User login
HIV does not appear to worsen COVID-19 outcomes
People living with HIV who are admitted to the hospital with COVID-19 are no more likely to die than those without HIV, an analysis conducted in New York City shows. This is despite the fact that comorbidities associated with worse COVID-19 outcomes were more common in the HIV group.
“We don’t see any signs that people with HIV should take extra precautions” to protect themselves from COVID-19, said Keith Sigel, MD, associate professor of medicine and infectious diseases at the Icahn School of Medicine at Mount Sinai, New York, and the lead researcher on the study, published online June 28 in Clinical Infectious Diseases.
“We still don’t have a great explanation for why we’re seeing what we’re seeing,” he added. “But we’re glad we’re seeing it.”
The findings have changed how Dr. Sigel talks to his patients with HIV about protecting themselves from COVID-19. Some patients have so curtailed their behavior for fear of acquiring COVID-19 that they aren’t buying groceries or attending needed medical appointments. With these data, Dr. Sigel said he’s comfortable telling his patients, “COVID-19 is bad all by itself, but you don’t need to go crazy. Wear a mask, practice appropriate social distancing and hygiene, but your risk doesn’t appear to be greater.”
The findings conform with those on the lack of association between HIV and COVID-19 severity seen in a cohort study from Spain, a case study from China, and case series from New Jersey, New York City, and Spain.
One of the only regions reporting something different so far is South Africa. There, HIV is the third most common comorbidity associated with death from COVID-19, according to a cohort analysis conducted in the province of Western Cape.
Along with data from HIV prevention and treatment trials, the conference will feature updates on where the world stands in the control of HIV during the COVID-19 pandemic. And for an even more focused look, the IAS COVID-19 Conference will immediately follow that meeting.
The New York City cohort
For their study, Dr. Sigel and colleagues examined the 4402 COVID-19 cases at the Mount Sinai Health System’s five hospitals between March 12 and April 23.
They found 88 people with COVID-19 whose charts showed codes indicating they were living with HIV. All 88 were receiving treatment, and 81% of them had undetectable viral loads documented at COVID admission or in the 12 months prior to admission.
The median age was 61 years, and 40% of the cohort was black and 30% was Hispanic.
Patients in the comparison group – 405 people without HIV from the Veterans Aging Cohort Study who had been admitted to the hospital for COVID-19 – were matched in terms of age, race, and stage of COVID-19.
The study had an 80% power to detect a 15% increase in the absolute risk for death in people with COVID-19, with or without HIV.
Patients with HIV were almost three times as likely to have smoked and were more likely to have chronic obstructive pulmonary disease, cirrhosis, and a history of cancer.
“This was a group of patients that one might suspect would do worse,” Dr. Sigel said. And yet, “we didn’t see any difference in deaths. We didn’t see any difference in respiratory failure.”
In fact, people with HIV required mechanical ventilation less often than those without HIV (18% vs. 23%). And when it came to mortality, one in five people died from COVID-19 during follow-up whether they had HIV or not (21% vs. 20%).
The only factor associated with significantly worse outcomes was a history of organ transplantation, “suggesting that non-HIV causes of immunodeficiency may be more prominent risks for severe outcomes,” Dr. Sigel and colleagues explained.
A surprise association
What’s more, the researchers found a slight association between the use of nucleoside reverse-transcriptase inhibitors (NRTI) by people with HIV and better outcomes in COVID-19. That echoes findings published June 26 in Annals of Internal Medicine, which showed that people with HIV taking the combination of tenofovir disoproxil fumarate plus emtricitabine (Truvada, Gilead Sciences) were less likely to be diagnosed with COVID-19, less likely to be hospitalized, and less likely to die.
This has led some to wonder whether NRTIs have some effect on SARS-CoV-2, the virus that causes COVID-19. Dr. Sigel said he wonders that too, but right now, it’s just musings.
“These studies are not even remotely designed” to show that NRTIs are protective against COVID-19, he explained. “Ours was extremely underpowered to detect that and there was a high potential for confounding.”
“I’d be wary of any study in a subpopulation – which is what we’re dealing with here – that is looking for signals of protection with certain medications,” he added.
A “modest” increase
Using the South African data, released on June 22, public health officials estimate that people with HIV are 2.75 times more likely to die from COVID-19 than those without HIV, making it the third most common comorbidity in people who died from COVID-19, behind diabetes and hypertension. This held true regardless of whether the people with HIV were on treatment.
But when they looked at COVID-19 deaths in the sickest of the sick – those hospitalized with COVID-19 symptoms – HIV was associated with just a 28% increase in the risk for death. The South African researchers called this risk “modest.”
“While these findings may overestimate the effect of HIV on COVID-19 death due to the presence of residual confounding, people living with HIV should be considered a high-risk group for COVID-19 management, with modestly elevated risk of poor outcomes, irrespective of viral suppression,” they wrote.
Epidemiologist Gregorio Millett, MPH, has been tracking the effect of HIV on COVID-19 outcomes since the start of the pandemic in his role as vice president and head of policy at the American Foundation for AIDS Research (amFAR).
Back in April, he and his colleagues looked at rates of COVID-19 deaths and hospitalizations in counties with disproportionate levels of black residents. These areas often overlapped with the communities selected for the Ending the HIV Epidemic plan to control HIV by 2030. What they found was that there was more HIV and COVID-19 in those communities.
What they didn’t find was that people with HIV in those communities had worse outcomes with COVID-19. This remained true even when they reran the analysis after the number of cases of COVID-19 in the United States surpassed 100,000. Those data have yet to be published, Mr. Millett reported.
“HIV does not pop out,” he said. “It’s still social determinants of health. It’s still underlying conditions. It’s still age as a primary factor.”
“People living with HIV are mainly dying of underlying conditions – so all the things associated with COVID-19 – rather than the association being with HIV itself,” he added.
Although he’s not ruling out the possibility that an association like the one in South Africa could emerge, Mr. Millett, who will present a plenary on the context of the HIV epidemic at the IAS conference, said he suspects we won’t see one.
“If we didn’t see an association with the counties that are disproportionately African American, in the black belt where we see high rates of HIV, particularly where we see the social determinants of health that definitely make a difference – if we’re not seeing that association there, where we have a high proportion of African Americans who are at risk both for HIV and COVID-19 – I just don’t think it’s going to emerge,” he said.
This article first appeared on Medscape.com.
People living with HIV who are admitted to the hospital with COVID-19 are no more likely to die than those without HIV, an analysis conducted in New York City shows. This is despite the fact that comorbidities associated with worse COVID-19 outcomes were more common in the HIV group.
“We don’t see any signs that people with HIV should take extra precautions” to protect themselves from COVID-19, said Keith Sigel, MD, associate professor of medicine and infectious diseases at the Icahn School of Medicine at Mount Sinai, New York, and the lead researcher on the study, published online June 28 in Clinical Infectious Diseases.
“We still don’t have a great explanation for why we’re seeing what we’re seeing,” he added. “But we’re glad we’re seeing it.”
The findings have changed how Dr. Sigel talks to his patients with HIV about protecting themselves from COVID-19. Some patients have so curtailed their behavior for fear of acquiring COVID-19 that they aren’t buying groceries or attending needed medical appointments. With these data, Dr. Sigel said he’s comfortable telling his patients, “COVID-19 is bad all by itself, but you don’t need to go crazy. Wear a mask, practice appropriate social distancing and hygiene, but your risk doesn’t appear to be greater.”
The findings conform with those on the lack of association between HIV and COVID-19 severity seen in a cohort study from Spain, a case study from China, and case series from New Jersey, New York City, and Spain.
One of the only regions reporting something different so far is South Africa. There, HIV is the third most common comorbidity associated with death from COVID-19, according to a cohort analysis conducted in the province of Western Cape.
Along with data from HIV prevention and treatment trials, the conference will feature updates on where the world stands in the control of HIV during the COVID-19 pandemic. And for an even more focused look, the IAS COVID-19 Conference will immediately follow that meeting.
The New York City cohort
For their study, Dr. Sigel and colleagues examined the 4402 COVID-19 cases at the Mount Sinai Health System’s five hospitals between March 12 and April 23.
They found 88 people with COVID-19 whose charts showed codes indicating they were living with HIV. All 88 were receiving treatment, and 81% of them had undetectable viral loads documented at COVID admission or in the 12 months prior to admission.
The median age was 61 years, and 40% of the cohort was black and 30% was Hispanic.
Patients in the comparison group – 405 people without HIV from the Veterans Aging Cohort Study who had been admitted to the hospital for COVID-19 – were matched in terms of age, race, and stage of COVID-19.
The study had an 80% power to detect a 15% increase in the absolute risk for death in people with COVID-19, with or without HIV.
Patients with HIV were almost three times as likely to have smoked and were more likely to have chronic obstructive pulmonary disease, cirrhosis, and a history of cancer.
“This was a group of patients that one might suspect would do worse,” Dr. Sigel said. And yet, “we didn’t see any difference in deaths. We didn’t see any difference in respiratory failure.”
In fact, people with HIV required mechanical ventilation less often than those without HIV (18% vs. 23%). And when it came to mortality, one in five people died from COVID-19 during follow-up whether they had HIV or not (21% vs. 20%).
The only factor associated with significantly worse outcomes was a history of organ transplantation, “suggesting that non-HIV causes of immunodeficiency may be more prominent risks for severe outcomes,” Dr. Sigel and colleagues explained.
A surprise association
What’s more, the researchers found a slight association between the use of nucleoside reverse-transcriptase inhibitors (NRTI) by people with HIV and better outcomes in COVID-19. That echoes findings published June 26 in Annals of Internal Medicine, which showed that people with HIV taking the combination of tenofovir disoproxil fumarate plus emtricitabine (Truvada, Gilead Sciences) were less likely to be diagnosed with COVID-19, less likely to be hospitalized, and less likely to die.
This has led some to wonder whether NRTIs have some effect on SARS-CoV-2, the virus that causes COVID-19. Dr. Sigel said he wonders that too, but right now, it’s just musings.
“These studies are not even remotely designed” to show that NRTIs are protective against COVID-19, he explained. “Ours was extremely underpowered to detect that and there was a high potential for confounding.”
“I’d be wary of any study in a subpopulation – which is what we’re dealing with here – that is looking for signals of protection with certain medications,” he added.
A “modest” increase
Using the South African data, released on June 22, public health officials estimate that people with HIV are 2.75 times more likely to die from COVID-19 than those without HIV, making it the third most common comorbidity in people who died from COVID-19, behind diabetes and hypertension. This held true regardless of whether the people with HIV were on treatment.
But when they looked at COVID-19 deaths in the sickest of the sick – those hospitalized with COVID-19 symptoms – HIV was associated with just a 28% increase in the risk for death. The South African researchers called this risk “modest.”
“While these findings may overestimate the effect of HIV on COVID-19 death due to the presence of residual confounding, people living with HIV should be considered a high-risk group for COVID-19 management, with modestly elevated risk of poor outcomes, irrespective of viral suppression,” they wrote.
Epidemiologist Gregorio Millett, MPH, has been tracking the effect of HIV on COVID-19 outcomes since the start of the pandemic in his role as vice president and head of policy at the American Foundation for AIDS Research (amFAR).
Back in April, he and his colleagues looked at rates of COVID-19 deaths and hospitalizations in counties with disproportionate levels of black residents. These areas often overlapped with the communities selected for the Ending the HIV Epidemic plan to control HIV by 2030. What they found was that there was more HIV and COVID-19 in those communities.
What they didn’t find was that people with HIV in those communities had worse outcomes with COVID-19. This remained true even when they reran the analysis after the number of cases of COVID-19 in the United States surpassed 100,000. Those data have yet to be published, Mr. Millett reported.
“HIV does not pop out,” he said. “It’s still social determinants of health. It’s still underlying conditions. It’s still age as a primary factor.”
“People living with HIV are mainly dying of underlying conditions – so all the things associated with COVID-19 – rather than the association being with HIV itself,” he added.
Although he’s not ruling out the possibility that an association like the one in South Africa could emerge, Mr. Millett, who will present a plenary on the context of the HIV epidemic at the IAS conference, said he suspects we won’t see one.
“If we didn’t see an association with the counties that are disproportionately African American, in the black belt where we see high rates of HIV, particularly where we see the social determinants of health that definitely make a difference – if we’re not seeing that association there, where we have a high proportion of African Americans who are at risk both for HIV and COVID-19 – I just don’t think it’s going to emerge,” he said.
This article first appeared on Medscape.com.
People living with HIV who are admitted to the hospital with COVID-19 are no more likely to die than those without HIV, an analysis conducted in New York City shows. This is despite the fact that comorbidities associated with worse COVID-19 outcomes were more common in the HIV group.
“We don’t see any signs that people with HIV should take extra precautions” to protect themselves from COVID-19, said Keith Sigel, MD, associate professor of medicine and infectious diseases at the Icahn School of Medicine at Mount Sinai, New York, and the lead researcher on the study, published online June 28 in Clinical Infectious Diseases.
“We still don’t have a great explanation for why we’re seeing what we’re seeing,” he added. “But we’re glad we’re seeing it.”
The findings have changed how Dr. Sigel talks to his patients with HIV about protecting themselves from COVID-19. Some patients have so curtailed their behavior for fear of acquiring COVID-19 that they aren’t buying groceries or attending needed medical appointments. With these data, Dr. Sigel said he’s comfortable telling his patients, “COVID-19 is bad all by itself, but you don’t need to go crazy. Wear a mask, practice appropriate social distancing and hygiene, but your risk doesn’t appear to be greater.”
The findings conform with those on the lack of association between HIV and COVID-19 severity seen in a cohort study from Spain, a case study from China, and case series from New Jersey, New York City, and Spain.
One of the only regions reporting something different so far is South Africa. There, HIV is the third most common comorbidity associated with death from COVID-19, according to a cohort analysis conducted in the province of Western Cape.
Along with data from HIV prevention and treatment trials, the conference will feature updates on where the world stands in the control of HIV during the COVID-19 pandemic. And for an even more focused look, the IAS COVID-19 Conference will immediately follow that meeting.
The New York City cohort
For their study, Dr. Sigel and colleagues examined the 4402 COVID-19 cases at the Mount Sinai Health System’s five hospitals between March 12 and April 23.
They found 88 people with COVID-19 whose charts showed codes indicating they were living with HIV. All 88 were receiving treatment, and 81% of them had undetectable viral loads documented at COVID admission or in the 12 months prior to admission.
The median age was 61 years, and 40% of the cohort was black and 30% was Hispanic.
Patients in the comparison group – 405 people without HIV from the Veterans Aging Cohort Study who had been admitted to the hospital for COVID-19 – were matched in terms of age, race, and stage of COVID-19.
The study had an 80% power to detect a 15% increase in the absolute risk for death in people with COVID-19, with or without HIV.
Patients with HIV were almost three times as likely to have smoked and were more likely to have chronic obstructive pulmonary disease, cirrhosis, and a history of cancer.
“This was a group of patients that one might suspect would do worse,” Dr. Sigel said. And yet, “we didn’t see any difference in deaths. We didn’t see any difference in respiratory failure.”
In fact, people with HIV required mechanical ventilation less often than those without HIV (18% vs. 23%). And when it came to mortality, one in five people died from COVID-19 during follow-up whether they had HIV or not (21% vs. 20%).
The only factor associated with significantly worse outcomes was a history of organ transplantation, “suggesting that non-HIV causes of immunodeficiency may be more prominent risks for severe outcomes,” Dr. Sigel and colleagues explained.
A surprise association
What’s more, the researchers found a slight association between the use of nucleoside reverse-transcriptase inhibitors (NRTI) by people with HIV and better outcomes in COVID-19. That echoes findings published June 26 in Annals of Internal Medicine, which showed that people with HIV taking the combination of tenofovir disoproxil fumarate plus emtricitabine (Truvada, Gilead Sciences) were less likely to be diagnosed with COVID-19, less likely to be hospitalized, and less likely to die.
This has led some to wonder whether NRTIs have some effect on SARS-CoV-2, the virus that causes COVID-19. Dr. Sigel said he wonders that too, but right now, it’s just musings.
“These studies are not even remotely designed” to show that NRTIs are protective against COVID-19, he explained. “Ours was extremely underpowered to detect that and there was a high potential for confounding.”
“I’d be wary of any study in a subpopulation – which is what we’re dealing with here – that is looking for signals of protection with certain medications,” he added.
A “modest” increase
Using the South African data, released on June 22, public health officials estimate that people with HIV are 2.75 times more likely to die from COVID-19 than those without HIV, making it the third most common comorbidity in people who died from COVID-19, behind diabetes and hypertension. This held true regardless of whether the people with HIV were on treatment.
But when they looked at COVID-19 deaths in the sickest of the sick – those hospitalized with COVID-19 symptoms – HIV was associated with just a 28% increase in the risk for death. The South African researchers called this risk “modest.”
“While these findings may overestimate the effect of HIV on COVID-19 death due to the presence of residual confounding, people living with HIV should be considered a high-risk group for COVID-19 management, with modestly elevated risk of poor outcomes, irrespective of viral suppression,” they wrote.
Epidemiologist Gregorio Millett, MPH, has been tracking the effect of HIV on COVID-19 outcomes since the start of the pandemic in his role as vice president and head of policy at the American Foundation for AIDS Research (amFAR).
Back in April, he and his colleagues looked at rates of COVID-19 deaths and hospitalizations in counties with disproportionate levels of black residents. These areas often overlapped with the communities selected for the Ending the HIV Epidemic plan to control HIV by 2030. What they found was that there was more HIV and COVID-19 in those communities.
What they didn’t find was that people with HIV in those communities had worse outcomes with COVID-19. This remained true even when they reran the analysis after the number of cases of COVID-19 in the United States surpassed 100,000. Those data have yet to be published, Mr. Millett reported.
“HIV does not pop out,” he said. “It’s still social determinants of health. It’s still underlying conditions. It’s still age as a primary factor.”
“People living with HIV are mainly dying of underlying conditions – so all the things associated with COVID-19 – rather than the association being with HIV itself,” he added.
Although he’s not ruling out the possibility that an association like the one in South Africa could emerge, Mr. Millett, who will present a plenary on the context of the HIV epidemic at the IAS conference, said he suspects we won’t see one.
“If we didn’t see an association with the counties that are disproportionately African American, in the black belt where we see high rates of HIV, particularly where we see the social determinants of health that definitely make a difference – if we’re not seeing that association there, where we have a high proportion of African Americans who are at risk both for HIV and COVID-19 – I just don’t think it’s going to emerge,” he said.
This article first appeared on Medscape.com.
FROM AIDS 2020
‘COVID-sorting’: How we decide whom to get close to and whom to avoid
I was recently interviewed, as a gay psychiatrist treating gay patients who lived through the AIDS epidemic, about my perspectives on living through a COVID pandemic: Were there parallels and contrasts between the two? A month later, listening to patients remotely via teletherapy, I’m experiencing an unsettling similarity to serosorting, a phenomenon that emerged during the AIDS epidemic.
Serosorting is the practice of choosing a sexual partner based on their HIV serostatus. Sorting out who was positive from who was negative allowed people to give themselves permission to have unprotected sex without risk of getting HIV. However, it was not uncommon to make those decisions without really knowing a potential partner’s actual serostatus. In fact, a lot of people serosorted by guessing.
Why not just ask a potential partner, “What’s your serostatus?” Apparently, for some, introducing the subject of HIV was deemed a sexual buzzkill. Instead, assumptions were made based on outer appearances.
Did someone look healthy? Were they well built? Were they overweight, meaning not emaciated from AIDS? If so, they were presumed negative and safe to have risky, unprotected sex with them.
Some imagined age correlated with serostatus. Since anyone older than some arbitrary age – like 30, to pull a number out of a hat – was expected to be more likely to have HIV than someone under 30, they would use that guideline in choosing sexual partners. However, these decisions were made without factual knowledge, like a blood test, but using some internal reasoning process.
Which brings us to what might be called “COVID-sorting.”
Some of my patients believe they had COVID-19, although they’d not been tested to either confirm or disprove that belief. Others had positive COVID-19 antibody tests, which they believe provides immunity. Among that group, some had symptoms, others did not.
Yet regardless of what they actually know or don’t know, patients are making calculations about managing physical distancing using their own internal formulas. They make risk calculations having little to do with actual knowledge of public health precautions on preventing COVID’s spread.
For example, one patient was planning a Memorial Day weekend in a shared Fire Island house with five friends and acquaintances. All six live alone and, as far as he knows, all are physically distancing. Consequently, my patient doesn’t think house-sharing is anything to worry about, even though he doesn’t know how scrupulously others have followed distancing guidelines.
Another patient, recovering at home after being ill with COVID-19, felt safe inviting someone over for sex who had also been ill and recovered. He didn’t think they could infect each other, presuming, not altogether unreasonably, they were both immune.
Finally, there are those who don’t know whether they had COVID-19, but think they did because they experienced influenza-like symptoms. They are giving themselves permission to meet up with others who feel the same.
Yet a Mount Sinai study, which has not yet been peer-reviewed, raises fascinating issues about immunity. The study included 719 people who suspected they had COVID-19 based on some respiratory symptoms. The majority, 62%, had no antibodies. Researchers believe they mistook influenza, another viral infection, or allergies for COVID-19 (medRxiv. 2020 May 5. doi: 10.1101/2020.04.04.2008516).
The study also included 624 people who tested positive for the virus and recovered. All but three developed antibodies. Many assume those who are antibody-positive are now immune. They may be right. However, we don’t know definitively that they are, and if they are, we do not yet know how long immunity may last. Further, as reported in the New York Times, just because you test positive for antibodies, doesn’t mean you have them.
It should be underscored that COVID-sorting is not limited to gay men or psychiatric patients. And as many states have begun opening up restrictions on social gatherings, we are seeing an all-too-human psychological mindset with wider implications – rising numbers of cases. As we move forward, all of us will have to decide for ourselves, and not only in sexual situations, how to get on with our lives in a post–COVID-19 era.
Given how much is still unknown, it is likely each of us will come up with our own algorithm of risk assessment. It is likely that the formulas used will not necessarily be based on scientific facts, although that would be ideal. If past epidemic and recent pandemic behaviors are any indicators, people’s actions will reflect some combination of their own needs and desires, their own comfort level with risk-taking, and their relative understanding of complex subjects like virology, immunology, epidemiology, and public health. The challenge faced by public health officials today is to translate complex scientific and medical issues into messages average people can understand.
What exactly can be done? I’m not exactly sure, but I hope that improved education and communication can help. In the first 2 decades of the AIDS epidemic, efforts were made to change and tailor HIV-prevention messages to specific, at-risk demographic groups. Today, public health messages aimed at preventing COVID-19’s spread that resonate with older people can fall on a younger person’s deaf ears. One message size does not fit all. Hopefully, public health officials and government leaders will act on this sooner rather than later.
Dr. Drescher, a psychoanalyst, is clinical professor of psychiatry at Columbia University, and training and supervising analyst at the William A. White Institute, both in New York. He also is emeritus editor of the Journal of Gay & Lesbian Mental Health. Dr. Drescher has no other disclosures.
I was recently interviewed, as a gay psychiatrist treating gay patients who lived through the AIDS epidemic, about my perspectives on living through a COVID pandemic: Were there parallels and contrasts between the two? A month later, listening to patients remotely via teletherapy, I’m experiencing an unsettling similarity to serosorting, a phenomenon that emerged during the AIDS epidemic.
Serosorting is the practice of choosing a sexual partner based on their HIV serostatus. Sorting out who was positive from who was negative allowed people to give themselves permission to have unprotected sex without risk of getting HIV. However, it was not uncommon to make those decisions without really knowing a potential partner’s actual serostatus. In fact, a lot of people serosorted by guessing.
Why not just ask a potential partner, “What’s your serostatus?” Apparently, for some, introducing the subject of HIV was deemed a sexual buzzkill. Instead, assumptions were made based on outer appearances.
Did someone look healthy? Were they well built? Were they overweight, meaning not emaciated from AIDS? If so, they were presumed negative and safe to have risky, unprotected sex with them.
Some imagined age correlated with serostatus. Since anyone older than some arbitrary age – like 30, to pull a number out of a hat – was expected to be more likely to have HIV than someone under 30, they would use that guideline in choosing sexual partners. However, these decisions were made without factual knowledge, like a blood test, but using some internal reasoning process.
Which brings us to what might be called “COVID-sorting.”
Some of my patients believe they had COVID-19, although they’d not been tested to either confirm or disprove that belief. Others had positive COVID-19 antibody tests, which they believe provides immunity. Among that group, some had symptoms, others did not.
Yet regardless of what they actually know or don’t know, patients are making calculations about managing physical distancing using their own internal formulas. They make risk calculations having little to do with actual knowledge of public health precautions on preventing COVID’s spread.
For example, one patient was planning a Memorial Day weekend in a shared Fire Island house with five friends and acquaintances. All six live alone and, as far as he knows, all are physically distancing. Consequently, my patient doesn’t think house-sharing is anything to worry about, even though he doesn’t know how scrupulously others have followed distancing guidelines.
Another patient, recovering at home after being ill with COVID-19, felt safe inviting someone over for sex who had also been ill and recovered. He didn’t think they could infect each other, presuming, not altogether unreasonably, they were both immune.
Finally, there are those who don’t know whether they had COVID-19, but think they did because they experienced influenza-like symptoms. They are giving themselves permission to meet up with others who feel the same.
Yet a Mount Sinai study, which has not yet been peer-reviewed, raises fascinating issues about immunity. The study included 719 people who suspected they had COVID-19 based on some respiratory symptoms. The majority, 62%, had no antibodies. Researchers believe they mistook influenza, another viral infection, or allergies for COVID-19 (medRxiv. 2020 May 5. doi: 10.1101/2020.04.04.2008516).
The study also included 624 people who tested positive for the virus and recovered. All but three developed antibodies. Many assume those who are antibody-positive are now immune. They may be right. However, we don’t know definitively that they are, and if they are, we do not yet know how long immunity may last. Further, as reported in the New York Times, just because you test positive for antibodies, doesn’t mean you have them.
It should be underscored that COVID-sorting is not limited to gay men or psychiatric patients. And as many states have begun opening up restrictions on social gatherings, we are seeing an all-too-human psychological mindset with wider implications – rising numbers of cases. As we move forward, all of us will have to decide for ourselves, and not only in sexual situations, how to get on with our lives in a post–COVID-19 era.
Given how much is still unknown, it is likely each of us will come up with our own algorithm of risk assessment. It is likely that the formulas used will not necessarily be based on scientific facts, although that would be ideal. If past epidemic and recent pandemic behaviors are any indicators, people’s actions will reflect some combination of their own needs and desires, their own comfort level with risk-taking, and their relative understanding of complex subjects like virology, immunology, epidemiology, and public health. The challenge faced by public health officials today is to translate complex scientific and medical issues into messages average people can understand.
What exactly can be done? I’m not exactly sure, but I hope that improved education and communication can help. In the first 2 decades of the AIDS epidemic, efforts were made to change and tailor HIV-prevention messages to specific, at-risk demographic groups. Today, public health messages aimed at preventing COVID-19’s spread that resonate with older people can fall on a younger person’s deaf ears. One message size does not fit all. Hopefully, public health officials and government leaders will act on this sooner rather than later.
Dr. Drescher, a psychoanalyst, is clinical professor of psychiatry at Columbia University, and training and supervising analyst at the William A. White Institute, both in New York. He also is emeritus editor of the Journal of Gay & Lesbian Mental Health. Dr. Drescher has no other disclosures.
I was recently interviewed, as a gay psychiatrist treating gay patients who lived through the AIDS epidemic, about my perspectives on living through a COVID pandemic: Were there parallels and contrasts between the two? A month later, listening to patients remotely via teletherapy, I’m experiencing an unsettling similarity to serosorting, a phenomenon that emerged during the AIDS epidemic.
Serosorting is the practice of choosing a sexual partner based on their HIV serostatus. Sorting out who was positive from who was negative allowed people to give themselves permission to have unprotected sex without risk of getting HIV. However, it was not uncommon to make those decisions without really knowing a potential partner’s actual serostatus. In fact, a lot of people serosorted by guessing.
Why not just ask a potential partner, “What’s your serostatus?” Apparently, for some, introducing the subject of HIV was deemed a sexual buzzkill. Instead, assumptions were made based on outer appearances.
Did someone look healthy? Were they well built? Were they overweight, meaning not emaciated from AIDS? If so, they were presumed negative and safe to have risky, unprotected sex with them.
Some imagined age correlated with serostatus. Since anyone older than some arbitrary age – like 30, to pull a number out of a hat – was expected to be more likely to have HIV than someone under 30, they would use that guideline in choosing sexual partners. However, these decisions were made without factual knowledge, like a blood test, but using some internal reasoning process.
Which brings us to what might be called “COVID-sorting.”
Some of my patients believe they had COVID-19, although they’d not been tested to either confirm or disprove that belief. Others had positive COVID-19 antibody tests, which they believe provides immunity. Among that group, some had symptoms, others did not.
Yet regardless of what they actually know or don’t know, patients are making calculations about managing physical distancing using their own internal formulas. They make risk calculations having little to do with actual knowledge of public health precautions on preventing COVID’s spread.
For example, one patient was planning a Memorial Day weekend in a shared Fire Island house with five friends and acquaintances. All six live alone and, as far as he knows, all are physically distancing. Consequently, my patient doesn’t think house-sharing is anything to worry about, even though he doesn’t know how scrupulously others have followed distancing guidelines.
Another patient, recovering at home after being ill with COVID-19, felt safe inviting someone over for sex who had also been ill and recovered. He didn’t think they could infect each other, presuming, not altogether unreasonably, they were both immune.
Finally, there are those who don’t know whether they had COVID-19, but think they did because they experienced influenza-like symptoms. They are giving themselves permission to meet up with others who feel the same.
Yet a Mount Sinai study, which has not yet been peer-reviewed, raises fascinating issues about immunity. The study included 719 people who suspected they had COVID-19 based on some respiratory symptoms. The majority, 62%, had no antibodies. Researchers believe they mistook influenza, another viral infection, or allergies for COVID-19 (medRxiv. 2020 May 5. doi: 10.1101/2020.04.04.2008516).
The study also included 624 people who tested positive for the virus and recovered. All but three developed antibodies. Many assume those who are antibody-positive are now immune. They may be right. However, we don’t know definitively that they are, and if they are, we do not yet know how long immunity may last. Further, as reported in the New York Times, just because you test positive for antibodies, doesn’t mean you have them.
It should be underscored that COVID-sorting is not limited to gay men or psychiatric patients. And as many states have begun opening up restrictions on social gatherings, we are seeing an all-too-human psychological mindset with wider implications – rising numbers of cases. As we move forward, all of us will have to decide for ourselves, and not only in sexual situations, how to get on with our lives in a post–COVID-19 era.
Given how much is still unknown, it is likely each of us will come up with our own algorithm of risk assessment. It is likely that the formulas used will not necessarily be based on scientific facts, although that would be ideal. If past epidemic and recent pandemic behaviors are any indicators, people’s actions will reflect some combination of their own needs and desires, their own comfort level with risk-taking, and their relative understanding of complex subjects like virology, immunology, epidemiology, and public health. The challenge faced by public health officials today is to translate complex scientific and medical issues into messages average people can understand.
What exactly can be done? I’m not exactly sure, but I hope that improved education and communication can help. In the first 2 decades of the AIDS epidemic, efforts were made to change and tailor HIV-prevention messages to specific, at-risk demographic groups. Today, public health messages aimed at preventing COVID-19’s spread that resonate with older people can fall on a younger person’s deaf ears. One message size does not fit all. Hopefully, public health officials and government leaders will act on this sooner rather than later.
Dr. Drescher, a psychoanalyst, is clinical professor of psychiatry at Columbia University, and training and supervising analyst at the William A. White Institute, both in New York. He also is emeritus editor of the Journal of Gay & Lesbian Mental Health. Dr. Drescher has no other disclosures.
ID dermatology: Advancements, but new challenges, over 50 years
When Stephen Tyring, MD, PhD, an infectious disease dermatologist, started his career in the early 1980s, he said “we were diagnosing Kaposi’s sarcoma right and left. We would see a new case every day or two.”
It was the early days of the HIV/AIDS epidemic, and dermatologists were at the forefront because HIV/AIDS often presented with skin manifestations. Dr. Tyring, clinical professor in the departments of dermatology, microbiology & molecular genetics and internal medicine at the University of Texas Health Science Center, Houston, and his colleagues referred Kaposi’s patients for chemotherapy and radiation, but the outlook was often grim, especially if lesions developed in the lungs.
Dermatologist don’t see much Kaposi’s anymore because of highly effective treatments for HIV.
Members of the original editorial advisory board saw it coming. In a feature in which board members provided their prediction for the 1970s that appeared in the first issue, New York dermatologist Norman Orentreich, MD, counted the “probable introduction of virucidal agents” as one of the “significant advances or changes that I foresee in the next 10 years.” J. Lamar Callaway, MD, professor of dermatology at Duke University, Durham, N.C., predicted that “the next 10 years should develop effective anti-viral agents for warts, herpes simplex, and herpes zoster.”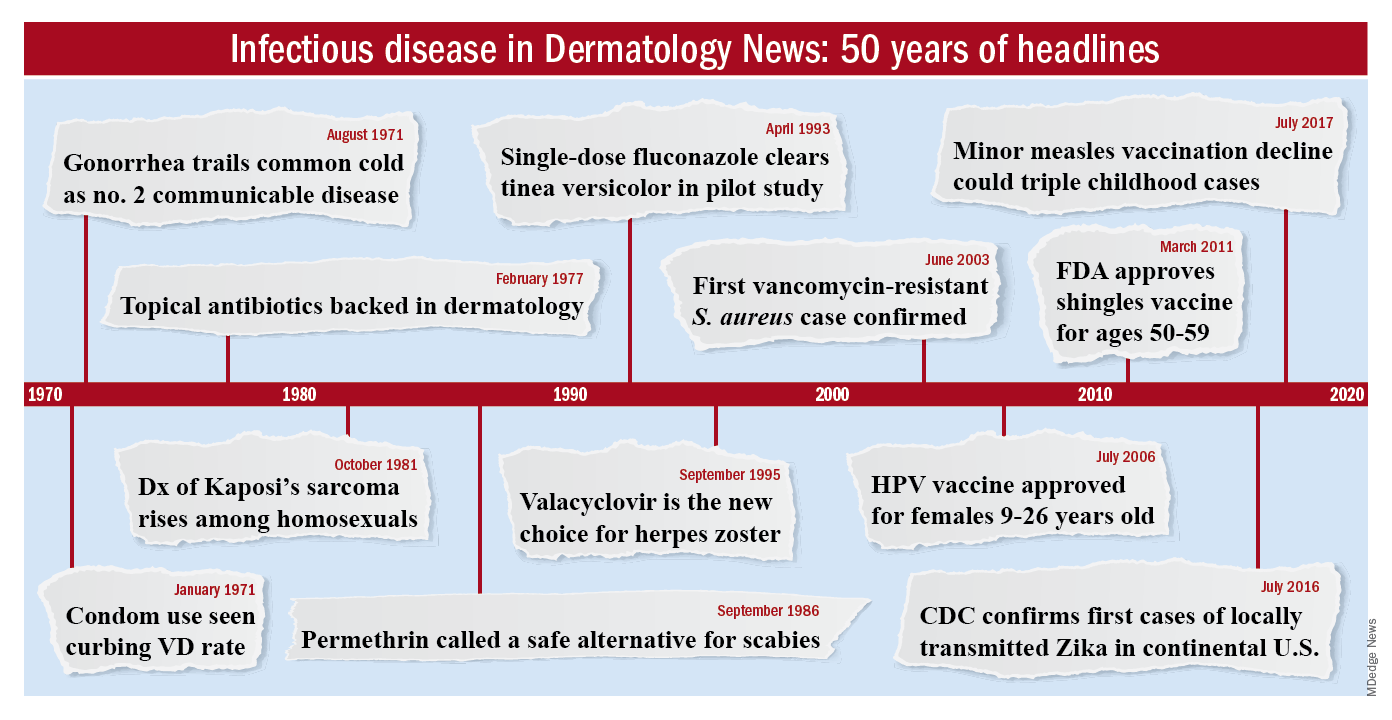
To celebrate the 50th anniversary of Dermatology News, we are looking back at how the field has changed since that first issue. The focus this month is infectious disease. There’s a lot to be grateful for but there are also challenges like antibiotic resistance that weren’t on the radar screens of Dr. Orentreich, Dr. Callaway, and their peers in 1970.
All in all, “the only thing I wish we did the old way is sit at the bedside and talk to patients more. We rely so much on technology now that we sometimes lose the art of medicine, which is comforting to the patient,” said Theodore Rosen, MD, an ID dermatologist and professor of dermatology at Baylor College of Medicine, Houston, who’s been in practice for 42 years.
“A lot of advancements against herpes viruses”
One of the biggest wins for ID dermatology over the last 5 decades has been the management of herpes, both herpes simplex virus 1 and 2, as well as herpes zoster virus. It started with the approval of acyclovir in 1981. Before then, “we had no direct therapy for genital herpes, herpes zoster, or disseminated herpes in immunosuppressed or cancer patients,” Dr. Rosen said.
“I can remember doing an interview with Good Morning America when I gave the first IV dose of acyclovir in the city of Houston for really bad disseminated herpes” in an HIV patient, he said, and it worked.
Two derivatives, valacyclovir and famciclovir, became available in the mid-1990s, so today “we have three drugs and some others at the periphery that are all highly effective not only” against herpes, but also for preventing outbreaks; valacyclovir can even prevent asymptomatic shedding, therefore possibly preventing new infections. “That’s a concept we didn’t even have 40 years ago,” Dr. Rosen said.
Cidofovir has also made a difference. The IV formulation was approved for AIDS-associated cytomegalovirus retinitis in 1996 but discontinued a few years later amid concerns of severe renal toxicity. It’s found a new home in dermatology since then, explained ID dermatologist Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
Dermatologists see acyclovir-resistant herpes “heaped up on the genitals in HIV patients,” and there weren’t many options in the past. A few years ago, “we [tried] injecting cidofovir directly into the skin lesions, and it’s been remarkably successful. It is a good way to treat these lesions” if dermatologists can get it compounded, she said.
Shingles vaccines, first the live attenuated zoster vaccine (Zostavax) approved by the Food and Drug Administration in 2006 and the more effective recombinant zoster vaccine (Shingrix) approved in 2017, have also had a significant impact.
Dr. Rosen remembers what it was like when he first started practicing over 40 years ago. Not uncommonly, “we saw horrible cases of shingles,” including one in his uncle, who was left with permanent hand pain long after the rash subsided.
Today, “I see much less shingles, and when I do see it, it’s in a much-attenuated form. [Shingrix], even if it doesn’t prevent the disease, often prevents postherpetic neuralgia,” he said.
Also, with pediatric vaccinations against chicken pox, “we’re probably going to see a whole new generation without shingles, which is huge. We’ve made a lot of advancements against herpes viruses,” Dr. Kovarik said.
“We finally found something that helps”
“We’ve [also] come a really long way with genital wart treatment,” Dr. Kovarik said.
It started with approval of topical imiquimod in 1997. “Before that, we were just killing one wart here and one wart there” but they would often come back and pop up in other areas. Injectable interferon was an option at the time, but people didn’t like all the needles.
With imiquimod, “we finally [had] a way to target HPV [human papillomavirus] and not just scrape” or freeze one wart at a time, and “we were able to generate an inflammatory response in the whole area to clear the virus.” Working with HIV patients, “I see sheets and sheets of confluent warts throughout the whole genital area; to try to freeze that is impossible. Now I have a way to get rid of [genital] warts and keep them away even if you have a big cluster,” she said.
“Sometimes, we’ll do both liquid nitrogen and imiquimod. That’s a good way to tackle people who have a high burden of warts,” Dr. Kovarik noted. Other effective treatments have come out as well, including an ointment formulation of sinecatechins, extracted from green tea, “but you have to put it on several times a day, and insurance companies don’t cover it often,” she said.
Intralesional cidofovir is also proving to be boon for potentially malignant refractory warts in HIV and transplant patients. “It’s an incredible treatment. We can inject that antiviral into warts and get rid of them. We finally found something that helps” these people, Dr. Kovarik said.
The HPV vaccine Gardasil is making a difference, as well. In addition to cervical dysplasia and anogenital cancers, it protects against two condyloma strains. Dr. Rosen said he’s seeing fewer cases of genital warts now than when he started practicing, likely because of the vaccine.
“Organisms that weren’t pathogens are now pathogens”
Antibiotic resistance probably tops the list for what’s changed in a bad way in ID dermatology since 1970. Dr. Rosen remembers at the start of his career that “we never worried about antibiotic resistance. We’d put people on antibiotics for acne, rosacea, and we’d keep them on them for 3 years, 6 years”; resistance wasn’t on the radar screen and was not mentioned once in the first issue of Dermatology News, which was packed with articles and ran 24 pages.
The situation is different now. Driven by decades of overuse in agriculture and the medical system, antibiotic resistance is a concern throughout medicine, and unfortunately, “we have not come nearly as far as fast with antibiotics,” at least the ones dermatologists use, “as we have with antivirals,” Dr. Tyring said.
For instance, methicillin-resistant Staphylococcus aureus (MRSA), first described in the United States in 1968, is “no longer the exception to the rule, but the rule” itself, he said, with carbuncles, furuncles, and abscesses not infrequently growing out MRSA. There are also new drug-resistant forms of old problems like gonorrhea and tuberculosis, among other developments, and impetigo has shifted since 1970 from mostly a Streptococcus infection easily treated with penicillin to often a Staphylococcus disease that’s resistant to it. There’s also been a steady march of new pathogens, including the latest one, SARS-CoV-2, the virus that causes COVID-19, which has been recognized as having a variety of skin manifestations.
“No matter how smart we think we are, nature has a way of putting us back in our place,” Dr. Rosen said.
The bright spot is that “we’ve become very adept at identifying and characterizing” microbes “based on techniques we didn’t even have when I started practicing,” such as polymerase chain reaction. “It has taken a lot of guess work out of treating infectious diseases,” he said.
The widespread use of immunosuppressives such as cyclophosphamide, mycophenolate, azathioprine, rituximab, and other agents used in conjunction with solid organ transplantation, has also been a challenge. “We are seeing infections with really odd organisms. Just recently, I had a patient with fusarium in the skin; it’s a fungus that lives in the dirt. I saw a patient with a species of algae” that normally lives in stagnant water, he commented. “We used to get [things like that] back on reports, and we’d throw them away. You can’t do that anymore. Organisms that weren’t pathogens in the past are now pathogens,” particularly in immunosuppressed people, Dr. Rosen said.
Venereologists no more
There’s been another big change in the field. “Back in the not too distant past, dermatologists in the U.S. were referred to as ‘dermatologist-venereologists.’ ” It goes back to the time when syphilis wasn’t diagnosed and treated early, so patients often presented with secondary skin complications and went to dermatologists for help. As a result, “dermatologists became the most experienced at treating it,” Dr. Tyring said.
That’s faded from practice. Part of the reason is that as late as 2000, syphilis seemed to be on the way out; the Centers for Disease and Control and Prevention even raised the possibility of elimination. Dermatologists turned their attention to other areas.
It might have been short-sighted, Dr. Rosen said. Syphilis has made a strong comeback, and drug-resistant gonorrhea has also emerged globally and in at least a few states. No other medical field has stepped in to take up the slack. “Ob.gyns. are busy delivering babies, ID [physicians are] concerned about HIV, and urologists are worried about kidney stones and cancer.” Other than herpes and genital warts, “we have not done well” with management of sexually transmitted diseases, he said.
“I could sense” his frustration
The first issue of Dermatology News carried an article and photospread about scabies that could run today, except that topical permethrin and oral ivermectin have largely replaced benzyl benzoate and sulfur ointments for treatment in the United States. In the article, Scottish dermatologist J. O’D. Alexander, MD, called scabies “the scourge of mankind” and blamed it’s prevalence on “an offhand attitude to the disease which makes control very difficult.”
“I could sense this man’s frustration that people were not recognizing scabies,” Dr. Kovarik said, and it’s no closer to being eradicated than it was in 1970. “It’s still around, and we see it in our clinics. It’s a horrible disease in kids we see in dermatology not infrequently,” and treatment has only advanced a bit.
The article highlights what hasn’t changed much in ID dermatology over the years. Common warts are another one. “With all the evolution in medicine, we don’t have any better treatments approved for common warts than we ever had.” Injecting cidofovir “works great,” but access is a problem, Dr. Tyring said.
Onychomycosis has also proven a tough nut to crack. Readers back in 1970 counted the introduction of the antifungal, griseofulvin, as a major advancement in the 1960s; it’s still a go-to for tinea capitis, but it didn’t work very well for toenail fungus. Terbinafine (Lamisil), approved in 1993, and subsequent developments have helped, but the field still awaits more effective options; a few potential new agents are in the pipeline.
Although there have been major advancements for serious systemic fungal infections, “we’ve mainly seen small steps forward” in ID dermatology, Dr. Tyring said.
Dr. Tyring, Dr. Kovarik, and Dr. Rosen said they had no relevant disclosures.
When Stephen Tyring, MD, PhD, an infectious disease dermatologist, started his career in the early 1980s, he said “we were diagnosing Kaposi’s sarcoma right and left. We would see a new case every day or two.”
It was the early days of the HIV/AIDS epidemic, and dermatologists were at the forefront because HIV/AIDS often presented with skin manifestations. Dr. Tyring, clinical professor in the departments of dermatology, microbiology & molecular genetics and internal medicine at the University of Texas Health Science Center, Houston, and his colleagues referred Kaposi’s patients for chemotherapy and radiation, but the outlook was often grim, especially if lesions developed in the lungs.
Dermatologist don’t see much Kaposi’s anymore because of highly effective treatments for HIV.
Members of the original editorial advisory board saw it coming. In a feature in which board members provided their prediction for the 1970s that appeared in the first issue, New York dermatologist Norman Orentreich, MD, counted the “probable introduction of virucidal agents” as one of the “significant advances or changes that I foresee in the next 10 years.” J. Lamar Callaway, MD, professor of dermatology at Duke University, Durham, N.C., predicted that “the next 10 years should develop effective anti-viral agents for warts, herpes simplex, and herpes zoster.”
To celebrate the 50th anniversary of Dermatology News, we are looking back at how the field has changed since that first issue. The focus this month is infectious disease. There’s a lot to be grateful for but there are also challenges like antibiotic resistance that weren’t on the radar screens of Dr. Orentreich, Dr. Callaway, and their peers in 1970.
All in all, “the only thing I wish we did the old way is sit at the bedside and talk to patients more. We rely so much on technology now that we sometimes lose the art of medicine, which is comforting to the patient,” said Theodore Rosen, MD, an ID dermatologist and professor of dermatology at Baylor College of Medicine, Houston, who’s been in practice for 42 years.
“A lot of advancements against herpes viruses”
One of the biggest wins for ID dermatology over the last 5 decades has been the management of herpes, both herpes simplex virus 1 and 2, as well as herpes zoster virus. It started with the approval of acyclovir in 1981. Before then, “we had no direct therapy for genital herpes, herpes zoster, or disseminated herpes in immunosuppressed or cancer patients,” Dr. Rosen said.
“I can remember doing an interview with Good Morning America when I gave the first IV dose of acyclovir in the city of Houston for really bad disseminated herpes” in an HIV patient, he said, and it worked.
Two derivatives, valacyclovir and famciclovir, became available in the mid-1990s, so today “we have three drugs and some others at the periphery that are all highly effective not only” against herpes, but also for preventing outbreaks; valacyclovir can even prevent asymptomatic shedding, therefore possibly preventing new infections. “That’s a concept we didn’t even have 40 years ago,” Dr. Rosen said.
Cidofovir has also made a difference. The IV formulation was approved for AIDS-associated cytomegalovirus retinitis in 1996 but discontinued a few years later amid concerns of severe renal toxicity. It’s found a new home in dermatology since then, explained ID dermatologist Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
Dermatologists see acyclovir-resistant herpes “heaped up on the genitals in HIV patients,” and there weren’t many options in the past. A few years ago, “we [tried] injecting cidofovir directly into the skin lesions, and it’s been remarkably successful. It is a good way to treat these lesions” if dermatologists can get it compounded, she said.
Shingles vaccines, first the live attenuated zoster vaccine (Zostavax) approved by the Food and Drug Administration in 2006 and the more effective recombinant zoster vaccine (Shingrix) approved in 2017, have also had a significant impact.
Dr. Rosen remembers what it was like when he first started practicing over 40 years ago. Not uncommonly, “we saw horrible cases of shingles,” including one in his uncle, who was left with permanent hand pain long after the rash subsided.
Today, “I see much less shingles, and when I do see it, it’s in a much-attenuated form. [Shingrix], even if it doesn’t prevent the disease, often prevents postherpetic neuralgia,” he said.
Also, with pediatric vaccinations against chicken pox, “we’re probably going to see a whole new generation without shingles, which is huge. We’ve made a lot of advancements against herpes viruses,” Dr. Kovarik said.
“We finally found something that helps”
“We’ve [also] come a really long way with genital wart treatment,” Dr. Kovarik said.
It started with approval of topical imiquimod in 1997. “Before that, we were just killing one wart here and one wart there” but they would often come back and pop up in other areas. Injectable interferon was an option at the time, but people didn’t like all the needles.
With imiquimod, “we finally [had] a way to target HPV [human papillomavirus] and not just scrape” or freeze one wart at a time, and “we were able to generate an inflammatory response in the whole area to clear the virus.” Working with HIV patients, “I see sheets and sheets of confluent warts throughout the whole genital area; to try to freeze that is impossible. Now I have a way to get rid of [genital] warts and keep them away even if you have a big cluster,” she said.
“Sometimes, we’ll do both liquid nitrogen and imiquimod. That’s a good way to tackle people who have a high burden of warts,” Dr. Kovarik noted. Other effective treatments have come out as well, including an ointment formulation of sinecatechins, extracted from green tea, “but you have to put it on several times a day, and insurance companies don’t cover it often,” she said.
Intralesional cidofovir is also proving to be boon for potentially malignant refractory warts in HIV and transplant patients. “It’s an incredible treatment. We can inject that antiviral into warts and get rid of them. We finally found something that helps” these people, Dr. Kovarik said.
The HPV vaccine Gardasil is making a difference, as well. In addition to cervical dysplasia and anogenital cancers, it protects against two condyloma strains. Dr. Rosen said he’s seeing fewer cases of genital warts now than when he started practicing, likely because of the vaccine.
“Organisms that weren’t pathogens are now pathogens”
Antibiotic resistance probably tops the list for what’s changed in a bad way in ID dermatology since 1970. Dr. Rosen remembers at the start of his career that “we never worried about antibiotic resistance. We’d put people on antibiotics for acne, rosacea, and we’d keep them on them for 3 years, 6 years”; resistance wasn’t on the radar screen and was not mentioned once in the first issue of Dermatology News, which was packed with articles and ran 24 pages.
The situation is different now. Driven by decades of overuse in agriculture and the medical system, antibiotic resistance is a concern throughout medicine, and unfortunately, “we have not come nearly as far as fast with antibiotics,” at least the ones dermatologists use, “as we have with antivirals,” Dr. Tyring said.
For instance, methicillin-resistant Staphylococcus aureus (MRSA), first described in the United States in 1968, is “no longer the exception to the rule, but the rule” itself, he said, with carbuncles, furuncles, and abscesses not infrequently growing out MRSA. There are also new drug-resistant forms of old problems like gonorrhea and tuberculosis, among other developments, and impetigo has shifted since 1970 from mostly a Streptococcus infection easily treated with penicillin to often a Staphylococcus disease that’s resistant to it. There’s also been a steady march of new pathogens, including the latest one, SARS-CoV-2, the virus that causes COVID-19, which has been recognized as having a variety of skin manifestations.
“No matter how smart we think we are, nature has a way of putting us back in our place,” Dr. Rosen said.
The bright spot is that “we’ve become very adept at identifying and characterizing” microbes “based on techniques we didn’t even have when I started practicing,” such as polymerase chain reaction. “It has taken a lot of guess work out of treating infectious diseases,” he said.
The widespread use of immunosuppressives such as cyclophosphamide, mycophenolate, azathioprine, rituximab, and other agents used in conjunction with solid organ transplantation, has also been a challenge. “We are seeing infections with really odd organisms. Just recently, I had a patient with fusarium in the skin; it’s a fungus that lives in the dirt. I saw a patient with a species of algae” that normally lives in stagnant water, he commented. “We used to get [things like that] back on reports, and we’d throw them away. You can’t do that anymore. Organisms that weren’t pathogens in the past are now pathogens,” particularly in immunosuppressed people, Dr. Rosen said.
Venereologists no more
There’s been another big change in the field. “Back in the not too distant past, dermatologists in the U.S. were referred to as ‘dermatologist-venereologists.’ ” It goes back to the time when syphilis wasn’t diagnosed and treated early, so patients often presented with secondary skin complications and went to dermatologists for help. As a result, “dermatologists became the most experienced at treating it,” Dr. Tyring said.
That’s faded from practice. Part of the reason is that as late as 2000, syphilis seemed to be on the way out; the Centers for Disease and Control and Prevention even raised the possibility of elimination. Dermatologists turned their attention to other areas.
It might have been short-sighted, Dr. Rosen said. Syphilis has made a strong comeback, and drug-resistant gonorrhea has also emerged globally and in at least a few states. No other medical field has stepped in to take up the slack. “Ob.gyns. are busy delivering babies, ID [physicians are] concerned about HIV, and urologists are worried about kidney stones and cancer.” Other than herpes and genital warts, “we have not done well” with management of sexually transmitted diseases, he said.
“I could sense” his frustration
The first issue of Dermatology News carried an article and photospread about scabies that could run today, except that topical permethrin and oral ivermectin have largely replaced benzyl benzoate and sulfur ointments for treatment in the United States. In the article, Scottish dermatologist J. O’D. Alexander, MD, called scabies “the scourge of mankind” and blamed it’s prevalence on “an offhand attitude to the disease which makes control very difficult.”
“I could sense this man’s frustration that people were not recognizing scabies,” Dr. Kovarik said, and it’s no closer to being eradicated than it was in 1970. “It’s still around, and we see it in our clinics. It’s a horrible disease in kids we see in dermatology not infrequently,” and treatment has only advanced a bit.
The article highlights what hasn’t changed much in ID dermatology over the years. Common warts are another one. “With all the evolution in medicine, we don’t have any better treatments approved for common warts than we ever had.” Injecting cidofovir “works great,” but access is a problem, Dr. Tyring said.
Onychomycosis has also proven a tough nut to crack. Readers back in 1970 counted the introduction of the antifungal, griseofulvin, as a major advancement in the 1960s; it’s still a go-to for tinea capitis, but it didn’t work very well for toenail fungus. Terbinafine (Lamisil), approved in 1993, and subsequent developments have helped, but the field still awaits more effective options; a few potential new agents are in the pipeline.
Although there have been major advancements for serious systemic fungal infections, “we’ve mainly seen small steps forward” in ID dermatology, Dr. Tyring said.
Dr. Tyring, Dr. Kovarik, and Dr. Rosen said they had no relevant disclosures.
When Stephen Tyring, MD, PhD, an infectious disease dermatologist, started his career in the early 1980s, he said “we were diagnosing Kaposi’s sarcoma right and left. We would see a new case every day or two.”
It was the early days of the HIV/AIDS epidemic, and dermatologists were at the forefront because HIV/AIDS often presented with skin manifestations. Dr. Tyring, clinical professor in the departments of dermatology, microbiology & molecular genetics and internal medicine at the University of Texas Health Science Center, Houston, and his colleagues referred Kaposi’s patients for chemotherapy and radiation, but the outlook was often grim, especially if lesions developed in the lungs.
Dermatologist don’t see much Kaposi’s anymore because of highly effective treatments for HIV.
Members of the original editorial advisory board saw it coming. In a feature in which board members provided their prediction for the 1970s that appeared in the first issue, New York dermatologist Norman Orentreich, MD, counted the “probable introduction of virucidal agents” as one of the “significant advances or changes that I foresee in the next 10 years.” J. Lamar Callaway, MD, professor of dermatology at Duke University, Durham, N.C., predicted that “the next 10 years should develop effective anti-viral agents for warts, herpes simplex, and herpes zoster.”
To celebrate the 50th anniversary of Dermatology News, we are looking back at how the field has changed since that first issue. The focus this month is infectious disease. There’s a lot to be grateful for but there are also challenges like antibiotic resistance that weren’t on the radar screens of Dr. Orentreich, Dr. Callaway, and their peers in 1970.
All in all, “the only thing I wish we did the old way is sit at the bedside and talk to patients more. We rely so much on technology now that we sometimes lose the art of medicine, which is comforting to the patient,” said Theodore Rosen, MD, an ID dermatologist and professor of dermatology at Baylor College of Medicine, Houston, who’s been in practice for 42 years.
“A lot of advancements against herpes viruses”
One of the biggest wins for ID dermatology over the last 5 decades has been the management of herpes, both herpes simplex virus 1 and 2, as well as herpes zoster virus. It started with the approval of acyclovir in 1981. Before then, “we had no direct therapy for genital herpes, herpes zoster, or disseminated herpes in immunosuppressed or cancer patients,” Dr. Rosen said.
“I can remember doing an interview with Good Morning America when I gave the first IV dose of acyclovir in the city of Houston for really bad disseminated herpes” in an HIV patient, he said, and it worked.
Two derivatives, valacyclovir and famciclovir, became available in the mid-1990s, so today “we have three drugs and some others at the periphery that are all highly effective not only” against herpes, but also for preventing outbreaks; valacyclovir can even prevent asymptomatic shedding, therefore possibly preventing new infections. “That’s a concept we didn’t even have 40 years ago,” Dr. Rosen said.
Cidofovir has also made a difference. The IV formulation was approved for AIDS-associated cytomegalovirus retinitis in 1996 but discontinued a few years later amid concerns of severe renal toxicity. It’s found a new home in dermatology since then, explained ID dermatologist Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
Dermatologists see acyclovir-resistant herpes “heaped up on the genitals in HIV patients,” and there weren’t many options in the past. A few years ago, “we [tried] injecting cidofovir directly into the skin lesions, and it’s been remarkably successful. It is a good way to treat these lesions” if dermatologists can get it compounded, she said.
Shingles vaccines, first the live attenuated zoster vaccine (Zostavax) approved by the Food and Drug Administration in 2006 and the more effective recombinant zoster vaccine (Shingrix) approved in 2017, have also had a significant impact.
Dr. Rosen remembers what it was like when he first started practicing over 40 years ago. Not uncommonly, “we saw horrible cases of shingles,” including one in his uncle, who was left with permanent hand pain long after the rash subsided.
Today, “I see much less shingles, and when I do see it, it’s in a much-attenuated form. [Shingrix], even if it doesn’t prevent the disease, often prevents postherpetic neuralgia,” he said.
Also, with pediatric vaccinations against chicken pox, “we’re probably going to see a whole new generation without shingles, which is huge. We’ve made a lot of advancements against herpes viruses,” Dr. Kovarik said.
“We finally found something that helps”
“We’ve [also] come a really long way with genital wart treatment,” Dr. Kovarik said.
It started with approval of topical imiquimod in 1997. “Before that, we were just killing one wart here and one wart there” but they would often come back and pop up in other areas. Injectable interferon was an option at the time, but people didn’t like all the needles.
With imiquimod, “we finally [had] a way to target HPV [human papillomavirus] and not just scrape” or freeze one wart at a time, and “we were able to generate an inflammatory response in the whole area to clear the virus.” Working with HIV patients, “I see sheets and sheets of confluent warts throughout the whole genital area; to try to freeze that is impossible. Now I have a way to get rid of [genital] warts and keep them away even if you have a big cluster,” she said.
“Sometimes, we’ll do both liquid nitrogen and imiquimod. That’s a good way to tackle people who have a high burden of warts,” Dr. Kovarik noted. Other effective treatments have come out as well, including an ointment formulation of sinecatechins, extracted from green tea, “but you have to put it on several times a day, and insurance companies don’t cover it often,” she said.
Intralesional cidofovir is also proving to be boon for potentially malignant refractory warts in HIV and transplant patients. “It’s an incredible treatment. We can inject that antiviral into warts and get rid of them. We finally found something that helps” these people, Dr. Kovarik said.
The HPV vaccine Gardasil is making a difference, as well. In addition to cervical dysplasia and anogenital cancers, it protects against two condyloma strains. Dr. Rosen said he’s seeing fewer cases of genital warts now than when he started practicing, likely because of the vaccine.
“Organisms that weren’t pathogens are now pathogens”
Antibiotic resistance probably tops the list for what’s changed in a bad way in ID dermatology since 1970. Dr. Rosen remembers at the start of his career that “we never worried about antibiotic resistance. We’d put people on antibiotics for acne, rosacea, and we’d keep them on them for 3 years, 6 years”; resistance wasn’t on the radar screen and was not mentioned once in the first issue of Dermatology News, which was packed with articles and ran 24 pages.
The situation is different now. Driven by decades of overuse in agriculture and the medical system, antibiotic resistance is a concern throughout medicine, and unfortunately, “we have not come nearly as far as fast with antibiotics,” at least the ones dermatologists use, “as we have with antivirals,” Dr. Tyring said.
For instance, methicillin-resistant Staphylococcus aureus (MRSA), first described in the United States in 1968, is “no longer the exception to the rule, but the rule” itself, he said, with carbuncles, furuncles, and abscesses not infrequently growing out MRSA. There are also new drug-resistant forms of old problems like gonorrhea and tuberculosis, among other developments, and impetigo has shifted since 1970 from mostly a Streptococcus infection easily treated with penicillin to often a Staphylococcus disease that’s resistant to it. There’s also been a steady march of new pathogens, including the latest one, SARS-CoV-2, the virus that causes COVID-19, which has been recognized as having a variety of skin manifestations.
“No matter how smart we think we are, nature has a way of putting us back in our place,” Dr. Rosen said.
The bright spot is that “we’ve become very adept at identifying and characterizing” microbes “based on techniques we didn’t even have when I started practicing,” such as polymerase chain reaction. “It has taken a lot of guess work out of treating infectious diseases,” he said.
The widespread use of immunosuppressives such as cyclophosphamide, mycophenolate, azathioprine, rituximab, and other agents used in conjunction with solid organ transplantation, has also been a challenge. “We are seeing infections with really odd organisms. Just recently, I had a patient with fusarium in the skin; it’s a fungus that lives in the dirt. I saw a patient with a species of algae” that normally lives in stagnant water, he commented. “We used to get [things like that] back on reports, and we’d throw them away. You can’t do that anymore. Organisms that weren’t pathogens in the past are now pathogens,” particularly in immunosuppressed people, Dr. Rosen said.
Venereologists no more
There’s been another big change in the field. “Back in the not too distant past, dermatologists in the U.S. were referred to as ‘dermatologist-venereologists.’ ” It goes back to the time when syphilis wasn’t diagnosed and treated early, so patients often presented with secondary skin complications and went to dermatologists for help. As a result, “dermatologists became the most experienced at treating it,” Dr. Tyring said.
That’s faded from practice. Part of the reason is that as late as 2000, syphilis seemed to be on the way out; the Centers for Disease and Control and Prevention even raised the possibility of elimination. Dermatologists turned their attention to other areas.
It might have been short-sighted, Dr. Rosen said. Syphilis has made a strong comeback, and drug-resistant gonorrhea has also emerged globally and in at least a few states. No other medical field has stepped in to take up the slack. “Ob.gyns. are busy delivering babies, ID [physicians are] concerned about HIV, and urologists are worried about kidney stones and cancer.” Other than herpes and genital warts, “we have not done well” with management of sexually transmitted diseases, he said.
“I could sense” his frustration
The first issue of Dermatology News carried an article and photospread about scabies that could run today, except that topical permethrin and oral ivermectin have largely replaced benzyl benzoate and sulfur ointments for treatment in the United States. In the article, Scottish dermatologist J. O’D. Alexander, MD, called scabies “the scourge of mankind” and blamed it’s prevalence on “an offhand attitude to the disease which makes control very difficult.”
“I could sense this man’s frustration that people were not recognizing scabies,” Dr. Kovarik said, and it’s no closer to being eradicated than it was in 1970. “It’s still around, and we see it in our clinics. It’s a horrible disease in kids we see in dermatology not infrequently,” and treatment has only advanced a bit.
The article highlights what hasn’t changed much in ID dermatology over the years. Common warts are another one. “With all the evolution in medicine, we don’t have any better treatments approved for common warts than we ever had.” Injecting cidofovir “works great,” but access is a problem, Dr. Tyring said.
Onychomycosis has also proven a tough nut to crack. Readers back in 1970 counted the introduction of the antifungal, griseofulvin, as a major advancement in the 1960s; it’s still a go-to for tinea capitis, but it didn’t work very well for toenail fungus. Terbinafine (Lamisil), approved in 1993, and subsequent developments have helped, but the field still awaits more effective options; a few potential new agents are in the pipeline.
Although there have been major advancements for serious systemic fungal infections, “we’ve mainly seen small steps forward” in ID dermatology, Dr. Tyring said.
Dr. Tyring, Dr. Kovarik, and Dr. Rosen said they had no relevant disclosures.
Infectious Diseases Board Review: Menopause in Women Living With HIV
More than half of the 37.9 million persons living with HIV (PLWH) worldwide are women.1 Between 2010 and 2016, 58% of women living with HIV (WLWH) in the United States were older than 45 years.2 As such, an increasing number of WLWH are entering menopause and living well beyond menopause. Despite this, health care providers expressed a lack of confidence in managing menopause in WLWH, and menopausal symptoms often are not recognized by providers.3 Enhancing our knowledge about menopause in WLWH is important, since the physiologic changes associated with menopause impact short- and long-term quality of life and mortality.
Test your knowledge of this topic HERE.
Amenorrhea
Menstrual irregularities, including amenorrhea and anovulation, are more frequently found in women of low socioeconomic status, presumably due to associated physical and emotional stress.4 In addition, women with low body mass index (BMI) have decreased serum estradiol levels, which lead to amenorrhea.4,5 Furthermore, low parity and many legal and illegal drugs are associated with amenorrhea, including hormonal contraceptives, opiates, stimulants, antipsychotics, and chemotherapeutic agents.6-8
Test your knowledge of this topic HERE.
Age at Menopause
In the United States, the median age of menopause is between 50 and 52 years in middle-class white women.11,12 Earlier menopause has been observed in women who are African American, are nulliparous, have a lower BMI, smoke tobacco, and have more stress, less education, and higher unemployment rates.11,13,14 Because 57% of women diagnosed with HIV in 2018 were African American and many WLWH have other risk factors associated with earlier menopause, studies examining the age of menopause in WLWH need to use a comparator group of women without HIV with similar characteristics and control for these factors to determine the influence of HIV on the age of menopause.
Menopause-Associated Symptoms
The perimenopausal period, which begins, on average, 4 years prior to the final menstrual period, is characterized by hormonal fluctuations leading to irregular menstrual cycles.19,20 Symptoms associated with these physiologic changes during the perimenopausal period include vasomotor symptoms (hot flashes), genitourinary symptoms (vaginal dryness and dyspareunia), anxiety, depression, sleep disturbances, and joint aches.21,22 Such menopausal symptoms can be distressing and negatively impact quality of life.23 In WLWH, severe menopausal symptoms have been associated with suboptimal adherence to antiretroviral therapy (ART).24
Vasomotor
In the United States, the most common symptom during perimenopause is hot flashes, which occur in 38% to 80% of women.32,33 Vasomotor symptoms are most common in women who smoke, use illicit substances, have a high BMI, are of lower socioeconomic status, and are African American.11 As expected, prior studies focusing on hot flash prevalence among premenopausal, perimenopausal, and postmenopausal WLWH found that postmenopausal women experience more hot flashes than premenopausal or perimenopausal women.27,28 In addition, a comparison of women with and without HIV demonstrated a higher prevalence of hot flashes among WLWH.26,29 Vasomotor symptoms can be severely distressing, with hot flashes contributing to increased risk of depression.25,34 In a cross-sectional analysis of 835 WLWH and 335 women without HIV from the WIHS cohort, persistent vasomotor symptoms predicted elevated depressive symptoms in both WLWH and women without HIV.34 In a similar cross-sectional analysis of 536 women, among whom 54% were WLWH and 37% were perimenopausal, psychological symptoms were prevalent in 61% of the women with vasomotor symptoms.29
Genitourinary
Estrogen deficiency, which accompanies the perimenopausal period, leads to vulvovaginal atrophy (VVA), manifesting with symptoms of vaginal dryness, itching, burning, urinary urgency, and dyspareunia (painful intercourse).33,35,36 Unlike vasomotor symptoms, which diminish with time, genitourinary symptoms generally worsen if left untreated.37 Furthermore, these symptoms are often underreported and underdiagnosed.38,39 VVA was found in 43% to 84% of postmenopausal women.36,40,41 In the AGATA study, the prevalence of VVA was associated with years since menopause. 36 Vaginal dryness and dyspareunia were common.
Psychiatric
Anxiety and depression are also common symptoms in perimenopausal women.46-48 Studies have shown that depression is diagnosed 2.5 times more frequently among perimenopausal women than premenopausal women.48 In a study by Miller et al that focused on 536 WLWH, among whom 37% were perimenopausal, 89% reported psychological symptoms.29 Ferreira et al found that perimenopausal WLWH had an increased incidence of psychological symptoms, such as depression and anxiety, compared to women without HIV infection.26 Whether this increased prevalence of psychological symptoms seen in WLWH can be attributed to menopause is unclear, since one third to one half of men and women living with HIV experience symptoms of depression.49 However, in the WIHS, which compared findings from 835 WLWH to findings from 335 women without HIV from all menopausal stages, elevated depressive symptoms were seen in the early perimenopausal period.34 There was no increased incidence of such symptoms during the premenopausal or postmenopausal stage, suggesting that factors related to menopause contribute to depressive symptoms during the perimenopausal stage.34
Other Symptoms
Sleep disturbances are common among perimenopausal women, with an estimated prevalence between 38% and 46%.52-54 Hot flashes, anxiety, and depression appear to be factors that contribute to sleep difficulty.52-54 In a cross-sectional study of 273 WLWH and 264 women without HIV between 40 and 60 years of age, insomnia was found in 51% of perimenopausal and 53% of postmenopausal WLWH. The prevalence of insomnia in WLWH and women without HIV was the same.55 Joint aches are also commonly reported in the perimenopausal period, with a prevalence as high as 50% to 60% among perimenopausal women in the United States.22,29 Miller and colleagues found that 63% of menopausal WLWH reported arthralgia.29
Test your knowledge of this topic HERE.
Treatment
Despite the increased severity of menopausal symptoms experienced among WLWH, menopausal replacement therapy (MRT) is used less frequently in WLWH than in women living without HIV.55 Topical treatment is recommended for women who are experiencing vaginal dryness. First-line treatment is topical nonhormonal therapy, such as moisturizers and lubricants.56 If symptoms are not relieved, then topical vaginal estrogen therapy is recommended.56 Randomized placebo-controlled studies have verified the safety and efficacy of topical estrogen in the general population, and there is no reason to expect different outcomes in WLWH.57,58
Cardiovascular Risk
Estrogen deficiency that occurs during menopause leads to an increased risk of cardiovascular disease, particularly with changes in lipid profiles, insulin resistance, and body composition (eg, increased fat mass and waist circumference).61 HIV infection also is associated with a higher risk of cardiovascular disease, with studies consistently reporting a 1.5- to 2-fold increase in the rate of cardiovascular events in PLWH compared to persons without HIV.62 The inflammatory effects of HIV as well as ART exposure, specifically to PIs and abacavir, increase the risk for cardiovascular disease.62 In addition, traditional risk factors, including dyslipidemia, contribute to cardiovascular disease risk in this population.63,64
Osteoporosis
Menopause, with its associated estrogen deficiency, is the most important risk factor linked to increased bone turnover and bone loss.70 In addition, HIV is associated with bone loss, with low bone mineral density (BMD) described even among men and premenopausal women with HIV infection.71 Although decreased BMD associated with HIV stabilizes or even improves after initiation of ART in the younger population,72-74 chronic inflammation caused by HIV stimulates osteoclast differentiation and resorption.71 Other factors that appear to contribute to decreased BMD among PLWH include ART; vitamin D deficiency; low BMI; poor nutrition; inactivity; use of tobacco, alcohol, and illicit drugs; hepatitis B and C coinfection; and frailty, defined as increased vulnerability to stresses related to aging.72-80 Among ARTs, tenofovir disoproxil fumarate is associated with an increased risk of osteoporosis, and switching from this agent to tenofovir alafenamide improves bone density.81 Prolonged amenorrhea is also an added risk factor for osteoporosis in WLWH.82
Cognition
Both men and women living with HIV are at higher risk for cognitive impairment, ranging from minor cognitive-motor disorder to HIV-associated dementia.91 In addition, the menopause transition is characterized by cognitive changes, such as memory loss and difficulty concentrating.92,93 Studies focusing on the effects of both HIV infection and menopause on cognition have been limited thus far. A cross-sectional study demonstrated that HIV infection, but not menopausal stage, was associated with worse performance on cognitive measures.94 While menopausal stage was not associated with cognitive decline, menopausal symptoms like depression, anxiety, and vasomotor symptoms were associated with lower cognitive performance, highlighting the importance of recognition and treatment of menopausal symptoms.94
Test your knowledge of this topic HERE.
Cervical Dysplasia
WLWH are at increased risk for low- and high-grade squamous intraepithelial lesions (SILs) and more rapid progression to cervical carcinoma, as compared to women without HIV.95 This increased risk of cervical disease is associated with age, human papillomavirus genotype, and degree of immunosuppression.96 In addition, menopause appears to affect the risk of cervical disease. Postmenopausal WLWH had a higher risk of progression of SILs and persistence of lower-grade SILs compared to premenopausal women.97,98 Although studies on progression to cervical cancer in postmenopausal WLWH remain limited, current data suggest that postmenopausal WLWH should continue to be monitored and screened similarly to premenopausal women.
HIV Acquisition and Transmission
Women aged 50 years and older are primarily exposed to HIV through heterosexual contact.99 While the lack of awareness of HIV risk and less frequent use of barrier protection can contribute to new HIV infection in older women, physiologic changes associated with menopause also may be playing a role.100 Vaginal wall thinning and immunologic changes of the cervix that occur during menopause may serve as a risk factor for HIV acquisition. The cervicovaginal mucosa of postmenopausal women had higher levels of p24 antigen after ex vivo HIV-1 infection, suggesting higher susceptibility to acquire HIV infection.101 Postmenopausal women have been shown to have increased cervical CCR5 expression, which serves as an entry point of HIV into target cells.102 Finally, anti-HIV-1 activity was significantly decreased in postmenopausal women compared to premenopausal women.103 In addition, ex vivo studies demonstrated reduced tenofovir disoproxil fumarate and emtricitabine triphosphate concentrations in cervical tissue of postmenopausal women, suggesting that postmenopausal women may need higher doses of pre-exposure prophylaxis to achieve protective efficacy.104
HIV Progression
With prior data suggesting that younger persons experience better immunologic and virologic responses to ART,107-109 it had previously been hypothesized that virologic and immunologic responses to ART will decline once WLWH reach menopause. However, current studies suggest that menopause does not affect the progression of HIV and that ART-naive women should respond to ART, regardless of their menopausal status. Treatment responses to ART, determined by the median changes in CD4 cell counts and percentages and viral load, in ART-naive individuals did not differ between premenopausal and postmenopausal women.110 In addition, there appear to be no significant changes in CD4 cell counts as WLWH progress through menopause.111
Conclusion
As individuals with HIV infection live longer, an increasing number of women will enter menopause and live many years beyond menopause. WLWH experience earlier and more severe menopausal symptoms, but evidence on the appropriate management of these symptoms is still lacking. These conditions require proper surveillance, and can be prevented with an improved understanding of the effects of menopause on WLWH. However, there remain significant gaps in our understanding of menopause in WLWH. As practitioners encounter an increasing number of perimenopausal and postmenopausal WLWH, studies of the effects of HIV on comorbidities and symptoms of menopause and their appropriate management are necessary to improve care of WLWH.
Test your knowledge of this topic HERE.
1. UNAIDS. AIDSInfo. 2019 estimates https://www.unaids.org/en/resources/infographics/girls-and-women-living-with-HIV. Accessed April 30, 2020.
2. Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report 2019;24(No. 1). www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed April 30, 2020.
3. Chirwa M, Ma R, Guallar C, et al. Managing menopause in women living with HIV: A survey of primary care practitioners. Post Reprod Health. 2017;23:111-115.
4. Munster K, Helm P, Schmidt L. Secondary amenorrhea: Prevalence and medical contract–A cross sectional study from a Danish county. Br J Obstet Gynecol. 1992;99:430-433.
5. Vyver E, Steinegger C, Katzman DK, et al. Eating disorders and menstrual dysfunction in adolescents. Ann N Y Acad Sci. 2008;1135: 253-264.
6. Cejtin HE, Evans CT, Greenblatt R, et al. Prolonged amenorrhea and resumption of menses in women with HIV. J Womens Health (Larchmt). 2018;27:1441‐1448.
7. Bai J, Greenwald E, Caterini H, et al. Drug-related menstrual aberrations. Obstet Gynecol. 1974;44:713-719.
8. Cejtin HE, Kalinowski A, Bacchetti P. Effects of human immunodeficiency virus on protracted amenorrhea and ovarian dysfunction. Obstet Gynecol. 2006;108:1423-1431.
9. King EM, Albert AY, Murray MCM. HIV and amenorrhea: a meta-analysis. AIDS. 2019;33:483‐491.
10. Watts DH, Spino C, Zaborski L. Comparison of gynecologic history and laboratory results in HIV-positive women with CDR+ lymphocyte counts between 200 and 500 cells/μl and below 100 cells/ μl. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:455-462.
11. Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178:70-83.
12. Thomas F, Renaud F, Benefice E, et al. International variability of ages at menarche and menopause: patterns and main determinants. Hum Biol. 2001;73:271-290.
13. Bromberger JT, Matthews KA, Kuller LH, et al. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997; 145:24-33.
14. Schoenbaum E, Hartel D, Lo Y, et al. HIV infection, drug use, and onset of natural menopause. Clinical Infect Dis. 2005;41: 1517-1524.
15. Research on the menopause in the 1990s. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1996;866:1-107.
16. de Pommerol M, Hessamfar M, Lawson-Ayayi S, et al. Menopause and HIV infection: age at onset and associated factors, ANRS CO3 Aquitaine cohort. Int J STD AIDS. 2011;22:67-72.
17. Calvet G, Grinsztejn G. Predictors of early menopause in HIV infected women: a prospective cohort study. Am J Obstet Gynecol. 2015;212:765.
18. Cejtin SH, Taylor R, Watts DH. Assessment of menopausal status among women in the Women’s Interagency HIV study (WIHS). Proceedings of the XV International AIDS Conference; July 11-16, 2004; Bangkok, Thailand.
19. Taffe JR, Dennerstein L. Menstrual patterns leading to the final menstrual period. Menopause. 2002;9:32-40.
20. Miro F, Parker SW, Aspinall LJ, et al. Origins and consequences of the elongation of the human menstrual cycle during the menopausal transition: the FREEDOM Study. J Clin Endocrinol Metab. 2004;89:4910-4915.
21. McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14:103-115.
22. Blümel JE, Chedraui P, Baron G, et al. Menopause could be involved in the pathogenesis of muscle and joint aches in mid-aged women. Maturitas. 2013;75:94-100.
23. Woods NF, Mitchell ES. Symptoms interference with work and relationships during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2011;18:654-661.
24. Duff PK, Money DM, Ogilvie GS, et al. Severe menopausal symptoms associated with reduced adherence to antiretroviral therapy among perimenopausal and menopausal women living with HIV in Metro Vancouver. Menopause. 2018;25:531-537.
25. Johnson TM, Cohen HW, Howard AA, et al. Attribution of menopause symptoms in human immunodeficiency virus–infected or at-risk drug-using women. Menopause. 2008;15:551-557.
26. Ferreira CE, Pinto-Neto AM, Conde DM, et al. Menopausal symptoms in women infected with HIV: prevalence and associated factors. Gynecol Endocrinol. 2007;23:198-205.
27. Fantry L, Zhan M, Taylor G, et al. Age at menopause and menopausal symptoms in HIV-infected women. AIDS Patient Care STD. 2005;19:703-711.
28. Boonyanurak P, Bunupuradah T, Wilawan K, et al. Age at menopause and menopause-related symptoms in human immunodeficiency virus-infected Thai women. Menopause. 2012;19: 820-824.
29. Miller SA, Santoro N, Lo Y. Menopausal symptoms in HIV-infected and drug-using women. Menopause. 2005;12:348-356.
30. Looby S, Shifren J, Corless I. Increased hot flash severity and related interference in perimenopausal HIV-infected women. Menopause. 2014;21:403-409.
31. Schnall R, Jia H, Olender S, et al. In people living with HIV (PLWH), menopause (natural or surgical) contributes to the greater symptom burden in women: results from an online US survey. Menopause. 2018;25:744-752.
32. Thurston RC, Joffe H. Vasomotor symptoms and menopause: findings from the Study of Women’s Health across the Nation. Obstet Gynecol Clin North Am. 2011;38:489-501.
33. Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 Suppl 12B:14-24.
34. Maki PM, Rubin LH, Cohen M, et al. Depressive symptoms are increased in the early perimenopausal stage in ethnically diverse human immunodeficiency virus-infected and human immunodeficiency virus-uninfected women. Menopause. 2012;19: 1215-1233.
35. Dennerstein L, Dudley EC, Hopper JL, et al. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
36. Palma F, Volpe A, Villa P, et al. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: The AGATA study. Maturitas. 2016;83:40-44.
37. Cutler WB, Garcia CR, McCoy N. Perimenopausal sexuality. Arch Sex Behav. 1987;16:225-234.
38. Moreira ED, Glasser DB, Nicolosi A, et al. GSSAB Investigators’ Group. Sexual problems and help-seeking behavior in adults in the United Kingdom and continental Europe. BJU Int. 2008;101:1005-1111.
39. MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85:87-94.
40. Nappi RE, Kokot-Kierepa M. Women’s voices in the menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67:233-238.
41. Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6:2133-2142.
42. Valadares AL, Pinto-Neto AM, Conde DM, et al. A population-based study of dyspareunia in a cohort of middle-aged Brazilian women. Menopause. 2008;15:1184-1190.
43. Latthe P, Migini L, Gray R, et al. Factors predisposing women to chronic pelvic pain: a systemic review. BMJ. 2006;332:749-755.
44. Potter DA, Moreno A, Luther MF, et al. Effects of follicular-phase cocaine administration on menstrual and ovarian cyclicity in rhesus monkeys. Am J Obstet Gynecol. 1998;178:118-125.
45. Valadares AL, Pinto-Neto AM, Gomes D, et al. Dyspareunia in HIV-positive and HIV-negative middle-aged women: a cross-sectional study. BMJ Open. 2014;4:e004974.
46. Bromberger JT, Meyer PM, Kravitz HM, et al. Psychologic distress and natural menopause: a multiethnic community study. Am J Public Health. 2001;91:1435-1442.
47. Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression. Results from the Massachusetts Women’s Health Study. Ann Epidemiol. 1994;4:214-220.
48. Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006; 63:375-382.
49. Eller LS, Corless I, Bunch EH, et al. Self-care strategies for depressive symptoms in people with HIV disease. J Adv Nurs. 2005;51:119-130.
50. Fuh JL, Wang SJ, Lee SJ, et al. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas. 2006;53:447-453.
51. Hinkin CH, Castellon SA, Atkinson JH, et al. Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol. 2001;54:S44-S52
52. Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19-28.
53. Freedman RR, Roehrs TA. Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause. 2006;13:576-583.
54. Erlik Y, Tataryn IV, Meldrum DR, et al. Association of waking episodes aspects of HIV infection among older adults. J Clin Epidemiol. 2001;54:S44–52.
55. Lui-Filho JF, Valadares AR, Gomes D, et al. Menopausal symptoms and associated factors in HIV-positive women. Maturitas. 2013;76:172-178.
56. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888‐904.
57. Fernandes T, Pedro AO, Baccaro LF, et al. Hormonal, metabolic, and endometrial safety of testosterone vaginal cream versus estrogens for the treatment of vulvovaginal atrophy in postmenopausal women: a randomized, placebo-controlled study. Menopause. 2018; 25:641‐647.
58. Kroll R, Archer DF, Lin Y, et al. A randomized, multicenter, double-blind study to evaluate the safety and efficacy of estradiol vaginal cream 0.003% in postmenopausal women with dyspareunia as the most bothersome symptom. Menopause. 2018;25:133‐138.
59. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Tables 21a-d.www.aidsinfo.nih.gov/ContentFiles/ AdultandAdolescentGL.pdf. Accessed May 4, 2020.
60. Tittle, V, Bull, L, Boffito, M. Pharmacokinetic and pharmacodynamics drug interactions between antiretrovirals and oral contraceptives. Clin Pharmacokinet. 2015;54:23-34.
61. Sower M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrin Metab. 2007;92:895- 901.
62. Eyawo O, Brockman G, Goldsmith CH, et al. Risk of myocardial infarction among people living with HIV: an updated systematic review and meta-analysis. BMJ Open. 2019;9:e025874.
63. Flooris-Moore M, Howard AA, Lo Y, et al. Increased serum lipids are associated with higher CD4 lymphocyte count in HIV-infected women. HIV Med. 2006;7:421-430.
64. Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130-139.
65. Grinspoon S, Carr A. Cardiovascular risk and body fat abnormalities in HIV-infected adults. N Engl J Med. 2005; 352:48–62.
66. Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM). Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42:562-571.
67. Alcaide ML, Parmigiani A, Pallikkuth S, et al. Immune activation in HIV-infected aging women on antiretrovirals--implications for age-associated comorbidities: a cross-sectional pilot study. PLoS One. 2013;8:e63804.
68. Karim R, Mack WJ, Kono N, et al. Gonadotropin and sex steroid levels in HIV-infected premenopausal women and their association with subclinical atherosclerosis in HIV-infected and -uninfected women in the women’s interagency HIV study (WIHS). J Clin Endocrinol Metab. 2013;98:E610‐E618.
69. Looby SE, Fitch KV, Srinivasa S, et al. Reduced ovarian reserve relates to monocyte activation and subclinical coronary atherosclerotic plaque in women with HIV. AIDS. 2016;30:383‐393.
70. Akhter MP, Lappe JM, Davies KM, et al. Transmenopausal changes in the trabecular bone structure. Bone. 2007;41:111-116.
71. Gibellini D, De Crignis E, Ponti C. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNF-alpha activation. J Med Virol. 2008;80:1507-1514.
72. Cassetti I, Madruga JV, Suleiman JM, et al. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV- 1-infected patients. HIV Clin Trials. 2007;8:164-172.
73. McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203: 1791-1801.
74. Hansen AB, Obel N, Nielsen H, et al. Bone mineral density changes in protease inhibitor-sparing vs. nucleoside reverse transcriptase inhibitor-sparing highly active antiretroviral therapy: Data from a randomized trial. HIV Med. 2011;12:157-165.
75. FDao CN, Patel P, Overton ET, et al. Study to understand the natural history of HIV and AIDS in the era of effective therapy (SUN) investigators. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D levels in cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396-405.
76. Jacobson DL, Spiegelman D, Know TK, Wilson IB. Evolution and predictors of change in total bone mineral density over time in HIV-infected men and women in the nutrition for healthy living study. J Acquir Immune Defic Syndr Hum Retrovirol. 2008;49:298-308.
77. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporosis Int. 2005;16:581-589.
78. Pedrazzoni M, Vescovi L, Maninetti M, et al. Effects of chronic heroine abuse on bone and mineral metabolism. Acta Endocrinol. 1993;129:42-45.
79. Lo Re V 3rd, Guaraldi G, Leonard MB, et al. Viral hepatitis is associated with reduced bone mineral density in HIV-infected women but not men. AIDS. 1990;23:2191-2198.
80. Bregigeon S, Galinier A, Zaegel-Faucher O, et al. Frailty in HIV infected people: a new risk factor for bone mineral density loss [published correction appears in AIDS. AIDS. 2017;31: 1573‐1577.
81. Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2015;16:43-45.
82. King EM, Nesbitt A, Albert AYK, et al. Prolonged amenorrhea and low hip bone mineral density in women living with HIV-a controlled cross-sectional study. J Acquir Immune Defic Syndr. 2020;83:
486‐495.
83. Yin MT, Mcmahon DJ, Ferris DC, et al. Low bone mass and high bone turnover in postmenopausal human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2010;95:620-629.
84. Yin MT, Modarresi R, Shane E, et al. Effects of HIV infection and antiretroviral therapy with ritonavir on induction of osteoclast-like cells in postmenopausal women. Osteoporos Int. 2011;22:1459-1466.
85. Sharma A, Cohen HW, Freeman R, et al. Prospective evaluation of bone mineral density among middle-aged HIV-infected and uninfected women: association between methadone use and bone loss. Maturitas. 2011;70:295-301.
86. Sharma A, Flom PL, Rosen CJ, et al. Racial differences in bone loss and relation to menopause among HIV-infected and uninfected women. Bone. 2015;77:24-30.
87. Aberg JA, Gallant JE, Ghanem KG, et al, Infectious Diseases Society of America. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:1‐10.
88. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis 2014. Washington, DC: National Osteoporosis Foundation; 2014.
89. Yin MT, Choudhury A, Bucovsky M, et al. A randomized placebo-controlled trial of low- versus moderate-dose vitamin d3 supplementation on bone mineral density in postmenopausal women with HIV. J Acquir Immune Defic Syndr. 2019;80:342-349.
90. McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937-946.
91. Price RW. Neurological complications of HIV infection. Lancet. 1996;348:445-452.
92. Soares CN, Maki PM. Menopausal transition, mood, and cognition: an integrated view to close the gaps. Menopause. 2010;17:812-814.
93. Greendale GA, Wight RG, Huang MH, et al. Menopause-associated symptoms and cognitive performance: results from the study of women’s health across the nation. Am J Epidemiol. 2010;171:1214-1224.
94. Rubin LH, Sundermann EE, Cook JA, et al. An investigation of menopausal stage and symptoms on cognition in HIV-infected women. Menopause. 2014;21:997-1006.
95. Ellerbrock TV, Chiasson MA, Bush TJ, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA. 2000;283:1031-1037.
96. Mandelblatt JS, Kanetsky P, Eggert L, et al. Is HIV infection a cofactor for cervical squamous cell neoplasia? Cancer Epidemiol Biomarkers Prev. 1999;8:97-106.
97. Kim SC, Messing S, Shah K, et al. Effects of highly active antiretroviral therapy (HAART) and menopause on risk of progression of cervical dysplasia in human immune deficiency virus (HIV) infected women. Infect Dis Obstet Gynecol. 2013;2013:784718.
98. Ceccaldi PF, Ferreira C, Coussy F, et al. Cervical disease in postmenopausal HIV-1 infected women. J Gynecol Obstet Biol Reprod. 2010;39:466-470.
99. Centers for Disease Control and Prevention. HIV and older Americans. www.cdc.gov/hiv/group/age/olderamericans/index.html. Accessed May 11, 2020.
100. Levy JA, Ory MG, Crystal S. HIV/AIDS interventions for midlife and older adults: current status and challenges. J Acquir Immune Defic Syndr. 2003;33 Suppl 2:S59-S67.
101. Thurman AR, Yousefieh N, Chandra N, et al. Comparison of mucosal markers of human immunodeficiency virus susceptibility in healthy premenopausal versus postmenopausal women. AIDS Res Hum Retroviruses. 2017;33:807-819.
102. Meditz AL, Moreau KL, MaWhinney S, et al. CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J Acquir Immune Defic Syndr. 2012;59:221-228.
103. Chappell CA, Isaacs CE, Xu W, et al. The effect of menopause on the innate antiviral activity of cervicovaginal lavage. Am J Obstet Gynecol. 2015;213:204.
104. Nicol MR, Brewers LM, Kashuba ADM, et al. The role of menopause in tenofovir diphosphate and emtricitabine triphosphate concentrations in cervical tissue. AIDS. 2018;32:11-15.
105. Melo KC, Melo MR, Ricci BV, Segurado AC. Correlates of human immunodeficiency virus cervicovaginal shedding among postmenopausal and fertile-aged women. Menopause. 2012;19:150-156.
106. Landolt NK, Do T, Kasipong N, et al. Low-level genital HIV shedding in Thai HIV-infected women with suppressed plasma viral load after menopause: a longitudinal study. J Virus Erad. 2017;3:204-207.
107. Viard JP, Mocroft A, Chiesi A, et al. Influence of age of CD4 cell recovery in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy: evidence from the Euro SIDA study. J Infect Dis. 2001;193:1290-1294.
108. Grabar S, Kousignian I, Sobel A, et al. Immunological and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS. 2004;18:2029-2038.
109. Cuzin L, Delpierre C, Gerard S, et al. Immunologic and clinical responses to highly active antiretroviral therapy in patients with HIV infection aged >50 years. Clin Infect Dis. 2007;45:654-657.
110. Patterson KB, Cohn SE, Uynik J, et al. Treatment responses in antiretroviral treatment-naïve premenopausal and postmenopausal HIV-1 infected women: an analysis from AIDS clinical trials group studies. Clin Infect Dis. 2009;49:473476.
111. van Benthem BH, Vernazza P, Coutinho RA, et al. The impact of pregnancy and menopause on CD4 lymphocyte count in HIV-infected women. AIDS. 2002;16:919-922.
More than half of the 37.9 million persons living with HIV (PLWH) worldwide are women.1 Between 2010 and 2016, 58% of women living with HIV (WLWH) in the United States were older than 45 years.2 As such, an increasing number of WLWH are entering menopause and living well beyond menopause. Despite this, health care providers expressed a lack of confidence in managing menopause in WLWH, and menopausal symptoms often are not recognized by providers.3 Enhancing our knowledge about menopause in WLWH is important, since the physiologic changes associated with menopause impact short- and long-term quality of life and mortality.
Test your knowledge of this topic HERE.
Amenorrhea
Menstrual irregularities, including amenorrhea and anovulation, are more frequently found in women of low socioeconomic status, presumably due to associated physical and emotional stress.4 In addition, women with low body mass index (BMI) have decreased serum estradiol levels, which lead to amenorrhea.4,5 Furthermore, low parity and many legal and illegal drugs are associated with amenorrhea, including hormonal contraceptives, opiates, stimulants, antipsychotics, and chemotherapeutic agents.6-8
Test your knowledge of this topic HERE.
Age at Menopause
In the United States, the median age of menopause is between 50 and 52 years in middle-class white women.11,12 Earlier menopause has been observed in women who are African American, are nulliparous, have a lower BMI, smoke tobacco, and have more stress, less education, and higher unemployment rates.11,13,14 Because 57% of women diagnosed with HIV in 2018 were African American and many WLWH have other risk factors associated with earlier menopause, studies examining the age of menopause in WLWH need to use a comparator group of women without HIV with similar characteristics and control for these factors to determine the influence of HIV on the age of menopause.
Menopause-Associated Symptoms
The perimenopausal period, which begins, on average, 4 years prior to the final menstrual period, is characterized by hormonal fluctuations leading to irregular menstrual cycles.19,20 Symptoms associated with these physiologic changes during the perimenopausal period include vasomotor symptoms (hot flashes), genitourinary symptoms (vaginal dryness and dyspareunia), anxiety, depression, sleep disturbances, and joint aches.21,22 Such menopausal symptoms can be distressing and negatively impact quality of life.23 In WLWH, severe menopausal symptoms have been associated with suboptimal adherence to antiretroviral therapy (ART).24
Vasomotor
In the United States, the most common symptom during perimenopause is hot flashes, which occur in 38% to 80% of women.32,33 Vasomotor symptoms are most common in women who smoke, use illicit substances, have a high BMI, are of lower socioeconomic status, and are African American.11 As expected, prior studies focusing on hot flash prevalence among premenopausal, perimenopausal, and postmenopausal WLWH found that postmenopausal women experience more hot flashes than premenopausal or perimenopausal women.27,28 In addition, a comparison of women with and without HIV demonstrated a higher prevalence of hot flashes among WLWH.26,29 Vasomotor symptoms can be severely distressing, with hot flashes contributing to increased risk of depression.25,34 In a cross-sectional analysis of 835 WLWH and 335 women without HIV from the WIHS cohort, persistent vasomotor symptoms predicted elevated depressive symptoms in both WLWH and women without HIV.34 In a similar cross-sectional analysis of 536 women, among whom 54% were WLWH and 37% were perimenopausal, psychological symptoms were prevalent in 61% of the women with vasomotor symptoms.29
Genitourinary
Estrogen deficiency, which accompanies the perimenopausal period, leads to vulvovaginal atrophy (VVA), manifesting with symptoms of vaginal dryness, itching, burning, urinary urgency, and dyspareunia (painful intercourse).33,35,36 Unlike vasomotor symptoms, which diminish with time, genitourinary symptoms generally worsen if left untreated.37 Furthermore, these symptoms are often underreported and underdiagnosed.38,39 VVA was found in 43% to 84% of postmenopausal women.36,40,41 In the AGATA study, the prevalence of VVA was associated with years since menopause. 36 Vaginal dryness and dyspareunia were common.
Psychiatric
Anxiety and depression are also common symptoms in perimenopausal women.46-48 Studies have shown that depression is diagnosed 2.5 times more frequently among perimenopausal women than premenopausal women.48 In a study by Miller et al that focused on 536 WLWH, among whom 37% were perimenopausal, 89% reported psychological symptoms.29 Ferreira et al found that perimenopausal WLWH had an increased incidence of psychological symptoms, such as depression and anxiety, compared to women without HIV infection.26 Whether this increased prevalence of psychological symptoms seen in WLWH can be attributed to menopause is unclear, since one third to one half of men and women living with HIV experience symptoms of depression.49 However, in the WIHS, which compared findings from 835 WLWH to findings from 335 women without HIV from all menopausal stages, elevated depressive symptoms were seen in the early perimenopausal period.34 There was no increased incidence of such symptoms during the premenopausal or postmenopausal stage, suggesting that factors related to menopause contribute to depressive symptoms during the perimenopausal stage.34
Other Symptoms
Sleep disturbances are common among perimenopausal women, with an estimated prevalence between 38% and 46%.52-54 Hot flashes, anxiety, and depression appear to be factors that contribute to sleep difficulty.52-54 In a cross-sectional study of 273 WLWH and 264 women without HIV between 40 and 60 years of age, insomnia was found in 51% of perimenopausal and 53% of postmenopausal WLWH. The prevalence of insomnia in WLWH and women without HIV was the same.55 Joint aches are also commonly reported in the perimenopausal period, with a prevalence as high as 50% to 60% among perimenopausal women in the United States.22,29 Miller and colleagues found that 63% of menopausal WLWH reported arthralgia.29
Test your knowledge of this topic HERE.
Treatment
Despite the increased severity of menopausal symptoms experienced among WLWH, menopausal replacement therapy (MRT) is used less frequently in WLWH than in women living without HIV.55 Topical treatment is recommended for women who are experiencing vaginal dryness. First-line treatment is topical nonhormonal therapy, such as moisturizers and lubricants.56 If symptoms are not relieved, then topical vaginal estrogen therapy is recommended.56 Randomized placebo-controlled studies have verified the safety and efficacy of topical estrogen in the general population, and there is no reason to expect different outcomes in WLWH.57,58
Cardiovascular Risk
Estrogen deficiency that occurs during menopause leads to an increased risk of cardiovascular disease, particularly with changes in lipid profiles, insulin resistance, and body composition (eg, increased fat mass and waist circumference).61 HIV infection also is associated with a higher risk of cardiovascular disease, with studies consistently reporting a 1.5- to 2-fold increase in the rate of cardiovascular events in PLWH compared to persons without HIV.62 The inflammatory effects of HIV as well as ART exposure, specifically to PIs and abacavir, increase the risk for cardiovascular disease.62 In addition, traditional risk factors, including dyslipidemia, contribute to cardiovascular disease risk in this population.63,64
Osteoporosis
Menopause, with its associated estrogen deficiency, is the most important risk factor linked to increased bone turnover and bone loss.70 In addition, HIV is associated with bone loss, with low bone mineral density (BMD) described even among men and premenopausal women with HIV infection.71 Although decreased BMD associated with HIV stabilizes or even improves after initiation of ART in the younger population,72-74 chronic inflammation caused by HIV stimulates osteoclast differentiation and resorption.71 Other factors that appear to contribute to decreased BMD among PLWH include ART; vitamin D deficiency; low BMI; poor nutrition; inactivity; use of tobacco, alcohol, and illicit drugs; hepatitis B and C coinfection; and frailty, defined as increased vulnerability to stresses related to aging.72-80 Among ARTs, tenofovir disoproxil fumarate is associated with an increased risk of osteoporosis, and switching from this agent to tenofovir alafenamide improves bone density.81 Prolonged amenorrhea is also an added risk factor for osteoporosis in WLWH.82
Cognition
Both men and women living with HIV are at higher risk for cognitive impairment, ranging from minor cognitive-motor disorder to HIV-associated dementia.91 In addition, the menopause transition is characterized by cognitive changes, such as memory loss and difficulty concentrating.92,93 Studies focusing on the effects of both HIV infection and menopause on cognition have been limited thus far. A cross-sectional study demonstrated that HIV infection, but not menopausal stage, was associated with worse performance on cognitive measures.94 While menopausal stage was not associated with cognitive decline, menopausal symptoms like depression, anxiety, and vasomotor symptoms were associated with lower cognitive performance, highlighting the importance of recognition and treatment of menopausal symptoms.94
Test your knowledge of this topic HERE.
Cervical Dysplasia
WLWH are at increased risk for low- and high-grade squamous intraepithelial lesions (SILs) and more rapid progression to cervical carcinoma, as compared to women without HIV.95 This increased risk of cervical disease is associated with age, human papillomavirus genotype, and degree of immunosuppression.96 In addition, menopause appears to affect the risk of cervical disease. Postmenopausal WLWH had a higher risk of progression of SILs and persistence of lower-grade SILs compared to premenopausal women.97,98 Although studies on progression to cervical cancer in postmenopausal WLWH remain limited, current data suggest that postmenopausal WLWH should continue to be monitored and screened similarly to premenopausal women.
HIV Acquisition and Transmission
Women aged 50 years and older are primarily exposed to HIV through heterosexual contact.99 While the lack of awareness of HIV risk and less frequent use of barrier protection can contribute to new HIV infection in older women, physiologic changes associated with menopause also may be playing a role.100 Vaginal wall thinning and immunologic changes of the cervix that occur during menopause may serve as a risk factor for HIV acquisition. The cervicovaginal mucosa of postmenopausal women had higher levels of p24 antigen after ex vivo HIV-1 infection, suggesting higher susceptibility to acquire HIV infection.101 Postmenopausal women have been shown to have increased cervical CCR5 expression, which serves as an entry point of HIV into target cells.102 Finally, anti-HIV-1 activity was significantly decreased in postmenopausal women compared to premenopausal women.103 In addition, ex vivo studies demonstrated reduced tenofovir disoproxil fumarate and emtricitabine triphosphate concentrations in cervical tissue of postmenopausal women, suggesting that postmenopausal women may need higher doses of pre-exposure prophylaxis to achieve protective efficacy.104
HIV Progression
With prior data suggesting that younger persons experience better immunologic and virologic responses to ART,107-109 it had previously been hypothesized that virologic and immunologic responses to ART will decline once WLWH reach menopause. However, current studies suggest that menopause does not affect the progression of HIV and that ART-naive women should respond to ART, regardless of their menopausal status. Treatment responses to ART, determined by the median changes in CD4 cell counts and percentages and viral load, in ART-naive individuals did not differ between premenopausal and postmenopausal women.110 In addition, there appear to be no significant changes in CD4 cell counts as WLWH progress through menopause.111
Conclusion
As individuals with HIV infection live longer, an increasing number of women will enter menopause and live many years beyond menopause. WLWH experience earlier and more severe menopausal symptoms, but evidence on the appropriate management of these symptoms is still lacking. These conditions require proper surveillance, and can be prevented with an improved understanding of the effects of menopause on WLWH. However, there remain significant gaps in our understanding of menopause in WLWH. As practitioners encounter an increasing number of perimenopausal and postmenopausal WLWH, studies of the effects of HIV on comorbidities and symptoms of menopause and their appropriate management are necessary to improve care of WLWH.
Test your knowledge of this topic HERE.
More than half of the 37.9 million persons living with HIV (PLWH) worldwide are women.1 Between 2010 and 2016, 58% of women living with HIV (WLWH) in the United States were older than 45 years.2 As such, an increasing number of WLWH are entering menopause and living well beyond menopause. Despite this, health care providers expressed a lack of confidence in managing menopause in WLWH, and menopausal symptoms often are not recognized by providers.3 Enhancing our knowledge about menopause in WLWH is important, since the physiologic changes associated with menopause impact short- and long-term quality of life and mortality.
Test your knowledge of this topic HERE.
Amenorrhea
Menstrual irregularities, including amenorrhea and anovulation, are more frequently found in women of low socioeconomic status, presumably due to associated physical and emotional stress.4 In addition, women with low body mass index (BMI) have decreased serum estradiol levels, which lead to amenorrhea.4,5 Furthermore, low parity and many legal and illegal drugs are associated with amenorrhea, including hormonal contraceptives, opiates, stimulants, antipsychotics, and chemotherapeutic agents.6-8
Test your knowledge of this topic HERE.
Age at Menopause
In the United States, the median age of menopause is between 50 and 52 years in middle-class white women.11,12 Earlier menopause has been observed in women who are African American, are nulliparous, have a lower BMI, smoke tobacco, and have more stress, less education, and higher unemployment rates.11,13,14 Because 57% of women diagnosed with HIV in 2018 were African American and many WLWH have other risk factors associated with earlier menopause, studies examining the age of menopause in WLWH need to use a comparator group of women without HIV with similar characteristics and control for these factors to determine the influence of HIV on the age of menopause.
Menopause-Associated Symptoms
The perimenopausal period, which begins, on average, 4 years prior to the final menstrual period, is characterized by hormonal fluctuations leading to irregular menstrual cycles.19,20 Symptoms associated with these physiologic changes during the perimenopausal period include vasomotor symptoms (hot flashes), genitourinary symptoms (vaginal dryness and dyspareunia), anxiety, depression, sleep disturbances, and joint aches.21,22 Such menopausal symptoms can be distressing and negatively impact quality of life.23 In WLWH, severe menopausal symptoms have been associated with suboptimal adherence to antiretroviral therapy (ART).24
Vasomotor
In the United States, the most common symptom during perimenopause is hot flashes, which occur in 38% to 80% of women.32,33 Vasomotor symptoms are most common in women who smoke, use illicit substances, have a high BMI, are of lower socioeconomic status, and are African American.11 As expected, prior studies focusing on hot flash prevalence among premenopausal, perimenopausal, and postmenopausal WLWH found that postmenopausal women experience more hot flashes than premenopausal or perimenopausal women.27,28 In addition, a comparison of women with and without HIV demonstrated a higher prevalence of hot flashes among WLWH.26,29 Vasomotor symptoms can be severely distressing, with hot flashes contributing to increased risk of depression.25,34 In a cross-sectional analysis of 835 WLWH and 335 women without HIV from the WIHS cohort, persistent vasomotor symptoms predicted elevated depressive symptoms in both WLWH and women without HIV.34 In a similar cross-sectional analysis of 536 women, among whom 54% were WLWH and 37% were perimenopausal, psychological symptoms were prevalent in 61% of the women with vasomotor symptoms.29
Genitourinary
Estrogen deficiency, which accompanies the perimenopausal period, leads to vulvovaginal atrophy (VVA), manifesting with symptoms of vaginal dryness, itching, burning, urinary urgency, and dyspareunia (painful intercourse).33,35,36 Unlike vasomotor symptoms, which diminish with time, genitourinary symptoms generally worsen if left untreated.37 Furthermore, these symptoms are often underreported and underdiagnosed.38,39 VVA was found in 43% to 84% of postmenopausal women.36,40,41 In the AGATA study, the prevalence of VVA was associated with years since menopause. 36 Vaginal dryness and dyspareunia were common.
Psychiatric
Anxiety and depression are also common symptoms in perimenopausal women.46-48 Studies have shown that depression is diagnosed 2.5 times more frequently among perimenopausal women than premenopausal women.48 In a study by Miller et al that focused on 536 WLWH, among whom 37% were perimenopausal, 89% reported psychological symptoms.29 Ferreira et al found that perimenopausal WLWH had an increased incidence of psychological symptoms, such as depression and anxiety, compared to women without HIV infection.26 Whether this increased prevalence of psychological symptoms seen in WLWH can be attributed to menopause is unclear, since one third to one half of men and women living with HIV experience symptoms of depression.49 However, in the WIHS, which compared findings from 835 WLWH to findings from 335 women without HIV from all menopausal stages, elevated depressive symptoms were seen in the early perimenopausal period.34 There was no increased incidence of such symptoms during the premenopausal or postmenopausal stage, suggesting that factors related to menopause contribute to depressive symptoms during the perimenopausal stage.34
Other Symptoms
Sleep disturbances are common among perimenopausal women, with an estimated prevalence between 38% and 46%.52-54 Hot flashes, anxiety, and depression appear to be factors that contribute to sleep difficulty.52-54 In a cross-sectional study of 273 WLWH and 264 women without HIV between 40 and 60 years of age, insomnia was found in 51% of perimenopausal and 53% of postmenopausal WLWH. The prevalence of insomnia in WLWH and women without HIV was the same.55 Joint aches are also commonly reported in the perimenopausal period, with a prevalence as high as 50% to 60% among perimenopausal women in the United States.22,29 Miller and colleagues found that 63% of menopausal WLWH reported arthralgia.29
Test your knowledge of this topic HERE.
Treatment
Despite the increased severity of menopausal symptoms experienced among WLWH, menopausal replacement therapy (MRT) is used less frequently in WLWH than in women living without HIV.55 Topical treatment is recommended for women who are experiencing vaginal dryness. First-line treatment is topical nonhormonal therapy, such as moisturizers and lubricants.56 If symptoms are not relieved, then topical vaginal estrogen therapy is recommended.56 Randomized placebo-controlled studies have verified the safety and efficacy of topical estrogen in the general population, and there is no reason to expect different outcomes in WLWH.57,58
Cardiovascular Risk
Estrogen deficiency that occurs during menopause leads to an increased risk of cardiovascular disease, particularly with changes in lipid profiles, insulin resistance, and body composition (eg, increased fat mass and waist circumference).61 HIV infection also is associated with a higher risk of cardiovascular disease, with studies consistently reporting a 1.5- to 2-fold increase in the rate of cardiovascular events in PLWH compared to persons without HIV.62 The inflammatory effects of HIV as well as ART exposure, specifically to PIs and abacavir, increase the risk for cardiovascular disease.62 In addition, traditional risk factors, including dyslipidemia, contribute to cardiovascular disease risk in this population.63,64
Osteoporosis
Menopause, with its associated estrogen deficiency, is the most important risk factor linked to increased bone turnover and bone loss.70 In addition, HIV is associated with bone loss, with low bone mineral density (BMD) described even among men and premenopausal women with HIV infection.71 Although decreased BMD associated with HIV stabilizes or even improves after initiation of ART in the younger population,72-74 chronic inflammation caused by HIV stimulates osteoclast differentiation and resorption.71 Other factors that appear to contribute to decreased BMD among PLWH include ART; vitamin D deficiency; low BMI; poor nutrition; inactivity; use of tobacco, alcohol, and illicit drugs; hepatitis B and C coinfection; and frailty, defined as increased vulnerability to stresses related to aging.72-80 Among ARTs, tenofovir disoproxil fumarate is associated with an increased risk of osteoporosis, and switching from this agent to tenofovir alafenamide improves bone density.81 Prolonged amenorrhea is also an added risk factor for osteoporosis in WLWH.82
Cognition
Both men and women living with HIV are at higher risk for cognitive impairment, ranging from minor cognitive-motor disorder to HIV-associated dementia.91 In addition, the menopause transition is characterized by cognitive changes, such as memory loss and difficulty concentrating.92,93 Studies focusing on the effects of both HIV infection and menopause on cognition have been limited thus far. A cross-sectional study demonstrated that HIV infection, but not menopausal stage, was associated with worse performance on cognitive measures.94 While menopausal stage was not associated with cognitive decline, menopausal symptoms like depression, anxiety, and vasomotor symptoms were associated with lower cognitive performance, highlighting the importance of recognition and treatment of menopausal symptoms.94
Test your knowledge of this topic HERE.
Cervical Dysplasia
WLWH are at increased risk for low- and high-grade squamous intraepithelial lesions (SILs) and more rapid progression to cervical carcinoma, as compared to women without HIV.95 This increased risk of cervical disease is associated with age, human papillomavirus genotype, and degree of immunosuppression.96 In addition, menopause appears to affect the risk of cervical disease. Postmenopausal WLWH had a higher risk of progression of SILs and persistence of lower-grade SILs compared to premenopausal women.97,98 Although studies on progression to cervical cancer in postmenopausal WLWH remain limited, current data suggest that postmenopausal WLWH should continue to be monitored and screened similarly to premenopausal women.
HIV Acquisition and Transmission
Women aged 50 years and older are primarily exposed to HIV through heterosexual contact.99 While the lack of awareness of HIV risk and less frequent use of barrier protection can contribute to new HIV infection in older women, physiologic changes associated with menopause also may be playing a role.100 Vaginal wall thinning and immunologic changes of the cervix that occur during menopause may serve as a risk factor for HIV acquisition. The cervicovaginal mucosa of postmenopausal women had higher levels of p24 antigen after ex vivo HIV-1 infection, suggesting higher susceptibility to acquire HIV infection.101 Postmenopausal women have been shown to have increased cervical CCR5 expression, which serves as an entry point of HIV into target cells.102 Finally, anti-HIV-1 activity was significantly decreased in postmenopausal women compared to premenopausal women.103 In addition, ex vivo studies demonstrated reduced tenofovir disoproxil fumarate and emtricitabine triphosphate concentrations in cervical tissue of postmenopausal women, suggesting that postmenopausal women may need higher doses of pre-exposure prophylaxis to achieve protective efficacy.104
HIV Progression
With prior data suggesting that younger persons experience better immunologic and virologic responses to ART,107-109 it had previously been hypothesized that virologic and immunologic responses to ART will decline once WLWH reach menopause. However, current studies suggest that menopause does not affect the progression of HIV and that ART-naive women should respond to ART, regardless of their menopausal status. Treatment responses to ART, determined by the median changes in CD4 cell counts and percentages and viral load, in ART-naive individuals did not differ between premenopausal and postmenopausal women.110 In addition, there appear to be no significant changes in CD4 cell counts as WLWH progress through menopause.111
Conclusion
As individuals with HIV infection live longer, an increasing number of women will enter menopause and live many years beyond menopause. WLWH experience earlier and more severe menopausal symptoms, but evidence on the appropriate management of these symptoms is still lacking. These conditions require proper surveillance, and can be prevented with an improved understanding of the effects of menopause on WLWH. However, there remain significant gaps in our understanding of menopause in WLWH. As practitioners encounter an increasing number of perimenopausal and postmenopausal WLWH, studies of the effects of HIV on comorbidities and symptoms of menopause and their appropriate management are necessary to improve care of WLWH.
Test your knowledge of this topic HERE.
1. UNAIDS. AIDSInfo. 2019 estimates https://www.unaids.org/en/resources/infographics/girls-and-women-living-with-HIV. Accessed April 30, 2020.
2. Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report 2019;24(No. 1). www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed April 30, 2020.
3. Chirwa M, Ma R, Guallar C, et al. Managing menopause in women living with HIV: A survey of primary care practitioners. Post Reprod Health. 2017;23:111-115.
4. Munster K, Helm P, Schmidt L. Secondary amenorrhea: Prevalence and medical contract–A cross sectional study from a Danish county. Br J Obstet Gynecol. 1992;99:430-433.
5. Vyver E, Steinegger C, Katzman DK, et al. Eating disorders and menstrual dysfunction in adolescents. Ann N Y Acad Sci. 2008;1135: 253-264.
6. Cejtin HE, Evans CT, Greenblatt R, et al. Prolonged amenorrhea and resumption of menses in women with HIV. J Womens Health (Larchmt). 2018;27:1441‐1448.
7. Bai J, Greenwald E, Caterini H, et al. Drug-related menstrual aberrations. Obstet Gynecol. 1974;44:713-719.
8. Cejtin HE, Kalinowski A, Bacchetti P. Effects of human immunodeficiency virus on protracted amenorrhea and ovarian dysfunction. Obstet Gynecol. 2006;108:1423-1431.
9. King EM, Albert AY, Murray MCM. HIV and amenorrhea: a meta-analysis. AIDS. 2019;33:483‐491.
10. Watts DH, Spino C, Zaborski L. Comparison of gynecologic history and laboratory results in HIV-positive women with CDR+ lymphocyte counts between 200 and 500 cells/μl and below 100 cells/ μl. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:455-462.
11. Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178:70-83.
12. Thomas F, Renaud F, Benefice E, et al. International variability of ages at menarche and menopause: patterns and main determinants. Hum Biol. 2001;73:271-290.
13. Bromberger JT, Matthews KA, Kuller LH, et al. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997; 145:24-33.
14. Schoenbaum E, Hartel D, Lo Y, et al. HIV infection, drug use, and onset of natural menopause. Clinical Infect Dis. 2005;41: 1517-1524.
15. Research on the menopause in the 1990s. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1996;866:1-107.
16. de Pommerol M, Hessamfar M, Lawson-Ayayi S, et al. Menopause and HIV infection: age at onset and associated factors, ANRS CO3 Aquitaine cohort. Int J STD AIDS. 2011;22:67-72.
17. Calvet G, Grinsztejn G. Predictors of early menopause in HIV infected women: a prospective cohort study. Am J Obstet Gynecol. 2015;212:765.
18. Cejtin SH, Taylor R, Watts DH. Assessment of menopausal status among women in the Women’s Interagency HIV study (WIHS). Proceedings of the XV International AIDS Conference; July 11-16, 2004; Bangkok, Thailand.
19. Taffe JR, Dennerstein L. Menstrual patterns leading to the final menstrual period. Menopause. 2002;9:32-40.
20. Miro F, Parker SW, Aspinall LJ, et al. Origins and consequences of the elongation of the human menstrual cycle during the menopausal transition: the FREEDOM Study. J Clin Endocrinol Metab. 2004;89:4910-4915.
21. McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14:103-115.
22. Blümel JE, Chedraui P, Baron G, et al. Menopause could be involved in the pathogenesis of muscle and joint aches in mid-aged women. Maturitas. 2013;75:94-100.
23. Woods NF, Mitchell ES. Symptoms interference with work and relationships during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2011;18:654-661.
24. Duff PK, Money DM, Ogilvie GS, et al. Severe menopausal symptoms associated with reduced adherence to antiretroviral therapy among perimenopausal and menopausal women living with HIV in Metro Vancouver. Menopause. 2018;25:531-537.
25. Johnson TM, Cohen HW, Howard AA, et al. Attribution of menopause symptoms in human immunodeficiency virus–infected or at-risk drug-using women. Menopause. 2008;15:551-557.
26. Ferreira CE, Pinto-Neto AM, Conde DM, et al. Menopausal symptoms in women infected with HIV: prevalence and associated factors. Gynecol Endocrinol. 2007;23:198-205.
27. Fantry L, Zhan M, Taylor G, et al. Age at menopause and menopausal symptoms in HIV-infected women. AIDS Patient Care STD. 2005;19:703-711.
28. Boonyanurak P, Bunupuradah T, Wilawan K, et al. Age at menopause and menopause-related symptoms in human immunodeficiency virus-infected Thai women. Menopause. 2012;19: 820-824.
29. Miller SA, Santoro N, Lo Y. Menopausal symptoms in HIV-infected and drug-using women. Menopause. 2005;12:348-356.
30. Looby S, Shifren J, Corless I. Increased hot flash severity and related interference in perimenopausal HIV-infected women. Menopause. 2014;21:403-409.
31. Schnall R, Jia H, Olender S, et al. In people living with HIV (PLWH), menopause (natural or surgical) contributes to the greater symptom burden in women: results from an online US survey. Menopause. 2018;25:744-752.
32. Thurston RC, Joffe H. Vasomotor symptoms and menopause: findings from the Study of Women’s Health across the Nation. Obstet Gynecol Clin North Am. 2011;38:489-501.
33. Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 Suppl 12B:14-24.
34. Maki PM, Rubin LH, Cohen M, et al. Depressive symptoms are increased in the early perimenopausal stage in ethnically diverse human immunodeficiency virus-infected and human immunodeficiency virus-uninfected women. Menopause. 2012;19: 1215-1233.
35. Dennerstein L, Dudley EC, Hopper JL, et al. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
36. Palma F, Volpe A, Villa P, et al. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: The AGATA study. Maturitas. 2016;83:40-44.
37. Cutler WB, Garcia CR, McCoy N. Perimenopausal sexuality. Arch Sex Behav. 1987;16:225-234.
38. Moreira ED, Glasser DB, Nicolosi A, et al. GSSAB Investigators’ Group. Sexual problems and help-seeking behavior in adults in the United Kingdom and continental Europe. BJU Int. 2008;101:1005-1111.
39. MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85:87-94.
40. Nappi RE, Kokot-Kierepa M. Women’s voices in the menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67:233-238.
41. Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6:2133-2142.
42. Valadares AL, Pinto-Neto AM, Conde DM, et al. A population-based study of dyspareunia in a cohort of middle-aged Brazilian women. Menopause. 2008;15:1184-1190.
43. Latthe P, Migini L, Gray R, et al. Factors predisposing women to chronic pelvic pain: a systemic review. BMJ. 2006;332:749-755.
44. Potter DA, Moreno A, Luther MF, et al. Effects of follicular-phase cocaine administration on menstrual and ovarian cyclicity in rhesus monkeys. Am J Obstet Gynecol. 1998;178:118-125.
45. Valadares AL, Pinto-Neto AM, Gomes D, et al. Dyspareunia in HIV-positive and HIV-negative middle-aged women: a cross-sectional study. BMJ Open. 2014;4:e004974.
46. Bromberger JT, Meyer PM, Kravitz HM, et al. Psychologic distress and natural menopause: a multiethnic community study. Am J Public Health. 2001;91:1435-1442.
47. Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression. Results from the Massachusetts Women’s Health Study. Ann Epidemiol. 1994;4:214-220.
48. Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006; 63:375-382.
49. Eller LS, Corless I, Bunch EH, et al. Self-care strategies for depressive symptoms in people with HIV disease. J Adv Nurs. 2005;51:119-130.
50. Fuh JL, Wang SJ, Lee SJ, et al. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas. 2006;53:447-453.
51. Hinkin CH, Castellon SA, Atkinson JH, et al. Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol. 2001;54:S44-S52
52. Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19-28.
53. Freedman RR, Roehrs TA. Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause. 2006;13:576-583.
54. Erlik Y, Tataryn IV, Meldrum DR, et al. Association of waking episodes aspects of HIV infection among older adults. J Clin Epidemiol. 2001;54:S44–52.
55. Lui-Filho JF, Valadares AR, Gomes D, et al. Menopausal symptoms and associated factors in HIV-positive women. Maturitas. 2013;76:172-178.
56. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888‐904.
57. Fernandes T, Pedro AO, Baccaro LF, et al. Hormonal, metabolic, and endometrial safety of testosterone vaginal cream versus estrogens for the treatment of vulvovaginal atrophy in postmenopausal women: a randomized, placebo-controlled study. Menopause. 2018; 25:641‐647.
58. Kroll R, Archer DF, Lin Y, et al. A randomized, multicenter, double-blind study to evaluate the safety and efficacy of estradiol vaginal cream 0.003% in postmenopausal women with dyspareunia as the most bothersome symptom. Menopause. 2018;25:133‐138.
59. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Tables 21a-d.www.aidsinfo.nih.gov/ContentFiles/ AdultandAdolescentGL.pdf. Accessed May 4, 2020.
60. Tittle, V, Bull, L, Boffito, M. Pharmacokinetic and pharmacodynamics drug interactions between antiretrovirals and oral contraceptives. Clin Pharmacokinet. 2015;54:23-34.
61. Sower M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrin Metab. 2007;92:895- 901.
62. Eyawo O, Brockman G, Goldsmith CH, et al. Risk of myocardial infarction among people living with HIV: an updated systematic review and meta-analysis. BMJ Open. 2019;9:e025874.
63. Flooris-Moore M, Howard AA, Lo Y, et al. Increased serum lipids are associated with higher CD4 lymphocyte count in HIV-infected women. HIV Med. 2006;7:421-430.
64. Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130-139.
65. Grinspoon S, Carr A. Cardiovascular risk and body fat abnormalities in HIV-infected adults. N Engl J Med. 2005; 352:48–62.
66. Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM). Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42:562-571.
67. Alcaide ML, Parmigiani A, Pallikkuth S, et al. Immune activation in HIV-infected aging women on antiretrovirals--implications for age-associated comorbidities: a cross-sectional pilot study. PLoS One. 2013;8:e63804.
68. Karim R, Mack WJ, Kono N, et al. Gonadotropin and sex steroid levels in HIV-infected premenopausal women and their association with subclinical atherosclerosis in HIV-infected and -uninfected women in the women’s interagency HIV study (WIHS). J Clin Endocrinol Metab. 2013;98:E610‐E618.
69. Looby SE, Fitch KV, Srinivasa S, et al. Reduced ovarian reserve relates to monocyte activation and subclinical coronary atherosclerotic plaque in women with HIV. AIDS. 2016;30:383‐393.
70. Akhter MP, Lappe JM, Davies KM, et al. Transmenopausal changes in the trabecular bone structure. Bone. 2007;41:111-116.
71. Gibellini D, De Crignis E, Ponti C. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNF-alpha activation. J Med Virol. 2008;80:1507-1514.
72. Cassetti I, Madruga JV, Suleiman JM, et al. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV- 1-infected patients. HIV Clin Trials. 2007;8:164-172.
73. McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203: 1791-1801.
74. Hansen AB, Obel N, Nielsen H, et al. Bone mineral density changes in protease inhibitor-sparing vs. nucleoside reverse transcriptase inhibitor-sparing highly active antiretroviral therapy: Data from a randomized trial. HIV Med. 2011;12:157-165.
75. FDao CN, Patel P, Overton ET, et al. Study to understand the natural history of HIV and AIDS in the era of effective therapy (SUN) investigators. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D levels in cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396-405.
76. Jacobson DL, Spiegelman D, Know TK, Wilson IB. Evolution and predictors of change in total bone mineral density over time in HIV-infected men and women in the nutrition for healthy living study. J Acquir Immune Defic Syndr Hum Retrovirol. 2008;49:298-308.
77. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporosis Int. 2005;16:581-589.
78. Pedrazzoni M, Vescovi L, Maninetti M, et al. Effects of chronic heroine abuse on bone and mineral metabolism. Acta Endocrinol. 1993;129:42-45.
79. Lo Re V 3rd, Guaraldi G, Leonard MB, et al. Viral hepatitis is associated with reduced bone mineral density in HIV-infected women but not men. AIDS. 1990;23:2191-2198.
80. Bregigeon S, Galinier A, Zaegel-Faucher O, et al. Frailty in HIV infected people: a new risk factor for bone mineral density loss [published correction appears in AIDS. AIDS. 2017;31: 1573‐1577.
81. Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2015;16:43-45.
82. King EM, Nesbitt A, Albert AYK, et al. Prolonged amenorrhea and low hip bone mineral density in women living with HIV-a controlled cross-sectional study. J Acquir Immune Defic Syndr. 2020;83:
486‐495.
83. Yin MT, Mcmahon DJ, Ferris DC, et al. Low bone mass and high bone turnover in postmenopausal human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2010;95:620-629.
84. Yin MT, Modarresi R, Shane E, et al. Effects of HIV infection and antiretroviral therapy with ritonavir on induction of osteoclast-like cells in postmenopausal women. Osteoporos Int. 2011;22:1459-1466.
85. Sharma A, Cohen HW, Freeman R, et al. Prospective evaluation of bone mineral density among middle-aged HIV-infected and uninfected women: association between methadone use and bone loss. Maturitas. 2011;70:295-301.
86. Sharma A, Flom PL, Rosen CJ, et al. Racial differences in bone loss and relation to menopause among HIV-infected and uninfected women. Bone. 2015;77:24-30.
87. Aberg JA, Gallant JE, Ghanem KG, et al, Infectious Diseases Society of America. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:1‐10.
88. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis 2014. Washington, DC: National Osteoporosis Foundation; 2014.
89. Yin MT, Choudhury A, Bucovsky M, et al. A randomized placebo-controlled trial of low- versus moderate-dose vitamin d3 supplementation on bone mineral density in postmenopausal women with HIV. J Acquir Immune Defic Syndr. 2019;80:342-349.
90. McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937-946.
91. Price RW. Neurological complications of HIV infection. Lancet. 1996;348:445-452.
92. Soares CN, Maki PM. Menopausal transition, mood, and cognition: an integrated view to close the gaps. Menopause. 2010;17:812-814.
93. Greendale GA, Wight RG, Huang MH, et al. Menopause-associated symptoms and cognitive performance: results from the study of women’s health across the nation. Am J Epidemiol. 2010;171:1214-1224.
94. Rubin LH, Sundermann EE, Cook JA, et al. An investigation of menopausal stage and symptoms on cognition in HIV-infected women. Menopause. 2014;21:997-1006.
95. Ellerbrock TV, Chiasson MA, Bush TJ, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA. 2000;283:1031-1037.
96. Mandelblatt JS, Kanetsky P, Eggert L, et al. Is HIV infection a cofactor for cervical squamous cell neoplasia? Cancer Epidemiol Biomarkers Prev. 1999;8:97-106.
97. Kim SC, Messing S, Shah K, et al. Effects of highly active antiretroviral therapy (HAART) and menopause on risk of progression of cervical dysplasia in human immune deficiency virus (HIV) infected women. Infect Dis Obstet Gynecol. 2013;2013:784718.
98. Ceccaldi PF, Ferreira C, Coussy F, et al. Cervical disease in postmenopausal HIV-1 infected women. J Gynecol Obstet Biol Reprod. 2010;39:466-470.
99. Centers for Disease Control and Prevention. HIV and older Americans. www.cdc.gov/hiv/group/age/olderamericans/index.html. Accessed May 11, 2020.
100. Levy JA, Ory MG, Crystal S. HIV/AIDS interventions for midlife and older adults: current status and challenges. J Acquir Immune Defic Syndr. 2003;33 Suppl 2:S59-S67.
101. Thurman AR, Yousefieh N, Chandra N, et al. Comparison of mucosal markers of human immunodeficiency virus susceptibility in healthy premenopausal versus postmenopausal women. AIDS Res Hum Retroviruses. 2017;33:807-819.
102. Meditz AL, Moreau KL, MaWhinney S, et al. CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J Acquir Immune Defic Syndr. 2012;59:221-228.
103. Chappell CA, Isaacs CE, Xu W, et al. The effect of menopause on the innate antiviral activity of cervicovaginal lavage. Am J Obstet Gynecol. 2015;213:204.
104. Nicol MR, Brewers LM, Kashuba ADM, et al. The role of menopause in tenofovir diphosphate and emtricitabine triphosphate concentrations in cervical tissue. AIDS. 2018;32:11-15.
105. Melo KC, Melo MR, Ricci BV, Segurado AC. Correlates of human immunodeficiency virus cervicovaginal shedding among postmenopausal and fertile-aged women. Menopause. 2012;19:150-156.
106. Landolt NK, Do T, Kasipong N, et al. Low-level genital HIV shedding in Thai HIV-infected women with suppressed plasma viral load after menopause: a longitudinal study. J Virus Erad. 2017;3:204-207.
107. Viard JP, Mocroft A, Chiesi A, et al. Influence of age of CD4 cell recovery in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy: evidence from the Euro SIDA study. J Infect Dis. 2001;193:1290-1294.
108. Grabar S, Kousignian I, Sobel A, et al. Immunological and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS. 2004;18:2029-2038.
109. Cuzin L, Delpierre C, Gerard S, et al. Immunologic and clinical responses to highly active antiretroviral therapy in patients with HIV infection aged >50 years. Clin Infect Dis. 2007;45:654-657.
110. Patterson KB, Cohn SE, Uynik J, et al. Treatment responses in antiretroviral treatment-naïve premenopausal and postmenopausal HIV-1 infected women: an analysis from AIDS clinical trials group studies. Clin Infect Dis. 2009;49:473476.
111. van Benthem BH, Vernazza P, Coutinho RA, et al. The impact of pregnancy and menopause on CD4 lymphocyte count in HIV-infected women. AIDS. 2002;16:919-922.
1. UNAIDS. AIDSInfo. 2019 estimates https://www.unaids.org/en/resources/infographics/girls-and-women-living-with-HIV. Accessed April 30, 2020.
2. Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report 2019;24(No. 1). www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed April 30, 2020.
3. Chirwa M, Ma R, Guallar C, et al. Managing menopause in women living with HIV: A survey of primary care practitioners. Post Reprod Health. 2017;23:111-115.
4. Munster K, Helm P, Schmidt L. Secondary amenorrhea: Prevalence and medical contract–A cross sectional study from a Danish county. Br J Obstet Gynecol. 1992;99:430-433.
5. Vyver E, Steinegger C, Katzman DK, et al. Eating disorders and menstrual dysfunction in adolescents. Ann N Y Acad Sci. 2008;1135: 253-264.
6. Cejtin HE, Evans CT, Greenblatt R, et al. Prolonged amenorrhea and resumption of menses in women with HIV. J Womens Health (Larchmt). 2018;27:1441‐1448.
7. Bai J, Greenwald E, Caterini H, et al. Drug-related menstrual aberrations. Obstet Gynecol. 1974;44:713-719.
8. Cejtin HE, Kalinowski A, Bacchetti P. Effects of human immunodeficiency virus on protracted amenorrhea and ovarian dysfunction. Obstet Gynecol. 2006;108:1423-1431.
9. King EM, Albert AY, Murray MCM. HIV and amenorrhea: a meta-analysis. AIDS. 2019;33:483‐491.
10. Watts DH, Spino C, Zaborski L. Comparison of gynecologic history and laboratory results in HIV-positive women with CDR+ lymphocyte counts between 200 and 500 cells/μl and below 100 cells/ μl. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:455-462.
11. Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178:70-83.
12. Thomas F, Renaud F, Benefice E, et al. International variability of ages at menarche and menopause: patterns and main determinants. Hum Biol. 2001;73:271-290.
13. Bromberger JT, Matthews KA, Kuller LH, et al. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997; 145:24-33.
14. Schoenbaum E, Hartel D, Lo Y, et al. HIV infection, drug use, and onset of natural menopause. Clinical Infect Dis. 2005;41: 1517-1524.
15. Research on the menopause in the 1990s. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1996;866:1-107.
16. de Pommerol M, Hessamfar M, Lawson-Ayayi S, et al. Menopause and HIV infection: age at onset and associated factors, ANRS CO3 Aquitaine cohort. Int J STD AIDS. 2011;22:67-72.
17. Calvet G, Grinsztejn G. Predictors of early menopause in HIV infected women: a prospective cohort study. Am J Obstet Gynecol. 2015;212:765.
18. Cejtin SH, Taylor R, Watts DH. Assessment of menopausal status among women in the Women’s Interagency HIV study (WIHS). Proceedings of the XV International AIDS Conference; July 11-16, 2004; Bangkok, Thailand.
19. Taffe JR, Dennerstein L. Menstrual patterns leading to the final menstrual period. Menopause. 2002;9:32-40.
20. Miro F, Parker SW, Aspinall LJ, et al. Origins and consequences of the elongation of the human menstrual cycle during the menopausal transition: the FREEDOM Study. J Clin Endocrinol Metab. 2004;89:4910-4915.
21. McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14:103-115.
22. Blümel JE, Chedraui P, Baron G, et al. Menopause could be involved in the pathogenesis of muscle and joint aches in mid-aged women. Maturitas. 2013;75:94-100.
23. Woods NF, Mitchell ES. Symptoms interference with work and relationships during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2011;18:654-661.
24. Duff PK, Money DM, Ogilvie GS, et al. Severe menopausal symptoms associated with reduced adherence to antiretroviral therapy among perimenopausal and menopausal women living with HIV in Metro Vancouver. Menopause. 2018;25:531-537.
25. Johnson TM, Cohen HW, Howard AA, et al. Attribution of menopause symptoms in human immunodeficiency virus–infected or at-risk drug-using women. Menopause. 2008;15:551-557.
26. Ferreira CE, Pinto-Neto AM, Conde DM, et al. Menopausal symptoms in women infected with HIV: prevalence and associated factors. Gynecol Endocrinol. 2007;23:198-205.
27. Fantry L, Zhan M, Taylor G, et al. Age at menopause and menopausal symptoms in HIV-infected women. AIDS Patient Care STD. 2005;19:703-711.
28. Boonyanurak P, Bunupuradah T, Wilawan K, et al. Age at menopause and menopause-related symptoms in human immunodeficiency virus-infected Thai women. Menopause. 2012;19: 820-824.
29. Miller SA, Santoro N, Lo Y. Menopausal symptoms in HIV-infected and drug-using women. Menopause. 2005;12:348-356.
30. Looby S, Shifren J, Corless I. Increased hot flash severity and related interference in perimenopausal HIV-infected women. Menopause. 2014;21:403-409.
31. Schnall R, Jia H, Olender S, et al. In people living with HIV (PLWH), menopause (natural or surgical) contributes to the greater symptom burden in women: results from an online US survey. Menopause. 2018;25:744-752.
32. Thurston RC, Joffe H. Vasomotor symptoms and menopause: findings from the Study of Women’s Health across the Nation. Obstet Gynecol Clin North Am. 2011;38:489-501.
33. Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 Suppl 12B:14-24.
34. Maki PM, Rubin LH, Cohen M, et al. Depressive symptoms are increased in the early perimenopausal stage in ethnically diverse human immunodeficiency virus-infected and human immunodeficiency virus-uninfected women. Menopause. 2012;19: 1215-1233.
35. Dennerstein L, Dudley EC, Hopper JL, et al. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
36. Palma F, Volpe A, Villa P, et al. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: The AGATA study. Maturitas. 2016;83:40-44.
37. Cutler WB, Garcia CR, McCoy N. Perimenopausal sexuality. Arch Sex Behav. 1987;16:225-234.
38. Moreira ED, Glasser DB, Nicolosi A, et al. GSSAB Investigators’ Group. Sexual problems and help-seeking behavior in adults in the United Kingdom and continental Europe. BJU Int. 2008;101:1005-1111.
39. MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85:87-94.
40. Nappi RE, Kokot-Kierepa M. Women’s voices in the menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67:233-238.
41. Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6:2133-2142.
42. Valadares AL, Pinto-Neto AM, Conde DM, et al. A population-based study of dyspareunia in a cohort of middle-aged Brazilian women. Menopause. 2008;15:1184-1190.
43. Latthe P, Migini L, Gray R, et al. Factors predisposing women to chronic pelvic pain: a systemic review. BMJ. 2006;332:749-755.
44. Potter DA, Moreno A, Luther MF, et al. Effects of follicular-phase cocaine administration on menstrual and ovarian cyclicity in rhesus monkeys. Am J Obstet Gynecol. 1998;178:118-125.
45. Valadares AL, Pinto-Neto AM, Gomes D, et al. Dyspareunia in HIV-positive and HIV-negative middle-aged women: a cross-sectional study. BMJ Open. 2014;4:e004974.
46. Bromberger JT, Meyer PM, Kravitz HM, et al. Psychologic distress and natural menopause: a multiethnic community study. Am J Public Health. 2001;91:1435-1442.
47. Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression. Results from the Massachusetts Women’s Health Study. Ann Epidemiol. 1994;4:214-220.
48. Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006; 63:375-382.
49. Eller LS, Corless I, Bunch EH, et al. Self-care strategies for depressive symptoms in people with HIV disease. J Adv Nurs. 2005;51:119-130.
50. Fuh JL, Wang SJ, Lee SJ, et al. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas. 2006;53:447-453.
51. Hinkin CH, Castellon SA, Atkinson JH, et al. Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol. 2001;54:S44-S52
52. Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19-28.
53. Freedman RR, Roehrs TA. Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause. 2006;13:576-583.
54. Erlik Y, Tataryn IV, Meldrum DR, et al. Association of waking episodes aspects of HIV infection among older adults. J Clin Epidemiol. 2001;54:S44–52.
55. Lui-Filho JF, Valadares AR, Gomes D, et al. Menopausal symptoms and associated factors in HIV-positive women. Maturitas. 2013;76:172-178.
56. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888‐904.
57. Fernandes T, Pedro AO, Baccaro LF, et al. Hormonal, metabolic, and endometrial safety of testosterone vaginal cream versus estrogens for the treatment of vulvovaginal atrophy in postmenopausal women: a randomized, placebo-controlled study. Menopause. 2018; 25:641‐647.
58. Kroll R, Archer DF, Lin Y, et al. A randomized, multicenter, double-blind study to evaluate the safety and efficacy of estradiol vaginal cream 0.003% in postmenopausal women with dyspareunia as the most bothersome symptom. Menopause. 2018;25:133‐138.
59. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Tables 21a-d.www.aidsinfo.nih.gov/ContentFiles/ AdultandAdolescentGL.pdf. Accessed May 4, 2020.
60. Tittle, V, Bull, L, Boffito, M. Pharmacokinetic and pharmacodynamics drug interactions between antiretrovirals and oral contraceptives. Clin Pharmacokinet. 2015;54:23-34.
61. Sower M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrin Metab. 2007;92:895- 901.
62. Eyawo O, Brockman G, Goldsmith CH, et al. Risk of myocardial infarction among people living with HIV: an updated systematic review and meta-analysis. BMJ Open. 2019;9:e025874.
63. Flooris-Moore M, Howard AA, Lo Y, et al. Increased serum lipids are associated with higher CD4 lymphocyte count in HIV-infected women. HIV Med. 2006;7:421-430.
64. Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130-139.
65. Grinspoon S, Carr A. Cardiovascular risk and body fat abnormalities in HIV-infected adults. N Engl J Med. 2005; 352:48–62.
66. Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM). Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42:562-571.
67. Alcaide ML, Parmigiani A, Pallikkuth S, et al. Immune activation in HIV-infected aging women on antiretrovirals--implications for age-associated comorbidities: a cross-sectional pilot study. PLoS One. 2013;8:e63804.
68. Karim R, Mack WJ, Kono N, et al. Gonadotropin and sex steroid levels in HIV-infected premenopausal women and their association with subclinical atherosclerosis in HIV-infected and -uninfected women in the women’s interagency HIV study (WIHS). J Clin Endocrinol Metab. 2013;98:E610‐E618.
69. Looby SE, Fitch KV, Srinivasa S, et al. Reduced ovarian reserve relates to monocyte activation and subclinical coronary atherosclerotic plaque in women with HIV. AIDS. 2016;30:383‐393.
70. Akhter MP, Lappe JM, Davies KM, et al. Transmenopausal changes in the trabecular bone structure. Bone. 2007;41:111-116.
71. Gibellini D, De Crignis E, Ponti C. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNF-alpha activation. J Med Virol. 2008;80:1507-1514.
72. Cassetti I, Madruga JV, Suleiman JM, et al. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV- 1-infected patients. HIV Clin Trials. 2007;8:164-172.
73. McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203: 1791-1801.
74. Hansen AB, Obel N, Nielsen H, et al. Bone mineral density changes in protease inhibitor-sparing vs. nucleoside reverse transcriptase inhibitor-sparing highly active antiretroviral therapy: Data from a randomized trial. HIV Med. 2011;12:157-165.
75. FDao CN, Patel P, Overton ET, et al. Study to understand the natural history of HIV and AIDS in the era of effective therapy (SUN) investigators. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D levels in cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396-405.
76. Jacobson DL, Spiegelman D, Know TK, Wilson IB. Evolution and predictors of change in total bone mineral density over time in HIV-infected men and women in the nutrition for healthy living study. J Acquir Immune Defic Syndr Hum Retrovirol. 2008;49:298-308.
77. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporosis Int. 2005;16:581-589.
78. Pedrazzoni M, Vescovi L, Maninetti M, et al. Effects of chronic heroine abuse on bone and mineral metabolism. Acta Endocrinol. 1993;129:42-45.
79. Lo Re V 3rd, Guaraldi G, Leonard MB, et al. Viral hepatitis is associated with reduced bone mineral density in HIV-infected women but not men. AIDS. 1990;23:2191-2198.
80. Bregigeon S, Galinier A, Zaegel-Faucher O, et al. Frailty in HIV infected people: a new risk factor for bone mineral density loss [published correction appears in AIDS. AIDS. 2017;31: 1573‐1577.
81. Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2015;16:43-45.
82. King EM, Nesbitt A, Albert AYK, et al. Prolonged amenorrhea and low hip bone mineral density in women living with HIV-a controlled cross-sectional study. J Acquir Immune Defic Syndr. 2020;83:
486‐495.
83. Yin MT, Mcmahon DJ, Ferris DC, et al. Low bone mass and high bone turnover in postmenopausal human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2010;95:620-629.
84. Yin MT, Modarresi R, Shane E, et al. Effects of HIV infection and antiretroviral therapy with ritonavir on induction of osteoclast-like cells in postmenopausal women. Osteoporos Int. 2011;22:1459-1466.
85. Sharma A, Cohen HW, Freeman R, et al. Prospective evaluation of bone mineral density among middle-aged HIV-infected and uninfected women: association between methadone use and bone loss. Maturitas. 2011;70:295-301.
86. Sharma A, Flom PL, Rosen CJ, et al. Racial differences in bone loss and relation to menopause among HIV-infected and uninfected women. Bone. 2015;77:24-30.
87. Aberg JA, Gallant JE, Ghanem KG, et al, Infectious Diseases Society of America. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:1‐10.
88. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis 2014. Washington, DC: National Osteoporosis Foundation; 2014.
89. Yin MT, Choudhury A, Bucovsky M, et al. A randomized placebo-controlled trial of low- versus moderate-dose vitamin d3 supplementation on bone mineral density in postmenopausal women with HIV. J Acquir Immune Defic Syndr. 2019;80:342-349.
90. McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937-946.
91. Price RW. Neurological complications of HIV infection. Lancet. 1996;348:445-452.
92. Soares CN, Maki PM. Menopausal transition, mood, and cognition: an integrated view to close the gaps. Menopause. 2010;17:812-814.
93. Greendale GA, Wight RG, Huang MH, et al. Menopause-associated symptoms and cognitive performance: results from the study of women’s health across the nation. Am J Epidemiol. 2010;171:1214-1224.
94. Rubin LH, Sundermann EE, Cook JA, et al. An investigation of menopausal stage and symptoms on cognition in HIV-infected women. Menopause. 2014;21:997-1006.
95. Ellerbrock TV, Chiasson MA, Bush TJ, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA. 2000;283:1031-1037.
96. Mandelblatt JS, Kanetsky P, Eggert L, et al. Is HIV infection a cofactor for cervical squamous cell neoplasia? Cancer Epidemiol Biomarkers Prev. 1999;8:97-106.
97. Kim SC, Messing S, Shah K, et al. Effects of highly active antiretroviral therapy (HAART) and menopause on risk of progression of cervical dysplasia in human immune deficiency virus (HIV) infected women. Infect Dis Obstet Gynecol. 2013;2013:784718.
98. Ceccaldi PF, Ferreira C, Coussy F, et al. Cervical disease in postmenopausal HIV-1 infected women. J Gynecol Obstet Biol Reprod. 2010;39:466-470.
99. Centers for Disease Control and Prevention. HIV and older Americans. www.cdc.gov/hiv/group/age/olderamericans/index.html. Accessed May 11, 2020.
100. Levy JA, Ory MG, Crystal S. HIV/AIDS interventions for midlife and older adults: current status and challenges. J Acquir Immune Defic Syndr. 2003;33 Suppl 2:S59-S67.
101. Thurman AR, Yousefieh N, Chandra N, et al. Comparison of mucosal markers of human immunodeficiency virus susceptibility in healthy premenopausal versus postmenopausal women. AIDS Res Hum Retroviruses. 2017;33:807-819.
102. Meditz AL, Moreau KL, MaWhinney S, et al. CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J Acquir Immune Defic Syndr. 2012;59:221-228.
103. Chappell CA, Isaacs CE, Xu W, et al. The effect of menopause on the innate antiviral activity of cervicovaginal lavage. Am J Obstet Gynecol. 2015;213:204.
104. Nicol MR, Brewers LM, Kashuba ADM, et al. The role of menopause in tenofovir diphosphate and emtricitabine triphosphate concentrations in cervical tissue. AIDS. 2018;32:11-15.
105. Melo KC, Melo MR, Ricci BV, Segurado AC. Correlates of human immunodeficiency virus cervicovaginal shedding among postmenopausal and fertile-aged women. Menopause. 2012;19:150-156.
106. Landolt NK, Do T, Kasipong N, et al. Low-level genital HIV shedding in Thai HIV-infected women with suppressed plasma viral load after menopause: a longitudinal study. J Virus Erad. 2017;3:204-207.
107. Viard JP, Mocroft A, Chiesi A, et al. Influence of age of CD4 cell recovery in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy: evidence from the Euro SIDA study. J Infect Dis. 2001;193:1290-1294.
108. Grabar S, Kousignian I, Sobel A, et al. Immunological and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS. 2004;18:2029-2038.
109. Cuzin L, Delpierre C, Gerard S, et al. Immunologic and clinical responses to highly active antiretroviral therapy in patients with HIV infection aged >50 years. Clin Infect Dis. 2007;45:654-657.
110. Patterson KB, Cohn SE, Uynik J, et al. Treatment responses in antiretroviral treatment-naïve premenopausal and postmenopausal HIV-1 infected women: an analysis from AIDS clinical trials group studies. Clin Infect Dis. 2009;49:473476.
111. van Benthem BH, Vernazza P, Coutinho RA, et al. The impact of pregnancy and menopause on CD4 lymphocyte count in HIV-infected women. AIDS. 2002;16:919-922.
MCC response varies based on immunosuppression type, especially CLL
Patients with Merkel cell carcinoma and chronic immunosuppression may fare better or worse on immunotherapy based on the reason for immunosuppression, according to recent research at the annual meeting of the Society for Investigative Dermatology, held virtually.
About 10% of patients with Merkel cell carcinoma (MCC) are immunosuppressed at diagnosis, and these patients tend to have a more aggressive disease course and worse disease-specific survival compared with immunocompetent patients, Lauren Zawacki, a research assistant in the Nghiem Lab at the University of Washington, Seattle, said in her presentation. Although patients are receiving immune checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 as treatments, the efficacy and side effects on immunosuppressed patients have not been well studied because many of these patients are not eligible for clinical trials.
Ms. Zawacki and colleagues analyzed data from a prospective Seattle registry of 1,442 patients with MCC, identifying 179 patients with MCC who had chronic immunosuppression due to chronic lymphocytic leukemia (CLL), solid organ transplants, autoimmune disorders, other hematological malignancies, and HIV and AIDS. Non-Hodgkin lymphoma comprised 7 of 8 patients in the group with other hematological malignancies, and Crohn’s disease made up 5 of 6 patients in the autoimmune disorder group. Of the 179 patients with MCC and immunosuppression, 31 patients were treated with either anti-PD-1 or anti-PD-L1 therapy.
There was an objective response rate of 52%, with 14 patients having a complete response, 2 patients having a partial response, and 15 patients experiencing disease progression. Of the patients with disease progression, 11 died of MCC. The response rate in immunocompromised patients is similar to results seen by her group in immunocompetent patients (Nghiem P et al. N Engl J Med 2016; 374:2542-52), said Ms. Zawacki. “While the overall objective response rate is comparable between immunocompetent and immunosuppressed patients, the response rates vary greatly between the different types of immunosuppression,” she said.
When grouping response rates by immunosuppression type, they found 2 of 11 patients with CLL (18%) and 2 of 6 patients with autoimmune disease (33%) had an objective response, while 2 of 3 patients with HIV/AIDS (66%) and 7 of 7 patients with other hematologic malignancies (100%) had an objective response.
“While the numbers of the cohort are small, there still seems to be a considerable difference in the response rate between the different types of immune suppression, which is critical when we’re treating patients who typically have a more aggressive disease course,” said Ms. Zawacki.
In particular, the finding of no patients with MCC and CLL achieving a complete response interested Ms. Zawacki and her colleagues, since about one-fourth of patients in the Seattle registry have this combination of disease. “Not only did none of the CLL patients have a complete response, but 7 out of the 11 patients with CLL died from MCC,” she explained. When examining further, the researchers found 45% of patients in this group discontinued because of side effects of immunotherapy and had a median time to recurrence of 1.5 months. “This finding suggests that CLL in particular plays a large role in impairing the function of the immune system, leading to not only a more aggressive disease course, but a poorer response to immunotherapy,” she said.
“There is a significant need for improved interventions for patients with CLL and autoimmune disorders,” she added. “Research for immunosuppressed patients is critical given the associated aggressive disease course and their lack of inclusion in clinical trials.”
Ms. Zawacki acknowledged the small number of patients in the study as a limitation, and patients who received follow-up at outside facilities may have received slightly different care, which could impact adverse event reporting or reasons for study discontinuation.
“A multi-institutional study would be beneficial to expand the number of patients in that cohort and to help confirm the trend observed in this study. In addition, future studies should assess the role of combination systemic therapy, such as neutron radiation and immunotherapy together in order to see if the objective response can be approved among immunosuppressed patients,” she said.
This study was supported by funding from the MCC Patient Gift Fund, the National Cancer Institute, and a grant from NIH. Ms. Zawacki reports no relevant conflicts of interest.
SOURCE: Zawacki L. SID 2020, Abstract 497.
Patients with Merkel cell carcinoma and chronic immunosuppression may fare better or worse on immunotherapy based on the reason for immunosuppression, according to recent research at the annual meeting of the Society for Investigative Dermatology, held virtually.
About 10% of patients with Merkel cell carcinoma (MCC) are immunosuppressed at diagnosis, and these patients tend to have a more aggressive disease course and worse disease-specific survival compared with immunocompetent patients, Lauren Zawacki, a research assistant in the Nghiem Lab at the University of Washington, Seattle, said in her presentation. Although patients are receiving immune checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 as treatments, the efficacy and side effects on immunosuppressed patients have not been well studied because many of these patients are not eligible for clinical trials.
Ms. Zawacki and colleagues analyzed data from a prospective Seattle registry of 1,442 patients with MCC, identifying 179 patients with MCC who had chronic immunosuppression due to chronic lymphocytic leukemia (CLL), solid organ transplants, autoimmune disorders, other hematological malignancies, and HIV and AIDS. Non-Hodgkin lymphoma comprised 7 of 8 patients in the group with other hematological malignancies, and Crohn’s disease made up 5 of 6 patients in the autoimmune disorder group. Of the 179 patients with MCC and immunosuppression, 31 patients were treated with either anti-PD-1 or anti-PD-L1 therapy.
There was an objective response rate of 52%, with 14 patients having a complete response, 2 patients having a partial response, and 15 patients experiencing disease progression. Of the patients with disease progression, 11 died of MCC. The response rate in immunocompromised patients is similar to results seen by her group in immunocompetent patients (Nghiem P et al. N Engl J Med 2016; 374:2542-52), said Ms. Zawacki. “While the overall objective response rate is comparable between immunocompetent and immunosuppressed patients, the response rates vary greatly between the different types of immunosuppression,” she said.
When grouping response rates by immunosuppression type, they found 2 of 11 patients with CLL (18%) and 2 of 6 patients with autoimmune disease (33%) had an objective response, while 2 of 3 patients with HIV/AIDS (66%) and 7 of 7 patients with other hematologic malignancies (100%) had an objective response.
“While the numbers of the cohort are small, there still seems to be a considerable difference in the response rate between the different types of immune suppression, which is critical when we’re treating patients who typically have a more aggressive disease course,” said Ms. Zawacki.
In particular, the finding of no patients with MCC and CLL achieving a complete response interested Ms. Zawacki and her colleagues, since about one-fourth of patients in the Seattle registry have this combination of disease. “Not only did none of the CLL patients have a complete response, but 7 out of the 11 patients with CLL died from MCC,” she explained. When examining further, the researchers found 45% of patients in this group discontinued because of side effects of immunotherapy and had a median time to recurrence of 1.5 months. “This finding suggests that CLL in particular plays a large role in impairing the function of the immune system, leading to not only a more aggressive disease course, but a poorer response to immunotherapy,” she said.
“There is a significant need for improved interventions for patients with CLL and autoimmune disorders,” she added. “Research for immunosuppressed patients is critical given the associated aggressive disease course and their lack of inclusion in clinical trials.”
Ms. Zawacki acknowledged the small number of patients in the study as a limitation, and patients who received follow-up at outside facilities may have received slightly different care, which could impact adverse event reporting or reasons for study discontinuation.
“A multi-institutional study would be beneficial to expand the number of patients in that cohort and to help confirm the trend observed in this study. In addition, future studies should assess the role of combination systemic therapy, such as neutron radiation and immunotherapy together in order to see if the objective response can be approved among immunosuppressed patients,” she said.
This study was supported by funding from the MCC Patient Gift Fund, the National Cancer Institute, and a grant from NIH. Ms. Zawacki reports no relevant conflicts of interest.
SOURCE: Zawacki L. SID 2020, Abstract 497.
Patients with Merkel cell carcinoma and chronic immunosuppression may fare better or worse on immunotherapy based on the reason for immunosuppression, according to recent research at the annual meeting of the Society for Investigative Dermatology, held virtually.
About 10% of patients with Merkel cell carcinoma (MCC) are immunosuppressed at diagnosis, and these patients tend to have a more aggressive disease course and worse disease-specific survival compared with immunocompetent patients, Lauren Zawacki, a research assistant in the Nghiem Lab at the University of Washington, Seattle, said in her presentation. Although patients are receiving immune checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 as treatments, the efficacy and side effects on immunosuppressed patients have not been well studied because many of these patients are not eligible for clinical trials.
Ms. Zawacki and colleagues analyzed data from a prospective Seattle registry of 1,442 patients with MCC, identifying 179 patients with MCC who had chronic immunosuppression due to chronic lymphocytic leukemia (CLL), solid organ transplants, autoimmune disorders, other hematological malignancies, and HIV and AIDS. Non-Hodgkin lymphoma comprised 7 of 8 patients in the group with other hematological malignancies, and Crohn’s disease made up 5 of 6 patients in the autoimmune disorder group. Of the 179 patients with MCC and immunosuppression, 31 patients were treated with either anti-PD-1 or anti-PD-L1 therapy.
There was an objective response rate of 52%, with 14 patients having a complete response, 2 patients having a partial response, and 15 patients experiencing disease progression. Of the patients with disease progression, 11 died of MCC. The response rate in immunocompromised patients is similar to results seen by her group in immunocompetent patients (Nghiem P et al. N Engl J Med 2016; 374:2542-52), said Ms. Zawacki. “While the overall objective response rate is comparable between immunocompetent and immunosuppressed patients, the response rates vary greatly between the different types of immunosuppression,” she said.
When grouping response rates by immunosuppression type, they found 2 of 11 patients with CLL (18%) and 2 of 6 patients with autoimmune disease (33%) had an objective response, while 2 of 3 patients with HIV/AIDS (66%) and 7 of 7 patients with other hematologic malignancies (100%) had an objective response.
“While the numbers of the cohort are small, there still seems to be a considerable difference in the response rate between the different types of immune suppression, which is critical when we’re treating patients who typically have a more aggressive disease course,” said Ms. Zawacki.
In particular, the finding of no patients with MCC and CLL achieving a complete response interested Ms. Zawacki and her colleagues, since about one-fourth of patients in the Seattle registry have this combination of disease. “Not only did none of the CLL patients have a complete response, but 7 out of the 11 patients with CLL died from MCC,” she explained. When examining further, the researchers found 45% of patients in this group discontinued because of side effects of immunotherapy and had a median time to recurrence of 1.5 months. “This finding suggests that CLL in particular plays a large role in impairing the function of the immune system, leading to not only a more aggressive disease course, but a poorer response to immunotherapy,” she said.
“There is a significant need for improved interventions for patients with CLL and autoimmune disorders,” she added. “Research for immunosuppressed patients is critical given the associated aggressive disease course and their lack of inclusion in clinical trials.”
Ms. Zawacki acknowledged the small number of patients in the study as a limitation, and patients who received follow-up at outside facilities may have received slightly different care, which could impact adverse event reporting or reasons for study discontinuation.
“A multi-institutional study would be beneficial to expand the number of patients in that cohort and to help confirm the trend observed in this study. In addition, future studies should assess the role of combination systemic therapy, such as neutron radiation and immunotherapy together in order to see if the objective response can be approved among immunosuppressed patients,” she said.
This study was supported by funding from the MCC Patient Gift Fund, the National Cancer Institute, and a grant from NIH. Ms. Zawacki reports no relevant conflicts of interest.
SOURCE: Zawacki L. SID 2020, Abstract 497.
FROM SID 2020
FDA approves pomalidomide for Kaposi sarcoma
The Food and Drug Administration has granted accelerated approval to pomalidomide (Pomalyst, Bristol-Myers Squibb) for the treatment of AIDS-related Kaposi sarcoma that is resistant to highly active antiretroviral therapy (HAART) or that occurs in HIV-negative patients.
Pomalidomide is the only oral agent and first new treatment option for Kaposi sarcoma in more than 20 years, according to the company.
The drug, a thalidomide analogue, is already marketed for the treatment of multiple myeloma.
Pomalidomide has “shown positive results in Kaposi sarcoma patients, regardless of their HIV status,” said Robert Yarchoan, MD, chief of the HIV and AIDS Malignancy Branch, National Cancer Institute, in a press statement.
The conditional approval is based on the 71% overall response rate observed in a phase 1/2 open-label, single-arm clinical trial that involved 28 patients, 18 of whom were HIV positive and 10 of whom were HIV negative.
Most of the responses were partial (57%; 16/28); 14% (4/28) were complete. Median duration of response was 12.1 months. Additionally, for half of the patients who showed a response, that response was maintained for more than 12 months.
Patients received 5 mg of pomalidomide once daily for 21 of 28-day cycles until disease progression or unacceptable toxicity occurred.
Permanent discontinuation because of an adverse reaction occurred in 11% (3/28) of patients.
Adverse reactions (≥20%) included maculopapular rash (71%), constipation (71%), fatigue (68%), nausea (36%), diarrhea (32%), cough (29%), dyspnea (29%), peripheral edema (29%), upper respiratory tract infection (29%), muscle spasms (25%), hypothyroidism (21%), dry skin (21%), and chills (21%).
Grade 3 or 4 adverse reactions included maculopapular rash (3.6%), diarrhea (3.6%), and peripheral edema (3.6%).
Grade 3 or 4 laboratory abnormalities (≥5%) that worsened from baseline included decreased absolute neutrophil count (50%), decreased phosphate level (25%), elevated glucose level (7%), and elevated creatine kinase level (7%).
As a thalidomide analogue, pomalidomide includes a boxed warning in the prescribing information; thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. Deep vein thrombosis, pulmonary embolism, myocardial infarction, and stroke can occur in patients treated with pomalidomide; thromboprophylaxis is recommended.
Pomalidomide is available only through a restricted distribution program, Pomalyst REMS.
This article first appeared on Medscape.com.
The Food and Drug Administration has granted accelerated approval to pomalidomide (Pomalyst, Bristol-Myers Squibb) for the treatment of AIDS-related Kaposi sarcoma that is resistant to highly active antiretroviral therapy (HAART) or that occurs in HIV-negative patients.
Pomalidomide is the only oral agent and first new treatment option for Kaposi sarcoma in more than 20 years, according to the company.
The drug, a thalidomide analogue, is already marketed for the treatment of multiple myeloma.
Pomalidomide has “shown positive results in Kaposi sarcoma patients, regardless of their HIV status,” said Robert Yarchoan, MD, chief of the HIV and AIDS Malignancy Branch, National Cancer Institute, in a press statement.
The conditional approval is based on the 71% overall response rate observed in a phase 1/2 open-label, single-arm clinical trial that involved 28 patients, 18 of whom were HIV positive and 10 of whom were HIV negative.
Most of the responses were partial (57%; 16/28); 14% (4/28) were complete. Median duration of response was 12.1 months. Additionally, for half of the patients who showed a response, that response was maintained for more than 12 months.
Patients received 5 mg of pomalidomide once daily for 21 of 28-day cycles until disease progression or unacceptable toxicity occurred.
Permanent discontinuation because of an adverse reaction occurred in 11% (3/28) of patients.
Adverse reactions (≥20%) included maculopapular rash (71%), constipation (71%), fatigue (68%), nausea (36%), diarrhea (32%), cough (29%), dyspnea (29%), peripheral edema (29%), upper respiratory tract infection (29%), muscle spasms (25%), hypothyroidism (21%), dry skin (21%), and chills (21%).
Grade 3 or 4 adverse reactions included maculopapular rash (3.6%), diarrhea (3.6%), and peripheral edema (3.6%).
Grade 3 or 4 laboratory abnormalities (≥5%) that worsened from baseline included decreased absolute neutrophil count (50%), decreased phosphate level (25%), elevated glucose level (7%), and elevated creatine kinase level (7%).
As a thalidomide analogue, pomalidomide includes a boxed warning in the prescribing information; thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. Deep vein thrombosis, pulmonary embolism, myocardial infarction, and stroke can occur in patients treated with pomalidomide; thromboprophylaxis is recommended.
Pomalidomide is available only through a restricted distribution program, Pomalyst REMS.
This article first appeared on Medscape.com.
The Food and Drug Administration has granted accelerated approval to pomalidomide (Pomalyst, Bristol-Myers Squibb) for the treatment of AIDS-related Kaposi sarcoma that is resistant to highly active antiretroviral therapy (HAART) or that occurs in HIV-negative patients.
Pomalidomide is the only oral agent and first new treatment option for Kaposi sarcoma in more than 20 years, according to the company.
The drug, a thalidomide analogue, is already marketed for the treatment of multiple myeloma.
Pomalidomide has “shown positive results in Kaposi sarcoma patients, regardless of their HIV status,” said Robert Yarchoan, MD, chief of the HIV and AIDS Malignancy Branch, National Cancer Institute, in a press statement.
The conditional approval is based on the 71% overall response rate observed in a phase 1/2 open-label, single-arm clinical trial that involved 28 patients, 18 of whom were HIV positive and 10 of whom were HIV negative.
Most of the responses were partial (57%; 16/28); 14% (4/28) were complete. Median duration of response was 12.1 months. Additionally, for half of the patients who showed a response, that response was maintained for more than 12 months.
Patients received 5 mg of pomalidomide once daily for 21 of 28-day cycles until disease progression or unacceptable toxicity occurred.
Permanent discontinuation because of an adverse reaction occurred in 11% (3/28) of patients.
Adverse reactions (≥20%) included maculopapular rash (71%), constipation (71%), fatigue (68%), nausea (36%), diarrhea (32%), cough (29%), dyspnea (29%), peripheral edema (29%), upper respiratory tract infection (29%), muscle spasms (25%), hypothyroidism (21%), dry skin (21%), and chills (21%).
Grade 3 or 4 adverse reactions included maculopapular rash (3.6%), diarrhea (3.6%), and peripheral edema (3.6%).
Grade 3 or 4 laboratory abnormalities (≥5%) that worsened from baseline included decreased absolute neutrophil count (50%), decreased phosphate level (25%), elevated glucose level (7%), and elevated creatine kinase level (7%).
As a thalidomide analogue, pomalidomide includes a boxed warning in the prescribing information; thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. Deep vein thrombosis, pulmonary embolism, myocardial infarction, and stroke can occur in patients treated with pomalidomide; thromboprophylaxis is recommended.
Pomalidomide is available only through a restricted distribution program, Pomalyst REMS.
This article first appeared on Medscape.com.
Evolocumab safe, well-tolerated in HIV+ patients
Evolocumab proved effective, well tolerated, and safe for the treatment of refractory dyslipidemia in persons living with HIV in the phase 3, randomized, double-blind BEIJERINCK study.
At 24 weeks, nearly three-quarters of patients randomized to evolocumab (Repatha) achieved at least a 50% reduction in LDL cholesterol while on maximally tolerated background lipid lowering with a statin and/or other drugs. This was accompanied by significant reductions in other atherogenic lipids, Franck Boccara, MD, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Evolocumab thus shows the potential to help fill a major unmet need for more effective treatment of dyslipidemia in HIV-positive patients, who number an estimated 38 million worldwide, including 1.1 million in the United States. Access to highly active antiretroviral therapies has transformed HIV infection into a chronic manageable disease, but this major advance has been accompanied by a rate of premature atherosclerotic cardiovascular disease that’s nearly twice that of the general population, observed Dr. Boccara, a cardiologist at Sorbonne University, Paris.
The BEIJERINCK study included 464 HIV-infected patients in the United States and 14 other countries on five continents. Participants had a mean baseline LDL cholesterol of 133 mg/dL and triglycerides of about 190 mg/dL while on maximally tolerated lipid-lowering therapy. They had been diagnosed with HIV an average of 18 years earlier. One-third of them had known atherosclerotic cardiovascular disease. More than one-quarter of participants were cigarette smokers. Patients were randomized 2:1 to 24 weeks of double-blind subcutaneous evolocumab at 420 mg once monthly or placebo, then an additional 24 weeks of open-label evolocumab for all.
The primary endpoint was change in LDL from baseline to week 24: a 56.2% reduction in the evolocumab group and a 0.7% increase with placebo. About 73% of patients on evolocumab achieved at least a 50% reduction in LDL cholesterol, as did less than 1% of controls. Likewise, 73% of the evolocumab group got their LDL cholesterol below 70 mg/dL, compared with 7.9% with placebo.
The evolocumab group also experienced favorable placebo-subtracted differences from baseline of 23% in triglycerides, 27% in lipoprotein(a), and 22% in very-low-density lipoprotein cholesterol.
As was the case in the earlier, much larger landmark clinical trials, evolocumab was well tolerated in BEIJERINCK, with a side effect profile similar to placebo. Notably, there was no increase in liver abnormalities in evolocumab-treated patients on highly active antiretroviral therapy, and no one developed evolocumab neutralizing antibodies.
Dr. Boccara reported receiving a research grant from Amgen, the study sponsor, as well as lecture fees from several other pharmaceutical companies.
Simultaneous with the presentation at ACC 2020, the primary results of the BEIJERINCK study were published online (J Am Coll Cardiol. 2020 Mar 19. doi: 10.1016/j.jacc.2020.03.025).
Evolocumab proved effective, well tolerated, and safe for the treatment of refractory dyslipidemia in persons living with HIV in the phase 3, randomized, double-blind BEIJERINCK study.
At 24 weeks, nearly three-quarters of patients randomized to evolocumab (Repatha) achieved at least a 50% reduction in LDL cholesterol while on maximally tolerated background lipid lowering with a statin and/or other drugs. This was accompanied by significant reductions in other atherogenic lipids, Franck Boccara, MD, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Evolocumab thus shows the potential to help fill a major unmet need for more effective treatment of dyslipidemia in HIV-positive patients, who number an estimated 38 million worldwide, including 1.1 million in the United States. Access to highly active antiretroviral therapies has transformed HIV infection into a chronic manageable disease, but this major advance has been accompanied by a rate of premature atherosclerotic cardiovascular disease that’s nearly twice that of the general population, observed Dr. Boccara, a cardiologist at Sorbonne University, Paris.
The BEIJERINCK study included 464 HIV-infected patients in the United States and 14 other countries on five continents. Participants had a mean baseline LDL cholesterol of 133 mg/dL and triglycerides of about 190 mg/dL while on maximally tolerated lipid-lowering therapy. They had been diagnosed with HIV an average of 18 years earlier. One-third of them had known atherosclerotic cardiovascular disease. More than one-quarter of participants were cigarette smokers. Patients were randomized 2:1 to 24 weeks of double-blind subcutaneous evolocumab at 420 mg once monthly or placebo, then an additional 24 weeks of open-label evolocumab for all.
The primary endpoint was change in LDL from baseline to week 24: a 56.2% reduction in the evolocumab group and a 0.7% increase with placebo. About 73% of patients on evolocumab achieved at least a 50% reduction in LDL cholesterol, as did less than 1% of controls. Likewise, 73% of the evolocumab group got their LDL cholesterol below 70 mg/dL, compared with 7.9% with placebo.
The evolocumab group also experienced favorable placebo-subtracted differences from baseline of 23% in triglycerides, 27% in lipoprotein(a), and 22% in very-low-density lipoprotein cholesterol.
As was the case in the earlier, much larger landmark clinical trials, evolocumab was well tolerated in BEIJERINCK, with a side effect profile similar to placebo. Notably, there was no increase in liver abnormalities in evolocumab-treated patients on highly active antiretroviral therapy, and no one developed evolocumab neutralizing antibodies.
Dr. Boccara reported receiving a research grant from Amgen, the study sponsor, as well as lecture fees from several other pharmaceutical companies.
Simultaneous with the presentation at ACC 2020, the primary results of the BEIJERINCK study were published online (J Am Coll Cardiol. 2020 Mar 19. doi: 10.1016/j.jacc.2020.03.025).
Evolocumab proved effective, well tolerated, and safe for the treatment of refractory dyslipidemia in persons living with HIV in the phase 3, randomized, double-blind BEIJERINCK study.
At 24 weeks, nearly three-quarters of patients randomized to evolocumab (Repatha) achieved at least a 50% reduction in LDL cholesterol while on maximally tolerated background lipid lowering with a statin and/or other drugs. This was accompanied by significant reductions in other atherogenic lipids, Franck Boccara, MD, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Evolocumab thus shows the potential to help fill a major unmet need for more effective treatment of dyslipidemia in HIV-positive patients, who number an estimated 38 million worldwide, including 1.1 million in the United States. Access to highly active antiretroviral therapies has transformed HIV infection into a chronic manageable disease, but this major advance has been accompanied by a rate of premature atherosclerotic cardiovascular disease that’s nearly twice that of the general population, observed Dr. Boccara, a cardiologist at Sorbonne University, Paris.
The BEIJERINCK study included 464 HIV-infected patients in the United States and 14 other countries on five continents. Participants had a mean baseline LDL cholesterol of 133 mg/dL and triglycerides of about 190 mg/dL while on maximally tolerated lipid-lowering therapy. They had been diagnosed with HIV an average of 18 years earlier. One-third of them had known atherosclerotic cardiovascular disease. More than one-quarter of participants were cigarette smokers. Patients were randomized 2:1 to 24 weeks of double-blind subcutaneous evolocumab at 420 mg once monthly or placebo, then an additional 24 weeks of open-label evolocumab for all.
The primary endpoint was change in LDL from baseline to week 24: a 56.2% reduction in the evolocumab group and a 0.7% increase with placebo. About 73% of patients on evolocumab achieved at least a 50% reduction in LDL cholesterol, as did less than 1% of controls. Likewise, 73% of the evolocumab group got their LDL cholesterol below 70 mg/dL, compared with 7.9% with placebo.
The evolocumab group also experienced favorable placebo-subtracted differences from baseline of 23% in triglycerides, 27% in lipoprotein(a), and 22% in very-low-density lipoprotein cholesterol.
As was the case in the earlier, much larger landmark clinical trials, evolocumab was well tolerated in BEIJERINCK, with a side effect profile similar to placebo. Notably, there was no increase in liver abnormalities in evolocumab-treated patients on highly active antiretroviral therapy, and no one developed evolocumab neutralizing antibodies.
Dr. Boccara reported receiving a research grant from Amgen, the study sponsor, as well as lecture fees from several other pharmaceutical companies.
Simultaneous with the presentation at ACC 2020, the primary results of the BEIJERINCK study were published online (J Am Coll Cardiol. 2020 Mar 19. doi: 10.1016/j.jacc.2020.03.025).
FROM ACC 2020
Should ART for HIV be initiated prior to tuberculosis testing results?
Tuberculosis symptoms as defined by the World Health Organization were effective in identifying patients with TB for the purposes of same-day antiretroviral therapy (ART) initiation in patients diagnosed with HIV, according to a pooled study of patients in two clinical trials. Guidelines suggest that patients with one or more TB symptoms be investigated for active TB before initiation of ART.
However, more than 80% of patients with TB symptoms did not have the disease and faced a delay of ART initiation, despite the many benefits of same-day ART initiation, according to the study presented online at the Conference on Retroviruses & Opportunistic Infections. This year CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
In her presentation, Alana T. Brennan, PhD, of the Boston University School of Public Health discussed the pooled results of 834 patients in the SLATE (Simple Algorithm for Treatment Eligibility) I and SLATE II trials. These two trials, conducted in South Africa and Kenya, respectively, assessed two variations of a simplified algorithm for eligibility for same-day ART initiation.
A total of 834 patients at baseline reported any self-described symptoms of TB using the WHO four-symptom TB screen (cough, fever, weight loss, night sweats). Those patients with any TB symptoms were assessed by sputum samples. The outcomes were prevalence of TB symptoms, TB diagnosis, and treatment.
Among the 834 patients, 493 (60%) reported no symptoms; 215 (26%) reported one to two symptoms, and 120 (14%) reported three to four symptoms. Only 66% of the patients with one to two symptoms were tested for TB; 78% of the patients with three to four symptoms were tested. Of these, only 1% of the patients with one to two symptoms tested positive for TB, and only 2% of the patients with three to four symptoms tested positive, according to Dr. Brennan.
“More than 80% of patients with TB symptoms did not have TB, but faced delay in ART initiation. No same-day [ART] initiators reported adverse events, so we hope that there would be some reconsideration of the requirement of TB testing prior to ART initiation due to any symptom of TB. … A potential consideration of the severity of the symptoms a patient has is necessary,” Dr. Brennan concluded.
Dr. Brennan reported that there were no disclosures.
SOURCE: Brennan AT et al. CROI 2020, Abstract 720.
Tuberculosis symptoms as defined by the World Health Organization were effective in identifying patients with TB for the purposes of same-day antiretroviral therapy (ART) initiation in patients diagnosed with HIV, according to a pooled study of patients in two clinical trials. Guidelines suggest that patients with one or more TB symptoms be investigated for active TB before initiation of ART.
However, more than 80% of patients with TB symptoms did not have the disease and faced a delay of ART initiation, despite the many benefits of same-day ART initiation, according to the study presented online at the Conference on Retroviruses & Opportunistic Infections. This year CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
In her presentation, Alana T. Brennan, PhD, of the Boston University School of Public Health discussed the pooled results of 834 patients in the SLATE (Simple Algorithm for Treatment Eligibility) I and SLATE II trials. These two trials, conducted in South Africa and Kenya, respectively, assessed two variations of a simplified algorithm for eligibility for same-day ART initiation.
A total of 834 patients at baseline reported any self-described symptoms of TB using the WHO four-symptom TB screen (cough, fever, weight loss, night sweats). Those patients with any TB symptoms were assessed by sputum samples. The outcomes were prevalence of TB symptoms, TB diagnosis, and treatment.
Among the 834 patients, 493 (60%) reported no symptoms; 215 (26%) reported one to two symptoms, and 120 (14%) reported three to four symptoms. Only 66% of the patients with one to two symptoms were tested for TB; 78% of the patients with three to four symptoms were tested. Of these, only 1% of the patients with one to two symptoms tested positive for TB, and only 2% of the patients with three to four symptoms tested positive, according to Dr. Brennan.
“More than 80% of patients with TB symptoms did not have TB, but faced delay in ART initiation. No same-day [ART] initiators reported adverse events, so we hope that there would be some reconsideration of the requirement of TB testing prior to ART initiation due to any symptom of TB. … A potential consideration of the severity of the symptoms a patient has is necessary,” Dr. Brennan concluded.
Dr. Brennan reported that there were no disclosures.
SOURCE: Brennan AT et al. CROI 2020, Abstract 720.
Tuberculosis symptoms as defined by the World Health Organization were effective in identifying patients with TB for the purposes of same-day antiretroviral therapy (ART) initiation in patients diagnosed with HIV, according to a pooled study of patients in two clinical trials. Guidelines suggest that patients with one or more TB symptoms be investigated for active TB before initiation of ART.
However, more than 80% of patients with TB symptoms did not have the disease and faced a delay of ART initiation, despite the many benefits of same-day ART initiation, according to the study presented online at the Conference on Retroviruses & Opportunistic Infections. This year CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
In her presentation, Alana T. Brennan, PhD, of the Boston University School of Public Health discussed the pooled results of 834 patients in the SLATE (Simple Algorithm for Treatment Eligibility) I and SLATE II trials. These two trials, conducted in South Africa and Kenya, respectively, assessed two variations of a simplified algorithm for eligibility for same-day ART initiation.
A total of 834 patients at baseline reported any self-described symptoms of TB using the WHO four-symptom TB screen (cough, fever, weight loss, night sweats). Those patients with any TB symptoms were assessed by sputum samples. The outcomes were prevalence of TB symptoms, TB diagnosis, and treatment.
Among the 834 patients, 493 (60%) reported no symptoms; 215 (26%) reported one to two symptoms, and 120 (14%) reported three to four symptoms. Only 66% of the patients with one to two symptoms were tested for TB; 78% of the patients with three to four symptoms were tested. Of these, only 1% of the patients with one to two symptoms tested positive for TB, and only 2% of the patients with three to four symptoms tested positive, according to Dr. Brennan.
“More than 80% of patients with TB symptoms did not have TB, but faced delay in ART initiation. No same-day [ART] initiators reported adverse events, so we hope that there would be some reconsideration of the requirement of TB testing prior to ART initiation due to any symptom of TB. … A potential consideration of the severity of the symptoms a patient has is necessary,” Dr. Brennan concluded.
Dr. Brennan reported that there were no disclosures.
SOURCE: Brennan AT et al. CROI 2020, Abstract 720.
FROM CROI 2020
Bone density slow to rebound after lactation in women with HIV
Women with HIV had more bone mobilization during lactation, and attenuated skeletal recovery after lactation, compared with HIV-negative women, according to research presented during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The study “demonstrated that there were reductions as expected in BMD during breastfeeding, and there was recovery at the end of breastfeeding, which was higher among women who were not HIV-infected compared to HIV-infected women,” said Mary Glenn Fowler, MD, speaking in a video presentation during the virtual conference. The differences between women who had HIV and the HIV-negative reference group were statistically significant (P = .003 for lumbar spine and P less than .001 for whole-body aBMD).
“We also saw that for whole-body BMD, there was recovery at the end of breastfeeding for women who were not HIV infected, but a dampened response of recovery for BMD for HIV-infected women,” she went on, adding: “These findings held after adjustment for parity, age, body mass, breastfeeding practices, duration of breastfeeding, use of [injectable medroxyprogesterone acetate], and resumption of menses.”
Dr. Fowler presented the study’s results on behalf of lead author Florence Nabwire, PhD, an investigator scientist in the nutrition and bone health group of the United Kingdom’s Medical Research Council (Cambridge).
Although it’s known that antiretroviral therapy (ART) is associated with bone loss, Dr. Fowler explained that there are only limited data in HIV-positive women who are lactating. It’s important to see what happens during lactation for this group of women because of the potential sequelae later in life of insufficient recovery from the physiological bone mobilization that occurs during lactation. The study looked at changes in areal bone mineral density (aBMD) both during and after lactation for women with HIV living in Uganda who were taking Option B+ ART, a regimen that includes tenofovir, 3TC, and efavirenz. These women were compared with a reference group of HIV-negative women.
In all, 95 women with HIV and 96 HIV negative women were recruited into the study during pregnancy. Participants were followed postpartum at weeks 2, 14, and 26, and at a final visit that occurred 14 weeks after lactation stopped.
In addition to lumbar spine, total hip, and femoral neck aBMD measurements, the investigators also obtained whole body-less-head reading.
For total hip and femoral neck aBMD, the nadir of density was seen at 26 postpartum, when a drop of about 6% was seen from baseline readings. By the final post-lactation visit, women without HIV had recovered to their baseline; for women with HIV, some recovery also occurred, but the effect was dampened, with a persistent bone density deficit of about 3% from baseline. The differences between HIV-positive and HIV-negative women in these measurements were also statistically significant, at P less than .001 for total hip aBMD differences and P = .0008 for femoral neck differences. Again, correction for multiple confounders didn’t attenuate the results, said Dr. Fowler.
“In conclusion, these data showed accentuated mobilization of hip and whole body aBMD during lactation,” said Dr. Fowler, who also noted “slower skeletal recovery post lactation for HIV-infected women.” Clinical implications of these findings aren’t currently known, she said. Further ongoing studies are aiming to tease out both mechanisms and longer-term consequences for the bone health of HIV-infected women and their children, who may also see differences in bone mineral accretion and growth.
Session moderator Risa Hoffman, MD, in introductory remarks, set the findings in some context. “As we know, HIV-positive adults have low bone mineral density, and this appears to be a result of interactions of HIV, traditional risk factors for loss of bone density, and antiretroviral therapy,” said Dr. Hoffman, director of the global health program at the University of California, Los Angeles. She added that previous work had shown that “middle-aged HIV-positive women have higher 10-year fracture incidence compared to their HIV-negative counterparts.” The current study, she said, “has both short- and long-term implications for women as they go through multiple pregnancies and multiple periods of breastfeeding.”
The study was funded by the United Kingdom’s Medical Research Council and Department for International Development as well as the Alborada Trust and the Gates Cambridge Scholarship. The authors reported no conflicts of interest.
SOURCE: Nabwire F et al. CROI 2020, Abstract 768.
Women with HIV had more bone mobilization during lactation, and attenuated skeletal recovery after lactation, compared with HIV-negative women, according to research presented during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The study “demonstrated that there were reductions as expected in BMD during breastfeeding, and there was recovery at the end of breastfeeding, which was higher among women who were not HIV-infected compared to HIV-infected women,” said Mary Glenn Fowler, MD, speaking in a video presentation during the virtual conference. The differences between women who had HIV and the HIV-negative reference group were statistically significant (P = .003 for lumbar spine and P less than .001 for whole-body aBMD).
“We also saw that for whole-body BMD, there was recovery at the end of breastfeeding for women who were not HIV infected, but a dampened response of recovery for BMD for HIV-infected women,” she went on, adding: “These findings held after adjustment for parity, age, body mass, breastfeeding practices, duration of breastfeeding, use of [injectable medroxyprogesterone acetate], and resumption of menses.”
Dr. Fowler presented the study’s results on behalf of lead author Florence Nabwire, PhD, an investigator scientist in the nutrition and bone health group of the United Kingdom’s Medical Research Council (Cambridge).
Although it’s known that antiretroviral therapy (ART) is associated with bone loss, Dr. Fowler explained that there are only limited data in HIV-positive women who are lactating. It’s important to see what happens during lactation for this group of women because of the potential sequelae later in life of insufficient recovery from the physiological bone mobilization that occurs during lactation. The study looked at changes in areal bone mineral density (aBMD) both during and after lactation for women with HIV living in Uganda who were taking Option B+ ART, a regimen that includes tenofovir, 3TC, and efavirenz. These women were compared with a reference group of HIV-negative women.
In all, 95 women with HIV and 96 HIV negative women were recruited into the study during pregnancy. Participants were followed postpartum at weeks 2, 14, and 26, and at a final visit that occurred 14 weeks after lactation stopped.
In addition to lumbar spine, total hip, and femoral neck aBMD measurements, the investigators also obtained whole body-less-head reading.
For total hip and femoral neck aBMD, the nadir of density was seen at 26 postpartum, when a drop of about 6% was seen from baseline readings. By the final post-lactation visit, women without HIV had recovered to their baseline; for women with HIV, some recovery also occurred, but the effect was dampened, with a persistent bone density deficit of about 3% from baseline. The differences between HIV-positive and HIV-negative women in these measurements were also statistically significant, at P less than .001 for total hip aBMD differences and P = .0008 for femoral neck differences. Again, correction for multiple confounders didn’t attenuate the results, said Dr. Fowler.
“In conclusion, these data showed accentuated mobilization of hip and whole body aBMD during lactation,” said Dr. Fowler, who also noted “slower skeletal recovery post lactation for HIV-infected women.” Clinical implications of these findings aren’t currently known, she said. Further ongoing studies are aiming to tease out both mechanisms and longer-term consequences for the bone health of HIV-infected women and their children, who may also see differences in bone mineral accretion and growth.
Session moderator Risa Hoffman, MD, in introductory remarks, set the findings in some context. “As we know, HIV-positive adults have low bone mineral density, and this appears to be a result of interactions of HIV, traditional risk factors for loss of bone density, and antiretroviral therapy,” said Dr. Hoffman, director of the global health program at the University of California, Los Angeles. She added that previous work had shown that “middle-aged HIV-positive women have higher 10-year fracture incidence compared to their HIV-negative counterparts.” The current study, she said, “has both short- and long-term implications for women as they go through multiple pregnancies and multiple periods of breastfeeding.”
The study was funded by the United Kingdom’s Medical Research Council and Department for International Development as well as the Alborada Trust and the Gates Cambridge Scholarship. The authors reported no conflicts of interest.
SOURCE: Nabwire F et al. CROI 2020, Abstract 768.
Women with HIV had more bone mobilization during lactation, and attenuated skeletal recovery after lactation, compared with HIV-negative women, according to research presented during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The study “demonstrated that there were reductions as expected in BMD during breastfeeding, and there was recovery at the end of breastfeeding, which was higher among women who were not HIV-infected compared to HIV-infected women,” said Mary Glenn Fowler, MD, speaking in a video presentation during the virtual conference. The differences between women who had HIV and the HIV-negative reference group were statistically significant (P = .003 for lumbar spine and P less than .001 for whole-body aBMD).
“We also saw that for whole-body BMD, there was recovery at the end of breastfeeding for women who were not HIV infected, but a dampened response of recovery for BMD for HIV-infected women,” she went on, adding: “These findings held after adjustment for parity, age, body mass, breastfeeding practices, duration of breastfeeding, use of [injectable medroxyprogesterone acetate], and resumption of menses.”
Dr. Fowler presented the study’s results on behalf of lead author Florence Nabwire, PhD, an investigator scientist in the nutrition and bone health group of the United Kingdom’s Medical Research Council (Cambridge).
Although it’s known that antiretroviral therapy (ART) is associated with bone loss, Dr. Fowler explained that there are only limited data in HIV-positive women who are lactating. It’s important to see what happens during lactation for this group of women because of the potential sequelae later in life of insufficient recovery from the physiological bone mobilization that occurs during lactation. The study looked at changes in areal bone mineral density (aBMD) both during and after lactation for women with HIV living in Uganda who were taking Option B+ ART, a regimen that includes tenofovir, 3TC, and efavirenz. These women were compared with a reference group of HIV-negative women.
In all, 95 women with HIV and 96 HIV negative women were recruited into the study during pregnancy. Participants were followed postpartum at weeks 2, 14, and 26, and at a final visit that occurred 14 weeks after lactation stopped.
In addition to lumbar spine, total hip, and femoral neck aBMD measurements, the investigators also obtained whole body-less-head reading.
For total hip and femoral neck aBMD, the nadir of density was seen at 26 postpartum, when a drop of about 6% was seen from baseline readings. By the final post-lactation visit, women without HIV had recovered to their baseline; for women with HIV, some recovery also occurred, but the effect was dampened, with a persistent bone density deficit of about 3% from baseline. The differences between HIV-positive and HIV-negative women in these measurements were also statistically significant, at P less than .001 for total hip aBMD differences and P = .0008 for femoral neck differences. Again, correction for multiple confounders didn’t attenuate the results, said Dr. Fowler.
“In conclusion, these data showed accentuated mobilization of hip and whole body aBMD during lactation,” said Dr. Fowler, who also noted “slower skeletal recovery post lactation for HIV-infected women.” Clinical implications of these findings aren’t currently known, she said. Further ongoing studies are aiming to tease out both mechanisms and longer-term consequences for the bone health of HIV-infected women and their children, who may also see differences in bone mineral accretion and growth.
Session moderator Risa Hoffman, MD, in introductory remarks, set the findings in some context. “As we know, HIV-positive adults have low bone mineral density, and this appears to be a result of interactions of HIV, traditional risk factors for loss of bone density, and antiretroviral therapy,” said Dr. Hoffman, director of the global health program at the University of California, Los Angeles. She added that previous work had shown that “middle-aged HIV-positive women have higher 10-year fracture incidence compared to their HIV-negative counterparts.” The current study, she said, “has both short- and long-term implications for women as they go through multiple pregnancies and multiple periods of breastfeeding.”
The study was funded by the United Kingdom’s Medical Research Council and Department for International Development as well as the Alborada Trust and the Gates Cambridge Scholarship. The authors reported no conflicts of interest.
SOURCE: Nabwire F et al. CROI 2020, Abstract 768.
FROM CROI 2020
A case of neutrophilic eccrine hidradenitis attributed to HIV treatment
arising in an affected patient, Jessica Kalen, MD, advised during a virtual meeting held by the George Washington University department of dermatology.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
In a presentation entitled, “When HAART [highly active antiretroviral therapy] Hurts,” Dr. Kalen, a dermatology resident at the university, presented a case report involving a 65-year-old man who presented with juicy red edematous papules and plaques on his scalp and ears. He was on the three-drug combination of rilpivirine (a non-nucleoside reverse transcriptase inhibitor), and the NRTIs tenofovir, and emtricitabine (Odefsey) for treatment of HIV infection, which was well controlled, with no detectable viral load.
The patient was also on insulin detemir for diabetes; pravastatin, amlodipine, and lisinopril for hypertension; and episodic acyclovir for recurrent herpes simplex outbreaks. However, none of those drugs has been associated with NEH. In contrast, Dr. Kalen found three published case reports describing a link between NRTIs and NEH.
Lesional biopsy of her patient showed the classic features of NEH: a dermal neutrophilic infiltrate surrounding the eccrine secretory coils and ducts, with vacuolar degeneration that spared the acrosyringium.
The most common causes of NEH, a rare dermatologic disorder first described in 1982, are hematologic malignancies and some of the chemotherapeutic agents used in treating them. Particularly prominent are acute myelogenous leukemia and cytarabine, which are often prescribed for that cancer. Carbamazepine, granulocyte-colony stimulating factor, and BRAF inhibitors have also been associated with NEH.
The pathogenesis of NEH is not fully worked out; however, NRTIs are secreted via eccrine structures, and that close contact could potentially promote an environment favoring inflammation and destruction of the eccrine coils. Also, NRTIs inhibit DNA polymerase, as does cytarabine, Dr. Kalen noted.
Her patient’s NEH was treated with triamcinolone. His skin condition resolved completely while he remained on NRTI therapy, with no relapses to date.
Dr. Kalen reported having no financial conflicts regarding her presentation.
arising in an affected patient, Jessica Kalen, MD, advised during a virtual meeting held by the George Washington University department of dermatology.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
In a presentation entitled, “When HAART [highly active antiretroviral therapy] Hurts,” Dr. Kalen, a dermatology resident at the university, presented a case report involving a 65-year-old man who presented with juicy red edematous papules and plaques on his scalp and ears. He was on the three-drug combination of rilpivirine (a non-nucleoside reverse transcriptase inhibitor), and the NRTIs tenofovir, and emtricitabine (Odefsey) for treatment of HIV infection, which was well controlled, with no detectable viral load.
The patient was also on insulin detemir for diabetes; pravastatin, amlodipine, and lisinopril for hypertension; and episodic acyclovir for recurrent herpes simplex outbreaks. However, none of those drugs has been associated with NEH. In contrast, Dr. Kalen found three published case reports describing a link between NRTIs and NEH.
Lesional biopsy of her patient showed the classic features of NEH: a dermal neutrophilic infiltrate surrounding the eccrine secretory coils and ducts, with vacuolar degeneration that spared the acrosyringium.
The most common causes of NEH, a rare dermatologic disorder first described in 1982, are hematologic malignancies and some of the chemotherapeutic agents used in treating them. Particularly prominent are acute myelogenous leukemia and cytarabine, which are often prescribed for that cancer. Carbamazepine, granulocyte-colony stimulating factor, and BRAF inhibitors have also been associated with NEH.
The pathogenesis of NEH is not fully worked out; however, NRTIs are secreted via eccrine structures, and that close contact could potentially promote an environment favoring inflammation and destruction of the eccrine coils. Also, NRTIs inhibit DNA polymerase, as does cytarabine, Dr. Kalen noted.
Her patient’s NEH was treated with triamcinolone. His skin condition resolved completely while he remained on NRTI therapy, with no relapses to date.
Dr. Kalen reported having no financial conflicts regarding her presentation.
arising in an affected patient, Jessica Kalen, MD, advised during a virtual meeting held by the George Washington University department of dermatology.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
In a presentation entitled, “When HAART [highly active antiretroviral therapy] Hurts,” Dr. Kalen, a dermatology resident at the university, presented a case report involving a 65-year-old man who presented with juicy red edematous papules and plaques on his scalp and ears. He was on the three-drug combination of rilpivirine (a non-nucleoside reverse transcriptase inhibitor), and the NRTIs tenofovir, and emtricitabine (Odefsey) for treatment of HIV infection, which was well controlled, with no detectable viral load.
The patient was also on insulin detemir for diabetes; pravastatin, amlodipine, and lisinopril for hypertension; and episodic acyclovir for recurrent herpes simplex outbreaks. However, none of those drugs has been associated with NEH. In contrast, Dr. Kalen found three published case reports describing a link between NRTIs and NEH.
Lesional biopsy of her patient showed the classic features of NEH: a dermal neutrophilic infiltrate surrounding the eccrine secretory coils and ducts, with vacuolar degeneration that spared the acrosyringium.
The most common causes of NEH, a rare dermatologic disorder first described in 1982, are hematologic malignancies and some of the chemotherapeutic agents used in treating them. Particularly prominent are acute myelogenous leukemia and cytarabine, which are often prescribed for that cancer. Carbamazepine, granulocyte-colony stimulating factor, and BRAF inhibitors have also been associated with NEH.
The pathogenesis of NEH is not fully worked out; however, NRTIs are secreted via eccrine structures, and that close contact could potentially promote an environment favoring inflammation and destruction of the eccrine coils. Also, NRTIs inhibit DNA polymerase, as does cytarabine, Dr. Kalen noted.
Her patient’s NEH was treated with triamcinolone. His skin condition resolved completely while he remained on NRTI therapy, with no relapses to date.
Dr. Kalen reported having no financial conflicts regarding her presentation.