference data in one template in lieu of searching for data in multiple places in the EHR.9
Results
The standardized H&P medicine template was implemented at MRVAMC and LCVAMC in June 2018 (the final month of the third quarter of FY 2018). As clinical providers throughout NF/SGVHS used the standardized template, acute LOC postimplementation data for CSI significantly improved. Although the national VHA average slightly decreased from 0.96 in the first quarter of FY 2017 to 0.89, in the first quarter of FY 2019, MRVAMC acute LOC CSI improved from 0.76 to 0.97, and LCVAMC acute LOC CSI improved from 0.81 to 1.07 during the same period.
In addition, compliance also was monitored within MRVAMC and LCVAMC for about 1 year after standardized H&P medicine template implementation. Compliance was determined by how often the standardized H&P medicine template was used for inpatient medicine admissions to the acute care wards vs other H&P notes used (such as personalized templates).
Methodology for compliance analysis included acquisition of completed H&P medicine notes from June 18, 2018 to June 30, 2019, within the VHA Veterans Information Systems and Technology Architecture (VistA) clinical and business information system using the search strings: “H&P admission history and physical” and “history of present illness.”10
A review identified 10,845 completed medicine H&P notes. Nine hundred eighteen notes were excluded as their search function yielded a location not corresponding to MRVAMC or LCVAMC. Of the 9,927 notes remaining, 8,025 of these were completed medicine H&P notes at MRVAMC and 1,902 were completed medicine H&P notes at LCVAMC (Figure 2).
From June 18, 2018 to June 30, 2019 at MRVAMC, compliance was reviewed monthly for the 8,025 completed H&P medicine notes. Of the completed H&P medicine notes, the standardized H&P medicine template was used 43.2% in June 2018. By June 2019, MRVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use at 89.9% (Figure 3). Total average compliance from June 18, 2018 to June 30, 2019, was 88.4%, which doubled compliance from the initial introduction of the standardized H&P medicine template.
Compliance was reviewed monthly for the 1,902 completed H&P medicine notes from June 18, 2018 to June 30, 2019, at LCVAMC. Of the completed H&P medicine notes, the standardized template was used 48.2% of the time in June 2018. By June 2019, LCVAMC clinical providers demonstrated significant improvement for standardized H&P medicine template use, which increased to 96.9%. Total average compliance from June 18, 2018 to June 30, 2019, was 93.8%, which was almost double the baseline compliance rate.
Discussion
Template design with clear objectives, strategic collaboration, and integrated informatics solutions has the potential to increase accuracy of documentation. As shown, the NF/SGVHS template design was associated with significant improvement in acute LOC CSI for both MRVAMC and LCVAMC due to more accurate documentation using the standardized H&P medicine template.
Numerous factors contributed to the success of this template design. First, a clear vision for application of the template was communicated with key stakeholders. In addition, the template design team was focused on specific goals rather than a one size fits all approach, which was crucial for sustainable execution. Although interdisciplinary teamwork has the potential to result in innovative practices, large multidisciplinary teams also may have difficulty establishing a shared vision that can result in barriers to achieving project goals.
Balancing standardization and customization was essential for user buy-in. As noted by Gardner and Pearce, inviting clinical providers to participate in template design and allowing for customization has the potential to increase acceptance and use of templates.11 Although the original design for the standardized H&P medicine template started with the medicine service at NF/SGVHS, the design framework is applicable to numerous services where various clinical care elements can be customized.
Explaining the informatics tools built into the template allowed clinicians to see opportunities to improve clinical documentation and the impact it has on reporting health care outcomes. When improvement work involves integrating clinical care delivery and administrative expectations, it is essential that health care systems understand and strategically execute project initiatives at this critical juncture.
Finally, incorporation of a sustainability plan when process improvement strategies are implemented is vital. In addition to collaboration with the clinical providers during design and implementation of the standardized template, leadership buy-in was key. Compliance with standardized H&P medicine template use was monitored monthly and reviewed by the NF/SGVHS Chief of Staff.
As noted, LCVAMC postimplementation acute LOC CSI was higher than that of MRVAMC despite being a smaller facility. This might be due to the MRVAMC designation as a teaching institution. Medicine is the only inpatient service at LCVAMC staffed by hospitalists with limited specialists available for consultation, whereas MRVAMC is a tertiary care teaching facility with numerous inpatient services and subspecialties. As LCVAMC has more continuity, house staff rotating at MRVAMC require continued training/education on new templates and process changes.
Limitations
Although standardized template design was successful at NF/SGVHS, limitations should be noted. Our clinical documentation improvement (CDI) program also was expanded about the same time as the new templates were released. The expansion of the CDI program in addition to new template design likely had a synergistic effect on acute LOC CSI.
CSI is a complex, risk-adjusted model that includes numerous factors, including but not limited to diagnosis and comorbid conditions. Other factors include age, marital status, procedures, source of admission, specific laboratory values, medical or surgical diagnosis-related group, intensive care unit stays, and immunosuppressive status. CSI also includes operative and nonoperative components that average into an overall CSI. As the majority of CSI is composed of nonoperative constituents within NF/SGVHS, we do not believe this had any substantial impact on reporting of CSI improvements.
In addition, template entry into VHA EHR requires a location selection (such as a clinic name or ward name following an inpatient admission). Of the 10,845 completed H&P medicine notes identified in VistA, 918 notes were excluded from analysis as their search function yielded a location not corresponding to MRVAMC or LCVAMC. For the 918 notes excluded, we believe this was likely due to user error where locations not related to MRVAMC or LCVAMC were selected during standardized H&P medicine template entry.
Conclusions
After the NF/SGVHS implementation of a uniquely designed template embedded with informatics solutions within the VHA EHR, the CSI increased due to more accurate documentation.
Next steps include determining the impact of the NF/SGVHS template design on potential reimbursement and expanding template design into the outpatient setting where there are additional opportunities to improve clinical documentation and reliable reporting of health care outcomes.
Acknowledgments
The authors thank the following individuals for their experience and contribution: Beverley White is the Clinical Documentation Improvement Coordinator at North Florida/South Georgia Veterans Health System and provided expertise on documentation requirements. Russell Jacobitz and Susan Rozelle provided technical expertise on electronic health record system enhancements and implemented the template design. Jess Delaune, MD, and Robert Carroll, MD, provided additional physician input during template design. We also acknowledge the Inpatient Evaluation Center (IPEC) within the Veterans Health Administration (VHA). IPEC developed the case severity index, a risk-adjusted formula incorporated into the VHA quality metric reporting system, Strategic Analytics for Improvement and Learning (SAIL).
1. Kang R, Kunkel S, Columbo J, et al. Association of Hospital Employee satisfaction with patient safety and satisfaction within Veterans Affairs Medical Centers. Am J Med. 2019;132(4):530-534.e1. doi: 10.1016/j.amjmed.2018.11.031
2. Valluru, N, Kang L, Gaidos JK. Health maintenance documentation improves for veterans with IBD using a template in the Computerized Patient Record System. Dig Dis Sci. 2018;63(7):1782-1786. doi:10.1007%2Fs10620-018-5093-5
3. Thaker VV, Lee F, Bottino CJ, et al. Impact of an electronic template on documentation of obesity in a primary care clinic. Clin Pediatr. 2016;55(12):1152-1159. doi:10.1177/0009922815621331
4. Grogan EL, Speroff T, Deppen S, et al. Improving documentation of patient acuity level using a progress note template. J Am Coll Surg. 2004;199(3):468-475. doi:10.1016/j.jamcollsurg.2004.05.254
5. Centers for Disease Control and Prevention. Classification of diseases, functioning, and disability. https://www .cdc.gov/nchs/icd/index.htm. Updated June 30, 2020. Accessed October 12, 2020.
6. Marill K A, Gauharou ES, Nelson BK, et al. Prospective, randomized trial of template-assisted versus undirected written recording of physician records in the emergency department. Ann Emerg Med. 1999;33(5):500- 509. doi:10.1016/S0196-0644(99)70336-7
7. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004
8. Hart KA, Steinfeldt BA, Braun RD. Formulation and applications of a probalistic Pareto chart. AIAA. 2015;0804. doi:10.2514/6.2015-0804
9. IBM. IBM knowledge center: overview of data objects. https://www.ibm.com/support/knowledgecenter /en/SSLTBW_2.3.0/com.ibm.zos.v2r3.cbclx01/data _objects.htm. Accessed October 12, 2020.
10. US Department of Veterans Affairs. History of IT at VA. https://www.oit.va.gov/about/history.cfm. Accessed October 18, 2020.
11. Gardner CL, Pearce PF. Customization of electronic medical record templates to improve end-user satisfaction. Comput Inform Nurs. 2013;31(3):115-121. doi:10.1097/NXN.0b013e3182771814
1. Kang R, Kunkel S, Columbo J, et al. Association of Hospital Employee satisfaction with patient safety and satisfaction within Veterans Affairs Medical Centers. Am J Med. 2019;132(4):530-534.e1. doi: 10.1016/j.amjmed.2018.11.031
2. Valluru, N, Kang L, Gaidos JK. Health maintenance documentation improves for veterans with IBD using a template in the Computerized Patient Record System. Dig Dis Sci. 2018;63(7):1782-1786. doi:10.1007%2Fs10620-018-5093-5
3. Thaker VV, Lee F, Bottino CJ, et al. Impact of an electronic template on documentation of obesity in a primary care clinic. Clin Pediatr. 2016;55(12):1152-1159. doi:10.1177/0009922815621331
4. Grogan EL, Speroff T, Deppen S, et al. Improving documentation of patient acuity level using a progress note template. J Am Coll Surg. 2004;199(3):468-475. doi:10.1016/j.jamcollsurg.2004.05.254
5. Centers for Disease Control and Prevention. Classification of diseases, functioning, and disability. https://www .cdc.gov/nchs/icd/index.htm. Updated June 30, 2020. Accessed October 12, 2020.
6. Marill K A, Gauharou ES, Nelson BK, et al. Prospective, randomized trial of template-assisted versus undirected written recording of physician records in the emergency department. Ann Emerg Med. 1999;33(5):500- 509. doi:10.1016/S0196-0644(99)70336-7
7. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004
8. Hart KA, Steinfeldt BA, Braun RD. Formulation and applications of a probalistic Pareto chart. AIAA. 2015;0804. doi:10.2514/6.2015-0804
9. IBM. IBM knowledge center: overview of data objects. https://www.ibm.com/support/knowledgecenter /en/SSLTBW_2.3.0/com.ibm.zos.v2r3.cbclx01/data _objects.htm. Accessed October 12, 2020.
10. US Department of Veterans Affairs. History of IT at VA. https://www.oit.va.gov/about/history.cfm. Accessed October 18, 2020.
11. Gardner CL, Pearce PF. Customization of electronic medical record templates to improve end-user satisfaction. Comput Inform Nurs. 2013;31(3):115-121. doi:10.1097/NXN.0b013e3182771814
Biden victory: What it means for COVID, health care
The former vice president has sketched out a big health agenda: ramping up the federal response to COVID-19, boosting the Affordable Care Act, creating a new “public option” to cover uninsured Americans, and expanding Medicare and Medicaid.
But the president-elect’s long to-do list on health is likely to face significant roadblocks in Congress and the courts, experts say.
For instance, Biden’s ambitious proposals on COVID-19 -- including his recent call for a national mask mandate -- could be waylaid by legal challenges and run into political hurdles on Capitol Hill, where he may face a divided Congress.
Joseph Antos, PhD, a health policy expert with the conservative American Enterprise Institute, predicts Biden will encounter the same type of congressional “gridlock situation” that President Barack Obama ran into during his second term.
“We have a situation that has been like this for a very, very long time -- lack of cooperation, lack of recognition that either party is capable of rising above their own electoral views to deal with problems that the country actually has.”
Antos also suggests that Biden may also face enormous political pressure to address the economic fallout from the coronavirus, including record unemployment and business closures, before anything else.
“I think it’s really going to be efforts that are intended to promote economic development and promote the economy,” he says.
In addition, Biden’s plans to expand Obamacare might face a new challenge from the Supreme Court in the year ahead. This month, the high court will take up a new case seeking to overturn the law.
Even so, experts say Biden’s plans on COVID-19 and expanding health care are likely to define his tenure in the White House as a central focus of his presidency.
“Health care will be at the very top of the list of the president’s priorities,” says Sabrina Corlette, JD, co-director of the Center on Health Insurance Reforms at Georgetown University’s McCourt School of Public Policy. “I do think, however, that the administration is going to be very preoccupied with the response to COVID-19 and the economic fallout … particularly in the first year.”
Here’s a closer look at what we can expect from a Biden presidency.
COVID-19: Federalizing response efforts
Biden will move to federalize the response to COVID-19. He has said he will take back major responsibilities from the states -- such as setting national policies on mask wearing, social distancing, and the reopening of schools and businesses, based on CDC guidance. In the days leading up to the election, Biden called for a national mask mandate, after waffling on the issue throughout the summer.
He has said he will let public health science drive political policy. Biden is also planning to create his own task force to advise officials during the transition on managing the new surge in COVID-19 cases, vaccine safety and protecting at-risk populations, Politico reported this week. He received a virtual briefing on the pandemic from a panel of experts as he awaited the election’s outcome.
“I think we will no longer have this confused and contradictory public messaging,” Corlette says, “but I also think there will be humility and the recognition that the evidence is evolving -- that we don’t have all the answers, but we’re learning as we go.”
But national mandates on masks and social distancing will be challenging to enforce, experts say. They are also likely to face pushback from business interests, opposition from public officials in GOP-led states, and even legal challenges.
Biden’s ability to work with Congress -- or not -- may determine whether he is able to implement some of the key components of his coronavirus action plan, which includes:
- Providing free COVID-19 testing for all Americans
- Hiring 100,000 contact tracers
- Eliminating out-of-pocket expenses for coronavirus treatment
- Delivering “sufficient” PPE for essential workers
- Supporting science-backed vaccines and medical treatments being developed
- Requiring the reopening of businesses, workplaces, and schools only after “sufficient” reductions in community transmission -- under evidence-based protocols put forward by the CDC
- Giving emergency paid leave for workers dislocated by the pandemic and more financial aid for workers, families, and small businesses
- Shoring up safeguards to protect at-risk Americans, including older people
- Boosting pay for health care workers on the front lines
Biden has not detailed how he would pay for many of these, beyond promising to force wealthy Americans to “pay their fair share” of taxes to help. He has proposed a tax increase on Americans making more than $400,000 a year, which would require congressional approval.
Antos says he expects Biden’s proposed COVID-19 action plan to be virtually the same as Trump’s in two areas: efforts to develop a vaccine and antiviral treatments.
The administration has spent some $225 million on COVID-19 testing efforts, with a particular focus on rural areas.
Trump launched Operation Warp Speed to fast-track a vaccine. As part of that, the federal government has contracted with six drug companies, spending nearly $11 billion. The operation aims to provide at least 300 million doses of a coronavirus vaccine by January 2021.
Antos would like to see “a more sophisticated approach to social distancing” from the president-elect that takes into account the different challenges facing Americans depending on their income, work situation, and other factors during the pandemic.
“There are a lot of people in this country where working from home is fine and their jobs are secure,” he notes. “It’s the person who used to work at a restaurant that closed, it’s the line worker at a factory that has severely cut back its hours. It’s basically lower-middle-class people, low-income people, middle-class people, and it’s not the elite.
“And the policies have not given enough consideration to the fact that their circumstances and their tradeoffs would differ from the tradeoffs of somebody who doesn’t have anything to worry about economically.
“So, what we need is a more supple policy [that] will give people the information they need and give them the financial support that they also need … so they can make good decisions for themselves and their families. And we basically haven’t done that.”
Obamacare on the blocks?
The Supreme Court’s decision to take up another case seeking to overturn the Affordable Care Act could hand Biden’s health agenda a major setback -- and put the medical care for millions of Americans in jeopardy.
On Nov. 10, the high court will hear oral arguments on a lawsuit that would strike down all of Obamacare. A decision is not expected until next year.
The court has previously upheld the 2010 law, which Biden helped usher through Congress as vice president. But the addition of right-leaning Supreme Court Justice Amy Coney Barrett to the bench last month gives the court a clear conservative majority that could mean the end of Obamacare, legal experts say.
Republicans have opposed the law since its passage, but they have been unable to muster the votes to repeal it, or to pass an alternative
Antos, from the American Enterprise Institute, notes conservatives believe the law has increased costs for health care and insurance over the past decade, in part because of its protections for Americans with preexisting conditions and requiring insurers to provide comprehensive “gold-plated” policies.
“It’s driven up costs, offers plans that are not very strong, put high-risk folks into the same [insurance pool], which has increased costs for everyone, the employer mandate … these are all the reasons,” he says.
The Supreme Court isn’t expected to deliver a decision on the Affordable Care Act before the middle of next year. But the uncertainty will likely push back Biden’s proposals to expand on the law.
Overturning Obamacare would have huge impacts on millions of Americans:
- As many as 133 million Americans -- roughly half the U.S. population -- with preexisting conditions could find it harder, if not impossible, to find affordable health insurance. That figure does not include Americans infected with COVID-19.
- About 165 million who require expensive treatments -- for cancer and other conditions -- would no longer be protected from huge costs for care by federal caps on out-of-pocket expenditures the Affordable Care Act requires.
- An estimated 21 million who now buy insurance through the Obamacare Marketplaces could lose their coverage.
- Another 12 million on Medicaid could find themselves without insurance.
- At least 2 million young adults ages 26 and under, now on their parents’ health policies, could be kicked off.
- Millions of people who use Medicare could face higher costs.
- Federal subsidies for lower-income Americans to buy policies would disappear.
Throughout the campaign, Biden repeatedly stressed the need to preserve the law’s provision barring insurance companies from refusing coverage for Americans with preexisting conditions, such as diabetes, cancer, and heart disease. It also outlaws charging higher premiums on the basis of health status, age, or gender.
Biden has also pledged to bolster the law as president.
He has proposed a variety of add-ons to the Affordable Care Act he says will “insure more than an estimated 97% of Americans,” according to the Biden campaign site.
Biden’s proposals include offering larger federal subsidies to help low- and middle-income Americans pay for policies purchased through Obamacare insurance Marketplaces.
The boldest of Biden’s proposals is the creation of a “public option” for insurance -- a Medicare-like program that small businesses and individuals could choose if they do not have coverage, cannot afford it, or don’t like their employer-based coverage.
It would also automatically enroll millions of uninsured Americans living in the 14 states that have not expanded Medicaid, which covers low-income people.
But such a plan would require congressional approval -- including a “super majority” of 60 Senate votes to block a likely GOP filibuster. That will be a significant challenge Biden will have to overcome, with Congress so evenly divided.
The White House would also have to defeat heavy lobbying from some of the most influential industry interest groups in Washington, Corlette says.
“I’m not even confident they would get all the Democrat votes,” she says.
“So, it’s a going to be an uphill battle to get a public option passed.”
Taken together, Biden’s plans for expanding Obamacare are projected to cost $750 billion over 10 years. He has said much of that financing would come from increasing taxes on the wealthy.
That means it would likely require congressional approval, which Antos suggests is unlikely given the polarization on Capitol Hill.
Medicare, Medicaid, and drug costs
Biden has called for a host of reforms targeting Medicare, Medicaid, and rising drug costs.
On Medicare, which primarily covers seniors 65 and older, Biden has proposed lowering the eligibility age from 65 to 60. That could extend Medicare to up to 20 million more Americans.
On Medicaid, the health care safety net for low-income and disabled Americans, the president-elect supports increased federal funding to states during the current economic crisis, and potentially beyond.
Medicare is likely to become a key focus of the new administration, in light of the pressures the pandemic is placing on Medicare funding.
In April, Medicare’s trustees said that the Part A trust fund for the program, which pays for hospital and inpatient care, could start to run dry in 2026.
But those projections did not include the impact of COVID-19. Some economists have since projected that Medicare Part A could become insolvent as early as 2022.
Medicare Part B, which pays for doctor and outpatient costs, is funded by general tax funding and beneficiary insurance premiums, so it is not in danger of drying up.
Adding to those pressures is an executive order Trump signed in August temporarily deferring payroll taxes, a primary funding vehicle for Medicare and Social Security.
Under these taxes, employees pay 6.2% of their earnings (on annual income up to $137,700) toward Social Security and 1.45% for Medicare taxes each pay period. Employers pay the same rate per paycheck, adding up to a combined 12.4% Social Security tax and 2.9% Medicare tax.
Biden has said he would reverse the tax cut when he takes office.
But to get a handle on Medicare and Medicaid funding issues, he is likely to need congressional support. Corlette and other experts say that could be a challenge while the nation remains in the grip of the coronavirus pandemic.
In addition to his Medicare and Medicaid reforms, Biden has proposed several plans to lower drug prices, a subset of rising health care and insurance costs.
U.S. spending on prescription drugs has increased nearly 42% over the past decade -- from $253.1 billion in 2010 to $358.7 billion in 2020 (projected) -- according to the Centers for Medicare & Medicaid Services.
In 2020, retail prices for 460 commonly prescribed drugs have spiked an average of 5.2%, according to new analysis by 3 Axis Advisors, a health research firm.
That’s more than double the projected rate of inflation.
To control drug costs, Biden supports legislation approved by the Democratic-led House of Representatives last year that would empower Medicare to negotiate drug prices with drug companies, as private insurers do.
Federal law now bars Medicare from negotiating prices on behalf of the 67.7 million Americans who use it. Drug companies and many GOP leaders argue that the current law is necessary to allow them to spend more on research and development of new medications.
In addition, Biden supports the idea of lifting bans on importing drugs from foreign countries with lower costs.
He also backs creating an independent review board to set price caps for new medications with no competitors; making high-quality generics more available; ending tax breaks for drug company advertising; and limiting their leeway in raising prices.
All of these proposals would likely require congressional approval and could face legal challenges in the courts.
This article first appeared on WebMD.com.
The former vice president has sketched out a big health agenda: ramping up the federal response to COVID-19, boosting the Affordable Care Act, creating a new “public option” to cover uninsured Americans, and expanding Medicare and Medicaid.
But the president-elect’s long to-do list on health is likely to face significant roadblocks in Congress and the courts, experts say.
For instance, Biden’s ambitious proposals on COVID-19 -- including his recent call for a national mask mandate -- could be waylaid by legal challenges and run into political hurdles on Capitol Hill, where he may face a divided Congress.
Joseph Antos, PhD, a health policy expert with the conservative American Enterprise Institute, predicts Biden will encounter the same type of congressional “gridlock situation” that President Barack Obama ran into during his second term.
“We have a situation that has been like this for a very, very long time -- lack of cooperation, lack of recognition that either party is capable of rising above their own electoral views to deal with problems that the country actually has.”
Antos also suggests that Biden may also face enormous political pressure to address the economic fallout from the coronavirus, including record unemployment and business closures, before anything else.
“I think it’s really going to be efforts that are intended to promote economic development and promote the economy,” he says.
In addition, Biden’s plans to expand Obamacare might face a new challenge from the Supreme Court in the year ahead. This month, the high court will take up a new case seeking to overturn the law.
Even so, experts say Biden’s plans on COVID-19 and expanding health care are likely to define his tenure in the White House as a central focus of his presidency.
“Health care will be at the very top of the list of the president’s priorities,” says Sabrina Corlette, JD, co-director of the Center on Health Insurance Reforms at Georgetown University’s McCourt School of Public Policy. “I do think, however, that the administration is going to be very preoccupied with the response to COVID-19 and the economic fallout … particularly in the first year.”
Here’s a closer look at what we can expect from a Biden presidency.
COVID-19: Federalizing response efforts
Biden will move to federalize the response to COVID-19. He has said he will take back major responsibilities from the states -- such as setting national policies on mask wearing, social distancing, and the reopening of schools and businesses, based on CDC guidance. In the days leading up to the election, Biden called for a national mask mandate, after waffling on the issue throughout the summer.
He has said he will let public health science drive political policy. Biden is also planning to create his own task force to advise officials during the transition on managing the new surge in COVID-19 cases, vaccine safety and protecting at-risk populations, Politico reported this week. He received a virtual briefing on the pandemic from a panel of experts as he awaited the election’s outcome.
“I think we will no longer have this confused and contradictory public messaging,” Corlette says, “but I also think there will be humility and the recognition that the evidence is evolving -- that we don’t have all the answers, but we’re learning as we go.”
But national mandates on masks and social distancing will be challenging to enforce, experts say. They are also likely to face pushback from business interests, opposition from public officials in GOP-led states, and even legal challenges.
Biden’s ability to work with Congress -- or not -- may determine whether he is able to implement some of the key components of his coronavirus action plan, which includes:
- Providing free COVID-19 testing for all Americans
- Hiring 100,000 contact tracers
- Eliminating out-of-pocket expenses for coronavirus treatment
- Delivering “sufficient” PPE for essential workers
- Supporting science-backed vaccines and medical treatments being developed
- Requiring the reopening of businesses, workplaces, and schools only after “sufficient” reductions in community transmission -- under evidence-based protocols put forward by the CDC
- Giving emergency paid leave for workers dislocated by the pandemic and more financial aid for workers, families, and small businesses
- Shoring up safeguards to protect at-risk Americans, including older people
- Boosting pay for health care workers on the front lines
Biden has not detailed how he would pay for many of these, beyond promising to force wealthy Americans to “pay their fair share” of taxes to help. He has proposed a tax increase on Americans making more than $400,000 a year, which would require congressional approval.
Antos says he expects Biden’s proposed COVID-19 action plan to be virtually the same as Trump’s in two areas: efforts to develop a vaccine and antiviral treatments.
The administration has spent some $225 million on COVID-19 testing efforts, with a particular focus on rural areas.
Trump launched Operation Warp Speed to fast-track a vaccine. As part of that, the federal government has contracted with six drug companies, spending nearly $11 billion. The operation aims to provide at least 300 million doses of a coronavirus vaccine by January 2021.
Antos would like to see “a more sophisticated approach to social distancing” from the president-elect that takes into account the different challenges facing Americans depending on their income, work situation, and other factors during the pandemic.
“There are a lot of people in this country where working from home is fine and their jobs are secure,” he notes. “It’s the person who used to work at a restaurant that closed, it’s the line worker at a factory that has severely cut back its hours. It’s basically lower-middle-class people, low-income people, middle-class people, and it’s not the elite.
“And the policies have not given enough consideration to the fact that their circumstances and their tradeoffs would differ from the tradeoffs of somebody who doesn’t have anything to worry about economically.
“So, what we need is a more supple policy [that] will give people the information they need and give them the financial support that they also need … so they can make good decisions for themselves and their families. And we basically haven’t done that.”
Obamacare on the blocks?
The Supreme Court’s decision to take up another case seeking to overturn the Affordable Care Act could hand Biden’s health agenda a major setback -- and put the medical care for millions of Americans in jeopardy.
On Nov. 10, the high court will hear oral arguments on a lawsuit that would strike down all of Obamacare. A decision is not expected until next year.
The court has previously upheld the 2010 law, which Biden helped usher through Congress as vice president. But the addition of right-leaning Supreme Court Justice Amy Coney Barrett to the bench last month gives the court a clear conservative majority that could mean the end of Obamacare, legal experts say.
Republicans have opposed the law since its passage, but they have been unable to muster the votes to repeal it, or to pass an alternative
Antos, from the American Enterprise Institute, notes conservatives believe the law has increased costs for health care and insurance over the past decade, in part because of its protections for Americans with preexisting conditions and requiring insurers to provide comprehensive “gold-plated” policies.
“It’s driven up costs, offers plans that are not very strong, put high-risk folks into the same [insurance pool], which has increased costs for everyone, the employer mandate … these are all the reasons,” he says.
The Supreme Court isn’t expected to deliver a decision on the Affordable Care Act before the middle of next year. But the uncertainty will likely push back Biden’s proposals to expand on the law.
Overturning Obamacare would have huge impacts on millions of Americans:
- As many as 133 million Americans -- roughly half the U.S. population -- with preexisting conditions could find it harder, if not impossible, to find affordable health insurance. That figure does not include Americans infected with COVID-19.
- About 165 million who require expensive treatments -- for cancer and other conditions -- would no longer be protected from huge costs for care by federal caps on out-of-pocket expenditures the Affordable Care Act requires.
- An estimated 21 million who now buy insurance through the Obamacare Marketplaces could lose their coverage.
- Another 12 million on Medicaid could find themselves without insurance.
- At least 2 million young adults ages 26 and under, now on their parents’ health policies, could be kicked off.
- Millions of people who use Medicare could face higher costs.
- Federal subsidies for lower-income Americans to buy policies would disappear.
Throughout the campaign, Biden repeatedly stressed the need to preserve the law’s provision barring insurance companies from refusing coverage for Americans with preexisting conditions, such as diabetes, cancer, and heart disease. It also outlaws charging higher premiums on the basis of health status, age, or gender.
Biden has also pledged to bolster the law as president.
He has proposed a variety of add-ons to the Affordable Care Act he says will “insure more than an estimated 97% of Americans,” according to the Biden campaign site.
Biden’s proposals include offering larger federal subsidies to help low- and middle-income Americans pay for policies purchased through Obamacare insurance Marketplaces.
The boldest of Biden’s proposals is the creation of a “public option” for insurance -- a Medicare-like program that small businesses and individuals could choose if they do not have coverage, cannot afford it, or don’t like their employer-based coverage.
It would also automatically enroll millions of uninsured Americans living in the 14 states that have not expanded Medicaid, which covers low-income people.
But such a plan would require congressional approval -- including a “super majority” of 60 Senate votes to block a likely GOP filibuster. That will be a significant challenge Biden will have to overcome, with Congress so evenly divided.
The White House would also have to defeat heavy lobbying from some of the most influential industry interest groups in Washington, Corlette says.
“I’m not even confident they would get all the Democrat votes,” she says.
“So, it’s a going to be an uphill battle to get a public option passed.”
Taken together, Biden’s plans for expanding Obamacare are projected to cost $750 billion over 10 years. He has said much of that financing would come from increasing taxes on the wealthy.
That means it would likely require congressional approval, which Antos suggests is unlikely given the polarization on Capitol Hill.
Medicare, Medicaid, and drug costs
Biden has called for a host of reforms targeting Medicare, Medicaid, and rising drug costs.
On Medicare, which primarily covers seniors 65 and older, Biden has proposed lowering the eligibility age from 65 to 60. That could extend Medicare to up to 20 million more Americans.
On Medicaid, the health care safety net for low-income and disabled Americans, the president-elect supports increased federal funding to states during the current economic crisis, and potentially beyond.
Medicare is likely to become a key focus of the new administration, in light of the pressures the pandemic is placing on Medicare funding.
In April, Medicare’s trustees said that the Part A trust fund for the program, which pays for hospital and inpatient care, could start to run dry in 2026.
But those projections did not include the impact of COVID-19. Some economists have since projected that Medicare Part A could become insolvent as early as 2022.
Medicare Part B, which pays for doctor and outpatient costs, is funded by general tax funding and beneficiary insurance premiums, so it is not in danger of drying up.
Adding to those pressures is an executive order Trump signed in August temporarily deferring payroll taxes, a primary funding vehicle for Medicare and Social Security.
Under these taxes, employees pay 6.2% of their earnings (on annual income up to $137,700) toward Social Security and 1.45% for Medicare taxes each pay period. Employers pay the same rate per paycheck, adding up to a combined 12.4% Social Security tax and 2.9% Medicare tax.
Biden has said he would reverse the tax cut when he takes office.
But to get a handle on Medicare and Medicaid funding issues, he is likely to need congressional support. Corlette and other experts say that could be a challenge while the nation remains in the grip of the coronavirus pandemic.
In addition to his Medicare and Medicaid reforms, Biden has proposed several plans to lower drug prices, a subset of rising health care and insurance costs.
U.S. spending on prescription drugs has increased nearly 42% over the past decade -- from $253.1 billion in 2010 to $358.7 billion in 2020 (projected) -- according to the Centers for Medicare & Medicaid Services.
In 2020, retail prices for 460 commonly prescribed drugs have spiked an average of 5.2%, according to new analysis by 3 Axis Advisors, a health research firm.
That’s more than double the projected rate of inflation.
To control drug costs, Biden supports legislation approved by the Democratic-led House of Representatives last year that would empower Medicare to negotiate drug prices with drug companies, as private insurers do.
Federal law now bars Medicare from negotiating prices on behalf of the 67.7 million Americans who use it. Drug companies and many GOP leaders argue that the current law is necessary to allow them to spend more on research and development of new medications.
In addition, Biden supports the idea of lifting bans on importing drugs from foreign countries with lower costs.
He also backs creating an independent review board to set price caps for new medications with no competitors; making high-quality generics more available; ending tax breaks for drug company advertising; and limiting their leeway in raising prices.
All of these proposals would likely require congressional approval and could face legal challenges in the courts.
This article first appeared on WebMD.com.
The former vice president has sketched out a big health agenda: ramping up the federal response to COVID-19, boosting the Affordable Care Act, creating a new “public option” to cover uninsured Americans, and expanding Medicare and Medicaid.
But the president-elect’s long to-do list on health is likely to face significant roadblocks in Congress and the courts, experts say.
For instance, Biden’s ambitious proposals on COVID-19 -- including his recent call for a national mask mandate -- could be waylaid by legal challenges and run into political hurdles on Capitol Hill, where he may face a divided Congress.
Joseph Antos, PhD, a health policy expert with the conservative American Enterprise Institute, predicts Biden will encounter the same type of congressional “gridlock situation” that President Barack Obama ran into during his second term.
“We have a situation that has been like this for a very, very long time -- lack of cooperation, lack of recognition that either party is capable of rising above their own electoral views to deal with problems that the country actually has.”
Antos also suggests that Biden may also face enormous political pressure to address the economic fallout from the coronavirus, including record unemployment and business closures, before anything else.
“I think it’s really going to be efforts that are intended to promote economic development and promote the economy,” he says.
In addition, Biden’s plans to expand Obamacare might face a new challenge from the Supreme Court in the year ahead. This month, the high court will take up a new case seeking to overturn the law.
Even so, experts say Biden’s plans on COVID-19 and expanding health care are likely to define his tenure in the White House as a central focus of his presidency.
“Health care will be at the very top of the list of the president’s priorities,” says Sabrina Corlette, JD, co-director of the Center on Health Insurance Reforms at Georgetown University’s McCourt School of Public Policy. “I do think, however, that the administration is going to be very preoccupied with the response to COVID-19 and the economic fallout … particularly in the first year.”
Here’s a closer look at what we can expect from a Biden presidency.
COVID-19: Federalizing response efforts
Biden will move to federalize the response to COVID-19. He has said he will take back major responsibilities from the states -- such as setting national policies on mask wearing, social distancing, and the reopening of schools and businesses, based on CDC guidance. In the days leading up to the election, Biden called for a national mask mandate, after waffling on the issue throughout the summer.
He has said he will let public health science drive political policy. Biden is also planning to create his own task force to advise officials during the transition on managing the new surge in COVID-19 cases, vaccine safety and protecting at-risk populations, Politico reported this week. He received a virtual briefing on the pandemic from a panel of experts as he awaited the election’s outcome.
“I think we will no longer have this confused and contradictory public messaging,” Corlette says, “but I also think there will be humility and the recognition that the evidence is evolving -- that we don’t have all the answers, but we’re learning as we go.”
But national mandates on masks and social distancing will be challenging to enforce, experts say. They are also likely to face pushback from business interests, opposition from public officials in GOP-led states, and even legal challenges.
Biden’s ability to work with Congress -- or not -- may determine whether he is able to implement some of the key components of his coronavirus action plan, which includes:
- Providing free COVID-19 testing for all Americans
- Hiring 100,000 contact tracers
- Eliminating out-of-pocket expenses for coronavirus treatment
- Delivering “sufficient” PPE for essential workers
- Supporting science-backed vaccines and medical treatments being developed
- Requiring the reopening of businesses, workplaces, and schools only after “sufficient” reductions in community transmission -- under evidence-based protocols put forward by the CDC
- Giving emergency paid leave for workers dislocated by the pandemic and more financial aid for workers, families, and small businesses
- Shoring up safeguards to protect at-risk Americans, including older people
- Boosting pay for health care workers on the front lines
Biden has not detailed how he would pay for many of these, beyond promising to force wealthy Americans to “pay their fair share” of taxes to help. He has proposed a tax increase on Americans making more than $400,000 a year, which would require congressional approval.
Antos says he expects Biden’s proposed COVID-19 action plan to be virtually the same as Trump’s in two areas: efforts to develop a vaccine and antiviral treatments.
The administration has spent some $225 million on COVID-19 testing efforts, with a particular focus on rural areas.
Trump launched Operation Warp Speed to fast-track a vaccine. As part of that, the federal government has contracted with six drug companies, spending nearly $11 billion. The operation aims to provide at least 300 million doses of a coronavirus vaccine by January 2021.
Antos would like to see “a more sophisticated approach to social distancing” from the president-elect that takes into account the different challenges facing Americans depending on their income, work situation, and other factors during the pandemic.
“There are a lot of people in this country where working from home is fine and their jobs are secure,” he notes. “It’s the person who used to work at a restaurant that closed, it’s the line worker at a factory that has severely cut back its hours. It’s basically lower-middle-class people, low-income people, middle-class people, and it’s not the elite.
“And the policies have not given enough consideration to the fact that their circumstances and their tradeoffs would differ from the tradeoffs of somebody who doesn’t have anything to worry about economically.
“So, what we need is a more supple policy [that] will give people the information they need and give them the financial support that they also need … so they can make good decisions for themselves and their families. And we basically haven’t done that.”
Obamacare on the blocks?
The Supreme Court’s decision to take up another case seeking to overturn the Affordable Care Act could hand Biden’s health agenda a major setback -- and put the medical care for millions of Americans in jeopardy.
On Nov. 10, the high court will hear oral arguments on a lawsuit that would strike down all of Obamacare. A decision is not expected until next year.
The court has previously upheld the 2010 law, which Biden helped usher through Congress as vice president. But the addition of right-leaning Supreme Court Justice Amy Coney Barrett to the bench last month gives the court a clear conservative majority that could mean the end of Obamacare, legal experts say.
Republicans have opposed the law since its passage, but they have been unable to muster the votes to repeal it, or to pass an alternative
Antos, from the American Enterprise Institute, notes conservatives believe the law has increased costs for health care and insurance over the past decade, in part because of its protections for Americans with preexisting conditions and requiring insurers to provide comprehensive “gold-plated” policies.
“It’s driven up costs, offers plans that are not very strong, put high-risk folks into the same [insurance pool], which has increased costs for everyone, the employer mandate … these are all the reasons,” he says.
The Supreme Court isn’t expected to deliver a decision on the Affordable Care Act before the middle of next year. But the uncertainty will likely push back Biden’s proposals to expand on the law.
Overturning Obamacare would have huge impacts on millions of Americans:
- As many as 133 million Americans -- roughly half the U.S. population -- with preexisting conditions could find it harder, if not impossible, to find affordable health insurance. That figure does not include Americans infected with COVID-19.
- About 165 million who require expensive treatments -- for cancer and other conditions -- would no longer be protected from huge costs for care by federal caps on out-of-pocket expenditures the Affordable Care Act requires.
- An estimated 21 million who now buy insurance through the Obamacare Marketplaces could lose their coverage.
- Another 12 million on Medicaid could find themselves without insurance.
- At least 2 million young adults ages 26 and under, now on their parents’ health policies, could be kicked off.
- Millions of people who use Medicare could face higher costs.
- Federal subsidies for lower-income Americans to buy policies would disappear.
Throughout the campaign, Biden repeatedly stressed the need to preserve the law’s provision barring insurance companies from refusing coverage for Americans with preexisting conditions, such as diabetes, cancer, and heart disease. It also outlaws charging higher premiums on the basis of health status, age, or gender.
Biden has also pledged to bolster the law as president.
He has proposed a variety of add-ons to the Affordable Care Act he says will “insure more than an estimated 97% of Americans,” according to the Biden campaign site.
Biden’s proposals include offering larger federal subsidies to help low- and middle-income Americans pay for policies purchased through Obamacare insurance Marketplaces.
The boldest of Biden’s proposals is the creation of a “public option” for insurance -- a Medicare-like program that small businesses and individuals could choose if they do not have coverage, cannot afford it, or don’t like their employer-based coverage.
It would also automatically enroll millions of uninsured Americans living in the 14 states that have not expanded Medicaid, which covers low-income people.
But such a plan would require congressional approval -- including a “super majority” of 60 Senate votes to block a likely GOP filibuster. That will be a significant challenge Biden will have to overcome, with Congress so evenly divided.
The White House would also have to defeat heavy lobbying from some of the most influential industry interest groups in Washington, Corlette says.
“I’m not even confident they would get all the Democrat votes,” she says.
“So, it’s a going to be an uphill battle to get a public option passed.”
Taken together, Biden’s plans for expanding Obamacare are projected to cost $750 billion over 10 years. He has said much of that financing would come from increasing taxes on the wealthy.
That means it would likely require congressional approval, which Antos suggests is unlikely given the polarization on Capitol Hill.
Medicare, Medicaid, and drug costs
Biden has called for a host of reforms targeting Medicare, Medicaid, and rising drug costs.
On Medicare, which primarily covers seniors 65 and older, Biden has proposed lowering the eligibility age from 65 to 60. That could extend Medicare to up to 20 million more Americans.
On Medicaid, the health care safety net for low-income and disabled Americans, the president-elect supports increased federal funding to states during the current economic crisis, and potentially beyond.
Medicare is likely to become a key focus of the new administration, in light of the pressures the pandemic is placing on Medicare funding.
In April, Medicare’s trustees said that the Part A trust fund for the program, which pays for hospital and inpatient care, could start to run dry in 2026.
But those projections did not include the impact of COVID-19. Some economists have since projected that Medicare Part A could become insolvent as early as 2022.
Medicare Part B, which pays for doctor and outpatient costs, is funded by general tax funding and beneficiary insurance premiums, so it is not in danger of drying up.
Adding to those pressures is an executive order Trump signed in August temporarily deferring payroll taxes, a primary funding vehicle for Medicare and Social Security.
Under these taxes, employees pay 6.2% of their earnings (on annual income up to $137,700) toward Social Security and 1.45% for Medicare taxes each pay period. Employers pay the same rate per paycheck, adding up to a combined 12.4% Social Security tax and 2.9% Medicare tax.
Biden has said he would reverse the tax cut when he takes office.
But to get a handle on Medicare and Medicaid funding issues, he is likely to need congressional support. Corlette and other experts say that could be a challenge while the nation remains in the grip of the coronavirus pandemic.
In addition to his Medicare and Medicaid reforms, Biden has proposed several plans to lower drug prices, a subset of rising health care and insurance costs.
U.S. spending on prescription drugs has increased nearly 42% over the past decade -- from $253.1 billion in 2010 to $358.7 billion in 2020 (projected) -- according to the Centers for Medicare & Medicaid Services.
In 2020, retail prices for 460 commonly prescribed drugs have spiked an average of 5.2%, according to new analysis by 3 Axis Advisors, a health research firm.
That’s more than double the projected rate of inflation.
To control drug costs, Biden supports legislation approved by the Democratic-led House of Representatives last year that would empower Medicare to negotiate drug prices with drug companies, as private insurers do.
Federal law now bars Medicare from negotiating prices on behalf of the 67.7 million Americans who use it. Drug companies and many GOP leaders argue that the current law is necessary to allow them to spend more on research and development of new medications.
In addition, Biden supports the idea of lifting bans on importing drugs from foreign countries with lower costs.
He also backs creating an independent review board to set price caps for new medications with no competitors; making high-quality generics more available; ending tax breaks for drug company advertising; and limiting their leeway in raising prices.
All of these proposals would likely require congressional approval and could face legal challenges in the courts.
This article first appeared on WebMD.com.
Why Accept a VA Detail or Short-Term Assignment? Benefits to Employees and the Service
In the Veterans Health Administration (VHA), there are frequent e-mails and requests for employees to accept a detail or short-term assignment across a wide range of positions from administrative to executive leadership. These opportunities afford an employee and the service line valuable benefits and growth opportunities; however, there are reasons why some may be reluctant to pursue these opportunities. In this article, we discuss the barriers to applying for and accepting detail positions and the benefits for the employee and the service lines during periods of standard operations as well as during emergencies requiring alternative staffing strategies.
Details are short-term assignments used to fill a vacant position while hiring for the permanent position or to fill a short-term need (eg, during a pandemic). Details usually last 30 to 120 days, though they may be extended, depending on the position, the number of people willing to serve in the detailed role, and the time to select a candidate for the permanent position. Details can be created for any skill level or type of position to meet an identified need, but they are most often needed for supervisory or leadership roles.
The COVID-19 pandemic has shed light on the importance of individuals’ flexibility and adaptability both within and between roles. Many US Department of Veterans Affairs (VA) facilities stood up Incident Command structures to support the changes required to adapt to the needs created by the pandemic. Establishing an Incident Command means that people within the organization must take on new responsibilities, and in many cases, they are detailed to new positions that were not needed or prioritized before the pandemic.
Barriers
An employee may be reluctant to apply for or accept a detail because he or she has little to no experience; feels uncomfortable stepping into an unfamiliar role; is concerned about making a leap from a clinical to administrative role; has uncertainty whether the job is a good professional fit; dislikes the lack of a pay increase during the detail period even if the new role has more responsibility; and has concern that serving in the detail may make them ineligible to apply for the permanent position due to a perception of being preselected. Additionally, the employee may recognize the added stress on colleagues because the same amount of work must be completed.
Benefits
Although leaving a position for a period of months can be stressful, serving in a detail position provides significant opportunities for professional growth. An employee can gain knowledge and experience in an unfamiliar role before applying for or committing to a permanent position. Those serving in temporary details are often given more support as colleagues and supervisors understand that the role was accepted on short notice with little time to prepare. Other benefits include expanding professional contacts, gaining perspective on a different part of the VHA, and working on skills, such as flexibility, time management, and perseverance. By succeeding in a detail, employees build professional acumen. After taking on additional challenges they become more competitive for future jobs. The VHA Executive Candidate Development Program requires a 120-day detail, serving as either assistant or associate director, chief of staff, or associate director for patient care services/nurse executive as part of the program.1
Temporarily leaving a service line to detail in a different service line has an impact on the home service because of the restrictions imposed. These restrictions guarantee that the employee can return to the original position at the end of a detail, thus providing a sense of job security; however, the home service line is down an employee.
Given these considerations, the following are key points to establish before undertaking the detail: (1) length of assignment; (2) once started, potential for the assignment to be extended; (3) will the employee be doing any of their prior job or just the new job or a blend of both; (4) possible changes in hours and site of work of the employee; (5) who will supervise the employee; (6) who will write the employee’s review; (7) training or skills needed prior to starting; (8) necessary paperwork; (9) how will the new assignment be communicated to others; (10) what happens if the detail ends sooner than planned; and (11) approval and support of all involved parties.
The employee’s home service may need a temporary plan to cover the employee’s workload, especially if the employee will be detailed to a different service line. The temporary plan may require creativity and flexibility and can be a way to trial the contingency plans for staffing the home service. One benefit to the home service is that the employee will have additional skills on returning that may benefit the home service, and the service will gain a potential leader.
When an employee goes to a different service, that service gains an employee who may bring a new perspective to help solve existing conflicts or problems. This can serve as a time to reset expectations or set new goals prior to the arrival of new leadership. If the detail is a good fit, then there is the chance that the employee may return in the future or refer others to it as a professional opportunity.
Conclusions
A detail can benefit the employee and the home and host services if planned in advance, and all parties support the process. A short-term leadership or administrative assignment can help an employee gain valuable experience for the future.
1. US Department of Veterans Affairs. Improve VA’s employee experience.obamaadministration.archives.performance.gov/node/65741.html. Published 2017. Accessed October 19, 2020.
In the Veterans Health Administration (VHA), there are frequent e-mails and requests for employees to accept a detail or short-term assignment across a wide range of positions from administrative to executive leadership. These opportunities afford an employee and the service line valuable benefits and growth opportunities; however, there are reasons why some may be reluctant to pursue these opportunities. In this article, we discuss the barriers to applying for and accepting detail positions and the benefits for the employee and the service lines during periods of standard operations as well as during emergencies requiring alternative staffing strategies.
Details are short-term assignments used to fill a vacant position while hiring for the permanent position or to fill a short-term need (eg, during a pandemic). Details usually last 30 to 120 days, though they may be extended, depending on the position, the number of people willing to serve in the detailed role, and the time to select a candidate for the permanent position. Details can be created for any skill level or type of position to meet an identified need, but they are most often needed for supervisory or leadership roles.
The COVID-19 pandemic has shed light on the importance of individuals’ flexibility and adaptability both within and between roles. Many US Department of Veterans Affairs (VA) facilities stood up Incident Command structures to support the changes required to adapt to the needs created by the pandemic. Establishing an Incident Command means that people within the organization must take on new responsibilities, and in many cases, they are detailed to new positions that were not needed or prioritized before the pandemic.
Barriers
An employee may be reluctant to apply for or accept a detail because he or she has little to no experience; feels uncomfortable stepping into an unfamiliar role; is concerned about making a leap from a clinical to administrative role; has uncertainty whether the job is a good professional fit; dislikes the lack of a pay increase during the detail period even if the new role has more responsibility; and has concern that serving in the detail may make them ineligible to apply for the permanent position due to a perception of being preselected. Additionally, the employee may recognize the added stress on colleagues because the same amount of work must be completed.
Benefits
Although leaving a position for a period of months can be stressful, serving in a detail position provides significant opportunities for professional growth. An employee can gain knowledge and experience in an unfamiliar role before applying for or committing to a permanent position. Those serving in temporary details are often given more support as colleagues and supervisors understand that the role was accepted on short notice with little time to prepare. Other benefits include expanding professional contacts, gaining perspective on a different part of the VHA, and working on skills, such as flexibility, time management, and perseverance. By succeeding in a detail, employees build professional acumen. After taking on additional challenges they become more competitive for future jobs. The VHA Executive Candidate Development Program requires a 120-day detail, serving as either assistant or associate director, chief of staff, or associate director for patient care services/nurse executive as part of the program.1
Temporarily leaving a service line to detail in a different service line has an impact on the home service because of the restrictions imposed. These restrictions guarantee that the employee can return to the original position at the end of a detail, thus providing a sense of job security; however, the home service line is down an employee.
Given these considerations, the following are key points to establish before undertaking the detail: (1) length of assignment; (2) once started, potential for the assignment to be extended; (3) will the employee be doing any of their prior job or just the new job or a blend of both; (4) possible changes in hours and site of work of the employee; (5) who will supervise the employee; (6) who will write the employee’s review; (7) training or skills needed prior to starting; (8) necessary paperwork; (9) how will the new assignment be communicated to others; (10) what happens if the detail ends sooner than planned; and (11) approval and support of all involved parties.
The employee’s home service may need a temporary plan to cover the employee’s workload, especially if the employee will be detailed to a different service line. The temporary plan may require creativity and flexibility and can be a way to trial the contingency plans for staffing the home service. One benefit to the home service is that the employee will have additional skills on returning that may benefit the home service, and the service will gain a potential leader.
When an employee goes to a different service, that service gains an employee who may bring a new perspective to help solve existing conflicts or problems. This can serve as a time to reset expectations or set new goals prior to the arrival of new leadership. If the detail is a good fit, then there is the chance that the employee may return in the future or refer others to it as a professional opportunity.
Conclusions
A detail can benefit the employee and the home and host services if planned in advance, and all parties support the process. A short-term leadership or administrative assignment can help an employee gain valuable experience for the future.
In the Veterans Health Administration (VHA), there are frequent e-mails and requests for employees to accept a detail or short-term assignment across a wide range of positions from administrative to executive leadership. These opportunities afford an employee and the service line valuable benefits and growth opportunities; however, there are reasons why some may be reluctant to pursue these opportunities. In this article, we discuss the barriers to applying for and accepting detail positions and the benefits for the employee and the service lines during periods of standard operations as well as during emergencies requiring alternative staffing strategies.
Details are short-term assignments used to fill a vacant position while hiring for the permanent position or to fill a short-term need (eg, during a pandemic). Details usually last 30 to 120 days, though they may be extended, depending on the position, the number of people willing to serve in the detailed role, and the time to select a candidate for the permanent position. Details can be created for any skill level or type of position to meet an identified need, but they are most often needed for supervisory or leadership roles.
The COVID-19 pandemic has shed light on the importance of individuals’ flexibility and adaptability both within and between roles. Many US Department of Veterans Affairs (VA) facilities stood up Incident Command structures to support the changes required to adapt to the needs created by the pandemic. Establishing an Incident Command means that people within the organization must take on new responsibilities, and in many cases, they are detailed to new positions that were not needed or prioritized before the pandemic.
Barriers
An employee may be reluctant to apply for or accept a detail because he or she has little to no experience; feels uncomfortable stepping into an unfamiliar role; is concerned about making a leap from a clinical to administrative role; has uncertainty whether the job is a good professional fit; dislikes the lack of a pay increase during the detail period even if the new role has more responsibility; and has concern that serving in the detail may make them ineligible to apply for the permanent position due to a perception of being preselected. Additionally, the employee may recognize the added stress on colleagues because the same amount of work must be completed.
Benefits
Although leaving a position for a period of months can be stressful, serving in a detail position provides significant opportunities for professional growth. An employee can gain knowledge and experience in an unfamiliar role before applying for or committing to a permanent position. Those serving in temporary details are often given more support as colleagues and supervisors understand that the role was accepted on short notice with little time to prepare. Other benefits include expanding professional contacts, gaining perspective on a different part of the VHA, and working on skills, such as flexibility, time management, and perseverance. By succeeding in a detail, employees build professional acumen. After taking on additional challenges they become more competitive for future jobs. The VHA Executive Candidate Development Program requires a 120-day detail, serving as either assistant or associate director, chief of staff, or associate director for patient care services/nurse executive as part of the program.1
Temporarily leaving a service line to detail in a different service line has an impact on the home service because of the restrictions imposed. These restrictions guarantee that the employee can return to the original position at the end of a detail, thus providing a sense of job security; however, the home service line is down an employee.
Given these considerations, the following are key points to establish before undertaking the detail: (1) length of assignment; (2) once started, potential for the assignment to be extended; (3) will the employee be doing any of their prior job or just the new job or a blend of both; (4) possible changes in hours and site of work of the employee; (5) who will supervise the employee; (6) who will write the employee’s review; (7) training or skills needed prior to starting; (8) necessary paperwork; (9) how will the new assignment be communicated to others; (10) what happens if the detail ends sooner than planned; and (11) approval and support of all involved parties.
The employee’s home service may need a temporary plan to cover the employee’s workload, especially if the employee will be detailed to a different service line. The temporary plan may require creativity and flexibility and can be a way to trial the contingency plans for staffing the home service. One benefit to the home service is that the employee will have additional skills on returning that may benefit the home service, and the service will gain a potential leader.
When an employee goes to a different service, that service gains an employee who may bring a new perspective to help solve existing conflicts or problems. This can serve as a time to reset expectations or set new goals prior to the arrival of new leadership. If the detail is a good fit, then there is the chance that the employee may return in the future or refer others to it as a professional opportunity.
Conclusions
A detail can benefit the employee and the home and host services if planned in advance, and all parties support the process. A short-term leadership or administrative assignment can help an employee gain valuable experience for the future.
1. US Department of Veterans Affairs. Improve VA’s employee experience.obamaadministration.archives.performance.gov/node/65741.html. Published 2017. Accessed October 19, 2020.
1. US Department of Veterans Affairs. Improve VA’s employee experience.obamaadministration.archives.performance.gov/node/65741.html. Published 2017. Accessed October 19, 2020.
Can an ‘unheard of’ approach up adherence to public health advice?
Using principles of psychoanalysis to craft public health messaging may be a novel and effective way of increasing adherence to public health advice during the COVID-19 pandemic, experts say.
In a letter published online Oct. 19 in The Lancet, coauthors Austin Ratner, MD, and Nisarg Gandhi, believe that, as expert communicators, psychoanalysts should be part of the public health care team to help battle the pandemic.
“The idea of using psychoanalysis in a public health setting is relatively unheard of,” Ratner, the author of a book titled “The Psychoanalyst’s Aversion to Proof,” told Medscape Medical News. Ratner earned his MD at John Hopkins School of Medicine but left medicine to become an author. Gandhi is a clinical research intern at Saint Barnabas Medical Center in Livingston, New Jersey.
Psychoanalysis postulates that defense mechanisms, such as denial, may play an important role in nonadherence to public health guidance regarding the pandemic, Ratner said.
including nonadherence to medical advice regarding COVID-19, as well as climate change and politics.
“By understanding that fear and anxiety underpin a lot of denial, the psychoanalytic viewpoint can help influence public health officials in recognizing the fear and anxiety, how to talk about the threat [of the pandemic], and what can be done about it,” he added.
“A new partnership”
“Psychoanalysts have historically resisted collaboration with disciplines such as social and experimental psychology,” Ratner said. This “insularity” results in “lost opportunities on the path for psychoanalysis to become part of the conversation regarding mass denial and mass nonadherence to medical advice.”
He noted that change is afoot in the psychoanalytic community. The American Psychoanalytic Association (APsaA) has begun to “empower constituents” who seek greater “integration with experimental science and greater involvement with public health.”
To that end, Ratner suggests a “new partnership” between three fields that have until now been disparate: experimental psychology, public health, and psychoanalysis.
Cognitive scientists have studied and documented denial, attributing it to “anxiety’s power to compromise rational thought,” but their approach has not focused on the psychoanalytic model of denial as a defense mechanism, Ratner observed.
Mark Smaller, PhD, past president of APsaA and board member of the International Psychoanalytical Association, elaborated.
“From a psychoanalytic perspective, I am interested in how a defense mechanism functions for individuals and groups,” Smaller told Medscape Medical News.
Denial as a defense mechanism often arises, whether in individuals or groups, from a sense of helplessness, explained Smaller, who is also the chair of the department of public advocacy at APsaA.
“People can only tolerate a certain amount of helplessness – in fact, I would suggest as an analyst that helplessness is the most difficult feeling for humans to come to terms with,” he said.
Helplessness can contribute to trauma and “I think we have a mass case of traumatic helplessness in our country right now because of the pandemic.”
Some people respond to a sense of helplessness with depression or hopelessness, while others “try to integrate the impact of the pandemic by focusing on things over which they have control, like wearing a mask, social distancing, and avoiding places with large numbers of people where the virus can be easily transmitted,” said Smaller.
However, “what seems to have occurred in our country is that, although many people have focused on what we do have control of, a large segment of our population are acting as if COVID-19 doesn’t exist, and we have leadership supporting this denial,” he added.
Is “denial” evidence-based?
Commenting for Medscape Medical News, Richard McAnulty, PhD, associate professor of psychology at the University of North Carolina at Charlotte expressed skepticism about the psychoanalytic view of denial, and its potential role in addressing the pandemic.
“A key criticism of psychoanalytic and psychodynamic viewpoints is that many – including the concept of a subconscious mind – are theoretical, not open to empirical research, and not measurable; and one of the most fundamental requirements in science is that all your constructs are measurable.”
For this reason, this approach is “limited in usefulness, although it might be an interesting source of speculation,” said McAnulty.
Ratner disagreed, noting that there is research corroborating the existence of an unconscious mind. Noted analyst Carl Jung, Ratner pointed out, conducted “some great experiments to prove some of the central tenets of psychoanalysis using word associations.”
Jung found that, if individuals were challenged with words that evoked painful associations, it took them longer to arrive at the answer to the test. They also made more mistakes.
Jung’s research “goes back to a core idea of psychoanalysis, which is that painful or difficult thoughts and feelings get distorted, pushed out of consciousness, forgotten, delayed, or suppressed,” Ratner said. These responses might account for “what we’re seeing the U.S. that people are resorting to irrational thinking without being aware of it.”
McAnulty suggested that the psychodynamic idea of denial as a defense mechanism is not relevant to mass nonadherence to pandemic-related medical advice.
Rather, the denial stems from “schemas and belief systems about the world, how people should operate and behave, and the role of government and the medical establishment,” he said.
“When certain recommendations are discrepant with the world view, it creates dissonance or a mismatch and the person will try to reconcile the mismatch,” McAnulty continued. “One way to do that is to say that these recommendations are invalid because they violate the individual’s political beliefs, world view, or religious ideas.”
Ultimately, “it depends on how we define denial,” said McAnulty. “If it means dismissing information that doesn’t fit an existing belief system, that’s denial, but the psychodynamic meaning of ‘denial’ is much deeper than that.”
Smaller, the past president of APsaA, emphasized the importance of empathy when addressing the public. “Psychoanalysts bring empathy to irrationality. Having a psychoanalyst as a team member can help public health officials to communicate better and craft the understanding of anxiety and fear into their message.”
Ratner said he is “not proposing a simplistic silver bullet as an answer to a very complex, multifaceted problem of nonadherence to medical advice.”
Instead, he is “proposing something that hasn’t happened yet, which is more research and more conversation, with psychoanalysis as part of the conversation, because the notion of denial is so relevant, despite how many other factors are involved.”
Ratner, Gandhi, Smaller, and McAnulty have disclosed no relevant financial relationships. Ratner is the author of The Psychoanalyst’s Aversion to Proof and the medical textbook Concepts in Medical Physiology.
This article first appeared on Medscape.com.
Using principles of psychoanalysis to craft public health messaging may be a novel and effective way of increasing adherence to public health advice during the COVID-19 pandemic, experts say.
In a letter published online Oct. 19 in The Lancet, coauthors Austin Ratner, MD, and Nisarg Gandhi, believe that, as expert communicators, psychoanalysts should be part of the public health care team to help battle the pandemic.
“The idea of using psychoanalysis in a public health setting is relatively unheard of,” Ratner, the author of a book titled “The Psychoanalyst’s Aversion to Proof,” told Medscape Medical News. Ratner earned his MD at John Hopkins School of Medicine but left medicine to become an author. Gandhi is a clinical research intern at Saint Barnabas Medical Center in Livingston, New Jersey.
Psychoanalysis postulates that defense mechanisms, such as denial, may play an important role in nonadherence to public health guidance regarding the pandemic, Ratner said.
including nonadherence to medical advice regarding COVID-19, as well as climate change and politics.
“By understanding that fear and anxiety underpin a lot of denial, the psychoanalytic viewpoint can help influence public health officials in recognizing the fear and anxiety, how to talk about the threat [of the pandemic], and what can be done about it,” he added.
“A new partnership”
“Psychoanalysts have historically resisted collaboration with disciplines such as social and experimental psychology,” Ratner said. This “insularity” results in “lost opportunities on the path for psychoanalysis to become part of the conversation regarding mass denial and mass nonadherence to medical advice.”
He noted that change is afoot in the psychoanalytic community. The American Psychoanalytic Association (APsaA) has begun to “empower constituents” who seek greater “integration with experimental science and greater involvement with public health.”
To that end, Ratner suggests a “new partnership” between three fields that have until now been disparate: experimental psychology, public health, and psychoanalysis.
Cognitive scientists have studied and documented denial, attributing it to “anxiety’s power to compromise rational thought,” but their approach has not focused on the psychoanalytic model of denial as a defense mechanism, Ratner observed.
Mark Smaller, PhD, past president of APsaA and board member of the International Psychoanalytical Association, elaborated.
“From a psychoanalytic perspective, I am interested in how a defense mechanism functions for individuals and groups,” Smaller told Medscape Medical News.
Denial as a defense mechanism often arises, whether in individuals or groups, from a sense of helplessness, explained Smaller, who is also the chair of the department of public advocacy at APsaA.
“People can only tolerate a certain amount of helplessness – in fact, I would suggest as an analyst that helplessness is the most difficult feeling for humans to come to terms with,” he said.
Helplessness can contribute to trauma and “I think we have a mass case of traumatic helplessness in our country right now because of the pandemic.”
Some people respond to a sense of helplessness with depression or hopelessness, while others “try to integrate the impact of the pandemic by focusing on things over which they have control, like wearing a mask, social distancing, and avoiding places with large numbers of people where the virus can be easily transmitted,” said Smaller.
However, “what seems to have occurred in our country is that, although many people have focused on what we do have control of, a large segment of our population are acting as if COVID-19 doesn’t exist, and we have leadership supporting this denial,” he added.
Is “denial” evidence-based?
Commenting for Medscape Medical News, Richard McAnulty, PhD, associate professor of psychology at the University of North Carolina at Charlotte expressed skepticism about the psychoanalytic view of denial, and its potential role in addressing the pandemic.
“A key criticism of psychoanalytic and psychodynamic viewpoints is that many – including the concept of a subconscious mind – are theoretical, not open to empirical research, and not measurable; and one of the most fundamental requirements in science is that all your constructs are measurable.”
For this reason, this approach is “limited in usefulness, although it might be an interesting source of speculation,” said McAnulty.
Ratner disagreed, noting that there is research corroborating the existence of an unconscious mind. Noted analyst Carl Jung, Ratner pointed out, conducted “some great experiments to prove some of the central tenets of psychoanalysis using word associations.”
Jung found that, if individuals were challenged with words that evoked painful associations, it took them longer to arrive at the answer to the test. They also made more mistakes.
Jung’s research “goes back to a core idea of psychoanalysis, which is that painful or difficult thoughts and feelings get distorted, pushed out of consciousness, forgotten, delayed, or suppressed,” Ratner said. These responses might account for “what we’re seeing the U.S. that people are resorting to irrational thinking without being aware of it.”
McAnulty suggested that the psychodynamic idea of denial as a defense mechanism is not relevant to mass nonadherence to pandemic-related medical advice.
Rather, the denial stems from “schemas and belief systems about the world, how people should operate and behave, and the role of government and the medical establishment,” he said.
“When certain recommendations are discrepant with the world view, it creates dissonance or a mismatch and the person will try to reconcile the mismatch,” McAnulty continued. “One way to do that is to say that these recommendations are invalid because they violate the individual’s political beliefs, world view, or religious ideas.”
Ultimately, “it depends on how we define denial,” said McAnulty. “If it means dismissing information that doesn’t fit an existing belief system, that’s denial, but the psychodynamic meaning of ‘denial’ is much deeper than that.”
Smaller, the past president of APsaA, emphasized the importance of empathy when addressing the public. “Psychoanalysts bring empathy to irrationality. Having a psychoanalyst as a team member can help public health officials to communicate better and craft the understanding of anxiety and fear into their message.”
Ratner said he is “not proposing a simplistic silver bullet as an answer to a very complex, multifaceted problem of nonadherence to medical advice.”
Instead, he is “proposing something that hasn’t happened yet, which is more research and more conversation, with psychoanalysis as part of the conversation, because the notion of denial is so relevant, despite how many other factors are involved.”
Ratner, Gandhi, Smaller, and McAnulty have disclosed no relevant financial relationships. Ratner is the author of The Psychoanalyst’s Aversion to Proof and the medical textbook Concepts in Medical Physiology.
This article first appeared on Medscape.com.
Using principles of psychoanalysis to craft public health messaging may be a novel and effective way of increasing adherence to public health advice during the COVID-19 pandemic, experts say.
In a letter published online Oct. 19 in The Lancet, coauthors Austin Ratner, MD, and Nisarg Gandhi, believe that, as expert communicators, psychoanalysts should be part of the public health care team to help battle the pandemic.
“The idea of using psychoanalysis in a public health setting is relatively unheard of,” Ratner, the author of a book titled “The Psychoanalyst’s Aversion to Proof,” told Medscape Medical News. Ratner earned his MD at John Hopkins School of Medicine but left medicine to become an author. Gandhi is a clinical research intern at Saint Barnabas Medical Center in Livingston, New Jersey.
Psychoanalysis postulates that defense mechanisms, such as denial, may play an important role in nonadherence to public health guidance regarding the pandemic, Ratner said.
including nonadherence to medical advice regarding COVID-19, as well as climate change and politics.
“By understanding that fear and anxiety underpin a lot of denial, the psychoanalytic viewpoint can help influence public health officials in recognizing the fear and anxiety, how to talk about the threat [of the pandemic], and what can be done about it,” he added.
“A new partnership”
“Psychoanalysts have historically resisted collaboration with disciplines such as social and experimental psychology,” Ratner said. This “insularity” results in “lost opportunities on the path for psychoanalysis to become part of the conversation regarding mass denial and mass nonadherence to medical advice.”
He noted that change is afoot in the psychoanalytic community. The American Psychoanalytic Association (APsaA) has begun to “empower constituents” who seek greater “integration with experimental science and greater involvement with public health.”
To that end, Ratner suggests a “new partnership” between three fields that have until now been disparate: experimental psychology, public health, and psychoanalysis.
Cognitive scientists have studied and documented denial, attributing it to “anxiety’s power to compromise rational thought,” but their approach has not focused on the psychoanalytic model of denial as a defense mechanism, Ratner observed.
Mark Smaller, PhD, past president of APsaA and board member of the International Psychoanalytical Association, elaborated.
“From a psychoanalytic perspective, I am interested in how a defense mechanism functions for individuals and groups,” Smaller told Medscape Medical News.
Denial as a defense mechanism often arises, whether in individuals or groups, from a sense of helplessness, explained Smaller, who is also the chair of the department of public advocacy at APsaA.
“People can only tolerate a certain amount of helplessness – in fact, I would suggest as an analyst that helplessness is the most difficult feeling for humans to come to terms with,” he said.
Helplessness can contribute to trauma and “I think we have a mass case of traumatic helplessness in our country right now because of the pandemic.”
Some people respond to a sense of helplessness with depression or hopelessness, while others “try to integrate the impact of the pandemic by focusing on things over which they have control, like wearing a mask, social distancing, and avoiding places with large numbers of people where the virus can be easily transmitted,” said Smaller.
However, “what seems to have occurred in our country is that, although many people have focused on what we do have control of, a large segment of our population are acting as if COVID-19 doesn’t exist, and we have leadership supporting this denial,” he added.
Is “denial” evidence-based?
Commenting for Medscape Medical News, Richard McAnulty, PhD, associate professor of psychology at the University of North Carolina at Charlotte expressed skepticism about the psychoanalytic view of denial, and its potential role in addressing the pandemic.
“A key criticism of psychoanalytic and psychodynamic viewpoints is that many – including the concept of a subconscious mind – are theoretical, not open to empirical research, and not measurable; and one of the most fundamental requirements in science is that all your constructs are measurable.”
For this reason, this approach is “limited in usefulness, although it might be an interesting source of speculation,” said McAnulty.
Ratner disagreed, noting that there is research corroborating the existence of an unconscious mind. Noted analyst Carl Jung, Ratner pointed out, conducted “some great experiments to prove some of the central tenets of psychoanalysis using word associations.”
Jung found that, if individuals were challenged with words that evoked painful associations, it took them longer to arrive at the answer to the test. They also made more mistakes.
Jung’s research “goes back to a core idea of psychoanalysis, which is that painful or difficult thoughts and feelings get distorted, pushed out of consciousness, forgotten, delayed, or suppressed,” Ratner said. These responses might account for “what we’re seeing the U.S. that people are resorting to irrational thinking without being aware of it.”
McAnulty suggested that the psychodynamic idea of denial as a defense mechanism is not relevant to mass nonadherence to pandemic-related medical advice.
Rather, the denial stems from “schemas and belief systems about the world, how people should operate and behave, and the role of government and the medical establishment,” he said.
“When certain recommendations are discrepant with the world view, it creates dissonance or a mismatch and the person will try to reconcile the mismatch,” McAnulty continued. “One way to do that is to say that these recommendations are invalid because they violate the individual’s political beliefs, world view, or religious ideas.”
Ultimately, “it depends on how we define denial,” said McAnulty. “If it means dismissing information that doesn’t fit an existing belief system, that’s denial, but the psychodynamic meaning of ‘denial’ is much deeper than that.”
Smaller, the past president of APsaA, emphasized the importance of empathy when addressing the public. “Psychoanalysts bring empathy to irrationality. Having a psychoanalyst as a team member can help public health officials to communicate better and craft the understanding of anxiety and fear into their message.”
Ratner said he is “not proposing a simplistic silver bullet as an answer to a very complex, multifaceted problem of nonadherence to medical advice.”
Instead, he is “proposing something that hasn’t happened yet, which is more research and more conversation, with psychoanalysis as part of the conversation, because the notion of denial is so relevant, despite how many other factors are involved.”
Ratner, Gandhi, Smaller, and McAnulty have disclosed no relevant financial relationships. Ratner is the author of The Psychoanalyst’s Aversion to Proof and the medical textbook Concepts in Medical Physiology.
This article first appeared on Medscape.com.
Syphilis: Cutting risk through primary prevention and prenatal screening
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
Apps for applying to ObGyn residency programs in the era of virtual interviews
The coronavirus disease 2019 (COVID-19) pandemic has upended the traditional 2020–2021 application season for ObGyn residency programs. In May 2020, the 2 national ObGyn education organizations, the Association of Professors of Gynecology and Obstetrics (APGO) and Council on Resident Education in ObGyn (CREOG), issued guidelines to ensure a fair and equitable application process.1 These guidelines are consistent with recommendations from the Association of American Medical Colleges (AAMC) and the Coalition for Physician Accountability. Important recommendations include:
- limiting away rotations
- being flexible in the number of specialty-specific letters of recommendation required
- encouraging residency programs to develop alternate means of conveying information about their curriculum.
In addition, these statements provide timing on when programs should release interview offers and when to begin interviews. Finally, programs are required to commit to online interviews and virtual visits for all applicants, including local students, rather than in-person interviews.
Here, we focus on identifying apps that students can use to help them with the application process—apps for the nuts and bolts of applying and interviewing and apps to learn more about individual programs.
Students must use the Electronic Residency Application Service (ERAS) platform from AAMC to enter their information and register with the National Resident Matching Program (NRMP). Students also must use the ERAS to submit their applications to their selected residency programs. The ERAS platform does not include an app to aid in the completion or submission of an application. The NRMP has developed the MATCH PRISM app, but this does not allow students to register for the match or submit their rank list. To learn about how to schedule interviews, residency programs may use one of the following sources: ERAS, Interview Broker, or Thalamus. Moreover, APGO/CREOG has partnered with Thalamus for the upcoming application cycle, which provides residency programs and applicants tools for application management, interview scheduling, and itinerary building. Thalamus offers a free app.
This year offers some unique challenges. The application process for ObGyn residencies is likely to be more competitive, and students face the added stress of having to navigate the interview season:
- without away rotations (audition interviews)
- without in-person visits of the city/hospital/program or social events before or after interview day
- with an all-virtual interview day.
Continue to: To find information on individual residency programs...
To find information on individual residency programs, the APGO website lists the FREIDA and APGO Residency Directories, which are not apps. Students are also aware of the Doximity Residency Navigator, which does include an app. The NRMP MATCH PRISM app is another resource, as it provides students with a directory of residency programs and information about each program.
The American College of Obstetricians and Gynecologists (ACOG) recognizes that residency program websites and social media will be crucial in helping applicants learn about individual programs, faculty, and residents. As such, ACOG hosted a Virtual Residency Showcase in September 2020 in which programs posted content on Instagram and Twitter using the hashtag #ACOG-ResWeek20.2 Similarly, APGO and CREOG produced a report containing a social media directory, which lists individual residency programs and whether or not they have a social media handle/account.3 In a recent webinar,4 Drs. Sarah Santiago and Elizabeth Southworth noted that the number of residency programs that have an Instagram account more than doubled (from 60 to 128) between May and September 2020.
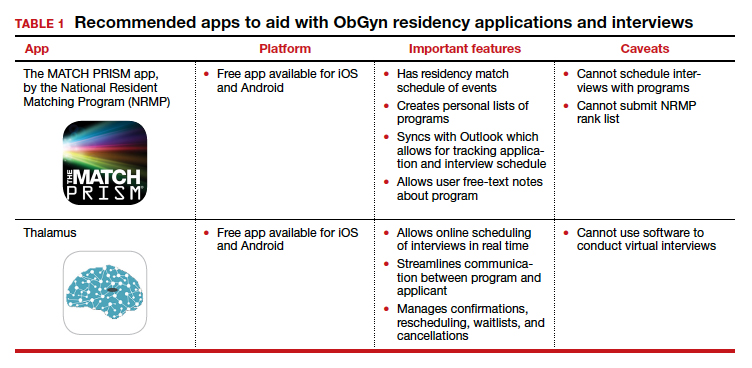
We present 2 tables describing the important features and caveats of apps available to students to assist them with residency applications this year—TABLE 1 summarizes apps to aid with applications and interviews; TABLE 2 lists apps designed for students to learn more about individual residency programs. We wish all of this year’s students every success in their search for the right program. ●
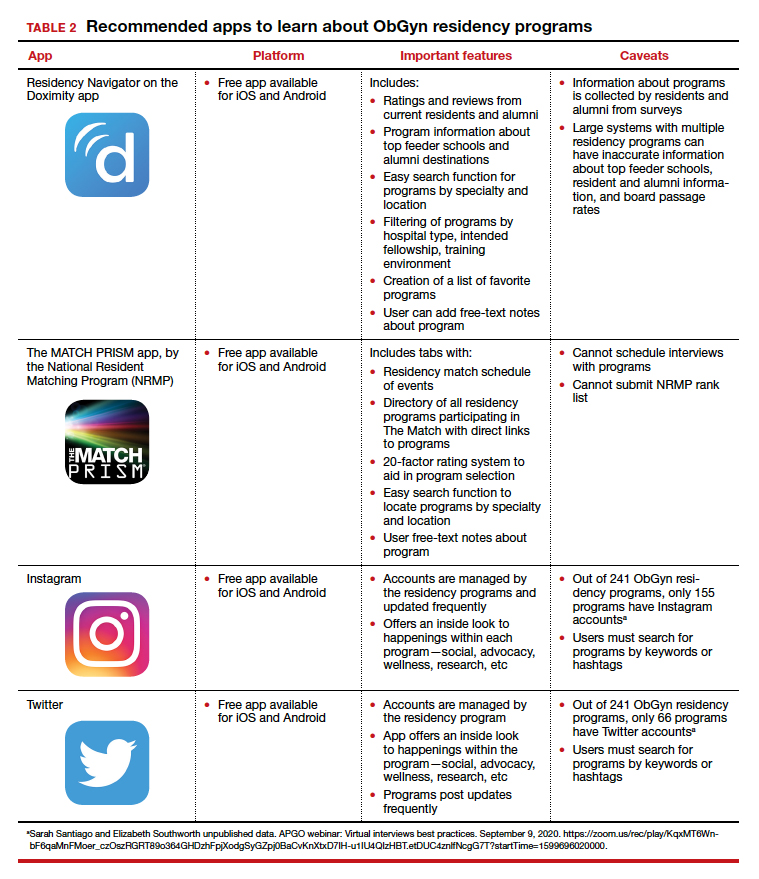
- Association of Professors of Gynecology and Obstetrics, Council on Resident Education in ObGyn. Updated APGO and CREOG Residency Application Response to COVID-19. https://www.apgo.org/wp-content/uploads/2020/05 /Updated-APGO-CREOG-Residency-Response-to -COVID-19-.pdf. Accessed October 27, 2020.
- https://www.acog.org/education-and-events/webinars /virtual-residency-showcase. Accessed October 4, 2020.
- Social media directory-ObGyn. https://docs.google.com /spreadsheets/d/e/2PACX-1vQ6boyn7FWV9tEhfQp1o3 XJgNIPNBQ3qCYf4IpV-rOPcd212J-HNR84p0r85nXrAz MvOmcNlgjywDP/pubhtml?gid=1472916499&single =true. Accessed October 27, 2020.
- APGO webinar: Virtual interviews best practices. September 9, 2020. https://zoom.us/rec/play/KqxMT6Wnb F6qaMnFMoer_czOszRGRT89o364GHDzhFpjXodgSyGZpj 0BaCvKnXtxD7IH-u1IU4QIzHBT.etDUC4znlfNcgG7T?start Time=1599696020000. Accessed October 4, 2020.
The coronavirus disease 2019 (COVID-19) pandemic has upended the traditional 2020–2021 application season for ObGyn residency programs. In May 2020, the 2 national ObGyn education organizations, the Association of Professors of Gynecology and Obstetrics (APGO) and Council on Resident Education in ObGyn (CREOG), issued guidelines to ensure a fair and equitable application process.1 These guidelines are consistent with recommendations from the Association of American Medical Colleges (AAMC) and the Coalition for Physician Accountability. Important recommendations include:
- limiting away rotations
- being flexible in the number of specialty-specific letters of recommendation required
- encouraging residency programs to develop alternate means of conveying information about their curriculum.
In addition, these statements provide timing on when programs should release interview offers and when to begin interviews. Finally, programs are required to commit to online interviews and virtual visits for all applicants, including local students, rather than in-person interviews.
Here, we focus on identifying apps that students can use to help them with the application process—apps for the nuts and bolts of applying and interviewing and apps to learn more about individual programs.
Students must use the Electronic Residency Application Service (ERAS) platform from AAMC to enter their information and register with the National Resident Matching Program (NRMP). Students also must use the ERAS to submit their applications to their selected residency programs. The ERAS platform does not include an app to aid in the completion or submission of an application. The NRMP has developed the MATCH PRISM app, but this does not allow students to register for the match or submit their rank list. To learn about how to schedule interviews, residency programs may use one of the following sources: ERAS, Interview Broker, or Thalamus. Moreover, APGO/CREOG has partnered with Thalamus for the upcoming application cycle, which provides residency programs and applicants tools for application management, interview scheduling, and itinerary building. Thalamus offers a free app.
This year offers some unique challenges. The application process for ObGyn residencies is likely to be more competitive, and students face the added stress of having to navigate the interview season:
- without away rotations (audition interviews)
- without in-person visits of the city/hospital/program or social events before or after interview day
- with an all-virtual interview day.
Continue to: To find information on individual residency programs...
To find information on individual residency programs, the APGO website lists the FREIDA and APGO Residency Directories, which are not apps. Students are also aware of the Doximity Residency Navigator, which does include an app. The NRMP MATCH PRISM app is another resource, as it provides students with a directory of residency programs and information about each program.
The American College of Obstetricians and Gynecologists (ACOG) recognizes that residency program websites and social media will be crucial in helping applicants learn about individual programs, faculty, and residents. As such, ACOG hosted a Virtual Residency Showcase in September 2020 in which programs posted content on Instagram and Twitter using the hashtag #ACOG-ResWeek20.2 Similarly, APGO and CREOG produced a report containing a social media directory, which lists individual residency programs and whether or not they have a social media handle/account.3 In a recent webinar,4 Drs. Sarah Santiago and Elizabeth Southworth noted that the number of residency programs that have an Instagram account more than doubled (from 60 to 128) between May and September 2020.

We present 2 tables describing the important features and caveats of apps available to students to assist them with residency applications this year—TABLE 1 summarizes apps to aid with applications and interviews; TABLE 2 lists apps designed for students to learn more about individual residency programs. We wish all of this year’s students every success in their search for the right program. ●

The coronavirus disease 2019 (COVID-19) pandemic has upended the traditional 2020–2021 application season for ObGyn residency programs. In May 2020, the 2 national ObGyn education organizations, the Association of Professors of Gynecology and Obstetrics (APGO) and Council on Resident Education in ObGyn (CREOG), issued guidelines to ensure a fair and equitable application process.1 These guidelines are consistent with recommendations from the Association of American Medical Colleges (AAMC) and the Coalition for Physician Accountability. Important recommendations include:
- limiting away rotations
- being flexible in the number of specialty-specific letters of recommendation required
- encouraging residency programs to develop alternate means of conveying information about their curriculum.
In addition, these statements provide timing on when programs should release interview offers and when to begin interviews. Finally, programs are required to commit to online interviews and virtual visits for all applicants, including local students, rather than in-person interviews.
Here, we focus on identifying apps that students can use to help them with the application process—apps for the nuts and bolts of applying and interviewing and apps to learn more about individual programs.
Students must use the Electronic Residency Application Service (ERAS) platform from AAMC to enter their information and register with the National Resident Matching Program (NRMP). Students also must use the ERAS to submit their applications to their selected residency programs. The ERAS platform does not include an app to aid in the completion or submission of an application. The NRMP has developed the MATCH PRISM app, but this does not allow students to register for the match or submit their rank list. To learn about how to schedule interviews, residency programs may use one of the following sources: ERAS, Interview Broker, or Thalamus. Moreover, APGO/CREOG has partnered with Thalamus for the upcoming application cycle, which provides residency programs and applicants tools for application management, interview scheduling, and itinerary building. Thalamus offers a free app.
This year offers some unique challenges. The application process for ObGyn residencies is likely to be more competitive, and students face the added stress of having to navigate the interview season:
- without away rotations (audition interviews)
- without in-person visits of the city/hospital/program or social events before or after interview day
- with an all-virtual interview day.
Continue to: To find information on individual residency programs...
To find information on individual residency programs, the APGO website lists the FREIDA and APGO Residency Directories, which are not apps. Students are also aware of the Doximity Residency Navigator, which does include an app. The NRMP MATCH PRISM app is another resource, as it provides students with a directory of residency programs and information about each program.
The American College of Obstetricians and Gynecologists (ACOG) recognizes that residency program websites and social media will be crucial in helping applicants learn about individual programs, faculty, and residents. As such, ACOG hosted a Virtual Residency Showcase in September 2020 in which programs posted content on Instagram and Twitter using the hashtag #ACOG-ResWeek20.2 Similarly, APGO and CREOG produced a report containing a social media directory, which lists individual residency programs and whether or not they have a social media handle/account.3 In a recent webinar,4 Drs. Sarah Santiago and Elizabeth Southworth noted that the number of residency programs that have an Instagram account more than doubled (from 60 to 128) between May and September 2020.

We present 2 tables describing the important features and caveats of apps available to students to assist them with residency applications this year—TABLE 1 summarizes apps to aid with applications and interviews; TABLE 2 lists apps designed for students to learn more about individual residency programs. We wish all of this year’s students every success in their search for the right program. ●

- Association of Professors of Gynecology and Obstetrics, Council on Resident Education in ObGyn. Updated APGO and CREOG Residency Application Response to COVID-19. https://www.apgo.org/wp-content/uploads/2020/05 /Updated-APGO-CREOG-Residency-Response-to -COVID-19-.pdf. Accessed October 27, 2020.
- https://www.acog.org/education-and-events/webinars /virtual-residency-showcase. Accessed October 4, 2020.
- Social media directory-ObGyn. https://docs.google.com /spreadsheets/d/e/2PACX-1vQ6boyn7FWV9tEhfQp1o3 XJgNIPNBQ3qCYf4IpV-rOPcd212J-HNR84p0r85nXrAz MvOmcNlgjywDP/pubhtml?gid=1472916499&single =true. Accessed October 27, 2020.
- APGO webinar: Virtual interviews best practices. September 9, 2020. https://zoom.us/rec/play/KqxMT6Wnb F6qaMnFMoer_czOszRGRT89o364GHDzhFpjXodgSyGZpj 0BaCvKnXtxD7IH-u1IU4QIzHBT.etDUC4znlfNcgG7T?start Time=1599696020000. Accessed October 4, 2020.
- Association of Professors of Gynecology and Obstetrics, Council on Resident Education in ObGyn. Updated APGO and CREOG Residency Application Response to COVID-19. https://www.apgo.org/wp-content/uploads/2020/05 /Updated-APGO-CREOG-Residency-Response-to -COVID-19-.pdf. Accessed October 27, 2020.
- https://www.acog.org/education-and-events/webinars /virtual-residency-showcase. Accessed October 4, 2020.
- Social media directory-ObGyn. https://docs.google.com /spreadsheets/d/e/2PACX-1vQ6boyn7FWV9tEhfQp1o3 XJgNIPNBQ3qCYf4IpV-rOPcd212J-HNR84p0r85nXrAz MvOmcNlgjywDP/pubhtml?gid=1472916499&single =true. Accessed October 27, 2020.
- APGO webinar: Virtual interviews best practices. September 9, 2020. https://zoom.us/rec/play/KqxMT6Wnb F6qaMnFMoer_czOszRGRT89o364GHDzhFpjXodgSyGZpj 0BaCvKnXtxD7IH-u1IU4QIzHBT.etDUC4znlfNcgG7T?start Time=1599696020000. Accessed October 4, 2020.
How mental health care would look under a Trump vs. Biden administration
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.