User login
Liberalized European sports cardiology guidelines break new ground
New guidelines on sports cardiology from the European Society of Cardiology break fresh ground by green-lighting participation in vigorous competitive sports by selected patients with stable coronary artery disease, heart failure, or mild arrhythmias.
These liberalized guidelines, released at the virtual annual congress of the European Society of Cardiology, thus move well beyond the standard exercise advice to engage in about 150 minutes per week of moderate physical activity, typically defined as brisk walking or its equivalent.
The guidelines reflect a conviction that exercise is powerful medicine for patients with cardiovascular disease and also affords a means to help curb the epidemics of diabetes and obesity that drive cardiovascular risk, according to Antonio Pelliccia, MD, who cochaired the 24-member task force of European and American experts that developed the guidelines.
In a session highlighting the new sports cardiology guidelines, Mats Borjesson, MD, head of the Center for Health and Performance at Gothenburg (Sweden) University, summarized the section devoted to patients with stable coronary artery disease: “If you have established CAD and a low risk of adverse events during exercise, you are eligible for high-intensity exercise and competitive sports. But if you have persistent ischemia despite medical treatment, or symptoms, then you’re only eligible for leisure-time subthreshold activity.”
Dr. Pelliccia put this new recommendation into context.
“We are not talking anymore in this particular disease just about cardiac rehabilitation or leisure-time activity, but we are also opening the border and talking about competitive sports activity in selected patients where you have the evidence for low risk of exercise-induced adverse events. This is a major achievement now for what is the major disease in our adult population,” said Dr. Pelliccia, chief of cardiology at the Institute of Sports Medicine and Science at the Italian National Olympic Committee and professor of sports cardiology at La Sapienza University of Rome.
The recommendation for individualized consideration of all types of exercise, even including vigorous competitive sports, in low-risk patients with CAD gets a class IIa, level of evidence (LOE) C recommendation in the new guidelines. That’s a big step down from a ringing class Ia endorsement, but since sports cardiology is a relatively young field with little evidence that’s based on randomized trials, the guidelines are rife with many other class IIa, LOE C recommendations as well.
“The level of evidence is rather low, so these guidelines are very much the personal perspective of the expert panel,” explained Martin Halle, MD, professor and head of the department of prevention, rehabilitation, and sports cardiology at Technical University of Munich.
The high-risk features for exercise-induced cardiac adverse events in patients with longstanding stable CAD, as cited in the guidelines, include a critical coronary stenosis, defined as a more than 70% lesion in a major coronary artery or a greater than 50% stenosis in the left main, and/or a fractional flow reserve score of less than 0.8; a left ventricular ejection fraction of 50% or less with wall-motion abnormalities; inducible myocardial ischemia on maximal exercise testing; nonsustained ventricular tachycardia; polymorphic or very frequent ventricular premature beats at rest and during maximum stress; and a recent acute coronary syndrome (ACS). These features call for an exercise prescription tailored to remain below the patient’s angina and ischemia thresholds.
“It’s important for cardiologists out there to understand that we definitely need a maximal exercise test. In somebody who is running and has an ACS and then wants to start running again, 200 watts on an ergometer is too low. We have to push them up to the end, and then if everything is okay – left ventricular function is okay, no ischemia, no arrhythmias under exercise testing – then it’s fine,” Dr. Halle said.
Dr. Pelliccia added that close follow-up is needed, because this is an evolving disease.”
Exercise and heart failure
Massimo F. Piepoli, MD, PhD, noted that the guidelines give a class IIb, LOE C recommendation for consideration of high-intensity recreational endurance and power sports in patients with heart failure with either midrange or preserved ejection fraction, provided they are stable, asymptomatic, on optimal guideline-directed medical therapy, and without abnormalities on a maximal exercise stress test.
However, such intense physical activity is not recommended in patients with heart failure with reduced ejection fraction, regardless of their symptom status, added Dr. Piepoli of Guglielmo da Saliceto Hospital in Placenza, Italy.
“We’re talking here, I think for the first time, about possible competitive sports participation in individuals with heart failure, depending on their clinical condition. We are really opening the barriers to sports participation, even in these patients in whom we never thought of it before,” Dr. Pelliccia observed.
Valvular heart disease and exercise
Guidelines panelist Sabiha Gati, MRCP, PhD, said asymptomatic individuals with mild valvular abnormalities can participate in all recreational and competitive sports; that’s a class I, LOE C recommendation.
“Moderate regurgitant lesions are better tolerated than stenotic lesions, and those with preserved systolic function, good functional capacity, without any exercise-induced arrhythmias or ischemia or abnormal hemodynamic response are considered to be low risk and can participate in all sports,” added Dr. Gati, a cardiologist at Royal Brompton Hospital, London.
The two most common valvular abnormalities encountered in clinical practice are bicuspid aortic valve and mitral valve prolapse. Dr. Gati noted that, while mitral valve prolapse has a benign prognosis in the great majority of affected individuals, the presence of specific features indicative of increased risk for sudden cardiac death precludes participation in strenuous exercise. These include T-wave inversion in the inferior leads on a 12-lead ECG, long QT, bileaflet mitral valve prolapse, basal inferolateral wall fibrosis, severe mitral regurgitation, or a family history of sudden cardiac death.
Bicuspid aortic valve has a prevalence of 1%-2% in the general population. It can be associated with aortic stenosis, aortic regurgitation, and increased risk of ascending aortic aneurysm and dissection. Since it remains unclear whether intensive exercise accelerates aortic dilatation, a cautious approach to sports participation is recommended in patients with an ascending aorta above the normal limit of 40 mm, she said.
The 80-page ESC sports cardiology guidelines, published online simultaneously with their presentation, cover a broad range of additional topics, including exercise recommendations for the general public, for the elderly, as well as for patients with cardiomyopathies, adult congenital heart disease, arrhythmias, and channelopathies. Gaps in evidence are also highlighted.
SOURCE: Pelliccia A. ESC 2020 and Eur Heart J. 2020 Aug 29. doi: 10.1093/eurheartj/ehaa605.
New guidelines on sports cardiology from the European Society of Cardiology break fresh ground by green-lighting participation in vigorous competitive sports by selected patients with stable coronary artery disease, heart failure, or mild arrhythmias.
These liberalized guidelines, released at the virtual annual congress of the European Society of Cardiology, thus move well beyond the standard exercise advice to engage in about 150 minutes per week of moderate physical activity, typically defined as brisk walking or its equivalent.
The guidelines reflect a conviction that exercise is powerful medicine for patients with cardiovascular disease and also affords a means to help curb the epidemics of diabetes and obesity that drive cardiovascular risk, according to Antonio Pelliccia, MD, who cochaired the 24-member task force of European and American experts that developed the guidelines.
In a session highlighting the new sports cardiology guidelines, Mats Borjesson, MD, head of the Center for Health and Performance at Gothenburg (Sweden) University, summarized the section devoted to patients with stable coronary artery disease: “If you have established CAD and a low risk of adverse events during exercise, you are eligible for high-intensity exercise and competitive sports. But if you have persistent ischemia despite medical treatment, or symptoms, then you’re only eligible for leisure-time subthreshold activity.”
Dr. Pelliccia put this new recommendation into context.
“We are not talking anymore in this particular disease just about cardiac rehabilitation or leisure-time activity, but we are also opening the border and talking about competitive sports activity in selected patients where you have the evidence for low risk of exercise-induced adverse events. This is a major achievement now for what is the major disease in our adult population,” said Dr. Pelliccia, chief of cardiology at the Institute of Sports Medicine and Science at the Italian National Olympic Committee and professor of sports cardiology at La Sapienza University of Rome.
The recommendation for individualized consideration of all types of exercise, even including vigorous competitive sports, in low-risk patients with CAD gets a class IIa, level of evidence (LOE) C recommendation in the new guidelines. That’s a big step down from a ringing class Ia endorsement, but since sports cardiology is a relatively young field with little evidence that’s based on randomized trials, the guidelines are rife with many other class IIa, LOE C recommendations as well.
“The level of evidence is rather low, so these guidelines are very much the personal perspective of the expert panel,” explained Martin Halle, MD, professor and head of the department of prevention, rehabilitation, and sports cardiology at Technical University of Munich.
The high-risk features for exercise-induced cardiac adverse events in patients with longstanding stable CAD, as cited in the guidelines, include a critical coronary stenosis, defined as a more than 70% lesion in a major coronary artery or a greater than 50% stenosis in the left main, and/or a fractional flow reserve score of less than 0.8; a left ventricular ejection fraction of 50% or less with wall-motion abnormalities; inducible myocardial ischemia on maximal exercise testing; nonsustained ventricular tachycardia; polymorphic or very frequent ventricular premature beats at rest and during maximum stress; and a recent acute coronary syndrome (ACS). These features call for an exercise prescription tailored to remain below the patient’s angina and ischemia thresholds.
“It’s important for cardiologists out there to understand that we definitely need a maximal exercise test. In somebody who is running and has an ACS and then wants to start running again, 200 watts on an ergometer is too low. We have to push them up to the end, and then if everything is okay – left ventricular function is okay, no ischemia, no arrhythmias under exercise testing – then it’s fine,” Dr. Halle said.
Dr. Pelliccia added that close follow-up is needed, because this is an evolving disease.”
Exercise and heart failure
Massimo F. Piepoli, MD, PhD, noted that the guidelines give a class IIb, LOE C recommendation for consideration of high-intensity recreational endurance and power sports in patients with heart failure with either midrange or preserved ejection fraction, provided they are stable, asymptomatic, on optimal guideline-directed medical therapy, and without abnormalities on a maximal exercise stress test.
However, such intense physical activity is not recommended in patients with heart failure with reduced ejection fraction, regardless of their symptom status, added Dr. Piepoli of Guglielmo da Saliceto Hospital in Placenza, Italy.
“We’re talking here, I think for the first time, about possible competitive sports participation in individuals with heart failure, depending on their clinical condition. We are really opening the barriers to sports participation, even in these patients in whom we never thought of it before,” Dr. Pelliccia observed.
Valvular heart disease and exercise
Guidelines panelist Sabiha Gati, MRCP, PhD, said asymptomatic individuals with mild valvular abnormalities can participate in all recreational and competitive sports; that’s a class I, LOE C recommendation.
“Moderate regurgitant lesions are better tolerated than stenotic lesions, and those with preserved systolic function, good functional capacity, without any exercise-induced arrhythmias or ischemia or abnormal hemodynamic response are considered to be low risk and can participate in all sports,” added Dr. Gati, a cardiologist at Royal Brompton Hospital, London.
The two most common valvular abnormalities encountered in clinical practice are bicuspid aortic valve and mitral valve prolapse. Dr. Gati noted that, while mitral valve prolapse has a benign prognosis in the great majority of affected individuals, the presence of specific features indicative of increased risk for sudden cardiac death precludes participation in strenuous exercise. These include T-wave inversion in the inferior leads on a 12-lead ECG, long QT, bileaflet mitral valve prolapse, basal inferolateral wall fibrosis, severe mitral regurgitation, or a family history of sudden cardiac death.
Bicuspid aortic valve has a prevalence of 1%-2% in the general population. It can be associated with aortic stenosis, aortic regurgitation, and increased risk of ascending aortic aneurysm and dissection. Since it remains unclear whether intensive exercise accelerates aortic dilatation, a cautious approach to sports participation is recommended in patients with an ascending aorta above the normal limit of 40 mm, she said.
The 80-page ESC sports cardiology guidelines, published online simultaneously with their presentation, cover a broad range of additional topics, including exercise recommendations for the general public, for the elderly, as well as for patients with cardiomyopathies, adult congenital heart disease, arrhythmias, and channelopathies. Gaps in evidence are also highlighted.
SOURCE: Pelliccia A. ESC 2020 and Eur Heart J. 2020 Aug 29. doi: 10.1093/eurheartj/ehaa605.
New guidelines on sports cardiology from the European Society of Cardiology break fresh ground by green-lighting participation in vigorous competitive sports by selected patients with stable coronary artery disease, heart failure, or mild arrhythmias.
These liberalized guidelines, released at the virtual annual congress of the European Society of Cardiology, thus move well beyond the standard exercise advice to engage in about 150 minutes per week of moderate physical activity, typically defined as brisk walking or its equivalent.
The guidelines reflect a conviction that exercise is powerful medicine for patients with cardiovascular disease and also affords a means to help curb the epidemics of diabetes and obesity that drive cardiovascular risk, according to Antonio Pelliccia, MD, who cochaired the 24-member task force of European and American experts that developed the guidelines.
In a session highlighting the new sports cardiology guidelines, Mats Borjesson, MD, head of the Center for Health and Performance at Gothenburg (Sweden) University, summarized the section devoted to patients with stable coronary artery disease: “If you have established CAD and a low risk of adverse events during exercise, you are eligible for high-intensity exercise and competitive sports. But if you have persistent ischemia despite medical treatment, or symptoms, then you’re only eligible for leisure-time subthreshold activity.”
Dr. Pelliccia put this new recommendation into context.
“We are not talking anymore in this particular disease just about cardiac rehabilitation or leisure-time activity, but we are also opening the border and talking about competitive sports activity in selected patients where you have the evidence for low risk of exercise-induced adverse events. This is a major achievement now for what is the major disease in our adult population,” said Dr. Pelliccia, chief of cardiology at the Institute of Sports Medicine and Science at the Italian National Olympic Committee and professor of sports cardiology at La Sapienza University of Rome.
The recommendation for individualized consideration of all types of exercise, even including vigorous competitive sports, in low-risk patients with CAD gets a class IIa, level of evidence (LOE) C recommendation in the new guidelines. That’s a big step down from a ringing class Ia endorsement, but since sports cardiology is a relatively young field with little evidence that’s based on randomized trials, the guidelines are rife with many other class IIa, LOE C recommendations as well.
“The level of evidence is rather low, so these guidelines are very much the personal perspective of the expert panel,” explained Martin Halle, MD, professor and head of the department of prevention, rehabilitation, and sports cardiology at Technical University of Munich.
The high-risk features for exercise-induced cardiac adverse events in patients with longstanding stable CAD, as cited in the guidelines, include a critical coronary stenosis, defined as a more than 70% lesion in a major coronary artery or a greater than 50% stenosis in the left main, and/or a fractional flow reserve score of less than 0.8; a left ventricular ejection fraction of 50% or less with wall-motion abnormalities; inducible myocardial ischemia on maximal exercise testing; nonsustained ventricular tachycardia; polymorphic or very frequent ventricular premature beats at rest and during maximum stress; and a recent acute coronary syndrome (ACS). These features call for an exercise prescription tailored to remain below the patient’s angina and ischemia thresholds.
“It’s important for cardiologists out there to understand that we definitely need a maximal exercise test. In somebody who is running and has an ACS and then wants to start running again, 200 watts on an ergometer is too low. We have to push them up to the end, and then if everything is okay – left ventricular function is okay, no ischemia, no arrhythmias under exercise testing – then it’s fine,” Dr. Halle said.
Dr. Pelliccia added that close follow-up is needed, because this is an evolving disease.”
Exercise and heart failure
Massimo F. Piepoli, MD, PhD, noted that the guidelines give a class IIb, LOE C recommendation for consideration of high-intensity recreational endurance and power sports in patients with heart failure with either midrange or preserved ejection fraction, provided they are stable, asymptomatic, on optimal guideline-directed medical therapy, and without abnormalities on a maximal exercise stress test.
However, such intense physical activity is not recommended in patients with heart failure with reduced ejection fraction, regardless of their symptom status, added Dr. Piepoli of Guglielmo da Saliceto Hospital in Placenza, Italy.
“We’re talking here, I think for the first time, about possible competitive sports participation in individuals with heart failure, depending on their clinical condition. We are really opening the barriers to sports participation, even in these patients in whom we never thought of it before,” Dr. Pelliccia observed.
Valvular heart disease and exercise
Guidelines panelist Sabiha Gati, MRCP, PhD, said asymptomatic individuals with mild valvular abnormalities can participate in all recreational and competitive sports; that’s a class I, LOE C recommendation.
“Moderate regurgitant lesions are better tolerated than stenotic lesions, and those with preserved systolic function, good functional capacity, without any exercise-induced arrhythmias or ischemia or abnormal hemodynamic response are considered to be low risk and can participate in all sports,” added Dr. Gati, a cardiologist at Royal Brompton Hospital, London.
The two most common valvular abnormalities encountered in clinical practice are bicuspid aortic valve and mitral valve prolapse. Dr. Gati noted that, while mitral valve prolapse has a benign prognosis in the great majority of affected individuals, the presence of specific features indicative of increased risk for sudden cardiac death precludes participation in strenuous exercise. These include T-wave inversion in the inferior leads on a 12-lead ECG, long QT, bileaflet mitral valve prolapse, basal inferolateral wall fibrosis, severe mitral regurgitation, or a family history of sudden cardiac death.
Bicuspid aortic valve has a prevalence of 1%-2% in the general population. It can be associated with aortic stenosis, aortic regurgitation, and increased risk of ascending aortic aneurysm and dissection. Since it remains unclear whether intensive exercise accelerates aortic dilatation, a cautious approach to sports participation is recommended in patients with an ascending aorta above the normal limit of 40 mm, she said.
The 80-page ESC sports cardiology guidelines, published online simultaneously with their presentation, cover a broad range of additional topics, including exercise recommendations for the general public, for the elderly, as well as for patients with cardiomyopathies, adult congenital heart disease, arrhythmias, and channelopathies. Gaps in evidence are also highlighted.
SOURCE: Pelliccia A. ESC 2020 and Eur Heart J. 2020 Aug 29. doi: 10.1093/eurheartj/ehaa605.
FROM ESC CONGRESS 2020
MIS-C cardiac evaluation requires more than EF
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
DAPA-CKD: SGLT2 inhibitor benefit extends to chronic kidney disease without diabetes
In the DAPA-CKD trial, treatment with the SGLT2 inhibitor dapagliflozin (Farxiga) cut the incidence of substantially worsened chronic kidney disease by an average of 39% compared with placebo when added to standard treatment, with a number needed to treat of 19 to prevent one primary outcome event after a median of 2.4 years.
The level of benefit was similar in both the one-third of enrolled patients without diabetes and in the two-thirds with diabetes, showing a statistically significant 50% cut in the primary endpoint among patients without diabetes, Hiddo J.L. Heerspink, MD, reported at the virtual annual congress of the European Society of Cardiology.
“We found that dapagliflozin delayed the initiation of dialysis, and reduced the number of deaths,” regardless of diabetes status, Dr. Heerspink, of University Medical Centre Groningen, the Netherlands, said during a press conference. “DAPA-CKD trial has shown dapagliflozin’s potential as a long-awaited new treatment for patients with chronic kidney disease.”
This finding ushers in a “completely new era in chronic kidney disease management,” said Janani Rangaswami, MD, a nephrologist and cardiorenal syndrome specialist at Einstein Medical Center in Philadelphia. “It’s good news” for these patients.
The results showed that dapagliflozin is the first “game changing” drug for chronic kidney disease in 2 decades, following the introduction of angiotensin converting enzyme inhibitors and angiotensin receptor blockers, she said in an interview. And given the consistency of the findings with the results from several other studies that documented meaningful renal protection by several different SGLT2 inhibitors, the results from this single trial also convincingly establish dapagliflozin as a standard-of-care agent to use on the types of patients the study enrolled, she said in an interview.
Representing many real-world patients
The DAPA-CKD trial enrolled 4,304 patients with albuminuria based on having a urinary albumin-to-creatinine ratio of at least 200 mg/g, and an estimated glomerular filtration rate (eGFR) of 25-75 mL/min per 1.73 m2 (with 90% of patients having an eGFR of less than 60 mL/min per 1.73 m2), and 97% were on treatment with a renin-angiotensin system–blocking drug. The primary endpoint was the combined rate of a drop in eGFR of at least 50% from baseline, progression to end stage renal disease, or renal or cardiovascular death; the between-group difference in this composite was driven primarily by both preserved eGFR and by prevention of end stage renal disease.
This represents both an appropriate target population, and meaningful endpoints, Dr. Rangaswami said. The study was “very representative of who we see in real-world practice,” a group that likely includes “hundreds of thousands” of U.S. patients with nondiabetic chronic kidney disease, she estimated.
Another notable finding was that 14% of the enrolled patients had eGFR values at baseline of 25-29 mL/min per 1.73 m2, pegging them as having stage 4 chronic kidney disease, and the median baseline eGFR was 43 mL/min per 1.73 m2, but dapagliflozin treatment was as safe and effective in these patients as it was in enrolled patients with a higher level of retained renal activity. This experience should give clinicians greater confidence about using dapagliflozin and other drugs in the sodium-glucose cotransporter (SGLT) 2 inhibitor class in patients with substantially depressed renal function, Dr. Rangaswami said.
“We now need to be more proactive about treating patients with more advanced kidney disease who can still benefit” from dapagliflozin treatment. “The sooner you intervene the better,” to slow further progression, but the new findings show “benefit even when treating patients with lower eGFRs. There is still hope to prevent or delay dialysis.”
A heart-kidney connection
Dapagliflozin treatment also cut all-cause mortality by a statistically significant, relative 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization, a benefit seen consistently in several prior studies of SGLT2 inhibitors, but possibly unexpected here because enrolled patients underwent no selection for a history of heart failure or any other cardiovascular disease. But the finding shouldn’t surprise, because “chronic kidney disease is an independent risk factor for cardiovascular disease across the board, and especially for heart failure,” noted Dr. Rangaswami.
“Heart and kidney disease is one big spectrum,” and the collected experience of several trials that have now proven the efficacy of SGLT2 inhibitors among patients with heart failure with reduced ejection fraction or with chronic kidney disease, regardless of their glycemic control, shows how broadly this drug class can benefit patients across the breadth of this spectrum, she said.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Heerspink has been a consultant to and received research funding from AstraZeneca and from several other companies. Dr. Rangaswami had no disclosures.
In the DAPA-CKD trial, treatment with the SGLT2 inhibitor dapagliflozin (Farxiga) cut the incidence of substantially worsened chronic kidney disease by an average of 39% compared with placebo when added to standard treatment, with a number needed to treat of 19 to prevent one primary outcome event after a median of 2.4 years.
The level of benefit was similar in both the one-third of enrolled patients without diabetes and in the two-thirds with diabetes, showing a statistically significant 50% cut in the primary endpoint among patients without diabetes, Hiddo J.L. Heerspink, MD, reported at the virtual annual congress of the European Society of Cardiology.
“We found that dapagliflozin delayed the initiation of dialysis, and reduced the number of deaths,” regardless of diabetes status, Dr. Heerspink, of University Medical Centre Groningen, the Netherlands, said during a press conference. “DAPA-CKD trial has shown dapagliflozin’s potential as a long-awaited new treatment for patients with chronic kidney disease.”
This finding ushers in a “completely new era in chronic kidney disease management,” said Janani Rangaswami, MD, a nephrologist and cardiorenal syndrome specialist at Einstein Medical Center in Philadelphia. “It’s good news” for these patients.
The results showed that dapagliflozin is the first “game changing” drug for chronic kidney disease in 2 decades, following the introduction of angiotensin converting enzyme inhibitors and angiotensin receptor blockers, she said in an interview. And given the consistency of the findings with the results from several other studies that documented meaningful renal protection by several different SGLT2 inhibitors, the results from this single trial also convincingly establish dapagliflozin as a standard-of-care agent to use on the types of patients the study enrolled, she said in an interview.
Representing many real-world patients
The DAPA-CKD trial enrolled 4,304 patients with albuminuria based on having a urinary albumin-to-creatinine ratio of at least 200 mg/g, and an estimated glomerular filtration rate (eGFR) of 25-75 mL/min per 1.73 m2 (with 90% of patients having an eGFR of less than 60 mL/min per 1.73 m2), and 97% were on treatment with a renin-angiotensin system–blocking drug. The primary endpoint was the combined rate of a drop in eGFR of at least 50% from baseline, progression to end stage renal disease, or renal or cardiovascular death; the between-group difference in this composite was driven primarily by both preserved eGFR and by prevention of end stage renal disease.
This represents both an appropriate target population, and meaningful endpoints, Dr. Rangaswami said. The study was “very representative of who we see in real-world practice,” a group that likely includes “hundreds of thousands” of U.S. patients with nondiabetic chronic kidney disease, she estimated.
Another notable finding was that 14% of the enrolled patients had eGFR values at baseline of 25-29 mL/min per 1.73 m2, pegging them as having stage 4 chronic kidney disease, and the median baseline eGFR was 43 mL/min per 1.73 m2, but dapagliflozin treatment was as safe and effective in these patients as it was in enrolled patients with a higher level of retained renal activity. This experience should give clinicians greater confidence about using dapagliflozin and other drugs in the sodium-glucose cotransporter (SGLT) 2 inhibitor class in patients with substantially depressed renal function, Dr. Rangaswami said.
“We now need to be more proactive about treating patients with more advanced kidney disease who can still benefit” from dapagliflozin treatment. “The sooner you intervene the better,” to slow further progression, but the new findings show “benefit even when treating patients with lower eGFRs. There is still hope to prevent or delay dialysis.”
A heart-kidney connection
Dapagliflozin treatment also cut all-cause mortality by a statistically significant, relative 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization, a benefit seen consistently in several prior studies of SGLT2 inhibitors, but possibly unexpected here because enrolled patients underwent no selection for a history of heart failure or any other cardiovascular disease. But the finding shouldn’t surprise, because “chronic kidney disease is an independent risk factor for cardiovascular disease across the board, and especially for heart failure,” noted Dr. Rangaswami.
“Heart and kidney disease is one big spectrum,” and the collected experience of several trials that have now proven the efficacy of SGLT2 inhibitors among patients with heart failure with reduced ejection fraction or with chronic kidney disease, regardless of their glycemic control, shows how broadly this drug class can benefit patients across the breadth of this spectrum, she said.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Heerspink has been a consultant to and received research funding from AstraZeneca and from several other companies. Dr. Rangaswami had no disclosures.
In the DAPA-CKD trial, treatment with the SGLT2 inhibitor dapagliflozin (Farxiga) cut the incidence of substantially worsened chronic kidney disease by an average of 39% compared with placebo when added to standard treatment, with a number needed to treat of 19 to prevent one primary outcome event after a median of 2.4 years.
The level of benefit was similar in both the one-third of enrolled patients without diabetes and in the two-thirds with diabetes, showing a statistically significant 50% cut in the primary endpoint among patients without diabetes, Hiddo J.L. Heerspink, MD, reported at the virtual annual congress of the European Society of Cardiology.
“We found that dapagliflozin delayed the initiation of dialysis, and reduced the number of deaths,” regardless of diabetes status, Dr. Heerspink, of University Medical Centre Groningen, the Netherlands, said during a press conference. “DAPA-CKD trial has shown dapagliflozin’s potential as a long-awaited new treatment for patients with chronic kidney disease.”
This finding ushers in a “completely new era in chronic kidney disease management,” said Janani Rangaswami, MD, a nephrologist and cardiorenal syndrome specialist at Einstein Medical Center in Philadelphia. “It’s good news” for these patients.
The results showed that dapagliflozin is the first “game changing” drug for chronic kidney disease in 2 decades, following the introduction of angiotensin converting enzyme inhibitors and angiotensin receptor blockers, she said in an interview. And given the consistency of the findings with the results from several other studies that documented meaningful renal protection by several different SGLT2 inhibitors, the results from this single trial also convincingly establish dapagliflozin as a standard-of-care agent to use on the types of patients the study enrolled, she said in an interview.
Representing many real-world patients
The DAPA-CKD trial enrolled 4,304 patients with albuminuria based on having a urinary albumin-to-creatinine ratio of at least 200 mg/g, and an estimated glomerular filtration rate (eGFR) of 25-75 mL/min per 1.73 m2 (with 90% of patients having an eGFR of less than 60 mL/min per 1.73 m2), and 97% were on treatment with a renin-angiotensin system–blocking drug. The primary endpoint was the combined rate of a drop in eGFR of at least 50% from baseline, progression to end stage renal disease, or renal or cardiovascular death; the between-group difference in this composite was driven primarily by both preserved eGFR and by prevention of end stage renal disease.
This represents both an appropriate target population, and meaningful endpoints, Dr. Rangaswami said. The study was “very representative of who we see in real-world practice,” a group that likely includes “hundreds of thousands” of U.S. patients with nondiabetic chronic kidney disease, she estimated.
Another notable finding was that 14% of the enrolled patients had eGFR values at baseline of 25-29 mL/min per 1.73 m2, pegging them as having stage 4 chronic kidney disease, and the median baseline eGFR was 43 mL/min per 1.73 m2, but dapagliflozin treatment was as safe and effective in these patients as it was in enrolled patients with a higher level of retained renal activity. This experience should give clinicians greater confidence about using dapagliflozin and other drugs in the sodium-glucose cotransporter (SGLT) 2 inhibitor class in patients with substantially depressed renal function, Dr. Rangaswami said.
“We now need to be more proactive about treating patients with more advanced kidney disease who can still benefit” from dapagliflozin treatment. “The sooner you intervene the better,” to slow further progression, but the new findings show “benefit even when treating patients with lower eGFRs. There is still hope to prevent or delay dialysis.”
A heart-kidney connection
Dapagliflozin treatment also cut all-cause mortality by a statistically significant, relative 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization, a benefit seen consistently in several prior studies of SGLT2 inhibitors, but possibly unexpected here because enrolled patients underwent no selection for a history of heart failure or any other cardiovascular disease. But the finding shouldn’t surprise, because “chronic kidney disease is an independent risk factor for cardiovascular disease across the board, and especially for heart failure,” noted Dr. Rangaswami.
“Heart and kidney disease is one big spectrum,” and the collected experience of several trials that have now proven the efficacy of SGLT2 inhibitors among patients with heart failure with reduced ejection fraction or with chronic kidney disease, regardless of their glycemic control, shows how broadly this drug class can benefit patients across the breadth of this spectrum, she said.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Heerspink has been a consultant to and received research funding from AstraZeneca and from several other companies. Dr. Rangaswami had no disclosures.
FROM ESC CONGRESS 2020
COVID-19 at home: What does optimal care look like?
Marilyn Stebbins, PharmD, fell ill at the end of February 2020. Initially diagnosed with multifocal pneumonia and treated with antibiotics, she later developed severe gastrointestinal symptoms, fatigue, and shortness of breath. She was hospitalized in early March and was diagnosed with COVID-19.
It was still early in the pandemic, and testing was not available for her husband. After she was discharged, her husband isolated himself as much as possible. But that limited the amount of care he could offer.
“When I came home after 8 days in the ICU, I felt completely alone and terrified of not being able to care for myself and not knowing how much care my husband could provide,” said Dr. Stebbins, professor of clinical pharmacy at the University of California, San Francisco.
“I can’t even imagine what it would have been like if I had been home alone without my husband in the house,” she said. “I think about the people who died at home and understand how that might happen.”
Dr. Stebbins is one of tens of thousands of people who, whether hospitalized and discharged or never admitted for inpatient care, needed to find ways to convalesce at home. Data from the Centers for Medicare & Medicaid Services show that, of 326,674 beneficiaries who tested positive for COVID-19 between May 16 and June 11, 2020, 109,607 were hospitalized, suggesting that two-thirds were outpatients.
Most attention has focused on the sickest patients, leaving less severe cases to fall through the cracks. Despite fever, cough, difficulty breathing, and a surfeit of other symptoms, there are few available resources and all too little support to help patients navigate the physical and emotional struggles of contending with COVID-19 at home.
No ‘cookie-cutter’ approach
The speed with which the pandemic progressed caught public health systems off guard, but now, “it is essential to put into place the infrastructure to care for the physical and mental health needs of patients at home because most are in the community and many, if not most, still aren’t receiving sufficient support at home,” said Dr. Stebbins.
said Gary LeRoy, MD, a family physician in Dayton, Ohio. He emphasized that there is “no cookie-cutter formula” for home care, because every patient’s situation is different.
“I begin by having a detailed conversation with each patient to ascertain whether their home environment is safe and to paint a picture of their circumstances,” Dr. LeRoy, who is the president of the American Academy of Family Physicians, said in an interview.
Dr. LeRoy suggested questions that constitute “not just a ‘medical’ checklist but a ‘whole life’ checklist.”
- Do you have access to food, water, medications, sanitation/cleaning supplies, a thermometer, and other necessities? If not, who might assist in providing those?
- Do you need help with activities of daily living and self-care?
- Who else lives in your household? Do they have signs and symptoms of the virus? Have they been tested?
- Do you have enough physical space between you and other household members?
- Do you have children? How are they being cared for?
- What type of work do you do? What are the implications for your employment if you are unable to work for an extended period?
- Do you have an emotional, social, and spiritual support system (e.g., family, friends, community, church)?
- Do you have concerns I haven’t mentioned?
Patients’ responses will inform the management plan and determine what medical and social resources are needed, he said.
Daily check-in
Dr. Stebbins said the nurse case manager from her insurance company called her daily after she came home from the hospital. She was told that a public health nurse would also call, but no one from the health department called for days – a situation she hopes has improved.
One way or another, she said, “health care providers [or their staff] should check in with patients daily, either telephonically or via video.” She noted that video is superior, because “someone who isn’t a family member needs to put eyes on a patient and might be able to detect warning signs that a family member without healthcare training might not notice.”
Dr. LeRoy, who is also an associate professor of medicine at Wright State University, Dayton, Ohio, said that, given his time constraints, a nurse or medical assistant in his practice conducts the daily check-ins and notifies him if the patient has fever or other symptoms.
“Under ordinary circumstances, when a patient comes to see me for some type of medical condition, I get to meet the patient, consider what might be going on, then order a test, wait for the results, and suggest a treatment plan. But these are anything but ordinary circumstances,” said Matthew Exline, MD, a pulmonary and critical care specialist at the Ohio State University Wexner Medical Center, Columbus.
“That traditional structure broke down with COVID-19, when we may have test results without even seeing the patient. And without this interaction, it is harder to know as a physician what course of action to take,” he said in an interview.
Once a diagnosis has been made, the physician has at least some data to help guide next steps, even if there has been no prior meeting with the patient.
For example, a positive test raises a host of issues, not the least of which is the risk of spreading the infection to other household members and questions about whether to go the hospital. Moreover, for patients, positive tests can have serious ramifications.
“Severe shortness of breath at rest is not typical of the flu, nor is loss of taste or smell,” said Dr. Exline. Practitioners must educate patients and families about specific symptoms of COVID-19, including shortness of breath, loss of taste or smell, and gastrointestinal or neurologic symptoms, and when to seek emergency care.
Dr. LeRoy suggests buying a pulse oximeter to gauge blood oxygen levels and pulse rate. Together with a thermometer, a portable blood pressure monitor, and, if indicated, a blood glucose monitor, these devices provide a comprehensive and accurate assessment of vital signs.
Dr. LeRoy also educates patients and their families about when to seek medical attention.
Dr. Stebbins takes a similar approach. “Family members are part of, not apart from, the care of patients with COVID-19, and it’s our responsibility as healthcare providers to consider them in the patient’s care plan.”
Keeping family safe
Beyond care, family members need a plan to keep themselves healthy, too.
“A patient with COVID-19 at home should self-quarantine as much as possible to keep other family members safe, if they continue to live in the same house,” Dr. Exline said.
Ideally, uninfected family members should stay with relatives or friends. When that’s not possible, everyone in the household should wear a mask, be vigilant about hand washing, and wipe down all surfaces – including doorknobs, light switches, faucet handles, cellphones, and utensils – regularly with bleach or an alcohol solution.
Caregivers should also minimize the amount of time they are exposed to the patient.
“Set food, water, and medication on the night table and leave the room rather than spending hours at the bedside, since limiting exposure to viral load reduces the chances of contagion,” said Dr. Exline.
The Centers for Disease Control and Prevention offers guidance for household members caring for COVID-19 patients at home. It provides tips on how to help patients follow the doctor’s instructions and ways to ensure adequate hydration and rest, among others.
Patients with COVID-19 who live alone face more formidable challenges.
Dr. LeRoy says physicians can help patients by educating themselves about available social services in their community so they can provide appropriate referrals and connections. Such initiatives can include meal programs, friendly visit and financial assistance programs, as well as childcare and home health agencies.
He noted that Aunt Bertha, a social care network, provides a guide to social services throughout the United States. Additional resources are available on USA.gov.
Comfort and support
Patients with COVID-19 need to be as comfortable and as supported as possible, both physically and emotionally.
“While I was sick, my dogs curled up next to me and didn’t leave my side, and they were my saving grace. There’s not enough to be said about emotional support,” Dr. Stebbins said.
Although important, emotional support is not enough. For patients with respiratory disorders, such as chronic obstructive pulmonary disease, asthma, heart failure, or pneumonia, their subjective symptoms of shortness of breath, air hunger, or cough may improve with supplemental oxygen at home. Other measures include repositioning of the patient to lessen the body weight over the lungs or the use of lung percussion, Leroy said.
He added that improvement may also come from drainage of sputum from the airway passages, the use of agents to liquefy thick sputum (mucolytics), or aerosolized bronchodilator medications.
However, Dr. LeRoy cautioned, “one remedy does not work for everyone – an individual can improve gradually by using these home support interventions, or their respiratory status can deteriorate rapidly despite all these interventions.”
For this reason, he says patients should consult their personal physician to determine which, if any, of these home treatments would be best for their particular situation.
Patients who need emotional support, psychotherapy, or psychotropic medications may find teletherapy helpful. Guidance for psychiatrists, psychologists, and social workers regarding the treatment of COVID-19 patients via teletherapy can be found on the American Psychiatric Association, the American Psychological Association, and the National Association of Social Workers websites.
Pharmacists can also help ensure patient safety, Dr. Stebbins said.
If a patient has not picked up their usual medications, Dr. Stebbins said, “they may need a check-in call. Some may be ill and alone and may need encouragement to seek medical attention, and some may have no means of getting to the pharmacy and may need medications delivered.”
A home healthcare agency may also be helpful for homebound patients. David Bersson, director of operations at Synergy Home Care of Bergen County, N.J., has arranged in-home caregivers for patients with COVID-19.
The amount of care that professional caregivers provide can range from several hours per week to full-time, depending on the patient’s needs and budget, and can include companionship, Mr. Bersson said in an interview.
Because patient and caregiver safety are paramount, caregivers are thoroughly trained in protection and decontamination procedures and are regularly tested for COVID-19 prior to being sent into a client’s home.
Health insurance companies do not cover this service, Mr. Bersson noted, but the VetAssist program covers home care for veterans and their spouses who meet income requirements.
Caregiving and companionship are both vital pieces of the at-home care puzzle. “It was the virtual emotional support I got from friends, family, coworkers, and healthcare professionals that meant so much to me, and I know they played an important part in my recovery,” Dr. Stebbins said.
Dr. LeRoy agreed, noting that he calls patients, even if they only have mild symptoms and his nurse has already spoken to them. “The call doesn’t take much time – maybe just a 5-minute conversation – but it makes patients aware that I care.”
Dr. Stebbins, Dr. Exline, and Dr. LeRoy report no relevant financial relationships. Mr. Bersson is the director of operations at Synergy Home Care of Bergen County, New Jersey.
This story first appeared on Medscape.com.
Marilyn Stebbins, PharmD, fell ill at the end of February 2020. Initially diagnosed with multifocal pneumonia and treated with antibiotics, she later developed severe gastrointestinal symptoms, fatigue, and shortness of breath. She was hospitalized in early March and was diagnosed with COVID-19.
It was still early in the pandemic, and testing was not available for her husband. After she was discharged, her husband isolated himself as much as possible. But that limited the amount of care he could offer.
“When I came home after 8 days in the ICU, I felt completely alone and terrified of not being able to care for myself and not knowing how much care my husband could provide,” said Dr. Stebbins, professor of clinical pharmacy at the University of California, San Francisco.
“I can’t even imagine what it would have been like if I had been home alone without my husband in the house,” she said. “I think about the people who died at home and understand how that might happen.”
Dr. Stebbins is one of tens of thousands of people who, whether hospitalized and discharged or never admitted for inpatient care, needed to find ways to convalesce at home. Data from the Centers for Medicare & Medicaid Services show that, of 326,674 beneficiaries who tested positive for COVID-19 between May 16 and June 11, 2020, 109,607 were hospitalized, suggesting that two-thirds were outpatients.
Most attention has focused on the sickest patients, leaving less severe cases to fall through the cracks. Despite fever, cough, difficulty breathing, and a surfeit of other symptoms, there are few available resources and all too little support to help patients navigate the physical and emotional struggles of contending with COVID-19 at home.
No ‘cookie-cutter’ approach
The speed with which the pandemic progressed caught public health systems off guard, but now, “it is essential to put into place the infrastructure to care for the physical and mental health needs of patients at home because most are in the community and many, if not most, still aren’t receiving sufficient support at home,” said Dr. Stebbins.
said Gary LeRoy, MD, a family physician in Dayton, Ohio. He emphasized that there is “no cookie-cutter formula” for home care, because every patient’s situation is different.
“I begin by having a detailed conversation with each patient to ascertain whether their home environment is safe and to paint a picture of their circumstances,” Dr. LeRoy, who is the president of the American Academy of Family Physicians, said in an interview.
Dr. LeRoy suggested questions that constitute “not just a ‘medical’ checklist but a ‘whole life’ checklist.”
- Do you have access to food, water, medications, sanitation/cleaning supplies, a thermometer, and other necessities? If not, who might assist in providing those?
- Do you need help with activities of daily living and self-care?
- Who else lives in your household? Do they have signs and symptoms of the virus? Have they been tested?
- Do you have enough physical space between you and other household members?
- Do you have children? How are they being cared for?
- What type of work do you do? What are the implications for your employment if you are unable to work for an extended period?
- Do you have an emotional, social, and spiritual support system (e.g., family, friends, community, church)?
- Do you have concerns I haven’t mentioned?
Patients’ responses will inform the management plan and determine what medical and social resources are needed, he said.
Daily check-in
Dr. Stebbins said the nurse case manager from her insurance company called her daily after she came home from the hospital. She was told that a public health nurse would also call, but no one from the health department called for days – a situation she hopes has improved.
One way or another, she said, “health care providers [or their staff] should check in with patients daily, either telephonically or via video.” She noted that video is superior, because “someone who isn’t a family member needs to put eyes on a patient and might be able to detect warning signs that a family member without healthcare training might not notice.”
Dr. LeRoy, who is also an associate professor of medicine at Wright State University, Dayton, Ohio, said that, given his time constraints, a nurse or medical assistant in his practice conducts the daily check-ins and notifies him if the patient has fever or other symptoms.
“Under ordinary circumstances, when a patient comes to see me for some type of medical condition, I get to meet the patient, consider what might be going on, then order a test, wait for the results, and suggest a treatment plan. But these are anything but ordinary circumstances,” said Matthew Exline, MD, a pulmonary and critical care specialist at the Ohio State University Wexner Medical Center, Columbus.
“That traditional structure broke down with COVID-19, when we may have test results without even seeing the patient. And without this interaction, it is harder to know as a physician what course of action to take,” he said in an interview.
Once a diagnosis has been made, the physician has at least some data to help guide next steps, even if there has been no prior meeting with the patient.
For example, a positive test raises a host of issues, not the least of which is the risk of spreading the infection to other household members and questions about whether to go the hospital. Moreover, for patients, positive tests can have serious ramifications.
“Severe shortness of breath at rest is not typical of the flu, nor is loss of taste or smell,” said Dr. Exline. Practitioners must educate patients and families about specific symptoms of COVID-19, including shortness of breath, loss of taste or smell, and gastrointestinal or neurologic symptoms, and when to seek emergency care.
Dr. LeRoy suggests buying a pulse oximeter to gauge blood oxygen levels and pulse rate. Together with a thermometer, a portable blood pressure monitor, and, if indicated, a blood glucose monitor, these devices provide a comprehensive and accurate assessment of vital signs.
Dr. LeRoy also educates patients and their families about when to seek medical attention.
Dr. Stebbins takes a similar approach. “Family members are part of, not apart from, the care of patients with COVID-19, and it’s our responsibility as healthcare providers to consider them in the patient’s care plan.”
Keeping family safe
Beyond care, family members need a plan to keep themselves healthy, too.
“A patient with COVID-19 at home should self-quarantine as much as possible to keep other family members safe, if they continue to live in the same house,” Dr. Exline said.
Ideally, uninfected family members should stay with relatives or friends. When that’s not possible, everyone in the household should wear a mask, be vigilant about hand washing, and wipe down all surfaces – including doorknobs, light switches, faucet handles, cellphones, and utensils – regularly with bleach or an alcohol solution.
Caregivers should also minimize the amount of time they are exposed to the patient.
“Set food, water, and medication on the night table and leave the room rather than spending hours at the bedside, since limiting exposure to viral load reduces the chances of contagion,” said Dr. Exline.
The Centers for Disease Control and Prevention offers guidance for household members caring for COVID-19 patients at home. It provides tips on how to help patients follow the doctor’s instructions and ways to ensure adequate hydration and rest, among others.
Patients with COVID-19 who live alone face more formidable challenges.
Dr. LeRoy says physicians can help patients by educating themselves about available social services in their community so they can provide appropriate referrals and connections. Such initiatives can include meal programs, friendly visit and financial assistance programs, as well as childcare and home health agencies.
He noted that Aunt Bertha, a social care network, provides a guide to social services throughout the United States. Additional resources are available on USA.gov.
Comfort and support
Patients with COVID-19 need to be as comfortable and as supported as possible, both physically and emotionally.
“While I was sick, my dogs curled up next to me and didn’t leave my side, and they were my saving grace. There’s not enough to be said about emotional support,” Dr. Stebbins said.
Although important, emotional support is not enough. For patients with respiratory disorders, such as chronic obstructive pulmonary disease, asthma, heart failure, or pneumonia, their subjective symptoms of shortness of breath, air hunger, or cough may improve with supplemental oxygen at home. Other measures include repositioning of the patient to lessen the body weight over the lungs or the use of lung percussion, Leroy said.
He added that improvement may also come from drainage of sputum from the airway passages, the use of agents to liquefy thick sputum (mucolytics), or aerosolized bronchodilator medications.
However, Dr. LeRoy cautioned, “one remedy does not work for everyone – an individual can improve gradually by using these home support interventions, or their respiratory status can deteriorate rapidly despite all these interventions.”
For this reason, he says patients should consult their personal physician to determine which, if any, of these home treatments would be best for their particular situation.
Patients who need emotional support, psychotherapy, or psychotropic medications may find teletherapy helpful. Guidance for psychiatrists, psychologists, and social workers regarding the treatment of COVID-19 patients via teletherapy can be found on the American Psychiatric Association, the American Psychological Association, and the National Association of Social Workers websites.
Pharmacists can also help ensure patient safety, Dr. Stebbins said.
If a patient has not picked up their usual medications, Dr. Stebbins said, “they may need a check-in call. Some may be ill and alone and may need encouragement to seek medical attention, and some may have no means of getting to the pharmacy and may need medications delivered.”
A home healthcare agency may also be helpful for homebound patients. David Bersson, director of operations at Synergy Home Care of Bergen County, N.J., has arranged in-home caregivers for patients with COVID-19.
The amount of care that professional caregivers provide can range from several hours per week to full-time, depending on the patient’s needs and budget, and can include companionship, Mr. Bersson said in an interview.
Because patient and caregiver safety are paramount, caregivers are thoroughly trained in protection and decontamination procedures and are regularly tested for COVID-19 prior to being sent into a client’s home.
Health insurance companies do not cover this service, Mr. Bersson noted, but the VetAssist program covers home care for veterans and their spouses who meet income requirements.
Caregiving and companionship are both vital pieces of the at-home care puzzle. “It was the virtual emotional support I got from friends, family, coworkers, and healthcare professionals that meant so much to me, and I know they played an important part in my recovery,” Dr. Stebbins said.
Dr. LeRoy agreed, noting that he calls patients, even if they only have mild symptoms and his nurse has already spoken to them. “The call doesn’t take much time – maybe just a 5-minute conversation – but it makes patients aware that I care.”
Dr. Stebbins, Dr. Exline, and Dr. LeRoy report no relevant financial relationships. Mr. Bersson is the director of operations at Synergy Home Care of Bergen County, New Jersey.
This story first appeared on Medscape.com.
Marilyn Stebbins, PharmD, fell ill at the end of February 2020. Initially diagnosed with multifocal pneumonia and treated with antibiotics, she later developed severe gastrointestinal symptoms, fatigue, and shortness of breath. She was hospitalized in early March and was diagnosed with COVID-19.
It was still early in the pandemic, and testing was not available for her husband. After she was discharged, her husband isolated himself as much as possible. But that limited the amount of care he could offer.
“When I came home after 8 days in the ICU, I felt completely alone and terrified of not being able to care for myself and not knowing how much care my husband could provide,” said Dr. Stebbins, professor of clinical pharmacy at the University of California, San Francisco.
“I can’t even imagine what it would have been like if I had been home alone without my husband in the house,” she said. “I think about the people who died at home and understand how that might happen.”
Dr. Stebbins is one of tens of thousands of people who, whether hospitalized and discharged or never admitted for inpatient care, needed to find ways to convalesce at home. Data from the Centers for Medicare & Medicaid Services show that, of 326,674 beneficiaries who tested positive for COVID-19 between May 16 and June 11, 2020, 109,607 were hospitalized, suggesting that two-thirds were outpatients.
Most attention has focused on the sickest patients, leaving less severe cases to fall through the cracks. Despite fever, cough, difficulty breathing, and a surfeit of other symptoms, there are few available resources and all too little support to help patients navigate the physical and emotional struggles of contending with COVID-19 at home.
No ‘cookie-cutter’ approach
The speed with which the pandemic progressed caught public health systems off guard, but now, “it is essential to put into place the infrastructure to care for the physical and mental health needs of patients at home because most are in the community and many, if not most, still aren’t receiving sufficient support at home,” said Dr. Stebbins.
said Gary LeRoy, MD, a family physician in Dayton, Ohio. He emphasized that there is “no cookie-cutter formula” for home care, because every patient’s situation is different.
“I begin by having a detailed conversation with each patient to ascertain whether their home environment is safe and to paint a picture of their circumstances,” Dr. LeRoy, who is the president of the American Academy of Family Physicians, said in an interview.
Dr. LeRoy suggested questions that constitute “not just a ‘medical’ checklist but a ‘whole life’ checklist.”
- Do you have access to food, water, medications, sanitation/cleaning supplies, a thermometer, and other necessities? If not, who might assist in providing those?
- Do you need help with activities of daily living and self-care?
- Who else lives in your household? Do they have signs and symptoms of the virus? Have they been tested?
- Do you have enough physical space between you and other household members?
- Do you have children? How are they being cared for?
- What type of work do you do? What are the implications for your employment if you are unable to work for an extended period?
- Do you have an emotional, social, and spiritual support system (e.g., family, friends, community, church)?
- Do you have concerns I haven’t mentioned?
Patients’ responses will inform the management plan and determine what medical and social resources are needed, he said.
Daily check-in
Dr. Stebbins said the nurse case manager from her insurance company called her daily after she came home from the hospital. She was told that a public health nurse would also call, but no one from the health department called for days – a situation she hopes has improved.
One way or another, she said, “health care providers [or their staff] should check in with patients daily, either telephonically or via video.” She noted that video is superior, because “someone who isn’t a family member needs to put eyes on a patient and might be able to detect warning signs that a family member without healthcare training might not notice.”
Dr. LeRoy, who is also an associate professor of medicine at Wright State University, Dayton, Ohio, said that, given his time constraints, a nurse or medical assistant in his practice conducts the daily check-ins and notifies him if the patient has fever or other symptoms.
“Under ordinary circumstances, when a patient comes to see me for some type of medical condition, I get to meet the patient, consider what might be going on, then order a test, wait for the results, and suggest a treatment plan. But these are anything but ordinary circumstances,” said Matthew Exline, MD, a pulmonary and critical care specialist at the Ohio State University Wexner Medical Center, Columbus.
“That traditional structure broke down with COVID-19, when we may have test results without even seeing the patient. And without this interaction, it is harder to know as a physician what course of action to take,” he said in an interview.
Once a diagnosis has been made, the physician has at least some data to help guide next steps, even if there has been no prior meeting with the patient.
For example, a positive test raises a host of issues, not the least of which is the risk of spreading the infection to other household members and questions about whether to go the hospital. Moreover, for patients, positive tests can have serious ramifications.
“Severe shortness of breath at rest is not typical of the flu, nor is loss of taste or smell,” said Dr. Exline. Practitioners must educate patients and families about specific symptoms of COVID-19, including shortness of breath, loss of taste or smell, and gastrointestinal or neurologic symptoms, and when to seek emergency care.
Dr. LeRoy suggests buying a pulse oximeter to gauge blood oxygen levels and pulse rate. Together with a thermometer, a portable blood pressure monitor, and, if indicated, a blood glucose monitor, these devices provide a comprehensive and accurate assessment of vital signs.
Dr. LeRoy also educates patients and their families about when to seek medical attention.
Dr. Stebbins takes a similar approach. “Family members are part of, not apart from, the care of patients with COVID-19, and it’s our responsibility as healthcare providers to consider them in the patient’s care plan.”
Keeping family safe
Beyond care, family members need a plan to keep themselves healthy, too.
“A patient with COVID-19 at home should self-quarantine as much as possible to keep other family members safe, if they continue to live in the same house,” Dr. Exline said.
Ideally, uninfected family members should stay with relatives or friends. When that’s not possible, everyone in the household should wear a mask, be vigilant about hand washing, and wipe down all surfaces – including doorknobs, light switches, faucet handles, cellphones, and utensils – regularly with bleach or an alcohol solution.
Caregivers should also minimize the amount of time they are exposed to the patient.
“Set food, water, and medication on the night table and leave the room rather than spending hours at the bedside, since limiting exposure to viral load reduces the chances of contagion,” said Dr. Exline.
The Centers for Disease Control and Prevention offers guidance for household members caring for COVID-19 patients at home. It provides tips on how to help patients follow the doctor’s instructions and ways to ensure adequate hydration and rest, among others.
Patients with COVID-19 who live alone face more formidable challenges.
Dr. LeRoy says physicians can help patients by educating themselves about available social services in their community so they can provide appropriate referrals and connections. Such initiatives can include meal programs, friendly visit and financial assistance programs, as well as childcare and home health agencies.
He noted that Aunt Bertha, a social care network, provides a guide to social services throughout the United States. Additional resources are available on USA.gov.
Comfort and support
Patients with COVID-19 need to be as comfortable and as supported as possible, both physically and emotionally.
“While I was sick, my dogs curled up next to me and didn’t leave my side, and they were my saving grace. There’s not enough to be said about emotional support,” Dr. Stebbins said.
Although important, emotional support is not enough. For patients with respiratory disorders, such as chronic obstructive pulmonary disease, asthma, heart failure, or pneumonia, their subjective symptoms of shortness of breath, air hunger, or cough may improve with supplemental oxygen at home. Other measures include repositioning of the patient to lessen the body weight over the lungs or the use of lung percussion, Leroy said.
He added that improvement may also come from drainage of sputum from the airway passages, the use of agents to liquefy thick sputum (mucolytics), or aerosolized bronchodilator medications.
However, Dr. LeRoy cautioned, “one remedy does not work for everyone – an individual can improve gradually by using these home support interventions, or their respiratory status can deteriorate rapidly despite all these interventions.”
For this reason, he says patients should consult their personal physician to determine which, if any, of these home treatments would be best for their particular situation.
Patients who need emotional support, psychotherapy, or psychotropic medications may find teletherapy helpful. Guidance for psychiatrists, psychologists, and social workers regarding the treatment of COVID-19 patients via teletherapy can be found on the American Psychiatric Association, the American Psychological Association, and the National Association of Social Workers websites.
Pharmacists can also help ensure patient safety, Dr. Stebbins said.
If a patient has not picked up their usual medications, Dr. Stebbins said, “they may need a check-in call. Some may be ill and alone and may need encouragement to seek medical attention, and some may have no means of getting to the pharmacy and may need medications delivered.”
A home healthcare agency may also be helpful for homebound patients. David Bersson, director of operations at Synergy Home Care of Bergen County, N.J., has arranged in-home caregivers for patients with COVID-19.
The amount of care that professional caregivers provide can range from several hours per week to full-time, depending on the patient’s needs and budget, and can include companionship, Mr. Bersson said in an interview.
Because patient and caregiver safety are paramount, caregivers are thoroughly trained in protection and decontamination procedures and are regularly tested for COVID-19 prior to being sent into a client’s home.
Health insurance companies do not cover this service, Mr. Bersson noted, but the VetAssist program covers home care for veterans and their spouses who meet income requirements.
Caregiving and companionship are both vital pieces of the at-home care puzzle. “It was the virtual emotional support I got from friends, family, coworkers, and healthcare professionals that meant so much to me, and I know they played an important part in my recovery,” Dr. Stebbins said.
Dr. LeRoy agreed, noting that he calls patients, even if they only have mild symptoms and his nurse has already spoken to them. “The call doesn’t take much time – maybe just a 5-minute conversation – but it makes patients aware that I care.”
Dr. Stebbins, Dr. Exline, and Dr. LeRoy report no relevant financial relationships. Mr. Bersson is the director of operations at Synergy Home Care of Bergen County, New Jersey.
This story first appeared on Medscape.com.
EXPLORER trial hints at potential new drug option in obstructive hypertrophic cardiomyopathy
An investigational drug that targets part of the molecular machinery underlying obstructive hypertrophic cardiomyopathy (HCM) can improve both symptoms and functional status in patients with the genetic disorder, suggests a placebo-controlled phase 3 trial.
Treatment with mavacamten (MyoKardia) worked partly by alleviating high-pressure gradients in the left ventricular outflow tract (LVOT), a key characteristic of obstructive HCM. Its effects appeared consistent across a wide range of objective and patient-assessed endpoints.
Mavacamten is “the first potential medical therapy addressing the underlying biology of symptoms in hypertrophic obstructive cardiomyopathy,” observed Iacopo Olivotto, MD, Careggi University Hospital, Florence, Italy.
Patients in the EXPLORER-HCM trial who took the new drug showed improvements in “every aspect of objective performance and subjective well-being,” Dr. Olivotto said at a preview for journalists before his formal online presentation of the results during the virtual European Society of Cardiology Congress 2020, staged in lieu of the traditional annual meeting because of the COVID-19 pandemic.
Dr. Olivotto, also lead author on the study’s same-day publication in The Lancet, was exuberant about the findings. “It is really hard to convey what this actually means for a scientific and clinical community that has spent over 60 years trying to understand and cure hypertrophic cardiomyopathy.”
MyoKardia released abbreviated top-line results of EXPLORER-HCM in May, which were reported by theheart.org | Medscape Cardiology at the time.
“I think it’s pretty exciting. We certainly need more and better drugs for this patient population,” Arnon Adler, MD, who is not associated with the trial but follows HCM at the Peter Munk Cardiac Centre, Toronto General Hospital, said in an interview.
The trial compared the new drug to placebo rather than full contemporary drug therapy for obstructive HCM, Adler cautioned, and had a fairly short follow-up time. But he was impressed that mavacamten’s apparent benefits seemed consistent not only for endpoints like change in New York Heart Association (NYHA) functional class and quality of life but also for more objective measures like peak VO2 and LVOT gradients.
“I think the results were promising across the board,” he told.
Unique mechanism of action
Mavacamten is described as a first-in-class, small-molecule, selective allosteric inhibitor of cardiac myosin adenosine triphosphatase that addresses the underlying pathophysiology of HCM by reducing actin–myosin cross-bridge formation. It thereby inhibits the excessive myocyte contractility that is a key mechanism of the disorder’s tell-tale hypertrophy, something the available HCM drug therapies don’t do.
Almost three-fourths of patients in the trial were initially in NYHA class 2. Such patients in practice tend to be treated pharmacologically, with more invasive but generally effective surgical myectomy and alcohol septal ablation performed more often for patients in NYHA class 3.
“In the EXPLORER-HCM trial, patients enrolled did not have any immediate indication for surgery,” although many of them in NYHA class 2 would likely progress to NYHA 3, Dr. Olivotto said in an interview.
Based on the trial, he said, it’s possible that mavacamten could lead to “earlier and broader treatment of obstruction symptoms in patients who would never have qualified for surgery in the first place because their symptoms may not be severe enough, but they are still limited.”
Notably, the published report notes, 27% of patients taking mavacamten achieved what was defined as a complete response – that is, a reduction of all LVOT gradients to less than 30 mm Hg in the total absence of symptoms.
Only 1% of patients in the placebo-treated control group met that goal, “showing that mavacamten might be capable of achieving marked relief of symptoms and LVOT obstruction,” the report states.
In the trial, “treatment with mavacamten led to clinically meaningful improvements in hemodynamic status, functional capacity, and subjective well-being in patients with obstructive hypertrophic cardiomyopathy,” agrees an editorial accompanying the EXPLORER-HCM publication.
Mavacamten might even compare favorably to surgery and ablative therapy, speculated the editorialists, Michael Papadakis, MBBS, MD, and colleagues of St. George’s University Hospitals NHS Foundation Trust, London. The drug appeared to reduce the peak LVOT gradient “to less than the guideline-based threshold for septal reduction therapy, 50 mm Hg, in 74% of patients, compared with 21% in the placebo group, indicating that mavacamten could represent a valid alternative to highly specialized invasive therapy,” they wrote.
Standard drug therapy
“There are approved drugs for obstructive hypertrophic cardiomyopathy, but they are ancient and were developed for other diseases,” observed Dr. Olivotto at the media briefing. Those drug options – primarily beta-blockers, nondihydropyridine calcium-channel blockers, and the sodium-channel blocker disopyramide – are often ineffective or cause onerous side effects, he said.
Notably in EXPLORER-HCM, patients in both the mavacamten and placebo groups could also be receiving beta-blockers and calcium-channel blockers, but no one in the trial could be receiving disopyramide, which can prolong the QT interval.
“By design,” mavacamten wasn’t compared to disopyramide, “a much more potent drug for lowering gradient and improving symptoms than beta-blockers or calcium-channel blockers,” said Martin S. Maron, MD, medical director at the Hypertrophic Cardiomyopathy Center and Research Institute, Tufts Medical Center, Boston.
Therefore, the trial’s results can’t be extrapolated to conclude that the new drug is superior to disopyramide “or the gold standard, surgical myectomy,” he said in an interview.
Dr. Adler agreed that observational studies suggest a benefit from disopyramide that may rival the apparent effect of mavacamten. “But of course, you can’t make direct comparisons because we never had a study like this for disopyramide.” Because it has many side effects and limitations, “it’s not a drug that I like using, but it is beneficial for some patients and I do use it quite a bit.”
What EXPLORER-HCM does seem to show, Dr. Maron said, “is that the mechanism of action of the drug does seem to play out. It lowers gradients in a pretty reliable and powerful way, and that translates into clinical improvement in many patients. So it starts to support the idea that this drug and the class of drugs, myosin inhibitors, may represent another medical therapy option for symptomatic obstructive HCM.”
And, he pointed out, about one-fifth of patients with obstructive HCM don’t respond to disopyramide with fewer symptoms, and in others the drug “starts to lose efficacy over time.” So disopyramide has limitations, and EXPLORER-HCM “provides the possibility of an additional drug option.”
EXPLORER-HCM randomly assigned 251 adults with obstructive HCM in 13 countries to receive mavacamten, titrated from a starting dosage of 5 mg/day to a possible 15 mg/day or to placebo for 30 weeks.
The patients were required to have a peak LVOT gradient at least 50 mm Hg, a left ventricular ejection fraction (LVEF) of at least 55%, and symptoms indicating NYHA class 2 or 3; ultimately, 73% started the trial in NYHA class 2.
In the intention-to-treat analysis, 36.6% of patients receiving mavacamten and 17.2% of control patients met the composite primary endpoint (P = .0005), which consisted of either a 1.5–mL/kg per minute or greater improvement in peak oxygen consumption (pVO2) and at least a one-step reduction in NYHA functional class or at least a 3.0–mL/kg per minute pVO2 increase without deterioration in NYHA class, by week 30.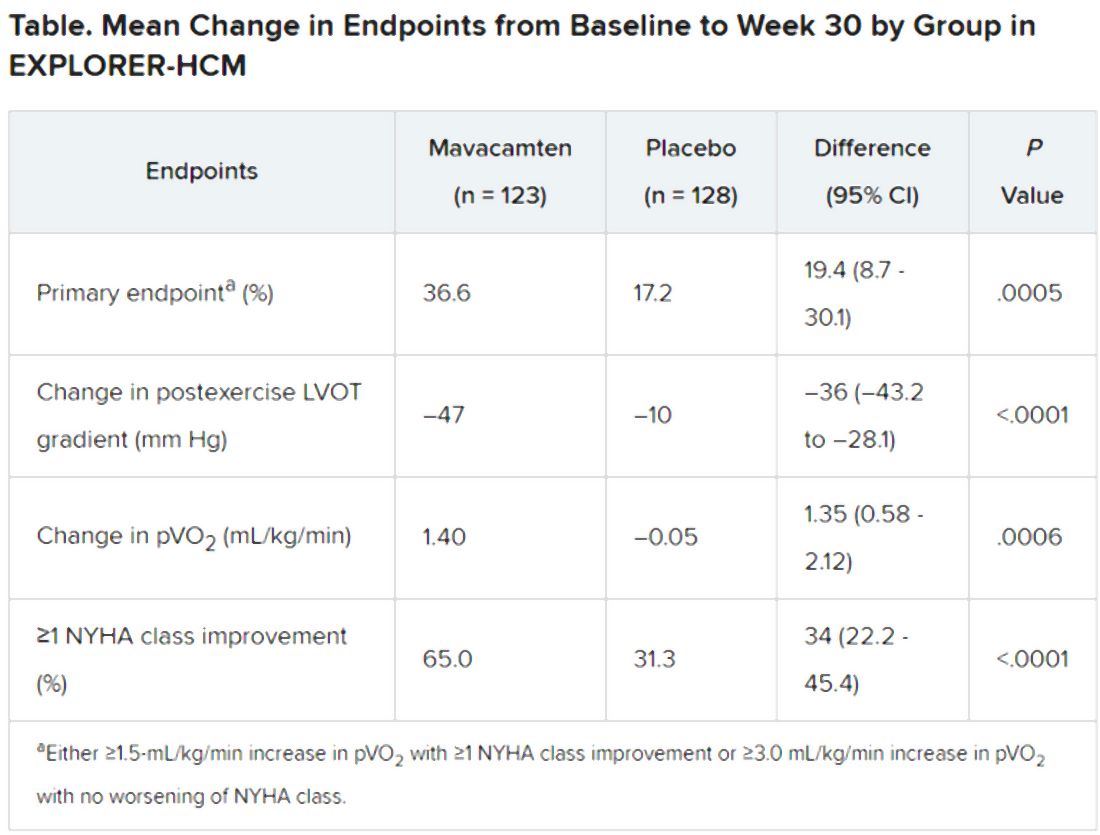
Patients receiving mavacamten also showed greater improvement in the individual endpoints of postexercise LVOT gradient, NYHA class, and two score-based symptom assessments – the Kansas City Cardiomyopathy Questionnaire-Clinical Summary and Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness-of-Breath domain – compared with control patients.
Safety and tolerability issues were similar in both groups, the reports notes. Ten patients in the mavacamten group reported 11 serious adverse events, compared with 20 such events reported by 11 patients in the control group.
“We can say from these results that mavacamten is a promising drug for symptom relief and functional class improvement associated with outflow gradient reduction in selected patients with hypertrophic obstructive cardiomyopathy,” and that, on the basis of this trial, it has potential as a drug of first choice, Franco Cecchi, MD, University of Florence (Italy), said as an invited discussant following Dr. Olivotto’s formal presentation of the trial.
Although serious adverse events were few, it was noteworthy that seven patients receiving mavacamten but only two patients receiving placebo showed LVEF reductions to below the 50% threshold during the trial, Dr. Cecchi observed. The LVEFs normalized once the drug was discontinued, but still, it may mean that mavacamten should be carefully uptitrated according to LVEF, he said.
Those LVEF reductions raise questions about “the reliability of being able to dose patients safely in the outpatient setting,” Dr. Maron said. “You have to ask, ‘Can this be extrapolated to the general practicing community without patients dropping their ejection fractions too much?’ ”
In addition, “we don’t have any idea about long-term efficacy for this drug, and we can draw very limited information about long-term safety here as well. That’s another other question mark,” Dr. Maron said.
“If I could have patients really become asymptomatic or mildly symptomatic without any surgery on a drug that is safe and can be taken for a prolonged period of time, that would be great,” Dr. Adler added. He noted that long-term follow-up of patients taking mavacamten in various trials has been underway and should help answer safety and efficacy questions about chronic therapy.
“Should mavacamten prove to be clinically effective and safe following long-term therapy in a larger and more diverse population, it would represent a much anticipated development in the treatment of hypertrophic cardiomyopathy,” the accompanying editorial states.
“Were the drug to realise its potential as a disease-modifying therapy in younger individuals, it would represent a great milestone in the area of inherited cardiomyopathies.”
MyoKardia funded EXPLORER-HCM. Dr. Olivotto discloses receiving grants from MyoKardia, Sanofi-Genzyme, Shire, Amicus, and Bayer; honoraria from Sanofi-Genzyme, Shire, and Bayer; and payments for consulting from MyoKardia. Disclosures for the other authors are in the report. Dr. Papadakis and the other editorialists report that they have no competing interests. Dr. Adler had no disclosures. Dr. Maron discloses consulting for and serving on a trial steering committee for Cytokinetics, sponsor of the 60-patient phase 2 placebo-controlled trial REDWOOD-HCM of patients with obstructive HCM treated with CK-3773274, a drug that works by a mechanism similar to that of mavacamten.
A version of this article originally appeared on Medscape.com.
An investigational drug that targets part of the molecular machinery underlying obstructive hypertrophic cardiomyopathy (HCM) can improve both symptoms and functional status in patients with the genetic disorder, suggests a placebo-controlled phase 3 trial.
Treatment with mavacamten (MyoKardia) worked partly by alleviating high-pressure gradients in the left ventricular outflow tract (LVOT), a key characteristic of obstructive HCM. Its effects appeared consistent across a wide range of objective and patient-assessed endpoints.
Mavacamten is “the first potential medical therapy addressing the underlying biology of symptoms in hypertrophic obstructive cardiomyopathy,” observed Iacopo Olivotto, MD, Careggi University Hospital, Florence, Italy.
Patients in the EXPLORER-HCM trial who took the new drug showed improvements in “every aspect of objective performance and subjective well-being,” Dr. Olivotto said at a preview for journalists before his formal online presentation of the results during the virtual European Society of Cardiology Congress 2020, staged in lieu of the traditional annual meeting because of the COVID-19 pandemic.
Dr. Olivotto, also lead author on the study’s same-day publication in The Lancet, was exuberant about the findings. “It is really hard to convey what this actually means for a scientific and clinical community that has spent over 60 years trying to understand and cure hypertrophic cardiomyopathy.”
MyoKardia released abbreviated top-line results of EXPLORER-HCM in May, which were reported by theheart.org | Medscape Cardiology at the time.
“I think it’s pretty exciting. We certainly need more and better drugs for this patient population,” Arnon Adler, MD, who is not associated with the trial but follows HCM at the Peter Munk Cardiac Centre, Toronto General Hospital, said in an interview.
The trial compared the new drug to placebo rather than full contemporary drug therapy for obstructive HCM, Adler cautioned, and had a fairly short follow-up time. But he was impressed that mavacamten’s apparent benefits seemed consistent not only for endpoints like change in New York Heart Association (NYHA) functional class and quality of life but also for more objective measures like peak VO2 and LVOT gradients.
“I think the results were promising across the board,” he told.
Unique mechanism of action
Mavacamten is described as a first-in-class, small-molecule, selective allosteric inhibitor of cardiac myosin adenosine triphosphatase that addresses the underlying pathophysiology of HCM by reducing actin–myosin cross-bridge formation. It thereby inhibits the excessive myocyte contractility that is a key mechanism of the disorder’s tell-tale hypertrophy, something the available HCM drug therapies don’t do.
Almost three-fourths of patients in the trial were initially in NYHA class 2. Such patients in practice tend to be treated pharmacologically, with more invasive but generally effective surgical myectomy and alcohol septal ablation performed more often for patients in NYHA class 3.
“In the EXPLORER-HCM trial, patients enrolled did not have any immediate indication for surgery,” although many of them in NYHA class 2 would likely progress to NYHA 3, Dr. Olivotto said in an interview.
Based on the trial, he said, it’s possible that mavacamten could lead to “earlier and broader treatment of obstruction symptoms in patients who would never have qualified for surgery in the first place because their symptoms may not be severe enough, but they are still limited.”
Notably, the published report notes, 27% of patients taking mavacamten achieved what was defined as a complete response – that is, a reduction of all LVOT gradients to less than 30 mm Hg in the total absence of symptoms.
Only 1% of patients in the placebo-treated control group met that goal, “showing that mavacamten might be capable of achieving marked relief of symptoms and LVOT obstruction,” the report states.
In the trial, “treatment with mavacamten led to clinically meaningful improvements in hemodynamic status, functional capacity, and subjective well-being in patients with obstructive hypertrophic cardiomyopathy,” agrees an editorial accompanying the EXPLORER-HCM publication.
Mavacamten might even compare favorably to surgery and ablative therapy, speculated the editorialists, Michael Papadakis, MBBS, MD, and colleagues of St. George’s University Hospitals NHS Foundation Trust, London. The drug appeared to reduce the peak LVOT gradient “to less than the guideline-based threshold for septal reduction therapy, 50 mm Hg, in 74% of patients, compared with 21% in the placebo group, indicating that mavacamten could represent a valid alternative to highly specialized invasive therapy,” they wrote.
Standard drug therapy
“There are approved drugs for obstructive hypertrophic cardiomyopathy, but they are ancient and were developed for other diseases,” observed Dr. Olivotto at the media briefing. Those drug options – primarily beta-blockers, nondihydropyridine calcium-channel blockers, and the sodium-channel blocker disopyramide – are often ineffective or cause onerous side effects, he said.
Notably in EXPLORER-HCM, patients in both the mavacamten and placebo groups could also be receiving beta-blockers and calcium-channel blockers, but no one in the trial could be receiving disopyramide, which can prolong the QT interval.
“By design,” mavacamten wasn’t compared to disopyramide, “a much more potent drug for lowering gradient and improving symptoms than beta-blockers or calcium-channel blockers,” said Martin S. Maron, MD, medical director at the Hypertrophic Cardiomyopathy Center and Research Institute, Tufts Medical Center, Boston.
Therefore, the trial’s results can’t be extrapolated to conclude that the new drug is superior to disopyramide “or the gold standard, surgical myectomy,” he said in an interview.
Dr. Adler agreed that observational studies suggest a benefit from disopyramide that may rival the apparent effect of mavacamten. “But of course, you can’t make direct comparisons because we never had a study like this for disopyramide.” Because it has many side effects and limitations, “it’s not a drug that I like using, but it is beneficial for some patients and I do use it quite a bit.”
What EXPLORER-HCM does seem to show, Dr. Maron said, “is that the mechanism of action of the drug does seem to play out. It lowers gradients in a pretty reliable and powerful way, and that translates into clinical improvement in many patients. So it starts to support the idea that this drug and the class of drugs, myosin inhibitors, may represent another medical therapy option for symptomatic obstructive HCM.”
And, he pointed out, about one-fifth of patients with obstructive HCM don’t respond to disopyramide with fewer symptoms, and in others the drug “starts to lose efficacy over time.” So disopyramide has limitations, and EXPLORER-HCM “provides the possibility of an additional drug option.”
EXPLORER-HCM randomly assigned 251 adults with obstructive HCM in 13 countries to receive mavacamten, titrated from a starting dosage of 5 mg/day to a possible 15 mg/day or to placebo for 30 weeks.
The patients were required to have a peak LVOT gradient at least 50 mm Hg, a left ventricular ejection fraction (LVEF) of at least 55%, and symptoms indicating NYHA class 2 or 3; ultimately, 73% started the trial in NYHA class 2.
In the intention-to-treat analysis, 36.6% of patients receiving mavacamten and 17.2% of control patients met the composite primary endpoint (P = .0005), which consisted of either a 1.5–mL/kg per minute or greater improvement in peak oxygen consumption (pVO2) and at least a one-step reduction in NYHA functional class or at least a 3.0–mL/kg per minute pVO2 increase without deterioration in NYHA class, by week 30.
Patients receiving mavacamten also showed greater improvement in the individual endpoints of postexercise LVOT gradient, NYHA class, and two score-based symptom assessments – the Kansas City Cardiomyopathy Questionnaire-Clinical Summary and Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness-of-Breath domain – compared with control patients.
Safety and tolerability issues were similar in both groups, the reports notes. Ten patients in the mavacamten group reported 11 serious adverse events, compared with 20 such events reported by 11 patients in the control group.
“We can say from these results that mavacamten is a promising drug for symptom relief and functional class improvement associated with outflow gradient reduction in selected patients with hypertrophic obstructive cardiomyopathy,” and that, on the basis of this trial, it has potential as a drug of first choice, Franco Cecchi, MD, University of Florence (Italy), said as an invited discussant following Dr. Olivotto’s formal presentation of the trial.
Although serious adverse events were few, it was noteworthy that seven patients receiving mavacamten but only two patients receiving placebo showed LVEF reductions to below the 50% threshold during the trial, Dr. Cecchi observed. The LVEFs normalized once the drug was discontinued, but still, it may mean that mavacamten should be carefully uptitrated according to LVEF, he said.
Those LVEF reductions raise questions about “the reliability of being able to dose patients safely in the outpatient setting,” Dr. Maron said. “You have to ask, ‘Can this be extrapolated to the general practicing community without patients dropping their ejection fractions too much?’ ”
In addition, “we don’t have any idea about long-term efficacy for this drug, and we can draw very limited information about long-term safety here as well. That’s another other question mark,” Dr. Maron said.
“If I could have patients really become asymptomatic or mildly symptomatic without any surgery on a drug that is safe and can be taken for a prolonged period of time, that would be great,” Dr. Adler added. He noted that long-term follow-up of patients taking mavacamten in various trials has been underway and should help answer safety and efficacy questions about chronic therapy.
“Should mavacamten prove to be clinically effective and safe following long-term therapy in a larger and more diverse population, it would represent a much anticipated development in the treatment of hypertrophic cardiomyopathy,” the accompanying editorial states.
“Were the drug to realise its potential as a disease-modifying therapy in younger individuals, it would represent a great milestone in the area of inherited cardiomyopathies.”
MyoKardia funded EXPLORER-HCM. Dr. Olivotto discloses receiving grants from MyoKardia, Sanofi-Genzyme, Shire, Amicus, and Bayer; honoraria from Sanofi-Genzyme, Shire, and Bayer; and payments for consulting from MyoKardia. Disclosures for the other authors are in the report. Dr. Papadakis and the other editorialists report that they have no competing interests. Dr. Adler had no disclosures. Dr. Maron discloses consulting for and serving on a trial steering committee for Cytokinetics, sponsor of the 60-patient phase 2 placebo-controlled trial REDWOOD-HCM of patients with obstructive HCM treated with CK-3773274, a drug that works by a mechanism similar to that of mavacamten.
A version of this article originally appeared on Medscape.com.
An investigational drug that targets part of the molecular machinery underlying obstructive hypertrophic cardiomyopathy (HCM) can improve both symptoms and functional status in patients with the genetic disorder, suggests a placebo-controlled phase 3 trial.
Treatment with mavacamten (MyoKardia) worked partly by alleviating high-pressure gradients in the left ventricular outflow tract (LVOT), a key characteristic of obstructive HCM. Its effects appeared consistent across a wide range of objective and patient-assessed endpoints.
Mavacamten is “the first potential medical therapy addressing the underlying biology of symptoms in hypertrophic obstructive cardiomyopathy,” observed Iacopo Olivotto, MD, Careggi University Hospital, Florence, Italy.
Patients in the EXPLORER-HCM trial who took the new drug showed improvements in “every aspect of objective performance and subjective well-being,” Dr. Olivotto said at a preview for journalists before his formal online presentation of the results during the virtual European Society of Cardiology Congress 2020, staged in lieu of the traditional annual meeting because of the COVID-19 pandemic.
Dr. Olivotto, also lead author on the study’s same-day publication in The Lancet, was exuberant about the findings. “It is really hard to convey what this actually means for a scientific and clinical community that has spent over 60 years trying to understand and cure hypertrophic cardiomyopathy.”
MyoKardia released abbreviated top-line results of EXPLORER-HCM in May, which were reported by theheart.org | Medscape Cardiology at the time.
“I think it’s pretty exciting. We certainly need more and better drugs for this patient population,” Arnon Adler, MD, who is not associated with the trial but follows HCM at the Peter Munk Cardiac Centre, Toronto General Hospital, said in an interview.
The trial compared the new drug to placebo rather than full contemporary drug therapy for obstructive HCM, Adler cautioned, and had a fairly short follow-up time. But he was impressed that mavacamten’s apparent benefits seemed consistent not only for endpoints like change in New York Heart Association (NYHA) functional class and quality of life but also for more objective measures like peak VO2 and LVOT gradients.
“I think the results were promising across the board,” he told.
Unique mechanism of action
Mavacamten is described as a first-in-class, small-molecule, selective allosteric inhibitor of cardiac myosin adenosine triphosphatase that addresses the underlying pathophysiology of HCM by reducing actin–myosin cross-bridge formation. It thereby inhibits the excessive myocyte contractility that is a key mechanism of the disorder’s tell-tale hypertrophy, something the available HCM drug therapies don’t do.
Almost three-fourths of patients in the trial were initially in NYHA class 2. Such patients in practice tend to be treated pharmacologically, with more invasive but generally effective surgical myectomy and alcohol septal ablation performed more often for patients in NYHA class 3.
“In the EXPLORER-HCM trial, patients enrolled did not have any immediate indication for surgery,” although many of them in NYHA class 2 would likely progress to NYHA 3, Dr. Olivotto said in an interview.
Based on the trial, he said, it’s possible that mavacamten could lead to “earlier and broader treatment of obstruction symptoms in patients who would never have qualified for surgery in the first place because their symptoms may not be severe enough, but they are still limited.”
Notably, the published report notes, 27% of patients taking mavacamten achieved what was defined as a complete response – that is, a reduction of all LVOT gradients to less than 30 mm Hg in the total absence of symptoms.
Only 1% of patients in the placebo-treated control group met that goal, “showing that mavacamten might be capable of achieving marked relief of symptoms and LVOT obstruction,” the report states.
In the trial, “treatment with mavacamten led to clinically meaningful improvements in hemodynamic status, functional capacity, and subjective well-being in patients with obstructive hypertrophic cardiomyopathy,” agrees an editorial accompanying the EXPLORER-HCM publication.
Mavacamten might even compare favorably to surgery and ablative therapy, speculated the editorialists, Michael Papadakis, MBBS, MD, and colleagues of St. George’s University Hospitals NHS Foundation Trust, London. The drug appeared to reduce the peak LVOT gradient “to less than the guideline-based threshold for septal reduction therapy, 50 mm Hg, in 74% of patients, compared with 21% in the placebo group, indicating that mavacamten could represent a valid alternative to highly specialized invasive therapy,” they wrote.
Standard drug therapy
“There are approved drugs for obstructive hypertrophic cardiomyopathy, but they are ancient and were developed for other diseases,” observed Dr. Olivotto at the media briefing. Those drug options – primarily beta-blockers, nondihydropyridine calcium-channel blockers, and the sodium-channel blocker disopyramide – are often ineffective or cause onerous side effects, he said.
Notably in EXPLORER-HCM, patients in both the mavacamten and placebo groups could also be receiving beta-blockers and calcium-channel blockers, but no one in the trial could be receiving disopyramide, which can prolong the QT interval.
“By design,” mavacamten wasn’t compared to disopyramide, “a much more potent drug for lowering gradient and improving symptoms than beta-blockers or calcium-channel blockers,” said Martin S. Maron, MD, medical director at the Hypertrophic Cardiomyopathy Center and Research Institute, Tufts Medical Center, Boston.
Therefore, the trial’s results can’t be extrapolated to conclude that the new drug is superior to disopyramide “or the gold standard, surgical myectomy,” he said in an interview.
Dr. Adler agreed that observational studies suggest a benefit from disopyramide that may rival the apparent effect of mavacamten. “But of course, you can’t make direct comparisons because we never had a study like this for disopyramide.” Because it has many side effects and limitations, “it’s not a drug that I like using, but it is beneficial for some patients and I do use it quite a bit.”
What EXPLORER-HCM does seem to show, Dr. Maron said, “is that the mechanism of action of the drug does seem to play out. It lowers gradients in a pretty reliable and powerful way, and that translates into clinical improvement in many patients. So it starts to support the idea that this drug and the class of drugs, myosin inhibitors, may represent another medical therapy option for symptomatic obstructive HCM.”
And, he pointed out, about one-fifth of patients with obstructive HCM don’t respond to disopyramide with fewer symptoms, and in others the drug “starts to lose efficacy over time.” So disopyramide has limitations, and EXPLORER-HCM “provides the possibility of an additional drug option.”
EXPLORER-HCM randomly assigned 251 adults with obstructive HCM in 13 countries to receive mavacamten, titrated from a starting dosage of 5 mg/day to a possible 15 mg/day or to placebo for 30 weeks.
The patients were required to have a peak LVOT gradient at least 50 mm Hg, a left ventricular ejection fraction (LVEF) of at least 55%, and symptoms indicating NYHA class 2 or 3; ultimately, 73% started the trial in NYHA class 2.
In the intention-to-treat analysis, 36.6% of patients receiving mavacamten and 17.2% of control patients met the composite primary endpoint (P = .0005), which consisted of either a 1.5–mL/kg per minute or greater improvement in peak oxygen consumption (pVO2) and at least a one-step reduction in NYHA functional class or at least a 3.0–mL/kg per minute pVO2 increase without deterioration in NYHA class, by week 30.
Patients receiving mavacamten also showed greater improvement in the individual endpoints of postexercise LVOT gradient, NYHA class, and two score-based symptom assessments – the Kansas City Cardiomyopathy Questionnaire-Clinical Summary and Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness-of-Breath domain – compared with control patients.
Safety and tolerability issues were similar in both groups, the reports notes. Ten patients in the mavacamten group reported 11 serious adverse events, compared with 20 such events reported by 11 patients in the control group.
“We can say from these results that mavacamten is a promising drug for symptom relief and functional class improvement associated with outflow gradient reduction in selected patients with hypertrophic obstructive cardiomyopathy,” and that, on the basis of this trial, it has potential as a drug of first choice, Franco Cecchi, MD, University of Florence (Italy), said as an invited discussant following Dr. Olivotto’s formal presentation of the trial.
Although serious adverse events were few, it was noteworthy that seven patients receiving mavacamten but only two patients receiving placebo showed LVEF reductions to below the 50% threshold during the trial, Dr. Cecchi observed. The LVEFs normalized once the drug was discontinued, but still, it may mean that mavacamten should be carefully uptitrated according to LVEF, he said.
Those LVEF reductions raise questions about “the reliability of being able to dose patients safely in the outpatient setting,” Dr. Maron said. “You have to ask, ‘Can this be extrapolated to the general practicing community without patients dropping their ejection fractions too much?’ ”
In addition, “we don’t have any idea about long-term efficacy for this drug, and we can draw very limited information about long-term safety here as well. That’s another other question mark,” Dr. Maron said.
“If I could have patients really become asymptomatic or mildly symptomatic without any surgery on a drug that is safe and can be taken for a prolonged period of time, that would be great,” Dr. Adler added. He noted that long-term follow-up of patients taking mavacamten in various trials has been underway and should help answer safety and efficacy questions about chronic therapy.
“Should mavacamten prove to be clinically effective and safe following long-term therapy in a larger and more diverse population, it would represent a much anticipated development in the treatment of hypertrophic cardiomyopathy,” the accompanying editorial states.
“Were the drug to realise its potential as a disease-modifying therapy in younger individuals, it would represent a great milestone in the area of inherited cardiomyopathies.”
MyoKardia funded EXPLORER-HCM. Dr. Olivotto discloses receiving grants from MyoKardia, Sanofi-Genzyme, Shire, Amicus, and Bayer; honoraria from Sanofi-Genzyme, Shire, and Bayer; and payments for consulting from MyoKardia. Disclosures for the other authors are in the report. Dr. Papadakis and the other editorialists report that they have no competing interests. Dr. Adler had no disclosures. Dr. Maron discloses consulting for and serving on a trial steering committee for Cytokinetics, sponsor of the 60-patient phase 2 placebo-controlled trial REDWOOD-HCM of patients with obstructive HCM treated with CK-3773274, a drug that works by a mechanism similar to that of mavacamten.
A version of this article originally appeared on Medscape.com.
EMPEROR-Reduced: Empagliflozin’s HFrEF benefit solidifies class effects
The SGLT2 inhibitor drug class solidified its role as a major, new treatment for patients with heart failure with reduced ejection fraction and no diabetes, with results from a second large, controlled trial showing clear efficacy and safety in this population.
Patients with heart failure with reduced ejection fraction (HFrEF) treated with the sodium glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) had a statistically significant 25% relative cut in their incidence of cardiovascular death or first heart failure hospitalization, compared with placebo-treated controls when added on top of standard HFrEF treatment, and this benefit was consistent regardless of whether the treated patients also had type 2 diabetes, Milton Packer, MD, reported at the virtual annual congress of the European Society of Cardiology.
This 25% drop in the primary endpoint with empagliflozin treatment in the EMPEROR-Reduced trial exactly matched the cut in incidence of cardiovascular death or heart failure hospitalization produced by treatment with a another SGLT2 inhibitor, dapagliflozin (Farxiga), in the DAPA-HF trial (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
The performance of these two SGLT2 inhibitors was “incredibly consistent” across the their respective trials run in HFrEF patients with and without type 2 diabetes, and the combined evidence base of the two trials makes for “really compelling evidence” of both safety and efficacy that should prompt a change to U.S. practice, with both of these drugs forming a new cornerstone of HFrEF treatment, Dr. Packer said.
Results plant drug class firmly as HFrEF treatment
Dr. Packer stressed in his presentation that optimal treatment of patients with HFrEF now demands use of one of these two SGLT2 inhibitors, as well as sacubitril plus valsartan (Entresto), a beta-blocker, and a mineralocorticoid receptor antagonist, plus a diuretic as a fifth drug class for the many HFrEF patients who also need treatment for fluid overload. He further advocated for rapid introduction of these four cornerstone agents with proven survival benefits once a patient receives a HFrEF diagnosis, suggesting that sacubitril plus valsartan, an SGLT2 inhibitor, a beta-blocker, and a mineralocorticoid receptor antagonist could all be initiated within 6 weeks or less while acknowledging that optimal up-titration of the beta-blocker would likely take longer.
The order in which a patient starts these drugs shouldn’t matter, and there currently seems to be no evidence that clearly points toward using either dapagliflozin or empagliflozin over the other, Dr. Packer added.
In recognition of the importance of sending a message to heart failure clinicians about the newly proven efficacy of SGLT2 inhibitors in HFrEF patients, the American College of Cardiology and American Heart Association are now drafting an “expert decision pathway” to help clinicians as they enter this new prescribing space. This interim guidance should come out before the end of 2020, prior to release of fully revised HFrEF management guidelines in 2021, said Athena Poppas, MD, president of the ACC, in an interview.
“There is clearly need for education” that can help guide physicians who care for HFrEF patients on how to introduce an SGLT2 inhibitor along with the additional, lengthy list of drug classes proven to benefit these patients, noted Dr. Poppas, who is also a professor and chief of cardiology at the Brown University in Providence, R.I. Physicians may find that they need extra backup for successfully starting both sacubitril plus valsartan and an SGLT2 inhibitor in HFrEF patients because recent history has shown substantial pushback from third-party payers in reimbursing for these relatively expensive drugs, Dr. Poppas noted. She added that this is a problem that may be compounded when patients should ideally get both drug classes.
Physicians who care for heart failure patients have their own history of dragging their feet when adding new drugs to the regimens HFrEF patients receive. The angiotensin converting enzyme inhibitors and beta-blockers took about 17 years each to start reaching a majority of U.S. HFrEF patients, and sacubitril plus valsartan is now used on perhaps a quarter to a third of HFrEF patients despite receiving Food and Drug Administration approval for these patients in mid 2015, noted Christopher M. O’Connor, MD, a heart failure specialist and president of the Inova Heart and Vascular Institute in Fairfax, Va.
Despite dapagliflozin receiving FDA approval in May 2020 for treating HFrEF in patients without diabetes, early uptake in U.S. practice has been very slow, with findings from large U.S. patient registries suggesting that perhaps 1% of suitable HFrEF patients currently get the drug, estimated Dr. O’Connor in an interview.
Given how strong the evidence now is for benefit and safety from dapagliflozin and empagliflozin, it may take as little as 5 years to reach greater than 50% penetration of one of these drugs into U.S. HFrEF patient populations, suggested Dr. Packer, a distinguished scholar in cardiovascular science at Baylor University Medical Center in Dallas.
EMPEROR-Reduced outcomes
The road to routine use of these SGLT2 inhibitor drugs should be hastened by empagliflozin’s impressive performance in EMPEROR-Reduced, in which the drug scored highly significant benefits over placebo for the prespecified primary and two major secondary endpoints, one of which was a measure of preserved renal function.
The trial randomized 3,730 patients at 520 sites in 20 countries during 2017-2019 and followed them on treatment for a median of 16 months. All patients had a left ventricular ejection fraction of 40% or less, and roughly three-quarters had New York Heart Association (NYHA) class II function, nearly one-quarter had class III function, and fewer than 1% of patients fell into the class IV category.
The primary endpoint occurred in 19% of the empagliflozin-treated patients and in 25% of those who received placebo. Among the half of patients with diabetes in the trial, the relative risk reduction by empagliflozin compared with placebo was a statistically significant 28%; among those without diabetes, it was a statistically significant 22%. Concurrently with Dr. Packer’s report, the results appeared in an article posted online (N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190).
The study also had two main prespecified secondary endpoints: the incidence of total hospitalizations for heart failure, both first and recurrent, which fell by 30% in the empagliflozin-treated patients, compared with placebo, and the rate of declining renal function during the 16 months of the study as measured by estimated glomerular filtration rate, which dropped by roughly 1 mL/min per 1.73 m2 among the empagliflozin recipients and by about 4 mL/min/ per 1.73 m2 in the placebo patients.
Treatment with empagliflozin also achieved a notable, statistically significant 50% drop in major adverse renal events, consistent with the performance of other drugs in the class.
“Renal protection is a big plus” of empagliflozin in this trial and from the other SGLT2 inhibitors in prior studies, noted Dr. O’Connor.
The EMPEROR-Reduced results also showed an important benefit for HFrEF patients from empagliflozin not previously seen as quickly with any other drug class, noted Dr. Packer. The SGLT2 inhibitor led to statistically a significant slowing in the progression of patients from NYHA class II function to class III, compared with placebo, and it also significantly promoted the recovery of patients from NYHA class III to class II, an effect that became apparent within the first month on treatment and a benefit that is a “big deal” for patients because it represents a “significant change in functional capacity.” This additional dimension of empagliflozin’s benefit “really impressed me,” Dr. Packer said.
EMPEROR-Reduced was funded by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin. Dr. Packer has received personal fees from Boehringer Ingelheim and Eli Lilly and from several other companies. Dr. Poppas and Dr. O’Connor had no relevant disclosures.
SOURCE: Packer M. ESC 2020. N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190.
The SGLT2 inhibitor drug class solidified its role as a major, new treatment for patients with heart failure with reduced ejection fraction and no diabetes, with results from a second large, controlled trial showing clear efficacy and safety in this population.
Patients with heart failure with reduced ejection fraction (HFrEF) treated with the sodium glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) had a statistically significant 25% relative cut in their incidence of cardiovascular death or first heart failure hospitalization, compared with placebo-treated controls when added on top of standard HFrEF treatment, and this benefit was consistent regardless of whether the treated patients also had type 2 diabetes, Milton Packer, MD, reported at the virtual annual congress of the European Society of Cardiology.
This 25% drop in the primary endpoint with empagliflozin treatment in the EMPEROR-Reduced trial exactly matched the cut in incidence of cardiovascular death or heart failure hospitalization produced by treatment with a another SGLT2 inhibitor, dapagliflozin (Farxiga), in the DAPA-HF trial (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
The performance of these two SGLT2 inhibitors was “incredibly consistent” across the their respective trials run in HFrEF patients with and without type 2 diabetes, and the combined evidence base of the two trials makes for “really compelling evidence” of both safety and efficacy that should prompt a change to U.S. practice, with both of these drugs forming a new cornerstone of HFrEF treatment, Dr. Packer said.
Results plant drug class firmly as HFrEF treatment
Dr. Packer stressed in his presentation that optimal treatment of patients with HFrEF now demands use of one of these two SGLT2 inhibitors, as well as sacubitril plus valsartan (Entresto), a beta-blocker, and a mineralocorticoid receptor antagonist, plus a diuretic as a fifth drug class for the many HFrEF patients who also need treatment for fluid overload. He further advocated for rapid introduction of these four cornerstone agents with proven survival benefits once a patient receives a HFrEF diagnosis, suggesting that sacubitril plus valsartan, an SGLT2 inhibitor, a beta-blocker, and a mineralocorticoid receptor antagonist could all be initiated within 6 weeks or less while acknowledging that optimal up-titration of the beta-blocker would likely take longer.
The order in which a patient starts these drugs shouldn’t matter, and there currently seems to be no evidence that clearly points toward using either dapagliflozin or empagliflozin over the other, Dr. Packer added.
In recognition of the importance of sending a message to heart failure clinicians about the newly proven efficacy of SGLT2 inhibitors in HFrEF patients, the American College of Cardiology and American Heart Association are now drafting an “expert decision pathway” to help clinicians as they enter this new prescribing space. This interim guidance should come out before the end of 2020, prior to release of fully revised HFrEF management guidelines in 2021, said Athena Poppas, MD, president of the ACC, in an interview.
“There is clearly need for education” that can help guide physicians who care for HFrEF patients on how to introduce an SGLT2 inhibitor along with the additional, lengthy list of drug classes proven to benefit these patients, noted Dr. Poppas, who is also a professor and chief of cardiology at the Brown University in Providence, R.I. Physicians may find that they need extra backup for successfully starting both sacubitril plus valsartan and an SGLT2 inhibitor in HFrEF patients because recent history has shown substantial pushback from third-party payers in reimbursing for these relatively expensive drugs, Dr. Poppas noted. She added that this is a problem that may be compounded when patients should ideally get both drug classes.
Physicians who care for heart failure patients have their own history of dragging their feet when adding new drugs to the regimens HFrEF patients receive. The angiotensin converting enzyme inhibitors and beta-blockers took about 17 years each to start reaching a majority of U.S. HFrEF patients, and sacubitril plus valsartan is now used on perhaps a quarter to a third of HFrEF patients despite receiving Food and Drug Administration approval for these patients in mid 2015, noted Christopher M. O’Connor, MD, a heart failure specialist and president of the Inova Heart and Vascular Institute in Fairfax, Va.
Despite dapagliflozin receiving FDA approval in May 2020 for treating HFrEF in patients without diabetes, early uptake in U.S. practice has been very slow, with findings from large U.S. patient registries suggesting that perhaps 1% of suitable HFrEF patients currently get the drug, estimated Dr. O’Connor in an interview.
Given how strong the evidence now is for benefit and safety from dapagliflozin and empagliflozin, it may take as little as 5 years to reach greater than 50% penetration of one of these drugs into U.S. HFrEF patient populations, suggested Dr. Packer, a distinguished scholar in cardiovascular science at Baylor University Medical Center in Dallas.
EMPEROR-Reduced outcomes
The road to routine use of these SGLT2 inhibitor drugs should be hastened by empagliflozin’s impressive performance in EMPEROR-Reduced, in which the drug scored highly significant benefits over placebo for the prespecified primary and two major secondary endpoints, one of which was a measure of preserved renal function.
The trial randomized 3,730 patients at 520 sites in 20 countries during 2017-2019 and followed them on treatment for a median of 16 months. All patients had a left ventricular ejection fraction of 40% or less, and roughly three-quarters had New York Heart Association (NYHA) class II function, nearly one-quarter had class III function, and fewer than 1% of patients fell into the class IV category.
The primary endpoint occurred in 19% of the empagliflozin-treated patients and in 25% of those who received placebo. Among the half of patients with diabetes in the trial, the relative risk reduction by empagliflozin compared with placebo was a statistically significant 28%; among those without diabetes, it was a statistically significant 22%. Concurrently with Dr. Packer’s report, the results appeared in an article posted online (N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190).
The study also had two main prespecified secondary endpoints: the incidence of total hospitalizations for heart failure, both first and recurrent, which fell by 30% in the empagliflozin-treated patients, compared with placebo, and the rate of declining renal function during the 16 months of the study as measured by estimated glomerular filtration rate, which dropped by roughly 1 mL/min per 1.73 m2 among the empagliflozin recipients and by about 4 mL/min/ per 1.73 m2 in the placebo patients.
Treatment with empagliflozin also achieved a notable, statistically significant 50% drop in major adverse renal events, consistent with the performance of other drugs in the class.
“Renal protection is a big plus” of empagliflozin in this trial and from the other SGLT2 inhibitors in prior studies, noted Dr. O’Connor.
The EMPEROR-Reduced results also showed an important benefit for HFrEF patients from empagliflozin not previously seen as quickly with any other drug class, noted Dr. Packer. The SGLT2 inhibitor led to statistically a significant slowing in the progression of patients from NYHA class II function to class III, compared with placebo, and it also significantly promoted the recovery of patients from NYHA class III to class II, an effect that became apparent within the first month on treatment and a benefit that is a “big deal” for patients because it represents a “significant change in functional capacity.” This additional dimension of empagliflozin’s benefit “really impressed me,” Dr. Packer said.
EMPEROR-Reduced was funded by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin. Dr. Packer has received personal fees from Boehringer Ingelheim and Eli Lilly and from several other companies. Dr. Poppas and Dr. O’Connor had no relevant disclosures.
SOURCE: Packer M. ESC 2020. N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190.
The SGLT2 inhibitor drug class solidified its role as a major, new treatment for patients with heart failure with reduced ejection fraction and no diabetes, with results from a second large, controlled trial showing clear efficacy and safety in this population.
Patients with heart failure with reduced ejection fraction (HFrEF) treated with the sodium glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) had a statistically significant 25% relative cut in their incidence of cardiovascular death or first heart failure hospitalization, compared with placebo-treated controls when added on top of standard HFrEF treatment, and this benefit was consistent regardless of whether the treated patients also had type 2 diabetes, Milton Packer, MD, reported at the virtual annual congress of the European Society of Cardiology.
This 25% drop in the primary endpoint with empagliflozin treatment in the EMPEROR-Reduced trial exactly matched the cut in incidence of cardiovascular death or heart failure hospitalization produced by treatment with a another SGLT2 inhibitor, dapagliflozin (Farxiga), in the DAPA-HF trial (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
The performance of these two SGLT2 inhibitors was “incredibly consistent” across the their respective trials run in HFrEF patients with and without type 2 diabetes, and the combined evidence base of the two trials makes for “really compelling evidence” of both safety and efficacy that should prompt a change to U.S. practice, with both of these drugs forming a new cornerstone of HFrEF treatment, Dr. Packer said.
Results plant drug class firmly as HFrEF treatment
Dr. Packer stressed in his presentation that optimal treatment of patients with HFrEF now demands use of one of these two SGLT2 inhibitors, as well as sacubitril plus valsartan (Entresto), a beta-blocker, and a mineralocorticoid receptor antagonist, plus a diuretic as a fifth drug class for the many HFrEF patients who also need treatment for fluid overload. He further advocated for rapid introduction of these four cornerstone agents with proven survival benefits once a patient receives a HFrEF diagnosis, suggesting that sacubitril plus valsartan, an SGLT2 inhibitor, a beta-blocker, and a mineralocorticoid receptor antagonist could all be initiated within 6 weeks or less while acknowledging that optimal up-titration of the beta-blocker would likely take longer.
The order in which a patient starts these drugs shouldn’t matter, and there currently seems to be no evidence that clearly points toward using either dapagliflozin or empagliflozin over the other, Dr. Packer added.
In recognition of the importance of sending a message to heart failure clinicians about the newly proven efficacy of SGLT2 inhibitors in HFrEF patients, the American College of Cardiology and American Heart Association are now drafting an “expert decision pathway” to help clinicians as they enter this new prescribing space. This interim guidance should come out before the end of 2020, prior to release of fully revised HFrEF management guidelines in 2021, said Athena Poppas, MD, president of the ACC, in an interview.
“There is clearly need for education” that can help guide physicians who care for HFrEF patients on how to introduce an SGLT2 inhibitor along with the additional, lengthy list of drug classes proven to benefit these patients, noted Dr. Poppas, who is also a professor and chief of cardiology at the Brown University in Providence, R.I. Physicians may find that they need extra backup for successfully starting both sacubitril plus valsartan and an SGLT2 inhibitor in HFrEF patients because recent history has shown substantial pushback from third-party payers in reimbursing for these relatively expensive drugs, Dr. Poppas noted. She added that this is a problem that may be compounded when patients should ideally get both drug classes.
Physicians who care for heart failure patients have their own history of dragging their feet when adding new drugs to the regimens HFrEF patients receive. The angiotensin converting enzyme inhibitors and beta-blockers took about 17 years each to start reaching a majority of U.S. HFrEF patients, and sacubitril plus valsartan is now used on perhaps a quarter to a third of HFrEF patients despite receiving Food and Drug Administration approval for these patients in mid 2015, noted Christopher M. O’Connor, MD, a heart failure specialist and president of the Inova Heart and Vascular Institute in Fairfax, Va.
Despite dapagliflozin receiving FDA approval in May 2020 for treating HFrEF in patients without diabetes, early uptake in U.S. practice has been very slow, with findings from large U.S. patient registries suggesting that perhaps 1% of suitable HFrEF patients currently get the drug, estimated Dr. O’Connor in an interview.
Given how strong the evidence now is for benefit and safety from dapagliflozin and empagliflozin, it may take as little as 5 years to reach greater than 50% penetration of one of these drugs into U.S. HFrEF patient populations, suggested Dr. Packer, a distinguished scholar in cardiovascular science at Baylor University Medical Center in Dallas.
EMPEROR-Reduced outcomes
The road to routine use of these SGLT2 inhibitor drugs should be hastened by empagliflozin’s impressive performance in EMPEROR-Reduced, in which the drug scored highly significant benefits over placebo for the prespecified primary and two major secondary endpoints, one of which was a measure of preserved renal function.
The trial randomized 3,730 patients at 520 sites in 20 countries during 2017-2019 and followed them on treatment for a median of 16 months. All patients had a left ventricular ejection fraction of 40% or less, and roughly three-quarters had New York Heart Association (NYHA) class II function, nearly one-quarter had class III function, and fewer than 1% of patients fell into the class IV category.
The primary endpoint occurred in 19% of the empagliflozin-treated patients and in 25% of those who received placebo. Among the half of patients with diabetes in the trial, the relative risk reduction by empagliflozin compared with placebo was a statistically significant 28%; among those without diabetes, it was a statistically significant 22%. Concurrently with Dr. Packer’s report, the results appeared in an article posted online (N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190).
The study also had two main prespecified secondary endpoints: the incidence of total hospitalizations for heart failure, both first and recurrent, which fell by 30% in the empagliflozin-treated patients, compared with placebo, and the rate of declining renal function during the 16 months of the study as measured by estimated glomerular filtration rate, which dropped by roughly 1 mL/min per 1.73 m2 among the empagliflozin recipients and by about 4 mL/min/ per 1.73 m2 in the placebo patients.
Treatment with empagliflozin also achieved a notable, statistically significant 50% drop in major adverse renal events, consistent with the performance of other drugs in the class.
“Renal protection is a big plus” of empagliflozin in this trial and from the other SGLT2 inhibitors in prior studies, noted Dr. O’Connor.
The EMPEROR-Reduced results also showed an important benefit for HFrEF patients from empagliflozin not previously seen as quickly with any other drug class, noted Dr. Packer. The SGLT2 inhibitor led to statistically a significant slowing in the progression of patients from NYHA class II function to class III, compared with placebo, and it also significantly promoted the recovery of patients from NYHA class III to class II, an effect that became apparent within the first month on treatment and a benefit that is a “big deal” for patients because it represents a “significant change in functional capacity.” This additional dimension of empagliflozin’s benefit “really impressed me,” Dr. Packer said.
EMPEROR-Reduced was funded by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin. Dr. Packer has received personal fees from Boehringer Ingelheim and Eli Lilly and from several other companies. Dr. Poppas and Dr. O’Connor had no relevant disclosures.
SOURCE: Packer M. ESC 2020. N Engl J Med. 2020 Aug 29. doi: 10.1056/NEJMoa2022190.
FROM ESC CONGRESS 2020
FDA pulls amputation boxed warning off canagliflozin label
The Food and Drug Administration has removed the boxed warning about the risk of leg and foot amputations for canagliflozin (Invokana, Invokamet, Janssen), a sodium-glucose cotransporter-2 (SGLT2) inhibitor for the treatment of type 2 diabetes, the agency announced Aug. 26.
As previously reported by Medscape Medical News, the FDA added the boxed warning to the canagliflozin label in May 2017, after an approximately doubled risk for lower-extremity amputations with the drug compared with placebo was seen during two trials.
The FDA said the decision to remove the boxed warning was made following a review of new data from three clinical trials, which demonstrated additional heart- and kidney-related benefits and led to additional approved uses for canagliflozin.
In 2018, canagliflozin was approved to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes who have established cardiovascular disease.
In 2019, canagliflozin was approved to reduce the risk of end-stage kidney disease, worsening of kidney function, cardiovascular death, and heart failure hospitalization, in adults with type 2 diabetes and diabetic kidney disease.
“Collectively, these newly identified effects of canagliflozin on heart and kidney disease show significantly enhanced benefit of this medicine,” the FDA said.
The safety information from these trials, the FDA said, suggests that the risk of amputation, “while still increased with canagliflozin, is lower than previously described, particularly when appropriately monitored.”
The agency added: “Based upon these considerations, FDA concluded that the boxed warning should be removed.”
The FDA announcement said clinicians and patients should continue to be aware of the importance of preventive foot care and to monitor for new pain, tenderness, sores, ulcers, and infections in the legs and feet. Risk factors that may predispose patients to amputation should be considered when choosing antidiabetic medicines.
Health care professionals are encouraged to report adverse reactions with canagliflozin to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has removed the boxed warning about the risk of leg and foot amputations for canagliflozin (Invokana, Invokamet, Janssen), a sodium-glucose cotransporter-2 (SGLT2) inhibitor for the treatment of type 2 diabetes, the agency announced Aug. 26.
As previously reported by Medscape Medical News, the FDA added the boxed warning to the canagliflozin label in May 2017, after an approximately doubled risk for lower-extremity amputations with the drug compared with placebo was seen during two trials.
The FDA said the decision to remove the boxed warning was made following a review of new data from three clinical trials, which demonstrated additional heart- and kidney-related benefits and led to additional approved uses for canagliflozin.
In 2018, canagliflozin was approved to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes who have established cardiovascular disease.
In 2019, canagliflozin was approved to reduce the risk of end-stage kidney disease, worsening of kidney function, cardiovascular death, and heart failure hospitalization, in adults with type 2 diabetes and diabetic kidney disease.
“Collectively, these newly identified effects of canagliflozin on heart and kidney disease show significantly enhanced benefit of this medicine,” the FDA said.
The safety information from these trials, the FDA said, suggests that the risk of amputation, “while still increased with canagliflozin, is lower than previously described, particularly when appropriately monitored.”
The agency added: “Based upon these considerations, FDA concluded that the boxed warning should be removed.”
The FDA announcement said clinicians and patients should continue to be aware of the importance of preventive foot care and to monitor for new pain, tenderness, sores, ulcers, and infections in the legs and feet. Risk factors that may predispose patients to amputation should be considered when choosing antidiabetic medicines.
Health care professionals are encouraged to report adverse reactions with canagliflozin to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has removed the boxed warning about the risk of leg and foot amputations for canagliflozin (Invokana, Invokamet, Janssen), a sodium-glucose cotransporter-2 (SGLT2) inhibitor for the treatment of type 2 diabetes, the agency announced Aug. 26.
As previously reported by Medscape Medical News, the FDA added the boxed warning to the canagliflozin label in May 2017, after an approximately doubled risk for lower-extremity amputations with the drug compared with placebo was seen during two trials.
The FDA said the decision to remove the boxed warning was made following a review of new data from three clinical trials, which demonstrated additional heart- and kidney-related benefits and led to additional approved uses for canagliflozin.
In 2018, canagliflozin was approved to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes who have established cardiovascular disease.
In 2019, canagliflozin was approved to reduce the risk of end-stage kidney disease, worsening of kidney function, cardiovascular death, and heart failure hospitalization, in adults with type 2 diabetes and diabetic kidney disease.
“Collectively, these newly identified effects of canagliflozin on heart and kidney disease show significantly enhanced benefit of this medicine,” the FDA said.
The safety information from these trials, the FDA said, suggests that the risk of amputation, “while still increased with canagliflozin, is lower than previously described, particularly when appropriately monitored.”
The agency added: “Based upon these considerations, FDA concluded that the boxed warning should be removed.”
The FDA announcement said clinicians and patients should continue to be aware of the importance of preventive foot care and to monitor for new pain, tenderness, sores, ulcers, and infections in the legs and feet. Risk factors that may predispose patients to amputation should be considered when choosing antidiabetic medicines.
Health care professionals are encouraged to report adverse reactions with canagliflozin to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
First evidence of SARS-CoV-2 in heart cells
SARS-CoV-2 has been found in cardiac tissue of a child from Brazil with multisystem inflammatory syndrome (MIS-C) related to COVID-19 who presented with myocarditis and died of heart failure.
It’s believed to be the first evidence of direct infection of heart muscle cells by the virus; viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.
The case was described in a report published online August 20 in The Lancet Child & Adolescent Health.
“The presence of the virus in various cell types of cardiac tissue, as evidenced by electron microscopy, shows that myocarditis in this case is likely a direct inflammatory response to the virus infection in the heart,” first author Marisa Dolhnikoff, MD, department of pathology, University of São Paulo, said in an interview.
There have been previous reports in adults with COVID-19 of both SARS-CoV-2 RNA by reverse transcription–polymerase chain reaction (RT-PCR) and viral particles by electron microscopy in cardiac tissue from endomyocardial specimens, the researchers noted. One of these reports, published in April by Tavazzi and colleagues, “detected viral particles in cardiac macrophages in an adult patient with acute cardiac injury associated with COVID-19; no viral particles were seen in cardiomyocytes or endothelial cells.
“Our case report is the first to our knowledge to document the presence of viral particles in the cardiac tissue of a child affected by MIS-C,” they added. “Moreover, viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.”
‘Concerning’ case report
“This is a concerning report as it shows for the first time that the virus can actually invade the heart muscle cells themselves,” C. Michael Gibson, MD, CEO of the Baim Institute for Clinical Research in Boston, said in an interview.
“Previous reports of COVID-19 and the heart found that the virus was in the area outside the heart muscle cells. We do not know yet the relative contribution of the inflammatory cells invading the heart, the release of blood-borne inflammatory mediators, and the virus inside the heart muscle cells themselves to heart damage,” Dr. Gibson said.
The patient was a previously healthy 11-year-old girl of African descent with MIS-C related to COVID-19. She developed cardiac failure and died after 1 day in the hospital, despite aggressive treatment.
SARS-CoV-2 RNA was detected on a postmortem nasopharyngeal swab and in cardiac and pulmonary tissues by RT-PCR.
Postmortem ultrasound examination of the heart showed a “hyperechogenic and diffusely thickened endocardium (mean thickness, 10 mm), a thickened myocardium (18 mm thick in the left ventricle), and a small pericardial effusion,” Dr. Dolhnikoff and colleagues reported.
Histopathologic exam revealed myocarditis, pericarditis, and endocarditis characterized by infiltration of inflammatory cells. Inflammation was mainly interstitial and perivascular, associated with foci of cardiomyocyte necrosis and was mainly composed of CD68+ macrophages, a few CD45+ lymphocytes, and a few neutrophils and eosinophils.
Electron microscopy of cardiac tissue revealed spherical viral particles in shape and size consistent with the Coronaviridae family in the extracellular compartment and within cardiomyocytes, capillary endothelial cells, endocardium endothelial cells, macrophages, neutrophils, and fibroblasts.
Microthrombi in the pulmonary arterioles and renal glomerular capillaries were also seen at autopsy. SARS-CoV-2–associated pneumonia was mild.
Lymphoid depletion and signs of hemophagocytosis were observed in the spleen and lymph nodes. Acute tubular necrosis in the kidneys and hepatic centrilobular necrosis, secondary to shock, were also seen. Brain tissue showed microglial reactivity.
“Fortunately, MIS-C is a rare event and, although it can be severe and life threatening, most children recover,” Dr. Dolhnikoff commented.
“This case report comes at a time when the scientific community around the world calls attention to MIS-C and the need for it to be quickly recognized and treated by the pediatric community. Evidence of a direct relation between the virus and myocarditis confirms that MIS-C is one of the possible forms of presentation of COVID-19 and that the heart may be the target organ. It also alerts clinicians to possible cardiac sequelae in these children,” she added.
Experts weigh in
Scott Aydin, MD, medical director of pediatric cardiac intensive care, Mount Sinai Kravis Children’s Hospital in New York City, said that this case report is “unfortunately not all that surprising.
“Since the initial presentations of MIS-C several months ago, we have suspected mechanisms of direct and indirect injury to the myocardium. This important work is just the next step in further understanding the mechanisms of how COVID-19 creates havoc in the human body and the choices of possible therapies we have to treat children with COVID-19 and MIS-C,” said Dr. Aydin, who was not involved with the case report.
Anish Koka, MD, a cardiologist in private practice in Philadelphia, noted that, in these cases, endomyocardial biopsy is “rarely done because it is fairly invasive, but even when it has been done, the pathologic findings are of widespread inflammation rather than virus-induced cell necrosis.”
“While reports like this are sure to spawn viral tweets, it’s vital to understand that it’s not unusual to find widespread organ dissemination of virus in very sick patients. This does not mean that the virus is causing dysfunction of the organ it happens to be found in,” Dr. Koka said in an interview.
He noted that, in the case of the young girl who died, it took high PCR-cycle threshold values to isolate virus from the lung and heart samples.
“This means there was a low viral load in both organs, supporting the theory of SARS-CoV-2 as a potential trigger of a widespread inflammatory response that results in organ damage, rather than the virus itself infecting and destroying organs,” said Dr. Koka, who was also not associated with the case report.
This research had no specific funding. The authors declared no competing interests. Dr. Aydin disclosed no relevant financial relationships. Dr. Koka disclosed financial relationships with Boehringer Ingelheim and Jardiance.
This article first appeared on Medscape.com.
SARS-CoV-2 has been found in cardiac tissue of a child from Brazil with multisystem inflammatory syndrome (MIS-C) related to COVID-19 who presented with myocarditis and died of heart failure.
It’s believed to be the first evidence of direct infection of heart muscle cells by the virus; viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.
The case was described in a report published online August 20 in The Lancet Child & Adolescent Health.
“The presence of the virus in various cell types of cardiac tissue, as evidenced by electron microscopy, shows that myocarditis in this case is likely a direct inflammatory response to the virus infection in the heart,” first author Marisa Dolhnikoff, MD, department of pathology, University of São Paulo, said in an interview.
There have been previous reports in adults with COVID-19 of both SARS-CoV-2 RNA by reverse transcription–polymerase chain reaction (RT-PCR) and viral particles by electron microscopy in cardiac tissue from endomyocardial specimens, the researchers noted. One of these reports, published in April by Tavazzi and colleagues, “detected viral particles in cardiac macrophages in an adult patient with acute cardiac injury associated with COVID-19; no viral particles were seen in cardiomyocytes or endothelial cells.
“Our case report is the first to our knowledge to document the presence of viral particles in the cardiac tissue of a child affected by MIS-C,” they added. “Moreover, viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.”
‘Concerning’ case report
“This is a concerning report as it shows for the first time that the virus can actually invade the heart muscle cells themselves,” C. Michael Gibson, MD, CEO of the Baim Institute for Clinical Research in Boston, said in an interview.
“Previous reports of COVID-19 and the heart found that the virus was in the area outside the heart muscle cells. We do not know yet the relative contribution of the inflammatory cells invading the heart, the release of blood-borne inflammatory mediators, and the virus inside the heart muscle cells themselves to heart damage,” Dr. Gibson said.
The patient was a previously healthy 11-year-old girl of African descent with MIS-C related to COVID-19. She developed cardiac failure and died after 1 day in the hospital, despite aggressive treatment.
SARS-CoV-2 RNA was detected on a postmortem nasopharyngeal swab and in cardiac and pulmonary tissues by RT-PCR.
Postmortem ultrasound examination of the heart showed a “hyperechogenic and diffusely thickened endocardium (mean thickness, 10 mm), a thickened myocardium (18 mm thick in the left ventricle), and a small pericardial effusion,” Dr. Dolhnikoff and colleagues reported.
Histopathologic exam revealed myocarditis, pericarditis, and endocarditis characterized by infiltration of inflammatory cells. Inflammation was mainly interstitial and perivascular, associated with foci of cardiomyocyte necrosis and was mainly composed of CD68+ macrophages, a few CD45+ lymphocytes, and a few neutrophils and eosinophils.
Electron microscopy of cardiac tissue revealed spherical viral particles in shape and size consistent with the Coronaviridae family in the extracellular compartment and within cardiomyocytes, capillary endothelial cells, endocardium endothelial cells, macrophages, neutrophils, and fibroblasts.
Microthrombi in the pulmonary arterioles and renal glomerular capillaries were also seen at autopsy. SARS-CoV-2–associated pneumonia was mild.
Lymphoid depletion and signs of hemophagocytosis were observed in the spleen and lymph nodes. Acute tubular necrosis in the kidneys and hepatic centrilobular necrosis, secondary to shock, were also seen. Brain tissue showed microglial reactivity.
“Fortunately, MIS-C is a rare event and, although it can be severe and life threatening, most children recover,” Dr. Dolhnikoff commented.
“This case report comes at a time when the scientific community around the world calls attention to MIS-C and the need for it to be quickly recognized and treated by the pediatric community. Evidence of a direct relation between the virus and myocarditis confirms that MIS-C is one of the possible forms of presentation of COVID-19 and that the heart may be the target organ. It also alerts clinicians to possible cardiac sequelae in these children,” she added.
Experts weigh in
Scott Aydin, MD, medical director of pediatric cardiac intensive care, Mount Sinai Kravis Children’s Hospital in New York City, said that this case report is “unfortunately not all that surprising.
“Since the initial presentations of MIS-C several months ago, we have suspected mechanisms of direct and indirect injury to the myocardium. This important work is just the next step in further understanding the mechanisms of how COVID-19 creates havoc in the human body and the choices of possible therapies we have to treat children with COVID-19 and MIS-C,” said Dr. Aydin, who was not involved with the case report.
Anish Koka, MD, a cardiologist in private practice in Philadelphia, noted that, in these cases, endomyocardial biopsy is “rarely done because it is fairly invasive, but even when it has been done, the pathologic findings are of widespread inflammation rather than virus-induced cell necrosis.”
“While reports like this are sure to spawn viral tweets, it’s vital to understand that it’s not unusual to find widespread organ dissemination of virus in very sick patients. This does not mean that the virus is causing dysfunction of the organ it happens to be found in,” Dr. Koka said in an interview.
He noted that, in the case of the young girl who died, it took high PCR-cycle threshold values to isolate virus from the lung and heart samples.
“This means there was a low viral load in both organs, supporting the theory of SARS-CoV-2 as a potential trigger of a widespread inflammatory response that results in organ damage, rather than the virus itself infecting and destroying organs,” said Dr. Koka, who was also not associated with the case report.
This research had no specific funding. The authors declared no competing interests. Dr. Aydin disclosed no relevant financial relationships. Dr. Koka disclosed financial relationships with Boehringer Ingelheim and Jardiance.
This article first appeared on Medscape.com.
SARS-CoV-2 has been found in cardiac tissue of a child from Brazil with multisystem inflammatory syndrome (MIS-C) related to COVID-19 who presented with myocarditis and died of heart failure.
It’s believed to be the first evidence of direct infection of heart muscle cells by the virus; viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.
The case was described in a report published online August 20 in The Lancet Child & Adolescent Health.
“The presence of the virus in various cell types of cardiac tissue, as evidenced by electron microscopy, shows that myocarditis in this case is likely a direct inflammatory response to the virus infection in the heart,” first author Marisa Dolhnikoff, MD, department of pathology, University of São Paulo, said in an interview.
There have been previous reports in adults with COVID-19 of both SARS-CoV-2 RNA by reverse transcription–polymerase chain reaction (RT-PCR) and viral particles by electron microscopy in cardiac tissue from endomyocardial specimens, the researchers noted. One of these reports, published in April by Tavazzi and colleagues, “detected viral particles in cardiac macrophages in an adult patient with acute cardiac injury associated with COVID-19; no viral particles were seen in cardiomyocytes or endothelial cells.
“Our case report is the first to our knowledge to document the presence of viral particles in the cardiac tissue of a child affected by MIS-C,” they added. “Moreover, viral particles were identified in different cell lineages of the heart, including cardiomyocytes, endothelial cells, mesenchymal cells, and inflammatory cells.”
‘Concerning’ case report
“This is a concerning report as it shows for the first time that the virus can actually invade the heart muscle cells themselves,” C. Michael Gibson, MD, CEO of the Baim Institute for Clinical Research in Boston, said in an interview.
“Previous reports of COVID-19 and the heart found that the virus was in the area outside the heart muscle cells. We do not know yet the relative contribution of the inflammatory cells invading the heart, the release of blood-borne inflammatory mediators, and the virus inside the heart muscle cells themselves to heart damage,” Dr. Gibson said.
The patient was a previously healthy 11-year-old girl of African descent with MIS-C related to COVID-19. She developed cardiac failure and died after 1 day in the hospital, despite aggressive treatment.
SARS-CoV-2 RNA was detected on a postmortem nasopharyngeal swab and in cardiac and pulmonary tissues by RT-PCR.
Postmortem ultrasound examination of the heart showed a “hyperechogenic and diffusely thickened endocardium (mean thickness, 10 mm), a thickened myocardium (18 mm thick in the left ventricle), and a small pericardial effusion,” Dr. Dolhnikoff and colleagues reported.
Histopathologic exam revealed myocarditis, pericarditis, and endocarditis characterized by infiltration of inflammatory cells. Inflammation was mainly interstitial and perivascular, associated with foci of cardiomyocyte necrosis and was mainly composed of CD68+ macrophages, a few CD45+ lymphocytes, and a few neutrophils and eosinophils.
Electron microscopy of cardiac tissue revealed spherical viral particles in shape and size consistent with the Coronaviridae family in the extracellular compartment and within cardiomyocytes, capillary endothelial cells, endocardium endothelial cells, macrophages, neutrophils, and fibroblasts.
Microthrombi in the pulmonary arterioles and renal glomerular capillaries were also seen at autopsy. SARS-CoV-2–associated pneumonia was mild.
Lymphoid depletion and signs of hemophagocytosis were observed in the spleen and lymph nodes. Acute tubular necrosis in the kidneys and hepatic centrilobular necrosis, secondary to shock, were also seen. Brain tissue showed microglial reactivity.
“Fortunately, MIS-C is a rare event and, although it can be severe and life threatening, most children recover,” Dr. Dolhnikoff commented.
“This case report comes at a time when the scientific community around the world calls attention to MIS-C and the need for it to be quickly recognized and treated by the pediatric community. Evidence of a direct relation between the virus and myocarditis confirms that MIS-C is one of the possible forms of presentation of COVID-19 and that the heart may be the target organ. It also alerts clinicians to possible cardiac sequelae in these children,” she added.
Experts weigh in
Scott Aydin, MD, medical director of pediatric cardiac intensive care, Mount Sinai Kravis Children’s Hospital in New York City, said that this case report is “unfortunately not all that surprising.
“Since the initial presentations of MIS-C several months ago, we have suspected mechanisms of direct and indirect injury to the myocardium. This important work is just the next step in further understanding the mechanisms of how COVID-19 creates havoc in the human body and the choices of possible therapies we have to treat children with COVID-19 and MIS-C,” said Dr. Aydin, who was not involved with the case report.
Anish Koka, MD, a cardiologist in private practice in Philadelphia, noted that, in these cases, endomyocardial biopsy is “rarely done because it is fairly invasive, but even when it has been done, the pathologic findings are of widespread inflammation rather than virus-induced cell necrosis.”
“While reports like this are sure to spawn viral tweets, it’s vital to understand that it’s not unusual to find widespread organ dissemination of virus in very sick patients. This does not mean that the virus is causing dysfunction of the organ it happens to be found in,” Dr. Koka said in an interview.
He noted that, in the case of the young girl who died, it took high PCR-cycle threshold values to isolate virus from the lung and heart samples.
“This means there was a low viral load in both organs, supporting the theory of SARS-CoV-2 as a potential trigger of a widespread inflammatory response that results in organ damage, rather than the virus itself infecting and destroying organs,” said Dr. Koka, who was also not associated with the case report.
This research had no specific funding. The authors declared no competing interests. Dr. Aydin disclosed no relevant financial relationships. Dr. Koka disclosed financial relationships with Boehringer Ingelheim and Jardiance.
This article first appeared on Medscape.com.
Pulmonary artery denervation eases PAH after endarterectomy
Pulmonary artery denervation (PADN) provides persistent and clinically significant hemodynamic improvements in patients with persistent chronic thromboembolic hypertension (CTEPH) after pulmonary endarterectomy (PEA), according to a randomized, sham-controlled trial.
“PADN in patients with CTEPH after PEA was safe and effective,” according to an investigating team led by Alexander Romanov, MD, PhD.
The mean reduction in pulmonary vascular resistance (PVR) was 258 dyn/sec per cm–5 for those randomized to PADN versus 149 dyn/sec per cm–5 (P = .001) for those randomized to the sham procedure, according to the newly published findings.
For the 6-minute walk test (6MWT), the mean distance was 470 m for the experimental group versus 399 m (P = .03) for the controls.
Several secondary endpoints measuring hemodynamics also favored PADN relative to the sham procedure at 12 months. This included the relative increase in tricuspid annular systolic excursion (P = .03) and the increase in the right ventricular fraction area (P < .001).
A total of 50 patients with residual CTEPH for at least 6 months after PEA despite medical therapy were enrolled and randomized. Entry criteria included a mean pulmonary artery pressure (PAP) of 25 mm Hg or greater or PVR greater than 400 dyn/sec per cm–5 on right heart catheterization. Patients with comorbidities associated with a life expectancy of less than 1 year were excluded.
Those randomized to the sham group were treated with riociguat over the course of follow-up. This therapy was not offered to patients in the PADN group, but all patients were blinded to the procedure and told that riociguat might or might not be administered.
Following the procedure, participating clinicians, who were also blinded to the procedure, were instructed to provide standard therapies for heart failure, such beta-blockers, diuretics, or digoxin, as needed. All patients were placed on an oral anticoagulant.
At 12 months the mean PAP (26 vs. 35 mm Hg; P < .001) and the mean systolic PAP (46 vs. 54 mm Hg; P = .01) were significantly lower in the PADN group versus those who underwent a sham procedure.
About 52% of the PADN group versus 12% of the sham group were classified as responders by the definition of a PVR reduction of at least 150 dyn/sec per cm–5 and 6MWT improvement of at least 20%, compared with baseline, reported Dr. Romanov, of the E. Meshalkin National Medical Research Center, ministry of health, Novosibirsk, Russia, and coinvestigators.
Of the three deaths caused by heart failure over the course of follow-up, two occurred in the sham group. Of the eight hospitalizations for heart failure, seven (29% of the sham group) occurred among controls versus one in those treated with PADN (4% of this group; P = .049).
There was one groin hematoma at the puncture site in each group. Both resolved without any consequences prior to hospital discharge. There were no other significant procedure-related complications in either group.
Larger multicenter trials are needed to confirm these findings, according to both the trial investigators and Marius M. Hoeper, MD, who is charge of the pulmonary hypertension program at the Hannover (Germany) Medical School.
In an editorial that accompanied publication of these findings, Dr. Hoeper identified the small sample size of this study as one of its limitations, but he said the results are consistent with several other small studies associating pulmonary artery denervation with benefit in pulmonary hypertension.
“It appears as if we are currently witnessing the emergence of a new treatment option for various forms of pulmonary hypertension,” Dr. Hoeper wrote. In his critique of the study, he suggested that it would have been “more informative” if both groups were on background riociguat, but the data from this and other studies so far indicates that ablation to achieve denervation “is safe and feasible.”
The PADN technique used in this study might be relevant to the results. Dr. Hoeper noted that the investigators employed catheter tip–based electroanatomic mapping with a novel remote navigation system with three-dimensional imaging of the right ventricle and central pulmonary arteries.
“Apparently, this approach minimizes radiation exposure and provides precise location of ablation sites,” Dr. Hoeper observed. However, he called for direct comparisons of this tool to the guidance systems used in other studies.
In an interview, Dr. Hoeper acknowledged that it is not yet clear that a large-scale trial of pulmonary artery denervation for the indication evaluated in this study is coming. He noted several strategies in CTEPH are widely used without trials confirming a reduction in clinical events.
“Balloon pulmonary angioplasty for CTEPH has become an established treatment around the world without any randomized, controlled trial and without demonstration of improved outcomes. A couple of well-conducted observational trials might be sufficient to convince physicians to introduce PADN as well,” he said. If such studies associated PADN with “improvements in hemodynamics, exercise capacity, and patient-reported outcomes, it might be sufficient.”
Currently, Dr. Hoeper is most concerned about obtaining further evidence of safety, which he characterized as a “major issue.”
If a multicenter trial is conducted “the primary endpoint should be focused on clinical events,” according to Dr. Romanov, who was asked to comment on the next steps in validating PADN for the treatment of CTEPH-associated pulmonary hypertension persisting after endarterectomy.
“The mortality rate during 1-year long-term follow-up is not so high, but heart failure progression is a problem. So in my view, the primary endpoint should be a composite of death and heart failure hospitalization,” he said. He called for follow-up duration of 2-3 years.
Jonathan Steinberg, MD, director of cardiac clinical trials and education, Summit Medical Group, Montclair, N.J., also called a trial with hard endpoints, such as death, the ideal.
In the meantime, hemodynamic and functional measures “are still quite valuable and move the ball forward for this intervention,” he said in an interview. Senior author of this trial and principle investigator of the recent ERADICATE-AF trial, which evaluated renal denervation in preventing recurrence of atrial fibrillation (JAMA. 2020;323:248-55), Dr. Steinberg predicted, “I do indeed suspect we will see trials that are more accomplishable [than a large-scale, randomized, controlled trial] in the not too distant future.”
Dr. Romanov received funding from Biosense Webster. Dr. Hoeper has received fees for lectures and/or consultations from Acceleron, Actelion, Bayer, Janssen, Merck Sharp & Dohme, and Pfizer.
SOURCE: Romanov A et al. J Am Coll Cardiol. 2020 Aug 17;76:916-26.
Pulmonary artery denervation (PADN) provides persistent and clinically significant hemodynamic improvements in patients with persistent chronic thromboembolic hypertension (CTEPH) after pulmonary endarterectomy (PEA), according to a randomized, sham-controlled trial.
“PADN in patients with CTEPH after PEA was safe and effective,” according to an investigating team led by Alexander Romanov, MD, PhD.
The mean reduction in pulmonary vascular resistance (PVR) was 258 dyn/sec per cm–5 for those randomized to PADN versus 149 dyn/sec per cm–5 (P = .001) for those randomized to the sham procedure, according to the newly published findings.
For the 6-minute walk test (6MWT), the mean distance was 470 m for the experimental group versus 399 m (P = .03) for the controls.
Several secondary endpoints measuring hemodynamics also favored PADN relative to the sham procedure at 12 months. This included the relative increase in tricuspid annular systolic excursion (P = .03) and the increase in the right ventricular fraction area (P < .001).
A total of 50 patients with residual CTEPH for at least 6 months after PEA despite medical therapy were enrolled and randomized. Entry criteria included a mean pulmonary artery pressure (PAP) of 25 mm Hg or greater or PVR greater than 400 dyn/sec per cm–5 on right heart catheterization. Patients with comorbidities associated with a life expectancy of less than 1 year were excluded.
Those randomized to the sham group were treated with riociguat over the course of follow-up. This therapy was not offered to patients in the PADN group, but all patients were blinded to the procedure and told that riociguat might or might not be administered.
Following the procedure, participating clinicians, who were also blinded to the procedure, were instructed to provide standard therapies for heart failure, such beta-blockers, diuretics, or digoxin, as needed. All patients were placed on an oral anticoagulant.
At 12 months the mean PAP (26 vs. 35 mm Hg; P < .001) and the mean systolic PAP (46 vs. 54 mm Hg; P = .01) were significantly lower in the PADN group versus those who underwent a sham procedure.
About 52% of the PADN group versus 12% of the sham group were classified as responders by the definition of a PVR reduction of at least 150 dyn/sec per cm–5 and 6MWT improvement of at least 20%, compared with baseline, reported Dr. Romanov, of the E. Meshalkin National Medical Research Center, ministry of health, Novosibirsk, Russia, and coinvestigators.
Of the three deaths caused by heart failure over the course of follow-up, two occurred in the sham group. Of the eight hospitalizations for heart failure, seven (29% of the sham group) occurred among controls versus one in those treated with PADN (4% of this group; P = .049).
There was one groin hematoma at the puncture site in each group. Both resolved without any consequences prior to hospital discharge. There were no other significant procedure-related complications in either group.
Larger multicenter trials are needed to confirm these findings, according to both the trial investigators and Marius M. Hoeper, MD, who is charge of the pulmonary hypertension program at the Hannover (Germany) Medical School.
In an editorial that accompanied publication of these findings, Dr. Hoeper identified the small sample size of this study as one of its limitations, but he said the results are consistent with several other small studies associating pulmonary artery denervation with benefit in pulmonary hypertension.
“It appears as if we are currently witnessing the emergence of a new treatment option for various forms of pulmonary hypertension,” Dr. Hoeper wrote. In his critique of the study, he suggested that it would have been “more informative” if both groups were on background riociguat, but the data from this and other studies so far indicates that ablation to achieve denervation “is safe and feasible.”
The PADN technique used in this study might be relevant to the results. Dr. Hoeper noted that the investigators employed catheter tip–based electroanatomic mapping with a novel remote navigation system with three-dimensional imaging of the right ventricle and central pulmonary arteries.
“Apparently, this approach minimizes radiation exposure and provides precise location of ablation sites,” Dr. Hoeper observed. However, he called for direct comparisons of this tool to the guidance systems used in other studies.
In an interview, Dr. Hoeper acknowledged that it is not yet clear that a large-scale trial of pulmonary artery denervation for the indication evaluated in this study is coming. He noted several strategies in CTEPH are widely used without trials confirming a reduction in clinical events.
“Balloon pulmonary angioplasty for CTEPH has become an established treatment around the world without any randomized, controlled trial and without demonstration of improved outcomes. A couple of well-conducted observational trials might be sufficient to convince physicians to introduce PADN as well,” he said. If such studies associated PADN with “improvements in hemodynamics, exercise capacity, and patient-reported outcomes, it might be sufficient.”
Currently, Dr. Hoeper is most concerned about obtaining further evidence of safety, which he characterized as a “major issue.”
If a multicenter trial is conducted “the primary endpoint should be focused on clinical events,” according to Dr. Romanov, who was asked to comment on the next steps in validating PADN for the treatment of CTEPH-associated pulmonary hypertension persisting after endarterectomy.
“The mortality rate during 1-year long-term follow-up is not so high, but heart failure progression is a problem. So in my view, the primary endpoint should be a composite of death and heart failure hospitalization,” he said. He called for follow-up duration of 2-3 years.
Jonathan Steinberg, MD, director of cardiac clinical trials and education, Summit Medical Group, Montclair, N.J., also called a trial with hard endpoints, such as death, the ideal.
In the meantime, hemodynamic and functional measures “are still quite valuable and move the ball forward for this intervention,” he said in an interview. Senior author of this trial and principle investigator of the recent ERADICATE-AF trial, which evaluated renal denervation in preventing recurrence of atrial fibrillation (JAMA. 2020;323:248-55), Dr. Steinberg predicted, “I do indeed suspect we will see trials that are more accomplishable [than a large-scale, randomized, controlled trial] in the not too distant future.”
Dr. Romanov received funding from Biosense Webster. Dr. Hoeper has received fees for lectures and/or consultations from Acceleron, Actelion, Bayer, Janssen, Merck Sharp & Dohme, and Pfizer.
SOURCE: Romanov A et al. J Am Coll Cardiol. 2020 Aug 17;76:916-26.
Pulmonary artery denervation (PADN) provides persistent and clinically significant hemodynamic improvements in patients with persistent chronic thromboembolic hypertension (CTEPH) after pulmonary endarterectomy (PEA), according to a randomized, sham-controlled trial.
“PADN in patients with CTEPH after PEA was safe and effective,” according to an investigating team led by Alexander Romanov, MD, PhD.
The mean reduction in pulmonary vascular resistance (PVR) was 258 dyn/sec per cm–5 for those randomized to PADN versus 149 dyn/sec per cm–5 (P = .001) for those randomized to the sham procedure, according to the newly published findings.
For the 6-minute walk test (6MWT), the mean distance was 470 m for the experimental group versus 399 m (P = .03) for the controls.
Several secondary endpoints measuring hemodynamics also favored PADN relative to the sham procedure at 12 months. This included the relative increase in tricuspid annular systolic excursion (P = .03) and the increase in the right ventricular fraction area (P < .001).
A total of 50 patients with residual CTEPH for at least 6 months after PEA despite medical therapy were enrolled and randomized. Entry criteria included a mean pulmonary artery pressure (PAP) of 25 mm Hg or greater or PVR greater than 400 dyn/sec per cm–5 on right heart catheterization. Patients with comorbidities associated with a life expectancy of less than 1 year were excluded.
Those randomized to the sham group were treated with riociguat over the course of follow-up. This therapy was not offered to patients in the PADN group, but all patients were blinded to the procedure and told that riociguat might or might not be administered.
Following the procedure, participating clinicians, who were also blinded to the procedure, were instructed to provide standard therapies for heart failure, such beta-blockers, diuretics, or digoxin, as needed. All patients were placed on an oral anticoagulant.
At 12 months the mean PAP (26 vs. 35 mm Hg; P < .001) and the mean systolic PAP (46 vs. 54 mm Hg; P = .01) were significantly lower in the PADN group versus those who underwent a sham procedure.
About 52% of the PADN group versus 12% of the sham group were classified as responders by the definition of a PVR reduction of at least 150 dyn/sec per cm–5 and 6MWT improvement of at least 20%, compared with baseline, reported Dr. Romanov, of the E. Meshalkin National Medical Research Center, ministry of health, Novosibirsk, Russia, and coinvestigators.
Of the three deaths caused by heart failure over the course of follow-up, two occurred in the sham group. Of the eight hospitalizations for heart failure, seven (29% of the sham group) occurred among controls versus one in those treated with PADN (4% of this group; P = .049).
There was one groin hematoma at the puncture site in each group. Both resolved without any consequences prior to hospital discharge. There were no other significant procedure-related complications in either group.
Larger multicenter trials are needed to confirm these findings, according to both the trial investigators and Marius M. Hoeper, MD, who is charge of the pulmonary hypertension program at the Hannover (Germany) Medical School.
In an editorial that accompanied publication of these findings, Dr. Hoeper identified the small sample size of this study as one of its limitations, but he said the results are consistent with several other small studies associating pulmonary artery denervation with benefit in pulmonary hypertension.
“It appears as if we are currently witnessing the emergence of a new treatment option for various forms of pulmonary hypertension,” Dr. Hoeper wrote. In his critique of the study, he suggested that it would have been “more informative” if both groups were on background riociguat, but the data from this and other studies so far indicates that ablation to achieve denervation “is safe and feasible.”
The PADN technique used in this study might be relevant to the results. Dr. Hoeper noted that the investigators employed catheter tip–based electroanatomic mapping with a novel remote navigation system with three-dimensional imaging of the right ventricle and central pulmonary arteries.
“Apparently, this approach minimizes radiation exposure and provides precise location of ablation sites,” Dr. Hoeper observed. However, he called for direct comparisons of this tool to the guidance systems used in other studies.
In an interview, Dr. Hoeper acknowledged that it is not yet clear that a large-scale trial of pulmonary artery denervation for the indication evaluated in this study is coming. He noted several strategies in CTEPH are widely used without trials confirming a reduction in clinical events.
“Balloon pulmonary angioplasty for CTEPH has become an established treatment around the world without any randomized, controlled trial and without demonstration of improved outcomes. A couple of well-conducted observational trials might be sufficient to convince physicians to introduce PADN as well,” he said. If such studies associated PADN with “improvements in hemodynamics, exercise capacity, and patient-reported outcomes, it might be sufficient.”
Currently, Dr. Hoeper is most concerned about obtaining further evidence of safety, which he characterized as a “major issue.”
If a multicenter trial is conducted “the primary endpoint should be focused on clinical events,” according to Dr. Romanov, who was asked to comment on the next steps in validating PADN for the treatment of CTEPH-associated pulmonary hypertension persisting after endarterectomy.
“The mortality rate during 1-year long-term follow-up is not so high, but heart failure progression is a problem. So in my view, the primary endpoint should be a composite of death and heart failure hospitalization,” he said. He called for follow-up duration of 2-3 years.
Jonathan Steinberg, MD, director of cardiac clinical trials and education, Summit Medical Group, Montclair, N.J., also called a trial with hard endpoints, such as death, the ideal.
In the meantime, hemodynamic and functional measures “are still quite valuable and move the ball forward for this intervention,” he said in an interview. Senior author of this trial and principle investigator of the recent ERADICATE-AF trial, which evaluated renal denervation in preventing recurrence of atrial fibrillation (JAMA. 2020;323:248-55), Dr. Steinberg predicted, “I do indeed suspect we will see trials that are more accomplishable [than a large-scale, randomized, controlled trial] in the not too distant future.”
Dr. Romanov received funding from Biosense Webster. Dr. Hoeper has received fees for lectures and/or consultations from Acceleron, Actelion, Bayer, Janssen, Merck Sharp & Dohme, and Pfizer.
SOURCE: Romanov A et al. J Am Coll Cardiol. 2020 Aug 17;76:916-26.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Machine learning shows ability to predict diastolic dysfunction with ECG
A machine-learning model that uses readily available clinical and electrocardiography data may have the potential to identify left ventricular (LV) diastolic dysfunction, a key biomarker in predicting heart failure, without echocardiography, but a workable clinical platform is still far off, a team of North American researchers reported.
“This cost-effective strategy may be a valuable first clinical step for assessing the presence of LV dysfunction and may potentially aid in the early diagnosis and management of heart failure patients,” Nobuyuki Kagiyama, MD, PhD, of West Virginia University, Morgantown, and colleagues, wrote in the Journal of the American Academy of Cardiology.
The researchers reported on a multicenter, prospective study that evaluated 1,202 patients from three centers in the United States and one in Canada. To develop machine-learning models, the study pooled 814 patients from the U.S. institutions as an internal cohort. They were then randomly divided into a training set and an internal test set on an 80:20 basis (651 and 163). The 388 Canadian patients were reserved as an external set to test the model.
All patients had 12-lead ECG and simultaneous body surface signal-processed ECG (spECG) along with comprehensive two-dimensional Doppler ECG on the same day.
How the model works
The machine-learning model estimated echocardiographic LV relaxation velocities (e’) values using traditional ECG and spECG features. The model also took into account 10 basic clinical features: age; sex; systolic and diastolic blood pressure; and comorbid conditions such as cerebrovascular and cardiovascular disease, diabetes, hypertension, dyslipidemia, and chronic kidney disease.
Patient characteristics were starkly different between the internal (United States) and external (Canadian) cohorts, with the latter being 10 years older on average (65 vs. 44; P < .001), predominantly male (58.2% vs. 47.3%; P < .001) and with significantly lower rates of coronary artery disease (1.8% vs. 21.1%; P < .001), although average blood pressure was similar between the two groups.
The study used area under the curve (AUC) to calculate the predictability of the machine-learning estimated e’ values versus the guideline-based reduced e’, finding close correlation between the internal (AUC, 0.83; sensitivity, 78%; specificity, 77%; negative predictive value, 73%; and positive predictive value, 82%) and external test sets (AUC, 0.84; sensitivity, 90%; specificity, 61%; NPV, 81%; and PPV, 77%).
Similar variations between the two cohorts were reported for global LV diastolic dysfunction and reduced LV ejection fraction.
The final model used 18 features in all, including 3 clinical features (age, dyslipidemia, and hypertension), 7 scores from spECG features, and 8 from traditional ECG features.
Interpreting the results
Dr. Kagiyama and colleagues noted that, because impaired myocardial relaxation is an early sign of cardiac tissue deterioration, screening for it can aid in early detection of subclinical LVDD and earlier treatment for hypertension and diabetes. But they acknowledged that further studies are needed.
In an invited editorial, Khurram Nasir, MD, MPH, MSc, of Houston Methodist DeBakey Heart and Vascular Center and Rohan Khera, MD, MS, of Yale University, New Haven, Conn., wrote that the machine-learning model has a way to go.
They noted that the 73%-77% accuracy of the model in identifying diastolic dysfunction impedes its imminent use. “Although we are excited about the prospects of such developments, we hold out for better evidence for their actual use,” they wrote, adding that the algorithms have limited use in the clinic because most patients already get “definitive testing” if they need it.
Developing a machine-learning model that obviates the need for ECG for evaluating LV diastolic dysfunction seems dubious at this time, said Luigi Di Biase, MD, PhD, section head of electrophysiology and director of arrhythmia services at Montefiore Medical Center and professor at Albert Einstein College of Medicine, both in New York. “The echo is not a difficult test. It’s the most proven usable tool that we have in cardiology because it’s easy to reproduce, low cost, and noninvasive – so we have all that we want in medicine.”
But machine learning does have potential, added Dr. Di Biase, who’s also a member of the American College of Cardiology’s Electrophysiology Section Leadership Council. “If this application could predict the people that would develop diastolic dysfunction that leads to heart failure – because an echo at that time may be negative but there may be other features that tell me this patient will develop disease – then it would have a much different clinical impact.”
The National Science Foundation provided funding for the study. Heart Test Laboratories, doing business as Heart Sciences, provided funding and spECG devices. Dr. Kagiyama reported receiving a research grant from Hitachi Healthcare. A coauthor disclosed financial relationships with Heart Sciences, Ultronics, and Kencor Health.
Dr. Nasir, Dr. Khera, and Dr. Di Biase have no relevant financial relationships to disclose.
SOURCE: Kagiyama N et al. J Am Coll Cardiol. 2020;76:930-41.
A machine-learning model that uses readily available clinical and electrocardiography data may have the potential to identify left ventricular (LV) diastolic dysfunction, a key biomarker in predicting heart failure, without echocardiography, but a workable clinical platform is still far off, a team of North American researchers reported.
“This cost-effective strategy may be a valuable first clinical step for assessing the presence of LV dysfunction and may potentially aid in the early diagnosis and management of heart failure patients,” Nobuyuki Kagiyama, MD, PhD, of West Virginia University, Morgantown, and colleagues, wrote in the Journal of the American Academy of Cardiology.
The researchers reported on a multicenter, prospective study that evaluated 1,202 patients from three centers in the United States and one in Canada. To develop machine-learning models, the study pooled 814 patients from the U.S. institutions as an internal cohort. They were then randomly divided into a training set and an internal test set on an 80:20 basis (651 and 163). The 388 Canadian patients were reserved as an external set to test the model.
All patients had 12-lead ECG and simultaneous body surface signal-processed ECG (spECG) along with comprehensive two-dimensional Doppler ECG on the same day.
How the model works
The machine-learning model estimated echocardiographic LV relaxation velocities (e’) values using traditional ECG and spECG features. The model also took into account 10 basic clinical features: age; sex; systolic and diastolic blood pressure; and comorbid conditions such as cerebrovascular and cardiovascular disease, diabetes, hypertension, dyslipidemia, and chronic kidney disease.
Patient characteristics were starkly different between the internal (United States) and external (Canadian) cohorts, with the latter being 10 years older on average (65 vs. 44; P < .001), predominantly male (58.2% vs. 47.3%; P < .001) and with significantly lower rates of coronary artery disease (1.8% vs. 21.1%; P < .001), although average blood pressure was similar between the two groups.
The study used area under the curve (AUC) to calculate the predictability of the machine-learning estimated e’ values versus the guideline-based reduced e’, finding close correlation between the internal (AUC, 0.83; sensitivity, 78%; specificity, 77%; negative predictive value, 73%; and positive predictive value, 82%) and external test sets (AUC, 0.84; sensitivity, 90%; specificity, 61%; NPV, 81%; and PPV, 77%).
Similar variations between the two cohorts were reported for global LV diastolic dysfunction and reduced LV ejection fraction.
The final model used 18 features in all, including 3 clinical features (age, dyslipidemia, and hypertension), 7 scores from spECG features, and 8 from traditional ECG features.
Interpreting the results
Dr. Kagiyama and colleagues noted that, because impaired myocardial relaxation is an early sign of cardiac tissue deterioration, screening for it can aid in early detection of subclinical LVDD and earlier treatment for hypertension and diabetes. But they acknowledged that further studies are needed.
In an invited editorial, Khurram Nasir, MD, MPH, MSc, of Houston Methodist DeBakey Heart and Vascular Center and Rohan Khera, MD, MS, of Yale University, New Haven, Conn., wrote that the machine-learning model has a way to go.
They noted that the 73%-77% accuracy of the model in identifying diastolic dysfunction impedes its imminent use. “Although we are excited about the prospects of such developments, we hold out for better evidence for their actual use,” they wrote, adding that the algorithms have limited use in the clinic because most patients already get “definitive testing” if they need it.
Developing a machine-learning model that obviates the need for ECG for evaluating LV diastolic dysfunction seems dubious at this time, said Luigi Di Biase, MD, PhD, section head of electrophysiology and director of arrhythmia services at Montefiore Medical Center and professor at Albert Einstein College of Medicine, both in New York. “The echo is not a difficult test. It’s the most proven usable tool that we have in cardiology because it’s easy to reproduce, low cost, and noninvasive – so we have all that we want in medicine.”
But machine learning does have potential, added Dr. Di Biase, who’s also a member of the American College of Cardiology’s Electrophysiology Section Leadership Council. “If this application could predict the people that would develop diastolic dysfunction that leads to heart failure – because an echo at that time may be negative but there may be other features that tell me this patient will develop disease – then it would have a much different clinical impact.”
The National Science Foundation provided funding for the study. Heart Test Laboratories, doing business as Heart Sciences, provided funding and spECG devices. Dr. Kagiyama reported receiving a research grant from Hitachi Healthcare. A coauthor disclosed financial relationships with Heart Sciences, Ultronics, and Kencor Health.
Dr. Nasir, Dr. Khera, and Dr. Di Biase have no relevant financial relationships to disclose.
SOURCE: Kagiyama N et al. J Am Coll Cardiol. 2020;76:930-41.
A machine-learning model that uses readily available clinical and electrocardiography data may have the potential to identify left ventricular (LV) diastolic dysfunction, a key biomarker in predicting heart failure, without echocardiography, but a workable clinical platform is still far off, a team of North American researchers reported.
“This cost-effective strategy may be a valuable first clinical step for assessing the presence of LV dysfunction and may potentially aid in the early diagnosis and management of heart failure patients,” Nobuyuki Kagiyama, MD, PhD, of West Virginia University, Morgantown, and colleagues, wrote in the Journal of the American Academy of Cardiology.
The researchers reported on a multicenter, prospective study that evaluated 1,202 patients from three centers in the United States and one in Canada. To develop machine-learning models, the study pooled 814 patients from the U.S. institutions as an internal cohort. They were then randomly divided into a training set and an internal test set on an 80:20 basis (651 and 163). The 388 Canadian patients were reserved as an external set to test the model.
All patients had 12-lead ECG and simultaneous body surface signal-processed ECG (spECG) along with comprehensive two-dimensional Doppler ECG on the same day.
How the model works
The machine-learning model estimated echocardiographic LV relaxation velocities (e’) values using traditional ECG and spECG features. The model also took into account 10 basic clinical features: age; sex; systolic and diastolic blood pressure; and comorbid conditions such as cerebrovascular and cardiovascular disease, diabetes, hypertension, dyslipidemia, and chronic kidney disease.
Patient characteristics were starkly different between the internal (United States) and external (Canadian) cohorts, with the latter being 10 years older on average (65 vs. 44; P < .001), predominantly male (58.2% vs. 47.3%; P < .001) and with significantly lower rates of coronary artery disease (1.8% vs. 21.1%; P < .001), although average blood pressure was similar between the two groups.
The study used area under the curve (AUC) to calculate the predictability of the machine-learning estimated e’ values versus the guideline-based reduced e’, finding close correlation between the internal (AUC, 0.83; sensitivity, 78%; specificity, 77%; negative predictive value, 73%; and positive predictive value, 82%) and external test sets (AUC, 0.84; sensitivity, 90%; specificity, 61%; NPV, 81%; and PPV, 77%).
Similar variations between the two cohorts were reported for global LV diastolic dysfunction and reduced LV ejection fraction.
The final model used 18 features in all, including 3 clinical features (age, dyslipidemia, and hypertension), 7 scores from spECG features, and 8 from traditional ECG features.
Interpreting the results
Dr. Kagiyama and colleagues noted that, because impaired myocardial relaxation is an early sign of cardiac tissue deterioration, screening for it can aid in early detection of subclinical LVDD and earlier treatment for hypertension and diabetes. But they acknowledged that further studies are needed.
In an invited editorial, Khurram Nasir, MD, MPH, MSc, of Houston Methodist DeBakey Heart and Vascular Center and Rohan Khera, MD, MS, of Yale University, New Haven, Conn., wrote that the machine-learning model has a way to go.
They noted that the 73%-77% accuracy of the model in identifying diastolic dysfunction impedes its imminent use. “Although we are excited about the prospects of such developments, we hold out for better evidence for their actual use,” they wrote, adding that the algorithms have limited use in the clinic because most patients already get “definitive testing” if they need it.
Developing a machine-learning model that obviates the need for ECG for evaluating LV diastolic dysfunction seems dubious at this time, said Luigi Di Biase, MD, PhD, section head of electrophysiology and director of arrhythmia services at Montefiore Medical Center and professor at Albert Einstein College of Medicine, both in New York. “The echo is not a difficult test. It’s the most proven usable tool that we have in cardiology because it’s easy to reproduce, low cost, and noninvasive – so we have all that we want in medicine.”
But machine learning does have potential, added Dr. Di Biase, who’s also a member of the American College of Cardiology’s Electrophysiology Section Leadership Council. “If this application could predict the people that would develop diastolic dysfunction that leads to heart failure – because an echo at that time may be negative but there may be other features that tell me this patient will develop disease – then it would have a much different clinical impact.”
The National Science Foundation provided funding for the study. Heart Test Laboratories, doing business as Heart Sciences, provided funding and spECG devices. Dr. Kagiyama reported receiving a research grant from Hitachi Healthcare. A coauthor disclosed financial relationships with Heart Sciences, Ultronics, and Kencor Health.
Dr. Nasir, Dr. Khera, and Dr. Di Biase have no relevant financial relationships to disclose.
SOURCE: Kagiyama N et al. J Am Coll Cardiol. 2020;76:930-41.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY














