User login
Focus on patient-level factors, postop complications to reduce readmissions
CHICAGO – Preadmission and postdischarge factors were important predictors of postoperative readmission in a large cohort of surgical patients, but the hospital course had little incremental impact on either readmissions or postdischarge complications in the cohort, according to a retrospective study of Veterans Affairs data.
The findings suggest that efforts to reduce postoperative readmissions should focus on enhanced postdischarge surveillance and early intervention, Dr. Melanie S. Morris of the University of Alabama at Birmingham reported at the annual meeting of the American Surgical Association.
To assess the relative contributions of patient factors, operative characteristics, and postoperative hospital course on readmissions, she and her colleagues evaluated 243,956 general, vascular, and orthopedic surgery patients in 121 VA hospitals. The overall readmission rate among the cohort was 11.1%, and for general, vascular, and orthopedic surgeries, the rates were 12.9%, 15.4%, and 7.6%, respectively; the average postoperative length of stay was 6.9 days, and 6.1% of patients experienced a predischarge complication.
Almost all readmissions occurred within 2 weeks of discharge, and for general surgery patients, most occurred within 1 week. The readmission rate for vascular surgery patients remained high beyond the 2-week mark.
An examination of the reasons for readmission showed that wound complications were the most common reason for readmission, and this was particularly true for vascular surgery patients, in whom 44% of readmissions were for wound complications, Dr. Morris said.
Gastrointestinal complications including ileus and obstruction were also common, accounting for nearly 28% of readmissions among general surgery patients, she said.
Importantly, when including preoperative data (such as demographics, comorbidities, social and behavioral factors, labs and vital signs, and planned procedure type), the variability in readmissions could only be explained 8.6% of the time, she said.
“Adding in operative data, such as procedure complexity and intraoperative blood transfusions, as well as postoperative course, added very little to our predictive ability. Including both of those groups, we could only explain 10% of the variation in readmission,” she said.
Including postdischarge data such as complications and emergency department utilization in the model increased predictive ability to 18%.
R2 and C-statistics comparing the sequentially built model showed that demographics and comorbidities contributed the most to predicting readmission risk, Dr. Morris said.
Modeling based on readmission reason and specialty improved predictive ability. For example, almost 12% of readmissions for wound complications among vascular surgery patients were predictable.
“Our best predictive ability was for orthopedic patients who were readmitted with pneumonia. We were able to predict that 14% of the time,” she said.
The findings were derived by merging VA Surgical Quality Improvement Program data from inpatient operations performed between 2007 and 2014 and involving at least a 2-day postoperative hospital stay, with clinical data including laboratory findings, vitals, prior health care utilization, and postoperative complications.
“We then grouped our variables of interest into the following categories: preoperative, operative, postoperative but predischarge, and postdischarge,” she explained, noting that logistic models predicting 30-day readmission were constructed by sequentially adding groups into the model. Models were compared by way of adjusted R2 and C-statistics.
Assuming postoperative readmissions are preventable suggests that they are linked to the quality of care during the index hospitalization. The current findings demonstrate the challenges in predicting readmissions, and are important given that hospitals with higher-than-expected readmission rates for certain diagnoses and procedures are fined by the Centers for Medicare & Medicaid Services; 54% of hospitals were fined in 2015, she said.
“Readmission is difficult to predict at the time of discharge despite exhaustive statistical modeling with very granular clinical patient-level detail. Preoperative patient factors and postdischarge complications contribute the most to predictive models. Efforts to decrease readmissions should focus on modifiable patient-level factors, transitions of care, and minimizing postoperative complications,” she concluded.
Dr. Morris reported having no disclosures.
The complete manuscript of this presentation is anticipated to be published in Annals of Surgery pending editorial review.
CHICAGO – Preadmission and postdischarge factors were important predictors of postoperative readmission in a large cohort of surgical patients, but the hospital course had little incremental impact on either readmissions or postdischarge complications in the cohort, according to a retrospective study of Veterans Affairs data.
The findings suggest that efforts to reduce postoperative readmissions should focus on enhanced postdischarge surveillance and early intervention, Dr. Melanie S. Morris of the University of Alabama at Birmingham reported at the annual meeting of the American Surgical Association.
To assess the relative contributions of patient factors, operative characteristics, and postoperative hospital course on readmissions, she and her colleagues evaluated 243,956 general, vascular, and orthopedic surgery patients in 121 VA hospitals. The overall readmission rate among the cohort was 11.1%, and for general, vascular, and orthopedic surgeries, the rates were 12.9%, 15.4%, and 7.6%, respectively; the average postoperative length of stay was 6.9 days, and 6.1% of patients experienced a predischarge complication.
Almost all readmissions occurred within 2 weeks of discharge, and for general surgery patients, most occurred within 1 week. The readmission rate for vascular surgery patients remained high beyond the 2-week mark.
An examination of the reasons for readmission showed that wound complications were the most common reason for readmission, and this was particularly true for vascular surgery patients, in whom 44% of readmissions were for wound complications, Dr. Morris said.
Gastrointestinal complications including ileus and obstruction were also common, accounting for nearly 28% of readmissions among general surgery patients, she said.
Importantly, when including preoperative data (such as demographics, comorbidities, social and behavioral factors, labs and vital signs, and planned procedure type), the variability in readmissions could only be explained 8.6% of the time, she said.
“Adding in operative data, such as procedure complexity and intraoperative blood transfusions, as well as postoperative course, added very little to our predictive ability. Including both of those groups, we could only explain 10% of the variation in readmission,” she said.
Including postdischarge data such as complications and emergency department utilization in the model increased predictive ability to 18%.
R2 and C-statistics comparing the sequentially built model showed that demographics and comorbidities contributed the most to predicting readmission risk, Dr. Morris said.
Modeling based on readmission reason and specialty improved predictive ability. For example, almost 12% of readmissions for wound complications among vascular surgery patients were predictable.
“Our best predictive ability was for orthopedic patients who were readmitted with pneumonia. We were able to predict that 14% of the time,” she said.
The findings were derived by merging VA Surgical Quality Improvement Program data from inpatient operations performed between 2007 and 2014 and involving at least a 2-day postoperative hospital stay, with clinical data including laboratory findings, vitals, prior health care utilization, and postoperative complications.
“We then grouped our variables of interest into the following categories: preoperative, operative, postoperative but predischarge, and postdischarge,” she explained, noting that logistic models predicting 30-day readmission were constructed by sequentially adding groups into the model. Models were compared by way of adjusted R2 and C-statistics.
Assuming postoperative readmissions are preventable suggests that they are linked to the quality of care during the index hospitalization. The current findings demonstrate the challenges in predicting readmissions, and are important given that hospitals with higher-than-expected readmission rates for certain diagnoses and procedures are fined by the Centers for Medicare & Medicaid Services; 54% of hospitals were fined in 2015, she said.
“Readmission is difficult to predict at the time of discharge despite exhaustive statistical modeling with very granular clinical patient-level detail. Preoperative patient factors and postdischarge complications contribute the most to predictive models. Efforts to decrease readmissions should focus on modifiable patient-level factors, transitions of care, and minimizing postoperative complications,” she concluded.
Dr. Morris reported having no disclosures.
The complete manuscript of this presentation is anticipated to be published in Annals of Surgery pending editorial review.
CHICAGO – Preadmission and postdischarge factors were important predictors of postoperative readmission in a large cohort of surgical patients, but the hospital course had little incremental impact on either readmissions or postdischarge complications in the cohort, according to a retrospective study of Veterans Affairs data.
The findings suggest that efforts to reduce postoperative readmissions should focus on enhanced postdischarge surveillance and early intervention, Dr. Melanie S. Morris of the University of Alabama at Birmingham reported at the annual meeting of the American Surgical Association.
To assess the relative contributions of patient factors, operative characteristics, and postoperative hospital course on readmissions, she and her colleagues evaluated 243,956 general, vascular, and orthopedic surgery patients in 121 VA hospitals. The overall readmission rate among the cohort was 11.1%, and for general, vascular, and orthopedic surgeries, the rates were 12.9%, 15.4%, and 7.6%, respectively; the average postoperative length of stay was 6.9 days, and 6.1% of patients experienced a predischarge complication.
Almost all readmissions occurred within 2 weeks of discharge, and for general surgery patients, most occurred within 1 week. The readmission rate for vascular surgery patients remained high beyond the 2-week mark.
An examination of the reasons for readmission showed that wound complications were the most common reason for readmission, and this was particularly true for vascular surgery patients, in whom 44% of readmissions were for wound complications, Dr. Morris said.
Gastrointestinal complications including ileus and obstruction were also common, accounting for nearly 28% of readmissions among general surgery patients, she said.
Importantly, when including preoperative data (such as demographics, comorbidities, social and behavioral factors, labs and vital signs, and planned procedure type), the variability in readmissions could only be explained 8.6% of the time, she said.
“Adding in operative data, such as procedure complexity and intraoperative blood transfusions, as well as postoperative course, added very little to our predictive ability. Including both of those groups, we could only explain 10% of the variation in readmission,” she said.
Including postdischarge data such as complications and emergency department utilization in the model increased predictive ability to 18%.
R2 and C-statistics comparing the sequentially built model showed that demographics and comorbidities contributed the most to predicting readmission risk, Dr. Morris said.
Modeling based on readmission reason and specialty improved predictive ability. For example, almost 12% of readmissions for wound complications among vascular surgery patients were predictable.
“Our best predictive ability was for orthopedic patients who were readmitted with pneumonia. We were able to predict that 14% of the time,” she said.
The findings were derived by merging VA Surgical Quality Improvement Program data from inpatient operations performed between 2007 and 2014 and involving at least a 2-day postoperative hospital stay, with clinical data including laboratory findings, vitals, prior health care utilization, and postoperative complications.
“We then grouped our variables of interest into the following categories: preoperative, operative, postoperative but predischarge, and postdischarge,” she explained, noting that logistic models predicting 30-day readmission were constructed by sequentially adding groups into the model. Models were compared by way of adjusted R2 and C-statistics.
Assuming postoperative readmissions are preventable suggests that they are linked to the quality of care during the index hospitalization. The current findings demonstrate the challenges in predicting readmissions, and are important given that hospitals with higher-than-expected readmission rates for certain diagnoses and procedures are fined by the Centers for Medicare & Medicaid Services; 54% of hospitals were fined in 2015, she said.
“Readmission is difficult to predict at the time of discharge despite exhaustive statistical modeling with very granular clinical patient-level detail. Preoperative patient factors and postdischarge complications contribute the most to predictive models. Efforts to decrease readmissions should focus on modifiable patient-level factors, transitions of care, and minimizing postoperative complications,” she concluded.
Dr. Morris reported having no disclosures.
The complete manuscript of this presentation is anticipated to be published in Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: Preadmission and postdischarge factors were important predictors of postoperative readmission in a large cohort of surgical patients, but the hospital course had little incremental impact on either readmissions or postdischarge complications.
Major finding: Including both preoperative and operative data in the model predicted only 10% of the variability in readmission rates.
Data source: A retrospective study of data for nearly 244,000 VA patients.
Disclosures: Dr. Morris reported having no disclosures.
Robotic vascular surgery: Ready for prime time?
A single-center experience using the da Vinci robotic system to perform vascular procedures demonstrated the safety and feasibility of this technique in different areas of vascular surgery.
Dr. Petr Štádler and his colleagues at the No Homolce Hospital in Prague reported on 310 robotic-assisted vascular procedures performed between November 2005 and May 2014 with the aid of the da Vinci system. They concluded that robotic-assisted vascular procedures added to the speed and relative simplicity of construction of vascular anastomoses.
The patient cohort had procedures consisting of 224 robotic occlusive disease treatments (group 1), 65 robotic aorto-illiac aneurysm surgeries (group II), and 21 other robotic procedures (group III) as reported online in the European Journal of Vascular and Endovascular Surgery (2016. doi: 10.1016/j.ejvs.2016.02.016).
A total of 298 cases (96.1%) were successfully completed robotically, with conversion required in 10 cases; 2 patients were inoperable. The overall 30-day mortality rate was 0.3% for the entire cohort, and only two (0.6%) late prosthetic infections were seen. The median operating time was 204 min, the median anastomosis time was 29 min, and median blood loss was 571 mL.
In comparing groups I and II, group I required an operative time of 194 min, compared with 253 min in group II. Mean aortic cross-clamp time was 37 min in group I and 93 min in group II, while the mean blood loss was greater in group II (1,210 mL) as compared with group 1 (320 mL).
“The robotic system provides a real opportunity for minimally invasive surgery in the field of vascular surgery ... with all its advantages. Robotic AAA [abdominal aortic aneurysm] and aortofemoral bypass represent the standard operations in vascular surgery and they are not only possible, but safe and effective,” said Dr. Štádler and his colleagues. They added, however, that “further randomized studies are needed to ensure its benefits and the cost-effectiveness of robotic vascular surgery, compared with open and laparoscopic repair.”
Dr, Štádler and his colleagues reported that they had no disclosures.
When we examine the data presented by the authors of this paper closely, we see vast differences between the group I patients (bypasses for aortoiliac occlusive disease) and the group II patients (repair of aortoiliac aneurysms). In the occlusive group (group I), the operative time averaged 194 minutes. However, in the aneurysm group, the surgical repair of an aneurysm took over 4 hours. Another broad discrepancy between groups I and II is evident in examining cross-clamp time. In the occlusive group, the cross-clamp was 37 minutes; however, aneurysm patients required 93 minutes of cross-clamp to complete the proximal anastomosis. Similar disparities are seen in mean blood loss. Patients with occlusive disease lost an average of 320 mL of blood, while aneurysm patients lost 1,210 mL
 |
Dr. Mark A. Adelman |
The authors have been clever in combining these two groups as a single cohort.
However, I might argue that by segregating the groups, we might find that occlusive disease is well treated with robotically assisted surgery, but aneurysm repair should be left to open or endovascular techniques.
In addition to the data disparities, there are several practical limitations to performing robotic aortic surgery. Learning robotic techniques requires significant additional surgical training that is typically not within the skill set of a vascular surgeon. Who will devote the time and resources toward training vascular surgeons? Presently, there are increased hardware and operating room times associated with robotic-assisted surgeries. Because of the bulkiness of the robotic system and need for space for mechanical arms, large operating rooms must be utilized to perform robotic procedures.
Although I have not performed robotic surgery, I understand the tactile feedback, or haptics are significantly reduced when operating with the robot. Lastly, during this era of value-based medicine, is robotic surgery too expensive? The current cost of a robot approaches $2 million, and robotic arms have a limited life expectancy.
In summary, the authors have not demonstrated that this procedure is safe in aneurysm patients, or generalizable across all vascular surgeons given the lack of training paradigm. Further, robotic procedures are probably not cost effective in this very cost-sensitive health care environment. When I was training under Dr. Frank Cole Spencer in general surgery, he was fond of saying “just because you can teach a dog to ride a bicycle, it does not mean that you should.” As Dr. Juan Parodi will remind us, if the technology becomes more affordable, and changes significantly, we must all remain open minded. But currently, this technology is not yet ready for prime time. In its current state, this dog will not be learning to ride this robotic bike.
Dr. Mark A. Adelman is the Frank J. Veith, MD Professor, chief of vascular and endovascular surgery, and vice chair for strategy and business development, department of surgery, NYU Langone Medical Center, New York.
When we examine the data presented by the authors of this paper closely, we see vast differences between the group I patients (bypasses for aortoiliac occlusive disease) and the group II patients (repair of aortoiliac aneurysms). In the occlusive group (group I), the operative time averaged 194 minutes. However, in the aneurysm group, the surgical repair of an aneurysm took over 4 hours. Another broad discrepancy between groups I and II is evident in examining cross-clamp time. In the occlusive group, the cross-clamp was 37 minutes; however, aneurysm patients required 93 minutes of cross-clamp to complete the proximal anastomosis. Similar disparities are seen in mean blood loss. Patients with occlusive disease lost an average of 320 mL of blood, while aneurysm patients lost 1,210 mL
 |
Dr. Mark A. Adelman |
The authors have been clever in combining these two groups as a single cohort.
However, I might argue that by segregating the groups, we might find that occlusive disease is well treated with robotically assisted surgery, but aneurysm repair should be left to open or endovascular techniques.
In addition to the data disparities, there are several practical limitations to performing robotic aortic surgery. Learning robotic techniques requires significant additional surgical training that is typically not within the skill set of a vascular surgeon. Who will devote the time and resources toward training vascular surgeons? Presently, there are increased hardware and operating room times associated with robotic-assisted surgeries. Because of the bulkiness of the robotic system and need for space for mechanical arms, large operating rooms must be utilized to perform robotic procedures.
Although I have not performed robotic surgery, I understand the tactile feedback, or haptics are significantly reduced when operating with the robot. Lastly, during this era of value-based medicine, is robotic surgery too expensive? The current cost of a robot approaches $2 million, and robotic arms have a limited life expectancy.
In summary, the authors have not demonstrated that this procedure is safe in aneurysm patients, or generalizable across all vascular surgeons given the lack of training paradigm. Further, robotic procedures are probably not cost effective in this very cost-sensitive health care environment. When I was training under Dr. Frank Cole Spencer in general surgery, he was fond of saying “just because you can teach a dog to ride a bicycle, it does not mean that you should.” As Dr. Juan Parodi will remind us, if the technology becomes more affordable, and changes significantly, we must all remain open minded. But currently, this technology is not yet ready for prime time. In its current state, this dog will not be learning to ride this robotic bike.
Dr. Mark A. Adelman is the Frank J. Veith, MD Professor, chief of vascular and endovascular surgery, and vice chair for strategy and business development, department of surgery, NYU Langone Medical Center, New York.
When we examine the data presented by the authors of this paper closely, we see vast differences between the group I patients (bypasses for aortoiliac occlusive disease) and the group II patients (repair of aortoiliac aneurysms). In the occlusive group (group I), the operative time averaged 194 minutes. However, in the aneurysm group, the surgical repair of an aneurysm took over 4 hours. Another broad discrepancy between groups I and II is evident in examining cross-clamp time. In the occlusive group, the cross-clamp was 37 minutes; however, aneurysm patients required 93 minutes of cross-clamp to complete the proximal anastomosis. Similar disparities are seen in mean blood loss. Patients with occlusive disease lost an average of 320 mL of blood, while aneurysm patients lost 1,210 mL
 |
Dr. Mark A. Adelman |
The authors have been clever in combining these two groups as a single cohort.
However, I might argue that by segregating the groups, we might find that occlusive disease is well treated with robotically assisted surgery, but aneurysm repair should be left to open or endovascular techniques.
In addition to the data disparities, there are several practical limitations to performing robotic aortic surgery. Learning robotic techniques requires significant additional surgical training that is typically not within the skill set of a vascular surgeon. Who will devote the time and resources toward training vascular surgeons? Presently, there are increased hardware and operating room times associated with robotic-assisted surgeries. Because of the bulkiness of the robotic system and need for space for mechanical arms, large operating rooms must be utilized to perform robotic procedures.
Although I have not performed robotic surgery, I understand the tactile feedback, or haptics are significantly reduced when operating with the robot. Lastly, during this era of value-based medicine, is robotic surgery too expensive? The current cost of a robot approaches $2 million, and robotic arms have a limited life expectancy.
In summary, the authors have not demonstrated that this procedure is safe in aneurysm patients, or generalizable across all vascular surgeons given the lack of training paradigm. Further, robotic procedures are probably not cost effective in this very cost-sensitive health care environment. When I was training under Dr. Frank Cole Spencer in general surgery, he was fond of saying “just because you can teach a dog to ride a bicycle, it does not mean that you should.” As Dr. Juan Parodi will remind us, if the technology becomes more affordable, and changes significantly, we must all remain open minded. But currently, this technology is not yet ready for prime time. In its current state, this dog will not be learning to ride this robotic bike.
Dr. Mark A. Adelman is the Frank J. Veith, MD Professor, chief of vascular and endovascular surgery, and vice chair for strategy and business development, department of surgery, NYU Langone Medical Center, New York.
A single-center experience using the da Vinci robotic system to perform vascular procedures demonstrated the safety and feasibility of this technique in different areas of vascular surgery.
Dr. Petr Štádler and his colleagues at the No Homolce Hospital in Prague reported on 310 robotic-assisted vascular procedures performed between November 2005 and May 2014 with the aid of the da Vinci system. They concluded that robotic-assisted vascular procedures added to the speed and relative simplicity of construction of vascular anastomoses.
The patient cohort had procedures consisting of 224 robotic occlusive disease treatments (group 1), 65 robotic aorto-illiac aneurysm surgeries (group II), and 21 other robotic procedures (group III) as reported online in the European Journal of Vascular and Endovascular Surgery (2016. doi: 10.1016/j.ejvs.2016.02.016).
A total of 298 cases (96.1%) were successfully completed robotically, with conversion required in 10 cases; 2 patients were inoperable. The overall 30-day mortality rate was 0.3% for the entire cohort, and only two (0.6%) late prosthetic infections were seen. The median operating time was 204 min, the median anastomosis time was 29 min, and median blood loss was 571 mL.
In comparing groups I and II, group I required an operative time of 194 min, compared with 253 min in group II. Mean aortic cross-clamp time was 37 min in group I and 93 min in group II, while the mean blood loss was greater in group II (1,210 mL) as compared with group 1 (320 mL).
“The robotic system provides a real opportunity for minimally invasive surgery in the field of vascular surgery ... with all its advantages. Robotic AAA [abdominal aortic aneurysm] and aortofemoral bypass represent the standard operations in vascular surgery and they are not only possible, but safe and effective,” said Dr. Štádler and his colleagues. They added, however, that “further randomized studies are needed to ensure its benefits and the cost-effectiveness of robotic vascular surgery, compared with open and laparoscopic repair.”
Dr, Štádler and his colleagues reported that they had no disclosures.
A single-center experience using the da Vinci robotic system to perform vascular procedures demonstrated the safety and feasibility of this technique in different areas of vascular surgery.
Dr. Petr Štádler and his colleagues at the No Homolce Hospital in Prague reported on 310 robotic-assisted vascular procedures performed between November 2005 and May 2014 with the aid of the da Vinci system. They concluded that robotic-assisted vascular procedures added to the speed and relative simplicity of construction of vascular anastomoses.
The patient cohort had procedures consisting of 224 robotic occlusive disease treatments (group 1), 65 robotic aorto-illiac aneurysm surgeries (group II), and 21 other robotic procedures (group III) as reported online in the European Journal of Vascular and Endovascular Surgery (2016. doi: 10.1016/j.ejvs.2016.02.016).
A total of 298 cases (96.1%) were successfully completed robotically, with conversion required in 10 cases; 2 patients were inoperable. The overall 30-day mortality rate was 0.3% for the entire cohort, and only two (0.6%) late prosthetic infections were seen. The median operating time was 204 min, the median anastomosis time was 29 min, and median blood loss was 571 mL.
In comparing groups I and II, group I required an operative time of 194 min, compared with 253 min in group II. Mean aortic cross-clamp time was 37 min in group I and 93 min in group II, while the mean blood loss was greater in group II (1,210 mL) as compared with group 1 (320 mL).
“The robotic system provides a real opportunity for minimally invasive surgery in the field of vascular surgery ... with all its advantages. Robotic AAA [abdominal aortic aneurysm] and aortofemoral bypass represent the standard operations in vascular surgery and they are not only possible, but safe and effective,” said Dr. Štádler and his colleagues. They added, however, that “further randomized studies are needed to ensure its benefits and the cost-effectiveness of robotic vascular surgery, compared with open and laparoscopic repair.”
Dr, Štádler and his colleagues reported that they had no disclosures.
FROM EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY
Key clinical point: Robotic-assisted vascular surgery procedures appeared safe and provided benefits in speed and simplicity for vascular anastomoses.
Major finding: A total of 298 (96.1%) cases were successfully completed robotically, with a 30-day mortality of 0.3% and two (0.6%) late prosthetic infections seen.
Data source: A prospective study was performed assessing 310 robotic-assisted vascular procedures.
Disclosures: The authors reported that they had no disclosures.
How can I predict bleeding in my elderly patient taking anticoagulants?
We have tools to predict bleeding risk, but their predictive value is modest, and the estimated risk of bleeding is often outweighed by the benefits of anticoagulant therapy.
Anticoagulant therapy is commonly prescribed for conditions that disproportionately affect the elderly, including atrial fibrillation, venous thromboembolism, and valvular heart disease. Though anticoagulants are highly effective in preventing clots, they also significantly increase the risk of bleeding. Since older age is a risk factor for bleeding as well as thrombosis, it is essential to weigh the risks and benefits of anticoagulants for each patient.
WHAT KINDS OF BLEEDING DEVELOP IN PATIENTS ON ANTICOAGULANTS?
Patients taking anticoagulants have roughly double the risk of bleeding compared with patients not on anticoagulants.1 Bleeding rates tend to be slightly higher in patients taking anticoagulants for venous thromboembolism than in those taking them for atrial fibrillation. The average yearly risk of a “major” anticoagulant-associated bleeding event (eg, requiring transfusion or intervention or occurring in a critical anatomic site) is about 2% to 3%, with most of the bleeding being gastrointestinal.2
Intracranial hemorrhage is by far the most deadly complication of anticoagulant therapy: it causes 90% of deaths and disability from warfarin-associated hemorrhage and is associated with a death rate over 50%; however, it is much less common than gastrointestinal bleeding.3 Anticoagulant therapy increases the risk of intracranial hemorrhage by only 0.2% per year.1
RISK-PREDICTION TOOLS HAVE LIMITATIONS
Not all patients have the same risk of bleeding when taking anticoagulants. Many factors in addition to advanced age have been associated with increased bleeding risk, including coexisting medical conditions (such as malignancy, prior stroke or bleeding event, and renal insufficiency), medications (particularly aspirin, nonsteroidal anti-inflammatory drugs, and other antiplatelet drugs), and the timing and intensity of anticoagulation therapy.4
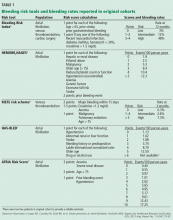
Scoring tools have been developed to identify patients at higher risk of bleeding (Table 1).4–9 The various schemes incorporate many of the same variables, such as older age, renal impairment, and history of bleeding, but some include additional risk factors while others are more parsimonious. They also differ in how individual risk factors are weighted to generate a final risk score.
In terms of predictive ability, none of the available risk schemes appears to be vastly superior, and their ability to predict hemorrhage is modest at best. There is also no universal or well-established threshold at which the risk of bleeding is so high that one would not consider anticoagulants. In fact, a “high-risk” patient may have an aggregate bleeding rate of only 4% to 6% per year. Using risk schemes such as ATRIA,5 HEMORR2HAGES,6 and HAS-BLED7 may be more useful because they provide an estimate of bleeding risk for each point on the scale.
Moreover, the current tools to predict bleeding risk have several other limitations. They were developed in patients already taking anticoagulants and so probably underestimate the actual risk of hemorrhage, as people who could not take anticoagulants were excluded, most likely because they were at high risk of bleeding. Therefore, bleeding risk tools probably apply best to a patient for whom anticoagulation can be considered.
Some clinical variables are necessarily broad. For example, “prior bleeding” is a risk factor included in several risk scores, but does not distinguish between massive variceal bleeding and minor hemorrhoidal bleeding.
Risk scores do not effectively predict intracranial hemorrhage.
Finally, these risk tools were developed in patients taking vitamin K antagonists, and it is not yet established that they can effectively predict hemorrhage related to other, newer anticoagulants.
WHEN DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
For patients with atrial fibrillation, the net clinical benefit of anticoagulation (strokes prevented minus bleeding events induced) increases as the risk of stroke rises. Updated guidelines for managing atrial fibrillation now recommend anticoagulation for most patients.10
For most older patients with atrial fibrillation, the decision to anticoagulate may not change even if a bleeding risk tool indicates a high bleeding risk.11 For example, a patient with a history of ischemic stroke will generally derive more benefit than harm from anticoagulants. The primary exception is in patients with prior lobar intracranial hemorrhage, because of the high risk of rebleeding and the worse outcomes associated with intracranial hemorrhage.12 As a general rule, most patients with atrial fibrillation and an additional risk factor for stroke should be considered for anticoagulant therapy unless they have a history of lobar intracranial hemorrhage.
Anticoagulation may be deferred if the patient is at the lower end of the stroke risk spectrum and if the bleeding risk is calculated to be high. However, as noted before, current bleeding risk tools probably do not capture the experiences of patients at the extremes of high bleeding risk, so clinical judgment continues to be important. In addition, forgoing anticoagulation could be reasonable even in patients at high risk for recurrent stroke if their life expectancy is limited, if anticoagulation is unacceptably burdensome, or if it is not within their goals and preferences.
WHAT ABOUT FALL RISK?
Fall risk commonly deters clinicians from prescribing anticoagulants because of the fear of causing intracranial hemorrhage. In particular, falls increase the risk for subdural hematoma, which has a death rate comparable to that of ischemic stroke.13
Studies have had difficulty quantifying the exact risk associated with falls because these patients are less likely to be prescribed anticoagulants. One decision analysis estimated that a person would have to fall about 300 times per year before the risk of intracranial hemorrhage outweighed the benefits from stroke reduction.14 Studies have found that patients at high risk of falls have a higher risk of intracranial hemorrhage, but that this risk is counterbalanced by an even greater risk of ischemic stroke.15
Therefore, if the baseline risk of ischemic stroke is high, anticoagulation is still favored.
WHEN SHOULD I USE A BLEEDING RISK TOOL?
Despite their limitations, bleeding risk tools are useful in clinical practice when estimates of bleeding risk affect clinical behavior. They are most helpful for patients at the lower end of the stroke or thromboembolism risk spectrum, where the decision to anticoagulate is strongly influenced by bleeding risk. Risk tools may also be helpful when counseling patients about their bleeding risk off and on anticoagulants.
Finally, recognizing that a patient is at high bleeding risk may lead the clinician to consider closer monitoring of anticoagulants or to implement strategies to reduce the risk.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Lopes LC, Spencer FA, Neumann I, et al. Bleeding risk in atrial fibrillation patients taking vitamin K antagonists: systematic review and meta-analysis. Clin Pharmacol Ther 2013; 94:367–375.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Lopes RD, Crowley MJ, Shah BR, et al. Stroke prevention in atrial fibrillation. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Aug. Report No.: 13-EHC113-EF.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J 2006; 151:713–719.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998; 105:91–99.
- Nieto JA, Solano R, Iglesias NT, et al, for the RIETE Investigators. Validation of a score for predicting fatal bleeding in patients receiving anticoagulation for venous thromboembolism. Thrombosis Res 2013; 132:175–179.
- January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130:2071–2104.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003; 34:1710–1716.
- Fang MC, Go AS, Chang Y, et al. Thirty-day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke 2012; 43:1795–1799.
- Man-Son-Hing M, Laupacis A. Anticoagulant-related bleeding in older persons with atrial fibrillation: physicians' fears often unfounded. Arch Intern Med 2003; 163:1580–1586.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
We have tools to predict bleeding risk, but their predictive value is modest, and the estimated risk of bleeding is often outweighed by the benefits of anticoagulant therapy.
Anticoagulant therapy is commonly prescribed for conditions that disproportionately affect the elderly, including atrial fibrillation, venous thromboembolism, and valvular heart disease. Though anticoagulants are highly effective in preventing clots, they also significantly increase the risk of bleeding. Since older age is a risk factor for bleeding as well as thrombosis, it is essential to weigh the risks and benefits of anticoagulants for each patient.
WHAT KINDS OF BLEEDING DEVELOP IN PATIENTS ON ANTICOAGULANTS?
Patients taking anticoagulants have roughly double the risk of bleeding compared with patients not on anticoagulants.1 Bleeding rates tend to be slightly higher in patients taking anticoagulants for venous thromboembolism than in those taking them for atrial fibrillation. The average yearly risk of a “major” anticoagulant-associated bleeding event (eg, requiring transfusion or intervention or occurring in a critical anatomic site) is about 2% to 3%, with most of the bleeding being gastrointestinal.2
Intracranial hemorrhage is by far the most deadly complication of anticoagulant therapy: it causes 90% of deaths and disability from warfarin-associated hemorrhage and is associated with a death rate over 50%; however, it is much less common than gastrointestinal bleeding.3 Anticoagulant therapy increases the risk of intracranial hemorrhage by only 0.2% per year.1
RISK-PREDICTION TOOLS HAVE LIMITATIONS
Not all patients have the same risk of bleeding when taking anticoagulants. Many factors in addition to advanced age have been associated with increased bleeding risk, including coexisting medical conditions (such as malignancy, prior stroke or bleeding event, and renal insufficiency), medications (particularly aspirin, nonsteroidal anti-inflammatory drugs, and other antiplatelet drugs), and the timing and intensity of anticoagulation therapy.4
Scoring tools have been developed to identify patients at higher risk of bleeding (Table 1).4–9 The various schemes incorporate many of the same variables, such as older age, renal impairment, and history of bleeding, but some include additional risk factors while others are more parsimonious. They also differ in how individual risk factors are weighted to generate a final risk score.
In terms of predictive ability, none of the available risk schemes appears to be vastly superior, and their ability to predict hemorrhage is modest at best. There is also no universal or well-established threshold at which the risk of bleeding is so high that one would not consider anticoagulants. In fact, a “high-risk” patient may have an aggregate bleeding rate of only 4% to 6% per year. Using risk schemes such as ATRIA,5 HEMORR2HAGES,6 and HAS-BLED7 may be more useful because they provide an estimate of bleeding risk for each point on the scale.
Moreover, the current tools to predict bleeding risk have several other limitations. They were developed in patients already taking anticoagulants and so probably underestimate the actual risk of hemorrhage, as people who could not take anticoagulants were excluded, most likely because they were at high risk of bleeding. Therefore, bleeding risk tools probably apply best to a patient for whom anticoagulation can be considered.
Some clinical variables are necessarily broad. For example, “prior bleeding” is a risk factor included in several risk scores, but does not distinguish between massive variceal bleeding and minor hemorrhoidal bleeding.
Risk scores do not effectively predict intracranial hemorrhage.
Finally, these risk tools were developed in patients taking vitamin K antagonists, and it is not yet established that they can effectively predict hemorrhage related to other, newer anticoagulants.
WHEN DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
For patients with atrial fibrillation, the net clinical benefit of anticoagulation (strokes prevented minus bleeding events induced) increases as the risk of stroke rises. Updated guidelines for managing atrial fibrillation now recommend anticoagulation for most patients.10
For most older patients with atrial fibrillation, the decision to anticoagulate may not change even if a bleeding risk tool indicates a high bleeding risk.11 For example, a patient with a history of ischemic stroke will generally derive more benefit than harm from anticoagulants. The primary exception is in patients with prior lobar intracranial hemorrhage, because of the high risk of rebleeding and the worse outcomes associated with intracranial hemorrhage.12 As a general rule, most patients with atrial fibrillation and an additional risk factor for stroke should be considered for anticoagulant therapy unless they have a history of lobar intracranial hemorrhage.
Anticoagulation may be deferred if the patient is at the lower end of the stroke risk spectrum and if the bleeding risk is calculated to be high. However, as noted before, current bleeding risk tools probably do not capture the experiences of patients at the extremes of high bleeding risk, so clinical judgment continues to be important. In addition, forgoing anticoagulation could be reasonable even in patients at high risk for recurrent stroke if their life expectancy is limited, if anticoagulation is unacceptably burdensome, or if it is not within their goals and preferences.
WHAT ABOUT FALL RISK?
Fall risk commonly deters clinicians from prescribing anticoagulants because of the fear of causing intracranial hemorrhage. In particular, falls increase the risk for subdural hematoma, which has a death rate comparable to that of ischemic stroke.13
Studies have had difficulty quantifying the exact risk associated with falls because these patients are less likely to be prescribed anticoagulants. One decision analysis estimated that a person would have to fall about 300 times per year before the risk of intracranial hemorrhage outweighed the benefits from stroke reduction.14 Studies have found that patients at high risk of falls have a higher risk of intracranial hemorrhage, but that this risk is counterbalanced by an even greater risk of ischemic stroke.15
Therefore, if the baseline risk of ischemic stroke is high, anticoagulation is still favored.
WHEN SHOULD I USE A BLEEDING RISK TOOL?
Despite their limitations, bleeding risk tools are useful in clinical practice when estimates of bleeding risk affect clinical behavior. They are most helpful for patients at the lower end of the stroke or thromboembolism risk spectrum, where the decision to anticoagulate is strongly influenced by bleeding risk. Risk tools may also be helpful when counseling patients about their bleeding risk off and on anticoagulants.
Finally, recognizing that a patient is at high bleeding risk may lead the clinician to consider closer monitoring of anticoagulants or to implement strategies to reduce the risk.
We have tools to predict bleeding risk, but their predictive value is modest, and the estimated risk of bleeding is often outweighed by the benefits of anticoagulant therapy.
Anticoagulant therapy is commonly prescribed for conditions that disproportionately affect the elderly, including atrial fibrillation, venous thromboembolism, and valvular heart disease. Though anticoagulants are highly effective in preventing clots, they also significantly increase the risk of bleeding. Since older age is a risk factor for bleeding as well as thrombosis, it is essential to weigh the risks and benefits of anticoagulants for each patient.
WHAT KINDS OF BLEEDING DEVELOP IN PATIENTS ON ANTICOAGULANTS?
Patients taking anticoagulants have roughly double the risk of bleeding compared with patients not on anticoagulants.1 Bleeding rates tend to be slightly higher in patients taking anticoagulants for venous thromboembolism than in those taking them for atrial fibrillation. The average yearly risk of a “major” anticoagulant-associated bleeding event (eg, requiring transfusion or intervention or occurring in a critical anatomic site) is about 2% to 3%, with most of the bleeding being gastrointestinal.2
Intracranial hemorrhage is by far the most deadly complication of anticoagulant therapy: it causes 90% of deaths and disability from warfarin-associated hemorrhage and is associated with a death rate over 50%; however, it is much less common than gastrointestinal bleeding.3 Anticoagulant therapy increases the risk of intracranial hemorrhage by only 0.2% per year.1
RISK-PREDICTION TOOLS HAVE LIMITATIONS
Not all patients have the same risk of bleeding when taking anticoagulants. Many factors in addition to advanced age have been associated with increased bleeding risk, including coexisting medical conditions (such as malignancy, prior stroke or bleeding event, and renal insufficiency), medications (particularly aspirin, nonsteroidal anti-inflammatory drugs, and other antiplatelet drugs), and the timing and intensity of anticoagulation therapy.4
Scoring tools have been developed to identify patients at higher risk of bleeding (Table 1).4–9 The various schemes incorporate many of the same variables, such as older age, renal impairment, and history of bleeding, but some include additional risk factors while others are more parsimonious. They also differ in how individual risk factors are weighted to generate a final risk score.
In terms of predictive ability, none of the available risk schemes appears to be vastly superior, and their ability to predict hemorrhage is modest at best. There is also no universal or well-established threshold at which the risk of bleeding is so high that one would not consider anticoagulants. In fact, a “high-risk” patient may have an aggregate bleeding rate of only 4% to 6% per year. Using risk schemes such as ATRIA,5 HEMORR2HAGES,6 and HAS-BLED7 may be more useful because they provide an estimate of bleeding risk for each point on the scale.
Moreover, the current tools to predict bleeding risk have several other limitations. They were developed in patients already taking anticoagulants and so probably underestimate the actual risk of hemorrhage, as people who could not take anticoagulants were excluded, most likely because they were at high risk of bleeding. Therefore, bleeding risk tools probably apply best to a patient for whom anticoagulation can be considered.
Some clinical variables are necessarily broad. For example, “prior bleeding” is a risk factor included in several risk scores, but does not distinguish between massive variceal bleeding and minor hemorrhoidal bleeding.
Risk scores do not effectively predict intracranial hemorrhage.
Finally, these risk tools were developed in patients taking vitamin K antagonists, and it is not yet established that they can effectively predict hemorrhage related to other, newer anticoagulants.
WHEN DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
For patients with atrial fibrillation, the net clinical benefit of anticoagulation (strokes prevented minus bleeding events induced) increases as the risk of stroke rises. Updated guidelines for managing atrial fibrillation now recommend anticoagulation for most patients.10
For most older patients with atrial fibrillation, the decision to anticoagulate may not change even if a bleeding risk tool indicates a high bleeding risk.11 For example, a patient with a history of ischemic stroke will generally derive more benefit than harm from anticoagulants. The primary exception is in patients with prior lobar intracranial hemorrhage, because of the high risk of rebleeding and the worse outcomes associated with intracranial hemorrhage.12 As a general rule, most patients with atrial fibrillation and an additional risk factor for stroke should be considered for anticoagulant therapy unless they have a history of lobar intracranial hemorrhage.
Anticoagulation may be deferred if the patient is at the lower end of the stroke risk spectrum and if the bleeding risk is calculated to be high. However, as noted before, current bleeding risk tools probably do not capture the experiences of patients at the extremes of high bleeding risk, so clinical judgment continues to be important. In addition, forgoing anticoagulation could be reasonable even in patients at high risk for recurrent stroke if their life expectancy is limited, if anticoagulation is unacceptably burdensome, or if it is not within their goals and preferences.
WHAT ABOUT FALL RISK?
Fall risk commonly deters clinicians from prescribing anticoagulants because of the fear of causing intracranial hemorrhage. In particular, falls increase the risk for subdural hematoma, which has a death rate comparable to that of ischemic stroke.13
Studies have had difficulty quantifying the exact risk associated with falls because these patients are less likely to be prescribed anticoagulants. One decision analysis estimated that a person would have to fall about 300 times per year before the risk of intracranial hemorrhage outweighed the benefits from stroke reduction.14 Studies have found that patients at high risk of falls have a higher risk of intracranial hemorrhage, but that this risk is counterbalanced by an even greater risk of ischemic stroke.15
Therefore, if the baseline risk of ischemic stroke is high, anticoagulation is still favored.
WHEN SHOULD I USE A BLEEDING RISK TOOL?
Despite their limitations, bleeding risk tools are useful in clinical practice when estimates of bleeding risk affect clinical behavior. They are most helpful for patients at the lower end of the stroke or thromboembolism risk spectrum, where the decision to anticoagulate is strongly influenced by bleeding risk. Risk tools may also be helpful when counseling patients about their bleeding risk off and on anticoagulants.
Finally, recognizing that a patient is at high bleeding risk may lead the clinician to consider closer monitoring of anticoagulants or to implement strategies to reduce the risk.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Lopes LC, Spencer FA, Neumann I, et al. Bleeding risk in atrial fibrillation patients taking vitamin K antagonists: systematic review and meta-analysis. Clin Pharmacol Ther 2013; 94:367–375.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Lopes RD, Crowley MJ, Shah BR, et al. Stroke prevention in atrial fibrillation. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Aug. Report No.: 13-EHC113-EF.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J 2006; 151:713–719.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998; 105:91–99.
- Nieto JA, Solano R, Iglesias NT, et al, for the RIETE Investigators. Validation of a score for predicting fatal bleeding in patients receiving anticoagulation for venous thromboembolism. Thrombosis Res 2013; 132:175–179.
- January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130:2071–2104.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003; 34:1710–1716.
- Fang MC, Go AS, Chang Y, et al. Thirty-day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke 2012; 43:1795–1799.
- Man-Son-Hing M, Laupacis A. Anticoagulant-related bleeding in older persons with atrial fibrillation: physicians' fears often unfounded. Arch Intern Med 2003; 163:1580–1586.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Lopes LC, Spencer FA, Neumann I, et al. Bleeding risk in atrial fibrillation patients taking vitamin K antagonists: systematic review and meta-analysis. Clin Pharmacol Ther 2013; 94:367–375.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Lopes RD, Crowley MJ, Shah BR, et al. Stroke prevention in atrial fibrillation. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Aug. Report No.: 13-EHC113-EF.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J 2006; 151:713–719.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998; 105:91–99.
- Nieto JA, Solano R, Iglesias NT, et al, for the RIETE Investigators. Validation of a score for predicting fatal bleeding in patients receiving anticoagulation for venous thromboembolism. Thrombosis Res 2013; 132:175–179.
- January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130:2071–2104.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003; 34:1710–1716.
- Fang MC, Go AS, Chang Y, et al. Thirty-day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke 2012; 43:1795–1799.
- Man-Son-Hing M, Laupacis A. Anticoagulant-related bleeding in older persons with atrial fibrillation: physicians' fears often unfounded. Arch Intern Med 2003; 163:1580–1586.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
Predicting is tough, especially about the future
Quinn and Fang, in this issue of the Journal discuss efforts to predict bleeding complications associated with anticoagulant therapy in elderly patients. They note, as others have suggested, that we may fear the risk of severe anticoagulant-associated bleeding more than is warranted based on the data. The level of that fear and the risk of bleeding depend on the specific need for anticoagulant therapy in a given patient and on the risk of serious adverse outcomes from thrombosis that the anticoagulation is supposed to prevent. All prediction models are based on an “average” patient with certain characteristics. But of course none of our patients are average.
The studies Quinn and Fang discuss focus on vitamin K antagonist therapy. There is probably not enough practice-based or trial-based evidence yet to evaluate the risks associated with the new generation of anticoagulants.
All prediction models have limitations. The recent discussion on establishing a risk-based strategy to guide institution of lipid-lowering therapy highlights the challenges inherent in trying to base therapeutic decisions on predictive models. But however imperfect, models are still widely used to predict fracture risk in patients being considered for bone antiresorptive therapy and to predict the need for anticoagulation therapy or further diagnostic testing in patients with potential deep vein thrombosis or atrial fibrillation.
The decision to start anticoagulation in an elderly patient is often informed by the possibility of an easily recognized and feared risk factor for bleeding complications—falling. Falls are certainly important and are a major contributor to subdural hematoma and complicated hip fracture. But there are more common causes of severe bleeding complications that are less easily predicted by functional assessment of the patient. Nonetheless, fall risk can be lessened by prescribing exercise programs such as tai chi to improve balance, limiting the use of drugs associated with falls in the elderly, perhaps correcting hyponatremia, and testing for orthostatic hypotension as part of the physical examination. (Mild compression stockings and medication adjustment may reduce orthostasis.) Some of these interventions are easily accomplished, and probably should be done with all of our elderly and frail patients.
As we build more risk calculators into our electronic medical records, we must continue to consider their limitations as well as their specific utility. To paraphrase Yogi Berra, making predictions is tough, especially about the future.
Quinn and Fang, in this issue of the Journal discuss efforts to predict bleeding complications associated with anticoagulant therapy in elderly patients. They note, as others have suggested, that we may fear the risk of severe anticoagulant-associated bleeding more than is warranted based on the data. The level of that fear and the risk of bleeding depend on the specific need for anticoagulant therapy in a given patient and on the risk of serious adverse outcomes from thrombosis that the anticoagulation is supposed to prevent. All prediction models are based on an “average” patient with certain characteristics. But of course none of our patients are average.
The studies Quinn and Fang discuss focus on vitamin K antagonist therapy. There is probably not enough practice-based or trial-based evidence yet to evaluate the risks associated with the new generation of anticoagulants.
All prediction models have limitations. The recent discussion on establishing a risk-based strategy to guide institution of lipid-lowering therapy highlights the challenges inherent in trying to base therapeutic decisions on predictive models. But however imperfect, models are still widely used to predict fracture risk in patients being considered for bone antiresorptive therapy and to predict the need for anticoagulation therapy or further diagnostic testing in patients with potential deep vein thrombosis or atrial fibrillation.
The decision to start anticoagulation in an elderly patient is often informed by the possibility of an easily recognized and feared risk factor for bleeding complications—falling. Falls are certainly important and are a major contributor to subdural hematoma and complicated hip fracture. But there are more common causes of severe bleeding complications that are less easily predicted by functional assessment of the patient. Nonetheless, fall risk can be lessened by prescribing exercise programs such as tai chi to improve balance, limiting the use of drugs associated with falls in the elderly, perhaps correcting hyponatremia, and testing for orthostatic hypotension as part of the physical examination. (Mild compression stockings and medication adjustment may reduce orthostasis.) Some of these interventions are easily accomplished, and probably should be done with all of our elderly and frail patients.
As we build more risk calculators into our electronic medical records, we must continue to consider their limitations as well as their specific utility. To paraphrase Yogi Berra, making predictions is tough, especially about the future.
Quinn and Fang, in this issue of the Journal discuss efforts to predict bleeding complications associated with anticoagulant therapy in elderly patients. They note, as others have suggested, that we may fear the risk of severe anticoagulant-associated bleeding more than is warranted based on the data. The level of that fear and the risk of bleeding depend on the specific need for anticoagulant therapy in a given patient and on the risk of serious adverse outcomes from thrombosis that the anticoagulation is supposed to prevent. All prediction models are based on an “average” patient with certain characteristics. But of course none of our patients are average.
The studies Quinn and Fang discuss focus on vitamin K antagonist therapy. There is probably not enough practice-based or trial-based evidence yet to evaluate the risks associated with the new generation of anticoagulants.
All prediction models have limitations. The recent discussion on establishing a risk-based strategy to guide institution of lipid-lowering therapy highlights the challenges inherent in trying to base therapeutic decisions on predictive models. But however imperfect, models are still widely used to predict fracture risk in patients being considered for bone antiresorptive therapy and to predict the need for anticoagulation therapy or further diagnostic testing in patients with potential deep vein thrombosis or atrial fibrillation.
The decision to start anticoagulation in an elderly patient is often informed by the possibility of an easily recognized and feared risk factor for bleeding complications—falling. Falls are certainly important and are a major contributor to subdural hematoma and complicated hip fracture. But there are more common causes of severe bleeding complications that are less easily predicted by functional assessment of the patient. Nonetheless, fall risk can be lessened by prescribing exercise programs such as tai chi to improve balance, limiting the use of drugs associated with falls in the elderly, perhaps correcting hyponatremia, and testing for orthostatic hypotension as part of the physical examination. (Mild compression stockings and medication adjustment may reduce orthostasis.) Some of these interventions are easily accomplished, and probably should be done with all of our elderly and frail patients.
As we build more risk calculators into our electronic medical records, we must continue to consider their limitations as well as their specific utility. To paraphrase Yogi Berra, making predictions is tough, especially about the future.
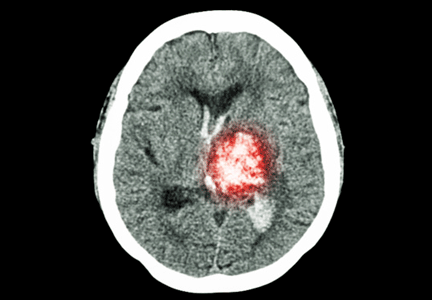
Endovascular thrombectomy procedure volume for stroke may not affect outcomes
VANCOUVER – The relationship between hospitals’ procedural volume and patient outcomes that has been observed for many cardiovascular interventions and other surgeries does not hold for endovascular mechanical thrombectomy procedures for acute ischemic stroke, according to an analysis of cases during 2008-2011 in the Nationwide Inpatient Sample.
In-hospital mortality and rates for any complications were not associated with high or low endovascular mechanical thrombectomy (EMT) volume at hospitals across the United States in the analysis of 13,502 adult patients hospitalized with a primary diagnosis of acute ischemic stroke and treated with EMT, neurology resident Dr. Abhishek Lunagariya of the University of Florida, Gainesville, reported at the annual meeting of the American Academy of Neurology.
A smaller prior study of 2,749 EMTs done in 296 hospitals in 2008 showed lower mortality in high-volume hospitals that performed 10 or more of the procedures per year (J Stroke Cerebrovasc Dis. 2013 Nov; 22[8]:1263-9).
Of the 13,502 EMTs in the study, 25% occurred at low-volume hospitals performing less than 10 per year. Low-volume hospitals had higher in-hospital mortality than did higher-volume centers performing 10 or more of the procedures per year in an unadjusted comparison (26% vs. 21%). A comparison of a combined endpoint for any complications (in-hospital mortality, intracerebral hemorrhage, and vascular complications) was also significantly in favor of high-volume hospitals (34% vs. 30%).
However, in a multivariate hierarchical model, low-volume hospitals were not associated with higher in-hospital mortality (odds ratio, 0.95; 95% confidence interval, 0.74-1.23) or rate of any complications (OR, 0.96; 95% CI, 0.76-1.21). These analyses were adjusted for age, gender, ethnicity, primary payer, median household income, hospital region/teaching status/location/bed size, Charlson Comorbidity Index, calendar year, and use of intravenous tissue plasminogen activator.
Dr. Lunagariya noted that he and his associates could not adjust the comparisons for National Institutes of Health Stroke Scale scores because they are not recorded in the National Inpatient Sample. They also could not examine what happened to patients after discharge.
Dr. Lunagariya suggested a variety of possible reasons that might help to explain the lack of an association between hospital procedure volume and outcomes after adjustment: the availability of better thrombectomy devices since the smaller 2008 study, lesser operator variability, favorable patient selection, and an increased skill set of operators working at low-volume hospitals.
One audience member noted that some endovascular interventionalists will operate at both high-volume and low-volume hospitals and could account for some of the findings. That indeed might be happening more often and needs to happen more often, Dr. Lunagariya said in an interview, in order to combat the “common belief” that it would be better to wait for a patient to undergo the procedure at a high- rather than low-volume hospital. Patients who receive initial care for stroke at a low-volume hospital but are not stable enough or do not have enough time to be transferred could benefit from EMT if an interventionalist who performs EMT drove there instead, he said.
With even newer devices now available that are thought to be easier to use, Dr. Lunagariya suggested that the similarity in outcomes at low- and higher-volume centers may not change in updated analyses of more recent EMT procedures for ischemic stroke.
The investigators received no funding for the study, and they reported having no financial disclosures.
VANCOUVER – The relationship between hospitals’ procedural volume and patient outcomes that has been observed for many cardiovascular interventions and other surgeries does not hold for endovascular mechanical thrombectomy procedures for acute ischemic stroke, according to an analysis of cases during 2008-2011 in the Nationwide Inpatient Sample.
In-hospital mortality and rates for any complications were not associated with high or low endovascular mechanical thrombectomy (EMT) volume at hospitals across the United States in the analysis of 13,502 adult patients hospitalized with a primary diagnosis of acute ischemic stroke and treated with EMT, neurology resident Dr. Abhishek Lunagariya of the University of Florida, Gainesville, reported at the annual meeting of the American Academy of Neurology.
A smaller prior study of 2,749 EMTs done in 296 hospitals in 2008 showed lower mortality in high-volume hospitals that performed 10 or more of the procedures per year (J Stroke Cerebrovasc Dis. 2013 Nov; 22[8]:1263-9).
Of the 13,502 EMTs in the study, 25% occurred at low-volume hospitals performing less than 10 per year. Low-volume hospitals had higher in-hospital mortality than did higher-volume centers performing 10 or more of the procedures per year in an unadjusted comparison (26% vs. 21%). A comparison of a combined endpoint for any complications (in-hospital mortality, intracerebral hemorrhage, and vascular complications) was also significantly in favor of high-volume hospitals (34% vs. 30%).
However, in a multivariate hierarchical model, low-volume hospitals were not associated with higher in-hospital mortality (odds ratio, 0.95; 95% confidence interval, 0.74-1.23) or rate of any complications (OR, 0.96; 95% CI, 0.76-1.21). These analyses were adjusted for age, gender, ethnicity, primary payer, median household income, hospital region/teaching status/location/bed size, Charlson Comorbidity Index, calendar year, and use of intravenous tissue plasminogen activator.
Dr. Lunagariya noted that he and his associates could not adjust the comparisons for National Institutes of Health Stroke Scale scores because they are not recorded in the National Inpatient Sample. They also could not examine what happened to patients after discharge.
Dr. Lunagariya suggested a variety of possible reasons that might help to explain the lack of an association between hospital procedure volume and outcomes after adjustment: the availability of better thrombectomy devices since the smaller 2008 study, lesser operator variability, favorable patient selection, and an increased skill set of operators working at low-volume hospitals.
One audience member noted that some endovascular interventionalists will operate at both high-volume and low-volume hospitals and could account for some of the findings. That indeed might be happening more often and needs to happen more often, Dr. Lunagariya said in an interview, in order to combat the “common belief” that it would be better to wait for a patient to undergo the procedure at a high- rather than low-volume hospital. Patients who receive initial care for stroke at a low-volume hospital but are not stable enough or do not have enough time to be transferred could benefit from EMT if an interventionalist who performs EMT drove there instead, he said.
With even newer devices now available that are thought to be easier to use, Dr. Lunagariya suggested that the similarity in outcomes at low- and higher-volume centers may not change in updated analyses of more recent EMT procedures for ischemic stroke.
The investigators received no funding for the study, and they reported having no financial disclosures.
VANCOUVER – The relationship between hospitals’ procedural volume and patient outcomes that has been observed for many cardiovascular interventions and other surgeries does not hold for endovascular mechanical thrombectomy procedures for acute ischemic stroke, according to an analysis of cases during 2008-2011 in the Nationwide Inpatient Sample.
In-hospital mortality and rates for any complications were not associated with high or low endovascular mechanical thrombectomy (EMT) volume at hospitals across the United States in the analysis of 13,502 adult patients hospitalized with a primary diagnosis of acute ischemic stroke and treated with EMT, neurology resident Dr. Abhishek Lunagariya of the University of Florida, Gainesville, reported at the annual meeting of the American Academy of Neurology.
A smaller prior study of 2,749 EMTs done in 296 hospitals in 2008 showed lower mortality in high-volume hospitals that performed 10 or more of the procedures per year (J Stroke Cerebrovasc Dis. 2013 Nov; 22[8]:1263-9).
Of the 13,502 EMTs in the study, 25% occurred at low-volume hospitals performing less than 10 per year. Low-volume hospitals had higher in-hospital mortality than did higher-volume centers performing 10 or more of the procedures per year in an unadjusted comparison (26% vs. 21%). A comparison of a combined endpoint for any complications (in-hospital mortality, intracerebral hemorrhage, and vascular complications) was also significantly in favor of high-volume hospitals (34% vs. 30%).
However, in a multivariate hierarchical model, low-volume hospitals were not associated with higher in-hospital mortality (odds ratio, 0.95; 95% confidence interval, 0.74-1.23) or rate of any complications (OR, 0.96; 95% CI, 0.76-1.21). These analyses were adjusted for age, gender, ethnicity, primary payer, median household income, hospital region/teaching status/location/bed size, Charlson Comorbidity Index, calendar year, and use of intravenous tissue plasminogen activator.
Dr. Lunagariya noted that he and his associates could not adjust the comparisons for National Institutes of Health Stroke Scale scores because they are not recorded in the National Inpatient Sample. They also could not examine what happened to patients after discharge.
Dr. Lunagariya suggested a variety of possible reasons that might help to explain the lack of an association between hospital procedure volume and outcomes after adjustment: the availability of better thrombectomy devices since the smaller 2008 study, lesser operator variability, favorable patient selection, and an increased skill set of operators working at low-volume hospitals.
One audience member noted that some endovascular interventionalists will operate at both high-volume and low-volume hospitals and could account for some of the findings. That indeed might be happening more often and needs to happen more often, Dr. Lunagariya said in an interview, in order to combat the “common belief” that it would be better to wait for a patient to undergo the procedure at a high- rather than low-volume hospital. Patients who receive initial care for stroke at a low-volume hospital but are not stable enough or do not have enough time to be transferred could benefit from EMT if an interventionalist who performs EMT drove there instead, he said.
With even newer devices now available that are thought to be easier to use, Dr. Lunagariya suggested that the similarity in outcomes at low- and higher-volume centers may not change in updated analyses of more recent EMT procedures for ischemic stroke.
The investigators received no funding for the study, and they reported having no financial disclosures.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: Patient outcomes after endovascular mechanical thrombectomy for acute ischemic stroke do not appear to be worse at low- versus high-volume hospitals.
Major finding: In a multivariate hierarchical model, low-volume hospitals (fewer than 10 thrombectomies per year) were not associated with higher in-hospital mortality (odds ratio, 0.95; 95% confidence interval, 0.74-1.23) or rate of any complications (OR, 0.96; 95% CI, 0.76-1.21).
Data source: A retrospective review of 13,502 patients with acute ischemic stroke who underwent endovascular mechanical thrombectomy in 2008-2011.
Disclosures: The investigators received no funding for the study, and they reported having no financial disclosures.
What is the best approach to a high systolic pulmonary artery pressure on echocardiography?
The incidental finding of high systolic pulmonary artery pressure on echocardiography is common. What we should do about it varies according to clinical presentation, comorbidities, and results of other tests, including assessment of the right ventricle. Thus, the optimal approach ranges from no further investigation to right heart catheterization and, in some cases, referral to a pulmonary hypertension center.
THE TWO MEASUREMENTS COMPARED
Although it raises concern, the finding of high systolic pulmonary artery pressure is not enough to diagnose pulmonary hypertension. In fact, several other conditions are associated with high systolic pulmonary artery pressure on echocardiography (Table 1). The diagnosis must be confirmed with right heart catheterization.1
Echocardiography provides an estimate of the systolic pulmonary artery pressure that is calculated from other values, whereas right heart catheterization gives a direct measurement of the mean pulmonary artery pressure, which is necessary for diagnosing pulmonary hypertension. The two values are correlated, but the differences are noteworthy.
WHAT IS PULMONARY HYPERTENSION?
Pulmonary hypertension is defined by a resting mean pulmonary artery pressure 25 mm Hg or greater during right heart catheterization.1 The large number of conditions associated with pulmonary hypertension can be divided into five groups2:
- Group 1, pulmonary artery hypertension
- Group 2, pulmonary hypertension associated with left heart disease
- Group 3, pulmonary hypertension due to chronic lung disease or hypoxia
- Group 4, chronic thromboembolic pulmonary hypertension
- Group 5, pulmonary hypertension due to unclear multifactorial mechanisms.2
Pulmonary artery hypertension (group 1) is a syndrome characterized by a restricted flow of small pulmonary arteries that can be idiopathic, heritable, or induced by anorexigens, connective tissue disease, congenital heart disease, portal hypertension, human immunodeficiency virus (HIV), or schistosomiasis.2,3 In spite of significant advances in therapy in the last 3 decades, pulmonary artery hypertension continues to lead to right heart failure and death,4 and the diagnosis has adverse prognostic implications. Therefore, it is essential to be attentive when reviewing the echocardiogram, since an elevated systolic pulmonary artery pressure may be an important clue to pulmonary hypertension.
ESTIMATED PRESSURE: HOW HIGH IS TOO HIGH?
There is no consensus on the optimal cutoff of echocardiographic systolic pulmonary artery pressure to trigger a further evaluation for pulmonary hypertension.
A retrospective evaluation of nearly 16,000 normal echocardiograms found that the 95% upper limit for systolic pulmonary artery pressure was 37 mm Hg.5
European guidelines6 propose that pulmonary hypertension is unlikely if the estimated systolic pulmonary artery pressure is 36 mm Hg or lower, possible if it is 37 to 50 mm Hg, and likely if it is higher than 50 mm Hg.6
The 2009 consensus document of the American College of Cardiology Foundation and American Heart Association3 recommends a systolic pulmonary artery pressure greater than 40 mm Hg as the threshold to suggest further evaluation in a patient with unexplained dyspnea.
Converting the systolic pulmonary artery pressure to the mean pressure
Although not validated to use with echocardiography, the most accurate estimate of mean pulmonary artery pressure was shown in one study7 to be obtained with the equation:
0.61 × systolic pulmonary artery pressure
+ 2 mm Hg
Using this formula, a systolic pulmonary artery pressure of 37 mm Hg would correspond to a mean pulmonary artery pressure of 24.6 mm Hg. A systolic pulmonary artery pressure of 40 mm Hg would correspond to a mean pulmonary artery pressure of 26.4 mm Hg.
Estimated systolic pulmonary artery pressure depends on several variables
Systolic pulmonary artery pressure is estimated using the simplified Bernoulli equation8:
4 × tricuspid regurgitation jet velocity2 (m/s)
+ right atrial pressure (mm Hg)
Tricuspid regurgitation is present in over 75% of the normal population. The regurgitation velocity across the tricuspid valve must be measured to estimate the pressure gradient between the right ventricle and the right atrium. The right atrial pressure is estimated from the diameter of the inferior vena cava and the degree of inspiratory collapse with the sniff test. As the right atrial pressure increases, the inferior vena cava dilates and inspiratory collapse decreases.8 If there is no gradient across the right ventricular outflow tract or pulmonary valve, the right ventricular systolic pressure is equal to the systolic pulmonary artery pressure.
Since tricuspid regurgitation velocity is squared and then multiplied by 4, small deviations of this measurement lead to markedly different systolic pulmonary artery pressure values. To avoid this problem, the tricuspid regurgitation velocity needs to be looked at in multiple echocardiographic views to find the best alignment with the flow and an adequate envelope.
Many causes of high estimated systolic pulmonary artery pressure
Table 1 shows conditions associated with a high estimated systolic pulmonary artery pressure. Echocardiographic limitations, constitutional factors, and high cardiac output states can lead to an apparent elevation in systolic pulmonary artery pressure, which is not confirmed later during right heart catheterization.
Systolic pulmonary artery pressure increases with age and body mass index as a result of worsening left ventricular diastolic dysfunction.8 In fact, an estimated pressure greater than 40 mm Hg is found5 in 6% of people over age 50 and in 5% of people with a body mass index greater than 30 kg/m2. It can also be high in conditions in which there is an increase in cardiac output, such as pregnancy, anemia (sickle cell disease, thalassemia), cirrhosis, and arteriovenous fistula.
The estimated systolic value often differs from the measured value
Studies have compared the systolic pulmonary artery pressure measured during right heart catheterization with the estimated value on echocardiography.9,10 These studies noted a reasonable degree of agreement between the tests but a substantial variability.
Both underestimation and overestimation of the systolic pulmonary artery pressure by echocardiography were common, with 95% limits of agreement ranging from minus 40 mm Hg to plus 40 mm Hg.9,10 A difference of plus or minus 10 mm Hg in systolic pulmonary artery pressure between echocardiography and catheterization was observed in 48% to 51% of patients with pulmonary hypertension, particularly in those with higher systolic pulmonary artery pressure.9,10
An important reason for overestimation of systolic pulmonary artery pressure is the inaccurate estimation of the right atrial pressure by echocardiography.9,10 Indeed, this factor may account for half of the cases in which the systolic pulmonary artery pressure is overestimated.10 Although the traditional methods to estimate the right atrial pressure have been revisited,8,11 this estimation is less reliable for intermediate pressure values, for patients on mechanical ventilation, and for young athletes.8
Other explanations for the variability between measured and estimated systolic pulmonary artery pressure include suboptimal alignment between the Doppler beam and the regurgitant jet, severe tricuspid regurgitation, arrhythmias, and limitations inherent to the simplified Bernoulli equation.12 The estimated value is particularly inaccurate in patients with advanced lung disease, possibly owing to lung hyperinflation and alteration in the thoracic cavity and position of the heart—all factors that limit visualization and measurement of the tricuspid regurgitant jet.13
OTHER SIGNS OF PULMONARY HYPERTENSION ON ECHOCARDIOGRAPHY
Echocardiography provides information that is useful in assessing the accuracy of the estimated systolic pulmonary artery pressure, particularly right ventricular size and function.
As pulmonary hypertension progresses, the right ventricle dilates, and its function is compromised. Therefore, it is important to determine the right ventricular size and function by using objective echocardiographic findings such as right ventricular diameters (basal, mid, apical) and area, right ventricular fractional area change, tricuspid annular plane systolic excursion, myocardial performance index, and the pulsed tissue Doppler tricuspid annular peak systolic excursion velocity.8
Other echocardiographic features that suggest pulmonary hypertension include a dilated right atrial area, flattening of the interventricular septum, notching of the right ventricular outflow tract flow, and dilation of the main pulmonary artery. Interestingly, left ventricular diastolic dysfunction of the impaired relaxation type (grade I) is commonly observed in pulmonary hypertension14; however, more advanced degrees of diastolic dysfunction, ie, pseudonormalization (grade II) or restrictive left ventricular filling (grade III),15 particularly when associated with a left atrial enlargement, suggest pulmonary hypertension associated with left heart disease and not pulmonary artery hypertension.
WHAT TO DO IF ECHOCARDIOGRAPHY INDICATES PULMONARY HYPERTENSION
An algorithm showing the approach to an elevated systolic pulmonary artery pressure on echocardiography is presented in Figure 1.
In the appropriate clinical setting, if the systolic pulmonary artery pressure is 40 mm Hg or greater or if other echocardiographic variables suggest pulmonary hypertension, our practice is to proceed with right heart catheterization.
Clinical variables that suggest pulmonary hypertension include progressive dyspnea, chest pain, presyncope-syncope, lower extremity edema, hepatomegaly, jugular vein distention, hepatojugular reflux, sternal heave, loud second heart sound (P2), murmur of tricuspid or pulmonary regurgitation, and right ventricular third heart sound.16 These are of particular interest when associated with conditions known to cause pulmonary hypertension,2such as connective tissue disease, portal hypertension, congenital heart disease, HIV infection, and certain drugs and toxins.
Other tests that raise suspicion of pulmonary hypertension are an electrocardiogram suggesting a dilated right atrium or ventricle, an elevated brain natriuretic peptide level, a low carbon monoxide diffusing capacity on pulmonary function testing, and an enlarged pulmonary artery diameter on imaging.
Given the high prevalence of pulmonary hypertension, the Fifth World Symposium on Pulmonary Hypertension recommended first considering heart or parenchymal lung disease when an echocardiogram suggests pulmonary hypertension.6 If there are signs of severe pulmonary hypertension or right ventricular dysfunction, referral to a center specializing in pulmonary hypertension is recommended. Referral is also appropriate when there is no major heart or lung disease and the echocardiogram shows an elevated systolic pulmonary artery pressure, particularly when the clinical presentation or results of other testing suggest pulmonary hypertension.
TAKE-HOME POINTS
In the appropriate context, a high systolic pulmonary artery pressure on echocardiography suggests pulmonary hypertension, but right heart catheterization is needed to confirm the diagnosis. Estimating the systolic pulmonary artery pressure with echocardiography has limitations, including false-positive results, predominantly when the pretest probability of pulmonary hypertension is low.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D42–D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D34–D41.
- McLaughlin VV, Archer SL, Badesch DB, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53:1573–1619.
- Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188:365–369.
- McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001; 104:2797–2802.
- Galiè N, Hoeper MM, Humbert M, et al; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30:2493–2537.
- Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Herve P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 2009; 135:760–768.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713.
- Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139:988–993.
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179:615–621.
- Brennan JM, Blair JE, Goonewardena S, et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20:857–861.
- Giardini A, Tacy TA. Non-invasive estimation of pressure gradients in regurgitant jets: an overdue consideration. Eur J Echocardiogr 2008; 9:578–584.
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167:735–740.
- Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 2012; 141:1457–1465.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–133.
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(suppl S):40S–47S.
The incidental finding of high systolic pulmonary artery pressure on echocardiography is common. What we should do about it varies according to clinical presentation, comorbidities, and results of other tests, including assessment of the right ventricle. Thus, the optimal approach ranges from no further investigation to right heart catheterization and, in some cases, referral to a pulmonary hypertension center.
THE TWO MEASUREMENTS COMPARED
Although it raises concern, the finding of high systolic pulmonary artery pressure is not enough to diagnose pulmonary hypertension. In fact, several other conditions are associated with high systolic pulmonary artery pressure on echocardiography (Table 1). The diagnosis must be confirmed with right heart catheterization.1
Echocardiography provides an estimate of the systolic pulmonary artery pressure that is calculated from other values, whereas right heart catheterization gives a direct measurement of the mean pulmonary artery pressure, which is necessary for diagnosing pulmonary hypertension. The two values are correlated, but the differences are noteworthy.
WHAT IS PULMONARY HYPERTENSION?
Pulmonary hypertension is defined by a resting mean pulmonary artery pressure 25 mm Hg or greater during right heart catheterization.1 The large number of conditions associated with pulmonary hypertension can be divided into five groups2:
- Group 1, pulmonary artery hypertension
- Group 2, pulmonary hypertension associated with left heart disease
- Group 3, pulmonary hypertension due to chronic lung disease or hypoxia
- Group 4, chronic thromboembolic pulmonary hypertension
- Group 5, pulmonary hypertension due to unclear multifactorial mechanisms.2
Pulmonary artery hypertension (group 1) is a syndrome characterized by a restricted flow of small pulmonary arteries that can be idiopathic, heritable, or induced by anorexigens, connective tissue disease, congenital heart disease, portal hypertension, human immunodeficiency virus (HIV), or schistosomiasis.2,3 In spite of significant advances in therapy in the last 3 decades, pulmonary artery hypertension continues to lead to right heart failure and death,4 and the diagnosis has adverse prognostic implications. Therefore, it is essential to be attentive when reviewing the echocardiogram, since an elevated systolic pulmonary artery pressure may be an important clue to pulmonary hypertension.
ESTIMATED PRESSURE: HOW HIGH IS TOO HIGH?
There is no consensus on the optimal cutoff of echocardiographic systolic pulmonary artery pressure to trigger a further evaluation for pulmonary hypertension.
A retrospective evaluation of nearly 16,000 normal echocardiograms found that the 95% upper limit for systolic pulmonary artery pressure was 37 mm Hg.5
European guidelines6 propose that pulmonary hypertension is unlikely if the estimated systolic pulmonary artery pressure is 36 mm Hg or lower, possible if it is 37 to 50 mm Hg, and likely if it is higher than 50 mm Hg.6
The 2009 consensus document of the American College of Cardiology Foundation and American Heart Association3 recommends a systolic pulmonary artery pressure greater than 40 mm Hg as the threshold to suggest further evaluation in a patient with unexplained dyspnea.
Converting the systolic pulmonary artery pressure to the mean pressure
Although not validated to use with echocardiography, the most accurate estimate of mean pulmonary artery pressure was shown in one study7 to be obtained with the equation:
0.61 × systolic pulmonary artery pressure
+ 2 mm Hg
Using this formula, a systolic pulmonary artery pressure of 37 mm Hg would correspond to a mean pulmonary artery pressure of 24.6 mm Hg. A systolic pulmonary artery pressure of 40 mm Hg would correspond to a mean pulmonary artery pressure of 26.4 mm Hg.
Estimated systolic pulmonary artery pressure depends on several variables
Systolic pulmonary artery pressure is estimated using the simplified Bernoulli equation8:
4 × tricuspid regurgitation jet velocity2 (m/s)
+ right atrial pressure (mm Hg)
Tricuspid regurgitation is present in over 75% of the normal population. The regurgitation velocity across the tricuspid valve must be measured to estimate the pressure gradient between the right ventricle and the right atrium. The right atrial pressure is estimated from the diameter of the inferior vena cava and the degree of inspiratory collapse with the sniff test. As the right atrial pressure increases, the inferior vena cava dilates and inspiratory collapse decreases.8 If there is no gradient across the right ventricular outflow tract or pulmonary valve, the right ventricular systolic pressure is equal to the systolic pulmonary artery pressure.
Since tricuspid regurgitation velocity is squared and then multiplied by 4, small deviations of this measurement lead to markedly different systolic pulmonary artery pressure values. To avoid this problem, the tricuspid regurgitation velocity needs to be looked at in multiple echocardiographic views to find the best alignment with the flow and an adequate envelope.
Many causes of high estimated systolic pulmonary artery pressure
Table 1 shows conditions associated with a high estimated systolic pulmonary artery pressure. Echocardiographic limitations, constitutional factors, and high cardiac output states can lead to an apparent elevation in systolic pulmonary artery pressure, which is not confirmed later during right heart catheterization.
Systolic pulmonary artery pressure increases with age and body mass index as a result of worsening left ventricular diastolic dysfunction.8 In fact, an estimated pressure greater than 40 mm Hg is found5 in 6% of people over age 50 and in 5% of people with a body mass index greater than 30 kg/m2. It can also be high in conditions in which there is an increase in cardiac output, such as pregnancy, anemia (sickle cell disease, thalassemia), cirrhosis, and arteriovenous fistula.
The estimated systolic value often differs from the measured value
Studies have compared the systolic pulmonary artery pressure measured during right heart catheterization with the estimated value on echocardiography.9,10 These studies noted a reasonable degree of agreement between the tests but a substantial variability.
Both underestimation and overestimation of the systolic pulmonary artery pressure by echocardiography were common, with 95% limits of agreement ranging from minus 40 mm Hg to plus 40 mm Hg.9,10 A difference of plus or minus 10 mm Hg in systolic pulmonary artery pressure between echocardiography and catheterization was observed in 48% to 51% of patients with pulmonary hypertension, particularly in those with higher systolic pulmonary artery pressure.9,10
An important reason for overestimation of systolic pulmonary artery pressure is the inaccurate estimation of the right atrial pressure by echocardiography.9,10 Indeed, this factor may account for half of the cases in which the systolic pulmonary artery pressure is overestimated.10 Although the traditional methods to estimate the right atrial pressure have been revisited,8,11 this estimation is less reliable for intermediate pressure values, for patients on mechanical ventilation, and for young athletes.8
Other explanations for the variability between measured and estimated systolic pulmonary artery pressure include suboptimal alignment between the Doppler beam and the regurgitant jet, severe tricuspid regurgitation, arrhythmias, and limitations inherent to the simplified Bernoulli equation.12 The estimated value is particularly inaccurate in patients with advanced lung disease, possibly owing to lung hyperinflation and alteration in the thoracic cavity and position of the heart—all factors that limit visualization and measurement of the tricuspid regurgitant jet.13
OTHER SIGNS OF PULMONARY HYPERTENSION ON ECHOCARDIOGRAPHY
Echocardiography provides information that is useful in assessing the accuracy of the estimated systolic pulmonary artery pressure, particularly right ventricular size and function.
As pulmonary hypertension progresses, the right ventricle dilates, and its function is compromised. Therefore, it is important to determine the right ventricular size and function by using objective echocardiographic findings such as right ventricular diameters (basal, mid, apical) and area, right ventricular fractional area change, tricuspid annular plane systolic excursion, myocardial performance index, and the pulsed tissue Doppler tricuspid annular peak systolic excursion velocity.8
Other echocardiographic features that suggest pulmonary hypertension include a dilated right atrial area, flattening of the interventricular septum, notching of the right ventricular outflow tract flow, and dilation of the main pulmonary artery. Interestingly, left ventricular diastolic dysfunction of the impaired relaxation type (grade I) is commonly observed in pulmonary hypertension14; however, more advanced degrees of diastolic dysfunction, ie, pseudonormalization (grade II) or restrictive left ventricular filling (grade III),15 particularly when associated with a left atrial enlargement, suggest pulmonary hypertension associated with left heart disease and not pulmonary artery hypertension.
WHAT TO DO IF ECHOCARDIOGRAPHY INDICATES PULMONARY HYPERTENSION
An algorithm showing the approach to an elevated systolic pulmonary artery pressure on echocardiography is presented in Figure 1.
In the appropriate clinical setting, if the systolic pulmonary artery pressure is 40 mm Hg or greater or if other echocardiographic variables suggest pulmonary hypertension, our practice is to proceed with right heart catheterization.
Clinical variables that suggest pulmonary hypertension include progressive dyspnea, chest pain, presyncope-syncope, lower extremity edema, hepatomegaly, jugular vein distention, hepatojugular reflux, sternal heave, loud second heart sound (P2), murmur of tricuspid or pulmonary regurgitation, and right ventricular third heart sound.16 These are of particular interest when associated with conditions known to cause pulmonary hypertension,2such as connective tissue disease, portal hypertension, congenital heart disease, HIV infection, and certain drugs and toxins.
Other tests that raise suspicion of pulmonary hypertension are an electrocardiogram suggesting a dilated right atrium or ventricle, an elevated brain natriuretic peptide level, a low carbon monoxide diffusing capacity on pulmonary function testing, and an enlarged pulmonary artery diameter on imaging.
Given the high prevalence of pulmonary hypertension, the Fifth World Symposium on Pulmonary Hypertension recommended first considering heart or parenchymal lung disease when an echocardiogram suggests pulmonary hypertension.6 If there are signs of severe pulmonary hypertension or right ventricular dysfunction, referral to a center specializing in pulmonary hypertension is recommended. Referral is also appropriate when there is no major heart or lung disease and the echocardiogram shows an elevated systolic pulmonary artery pressure, particularly when the clinical presentation or results of other testing suggest pulmonary hypertension.
TAKE-HOME POINTS
In the appropriate context, a high systolic pulmonary artery pressure on echocardiography suggests pulmonary hypertension, but right heart catheterization is needed to confirm the diagnosis. Estimating the systolic pulmonary artery pressure with echocardiography has limitations, including false-positive results, predominantly when the pretest probability of pulmonary hypertension is low.
The incidental finding of high systolic pulmonary artery pressure on echocardiography is common. What we should do about it varies according to clinical presentation, comorbidities, and results of other tests, including assessment of the right ventricle. Thus, the optimal approach ranges from no further investigation to right heart catheterization and, in some cases, referral to a pulmonary hypertension center.
THE TWO MEASUREMENTS COMPARED
Although it raises concern, the finding of high systolic pulmonary artery pressure is not enough to diagnose pulmonary hypertension. In fact, several other conditions are associated with high systolic pulmonary artery pressure on echocardiography (Table 1). The diagnosis must be confirmed with right heart catheterization.1
Echocardiography provides an estimate of the systolic pulmonary artery pressure that is calculated from other values, whereas right heart catheterization gives a direct measurement of the mean pulmonary artery pressure, which is necessary for diagnosing pulmonary hypertension. The two values are correlated, but the differences are noteworthy.
WHAT IS PULMONARY HYPERTENSION?
Pulmonary hypertension is defined by a resting mean pulmonary artery pressure 25 mm Hg or greater during right heart catheterization.1 The large number of conditions associated with pulmonary hypertension can be divided into five groups2:
- Group 1, pulmonary artery hypertension
- Group 2, pulmonary hypertension associated with left heart disease
- Group 3, pulmonary hypertension due to chronic lung disease or hypoxia
- Group 4, chronic thromboembolic pulmonary hypertension
- Group 5, pulmonary hypertension due to unclear multifactorial mechanisms.2
Pulmonary artery hypertension (group 1) is a syndrome characterized by a restricted flow of small pulmonary arteries that can be idiopathic, heritable, or induced by anorexigens, connective tissue disease, congenital heart disease, portal hypertension, human immunodeficiency virus (HIV), or schistosomiasis.2,3 In spite of significant advances in therapy in the last 3 decades, pulmonary artery hypertension continues to lead to right heart failure and death,4 and the diagnosis has adverse prognostic implications. Therefore, it is essential to be attentive when reviewing the echocardiogram, since an elevated systolic pulmonary artery pressure may be an important clue to pulmonary hypertension.
ESTIMATED PRESSURE: HOW HIGH IS TOO HIGH?
There is no consensus on the optimal cutoff of echocardiographic systolic pulmonary artery pressure to trigger a further evaluation for pulmonary hypertension.
A retrospective evaluation of nearly 16,000 normal echocardiograms found that the 95% upper limit for systolic pulmonary artery pressure was 37 mm Hg.5
European guidelines6 propose that pulmonary hypertension is unlikely if the estimated systolic pulmonary artery pressure is 36 mm Hg or lower, possible if it is 37 to 50 mm Hg, and likely if it is higher than 50 mm Hg.6
The 2009 consensus document of the American College of Cardiology Foundation and American Heart Association3 recommends a systolic pulmonary artery pressure greater than 40 mm Hg as the threshold to suggest further evaluation in a patient with unexplained dyspnea.
Converting the systolic pulmonary artery pressure to the mean pressure
Although not validated to use with echocardiography, the most accurate estimate of mean pulmonary artery pressure was shown in one study7 to be obtained with the equation:
0.61 × systolic pulmonary artery pressure
+ 2 mm Hg
Using this formula, a systolic pulmonary artery pressure of 37 mm Hg would correspond to a mean pulmonary artery pressure of 24.6 mm Hg. A systolic pulmonary artery pressure of 40 mm Hg would correspond to a mean pulmonary artery pressure of 26.4 mm Hg.
Estimated systolic pulmonary artery pressure depends on several variables
Systolic pulmonary artery pressure is estimated using the simplified Bernoulli equation8:
4 × tricuspid regurgitation jet velocity2 (m/s)
+ right atrial pressure (mm Hg)
Tricuspid regurgitation is present in over 75% of the normal population. The regurgitation velocity across the tricuspid valve must be measured to estimate the pressure gradient between the right ventricle and the right atrium. The right atrial pressure is estimated from the diameter of the inferior vena cava and the degree of inspiratory collapse with the sniff test. As the right atrial pressure increases, the inferior vena cava dilates and inspiratory collapse decreases.8 If there is no gradient across the right ventricular outflow tract or pulmonary valve, the right ventricular systolic pressure is equal to the systolic pulmonary artery pressure.
Since tricuspid regurgitation velocity is squared and then multiplied by 4, small deviations of this measurement lead to markedly different systolic pulmonary artery pressure values. To avoid this problem, the tricuspid regurgitation velocity needs to be looked at in multiple echocardiographic views to find the best alignment with the flow and an adequate envelope.
Many causes of high estimated systolic pulmonary artery pressure
Table 1 shows conditions associated with a high estimated systolic pulmonary artery pressure. Echocardiographic limitations, constitutional factors, and high cardiac output states can lead to an apparent elevation in systolic pulmonary artery pressure, which is not confirmed later during right heart catheterization.
Systolic pulmonary artery pressure increases with age and body mass index as a result of worsening left ventricular diastolic dysfunction.8 In fact, an estimated pressure greater than 40 mm Hg is found5 in 6% of people over age 50 and in 5% of people with a body mass index greater than 30 kg/m2. It can also be high in conditions in which there is an increase in cardiac output, such as pregnancy, anemia (sickle cell disease, thalassemia), cirrhosis, and arteriovenous fistula.
The estimated systolic value often differs from the measured value
Studies have compared the systolic pulmonary artery pressure measured during right heart catheterization with the estimated value on echocardiography.9,10 These studies noted a reasonable degree of agreement between the tests but a substantial variability.
Both underestimation and overestimation of the systolic pulmonary artery pressure by echocardiography were common, with 95% limits of agreement ranging from minus 40 mm Hg to plus 40 mm Hg.9,10 A difference of plus or minus 10 mm Hg in systolic pulmonary artery pressure between echocardiography and catheterization was observed in 48% to 51% of patients with pulmonary hypertension, particularly in those with higher systolic pulmonary artery pressure.9,10
An important reason for overestimation of systolic pulmonary artery pressure is the inaccurate estimation of the right atrial pressure by echocardiography.9,10 Indeed, this factor may account for half of the cases in which the systolic pulmonary artery pressure is overestimated.10 Although the traditional methods to estimate the right atrial pressure have been revisited,8,11 this estimation is less reliable for intermediate pressure values, for patients on mechanical ventilation, and for young athletes.8
Other explanations for the variability between measured and estimated systolic pulmonary artery pressure include suboptimal alignment between the Doppler beam and the regurgitant jet, severe tricuspid regurgitation, arrhythmias, and limitations inherent to the simplified Bernoulli equation.12 The estimated value is particularly inaccurate in patients with advanced lung disease, possibly owing to lung hyperinflation and alteration in the thoracic cavity and position of the heart—all factors that limit visualization and measurement of the tricuspid regurgitant jet.13
OTHER SIGNS OF PULMONARY HYPERTENSION ON ECHOCARDIOGRAPHY
Echocardiography provides information that is useful in assessing the accuracy of the estimated systolic pulmonary artery pressure, particularly right ventricular size and function.
As pulmonary hypertension progresses, the right ventricle dilates, and its function is compromised. Therefore, it is important to determine the right ventricular size and function by using objective echocardiographic findings such as right ventricular diameters (basal, mid, apical) and area, right ventricular fractional area change, tricuspid annular plane systolic excursion, myocardial performance index, and the pulsed tissue Doppler tricuspid annular peak systolic excursion velocity.8
Other echocardiographic features that suggest pulmonary hypertension include a dilated right atrial area, flattening of the interventricular septum, notching of the right ventricular outflow tract flow, and dilation of the main pulmonary artery. Interestingly, left ventricular diastolic dysfunction of the impaired relaxation type (grade I) is commonly observed in pulmonary hypertension14; however, more advanced degrees of diastolic dysfunction, ie, pseudonormalization (grade II) or restrictive left ventricular filling (grade III),15 particularly when associated with a left atrial enlargement, suggest pulmonary hypertension associated with left heart disease and not pulmonary artery hypertension.
WHAT TO DO IF ECHOCARDIOGRAPHY INDICATES PULMONARY HYPERTENSION
An algorithm showing the approach to an elevated systolic pulmonary artery pressure on echocardiography is presented in Figure 1.
In the appropriate clinical setting, if the systolic pulmonary artery pressure is 40 mm Hg or greater or if other echocardiographic variables suggest pulmonary hypertension, our practice is to proceed with right heart catheterization.
Clinical variables that suggest pulmonary hypertension include progressive dyspnea, chest pain, presyncope-syncope, lower extremity edema, hepatomegaly, jugular vein distention, hepatojugular reflux, sternal heave, loud second heart sound (P2), murmur of tricuspid or pulmonary regurgitation, and right ventricular third heart sound.16 These are of particular interest when associated with conditions known to cause pulmonary hypertension,2such as connective tissue disease, portal hypertension, congenital heart disease, HIV infection, and certain drugs and toxins.
Other tests that raise suspicion of pulmonary hypertension are an electrocardiogram suggesting a dilated right atrium or ventricle, an elevated brain natriuretic peptide level, a low carbon monoxide diffusing capacity on pulmonary function testing, and an enlarged pulmonary artery diameter on imaging.
Given the high prevalence of pulmonary hypertension, the Fifth World Symposium on Pulmonary Hypertension recommended first considering heart or parenchymal lung disease when an echocardiogram suggests pulmonary hypertension.6 If there are signs of severe pulmonary hypertension or right ventricular dysfunction, referral to a center specializing in pulmonary hypertension is recommended. Referral is also appropriate when there is no major heart or lung disease and the echocardiogram shows an elevated systolic pulmonary artery pressure, particularly when the clinical presentation or results of other testing suggest pulmonary hypertension.
TAKE-HOME POINTS
In the appropriate context, a high systolic pulmonary artery pressure on echocardiography suggests pulmonary hypertension, but right heart catheterization is needed to confirm the diagnosis. Estimating the systolic pulmonary artery pressure with echocardiography has limitations, including false-positive results, predominantly when the pretest probability of pulmonary hypertension is low.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D42–D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D34–D41.
- McLaughlin VV, Archer SL, Badesch DB, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53:1573–1619.
- Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188:365–369.
- McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001; 104:2797–2802.
- Galiè N, Hoeper MM, Humbert M, et al; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30:2493–2537.
- Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Herve P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 2009; 135:760–768.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713.
- Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139:988–993.
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179:615–621.
- Brennan JM, Blair JE, Goonewardena S, et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20:857–861.
- Giardini A, Tacy TA. Non-invasive estimation of pressure gradients in regurgitant jets: an overdue consideration. Eur J Echocardiogr 2008; 9:578–584.
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167:735–740.
- Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 2012; 141:1457–1465.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–133.
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(suppl S):40S–47S.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D42–D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D34–D41.
- McLaughlin VV, Archer SL, Badesch DB, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53:1573–1619.
- Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188:365–369.
- McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001; 104:2797–2802.
- Galiè N, Hoeper MM, Humbert M, et al; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30:2493–2537.
- Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Herve P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 2009; 135:760–768.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713.
- Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139:988–993.
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179:615–621.
- Brennan JM, Blair JE, Goonewardena S, et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20:857–861.
- Giardini A, Tacy TA. Non-invasive estimation of pressure gradients in regurgitant jets: an overdue consideration. Eur J Echocardiogr 2008; 9:578–584.
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167:735–740.
- Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 2012; 141:1457–1465.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–133.
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(suppl S):40S–47S.
Cocaine-induced ecchymotic rash
A 50-year-old man presented with a painful rash over his extremities for the past 2 days (Figure 1). He said he had been in his usual state of health until the day he woke up with the rash. The rash was initially limited to his upper and lower extremities, but the next day he noticed similar lesions over his cheek and hard palate. He was a smoker and was known to have hepatitis C virus infection. He denied recent trauma, fever, or chills. He said he had snorted cocaine about 24 hours before the rash first appeared.
On examination, his vital signs were normal. He had an extensive retiform rash involving the upper and lower extremities, earlobes, right cheek, and hard palate. Otherwise, the physical examination was normal.
Initial laboratory evaluation showed:
- Hemoglobin 11.5 g/dL (reference range 14.0–17.5)
- White blood cell count 2.1 × 109/L (4.5–11.0)
- Platelet count 168 × 109/L (150–350)
- Absolute neutrophil count 0.9 × 109/L (≥ 1.5)
- Urine toxicology screen positive for cocaine.
He was admitted to the hospital and was started on intravenous vancomycin and piperacillin-sulbactam for a presumed infectious cause of the rash.
One day later, testing for myeloperoxidase-specific antineutrophil cytoplasmic antibodies (p-ANCA) was strongly positive. Skin biopsy revealed leukocytoclastic vasculitis with small-vessel thrombosis. These findings, along with the timing of the appearance of the rash after his cocaine use, led to a diagnosis of levamisole-adulterated cocaine-induced vasculitis.
LEVAMISOLE AND VASCULITIS
Levamisole used to be used as an antihelminthic and as an adjuvant chemotherapeutic agent, but it is also added to cocaine to increase its euphoric and psychotropic effects.1 It has been withdrawn from the market for human use because of toxic side effects including agranulocytosis, vasculitis, and autoantibody positivity.
Levamisole-adulterated cocaine has been known to induce ANCA-associated vasculitis.2 Symptoms of levamisole-induced vasculitis usually start a few hours to a few days after the last dose of cocaine. Almost all patients with this condition present with a characteristic retiform purpuric rash, which has a predilection for the ears, nose, cheeks, and extremities. It also causes neutropenia and agranulocytosis (as well as autoantibodies, including antinuclear antibodies and antiphospholipid antibodies).
The characteristic lesions tend to be in a stellate pattern with erythematous borders. They often but not always have a central necrotic area. The location of the rash and the fact that it resolves after discontinuation of the offending agent help distinguish this condition from other types of vasculitis. Usually, antibodies against myeloperoxidase are present.3
Leukopenia does not typically occur in patients with primary vasculitis syndromes. The literature is mixed on the presence or absence of specific ANCAs.
Inflammatory and noninflammatory vasculopathic disorders that can present similarly include disseminated intravascular coagulation, antiphospholipid syndrome, warfarin-induced skin necrosis, paroxysmal nocturnal hemoglobinuria, cryoglobulinemia, vasculitis, and calciphylaxis.
Treatment is mainly supportive, but emollients and steroids4 have been reported to alleviate the symptoms. Surgical debridement is rarely needed unless skin necrosis is extensive.
Levamisole-induced vasculitis is a diagnosis of exclusion but should be strongly considered when the rash is associated with recent cocaine use, retiform purpura, or bullae with skin involvement, leukopenia, and positive ANCA in high titers.3 If cocaine use is discontinued, the syndrome is expected to resolve.
- Tervaert JW, Stegeman CA. A difficult diagnosis. Lancet 2004; 364:1313–1314.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther 2010; 88:408–411.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol 2011; 30:1385–1392.
- Carter MR, Amirhaeri S. p-ANCA-associated vasculitis caused by levamisole-adulterated cocaine: a case report. Case Rep Emerg Med 2013; 2013:878903.
A 50-year-old man presented with a painful rash over his extremities for the past 2 days (Figure 1). He said he had been in his usual state of health until the day he woke up with the rash. The rash was initially limited to his upper and lower extremities, but the next day he noticed similar lesions over his cheek and hard palate. He was a smoker and was known to have hepatitis C virus infection. He denied recent trauma, fever, or chills. He said he had snorted cocaine about 24 hours before the rash first appeared.
On examination, his vital signs were normal. He had an extensive retiform rash involving the upper and lower extremities, earlobes, right cheek, and hard palate. Otherwise, the physical examination was normal.
Initial laboratory evaluation showed:
- Hemoglobin 11.5 g/dL (reference range 14.0–17.5)
- White blood cell count 2.1 × 109/L (4.5–11.0)
- Platelet count 168 × 109/L (150–350)
- Absolute neutrophil count 0.9 × 109/L (≥ 1.5)
- Urine toxicology screen positive for cocaine.
He was admitted to the hospital and was started on intravenous vancomycin and piperacillin-sulbactam for a presumed infectious cause of the rash.
One day later, testing for myeloperoxidase-specific antineutrophil cytoplasmic antibodies (p-ANCA) was strongly positive. Skin biopsy revealed leukocytoclastic vasculitis with small-vessel thrombosis. These findings, along with the timing of the appearance of the rash after his cocaine use, led to a diagnosis of levamisole-adulterated cocaine-induced vasculitis.
LEVAMISOLE AND VASCULITIS
Levamisole used to be used as an antihelminthic and as an adjuvant chemotherapeutic agent, but it is also added to cocaine to increase its euphoric and psychotropic effects.1 It has been withdrawn from the market for human use because of toxic side effects including agranulocytosis, vasculitis, and autoantibody positivity.
Levamisole-adulterated cocaine has been known to induce ANCA-associated vasculitis.2 Symptoms of levamisole-induced vasculitis usually start a few hours to a few days after the last dose of cocaine. Almost all patients with this condition present with a characteristic retiform purpuric rash, which has a predilection for the ears, nose, cheeks, and extremities. It also causes neutropenia and agranulocytosis (as well as autoantibodies, including antinuclear antibodies and antiphospholipid antibodies).
The characteristic lesions tend to be in a stellate pattern with erythematous borders. They often but not always have a central necrotic area. The location of the rash and the fact that it resolves after discontinuation of the offending agent help distinguish this condition from other types of vasculitis. Usually, antibodies against myeloperoxidase are present.3
Leukopenia does not typically occur in patients with primary vasculitis syndromes. The literature is mixed on the presence or absence of specific ANCAs.
Inflammatory and noninflammatory vasculopathic disorders that can present similarly include disseminated intravascular coagulation, antiphospholipid syndrome, warfarin-induced skin necrosis, paroxysmal nocturnal hemoglobinuria, cryoglobulinemia, vasculitis, and calciphylaxis.
Treatment is mainly supportive, but emollients and steroids4 have been reported to alleviate the symptoms. Surgical debridement is rarely needed unless skin necrosis is extensive.
Levamisole-induced vasculitis is a diagnosis of exclusion but should be strongly considered when the rash is associated with recent cocaine use, retiform purpura, or bullae with skin involvement, leukopenia, and positive ANCA in high titers.3 If cocaine use is discontinued, the syndrome is expected to resolve.
A 50-year-old man presented with a painful rash over his extremities for the past 2 days (Figure 1). He said he had been in his usual state of health until the day he woke up with the rash. The rash was initially limited to his upper and lower extremities, but the next day he noticed similar lesions over his cheek and hard palate. He was a smoker and was known to have hepatitis C virus infection. He denied recent trauma, fever, or chills. He said he had snorted cocaine about 24 hours before the rash first appeared.
On examination, his vital signs were normal. He had an extensive retiform rash involving the upper and lower extremities, earlobes, right cheek, and hard palate. Otherwise, the physical examination was normal.
Initial laboratory evaluation showed:
- Hemoglobin 11.5 g/dL (reference range 14.0–17.5)
- White blood cell count 2.1 × 109/L (4.5–11.0)
- Platelet count 168 × 109/L (150–350)
- Absolute neutrophil count 0.9 × 109/L (≥ 1.5)
- Urine toxicology screen positive for cocaine.
He was admitted to the hospital and was started on intravenous vancomycin and piperacillin-sulbactam for a presumed infectious cause of the rash.
One day later, testing for myeloperoxidase-specific antineutrophil cytoplasmic antibodies (p-ANCA) was strongly positive. Skin biopsy revealed leukocytoclastic vasculitis with small-vessel thrombosis. These findings, along with the timing of the appearance of the rash after his cocaine use, led to a diagnosis of levamisole-adulterated cocaine-induced vasculitis.
LEVAMISOLE AND VASCULITIS
Levamisole used to be used as an antihelminthic and as an adjuvant chemotherapeutic agent, but it is also added to cocaine to increase its euphoric and psychotropic effects.1 It has been withdrawn from the market for human use because of toxic side effects including agranulocytosis, vasculitis, and autoantibody positivity.
Levamisole-adulterated cocaine has been known to induce ANCA-associated vasculitis.2 Symptoms of levamisole-induced vasculitis usually start a few hours to a few days after the last dose of cocaine. Almost all patients with this condition present with a characteristic retiform purpuric rash, which has a predilection for the ears, nose, cheeks, and extremities. It also causes neutropenia and agranulocytosis (as well as autoantibodies, including antinuclear antibodies and antiphospholipid antibodies).
The characteristic lesions tend to be in a stellate pattern with erythematous borders. They often but not always have a central necrotic area. The location of the rash and the fact that it resolves after discontinuation of the offending agent help distinguish this condition from other types of vasculitis. Usually, antibodies against myeloperoxidase are present.3
Leukopenia does not typically occur in patients with primary vasculitis syndromes. The literature is mixed on the presence or absence of specific ANCAs.
Inflammatory and noninflammatory vasculopathic disorders that can present similarly include disseminated intravascular coagulation, antiphospholipid syndrome, warfarin-induced skin necrosis, paroxysmal nocturnal hemoglobinuria, cryoglobulinemia, vasculitis, and calciphylaxis.
Treatment is mainly supportive, but emollients and steroids4 have been reported to alleviate the symptoms. Surgical debridement is rarely needed unless skin necrosis is extensive.
Levamisole-induced vasculitis is a diagnosis of exclusion but should be strongly considered when the rash is associated with recent cocaine use, retiform purpura, or bullae with skin involvement, leukopenia, and positive ANCA in high titers.3 If cocaine use is discontinued, the syndrome is expected to resolve.
- Tervaert JW, Stegeman CA. A difficult diagnosis. Lancet 2004; 364:1313–1314.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther 2010; 88:408–411.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol 2011; 30:1385–1392.
- Carter MR, Amirhaeri S. p-ANCA-associated vasculitis caused by levamisole-adulterated cocaine: a case report. Case Rep Emerg Med 2013; 2013:878903.
- Tervaert JW, Stegeman CA. A difficult diagnosis. Lancet 2004; 364:1313–1314.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther 2010; 88:408–411.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol 2011; 30:1385–1392.
- Carter MR, Amirhaeri S. p-ANCA-associated vasculitis caused by levamisole-adulterated cocaine: a case report. Case Rep Emerg Med 2013; 2013:878903.
Endovascular surges over surgery for patients hospitalized for CLI
Even though there was a steady rate of patients with critical limb ischemia (CLI) admitted to hospitals from 2003 to 2011, surgical revascularization decreased and endovascular treatment increased significantly, with concomitant decreases in in-hospital mortality and major amputation, according to the results of an analysis of the Nationwide Inpatient Sample of 642,433 patients hospitalized with CLI.
In addition, despite multiple adjustments, endovascular revascularization was associated with reduced in-hospital mortality, compared with surgical revascularization over the same period, according to a report online in the Journal of the American College of Cardiology.
The annual in-hospital mortality rate decreased from 5.4% in 2003 to 3.4% in 2011 (P less than .001), and the major amputation rate dropped from 16.7% to 10.8%. There also was a significant decrease in length-of-stay (LOS) from 10 days to 8.4 days over the same period (P less than .001); however this did not translate to a significant difference in the cost of hospitalization, according to Dr. Shikhar Agarwal and colleagues at the Cleveland Clinic [doi:10.1016/j.jacc.2016.02.040].
Significant predictive factors of in-hospital mortality after multivariate regression analysis were female sex, older age, emergent admission, a primary indication of septicemia, heart failure, and respiratory disease, as well any stump complications present during admission. In contrast, any form of revascularization was associated with significantly reduced in-hospital mortality.
A comparison of revascularization methods showed that surgical revascularization significantly decreased from 13.9% in 2003 to 8.8% in 2011, while endovascular revascularization increased from 5.1% to 11%. Also, endovascular revascularization was associated with a significant decrease in in-hospital mortality compared with surgical revascularization over the study period (2.34% vs. 2.73%, respectively; odds ratio = .69). Major amputation rates were not significantly different between the two treatments (6.5% vs. 5.7%; OR = .99).
Length of stay was significantly lower with endovascular treatment compared with surgical (8.7 vs. 10.7 days) as were costs ($31,679 vs. $32,485, respectively).
Women had a higher rate of in-hospital mortality, but a lower rate of major amputation. Although race was not seen as a factor in predicting in-hospital mortality, blacks and other nonwhite races had significantly higher rates of amputation and lower rates of revascularization, compared with whites.
Approximately half of the patients assessed were admitted for primary CLI-related diagnoses. The other, non–CLI-related conditions – such as acute MI, cerebrovascular events, respiratory disease, heart failure, and acute kidney disease – have all been independently associated with increased in-hospital mortality and may be confounding, according to the authors. These are still relevant because CLI patients have an overall elevated cardiovascular risk in multiple vascular beds.
In terms of limitations, the authors noted the possibility of selection bias in the database, the rise of standalone outpatient centers in more recent years, which might funnel off select patients, and the lack of anatomical information in the NIS database, which precludes a determination of the appropriateness of treatment choice. Also, the type and invasiveness of the endovascular therapy cannot be determined. “It is possible that simple lesions were preferentially treated with endovascular therapy, whereas more complex lesions were treated by surgical techniques, leading to obvious differences in outcomes. Alternatively, it may be likely that the findings underestimate the impact of endovascular therapy, as sicker patients with higher comorbidities and poor targets were more likely to undergo endovascular revascularization,” the researchers pointed out.
“Despite similar rates of major amputation, endovascular revascularization was associated with reduced in-hospital mortality, mean LOS, and mean cost of hospitalization. Although the results are encouraging, there remain significant disparities and gaps that must be addressed,” Dr. Agarwal and his colleagues concluded.
The authors reported that they had no relevant disclosures.
Many of the unanswered questions regarding the optimal approach to CLI are being addressed by the National Heart, Lung, and Blood Institute–sponsored, multicenter, randomized BEST-CLI (Best Endovascular vs. Best Surgical Therapy in Patients with Critical Limb Ischemia) trial. The BEST-CLI trial will hopefully be completed in 2017. Until that time, clinicians will continue to rely on the best available data to guide revascularization strategies for the management of CLI.
Consistent with prior investigations, Dr. Agarwal et al. demonstrated a significant reduction in the proportion of patients undergoing surgical revascularization with a concomitant rise in endovascular revascularization during the same time period. This was accompanied by a steady decline in the incidence of in-hospital mortality and major amputation. Endovascular therapy was associated with a shorter mean length of stay and reduced hospital costs, despite a similar rate of in-hospital major amputation. As the authors correctly point out, the decreasing amputation and mortality rates cannot be directly attributable to a rise in endovascular therapy, as these studies cannot provide causal conclusions. Numerous other factors can influence mortality and amputation rates, including better medical care, aggressive risk factor modification, and appropriate wound care. Still, these associations are powerful and hypothesis generating, and they warrant further investigation.
Whether the improving CLI outcomes can be explained by the growth of these endovascular therapies is yet to be proved. We await the results of the landmark BEST-CLI trial to provide clarity regarding this issue and to further clarify the future role of surgical versus endovascular revascularization.
Dr. John R. Laird and Dr. Gagan D. Singh of the University of California, Davis Medical Center, Sacramento, and Dr. Ehrin J. Armstrong of the University of Colorado, Denver, made their comments in an invited editorial published online in the Journal of the American College of Cardiology (doi: 10.1016/j.jacc.2016.02.041). Dr. Laird has served as a consultant or advisory board member for Bard Peripheral Vascular, Boston Scientific, Cordis, Medtronic, and Abbott Vascular; and has received research support from WL Gore. Dr. Armstrong has served as a consultant or advisory board member for Abbott Vascular, Boston Scientific, Medtronic, Merck, and Spectranetics. Dr. Singh reported that he has no relevant disclosures.
Many of the unanswered questions regarding the optimal approach to CLI are being addressed by the National Heart, Lung, and Blood Institute–sponsored, multicenter, randomized BEST-CLI (Best Endovascular vs. Best Surgical Therapy in Patients with Critical Limb Ischemia) trial. The BEST-CLI trial will hopefully be completed in 2017. Until that time, clinicians will continue to rely on the best available data to guide revascularization strategies for the management of CLI.
Consistent with prior investigations, Dr. Agarwal et al. demonstrated a significant reduction in the proportion of patients undergoing surgical revascularization with a concomitant rise in endovascular revascularization during the same time period. This was accompanied by a steady decline in the incidence of in-hospital mortality and major amputation. Endovascular therapy was associated with a shorter mean length of stay and reduced hospital costs, despite a similar rate of in-hospital major amputation. As the authors correctly point out, the decreasing amputation and mortality rates cannot be directly attributable to a rise in endovascular therapy, as these studies cannot provide causal conclusions. Numerous other factors can influence mortality and amputation rates, including better medical care, aggressive risk factor modification, and appropriate wound care. Still, these associations are powerful and hypothesis generating, and they warrant further investigation.
Whether the improving CLI outcomes can be explained by the growth of these endovascular therapies is yet to be proved. We await the results of the landmark BEST-CLI trial to provide clarity regarding this issue and to further clarify the future role of surgical versus endovascular revascularization.
Dr. John R. Laird and Dr. Gagan D. Singh of the University of California, Davis Medical Center, Sacramento, and Dr. Ehrin J. Armstrong of the University of Colorado, Denver, made their comments in an invited editorial published online in the Journal of the American College of Cardiology (doi: 10.1016/j.jacc.2016.02.041). Dr. Laird has served as a consultant or advisory board member for Bard Peripheral Vascular, Boston Scientific, Cordis, Medtronic, and Abbott Vascular; and has received research support from WL Gore. Dr. Armstrong has served as a consultant or advisory board member for Abbott Vascular, Boston Scientific, Medtronic, Merck, and Spectranetics. Dr. Singh reported that he has no relevant disclosures.
Many of the unanswered questions regarding the optimal approach to CLI are being addressed by the National Heart, Lung, and Blood Institute–sponsored, multicenter, randomized BEST-CLI (Best Endovascular vs. Best Surgical Therapy in Patients with Critical Limb Ischemia) trial. The BEST-CLI trial will hopefully be completed in 2017. Until that time, clinicians will continue to rely on the best available data to guide revascularization strategies for the management of CLI.
Consistent with prior investigations, Dr. Agarwal et al. demonstrated a significant reduction in the proportion of patients undergoing surgical revascularization with a concomitant rise in endovascular revascularization during the same time period. This was accompanied by a steady decline in the incidence of in-hospital mortality and major amputation. Endovascular therapy was associated with a shorter mean length of stay and reduced hospital costs, despite a similar rate of in-hospital major amputation. As the authors correctly point out, the decreasing amputation and mortality rates cannot be directly attributable to a rise in endovascular therapy, as these studies cannot provide causal conclusions. Numerous other factors can influence mortality and amputation rates, including better medical care, aggressive risk factor modification, and appropriate wound care. Still, these associations are powerful and hypothesis generating, and they warrant further investigation.
Whether the improving CLI outcomes can be explained by the growth of these endovascular therapies is yet to be proved. We await the results of the landmark BEST-CLI trial to provide clarity regarding this issue and to further clarify the future role of surgical versus endovascular revascularization.
Dr. John R. Laird and Dr. Gagan D. Singh of the University of California, Davis Medical Center, Sacramento, and Dr. Ehrin J. Armstrong of the University of Colorado, Denver, made their comments in an invited editorial published online in the Journal of the American College of Cardiology (doi: 10.1016/j.jacc.2016.02.041). Dr. Laird has served as a consultant or advisory board member for Bard Peripheral Vascular, Boston Scientific, Cordis, Medtronic, and Abbott Vascular; and has received research support from WL Gore. Dr. Armstrong has served as a consultant or advisory board member for Abbott Vascular, Boston Scientific, Medtronic, Merck, and Spectranetics. Dr. Singh reported that he has no relevant disclosures.
Even though there was a steady rate of patients with critical limb ischemia (CLI) admitted to hospitals from 2003 to 2011, surgical revascularization decreased and endovascular treatment increased significantly, with concomitant decreases in in-hospital mortality and major amputation, according to the results of an analysis of the Nationwide Inpatient Sample of 642,433 patients hospitalized with CLI.
In addition, despite multiple adjustments, endovascular revascularization was associated with reduced in-hospital mortality, compared with surgical revascularization over the same period, according to a report online in the Journal of the American College of Cardiology.
The annual in-hospital mortality rate decreased from 5.4% in 2003 to 3.4% in 2011 (P less than .001), and the major amputation rate dropped from 16.7% to 10.8%. There also was a significant decrease in length-of-stay (LOS) from 10 days to 8.4 days over the same period (P less than .001); however this did not translate to a significant difference in the cost of hospitalization, according to Dr. Shikhar Agarwal and colleagues at the Cleveland Clinic [doi:10.1016/j.jacc.2016.02.040].
Significant predictive factors of in-hospital mortality after multivariate regression analysis were female sex, older age, emergent admission, a primary indication of septicemia, heart failure, and respiratory disease, as well any stump complications present during admission. In contrast, any form of revascularization was associated with significantly reduced in-hospital mortality.
A comparison of revascularization methods showed that surgical revascularization significantly decreased from 13.9% in 2003 to 8.8% in 2011, while endovascular revascularization increased from 5.1% to 11%. Also, endovascular revascularization was associated with a significant decrease in in-hospital mortality compared with surgical revascularization over the study period (2.34% vs. 2.73%, respectively; odds ratio = .69). Major amputation rates were not significantly different between the two treatments (6.5% vs. 5.7%; OR = .99).
Length of stay was significantly lower with endovascular treatment compared with surgical (8.7 vs. 10.7 days) as were costs ($31,679 vs. $32,485, respectively).
Women had a higher rate of in-hospital mortality, but a lower rate of major amputation. Although race was not seen as a factor in predicting in-hospital mortality, blacks and other nonwhite races had significantly higher rates of amputation and lower rates of revascularization, compared with whites.
Approximately half of the patients assessed were admitted for primary CLI-related diagnoses. The other, non–CLI-related conditions – such as acute MI, cerebrovascular events, respiratory disease, heart failure, and acute kidney disease – have all been independently associated with increased in-hospital mortality and may be confounding, according to the authors. These are still relevant because CLI patients have an overall elevated cardiovascular risk in multiple vascular beds.
In terms of limitations, the authors noted the possibility of selection bias in the database, the rise of standalone outpatient centers in more recent years, which might funnel off select patients, and the lack of anatomical information in the NIS database, which precludes a determination of the appropriateness of treatment choice. Also, the type and invasiveness of the endovascular therapy cannot be determined. “It is possible that simple lesions were preferentially treated with endovascular therapy, whereas more complex lesions were treated by surgical techniques, leading to obvious differences in outcomes. Alternatively, it may be likely that the findings underestimate the impact of endovascular therapy, as sicker patients with higher comorbidities and poor targets were more likely to undergo endovascular revascularization,” the researchers pointed out.
“Despite similar rates of major amputation, endovascular revascularization was associated with reduced in-hospital mortality, mean LOS, and mean cost of hospitalization. Although the results are encouraging, there remain significant disparities and gaps that must be addressed,” Dr. Agarwal and his colleagues concluded.
The authors reported that they had no relevant disclosures.
Even though there was a steady rate of patients with critical limb ischemia (CLI) admitted to hospitals from 2003 to 2011, surgical revascularization decreased and endovascular treatment increased significantly, with concomitant decreases in in-hospital mortality and major amputation, according to the results of an analysis of the Nationwide Inpatient Sample of 642,433 patients hospitalized with CLI.
In addition, despite multiple adjustments, endovascular revascularization was associated with reduced in-hospital mortality, compared with surgical revascularization over the same period, according to a report online in the Journal of the American College of Cardiology.
The annual in-hospital mortality rate decreased from 5.4% in 2003 to 3.4% in 2011 (P less than .001), and the major amputation rate dropped from 16.7% to 10.8%. There also was a significant decrease in length-of-stay (LOS) from 10 days to 8.4 days over the same period (P less than .001); however this did not translate to a significant difference in the cost of hospitalization, according to Dr. Shikhar Agarwal and colleagues at the Cleveland Clinic [doi:10.1016/j.jacc.2016.02.040].
Significant predictive factors of in-hospital mortality after multivariate regression analysis were female sex, older age, emergent admission, a primary indication of septicemia, heart failure, and respiratory disease, as well any stump complications present during admission. In contrast, any form of revascularization was associated with significantly reduced in-hospital mortality.
A comparison of revascularization methods showed that surgical revascularization significantly decreased from 13.9% in 2003 to 8.8% in 2011, while endovascular revascularization increased from 5.1% to 11%. Also, endovascular revascularization was associated with a significant decrease in in-hospital mortality compared with surgical revascularization over the study period (2.34% vs. 2.73%, respectively; odds ratio = .69). Major amputation rates were not significantly different between the two treatments (6.5% vs. 5.7%; OR = .99).
Length of stay was significantly lower with endovascular treatment compared with surgical (8.7 vs. 10.7 days) as were costs ($31,679 vs. $32,485, respectively).
Women had a higher rate of in-hospital mortality, but a lower rate of major amputation. Although race was not seen as a factor in predicting in-hospital mortality, blacks and other nonwhite races had significantly higher rates of amputation and lower rates of revascularization, compared with whites.
Approximately half of the patients assessed were admitted for primary CLI-related diagnoses. The other, non–CLI-related conditions – such as acute MI, cerebrovascular events, respiratory disease, heart failure, and acute kidney disease – have all been independently associated with increased in-hospital mortality and may be confounding, according to the authors. These are still relevant because CLI patients have an overall elevated cardiovascular risk in multiple vascular beds.
In terms of limitations, the authors noted the possibility of selection bias in the database, the rise of standalone outpatient centers in more recent years, which might funnel off select patients, and the lack of anatomical information in the NIS database, which precludes a determination of the appropriateness of treatment choice. Also, the type and invasiveness of the endovascular therapy cannot be determined. “It is possible that simple lesions were preferentially treated with endovascular therapy, whereas more complex lesions were treated by surgical techniques, leading to obvious differences in outcomes. Alternatively, it may be likely that the findings underestimate the impact of endovascular therapy, as sicker patients with higher comorbidities and poor targets were more likely to undergo endovascular revascularization,” the researchers pointed out.
“Despite similar rates of major amputation, endovascular revascularization was associated with reduced in-hospital mortality, mean LOS, and mean cost of hospitalization. Although the results are encouraging, there remain significant disparities and gaps that must be addressed,” Dr. Agarwal and his colleagues concluded.
The authors reported that they had no relevant disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Surgery in hospitalized CLI patients decreased and endovascular treatment increased from 2003 to 2011 with a concomitant decrease in in-hospital mortality and major amputation.
Major finding: Surgical revascularization significantly decreased from 13.9% in 2003 to 8.8% in 2011, while endovascular revascularization increased from 5.1% to 11%.
Data source: A retrospective database analysis of 642,433 patients hospitalized with CLI from 2003 to 2011 who were included in the Nationwide Inpatient Sample.
Disclosures: The authors reported that they had no relevant disclosures.
Flu vaccination found safe in surgical patients
Immunizing surgical patients against seasonal influenza before they are discharged from the hospital appears safe and is a sound strategy for expanding vaccine coverage, especially among people at high risk, according to a report published online March 14 in Annals of Internal Medicine.
All health care contacts, including hospitalizations, are considered excellent opportunities for influenza vaccination, and current recommendations advise that eligible inpatients receive the immunization before discharge. However, surgical patients don’t often get the flu vaccine before they leave the hospital, likely because of concerns that potential adverse effects like fever and myalgia could be falsely attributed to surgical complications. This would lead to unnecessary patient evaluations and could interfere with postsurgical care, said Sara Y. Tartof, Ph.D., and her associates in the department of research and evaluation, Kaiser Permanente Southern California, Pasadena.
“Although this concern is understandable, few clinical data support it,” they noted.
“To provide clinical evidence that would either substantiate or refute” these concerns about perioperative flu vaccination, the investigators analyzed data in the electronic health records for 81,647 surgeries. All the study participants were deemed eligible for flu vaccination. They were socioeconomically and ethnically diverse, ranged in age from 6 months to 106 years, and underwent surgery at 14 hospitals during three consecutive flu seasons. Operations included general, cardiac, eye, dermatologic, ENT, neurologic, ob.gyn., oral/maxillofacial, orthopedic, plastic, podiatric, urologic, and vascular procedures.
Patients received a flu vaccine in 6,420 hospital stays for surgery – only 15% of 42,777 eligible hospitalizations – usually on the day of discharge. (The remaining 38,870 patients either had been vaccinated before hospital admission or were vaccinated more than a week after discharge and were not included in further analyses.)
Compared with eligible patients who didn’t receive a flu vaccine during hospitalization for surgery, those who did showed no increased risk for subsequent inpatient visits, ED visits, or clinical work-ups for infection. Patients who received the flu vaccine before discharge showed a minimally increased risk for outpatient visits during the week following hospitalization, but this was considered unlikely “to translate into substantial clinical impact,” especially when balanced against the benefit of immunization, Dr. Tartof and her associates said (Ann Intern Med. 2016 Mar 14. doi: 10.7326/M15-1667).
Giving the flu vaccine during a surgical hospitalization “is an opportunity to protect a high-risk population,” because surgery patients tend to be of an age, and to have comorbid conditions, that raise their risk for flu complications. In addition, previous research has reported that 39%-46% of adults hospitalized for influenza-related disease in a given year had been hospitalized during the preceding autumn, indicating that recent hospitalization also raises the risk for flu complications, the investigators said.
“Our data support the rationale for increasing vaccination rates among surgical inpatients,” they said.
This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
Immunizing surgical patients against seasonal influenza before they are discharged from the hospital appears safe and is a sound strategy for expanding vaccine coverage, especially among people at high risk, according to a report published online March 14 in Annals of Internal Medicine.
All health care contacts, including hospitalizations, are considered excellent opportunities for influenza vaccination, and current recommendations advise that eligible inpatients receive the immunization before discharge. However, surgical patients don’t often get the flu vaccine before they leave the hospital, likely because of concerns that potential adverse effects like fever and myalgia could be falsely attributed to surgical complications. This would lead to unnecessary patient evaluations and could interfere with postsurgical care, said Sara Y. Tartof, Ph.D., and her associates in the department of research and evaluation, Kaiser Permanente Southern California, Pasadena.
“Although this concern is understandable, few clinical data support it,” they noted.
“To provide clinical evidence that would either substantiate or refute” these concerns about perioperative flu vaccination, the investigators analyzed data in the electronic health records for 81,647 surgeries. All the study participants were deemed eligible for flu vaccination. They were socioeconomically and ethnically diverse, ranged in age from 6 months to 106 years, and underwent surgery at 14 hospitals during three consecutive flu seasons. Operations included general, cardiac, eye, dermatologic, ENT, neurologic, ob.gyn., oral/maxillofacial, orthopedic, plastic, podiatric, urologic, and vascular procedures.
Patients received a flu vaccine in 6,420 hospital stays for surgery – only 15% of 42,777 eligible hospitalizations – usually on the day of discharge. (The remaining 38,870 patients either had been vaccinated before hospital admission or were vaccinated more than a week after discharge and were not included in further analyses.)
Compared with eligible patients who didn’t receive a flu vaccine during hospitalization for surgery, those who did showed no increased risk for subsequent inpatient visits, ED visits, or clinical work-ups for infection. Patients who received the flu vaccine before discharge showed a minimally increased risk for outpatient visits during the week following hospitalization, but this was considered unlikely “to translate into substantial clinical impact,” especially when balanced against the benefit of immunization, Dr. Tartof and her associates said (Ann Intern Med. 2016 Mar 14. doi: 10.7326/M15-1667).
Giving the flu vaccine during a surgical hospitalization “is an opportunity to protect a high-risk population,” because surgery patients tend to be of an age, and to have comorbid conditions, that raise their risk for flu complications. In addition, previous research has reported that 39%-46% of adults hospitalized for influenza-related disease in a given year had been hospitalized during the preceding autumn, indicating that recent hospitalization also raises the risk for flu complications, the investigators said.
“Our data support the rationale for increasing vaccination rates among surgical inpatients,” they said.
This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
Immunizing surgical patients against seasonal influenza before they are discharged from the hospital appears safe and is a sound strategy for expanding vaccine coverage, especially among people at high risk, according to a report published online March 14 in Annals of Internal Medicine.
All health care contacts, including hospitalizations, are considered excellent opportunities for influenza vaccination, and current recommendations advise that eligible inpatients receive the immunization before discharge. However, surgical patients don’t often get the flu vaccine before they leave the hospital, likely because of concerns that potential adverse effects like fever and myalgia could be falsely attributed to surgical complications. This would lead to unnecessary patient evaluations and could interfere with postsurgical care, said Sara Y. Tartof, Ph.D., and her associates in the department of research and evaluation, Kaiser Permanente Southern California, Pasadena.
“Although this concern is understandable, few clinical data support it,” they noted.
“To provide clinical evidence that would either substantiate or refute” these concerns about perioperative flu vaccination, the investigators analyzed data in the electronic health records for 81,647 surgeries. All the study participants were deemed eligible for flu vaccination. They were socioeconomically and ethnically diverse, ranged in age from 6 months to 106 years, and underwent surgery at 14 hospitals during three consecutive flu seasons. Operations included general, cardiac, eye, dermatologic, ENT, neurologic, ob.gyn., oral/maxillofacial, orthopedic, plastic, podiatric, urologic, and vascular procedures.
Patients received a flu vaccine in 6,420 hospital stays for surgery – only 15% of 42,777 eligible hospitalizations – usually on the day of discharge. (The remaining 38,870 patients either had been vaccinated before hospital admission or were vaccinated more than a week after discharge and were not included in further analyses.)
Compared with eligible patients who didn’t receive a flu vaccine during hospitalization for surgery, those who did showed no increased risk for subsequent inpatient visits, ED visits, or clinical work-ups for infection. Patients who received the flu vaccine before discharge showed a minimally increased risk for outpatient visits during the week following hospitalization, but this was considered unlikely “to translate into substantial clinical impact,” especially when balanced against the benefit of immunization, Dr. Tartof and her associates said (Ann Intern Med. 2016 Mar 14. doi: 10.7326/M15-1667).
Giving the flu vaccine during a surgical hospitalization “is an opportunity to protect a high-risk population,” because surgery patients tend to be of an age, and to have comorbid conditions, that raise their risk for flu complications. In addition, previous research has reported that 39%-46% of adults hospitalized for influenza-related disease in a given year had been hospitalized during the preceding autumn, indicating that recent hospitalization also raises the risk for flu complications, the investigators said.
“Our data support the rationale for increasing vaccination rates among surgical inpatients,” they said.
This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Immunizing surgical patients against seasonal influenza before they leave the hospital appears safe.
Major finding: Patients received a flu vaccine in only 6,420 hospital stays for surgery, comprising only 15% of the patient hospitalizations that were eligible.
Data source: A retrospective cohort study involving 81,647 surgeries at 14 California hospitals during three consecutive flu seasons.
Disclosures: This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
Outcomes in major surgery unchanged by continuing clopidogrel
MONTREAL – Patients who stayed on antiplatelet therapy close to – or even up until – major surgery fared just as well as those who stopped their medication earlier in a retrospective, single-center study.
The study found no difference in blood product administration, adverse perioperative events, or all-cause 30-day mortality regardless of whether patients stopped clopidogrel (Plavix) the recommended 5 days before surgery.
“We believe that continuing clopidogrel in elective and emergent surgical situations appears to be safe, and may challenge the current recommendations,” said presenter Dr. David Strosberg.
The study addressed a thorny question for surgeons, Dr. Strosberg said. “As surgeons, we face a dilemma: Do we take the risk of thrombotic complications in stopping the antiplatelet drugs, or do we take the risk of increased surgical bleeding with continuing therapy?”
The package insert for clopidogrel advises discontinuation 5 days prior to surgery. However, manufacturer labeling also states that discontinuation of clopidogrel can lead to adverse cardiac events, said Dr. Strosberg, a general surgery resident at Ohio State University in Columbus.
The aim of the study, presented at the annual meeting of the Central Surgical Association, was to ascertain whether continuing antiplatelet therapy increased the rate of adverse surgical outcomes in those undergoing major emergent or elective surgery.
Dr. Strosberg and his colleagues retrospectively reviewed the record of patients over a 4-year period at a single institution and included those undergoing major general, thoracic, or vascular surgery who were taking clopidogrel at the time of presentation.
Data collected included patient characteristics, including demographic data and comorbidities, as well as transfusion requirements and perioperative events.
A total of 200 patients who had 205 qualifying procedures and were taking clopidogrel were included in the study. Of these, 116 patients (Group A) had their clopidogrel held for at least 5 days preoperatively. The remaining 89 patients (Group B) had their clopidogrel held for less than 5 days, or not at all.
Patient demographics were similar between the two groups. Patients in Group A were more likely to have emergency surgery, to have peripheral stents placed, to have COPD or peripheral vascular disease, to have a malignancy, and to have received aspirin within five days of surgery (P less than .01 for all).
Blood product administration rates and volumes did not differ significantly between the two groups, and there was no difference between the groups in the incidence of myocardial infarctions, cerebrovascular events, or acute visceral or lower extremity ischemia.
Three patients in each group died within 30 days of the procedure, a nonsignificant difference. However, in the group that had clopidogrel held, three patients had perioperative myocardial infarctions, and two of these patients died. In discussing the study, Dr. Michael Dalsing said, “I think a lot of us would accept bleeding more over myocardial infarction.”
A subgroup analysis of the group who had clopidogrel held for fewer than 5 days compared outcomes for emergent vs. non-emergent surgery. The emergent surgery subgroup had a significantly higher rate of preoperative platelet transfusions, although numbers overall were small (2/17, 11.8%, vs. 0/72; P = .03).
Dr. Strosberg noted study limitations that included the retrospective, single-center nature of the study, and the fact that one variable, estimated blood loss, is notoriously subjective and inaccurate.
Dr. Dalsing, chief of vascular surgery at Indiana University, Indianapolis, said that he “was surprised that not even one patient went back for postoperative bleeding in this high-risk group of patients,” and raised the question of potential selection bias. Dr. Strosberg replied that comorbidities were ascertained by the physician at the time of surgery planning; since no differences were seen between study groups, investigators didn’t go back and parse out details about comorbid conditions.
In discussion following the presentation, surgeons spoke to the real-world challenges of performing surgery on a patient with antiplatelet therapy on board.
“Overall, I think your data support kind of a bias I have. Since I’m a vascular surgeon, we almost always operate on clopidogrel, and I don’t know if our bleeding risk is worse or better. But it’s something we almost have to do to keep our grafts going,” Dr. Dalsing said.
Dr. Peter Henke, professor of vascular surgery at the University of Michigan, Ann Arbor, said, “I’d be a little bit cautious with this. If you’ve ever done a big aortic procedure on someone on Plavix, I’ve seen them lose up to a couple of liters of blood just with oozing.”
“Those of us who do open aortic surgery know that very few things bleed like the back wall of an aortic anastomosis of a patient on Plavix,” echoed Dr. Peter Rossi, associate professor of vascular surgery at the Medical College of Wisconsin, Milwaukee.
The study authors reported no relevant disclosures.
On Twitter @karioakes
MONTREAL – Patients who stayed on antiplatelet therapy close to – or even up until – major surgery fared just as well as those who stopped their medication earlier in a retrospective, single-center study.
The study found no difference in blood product administration, adverse perioperative events, or all-cause 30-day mortality regardless of whether patients stopped clopidogrel (Plavix) the recommended 5 days before surgery.
“We believe that continuing clopidogrel in elective and emergent surgical situations appears to be safe, and may challenge the current recommendations,” said presenter Dr. David Strosberg.
The study addressed a thorny question for surgeons, Dr. Strosberg said. “As surgeons, we face a dilemma: Do we take the risk of thrombotic complications in stopping the antiplatelet drugs, or do we take the risk of increased surgical bleeding with continuing therapy?”
The package insert for clopidogrel advises discontinuation 5 days prior to surgery. However, manufacturer labeling also states that discontinuation of clopidogrel can lead to adverse cardiac events, said Dr. Strosberg, a general surgery resident at Ohio State University in Columbus.
The aim of the study, presented at the annual meeting of the Central Surgical Association, was to ascertain whether continuing antiplatelet therapy increased the rate of adverse surgical outcomes in those undergoing major emergent or elective surgery.
Dr. Strosberg and his colleagues retrospectively reviewed the record of patients over a 4-year period at a single institution and included those undergoing major general, thoracic, or vascular surgery who were taking clopidogrel at the time of presentation.
Data collected included patient characteristics, including demographic data and comorbidities, as well as transfusion requirements and perioperative events.
A total of 200 patients who had 205 qualifying procedures and were taking clopidogrel were included in the study. Of these, 116 patients (Group A) had their clopidogrel held for at least 5 days preoperatively. The remaining 89 patients (Group B) had their clopidogrel held for less than 5 days, or not at all.
Patient demographics were similar between the two groups. Patients in Group A were more likely to have emergency surgery, to have peripheral stents placed, to have COPD or peripheral vascular disease, to have a malignancy, and to have received aspirin within five days of surgery (P less than .01 for all).
Blood product administration rates and volumes did not differ significantly between the two groups, and there was no difference between the groups in the incidence of myocardial infarctions, cerebrovascular events, or acute visceral or lower extremity ischemia.
Three patients in each group died within 30 days of the procedure, a nonsignificant difference. However, in the group that had clopidogrel held, three patients had perioperative myocardial infarctions, and two of these patients died. In discussing the study, Dr. Michael Dalsing said, “I think a lot of us would accept bleeding more over myocardial infarction.”
A subgroup analysis of the group who had clopidogrel held for fewer than 5 days compared outcomes for emergent vs. non-emergent surgery. The emergent surgery subgroup had a significantly higher rate of preoperative platelet transfusions, although numbers overall were small (2/17, 11.8%, vs. 0/72; P = .03).
Dr. Strosberg noted study limitations that included the retrospective, single-center nature of the study, and the fact that one variable, estimated blood loss, is notoriously subjective and inaccurate.
Dr. Dalsing, chief of vascular surgery at Indiana University, Indianapolis, said that he “was surprised that not even one patient went back for postoperative bleeding in this high-risk group of patients,” and raised the question of potential selection bias. Dr. Strosberg replied that comorbidities were ascertained by the physician at the time of surgery planning; since no differences were seen between study groups, investigators didn’t go back and parse out details about comorbid conditions.
In discussion following the presentation, surgeons spoke to the real-world challenges of performing surgery on a patient with antiplatelet therapy on board.
“Overall, I think your data support kind of a bias I have. Since I’m a vascular surgeon, we almost always operate on clopidogrel, and I don’t know if our bleeding risk is worse or better. But it’s something we almost have to do to keep our grafts going,” Dr. Dalsing said.
Dr. Peter Henke, professor of vascular surgery at the University of Michigan, Ann Arbor, said, “I’d be a little bit cautious with this. If you’ve ever done a big aortic procedure on someone on Plavix, I’ve seen them lose up to a couple of liters of blood just with oozing.”
“Those of us who do open aortic surgery know that very few things bleed like the back wall of an aortic anastomosis of a patient on Plavix,” echoed Dr. Peter Rossi, associate professor of vascular surgery at the Medical College of Wisconsin, Milwaukee.
The study authors reported no relevant disclosures.
On Twitter @karioakes
MONTREAL – Patients who stayed on antiplatelet therapy close to – or even up until – major surgery fared just as well as those who stopped their medication earlier in a retrospective, single-center study.
The study found no difference in blood product administration, adverse perioperative events, or all-cause 30-day mortality regardless of whether patients stopped clopidogrel (Plavix) the recommended 5 days before surgery.
“We believe that continuing clopidogrel in elective and emergent surgical situations appears to be safe, and may challenge the current recommendations,” said presenter Dr. David Strosberg.
The study addressed a thorny question for surgeons, Dr. Strosberg said. “As surgeons, we face a dilemma: Do we take the risk of thrombotic complications in stopping the antiplatelet drugs, or do we take the risk of increased surgical bleeding with continuing therapy?”
The package insert for clopidogrel advises discontinuation 5 days prior to surgery. However, manufacturer labeling also states that discontinuation of clopidogrel can lead to adverse cardiac events, said Dr. Strosberg, a general surgery resident at Ohio State University in Columbus.
The aim of the study, presented at the annual meeting of the Central Surgical Association, was to ascertain whether continuing antiplatelet therapy increased the rate of adverse surgical outcomes in those undergoing major emergent or elective surgery.
Dr. Strosberg and his colleagues retrospectively reviewed the record of patients over a 4-year period at a single institution and included those undergoing major general, thoracic, or vascular surgery who were taking clopidogrel at the time of presentation.
Data collected included patient characteristics, including demographic data and comorbidities, as well as transfusion requirements and perioperative events.
A total of 200 patients who had 205 qualifying procedures and were taking clopidogrel were included in the study. Of these, 116 patients (Group A) had their clopidogrel held for at least 5 days preoperatively. The remaining 89 patients (Group B) had their clopidogrel held for less than 5 days, or not at all.
Patient demographics were similar between the two groups. Patients in Group A were more likely to have emergency surgery, to have peripheral stents placed, to have COPD or peripheral vascular disease, to have a malignancy, and to have received aspirin within five days of surgery (P less than .01 for all).
Blood product administration rates and volumes did not differ significantly between the two groups, and there was no difference between the groups in the incidence of myocardial infarctions, cerebrovascular events, or acute visceral or lower extremity ischemia.
Three patients in each group died within 30 days of the procedure, a nonsignificant difference. However, in the group that had clopidogrel held, three patients had perioperative myocardial infarctions, and two of these patients died. In discussing the study, Dr. Michael Dalsing said, “I think a lot of us would accept bleeding more over myocardial infarction.”
A subgroup analysis of the group who had clopidogrel held for fewer than 5 days compared outcomes for emergent vs. non-emergent surgery. The emergent surgery subgroup had a significantly higher rate of preoperative platelet transfusions, although numbers overall were small (2/17, 11.8%, vs. 0/72; P = .03).
Dr. Strosberg noted study limitations that included the retrospective, single-center nature of the study, and the fact that one variable, estimated blood loss, is notoriously subjective and inaccurate.
Dr. Dalsing, chief of vascular surgery at Indiana University, Indianapolis, said that he “was surprised that not even one patient went back for postoperative bleeding in this high-risk group of patients,” and raised the question of potential selection bias. Dr. Strosberg replied that comorbidities were ascertained by the physician at the time of surgery planning; since no differences were seen between study groups, investigators didn’t go back and parse out details about comorbid conditions.
In discussion following the presentation, surgeons spoke to the real-world challenges of performing surgery on a patient with antiplatelet therapy on board.
“Overall, I think your data support kind of a bias I have. Since I’m a vascular surgeon, we almost always operate on clopidogrel, and I don’t know if our bleeding risk is worse or better. But it’s something we almost have to do to keep our grafts going,” Dr. Dalsing said.
Dr. Peter Henke, professor of vascular surgery at the University of Michigan, Ann Arbor, said, “I’d be a little bit cautious with this. If you’ve ever done a big aortic procedure on someone on Plavix, I’ve seen them lose up to a couple of liters of blood just with oozing.”
“Those of us who do open aortic surgery know that very few things bleed like the back wall of an aortic anastomosis of a patient on Plavix,” echoed Dr. Peter Rossi, associate professor of vascular surgery at the Medical College of Wisconsin, Milwaukee.
The study authors reported no relevant disclosures.
On Twitter @karioakes
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: Outcomes were similar whether patients did or didn’t stop clopidogrel before major surgery.
Major finding: No significant differences in blood product use, adverse events, or death were seen with continuing clopidogrel.
Data source: Retrospective, single-center review of 200 patients undergoing major elective or emergent surgery and taking clopidogrel.
Disclosures: The study authors reported no relevant disclosures.