User login
Left Arm Pain, Numbness, and Weakness
ANSWER
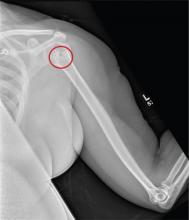
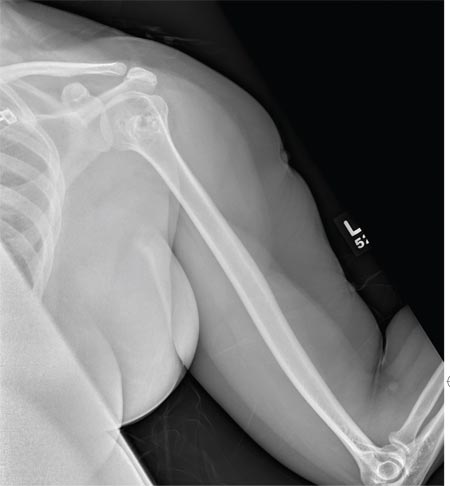
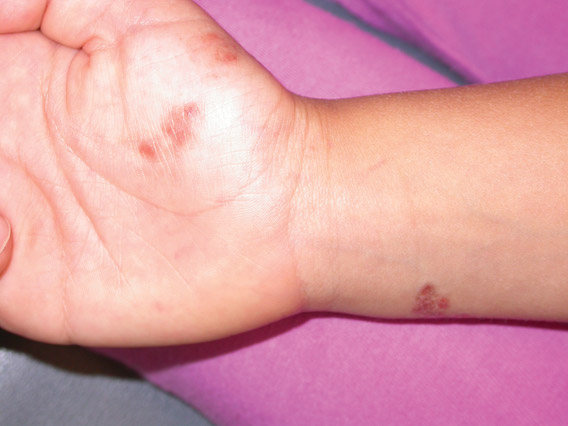
The radiograph shows no evidence of a fracture. However, there is a 2-cm focal sclerotic area noted within the juncture of the humeral neck and head. This finding could represent an enchondroma, a bone cyst, or a bone infarct. Additional imaging, including MRI and bone scan, is warranted, as is orthopedic evaluation. This finding is likely incidental, as the patient’s clinical exam is suggestive of a cervical radiculitis referable to the herniated disc in her neck.
ANSWER
The radiograph shows no evidence of a fracture. However, there is a 2-cm focal sclerotic area noted within the juncture of the humeral neck and head. This finding could represent an enchondroma, a bone cyst, or a bone infarct. Additional imaging, including MRI and bone scan, is warranted, as is orthopedic evaluation. This finding is likely incidental, as the patient’s clinical exam is suggestive of a cervical radiculitis referable to the herniated disc in her neck.
ANSWER
The radiograph shows no evidence of a fracture. However, there is a 2-cm focal sclerotic area noted within the juncture of the humeral neck and head. This finding could represent an enchondroma, a bone cyst, or a bone infarct. Additional imaging, including MRI and bone scan, is warranted, as is orthopedic evaluation. This finding is likely incidental, as the patient’s clinical exam is suggestive of a cervical radiculitis referable to the herniated disc in her neck.
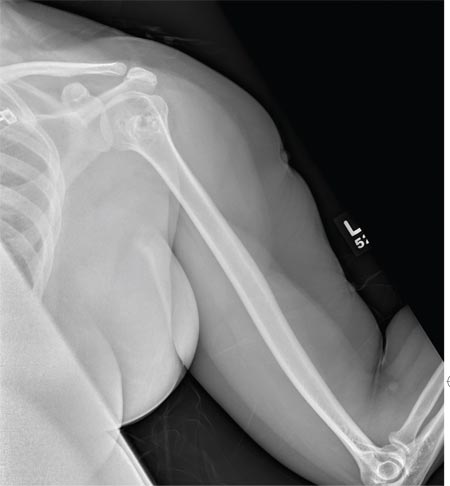
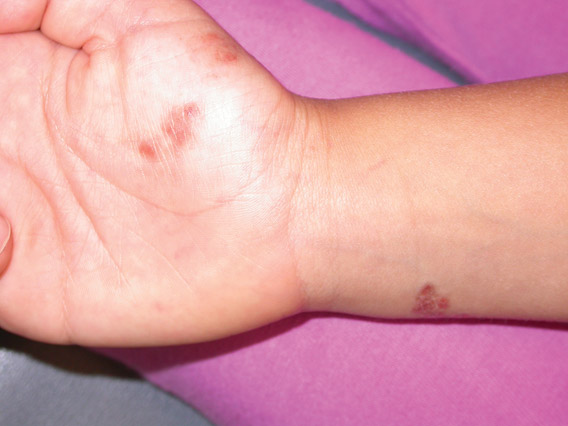
A 40-year-old woman presents to the urgent care clinic complaining of left arm pain with associated numbness and weakness. She denies any injury or trauma, adding that the pain manifested several months ago but has recently progressed. She has already undergone outpatient MRI of her neck; she was told she had some “herniated discs” and would need to see a specialist. Her medical history is significant for hypertension. On physical examination, the patient appears uncomfortable but in no obvious distress. Vital signs are normal. Tenderness is present at the left trapezius and the left shoulder. Mild weakness is present in the left arm; strength is 4/5 and grip strength, 3/5. Pulses are normal, and sensation is intact. Available medical records include a report from her recent MRI of the cervical spine. Findings include a moderate left-sided disc osteophyte at the C6-C7 level and resultant cervical stenosis. A radiograph of the left shoulder is obtained. What is your impression?
Lesions’ Pattern Helps Line Up Diagnosis
ANSWER
The correct answer is lichen nitidus (choice “b”), a harmless, self-limited condition of unknown origin. The lesions’ flat-topped (planar) surfaces and tendency to form in linear configurations along lines of trauma (so-called Koebner phenomenon) are also features seen in lichen planus (choice “d”) lesions; however, the latter are almost always pruritic and purple in color. Ironically, the histologic pattern seen in both is almost identical.
An extremely common condition, molluscum contagiosum (choice “a”) presents with multiple tiny papules. But these are not planar, and most will have an umbilicated center. See the Discussion for ways to distinguish it from lichen nitidus.
Flat warts (choice “c”), known as verruca plana, can strongly resemble lichen nitidus, but they are not as flat-topped and do not appear white. They do Koebnerize, however, which occasionally makes the distinction difficult.
DISCUSSION
Lichen nitidus (LN) is an unusual but benign condition primarily affecting children and young adults. Due to the contrast, the white planar papules are easier to see on darker skin. As is the case with many dermatologic diagnoses, LN is easily identified if you’ve heard of it and therefore know what to expect—but much more difficult if you haven’t.
LN’s unique manifestation distinguishes it from other items in the differential. For example, molluscum and LN can easily be confused, especially since both primarily affect children. But the pathognomic central umbilication of molluscum lesions is the key distinguishing feature; the best way to highlight it is with a short blast of liquid nitrogen. (Usually, though, the planar surfaces of LN are sufficient to distinguish it from other conditions.)
In the United States, the term Koebner phenomenon refers to the tendency for lesions to form along areas of trauma, usually in a linear configuration. All four items in our differential can present in this way. However, the term auto-inoculation might be more properly applied to conditions such as warts and molluscum, since the trauma has merely inoculated the organism into the skin. Inflammatory conditions such as LN and lichen planus are not truly “spread” by the trauma.
Linearly configured lesions are sufficiently unusual in dermatology to warrant their own differential. Among those that can present in this manner are psoriasis, lichen sclerosus et atrophicus, and vitiligo.
TREATMENT/PROGNOSIS
Our LN patient did not require any treatment, nor was any possible. The condition is quite likely to clear on its own, leaving little if any evidence in its wake.
I often show affected patients and/or their parents pictures of these types of conditions from our textbooks, for added reassurance. And in this day and age, I direct them to websites where they can do more investigation on their own time.
The effective practice of dermatology (and of all medicine, for that matter) includes more than merely making a correct diagnosis: I believe we’re obliged to “sell” it as well.
ANSWER
The correct answer is lichen nitidus (choice “b”), a harmless, self-limited condition of unknown origin. The lesions’ flat-topped (planar) surfaces and tendency to form in linear configurations along lines of trauma (so-called Koebner phenomenon) are also features seen in lichen planus (choice “d”) lesions; however, the latter are almost always pruritic and purple in color. Ironically, the histologic pattern seen in both is almost identical.
An extremely common condition, molluscum contagiosum (choice “a”) presents with multiple tiny papules. But these are not planar, and most will have an umbilicated center. See the Discussion for ways to distinguish it from lichen nitidus.
Flat warts (choice “c”), known as verruca plana, can strongly resemble lichen nitidus, but they are not as flat-topped and do not appear white. They do Koebnerize, however, which occasionally makes the distinction difficult.
DISCUSSION
Lichen nitidus (LN) is an unusual but benign condition primarily affecting children and young adults. Due to the contrast, the white planar papules are easier to see on darker skin. As is the case with many dermatologic diagnoses, LN is easily identified if you’ve heard of it and therefore know what to expect—but much more difficult if you haven’t.
LN’s unique manifestation distinguishes it from other items in the differential. For example, molluscum and LN can easily be confused, especially since both primarily affect children. But the pathognomic central umbilication of molluscum lesions is the key distinguishing feature; the best way to highlight it is with a short blast of liquid nitrogen. (Usually, though, the planar surfaces of LN are sufficient to distinguish it from other conditions.)
In the United States, the term Koebner phenomenon refers to the tendency for lesions to form along areas of trauma, usually in a linear configuration. All four items in our differential can present in this way. However, the term auto-inoculation might be more properly applied to conditions such as warts and molluscum, since the trauma has merely inoculated the organism into the skin. Inflammatory conditions such as LN and lichen planus are not truly “spread” by the trauma.
Linearly configured lesions are sufficiently unusual in dermatology to warrant their own differential. Among those that can present in this manner are psoriasis, lichen sclerosus et atrophicus, and vitiligo.
TREATMENT/PROGNOSIS
Our LN patient did not require any treatment, nor was any possible. The condition is quite likely to clear on its own, leaving little if any evidence in its wake.
I often show affected patients and/or their parents pictures of these types of conditions from our textbooks, for added reassurance. And in this day and age, I direct them to websites where they can do more investigation on their own time.
The effective practice of dermatology (and of all medicine, for that matter) includes more than merely making a correct diagnosis: I believe we’re obliged to “sell” it as well.
ANSWER
The correct answer is lichen nitidus (choice “b”), a harmless, self-limited condition of unknown origin. The lesions’ flat-topped (planar) surfaces and tendency to form in linear configurations along lines of trauma (so-called Koebner phenomenon) are also features seen in lichen planus (choice “d”) lesions; however, the latter are almost always pruritic and purple in color. Ironically, the histologic pattern seen in both is almost identical.
An extremely common condition, molluscum contagiosum (choice “a”) presents with multiple tiny papules. But these are not planar, and most will have an umbilicated center. See the Discussion for ways to distinguish it from lichen nitidus.
Flat warts (choice “c”), known as verruca plana, can strongly resemble lichen nitidus, but they are not as flat-topped and do not appear white. They do Koebnerize, however, which occasionally makes the distinction difficult.
DISCUSSION
Lichen nitidus (LN) is an unusual but benign condition primarily affecting children and young adults. Due to the contrast, the white planar papules are easier to see on darker skin. As is the case with many dermatologic diagnoses, LN is easily identified if you’ve heard of it and therefore know what to expect—but much more difficult if you haven’t.
LN’s unique manifestation distinguishes it from other items in the differential. For example, molluscum and LN can easily be confused, especially since both primarily affect children. But the pathognomic central umbilication of molluscum lesions is the key distinguishing feature; the best way to highlight it is with a short blast of liquid nitrogen. (Usually, though, the planar surfaces of LN are sufficient to distinguish it from other conditions.)
In the United States, the term Koebner phenomenon refers to the tendency for lesions to form along areas of trauma, usually in a linear configuration. All four items in our differential can present in this way. However, the term auto-inoculation might be more properly applied to conditions such as warts and molluscum, since the trauma has merely inoculated the organism into the skin. Inflammatory conditions such as LN and lichen planus are not truly “spread” by the trauma.
Linearly configured lesions are sufficiently unusual in dermatology to warrant their own differential. Among those that can present in this manner are psoriasis, lichen sclerosus et atrophicus, and vitiligo.
TREATMENT/PROGNOSIS
Our LN patient did not require any treatment, nor was any possible. The condition is quite likely to clear on its own, leaving little if any evidence in its wake.
I often show affected patients and/or their parents pictures of these types of conditions from our textbooks, for added reassurance. And in this day and age, I direct them to websites where they can do more investigation on their own time.
The effective practice of dermatology (and of all medicine, for that matter) includes more than merely making a correct diagnosis: I believe we’re obliged to “sell” it as well.
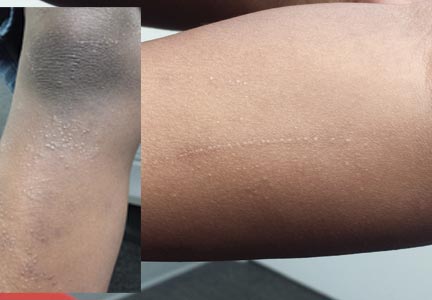
Six months ago, a 6-year-old boy developed asymptomatic lesions on his elbows, then his knees. They slowly spread to other areas, including his forearms. One primary care provider diagnosed probable warts; another, molluscum. The prescribed treatments—liquid nitrogen and tretinoin, respectively—had no effect. The boy’s mother became alarmed when the lesions started to form in long lines on his arms. At that point, she decided to bring him to dermatology for evaluation. Aside from his skin condition, the child is healthy, according to both his mother and the records provided by his primary care provider’s office. The lesions are particularly numerous over the extensor surfaces of the legs—especially the knees—but are also seen on the extensor forearms and elbows. The lesions are exquisitely discrete, identical, tiny white pinpoint papules, all with flat tops. None are umbilicated. In several areas of the arms, linear collections of lesions, some extending as long as 6 cm, are noted. The rest of his exposed type V skin is unremarkable.
Lobular-Appearing Nodule on the Scalp
The Diagnosis: Dermal Cylindroma
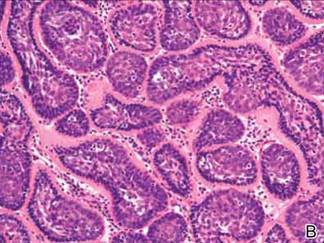
Microsopic evaluation of a tangential biopsy revealed findings of a dermal process consisting of well-circumscribed islands of pale and darker blue cells with little cytoplasm outlined by a hyaline basement membrane (Figure). These cellular islands were arranged in a jigsawlike configuration. These findings were thought to be consistent with a diagnosis of cylindroma.
|
Cylindromas are benign appendageal neoplasms with a somewhat controversial histogenesis. Munger and colleagues1 investigated the pattern of acid mucopolysaccharide secretion by these tumors in association with prosecretory vacuoles in proximity to the Golgi apparatus, which led to their impression that cylindromas most resemble eccrine rather than apocrine sweat glands. Other researchers, however, have concluded that cylindromas are of apocrine derivation.2
Clinically, cylindromas appear most often in 2 settings: isolated or as a manifestation of one of several inherited familial syndromes. One such syndrome is familial cylindromatosis, a rare autosomal-dominant disorder in which affected individuals develop multiple cylindromas, usually on the head and neck. The merging of multiple lesions gives rise to the often-employed term turban tumor.3 This syndrome has been linked to mutations in the cylindromatosis gene, CYLD.4 Brooke-Spiegler syndrome also has been associated with the development of multiple cylindromas. Similar to familial cylindromatosis, it is inherited in an autosomal-dominant fashion. Brooke-Spiegler syndrome is typified by the appearance of multiple cylindromas, trichoepitheliomas, and less commonly spiradenomas. Mutations in the CYLD gene also have been linked to Brooke-Spiegler syndrome in some cases.5
Although considered a benign entity, in rare cases cylindromas have shown evidence of malignant transformation to cylindrocarcinoma. This more aggressive tumor may occur in the setting of isolated cylindromas or more commonly in individuals with numerous lesions, as with both familial cylindromatosis and Brooke-Spiegler syndrome. These lesions may appear to grow rapidly, ulcerate, or bleed, traits that are not associated with their benign counterparts.
Diagnosis of cylindromas rests on histopathologic confirmation, which demonstrates well-defined dermal islands of epithelial cells comprised of dark- and pale-staining nuclei. These tumor islands are surrounded by a hyaline basement membrane and often take on the appearance of a jigsaw puzzle. Cylindrocarcinomas exhibit greater cellular pleomorphism and higher mitotic rates.
Dermal cylindromas require no further treatment but can be electively excised, while treatment of cylindrocarcinoma with excision is curative.6 Definitive excision was offered to our patient, but she declined treatment.
1. Munger BL, Graham JH, Helwig EB. Ultrastructure and histochemical characteristics of dermal eccrine cylindroma (turban tumor). J Invest Dermatol. 1962;39:577-595.
2. Tellechea O, Reis JP, Ilheu O, et al. Dermal cylindroma. an immunohistochemical study of thirteen cases. Am J Dermatopathol. 1995;17:260-265.
3. Biggs PJ, Wooster R, Ford D, et al. Familial cylindromatosis (turban tumour syndrome) gene localised to chromosome 16q12-q13: evidence for its role as a tumour suppressor gene. Nat Genet. 1995;11:441-443.
4. Bignell GR, Warren W, Seal S, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160-165.
5. Bowen S, Gill M, Lee DA, et al. Mutations in the CYLD gene in Brooke-Spiegler syndrome, familial cylindromatosis, and multiple familial trichoepithelioma: lack of genotype-phenotype correlation. J Invest Dermatol. 2005;124:919-920.
6. Gerretsen AL, van der Putte SC, Deenstra W, et al. Cutaneous cylindroma with malignant transformation. Cancer. 1993;72:1618-1623.
The Diagnosis: Dermal Cylindroma
Microsopic evaluation of a tangential biopsy revealed findings of a dermal process consisting of well-circumscribed islands of pale and darker blue cells with little cytoplasm outlined by a hyaline basement membrane (Figure). These cellular islands were arranged in a jigsawlike configuration. These findings were thought to be consistent with a diagnosis of cylindroma.
|
Cylindromas are benign appendageal neoplasms with a somewhat controversial histogenesis. Munger and colleagues1 investigated the pattern of acid mucopolysaccharide secretion by these tumors in association with prosecretory vacuoles in proximity to the Golgi apparatus, which led to their impression that cylindromas most resemble eccrine rather than apocrine sweat glands. Other researchers, however, have concluded that cylindromas are of apocrine derivation.2
Clinically, cylindromas appear most often in 2 settings: isolated or as a manifestation of one of several inherited familial syndromes. One such syndrome is familial cylindromatosis, a rare autosomal-dominant disorder in which affected individuals develop multiple cylindromas, usually on the head and neck. The merging of multiple lesions gives rise to the often-employed term turban tumor.3 This syndrome has been linked to mutations in the cylindromatosis gene, CYLD.4 Brooke-Spiegler syndrome also has been associated with the development of multiple cylindromas. Similar to familial cylindromatosis, it is inherited in an autosomal-dominant fashion. Brooke-Spiegler syndrome is typified by the appearance of multiple cylindromas, trichoepitheliomas, and less commonly spiradenomas. Mutations in the CYLD gene also have been linked to Brooke-Spiegler syndrome in some cases.5
Although considered a benign entity, in rare cases cylindromas have shown evidence of malignant transformation to cylindrocarcinoma. This more aggressive tumor may occur in the setting of isolated cylindromas or more commonly in individuals with numerous lesions, as with both familial cylindromatosis and Brooke-Spiegler syndrome. These lesions may appear to grow rapidly, ulcerate, or bleed, traits that are not associated with their benign counterparts.
Diagnosis of cylindromas rests on histopathologic confirmation, which demonstrates well-defined dermal islands of epithelial cells comprised of dark- and pale-staining nuclei. These tumor islands are surrounded by a hyaline basement membrane and often take on the appearance of a jigsaw puzzle. Cylindrocarcinomas exhibit greater cellular pleomorphism and higher mitotic rates.
Dermal cylindromas require no further treatment but can be electively excised, while treatment of cylindrocarcinoma with excision is curative.6 Definitive excision was offered to our patient, but she declined treatment.
The Diagnosis: Dermal Cylindroma
Microsopic evaluation of a tangential biopsy revealed findings of a dermal process consisting of well-circumscribed islands of pale and darker blue cells with little cytoplasm outlined by a hyaline basement membrane (Figure). These cellular islands were arranged in a jigsawlike configuration. These findings were thought to be consistent with a diagnosis of cylindroma.
|
Cylindromas are benign appendageal neoplasms with a somewhat controversial histogenesis. Munger and colleagues1 investigated the pattern of acid mucopolysaccharide secretion by these tumors in association with prosecretory vacuoles in proximity to the Golgi apparatus, which led to their impression that cylindromas most resemble eccrine rather than apocrine sweat glands. Other researchers, however, have concluded that cylindromas are of apocrine derivation.2
Clinically, cylindromas appear most often in 2 settings: isolated or as a manifestation of one of several inherited familial syndromes. One such syndrome is familial cylindromatosis, a rare autosomal-dominant disorder in which affected individuals develop multiple cylindromas, usually on the head and neck. The merging of multiple lesions gives rise to the often-employed term turban tumor.3 This syndrome has been linked to mutations in the cylindromatosis gene, CYLD.4 Brooke-Spiegler syndrome also has been associated with the development of multiple cylindromas. Similar to familial cylindromatosis, it is inherited in an autosomal-dominant fashion. Brooke-Spiegler syndrome is typified by the appearance of multiple cylindromas, trichoepitheliomas, and less commonly spiradenomas. Mutations in the CYLD gene also have been linked to Brooke-Spiegler syndrome in some cases.5
Although considered a benign entity, in rare cases cylindromas have shown evidence of malignant transformation to cylindrocarcinoma. This more aggressive tumor may occur in the setting of isolated cylindromas or more commonly in individuals with numerous lesions, as with both familial cylindromatosis and Brooke-Spiegler syndrome. These lesions may appear to grow rapidly, ulcerate, or bleed, traits that are not associated with their benign counterparts.
Diagnosis of cylindromas rests on histopathologic confirmation, which demonstrates well-defined dermal islands of epithelial cells comprised of dark- and pale-staining nuclei. These tumor islands are surrounded by a hyaline basement membrane and often take on the appearance of a jigsaw puzzle. Cylindrocarcinomas exhibit greater cellular pleomorphism and higher mitotic rates.
Dermal cylindromas require no further treatment but can be electively excised, while treatment of cylindrocarcinoma with excision is curative.6 Definitive excision was offered to our patient, but she declined treatment.
1. Munger BL, Graham JH, Helwig EB. Ultrastructure and histochemical characteristics of dermal eccrine cylindroma (turban tumor). J Invest Dermatol. 1962;39:577-595.
2. Tellechea O, Reis JP, Ilheu O, et al. Dermal cylindroma. an immunohistochemical study of thirteen cases. Am J Dermatopathol. 1995;17:260-265.
3. Biggs PJ, Wooster R, Ford D, et al. Familial cylindromatosis (turban tumour syndrome) gene localised to chromosome 16q12-q13: evidence for its role as a tumour suppressor gene. Nat Genet. 1995;11:441-443.
4. Bignell GR, Warren W, Seal S, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160-165.
5. Bowen S, Gill M, Lee DA, et al. Mutations in the CYLD gene in Brooke-Spiegler syndrome, familial cylindromatosis, and multiple familial trichoepithelioma: lack of genotype-phenotype correlation. J Invest Dermatol. 2005;124:919-920.
6. Gerretsen AL, van der Putte SC, Deenstra W, et al. Cutaneous cylindroma with malignant transformation. Cancer. 1993;72:1618-1623.
1. Munger BL, Graham JH, Helwig EB. Ultrastructure and histochemical characteristics of dermal eccrine cylindroma (turban tumor). J Invest Dermatol. 1962;39:577-595.
2. Tellechea O, Reis JP, Ilheu O, et al. Dermal cylindroma. an immunohistochemical study of thirteen cases. Am J Dermatopathol. 1995;17:260-265.
3. Biggs PJ, Wooster R, Ford D, et al. Familial cylindromatosis (turban tumour syndrome) gene localised to chromosome 16q12-q13: evidence for its role as a tumour suppressor gene. Nat Genet. 1995;11:441-443.
4. Bignell GR, Warren W, Seal S, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160-165.
5. Bowen S, Gill M, Lee DA, et al. Mutations in the CYLD gene in Brooke-Spiegler syndrome, familial cylindromatosis, and multiple familial trichoepithelioma: lack of genotype-phenotype correlation. J Invest Dermatol. 2005;124:919-920.
6. Gerretsen AL, van der Putte SC, Deenstra W, et al. Cutaneous cylindroma with malignant transformation. Cancer. 1993;72:1618-1623.
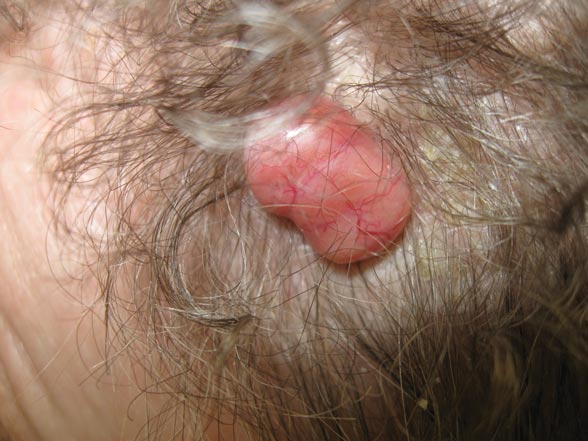
A 79-year-old woman presented with a lesion on the left side of the scalp of several years’ duration that had slowly increased in size. Despite its growth, the lesion remained asymptomatic. Physical examination revealed an exophytic, lobular-appearing nodule on the left side of the temporoparietal scalp, measuring 1.5 cm in size.
Lesions With a Distinct Fingerprint Presentation
The Diagnosis: Phytophotodermatitis
Phytophotodermatitis (PPD) is a nonimmunologic cutaneous phototoxic inflammatory reaction resulting from the activation of photosensitizing botanical agents such as furanocoumarins in contact with the skin by exposure to UVA light.1,2 Furanocoumarins, including psoralens and angelicins, become photoexcited and covalently bind to pyrimidine bases on DNA strands, resulting in acute damage to epidermal, dermal, and endothelial cells.1,3
Vegetation most commonly implicated in this plant solar dermatitis are celery, fennel, parsnip, parsley, and hogweed (Apiaceae [formerly known as the Umbelliferae family]), as well as oranges, lemons, limes, and grapefruits (Rutaceae or citrus family).1,3 Psoralens found in the Persian lime have been noted to cause phototoxic eruptions in the United States, with the rind containing higher concentrations than the pulp.4
Clinical features of PPD include erythema, edema, and vesicle or bullae formation 12 to 36 hours after psoralen and UV light exposure. Burning and pain may be present, but pruritus is not a common characteristic of the eruptions, distinguishing PPD from allergic phytodermatitis.
Hyperpigmentation appears on resolution of the lesions and slowly fades over months to years.1,3,5 Mild exposure may lead to hyperpigmentation without a vesicular or erythematous eruption.1 Phytophotodermatitis follows a benign course and often spontaneously resolves; however, prolonged hyperpigmentation may cause concern for these patients.
Phytophotodermatitis is common among patients preparing drinks and foods with citrus juices or after gardening. Our patient had prepared limeade 3 weeks prior to presentation. The distribution of cutaneous exposure to furanocoumarins influences clinical presentation and may range from blotches and streaks to distinct fingerprint smudges and handprints, as seen in our patient. The distinct full handprint on the right arm was striking. The bullous lesions and resulting hyperpigmentation may mimic burns and healing bruises. In children, PPD often is mistaken for child abuse.1,6,7 In adults, it often is misdiagnosed as poison oak dermatitis, erythema multiforme, and thrombocytopenic purpura.1,3 It is important to recognize PPD to avoid delay in or misdiagnosis and to better counsel patients on how to avoid recurrent episodes of PPD.
1. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Maryland Heights, MO: Mosby; 2008.
2. Pomeranz MK, Karen JK. Phytophotodermatitis and limes. N Engl J Med. 2007;357:e1.
3. Sassiville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
4. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
5. Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
6. Mill J, Wallis B, Cuttle L, et al. Phytophotodermatitis: case reports of children presenting with blistering after preparing lime juice. Burns. 2008;34:731-733.
7. Carlsen K, Weismann K. Phytophotodermatitis in 19 children admitted to hospital and their differential diagnoses: child abuse and herpes simplex virus infection. J Am Acad Dermatol. 2007;57(suppl):S88-S91.
The Diagnosis: Phytophotodermatitis
Phytophotodermatitis (PPD) is a nonimmunologic cutaneous phototoxic inflammatory reaction resulting from the activation of photosensitizing botanical agents such as furanocoumarins in contact with the skin by exposure to UVA light.1,2 Furanocoumarins, including psoralens and angelicins, become photoexcited and covalently bind to pyrimidine bases on DNA strands, resulting in acute damage to epidermal, dermal, and endothelial cells.1,3
Vegetation most commonly implicated in this plant solar dermatitis are celery, fennel, parsnip, parsley, and hogweed (Apiaceae [formerly known as the Umbelliferae family]), as well as oranges, lemons, limes, and grapefruits (Rutaceae or citrus family).1,3 Psoralens found in the Persian lime have been noted to cause phototoxic eruptions in the United States, with the rind containing higher concentrations than the pulp.4
Clinical features of PPD include erythema, edema, and vesicle or bullae formation 12 to 36 hours after psoralen and UV light exposure. Burning and pain may be present, but pruritus is not a common characteristic of the eruptions, distinguishing PPD from allergic phytodermatitis.
Hyperpigmentation appears on resolution of the lesions and slowly fades over months to years.1,3,5 Mild exposure may lead to hyperpigmentation without a vesicular or erythematous eruption.1 Phytophotodermatitis follows a benign course and often spontaneously resolves; however, prolonged hyperpigmentation may cause concern for these patients.
Phytophotodermatitis is common among patients preparing drinks and foods with citrus juices or after gardening. Our patient had prepared limeade 3 weeks prior to presentation. The distribution of cutaneous exposure to furanocoumarins influences clinical presentation and may range from blotches and streaks to distinct fingerprint smudges and handprints, as seen in our patient. The distinct full handprint on the right arm was striking. The bullous lesions and resulting hyperpigmentation may mimic burns and healing bruises. In children, PPD often is mistaken for child abuse.1,6,7 In adults, it often is misdiagnosed as poison oak dermatitis, erythema multiforme, and thrombocytopenic purpura.1,3 It is important to recognize PPD to avoid delay in or misdiagnosis and to better counsel patients on how to avoid recurrent episodes of PPD.
The Diagnosis: Phytophotodermatitis
Phytophotodermatitis (PPD) is a nonimmunologic cutaneous phototoxic inflammatory reaction resulting from the activation of photosensitizing botanical agents such as furanocoumarins in contact with the skin by exposure to UVA light.1,2 Furanocoumarins, including psoralens and angelicins, become photoexcited and covalently bind to pyrimidine bases on DNA strands, resulting in acute damage to epidermal, dermal, and endothelial cells.1,3
Vegetation most commonly implicated in this plant solar dermatitis are celery, fennel, parsnip, parsley, and hogweed (Apiaceae [formerly known as the Umbelliferae family]), as well as oranges, lemons, limes, and grapefruits (Rutaceae or citrus family).1,3 Psoralens found in the Persian lime have been noted to cause phototoxic eruptions in the United States, with the rind containing higher concentrations than the pulp.4
Clinical features of PPD include erythema, edema, and vesicle or bullae formation 12 to 36 hours after psoralen and UV light exposure. Burning and pain may be present, but pruritus is not a common characteristic of the eruptions, distinguishing PPD from allergic phytodermatitis.
Hyperpigmentation appears on resolution of the lesions and slowly fades over months to years.1,3,5 Mild exposure may lead to hyperpigmentation without a vesicular or erythematous eruption.1 Phytophotodermatitis follows a benign course and often spontaneously resolves; however, prolonged hyperpigmentation may cause concern for these patients.
Phytophotodermatitis is common among patients preparing drinks and foods with citrus juices or after gardening. Our patient had prepared limeade 3 weeks prior to presentation. The distribution of cutaneous exposure to furanocoumarins influences clinical presentation and may range from blotches and streaks to distinct fingerprint smudges and handprints, as seen in our patient. The distinct full handprint on the right arm was striking. The bullous lesions and resulting hyperpigmentation may mimic burns and healing bruises. In children, PPD often is mistaken for child abuse.1,6,7 In adults, it often is misdiagnosed as poison oak dermatitis, erythema multiforme, and thrombocytopenic purpura.1,3 It is important to recognize PPD to avoid delay in or misdiagnosis and to better counsel patients on how to avoid recurrent episodes of PPD.
1. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Maryland Heights, MO: Mosby; 2008.
2. Pomeranz MK, Karen JK. Phytophotodermatitis and limes. N Engl J Med. 2007;357:e1.
3. Sassiville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
4. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
5. Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
6. Mill J, Wallis B, Cuttle L, et al. Phytophotodermatitis: case reports of children presenting with blistering after preparing lime juice. Burns. 2008;34:731-733.
7. Carlsen K, Weismann K. Phytophotodermatitis in 19 children admitted to hospital and their differential diagnoses: child abuse and herpes simplex virus infection. J Am Acad Dermatol. 2007;57(suppl):S88-S91.
1. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Maryland Heights, MO: Mosby; 2008.
2. Pomeranz MK, Karen JK. Phytophotodermatitis and limes. N Engl J Med. 2007;357:e1.
3. Sassiville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
4. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
5. Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
6. Mill J, Wallis B, Cuttle L, et al. Phytophotodermatitis: case reports of children presenting with blistering after preparing lime juice. Burns. 2008;34:731-733.
7. Carlsen K, Weismann K. Phytophotodermatitis in 19 children admitted to hospital and their differential diagnoses: child abuse and herpes simplex virus infection. J Am Acad Dermatol. 2007;57(suppl):S88-S91.

A 17-year-old adolescent girl presented with scattered brown macules over the dorsal aspect of the hands bilaterally and a brown patch in the shape of a hand on the right upper arm of 3 weeks’ duration.
Multiple Firm Pink Papules and Nodules
The Diagnosis: Myeloid Leukemia Cutis
Leukemia cutis represents the infiltration of leukemic cells into the skin. It has been described in the setting of both myeloid and lymphoid leukemia. In the setting of acute myeloid leukemia, it has been reported to occur in 2% to 13% of patients overall,1,2 but it may occur in 31% of patients with the acute myelomonocytic or acute monocytic leukemia subtypes.3 Leukemia cutis is less common, with chronic myeloid leukemia occurring in 2.7% of patients in one study.4 In another study, 65% of patients with myeloid leukemia cutis had an acute myeloid leukemia.5
Myeloid leukemia cutis has been reported in patients aged 22 days to 90 years, with a median age of 62 years. There is a male predominance (1.4:1 ratio).5,6 The diagnosis of leukemia cutis is made concurrently with the diagnosis of leukemia in approximately 30% of cases, subsequent to the diagnosis of leukemia in approximately 60% of cases, and prior to the diagnosis of leukemia in approximately 10% of cases.5
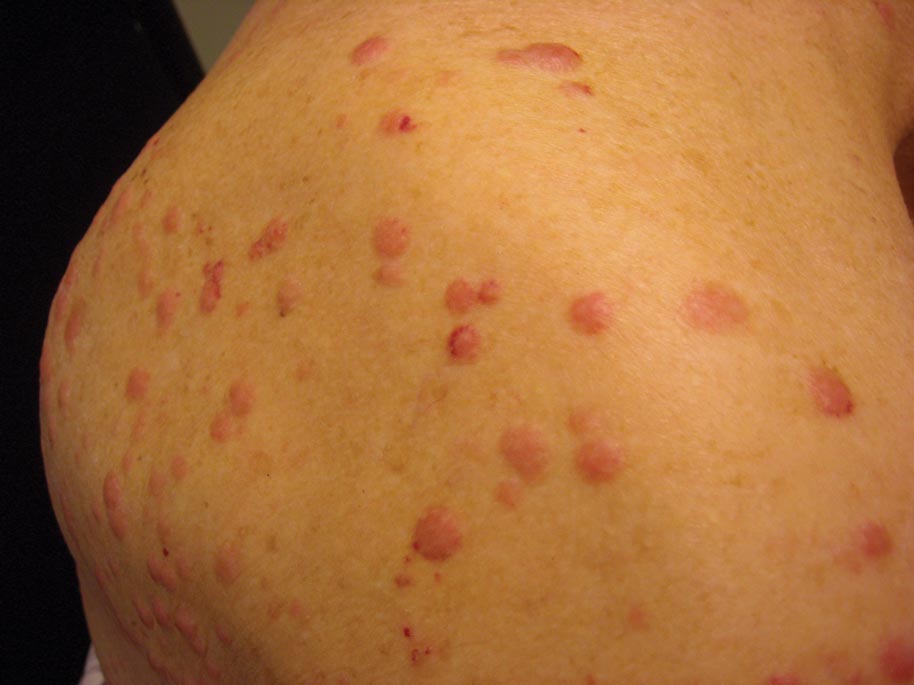
Clinically, myeloid leukemia cutis presents as an asymptomatic solitary lesion in 23% of cases or as multiple lesions in 77% of cases. Lesions consist of pink to red to violaceous papules, nodules, and macules that are occasionally purpuric and involve any cutaneous surface.5
Histologically, the epidermis is unremarkable. Beneath a grenz zone within the dermis and usually extending into the subcutis there is a diffuse or nodular proliferation of neoplastic cells, often with perivascular and periadnexal accentuation and sometimes single filing of cells between collagen bundles (Figure 1). The cells are immature myeloid cells with irregular nuclear contours that may be indented or reniform (Figure 2). Nuclei contain finely dispersed chromatin with variably prominent nucleoli.5,6 Immunohistochemically, CD68 is positive in approximately 97% of cases, myeloperoxidase in 62%, and lysozyme in 85%. CD168, CD14, CD4, CD33, CD117, CD34, CD56, CD123, and CD303 are variably positive. CD3 and CD20, markers of lymphoid leukemia, are negative.5-8
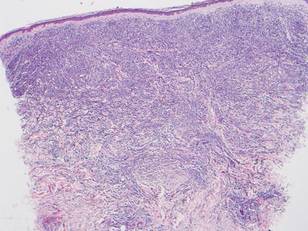
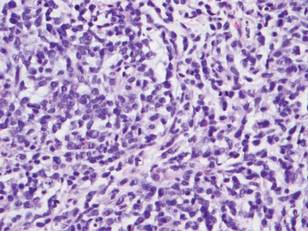
Leukemia cutis in the setting of a myeloid leukemia portends a grave prognosis. In a series of 18 patients, 16 had additional extramedullary leukemia, including meningeal leukemia in 6 patients.2 Most patients with myeloid leukemia cutis die within an average of 1 to 8 months of diagnosis.9
- Boggs DR, Wintrobe MM, Cartwright GE. The acute leukemias. analysis of 322 cases and review of the literature. Medicine (Baltimore). 1962;41:163-225.
- Baer MR, Barcos M, Farrell H, et al. Acute myelogenous leukemia with leukemia cutis. eighteen cases seen between 1969 and 1986. Cancer. 1989;63:2192-2200.
- Straus DJ, Mertelsmann R, Koziner B, et al. The acute monocytic leukemias: multidisciplinary studies in 45 patients. Medicine (Baltimore). 1980;59:409-425.
- Rosenthal S, Canellos GP, DeVita VT Jr, et al. Characteristics of blast crisis in chronic granulocytic leukemia. Blood. 1977;49:705-714.
- Bénet C, Gomez A, Aguilar C, et al. Histologic and immunohistologic characterization of skin localization of myeloid disorders: a study of 173 cases. Am J Clin Pathol. 2011;135:278-290.
- Cronin DM, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol. 2009;132:101-110.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Su WP, Buechner SA, Li CY. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
The Diagnosis: Myeloid Leukemia Cutis
Leukemia cutis represents the infiltration of leukemic cells into the skin. It has been described in the setting of both myeloid and lymphoid leukemia. In the setting of acute myeloid leukemia, it has been reported to occur in 2% to 13% of patients overall,1,2 but it may occur in 31% of patients with the acute myelomonocytic or acute monocytic leukemia subtypes.3 Leukemia cutis is less common, with chronic myeloid leukemia occurring in 2.7% of patients in one study.4 In another study, 65% of patients with myeloid leukemia cutis had an acute myeloid leukemia.5
Myeloid leukemia cutis has been reported in patients aged 22 days to 90 years, with a median age of 62 years. There is a male predominance (1.4:1 ratio).5,6 The diagnosis of leukemia cutis is made concurrently with the diagnosis of leukemia in approximately 30% of cases, subsequent to the diagnosis of leukemia in approximately 60% of cases, and prior to the diagnosis of leukemia in approximately 10% of cases.5
Clinically, myeloid leukemia cutis presents as an asymptomatic solitary lesion in 23% of cases or as multiple lesions in 77% of cases. Lesions consist of pink to red to violaceous papules, nodules, and macules that are occasionally purpuric and involve any cutaneous surface.5
Histologically, the epidermis is unremarkable. Beneath a grenz zone within the dermis and usually extending into the subcutis there is a diffuse or nodular proliferation of neoplastic cells, often with perivascular and periadnexal accentuation and sometimes single filing of cells between collagen bundles (Figure 1). The cells are immature myeloid cells with irregular nuclear contours that may be indented or reniform (Figure 2). Nuclei contain finely dispersed chromatin with variably prominent nucleoli.5,6 Immunohistochemically, CD68 is positive in approximately 97% of cases, myeloperoxidase in 62%, and lysozyme in 85%. CD168, CD14, CD4, CD33, CD117, CD34, CD56, CD123, and CD303 are variably positive. CD3 and CD20, markers of lymphoid leukemia, are negative.5-8


Leukemia cutis in the setting of a myeloid leukemia portends a grave prognosis. In a series of 18 patients, 16 had additional extramedullary leukemia, including meningeal leukemia in 6 patients.2 Most patients with myeloid leukemia cutis die within an average of 1 to 8 months of diagnosis.9
The Diagnosis: Myeloid Leukemia Cutis
Leukemia cutis represents the infiltration of leukemic cells into the skin. It has been described in the setting of both myeloid and lymphoid leukemia. In the setting of acute myeloid leukemia, it has been reported to occur in 2% to 13% of patients overall,1,2 but it may occur in 31% of patients with the acute myelomonocytic or acute monocytic leukemia subtypes.3 Leukemia cutis is less common, with chronic myeloid leukemia occurring in 2.7% of patients in one study.4 In another study, 65% of patients with myeloid leukemia cutis had an acute myeloid leukemia.5
Myeloid leukemia cutis has been reported in patients aged 22 days to 90 years, with a median age of 62 years. There is a male predominance (1.4:1 ratio).5,6 The diagnosis of leukemia cutis is made concurrently with the diagnosis of leukemia in approximately 30% of cases, subsequent to the diagnosis of leukemia in approximately 60% of cases, and prior to the diagnosis of leukemia in approximately 10% of cases.5
Clinically, myeloid leukemia cutis presents as an asymptomatic solitary lesion in 23% of cases or as multiple lesions in 77% of cases. Lesions consist of pink to red to violaceous papules, nodules, and macules that are occasionally purpuric and involve any cutaneous surface.5
Histologically, the epidermis is unremarkable. Beneath a grenz zone within the dermis and usually extending into the subcutis there is a diffuse or nodular proliferation of neoplastic cells, often with perivascular and periadnexal accentuation and sometimes single filing of cells between collagen bundles (Figure 1). The cells are immature myeloid cells with irregular nuclear contours that may be indented or reniform (Figure 2). Nuclei contain finely dispersed chromatin with variably prominent nucleoli.5,6 Immunohistochemically, CD68 is positive in approximately 97% of cases, myeloperoxidase in 62%, and lysozyme in 85%. CD168, CD14, CD4, CD33, CD117, CD34, CD56, CD123, and CD303 are variably positive. CD3 and CD20, markers of lymphoid leukemia, are negative.5-8


Leukemia cutis in the setting of a myeloid leukemia portends a grave prognosis. In a series of 18 patients, 16 had additional extramedullary leukemia, including meningeal leukemia in 6 patients.2 Most patients with myeloid leukemia cutis die within an average of 1 to 8 months of diagnosis.9
- Boggs DR, Wintrobe MM, Cartwright GE. The acute leukemias. analysis of 322 cases and review of the literature. Medicine (Baltimore). 1962;41:163-225.
- Baer MR, Barcos M, Farrell H, et al. Acute myelogenous leukemia with leukemia cutis. eighteen cases seen between 1969 and 1986. Cancer. 1989;63:2192-2200.
- Straus DJ, Mertelsmann R, Koziner B, et al. The acute monocytic leukemias: multidisciplinary studies in 45 patients. Medicine (Baltimore). 1980;59:409-425.
- Rosenthal S, Canellos GP, DeVita VT Jr, et al. Characteristics of blast crisis in chronic granulocytic leukemia. Blood. 1977;49:705-714.
- Bénet C, Gomez A, Aguilar C, et al. Histologic and immunohistologic characterization of skin localization of myeloid disorders: a study of 173 cases. Am J Clin Pathol. 2011;135:278-290.
- Cronin DM, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol. 2009;132:101-110.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Su WP, Buechner SA, Li CY. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
- Boggs DR, Wintrobe MM, Cartwright GE. The acute leukemias. analysis of 322 cases and review of the literature. Medicine (Baltimore). 1962;41:163-225.
- Baer MR, Barcos M, Farrell H, et al. Acute myelogenous leukemia with leukemia cutis. eighteen cases seen between 1969 and 1986. Cancer. 1989;63:2192-2200.
- Straus DJ, Mertelsmann R, Koziner B, et al. The acute monocytic leukemias: multidisciplinary studies in 45 patients. Medicine (Baltimore). 1980;59:409-425.
- Rosenthal S, Canellos GP, DeVita VT Jr, et al. Characteristics of blast crisis in chronic granulocytic leukemia. Blood. 1977;49:705-714.
- Bénet C, Gomez A, Aguilar C, et al. Histologic and immunohistologic characterization of skin localization of myeloid disorders: a study of 173 cases. Am J Clin Pathol. 2011;135:278-290.
- Cronin DM, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol. 2009;132:101-110.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Su WP, Buechner SA, Li CY. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
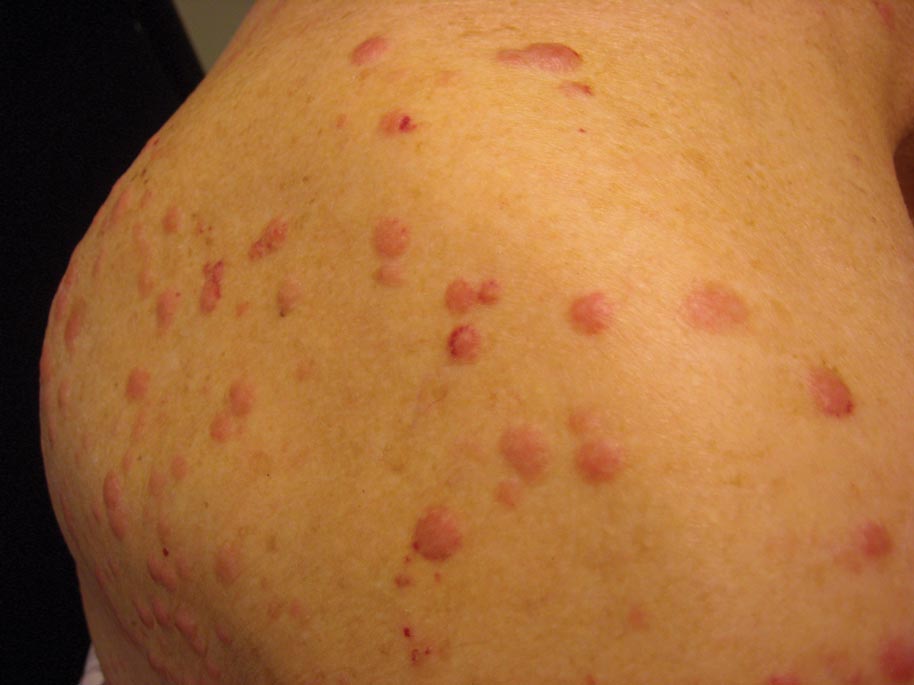
A 91-year-old man presented with numerous, scattered, asymptomatic, 3- to 9-mm, smooth, firm, pink papules and nodules involving the neck, trunk, and arms and legs of 1 week’s duration.
Man With Diverticulitis Undergoes Precolonoscopy Evaluation
ANSWER
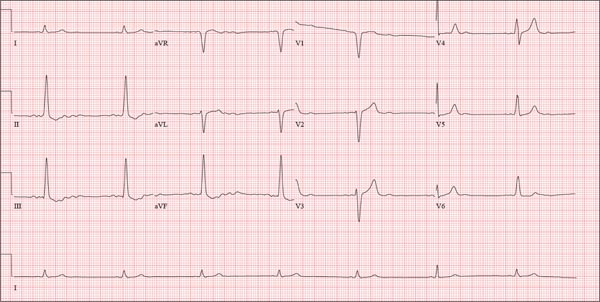
This ECG shows probable ectopic rhythm with second-degree atrioventricular (AV) block and a nonspecific intraventricular conduction block.
The P-wave morphology is unusual; rather than being upright and positive in leads II and aVF, the P waves are biphasic and prolonged, suggesting they originate from an atrial source other than the sinus node.
The rhythm strip of lead I in this ECG isn’t of much help in determining the atrial rhythm, as the P waves are small. However, if you look at the strip beginning with either lead II or III and keep in mind that the strip is continuous even though the leads change (eg, lead III becomes lead aVF which becomes V3, etc), you can see an atrial complex immediately following the T wave that is very similar to the P wave prior to the QRS complex. The rate of the P waves is 84 beats/min, which is twice that of the QRS complex (42 beats/min) and therefore consistent with a 2:1 heart block.
A nonspecific AV conduction block is evidenced by a QRS duration > 120 ms that does not have the appearance of a right or left bundle branch block.
Finally, while the QT interval of 514 ms is worrisome for long QT interval, it is normal when corrected for rate.
ANSWER
This ECG shows probable ectopic rhythm with second-degree atrioventricular (AV) block and a nonspecific intraventricular conduction block.
The P-wave morphology is unusual; rather than being upright and positive in leads II and aVF, the P waves are biphasic and prolonged, suggesting they originate from an atrial source other than the sinus node.
The rhythm strip of lead I in this ECG isn’t of much help in determining the atrial rhythm, as the P waves are small. However, if you look at the strip beginning with either lead II or III and keep in mind that the strip is continuous even though the leads change (eg, lead III becomes lead aVF which becomes V3, etc), you can see an atrial complex immediately following the T wave that is very similar to the P wave prior to the QRS complex. The rate of the P waves is 84 beats/min, which is twice that of the QRS complex (42 beats/min) and therefore consistent with a 2:1 heart block.
A nonspecific AV conduction block is evidenced by a QRS duration > 120 ms that does not have the appearance of a right or left bundle branch block.
Finally, while the QT interval of 514 ms is worrisome for long QT interval, it is normal when corrected for rate.
ANSWER
This ECG shows probable ectopic rhythm with second-degree atrioventricular (AV) block and a nonspecific intraventricular conduction block.
The P-wave morphology is unusual; rather than being upright and positive in leads II and aVF, the P waves are biphasic and prolonged, suggesting they originate from an atrial source other than the sinus node.
The rhythm strip of lead I in this ECG isn’t of much help in determining the atrial rhythm, as the P waves are small. However, if you look at the strip beginning with either lead II or III and keep in mind that the strip is continuous even though the leads change (eg, lead III becomes lead aVF which becomes V3, etc), you can see an atrial complex immediately following the T wave that is very similar to the P wave prior to the QRS complex. The rate of the P waves is 84 beats/min, which is twice that of the QRS complex (42 beats/min) and therefore consistent with a 2:1 heart block.
A nonspecific AV conduction block is evidenced by a QRS duration > 120 ms that does not have the appearance of a right or left bundle branch block.
Finally, while the QT interval of 514 ms is worrisome for long QT interval, it is normal when corrected for rate.
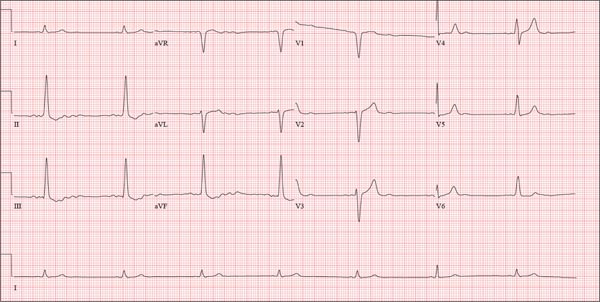
A 74-year-old man with recurring episodes of melena presents for a preoperative evaluation prior to colonoscopy. He has had three such procedures in the past five years, all of which indicated diverticulitis. The current episode began about a month ago, but the patient delayed seeking care until last week due to work obligations. The patient reports feeling more lethargic and becoming more easily tired than he has with previous episodes, which concerns him. He doesn’t think he has lost more blood than before but admits he’s been “too busy” to notice. He denies chest pain, shortness of breath, palpitations, peripheral extremity swelling, or recent weight change (gain or loss). He has not experienced loss of appetite or abdominal pain. Medical history is remarkable for hypertension, cholecystitis, and diverticulitis. There is no history of coronary artery disease, diabetes, or chronic obstructive pulmonary disease. Surgical history is remarkable for cholecystectomy and surgical repair of a high fracture of the left ankle. The patient owns a 475-acre farm, where he has lived his entire life. He is a widower who relies on his four sons to help with chores, although he insists on driving the combine himself during harvest (which is why he delayed seeking care this time). He does not smoke or drink. His current medications include hydrochlorothiazide and naproxen as needed for musculoskeletal discomfort. The review of systems is remarkable for fatigue and “the usual aches and pains of working on a farm.” The remainder of the review is noncontributory. The physical exam reveals a thin, weather-worn male in no distress. His height is 76 in and his weight, 172 lb. Both are unchanged from his previous clinic visit (six months ago). Vital signs include a blood pressure of 138/78 mm Hg; pulse, 46 beats/min; O2 saturation, 96%; and temperature, 98.2°F. Pertinent findings include normal breath sounds, a regular (albeit slow at 46 beats/min) rhythm, an early grade II/VI systolic murmur heard at the left upper sternal border, and a soft, nontender abdomen. There is no peripheral edema and no femoral or carotid bruits. The neurologic exam is intact. While the patient is undergoing preoperative laboratory tests and ECG, you review his medical record. Of note, the bradycardia found during today’s physical was not present six months ago. Laboratory data include a normal chemistry panel and a hematocrit of 38%. The ECG reveals a ventricular rate of 42 beats/min; PR interval, not reported; QRS duration, 130 ms; QT/QTc interval, 514/429 ms; P axis, 83°; R axis, 84°; and T axis, –43°. What is your interpretation of this ECG?
Man Unresponsive After Being Struck by Car
ANSWER
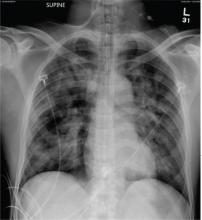
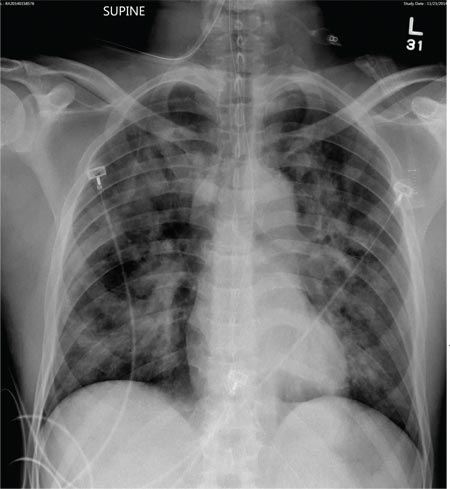
The radiograph demonstrates bilateral patchy, fluffy infiltrates as well as what is sometimes referred to as ground-glass opacities. In the setting of trauma and respiratory compromise, these areas are most suggestive of pulmonary contusions and early acute respiratory distress syndrome. Other possibilities in the differential diagnosis include pulmonary edema, atypical pneumonia, and pulmonary metastases.
ANSWER
The radiograph demonstrates bilateral patchy, fluffy infiltrates as well as what is sometimes referred to as ground-glass opacities. In the setting of trauma and respiratory compromise, these areas are most suggestive of pulmonary contusions and early acute respiratory distress syndrome. Other possibilities in the differential diagnosis include pulmonary edema, atypical pneumonia, and pulmonary metastases.
ANSWER
The radiograph demonstrates bilateral patchy, fluffy infiltrates as well as what is sometimes referred to as ground-glass opacities. In the setting of trauma and respiratory compromise, these areas are most suggestive of pulmonary contusions and early acute respiratory distress syndrome. Other possibilities in the differential diagnosis include pulmonary edema, atypical pneumonia, and pulmonary metastases.
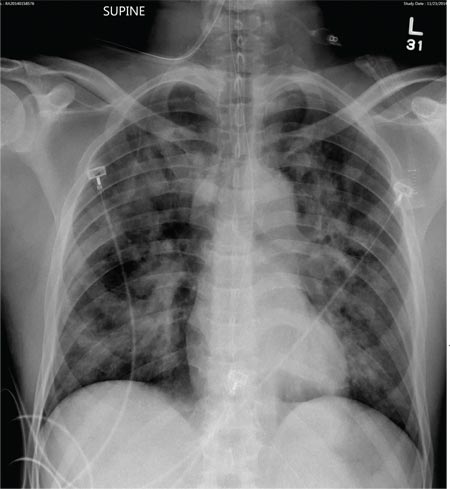
A 50-year-old man is transferred to your facility from an outlying community hospital. He is purportedly a pedestrian who was struck by a car. EMS personnel reported him to be unresponsive at the scene. He was intubated for airway protection and stabilized at the outside facility prior to transfer. Upon arrival at your facility, he is still intubated and unresponsive, and his Glasgow Coma Scale score is 3T. His heart rate is 150 beats/min and his blood pressure, 105/56 mm Hg. No additional history is available. Primary survey reveals a large scalp laceration with currently controlled bleeding. His pupils are nonreactive bilaterally. The patient is tachycardic with bilateral crackles. He also has a laceration and deformity of his right lower extremity. No imaging was provided in the transfer, so you obtain a portable chest radiograph. What is your impression?
Seven Years of Pain Between the Toes
ANSWER
The correct answer is soft corn (choice “c”). They are caused by bony friction and almost always found between the fourth and fifth toes.
Soft corns are often mistaken for warts (choice “a”). But warts don’t present as painful, macerated lesions between the toes.
Morton neuroma (choice “b”) is actually a neurofibroma, not a virtual tumor. It is usually found on the plantar forefoot between the second and third toes.
Interdigital fungal infections (choice “d”) often develop between the fourth and fifth toes and are often macerated. However, they do not take the form of lesions and do not hurt.
DISCUSSION
Soft corns are known in podiatric circles as heloma molle but are sometimes called kissing corns because they’re caused by friction between bony prominences on the fourth and fifth phalanges, which rub together with every step. Normally, these toes are hourglass shaped, but in patients prone to develop soft corns, the proximal bases of the toes are too wide. The type of shoe the patient wears can be an important factor as well, especially when high heels and/or narrow toe boxes are involved.
The treatment of soft corns can be nonsurgical—sometimes as simple as separating the toes with a tuft of lambswool. However, surgical intervention is often required. In such cases, the head of the proximal phalanx is cut and removed to make the adjacent bones more parallel. Occasionally, the skin is so damaged that it too must be removed and the toes sewn together.
Removing corns with chemicals, shaving, or excision provides no lasting relief, since these methods do not address the underlying structural issues.
Hard corns, also known as heloma durum, tend to develop on the dorsal aspect of the fifth toe secondary to pressure from shoes. Changing the type of shoe worn is one solution, but often, as with soft corns, the underlying bony prominence must be addressed.
There is a third type of corn, the periungual corn, which develops on or near the edge of a nail. These corns are often erroneously called warts.
This patient was referred to a podiatrist, who will likely solve the problem. There is no topical product that can help, and nonsurgical approaches will provide temporary relief at best.
ANSWER
The correct answer is soft corn (choice “c”). They are caused by bony friction and almost always found between the fourth and fifth toes.
Soft corns are often mistaken for warts (choice “a”). But warts don’t present as painful, macerated lesions between the toes.
Morton neuroma (choice “b”) is actually a neurofibroma, not a virtual tumor. It is usually found on the plantar forefoot between the second and third toes.
Interdigital fungal infections (choice “d”) often develop between the fourth and fifth toes and are often macerated. However, they do not take the form of lesions and do not hurt.
DISCUSSION
Soft corns are known in podiatric circles as heloma molle but are sometimes called kissing corns because they’re caused by friction between bony prominences on the fourth and fifth phalanges, which rub together with every step. Normally, these toes are hourglass shaped, but in patients prone to develop soft corns, the proximal bases of the toes are too wide. The type of shoe the patient wears can be an important factor as well, especially when high heels and/or narrow toe boxes are involved.
The treatment of soft corns can be nonsurgical—sometimes as simple as separating the toes with a tuft of lambswool. However, surgical intervention is often required. In such cases, the head of the proximal phalanx is cut and removed to make the adjacent bones more parallel. Occasionally, the skin is so damaged that it too must be removed and the toes sewn together.
Removing corns with chemicals, shaving, or excision provides no lasting relief, since these methods do not address the underlying structural issues.
Hard corns, also known as heloma durum, tend to develop on the dorsal aspect of the fifth toe secondary to pressure from shoes. Changing the type of shoe worn is one solution, but often, as with soft corns, the underlying bony prominence must be addressed.
There is a third type of corn, the periungual corn, which develops on or near the edge of a nail. These corns are often erroneously called warts.
This patient was referred to a podiatrist, who will likely solve the problem. There is no topical product that can help, and nonsurgical approaches will provide temporary relief at best.
ANSWER
The correct answer is soft corn (choice “c”). They are caused by bony friction and almost always found between the fourth and fifth toes.
Soft corns are often mistaken for warts (choice “a”). But warts don’t present as painful, macerated lesions between the toes.
Morton neuroma (choice “b”) is actually a neurofibroma, not a virtual tumor. It is usually found on the plantar forefoot between the second and third toes.
Interdigital fungal infections (choice “d”) often develop between the fourth and fifth toes and are often macerated. However, they do not take the form of lesions and do not hurt.
DISCUSSION
Soft corns are known in podiatric circles as heloma molle but are sometimes called kissing corns because they’re caused by friction between bony prominences on the fourth and fifth phalanges, which rub together with every step. Normally, these toes are hourglass shaped, but in patients prone to develop soft corns, the proximal bases of the toes are too wide. The type of shoe the patient wears can be an important factor as well, especially when high heels and/or narrow toe boxes are involved.
The treatment of soft corns can be nonsurgical—sometimes as simple as separating the toes with a tuft of lambswool. However, surgical intervention is often required. In such cases, the head of the proximal phalanx is cut and removed to make the adjacent bones more parallel. Occasionally, the skin is so damaged that it too must be removed and the toes sewn together.
Removing corns with chemicals, shaving, or excision provides no lasting relief, since these methods do not address the underlying structural issues.
Hard corns, also known as heloma durum, tend to develop on the dorsal aspect of the fifth toe secondary to pressure from shoes. Changing the type of shoe worn is one solution, but often, as with soft corns, the underlying bony prominence must be addressed.
There is a third type of corn, the periungual corn, which develops on or near the edge of a nail. These corns are often erroneously called warts.
This patient was referred to a podiatrist, who will likely solve the problem. There is no topical product that can help, and nonsurgical approaches will provide temporary relief at best.
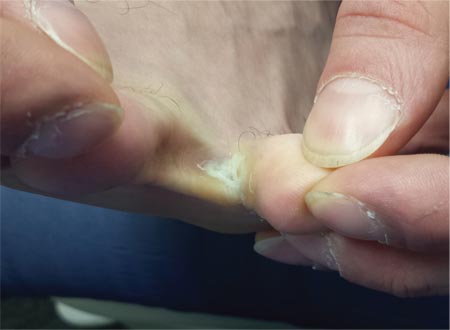
For at least seven years, this 40-year-old man has had pain in the area between the fourth and fifth toes on his left foot. During that time, he has consulted clinicians in a number of settings—including urgent care centers and the emergency department—and received “at least 30” prescriptions for oral antibiotics. Given his persistent pain, none of these treatment attempts has helped. He spends a great deal of time on his feet at work, which worsens the pain. The only relief he experiences is when he goes home at night and removes his socks and shoes. Walking barefoot, he reports, results in relatively little discomfort. The patient claims to be in good health otherwise, specifically denying diabetes. He takes no medications regularly. The skin in the lowest point of the webspace between his fourth and fifth toes is focally thickened, white, and macerated, but there is no redness. The area is exquisitely tender to touch. Examination of the rest of his foot is unremarkable.
Erythematous Nodular Plaque Encircling the Lower Leg
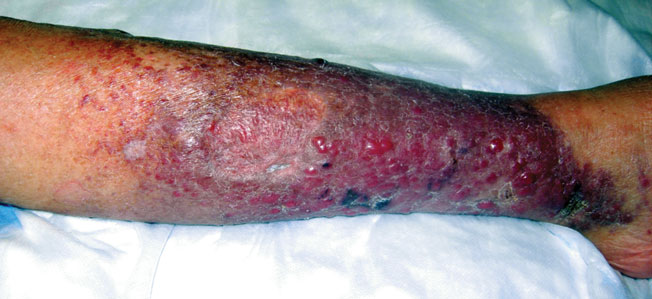
The Diagnosis: Merkel Cell Carcinoma
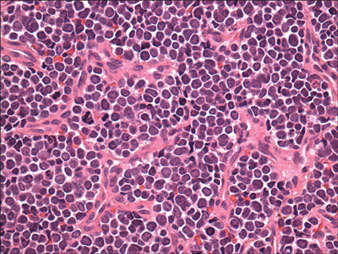
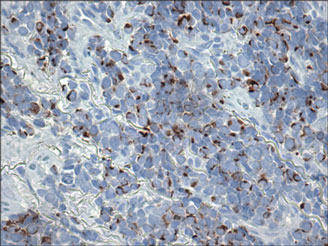
Histopathology showed small round cells (Figure 1) that stained positive for cytokeratin 20 in a paranuclear dotlike pattern (Figure 2). The tumor stained negative for lymphoma (CD45) marker. Fluorodeoxyglucose positron emission tomography showed focally increased activity at cutaneous sites corresponding to the nodules, but lymph nodes and visceral sites did not show areas of increased metabolic activity. She underwent an above-knee amputation. She was started on a chemotherapy regimen of etoposide and carboplatin given that the pathology of the excised limb demonstrated vascular and lymphatic invasion by the tumor cells in the proximal skin margin. After 4 months she presented with gangrenous changes of the amputated limb and evidence of metastasis to the region of the skin flap. Similar tumors presented on the ipsilateral hip. Given her general poor condition and aggressive nature of the tumor, the patient decided to pursue hospice care 6 months after her diagnosis of Merkel cell carcinoma (MCC).
Figure 1. The tumor was composed of sheets of cells with a high nuclear-cytoplasmic ratio and fine chromatin without nucleoli (H&E, original magnification ×40). |
Figure 2. Immunohistochemical staining for cytokera-tin 20 showed positivity in a paranuclear dotlike pattern (original magnification ×40). |
Merkel cell carcinoma usually presents as firm, red to purple papules on sun-exposed skin in older patients with light skin. Factors strongly associated with the development of MCC are age (>65 years), lighter skin types, history of extensive sun exposure, and chronic immune suppression (eg, kidney or heart transplantation, human immunodeficiency virus).1 The rate of MCC has increased 3-fold between 1986 and 2001; the rate of MCC was 0.15 cases per 100,000 individuals in 1986, climbing up to 0.44 cases per 100,000 individuals in 2001.2 Our patient had been on immunosuppressants—prednisone, cyclosporine, and sirolimus—for nearly a decade following kidney transplants, which had been discontinued 2 years prior to presentation.
Heath et al3 defined an acronym AEIOU (asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than 50 years, UV-exposed site on a person with fair skin) for MCC features derived from 195 patients. They advised that a biopsy is warranted if the patient presents with more than 3 of these features.3
The 1991 MCC staging system was revised in 1999 and 2005 based on experience at Memorial Sloan Kettering Cancer Center (New York, New York).4 In 2010 the American Joint Committee on Cancer staging was introduced for MCC, which follows other skin malignancies.5 Using this TNM staging system for primary tumors, regional lymph nodes, and distant metastasis, our patient at the time of presentation was stage IIB (T3N0M0), with tumor size greater than 5 cm, nodes negative by clinical examination, and no distant metastasis. In a span of 3 months, she had metastasis to the skin, subcutaneous tissue, and distant lymph nodes, which resulted in classification as stage IV, proving the aggressive nature of the tumor.
The newly discovered Merkel cell polyomavirus (MCPyV) is found integrating into the Merkel cell genome. Merkel cell polyomavirus is present in 80% of cancers and is expressed in a clonal pattern, while 90% of MCC patients are seropositive for the same. Unlike antibodies to MCPyV VP1 protein, antibodies to the T antigen for MCPyV track disease burden and may be a useful biomarker for MCC in the future.6
A study of 251 patients in 1970-2002 showed that pathologic nodal staging identifies a group of patients with excellent long-term survival.1 Our patient preferred to undergo positron emission tomography rather than a sentinel lymph node biopsy prior to surgery. Also, after margin-negative excision and pathologic nodal staging, local and nodal recurrence rates were low. Adjuvant chemotherapy for stage III patients showed a trend (P=.08) to decreased survival compared with stage II patients who did not receive chemotherapy.7 A multidisciplinary approach to treatment including surgery, radiation,8 and chemotherapy needs to be created for each individual patient. Merkel cell carcinoma is the cause of death in 35% of patients within 3 years of diagnosis.9
Merkel cell carcinoma is a rare orphan tumor with rapidly increasing incidence in an era of immunosuppression. It has a grave prognosis, as demonstrated in our case, if not detected early. People at increased risk for MCC must have regular skin checks. Unfortunately, our patient was a nursing home resident and had not had a skin check for 2 years prior to presentation.
1. Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309.
2. Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
3. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
4. Edge S, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. New York, NY: Springer; 2010.
5. Assouline A, Tai P, Joseph K, et al. Merkel cell carcinoma of skin-current controversies and recommendations.Rare Tumors. 2011;4:e23.
6. Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to Merkel cell polyomavirus T-antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 2010;70:8388-8397.
7. Poulsen M, Rischin D, Walpole E, et al; Trans-Tasman Radiation Oncology Group. High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study—TROG 96:07. J Clin Oncol. 2003;21:4371-4376.
8. Lewis KG, Weinstock MA, Weaver AL, et al. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142:693-700.
9. Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
The Diagnosis: Merkel Cell Carcinoma
Histopathology showed small round cells (Figure 1) that stained positive for cytokeratin 20 in a paranuclear dotlike pattern (Figure 2). The tumor stained negative for lymphoma (CD45) marker. Fluorodeoxyglucose positron emission tomography showed focally increased activity at cutaneous sites corresponding to the nodules, but lymph nodes and visceral sites did not show areas of increased metabolic activity. She underwent an above-knee amputation. She was started on a chemotherapy regimen of etoposide and carboplatin given that the pathology of the excised limb demonstrated vascular and lymphatic invasion by the tumor cells in the proximal skin margin. After 4 months she presented with gangrenous changes of the amputated limb and evidence of metastasis to the region of the skin flap. Similar tumors presented on the ipsilateral hip. Given her general poor condition and aggressive nature of the tumor, the patient decided to pursue hospice care 6 months after her diagnosis of Merkel cell carcinoma (MCC).
Figure 1. The tumor was composed of sheets of cells with a high nuclear-cytoplasmic ratio and fine chromatin without nucleoli (H&E, original magnification ×40). |
Figure 2. Immunohistochemical staining for cytokera-tin 20 showed positivity in a paranuclear dotlike pattern (original magnification ×40). |
Merkel cell carcinoma usually presents as firm, red to purple papules on sun-exposed skin in older patients with light skin. Factors strongly associated with the development of MCC are age (>65 years), lighter skin types, history of extensive sun exposure, and chronic immune suppression (eg, kidney or heart transplantation, human immunodeficiency virus).1 The rate of MCC has increased 3-fold between 1986 and 2001; the rate of MCC was 0.15 cases per 100,000 individuals in 1986, climbing up to 0.44 cases per 100,000 individuals in 2001.2 Our patient had been on immunosuppressants—prednisone, cyclosporine, and sirolimus—for nearly a decade following kidney transplants, which had been discontinued 2 years prior to presentation.
Heath et al3 defined an acronym AEIOU (asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than 50 years, UV-exposed site on a person with fair skin) for MCC features derived from 195 patients. They advised that a biopsy is warranted if the patient presents with more than 3 of these features.3
The 1991 MCC staging system was revised in 1999 and 2005 based on experience at Memorial Sloan Kettering Cancer Center (New York, New York).4 In 2010 the American Joint Committee on Cancer staging was introduced for MCC, which follows other skin malignancies.5 Using this TNM staging system for primary tumors, regional lymph nodes, and distant metastasis, our patient at the time of presentation was stage IIB (T3N0M0), with tumor size greater than 5 cm, nodes negative by clinical examination, and no distant metastasis. In a span of 3 months, she had metastasis to the skin, subcutaneous tissue, and distant lymph nodes, which resulted in classification as stage IV, proving the aggressive nature of the tumor.
The newly discovered Merkel cell polyomavirus (MCPyV) is found integrating into the Merkel cell genome. Merkel cell polyomavirus is present in 80% of cancers and is expressed in a clonal pattern, while 90% of MCC patients are seropositive for the same. Unlike antibodies to MCPyV VP1 protein, antibodies to the T antigen for MCPyV track disease burden and may be a useful biomarker for MCC in the future.6
A study of 251 patients in 1970-2002 showed that pathologic nodal staging identifies a group of patients with excellent long-term survival.1 Our patient preferred to undergo positron emission tomography rather than a sentinel lymph node biopsy prior to surgery. Also, after margin-negative excision and pathologic nodal staging, local and nodal recurrence rates were low. Adjuvant chemotherapy for stage III patients showed a trend (P=.08) to decreased survival compared with stage II patients who did not receive chemotherapy.7 A multidisciplinary approach to treatment including surgery, radiation,8 and chemotherapy needs to be created for each individual patient. Merkel cell carcinoma is the cause of death in 35% of patients within 3 years of diagnosis.9
Merkel cell carcinoma is a rare orphan tumor with rapidly increasing incidence in an era of immunosuppression. It has a grave prognosis, as demonstrated in our case, if not detected early. People at increased risk for MCC must have regular skin checks. Unfortunately, our patient was a nursing home resident and had not had a skin check for 2 years prior to presentation.
The Diagnosis: Merkel Cell Carcinoma
Histopathology showed small round cells (Figure 1) that stained positive for cytokeratin 20 in a paranuclear dotlike pattern (Figure 2). The tumor stained negative for lymphoma (CD45) marker. Fluorodeoxyglucose positron emission tomography showed focally increased activity at cutaneous sites corresponding to the nodules, but lymph nodes and visceral sites did not show areas of increased metabolic activity. She underwent an above-knee amputation. She was started on a chemotherapy regimen of etoposide and carboplatin given that the pathology of the excised limb demonstrated vascular and lymphatic invasion by the tumor cells in the proximal skin margin. After 4 months she presented with gangrenous changes of the amputated limb and evidence of metastasis to the region of the skin flap. Similar tumors presented on the ipsilateral hip. Given her general poor condition and aggressive nature of the tumor, the patient decided to pursue hospice care 6 months after her diagnosis of Merkel cell carcinoma (MCC).
Figure 1. The tumor was composed of sheets of cells with a high nuclear-cytoplasmic ratio and fine chromatin without nucleoli (H&E, original magnification ×40). |
Figure 2. Immunohistochemical staining for cytokera-tin 20 showed positivity in a paranuclear dotlike pattern (original magnification ×40). |
Merkel cell carcinoma usually presents as firm, red to purple papules on sun-exposed skin in older patients with light skin. Factors strongly associated with the development of MCC are age (>65 years), lighter skin types, history of extensive sun exposure, and chronic immune suppression (eg, kidney or heart transplantation, human immunodeficiency virus).1 The rate of MCC has increased 3-fold between 1986 and 2001; the rate of MCC was 0.15 cases per 100,000 individuals in 1986, climbing up to 0.44 cases per 100,000 individuals in 2001.2 Our patient had been on immunosuppressants—prednisone, cyclosporine, and sirolimus—for nearly a decade following kidney transplants, which had been discontinued 2 years prior to presentation.
Heath et al3 defined an acronym AEIOU (asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than 50 years, UV-exposed site on a person with fair skin) for MCC features derived from 195 patients. They advised that a biopsy is warranted if the patient presents with more than 3 of these features.3
The 1991 MCC staging system was revised in 1999 and 2005 based on experience at Memorial Sloan Kettering Cancer Center (New York, New York).4 In 2010 the American Joint Committee on Cancer staging was introduced for MCC, which follows other skin malignancies.5 Using this TNM staging system for primary tumors, regional lymph nodes, and distant metastasis, our patient at the time of presentation was stage IIB (T3N0M0), with tumor size greater than 5 cm, nodes negative by clinical examination, and no distant metastasis. In a span of 3 months, she had metastasis to the skin, subcutaneous tissue, and distant lymph nodes, which resulted in classification as stage IV, proving the aggressive nature of the tumor.
The newly discovered Merkel cell polyomavirus (MCPyV) is found integrating into the Merkel cell genome. Merkel cell polyomavirus is present in 80% of cancers and is expressed in a clonal pattern, while 90% of MCC patients are seropositive for the same. Unlike antibodies to MCPyV VP1 protein, antibodies to the T antigen for MCPyV track disease burden and may be a useful biomarker for MCC in the future.6
A study of 251 patients in 1970-2002 showed that pathologic nodal staging identifies a group of patients with excellent long-term survival.1 Our patient preferred to undergo positron emission tomography rather than a sentinel lymph node biopsy prior to surgery. Also, after margin-negative excision and pathologic nodal staging, local and nodal recurrence rates were low. Adjuvant chemotherapy for stage III patients showed a trend (P=.08) to decreased survival compared with stage II patients who did not receive chemotherapy.7 A multidisciplinary approach to treatment including surgery, radiation,8 and chemotherapy needs to be created for each individual patient. Merkel cell carcinoma is the cause of death in 35% of patients within 3 years of diagnosis.9
Merkel cell carcinoma is a rare orphan tumor with rapidly increasing incidence in an era of immunosuppression. It has a grave prognosis, as demonstrated in our case, if not detected early. People at increased risk for MCC must have regular skin checks. Unfortunately, our patient was a nursing home resident and had not had a skin check for 2 years prior to presentation.
1. Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309.
2. Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
3. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
4. Edge S, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. New York, NY: Springer; 2010.
5. Assouline A, Tai P, Joseph K, et al. Merkel cell carcinoma of skin-current controversies and recommendations.Rare Tumors. 2011;4:e23.
6. Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to Merkel cell polyomavirus T-antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 2010;70:8388-8397.
7. Poulsen M, Rischin D, Walpole E, et al; Trans-Tasman Radiation Oncology Group. High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study—TROG 96:07. J Clin Oncol. 2003;21:4371-4376.
8. Lewis KG, Weinstock MA, Weaver AL, et al. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142:693-700.
9. Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
1. Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309.
2. Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
3. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
4. Edge S, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. New York, NY: Springer; 2010.
5. Assouline A, Tai P, Joseph K, et al. Merkel cell carcinoma of skin-current controversies and recommendations.Rare Tumors. 2011;4:e23.
6. Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to Merkel cell polyomavirus T-antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 2010;70:8388-8397.
7. Poulsen M, Rischin D, Walpole E, et al; Trans-Tasman Radiation Oncology Group. High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study—TROG 96:07. J Clin Oncol. 2003;21:4371-4376.
8. Lewis KG, Weinstock MA, Weaver AL, et al. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142:693-700.
9. Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
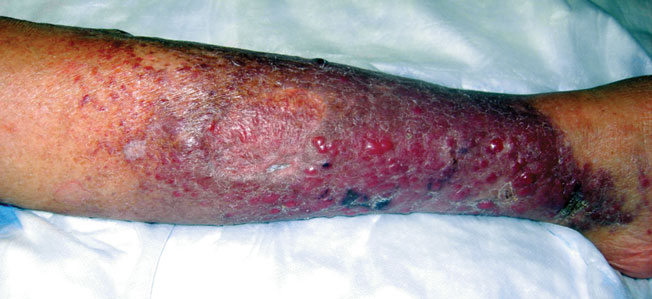
A 66-year-old woman presented with red to violaceous, rapidly growing nodules on the skin. Her medical history was remarkable for diabetes mellitus, hypertension, dyslipidemia, and renal failure. She had 2 rejected kidney transplants and was on hemodialysis at the time of presentation. She noticed asymptomatic nodules present on the left lower leg that progressively coalesced, finally encroaching the whole girth of the limb, spreading from the foot to the knee in a short duration of 3 months. The regional lymph nodes were not clinically palpable.
Erythematous Plaques on the Hand and Wrist
The Diagnosis: Lichen Aureus
Lichen aureus (LA) is classified as a pigmented purpuric dermatosis (PPD), a collection of conditions that are characterized by petechiae, pigmentation, and occasionally telangiectasia without a causative underlying disorder. As in our case, the lesions of LA are usually asymptomatic. They appear as circumscribed areas of discrete or confluent macules and papules that can range in color from gold or copper to purple (Figure 1). The lesions typically occur unilaterally on the lower extremities but can occur on all body regions. The etiology is unknown, but explanations such as venous insufficiency,1 contact allergens,2 and drugs3,4 have been proposed. Unlike other PPDs, LA tends to occur abruptly and then either stabilizes or progresses slowly over years. Studies have reported resolution in 2 to 7 years.5 The average age of onset is in the 20s and 30s, with pediatric cases accounting for only 17%.6 Pediatric cases are more likely to be self-limited and occur in uncommon sites such as the trunk and arms.7
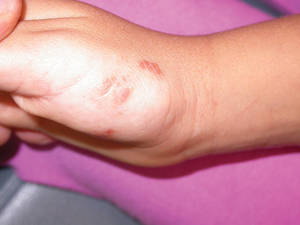
Lichen aureus is characterized histopathologically by a dense, bandlike, dermal inflammatory infiltrate (Figure 2). Additionally, there is variable exocytosis of lymphocytes and marked accumulation of siderotic macrophages (Figure 3). These qualities in the proper clinical setting help differentiate LA from other PPDs that share findings of capillaritis, hemosiderin deposition, and erythrocyte extravasation near dermal vessels. An iron stain assists in the diagnosis of LA (Figure 3), as it differentiates the disease from other lichenoid conditions such as lichen planus. Zaballos et al8 also demonstrated a role for dermoscopy to clinically differentiate LA from other similar-appearing lesions such as lichen planus.
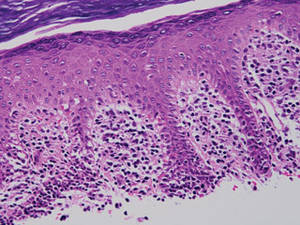
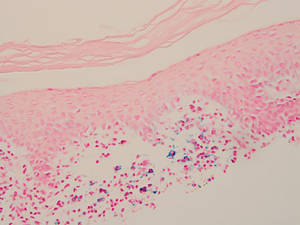
The lesions of LA are benign. Because the predominantly T-cell infiltrate is monoclonal in approximately 50% of cases,2,9-11 authors have suggested the possibility of progression to cutaneous T-cell lymphoma.9,12 Guitart and Magro13 classified LA as a T-cell lymphoid dyscrasia with potential for progression. Despite these reports, the general consensus is that LA is a benign, self-limiting condition. The benign nature of LA is supported by Fink-Puches et al2 who followed 23 patients for a mean 102.1 months and did not observe a single case of progression to malignancy.
There have been many treatment regimens attempted for patients with LA. Topical corticosteroids have not been found to be beneficial14; however, there have been isolated cases reporting its efficacy in children.7,15 Other medications that have been effective in small trials include psoralen plus UVA,16 topical pimecrolimus,5 calcium dobesilate,17 and combination therapy with pentoxifylline and prostacyclin.18 Despite some reported benefit, the use of potent immunomodulating medications is not indicated due to the benign nature of the disease. Alternative supplements including oral bioflavonoids and ascorbic acid have also been explored with modest benefit.19
1. Reinhardt L, Wilkin JK, Tausend R. Vascular abnormalities in lichen aureus. J Am Acad Dermatol. 1983;8:417-420.
2. Fink-Puches R, Wolf P, Kerl H, et al. Lichen aureus: clinicopathologic features, natural history, and relationship to mycosis fungoides. Arch Dermatol. 2008;144:1169-1173.
3. Nishioka K, Katayama I, Masuzawa M, et al. Drug-induced chronic pigmented purpura. J Dermatol. 1989;16:220-222.
4. Yazdi AS, Mayser P, Sander CA. Lichen aureus with clonal T cells in a child possibly induced by regular consumption of an energy drink. J Cutan Pathol. 2008;35:960-962.
5. Bohm M, Bonsmann G, Luger TA. Resolution of lichen aureus in a 10-year-old child after topical pimecrolimus. Br J Dermatol. 2004;151:519-520.
6. Gelmetti C, Cerri D, Grimalt R. Lichen aureus in childhood. Pediatr Dermatol. 1991;8:280-283.
7. Kim MJ, Kim BY, Park KC, et al. A case of childhood lichen aureus. Ann Dermatol. 2009;21:393-395.
8. Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
9. Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
10. Magro CM, Schaefer JT, Crowson AN, et al. Pigmented purpuric dermatosis: classification by phenotypic and molecular profiles. Am J Clin Pathol. 2007;128:218-229.
11. Crowson AN, Magro CM, Zahorchak R. Atypical pigmentary purpura: a clinical, histopathologic, and genotypic study. Hum Pathol. 1999;30:1004-1012.
12. Barnhill RL, Braverman IM. Progression of pigmented purpura-like eruptions to mycosis fungoides: report of three cases. J Am Acad Dermatol. 1988;19(1, pt 1):25-31.
13. Guitart J, Magro C. Cutaneous T-cell lymphoid dyscrasia: a unifying term for idiopathic chronic dermatoses with persistent T-cell clones. Arch Dermatol. 2007;143:921-932.
14. Graham RM, English JS, Emmerson RW. Lichen aureus—a study of twelve cases. Clin Exp Dermatol. 1984;9:393-401.
15. Fujita H, Iguchi M, Ikari Y, et al. Lichen aureus on the back in a 6-year-old girl. J Dermatol. 2007;34:148-149.
16. Ling TC, Goulden V, Goodfield MJ. PUVA therapy in lichen aureus. J Am Acad Dermatol. 2001;45:145-146.
17. Agrawal SK, Gandhi V, Bhattacharya SN. Calcium dobesilate (Cd) in pigmented purpuric dermatosis (PPD): a pilot evaluation. J Dermatol. 2004;31:98-103.
18. Lee HW, Lee DK, Chang SE, et al. Segmental lichen aureus: combination therapy with pentoxifylline and prostacyclin. J Eur Acad Dermatol Venereol. 2006;20:1378-1380.
19. Reinhold U, Seiter S, Ugurel S, et al. Treatment of progressive pigmented purpura with oral bioflavonoids and ascorbic acid: an open pilot study in 3 patients. J Am Acad Dermatol. 1999;4(2, pt 1):207-208.
The Diagnosis: Lichen Aureus
Lichen aureus (LA) is classified as a pigmented purpuric dermatosis (PPD), a collection of conditions that are characterized by petechiae, pigmentation, and occasionally telangiectasia without a causative underlying disorder. As in our case, the lesions of LA are usually asymptomatic. They appear as circumscribed areas of discrete or confluent macules and papules that can range in color from gold or copper to purple (Figure 1). The lesions typically occur unilaterally on the lower extremities but can occur on all body regions. The etiology is unknown, but explanations such as venous insufficiency,1 contact allergens,2 and drugs3,4 have been proposed. Unlike other PPDs, LA tends to occur abruptly and then either stabilizes or progresses slowly over years. Studies have reported resolution in 2 to 7 years.5 The average age of onset is in the 20s and 30s, with pediatric cases accounting for only 17%.6 Pediatric cases are more likely to be self-limited and occur in uncommon sites such as the trunk and arms.7

Lichen aureus is characterized histopathologically by a dense, bandlike, dermal inflammatory infiltrate (Figure 2). Additionally, there is variable exocytosis of lymphocytes and marked accumulation of siderotic macrophages (Figure 3). These qualities in the proper clinical setting help differentiate LA from other PPDs that share findings of capillaritis, hemosiderin deposition, and erythrocyte extravasation near dermal vessels. An iron stain assists in the diagnosis of LA (Figure 3), as it differentiates the disease from other lichenoid conditions such as lichen planus. Zaballos et al8 also demonstrated a role for dermoscopy to clinically differentiate LA from other similar-appearing lesions such as lichen planus.


The lesions of LA are benign. Because the predominantly T-cell infiltrate is monoclonal in approximately 50% of cases,2,9-11 authors have suggested the possibility of progression to cutaneous T-cell lymphoma.9,12 Guitart and Magro13 classified LA as a T-cell lymphoid dyscrasia with potential for progression. Despite these reports, the general consensus is that LA is a benign, self-limiting condition. The benign nature of LA is supported by Fink-Puches et al2 who followed 23 patients for a mean 102.1 months and did not observe a single case of progression to malignancy.
There have been many treatment regimens attempted for patients with LA. Topical corticosteroids have not been found to be beneficial14; however, there have been isolated cases reporting its efficacy in children.7,15 Other medications that have been effective in small trials include psoralen plus UVA,16 topical pimecrolimus,5 calcium dobesilate,17 and combination therapy with pentoxifylline and prostacyclin.18 Despite some reported benefit, the use of potent immunomodulating medications is not indicated due to the benign nature of the disease. Alternative supplements including oral bioflavonoids and ascorbic acid have also been explored with modest benefit.19
The Diagnosis: Lichen Aureus
Lichen aureus (LA) is classified as a pigmented purpuric dermatosis (PPD), a collection of conditions that are characterized by petechiae, pigmentation, and occasionally telangiectasia without a causative underlying disorder. As in our case, the lesions of LA are usually asymptomatic. They appear as circumscribed areas of discrete or confluent macules and papules that can range in color from gold or copper to purple (Figure 1). The lesions typically occur unilaterally on the lower extremities but can occur on all body regions. The etiology is unknown, but explanations such as venous insufficiency,1 contact allergens,2 and drugs3,4 have been proposed. Unlike other PPDs, LA tends to occur abruptly and then either stabilizes or progresses slowly over years. Studies have reported resolution in 2 to 7 years.5 The average age of onset is in the 20s and 30s, with pediatric cases accounting for only 17%.6 Pediatric cases are more likely to be self-limited and occur in uncommon sites such as the trunk and arms.7

Lichen aureus is characterized histopathologically by a dense, bandlike, dermal inflammatory infiltrate (Figure 2). Additionally, there is variable exocytosis of lymphocytes and marked accumulation of siderotic macrophages (Figure 3). These qualities in the proper clinical setting help differentiate LA from other PPDs that share findings of capillaritis, hemosiderin deposition, and erythrocyte extravasation near dermal vessels. An iron stain assists in the diagnosis of LA (Figure 3), as it differentiates the disease from other lichenoid conditions such as lichen planus. Zaballos et al8 also demonstrated a role for dermoscopy to clinically differentiate LA from other similar-appearing lesions such as lichen planus.


The lesions of LA are benign. Because the predominantly T-cell infiltrate is monoclonal in approximately 50% of cases,2,9-11 authors have suggested the possibility of progression to cutaneous T-cell lymphoma.9,12 Guitart and Magro13 classified LA as a T-cell lymphoid dyscrasia with potential for progression. Despite these reports, the general consensus is that LA is a benign, self-limiting condition. The benign nature of LA is supported by Fink-Puches et al2 who followed 23 patients for a mean 102.1 months and did not observe a single case of progression to malignancy.
There have been many treatment regimens attempted for patients with LA. Topical corticosteroids have not been found to be beneficial14; however, there have been isolated cases reporting its efficacy in children.7,15 Other medications that have been effective in small trials include psoralen plus UVA,16 topical pimecrolimus,5 calcium dobesilate,17 and combination therapy with pentoxifylline and prostacyclin.18 Despite some reported benefit, the use of potent immunomodulating medications is not indicated due to the benign nature of the disease. Alternative supplements including oral bioflavonoids and ascorbic acid have also been explored with modest benefit.19
1. Reinhardt L, Wilkin JK, Tausend R. Vascular abnormalities in lichen aureus. J Am Acad Dermatol. 1983;8:417-420.
2. Fink-Puches R, Wolf P, Kerl H, et al. Lichen aureus: clinicopathologic features, natural history, and relationship to mycosis fungoides. Arch Dermatol. 2008;144:1169-1173.
3. Nishioka K, Katayama I, Masuzawa M, et al. Drug-induced chronic pigmented purpura. J Dermatol. 1989;16:220-222.
4. Yazdi AS, Mayser P, Sander CA. Lichen aureus with clonal T cells in a child possibly induced by regular consumption of an energy drink. J Cutan Pathol. 2008;35:960-962.
5. Bohm M, Bonsmann G, Luger TA. Resolution of lichen aureus in a 10-year-old child after topical pimecrolimus. Br J Dermatol. 2004;151:519-520.
6. Gelmetti C, Cerri D, Grimalt R. Lichen aureus in childhood. Pediatr Dermatol. 1991;8:280-283.
7. Kim MJ, Kim BY, Park KC, et al. A case of childhood lichen aureus. Ann Dermatol. 2009;21:393-395.
8. Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
9. Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
10. Magro CM, Schaefer JT, Crowson AN, et al. Pigmented purpuric dermatosis: classification by phenotypic and molecular profiles. Am J Clin Pathol. 2007;128:218-229.
11. Crowson AN, Magro CM, Zahorchak R. Atypical pigmentary purpura: a clinical, histopathologic, and genotypic study. Hum Pathol. 1999;30:1004-1012.
12. Barnhill RL, Braverman IM. Progression of pigmented purpura-like eruptions to mycosis fungoides: report of three cases. J Am Acad Dermatol. 1988;19(1, pt 1):25-31.
13. Guitart J, Magro C. Cutaneous T-cell lymphoid dyscrasia: a unifying term for idiopathic chronic dermatoses with persistent T-cell clones. Arch Dermatol. 2007;143:921-932.
14. Graham RM, English JS, Emmerson RW. Lichen aureus—a study of twelve cases. Clin Exp Dermatol. 1984;9:393-401.
15. Fujita H, Iguchi M, Ikari Y, et al. Lichen aureus on the back in a 6-year-old girl. J Dermatol. 2007;34:148-149.
16. Ling TC, Goulden V, Goodfield MJ. PUVA therapy in lichen aureus. J Am Acad Dermatol. 2001;45:145-146.
17. Agrawal SK, Gandhi V, Bhattacharya SN. Calcium dobesilate (Cd) in pigmented purpuric dermatosis (PPD): a pilot evaluation. J Dermatol. 2004;31:98-103.
18. Lee HW, Lee DK, Chang SE, et al. Segmental lichen aureus: combination therapy with pentoxifylline and prostacyclin. J Eur Acad Dermatol Venereol. 2006;20:1378-1380.
19. Reinhold U, Seiter S, Ugurel S, et al. Treatment of progressive pigmented purpura with oral bioflavonoids and ascorbic acid: an open pilot study in 3 patients. J Am Acad Dermatol. 1999;4(2, pt 1):207-208.
1. Reinhardt L, Wilkin JK, Tausend R. Vascular abnormalities in lichen aureus. J Am Acad Dermatol. 1983;8:417-420.
2. Fink-Puches R, Wolf P, Kerl H, et al. Lichen aureus: clinicopathologic features, natural history, and relationship to mycosis fungoides. Arch Dermatol. 2008;144:1169-1173.
3. Nishioka K, Katayama I, Masuzawa M, et al. Drug-induced chronic pigmented purpura. J Dermatol. 1989;16:220-222.
4. Yazdi AS, Mayser P, Sander CA. Lichen aureus with clonal T cells in a child possibly induced by regular consumption of an energy drink. J Cutan Pathol. 2008;35:960-962.
5. Bohm M, Bonsmann G, Luger TA. Resolution of lichen aureus in a 10-year-old child after topical pimecrolimus. Br J Dermatol. 2004;151:519-520.
6. Gelmetti C, Cerri D, Grimalt R. Lichen aureus in childhood. Pediatr Dermatol. 1991;8:280-283.
7. Kim MJ, Kim BY, Park KC, et al. A case of childhood lichen aureus. Ann Dermatol. 2009;21:393-395.
8. Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290-1291.
9. Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? a study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118.
10. Magro CM, Schaefer JT, Crowson AN, et al. Pigmented purpuric dermatosis: classification by phenotypic and molecular profiles. Am J Clin Pathol. 2007;128:218-229.
11. Crowson AN, Magro CM, Zahorchak R. Atypical pigmentary purpura: a clinical, histopathologic, and genotypic study. Hum Pathol. 1999;30:1004-1012.
12. Barnhill RL, Braverman IM. Progression of pigmented purpura-like eruptions to mycosis fungoides: report of three cases. J Am Acad Dermatol. 1988;19(1, pt 1):25-31.
13. Guitart J, Magro C. Cutaneous T-cell lymphoid dyscrasia: a unifying term for idiopathic chronic dermatoses with persistent T-cell clones. Arch Dermatol. 2007;143:921-932.
14. Graham RM, English JS, Emmerson RW. Lichen aureus—a study of twelve cases. Clin Exp Dermatol. 1984;9:393-401.
15. Fujita H, Iguchi M, Ikari Y, et al. Lichen aureus on the back in a 6-year-old girl. J Dermatol. 2007;34:148-149.
16. Ling TC, Goulden V, Goodfield MJ. PUVA therapy in lichen aureus. J Am Acad Dermatol. 2001;45:145-146.
17. Agrawal SK, Gandhi V, Bhattacharya SN. Calcium dobesilate (Cd) in pigmented purpuric dermatosis (PPD): a pilot evaluation. J Dermatol. 2004;31:98-103.
18. Lee HW, Lee DK, Chang SE, et al. Segmental lichen aureus: combination therapy with pentoxifylline and prostacyclin. J Eur Acad Dermatol Venereol. 2006;20:1378-1380.
19. Reinhold U, Seiter S, Ugurel S, et al. Treatment of progressive pigmented purpura with oral bioflavonoids and ascorbic acid: an open pilot study in 3 patients. J Am Acad Dermatol. 1999;4(2, pt 1):207-208.