User login
New CDC guidance on prescribing opioids for pain
The 2022 Clinical Practice Guideline provides guidance on determining whether to initiate opioids for pain; selecting opioids and determining opioid dosages; deciding duration of initial opioid prescription and conducting follow-up; and assessing risk and addressing potential harms of opioid use.
“Patients with pain should receive compassionate, safe, and effective pain care. We want clinicians and patients to have the information they need to weigh the benefits of different approaches to pain care, with the goal of helping people reduce their pain and improve their quality of life,” Christopher M. Jones, PharmD, DrPH, acting director for the CDC’s National Center for Injury Prevention and Control, said in a news release.
How to taper safely
The last guideline on the topic was released by CDC in 2016. Since then, new evidence has emerged regarding the benefits and risks of prescription opioids for acute and chronic pain, comparisons with nonopioid pain treatments, dosing strategies, opioid dose-dependent effects, risk mitigation strategies, and opioid tapering and discontinuation, the CDC says.
A “critical” addition to the 2022 guideline is advice on tapering opioids, Dr. Jones said during a press briefing.
“Practical tips on how to taper in an individualized patient-centered manner have been added to help clinicians if the decision is made to taper opioids, and the guideline explicitly advises against abrupt discontinuation or rapid dose reductions of opioids,” Dr. Jones said.
“That is based on lessons learned over the last several years as well as new science about how we approach tapering and the real harms that can result when patients are abruptly discontinued or rapidly tapered,” he added.
The updated guideline was published online Nov. 3 in the Morbidity and Mortality Weekly Report.
Key recommendations in the 100-page document include the following:
- In determining whether or not to initiate opioids, nonopioid therapies are at least as effective as opioids for many common types of acute pain. Use of nondrug and nonopioid drug therapies should be maximized as appropriate, and opioid therapy should only be considered for acute pain if it is anticipated that benefits outweigh risks to the patient.
- Before starting opioid therapy, providers should discuss with patients the realistic benefits and known risks of opioid therapy.
- Before starting ongoing opioid therapy for patients with subacute pain lasting 1 to 3 months or chronic pain lasting more than 3 months, providers should work with patients to establish treatment goals for pain and function, and consideration should be given as to how opioid therapy will be discontinued if benefits do not outweigh risks.
- Once opioids are started, the lowest effective dose of immediate-release opioids should be prescribed for no longer than needed for the expected duration of pain severe enough to require opioids.
- Within 1 to 4 weeks of starting opioid therapy for subacute or chronic pain, providers should work with patients to evaluate and carefully weigh benefits and risks of continuing opioid therapy; care should be exercised when increasing, continuing, or reducing opioid dosage.
- Before starting and periodically during ongoing opioid therapy, providers should evaluate risk for opioid-related harms and should work with patients to incorporate relevant strategies to mitigate risk, including offering naloxone and reviewing potential interactions with any other prescribed medications or substance used.
- Abrupt discontinuation of opioids should be avoided, especially for patients receiving high doses.
- For treating patients with opioid use disorder, treatment with evidence-based medications should be provided, or arrangements for such treatment should be made.
Dr. Jones emphasized that the guideline is “voluntary and meant to guide shared decision-making between a clinician and patient. It’s not meant to be implemented as absolute limits of policy or practice by clinicians, health systems, insurance companies, governmental entities.”
He also noted that the “current state of the overdose crisis, which is very much driven by illicit synthetic opioids, is not the aim of this guideline.
“The release of this guideline is really about advancing pain care and improving the lives of patients living with pain,” he said.
“We know that at least 1 in 5 people in the country have chronic pain. It’s one of the most common reasons why people present to their health care provider, and the goal here is to advance pain care, function, and quality of life for that patient population, while also reducing misuse, diversion, and consequences of prescription opioid misuse,” Dr. Jones added.
A version of this article first appeared on Medscape.com.
The 2022 Clinical Practice Guideline provides guidance on determining whether to initiate opioids for pain; selecting opioids and determining opioid dosages; deciding duration of initial opioid prescription and conducting follow-up; and assessing risk and addressing potential harms of opioid use.
“Patients with pain should receive compassionate, safe, and effective pain care. We want clinicians and patients to have the information they need to weigh the benefits of different approaches to pain care, with the goal of helping people reduce their pain and improve their quality of life,” Christopher M. Jones, PharmD, DrPH, acting director for the CDC’s National Center for Injury Prevention and Control, said in a news release.
How to taper safely
The last guideline on the topic was released by CDC in 2016. Since then, new evidence has emerged regarding the benefits and risks of prescription opioids for acute and chronic pain, comparisons with nonopioid pain treatments, dosing strategies, opioid dose-dependent effects, risk mitigation strategies, and opioid tapering and discontinuation, the CDC says.
A “critical” addition to the 2022 guideline is advice on tapering opioids, Dr. Jones said during a press briefing.
“Practical tips on how to taper in an individualized patient-centered manner have been added to help clinicians if the decision is made to taper opioids, and the guideline explicitly advises against abrupt discontinuation or rapid dose reductions of opioids,” Dr. Jones said.
“That is based on lessons learned over the last several years as well as new science about how we approach tapering and the real harms that can result when patients are abruptly discontinued or rapidly tapered,” he added.
The updated guideline was published online Nov. 3 in the Morbidity and Mortality Weekly Report.
Key recommendations in the 100-page document include the following:
- In determining whether or not to initiate opioids, nonopioid therapies are at least as effective as opioids for many common types of acute pain. Use of nondrug and nonopioid drug therapies should be maximized as appropriate, and opioid therapy should only be considered for acute pain if it is anticipated that benefits outweigh risks to the patient.
- Before starting opioid therapy, providers should discuss with patients the realistic benefits and known risks of opioid therapy.
- Before starting ongoing opioid therapy for patients with subacute pain lasting 1 to 3 months or chronic pain lasting more than 3 months, providers should work with patients to establish treatment goals for pain and function, and consideration should be given as to how opioid therapy will be discontinued if benefits do not outweigh risks.
- Once opioids are started, the lowest effective dose of immediate-release opioids should be prescribed for no longer than needed for the expected duration of pain severe enough to require opioids.
- Within 1 to 4 weeks of starting opioid therapy for subacute or chronic pain, providers should work with patients to evaluate and carefully weigh benefits and risks of continuing opioid therapy; care should be exercised when increasing, continuing, or reducing opioid dosage.
- Before starting and periodically during ongoing opioid therapy, providers should evaluate risk for opioid-related harms and should work with patients to incorporate relevant strategies to mitigate risk, including offering naloxone and reviewing potential interactions with any other prescribed medications or substance used.
- Abrupt discontinuation of opioids should be avoided, especially for patients receiving high doses.
- For treating patients with opioid use disorder, treatment with evidence-based medications should be provided, or arrangements for such treatment should be made.
Dr. Jones emphasized that the guideline is “voluntary and meant to guide shared decision-making between a clinician and patient. It’s not meant to be implemented as absolute limits of policy or practice by clinicians, health systems, insurance companies, governmental entities.”
He also noted that the “current state of the overdose crisis, which is very much driven by illicit synthetic opioids, is not the aim of this guideline.
“The release of this guideline is really about advancing pain care and improving the lives of patients living with pain,” he said.
“We know that at least 1 in 5 people in the country have chronic pain. It’s one of the most common reasons why people present to their health care provider, and the goal here is to advance pain care, function, and quality of life for that patient population, while also reducing misuse, diversion, and consequences of prescription opioid misuse,” Dr. Jones added.
A version of this article first appeared on Medscape.com.
The 2022 Clinical Practice Guideline provides guidance on determining whether to initiate opioids for pain; selecting opioids and determining opioid dosages; deciding duration of initial opioid prescription and conducting follow-up; and assessing risk and addressing potential harms of opioid use.
“Patients with pain should receive compassionate, safe, and effective pain care. We want clinicians and patients to have the information they need to weigh the benefits of different approaches to pain care, with the goal of helping people reduce their pain and improve their quality of life,” Christopher M. Jones, PharmD, DrPH, acting director for the CDC’s National Center for Injury Prevention and Control, said in a news release.
How to taper safely
The last guideline on the topic was released by CDC in 2016. Since then, new evidence has emerged regarding the benefits and risks of prescription opioids for acute and chronic pain, comparisons with nonopioid pain treatments, dosing strategies, opioid dose-dependent effects, risk mitigation strategies, and opioid tapering and discontinuation, the CDC says.
A “critical” addition to the 2022 guideline is advice on tapering opioids, Dr. Jones said during a press briefing.
“Practical tips on how to taper in an individualized patient-centered manner have been added to help clinicians if the decision is made to taper opioids, and the guideline explicitly advises against abrupt discontinuation or rapid dose reductions of opioids,” Dr. Jones said.
“That is based on lessons learned over the last several years as well as new science about how we approach tapering and the real harms that can result when patients are abruptly discontinued or rapidly tapered,” he added.
The updated guideline was published online Nov. 3 in the Morbidity and Mortality Weekly Report.
Key recommendations in the 100-page document include the following:
- In determining whether or not to initiate opioids, nonopioid therapies are at least as effective as opioids for many common types of acute pain. Use of nondrug and nonopioid drug therapies should be maximized as appropriate, and opioid therapy should only be considered for acute pain if it is anticipated that benefits outweigh risks to the patient.
- Before starting opioid therapy, providers should discuss with patients the realistic benefits and known risks of opioid therapy.
- Before starting ongoing opioid therapy for patients with subacute pain lasting 1 to 3 months or chronic pain lasting more than 3 months, providers should work with patients to establish treatment goals for pain and function, and consideration should be given as to how opioid therapy will be discontinued if benefits do not outweigh risks.
- Once opioids are started, the lowest effective dose of immediate-release opioids should be prescribed for no longer than needed for the expected duration of pain severe enough to require opioids.
- Within 1 to 4 weeks of starting opioid therapy for subacute or chronic pain, providers should work with patients to evaluate and carefully weigh benefits and risks of continuing opioid therapy; care should be exercised when increasing, continuing, or reducing opioid dosage.
- Before starting and periodically during ongoing opioid therapy, providers should evaluate risk for opioid-related harms and should work with patients to incorporate relevant strategies to mitigate risk, including offering naloxone and reviewing potential interactions with any other prescribed medications or substance used.
- Abrupt discontinuation of opioids should be avoided, especially for patients receiving high doses.
- For treating patients with opioid use disorder, treatment with evidence-based medications should be provided, or arrangements for such treatment should be made.
Dr. Jones emphasized that the guideline is “voluntary and meant to guide shared decision-making between a clinician and patient. It’s not meant to be implemented as absolute limits of policy or practice by clinicians, health systems, insurance companies, governmental entities.”
He also noted that the “current state of the overdose crisis, which is very much driven by illicit synthetic opioids, is not the aim of this guideline.
“The release of this guideline is really about advancing pain care and improving the lives of patients living with pain,” he said.
“We know that at least 1 in 5 people in the country have chronic pain. It’s one of the most common reasons why people present to their health care provider, and the goal here is to advance pain care, function, and quality of life for that patient population, while also reducing misuse, diversion, and consequences of prescription opioid misuse,” Dr. Jones added.
A version of this article first appeared on Medscape.com.
DoD will cover travel expenses for abortion care
Some 80,000 active-duty women are stationed in states with abortion restrictions or bans. That’s 40% of active-duty service women in the continental United States, according to research sponsored by the US Department of Defense (DoD) and released in September. Nearly all (95%) are of reproductive age. Annually, an estimated 2573 to 4126 women have an abortion, but just a handful of those are done at military treatment facilities. Moreover, roughly 275,000 DoD civilians also live in states with a full ban or extreme restrictions on access to abortion. Of those, more than 81,000 are women. Nearly 43% have no access to abortion or drastically abridged access.
The recent Supreme Court ruling in Dobbs v Jackson Women’s Health Organization has created uncertainty for those women and their families, and potential legal and financial risk for the health care practitioners who would provide reproductive care, Defense Secretary Lloyd Austin said in an October 20, 2022 memo.
Therefore, he has directed the DoD to take “all appropriate action… as soon as possible to ensure that our service members and their families can access reproductive health care and our health care providers can operate effectively.”
Among the actions he has approved: Paying for travel to reproductive health care—essentially, making it more feasible for members to cross state lines. Service members, he noted in the memo, are often required to travel or move to meet staffing, operational, and training requirements. The “practical effects,” he said, are that significant numbers of service members and their families “may be forced to travel greater distances, take more time off from work, and pay more out-of-pocket expenses to receive reproductive health care.”
Those effects, Austin said, “qualify as unusual, extraordinary, hardship, or emergency circumstances for service members and their dependents and will interfere with our ability to recruit, retain, and maintain the readiness of a highly qualified force.”
Women, who comprise 17% of the active-duty force, are the fastest-growing subpopulation in the military. For the past several years, according to the DoD research report, the military services have been “deliberately recruiting women”—who perform essential duties in every sector: health care and electrical and mechanical equipment repair, for example.
“The full effects of Dobbs on military readiness are yet to be known,” the report says, but it notes several potential problems: Women may not join the service knowing that they could end up in a state with restrictions. If already serving, they may leave. In some states, women face criminal prosecution.
The long arm of Dobbs reaches far into the future, too. For instance, if unintended pregnancies are carried to term, the DoD will need to provide care to women during pregnancy, delivery, and the postpartum period—and the family will need to care for the child. Looking only at women in states with restricted access or bans, the DoD estimates the number of unintended pregnancies annually would be 2800 among civilian employees and between 4400 and 4700 among active-duty service women.
Men are also directly affected: More than 40% of male service members are married to a civilian woman who is a TRICARE dependent, 20% of active-duty service women are married to a fellow service member, and active-duty service men might be responsible for pregnancies among women who are not DoD dependents but who might be unable to get an abortion, the DoD report notes.
Austin has directed the DoD to create a uniform policy that allows for appropriate administrative absence, to establish travel and transportation allowances, and to amend any applicable travel regulations to facilitate official travel to access noncovered reproductive health care that is unavailable within the local area of the service member’s permanent duty station.
So that health care practitioners do not have to face criminal or civil liability or risk losing their licenses, Austin directed the DoD to develop a program to reimburse applicable fees, as appropriate and consistent with applicable federal law, for DoD health care practitioners who wish to become licensed in a state other than that in which they are currently licensed. He also directed the DoD to develop a program to support DoD practitioners who are subject to adverse action, including indemnification of any verdict, judgment, or other monetary award consistent with applicable law.
“Our greatest strength is our people,” Austin wrote. “There is no higher priority than taking care of our people, and ensuring their health and well-being.” He directed that the actions outlined in the memorandum “be executed as soon as possible.”
Some 80,000 active-duty women are stationed in states with abortion restrictions or bans. That’s 40% of active-duty service women in the continental United States, according to research sponsored by the US Department of Defense (DoD) and released in September. Nearly all (95%) are of reproductive age. Annually, an estimated 2573 to 4126 women have an abortion, but just a handful of those are done at military treatment facilities. Moreover, roughly 275,000 DoD civilians also live in states with a full ban or extreme restrictions on access to abortion. Of those, more than 81,000 are women. Nearly 43% have no access to abortion or drastically abridged access.
The recent Supreme Court ruling in Dobbs v Jackson Women’s Health Organization has created uncertainty for those women and their families, and potential legal and financial risk for the health care practitioners who would provide reproductive care, Defense Secretary Lloyd Austin said in an October 20, 2022 memo.
Therefore, he has directed the DoD to take “all appropriate action… as soon as possible to ensure that our service members and their families can access reproductive health care and our health care providers can operate effectively.”
Among the actions he has approved: Paying for travel to reproductive health care—essentially, making it more feasible for members to cross state lines. Service members, he noted in the memo, are often required to travel or move to meet staffing, operational, and training requirements. The “practical effects,” he said, are that significant numbers of service members and their families “may be forced to travel greater distances, take more time off from work, and pay more out-of-pocket expenses to receive reproductive health care.”
Those effects, Austin said, “qualify as unusual, extraordinary, hardship, or emergency circumstances for service members and their dependents and will interfere with our ability to recruit, retain, and maintain the readiness of a highly qualified force.”
Women, who comprise 17% of the active-duty force, are the fastest-growing subpopulation in the military. For the past several years, according to the DoD research report, the military services have been “deliberately recruiting women”—who perform essential duties in every sector: health care and electrical and mechanical equipment repair, for example.
“The full effects of Dobbs on military readiness are yet to be known,” the report says, but it notes several potential problems: Women may not join the service knowing that they could end up in a state with restrictions. If already serving, they may leave. In some states, women face criminal prosecution.
The long arm of Dobbs reaches far into the future, too. For instance, if unintended pregnancies are carried to term, the DoD will need to provide care to women during pregnancy, delivery, and the postpartum period—and the family will need to care for the child. Looking only at women in states with restricted access or bans, the DoD estimates the number of unintended pregnancies annually would be 2800 among civilian employees and between 4400 and 4700 among active-duty service women.
Men are also directly affected: More than 40% of male service members are married to a civilian woman who is a TRICARE dependent, 20% of active-duty service women are married to a fellow service member, and active-duty service men might be responsible for pregnancies among women who are not DoD dependents but who might be unable to get an abortion, the DoD report notes.
Austin has directed the DoD to create a uniform policy that allows for appropriate administrative absence, to establish travel and transportation allowances, and to amend any applicable travel regulations to facilitate official travel to access noncovered reproductive health care that is unavailable within the local area of the service member’s permanent duty station.
So that health care practitioners do not have to face criminal or civil liability or risk losing their licenses, Austin directed the DoD to develop a program to reimburse applicable fees, as appropriate and consistent with applicable federal law, for DoD health care practitioners who wish to become licensed in a state other than that in which they are currently licensed. He also directed the DoD to develop a program to support DoD practitioners who are subject to adverse action, including indemnification of any verdict, judgment, or other monetary award consistent with applicable law.
“Our greatest strength is our people,” Austin wrote. “There is no higher priority than taking care of our people, and ensuring their health and well-being.” He directed that the actions outlined in the memorandum “be executed as soon as possible.”
Some 80,000 active-duty women are stationed in states with abortion restrictions or bans. That’s 40% of active-duty service women in the continental United States, according to research sponsored by the US Department of Defense (DoD) and released in September. Nearly all (95%) are of reproductive age. Annually, an estimated 2573 to 4126 women have an abortion, but just a handful of those are done at military treatment facilities. Moreover, roughly 275,000 DoD civilians also live in states with a full ban or extreme restrictions on access to abortion. Of those, more than 81,000 are women. Nearly 43% have no access to abortion or drastically abridged access.
The recent Supreme Court ruling in Dobbs v Jackson Women’s Health Organization has created uncertainty for those women and their families, and potential legal and financial risk for the health care practitioners who would provide reproductive care, Defense Secretary Lloyd Austin said in an October 20, 2022 memo.
Therefore, he has directed the DoD to take “all appropriate action… as soon as possible to ensure that our service members and their families can access reproductive health care and our health care providers can operate effectively.”
Among the actions he has approved: Paying for travel to reproductive health care—essentially, making it more feasible for members to cross state lines. Service members, he noted in the memo, are often required to travel or move to meet staffing, operational, and training requirements. The “practical effects,” he said, are that significant numbers of service members and their families “may be forced to travel greater distances, take more time off from work, and pay more out-of-pocket expenses to receive reproductive health care.”
Those effects, Austin said, “qualify as unusual, extraordinary, hardship, or emergency circumstances for service members and their dependents and will interfere with our ability to recruit, retain, and maintain the readiness of a highly qualified force.”
Women, who comprise 17% of the active-duty force, are the fastest-growing subpopulation in the military. For the past several years, according to the DoD research report, the military services have been “deliberately recruiting women”—who perform essential duties in every sector: health care and electrical and mechanical equipment repair, for example.
“The full effects of Dobbs on military readiness are yet to be known,” the report says, but it notes several potential problems: Women may not join the service knowing that they could end up in a state with restrictions. If already serving, they may leave. In some states, women face criminal prosecution.
The long arm of Dobbs reaches far into the future, too. For instance, if unintended pregnancies are carried to term, the DoD will need to provide care to women during pregnancy, delivery, and the postpartum period—and the family will need to care for the child. Looking only at women in states with restricted access or bans, the DoD estimates the number of unintended pregnancies annually would be 2800 among civilian employees and between 4400 and 4700 among active-duty service women.
Men are also directly affected: More than 40% of male service members are married to a civilian woman who is a TRICARE dependent, 20% of active-duty service women are married to a fellow service member, and active-duty service men might be responsible for pregnancies among women who are not DoD dependents but who might be unable to get an abortion, the DoD report notes.
Austin has directed the DoD to create a uniform policy that allows for appropriate administrative absence, to establish travel and transportation allowances, and to amend any applicable travel regulations to facilitate official travel to access noncovered reproductive health care that is unavailable within the local area of the service member’s permanent duty station.
So that health care practitioners do not have to face criminal or civil liability or risk losing their licenses, Austin directed the DoD to develop a program to reimburse applicable fees, as appropriate and consistent with applicable federal law, for DoD health care practitioners who wish to become licensed in a state other than that in which they are currently licensed. He also directed the DoD to develop a program to support DoD practitioners who are subject to adverse action, including indemnification of any verdict, judgment, or other monetary award consistent with applicable law.
“Our greatest strength is our people,” Austin wrote. “There is no higher priority than taking care of our people, and ensuring their health and well-being.” He directed that the actions outlined in the memorandum “be executed as soon as possible.”
VA Fast-Tracks Hiring to Address Critical Shortages
In an intensive push to fill acute workforce shortages, the US Department of Veterans Affairs (VA) is holding a “national onboarding surge event” the week of November 14. The goal is to get people who have already said yes to a job in the VA on that job more quickly. Every VA facility has been asked to submit a list of the highest-priority candidates, regardless of the position.
One of the most pressing reasons for getting more workers into the pipeline faster is that more and more veterans are entering VA care. As of October 1, tens of thousands of veterans will be eligible for VA health care, thanks to the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics Act of 2022 (PACT Act), passed in August, which expanded benefits for post-9/11 service members with illnesses due to toxic exposures.
Another reason is the need to fill the gaps left by attrition. In an October 19 press briefing, VA Undersecretary for Health Shereef Elnahal said the agency needs to hire about 52,000 employees per year just to keep up with the rate of health care professionals (HCPs) leaving the agency. At a September breakfast meeting with the Defense Writers Group, VA Secretary Denis McDonough said July 2022 marked the first month this year that the VA hired more nurses than it lost to retirement. He said the VA needs to hire 45,000 nurses over the next 3 years to keep up with attrition and growing demand for veteran care.
“We have to do a better job on hiring,” McDonough said. Streamlining the process is a major goal. Hiring rules loosened during the pandemic have since tightened back up. He pointed out that in many cases, the VA takes 90 to 100 days to onboard candidates and called the long-drawn-out process “being dragged through a bureaucratic morass.” During that time, he said, “They’re not being paid, they’re filling out paperwork… That’s disastrous.” In his press briefing, Elnahal said “we lose folks after we’ve made the selection” because the process is so long.
Moreover, the agency has a critical shortage not only of HCPs but the human resources professionals needed to fast-track the hirees’ progress. McDonough called it a “supply chain issue.” “We have the lowest ratio of human resource professionals per employee in the federal government by a long shot.” Partly, he said, because “a lot of our people end up hired away to other federal agencies.”
McDonough said the VA is also interested in transitioning more active-duty service members with in-demand skills, certifications, and talent into the VA workforce. “Cross-walking active duty into VA service much more aggressively,” he said, is another way to “grow that supply of ready, deployable, trained personnel.” The PACT Act gives the VA new incentives to entice workers, such as expanded recruitment, retention bonuses, and student loan repayment. The VA already provides training to about 1500 nurse and nurse residency programs across the VA, McDonough said but has plans for expanding to 5 times its current scope. He also addressed the question of a looming physician shortage: “Roughly 7 in 10 doctors in the United States will have had some portion of their training in a VA facility. We have to maintain that training function going forward.” The VA trains doctors, he added, “better than anybody else.”
The onboarding event will serve as a “national signal that we take this priority very seriously,” Elnahal said. “This will be not only a chance to have a step function improvement in the number of folks on board, which is an urgent priority, but to also set the groundwork for the more longitudinal work that we will need to do to improve the hiring process.”
Bulking up the workforce, he said, is “still far and away among our first priorities. Because if we don’t get our hospitals and facility staffed, it’s going to be a really hard effort to make process on the other priorities.”
In an intensive push to fill acute workforce shortages, the US Department of Veterans Affairs (VA) is holding a “national onboarding surge event” the week of November 14. The goal is to get people who have already said yes to a job in the VA on that job more quickly. Every VA facility has been asked to submit a list of the highest-priority candidates, regardless of the position.
One of the most pressing reasons for getting more workers into the pipeline faster is that more and more veterans are entering VA care. As of October 1, tens of thousands of veterans will be eligible for VA health care, thanks to the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics Act of 2022 (PACT Act), passed in August, which expanded benefits for post-9/11 service members with illnesses due to toxic exposures.
Another reason is the need to fill the gaps left by attrition. In an October 19 press briefing, VA Undersecretary for Health Shereef Elnahal said the agency needs to hire about 52,000 employees per year just to keep up with the rate of health care professionals (HCPs) leaving the agency. At a September breakfast meeting with the Defense Writers Group, VA Secretary Denis McDonough said July 2022 marked the first month this year that the VA hired more nurses than it lost to retirement. He said the VA needs to hire 45,000 nurses over the next 3 years to keep up with attrition and growing demand for veteran care.
“We have to do a better job on hiring,” McDonough said. Streamlining the process is a major goal. Hiring rules loosened during the pandemic have since tightened back up. He pointed out that in many cases, the VA takes 90 to 100 days to onboard candidates and called the long-drawn-out process “being dragged through a bureaucratic morass.” During that time, he said, “They’re not being paid, they’re filling out paperwork… That’s disastrous.” In his press briefing, Elnahal said “we lose folks after we’ve made the selection” because the process is so long.
Moreover, the agency has a critical shortage not only of HCPs but the human resources professionals needed to fast-track the hirees’ progress. McDonough called it a “supply chain issue.” “We have the lowest ratio of human resource professionals per employee in the federal government by a long shot.” Partly, he said, because “a lot of our people end up hired away to other federal agencies.”
McDonough said the VA is also interested in transitioning more active-duty service members with in-demand skills, certifications, and talent into the VA workforce. “Cross-walking active duty into VA service much more aggressively,” he said, is another way to “grow that supply of ready, deployable, trained personnel.” The PACT Act gives the VA new incentives to entice workers, such as expanded recruitment, retention bonuses, and student loan repayment. The VA already provides training to about 1500 nurse and nurse residency programs across the VA, McDonough said but has plans for expanding to 5 times its current scope. He also addressed the question of a looming physician shortage: “Roughly 7 in 10 doctors in the United States will have had some portion of their training in a VA facility. We have to maintain that training function going forward.” The VA trains doctors, he added, “better than anybody else.”
The onboarding event will serve as a “national signal that we take this priority very seriously,” Elnahal said. “This will be not only a chance to have a step function improvement in the number of folks on board, which is an urgent priority, but to also set the groundwork for the more longitudinal work that we will need to do to improve the hiring process.”
Bulking up the workforce, he said, is “still far and away among our first priorities. Because if we don’t get our hospitals and facility staffed, it’s going to be a really hard effort to make process on the other priorities.”
In an intensive push to fill acute workforce shortages, the US Department of Veterans Affairs (VA) is holding a “national onboarding surge event” the week of November 14. The goal is to get people who have already said yes to a job in the VA on that job more quickly. Every VA facility has been asked to submit a list of the highest-priority candidates, regardless of the position.
One of the most pressing reasons for getting more workers into the pipeline faster is that more and more veterans are entering VA care. As of October 1, tens of thousands of veterans will be eligible for VA health care, thanks to the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics Act of 2022 (PACT Act), passed in August, which expanded benefits for post-9/11 service members with illnesses due to toxic exposures.
Another reason is the need to fill the gaps left by attrition. In an October 19 press briefing, VA Undersecretary for Health Shereef Elnahal said the agency needs to hire about 52,000 employees per year just to keep up with the rate of health care professionals (HCPs) leaving the agency. At a September breakfast meeting with the Defense Writers Group, VA Secretary Denis McDonough said July 2022 marked the first month this year that the VA hired more nurses than it lost to retirement. He said the VA needs to hire 45,000 nurses over the next 3 years to keep up with attrition and growing demand for veteran care.
“We have to do a better job on hiring,” McDonough said. Streamlining the process is a major goal. Hiring rules loosened during the pandemic have since tightened back up. He pointed out that in many cases, the VA takes 90 to 100 days to onboard candidates and called the long-drawn-out process “being dragged through a bureaucratic morass.” During that time, he said, “They’re not being paid, they’re filling out paperwork… That’s disastrous.” In his press briefing, Elnahal said “we lose folks after we’ve made the selection” because the process is so long.
Moreover, the agency has a critical shortage not only of HCPs but the human resources professionals needed to fast-track the hirees’ progress. McDonough called it a “supply chain issue.” “We have the lowest ratio of human resource professionals per employee in the federal government by a long shot.” Partly, he said, because “a lot of our people end up hired away to other federal agencies.”
McDonough said the VA is also interested in transitioning more active-duty service members with in-demand skills, certifications, and talent into the VA workforce. “Cross-walking active duty into VA service much more aggressively,” he said, is another way to “grow that supply of ready, deployable, trained personnel.” The PACT Act gives the VA new incentives to entice workers, such as expanded recruitment, retention bonuses, and student loan repayment. The VA already provides training to about 1500 nurse and nurse residency programs across the VA, McDonough said but has plans for expanding to 5 times its current scope. He also addressed the question of a looming physician shortage: “Roughly 7 in 10 doctors in the United States will have had some portion of their training in a VA facility. We have to maintain that training function going forward.” The VA trains doctors, he added, “better than anybody else.”
The onboarding event will serve as a “national signal that we take this priority very seriously,” Elnahal said. “This will be not only a chance to have a step function improvement in the number of folks on board, which is an urgent priority, but to also set the groundwork for the more longitudinal work that we will need to do to improve the hiring process.”
Bulking up the workforce, he said, is “still far and away among our first priorities. Because if we don’t get our hospitals and facility staffed, it’s going to be a really hard effort to make process on the other priorities.”
VA Gets it Right on Suicide
For years, the US Department of Veterans Affairs (VA) has painstakingly labored to track, research, and address veteran suicide. Their exceptional work was dealt an unwarranted blow a month ago with the publication of an incomplete report entitled Operation Deep Dive (OpDD). The $3.9 million study from America’s Warrior Partnership (AWP) examined death data of former service members in 8 states between 2014 and 2018. The interim report criticized the VA for minimizing the extent of veteran suicide, asserting, “former service members take their own lives each year at a rate approximately 2.4 times greater than previously reported by the VA.”
The sensational results were accepted at face value and immediately garnered negative nationwide headlines, with lawmakers, media outlets, and veterans rushing to impugn the VA. Senate Committee on Veterans’ Affairs Ranking Republican Member Jerry Moran of Kansas opined, “The disparity between the numbers of veteran suicides reported by the VA and [OpDD] is concerning. We need an honest assessment of the scope of the problem.” A U.S. Medicine headline stated “VA undercounted thousands of veteran suicides. [OpDD] posited daily suicide rate is 240% higher.” Fox News declared, “Veterans committing suicide at rate 2 times higher than VA data show: study,” as did Military Times, “Veterans suicide rate may be double federal estimates, study suggests.”
Disturbingly, those who echoed AWP’s claims got the story backward. It’s AWP, not VA, whose suicide data and conclusions are faulty.
For starters, the VA data encompasses veterans across all 50 states, the District of Columbia, Puerto Rico, and the US Virgin Islands. In contrast, AWP inferred national veteran suicide figures based on partial, skewed data. As delineated by researchers in an in-press Military Medicine letter to the Editor, 7 of the 8 states sampled (Alabama, Florida, Maine, Massachusetts, Michigan, Minnesota, Montana, and Oregon) had suicide rates above the national average for the years under investigation. This factor alone overinflates AWP’s purported suicide numbers.
Additionally, AWP altered the definition of “taking one’s life” and then misapplied that designation. Conventionally, the term refers to suicide, but AWP used it to also include nonnatural deaths assessed by coroners and medical examiners as accidental or undetermined. Two examples of this self-injury mortality (SIM) are opioid overdoses and single-driver car crash deaths. AWP added suicides and SIMs to derive a total number of veterans who took their life and falsely contrasted that aggregate against the VA count of suicides. That’s like comparing the whole category of fruit to the subcategory of apples.
AWP should be applauded for drawing attention to and accounting for accidental and undetermined deaths. However, the standard protocol is to consider SIMs distinctly from suicides. Among the many reasons for precise labeling is so that grieving family members aren’t mistakenly informed that their loved one died by suicide. VA conveys the rate of veteran overdose deaths in separate reports, for example, the Veteran Drug Overdose Mortality, 2010-2019 publication. Those numbers were ignored in AWP’s calculations.
AWP was neglectful in another way. The second phase of the project—a deep examination of community-level factors preceding suicides and nonnatural deaths—began in 2019. This information was collected and analyzed through sociocultural death investigation (SDI) interviews of 3 to 4 family members, friends, and colleagues of the deceased. SDIs consisted of 19 factors, such as history of the veteran’s mental health problems, social connectedness, finances, group memberships, and access to firearms. However, the interim report omitted the preliminary analysis of these factors, which AWP stated would be made available this year.
OpDD conclusions were so unfounded that AWP’s analytic research partner, the University of Alabama, distanced itself from the interim report. “We were not consulted on the released figures,” Dr. Karl Hamner, the University of Alabama principal investigator on the study, told me. “We did not make any conclusions and we don’t endorse the reported findings about national rates or numbers per day. Nor did we make any statements about the VA’s data.”
As it happens, the VA’s 2022 National Veteran Suicide Prevention Annual Report was issued the same week as the OpDD report. VA found that veteran suicides decreased by 9.7% over the last 2 years, nearly twice the decrease for nonveterans. Yet, in a contemporaneous hearing of the House Committee on Veterans’ Affairs, AWP’s President and CEO Jim Lorraine testified that the progress preventing veteran suicide was “a disgrace” and “a failure.” He misattributed that it was VA (not AWP) that “must be more open and transparent about their data.”
Unsupported denigration of the VA tarnishes its reputation, undermining veterans’ trust in the health care system and increasing barriers to seeking needed services. More broadly, it fortifies those forces who wish to redirect allocations away from VA and towards non-VA veterans’ entities like AWP. The media and other stakeholders must take a lesson about getting the story straight before reflexively amplifying false accusations about the VA. Veterans deserve better.
For years, the US Department of Veterans Affairs (VA) has painstakingly labored to track, research, and address veteran suicide. Their exceptional work was dealt an unwarranted blow a month ago with the publication of an incomplete report entitled Operation Deep Dive (OpDD). The $3.9 million study from America’s Warrior Partnership (AWP) examined death data of former service members in 8 states between 2014 and 2018. The interim report criticized the VA for minimizing the extent of veteran suicide, asserting, “former service members take their own lives each year at a rate approximately 2.4 times greater than previously reported by the VA.”
The sensational results were accepted at face value and immediately garnered negative nationwide headlines, with lawmakers, media outlets, and veterans rushing to impugn the VA. Senate Committee on Veterans’ Affairs Ranking Republican Member Jerry Moran of Kansas opined, “The disparity between the numbers of veteran suicides reported by the VA and [OpDD] is concerning. We need an honest assessment of the scope of the problem.” A U.S. Medicine headline stated “VA undercounted thousands of veteran suicides. [OpDD] posited daily suicide rate is 240% higher.” Fox News declared, “Veterans committing suicide at rate 2 times higher than VA data show: study,” as did Military Times, “Veterans suicide rate may be double federal estimates, study suggests.”
Disturbingly, those who echoed AWP’s claims got the story backward. It’s AWP, not VA, whose suicide data and conclusions are faulty.
For starters, the VA data encompasses veterans across all 50 states, the District of Columbia, Puerto Rico, and the US Virgin Islands. In contrast, AWP inferred national veteran suicide figures based on partial, skewed data. As delineated by researchers in an in-press Military Medicine letter to the Editor, 7 of the 8 states sampled (Alabama, Florida, Maine, Massachusetts, Michigan, Minnesota, Montana, and Oregon) had suicide rates above the national average for the years under investigation. This factor alone overinflates AWP’s purported suicide numbers.
Additionally, AWP altered the definition of “taking one’s life” and then misapplied that designation. Conventionally, the term refers to suicide, but AWP used it to also include nonnatural deaths assessed by coroners and medical examiners as accidental or undetermined. Two examples of this self-injury mortality (SIM) are opioid overdoses and single-driver car crash deaths. AWP added suicides and SIMs to derive a total number of veterans who took their life and falsely contrasted that aggregate against the VA count of suicides. That’s like comparing the whole category of fruit to the subcategory of apples.
AWP should be applauded for drawing attention to and accounting for accidental and undetermined deaths. However, the standard protocol is to consider SIMs distinctly from suicides. Among the many reasons for precise labeling is so that grieving family members aren’t mistakenly informed that their loved one died by suicide. VA conveys the rate of veteran overdose deaths in separate reports, for example, the Veteran Drug Overdose Mortality, 2010-2019 publication. Those numbers were ignored in AWP’s calculations.
AWP was neglectful in another way. The second phase of the project—a deep examination of community-level factors preceding suicides and nonnatural deaths—began in 2019. This information was collected and analyzed through sociocultural death investigation (SDI) interviews of 3 to 4 family members, friends, and colleagues of the deceased. SDIs consisted of 19 factors, such as history of the veteran’s mental health problems, social connectedness, finances, group memberships, and access to firearms. However, the interim report omitted the preliminary analysis of these factors, which AWP stated would be made available this year.
OpDD conclusions were so unfounded that AWP’s analytic research partner, the University of Alabama, distanced itself from the interim report. “We were not consulted on the released figures,” Dr. Karl Hamner, the University of Alabama principal investigator on the study, told me. “We did not make any conclusions and we don’t endorse the reported findings about national rates or numbers per day. Nor did we make any statements about the VA’s data.”
As it happens, the VA’s 2022 National Veteran Suicide Prevention Annual Report was issued the same week as the OpDD report. VA found that veteran suicides decreased by 9.7% over the last 2 years, nearly twice the decrease for nonveterans. Yet, in a contemporaneous hearing of the House Committee on Veterans’ Affairs, AWP’s President and CEO Jim Lorraine testified that the progress preventing veteran suicide was “a disgrace” and “a failure.” He misattributed that it was VA (not AWP) that “must be more open and transparent about their data.”
Unsupported denigration of the VA tarnishes its reputation, undermining veterans’ trust in the health care system and increasing barriers to seeking needed services. More broadly, it fortifies those forces who wish to redirect allocations away from VA and towards non-VA veterans’ entities like AWP. The media and other stakeholders must take a lesson about getting the story straight before reflexively amplifying false accusations about the VA. Veterans deserve better.
For years, the US Department of Veterans Affairs (VA) has painstakingly labored to track, research, and address veteran suicide. Their exceptional work was dealt an unwarranted blow a month ago with the publication of an incomplete report entitled Operation Deep Dive (OpDD). The $3.9 million study from America’s Warrior Partnership (AWP) examined death data of former service members in 8 states between 2014 and 2018. The interim report criticized the VA for minimizing the extent of veteran suicide, asserting, “former service members take their own lives each year at a rate approximately 2.4 times greater than previously reported by the VA.”
The sensational results were accepted at face value and immediately garnered negative nationwide headlines, with lawmakers, media outlets, and veterans rushing to impugn the VA. Senate Committee on Veterans’ Affairs Ranking Republican Member Jerry Moran of Kansas opined, “The disparity between the numbers of veteran suicides reported by the VA and [OpDD] is concerning. We need an honest assessment of the scope of the problem.” A U.S. Medicine headline stated “VA undercounted thousands of veteran suicides. [OpDD] posited daily suicide rate is 240% higher.” Fox News declared, “Veterans committing suicide at rate 2 times higher than VA data show: study,” as did Military Times, “Veterans suicide rate may be double federal estimates, study suggests.”
Disturbingly, those who echoed AWP’s claims got the story backward. It’s AWP, not VA, whose suicide data and conclusions are faulty.
For starters, the VA data encompasses veterans across all 50 states, the District of Columbia, Puerto Rico, and the US Virgin Islands. In contrast, AWP inferred national veteran suicide figures based on partial, skewed data. As delineated by researchers in an in-press Military Medicine letter to the Editor, 7 of the 8 states sampled (Alabama, Florida, Maine, Massachusetts, Michigan, Minnesota, Montana, and Oregon) had suicide rates above the national average for the years under investigation. This factor alone overinflates AWP’s purported suicide numbers.
Additionally, AWP altered the definition of “taking one’s life” and then misapplied that designation. Conventionally, the term refers to suicide, but AWP used it to also include nonnatural deaths assessed by coroners and medical examiners as accidental or undetermined. Two examples of this self-injury mortality (SIM) are opioid overdoses and single-driver car crash deaths. AWP added suicides and SIMs to derive a total number of veterans who took their life and falsely contrasted that aggregate against the VA count of suicides. That’s like comparing the whole category of fruit to the subcategory of apples.
AWP should be applauded for drawing attention to and accounting for accidental and undetermined deaths. However, the standard protocol is to consider SIMs distinctly from suicides. Among the many reasons for precise labeling is so that grieving family members aren’t mistakenly informed that their loved one died by suicide. VA conveys the rate of veteran overdose deaths in separate reports, for example, the Veteran Drug Overdose Mortality, 2010-2019 publication. Those numbers were ignored in AWP’s calculations.
AWP was neglectful in another way. The second phase of the project—a deep examination of community-level factors preceding suicides and nonnatural deaths—began in 2019. This information was collected and analyzed through sociocultural death investigation (SDI) interviews of 3 to 4 family members, friends, and colleagues of the deceased. SDIs consisted of 19 factors, such as history of the veteran’s mental health problems, social connectedness, finances, group memberships, and access to firearms. However, the interim report omitted the preliminary analysis of these factors, which AWP stated would be made available this year.
OpDD conclusions were so unfounded that AWP’s analytic research partner, the University of Alabama, distanced itself from the interim report. “We were not consulted on the released figures,” Dr. Karl Hamner, the University of Alabama principal investigator on the study, told me. “We did not make any conclusions and we don’t endorse the reported findings about national rates or numbers per day. Nor did we make any statements about the VA’s data.”
As it happens, the VA’s 2022 National Veteran Suicide Prevention Annual Report was issued the same week as the OpDD report. VA found that veteran suicides decreased by 9.7% over the last 2 years, nearly twice the decrease for nonveterans. Yet, in a contemporaneous hearing of the House Committee on Veterans’ Affairs, AWP’s President and CEO Jim Lorraine testified that the progress preventing veteran suicide was “a disgrace” and “a failure.” He misattributed that it was VA (not AWP) that “must be more open and transparent about their data.”
Unsupported denigration of the VA tarnishes its reputation, undermining veterans’ trust in the health care system and increasing barriers to seeking needed services. More broadly, it fortifies those forces who wish to redirect allocations away from VA and towards non-VA veterans’ entities like AWP. The media and other stakeholders must take a lesson about getting the story straight before reflexively amplifying false accusations about the VA. Veterans deserve better.
Climate change: Commentary in four dermatology journals calls for emergency action
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
Dermatologists fear effects of Dobbs decision for patients on isotretinoin, methotrexate
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The marked contrast in pandemic outcomes between Japan and the United States
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
A farewell to arms? Drug approvals based on single-arm trials can be flawed
PARIS – with results that should only be used, under certain conditions, for accelerated approvals that should then be followed by confirmatory studies.
In fact, many drugs approved over the last decade based solely on data from single-arm trials have been subsequently withdrawn when put through the rigors of a head-to-head randomized controlled trial, according to Bishal Gyawali, MD, PhD, from the department of oncology at Queen’s University, Kingston, Ont.
“Single-arm trials are not meant to provide confirmatory evidence sufficient for approval; However, that ship has sailed, and we have several drugs that are approved on the basis of single-arm trials, but we need to make sure that those approvals are accelerated or conditional approvals, not regular approval,” he said in a presentation included in a special session on drug approvals at the European Society for Medical Oncology Congress.
“We should not allow premature regular approval based on single-arm trials, because once a drug gets conditional approval, access is not an issue. Patients will have access to the drug anyway, but we should ensure that robust evidence follows, and long-term follow-up data are needed to develop confidence in the efficacy outcomes that are seen in single-arm trials,” he said.
In many cases, single-arm trials are large enough or of long enough duration that investigators could have reasonably performed a randomized controlled trial (RCT) in the first place, Dr. Gyawali added.
Why do single-arm trials?
The term “single-arm registration trial” is something of an oxymoron, he said, noting that the purpose of such trials should be whether to take the drug to a phase 3, randomized trial. But as authors of a 2019 study in JAMA Network Open showed, of a sample of phase 3 RCTs, 42% did not have a prior phase 2 trial, and 28% had a negative phase 2 trial. Single-arm trials may be acceptable for conditional drug approvals if all of the following conditions are met:
- A RCT is not possible because the disease is rare or randomization would be unethical.
- The safety of the drug is established and its potential benefits outweigh its risks.
- The drug is associated with a high and durable overall or objective response rate.
- The mechanism of action is supported by a strong scientific rationale, and if the drug may meet an unmet medical need.
Survival endpoints won’t do
Efficacy endpoints typically used in RCTs, such as progression-free survival (PFS) and overall survival (OS) can be misleading because they may be a result of the natural history of the disease and not the drug being tested, whereas ORRs are almost certainly reflective of the action of the drug itself, because spontaneous tumor regression is a rare phenomenon, Dr. Gyawali said.
He cautioned, however, that the ORR of placebo is not zero percent. For example in a 2018 study of sorafenib (Nexavar) versus placebo for advanced or refractory desmoid tumors, the ORR with the active drug was 33%, and the ORR for placebo was 20%.
It’s also open to question, he said, what constitutes an acceptably high ORR and duration of response, pointing to Food and Drug Administration accelerated approval of an indication for nivolumab (Opdivo) for treatment of patients with hepatocellular carcinoma (HCC) that had progressed on sorafenib. In the single-arm trial used as the basis for approval, the ORRs as assessed by an independent central review committee blinded to the results was 14.3%.
“So, nivolumab in hepatocellular cancer was approved on the basis of a response rate lower than that of placebo, albeit in a different tumor. But the point I’m trying to show here is we don’t have a good definition of what is a good response rate,” he said.
In July 2021, Bristol-Myers Squibb voluntarily withdrew the HCC indication for nivolumab, following negative results of the CheckMate 459 trial and a 5-4 vote against continuing the accelerated approval.
On second thought ...
Citing data compiled by Nathan I. Cherny, MD, from Shaare Zedek Medical Center, Jerusalem, Dr. Gyawali noted that 58 of 161 FDA approvals from 2017 to 2021 of drugs for adult solid tumors were based on single-arm trials. Of the 58 drugs, 39 received accelerated approvals, and 19 received regular approvals; of the 39 that received accelerated approvals, 4 were subsequently withdrawn, 8 were converted to regular approvals, and the remainder continued as accelerated approvals.
Interestingly, the median response rate among all the drugs was 40%, and did not differ between the type of approval received, suggesting that response rates are not predictive of whether a drug will receive a conditional or full-fledged go-ahead.
What’s rare and safe?
The definition of a rare disease in the United States is one that affects fewer than 40,000 per year, and in Europe it’s an incidence rate of less than 6 per 100,000 population, Dr. Gyawali noted. But he argued that even non–small cell lung cancer, the most common form of cancer in the world, could be considered rare if it is broken down into subtypes that are treated according to specific mutations that may occur in a relatively small number of patients.
He also noted that a specific drug’s safety, one of the most important criteria for granting approval to a drug based on a single-arm trial, can be difficult to judge without adequate controls for comparison.
Cherry-picking patients
Winette van der Graaf, MD, president of the European Organization for the Research and Treatment of Cancer, who attended the session where Dr. Gyawali’s presentation was played, said in an interview that clinicians should cast a critical eye on how trials are designed and conducted, including patient selection and choice of endpoints.
“One of the most obvious things to be concerned about is that we’re still having patients with good performance status enrolled, mostly PS 0 or 1, so how representative are these clinical trials for the patients we see in front of us on a daily basis?” she said.
“The other question is radiological endpoints, which we focus on with OS and PFS are most important for patients, especially if you consider that if patients may have asymptomatic disease, and we are only treating them with potentially toxic medication, what are we doing for them? Median overall survival when you look at all of these trials is only 4 months, so we really need to take into account how we affect patients in clinical trials,” she added.
Dr. van der Graaf emphasized that clinical trial investigators need to more routinely incorporate quality of life measures and other patient-reported outcomes in clinical trial results to help regulators and clinicians in practice get a better sense of the true clinical benefit of a new drug.
Dr. Gyawali did not disclose a funding source for his presentation. He reported consulting fees from Vivio Health and research grants from the American Society of Clinical Oncology. Dr. van der Graaf reported no conflicts of interest.
PARIS – with results that should only be used, under certain conditions, for accelerated approvals that should then be followed by confirmatory studies.
In fact, many drugs approved over the last decade based solely on data from single-arm trials have been subsequently withdrawn when put through the rigors of a head-to-head randomized controlled trial, according to Bishal Gyawali, MD, PhD, from the department of oncology at Queen’s University, Kingston, Ont.
“Single-arm trials are not meant to provide confirmatory evidence sufficient for approval; However, that ship has sailed, and we have several drugs that are approved on the basis of single-arm trials, but we need to make sure that those approvals are accelerated or conditional approvals, not regular approval,” he said in a presentation included in a special session on drug approvals at the European Society for Medical Oncology Congress.
“We should not allow premature regular approval based on single-arm trials, because once a drug gets conditional approval, access is not an issue. Patients will have access to the drug anyway, but we should ensure that robust evidence follows, and long-term follow-up data are needed to develop confidence in the efficacy outcomes that are seen in single-arm trials,” he said.
In many cases, single-arm trials are large enough or of long enough duration that investigators could have reasonably performed a randomized controlled trial (RCT) in the first place, Dr. Gyawali added.
Why do single-arm trials?
The term “single-arm registration trial” is something of an oxymoron, he said, noting that the purpose of such trials should be whether to take the drug to a phase 3, randomized trial. But as authors of a 2019 study in JAMA Network Open showed, of a sample of phase 3 RCTs, 42% did not have a prior phase 2 trial, and 28% had a negative phase 2 trial. Single-arm trials may be acceptable for conditional drug approvals if all of the following conditions are met:
- A RCT is not possible because the disease is rare or randomization would be unethical.
- The safety of the drug is established and its potential benefits outweigh its risks.
- The drug is associated with a high and durable overall or objective response rate.
- The mechanism of action is supported by a strong scientific rationale, and if the drug may meet an unmet medical need.
Survival endpoints won’t do
Efficacy endpoints typically used in RCTs, such as progression-free survival (PFS) and overall survival (OS) can be misleading because they may be a result of the natural history of the disease and not the drug being tested, whereas ORRs are almost certainly reflective of the action of the drug itself, because spontaneous tumor regression is a rare phenomenon, Dr. Gyawali said.
He cautioned, however, that the ORR of placebo is not zero percent. For example in a 2018 study of sorafenib (Nexavar) versus placebo for advanced or refractory desmoid tumors, the ORR with the active drug was 33%, and the ORR for placebo was 20%.
It’s also open to question, he said, what constitutes an acceptably high ORR and duration of response, pointing to Food and Drug Administration accelerated approval of an indication for nivolumab (Opdivo) for treatment of patients with hepatocellular carcinoma (HCC) that had progressed on sorafenib. In the single-arm trial used as the basis for approval, the ORRs as assessed by an independent central review committee blinded to the results was 14.3%.
“So, nivolumab in hepatocellular cancer was approved on the basis of a response rate lower than that of placebo, albeit in a different tumor. But the point I’m trying to show here is we don’t have a good definition of what is a good response rate,” he said.
In July 2021, Bristol-Myers Squibb voluntarily withdrew the HCC indication for nivolumab, following negative results of the CheckMate 459 trial and a 5-4 vote against continuing the accelerated approval.
On second thought ...
Citing data compiled by Nathan I. Cherny, MD, from Shaare Zedek Medical Center, Jerusalem, Dr. Gyawali noted that 58 of 161 FDA approvals from 2017 to 2021 of drugs for adult solid tumors were based on single-arm trials. Of the 58 drugs, 39 received accelerated approvals, and 19 received regular approvals; of the 39 that received accelerated approvals, 4 were subsequently withdrawn, 8 were converted to regular approvals, and the remainder continued as accelerated approvals.
Interestingly, the median response rate among all the drugs was 40%, and did not differ between the type of approval received, suggesting that response rates are not predictive of whether a drug will receive a conditional or full-fledged go-ahead.
What’s rare and safe?
The definition of a rare disease in the United States is one that affects fewer than 40,000 per year, and in Europe it’s an incidence rate of less than 6 per 100,000 population, Dr. Gyawali noted. But he argued that even non–small cell lung cancer, the most common form of cancer in the world, could be considered rare if it is broken down into subtypes that are treated according to specific mutations that may occur in a relatively small number of patients.
He also noted that a specific drug’s safety, one of the most important criteria for granting approval to a drug based on a single-arm trial, can be difficult to judge without adequate controls for comparison.
Cherry-picking patients
Winette van der Graaf, MD, president of the European Organization for the Research and Treatment of Cancer, who attended the session where Dr. Gyawali’s presentation was played, said in an interview that clinicians should cast a critical eye on how trials are designed and conducted, including patient selection and choice of endpoints.
“One of the most obvious things to be concerned about is that we’re still having patients with good performance status enrolled, mostly PS 0 or 1, so how representative are these clinical trials for the patients we see in front of us on a daily basis?” she said.
“The other question is radiological endpoints, which we focus on with OS and PFS are most important for patients, especially if you consider that if patients may have asymptomatic disease, and we are only treating them with potentially toxic medication, what are we doing for them? Median overall survival when you look at all of these trials is only 4 months, so we really need to take into account how we affect patients in clinical trials,” she added.
Dr. van der Graaf emphasized that clinical trial investigators need to more routinely incorporate quality of life measures and other patient-reported outcomes in clinical trial results to help regulators and clinicians in practice get a better sense of the true clinical benefit of a new drug.
Dr. Gyawali did not disclose a funding source for his presentation. He reported consulting fees from Vivio Health and research grants from the American Society of Clinical Oncology. Dr. van der Graaf reported no conflicts of interest.
PARIS – with results that should only be used, under certain conditions, for accelerated approvals that should then be followed by confirmatory studies.
In fact, many drugs approved over the last decade based solely on data from single-arm trials have been subsequently withdrawn when put through the rigors of a head-to-head randomized controlled trial, according to Bishal Gyawali, MD, PhD, from the department of oncology at Queen’s University, Kingston, Ont.
“Single-arm trials are not meant to provide confirmatory evidence sufficient for approval; However, that ship has sailed, and we have several drugs that are approved on the basis of single-arm trials, but we need to make sure that those approvals are accelerated or conditional approvals, not regular approval,” he said in a presentation included in a special session on drug approvals at the European Society for Medical Oncology Congress.
“We should not allow premature regular approval based on single-arm trials, because once a drug gets conditional approval, access is not an issue. Patients will have access to the drug anyway, but we should ensure that robust evidence follows, and long-term follow-up data are needed to develop confidence in the efficacy outcomes that are seen in single-arm trials,” he said.
In many cases, single-arm trials are large enough or of long enough duration that investigators could have reasonably performed a randomized controlled trial (RCT) in the first place, Dr. Gyawali added.
Why do single-arm trials?
The term “single-arm registration trial” is something of an oxymoron, he said, noting that the purpose of such trials should be whether to take the drug to a phase 3, randomized trial. But as authors of a 2019 study in JAMA Network Open showed, of a sample of phase 3 RCTs, 42% did not have a prior phase 2 trial, and 28% had a negative phase 2 trial. Single-arm trials may be acceptable for conditional drug approvals if all of the following conditions are met:
- A RCT is not possible because the disease is rare or randomization would be unethical.
- The safety of the drug is established and its potential benefits outweigh its risks.
- The drug is associated with a high and durable overall or objective response rate.
- The mechanism of action is supported by a strong scientific rationale, and if the drug may meet an unmet medical need.
Survival endpoints won’t do
Efficacy endpoints typically used in RCTs, such as progression-free survival (PFS) and overall survival (OS) can be misleading because they may be a result of the natural history of the disease and not the drug being tested, whereas ORRs are almost certainly reflective of the action of the drug itself, because spontaneous tumor regression is a rare phenomenon, Dr. Gyawali said.
He cautioned, however, that the ORR of placebo is not zero percent. For example in a 2018 study of sorafenib (Nexavar) versus placebo for advanced or refractory desmoid tumors, the ORR with the active drug was 33%, and the ORR for placebo was 20%.
It’s also open to question, he said, what constitutes an acceptably high ORR and duration of response, pointing to Food and Drug Administration accelerated approval of an indication for nivolumab (Opdivo) for treatment of patients with hepatocellular carcinoma (HCC) that had progressed on sorafenib. In the single-arm trial used as the basis for approval, the ORRs as assessed by an independent central review committee blinded to the results was 14.3%.
“So, nivolumab in hepatocellular cancer was approved on the basis of a response rate lower than that of placebo, albeit in a different tumor. But the point I’m trying to show here is we don’t have a good definition of what is a good response rate,” he said.
In July 2021, Bristol-Myers Squibb voluntarily withdrew the HCC indication for nivolumab, following negative results of the CheckMate 459 trial and a 5-4 vote against continuing the accelerated approval.
On second thought ...
Citing data compiled by Nathan I. Cherny, MD, from Shaare Zedek Medical Center, Jerusalem, Dr. Gyawali noted that 58 of 161 FDA approvals from 2017 to 2021 of drugs for adult solid tumors were based on single-arm trials. Of the 58 drugs, 39 received accelerated approvals, and 19 received regular approvals; of the 39 that received accelerated approvals, 4 were subsequently withdrawn, 8 were converted to regular approvals, and the remainder continued as accelerated approvals.
Interestingly, the median response rate among all the drugs was 40%, and did not differ between the type of approval received, suggesting that response rates are not predictive of whether a drug will receive a conditional or full-fledged go-ahead.
What’s rare and safe?
The definition of a rare disease in the United States is one that affects fewer than 40,000 per year, and in Europe it’s an incidence rate of less than 6 per 100,000 population, Dr. Gyawali noted. But he argued that even non–small cell lung cancer, the most common form of cancer in the world, could be considered rare if it is broken down into subtypes that are treated according to specific mutations that may occur in a relatively small number of patients.
He also noted that a specific drug’s safety, one of the most important criteria for granting approval to a drug based on a single-arm trial, can be difficult to judge without adequate controls for comparison.
Cherry-picking patients
Winette van der Graaf, MD, president of the European Organization for the Research and Treatment of Cancer, who attended the session where Dr. Gyawali’s presentation was played, said in an interview that clinicians should cast a critical eye on how trials are designed and conducted, including patient selection and choice of endpoints.
“One of the most obvious things to be concerned about is that we’re still having patients with good performance status enrolled, mostly PS 0 or 1, so how representative are these clinical trials for the patients we see in front of us on a daily basis?” she said.
“The other question is radiological endpoints, which we focus on with OS and PFS are most important for patients, especially if you consider that if patients may have asymptomatic disease, and we are only treating them with potentially toxic medication, what are we doing for them? Median overall survival when you look at all of these trials is only 4 months, so we really need to take into account how we affect patients in clinical trials,” she added.
Dr. van der Graaf emphasized that clinical trial investigators need to more routinely incorporate quality of life measures and other patient-reported outcomes in clinical trial results to help regulators and clinicians in practice get a better sense of the true clinical benefit of a new drug.
Dr. Gyawali did not disclose a funding source for his presentation. He reported consulting fees from Vivio Health and research grants from the American Society of Clinical Oncology. Dr. van der Graaf reported no conflicts of interest.
AT ESMO CONGRESS 2022
Biden’s Cancer Moonshot turns its focus to early-detection blood tests
There’s big buzz about the hot prospects for blood tests designed to detect multiple kinds of cancer. President Biden highlighted them in a speech about the Cancer Moonshot program on Sept. 12, just a day after study results touted an experimental test’s ability to detect dozens of kinds of cancer. Meanwhile, the federal government is heralding an upcoming trial that will eventually enroll as many as 225,000 subjects.
There are plenty of reasons to be cautious, however. And if these tests become standard, the oncology field will need to figure out how to navigate a thicket of new challenges.
“Our friends in internal medicine and primary care will be looking to us for guidance. We need to make sure that we’re coming at this without too much optimism before we really have the data,” said Jyoti D. Patel, MD, medical director of thoracic oncology and assistant director for clinical research at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago.
Dr. Patel is a member of the communications workgroup of the Multicancer Early Detection Consortium, a nonprofit, public-private organization that’s providing insight and guidance into the development of screening tests. The consortium published a position paper earlier this year.
According to Dr. Patel, early cancer screening today can detect only five types of cancer: prostate, breast, lung, cervical, and colon. The Cancer Moonshot program has prioritized research into greatly expanding this number. President Biden referred to this goal in his Sept. 12 speech: “Imagine a simple blood test during an annual physical that could detect cancer early, where the chances of a cure are best.”
Biden said the National Cancer Institute is launching a major trial as part of the Cancer Moonshot program. The Vanguard Study on Multi-Cancer Detection plans to enlist 25,000 healthy women and men between 45 and 70 years old in 2024, then later enroll as many as 225,000 people.
Meanwhile, researchers reported on Sept. 11 that the Galleri multicancer detection blood test found positive cancer signals in 1.4% of 6,621 healthy subjects, and cancer was ultimately confirmed in 38% of those in that group. Nineteen solid tumors and 17 hematologic cancers were diagnosed; 26 of these were cancer types that don’t have routine screening available.
The Galleri test is widely available in the United States, although the $950 cost is not covered by insurance.
While the data is exciting, the high false-positive rate is worrisome, Dr. Patel said. “Are there ways that we can further define that by cancer-risk assessment or by having better captures in our technology that reflect RNA methylation or epigenetic changes that may lead to susceptibility to cancers?”
Additional research is essential
Ernest Hawk, MD, vice president and division head of cancer prevention and population sciences at the University of Texas MD Anderson Cancer Center, Houston, said it’s “absolutely essential” that research into screening tests clearly demonstrates improved patient outcomes over time.
“We need to have much longer follow-up of all participants – whether the screening results are positive or negative – and mitigate the potential risks of such testing,” said Dr. Hawk, who’s worked with the Multicancer Early Detection Consortium.
On another front, Northwestern University’s Dr. Patel highlighted that while easy-to-access cancer screening could create tremendous opportunities to treat early cancer and shrink disparities in care, it may produce “an onslaught of patients with early-stage disease. Do we have the workforce to help us?” Also, she said, “if we find a patient with early-stage disease, how are we going to risk-stratify their follow-up and adjuvant therapy? Are there ways to prognosticate with more granularity than we do now?”
What’s next? “Multicancer early-detection tests could truly revolutionize cancer care if they work as we hope they will, but only time, extensive participation in research, and hard work will prove whether that is true or not,” said MD Anderson’s Dr. Hawk. “I anticipate that we’ll have reasonable answers within the next decade, given the pace of existing company-sponsored research and NCI’s planned involvement in testing various technologies available.”
For her part, Dr. Patel said oncologists should be aware that multicancer screening tests are available and be ready to address questions about them. “Think about how you can advise patients in the absence of data,” she said.
Dr. Patel and Dr. Hawk have no relevant disclosures.
There’s big buzz about the hot prospects for blood tests designed to detect multiple kinds of cancer. President Biden highlighted them in a speech about the Cancer Moonshot program on Sept. 12, just a day after study results touted an experimental test’s ability to detect dozens of kinds of cancer. Meanwhile, the federal government is heralding an upcoming trial that will eventually enroll as many as 225,000 subjects.
There are plenty of reasons to be cautious, however. And if these tests become standard, the oncology field will need to figure out how to navigate a thicket of new challenges.
“Our friends in internal medicine and primary care will be looking to us for guidance. We need to make sure that we’re coming at this without too much optimism before we really have the data,” said Jyoti D. Patel, MD, medical director of thoracic oncology and assistant director for clinical research at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago.
Dr. Patel is a member of the communications workgroup of the Multicancer Early Detection Consortium, a nonprofit, public-private organization that’s providing insight and guidance into the development of screening tests. The consortium published a position paper earlier this year.
According to Dr. Patel, early cancer screening today can detect only five types of cancer: prostate, breast, lung, cervical, and colon. The Cancer Moonshot program has prioritized research into greatly expanding this number. President Biden referred to this goal in his Sept. 12 speech: “Imagine a simple blood test during an annual physical that could detect cancer early, where the chances of a cure are best.”
Biden said the National Cancer Institute is launching a major trial as part of the Cancer Moonshot program. The Vanguard Study on Multi-Cancer Detection plans to enlist 25,000 healthy women and men between 45 and 70 years old in 2024, then later enroll as many as 225,000 people.
Meanwhile, researchers reported on Sept. 11 that the Galleri multicancer detection blood test found positive cancer signals in 1.4% of 6,621 healthy subjects, and cancer was ultimately confirmed in 38% of those in that group. Nineteen solid tumors and 17 hematologic cancers were diagnosed; 26 of these were cancer types that don’t have routine screening available.
The Galleri test is widely available in the United States, although the $950 cost is not covered by insurance.
While the data is exciting, the high false-positive rate is worrisome, Dr. Patel said. “Are there ways that we can further define that by cancer-risk assessment or by having better captures in our technology that reflect RNA methylation or epigenetic changes that may lead to susceptibility to cancers?”
Additional research is essential
Ernest Hawk, MD, vice president and division head of cancer prevention and population sciences at the University of Texas MD Anderson Cancer Center, Houston, said it’s “absolutely essential” that research into screening tests clearly demonstrates improved patient outcomes over time.
“We need to have much longer follow-up of all participants – whether the screening results are positive or negative – and mitigate the potential risks of such testing,” said Dr. Hawk, who’s worked with the Multicancer Early Detection Consortium.
On another front, Northwestern University’s Dr. Patel highlighted that while easy-to-access cancer screening could create tremendous opportunities to treat early cancer and shrink disparities in care, it may produce “an onslaught of patients with early-stage disease. Do we have the workforce to help us?” Also, she said, “if we find a patient with early-stage disease, how are we going to risk-stratify their follow-up and adjuvant therapy? Are there ways to prognosticate with more granularity than we do now?”
What’s next? “Multicancer early-detection tests could truly revolutionize cancer care if they work as we hope they will, but only time, extensive participation in research, and hard work will prove whether that is true or not,” said MD Anderson’s Dr. Hawk. “I anticipate that we’ll have reasonable answers within the next decade, given the pace of existing company-sponsored research and NCI’s planned involvement in testing various technologies available.”
For her part, Dr. Patel said oncologists should be aware that multicancer screening tests are available and be ready to address questions about them. “Think about how you can advise patients in the absence of data,” she said.
Dr. Patel and Dr. Hawk have no relevant disclosures.
There’s big buzz about the hot prospects for blood tests designed to detect multiple kinds of cancer. President Biden highlighted them in a speech about the Cancer Moonshot program on Sept. 12, just a day after study results touted an experimental test’s ability to detect dozens of kinds of cancer. Meanwhile, the federal government is heralding an upcoming trial that will eventually enroll as many as 225,000 subjects.
There are plenty of reasons to be cautious, however. And if these tests become standard, the oncology field will need to figure out how to navigate a thicket of new challenges.
“Our friends in internal medicine and primary care will be looking to us for guidance. We need to make sure that we’re coming at this without too much optimism before we really have the data,” said Jyoti D. Patel, MD, medical director of thoracic oncology and assistant director for clinical research at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago.
Dr. Patel is a member of the communications workgroup of the Multicancer Early Detection Consortium, a nonprofit, public-private organization that’s providing insight and guidance into the development of screening tests. The consortium published a position paper earlier this year.
According to Dr. Patel, early cancer screening today can detect only five types of cancer: prostate, breast, lung, cervical, and colon. The Cancer Moonshot program has prioritized research into greatly expanding this number. President Biden referred to this goal in his Sept. 12 speech: “Imagine a simple blood test during an annual physical that could detect cancer early, where the chances of a cure are best.”
Biden said the National Cancer Institute is launching a major trial as part of the Cancer Moonshot program. The Vanguard Study on Multi-Cancer Detection plans to enlist 25,000 healthy women and men between 45 and 70 years old in 2024, then later enroll as many as 225,000 people.
Meanwhile, researchers reported on Sept. 11 that the Galleri multicancer detection blood test found positive cancer signals in 1.4% of 6,621 healthy subjects, and cancer was ultimately confirmed in 38% of those in that group. Nineteen solid tumors and 17 hematologic cancers were diagnosed; 26 of these were cancer types that don’t have routine screening available.
The Galleri test is widely available in the United States, although the $950 cost is not covered by insurance.
While the data is exciting, the high false-positive rate is worrisome, Dr. Patel said. “Are there ways that we can further define that by cancer-risk assessment or by having better captures in our technology that reflect RNA methylation or epigenetic changes that may lead to susceptibility to cancers?”
Additional research is essential
Ernest Hawk, MD, vice president and division head of cancer prevention and population sciences at the University of Texas MD Anderson Cancer Center, Houston, said it’s “absolutely essential” that research into screening tests clearly demonstrates improved patient outcomes over time.
“We need to have much longer follow-up of all participants – whether the screening results are positive or negative – and mitigate the potential risks of such testing,” said Dr. Hawk, who’s worked with the Multicancer Early Detection Consortium.
On another front, Northwestern University’s Dr. Patel highlighted that while easy-to-access cancer screening could create tremendous opportunities to treat early cancer and shrink disparities in care, it may produce “an onslaught of patients with early-stage disease. Do we have the workforce to help us?” Also, she said, “if we find a patient with early-stage disease, how are we going to risk-stratify their follow-up and adjuvant therapy? Are there ways to prognosticate with more granularity than we do now?”
What’s next? “Multicancer early-detection tests could truly revolutionize cancer care if they work as we hope they will, but only time, extensive participation in research, and hard work will prove whether that is true or not,” said MD Anderson’s Dr. Hawk. “I anticipate that we’ll have reasonable answers within the next decade, given the pace of existing company-sponsored research and NCI’s planned involvement in testing various technologies available.”
For her part, Dr. Patel said oncologists should be aware that multicancer screening tests are available and be ready to address questions about them. “Think about how you can advise patients in the absence of data,” she said.
Dr. Patel and Dr. Hawk have no relevant disclosures.
Improving Inpatient COVID-19 Vaccination Rates Among Adult Patients at a Tertiary Academic Medical Center
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.
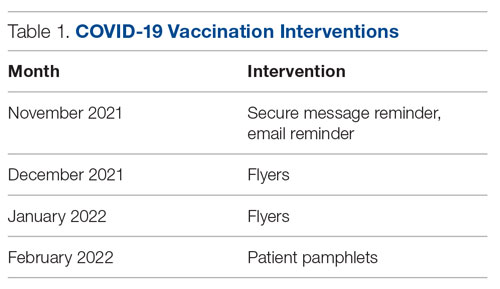
In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.
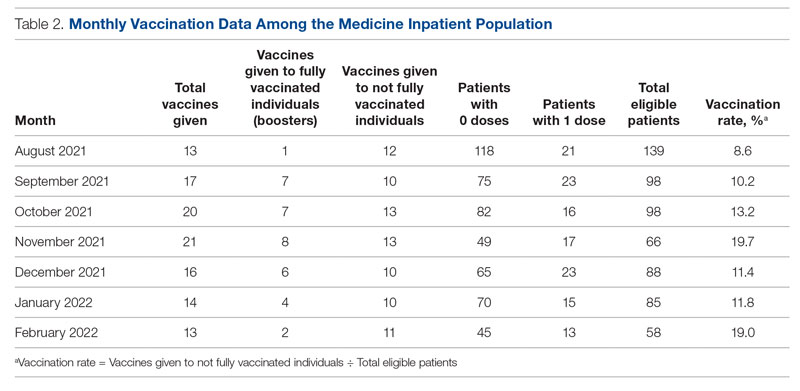
Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).
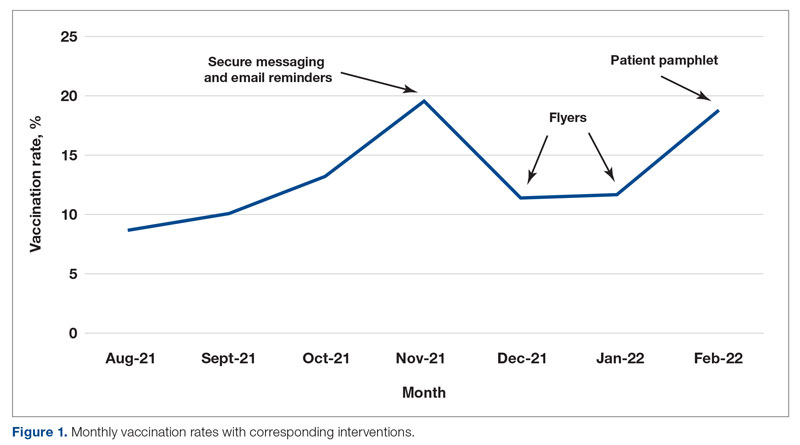
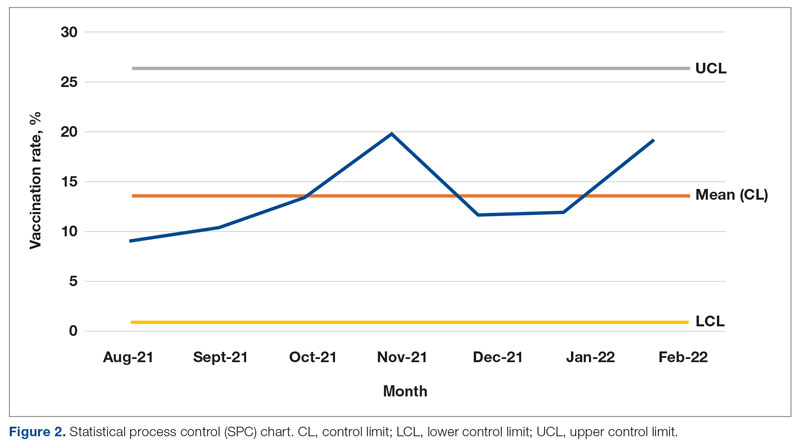
Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; arubin@mfa.gwu.edu
Disclosures: None reported.
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.

In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.

Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).


Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; arubin@mfa.gwu.edu
Disclosures: None reported.
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.

In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.

Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).


Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; arubin@mfa.gwu.edu
Disclosures: None reported.
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1









