User login
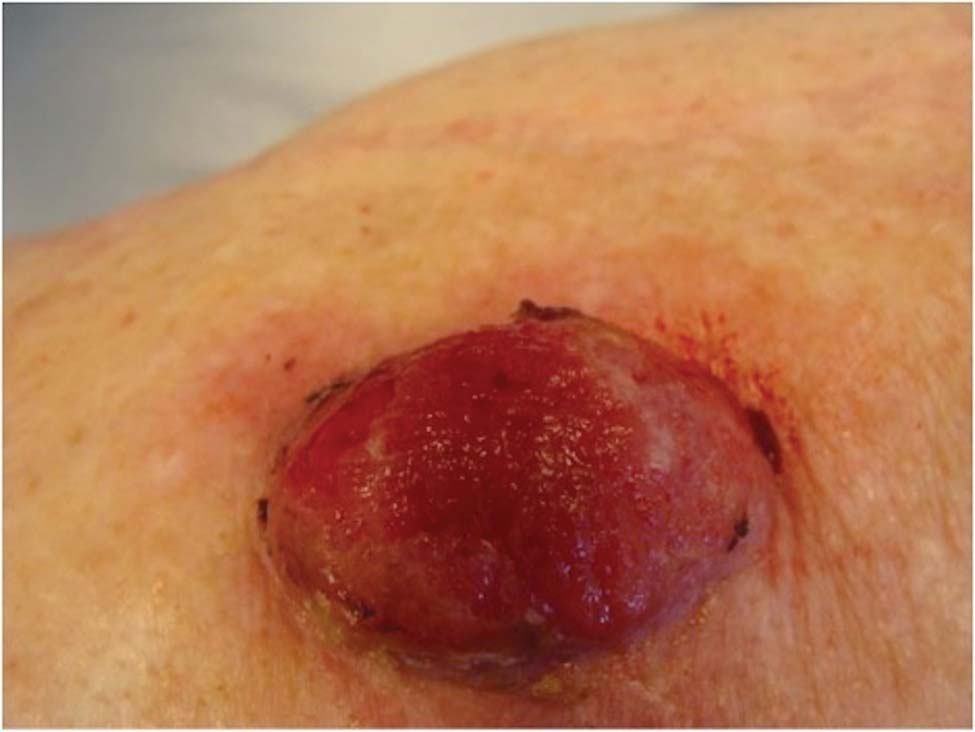
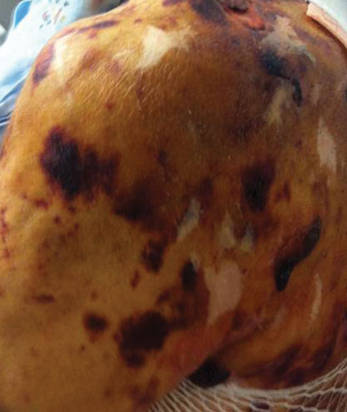
Friable Nodule on the Back
The Diagnosis: Spindle Cell Malignant Melanoma With Perineural Invasion
The incidence of melanoma has steadily increased in the United States since the 1930s when the incidence was reported at 1.0 per 100,000.1 In 1973 melanoma incidence was 6.8 per 100,000, and by 2007 the rate increased to 20.1 per 100,000.2 The American Cancer Society projects 73,870 new cases of melanoma in 2015, with melanoma as the fifth most common cancer in males and the seventh most common in females.3 Melanoma-related deaths are projected to be 9940. The lifetime probability of developing melanoma is 1 in 34 for males and 1 in 53 for females. Twice as many males are estimated to have melanoma-related deaths compared to females. The 5-year relative survival rate is 93% for white individuals and 75% for black individuals.3
Spindle cell melanoma is a rare variant of melanoma that was originally described by Conley et al4 in 1971. The lesion represents 2% to 4% of all melanomas and presents in older patients on sun-exposed skin as a pink or variably pigmented nodule measuring an average of 2 cm.5 Males are affected more than females, and prominent neural invasion is present in 30% to 100% of cases.6-10 Neural invasion can result in nerve palsies and/or dysesthesia. Because half of these lesions are amelanotic, they are often clinically misdiagnosed prior to biopsy.11
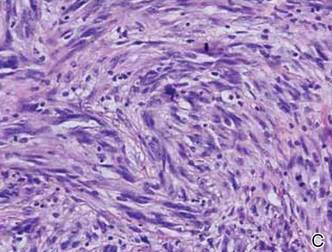
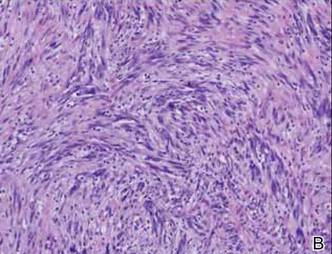
Histopathologically, these lesions can be quite challenging. Spindle cell melanoma histologically is an intradermal lesion composed of spindle cells distributed in bundles, fascicles, or nests, or singly between collagen fibers of the dermis. Other spindle tumors such as spindle cell squamous cell carcinoma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, angiosarcoma, and leiomyosarcoma have a similar presentation with hematoxylin and eosin stain. The diagnostic process for spindle cell tumors is greatly aided by immunohistologic analysis. Spindle cell melanoma usually shows immunoreactivity with S-100. Human melanoma black 45, CD57, and neuron-specific enolase usually do not stain, and CD68 has been demonstrated in a minority of cases.
The initial biopsy specimen in our case displayed a dense dermal atypical spindle cell proliferation with hematoxylin and eosin stain. The differential diagnosis of the proliferation included desmoplastic melanoma, spindle cell squamous cell carcinoma, leiomyosarcoma, angiosarcoma, and atypical fibroxanthoma. Immunostains were used to further study the lesional biopsy. Cytokeratin 34bE12, CK5/6, cerium ammonium molybdate 5.2, CK7, epithelial membrane antigen, CK18, high-molecular-weight cytokeratin, S-100, Melan-A, human melanoma black 45, smooth muscle actin, desmin, CD68, CD34, CD10, and p63 were studied. The atypical dermal spindle cells were positive for S-100. S-100 and Melan-A highlighted an increased number of single melanocytes at the dermoepidermal junction. Other markers were negative.
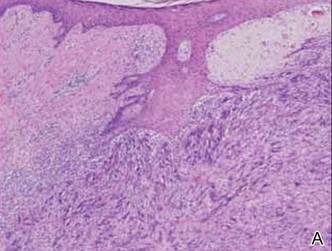
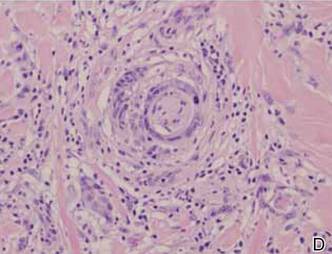
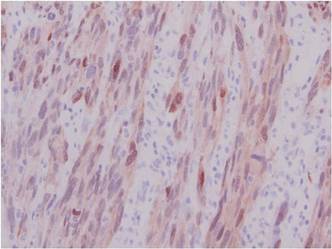
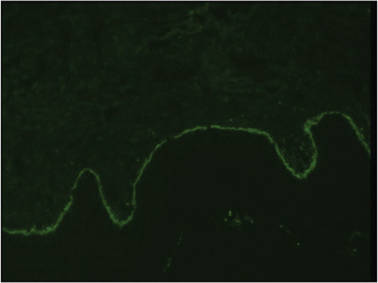
A 1.0-cm wide excision to fascia was performed. Routine hematoxylin and eosin stain showed atypical dermal spindle cells with perineural invasion (Figure 1). The malignant spindle cells were S-100 positive (Figure 2). The spindle cells were negative for high-molecular-weight cytokeratin, CK5/6, p63, Melan-A, A103, microphthalmia, and tyrosinase. These findings confirm melanoma of the spindle cell type.
|
The lesion was a Clark level V melanoma with a Breslow thickness of at least 12 mm. Perineural invasion was noted and the mitotic index was 7 cells/mm2. Vascular and lymphatic invasion was negative and ulceration was present. Tumor-infiltrating lymphocytes were brisk, resulting in a pathologic staging of pT4NXMX.
On initial histologic study with hematoxylin and eosin stain, spindle cell melanoma lesions tend to be generally quite thick. Manganoni et al12 reported in their series a Breslow thickness ranging from 2.1 to 12 mm with a mean of 5.8 mm.
Treatment in our case involved a second wide incision to fascia with an additional 2-cm margin. The role of sentinel lymph node biopsy (SLNB) remains undefined. Patients with spindle cell melanoma have a lower frequency of positive sentinel lymph nodes than nondesmoplastic melanomas.13 As such, the need to perform SLNB has not been determined.13-15 Our patient had a history of breast cancer. The lesion appeared on the left side of the back and she pre-viously had a complete axillary lymphadenectomy on the left axillae. She declined SLNB. A systematic workup by the oncology service was negative. Continued follow-up has revealed no recurrent disease and recent workup was negative for metastatic disease.
Many studies report the increased incidence of local recurrence after excision for spindle cell melanoma as compared to non–spindle cell melanoma,16-18 which is likely related to perineural invasion as in our patient.
Spindle cell melanoma is a rare tumor that is often amelanotic and difficult to diagnose clinically. Routine hematoxylin and eosin staining shows a dermal spindle cell tumor. Immunohistochemical study is of great aid in defining the tumor. The clinician and pathologist must work together to correctly diagnose and treat this lesion.
1. Mikkilineni R, Weinstock MA. Epidemiology. In: Sober AJ, Haluska FG, eds. Atlas of Clinical Oncology: Skin Cancer. London, England: BC Decker; 2001:1-15.
2. Rigel D. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29:204-209.
3. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed February 10, 2015.
4. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of malignant melanoma. Cancer. 1971;28:914-936.
5. Repertinger SK, Teruya B, Sarma DP. Common spindle cell malignant neoplasms of the skin: differential diagnosis and review of the literature. Internet J Dermatol. 2009;7. https://ispub.com/IJD/7/2/11747. Accessed February 10, 2015.
6. Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradial. 2004;25:5-11.
7. Cruz J, Reis-Filho JS, Lopes JM. Malignant peripheral nerve sheath tumor-like primary cutaneous melanoma. J Clin Pathol. 2004;57:218-220.
8. Tsao H, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. Semin Cutan Med Surg. 1997;16:45-47.
9. Kossard S, Doherty E, Murray E. Neurotropic melanoma. a variant of desmoplastic melanoma. Arch Dermatol. 1997;7:907-912.
10. Bruijn JA, Salasche S, Sober AJ, et al. Desmoplastic melanoma: clinicopathologic aspects of six cases. Dermatology. 1992;185:3-8.
11. Jesitus J. Desmoplastic melanoma. Dermatology Times. March 2009:1-2.
12. Manganoni AM, Farisoglio C, Bassissi S, et al. Desmoplastic melanoma: report of 5 cases. Dermatol Res Pract. 2009;2009:679010.
13. Gyorki DE, Busam K, Panageas K, et al. Sentinel lymph node biopsy for patients with desmoplastic melanoma. Ann Surg Oncol. 2003;10:403-407.
14. Livestro DP, Muzikansky A, Kaine EM, et al. of desmoplastic melanoma: a case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739-6746.
15. Pawlik TM, Ross MI, Prieto VG, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106:900-906.
16. Smithers HM, McLeod GR, Little JH. Desmoplastic melanoma: patterns of recurrence. World J Surg. 1992;16:186-190.
17. McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 2004;36:445-451.
18. Bruijn JA, Mihm MC Jr, Barnhill RL. Desmoplastic melanoma. Histopathology. 1992;20:197-205.
The Diagnosis: Spindle Cell Malignant Melanoma With Perineural Invasion
The incidence of melanoma has steadily increased in the United States since the 1930s when the incidence was reported at 1.0 per 100,000.1 In 1973 melanoma incidence was 6.8 per 100,000, and by 2007 the rate increased to 20.1 per 100,000.2 The American Cancer Society projects 73,870 new cases of melanoma in 2015, with melanoma as the fifth most common cancer in males and the seventh most common in females.3 Melanoma-related deaths are projected to be 9940. The lifetime probability of developing melanoma is 1 in 34 for males and 1 in 53 for females. Twice as many males are estimated to have melanoma-related deaths compared to females. The 5-year relative survival rate is 93% for white individuals and 75% for black individuals.3
Spindle cell melanoma is a rare variant of melanoma that was originally described by Conley et al4 in 1971. The lesion represents 2% to 4% of all melanomas and presents in older patients on sun-exposed skin as a pink or variably pigmented nodule measuring an average of 2 cm.5 Males are affected more than females, and prominent neural invasion is present in 30% to 100% of cases.6-10 Neural invasion can result in nerve palsies and/or dysesthesia. Because half of these lesions are amelanotic, they are often clinically misdiagnosed prior to biopsy.11
Histopathologically, these lesions can be quite challenging. Spindle cell melanoma histologically is an intradermal lesion composed of spindle cells distributed in bundles, fascicles, or nests, or singly between collagen fibers of the dermis. Other spindle tumors such as spindle cell squamous cell carcinoma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, angiosarcoma, and leiomyosarcoma have a similar presentation with hematoxylin and eosin stain. The diagnostic process for spindle cell tumors is greatly aided by immunohistologic analysis. Spindle cell melanoma usually shows immunoreactivity with S-100. Human melanoma black 45, CD57, and neuron-specific enolase usually do not stain, and CD68 has been demonstrated in a minority of cases.
The initial biopsy specimen in our case displayed a dense dermal atypical spindle cell proliferation with hematoxylin and eosin stain. The differential diagnosis of the proliferation included desmoplastic melanoma, spindle cell squamous cell carcinoma, leiomyosarcoma, angiosarcoma, and atypical fibroxanthoma. Immunostains were used to further study the lesional biopsy. Cytokeratin 34bE12, CK5/6, cerium ammonium molybdate 5.2, CK7, epithelial membrane antigen, CK18, high-molecular-weight cytokeratin, S-100, Melan-A, human melanoma black 45, smooth muscle actin, desmin, CD68, CD34, CD10, and p63 were studied. The atypical dermal spindle cells were positive for S-100. S-100 and Melan-A highlighted an increased number of single melanocytes at the dermoepidermal junction. Other markers were negative.
A 1.0-cm wide excision to fascia was performed. Routine hematoxylin and eosin stain showed atypical dermal spindle cells with perineural invasion (Figure 1). The malignant spindle cells were S-100 positive (Figure 2). The spindle cells were negative for high-molecular-weight cytokeratin, CK5/6, p63, Melan-A, A103, microphthalmia, and tyrosinase. These findings confirm melanoma of the spindle cell type.
|
The lesion was a Clark level V melanoma with a Breslow thickness of at least 12 mm. Perineural invasion was noted and the mitotic index was 7 cells/mm2. Vascular and lymphatic invasion was negative and ulceration was present. Tumor-infiltrating lymphocytes were brisk, resulting in a pathologic staging of pT4NXMX.
On initial histologic study with hematoxylin and eosin stain, spindle cell melanoma lesions tend to be generally quite thick. Manganoni et al12 reported in their series a Breslow thickness ranging from 2.1 to 12 mm with a mean of 5.8 mm.
Treatment in our case involved a second wide incision to fascia with an additional 2-cm margin. The role of sentinel lymph node biopsy (SLNB) remains undefined. Patients with spindle cell melanoma have a lower frequency of positive sentinel lymph nodes than nondesmoplastic melanomas.13 As such, the need to perform SLNB has not been determined.13-15 Our patient had a history of breast cancer. The lesion appeared on the left side of the back and she pre-viously had a complete axillary lymphadenectomy on the left axillae. She declined SLNB. A systematic workup by the oncology service was negative. Continued follow-up has revealed no recurrent disease and recent workup was negative for metastatic disease.
Many studies report the increased incidence of local recurrence after excision for spindle cell melanoma as compared to non–spindle cell melanoma,16-18 which is likely related to perineural invasion as in our patient.
Spindle cell melanoma is a rare tumor that is often amelanotic and difficult to diagnose clinically. Routine hematoxylin and eosin staining shows a dermal spindle cell tumor. Immunohistochemical study is of great aid in defining the tumor. The clinician and pathologist must work together to correctly diagnose and treat this lesion.
The Diagnosis: Spindle Cell Malignant Melanoma With Perineural Invasion
The incidence of melanoma has steadily increased in the United States since the 1930s when the incidence was reported at 1.0 per 100,000.1 In 1973 melanoma incidence was 6.8 per 100,000, and by 2007 the rate increased to 20.1 per 100,000.2 The American Cancer Society projects 73,870 new cases of melanoma in 2015, with melanoma as the fifth most common cancer in males and the seventh most common in females.3 Melanoma-related deaths are projected to be 9940. The lifetime probability of developing melanoma is 1 in 34 for males and 1 in 53 for females. Twice as many males are estimated to have melanoma-related deaths compared to females. The 5-year relative survival rate is 93% for white individuals and 75% for black individuals.3
Spindle cell melanoma is a rare variant of melanoma that was originally described by Conley et al4 in 1971. The lesion represents 2% to 4% of all melanomas and presents in older patients on sun-exposed skin as a pink or variably pigmented nodule measuring an average of 2 cm.5 Males are affected more than females, and prominent neural invasion is present in 30% to 100% of cases.6-10 Neural invasion can result in nerve palsies and/or dysesthesia. Because half of these lesions are amelanotic, they are often clinically misdiagnosed prior to biopsy.11
Histopathologically, these lesions can be quite challenging. Spindle cell melanoma histologically is an intradermal lesion composed of spindle cells distributed in bundles, fascicles, or nests, or singly between collagen fibers of the dermis. Other spindle tumors such as spindle cell squamous cell carcinoma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, angiosarcoma, and leiomyosarcoma have a similar presentation with hematoxylin and eosin stain. The diagnostic process for spindle cell tumors is greatly aided by immunohistologic analysis. Spindle cell melanoma usually shows immunoreactivity with S-100. Human melanoma black 45, CD57, and neuron-specific enolase usually do not stain, and CD68 has been demonstrated in a minority of cases.
The initial biopsy specimen in our case displayed a dense dermal atypical spindle cell proliferation with hematoxylin and eosin stain. The differential diagnosis of the proliferation included desmoplastic melanoma, spindle cell squamous cell carcinoma, leiomyosarcoma, angiosarcoma, and atypical fibroxanthoma. Immunostains were used to further study the lesional biopsy. Cytokeratin 34bE12, CK5/6, cerium ammonium molybdate 5.2, CK7, epithelial membrane antigen, CK18, high-molecular-weight cytokeratin, S-100, Melan-A, human melanoma black 45, smooth muscle actin, desmin, CD68, CD34, CD10, and p63 were studied. The atypical dermal spindle cells were positive for S-100. S-100 and Melan-A highlighted an increased number of single melanocytes at the dermoepidermal junction. Other markers were negative.
A 1.0-cm wide excision to fascia was performed. Routine hematoxylin and eosin stain showed atypical dermal spindle cells with perineural invasion (Figure 1). The malignant spindle cells were S-100 positive (Figure 2). The spindle cells were negative for high-molecular-weight cytokeratin, CK5/6, p63, Melan-A, A103, microphthalmia, and tyrosinase. These findings confirm melanoma of the spindle cell type.
|
The lesion was a Clark level V melanoma with a Breslow thickness of at least 12 mm. Perineural invasion was noted and the mitotic index was 7 cells/mm2. Vascular and lymphatic invasion was negative and ulceration was present. Tumor-infiltrating lymphocytes were brisk, resulting in a pathologic staging of pT4NXMX.
On initial histologic study with hematoxylin and eosin stain, spindle cell melanoma lesions tend to be generally quite thick. Manganoni et al12 reported in their series a Breslow thickness ranging from 2.1 to 12 mm with a mean of 5.8 mm.
Treatment in our case involved a second wide incision to fascia with an additional 2-cm margin. The role of sentinel lymph node biopsy (SLNB) remains undefined. Patients with spindle cell melanoma have a lower frequency of positive sentinel lymph nodes than nondesmoplastic melanomas.13 As such, the need to perform SLNB has not been determined.13-15 Our patient had a history of breast cancer. The lesion appeared on the left side of the back and she pre-viously had a complete axillary lymphadenectomy on the left axillae. She declined SLNB. A systematic workup by the oncology service was negative. Continued follow-up has revealed no recurrent disease and recent workup was negative for metastatic disease.
Many studies report the increased incidence of local recurrence after excision for spindle cell melanoma as compared to non–spindle cell melanoma,16-18 which is likely related to perineural invasion as in our patient.
Spindle cell melanoma is a rare tumor that is often amelanotic and difficult to diagnose clinically. Routine hematoxylin and eosin staining shows a dermal spindle cell tumor. Immunohistochemical study is of great aid in defining the tumor. The clinician and pathologist must work together to correctly diagnose and treat this lesion.
1. Mikkilineni R, Weinstock MA. Epidemiology. In: Sober AJ, Haluska FG, eds. Atlas of Clinical Oncology: Skin Cancer. London, England: BC Decker; 2001:1-15.
2. Rigel D. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29:204-209.
3. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed February 10, 2015.
4. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of malignant melanoma. Cancer. 1971;28:914-936.
5. Repertinger SK, Teruya B, Sarma DP. Common spindle cell malignant neoplasms of the skin: differential diagnosis and review of the literature. Internet J Dermatol. 2009;7. https://ispub.com/IJD/7/2/11747. Accessed February 10, 2015.
6. Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradial. 2004;25:5-11.
7. Cruz J, Reis-Filho JS, Lopes JM. Malignant peripheral nerve sheath tumor-like primary cutaneous melanoma. J Clin Pathol. 2004;57:218-220.
8. Tsao H, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. Semin Cutan Med Surg. 1997;16:45-47.
9. Kossard S, Doherty E, Murray E. Neurotropic melanoma. a variant of desmoplastic melanoma. Arch Dermatol. 1997;7:907-912.
10. Bruijn JA, Salasche S, Sober AJ, et al. Desmoplastic melanoma: clinicopathologic aspects of six cases. Dermatology. 1992;185:3-8.
11. Jesitus J. Desmoplastic melanoma. Dermatology Times. March 2009:1-2.
12. Manganoni AM, Farisoglio C, Bassissi S, et al. Desmoplastic melanoma: report of 5 cases. Dermatol Res Pract. 2009;2009:679010.
13. Gyorki DE, Busam K, Panageas K, et al. Sentinel lymph node biopsy for patients with desmoplastic melanoma. Ann Surg Oncol. 2003;10:403-407.
14. Livestro DP, Muzikansky A, Kaine EM, et al. of desmoplastic melanoma: a case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739-6746.
15. Pawlik TM, Ross MI, Prieto VG, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106:900-906.
16. Smithers HM, McLeod GR, Little JH. Desmoplastic melanoma: patterns of recurrence. World J Surg. 1992;16:186-190.
17. McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 2004;36:445-451.
18. Bruijn JA, Mihm MC Jr, Barnhill RL. Desmoplastic melanoma. Histopathology. 1992;20:197-205.
1. Mikkilineni R, Weinstock MA. Epidemiology. In: Sober AJ, Haluska FG, eds. Atlas of Clinical Oncology: Skin Cancer. London, England: BC Decker; 2001:1-15.
2. Rigel D. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29:204-209.
3. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed February 10, 2015.
4. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of malignant melanoma. Cancer. 1971;28:914-936.
5. Repertinger SK, Teruya B, Sarma DP. Common spindle cell malignant neoplasms of the skin: differential diagnosis and review of the literature. Internet J Dermatol. 2009;7. https://ispub.com/IJD/7/2/11747. Accessed February 10, 2015.
6. Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradial. 2004;25:5-11.
7. Cruz J, Reis-Filho JS, Lopes JM. Malignant peripheral nerve sheath tumor-like primary cutaneous melanoma. J Clin Pathol. 2004;57:218-220.
8. Tsao H, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. Semin Cutan Med Surg. 1997;16:45-47.
9. Kossard S, Doherty E, Murray E. Neurotropic melanoma. a variant of desmoplastic melanoma. Arch Dermatol. 1997;7:907-912.
10. Bruijn JA, Salasche S, Sober AJ, et al. Desmoplastic melanoma: clinicopathologic aspects of six cases. Dermatology. 1992;185:3-8.
11. Jesitus J. Desmoplastic melanoma. Dermatology Times. March 2009:1-2.
12. Manganoni AM, Farisoglio C, Bassissi S, et al. Desmoplastic melanoma: report of 5 cases. Dermatol Res Pract. 2009;2009:679010.
13. Gyorki DE, Busam K, Panageas K, et al. Sentinel lymph node biopsy for patients with desmoplastic melanoma. Ann Surg Oncol. 2003;10:403-407.
14. Livestro DP, Muzikansky A, Kaine EM, et al. of desmoplastic melanoma: a case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739-6746.
15. Pawlik TM, Ross MI, Prieto VG, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106:900-906.
16. Smithers HM, McLeod GR, Little JH. Desmoplastic melanoma: patterns of recurrence. World J Surg. 1992;16:186-190.
17. McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 2004;36:445-451.
18. Bruijn JA, Mihm MC Jr, Barnhill RL. Desmoplastic melanoma. Histopathology. 1992;20:197-205.
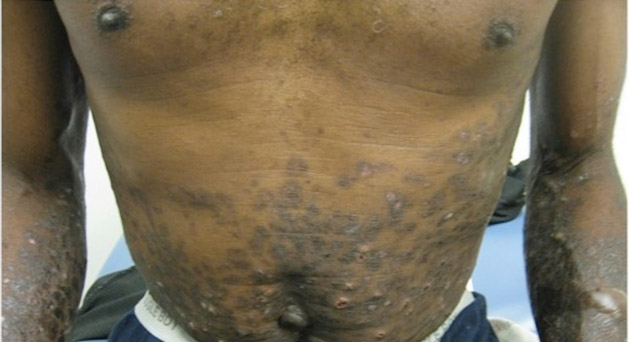
A 78-year-old woman presented with a large friable, sharply demarcated nodule of 3 months’ duration on the left side of the back. The lesion occasionally bled but was otherwise asymptomatic. There was no perilesional paresthesia. The patient’s medical history included hypertension, depression, chronic obstructive pulmonary disease, breast cancer, osteoporosis, and aortic valve disease.
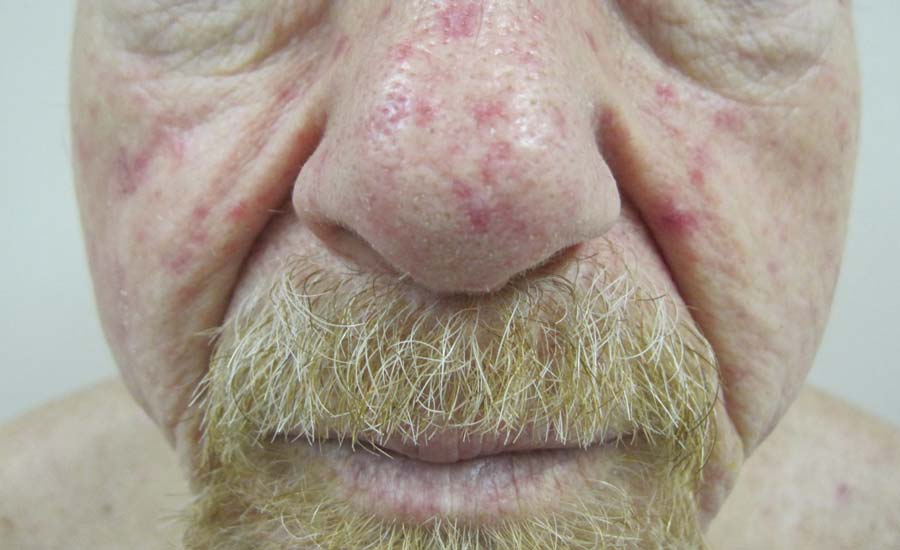
Telangiectases on the Cheeks and Nose
The Diagnosis: Hereditary Hemorrhagic Telangiectasia
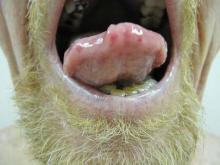
Physical examination of our patient revealed multiple fine punctate telangiectases on the bilateral cheeks and the dorsum of the nose. Further examination revealed matlike telangiectases on the distal aspect of the tongue (Figure), buccal mucosa, palms, and fingers. Since diagnosis he has experienced several episodes of severe gastrointestinal tract bleeding. He underwent an esophagogastroduodenoscopy and was found to have bleeding gastric antral vascular ectasia that was treated with argon plasma coagulation. One year later he had a second esophagogastroduodenoscopy performed because of heme-positive stools and was found to have additional bleeding vascular ectasia that was treated again with argon plasma coagulation.
Hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome, is an autosomal-dominant disorder that is characterized by mucocutaneous and visceral telangiectases, recurrent hemorrhages, and a familial occurrence.1 In North America, the overall incidence is approximately 1 per 5000 to 10,000 individuals per year.2 Although this disorder generally has an autosomal-dominant transmission, approximately 20% of cases do not have a familial component.2
Clinically these patients most commonly present with a recurrent episode of epistaxis that occurs within the first 2 decades of life. Shortly after the onset of these recurrent episodes, patients begin to develop punctate or splinterlike telangiectases on the lips, oral mucosa, upper extremities, nail beds, and trunk. These cutaneous telangiectases are a cosmetic problem and can sometimes cause hemorrhage, especially from the tongue and fingers.1 The serious complications of HHT arise from internal organ involvement. Gastrointestinal telangiectases and arteriovenous malformations (AVMs) can result in acute gastrointestinal hemorrhages or iron deficiency anemia in approximately 16% of patients. Central nervous system AVMs result in migraines, brain abscesses, paraparesis, ischemia, strokes, transient ischemic attacks, seizures, and both intracerebral and subarachnoid hemorrhage. Pulmonary AVMs can cause a right-to-left shunt, leading to hypoxemia and embolic events due to the bypass of the lungs, which act as a filtering capillary bed.3
Genetic linkage analysis has revealed 2 genes that are responsible for HHT. The first gene, ENG, encodes endoglin and is found on band 9q33-34.3 The second gene, ALK1, encodes activin receptorlike kinase 1 and is found on band 12q11-12. Both of these gene products are involved with vascular remodeling. They are integral membrane glycoproteins mainly expressed on vascular endothelial cells and act as surface receptors for transforming growth factor b. Mutations in ALK1 are associated with a generally more benign course, whereas mutations in ENG more commonly have pulmonary AVMs.3
The diagnosis of HHT is established if 3 of the following features are present: (1) epistaxis (ie, spontaneous recurrent nosebleeds); (2) telangiectases in characteristic sites (ie, oral cavity, lips, nose, fingers); (3) visceral lesions, such as gastrointestinal telangiectases (with or without bleeding), pulmonary AVM, hepatic AVM, cerebral AVM, or spinal AVM; (4) family history (ie, a first-degree relative with HHT).3 Similar diseases with telangiectases should be considered in the differential diagnosis including CREST syndrome (characterized by calcinosis, Raynaud phenomenon, esophageal motility disorders, sclerodactyly, and telangiectasia), generalized essential telangiectasia, and ataxia telangiectasia.2
Patients diagnosed with HHT who have a family history of the disease should be evaluated for pulmonary AVM through chest computed tomography and pulmonary angiography because they are at the highest risk. Magnetic resonance imaging is useful to rule out central nervous system involvement. Treatment of cutaneous telangiectases consists of electrocauterization; sclerotherapy; and a variety of lasers and light sources including the Nd:YAG laser, intense pulsed light, argon laser, or pulsed dye laser.1,2,4 An extremely useful resource for patients with HHT and family members is the HHT Foundation (http://curehht.org).
1. Fernández-Jorge B, Del Pozo Losada J, Paradela S, et al. Treatment of cutaneous and mucosal telangiectases in hereditary hemorrhagic telangiectasia: report of three cases. J Cosmet Laser Ther. 2007;9:29-33.
2. Lee HE, Sagong C, Yeo KY, et al. A case of hereditary hemorrhagic telangiectasia. Ann Dermatol. 2009;21:206-208.
3. Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations. part II: associated syndromes. J Am Acad Dermatol. 2007;56:541-564.
4. Sato Y, Takayama T, Takahari D, et al. Successful treatment for gastro-intestinal bleeding of Osler-Weber-Rendu disease by argon plasma coagulation using double-balloon enteroscopy. Endoscopy. 2008;40(suppl 2):E228-E229.
The Diagnosis: Hereditary Hemorrhagic Telangiectasia
Physical examination of our patient revealed multiple fine punctate telangiectases on the bilateral cheeks and the dorsum of the nose. Further examination revealed matlike telangiectases on the distal aspect of the tongue (Figure), buccal mucosa, palms, and fingers. Since diagnosis he has experienced several episodes of severe gastrointestinal tract bleeding. He underwent an esophagogastroduodenoscopy and was found to have bleeding gastric antral vascular ectasia that was treated with argon plasma coagulation. One year later he had a second esophagogastroduodenoscopy performed because of heme-positive stools and was found to have additional bleeding vascular ectasia that was treated again with argon plasma coagulation.
Hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome, is an autosomal-dominant disorder that is characterized by mucocutaneous and visceral telangiectases, recurrent hemorrhages, and a familial occurrence.1 In North America, the overall incidence is approximately 1 per 5000 to 10,000 individuals per year.2 Although this disorder generally has an autosomal-dominant transmission, approximately 20% of cases do not have a familial component.2
Clinically these patients most commonly present with a recurrent episode of epistaxis that occurs within the first 2 decades of life. Shortly after the onset of these recurrent episodes, patients begin to develop punctate or splinterlike telangiectases on the lips, oral mucosa, upper extremities, nail beds, and trunk. These cutaneous telangiectases are a cosmetic problem and can sometimes cause hemorrhage, especially from the tongue and fingers.1 The serious complications of HHT arise from internal organ involvement. Gastrointestinal telangiectases and arteriovenous malformations (AVMs) can result in acute gastrointestinal hemorrhages or iron deficiency anemia in approximately 16% of patients. Central nervous system AVMs result in migraines, brain abscesses, paraparesis, ischemia, strokes, transient ischemic attacks, seizures, and both intracerebral and subarachnoid hemorrhage. Pulmonary AVMs can cause a right-to-left shunt, leading to hypoxemia and embolic events due to the bypass of the lungs, which act as a filtering capillary bed.3
Genetic linkage analysis has revealed 2 genes that are responsible for HHT. The first gene, ENG, encodes endoglin and is found on band 9q33-34.3 The second gene, ALK1, encodes activin receptorlike kinase 1 and is found on band 12q11-12. Both of these gene products are involved with vascular remodeling. They are integral membrane glycoproteins mainly expressed on vascular endothelial cells and act as surface receptors for transforming growth factor b. Mutations in ALK1 are associated with a generally more benign course, whereas mutations in ENG more commonly have pulmonary AVMs.3
The diagnosis of HHT is established if 3 of the following features are present: (1) epistaxis (ie, spontaneous recurrent nosebleeds); (2) telangiectases in characteristic sites (ie, oral cavity, lips, nose, fingers); (3) visceral lesions, such as gastrointestinal telangiectases (with or without bleeding), pulmonary AVM, hepatic AVM, cerebral AVM, or spinal AVM; (4) family history (ie, a first-degree relative with HHT).3 Similar diseases with telangiectases should be considered in the differential diagnosis including CREST syndrome (characterized by calcinosis, Raynaud phenomenon, esophageal motility disorders, sclerodactyly, and telangiectasia), generalized essential telangiectasia, and ataxia telangiectasia.2
Patients diagnosed with HHT who have a family history of the disease should be evaluated for pulmonary AVM through chest computed tomography and pulmonary angiography because they are at the highest risk. Magnetic resonance imaging is useful to rule out central nervous system involvement. Treatment of cutaneous telangiectases consists of electrocauterization; sclerotherapy; and a variety of lasers and light sources including the Nd:YAG laser, intense pulsed light, argon laser, or pulsed dye laser.1,2,4 An extremely useful resource for patients with HHT and family members is the HHT Foundation (http://curehht.org).
The Diagnosis: Hereditary Hemorrhagic Telangiectasia
Physical examination of our patient revealed multiple fine punctate telangiectases on the bilateral cheeks and the dorsum of the nose. Further examination revealed matlike telangiectases on the distal aspect of the tongue (Figure), buccal mucosa, palms, and fingers. Since diagnosis he has experienced several episodes of severe gastrointestinal tract bleeding. He underwent an esophagogastroduodenoscopy and was found to have bleeding gastric antral vascular ectasia that was treated with argon plasma coagulation. One year later he had a second esophagogastroduodenoscopy performed because of heme-positive stools and was found to have additional bleeding vascular ectasia that was treated again with argon plasma coagulation.
Hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome, is an autosomal-dominant disorder that is characterized by mucocutaneous and visceral telangiectases, recurrent hemorrhages, and a familial occurrence.1 In North America, the overall incidence is approximately 1 per 5000 to 10,000 individuals per year.2 Although this disorder generally has an autosomal-dominant transmission, approximately 20% of cases do not have a familial component.2
Clinically these patients most commonly present with a recurrent episode of epistaxis that occurs within the first 2 decades of life. Shortly after the onset of these recurrent episodes, patients begin to develop punctate or splinterlike telangiectases on the lips, oral mucosa, upper extremities, nail beds, and trunk. These cutaneous telangiectases are a cosmetic problem and can sometimes cause hemorrhage, especially from the tongue and fingers.1 The serious complications of HHT arise from internal organ involvement. Gastrointestinal telangiectases and arteriovenous malformations (AVMs) can result in acute gastrointestinal hemorrhages or iron deficiency anemia in approximately 16% of patients. Central nervous system AVMs result in migraines, brain abscesses, paraparesis, ischemia, strokes, transient ischemic attacks, seizures, and both intracerebral and subarachnoid hemorrhage. Pulmonary AVMs can cause a right-to-left shunt, leading to hypoxemia and embolic events due to the bypass of the lungs, which act as a filtering capillary bed.3
Genetic linkage analysis has revealed 2 genes that are responsible for HHT. The first gene, ENG, encodes endoglin and is found on band 9q33-34.3 The second gene, ALK1, encodes activin receptorlike kinase 1 and is found on band 12q11-12. Both of these gene products are involved with vascular remodeling. They are integral membrane glycoproteins mainly expressed on vascular endothelial cells and act as surface receptors for transforming growth factor b. Mutations in ALK1 are associated with a generally more benign course, whereas mutations in ENG more commonly have pulmonary AVMs.3
The diagnosis of HHT is established if 3 of the following features are present: (1) epistaxis (ie, spontaneous recurrent nosebleeds); (2) telangiectases in characteristic sites (ie, oral cavity, lips, nose, fingers); (3) visceral lesions, such as gastrointestinal telangiectases (with or without bleeding), pulmonary AVM, hepatic AVM, cerebral AVM, or spinal AVM; (4) family history (ie, a first-degree relative with HHT).3 Similar diseases with telangiectases should be considered in the differential diagnosis including CREST syndrome (characterized by calcinosis, Raynaud phenomenon, esophageal motility disorders, sclerodactyly, and telangiectasia), generalized essential telangiectasia, and ataxia telangiectasia.2
Patients diagnosed with HHT who have a family history of the disease should be evaluated for pulmonary AVM through chest computed tomography and pulmonary angiography because they are at the highest risk. Magnetic resonance imaging is useful to rule out central nervous system involvement. Treatment of cutaneous telangiectases consists of electrocauterization; sclerotherapy; and a variety of lasers and light sources including the Nd:YAG laser, intense pulsed light, argon laser, or pulsed dye laser.1,2,4 An extremely useful resource for patients with HHT and family members is the HHT Foundation (http://curehht.org).
1. Fernández-Jorge B, Del Pozo Losada J, Paradela S, et al. Treatment of cutaneous and mucosal telangiectases in hereditary hemorrhagic telangiectasia: report of three cases. J Cosmet Laser Ther. 2007;9:29-33.
2. Lee HE, Sagong C, Yeo KY, et al. A case of hereditary hemorrhagic telangiectasia. Ann Dermatol. 2009;21:206-208.
3. Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations. part II: associated syndromes. J Am Acad Dermatol. 2007;56:541-564.
4. Sato Y, Takayama T, Takahari D, et al. Successful treatment for gastro-intestinal bleeding of Osler-Weber-Rendu disease by argon plasma coagulation using double-balloon enteroscopy. Endoscopy. 2008;40(suppl 2):E228-E229.
1. Fernández-Jorge B, Del Pozo Losada J, Paradela S, et al. Treatment of cutaneous and mucosal telangiectases in hereditary hemorrhagic telangiectasia: report of three cases. J Cosmet Laser Ther. 2007;9:29-33.
2. Lee HE, Sagong C, Yeo KY, et al. A case of hereditary hemorrhagic telangiectasia. Ann Dermatol. 2009;21:206-208.
3. Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations. part II: associated syndromes. J Am Acad Dermatol. 2007;56:541-564.
4. Sato Y, Takayama T, Takahari D, et al. Successful treatment for gastro-intestinal bleeding of Osler-Weber-Rendu disease by argon plasma coagulation using double-balloon enteroscopy. Endoscopy. 2008;40(suppl 2):E228-E229.
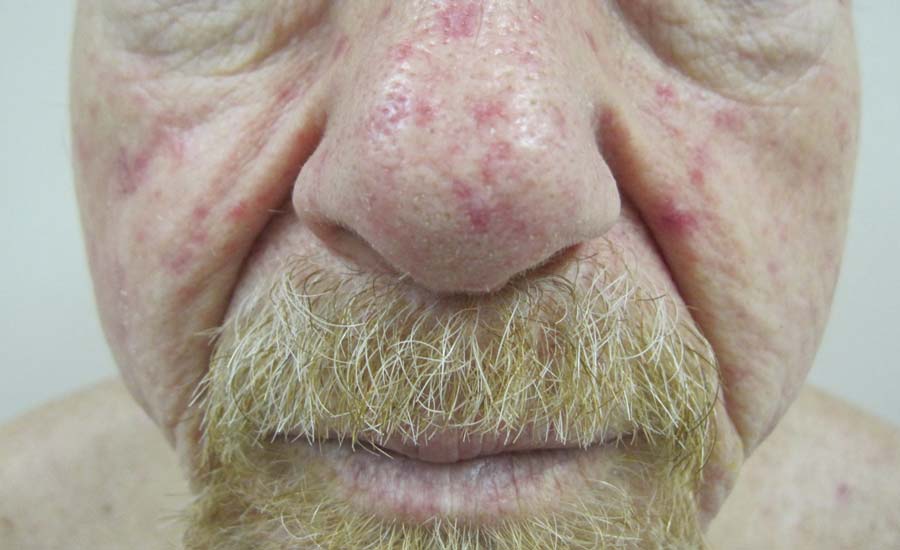
A 68-year-old man presented for evaluation of numerous telangiectases that developed on the cheeks, nose, lips, tongue, and fingers. He denied any history of gastrointestinal tract bleeding but admitted to numerous nosebleeds in the recent past. Family history indicated that his maternal grandmother died of an aneurysm, his mother died of a cerebral hemorrhage, 2 paternal cousins had hemochromatosis, and 2 brothers were healthy.
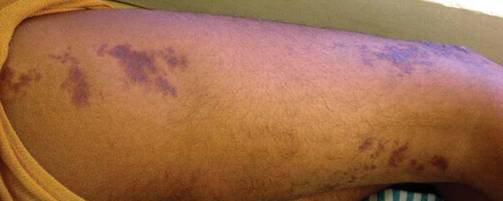
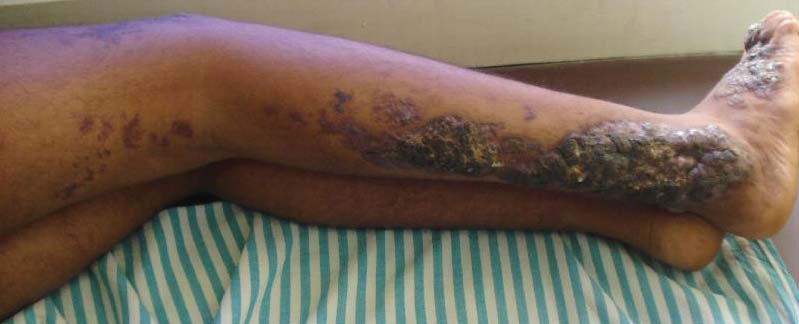
Bluish Red Verrucous Lesions on the Leg
The Diagnosis: Linear Verrucous Hemangioma
Verrucous hemangioma is a rare congenital vascular malformation of the cutaneous and subcutaneous tissues. Although almost invariably present at birth, it may appear later in childhood or even in adulthood. Lesions commonly are found on the legs and may be linear, multiple, and disseminated or sometimes confined to the digits. In the early phase of evolution, the lesions are nonkeratotic, soft, blue-red plaques, but they gradually become increasingly hyperkeratotic.1 Linear verrucous hemangioma is even more rare with few published reports.
In 1937, Halter2 first used the term verrucous hemangioma to describe a 16-year-old adolescent boy who presented with a linear purpuric cluster of plaques extending from the right buttock to the toes. Imperial and Helwig3 later described it as a distinct entity. Since then, similar lesions have been described using a variety of names such as angiokeratoma circumscriptum neviforme, angiokeratoma circumscriptum, angiokeratoma corporis neviforme, keratotic hemangioma, nevus vascularis unius lateralis, and nevus keratoangiomatosus.3 Therefore, the exact incidence is difficult to determine.
Lesions generally are noted at birth or in early childhood and are often located on the lower extremities. The early lesions are bluish red in color. Secondary infection is a frequent complication, resulting in reactive papillomatosis and hyperkeratosis; thus, the older lesions acquire a verrucous or warty surface.3 Clinically, they may resemble angiokeratoma, lym-phangioma circumscriptum, verrucous epidermal nevus, verrucous cancer, or even malignant melanoma. Lesions initially resemble port-wine stains and later may become soft, bluish red vascular swellings that tend to grow in size and become verrucous.1
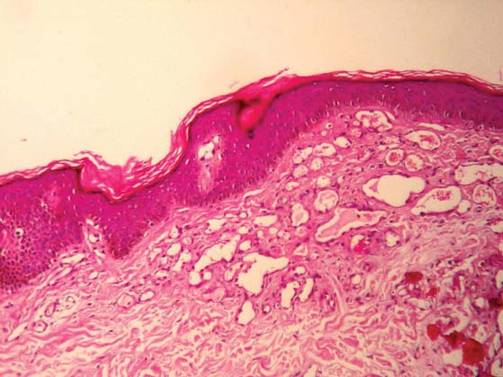
The histologic appearance closely resembles angiokeratoma, as both lesions show vascular spaces beneath a papillomatous and hyperkeratotic epidermis.4 However, in contrast to angiokeratoma, the vascular spaces in verrucous hemangioma also involve the lower dermis and subcutaneous tissues.
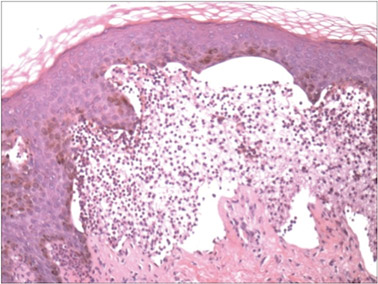
Although cases of linear verrucous hemangioma have been reported, its distribution along the lines of Blaschko is rare.5,6 It has been proposed that these lesions may actually be following dermatomal patterns or that the linear arrangement represents genetic mosaicism.5 In our case, the lesion started as a small plaque in childhood and gradually spread linearly to the buttock (Figure 1). A biopsy was taken from the lesion on the right buttock, which showed numerous dilated capillaries in the dermis (Figure 2).
|
Verrucous hemangiomas are best treated by excision. Larger lesions will need grafting. There is a tendency for recurrence to occur unless excision is complete.1 Yang and Ohara7 reported 14 patients with small localized lesions that were cured by 1 session of surgery without recurrence; 9 patients with wider and more extensive lesions required combination therapy in several stages for optimal results.
1. Atherton DJ, Moss C. Naevi and other developmental defects. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. 7th ed. London, England: Blackwell Scientific Publications; 2004:15-60.
2. Halter K. Haemangioma verrucosum mit Osteoatrophie. Dermatol Z. 1937;75:271-279.
3. Imperial R, Helwig EB. Verrucous hemangioma: a clinicopathological study of 21 cases. Arch Dermatol. 1967;96:247-253.
4. Calduch L, Ortega C, Navarro V, et al. Verrucous hemangioma: Report of two cases and review of the literature. Pediatr Dermatol. 2000;17:213-217.
5. Wentscher U, Happle R. Linear verrucous hemangioma. J Am Acad Dermatol. 2000;42:516-518.
6. Jain VK, Aggarwal K, Jain S. Linear verrucous hemangioma on the leg. Indian J Dermatol Venereol Leprol. 2008;74:656-658.
7. Yang CH, Ohara K. Successful surgical treatment of verrucous hemangioma: a combined approach. Dermatol Surg. 2002;28:913-919.
The Diagnosis: Linear Verrucous Hemangioma
Verrucous hemangioma is a rare congenital vascular malformation of the cutaneous and subcutaneous tissues. Although almost invariably present at birth, it may appear later in childhood or even in adulthood. Lesions commonly are found on the legs and may be linear, multiple, and disseminated or sometimes confined to the digits. In the early phase of evolution, the lesions are nonkeratotic, soft, blue-red plaques, but they gradually become increasingly hyperkeratotic.1 Linear verrucous hemangioma is even more rare with few published reports.
In 1937, Halter2 first used the term verrucous hemangioma to describe a 16-year-old adolescent boy who presented with a linear purpuric cluster of plaques extending from the right buttock to the toes. Imperial and Helwig3 later described it as a distinct entity. Since then, similar lesions have been described using a variety of names such as angiokeratoma circumscriptum neviforme, angiokeratoma circumscriptum, angiokeratoma corporis neviforme, keratotic hemangioma, nevus vascularis unius lateralis, and nevus keratoangiomatosus.3 Therefore, the exact incidence is difficult to determine.
Lesions generally are noted at birth or in early childhood and are often located on the lower extremities. The early lesions are bluish red in color. Secondary infection is a frequent complication, resulting in reactive papillomatosis and hyperkeratosis; thus, the older lesions acquire a verrucous or warty surface.3 Clinically, they may resemble angiokeratoma, lym-phangioma circumscriptum, verrucous epidermal nevus, verrucous cancer, or even malignant melanoma. Lesions initially resemble port-wine stains and later may become soft, bluish red vascular swellings that tend to grow in size and become verrucous.1
The histologic appearance closely resembles angiokeratoma, as both lesions show vascular spaces beneath a papillomatous and hyperkeratotic epidermis.4 However, in contrast to angiokeratoma, the vascular spaces in verrucous hemangioma also involve the lower dermis and subcutaneous tissues.
Although cases of linear verrucous hemangioma have been reported, its distribution along the lines of Blaschko is rare.5,6 It has been proposed that these lesions may actually be following dermatomal patterns or that the linear arrangement represents genetic mosaicism.5 In our case, the lesion started as a small plaque in childhood and gradually spread linearly to the buttock (Figure 1). A biopsy was taken from the lesion on the right buttock, which showed numerous dilated capillaries in the dermis (Figure 2).
|
Verrucous hemangiomas are best treated by excision. Larger lesions will need grafting. There is a tendency for recurrence to occur unless excision is complete.1 Yang and Ohara7 reported 14 patients with small localized lesions that were cured by 1 session of surgery without recurrence; 9 patients with wider and more extensive lesions required combination therapy in several stages for optimal results.
The Diagnosis: Linear Verrucous Hemangioma
Verrucous hemangioma is a rare congenital vascular malformation of the cutaneous and subcutaneous tissues. Although almost invariably present at birth, it may appear later in childhood or even in adulthood. Lesions commonly are found on the legs and may be linear, multiple, and disseminated or sometimes confined to the digits. In the early phase of evolution, the lesions are nonkeratotic, soft, blue-red plaques, but they gradually become increasingly hyperkeratotic.1 Linear verrucous hemangioma is even more rare with few published reports.
In 1937, Halter2 first used the term verrucous hemangioma to describe a 16-year-old adolescent boy who presented with a linear purpuric cluster of plaques extending from the right buttock to the toes. Imperial and Helwig3 later described it as a distinct entity. Since then, similar lesions have been described using a variety of names such as angiokeratoma circumscriptum neviforme, angiokeratoma circumscriptum, angiokeratoma corporis neviforme, keratotic hemangioma, nevus vascularis unius lateralis, and nevus keratoangiomatosus.3 Therefore, the exact incidence is difficult to determine.
Lesions generally are noted at birth or in early childhood and are often located on the lower extremities. The early lesions are bluish red in color. Secondary infection is a frequent complication, resulting in reactive papillomatosis and hyperkeratosis; thus, the older lesions acquire a verrucous or warty surface.3 Clinically, they may resemble angiokeratoma, lym-phangioma circumscriptum, verrucous epidermal nevus, verrucous cancer, or even malignant melanoma. Lesions initially resemble port-wine stains and later may become soft, bluish red vascular swellings that tend to grow in size and become verrucous.1
The histologic appearance closely resembles angiokeratoma, as both lesions show vascular spaces beneath a papillomatous and hyperkeratotic epidermis.4 However, in contrast to angiokeratoma, the vascular spaces in verrucous hemangioma also involve the lower dermis and subcutaneous tissues.
Although cases of linear verrucous hemangioma have been reported, its distribution along the lines of Blaschko is rare.5,6 It has been proposed that these lesions may actually be following dermatomal patterns or that the linear arrangement represents genetic mosaicism.5 In our case, the lesion started as a small plaque in childhood and gradually spread linearly to the buttock (Figure 1). A biopsy was taken from the lesion on the right buttock, which showed numerous dilated capillaries in the dermis (Figure 2).
|
Verrucous hemangiomas are best treated by excision. Larger lesions will need grafting. There is a tendency for recurrence to occur unless excision is complete.1 Yang and Ohara7 reported 14 patients with small localized lesions that were cured by 1 session of surgery without recurrence; 9 patients with wider and more extensive lesions required combination therapy in several stages for optimal results.
1. Atherton DJ, Moss C. Naevi and other developmental defects. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. 7th ed. London, England: Blackwell Scientific Publications; 2004:15-60.
2. Halter K. Haemangioma verrucosum mit Osteoatrophie. Dermatol Z. 1937;75:271-279.
3. Imperial R, Helwig EB. Verrucous hemangioma: a clinicopathological study of 21 cases. Arch Dermatol. 1967;96:247-253.
4. Calduch L, Ortega C, Navarro V, et al. Verrucous hemangioma: Report of two cases and review of the literature. Pediatr Dermatol. 2000;17:213-217.
5. Wentscher U, Happle R. Linear verrucous hemangioma. J Am Acad Dermatol. 2000;42:516-518.
6. Jain VK, Aggarwal K, Jain S. Linear verrucous hemangioma on the leg. Indian J Dermatol Venereol Leprol. 2008;74:656-658.
7. Yang CH, Ohara K. Successful surgical treatment of verrucous hemangioma: a combined approach. Dermatol Surg. 2002;28:913-919.
1. Atherton DJ, Moss C. Naevi and other developmental defects. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. 7th ed. London, England: Blackwell Scientific Publications; 2004:15-60.
2. Halter K. Haemangioma verrucosum mit Osteoatrophie. Dermatol Z. 1937;75:271-279.
3. Imperial R, Helwig EB. Verrucous hemangioma: a clinicopathological study of 21 cases. Arch Dermatol. 1967;96:247-253.
4. Calduch L, Ortega C, Navarro V, et al. Verrucous hemangioma: Report of two cases and review of the literature. Pediatr Dermatol. 2000;17:213-217.
5. Wentscher U, Happle R. Linear verrucous hemangioma. J Am Acad Dermatol. 2000;42:516-518.
6. Jain VK, Aggarwal K, Jain S. Linear verrucous hemangioma on the leg. Indian J Dermatol Venereol Leprol. 2008;74:656-658.
7. Yang CH, Ohara K. Successful surgical treatment of verrucous hemangioma: a combined approach. Dermatol Surg. 2002;28:913-919.
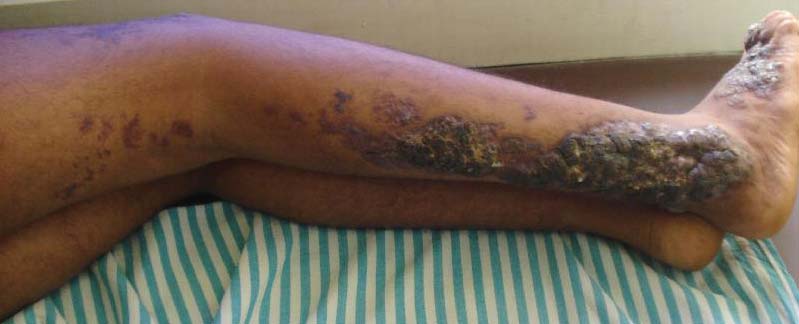
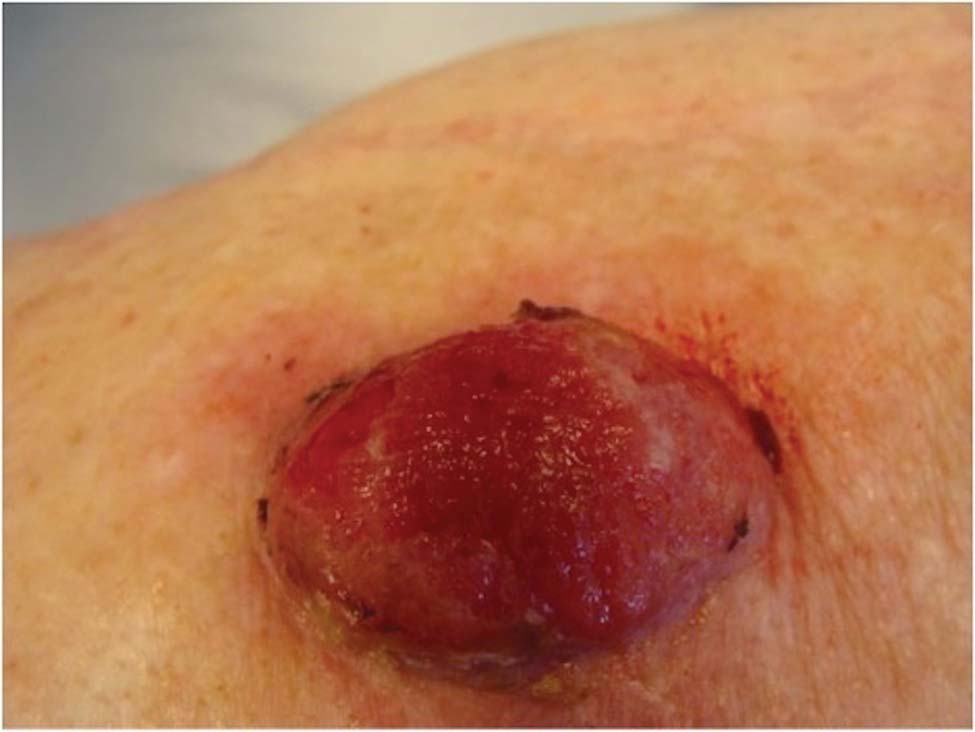
An 18-year-old man presented with a history of multiple bluish verrucous lesions over the right leg. A small nodule on the lateral aspect of the right ankle was present at birth; it increased in size and number and gradually extended to the right buttock. He had recurrent bleeding and infection over the lesions. No other remarkable comorbidities were noted. Dermatologic examination revealed multiple well-circumscribed, bluish red, verrucous lesions distributed linearly along the lateral aspect of the leg. The surface of the lesions was verruciform and showed crusting at places. The second and third toes on the right foot were involved. On the buttock, multiple well-defined, bluish red plaques were present. Both limbs were of equal length.
An Incidental Finding During Neuro Evaluation
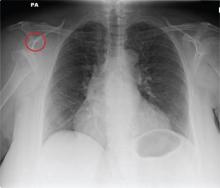
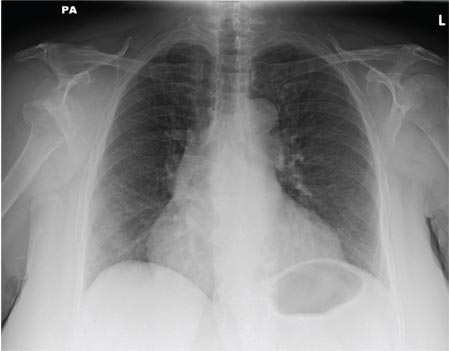
ANSWER
The radiograph shows a normal-appearing chest. Of note, though, is an anterior dislocation of the right shoulder. In addition, there is a fracture within the greater tuberosity of the right humerus.
Prompt orthopedic evaluation is obtained. In further discussion with the family, it was revealed that the patient had been experiencing falls recently; this injury was most likely the result of one.
ANSWER
The radiograph shows a normal-appearing chest. Of note, though, is an anterior dislocation of the right shoulder. In addition, there is a fracture within the greater tuberosity of the right humerus.
Prompt orthopedic evaluation is obtained. In further discussion with the family, it was revealed that the patient had been experiencing falls recently; this injury was most likely the result of one.
ANSWER
The radiograph shows a normal-appearing chest. Of note, though, is an anterior dislocation of the right shoulder. In addition, there is a fracture within the greater tuberosity of the right humerus.
Prompt orthopedic evaluation is obtained. In further discussion with the family, it was revealed that the patient had been experiencing falls recently; this injury was most likely the result of one.
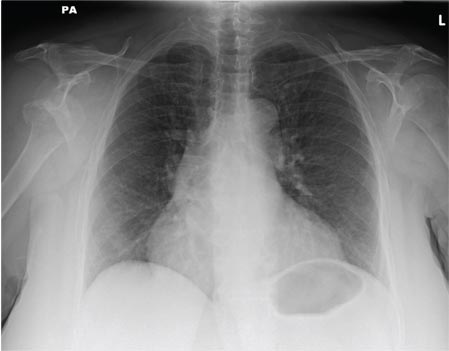
A 65-year-old woman is transferred to your facility from an outlying hospital for evaluation of a brain tumor. Family members found the patient sitting on the sofa, with a decreased level of consciousness. There was reported “seizure-type activity.” When she arrived at the outlying hospital, the patient was noted to have right-side weakness. Stat CT of the head demonstrated a fairly large parasagittal mass, and the patient was urgently transferred to your facility for neurosurgical evaluation. Primary survey on arrival shows an older female who is awake, alert, and in no obvious distress. Vital signs are normal. She has fairly pronounced right upper extremity weakness, more proximally than distally. Otherwise, the exam grossly appears normal. The patient’s initial imaging studies were sent with her on a CD. As you are trying to view the images of the brain, a chest radiograph pops up on your screen. What is your impression?
What Does This Man Need (Besides Milk & Cookies)?
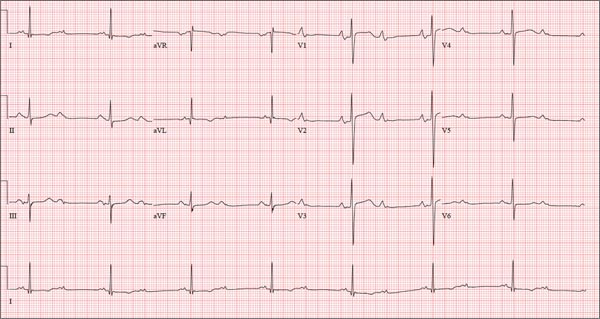
ANSWER
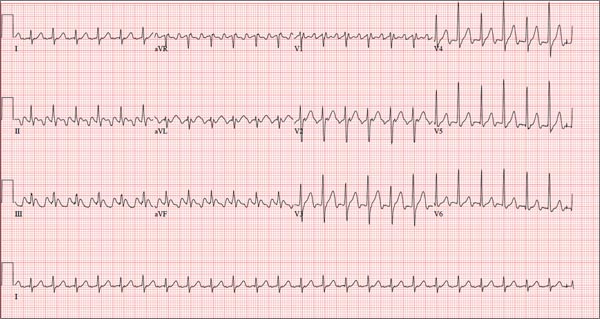
This ECG is representative of sinus rhythm with second-degree atrioventricular block with 2:1 conduction; possible left atrial enlargement; and ST-T wave abnormalities suspicious for lateral ischemia.
Sinus rhythm is evidenced by the P waves that march through at a rate that is consistently double that of the QRS rate (82 beats/min). The PR interval in the conducted beats remains constant, with every other P wave blocked from conducting into the ventricles.
The biphasic P wave seen in lead V1 does not meet criteria for left atrial enlargement (P wave in lead I ≥ 110 ms, terminal negative P wave in lead V1 ≥ 1 mm2) but is suspicious. Finally, ST-T wave elevations in leads V2-V4 are suspicious for ventricular septal ischemia.
The patient underwent placement of a dual-chamber permanent pacemaker. He has done well since.
ANSWER
This ECG is representative of sinus rhythm with second-degree atrioventricular block with 2:1 conduction; possible left atrial enlargement; and ST-T wave abnormalities suspicious for lateral ischemia.
Sinus rhythm is evidenced by the P waves that march through at a rate that is consistently double that of the QRS rate (82 beats/min). The PR interval in the conducted beats remains constant, with every other P wave blocked from conducting into the ventricles.
The biphasic P wave seen in lead V1 does not meet criteria for left atrial enlargement (P wave in lead I ≥ 110 ms, terminal negative P wave in lead V1 ≥ 1 mm2) but is suspicious. Finally, ST-T wave elevations in leads V2-V4 are suspicious for ventricular septal ischemia.
The patient underwent placement of a dual-chamber permanent pacemaker. He has done well since.
ANSWER
This ECG is representative of sinus rhythm with second-degree atrioventricular block with 2:1 conduction; possible left atrial enlargement; and ST-T wave abnormalities suspicious for lateral ischemia.
Sinus rhythm is evidenced by the P waves that march through at a rate that is consistently double that of the QRS rate (82 beats/min). The PR interval in the conducted beats remains constant, with every other P wave blocked from conducting into the ventricles.
The biphasic P wave seen in lead V1 does not meet criteria for left atrial enlargement (P wave in lead I ≥ 110 ms, terminal negative P wave in lead V1 ≥ 1 mm2) but is suspicious. Finally, ST-T wave elevations in leads V2-V4 are suspicious for ventricular septal ischemia.
The patient underwent placement of a dual-chamber permanent pacemaker. He has done well since.

A 74-year-old man has been a resident of a skilled nursing facility for seven years and is well known to the staff. This morning, when the medical assistant performed a routine vital sign check, she noticed the patient’s heart rate was in the 40s. This newly discovered bradycardia, coupled with a four-day history of lethargy, prompts the facility to transfer the patient to your emergency department for evaluation. The patient has a history of hypertension, hypothyroidism, chronic obstructive pulmonary disease, GERD, osteoarthritis, and dementia. Surgical history includes appendectomy, cholecystectomy, and left hip replacement. The patient’s multiple chronic conditions are well managed with medications, including a b-blocker, hydrochlorothiazide, levothyroxine, and an inhaler. He receives protein and vitamin supplements daily and is allergic to penicillin. There is a remote history of smoking (from his youth and tour of duty in the Korean War), although the patient says he hasn’t smoked in 30 years. He has “never touched” alcohol, because his father died of complications from alcoholism at age 45. The patient’s wife died of a stroke 11 years ago. His son (and family) visit him twice weekly, bringing chocolate milk and cookies that the patient anxiously awaits. The review of systems is remarkable for a recent cold (resolved), urinary retention, and loose stools. The patient’s appetite is intact. He also exhibits evidence of short-term memory loss; however, this is sporadic. Vital signs on arrival include a blood pressure of 158/88 mm Hg; pulse, 48 beats/min and regular; respiratory rate, 14 breaths/min; and temperature, 97.6°F. His weight is 174 lb and his height, 69 in. Pertinent findings on the physical exam include mild cataracts bilaterally, a right carotid bruit, no evidence of elevated neck veins, and late expiratory wheezes in both bases. The cardiac exam is remarkable for a regular rhythm with a heart rate of 42 beats/min. There is a grade II/VI early systolic murmur at the left upper sternal border but no radiation, extra heart sounds, or rubs. The abdomen is soft and nontender, with old surgical scars, and the abdominal aorta is easily palpable. The extremities exhibit full range of motion without peripheral edema, and osteoarthritic changes are evident in both hands. An ECG shows a ventricular rate of 43 beats/min; PR interval, 198 ms; QRS duration, 96 ms; QT/QTc interval, 464/392 ms; P axis, 60°; R axis, 4°; and T axis, 107°. What is your interpretation of this ECG?
Boy Has Had “Bald Spot” Since Birth
ANSWER
The answer is temporal triangular alopecia (choice “d”), an unusual form of permanent hair loss preferentially affecting the exact area depicted in this case.
Alopecia areata (choice “a”) involves localized hair loss. By contrast, this patient never had hair in this area to lose.
Nevus sebaceous (choice “b”) is a congenital hamartoma that is typically hairless; there are no follicles, and the bumpy, rough surface is composed of sebaceous globules.
Cutis aplasia (choice “c”) manifests with hairless lesions, but there is marked aplasia of the skin as well and no surface adnexae, let alone hairs or follicles.
DISCUSSION
Temporal triangular alopecia (TTA) is an unusual type of alopecia. Of unknown origin, it usually affects this area of the scalp—and usually unilaterally. Approximately one-third of TTA patients are born with the condition; the rest develop it in the first two to three years of life. As in this case, it is often wrongly attributed to the use of forceps but has nothing to do with trauma. One school of thought holds that TTA is probably an inherited condition—but others disagree.
TTA was originally known as congenital triangular alopecia. However, when enough cases had been accumulated to accurately determine the nature of the condition, it was realized that TTA is not always congenital or triangular. Thus, a new name was bestowed.
The hallmark of TTA is the normal number of hair follicles that only grow vellus hairs. The solitary peripheral tuft of terminal dark hairs is typical of TTA and thus a confirmatory finding.
TREATMENT/PROGNOSIS
TTA is by definition permanent. Since there’s no inflammation (a key difference from alopecia areata), steroids are useless. The only successful treatment for TTA, if any is attempted, is hair transplantation. As of this writing, the family is mulling this treatment option.
ANSWER
The answer is temporal triangular alopecia (choice “d”), an unusual form of permanent hair loss preferentially affecting the exact area depicted in this case.
Alopecia areata (choice “a”) involves localized hair loss. By contrast, this patient never had hair in this area to lose.
Nevus sebaceous (choice “b”) is a congenital hamartoma that is typically hairless; there are no follicles, and the bumpy, rough surface is composed of sebaceous globules.
Cutis aplasia (choice “c”) manifests with hairless lesions, but there is marked aplasia of the skin as well and no surface adnexae, let alone hairs or follicles.
DISCUSSION
Temporal triangular alopecia (TTA) is an unusual type of alopecia. Of unknown origin, it usually affects this area of the scalp—and usually unilaterally. Approximately one-third of TTA patients are born with the condition; the rest develop it in the first two to three years of life. As in this case, it is often wrongly attributed to the use of forceps but has nothing to do with trauma. One school of thought holds that TTA is probably an inherited condition—but others disagree.
TTA was originally known as congenital triangular alopecia. However, when enough cases had been accumulated to accurately determine the nature of the condition, it was realized that TTA is not always congenital or triangular. Thus, a new name was bestowed.
The hallmark of TTA is the normal number of hair follicles that only grow vellus hairs. The solitary peripheral tuft of terminal dark hairs is typical of TTA and thus a confirmatory finding.
TREATMENT/PROGNOSIS
TTA is by definition permanent. Since there’s no inflammation (a key difference from alopecia areata), steroids are useless. The only successful treatment for TTA, if any is attempted, is hair transplantation. As of this writing, the family is mulling this treatment option.
ANSWER
The answer is temporal triangular alopecia (choice “d”), an unusual form of permanent hair loss preferentially affecting the exact area depicted in this case.
Alopecia areata (choice “a”) involves localized hair loss. By contrast, this patient never had hair in this area to lose.
Nevus sebaceous (choice “b”) is a congenital hamartoma that is typically hairless; there are no follicles, and the bumpy, rough surface is composed of sebaceous globules.
Cutis aplasia (choice “c”) manifests with hairless lesions, but there is marked aplasia of the skin as well and no surface adnexae, let alone hairs or follicles.
DISCUSSION
Temporal triangular alopecia (TTA) is an unusual type of alopecia. Of unknown origin, it usually affects this area of the scalp—and usually unilaterally. Approximately one-third of TTA patients are born with the condition; the rest develop it in the first two to three years of life. As in this case, it is often wrongly attributed to the use of forceps but has nothing to do with trauma. One school of thought holds that TTA is probably an inherited condition—but others disagree.
TTA was originally known as congenital triangular alopecia. However, when enough cases had been accumulated to accurately determine the nature of the condition, it was realized that TTA is not always congenital or triangular. Thus, a new name was bestowed.
The hallmark of TTA is the normal number of hair follicles that only grow vellus hairs. The solitary peripheral tuft of terminal dark hairs is typical of TTA and thus a confirmatory finding.
TREATMENT/PROGNOSIS
TTA is by definition permanent. Since there’s no inflammation (a key difference from alopecia areata), steroids are useless. The only successful treatment for TTA, if any is attempted, is hair transplantation. As of this writing, the family is mulling this treatment option.
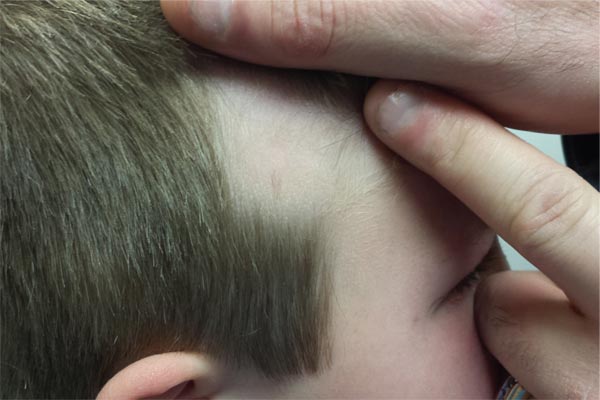
Since birth, this 8-year-old boy has had a “bald spot” on his scalp. The pediatrician who attended the birth suggested trauma as the cause, since forceps were used to facilitate delivery. But the problem has failed to resolve, leaving the boy an object of ridicule among his classmates. According to the patient’s parents, there has never been any broken skin or hair growth in the area. There is no family history of similar problems, and the child’s health history is unremarkable. The child’s current pediatrician, who made the referral to dermatology, suggested the lesion might be a form of nevus sebaceous. The affected site is roughly triangular, measures about 3 cm on each side, and is located just inside the temporal scalp. The hair loss in this sharply circumscribed area is almost complete, with a lone tuft of darker terminal hairs on the inferior aspect of the site. No redness or epidermal disturbance (eg, scaling) is noted. Dermatoscopic examination (with 10x magnification) reveals a normal number of follicles and hairs. The latter are vellus hairs, except for the aforementioned solitary tuft. The rest of the scalp, including the same location on the opposite side, is free of any significant changes.
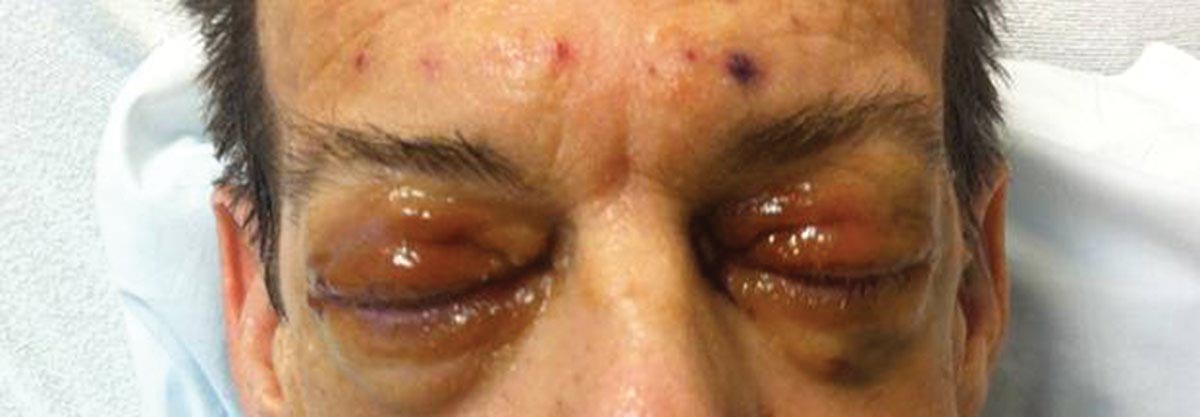
Waxy Indurated Plaques on the Eyelids
The Diagnosis: Primary (Myeloma-Associated) Systemic Amyloidosis
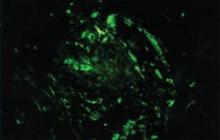
Amyloidosis is a broad term describing the abnormal autoaggregation of normally soluble proteins as extracellular deposits. More than 2 dozen protein precursors to these amyloid fibrils have been characterized; however, all amyloid deposits invariably act to disrupt normal tissue structure and function. The term amyloid dates back to the 19th century when it was used by Rudolph Virchow to describe the lardaceous appearance of affected tissue. In the 1920s amyloid was found to avidly stain with Congo red, and shortly thereafter the classical apple green birefringence was described when these plaques were exposed to polarized light1 (Figure 1). Currently, amyloidosis is classified by the World Health Organization based on the type of protein deposited; there are more than 20 types of low-molecular-weight proteins that form amyloid fibrils. Generally, amyloidosis can be viewed as primary (AL), secondary (AA), or hereditary in origin. Hereditary amyloidosis usually arises in the setting of familial mutations that potentiate the aggregation of plasma proteins, while secondary amyloidosis develops in the setting of chronic inflammatory diseases (eg, rheumatoid arthritis, lupus). Primary systemic amyloidosis, which presented in our patient, is caused by the overproduction and deposition of clonal immunoglobulin light chain fragments.
Prevalence
Primary systemic amyloidosis (AL amyloidosis) is the most common cause of amyloidosis in the United States, with an annual incidence of 6 to 7 cases per million. The median age at diagnosis is 73.5 years and approximately 60% of patients are male.2 Due to the rare nature of this disease, few large-scale studies have been conducted looking at the pathogenesis, presentation, and best treatment options for AL amyloidosis. However, the following trends were compiled based on the available literature and serve as a useful clinical guide. Primary systemic AL amyloidosis is characterized by an underlying plasma cell dyscrasia, such as multiple myeloma or monoclonal gammopathy of undetermined significance.3 Although AL amyloidosis frequently is associated with multiple myeloma, only 10% of these patients have coexisting multiple myeloma. Conversely, 12% to 15% of multiple myeloma patients are eventually diagnosed with AL amyloi-dosis. The plasma cells in AL amyloidosis are clonal and produce immunoglobulin κ and λ light chains. Interestingly, the ratio of λ to κ light chains in AL amyloidosis is 3 to 1, which is the reverse of the ratio usually seen in multiple myeloma.4
Clinical Presentation
Because AL amyloidosis can affect virtually every organ system except the brain parenchyma, the clinical presentation is extremely variable and initial symptoms are nonspecific, including fatigue and weight loss. Therefore, the diagnosis is frequently delayed until end-organ dysfunction becomes evident.5 At presentation, three-quarters of patients have involvement of 2 or more organs. In a study of 445 patients with AL amyloidosis, the most commonly involved organs included the kidneys (40%–70%), heart (30%–60%), liver (10%–25%), gastrointestinal tract (10%–20%), peripheral nervous system (5%–20%), and soft tissue infiltration (15%), with macroglossia being a pathognomonic finding. Macroglossia arises from the direct deposition of monoclonal immunoglobulin light chains within the tongue and is observed in up to 40% of cases.6 Dental indentations can be observed on the lateral aspects of the tongue. Macroglossia can lead to dysphagia as well as respiratory complications.
Diagnosis
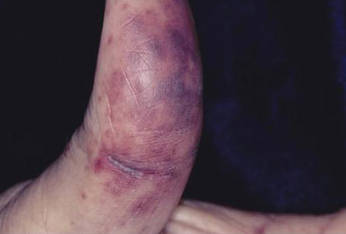
Distinct cutaneous manifestations of amyloidosis are evident in 29% to 40% of cases and can prompt the astute dermatologist to test for underlying disease. Purpura and ecchymotic lesions occur in 15% of patients and are usually preceded by minor trauma (pinch purpura) or maneuvers that increase intravascular pressure (eg, Valsalva maneuver, vomiting).7 Periorbital distribution is the most classic finding, as was evident in our patient; however, all flexural regions including the neck, axillae, and anogenital regions can be involved. Our patient also had purpura present on the thumb (Figure 2) and shoulder (Figure 3), demonstrating the variable presentation of amyloidosis on the skin. Mechanistically, AL amyloidosis is thought to contribute to purpura by infiltrating blood vessels, thus making them more fragile. Furthermore, AL amyloidosis can potentiate a bleeding diathesis by depleting the body of factor X secondary to the binding of vitamin K–dependent factors to amyloid deposits. Additional cutaneous findings in AL amyloidosis may include smooth, shiny, waxy papules that may coalesce to form nodules and plaques of various sizes. Lesions typically are distributed in the flexural areas, including the mouth, eyes, neck, inguinal folds, and axillae. Lesions occurring in the perianal and vulvar skin may resemble giant condylomata or xanthomas.8 Although rare, nail dystrophy characterized by brittle nail plates and longitudinal ridging also can occur in patients with systemic AL amyloidosis. Another rare but extremely important cutaneous manifestation is hemorrhagic or clear subepidermal bullae. Histopathology can aid in the diagnosis of bullous amyloidosis.9 Diagnosis is usually accomplished by tissue biopsy of an affected organ or surrogate site as well as screening of the serum and urine for monoclonal immunoglobulins (detected in >90% of cases). The choice of a suitable biopsy site is at times complicated by accessibility and morbidity of the procedure as well as the varying yields. The biopsy sites with the highest yield in AL amyloidosis are the kidneys and liver (90%), followed by abdominal fat-pad (60%–84%), rectum (50%–80%), bone marrow (50%–55%), and skin (50%).10 Given the invasive nature of kidney and liver biopsies, the abdominal fat-pad frequently is sampled first, followed by more invasive procedures if the initial biopsy is negative and clinical suspicion remains high. In the presence of clinically evident mucocutaneous lesions, skin biopsy also can be used for initial diagnosis; however, the yield drops precipitously when skin involvement is absent. Amyloid has an eosinophilic amorphous appearance on hematoxylin and eosin stains and is definitively diagnosed by Congo red staining (apple green birefringence under polarized light). Because Congo red staining only confirms the presence of amyloid and not the precipitated protein, typing of the amyloid deposits should be done either by immunohistochemical staining or electron microscopy. Because of the association of AL amyloidosis with plasma cell dyscrasia, a bone marrow biopsy is warranted to grade the degree of plasma cell infiltration of the marrow. If no plasma cell dyscrasia is identified in the setting of a biopsy suggestive of amyloid, other causes of amyloidosis should be considered including familial (10% of cases) and secondary variants.11 End-organ involvement also should be investigated with echocardiography, electrocardiography peripheral nerve studies, skeletal survey, and renal and liver function testing. Prognosis is poor with expected survival of 12 to 14 months and is heavily dependent on the degree and type of end-organ involvement at the time of diagnosis, with median survival ranging from 4 to 6 months in patients with congestive heart failure to as long as 2 years in patients with primarily renal involvement.5,10,12,13 Poor prognosticating factors in AL amyloidosis involve dominant cardiac involvement, coexisting multiple myeloma, and increased peripheral plasma cell counts.14
|
Treatment
Treatment regimens for AL amyloidosis have historically been adapted from those used to treat multiple myeloma, with the primary focus being the reduction of the amyloidogenic clone. The first effective therapy for AL amyloidosis was melphalan and prednisone. Although this regimen extended survival from half a year (6–8 months) to a year (12–18 months), it also was plagued by severe side effects and delayed response times. Increased response rates and fewer side effects have been achieved by replacing prednisone with dexamethasone in the aforementioned regimen.15 High-dose melphalan conditioning followed by autologous stem cell transplant has shown tremendous promise, with complete remission rates approaching 40% and median survival exceeding 4 years in some studies.16 However, the treatment-related mortality of stem cell transplant regimens is 10% to 20%, thus limiting this approach to younger and healthier patients. Exciting early work is emerging to support the efficacy of novel agents in the treatment of AL amyloidosis, including thalidomide, lenalidomide, and bortezomib. Because these agents do not have many of the traditional side effects associated with chemotherapy, they are increasingly being used in patients who are not candidates for stem cell transplant or melphalan-dexamethasone. They also can be used as salvage therapy in patients with relapsed disease.
Conclusion
Primary AL amyloidosis is a rare disorder that carries a poor prognosis. One of the major challenges for clinicians is diagnosis, as nearly any organ system can be affected, resulting in variable presentations. Although cutaneous manifestations are highly characteristic of AL amyloidosis and may help aid in its diagnosis, only a limited number of patients have involvement of the skin. As such, further research is needed to facilitate earlier diagnosis of primary AL amyloidosis as well as to develop novel therapies for this devastating disease.
1. Kyle RA. Amyloidosis: a convoluted story. Br J Haematol. 2001;114:529-538.
2. Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79:1817-1822.
3. Glenner GG, Terry W, Harada M, et al. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971;172:1150-1151.
4. Solomon AB. Frangione B, Franklin EC. Bence Jones proteins and light chains of immunoglobulins. preferential association of the V lambda VI subgroup of human light chains with amyloidosis AL (lambda). J Clin Invest. 1982;70:453-460.
5. Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45-59.
6. Thibault I, Vallieres I. Macroglossia due to systemic amyloidosis: is there a role for radiotherapy? Case Rep Oncol. 2011;4:392-399.
7. Breathnach SM. Amyloid and amyloidosis. J Am Acad Dermatol. 1988;18:1-16.
8. Chapman RS, Neville EA, Lawson JW. Xanthoma-like skin lesions as a presenting feature in primary systemic amyloidosis. Br J Clin Pract. 1973;27:271-273.
9. Asahina A, Hasegawa K, Ishiyama M, et al. Bullous amyloidosis mimicking bullous pemphigoid: usefulness of electron microscopic examination. Acta Derm Venereol. 2010;90:427-428.
10. Kyle RA, Bayrd ED. Amyloidosis: review of 236 cases. Medicine. 1975;54:271-299.
11. Lachmann HJ, Booth DR, Booth SE, et al. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med. 2002;346:1786-1791.
12. Kyle RA, Greipp PR. Amyloidosis (AL). clinical and laboratory features in 229 cases. Mayo Clin Proc. 1983;58:665-683.
13. Madan SA, Dispenzieri A, Lacy MQ, et al. Clinical features and treatment response of light chain (AL) amyloidosis diagnosed in patients with previous diagnosis of multiple myeloma. Mayo Clin Proc. 2010;85:232-238.
14. Pardanani A, Witzig TE, Schroeder G, et al. Circulating peripheral blood plasma cells as a prognostic indicator in patients with primary systemic amyloidosis. Blood. 2003;101:827-830.
15. Palladini G, Perfetti V, Obici L, et al. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood. 2004;103:2936-2938.
16. Skinner M, Sanchorawala V, Seldin DC, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004;140:85-93.
The Diagnosis: Primary (Myeloma-Associated) Systemic Amyloidosis
Amyloidosis is a broad term describing the abnormal autoaggregation of normally soluble proteins as extracellular deposits. More than 2 dozen protein precursors to these amyloid fibrils have been characterized; however, all amyloid deposits invariably act to disrupt normal tissue structure and function. The term amyloid dates back to the 19th century when it was used by Rudolph Virchow to describe the lardaceous appearance of affected tissue. In the 1920s amyloid was found to avidly stain with Congo red, and shortly thereafter the classical apple green birefringence was described when these plaques were exposed to polarized light1 (Figure 1). Currently, amyloidosis is classified by the World Health Organization based on the type of protein deposited; there are more than 20 types of low-molecular-weight proteins that form amyloid fibrils. Generally, amyloidosis can be viewed as primary (AL), secondary (AA), or hereditary in origin. Hereditary amyloidosis usually arises in the setting of familial mutations that potentiate the aggregation of plasma proteins, while secondary amyloidosis develops in the setting of chronic inflammatory diseases (eg, rheumatoid arthritis, lupus). Primary systemic amyloidosis, which presented in our patient, is caused by the overproduction and deposition of clonal immunoglobulin light chain fragments.
Prevalence
Primary systemic amyloidosis (AL amyloidosis) is the most common cause of amyloidosis in the United States, with an annual incidence of 6 to 7 cases per million. The median age at diagnosis is 73.5 years and approximately 60% of patients are male.2 Due to the rare nature of this disease, few large-scale studies have been conducted looking at the pathogenesis, presentation, and best treatment options for AL amyloidosis. However, the following trends were compiled based on the available literature and serve as a useful clinical guide. Primary systemic AL amyloidosis is characterized by an underlying plasma cell dyscrasia, such as multiple myeloma or monoclonal gammopathy of undetermined significance.3 Although AL amyloidosis frequently is associated with multiple myeloma, only 10% of these patients have coexisting multiple myeloma. Conversely, 12% to 15% of multiple myeloma patients are eventually diagnosed with AL amyloi-dosis. The plasma cells in AL amyloidosis are clonal and produce immunoglobulin κ and λ light chains. Interestingly, the ratio of λ to κ light chains in AL amyloidosis is 3 to 1, which is the reverse of the ratio usually seen in multiple myeloma.4
Clinical Presentation
Because AL amyloidosis can affect virtually every organ system except the brain parenchyma, the clinical presentation is extremely variable and initial symptoms are nonspecific, including fatigue and weight loss. Therefore, the diagnosis is frequently delayed until end-organ dysfunction becomes evident.5 At presentation, three-quarters of patients have involvement of 2 or more organs. In a study of 445 patients with AL amyloidosis, the most commonly involved organs included the kidneys (40%–70%), heart (30%–60%), liver (10%–25%), gastrointestinal tract (10%–20%), peripheral nervous system (5%–20%), and soft tissue infiltration (15%), with macroglossia being a pathognomonic finding. Macroglossia arises from the direct deposition of monoclonal immunoglobulin light chains within the tongue and is observed in up to 40% of cases.6 Dental indentations can be observed on the lateral aspects of the tongue. Macroglossia can lead to dysphagia as well as respiratory complications.
Diagnosis
Distinct cutaneous manifestations of amyloidosis are evident in 29% to 40% of cases and can prompt the astute dermatologist to test for underlying disease. Purpura and ecchymotic lesions occur in 15% of patients and are usually preceded by minor trauma (pinch purpura) or maneuvers that increase intravascular pressure (eg, Valsalva maneuver, vomiting).7 Periorbital distribution is the most classic finding, as was evident in our patient; however, all flexural regions including the neck, axillae, and anogenital regions can be involved. Our patient also had purpura present on the thumb (Figure 2) and shoulder (Figure 3), demonstrating the variable presentation of amyloidosis on the skin. Mechanistically, AL amyloidosis is thought to contribute to purpura by infiltrating blood vessels, thus making them more fragile. Furthermore, AL amyloidosis can potentiate a bleeding diathesis by depleting the body of factor X secondary to the binding of vitamin K–dependent factors to amyloid deposits. Additional cutaneous findings in AL amyloidosis may include smooth, shiny, waxy papules that may coalesce to form nodules and plaques of various sizes. Lesions typically are distributed in the flexural areas, including the mouth, eyes, neck, inguinal folds, and axillae. Lesions occurring in the perianal and vulvar skin may resemble giant condylomata or xanthomas.8 Although rare, nail dystrophy characterized by brittle nail plates and longitudinal ridging also can occur in patients with systemic AL amyloidosis. Another rare but extremely important cutaneous manifestation is hemorrhagic or clear subepidermal bullae. Histopathology can aid in the diagnosis of bullous amyloidosis.9 Diagnosis is usually accomplished by tissue biopsy of an affected organ or surrogate site as well as screening of the serum and urine for monoclonal immunoglobulins (detected in >90% of cases). The choice of a suitable biopsy site is at times complicated by accessibility and morbidity of the procedure as well as the varying yields. The biopsy sites with the highest yield in AL amyloidosis are the kidneys and liver (90%), followed by abdominal fat-pad (60%–84%), rectum (50%–80%), bone marrow (50%–55%), and skin (50%).10 Given the invasive nature of kidney and liver biopsies, the abdominal fat-pad frequently is sampled first, followed by more invasive procedures if the initial biopsy is negative and clinical suspicion remains high. In the presence of clinically evident mucocutaneous lesions, skin biopsy also can be used for initial diagnosis; however, the yield drops precipitously when skin involvement is absent. Amyloid has an eosinophilic amorphous appearance on hematoxylin and eosin stains and is definitively diagnosed by Congo red staining (apple green birefringence under polarized light). Because Congo red staining only confirms the presence of amyloid and not the precipitated protein, typing of the amyloid deposits should be done either by immunohistochemical staining or electron microscopy. Because of the association of AL amyloidosis with plasma cell dyscrasia, a bone marrow biopsy is warranted to grade the degree of plasma cell infiltration of the marrow. If no plasma cell dyscrasia is identified in the setting of a biopsy suggestive of amyloid, other causes of amyloidosis should be considered including familial (10% of cases) and secondary variants.11 End-organ involvement also should be investigated with echocardiography, electrocardiography peripheral nerve studies, skeletal survey, and renal and liver function testing. Prognosis is poor with expected survival of 12 to 14 months and is heavily dependent on the degree and type of end-organ involvement at the time of diagnosis, with median survival ranging from 4 to 6 months in patients with congestive heart failure to as long as 2 years in patients with primarily renal involvement.5,10,12,13 Poor prognosticating factors in AL amyloidosis involve dominant cardiac involvement, coexisting multiple myeloma, and increased peripheral plasma cell counts.14
|
Treatment
Treatment regimens for AL amyloidosis have historically been adapted from those used to treat multiple myeloma, with the primary focus being the reduction of the amyloidogenic clone. The first effective therapy for AL amyloidosis was melphalan and prednisone. Although this regimen extended survival from half a year (6–8 months) to a year (12–18 months), it also was plagued by severe side effects and delayed response times. Increased response rates and fewer side effects have been achieved by replacing prednisone with dexamethasone in the aforementioned regimen.15 High-dose melphalan conditioning followed by autologous stem cell transplant has shown tremendous promise, with complete remission rates approaching 40% and median survival exceeding 4 years in some studies.16 However, the treatment-related mortality of stem cell transplant regimens is 10% to 20%, thus limiting this approach to younger and healthier patients. Exciting early work is emerging to support the efficacy of novel agents in the treatment of AL amyloidosis, including thalidomide, lenalidomide, and bortezomib. Because these agents do not have many of the traditional side effects associated with chemotherapy, they are increasingly being used in patients who are not candidates for stem cell transplant or melphalan-dexamethasone. They also can be used as salvage therapy in patients with relapsed disease.
Conclusion
Primary AL amyloidosis is a rare disorder that carries a poor prognosis. One of the major challenges for clinicians is diagnosis, as nearly any organ system can be affected, resulting in variable presentations. Although cutaneous manifestations are highly characteristic of AL amyloidosis and may help aid in its diagnosis, only a limited number of patients have involvement of the skin. As such, further research is needed to facilitate earlier diagnosis of primary AL amyloidosis as well as to develop novel therapies for this devastating disease.
The Diagnosis: Primary (Myeloma-Associated) Systemic Amyloidosis
Amyloidosis is a broad term describing the abnormal autoaggregation of normally soluble proteins as extracellular deposits. More than 2 dozen protein precursors to these amyloid fibrils have been characterized; however, all amyloid deposits invariably act to disrupt normal tissue structure and function. The term amyloid dates back to the 19th century when it was used by Rudolph Virchow to describe the lardaceous appearance of affected tissue. In the 1920s amyloid was found to avidly stain with Congo red, and shortly thereafter the classical apple green birefringence was described when these plaques were exposed to polarized light1 (Figure 1). Currently, amyloidosis is classified by the World Health Organization based on the type of protein deposited; there are more than 20 types of low-molecular-weight proteins that form amyloid fibrils. Generally, amyloidosis can be viewed as primary (AL), secondary (AA), or hereditary in origin. Hereditary amyloidosis usually arises in the setting of familial mutations that potentiate the aggregation of plasma proteins, while secondary amyloidosis develops in the setting of chronic inflammatory diseases (eg, rheumatoid arthritis, lupus). Primary systemic amyloidosis, which presented in our patient, is caused by the overproduction and deposition of clonal immunoglobulin light chain fragments.
Prevalence
Primary systemic amyloidosis (AL amyloidosis) is the most common cause of amyloidosis in the United States, with an annual incidence of 6 to 7 cases per million. The median age at diagnosis is 73.5 years and approximately 60% of patients are male.2 Due to the rare nature of this disease, few large-scale studies have been conducted looking at the pathogenesis, presentation, and best treatment options for AL amyloidosis. However, the following trends were compiled based on the available literature and serve as a useful clinical guide. Primary systemic AL amyloidosis is characterized by an underlying plasma cell dyscrasia, such as multiple myeloma or monoclonal gammopathy of undetermined significance.3 Although AL amyloidosis frequently is associated with multiple myeloma, only 10% of these patients have coexisting multiple myeloma. Conversely, 12% to 15% of multiple myeloma patients are eventually diagnosed with AL amyloi-dosis. The plasma cells in AL amyloidosis are clonal and produce immunoglobulin κ and λ light chains. Interestingly, the ratio of λ to κ light chains in AL amyloidosis is 3 to 1, which is the reverse of the ratio usually seen in multiple myeloma.4
Clinical Presentation
Because AL amyloidosis can affect virtually every organ system except the brain parenchyma, the clinical presentation is extremely variable and initial symptoms are nonspecific, including fatigue and weight loss. Therefore, the diagnosis is frequently delayed until end-organ dysfunction becomes evident.5 At presentation, three-quarters of patients have involvement of 2 or more organs. In a study of 445 patients with AL amyloidosis, the most commonly involved organs included the kidneys (40%–70%), heart (30%–60%), liver (10%–25%), gastrointestinal tract (10%–20%), peripheral nervous system (5%–20%), and soft tissue infiltration (15%), with macroglossia being a pathognomonic finding. Macroglossia arises from the direct deposition of monoclonal immunoglobulin light chains within the tongue and is observed in up to 40% of cases.6 Dental indentations can be observed on the lateral aspects of the tongue. Macroglossia can lead to dysphagia as well as respiratory complications.
Diagnosis
Distinct cutaneous manifestations of amyloidosis are evident in 29% to 40% of cases and can prompt the astute dermatologist to test for underlying disease. Purpura and ecchymotic lesions occur in 15% of patients and are usually preceded by minor trauma (pinch purpura) or maneuvers that increase intravascular pressure (eg, Valsalva maneuver, vomiting).7 Periorbital distribution is the most classic finding, as was evident in our patient; however, all flexural regions including the neck, axillae, and anogenital regions can be involved. Our patient also had purpura present on the thumb (Figure 2) and shoulder (Figure 3), demonstrating the variable presentation of amyloidosis on the skin. Mechanistically, AL amyloidosis is thought to contribute to purpura by infiltrating blood vessels, thus making them more fragile. Furthermore, AL amyloidosis can potentiate a bleeding diathesis by depleting the body of factor X secondary to the binding of vitamin K–dependent factors to amyloid deposits. Additional cutaneous findings in AL amyloidosis may include smooth, shiny, waxy papules that may coalesce to form nodules and plaques of various sizes. Lesions typically are distributed in the flexural areas, including the mouth, eyes, neck, inguinal folds, and axillae. Lesions occurring in the perianal and vulvar skin may resemble giant condylomata or xanthomas.8 Although rare, nail dystrophy characterized by brittle nail plates and longitudinal ridging also can occur in patients with systemic AL amyloidosis. Another rare but extremely important cutaneous manifestation is hemorrhagic or clear subepidermal bullae. Histopathology can aid in the diagnosis of bullous amyloidosis.9 Diagnosis is usually accomplished by tissue biopsy of an affected organ or surrogate site as well as screening of the serum and urine for monoclonal immunoglobulins (detected in >90% of cases). The choice of a suitable biopsy site is at times complicated by accessibility and morbidity of the procedure as well as the varying yields. The biopsy sites with the highest yield in AL amyloidosis are the kidneys and liver (90%), followed by abdominal fat-pad (60%–84%), rectum (50%–80%), bone marrow (50%–55%), and skin (50%).10 Given the invasive nature of kidney and liver biopsies, the abdominal fat-pad frequently is sampled first, followed by more invasive procedures if the initial biopsy is negative and clinical suspicion remains high. In the presence of clinically evident mucocutaneous lesions, skin biopsy also can be used for initial diagnosis; however, the yield drops precipitously when skin involvement is absent. Amyloid has an eosinophilic amorphous appearance on hematoxylin and eosin stains and is definitively diagnosed by Congo red staining (apple green birefringence under polarized light). Because Congo red staining only confirms the presence of amyloid and not the precipitated protein, typing of the amyloid deposits should be done either by immunohistochemical staining or electron microscopy. Because of the association of AL amyloidosis with plasma cell dyscrasia, a bone marrow biopsy is warranted to grade the degree of plasma cell infiltration of the marrow. If no plasma cell dyscrasia is identified in the setting of a biopsy suggestive of amyloid, other causes of amyloidosis should be considered including familial (10% of cases) and secondary variants.11 End-organ involvement also should be investigated with echocardiography, electrocardiography peripheral nerve studies, skeletal survey, and renal and liver function testing. Prognosis is poor with expected survival of 12 to 14 months and is heavily dependent on the degree and type of end-organ involvement at the time of diagnosis, with median survival ranging from 4 to 6 months in patients with congestive heart failure to as long as 2 years in patients with primarily renal involvement.5,10,12,13 Poor prognosticating factors in AL amyloidosis involve dominant cardiac involvement, coexisting multiple myeloma, and increased peripheral plasma cell counts.14
|
Treatment
Treatment regimens for AL amyloidosis have historically been adapted from those used to treat multiple myeloma, with the primary focus being the reduction of the amyloidogenic clone. The first effective therapy for AL amyloidosis was melphalan and prednisone. Although this regimen extended survival from half a year (6–8 months) to a year (12–18 months), it also was plagued by severe side effects and delayed response times. Increased response rates and fewer side effects have been achieved by replacing prednisone with dexamethasone in the aforementioned regimen.15 High-dose melphalan conditioning followed by autologous stem cell transplant has shown tremendous promise, with complete remission rates approaching 40% and median survival exceeding 4 years in some studies.16 However, the treatment-related mortality of stem cell transplant regimens is 10% to 20%, thus limiting this approach to younger and healthier patients. Exciting early work is emerging to support the efficacy of novel agents in the treatment of AL amyloidosis, including thalidomide, lenalidomide, and bortezomib. Because these agents do not have many of the traditional side effects associated with chemotherapy, they are increasingly being used in patients who are not candidates for stem cell transplant or melphalan-dexamethasone. They also can be used as salvage therapy in patients with relapsed disease.
Conclusion
Primary AL amyloidosis is a rare disorder that carries a poor prognosis. One of the major challenges for clinicians is diagnosis, as nearly any organ system can be affected, resulting in variable presentations. Although cutaneous manifestations are highly characteristic of AL amyloidosis and may help aid in its diagnosis, only a limited number of patients have involvement of the skin. As such, further research is needed to facilitate earlier diagnosis of primary AL amyloidosis as well as to develop novel therapies for this devastating disease.
1. Kyle RA. Amyloidosis: a convoluted story. Br J Haematol. 2001;114:529-538.
2. Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79:1817-1822.
3. Glenner GG, Terry W, Harada M, et al. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971;172:1150-1151.
4. Solomon AB. Frangione B, Franklin EC. Bence Jones proteins and light chains of immunoglobulins. preferential association of the V lambda VI subgroup of human light chains with amyloidosis AL (lambda). J Clin Invest. 1982;70:453-460.
5. Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45-59.
6. Thibault I, Vallieres I. Macroglossia due to systemic amyloidosis: is there a role for radiotherapy? Case Rep Oncol. 2011;4:392-399.
7. Breathnach SM. Amyloid and amyloidosis. J Am Acad Dermatol. 1988;18:1-16.
8. Chapman RS, Neville EA, Lawson JW. Xanthoma-like skin lesions as a presenting feature in primary systemic amyloidosis. Br J Clin Pract. 1973;27:271-273.
9. Asahina A, Hasegawa K, Ishiyama M, et al. Bullous amyloidosis mimicking bullous pemphigoid: usefulness of electron microscopic examination. Acta Derm Venereol. 2010;90:427-428.
10. Kyle RA, Bayrd ED. Amyloidosis: review of 236 cases. Medicine. 1975;54:271-299.
11. Lachmann HJ, Booth DR, Booth SE, et al. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med. 2002;346:1786-1791.
12. Kyle RA, Greipp PR. Amyloidosis (AL). clinical and laboratory features in 229 cases. Mayo Clin Proc. 1983;58:665-683.
13. Madan SA, Dispenzieri A, Lacy MQ, et al. Clinical features and treatment response of light chain (AL) amyloidosis diagnosed in patients with previous diagnosis of multiple myeloma. Mayo Clin Proc. 2010;85:232-238.
14. Pardanani A, Witzig TE, Schroeder G, et al. Circulating peripheral blood plasma cells as a prognostic indicator in patients with primary systemic amyloidosis. Blood. 2003;101:827-830.
15. Palladini G, Perfetti V, Obici L, et al. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood. 2004;103:2936-2938.
16. Skinner M, Sanchorawala V, Seldin DC, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004;140:85-93.
1. Kyle RA. Amyloidosis: a convoluted story. Br J Haematol. 2001;114:529-538.
2. Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79:1817-1822.
3. Glenner GG, Terry W, Harada M, et al. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971;172:1150-1151.
4. Solomon AB. Frangione B, Franklin EC. Bence Jones proteins and light chains of immunoglobulins. preferential association of the V lambda VI subgroup of human light chains with amyloidosis AL (lambda). J Clin Invest. 1982;70:453-460.
5. Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45-59.
6. Thibault I, Vallieres I. Macroglossia due to systemic amyloidosis: is there a role for radiotherapy? Case Rep Oncol. 2011;4:392-399.
7. Breathnach SM. Amyloid and amyloidosis. J Am Acad Dermatol. 1988;18:1-16.
8. Chapman RS, Neville EA, Lawson JW. Xanthoma-like skin lesions as a presenting feature in primary systemic amyloidosis. Br J Clin Pract. 1973;27:271-273.
9. Asahina A, Hasegawa K, Ishiyama M, et al. Bullous amyloidosis mimicking bullous pemphigoid: usefulness of electron microscopic examination. Acta Derm Venereol. 2010;90:427-428.
10. Kyle RA, Bayrd ED. Amyloidosis: review of 236 cases. Medicine. 1975;54:271-299.
11. Lachmann HJ, Booth DR, Booth SE, et al. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med. 2002;346:1786-1791.
12. Kyle RA, Greipp PR. Amyloidosis (AL). clinical and laboratory features in 229 cases. Mayo Clin Proc. 1983;58:665-683.
13. Madan SA, Dispenzieri A, Lacy MQ, et al. Clinical features and treatment response of light chain (AL) amyloidosis diagnosed in patients with previous diagnosis of multiple myeloma. Mayo Clin Proc. 2010;85:232-238.
14. Pardanani A, Witzig TE, Schroeder G, et al. Circulating peripheral blood plasma cells as a prognostic indicator in patients with primary systemic amyloidosis. Blood. 2003;101:827-830.
15. Palladini G, Perfetti V, Obici L, et al. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood. 2004;103:2936-2938.
16. Skinner M, Sanchorawala V, Seldin DC, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004;140:85-93.
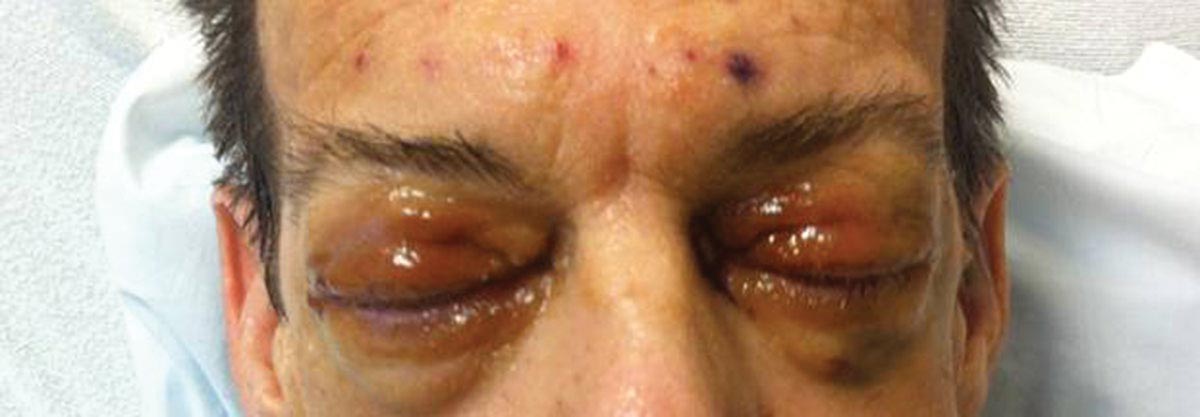
A 57-year-old man who underwent an orthotopic liver transplant 10 months prior for autoimmune hepatitis developed a rash on the trunk, arms, and legs. A dermatology consultation was requested. On evaluation, the patient was noted to have purpuric patches on the trunk and extremities, as well as prominent, bilateral, waxy and indurated periorbital plaques. The patient’s medical history revealed anemia, osteopenia, multiple bone fractures, and renal impairment.
Multiple Papules on the Eyelid Margin
The Diagnosis: Molluscum Contagiosum
Dermoscopy showed multiple whitish amorphous structures with peripheral blood vessels (Figure 1). A skin biopsy specimen from the lower eyelid revealed loculated and endophytic epidermal hyperplasia. The keratinocytes contained large eosinophilic intracytoplasmic inclusion bodies, and the diagnosis of molluscum contagiosum (MC) was confirmed (Figure 2). Laboratory results were positive for human immunodeficiency virus (HIV) infection with a CD4 lymphocyte count of 18 cells/mm3 and viral load of 199,686 copies/mL. The patient was treated with CO2 laser therapy for eyelid lesions. A regimen of highly active antiretroviral therapy (HAART) was later started using a combination of lamivudine-zidovudine (150 mg and 300 mg) as well as lopinavir-ritonavir (400 mg and 100 mg), both twice daily. There was no recurrence at 3-month follow-up.
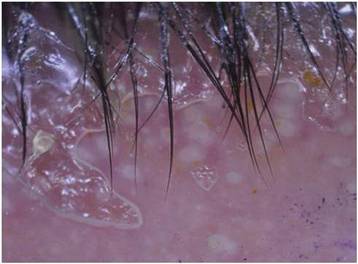 |
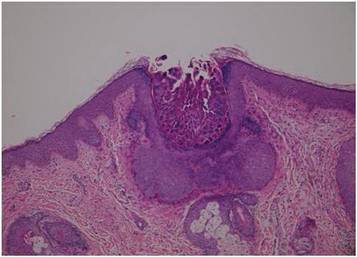 |
Molluscum contagiosum is a common cutaneous infection that is caused by a double-stranded DNA poxvirus. The clinical manifestations of MC are solitary or multiple, tiny, dome-shaped, pale, waxy or flesh-colored papules with central umbilication. The skin lesions can be located anywhere on the body. It occurs mostly in children, but adults also may be affected. In patients with atopic dermatitis or immunocompromised status such as AIDS, acute lymphoblastic leukemia, multiple myeloma, hyperimmunoglobulin E syndrome, or treatment with prednisone and methotrexate, the cutaneous lesions may be more extensive with an atypical presentation.1-6
The diagnosis of MC is mainly made by clinical inspection. However, Giemsa staining, Papanicolaou tests, and histopathology are useful for diagnosis of atypical MC.7 Dermoscopy is a noninvasive and fast diagnostic tool for MC.8,9 In dermoscopy, MC is characterized by multiple spherical, whitish, amorphous structures with a crown of blood vessels surrounding the periphery, termed red corona.8,9 The whitish amorphous structures and red corona correlate with inclusion bodies and dermal dilated blood vessels, respectively.
Approximately 13% of HIV patients have cutaneous MC, and the lesions tend to be more diffuse and refractory to treatment.10 A giant variant and abscess formation also have been described.11,12 Molluscum contagiosum of the eyelids often occurs in advanced HIV infection with a CD4 count less than 80 cells/mm3.1 These patients often have been diagnosed with HIV before developing eyelid MC. The severity of MC in immunocompromised patients may be related to the deficits of cell-mediated immunity, especially the T helper 1 (TH1) cytokine pathway. One case report also showed the clinical remission of MC after restoration of CD4 count with HAART.13 Our patient was not previously diagnosed with HIV and the MC of the eyelid margin was the early presentation of AIDS.
Molluscum contagiosum of the eyelids may cause chronic keratoconjunctivitis or even vascular infiltration and scarring of the peripheral cornea.14 These manifestations may be attributed to a hypersensitivity reaction to viral protein in tear film. Therefore, individuals with eyelid MC should accept thorough examination of the conjunctiva and cornea.
Treatment options include surgical excision, CO2 laser, curettage, and hyperfocal cryotherapy.15 Several reports also have demonstrated effectiveness of cidofovir for treatment of extensive MC lesions.16 Highly active antiretroviral therapy may play a role in the treatment of patients with AIDS by restoring the CD4 count.13 However, a few patients may develop immune reconstitution inflammatory syndrome, an intensive inflammatory reaction to pathogens after HAART, leading to paradoxical worsening of existing infection. Spontaneous corneal perforation due to immune reconstitution inflammatory syndrome in a case with eyelid and conjunctival MC has been reported.17 Therefore, physicians should perform MC therapy before HAART and mucocutaneous lesions should be followed regularly to prevent possible morbidity.
In summary, we report a case of AIDS with the initial presentation of MC on the eyelid margin. Physicians should test for HIV infection in patients with an atypical presentation of MC. The ocular mucosa also should be examined in patients with MC of the eyelid to prevent possible complications.
1. Pérez-Blázquez E, Villafruela I, Madero S. Eyelid molluscum contagiosum in patients with human immunodeficiency virus infection. Orbit. 1999;18:75-81.
2. Ozyürek E, Sentürk N, Kefeli M, et al. Ulcerating molluscum contagiosum in a boy with relapsed acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33:e114-e116.
3. Moradi P, Bhogal M, Thaung C, et al. Epibulbar molluscum contagiosum lesions in multiple myeloma. Cornea. 2011;30:910-911.
4. Rosenberg EW, Yusk JW. Molluscum contagiosum. eruption following treatment with prednisone and methotrexate. Arch Dermatol. 1970;101:439-441.
5. Yang CH, Lee WI, Hsu TS. Disseminated white papules. Arch Dermatol. 2006;142:775-780.
6. Fotiadou C, Lazaridou E, Lekkas D, et al. Disseminated, eruptive molluscum contagiosum lesions in a psoriasis patient under treatment with methotrexate and cyclosporine. Eur J Dermatol. 2012;22:147-148.
7. Kumar N, Okiro P, Wasike R. Cytological diagnosis of molluscum contagiosum with an unusual clinical presentation at an unusual site. J Dermatol Case Rep. 2010;4:63-65.
8. Micali G, Lacarrubba F. Augmented diagnostic capability using videodermatoscopy on selected infectious and non-infectious penile growths. Int J Dermatol. 2011;50:1501-1505.
9. Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146.
10. Tzung TY, Yang CY, Chao SC, et al. Cutaneous manifestations of human immunodeficiency virus infection in Taiwan. Kaohsiung J Med Sci. 2004;20:216-224.
11. Chang W-Y, Chang C-P, Yang S-A, et al. Giant molluscum contagiosum with concurrence of molluscum dermatitis. Dermatol Sinica. 2005;23:81-85.
12. Bates CM, Carey PB, Dhar J, et al. Molluscum contagiosum—a novel presentation. Int J STD AIDS. 2001;12:614-615.
13. Schulz D, Sarra GM, Koerner UB, et al. Evolution of HIV-1-related conjunctival molluscum contagiosum under HAART: report of a bilaterally manifesting case and literature review. Graefes Arch Clin Exp Ophthalmol. 2004;242:951-955.
14. Redmond RM. Molluscum contagiosum is not always benign. BMJ. 2004;329:403.
15. Bardenstein DS, Elmets C. Hyperfocal cryotherapy of multiple molluscum contagiosum lesions in patients with the acquired immune deficiency syndrome. Ophthalmology. 1995;102:131-134.
16. Erickson C, Driscoll M, Gaspari A. Efficacy of intravenous cidofovir in the treatment of giant molluscum contagiosum in a patient with human immunodeficiency virus. Arch Dermatol. 2011;147:652-654.
17. Williamson W, Dorot N, Mortemousque B, et al. Spontaneous corneal perforation and conjunctival molluscum contagiosum in a AIDS patient [in French]. J Fr Ophtalmol. 1995;18:703-707.
The Diagnosis: Molluscum Contagiosum
Dermoscopy showed multiple whitish amorphous structures with peripheral blood vessels (Figure 1). A skin biopsy specimen from the lower eyelid revealed loculated and endophytic epidermal hyperplasia. The keratinocytes contained large eosinophilic intracytoplasmic inclusion bodies, and the diagnosis of molluscum contagiosum (MC) was confirmed (Figure 2). Laboratory results were positive for human immunodeficiency virus (HIV) infection with a CD4 lymphocyte count of 18 cells/mm3 and viral load of 199,686 copies/mL. The patient was treated with CO2 laser therapy for eyelid lesions. A regimen of highly active antiretroviral therapy (HAART) was later started using a combination of lamivudine-zidovudine (150 mg and 300 mg) as well as lopinavir-ritonavir (400 mg and 100 mg), both twice daily. There was no recurrence at 3-month follow-up.
 |
 |
Molluscum contagiosum is a common cutaneous infection that is caused by a double-stranded DNA poxvirus. The clinical manifestations of MC are solitary or multiple, tiny, dome-shaped, pale, waxy or flesh-colored papules with central umbilication. The skin lesions can be located anywhere on the body. It occurs mostly in children, but adults also may be affected. In patients with atopic dermatitis or immunocompromised status such as AIDS, acute lymphoblastic leukemia, multiple myeloma, hyperimmunoglobulin E syndrome, or treatment with prednisone and methotrexate, the cutaneous lesions may be more extensive with an atypical presentation.1-6
The diagnosis of MC is mainly made by clinical inspection. However, Giemsa staining, Papanicolaou tests, and histopathology are useful for diagnosis of atypical MC.7 Dermoscopy is a noninvasive and fast diagnostic tool for MC.8,9 In dermoscopy, MC is characterized by multiple spherical, whitish, amorphous structures with a crown of blood vessels surrounding the periphery, termed red corona.8,9 The whitish amorphous structures and red corona correlate with inclusion bodies and dermal dilated blood vessels, respectively.
Approximately 13% of HIV patients have cutaneous MC, and the lesions tend to be more diffuse and refractory to treatment.10 A giant variant and abscess formation also have been described.11,12 Molluscum contagiosum of the eyelids often occurs in advanced HIV infection with a CD4 count less than 80 cells/mm3.1 These patients often have been diagnosed with HIV before developing eyelid MC. The severity of MC in immunocompromised patients may be related to the deficits of cell-mediated immunity, especially the T helper 1 (TH1) cytokine pathway. One case report also showed the clinical remission of MC after restoration of CD4 count with HAART.13 Our patient was not previously diagnosed with HIV and the MC of the eyelid margin was the early presentation of AIDS.
Molluscum contagiosum of the eyelids may cause chronic keratoconjunctivitis or even vascular infiltration and scarring of the peripheral cornea.14 These manifestations may be attributed to a hypersensitivity reaction to viral protein in tear film. Therefore, individuals with eyelid MC should accept thorough examination of the conjunctiva and cornea.
Treatment options include surgical excision, CO2 laser, curettage, and hyperfocal cryotherapy.15 Several reports also have demonstrated effectiveness of cidofovir for treatment of extensive MC lesions.16 Highly active antiretroviral therapy may play a role in the treatment of patients with AIDS by restoring the CD4 count.13 However, a few patients may develop immune reconstitution inflammatory syndrome, an intensive inflammatory reaction to pathogens after HAART, leading to paradoxical worsening of existing infection. Spontaneous corneal perforation due to immune reconstitution inflammatory syndrome in a case with eyelid and conjunctival MC has been reported.17 Therefore, physicians should perform MC therapy before HAART and mucocutaneous lesions should be followed regularly to prevent possible morbidity.
In summary, we report a case of AIDS with the initial presentation of MC on the eyelid margin. Physicians should test for HIV infection in patients with an atypical presentation of MC. The ocular mucosa also should be examined in patients with MC of the eyelid to prevent possible complications.
The Diagnosis: Molluscum Contagiosum
Dermoscopy showed multiple whitish amorphous structures with peripheral blood vessels (Figure 1). A skin biopsy specimen from the lower eyelid revealed loculated and endophytic epidermal hyperplasia. The keratinocytes contained large eosinophilic intracytoplasmic inclusion bodies, and the diagnosis of molluscum contagiosum (MC) was confirmed (Figure 2). Laboratory results were positive for human immunodeficiency virus (HIV) infection with a CD4 lymphocyte count of 18 cells/mm3 and viral load of 199,686 copies/mL. The patient was treated with CO2 laser therapy for eyelid lesions. A regimen of highly active antiretroviral therapy (HAART) was later started using a combination of lamivudine-zidovudine (150 mg and 300 mg) as well as lopinavir-ritonavir (400 mg and 100 mg), both twice daily. There was no recurrence at 3-month follow-up.
 |
 |
Molluscum contagiosum is a common cutaneous infection that is caused by a double-stranded DNA poxvirus. The clinical manifestations of MC are solitary or multiple, tiny, dome-shaped, pale, waxy or flesh-colored papules with central umbilication. The skin lesions can be located anywhere on the body. It occurs mostly in children, but adults also may be affected. In patients with atopic dermatitis or immunocompromised status such as AIDS, acute lymphoblastic leukemia, multiple myeloma, hyperimmunoglobulin E syndrome, or treatment with prednisone and methotrexate, the cutaneous lesions may be more extensive with an atypical presentation.1-6
The diagnosis of MC is mainly made by clinical inspection. However, Giemsa staining, Papanicolaou tests, and histopathology are useful for diagnosis of atypical MC.7 Dermoscopy is a noninvasive and fast diagnostic tool for MC.8,9 In dermoscopy, MC is characterized by multiple spherical, whitish, amorphous structures with a crown of blood vessels surrounding the periphery, termed red corona.8,9 The whitish amorphous structures and red corona correlate with inclusion bodies and dermal dilated blood vessels, respectively.
Approximately 13% of HIV patients have cutaneous MC, and the lesions tend to be more diffuse and refractory to treatment.10 A giant variant and abscess formation also have been described.11,12 Molluscum contagiosum of the eyelids often occurs in advanced HIV infection with a CD4 count less than 80 cells/mm3.1 These patients often have been diagnosed with HIV before developing eyelid MC. The severity of MC in immunocompromised patients may be related to the deficits of cell-mediated immunity, especially the T helper 1 (TH1) cytokine pathway. One case report also showed the clinical remission of MC after restoration of CD4 count with HAART.13 Our patient was not previously diagnosed with HIV and the MC of the eyelid margin was the early presentation of AIDS.
Molluscum contagiosum of the eyelids may cause chronic keratoconjunctivitis or even vascular infiltration and scarring of the peripheral cornea.14 These manifestations may be attributed to a hypersensitivity reaction to viral protein in tear film. Therefore, individuals with eyelid MC should accept thorough examination of the conjunctiva and cornea.
Treatment options include surgical excision, CO2 laser, curettage, and hyperfocal cryotherapy.15 Several reports also have demonstrated effectiveness of cidofovir for treatment of extensive MC lesions.16 Highly active antiretroviral therapy may play a role in the treatment of patients with AIDS by restoring the CD4 count.13 However, a few patients may develop immune reconstitution inflammatory syndrome, an intensive inflammatory reaction to pathogens after HAART, leading to paradoxical worsening of existing infection. Spontaneous corneal perforation due to immune reconstitution inflammatory syndrome in a case with eyelid and conjunctival MC has been reported.17 Therefore, physicians should perform MC therapy before HAART and mucocutaneous lesions should be followed regularly to prevent possible morbidity.
In summary, we report a case of AIDS with the initial presentation of MC on the eyelid margin. Physicians should test for HIV infection in patients with an atypical presentation of MC. The ocular mucosa also should be examined in patients with MC of the eyelid to prevent possible complications.
1. Pérez-Blázquez E, Villafruela I, Madero S. Eyelid molluscum contagiosum in patients with human immunodeficiency virus infection. Orbit. 1999;18:75-81.
2. Ozyürek E, Sentürk N, Kefeli M, et al. Ulcerating molluscum contagiosum in a boy with relapsed acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33:e114-e116.
3. Moradi P, Bhogal M, Thaung C, et al. Epibulbar molluscum contagiosum lesions in multiple myeloma. Cornea. 2011;30:910-911.
4. Rosenberg EW, Yusk JW. Molluscum contagiosum. eruption following treatment with prednisone and methotrexate. Arch Dermatol. 1970;101:439-441.
5. Yang CH, Lee WI, Hsu TS. Disseminated white papules. Arch Dermatol. 2006;142:775-780.
6. Fotiadou C, Lazaridou E, Lekkas D, et al. Disseminated, eruptive molluscum contagiosum lesions in a psoriasis patient under treatment with methotrexate and cyclosporine. Eur J Dermatol. 2012;22:147-148.
7. Kumar N, Okiro P, Wasike R. Cytological diagnosis of molluscum contagiosum with an unusual clinical presentation at an unusual site. J Dermatol Case Rep. 2010;4:63-65.
8. Micali G, Lacarrubba F. Augmented diagnostic capability using videodermatoscopy on selected infectious and non-infectious penile growths. Int J Dermatol. 2011;50:1501-1505.
9. Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146.
10. Tzung TY, Yang CY, Chao SC, et al. Cutaneous manifestations of human immunodeficiency virus infection in Taiwan. Kaohsiung J Med Sci. 2004;20:216-224.
11. Chang W-Y, Chang C-P, Yang S-A, et al. Giant molluscum contagiosum with concurrence of molluscum dermatitis. Dermatol Sinica. 2005;23:81-85.
12. Bates CM, Carey PB, Dhar J, et al. Molluscum contagiosum—a novel presentation. Int J STD AIDS. 2001;12:614-615.
13. Schulz D, Sarra GM, Koerner UB, et al. Evolution of HIV-1-related conjunctival molluscum contagiosum under HAART: report of a bilaterally manifesting case and literature review. Graefes Arch Clin Exp Ophthalmol. 2004;242:951-955.
14. Redmond RM. Molluscum contagiosum is not always benign. BMJ. 2004;329:403.
15. Bardenstein DS, Elmets C. Hyperfocal cryotherapy of multiple molluscum contagiosum lesions in patients with the acquired immune deficiency syndrome. Ophthalmology. 1995;102:131-134.
16. Erickson C, Driscoll M, Gaspari A. Efficacy of intravenous cidofovir in the treatment of giant molluscum contagiosum in a patient with human immunodeficiency virus. Arch Dermatol. 2011;147:652-654.
17. Williamson W, Dorot N, Mortemousque B, et al. Spontaneous corneal perforation and conjunctival molluscum contagiosum in a AIDS patient [in French]. J Fr Ophtalmol. 1995;18:703-707.
1. Pérez-Blázquez E, Villafruela I, Madero S. Eyelid molluscum contagiosum in patients with human immunodeficiency virus infection. Orbit. 1999;18:75-81.
2. Ozyürek E, Sentürk N, Kefeli M, et al. Ulcerating molluscum contagiosum in a boy with relapsed acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33:e114-e116.
3. Moradi P, Bhogal M, Thaung C, et al. Epibulbar molluscum contagiosum lesions in multiple myeloma. Cornea. 2011;30:910-911.
4. Rosenberg EW, Yusk JW. Molluscum contagiosum. eruption following treatment with prednisone and methotrexate. Arch Dermatol. 1970;101:439-441.
5. Yang CH, Lee WI, Hsu TS. Disseminated white papules. Arch Dermatol. 2006;142:775-780.
6. Fotiadou C, Lazaridou E, Lekkas D, et al. Disseminated, eruptive molluscum contagiosum lesions in a psoriasis patient under treatment with methotrexate and cyclosporine. Eur J Dermatol. 2012;22:147-148.
7. Kumar N, Okiro P, Wasike R. Cytological diagnosis of molluscum contagiosum with an unusual clinical presentation at an unusual site. J Dermatol Case Rep. 2010;4:63-65.
8. Micali G, Lacarrubba F. Augmented diagnostic capability using videodermatoscopy on selected infectious and non-infectious penile growths. Int J Dermatol. 2011;50:1501-1505.
9. Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146.
10. Tzung TY, Yang CY, Chao SC, et al. Cutaneous manifestations of human immunodeficiency virus infection in Taiwan. Kaohsiung J Med Sci. 2004;20:216-224.
11. Chang W-Y, Chang C-P, Yang S-A, et al. Giant molluscum contagiosum with concurrence of molluscum dermatitis. Dermatol Sinica. 2005;23:81-85.
12. Bates CM, Carey PB, Dhar J, et al. Molluscum contagiosum—a novel presentation. Int J STD AIDS. 2001;12:614-615.
13. Schulz D, Sarra GM, Koerner UB, et al. Evolution of HIV-1-related conjunctival molluscum contagiosum under HAART: report of a bilaterally manifesting case and literature review. Graefes Arch Clin Exp Ophthalmol. 2004;242:951-955.
14. Redmond RM. Molluscum contagiosum is not always benign. BMJ. 2004;329:403.
15. Bardenstein DS, Elmets C. Hyperfocal cryotherapy of multiple molluscum contagiosum lesions in patients with the acquired immune deficiency syndrome. Ophthalmology. 1995;102:131-134.
16. Erickson C, Driscoll M, Gaspari A. Efficacy of intravenous cidofovir in the treatment of giant molluscum contagiosum in a patient with human immunodeficiency virus. Arch Dermatol. 2011;147:652-654.
17. Williamson W, Dorot N, Mortemousque B, et al. Spontaneous corneal perforation and conjunctival molluscum contagiosum in a AIDS patient [in French]. J Fr Ophtalmol. 1995;18:703-707.
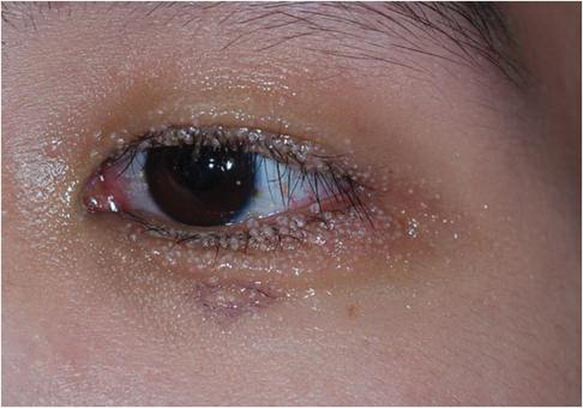
A 24-year-old man presented with multiple tiny papules over the left eyelid margin of 2 to 3 months’ duration. There were a couple of papules on the left upper eyelid initially, but they progressed to the upper and lower eyelid margin after scratching. On physical examination, multiple whitish to flesh-colored pearly papules measuring 1 to 2 mm were located on the left eyelid margin. Palpebral follicular conjunctivitis also was noted.
Tense Bullae With Widespread Erosions
The Diagnosis: Linear IgA Bullous Dermatosis
A biopsy specimen from an intact vesicle was obtained. Histologic findings showed a basket weave stratum corneum suggestive of an acute process. There was subepidermal separation with an inflammatory infiltrate of neutrophils (Figure 1). Direct immunofluorescence yielded a pattern of IgA deposition along the dermoepidermal junction (Figure 2). A diagnosis of linear IgA bullous dermatosis (LABD) was made. The patient was started on 100 mg daily of dapsone. The dose was subsequently increased to 175 mg twice daily, resulting in complete clearance. He became dermatologically disease free after 10 months and the dapsone was successfully tapered.
|
Linear IgA bullous dermatosis is an autoimmune subepidermal blistering disease with linear IgA deposits found along the basement membrane of the skin. There are 3 major categories of LABD: drug induced, systemic disorder related, and idiopathic.1 Patients with LABD present with a pruritic vesicobullous eruption that tends to favor the trunk, proximal extremities, and acral regions of the body. Mucous membrane lesions are present in less than 50% of patients.2 Linear IgA bullous dermatosis may resemble bullous pemphigoid, erythema multiforme, dermatitis herpetiformis, or toxic epidermal necrolysis. The gold standard for diagnosis is immunofluorescence staining that shows linear IgA deposition along the skin’s basement membrane.1 Prognosis for LABD is variable; there is risk for persistence and scarring.2 The drug-induced form of LABD is associated with clearance with the removal of the inciting agent.1
There are several autoimmune disorders that have been described in association with human immunodeficiency virus (HIV).3 Autoimmune bullous dermatoses, while described, are very uncommon in the setting of HIV infection. Previously reported cases include bullous pemphigoid, epidermolysis bullosa acquisita, pemphigus herpetiformis, pemphigus vegetans, pemphigus vulgaris, and cicatricial pemphigoid.4-12 The presentation of LABD in an HIV-positive patient is extremely rare.
There are 3 proposed mechanisms by which HIV and autoimmune bullous dermatoses coexist: unregulated B-cell activation, loss of T-suppressor cell regulation, and molecular mimicry. In patients with HIV, infected macrophages increase production of IL-1 and IL-6, causing nonspecific stimulation of B cells. Further production of tumor necrosis factor and other lymphotoxins may kill CD8+ T-suppressor cells, which further reduces B-cell regulation and production of nonspecific antibodies. Unregulated B-cell activation could lead to proliferation of antiself-specific B cells and autoantibodies. Additionally, various autoantibodies may arise due to mimicry between HIV antigens and human proteins. Some of the antibodies produced may be cytotoxic antilymphocyte antibodies that further disrupt B-cell regulation.13,14
Zandman-Goddard and Shoenfeld14 proposed a staging system of autoimmune disease and HIV with respect to CD4 count and viral load. Stage I is clinical latency of HIV, with a high CD4 count (>500 cells/mm3) and high viral load, which correlates with an acute infection of HIV and an intact immune system. Autoimmune disease can be seen in this stage. Stage II is cellular response, a quiescent period without overt manifestations of AIDS. The CD4 count is declining (200–499 cells/mm3), indicating immunosuppression, and the viral count is high. Autoimmune disease can occur and typically includes immune complex–mediated disease and vasculitis. Stage III is immune deficiency. The CD4 count is low (<200 cells/mm3), viral load is high, and AIDS develops. Autoimmune disease is not seen during this stage. Stage IV is the period of immune restoration following the advent of highly active antiretroviral therapy. There is a high CD4 count (>500 cells/mm3) and low viral load. There is a resurgence of autoimmune disease in this stage. Autoimmune disease can occur with an immune system capable of B- and T-cell interactions and a normal CD4 count. Autoimmunity is possible in stages I, II, and IV.14 Our patient developed bullous disease in stage II.
Although uncommon, autoimmune disease is possible in the setting of immune deficiency. The presence of autoimmune disease in a patient with HIV can only be seen during certain stages of infection. Knowledge of the possible scenarios of autoimmune disease can assist the clinician with monitoring status of the HIV infection or immune reconstitution.
1. Bouldin MB, Clowers-Webb HE, Davis JL, et al. Naproxen-associated linear IgA bullous dermatosis: case report and review. Mayo Clin Proc. 2000;75:967-970.
2. Nousari HC, Kimyai-Asadi A, Caeiro JP, et al. Clinical, demographic, and immunohistologic features of vancomycin-induced linear IgA bullous disease of the skin: report of 2 cases and review of the literature. Medicine. 1999;78:1-8.
3. Gala S, Fulcher DA. How HIV leads to autoimmune disorders. Med J Aust. 1996;164:224-226.
4. Lateef A, Packles MR, White SM, et al. Pemphigus vegetans in association with human immunodeficiency virus. Int J Dermatol. 1999;38:778-781.
5. Levy PM, Balavoine JF, Salomon D, et al. Ritodrine-responsive bullous pemphigoing in a patient with AIDS-related complex. Br J Dermatol. 1986;114:635-636.
6. Bull RH, Fallowfield ME, Marsden RA. Autoimmune blistering diseases associated with HIV infection. Clin Exp Dermatol. 1994;19:47-50.
7. Chou K, Kauh YC, Jacoby RA, et al. Autoimmune bullous disease in a patient with HIV infection. J Am Acad Dermatol. 1991;24:1022-1023.
8. Mahé A, Flageul B, Prost C, et al. Pemphigus vegetans in an HIV-1-infected man. Clin Exp Dermatol. 1994;19:447.
9. Capizzi R, Marasca G, De Luca A, et al. Pemphigus vulgaris in a human-immunodeficiency-virus-infected patient. Dermatology. 1998;197:97-98.
10. Splaver A, Silos S, Lowell B, et al. Case report: pemphigus vulgaris in a patient infected with HIV. AIDS Patient Care STDS. 2000;14:295-296.
11. Hodgson TA, Fidler SJ, Speight PM, et al. Oral pemphigus vulgaris associated with HIV infection. J Am Acad Dermatol. 2003;49:313-315.
12. Demathé A, Arede LT, Miyahara GI. Mucous membrane pemphigoid in HIV patient: a case report. Cases J. 2008;1:345.
13. Etzioni A. Immune deficiency and autoimmunity. Autoimmun Rev. 2003;2:364-369.
14. Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329-337.
The Diagnosis: Linear IgA Bullous Dermatosis
A biopsy specimen from an intact vesicle was obtained. Histologic findings showed a basket weave stratum corneum suggestive of an acute process. There was subepidermal separation with an inflammatory infiltrate of neutrophils (Figure 1). Direct immunofluorescence yielded a pattern of IgA deposition along the dermoepidermal junction (Figure 2). A diagnosis of linear IgA bullous dermatosis (LABD) was made. The patient was started on 100 mg daily of dapsone. The dose was subsequently increased to 175 mg twice daily, resulting in complete clearance. He became dermatologically disease free after 10 months and the dapsone was successfully tapered.
|
Linear IgA bullous dermatosis is an autoimmune subepidermal blistering disease with linear IgA deposits found along the basement membrane of the skin. There are 3 major categories of LABD: drug induced, systemic disorder related, and idiopathic.1 Patients with LABD present with a pruritic vesicobullous eruption that tends to favor the trunk, proximal extremities, and acral regions of the body. Mucous membrane lesions are present in less than 50% of patients.2 Linear IgA bullous dermatosis may resemble bullous pemphigoid, erythema multiforme, dermatitis herpetiformis, or toxic epidermal necrolysis. The gold standard for diagnosis is immunofluorescence staining that shows linear IgA deposition along the skin’s basement membrane.1 Prognosis for LABD is variable; there is risk for persistence and scarring.2 The drug-induced form of LABD is associated with clearance with the removal of the inciting agent.1
There are several autoimmune disorders that have been described in association with human immunodeficiency virus (HIV).3 Autoimmune bullous dermatoses, while described, are very uncommon in the setting of HIV infection. Previously reported cases include bullous pemphigoid, epidermolysis bullosa acquisita, pemphigus herpetiformis, pemphigus vegetans, pemphigus vulgaris, and cicatricial pemphigoid.4-12 The presentation of LABD in an HIV-positive patient is extremely rare.
There are 3 proposed mechanisms by which HIV and autoimmune bullous dermatoses coexist: unregulated B-cell activation, loss of T-suppressor cell regulation, and molecular mimicry. In patients with HIV, infected macrophages increase production of IL-1 and IL-6, causing nonspecific stimulation of B cells. Further production of tumor necrosis factor and other lymphotoxins may kill CD8+ T-suppressor cells, which further reduces B-cell regulation and production of nonspecific antibodies. Unregulated B-cell activation could lead to proliferation of antiself-specific B cells and autoantibodies. Additionally, various autoantibodies may arise due to mimicry between HIV antigens and human proteins. Some of the antibodies produced may be cytotoxic antilymphocyte antibodies that further disrupt B-cell regulation.13,14
Zandman-Goddard and Shoenfeld14 proposed a staging system of autoimmune disease and HIV with respect to CD4 count and viral load. Stage I is clinical latency of HIV, with a high CD4 count (>500 cells/mm3) and high viral load, which correlates with an acute infection of HIV and an intact immune system. Autoimmune disease can be seen in this stage. Stage II is cellular response, a quiescent period without overt manifestations of AIDS. The CD4 count is declining (200–499 cells/mm3), indicating immunosuppression, and the viral count is high. Autoimmune disease can occur and typically includes immune complex–mediated disease and vasculitis. Stage III is immune deficiency. The CD4 count is low (<200 cells/mm3), viral load is high, and AIDS develops. Autoimmune disease is not seen during this stage. Stage IV is the period of immune restoration following the advent of highly active antiretroviral therapy. There is a high CD4 count (>500 cells/mm3) and low viral load. There is a resurgence of autoimmune disease in this stage. Autoimmune disease can occur with an immune system capable of B- and T-cell interactions and a normal CD4 count. Autoimmunity is possible in stages I, II, and IV.14 Our patient developed bullous disease in stage II.
Although uncommon, autoimmune disease is possible in the setting of immune deficiency. The presence of autoimmune disease in a patient with HIV can only be seen during certain stages of infection. Knowledge of the possible scenarios of autoimmune disease can assist the clinician with monitoring status of the HIV infection or immune reconstitution.
The Diagnosis: Linear IgA Bullous Dermatosis
A biopsy specimen from an intact vesicle was obtained. Histologic findings showed a basket weave stratum corneum suggestive of an acute process. There was subepidermal separation with an inflammatory infiltrate of neutrophils (Figure 1). Direct immunofluorescence yielded a pattern of IgA deposition along the dermoepidermal junction (Figure 2). A diagnosis of linear IgA bullous dermatosis (LABD) was made. The patient was started on 100 mg daily of dapsone. The dose was subsequently increased to 175 mg twice daily, resulting in complete clearance. He became dermatologically disease free after 10 months and the dapsone was successfully tapered.
|
Linear IgA bullous dermatosis is an autoimmune subepidermal blistering disease with linear IgA deposits found along the basement membrane of the skin. There are 3 major categories of LABD: drug induced, systemic disorder related, and idiopathic.1 Patients with LABD present with a pruritic vesicobullous eruption that tends to favor the trunk, proximal extremities, and acral regions of the body. Mucous membrane lesions are present in less than 50% of patients.2 Linear IgA bullous dermatosis may resemble bullous pemphigoid, erythema multiforme, dermatitis herpetiformis, or toxic epidermal necrolysis. The gold standard for diagnosis is immunofluorescence staining that shows linear IgA deposition along the skin’s basement membrane.1 Prognosis for LABD is variable; there is risk for persistence and scarring.2 The drug-induced form of LABD is associated with clearance with the removal of the inciting agent.1
There are several autoimmune disorders that have been described in association with human immunodeficiency virus (HIV).3 Autoimmune bullous dermatoses, while described, are very uncommon in the setting of HIV infection. Previously reported cases include bullous pemphigoid, epidermolysis bullosa acquisita, pemphigus herpetiformis, pemphigus vegetans, pemphigus vulgaris, and cicatricial pemphigoid.4-12 The presentation of LABD in an HIV-positive patient is extremely rare.
There are 3 proposed mechanisms by which HIV and autoimmune bullous dermatoses coexist: unregulated B-cell activation, loss of T-suppressor cell regulation, and molecular mimicry. In patients with HIV, infected macrophages increase production of IL-1 and IL-6, causing nonspecific stimulation of B cells. Further production of tumor necrosis factor and other lymphotoxins may kill CD8+ T-suppressor cells, which further reduces B-cell regulation and production of nonspecific antibodies. Unregulated B-cell activation could lead to proliferation of antiself-specific B cells and autoantibodies. Additionally, various autoantibodies may arise due to mimicry between HIV antigens and human proteins. Some of the antibodies produced may be cytotoxic antilymphocyte antibodies that further disrupt B-cell regulation.13,14
Zandman-Goddard and Shoenfeld14 proposed a staging system of autoimmune disease and HIV with respect to CD4 count and viral load. Stage I is clinical latency of HIV, with a high CD4 count (>500 cells/mm3) and high viral load, which correlates with an acute infection of HIV and an intact immune system. Autoimmune disease can be seen in this stage. Stage II is cellular response, a quiescent period without overt manifestations of AIDS. The CD4 count is declining (200–499 cells/mm3), indicating immunosuppression, and the viral count is high. Autoimmune disease can occur and typically includes immune complex–mediated disease and vasculitis. Stage III is immune deficiency. The CD4 count is low (<200 cells/mm3), viral load is high, and AIDS develops. Autoimmune disease is not seen during this stage. Stage IV is the period of immune restoration following the advent of highly active antiretroviral therapy. There is a high CD4 count (>500 cells/mm3) and low viral load. There is a resurgence of autoimmune disease in this stage. Autoimmune disease can occur with an immune system capable of B- and T-cell interactions and a normal CD4 count. Autoimmunity is possible in stages I, II, and IV.14 Our patient developed bullous disease in stage II.
Although uncommon, autoimmune disease is possible in the setting of immune deficiency. The presence of autoimmune disease in a patient with HIV can only be seen during certain stages of infection. Knowledge of the possible scenarios of autoimmune disease can assist the clinician with monitoring status of the HIV infection or immune reconstitution.
1. Bouldin MB, Clowers-Webb HE, Davis JL, et al. Naproxen-associated linear IgA bullous dermatosis: case report and review. Mayo Clin Proc. 2000;75:967-970.
2. Nousari HC, Kimyai-Asadi A, Caeiro JP, et al. Clinical, demographic, and immunohistologic features of vancomycin-induced linear IgA bullous disease of the skin: report of 2 cases and review of the literature. Medicine. 1999;78:1-8.
3. Gala S, Fulcher DA. How HIV leads to autoimmune disorders. Med J Aust. 1996;164:224-226.
4. Lateef A, Packles MR, White SM, et al. Pemphigus vegetans in association with human immunodeficiency virus. Int J Dermatol. 1999;38:778-781.
5. Levy PM, Balavoine JF, Salomon D, et al. Ritodrine-responsive bullous pemphigoing in a patient with AIDS-related complex. Br J Dermatol. 1986;114:635-636.
6. Bull RH, Fallowfield ME, Marsden RA. Autoimmune blistering diseases associated with HIV infection. Clin Exp Dermatol. 1994;19:47-50.
7. Chou K, Kauh YC, Jacoby RA, et al. Autoimmune bullous disease in a patient with HIV infection. J Am Acad Dermatol. 1991;24:1022-1023.
8. Mahé A, Flageul B, Prost C, et al. Pemphigus vegetans in an HIV-1-infected man. Clin Exp Dermatol. 1994;19:447.
9. Capizzi R, Marasca G, De Luca A, et al. Pemphigus vulgaris in a human-immunodeficiency-virus-infected patient. Dermatology. 1998;197:97-98.
10. Splaver A, Silos S, Lowell B, et al. Case report: pemphigus vulgaris in a patient infected with HIV. AIDS Patient Care STDS. 2000;14:295-296.
11. Hodgson TA, Fidler SJ, Speight PM, et al. Oral pemphigus vulgaris associated with HIV infection. J Am Acad Dermatol. 2003;49:313-315.
12. Demathé A, Arede LT, Miyahara GI. Mucous membrane pemphigoid in HIV patient: a case report. Cases J. 2008;1:345.
13. Etzioni A. Immune deficiency and autoimmunity. Autoimmun Rev. 2003;2:364-369.
14. Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329-337.
1. Bouldin MB, Clowers-Webb HE, Davis JL, et al. Naproxen-associated linear IgA bullous dermatosis: case report and review. Mayo Clin Proc. 2000;75:967-970.
2. Nousari HC, Kimyai-Asadi A, Caeiro JP, et al. Clinical, demographic, and immunohistologic features of vancomycin-induced linear IgA bullous disease of the skin: report of 2 cases and review of the literature. Medicine. 1999;78:1-8.
3. Gala S, Fulcher DA. How HIV leads to autoimmune disorders. Med J Aust. 1996;164:224-226.
4. Lateef A, Packles MR, White SM, et al. Pemphigus vegetans in association with human immunodeficiency virus. Int J Dermatol. 1999;38:778-781.
5. Levy PM, Balavoine JF, Salomon D, et al. Ritodrine-responsive bullous pemphigoing in a patient with AIDS-related complex. Br J Dermatol. 1986;114:635-636.
6. Bull RH, Fallowfield ME, Marsden RA. Autoimmune blistering diseases associated with HIV infection. Clin Exp Dermatol. 1994;19:47-50.
7. Chou K, Kauh YC, Jacoby RA, et al. Autoimmune bullous disease in a patient with HIV infection. J Am Acad Dermatol. 1991;24:1022-1023.
8. Mahé A, Flageul B, Prost C, et al. Pemphigus vegetans in an HIV-1-infected man. Clin Exp Dermatol. 1994;19:447.
9. Capizzi R, Marasca G, De Luca A, et al. Pemphigus vulgaris in a human-immunodeficiency-virus-infected patient. Dermatology. 1998;197:97-98.
10. Splaver A, Silos S, Lowell B, et al. Case report: pemphigus vulgaris in a patient infected with HIV. AIDS Patient Care STDS. 2000;14:295-296.
11. Hodgson TA, Fidler SJ, Speight PM, et al. Oral pemphigus vulgaris associated with HIV infection. J Am Acad Dermatol. 2003;49:313-315.
12. Demathé A, Arede LT, Miyahara GI. Mucous membrane pemphigoid in HIV patient: a case report. Cases J. 2008;1:345.
13. Etzioni A. Immune deficiency and autoimmunity. Autoimmun Rev. 2003;2:364-369.
14. Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329-337.
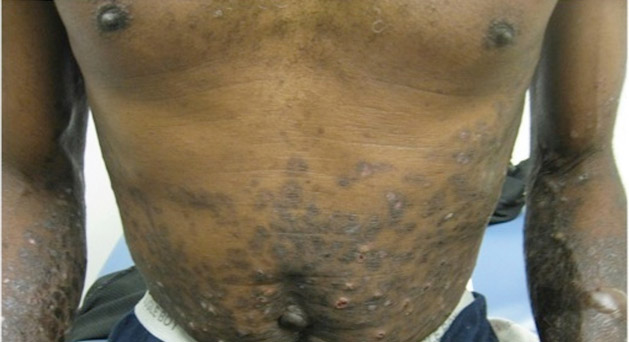
A 50-year-old black man presented with a new-onset widespread pruritic bullous eruption 7 months after being diagnosed with human immunodeficiency virus. The CD4 lymphocyte count was 421 cells/mm3 and viral load was 7818 copies/mL. Results of a viral culture were negative for herpes simplex virus. Dermatologic examination revealed numerous intact tense bullae as well as scattered erosions on the trunk and extremities. Postinflammatory hyperpigmentation was prominent, with some areas of hypopigmentation and depigmentation.
Woman Awakens With Rapid Heart Rate
ANSWER
The correct interpretation of this ECG is atrial flutter with a 2:1 block. Careful inspection of lead I reveals a P wave at the terminal portion of the QRS complex, in addition to the P wave seen with a consistent PR interval of 150 ms. This results in two P waves for each QRS complex. Given the presence of the flutter waves, an accurate assessment of the ST segment is not possible.
ANSWER
The correct interpretation of this ECG is atrial flutter with a 2:1 block. Careful inspection of lead I reveals a P wave at the terminal portion of the QRS complex, in addition to the P wave seen with a consistent PR interval of 150 ms. This results in two P waves for each QRS complex. Given the presence of the flutter waves, an accurate assessment of the ST segment is not possible.
ANSWER
The correct interpretation of this ECG is atrial flutter with a 2:1 block. Careful inspection of lead I reveals a P wave at the terminal portion of the QRS complex, in addition to the P wave seen with a consistent PR interval of 150 ms. This results in two P waves for each QRS complex. Given the presence of the flutter waves, an accurate assessment of the ST segment is not possible.
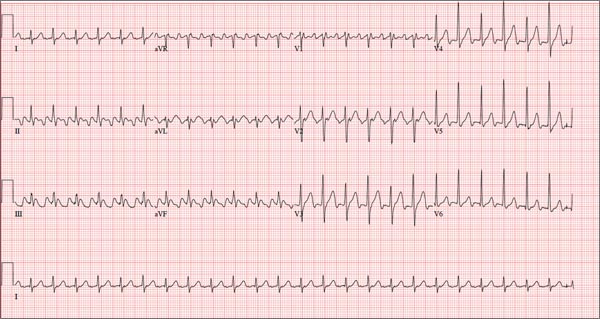
Three nights ago, a 44-year-old woman awoke with a regular, rapid heart rate that lasted about 15 minutes before abruptly terminating. The next morning, at the hospital where she works as an emergency department (ED) nurse, she had a colleague perform an undocumented ECG that, by the patient’s account, was normal. Early this morning, she was again awakened by a similar regular but rapid heart rate. Not wanting anyone at her facility to know about the problem, she presents to your ED instead. She denies chest pain but admits that she is slightly short of breath, adding that her symptoms remind her of how she feels when finishing a 10K run. The patient has been in excellent health with no underlying medical problems and no prior cardiac history. She is an avid runner, averaging three miles a day, and does not smoke. She does report drinking two or three glasses of wine in the evenings and admits she likes to party on the weekends, frequently consuming three or four margaritas with her coworkers on Saturday nights. She experimented with cannabis in college but hasn’t used illicit or recreational drugs since graduating. The patient is divorced, has a steady boyfriend, and has no children or siblings. Her parents are alive and well, with no history of arrhythmias, cardiovascular disease, or diabetes. She has no known drug allergies. Her medications include an oral contraceptive and occasional ibuprofen for soreness following exercise. The review of systems is remarkable for menses, which began yesterday. She denies that she is pregnant. Her vision is corrected with contact lenses. Physical examination reveals a thin, athletic-appearing woman who seems anxious. Her height is 67 in and her weight, 132 lb. Vital signs include a blood pressure of 118/68 mm Hg; pulse, 150 beats/min; respiratory rate, 14 breaths/min-1; and temperature, 37.8°C. The HEENT exam is normal with the presence of contact lenses. There is no thyromegaly. The lungs are clear in all fields. Her cardiac exam reveals a regular, rapid rate of 150 beats/min, without murmurs, rubs, or extra heart sounds. The abdomen is soft and nontender without palpable masses. The peripheral pulses are strong and equal bilaterally. There is no peripheral edema. The neurologic exam is intact. Laboratory tests, including a complete blood count, thyroid panel, and chemistry panel, are performed. All values are within normal limits. An ECG reveals a ventricular rate of 149 beats/min; PR interval, 150 ms; QRS interval, 102 ms; QT/QTc interval, 270/425 ms; P axis, 103°; R axis, 78°; and T axis, –18°. What is your interpretation of this ECG?