User login
Whether to anticoagulate: Toward a more reasoned approach
The article by Hagerty and Rich in this issue of the Cleveland Clinic Journal of Medicine1 covers an important topic—whether elderly patients with atrial fibrillation should receive anticoagulant therapy for it, or whether the risk of bleeding with this therapy outweighs the benefit of preventing stroke.
BETTER RISK PREDICTORS ARE NEEDED
Prediction tools are available for estimating the risk of stroke in patients with atrial fibrillation without anticoagulation2,3 and to estimate bleeding risk from anticoagulation4–7 (Table 1). Both tools have limitations, but as Hagerty and Rich point out, the stroke risk scales are likely better than the bleeding risk scales.
For example, Fang et al8 note that the risk of intracranial hemorrhage increases significantly after age 85. The bleeding risk scales use lower age cutoffs than this, perhaps increasing their sensitivity but decreasing their specificity.
Although HAS-BLED5,6 includes antiplatelet drugs such as nonsteroidal anti-inflammatory drugs and aspirin as risk factors for bleeding, ATRIA4 and HEMORR2HAGES7 do not.
Other drugs such as macrolides, quinolones, and high-dose corticosteroids raise the international normalized ratio (INR). These are typically used short-term, but can cause major fluctuations in the INR that may not be detected by monthly INR checks. Incorporating the short-term use of such drugs into bleeding risk scales would be difficult if not impossible a priori. Yet clinicians should be aware that these drugs can affect bleeding risk.
As Hagerty and Rich note,1 the bleeding risk scores were developed for warfarin, and their applicability to patients treated with novel oral anticoagulants is uncertain.
All three of the available bleeding risk scales consider prior bleeding as a risk factor, but the severity of the prior bleeding varies. Although it is understandable to include major bleeding as a risk factor since it carries an increased risk of death, minor bleeding can affect morbidity and quality of life. Only the ATRIA score4 considers both major and minor bleeding, while HEMORR2HAGES7 does not specify bleeding severity, and HAS-BLED5,6 considers only major bleeding. Clearly, there is a need to update these existing bleeding risk scores so that they can apply to novel oral anticoagulants and consider both major and minor bleeding.
As the authors note, for predicting the risk of stroke, the CHA2DS2-VASc score3 provides more precision than the CHADS2 score2 at the lower end of the benefit spectrum. Unfortunately, there is no similar screening tool to predict bleeding risk from anticoagulation with greater precision in the middle to lower part of the risk spectrum.
THE PATIENT’S PREFERENCES MATTER
The patient’s life expectancy and personal preferences are important independent factors that affect the decision of whether to anticoagulate or not. It is the responsibility of clinicians who care for older adults to make sure that these two important considerations are included in any anticoagulation decision-making for this group of patients.
- Hagerty T, Rich MW. Fall risk and anticoagulation for atrial fibrillation in the elderly: a delicate balance. Cleve Clin J Med 2017; 84:35–40.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010; 137:263–272.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol 2011; 58:395–401.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011; 57:173–180.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med 2004; 141:745–752.
The article by Hagerty and Rich in this issue of the Cleveland Clinic Journal of Medicine1 covers an important topic—whether elderly patients with atrial fibrillation should receive anticoagulant therapy for it, or whether the risk of bleeding with this therapy outweighs the benefit of preventing stroke.
BETTER RISK PREDICTORS ARE NEEDED
Prediction tools are available for estimating the risk of stroke in patients with atrial fibrillation without anticoagulation2,3 and to estimate bleeding risk from anticoagulation4–7 (Table 1). Both tools have limitations, but as Hagerty and Rich point out, the stroke risk scales are likely better than the bleeding risk scales.
For example, Fang et al8 note that the risk of intracranial hemorrhage increases significantly after age 85. The bleeding risk scales use lower age cutoffs than this, perhaps increasing their sensitivity but decreasing their specificity.
Although HAS-BLED5,6 includes antiplatelet drugs such as nonsteroidal anti-inflammatory drugs and aspirin as risk factors for bleeding, ATRIA4 and HEMORR2HAGES7 do not.
Other drugs such as macrolides, quinolones, and high-dose corticosteroids raise the international normalized ratio (INR). These are typically used short-term, but can cause major fluctuations in the INR that may not be detected by monthly INR checks. Incorporating the short-term use of such drugs into bleeding risk scales would be difficult if not impossible a priori. Yet clinicians should be aware that these drugs can affect bleeding risk.
As Hagerty and Rich note,1 the bleeding risk scores were developed for warfarin, and their applicability to patients treated with novel oral anticoagulants is uncertain.
All three of the available bleeding risk scales consider prior bleeding as a risk factor, but the severity of the prior bleeding varies. Although it is understandable to include major bleeding as a risk factor since it carries an increased risk of death, minor bleeding can affect morbidity and quality of life. Only the ATRIA score4 considers both major and minor bleeding, while HEMORR2HAGES7 does not specify bleeding severity, and HAS-BLED5,6 considers only major bleeding. Clearly, there is a need to update these existing bleeding risk scores so that they can apply to novel oral anticoagulants and consider both major and minor bleeding.
As the authors note, for predicting the risk of stroke, the CHA2DS2-VASc score3 provides more precision than the CHADS2 score2 at the lower end of the benefit spectrum. Unfortunately, there is no similar screening tool to predict bleeding risk from anticoagulation with greater precision in the middle to lower part of the risk spectrum.
THE PATIENT’S PREFERENCES MATTER
The patient’s life expectancy and personal preferences are important independent factors that affect the decision of whether to anticoagulate or not. It is the responsibility of clinicians who care for older adults to make sure that these two important considerations are included in any anticoagulation decision-making for this group of patients.
The article by Hagerty and Rich in this issue of the Cleveland Clinic Journal of Medicine1 covers an important topic—whether elderly patients with atrial fibrillation should receive anticoagulant therapy for it, or whether the risk of bleeding with this therapy outweighs the benefit of preventing stroke.
BETTER RISK PREDICTORS ARE NEEDED
Prediction tools are available for estimating the risk of stroke in patients with atrial fibrillation without anticoagulation2,3 and to estimate bleeding risk from anticoagulation4–7 (Table 1). Both tools have limitations, but as Hagerty and Rich point out, the stroke risk scales are likely better than the bleeding risk scales.
For example, Fang et al8 note that the risk of intracranial hemorrhage increases significantly after age 85. The bleeding risk scales use lower age cutoffs than this, perhaps increasing their sensitivity but decreasing their specificity.
Although HAS-BLED5,6 includes antiplatelet drugs such as nonsteroidal anti-inflammatory drugs and aspirin as risk factors for bleeding, ATRIA4 and HEMORR2HAGES7 do not.
Other drugs such as macrolides, quinolones, and high-dose corticosteroids raise the international normalized ratio (INR). These are typically used short-term, but can cause major fluctuations in the INR that may not be detected by monthly INR checks. Incorporating the short-term use of such drugs into bleeding risk scales would be difficult if not impossible a priori. Yet clinicians should be aware that these drugs can affect bleeding risk.
As Hagerty and Rich note,1 the bleeding risk scores were developed for warfarin, and their applicability to patients treated with novel oral anticoagulants is uncertain.
All three of the available bleeding risk scales consider prior bleeding as a risk factor, but the severity of the prior bleeding varies. Although it is understandable to include major bleeding as a risk factor since it carries an increased risk of death, minor bleeding can affect morbidity and quality of life. Only the ATRIA score4 considers both major and minor bleeding, while HEMORR2HAGES7 does not specify bleeding severity, and HAS-BLED5,6 considers only major bleeding. Clearly, there is a need to update these existing bleeding risk scores so that they can apply to novel oral anticoagulants and consider both major and minor bleeding.
As the authors note, for predicting the risk of stroke, the CHA2DS2-VASc score3 provides more precision than the CHADS2 score2 at the lower end of the benefit spectrum. Unfortunately, there is no similar screening tool to predict bleeding risk from anticoagulation with greater precision in the middle to lower part of the risk spectrum.
THE PATIENT’S PREFERENCES MATTER
The patient’s life expectancy and personal preferences are important independent factors that affect the decision of whether to anticoagulate or not. It is the responsibility of clinicians who care for older adults to make sure that these two important considerations are included in any anticoagulation decision-making for this group of patients.
- Hagerty T, Rich MW. Fall risk and anticoagulation for atrial fibrillation in the elderly: a delicate balance. Cleve Clin J Med 2017; 84:35–40.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010; 137:263–272.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol 2011; 58:395–401.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011; 57:173–180.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med 2004; 141:745–752.
- Hagerty T, Rich MW. Fall risk and anticoagulation for atrial fibrillation in the elderly: a delicate balance. Cleve Clin J Med 2017; 84:35–40.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010; 137:263–272.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol 2011; 58:395–401.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011; 57:173–180.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med 2004; 141:745–752.
Imaging suggestive, but symptoms atypical
A 66-year-old man with chronic obstructive pulmonary disease (COPD) was brought to the emergency department with a 2-week history of progressive dyspnea followed by altered mental status. He had no history of diabetes mellitus, hypertension, or drug abuse.
On physical examination, he was stuporous. He had no fever or hypotension, but his pulse and breathing were rapid, and he had central cyanosis, bilateral conjunctival congestion, a puffy face, generalized wheezing, basilar crackles in both lungs, and leg edema.
Laboratory testing showed hypoxia and severe hypercarbia. His hematocrit was 65% (reference range 39–51) and his hemoglobin level was 21.5 g/dL (13–17).
The patient was diagnosed with an exacerbation of COPD. He was intubated, placed on mechanical ventilation, and admitted to the intensive care unit.
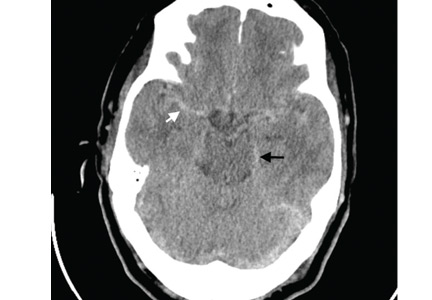
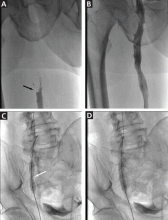
Computed tomography (CT) performed because of his decreased level of consciousness (Figure 1) showed increased attenuation in the ambient cistern and the lateral aspect of the lateral cerebral fissure, suggesting subarachnoid hemorrhage. The attenuation value in these areas was 89 Hounsfield units (typical values for brain tissue are in the 20s to 30s, and for blood in the 30s to 40s). To further evaluate for subarachnoid hemorrhage, lumbar puncture was performed, but analysis of the fluid sample showed normal protein and glucose levels and no cells.
Based on the results of cerebrospinal fluid evaluation and on the CT attenuation value, a diagnosis of pseudosubarachnoid hemorrhage due to polycythemia was made.
SUBARACHNOID VS PSEUDOSUBARACHNOID HEMORRHAGE
Subarachnoid hemorrhage typically begins with a “thunder-clap” headache (beginning suddenly and described by patients as “the worst headache ever.”) While not all patients have this presentation, if imaging suggests subarachnoid hemorrhage but the patient has atypical signs and symptoms (eg, other than headache), then pseudosubarachnoid hemorrhage should be considered.
Brain CT is one of the most reliable tools for diagnosing subarachnoid hemorrhage in the emergency department. Done within 6 hours of symptom onset, it has a sensitivity of 98.7% and a specificity of 99.9%.1 Magnetic resonance imaging can also visualize subarachnoid hemorrhage within the first 12 hours, typically as a hyperintensity in the subarachnoid space on fluid-attenuated inversion-recovery sequences2 and on susceptibility-weighted sequences.
Lumbar puncture is also an important diagnostic tool but carries a risk of brain herniation in patients with brain edema.
Pseudosubarachnoid hemorrhage is an artifact of CT imaging. It is rare, and its prevalence is unknown.3 However, it may be seen in up to 20% of patients after resuscitation, as a result of diffuse cerebral edema that lowers the attenuation of brain tissue on CT, making the vessels relatively conspicuous. It can also be seen in purulent meningitis (due to proteinaceous influx after blood-brain barrier disruption),4 in meningeal leukemia (due to increased cellularity in the leptomeninges), and in severe polycythemia (from a higher concentration of blood and hemoglobin in the vessels).3,5–7
Although the level of attenuation on CT may help distinguish subarachnoid from pseudosubarachnoid hemorrhage, its accuracy has not been defined. Inspecting the CT images may clarify whether areas with high attenuation look like blood vessels vs subarachnoid hemorrhage.
Our patient recovered and had an uneventful follow-up. The cause of his elevated hematocrit was likely chronic hypoxia from COPD.
Acknowledgment: We thank Dr. Saeide Khanbagi and Dr. Azade Nasr-lari for their cooperation.
- Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke 2016; 47:750–755.
- Sohn CH, Baik SK, Lee HJ, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol 2005; 26:662–665.
- Yuzawa H, Higano S, Mugikura S, et al. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol 2008; 29:1544–1549.
- Given CA 2nd, Burdette JH, Elster AD, Williams DW 3rd. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol 2003; 24:254–256.
- Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol 1998; 28:222–225.
- Ben Salem D, Osseby GV, Rezaizadeh-Bourdariat K, et al. Spontaneous hyperdense intracranial vessels seen on CT scan in polycythemia cases. J Radiol 2003; 84:605–608. French.
- Hsieh SW, Khor GT, Chen CN, Huang P. Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med 2012; 42:e109–e111.
A 66-year-old man with chronic obstructive pulmonary disease (COPD) was brought to the emergency department with a 2-week history of progressive dyspnea followed by altered mental status. He had no history of diabetes mellitus, hypertension, or drug abuse.
On physical examination, he was stuporous. He had no fever or hypotension, but his pulse and breathing were rapid, and he had central cyanosis, bilateral conjunctival congestion, a puffy face, generalized wheezing, basilar crackles in both lungs, and leg edema.
Laboratory testing showed hypoxia and severe hypercarbia. His hematocrit was 65% (reference range 39–51) and his hemoglobin level was 21.5 g/dL (13–17).
The patient was diagnosed with an exacerbation of COPD. He was intubated, placed on mechanical ventilation, and admitted to the intensive care unit.
Computed tomography (CT) performed because of his decreased level of consciousness (Figure 1) showed increased attenuation in the ambient cistern and the lateral aspect of the lateral cerebral fissure, suggesting subarachnoid hemorrhage. The attenuation value in these areas was 89 Hounsfield units (typical values for brain tissue are in the 20s to 30s, and for blood in the 30s to 40s). To further evaluate for subarachnoid hemorrhage, lumbar puncture was performed, but analysis of the fluid sample showed normal protein and glucose levels and no cells.
Based on the results of cerebrospinal fluid evaluation and on the CT attenuation value, a diagnosis of pseudosubarachnoid hemorrhage due to polycythemia was made.
SUBARACHNOID VS PSEUDOSUBARACHNOID HEMORRHAGE
Subarachnoid hemorrhage typically begins with a “thunder-clap” headache (beginning suddenly and described by patients as “the worst headache ever.”) While not all patients have this presentation, if imaging suggests subarachnoid hemorrhage but the patient has atypical signs and symptoms (eg, other than headache), then pseudosubarachnoid hemorrhage should be considered.
Brain CT is one of the most reliable tools for diagnosing subarachnoid hemorrhage in the emergency department. Done within 6 hours of symptom onset, it has a sensitivity of 98.7% and a specificity of 99.9%.1 Magnetic resonance imaging can also visualize subarachnoid hemorrhage within the first 12 hours, typically as a hyperintensity in the subarachnoid space on fluid-attenuated inversion-recovery sequences2 and on susceptibility-weighted sequences.
Lumbar puncture is also an important diagnostic tool but carries a risk of brain herniation in patients with brain edema.
Pseudosubarachnoid hemorrhage is an artifact of CT imaging. It is rare, and its prevalence is unknown.3 However, it may be seen in up to 20% of patients after resuscitation, as a result of diffuse cerebral edema that lowers the attenuation of brain tissue on CT, making the vessels relatively conspicuous. It can also be seen in purulent meningitis (due to proteinaceous influx after blood-brain barrier disruption),4 in meningeal leukemia (due to increased cellularity in the leptomeninges), and in severe polycythemia (from a higher concentration of blood and hemoglobin in the vessels).3,5–7
Although the level of attenuation on CT may help distinguish subarachnoid from pseudosubarachnoid hemorrhage, its accuracy has not been defined. Inspecting the CT images may clarify whether areas with high attenuation look like blood vessels vs subarachnoid hemorrhage.
Our patient recovered and had an uneventful follow-up. The cause of his elevated hematocrit was likely chronic hypoxia from COPD.
Acknowledgment: We thank Dr. Saeide Khanbagi and Dr. Azade Nasr-lari for their cooperation.
A 66-year-old man with chronic obstructive pulmonary disease (COPD) was brought to the emergency department with a 2-week history of progressive dyspnea followed by altered mental status. He had no history of diabetes mellitus, hypertension, or drug abuse.
On physical examination, he was stuporous. He had no fever or hypotension, but his pulse and breathing were rapid, and he had central cyanosis, bilateral conjunctival congestion, a puffy face, generalized wheezing, basilar crackles in both lungs, and leg edema.
Laboratory testing showed hypoxia and severe hypercarbia. His hematocrit was 65% (reference range 39–51) and his hemoglobin level was 21.5 g/dL (13–17).
The patient was diagnosed with an exacerbation of COPD. He was intubated, placed on mechanical ventilation, and admitted to the intensive care unit.
Computed tomography (CT) performed because of his decreased level of consciousness (Figure 1) showed increased attenuation in the ambient cistern and the lateral aspect of the lateral cerebral fissure, suggesting subarachnoid hemorrhage. The attenuation value in these areas was 89 Hounsfield units (typical values for brain tissue are in the 20s to 30s, and for blood in the 30s to 40s). To further evaluate for subarachnoid hemorrhage, lumbar puncture was performed, but analysis of the fluid sample showed normal protein and glucose levels and no cells.
Based on the results of cerebrospinal fluid evaluation and on the CT attenuation value, a diagnosis of pseudosubarachnoid hemorrhage due to polycythemia was made.
SUBARACHNOID VS PSEUDOSUBARACHNOID HEMORRHAGE
Subarachnoid hemorrhage typically begins with a “thunder-clap” headache (beginning suddenly and described by patients as “the worst headache ever.”) While not all patients have this presentation, if imaging suggests subarachnoid hemorrhage but the patient has atypical signs and symptoms (eg, other than headache), then pseudosubarachnoid hemorrhage should be considered.
Brain CT is one of the most reliable tools for diagnosing subarachnoid hemorrhage in the emergency department. Done within 6 hours of symptom onset, it has a sensitivity of 98.7% and a specificity of 99.9%.1 Magnetic resonance imaging can also visualize subarachnoid hemorrhage within the first 12 hours, typically as a hyperintensity in the subarachnoid space on fluid-attenuated inversion-recovery sequences2 and on susceptibility-weighted sequences.
Lumbar puncture is also an important diagnostic tool but carries a risk of brain herniation in patients with brain edema.
Pseudosubarachnoid hemorrhage is an artifact of CT imaging. It is rare, and its prevalence is unknown.3 However, it may be seen in up to 20% of patients after resuscitation, as a result of diffuse cerebral edema that lowers the attenuation of brain tissue on CT, making the vessels relatively conspicuous. It can also be seen in purulent meningitis (due to proteinaceous influx after blood-brain barrier disruption),4 in meningeal leukemia (due to increased cellularity in the leptomeninges), and in severe polycythemia (from a higher concentration of blood and hemoglobin in the vessels).3,5–7
Although the level of attenuation on CT may help distinguish subarachnoid from pseudosubarachnoid hemorrhage, its accuracy has not been defined. Inspecting the CT images may clarify whether areas with high attenuation look like blood vessels vs subarachnoid hemorrhage.
Our patient recovered and had an uneventful follow-up. The cause of his elevated hematocrit was likely chronic hypoxia from COPD.
Acknowledgment: We thank Dr. Saeide Khanbagi and Dr. Azade Nasr-lari for their cooperation.
- Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke 2016; 47:750–755.
- Sohn CH, Baik SK, Lee HJ, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol 2005; 26:662–665.
- Yuzawa H, Higano S, Mugikura S, et al. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol 2008; 29:1544–1549.
- Given CA 2nd, Burdette JH, Elster AD, Williams DW 3rd. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol 2003; 24:254–256.
- Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol 1998; 28:222–225.
- Ben Salem D, Osseby GV, Rezaizadeh-Bourdariat K, et al. Spontaneous hyperdense intracranial vessels seen on CT scan in polycythemia cases. J Radiol 2003; 84:605–608. French.
- Hsieh SW, Khor GT, Chen CN, Huang P. Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med 2012; 42:e109–e111.
- Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke 2016; 47:750–755.
- Sohn CH, Baik SK, Lee HJ, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol 2005; 26:662–665.
- Yuzawa H, Higano S, Mugikura S, et al. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol 2008; 29:1544–1549.
- Given CA 2nd, Burdette JH, Elster AD, Williams DW 3rd. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol 2003; 24:254–256.
- Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol 1998; 28:222–225.
- Ben Salem D, Osseby GV, Rezaizadeh-Bourdariat K, et al. Spontaneous hyperdense intracranial vessels seen on CT scan in polycythemia cases. J Radiol 2003; 84:605–608. French.
- Hsieh SW, Khor GT, Chen CN, Huang P. Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med 2012; 42:e109–e111.
Fall risk and anticoagulation for atrial fibrillation in the elderly: A delicate balance
An 86-year-old woman with hypertension, osteoporosis, and mild cognitive impairment presents with episodes of palpitations and heart “fluttering.” These episodes occur 1 to 2 times per week, last for up to several hours, and are associated with mild shortness of breath and reduced activity tolerance. She is widowed and lives in a retirement facility, but she is independent in activities of daily living. She has fallen twice in the past year without significant injury.
Physical examination is unremarkable. An electrocardiogram demonstrates sinus rhythm with left ventricular hypertrophy. A 30-day event monitor reveals several episodes of paroxysmal atrial fibrillation that correspond with her symptoms. A subsequent echocardiogram shows normal left ventricular systolic function, mild diastolic dysfunction, and no significant valvular abnormalities. Laboratory studies, including thyroid-stimulating hormone, are normal.
What is this patient’s risk of stroke? What is her risk of major bleeding from anticoagulation? How should fall risk be addressed in the decision-making process? What other factors should be considered?
AGE, ATRIAL FIBRILLATION, AND STROKE RISK
The prevalence of atrial fibrillation increases with age, and nearly half of patients with atrial fibrillation in the United States are 75 or older.1 In addition, older age is an independent risk factor for stroke in patients with atrial fibrillation, and the proportion of strokes attributable to atrial fibrillation increases exponentially with age:
- 1.5% at age 50 to 59
- 2.8% at age 60 to 69
- 9.9% at age 70 to 79
- 23.5% at age 80 to 89.2
Numerous large randomized trials have shown that anticoagulation with warfarin reduces the risk of stroke by about two-thirds in patients with atrial fibrillation, and that this benefit extends to the elderly.
In the Birmingham Atrial Fibrillation Treatment of the Aged trial,3 973 patients at least 75 years old (mean age 81.5, 55% male) were randomized to receive either warfarin with a target international normalized ratio of 2.0 to 3.0 or aspirin 75 mg/day. Over an average follow-up of 2.7 years, the composite outcome of fatal or disabling stroke, arterial embolism, or intracranial hemorrhage occurred in 24 (4.9%) of the 488 patients in the warfarin group and 48 (9.9%) of the 485 patients in the aspirin group (absolute yearly risk reduction 2%, 95% confidence interval 0.7–3.2, number needed to treat 50 for 1 year). Importantly, the benefit of warfarin was similar in men and women, and in patients ages 75 to 79, 80 to 84, and 85 and older.
More recently, the oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban have been shown to be at least as effective as warfarin with respect to both stroke prevention and major bleeding complications, and subgroup analyses have confirmed similar outcomes in older and younger patients.4,5
But despite the proven value of anticoagulation for stroke prevention in older adults, only 40% to 60% of older patients who are suitable candidates for anticoagulation actually receive it.6 Moreover, the proportion of patients who are treated declines progressively with age. The most frequently cited reason for nontreatment is perception of a high risk of falls and associated concerns about bleeding, especially intracranial hemorrhage.7–10
BALANCING STROKE RISK VS BLEEDING RISK
Balancing the risk of stroke against the risk of bleeding related to falls is a commonly encountered conundrum in older patients with atrial fibrillation.
Stroke risk
The CHADS2 score was, until recently, the most widely used method for assessing stroke risk in patients with nonvalvular atrial fibrillation. CHADS2 assigns 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes, and 2 points for prior stroke or transient ischemic attack (range 0–6 points). Annual stroke risk based on the CHADS2 score ranges from about 2% to about 18%
(Table 1).11
The CHA2DS2-VASc score,12 a modification of CHADS2, appears to assess the risk of stroke more accurately, especially at the lower end of the scale, and recent guidelines for managing atrial fibrillation recommend using the CHA2DS2-VASc algorithm.13 CHA2DS2-VASc is similar to CHADS2, except that it assigns 1 point for ages 65 to 74, 2 points for ages 75 and older, 1 point for vascular disease (coronary artery disease, peripheral arterial disease, aortic aneurysm), and 1 point for female sex (Table 1).11,12
For both CHADS2 and CHA2DS2-VASc, systemic anticoagulation is recommended for patients who have a score of 2 or higher. Our patient’s CHADS2 score is 2, and her CHA2DS2-VASc score is 4, corresponding to an annual estimated stroke risk of 4% with both scores (Table 1). Note, however, that the CHA2DS2-VASc score provides more information at the lower end of the spectrum.
Bleeding risk
Several scoring systems for assessing bleeding risk have also been developed (Table 2).14–16 Of these, the HAS-BLED score has come to be used more widely in recent years.
Perhaps not surprisingly, some of the same factors associated with risk of stroke also predict increased risk of bleeding (eg, older age, hypertension, prior stroke).14 Note, however, that history of falling or high risk of falling is only included in one of the bleeding risk models (HEMORR2HAGES).15
These tools are somewhat limited by their lack of consideration of concomitant antiplatelet therapy (only included in HAS-BLED) or history of bleeding (only ATRIA16 considers major and minor bleeding, HEMORR2HAGES does not specify bleeding severity, and HAS-BLED only considers major bleeding). The models also fail to include medications such as antibiotics or antiarrhythmic agents, which are commonly used by older patients with atrial fibrillation and may increase bleeding risk. In addition, all bleeding risk scores were developed for warfarin, and their applicability to patients treated with the newer oral anticoagulants has not been established.
At the time of presentation, our patient has a HAS-BLED score of 2 (1 point each for age and hypertension), placing her at intermediate risk of bleeding.14
Fear the clot, not the bleed
So how does one balance the risk of stroke vs the risk of bleeding? An adage from the early days of thrombolytic therapy for acute myocardial infarction was “fear the clot, not the bleed.” In other words, in the present context the consequences of a thrombus embolizing from the heart to the brain are likely to be more devastating and more permanent than the consequences of anticoagulation-associated hemorrhage.
Support for this view is underscored by a 2015 study by Lip et al,17 who examined stroke and bleeding risks and outcomes in a large real-world population of patients age 75 and older. The analysis included 819 patients ages 85 to 89 and 386 patients age 90 and older. The key finding was that the oldest patients derived the greatest net benefit from anticoagulation.
Moreover, the Canadian stroke registry of 3,197 patients, mean age 79, showed that advanced age was a more potent risk factor for ischemic stroke than it was for hemorrhagic stroke.18
Thus, the benefit from anticoagulation in patients with atrial fibrillation does not appear to have an upper age limit.
FALLS AND ANTICOAGULATION
Falls are an important source of morbidity, disability, and activity curtailment in older adults and, like atrial fibrillation, the incidence and prevalence of falls increase with age. In community-dwelling adults age 65 and older, the overall proportion with at least 1 fall in the preceding year ranges from about 30% to 40%.19 However, the rate increases with age and exceeds 50% in nursing home residents.20
Although anticoagulation is associated with a higher risk of bleeding in patients who fall, the absolute risk is small.
In a study of older adults with nonvalvular atrial fibrillation, a history of falls or documented high risk of falling was associated with a risk of intracranial hemorrhage during follow-up that was 1.9 times higher.21 Importantly, however, this risk did not differ among patients treated with warfarin, aspirin, or no antithrombotic therapy. In this analysis, patients with a CHADS2 score of 2 or higher benefited from anticoagulation, whether or not they were considered to be at risk for falls.
In another study,22 it was estimated that an individual would have to fall 295 times in 1 year for the risk of fall-related major bleeding to outweigh the benefit of warfarin in reducing the risk of stroke.
Thus, based on available evidence, perception of a high risk of falling should not be construed as justification for withholding anticoagulation in older patients who are otherwise suitable candidates for such therapy.
AT WHAT POINT DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
Absolute contraindications to anticoagulation include an intracranial hemorrhage or neurosurgical procedure with high risk for bleeding within the past 30 days, an intracranial neoplasm or vascular abnormality with high risk of bleeding, recurrent life-threatening gastrointestinal or other bleeding events, and severe bleeding disorders, including severe thrombocytopenia.
In patients with atrial fibrillation at high risk of bleeding as assessed by one of the bleeding risk scores and relatively low risk of ischemic stroke, the risk of anticoagulation may outweigh the benefit, although no studies have specifically addressed this issue.
In patients with frequent falls, including injurious falls, the benefits of anticoagulation usually outweigh the risks of bleeding, but management should incorporate interventions designed to mitigate fall risk.
Finally, in patients with a poor prognosis approaching the end of life, the risks and burdens of anticoagulation may exceed the perceived benefits, in which case discontinuation of anticoagulation may be appropriate.
SHOULD OUR PATIENT RECEIVE ANTICOAGULATION?
As noted above, our patient has a high risk of stroke and a moderate risk of bleeding, and multiple lines of evidence indicate that the benefits of anticoagulation (ie, prevention of stroke and systemic embolization) substantially outweigh the risks of bleeding. Although she has a history of falls, which may seem to muddy the waters, this factor should not play a major role in decision-making. Moreover, her advanced age should, if anything, be considered a point in favor of anticoagulation. So from the scientific standpoint, anticoagulation is the clear winner.
A shared decision
But that is not the end of the story. Since there is tension between benefits and risks with either approach (ie, anticoagulation or no anticoagulation), it is important to discuss the issues and options with the patient and relevant caregivers. Most older adults have witnessed the ravages of stroke in a friend or relative, and a recent study showed that most would be willing to accept a modest risk of bleeding to prevent a stroke.23
However, this is ultimately a personal decision for each patient, and in accordance with the principle of patient autonomy, the patient’s expressed wishes should be honored by using a process of shared decision-making.
Which anticoagulant?
Finally, what about the choice of anticoagulation? The complexities of using warfarin, including its narrow therapeutic range and myriad interactions with other medications and foods, can make it a less appealing option for both patient and provider.
We recommend a novel oral anticoagulant as first-line therapy in the absence of contraindications such as severe renal insufficiency, and prefer apixaban because it is the only agent shown to be superior to warfarin with respect to both stroke prevention and bleeding risk.24
Important disadvantages of the novel oral anticoagulants include their higher cost and lack of an effective antidote in the event of clinically significant bleeding (with the exception of idarucizumab, which was recently approved for reversal of serious bleeding associated with dabigatran), issues that may be of particular concern to older adults. While there is no therapeutic range to monitor for the newer agents, more frequent monitoring for occult anemia may be needed.
Thus, selection of an anticoagulant should also be individualized through shared decision-making.
Is aspirin alone an alternative?
And what if the patient chooses to forgo anticoagulation? In that case, aspirin 75 to 325 mg/day may seem reasonable, but there is scant evidence that aspirin is beneficial for stroke prevention in patients with atrial fibrillation in this age group, and aspirin, too, is associated with an increased risk of bleeding.25
As a result, current US and European guidelines recommend a very limited role for aspirin as a single agent in the management of atrial fibrillation.26 The joint 2014 guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society give aspirin a class IIB recommendation (ie, it “may” be considered), level of evidence C (ie, very limited) for use as an alternative to no antithrombotic therapy or systemic anticoagulation only in patients with a CHA2DS2-VASc score of 1, thereby excluding all patients age 75 and older.13
In most cases, aspirin as sole prophylaxis against stroke in atrial fibrillation should be avoided in the absence of another indication for its use, such as coexisting coronary artery disease or peripheral arterial disease.
A COMPLEX DECISION
In summary, the decisions surrounding anticoagulation of elderly patients with nonvalvular atrial fibrillation are complex. Accurate assessment of stroke risk is key, and although bleeding risk is also an essential consideration, it is important not to overemphasize bleeding and fall risks in the decision-making process.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Mant J, Hobbs FD, Fletcher K, et al; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurology 2013; 70:1486–1490.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Rich MW. Atrial fibrillation in long term care. J Am Med Dir Assoc 2012; 13:688–691.
- McCrory DC, Matchar DB, Samsa G, Sanders LL, Pritchett EL. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med 1995; 155:277–281.
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011; 40:675–683.
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 2011; 161:241–246.
- Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc 2015; 63:71–76.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥ 75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke 2015; 46:143–150.
- McGrath ER, Kapral MK, Fang J, et al; Investigators of the Registry of the Canadian Stroke Network. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 2012; 43:2048–2054.
- Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am 2015; 99:281–293.
- Deandrea S, Bravi F, Turati F, Lucenteforte E, La Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr 2013; 56:407–415.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Riva N, Smith DE, Lip GY, Lane DA. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age Ageing 2011; 40:653–655.
- De Caterina R, Andersson U, Alexander JH, et al; ARISTOTLE Investigators. History of bleeding and outcomes with apixaban versus warfarin in patients with atrial fibrillation in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2016; 175:175–183.
- Ben Freedman S, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015; 36:653–656.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
An 86-year-old woman with hypertension, osteoporosis, and mild cognitive impairment presents with episodes of palpitations and heart “fluttering.” These episodes occur 1 to 2 times per week, last for up to several hours, and are associated with mild shortness of breath and reduced activity tolerance. She is widowed and lives in a retirement facility, but she is independent in activities of daily living. She has fallen twice in the past year without significant injury.
Physical examination is unremarkable. An electrocardiogram demonstrates sinus rhythm with left ventricular hypertrophy. A 30-day event monitor reveals several episodes of paroxysmal atrial fibrillation that correspond with her symptoms. A subsequent echocardiogram shows normal left ventricular systolic function, mild diastolic dysfunction, and no significant valvular abnormalities. Laboratory studies, including thyroid-stimulating hormone, are normal.
What is this patient’s risk of stroke? What is her risk of major bleeding from anticoagulation? How should fall risk be addressed in the decision-making process? What other factors should be considered?
AGE, ATRIAL FIBRILLATION, AND STROKE RISK
The prevalence of atrial fibrillation increases with age, and nearly half of patients with atrial fibrillation in the United States are 75 or older.1 In addition, older age is an independent risk factor for stroke in patients with atrial fibrillation, and the proportion of strokes attributable to atrial fibrillation increases exponentially with age:
- 1.5% at age 50 to 59
- 2.8% at age 60 to 69
- 9.9% at age 70 to 79
- 23.5% at age 80 to 89.2
Numerous large randomized trials have shown that anticoagulation with warfarin reduces the risk of stroke by about two-thirds in patients with atrial fibrillation, and that this benefit extends to the elderly.
In the Birmingham Atrial Fibrillation Treatment of the Aged trial,3 973 patients at least 75 years old (mean age 81.5, 55% male) were randomized to receive either warfarin with a target international normalized ratio of 2.0 to 3.0 or aspirin 75 mg/day. Over an average follow-up of 2.7 years, the composite outcome of fatal or disabling stroke, arterial embolism, or intracranial hemorrhage occurred in 24 (4.9%) of the 488 patients in the warfarin group and 48 (9.9%) of the 485 patients in the aspirin group (absolute yearly risk reduction 2%, 95% confidence interval 0.7–3.2, number needed to treat 50 for 1 year). Importantly, the benefit of warfarin was similar in men and women, and in patients ages 75 to 79, 80 to 84, and 85 and older.
More recently, the oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban have been shown to be at least as effective as warfarin with respect to both stroke prevention and major bleeding complications, and subgroup analyses have confirmed similar outcomes in older and younger patients.4,5
But despite the proven value of anticoagulation for stroke prevention in older adults, only 40% to 60% of older patients who are suitable candidates for anticoagulation actually receive it.6 Moreover, the proportion of patients who are treated declines progressively with age. The most frequently cited reason for nontreatment is perception of a high risk of falls and associated concerns about bleeding, especially intracranial hemorrhage.7–10
BALANCING STROKE RISK VS BLEEDING RISK
Balancing the risk of stroke against the risk of bleeding related to falls is a commonly encountered conundrum in older patients with atrial fibrillation.
Stroke risk
The CHADS2 score was, until recently, the most widely used method for assessing stroke risk in patients with nonvalvular atrial fibrillation. CHADS2 assigns 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes, and 2 points for prior stroke or transient ischemic attack (range 0–6 points). Annual stroke risk based on the CHADS2 score ranges from about 2% to about 18%
(Table 1).11
The CHA2DS2-VASc score,12 a modification of CHADS2, appears to assess the risk of stroke more accurately, especially at the lower end of the scale, and recent guidelines for managing atrial fibrillation recommend using the CHA2DS2-VASc algorithm.13 CHA2DS2-VASc is similar to CHADS2, except that it assigns 1 point for ages 65 to 74, 2 points for ages 75 and older, 1 point for vascular disease (coronary artery disease, peripheral arterial disease, aortic aneurysm), and 1 point for female sex (Table 1).11,12
For both CHADS2 and CHA2DS2-VASc, systemic anticoagulation is recommended for patients who have a score of 2 or higher. Our patient’s CHADS2 score is 2, and her CHA2DS2-VASc score is 4, corresponding to an annual estimated stroke risk of 4% with both scores (Table 1). Note, however, that the CHA2DS2-VASc score provides more information at the lower end of the spectrum.
Bleeding risk
Several scoring systems for assessing bleeding risk have also been developed (Table 2).14–16 Of these, the HAS-BLED score has come to be used more widely in recent years.
Perhaps not surprisingly, some of the same factors associated with risk of stroke also predict increased risk of bleeding (eg, older age, hypertension, prior stroke).14 Note, however, that history of falling or high risk of falling is only included in one of the bleeding risk models (HEMORR2HAGES).15
These tools are somewhat limited by their lack of consideration of concomitant antiplatelet therapy (only included in HAS-BLED) or history of bleeding (only ATRIA16 considers major and minor bleeding, HEMORR2HAGES does not specify bleeding severity, and HAS-BLED only considers major bleeding). The models also fail to include medications such as antibiotics or antiarrhythmic agents, which are commonly used by older patients with atrial fibrillation and may increase bleeding risk. In addition, all bleeding risk scores were developed for warfarin, and their applicability to patients treated with the newer oral anticoagulants has not been established.
At the time of presentation, our patient has a HAS-BLED score of 2 (1 point each for age and hypertension), placing her at intermediate risk of bleeding.14
Fear the clot, not the bleed
So how does one balance the risk of stroke vs the risk of bleeding? An adage from the early days of thrombolytic therapy for acute myocardial infarction was “fear the clot, not the bleed.” In other words, in the present context the consequences of a thrombus embolizing from the heart to the brain are likely to be more devastating and more permanent than the consequences of anticoagulation-associated hemorrhage.
Support for this view is underscored by a 2015 study by Lip et al,17 who examined stroke and bleeding risks and outcomes in a large real-world population of patients age 75 and older. The analysis included 819 patients ages 85 to 89 and 386 patients age 90 and older. The key finding was that the oldest patients derived the greatest net benefit from anticoagulation.
Moreover, the Canadian stroke registry of 3,197 patients, mean age 79, showed that advanced age was a more potent risk factor for ischemic stroke than it was for hemorrhagic stroke.18
Thus, the benefit from anticoagulation in patients with atrial fibrillation does not appear to have an upper age limit.
FALLS AND ANTICOAGULATION
Falls are an important source of morbidity, disability, and activity curtailment in older adults and, like atrial fibrillation, the incidence and prevalence of falls increase with age. In community-dwelling adults age 65 and older, the overall proportion with at least 1 fall in the preceding year ranges from about 30% to 40%.19 However, the rate increases with age and exceeds 50% in nursing home residents.20
Although anticoagulation is associated with a higher risk of bleeding in patients who fall, the absolute risk is small.
In a study of older adults with nonvalvular atrial fibrillation, a history of falls or documented high risk of falling was associated with a risk of intracranial hemorrhage during follow-up that was 1.9 times higher.21 Importantly, however, this risk did not differ among patients treated with warfarin, aspirin, or no antithrombotic therapy. In this analysis, patients with a CHADS2 score of 2 or higher benefited from anticoagulation, whether or not they were considered to be at risk for falls.
In another study,22 it was estimated that an individual would have to fall 295 times in 1 year for the risk of fall-related major bleeding to outweigh the benefit of warfarin in reducing the risk of stroke.
Thus, based on available evidence, perception of a high risk of falling should not be construed as justification for withholding anticoagulation in older patients who are otherwise suitable candidates for such therapy.
AT WHAT POINT DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
Absolute contraindications to anticoagulation include an intracranial hemorrhage or neurosurgical procedure with high risk for bleeding within the past 30 days, an intracranial neoplasm or vascular abnormality with high risk of bleeding, recurrent life-threatening gastrointestinal or other bleeding events, and severe bleeding disorders, including severe thrombocytopenia.
In patients with atrial fibrillation at high risk of bleeding as assessed by one of the bleeding risk scores and relatively low risk of ischemic stroke, the risk of anticoagulation may outweigh the benefit, although no studies have specifically addressed this issue.
In patients with frequent falls, including injurious falls, the benefits of anticoagulation usually outweigh the risks of bleeding, but management should incorporate interventions designed to mitigate fall risk.
Finally, in patients with a poor prognosis approaching the end of life, the risks and burdens of anticoagulation may exceed the perceived benefits, in which case discontinuation of anticoagulation may be appropriate.
SHOULD OUR PATIENT RECEIVE ANTICOAGULATION?
As noted above, our patient has a high risk of stroke and a moderate risk of bleeding, and multiple lines of evidence indicate that the benefits of anticoagulation (ie, prevention of stroke and systemic embolization) substantially outweigh the risks of bleeding. Although she has a history of falls, which may seem to muddy the waters, this factor should not play a major role in decision-making. Moreover, her advanced age should, if anything, be considered a point in favor of anticoagulation. So from the scientific standpoint, anticoagulation is the clear winner.
A shared decision
But that is not the end of the story. Since there is tension between benefits and risks with either approach (ie, anticoagulation or no anticoagulation), it is important to discuss the issues and options with the patient and relevant caregivers. Most older adults have witnessed the ravages of stroke in a friend or relative, and a recent study showed that most would be willing to accept a modest risk of bleeding to prevent a stroke.23
However, this is ultimately a personal decision for each patient, and in accordance with the principle of patient autonomy, the patient’s expressed wishes should be honored by using a process of shared decision-making.
Which anticoagulant?
Finally, what about the choice of anticoagulation? The complexities of using warfarin, including its narrow therapeutic range and myriad interactions with other medications and foods, can make it a less appealing option for both patient and provider.
We recommend a novel oral anticoagulant as first-line therapy in the absence of contraindications such as severe renal insufficiency, and prefer apixaban because it is the only agent shown to be superior to warfarin with respect to both stroke prevention and bleeding risk.24
Important disadvantages of the novel oral anticoagulants include their higher cost and lack of an effective antidote in the event of clinically significant bleeding (with the exception of idarucizumab, which was recently approved for reversal of serious bleeding associated with dabigatran), issues that may be of particular concern to older adults. While there is no therapeutic range to monitor for the newer agents, more frequent monitoring for occult anemia may be needed.
Thus, selection of an anticoagulant should also be individualized through shared decision-making.
Is aspirin alone an alternative?
And what if the patient chooses to forgo anticoagulation? In that case, aspirin 75 to 325 mg/day may seem reasonable, but there is scant evidence that aspirin is beneficial for stroke prevention in patients with atrial fibrillation in this age group, and aspirin, too, is associated with an increased risk of bleeding.25
As a result, current US and European guidelines recommend a very limited role for aspirin as a single agent in the management of atrial fibrillation.26 The joint 2014 guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society give aspirin a class IIB recommendation (ie, it “may” be considered), level of evidence C (ie, very limited) for use as an alternative to no antithrombotic therapy or systemic anticoagulation only in patients with a CHA2DS2-VASc score of 1, thereby excluding all patients age 75 and older.13
In most cases, aspirin as sole prophylaxis against stroke in atrial fibrillation should be avoided in the absence of another indication for its use, such as coexisting coronary artery disease or peripheral arterial disease.
A COMPLEX DECISION
In summary, the decisions surrounding anticoagulation of elderly patients with nonvalvular atrial fibrillation are complex. Accurate assessment of stroke risk is key, and although bleeding risk is also an essential consideration, it is important not to overemphasize bleeding and fall risks in the decision-making process.
An 86-year-old woman with hypertension, osteoporosis, and mild cognitive impairment presents with episodes of palpitations and heart “fluttering.” These episodes occur 1 to 2 times per week, last for up to several hours, and are associated with mild shortness of breath and reduced activity tolerance. She is widowed and lives in a retirement facility, but she is independent in activities of daily living. She has fallen twice in the past year without significant injury.
Physical examination is unremarkable. An electrocardiogram demonstrates sinus rhythm with left ventricular hypertrophy. A 30-day event monitor reveals several episodes of paroxysmal atrial fibrillation that correspond with her symptoms. A subsequent echocardiogram shows normal left ventricular systolic function, mild diastolic dysfunction, and no significant valvular abnormalities. Laboratory studies, including thyroid-stimulating hormone, are normal.
What is this patient’s risk of stroke? What is her risk of major bleeding from anticoagulation? How should fall risk be addressed in the decision-making process? What other factors should be considered?
AGE, ATRIAL FIBRILLATION, AND STROKE RISK
The prevalence of atrial fibrillation increases with age, and nearly half of patients with atrial fibrillation in the United States are 75 or older.1 In addition, older age is an independent risk factor for stroke in patients with atrial fibrillation, and the proportion of strokes attributable to atrial fibrillation increases exponentially with age:
- 1.5% at age 50 to 59
- 2.8% at age 60 to 69
- 9.9% at age 70 to 79
- 23.5% at age 80 to 89.2
Numerous large randomized trials have shown that anticoagulation with warfarin reduces the risk of stroke by about two-thirds in patients with atrial fibrillation, and that this benefit extends to the elderly.
In the Birmingham Atrial Fibrillation Treatment of the Aged trial,3 973 patients at least 75 years old (mean age 81.5, 55% male) were randomized to receive either warfarin with a target international normalized ratio of 2.0 to 3.0 or aspirin 75 mg/day. Over an average follow-up of 2.7 years, the composite outcome of fatal or disabling stroke, arterial embolism, or intracranial hemorrhage occurred in 24 (4.9%) of the 488 patients in the warfarin group and 48 (9.9%) of the 485 patients in the aspirin group (absolute yearly risk reduction 2%, 95% confidence interval 0.7–3.2, number needed to treat 50 for 1 year). Importantly, the benefit of warfarin was similar in men and women, and in patients ages 75 to 79, 80 to 84, and 85 and older.
More recently, the oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban have been shown to be at least as effective as warfarin with respect to both stroke prevention and major bleeding complications, and subgroup analyses have confirmed similar outcomes in older and younger patients.4,5
But despite the proven value of anticoagulation for stroke prevention in older adults, only 40% to 60% of older patients who are suitable candidates for anticoagulation actually receive it.6 Moreover, the proportion of patients who are treated declines progressively with age. The most frequently cited reason for nontreatment is perception of a high risk of falls and associated concerns about bleeding, especially intracranial hemorrhage.7–10
BALANCING STROKE RISK VS BLEEDING RISK
Balancing the risk of stroke against the risk of bleeding related to falls is a commonly encountered conundrum in older patients with atrial fibrillation.
Stroke risk
The CHADS2 score was, until recently, the most widely used method for assessing stroke risk in patients with nonvalvular atrial fibrillation. CHADS2 assigns 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes, and 2 points for prior stroke or transient ischemic attack (range 0–6 points). Annual stroke risk based on the CHADS2 score ranges from about 2% to about 18%
(Table 1).11
The CHA2DS2-VASc score,12 a modification of CHADS2, appears to assess the risk of stroke more accurately, especially at the lower end of the scale, and recent guidelines for managing atrial fibrillation recommend using the CHA2DS2-VASc algorithm.13 CHA2DS2-VASc is similar to CHADS2, except that it assigns 1 point for ages 65 to 74, 2 points for ages 75 and older, 1 point for vascular disease (coronary artery disease, peripheral arterial disease, aortic aneurysm), and 1 point for female sex (Table 1).11,12
For both CHADS2 and CHA2DS2-VASc, systemic anticoagulation is recommended for patients who have a score of 2 or higher. Our patient’s CHADS2 score is 2, and her CHA2DS2-VASc score is 4, corresponding to an annual estimated stroke risk of 4% with both scores (Table 1). Note, however, that the CHA2DS2-VASc score provides more information at the lower end of the spectrum.
Bleeding risk
Several scoring systems for assessing bleeding risk have also been developed (Table 2).14–16 Of these, the HAS-BLED score has come to be used more widely in recent years.
Perhaps not surprisingly, some of the same factors associated with risk of stroke also predict increased risk of bleeding (eg, older age, hypertension, prior stroke).14 Note, however, that history of falling or high risk of falling is only included in one of the bleeding risk models (HEMORR2HAGES).15
These tools are somewhat limited by their lack of consideration of concomitant antiplatelet therapy (only included in HAS-BLED) or history of bleeding (only ATRIA16 considers major and minor bleeding, HEMORR2HAGES does not specify bleeding severity, and HAS-BLED only considers major bleeding). The models also fail to include medications such as antibiotics or antiarrhythmic agents, which are commonly used by older patients with atrial fibrillation and may increase bleeding risk. In addition, all bleeding risk scores were developed for warfarin, and their applicability to patients treated with the newer oral anticoagulants has not been established.
At the time of presentation, our patient has a HAS-BLED score of 2 (1 point each for age and hypertension), placing her at intermediate risk of bleeding.14
Fear the clot, not the bleed
So how does one balance the risk of stroke vs the risk of bleeding? An adage from the early days of thrombolytic therapy for acute myocardial infarction was “fear the clot, not the bleed.” In other words, in the present context the consequences of a thrombus embolizing from the heart to the brain are likely to be more devastating and more permanent than the consequences of anticoagulation-associated hemorrhage.
Support for this view is underscored by a 2015 study by Lip et al,17 who examined stroke and bleeding risks and outcomes in a large real-world population of patients age 75 and older. The analysis included 819 patients ages 85 to 89 and 386 patients age 90 and older. The key finding was that the oldest patients derived the greatest net benefit from anticoagulation.
Moreover, the Canadian stroke registry of 3,197 patients, mean age 79, showed that advanced age was a more potent risk factor for ischemic stroke than it was for hemorrhagic stroke.18
Thus, the benefit from anticoagulation in patients with atrial fibrillation does not appear to have an upper age limit.
FALLS AND ANTICOAGULATION
Falls are an important source of morbidity, disability, and activity curtailment in older adults and, like atrial fibrillation, the incidence and prevalence of falls increase with age. In community-dwelling adults age 65 and older, the overall proportion with at least 1 fall in the preceding year ranges from about 30% to 40%.19 However, the rate increases with age and exceeds 50% in nursing home residents.20
Although anticoagulation is associated with a higher risk of bleeding in patients who fall, the absolute risk is small.
In a study of older adults with nonvalvular atrial fibrillation, a history of falls or documented high risk of falling was associated with a risk of intracranial hemorrhage during follow-up that was 1.9 times higher.21 Importantly, however, this risk did not differ among patients treated with warfarin, aspirin, or no antithrombotic therapy. In this analysis, patients with a CHADS2 score of 2 or higher benefited from anticoagulation, whether or not they were considered to be at risk for falls.
In another study,22 it was estimated that an individual would have to fall 295 times in 1 year for the risk of fall-related major bleeding to outweigh the benefit of warfarin in reducing the risk of stroke.
Thus, based on available evidence, perception of a high risk of falling should not be construed as justification for withholding anticoagulation in older patients who are otherwise suitable candidates for such therapy.
AT WHAT POINT DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
Absolute contraindications to anticoagulation include an intracranial hemorrhage or neurosurgical procedure with high risk for bleeding within the past 30 days, an intracranial neoplasm or vascular abnormality with high risk of bleeding, recurrent life-threatening gastrointestinal or other bleeding events, and severe bleeding disorders, including severe thrombocytopenia.
In patients with atrial fibrillation at high risk of bleeding as assessed by one of the bleeding risk scores and relatively low risk of ischemic stroke, the risk of anticoagulation may outweigh the benefit, although no studies have specifically addressed this issue.
In patients with frequent falls, including injurious falls, the benefits of anticoagulation usually outweigh the risks of bleeding, but management should incorporate interventions designed to mitigate fall risk.
Finally, in patients with a poor prognosis approaching the end of life, the risks and burdens of anticoagulation may exceed the perceived benefits, in which case discontinuation of anticoagulation may be appropriate.
SHOULD OUR PATIENT RECEIVE ANTICOAGULATION?
As noted above, our patient has a high risk of stroke and a moderate risk of bleeding, and multiple lines of evidence indicate that the benefits of anticoagulation (ie, prevention of stroke and systemic embolization) substantially outweigh the risks of bleeding. Although she has a history of falls, which may seem to muddy the waters, this factor should not play a major role in decision-making. Moreover, her advanced age should, if anything, be considered a point in favor of anticoagulation. So from the scientific standpoint, anticoagulation is the clear winner.
A shared decision
But that is not the end of the story. Since there is tension between benefits and risks with either approach (ie, anticoagulation or no anticoagulation), it is important to discuss the issues and options with the patient and relevant caregivers. Most older adults have witnessed the ravages of stroke in a friend or relative, and a recent study showed that most would be willing to accept a modest risk of bleeding to prevent a stroke.23
However, this is ultimately a personal decision for each patient, and in accordance with the principle of patient autonomy, the patient’s expressed wishes should be honored by using a process of shared decision-making.
Which anticoagulant?
Finally, what about the choice of anticoagulation? The complexities of using warfarin, including its narrow therapeutic range and myriad interactions with other medications and foods, can make it a less appealing option for both patient and provider.
We recommend a novel oral anticoagulant as first-line therapy in the absence of contraindications such as severe renal insufficiency, and prefer apixaban because it is the only agent shown to be superior to warfarin with respect to both stroke prevention and bleeding risk.24
Important disadvantages of the novel oral anticoagulants include their higher cost and lack of an effective antidote in the event of clinically significant bleeding (with the exception of idarucizumab, which was recently approved for reversal of serious bleeding associated with dabigatran), issues that may be of particular concern to older adults. While there is no therapeutic range to monitor for the newer agents, more frequent monitoring for occult anemia may be needed.
Thus, selection of an anticoagulant should also be individualized through shared decision-making.
Is aspirin alone an alternative?
And what if the patient chooses to forgo anticoagulation? In that case, aspirin 75 to 325 mg/day may seem reasonable, but there is scant evidence that aspirin is beneficial for stroke prevention in patients with atrial fibrillation in this age group, and aspirin, too, is associated with an increased risk of bleeding.25
As a result, current US and European guidelines recommend a very limited role for aspirin as a single agent in the management of atrial fibrillation.26 The joint 2014 guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society give aspirin a class IIB recommendation (ie, it “may” be considered), level of evidence C (ie, very limited) for use as an alternative to no antithrombotic therapy or systemic anticoagulation only in patients with a CHA2DS2-VASc score of 1, thereby excluding all patients age 75 and older.13
In most cases, aspirin as sole prophylaxis against stroke in atrial fibrillation should be avoided in the absence of another indication for its use, such as coexisting coronary artery disease or peripheral arterial disease.
A COMPLEX DECISION
In summary, the decisions surrounding anticoagulation of elderly patients with nonvalvular atrial fibrillation are complex. Accurate assessment of stroke risk is key, and although bleeding risk is also an essential consideration, it is important not to overemphasize bleeding and fall risks in the decision-making process.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Mant J, Hobbs FD, Fletcher K, et al; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurology 2013; 70:1486–1490.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Rich MW. Atrial fibrillation in long term care. J Am Med Dir Assoc 2012; 13:688–691.
- McCrory DC, Matchar DB, Samsa G, Sanders LL, Pritchett EL. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med 1995; 155:277–281.
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011; 40:675–683.
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 2011; 161:241–246.
- Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc 2015; 63:71–76.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥ 75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke 2015; 46:143–150.
- McGrath ER, Kapral MK, Fang J, et al; Investigators of the Registry of the Canadian Stroke Network. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 2012; 43:2048–2054.
- Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am 2015; 99:281–293.
- Deandrea S, Bravi F, Turati F, Lucenteforte E, La Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr 2013; 56:407–415.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Riva N, Smith DE, Lip GY, Lane DA. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age Ageing 2011; 40:653–655.
- De Caterina R, Andersson U, Alexander JH, et al; ARISTOTLE Investigators. History of bleeding and outcomes with apixaban versus warfarin in patients with atrial fibrillation in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2016; 175:175–183.
- Ben Freedman S, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015; 36:653–656.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Mant J, Hobbs FD, Fletcher K, et al; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurology 2013; 70:1486–1490.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Rich MW. Atrial fibrillation in long term care. J Am Med Dir Assoc 2012; 13:688–691.
- McCrory DC, Matchar DB, Samsa G, Sanders LL, Pritchett EL. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med 1995; 155:277–281.
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011; 40:675–683.
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 2011; 161:241–246.
- Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc 2015; 63:71–76.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥ 75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke 2015; 46:143–150.
- McGrath ER, Kapral MK, Fang J, et al; Investigators of the Registry of the Canadian Stroke Network. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 2012; 43:2048–2054.
- Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am 2015; 99:281–293.
- Deandrea S, Bravi F, Turati F, Lucenteforte E, La Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr 2013; 56:407–415.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Riva N, Smith DE, Lip GY, Lane DA. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age Ageing 2011; 40:653–655.
- De Caterina R, Andersson U, Alexander JH, et al; ARISTOTLE Investigators. History of bleeding and outcomes with apixaban versus warfarin in patients with atrial fibrillation in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2016; 175:175–183.
- Ben Freedman S, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015; 36:653–656.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
KEY POINTS
- For most patients in this category, the benefits of anticoagulation outweigh the risks.
- Although they are not perfect, scoring systems have been developed to predict the risk of stroke without anticoagulation and the risk of bleeding with anticoagulation.
- The decision-making process is complex and should be shared with the patient and the patient’s family and caregivers.
Covered-stent TIPS tops large-volume paracentesis for cirrhosis survival
One-year survival without liver transplant was far more likely when transjugular intrahepatic portosystemic shunts (TIPS) with covered stents were used to treat cirrhosis with recurrent ascites, instead of ongoing large-volume paracenteses with albumin, in a 62-patient randomized trial from France.
“TIPS with covered stents ... should therefore be preferred to LVP [large-volume paracenteses] with volume expansion... These findings support TIPS as the first-line intervention,” said investigators led by gastroenterologist Christophe Bureau, MD, of Toulouse (France) University in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2016.09.016).
All 62 patients had at least two LVPs prior to the study; 29 were then randomized to covered transjugular intrahepatic portosystemic shunt (TIPS), and 33 to LVP and albumin as needed. All the patients were on a low-salt diet.
Twenty-seven TIPS patients (93%) were alive without a liver transplant at 1 year, versus 17 (52%) in the LVP group (P = .003). TIPS patients had a total of 32 paracenteses in the first year, versus 320 in the LVP group. Six paracentesis patients (18%) had portal hypertension–related bleeding, and six had hernia-related complications; none of the TIPS patients had either. LVP patients spent a mean of 35 days in the hospital, versus 17 days for the TIPS group (P = .04). The probability of remaining free of encephalopathy at 1 year was the same in both groups, at 65%.
It has been shown before that TIPS has the edge on LVP for reducing recurrence of tense ascites. However, early studies used uncovered stents and, due to their almost 80% risk of dysfunction, they did not show a significant benefit for survival. As a result, repeated paracenteses have been recommended as first-line treatment, with TIPS held in reserve for patients who need very frequent LVP.
Polytetrafluoroethylene-covered stents appear to have changed the equation, “owing to a substantial decrease in the rate of shunt dysfunction,” the investigators said.
The French results are a bit better than previous reports of covered TIPS. “This could be related to greater experience with the TIPS procedure;” there were no technical failures. The study also mostly included patients younger than 65 years with Child-Pugh class B disease and no prior encephalopathy – favorable factors that also may have contributed to the results. However, “we believe that the use of covered stents was the main determinant of the observed improvement in outcomes... TIPS with uncovered stent[s] should not be considered effective or recommended any longer for the long-term treatment of” portal hypertension, they said.
Cirrhosis in the trial was due almost entirely to alcohol abuse. About three-quarters of both groups reported abstinence while enrolled. The mean age was 56 years, and the majority of subjects were men.
The work was funded by the French Ministry of Health and supported by Gore, maker of the covered stent used in the study. Dr. Bureau and another author are Gore consultants.
One-year survival without liver transplant was far more likely when transjugular intrahepatic portosystemic shunts (TIPS) with covered stents were used to treat cirrhosis with recurrent ascites, instead of ongoing large-volume paracenteses with albumin, in a 62-patient randomized trial from France.
“TIPS with covered stents ... should therefore be preferred to LVP [large-volume paracenteses] with volume expansion... These findings support TIPS as the first-line intervention,” said investigators led by gastroenterologist Christophe Bureau, MD, of Toulouse (France) University in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2016.09.016).
All 62 patients had at least two LVPs prior to the study; 29 were then randomized to covered transjugular intrahepatic portosystemic shunt (TIPS), and 33 to LVP and albumin as needed. All the patients were on a low-salt diet.
Twenty-seven TIPS patients (93%) were alive without a liver transplant at 1 year, versus 17 (52%) in the LVP group (P = .003). TIPS patients had a total of 32 paracenteses in the first year, versus 320 in the LVP group. Six paracentesis patients (18%) had portal hypertension–related bleeding, and six had hernia-related complications; none of the TIPS patients had either. LVP patients spent a mean of 35 days in the hospital, versus 17 days for the TIPS group (P = .04). The probability of remaining free of encephalopathy at 1 year was the same in both groups, at 65%.
It has been shown before that TIPS has the edge on LVP for reducing recurrence of tense ascites. However, early studies used uncovered stents and, due to their almost 80% risk of dysfunction, they did not show a significant benefit for survival. As a result, repeated paracenteses have been recommended as first-line treatment, with TIPS held in reserve for patients who need very frequent LVP.
Polytetrafluoroethylene-covered stents appear to have changed the equation, “owing to a substantial decrease in the rate of shunt dysfunction,” the investigators said.
The French results are a bit better than previous reports of covered TIPS. “This could be related to greater experience with the TIPS procedure;” there were no technical failures. The study also mostly included patients younger than 65 years with Child-Pugh class B disease and no prior encephalopathy – favorable factors that also may have contributed to the results. However, “we believe that the use of covered stents was the main determinant of the observed improvement in outcomes... TIPS with uncovered stent[s] should not be considered effective or recommended any longer for the long-term treatment of” portal hypertension, they said.
Cirrhosis in the trial was due almost entirely to alcohol abuse. About three-quarters of both groups reported abstinence while enrolled. The mean age was 56 years, and the majority of subjects were men.
The work was funded by the French Ministry of Health and supported by Gore, maker of the covered stent used in the study. Dr. Bureau and another author are Gore consultants.
One-year survival without liver transplant was far more likely when transjugular intrahepatic portosystemic shunts (TIPS) with covered stents were used to treat cirrhosis with recurrent ascites, instead of ongoing large-volume paracenteses with albumin, in a 62-patient randomized trial from France.
“TIPS with covered stents ... should therefore be preferred to LVP [large-volume paracenteses] with volume expansion... These findings support TIPS as the first-line intervention,” said investigators led by gastroenterologist Christophe Bureau, MD, of Toulouse (France) University in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2016.09.016).
All 62 patients had at least two LVPs prior to the study; 29 were then randomized to covered transjugular intrahepatic portosystemic shunt (TIPS), and 33 to LVP and albumin as needed. All the patients were on a low-salt diet.
Twenty-seven TIPS patients (93%) were alive without a liver transplant at 1 year, versus 17 (52%) in the LVP group (P = .003). TIPS patients had a total of 32 paracenteses in the first year, versus 320 in the LVP group. Six paracentesis patients (18%) had portal hypertension–related bleeding, and six had hernia-related complications; none of the TIPS patients had either. LVP patients spent a mean of 35 days in the hospital, versus 17 days for the TIPS group (P = .04). The probability of remaining free of encephalopathy at 1 year was the same in both groups, at 65%.
It has been shown before that TIPS has the edge on LVP for reducing recurrence of tense ascites. However, early studies used uncovered stents and, due to their almost 80% risk of dysfunction, they did not show a significant benefit for survival. As a result, repeated paracenteses have been recommended as first-line treatment, with TIPS held in reserve for patients who need very frequent LVP.
Polytetrafluoroethylene-covered stents appear to have changed the equation, “owing to a substantial decrease in the rate of shunt dysfunction,” the investigators said.
The French results are a bit better than previous reports of covered TIPS. “This could be related to greater experience with the TIPS procedure;” there were no technical failures. The study also mostly included patients younger than 65 years with Child-Pugh class B disease and no prior encephalopathy – favorable factors that also may have contributed to the results. However, “we believe that the use of covered stents was the main determinant of the observed improvement in outcomes... TIPS with uncovered stent[s] should not be considered effective or recommended any longer for the long-term treatment of” portal hypertension, they said.
Cirrhosis in the trial was due almost entirely to alcohol abuse. About three-quarters of both groups reported abstinence while enrolled. The mean age was 56 years, and the majority of subjects were men.
The work was funded by the French Ministry of Health and supported by Gore, maker of the covered stent used in the study. Dr. Bureau and another author are Gore consultants.
FROM GASTROENTEROLOGY
Key clinical point:
Major finding: Twenty-seven TIPS patients (93%) were alive without a liver transplant at 1 year, versus 17 (52%) in the LVP group (P = .003).
Data source: Randomized trial with 62 patients.
Disclosures: The work was funded by the French Ministry of Health and supported by Gore, maker of the covered stent used in the study. The lead and one other investigator are Gore consultants.
Do patients with submassive pulmonary embolism benefit from thrombolytic therapy?
For patients with submassive acute pulmonary embolism—the intermediate category between massive and low-risk—whether to give thrombolytic therapy is controversial. In general, patients with massive pulmonary embolism need this therapy, whereas those with low-risk pulmonary embolism do not—and neither do most of those with submassive embolism. But where should we draw the line?
More than 600,000 patients suffer pulmonary embolisms every year in the United States, and 50,000 to 200,000 people die of them.1–3 In various studies,4–6 within 1 year, 12.9% of patients had another pulmonary embolism, 7.3% developed chronic venous insufficiency, and 3.8% developed chronic thromboembolic pulmonary hypertension.
THREE CATEGORIES OF RISK
Episodes of acute pulmonary embolism are classified as low-risk (about 70% of cases), hemodynamically unstable or massive (5%), or submassive (25%).7,8
Low-risk acute pulmonary embolism is defined by the absence of right ventricular dysfunction and the absence of myocardial necrosis. The death rate in such cases is less than 1%.9 Its pharmacologic management includes parenteral anticoagulation and early initiation of long-term anticoagulation therapy, which the American College of Chest Physicians (ACCP) gives a grade IB recommendation (strong, based on moderate-quality evidence).10
Massive or hemodynamically unstable pulmonary embolism is characterized by any of the following, in the absence of other causes8:
- Sustained hypotension (systolic blood pressure < 90 mm Hg for ≥ 15 minutes)
- An absolute decrease in systolic blood pressure of 40 mm Hg or more
- Need for inotropic support
- Cardiac arrest
- Bradycardia (heart rate < 40 beats per minute).
The death rate is more than 30% in patients presenting with shock and approaches 70% in those presenting with cardiac arrest.11,12 Therefore, the consensus is that this category of pulmonary embolism merits aggressive treatment. Systemic thrombolytic therapy is recommended in those who are not at high risk of major bleeding, though the ACCP gives it only a grade 2C recommendation (weak, based on low-quality evidence).10
Submassive pulmonary embolism is defined by evidence of right ventricular dysfunction with normal blood pressure. According to the ACCP guidelines, thrombolytic therapy should be considered (grade 2C recommendation) for patients with acute pulmonary embolism without hypotension and with a low bleeding risk (with no renal failure and not on dual antiplatelet therapy), but at high risk of developing hypotension.10
DIAGNOSING SUBMASSIVE PULMONARY EMBOLISM, DELINEATING ITS SEVERITY
In managing acute pulmonary embolism, it is critical to recognize whether a patient is at high risk of clinical deterioration.
Blood pressure
The systolic blood pressure not only indicates whether the patient has hypotension (systolic blood pressure < 90 mm Hg) and therefore massive rather than submassive or low-risk pulmonary embolism; it is also important as a baseline value. A decrease in systolic blood pressure of 40 mm Hg or more is associated with worse outcomes.12
Right ventricular dysfunction
The physiologic response to occlusion of the pulmonary arteries can result in early myocardial injury and right ventricular dysfunction, which can be assessed by various methods (Table 1).
Electrocardiographic signs. Right heart strain may be recognized on electrocardiography as:
- Evidence of new complete or incomplete right bundle branch block
- T-wave inversion in the anterolateral leads V1 to V4
- S1Q3T3 (a large S wave in lead I, a Q wave in lead III, and an inverted T wave in lead III, the classic pattern of acute cor pulmonale).13
These findings add incremental prognostic value to echocardiographic findings in patients with submassive pulmonary embolism.14
Cardiac biomarkers such as B-type natriuretic peptide (BNP), N-terminal-pro-BNP (NT-pro-BNP), cardiac troponins, and heart-type fatty acid-binding protein (H-FABP) are also markers of right-sided myocardial damage and strain and can be used to identify patients with submassive pulmonary embolism.15 Abnormal levels of these substances are as follows:
- Troponin T greater than 0.1 ng/mL
- Troponin I greater than 0.4 ng/mL
- BNP greater than 90 pg/mL
- NT-pro-BNP greater than 500 pg/mL
- H-FABP less than 6 ng/mL.
These levels have prognostic value, identifying patients with submassive pulmonary embolism at risk of deterioration or death,14,16,17
Echocardiographic signs. Right ventricular dysfunction can be assessed quickly at the bedside with portable transthoracic echocardiography. A meta-analysis showed that close to 37% of hemodynamically stable patients with pulmonary embolism had echocardiographic evidence of right ventricular dysfunction on presentation and a higher short-term mortality rate.18 Evidence of right ventricular dysfunction includes the following:
- New-onset hypokinesis or akinesis
- Right ventricular dilation
- Right ventricular free-wall hypokinesis with apical sparing (the McConnell sign)
- Paradoxical movement of the interventricular septum
- Newly increased right ventricular systolic pressure
- Pulmonary hypertension, defined as tricuspid regurgitation jet velocity greater than 2.8 m/s.15,19
Computed tomographic (CT) angiography is widely available. Findings that have prognostic value in determining those at higher risk of death include the following20,21:
- A dilated right ventricle—ratio of right ventricle to left ventricle diameter (RV:LV ratio) greater than 0.9
- Interventricular septal bowing.
PESI and sPESI scores. The European Society of Cardiology 2014 guidelines stratify the risk in normotensive patients with pulmonary embolism according to their score on the Pulmonary Embolism Severity Index (PESI) or the simplified PESI (sPESI). There are five PESI classes. Those in PESI class III or IV or with an sPESI score of 1 or more (on a scale of 0 to 6) are considered at intermediate risk of clinical deterioration and are then further risk-stratified according to whether they have right ventricular dysfunction (based on echocardiography or computed tomography) and elevated cardiac biomarkers. These scoring systems are based on easily obtainable clinical information such as age, male sex, history of cancer, history of heart failure, history of chronic lung disease, heart rate, systolic blood pressure, respiratory rate, temperature, and altered mental status, and calculators are readily available.
Anticoagulation for all, plus thrombolysis for some
Patients with neither right ventricular dysfunction nor elevated cardiac biomarkers are at intermediate to low risk of clinical deterioration, and it is recommended that they be given anticoagulation therapy in an inpatient setting.
On the other hand, patients with both right ventricular dysfunction and elevated cardiac biomarkers are considered at intermediate to high risk of clinical deterioration; they should also be managed with anticoagulation and monitored closely for the need for rescue reperfusion therapy with thrombolytics.22
THROMBOLYTIC AGENTS
Thrombolytic agents are the cornerstone of management for patients presenting with pulmonary embolism who are at high risk. As noted above, these agents are recommended in massive pulmonary embolism, but their role in submassive pulmonary embolism remains controversial.
Thrombolytics work by activating endogenous plasminogen. The resulting plasmin promotes clot lysis, reducing the size of the thrombus, decreasing pulmonary vasculature resistance, and improving right ventricular function.23
To date, three thrombolytic agents have received US Food and Drug Administration approval for use in massive pulmonary embolism: alteplase, urokinase, and streptokinase. But only alteplase is still available in the United States. Alteplase is also the best tolerated, whereas streptokinase is highly antigenic and may cause further deterioration in an already unstable patient. Alteplase is also the most fibrin-specific and is considered the most potent of the three agents.24
Additional thrombolytic agents under investigation for use in acute pulmonary embolism include reteplase, tenecteplase, and desmoteplase. These agents are more fibrin-specific than alteplase. Reteplase is a second-generation recombinant tissue-type plasminogen activator with a quicker onset of action and longer half-life than alteplase, allowing for bolus dosing. Tenecteplase, a variant of alteplase, is cleared more slowly and is 14 times more fibrin-specific than alteplase, also allowing for bolus dosing. Desmoteplase, a fibrin-specific agent currently in phase 2 trials, also has a longer half-life and appears to be more potent than alteplase. Table 2 lists the dosing and the degree of fibrin specificity of these agents.
Complications of thrombolytic therapy
Submassive pulmonary embolism has a low death rate, and the benefit of systemic thrombolytic therapy for this condition is controversial. Therefore, risk stratification is very important before pursuing this therapy.
A meta-analysis25 of 16 randomized controlled trials included 2,125 patients with pulmonary embolism:
- 210 (9.88%) in the low-risk category
- 1,499 (70.54%) in the submassive category
- 31 (1.46%) in the massive category
- 385 (18.11%) whose disease severity could not be determined.
Major bleeding occurred in:
- 98 (9.24%) of 1,061 patients receiving anticoagulation plus thrombolytics
- 36 (3.42%) of 1,054 patients receiving anticoagulation without thrombolytics (odds ratio [OR] 2.73, 95% confidence interval [CI] 1.91–3.91; number needed to harm [NNH] 18, 95% CI 13–27).
Intracranial hemorrhage occurred in:
- 15 (1.46%) of 2,014 patients on thrombolytic therapy
- 2 (0.19%) of 1,019 patients not on thrombolytic therapy (OR 4.63, 95% CI 1.78–12.04; NNH 78, 95% CI 48–206).
Of note, the incidence of major bleeding was not significantly increased in those age 65 or younger receiving thrombolytics (OR 1.25, 95% CI 0.5–3.14).
Comments. Definitions of major bleeding varied in the individual trials. Additionally, intracranial hemorrhage was included as a major bleeding end point in any trial in which it was not prespecified.
These findings emphasize the importance of risk stratification before pursuing thrombolytic therapy in submassive pulmonary embolism.
Table 3 lists absolute and relative contraindications to thrombolytic therapy.
MAJOR STUDIES IN SUBMASSIVE PULMONARY EMBOLISM
The MAPPET-3 trial
The Management Strategies and Prognosis of Pulmonary Embolism-3 (MAPPET-3) trial,26 in 2002, was the first major trial to study thrombolytic therapy in submassive pulmonary embolism.
In this prospective, randomized, double-blinded trial conducted in Germany, 118 patients received heparin with alteplase (100 mg over 2 hours) and 138 received heparin with placebo. The primary end point was in-hospital death or clinical deterioration requiring escalation of treatment. Secondary outcomes included recurrent pulmonary embolism, major bleeding, and stroke. Major bleeding was defined as fatal bleeding, hemorrhagic stroke, or drop in the hemoglobin concentration by more than 4 g/dL, with or without the need for red blood cell transfusion.
Right ventricular dysfunction was diagnosed by echocardiography in 30% of the participants, and the rest of the patients were classified as having submassive pulmonary embolism on the basis of electrocardiographic criteria alone. It is likely that the latter group had a less severe form of the disease and did not benefit from thrombolytic therapy as much as patients with echocardiographic findings of right ventricular dysfunction and elevated serum cardiac biomarkers.
Results. At 30 days, 11% of the alteplase-plus-heparin group had met the primary end point, compared with 24.6% of the placebo-plus-heparin group (P = .006). The difference was mostly driven by the need for secondary thrombolysis (7.6% vs 23.2%, P = .001), since 32 (23.2%) of the 138 patients in the control group required secondary thrombolysis, accounting for 94% of the 34 patients in this group who required escalation of treatment. Most cases of clinical deterioration in this group occurred within the first 5 days.
Mortality rates were 3.4% in the heparin-plus-alteplase group and 2.2% in the heparin-plus-placebo group, but the difference was not statistically significant (P = .71).
Major bleeding occurred in 1 patient in the heparin-plus-alteplase group and 5 patients in the heparin-plus-placebo group, but the trial’s definition of major bleeding may have resulted in underestimation of this event. The definition put forth by the International Society on Thrombosis and Haemostasis is less strict, defining bleeding in nonsurgical patients as major if it is fatal, symptomatic in a critical area or organ, or causing a fall in hemoglobin level of 2.0 g/dL or more, leading to transfusion of two or more units of whole blood or red cells.27
MOPETT trial
The Moderate Pulmonary Embolism Treated with Thrombolysis (MOPETT) trial28 was a single-center, randomized trial in 121 normotensive patients with “moderate” pulmonary embolism and right ventricular dysfunction. Moderate pulmonary embolism was defined as signs and symptoms of pulmonary embolism with evidence on computed tomographic angiography of greater than 70% involvement with thrombus in two or more lobes or left or right main pulmonary arteries, or by a high-probability ventilation-perfusion scan showing a mismatch in two or more lobes.
The authors defined right ventricular dysfunction by elevated cardiac markers or by findings on echocardiography. Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.
The primary outcome was the development of pulmonary hypertension, based on echocardiography.
Patients were randomized to either anticoagulation alone (unfractionated heparin or low-molecular-weight heparin) or anticoagulation plus half-dose alteplase (0.5 mg/kg, to a maximum of 50 mg). Echocardiography was performed within 2 hours of study entry, at 48 hours, and every 6 months thereafter. The mean duration of follow-up was 28 months.
Results. Pulmonary hypertension developed in 16% of the anticoagulation-plus-alteplase group vs 57% of the anticoagulation-only group (P < .001). However, the clinical relevance of elevated right-sided pressures observed by echocardiography in asymptomatic patients is uncertain. Alteplase had no impact on the rates of death or recurrent pulmonary embolism.
PEITHO trial
The 2014 Pulmonary Embolism Thrombolysis (PEITHO) trial29 was a prospective, randomized, double-blinded, placebo-controlled trial conducted in 13 countries between 2007 and 2012. A total of 1,005 patients with submassive pulmonary embolism received unfractionated heparin and were randomized to also receive either tenecteplase or placebo.
The primary end point was death from any cause or hemodynamic compromise within 7 days of randomization. Secondary end points included death within 30 days, recurrence of pulmonary embolism, major bleeding, and stroke.
Echocardiography was strongly recommended for diagnosing right ventricular dysfunction in all patients. When this was unavailable, computed tomographic images were used to assess right ventricular dysfunction. Major bleeding was characterized as moderate or severe, and bleeding events were reported using the International Society on Thrombosis and Haemostasis criteria.
Results. The tenecteplase group had a lower rate of the primary end point at 7 days (2.6% vs 5.6%, P = .02), but no significant reduction in all-cause mortality at 30 days (2.4% vs 3.2%, P = .42). In addition, the tenecteplase group had higher rates of major extracranial bleeding (6.3% vs 1.2%, P < .001) and stroke (2.4% vs 0.2%, P = .004) at 7 days.
Although the PEITHO trial showed no reduction in mortality rates and showed a higher rate of major bleeding, this may have been related to using a higher dose of tenecteplase than needed in this population. Further studies should be conducted to confirm this theory.
TOPCOAT trial
The Tenecteplase or Placebo, Cardiopulmonary Outcomes at Three months (TOPCOAT) trial,30 published in 2014, was a multicenter, double-blind, intention-to-treat, randomized trial carried out in eight centers in the United States. The authors evaluated a composite outcome (death, circulatory shock, intubation, major bleeding, recurrent pulmonary embolism, and functional capacity) with the use of tenecteplase in submassive pulmonary embolism.
A total of 83 patients received low-molecular-weight heparin and were randomized to also receive either tenecteplase or placebo. Submassive pulmonary embolism was defined as evidence of right ventricular strain based on echocardiographic findings and elevated cardiac markers (troponin, BNP, or NT-pro-BNP).
Results. Adverse outcomes occurred in 37% of the patients in the placebo group compared with 15% of those in the tenecteplase group (P = .017). The study was terminated early because the lead author relocated to another institution.
Wang et al
In a prospective, randomized, open-label, multicenter study31 conducted in China between 2002 and 2006, 118 patients received low-molecular-weight heparin plus alteplase in a dose of either 100 mg or 50 mg over 2 hours.
Results. There were significantly fewer bleeding episodes in patients receiving half-dose alteplase in the subgroups that weighed less than 65 kg (14.8% vs 41.2%, P = .049) or who had a body mass index less than 24 kg/m2 (8.7% vs 42.9%, P = .014).
Meta-analysis
A subgroup analysis25 of patients with submassive pulmonary embolism from a 2014 meta-analysis of randomized controlled trials of thrombolytic therapy in pulmonary embolism found that thrombolysis was associated with a lower mortality rate (OR 0.48; 95% CI 0.25–0.92) but a higher rate of major bleeding (OR 3.19, 95% CI 2.07–4.92).
Is there a role for low-dose thrombolytic therapy?
The MOPETT study, discussed above, evaluated the effect of thrombolysis in a low (“safe”) dose in reducing pulmonary artery pressure in moderate pulmonary embolism.28 The primary end points were pulmonary hypertension and the composite end point of pulmonary hypertension and recurrent pulmonary embolism. In the thrombolysis group, the pulmonary arterial pressure fell immediately and was still lower at 28 months. As mentioned, although the incidence of pulmonary hypertension was lower with thrombolysis, no significant differences were noted in the rate of individual outcomes of death and recurrent pulmonary embolism when assessed independently. Furthermore, the definition of moderate pulmonary embolism used in this study is different from the submassive criteria.
Wang et al31 enrolled patients to receive low-molecular-weight heparin plus alteplase in a dose of either 50 or 100 mg. The rate of bleeding was lower with the 50-mg dose, but only in the subset of patients with lower weight and body mass index.
What is the role of catheter-guided therapy?
Catheter-directed therapy involves infusing thrombolytic agents directly into the pulmonary arteries where the clots are. The idea is to expose the patient to lower doses of systemic thrombolytics and thus decrease the risk of bleeding.
The ULTIMA study32 (Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism) evaluated whether this treatment would reverse right ventricular dilation in intermediate-risk patients, compared with anticoagulation. Intermediate-risk pulmonary embolism was defined as an embolus located in at least one main or proximal lower lobe pulmonary artery and an RV:LV ratio of at least 1.0 obtained from the echocardiographic apical four-chamber view.
The study showed hemodynamic improvement as evidenced by a lower RV:LV ratio. However, at 90 days the mortality rate was not significantly lower in the treatment group than in the control group. Of note, no major bleeding events were reported in the treatment group.
The SEATTLE II trial,33 a nonrandomized study completed in April 2013, evaluated the efficacy and safety of ultrasonographically guided, catheter-based, low-dose fibrinolysis for patients with massive and submassive pulmonary embolism. Patients had CT evidence of proximal pulmonary embolism and a dilated right ventricle (RV:LV ratio ≥ 0.9). Patients received alteplase 24 mg, either as 1 mg/hour for 24 hours with a unilateral catheter or 1 mg/hour in each of two catheters for 12 hours.
At 48 hours after the procedure, the mean RV:LV ratio had decreased from 1.55 to 1.13, the mean pulmonary arterial systolic pressure had fallen, and the anatomical clot burden had decreased. A total of 15 patients (10%) experienced major bleeding but there were no reports of any fatal or intracranial bleeding. Patients with massive pulmonary embolism were more likely to experience major bleeding episodes than those with submassive pulmonary embolism (23% vs 7%, P = .02).
The weakness of this study is that it was a single-arm study and therefore limits comparisons with other therapies such as tissue plasminogen activator for massive pulmonary embolism or anticoagulation. Also, although there was an acute improvement in hemodynamics, it is unclear if that translates to improvement in mortality rate.
Based on the available literature,29,31,33 patients presenting with submassive pulmonary embolism who are of low body weight (body mass index < 24 kg/m2 or weight < 65 kg) or are over age 75 may benefit from low-dose catheter-guided thrombolysis therapy or low-dose systemic alteplase (50 mg). Further studies should be conducted comparing these two therapeutic strategies.
SURGICAL EMBOLECTOMY: STILL THE LAST RESORT
Surgery has been the last resort for patients with pulmonary embolism. Although recent reports show a decrease in mortality from advances in surgical embolectomy, the mortality rate is greater than 10%.34
- Indications for surgical embolectomy are35:
- Failure of or contraindications to thrombolytic therapy
- Continued hemodynamic instability despite maximal medical therapy
- Associated cardiac pathology such as patent foramen ovale, atrial septal defect, and free-floating right heart thrombi
- Inadequate time for systemic thrombolytics to take effect.
No large or randomized controlled trials of surgical embolectomy for submassive pulmonary embolism have been done. In one study, of 47 patients undergoing surgical embolectomy, 15 (32%) met the criteria for submassive pulmonary embolism based on right ventricular hemodynamic dysfunction. The report did not mention if biomarkers such as troponin and BNP were considered in the decision to operate.36
At this time, surgical embolectomy remains a last resort for patients with acute massive pulmonary embolism who have contraindications to thrombolysis or for whom it has failed. Given the risk of death associated with surgical embolectomy, large randomized controlled trials need to be done to see if there is any benefit in the submassive pulmonary embolism population.
ONE TREATMENT DOES NOT FIT ALL
Given the evidence to date, we do not recommend thrombolytic therapy for all patients with submassive pulmonary embolism. The risk of complications (hemorrhage) is significant, and the benefit is unclear. A one-treatment-for-all approach cannot be applied in this situation.
Furthermore, each of the trials performed so far defined submassive pulmonary embolism slightly differently (Table 4), and many were underpowered to detect a difference in mortality rates between the treatment groups. Further studies are needed to determine the exact subset of patients with submassive pulmonary embolism that may truly benefit from thrombolytic therapy.
As such, patients with submassive pulmonary embolism should be managed by a multidisciplinary team to determine the need for thrombolytic therapy, especially in low doses, on a case-by-case basis according to the patient’s risk of further clinical deterioration.
- Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998; 158:585–593.
- Stein PD, Matta F, Keyes DC, Willyerd GL. Impact of vena cava filters on in-hospital case fatality rate from pulmonary embolism. Am J Med 2012; 125:478–484.
- Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest 2002; 121:877–905.
- Heit JA, Mohr DN, Silverstein MD, Petterson TM, O’Fallon WM, Melton LJ 3rd. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med 2000; 160:761–768.
- Mohr DN, Silverstein MD, Heit JA, Petterson TM, O’Fallon WM, Melton LJ. The venous stasis syndrome after deep venous thrombosis or pulmonary embolism: a population-based study. Mayo Clin Proc 2000; 75:1249–1256.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Tapson VF. Acute pulmonary embolism. N Engl J Med 2008; 358:1037–1052.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Kreit JW. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest 2004; 125:1539–1545.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Comess KA, DeRook FA, Russell ML, Tognazzi-Evans TA, Beach KW. The incidence of pulmonary embolism in unexplained sudden cardiac arrest with pulseless electrical activity. Am J Med 2000; 109:351–356.
- Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol 1997; 30:1165–1171.
- Piazza G. Submassive pulmonary embolism. JAMA 2013; 309:171–180.
- Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 2008; 178:425–430.
- Vanni S, Polidori G, Vergara R, et al. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med 2009; 122:257–264.
- Amorim S, Dias P, Rodrigues RA, et al. Troponin I as a marker of right ventricular dysfunction and severity of pulmonary embolism. Rev Port Cardiol 2006; 25:181–186.
- Dellas C, Puls M, Lankeit M, et al. Elevated heart-type fatty acid-binding protein levels on admission predict an adverse outcome in normotensive patients with acute pulmonary embolism. J Am Coll Cardiol 2010; 55:2150–2157.
- Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord 2014; 14:64.
- Nazeyrollas P, Metz D, Jolly D, et al. Use of transthoracic Doppler echocardiography combined with clinical and electrocardiographic data to predict acute pulmonary embolism. Eur Heart J 1996;17: 779–786.
- Wake N, Kumamaru KK, George E, et al. Computed tomography and echocardiography in patients with acute pulmonary embolism: part 1: correlation of findings of right ventricular enlargement. J Thorac Imaging 2014; 29:W1–W6.
- Becattini C, Agnelli G, Germini F, Vedovati MC. Computed tomography to assess risk of death in acute pulmonary embolism: a meta-analysis. Eur Respir J 2014; 43:1678–1690.
- Konstantinides SV, Torbicki A, Agnelli G, et al; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069, 69a–69k.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Daley MJ, Lat I. Clinical controversies in thrombolytic therapy for the management of acute pulmonary embolism. Pharmacotherapy 2012; 32:158–172.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311:2414–2421.
- Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W; Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 2002; 347:1143–1150.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Wang C, Zhai Z, Yang Y, et al; China Venous Thromboembolism (VTE) Study Group. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest 2010; 137:254–262.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
- Stein PD, Alnas M, Beemath A, Patel NR. Outcome of pulmonary embolectomy. Am J Cardiol 2007; 99:421–423.
- He C, Von Segesser LK, Kappetein PA, Mestres CA, Smith JA, Choong CK. Acute pulmonary embolectomy. Eur J Cardiothorac Surg 2013; 43:1087–1095.
- Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg 2005; 129:1018–1023.
For patients with submassive acute pulmonary embolism—the intermediate category between massive and low-risk—whether to give thrombolytic therapy is controversial. In general, patients with massive pulmonary embolism need this therapy, whereas those with low-risk pulmonary embolism do not—and neither do most of those with submassive embolism. But where should we draw the line?
More than 600,000 patients suffer pulmonary embolisms every year in the United States, and 50,000 to 200,000 people die of them.1–3 In various studies,4–6 within 1 year, 12.9% of patients had another pulmonary embolism, 7.3% developed chronic venous insufficiency, and 3.8% developed chronic thromboembolic pulmonary hypertension.
THREE CATEGORIES OF RISK
Episodes of acute pulmonary embolism are classified as low-risk (about 70% of cases), hemodynamically unstable or massive (5%), or submassive (25%).7,8
Low-risk acute pulmonary embolism is defined by the absence of right ventricular dysfunction and the absence of myocardial necrosis. The death rate in such cases is less than 1%.9 Its pharmacologic management includes parenteral anticoagulation and early initiation of long-term anticoagulation therapy, which the American College of Chest Physicians (ACCP) gives a grade IB recommendation (strong, based on moderate-quality evidence).10
Massive or hemodynamically unstable pulmonary embolism is characterized by any of the following, in the absence of other causes8:
- Sustained hypotension (systolic blood pressure < 90 mm Hg for ≥ 15 minutes)
- An absolute decrease in systolic blood pressure of 40 mm Hg or more
- Need for inotropic support
- Cardiac arrest
- Bradycardia (heart rate < 40 beats per minute).
The death rate is more than 30% in patients presenting with shock and approaches 70% in those presenting with cardiac arrest.11,12 Therefore, the consensus is that this category of pulmonary embolism merits aggressive treatment. Systemic thrombolytic therapy is recommended in those who are not at high risk of major bleeding, though the ACCP gives it only a grade 2C recommendation (weak, based on low-quality evidence).10
Submassive pulmonary embolism is defined by evidence of right ventricular dysfunction with normal blood pressure. According to the ACCP guidelines, thrombolytic therapy should be considered (grade 2C recommendation) for patients with acute pulmonary embolism without hypotension and with a low bleeding risk (with no renal failure and not on dual antiplatelet therapy), but at high risk of developing hypotension.10
DIAGNOSING SUBMASSIVE PULMONARY EMBOLISM, DELINEATING ITS SEVERITY
In managing acute pulmonary embolism, it is critical to recognize whether a patient is at high risk of clinical deterioration.
Blood pressure
The systolic blood pressure not only indicates whether the patient has hypotension (systolic blood pressure < 90 mm Hg) and therefore massive rather than submassive or low-risk pulmonary embolism; it is also important as a baseline value. A decrease in systolic blood pressure of 40 mm Hg or more is associated with worse outcomes.12
Right ventricular dysfunction
The physiologic response to occlusion of the pulmonary arteries can result in early myocardial injury and right ventricular dysfunction, which can be assessed by various methods (Table 1).
Electrocardiographic signs. Right heart strain may be recognized on electrocardiography as:
- Evidence of new complete or incomplete right bundle branch block
- T-wave inversion in the anterolateral leads V1 to V4
- S1Q3T3 (a large S wave in lead I, a Q wave in lead III, and an inverted T wave in lead III, the classic pattern of acute cor pulmonale).13
These findings add incremental prognostic value to echocardiographic findings in patients with submassive pulmonary embolism.14
Cardiac biomarkers such as B-type natriuretic peptide (BNP), N-terminal-pro-BNP (NT-pro-BNP), cardiac troponins, and heart-type fatty acid-binding protein (H-FABP) are also markers of right-sided myocardial damage and strain and can be used to identify patients with submassive pulmonary embolism.15 Abnormal levels of these substances are as follows:
- Troponin T greater than 0.1 ng/mL
- Troponin I greater than 0.4 ng/mL
- BNP greater than 90 pg/mL
- NT-pro-BNP greater than 500 pg/mL
- H-FABP less than 6 ng/mL.
These levels have prognostic value, identifying patients with submassive pulmonary embolism at risk of deterioration or death,14,16,17
Echocardiographic signs. Right ventricular dysfunction can be assessed quickly at the bedside with portable transthoracic echocardiography. A meta-analysis showed that close to 37% of hemodynamically stable patients with pulmonary embolism had echocardiographic evidence of right ventricular dysfunction on presentation and a higher short-term mortality rate.18 Evidence of right ventricular dysfunction includes the following:
- New-onset hypokinesis or akinesis
- Right ventricular dilation
- Right ventricular free-wall hypokinesis with apical sparing (the McConnell sign)
- Paradoxical movement of the interventricular septum
- Newly increased right ventricular systolic pressure
- Pulmonary hypertension, defined as tricuspid regurgitation jet velocity greater than 2.8 m/s.15,19
Computed tomographic (CT) angiography is widely available. Findings that have prognostic value in determining those at higher risk of death include the following20,21:
- A dilated right ventricle—ratio of right ventricle to left ventricle diameter (RV:LV ratio) greater than 0.9
- Interventricular septal bowing.
PESI and sPESI scores. The European Society of Cardiology 2014 guidelines stratify the risk in normotensive patients with pulmonary embolism according to their score on the Pulmonary Embolism Severity Index (PESI) or the simplified PESI (sPESI). There are five PESI classes. Those in PESI class III or IV or with an sPESI score of 1 or more (on a scale of 0 to 6) are considered at intermediate risk of clinical deterioration and are then further risk-stratified according to whether they have right ventricular dysfunction (based on echocardiography or computed tomography) and elevated cardiac biomarkers. These scoring systems are based on easily obtainable clinical information such as age, male sex, history of cancer, history of heart failure, history of chronic lung disease, heart rate, systolic blood pressure, respiratory rate, temperature, and altered mental status, and calculators are readily available.
Anticoagulation for all, plus thrombolysis for some
Patients with neither right ventricular dysfunction nor elevated cardiac biomarkers are at intermediate to low risk of clinical deterioration, and it is recommended that they be given anticoagulation therapy in an inpatient setting.
On the other hand, patients with both right ventricular dysfunction and elevated cardiac biomarkers are considered at intermediate to high risk of clinical deterioration; they should also be managed with anticoagulation and monitored closely for the need for rescue reperfusion therapy with thrombolytics.22
THROMBOLYTIC AGENTS
Thrombolytic agents are the cornerstone of management for patients presenting with pulmonary embolism who are at high risk. As noted above, these agents are recommended in massive pulmonary embolism, but their role in submassive pulmonary embolism remains controversial.
Thrombolytics work by activating endogenous plasminogen. The resulting plasmin promotes clot lysis, reducing the size of the thrombus, decreasing pulmonary vasculature resistance, and improving right ventricular function.23
To date, three thrombolytic agents have received US Food and Drug Administration approval for use in massive pulmonary embolism: alteplase, urokinase, and streptokinase. But only alteplase is still available in the United States. Alteplase is also the best tolerated, whereas streptokinase is highly antigenic and may cause further deterioration in an already unstable patient. Alteplase is also the most fibrin-specific and is considered the most potent of the three agents.24
Additional thrombolytic agents under investigation for use in acute pulmonary embolism include reteplase, tenecteplase, and desmoteplase. These agents are more fibrin-specific than alteplase. Reteplase is a second-generation recombinant tissue-type plasminogen activator with a quicker onset of action and longer half-life than alteplase, allowing for bolus dosing. Tenecteplase, a variant of alteplase, is cleared more slowly and is 14 times more fibrin-specific than alteplase, also allowing for bolus dosing. Desmoteplase, a fibrin-specific agent currently in phase 2 trials, also has a longer half-life and appears to be more potent than alteplase. Table 2 lists the dosing and the degree of fibrin specificity of these agents.
Complications of thrombolytic therapy
Submassive pulmonary embolism has a low death rate, and the benefit of systemic thrombolytic therapy for this condition is controversial. Therefore, risk stratification is very important before pursuing this therapy.
A meta-analysis25 of 16 randomized controlled trials included 2,125 patients with pulmonary embolism:
- 210 (9.88%) in the low-risk category
- 1,499 (70.54%) in the submassive category
- 31 (1.46%) in the massive category
- 385 (18.11%) whose disease severity could not be determined.
Major bleeding occurred in:
- 98 (9.24%) of 1,061 patients receiving anticoagulation plus thrombolytics
- 36 (3.42%) of 1,054 patients receiving anticoagulation without thrombolytics (odds ratio [OR] 2.73, 95% confidence interval [CI] 1.91–3.91; number needed to harm [NNH] 18, 95% CI 13–27).
Intracranial hemorrhage occurred in:
- 15 (1.46%) of 2,014 patients on thrombolytic therapy
- 2 (0.19%) of 1,019 patients not on thrombolytic therapy (OR 4.63, 95% CI 1.78–12.04; NNH 78, 95% CI 48–206).
Of note, the incidence of major bleeding was not significantly increased in those age 65 or younger receiving thrombolytics (OR 1.25, 95% CI 0.5–3.14).
Comments. Definitions of major bleeding varied in the individual trials. Additionally, intracranial hemorrhage was included as a major bleeding end point in any trial in which it was not prespecified.
These findings emphasize the importance of risk stratification before pursuing thrombolytic therapy in submassive pulmonary embolism.
Table 3 lists absolute and relative contraindications to thrombolytic therapy.
MAJOR STUDIES IN SUBMASSIVE PULMONARY EMBOLISM
The MAPPET-3 trial
The Management Strategies and Prognosis of Pulmonary Embolism-3 (MAPPET-3) trial,26 in 2002, was the first major trial to study thrombolytic therapy in submassive pulmonary embolism.
In this prospective, randomized, double-blinded trial conducted in Germany, 118 patients received heparin with alteplase (100 mg over 2 hours) and 138 received heparin with placebo. The primary end point was in-hospital death or clinical deterioration requiring escalation of treatment. Secondary outcomes included recurrent pulmonary embolism, major bleeding, and stroke. Major bleeding was defined as fatal bleeding, hemorrhagic stroke, or drop in the hemoglobin concentration by more than 4 g/dL, with or without the need for red blood cell transfusion.
Right ventricular dysfunction was diagnosed by echocardiography in 30% of the participants, and the rest of the patients were classified as having submassive pulmonary embolism on the basis of electrocardiographic criteria alone. It is likely that the latter group had a less severe form of the disease and did not benefit from thrombolytic therapy as much as patients with echocardiographic findings of right ventricular dysfunction and elevated serum cardiac biomarkers.
Results. At 30 days, 11% of the alteplase-plus-heparin group had met the primary end point, compared with 24.6% of the placebo-plus-heparin group (P = .006). The difference was mostly driven by the need for secondary thrombolysis (7.6% vs 23.2%, P = .001), since 32 (23.2%) of the 138 patients in the control group required secondary thrombolysis, accounting for 94% of the 34 patients in this group who required escalation of treatment. Most cases of clinical deterioration in this group occurred within the first 5 days.
Mortality rates were 3.4% in the heparin-plus-alteplase group and 2.2% in the heparin-plus-placebo group, but the difference was not statistically significant (P = .71).
Major bleeding occurred in 1 patient in the heparin-plus-alteplase group and 5 patients in the heparin-plus-placebo group, but the trial’s definition of major bleeding may have resulted in underestimation of this event. The definition put forth by the International Society on Thrombosis and Haemostasis is less strict, defining bleeding in nonsurgical patients as major if it is fatal, symptomatic in a critical area or organ, or causing a fall in hemoglobin level of 2.0 g/dL or more, leading to transfusion of two or more units of whole blood or red cells.27
MOPETT trial
The Moderate Pulmonary Embolism Treated with Thrombolysis (MOPETT) trial28 was a single-center, randomized trial in 121 normotensive patients with “moderate” pulmonary embolism and right ventricular dysfunction. Moderate pulmonary embolism was defined as signs and symptoms of pulmonary embolism with evidence on computed tomographic angiography of greater than 70% involvement with thrombus in two or more lobes or left or right main pulmonary arteries, or by a high-probability ventilation-perfusion scan showing a mismatch in two or more lobes.
The authors defined right ventricular dysfunction by elevated cardiac markers or by findings on echocardiography. Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.
The primary outcome was the development of pulmonary hypertension, based on echocardiography.
Patients were randomized to either anticoagulation alone (unfractionated heparin or low-molecular-weight heparin) or anticoagulation plus half-dose alteplase (0.5 mg/kg, to a maximum of 50 mg). Echocardiography was performed within 2 hours of study entry, at 48 hours, and every 6 months thereafter. The mean duration of follow-up was 28 months.
Results. Pulmonary hypertension developed in 16% of the anticoagulation-plus-alteplase group vs 57% of the anticoagulation-only group (P < .001). However, the clinical relevance of elevated right-sided pressures observed by echocardiography in asymptomatic patients is uncertain. Alteplase had no impact on the rates of death or recurrent pulmonary embolism.
PEITHO trial
The 2014 Pulmonary Embolism Thrombolysis (PEITHO) trial29 was a prospective, randomized, double-blinded, placebo-controlled trial conducted in 13 countries between 2007 and 2012. A total of 1,005 patients with submassive pulmonary embolism received unfractionated heparin and were randomized to also receive either tenecteplase or placebo.
The primary end point was death from any cause or hemodynamic compromise within 7 days of randomization. Secondary end points included death within 30 days, recurrence of pulmonary embolism, major bleeding, and stroke.
Echocardiography was strongly recommended for diagnosing right ventricular dysfunction in all patients. When this was unavailable, computed tomographic images were used to assess right ventricular dysfunction. Major bleeding was characterized as moderate or severe, and bleeding events were reported using the International Society on Thrombosis and Haemostasis criteria.
Results. The tenecteplase group had a lower rate of the primary end point at 7 days (2.6% vs 5.6%, P = .02), but no significant reduction in all-cause mortality at 30 days (2.4% vs 3.2%, P = .42). In addition, the tenecteplase group had higher rates of major extracranial bleeding (6.3% vs 1.2%, P < .001) and stroke (2.4% vs 0.2%, P = .004) at 7 days.
Although the PEITHO trial showed no reduction in mortality rates and showed a higher rate of major bleeding, this may have been related to using a higher dose of tenecteplase than needed in this population. Further studies should be conducted to confirm this theory.
TOPCOAT trial
The Tenecteplase or Placebo, Cardiopulmonary Outcomes at Three months (TOPCOAT) trial,30 published in 2014, was a multicenter, double-blind, intention-to-treat, randomized trial carried out in eight centers in the United States. The authors evaluated a composite outcome (death, circulatory shock, intubation, major bleeding, recurrent pulmonary embolism, and functional capacity) with the use of tenecteplase in submassive pulmonary embolism.
A total of 83 patients received low-molecular-weight heparin and were randomized to also receive either tenecteplase or placebo. Submassive pulmonary embolism was defined as evidence of right ventricular strain based on echocardiographic findings and elevated cardiac markers (troponin, BNP, or NT-pro-BNP).
Results. Adverse outcomes occurred in 37% of the patients in the placebo group compared with 15% of those in the tenecteplase group (P = .017). The study was terminated early because the lead author relocated to another institution.
Wang et al
In a prospective, randomized, open-label, multicenter study31 conducted in China between 2002 and 2006, 118 patients received low-molecular-weight heparin plus alteplase in a dose of either 100 mg or 50 mg over 2 hours.
Results. There were significantly fewer bleeding episodes in patients receiving half-dose alteplase in the subgroups that weighed less than 65 kg (14.8% vs 41.2%, P = .049) or who had a body mass index less than 24 kg/m2 (8.7% vs 42.9%, P = .014).
Meta-analysis
A subgroup analysis25 of patients with submassive pulmonary embolism from a 2014 meta-analysis of randomized controlled trials of thrombolytic therapy in pulmonary embolism found that thrombolysis was associated with a lower mortality rate (OR 0.48; 95% CI 0.25–0.92) but a higher rate of major bleeding (OR 3.19, 95% CI 2.07–4.92).
Is there a role for low-dose thrombolytic therapy?
The MOPETT study, discussed above, evaluated the effect of thrombolysis in a low (“safe”) dose in reducing pulmonary artery pressure in moderate pulmonary embolism.28 The primary end points were pulmonary hypertension and the composite end point of pulmonary hypertension and recurrent pulmonary embolism. In the thrombolysis group, the pulmonary arterial pressure fell immediately and was still lower at 28 months. As mentioned, although the incidence of pulmonary hypertension was lower with thrombolysis, no significant differences were noted in the rate of individual outcomes of death and recurrent pulmonary embolism when assessed independently. Furthermore, the definition of moderate pulmonary embolism used in this study is different from the submassive criteria.
Wang et al31 enrolled patients to receive low-molecular-weight heparin plus alteplase in a dose of either 50 or 100 mg. The rate of bleeding was lower with the 50-mg dose, but only in the subset of patients with lower weight and body mass index.
What is the role of catheter-guided therapy?
Catheter-directed therapy involves infusing thrombolytic agents directly into the pulmonary arteries where the clots are. The idea is to expose the patient to lower doses of systemic thrombolytics and thus decrease the risk of bleeding.
The ULTIMA study32 (Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism) evaluated whether this treatment would reverse right ventricular dilation in intermediate-risk patients, compared with anticoagulation. Intermediate-risk pulmonary embolism was defined as an embolus located in at least one main or proximal lower lobe pulmonary artery and an RV:LV ratio of at least 1.0 obtained from the echocardiographic apical four-chamber view.
The study showed hemodynamic improvement as evidenced by a lower RV:LV ratio. However, at 90 days the mortality rate was not significantly lower in the treatment group than in the control group. Of note, no major bleeding events were reported in the treatment group.
The SEATTLE II trial,33 a nonrandomized study completed in April 2013, evaluated the efficacy and safety of ultrasonographically guided, catheter-based, low-dose fibrinolysis for patients with massive and submassive pulmonary embolism. Patients had CT evidence of proximal pulmonary embolism and a dilated right ventricle (RV:LV ratio ≥ 0.9). Patients received alteplase 24 mg, either as 1 mg/hour for 24 hours with a unilateral catheter or 1 mg/hour in each of two catheters for 12 hours.
At 48 hours after the procedure, the mean RV:LV ratio had decreased from 1.55 to 1.13, the mean pulmonary arterial systolic pressure had fallen, and the anatomical clot burden had decreased. A total of 15 patients (10%) experienced major bleeding but there were no reports of any fatal or intracranial bleeding. Patients with massive pulmonary embolism were more likely to experience major bleeding episodes than those with submassive pulmonary embolism (23% vs 7%, P = .02).
The weakness of this study is that it was a single-arm study and therefore limits comparisons with other therapies such as tissue plasminogen activator for massive pulmonary embolism or anticoagulation. Also, although there was an acute improvement in hemodynamics, it is unclear if that translates to improvement in mortality rate.
Based on the available literature,29,31,33 patients presenting with submassive pulmonary embolism who are of low body weight (body mass index < 24 kg/m2 or weight < 65 kg) or are over age 75 may benefit from low-dose catheter-guided thrombolysis therapy or low-dose systemic alteplase (50 mg). Further studies should be conducted comparing these two therapeutic strategies.
SURGICAL EMBOLECTOMY: STILL THE LAST RESORT
Surgery has been the last resort for patients with pulmonary embolism. Although recent reports show a decrease in mortality from advances in surgical embolectomy, the mortality rate is greater than 10%.34
- Indications for surgical embolectomy are35:
- Failure of or contraindications to thrombolytic therapy
- Continued hemodynamic instability despite maximal medical therapy
- Associated cardiac pathology such as patent foramen ovale, atrial septal defect, and free-floating right heart thrombi
- Inadequate time for systemic thrombolytics to take effect.
No large or randomized controlled trials of surgical embolectomy for submassive pulmonary embolism have been done. In one study, of 47 patients undergoing surgical embolectomy, 15 (32%) met the criteria for submassive pulmonary embolism based on right ventricular hemodynamic dysfunction. The report did not mention if biomarkers such as troponin and BNP were considered in the decision to operate.36
At this time, surgical embolectomy remains a last resort for patients with acute massive pulmonary embolism who have contraindications to thrombolysis or for whom it has failed. Given the risk of death associated with surgical embolectomy, large randomized controlled trials need to be done to see if there is any benefit in the submassive pulmonary embolism population.
ONE TREATMENT DOES NOT FIT ALL
Given the evidence to date, we do not recommend thrombolytic therapy for all patients with submassive pulmonary embolism. The risk of complications (hemorrhage) is significant, and the benefit is unclear. A one-treatment-for-all approach cannot be applied in this situation.
Furthermore, each of the trials performed so far defined submassive pulmonary embolism slightly differently (Table 4), and many were underpowered to detect a difference in mortality rates between the treatment groups. Further studies are needed to determine the exact subset of patients with submassive pulmonary embolism that may truly benefit from thrombolytic therapy.
As such, patients with submassive pulmonary embolism should be managed by a multidisciplinary team to determine the need for thrombolytic therapy, especially in low doses, on a case-by-case basis according to the patient’s risk of further clinical deterioration.
For patients with submassive acute pulmonary embolism—the intermediate category between massive and low-risk—whether to give thrombolytic therapy is controversial. In general, patients with massive pulmonary embolism need this therapy, whereas those with low-risk pulmonary embolism do not—and neither do most of those with submassive embolism. But where should we draw the line?
More than 600,000 patients suffer pulmonary embolisms every year in the United States, and 50,000 to 200,000 people die of them.1–3 In various studies,4–6 within 1 year, 12.9% of patients had another pulmonary embolism, 7.3% developed chronic venous insufficiency, and 3.8% developed chronic thromboembolic pulmonary hypertension.
THREE CATEGORIES OF RISK
Episodes of acute pulmonary embolism are classified as low-risk (about 70% of cases), hemodynamically unstable or massive (5%), or submassive (25%).7,8
Low-risk acute pulmonary embolism is defined by the absence of right ventricular dysfunction and the absence of myocardial necrosis. The death rate in such cases is less than 1%.9 Its pharmacologic management includes parenteral anticoagulation and early initiation of long-term anticoagulation therapy, which the American College of Chest Physicians (ACCP) gives a grade IB recommendation (strong, based on moderate-quality evidence).10
Massive or hemodynamically unstable pulmonary embolism is characterized by any of the following, in the absence of other causes8:
- Sustained hypotension (systolic blood pressure < 90 mm Hg for ≥ 15 minutes)
- An absolute decrease in systolic blood pressure of 40 mm Hg or more
- Need for inotropic support
- Cardiac arrest
- Bradycardia (heart rate < 40 beats per minute).
The death rate is more than 30% in patients presenting with shock and approaches 70% in those presenting with cardiac arrest.11,12 Therefore, the consensus is that this category of pulmonary embolism merits aggressive treatment. Systemic thrombolytic therapy is recommended in those who are not at high risk of major bleeding, though the ACCP gives it only a grade 2C recommendation (weak, based on low-quality evidence).10
Submassive pulmonary embolism is defined by evidence of right ventricular dysfunction with normal blood pressure. According to the ACCP guidelines, thrombolytic therapy should be considered (grade 2C recommendation) for patients with acute pulmonary embolism without hypotension and with a low bleeding risk (with no renal failure and not on dual antiplatelet therapy), but at high risk of developing hypotension.10
DIAGNOSING SUBMASSIVE PULMONARY EMBOLISM, DELINEATING ITS SEVERITY
In managing acute pulmonary embolism, it is critical to recognize whether a patient is at high risk of clinical deterioration.
Blood pressure
The systolic blood pressure not only indicates whether the patient has hypotension (systolic blood pressure < 90 mm Hg) and therefore massive rather than submassive or low-risk pulmonary embolism; it is also important as a baseline value. A decrease in systolic blood pressure of 40 mm Hg or more is associated with worse outcomes.12
Right ventricular dysfunction
The physiologic response to occlusion of the pulmonary arteries can result in early myocardial injury and right ventricular dysfunction, which can be assessed by various methods (Table 1).
Electrocardiographic signs. Right heart strain may be recognized on electrocardiography as:
- Evidence of new complete or incomplete right bundle branch block
- T-wave inversion in the anterolateral leads V1 to V4
- S1Q3T3 (a large S wave in lead I, a Q wave in lead III, and an inverted T wave in lead III, the classic pattern of acute cor pulmonale).13
These findings add incremental prognostic value to echocardiographic findings in patients with submassive pulmonary embolism.14
Cardiac biomarkers such as B-type natriuretic peptide (BNP), N-terminal-pro-BNP (NT-pro-BNP), cardiac troponins, and heart-type fatty acid-binding protein (H-FABP) are also markers of right-sided myocardial damage and strain and can be used to identify patients with submassive pulmonary embolism.15 Abnormal levels of these substances are as follows:
- Troponin T greater than 0.1 ng/mL
- Troponin I greater than 0.4 ng/mL
- BNP greater than 90 pg/mL
- NT-pro-BNP greater than 500 pg/mL
- H-FABP less than 6 ng/mL.
These levels have prognostic value, identifying patients with submassive pulmonary embolism at risk of deterioration or death,14,16,17
Echocardiographic signs. Right ventricular dysfunction can be assessed quickly at the bedside with portable transthoracic echocardiography. A meta-analysis showed that close to 37% of hemodynamically stable patients with pulmonary embolism had echocardiographic evidence of right ventricular dysfunction on presentation and a higher short-term mortality rate.18 Evidence of right ventricular dysfunction includes the following:
- New-onset hypokinesis or akinesis
- Right ventricular dilation
- Right ventricular free-wall hypokinesis with apical sparing (the McConnell sign)
- Paradoxical movement of the interventricular septum
- Newly increased right ventricular systolic pressure
- Pulmonary hypertension, defined as tricuspid regurgitation jet velocity greater than 2.8 m/s.15,19
Computed tomographic (CT) angiography is widely available. Findings that have prognostic value in determining those at higher risk of death include the following20,21:
- A dilated right ventricle—ratio of right ventricle to left ventricle diameter (RV:LV ratio) greater than 0.9
- Interventricular septal bowing.
PESI and sPESI scores. The European Society of Cardiology 2014 guidelines stratify the risk in normotensive patients with pulmonary embolism according to their score on the Pulmonary Embolism Severity Index (PESI) or the simplified PESI (sPESI). There are five PESI classes. Those in PESI class III or IV or with an sPESI score of 1 or more (on a scale of 0 to 6) are considered at intermediate risk of clinical deterioration and are then further risk-stratified according to whether they have right ventricular dysfunction (based on echocardiography or computed tomography) and elevated cardiac biomarkers. These scoring systems are based on easily obtainable clinical information such as age, male sex, history of cancer, history of heart failure, history of chronic lung disease, heart rate, systolic blood pressure, respiratory rate, temperature, and altered mental status, and calculators are readily available.
Anticoagulation for all, plus thrombolysis for some
Patients with neither right ventricular dysfunction nor elevated cardiac biomarkers are at intermediate to low risk of clinical deterioration, and it is recommended that they be given anticoagulation therapy in an inpatient setting.
On the other hand, patients with both right ventricular dysfunction and elevated cardiac biomarkers are considered at intermediate to high risk of clinical deterioration; they should also be managed with anticoagulation and monitored closely for the need for rescue reperfusion therapy with thrombolytics.22
THROMBOLYTIC AGENTS
Thrombolytic agents are the cornerstone of management for patients presenting with pulmonary embolism who are at high risk. As noted above, these agents are recommended in massive pulmonary embolism, but their role in submassive pulmonary embolism remains controversial.
Thrombolytics work by activating endogenous plasminogen. The resulting plasmin promotes clot lysis, reducing the size of the thrombus, decreasing pulmonary vasculature resistance, and improving right ventricular function.23
To date, three thrombolytic agents have received US Food and Drug Administration approval for use in massive pulmonary embolism: alteplase, urokinase, and streptokinase. But only alteplase is still available in the United States. Alteplase is also the best tolerated, whereas streptokinase is highly antigenic and may cause further deterioration in an already unstable patient. Alteplase is also the most fibrin-specific and is considered the most potent of the three agents.24
Additional thrombolytic agents under investigation for use in acute pulmonary embolism include reteplase, tenecteplase, and desmoteplase. These agents are more fibrin-specific than alteplase. Reteplase is a second-generation recombinant tissue-type plasminogen activator with a quicker onset of action and longer half-life than alteplase, allowing for bolus dosing. Tenecteplase, a variant of alteplase, is cleared more slowly and is 14 times more fibrin-specific than alteplase, also allowing for bolus dosing. Desmoteplase, a fibrin-specific agent currently in phase 2 trials, also has a longer half-life and appears to be more potent than alteplase. Table 2 lists the dosing and the degree of fibrin specificity of these agents.
Complications of thrombolytic therapy
Submassive pulmonary embolism has a low death rate, and the benefit of systemic thrombolytic therapy for this condition is controversial. Therefore, risk stratification is very important before pursuing this therapy.
A meta-analysis25 of 16 randomized controlled trials included 2,125 patients with pulmonary embolism:
- 210 (9.88%) in the low-risk category
- 1,499 (70.54%) in the submassive category
- 31 (1.46%) in the massive category
- 385 (18.11%) whose disease severity could not be determined.
Major bleeding occurred in:
- 98 (9.24%) of 1,061 patients receiving anticoagulation plus thrombolytics
- 36 (3.42%) of 1,054 patients receiving anticoagulation without thrombolytics (odds ratio [OR] 2.73, 95% confidence interval [CI] 1.91–3.91; number needed to harm [NNH] 18, 95% CI 13–27).
Intracranial hemorrhage occurred in:
- 15 (1.46%) of 2,014 patients on thrombolytic therapy
- 2 (0.19%) of 1,019 patients not on thrombolytic therapy (OR 4.63, 95% CI 1.78–12.04; NNH 78, 95% CI 48–206).
Of note, the incidence of major bleeding was not significantly increased in those age 65 or younger receiving thrombolytics (OR 1.25, 95% CI 0.5–3.14).
Comments. Definitions of major bleeding varied in the individual trials. Additionally, intracranial hemorrhage was included as a major bleeding end point in any trial in which it was not prespecified.
These findings emphasize the importance of risk stratification before pursuing thrombolytic therapy in submassive pulmonary embolism.
Table 3 lists absolute and relative contraindications to thrombolytic therapy.
MAJOR STUDIES IN SUBMASSIVE PULMONARY EMBOLISM
The MAPPET-3 trial
The Management Strategies and Prognosis of Pulmonary Embolism-3 (MAPPET-3) trial,26 in 2002, was the first major trial to study thrombolytic therapy in submassive pulmonary embolism.
In this prospective, randomized, double-blinded trial conducted in Germany, 118 patients received heparin with alteplase (100 mg over 2 hours) and 138 received heparin with placebo. The primary end point was in-hospital death or clinical deterioration requiring escalation of treatment. Secondary outcomes included recurrent pulmonary embolism, major bleeding, and stroke. Major bleeding was defined as fatal bleeding, hemorrhagic stroke, or drop in the hemoglobin concentration by more than 4 g/dL, with or without the need for red blood cell transfusion.
Right ventricular dysfunction was diagnosed by echocardiography in 30% of the participants, and the rest of the patients were classified as having submassive pulmonary embolism on the basis of electrocardiographic criteria alone. It is likely that the latter group had a less severe form of the disease and did not benefit from thrombolytic therapy as much as patients with echocardiographic findings of right ventricular dysfunction and elevated serum cardiac biomarkers.
Results. At 30 days, 11% of the alteplase-plus-heparin group had met the primary end point, compared with 24.6% of the placebo-plus-heparin group (P = .006). The difference was mostly driven by the need for secondary thrombolysis (7.6% vs 23.2%, P = .001), since 32 (23.2%) of the 138 patients in the control group required secondary thrombolysis, accounting for 94% of the 34 patients in this group who required escalation of treatment. Most cases of clinical deterioration in this group occurred within the first 5 days.
Mortality rates were 3.4% in the heparin-plus-alteplase group and 2.2% in the heparin-plus-placebo group, but the difference was not statistically significant (P = .71).
Major bleeding occurred in 1 patient in the heparin-plus-alteplase group and 5 patients in the heparin-plus-placebo group, but the trial’s definition of major bleeding may have resulted in underestimation of this event. The definition put forth by the International Society on Thrombosis and Haemostasis is less strict, defining bleeding in nonsurgical patients as major if it is fatal, symptomatic in a critical area or organ, or causing a fall in hemoglobin level of 2.0 g/dL or more, leading to transfusion of two or more units of whole blood or red cells.27
MOPETT trial
The Moderate Pulmonary Embolism Treated with Thrombolysis (MOPETT) trial28 was a single-center, randomized trial in 121 normotensive patients with “moderate” pulmonary embolism and right ventricular dysfunction. Moderate pulmonary embolism was defined as signs and symptoms of pulmonary embolism with evidence on computed tomographic angiography of greater than 70% involvement with thrombus in two or more lobes or left or right main pulmonary arteries, or by a high-probability ventilation-perfusion scan showing a mismatch in two or more lobes.
The authors defined right ventricular dysfunction by elevated cardiac markers or by findings on echocardiography. Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.
The primary outcome was the development of pulmonary hypertension, based on echocardiography.
Patients were randomized to either anticoagulation alone (unfractionated heparin or low-molecular-weight heparin) or anticoagulation plus half-dose alteplase (0.5 mg/kg, to a maximum of 50 mg). Echocardiography was performed within 2 hours of study entry, at 48 hours, and every 6 months thereafter. The mean duration of follow-up was 28 months.
Results. Pulmonary hypertension developed in 16% of the anticoagulation-plus-alteplase group vs 57% of the anticoagulation-only group (P < .001). However, the clinical relevance of elevated right-sided pressures observed by echocardiography in asymptomatic patients is uncertain. Alteplase had no impact on the rates of death or recurrent pulmonary embolism.
PEITHO trial
The 2014 Pulmonary Embolism Thrombolysis (PEITHO) trial29 was a prospective, randomized, double-blinded, placebo-controlled trial conducted in 13 countries between 2007 and 2012. A total of 1,005 patients with submassive pulmonary embolism received unfractionated heparin and were randomized to also receive either tenecteplase or placebo.
The primary end point was death from any cause or hemodynamic compromise within 7 days of randomization. Secondary end points included death within 30 days, recurrence of pulmonary embolism, major bleeding, and stroke.
Echocardiography was strongly recommended for diagnosing right ventricular dysfunction in all patients. When this was unavailable, computed tomographic images were used to assess right ventricular dysfunction. Major bleeding was characterized as moderate or severe, and bleeding events were reported using the International Society on Thrombosis and Haemostasis criteria.
Results. The tenecteplase group had a lower rate of the primary end point at 7 days (2.6% vs 5.6%, P = .02), but no significant reduction in all-cause mortality at 30 days (2.4% vs 3.2%, P = .42). In addition, the tenecteplase group had higher rates of major extracranial bleeding (6.3% vs 1.2%, P < .001) and stroke (2.4% vs 0.2%, P = .004) at 7 days.
Although the PEITHO trial showed no reduction in mortality rates and showed a higher rate of major bleeding, this may have been related to using a higher dose of tenecteplase than needed in this population. Further studies should be conducted to confirm this theory.
TOPCOAT trial
The Tenecteplase or Placebo, Cardiopulmonary Outcomes at Three months (TOPCOAT) trial,30 published in 2014, was a multicenter, double-blind, intention-to-treat, randomized trial carried out in eight centers in the United States. The authors evaluated a composite outcome (death, circulatory shock, intubation, major bleeding, recurrent pulmonary embolism, and functional capacity) with the use of tenecteplase in submassive pulmonary embolism.
A total of 83 patients received low-molecular-weight heparin and were randomized to also receive either tenecteplase or placebo. Submassive pulmonary embolism was defined as evidence of right ventricular strain based on echocardiographic findings and elevated cardiac markers (troponin, BNP, or NT-pro-BNP).
Results. Adverse outcomes occurred in 37% of the patients in the placebo group compared with 15% of those in the tenecteplase group (P = .017). The study was terminated early because the lead author relocated to another institution.
Wang et al
In a prospective, randomized, open-label, multicenter study31 conducted in China between 2002 and 2006, 118 patients received low-molecular-weight heparin plus alteplase in a dose of either 100 mg or 50 mg over 2 hours.
Results. There were significantly fewer bleeding episodes in patients receiving half-dose alteplase in the subgroups that weighed less than 65 kg (14.8% vs 41.2%, P = .049) or who had a body mass index less than 24 kg/m2 (8.7% vs 42.9%, P = .014).
Meta-analysis
A subgroup analysis25 of patients with submassive pulmonary embolism from a 2014 meta-analysis of randomized controlled trials of thrombolytic therapy in pulmonary embolism found that thrombolysis was associated with a lower mortality rate (OR 0.48; 95% CI 0.25–0.92) but a higher rate of major bleeding (OR 3.19, 95% CI 2.07–4.92).
Is there a role for low-dose thrombolytic therapy?
The MOPETT study, discussed above, evaluated the effect of thrombolysis in a low (“safe”) dose in reducing pulmonary artery pressure in moderate pulmonary embolism.28 The primary end points were pulmonary hypertension and the composite end point of pulmonary hypertension and recurrent pulmonary embolism. In the thrombolysis group, the pulmonary arterial pressure fell immediately and was still lower at 28 months. As mentioned, although the incidence of pulmonary hypertension was lower with thrombolysis, no significant differences were noted in the rate of individual outcomes of death and recurrent pulmonary embolism when assessed independently. Furthermore, the definition of moderate pulmonary embolism used in this study is different from the submassive criteria.
Wang et al31 enrolled patients to receive low-molecular-weight heparin plus alteplase in a dose of either 50 or 100 mg. The rate of bleeding was lower with the 50-mg dose, but only in the subset of patients with lower weight and body mass index.
What is the role of catheter-guided therapy?
Catheter-directed therapy involves infusing thrombolytic agents directly into the pulmonary arteries where the clots are. The idea is to expose the patient to lower doses of systemic thrombolytics and thus decrease the risk of bleeding.
The ULTIMA study32 (Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism) evaluated whether this treatment would reverse right ventricular dilation in intermediate-risk patients, compared with anticoagulation. Intermediate-risk pulmonary embolism was defined as an embolus located in at least one main or proximal lower lobe pulmonary artery and an RV:LV ratio of at least 1.0 obtained from the echocardiographic apical four-chamber view.
The study showed hemodynamic improvement as evidenced by a lower RV:LV ratio. However, at 90 days the mortality rate was not significantly lower in the treatment group than in the control group. Of note, no major bleeding events were reported in the treatment group.
The SEATTLE II trial,33 a nonrandomized study completed in April 2013, evaluated the efficacy and safety of ultrasonographically guided, catheter-based, low-dose fibrinolysis for patients with massive and submassive pulmonary embolism. Patients had CT evidence of proximal pulmonary embolism and a dilated right ventricle (RV:LV ratio ≥ 0.9). Patients received alteplase 24 mg, either as 1 mg/hour for 24 hours with a unilateral catheter or 1 mg/hour in each of two catheters for 12 hours.
At 48 hours after the procedure, the mean RV:LV ratio had decreased from 1.55 to 1.13, the mean pulmonary arterial systolic pressure had fallen, and the anatomical clot burden had decreased. A total of 15 patients (10%) experienced major bleeding but there were no reports of any fatal or intracranial bleeding. Patients with massive pulmonary embolism were more likely to experience major bleeding episodes than those with submassive pulmonary embolism (23% vs 7%, P = .02).
The weakness of this study is that it was a single-arm study and therefore limits comparisons with other therapies such as tissue plasminogen activator for massive pulmonary embolism or anticoagulation. Also, although there was an acute improvement in hemodynamics, it is unclear if that translates to improvement in mortality rate.
Based on the available literature,29,31,33 patients presenting with submassive pulmonary embolism who are of low body weight (body mass index < 24 kg/m2 or weight < 65 kg) or are over age 75 may benefit from low-dose catheter-guided thrombolysis therapy or low-dose systemic alteplase (50 mg). Further studies should be conducted comparing these two therapeutic strategies.
SURGICAL EMBOLECTOMY: STILL THE LAST RESORT
Surgery has been the last resort for patients with pulmonary embolism. Although recent reports show a decrease in mortality from advances in surgical embolectomy, the mortality rate is greater than 10%.34
- Indications for surgical embolectomy are35:
- Failure of or contraindications to thrombolytic therapy
- Continued hemodynamic instability despite maximal medical therapy
- Associated cardiac pathology such as patent foramen ovale, atrial septal defect, and free-floating right heart thrombi
- Inadequate time for systemic thrombolytics to take effect.
No large or randomized controlled trials of surgical embolectomy for submassive pulmonary embolism have been done. In one study, of 47 patients undergoing surgical embolectomy, 15 (32%) met the criteria for submassive pulmonary embolism based on right ventricular hemodynamic dysfunction. The report did not mention if biomarkers such as troponin and BNP were considered in the decision to operate.36
At this time, surgical embolectomy remains a last resort for patients with acute massive pulmonary embolism who have contraindications to thrombolysis or for whom it has failed. Given the risk of death associated with surgical embolectomy, large randomized controlled trials need to be done to see if there is any benefit in the submassive pulmonary embolism population.
ONE TREATMENT DOES NOT FIT ALL
Given the evidence to date, we do not recommend thrombolytic therapy for all patients with submassive pulmonary embolism. The risk of complications (hemorrhage) is significant, and the benefit is unclear. A one-treatment-for-all approach cannot be applied in this situation.
Furthermore, each of the trials performed so far defined submassive pulmonary embolism slightly differently (Table 4), and many were underpowered to detect a difference in mortality rates between the treatment groups. Further studies are needed to determine the exact subset of patients with submassive pulmonary embolism that may truly benefit from thrombolytic therapy.
As such, patients with submassive pulmonary embolism should be managed by a multidisciplinary team to determine the need for thrombolytic therapy, especially in low doses, on a case-by-case basis according to the patient’s risk of further clinical deterioration.
- Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998; 158:585–593.
- Stein PD, Matta F, Keyes DC, Willyerd GL. Impact of vena cava filters on in-hospital case fatality rate from pulmonary embolism. Am J Med 2012; 125:478–484.
- Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest 2002; 121:877–905.
- Heit JA, Mohr DN, Silverstein MD, Petterson TM, O’Fallon WM, Melton LJ 3rd. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med 2000; 160:761–768.
- Mohr DN, Silverstein MD, Heit JA, Petterson TM, O’Fallon WM, Melton LJ. The venous stasis syndrome after deep venous thrombosis or pulmonary embolism: a population-based study. Mayo Clin Proc 2000; 75:1249–1256.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Tapson VF. Acute pulmonary embolism. N Engl J Med 2008; 358:1037–1052.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Kreit JW. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest 2004; 125:1539–1545.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Comess KA, DeRook FA, Russell ML, Tognazzi-Evans TA, Beach KW. The incidence of pulmonary embolism in unexplained sudden cardiac arrest with pulseless electrical activity. Am J Med 2000; 109:351–356.
- Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol 1997; 30:1165–1171.
- Piazza G. Submassive pulmonary embolism. JAMA 2013; 309:171–180.
- Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 2008; 178:425–430.
- Vanni S, Polidori G, Vergara R, et al. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med 2009; 122:257–264.
- Amorim S, Dias P, Rodrigues RA, et al. Troponin I as a marker of right ventricular dysfunction and severity of pulmonary embolism. Rev Port Cardiol 2006; 25:181–186.
- Dellas C, Puls M, Lankeit M, et al. Elevated heart-type fatty acid-binding protein levels on admission predict an adverse outcome in normotensive patients with acute pulmonary embolism. J Am Coll Cardiol 2010; 55:2150–2157.
- Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord 2014; 14:64.
- Nazeyrollas P, Metz D, Jolly D, et al. Use of transthoracic Doppler echocardiography combined with clinical and electrocardiographic data to predict acute pulmonary embolism. Eur Heart J 1996;17: 779–786.
- Wake N, Kumamaru KK, George E, et al. Computed tomography and echocardiography in patients with acute pulmonary embolism: part 1: correlation of findings of right ventricular enlargement. J Thorac Imaging 2014; 29:W1–W6.
- Becattini C, Agnelli G, Germini F, Vedovati MC. Computed tomography to assess risk of death in acute pulmonary embolism: a meta-analysis. Eur Respir J 2014; 43:1678–1690.
- Konstantinides SV, Torbicki A, Agnelli G, et al; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069, 69a–69k.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Daley MJ, Lat I. Clinical controversies in thrombolytic therapy for the management of acute pulmonary embolism. Pharmacotherapy 2012; 32:158–172.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311:2414–2421.
- Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W; Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 2002; 347:1143–1150.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Wang C, Zhai Z, Yang Y, et al; China Venous Thromboembolism (VTE) Study Group. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest 2010; 137:254–262.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
- Stein PD, Alnas M, Beemath A, Patel NR. Outcome of pulmonary embolectomy. Am J Cardiol 2007; 99:421–423.
- He C, Von Segesser LK, Kappetein PA, Mestres CA, Smith JA, Choong CK. Acute pulmonary embolectomy. Eur J Cardiothorac Surg 2013; 43:1087–1095.
- Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg 2005; 129:1018–1023.
- Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998; 158:585–593.
- Stein PD, Matta F, Keyes DC, Willyerd GL. Impact of vena cava filters on in-hospital case fatality rate from pulmonary embolism. Am J Med 2012; 125:478–484.
- Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest 2002; 121:877–905.
- Heit JA, Mohr DN, Silverstein MD, Petterson TM, O’Fallon WM, Melton LJ 3rd. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med 2000; 160:761–768.
- Mohr DN, Silverstein MD, Heit JA, Petterson TM, O’Fallon WM, Melton LJ. The venous stasis syndrome after deep venous thrombosis or pulmonary embolism: a population-based study. Mayo Clin Proc 2000; 75:1249–1256.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Tapson VF. Acute pulmonary embolism. N Engl J Med 2008; 358:1037–1052.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Kreit JW. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest 2004; 125:1539–1545.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Comess KA, DeRook FA, Russell ML, Tognazzi-Evans TA, Beach KW. The incidence of pulmonary embolism in unexplained sudden cardiac arrest with pulseless electrical activity. Am J Med 2000; 109:351–356.
- Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol 1997; 30:1165–1171.
- Piazza G. Submassive pulmonary embolism. JAMA 2013; 309:171–180.
- Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 2008; 178:425–430.
- Vanni S, Polidori G, Vergara R, et al. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med 2009; 122:257–264.
- Amorim S, Dias P, Rodrigues RA, et al. Troponin I as a marker of right ventricular dysfunction and severity of pulmonary embolism. Rev Port Cardiol 2006; 25:181–186.
- Dellas C, Puls M, Lankeit M, et al. Elevated heart-type fatty acid-binding protein levels on admission predict an adverse outcome in normotensive patients with acute pulmonary embolism. J Am Coll Cardiol 2010; 55:2150–2157.
- Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord 2014; 14:64.
- Nazeyrollas P, Metz D, Jolly D, et al. Use of transthoracic Doppler echocardiography combined with clinical and electrocardiographic data to predict acute pulmonary embolism. Eur Heart J 1996;17: 779–786.
- Wake N, Kumamaru KK, George E, et al. Computed tomography and echocardiography in patients with acute pulmonary embolism: part 1: correlation of findings of right ventricular enlargement. J Thorac Imaging 2014; 29:W1–W6.
- Becattini C, Agnelli G, Germini F, Vedovati MC. Computed tomography to assess risk of death in acute pulmonary embolism: a meta-analysis. Eur Respir J 2014; 43:1678–1690.
- Konstantinides SV, Torbicki A, Agnelli G, et al; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069, 69a–69k.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Daley MJ, Lat I. Clinical controversies in thrombolytic therapy for the management of acute pulmonary embolism. Pharmacotherapy 2012; 32:158–172.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311:2414–2421.
- Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W; Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 2002; 347:1143–1150.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Wang C, Zhai Z, Yang Y, et al; China Venous Thromboembolism (VTE) Study Group. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest 2010; 137:254–262.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
- Stein PD, Alnas M, Beemath A, Patel NR. Outcome of pulmonary embolectomy. Am J Cardiol 2007; 99:421–423.
- He C, Von Segesser LK, Kappetein PA, Mestres CA, Smith JA, Choong CK. Acute pulmonary embolectomy. Eur J Cardiothorac Surg 2013; 43:1087–1095.
- Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg 2005; 129:1018–1023.
KEY POINTS
- Most patients with submassive pulmonary embolism do not need thrombolytic therapy.
- Identifying patients with submassive pulmonary embolism at highest risk of clinical deterioration can guide physicians to consider thrombolytic therapy.
- In clinical trials, thrombolytic therapy reduced the rates of secondary outcomes but did not reduce the rate of death in this patient population.
Phlegmasia cerulea dolens from radiation-induced venous stenosis
A 77-year-old man presented with a 5-day history of painful swelling of his right leg. He reported no trauma, no recent surgery, no history of thrombophilic disorder, and no prolonged immobilization. However, he had a history of prostate cancer, treated 10 years earlier with pelvic radiation.
Examination revealed massive right leg swelling extending from the thigh to the ankle, along with bluish-red skin discoloration (Figure 1). Doppler ultrasonography demonstrated acute thrombosis involving the right iliofemoral veins. These findings were consistent with phlegmasia cerulea dolens.
Urgent percutaneous catheter-directed thrombolysis was performed. Venography revealed extensive thrombosis of the femoral vein (Figure 2A) extending into the right external iliac vein. This was treated with catheter-directed pharmacomechanical thrombectomy.
Venography after this procedure showed significant improvement in venous blood flow (Figure 2B). However, stenosis of the right external iliac vein was also noted (Figure 2C) and was treated with balloon angioplasty (Figure 2D) followed by placement of a stent (14 × 40 mm).
In the immediate postprocedural period, there was marked reduction in swelling and normalization of skin color (Figure 3). The patient did not experience significant bleeding during or after the procedure. Treatment with intravenous unfractionated heparin was continued during the hospital stay, and he was discharged on warfarin with a therapeutic international normalized ratio. At a follow-up visit 3 months later, he was asymptomatic.
A RARE BUT SEVERE TYPE OF ACUTE DEEP VEIN THROMBOSIS
Phlegmasia cerulea dolens (painful cyanotic swollen leg) is a rare and severe form of acute deep vein thrombosis (DVT) characterized by marked limb pain, swelling, and blue discoloration.1 DVT is the most common cause of acute-onset unilateral leg pain, swelling, and skin discoloration.2
The differential diagnosis
The differential diagnosis includes infection (cellulitis, necrotizing fasciitis), compartment syndrome from limb injury, musculoskeletal conditions such as ruptured Baker cyst, venous stasis due to external compression (May-Thurner syndrome, iliac vein compression syndrome, pelvic tumor), acute limb ischemia from arterial obstruction, and complex regional pain syndrome (reflex sympathetic dystrophy).
Management recommendations
As in most cases of DVT, initial treatment of phlegmasia cerulea dolens involves systemic anticoagulation with heparin, elevation of the affected extremity, and fluid resuscitation if the patient is hypotensive. However, phlegmasia cerulea dolens is a major indication for catheter-directed thrombolysis,3,4 so an urgent vascular surgery or interventional cardiology consultation is also required. The American College of Chest Physicians recommends catheter-directed thrombolysis for acute DVT of the iliofemoral veins in patients with symptoms for less than 14 days, good functional capacity, and a life expectancy beyond 1 year.5 This intervention results in reduced incidence of postthrombotic syndrome and improved quality of life5,6 compared with anticoagulation therapy alone.
Who is at risk?
Risk factors for phlegmasia cerulea dolens include a history of malignancy, inherited or acquired thrombophilia, surgery, radiation therapy, trauma, placement of an inferior vena cava filter, and pregnancy. In our patient, the iliac vein stenosis most likely was the result of the radiation therapy he had undergone for prostate cancer.
Arterial stenosis is a well-known complication of radiation therapy and is associated with an increased risk of cardiovascular events.7,8 Radiation induces endothelial damage followed by proliferation of smooth muscle cells, resulting in luminal stenosis and thrombosis. At the cellular level, radiation leads to an acute increase in pro-inflammatory cytokines and endothelial adhesion molecules, causing the recruitment of inflammatory cells to radiation-exposed vessels and chronic activation of transcription factor NF-kappa B, leading to long-term inflammation and angiogenesis.9
Carotid, coronary, and iliac artery stenosis are known to occur around 10 years after radiation therapy to the head, neck, breast, and pelvis. Radiation-induced iliac vein stenosis is rare and can manifest as acute proximal DVT.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to leg edema of unclear etiology. J Am Board Fam Med 2006; 19:148–160.
- Casey ET, Murad MH, Zumaeta-Garcia M, et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012; 55:1463–1473.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99:365–375.
- Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol 2010; 55:1237–1239.
- Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010; 55:1227–1236.
A 77-year-old man presented with a 5-day history of painful swelling of his right leg. He reported no trauma, no recent surgery, no history of thrombophilic disorder, and no prolonged immobilization. However, he had a history of prostate cancer, treated 10 years earlier with pelvic radiation.
Examination revealed massive right leg swelling extending from the thigh to the ankle, along with bluish-red skin discoloration (Figure 1). Doppler ultrasonography demonstrated acute thrombosis involving the right iliofemoral veins. These findings were consistent with phlegmasia cerulea dolens.
Urgent percutaneous catheter-directed thrombolysis was performed. Venography revealed extensive thrombosis of the femoral vein (Figure 2A) extending into the right external iliac vein. This was treated with catheter-directed pharmacomechanical thrombectomy.
Venography after this procedure showed significant improvement in venous blood flow (Figure 2B). However, stenosis of the right external iliac vein was also noted (Figure 2C) and was treated with balloon angioplasty (Figure 2D) followed by placement of a stent (14 × 40 mm).
In the immediate postprocedural period, there was marked reduction in swelling and normalization of skin color (Figure 3). The patient did not experience significant bleeding during or after the procedure. Treatment with intravenous unfractionated heparin was continued during the hospital stay, and he was discharged on warfarin with a therapeutic international normalized ratio. At a follow-up visit 3 months later, he was asymptomatic.
A RARE BUT SEVERE TYPE OF ACUTE DEEP VEIN THROMBOSIS
Phlegmasia cerulea dolens (painful cyanotic swollen leg) is a rare and severe form of acute deep vein thrombosis (DVT) characterized by marked limb pain, swelling, and blue discoloration.1 DVT is the most common cause of acute-onset unilateral leg pain, swelling, and skin discoloration.2
The differential diagnosis
The differential diagnosis includes infection (cellulitis, necrotizing fasciitis), compartment syndrome from limb injury, musculoskeletal conditions such as ruptured Baker cyst, venous stasis due to external compression (May-Thurner syndrome, iliac vein compression syndrome, pelvic tumor), acute limb ischemia from arterial obstruction, and complex regional pain syndrome (reflex sympathetic dystrophy).
Management recommendations
As in most cases of DVT, initial treatment of phlegmasia cerulea dolens involves systemic anticoagulation with heparin, elevation of the affected extremity, and fluid resuscitation if the patient is hypotensive. However, phlegmasia cerulea dolens is a major indication for catheter-directed thrombolysis,3,4 so an urgent vascular surgery or interventional cardiology consultation is also required. The American College of Chest Physicians recommends catheter-directed thrombolysis for acute DVT of the iliofemoral veins in patients with symptoms for less than 14 days, good functional capacity, and a life expectancy beyond 1 year.5 This intervention results in reduced incidence of postthrombotic syndrome and improved quality of life5,6 compared with anticoagulation therapy alone.
Who is at risk?
Risk factors for phlegmasia cerulea dolens include a history of malignancy, inherited or acquired thrombophilia, surgery, radiation therapy, trauma, placement of an inferior vena cava filter, and pregnancy. In our patient, the iliac vein stenosis most likely was the result of the radiation therapy he had undergone for prostate cancer.
Arterial stenosis is a well-known complication of radiation therapy and is associated with an increased risk of cardiovascular events.7,8 Radiation induces endothelial damage followed by proliferation of smooth muscle cells, resulting in luminal stenosis and thrombosis. At the cellular level, radiation leads to an acute increase in pro-inflammatory cytokines and endothelial adhesion molecules, causing the recruitment of inflammatory cells to radiation-exposed vessels and chronic activation of transcription factor NF-kappa B, leading to long-term inflammation and angiogenesis.9
Carotid, coronary, and iliac artery stenosis are known to occur around 10 years after radiation therapy to the head, neck, breast, and pelvis. Radiation-induced iliac vein stenosis is rare and can manifest as acute proximal DVT.
A 77-year-old man presented with a 5-day history of painful swelling of his right leg. He reported no trauma, no recent surgery, no history of thrombophilic disorder, and no prolonged immobilization. However, he had a history of prostate cancer, treated 10 years earlier with pelvic radiation.
Examination revealed massive right leg swelling extending from the thigh to the ankle, along with bluish-red skin discoloration (Figure 1). Doppler ultrasonography demonstrated acute thrombosis involving the right iliofemoral veins. These findings were consistent with phlegmasia cerulea dolens.
Urgent percutaneous catheter-directed thrombolysis was performed. Venography revealed extensive thrombosis of the femoral vein (Figure 2A) extending into the right external iliac vein. This was treated with catheter-directed pharmacomechanical thrombectomy.
Venography after this procedure showed significant improvement in venous blood flow (Figure 2B). However, stenosis of the right external iliac vein was also noted (Figure 2C) and was treated with balloon angioplasty (Figure 2D) followed by placement of a stent (14 × 40 mm).
In the immediate postprocedural period, there was marked reduction in swelling and normalization of skin color (Figure 3). The patient did not experience significant bleeding during or after the procedure. Treatment with intravenous unfractionated heparin was continued during the hospital stay, and he was discharged on warfarin with a therapeutic international normalized ratio. At a follow-up visit 3 months later, he was asymptomatic.
A RARE BUT SEVERE TYPE OF ACUTE DEEP VEIN THROMBOSIS
Phlegmasia cerulea dolens (painful cyanotic swollen leg) is a rare and severe form of acute deep vein thrombosis (DVT) characterized by marked limb pain, swelling, and blue discoloration.1 DVT is the most common cause of acute-onset unilateral leg pain, swelling, and skin discoloration.2
The differential diagnosis
The differential diagnosis includes infection (cellulitis, necrotizing fasciitis), compartment syndrome from limb injury, musculoskeletal conditions such as ruptured Baker cyst, venous stasis due to external compression (May-Thurner syndrome, iliac vein compression syndrome, pelvic tumor), acute limb ischemia from arterial obstruction, and complex regional pain syndrome (reflex sympathetic dystrophy).
Management recommendations
As in most cases of DVT, initial treatment of phlegmasia cerulea dolens involves systemic anticoagulation with heparin, elevation of the affected extremity, and fluid resuscitation if the patient is hypotensive. However, phlegmasia cerulea dolens is a major indication for catheter-directed thrombolysis,3,4 so an urgent vascular surgery or interventional cardiology consultation is also required. The American College of Chest Physicians recommends catheter-directed thrombolysis for acute DVT of the iliofemoral veins in patients with symptoms for less than 14 days, good functional capacity, and a life expectancy beyond 1 year.5 This intervention results in reduced incidence of postthrombotic syndrome and improved quality of life5,6 compared with anticoagulation therapy alone.
Who is at risk?
Risk factors for phlegmasia cerulea dolens include a history of malignancy, inherited or acquired thrombophilia, surgery, radiation therapy, trauma, placement of an inferior vena cava filter, and pregnancy. In our patient, the iliac vein stenosis most likely was the result of the radiation therapy he had undergone for prostate cancer.
Arterial stenosis is a well-known complication of radiation therapy and is associated with an increased risk of cardiovascular events.7,8 Radiation induces endothelial damage followed by proliferation of smooth muscle cells, resulting in luminal stenosis and thrombosis. At the cellular level, radiation leads to an acute increase in pro-inflammatory cytokines and endothelial adhesion molecules, causing the recruitment of inflammatory cells to radiation-exposed vessels and chronic activation of transcription factor NF-kappa B, leading to long-term inflammation and angiogenesis.9
Carotid, coronary, and iliac artery stenosis are known to occur around 10 years after radiation therapy to the head, neck, breast, and pelvis. Radiation-induced iliac vein stenosis is rare and can manifest as acute proximal DVT.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to leg edema of unclear etiology. J Am Board Fam Med 2006; 19:148–160.
- Casey ET, Murad MH, Zumaeta-Garcia M, et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012; 55:1463–1473.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99:365–375.
- Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol 2010; 55:1237–1239.
- Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010; 55:1227–1236.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to leg edema of unclear etiology. J Am Board Fam Med 2006; 19:148–160.
- Casey ET, Murad MH, Zumaeta-Garcia M, et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012; 55:1463–1473.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99:365–375.
- Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol 2010; 55:1237–1239.
- Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010; 55:1227–1236.
Thrombolysis in submassive pulmonary embolism: Finding the balance
In this issue of the Journal, Ataya et al1 provide a comprehensive review of thrombolysis in submassive pulmonary embolism, a subject of much debate. In massive pulmonary embolism, thrombolytic therapy is usually indicated2; in submassive pulmonary embolism, the decision is not so clear. Which patients with submassive embolism would benefit from thrombolysis, and which patients require only anticoagulant therapy? The answer lies in finding the balance between the potential benefit of thrombolytic therapy—preventing death or hemodynamic collapse—and the numerically low but potentially catastrophic risk of intracranial bleeding.
In general, submassive pulmonary embolism refers to an acute pulmonary embolus serious enough to cause evidence of right ventricular dysfunction or necrosis but not hemodynamic instability (ie, with systolic blood pressure > 90 mm Hg) on presentation.3 It accounts for about 25% of cases of pulmonary embolism,4,5 and perhaps 0.5 to 1% of patients admitted to intensive care units across the country.6 The 30-day mortality rate can be as high as 30%, making it a condition that requires prompt identification and appropriate management.
But clinical trials have failed to demonstrate a substantial improvement in mortality rates with thrombolytic therapy in patients with submassive pulmonary embolism, and have shown improvement only in other clinical end points.7 Part of the problem is that this is a heterogeneous condition, posing a challenge for the optimal design and interpretation of studies.
WHO IS AT RISK OF DEATH OR DETERIORATION?
If clinicians could ascertain in each patient whether the risk-benefit ratio is favorable for thrombolytic therapy, it would be easier to provide optimal care. This is not a straightforward task, and it requires integration of clinical judgment, high index of suspicion for deterioration, and clinical tools.
One of the challenges is that it is difficult to identify normotensive patients at the highest risk of poor outcomes. Several factors are associated with a higher risk of death within 30 days (Table 1). While each of these has a negative predictive value of about 95% or even higher (meaning that it is very good at predicting who will not die), they all have very low positive predictive values (meaning that none of them, by itself, is very good at predicting who will die).
For this reason, a multimodal approach to risk stratification has emerged. For example, Jiménez et al8 showed that normotensive patients with acute pulmonary embolism and a combination of abnormal Simplified Pulmonary Embolism Severity Index, elevated B-type natriuretic peptide level, elevated troponin level, and lower-extremity deep vein thrombosis had a 26% rate of complications (death, hemodynamic collapse, or recurrent pulmonary embolism) within 30 days.
Bova et al9 showed that the combination of borderline low systolic blood pressure (90–100 mm Hg), tachycardia (heart rate ≥ 110 beats per minute), elevated troponin, and right ventricular dysfunction by echocardiography or computed tomography allowed for the separation of three groups with significantly different rates of poor outcomes.
WHO IS AT RISK OF BLEEDING?
Estimation of the risk of bleeding is currently less sophisticated, and we need a bleeding score to use in the setting of acute pulmonary embolism. A few studies have shed some light on this issue beyond the known absolute and relative contraindications to thrombolysis.
Ataya et al1 note a meta-analysis10 showing that systemic thrombolytic therapy was not associated with an increased risk of major bleeding in patients age 65 or younger. Similarly, a large observational study showed a strong association between the risk of intracerebral hemorrhage and increasing age11 and also identified comorbidities such as kidney disease as risk factors. While the frequently cited Pulmonary Embolism Thrombolysis trial12 showed a significantly higher risk of stroke with tenecteplase, careful review of its data reveals that all 10 of the 506 patients in the tenecteplase group who sustained a hemorrhagic stroke were age 65 or older.12
A TEAM APPROACH
Thus, in patients with acute pulmonary embolism, clinicians face the difficult task of assessing the patient’s risk of death and clinical worsening and balancing that risk against the risk of bleeding, to identify those who may benefit from early reperfusion therapies, including systemic thrombolysis, catheter-directed thrombolysis, mechanical treatment, and surgical embolectomy.
Given the absence of high-quality evidence to guide these decisions, several institutions have developed multidisciplinary pulmonary embolism response teams to provide rapid evaluation and risk stratification and to recommend and implement advanced therapies, as appropriate. This is a novel concept that is still evolving but holds promise, as it integrates the experience and expertise of physicians in multiple specialties, such as pulmonary and critical care medicine, vascular medicine, interventional radiology, interventional cardiology, emergency medicine, and cardiothoracic surgery, who can then fill the currently existing knowledge gaps for clinical care and, possibly, research.13
Early published experience has documented the feasibility of this multidisciplinary approach.14 The first 95 patients treated at Cleveland Clinic had a 30-day mortality rate of 3.2%, which was lower than the expected 9% rate predicted by the Pulmonary Embolism Severity Index score (unpublished observation).
Figure 1 shows the algorithm currently used by Cleveland Clinic’s pulmonary embolism response team, with the caveat that no algorithm can fully capture the extent of the complexities and discussions that each case triggers within the team.
TOWARD BETTER UNDERSTANDING
As Ataya et al point out,1 the current state of the evidence does not allow a clear, simplistic, one-size-fits-all approach. A question that arises from this controversial topic is whether we should look for markers of right ventricular dysfunction in every patient admitted with a diagnosis of pulmonary embolism, or only in those with a significant anatomic burden of clot on imaging. Would testing everyone be an appropriate way to identify patients at risk of further deterioration early and therefore prevent adverse outcomes in a timely manner? Or would it not be cost-effective and translate into ordering more diagnostic testing, as well as an increase in downstream workup with higher healthcare costs?
Once we better understand this condition and the factors that predict a higher risk of deterioration, we should be able to design prospective studies that can help elucidate the most appropriate diagnostic and therapeutic approach for such challenging cases. In the meantime, it is important to appraise the evidence in a critical way, as Ataya et al have done in their review.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. The role of thrombolytic therapy in patients with submassive pulmonary embolism. Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation 2005; 112:e28–e32.
- Busse LW, Vourlekis JS. Submassive pulmonary embolism. Crit Care Clin 2014; 30:447–473.
- Tapson VF. Acute pulmonary embolism. N Engl J Med 2008; 358:1037–1052.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Bahloul M, Chaari A, Kallel H, et al. Pulmonary embolism in intensive care unit: predictive factors, clinical manifestations and outcome. Ann Thorac Med 2010; 5:97–103.
- Piazza G, Goldhaber SZ. Fibrinolysis for acute pulmonary embolism. Vasc Med 2010; 15:419–428.
- Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med 2014; 189:718–726.
- Bova C, Sanchez O, Prandoni P, et al. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur Respir J 2014; 44:694–703.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311:2414–2421.
- Stein PD, Matta F, Steinberger DS, Keyes DC. Intracerebral hemorrhage with thrombolytic therapy for acute pulmonary embolism. Am J Med 2012; 125:50–56.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation 2016; 133:98–103.
- Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest 2016; 150:384–393.
In this issue of the Journal, Ataya et al1 provide a comprehensive review of thrombolysis in submassive pulmonary embolism, a subject of much debate. In massive pulmonary embolism, thrombolytic therapy is usually indicated2; in submassive pulmonary embolism, the decision is not so clear. Which patients with submassive embolism would benefit from thrombolysis, and which patients require only anticoagulant therapy? The answer lies in finding the balance between the potential benefit of thrombolytic therapy—preventing death or hemodynamic collapse—and the numerically low but potentially catastrophic risk of intracranial bleeding.
In general, submassive pulmonary embolism refers to an acute pulmonary embolus serious enough to cause evidence of right ventricular dysfunction or necrosis but not hemodynamic instability (ie, with systolic blood pressure > 90 mm Hg) on presentation.3 It accounts for about 25% of cases of pulmonary embolism,4,5 and perhaps 0.5 to 1% of patients admitted to intensive care units across the country.6 The 30-day mortality rate can be as high as 30%, making it a condition that requires prompt identification and appropriate management.
But clinical trials have failed to demonstrate a substantial improvement in mortality rates with thrombolytic therapy in patients with submassive pulmonary embolism, and have shown improvement only in other clinical end points.7 Part of the problem is that this is a heterogeneous condition, posing a challenge for the optimal design and interpretation of studies.
WHO IS AT RISK OF DEATH OR DETERIORATION?
If clinicians could ascertain in each patient whether the risk-benefit ratio is favorable for thrombolytic therapy, it would be easier to provide optimal care. This is not a straightforward task, and it requires integration of clinical judgment, high index of suspicion for deterioration, and clinical tools.
One of the challenges is that it is difficult to identify normotensive patients at the highest risk of poor outcomes. Several factors are associated with a higher risk of death within 30 days (Table 1). While each of these has a negative predictive value of about 95% or even higher (meaning that it is very good at predicting who will not die), they all have very low positive predictive values (meaning that none of them, by itself, is very good at predicting who will die).
For this reason, a multimodal approach to risk stratification has emerged. For example, Jiménez et al8 showed that normotensive patients with acute pulmonary embolism and a combination of abnormal Simplified Pulmonary Embolism Severity Index, elevated B-type natriuretic peptide level, elevated troponin level, and lower-extremity deep vein thrombosis had a 26% rate of complications (death, hemodynamic collapse, or recurrent pulmonary embolism) within 30 days.
Bova et al9 showed that the combination of borderline low systolic blood pressure (90–100 mm Hg), tachycardia (heart rate ≥ 110 beats per minute), elevated troponin, and right ventricular dysfunction by echocardiography or computed tomography allowed for the separation of three groups with significantly different rates of poor outcomes.
WHO IS AT RISK OF BLEEDING?
Estimation of the risk of bleeding is currently less sophisticated, and we need a bleeding score to use in the setting of acute pulmonary embolism. A few studies have shed some light on this issue beyond the known absolute and relative contraindications to thrombolysis.
Ataya et al1 note a meta-analysis10 showing that systemic thrombolytic therapy was not associated with an increased risk of major bleeding in patients age 65 or younger. Similarly, a large observational study showed a strong association between the risk of intracerebral hemorrhage and increasing age11 and also identified comorbidities such as kidney disease as risk factors. While the frequently cited Pulmonary Embolism Thrombolysis trial12 showed a significantly higher risk of stroke with tenecteplase, careful review of its data reveals that all 10 of the 506 patients in the tenecteplase group who sustained a hemorrhagic stroke were age 65 or older.12
A TEAM APPROACH
Thus, in patients with acute pulmonary embolism, clinicians face the difficult task of assessing the patient’s risk of death and clinical worsening and balancing that risk against the risk of bleeding, to identify those who may benefit from early reperfusion therapies, including systemic thrombolysis, catheter-directed thrombolysis, mechanical treatment, and surgical embolectomy.
Given the absence of high-quality evidence to guide these decisions, several institutions have developed multidisciplinary pulmonary embolism response teams to provide rapid evaluation and risk stratification and to recommend and implement advanced therapies, as appropriate. This is a novel concept that is still evolving but holds promise, as it integrates the experience and expertise of physicians in multiple specialties, such as pulmonary and critical care medicine, vascular medicine, interventional radiology, interventional cardiology, emergency medicine, and cardiothoracic surgery, who can then fill the currently existing knowledge gaps for clinical care and, possibly, research.13
Early published experience has documented the feasibility of this multidisciplinary approach.14 The first 95 patients treated at Cleveland Clinic had a 30-day mortality rate of 3.2%, which was lower than the expected 9% rate predicted by the Pulmonary Embolism Severity Index score (unpublished observation).
Figure 1 shows the algorithm currently used by Cleveland Clinic’s pulmonary embolism response team, with the caveat that no algorithm can fully capture the extent of the complexities and discussions that each case triggers within the team.
TOWARD BETTER UNDERSTANDING
As Ataya et al point out,1 the current state of the evidence does not allow a clear, simplistic, one-size-fits-all approach. A question that arises from this controversial topic is whether we should look for markers of right ventricular dysfunction in every patient admitted with a diagnosis of pulmonary embolism, or only in those with a significant anatomic burden of clot on imaging. Would testing everyone be an appropriate way to identify patients at risk of further deterioration early and therefore prevent adverse outcomes in a timely manner? Or would it not be cost-effective and translate into ordering more diagnostic testing, as well as an increase in downstream workup with higher healthcare costs?
Once we better understand this condition and the factors that predict a higher risk of deterioration, we should be able to design prospective studies that can help elucidate the most appropriate diagnostic and therapeutic approach for such challenging cases. In the meantime, it is important to appraise the evidence in a critical way, as Ataya et al have done in their review.
In this issue of the Journal, Ataya et al1 provide a comprehensive review of thrombolysis in submassive pulmonary embolism, a subject of much debate. In massive pulmonary embolism, thrombolytic therapy is usually indicated2; in submassive pulmonary embolism, the decision is not so clear. Which patients with submassive embolism would benefit from thrombolysis, and which patients require only anticoagulant therapy? The answer lies in finding the balance between the potential benefit of thrombolytic therapy—preventing death or hemodynamic collapse—and the numerically low but potentially catastrophic risk of intracranial bleeding.
In general, submassive pulmonary embolism refers to an acute pulmonary embolus serious enough to cause evidence of right ventricular dysfunction or necrosis but not hemodynamic instability (ie, with systolic blood pressure > 90 mm Hg) on presentation.3 It accounts for about 25% of cases of pulmonary embolism,4,5 and perhaps 0.5 to 1% of patients admitted to intensive care units across the country.6 The 30-day mortality rate can be as high as 30%, making it a condition that requires prompt identification and appropriate management.
But clinical trials have failed to demonstrate a substantial improvement in mortality rates with thrombolytic therapy in patients with submassive pulmonary embolism, and have shown improvement only in other clinical end points.7 Part of the problem is that this is a heterogeneous condition, posing a challenge for the optimal design and interpretation of studies.
WHO IS AT RISK OF DEATH OR DETERIORATION?
If clinicians could ascertain in each patient whether the risk-benefit ratio is favorable for thrombolytic therapy, it would be easier to provide optimal care. This is not a straightforward task, and it requires integration of clinical judgment, high index of suspicion for deterioration, and clinical tools.
One of the challenges is that it is difficult to identify normotensive patients at the highest risk of poor outcomes. Several factors are associated with a higher risk of death within 30 days (Table 1). While each of these has a negative predictive value of about 95% or even higher (meaning that it is very good at predicting who will not die), they all have very low positive predictive values (meaning that none of them, by itself, is very good at predicting who will die).
For this reason, a multimodal approach to risk stratification has emerged. For example, Jiménez et al8 showed that normotensive patients with acute pulmonary embolism and a combination of abnormal Simplified Pulmonary Embolism Severity Index, elevated B-type natriuretic peptide level, elevated troponin level, and lower-extremity deep vein thrombosis had a 26% rate of complications (death, hemodynamic collapse, or recurrent pulmonary embolism) within 30 days.
Bova et al9 showed that the combination of borderline low systolic blood pressure (90–100 mm Hg), tachycardia (heart rate ≥ 110 beats per minute), elevated troponin, and right ventricular dysfunction by echocardiography or computed tomography allowed for the separation of three groups with significantly different rates of poor outcomes.
WHO IS AT RISK OF BLEEDING?
Estimation of the risk of bleeding is currently less sophisticated, and we need a bleeding score to use in the setting of acute pulmonary embolism. A few studies have shed some light on this issue beyond the known absolute and relative contraindications to thrombolysis.
Ataya et al1 note a meta-analysis10 showing that systemic thrombolytic therapy was not associated with an increased risk of major bleeding in patients age 65 or younger. Similarly, a large observational study showed a strong association between the risk of intracerebral hemorrhage and increasing age11 and also identified comorbidities such as kidney disease as risk factors. While the frequently cited Pulmonary Embolism Thrombolysis trial12 showed a significantly higher risk of stroke with tenecteplase, careful review of its data reveals that all 10 of the 506 patients in the tenecteplase group who sustained a hemorrhagic stroke were age 65 or older.12
A TEAM APPROACH
Thus, in patients with acute pulmonary embolism, clinicians face the difficult task of assessing the patient’s risk of death and clinical worsening and balancing that risk against the risk of bleeding, to identify those who may benefit from early reperfusion therapies, including systemic thrombolysis, catheter-directed thrombolysis, mechanical treatment, and surgical embolectomy.
Given the absence of high-quality evidence to guide these decisions, several institutions have developed multidisciplinary pulmonary embolism response teams to provide rapid evaluation and risk stratification and to recommend and implement advanced therapies, as appropriate. This is a novel concept that is still evolving but holds promise, as it integrates the experience and expertise of physicians in multiple specialties, such as pulmonary and critical care medicine, vascular medicine, interventional radiology, interventional cardiology, emergency medicine, and cardiothoracic surgery, who can then fill the currently existing knowledge gaps for clinical care and, possibly, research.13
Early published experience has documented the feasibility of this multidisciplinary approach.14 The first 95 patients treated at Cleveland Clinic had a 30-day mortality rate of 3.2%, which was lower than the expected 9% rate predicted by the Pulmonary Embolism Severity Index score (unpublished observation).
Figure 1 shows the algorithm currently used by Cleveland Clinic’s pulmonary embolism response team, with the caveat that no algorithm can fully capture the extent of the complexities and discussions that each case triggers within the team.
TOWARD BETTER UNDERSTANDING
As Ataya et al point out,1 the current state of the evidence does not allow a clear, simplistic, one-size-fits-all approach. A question that arises from this controversial topic is whether we should look for markers of right ventricular dysfunction in every patient admitted with a diagnosis of pulmonary embolism, or only in those with a significant anatomic burden of clot on imaging. Would testing everyone be an appropriate way to identify patients at risk of further deterioration early and therefore prevent adverse outcomes in a timely manner? Or would it not be cost-effective and translate into ordering more diagnostic testing, as well as an increase in downstream workup with higher healthcare costs?
Once we better understand this condition and the factors that predict a higher risk of deterioration, we should be able to design prospective studies that can help elucidate the most appropriate diagnostic and therapeutic approach for such challenging cases. In the meantime, it is important to appraise the evidence in a critical way, as Ataya et al have done in their review.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. The role of thrombolytic therapy in patients with submassive pulmonary embolism. Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation 2005; 112:e28–e32.
- Busse LW, Vourlekis JS. Submassive pulmonary embolism. Crit Care Clin 2014; 30:447–473.
- Tapson VF. Acute pulmonary embolism. N Engl J Med 2008; 358:1037–1052.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Bahloul M, Chaari A, Kallel H, et al. Pulmonary embolism in intensive care unit: predictive factors, clinical manifestations and outcome. Ann Thorac Med 2010; 5:97–103.
- Piazza G, Goldhaber SZ. Fibrinolysis for acute pulmonary embolism. Vasc Med 2010; 15:419–428.
- Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med 2014; 189:718–726.
- Bova C, Sanchez O, Prandoni P, et al. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur Respir J 2014; 44:694–703.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311:2414–2421.
- Stein PD, Matta F, Steinberger DS, Keyes DC. Intracerebral hemorrhage with thrombolytic therapy for acute pulmonary embolism. Am J Med 2012; 125:50–56.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation 2016; 133:98–103.
- Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest 2016; 150:384–393.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. The role of thrombolytic therapy in patients with submassive pulmonary embolism. Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation 2005; 112:e28–e32.
- Busse LW, Vourlekis JS. Submassive pulmonary embolism. Crit Care Clin 2014; 30:447–473.
- Tapson VF. Acute pulmonary embolism. N Engl J Med 2008; 358:1037–1052.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Bahloul M, Chaari A, Kallel H, et al. Pulmonary embolism in intensive care unit: predictive factors, clinical manifestations and outcome. Ann Thorac Med 2010; 5:97–103.
- Piazza G, Goldhaber SZ. Fibrinolysis for acute pulmonary embolism. Vasc Med 2010; 15:419–428.
- Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med 2014; 189:718–726.
- Bova C, Sanchez O, Prandoni P, et al. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur Respir J 2014; 44:694–703.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311:2414–2421.
- Stein PD, Matta F, Steinberger DS, Keyes DC. Intracerebral hemorrhage with thrombolytic therapy for acute pulmonary embolism. Am J Med 2012; 125:50–56.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation 2016; 133:98–103.
- Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest 2016; 150:384–393.
Early TIPS effective in high-risk cirrhosis patients, but still underutilized
BOSTON – High-risk cirrhosis patients treated early with a transjugular intrahepatic portosystemic shunt (TIPS) showed increased survival rates and reduced rates of adverse events, according to a study.
The data were presented at the American Association for the Study of Liver Diseases by Virginia Hernandez-Gea, MD, a hepatologist at the Hospital Clinic in Barcelona.
In each study arm, three-quarters were men in their mid-50s. Cirrhosis in the non-TIPS group was alcohol-related in 57.4% of the cohort, compared with 71.2% of the group given early TIPS; roughly half of each group mentioned alcohol use in the past 3 months.
Also similar were Model for End-stage Liver Disease (MELD) scores: an average of 15.5 in the non-TIPS group, compared with 15 on average in the TIPS group. Nearly three-quarters of the TIPS group had a Child-Pugh C score, compared with 64% in the non-TIPS group. A Child-Pugh score with active bleeding was recorded in 28.8% of the TIPS group, compared with 36% in the non-TIPS group.
The transplant-free survival rate at 1 year in the standard care group was 61%, compared with 76% in the early TIPS group (P = .0175). The failure and bleeding rate at 1 year was significantly higher in the standard care group: 91%, compared with 68% in the early TIPS group (P = .004). Failure and bleeding rates in the Child-Pugh B and C groups across the study were similar.
Ascites at 1 year was seen in 88% of the standard care group, compared with in 64% of the study group. Rates of hepatic encephalopathy were similar in those with Child-Pugh B with active bleeding, and Child-Pugh C across both groups: 22% in the standard care group vs. 25% in the early TIPS group.
That there was no associated significant risk of hepatic encephalopathy in persons with acute variceal bleeding who were given early TIPS “strongly suggests that early TIPS should be included in clinical practice,” Dr. Hernandez-Gea said, noting that only 10% of the 34 sites in the study had used early TIPS. “We don’t really know why centers are not using this, since it is very difficult to find treatments that extend survival rates in this population.”
BOSTON – High-risk cirrhosis patients treated early with a transjugular intrahepatic portosystemic shunt (TIPS) showed increased survival rates and reduced rates of adverse events, according to a study.
The data were presented at the American Association for the Study of Liver Diseases by Virginia Hernandez-Gea, MD, a hepatologist at the Hospital Clinic in Barcelona.
In each study arm, three-quarters were men in their mid-50s. Cirrhosis in the non-TIPS group was alcohol-related in 57.4% of the cohort, compared with 71.2% of the group given early TIPS; roughly half of each group mentioned alcohol use in the past 3 months.
Also similar were Model for End-stage Liver Disease (MELD) scores: an average of 15.5 in the non-TIPS group, compared with 15 on average in the TIPS group. Nearly three-quarters of the TIPS group had a Child-Pugh C score, compared with 64% in the non-TIPS group. A Child-Pugh score with active bleeding was recorded in 28.8% of the TIPS group, compared with 36% in the non-TIPS group.
The transplant-free survival rate at 1 year in the standard care group was 61%, compared with 76% in the early TIPS group (P = .0175). The failure and bleeding rate at 1 year was significantly higher in the standard care group: 91%, compared with 68% in the early TIPS group (P = .004). Failure and bleeding rates in the Child-Pugh B and C groups across the study were similar.
Ascites at 1 year was seen in 88% of the standard care group, compared with in 64% of the study group. Rates of hepatic encephalopathy were similar in those with Child-Pugh B with active bleeding, and Child-Pugh C across both groups: 22% in the standard care group vs. 25% in the early TIPS group.
That there was no associated significant risk of hepatic encephalopathy in persons with acute variceal bleeding who were given early TIPS “strongly suggests that early TIPS should be included in clinical practice,” Dr. Hernandez-Gea said, noting that only 10% of the 34 sites in the study had used early TIPS. “We don’t really know why centers are not using this, since it is very difficult to find treatments that extend survival rates in this population.”
BOSTON – High-risk cirrhosis patients treated early with a transjugular intrahepatic portosystemic shunt (TIPS) showed increased survival rates and reduced rates of adverse events, according to a study.
The data were presented at the American Association for the Study of Liver Diseases by Virginia Hernandez-Gea, MD, a hepatologist at the Hospital Clinic in Barcelona.
In each study arm, three-quarters were men in their mid-50s. Cirrhosis in the non-TIPS group was alcohol-related in 57.4% of the cohort, compared with 71.2% of the group given early TIPS; roughly half of each group mentioned alcohol use in the past 3 months.
Also similar were Model for End-stage Liver Disease (MELD) scores: an average of 15.5 in the non-TIPS group, compared with 15 on average in the TIPS group. Nearly three-quarters of the TIPS group had a Child-Pugh C score, compared with 64% in the non-TIPS group. A Child-Pugh score with active bleeding was recorded in 28.8% of the TIPS group, compared with 36% in the non-TIPS group.
The transplant-free survival rate at 1 year in the standard care group was 61%, compared with 76% in the early TIPS group (P = .0175). The failure and bleeding rate at 1 year was significantly higher in the standard care group: 91%, compared with 68% in the early TIPS group (P = .004). Failure and bleeding rates in the Child-Pugh B and C groups across the study were similar.
Ascites at 1 year was seen in 88% of the standard care group, compared with in 64% of the study group. Rates of hepatic encephalopathy were similar in those with Child-Pugh B with active bleeding, and Child-Pugh C across both groups: 22% in the standard care group vs. 25% in the early TIPS group.
That there was no associated significant risk of hepatic encephalopathy in persons with acute variceal bleeding who were given early TIPS “strongly suggests that early TIPS should be included in clinical practice,” Dr. Hernandez-Gea said, noting that only 10% of the 34 sites in the study had used early TIPS. “We don’t really know why centers are not using this, since it is very difficult to find treatments that extend survival rates in this population.”
AT THE LIVER MEETING
Key clinical point:
Major finding: At 1 year post procedure, early TIPS was associated with better rates of survival and lower rates of adverse events, compared with those who did not receive early TIPS.
Data source: Multicenter, international observational study between 2011 and 2015 of 671 high-risk patients with cirrhosis managed according to current guidelines.
Disclosures: Dr. Hernandez-Gea did not have any relevant disclosures.
Is regional anesthesia safer in CEA?
COLUMBUS, OHIO – General anesthesia during carotid endarterectomy carries almost twice the risk of complications and unplanned intubation as regional anesthesia, but the latter approach, which is not available in all hospitals, has its own issues, an analysis of procedures from a statewide database in Michigan found.
“This study is timely because of CMS [Center for Medicare & Medicaid Services] initiatives tying reimbursement to specific quality measures,” Ahmad S Hussain, MD, of Wayne State University in Detroit said in reporting the study results at the annual meeting of the Midwestern Vascular Surgery Society.
Regional anesthesia in CEA emerged in the 1990s, Dr. Hussain said, and allows for more reliable neurologic monitoring and more direct evaluation of the need for stenting during CEA than general anesthesia, which requires continuous monitoring of cerebral perfusion with carotid stump pressures, electroencephalogram, and transcranial doppler.
The researchers retrospectively analyzed 4,558 patients who had CEA at hospitals participating in the Michigan Surgical Quality Cooperative from 2012 to 2014 – 4,008 of whom had general anesthesia and 550 regional anesthesia.
“Advocates for carotid endarterectomy with regional anesthesia cite a reduction in hemodynamic instability and the ability for neurological monitoring, but many still prefer general anesthesia because the benefits of regional anesthesia have not been clearly demonstrated, allowing that regional anesthesia may not be available in all centers and allowing that a certain amount of patient movement during the procedure may not be uniformly tolerated,” Dr. Hussain said.
General anesthesia patients in the study had more than twice the rate of any morbidity at 30 days than those who had regional, 8.7% vs. 4.2%, and significantly higher rates of unplanned intervention, 2.1% vs. 0.6%. Dr. Hussain said. However, the study could not determine differences in 30-day mortality or other key outcomes, such as rates of pneumonia, sepsis, deep vein thrombosis, or pulmonary embolism, becauseof insufficient sample sizes, Dr. Hussain said
The study found less significant differences between general and regional anesthesia techniques, respectively, in rates of extended length of stay, 12.1% vs. 9.5%; readmissions, 9.2% vs. 6.1%; and reoperation, 4.5% vs. 3%.
The retrospective study used two models to analyze odds ratios: Model 1 adjusted for case mix; and model 2 adjusted for case mix as fixed effects and site as a random effect. While the retrospective nature of the study may be a limitation, the findings support the use of regional anesthesia for CEA when available, Dr. Hussain said.
Dr. Hussain had no relationships to disclose.
COLUMBUS, OHIO – General anesthesia during carotid endarterectomy carries almost twice the risk of complications and unplanned intubation as regional anesthesia, but the latter approach, which is not available in all hospitals, has its own issues, an analysis of procedures from a statewide database in Michigan found.
“This study is timely because of CMS [Center for Medicare & Medicaid Services] initiatives tying reimbursement to specific quality measures,” Ahmad S Hussain, MD, of Wayne State University in Detroit said in reporting the study results at the annual meeting of the Midwestern Vascular Surgery Society.
Regional anesthesia in CEA emerged in the 1990s, Dr. Hussain said, and allows for more reliable neurologic monitoring and more direct evaluation of the need for stenting during CEA than general anesthesia, which requires continuous monitoring of cerebral perfusion with carotid stump pressures, electroencephalogram, and transcranial doppler.
The researchers retrospectively analyzed 4,558 patients who had CEA at hospitals participating in the Michigan Surgical Quality Cooperative from 2012 to 2014 – 4,008 of whom had general anesthesia and 550 regional anesthesia.
“Advocates for carotid endarterectomy with regional anesthesia cite a reduction in hemodynamic instability and the ability for neurological monitoring, but many still prefer general anesthesia because the benefits of regional anesthesia have not been clearly demonstrated, allowing that regional anesthesia may not be available in all centers and allowing that a certain amount of patient movement during the procedure may not be uniformly tolerated,” Dr. Hussain said.
General anesthesia patients in the study had more than twice the rate of any morbidity at 30 days than those who had regional, 8.7% vs. 4.2%, and significantly higher rates of unplanned intervention, 2.1% vs. 0.6%. Dr. Hussain said. However, the study could not determine differences in 30-day mortality or other key outcomes, such as rates of pneumonia, sepsis, deep vein thrombosis, or pulmonary embolism, becauseof insufficient sample sizes, Dr. Hussain said
The study found less significant differences between general and regional anesthesia techniques, respectively, in rates of extended length of stay, 12.1% vs. 9.5%; readmissions, 9.2% vs. 6.1%; and reoperation, 4.5% vs. 3%.
The retrospective study used two models to analyze odds ratios: Model 1 adjusted for case mix; and model 2 adjusted for case mix as fixed effects and site as a random effect. While the retrospective nature of the study may be a limitation, the findings support the use of regional anesthesia for CEA when available, Dr. Hussain said.
Dr. Hussain had no relationships to disclose.
COLUMBUS, OHIO – General anesthesia during carotid endarterectomy carries almost twice the risk of complications and unplanned intubation as regional anesthesia, but the latter approach, which is not available in all hospitals, has its own issues, an analysis of procedures from a statewide database in Michigan found.
“This study is timely because of CMS [Center for Medicare & Medicaid Services] initiatives tying reimbursement to specific quality measures,” Ahmad S Hussain, MD, of Wayne State University in Detroit said in reporting the study results at the annual meeting of the Midwestern Vascular Surgery Society.
Regional anesthesia in CEA emerged in the 1990s, Dr. Hussain said, and allows for more reliable neurologic monitoring and more direct evaluation of the need for stenting during CEA than general anesthesia, which requires continuous monitoring of cerebral perfusion with carotid stump pressures, electroencephalogram, and transcranial doppler.
The researchers retrospectively analyzed 4,558 patients who had CEA at hospitals participating in the Michigan Surgical Quality Cooperative from 2012 to 2014 – 4,008 of whom had general anesthesia and 550 regional anesthesia.
“Advocates for carotid endarterectomy with regional anesthesia cite a reduction in hemodynamic instability and the ability for neurological monitoring, but many still prefer general anesthesia because the benefits of regional anesthesia have not been clearly demonstrated, allowing that regional anesthesia may not be available in all centers and allowing that a certain amount of patient movement during the procedure may not be uniformly tolerated,” Dr. Hussain said.
General anesthesia patients in the study had more than twice the rate of any morbidity at 30 days than those who had regional, 8.7% vs. 4.2%, and significantly higher rates of unplanned intervention, 2.1% vs. 0.6%. Dr. Hussain said. However, the study could not determine differences in 30-day mortality or other key outcomes, such as rates of pneumonia, sepsis, deep vein thrombosis, or pulmonary embolism, becauseof insufficient sample sizes, Dr. Hussain said
The study found less significant differences between general and regional anesthesia techniques, respectively, in rates of extended length of stay, 12.1% vs. 9.5%; readmissions, 9.2% vs. 6.1%; and reoperation, 4.5% vs. 3%.
The retrospective study used two models to analyze odds ratios: Model 1 adjusted for case mix; and model 2 adjusted for case mix as fixed effects and site as a random effect. While the retrospective nature of the study may be a limitation, the findings support the use of regional anesthesia for CEA when available, Dr. Hussain said.
Dr. Hussain had no relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: General anesthesia for carotid endarterectomy carries a higher risk of complications and readmissions than regional anesthesia.
Major finding: Any morbidity after CEA with general anesthesia was 8.7% vs. 4.2% for regional anesthesia, and readmissions rates were 9.2% vs. 6.1%.
Data source: Retrospective analysis of 4,558 patients who had CEA between 2012 and 2014 at hospitals participating in the Michigan Surgical Quality Collaborative database.
Disclosures: Dr. Hussain reported having no financial disclosures.
Infection, readmission linked after open lower-extremity procedures
COLUMBUS, OHIO – Infections account for more than one-third of readmissions after endovascular lower-extremity procedures, but an analysis of these procedures over a 6-year period has identified a handful of factors, including an extended hospital stay, that may help vascular surgeons identify patients at greatest risk and reduce infection-related readmissions.
“Of a little over 7,000 patients that we evaluated with peripheral artery disease who underwent an elective lower-extremity procedure, we found an overall readmission rate of 10.9%; about 9.5% for those who underwent an open procedure and just over 12% for those who underwent an endovascular procedure,” Joseph C. Melvin, MD, of the University of Missouri Hospitals & Clinics in Columbia said at the annual meeting of the Midwestern Vascular Surgery Society.
While the readmission rate for open operations was lower, the infection rate at readmission was higher for open procedures: 45.5% (157 of 345 readmissions) vs. 31.1% (132 of 425 readmissions), Dr. Melvin said.
“The risk factors for diagnosis of infection at readmission we found to be significant were anemia, chronic kidney disease, and end-stage renal disease, any infection at the time of the index admission, specifically cellulitis or abscess of the lower extremity given the patient’s peripheral artery disease status, diabetes, and then complications including posthemorrhagic anemia,” Dr. Melvin said. Laboratory testing values at the time of index admissions confirmed the risk factors.
The investigators also used multivariable logistic regression models in the analysis and found that factors most predictive of an infection-related readmission were length of stay, having the procedure at a teaching facility, anemia, and infection at the index admission, Dr. Melvin said.
The surgical site was the most common source of the infection, and Staphylococcus “not surprisingly” accounted for 25% of pathogens, Dr. Melvin said. “But what we did find to be interesting was that just over 40% of patients were found to have a gram-negative bacteria isolated, which would come into play with our decision with regards to antibiotic treatment,” he said.
The data suggest that further evaluation of ways to decrease postoperative infections and use of broad-spectrum antibiotics during readmissions may improve outcomes after open lower-extremity procedures, Dr. Melvin said.
Dr. Melvin had no financial relationships to disclose.
COLUMBUS, OHIO – Infections account for more than one-third of readmissions after endovascular lower-extremity procedures, but an analysis of these procedures over a 6-year period has identified a handful of factors, including an extended hospital stay, that may help vascular surgeons identify patients at greatest risk and reduce infection-related readmissions.
“Of a little over 7,000 patients that we evaluated with peripheral artery disease who underwent an elective lower-extremity procedure, we found an overall readmission rate of 10.9%; about 9.5% for those who underwent an open procedure and just over 12% for those who underwent an endovascular procedure,” Joseph C. Melvin, MD, of the University of Missouri Hospitals & Clinics in Columbia said at the annual meeting of the Midwestern Vascular Surgery Society.
While the readmission rate for open operations was lower, the infection rate at readmission was higher for open procedures: 45.5% (157 of 345 readmissions) vs. 31.1% (132 of 425 readmissions), Dr. Melvin said.
“The risk factors for diagnosis of infection at readmission we found to be significant were anemia, chronic kidney disease, and end-stage renal disease, any infection at the time of the index admission, specifically cellulitis or abscess of the lower extremity given the patient’s peripheral artery disease status, diabetes, and then complications including posthemorrhagic anemia,” Dr. Melvin said. Laboratory testing values at the time of index admissions confirmed the risk factors.
The investigators also used multivariable logistic regression models in the analysis and found that factors most predictive of an infection-related readmission were length of stay, having the procedure at a teaching facility, anemia, and infection at the index admission, Dr. Melvin said.
The surgical site was the most common source of the infection, and Staphylococcus “not surprisingly” accounted for 25% of pathogens, Dr. Melvin said. “But what we did find to be interesting was that just over 40% of patients were found to have a gram-negative bacteria isolated, which would come into play with our decision with regards to antibiotic treatment,” he said.
The data suggest that further evaluation of ways to decrease postoperative infections and use of broad-spectrum antibiotics during readmissions may improve outcomes after open lower-extremity procedures, Dr. Melvin said.
Dr. Melvin had no financial relationships to disclose.
COLUMBUS, OHIO – Infections account for more than one-third of readmissions after endovascular lower-extremity procedures, but an analysis of these procedures over a 6-year period has identified a handful of factors, including an extended hospital stay, that may help vascular surgeons identify patients at greatest risk and reduce infection-related readmissions.
“Of a little over 7,000 patients that we evaluated with peripheral artery disease who underwent an elective lower-extremity procedure, we found an overall readmission rate of 10.9%; about 9.5% for those who underwent an open procedure and just over 12% for those who underwent an endovascular procedure,” Joseph C. Melvin, MD, of the University of Missouri Hospitals & Clinics in Columbia said at the annual meeting of the Midwestern Vascular Surgery Society.
While the readmission rate for open operations was lower, the infection rate at readmission was higher for open procedures: 45.5% (157 of 345 readmissions) vs. 31.1% (132 of 425 readmissions), Dr. Melvin said.
“The risk factors for diagnosis of infection at readmission we found to be significant were anemia, chronic kidney disease, and end-stage renal disease, any infection at the time of the index admission, specifically cellulitis or abscess of the lower extremity given the patient’s peripheral artery disease status, diabetes, and then complications including posthemorrhagic anemia,” Dr. Melvin said. Laboratory testing values at the time of index admissions confirmed the risk factors.
The investigators also used multivariable logistic regression models in the analysis and found that factors most predictive of an infection-related readmission were length of stay, having the procedure at a teaching facility, anemia, and infection at the index admission, Dr. Melvin said.
The surgical site was the most common source of the infection, and Staphylococcus “not surprisingly” accounted for 25% of pathogens, Dr. Melvin said. “But what we did find to be interesting was that just over 40% of patients were found to have a gram-negative bacteria isolated, which would come into play with our decision with regards to antibiotic treatment,” he said.
The data suggest that further evaluation of ways to decrease postoperative infections and use of broad-spectrum antibiotics during readmissions may improve outcomes after open lower-extremity procedures, Dr. Melvin said.
Dr. Melvin had no financial relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: Extended hospital stay and other factors can help identify patients at greatest risk for readmission due to infection.
Major finding: More than one-third of readmissions from lower-extremity procedures are the result of infections.
Data source: 7,089 elective lower extremity procedures selected from the Cerner Health Facts database.
Disclosures: Dr. Melvin reported having no financial disclosures.