User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
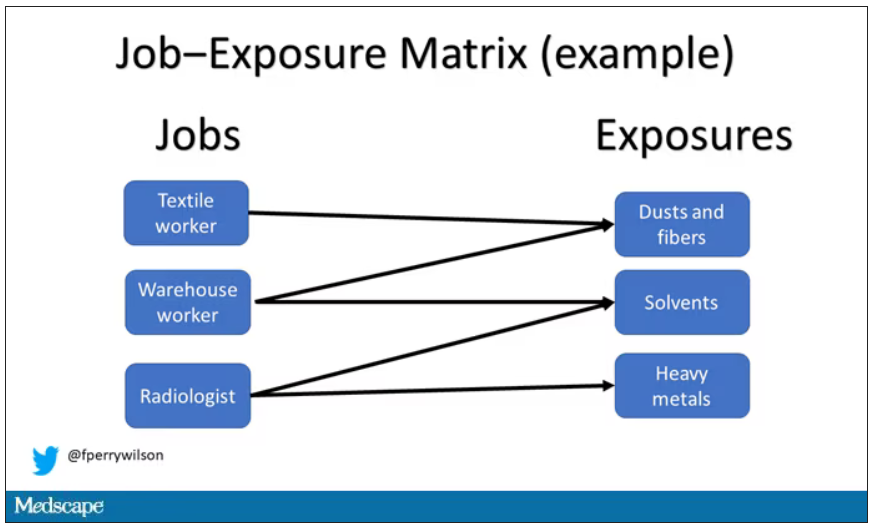
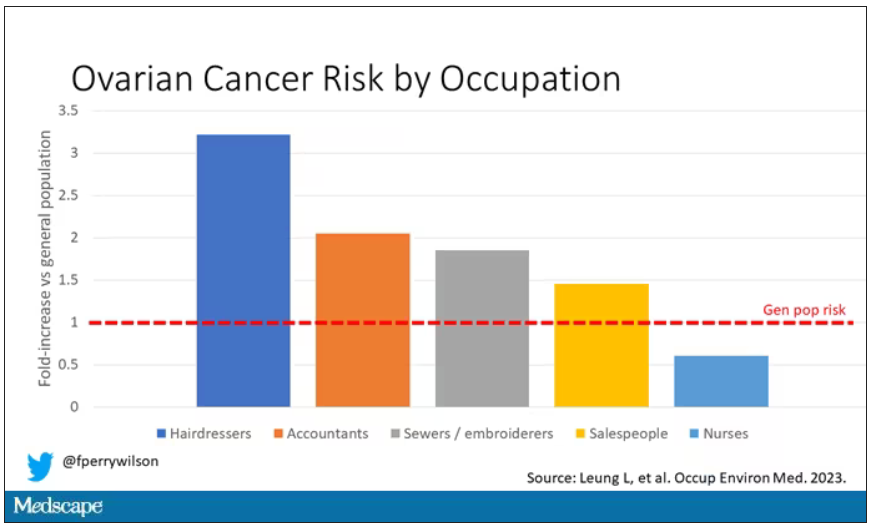
Link between low co-pays for new diabetes drugs and patient adherence
Findings from a recent study indicate that the less U.S. patients pay out of pocket for drugs that often have high co-pays, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagonlike peptide-1 (GLP-1) agonists, the more they adhere to taking these medications.
The study, led by Utibe R. Essien, MD, from University of California, Los Angeles, and Balvindar Singh, MD, PhD, from University of Pittsburgh, was published online in JAMA Cardiology.
Patient data from Clinformatics Data Mart, a health insurance claims database, was analyzed for the study. The information for 90,041 adults from the United States who had commercial and Medicare health insurance, and who started taking a GLP-1 agonist or SGLT2 inhibitor between 2014 and 2020 was reviewed. Participants had type 2 diabetes, heart failure, or both.
The primary outcome showed patients with a lower drug co-pay had significantly higher odds of 12-month adherence to GLP-1 agonists and SGLT2 inhibitors, compared with those with a higher co-pay. These differences persisted after controlling for patient demographic, clinical, and socioeconomic covariates.
After full adjustments were made and after the 12 months, patients with a high co-pay of $50 per month or more were 53% less likely to adhere to an SGLT2 inhibitor and 32% less likely to adhere to a GLP-1 agonist, compared with patients with a co-pay of less than $10 per month for these agents.
“Lowering high out-of-pocket prescription costs may be key to improving adherence to guideline-recommended therapies and advancing overall quality of care in patients with type 2 diabetes and heart failure,” the authors conclude.
The authors acknowledge the study’s limitations, including the inability to exclude residual confounding, uncertain generalizability for those without health insurance or with public insurance and possible misclassifications of type 2 diabetes and heart failure diagnoses or medical comorbidities. Additionally, this study did not have information on patients’ preferences associated with medication use, including specific reasons for poor adherence, and could not assess how co-payments influenced initial prescription receipt or abandonment at the pharmacy, or other factors including possible price inflation.
The study received no commercial funding. One author (not a lead author) is an adviser to several drug companies including ones that market SGLT2 inhibitors or GLP-1 agonists.
Findings from a recent study indicate that the less U.S. patients pay out of pocket for drugs that often have high co-pays, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagonlike peptide-1 (GLP-1) agonists, the more they adhere to taking these medications.
The study, led by Utibe R. Essien, MD, from University of California, Los Angeles, and Balvindar Singh, MD, PhD, from University of Pittsburgh, was published online in JAMA Cardiology.
Patient data from Clinformatics Data Mart, a health insurance claims database, was analyzed for the study. The information for 90,041 adults from the United States who had commercial and Medicare health insurance, and who started taking a GLP-1 agonist or SGLT2 inhibitor between 2014 and 2020 was reviewed. Participants had type 2 diabetes, heart failure, or both.
The primary outcome showed patients with a lower drug co-pay had significantly higher odds of 12-month adherence to GLP-1 agonists and SGLT2 inhibitors, compared with those with a higher co-pay. These differences persisted after controlling for patient demographic, clinical, and socioeconomic covariates.
After full adjustments were made and after the 12 months, patients with a high co-pay of $50 per month or more were 53% less likely to adhere to an SGLT2 inhibitor and 32% less likely to adhere to a GLP-1 agonist, compared with patients with a co-pay of less than $10 per month for these agents.
“Lowering high out-of-pocket prescription costs may be key to improving adherence to guideline-recommended therapies and advancing overall quality of care in patients with type 2 diabetes and heart failure,” the authors conclude.
The authors acknowledge the study’s limitations, including the inability to exclude residual confounding, uncertain generalizability for those without health insurance or with public insurance and possible misclassifications of type 2 diabetes and heart failure diagnoses or medical comorbidities. Additionally, this study did not have information on patients’ preferences associated with medication use, including specific reasons for poor adherence, and could not assess how co-payments influenced initial prescription receipt or abandonment at the pharmacy, or other factors including possible price inflation.
The study received no commercial funding. One author (not a lead author) is an adviser to several drug companies including ones that market SGLT2 inhibitors or GLP-1 agonists.
Findings from a recent study indicate that the less U.S. patients pay out of pocket for drugs that often have high co-pays, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagonlike peptide-1 (GLP-1) agonists, the more they adhere to taking these medications.
The study, led by Utibe R. Essien, MD, from University of California, Los Angeles, and Balvindar Singh, MD, PhD, from University of Pittsburgh, was published online in JAMA Cardiology.
Patient data from Clinformatics Data Mart, a health insurance claims database, was analyzed for the study. The information for 90,041 adults from the United States who had commercial and Medicare health insurance, and who started taking a GLP-1 agonist or SGLT2 inhibitor between 2014 and 2020 was reviewed. Participants had type 2 diabetes, heart failure, or both.
The primary outcome showed patients with a lower drug co-pay had significantly higher odds of 12-month adherence to GLP-1 agonists and SGLT2 inhibitors, compared with those with a higher co-pay. These differences persisted after controlling for patient demographic, clinical, and socioeconomic covariates.
After full adjustments were made and after the 12 months, patients with a high co-pay of $50 per month or more were 53% less likely to adhere to an SGLT2 inhibitor and 32% less likely to adhere to a GLP-1 agonist, compared with patients with a co-pay of less than $10 per month for these agents.
“Lowering high out-of-pocket prescription costs may be key to improving adherence to guideline-recommended therapies and advancing overall quality of care in patients with type 2 diabetes and heart failure,” the authors conclude.
The authors acknowledge the study’s limitations, including the inability to exclude residual confounding, uncertain generalizability for those without health insurance or with public insurance and possible misclassifications of type 2 diabetes and heart failure diagnoses or medical comorbidities. Additionally, this study did not have information on patients’ preferences associated with medication use, including specific reasons for poor adherence, and could not assess how co-payments influenced initial prescription receipt or abandonment at the pharmacy, or other factors including possible price inflation.
The study received no commercial funding. One author (not a lead author) is an adviser to several drug companies including ones that market SGLT2 inhibitors or GLP-1 agonists.
FROM JAMA CARDIOLOGY
FDA approves cognitive-behavioral app for adults with type 2 diabetes
A smartphone-based app designed to deliver cognitive-behavioral therapy (CBT) to adults with type 2 diabetes received marketing approval as a class II medical device from the Food and Drug Administration on July 10, becoming the first digital behavioral therapeutic device for people with diabetes to receive this designation for U.S. patients.
Better Therapeutics representatives said that the app, formerly known as BT-001, will be called AspyreRX, with U.S. sales planned to launch in October-December 2023.
The app will be available to patients exclusively by prescription, with a planned 90-day use duration and an option for a second 90-day prescription. A company official said the price per prescription will be about $500-800, although this is not yet finalized. The app is intended for use in concert with the conventional pillars of glycemic control in people with type 2 diabetes: lifestyle modification and treatment with antidiabetes medications.
Senior staff members of Better Therapeutics acknowledged the critical need for an education program, which they will now launch for clinicians, payers, and patients to get across the message of the potential benefit and safety associated with using the CBT app. Their initial marketing will target patients with type 2 diabetes and poorly controlled hemoglobin A1c levels in five to six U.S. regions with high numbers of these patients. The company will also attempt to make the app available through the Department of Veterans Affairs health system and try to secure coverage by Medicare and commercial health-insurance providers.
Approval based on pivotal trial results
The FDA approval focused on data collected in the BT-001 randomized, controlled trial, which included 669 U.S. adults with poorly controlled type 2 diabetes. Results, published in 2022 in Diabetes Care, showed that after 90 days, people using the app had an average incremental reduction in A1c of 0.39 percentage points, compared with control patients who didn’t use the app, the primary endpoint. Use of the app also appeared safe.
Subsequent meeting presentations of study findings showed that A1c-lowering linked with app use was durable during continued use for a total of 180 days, that the effectiveness of the app in helping to lower A1c levels was “dose dependent” relative to the number of lessons a person completed, and that using the app significantly linked with a reduced need for intensified glycemic control through added medications.
Another finding of the extended-use phase of the study was that 81% of patients assigned to the app-using group continued to regularly use the app after 180 days, a level of durable engagement by patients that “exceeded our expectations,” said Diane Gomez-Thinnes, chief commercial officer of Better Therapeutics, during a press conference.
The company plans to tweak the app prior to its launch based on additional analyses of results from the pivotal study to further improve patient engagement and app ease of use. The company is also planning to expand the range of smartphones that can support the app, although about 90%-95% of U.S. smartphones have this capability.
Better Therapeutics is also actively developing and testing other modifications to the basic CBT app to make it usable by people with other cardiometabolic disorders such as hypertension, obesity, and fatty liver disease.
The BT-001 study was funded by Better Therapeutics.
A version of this article first appeared on Medscape.com.
A smartphone-based app designed to deliver cognitive-behavioral therapy (CBT) to adults with type 2 diabetes received marketing approval as a class II medical device from the Food and Drug Administration on July 10, becoming the first digital behavioral therapeutic device for people with diabetes to receive this designation for U.S. patients.
Better Therapeutics representatives said that the app, formerly known as BT-001, will be called AspyreRX, with U.S. sales planned to launch in October-December 2023.
The app will be available to patients exclusively by prescription, with a planned 90-day use duration and an option for a second 90-day prescription. A company official said the price per prescription will be about $500-800, although this is not yet finalized. The app is intended for use in concert with the conventional pillars of glycemic control in people with type 2 diabetes: lifestyle modification and treatment with antidiabetes medications.
Senior staff members of Better Therapeutics acknowledged the critical need for an education program, which they will now launch for clinicians, payers, and patients to get across the message of the potential benefit and safety associated with using the CBT app. Their initial marketing will target patients with type 2 diabetes and poorly controlled hemoglobin A1c levels in five to six U.S. regions with high numbers of these patients. The company will also attempt to make the app available through the Department of Veterans Affairs health system and try to secure coverage by Medicare and commercial health-insurance providers.
Approval based on pivotal trial results
The FDA approval focused on data collected in the BT-001 randomized, controlled trial, which included 669 U.S. adults with poorly controlled type 2 diabetes. Results, published in 2022 in Diabetes Care, showed that after 90 days, people using the app had an average incremental reduction in A1c of 0.39 percentage points, compared with control patients who didn’t use the app, the primary endpoint. Use of the app also appeared safe.
Subsequent meeting presentations of study findings showed that A1c-lowering linked with app use was durable during continued use for a total of 180 days, that the effectiveness of the app in helping to lower A1c levels was “dose dependent” relative to the number of lessons a person completed, and that using the app significantly linked with a reduced need for intensified glycemic control through added medications.
Another finding of the extended-use phase of the study was that 81% of patients assigned to the app-using group continued to regularly use the app after 180 days, a level of durable engagement by patients that “exceeded our expectations,” said Diane Gomez-Thinnes, chief commercial officer of Better Therapeutics, during a press conference.
The company plans to tweak the app prior to its launch based on additional analyses of results from the pivotal study to further improve patient engagement and app ease of use. The company is also planning to expand the range of smartphones that can support the app, although about 90%-95% of U.S. smartphones have this capability.
Better Therapeutics is also actively developing and testing other modifications to the basic CBT app to make it usable by people with other cardiometabolic disorders such as hypertension, obesity, and fatty liver disease.
The BT-001 study was funded by Better Therapeutics.
A version of this article first appeared on Medscape.com.
A smartphone-based app designed to deliver cognitive-behavioral therapy (CBT) to adults with type 2 diabetes received marketing approval as a class II medical device from the Food and Drug Administration on July 10, becoming the first digital behavioral therapeutic device for people with diabetes to receive this designation for U.S. patients.
Better Therapeutics representatives said that the app, formerly known as BT-001, will be called AspyreRX, with U.S. sales planned to launch in October-December 2023.
The app will be available to patients exclusively by prescription, with a planned 90-day use duration and an option for a second 90-day prescription. A company official said the price per prescription will be about $500-800, although this is not yet finalized. The app is intended for use in concert with the conventional pillars of glycemic control in people with type 2 diabetes: lifestyle modification and treatment with antidiabetes medications.
Senior staff members of Better Therapeutics acknowledged the critical need for an education program, which they will now launch for clinicians, payers, and patients to get across the message of the potential benefit and safety associated with using the CBT app. Their initial marketing will target patients with type 2 diabetes and poorly controlled hemoglobin A1c levels in five to six U.S. regions with high numbers of these patients. The company will also attempt to make the app available through the Department of Veterans Affairs health system and try to secure coverage by Medicare and commercial health-insurance providers.
Approval based on pivotal trial results
The FDA approval focused on data collected in the BT-001 randomized, controlled trial, which included 669 U.S. adults with poorly controlled type 2 diabetes. Results, published in 2022 in Diabetes Care, showed that after 90 days, people using the app had an average incremental reduction in A1c of 0.39 percentage points, compared with control patients who didn’t use the app, the primary endpoint. Use of the app also appeared safe.
Subsequent meeting presentations of study findings showed that A1c-lowering linked with app use was durable during continued use for a total of 180 days, that the effectiveness of the app in helping to lower A1c levels was “dose dependent” relative to the number of lessons a person completed, and that using the app significantly linked with a reduced need for intensified glycemic control through added medications.
Another finding of the extended-use phase of the study was that 81% of patients assigned to the app-using group continued to regularly use the app after 180 days, a level of durable engagement by patients that “exceeded our expectations,” said Diane Gomez-Thinnes, chief commercial officer of Better Therapeutics, during a press conference.
The company plans to tweak the app prior to its launch based on additional analyses of results from the pivotal study to further improve patient engagement and app ease of use. The company is also planning to expand the range of smartphones that can support the app, although about 90%-95% of U.S. smartphones have this capability.
Better Therapeutics is also actively developing and testing other modifications to the basic CBT app to make it usable by people with other cardiometabolic disorders such as hypertension, obesity, and fatty liver disease.
The BT-001 study was funded by Better Therapeutics.
A version of this article first appeared on Medscape.com.
Aging and type 1 diabetes: ‘Complete picture’ 40 years on
SAN DIEGO –
New funding for 2022-2027 for the DCCT long-term observational follow-up study, the Epidemiology of Diabetes Interventions and Complications (EDIC) will go toward investigating aspects of type 1 diabetes that are associated with aging and are also common in type 2 diabetes, including cardiovascular disease, fatty liver disease, and sleep apnea.
The original randomized DCCT clinical trial results, published in 1993 in the New England Journal of Medicine, proved that early intensive glycemic control was the key to preventing or slowing the progression of long-term eye, kidney, and nerve complications of type 1 diabetes. Subsequently, EDIC has yielded many more major findings including that early tight glycemic control also reduces cardiovascular risk and prolongs survival in type 1 diabetes.
And although the phenomenon of metabolic memory initially seen in EDIC means that early glycemic control is important, subsequent EDIC data also have suggested that it is never too late to initiate intensive glycemic control, speakers emphasized during a special symposium commemorating 40 years since the start of DCCT, held during the annual scientific sessions of the American Diabetes Association. As with the 30-year DCCT/EDIC commemorative symposium held in 2013, local study participants were in the audience and were acknowledged with long applause.
Together, DCCT and EDIC – both funded by the National Institutes of Health at 27 sites in the United States and Canada – have changed the standard of care for people with type 1 diabetes and continue to inform clinical practice. Prior to the DCCT, between 1930 and 1970, about a third of people with type 1 diabetes developed vision loss and one in five experienced kidney failure and/or myocardial infarction. Stroke and amputation were also common, DCCT/EDIC chair David M. Nathan, MD, said while introducing the symposium.
“All of the advances in care of type 1 diabetes have developed because this study demonstrated that it was important – continuous glucose monitoring (CGM), new insulins, better [insulin] pumps. ... I think the most profound finding is that mortality in our intensively treated cohort is the same as in the general population. That says it all,” Dr. Nathan said in an interview.
And now, “what we still have yet to contribute is what happens to type 1 diabetes as people get older,” added Dr. Nathan, a professor of medicine at Harvard Medical School and director of the Diabetes Center at Massachusetts General Hospital, both in Boston.
‘Something that heretofore none of us could have imagined’
The 1,441 DCCT participants had a mean age of 27 years at baseline in 1983, when they were randomized to intensive insulin therapy or usual care. The 1,375 participants (96%) who continued into EDIC in 1994 were an average of 35 years old at that point, when the usual care group was taught intensive glycemic management and all participants returned to their personal health care teams. The 1,075 participants in EDIC today are an average age of 63 years.
Only 11 participants had died at the start of EDIC, and just 250 (17%) have died as of 2023, said study coordinator cochair Gayle Lorenzi, RN, who is a certified diabetes care and education specialist at the University of California, San Diego.
“DCCT/EDIC because of its longevity represents a unique opportunity to explore aging in long duration of type 1 diabetes, something that heretofore none of us could have imagined, especially for those of you in the audience who started your careers in the 70s and 80s,” Ms. Lorenzi commented.
About 36% of the cohort now has overweight and 40% have obesity, mirroring the general population. And they now have a mean hemoglobin A1c of 7.3%.
According to Barbara H. Braffett, PhD, co–principal investigator at the DCCT/EDIC data coordinating center: “The EDIC study is now shifting its focus during the next 5 years to understand the clinical course of type 1 diabetes in the setting of advancing duration and age, as well as increasing adiposity, which has progressively affected individuals with type 1 diabetes and has potential long-term adverse consequences.”
Dr. Braffett outlined the new study approaches added in 2022-2027. Cardiopulmonary exercise testing, two-dimensional Doppler echocardiography, and carotid-femoral pulse wave velocity will be used to quantify functional and structural changes central to heart failure.
Dr. Nathan commented that, although enough cardiovascular events were available in EDIC by 2006 to demonstrate a significant 58% reduction in the intensive therapy group, “now we can start looking at the aging heart. We have a bunch of great cardiologists working with us who will be guiding us on measuring everything.”
Fatty liver disease in the setting of increasing adiposity will also be investigated using transient elastography (FibroScan) and the Fibrosis-4 index, a quantification of liver enzymes and platelet count.
Dr. Nathan noted that the study participants have had “this kind of funny metabolic milieu in their liver for decades. They don’t make insulin in their pancreas, and therefore, the insulin they get is peripheral and then it goes to their liver. Well, what does that do to them?”
Participants will also complete three symptom questionnaires assessing obstructive sleep apnea, aimed at guiding future sleep studies in those found to be at high risk, Dr. Braffett said.
DCCT/EDIC over 40 years: ‘Incredibly complete picture’
As of 2023, the DCCT/EDIC participants have been studied for longer than 60% of their lifespans and for over 80% of their diabetes duration, Dr. Braffett noted.
During the EDIC 2017-2022 cycle, Dr. Braffett and other speakers summarized, prior EDIC efforts had focused on aspects of cognitive function, physical function, and cheiroarthropathy.
Other DCCT/EDIC studies examined the relationship of A1c and diabetes duration in cardiovascular disease risk, the association of microvascular complications with the risk of cardiovascular disease beyond traditional risk factors, and the risk of severe hypoglycemia over the first 30 years of DCCT/EDIC follow-up.
Moreover, the longitudinal eye and kidney assessments over the 40 years have informed screening guidelines for retinopathy and urinary albumin.
Dr. Nathan said: “Today, the number with horrible complications is very few, but we haven’t erased complications entirely. ... We have this incredibly complete picture of type 1 diabetes that allows us to explore everything. We welcome people to come to us with ideas. That’s the value of this research.”
Dr. Nathan, Ms. Lorenzi, and Dr. Braffett reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO –
New funding for 2022-2027 for the DCCT long-term observational follow-up study, the Epidemiology of Diabetes Interventions and Complications (EDIC) will go toward investigating aspects of type 1 diabetes that are associated with aging and are also common in type 2 diabetes, including cardiovascular disease, fatty liver disease, and sleep apnea.
The original randomized DCCT clinical trial results, published in 1993 in the New England Journal of Medicine, proved that early intensive glycemic control was the key to preventing or slowing the progression of long-term eye, kidney, and nerve complications of type 1 diabetes. Subsequently, EDIC has yielded many more major findings including that early tight glycemic control also reduces cardiovascular risk and prolongs survival in type 1 diabetes.
And although the phenomenon of metabolic memory initially seen in EDIC means that early glycemic control is important, subsequent EDIC data also have suggested that it is never too late to initiate intensive glycemic control, speakers emphasized during a special symposium commemorating 40 years since the start of DCCT, held during the annual scientific sessions of the American Diabetes Association. As with the 30-year DCCT/EDIC commemorative symposium held in 2013, local study participants were in the audience and were acknowledged with long applause.
Together, DCCT and EDIC – both funded by the National Institutes of Health at 27 sites in the United States and Canada – have changed the standard of care for people with type 1 diabetes and continue to inform clinical practice. Prior to the DCCT, between 1930 and 1970, about a third of people with type 1 diabetes developed vision loss and one in five experienced kidney failure and/or myocardial infarction. Stroke and amputation were also common, DCCT/EDIC chair David M. Nathan, MD, said while introducing the symposium.
“All of the advances in care of type 1 diabetes have developed because this study demonstrated that it was important – continuous glucose monitoring (CGM), new insulins, better [insulin] pumps. ... I think the most profound finding is that mortality in our intensively treated cohort is the same as in the general population. That says it all,” Dr. Nathan said in an interview.
And now, “what we still have yet to contribute is what happens to type 1 diabetes as people get older,” added Dr. Nathan, a professor of medicine at Harvard Medical School and director of the Diabetes Center at Massachusetts General Hospital, both in Boston.
‘Something that heretofore none of us could have imagined’
The 1,441 DCCT participants had a mean age of 27 years at baseline in 1983, when they were randomized to intensive insulin therapy or usual care. The 1,375 participants (96%) who continued into EDIC in 1994 were an average of 35 years old at that point, when the usual care group was taught intensive glycemic management and all participants returned to their personal health care teams. The 1,075 participants in EDIC today are an average age of 63 years.
Only 11 participants had died at the start of EDIC, and just 250 (17%) have died as of 2023, said study coordinator cochair Gayle Lorenzi, RN, who is a certified diabetes care and education specialist at the University of California, San Diego.
“DCCT/EDIC because of its longevity represents a unique opportunity to explore aging in long duration of type 1 diabetes, something that heretofore none of us could have imagined, especially for those of you in the audience who started your careers in the 70s and 80s,” Ms. Lorenzi commented.
About 36% of the cohort now has overweight and 40% have obesity, mirroring the general population. And they now have a mean hemoglobin A1c of 7.3%.
According to Barbara H. Braffett, PhD, co–principal investigator at the DCCT/EDIC data coordinating center: “The EDIC study is now shifting its focus during the next 5 years to understand the clinical course of type 1 diabetes in the setting of advancing duration and age, as well as increasing adiposity, which has progressively affected individuals with type 1 diabetes and has potential long-term adverse consequences.”
Dr. Braffett outlined the new study approaches added in 2022-2027. Cardiopulmonary exercise testing, two-dimensional Doppler echocardiography, and carotid-femoral pulse wave velocity will be used to quantify functional and structural changes central to heart failure.
Dr. Nathan commented that, although enough cardiovascular events were available in EDIC by 2006 to demonstrate a significant 58% reduction in the intensive therapy group, “now we can start looking at the aging heart. We have a bunch of great cardiologists working with us who will be guiding us on measuring everything.”
Fatty liver disease in the setting of increasing adiposity will also be investigated using transient elastography (FibroScan) and the Fibrosis-4 index, a quantification of liver enzymes and platelet count.
Dr. Nathan noted that the study participants have had “this kind of funny metabolic milieu in their liver for decades. They don’t make insulin in their pancreas, and therefore, the insulin they get is peripheral and then it goes to their liver. Well, what does that do to them?”
Participants will also complete three symptom questionnaires assessing obstructive sleep apnea, aimed at guiding future sleep studies in those found to be at high risk, Dr. Braffett said.
DCCT/EDIC over 40 years: ‘Incredibly complete picture’
As of 2023, the DCCT/EDIC participants have been studied for longer than 60% of their lifespans and for over 80% of their diabetes duration, Dr. Braffett noted.
During the EDIC 2017-2022 cycle, Dr. Braffett and other speakers summarized, prior EDIC efforts had focused on aspects of cognitive function, physical function, and cheiroarthropathy.
Other DCCT/EDIC studies examined the relationship of A1c and diabetes duration in cardiovascular disease risk, the association of microvascular complications with the risk of cardiovascular disease beyond traditional risk factors, and the risk of severe hypoglycemia over the first 30 years of DCCT/EDIC follow-up.
Moreover, the longitudinal eye and kidney assessments over the 40 years have informed screening guidelines for retinopathy and urinary albumin.
Dr. Nathan said: “Today, the number with horrible complications is very few, but we haven’t erased complications entirely. ... We have this incredibly complete picture of type 1 diabetes that allows us to explore everything. We welcome people to come to us with ideas. That’s the value of this research.”
Dr. Nathan, Ms. Lorenzi, and Dr. Braffett reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO –
New funding for 2022-2027 for the DCCT long-term observational follow-up study, the Epidemiology of Diabetes Interventions and Complications (EDIC) will go toward investigating aspects of type 1 diabetes that are associated with aging and are also common in type 2 diabetes, including cardiovascular disease, fatty liver disease, and sleep apnea.
The original randomized DCCT clinical trial results, published in 1993 in the New England Journal of Medicine, proved that early intensive glycemic control was the key to preventing or slowing the progression of long-term eye, kidney, and nerve complications of type 1 diabetes. Subsequently, EDIC has yielded many more major findings including that early tight glycemic control also reduces cardiovascular risk and prolongs survival in type 1 diabetes.
And although the phenomenon of metabolic memory initially seen in EDIC means that early glycemic control is important, subsequent EDIC data also have suggested that it is never too late to initiate intensive glycemic control, speakers emphasized during a special symposium commemorating 40 years since the start of DCCT, held during the annual scientific sessions of the American Diabetes Association. As with the 30-year DCCT/EDIC commemorative symposium held in 2013, local study participants were in the audience and were acknowledged with long applause.
Together, DCCT and EDIC – both funded by the National Institutes of Health at 27 sites in the United States and Canada – have changed the standard of care for people with type 1 diabetes and continue to inform clinical practice. Prior to the DCCT, between 1930 and 1970, about a third of people with type 1 diabetes developed vision loss and one in five experienced kidney failure and/or myocardial infarction. Stroke and amputation were also common, DCCT/EDIC chair David M. Nathan, MD, said while introducing the symposium.
“All of the advances in care of type 1 diabetes have developed because this study demonstrated that it was important – continuous glucose monitoring (CGM), new insulins, better [insulin] pumps. ... I think the most profound finding is that mortality in our intensively treated cohort is the same as in the general population. That says it all,” Dr. Nathan said in an interview.
And now, “what we still have yet to contribute is what happens to type 1 diabetes as people get older,” added Dr. Nathan, a professor of medicine at Harvard Medical School and director of the Diabetes Center at Massachusetts General Hospital, both in Boston.
‘Something that heretofore none of us could have imagined’
The 1,441 DCCT participants had a mean age of 27 years at baseline in 1983, when they were randomized to intensive insulin therapy or usual care. The 1,375 participants (96%) who continued into EDIC in 1994 were an average of 35 years old at that point, when the usual care group was taught intensive glycemic management and all participants returned to their personal health care teams. The 1,075 participants in EDIC today are an average age of 63 years.
Only 11 participants had died at the start of EDIC, and just 250 (17%) have died as of 2023, said study coordinator cochair Gayle Lorenzi, RN, who is a certified diabetes care and education specialist at the University of California, San Diego.
“DCCT/EDIC because of its longevity represents a unique opportunity to explore aging in long duration of type 1 diabetes, something that heretofore none of us could have imagined, especially for those of you in the audience who started your careers in the 70s and 80s,” Ms. Lorenzi commented.
About 36% of the cohort now has overweight and 40% have obesity, mirroring the general population. And they now have a mean hemoglobin A1c of 7.3%.
According to Barbara H. Braffett, PhD, co–principal investigator at the DCCT/EDIC data coordinating center: “The EDIC study is now shifting its focus during the next 5 years to understand the clinical course of type 1 diabetes in the setting of advancing duration and age, as well as increasing adiposity, which has progressively affected individuals with type 1 diabetes and has potential long-term adverse consequences.”
Dr. Braffett outlined the new study approaches added in 2022-2027. Cardiopulmonary exercise testing, two-dimensional Doppler echocardiography, and carotid-femoral pulse wave velocity will be used to quantify functional and structural changes central to heart failure.
Dr. Nathan commented that, although enough cardiovascular events were available in EDIC by 2006 to demonstrate a significant 58% reduction in the intensive therapy group, “now we can start looking at the aging heart. We have a bunch of great cardiologists working with us who will be guiding us on measuring everything.”
Fatty liver disease in the setting of increasing adiposity will also be investigated using transient elastography (FibroScan) and the Fibrosis-4 index, a quantification of liver enzymes and platelet count.
Dr. Nathan noted that the study participants have had “this kind of funny metabolic milieu in their liver for decades. They don’t make insulin in their pancreas, and therefore, the insulin they get is peripheral and then it goes to their liver. Well, what does that do to them?”
Participants will also complete three symptom questionnaires assessing obstructive sleep apnea, aimed at guiding future sleep studies in those found to be at high risk, Dr. Braffett said.
DCCT/EDIC over 40 years: ‘Incredibly complete picture’
As of 2023, the DCCT/EDIC participants have been studied for longer than 60% of their lifespans and for over 80% of their diabetes duration, Dr. Braffett noted.
During the EDIC 2017-2022 cycle, Dr. Braffett and other speakers summarized, prior EDIC efforts had focused on aspects of cognitive function, physical function, and cheiroarthropathy.
Other DCCT/EDIC studies examined the relationship of A1c and diabetes duration in cardiovascular disease risk, the association of microvascular complications with the risk of cardiovascular disease beyond traditional risk factors, and the risk of severe hypoglycemia over the first 30 years of DCCT/EDIC follow-up.
Moreover, the longitudinal eye and kidney assessments over the 40 years have informed screening guidelines for retinopathy and urinary albumin.
Dr. Nathan said: “Today, the number with horrible complications is very few, but we haven’t erased complications entirely. ... We have this incredibly complete picture of type 1 diabetes that allows us to explore everything. We welcome people to come to us with ideas. That’s the value of this research.”
Dr. Nathan, Ms. Lorenzi, and Dr. Braffett reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADA 2023
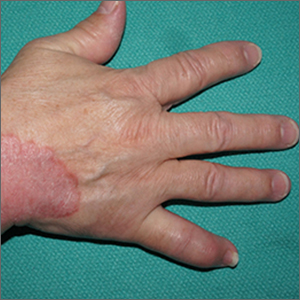
Oral IL-23 receptor antagonist for psoriasis promising: Phase 2b study
SINGAPORE – across all doses, compared with placebo, according to results of the FRONTIER 1 trial.
In the 16-week phase 2b study, 255 adults with moderate to severe plaque psoriasis were randomly assigned into six treatment groups: placebo (n = 43), JNJ-2113 25 mg daily (n = 43), 25 mg twice daily (n = 41), 50 mg daily (n = 43), 100 mg daily (n = 43), or 100 mg twice daily (n = 42).
Of those who took the placebo, only 9.3% achieved the study’s primary endpoint of a 75% or greater improvement in the Psoriasis Area and Severity Index (PASI-75) by week 16. This was compared with 78.6% in the group that took the highest dose.
“Additionally, the onset of action was fairly fast: at week 4, more than 20% of patients had achieved PASI 75,” said Robert Bissonnette, MD, CEO of Innovaderm Research in Montreal, who presented the findings during a late-breaker session at the World Congress of Dermatology.
Patients in the remaining groups demonstrated a response that corresponded to dosing level: with 37.2%, 51.2%, 58.1%, and 65.1% achieving PASI-75 in the 25 mg daily, 25 mg twice-daily, 50 mg daily, and 100 mg daily groups, respectively.
“These results are very interesting because in terms of psoriasis treatment, if this is confirmed in phase 3, it would give us an oral alternative that would be selective for IL-23,” said Dr. Bissonnette, referring to the signaling pathway that plays a critical role in the pathogenesis of several immune-mediated inflammatory diseases, including plaque psoriasis.
Although rarely life-threatening, the skin disorder is often intractable to treatment. In recent years, therapies that block IL-23 signaling and downstream inflammatory cytokine production have proven useful. “We have on the market a number of biological agents targeting IL-23 that we use on a regular basis,” said Dr. Bissonnette. “However, there are currently no orally delivered therapies.”
If successful, JNJ-2113 – a first-in-class oral IL-23 antagonist peptide developed by Janssen – could change the treatment paradigm for patients with moderate to severe plaque psoriasis. “When I was first introduced to the concept, I thought it wouldn’t work as it’s a peptide, that it would be digested by the stomach,” he told the audience. “But because of its GI stability and its potency, when you administer it orally, you can detect pharmacological activity.”
A well-tolerated alternative
Participants in the FRONTIER 1 trial were on average about 44 years old and weighed 88.9 kg (195 lb). Most had been living with psoriasis for about 18 years, with a total PASI score of 19.05. In addition, 43.1% had been treated with phototherapy in the past, 22% with biologics, and 78.4% with systemics.
PASI 90 and 100 were among some of the secondary outcomes measured. Similar to the primary outcome of PASI 75, all treatment groups demonstrated a statistically significant dose-response in PASI 90, compared with placebo. For those on the highest dose of JNJ-2113, 59.5% and 40.5% achieved PASI 90 and PASI 100, respectively, by week 16. The corresponding figures for those receiving placebo were 2.3% and 0%.
The safety profile for JNJ-2113 across all doses was similar to that of placebo, with no evidence of a dose-dependent increase in the occurrence of adverse events (AEs). The most frequently reported AEs were COVID-19 and nasopharyngitis. There were three serious AEs (COVID-19, infected cyst, suicide attempt) among those on the active drug, but the investigators assessed that they were not related to the study intervention. No deaths, major adverse cardiac events, or malignancies were reported during the study.
Approached for an independent comment, Marius-Anton Ionescu, MD, PhD, from the University Hospital Saint Louis, Paris, who specializes in psoriasis, told this news organization that the new development with JNJ-2113 “is really promising.”
Treatment for plaque psoriasis has improved to the point where some biologics, such as risankizumab (Skyrizi), only require patients to have “four shots a year,” he says. “This is the future of psoriasis treatment; it might go down to two shots a year” – a regimen that will be easier than taking an oral medication once or twice a day.
“But it’s good to have an oral option because you will always have some patients who say: ‘Shots are not for me, I’m afraid,’ ” he says.
However, Dr. Ionescu noted that if JNJ-2113 were to pass phase 3 trials, it might face stiff competition from the selective tyrosine kinase 2 (TYK2) inhibitor deucravacitinib (Sotyktu), which the U.S. Food and Drug Administration approved for use in adults with moderate to severe plaque psoriasis last September. “It has very good results and is the first oral therapy that is comparable with biologics for plaque psoriasis,” he says.
But Dr. Bissonnette remains hopeful for the future. “I think JNJ-2113 goes way beyond psoriasis because this type of strategy using oral peptide–blocking receptors could be used in other immune-mediated diseases, including atopic dermatitis and other diseases outside of dermatology.” In addition to running a phase 3 study for moderate to severe plaque psoriasis, Janssen is planning to initiate a phase 2b clinical trial of JNJ-2113 in adults with ulcerative colitis.
The study was funded by Janssen. Dr. Bissonnette reports consulting and investigating for Janssen, and being on advisory panels and receiving research funding from multiple other pharmaceutical companies. Dr. Ionescu is an investigator for Psoriasis National Register France Psobioteq (no honoraria), and an investigator and speaker for Uriage cosmetics (honoraria).
A version of this article first appeared on Medscape.com.
SINGAPORE – across all doses, compared with placebo, according to results of the FRONTIER 1 trial.
In the 16-week phase 2b study, 255 adults with moderate to severe plaque psoriasis were randomly assigned into six treatment groups: placebo (n = 43), JNJ-2113 25 mg daily (n = 43), 25 mg twice daily (n = 41), 50 mg daily (n = 43), 100 mg daily (n = 43), or 100 mg twice daily (n = 42).
Of those who took the placebo, only 9.3% achieved the study’s primary endpoint of a 75% or greater improvement in the Psoriasis Area and Severity Index (PASI-75) by week 16. This was compared with 78.6% in the group that took the highest dose.
“Additionally, the onset of action was fairly fast: at week 4, more than 20% of patients had achieved PASI 75,” said Robert Bissonnette, MD, CEO of Innovaderm Research in Montreal, who presented the findings during a late-breaker session at the World Congress of Dermatology.
Patients in the remaining groups demonstrated a response that corresponded to dosing level: with 37.2%, 51.2%, 58.1%, and 65.1% achieving PASI-75 in the 25 mg daily, 25 mg twice-daily, 50 mg daily, and 100 mg daily groups, respectively.
“These results are very interesting because in terms of psoriasis treatment, if this is confirmed in phase 3, it would give us an oral alternative that would be selective for IL-23,” said Dr. Bissonnette, referring to the signaling pathway that plays a critical role in the pathogenesis of several immune-mediated inflammatory diseases, including plaque psoriasis.
Although rarely life-threatening, the skin disorder is often intractable to treatment. In recent years, therapies that block IL-23 signaling and downstream inflammatory cytokine production have proven useful. “We have on the market a number of biological agents targeting IL-23 that we use on a regular basis,” said Dr. Bissonnette. “However, there are currently no orally delivered therapies.”
If successful, JNJ-2113 – a first-in-class oral IL-23 antagonist peptide developed by Janssen – could change the treatment paradigm for patients with moderate to severe plaque psoriasis. “When I was first introduced to the concept, I thought it wouldn’t work as it’s a peptide, that it would be digested by the stomach,” he told the audience. “But because of its GI stability and its potency, when you administer it orally, you can detect pharmacological activity.”
A well-tolerated alternative
Participants in the FRONTIER 1 trial were on average about 44 years old and weighed 88.9 kg (195 lb). Most had been living with psoriasis for about 18 years, with a total PASI score of 19.05. In addition, 43.1% had been treated with phototherapy in the past, 22% with biologics, and 78.4% with systemics.
PASI 90 and 100 were among some of the secondary outcomes measured. Similar to the primary outcome of PASI 75, all treatment groups demonstrated a statistically significant dose-response in PASI 90, compared with placebo. For those on the highest dose of JNJ-2113, 59.5% and 40.5% achieved PASI 90 and PASI 100, respectively, by week 16. The corresponding figures for those receiving placebo were 2.3% and 0%.
The safety profile for JNJ-2113 across all doses was similar to that of placebo, with no evidence of a dose-dependent increase in the occurrence of adverse events (AEs). The most frequently reported AEs were COVID-19 and nasopharyngitis. There were three serious AEs (COVID-19, infected cyst, suicide attempt) among those on the active drug, but the investigators assessed that they were not related to the study intervention. No deaths, major adverse cardiac events, or malignancies were reported during the study.
Approached for an independent comment, Marius-Anton Ionescu, MD, PhD, from the University Hospital Saint Louis, Paris, who specializes in psoriasis, told this news organization that the new development with JNJ-2113 “is really promising.”
Treatment for plaque psoriasis has improved to the point where some biologics, such as risankizumab (Skyrizi), only require patients to have “four shots a year,” he says. “This is the future of psoriasis treatment; it might go down to two shots a year” – a regimen that will be easier than taking an oral medication once or twice a day.
“But it’s good to have an oral option because you will always have some patients who say: ‘Shots are not for me, I’m afraid,’ ” he says.
However, Dr. Ionescu noted that if JNJ-2113 were to pass phase 3 trials, it might face stiff competition from the selective tyrosine kinase 2 (TYK2) inhibitor deucravacitinib (Sotyktu), which the U.S. Food and Drug Administration approved for use in adults with moderate to severe plaque psoriasis last September. “It has very good results and is the first oral therapy that is comparable with biologics for plaque psoriasis,” he says.
But Dr. Bissonnette remains hopeful for the future. “I think JNJ-2113 goes way beyond psoriasis because this type of strategy using oral peptide–blocking receptors could be used in other immune-mediated diseases, including atopic dermatitis and other diseases outside of dermatology.” In addition to running a phase 3 study for moderate to severe plaque psoriasis, Janssen is planning to initiate a phase 2b clinical trial of JNJ-2113 in adults with ulcerative colitis.
The study was funded by Janssen. Dr. Bissonnette reports consulting and investigating for Janssen, and being on advisory panels and receiving research funding from multiple other pharmaceutical companies. Dr. Ionescu is an investigator for Psoriasis National Register France Psobioteq (no honoraria), and an investigator and speaker for Uriage cosmetics (honoraria).
A version of this article first appeared on Medscape.com.
SINGAPORE – across all doses, compared with placebo, according to results of the FRONTIER 1 trial.
In the 16-week phase 2b study, 255 adults with moderate to severe plaque psoriasis were randomly assigned into six treatment groups: placebo (n = 43), JNJ-2113 25 mg daily (n = 43), 25 mg twice daily (n = 41), 50 mg daily (n = 43), 100 mg daily (n = 43), or 100 mg twice daily (n = 42).
Of those who took the placebo, only 9.3% achieved the study’s primary endpoint of a 75% or greater improvement in the Psoriasis Area and Severity Index (PASI-75) by week 16. This was compared with 78.6% in the group that took the highest dose.
“Additionally, the onset of action was fairly fast: at week 4, more than 20% of patients had achieved PASI 75,” said Robert Bissonnette, MD, CEO of Innovaderm Research in Montreal, who presented the findings during a late-breaker session at the World Congress of Dermatology.
Patients in the remaining groups demonstrated a response that corresponded to dosing level: with 37.2%, 51.2%, 58.1%, and 65.1% achieving PASI-75 in the 25 mg daily, 25 mg twice-daily, 50 mg daily, and 100 mg daily groups, respectively.
“These results are very interesting because in terms of psoriasis treatment, if this is confirmed in phase 3, it would give us an oral alternative that would be selective for IL-23,” said Dr. Bissonnette, referring to the signaling pathway that plays a critical role in the pathogenesis of several immune-mediated inflammatory diseases, including plaque psoriasis.
Although rarely life-threatening, the skin disorder is often intractable to treatment. In recent years, therapies that block IL-23 signaling and downstream inflammatory cytokine production have proven useful. “We have on the market a number of biological agents targeting IL-23 that we use on a regular basis,” said Dr. Bissonnette. “However, there are currently no orally delivered therapies.”
If successful, JNJ-2113 – a first-in-class oral IL-23 antagonist peptide developed by Janssen – could change the treatment paradigm for patients with moderate to severe plaque psoriasis. “When I was first introduced to the concept, I thought it wouldn’t work as it’s a peptide, that it would be digested by the stomach,” he told the audience. “But because of its GI stability and its potency, when you administer it orally, you can detect pharmacological activity.”
A well-tolerated alternative
Participants in the FRONTIER 1 trial were on average about 44 years old and weighed 88.9 kg (195 lb). Most had been living with psoriasis for about 18 years, with a total PASI score of 19.05. In addition, 43.1% had been treated with phototherapy in the past, 22% with biologics, and 78.4% with systemics.
PASI 90 and 100 were among some of the secondary outcomes measured. Similar to the primary outcome of PASI 75, all treatment groups demonstrated a statistically significant dose-response in PASI 90, compared with placebo. For those on the highest dose of JNJ-2113, 59.5% and 40.5% achieved PASI 90 and PASI 100, respectively, by week 16. The corresponding figures for those receiving placebo were 2.3% and 0%.
The safety profile for JNJ-2113 across all doses was similar to that of placebo, with no evidence of a dose-dependent increase in the occurrence of adverse events (AEs). The most frequently reported AEs were COVID-19 and nasopharyngitis. There were three serious AEs (COVID-19, infected cyst, suicide attempt) among those on the active drug, but the investigators assessed that they were not related to the study intervention. No deaths, major adverse cardiac events, or malignancies were reported during the study.
Approached for an independent comment, Marius-Anton Ionescu, MD, PhD, from the University Hospital Saint Louis, Paris, who specializes in psoriasis, told this news organization that the new development with JNJ-2113 “is really promising.”
Treatment for plaque psoriasis has improved to the point where some biologics, such as risankizumab (Skyrizi), only require patients to have “four shots a year,” he says. “This is the future of psoriasis treatment; it might go down to two shots a year” – a regimen that will be easier than taking an oral medication once or twice a day.
“But it’s good to have an oral option because you will always have some patients who say: ‘Shots are not for me, I’m afraid,’ ” he says.
However, Dr. Ionescu noted that if JNJ-2113 were to pass phase 3 trials, it might face stiff competition from the selective tyrosine kinase 2 (TYK2) inhibitor deucravacitinib (Sotyktu), which the U.S. Food and Drug Administration approved for use in adults with moderate to severe plaque psoriasis last September. “It has very good results and is the first oral therapy that is comparable with biologics for plaque psoriasis,” he says.
But Dr. Bissonnette remains hopeful for the future. “I think JNJ-2113 goes way beyond psoriasis because this type of strategy using oral peptide–blocking receptors could be used in other immune-mediated diseases, including atopic dermatitis and other diseases outside of dermatology.” In addition to running a phase 3 study for moderate to severe plaque psoriasis, Janssen is planning to initiate a phase 2b clinical trial of JNJ-2113 in adults with ulcerative colitis.
The study was funded by Janssen. Dr. Bissonnette reports consulting and investigating for Janssen, and being on advisory panels and receiving research funding from multiple other pharmaceutical companies. Dr. Ionescu is an investigator for Psoriasis National Register France Psobioteq (no honoraria), and an investigator and speaker for Uriage cosmetics (honoraria).
A version of this article first appeared on Medscape.com.
AT WCD 2023
JAK inhibitors efficacious for atopic dermatitis in Asian patients, study finds
SINGAPORE – conducted in Singapore has found.
“Abrocitinib and upadacitinib surprisingly appeared to have better treatment efficacy compared to baricitinib,” said study lead Yik Weng Yew, MD, PhD, MPH, deputy head of research at Singapore’s National Skin Centre (NSC), who presented the results at the 25th World Congress of Dermatology. “But overall, as a group, I think they show a very good treatment response, as well as a good effect on itch response.”
JAK inhibitors are used to treat a variety of inflammatory diseases including alopecia areata, rheumatoid arthritis, and inflammatory bowel disease. Although treatment for severe eczema was previously limited to topical steroids and oral immunosuppressants, there are now two oral JAK inhibitors – abrocitinib and upadacitinib – approved in 2022 by the Food and Drug Administration for treating AD, which affects up to 2.4% of the global population. (A topical formulation of ruxolitinib, a JAK inhibitor, was approved for AD in 2021.)
The Singapore study is one of the few that have examined the safety and efficacy of JAK inhibitors for treatment of AD in a non-White population.
Chinese population
For the 12-week trial, conducted in 2022, Dr. Yew and associates recruited 35 patients from the NSC. More than half of participants (64%) were men and most (96%) were of Chinese ethnicity. Four of every five patients had previously received systemic agents: 17% had been treated with one systemic agent, 18.9% with two, 15.1% with three, 22.6% with four, and 3.8% with five. The most commonly used agents were cyclosporine (62.3%), methotrexate (47.2%), azathioprine (39.6%), and dupilumab (35.8%).
“The switch in therapy could have been a result of inadequate efficacy or cost reasons because in Singapore patients pay out of pocket for AD treatments,” said Dr. Yew.
Additionally, he offered a caveat on the profile of participants: “Perhaps they were more difficult atopic eczema patients, and therefore, the efficacy [of JAK inhibitors] might be a bit different.”
Clearer skin, less itch
Patients received one of the three study drugs: baricitinib (66%), abrocitinib (21%), and upadacitinib (13%). The distribution was “affected by reimbursement patterns and availability of the drug,” explained Dr. Yew.
They were assessed at weeks 4 and 12. By study end, the proportion of patients who self-reported an improvement in their condition was 100% for upadacitinib, 90% for abrocitinib, and 69% for baricitinib.
Scores on the Investigator Global Assessment (IGA) also improved with treatment. Patients in the baricitinib group saw their mean score fall from 4.0 to 3.0 by week 4, then to 2.0 by week 12. With upadacitinib and abrocitinib, “you can see that there is a nice decrease in IGA responses,” said Dr. Yew, referring to the larger improvement in scores experienced by patients on those two treatments. For patients on upadacitinib, IGA decreased from 3.5 to 2 at 4 weeks, then to 0.5 at 12 weeks, while those taking abrocitinib had their scores drop from 4.0 to 2.0 at 4 weeks, then to 1.0 at 12 weeks.
When it came to itch reduction, the abrocitinib group experienced the biggest reduction, with a median reduction of 5.5 points in itch score. Median reduction in itch score was 4 points for the other two groups. “Oral JAK inhibitors appear to have a good effect on itch response,” said Dr. Yew.
However, the researchers observed no significant reduction in percentage of body surface area affected, the last outcome assessed.
The most commonly reported adverse events were increased creatine kinase levels (11.3% of patients), increased LDL cholesterol levels (9.4%), and herpes zoster (9.4%). Those in the abrocitinib reported a higher number of these adverse events, compared with the other two treatment groups. (There were no herpes zoster cases among those taking baricitinib.)
For herpes zoster, Dr. Yew said “the common recommendation” is to give the inactivated shingles vaccine. “But the problem is that, number one, these patients would have probably failed multiple agents so they probably can’t wait for you to vaccinate before you initiate treatment.”
In addition, people in Singapore have to pay out-of-pocket for the two vaccine doses, “which is probably a month’s worth of medication,” he noted. “So we have a lot of resistance from patients.”
Additionally, Dr. Yew noted that contrary to what has previously been reported in the literature, there were few complaints of acne as a side effect in the Singaporean study population.
Toward greater representation
Dr. Yew pointed out that the study was limited by a few factors: neither the Eczema Area and Severity Index or Scoring of Atopic Dermatitis index data was used, and the study population was small and not representative of the real world.
Still, the new findings contribute to the overall safety and efficacy profile of JAK inhibitors in AD, which has so far been scarce in non-White populations.
“In Western studies, unfortunately, the representation of the population of skin of color or different ethnicities is underrepresented,” said Yousef Binamer, MD, chair of the dermatology department at King Faisal Specialist Hospital, Riyadh, Saudi Arabia, when approached for an independent comment on the results.
“This is now why researchers are looking into specific groups to study them,” which he pointed out, is crucial because “the immunophenotyping of AD is different for each background.”
The incidence and severity of AD tend to be higher in Asian and Middle Eastern populations, for instance, he noted. “It’s very common in Asia, and not so common in very white skin. I did my training in Canada so I see the difference,” said Dr. Binamer. “Asian people tend to be more itchy and have a tendency to scar on pigmentation.” Whereas White people “usually do not have this issue.”
“So I think real-world evidence of JAK inhibitors in the other populations is important,” he said. Studies such as the one conducted in Singapore, as well as the recently reported QUARTZ3 study, which examined the use of the JAK inhibitor ivarmacitinib in 256 Chinese patients with AD, are helping to pave the way.
The study was independently supported. Dr. Yew and Dr. Binamer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SINGAPORE – conducted in Singapore has found.
“Abrocitinib and upadacitinib surprisingly appeared to have better treatment efficacy compared to baricitinib,” said study lead Yik Weng Yew, MD, PhD, MPH, deputy head of research at Singapore’s National Skin Centre (NSC), who presented the results at the 25th World Congress of Dermatology. “But overall, as a group, I think they show a very good treatment response, as well as a good effect on itch response.”
JAK inhibitors are used to treat a variety of inflammatory diseases including alopecia areata, rheumatoid arthritis, and inflammatory bowel disease. Although treatment for severe eczema was previously limited to topical steroids and oral immunosuppressants, there are now two oral JAK inhibitors – abrocitinib and upadacitinib – approved in 2022 by the Food and Drug Administration for treating AD, which affects up to 2.4% of the global population. (A topical formulation of ruxolitinib, a JAK inhibitor, was approved for AD in 2021.)
The Singapore study is one of the few that have examined the safety and efficacy of JAK inhibitors for treatment of AD in a non-White population.
Chinese population
For the 12-week trial, conducted in 2022, Dr. Yew and associates recruited 35 patients from the NSC. More than half of participants (64%) were men and most (96%) were of Chinese ethnicity. Four of every five patients had previously received systemic agents: 17% had been treated with one systemic agent, 18.9% with two, 15.1% with three, 22.6% with four, and 3.8% with five. The most commonly used agents were cyclosporine (62.3%), methotrexate (47.2%), azathioprine (39.6%), and dupilumab (35.8%).
“The switch in therapy could have been a result of inadequate efficacy or cost reasons because in Singapore patients pay out of pocket for AD treatments,” said Dr. Yew.
Additionally, he offered a caveat on the profile of participants: “Perhaps they were more difficult atopic eczema patients, and therefore, the efficacy [of JAK inhibitors] might be a bit different.”
Clearer skin, less itch
Patients received one of the three study drugs: baricitinib (66%), abrocitinib (21%), and upadacitinib (13%). The distribution was “affected by reimbursement patterns and availability of the drug,” explained Dr. Yew.
They were assessed at weeks 4 and 12. By study end, the proportion of patients who self-reported an improvement in their condition was 100% for upadacitinib, 90% for abrocitinib, and 69% for baricitinib.
Scores on the Investigator Global Assessment (IGA) also improved with treatment. Patients in the baricitinib group saw their mean score fall from 4.0 to 3.0 by week 4, then to 2.0 by week 12. With upadacitinib and abrocitinib, “you can see that there is a nice decrease in IGA responses,” said Dr. Yew, referring to the larger improvement in scores experienced by patients on those two treatments. For patients on upadacitinib, IGA decreased from 3.5 to 2 at 4 weeks, then to 0.5 at 12 weeks, while those taking abrocitinib had their scores drop from 4.0 to 2.0 at 4 weeks, then to 1.0 at 12 weeks.
When it came to itch reduction, the abrocitinib group experienced the biggest reduction, with a median reduction of 5.5 points in itch score. Median reduction in itch score was 4 points for the other two groups. “Oral JAK inhibitors appear to have a good effect on itch response,” said Dr. Yew.
However, the researchers observed no significant reduction in percentage of body surface area affected, the last outcome assessed.
The most commonly reported adverse events were increased creatine kinase levels (11.3% of patients), increased LDL cholesterol levels (9.4%), and herpes zoster (9.4%). Those in the abrocitinib reported a higher number of these adverse events, compared with the other two treatment groups. (There were no herpes zoster cases among those taking baricitinib.)
For herpes zoster, Dr. Yew said “the common recommendation” is to give the inactivated shingles vaccine. “But the problem is that, number one, these patients would have probably failed multiple agents so they probably can’t wait for you to vaccinate before you initiate treatment.”
In addition, people in Singapore have to pay out-of-pocket for the two vaccine doses, “which is probably a month’s worth of medication,” he noted. “So we have a lot of resistance from patients.”
Additionally, Dr. Yew noted that contrary to what has previously been reported in the literature, there were few complaints of acne as a side effect in the Singaporean study population.
Toward greater representation
Dr. Yew pointed out that the study was limited by a few factors: neither the Eczema Area and Severity Index or Scoring of Atopic Dermatitis index data was used, and the study population was small and not representative of the real world.
Still, the new findings contribute to the overall safety and efficacy profile of JAK inhibitors in AD, which has so far been scarce in non-White populations.
“In Western studies, unfortunately, the representation of the population of skin of color or different ethnicities is underrepresented,” said Yousef Binamer, MD, chair of the dermatology department at King Faisal Specialist Hospital, Riyadh, Saudi Arabia, when approached for an independent comment on the results.
“This is now why researchers are looking into specific groups to study them,” which he pointed out, is crucial because “the immunophenotyping of AD is different for each background.”
The incidence and severity of AD tend to be higher in Asian and Middle Eastern populations, for instance, he noted. “It’s very common in Asia, and not so common in very white skin. I did my training in Canada so I see the difference,” said Dr. Binamer. “Asian people tend to be more itchy and have a tendency to scar on pigmentation.” Whereas White people “usually do not have this issue.”
“So I think real-world evidence of JAK inhibitors in the other populations is important,” he said. Studies such as the one conducted in Singapore, as well as the recently reported QUARTZ3 study, which examined the use of the JAK inhibitor ivarmacitinib in 256 Chinese patients with AD, are helping to pave the way.
The study was independently supported. Dr. Yew and Dr. Binamer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SINGAPORE – conducted in Singapore has found.
“Abrocitinib and upadacitinib surprisingly appeared to have better treatment efficacy compared to baricitinib,” said study lead Yik Weng Yew, MD, PhD, MPH, deputy head of research at Singapore’s National Skin Centre (NSC), who presented the results at the 25th World Congress of Dermatology. “But overall, as a group, I think they show a very good treatment response, as well as a good effect on itch response.”
JAK inhibitors are used to treat a variety of inflammatory diseases including alopecia areata, rheumatoid arthritis, and inflammatory bowel disease. Although treatment for severe eczema was previously limited to topical steroids and oral immunosuppressants, there are now two oral JAK inhibitors – abrocitinib and upadacitinib – approved in 2022 by the Food and Drug Administration for treating AD, which affects up to 2.4% of the global population. (A topical formulation of ruxolitinib, a JAK inhibitor, was approved for AD in 2021.)
The Singapore study is one of the few that have examined the safety and efficacy of JAK inhibitors for treatment of AD in a non-White population.
Chinese population
For the 12-week trial, conducted in 2022, Dr. Yew and associates recruited 35 patients from the NSC. More than half of participants (64%) were men and most (96%) were of Chinese ethnicity. Four of every five patients had previously received systemic agents: 17% had been treated with one systemic agent, 18.9% with two, 15.1% with three, 22.6% with four, and 3.8% with five. The most commonly used agents were cyclosporine (62.3%), methotrexate (47.2%), azathioprine (39.6%), and dupilumab (35.8%).
“The switch in therapy could have been a result of inadequate efficacy or cost reasons because in Singapore patients pay out of pocket for AD treatments,” said Dr. Yew.
Additionally, he offered a caveat on the profile of participants: “Perhaps they were more difficult atopic eczema patients, and therefore, the efficacy [of JAK inhibitors] might be a bit different.”
Clearer skin, less itch
Patients received one of the three study drugs: baricitinib (66%), abrocitinib (21%), and upadacitinib (13%). The distribution was “affected by reimbursement patterns and availability of the drug,” explained Dr. Yew.
They were assessed at weeks 4 and 12. By study end, the proportion of patients who self-reported an improvement in their condition was 100% for upadacitinib, 90% for abrocitinib, and 69% for baricitinib.
Scores on the Investigator Global Assessment (IGA) also improved with treatment. Patients in the baricitinib group saw their mean score fall from 4.0 to 3.0 by week 4, then to 2.0 by week 12. With upadacitinib and abrocitinib, “you can see that there is a nice decrease in IGA responses,” said Dr. Yew, referring to the larger improvement in scores experienced by patients on those two treatments. For patients on upadacitinib, IGA decreased from 3.5 to 2 at 4 weeks, then to 0.5 at 12 weeks, while those taking abrocitinib had their scores drop from 4.0 to 2.0 at 4 weeks, then to 1.0 at 12 weeks.
When it came to itch reduction, the abrocitinib group experienced the biggest reduction, with a median reduction of 5.5 points in itch score. Median reduction in itch score was 4 points for the other two groups. “Oral JAK inhibitors appear to have a good effect on itch response,” said Dr. Yew.
However, the researchers observed no significant reduction in percentage of body surface area affected, the last outcome assessed.
The most commonly reported adverse events were increased creatine kinase levels (11.3% of patients), increased LDL cholesterol levels (9.4%), and herpes zoster (9.4%). Those in the abrocitinib reported a higher number of these adverse events, compared with the other two treatment groups. (There were no herpes zoster cases among those taking baricitinib.)
For herpes zoster, Dr. Yew said “the common recommendation” is to give the inactivated shingles vaccine. “But the problem is that, number one, these patients would have probably failed multiple agents so they probably can’t wait for you to vaccinate before you initiate treatment.”
In addition, people in Singapore have to pay out-of-pocket for the two vaccine doses, “which is probably a month’s worth of medication,” he noted. “So we have a lot of resistance from patients.”
Additionally, Dr. Yew noted that contrary to what has previously been reported in the literature, there were few complaints of acne as a side effect in the Singaporean study population.
Toward greater representation
Dr. Yew pointed out that the study was limited by a few factors: neither the Eczema Area and Severity Index or Scoring of Atopic Dermatitis index data was used, and the study population was small and not representative of the real world.
Still, the new findings contribute to the overall safety and efficacy profile of JAK inhibitors in AD, which has so far been scarce in non-White populations.
“In Western studies, unfortunately, the representation of the population of skin of color or different ethnicities is underrepresented,” said Yousef Binamer, MD, chair of the dermatology department at King Faisal Specialist Hospital, Riyadh, Saudi Arabia, when approached for an independent comment on the results.
“This is now why researchers are looking into specific groups to study them,” which he pointed out, is crucial because “the immunophenotyping of AD is different for each background.”
The incidence and severity of AD tend to be higher in Asian and Middle Eastern populations, for instance, he noted. “It’s very common in Asia, and not so common in very white skin. I did my training in Canada so I see the difference,” said Dr. Binamer. “Asian people tend to be more itchy and have a tendency to scar on pigmentation.” Whereas White people “usually do not have this issue.”
“So I think real-world evidence of JAK inhibitors in the other populations is important,” he said. Studies such as the one conducted in Singapore, as well as the recently reported QUARTZ3 study, which examined the use of the JAK inhibitor ivarmacitinib in 256 Chinese patients with AD, are helping to pave the way.
The study was independently supported. Dr. Yew and Dr. Binamer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT WCD 2023
Heart-protective diet in PURE study allows whole-fat dairy
Most of the protective food categories are in line with standard dietary guidelines for good health, but one that may be heart-protective is not usually included in such recommendations.
The food categories that were found to be protective include fruit, vegetables, nuts, legumes, and fish but also dairy, “mainly whole-fat,” in an analysis based on the international Prospective Urban and Rural Epidemiological (PURE) study and data from five other international trials that encompassed more than 240,000 people.
A healthy diet scoring system was derived from dietary patterns and clinical events observed in the PURE study and was applied to the populations of the other trials. Higher scores, corresponding to greater consumption of the six food categories, tracked with significantly reduced risks for death, myocardial infarction (MI), and stroke.
Reductions in mortality and CV-disease risk that were linked to the higher scores were especially pronounced in lower-income countries in the study published onlinein the European Heart Journal with lead author Andrew Mente, PhD, Population Health Research Institute, McMaster University, Hamilton, Ont.
The study in part refutes the frequent preference for low-fat or no-fat dairy foods over whole-fat dairy in healthy-diet recommendations. But it is consistent with earlier findings from PURE of reduced mortality risk with increased consumption of dietary fat, including saturated fat.
Whereas healthy-diet recommendations tend to emphasize reduced intake of fat, especially saturated fat, the report notes that “there are almost no national or international strategies and policies to increase a number of protective foods,” such as nuts, fish, and dairy.
“Therefore, while the findings from PURE are largely consistent with the nutrition science and modern dietary recommendations to focus on protective foods, the public’s understanding of healthy eating and relevant global policies have not yet caught up to this science,” it states.
“Guidelines and policy actions need to be updated with this newer evidence,” Dr. Mente said in an interview. “For example, the World Health Organization remains mainly focused on reducing certain nutrients, such as fat, saturated fat, added sugar, and salt,” he said. “These recommendations are echoed by government policy actions and industry, as evident by the continued focus on the usual nutrients in food labels of many countries.”
The current findings, Dr. Mente said, “can be used to ensure that the public’s understanding of healthy eating and relevant global policies are able to catch up to the science.”
Healthy diet score
PURE investigators developed their healthy diet score using data from 147,642 people from the general population in 21 countries. The investigators compared self-reported dietary intakes with long-term clinical outcomes.
The scoring system assigned a value of 1 for each of the six health-food categories when individuals’ intake exceeded the entire cohort’s median intake. It assigned a 0 when intake was below the median. The total PURE healthy diet score consisted of the sum of the six values, with higher scores corresponding to a healthier diet. The mean score for cohort was 2.95.
There were 15,707 deaths and 40,764 CV events during a median follow-up of 9.3 years. A score of at least 5 points, compared with 0 or 1 point, was associated with significantly reduced hazard ratios for mortality, MI, and stroke in multivariable analysis:
- Mortality: HR, 0.70 (95% CI, 0.63-0.77; P < .0001).
- Major CV disease: HR, 0.82 (95% CI, 0.75-0.91; P < .0001).
- MI: HR, 0.86 (95% CI, 0.75-0.99; P = .0014).
- Stroke: HR, 0.81 (95% CI, 0.71-0.93; P = .0034).
The healthy diet score’s relationship to clinical outcomes was explored in five other large independent studies, including three prospective trials of patients with CV disease that spanned 50 countries, a case-control study with MI patients in 52 countries, and a case-control study with stroke patients in 33 countries.
In the three prospective trials, higher scores were associated with reduced mortality, CV disease events, and MI:
- Mortality: HR, 0.73 (95% CI, 0.66-0.81).
- Major CV disease: HR, 0.79 (95% CI, 0.72-0.87).
- MI: HR, 0.85 (95% CI, 0.71-0.99).
In the two case-control studies, a higher diet score was associated with reduced odds ratios for first MI and for stroke:
- MI: OR, 0.72 (95% CI, 0.65-0.80).
- Stroke: OR, 0.57 (95% CI, 0.50-0.65).
In an analysis based on the PURE cohort, incorporation of unprocessed red meat or whole grains into the health diet score produced similar results, suggesting that a “modest amount” of meat or whole grains can be part of a healthy diet, the authors contend.
The results were similar in a combined analysis of all the prospective studies. In particular, improvement in diet score by one quintile was associated with significantly reduced risks for the following:
- Mortality: HR, 0.92 (95% CI, 0.90-0.93).
- Major CV disease: HR, 0.94 (95% CI, 0.93-0.95).
- MI: HR, 0.94 (95% CI, 0.92-0.96).
- Stroke: HR, 0.94 (95% CI, 0.89-0.99).
- Death or CV disease: HR, 0.93 (95% CI, 0.92-0.94).
“This strongly indicates that the take-home message for patients is the same as for general populations,” Dr. Mente said. “Eat plenty of fruits, vegetables, nuts, legumes, and a moderate amount of fish and whole-fat dairy to lower risk of CV disease and mortality.”
Dairy foods are not widely consumed in some cultures, he said, “but availability and cost are also factors in determining consumption.” Nonetheless, a high-quality diet can be achieved without including or excluding dairy foods. Context-specific policies and priorities are needed for different populations, “rather than a one-size-fits-all global policy.”
Food labels in many countries mainly focus on “reducing certain nutrients as the end-all, be-all,” Dr. Mente observed. “Our findings can be used as a basis for recommendations regarding what a healthy diet should be globally and then modified for each region based on the specific types of foods that are available and affordable in each region.”
Moreover, he said, “targeted food policies are needed to increase the availability and affordability of healthy foods, especially in lower-income countries where intakes are low.”
Common human biology
The current results from PURE “confirm prior observations from mostly Western nations that low intakes of fruits, vegetables, nuts, legumes, and fish are major risk factors for poor health,” observes Dariush Mozaffarian, MD, DrPH, MPH, Tufts University, Boston, in an accompanying editorial. “This suggests that common human biology, not merely confounding, explains these observed diet–disease relationships, strengthening causal inference on the power of nutrition.”
Moreover, “These findings provide further support that dairy foods, including whole-fat dairy, can be part of a healthy diet,” Dr. Mozaffarian writes. “The new results in PURE, in combination with prior reports, call for a re-evaluation of unrelenting guidelines to avoid whole-fat dairy products.”
Such studies “remind us of the continuing and devastating rise in diet-related chronic diseases globally, and of the power of protective foods to help address these burdens,” the editorial continues. “It is time for national nutrition guidelines, private sector innovations, government tax policy and agricultural incentives, food procurement policies, labeling and other regulatory priorities, and food-based health care interventions to catch up to the science.”
Not automatically superior
“I do not believe guidelines should be changed based on this single study,” contends Howard D. Sesso, ScD, MPH, associate director of the division of preventive medicine at Brigham and Women’s Hospital, Boston, who isn’t part of PURE. “But I welcome the scientific dialog that should come out of any study that challenges what we think we know,” he told this news organization.
“Many other dietary patterns have been identified over the years that also do a great job in predicting disease risk in observational studies,” observed Dr. Sesso. “Is PURE that much better? Maybe, maybe not. But not enough to dismiss other dietary patterns that are already the basis of dietary recommendations in the U.S., Europe, and worldwide.”
The PURE healthy diet score, he said, “appears to work well within the confines of their large pooling of studies around the world, but that doesn’t automatically make it superior to other dietary patterns.” The score “was only modestly, but not greatly, better than existing dietary patterns evaluated.”
Randomized controlled trials are needed, Dr. Sesso said, to “delve into more specific dietary components,” including unprocessed red meat, whole grains, and high-fat dairy foods. And, he said, more observational studies are needed to examine the score’s association with other cardiometabolic outcomes.
The PURE study is funded by the Population Health Research Institute, the Hamilton Health Sciences Research Institute, the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario; with support from Canadian Institutes of Health Research’s Strategy for Patient Oriented Research through the Ontario SPOR Support Unit, as well as the Ontario Ministry of Health and Long-Term Care; and through unrestricted grants from several pharmaceutical companies, with major contributions from AstraZeneca, Sanofi-Aventis, Boehringer Ingelheim, Servier, and GlaxoSmithKline. Additional contributions are from Novartis and King Pharma. Dr. Mente, Dr. Mozaffarian, and Dr. Sesso have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most of the protective food categories are in line with standard dietary guidelines for good health, but one that may be heart-protective is not usually included in such recommendations.
The food categories that were found to be protective include fruit, vegetables, nuts, legumes, and fish but also dairy, “mainly whole-fat,” in an analysis based on the international Prospective Urban and Rural Epidemiological (PURE) study and data from five other international trials that encompassed more than 240,000 people.
A healthy diet scoring system was derived from dietary patterns and clinical events observed in the PURE study and was applied to the populations of the other trials. Higher scores, corresponding to greater consumption of the six food categories, tracked with significantly reduced risks for death, myocardial infarction (MI), and stroke.
Reductions in mortality and CV-disease risk that were linked to the higher scores were especially pronounced in lower-income countries in the study published onlinein the European Heart Journal with lead author Andrew Mente, PhD, Population Health Research Institute, McMaster University, Hamilton, Ont.
The study in part refutes the frequent preference for low-fat or no-fat dairy foods over whole-fat dairy in healthy-diet recommendations. But it is consistent with earlier findings from PURE of reduced mortality risk with increased consumption of dietary fat, including saturated fat.
Whereas healthy-diet recommendations tend to emphasize reduced intake of fat, especially saturated fat, the report notes that “there are almost no national or international strategies and policies to increase a number of protective foods,” such as nuts, fish, and dairy.
“Therefore, while the findings from PURE are largely consistent with the nutrition science and modern dietary recommendations to focus on protective foods, the public’s understanding of healthy eating and relevant global policies have not yet caught up to this science,” it states.
“Guidelines and policy actions need to be updated with this newer evidence,” Dr. Mente said in an interview. “For example, the World Health Organization remains mainly focused on reducing certain nutrients, such as fat, saturated fat, added sugar, and salt,” he said. “These recommendations are echoed by government policy actions and industry, as evident by the continued focus on the usual nutrients in food labels of many countries.”
The current findings, Dr. Mente said, “can be used to ensure that the public’s understanding of healthy eating and relevant global policies are able to catch up to the science.”
Healthy diet score
PURE investigators developed their healthy diet score using data from 147,642 people from the general population in 21 countries. The investigators compared self-reported dietary intakes with long-term clinical outcomes.
The scoring system assigned a value of 1 for each of the six health-food categories when individuals’ intake exceeded the entire cohort’s median intake. It assigned a 0 when intake was below the median. The total PURE healthy diet score consisted of the sum of the six values, with higher scores corresponding to a healthier diet. The mean score for cohort was 2.95.
There were 15,707 deaths and 40,764 CV events during a median follow-up of 9.3 years. A score of at least 5 points, compared with 0 or 1 point, was associated with significantly reduced hazard ratios for mortality, MI, and stroke in multivariable analysis:
- Mortality: HR, 0.70 (95% CI, 0.63-0.77; P < .0001).
- Major CV disease: HR, 0.82 (95% CI, 0.75-0.91; P < .0001).
- MI: HR, 0.86 (95% CI, 0.75-0.99; P = .0014).
- Stroke: HR, 0.81 (95% CI, 0.71-0.93; P = .0034).
The healthy diet score’s relationship to clinical outcomes was explored in five other large independent studies, including three prospective trials of patients with CV disease that spanned 50 countries, a case-control study with MI patients in 52 countries, and a case-control study with stroke patients in 33 countries.
In the three prospective trials, higher scores were associated with reduced mortality, CV disease events, and MI:
- Mortality: HR, 0.73 (95% CI, 0.66-0.81).
- Major CV disease: HR, 0.79 (95% CI, 0.72-0.87).
- MI: HR, 0.85 (95% CI, 0.71-0.99).
In the two case-control studies, a higher diet score was associated with reduced odds ratios for first MI and for stroke:
- MI: OR, 0.72 (95% CI, 0.65-0.80).
- Stroke: OR, 0.57 (95% CI, 0.50-0.65).
In an analysis based on the PURE cohort, incorporation of unprocessed red meat or whole grains into the health diet score produced similar results, suggesting that a “modest amount” of meat or whole grains can be part of a healthy diet, the authors contend.
The results were similar in a combined analysis of all the prospective studies. In particular, improvement in diet score by one quintile was associated with significantly reduced risks for the following:
- Mortality: HR, 0.92 (95% CI, 0.90-0.93).
- Major CV disease: HR, 0.94 (95% CI, 0.93-0.95).
- MI: HR, 0.94 (95% CI, 0.92-0.96).
- Stroke: HR, 0.94 (95% CI, 0.89-0.99).
- Death or CV disease: HR, 0.93 (95% CI, 0.92-0.94).
“This strongly indicates that the take-home message for patients is the same as for general populations,” Dr. Mente said. “Eat plenty of fruits, vegetables, nuts, legumes, and a moderate amount of fish and whole-fat dairy to lower risk of CV disease and mortality.”
Dairy foods are not widely consumed in some cultures, he said, “but availability and cost are also factors in determining consumption.” Nonetheless, a high-quality diet can be achieved without including or excluding dairy foods. Context-specific policies and priorities are needed for different populations, “rather than a one-size-fits-all global policy.”
Food labels in many countries mainly focus on “reducing certain nutrients as the end-all, be-all,” Dr. Mente observed. “Our findings can be used as a basis for recommendations regarding what a healthy diet should be globally and then modified for each region based on the specific types of foods that are available and affordable in each region.”
Moreover, he said, “targeted food policies are needed to increase the availability and affordability of healthy foods, especially in lower-income countries where intakes are low.”
Common human biology
The current results from PURE “confirm prior observations from mostly Western nations that low intakes of fruits, vegetables, nuts, legumes, and fish are major risk factors for poor health,” observes Dariush Mozaffarian, MD, DrPH, MPH, Tufts University, Boston, in an accompanying editorial. “This suggests that common human biology, not merely confounding, explains these observed diet–disease relationships, strengthening causal inference on the power of nutrition.”
Moreover, “These findings provide further support that dairy foods, including whole-fat dairy, can be part of a healthy diet,” Dr. Mozaffarian writes. “The new results in PURE, in combination with prior reports, call for a re-evaluation of unrelenting guidelines to avoid whole-fat dairy products.”
Such studies “remind us of the continuing and devastating rise in diet-related chronic diseases globally, and of the power of protective foods to help address these burdens,” the editorial continues. “It is time for national nutrition guidelines, private sector innovations, government tax policy and agricultural incentives, food procurement policies, labeling and other regulatory priorities, and food-based health care interventions to catch up to the science.”
Not automatically superior
“I do not believe guidelines should be changed based on this single study,” contends Howard D. Sesso, ScD, MPH, associate director of the division of preventive medicine at Brigham and Women’s Hospital, Boston, who isn’t part of PURE. “But I welcome the scientific dialog that should come out of any study that challenges what we think we know,” he told this news organization.
“Many other dietary patterns have been identified over the years that also do a great job in predicting disease risk in observational studies,” observed Dr. Sesso. “Is PURE that much better? Maybe, maybe not. But not enough to dismiss other dietary patterns that are already the basis of dietary recommendations in the U.S., Europe, and worldwide.”
The PURE healthy diet score, he said, “appears to work well within the confines of their large pooling of studies around the world, but that doesn’t automatically make it superior to other dietary patterns.” The score “was only modestly, but not greatly, better than existing dietary patterns evaluated.”
Randomized controlled trials are needed, Dr. Sesso said, to “delve into more specific dietary components,” including unprocessed red meat, whole grains, and high-fat dairy foods. And, he said, more observational studies are needed to examine the score’s association with other cardiometabolic outcomes.
The PURE study is funded by the Population Health Research Institute, the Hamilton Health Sciences Research Institute, the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario; with support from Canadian Institutes of Health Research’s Strategy for Patient Oriented Research through the Ontario SPOR Support Unit, as well as the Ontario Ministry of Health and Long-Term Care; and through unrestricted grants from several pharmaceutical companies, with major contributions from AstraZeneca, Sanofi-Aventis, Boehringer Ingelheim, Servier, and GlaxoSmithKline. Additional contributions are from Novartis and King Pharma. Dr. Mente, Dr. Mozaffarian, and Dr. Sesso have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most of the protective food categories are in line with standard dietary guidelines for good health, but one that may be heart-protective is not usually included in such recommendations.
The food categories that were found to be protective include fruit, vegetables, nuts, legumes, and fish but also dairy, “mainly whole-fat,” in an analysis based on the international Prospective Urban and Rural Epidemiological (PURE) study and data from five other international trials that encompassed more than 240,000 people.
A healthy diet scoring system was derived from dietary patterns and clinical events observed in the PURE study and was applied to the populations of the other trials. Higher scores, corresponding to greater consumption of the six food categories, tracked with significantly reduced risks for death, myocardial infarction (MI), and stroke.
Reductions in mortality and CV-disease risk that were linked to the higher scores were especially pronounced in lower-income countries in the study published onlinein the European Heart Journal with lead author Andrew Mente, PhD, Population Health Research Institute, McMaster University, Hamilton, Ont.
The study in part refutes the frequent preference for low-fat or no-fat dairy foods over whole-fat dairy in healthy-diet recommendations. But it is consistent with earlier findings from PURE of reduced mortality risk with increased consumption of dietary fat, including saturated fat.
Whereas healthy-diet recommendations tend to emphasize reduced intake of fat, especially saturated fat, the report notes that “there are almost no national or international strategies and policies to increase a number of protective foods,” such as nuts, fish, and dairy.
“Therefore, while the findings from PURE are largely consistent with the nutrition science and modern dietary recommendations to focus on protective foods, the public’s understanding of healthy eating and relevant global policies have not yet caught up to this science,” it states.
“Guidelines and policy actions need to be updated with this newer evidence,” Dr. Mente said in an interview. “For example, the World Health Organization remains mainly focused on reducing certain nutrients, such as fat, saturated fat, added sugar, and salt,” he said. “These recommendations are echoed by government policy actions and industry, as evident by the continued focus on the usual nutrients in food labels of many countries.”
The current findings, Dr. Mente said, “can be used to ensure that the public’s understanding of healthy eating and relevant global policies are able to catch up to the science.”
Healthy diet score
PURE investigators developed their healthy diet score using data from 147,642 people from the general population in 21 countries. The investigators compared self-reported dietary intakes with long-term clinical outcomes.
The scoring system assigned a value of 1 for each of the six health-food categories when individuals’ intake exceeded the entire cohort’s median intake. It assigned a 0 when intake was below the median. The total PURE healthy diet score consisted of the sum of the six values, with higher scores corresponding to a healthier diet. The mean score for cohort was 2.95.
There were 15,707 deaths and 40,764 CV events during a median follow-up of 9.3 years. A score of at least 5 points, compared with 0 or 1 point, was associated with significantly reduced hazard ratios for mortality, MI, and stroke in multivariable analysis:
- Mortality: HR, 0.70 (95% CI, 0.63-0.77; P < .0001).
- Major CV disease: HR, 0.82 (95% CI, 0.75-0.91; P < .0001).
- MI: HR, 0.86 (95% CI, 0.75-0.99; P = .0014).
- Stroke: HR, 0.81 (95% CI, 0.71-0.93; P = .0034).
The healthy diet score’s relationship to clinical outcomes was explored in five other large independent studies, including three prospective trials of patients with CV disease that spanned 50 countries, a case-control study with MI patients in 52 countries, and a case-control study with stroke patients in 33 countries.
In the three prospective trials, higher scores were associated with reduced mortality, CV disease events, and MI:
- Mortality: HR, 0.73 (95% CI, 0.66-0.81).
- Major CV disease: HR, 0.79 (95% CI, 0.72-0.87).
- MI: HR, 0.85 (95% CI, 0.71-0.99).
In the two case-control studies, a higher diet score was associated with reduced odds ratios for first MI and for stroke:
- MI: OR, 0.72 (95% CI, 0.65-0.80).
- Stroke: OR, 0.57 (95% CI, 0.50-0.65).
In an analysis based on the PURE cohort, incorporation of unprocessed red meat or whole grains into the health diet score produced similar results, suggesting that a “modest amount” of meat or whole grains can be part of a healthy diet, the authors contend.
The results were similar in a combined analysis of all the prospective studies. In particular, improvement in diet score by one quintile was associated with significantly reduced risks for the following:
- Mortality: HR, 0.92 (95% CI, 0.90-0.93).
- Major CV disease: HR, 0.94 (95% CI, 0.93-0.95).
- MI: HR, 0.94 (95% CI, 0.92-0.96).
- Stroke: HR, 0.94 (95% CI, 0.89-0.99).
- Death or CV disease: HR, 0.93 (95% CI, 0.92-0.94).
“This strongly indicates that the take-home message for patients is the same as for general populations,” Dr. Mente said. “Eat plenty of fruits, vegetables, nuts, legumes, and a moderate amount of fish and whole-fat dairy to lower risk of CV disease and mortality.”
Dairy foods are not widely consumed in some cultures, he said, “but availability and cost are also factors in determining consumption.” Nonetheless, a high-quality diet can be achieved without including or excluding dairy foods. Context-specific policies and priorities are needed for different populations, “rather than a one-size-fits-all global policy.”
Food labels in many countries mainly focus on “reducing certain nutrients as the end-all, be-all,” Dr. Mente observed. “Our findings can be used as a basis for recommendations regarding what a healthy diet should be globally and then modified for each region based on the specific types of foods that are available and affordable in each region.”
Moreover, he said, “targeted food policies are needed to increase the availability and affordability of healthy foods, especially in lower-income countries where intakes are low.”
Common human biology
The current results from PURE “confirm prior observations from mostly Western nations that low intakes of fruits, vegetables, nuts, legumes, and fish are major risk factors for poor health,” observes Dariush Mozaffarian, MD, DrPH, MPH, Tufts University, Boston, in an accompanying editorial. “This suggests that common human biology, not merely confounding, explains these observed diet–disease relationships, strengthening causal inference on the power of nutrition.”
Moreover, “These findings provide further support that dairy foods, including whole-fat dairy, can be part of a healthy diet,” Dr. Mozaffarian writes. “The new results in PURE, in combination with prior reports, call for a re-evaluation of unrelenting guidelines to avoid whole-fat dairy products.”
Such studies “remind us of the continuing and devastating rise in diet-related chronic diseases globally, and of the power of protective foods to help address these burdens,” the editorial continues. “It is time for national nutrition guidelines, private sector innovations, government tax policy and agricultural incentives, food procurement policies, labeling and other regulatory priorities, and food-based health care interventions to catch up to the science.”
Not automatically superior
“I do not believe guidelines should be changed based on this single study,” contends Howard D. Sesso, ScD, MPH, associate director of the division of preventive medicine at Brigham and Women’s Hospital, Boston, who isn’t part of PURE. “But I welcome the scientific dialog that should come out of any study that challenges what we think we know,” he told this news organization.
“Many other dietary patterns have been identified over the years that also do a great job in predicting disease risk in observational studies,” observed Dr. Sesso. “Is PURE that much better? Maybe, maybe not. But not enough to dismiss other dietary patterns that are already the basis of dietary recommendations in the U.S., Europe, and worldwide.”
The PURE healthy diet score, he said, “appears to work well within the confines of their large pooling of studies around the world, but that doesn’t automatically make it superior to other dietary patterns.” The score “was only modestly, but not greatly, better than existing dietary patterns evaluated.”
Randomized controlled trials are needed, Dr. Sesso said, to “delve into more specific dietary components,” including unprocessed red meat, whole grains, and high-fat dairy foods. And, he said, more observational studies are needed to examine the score’s association with other cardiometabolic outcomes.
The PURE study is funded by the Population Health Research Institute, the Hamilton Health Sciences Research Institute, the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario; with support from Canadian Institutes of Health Research’s Strategy for Patient Oriented Research through the Ontario SPOR Support Unit, as well as the Ontario Ministry of Health and Long-Term Care; and through unrestricted grants from several pharmaceutical companies, with major contributions from AstraZeneca, Sanofi-Aventis, Boehringer Ingelheim, Servier, and GlaxoSmithKline. Additional contributions are from Novartis and King Pharma. Dr. Mente, Dr. Mozaffarian, and Dr. Sesso have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN HEART CENTER
FDA expands inclisiran statin-adjunct indication to include primary prevention
Novartis has announced.
The first-in-class small interfering RNA (siRNA) agent was approved in 2021 as an adjunct to statins for patients with clinical cardiovascular disease or heterozygous familial hypercholesterolemia. The indications now include patients taking statins for primary dyslipidemia who have high-risk comorbidities such as diabetes but who do not have a history of cardiovascular events, the company said.
Inclisiran, with a mechanism of action unique among drugs for dyslipidemia, works by “silencing” RNA involved in the synthesis of proprotein convertase subtilisin/kexin type 9. The protein helps regulate the number of LDL cholesterol cell-surface receptors.
Novartis said it has “global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.”
A version of this article first appeared on Medscape.com.
Novartis has announced.
The first-in-class small interfering RNA (siRNA) agent was approved in 2021 as an adjunct to statins for patients with clinical cardiovascular disease or heterozygous familial hypercholesterolemia. The indications now include patients taking statins for primary dyslipidemia who have high-risk comorbidities such as diabetes but who do not have a history of cardiovascular events, the company said.
Inclisiran, with a mechanism of action unique among drugs for dyslipidemia, works by “silencing” RNA involved in the synthesis of proprotein convertase subtilisin/kexin type 9. The protein helps regulate the number of LDL cholesterol cell-surface receptors.
Novartis said it has “global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.”
A version of this article first appeared on Medscape.com.
Novartis has announced.
The first-in-class small interfering RNA (siRNA) agent was approved in 2021 as an adjunct to statins for patients with clinical cardiovascular disease or heterozygous familial hypercholesterolemia. The indications now include patients taking statins for primary dyslipidemia who have high-risk comorbidities such as diabetes but who do not have a history of cardiovascular events, the company said.
Inclisiran, with a mechanism of action unique among drugs for dyslipidemia, works by “silencing” RNA involved in the synthesis of proprotein convertase subtilisin/kexin type 9. The protein helps regulate the number of LDL cholesterol cell-surface receptors.
Novartis said it has “global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.”
A version of this article first appeared on Medscape.com.
The surprising occupations with higher-than-expected ovarian cancer rates
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
Basically, all cancers are caused by a mix of genetic and environmental factors, with some cancers driven more strongly by one or the other. When it comes to ovarian cancer, which kills more than 13,000 women per year in the United States, genetic factors like the BRCA gene mutations are well described.
Other risk factors, like early menarche and nulliparity, are difficult to modify. The only slam-dunk environmental toxin to be linked to ovarian cancer is asbestos. Still, the vast majority of women who develop ovarian cancer do not have a known high-risk gene or asbestos exposure, so other triggers may be out there. How do we find them? The answer may just be good old-fashioned epidemiology.
That’s just what researchers, led by Anita Koushik at the University of Montreal, did in a new study appearing in the journal Occupational and Environmental Medicine.
They identified 497 women in Montreal who had recently been diagnosed with ovarian cancer. They then matched those women to 897 women without ovarian cancer, based on age and address. (This approach would not work well in the United States, as diagnosis of ovarian cancer might depend on access to medical care, which is not universal here. In Canada, however, it’s safer to assume that anyone who could have gotten ovarian cancer in Montreal would have been detected.)
Cases and controls identified, the researchers took a detailed occupational history for each participant: every job they ever worked, and when, and for how long. Each occupation was mapped to a standardized set of industries and, interestingly, to a set of environmental exposures ranging from cosmetic talc to cooking fumes to cotton dust, in what is known as a job-exposure matrix. Of course, they also collected data on other ovarian cancer risk factors.
After that, it’s a simple matter of looking at the rate of ovarian cancer by occupation and occupation-associated exposures, accounting for differences in things like pregnancy rates.
A brief aside here. I was at dinner with my wife the other night and telling her about this study, and I asked, “What do you think the occupation with the highest rate of ovarian cancer is?” And without missing a beat, she said: “Hairdressers.” Which blew my mind because of how random that was, but she was also – as usual – 100% correct.
Hairdressers, at least those who had been in the industry for more than 10 years, had a threefold higher risk for ovarian cancer than matched controls who had never been hairdressers.
Of course, my wife is a cancer surgeon, so she has a bit of a leg up on me here. Many of you may also know that there is actually a decent body of literature showing higher rates of various cancers among hairdressers, presumably due to the variety of chemicals they are exposed to on a continuous basis.
The No. 2 highest-risk profession on the list? Accountants, with about a twofold higher risk. That one is more of a puzzler. It could be a false positive; after all, there were multiple occupations checked and random error might give a few hits that are meaningless. But there are certainly some occupational factors unique to accountants that might bear further investigation – maybe exposure to volatile organic compounds from office printers, or just a particularly sedentary office environment.
In terms of specific exposures, there were high risks seen with mononuclear aromatic hydrocarbons, bleaches, ethanol, and fluorocarbons, among others, but we have to be a bit more careful here. These exposures were not directly measured. Rather, based on the job category a woman described, the exposures were imputed based on the job-exposure matrix. As such, the correlations between the job and the particular exposure are really quite high, making it essentially impossible to tease out whether it is, for example, being a hairdresser, or being exposed to fluorocarbons as a hairdresser, or being exposed to something else as a hairdresser, that is the problem.
This is how these types of studies work; they tend to raise more questions than they answer. But in a world where a cancer diagnosis can seem to come completely out of the blue, they provide the starting point that someday may lead to a more definitive culprit agent or group of agents. Until then, it might be wise for hairdressers to make sure their workplace is well ventilated.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
Basically, all cancers are caused by a mix of genetic and environmental factors, with some cancers driven more strongly by one or the other. When it comes to ovarian cancer, which kills more than 13,000 women per year in the United States, genetic factors like the BRCA gene mutations are well described.
Other risk factors, like early menarche and nulliparity, are difficult to modify. The only slam-dunk environmental toxin to be linked to ovarian cancer is asbestos. Still, the vast majority of women who develop ovarian cancer do not have a known high-risk gene or asbestos exposure, so other triggers may be out there. How do we find them? The answer may just be good old-fashioned epidemiology.
That’s just what researchers, led by Anita Koushik at the University of Montreal, did in a new study appearing in the journal Occupational and Environmental Medicine.
They identified 497 women in Montreal who had recently been diagnosed with ovarian cancer. They then matched those women to 897 women without ovarian cancer, based on age and address. (This approach would not work well in the United States, as diagnosis of ovarian cancer might depend on access to medical care, which is not universal here. In Canada, however, it’s safer to assume that anyone who could have gotten ovarian cancer in Montreal would have been detected.)
Cases and controls identified, the researchers took a detailed occupational history for each participant: every job they ever worked, and when, and for how long. Each occupation was mapped to a standardized set of industries and, interestingly, to a set of environmental exposures ranging from cosmetic talc to cooking fumes to cotton dust, in what is known as a job-exposure matrix. Of course, they also collected data on other ovarian cancer risk factors.
After that, it’s a simple matter of looking at the rate of ovarian cancer by occupation and occupation-associated exposures, accounting for differences in things like pregnancy rates.
A brief aside here. I was at dinner with my wife the other night and telling her about this study, and I asked, “What do you think the occupation with the highest rate of ovarian cancer is?” And without missing a beat, she said: “Hairdressers.” Which blew my mind because of how random that was, but she was also – as usual – 100% correct.
Hairdressers, at least those who had been in the industry for more than 10 years, had a threefold higher risk for ovarian cancer than matched controls who had never been hairdressers.
Of course, my wife is a cancer surgeon, so she has a bit of a leg up on me here. Many of you may also know that there is actually a decent body of literature showing higher rates of various cancers among hairdressers, presumably due to the variety of chemicals they are exposed to on a continuous basis.
The No. 2 highest-risk profession on the list? Accountants, with about a twofold higher risk. That one is more of a puzzler. It could be a false positive; after all, there were multiple occupations checked and random error might give a few hits that are meaningless. But there are certainly some occupational factors unique to accountants that might bear further investigation – maybe exposure to volatile organic compounds from office printers, or just a particularly sedentary office environment.
In terms of specific exposures, there were high risks seen with mononuclear aromatic hydrocarbons, bleaches, ethanol, and fluorocarbons, among others, but we have to be a bit more careful here. These exposures were not directly measured. Rather, based on the job category a woman described, the exposures were imputed based on the job-exposure matrix. As such, the correlations between the job and the particular exposure are really quite high, making it essentially impossible to tease out whether it is, for example, being a hairdresser, or being exposed to fluorocarbons as a hairdresser, or being exposed to something else as a hairdresser, that is the problem.
This is how these types of studies work; they tend to raise more questions than they answer. But in a world where a cancer diagnosis can seem to come completely out of the blue, they provide the starting point that someday may lead to a more definitive culprit agent or group of agents. Until then, it might be wise for hairdressers to make sure their workplace is well ventilated.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
Basically, all cancers are caused by a mix of genetic and environmental factors, with some cancers driven more strongly by one or the other. When it comes to ovarian cancer, which kills more than 13,000 women per year in the United States, genetic factors like the BRCA gene mutations are well described.
Other risk factors, like early menarche and nulliparity, are difficult to modify. The only slam-dunk environmental toxin to be linked to ovarian cancer is asbestos. Still, the vast majority of women who develop ovarian cancer do not have a known high-risk gene or asbestos exposure, so other triggers may be out there. How do we find them? The answer may just be good old-fashioned epidemiology.
That’s just what researchers, led by Anita Koushik at the University of Montreal, did in a new study appearing in the journal Occupational and Environmental Medicine.
They identified 497 women in Montreal who had recently been diagnosed with ovarian cancer. They then matched those women to 897 women without ovarian cancer, based on age and address. (This approach would not work well in the United States, as diagnosis of ovarian cancer might depend on access to medical care, which is not universal here. In Canada, however, it’s safer to assume that anyone who could have gotten ovarian cancer in Montreal would have been detected.)
Cases and controls identified, the researchers took a detailed occupational history for each participant: every job they ever worked, and when, and for how long. Each occupation was mapped to a standardized set of industries and, interestingly, to a set of environmental exposures ranging from cosmetic talc to cooking fumes to cotton dust, in what is known as a job-exposure matrix. Of course, they also collected data on other ovarian cancer risk factors.
After that, it’s a simple matter of looking at the rate of ovarian cancer by occupation and occupation-associated exposures, accounting for differences in things like pregnancy rates.
A brief aside here. I was at dinner with my wife the other night and telling her about this study, and I asked, “What do you think the occupation with the highest rate of ovarian cancer is?” And without missing a beat, she said: “Hairdressers.” Which blew my mind because of how random that was, but she was also – as usual – 100% correct.
Hairdressers, at least those who had been in the industry for more than 10 years, had a threefold higher risk for ovarian cancer than matched controls who had never been hairdressers.
Of course, my wife is a cancer surgeon, so she has a bit of a leg up on me here. Many of you may also know that there is actually a decent body of literature showing higher rates of various cancers among hairdressers, presumably due to the variety of chemicals they are exposed to on a continuous basis.
The No. 2 highest-risk profession on the list? Accountants, with about a twofold higher risk. That one is more of a puzzler. It could be a false positive; after all, there were multiple occupations checked and random error might give a few hits that are meaningless. But there are certainly some occupational factors unique to accountants that might bear further investigation – maybe exposure to volatile organic compounds from office printers, or just a particularly sedentary office environment.
In terms of specific exposures, there were high risks seen with mononuclear aromatic hydrocarbons, bleaches, ethanol, and fluorocarbons, among others, but we have to be a bit more careful here. These exposures were not directly measured. Rather, based on the job category a woman described, the exposures were imputed based on the job-exposure matrix. As such, the correlations between the job and the particular exposure are really quite high, making it essentially impossible to tease out whether it is, for example, being a hairdresser, or being exposed to fluorocarbons as a hairdresser, or being exposed to something else as a hairdresser, that is the problem.
This is how these types of studies work; they tend to raise more questions than they answer. But in a world where a cancer diagnosis can seem to come completely out of the blue, they provide the starting point that someday may lead to a more definitive culprit agent or group of agents. Until then, it might be wise for hairdressers to make sure their workplace is well ventilated.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Focal plaques and finger swelling
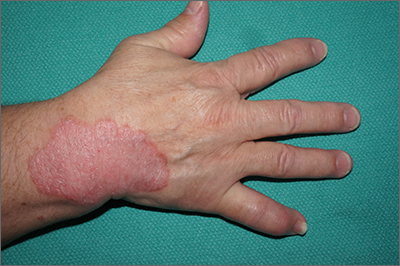
Well-demarcated symmetrical scaly plaques and dactylitis are consistent with psoriasis and psoriatic arthritis (PsA). Even in the absence of significant skin disease, a patient like this should be evaluated by Rheumatology for initiation of disease-modifying antirheumatic drugs (DMARDs).
Psoriatic arthritis manifests as a peripheral arthritis affecting the small joints of the wrists and hands, pain at the insertion of tendons and ligaments (enthesitis), or as axial arthritis. This variable presentation and the lack of specific serological marker can make diagnosis challenging. Associated symptoms beyond the musculoskeletal system include uveitis, inflammatory bowel disease, and cutaneous psoriasis.1 In contrast to osteoarthritis, PsA symptoms are often worse in the morning and improve over the course of the day. Patients with a history of psoriasis on the skin have about a 10% chance of developing PsA, with increased rates in patients who have more widespread plaques and patients with psoriasis at a young age.2 Although not pathognomonic for PsA, pitting of the fingernails may reflect episodic enthesitis in the extensor tendons of the fingers.3 Radiographs of the hands in severe cases may demonstrate narrowing of the proximal portion of the distal or proximal interphalangeal joints with a cup-like concavity of the distal half of the joint.
Conventional DMARDs (such as methotrexate and azathioprine) and biologic DMARDs (including TNF-alpha inhibitors, IL-17 inhibitors, IL-23 inhibitors) are first-line treatments and can stop or slow the progression of disease but will not reverse existing damage. For this reason, it is important to promptly start DMARD therapy after the diagnosis has been established.4
This patient was initiated on adalimumab 40 mg subcutaneously every other week. Her pain improved after 2 months of therapy and her skin plaques almost entirely resolved at 6 months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020;214:108390. doi: 10.1016/j.clim.2020.108390
2. Ogdie A, Gelfand JM. Clinical risk factors for the development of psoriatic arthritis among patients with psoriasis: a review of available evidence. Curr Rheumatol Rep. 2015;17:64. doi: 10.1007/s11926-015-0540-1
3. Elliott A, Pendleton A, Wright G, et al. The relationship between the nail and systemic enthesitis in psoriatic arthritis. Rheumatol Adv Pract. 2021;5:rkab088. doi: 10.1093/rap/rkab088
4. Coates LC, Soriano ER, Corp N, et al. GRAPPA treatment recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465-479. doi: 10.1038/s41584-022-00798-0

Well-demarcated symmetrical scaly plaques and dactylitis are consistent with psoriasis and psoriatic arthritis (PsA). Even in the absence of significant skin disease, a patient like this should be evaluated by Rheumatology for initiation of disease-modifying antirheumatic drugs (DMARDs).
Psoriatic arthritis manifests as a peripheral arthritis affecting the small joints of the wrists and hands, pain at the insertion of tendons and ligaments (enthesitis), or as axial arthritis. This variable presentation and the lack of specific serological marker can make diagnosis challenging. Associated symptoms beyond the musculoskeletal system include uveitis, inflammatory bowel disease, and cutaneous psoriasis.1 In contrast to osteoarthritis, PsA symptoms are often worse in the morning and improve over the course of the day. Patients with a history of psoriasis on the skin have about a 10% chance of developing PsA, with increased rates in patients who have more widespread plaques and patients with psoriasis at a young age.2 Although not pathognomonic for PsA, pitting of the fingernails may reflect episodic enthesitis in the extensor tendons of the fingers.3 Radiographs of the hands in severe cases may demonstrate narrowing of the proximal portion of the distal or proximal interphalangeal joints with a cup-like concavity of the distal half of the joint.
Conventional DMARDs (such as methotrexate and azathioprine) and biologic DMARDs (including TNF-alpha inhibitors, IL-17 inhibitors, IL-23 inhibitors) are first-line treatments and can stop or slow the progression of disease but will not reverse existing damage. For this reason, it is important to promptly start DMARD therapy after the diagnosis has been established.4
This patient was initiated on adalimumab 40 mg subcutaneously every other week. Her pain improved after 2 months of therapy and her skin plaques almost entirely resolved at 6 months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Well-demarcated symmetrical scaly plaques and dactylitis are consistent with psoriasis and psoriatic arthritis (PsA). Even in the absence of significant skin disease, a patient like this should be evaluated by Rheumatology for initiation of disease-modifying antirheumatic drugs (DMARDs).
Psoriatic arthritis manifests as a peripheral arthritis affecting the small joints of the wrists and hands, pain at the insertion of tendons and ligaments (enthesitis), or as axial arthritis. This variable presentation and the lack of specific serological marker can make diagnosis challenging. Associated symptoms beyond the musculoskeletal system include uveitis, inflammatory bowel disease, and cutaneous psoriasis.1 In contrast to osteoarthritis, PsA symptoms are often worse in the morning and improve over the course of the day. Patients with a history of psoriasis on the skin have about a 10% chance of developing PsA, with increased rates in patients who have more widespread plaques and patients with psoriasis at a young age.2 Although not pathognomonic for PsA, pitting of the fingernails may reflect episodic enthesitis in the extensor tendons of the fingers.3 Radiographs of the hands in severe cases may demonstrate narrowing of the proximal portion of the distal or proximal interphalangeal joints with a cup-like concavity of the distal half of the joint.
Conventional DMARDs (such as methotrexate and azathioprine) and biologic DMARDs (including TNF-alpha inhibitors, IL-17 inhibitors, IL-23 inhibitors) are first-line treatments and can stop or slow the progression of disease but will not reverse existing damage. For this reason, it is important to promptly start DMARD therapy after the diagnosis has been established.4
This patient was initiated on adalimumab 40 mg subcutaneously every other week. Her pain improved after 2 months of therapy and her skin plaques almost entirely resolved at 6 months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020;214:108390. doi: 10.1016/j.clim.2020.108390
2. Ogdie A, Gelfand JM. Clinical risk factors for the development of psoriatic arthritis among patients with psoriasis: a review of available evidence. Curr Rheumatol Rep. 2015;17:64. doi: 10.1007/s11926-015-0540-1
3. Elliott A, Pendleton A, Wright G, et al. The relationship between the nail and systemic enthesitis in psoriatic arthritis. Rheumatol Adv Pract. 2021;5:rkab088. doi: 10.1093/rap/rkab088
4. Coates LC, Soriano ER, Corp N, et al. GRAPPA treatment recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465-479. doi: 10.1038/s41584-022-00798-0
1. Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020;214:108390. doi: 10.1016/j.clim.2020.108390
2. Ogdie A, Gelfand JM. Clinical risk factors for the development of psoriatic arthritis among patients with psoriasis: a review of available evidence. Curr Rheumatol Rep. 2015;17:64. doi: 10.1007/s11926-015-0540-1
3. Elliott A, Pendleton A, Wright G, et al. The relationship between the nail and systemic enthesitis in psoriatic arthritis. Rheumatol Adv Pract. 2021;5:rkab088. doi: 10.1093/rap/rkab088
4. Coates LC, Soriano ER, Corp N, et al. GRAPPA treatment recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465-479. doi: 10.1038/s41584-022-00798-0
Long COVID patients turn to doctors for help with disability claims
As millions of Americans face another year of long COVID, some are finding they are unable to return to work or cannot work as they did before they got sick and are turning to doctors for help with documenting their disability.
For those who can return to work, a doctor’s diagnosis of long COVID is key to gaining access to workplace accommodations, such as working flex hours or remotely. For those who cannot work, a note from the doctor is the first step to collecting disability payments.
With no definitive blood tests or scans for long COVID that could confirm a diagnosis, some say doctors may feel uncomfortable in this role, which puts them in a tough spot, said Wes Ely, MD, MPH, codirector of the critical illness, brain dysfunction and survivorship center at Vanderbilt University, Nashville, Tenn.
Doctors typically are not taught to deal with vagueness in diagnostics.
“Long COVID falls straight into the gray zone,” he said. There are no tests and a long list of common symptoms. “It makes a lot of doctors feel super insecure,” he said.
Now, patients and their advocates are calling for doctors to be more open-minded about how they assess those with long COVID and other chronic illnesses. Although their disability may not be visible, many with long COVID struggle to function. If they need help, they say, they need a doctor to confirm their limitations – test results or no test results.
Better documentation of patient-reported symptoms would go a long way, according to a perspective published in The New England Journal of Medicine.
“There’s a long history of people with disabilities being forced to ask doctors to legitimize their symptoms,” said study author Zackary Berger, MD, PhD, Johns Hopkins University, Baltimore, Md. Dr. Berger believes doctors should learn to listen more closely to patients, turn their narratives into patient notes, and use the new International Classification of Diseases 10 (ICD-10) code, a worldwide system for identifying and generating data on diseases, when they diagnose long COVID. He also thinks doctors should become advocates for their patients.
The Americans With Disabilities Act allows employers to request medical proof of disability, “and thereby assigns physicians the gate-keeping role of determining patients’ eligibility for reasonable accommodations,” according to the analysis. Those accommodations may mean a handicapped parking space or extra days working remotely.
Without a definitive diagnostic test, long COVID joins fibromyalgia and ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome), which lack biomarkers or imaging tests to support a diagnosis, they write.
“These diagnoses are therefore contentious, and government agencies, employers, and many physicians do not accept these conditions as real,” they write.
Physicians make a good faith effort in trying to understand long COVID, but both doctors and the courts like to see evidence, said Michael Ashley Stein, JD, PHD, director of the Harvard Law School Project on Disability. Dr. Stein and others say that doctors should listen closely to their patients’ descriptions of their symptoms.
“In the absence of agreed-upon biomarkers, doctors need to listen to their patients and look for other [indications] and other consistent evidence of conditions, and then work from there rather than dismiss the existence of these conditions,” he said.
Dr. Ely said he and others were taught in medical school that if it doesn’t come up on a diagnostic test, there’s no problem. “I am absolutely complicit,” he said. “I’m part of the community that did that for so many years.”
Dr. Ely agreed that the demand for clinical test results does not work for long COVID and chronic diseases such as ME/CFS. People come in with complaints and they get a typical medical workup with labs, he said, and the labs look normal on paper.
“And [the doctor is] thinking: ‘I don’t know what is wrong with this person and there’s nothing on paper I can treat. I don’t know if I even believe in long COVID.’ ”
At the same time, patients might need support from a doctor to get accommodations at work under the ADA, such as flexible hours. Or doctors’ notes may be required if a patient is trying to collect private disability insurance, workers compensation, or federal disability payments through Social Security.
The U.S. Centers for Disease Control and Prevention guidelines on diagnosing long COVID, updated last December, point out that normal laboratory or imaging findings do not rule out long COVID.
In addition, 12 key symptoms of long COVID were identified in May by scientists working with the RECOVER Initiative, the federal government’s long COVID research program. These symptoms include fatigue, brain fog, dizziness, gastrointestinal symptoms, loss of or change in smell or taste, chest pain, and abnormal movements.
Still, patients with long COVID seeking help also face the “disability con,” a term coined by the second author of the NEJM article, Doron Dorfman, a professor at Seton Hall Law School in Newark, N.J.
“Nowadays, when people think disability, they immediately think fraud,” he said.
Prof. Dorfman thinks the perception that many people are faking disabilities to gain an unfair advantage is the biggest barrier for anyone seeking help. The disability system is “preventing people who deserve legal rights from actually obtaining them,” he said.
He urged doctors to believe their patients. One way is to try to “translate the person’s narrative into medical language.”
His coauthor Dr. Berger did not agree with the argument that doctors cannot diagnose without tests.
“Any clinician knows that lab tests are not everything,” he said. “There are conditions that don’t have specific biomarkers that we diagnose all the time.” He cited acquired pneumonia and urinary tract infections as examples.
Benefits lawyers have taken note of the complexities for people with long COVID who seek help through the ADA and federal disability program.
One law firm noted: “The government safety net is not designed to help an emerging disease with no clear diagnosis or treatment plans. Insurance carriers are denying claims, and long-term disability benefits are being denied.”
About 16 million working-age Americans have long COVID, according to an update of a 2022 report by the Brookings Institute. Up to 4 million of these people are out of work because of the condition, the study found. The research is based on newly collected U.S. Census Bureau data that show 24% of those with long COVID report “significant activity limitations.”
Dr. Ely said he sees progress in this area. Many of these issues have come up at the committee convened by the National Academy of Science to look at the working definition of long COVID. NAS, a Washington research group, held a public meeting on their findings on June 22.
A version of this article first appeared on Medscape.com.
As millions of Americans face another year of long COVID, some are finding they are unable to return to work or cannot work as they did before they got sick and are turning to doctors for help with documenting their disability.
For those who can return to work, a doctor’s diagnosis of long COVID is key to gaining access to workplace accommodations, such as working flex hours or remotely. For those who cannot work, a note from the doctor is the first step to collecting disability payments.
With no definitive blood tests or scans for long COVID that could confirm a diagnosis, some say doctors may feel uncomfortable in this role, which puts them in a tough spot, said Wes Ely, MD, MPH, codirector of the critical illness, brain dysfunction and survivorship center at Vanderbilt University, Nashville, Tenn.
Doctors typically are not taught to deal with vagueness in diagnostics.
“Long COVID falls straight into the gray zone,” he said. There are no tests and a long list of common symptoms. “It makes a lot of doctors feel super insecure,” he said.
Now, patients and their advocates are calling for doctors to be more open-minded about how they assess those with long COVID and other chronic illnesses. Although their disability may not be visible, many with long COVID struggle to function. If they need help, they say, they need a doctor to confirm their limitations – test results or no test results.
Better documentation of patient-reported symptoms would go a long way, according to a perspective published in The New England Journal of Medicine.
“There’s a long history of people with disabilities being forced to ask doctors to legitimize their symptoms,” said study author Zackary Berger, MD, PhD, Johns Hopkins University, Baltimore, Md. Dr. Berger believes doctors should learn to listen more closely to patients, turn their narratives into patient notes, and use the new International Classification of Diseases 10 (ICD-10) code, a worldwide system for identifying and generating data on diseases, when they diagnose long COVID. He also thinks doctors should become advocates for their patients.
The Americans With Disabilities Act allows employers to request medical proof of disability, “and thereby assigns physicians the gate-keeping role of determining patients’ eligibility for reasonable accommodations,” according to the analysis. Those accommodations may mean a handicapped parking space or extra days working remotely.
Without a definitive diagnostic test, long COVID joins fibromyalgia and ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome), which lack biomarkers or imaging tests to support a diagnosis, they write.
“These diagnoses are therefore contentious, and government agencies, employers, and many physicians do not accept these conditions as real,” they write.
Physicians make a good faith effort in trying to understand long COVID, but both doctors and the courts like to see evidence, said Michael Ashley Stein, JD, PHD, director of the Harvard Law School Project on Disability. Dr. Stein and others say that doctors should listen closely to their patients’ descriptions of their symptoms.
“In the absence of agreed-upon biomarkers, doctors need to listen to their patients and look for other [indications] and other consistent evidence of conditions, and then work from there rather than dismiss the existence of these conditions,” he said.
Dr. Ely said he and others were taught in medical school that if it doesn’t come up on a diagnostic test, there’s no problem. “I am absolutely complicit,” he said. “I’m part of the community that did that for so many years.”
Dr. Ely agreed that the demand for clinical test results does not work for long COVID and chronic diseases such as ME/CFS. People come in with complaints and they get a typical medical workup with labs, he said, and the labs look normal on paper.
“And [the doctor is] thinking: ‘I don’t know what is wrong with this person and there’s nothing on paper I can treat. I don’t know if I even believe in long COVID.’ ”
At the same time, patients might need support from a doctor to get accommodations at work under the ADA, such as flexible hours. Or doctors’ notes may be required if a patient is trying to collect private disability insurance, workers compensation, or federal disability payments through Social Security.
The U.S. Centers for Disease Control and Prevention guidelines on diagnosing long COVID, updated last December, point out that normal laboratory or imaging findings do not rule out long COVID.
In addition, 12 key symptoms of long COVID were identified in May by scientists working with the RECOVER Initiative, the federal government’s long COVID research program. These symptoms include fatigue, brain fog, dizziness, gastrointestinal symptoms, loss of or change in smell or taste, chest pain, and abnormal movements.
Still, patients with long COVID seeking help also face the “disability con,” a term coined by the second author of the NEJM article, Doron Dorfman, a professor at Seton Hall Law School in Newark, N.J.
“Nowadays, when people think disability, they immediately think fraud,” he said.
Prof. Dorfman thinks the perception that many people are faking disabilities to gain an unfair advantage is the biggest barrier for anyone seeking help. The disability system is “preventing people who deserve legal rights from actually obtaining them,” he said.
He urged doctors to believe their patients. One way is to try to “translate the person’s narrative into medical language.”
His coauthor Dr. Berger did not agree with the argument that doctors cannot diagnose without tests.
“Any clinician knows that lab tests are not everything,” he said. “There are conditions that don’t have specific biomarkers that we diagnose all the time.” He cited acquired pneumonia and urinary tract infections as examples.
Benefits lawyers have taken note of the complexities for people with long COVID who seek help through the ADA and federal disability program.
One law firm noted: “The government safety net is not designed to help an emerging disease with no clear diagnosis or treatment plans. Insurance carriers are denying claims, and long-term disability benefits are being denied.”
About 16 million working-age Americans have long COVID, according to an update of a 2022 report by the Brookings Institute. Up to 4 million of these people are out of work because of the condition, the study found. The research is based on newly collected U.S. Census Bureau data that show 24% of those with long COVID report “significant activity limitations.”
Dr. Ely said he sees progress in this area. Many of these issues have come up at the committee convened by the National Academy of Science to look at the working definition of long COVID. NAS, a Washington research group, held a public meeting on their findings on June 22.
A version of this article first appeared on Medscape.com.
As millions of Americans face another year of long COVID, some are finding they are unable to return to work or cannot work as they did before they got sick and are turning to doctors for help with documenting their disability.
For those who can return to work, a doctor’s diagnosis of long COVID is key to gaining access to workplace accommodations, such as working flex hours or remotely. For those who cannot work, a note from the doctor is the first step to collecting disability payments.
With no definitive blood tests or scans for long COVID that could confirm a diagnosis, some say doctors may feel uncomfortable in this role, which puts them in a tough spot, said Wes Ely, MD, MPH, codirector of the critical illness, brain dysfunction and survivorship center at Vanderbilt University, Nashville, Tenn.
Doctors typically are not taught to deal with vagueness in diagnostics.
“Long COVID falls straight into the gray zone,” he said. There are no tests and a long list of common symptoms. “It makes a lot of doctors feel super insecure,” he said.
Now, patients and their advocates are calling for doctors to be more open-minded about how they assess those with long COVID and other chronic illnesses. Although their disability may not be visible, many with long COVID struggle to function. If they need help, they say, they need a doctor to confirm their limitations – test results or no test results.
Better documentation of patient-reported symptoms would go a long way, according to a perspective published in The New England Journal of Medicine.
“There’s a long history of people with disabilities being forced to ask doctors to legitimize their symptoms,” said study author Zackary Berger, MD, PhD, Johns Hopkins University, Baltimore, Md. Dr. Berger believes doctors should learn to listen more closely to patients, turn their narratives into patient notes, and use the new International Classification of Diseases 10 (ICD-10) code, a worldwide system for identifying and generating data on diseases, when they diagnose long COVID. He also thinks doctors should become advocates for their patients.
The Americans With Disabilities Act allows employers to request medical proof of disability, “and thereby assigns physicians the gate-keeping role of determining patients’ eligibility for reasonable accommodations,” according to the analysis. Those accommodations may mean a handicapped parking space or extra days working remotely.
Without a definitive diagnostic test, long COVID joins fibromyalgia and ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome), which lack biomarkers or imaging tests to support a diagnosis, they write.
“These diagnoses are therefore contentious, and government agencies, employers, and many physicians do not accept these conditions as real,” they write.
Physicians make a good faith effort in trying to understand long COVID, but both doctors and the courts like to see evidence, said Michael Ashley Stein, JD, PHD, director of the Harvard Law School Project on Disability. Dr. Stein and others say that doctors should listen closely to their patients’ descriptions of their symptoms.
“In the absence of agreed-upon biomarkers, doctors need to listen to their patients and look for other [indications] and other consistent evidence of conditions, and then work from there rather than dismiss the existence of these conditions,” he said.
Dr. Ely said he and others were taught in medical school that if it doesn’t come up on a diagnostic test, there’s no problem. “I am absolutely complicit,” he said. “I’m part of the community that did that for so many years.”
Dr. Ely agreed that the demand for clinical test results does not work for long COVID and chronic diseases such as ME/CFS. People come in with complaints and they get a typical medical workup with labs, he said, and the labs look normal on paper.
“And [the doctor is] thinking: ‘I don’t know what is wrong with this person and there’s nothing on paper I can treat. I don’t know if I even believe in long COVID.’ ”
At the same time, patients might need support from a doctor to get accommodations at work under the ADA, such as flexible hours. Or doctors’ notes may be required if a patient is trying to collect private disability insurance, workers compensation, or federal disability payments through Social Security.
The U.S. Centers for Disease Control and Prevention guidelines on diagnosing long COVID, updated last December, point out that normal laboratory or imaging findings do not rule out long COVID.
In addition, 12 key symptoms of long COVID were identified in May by scientists working with the RECOVER Initiative, the federal government’s long COVID research program. These symptoms include fatigue, brain fog, dizziness, gastrointestinal symptoms, loss of or change in smell or taste, chest pain, and abnormal movements.
Still, patients with long COVID seeking help also face the “disability con,” a term coined by the second author of the NEJM article, Doron Dorfman, a professor at Seton Hall Law School in Newark, N.J.
“Nowadays, when people think disability, they immediately think fraud,” he said.
Prof. Dorfman thinks the perception that many people are faking disabilities to gain an unfair advantage is the biggest barrier for anyone seeking help. The disability system is “preventing people who deserve legal rights from actually obtaining them,” he said.
He urged doctors to believe their patients. One way is to try to “translate the person’s narrative into medical language.”
His coauthor Dr. Berger did not agree with the argument that doctors cannot diagnose without tests.
“Any clinician knows that lab tests are not everything,” he said. “There are conditions that don’t have specific biomarkers that we diagnose all the time.” He cited acquired pneumonia and urinary tract infections as examples.
Benefits lawyers have taken note of the complexities for people with long COVID who seek help through the ADA and federal disability program.
One law firm noted: “The government safety net is not designed to help an emerging disease with no clear diagnosis or treatment plans. Insurance carriers are denying claims, and long-term disability benefits are being denied.”
About 16 million working-age Americans have long COVID, according to an update of a 2022 report by the Brookings Institute. Up to 4 million of these people are out of work because of the condition, the study found. The research is based on newly collected U.S. Census Bureau data that show 24% of those with long COVID report “significant activity limitations.”
Dr. Ely said he sees progress in this area. Many of these issues have come up at the committee convened by the National Academy of Science to look at the working definition of long COVID. NAS, a Washington research group, held a public meeting on their findings on June 22.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE