User login
Glucocorticoid-Induced Bone Loss: Dietary Supplementation Recommendations to Reduce the Risk for Osteoporosis and Osteoporotic Fractures
Glucocorticoids (GCs) are among the most widely prescribed medications in dermatologic practice. Although GCs are highly effective anti-inflammatory agents, long-term systemic therapy can result in dangerous adverse effects, including GC-induced osteoporosis (GIO), a bone disease associated with a heightened risk for fragility fractures.1,2 In the United States, an estimated 10.2 million adults have osteoporosis—defined as a T-score lower than −2.5 measured via a bone densitometry scan—and 43.4 million adults have low bone mineral density (BMD).3,4 The prevalence of osteoporosis is increasing, and the diagnosis is more common in females and adults 55 years and older.2 More than 2 million individuals have osteoporosis-related fractures annually, and the mortality risk is increased at 5 and 10 years following low-energy osteoporosis-related fractures.3-5
Glucocorticoid therapy is the leading iatrogenic cause of secondary osteoporosis. As many as 30% of all patients treated with systemic GCs for more than 6 months develop GIO.1,6,7 Glucocorticoid-induced BMD loss occurs at a rate of 6% to 12% of total BMD during the first year, slowing to approximately 3% per year during subsequent therapy.1 The risk for insufficiency fractures increases by as much as 75% from baseline in adults with rheumatic, pulmonary, and skin disorders within the first 3 months of therapy and peaks at approximately 12 months.1,2
Despite the risks, many long-term GC users never receive therapy to prevent bone loss; others are only started on therapy once they have sustained an insufficiency fracture. A 5-year international observational study including more than 40,000 postmenopausal women found that only 51% of patients who were on continuous GC therapy were undergoing BMD testing and appropriate medical management.8 This review highlights the existing evidence on the risks of osteoporosis and osteoporotic (OP) fractures in the setting of topical, intralesional, intramuscular, and systemic GC treatment, as well as recommendations for nutritional supplementation to reduce these risks.
Pathophysiology
The pathophysiology of GIO is multifactorial and occurs in both early and late phases.9,10 The early phase is characterized by rapid BMD reduction due to excessive bone resorption. The late phase is characterized by slower and more progressive BMD reduction due to impaired bone formation.9 At the osteocyte level, GCs decrease cell viability and induce apoptosis.11 At the osteoblast level, GCs impair cell replication and differentiation and have proapoptotic effects, resulting in decreased cell numbers and subsequent bone formation.10 At the osteoclast level, GCs increase expression of pro-osteoclastic cytokines and decrease mature osteoclast apoptosis, resulting in an expanded osteoclastic life span and prolonged bone resorption.12,13 Indirectly, GCs alter calcium metabolism by decreasing gastrointestinal calcium absorption and impairing renal absorption.14,15
GCs and Osteoporosis
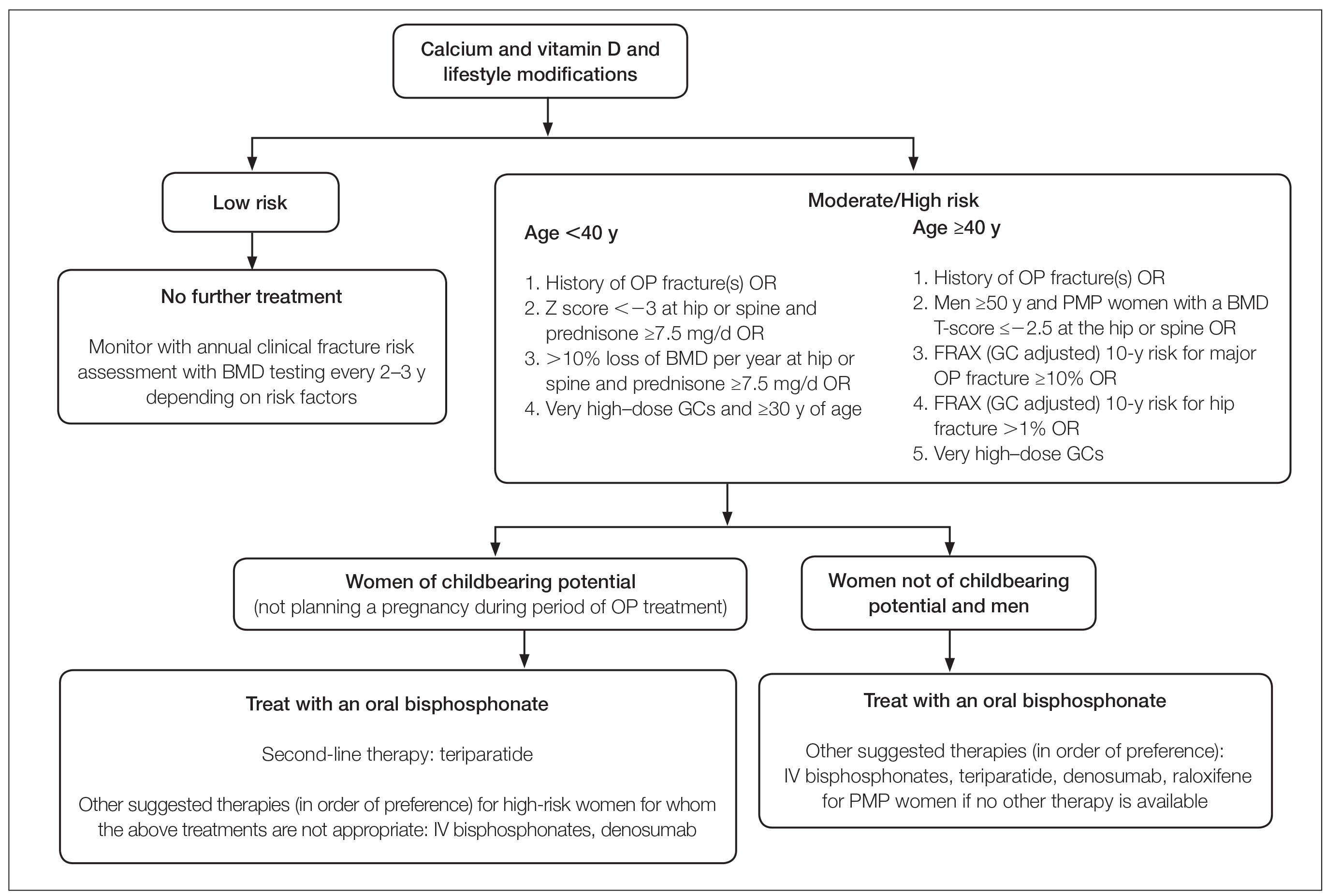
Oral GCs—Glucocorticoid-induced osteoporosis and fracture risk are dose and duration dependent.6 A study of 244,235 patients taking GCs and 244,235 controls found the relative risk of vertebral fracture was 1.55 (range, 1.20–2.01) for daily prednisone use at less than 2.5 mg, 2.59 (range, 2.16–3.10) for daily prednisone use from 2.5 to 7.4 mg, and 5.18 (range, 4.25–6.31) for daily doses of 7.5 mg or higher; the relative risk for hip fractures was 0.99 (range, 0.82–1.20), 1.77 (range, 1.55–2.02), and 2.27 (range, 1.94–2.66), respectively.16 Another large retrospective cohort study found that continuous treatment with prednisone 10 mg/d for more than 90 days compared to no GC exposure increased the risk for hip fractures 7-fold and 17-fold for vertebral fractures.17 Although the minimum cumulative dose of GCs known to cause osteoporosis is not clearly established, the American College of Rheumatology has proposed an algorithm as a basic approach to anticipate, prevent, and treat GIO (Figure).18,19 Fracture risk should be assessed in all patients who are prescribed prednisone 2.5 mg/d for 3 months or longer or an anticipated cumulative dose of more than 1 g per year. Patients 40 years and older with anticipated GC use of 3 months or longer should have both a bone densitometry scan and a Fracture Risk Assessment (FRAX) score. The FRAX tool estimates the 10-year probability of fracture in patients aged 40 to 80 years, and those patients can be further risk stratified as low (FRAX <10%), moderate (FRAX 10%–19%), or high (FRAX ≥20%) risk. In patients with moderate to high risk of fracture (FRAX >10%), initiation of pharmacologic treatment or referral to a metabolic bone specialist should be considered.18,19 First-line therapy is an oral bisphosphonate, and second-line therapies include intravenous bisphosphonates, teriparatide, denosumab, or raloxifene for patients at high risk for GIO.19 Adults younger than 40 years with a history of OP fracture or considerable risk factors for OP fractures should have a bone densitometry scan, and, if results are abnormal, the patient should be referred to a metabolic bone specialist. Those with low fracture risk based on bone densitometry and FRAX and those with no risk factors should be assessed annually for bone health (additional risk factors, GC dose and duration, bone densitometry/FRAX if indicated).18 In addition to GC dose and duration, additional risk factors for GIO, which are factored into the FRAX tool, include advanced age, low body mass index, history of bone fracture, smoking, excessive alcohol use (≥3 drinks/d), history of falls, low BMD, family history of bone fracture, and hypovitaminosis D.6
Topical GCs—Although there is strong evidence and clear guidelines regarding oral GIO, there is a dearth of data surrounding OP risk due to treatment with topical GCs. A recent retrospective nationwide Danish study evaluating the risk of osteoporosis and major OP fracture in 723,251 adults treated with potent or very potent topical steroids sought to evaluate these risks.20 Patients were included if they had filled prescriptions of at least 500 g of topical mometasone or an equivalent alternative. The investigators reported a 3% increase in relative risk of osteoporosis and major OP fracture with doubling of the cumulative topical GC dose (hazard ratio [HR], 1.03 [95% CI, 1.02-1.04] for both). The overall population-attributable risk was 4.3% (95% CI, 2.7%-5.8%) for osteoporosis and 2.7% (95% CI, 1.7%-3.8%) for major OP fracture. Notably, at least 10,000 g of mometasone was required for 1 additional patient to have a major OP fracture.20 In a commentary based on this study, Jackson21 noted that the number of patient-years of topical GC use needed for 1 fracture was 4-fold higher than that for high-dose oral GCs (40 mg/d prednisolone for ≥30 days). Another study assessed the effects of topical GCs on BMD in adults with moderate to severe atopic dermatitis over a 2-year period.22 No significant difference in BMD assessed via bone densitometry of either the lumbar spine or total hip at baseline or at 2-year follow-up was reported for either group treated with corticosteroids (<75 g per month or ≥75 g per month). Of note, the authors did not account for steroid potency, which ranged from class 1 through class 4.22 Although limited data exist, these studies suggest topical GCs used at conventional doses with appropriate breaks in therapy will not substantially increase risk for GIO or OP fracture; however, in the small subset of patients requiring chronic use of superpotent topical corticosteroids with other OP risk factors, transitioning to non–GC-based therapy or initiating bone health therapy may be advised to improve patient outcomes. Risk assessment, as in cases of chronic topical GC use, may be beneficial.
Intralesional GCs—Intralesional GCs are indicated for numerous inflammatory conditions including alopecia areata, discoid lupus erythematosus, keloids, and granuloma annulare. It generally is accepted that doses of triamcinolone acetonide should not exceed 20 mg per session spaced at least 3 weeks apart or up to 40 mg per month.18 One study demonstrated that doses of triamcinolone diacetate of 25 mg or less were unlikely to produce systemic effects and were determined to be a safe dose for intralesional injections.23 A retrospective cross-sectional case series including 18 patients with alopecia areata reported decreased BMD in 9 patients receiving intralesional triamcinolone acetonide 10 mg/mL at 4- to 8-week intervals for at least 20 months, with cumulative doses greater than 500 mg. This was particularly notable in postmenopausal women and men older than 50 years; participants with a body mass index less than 18.5 kg/m2, history of a stress fracture, family history of osteopenia or osteoporosis, and history of smoking; and those who did not regularly engage in weight-bearing exercises.24 Patients receiving long-term (ie, >1 year) intralesional steroids should be evaluated for osteoporosis risk and preventative strategies should be considered (ie, regular weight-bearing exercises, calcium and vitamin D supplementation, bisphosphate therapy). As with topical GCs, there are no clear guidelines for risk assessment or treatment recommendations for GIO.
Intramuscular GCs—The data regarding intramuscular (IM) GCs and dermatologic disease is severely limited, and to the best of our knowledge, no studies specifically assess the risk for GIO or fracture secondary to intramuscular GCs; however, a retrospective study of 27 patients (4 female, 23 male; mean age, 33 years [range, 12–61 years]) with refractory alopecia areata receiving IM triamcinolone acetonide (40 mg every 4 weeks for 3–6 months) reported 1 patient (a 56-year-old woman) with notably decreased bone densitometry from baseline requiring treatment discontinuation.25 No other patients at risk for osteoporosis had decreased BMD from treatment with IM triamcinolone; however, it was noted that 1 month following treatment, 10 of 11 assessed patients demonstrated decreased levels of morning serum cortisol and plasma adrenocorticotropic hormone—despite baseline levels within reference range—that resolved 3 months after treatment completion,25 which suggests a prolonged release of IM triamcinolone and sustained systemic effect. One systematic review of 342 patients with dermatologic diseases treated with IM corticosteroids found the primary side effects included dysmenorrhea, injection-site lipoatrophy, and adrenocortical suppression, with only a single reported case of low BMD.26 Given the paucity of evidence, additional studies are required to assess the effect of IM triamcinolone on BMD and risk for major OP fractures with regard to dosing and frequency. As there are no clear guidelines for osteoporosis evaluation in the setting of intramuscular GCs, it may be prudent to follow the algorithmic model recommended for oral steroids when anticipating at least 3 months of intramuscular GCs.
Diet and Prevention of Bone Loss
Given the profound impact that systemic GCs have on osteoporosis and fracture risk and the sparse data regarding risk from topical, intralesional, or intramuscular GCs, diet and nutrition represent a simple, safe, and potentially preventative method of slowing BMD loss and minimizing fracture risk. In higher-risk patients, nutritional assessment in combination with medical therapy also is likely warranted.
Calcium and Vitamin D3—Patients treated with any GC dose longer than 3 months should undergo calcium and vitamin D optimization.19 Exceptions for supplementation include certain patients with sarcoidosis, which can be associated with high vitamin D levels; patients with a history of hypercalcemia or hypercalciuria; and patients with chronic kidney disease.6 In a meta-analysis including 30,970 patients in 8 randomized controlled trials, calcium (500–1200 mg/d) and vitamin D (400–800 IU/d) supplementation reduced the risk of total fractures by 15% (summary relative risk estimate, 0.85 [95% CI, 0.73-0.98]) and hip fractures by 30% (summary relative risk estimate, 0.70 [95% CI, 0.56-0.87]).4 One double-blind, placebo-controlled clinical trial conducted by the Women’s Health Initiative that included 36,282 postmenopausal women who were taking 1000 mg of calcium and 400 IU of vitamin D3 daily for more than 5 years reported an HR of 0.62 (95% CI, 0.38-1.00) for hip fracture for supplementation vs placebo.27 Lastly, a 2016 Cochrane Review including 12 randomized trials and 1343 participants reported a 43% lower risk of new vertebral fractures following supplementation with calcium, vitamin D, or both compared with controls.28
Specific recommendations for calcium and vitamin D3 supplementation vary based on age and sex. The US Preventive Services Task Force concluded that insufficient evidence exists to support calcium and vitamin D3 supplementation in asymptomatic men and premenopausal women.29 The National Osteoporosis Foundation (NOF) supports the use of calcium supplementation for fracture risk reduction in middle-aged and older adults.4 Furthermore, the NOF supports the Institute of Medicine recommendations31 that men aged 50 to 70 years consume 1000 mg/d of calcium and that women 51 years and older as well as men 71 years and older consume 1200 mg/d of calcium.30 The NOF recommends 800 to 1000 IU/d of vitamin D in adults 50 years and older, while the Institute of Medicine recommends 600 IU/d in adults 70 years and younger and 800 IU/d in adults 71 years and older.31 These recommendations are similar to both the Endocrine Society and the American Geriatric Society.32,33 Total calcium should not exceed 2000 mg/d due to risk of adverse effects.
Dietary sources of vitamin D include fatty fish, mushrooms, and fortified dairy products, though recommended doses rarely can be achieved through diet alone.34 Dairy products are the primary source of dietary calcium. Other high-calcium foods include green leafy vegetables, nuts and seeds, soft-boned fish, and fortified beverages and cereals.35
Probiotics—A growing body of evidence suggests that probiotics may be beneficial in promoting bone health by improving calcium homeostasis, reducing risk for hyperparathyroidism secondary to GC therapy, and decreasing age-related bone resorption.36 An animal study demonstrated that probiotics can regulate bone resorption and formation as well as reduce bone loss secondary to GC therapy.37 A randomized, double-blind, placebo-controlled, multicenter trial randomly assigned 249 healthy, early postmenopausal women to receive probiotic treatment containing 3 lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and L plantarum DSM 15313) or placebo once daily for 12 months.38 Bone mineral density was measured at baseline and at 12 months. Of the 234 participants who completed the study, lactobacillus treatment reduced lumbosacral BMD loss compared to the placebo group (mean difference, 0.71% [95% CI, 0.06-1.35]). They also reported significant lumbosacral BMD loss in the placebo group (−0.72% [95% CI, −1.22 to −0.22]) compared to no BMD loss in the group treated with lactobacillus (−0.01% [95% CI, −0.50 to 0.48]).38 Although the data may be encouraging, more studies are needed to determine if probiotics should be regarded as an adjuvant treatment to calcium, vitamin D, and pharmacologic therapy for long-term prevention of bone loss in the setting of GIO.39 Because existing studies on probiotics include varying compositions and doses, larger studies with consistent supplementation are required. Encouraging probiotic intake through fermented dairy products may represent a simple low-risk intervention to support bone health.
Anti-inflammatory Diet—The traditional Mediterranean diet is rich in fruits, vegetables, fish, nuts, whole grains, legumes, and monounsaturated fats and low in meat and dairy products. The Mediterranean diet has been shown to be modestly protective against osteoporosis and fracture risk. A large US observational study including 93,676 women showed that those with the highest quintile of the alternate Mediterranean diet score had a lower risk for hip fracture (HR, 0.80 [95% CI, 0.66-0.97]), with an absolute risk reduction of 0.29% and number needed to treat at 342.40 A multicenter study involving adults from 8 European countries found that increased adherence to the Mediterranean diet was associated with a 7% reduction in hip fracture incidence (HR per 1 unit increase in Mediterranean diet, 0.93 [95% CI, 0.89-0.98]). High vegetable and fruit intake was associated with decreased hip fracture incidence (HR, 0.86 and 0.89 [95% CI, 0.79-0.94 and 0.82-0.97, respectively]), and high meat and excessive ethanol consumption were associated with increased fracture incidence (HR, 1.18 and 1.74 [95% CI, 1.06-1.31 and 1.32-2.31, respectively]).41 Similarly, a large observational study in Sweden that included 37,903 men and 33,403 women reported similar findings, noting a 6% lower hip fracture rate per one unit increase in alternate Mediterranean diet score (adjusted HR, 0.94 [95% CI, 0.92-0.96]).42 This is thought to be due in part to higher levels of dietary vitamin D present in many foods traditionally included in the Mediterranean diet.43 Additionally, olive oil, a staple in the Mediterranean diet, appears to reduce bone loss by promoting osteoblast proliferation and maturation, inhibiting bone resorption, suppressing oxidative stress and inflammation, and increasing calcium deposition in the extracellular matrix.44,45 Fruits, vegetables, legumes, and nuts also are rich in minerals including potassium and magnesium, which are important in bone health to promote osteoblast proliferation and vitamin D activation.36,46-48
Final Thoughts
Osteoporosis-related fractures are common and are associated with high morbidity and health care costs. Dermatologists using and prescribing corticosteroids must be aware of the risk for GIO, particularly in patients with a pre-existing diagnosis of osteopenia or osteoporosis. There likely is no oral corticosteroid dose that does not increase a patient’s risk for osteoporosis; therefore, oral GCs should be used at the lowest effective daily dose for the shortest duration possible. Patients with an anticipated duration of at least 3 months—regardless of dose—should be assessed for their risk for GIO. Patients using topical and intralesional corticosteroids are unlikely to develop GIO; however, those with risk factors and a considerable cumulative dose may warrant further evaluation. In all cases, we advocate for supplementing with calcium and vitamin D as well as promoting probiotic intake and the Mediterranean diet. Those at moderate to high risk for fracture may require additional medical therapy. Dermatologists are uniquely positioned to identify this at-risk population, and because osteoporosis is a chronic illness, primary care providers should be notified of prolonged GC therapy to help with risk assessment, initiation of vitamin and mineral supplementation, and follow-up with metabolic bone health specialists. Through a multidisciplinary approach and patient education, GIO and the potential risk for fracture can be successfully mitigated in most patients.
- Weinstein RS. Clinical practice. glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62-70.
- Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547-2556.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376.
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
- Caplan A, Fett N, Rosenbach M, et al. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1-9.
- Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32-36.
- Silverman S, Curtis J, Saag K, et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2015;26:419-420.
- Canalis E, Bilezikian JP, Angeli A, et al. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593-598.
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328.
- Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466-476.
- Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382-4389.
- Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592-5599.
- Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144-149.
- Huybers S, Naber TH, Bindels RJ, et al. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92-G97.
- Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993-1000.
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323-328.
- Lupsa BC, Insogna KL, Micheletti RG, et al. Corticosteroid use in chronic dermatologic disorders and osteoporosis. Int J Womens Dermatol. 2021;7:545-551.
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095-1110.
- Egeberg A, Schwarz P, Harsløf T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157:275-282.
- Jackson RD. Topical corticosteroids and glucocorticoid-induced osteoporosis-cumulative dose and duration matter. JAMA Dermatol. 2021;157:269-270.
- van Velsen SG, Haeck IM, Knol MJ, et al. Two-year assessment of effect of topical corticosteroids on bone mineral density in adults with moderate to severe atopic dermatitis. J Am Acad Dermatol. 2012;66:691-693.
- McGugan AD, Shuster S, Bottoms E. Adrenal suppression from intradermal triamcinolone. J Invest Dermatol. 1963;40:271-272.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Seo J, Lee YI, Hwang S, et al. Intramuscular triamcinolone acetonide: an undervalued option for refractory alopecia areata. J Dermatol. 2017;44:173-179.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567-580.
- Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi:10.1002/14651858.CD001347.pub2
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Vitamin D fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated August 12, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Calcium fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated June 2, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986.
- Schepper JD, Collins F, Rios-Arce ND, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801-820.
- Jansson PA, Curiac D, Ahrén IL, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:E154-E162.
- Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273-284.
- Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the Women’s Health Initiative. JAMA Intern Med. 2016;176:645-652.
- Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24:1587-1598.
- Byberg L, Bellavia A, Larsson SC, et al. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31:2098-2105.
- Zupo R, Lampignano L, Lattanzio A, et al. Association between adherence to the Mediterranean diet and circulating vitamin D levels. Int J Food Sci Nutr. 2020;71:884-890.
- Chin KY, Ima-Nirwana S. Olives and bone: a green osteoporosis prevention option. Int J Environ Res Public Health. 2016;13:755.
- García-Martínez O, Rivas A, Ramos-Torrecillas J, et al. The effect of olive oil on osteoporosis prevention. Int J Food Sci Nutr. 2014;65:834-840.
- Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181-189.
- Veronese N, Stubbs B, Solmi M, et al. Dietary magnesium intake and fracture risk: data from a large prospective study. Br J Nutr. 2017;117:1570-1576.
- Kong SH, Kim JH, Hong AR, et al. Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES)(2008-2011). Osteoporos Int. 2017;28:1577-1585.
Glucocorticoids (GCs) are among the most widely prescribed medications in dermatologic practice. Although GCs are highly effective anti-inflammatory agents, long-term systemic therapy can result in dangerous adverse effects, including GC-induced osteoporosis (GIO), a bone disease associated with a heightened risk for fragility fractures.1,2 In the United States, an estimated 10.2 million adults have osteoporosis—defined as a T-score lower than −2.5 measured via a bone densitometry scan—and 43.4 million adults have low bone mineral density (BMD).3,4 The prevalence of osteoporosis is increasing, and the diagnosis is more common in females and adults 55 years and older.2 More than 2 million individuals have osteoporosis-related fractures annually, and the mortality risk is increased at 5 and 10 years following low-energy osteoporosis-related fractures.3-5
Glucocorticoid therapy is the leading iatrogenic cause of secondary osteoporosis. As many as 30% of all patients treated with systemic GCs for more than 6 months develop GIO.1,6,7 Glucocorticoid-induced BMD loss occurs at a rate of 6% to 12% of total BMD during the first year, slowing to approximately 3% per year during subsequent therapy.1 The risk for insufficiency fractures increases by as much as 75% from baseline in adults with rheumatic, pulmonary, and skin disorders within the first 3 months of therapy and peaks at approximately 12 months.1,2
Despite the risks, many long-term GC users never receive therapy to prevent bone loss; others are only started on therapy once they have sustained an insufficiency fracture. A 5-year international observational study including more than 40,000 postmenopausal women found that only 51% of patients who were on continuous GC therapy were undergoing BMD testing and appropriate medical management.8 This review highlights the existing evidence on the risks of osteoporosis and osteoporotic (OP) fractures in the setting of topical, intralesional, intramuscular, and systemic GC treatment, as well as recommendations for nutritional supplementation to reduce these risks.
Pathophysiology
The pathophysiology of GIO is multifactorial and occurs in both early and late phases.9,10 The early phase is characterized by rapid BMD reduction due to excessive bone resorption. The late phase is characterized by slower and more progressive BMD reduction due to impaired bone formation.9 At the osteocyte level, GCs decrease cell viability and induce apoptosis.11 At the osteoblast level, GCs impair cell replication and differentiation and have proapoptotic effects, resulting in decreased cell numbers and subsequent bone formation.10 At the osteoclast level, GCs increase expression of pro-osteoclastic cytokines and decrease mature osteoclast apoptosis, resulting in an expanded osteoclastic life span and prolonged bone resorption.12,13 Indirectly, GCs alter calcium metabolism by decreasing gastrointestinal calcium absorption and impairing renal absorption.14,15
GCs and Osteoporosis
Oral GCs—Glucocorticoid-induced osteoporosis and fracture risk are dose and duration dependent.6 A study of 244,235 patients taking GCs and 244,235 controls found the relative risk of vertebral fracture was 1.55 (range, 1.20–2.01) for daily prednisone use at less than 2.5 mg, 2.59 (range, 2.16–3.10) for daily prednisone use from 2.5 to 7.4 mg, and 5.18 (range, 4.25–6.31) for daily doses of 7.5 mg or higher; the relative risk for hip fractures was 0.99 (range, 0.82–1.20), 1.77 (range, 1.55–2.02), and 2.27 (range, 1.94–2.66), respectively.16 Another large retrospective cohort study found that continuous treatment with prednisone 10 mg/d for more than 90 days compared to no GC exposure increased the risk for hip fractures 7-fold and 17-fold for vertebral fractures.17 Although the minimum cumulative dose of GCs known to cause osteoporosis is not clearly established, the American College of Rheumatology has proposed an algorithm as a basic approach to anticipate, prevent, and treat GIO (Figure).18,19 Fracture risk should be assessed in all patients who are prescribed prednisone 2.5 mg/d for 3 months or longer or an anticipated cumulative dose of more than 1 g per year. Patients 40 years and older with anticipated GC use of 3 months or longer should have both a bone densitometry scan and a Fracture Risk Assessment (FRAX) score. The FRAX tool estimates the 10-year probability of fracture in patients aged 40 to 80 years, and those patients can be further risk stratified as low (FRAX <10%), moderate (FRAX 10%–19%), or high (FRAX ≥20%) risk. In patients with moderate to high risk of fracture (FRAX >10%), initiation of pharmacologic treatment or referral to a metabolic bone specialist should be considered.18,19 First-line therapy is an oral bisphosphonate, and second-line therapies include intravenous bisphosphonates, teriparatide, denosumab, or raloxifene for patients at high risk for GIO.19 Adults younger than 40 years with a history of OP fracture or considerable risk factors for OP fractures should have a bone densitometry scan, and, if results are abnormal, the patient should be referred to a metabolic bone specialist. Those with low fracture risk based on bone densitometry and FRAX and those with no risk factors should be assessed annually for bone health (additional risk factors, GC dose and duration, bone densitometry/FRAX if indicated).18 In addition to GC dose and duration, additional risk factors for GIO, which are factored into the FRAX tool, include advanced age, low body mass index, history of bone fracture, smoking, excessive alcohol use (≥3 drinks/d), history of falls, low BMD, family history of bone fracture, and hypovitaminosis D.6

Topical GCs—Although there is strong evidence and clear guidelines regarding oral GIO, there is a dearth of data surrounding OP risk due to treatment with topical GCs. A recent retrospective nationwide Danish study evaluating the risk of osteoporosis and major OP fracture in 723,251 adults treated with potent or very potent topical steroids sought to evaluate these risks.20 Patients were included if they had filled prescriptions of at least 500 g of topical mometasone or an equivalent alternative. The investigators reported a 3% increase in relative risk of osteoporosis and major OP fracture with doubling of the cumulative topical GC dose (hazard ratio [HR], 1.03 [95% CI, 1.02-1.04] for both). The overall population-attributable risk was 4.3% (95% CI, 2.7%-5.8%) for osteoporosis and 2.7% (95% CI, 1.7%-3.8%) for major OP fracture. Notably, at least 10,000 g of mometasone was required for 1 additional patient to have a major OP fracture.20 In a commentary based on this study, Jackson21 noted that the number of patient-years of topical GC use needed for 1 fracture was 4-fold higher than that for high-dose oral GCs (40 mg/d prednisolone for ≥30 days). Another study assessed the effects of topical GCs on BMD in adults with moderate to severe atopic dermatitis over a 2-year period.22 No significant difference in BMD assessed via bone densitometry of either the lumbar spine or total hip at baseline or at 2-year follow-up was reported for either group treated with corticosteroids (<75 g per month or ≥75 g per month). Of note, the authors did not account for steroid potency, which ranged from class 1 through class 4.22 Although limited data exist, these studies suggest topical GCs used at conventional doses with appropriate breaks in therapy will not substantially increase risk for GIO or OP fracture; however, in the small subset of patients requiring chronic use of superpotent topical corticosteroids with other OP risk factors, transitioning to non–GC-based therapy or initiating bone health therapy may be advised to improve patient outcomes. Risk assessment, as in cases of chronic topical GC use, may be beneficial.
Intralesional GCs—Intralesional GCs are indicated for numerous inflammatory conditions including alopecia areata, discoid lupus erythematosus, keloids, and granuloma annulare. It generally is accepted that doses of triamcinolone acetonide should not exceed 20 mg per session spaced at least 3 weeks apart or up to 40 mg per month.18 One study demonstrated that doses of triamcinolone diacetate of 25 mg or less were unlikely to produce systemic effects and were determined to be a safe dose for intralesional injections.23 A retrospective cross-sectional case series including 18 patients with alopecia areata reported decreased BMD in 9 patients receiving intralesional triamcinolone acetonide 10 mg/mL at 4- to 8-week intervals for at least 20 months, with cumulative doses greater than 500 mg. This was particularly notable in postmenopausal women and men older than 50 years; participants with a body mass index less than 18.5 kg/m2, history of a stress fracture, family history of osteopenia or osteoporosis, and history of smoking; and those who did not regularly engage in weight-bearing exercises.24 Patients receiving long-term (ie, >1 year) intralesional steroids should be evaluated for osteoporosis risk and preventative strategies should be considered (ie, regular weight-bearing exercises, calcium and vitamin D supplementation, bisphosphate therapy). As with topical GCs, there are no clear guidelines for risk assessment or treatment recommendations for GIO.
Intramuscular GCs—The data regarding intramuscular (IM) GCs and dermatologic disease is severely limited, and to the best of our knowledge, no studies specifically assess the risk for GIO or fracture secondary to intramuscular GCs; however, a retrospective study of 27 patients (4 female, 23 male; mean age, 33 years [range, 12–61 years]) with refractory alopecia areata receiving IM triamcinolone acetonide (40 mg every 4 weeks for 3–6 months) reported 1 patient (a 56-year-old woman) with notably decreased bone densitometry from baseline requiring treatment discontinuation.25 No other patients at risk for osteoporosis had decreased BMD from treatment with IM triamcinolone; however, it was noted that 1 month following treatment, 10 of 11 assessed patients demonstrated decreased levels of morning serum cortisol and plasma adrenocorticotropic hormone—despite baseline levels within reference range—that resolved 3 months after treatment completion,25 which suggests a prolonged release of IM triamcinolone and sustained systemic effect. One systematic review of 342 patients with dermatologic diseases treated with IM corticosteroids found the primary side effects included dysmenorrhea, injection-site lipoatrophy, and adrenocortical suppression, with only a single reported case of low BMD.26 Given the paucity of evidence, additional studies are required to assess the effect of IM triamcinolone on BMD and risk for major OP fractures with regard to dosing and frequency. As there are no clear guidelines for osteoporosis evaluation in the setting of intramuscular GCs, it may be prudent to follow the algorithmic model recommended for oral steroids when anticipating at least 3 months of intramuscular GCs.
Diet and Prevention of Bone Loss
Given the profound impact that systemic GCs have on osteoporosis and fracture risk and the sparse data regarding risk from topical, intralesional, or intramuscular GCs, diet and nutrition represent a simple, safe, and potentially preventative method of slowing BMD loss and minimizing fracture risk. In higher-risk patients, nutritional assessment in combination with medical therapy also is likely warranted.
Calcium and Vitamin D3—Patients treated with any GC dose longer than 3 months should undergo calcium and vitamin D optimization.19 Exceptions for supplementation include certain patients with sarcoidosis, which can be associated with high vitamin D levels; patients with a history of hypercalcemia or hypercalciuria; and patients with chronic kidney disease.6 In a meta-analysis including 30,970 patients in 8 randomized controlled trials, calcium (500–1200 mg/d) and vitamin D (400–800 IU/d) supplementation reduced the risk of total fractures by 15% (summary relative risk estimate, 0.85 [95% CI, 0.73-0.98]) and hip fractures by 30% (summary relative risk estimate, 0.70 [95% CI, 0.56-0.87]).4 One double-blind, placebo-controlled clinical trial conducted by the Women’s Health Initiative that included 36,282 postmenopausal women who were taking 1000 mg of calcium and 400 IU of vitamin D3 daily for more than 5 years reported an HR of 0.62 (95% CI, 0.38-1.00) for hip fracture for supplementation vs placebo.27 Lastly, a 2016 Cochrane Review including 12 randomized trials and 1343 participants reported a 43% lower risk of new vertebral fractures following supplementation with calcium, vitamin D, or both compared with controls.28
Specific recommendations for calcium and vitamin D3 supplementation vary based on age and sex. The US Preventive Services Task Force concluded that insufficient evidence exists to support calcium and vitamin D3 supplementation in asymptomatic men and premenopausal women.29 The National Osteoporosis Foundation (NOF) supports the use of calcium supplementation for fracture risk reduction in middle-aged and older adults.4 Furthermore, the NOF supports the Institute of Medicine recommendations31 that men aged 50 to 70 years consume 1000 mg/d of calcium and that women 51 years and older as well as men 71 years and older consume 1200 mg/d of calcium.30 The NOF recommends 800 to 1000 IU/d of vitamin D in adults 50 years and older, while the Institute of Medicine recommends 600 IU/d in adults 70 years and younger and 800 IU/d in adults 71 years and older.31 These recommendations are similar to both the Endocrine Society and the American Geriatric Society.32,33 Total calcium should not exceed 2000 mg/d due to risk of adverse effects.
Dietary sources of vitamin D include fatty fish, mushrooms, and fortified dairy products, though recommended doses rarely can be achieved through diet alone.34 Dairy products are the primary source of dietary calcium. Other high-calcium foods include green leafy vegetables, nuts and seeds, soft-boned fish, and fortified beverages and cereals.35
Probiotics—A growing body of evidence suggests that probiotics may be beneficial in promoting bone health by improving calcium homeostasis, reducing risk for hyperparathyroidism secondary to GC therapy, and decreasing age-related bone resorption.36 An animal study demonstrated that probiotics can regulate bone resorption and formation as well as reduce bone loss secondary to GC therapy.37 A randomized, double-blind, placebo-controlled, multicenter trial randomly assigned 249 healthy, early postmenopausal women to receive probiotic treatment containing 3 lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and L plantarum DSM 15313) or placebo once daily for 12 months.38 Bone mineral density was measured at baseline and at 12 months. Of the 234 participants who completed the study, lactobacillus treatment reduced lumbosacral BMD loss compared to the placebo group (mean difference, 0.71% [95% CI, 0.06-1.35]). They also reported significant lumbosacral BMD loss in the placebo group (−0.72% [95% CI, −1.22 to −0.22]) compared to no BMD loss in the group treated with lactobacillus (−0.01% [95% CI, −0.50 to 0.48]).38 Although the data may be encouraging, more studies are needed to determine if probiotics should be regarded as an adjuvant treatment to calcium, vitamin D, and pharmacologic therapy for long-term prevention of bone loss in the setting of GIO.39 Because existing studies on probiotics include varying compositions and doses, larger studies with consistent supplementation are required. Encouraging probiotic intake through fermented dairy products may represent a simple low-risk intervention to support bone health.
Anti-inflammatory Diet—The traditional Mediterranean diet is rich in fruits, vegetables, fish, nuts, whole grains, legumes, and monounsaturated fats and low in meat and dairy products. The Mediterranean diet has been shown to be modestly protective against osteoporosis and fracture risk. A large US observational study including 93,676 women showed that those with the highest quintile of the alternate Mediterranean diet score had a lower risk for hip fracture (HR, 0.80 [95% CI, 0.66-0.97]), with an absolute risk reduction of 0.29% and number needed to treat at 342.40 A multicenter study involving adults from 8 European countries found that increased adherence to the Mediterranean diet was associated with a 7% reduction in hip fracture incidence (HR per 1 unit increase in Mediterranean diet, 0.93 [95% CI, 0.89-0.98]). High vegetable and fruit intake was associated with decreased hip fracture incidence (HR, 0.86 and 0.89 [95% CI, 0.79-0.94 and 0.82-0.97, respectively]), and high meat and excessive ethanol consumption were associated with increased fracture incidence (HR, 1.18 and 1.74 [95% CI, 1.06-1.31 and 1.32-2.31, respectively]).41 Similarly, a large observational study in Sweden that included 37,903 men and 33,403 women reported similar findings, noting a 6% lower hip fracture rate per one unit increase in alternate Mediterranean diet score (adjusted HR, 0.94 [95% CI, 0.92-0.96]).42 This is thought to be due in part to higher levels of dietary vitamin D present in many foods traditionally included in the Mediterranean diet.43 Additionally, olive oil, a staple in the Mediterranean diet, appears to reduce bone loss by promoting osteoblast proliferation and maturation, inhibiting bone resorption, suppressing oxidative stress and inflammation, and increasing calcium deposition in the extracellular matrix.44,45 Fruits, vegetables, legumes, and nuts also are rich in minerals including potassium and magnesium, which are important in bone health to promote osteoblast proliferation and vitamin D activation.36,46-48
Final Thoughts
Osteoporosis-related fractures are common and are associated with high morbidity and health care costs. Dermatologists using and prescribing corticosteroids must be aware of the risk for GIO, particularly in patients with a pre-existing diagnosis of osteopenia or osteoporosis. There likely is no oral corticosteroid dose that does not increase a patient’s risk for osteoporosis; therefore, oral GCs should be used at the lowest effective daily dose for the shortest duration possible. Patients with an anticipated duration of at least 3 months—regardless of dose—should be assessed for their risk for GIO. Patients using topical and intralesional corticosteroids are unlikely to develop GIO; however, those with risk factors and a considerable cumulative dose may warrant further evaluation. In all cases, we advocate for supplementing with calcium and vitamin D as well as promoting probiotic intake and the Mediterranean diet. Those at moderate to high risk for fracture may require additional medical therapy. Dermatologists are uniquely positioned to identify this at-risk population, and because osteoporosis is a chronic illness, primary care providers should be notified of prolonged GC therapy to help with risk assessment, initiation of vitamin and mineral supplementation, and follow-up with metabolic bone health specialists. Through a multidisciplinary approach and patient education, GIO and the potential risk for fracture can be successfully mitigated in most patients.
Glucocorticoids (GCs) are among the most widely prescribed medications in dermatologic practice. Although GCs are highly effective anti-inflammatory agents, long-term systemic therapy can result in dangerous adverse effects, including GC-induced osteoporosis (GIO), a bone disease associated with a heightened risk for fragility fractures.1,2 In the United States, an estimated 10.2 million adults have osteoporosis—defined as a T-score lower than −2.5 measured via a bone densitometry scan—and 43.4 million adults have low bone mineral density (BMD).3,4 The prevalence of osteoporosis is increasing, and the diagnosis is more common in females and adults 55 years and older.2 More than 2 million individuals have osteoporosis-related fractures annually, and the mortality risk is increased at 5 and 10 years following low-energy osteoporosis-related fractures.3-5
Glucocorticoid therapy is the leading iatrogenic cause of secondary osteoporosis. As many as 30% of all patients treated with systemic GCs for more than 6 months develop GIO.1,6,7 Glucocorticoid-induced BMD loss occurs at a rate of 6% to 12% of total BMD during the first year, slowing to approximately 3% per year during subsequent therapy.1 The risk for insufficiency fractures increases by as much as 75% from baseline in adults with rheumatic, pulmonary, and skin disorders within the first 3 months of therapy and peaks at approximately 12 months.1,2
Despite the risks, many long-term GC users never receive therapy to prevent bone loss; others are only started on therapy once they have sustained an insufficiency fracture. A 5-year international observational study including more than 40,000 postmenopausal women found that only 51% of patients who were on continuous GC therapy were undergoing BMD testing and appropriate medical management.8 This review highlights the existing evidence on the risks of osteoporosis and osteoporotic (OP) fractures in the setting of topical, intralesional, intramuscular, and systemic GC treatment, as well as recommendations for nutritional supplementation to reduce these risks.
Pathophysiology
The pathophysiology of GIO is multifactorial and occurs in both early and late phases.9,10 The early phase is characterized by rapid BMD reduction due to excessive bone resorption. The late phase is characterized by slower and more progressive BMD reduction due to impaired bone formation.9 At the osteocyte level, GCs decrease cell viability and induce apoptosis.11 At the osteoblast level, GCs impair cell replication and differentiation and have proapoptotic effects, resulting in decreased cell numbers and subsequent bone formation.10 At the osteoclast level, GCs increase expression of pro-osteoclastic cytokines and decrease mature osteoclast apoptosis, resulting in an expanded osteoclastic life span and prolonged bone resorption.12,13 Indirectly, GCs alter calcium metabolism by decreasing gastrointestinal calcium absorption and impairing renal absorption.14,15
GCs and Osteoporosis
Oral GCs—Glucocorticoid-induced osteoporosis and fracture risk are dose and duration dependent.6 A study of 244,235 patients taking GCs and 244,235 controls found the relative risk of vertebral fracture was 1.55 (range, 1.20–2.01) for daily prednisone use at less than 2.5 mg, 2.59 (range, 2.16–3.10) for daily prednisone use from 2.5 to 7.4 mg, and 5.18 (range, 4.25–6.31) for daily doses of 7.5 mg or higher; the relative risk for hip fractures was 0.99 (range, 0.82–1.20), 1.77 (range, 1.55–2.02), and 2.27 (range, 1.94–2.66), respectively.16 Another large retrospective cohort study found that continuous treatment with prednisone 10 mg/d for more than 90 days compared to no GC exposure increased the risk for hip fractures 7-fold and 17-fold for vertebral fractures.17 Although the minimum cumulative dose of GCs known to cause osteoporosis is not clearly established, the American College of Rheumatology has proposed an algorithm as a basic approach to anticipate, prevent, and treat GIO (Figure).18,19 Fracture risk should be assessed in all patients who are prescribed prednisone 2.5 mg/d for 3 months or longer or an anticipated cumulative dose of more than 1 g per year. Patients 40 years and older with anticipated GC use of 3 months or longer should have both a bone densitometry scan and a Fracture Risk Assessment (FRAX) score. The FRAX tool estimates the 10-year probability of fracture in patients aged 40 to 80 years, and those patients can be further risk stratified as low (FRAX <10%), moderate (FRAX 10%–19%), or high (FRAX ≥20%) risk. In patients with moderate to high risk of fracture (FRAX >10%), initiation of pharmacologic treatment or referral to a metabolic bone specialist should be considered.18,19 First-line therapy is an oral bisphosphonate, and second-line therapies include intravenous bisphosphonates, teriparatide, denosumab, or raloxifene for patients at high risk for GIO.19 Adults younger than 40 years with a history of OP fracture or considerable risk factors for OP fractures should have a bone densitometry scan, and, if results are abnormal, the patient should be referred to a metabolic bone specialist. Those with low fracture risk based on bone densitometry and FRAX and those with no risk factors should be assessed annually for bone health (additional risk factors, GC dose and duration, bone densitometry/FRAX if indicated).18 In addition to GC dose and duration, additional risk factors for GIO, which are factored into the FRAX tool, include advanced age, low body mass index, history of bone fracture, smoking, excessive alcohol use (≥3 drinks/d), history of falls, low BMD, family history of bone fracture, and hypovitaminosis D.6

Topical GCs—Although there is strong evidence and clear guidelines regarding oral GIO, there is a dearth of data surrounding OP risk due to treatment with topical GCs. A recent retrospective nationwide Danish study evaluating the risk of osteoporosis and major OP fracture in 723,251 adults treated with potent or very potent topical steroids sought to evaluate these risks.20 Patients were included if they had filled prescriptions of at least 500 g of topical mometasone or an equivalent alternative. The investigators reported a 3% increase in relative risk of osteoporosis and major OP fracture with doubling of the cumulative topical GC dose (hazard ratio [HR], 1.03 [95% CI, 1.02-1.04] for both). The overall population-attributable risk was 4.3% (95% CI, 2.7%-5.8%) for osteoporosis and 2.7% (95% CI, 1.7%-3.8%) for major OP fracture. Notably, at least 10,000 g of mometasone was required for 1 additional patient to have a major OP fracture.20 In a commentary based on this study, Jackson21 noted that the number of patient-years of topical GC use needed for 1 fracture was 4-fold higher than that for high-dose oral GCs (40 mg/d prednisolone for ≥30 days). Another study assessed the effects of topical GCs on BMD in adults with moderate to severe atopic dermatitis over a 2-year period.22 No significant difference in BMD assessed via bone densitometry of either the lumbar spine or total hip at baseline or at 2-year follow-up was reported for either group treated with corticosteroids (<75 g per month or ≥75 g per month). Of note, the authors did not account for steroid potency, which ranged from class 1 through class 4.22 Although limited data exist, these studies suggest topical GCs used at conventional doses with appropriate breaks in therapy will not substantially increase risk for GIO or OP fracture; however, in the small subset of patients requiring chronic use of superpotent topical corticosteroids with other OP risk factors, transitioning to non–GC-based therapy or initiating bone health therapy may be advised to improve patient outcomes. Risk assessment, as in cases of chronic topical GC use, may be beneficial.
Intralesional GCs—Intralesional GCs are indicated for numerous inflammatory conditions including alopecia areata, discoid lupus erythematosus, keloids, and granuloma annulare. It generally is accepted that doses of triamcinolone acetonide should not exceed 20 mg per session spaced at least 3 weeks apart or up to 40 mg per month.18 One study demonstrated that doses of triamcinolone diacetate of 25 mg or less were unlikely to produce systemic effects and were determined to be a safe dose for intralesional injections.23 A retrospective cross-sectional case series including 18 patients with alopecia areata reported decreased BMD in 9 patients receiving intralesional triamcinolone acetonide 10 mg/mL at 4- to 8-week intervals for at least 20 months, with cumulative doses greater than 500 mg. This was particularly notable in postmenopausal women and men older than 50 years; participants with a body mass index less than 18.5 kg/m2, history of a stress fracture, family history of osteopenia or osteoporosis, and history of smoking; and those who did not regularly engage in weight-bearing exercises.24 Patients receiving long-term (ie, >1 year) intralesional steroids should be evaluated for osteoporosis risk and preventative strategies should be considered (ie, regular weight-bearing exercises, calcium and vitamin D supplementation, bisphosphate therapy). As with topical GCs, there are no clear guidelines for risk assessment or treatment recommendations for GIO.
Intramuscular GCs—The data regarding intramuscular (IM) GCs and dermatologic disease is severely limited, and to the best of our knowledge, no studies specifically assess the risk for GIO or fracture secondary to intramuscular GCs; however, a retrospective study of 27 patients (4 female, 23 male; mean age, 33 years [range, 12–61 years]) with refractory alopecia areata receiving IM triamcinolone acetonide (40 mg every 4 weeks for 3–6 months) reported 1 patient (a 56-year-old woman) with notably decreased bone densitometry from baseline requiring treatment discontinuation.25 No other patients at risk for osteoporosis had decreased BMD from treatment with IM triamcinolone; however, it was noted that 1 month following treatment, 10 of 11 assessed patients demonstrated decreased levels of morning serum cortisol and plasma adrenocorticotropic hormone—despite baseline levels within reference range—that resolved 3 months after treatment completion,25 which suggests a prolonged release of IM triamcinolone and sustained systemic effect. One systematic review of 342 patients with dermatologic diseases treated with IM corticosteroids found the primary side effects included dysmenorrhea, injection-site lipoatrophy, and adrenocortical suppression, with only a single reported case of low BMD.26 Given the paucity of evidence, additional studies are required to assess the effect of IM triamcinolone on BMD and risk for major OP fractures with regard to dosing and frequency. As there are no clear guidelines for osteoporosis evaluation in the setting of intramuscular GCs, it may be prudent to follow the algorithmic model recommended for oral steroids when anticipating at least 3 months of intramuscular GCs.
Diet and Prevention of Bone Loss
Given the profound impact that systemic GCs have on osteoporosis and fracture risk and the sparse data regarding risk from topical, intralesional, or intramuscular GCs, diet and nutrition represent a simple, safe, and potentially preventative method of slowing BMD loss and minimizing fracture risk. In higher-risk patients, nutritional assessment in combination with medical therapy also is likely warranted.
Calcium and Vitamin D3—Patients treated with any GC dose longer than 3 months should undergo calcium and vitamin D optimization.19 Exceptions for supplementation include certain patients with sarcoidosis, which can be associated with high vitamin D levels; patients with a history of hypercalcemia or hypercalciuria; and patients with chronic kidney disease.6 In a meta-analysis including 30,970 patients in 8 randomized controlled trials, calcium (500–1200 mg/d) and vitamin D (400–800 IU/d) supplementation reduced the risk of total fractures by 15% (summary relative risk estimate, 0.85 [95% CI, 0.73-0.98]) and hip fractures by 30% (summary relative risk estimate, 0.70 [95% CI, 0.56-0.87]).4 One double-blind, placebo-controlled clinical trial conducted by the Women’s Health Initiative that included 36,282 postmenopausal women who were taking 1000 mg of calcium and 400 IU of vitamin D3 daily for more than 5 years reported an HR of 0.62 (95% CI, 0.38-1.00) for hip fracture for supplementation vs placebo.27 Lastly, a 2016 Cochrane Review including 12 randomized trials and 1343 participants reported a 43% lower risk of new vertebral fractures following supplementation with calcium, vitamin D, or both compared with controls.28
Specific recommendations for calcium and vitamin D3 supplementation vary based on age and sex. The US Preventive Services Task Force concluded that insufficient evidence exists to support calcium and vitamin D3 supplementation in asymptomatic men and premenopausal women.29 The National Osteoporosis Foundation (NOF) supports the use of calcium supplementation for fracture risk reduction in middle-aged and older adults.4 Furthermore, the NOF supports the Institute of Medicine recommendations31 that men aged 50 to 70 years consume 1000 mg/d of calcium and that women 51 years and older as well as men 71 years and older consume 1200 mg/d of calcium.30 The NOF recommends 800 to 1000 IU/d of vitamin D in adults 50 years and older, while the Institute of Medicine recommends 600 IU/d in adults 70 years and younger and 800 IU/d in adults 71 years and older.31 These recommendations are similar to both the Endocrine Society and the American Geriatric Society.32,33 Total calcium should not exceed 2000 mg/d due to risk of adverse effects.
Dietary sources of vitamin D include fatty fish, mushrooms, and fortified dairy products, though recommended doses rarely can be achieved through diet alone.34 Dairy products are the primary source of dietary calcium. Other high-calcium foods include green leafy vegetables, nuts and seeds, soft-boned fish, and fortified beverages and cereals.35
Probiotics—A growing body of evidence suggests that probiotics may be beneficial in promoting bone health by improving calcium homeostasis, reducing risk for hyperparathyroidism secondary to GC therapy, and decreasing age-related bone resorption.36 An animal study demonstrated that probiotics can regulate bone resorption and formation as well as reduce bone loss secondary to GC therapy.37 A randomized, double-blind, placebo-controlled, multicenter trial randomly assigned 249 healthy, early postmenopausal women to receive probiotic treatment containing 3 lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and L plantarum DSM 15313) or placebo once daily for 12 months.38 Bone mineral density was measured at baseline and at 12 months. Of the 234 participants who completed the study, lactobacillus treatment reduced lumbosacral BMD loss compared to the placebo group (mean difference, 0.71% [95% CI, 0.06-1.35]). They also reported significant lumbosacral BMD loss in the placebo group (−0.72% [95% CI, −1.22 to −0.22]) compared to no BMD loss in the group treated with lactobacillus (−0.01% [95% CI, −0.50 to 0.48]).38 Although the data may be encouraging, more studies are needed to determine if probiotics should be regarded as an adjuvant treatment to calcium, vitamin D, and pharmacologic therapy for long-term prevention of bone loss in the setting of GIO.39 Because existing studies on probiotics include varying compositions and doses, larger studies with consistent supplementation are required. Encouraging probiotic intake through fermented dairy products may represent a simple low-risk intervention to support bone health.
Anti-inflammatory Diet—The traditional Mediterranean diet is rich in fruits, vegetables, fish, nuts, whole grains, legumes, and monounsaturated fats and low in meat and dairy products. The Mediterranean diet has been shown to be modestly protective against osteoporosis and fracture risk. A large US observational study including 93,676 women showed that those with the highest quintile of the alternate Mediterranean diet score had a lower risk for hip fracture (HR, 0.80 [95% CI, 0.66-0.97]), with an absolute risk reduction of 0.29% and number needed to treat at 342.40 A multicenter study involving adults from 8 European countries found that increased adherence to the Mediterranean diet was associated with a 7% reduction in hip fracture incidence (HR per 1 unit increase in Mediterranean diet, 0.93 [95% CI, 0.89-0.98]). High vegetable and fruit intake was associated with decreased hip fracture incidence (HR, 0.86 and 0.89 [95% CI, 0.79-0.94 and 0.82-0.97, respectively]), and high meat and excessive ethanol consumption were associated with increased fracture incidence (HR, 1.18 and 1.74 [95% CI, 1.06-1.31 and 1.32-2.31, respectively]).41 Similarly, a large observational study in Sweden that included 37,903 men and 33,403 women reported similar findings, noting a 6% lower hip fracture rate per one unit increase in alternate Mediterranean diet score (adjusted HR, 0.94 [95% CI, 0.92-0.96]).42 This is thought to be due in part to higher levels of dietary vitamin D present in many foods traditionally included in the Mediterranean diet.43 Additionally, olive oil, a staple in the Mediterranean diet, appears to reduce bone loss by promoting osteoblast proliferation and maturation, inhibiting bone resorption, suppressing oxidative stress and inflammation, and increasing calcium deposition in the extracellular matrix.44,45 Fruits, vegetables, legumes, and nuts also are rich in minerals including potassium and magnesium, which are important in bone health to promote osteoblast proliferation and vitamin D activation.36,46-48
Final Thoughts
Osteoporosis-related fractures are common and are associated with high morbidity and health care costs. Dermatologists using and prescribing corticosteroids must be aware of the risk for GIO, particularly in patients with a pre-existing diagnosis of osteopenia or osteoporosis. There likely is no oral corticosteroid dose that does not increase a patient’s risk for osteoporosis; therefore, oral GCs should be used at the lowest effective daily dose for the shortest duration possible. Patients with an anticipated duration of at least 3 months—regardless of dose—should be assessed for their risk for GIO. Patients using topical and intralesional corticosteroids are unlikely to develop GIO; however, those with risk factors and a considerable cumulative dose may warrant further evaluation. In all cases, we advocate for supplementing with calcium and vitamin D as well as promoting probiotic intake and the Mediterranean diet. Those at moderate to high risk for fracture may require additional medical therapy. Dermatologists are uniquely positioned to identify this at-risk population, and because osteoporosis is a chronic illness, primary care providers should be notified of prolonged GC therapy to help with risk assessment, initiation of vitamin and mineral supplementation, and follow-up with metabolic bone health specialists. Through a multidisciplinary approach and patient education, GIO and the potential risk for fracture can be successfully mitigated in most patients.
- Weinstein RS. Clinical practice. glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62-70.
- Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547-2556.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376.
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
- Caplan A, Fett N, Rosenbach M, et al. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1-9.
- Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32-36.
- Silverman S, Curtis J, Saag K, et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2015;26:419-420.
- Canalis E, Bilezikian JP, Angeli A, et al. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593-598.
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328.
- Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466-476.
- Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382-4389.
- Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592-5599.
- Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144-149.
- Huybers S, Naber TH, Bindels RJ, et al. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92-G97.
- Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993-1000.
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323-328.
- Lupsa BC, Insogna KL, Micheletti RG, et al. Corticosteroid use in chronic dermatologic disorders and osteoporosis. Int J Womens Dermatol. 2021;7:545-551.
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095-1110.
- Egeberg A, Schwarz P, Harsløf T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157:275-282.
- Jackson RD. Topical corticosteroids and glucocorticoid-induced osteoporosis-cumulative dose and duration matter. JAMA Dermatol. 2021;157:269-270.
- van Velsen SG, Haeck IM, Knol MJ, et al. Two-year assessment of effect of topical corticosteroids on bone mineral density in adults with moderate to severe atopic dermatitis. J Am Acad Dermatol. 2012;66:691-693.
- McGugan AD, Shuster S, Bottoms E. Adrenal suppression from intradermal triamcinolone. J Invest Dermatol. 1963;40:271-272.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Seo J, Lee YI, Hwang S, et al. Intramuscular triamcinolone acetonide: an undervalued option for refractory alopecia areata. J Dermatol. 2017;44:173-179.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567-580.
- Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi:10.1002/14651858.CD001347.pub2
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Vitamin D fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated August 12, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Calcium fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated June 2, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986.
- Schepper JD, Collins F, Rios-Arce ND, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801-820.
- Jansson PA, Curiac D, Ahrén IL, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:E154-E162.
- Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273-284.
- Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the Women’s Health Initiative. JAMA Intern Med. 2016;176:645-652.
- Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24:1587-1598.
- Byberg L, Bellavia A, Larsson SC, et al. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31:2098-2105.
- Zupo R, Lampignano L, Lattanzio A, et al. Association between adherence to the Mediterranean diet and circulating vitamin D levels. Int J Food Sci Nutr. 2020;71:884-890.
- Chin KY, Ima-Nirwana S. Olives and bone: a green osteoporosis prevention option. Int J Environ Res Public Health. 2016;13:755.
- García-Martínez O, Rivas A, Ramos-Torrecillas J, et al. The effect of olive oil on osteoporosis prevention. Int J Food Sci Nutr. 2014;65:834-840.
- Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181-189.
- Veronese N, Stubbs B, Solmi M, et al. Dietary magnesium intake and fracture risk: data from a large prospective study. Br J Nutr. 2017;117:1570-1576.
- Kong SH, Kim JH, Hong AR, et al. Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES)(2008-2011). Osteoporos Int. 2017;28:1577-1585.
- Weinstein RS. Clinical practice. glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62-70.
- Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547-2556.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376.
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
- Caplan A, Fett N, Rosenbach M, et al. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1-9.
- Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32-36.
- Silverman S, Curtis J, Saag K, et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2015;26:419-420.
- Canalis E, Bilezikian JP, Angeli A, et al. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593-598.
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328.
- Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466-476.
- Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382-4389.
- Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592-5599.
- Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144-149.
- Huybers S, Naber TH, Bindels RJ, et al. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92-G97.
- Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993-1000.
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323-328.
- Lupsa BC, Insogna KL, Micheletti RG, et al. Corticosteroid use in chronic dermatologic disorders and osteoporosis. Int J Womens Dermatol. 2021;7:545-551.
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095-1110.
- Egeberg A, Schwarz P, Harsløf T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157:275-282.
- Jackson RD. Topical corticosteroids and glucocorticoid-induced osteoporosis-cumulative dose and duration matter. JAMA Dermatol. 2021;157:269-270.
- van Velsen SG, Haeck IM, Knol MJ, et al. Two-year assessment of effect of topical corticosteroids on bone mineral density in adults with moderate to severe atopic dermatitis. J Am Acad Dermatol. 2012;66:691-693.
- McGugan AD, Shuster S, Bottoms E. Adrenal suppression from intradermal triamcinolone. J Invest Dermatol. 1963;40:271-272.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Seo J, Lee YI, Hwang S, et al. Intramuscular triamcinolone acetonide: an undervalued option for refractory alopecia areata. J Dermatol. 2017;44:173-179.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567-580.
- Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi:10.1002/14651858.CD001347.pub2
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Vitamin D fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated August 12, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Calcium fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated June 2, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986.
- Schepper JD, Collins F, Rios-Arce ND, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801-820.
- Jansson PA, Curiac D, Ahrén IL, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:E154-E162.
- Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273-284.
- Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the Women’s Health Initiative. JAMA Intern Med. 2016;176:645-652.
- Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24:1587-1598.
- Byberg L, Bellavia A, Larsson SC, et al. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31:2098-2105.
- Zupo R, Lampignano L, Lattanzio A, et al. Association between adherence to the Mediterranean diet and circulating vitamin D levels. Int J Food Sci Nutr. 2020;71:884-890.
- Chin KY, Ima-Nirwana S. Olives and bone: a green osteoporosis prevention option. Int J Environ Res Public Health. 2016;13:755.
- García-Martínez O, Rivas A, Ramos-Torrecillas J, et al. The effect of olive oil on osteoporosis prevention. Int J Food Sci Nutr. 2014;65:834-840.
- Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181-189.
- Veronese N, Stubbs B, Solmi M, et al. Dietary magnesium intake and fracture risk: data from a large prospective study. Br J Nutr. 2017;117:1570-1576.
- Kong SH, Kim JH, Hong AR, et al. Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES)(2008-2011). Osteoporos Int. 2017;28:1577-1585.
Practice Points
- Many long-term glucocorticoid (GC) users never receive therapy to prevent bone loss, and others are only started on therapy once they have sustained an insufficiency fracture.
- Oral GCs should be used at the lowest effective daily dose for the shortest duration possible.
- Patients using topical and intralesional corticosteroids are unlikely to develop GC-induced osteoporosis.
Natural fertility: When less can be more
As reproductive specialists, part of our obligation is to improve a woman’s or couple’s ability to conceive in the most cost-effective manner, ideally through natural attempts at conception. While assisted reproductive technologies (ART) have provided impressive pregnancy rates across many diagnoses, including unexplained infertility, this advanced procedure comes with a significant financial cost to those without insurance and an emotional burden from the lack of a guaranteed outcome. Infertility procedures have minimal associated but potentially significant risks, most importantly multiple gestations. Contrary to popular belief, ovulation induction with intrauterine insemination (IUI) treatment has a greater risk of high-order multiple gestation when compared with IVF, given the inability of the former to control the number of embryos that may enter and implant in the endometrial cavity and the increased use of single embryo transfers with the latter. The specialist should evaluate the woman or couple for the basic issues of ovulation, tubal, and sperm function, as well as for lifestyle and environmental factors that can impede reproduction. As a result, “one size fits all” should not apply to patients, specifically those with infertility. This month’s column will present the detrimental effect of environmental and lifestyle factors on the goal of enhancing fertility through natural cycles of urine luteinizing-hormone timed intercourse.
Nutrition
Often overlooked in the infertility evaluation, an optimal diet improves fertility for both partners. Processed meat has been associated with reduced sperm quality. In ART, red meat has been associated with decreased embryo blastocyst formation. Lower trans fatty acids and higher omega-3s may improve fecundity. Considered one of the best overall diets, the Mediterranean diet consists of plant-based foods, such as whole grains, vegetables, legumes, fruits, nuts, seeds, herbs, and spices. Olive oil is the main source of added fat whereas fish, seafood, dairy, and poultry should be eaten in moderation. Fatty fish, such as mackerel, herring, sardines, albacore tuna, and salmon, are rich in omega-3 fatty acids, which have been shown to improve fecundity and IVF success, and have a positive association with blastocyst embryo development.1-3
Stress
The emotional effect of an infertility diagnosis has been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities.4 Whether stress causes or is a result of infertility has been a longstanding debate.5 Nevertheless, stress is the number-one reason patients discontinue fertility treatment.6 As fertility specialists, we must be cognizant of the devastation endured by infertility patients and maintain an open dialogue, as well as provide resources for coping strategies and counseling.
One popular method of improving mental health and fertility has been acupuncture. Initial enthusiasm originated from one of the first studies to explore the use of acupuncture during IVF. This was a prospective randomized study that showed treated patients had an approximately 100% improvement in clinical pregnancy rate. Unfortunately, there was no appropriate control group, just untreated controls.7 A subsequent study by the same investigator added a placebo acupuncture control group and did not show a statistically significant increase in pregnancy rates.8 Finally, a meta-analysis and reanalysis did not demonstrate any improvement in pregnancy outcome, whereas three of the studies analyzed suggested a possible reduction in pregnancies; placebo acupuncture was shown to have a higher success rate.9-11 While acupuncture is relatively safe, there appears to be only a placebo effect that may be helpful.
The effect of stress on reproduction has been addressed in one of my previous columns.
Alcohol and caffeine
The damaging effects of alcohol on the fetus during pregnancy are legion – abnormal facial features, microcephaly, low birth weight, hyperactive behavior, vision or hearing deficits, speech and language delays, and intellectual disability. Less known is the amount of alcohol that may have an effect during preconception. One of the first reports on the effect of alcohol on IVF concluded: a 13% decrease in the number of eggs aspirated; a 2.86 times increase in risk of not achieving pregnancy; and a 2.21 times increase in risk of miscarriage. For men, one additional drink per day increased the risk of not achieving a live birth from 2.28 to 8.32 times.12 Subsequent studies demonstrate a 16% reduction in IVF pregnancies in women who have at least four drinks per week; when the couple drank at least four drinks per week, the pregnancy rate decreased by 21%.13
However, a study from Denmark did not demonstrate a negative effect of low to moderate pretreatment amounts of alcohol and caffeine on IVF outcomes.14 Nevertheless, there is evidence that reducing or abstaining from alcohol intake may improve IVF outcomes.15 While there have been reports of higher miscarriage rates from caffeine,16,17 not all reports support a negative association.18
Smoking
The use of tobacco has been estimated to contribute to 13% of female infertility in a dose-response manner, including secondhand smoke. During ART, smoking reduces ovarian response to gonadotropins and decreases IVF success by up to 50%. Discontinuing smoking for 6 months beforehand appears to restore normal outcomes.19-20
The American Society for Reproductive Medicine Practice Committee on smoking provides the following invaluable information to share with patients on the harmful reproductive effects of smoking:21
- Early menopause by accelerating the loss of eggs.
- Higher rates of miscarriage and ectopic pregnancy.
- A decrease in sperm function.
- Possible genetic damage to eggs and sperm.
- Reduced sperm in son from maternal smoking.
Weight and exercise
Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction;22 a lower chance for conception;23 and infertility.24 Obese women have higher rates of miscarriage and recurrent miscarriage, reduced success with ART, an increased number of canceled cycles, and poorer quality oocytes retrieved. During pregnancy, obese women have three to four times higher rates of gestational diabetes and preeclampsia,25 as well as likelihood of having a fetus with macrosomia and birth defects, and a 1.3-2.1 times higher risk of stillbirth.26
Regarding physical activity, the rate of pregnancies (39.0% vs. 16.0%, P = .002) and live births (24.4% vs. 7.4% (P = .004) were higher with regular exercise vs. being sedentary. Obese women who exercised regularly had a live birth rate over threefold higher compared with those who were not active.27 Moderation should be employed given that women who exercise to exhaustion have 2.3 times the odds of fertility problems.28 In men, obesity has been shown to increase estrogens and reduce spermatogenesis. Exercise has improved semen parameters and testosterone. Paternal physical and sedentary activities were not related to clinical pregnancy or live birth rates following infertility treatment.29 As in women, men experience negative effects from high-intensity exercise, including bicycling, which can result in decreased semen parameters, follicle-stimulating hormone, LH, and testosterone levels.30
In couples desiring a more natural approach to infertility, fertility specialists can address environmental and lifestyle factors that may improve reproduction. When natural attempts at conception are not applicable or successful, IUI and ART are appropriate treatment options after considering estimated success rates as well as the physical, emotional, and financial investment of patients.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Wise LA et al. Am J Epidemiol. 2018;187:60-74.
2. Chui Y-H. Hum Reprod. 2018;33:156-65.
3. Ferreira Braga DPA et al. Reprod Biomed Online. 2015;31:30-8.
4. Domar AD et al. J Psychosom Obstet Gynaecol. 1993;14[suppl]:45-52.
5. Trolice MP. J Assist Reprod Genet. 2021 Apr;38[4]:873-5.
6. Gameiro S et al. Hum Reprod Update. 2012;18[6]:652-69.
7. Paulus WE et al. Fertil Steril. 2002;77:721-4.
8. Paulus WE et al. Hum Reprod. 2003;18:S18(abstr).
9. Wing SSE et al. Hum Reprod. 2009;24:341-8.
10. Hong Zheng C et al. Fertil Steril. 2012;97:599-611.
11. Meldrum DR et al. Fertil Steril. 2013;99:1821-4.
12. Klonoff-Cohen H et al. Fertil Steril. 2003;79:330-9.
13. Rossi BV et al. Obstet Gynecol. 2011;117:136-42.
14. Abadia L et al. Hum Reprod. 2017;32:1846-54.
15. Gormack AA et al. Hum Reprod. 2015;30:1617.
16. James JE. BMJ Evid Based Med. 2021;26:114-15.
17. Gaskins AJ et al. Eur J Nutr. 2018 Feb;57:107-17.
18. Machtinger R et al. Fertil Steril. 2017;108:1026-33.
19. Hughes EG et al. Fertil Steril. 1994;62:807.
20. de Ziegler D et al. Fertil Steril. 2013;100:927-8.
21. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2018;110:611-8.
22. Brewer CJ, Balen AH. Reproduction. 2010;140:347-64.
23. Wise LA et al. Hum Reprod. 2010;25:253-64.
24. Silvestris S et al. Reprod Biol Endocrinol. 2018;16[1]:22.
25. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
26. Aune D et al. JAMA. 2014;311:1536-46.
27. Palomba S et al. Reprod Biomed Online. 2014;29:72-9.
28. Gudmundsdottir SL et al. Hum Reprod. 2009;24[12]:3196-204.
29. Gaskins AJ et al. Hum Reprod. 2014;29:2575-82.
30. Wise LA et al. Fertil Steril. 2011;95:1025-30.
As reproductive specialists, part of our obligation is to improve a woman’s or couple’s ability to conceive in the most cost-effective manner, ideally through natural attempts at conception. While assisted reproductive technologies (ART) have provided impressive pregnancy rates across many diagnoses, including unexplained infertility, this advanced procedure comes with a significant financial cost to those without insurance and an emotional burden from the lack of a guaranteed outcome. Infertility procedures have minimal associated but potentially significant risks, most importantly multiple gestations. Contrary to popular belief, ovulation induction with intrauterine insemination (IUI) treatment has a greater risk of high-order multiple gestation when compared with IVF, given the inability of the former to control the number of embryos that may enter and implant in the endometrial cavity and the increased use of single embryo transfers with the latter. The specialist should evaluate the woman or couple for the basic issues of ovulation, tubal, and sperm function, as well as for lifestyle and environmental factors that can impede reproduction. As a result, “one size fits all” should not apply to patients, specifically those with infertility. This month’s column will present the detrimental effect of environmental and lifestyle factors on the goal of enhancing fertility through natural cycles of urine luteinizing-hormone timed intercourse.
Nutrition
Often overlooked in the infertility evaluation, an optimal diet improves fertility for both partners. Processed meat has been associated with reduced sperm quality. In ART, red meat has been associated with decreased embryo blastocyst formation. Lower trans fatty acids and higher omega-3s may improve fecundity. Considered one of the best overall diets, the Mediterranean diet consists of plant-based foods, such as whole grains, vegetables, legumes, fruits, nuts, seeds, herbs, and spices. Olive oil is the main source of added fat whereas fish, seafood, dairy, and poultry should be eaten in moderation. Fatty fish, such as mackerel, herring, sardines, albacore tuna, and salmon, are rich in omega-3 fatty acids, which have been shown to improve fecundity and IVF success, and have a positive association with blastocyst embryo development.1-3
Stress
The emotional effect of an infertility diagnosis has been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities.4 Whether stress causes or is a result of infertility has been a longstanding debate.5 Nevertheless, stress is the number-one reason patients discontinue fertility treatment.6 As fertility specialists, we must be cognizant of the devastation endured by infertility patients and maintain an open dialogue, as well as provide resources for coping strategies and counseling.
One popular method of improving mental health and fertility has been acupuncture. Initial enthusiasm originated from one of the first studies to explore the use of acupuncture during IVF. This was a prospective randomized study that showed treated patients had an approximately 100% improvement in clinical pregnancy rate. Unfortunately, there was no appropriate control group, just untreated controls.7 A subsequent study by the same investigator added a placebo acupuncture control group and did not show a statistically significant increase in pregnancy rates.8 Finally, a meta-analysis and reanalysis did not demonstrate any improvement in pregnancy outcome, whereas three of the studies analyzed suggested a possible reduction in pregnancies; placebo acupuncture was shown to have a higher success rate.9-11 While acupuncture is relatively safe, there appears to be only a placebo effect that may be helpful.
The effect of stress on reproduction has been addressed in one of my previous columns.
Alcohol and caffeine
The damaging effects of alcohol on the fetus during pregnancy are legion – abnormal facial features, microcephaly, low birth weight, hyperactive behavior, vision or hearing deficits, speech and language delays, and intellectual disability. Less known is the amount of alcohol that may have an effect during preconception. One of the first reports on the effect of alcohol on IVF concluded: a 13% decrease in the number of eggs aspirated; a 2.86 times increase in risk of not achieving pregnancy; and a 2.21 times increase in risk of miscarriage. For men, one additional drink per day increased the risk of not achieving a live birth from 2.28 to 8.32 times.12 Subsequent studies demonstrate a 16% reduction in IVF pregnancies in women who have at least four drinks per week; when the couple drank at least four drinks per week, the pregnancy rate decreased by 21%.13
However, a study from Denmark did not demonstrate a negative effect of low to moderate pretreatment amounts of alcohol and caffeine on IVF outcomes.14 Nevertheless, there is evidence that reducing or abstaining from alcohol intake may improve IVF outcomes.15 While there have been reports of higher miscarriage rates from caffeine,16,17 not all reports support a negative association.18
Smoking
The use of tobacco has been estimated to contribute to 13% of female infertility in a dose-response manner, including secondhand smoke. During ART, smoking reduces ovarian response to gonadotropins and decreases IVF success by up to 50%. Discontinuing smoking for 6 months beforehand appears to restore normal outcomes.19-20
The American Society for Reproductive Medicine Practice Committee on smoking provides the following invaluable information to share with patients on the harmful reproductive effects of smoking:21
- Early menopause by accelerating the loss of eggs.
- Higher rates of miscarriage and ectopic pregnancy.
- A decrease in sperm function.
- Possible genetic damage to eggs and sperm.
- Reduced sperm in son from maternal smoking.
Weight and exercise
Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction;22 a lower chance for conception;23 and infertility.24 Obese women have higher rates of miscarriage and recurrent miscarriage, reduced success with ART, an increased number of canceled cycles, and poorer quality oocytes retrieved. During pregnancy, obese women have three to four times higher rates of gestational diabetes and preeclampsia,25 as well as likelihood of having a fetus with macrosomia and birth defects, and a 1.3-2.1 times higher risk of stillbirth.26
Regarding physical activity, the rate of pregnancies (39.0% vs. 16.0%, P = .002) and live births (24.4% vs. 7.4% (P = .004) were higher with regular exercise vs. being sedentary. Obese women who exercised regularly had a live birth rate over threefold higher compared with those who were not active.27 Moderation should be employed given that women who exercise to exhaustion have 2.3 times the odds of fertility problems.28 In men, obesity has been shown to increase estrogens and reduce spermatogenesis. Exercise has improved semen parameters and testosterone. Paternal physical and sedentary activities were not related to clinical pregnancy or live birth rates following infertility treatment.29 As in women, men experience negative effects from high-intensity exercise, including bicycling, which can result in decreased semen parameters, follicle-stimulating hormone, LH, and testosterone levels.30
In couples desiring a more natural approach to infertility, fertility specialists can address environmental and lifestyle factors that may improve reproduction. When natural attempts at conception are not applicable or successful, IUI and ART are appropriate treatment options after considering estimated success rates as well as the physical, emotional, and financial investment of patients.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Wise LA et al. Am J Epidemiol. 2018;187:60-74.
2. Chui Y-H. Hum Reprod. 2018;33:156-65.
3. Ferreira Braga DPA et al. Reprod Biomed Online. 2015;31:30-8.
4. Domar AD et al. J Psychosom Obstet Gynaecol. 1993;14[suppl]:45-52.
5. Trolice MP. J Assist Reprod Genet. 2021 Apr;38[4]:873-5.
6. Gameiro S et al. Hum Reprod Update. 2012;18[6]:652-69.
7. Paulus WE et al. Fertil Steril. 2002;77:721-4.
8. Paulus WE et al. Hum Reprod. 2003;18:S18(abstr).
9. Wing SSE et al. Hum Reprod. 2009;24:341-8.
10. Hong Zheng C et al. Fertil Steril. 2012;97:599-611.
11. Meldrum DR et al. Fertil Steril. 2013;99:1821-4.
12. Klonoff-Cohen H et al. Fertil Steril. 2003;79:330-9.
13. Rossi BV et al. Obstet Gynecol. 2011;117:136-42.
14. Abadia L et al. Hum Reprod. 2017;32:1846-54.
15. Gormack AA et al. Hum Reprod. 2015;30:1617.
16. James JE. BMJ Evid Based Med. 2021;26:114-15.
17. Gaskins AJ et al. Eur J Nutr. 2018 Feb;57:107-17.
18. Machtinger R et al. Fertil Steril. 2017;108:1026-33.
19. Hughes EG et al. Fertil Steril. 1994;62:807.
20. de Ziegler D et al. Fertil Steril. 2013;100:927-8.
21. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2018;110:611-8.
22. Brewer CJ, Balen AH. Reproduction. 2010;140:347-64.
23. Wise LA et al. Hum Reprod. 2010;25:253-64.
24. Silvestris S et al. Reprod Biol Endocrinol. 2018;16[1]:22.
25. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
26. Aune D et al. JAMA. 2014;311:1536-46.
27. Palomba S et al. Reprod Biomed Online. 2014;29:72-9.
28. Gudmundsdottir SL et al. Hum Reprod. 2009;24[12]:3196-204.
29. Gaskins AJ et al. Hum Reprod. 2014;29:2575-82.
30. Wise LA et al. Fertil Steril. 2011;95:1025-30.
As reproductive specialists, part of our obligation is to improve a woman’s or couple’s ability to conceive in the most cost-effective manner, ideally through natural attempts at conception. While assisted reproductive technologies (ART) have provided impressive pregnancy rates across many diagnoses, including unexplained infertility, this advanced procedure comes with a significant financial cost to those without insurance and an emotional burden from the lack of a guaranteed outcome. Infertility procedures have minimal associated but potentially significant risks, most importantly multiple gestations. Contrary to popular belief, ovulation induction with intrauterine insemination (IUI) treatment has a greater risk of high-order multiple gestation when compared with IVF, given the inability of the former to control the number of embryos that may enter and implant in the endometrial cavity and the increased use of single embryo transfers with the latter. The specialist should evaluate the woman or couple for the basic issues of ovulation, tubal, and sperm function, as well as for lifestyle and environmental factors that can impede reproduction. As a result, “one size fits all” should not apply to patients, specifically those with infertility. This month’s column will present the detrimental effect of environmental and lifestyle factors on the goal of enhancing fertility through natural cycles of urine luteinizing-hormone timed intercourse.
Nutrition
Often overlooked in the infertility evaluation, an optimal diet improves fertility for both partners. Processed meat has been associated with reduced sperm quality. In ART, red meat has been associated with decreased embryo blastocyst formation. Lower trans fatty acids and higher omega-3s may improve fecundity. Considered one of the best overall diets, the Mediterranean diet consists of plant-based foods, such as whole grains, vegetables, legumes, fruits, nuts, seeds, herbs, and spices. Olive oil is the main source of added fat whereas fish, seafood, dairy, and poultry should be eaten in moderation. Fatty fish, such as mackerel, herring, sardines, albacore tuna, and salmon, are rich in omega-3 fatty acids, which have been shown to improve fecundity and IVF success, and have a positive association with blastocyst embryo development.1-3
Stress
The emotional effect of an infertility diagnosis has been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities.4 Whether stress causes or is a result of infertility has been a longstanding debate.5 Nevertheless, stress is the number-one reason patients discontinue fertility treatment.6 As fertility specialists, we must be cognizant of the devastation endured by infertility patients and maintain an open dialogue, as well as provide resources for coping strategies and counseling.
One popular method of improving mental health and fertility has been acupuncture. Initial enthusiasm originated from one of the first studies to explore the use of acupuncture during IVF. This was a prospective randomized study that showed treated patients had an approximately 100% improvement in clinical pregnancy rate. Unfortunately, there was no appropriate control group, just untreated controls.7 A subsequent study by the same investigator added a placebo acupuncture control group and did not show a statistically significant increase in pregnancy rates.8 Finally, a meta-analysis and reanalysis did not demonstrate any improvement in pregnancy outcome, whereas three of the studies analyzed suggested a possible reduction in pregnancies; placebo acupuncture was shown to have a higher success rate.9-11 While acupuncture is relatively safe, there appears to be only a placebo effect that may be helpful.
The effect of stress on reproduction has been addressed in one of my previous columns.
Alcohol and caffeine
The damaging effects of alcohol on the fetus during pregnancy are legion – abnormal facial features, microcephaly, low birth weight, hyperactive behavior, vision or hearing deficits, speech and language delays, and intellectual disability. Less known is the amount of alcohol that may have an effect during preconception. One of the first reports on the effect of alcohol on IVF concluded: a 13% decrease in the number of eggs aspirated; a 2.86 times increase in risk of not achieving pregnancy; and a 2.21 times increase in risk of miscarriage. For men, one additional drink per day increased the risk of not achieving a live birth from 2.28 to 8.32 times.12 Subsequent studies demonstrate a 16% reduction in IVF pregnancies in women who have at least four drinks per week; when the couple drank at least four drinks per week, the pregnancy rate decreased by 21%.13
However, a study from Denmark did not demonstrate a negative effect of low to moderate pretreatment amounts of alcohol and caffeine on IVF outcomes.14 Nevertheless, there is evidence that reducing or abstaining from alcohol intake may improve IVF outcomes.15 While there have been reports of higher miscarriage rates from caffeine,16,17 not all reports support a negative association.18
Smoking
The use of tobacco has been estimated to contribute to 13% of female infertility in a dose-response manner, including secondhand smoke. During ART, smoking reduces ovarian response to gonadotropins and decreases IVF success by up to 50%. Discontinuing smoking for 6 months beforehand appears to restore normal outcomes.19-20
The American Society for Reproductive Medicine Practice Committee on smoking provides the following invaluable information to share with patients on the harmful reproductive effects of smoking:21
- Early menopause by accelerating the loss of eggs.
- Higher rates of miscarriage and ectopic pregnancy.
- A decrease in sperm function.
- Possible genetic damage to eggs and sperm.
- Reduced sperm in son from maternal smoking.
Weight and exercise
Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction;22 a lower chance for conception;23 and infertility.24 Obese women have higher rates of miscarriage and recurrent miscarriage, reduced success with ART, an increased number of canceled cycles, and poorer quality oocytes retrieved. During pregnancy, obese women have three to four times higher rates of gestational diabetes and preeclampsia,25 as well as likelihood of having a fetus with macrosomia and birth defects, and a 1.3-2.1 times higher risk of stillbirth.26
Regarding physical activity, the rate of pregnancies (39.0% vs. 16.0%, P = .002) and live births (24.4% vs. 7.4% (P = .004) were higher with regular exercise vs. being sedentary. Obese women who exercised regularly had a live birth rate over threefold higher compared with those who were not active.27 Moderation should be employed given that women who exercise to exhaustion have 2.3 times the odds of fertility problems.28 In men, obesity has been shown to increase estrogens and reduce spermatogenesis. Exercise has improved semen parameters and testosterone. Paternal physical and sedentary activities were not related to clinical pregnancy or live birth rates following infertility treatment.29 As in women, men experience negative effects from high-intensity exercise, including bicycling, which can result in decreased semen parameters, follicle-stimulating hormone, LH, and testosterone levels.30
In couples desiring a more natural approach to infertility, fertility specialists can address environmental and lifestyle factors that may improve reproduction. When natural attempts at conception are not applicable or successful, IUI and ART are appropriate treatment options after considering estimated success rates as well as the physical, emotional, and financial investment of patients.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Wise LA et al. Am J Epidemiol. 2018;187:60-74.
2. Chui Y-H. Hum Reprod. 2018;33:156-65.
3. Ferreira Braga DPA et al. Reprod Biomed Online. 2015;31:30-8.
4. Domar AD et al. J Psychosom Obstet Gynaecol. 1993;14[suppl]:45-52.
5. Trolice MP. J Assist Reprod Genet. 2021 Apr;38[4]:873-5.
6. Gameiro S et al. Hum Reprod Update. 2012;18[6]:652-69.
7. Paulus WE et al. Fertil Steril. 2002;77:721-4.
8. Paulus WE et al. Hum Reprod. 2003;18:S18(abstr).
9. Wing SSE et al. Hum Reprod. 2009;24:341-8.
10. Hong Zheng C et al. Fertil Steril. 2012;97:599-611.
11. Meldrum DR et al. Fertil Steril. 2013;99:1821-4.
12. Klonoff-Cohen H et al. Fertil Steril. 2003;79:330-9.
13. Rossi BV et al. Obstet Gynecol. 2011;117:136-42.
14. Abadia L et al. Hum Reprod. 2017;32:1846-54.
15. Gormack AA et al. Hum Reprod. 2015;30:1617.
16. James JE. BMJ Evid Based Med. 2021;26:114-15.
17. Gaskins AJ et al. Eur J Nutr. 2018 Feb;57:107-17.
18. Machtinger R et al. Fertil Steril. 2017;108:1026-33.
19. Hughes EG et al. Fertil Steril. 1994;62:807.
20. de Ziegler D et al. Fertil Steril. 2013;100:927-8.
21. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2018;110:611-8.
22. Brewer CJ, Balen AH. Reproduction. 2010;140:347-64.
23. Wise LA et al. Hum Reprod. 2010;25:253-64.
24. Silvestris S et al. Reprod Biol Endocrinol. 2018;16[1]:22.
25. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
26. Aune D et al. JAMA. 2014;311:1536-46.
27. Palomba S et al. Reprod Biomed Online. 2014;29:72-9.
28. Gudmundsdottir SL et al. Hum Reprod. 2009;24[12]:3196-204.
29. Gaskins AJ et al. Hum Reprod. 2014;29:2575-82.
30. Wise LA et al. Fertil Steril. 2011;95:1025-30.
AI and reality – diagnosing otitis media is a real challenge
Let’s pretend for a moment that you receive a call from one of your college roommates who thanks to his family connections has become a venture capitalist in California. His group is considering investing in a start-up that is developing a handheld instrument that it claims will use artificial intelligence to diagnose ear infections far more accurately than the human eye. He wonders if you would like to help him evaluate the company’s proposal and offers you a small percentage of the profits for your efforts should they choose to invest.
Your former roommate has done enough research on his own to understand that otitis media makes up a large chunk of a pediatrician’s workload and that making an accurate diagnosis can often be difficult in a struggling child. He describes his own experience watching a frustrated pediatrician attempting to remove wax from his child’s ear and eventually prescribing antibiotics “to be safe.”
You agree and review the prospectus, which includes a paper from a peer-reviewed journal. What you discover is that the investigators used more than 600 high-resolution images of tympanic membranes taken “during operative myringotomy and tympanostomy tube placement” and the findings at tympanocentesis to train a neural network.
Once trained, the model they developed could differentiate with 95% accuracy between an image of a tympanic membrane that covered a normal middle ear from one that merely contained fluid and from one that contained infected fluid. When these same images were shown to 39 clinicians, more than half of which were pediatricians and included both faculty-level staff and trainees, the average diagnostic accuracy was 65%.
The prospectus includes prediction that this technology could easily be developed into a handheld instrument similar to a traditional otoscope, which could then be linked to the operator’s smartphone, giving the clinician an instant treat or no-treat answer.
Now, remember you have nothing to lose except maybe a friendship. How would you advise your old college roommate?
My advice to your college buddy would be one of caution! Yes, there is a potential big upside because there is a real need for a device that could provide a diagnostic accuracy that this AI model promises. While I suspect that AI will always be more accurate in diagnosis using static images, I bet that most people, clinicians and nonclinicians, could improve their accuracy by linking photos with diagnoses with an hour of practice.
However, evaluating a high-resolution photograph taken through an operative scope inserted into the cerumenless ear canal of a sedated, afrebrile child is several orders of magnitude less difficult than the real-world environment in which the diagnosis of otitis media is usually made.
If the venture capitalists were still interested in getting into the otitis media marketplace, you might suggest they look into companies that have already developed image capture otoscopes. At this point I could only find one on the Internet that was portable and it certainly isn’t small-child friendly. Once we have a tool that can capture images in real-world situations, the next step is to train AI systems to interpret them using the approach these researchers have developed. I bet it can be done. It will be only a matter of time ... and money.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Let’s pretend for a moment that you receive a call from one of your college roommates who thanks to his family connections has become a venture capitalist in California. His group is considering investing in a start-up that is developing a handheld instrument that it claims will use artificial intelligence to diagnose ear infections far more accurately than the human eye. He wonders if you would like to help him evaluate the company’s proposal and offers you a small percentage of the profits for your efforts should they choose to invest.
Your former roommate has done enough research on his own to understand that otitis media makes up a large chunk of a pediatrician’s workload and that making an accurate diagnosis can often be difficult in a struggling child. He describes his own experience watching a frustrated pediatrician attempting to remove wax from his child’s ear and eventually prescribing antibiotics “to be safe.”
You agree and review the prospectus, which includes a paper from a peer-reviewed journal. What you discover is that the investigators used more than 600 high-resolution images of tympanic membranes taken “during operative myringotomy and tympanostomy tube placement” and the findings at tympanocentesis to train a neural network.
Once trained, the model they developed could differentiate with 95% accuracy between an image of a tympanic membrane that covered a normal middle ear from one that merely contained fluid and from one that contained infected fluid. When these same images were shown to 39 clinicians, more than half of which were pediatricians and included both faculty-level staff and trainees, the average diagnostic accuracy was 65%.
The prospectus includes prediction that this technology could easily be developed into a handheld instrument similar to a traditional otoscope, which could then be linked to the operator’s smartphone, giving the clinician an instant treat or no-treat answer.
Now, remember you have nothing to lose except maybe a friendship. How would you advise your old college roommate?
My advice to your college buddy would be one of caution! Yes, there is a potential big upside because there is a real need for a device that could provide a diagnostic accuracy that this AI model promises. While I suspect that AI will always be more accurate in diagnosis using static images, I bet that most people, clinicians and nonclinicians, could improve their accuracy by linking photos with diagnoses with an hour of practice.
However, evaluating a high-resolution photograph taken through an operative scope inserted into the cerumenless ear canal of a sedated, afrebrile child is several orders of magnitude less difficult than the real-world environment in which the diagnosis of otitis media is usually made.
If the venture capitalists were still interested in getting into the otitis media marketplace, you might suggest they look into companies that have already developed image capture otoscopes. At this point I could only find one on the Internet that was portable and it certainly isn’t small-child friendly. Once we have a tool that can capture images in real-world situations, the next step is to train AI systems to interpret them using the approach these researchers have developed. I bet it can be done. It will be only a matter of time ... and money.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Let’s pretend for a moment that you receive a call from one of your college roommates who thanks to his family connections has become a venture capitalist in California. His group is considering investing in a start-up that is developing a handheld instrument that it claims will use artificial intelligence to diagnose ear infections far more accurately than the human eye. He wonders if you would like to help him evaluate the company’s proposal and offers you a small percentage of the profits for your efforts should they choose to invest.
Your former roommate has done enough research on his own to understand that otitis media makes up a large chunk of a pediatrician’s workload and that making an accurate diagnosis can often be difficult in a struggling child. He describes his own experience watching a frustrated pediatrician attempting to remove wax from his child’s ear and eventually prescribing antibiotics “to be safe.”
You agree and review the prospectus, which includes a paper from a peer-reviewed journal. What you discover is that the investigators used more than 600 high-resolution images of tympanic membranes taken “during operative myringotomy and tympanostomy tube placement” and the findings at tympanocentesis to train a neural network.
Once trained, the model they developed could differentiate with 95% accuracy between an image of a tympanic membrane that covered a normal middle ear from one that merely contained fluid and from one that contained infected fluid. When these same images were shown to 39 clinicians, more than half of which were pediatricians and included both faculty-level staff and trainees, the average diagnostic accuracy was 65%.
The prospectus includes prediction that this technology could easily be developed into a handheld instrument similar to a traditional otoscope, which could then be linked to the operator’s smartphone, giving the clinician an instant treat or no-treat answer.
Now, remember you have nothing to lose except maybe a friendship. How would you advise your old college roommate?
My advice to your college buddy would be one of caution! Yes, there is a potential big upside because there is a real need for a device that could provide a diagnostic accuracy that this AI model promises. While I suspect that AI will always be more accurate in diagnosis using static images, I bet that most people, clinicians and nonclinicians, could improve their accuracy by linking photos with diagnoses with an hour of practice.
However, evaluating a high-resolution photograph taken through an operative scope inserted into the cerumenless ear canal of a sedated, afrebrile child is several orders of magnitude less difficult than the real-world environment in which the diagnosis of otitis media is usually made.
If the venture capitalists were still interested in getting into the otitis media marketplace, you might suggest they look into companies that have already developed image capture otoscopes. At this point I could only find one on the Internet that was portable and it certainly isn’t small-child friendly. Once we have a tool that can capture images in real-world situations, the next step is to train AI systems to interpret them using the approach these researchers have developed. I bet it can be done. It will be only a matter of time ... and money.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Consensus Statement Supporting the Presence of Onsite Radiation Oncology Departments at VHA Medical Centers
Radiation therapy, along with surgery and systemic therapy, is a primary therapeutic modality for cancer management. At least half of cancer patients receive radiation as part of their treatment regimen.1 Multiple studies demonstrate that radiotherapy is underutilized worldwide.2 One reason for underutilization of radiotherapy globally is poor access to this treatment modality. Factors that contribute to poor access include long wait times for consultation, delays in treatment initiation, distance to a treatment facility, and poor coordination of care.
Taskforce Findings
The presence of onsite radiation oncology and its impact on utilization of radiotherapy is poorly studied. The Veterans Health Administration (VHA) Palliative Radiotherapy Taskforce recently conducted a survey to determine the barriers to referral and timeliness of treatment for palliative radiotherapy within the VHA.3 Key findings of this study comparing centers with onsite radiation departments with centers without onsite radiation departments include:
a. Radiation consults are more likely to be completed within 1 week of consult request at centers with onsite radiation therapy (68% vs 31%, respectively; P = .01).
b. Centers with onsite radiation therapy more frequently deliver emergent treatment within 24 hours for patients with spinal cord compression, an emergency condition in which prompt radiation can prevent or minimize long-term neurologic disability (94% vs 70%, respectively; P = .01).
c. Referring practitioners with onsite radiation departments are less likely to report difficulty contacting a radiation oncologist as a barrier to referral for palliative radiotherapy (0% vs 20%, respectively; P = .006).
d. Referring practitioners with onsite radiotherapy report patient travel as a barrier to referral for palliative radiotherapy less frequently (28% vs 71%, respectively; P < .001).
e. Practitioners with onsite radiation oncology departments are more likely to have multidisciplinary tumor boards (31% vs 3%, respectively; P = .01) and are more likely to be influenced by radiation oncology recommendations at tumor boards (69% vs 44%, respectively; P = .02).
Based on the findings of this study, the VHA Palliative Radiotherapy Taskforce has prepared this consensus statement regarding the importance of onsite radiation oncology departments at VHA medical centers. More information regarding our 5 key findings and their implications for patient care are as follows:
Timeliness of Radiation Oncology Consultation
Delays in radiation oncology consultation, which can also delay treatment initiation, are associated with poor satisfaction among both patients and referring clinicians.4 Wait times have been identified as a barrier to utilization of radiotherapy by both patients and clinicians.5,6 Furthermore, delays in initiation of definitive therapy have been associated with worse outcomes, including worse overall survival.7,8 Our survey study demonstrates that consults for palliative radiotherapy are occurring in a more timely manner at centers with onsite radiation departments. Radiation oncology consults are more frequently completed within 1 week at centers with onsite radiation oncology departments compared with centers without onsite radiation oncology departments (68% vs 31%, P = .01). This trend would likely be seen for nonpalliative, definitive cases as well. The presence of radiation oncology departments onsite at VHA medical centers is an important component of timely care for veterans to optimize outcomes of cancer treatment.
Timely Delivery of Radiotherapy for Oncologic Emergencies
There are a few scenarios in which emergent radiation treatment, within 24 hours, is indicated. These include malignant spinal cord compression, uncal herniation from brain metastasis, superior vena cava syndrome, and tumor hemorrhage.9 Studies on management of metastatic spinal cord compression demonstrate that delays in treatment are associated with reduced ambulation10 as well as loss of sphincter function and incontinence.11
Our study demonstrates that VHA medical centers with onsite radiotherapy more frequently deliver radiotherapy within 24 hours for patients with metastatic spinal cord compression. This timely delivery of treatment is critical to optimizing functional status and quality of life in patients requiring treatment for oncologic emergencies. Revisiting treatment pathways for such situations at regular intervals is crucial given that residents and staff may rotate and be unfamiliar with emergency protocols.
Communication With Radiation Oncologists
Several studies have demonstrated that the inability to contact a radiation oncologist and poor communication result in decreased referrals for palliative radiotherapy.12,13 Our study demonstrates that onsite radiation oncology is associated with improved ability to contact a radiation oncologist. About 20% of clinicians at facilities without onsite radiation oncology reported difficulty contacting a radiation oncologist, compared with 0% at facilities with onsite radiation departments (P = .006).
It is possible that increased radiation oncology presence at VHA medical centers, through attenuation of barriers related to contacting a radiation oncologist and improved communication, would lead to increased use of radiotherapy. Increased communication between referring clinicians and radiation oncologists also can help with education of those clinicians making the referral. Since knowledge gaps have been identified in multiple studies as a barrier to referral for radiotherapy, such communication and increased education on the role of radiotherapy could increase use.12-14
Patient Travel
Patient ability to travel was the most commonly reported barrier (81%) to referral for palliative radiotherapy in our study. Travel time and transportation difficulties have been established in multiple studies as barriers to radiotherapy for both definitive and palliative management.15-18 Travel for radiotherapy was much less frequently reported as a barrier among respondents with onsite radiation oncology departments compared with those without onsite radiation departments (28% vs 71%, respectively; P < .001).
It is therefore possible that expansion of VHA radiation oncology services, allowing for provision of onsite radiotherapy at more VHA facilities, would reduce travel burden. Increasing travel accommodations for patients and provision of patient lodging on hospital campuses, which is already offered at some VHA medical centers (ie, Fisher House Foundation), could also help attenuate this barrier.
Multidisciplinary Tumor Boards
Our study demonstrates that centers with onsite radiation departments more frequently hold multidisciplinary tumor boards compared with centers without radiation departments (31% vs 3%, respectively; P = .01). Multidisciplinary tumor boards allow subspecialties to meet regularly to communicate about patient care and can help mitigate barriers related to communication and education of the referring health care practitioners.
As cases are discussed in multidisciplinary tumor boards, health care practitioners have the opportunity to make recommendations and provide education on potential benefits and/or downsides of treatments offered by their respective specialties. Several studies have demonstrated that cases discussed at multidisciplinary tumor boards are more likely to be referred for radiation therapy.19-21 Furthermore, multidisciplinary tumor boards have been associated with improved treatment outcomes.22
Conclusions
In this consensus statement the VHA Palliative Radiotherapy Taskforce recommends the optimization of use of radiotherapy within the VHA. Radiation oncology services should be maintained where present in the VHA, with consideration for expansion of services to additional facilities. Telehealth should be used to expedite consults and treatment. Hypofractionation should be used, when appropriate, to ease travel burden. Options for transportation services and onsite housing, or hospitalization, should be understood by practitioners and offered to patients to mitigate barriers related to travel.
1. Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144. doi:10.1016/j.radonc.2014.03.024
2. Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153-1186. doi:10.1016/S1470-2045(15)00222-3
3. Gutt R, Malhotra S, Hagan MP, et al. Palliative radiotherapy within the Veterans Health Administration: barriers to referral and timeliness of treatment. JCO Oncol Pract. 2021;17(12):e1913-e1922. doi:10.1200/OP.20.00981
4. Agazaryan N, Chow P, Lamb J, et al. The timeliness initiative: continuous process improvement for prompt initiation of radiation therapy treatment. Adv Radiat Oncol. 2020;5(5):1014-1021. Published 2020 Mar 10. doi:10.1016/j.adro.2020.01.007
5. Gillan C, Briggs K, Goytisolo Pazos A, et al. Barriers to accessing radiation therapy in Canada: a systematic review. Radiat Oncol. 2012;7:167. Published 2012 Oct 12. doi:10.1186/1748-717X-7-167
6. Hanna TP, Richardson H, Peng Y, Kong W, Zhang-Salomons J, Mackillop WJ. A population-based study of factors affecting the use of radiotherapy for endometrial cancer. Clin Oncol (R Coll Radiol). 2012;24(8):e113-e124. doi:10.1016/j.clon.2012.01.007
7. Ho AS, Kim S, Tighiouart M, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124(15):3154-3162. doi:10.1002/cncr.31533
8. Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. Published 2020 Dec 1. doi:10.1001/jamanetworkopen.2020.30072
9. Mitera G, Swaminath A, Wong S, et al. Radiotherapy for oncologic emergencies on weekends: examining reasons for treatment and patterns of practice at a Canadian cancer centre. Curr Oncol. 2009;16(4):55-60. doi:10.3747/co.v16i4.352
10. Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine (Phila Pa 1976). 2016;41 (Suppl 20):S224-S230. doi:10.1097/BRS.0000000000001827
11. Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21. doi:10.1136/bmj.317.7150.18
12. Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol. 2006;78(1):101-106. doi:10.1016/j.radonc.2005.11.008
13. McCloskey SA, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13(2):130-137. doi:10.1097/PPO.0b013e31804675d4
14. Chierchini S, Ingrosso G, Saldi S, Stracci F, Aristei C. Physician and patient barriers to radiotherapy service access: treatment referral implications. Cancer Manag Res. 2019;11:8829-8833. Published 2019 Oct 7. doi:10.2147/CMAR.S168941
15. Longacre CF, Neprash HT, Shippee ND, Tuttle TM, Virnig BA. Travel, treatment choice, and survival among breast cancer patients: a population-based analysis. Womens Health Rep (New Rochelle). 2021;2(1):1-10. Published 2021 Jan 11. doi:10.1089/whr.2020.0094
16. Yang DD, Muralidhar V, Mahal BA, et al. Travel distance as a barrier to receipt of adjuvant radiation therapy after radical Prostatectomy. Am J Clin Oncol. 2018;41(10):953-959. doi:10.1097/COC.0000000000000410
17. Sundaresan P, King M, Stockler M, Costa D, Milross C. Barriers to radiotherapy utilization: Consumer perceptions of issues influencing radiotherapy-related decisions. Asia Pac J Clin Oncol. 2017;13(5):e489-e496. doi:10.1111/ajco.12579
18. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378-1385. doi:10.1634/theoncologist.2015-0110
19. Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39(12):838-841. doi:10.1111/j.1445-5994.2009.02019.x
20. Brännström F, Bjerregaard JK, Winbladh A, et al. Multidisciplinary team conferences promote treatment according to guidelines in rectal cancer. Acta Oncol. 2015;54(4):447-453. doi:10.3109/0284186X.2014.952387
21. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72. doi:10.1016/j.ctrv.2015.11.007
22. Freytag M, Herrlinger U, Hauser S, et al. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC Cancer. 2020;20(1):355. Published 2020 Apr 28. doi:10.1186/s12885-020-06809-1
Radiation therapy, along with surgery and systemic therapy, is a primary therapeutic modality for cancer management. At least half of cancer patients receive radiation as part of their treatment regimen.1 Multiple studies demonstrate that radiotherapy is underutilized worldwide.2 One reason for underutilization of radiotherapy globally is poor access to this treatment modality. Factors that contribute to poor access include long wait times for consultation, delays in treatment initiation, distance to a treatment facility, and poor coordination of care.
Taskforce Findings
The presence of onsite radiation oncology and its impact on utilization of radiotherapy is poorly studied. The Veterans Health Administration (VHA) Palliative Radiotherapy Taskforce recently conducted a survey to determine the barriers to referral and timeliness of treatment for palliative radiotherapy within the VHA.3 Key findings of this study comparing centers with onsite radiation departments with centers without onsite radiation departments include:
a. Radiation consults are more likely to be completed within 1 week of consult request at centers with onsite radiation therapy (68% vs 31%, respectively; P = .01).
b. Centers with onsite radiation therapy more frequently deliver emergent treatment within 24 hours for patients with spinal cord compression, an emergency condition in which prompt radiation can prevent or minimize long-term neurologic disability (94% vs 70%, respectively; P = .01).
c. Referring practitioners with onsite radiation departments are less likely to report difficulty contacting a radiation oncologist as a barrier to referral for palliative radiotherapy (0% vs 20%, respectively; P = .006).
d. Referring practitioners with onsite radiotherapy report patient travel as a barrier to referral for palliative radiotherapy less frequently (28% vs 71%, respectively; P < .001).
e. Practitioners with onsite radiation oncology departments are more likely to have multidisciplinary tumor boards (31% vs 3%, respectively; P = .01) and are more likely to be influenced by radiation oncology recommendations at tumor boards (69% vs 44%, respectively; P = .02).
Based on the findings of this study, the VHA Palliative Radiotherapy Taskforce has prepared this consensus statement regarding the importance of onsite radiation oncology departments at VHA medical centers. More information regarding our 5 key findings and their implications for patient care are as follows:
Timeliness of Radiation Oncology Consultation
Delays in radiation oncology consultation, which can also delay treatment initiation, are associated with poor satisfaction among both patients and referring clinicians.4 Wait times have been identified as a barrier to utilization of radiotherapy by both patients and clinicians.5,6 Furthermore, delays in initiation of definitive therapy have been associated with worse outcomes, including worse overall survival.7,8 Our survey study demonstrates that consults for palliative radiotherapy are occurring in a more timely manner at centers with onsite radiation departments. Radiation oncology consults are more frequently completed within 1 week at centers with onsite radiation oncology departments compared with centers without onsite radiation oncology departments (68% vs 31%, P = .01). This trend would likely be seen for nonpalliative, definitive cases as well. The presence of radiation oncology departments onsite at VHA medical centers is an important component of timely care for veterans to optimize outcomes of cancer treatment.
Timely Delivery of Radiotherapy for Oncologic Emergencies
There are a few scenarios in which emergent radiation treatment, within 24 hours, is indicated. These include malignant spinal cord compression, uncal herniation from brain metastasis, superior vena cava syndrome, and tumor hemorrhage.9 Studies on management of metastatic spinal cord compression demonstrate that delays in treatment are associated with reduced ambulation10 as well as loss of sphincter function and incontinence.11
Our study demonstrates that VHA medical centers with onsite radiotherapy more frequently deliver radiotherapy within 24 hours for patients with metastatic spinal cord compression. This timely delivery of treatment is critical to optimizing functional status and quality of life in patients requiring treatment for oncologic emergencies. Revisiting treatment pathways for such situations at regular intervals is crucial given that residents and staff may rotate and be unfamiliar with emergency protocols.
Communication With Radiation Oncologists
Several studies have demonstrated that the inability to contact a radiation oncologist and poor communication result in decreased referrals for palliative radiotherapy.12,13 Our study demonstrates that onsite radiation oncology is associated with improved ability to contact a radiation oncologist. About 20% of clinicians at facilities without onsite radiation oncology reported difficulty contacting a radiation oncologist, compared with 0% at facilities with onsite radiation departments (P = .006).
It is possible that increased radiation oncology presence at VHA medical centers, through attenuation of barriers related to contacting a radiation oncologist and improved communication, would lead to increased use of radiotherapy. Increased communication between referring clinicians and radiation oncologists also can help with education of those clinicians making the referral. Since knowledge gaps have been identified in multiple studies as a barrier to referral for radiotherapy, such communication and increased education on the role of radiotherapy could increase use.12-14
Patient Travel
Patient ability to travel was the most commonly reported barrier (81%) to referral for palliative radiotherapy in our study. Travel time and transportation difficulties have been established in multiple studies as barriers to radiotherapy for both definitive and palliative management.15-18 Travel for radiotherapy was much less frequently reported as a barrier among respondents with onsite radiation oncology departments compared with those without onsite radiation departments (28% vs 71%, respectively; P < .001).
It is therefore possible that expansion of VHA radiation oncology services, allowing for provision of onsite radiotherapy at more VHA facilities, would reduce travel burden. Increasing travel accommodations for patients and provision of patient lodging on hospital campuses, which is already offered at some VHA medical centers (ie, Fisher House Foundation), could also help attenuate this barrier.
Multidisciplinary Tumor Boards
Our study demonstrates that centers with onsite radiation departments more frequently hold multidisciplinary tumor boards compared with centers without radiation departments (31% vs 3%, respectively; P = .01). Multidisciplinary tumor boards allow subspecialties to meet regularly to communicate about patient care and can help mitigate barriers related to communication and education of the referring health care practitioners.
As cases are discussed in multidisciplinary tumor boards, health care practitioners have the opportunity to make recommendations and provide education on potential benefits and/or downsides of treatments offered by their respective specialties. Several studies have demonstrated that cases discussed at multidisciplinary tumor boards are more likely to be referred for radiation therapy.19-21 Furthermore, multidisciplinary tumor boards have been associated with improved treatment outcomes.22
Conclusions
In this consensus statement the VHA Palliative Radiotherapy Taskforce recommends the optimization of use of radiotherapy within the VHA. Radiation oncology services should be maintained where present in the VHA, with consideration for expansion of services to additional facilities. Telehealth should be used to expedite consults and treatment. Hypofractionation should be used, when appropriate, to ease travel burden. Options for transportation services and onsite housing, or hospitalization, should be understood by practitioners and offered to patients to mitigate barriers related to travel.
Radiation therapy, along with surgery and systemic therapy, is a primary therapeutic modality for cancer management. At least half of cancer patients receive radiation as part of their treatment regimen.1 Multiple studies demonstrate that radiotherapy is underutilized worldwide.2 One reason for underutilization of radiotherapy globally is poor access to this treatment modality. Factors that contribute to poor access include long wait times for consultation, delays in treatment initiation, distance to a treatment facility, and poor coordination of care.
Taskforce Findings
The presence of onsite radiation oncology and its impact on utilization of radiotherapy is poorly studied. The Veterans Health Administration (VHA) Palliative Radiotherapy Taskforce recently conducted a survey to determine the barriers to referral and timeliness of treatment for palliative radiotherapy within the VHA.3 Key findings of this study comparing centers with onsite radiation departments with centers without onsite radiation departments include:
a. Radiation consults are more likely to be completed within 1 week of consult request at centers with onsite radiation therapy (68% vs 31%, respectively; P = .01).
b. Centers with onsite radiation therapy more frequently deliver emergent treatment within 24 hours for patients with spinal cord compression, an emergency condition in which prompt radiation can prevent or minimize long-term neurologic disability (94% vs 70%, respectively; P = .01).
c. Referring practitioners with onsite radiation departments are less likely to report difficulty contacting a radiation oncologist as a barrier to referral for palliative radiotherapy (0% vs 20%, respectively; P = .006).
d. Referring practitioners with onsite radiotherapy report patient travel as a barrier to referral for palliative radiotherapy less frequently (28% vs 71%, respectively; P < .001).
e. Practitioners with onsite radiation oncology departments are more likely to have multidisciplinary tumor boards (31% vs 3%, respectively; P = .01) and are more likely to be influenced by radiation oncology recommendations at tumor boards (69% vs 44%, respectively; P = .02).
Based on the findings of this study, the VHA Palliative Radiotherapy Taskforce has prepared this consensus statement regarding the importance of onsite radiation oncology departments at VHA medical centers. More information regarding our 5 key findings and their implications for patient care are as follows:
Timeliness of Radiation Oncology Consultation
Delays in radiation oncology consultation, which can also delay treatment initiation, are associated with poor satisfaction among both patients and referring clinicians.4 Wait times have been identified as a barrier to utilization of radiotherapy by both patients and clinicians.5,6 Furthermore, delays in initiation of definitive therapy have been associated with worse outcomes, including worse overall survival.7,8 Our survey study demonstrates that consults for palliative radiotherapy are occurring in a more timely manner at centers with onsite radiation departments. Radiation oncology consults are more frequently completed within 1 week at centers with onsite radiation oncology departments compared with centers without onsite radiation oncology departments (68% vs 31%, P = .01). This trend would likely be seen for nonpalliative, definitive cases as well. The presence of radiation oncology departments onsite at VHA medical centers is an important component of timely care for veterans to optimize outcomes of cancer treatment.
Timely Delivery of Radiotherapy for Oncologic Emergencies
There are a few scenarios in which emergent radiation treatment, within 24 hours, is indicated. These include malignant spinal cord compression, uncal herniation from brain metastasis, superior vena cava syndrome, and tumor hemorrhage.9 Studies on management of metastatic spinal cord compression demonstrate that delays in treatment are associated with reduced ambulation10 as well as loss of sphincter function and incontinence.11
Our study demonstrates that VHA medical centers with onsite radiotherapy more frequently deliver radiotherapy within 24 hours for patients with metastatic spinal cord compression. This timely delivery of treatment is critical to optimizing functional status and quality of life in patients requiring treatment for oncologic emergencies. Revisiting treatment pathways for such situations at regular intervals is crucial given that residents and staff may rotate and be unfamiliar with emergency protocols.
Communication With Radiation Oncologists
Several studies have demonstrated that the inability to contact a radiation oncologist and poor communication result in decreased referrals for palliative radiotherapy.12,13 Our study demonstrates that onsite radiation oncology is associated with improved ability to contact a radiation oncologist. About 20% of clinicians at facilities without onsite radiation oncology reported difficulty contacting a radiation oncologist, compared with 0% at facilities with onsite radiation departments (P = .006).
It is possible that increased radiation oncology presence at VHA medical centers, through attenuation of barriers related to contacting a radiation oncologist and improved communication, would lead to increased use of radiotherapy. Increased communication between referring clinicians and radiation oncologists also can help with education of those clinicians making the referral. Since knowledge gaps have been identified in multiple studies as a barrier to referral for radiotherapy, such communication and increased education on the role of radiotherapy could increase use.12-14
Patient Travel
Patient ability to travel was the most commonly reported barrier (81%) to referral for palliative radiotherapy in our study. Travel time and transportation difficulties have been established in multiple studies as barriers to radiotherapy for both definitive and palliative management.15-18 Travel for radiotherapy was much less frequently reported as a barrier among respondents with onsite radiation oncology departments compared with those without onsite radiation departments (28% vs 71%, respectively; P < .001).
It is therefore possible that expansion of VHA radiation oncology services, allowing for provision of onsite radiotherapy at more VHA facilities, would reduce travel burden. Increasing travel accommodations for patients and provision of patient lodging on hospital campuses, which is already offered at some VHA medical centers (ie, Fisher House Foundation), could also help attenuate this barrier.
Multidisciplinary Tumor Boards
Our study demonstrates that centers with onsite radiation departments more frequently hold multidisciplinary tumor boards compared with centers without radiation departments (31% vs 3%, respectively; P = .01). Multidisciplinary tumor boards allow subspecialties to meet regularly to communicate about patient care and can help mitigate barriers related to communication and education of the referring health care practitioners.
As cases are discussed in multidisciplinary tumor boards, health care practitioners have the opportunity to make recommendations and provide education on potential benefits and/or downsides of treatments offered by their respective specialties. Several studies have demonstrated that cases discussed at multidisciplinary tumor boards are more likely to be referred for radiation therapy.19-21 Furthermore, multidisciplinary tumor boards have been associated with improved treatment outcomes.22
Conclusions
In this consensus statement the VHA Palliative Radiotherapy Taskforce recommends the optimization of use of radiotherapy within the VHA. Radiation oncology services should be maintained where present in the VHA, with consideration for expansion of services to additional facilities. Telehealth should be used to expedite consults and treatment. Hypofractionation should be used, when appropriate, to ease travel burden. Options for transportation services and onsite housing, or hospitalization, should be understood by practitioners and offered to patients to mitigate barriers related to travel.
1. Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144. doi:10.1016/j.radonc.2014.03.024
2. Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153-1186. doi:10.1016/S1470-2045(15)00222-3
3. Gutt R, Malhotra S, Hagan MP, et al. Palliative radiotherapy within the Veterans Health Administration: barriers to referral and timeliness of treatment. JCO Oncol Pract. 2021;17(12):e1913-e1922. doi:10.1200/OP.20.00981
4. Agazaryan N, Chow P, Lamb J, et al. The timeliness initiative: continuous process improvement for prompt initiation of radiation therapy treatment. Adv Radiat Oncol. 2020;5(5):1014-1021. Published 2020 Mar 10. doi:10.1016/j.adro.2020.01.007
5. Gillan C, Briggs K, Goytisolo Pazos A, et al. Barriers to accessing radiation therapy in Canada: a systematic review. Radiat Oncol. 2012;7:167. Published 2012 Oct 12. doi:10.1186/1748-717X-7-167
6. Hanna TP, Richardson H, Peng Y, Kong W, Zhang-Salomons J, Mackillop WJ. A population-based study of factors affecting the use of radiotherapy for endometrial cancer. Clin Oncol (R Coll Radiol). 2012;24(8):e113-e124. doi:10.1016/j.clon.2012.01.007
7. Ho AS, Kim S, Tighiouart M, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124(15):3154-3162. doi:10.1002/cncr.31533
8. Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. Published 2020 Dec 1. doi:10.1001/jamanetworkopen.2020.30072
9. Mitera G, Swaminath A, Wong S, et al. Radiotherapy for oncologic emergencies on weekends: examining reasons for treatment and patterns of practice at a Canadian cancer centre. Curr Oncol. 2009;16(4):55-60. doi:10.3747/co.v16i4.352
10. Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine (Phila Pa 1976). 2016;41 (Suppl 20):S224-S230. doi:10.1097/BRS.0000000000001827
11. Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21. doi:10.1136/bmj.317.7150.18
12. Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol. 2006;78(1):101-106. doi:10.1016/j.radonc.2005.11.008
13. McCloskey SA, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13(2):130-137. doi:10.1097/PPO.0b013e31804675d4
14. Chierchini S, Ingrosso G, Saldi S, Stracci F, Aristei C. Physician and patient barriers to radiotherapy service access: treatment referral implications. Cancer Manag Res. 2019;11:8829-8833. Published 2019 Oct 7. doi:10.2147/CMAR.S168941
15. Longacre CF, Neprash HT, Shippee ND, Tuttle TM, Virnig BA. Travel, treatment choice, and survival among breast cancer patients: a population-based analysis. Womens Health Rep (New Rochelle). 2021;2(1):1-10. Published 2021 Jan 11. doi:10.1089/whr.2020.0094
16. Yang DD, Muralidhar V, Mahal BA, et al. Travel distance as a barrier to receipt of adjuvant radiation therapy after radical Prostatectomy. Am J Clin Oncol. 2018;41(10):953-959. doi:10.1097/COC.0000000000000410
17. Sundaresan P, King M, Stockler M, Costa D, Milross C. Barriers to radiotherapy utilization: Consumer perceptions of issues influencing radiotherapy-related decisions. Asia Pac J Clin Oncol. 2017;13(5):e489-e496. doi:10.1111/ajco.12579
18. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378-1385. doi:10.1634/theoncologist.2015-0110
19. Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39(12):838-841. doi:10.1111/j.1445-5994.2009.02019.x
20. Brännström F, Bjerregaard JK, Winbladh A, et al. Multidisciplinary team conferences promote treatment according to guidelines in rectal cancer. Acta Oncol. 2015;54(4):447-453. doi:10.3109/0284186X.2014.952387
21. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72. doi:10.1016/j.ctrv.2015.11.007
22. Freytag M, Herrlinger U, Hauser S, et al. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC Cancer. 2020;20(1):355. Published 2020 Apr 28. doi:10.1186/s12885-020-06809-1
1. Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144. doi:10.1016/j.radonc.2014.03.024
2. Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153-1186. doi:10.1016/S1470-2045(15)00222-3
3. Gutt R, Malhotra S, Hagan MP, et al. Palliative radiotherapy within the Veterans Health Administration: barriers to referral and timeliness of treatment. JCO Oncol Pract. 2021;17(12):e1913-e1922. doi:10.1200/OP.20.00981
4. Agazaryan N, Chow P, Lamb J, et al. The timeliness initiative: continuous process improvement for prompt initiation of radiation therapy treatment. Adv Radiat Oncol. 2020;5(5):1014-1021. Published 2020 Mar 10. doi:10.1016/j.adro.2020.01.007
5. Gillan C, Briggs K, Goytisolo Pazos A, et al. Barriers to accessing radiation therapy in Canada: a systematic review. Radiat Oncol. 2012;7:167. Published 2012 Oct 12. doi:10.1186/1748-717X-7-167
6. Hanna TP, Richardson H, Peng Y, Kong W, Zhang-Salomons J, Mackillop WJ. A population-based study of factors affecting the use of radiotherapy for endometrial cancer. Clin Oncol (R Coll Radiol). 2012;24(8):e113-e124. doi:10.1016/j.clon.2012.01.007
7. Ho AS, Kim S, Tighiouart M, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124(15):3154-3162. doi:10.1002/cncr.31533
8. Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. Published 2020 Dec 1. doi:10.1001/jamanetworkopen.2020.30072
9. Mitera G, Swaminath A, Wong S, et al. Radiotherapy for oncologic emergencies on weekends: examining reasons for treatment and patterns of practice at a Canadian cancer centre. Curr Oncol. 2009;16(4):55-60. doi:10.3747/co.v16i4.352
10. Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine (Phila Pa 1976). 2016;41 (Suppl 20):S224-S230. doi:10.1097/BRS.0000000000001827
11. Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21. doi:10.1136/bmj.317.7150.18
12. Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol. 2006;78(1):101-106. doi:10.1016/j.radonc.2005.11.008
13. McCloskey SA, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13(2):130-137. doi:10.1097/PPO.0b013e31804675d4
14. Chierchini S, Ingrosso G, Saldi S, Stracci F, Aristei C. Physician and patient barriers to radiotherapy service access: treatment referral implications. Cancer Manag Res. 2019;11:8829-8833. Published 2019 Oct 7. doi:10.2147/CMAR.S168941
15. Longacre CF, Neprash HT, Shippee ND, Tuttle TM, Virnig BA. Travel, treatment choice, and survival among breast cancer patients: a population-based analysis. Womens Health Rep (New Rochelle). 2021;2(1):1-10. Published 2021 Jan 11. doi:10.1089/whr.2020.0094
16. Yang DD, Muralidhar V, Mahal BA, et al. Travel distance as a barrier to receipt of adjuvant radiation therapy after radical Prostatectomy. Am J Clin Oncol. 2018;41(10):953-959. doi:10.1097/COC.0000000000000410
17. Sundaresan P, King M, Stockler M, Costa D, Milross C. Barriers to radiotherapy utilization: Consumer perceptions of issues influencing radiotherapy-related decisions. Asia Pac J Clin Oncol. 2017;13(5):e489-e496. doi:10.1111/ajco.12579
18. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378-1385. doi:10.1634/theoncologist.2015-0110
19. Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39(12):838-841. doi:10.1111/j.1445-5994.2009.02019.x
20. Brännström F, Bjerregaard JK, Winbladh A, et al. Multidisciplinary team conferences promote treatment according to guidelines in rectal cancer. Acta Oncol. 2015;54(4):447-453. doi:10.3109/0284186X.2014.952387
21. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72. doi:10.1016/j.ctrv.2015.11.007
22. Freytag M, Herrlinger U, Hauser S, et al. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC Cancer. 2020;20(1):355. Published 2020 Apr 28. doi:10.1186/s12885-020-06809-1
A Learning Health System Approach to Long COVID Care
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
A Practical and Cost-Effective Approach to the Diagnosis of Heparin-Induced Thrombocytopenia: A Single-Center Quality Improvement Study
From the Veterans Affairs Ann Arbor Healthcare System Medicine Service (Dr. Cusick), University of Michigan College of Pharmacy, Clinical Pharmacy Service, Michigan Medicine (Dr. Hanigan), Department of Internal Medicine Clinical Experience and Quality, Michigan Medicine (Linda Bashaw), Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI (Dr. Heidemann), and the Operational Excellence Department, Sparrow Health System, Lansing, MI (Matthew Johnson).
Abstract
Background: Diagnosis of heparin-induced thrombocytopenia (HIT) requires completion of an enzyme-linked immunosorbent assay (ELISA)–based heparin-platelet factor 4 (PF4) antibody test. If this test is negative, HIT is excluded. If positive, a serotonin-release assay (SRA) test is indicated. The SRA is expensive and sometimes inappropriately ordered despite negative PF4 results, leading to unnecessary treatment with argatroban while awaiting SRA results.
Objectives: The primary objectives of this project were to reduce unnecessary SRA testing and argatroban utilization in patients with suspected HIT.
Methods: The authors implemented an intervention at a tertiary care academic hospital in November 2017 targeting patients hospitalized with suspected HIT. The intervention was controlled at the level of the laboratory and prevented ordering of SRA tests in the absence of a positive PF4 test. The number of SRA tests performed and argatroban bags administered were identified retrospectively via chart review before the intervention (January 2016 to November 2017) and post intervention (December 2017 to March 2020). Associated costs were calculated based on institutional SRA testing cost as well as the average wholesale price of argatroban.
Results: SRA testing decreased from an average of 3.7 SRA results per 1000 admissions before the intervention to an average of 0.6 results per 1000 admissions post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 prior to the intervention to 14.3 post intervention. Total estimated cost savings per 1000 admissions was $2361.20.
Conclusion: An evidence-based testing strategy for HIT can be effectively implemented at the level of the laboratory. This approach led to reductions in SRA testing and argatroban utilization with resultant cost savings.
Keywords: HIT, argatroban, anticoagulation, serotonin-release assay.
Thrombocytopenia is a common finding in hospitalized patients.1,2 Heparin-induced thrombocytopenia (HIT) is one of the many potential causes of thrombocytopenia in hospitalized patients and occurs when antibodies to the heparin-platelet factor 4 (PF4) complex develop after heparin exposure. This triggers a cascade of events, leading to platelet activation, platelet consumption, and thrombosis. While HIT is relatively rare, occurring in 0.3% to 0.5% of critically ill patients, many patients will be tested to rule out this potentially life-threatening cause of thrombocytopenia.3
The diagnosis of HIT utilizes a combination of both clinical suspicion and laboratory testing.4 The 4T score (Table) was developed to evaluate the clinical probability of HIT and involves assessing the degree and timing of thrombocytopenia, the presence or absence of thrombosis, and other potential causes of the thrombocytopenia.5 The 4T score is designed to be utilized to identify patients who require laboratory testing for HIT; however, it has low inter-rater agreement in patients undergoing evaluation for HIT,6 and, in our experience, completion of this scoring is time-consuming.
The enzyme-linked immunosorbent assay (ELISA) is a commonly used laboratory test to diagnose HIT that detects antibodies to the heparin-PF4 complex utilizing optical density (OD) units. When using an OD cutoff of 0.400, ELISA PF4 (PF4) tests have a sensitivity of 99.6%, but poor specificity at 69.3%.7 When the PF4 antibody test is positive with an OD ≥0.400, then a functional test is used to determine whether the antibodies detected will activate platelets. The serotonin-release assay (SRA) is a functional test that measures 14C-labeled serotonin release from donor platelets when mixed with patient serum or plasma containing HIT antibodies. In the correct clinical context, a positive ELISA PF4 antibody test along with a positive SRA is diagnostic of HIT.8
The process of diagnosing HIT in a timely and cost-effective manner is dependent on the clinician’s experience in diagnosing HIT as well as access to the laboratory testing necessary to confirm the diagnosis. PF4 antibody tests are time-consuming and not always available daily and/or are not available onsite. The SRA requires access to donor platelets and specialized radioactivity counting equipment, making it available only at particular centers.
The treatment of HIT is more straightforward and involves stopping all heparin products and starting a nonheparin anticoagulant. The direct thrombin inhibitor argatroban is one of the standard nonheparin anticoagulants used in patients with suspected HIT.4 While it is expensive, its short half-life and lack of renal clearance make it ideal for treatment of hospitalized patients with suspected HIT, many of whom need frequent procedures and/or have renal disease.
At our academic tertiary care center, we performed a retrospective analysis that showed inappropriate ordering of diagnostic HIT testing as well as unnecessary use of argatroban even when there was low suspicion for HIT based on laboratory findings. The aim of our project was to reduce unnecessary HIT testing and argatroban utilization without overburdening providers or interfering with established workflows.
Methods
Setting
The University of Michigan (UM) hospital is a 1000-bed tertiary care center in Ann Arbor, Michigan. The UM guidelines reflect evidence-based guidelines for the diagnosis and treatment of HIT.4 In 2016 the UM guidelines for laboratory testing included sending the PF4 antibody test first when there was clinical suspicion of HIT. The SRA was to be sent separately only when the PF4 returned positive (OD ≥ 0.400). Standard guidelines at UM also included switching patients with suspected HIT from heparin to a nonheparin anticoagulant and stopping all heparin products while awaiting the SRA results. The direct thrombin inhibitor argatroban is utilized at UM and monitored with anti-IIa levels. University of Michigan Hospital utilizes the Immucor PF4 IgG ELISA for detecting heparin-associated antibodies.9 In 2016, this PF4 test was performed in the UM onsite laboratory Monday through Friday. At UM the SRA is performed off site, with a turnaround time of 3 to 5 business days.
Baseline Data
We retrospectively reviewed PF4 and SRA testing as well as argatroban usage from December 2016 to May 2017. Despite the institutional guidelines, providers were sending PF4 and SRA simultaneously as soon as HIT was suspected; 62% of PF4 tests were ordered simultaneously with the SRA, but only 8% of these PF4 tests were positive with an OD ≥0.400. Of those patients with negative PF4 testing, argatroban was continued until the SRA returned negative, leading to many days of unnecessary argatroban usage. An informal survey of the anticoagulation pharmacists revealed that many recommended discontinuing argatroban when the PF4 test was negative, but providers routinely did not feel comfortable with this approach. This suggested many providers misunderstood the performance characteristics of the PF4 test.
Intervention
Our team consisted of hematology and internal medicine faculty, pharmacists, coagulation laboratory personnel, and quality improvement specialists. We designed and implemented an intervention in November 2017 focused on controlling the ordering of the SRA test. We chose to focus on this step due to the excellent sensitivity of the PF4 test with a cutoff of OD <0.400 and the significant expense of the SRA test. Under direction of the Coagulation Laboratory Director, a standard operating procedure was developed where the coagulation laboratory personnel did not send out the SRA until a positive PF4 test (OD ≥ 0.400) was reported. If the PF4 was negative, the SRA was canceled and the ordering provider received notification of the cancelled test via the electronic medical record, accompanied by education about HIT testing (Figure 1). In addition, the lab increased the availability of PF4 testing from 5 days to 7 days a week so there were no delays in tests ordered on Fridays or weekends.

Outcomes
Our primary goals were to decrease both SRA testing and argatroban use. Secondarily, we examined the cost-effectiveness of this intervention. We hypothesized that controlling the SRA testing at the laboratory level would decrease both SRA testing and argatroban use.
Data Collection
Pre- and postintervention data were collected retrospectively. Pre-intervention data were from January 2016 through November 2017, and postintervention data were from December 2017 through March 2020. The number of SRA tests performed were identified retrospectively via review of electronic ordering records. All patients who had a hospital admission after January 1, 2016, were included. These patients were filtered to include only those who had a result for an SRA test. In order to calculate cost-savings, we identified both the number of SRA tests ordered retrospectively as well as patients who had both an SRA resulted and had been administered argatroban. Cost-savings were calculated based on our institutional cost of $357 per SRA test.
At our institution, argatroban is supplied in 50-mL bags; therefore, we utilized the number of bags to identify argatroban usage. Savings were calculated using the average wholesale price (AWP) of $292.50 per 50-mL bag. The amounts billed or collected for the SRA testing or argatroban treatment were not collected. Costs were estimated using only direct costs to the institution. Safety data were not collected. As the intent of our project was a quality improvement activity, this project did not require institutional review board regulation per our institutional guidance.
Results
During the pre-intervention period, the average number of admissions (adults and children) at UM was 5863 per month. Post intervention there was an average of 5842 admissions per month. A total of 1192 PF4 tests were ordered before the intervention and 1148 were ordered post intervention. Prior to the intervention, 481 SRA tests were completed, while post intervention 105 were completed. Serotonin-release testing decreased from an average of 3.7 SRA results per 1000 admissions during the pre-intervention period to an average of 0.6 per 1000 admissions post intervention (Figure 2). Cost-savings were $1045 per 1000 admissions.

During the pre-intervention period, 2539 bags of argatroban were used, while 2337 bags were used post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 before the intervention to 14.3 post intervention. Cost-savings were $1316.20 per 1000 admissions. Figure 3 illustrates the monthly argatroban utilization per 1000 admissions during each quarter from January 2016 through March 2020.

Discussion
We designed and implemented an evidence-based strategy for HIT at our academic institution which led to a decrease in unnecessary SRA testing and argatroban utilization, with associated cost savings. By focusing on a single point of intervention at the laboratory level where SRA tests were held and canceled if the PF4 test was negative, we helped offload the decision-making from the provider while simultaneously providing just-in-time education to the provider. This intervention was designed with input from multiple stakeholders, including physicians, quality improvement specialists, pharmacists, and coagulation laboratory personnel.
Serotonin-release testing dramatically decreased post intervention even though a similar number of PF4 tests were performed before and after the intervention. This suggests that the decrease in SRA testing was a direct consequence of our intervention. Post intervention the number of completed SRA tests was 9% of the number of PF4 tests sent. This is consistent with our baseline pre-intervention data showing that only 8% of all PF4 tests sent were positive.
While the absolute number of argatroban bags utilized did not dramatically decrease after the intervention, the quarterly rate did, particularly after 2018. Given that argatroban data were only drawn from patients with a concurrent SRA test, this decrease is clearly from decreased usage in patients with suspected HIT. We suspect the decrease occurred because argatroban was not being continued while awaiting an SRA test in patients with a negative PF4 test. Decreasing the utilization of argatroban not only saved money but also reduced days of exposure to argatroban. While we do not have data regarding adverse events related to argatroban prior to the intervention, it is logical to conclude that reducing unnecessary exposure to argatroban reduces the risk of adverse events related to bleeding. Future studies would ideally address specific safety outcome metrics such as adverse events, bleeding risk, or missed diagnoses of HIT.
Our institutional guidelines for the diagnosis of HIT are evidence-based and helpful but are rarely followed by busy inpatient providers. Controlling the utilization of the SRA at the laboratory level had several advantages. First, removing SRA decision-making from providers who are not experts in the diagnosis of HIT guaranteed adherence to evidence-based guidelines. Second, pharmacists could safely recommend discontinuing argatroban when the PF4 test was negative as there was no SRA pending. Third, with cancellation at the laboratory level there was no need to further burden providers with yet another alert in the electronic health record. Fourth, just-in-time education was provided to the providers with justification for why the SRA test was canceled. Last, ruling out HIT within 24 hours with the PF4 test alone allowed providers to evaluate patients for other causes of thrombocytopenia much earlier than the 3 to 5 business days before the SRA results returned.
A limitation of this study is that it was conducted at a single center. Our approach is also limited by the lack of universal applicability. At our institution we are fortunate to have PF4 testing available in our coagulation laboratory 7 days a week. In addition, the coagulation laboratory controls sending the SRA to the reference laboratory. The specific intervention of controlling the SRA testing is therefore applicable only to institutions similar to ours; however, the concept of removing control of specialized testing from the provider is not unique. Inpatient thrombophilia testing has been a successful target of this approach.11-13 While electronic alerts and education of individual providers can also be effective initially, the effectiveness of these interventions has been repeatedly shown to wane over time.14-16
Conclusion
At our institution we were able to implement practical, evidence-based testing for HIT by implementing control over SRA testing at the level of the laboratory. This approach led to decreased argatroban utilization and cost savings.
Corresponding author: Alice Cusick, MD; LTC Charles S Kettles VA Medical Center, 2215 Fuller Road, Ann Arbor, MI 48105; mccoyag@med.umich.edu
Disclosures: None reported.
doi: 10.12788/jcom.0087
1. Fountain E, Arepally GM. Thrombocytopenia in hospitalized non-ICU patients. Blood. 2015;126(23):1060. doi:10.1182/blood.v126.23.1060.1060
2. Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139(2):271-278. doi:10.1378/chest.10-2243
3. Warkentin TE. Heparin-induced thrombocytopenia. Curr Opin Crit Care. 2015;21(6):576-585. doi:10.1097/MCC.0000000000000259
4. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. doi:10.1182/bloodadvances.2018024489
5. Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. doi:10.1182/blood-2012-07-443051
6. Northam KA, Parker WF, Chen S-L, et al. Evaluation of 4Ts score inter-rater agreement in patients undergoing evaluation for heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2021;32(5):328-334. doi:10.1097/MBC.0000000000001042
7. Raschke RA, Curry SC, Warkentin TE, Gerkin RD. Improving clinical interpretation of the anti-platelet factor 4/heparin enzyme-linked immunosorbent assay for the diagnosis of heparin-induced thrombocytopenia through the use of receiver operating characteristic analysis, stratum-specific likelihood ratios, and Bayes theorem. Chest. 2013;144(4):1269-1275. doi:10.1378/chest.12-2712
8. Warkentin TE, Arnold DM, Nazi I, Kelton JG. The platelet serotonin-release assay. Am J Hematol. 2015;90(6):564-572. doi:10.1002/ajh.24006
9. Use IFOR, Contents TOF. LIFECODES ® PF4 IgG assay:1-9.
10. Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):1-9. doi:10.1186/s12911-017-0430-8
11. O’Connor N, Carter-Johnson R. Effective screening of pathology tests controls costs: thrombophilia testing. J Clin Pathol. 2006;59(5):556. doi:10.1136/jcp.2005.030700
12. Lim MY, Greenberg CS. Inpatient thrombophilia testing: Impact of healthcare system technology and targeted clinician education on changing practice patterns. Vasc Med (United Kingdom). 2018;23(1):78-79. doi:10.1177/1358863X17742509
13. Cox JL, Shunkwiler SM, Koepsell SA. Requirement for a pathologist’s second signature limits inappropriate inpatient thrombophilia testing. Lab Med. 2017;48(4):367-371. doi:10.1093/labmed/lmx040
14. Kwang H, Mou E, Richman I, et al. Thrombophilia testing in the inpatient setting: impact of an educational intervention. BMC Med Inform Decis Mak. 2019;19(1):167. doi:10.1186/s12911-019-0889-6
15. Shah T, Patel-Teague S, Kroupa L, Meyer AND, Singh H. Impact of a national QI programme on reducing electronic health record notifications to clinicians. BMJ Qual Saf. 2019;28(1):10-14. doi:10.1136/bmjqs-2017-007447
16. Singh H, Spitzmueller C, Petersen NJ, Sawhney MK, Sittig DF. Information overload and missed test results in electronic health record-based settings. JAMA Intern Med. 2013;173(8):702-704. doi:10.1001/2013.jamainternmed.61
From the Veterans Affairs Ann Arbor Healthcare System Medicine Service (Dr. Cusick), University of Michigan College of Pharmacy, Clinical Pharmacy Service, Michigan Medicine (Dr. Hanigan), Department of Internal Medicine Clinical Experience and Quality, Michigan Medicine (Linda Bashaw), Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI (Dr. Heidemann), and the Operational Excellence Department, Sparrow Health System, Lansing, MI (Matthew Johnson).
Abstract
Background: Diagnosis of heparin-induced thrombocytopenia (HIT) requires completion of an enzyme-linked immunosorbent assay (ELISA)–based heparin-platelet factor 4 (PF4) antibody test. If this test is negative, HIT is excluded. If positive, a serotonin-release assay (SRA) test is indicated. The SRA is expensive and sometimes inappropriately ordered despite negative PF4 results, leading to unnecessary treatment with argatroban while awaiting SRA results.
Objectives: The primary objectives of this project were to reduce unnecessary SRA testing and argatroban utilization in patients with suspected HIT.
Methods: The authors implemented an intervention at a tertiary care academic hospital in November 2017 targeting patients hospitalized with suspected HIT. The intervention was controlled at the level of the laboratory and prevented ordering of SRA tests in the absence of a positive PF4 test. The number of SRA tests performed and argatroban bags administered were identified retrospectively via chart review before the intervention (January 2016 to November 2017) and post intervention (December 2017 to March 2020). Associated costs were calculated based on institutional SRA testing cost as well as the average wholesale price of argatroban.
Results: SRA testing decreased from an average of 3.7 SRA results per 1000 admissions before the intervention to an average of 0.6 results per 1000 admissions post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 prior to the intervention to 14.3 post intervention. Total estimated cost savings per 1000 admissions was $2361.20.
Conclusion: An evidence-based testing strategy for HIT can be effectively implemented at the level of the laboratory. This approach led to reductions in SRA testing and argatroban utilization with resultant cost savings.
Keywords: HIT, argatroban, anticoagulation, serotonin-release assay.
Thrombocytopenia is a common finding in hospitalized patients.1,2 Heparin-induced thrombocytopenia (HIT) is one of the many potential causes of thrombocytopenia in hospitalized patients and occurs when antibodies to the heparin-platelet factor 4 (PF4) complex develop after heparin exposure. This triggers a cascade of events, leading to platelet activation, platelet consumption, and thrombosis. While HIT is relatively rare, occurring in 0.3% to 0.5% of critically ill patients, many patients will be tested to rule out this potentially life-threatening cause of thrombocytopenia.3
The diagnosis of HIT utilizes a combination of both clinical suspicion and laboratory testing.4 The 4T score (Table) was developed to evaluate the clinical probability of HIT and involves assessing the degree and timing of thrombocytopenia, the presence or absence of thrombosis, and other potential causes of the thrombocytopenia.5 The 4T score is designed to be utilized to identify patients who require laboratory testing for HIT; however, it has low inter-rater agreement in patients undergoing evaluation for HIT,6 and, in our experience, completion of this scoring is time-consuming.
The enzyme-linked immunosorbent assay (ELISA) is a commonly used laboratory test to diagnose HIT that detects antibodies to the heparin-PF4 complex utilizing optical density (OD) units. When using an OD cutoff of 0.400, ELISA PF4 (PF4) tests have a sensitivity of 99.6%, but poor specificity at 69.3%.7 When the PF4 antibody test is positive with an OD ≥0.400, then a functional test is used to determine whether the antibodies detected will activate platelets. The serotonin-release assay (SRA) is a functional test that measures 14C-labeled serotonin release from donor platelets when mixed with patient serum or plasma containing HIT antibodies. In the correct clinical context, a positive ELISA PF4 antibody test along with a positive SRA is diagnostic of HIT.8
The process of diagnosing HIT in a timely and cost-effective manner is dependent on the clinician’s experience in diagnosing HIT as well as access to the laboratory testing necessary to confirm the diagnosis. PF4 antibody tests are time-consuming and not always available daily and/or are not available onsite. The SRA requires access to donor platelets and specialized radioactivity counting equipment, making it available only at particular centers.
The treatment of HIT is more straightforward and involves stopping all heparin products and starting a nonheparin anticoagulant. The direct thrombin inhibitor argatroban is one of the standard nonheparin anticoagulants used in patients with suspected HIT.4 While it is expensive, its short half-life and lack of renal clearance make it ideal for treatment of hospitalized patients with suspected HIT, many of whom need frequent procedures and/or have renal disease.
At our academic tertiary care center, we performed a retrospective analysis that showed inappropriate ordering of diagnostic HIT testing as well as unnecessary use of argatroban even when there was low suspicion for HIT based on laboratory findings. The aim of our project was to reduce unnecessary HIT testing and argatroban utilization without overburdening providers or interfering with established workflows.
Methods
Setting
The University of Michigan (UM) hospital is a 1000-bed tertiary care center in Ann Arbor, Michigan. The UM guidelines reflect evidence-based guidelines for the diagnosis and treatment of HIT.4 In 2016 the UM guidelines for laboratory testing included sending the PF4 antibody test first when there was clinical suspicion of HIT. The SRA was to be sent separately only when the PF4 returned positive (OD ≥ 0.400). Standard guidelines at UM also included switching patients with suspected HIT from heparin to a nonheparin anticoagulant and stopping all heparin products while awaiting the SRA results. The direct thrombin inhibitor argatroban is utilized at UM and monitored with anti-IIa levels. University of Michigan Hospital utilizes the Immucor PF4 IgG ELISA for detecting heparin-associated antibodies.9 In 2016, this PF4 test was performed in the UM onsite laboratory Monday through Friday. At UM the SRA is performed off site, with a turnaround time of 3 to 5 business days.
Baseline Data
We retrospectively reviewed PF4 and SRA testing as well as argatroban usage from December 2016 to May 2017. Despite the institutional guidelines, providers were sending PF4 and SRA simultaneously as soon as HIT was suspected; 62% of PF4 tests were ordered simultaneously with the SRA, but only 8% of these PF4 tests were positive with an OD ≥0.400. Of those patients with negative PF4 testing, argatroban was continued until the SRA returned negative, leading to many days of unnecessary argatroban usage. An informal survey of the anticoagulation pharmacists revealed that many recommended discontinuing argatroban when the PF4 test was negative, but providers routinely did not feel comfortable with this approach. This suggested many providers misunderstood the performance characteristics of the PF4 test.
Intervention
Our team consisted of hematology and internal medicine faculty, pharmacists, coagulation laboratory personnel, and quality improvement specialists. We designed and implemented an intervention in November 2017 focused on controlling the ordering of the SRA test. We chose to focus on this step due to the excellent sensitivity of the PF4 test with a cutoff of OD <0.400 and the significant expense of the SRA test. Under direction of the Coagulation Laboratory Director, a standard operating procedure was developed where the coagulation laboratory personnel did not send out the SRA until a positive PF4 test (OD ≥ 0.400) was reported. If the PF4 was negative, the SRA was canceled and the ordering provider received notification of the cancelled test via the electronic medical record, accompanied by education about HIT testing (Figure 1). In addition, the lab increased the availability of PF4 testing from 5 days to 7 days a week so there were no delays in tests ordered on Fridays or weekends.

Outcomes
Our primary goals were to decrease both SRA testing and argatroban use. Secondarily, we examined the cost-effectiveness of this intervention. We hypothesized that controlling the SRA testing at the laboratory level would decrease both SRA testing and argatroban use.
Data Collection
Pre- and postintervention data were collected retrospectively. Pre-intervention data were from January 2016 through November 2017, and postintervention data were from December 2017 through March 2020. The number of SRA tests performed were identified retrospectively via review of electronic ordering records. All patients who had a hospital admission after January 1, 2016, were included. These patients were filtered to include only those who had a result for an SRA test. In order to calculate cost-savings, we identified both the number of SRA tests ordered retrospectively as well as patients who had both an SRA resulted and had been administered argatroban. Cost-savings were calculated based on our institutional cost of $357 per SRA test.
At our institution, argatroban is supplied in 50-mL bags; therefore, we utilized the number of bags to identify argatroban usage. Savings were calculated using the average wholesale price (AWP) of $292.50 per 50-mL bag. The amounts billed or collected for the SRA testing or argatroban treatment were not collected. Costs were estimated using only direct costs to the institution. Safety data were not collected. As the intent of our project was a quality improvement activity, this project did not require institutional review board regulation per our institutional guidance.
Results
During the pre-intervention period, the average number of admissions (adults and children) at UM was 5863 per month. Post intervention there was an average of 5842 admissions per month. A total of 1192 PF4 tests were ordered before the intervention and 1148 were ordered post intervention. Prior to the intervention, 481 SRA tests were completed, while post intervention 105 were completed. Serotonin-release testing decreased from an average of 3.7 SRA results per 1000 admissions during the pre-intervention period to an average of 0.6 per 1000 admissions post intervention (Figure 2). Cost-savings were $1045 per 1000 admissions.

During the pre-intervention period, 2539 bags of argatroban were used, while 2337 bags were used post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 before the intervention to 14.3 post intervention. Cost-savings were $1316.20 per 1000 admissions. Figure 3 illustrates the monthly argatroban utilization per 1000 admissions during each quarter from January 2016 through March 2020.

Discussion
We designed and implemented an evidence-based strategy for HIT at our academic institution which led to a decrease in unnecessary SRA testing and argatroban utilization, with associated cost savings. By focusing on a single point of intervention at the laboratory level where SRA tests were held and canceled if the PF4 test was negative, we helped offload the decision-making from the provider while simultaneously providing just-in-time education to the provider. This intervention was designed with input from multiple stakeholders, including physicians, quality improvement specialists, pharmacists, and coagulation laboratory personnel.
Serotonin-release testing dramatically decreased post intervention even though a similar number of PF4 tests were performed before and after the intervention. This suggests that the decrease in SRA testing was a direct consequence of our intervention. Post intervention the number of completed SRA tests was 9% of the number of PF4 tests sent. This is consistent with our baseline pre-intervention data showing that only 8% of all PF4 tests sent were positive.
While the absolute number of argatroban bags utilized did not dramatically decrease after the intervention, the quarterly rate did, particularly after 2018. Given that argatroban data were only drawn from patients with a concurrent SRA test, this decrease is clearly from decreased usage in patients with suspected HIT. We suspect the decrease occurred because argatroban was not being continued while awaiting an SRA test in patients with a negative PF4 test. Decreasing the utilization of argatroban not only saved money but also reduced days of exposure to argatroban. While we do not have data regarding adverse events related to argatroban prior to the intervention, it is logical to conclude that reducing unnecessary exposure to argatroban reduces the risk of adverse events related to bleeding. Future studies would ideally address specific safety outcome metrics such as adverse events, bleeding risk, or missed diagnoses of HIT.
Our institutional guidelines for the diagnosis of HIT are evidence-based and helpful but are rarely followed by busy inpatient providers. Controlling the utilization of the SRA at the laboratory level had several advantages. First, removing SRA decision-making from providers who are not experts in the diagnosis of HIT guaranteed adherence to evidence-based guidelines. Second, pharmacists could safely recommend discontinuing argatroban when the PF4 test was negative as there was no SRA pending. Third, with cancellation at the laboratory level there was no need to further burden providers with yet another alert in the electronic health record. Fourth, just-in-time education was provided to the providers with justification for why the SRA test was canceled. Last, ruling out HIT within 24 hours with the PF4 test alone allowed providers to evaluate patients for other causes of thrombocytopenia much earlier than the 3 to 5 business days before the SRA results returned.
A limitation of this study is that it was conducted at a single center. Our approach is also limited by the lack of universal applicability. At our institution we are fortunate to have PF4 testing available in our coagulation laboratory 7 days a week. In addition, the coagulation laboratory controls sending the SRA to the reference laboratory. The specific intervention of controlling the SRA testing is therefore applicable only to institutions similar to ours; however, the concept of removing control of specialized testing from the provider is not unique. Inpatient thrombophilia testing has been a successful target of this approach.11-13 While electronic alerts and education of individual providers can also be effective initially, the effectiveness of these interventions has been repeatedly shown to wane over time.14-16
Conclusion
At our institution we were able to implement practical, evidence-based testing for HIT by implementing control over SRA testing at the level of the laboratory. This approach led to decreased argatroban utilization and cost savings.
Corresponding author: Alice Cusick, MD; LTC Charles S Kettles VA Medical Center, 2215 Fuller Road, Ann Arbor, MI 48105; mccoyag@med.umich.edu
Disclosures: None reported.
doi: 10.12788/jcom.0087
From the Veterans Affairs Ann Arbor Healthcare System Medicine Service (Dr. Cusick), University of Michigan College of Pharmacy, Clinical Pharmacy Service, Michigan Medicine (Dr. Hanigan), Department of Internal Medicine Clinical Experience and Quality, Michigan Medicine (Linda Bashaw), Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI (Dr. Heidemann), and the Operational Excellence Department, Sparrow Health System, Lansing, MI (Matthew Johnson).
Abstract
Background: Diagnosis of heparin-induced thrombocytopenia (HIT) requires completion of an enzyme-linked immunosorbent assay (ELISA)–based heparin-platelet factor 4 (PF4) antibody test. If this test is negative, HIT is excluded. If positive, a serotonin-release assay (SRA) test is indicated. The SRA is expensive and sometimes inappropriately ordered despite negative PF4 results, leading to unnecessary treatment with argatroban while awaiting SRA results.
Objectives: The primary objectives of this project were to reduce unnecessary SRA testing and argatroban utilization in patients with suspected HIT.
Methods: The authors implemented an intervention at a tertiary care academic hospital in November 2017 targeting patients hospitalized with suspected HIT. The intervention was controlled at the level of the laboratory and prevented ordering of SRA tests in the absence of a positive PF4 test. The number of SRA tests performed and argatroban bags administered were identified retrospectively via chart review before the intervention (January 2016 to November 2017) and post intervention (December 2017 to March 2020). Associated costs were calculated based on institutional SRA testing cost as well as the average wholesale price of argatroban.
Results: SRA testing decreased from an average of 3.7 SRA results per 1000 admissions before the intervention to an average of 0.6 results per 1000 admissions post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 prior to the intervention to 14.3 post intervention. Total estimated cost savings per 1000 admissions was $2361.20.
Conclusion: An evidence-based testing strategy for HIT can be effectively implemented at the level of the laboratory. This approach led to reductions in SRA testing and argatroban utilization with resultant cost savings.
Keywords: HIT, argatroban, anticoagulation, serotonin-release assay.
Thrombocytopenia is a common finding in hospitalized patients.1,2 Heparin-induced thrombocytopenia (HIT) is one of the many potential causes of thrombocytopenia in hospitalized patients and occurs when antibodies to the heparin-platelet factor 4 (PF4) complex develop after heparin exposure. This triggers a cascade of events, leading to platelet activation, platelet consumption, and thrombosis. While HIT is relatively rare, occurring in 0.3% to 0.5% of critically ill patients, many patients will be tested to rule out this potentially life-threatening cause of thrombocytopenia.3
The diagnosis of HIT utilizes a combination of both clinical suspicion and laboratory testing.4 The 4T score (Table) was developed to evaluate the clinical probability of HIT and involves assessing the degree and timing of thrombocytopenia, the presence or absence of thrombosis, and other potential causes of the thrombocytopenia.5 The 4T score is designed to be utilized to identify patients who require laboratory testing for HIT; however, it has low inter-rater agreement in patients undergoing evaluation for HIT,6 and, in our experience, completion of this scoring is time-consuming.
The enzyme-linked immunosorbent assay (ELISA) is a commonly used laboratory test to diagnose HIT that detects antibodies to the heparin-PF4 complex utilizing optical density (OD) units. When using an OD cutoff of 0.400, ELISA PF4 (PF4) tests have a sensitivity of 99.6%, but poor specificity at 69.3%.7 When the PF4 antibody test is positive with an OD ≥0.400, then a functional test is used to determine whether the antibodies detected will activate platelets. The serotonin-release assay (SRA) is a functional test that measures 14C-labeled serotonin release from donor platelets when mixed with patient serum or plasma containing HIT antibodies. In the correct clinical context, a positive ELISA PF4 antibody test along with a positive SRA is diagnostic of HIT.8
The process of diagnosing HIT in a timely and cost-effective manner is dependent on the clinician’s experience in diagnosing HIT as well as access to the laboratory testing necessary to confirm the diagnosis. PF4 antibody tests are time-consuming and not always available daily and/or are not available onsite. The SRA requires access to donor platelets and specialized radioactivity counting equipment, making it available only at particular centers.
The treatment of HIT is more straightforward and involves stopping all heparin products and starting a nonheparin anticoagulant. The direct thrombin inhibitor argatroban is one of the standard nonheparin anticoagulants used in patients with suspected HIT.4 While it is expensive, its short half-life and lack of renal clearance make it ideal for treatment of hospitalized patients with suspected HIT, many of whom need frequent procedures and/or have renal disease.
At our academic tertiary care center, we performed a retrospective analysis that showed inappropriate ordering of diagnostic HIT testing as well as unnecessary use of argatroban even when there was low suspicion for HIT based on laboratory findings. The aim of our project was to reduce unnecessary HIT testing and argatroban utilization without overburdening providers or interfering with established workflows.
Methods
Setting
The University of Michigan (UM) hospital is a 1000-bed tertiary care center in Ann Arbor, Michigan. The UM guidelines reflect evidence-based guidelines for the diagnosis and treatment of HIT.4 In 2016 the UM guidelines for laboratory testing included sending the PF4 antibody test first when there was clinical suspicion of HIT. The SRA was to be sent separately only when the PF4 returned positive (OD ≥ 0.400). Standard guidelines at UM also included switching patients with suspected HIT from heparin to a nonheparin anticoagulant and stopping all heparin products while awaiting the SRA results. The direct thrombin inhibitor argatroban is utilized at UM and monitored with anti-IIa levels. University of Michigan Hospital utilizes the Immucor PF4 IgG ELISA for detecting heparin-associated antibodies.9 In 2016, this PF4 test was performed in the UM onsite laboratory Monday through Friday. At UM the SRA is performed off site, with a turnaround time of 3 to 5 business days.
Baseline Data
We retrospectively reviewed PF4 and SRA testing as well as argatroban usage from December 2016 to May 2017. Despite the institutional guidelines, providers were sending PF4 and SRA simultaneously as soon as HIT was suspected; 62% of PF4 tests were ordered simultaneously with the SRA, but only 8% of these PF4 tests were positive with an OD ≥0.400. Of those patients with negative PF4 testing, argatroban was continued until the SRA returned negative, leading to many days of unnecessary argatroban usage. An informal survey of the anticoagulation pharmacists revealed that many recommended discontinuing argatroban when the PF4 test was negative, but providers routinely did not feel comfortable with this approach. This suggested many providers misunderstood the performance characteristics of the PF4 test.
Intervention
Our team consisted of hematology and internal medicine faculty, pharmacists, coagulation laboratory personnel, and quality improvement specialists. We designed and implemented an intervention in November 2017 focused on controlling the ordering of the SRA test. We chose to focus on this step due to the excellent sensitivity of the PF4 test with a cutoff of OD <0.400 and the significant expense of the SRA test. Under direction of the Coagulation Laboratory Director, a standard operating procedure was developed where the coagulation laboratory personnel did not send out the SRA until a positive PF4 test (OD ≥ 0.400) was reported. If the PF4 was negative, the SRA was canceled and the ordering provider received notification of the cancelled test via the electronic medical record, accompanied by education about HIT testing (Figure 1). In addition, the lab increased the availability of PF4 testing from 5 days to 7 days a week so there were no delays in tests ordered on Fridays or weekends.

Outcomes
Our primary goals were to decrease both SRA testing and argatroban use. Secondarily, we examined the cost-effectiveness of this intervention. We hypothesized that controlling the SRA testing at the laboratory level would decrease both SRA testing and argatroban use.
Data Collection
Pre- and postintervention data were collected retrospectively. Pre-intervention data were from January 2016 through November 2017, and postintervention data were from December 2017 through March 2020. The number of SRA tests performed were identified retrospectively via review of electronic ordering records. All patients who had a hospital admission after January 1, 2016, were included. These patients were filtered to include only those who had a result for an SRA test. In order to calculate cost-savings, we identified both the number of SRA tests ordered retrospectively as well as patients who had both an SRA resulted and had been administered argatroban. Cost-savings were calculated based on our institutional cost of $357 per SRA test.
At our institution, argatroban is supplied in 50-mL bags; therefore, we utilized the number of bags to identify argatroban usage. Savings were calculated using the average wholesale price (AWP) of $292.50 per 50-mL bag. The amounts billed or collected for the SRA testing or argatroban treatment were not collected. Costs were estimated using only direct costs to the institution. Safety data were not collected. As the intent of our project was a quality improvement activity, this project did not require institutional review board regulation per our institutional guidance.
Results
During the pre-intervention period, the average number of admissions (adults and children) at UM was 5863 per month. Post intervention there was an average of 5842 admissions per month. A total of 1192 PF4 tests were ordered before the intervention and 1148 were ordered post intervention. Prior to the intervention, 481 SRA tests were completed, while post intervention 105 were completed. Serotonin-release testing decreased from an average of 3.7 SRA results per 1000 admissions during the pre-intervention period to an average of 0.6 per 1000 admissions post intervention (Figure 2). Cost-savings were $1045 per 1000 admissions.

During the pre-intervention period, 2539 bags of argatroban were used, while 2337 bags were used post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 before the intervention to 14.3 post intervention. Cost-savings were $1316.20 per 1000 admissions. Figure 3 illustrates the monthly argatroban utilization per 1000 admissions during each quarter from January 2016 through March 2020.

Discussion
We designed and implemented an evidence-based strategy for HIT at our academic institution which led to a decrease in unnecessary SRA testing and argatroban utilization, with associated cost savings. By focusing on a single point of intervention at the laboratory level where SRA tests were held and canceled if the PF4 test was negative, we helped offload the decision-making from the provider while simultaneously providing just-in-time education to the provider. This intervention was designed with input from multiple stakeholders, including physicians, quality improvement specialists, pharmacists, and coagulation laboratory personnel.
Serotonin-release testing dramatically decreased post intervention even though a similar number of PF4 tests were performed before and after the intervention. This suggests that the decrease in SRA testing was a direct consequence of our intervention. Post intervention the number of completed SRA tests was 9% of the number of PF4 tests sent. This is consistent with our baseline pre-intervention data showing that only 8% of all PF4 tests sent were positive.
While the absolute number of argatroban bags utilized did not dramatically decrease after the intervention, the quarterly rate did, particularly after 2018. Given that argatroban data were only drawn from patients with a concurrent SRA test, this decrease is clearly from decreased usage in patients with suspected HIT. We suspect the decrease occurred because argatroban was not being continued while awaiting an SRA test in patients with a negative PF4 test. Decreasing the utilization of argatroban not only saved money but also reduced days of exposure to argatroban. While we do not have data regarding adverse events related to argatroban prior to the intervention, it is logical to conclude that reducing unnecessary exposure to argatroban reduces the risk of adverse events related to bleeding. Future studies would ideally address specific safety outcome metrics such as adverse events, bleeding risk, or missed diagnoses of HIT.
Our institutional guidelines for the diagnosis of HIT are evidence-based and helpful but are rarely followed by busy inpatient providers. Controlling the utilization of the SRA at the laboratory level had several advantages. First, removing SRA decision-making from providers who are not experts in the diagnosis of HIT guaranteed adherence to evidence-based guidelines. Second, pharmacists could safely recommend discontinuing argatroban when the PF4 test was negative as there was no SRA pending. Third, with cancellation at the laboratory level there was no need to further burden providers with yet another alert in the electronic health record. Fourth, just-in-time education was provided to the providers with justification for why the SRA test was canceled. Last, ruling out HIT within 24 hours with the PF4 test alone allowed providers to evaluate patients for other causes of thrombocytopenia much earlier than the 3 to 5 business days before the SRA results returned.
A limitation of this study is that it was conducted at a single center. Our approach is also limited by the lack of universal applicability. At our institution we are fortunate to have PF4 testing available in our coagulation laboratory 7 days a week. In addition, the coagulation laboratory controls sending the SRA to the reference laboratory. The specific intervention of controlling the SRA testing is therefore applicable only to institutions similar to ours; however, the concept of removing control of specialized testing from the provider is not unique. Inpatient thrombophilia testing has been a successful target of this approach.11-13 While electronic alerts and education of individual providers can also be effective initially, the effectiveness of these interventions has been repeatedly shown to wane over time.14-16
Conclusion
At our institution we were able to implement practical, evidence-based testing for HIT by implementing control over SRA testing at the level of the laboratory. This approach led to decreased argatroban utilization and cost savings.
Corresponding author: Alice Cusick, MD; LTC Charles S Kettles VA Medical Center, 2215 Fuller Road, Ann Arbor, MI 48105; mccoyag@med.umich.edu
Disclosures: None reported.
doi: 10.12788/jcom.0087
1. Fountain E, Arepally GM. Thrombocytopenia in hospitalized non-ICU patients. Blood. 2015;126(23):1060. doi:10.1182/blood.v126.23.1060.1060
2. Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139(2):271-278. doi:10.1378/chest.10-2243
3. Warkentin TE. Heparin-induced thrombocytopenia. Curr Opin Crit Care. 2015;21(6):576-585. doi:10.1097/MCC.0000000000000259
4. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. doi:10.1182/bloodadvances.2018024489
5. Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. doi:10.1182/blood-2012-07-443051
6. Northam KA, Parker WF, Chen S-L, et al. Evaluation of 4Ts score inter-rater agreement in patients undergoing evaluation for heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2021;32(5):328-334. doi:10.1097/MBC.0000000000001042
7. Raschke RA, Curry SC, Warkentin TE, Gerkin RD. Improving clinical interpretation of the anti-platelet factor 4/heparin enzyme-linked immunosorbent assay for the diagnosis of heparin-induced thrombocytopenia through the use of receiver operating characteristic analysis, stratum-specific likelihood ratios, and Bayes theorem. Chest. 2013;144(4):1269-1275. doi:10.1378/chest.12-2712
8. Warkentin TE, Arnold DM, Nazi I, Kelton JG. The platelet serotonin-release assay. Am J Hematol. 2015;90(6):564-572. doi:10.1002/ajh.24006
9. Use IFOR, Contents TOF. LIFECODES ® PF4 IgG assay:1-9.
10. Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):1-9. doi:10.1186/s12911-017-0430-8
11. O’Connor N, Carter-Johnson R. Effective screening of pathology tests controls costs: thrombophilia testing. J Clin Pathol. 2006;59(5):556. doi:10.1136/jcp.2005.030700
12. Lim MY, Greenberg CS. Inpatient thrombophilia testing: Impact of healthcare system technology and targeted clinician education on changing practice patterns. Vasc Med (United Kingdom). 2018;23(1):78-79. doi:10.1177/1358863X17742509
13. Cox JL, Shunkwiler SM, Koepsell SA. Requirement for a pathologist’s second signature limits inappropriate inpatient thrombophilia testing. Lab Med. 2017;48(4):367-371. doi:10.1093/labmed/lmx040
14. Kwang H, Mou E, Richman I, et al. Thrombophilia testing in the inpatient setting: impact of an educational intervention. BMC Med Inform Decis Mak. 2019;19(1):167. doi:10.1186/s12911-019-0889-6
15. Shah T, Patel-Teague S, Kroupa L, Meyer AND, Singh H. Impact of a national QI programme on reducing electronic health record notifications to clinicians. BMJ Qual Saf. 2019;28(1):10-14. doi:10.1136/bmjqs-2017-007447
16. Singh H, Spitzmueller C, Petersen NJ, Sawhney MK, Sittig DF. Information overload and missed test results in electronic health record-based settings. JAMA Intern Med. 2013;173(8):702-704. doi:10.1001/2013.jamainternmed.61
1. Fountain E, Arepally GM. Thrombocytopenia in hospitalized non-ICU patients. Blood. 2015;126(23):1060. doi:10.1182/blood.v126.23.1060.1060
2. Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139(2):271-278. doi:10.1378/chest.10-2243
3. Warkentin TE. Heparin-induced thrombocytopenia. Curr Opin Crit Care. 2015;21(6):576-585. doi:10.1097/MCC.0000000000000259
4. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. doi:10.1182/bloodadvances.2018024489
5. Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. doi:10.1182/blood-2012-07-443051
6. Northam KA, Parker WF, Chen S-L, et al. Evaluation of 4Ts score inter-rater agreement in patients undergoing evaluation for heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2021;32(5):328-334. doi:10.1097/MBC.0000000000001042
7. Raschke RA, Curry SC, Warkentin TE, Gerkin RD. Improving clinical interpretation of the anti-platelet factor 4/heparin enzyme-linked immunosorbent assay for the diagnosis of heparin-induced thrombocytopenia through the use of receiver operating characteristic analysis, stratum-specific likelihood ratios, and Bayes theorem. Chest. 2013;144(4):1269-1275. doi:10.1378/chest.12-2712
8. Warkentin TE, Arnold DM, Nazi I, Kelton JG. The platelet serotonin-release assay. Am J Hematol. 2015;90(6):564-572. doi:10.1002/ajh.24006
9. Use IFOR, Contents TOF. LIFECODES ® PF4 IgG assay:1-9.
10. Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):1-9. doi:10.1186/s12911-017-0430-8
11. O’Connor N, Carter-Johnson R. Effective screening of pathology tests controls costs: thrombophilia testing. J Clin Pathol. 2006;59(5):556. doi:10.1136/jcp.2005.030700
12. Lim MY, Greenberg CS. Inpatient thrombophilia testing: Impact of healthcare system technology and targeted clinician education on changing practice patterns. Vasc Med (United Kingdom). 2018;23(1):78-79. doi:10.1177/1358863X17742509
13. Cox JL, Shunkwiler SM, Koepsell SA. Requirement for a pathologist’s second signature limits inappropriate inpatient thrombophilia testing. Lab Med. 2017;48(4):367-371. doi:10.1093/labmed/lmx040
14. Kwang H, Mou E, Richman I, et al. Thrombophilia testing in the inpatient setting: impact of an educational intervention. BMC Med Inform Decis Mak. 2019;19(1):167. doi:10.1186/s12911-019-0889-6
15. Shah T, Patel-Teague S, Kroupa L, Meyer AND, Singh H. Impact of a national QI programme on reducing electronic health record notifications to clinicians. BMJ Qual Saf. 2019;28(1):10-14. doi:10.1136/bmjqs-2017-007447
16. Singh H, Spitzmueller C, Petersen NJ, Sawhney MK, Sittig DF. Information overload and missed test results in electronic health record-based settings. JAMA Intern Med. 2013;173(8):702-704. doi:10.1001/2013.jamainternmed.61
Headache and Covid-19: What clinicians should know
Edoardo Caronna, MD and Patricia Pozo-Rosich, MD, PhD, Neurology Department, Hospital Universitari Vall d’Hebron, Department of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain; and Headache and Neurological Pain Research Group, Vall d’Hebron Research Institute, Department of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain. Dr. Pozo-Rosich also serves on the boards of the International Headache Society and Council of the European Headache Federation and is an editor for various peer-reviewed journals, including Cephalalgia and Headache.
Headache is a symptom of the coronavirus disease 2019 (Covid-19), caused by the novel, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the pandemic began, researchers have tried to describe, understand, and help clinicians manage headache in the setting of Covid-19.
The reason is simple: Headache is common, often debilitating, and difficult to treat.1
Moreover, headache could manifest both in the acute phase of the infection and, once the infection has resolved, in the post-acute phase.1 Therefore, it is critical for clinicians to know more about headache, as headache can be a common reason that patients seek help, both in the specialized and non-specialized medical care setting.
Definitions and manifestations
While the first step in such a communication would be to define headache attributed to Covid-19, no specific definition exists, as this is a new disease. Therefore, headache attributed to Covid-19 should be defined under the diagnostic criteria, as contained in the International Classification of Headache Disorders-3, as headache attributed to a systemic viral infection.2 As this is a secondary headache appearing with an infection, the treating physician needs to rule out possible underlying meningitis and/or encephalitis in the diagnosis. Moreover, other secondary headaches (eg, cerebral venous thrombosis) may appear, so clinicians need to carefully evaluate patients with headache during Covid-19 to detect signs or symptoms that point to other etiologies.
It is also advisable to know the clinical manifestations of headache attributed to Covid-19. Studies published so far have observed two main phenotypes of headache in the acute phase of the infection: one resembles migraine, the other, a tension-type headache.1,3 Although patients with history of migraine who contract Covid-19 report headache that is more similar to primary headache disorder,4 two relevant aspects should be considered. Namely, migraine-like features can be observed in patients without personal migraine history; and Covid-19 patients with such history may perceive that headache they experience in the infection’s acute phase differs from their usual experience, especially regarding increased severity or duration.5,6 Of note, headache can be a prodromal symptom of the SARS-CoV-2 infection.1
Evolution of a headache
Because headache appearing after the acute phase of the infection can persist, often manifesting migraine-like features, it is inordinately helpful for clinicians to know its evolution.1 This persistent headache, sometimes referred to as post-covid headache, is not aptly named because the post-covid headache is not just one type of headache, but instead can manifest as different headache types.
A recently published case series in Headache discussed three Covid patients who all experienced persistent headache during the infection’s post-acute phase.7 These patients experienced a migraine-like phenotype as have others with mild Covid-19, but their personal history of migraine, as well as their experience with Covid-19 related headache, were substantially different. Some patients had personal migraine history while others did not; some patients experienced no headache in the acute phase but did so in the post-acute phase; and the concomitant symptoms of the post-acute phase, such as insomnia, memory loss, dizziness, fatigue, and brain fog, were differentially expressed by patients.7
This case series introduces the concept that patients with no prior history of migraine or any other primary headache disorder can develop a de novo headache because of their SARS-CoV-2 infection. Moreover, it could manifest as a new daily persistent headache. And patients with personal history of migraine may experience sudden chronification in their headache’s characteristics, rather than develop a new type of headache.7
In another study, soon to be published in Cephalalgia, researchers observed that the median duration of headache in the acute phase is 2 weeks. This multicenter Spanish study, in which data on headache duration were available for 874 patients, found that 16% of these particular patients had persistent headache after 9 months. According to this study, headache that does not resolve within the first 3 months is less likely to do so later on.
Treatment
For clinicians, the significance of these findings is straightforward: Patients with headache experienced in the infection’s acute phase that does not seem to resolve post-infection requires continued medical attention. Patients should be monitored, carefully managed, and treated to avoid the onset of a persisting headache. This applies to patients with or without personal migraine history.
But which treatments should be prescribed? As there are no specific therapies for headache attributed to Covid-19, either in the acute or post-acute phase of the infection, clinicians must turn to existing therapies.
As with patients with migraine, patients with persistent headache post-Covid infection need a headache prevention strategy.
The strategy should be based on the following principles:
- treat headache
- treat comorbidities including mood disorders, insomnia, and so on
- avoid complications such as medication overuse, which may be very common in these patients.
Acute medications
Despite the lack of specific literature on this matter, migraine-like phenotypes may respond to triptans and probably, where available, lasmiditan and gepants. These medications probably represent a therapeutic option for Covid patients with headache, but before prescribing them clinicians should carefully evaluate their use.
Before deciding on the prescription, clinicians should consider not only the medications’ most common contraindications, but also those that are related to Covid-19: the phase of the infection (acute/post-acute); the infection’s severity; and the presence of other Covid-related health problems. The concerns over the use of nonsteroidal anti-inflammatory medications (NSAIDs) and corticosteroids, raised when the pandemic first struck, have greatly dissipated.8,9 Some patients with prolonged headache may benefit from a brief cycle of corticosteroids, similar to the treatment given to those patients with status migrainosus. Nerve blocks could also be considered.
Preventive medications
Drugs can be prescribed according to the headache phenotype too, but there are no published studies that specifically evaluate headache prevention treatments in patients with persistent headache post-infection. The case series mentioned earlier in this article recorded that patients whose headaches were treated with amitriptyline and onabotulinumtoxinA had reported variable treatment responses to this regimen, according to the patients’ characteristics.7
However, one important question regarding the safety of Covid patients with migraine – specifically patients on preventive treatments during the infection’s acute phase – has been somewhat resolved.
Medications such as renin-angiotensin system (RAS) blockers, suspected of possible involvement in the SARs-CoV-2 pathogenicity, seem to be safe.8,10 And, in another multicenter Spanish study, researchers found that the use of anti-CGRP monoclonal antibodies did not seem to be associated with worse Covid-19 outcomes despite the possible implication of CGRP in modulating inflammatory responses during a viral infection.11
The study of anti-CGRP monoclonal antibodies could be important in the future for another reason: To see whether these medications could be effective as a preventive treatment in patients with persistent headache after Covid-19, regardless of whether these patients have personal migraine history.
An interesting and important message to close this article. Although headache experienced in the infection’s acute phase could be extremely disabling for patients, the evidence points to the presence of headache as a marker of a better Covid-19 prognosis, in terms of a shorter infection period and a lower risk of mortality among hospitalized patients.1,3,12
This brief communication contains current information to help clinicians treat and inform their patients with Covid-sourced headache. Yet, we must keep in mind that the majority of the data reported here and published in the literature refer to studies conducted during the first wave of the pandemic. The emergence of new SARS-CoV-2 variants and vaccines have enormously changed the disease’s clinical presentation and course, so future studies are warranted to re-assess the validity of these findings under new conditions.
References
1. Caronna E, Ballvé A, Llauradó A, Gallardo VJ, et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020; Nov;40(13):1410-1421.
2. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018; Jan;38(1):1-211.
3. Trigo J, García-Azorín D, Planchuelo-Gómez Á, et al. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: A retrospective cohort study. J Headache Pain. 2020;21(1):94. https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-020-01165-8
4. Porta-Etessam J, Matías-Guiu JA, González-García N, et al. Spectrum of Headaches Associated With SARS-CoV-2 Infection: Study of Healthcare Professionals. Headache. 2020;60(8):1697–1704.
5. Singh J, Ali A. Headache as the Presenting Symptom in 2 Patients With COVID-19 and a History of Migraine: 2 Case Reports. Headache. 2020;60(8):1773–1776.
6. Membrilla JA, de Lorenzo Í, Sastre M, Díaz de Terán J. Headache as a Cardinal Symptom of Coronavirus Disease 2019: A Cross-Sectional Study. Headache. 2020; Nov;60(10):2176-2191.
7. Caronna E, Alpuente A, Torres-Ferrus M, Pozo-Rosich P. Toward a better understanding of persistent headache after mild COVID-19: Three migraine-like yet distinct scenarios. Headache. 2021. https://doi.org/10.1111/head.14197
8. Maassenvandenbrink A, De Vries T, Danser AHJ. Headache medication and the COVID-19 pandemic. J Headache Pain. 2020;21(1). https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-020-01106-5
9. Arca KN, Smith JH, Chiang CC, et al. COVID-19 and Headache Medicine: A Narrative Review of Non-Steroidal Anti-Inflammatory Drug (NSAID) and Corticosteroid Use. Headache. 2020; Sep;60(8): 1558–1568.
10. Hippisley-Cox J, Young D, Coupland C, et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: Cohort study including 8.3 million people. Heart. 2020;Oct;106(19):1503-1511.
11. Caronna E, José Gallardo V, Alpuente A, Torres-Ferrus M, Sánchez-Mateo NM, Viguera-Romero J, et al. Safety of anti-CGRP monoclonal antibodies in patients with migraine during the COVID-19 pandemic: Present and future implications. Neurologia. 2021; Mar 19;36(8):611-617.
12. Gonzalez-Martinez A, Fanjul V, Ramos C, Serrano Ballesteros J, et al. Headache during SARS-CoV-2 infection as an early symptom associated with a more benign course of disease: a case–control study. Eur J Neurol. 2021;28(10):3426–36.
Edoardo Caronna, MD and Patricia Pozo-Rosich, MD, PhD, Neurology Department, Hospital Universitari Vall d’Hebron, Department of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain; and Headache and Neurological Pain Research Group, Vall d’Hebron Research Institute, Department of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain. Dr. Pozo-Rosich also serves on the boards of the International Headache Society and Council of the European Headache Federation and is an editor for various peer-reviewed journals, including Cephalalgia and Headache.
Headache is a symptom of the coronavirus disease 2019 (Covid-19), caused by the novel, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the pandemic began, researchers have tried to describe, understand, and help clinicians manage headache in the setting of Covid-19.
The reason is simple: Headache is common, often debilitating, and difficult to treat.1
Moreover, headache could manifest both in the acute phase of the infection and, once the infection has resolved, in the post-acute phase.1 Therefore, it is critical for clinicians to know more about headache, as headache can be a common reason that patients seek help, both in the specialized and non-specialized medical care setting.
Definitions and manifestations
While the first step in such a communication would be to define headache attributed to Covid-19, no specific definition exists, as this is a new disease. Therefore, headache attributed to Covid-19 should be defined under the diagnostic criteria, as contained in the International Classification of Headache Disorders-3, as headache attributed to a systemic viral infection.2 As this is a secondary headache appearing with an infection, the treating physician needs to rule out possible underlying meningitis and/or encephalitis in the diagnosis. Moreover, other secondary headaches (eg, cerebral venous thrombosis) may appear, so clinicians need to carefully evaluate patients with headache during Covid-19 to detect signs or symptoms that point to other etiologies.
It is also advisable to know the clinical manifestations of headache attributed to Covid-19. Studies published so far have observed two main phenotypes of headache in the acute phase of the infection: one resembles migraine, the other, a tension-type headache.1,3 Although patients with history of migraine who contract Covid-19 report headache that is more similar to primary headache disorder,4 two relevant aspects should be considered. Namely, migraine-like features can be observed in patients without personal migraine history; and Covid-19 patients with such history may perceive that headache they experience in the infection’s acute phase differs from their usual experience, especially regarding increased severity or duration.5,6 Of note, headache can be a prodromal symptom of the SARS-CoV-2 infection.1
Evolution of a headache
Because headache appearing after the acute phase of the infection can persist, often manifesting migraine-like features, it is inordinately helpful for clinicians to know its evolution.1 This persistent headache, sometimes referred to as post-covid headache, is not aptly named because the post-covid headache is not just one type of headache, but instead can manifest as different headache types.
A recently published case series in Headache discussed three Covid patients who all experienced persistent headache during the infection’s post-acute phase.7 These patients experienced a migraine-like phenotype as have others with mild Covid-19, but their personal history of migraine, as well as their experience with Covid-19 related headache, were substantially different. Some patients had personal migraine history while others did not; some patients experienced no headache in the acute phase but did so in the post-acute phase; and the concomitant symptoms of the post-acute phase, such as insomnia, memory loss, dizziness, fatigue, and brain fog, were differentially expressed by patients.7
This case series introduces the concept that patients with no prior history of migraine or any other primary headache disorder can develop a de novo headache because of their SARS-CoV-2 infection. Moreover, it could manifest as a new daily persistent headache. And patients with personal history of migraine may experience sudden chronification in their headache’s characteristics, rather than develop a new type of headache.7
In another study, soon to be published in Cephalalgia, researchers observed that the median duration of headache in the acute phase is 2 weeks. This multicenter Spanish study, in which data on headache duration were available for 874 patients, found that 16% of these particular patients had persistent headache after 9 months. According to this study, headache that does not resolve within the first 3 months is less likely to do so later on.
Treatment
For clinicians, the significance of these findings is straightforward: Patients with headache experienced in the infection’s acute phase that does not seem to resolve post-infection requires continued medical attention. Patients should be monitored, carefully managed, and treated to avoid the onset of a persisting headache. This applies to patients with or without personal migraine history.
But which treatments should be prescribed? As there are no specific therapies for headache attributed to Covid-19, either in the acute or post-acute phase of the infection, clinicians must turn to existing therapies.
As with patients with migraine, patients with persistent headache post-Covid infection need a headache prevention strategy.
The strategy should be based on the following principles:
- treat headache
- treat comorbidities including mood disorders, insomnia, and so on
- avoid complications such as medication overuse, which may be very common in these patients.
Acute medications
Despite the lack of specific literature on this matter, migraine-like phenotypes may respond to triptans and probably, where available, lasmiditan and gepants. These medications probably represent a therapeutic option for Covid patients with headache, but before prescribing them clinicians should carefully evaluate their use.
Before deciding on the prescription, clinicians should consider not only the medications’ most common contraindications, but also those that are related to Covid-19: the phase of the infection (acute/post-acute); the infection’s severity; and the presence of other Covid-related health problems. The concerns over the use of nonsteroidal anti-inflammatory medications (NSAIDs) and corticosteroids, raised when the pandemic first struck, have greatly dissipated.8,9 Some patients with prolonged headache may benefit from a brief cycle of corticosteroids, similar to the treatment given to those patients with status migrainosus. Nerve blocks could also be considered.
Preventive medications
Drugs can be prescribed according to the headache phenotype too, but there are no published studies that specifically evaluate headache prevention treatments in patients with persistent headache post-infection. The case series mentioned earlier in this article recorded that patients whose headaches were treated with amitriptyline and onabotulinumtoxinA had reported variable treatment responses to this regimen, according to the patients’ characteristics.7
However, one important question regarding the safety of Covid patients with migraine – specifically patients on preventive treatments during the infection’s acute phase – has been somewhat resolved.
Medications such as renin-angiotensin system (RAS) blockers, suspected of possible involvement in the SARs-CoV-2 pathogenicity, seem to be safe.8,10 And, in another multicenter Spanish study, researchers found that the use of anti-CGRP monoclonal antibodies did not seem to be associated with worse Covid-19 outcomes despite the possible implication of CGRP in modulating inflammatory responses during a viral infection.11
The study of anti-CGRP monoclonal antibodies could be important in the future for another reason: To see whether these medications could be effective as a preventive treatment in patients with persistent headache after Covid-19, regardless of whether these patients have personal migraine history.
An interesting and important message to close this article. Although headache experienced in the infection’s acute phase could be extremely disabling for patients, the evidence points to the presence of headache as a marker of a better Covid-19 prognosis, in terms of a shorter infection period and a lower risk of mortality among hospitalized patients.1,3,12
This brief communication contains current information to help clinicians treat and inform their patients with Covid-sourced headache. Yet, we must keep in mind that the majority of the data reported here and published in the literature refer to studies conducted during the first wave of the pandemic. The emergence of new SARS-CoV-2 variants and vaccines have enormously changed the disease’s clinical presentation and course, so future studies are warranted to re-assess the validity of these findings under new conditions.
Edoardo Caronna, MD and Patricia Pozo-Rosich, MD, PhD, Neurology Department, Hospital Universitari Vall d’Hebron, Department of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain; and Headache and Neurological Pain Research Group, Vall d’Hebron Research Institute, Department of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain. Dr. Pozo-Rosich also serves on the boards of the International Headache Society and Council of the European Headache Federation and is an editor for various peer-reviewed journals, including Cephalalgia and Headache.
Headache is a symptom of the coronavirus disease 2019 (Covid-19), caused by the novel, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the pandemic began, researchers have tried to describe, understand, and help clinicians manage headache in the setting of Covid-19.
The reason is simple: Headache is common, often debilitating, and difficult to treat.1
Moreover, headache could manifest both in the acute phase of the infection and, once the infection has resolved, in the post-acute phase.1 Therefore, it is critical for clinicians to know more about headache, as headache can be a common reason that patients seek help, both in the specialized and non-specialized medical care setting.
Definitions and manifestations
While the first step in such a communication would be to define headache attributed to Covid-19, no specific definition exists, as this is a new disease. Therefore, headache attributed to Covid-19 should be defined under the diagnostic criteria, as contained in the International Classification of Headache Disorders-3, as headache attributed to a systemic viral infection.2 As this is a secondary headache appearing with an infection, the treating physician needs to rule out possible underlying meningitis and/or encephalitis in the diagnosis. Moreover, other secondary headaches (eg, cerebral venous thrombosis) may appear, so clinicians need to carefully evaluate patients with headache during Covid-19 to detect signs or symptoms that point to other etiologies.
It is also advisable to know the clinical manifestations of headache attributed to Covid-19. Studies published so far have observed two main phenotypes of headache in the acute phase of the infection: one resembles migraine, the other, a tension-type headache.1,3 Although patients with history of migraine who contract Covid-19 report headache that is more similar to primary headache disorder,4 two relevant aspects should be considered. Namely, migraine-like features can be observed in patients without personal migraine history; and Covid-19 patients with such history may perceive that headache they experience in the infection’s acute phase differs from their usual experience, especially regarding increased severity or duration.5,6 Of note, headache can be a prodromal symptom of the SARS-CoV-2 infection.1
Evolution of a headache
Because headache appearing after the acute phase of the infection can persist, often manifesting migraine-like features, it is inordinately helpful for clinicians to know its evolution.1 This persistent headache, sometimes referred to as post-covid headache, is not aptly named because the post-covid headache is not just one type of headache, but instead can manifest as different headache types.
A recently published case series in Headache discussed three Covid patients who all experienced persistent headache during the infection’s post-acute phase.7 These patients experienced a migraine-like phenotype as have others with mild Covid-19, but their personal history of migraine, as well as their experience with Covid-19 related headache, were substantially different. Some patients had personal migraine history while others did not; some patients experienced no headache in the acute phase but did so in the post-acute phase; and the concomitant symptoms of the post-acute phase, such as insomnia, memory loss, dizziness, fatigue, and brain fog, were differentially expressed by patients.7
This case series introduces the concept that patients with no prior history of migraine or any other primary headache disorder can develop a de novo headache because of their SARS-CoV-2 infection. Moreover, it could manifest as a new daily persistent headache. And patients with personal history of migraine may experience sudden chronification in their headache’s characteristics, rather than develop a new type of headache.7
In another study, soon to be published in Cephalalgia, researchers observed that the median duration of headache in the acute phase is 2 weeks. This multicenter Spanish study, in which data on headache duration were available for 874 patients, found that 16% of these particular patients had persistent headache after 9 months. According to this study, headache that does not resolve within the first 3 months is less likely to do so later on.
Treatment
For clinicians, the significance of these findings is straightforward: Patients with headache experienced in the infection’s acute phase that does not seem to resolve post-infection requires continued medical attention. Patients should be monitored, carefully managed, and treated to avoid the onset of a persisting headache. This applies to patients with or without personal migraine history.
But which treatments should be prescribed? As there are no specific therapies for headache attributed to Covid-19, either in the acute or post-acute phase of the infection, clinicians must turn to existing therapies.
As with patients with migraine, patients with persistent headache post-Covid infection need a headache prevention strategy.
The strategy should be based on the following principles:
- treat headache
- treat comorbidities including mood disorders, insomnia, and so on
- avoid complications such as medication overuse, which may be very common in these patients.
Acute medications
Despite the lack of specific literature on this matter, migraine-like phenotypes may respond to triptans and probably, where available, lasmiditan and gepants. These medications probably represent a therapeutic option for Covid patients with headache, but before prescribing them clinicians should carefully evaluate their use.
Before deciding on the prescription, clinicians should consider not only the medications’ most common contraindications, but also those that are related to Covid-19: the phase of the infection (acute/post-acute); the infection’s severity; and the presence of other Covid-related health problems. The concerns over the use of nonsteroidal anti-inflammatory medications (NSAIDs) and corticosteroids, raised when the pandemic first struck, have greatly dissipated.8,9 Some patients with prolonged headache may benefit from a brief cycle of corticosteroids, similar to the treatment given to those patients with status migrainosus. Nerve blocks could also be considered.
Preventive medications
Drugs can be prescribed according to the headache phenotype too, but there are no published studies that specifically evaluate headache prevention treatments in patients with persistent headache post-infection. The case series mentioned earlier in this article recorded that patients whose headaches were treated with amitriptyline and onabotulinumtoxinA had reported variable treatment responses to this regimen, according to the patients’ characteristics.7
However, one important question regarding the safety of Covid patients with migraine – specifically patients on preventive treatments during the infection’s acute phase – has been somewhat resolved.
Medications such as renin-angiotensin system (RAS) blockers, suspected of possible involvement in the SARs-CoV-2 pathogenicity, seem to be safe.8,10 And, in another multicenter Spanish study, researchers found that the use of anti-CGRP monoclonal antibodies did not seem to be associated with worse Covid-19 outcomes despite the possible implication of CGRP in modulating inflammatory responses during a viral infection.11
The study of anti-CGRP monoclonal antibodies could be important in the future for another reason: To see whether these medications could be effective as a preventive treatment in patients with persistent headache after Covid-19, regardless of whether these patients have personal migraine history.
An interesting and important message to close this article. Although headache experienced in the infection’s acute phase could be extremely disabling for patients, the evidence points to the presence of headache as a marker of a better Covid-19 prognosis, in terms of a shorter infection period and a lower risk of mortality among hospitalized patients.1,3,12
This brief communication contains current information to help clinicians treat and inform their patients with Covid-sourced headache. Yet, we must keep in mind that the majority of the data reported here and published in the literature refer to studies conducted during the first wave of the pandemic. The emergence of new SARS-CoV-2 variants and vaccines have enormously changed the disease’s clinical presentation and course, so future studies are warranted to re-assess the validity of these findings under new conditions.
References
1. Caronna E, Ballvé A, Llauradó A, Gallardo VJ, et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020; Nov;40(13):1410-1421.
2. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018; Jan;38(1):1-211.
3. Trigo J, García-Azorín D, Planchuelo-Gómez Á, et al. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: A retrospective cohort study. J Headache Pain. 2020;21(1):94. https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-020-01165-8
4. Porta-Etessam J, Matías-Guiu JA, González-García N, et al. Spectrum of Headaches Associated With SARS-CoV-2 Infection: Study of Healthcare Professionals. Headache. 2020;60(8):1697–1704.
5. Singh J, Ali A. Headache as the Presenting Symptom in 2 Patients With COVID-19 and a History of Migraine: 2 Case Reports. Headache. 2020;60(8):1773–1776.
6. Membrilla JA, de Lorenzo Í, Sastre M, Díaz de Terán J. Headache as a Cardinal Symptom of Coronavirus Disease 2019: A Cross-Sectional Study. Headache. 2020; Nov;60(10):2176-2191.
7. Caronna E, Alpuente A, Torres-Ferrus M, Pozo-Rosich P. Toward a better understanding of persistent headache after mild COVID-19: Three migraine-like yet distinct scenarios. Headache. 2021. https://doi.org/10.1111/head.14197
8. Maassenvandenbrink A, De Vries T, Danser AHJ. Headache medication and the COVID-19 pandemic. J Headache Pain. 2020;21(1). https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-020-01106-5
9. Arca KN, Smith JH, Chiang CC, et al. COVID-19 and Headache Medicine: A Narrative Review of Non-Steroidal Anti-Inflammatory Drug (NSAID) and Corticosteroid Use. Headache. 2020; Sep;60(8): 1558–1568.
10. Hippisley-Cox J, Young D, Coupland C, et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: Cohort study including 8.3 million people. Heart. 2020;Oct;106(19):1503-1511.
11. Caronna E, José Gallardo V, Alpuente A, Torres-Ferrus M, Sánchez-Mateo NM, Viguera-Romero J, et al. Safety of anti-CGRP monoclonal antibodies in patients with migraine during the COVID-19 pandemic: Present and future implications. Neurologia. 2021; Mar 19;36(8):611-617.
12. Gonzalez-Martinez A, Fanjul V, Ramos C, Serrano Ballesteros J, et al. Headache during SARS-CoV-2 infection as an early symptom associated with a more benign course of disease: a case–control study. Eur J Neurol. 2021;28(10):3426–36.
References
1. Caronna E, Ballvé A, Llauradó A, Gallardo VJ, et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020; Nov;40(13):1410-1421.
2. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018; Jan;38(1):1-211.
3. Trigo J, García-Azorín D, Planchuelo-Gómez Á, et al. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: A retrospective cohort study. J Headache Pain. 2020;21(1):94. https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-020-01165-8
4. Porta-Etessam J, Matías-Guiu JA, González-García N, et al. Spectrum of Headaches Associated With SARS-CoV-2 Infection: Study of Healthcare Professionals. Headache. 2020;60(8):1697–1704.
5. Singh J, Ali A. Headache as the Presenting Symptom in 2 Patients With COVID-19 and a History of Migraine: 2 Case Reports. Headache. 2020;60(8):1773–1776.
6. Membrilla JA, de Lorenzo Í, Sastre M, Díaz de Terán J. Headache as a Cardinal Symptom of Coronavirus Disease 2019: A Cross-Sectional Study. Headache. 2020; Nov;60(10):2176-2191.
7. Caronna E, Alpuente A, Torres-Ferrus M, Pozo-Rosich P. Toward a better understanding of persistent headache after mild COVID-19: Three migraine-like yet distinct scenarios. Headache. 2021. https://doi.org/10.1111/head.14197
8. Maassenvandenbrink A, De Vries T, Danser AHJ. Headache medication and the COVID-19 pandemic. J Headache Pain. 2020;21(1). https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-020-01106-5
9. Arca KN, Smith JH, Chiang CC, et al. COVID-19 and Headache Medicine: A Narrative Review of Non-Steroidal Anti-Inflammatory Drug (NSAID) and Corticosteroid Use. Headache. 2020; Sep;60(8): 1558–1568.
10. Hippisley-Cox J, Young D, Coupland C, et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: Cohort study including 8.3 million people. Heart. 2020;Oct;106(19):1503-1511.
11. Caronna E, José Gallardo V, Alpuente A, Torres-Ferrus M, Sánchez-Mateo NM, Viguera-Romero J, et al. Safety of anti-CGRP monoclonal antibodies in patients with migraine during the COVID-19 pandemic: Present and future implications. Neurologia. 2021; Mar 19;36(8):611-617.
12. Gonzalez-Martinez A, Fanjul V, Ramos C, Serrano Ballesteros J, et al. Headache during SARS-CoV-2 infection as an early symptom associated with a more benign course of disease: a case–control study. Eur J Neurol. 2021;28(10):3426–36.
Oral step-down therapy for infective endocarditis
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: The standard of care for IE has been a prolonged course of IV antibiotics. Recent literature has suggested that oral antibiotics might be a safe and effective step-down therapy for IE.
Study design: Systematic review.
Setting: Literature review in October 2019, with update in February 2020, consisting of 21 observational studies and 3 randomized controlled trials.
Synopsis: Three RCTs and 21 observational studies were reviewed, with a focus on the effectiveness of antibiotics administered orally for part of the therapeutic course for IE patients. Patients included in the study had left- or right-sided IE. Pathogens included viridians streptococci, staphylococci, and enterococci, with a minority of patients infected with methicillin-resistant Staphylococcus aureus. Treatment regimens included beta-lactams, linezolid, fluoroquinolones, trimethoprim-sulfamethoxazole, or clindamycin, with or without rifampin.
In studies wherein IV antibiotics alone were compared with IV antibiotics with oral step-down therapy, there was no difference in clinical cure rate. Those given oral step-down therapy had a statistically significant lower mortality rate than patients who received only IV therapy.
Limitations include inconclusive data regarding duration of IV lead-in therapy, with the variance before conversion to oral antibiotics amongst the studies ranging from 0 to 24 days. The limited number of patients with MRSA infections makes it difficult to draw conclusions regarding this particular pathogen.
Bottom line: Highly orally bioavailable antibiotics should be considered for patients with IE who have cleared bacteremia and achieved clinical stability with IV regimens.
Citation: Spellberg B et al. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med. 2020;180(5):769-77. doi: 10.1001/jamainternmed.2020.0555.
Dr. Yoo is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Anticoagulant choice in antiphospholipid syndrome–associated thrombosis
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Timing of initiation of renal-replacement therapy in acute kidney injury
Background: Acute kidney injury (AKI) is a common complication that occurs in seriously ill patients admitted to the ICU, and many of these patients eventually require RRT. When complicated by major metabolic disorders, it is usually clear when therapy should be initiated. However, when these complications are absent, the most appropriate time to initiate RRT is unclear. There are potential advantages to performing early RRT in patients with severe AKI, such as restoring acid-base balance, preventing fluid accumulation, and preventing major electrolyte disturbances.
Study design: Multinational, randomized, controlled trial.
Setting: 168 hospitals in 15 countries.
Synopsis: Eligible patients were adults admitted to an ICU with severe AKI. Patients were randomly assigned to an accelerated strategy of RRT (initiated within 12 hours, 1,465 patients) or a standard strategy of RRT (held until conventional indications developed or AKI lasted more than 72 hours, 1,462 patients). RRT was performed in 1,418 (96.8%) in the accelerated group and 903 (61.8%) in the standard group. At 90 days, 643 deaths (43.9%) occurred in the accelerated group and 639 deaths (43.7%) occurred in the standard group (RR, 1.00; 95% CI, 0.93-1.09; P = .92). Among survivors at 90 days, 85 out of 814 accelerated patients (10.4%) and 49 of 815 standard patients (6.0%) continued to require RRT (RR, 1.75; 95% CI, 1.24-2.43), suggesting the possibility of increased dependence on long-term RRT if introduced early. Limitations include use of clinical equipoise to confirm full eligibility, introducing possible patient heterogeneity into the trial. In addition, broad discretion was given to clinicians on when to start RRT in the standard group resulting in variable initiation times.
Bottom line: In critically ill patients with severe AKI, earlier RRT did not result in lower mortality at 90 days compared with standard therapy and increased the risk of requiring RRT at 90 days.
Citation: Bagshaw SM et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383:240-51. doi: 10.1056/NEJMoa2000741.
Dr. Kim is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Acute kidney injury (AKI) is a common complication that occurs in seriously ill patients admitted to the ICU, and many of these patients eventually require RRT. When complicated by major metabolic disorders, it is usually clear when therapy should be initiated. However, when these complications are absent, the most appropriate time to initiate RRT is unclear. There are potential advantages to performing early RRT in patients with severe AKI, such as restoring acid-base balance, preventing fluid accumulation, and preventing major electrolyte disturbances.
Study design: Multinational, randomized, controlled trial.
Setting: 168 hospitals in 15 countries.
Synopsis: Eligible patients were adults admitted to an ICU with severe AKI. Patients were randomly assigned to an accelerated strategy of RRT (initiated within 12 hours, 1,465 patients) or a standard strategy of RRT (held until conventional indications developed or AKI lasted more than 72 hours, 1,462 patients). RRT was performed in 1,418 (96.8%) in the accelerated group and 903 (61.8%) in the standard group. At 90 days, 643 deaths (43.9%) occurred in the accelerated group and 639 deaths (43.7%) occurred in the standard group (RR, 1.00; 95% CI, 0.93-1.09; P = .92). Among survivors at 90 days, 85 out of 814 accelerated patients (10.4%) and 49 of 815 standard patients (6.0%) continued to require RRT (RR, 1.75; 95% CI, 1.24-2.43), suggesting the possibility of increased dependence on long-term RRT if introduced early. Limitations include use of clinical equipoise to confirm full eligibility, introducing possible patient heterogeneity into the trial. In addition, broad discretion was given to clinicians on when to start RRT in the standard group resulting in variable initiation times.
Bottom line: In critically ill patients with severe AKI, earlier RRT did not result in lower mortality at 90 days compared with standard therapy and increased the risk of requiring RRT at 90 days.
Citation: Bagshaw SM et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383:240-51. doi: 10.1056/NEJMoa2000741.
Dr. Kim is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Acute kidney injury (AKI) is a common complication that occurs in seriously ill patients admitted to the ICU, and many of these patients eventually require RRT. When complicated by major metabolic disorders, it is usually clear when therapy should be initiated. However, when these complications are absent, the most appropriate time to initiate RRT is unclear. There are potential advantages to performing early RRT in patients with severe AKI, such as restoring acid-base balance, preventing fluid accumulation, and preventing major electrolyte disturbances.
Study design: Multinational, randomized, controlled trial.
Setting: 168 hospitals in 15 countries.
Synopsis: Eligible patients were adults admitted to an ICU with severe AKI. Patients were randomly assigned to an accelerated strategy of RRT (initiated within 12 hours, 1,465 patients) or a standard strategy of RRT (held until conventional indications developed or AKI lasted more than 72 hours, 1,462 patients). RRT was performed in 1,418 (96.8%) in the accelerated group and 903 (61.8%) in the standard group. At 90 days, 643 deaths (43.9%) occurred in the accelerated group and 639 deaths (43.7%) occurred in the standard group (RR, 1.00; 95% CI, 0.93-1.09; P = .92). Among survivors at 90 days, 85 out of 814 accelerated patients (10.4%) and 49 of 815 standard patients (6.0%) continued to require RRT (RR, 1.75; 95% CI, 1.24-2.43), suggesting the possibility of increased dependence on long-term RRT if introduced early. Limitations include use of clinical equipoise to confirm full eligibility, introducing possible patient heterogeneity into the trial. In addition, broad discretion was given to clinicians on when to start RRT in the standard group resulting in variable initiation times.
Bottom line: In critically ill patients with severe AKI, earlier RRT did not result in lower mortality at 90 days compared with standard therapy and increased the risk of requiring RRT at 90 days.
Citation: Bagshaw SM et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383:240-51. doi: 10.1056/NEJMoa2000741.
Dr. Kim is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.