User login
Hospital Mergers in 2024: Five Things to Know
Hospital mergers and acquisitions continue to garner intense scrutiny from lawmakers, with pressure likely to hold steady following the recent announcement of new antitrust guidelines and state and federal investigations into potential healthcare monopolies.
In December, the US Department of Justice (DOJ) and the Federal Trade Commission (FTC) released updated guidelines outlining the factors they consider when determining if a merger illegally monopolizes a local healthcare market or jeopardizes access to critical healthcare services.
Last week, the DOJ also announced a UnitedHealth Group antitrust probe, just months after the healthcare conglomerate’s workforce numbers indicated it is now affiliated with or employs 10% of the US physician workforce.
While the impact of the latest guidelines is yet to be seen, concerns over healthcare market consolidation are not new. Over the past two decades, mergers have attracted attention for contributing to a decline in independent hospitals, said Rachel M. Werner, MD, PhD, executive director of the Leonard Davis Institute of Health Economics at the University of Pennsylvania, Philadelphia, Pennsylvania.
“At this point, most hospitals are operating in a pretty concentrated market,” she said.
Here are five things to know about the current state of hospital mergers.
1. Record-Breaking Merger Enforcements
The DOJ and FTC reported the highest level of enforcement activity in over 20 years in fiscal year 2022 — the latest available data. Together, the agencies filed 50 merger enforcement actions and brought a record-breaking number of merger enforcement challenges, resulting in 11 approved actions, the restructuring or abandonment of seven mergers, and six business deals entering litigation.
Included in those statistics was a proposed merger between the two largest health systems in Rhode Island, Lifespan and Care New England Health System, which was abandoned after the FTC and the state Attorney General took steps to block it. the HCA branch in Utah Healthcare abandoned plans for to acquire five Salt Lake City area hospitals from competitor Steward Health Care System, as did RWJBarnabas Health after exploring a merger with Saint Peter's Healthcare System in New Jersey.
2. New Antitrust Guidelines Consider Labor Market
The new guidelines notably focus on labor competition, said Jody Boudreault, JD, attorney and chair of the Antitrust Life Sciences and Healthcare Group at Baker Botts law firm in Washington, DC. Health professionals typically have more employment opportunities in an urban area, unless hindered by restrictive noncompete agreements, and fewer options in rural settings.
In the Lifespan merger that fell through, Ms. Boudreault said that the newly created hospital system would have employed two thirds of Rhode Island's full-time nurses, limiting opportunities for local employment elsewhere.
“Going forward, I would expect federal authorities to review not only the competitive impact of the hospitals merging but also the competitive impact of the physician, and especially nursing, workforce,” she said.
FTC Chair Lina M. Khan noted similar labor market concerns.
In a statement to Congress, she said that hospital consolidation reduces options for employees, who fear “being blacklisted from further hiring in a system that controls many of the hospitals in the area” and “makes workers afraid to file complaints, organize their workplace, or leave before the end of a contract.”
3. Mergers Can Drive Care Costs Higher
When hospital markets become less competitive, the cost of care often increases. In Indiana, inpatient prices rose 13% in hospitals that merged. Another study found that prices at monopoly hospitals are 12% higher than in markets with four or more rivals. Even cross-market mergers, when hospitals in different geographic locations combine, can drive prices higher.
Dr. Werner told this news organization that more significant price hikes of 20-30% aren’t unheard of, with reimbursements by some commercial insurance companies rising as much as 50%. “That’s the direct price that the insurers pay, but the burden of those higher prices ultimately falls on patients through higher premiums,” she said.
Still, the American Hospital Association (AHA) says that mergers and acquisitions can significantly lower annual operating expenses per admission and reduce inpatient readmission rates and mortality measures. In comments to the FTC, the AHA stated that mergers could provide a lifeline for rural and community hospitals struggling with shrinking payer reimbursement and rising labor and supply costs. The business arrangements also could ensure these communities maintain continuity of care.
Although a cross-market merger may initially benefit cash-strapped rural hospitals, Dr. Werner urged caution.
“In the long run, it’s not clear that it is good for patients because we start to see decreased access to some types of service, like labor and delivery, which are services needed in rural markets,” she said.
4. Mergers to Watch in 2024
Ms. Boudreault, who represented RWJBarnabas in the abandoned Saint Peter’s transaction, says the courts widely accepted the old merger guidelines, and it will take time to see how the new measures are interpreted. “The guidelines don’t yet have the force of law, but they can be persuasive to a court.”
Looking ahead, she is watching how Steward Health Care navigates its impending financial collapse. The nation’s largest private for-profit health system was previously owned by private equity firm Cerberus Capital Management and includes nine Massachusetts hospitals plus entities in at least seven other states.
Ms. Boudreault also plans to monitor Jefferson Health’s intent to merge with Lehigh Valley Health Network. “It’s a pretty big deal because they would become a 30-hospital system,” said Ms. Boudreault. The newly formed network would become the largest employer in Philadelphia.
5. Merger and Acquisition Reversals Unlikely
Dr. Werner said that mergers and acquisitions are complicated business moves that are nearly impossible to undo once approved, so it makes sense for agencies to continue to evaluate them closely.
“The costs of healthcare are borne by us as a society,” she said. “We’re going to have to live with the ill effects of a consolidated market once we let hospitals merge, so they deserve additional scrutiny.”
A version of this article appeared on Medscape.com.
Hospital mergers and acquisitions continue to garner intense scrutiny from lawmakers, with pressure likely to hold steady following the recent announcement of new antitrust guidelines and state and federal investigations into potential healthcare monopolies.
In December, the US Department of Justice (DOJ) and the Federal Trade Commission (FTC) released updated guidelines outlining the factors they consider when determining if a merger illegally monopolizes a local healthcare market or jeopardizes access to critical healthcare services.
Last week, the DOJ also announced a UnitedHealth Group antitrust probe, just months after the healthcare conglomerate’s workforce numbers indicated it is now affiliated with or employs 10% of the US physician workforce.
While the impact of the latest guidelines is yet to be seen, concerns over healthcare market consolidation are not new. Over the past two decades, mergers have attracted attention for contributing to a decline in independent hospitals, said Rachel M. Werner, MD, PhD, executive director of the Leonard Davis Institute of Health Economics at the University of Pennsylvania, Philadelphia, Pennsylvania.
“At this point, most hospitals are operating in a pretty concentrated market,” she said.
Here are five things to know about the current state of hospital mergers.
1. Record-Breaking Merger Enforcements
The DOJ and FTC reported the highest level of enforcement activity in over 20 years in fiscal year 2022 — the latest available data. Together, the agencies filed 50 merger enforcement actions and brought a record-breaking number of merger enforcement challenges, resulting in 11 approved actions, the restructuring or abandonment of seven mergers, and six business deals entering litigation.
Included in those statistics was a proposed merger between the two largest health systems in Rhode Island, Lifespan and Care New England Health System, which was abandoned after the FTC and the state Attorney General took steps to block it. the HCA branch in Utah Healthcare abandoned plans for to acquire five Salt Lake City area hospitals from competitor Steward Health Care System, as did RWJBarnabas Health after exploring a merger with Saint Peter's Healthcare System in New Jersey.
2. New Antitrust Guidelines Consider Labor Market
The new guidelines notably focus on labor competition, said Jody Boudreault, JD, attorney and chair of the Antitrust Life Sciences and Healthcare Group at Baker Botts law firm in Washington, DC. Health professionals typically have more employment opportunities in an urban area, unless hindered by restrictive noncompete agreements, and fewer options in rural settings.
In the Lifespan merger that fell through, Ms. Boudreault said that the newly created hospital system would have employed two thirds of Rhode Island's full-time nurses, limiting opportunities for local employment elsewhere.
“Going forward, I would expect federal authorities to review not only the competitive impact of the hospitals merging but also the competitive impact of the physician, and especially nursing, workforce,” she said.
FTC Chair Lina M. Khan noted similar labor market concerns.
In a statement to Congress, she said that hospital consolidation reduces options for employees, who fear “being blacklisted from further hiring in a system that controls many of the hospitals in the area” and “makes workers afraid to file complaints, organize their workplace, or leave before the end of a contract.”
3. Mergers Can Drive Care Costs Higher
When hospital markets become less competitive, the cost of care often increases. In Indiana, inpatient prices rose 13% in hospitals that merged. Another study found that prices at monopoly hospitals are 12% higher than in markets with four or more rivals. Even cross-market mergers, when hospitals in different geographic locations combine, can drive prices higher.
Dr. Werner told this news organization that more significant price hikes of 20-30% aren’t unheard of, with reimbursements by some commercial insurance companies rising as much as 50%. “That’s the direct price that the insurers pay, but the burden of those higher prices ultimately falls on patients through higher premiums,” she said.
Still, the American Hospital Association (AHA) says that mergers and acquisitions can significantly lower annual operating expenses per admission and reduce inpatient readmission rates and mortality measures. In comments to the FTC, the AHA stated that mergers could provide a lifeline for rural and community hospitals struggling with shrinking payer reimbursement and rising labor and supply costs. The business arrangements also could ensure these communities maintain continuity of care.
Although a cross-market merger may initially benefit cash-strapped rural hospitals, Dr. Werner urged caution.
“In the long run, it’s not clear that it is good for patients because we start to see decreased access to some types of service, like labor and delivery, which are services needed in rural markets,” she said.
4. Mergers to Watch in 2024
Ms. Boudreault, who represented RWJBarnabas in the abandoned Saint Peter’s transaction, says the courts widely accepted the old merger guidelines, and it will take time to see how the new measures are interpreted. “The guidelines don’t yet have the force of law, but they can be persuasive to a court.”
Looking ahead, she is watching how Steward Health Care navigates its impending financial collapse. The nation’s largest private for-profit health system was previously owned by private equity firm Cerberus Capital Management and includes nine Massachusetts hospitals plus entities in at least seven other states.
Ms. Boudreault also plans to monitor Jefferson Health’s intent to merge with Lehigh Valley Health Network. “It’s a pretty big deal because they would become a 30-hospital system,” said Ms. Boudreault. The newly formed network would become the largest employer in Philadelphia.
5. Merger and Acquisition Reversals Unlikely
Dr. Werner said that mergers and acquisitions are complicated business moves that are nearly impossible to undo once approved, so it makes sense for agencies to continue to evaluate them closely.
“The costs of healthcare are borne by us as a society,” she said. “We’re going to have to live with the ill effects of a consolidated market once we let hospitals merge, so they deserve additional scrutiny.”
A version of this article appeared on Medscape.com.
Hospital mergers and acquisitions continue to garner intense scrutiny from lawmakers, with pressure likely to hold steady following the recent announcement of new antitrust guidelines and state and federal investigations into potential healthcare monopolies.
In December, the US Department of Justice (DOJ) and the Federal Trade Commission (FTC) released updated guidelines outlining the factors they consider when determining if a merger illegally monopolizes a local healthcare market or jeopardizes access to critical healthcare services.
Last week, the DOJ also announced a UnitedHealth Group antitrust probe, just months after the healthcare conglomerate’s workforce numbers indicated it is now affiliated with or employs 10% of the US physician workforce.
While the impact of the latest guidelines is yet to be seen, concerns over healthcare market consolidation are not new. Over the past two decades, mergers have attracted attention for contributing to a decline in independent hospitals, said Rachel M. Werner, MD, PhD, executive director of the Leonard Davis Institute of Health Economics at the University of Pennsylvania, Philadelphia, Pennsylvania.
“At this point, most hospitals are operating in a pretty concentrated market,” she said.
Here are five things to know about the current state of hospital mergers.
1. Record-Breaking Merger Enforcements
The DOJ and FTC reported the highest level of enforcement activity in over 20 years in fiscal year 2022 — the latest available data. Together, the agencies filed 50 merger enforcement actions and brought a record-breaking number of merger enforcement challenges, resulting in 11 approved actions, the restructuring or abandonment of seven mergers, and six business deals entering litigation.
Included in those statistics was a proposed merger between the two largest health systems in Rhode Island, Lifespan and Care New England Health System, which was abandoned after the FTC and the state Attorney General took steps to block it. the HCA branch in Utah Healthcare abandoned plans for to acquire five Salt Lake City area hospitals from competitor Steward Health Care System, as did RWJBarnabas Health after exploring a merger with Saint Peter's Healthcare System in New Jersey.
2. New Antitrust Guidelines Consider Labor Market
The new guidelines notably focus on labor competition, said Jody Boudreault, JD, attorney and chair of the Antitrust Life Sciences and Healthcare Group at Baker Botts law firm in Washington, DC. Health professionals typically have more employment opportunities in an urban area, unless hindered by restrictive noncompete agreements, and fewer options in rural settings.
In the Lifespan merger that fell through, Ms. Boudreault said that the newly created hospital system would have employed two thirds of Rhode Island's full-time nurses, limiting opportunities for local employment elsewhere.
“Going forward, I would expect federal authorities to review not only the competitive impact of the hospitals merging but also the competitive impact of the physician, and especially nursing, workforce,” she said.
FTC Chair Lina M. Khan noted similar labor market concerns.
In a statement to Congress, she said that hospital consolidation reduces options for employees, who fear “being blacklisted from further hiring in a system that controls many of the hospitals in the area” and “makes workers afraid to file complaints, organize their workplace, or leave before the end of a contract.”
3. Mergers Can Drive Care Costs Higher
When hospital markets become less competitive, the cost of care often increases. In Indiana, inpatient prices rose 13% in hospitals that merged. Another study found that prices at monopoly hospitals are 12% higher than in markets with four or more rivals. Even cross-market mergers, when hospitals in different geographic locations combine, can drive prices higher.
Dr. Werner told this news organization that more significant price hikes of 20-30% aren’t unheard of, with reimbursements by some commercial insurance companies rising as much as 50%. “That’s the direct price that the insurers pay, but the burden of those higher prices ultimately falls on patients through higher premiums,” she said.
Still, the American Hospital Association (AHA) says that mergers and acquisitions can significantly lower annual operating expenses per admission and reduce inpatient readmission rates and mortality measures. In comments to the FTC, the AHA stated that mergers could provide a lifeline for rural and community hospitals struggling with shrinking payer reimbursement and rising labor and supply costs. The business arrangements also could ensure these communities maintain continuity of care.
Although a cross-market merger may initially benefit cash-strapped rural hospitals, Dr. Werner urged caution.
“In the long run, it’s not clear that it is good for patients because we start to see decreased access to some types of service, like labor and delivery, which are services needed in rural markets,” she said.
4. Mergers to Watch in 2024
Ms. Boudreault, who represented RWJBarnabas in the abandoned Saint Peter’s transaction, says the courts widely accepted the old merger guidelines, and it will take time to see how the new measures are interpreted. “The guidelines don’t yet have the force of law, but they can be persuasive to a court.”
Looking ahead, she is watching how Steward Health Care navigates its impending financial collapse. The nation’s largest private for-profit health system was previously owned by private equity firm Cerberus Capital Management and includes nine Massachusetts hospitals plus entities in at least seven other states.
Ms. Boudreault also plans to monitor Jefferson Health’s intent to merge with Lehigh Valley Health Network. “It’s a pretty big deal because they would become a 30-hospital system,” said Ms. Boudreault. The newly formed network would become the largest employer in Philadelphia.
5. Merger and Acquisition Reversals Unlikely
Dr. Werner said that mergers and acquisitions are complicated business moves that are nearly impossible to undo once approved, so it makes sense for agencies to continue to evaluate them closely.
“The costs of healthcare are borne by us as a society,” she said. “We’re going to have to live with the ill effects of a consolidated market once we let hospitals merge, so they deserve additional scrutiny.”
A version of this article appeared on Medscape.com.
E-Consults in Dermatology: A Retrospective Analysis
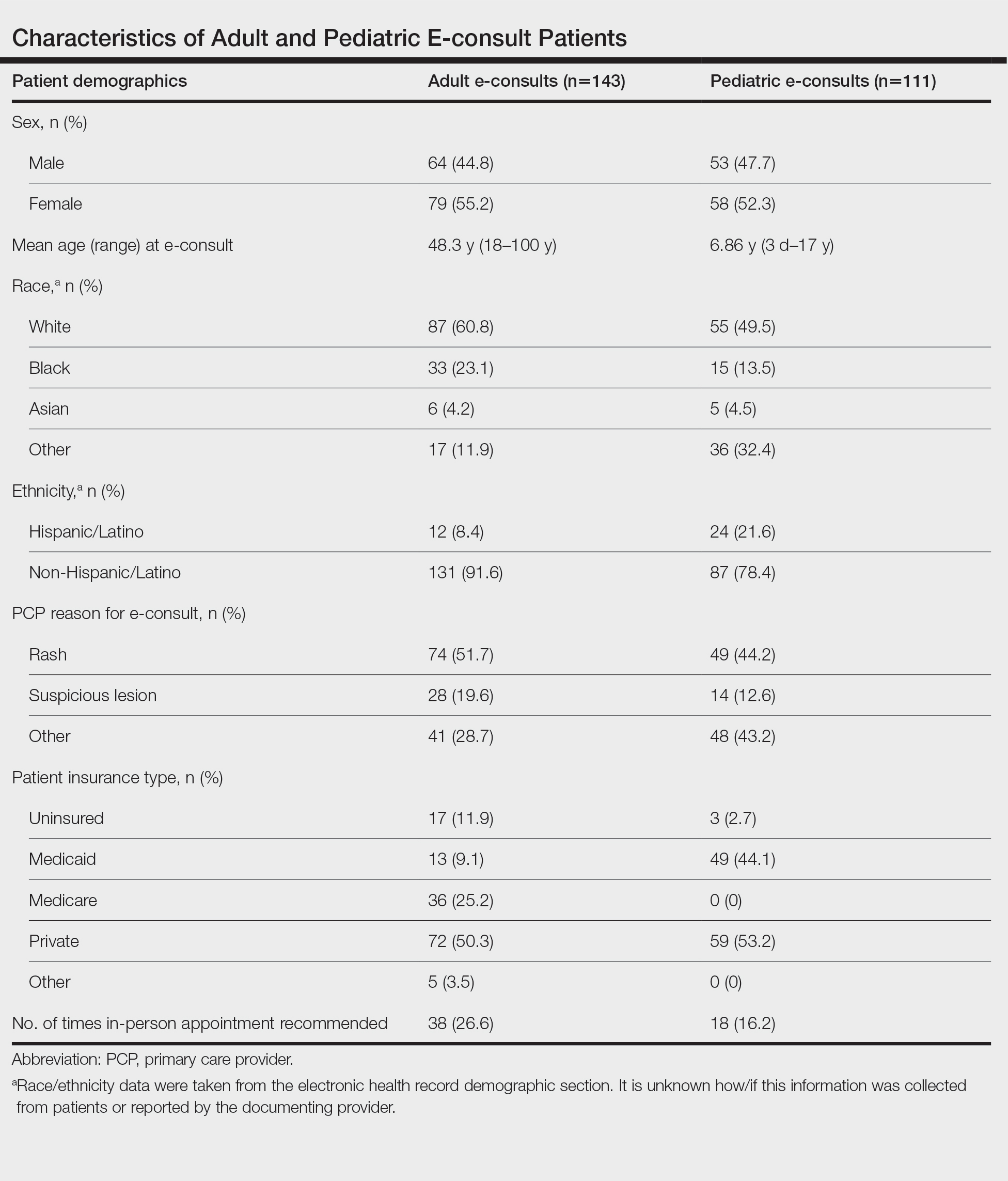
Dermatologic conditions affect approximately one-third of individuals in the United States.1,2 Nearly 1 in 4 physician office visits in the United States are for skin conditions, and less than one-third of these visits are with dermatologists. Although many of these patients may prefer to see a dermatologist for their concerns, they may not be able to access specialist care.3 The limited supply and urban-focused distribution of dermatologists along with reduced acceptance of state-funded insurance plans and long appointment wait times all pose considerable challenges to individuals seeking dermatologic care.2 Electronic consultations (e-consults) have emerged as a promising solution to overcoming these barriers while providing high-quality dermatologic care to a large diverse patient population.2,4 Although e-consults can be of service to all dermatology patients, this modality may be especially beneficial to underserved populations, such as the uninsured and Medicaid patients—groups that historically have experienced limited access to dermatology care due to the low reimbursement rates and high administrative burdens accompanying care delivery.4 This limited access leads to inequity in care, as timely access to dermatology is associated with improved diagnostic accuracy and disease outcomes.3 E-consult implementation can facilitate timely access for these underserved populations and bypass additional barriers to care such as lack of transportation or time off work. Prior e-consult studies have demonstrated relatively high numbers of Medicaid patients utilizing e-consult services.3,5
Although in-person visits remain the gold standard for diagnosis and treatment of dermatologic conditions, e-consults placed by primary care providers (PCPs) can improve access and help triage patients who require in-person dermatology visits.6 In this study, we conducted a retrospective chart review to characterize the e-consults requested of the dermatology department at a large tertiary care medical center in Winston-Salem, North Carolina.
Methods
The electronic health record (EHR) of Atrium Health Wake Forest Baptist (Winston-Salem, North Carolina) was screened for eligible patients from January 1, 2020, to May 31, 2021. Patients—both adult (aged ≥18 years) and pediatric (aged <18 years)—were included if they underwent a dermatology e-consult within this time frame. Provider notes in the medical records were reviewed to determine the nature of the lesion, how long the dermatologist took to complete the e-consult, whether an in-person appointment was recommended, and whether the patient was seen by dermatology within 90 days of the e-consult. Institutional review board approval was obtained.
For each e-consult, the PCP obtained clinical photographs of the lesion in question either through the EHR mobile application or by having patients upload their own photographs directly to their medical records. The referring PCP then completed a brief template regarding the patient’s clinical question and medical history and then sent the completed information to the consulting dermatologist’s EHR inbox. From there, the dermatologist could view the clinical question, documented photographs, and patient medical record to create a brief consult note with recommendations. The note was then sent back via EHR to the PCP to follow up with the patient. Patients were not charged for the e-consult.
Results
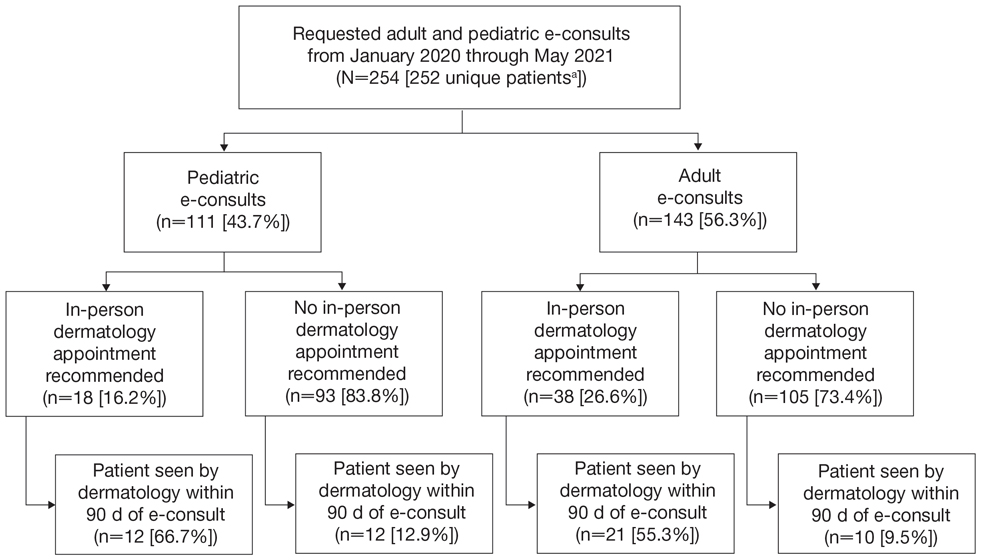
Two hundred fifty-four dermatology e-consults were requested by providers at the study center (eTable), which included 252 unique patients (2 patients had 2 separate e-consults regarding different clinical questions). The median time for completion of the e-consult—from submission of the PCP’s e-consult request to dermatologist completion—was 0.37 days. Fifty-six patients (22.0%) were recommended for an in-person appointment (Figure), 33 (58.9%) of whom ultimately scheduled the in-person appointment, and the median length of time between the completion of the e-consult and the in-person appointment was 16.5 days. The remaining 198 patients (78.0%) were not triaged to receive an in-person appointment following the e-consult,but 2 patients (8.7%) were ultimately seen in-person anyway via other referral pathways, with a median length of 33 days between e-consult completion and the in-person appointment. One hundred seventy-six patients (69.8%) avoided an in-person dermatology visit, although 38 (21.6%) of those patients were fewer than 90 days out from their e-consults at the time of data collection. The 254 e-consults included patients from 50 different zip codes, 49 (98.0%) of which were in North Carolina.
Comment
An e-consult is an asynchronous telehealth modality through which PCPs can request specialty evaluation to provide diagnostic and therapeutic guidance, facilitate PCP-specialist coordination of care, and increase access to specialty care with reduced wait times.7,8 Increased care access is especially important, as specialty referral can decrease overall health care expenditure; however, the demand for specialists often exceeds the availability.8 Our e-consult program drastically reduced the time from patients’ initial presentation at their PCP’s office to dermatologist recommendations for treatment or need for in-person dermatology follow-up.
In our analysis, patients were of different racial, ethnic, and socioeconomic backgrounds and lived across a variety of zip codes, predominantly in central and western North Carolina. Almost three-quarters of the patients resided in zip codes where the average income was less than the North Carolina median household income ($66,196).9 Additionally, 82 patients (32.3%) were uninsured or on Medicaid (eTable). These economically disadvantaged patient populations historically have had limited access to dermatologic care.4 One study showed that privately insured individuals were accepted as new patients by dermatologists 91% of the time compared to a 29.8% acceptance rate for publicly insured individuals.10 Uninsured and Medicaid patients also have to wait 34% longer for an appointment compared to individuals with Medicare or private insurance.2 Considering these patients may already be at an economic disadvantage when it comes to seeing and paying for dermatologic services, e-consults may reduce patient travel and appointment expenses while increasing access to specialty care. Based on a 2020 study, each e-consult generates an estimated savings of $80 out-of-pocket per patient per avoided in-person visit.11
In our study, the most common condition for an e-consult in both adult and pediatric patients was rash, which is consistent with prior e-consult studies.5,11 We found that most e-consult patients were not recommended for an in-person dermatology visit, and for those who were recommended to have an in-person visit, the wait time was reduced (Figure). These results corroborate that e-consults may be used as an important triage tool for determining whether a specialist appointment is indicated as well as a public health tool, as timely evaluation is associated with better dermatologic health care outcomes.3 However, the number of patients who did not present for an in-person appointment in our study may be overestimated, as 38 patients’ (21.6%) e-consults were conducted fewer than 90 days before our data collection. Although none of these patients had been seen in person, it is possible they requested an in-person visit after their medical records were reviewed for this study. Additionally, it is possible patients sought care from outside providers not documented in the EHR.
With regard to the payment model for the e-consult program, Atrium Health Wake Forest Baptist initially piloted the e-consult system through a partnership with the American Academy of Medical Colleges’ Project CORE: Coordinating Optimal Referral Experiences (https://www.aamc.org/what-we-do/mission-areas/health-care/project-core). Grant funding through Project CORE allowed both the referring PCP and the specialist completing the e-consult to each receive approximately 0.5 relative value units in payment for each consult completed. Based on early adoption successes, the institution has created additional internal funding to support the continued expansion of the e-consult system and is incentivized to continue funding, as proper utilization of e-consults improves patient access to timely specialist care, avoids no-shows or last-minute cancellations for specialist appointments, and decreases back-door access to specialist care through the emergency department and urgent care facilities.5 Although 0.5 relative value units is not equivalent compensation to an in-person office visit, our study showed that e-consults can be completed much more quickly and efficiently and do not utilize nursing staff or other office resources.
Conclusion
E-consults are an effective telehealth modality that can increase patients’ access to dermatologic specialty care.
Acknowledgments—The authors thank the Wake Forest University School of Medicine Department of Medical Education and Department of Dermatology (Winston-Salem, North Carolina) for their contributions to this research study as well as the Wake Forest Clinical and Translational Science Institute (Winston-Salem, North Carolina) for their help extracting EHR data.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Naka F, Lu J, Porto A, et al. Impact of dermatology econsults on access to care and skin cancer screening in underserved populations: a model for teledermatology services in community health centers. J Am Acad Dermatol. 2018;78:293-302.
- Mulcahy A, Mehrotra A, Edison K, et al. Variation in dermatologist visits by sociodemographic characteristics. J Am Acad Dermatol. 2017;76:918-924.
- Yang X, Barbieri JS, Kovarik CL. Cost analysis of a store-and-forward teledermatology consult system in Philadelphia. J Am Acad Dermatol. 2019;81:758-764.
- Wang RF, Trinidad J, Lawrence J, et al. Improved patient access and outcomes with the integration of an econsult program (teledermatology) within a large academic medical center. J Am Acad Dermatol. 2020;83:1633-1638.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Parikh PJ, Mowrey C, Gallimore J, et al. Evaluating e-consultation implementations based on use and time-line across various specialties. Int J Med Inform. 2017;108:42-48.
- Wasfy JH, Rao SK, Kalwani N, et al. Longer-term impact of cardiology e-consults. Am Heart J. 2016;173:86-93.
- United States Census Bureau. QuickFacts: North Carolina; United States. Accessed February 26, 2024. https://www.census.gov/quickfacts/fact/table/NC,US/PST045222
- Alghothani L, Jacks SK, Vander Horst A, et al. Disparities in access to dermatologic care according to insurance type. Arch Dermatol. 2012;148:956-957.
- Seiger K, Hawryluk EB, Kroshinsky D, et al. Pediatric dermatology econsults: reduced wait times and dermatology office visits. Pediatr Dermatol. 2020;37:804-810.
Dermatologic conditions affect approximately one-third of individuals in the United States.1,2 Nearly 1 in 4 physician office visits in the United States are for skin conditions, and less than one-third of these visits are with dermatologists. Although many of these patients may prefer to see a dermatologist for their concerns, they may not be able to access specialist care.3 The limited supply and urban-focused distribution of dermatologists along with reduced acceptance of state-funded insurance plans and long appointment wait times all pose considerable challenges to individuals seeking dermatologic care.2 Electronic consultations (e-consults) have emerged as a promising solution to overcoming these barriers while providing high-quality dermatologic care to a large diverse patient population.2,4 Although e-consults can be of service to all dermatology patients, this modality may be especially beneficial to underserved populations, such as the uninsured and Medicaid patients—groups that historically have experienced limited access to dermatology care due to the low reimbursement rates and high administrative burdens accompanying care delivery.4 This limited access leads to inequity in care, as timely access to dermatology is associated with improved diagnostic accuracy and disease outcomes.3 E-consult implementation can facilitate timely access for these underserved populations and bypass additional barriers to care such as lack of transportation or time off work. Prior e-consult studies have demonstrated relatively high numbers of Medicaid patients utilizing e-consult services.3,5
Although in-person visits remain the gold standard for diagnosis and treatment of dermatologic conditions, e-consults placed by primary care providers (PCPs) can improve access and help triage patients who require in-person dermatology visits.6 In this study, we conducted a retrospective chart review to characterize the e-consults requested of the dermatology department at a large tertiary care medical center in Winston-Salem, North Carolina.
Methods
The electronic health record (EHR) of Atrium Health Wake Forest Baptist (Winston-Salem, North Carolina) was screened for eligible patients from January 1, 2020, to May 31, 2021. Patients—both adult (aged ≥18 years) and pediatric (aged <18 years)—were included if they underwent a dermatology e-consult within this time frame. Provider notes in the medical records were reviewed to determine the nature of the lesion, how long the dermatologist took to complete the e-consult, whether an in-person appointment was recommended, and whether the patient was seen by dermatology within 90 days of the e-consult. Institutional review board approval was obtained.
For each e-consult, the PCP obtained clinical photographs of the lesion in question either through the EHR mobile application or by having patients upload their own photographs directly to their medical records. The referring PCP then completed a brief template regarding the patient’s clinical question and medical history and then sent the completed information to the consulting dermatologist’s EHR inbox. From there, the dermatologist could view the clinical question, documented photographs, and patient medical record to create a brief consult note with recommendations. The note was then sent back via EHR to the PCP to follow up with the patient. Patients were not charged for the e-consult.

Results
Two hundred fifty-four dermatology e-consults were requested by providers at the study center (eTable), which included 252 unique patients (2 patients had 2 separate e-consults regarding different clinical questions). The median time for completion of the e-consult—from submission of the PCP’s e-consult request to dermatologist completion—was 0.37 days. Fifty-six patients (22.0%) were recommended for an in-person appointment (Figure), 33 (58.9%) of whom ultimately scheduled the in-person appointment, and the median length of time between the completion of the e-consult and the in-person appointment was 16.5 days. The remaining 198 patients (78.0%) were not triaged to receive an in-person appointment following the e-consult,but 2 patients (8.7%) were ultimately seen in-person anyway via other referral pathways, with a median length of 33 days between e-consult completion and the in-person appointment. One hundred seventy-six patients (69.8%) avoided an in-person dermatology visit, although 38 (21.6%) of those patients were fewer than 90 days out from their e-consults at the time of data collection. The 254 e-consults included patients from 50 different zip codes, 49 (98.0%) of which were in North Carolina.

Comment
An e-consult is an asynchronous telehealth modality through which PCPs can request specialty evaluation to provide diagnostic and therapeutic guidance, facilitate PCP-specialist coordination of care, and increase access to specialty care with reduced wait times.7,8 Increased care access is especially important, as specialty referral can decrease overall health care expenditure; however, the demand for specialists often exceeds the availability.8 Our e-consult program drastically reduced the time from patients’ initial presentation at their PCP’s office to dermatologist recommendations for treatment or need for in-person dermatology follow-up.
In our analysis, patients were of different racial, ethnic, and socioeconomic backgrounds and lived across a variety of zip codes, predominantly in central and western North Carolina. Almost three-quarters of the patients resided in zip codes where the average income was less than the North Carolina median household income ($66,196).9 Additionally, 82 patients (32.3%) were uninsured or on Medicaid (eTable). These economically disadvantaged patient populations historically have had limited access to dermatologic care.4 One study showed that privately insured individuals were accepted as new patients by dermatologists 91% of the time compared to a 29.8% acceptance rate for publicly insured individuals.10 Uninsured and Medicaid patients also have to wait 34% longer for an appointment compared to individuals with Medicare or private insurance.2 Considering these patients may already be at an economic disadvantage when it comes to seeing and paying for dermatologic services, e-consults may reduce patient travel and appointment expenses while increasing access to specialty care. Based on a 2020 study, each e-consult generates an estimated savings of $80 out-of-pocket per patient per avoided in-person visit.11
In our study, the most common condition for an e-consult in both adult and pediatric patients was rash, which is consistent with prior e-consult studies.5,11 We found that most e-consult patients were not recommended for an in-person dermatology visit, and for those who were recommended to have an in-person visit, the wait time was reduced (Figure). These results corroborate that e-consults may be used as an important triage tool for determining whether a specialist appointment is indicated as well as a public health tool, as timely evaluation is associated with better dermatologic health care outcomes.3 However, the number of patients who did not present for an in-person appointment in our study may be overestimated, as 38 patients’ (21.6%) e-consults were conducted fewer than 90 days before our data collection. Although none of these patients had been seen in person, it is possible they requested an in-person visit after their medical records were reviewed for this study. Additionally, it is possible patients sought care from outside providers not documented in the EHR.
With regard to the payment model for the e-consult program, Atrium Health Wake Forest Baptist initially piloted the e-consult system through a partnership with the American Academy of Medical Colleges’ Project CORE: Coordinating Optimal Referral Experiences (https://www.aamc.org/what-we-do/mission-areas/health-care/project-core). Grant funding through Project CORE allowed both the referring PCP and the specialist completing the e-consult to each receive approximately 0.5 relative value units in payment for each consult completed. Based on early adoption successes, the institution has created additional internal funding to support the continued expansion of the e-consult system and is incentivized to continue funding, as proper utilization of e-consults improves patient access to timely specialist care, avoids no-shows or last-minute cancellations for specialist appointments, and decreases back-door access to specialist care through the emergency department and urgent care facilities.5 Although 0.5 relative value units is not equivalent compensation to an in-person office visit, our study showed that e-consults can be completed much more quickly and efficiently and do not utilize nursing staff or other office resources.
Conclusion
E-consults are an effective telehealth modality that can increase patients’ access to dermatologic specialty care.
Acknowledgments—The authors thank the Wake Forest University School of Medicine Department of Medical Education and Department of Dermatology (Winston-Salem, North Carolina) for their contributions to this research study as well as the Wake Forest Clinical and Translational Science Institute (Winston-Salem, North Carolina) for their help extracting EHR data.
Dermatologic conditions affect approximately one-third of individuals in the United States.1,2 Nearly 1 in 4 physician office visits in the United States are for skin conditions, and less than one-third of these visits are with dermatologists. Although many of these patients may prefer to see a dermatologist for their concerns, they may not be able to access specialist care.3 The limited supply and urban-focused distribution of dermatologists along with reduced acceptance of state-funded insurance plans and long appointment wait times all pose considerable challenges to individuals seeking dermatologic care.2 Electronic consultations (e-consults) have emerged as a promising solution to overcoming these barriers while providing high-quality dermatologic care to a large diverse patient population.2,4 Although e-consults can be of service to all dermatology patients, this modality may be especially beneficial to underserved populations, such as the uninsured and Medicaid patients—groups that historically have experienced limited access to dermatology care due to the low reimbursement rates and high administrative burdens accompanying care delivery.4 This limited access leads to inequity in care, as timely access to dermatology is associated with improved diagnostic accuracy and disease outcomes.3 E-consult implementation can facilitate timely access for these underserved populations and bypass additional barriers to care such as lack of transportation or time off work. Prior e-consult studies have demonstrated relatively high numbers of Medicaid patients utilizing e-consult services.3,5
Although in-person visits remain the gold standard for diagnosis and treatment of dermatologic conditions, e-consults placed by primary care providers (PCPs) can improve access and help triage patients who require in-person dermatology visits.6 In this study, we conducted a retrospective chart review to characterize the e-consults requested of the dermatology department at a large tertiary care medical center in Winston-Salem, North Carolina.
Methods
The electronic health record (EHR) of Atrium Health Wake Forest Baptist (Winston-Salem, North Carolina) was screened for eligible patients from January 1, 2020, to May 31, 2021. Patients—both adult (aged ≥18 years) and pediatric (aged <18 years)—were included if they underwent a dermatology e-consult within this time frame. Provider notes in the medical records were reviewed to determine the nature of the lesion, how long the dermatologist took to complete the e-consult, whether an in-person appointment was recommended, and whether the patient was seen by dermatology within 90 days of the e-consult. Institutional review board approval was obtained.
For each e-consult, the PCP obtained clinical photographs of the lesion in question either through the EHR mobile application or by having patients upload their own photographs directly to their medical records. The referring PCP then completed a brief template regarding the patient’s clinical question and medical history and then sent the completed information to the consulting dermatologist’s EHR inbox. From there, the dermatologist could view the clinical question, documented photographs, and patient medical record to create a brief consult note with recommendations. The note was then sent back via EHR to the PCP to follow up with the patient. Patients were not charged for the e-consult.

Results
Two hundred fifty-four dermatology e-consults were requested by providers at the study center (eTable), which included 252 unique patients (2 patients had 2 separate e-consults regarding different clinical questions). The median time for completion of the e-consult—from submission of the PCP’s e-consult request to dermatologist completion—was 0.37 days. Fifty-six patients (22.0%) were recommended for an in-person appointment (Figure), 33 (58.9%) of whom ultimately scheduled the in-person appointment, and the median length of time between the completion of the e-consult and the in-person appointment was 16.5 days. The remaining 198 patients (78.0%) were not triaged to receive an in-person appointment following the e-consult,but 2 patients (8.7%) were ultimately seen in-person anyway via other referral pathways, with a median length of 33 days between e-consult completion and the in-person appointment. One hundred seventy-six patients (69.8%) avoided an in-person dermatology visit, although 38 (21.6%) of those patients were fewer than 90 days out from their e-consults at the time of data collection. The 254 e-consults included patients from 50 different zip codes, 49 (98.0%) of which were in North Carolina.

Comment
An e-consult is an asynchronous telehealth modality through which PCPs can request specialty evaluation to provide diagnostic and therapeutic guidance, facilitate PCP-specialist coordination of care, and increase access to specialty care with reduced wait times.7,8 Increased care access is especially important, as specialty referral can decrease overall health care expenditure; however, the demand for specialists often exceeds the availability.8 Our e-consult program drastically reduced the time from patients’ initial presentation at their PCP’s office to dermatologist recommendations for treatment or need for in-person dermatology follow-up.
In our analysis, patients were of different racial, ethnic, and socioeconomic backgrounds and lived across a variety of zip codes, predominantly in central and western North Carolina. Almost three-quarters of the patients resided in zip codes where the average income was less than the North Carolina median household income ($66,196).9 Additionally, 82 patients (32.3%) were uninsured or on Medicaid (eTable). These economically disadvantaged patient populations historically have had limited access to dermatologic care.4 One study showed that privately insured individuals were accepted as new patients by dermatologists 91% of the time compared to a 29.8% acceptance rate for publicly insured individuals.10 Uninsured and Medicaid patients also have to wait 34% longer for an appointment compared to individuals with Medicare or private insurance.2 Considering these patients may already be at an economic disadvantage when it comes to seeing and paying for dermatologic services, e-consults may reduce patient travel and appointment expenses while increasing access to specialty care. Based on a 2020 study, each e-consult generates an estimated savings of $80 out-of-pocket per patient per avoided in-person visit.11
In our study, the most common condition for an e-consult in both adult and pediatric patients was rash, which is consistent with prior e-consult studies.5,11 We found that most e-consult patients were not recommended for an in-person dermatology visit, and for those who were recommended to have an in-person visit, the wait time was reduced (Figure). These results corroborate that e-consults may be used as an important triage tool for determining whether a specialist appointment is indicated as well as a public health tool, as timely evaluation is associated with better dermatologic health care outcomes.3 However, the number of patients who did not present for an in-person appointment in our study may be overestimated, as 38 patients’ (21.6%) e-consults were conducted fewer than 90 days before our data collection. Although none of these patients had been seen in person, it is possible they requested an in-person visit after their medical records were reviewed for this study. Additionally, it is possible patients sought care from outside providers not documented in the EHR.
With regard to the payment model for the e-consult program, Atrium Health Wake Forest Baptist initially piloted the e-consult system through a partnership with the American Academy of Medical Colleges’ Project CORE: Coordinating Optimal Referral Experiences (https://www.aamc.org/what-we-do/mission-areas/health-care/project-core). Grant funding through Project CORE allowed both the referring PCP and the specialist completing the e-consult to each receive approximately 0.5 relative value units in payment for each consult completed. Based on early adoption successes, the institution has created additional internal funding to support the continued expansion of the e-consult system and is incentivized to continue funding, as proper utilization of e-consults improves patient access to timely specialist care, avoids no-shows or last-minute cancellations for specialist appointments, and decreases back-door access to specialist care through the emergency department and urgent care facilities.5 Although 0.5 relative value units is not equivalent compensation to an in-person office visit, our study showed that e-consults can be completed much more quickly and efficiently and do not utilize nursing staff or other office resources.
Conclusion
E-consults are an effective telehealth modality that can increase patients’ access to dermatologic specialty care.
Acknowledgments—The authors thank the Wake Forest University School of Medicine Department of Medical Education and Department of Dermatology (Winston-Salem, North Carolina) for their contributions to this research study as well as the Wake Forest Clinical and Translational Science Institute (Winston-Salem, North Carolina) for their help extracting EHR data.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Naka F, Lu J, Porto A, et al. Impact of dermatology econsults on access to care and skin cancer screening in underserved populations: a model for teledermatology services in community health centers. J Am Acad Dermatol. 2018;78:293-302.
- Mulcahy A, Mehrotra A, Edison K, et al. Variation in dermatologist visits by sociodemographic characteristics. J Am Acad Dermatol. 2017;76:918-924.
- Yang X, Barbieri JS, Kovarik CL. Cost analysis of a store-and-forward teledermatology consult system in Philadelphia. J Am Acad Dermatol. 2019;81:758-764.
- Wang RF, Trinidad J, Lawrence J, et al. Improved patient access and outcomes with the integration of an econsult program (teledermatology) within a large academic medical center. J Am Acad Dermatol. 2020;83:1633-1638.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Parikh PJ, Mowrey C, Gallimore J, et al. Evaluating e-consultation implementations based on use and time-line across various specialties. Int J Med Inform. 2017;108:42-48.
- Wasfy JH, Rao SK, Kalwani N, et al. Longer-term impact of cardiology e-consults. Am Heart J. 2016;173:86-93.
- United States Census Bureau. QuickFacts: North Carolina; United States. Accessed February 26, 2024. https://www.census.gov/quickfacts/fact/table/NC,US/PST045222
- Alghothani L, Jacks SK, Vander Horst A, et al. Disparities in access to dermatologic care according to insurance type. Arch Dermatol. 2012;148:956-957.
- Seiger K, Hawryluk EB, Kroshinsky D, et al. Pediatric dermatology econsults: reduced wait times and dermatology office visits. Pediatr Dermatol. 2020;37:804-810.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Naka F, Lu J, Porto A, et al. Impact of dermatology econsults on access to care and skin cancer screening in underserved populations: a model for teledermatology services in community health centers. J Am Acad Dermatol. 2018;78:293-302.
- Mulcahy A, Mehrotra A, Edison K, et al. Variation in dermatologist visits by sociodemographic characteristics. J Am Acad Dermatol. 2017;76:918-924.
- Yang X, Barbieri JS, Kovarik CL. Cost analysis of a store-and-forward teledermatology consult system in Philadelphia. J Am Acad Dermatol. 2019;81:758-764.
- Wang RF, Trinidad J, Lawrence J, et al. Improved patient access and outcomes with the integration of an econsult program (teledermatology) within a large academic medical center. J Am Acad Dermatol. 2020;83:1633-1638.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Parikh PJ, Mowrey C, Gallimore J, et al. Evaluating e-consultation implementations based on use and time-line across various specialties. Int J Med Inform. 2017;108:42-48.
- Wasfy JH, Rao SK, Kalwani N, et al. Longer-term impact of cardiology e-consults. Am Heart J. 2016;173:86-93.
- United States Census Bureau. QuickFacts: North Carolina; United States. Accessed February 26, 2024. https://www.census.gov/quickfacts/fact/table/NC,US/PST045222
- Alghothani L, Jacks SK, Vander Horst A, et al. Disparities in access to dermatologic care according to insurance type. Arch Dermatol. 2012;148:956-957.
- Seiger K, Hawryluk EB, Kroshinsky D, et al. Pediatric dermatology econsults: reduced wait times and dermatology office visits. Pediatr Dermatol. 2020;37:804-810.
Practice Points
- Most electronic consult patients may be able to avoid in-person dermatology appointments.
- E-consults can increase patient access to dermatologic specialty care.
Outside the Guidelines: Denosumab Overuse in Prostate Cancer
Bone-modifying agents — most notably denosumab — are often prescribed to prevent skeletal-related complications in patients with metastatic castration-sensitive prostate cancer, but the drugs are not recommended for this indication and can lead to severe toxicities.
How much does Medicare spend each year on non-recommended bone therapy?
The answer, according to a new analysis in JCO Oncology Practice, is more than $44 million, with about $43 million coming from denosumab alone.
Overall, this study found that “the Medicare program pays tens of millions of dollars each year” for bone-modifying agents in patients with metastatic castration-sensitive prostate cancer, “which is not effective and may cause side effects,” lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center, New York City, and colleagues concluded.
“These findings suggest reducing bone agent overuse could be a rare healthcare ‘win-win.’ Lower costs AND improved patient outcomes,” tweeted Dr. Mitchell. “If I were a payer, I’d be paying attention!”
In Prostate Cancer, Bone-Modifying Drug Indications Vary
Bone-modifying drugs are indicated for some patients with prostate cancer.
The American Society of Clinical Oncology has endorsed guidelines that recommend the use of denosumab in men with nonmetastatic prostate cancer at high risk for fracture while taking androgen deprivation therapy.
Among men with metastatic castration-resistant prostate cancer, guidelines also recommend zoledronic acid or denosumab for preventing or delaying skeletal-related events, such as pathologic fractures and spinal cord compression.
For patients with metastatic castration-sensitive disease, however, the bone-modifying agents show no benefit in preventing skeletal-related events and are not recommended for that indication.
In this population, “treatment with bone agents results only in avoidable toxicity and financial cost,” Dr. Mitchell tweeted. In its higher-dose formulation, denosumab comes with a price tag of approximately $40,000 per year in the United States.
An earlier study from Dr. Mitchell and colleagues revealed that the use of bone-modifying drugs to prevent skeletal events in metastatic castration-sensitive prostate cancer is common.
To better understand the costs associated with this inappropriate use, the researchers reviewed Surveillance, Epidemiology, and End Results Program Medicare data from 2011 to 2015. The team identified the frequency and number of doses of zoledronic acid and denosumab prescribed against recommendations in the metastatic castration-sensitive setting, making sure to distinguish between the use of denosumab to prevent osteoporotic fractures (appropriate use) and to prevent skeletal-related events (non-recommended use).
The team found that, among 2627 patients with metastatic castration-sensitive prostate cancer, 42% received at least one dose of denosumab and 18% received at least one dose of zoledronic acid.
The authors also found that unnecessary use of these drugs increased over time — with a little over 17% of patients receiving zoledronic acid between 2007 and 2009 and just over 28% receiving either denosumab (20.3%) or zoledronic acid (8.4%) from 2012 to 2015.
The annual costs to Medicare from non-recommended prescribing came to $44,105,041 for both agents, with the costs associated with denosumab representing the lion’s share at $43,303,078.
Non-recommended use of these agents also came with adverse events, such as femur fracture and hypocalcemia, which cost an estimated $758,450 to treat annually — $682,865 for denosumab and $75,585 for zoledronic acid.
The study focused on the Medicare-age population, which means the estimates are conservative. “Denosumab overuse for younger patients with castration-sensitive prostate cancer would add substantially to this total,” the authors wrote.
“This study contributes new evidence of overuse in the metastatic castrate-sensitive prostate cancer setting, which I must admit reflects my clinical experience in seeing patients for second opinions who are treated in the community,” said Samuel U. Takvorian, MD, of the Division of Hematology and Oncology, Perelman School of Medicine, Philadelphia, who wasn’t involved in the research. “While there are some circumstances in which one would consider using a bone-modifying agent in the metastatic castrate-sensitive prostate cancer setting, most [of these] men don’t need them upfront.”
Why Is the Overuse Happening?
One reason for the inappropriate use of bone-modifying drugs could be confusion surrounding the recommendations because the drugs are recommended for some patients with prostate cancer.
Michael R. Laurent, MD, PhD, of Imelda Hospital, Bonheiden, Belgium, explained that the use of bone-modifying drugs is, paradoxically, often overlooked in settings where they are recommended — when patients have an elevated risk for osteoporosis or fracture.
“Guidelines are quite unequivocal in their recommendations to prevent osteoporosis in mostly older men who receive androgen deprivation therapy,” but “I think there is significant undertreatment” in these patients, Dr. Laurent told this news organization.
However, the recommendation for patients at risk for osteoporosis or bone fracture calls for less intense regimens, which may include lower-dose denosumab, administered once every 6 months, zoledronic acid, given yearly, or another lower potency agent, such as oral alendronate weekly, explained Philip J. Saylor, MD, an attending physician at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
Meanwhile, “monthly high-intensity therapy to prevent skeletal events should be reserved specifically for bone metastatic castration-resistant prostate cancer for more than just cost reasons,” Dr. Saylor said.
When it comes to the higher dose, monthly therapy in castration-sensitive prostate cancer, “we have no evidence that it is beneficial,” he said, adding that “when the prostate cancer itself is well controlled by hormonal therapy, there just aren’t very many pathologic fractures or other bone complications.”
Alongside possible confusion over the recommendations, many physicians also likely don’t know how much denosumab costs.
“In our recent physician interview study, we did find that most physicians were very much unaware of the cost of this drug, or the cost difference between denosumab and zoledronic acid, so I do think that lack of cost awareness is a factor,” Dr. Mitchell said.
Part of the reason may be how Medicare covers these agents. Typically, Medicare would not cover non-recommended indications, but “in this case, Medicare coverage is broader and includes both the guideline-recommended and non-recommended uses,” Dr. Mitchell explained.
However, the authors also identified a more cynical reason for non-recommended prescribing — promotional payments from drug makers to physicians.
In another recent paper, Dr. Mitchell said he found about “30% of doctors treating prostate cancer had received payments from Amgen for Xgeva [denosumab] promotion during the last year.”
These payments appeared to influence non-recommended prescribing: Among patients whose doctor had not received payments, 31.4% received non-recommended denosumab, which increased to nearly 50% of patients among doctors who had received payments.
Dr. Mitchell suggested a few ways to help curb inappropriate prescribing.
Medicare could, for instance, change its coverage policy to include only the recommended uses of these agents, Dr. Mitchell said.
More physician education would be another solution. “I think that physician education would be one ‘bottom-up’ approach that could work,” Dr. Mitchell added.
Dr. Mitchell, Dr. Takvorian, and Dr. Saylor had no disclosures to report. Dr. Laurent has received lecture and consultancy fees from Alexion, AM Pharma, Amgen, Galapagos, Kyowa Kirin, Menarini, Orifarm, Pharmanovia, Takeda, UCB, and Will Pharma.
A version of this article appeared on Medscape.com.
Bone-modifying agents — most notably denosumab — are often prescribed to prevent skeletal-related complications in patients with metastatic castration-sensitive prostate cancer, but the drugs are not recommended for this indication and can lead to severe toxicities.
How much does Medicare spend each year on non-recommended bone therapy?
The answer, according to a new analysis in JCO Oncology Practice, is more than $44 million, with about $43 million coming from denosumab alone.
Overall, this study found that “the Medicare program pays tens of millions of dollars each year” for bone-modifying agents in patients with metastatic castration-sensitive prostate cancer, “which is not effective and may cause side effects,” lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center, New York City, and colleagues concluded.
“These findings suggest reducing bone agent overuse could be a rare healthcare ‘win-win.’ Lower costs AND improved patient outcomes,” tweeted Dr. Mitchell. “If I were a payer, I’d be paying attention!”
In Prostate Cancer, Bone-Modifying Drug Indications Vary
Bone-modifying drugs are indicated for some patients with prostate cancer.
The American Society of Clinical Oncology has endorsed guidelines that recommend the use of denosumab in men with nonmetastatic prostate cancer at high risk for fracture while taking androgen deprivation therapy.
Among men with metastatic castration-resistant prostate cancer, guidelines also recommend zoledronic acid or denosumab for preventing or delaying skeletal-related events, such as pathologic fractures and spinal cord compression.
For patients with metastatic castration-sensitive disease, however, the bone-modifying agents show no benefit in preventing skeletal-related events and are not recommended for that indication.
In this population, “treatment with bone agents results only in avoidable toxicity and financial cost,” Dr. Mitchell tweeted. In its higher-dose formulation, denosumab comes with a price tag of approximately $40,000 per year in the United States.
An earlier study from Dr. Mitchell and colleagues revealed that the use of bone-modifying drugs to prevent skeletal events in metastatic castration-sensitive prostate cancer is common.
To better understand the costs associated with this inappropriate use, the researchers reviewed Surveillance, Epidemiology, and End Results Program Medicare data from 2011 to 2015. The team identified the frequency and number of doses of zoledronic acid and denosumab prescribed against recommendations in the metastatic castration-sensitive setting, making sure to distinguish between the use of denosumab to prevent osteoporotic fractures (appropriate use) and to prevent skeletal-related events (non-recommended use).
The team found that, among 2627 patients with metastatic castration-sensitive prostate cancer, 42% received at least one dose of denosumab and 18% received at least one dose of zoledronic acid.
The authors also found that unnecessary use of these drugs increased over time — with a little over 17% of patients receiving zoledronic acid between 2007 and 2009 and just over 28% receiving either denosumab (20.3%) or zoledronic acid (8.4%) from 2012 to 2015.
The annual costs to Medicare from non-recommended prescribing came to $44,105,041 for both agents, with the costs associated with denosumab representing the lion’s share at $43,303,078.
Non-recommended use of these agents also came with adverse events, such as femur fracture and hypocalcemia, which cost an estimated $758,450 to treat annually — $682,865 for denosumab and $75,585 for zoledronic acid.
The study focused on the Medicare-age population, which means the estimates are conservative. “Denosumab overuse for younger patients with castration-sensitive prostate cancer would add substantially to this total,” the authors wrote.
“This study contributes new evidence of overuse in the metastatic castrate-sensitive prostate cancer setting, which I must admit reflects my clinical experience in seeing patients for second opinions who are treated in the community,” said Samuel U. Takvorian, MD, of the Division of Hematology and Oncology, Perelman School of Medicine, Philadelphia, who wasn’t involved in the research. “While there are some circumstances in which one would consider using a bone-modifying agent in the metastatic castrate-sensitive prostate cancer setting, most [of these] men don’t need them upfront.”
Why Is the Overuse Happening?
One reason for the inappropriate use of bone-modifying drugs could be confusion surrounding the recommendations because the drugs are recommended for some patients with prostate cancer.
Michael R. Laurent, MD, PhD, of Imelda Hospital, Bonheiden, Belgium, explained that the use of bone-modifying drugs is, paradoxically, often overlooked in settings where they are recommended — when patients have an elevated risk for osteoporosis or fracture.
“Guidelines are quite unequivocal in their recommendations to prevent osteoporosis in mostly older men who receive androgen deprivation therapy,” but “I think there is significant undertreatment” in these patients, Dr. Laurent told this news organization.
However, the recommendation for patients at risk for osteoporosis or bone fracture calls for less intense regimens, which may include lower-dose denosumab, administered once every 6 months, zoledronic acid, given yearly, or another lower potency agent, such as oral alendronate weekly, explained Philip J. Saylor, MD, an attending physician at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
Meanwhile, “monthly high-intensity therapy to prevent skeletal events should be reserved specifically for bone metastatic castration-resistant prostate cancer for more than just cost reasons,” Dr. Saylor said.
When it comes to the higher dose, monthly therapy in castration-sensitive prostate cancer, “we have no evidence that it is beneficial,” he said, adding that “when the prostate cancer itself is well controlled by hormonal therapy, there just aren’t very many pathologic fractures or other bone complications.”
Alongside possible confusion over the recommendations, many physicians also likely don’t know how much denosumab costs.
“In our recent physician interview study, we did find that most physicians were very much unaware of the cost of this drug, or the cost difference between denosumab and zoledronic acid, so I do think that lack of cost awareness is a factor,” Dr. Mitchell said.
Part of the reason may be how Medicare covers these agents. Typically, Medicare would not cover non-recommended indications, but “in this case, Medicare coverage is broader and includes both the guideline-recommended and non-recommended uses,” Dr. Mitchell explained.
However, the authors also identified a more cynical reason for non-recommended prescribing — promotional payments from drug makers to physicians.
In another recent paper, Dr. Mitchell said he found about “30% of doctors treating prostate cancer had received payments from Amgen for Xgeva [denosumab] promotion during the last year.”
These payments appeared to influence non-recommended prescribing: Among patients whose doctor had not received payments, 31.4% received non-recommended denosumab, which increased to nearly 50% of patients among doctors who had received payments.
Dr. Mitchell suggested a few ways to help curb inappropriate prescribing.
Medicare could, for instance, change its coverage policy to include only the recommended uses of these agents, Dr. Mitchell said.
More physician education would be another solution. “I think that physician education would be one ‘bottom-up’ approach that could work,” Dr. Mitchell added.
Dr. Mitchell, Dr. Takvorian, and Dr. Saylor had no disclosures to report. Dr. Laurent has received lecture and consultancy fees from Alexion, AM Pharma, Amgen, Galapagos, Kyowa Kirin, Menarini, Orifarm, Pharmanovia, Takeda, UCB, and Will Pharma.
A version of this article appeared on Medscape.com.
Bone-modifying agents — most notably denosumab — are often prescribed to prevent skeletal-related complications in patients with metastatic castration-sensitive prostate cancer, but the drugs are not recommended for this indication and can lead to severe toxicities.
How much does Medicare spend each year on non-recommended bone therapy?
The answer, according to a new analysis in JCO Oncology Practice, is more than $44 million, with about $43 million coming from denosumab alone.
Overall, this study found that “the Medicare program pays tens of millions of dollars each year” for bone-modifying agents in patients with metastatic castration-sensitive prostate cancer, “which is not effective and may cause side effects,” lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center, New York City, and colleagues concluded.
“These findings suggest reducing bone agent overuse could be a rare healthcare ‘win-win.’ Lower costs AND improved patient outcomes,” tweeted Dr. Mitchell. “If I were a payer, I’d be paying attention!”
In Prostate Cancer, Bone-Modifying Drug Indications Vary
Bone-modifying drugs are indicated for some patients with prostate cancer.
The American Society of Clinical Oncology has endorsed guidelines that recommend the use of denosumab in men with nonmetastatic prostate cancer at high risk for fracture while taking androgen deprivation therapy.
Among men with metastatic castration-resistant prostate cancer, guidelines also recommend zoledronic acid or denosumab for preventing or delaying skeletal-related events, such as pathologic fractures and spinal cord compression.
For patients with metastatic castration-sensitive disease, however, the bone-modifying agents show no benefit in preventing skeletal-related events and are not recommended for that indication.
In this population, “treatment with bone agents results only in avoidable toxicity and financial cost,” Dr. Mitchell tweeted. In its higher-dose formulation, denosumab comes with a price tag of approximately $40,000 per year in the United States.
An earlier study from Dr. Mitchell and colleagues revealed that the use of bone-modifying drugs to prevent skeletal events in metastatic castration-sensitive prostate cancer is common.
To better understand the costs associated with this inappropriate use, the researchers reviewed Surveillance, Epidemiology, and End Results Program Medicare data from 2011 to 2015. The team identified the frequency and number of doses of zoledronic acid and denosumab prescribed against recommendations in the metastatic castration-sensitive setting, making sure to distinguish between the use of denosumab to prevent osteoporotic fractures (appropriate use) and to prevent skeletal-related events (non-recommended use).
The team found that, among 2627 patients with metastatic castration-sensitive prostate cancer, 42% received at least one dose of denosumab and 18% received at least one dose of zoledronic acid.
The authors also found that unnecessary use of these drugs increased over time — with a little over 17% of patients receiving zoledronic acid between 2007 and 2009 and just over 28% receiving either denosumab (20.3%) or zoledronic acid (8.4%) from 2012 to 2015.
The annual costs to Medicare from non-recommended prescribing came to $44,105,041 for both agents, with the costs associated with denosumab representing the lion’s share at $43,303,078.
Non-recommended use of these agents also came with adverse events, such as femur fracture and hypocalcemia, which cost an estimated $758,450 to treat annually — $682,865 for denosumab and $75,585 for zoledronic acid.
The study focused on the Medicare-age population, which means the estimates are conservative. “Denosumab overuse for younger patients with castration-sensitive prostate cancer would add substantially to this total,” the authors wrote.
“This study contributes new evidence of overuse in the metastatic castrate-sensitive prostate cancer setting, which I must admit reflects my clinical experience in seeing patients for second opinions who are treated in the community,” said Samuel U. Takvorian, MD, of the Division of Hematology and Oncology, Perelman School of Medicine, Philadelphia, who wasn’t involved in the research. “While there are some circumstances in which one would consider using a bone-modifying agent in the metastatic castrate-sensitive prostate cancer setting, most [of these] men don’t need them upfront.”
Why Is the Overuse Happening?
One reason for the inappropriate use of bone-modifying drugs could be confusion surrounding the recommendations because the drugs are recommended for some patients with prostate cancer.
Michael R. Laurent, MD, PhD, of Imelda Hospital, Bonheiden, Belgium, explained that the use of bone-modifying drugs is, paradoxically, often overlooked in settings where they are recommended — when patients have an elevated risk for osteoporosis or fracture.
“Guidelines are quite unequivocal in their recommendations to prevent osteoporosis in mostly older men who receive androgen deprivation therapy,” but “I think there is significant undertreatment” in these patients, Dr. Laurent told this news organization.
However, the recommendation for patients at risk for osteoporosis or bone fracture calls for less intense regimens, which may include lower-dose denosumab, administered once every 6 months, zoledronic acid, given yearly, or another lower potency agent, such as oral alendronate weekly, explained Philip J. Saylor, MD, an attending physician at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
Meanwhile, “monthly high-intensity therapy to prevent skeletal events should be reserved specifically for bone metastatic castration-resistant prostate cancer for more than just cost reasons,” Dr. Saylor said.
When it comes to the higher dose, monthly therapy in castration-sensitive prostate cancer, “we have no evidence that it is beneficial,” he said, adding that “when the prostate cancer itself is well controlled by hormonal therapy, there just aren’t very many pathologic fractures or other bone complications.”
Alongside possible confusion over the recommendations, many physicians also likely don’t know how much denosumab costs.
“In our recent physician interview study, we did find that most physicians were very much unaware of the cost of this drug, or the cost difference between denosumab and zoledronic acid, so I do think that lack of cost awareness is a factor,” Dr. Mitchell said.
Part of the reason may be how Medicare covers these agents. Typically, Medicare would not cover non-recommended indications, but “in this case, Medicare coverage is broader and includes both the guideline-recommended and non-recommended uses,” Dr. Mitchell explained.
However, the authors also identified a more cynical reason for non-recommended prescribing — promotional payments from drug makers to physicians.
In another recent paper, Dr. Mitchell said he found about “30% of doctors treating prostate cancer had received payments from Amgen for Xgeva [denosumab] promotion during the last year.”
These payments appeared to influence non-recommended prescribing: Among patients whose doctor had not received payments, 31.4% received non-recommended denosumab, which increased to nearly 50% of patients among doctors who had received payments.
Dr. Mitchell suggested a few ways to help curb inappropriate prescribing.
Medicare could, for instance, change its coverage policy to include only the recommended uses of these agents, Dr. Mitchell said.
More physician education would be another solution. “I think that physician education would be one ‘bottom-up’ approach that could work,” Dr. Mitchell added.
Dr. Mitchell, Dr. Takvorian, and Dr. Saylor had no disclosures to report. Dr. Laurent has received lecture and consultancy fees from Alexion, AM Pharma, Amgen, Galapagos, Kyowa Kirin, Menarini, Orifarm, Pharmanovia, Takeda, UCB, and Will Pharma.
A version of this article appeared on Medscape.com.
FDA Removes Harmful Chemicals From Food Packaging
Issued on February 28, 2024, “this means the major source of dietary exposure to PFAS from food packaging like fast-food wrappers, microwave popcorn bags, take-out paperboard containers, and pet food bags is being eliminated,” the FDA said in a statement.
In 2020, the FDA had secured commitments from manufacturers to stop selling products containing PFAS used in the food packaging for grease-proofing. “Today’s announcement marks the fulfillment of these voluntary commitments,” according to the agency.
PFAS, a class of thousands of chemicals also called “forever chemicals” are widely used in consumer and industrial products. People may be exposed via contaminated food packaging (although perhaps no longer in the United States) or occupationally. Studies have found that some PFAS disrupt hormones including estrogen and testosterone, whereas others may impair thyroid function.
Endocrine Society Report Sounds the Alarm About PFAS and Others
The FDA’s announcement came just 2 days after the Endocrine Society issued a new alarm about the human health dangers from environmental EDCs including PFAS in a report covering the latest science.
“Endocrine disrupting chemicals” are individual substances or mixtures that can interfere with natural hormonal function, leading to disease or even death. Many are ubiquitous in the modern environment and contribute to a wide range of human diseases.
The new report Endocrine Disrupting Chemicals: Threats to Human Health was issued jointly with the International Pollutants Elimination Network (IPEN), a global advocacy organization. It’s an update to the Endocrine Society’s 2015 report, providing new data on the endocrine-disrupting substances previously covered and adding four EDCs not discussed in that document: Pesticides, plastics, PFAS, and children’s products containing arsenic.
At a briefing held during the United Nations Environment Assembly meeting in Nairobi, Kenya, last week, the new report’s lead author Andrea C. Gore, PhD, of the University of Texas at Austin, noted, “A well-established body of scientific research indicates that endocrine-disrupting chemicals that are part of our daily lives are making us more susceptible to reproductive disorders, cancer, diabetes, obesity, heart disease, and other serious health conditions.”
Added Dr. Gore, who is also a member of the Endocrine Society’s Board of Directors, “These chemicals pose particularly serious risks to pregnant women and children. Now is the time for the UN Environment Assembly and other global policymakers to take action to address this threat to public health.”
While the science has been emerging rapidly, global and national chemical control policies haven’t kept up, the authors said. Of particular concern is that EDCs behave differently from other chemicals in many ways, including that even very low-dose exposures can pose health threats, but policies thus far haven’t dealt with that aspect.
Moreover, “the effects of low doses cannot be predicted by the effects observed at high doses. This means there may be no safe dose for exposure to EDCs,” according to the report.
Exposures can come from household products, including furniture, toys, and food packages, as well as electronics building materials and cosmetics. These chemicals are also in the outdoor environment, via pesticides, air pollution, and industrial waste.
“IPEN and the Endocrine Society call for chemical regulations based on the most modern scientific understanding of how hormones act and how EDCs can perturb these actions. We work to educate policy makers in global, regional, and national government assemblies and help ensure that regulations correlate with current scientific understanding,” they said in the report.
New Data on Four Classes of EDCs
Chapters of the report summarized the latest information about the science of EDCs and their links to endocrine disease and real-world exposure. It included a special section about “EDCs throughout the plastics life cycle” and a summary of the links between EDCs and climate change.
The report reviewed three pesticides, including the world’s most heavily applied herbicide, glycophosphate. Exposures can occur directly from the air, water, dust, and food residues. Recent data linked glycophosphate to adverse reproductive health outcomes.
Two toxic plastic chemicals, phthalates and bisphenols, are present in personal care products, among others. Emerging evidence links them with impaired neurodevelopment, leading to impaired cognitive function, learning, attention, and impulsivity.
Arsenic has long been linked to human health conditions including cancer, but more recent evidence finds it can disrupt multiple endocrine systems and lead to metabolic conditions including diabetes, reproductive dysfunction, and cardiovascular and neurocognitive conditions.
The special section about plastics noted that they are made from fossil fuels and chemicals, including many toxic substances that are known or suspected EDCs. People who live near plastic production facilities or waste dumps may be at greatest risk, but anyone can be exposed using any plastic product. Plastic waste disposal is increasingly problematic and often foisted on lower- and middle-income countries.
‘Additional Education and Awareness-Raising Among Stakeholders Remain Necessary’
Policies aimed at reducing human health risks from EDCs have included the 2022 Plastics Treaty, a resolution adopted by 175 countries at the United Nations Environmental Assembly that “may be a significant step toward global control of plastics and elimination of threats from exposures to EDCs in plastics,” the report said.
The authors added, “While significant progress has been made in recent years connecting scientific advances on EDCs with health-protective policies, additional education and awareness-raising among stakeholders remain necessary to achieve a safer and more sustainable environment that minimizes exposure to these harmful chemicals.”
The document was produced with financial contributions from the Government of Sweden, the Tides Foundation, Passport Foundation, and other donors.
A version of this article appeared on Medscape.com.
Issued on February 28, 2024, “this means the major source of dietary exposure to PFAS from food packaging like fast-food wrappers, microwave popcorn bags, take-out paperboard containers, and pet food bags is being eliminated,” the FDA said in a statement.
In 2020, the FDA had secured commitments from manufacturers to stop selling products containing PFAS used in the food packaging for grease-proofing. “Today’s announcement marks the fulfillment of these voluntary commitments,” according to the agency.
PFAS, a class of thousands of chemicals also called “forever chemicals” are widely used in consumer and industrial products. People may be exposed via contaminated food packaging (although perhaps no longer in the United States) or occupationally. Studies have found that some PFAS disrupt hormones including estrogen and testosterone, whereas others may impair thyroid function.
Endocrine Society Report Sounds the Alarm About PFAS and Others
The FDA’s announcement came just 2 days after the Endocrine Society issued a new alarm about the human health dangers from environmental EDCs including PFAS in a report covering the latest science.
“Endocrine disrupting chemicals” are individual substances or mixtures that can interfere with natural hormonal function, leading to disease or even death. Many are ubiquitous in the modern environment and contribute to a wide range of human diseases.
The new report Endocrine Disrupting Chemicals: Threats to Human Health was issued jointly with the International Pollutants Elimination Network (IPEN), a global advocacy organization. It’s an update to the Endocrine Society’s 2015 report, providing new data on the endocrine-disrupting substances previously covered and adding four EDCs not discussed in that document: Pesticides, plastics, PFAS, and children’s products containing arsenic.
At a briefing held during the United Nations Environment Assembly meeting in Nairobi, Kenya, last week, the new report’s lead author Andrea C. Gore, PhD, of the University of Texas at Austin, noted, “A well-established body of scientific research indicates that endocrine-disrupting chemicals that are part of our daily lives are making us more susceptible to reproductive disorders, cancer, diabetes, obesity, heart disease, and other serious health conditions.”
Added Dr. Gore, who is also a member of the Endocrine Society’s Board of Directors, “These chemicals pose particularly serious risks to pregnant women and children. Now is the time for the UN Environment Assembly and other global policymakers to take action to address this threat to public health.”
While the science has been emerging rapidly, global and national chemical control policies haven’t kept up, the authors said. Of particular concern is that EDCs behave differently from other chemicals in many ways, including that even very low-dose exposures can pose health threats, but policies thus far haven’t dealt with that aspect.
Moreover, “the effects of low doses cannot be predicted by the effects observed at high doses. This means there may be no safe dose for exposure to EDCs,” according to the report.
Exposures can come from household products, including furniture, toys, and food packages, as well as electronics building materials and cosmetics. These chemicals are also in the outdoor environment, via pesticides, air pollution, and industrial waste.
“IPEN and the Endocrine Society call for chemical regulations based on the most modern scientific understanding of how hormones act and how EDCs can perturb these actions. We work to educate policy makers in global, regional, and national government assemblies and help ensure that regulations correlate with current scientific understanding,” they said in the report.
New Data on Four Classes of EDCs
Chapters of the report summarized the latest information about the science of EDCs and their links to endocrine disease and real-world exposure. It included a special section about “EDCs throughout the plastics life cycle” and a summary of the links between EDCs and climate change.
The report reviewed three pesticides, including the world’s most heavily applied herbicide, glycophosphate. Exposures can occur directly from the air, water, dust, and food residues. Recent data linked glycophosphate to adverse reproductive health outcomes.
Two toxic plastic chemicals, phthalates and bisphenols, are present in personal care products, among others. Emerging evidence links them with impaired neurodevelopment, leading to impaired cognitive function, learning, attention, and impulsivity.
Arsenic has long been linked to human health conditions including cancer, but more recent evidence finds it can disrupt multiple endocrine systems and lead to metabolic conditions including diabetes, reproductive dysfunction, and cardiovascular and neurocognitive conditions.
The special section about plastics noted that they are made from fossil fuels and chemicals, including many toxic substances that are known or suspected EDCs. People who live near plastic production facilities or waste dumps may be at greatest risk, but anyone can be exposed using any plastic product. Plastic waste disposal is increasingly problematic and often foisted on lower- and middle-income countries.
‘Additional Education and Awareness-Raising Among Stakeholders Remain Necessary’
Policies aimed at reducing human health risks from EDCs have included the 2022 Plastics Treaty, a resolution adopted by 175 countries at the United Nations Environmental Assembly that “may be a significant step toward global control of plastics and elimination of threats from exposures to EDCs in plastics,” the report said.
The authors added, “While significant progress has been made in recent years connecting scientific advances on EDCs with health-protective policies, additional education and awareness-raising among stakeholders remain necessary to achieve a safer and more sustainable environment that minimizes exposure to these harmful chemicals.”
The document was produced with financial contributions from the Government of Sweden, the Tides Foundation, Passport Foundation, and other donors.
A version of this article appeared on Medscape.com.
Issued on February 28, 2024, “this means the major source of dietary exposure to PFAS from food packaging like fast-food wrappers, microwave popcorn bags, take-out paperboard containers, and pet food bags is being eliminated,” the FDA said in a statement.
In 2020, the FDA had secured commitments from manufacturers to stop selling products containing PFAS used in the food packaging for grease-proofing. “Today’s announcement marks the fulfillment of these voluntary commitments,” according to the agency.
PFAS, a class of thousands of chemicals also called “forever chemicals” are widely used in consumer and industrial products. People may be exposed via contaminated food packaging (although perhaps no longer in the United States) or occupationally. Studies have found that some PFAS disrupt hormones including estrogen and testosterone, whereas others may impair thyroid function.
Endocrine Society Report Sounds the Alarm About PFAS and Others
The FDA’s announcement came just 2 days after the Endocrine Society issued a new alarm about the human health dangers from environmental EDCs including PFAS in a report covering the latest science.
“Endocrine disrupting chemicals” are individual substances or mixtures that can interfere with natural hormonal function, leading to disease or even death. Many are ubiquitous in the modern environment and contribute to a wide range of human diseases.
The new report Endocrine Disrupting Chemicals: Threats to Human Health was issued jointly with the International Pollutants Elimination Network (IPEN), a global advocacy organization. It’s an update to the Endocrine Society’s 2015 report, providing new data on the endocrine-disrupting substances previously covered and adding four EDCs not discussed in that document: Pesticides, plastics, PFAS, and children’s products containing arsenic.
At a briefing held during the United Nations Environment Assembly meeting in Nairobi, Kenya, last week, the new report’s lead author Andrea C. Gore, PhD, of the University of Texas at Austin, noted, “A well-established body of scientific research indicates that endocrine-disrupting chemicals that are part of our daily lives are making us more susceptible to reproductive disorders, cancer, diabetes, obesity, heart disease, and other serious health conditions.”
Added Dr. Gore, who is also a member of the Endocrine Society’s Board of Directors, “These chemicals pose particularly serious risks to pregnant women and children. Now is the time for the UN Environment Assembly and other global policymakers to take action to address this threat to public health.”
While the science has been emerging rapidly, global and national chemical control policies haven’t kept up, the authors said. Of particular concern is that EDCs behave differently from other chemicals in many ways, including that even very low-dose exposures can pose health threats, but policies thus far haven’t dealt with that aspect.
Moreover, “the effects of low doses cannot be predicted by the effects observed at high doses. This means there may be no safe dose for exposure to EDCs,” according to the report.
Exposures can come from household products, including furniture, toys, and food packages, as well as electronics building materials and cosmetics. These chemicals are also in the outdoor environment, via pesticides, air pollution, and industrial waste.
“IPEN and the Endocrine Society call for chemical regulations based on the most modern scientific understanding of how hormones act and how EDCs can perturb these actions. We work to educate policy makers in global, regional, and national government assemblies and help ensure that regulations correlate with current scientific understanding,” they said in the report.
New Data on Four Classes of EDCs
Chapters of the report summarized the latest information about the science of EDCs and their links to endocrine disease and real-world exposure. It included a special section about “EDCs throughout the plastics life cycle” and a summary of the links between EDCs and climate change.
The report reviewed three pesticides, including the world’s most heavily applied herbicide, glycophosphate. Exposures can occur directly from the air, water, dust, and food residues. Recent data linked glycophosphate to adverse reproductive health outcomes.
Two toxic plastic chemicals, phthalates and bisphenols, are present in personal care products, among others. Emerging evidence links them with impaired neurodevelopment, leading to impaired cognitive function, learning, attention, and impulsivity.
Arsenic has long been linked to human health conditions including cancer, but more recent evidence finds it can disrupt multiple endocrine systems and lead to metabolic conditions including diabetes, reproductive dysfunction, and cardiovascular and neurocognitive conditions.
The special section about plastics noted that they are made from fossil fuels and chemicals, including many toxic substances that are known or suspected EDCs. People who live near plastic production facilities or waste dumps may be at greatest risk, but anyone can be exposed using any plastic product. Plastic waste disposal is increasingly problematic and often foisted on lower- and middle-income countries.
‘Additional Education and Awareness-Raising Among Stakeholders Remain Necessary’
Policies aimed at reducing human health risks from EDCs have included the 2022 Plastics Treaty, a resolution adopted by 175 countries at the United Nations Environmental Assembly that “may be a significant step toward global control of plastics and elimination of threats from exposures to EDCs in plastics,” the report said.
The authors added, “While significant progress has been made in recent years connecting scientific advances on EDCs with health-protective policies, additional education and awareness-raising among stakeholders remain necessary to achieve a safer and more sustainable environment that minimizes exposure to these harmful chemicals.”
The document was produced with financial contributions from the Government of Sweden, the Tides Foundation, Passport Foundation, and other donors.
A version of this article appeared on Medscape.com.
Private Equity in GI
Dear colleagues,
In this issue of Perspectives we will explore the business of medicine. With changes in reimbursement models and health care regulation over the past decades, private practice gastroenterology has evolved. Many gastroenterologists are now employed or are part of larger consolidated organizations. A key part of this evolution has been the influx of private equity in GI. The impact of private equity is still being written, and while many have embraced this business model, others have been critical of its influence.
In this issue, Dr. Paul J. Berggreen discusses his group’s experience with private equity and how it has helped improve the quality of patient care that they provide.
Dr. Michael L. Weinstein provides the counterpoint, discussing potential issues with the private equity model, and also highlighting an alternative path taken by his practice. An important topic for gastroenterologists of all ages. We welcome your experience with this issue. Please share with us on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
The Future of Medical Practice
BY DR. PAUL J. BERGGREEN
The future of medicine is being written as we speak. Trends that began in past decades have accelerated. Consolidation among massive hospital systems and health insurance conglomerates has gained momentum.
Physicians have been slow to organize and slower to mobilize. We spend our time caring for patients while national forces shape the future of our profession.
These trends have motivated many physicians to explore vehicles that allow them to remain independent. Creating business relationships with financial entities, including private equity, is one of those methods.
Before exploring those models, some background is instructive.
More than 100,000 doctors have left private practice and become employees of hospitals and other corporate entities since 2019. Today, approximately 75% of physicians are employees of larger health care entities – a record high.
This trend ought to alarm patients and policymakers. Research shows that independent medical practices often deliver better outcomes for patients than hospitals. Physician-owned practices also have lower per-patient costs, fewer preventable hospital admissions, and fewer re-admissions than their larger hospital-owned counterparts.
The business of medicine is very different than it was 40 years ago, when more than three in four doctors cared for patients in their own medical practices. The cost of managing a practice has surged. Labor, rent, and malpractice insurance have grown more expensive. Physicians have had to make significant investments in information technology and electronic health records.
Medicare’s reimbursement rates have not kept pace with these higher operational costs. In fact, Medicare payments to doctors have declined more than 25% in the last two decades after accounting for inflation.
By contrast, reimbursement for inpatient and outpatient hospital services as well as skilled nursing facilities has outpaced inflation since 2001.
Given these economic headwinds – and the mounting administrative and financial burdens that government regulation poses – many independent practitioners have concluded that they have little choice but to sell to larger entities like hospitals, health systems, or insurers.
If they do, they lose autonomy. Patients lose the personal touch an independent practice can offer.
To stay independent, many physicians are partnering with management services organizations (MSOs), which provide nonclinical services such as compliance, contracting, legal and IT support, cybersecurity, marketing, community outreach, recruiting assistance, billing, accounts payable, and guidance on the transition to value-based care.
MSOs are typically backed by investors: perhaps a public company, or a private equity firm. But it’s important to note that the clinical entity – the practice – remains separate from the MSO. Physicians retain control over clinical decision-making after partnering with an MSO.
Private equity is best viewed as a neutral financing mechanism that provides independent practices access to capital so they can build the business, clinical, and technological infrastructure to compete against the vertically integrated health systems that dominate medicine.
Private equity firms don’t “acquire” independent practices. A partnership with a private equity-backed MSO is often what empowers a practice to resist acquisition by a larger hospital or health care system.
The experience of my own practice, Arizona Digestive Health, is instructive. We partnered with GI Alliance – a private equity-backed, gastroenterologist-led MSO – in 2019.
My physician colleagues and I have retained complete clinical autonomy. But we now have the financial and operational support we need to remain independent – and deliver better care for our patients.
For example, we led the development of a GI-focused, population-based clinical dashboard that aggregates real-time data from almost 3 million patients across 16 states who are treated by practices affiliated with GI Alliance.
By drawing on that data, we’ve been able to implement comprehensive care-management programs. In the case of inflammatory bowel disease, for instance, we’ve been able to identify the highest-cost, most at-risk patients and implement more proactive treatment protocols, including dedicated care managers and hotlines. We’ve replicated this model in other disease states as well.
This kind of ongoing, high-touch intervention improves patient outcomes and reduces overall cost by minimizing unplanned episodes of care – like visits to the emergency room.
It’s not possible to provide this level of care in a smaller setting. I should know. I tried to implement a similar approach for the 55 doctors that comprise Arizona Digestive Health in Phoenix. We simply didn’t have the capital or resources to succeed.
Our experience at Arizona Digestive Health is not an outlier. I have seen numerous independent practices in gastroenterology and other specialties throughout the country leverage the resources of private equity-backed MSOs to enhance the level of care they provide – and improve patient outcomes and experiences.
In 2022, the physician leadership of GI Alliance spearheaded a transaction that resulted in the nearly 700 physicians whose independent gastroenterology practices were part of the alliance to grow their collective equity stake in the MSO to more than 85%. Our independent physicians now have voting control of the MSO board of directors.
This evolution of GI Alliance has enabled us to remain true to our mission of putting patients first while enhancing our ability to shape the business support our partnered gastroenterology practices need to expand access to the highest-quality, most affordable care in our communities.
Doctors caring for patients in their own practices used to be the foundation of the U.S. health care system – and for good reason. The model enables patients to receive more personalized care and build deeper, more longitudinal, more trusting relationships with their doctors. That remains the goal of physicians who value autonomy and independence.
Inaction will result in more of the same, with hospitals and insurance companies snapping up independent practices. It’s encouraging to see physicians take back control of their profession. But the climb remains steep.
The easiest way to predict the future is to invent it. Doing so in a patient-centric, physician-led, and physician-owned group is a great start to that journey.
Dr. Paul Berggreen is board chair and president of the American Independent Medical Practice Association. He is founder and president of Arizona Digestive Health, chief strategy officer for the GI Alliance, and chair of data analytics for the Digestive Health Physicians Association. He is also a consultant to Specialty Networks, which is not directly relevant to this article.
Thinking Strategically About Gastroenterology Practice
BY MICHAEL L. WEINSTEIN, MD
Whether you are a young gastroenterologist assessing your career opportunities, or a gastroenterology practice trying to assure your future success, you are likely considering how a private equity transaction might influence your options. In this column, I am going to share what I’ve learned and why my practice chose not to go the route of a private equity investment partner.
In 2018, Capital Digestive Care was an independent practice of 70 physicians centered around Washington, DC. Private equity firms were increasingly investing in health care, seeking to capitalize on the industry’s fragmentation, recession-proof business, and ability to leverage consolidation. Our leadership chose to spend a weekend on a strategic planning retreat to agree on our priorities and long-term goals. I highly recommend that you and/or your practice sit down to list your priorities as your first task.
After defining priorities, a SWOT analysis of your position today and what you project over the next decade will determine a strategy. There is a current shortage of more than 1,400 gastroenterologists in the United States. That gives us a pretty powerful “strength.” However, the consolidation of commercial payers and hospital systems is forcing physicians to accept low reimbursement and navigate a maze of administrative burdens. The mountain of regulatory, administrative, and financial functions can push physicians away from independent practice. Additionally, recruiting, training, and managing an office of medical personnel is not what most gastroenterologists want to do with their time.
The common denominator to achieve success with all of these practice management issues is size. So before providing thoughts about private equity, I recommend consolidation of medical practices as the strategy to achieving long-term goals. Practice size will allow physicians to spread out the administrative work, the cost of the business personnel, the IT systems, and the specialized resources. Purchasing power and negotiation relevance is achieved with size. Our priorities are taking care of our patients, our staff, and our practice colleagues. If we are providing high-value service and have a size relevant to the insurance companies, then we can negotiate value-based contracts, and at the end of the day, we will be financially well-off.
In contrast to the list of priorities a physician would create, the private equity fund manager’s goal is to generate wealth for themselves and their investors. Everything else, like innovation, enhanced service, employee satisfaction, and great quality, takes a back seat to accumulating profit. Their investments are made with a life-cycle of 4-6 years during which money is deployed by acquiring companies, improving the company bottom line profit through cost cutting or bolt-on acquisitions, increasing company profit distributions by adding leveraged debt to the corporate ledger, and then selling the companies often to another private equity fund. Physicians are trained to provide care to patients, and fund managers are trained to create wealth.
The medical practice as a business can grow over a career and provides physicians with top tier incomes. We are proud of the businesses we build and believe they are valuable. Private equity funds acquire medical practices for the future revenue and not the past results. They value a medical practice based on a multiple of the portion of future income the practice wants to sell. They ensure their future revenue through agreements that provide them management fees plus 25%-35% of future physician income for the current and all future physicians. The private equity company will say that the physicians are still independent but in reality all providers become employees of the company with wages defined by a formula. The private equity-owned Management Services Organization (MSO) controls decisions on carrier contracts, practice investments, purchasing, hiring, and the operations of the medical office. To get around corporate practice of medicine regulations, the ownership of the medical practice is placed in the hands of a single friendly physician who has a unique relationship to the MSO.
In my opinion, private equity is not the best strategy to achieve a successful medical practice, including acquiring the needed technology and human resources. It comes at a steep price, including loss of control and a permanent forfeiture of income (“the scrape”). The rhetoric professes that there will be income repair, monetization of practice value, and opportunity for a “second bite of the apple” when the private equity managers sell your practice to the next owner. Private equity’s main contribution for their outsized gains is the capital they bring to the practice. Everything else they bring can be found without selling the income of future partners to create a little more wealth for current partners.
The long-term results of private equity investment in gastroenterology practices has yet to be written. The experience in other specialties is partly documented in literature but the real stories are often hidden behind non-disparagement and non-disclosure clauses. Several investigations show that private equity ownership of health care providers leads to higher costs to patients and payers, employee dissatisfaction, diminished patient access, and worse health outcomes. The Federal Trade Commission and Department of Justice have vowed to scrutinize private equity deals because of mounting evidence that the motive for profit can conflict with maintaining quality.
In 2019, Capital Digestive Care chose Physicians Endoscopy as our strategic partner with the goal of separating and expanding our back-office functions into an MSO capable of providing business services to a larger practice and services to other practices outside of our own. Physicians Endoscopy has since been acquired by Optum/SCA. PE GI Solutions, the MSO, is now a partnership of CDC physician partners and Optum/SCA. Capital Digestive Care remains a practice owned 100% by the physicians. A Business Support Services Agreement defines the services CDC receives and the fees paid to the PE GI Solutions. We maintain MSO Board seats and have input into the operations of the MSO.
Consider your motivations and the degree of control you need. Do you recognize your gaps of knowledge and are you willing to hire people to advise you? Will your practice achieve a balance between the interests of older and younger physicians? Becoming an employed physician in a large practice is an option to manage the concerns about future career stability. Improved quality, expanded service offerings, clout to negotiate value-based payment deals with payers, and back-office business efficiency does not require selling yourself to a private equity fund.
Dr. Michael L. Weinstein is a founder and now chief executive officer of Capital Digestive Care. He is a founder and past president of the Digestive Health Physicians Association, previous counselor on the Governing Board of the American Gastroenterological Association. He reports no relevant conflicts.
References
The FTC and DOJ have vowed to scrutinize private equity deals. Here’s what it means for health care. FIERCE Healthcare, 2022, Oct. 21.
The Effect of Private Equity Investment in Health Care. Penn LDI. 2023 Mar. 10.
Olson, LK. Ethically challenged: Private equity storms US health care. Baltimore: Johns Hopkins University Press. 2022.
Dear colleagues,
In this issue of Perspectives we will explore the business of medicine. With changes in reimbursement models and health care regulation over the past decades, private practice gastroenterology has evolved. Many gastroenterologists are now employed or are part of larger consolidated organizations. A key part of this evolution has been the influx of private equity in GI. The impact of private equity is still being written, and while many have embraced this business model, others have been critical of its influence.
In this issue, Dr. Paul J. Berggreen discusses his group’s experience with private equity and how it has helped improve the quality of patient care that they provide.
Dr. Michael L. Weinstein provides the counterpoint, discussing potential issues with the private equity model, and also highlighting an alternative path taken by his practice. An important topic for gastroenterologists of all ages. We welcome your experience with this issue. Please share with us on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
The Future of Medical Practice
BY DR. PAUL J. BERGGREEN
The future of medicine is being written as we speak. Trends that began in past decades have accelerated. Consolidation among massive hospital systems and health insurance conglomerates has gained momentum.
Physicians have been slow to organize and slower to mobilize. We spend our time caring for patients while national forces shape the future of our profession.
These trends have motivated many physicians to explore vehicles that allow them to remain independent. Creating business relationships with financial entities, including private equity, is one of those methods.
Before exploring those models, some background is instructive.
More than 100,000 doctors have left private practice and become employees of hospitals and other corporate entities since 2019. Today, approximately 75% of physicians are employees of larger health care entities – a record high.
This trend ought to alarm patients and policymakers. Research shows that independent medical practices often deliver better outcomes for patients than hospitals. Physician-owned practices also have lower per-patient costs, fewer preventable hospital admissions, and fewer re-admissions than their larger hospital-owned counterparts.
The business of medicine is very different than it was 40 years ago, when more than three in four doctors cared for patients in their own medical practices. The cost of managing a practice has surged. Labor, rent, and malpractice insurance have grown more expensive. Physicians have had to make significant investments in information technology and electronic health records.
Medicare’s reimbursement rates have not kept pace with these higher operational costs. In fact, Medicare payments to doctors have declined more than 25% in the last two decades after accounting for inflation.
By contrast, reimbursement for inpatient and outpatient hospital services as well as skilled nursing facilities has outpaced inflation since 2001.
Given these economic headwinds – and the mounting administrative and financial burdens that government regulation poses – many independent practitioners have concluded that they have little choice but to sell to larger entities like hospitals, health systems, or insurers.
If they do, they lose autonomy. Patients lose the personal touch an independent practice can offer.
To stay independent, many physicians are partnering with management services organizations (MSOs), which provide nonclinical services such as compliance, contracting, legal and IT support, cybersecurity, marketing, community outreach, recruiting assistance, billing, accounts payable, and guidance on the transition to value-based care.
MSOs are typically backed by investors: perhaps a public company, or a private equity firm. But it’s important to note that the clinical entity – the practice – remains separate from the MSO. Physicians retain control over clinical decision-making after partnering with an MSO.
Private equity is best viewed as a neutral financing mechanism that provides independent practices access to capital so they can build the business, clinical, and technological infrastructure to compete against the vertically integrated health systems that dominate medicine.
Private equity firms don’t “acquire” independent practices. A partnership with a private equity-backed MSO is often what empowers a practice to resist acquisition by a larger hospital or health care system.
The experience of my own practice, Arizona Digestive Health, is instructive. We partnered with GI Alliance – a private equity-backed, gastroenterologist-led MSO – in 2019.
My physician colleagues and I have retained complete clinical autonomy. But we now have the financial and operational support we need to remain independent – and deliver better care for our patients.
For example, we led the development of a GI-focused, population-based clinical dashboard that aggregates real-time data from almost 3 million patients across 16 states who are treated by practices affiliated with GI Alliance.
By drawing on that data, we’ve been able to implement comprehensive care-management programs. In the case of inflammatory bowel disease, for instance, we’ve been able to identify the highest-cost, most at-risk patients and implement more proactive treatment protocols, including dedicated care managers and hotlines. We’ve replicated this model in other disease states as well.
This kind of ongoing, high-touch intervention improves patient outcomes and reduces overall cost by minimizing unplanned episodes of care – like visits to the emergency room.
It’s not possible to provide this level of care in a smaller setting. I should know. I tried to implement a similar approach for the 55 doctors that comprise Arizona Digestive Health in Phoenix. We simply didn’t have the capital or resources to succeed.
Our experience at Arizona Digestive Health is not an outlier. I have seen numerous independent practices in gastroenterology and other specialties throughout the country leverage the resources of private equity-backed MSOs to enhance the level of care they provide – and improve patient outcomes and experiences.
In 2022, the physician leadership of GI Alliance spearheaded a transaction that resulted in the nearly 700 physicians whose independent gastroenterology practices were part of the alliance to grow their collective equity stake in the MSO to more than 85%. Our independent physicians now have voting control of the MSO board of directors.
This evolution of GI Alliance has enabled us to remain true to our mission of putting patients first while enhancing our ability to shape the business support our partnered gastroenterology practices need to expand access to the highest-quality, most affordable care in our communities.
Doctors caring for patients in their own practices used to be the foundation of the U.S. health care system – and for good reason. The model enables patients to receive more personalized care and build deeper, more longitudinal, more trusting relationships with their doctors. That remains the goal of physicians who value autonomy and independence.
Inaction will result in more of the same, with hospitals and insurance companies snapping up independent practices. It’s encouraging to see physicians take back control of their profession. But the climb remains steep.
The easiest way to predict the future is to invent it. Doing so in a patient-centric, physician-led, and physician-owned group is a great start to that journey.
Dr. Paul Berggreen is board chair and president of the American Independent Medical Practice Association. He is founder and president of Arizona Digestive Health, chief strategy officer for the GI Alliance, and chair of data analytics for the Digestive Health Physicians Association. He is also a consultant to Specialty Networks, which is not directly relevant to this article.
Thinking Strategically About Gastroenterology Practice
BY MICHAEL L. WEINSTEIN, MD
Whether you are a young gastroenterologist assessing your career opportunities, or a gastroenterology practice trying to assure your future success, you are likely considering how a private equity transaction might influence your options. In this column, I am going to share what I’ve learned and why my practice chose not to go the route of a private equity investment partner.
In 2018, Capital Digestive Care was an independent practice of 70 physicians centered around Washington, DC. Private equity firms were increasingly investing in health care, seeking to capitalize on the industry’s fragmentation, recession-proof business, and ability to leverage consolidation. Our leadership chose to spend a weekend on a strategic planning retreat to agree on our priorities and long-term goals. I highly recommend that you and/or your practice sit down to list your priorities as your first task.
After defining priorities, a SWOT analysis of your position today and what you project over the next decade will determine a strategy. There is a current shortage of more than 1,400 gastroenterologists in the United States. That gives us a pretty powerful “strength.” However, the consolidation of commercial payers and hospital systems is forcing physicians to accept low reimbursement and navigate a maze of administrative burdens. The mountain of regulatory, administrative, and financial functions can push physicians away from independent practice. Additionally, recruiting, training, and managing an office of medical personnel is not what most gastroenterologists want to do with their time.
The common denominator to achieve success with all of these practice management issues is size. So before providing thoughts about private equity, I recommend consolidation of medical practices as the strategy to achieving long-term goals. Practice size will allow physicians to spread out the administrative work, the cost of the business personnel, the IT systems, and the specialized resources. Purchasing power and negotiation relevance is achieved with size. Our priorities are taking care of our patients, our staff, and our practice colleagues. If we are providing high-value service and have a size relevant to the insurance companies, then we can negotiate value-based contracts, and at the end of the day, we will be financially well-off.
In contrast to the list of priorities a physician would create, the private equity fund manager’s goal is to generate wealth for themselves and their investors. Everything else, like innovation, enhanced service, employee satisfaction, and great quality, takes a back seat to accumulating profit. Their investments are made with a life-cycle of 4-6 years during which money is deployed by acquiring companies, improving the company bottom line profit through cost cutting or bolt-on acquisitions, increasing company profit distributions by adding leveraged debt to the corporate ledger, and then selling the companies often to another private equity fund. Physicians are trained to provide care to patients, and fund managers are trained to create wealth.
The medical practice as a business can grow over a career and provides physicians with top tier incomes. We are proud of the businesses we build and believe they are valuable. Private equity funds acquire medical practices for the future revenue and not the past results. They value a medical practice based on a multiple of the portion of future income the practice wants to sell. They ensure their future revenue through agreements that provide them management fees plus 25%-35% of future physician income for the current and all future physicians. The private equity company will say that the physicians are still independent but in reality all providers become employees of the company with wages defined by a formula. The private equity-owned Management Services Organization (MSO) controls decisions on carrier contracts, practice investments, purchasing, hiring, and the operations of the medical office. To get around corporate practice of medicine regulations, the ownership of the medical practice is placed in the hands of a single friendly physician who has a unique relationship to the MSO.
In my opinion, private equity is not the best strategy to achieve a successful medical practice, including acquiring the needed technology and human resources. It comes at a steep price, including loss of control and a permanent forfeiture of income (“the scrape”). The rhetoric professes that there will be income repair, monetization of practice value, and opportunity for a “second bite of the apple” when the private equity managers sell your practice to the next owner. Private equity’s main contribution for their outsized gains is the capital they bring to the practice. Everything else they bring can be found without selling the income of future partners to create a little more wealth for current partners.
The long-term results of private equity investment in gastroenterology practices has yet to be written. The experience in other specialties is partly documented in literature but the real stories are often hidden behind non-disparagement and non-disclosure clauses. Several investigations show that private equity ownership of health care providers leads to higher costs to patients and payers, employee dissatisfaction, diminished patient access, and worse health outcomes. The Federal Trade Commission and Department of Justice have vowed to scrutinize private equity deals because of mounting evidence that the motive for profit can conflict with maintaining quality.
In 2019, Capital Digestive Care chose Physicians Endoscopy as our strategic partner with the goal of separating and expanding our back-office functions into an MSO capable of providing business services to a larger practice and services to other practices outside of our own. Physicians Endoscopy has since been acquired by Optum/SCA. PE GI Solutions, the MSO, is now a partnership of CDC physician partners and Optum/SCA. Capital Digestive Care remains a practice owned 100% by the physicians. A Business Support Services Agreement defines the services CDC receives and the fees paid to the PE GI Solutions. We maintain MSO Board seats and have input into the operations of the MSO.
Consider your motivations and the degree of control you need. Do you recognize your gaps of knowledge and are you willing to hire people to advise you? Will your practice achieve a balance between the interests of older and younger physicians? Becoming an employed physician in a large practice is an option to manage the concerns about future career stability. Improved quality, expanded service offerings, clout to negotiate value-based payment deals with payers, and back-office business efficiency does not require selling yourself to a private equity fund.
Dr. Michael L. Weinstein is a founder and now chief executive officer of Capital Digestive Care. He is a founder and past president of the Digestive Health Physicians Association, previous counselor on the Governing Board of the American Gastroenterological Association. He reports no relevant conflicts.
References
The FTC and DOJ have vowed to scrutinize private equity deals. Here’s what it means for health care. FIERCE Healthcare, 2022, Oct. 21.
The Effect of Private Equity Investment in Health Care. Penn LDI. 2023 Mar. 10.
Olson, LK. Ethically challenged: Private equity storms US health care. Baltimore: Johns Hopkins University Press. 2022.
Dear colleagues,
In this issue of Perspectives we will explore the business of medicine. With changes in reimbursement models and health care regulation over the past decades, private practice gastroenterology has evolved. Many gastroenterologists are now employed or are part of larger consolidated organizations. A key part of this evolution has been the influx of private equity in GI. The impact of private equity is still being written, and while many have embraced this business model, others have been critical of its influence.
In this issue, Dr. Paul J. Berggreen discusses his group’s experience with private equity and how it has helped improve the quality of patient care that they provide.
Dr. Michael L. Weinstein provides the counterpoint, discussing potential issues with the private equity model, and also highlighting an alternative path taken by his practice. An important topic for gastroenterologists of all ages. We welcome your experience with this issue. Please share with us on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
The Future of Medical Practice
BY DR. PAUL J. BERGGREEN
The future of medicine is being written as we speak. Trends that began in past decades have accelerated. Consolidation among massive hospital systems and health insurance conglomerates has gained momentum.
Physicians have been slow to organize and slower to mobilize. We spend our time caring for patients while national forces shape the future of our profession.
These trends have motivated many physicians to explore vehicles that allow them to remain independent. Creating business relationships with financial entities, including private equity, is one of those methods.
Before exploring those models, some background is instructive.
More than 100,000 doctors have left private practice and become employees of hospitals and other corporate entities since 2019. Today, approximately 75% of physicians are employees of larger health care entities – a record high.
This trend ought to alarm patients and policymakers. Research shows that independent medical practices often deliver better outcomes for patients than hospitals. Physician-owned practices also have lower per-patient costs, fewer preventable hospital admissions, and fewer re-admissions than their larger hospital-owned counterparts.
The business of medicine is very different than it was 40 years ago, when more than three in four doctors cared for patients in their own medical practices. The cost of managing a practice has surged. Labor, rent, and malpractice insurance have grown more expensive. Physicians have had to make significant investments in information technology and electronic health records.
Medicare’s reimbursement rates have not kept pace with these higher operational costs. In fact, Medicare payments to doctors have declined more than 25% in the last two decades after accounting for inflation.
By contrast, reimbursement for inpatient and outpatient hospital services as well as skilled nursing facilities has outpaced inflation since 2001.
Given these economic headwinds – and the mounting administrative and financial burdens that government regulation poses – many independent practitioners have concluded that they have little choice but to sell to larger entities like hospitals, health systems, or insurers.
If they do, they lose autonomy. Patients lose the personal touch an independent practice can offer.
To stay independent, many physicians are partnering with management services organizations (MSOs), which provide nonclinical services such as compliance, contracting, legal and IT support, cybersecurity, marketing, community outreach, recruiting assistance, billing, accounts payable, and guidance on the transition to value-based care.
MSOs are typically backed by investors: perhaps a public company, or a private equity firm. But it’s important to note that the clinical entity – the practice – remains separate from the MSO. Physicians retain control over clinical decision-making after partnering with an MSO.
Private equity is best viewed as a neutral financing mechanism that provides independent practices access to capital so they can build the business, clinical, and technological infrastructure to compete against the vertically integrated health systems that dominate medicine.
Private equity firms don’t “acquire” independent practices. A partnership with a private equity-backed MSO is often what empowers a practice to resist acquisition by a larger hospital or health care system.
The experience of my own practice, Arizona Digestive Health, is instructive. We partnered with GI Alliance – a private equity-backed, gastroenterologist-led MSO – in 2019.
My physician colleagues and I have retained complete clinical autonomy. But we now have the financial and operational support we need to remain independent – and deliver better care for our patients.
For example, we led the development of a GI-focused, population-based clinical dashboard that aggregates real-time data from almost 3 million patients across 16 states who are treated by practices affiliated with GI Alliance.
By drawing on that data, we’ve been able to implement comprehensive care-management programs. In the case of inflammatory bowel disease, for instance, we’ve been able to identify the highest-cost, most at-risk patients and implement more proactive treatment protocols, including dedicated care managers and hotlines. We’ve replicated this model in other disease states as well.
This kind of ongoing, high-touch intervention improves patient outcomes and reduces overall cost by minimizing unplanned episodes of care – like visits to the emergency room.
It’s not possible to provide this level of care in a smaller setting. I should know. I tried to implement a similar approach for the 55 doctors that comprise Arizona Digestive Health in Phoenix. We simply didn’t have the capital or resources to succeed.
Our experience at Arizona Digestive Health is not an outlier. I have seen numerous independent practices in gastroenterology and other specialties throughout the country leverage the resources of private equity-backed MSOs to enhance the level of care they provide – and improve patient outcomes and experiences.
In 2022, the physician leadership of GI Alliance spearheaded a transaction that resulted in the nearly 700 physicians whose independent gastroenterology practices were part of the alliance to grow their collective equity stake in the MSO to more than 85%. Our independent physicians now have voting control of the MSO board of directors.
This evolution of GI Alliance has enabled us to remain true to our mission of putting patients first while enhancing our ability to shape the business support our partnered gastroenterology practices need to expand access to the highest-quality, most affordable care in our communities.
Doctors caring for patients in their own practices used to be the foundation of the U.S. health care system – and for good reason. The model enables patients to receive more personalized care and build deeper, more longitudinal, more trusting relationships with their doctors. That remains the goal of physicians who value autonomy and independence.
Inaction will result in more of the same, with hospitals and insurance companies snapping up independent practices. It’s encouraging to see physicians take back control of their profession. But the climb remains steep.
The easiest way to predict the future is to invent it. Doing so in a patient-centric, physician-led, and physician-owned group is a great start to that journey.
Dr. Paul Berggreen is board chair and president of the American Independent Medical Practice Association. He is founder and president of Arizona Digestive Health, chief strategy officer for the GI Alliance, and chair of data analytics for the Digestive Health Physicians Association. He is also a consultant to Specialty Networks, which is not directly relevant to this article.
Thinking Strategically About Gastroenterology Practice
BY MICHAEL L. WEINSTEIN, MD
Whether you are a young gastroenterologist assessing your career opportunities, or a gastroenterology practice trying to assure your future success, you are likely considering how a private equity transaction might influence your options. In this column, I am going to share what I’ve learned and why my practice chose not to go the route of a private equity investment partner.
In 2018, Capital Digestive Care was an independent practice of 70 physicians centered around Washington, DC. Private equity firms were increasingly investing in health care, seeking to capitalize on the industry’s fragmentation, recession-proof business, and ability to leverage consolidation. Our leadership chose to spend a weekend on a strategic planning retreat to agree on our priorities and long-term goals. I highly recommend that you and/or your practice sit down to list your priorities as your first task.
After defining priorities, a SWOT analysis of your position today and what you project over the next decade will determine a strategy. There is a current shortage of more than 1,400 gastroenterologists in the United States. That gives us a pretty powerful “strength.” However, the consolidation of commercial payers and hospital systems is forcing physicians to accept low reimbursement and navigate a maze of administrative burdens. The mountain of regulatory, administrative, and financial functions can push physicians away from independent practice. Additionally, recruiting, training, and managing an office of medical personnel is not what most gastroenterologists want to do with their time.
The common denominator to achieve success with all of these practice management issues is size. So before providing thoughts about private equity, I recommend consolidation of medical practices as the strategy to achieving long-term goals. Practice size will allow physicians to spread out the administrative work, the cost of the business personnel, the IT systems, and the specialized resources. Purchasing power and negotiation relevance is achieved with size. Our priorities are taking care of our patients, our staff, and our practice colleagues. If we are providing high-value service and have a size relevant to the insurance companies, then we can negotiate value-based contracts, and at the end of the day, we will be financially well-off.
In contrast to the list of priorities a physician would create, the private equity fund manager’s goal is to generate wealth for themselves and their investors. Everything else, like innovation, enhanced service, employee satisfaction, and great quality, takes a back seat to accumulating profit. Their investments are made with a life-cycle of 4-6 years during which money is deployed by acquiring companies, improving the company bottom line profit through cost cutting or bolt-on acquisitions, increasing company profit distributions by adding leveraged debt to the corporate ledger, and then selling the companies often to another private equity fund. Physicians are trained to provide care to patients, and fund managers are trained to create wealth.
The medical practice as a business can grow over a career and provides physicians with top tier incomes. We are proud of the businesses we build and believe they are valuable. Private equity funds acquire medical practices for the future revenue and not the past results. They value a medical practice based on a multiple of the portion of future income the practice wants to sell. They ensure their future revenue through agreements that provide them management fees plus 25%-35% of future physician income for the current and all future physicians. The private equity company will say that the physicians are still independent but in reality all providers become employees of the company with wages defined by a formula. The private equity-owned Management Services Organization (MSO) controls decisions on carrier contracts, practice investments, purchasing, hiring, and the operations of the medical office. To get around corporate practice of medicine regulations, the ownership of the medical practice is placed in the hands of a single friendly physician who has a unique relationship to the MSO.
In my opinion, private equity is not the best strategy to achieve a successful medical practice, including acquiring the needed technology and human resources. It comes at a steep price, including loss of control and a permanent forfeiture of income (“the scrape”). The rhetoric professes that there will be income repair, monetization of practice value, and opportunity for a “second bite of the apple” when the private equity managers sell your practice to the next owner. Private equity’s main contribution for their outsized gains is the capital they bring to the practice. Everything else they bring can be found without selling the income of future partners to create a little more wealth for current partners.
The long-term results of private equity investment in gastroenterology practices has yet to be written. The experience in other specialties is partly documented in literature but the real stories are often hidden behind non-disparagement and non-disclosure clauses. Several investigations show that private equity ownership of health care providers leads to higher costs to patients and payers, employee dissatisfaction, diminished patient access, and worse health outcomes. The Federal Trade Commission and Department of Justice have vowed to scrutinize private equity deals because of mounting evidence that the motive for profit can conflict with maintaining quality.
In 2019, Capital Digestive Care chose Physicians Endoscopy as our strategic partner with the goal of separating and expanding our back-office functions into an MSO capable of providing business services to a larger practice and services to other practices outside of our own. Physicians Endoscopy has since been acquired by Optum/SCA. PE GI Solutions, the MSO, is now a partnership of CDC physician partners and Optum/SCA. Capital Digestive Care remains a practice owned 100% by the physicians. A Business Support Services Agreement defines the services CDC receives and the fees paid to the PE GI Solutions. We maintain MSO Board seats and have input into the operations of the MSO.
Consider your motivations and the degree of control you need. Do you recognize your gaps of knowledge and are you willing to hire people to advise you? Will your practice achieve a balance between the interests of older and younger physicians? Becoming an employed physician in a large practice is an option to manage the concerns about future career stability. Improved quality, expanded service offerings, clout to negotiate value-based payment deals with payers, and back-office business efficiency does not require selling yourself to a private equity fund.
Dr. Michael L. Weinstein is a founder and now chief executive officer of Capital Digestive Care. He is a founder and past president of the Digestive Health Physicians Association, previous counselor on the Governing Board of the American Gastroenterological Association. He reports no relevant conflicts.
References
The FTC and DOJ have vowed to scrutinize private equity deals. Here’s what it means for health care. FIERCE Healthcare, 2022, Oct. 21.
The Effect of Private Equity Investment in Health Care. Penn LDI. 2023 Mar. 10.
Olson, LK. Ethically challenged: Private equity storms US health care. Baltimore: Johns Hopkins University Press. 2022.
Receiving Unfair Negative Patient Reviews Online? These Apps Pledge Relief
Physicians’ negative online reviews — fair or unfair — can scare away new patients. But practices don’t have to sit idly by and watch their revenue shrink.
Increasingly, they’re turning to
Not all of these systems are effective, according to physicians who’ve used them. Asking patients for reviews is still not fully accepted, either. Still, some apps have proved their worth, doctors say.
Karen Horton, MD, a plastic surgeon in San Francisco, California, has used an automated system for 3 years. Even though reviews from plastic surgery patients can be difficult to get, Dr. Horton said, she has accumulated 535, with an average rating of just under 5 stars on a 1- to 5-star scale.
Dr. Horton, who speaks on the topic, said unfair negative reviews are a problem that needs addressing.
“A bad review sometimes says more about the patient than the provider,” she said. “Patients can use online reviews to vent about some perceived misgiving.”
Automated requests can address this problem. “The best way to deal with negative reviews is to ask average patients to post reviews,” she said. “These patients are more likely to be positive, but they wouldn’t leave a review unless asked.”
How Automated Systems Work
A variety of vendors provide an automated review request process to practices and hospitals. DearDoc, Loyal Health, Rater8, and Simple Interact work with healthcare providers, while Birdeye, Reputation, and Thrive Management work with all businesses.
Typically, these vendors access the practice’s electronic health record to get patients’ contact information and the daily appointment schedule to know which patients to contact. Patients are contacted after their appointment and are given the opportunity to go directly to a review site and post.
Inviting patients digitally rather than in person may seem unwelcoming, but many people prefer it, said Fred Horton, president of AMGA consulting in Alexandria, Virginia, a subsidiary of the American Medical Group Association. (He is not related to Karen Horton.)
“People tend to be more honest and detailed when responding to an automated message than to a person,” Mr. Horton told this news organization. “And younger patients actually prefer digital communications.”
But Mike Coppola, vice president of AMGA consulting, isn’t keen about automation.
He said practices can instead assign staff to ask patients to post reviews or an office can use signage displaying a Quick Response (QR) code, a two-dimensional matrix often used in restaurants to access a menu. Patients who put their smartphone cameras over the code are taken directly to a review site.
Still, staff would still need to help each patient access the site to be as effective as automation, and a QR invitation may be ignored. Pat Pazmino, MD, a plastic surgeon in Miami, Florida, told this news organization his office displays QR codes for reviews, but “I’m not sure many patients really use them.”
Some automated systems can go too far. Dr. Pazmino said a vendor he hired several years ago contacted “every patient who had ever called my office. A lot of them were annoyed.”
He said the service generated only 20 or 30 reviews, and some were negative. He did not like that he was soliciting patients to make negative reviews. He canceled the service.
What Is the Cost and Return on Investment?
“Our system makes it as easy as possible for patients to place reviews,” said Ravi Kalidindi, CEO of Simple Interact, a Dallas-based vendor that markets to doctors.
Dr. Kalidindi said Simple Interact charges $95-$145 per provider per month, depending on how the tool is used. For each dollar in cost, the practice typically earns $10 in extra revenue, he said.
Orrin Franko, MD, a hand surgeon in San Leandro, California, started using an automated patient review tool several years ago. He said that after installation received 10 reviews per month, all 5-star. “Now we have well over 700 reviews that generate close to $500,000 a year for our three-doctor practice,” he said.
Karen Horton reports more modest results. One new review comes in every 3-4 weeks. “Getting online reviews is a challenge for plastic surgeons,” he said. “Most patients are very private about having work done.”
Dr. Kalidindi reported that very few patients respond to Simple Interact’s invitation, but the numbers add up. “Typically, 3 of 100 patients contacted will ultimately post a positive review,” he said. “That means that a practice that sees 600 patients a month could get 18 positive reviews a month.”
Practices can also build their own systems and avoid vendors’ monthly fees. Dr. Franko built his own system, while Dr. Horton contracted with SILVR Agency, a digital marketing company in Solana Beach, California, to build hers for a one-time cost of about $3000.
Why Should Doctors Care About Online Reviews?
Online review sites for doctors include HealthGrades, RateMDs, Realself, Vitals, WebMD, and Zocdoc. (Medscape Medical News is part of WebMD.) Potential patients also consult general review sites like Facebook, Google My Business, and Yelp.
Consumers tend to prefer doctors who have many reviews, but most doctors get very few. One survey found that the average doctor has only seven online reviews, while competitors may have hundreds.
Having too few reviews also means that just one or two negative reviews can produce a poor average rating. It’s virtually impossible to remove negative reviews, and they can have a big impact. A 1-star rating reduces consumers’ clicks by 11%, according to Brightlocal, a company that surveys consumers’ use of online ratings.
Online reviews also influence Google searches, even when consumers never access a review site, said Lee Rensch, product director at Loyal Health, an Atlanta, Georgia–based vendor that works exclusively with hospitals.
By far the most common way to find a doctor is to use Google to search for doctors “near me,” Mr. Rensch told this news organization. The Google search brings up a ranked list of doctors, based partly on each doctor’s ratings on review sites.
Mr. Rensch said 15%-20% of Google’s ranking involves the number of reviews the doctor has, the average star rating, and the newness of the reviews. Other factors include whether the provider has responded to reviews and the description of the practice, he said.
How many people use the internet to find doctors? One survey found that 72% of healthcare consumers do so. Furthermore, healthcare ranks second in the most common use of reviews, after service businesses and before restaurants, according to a Brightlocal survey.
Is it OK to Ask for Reviews?
Dr. Franko said asking for reviews is still not fully accepted. “There remains a spectrum of opinions and emotions regarding the appropriateness of ‘soliciting’ online reviews from patients,” he said.
Dr. Horton said review sites are also divided. “Google encourages businesses to remind customers to leave reviews, but Yelp discourages it,” she said. “It wants reviews to be organic and spontaneous.”
“I don’t think this is a problem,” said E. Scot Davis, a practice management consultant in Little Rock, Arkansas, and a board member of the Large Urology Group Practice Association. “Not enough people leave positive reviews, so it’s a way of balancing out the impact of a few people who make negative reviews.”
Indeed, other businesses routinely ask for online reviews and customers are often willing to oblige. Brightlocal reported that in 2022, 80% of consumers said they were prompted by local businesses to leave a review and 65% did so.
Some physicians may wonder whether it’s ethical to limit requests for reviews to patients who had positive experiences. Some vendors first ask patients about their experiences and then invite only those with positive ones to post.
Dr. Kalidindi said Simple Interact asks patients about their experiences as a way to help practices improve their services. He said patients’ experiences aren’t normally used to cull out dissatisfied patients unless the customer asks for it.
Loyal Health’s tool does not ask patients about their experiences, according to Loyal Health President Brian Gresh. He told this news organization he is opposed to culling negative reviewers and said it’s against Google policy.
Mr. Coppola at AMGA Consulting also opposes the practice. “It’s misleading not to ask people who had a bad experience,” he said. “Besides, if you only have glowing reviews, consumers would be suspicious.”
Meanwhile, everyone agrees that practices shouldn’t pay for online reviews. Dr. Horton said she believes this would be considered unprofessional conduct by the Medical Board of California.
Conclusion
Automated systems have helped practices attain more and better online reviews, boosting their revenue. Although some frown on the idea of prompting patients to leave reviews, others say it is necessary because some negative online reviews can be unfair and harm practices.
A version of this article appeared on Medscape.com.
Physicians’ negative online reviews — fair or unfair — can scare away new patients. But practices don’t have to sit idly by and watch their revenue shrink.
Increasingly, they’re turning to
Not all of these systems are effective, according to physicians who’ve used them. Asking patients for reviews is still not fully accepted, either. Still, some apps have proved their worth, doctors say.
Karen Horton, MD, a plastic surgeon in San Francisco, California, has used an automated system for 3 years. Even though reviews from plastic surgery patients can be difficult to get, Dr. Horton said, she has accumulated 535, with an average rating of just under 5 stars on a 1- to 5-star scale.
Dr. Horton, who speaks on the topic, said unfair negative reviews are a problem that needs addressing.
“A bad review sometimes says more about the patient than the provider,” she said. “Patients can use online reviews to vent about some perceived misgiving.”
Automated requests can address this problem. “The best way to deal with negative reviews is to ask average patients to post reviews,” she said. “These patients are more likely to be positive, but they wouldn’t leave a review unless asked.”
How Automated Systems Work
A variety of vendors provide an automated review request process to practices and hospitals. DearDoc, Loyal Health, Rater8, and Simple Interact work with healthcare providers, while Birdeye, Reputation, and Thrive Management work with all businesses.
Typically, these vendors access the practice’s electronic health record to get patients’ contact information and the daily appointment schedule to know which patients to contact. Patients are contacted after their appointment and are given the opportunity to go directly to a review site and post.
Inviting patients digitally rather than in person may seem unwelcoming, but many people prefer it, said Fred Horton, president of AMGA consulting in Alexandria, Virginia, a subsidiary of the American Medical Group Association. (He is not related to Karen Horton.)
“People tend to be more honest and detailed when responding to an automated message than to a person,” Mr. Horton told this news organization. “And younger patients actually prefer digital communications.”
But Mike Coppola, vice president of AMGA consulting, isn’t keen about automation.
He said practices can instead assign staff to ask patients to post reviews or an office can use signage displaying a Quick Response (QR) code, a two-dimensional matrix often used in restaurants to access a menu. Patients who put their smartphone cameras over the code are taken directly to a review site.
Still, staff would still need to help each patient access the site to be as effective as automation, and a QR invitation may be ignored. Pat Pazmino, MD, a plastic surgeon in Miami, Florida, told this news organization his office displays QR codes for reviews, but “I’m not sure many patients really use them.”
Some automated systems can go too far. Dr. Pazmino said a vendor he hired several years ago contacted “every patient who had ever called my office. A lot of them were annoyed.”
He said the service generated only 20 or 30 reviews, and some were negative. He did not like that he was soliciting patients to make negative reviews. He canceled the service.
What Is the Cost and Return on Investment?
“Our system makes it as easy as possible for patients to place reviews,” said Ravi Kalidindi, CEO of Simple Interact, a Dallas-based vendor that markets to doctors.
Dr. Kalidindi said Simple Interact charges $95-$145 per provider per month, depending on how the tool is used. For each dollar in cost, the practice typically earns $10 in extra revenue, he said.
Orrin Franko, MD, a hand surgeon in San Leandro, California, started using an automated patient review tool several years ago. He said that after installation received 10 reviews per month, all 5-star. “Now we have well over 700 reviews that generate close to $500,000 a year for our three-doctor practice,” he said.
Karen Horton reports more modest results. One new review comes in every 3-4 weeks. “Getting online reviews is a challenge for plastic surgeons,” he said. “Most patients are very private about having work done.”
Dr. Kalidindi reported that very few patients respond to Simple Interact’s invitation, but the numbers add up. “Typically, 3 of 100 patients contacted will ultimately post a positive review,” he said. “That means that a practice that sees 600 patients a month could get 18 positive reviews a month.”
Practices can also build their own systems and avoid vendors’ monthly fees. Dr. Franko built his own system, while Dr. Horton contracted with SILVR Agency, a digital marketing company in Solana Beach, California, to build hers for a one-time cost of about $3000.
Why Should Doctors Care About Online Reviews?
Online review sites for doctors include HealthGrades, RateMDs, Realself, Vitals, WebMD, and Zocdoc. (Medscape Medical News is part of WebMD.) Potential patients also consult general review sites like Facebook, Google My Business, and Yelp.
Consumers tend to prefer doctors who have many reviews, but most doctors get very few. One survey found that the average doctor has only seven online reviews, while competitors may have hundreds.
Having too few reviews also means that just one or two negative reviews can produce a poor average rating. It’s virtually impossible to remove negative reviews, and they can have a big impact. A 1-star rating reduces consumers’ clicks by 11%, according to Brightlocal, a company that surveys consumers’ use of online ratings.
Online reviews also influence Google searches, even when consumers never access a review site, said Lee Rensch, product director at Loyal Health, an Atlanta, Georgia–based vendor that works exclusively with hospitals.
By far the most common way to find a doctor is to use Google to search for doctors “near me,” Mr. Rensch told this news organization. The Google search brings up a ranked list of doctors, based partly on each doctor’s ratings on review sites.
Mr. Rensch said 15%-20% of Google’s ranking involves the number of reviews the doctor has, the average star rating, and the newness of the reviews. Other factors include whether the provider has responded to reviews and the description of the practice, he said.
How many people use the internet to find doctors? One survey found that 72% of healthcare consumers do so. Furthermore, healthcare ranks second in the most common use of reviews, after service businesses and before restaurants, according to a Brightlocal survey.
Is it OK to Ask for Reviews?
Dr. Franko said asking for reviews is still not fully accepted. “There remains a spectrum of opinions and emotions regarding the appropriateness of ‘soliciting’ online reviews from patients,” he said.
Dr. Horton said review sites are also divided. “Google encourages businesses to remind customers to leave reviews, but Yelp discourages it,” she said. “It wants reviews to be organic and spontaneous.”
“I don’t think this is a problem,” said E. Scot Davis, a practice management consultant in Little Rock, Arkansas, and a board member of the Large Urology Group Practice Association. “Not enough people leave positive reviews, so it’s a way of balancing out the impact of a few people who make negative reviews.”
Indeed, other businesses routinely ask for online reviews and customers are often willing to oblige. Brightlocal reported that in 2022, 80% of consumers said they were prompted by local businesses to leave a review and 65% did so.
Some physicians may wonder whether it’s ethical to limit requests for reviews to patients who had positive experiences. Some vendors first ask patients about their experiences and then invite only those with positive ones to post.
Dr. Kalidindi said Simple Interact asks patients about their experiences as a way to help practices improve their services. He said patients’ experiences aren’t normally used to cull out dissatisfied patients unless the customer asks for it.
Loyal Health’s tool does not ask patients about their experiences, according to Loyal Health President Brian Gresh. He told this news organization he is opposed to culling negative reviewers and said it’s against Google policy.
Mr. Coppola at AMGA Consulting also opposes the practice. “It’s misleading not to ask people who had a bad experience,” he said. “Besides, if you only have glowing reviews, consumers would be suspicious.”
Meanwhile, everyone agrees that practices shouldn’t pay for online reviews. Dr. Horton said she believes this would be considered unprofessional conduct by the Medical Board of California.
Conclusion
Automated systems have helped practices attain more and better online reviews, boosting their revenue. Although some frown on the idea of prompting patients to leave reviews, others say it is necessary because some negative online reviews can be unfair and harm practices.
A version of this article appeared on Medscape.com.
Physicians’ negative online reviews — fair or unfair — can scare away new patients. But practices don’t have to sit idly by and watch their revenue shrink.
Increasingly, they’re turning to
Not all of these systems are effective, according to physicians who’ve used them. Asking patients for reviews is still not fully accepted, either. Still, some apps have proved their worth, doctors say.
Karen Horton, MD, a plastic surgeon in San Francisco, California, has used an automated system for 3 years. Even though reviews from plastic surgery patients can be difficult to get, Dr. Horton said, she has accumulated 535, with an average rating of just under 5 stars on a 1- to 5-star scale.
Dr. Horton, who speaks on the topic, said unfair negative reviews are a problem that needs addressing.
“A bad review sometimes says more about the patient than the provider,” she said. “Patients can use online reviews to vent about some perceived misgiving.”
Automated requests can address this problem. “The best way to deal with negative reviews is to ask average patients to post reviews,” she said. “These patients are more likely to be positive, but they wouldn’t leave a review unless asked.”
How Automated Systems Work
A variety of vendors provide an automated review request process to practices and hospitals. DearDoc, Loyal Health, Rater8, and Simple Interact work with healthcare providers, while Birdeye, Reputation, and Thrive Management work with all businesses.
Typically, these vendors access the practice’s electronic health record to get patients’ contact information and the daily appointment schedule to know which patients to contact. Patients are contacted after their appointment and are given the opportunity to go directly to a review site and post.
Inviting patients digitally rather than in person may seem unwelcoming, but many people prefer it, said Fred Horton, president of AMGA consulting in Alexandria, Virginia, a subsidiary of the American Medical Group Association. (He is not related to Karen Horton.)
“People tend to be more honest and detailed when responding to an automated message than to a person,” Mr. Horton told this news organization. “And younger patients actually prefer digital communications.”
But Mike Coppola, vice president of AMGA consulting, isn’t keen about automation.
He said practices can instead assign staff to ask patients to post reviews or an office can use signage displaying a Quick Response (QR) code, a two-dimensional matrix often used in restaurants to access a menu. Patients who put their smartphone cameras over the code are taken directly to a review site.
Still, staff would still need to help each patient access the site to be as effective as automation, and a QR invitation may be ignored. Pat Pazmino, MD, a plastic surgeon in Miami, Florida, told this news organization his office displays QR codes for reviews, but “I’m not sure many patients really use them.”
Some automated systems can go too far. Dr. Pazmino said a vendor he hired several years ago contacted “every patient who had ever called my office. A lot of them were annoyed.”
He said the service generated only 20 or 30 reviews, and some were negative. He did not like that he was soliciting patients to make negative reviews. He canceled the service.
What Is the Cost and Return on Investment?
“Our system makes it as easy as possible for patients to place reviews,” said Ravi Kalidindi, CEO of Simple Interact, a Dallas-based vendor that markets to doctors.
Dr. Kalidindi said Simple Interact charges $95-$145 per provider per month, depending on how the tool is used. For each dollar in cost, the practice typically earns $10 in extra revenue, he said.
Orrin Franko, MD, a hand surgeon in San Leandro, California, started using an automated patient review tool several years ago. He said that after installation received 10 reviews per month, all 5-star. “Now we have well over 700 reviews that generate close to $500,000 a year for our three-doctor practice,” he said.
Karen Horton reports more modest results. One new review comes in every 3-4 weeks. “Getting online reviews is a challenge for plastic surgeons,” he said. “Most patients are very private about having work done.”
Dr. Kalidindi reported that very few patients respond to Simple Interact’s invitation, but the numbers add up. “Typically, 3 of 100 patients contacted will ultimately post a positive review,” he said. “That means that a practice that sees 600 patients a month could get 18 positive reviews a month.”
Practices can also build their own systems and avoid vendors’ monthly fees. Dr. Franko built his own system, while Dr. Horton contracted with SILVR Agency, a digital marketing company in Solana Beach, California, to build hers for a one-time cost of about $3000.
Why Should Doctors Care About Online Reviews?
Online review sites for doctors include HealthGrades, RateMDs, Realself, Vitals, WebMD, and Zocdoc. (Medscape Medical News is part of WebMD.) Potential patients also consult general review sites like Facebook, Google My Business, and Yelp.
Consumers tend to prefer doctors who have many reviews, but most doctors get very few. One survey found that the average doctor has only seven online reviews, while competitors may have hundreds.
Having too few reviews also means that just one or two negative reviews can produce a poor average rating. It’s virtually impossible to remove negative reviews, and they can have a big impact. A 1-star rating reduces consumers’ clicks by 11%, according to Brightlocal, a company that surveys consumers’ use of online ratings.
Online reviews also influence Google searches, even when consumers never access a review site, said Lee Rensch, product director at Loyal Health, an Atlanta, Georgia–based vendor that works exclusively with hospitals.
By far the most common way to find a doctor is to use Google to search for doctors “near me,” Mr. Rensch told this news organization. The Google search brings up a ranked list of doctors, based partly on each doctor’s ratings on review sites.
Mr. Rensch said 15%-20% of Google’s ranking involves the number of reviews the doctor has, the average star rating, and the newness of the reviews. Other factors include whether the provider has responded to reviews and the description of the practice, he said.
How many people use the internet to find doctors? One survey found that 72% of healthcare consumers do so. Furthermore, healthcare ranks second in the most common use of reviews, after service businesses and before restaurants, according to a Brightlocal survey.
Is it OK to Ask for Reviews?
Dr. Franko said asking for reviews is still not fully accepted. “There remains a spectrum of opinions and emotions regarding the appropriateness of ‘soliciting’ online reviews from patients,” he said.
Dr. Horton said review sites are also divided. “Google encourages businesses to remind customers to leave reviews, but Yelp discourages it,” she said. “It wants reviews to be organic and spontaneous.”
“I don’t think this is a problem,” said E. Scot Davis, a practice management consultant in Little Rock, Arkansas, and a board member of the Large Urology Group Practice Association. “Not enough people leave positive reviews, so it’s a way of balancing out the impact of a few people who make negative reviews.”
Indeed, other businesses routinely ask for online reviews and customers are often willing to oblige. Brightlocal reported that in 2022, 80% of consumers said they were prompted by local businesses to leave a review and 65% did so.
Some physicians may wonder whether it’s ethical to limit requests for reviews to patients who had positive experiences. Some vendors first ask patients about their experiences and then invite only those with positive ones to post.
Dr. Kalidindi said Simple Interact asks patients about their experiences as a way to help practices improve their services. He said patients’ experiences aren’t normally used to cull out dissatisfied patients unless the customer asks for it.
Loyal Health’s tool does not ask patients about their experiences, according to Loyal Health President Brian Gresh. He told this news organization he is opposed to culling negative reviewers and said it’s against Google policy.
Mr. Coppola at AMGA Consulting also opposes the practice. “It’s misleading not to ask people who had a bad experience,” he said. “Besides, if you only have glowing reviews, consumers would be suspicious.”
Meanwhile, everyone agrees that practices shouldn’t pay for online reviews. Dr. Horton said she believes this would be considered unprofessional conduct by the Medical Board of California.
Conclusion
Automated systems have helped practices attain more and better online reviews, boosting their revenue. Although some frown on the idea of prompting patients to leave reviews, others say it is necessary because some negative online reviews can be unfair and harm practices.
A version of this article appeared on Medscape.com.
FDA Withdraws Melflufen Approval, but EMA Still Allows Its Use
But the European Medicines Agency (EMA) still authorizes the drug’s manufacturer Oncopeptides AB to market the drug, also called Pepaxti, in Europe, Iceland, Lichtenstein, Norway, and the United Kingdom.
Amol Akhade, MBBS, who describes himself as a senior consultant medical and hemato oncologist–bone marrow transplant physician on LinkedIn, raised questions about the inconsistencies between the FDA and EMA’s opinions about these drugs. Dr. Akhad, of Suyog Cancer Clinics in India, posted via the following handle @SuyogCancer on X (Twitter):
“How can one drug and one trial data [have] two diagonally different outcomes from two different drug approval agencies?
Melphalan Flufenamide is finally completely withdrawn by @US_FDA
But approval by @EMA_News stays.
How can be one drug be harmful across one side of Atlantic Ocean and becomes safe and useful on the other side of Atlantic Ocean?
Modern day miracle?”
EMA: Pepaxti’s Benefits Exceed Its Risks
The EMA, which could not be reached for comment regarding why the agency was still allowing patients to use the drug, said the following about Pepaxti on its website:
“The European Medicines Agency decided that Pepaxti’s benefits are greater than its risks and it can be authorised for use in the EU. The Agency noted the unmet medical need for patients with multiple myeloma who no longer improve with the available therapies. Despite some limitations in the studies, the results were considered clinically relevant, with the exception of the subgroup of patients who had an autologous stem cell transplant and whose disease progressed within three years of transplantation.
Regarding safety, although side effects, including severe effects, were seen with treatment involving Pepaxti, these were considered acceptable and manageable,” the agency wrote.
“Recommendations and precautions to be followed by healthcare professionals and patients for the safe and effective use of Pepaxti have been included in the summary of product characteristics and the package leaflet.
As for all medicines, data on the use of Pepaxti are continuously monitored. Suspected side effects reported with Pepaxti are carefully evaluated and any necessary action taken to protect patients,” according to the EMA.
The FDA’s final decision, issued on February 23, 2024, follows its warning in 2021 that meflufen plus dexamethasone exposed patients with multiple myeloma to increased risk for death, and its call for withdrawal of the drug in 2022.
“The grounds for withdrawing approval have been met because: (1) the confirmatory study conducted as a condition of accelerated approval did not confirm Pepaxto’s clinical benefit and (2) the available evidence demonstrates that Pepaxto is not shown to be safe or effective under its conditions of use,” Peter Marks, MD, PhD, Director of the FDA Center for Biologics Evaluation and Research, wrote in the final decision document.
Oncopeptides AB: Drug ‘Caters to a Large Unmet Need’
David Augustsson, Director of Corporate Affairs, Oncopeptides AB, explained in an interview why he thinks the EMA and FDA’s actions regarding the drug differ from each other.
“The European Medicines Agency had the opinion that the OCEAN study met its primary endpoint by demonstrating superior progression-free survival and it agreed that the potential detriment of overall survival was limited to patients progressing less than 36 months after an autologous stem cell transplant,” he said.“The FDA was not willing to acknowledge the observed clinically relevant differences across patient subgroups in the OCEAN study as confirmed.”
Mr. Augustsson added that this decision will deprive US patients of access to “a drug we believe caters to a large unmet need among elderly multiple myeloma patients with few treatment options left.”
“While we remain confident that we have science on our side we are of course disappointed in the decision [to remove Pepaxto from the US market],” Oncopeptides AB CEO Sofia Heigis said in a statement. “At the same time this is no change to our plans and we will continue to focus all our attention on the commercialization in Europe, progression of our pipeline and rest of world opportunities.”
FDA 'Took Swift Action' to Ensure Users of Pepaxto Were Informed of Risks
In February 2021, the FDA used the Accelerated Approval Program to enable certain patients with multiple myeloma to be treated with the peptide conjugated alkylating drug melflufen plus dexamethasone. Under the program, Oncopeptides was required to conduct the phase III randomized, controlled OCEAN clinical trial.
OCEAN enrolled 495 patients with relapsed/refractory multiple myeloma who had 2 to 4 lines of prior therapy and who were refractory to lenalidomide in the last line of therapy. Participants in the multinational study received either melflufen plus dexamethasone or pomalidomide plus dexamethasone until disease progression, unacceptable toxicity, or lack of benefit.
In July 2021, the FDA issued an alert that the study results showed increased risk for death in participants treated with melflufen. In October that year, at FDA request, Oncopeptides removed the drug from the US market but continued to provide it to patients already receiving it. In December 2022, the FDA requested that the company withdraw melflufen’s US marketing authorization.
Responding to questions about the timing of the FDA’s most recent decision about Pepaxto and how the decision will affect patient care in the US, the FDA emailed the following statement to this news organization:
“Since the OCEAN trial results for Pepaxto in 2021, the FDA has responded to safety concerns about Pepaxto by issuing a CDER Alert, communicating concerns to Oncopeptides, holding an Oncologic Drugs Advisory Committee meeting in September 2022, and issuing a letter of notice to Oncopeptides in July 2023, proposing to withdraw Pepaxto (NDA 214383). After receiving the notice, Oncopeptides appealed the withdrawal in August 2023. A meeting was held with the Commissioner’s designee, Dr. Peter Marks, Oncopeptides, and others from FDA in October 2023. Dr. Marks reviewed the record and considered the arguments made on appeal and issued a final decision on February 23, 2024. Prior to reaching a decision, the FDA took swift action to ensure those receiving Pepaxto in the post-confirmatory clinical trial were informed of the risks and that no new patients were enrolled in the trial. We also note that it is our understanding that Pepaxto has not been marketed in the U.S. since October 22, 2021.”
“This is the first time FDA has used the amended procedures for withdrawal of accelerated approval that were enacted in 2023, as part of the Food and Drug Omnibus Report Act of 2022 (FDORA),” the agency wrote in a Feb 23 statement. The agency will also remove melflufen from the Approved Drug Products with Therapeutic Equivalence Evaluations, also called the Orange Book.
But the European Medicines Agency (EMA) still authorizes the drug’s manufacturer Oncopeptides AB to market the drug, also called Pepaxti, in Europe, Iceland, Lichtenstein, Norway, and the United Kingdom.
Amol Akhade, MBBS, who describes himself as a senior consultant medical and hemato oncologist–bone marrow transplant physician on LinkedIn, raised questions about the inconsistencies between the FDA and EMA’s opinions about these drugs. Dr. Akhad, of Suyog Cancer Clinics in India, posted via the following handle @SuyogCancer on X (Twitter):
“How can one drug and one trial data [have] two diagonally different outcomes from two different drug approval agencies?
Melphalan Flufenamide is finally completely withdrawn by @US_FDA
But approval by @EMA_News stays.
How can be one drug be harmful across one side of Atlantic Ocean and becomes safe and useful on the other side of Atlantic Ocean?
Modern day miracle?”
EMA: Pepaxti’s Benefits Exceed Its Risks
The EMA, which could not be reached for comment regarding why the agency was still allowing patients to use the drug, said the following about Pepaxti on its website:
“The European Medicines Agency decided that Pepaxti’s benefits are greater than its risks and it can be authorised for use in the EU. The Agency noted the unmet medical need for patients with multiple myeloma who no longer improve with the available therapies. Despite some limitations in the studies, the results were considered clinically relevant, with the exception of the subgroup of patients who had an autologous stem cell transplant and whose disease progressed within three years of transplantation.
Regarding safety, although side effects, including severe effects, were seen with treatment involving Pepaxti, these were considered acceptable and manageable,” the agency wrote.
“Recommendations and precautions to be followed by healthcare professionals and patients for the safe and effective use of Pepaxti have been included in the summary of product characteristics and the package leaflet.
As for all medicines, data on the use of Pepaxti are continuously monitored. Suspected side effects reported with Pepaxti are carefully evaluated and any necessary action taken to protect patients,” according to the EMA.
The FDA’s final decision, issued on February 23, 2024, follows its warning in 2021 that meflufen plus dexamethasone exposed patients with multiple myeloma to increased risk for death, and its call for withdrawal of the drug in 2022.
“The grounds for withdrawing approval have been met because: (1) the confirmatory study conducted as a condition of accelerated approval did not confirm Pepaxto’s clinical benefit and (2) the available evidence demonstrates that Pepaxto is not shown to be safe or effective under its conditions of use,” Peter Marks, MD, PhD, Director of the FDA Center for Biologics Evaluation and Research, wrote in the final decision document.
Oncopeptides AB: Drug ‘Caters to a Large Unmet Need’
David Augustsson, Director of Corporate Affairs, Oncopeptides AB, explained in an interview why he thinks the EMA and FDA’s actions regarding the drug differ from each other.
“The European Medicines Agency had the opinion that the OCEAN study met its primary endpoint by demonstrating superior progression-free survival and it agreed that the potential detriment of overall survival was limited to patients progressing less than 36 months after an autologous stem cell transplant,” he said.“The FDA was not willing to acknowledge the observed clinically relevant differences across patient subgroups in the OCEAN study as confirmed.”
Mr. Augustsson added that this decision will deprive US patients of access to “a drug we believe caters to a large unmet need among elderly multiple myeloma patients with few treatment options left.”
“While we remain confident that we have science on our side we are of course disappointed in the decision [to remove Pepaxto from the US market],” Oncopeptides AB CEO Sofia Heigis said in a statement. “At the same time this is no change to our plans and we will continue to focus all our attention on the commercialization in Europe, progression of our pipeline and rest of world opportunities.”
FDA 'Took Swift Action' to Ensure Users of Pepaxto Were Informed of Risks
In February 2021, the FDA used the Accelerated Approval Program to enable certain patients with multiple myeloma to be treated with the peptide conjugated alkylating drug melflufen plus dexamethasone. Under the program, Oncopeptides was required to conduct the phase III randomized, controlled OCEAN clinical trial.
OCEAN enrolled 495 patients with relapsed/refractory multiple myeloma who had 2 to 4 lines of prior therapy and who were refractory to lenalidomide in the last line of therapy. Participants in the multinational study received either melflufen plus dexamethasone or pomalidomide plus dexamethasone until disease progression, unacceptable toxicity, or lack of benefit.
In July 2021, the FDA issued an alert that the study results showed increased risk for death in participants treated with melflufen. In October that year, at FDA request, Oncopeptides removed the drug from the US market but continued to provide it to patients already receiving it. In December 2022, the FDA requested that the company withdraw melflufen’s US marketing authorization.
Responding to questions about the timing of the FDA’s most recent decision about Pepaxto and how the decision will affect patient care in the US, the FDA emailed the following statement to this news organization:
“Since the OCEAN trial results for Pepaxto in 2021, the FDA has responded to safety concerns about Pepaxto by issuing a CDER Alert, communicating concerns to Oncopeptides, holding an Oncologic Drugs Advisory Committee meeting in September 2022, and issuing a letter of notice to Oncopeptides in July 2023, proposing to withdraw Pepaxto (NDA 214383). After receiving the notice, Oncopeptides appealed the withdrawal in August 2023. A meeting was held with the Commissioner’s designee, Dr. Peter Marks, Oncopeptides, and others from FDA in October 2023. Dr. Marks reviewed the record and considered the arguments made on appeal and issued a final decision on February 23, 2024. Prior to reaching a decision, the FDA took swift action to ensure those receiving Pepaxto in the post-confirmatory clinical trial were informed of the risks and that no new patients were enrolled in the trial. We also note that it is our understanding that Pepaxto has not been marketed in the U.S. since October 22, 2021.”
“This is the first time FDA has used the amended procedures for withdrawal of accelerated approval that were enacted in 2023, as part of the Food and Drug Omnibus Report Act of 2022 (FDORA),” the agency wrote in a Feb 23 statement. The agency will also remove melflufen from the Approved Drug Products with Therapeutic Equivalence Evaluations, also called the Orange Book.
But the European Medicines Agency (EMA) still authorizes the drug’s manufacturer Oncopeptides AB to market the drug, also called Pepaxti, in Europe, Iceland, Lichtenstein, Norway, and the United Kingdom.
Amol Akhade, MBBS, who describes himself as a senior consultant medical and hemato oncologist–bone marrow transplant physician on LinkedIn, raised questions about the inconsistencies between the FDA and EMA’s opinions about these drugs. Dr. Akhad, of Suyog Cancer Clinics in India, posted via the following handle @SuyogCancer on X (Twitter):
“How can one drug and one trial data [have] two diagonally different outcomes from two different drug approval agencies?
Melphalan Flufenamide is finally completely withdrawn by @US_FDA
But approval by @EMA_News stays.
How can be one drug be harmful across one side of Atlantic Ocean and becomes safe and useful on the other side of Atlantic Ocean?
Modern day miracle?”
EMA: Pepaxti’s Benefits Exceed Its Risks
The EMA, which could not be reached for comment regarding why the agency was still allowing patients to use the drug, said the following about Pepaxti on its website:
“The European Medicines Agency decided that Pepaxti’s benefits are greater than its risks and it can be authorised for use in the EU. The Agency noted the unmet medical need for patients with multiple myeloma who no longer improve with the available therapies. Despite some limitations in the studies, the results were considered clinically relevant, with the exception of the subgroup of patients who had an autologous stem cell transplant and whose disease progressed within three years of transplantation.
Regarding safety, although side effects, including severe effects, were seen with treatment involving Pepaxti, these were considered acceptable and manageable,” the agency wrote.
“Recommendations and precautions to be followed by healthcare professionals and patients for the safe and effective use of Pepaxti have been included in the summary of product characteristics and the package leaflet.
As for all medicines, data on the use of Pepaxti are continuously monitored. Suspected side effects reported with Pepaxti are carefully evaluated and any necessary action taken to protect patients,” according to the EMA.
The FDA’s final decision, issued on February 23, 2024, follows its warning in 2021 that meflufen plus dexamethasone exposed patients with multiple myeloma to increased risk for death, and its call for withdrawal of the drug in 2022.
“The grounds for withdrawing approval have been met because: (1) the confirmatory study conducted as a condition of accelerated approval did not confirm Pepaxto’s clinical benefit and (2) the available evidence demonstrates that Pepaxto is not shown to be safe or effective under its conditions of use,” Peter Marks, MD, PhD, Director of the FDA Center for Biologics Evaluation and Research, wrote in the final decision document.
Oncopeptides AB: Drug ‘Caters to a Large Unmet Need’
David Augustsson, Director of Corporate Affairs, Oncopeptides AB, explained in an interview why he thinks the EMA and FDA’s actions regarding the drug differ from each other.
“The European Medicines Agency had the opinion that the OCEAN study met its primary endpoint by demonstrating superior progression-free survival and it agreed that the potential detriment of overall survival was limited to patients progressing less than 36 months after an autologous stem cell transplant,” he said.“The FDA was not willing to acknowledge the observed clinically relevant differences across patient subgroups in the OCEAN study as confirmed.”
Mr. Augustsson added that this decision will deprive US patients of access to “a drug we believe caters to a large unmet need among elderly multiple myeloma patients with few treatment options left.”
“While we remain confident that we have science on our side we are of course disappointed in the decision [to remove Pepaxto from the US market],” Oncopeptides AB CEO Sofia Heigis said in a statement. “At the same time this is no change to our plans and we will continue to focus all our attention on the commercialization in Europe, progression of our pipeline and rest of world opportunities.”
FDA 'Took Swift Action' to Ensure Users of Pepaxto Were Informed of Risks
In February 2021, the FDA used the Accelerated Approval Program to enable certain patients with multiple myeloma to be treated with the peptide conjugated alkylating drug melflufen plus dexamethasone. Under the program, Oncopeptides was required to conduct the phase III randomized, controlled OCEAN clinical trial.
OCEAN enrolled 495 patients with relapsed/refractory multiple myeloma who had 2 to 4 lines of prior therapy and who were refractory to lenalidomide in the last line of therapy. Participants in the multinational study received either melflufen plus dexamethasone or pomalidomide plus dexamethasone until disease progression, unacceptable toxicity, or lack of benefit.
In July 2021, the FDA issued an alert that the study results showed increased risk for death in participants treated with melflufen. In October that year, at FDA request, Oncopeptides removed the drug from the US market but continued to provide it to patients already receiving it. In December 2022, the FDA requested that the company withdraw melflufen’s US marketing authorization.
Responding to questions about the timing of the FDA’s most recent decision about Pepaxto and how the decision will affect patient care in the US, the FDA emailed the following statement to this news organization:
“Since the OCEAN trial results for Pepaxto in 2021, the FDA has responded to safety concerns about Pepaxto by issuing a CDER Alert, communicating concerns to Oncopeptides, holding an Oncologic Drugs Advisory Committee meeting in September 2022, and issuing a letter of notice to Oncopeptides in July 2023, proposing to withdraw Pepaxto (NDA 214383). After receiving the notice, Oncopeptides appealed the withdrawal in August 2023. A meeting was held with the Commissioner’s designee, Dr. Peter Marks, Oncopeptides, and others from FDA in October 2023. Dr. Marks reviewed the record and considered the arguments made on appeal and issued a final decision on February 23, 2024. Prior to reaching a decision, the FDA took swift action to ensure those receiving Pepaxto in the post-confirmatory clinical trial were informed of the risks and that no new patients were enrolled in the trial. We also note that it is our understanding that Pepaxto has not been marketed in the U.S. since October 22, 2021.”
“This is the first time FDA has used the amended procedures for withdrawal of accelerated approval that were enacted in 2023, as part of the Food and Drug Omnibus Report Act of 2022 (FDORA),” the agency wrote in a Feb 23 statement. The agency will also remove melflufen from the Approved Drug Products with Therapeutic Equivalence Evaluations, also called the Orange Book.
Judge Won’t Overturn Invalidated USMLE Scores
(USMLE).
In a February 23 order, Judge Christopher R. Cooper, of the US District Court for the District of Columbia, denied Latika Giri’s emergency motion to block the National Board of Medical Examiners (NBME) from invalidating the scores, ruling the public interest plainly weighs against granting the request.
“First and foremost, is the overriding interest in public safety,” Cooper wrote in his 32-page order. “This is a case about the credentials of doctors applying to medical residency programs…Granting the preliminary injunction would create an unacceptable risk that individuals who lack the requisite knowledge and skills they purport to possess because they achieved their exam scores fraudulently will be administering medical care to unsuspecting patients across the nation.”
Attorneys for Giri did not return messages seeking comment about the order.
The NBME also did not return messages seeking comment. The board previously said it does not comment on pending litigation.
The decision is the latest development in a widespread cheating scandal. Giri, an international medical graduate (IMG) from Kathmandu, sued NBME earlier this month claiming the board discriminated against Nepali medical graduates when it invalidated hundreds of exam scores linked to the country.
Giri also accused NBME of violating its own procedures when it voided the scores before giving examinees a chance to argue and appeal. She asked the district court to block NBME from invalidating her exam scores while the lawsuit continues and restore her original results.
In court documents, NBME argued that it did not invalidate the scores because the examinees were Nepali but because staff concluded that there was “a good faith basis for questioning the validity of the scores.”
The invalidations were based on concerns that the results reflected prior access to secure exam content rather than knowledge and understanding of the medical principles and skills the exams are intended to assess, according to the NBME’s legal response.
“The USMLE program took reasonable and appropriate actions to prevent the significant harm and disruption that would result from allowing potentially unqualified individuals to participate in the 2024 residency Match,” the NBME stated in court documents. “If granted, the requested injunction would cause enormous harm not only to NBME… but also to state licensing authorities, which rely upon USMLE results to help ensure that physicians have the minimum competencies needed to provide safe and effective health care.”
In his order, Cooper wrote that Giri has not proven the board’s actions were discriminatory against Nepali doctors.
“Nothing in the present record suggests that NBME went looking for a problem in Nepal out of ethnicity-or national-origin based [sic] suspicion,” Cooper wrote. “[It] followed the trail of evidence, including tips about organized cheating taking place in medical schools and at a testing center located in Nepal, and on an online forum for which a ‘nexus to Nepal’ was a ticket to admission.”
NBME: Nepal Outperformed All Other Countries on USMLE
Court documents shed more light on NBME’s investigation into the suspected cheating and on the anomalous patterns the board allegedly discovered from Nepal medical graduates.
In response to anonymous tips, the USMLE program in early 2023 asked the NBME Psychometrics and Data Analysis (PADA) unit to analyze examinee performance data for test centers in Jordan, Nepal, and Pakistan, according to court records. Within the initial data analysis, the data involving the single test center in Nepal was “the most extreme,” the unit found.
Out of more than 400 test centers across the world, including those in the United States, the test center in Nepal produced the highest test scores in the world for Step 1 in 2021 and 2022 and the highest test scores in the world for Step 2 CK in 2022, according to court documents. For the 2022 Step 1 exam for example, the average score of examinees testing in the Nepal test center was 240. No other test center in the world had an average examinee score above 227, according to the NBME’s legal response.
The median item response time for examinees who tested at the Nepal test center in 2022 was also among the fastest of all international test centers for Step 1 and Step 2 CK, investigators found.
In addition, the volume of examinees taking the USMLE Step 1 and Step 2 CK at the Nepal test center in Nepal had sharply increased. Step 1 volume more than doubled in the Nepal test center from 281 examinees in 2019 to 662 examinees in 2022, according to court documents.
The rapid increase continued in 2023, when examinee volume was nearly three-and-a-half times higher than the 2019 volume. The data were consistent with anonymous tips received by the USMLE program office, suggesting there may be wide-scale collection and sharing of live USMLE exam content within Nepal.
Investigation Finds Similar Correct and Incorrect Answers
Agreement similarity among the exams analyzed also raised red flags. Investigators ran an “agreement analysis” for all examinees who tested at centers in Jordan, Nepal, and Pakistan as well as two centers in India, according to court documents.
For the 2022 Step 1 exam and the 2021 and 2022 Step 2 CK exam, the analysis showed a substantially higher percentage of examinees with a statistically significant level of agreement matches in the examine group that tested at centers in Jordan, Nepal, Pakistan, and India compared with the baseline group, according to legal records.
The vast majority of examinees with a statistically significant number of matching incorrect answers tested at the Nepal test center, data showed.
Further analysis found that examinee volumes increased considerably at the Nepal test center in the months prior to the USMLE program releasing new test items, “suggesting that candidates who had prior access to disclosed exam questions wanted to test before new questions came into the item pool.”
Investigators also identified posts on social medial and in online chat rooms suggesting groups were collecting and sharing large amounts of secure exam material in private groups. Some posts advised examinees to use the full examination time when taking the USMLE “to avoid raising suspicion about having had prior access to secure exam materials,” according to court documents.
From its investigation and analysis, the USMLE program identified 832 examinees who had passing level exam results whose validity the USMLE program had a significant and good faith basis for questioning, according to court records.
Of the total, 618 examinees had one Step score flagged as being of questioned validity, 202 examinees had two Step exam scores flagged, and 12 examinees had scores flagged on all three Step exams.
NBME Defends Departure From Traditional Procedures
In court documents, NBME disputed claims that it violated its own procedures by invalidating the exam scores. Giri’s report contends that examinees suspected of cheating are typically first advised of the matter, given an opportunity to share relevant information, and provided the right to appeal — during which time, their scores are treated as valid.
But the NBME said the USMLE program is authorized to take any actions it deems appropriate in response to concerns regarding score validity if the USMLE Committee for Individualized Review or the USMLE Composite Committee concludes that alternative or supplemental procedures are warranted in response to a given set of facts or circumstances.
“Following the month-long investigation and analysis…the USMLE program concluded that alternative procedures were warranted to address the score invalidity concerns identified in the interest of providing a process that is timely, efficient, effective, and fair, and given the large number of examinees involved in the investigation,” the board stated in its legal response.
In his order, Cooper wrote the current scenario, which implicates that more than 800 test-takers, is “clearly a situation calling for a procedure geared toward efficiency.” No evidence shows the board would not have taken similarly swift action if confronted with evidence of cheating on a comparable scale elsewhere, he wrote.
The judge also denied Giri’s motion to certify the lawsuit as a class action. The motion was denied without prejudice, meaning the plaintiff has the option to renew the motion should the case proceed.
A version of this article appeared on Medscape.com.
(USMLE).
In a February 23 order, Judge Christopher R. Cooper, of the US District Court for the District of Columbia, denied Latika Giri’s emergency motion to block the National Board of Medical Examiners (NBME) from invalidating the scores, ruling the public interest plainly weighs against granting the request.
“First and foremost, is the overriding interest in public safety,” Cooper wrote in his 32-page order. “This is a case about the credentials of doctors applying to medical residency programs…Granting the preliminary injunction would create an unacceptable risk that individuals who lack the requisite knowledge and skills they purport to possess because they achieved their exam scores fraudulently will be administering medical care to unsuspecting patients across the nation.”
Attorneys for Giri did not return messages seeking comment about the order.
The NBME also did not return messages seeking comment. The board previously said it does not comment on pending litigation.
The decision is the latest development in a widespread cheating scandal. Giri, an international medical graduate (IMG) from Kathmandu, sued NBME earlier this month claiming the board discriminated against Nepali medical graduates when it invalidated hundreds of exam scores linked to the country.
Giri also accused NBME of violating its own procedures when it voided the scores before giving examinees a chance to argue and appeal. She asked the district court to block NBME from invalidating her exam scores while the lawsuit continues and restore her original results.
In court documents, NBME argued that it did not invalidate the scores because the examinees were Nepali but because staff concluded that there was “a good faith basis for questioning the validity of the scores.”
The invalidations were based on concerns that the results reflected prior access to secure exam content rather than knowledge and understanding of the medical principles and skills the exams are intended to assess, according to the NBME’s legal response.
“The USMLE program took reasonable and appropriate actions to prevent the significant harm and disruption that would result from allowing potentially unqualified individuals to participate in the 2024 residency Match,” the NBME stated in court documents. “If granted, the requested injunction would cause enormous harm not only to NBME… but also to state licensing authorities, which rely upon USMLE results to help ensure that physicians have the minimum competencies needed to provide safe and effective health care.”
In his order, Cooper wrote that Giri has not proven the board’s actions were discriminatory against Nepali doctors.
“Nothing in the present record suggests that NBME went looking for a problem in Nepal out of ethnicity-or national-origin based [sic] suspicion,” Cooper wrote. “[It] followed the trail of evidence, including tips about organized cheating taking place in medical schools and at a testing center located in Nepal, and on an online forum for which a ‘nexus to Nepal’ was a ticket to admission.”
NBME: Nepal Outperformed All Other Countries on USMLE
Court documents shed more light on NBME’s investigation into the suspected cheating and on the anomalous patterns the board allegedly discovered from Nepal medical graduates.
In response to anonymous tips, the USMLE program in early 2023 asked the NBME Psychometrics and Data Analysis (PADA) unit to analyze examinee performance data for test centers in Jordan, Nepal, and Pakistan, according to court records. Within the initial data analysis, the data involving the single test center in Nepal was “the most extreme,” the unit found.
Out of more than 400 test centers across the world, including those in the United States, the test center in Nepal produced the highest test scores in the world for Step 1 in 2021 and 2022 and the highest test scores in the world for Step 2 CK in 2022, according to court documents. For the 2022 Step 1 exam for example, the average score of examinees testing in the Nepal test center was 240. No other test center in the world had an average examinee score above 227, according to the NBME’s legal response.
The median item response time for examinees who tested at the Nepal test center in 2022 was also among the fastest of all international test centers for Step 1 and Step 2 CK, investigators found.
In addition, the volume of examinees taking the USMLE Step 1 and Step 2 CK at the Nepal test center in Nepal had sharply increased. Step 1 volume more than doubled in the Nepal test center from 281 examinees in 2019 to 662 examinees in 2022, according to court documents.
The rapid increase continued in 2023, when examinee volume was nearly three-and-a-half times higher than the 2019 volume. The data were consistent with anonymous tips received by the USMLE program office, suggesting there may be wide-scale collection and sharing of live USMLE exam content within Nepal.
Investigation Finds Similar Correct and Incorrect Answers
Agreement similarity among the exams analyzed also raised red flags. Investigators ran an “agreement analysis” for all examinees who tested at centers in Jordan, Nepal, and Pakistan as well as two centers in India, according to court documents.
For the 2022 Step 1 exam and the 2021 and 2022 Step 2 CK exam, the analysis showed a substantially higher percentage of examinees with a statistically significant level of agreement matches in the examine group that tested at centers in Jordan, Nepal, Pakistan, and India compared with the baseline group, according to legal records.
The vast majority of examinees with a statistically significant number of matching incorrect answers tested at the Nepal test center, data showed.
Further analysis found that examinee volumes increased considerably at the Nepal test center in the months prior to the USMLE program releasing new test items, “suggesting that candidates who had prior access to disclosed exam questions wanted to test before new questions came into the item pool.”
Investigators also identified posts on social medial and in online chat rooms suggesting groups were collecting and sharing large amounts of secure exam material in private groups. Some posts advised examinees to use the full examination time when taking the USMLE “to avoid raising suspicion about having had prior access to secure exam materials,” according to court documents.
From its investigation and analysis, the USMLE program identified 832 examinees who had passing level exam results whose validity the USMLE program had a significant and good faith basis for questioning, according to court records.
Of the total, 618 examinees had one Step score flagged as being of questioned validity, 202 examinees had two Step exam scores flagged, and 12 examinees had scores flagged on all three Step exams.
NBME Defends Departure From Traditional Procedures
In court documents, NBME disputed claims that it violated its own procedures by invalidating the exam scores. Giri’s report contends that examinees suspected of cheating are typically first advised of the matter, given an opportunity to share relevant information, and provided the right to appeal — during which time, their scores are treated as valid.
But the NBME said the USMLE program is authorized to take any actions it deems appropriate in response to concerns regarding score validity if the USMLE Committee for Individualized Review or the USMLE Composite Committee concludes that alternative or supplemental procedures are warranted in response to a given set of facts or circumstances.
“Following the month-long investigation and analysis…the USMLE program concluded that alternative procedures were warranted to address the score invalidity concerns identified in the interest of providing a process that is timely, efficient, effective, and fair, and given the large number of examinees involved in the investigation,” the board stated in its legal response.
In his order, Cooper wrote the current scenario, which implicates that more than 800 test-takers, is “clearly a situation calling for a procedure geared toward efficiency.” No evidence shows the board would not have taken similarly swift action if confronted with evidence of cheating on a comparable scale elsewhere, he wrote.
The judge also denied Giri’s motion to certify the lawsuit as a class action. The motion was denied without prejudice, meaning the plaintiff has the option to renew the motion should the case proceed.
A version of this article appeared on Medscape.com.
(USMLE).
In a February 23 order, Judge Christopher R. Cooper, of the US District Court for the District of Columbia, denied Latika Giri’s emergency motion to block the National Board of Medical Examiners (NBME) from invalidating the scores, ruling the public interest plainly weighs against granting the request.
“First and foremost, is the overriding interest in public safety,” Cooper wrote in his 32-page order. “This is a case about the credentials of doctors applying to medical residency programs…Granting the preliminary injunction would create an unacceptable risk that individuals who lack the requisite knowledge and skills they purport to possess because they achieved their exam scores fraudulently will be administering medical care to unsuspecting patients across the nation.”
Attorneys for Giri did not return messages seeking comment about the order.
The NBME also did not return messages seeking comment. The board previously said it does not comment on pending litigation.
The decision is the latest development in a widespread cheating scandal. Giri, an international medical graduate (IMG) from Kathmandu, sued NBME earlier this month claiming the board discriminated against Nepali medical graduates when it invalidated hundreds of exam scores linked to the country.
Giri also accused NBME of violating its own procedures when it voided the scores before giving examinees a chance to argue and appeal. She asked the district court to block NBME from invalidating her exam scores while the lawsuit continues and restore her original results.
In court documents, NBME argued that it did not invalidate the scores because the examinees were Nepali but because staff concluded that there was “a good faith basis for questioning the validity of the scores.”
The invalidations were based on concerns that the results reflected prior access to secure exam content rather than knowledge and understanding of the medical principles and skills the exams are intended to assess, according to the NBME’s legal response.
“The USMLE program took reasonable and appropriate actions to prevent the significant harm and disruption that would result from allowing potentially unqualified individuals to participate in the 2024 residency Match,” the NBME stated in court documents. “If granted, the requested injunction would cause enormous harm not only to NBME… but also to state licensing authorities, which rely upon USMLE results to help ensure that physicians have the minimum competencies needed to provide safe and effective health care.”
In his order, Cooper wrote that Giri has not proven the board’s actions were discriminatory against Nepali doctors.
“Nothing in the present record suggests that NBME went looking for a problem in Nepal out of ethnicity-or national-origin based [sic] suspicion,” Cooper wrote. “[It] followed the trail of evidence, including tips about organized cheating taking place in medical schools and at a testing center located in Nepal, and on an online forum for which a ‘nexus to Nepal’ was a ticket to admission.”
NBME: Nepal Outperformed All Other Countries on USMLE
Court documents shed more light on NBME’s investigation into the suspected cheating and on the anomalous patterns the board allegedly discovered from Nepal medical graduates.
In response to anonymous tips, the USMLE program in early 2023 asked the NBME Psychometrics and Data Analysis (PADA) unit to analyze examinee performance data for test centers in Jordan, Nepal, and Pakistan, according to court records. Within the initial data analysis, the data involving the single test center in Nepal was “the most extreme,” the unit found.
Out of more than 400 test centers across the world, including those in the United States, the test center in Nepal produced the highest test scores in the world for Step 1 in 2021 and 2022 and the highest test scores in the world for Step 2 CK in 2022, according to court documents. For the 2022 Step 1 exam for example, the average score of examinees testing in the Nepal test center was 240. No other test center in the world had an average examinee score above 227, according to the NBME’s legal response.
The median item response time for examinees who tested at the Nepal test center in 2022 was also among the fastest of all international test centers for Step 1 and Step 2 CK, investigators found.
In addition, the volume of examinees taking the USMLE Step 1 and Step 2 CK at the Nepal test center in Nepal had sharply increased. Step 1 volume more than doubled in the Nepal test center from 281 examinees in 2019 to 662 examinees in 2022, according to court documents.
The rapid increase continued in 2023, when examinee volume was nearly three-and-a-half times higher than the 2019 volume. The data were consistent with anonymous tips received by the USMLE program office, suggesting there may be wide-scale collection and sharing of live USMLE exam content within Nepal.
Investigation Finds Similar Correct and Incorrect Answers
Agreement similarity among the exams analyzed also raised red flags. Investigators ran an “agreement analysis” for all examinees who tested at centers in Jordan, Nepal, and Pakistan as well as two centers in India, according to court documents.
For the 2022 Step 1 exam and the 2021 and 2022 Step 2 CK exam, the analysis showed a substantially higher percentage of examinees with a statistically significant level of agreement matches in the examine group that tested at centers in Jordan, Nepal, Pakistan, and India compared with the baseline group, according to legal records.
The vast majority of examinees with a statistically significant number of matching incorrect answers tested at the Nepal test center, data showed.
Further analysis found that examinee volumes increased considerably at the Nepal test center in the months prior to the USMLE program releasing new test items, “suggesting that candidates who had prior access to disclosed exam questions wanted to test before new questions came into the item pool.”
Investigators also identified posts on social medial and in online chat rooms suggesting groups were collecting and sharing large amounts of secure exam material in private groups. Some posts advised examinees to use the full examination time when taking the USMLE “to avoid raising suspicion about having had prior access to secure exam materials,” according to court documents.
From its investigation and analysis, the USMLE program identified 832 examinees who had passing level exam results whose validity the USMLE program had a significant and good faith basis for questioning, according to court records.
Of the total, 618 examinees had one Step score flagged as being of questioned validity, 202 examinees had two Step exam scores flagged, and 12 examinees had scores flagged on all three Step exams.
NBME Defends Departure From Traditional Procedures
In court documents, NBME disputed claims that it violated its own procedures by invalidating the exam scores. Giri’s report contends that examinees suspected of cheating are typically first advised of the matter, given an opportunity to share relevant information, and provided the right to appeal — during which time, their scores are treated as valid.
But the NBME said the USMLE program is authorized to take any actions it deems appropriate in response to concerns regarding score validity if the USMLE Committee for Individualized Review or the USMLE Composite Committee concludes that alternative or supplemental procedures are warranted in response to a given set of facts or circumstances.
“Following the month-long investigation and analysis…the USMLE program concluded that alternative procedures were warranted to address the score invalidity concerns identified in the interest of providing a process that is timely, efficient, effective, and fair, and given the large number of examinees involved in the investigation,” the board stated in its legal response.
In his order, Cooper wrote the current scenario, which implicates that more than 800 test-takers, is “clearly a situation calling for a procedure geared toward efficiency.” No evidence shows the board would not have taken similarly swift action if confronted with evidence of cheating on a comparable scale elsewhere, he wrote.
The judge also denied Giri’s motion to certify the lawsuit as a class action. The motion was denied without prejudice, meaning the plaintiff has the option to renew the motion should the case proceed.
A version of this article appeared on Medscape.com.
MOC Woes? This System Might Be the Solution
, and what he hopes will prove less stressful approach to maintaining his credentials: The Longitudinal Knowledge Assessment (LKA).
Dr. Ali, assistant professor at the Icahn School of Medicine at Mount Sinai in New York City, is far from alone. Since the American Board of Internal Medicine (ABIM) launched the new method in 2022, approximately 80% of internists have chosen the LKA to maintain their board certification over the 10-year Maintenance of Certification (MOC) exam coupled with continuing education requirements.
“You have to keep learning. I think the LKA is good in that regard, as long as the questions are relevantly updated,” said Dr. Ali, who was first board-certified in 2018 and obtained his geriatrics certification in 2020.
Many other internists contend the MOC is too time-consuming and expensive and have taken action.
Some specialists, including a group of oncologists, argue the exam contains too much information that has become irrelevant to clinical practice. Members of the American College of Cardiology have even left ABIM over the certification process, as this news organization previously reported. After receiving criticism, the ABIM introduced longitudinal assessment as a less onerous means to maintain certification — although the group denies it succumbed to negative feedback.
One and Done, or More Flexibility?
Both the traditional 10-year exam and the LKA have their advantages and disadvantages, according to Helen Chen, MD, the chair of the Geriatric Medicine Board Exam–Writing Committee at ABIM.
The LKA is arguably easier to access and available for most internal medicine disciplines. It requires no preparation for studying, and internists can complete exam questions on their phone, computer, or tablet.
Participants receive 30 questions per quarter for 5 years. Feedback is immediate and includes links to references for further learning. Once the process is completed and a physician meets the performance standard, the next 5-year cycle begins.
Still, some physicians still prefer the traditional 10-year, long-form exam. Studying for the test can be intense and take months. Physicians also must travel to an exam center on a designated date. However, once the test is over, the certification test does not roll around for another decade.
“It’s really about choice. Some doctors want to sit down and do it all at once and get it over with; others prefer to do a few questions at a time and never feel rushed,” said Dr. Chen, who is triple-boarded in geriatrics, internal medicine, and hospice and palliative medicine.
In 2022, Dr. Chen opted to begin the LKA cycle; a cross-country move and new job would not have allowed her enough time to prepare for the long-form exam, she said.
The new exam challenged her knowledge in smaller bites, provided immediate feedback, and allowed her to satisfy her curiosity through additional reading, she said, even if some questions were not relevant to her clinical practice.
The LKA is not yet as specialized, and ABIM is working to refine questions to be more relevant for some subspecialties.
Questions for both the LKA and long-form exam are developed from physician input, according to Dr. Chen. They are regularly assessed for relevance, accuracy, and changes to practice guidelines.
She acknowledged that questions can sometimes become outdated in a relatively short time, particularly for those taking the 10-year exam. But feedback from physicians helps committees analyze the relevancy of questions and how intensely an area should be tested. Committee members will even throw out questions if the literature changes significantly.
An Unnecessary Exercise
As criticism has mounted over the MOC, physicians have questioned whether recertification is necessary.
According to a survey of 1700 members of the American Society of Clinical Oncology (ASCO), most (64%) backed initial ABIM certification, but three quarters said the recertification process did not benefit their knowledge of clinical practice. More than 80% reported that Continuing Medical Education (CME) credits should suffice for ongoing learning, without having to be supplemented by the MOC exam. ASCO is considering alternative pathways to the current process based on their member feedback and plans to release a proposal to members in the first half of 2024.
Meanwhile, some cardiologists have called the MOC process “an onerous and unnecessary addition to continuing medical education requirements they already must meet at the state and hospital levels.”
The ABIM responded in part in a recent JAMA Viewpoint written by several members of the ABIM board of directors. They said board-certified physicians save the health system about $5 billion annually, compared with those who are not.
“Patients who are cared for by physicians who demonstrate more medical knowledge through certification and MOC have a better prognosis for a host of important outcomes including lower mortality from cardiovascular disease, fewer emergency department visits, and fewer unplanned hospitalizations,” the group wrote.
Certification provides a significant benefit, according to Dr. Ali. Some of his patients do ask about his credentials. He said he also finds keeping up with the latest information essential. Ongoing learning shows patients he is committed to providing the best care, he said. “It benefits me, and I’ve benefited my patients. When they come in with questions, I can speak knowledgeably,” he said.
Maintaining board certification is also not unique to internal medicine physicians or subspecialists. Other physician specialties mandate more frequent exams, include both oral and written portions, or administer exams totally online. The American Academy of Family Physicians (AAFP) has a longitudinal option, similar to the LKA, as an alternative to their 1-day exam.
Margo Savoy, MD, MPH, senior vice president of education, inclusiveness, and physician well-being at AAFP, said physicians should make the best choice for them.
“The AAFP welcomes the opportunity for family physicians to have options for how to demonstrate their competence and strongly encourages a balanced approach that avoids undue administrative burdens and fosters a culture of physician well-being and high-quality care,” Dr. Savoy said.
The ABIM has also been criticized for the fee structure for MOC, which some physicians consider excessive: $220 per year for the first certification and $120 for each additional certification. Physicians choosing to take the 10-year exam are charged an additional $700 testing center fee. Those charges do not include the cost of attending CME-related activities. One analysis estimated the cost of maintaining certification could reach into the tens of thousands of dollars, primarily from the time physicians must spend preparing for the long-form exam.
Dr. Chen pushed back on the contention that the ABIM is making a huge profit off of the 10-year exam. She called MOC fees reasonable when amortized over a 10-year cycle and noted the costs for longitudinal assessment are included in those charges.
Meanwhile, she encouraged physicians who were on the fence about maintaining board certification at all to consider both the benefit to their practice and to their patients, especially since the LKA has already demonstrated such popularity.
“There’s nothing like continuous learning to keep you humble,” Dr. Chen said. “You just don’t know everything.”
A version of this article appeared on Medscape.com.
, and what he hopes will prove less stressful approach to maintaining his credentials: The Longitudinal Knowledge Assessment (LKA).
Dr. Ali, assistant professor at the Icahn School of Medicine at Mount Sinai in New York City, is far from alone. Since the American Board of Internal Medicine (ABIM) launched the new method in 2022, approximately 80% of internists have chosen the LKA to maintain their board certification over the 10-year Maintenance of Certification (MOC) exam coupled with continuing education requirements.
“You have to keep learning. I think the LKA is good in that regard, as long as the questions are relevantly updated,” said Dr. Ali, who was first board-certified in 2018 and obtained his geriatrics certification in 2020.
Many other internists contend the MOC is too time-consuming and expensive and have taken action.
Some specialists, including a group of oncologists, argue the exam contains too much information that has become irrelevant to clinical practice. Members of the American College of Cardiology have even left ABIM over the certification process, as this news organization previously reported. After receiving criticism, the ABIM introduced longitudinal assessment as a less onerous means to maintain certification — although the group denies it succumbed to negative feedback.
One and Done, or More Flexibility?
Both the traditional 10-year exam and the LKA have their advantages and disadvantages, according to Helen Chen, MD, the chair of the Geriatric Medicine Board Exam–Writing Committee at ABIM.
The LKA is arguably easier to access and available for most internal medicine disciplines. It requires no preparation for studying, and internists can complete exam questions on their phone, computer, or tablet.
Participants receive 30 questions per quarter for 5 years. Feedback is immediate and includes links to references for further learning. Once the process is completed and a physician meets the performance standard, the next 5-year cycle begins.
Still, some physicians still prefer the traditional 10-year, long-form exam. Studying for the test can be intense and take months. Physicians also must travel to an exam center on a designated date. However, once the test is over, the certification test does not roll around for another decade.
“It’s really about choice. Some doctors want to sit down and do it all at once and get it over with; others prefer to do a few questions at a time and never feel rushed,” said Dr. Chen, who is triple-boarded in geriatrics, internal medicine, and hospice and palliative medicine.
In 2022, Dr. Chen opted to begin the LKA cycle; a cross-country move and new job would not have allowed her enough time to prepare for the long-form exam, she said.
The new exam challenged her knowledge in smaller bites, provided immediate feedback, and allowed her to satisfy her curiosity through additional reading, she said, even if some questions were not relevant to her clinical practice.
The LKA is not yet as specialized, and ABIM is working to refine questions to be more relevant for some subspecialties.
Questions for both the LKA and long-form exam are developed from physician input, according to Dr. Chen. They are regularly assessed for relevance, accuracy, and changes to practice guidelines.
She acknowledged that questions can sometimes become outdated in a relatively short time, particularly for those taking the 10-year exam. But feedback from physicians helps committees analyze the relevancy of questions and how intensely an area should be tested. Committee members will even throw out questions if the literature changes significantly.
An Unnecessary Exercise
As criticism has mounted over the MOC, physicians have questioned whether recertification is necessary.
According to a survey of 1700 members of the American Society of Clinical Oncology (ASCO), most (64%) backed initial ABIM certification, but three quarters said the recertification process did not benefit their knowledge of clinical practice. More than 80% reported that Continuing Medical Education (CME) credits should suffice for ongoing learning, without having to be supplemented by the MOC exam. ASCO is considering alternative pathways to the current process based on their member feedback and plans to release a proposal to members in the first half of 2024.
Meanwhile, some cardiologists have called the MOC process “an onerous and unnecessary addition to continuing medical education requirements they already must meet at the state and hospital levels.”
The ABIM responded in part in a recent JAMA Viewpoint written by several members of the ABIM board of directors. They said board-certified physicians save the health system about $5 billion annually, compared with those who are not.
“Patients who are cared for by physicians who demonstrate more medical knowledge through certification and MOC have a better prognosis for a host of important outcomes including lower mortality from cardiovascular disease, fewer emergency department visits, and fewer unplanned hospitalizations,” the group wrote.
Certification provides a significant benefit, according to Dr. Ali. Some of his patients do ask about his credentials. He said he also finds keeping up with the latest information essential. Ongoing learning shows patients he is committed to providing the best care, he said. “It benefits me, and I’ve benefited my patients. When they come in with questions, I can speak knowledgeably,” he said.
Maintaining board certification is also not unique to internal medicine physicians or subspecialists. Other physician specialties mandate more frequent exams, include both oral and written portions, or administer exams totally online. The American Academy of Family Physicians (AAFP) has a longitudinal option, similar to the LKA, as an alternative to their 1-day exam.
Margo Savoy, MD, MPH, senior vice president of education, inclusiveness, and physician well-being at AAFP, said physicians should make the best choice for them.
“The AAFP welcomes the opportunity for family physicians to have options for how to demonstrate their competence and strongly encourages a balanced approach that avoids undue administrative burdens and fosters a culture of physician well-being and high-quality care,” Dr. Savoy said.
The ABIM has also been criticized for the fee structure for MOC, which some physicians consider excessive: $220 per year for the first certification and $120 for each additional certification. Physicians choosing to take the 10-year exam are charged an additional $700 testing center fee. Those charges do not include the cost of attending CME-related activities. One analysis estimated the cost of maintaining certification could reach into the tens of thousands of dollars, primarily from the time physicians must spend preparing for the long-form exam.
Dr. Chen pushed back on the contention that the ABIM is making a huge profit off of the 10-year exam. She called MOC fees reasonable when amortized over a 10-year cycle and noted the costs for longitudinal assessment are included in those charges.
Meanwhile, she encouraged physicians who were on the fence about maintaining board certification at all to consider both the benefit to their practice and to their patients, especially since the LKA has already demonstrated such popularity.
“There’s nothing like continuous learning to keep you humble,” Dr. Chen said. “You just don’t know everything.”
A version of this article appeared on Medscape.com.
, and what he hopes will prove less stressful approach to maintaining his credentials: The Longitudinal Knowledge Assessment (LKA).
Dr. Ali, assistant professor at the Icahn School of Medicine at Mount Sinai in New York City, is far from alone. Since the American Board of Internal Medicine (ABIM) launched the new method in 2022, approximately 80% of internists have chosen the LKA to maintain their board certification over the 10-year Maintenance of Certification (MOC) exam coupled with continuing education requirements.
“You have to keep learning. I think the LKA is good in that regard, as long as the questions are relevantly updated,” said Dr. Ali, who was first board-certified in 2018 and obtained his geriatrics certification in 2020.
Many other internists contend the MOC is too time-consuming and expensive and have taken action.
Some specialists, including a group of oncologists, argue the exam contains too much information that has become irrelevant to clinical practice. Members of the American College of Cardiology have even left ABIM over the certification process, as this news organization previously reported. After receiving criticism, the ABIM introduced longitudinal assessment as a less onerous means to maintain certification — although the group denies it succumbed to negative feedback.
One and Done, or More Flexibility?
Both the traditional 10-year exam and the LKA have their advantages and disadvantages, according to Helen Chen, MD, the chair of the Geriatric Medicine Board Exam–Writing Committee at ABIM.
The LKA is arguably easier to access and available for most internal medicine disciplines. It requires no preparation for studying, and internists can complete exam questions on their phone, computer, or tablet.
Participants receive 30 questions per quarter for 5 years. Feedback is immediate and includes links to references for further learning. Once the process is completed and a physician meets the performance standard, the next 5-year cycle begins.
Still, some physicians still prefer the traditional 10-year, long-form exam. Studying for the test can be intense and take months. Physicians also must travel to an exam center on a designated date. However, once the test is over, the certification test does not roll around for another decade.
“It’s really about choice. Some doctors want to sit down and do it all at once and get it over with; others prefer to do a few questions at a time and never feel rushed,” said Dr. Chen, who is triple-boarded in geriatrics, internal medicine, and hospice and palliative medicine.
In 2022, Dr. Chen opted to begin the LKA cycle; a cross-country move and new job would not have allowed her enough time to prepare for the long-form exam, she said.
The new exam challenged her knowledge in smaller bites, provided immediate feedback, and allowed her to satisfy her curiosity through additional reading, she said, even if some questions were not relevant to her clinical practice.
The LKA is not yet as specialized, and ABIM is working to refine questions to be more relevant for some subspecialties.
Questions for both the LKA and long-form exam are developed from physician input, according to Dr. Chen. They are regularly assessed for relevance, accuracy, and changes to practice guidelines.
She acknowledged that questions can sometimes become outdated in a relatively short time, particularly for those taking the 10-year exam. But feedback from physicians helps committees analyze the relevancy of questions and how intensely an area should be tested. Committee members will even throw out questions if the literature changes significantly.
An Unnecessary Exercise
As criticism has mounted over the MOC, physicians have questioned whether recertification is necessary.
According to a survey of 1700 members of the American Society of Clinical Oncology (ASCO), most (64%) backed initial ABIM certification, but three quarters said the recertification process did not benefit their knowledge of clinical practice. More than 80% reported that Continuing Medical Education (CME) credits should suffice for ongoing learning, without having to be supplemented by the MOC exam. ASCO is considering alternative pathways to the current process based on their member feedback and plans to release a proposal to members in the first half of 2024.
Meanwhile, some cardiologists have called the MOC process “an onerous and unnecessary addition to continuing medical education requirements they already must meet at the state and hospital levels.”
The ABIM responded in part in a recent JAMA Viewpoint written by several members of the ABIM board of directors. They said board-certified physicians save the health system about $5 billion annually, compared with those who are not.
“Patients who are cared for by physicians who demonstrate more medical knowledge through certification and MOC have a better prognosis for a host of important outcomes including lower mortality from cardiovascular disease, fewer emergency department visits, and fewer unplanned hospitalizations,” the group wrote.
Certification provides a significant benefit, according to Dr. Ali. Some of his patients do ask about his credentials. He said he also finds keeping up with the latest information essential. Ongoing learning shows patients he is committed to providing the best care, he said. “It benefits me, and I’ve benefited my patients. When they come in with questions, I can speak knowledgeably,” he said.
Maintaining board certification is also not unique to internal medicine physicians or subspecialists. Other physician specialties mandate more frequent exams, include both oral and written portions, or administer exams totally online. The American Academy of Family Physicians (AAFP) has a longitudinal option, similar to the LKA, as an alternative to their 1-day exam.
Margo Savoy, MD, MPH, senior vice president of education, inclusiveness, and physician well-being at AAFP, said physicians should make the best choice for them.
“The AAFP welcomes the opportunity for family physicians to have options for how to demonstrate their competence and strongly encourages a balanced approach that avoids undue administrative burdens and fosters a culture of physician well-being and high-quality care,” Dr. Savoy said.
The ABIM has also been criticized for the fee structure for MOC, which some physicians consider excessive: $220 per year for the first certification and $120 for each additional certification. Physicians choosing to take the 10-year exam are charged an additional $700 testing center fee. Those charges do not include the cost of attending CME-related activities. One analysis estimated the cost of maintaining certification could reach into the tens of thousands of dollars, primarily from the time physicians must spend preparing for the long-form exam.
Dr. Chen pushed back on the contention that the ABIM is making a huge profit off of the 10-year exam. She called MOC fees reasonable when amortized over a 10-year cycle and noted the costs for longitudinal assessment are included in those charges.
Meanwhile, she encouraged physicians who were on the fence about maintaining board certification at all to consider both the benefit to their practice and to their patients, especially since the LKA has already demonstrated such popularity.
“There’s nothing like continuous learning to keep you humble,” Dr. Chen said. “You just don’t know everything.”
A version of this article appeared on Medscape.com.
Poor Quality of Cancer Content on Social Media
This transcript has been edited for clarity.
I’m delighted to talk about a very interesting topic in this commentary. This is an area that we generally don’t discuss, but it’s one that’s obviously very topical, which includes the question of social media.
The paper I’m referring to is entitled, “More Than a Song and Dance”: Exploration of Patient Perspectives and Educational Quality of Gynecologic Cancer Content on TikTok. The paper was published in Gynecologic Oncology in 2023.
They had a total of 466.7 million views. They looked at 430 of the 500 top posts that were eligible, looked at 11 central themes, did an objective analysis of educational content based on published strategy for looking at this.
What they found, unfortunately but not surprisingly, overall was that the educational quality and reliability were quite poor. They also noticed considerable differences in disparities based on racial background and really emphasized in their analysis not only how common it is for individuals to look at this content on TikTok but also concerns about what it is that the public, patients, and their families are actually seeing.
This, of course, specifically relates to gynecologic cancers, but almost certainly relates to other cancers as well. Clearly, this is a topic that needs to be discussed widely. It’s very complex and very controversial, but when you think about the information that might be provided to our patients and their families going to social media, it’s important that we understand what they’re seeing, what they’re hearing, what they’re viewing, and the impact this might have on their care and outcomes.
I encourage you to read this very interesting paper if you have an interest in this topic. Again, it was recently published in Gynecologic Oncology. I thank you for your attention.
Dr. Markman is professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California; president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m delighted to talk about a very interesting topic in this commentary. This is an area that we generally don’t discuss, but it’s one that’s obviously very topical, which includes the question of social media.
The paper I’m referring to is entitled, “More Than a Song and Dance”: Exploration of Patient Perspectives and Educational Quality of Gynecologic Cancer Content on TikTok. The paper was published in Gynecologic Oncology in 2023.
They had a total of 466.7 million views. They looked at 430 of the 500 top posts that were eligible, looked at 11 central themes, did an objective analysis of educational content based on published strategy for looking at this.
What they found, unfortunately but not surprisingly, overall was that the educational quality and reliability were quite poor. They also noticed considerable differences in disparities based on racial background and really emphasized in their analysis not only how common it is for individuals to look at this content on TikTok but also concerns about what it is that the public, patients, and their families are actually seeing.
This, of course, specifically relates to gynecologic cancers, but almost certainly relates to other cancers as well. Clearly, this is a topic that needs to be discussed widely. It’s very complex and very controversial, but when you think about the information that might be provided to our patients and their families going to social media, it’s important that we understand what they’re seeing, what they’re hearing, what they’re viewing, and the impact this might have on their care and outcomes.
I encourage you to read this very interesting paper if you have an interest in this topic. Again, it was recently published in Gynecologic Oncology. I thank you for your attention.
Dr. Markman is professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California; president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m delighted to talk about a very interesting topic in this commentary. This is an area that we generally don’t discuss, but it’s one that’s obviously very topical, which includes the question of social media.
The paper I’m referring to is entitled, “More Than a Song and Dance”: Exploration of Patient Perspectives and Educational Quality of Gynecologic Cancer Content on TikTok. The paper was published in Gynecologic Oncology in 2023.
They had a total of 466.7 million views. They looked at 430 of the 500 top posts that were eligible, looked at 11 central themes, did an objective analysis of educational content based on published strategy for looking at this.
What they found, unfortunately but not surprisingly, overall was that the educational quality and reliability were quite poor. They also noticed considerable differences in disparities based on racial background and really emphasized in their analysis not only how common it is for individuals to look at this content on TikTok but also concerns about what it is that the public, patients, and their families are actually seeing.
This, of course, specifically relates to gynecologic cancers, but almost certainly relates to other cancers as well. Clearly, this is a topic that needs to be discussed widely. It’s very complex and very controversial, but when you think about the information that might be provided to our patients and their families going to social media, it’s important that we understand what they’re seeing, what they’re hearing, what they’re viewing, and the impact this might have on their care and outcomes.
I encourage you to read this very interesting paper if you have an interest in this topic. Again, it was recently published in Gynecologic Oncology. I thank you for your attention.
Dr. Markman is professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California; president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.





