User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
BMI has greater impact on survival in younger breast cancer patients
new data suggest.
Obesity is a well-known risk factor for breast cancer in postmenopausal women and has been associated with adverse prognosis, said Senna W.M. Lammers, MD, of Maastricht (the Netherlands) University during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. In addition, some studies suggest that patients with higher body mass index (BMI) experience reduced benefits from endocrine therapy, she said.
Dr. Lammers and colleagues conducted a study to determine the prognostic and predictive effect of BMI on disease-free survival in postmenopausal women with hormone receptor–positive (HR+) breast cancer who were treated with extended endocrine therapy.
The study population included participants in the randomized, phase III DATA trial, which evaluated the use of 6 years vs. 3 years of anastrozole in postmenopausal women with HR+ breast cancer who were disease-free after 2-3 years of adjuvant tamoxifen therapy.
Patients were categorized based on BMI as having normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), or obese (30 kg/m2 or higher). The primary outcome was disease-free survival (DFS); the median follow-up period was 13.1 years.
DFS for patients with normal weight, overweight, and obesity was 66.2%, 59.5%, and 52.4%, with a P value of less than .001 for the trend, Dr. Lammers said. “These results were confirmed in multivariable analysis,” she said. Overall, patients with overweight and obesity had a worse DFS when compared with patients with normal weight (hazard ratio, 1.16; P = .10, for patients with overweight and HR, 1.26; P = .03 for patients with obesity).
“Next, we aimed to determine whether the prognostic effect of BMI differed by age,” Dr. Lammers said.
In women younger than 60 years, overweight and obesity were significantly associated with worse DFS (HR, 1.29; P = .05 and HR 1.83, P less than .001, respectively). However, this effect was not observed in women aged 60 years and older.
The researchers also examined the treatment effect of extended anastrozole on adapted DFS by weight, and found no significant differences among patients with normal weight, overweight, and obesity (HR, 1.00; HR, 0.74; and HR, 0.97, respectively), said Dr. Lammers.
In the question and answer session, Dr. Lammers was asked about possible explanations for the difference in DFS by age. Potential explanations include possible survival bias “as only the healthier [patients with obesity] survive to old age,” she said. Other potential explanations are biological, such as the potentially higher levels of bone density in older [patients with obesity], she said.
When asked about additional clinical implications, Dr. Lammers emphasized the importance of maintaining a healthy BMI for breast cancer patients of all ages. Other research areas might involve the use of lifestyle interventions, although these are challenging to implement, she noted.
Data draw attention to quality of life and lifestyle factors
The need to “look at drug development with new eyes” is particularly important when reviewing patient-reported outcomes, said Otto Metzger, MD, of the Dana Farber Cancer Institute, Boston, who served as the discussant for the session.
Dr. Metzger brought up the association between age and the effect of BMI on DFS, specifically.
Based on data from multiple studies and meta-analyses, “I do believe that obesity does play a role in prognosis,” he said, but the question is how long will researchers continue to simply record data without acting to add lifestyle interventions while also trying to develop new drugs, he said. Although convincing patients to make lifestyle changes remains a challenge, patients are often more motivated to make such changes after a cancer diagnosis, Dr. Metzger noted.
“I am a firm believer in the use of digital therapeutics in the context of clinical trials,” said Dr. Metzger. Digital technology offers great potential to educate patients on [adverse effects] and also to improve treatment adherence and quality of life, he concluded.
The study was supported by AstraZeneca, and Dr. Lammers disclosed financial relationships with AstraZeneca and Eli Lilly. Dr. Metzger disclosed receiving research funding to his institution from Pfizer, Genentech/Roche, and Sanofi, and serving as an adviser/consultant to AstraZeneca, Merck, Oncoclinicas, Resilience, and Roche.
new data suggest.
Obesity is a well-known risk factor for breast cancer in postmenopausal women and has been associated with adverse prognosis, said Senna W.M. Lammers, MD, of Maastricht (the Netherlands) University during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. In addition, some studies suggest that patients with higher body mass index (BMI) experience reduced benefits from endocrine therapy, she said.
Dr. Lammers and colleagues conducted a study to determine the prognostic and predictive effect of BMI on disease-free survival in postmenopausal women with hormone receptor–positive (HR+) breast cancer who were treated with extended endocrine therapy.
The study population included participants in the randomized, phase III DATA trial, which evaluated the use of 6 years vs. 3 years of anastrozole in postmenopausal women with HR+ breast cancer who were disease-free after 2-3 years of adjuvant tamoxifen therapy.
Patients were categorized based on BMI as having normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), or obese (30 kg/m2 or higher). The primary outcome was disease-free survival (DFS); the median follow-up period was 13.1 years.
DFS for patients with normal weight, overweight, and obesity was 66.2%, 59.5%, and 52.4%, with a P value of less than .001 for the trend, Dr. Lammers said. “These results were confirmed in multivariable analysis,” she said. Overall, patients with overweight and obesity had a worse DFS when compared with patients with normal weight (hazard ratio, 1.16; P = .10, for patients with overweight and HR, 1.26; P = .03 for patients with obesity).
“Next, we aimed to determine whether the prognostic effect of BMI differed by age,” Dr. Lammers said.
In women younger than 60 years, overweight and obesity were significantly associated with worse DFS (HR, 1.29; P = .05 and HR 1.83, P less than .001, respectively). However, this effect was not observed in women aged 60 years and older.
The researchers also examined the treatment effect of extended anastrozole on adapted DFS by weight, and found no significant differences among patients with normal weight, overweight, and obesity (HR, 1.00; HR, 0.74; and HR, 0.97, respectively), said Dr. Lammers.
In the question and answer session, Dr. Lammers was asked about possible explanations for the difference in DFS by age. Potential explanations include possible survival bias “as only the healthier [patients with obesity] survive to old age,” she said. Other potential explanations are biological, such as the potentially higher levels of bone density in older [patients with obesity], she said.
When asked about additional clinical implications, Dr. Lammers emphasized the importance of maintaining a healthy BMI for breast cancer patients of all ages. Other research areas might involve the use of lifestyle interventions, although these are challenging to implement, she noted.
Data draw attention to quality of life and lifestyle factors
The need to “look at drug development with new eyes” is particularly important when reviewing patient-reported outcomes, said Otto Metzger, MD, of the Dana Farber Cancer Institute, Boston, who served as the discussant for the session.
Dr. Metzger brought up the association between age and the effect of BMI on DFS, specifically.
Based on data from multiple studies and meta-analyses, “I do believe that obesity does play a role in prognosis,” he said, but the question is how long will researchers continue to simply record data without acting to add lifestyle interventions while also trying to develop new drugs, he said. Although convincing patients to make lifestyle changes remains a challenge, patients are often more motivated to make such changes after a cancer diagnosis, Dr. Metzger noted.
“I am a firm believer in the use of digital therapeutics in the context of clinical trials,” said Dr. Metzger. Digital technology offers great potential to educate patients on [adverse effects] and also to improve treatment adherence and quality of life, he concluded.
The study was supported by AstraZeneca, and Dr. Lammers disclosed financial relationships with AstraZeneca and Eli Lilly. Dr. Metzger disclosed receiving research funding to his institution from Pfizer, Genentech/Roche, and Sanofi, and serving as an adviser/consultant to AstraZeneca, Merck, Oncoclinicas, Resilience, and Roche.
new data suggest.
Obesity is a well-known risk factor for breast cancer in postmenopausal women and has been associated with adverse prognosis, said Senna W.M. Lammers, MD, of Maastricht (the Netherlands) University during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. In addition, some studies suggest that patients with higher body mass index (BMI) experience reduced benefits from endocrine therapy, she said.
Dr. Lammers and colleagues conducted a study to determine the prognostic and predictive effect of BMI on disease-free survival in postmenopausal women with hormone receptor–positive (HR+) breast cancer who were treated with extended endocrine therapy.
The study population included participants in the randomized, phase III DATA trial, which evaluated the use of 6 years vs. 3 years of anastrozole in postmenopausal women with HR+ breast cancer who were disease-free after 2-3 years of adjuvant tamoxifen therapy.
Patients were categorized based on BMI as having normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), or obese (30 kg/m2 or higher). The primary outcome was disease-free survival (DFS); the median follow-up period was 13.1 years.
DFS for patients with normal weight, overweight, and obesity was 66.2%, 59.5%, and 52.4%, with a P value of less than .001 for the trend, Dr. Lammers said. “These results were confirmed in multivariable analysis,” she said. Overall, patients with overweight and obesity had a worse DFS when compared with patients with normal weight (hazard ratio, 1.16; P = .10, for patients with overweight and HR, 1.26; P = .03 for patients with obesity).
“Next, we aimed to determine whether the prognostic effect of BMI differed by age,” Dr. Lammers said.
In women younger than 60 years, overweight and obesity were significantly associated with worse DFS (HR, 1.29; P = .05 and HR 1.83, P less than .001, respectively). However, this effect was not observed in women aged 60 years and older.
The researchers also examined the treatment effect of extended anastrozole on adapted DFS by weight, and found no significant differences among patients with normal weight, overweight, and obesity (HR, 1.00; HR, 0.74; and HR, 0.97, respectively), said Dr. Lammers.
In the question and answer session, Dr. Lammers was asked about possible explanations for the difference in DFS by age. Potential explanations include possible survival bias “as only the healthier [patients with obesity] survive to old age,” she said. Other potential explanations are biological, such as the potentially higher levels of bone density in older [patients with obesity], she said.
When asked about additional clinical implications, Dr. Lammers emphasized the importance of maintaining a healthy BMI for breast cancer patients of all ages. Other research areas might involve the use of lifestyle interventions, although these are challenging to implement, she noted.
Data draw attention to quality of life and lifestyle factors
The need to “look at drug development with new eyes” is particularly important when reviewing patient-reported outcomes, said Otto Metzger, MD, of the Dana Farber Cancer Institute, Boston, who served as the discussant for the session.
Dr. Metzger brought up the association between age and the effect of BMI on DFS, specifically.
Based on data from multiple studies and meta-analyses, “I do believe that obesity does play a role in prognosis,” he said, but the question is how long will researchers continue to simply record data without acting to add lifestyle interventions while also trying to develop new drugs, he said. Although convincing patients to make lifestyle changes remains a challenge, patients are often more motivated to make such changes after a cancer diagnosis, Dr. Metzger noted.
“I am a firm believer in the use of digital therapeutics in the context of clinical trials,” said Dr. Metzger. Digital technology offers great potential to educate patients on [adverse effects] and also to improve treatment adherence and quality of life, he concluded.
The study was supported by AstraZeneca, and Dr. Lammers disclosed financial relationships with AstraZeneca and Eli Lilly. Dr. Metzger disclosed receiving research funding to his institution from Pfizer, Genentech/Roche, and Sanofi, and serving as an adviser/consultant to AstraZeneca, Merck, Oncoclinicas, Resilience, and Roche.
FROM ESMO BREAST CANCER 2023
Fatigue is a monster for patients with pulmonary disease
If you’re looking for it, you’ll find fatigue almost everywhere. It’s so common that it hides in plain sight, never dealt with because it’s present for good reason: the inevitable consequence of age, whatever disease you’re treating, poor lifestyle choices, and the daily grind of twenty-first–century life. Its impact is so ubiquitous and pernicious that it’s considered acceptable.
Is it though? After all, fatigue can be debilitating. Not every symptom is worthy of a chronic syndrome bearing its name. Furthermore, what if its relationship to the disease you’re treating is bidirectional?
Outside of sleep medicine, I see little focus on fatigue among pulmonologists. This despite the existing data on fatigue related to sarcoidosis, chronic obstructive pulmonary disease (COPD), and interstitial lung disease. Even when we do pay it lip service, “addressing” fatigue or sleep is essentially a euphemism for ordering a sleep study.
As with fatigue, if you look for obstructive sleep apnea, it’ll be there, although with OSA, it’s related to the incredibly low, nonevidence-based threshold the American Academy of Sleep Medicine has established for making the diagnosis. With continuous positive airway pressure (CPAP) in hand, the patient has a new disease to worry about and a difficult behavioral change (wearing, cleaning, and resupplying their CPAP equipment) to make. Too often, the CPAP isn’t used – or is – and the fatigue persists. But it’s okay, because we followed somebody’s guideline.
The American Thoracic Society just published a research statement on cancer-related fatigue. It is comprehensive and highlights the high prevalence and poor recognition of cancer-related fatigue. The authors note that among cancers, those of the lung are associated with a higher comorbid disease burden, older age, and cigarette smoking. All these factors make patients with lung cancer particularly prone to fatigue. Interactions between these factors, lung cancer histology, and specific chemotherapy regimens are poorly understood. True to its title, the “research statement” serves more as a call to action than an evidence-based blueprint for diagnosis and management.
The cancer-related fatigue data that does exist suggests treatment starts with recognition followed by a focus on sleep, exercise, and nutrition. This should surprise no one. The data on fatigue in general (not specific to cancer-related fatigue) shows that although fatigue is not synonymous with poor quality or insufficient sleep, sleep is usually a major factor. The cancer-related conditions affecting sleep include anxiety, depression, insufficient sleep, insomnia, medication side effects, and OSA. The intersecting web is complex, but across underlying conditions (cancer or otherwise), the quickest most efficient method for mitigating fatigue is optimizing sleep.
Exercise and nutrition are also important. Again, across disease processes (interstitial lung disease, COPD, lung cancer, and so on), no drug comes close to aerobic exercise for reducing symptoms, including fatigue. If an exercise prescription could be delivered in pill-form, it’d be a blockbuster. But it can’t be, and the ATS lung cancer–related fatigue research statement nicely outlines the evidence for increased activity levels and the barriers to obtaining support and compliance. As is the case with exercise, support for improving nutrition is limited by cost, access, and patient education.
Perhaps most importantly, sleep, exercise, and nutrition require time for counseling and a behavior change for the physician and patient. Both are in short supply, and commitment is always ephemeral. Incentivization could perhaps be re-structured, but the ATS document notes this will be challenging. With respect to pulmonary rehabilitation (about 50% of patients with lung cancer have comorbid COPD), for example, reimbursement is poor, which serves as a disincentive. Their suggestions? Early integration and repeated introduction to rehabilitation and exercise concepts. Sounds great.
In summary, in my opinion, fatigue doesn’t receive the attention level commensurate with its impact. It’s easy to understand why, but I’m glad the ATS is highlighting the problem. Unbeknownst to me, multiple cancer guidelines already recommend screening for fatigue. The recent sarcoidosis treatment guideline published by the European Respiratory Society dedicated a PICO (Patients, Intervention, Comparison, Outcomes) to the topic and recommended exercise (pulmonary rehabilitation). That said, consensus statements on COPD mention it only in passing in relation to severe disease and end-of-life care, and idiopathic pulmonary fibrosis guidelines ignore it entirely. So, recognition is improving, but we’ve got ways to go.
Dr. Holley is professor of medicine at Uniformed Services University, Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He disclosed ties with Metapharm, CHEST College, and WebMD.
A version of this article originally appeared on Medscape.com.
If you’re looking for it, you’ll find fatigue almost everywhere. It’s so common that it hides in plain sight, never dealt with because it’s present for good reason: the inevitable consequence of age, whatever disease you’re treating, poor lifestyle choices, and the daily grind of twenty-first–century life. Its impact is so ubiquitous and pernicious that it’s considered acceptable.
Is it though? After all, fatigue can be debilitating. Not every symptom is worthy of a chronic syndrome bearing its name. Furthermore, what if its relationship to the disease you’re treating is bidirectional?
Outside of sleep medicine, I see little focus on fatigue among pulmonologists. This despite the existing data on fatigue related to sarcoidosis, chronic obstructive pulmonary disease (COPD), and interstitial lung disease. Even when we do pay it lip service, “addressing” fatigue or sleep is essentially a euphemism for ordering a sleep study.
As with fatigue, if you look for obstructive sleep apnea, it’ll be there, although with OSA, it’s related to the incredibly low, nonevidence-based threshold the American Academy of Sleep Medicine has established for making the diagnosis. With continuous positive airway pressure (CPAP) in hand, the patient has a new disease to worry about and a difficult behavioral change (wearing, cleaning, and resupplying their CPAP equipment) to make. Too often, the CPAP isn’t used – or is – and the fatigue persists. But it’s okay, because we followed somebody’s guideline.
The American Thoracic Society just published a research statement on cancer-related fatigue. It is comprehensive and highlights the high prevalence and poor recognition of cancer-related fatigue. The authors note that among cancers, those of the lung are associated with a higher comorbid disease burden, older age, and cigarette smoking. All these factors make patients with lung cancer particularly prone to fatigue. Interactions between these factors, lung cancer histology, and specific chemotherapy regimens are poorly understood. True to its title, the “research statement” serves more as a call to action than an evidence-based blueprint for diagnosis and management.
The cancer-related fatigue data that does exist suggests treatment starts with recognition followed by a focus on sleep, exercise, and nutrition. This should surprise no one. The data on fatigue in general (not specific to cancer-related fatigue) shows that although fatigue is not synonymous with poor quality or insufficient sleep, sleep is usually a major factor. The cancer-related conditions affecting sleep include anxiety, depression, insufficient sleep, insomnia, medication side effects, and OSA. The intersecting web is complex, but across underlying conditions (cancer or otherwise), the quickest most efficient method for mitigating fatigue is optimizing sleep.
Exercise and nutrition are also important. Again, across disease processes (interstitial lung disease, COPD, lung cancer, and so on), no drug comes close to aerobic exercise for reducing symptoms, including fatigue. If an exercise prescription could be delivered in pill-form, it’d be a blockbuster. But it can’t be, and the ATS lung cancer–related fatigue research statement nicely outlines the evidence for increased activity levels and the barriers to obtaining support and compliance. As is the case with exercise, support for improving nutrition is limited by cost, access, and patient education.
Perhaps most importantly, sleep, exercise, and nutrition require time for counseling and a behavior change for the physician and patient. Both are in short supply, and commitment is always ephemeral. Incentivization could perhaps be re-structured, but the ATS document notes this will be challenging. With respect to pulmonary rehabilitation (about 50% of patients with lung cancer have comorbid COPD), for example, reimbursement is poor, which serves as a disincentive. Their suggestions? Early integration and repeated introduction to rehabilitation and exercise concepts. Sounds great.
In summary, in my opinion, fatigue doesn’t receive the attention level commensurate with its impact. It’s easy to understand why, but I’m glad the ATS is highlighting the problem. Unbeknownst to me, multiple cancer guidelines already recommend screening for fatigue. The recent sarcoidosis treatment guideline published by the European Respiratory Society dedicated a PICO (Patients, Intervention, Comparison, Outcomes) to the topic and recommended exercise (pulmonary rehabilitation). That said, consensus statements on COPD mention it only in passing in relation to severe disease and end-of-life care, and idiopathic pulmonary fibrosis guidelines ignore it entirely. So, recognition is improving, but we’ve got ways to go.
Dr. Holley is professor of medicine at Uniformed Services University, Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He disclosed ties with Metapharm, CHEST College, and WebMD.
A version of this article originally appeared on Medscape.com.
If you’re looking for it, you’ll find fatigue almost everywhere. It’s so common that it hides in plain sight, never dealt with because it’s present for good reason: the inevitable consequence of age, whatever disease you’re treating, poor lifestyle choices, and the daily grind of twenty-first–century life. Its impact is so ubiquitous and pernicious that it’s considered acceptable.
Is it though? After all, fatigue can be debilitating. Not every symptom is worthy of a chronic syndrome bearing its name. Furthermore, what if its relationship to the disease you’re treating is bidirectional?
Outside of sleep medicine, I see little focus on fatigue among pulmonologists. This despite the existing data on fatigue related to sarcoidosis, chronic obstructive pulmonary disease (COPD), and interstitial lung disease. Even when we do pay it lip service, “addressing” fatigue or sleep is essentially a euphemism for ordering a sleep study.
As with fatigue, if you look for obstructive sleep apnea, it’ll be there, although with OSA, it’s related to the incredibly low, nonevidence-based threshold the American Academy of Sleep Medicine has established for making the diagnosis. With continuous positive airway pressure (CPAP) in hand, the patient has a new disease to worry about and a difficult behavioral change (wearing, cleaning, and resupplying their CPAP equipment) to make. Too often, the CPAP isn’t used – or is – and the fatigue persists. But it’s okay, because we followed somebody’s guideline.
The American Thoracic Society just published a research statement on cancer-related fatigue. It is comprehensive and highlights the high prevalence and poor recognition of cancer-related fatigue. The authors note that among cancers, those of the lung are associated with a higher comorbid disease burden, older age, and cigarette smoking. All these factors make patients with lung cancer particularly prone to fatigue. Interactions between these factors, lung cancer histology, and specific chemotherapy regimens are poorly understood. True to its title, the “research statement” serves more as a call to action than an evidence-based blueprint for diagnosis and management.
The cancer-related fatigue data that does exist suggests treatment starts with recognition followed by a focus on sleep, exercise, and nutrition. This should surprise no one. The data on fatigue in general (not specific to cancer-related fatigue) shows that although fatigue is not synonymous with poor quality or insufficient sleep, sleep is usually a major factor. The cancer-related conditions affecting sleep include anxiety, depression, insufficient sleep, insomnia, medication side effects, and OSA. The intersecting web is complex, but across underlying conditions (cancer or otherwise), the quickest most efficient method for mitigating fatigue is optimizing sleep.
Exercise and nutrition are also important. Again, across disease processes (interstitial lung disease, COPD, lung cancer, and so on), no drug comes close to aerobic exercise for reducing symptoms, including fatigue. If an exercise prescription could be delivered in pill-form, it’d be a blockbuster. But it can’t be, and the ATS lung cancer–related fatigue research statement nicely outlines the evidence for increased activity levels and the barriers to obtaining support and compliance. As is the case with exercise, support for improving nutrition is limited by cost, access, and patient education.
Perhaps most importantly, sleep, exercise, and nutrition require time for counseling and a behavior change for the physician and patient. Both are in short supply, and commitment is always ephemeral. Incentivization could perhaps be re-structured, but the ATS document notes this will be challenging. With respect to pulmonary rehabilitation (about 50% of patients with lung cancer have comorbid COPD), for example, reimbursement is poor, which serves as a disincentive. Their suggestions? Early integration and repeated introduction to rehabilitation and exercise concepts. Sounds great.
In summary, in my opinion, fatigue doesn’t receive the attention level commensurate with its impact. It’s easy to understand why, but I’m glad the ATS is highlighting the problem. Unbeknownst to me, multiple cancer guidelines already recommend screening for fatigue. The recent sarcoidosis treatment guideline published by the European Respiratory Society dedicated a PICO (Patients, Intervention, Comparison, Outcomes) to the topic and recommended exercise (pulmonary rehabilitation). That said, consensus statements on COPD mention it only in passing in relation to severe disease and end-of-life care, and idiopathic pulmonary fibrosis guidelines ignore it entirely. So, recognition is improving, but we’ve got ways to go.
Dr. Holley is professor of medicine at Uniformed Services University, Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He disclosed ties with Metapharm, CHEST College, and WebMD.
A version of this article originally appeared on Medscape.com.
Early gestational diabetes treatment may improve neonatal outcomes
Screening and treatment for gestational diabetes are currently recommended at 24-28 weeks’ gestation, with earlier testing recommended for women at increased risk, but the potential benefits of earlier intervention remain debatable, wrote David Simmons, MD, of Western Sydney University, Campbelltown, Australia, and colleagues.
“Until now, there has been complete equipoise over whether to treat hyperglycemia below that of overt diabetes early in pregnancy,” Dr. Simmons said in an interview. The conflicting questions: “Would early treatment reduce the excess deposition of fat on the baby with all of its sequelae; but would early treatment reduce fuel supply to some babies at a critical time and lead to SGA [small for gestational age]?” Dr. Simmons noted.
In a study published in the New England Journal of Medicine, Dr. Simmons and colleagues randomized 406 women aged 18 years and older with singleton pregnancies to immediate treatment for gestational diabetes. Another 396 women were randomized to a control group for deferred treatment or no treatment, based on results of an oral glucose tolerance test at 24-28 weeks’ gestation. All participants had at least one risk factor for hyperglycemia, and met the World Health Organization criteria for gestational diabetes. Women with preexisting diabetes or contraindicating comorbid medical conditions were excluded.
The study had three primary outcomes. The first was a composite of neonatal outcomes including birth before 37 weeks’ gestation, birth weight of 4,500 g or higher, birth trauma, neonatal respiratory distress, phototherapy, stillbirth or neonatal death, or shoulder dystocia.
The final sample included 748 women for adverse neonatal outcomes, 750 for pregnancy-related hypertension, and 492 for neonatal lean body mass. The mean age of the participants was 32 years; approximately one-third were white European and another third were South Asian. Overall baseline demographics were similar between the groups, and the initial oral glucose tolerance tests were performed at a mean of 15.6 weeks’ gestation.
Overall, 24.9% of women in the early treatment group experienced an adverse neonatal event vs. 30.5% of controls, for an adjusted risk difference of –5.6% and adjusted relative risk of 0.82.
Notably, in an exploratory subgroup analysis, respiratory distress occurred in 9.8% of infants born to women in the immediate treatment group vs. 17.0% of infants in the control group. “Neonatal respiratory distress was the main driver of the between-group difference observed for the first primary outcome,” the researchers wrote. A prespecified subgroup analysis suggested that the impact of an earlier intervention on adverse neonatal outcomes might be greater among women with a higher glycemic value and those whose oral glucose tolerance tests occurred at less than 14 weeks’ gestation, they noted. Stillbirths or neonatal deaths were similar and infrequent in both groups.
Pregnancy-related hypertension occurred in 10.6% of the immediate-treatment group and 9.9% of the controls group (adjusted risk difference, 0.7%). For the third outcome, the mean neonatal lean body mass was 2.86 g in the immediate-treatment group and 2.91 g for the controls (adjusted mean difference, −0.04 g).
No differences in serious adverse events related to either screening or treatment were noted between the groups.
Impact on neonatal outcomes merits further study
Dr. Simmons said that he was surprised by the study findings. “We thought if there was an effect, it would be small, but it isn’t,” he told this publication.
“If you combine the severe adverse outcomes, the perineal trauma and the reduction in days in NICU/special care unit, this is a significant impact on morbidity and likely on cost,” and researchers are currently examining data for cost-effectiveness, he said.
“We did not expect the likely large impact on reducing respiratory distress and perineal trauma,” he noted. “These findings have not been previously reported, perhaps because they were not looked for.” By contrast, “we thought here might be reductions in lower gestational age and cesarean delivery, but there was not,” he added.
The findings were limited by several factors including the nonstandardized approach to gestational diabetes treatment and the use of third-trimester treatment targets that had not been tested in earlier trimesters, the researchers noted. Other limitations included the focus on women already at high risk for hyperglycemia; therefore, the results might not generalize to women not at risk, they wrote.
The current study represents a beginning of answers, with data suggesting that early treatment for gestational diabetes reduces severe adverse pregnancy outcomes, days in NICU/special care unit, and perineal trauma, likely from the first trimester, said Dr. Simmons. However, the findings must be interpreted with caution, as criteria that are too low “might lead to more small babies,” he said. “We look forward to working with others to translate these findings into practice,” he added.
Much more research is needed to answer the many questions prompted by the current study, including who did and did not have complications, Dr. Simmons told this publication. Other studies are needed to collect data on cost-effectiveness, as well as consumer views, especially “different perspectives from different parts of the globe,” he said. Although there is not enough evidence yet to draw conclusions about the role of continuous glucose monitoring (CGM) in managing gestational diabetes, many studies are underway; “we look forward to the results,” of these studies, Dr. Simmons added.
Findings support early screening
Gestational diabetes is one of the most common medical complications of pregnancy, and accounts for more than 80% of diabetes-related diagnoses in pregnancy, said Emily Fay, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, in an interview.
“Previous studies have found that women with gestational diabetes are at higher risk in their pregnancy, including higher chance of developing preeclampsia, higher chance of cesarean delivery, and higher risks for their baby, including risk of shoulder dystocia, birth trauma, and jaundice, and higher birth weights,” she said. “Fortunately, studies have also shown that treatment of gestational diabetes helps lower these risks,” she noted. Currently, patients undergo routine screening for gestational diabetes between 24 and 28 weeks of pregnancy, but some who have risk factors for gestational diabetes may have screening in the early part of pregnancy, said Dr. Fay.
The current findings were not surprising overall, said Dr. Fay, who was not involved in the study. “The study authors looked at a variety of outcomes including neonatal adverse outcomes, neonatal body weight, and pregnancy-related hypertension,” she said.
The researchers found that patients treated early had a lower rate of adverse neonatal outcomes, which was to be expected, Dr. Fay said. “They did not find a difference in neonatal body weight; this also was not surprising, as the women who were not in the early treatment group still received treatment at the time of diagnosis later in pregnancy, which likely helped normalize the weights,” she explained.
“My takeaway from this study is that we should continue to screen patients with risk factors for gestational diabetes early in pregnancy and treat them at the time of diagnosis,” Dr. Fay told this publication. However, barriers that may exist to early treatment involve access to care, including being able to see a provider early in pregnancy, she said. “The treatment for gestational diabetes includes dietary education with diet changes and checking blood sugars frequently. Access to nutrition education can be limited and access to healthy foods can be expensive and difficult to obtain,” she noted. “Checking blood sugars throughout the day can also be difficult for those who are busy or working and who may not have the ability to take time to do this,” she said. However, “these barriers may be overcome by health care reform that improves patient access to and coverage of pregnancy care, improved access and affordability of healthy foods, and employer flexibility to allow the time and space to check blood sugars if needed,” she added.
Looking ahead, the use of continuous glucose monitors in pregnancy is an expanding area of research, said Dr. Fay. “Patients can quickly view their blood sugar without the use of finger sticks, which may help overcome some of the barriers patients may have with using finger sticks,” she noted. “Continuous glucose monitors have been used for those with type 1 and type 2 diabetes with success, and we need to better understand if these can also be helpful in gestational diabetes,” she said. Dr. Fay and colleagues at the University of Washington are currently conducting an ongoing study to explore the use of CGM in gestational diabetes.
The study was supported by the National Health and Medical Research Council, the Region Örebro Research Committee, the Medical Scientific Fund of the Mayor of Vienna, the South Western Sydney Local Health District Academic Unit, and a Western Sydney University Ainsworth Trust Grant. The researchers had no financial conflicts to disclose. Dr. Fay had no relevant financial conflicts to disclose.
Screening and treatment for gestational diabetes are currently recommended at 24-28 weeks’ gestation, with earlier testing recommended for women at increased risk, but the potential benefits of earlier intervention remain debatable, wrote David Simmons, MD, of Western Sydney University, Campbelltown, Australia, and colleagues.
“Until now, there has been complete equipoise over whether to treat hyperglycemia below that of overt diabetes early in pregnancy,” Dr. Simmons said in an interview. The conflicting questions: “Would early treatment reduce the excess deposition of fat on the baby with all of its sequelae; but would early treatment reduce fuel supply to some babies at a critical time and lead to SGA [small for gestational age]?” Dr. Simmons noted.
In a study published in the New England Journal of Medicine, Dr. Simmons and colleagues randomized 406 women aged 18 years and older with singleton pregnancies to immediate treatment for gestational diabetes. Another 396 women were randomized to a control group for deferred treatment or no treatment, based on results of an oral glucose tolerance test at 24-28 weeks’ gestation. All participants had at least one risk factor for hyperglycemia, and met the World Health Organization criteria for gestational diabetes. Women with preexisting diabetes or contraindicating comorbid medical conditions were excluded.
The study had three primary outcomes. The first was a composite of neonatal outcomes including birth before 37 weeks’ gestation, birth weight of 4,500 g or higher, birth trauma, neonatal respiratory distress, phototherapy, stillbirth or neonatal death, or shoulder dystocia.
The final sample included 748 women for adverse neonatal outcomes, 750 for pregnancy-related hypertension, and 492 for neonatal lean body mass. The mean age of the participants was 32 years; approximately one-third were white European and another third were South Asian. Overall baseline demographics were similar between the groups, and the initial oral glucose tolerance tests were performed at a mean of 15.6 weeks’ gestation.
Overall, 24.9% of women in the early treatment group experienced an adverse neonatal event vs. 30.5% of controls, for an adjusted risk difference of –5.6% and adjusted relative risk of 0.82.
Notably, in an exploratory subgroup analysis, respiratory distress occurred in 9.8% of infants born to women in the immediate treatment group vs. 17.0% of infants in the control group. “Neonatal respiratory distress was the main driver of the between-group difference observed for the first primary outcome,” the researchers wrote. A prespecified subgroup analysis suggested that the impact of an earlier intervention on adverse neonatal outcomes might be greater among women with a higher glycemic value and those whose oral glucose tolerance tests occurred at less than 14 weeks’ gestation, they noted. Stillbirths or neonatal deaths were similar and infrequent in both groups.
Pregnancy-related hypertension occurred in 10.6% of the immediate-treatment group and 9.9% of the controls group (adjusted risk difference, 0.7%). For the third outcome, the mean neonatal lean body mass was 2.86 g in the immediate-treatment group and 2.91 g for the controls (adjusted mean difference, −0.04 g).
No differences in serious adverse events related to either screening or treatment were noted between the groups.
Impact on neonatal outcomes merits further study
Dr. Simmons said that he was surprised by the study findings. “We thought if there was an effect, it would be small, but it isn’t,” he told this publication.
“If you combine the severe adverse outcomes, the perineal trauma and the reduction in days in NICU/special care unit, this is a significant impact on morbidity and likely on cost,” and researchers are currently examining data for cost-effectiveness, he said.
“We did not expect the likely large impact on reducing respiratory distress and perineal trauma,” he noted. “These findings have not been previously reported, perhaps because they were not looked for.” By contrast, “we thought here might be reductions in lower gestational age and cesarean delivery, but there was not,” he added.
The findings were limited by several factors including the nonstandardized approach to gestational diabetes treatment and the use of third-trimester treatment targets that had not been tested in earlier trimesters, the researchers noted. Other limitations included the focus on women already at high risk for hyperglycemia; therefore, the results might not generalize to women not at risk, they wrote.
The current study represents a beginning of answers, with data suggesting that early treatment for gestational diabetes reduces severe adverse pregnancy outcomes, days in NICU/special care unit, and perineal trauma, likely from the first trimester, said Dr. Simmons. However, the findings must be interpreted with caution, as criteria that are too low “might lead to more small babies,” he said. “We look forward to working with others to translate these findings into practice,” he added.
Much more research is needed to answer the many questions prompted by the current study, including who did and did not have complications, Dr. Simmons told this publication. Other studies are needed to collect data on cost-effectiveness, as well as consumer views, especially “different perspectives from different parts of the globe,” he said. Although there is not enough evidence yet to draw conclusions about the role of continuous glucose monitoring (CGM) in managing gestational diabetes, many studies are underway; “we look forward to the results,” of these studies, Dr. Simmons added.
Findings support early screening
Gestational diabetes is one of the most common medical complications of pregnancy, and accounts for more than 80% of diabetes-related diagnoses in pregnancy, said Emily Fay, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, in an interview.
“Previous studies have found that women with gestational diabetes are at higher risk in their pregnancy, including higher chance of developing preeclampsia, higher chance of cesarean delivery, and higher risks for their baby, including risk of shoulder dystocia, birth trauma, and jaundice, and higher birth weights,” she said. “Fortunately, studies have also shown that treatment of gestational diabetes helps lower these risks,” she noted. Currently, patients undergo routine screening for gestational diabetes between 24 and 28 weeks of pregnancy, but some who have risk factors for gestational diabetes may have screening in the early part of pregnancy, said Dr. Fay.
The current findings were not surprising overall, said Dr. Fay, who was not involved in the study. “The study authors looked at a variety of outcomes including neonatal adverse outcomes, neonatal body weight, and pregnancy-related hypertension,” she said.
The researchers found that patients treated early had a lower rate of adverse neonatal outcomes, which was to be expected, Dr. Fay said. “They did not find a difference in neonatal body weight; this also was not surprising, as the women who were not in the early treatment group still received treatment at the time of diagnosis later in pregnancy, which likely helped normalize the weights,” she explained.
“My takeaway from this study is that we should continue to screen patients with risk factors for gestational diabetes early in pregnancy and treat them at the time of diagnosis,” Dr. Fay told this publication. However, barriers that may exist to early treatment involve access to care, including being able to see a provider early in pregnancy, she said. “The treatment for gestational diabetes includes dietary education with diet changes and checking blood sugars frequently. Access to nutrition education can be limited and access to healthy foods can be expensive and difficult to obtain,” she noted. “Checking blood sugars throughout the day can also be difficult for those who are busy or working and who may not have the ability to take time to do this,” she said. However, “these barriers may be overcome by health care reform that improves patient access to and coverage of pregnancy care, improved access and affordability of healthy foods, and employer flexibility to allow the time and space to check blood sugars if needed,” she added.
Looking ahead, the use of continuous glucose monitors in pregnancy is an expanding area of research, said Dr. Fay. “Patients can quickly view their blood sugar without the use of finger sticks, which may help overcome some of the barriers patients may have with using finger sticks,” she noted. “Continuous glucose monitors have been used for those with type 1 and type 2 diabetes with success, and we need to better understand if these can also be helpful in gestational diabetes,” she said. Dr. Fay and colleagues at the University of Washington are currently conducting an ongoing study to explore the use of CGM in gestational diabetes.
The study was supported by the National Health and Medical Research Council, the Region Örebro Research Committee, the Medical Scientific Fund of the Mayor of Vienna, the South Western Sydney Local Health District Academic Unit, and a Western Sydney University Ainsworth Trust Grant. The researchers had no financial conflicts to disclose. Dr. Fay had no relevant financial conflicts to disclose.
Screening and treatment for gestational diabetes are currently recommended at 24-28 weeks’ gestation, with earlier testing recommended for women at increased risk, but the potential benefits of earlier intervention remain debatable, wrote David Simmons, MD, of Western Sydney University, Campbelltown, Australia, and colleagues.
“Until now, there has been complete equipoise over whether to treat hyperglycemia below that of overt diabetes early in pregnancy,” Dr. Simmons said in an interview. The conflicting questions: “Would early treatment reduce the excess deposition of fat on the baby with all of its sequelae; but would early treatment reduce fuel supply to some babies at a critical time and lead to SGA [small for gestational age]?” Dr. Simmons noted.
In a study published in the New England Journal of Medicine, Dr. Simmons and colleagues randomized 406 women aged 18 years and older with singleton pregnancies to immediate treatment for gestational diabetes. Another 396 women were randomized to a control group for deferred treatment or no treatment, based on results of an oral glucose tolerance test at 24-28 weeks’ gestation. All participants had at least one risk factor for hyperglycemia, and met the World Health Organization criteria for gestational diabetes. Women with preexisting diabetes or contraindicating comorbid medical conditions were excluded.
The study had three primary outcomes. The first was a composite of neonatal outcomes including birth before 37 weeks’ gestation, birth weight of 4,500 g or higher, birth trauma, neonatal respiratory distress, phototherapy, stillbirth or neonatal death, or shoulder dystocia.
The final sample included 748 women for adverse neonatal outcomes, 750 for pregnancy-related hypertension, and 492 for neonatal lean body mass. The mean age of the participants was 32 years; approximately one-third were white European and another third were South Asian. Overall baseline demographics were similar between the groups, and the initial oral glucose tolerance tests were performed at a mean of 15.6 weeks’ gestation.
Overall, 24.9% of women in the early treatment group experienced an adverse neonatal event vs. 30.5% of controls, for an adjusted risk difference of –5.6% and adjusted relative risk of 0.82.
Notably, in an exploratory subgroup analysis, respiratory distress occurred in 9.8% of infants born to women in the immediate treatment group vs. 17.0% of infants in the control group. “Neonatal respiratory distress was the main driver of the between-group difference observed for the first primary outcome,” the researchers wrote. A prespecified subgroup analysis suggested that the impact of an earlier intervention on adverse neonatal outcomes might be greater among women with a higher glycemic value and those whose oral glucose tolerance tests occurred at less than 14 weeks’ gestation, they noted. Stillbirths or neonatal deaths were similar and infrequent in both groups.
Pregnancy-related hypertension occurred in 10.6% of the immediate-treatment group and 9.9% of the controls group (adjusted risk difference, 0.7%). For the third outcome, the mean neonatal lean body mass was 2.86 g in the immediate-treatment group and 2.91 g for the controls (adjusted mean difference, −0.04 g).
No differences in serious adverse events related to either screening or treatment were noted between the groups.
Impact on neonatal outcomes merits further study
Dr. Simmons said that he was surprised by the study findings. “We thought if there was an effect, it would be small, but it isn’t,” he told this publication.
“If you combine the severe adverse outcomes, the perineal trauma and the reduction in days in NICU/special care unit, this is a significant impact on morbidity and likely on cost,” and researchers are currently examining data for cost-effectiveness, he said.
“We did not expect the likely large impact on reducing respiratory distress and perineal trauma,” he noted. “These findings have not been previously reported, perhaps because they were not looked for.” By contrast, “we thought here might be reductions in lower gestational age and cesarean delivery, but there was not,” he added.
The findings were limited by several factors including the nonstandardized approach to gestational diabetes treatment and the use of third-trimester treatment targets that had not been tested in earlier trimesters, the researchers noted. Other limitations included the focus on women already at high risk for hyperglycemia; therefore, the results might not generalize to women not at risk, they wrote.
The current study represents a beginning of answers, with data suggesting that early treatment for gestational diabetes reduces severe adverse pregnancy outcomes, days in NICU/special care unit, and perineal trauma, likely from the first trimester, said Dr. Simmons. However, the findings must be interpreted with caution, as criteria that are too low “might lead to more small babies,” he said. “We look forward to working with others to translate these findings into practice,” he added.
Much more research is needed to answer the many questions prompted by the current study, including who did and did not have complications, Dr. Simmons told this publication. Other studies are needed to collect data on cost-effectiveness, as well as consumer views, especially “different perspectives from different parts of the globe,” he said. Although there is not enough evidence yet to draw conclusions about the role of continuous glucose monitoring (CGM) in managing gestational diabetes, many studies are underway; “we look forward to the results,” of these studies, Dr. Simmons added.
Findings support early screening
Gestational diabetes is one of the most common medical complications of pregnancy, and accounts for more than 80% of diabetes-related diagnoses in pregnancy, said Emily Fay, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, in an interview.
“Previous studies have found that women with gestational diabetes are at higher risk in their pregnancy, including higher chance of developing preeclampsia, higher chance of cesarean delivery, and higher risks for their baby, including risk of shoulder dystocia, birth trauma, and jaundice, and higher birth weights,” she said. “Fortunately, studies have also shown that treatment of gestational diabetes helps lower these risks,” she noted. Currently, patients undergo routine screening for gestational diabetes between 24 and 28 weeks of pregnancy, but some who have risk factors for gestational diabetes may have screening in the early part of pregnancy, said Dr. Fay.
The current findings were not surprising overall, said Dr. Fay, who was not involved in the study. “The study authors looked at a variety of outcomes including neonatal adverse outcomes, neonatal body weight, and pregnancy-related hypertension,” she said.
The researchers found that patients treated early had a lower rate of adverse neonatal outcomes, which was to be expected, Dr. Fay said. “They did not find a difference in neonatal body weight; this also was not surprising, as the women who were not in the early treatment group still received treatment at the time of diagnosis later in pregnancy, which likely helped normalize the weights,” she explained.
“My takeaway from this study is that we should continue to screen patients with risk factors for gestational diabetes early in pregnancy and treat them at the time of diagnosis,” Dr. Fay told this publication. However, barriers that may exist to early treatment involve access to care, including being able to see a provider early in pregnancy, she said. “The treatment for gestational diabetes includes dietary education with diet changes and checking blood sugars frequently. Access to nutrition education can be limited and access to healthy foods can be expensive and difficult to obtain,” she noted. “Checking blood sugars throughout the day can also be difficult for those who are busy or working and who may not have the ability to take time to do this,” she said. However, “these barriers may be overcome by health care reform that improves patient access to and coverage of pregnancy care, improved access and affordability of healthy foods, and employer flexibility to allow the time and space to check blood sugars if needed,” she added.
Looking ahead, the use of continuous glucose monitors in pregnancy is an expanding area of research, said Dr. Fay. “Patients can quickly view their blood sugar without the use of finger sticks, which may help overcome some of the barriers patients may have with using finger sticks,” she noted. “Continuous glucose monitors have been used for those with type 1 and type 2 diabetes with success, and we need to better understand if these can also be helpful in gestational diabetes,” she said. Dr. Fay and colleagues at the University of Washington are currently conducting an ongoing study to explore the use of CGM in gestational diabetes.
The study was supported by the National Health and Medical Research Council, the Region Örebro Research Committee, the Medical Scientific Fund of the Mayor of Vienna, the South Western Sydney Local Health District Academic Unit, and a Western Sydney University Ainsworth Trust Grant. The researchers had no financial conflicts to disclose. Dr. Fay had no relevant financial conflicts to disclose.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
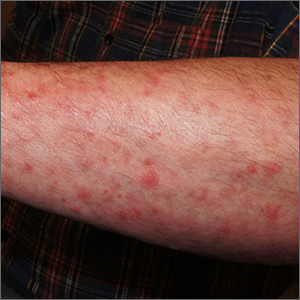
Cutaneous vasculitis curtails quality of life
, and its measurement with an organ-specific instrument may catch important disease outcomes better than a generic health-related quality of life index, according to survey responses from participants in the Vasculitis Patient-Powered Research Network (VPPRN).
Although cutaneous vasculitis often causes itching, pain, and ulceration, the impact of the disease on specific health-related quality of life (HRQOL) outcomes has not been systematically assessed, wrote Sarah Mann, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Dermatology, the researchers used the VPPRN to conduct an online survey of adults aged 18 years and older with cutaneous manifestations of vasculitis. The survey was conducted between January 2020 and August 2021.
The primary outcomes of HRQOL were determined using two validated measures. One measured skin-related HRQOL (the Effects of Skin Disease on Quality-of-Life Survey [Skindex-29]), and the other measured general health and well-being (36-Item Short Form Health Survey [SF-36]).
The final analysis included 190 survey responses. The mean age of the respondents was 50.5 years, 84.1% were female, and approximately two-thirds reported a duration of vasculitis of at least 5 years. Respondents’ vasculitides included cutaneous small-vessel vasculitis (14%), IgA vasculitis (6.5%), urticarial vasculitis (8.4%), granulomatosis with polyangiitis (17.6%), microscopic polyangiitis (10.3%), eosinophilic vasculitis (15%), polyarteritis nodosa (3.7%), and other vasculitis types (24.2%).
On the Skindex-29 domains, severely or very severely diminished HRQOL was reported by 77.6% of respondents for emotions, 78.5% for symptoms, 60.7% for functioning, and 75.7% for overall HRQOL.
On the SF-36, the HRQOL was below average on six of eight domains, and approximately half of the patients had summative physical component scores (56%) and mental component scores (52%) below 50.
The HRQOL outcomes of cutaneous vasculitis were worse on the Skindex-29 than the SF-36, the researchers noted. “This discordance may reflect the value of disease or organ-specific measures, which may be able to capture important outcomes of disease even when generic measures do not,” they said.
The study findings were limited by several factors, including the potential lack of generalizability to broader populations of vasculitis patients, the researchers noted. Other limitations included the underrepresentation of male patients and the lack of a disease-specific patient-reported outcome measure, they said.
In addition, “Because half of patients reported having disease which was in remission or mildly active, the study findings may underestimate the true role of active cutaneous vasculitis on HRQOL,” the researchers said.
More studies are needed to assess how HRQOL measures respond to disease treatment and control, the researchers wrote in their discussion. However, the results suggest that cutaneous vasculitis has a significant effect on patients’ perception of their health, as well as on their well-being and symptoms, they said.
The study was supported by the Patient-Centered Outcomes Research Institute and GlaxoSmithKline. Dr. Mann had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including GlaxoSmithKline.
, and its measurement with an organ-specific instrument may catch important disease outcomes better than a generic health-related quality of life index, according to survey responses from participants in the Vasculitis Patient-Powered Research Network (VPPRN).
Although cutaneous vasculitis often causes itching, pain, and ulceration, the impact of the disease on specific health-related quality of life (HRQOL) outcomes has not been systematically assessed, wrote Sarah Mann, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Dermatology, the researchers used the VPPRN to conduct an online survey of adults aged 18 years and older with cutaneous manifestations of vasculitis. The survey was conducted between January 2020 and August 2021.
The primary outcomes of HRQOL were determined using two validated measures. One measured skin-related HRQOL (the Effects of Skin Disease on Quality-of-Life Survey [Skindex-29]), and the other measured general health and well-being (36-Item Short Form Health Survey [SF-36]).
The final analysis included 190 survey responses. The mean age of the respondents was 50.5 years, 84.1% were female, and approximately two-thirds reported a duration of vasculitis of at least 5 years. Respondents’ vasculitides included cutaneous small-vessel vasculitis (14%), IgA vasculitis (6.5%), urticarial vasculitis (8.4%), granulomatosis with polyangiitis (17.6%), microscopic polyangiitis (10.3%), eosinophilic vasculitis (15%), polyarteritis nodosa (3.7%), and other vasculitis types (24.2%).
On the Skindex-29 domains, severely or very severely diminished HRQOL was reported by 77.6% of respondents for emotions, 78.5% for symptoms, 60.7% for functioning, and 75.7% for overall HRQOL.
On the SF-36, the HRQOL was below average on six of eight domains, and approximately half of the patients had summative physical component scores (56%) and mental component scores (52%) below 50.
The HRQOL outcomes of cutaneous vasculitis were worse on the Skindex-29 than the SF-36, the researchers noted. “This discordance may reflect the value of disease or organ-specific measures, which may be able to capture important outcomes of disease even when generic measures do not,” they said.
The study findings were limited by several factors, including the potential lack of generalizability to broader populations of vasculitis patients, the researchers noted. Other limitations included the underrepresentation of male patients and the lack of a disease-specific patient-reported outcome measure, they said.
In addition, “Because half of patients reported having disease which was in remission or mildly active, the study findings may underestimate the true role of active cutaneous vasculitis on HRQOL,” the researchers said.
More studies are needed to assess how HRQOL measures respond to disease treatment and control, the researchers wrote in their discussion. However, the results suggest that cutaneous vasculitis has a significant effect on patients’ perception of their health, as well as on their well-being and symptoms, they said.
The study was supported by the Patient-Centered Outcomes Research Institute and GlaxoSmithKline. Dr. Mann had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including GlaxoSmithKline.
, and its measurement with an organ-specific instrument may catch important disease outcomes better than a generic health-related quality of life index, according to survey responses from participants in the Vasculitis Patient-Powered Research Network (VPPRN).
Although cutaneous vasculitis often causes itching, pain, and ulceration, the impact of the disease on specific health-related quality of life (HRQOL) outcomes has not been systematically assessed, wrote Sarah Mann, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Dermatology, the researchers used the VPPRN to conduct an online survey of adults aged 18 years and older with cutaneous manifestations of vasculitis. The survey was conducted between January 2020 and August 2021.
The primary outcomes of HRQOL were determined using two validated measures. One measured skin-related HRQOL (the Effects of Skin Disease on Quality-of-Life Survey [Skindex-29]), and the other measured general health and well-being (36-Item Short Form Health Survey [SF-36]).
The final analysis included 190 survey responses. The mean age of the respondents was 50.5 years, 84.1% were female, and approximately two-thirds reported a duration of vasculitis of at least 5 years. Respondents’ vasculitides included cutaneous small-vessel vasculitis (14%), IgA vasculitis (6.5%), urticarial vasculitis (8.4%), granulomatosis with polyangiitis (17.6%), microscopic polyangiitis (10.3%), eosinophilic vasculitis (15%), polyarteritis nodosa (3.7%), and other vasculitis types (24.2%).
On the Skindex-29 domains, severely or very severely diminished HRQOL was reported by 77.6% of respondents for emotions, 78.5% for symptoms, 60.7% for functioning, and 75.7% for overall HRQOL.
On the SF-36, the HRQOL was below average on six of eight domains, and approximately half of the patients had summative physical component scores (56%) and mental component scores (52%) below 50.
The HRQOL outcomes of cutaneous vasculitis were worse on the Skindex-29 than the SF-36, the researchers noted. “This discordance may reflect the value of disease or organ-specific measures, which may be able to capture important outcomes of disease even when generic measures do not,” they said.
The study findings were limited by several factors, including the potential lack of generalizability to broader populations of vasculitis patients, the researchers noted. Other limitations included the underrepresentation of male patients and the lack of a disease-specific patient-reported outcome measure, they said.
In addition, “Because half of patients reported having disease which was in remission or mildly active, the study findings may underestimate the true role of active cutaneous vasculitis on HRQOL,” the researchers said.
More studies are needed to assess how HRQOL measures respond to disease treatment and control, the researchers wrote in their discussion. However, the results suggest that cutaneous vasculitis has a significant effect on patients’ perception of their health, as well as on their well-being and symptoms, they said.
The study was supported by the Patient-Centered Outcomes Research Institute and GlaxoSmithKline. Dr. Mann had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including GlaxoSmithKline.
FROM JAMA DERMATOLOGY
High cholesterol in seniors: Use statins for primary prevention?
LONG BEACH, CALIF. – For years, clinicians have debated whether prescribing statins to patients older than 75 for the prevention of cardiovascular events is appropriate.
In 2022, the U.S. Preventive Services Task Force concluded that scientific evidence was insufficient to assess the balance between the benefits and harms of the therapy for this older population.
At a session of the annual meeting of the American Geriatrics Society, experts laid out new preliminary recommendations of the AGS and the National Lipid Association on assessing risk and deciding on treatment.
The group concluded that LDL cholesterol levels are associated with incident arteriosclerotic cardiovascular disease (ASCVD), that the coronary artery calcium (CAC) score can be a valuable measure, and that statins may be reasonable to prescribe, even given the risks that have been linked to statins, such as that for muscle pain. Final recommendations are expected by fall 2023.
“This is still a work in progress,” said Daniel E. Forman, MD, professor of medicine and chair of geriatric cardiology at the University of Pittsburgh.
The AGS-NLA panel concluded that, for those aged 75 or older without established ASCVD, LDL cholesterol is associated with incident ASCVD, the only recommendation to be given a class I (strong) rating; others were classed as moderate or weak.
Dr. Forman reviewed the evidence for lowering LDL cholesterol to decrease ASCVD, citing a 2018 study that concluded, “Reverse causation may contribute to the association of lower TC with higher mortality in nonrandomized studies.”
However, research overall shows that, as LDL cholesterol levels increase, patients are more likely to experience a heart event.
Dr. Forman noted that the utility of equations for assessing 5- or 10-year risk of ASCVD is uncertain. However, he said, traditional risk factors, such as family history and ethnicity, still have value.
Assessing risk “has been enriched in the past few years by the introduction of the coronary artery calcium [CAC] score,” he said.
Lower scores predict lower rates of CVD events, Forman said. The AGS-NLA recommends measuring CAC if clinical uncertainty exists about the value of statins.
“It’s reasonable to measure CAC and to withhold statins when the CAC is zero,” Dr. Forman said. “When the CAC score is zero ... the risk of having a cardiovascular event is really next to nil. Patients are happy to know they have a CAC of zero.”
Likewise, patients appreciate knowing whether their score is high, which would indicate increased risk. He said the CAC score is underused by geriatric physicians.
The group also determined, after reviewing the research, that starting treatment is reasonable for patients with an LDL cholesterol level of 70-189 if they have no life-limiting illness and their life expectancy is over 5 years.
Other preliminary recommendations include the use of statins for those aged 75 and older, irrespective of risk for statin-associated muscle symptoms, type 2 diabetes, or impaired cognition. These associations are often weak, Dr. Forman added.
Focusing on person-centered decisions
Ariel Green, MD, MPH, PhD, associate professor of medicine at Johns Hopkins University, Baltimore, said statin therapy “should be individualized” to weigh benefits, noncardiac risks, and other considerations.
Clinicians can incorporate life expectancy into prevention decisions using tools such as ePrognosis, from the University of California, San Francisco, Dr. Green said.
If life expectancy is greater than the time to benefit, statin therapy may help. Dr. Green cited research that showed that 2.5 years of statin therapy was needed to prevent one major adverse cardiovascular event (MACE) per 100 patients in a population aged 50-75. Other data show reductions in MACE for those older than 75, but overall, the data are limited in this population.
The proposed recommendation is to use tools such as life tables that include comorbid conditions and functional status to guide clinical decisions.
“Another aspect of assessing net benefits of statin therapy is to consider competing health risks,” Dr. Green said.
The group recommends considering using competing risk-adjusted CVD models, though these are not widely used.
The group also recommends integrating screenings for frailty (Clinical Frailty Scale), dementia (Mini-Cog), and functional status (Vulnerable Elders Scale–13) into assessments.
“The presence of these syndromes should prompt elicitation of patient values and preferences related to prevention and medication use,” Dr. Green said.
Clinicians can use decision aids, but these are not always practical, owing to obstacles such as patients’ cognitive problems, Dr. Green said.
“Another approach is asking people to prioritize a set of universal health outcomes that apply across health conditions, such as maintaining independence, staying alive, reducing, or eliminating symptoms and focusing on comfort,” Dr. Green said.
She addressed the evidence about deprescribing statins, with a focus on those with a life expectancy of less than a year. Researchers have found an increase in quality of life and no increases in cardiovascular events or death when statins were deprescribed.
A welcome framework
Cory Krueger, MD, an internal medicine and geriatric physician in Cornville, Ariz., who attended the talk, said he welcomed the presentation, in which preliminary recommendations were explained.
“This has been a controversial area in geriatrics,” Dr. Krueger said. “At least this gave me a framework for discussing this with my patients in a reasonable way.”
Dr. Forman and Dr. Krueger disclosed no relevant financial relationships. Dr. Green receives funding from the National Institute of Aging and Impact Collaboratory.
A version of this article first appeared on Medscape.com.
LONG BEACH, CALIF. – For years, clinicians have debated whether prescribing statins to patients older than 75 for the prevention of cardiovascular events is appropriate.
In 2022, the U.S. Preventive Services Task Force concluded that scientific evidence was insufficient to assess the balance between the benefits and harms of the therapy for this older population.
At a session of the annual meeting of the American Geriatrics Society, experts laid out new preliminary recommendations of the AGS and the National Lipid Association on assessing risk and deciding on treatment.
The group concluded that LDL cholesterol levels are associated with incident arteriosclerotic cardiovascular disease (ASCVD), that the coronary artery calcium (CAC) score can be a valuable measure, and that statins may be reasonable to prescribe, even given the risks that have been linked to statins, such as that for muscle pain. Final recommendations are expected by fall 2023.
“This is still a work in progress,” said Daniel E. Forman, MD, professor of medicine and chair of geriatric cardiology at the University of Pittsburgh.
The AGS-NLA panel concluded that, for those aged 75 or older without established ASCVD, LDL cholesterol is associated with incident ASCVD, the only recommendation to be given a class I (strong) rating; others were classed as moderate or weak.
Dr. Forman reviewed the evidence for lowering LDL cholesterol to decrease ASCVD, citing a 2018 study that concluded, “Reverse causation may contribute to the association of lower TC with higher mortality in nonrandomized studies.”
However, research overall shows that, as LDL cholesterol levels increase, patients are more likely to experience a heart event.
Dr. Forman noted that the utility of equations for assessing 5- or 10-year risk of ASCVD is uncertain. However, he said, traditional risk factors, such as family history and ethnicity, still have value.
Assessing risk “has been enriched in the past few years by the introduction of the coronary artery calcium [CAC] score,” he said.
Lower scores predict lower rates of CVD events, Forman said. The AGS-NLA recommends measuring CAC if clinical uncertainty exists about the value of statins.
“It’s reasonable to measure CAC and to withhold statins when the CAC is zero,” Dr. Forman said. “When the CAC score is zero ... the risk of having a cardiovascular event is really next to nil. Patients are happy to know they have a CAC of zero.”
Likewise, patients appreciate knowing whether their score is high, which would indicate increased risk. He said the CAC score is underused by geriatric physicians.
The group also determined, after reviewing the research, that starting treatment is reasonable for patients with an LDL cholesterol level of 70-189 if they have no life-limiting illness and their life expectancy is over 5 years.
Other preliminary recommendations include the use of statins for those aged 75 and older, irrespective of risk for statin-associated muscle symptoms, type 2 diabetes, or impaired cognition. These associations are often weak, Dr. Forman added.
Focusing on person-centered decisions
Ariel Green, MD, MPH, PhD, associate professor of medicine at Johns Hopkins University, Baltimore, said statin therapy “should be individualized” to weigh benefits, noncardiac risks, and other considerations.
Clinicians can incorporate life expectancy into prevention decisions using tools such as ePrognosis, from the University of California, San Francisco, Dr. Green said.
If life expectancy is greater than the time to benefit, statin therapy may help. Dr. Green cited research that showed that 2.5 years of statin therapy was needed to prevent one major adverse cardiovascular event (MACE) per 100 patients in a population aged 50-75. Other data show reductions in MACE for those older than 75, but overall, the data are limited in this population.
The proposed recommendation is to use tools such as life tables that include comorbid conditions and functional status to guide clinical decisions.
“Another aspect of assessing net benefits of statin therapy is to consider competing health risks,” Dr. Green said.
The group recommends considering using competing risk-adjusted CVD models, though these are not widely used.
The group also recommends integrating screenings for frailty (Clinical Frailty Scale), dementia (Mini-Cog), and functional status (Vulnerable Elders Scale–13) into assessments.
“The presence of these syndromes should prompt elicitation of patient values and preferences related to prevention and medication use,” Dr. Green said.
Clinicians can use decision aids, but these are not always practical, owing to obstacles such as patients’ cognitive problems, Dr. Green said.
“Another approach is asking people to prioritize a set of universal health outcomes that apply across health conditions, such as maintaining independence, staying alive, reducing, or eliminating symptoms and focusing on comfort,” Dr. Green said.
She addressed the evidence about deprescribing statins, with a focus on those with a life expectancy of less than a year. Researchers have found an increase in quality of life and no increases in cardiovascular events or death when statins were deprescribed.
A welcome framework
Cory Krueger, MD, an internal medicine and geriatric physician in Cornville, Ariz., who attended the talk, said he welcomed the presentation, in which preliminary recommendations were explained.
“This has been a controversial area in geriatrics,” Dr. Krueger said. “At least this gave me a framework for discussing this with my patients in a reasonable way.”
Dr. Forman and Dr. Krueger disclosed no relevant financial relationships. Dr. Green receives funding from the National Institute of Aging and Impact Collaboratory.
A version of this article first appeared on Medscape.com.
LONG BEACH, CALIF. – For years, clinicians have debated whether prescribing statins to patients older than 75 for the prevention of cardiovascular events is appropriate.
In 2022, the U.S. Preventive Services Task Force concluded that scientific evidence was insufficient to assess the balance between the benefits and harms of the therapy for this older population.
At a session of the annual meeting of the American Geriatrics Society, experts laid out new preliminary recommendations of the AGS and the National Lipid Association on assessing risk and deciding on treatment.
The group concluded that LDL cholesterol levels are associated with incident arteriosclerotic cardiovascular disease (ASCVD), that the coronary artery calcium (CAC) score can be a valuable measure, and that statins may be reasonable to prescribe, even given the risks that have been linked to statins, such as that for muscle pain. Final recommendations are expected by fall 2023.
“This is still a work in progress,” said Daniel E. Forman, MD, professor of medicine and chair of geriatric cardiology at the University of Pittsburgh.
The AGS-NLA panel concluded that, for those aged 75 or older without established ASCVD, LDL cholesterol is associated with incident ASCVD, the only recommendation to be given a class I (strong) rating; others were classed as moderate or weak.
Dr. Forman reviewed the evidence for lowering LDL cholesterol to decrease ASCVD, citing a 2018 study that concluded, “Reverse causation may contribute to the association of lower TC with higher mortality in nonrandomized studies.”
However, research overall shows that, as LDL cholesterol levels increase, patients are more likely to experience a heart event.
Dr. Forman noted that the utility of equations for assessing 5- or 10-year risk of ASCVD is uncertain. However, he said, traditional risk factors, such as family history and ethnicity, still have value.
Assessing risk “has been enriched in the past few years by the introduction of the coronary artery calcium [CAC] score,” he said.
Lower scores predict lower rates of CVD events, Forman said. The AGS-NLA recommends measuring CAC if clinical uncertainty exists about the value of statins.
“It’s reasonable to measure CAC and to withhold statins when the CAC is zero,” Dr. Forman said. “When the CAC score is zero ... the risk of having a cardiovascular event is really next to nil. Patients are happy to know they have a CAC of zero.”
Likewise, patients appreciate knowing whether their score is high, which would indicate increased risk. He said the CAC score is underused by geriatric physicians.
The group also determined, after reviewing the research, that starting treatment is reasonable for patients with an LDL cholesterol level of 70-189 if they have no life-limiting illness and their life expectancy is over 5 years.
Other preliminary recommendations include the use of statins for those aged 75 and older, irrespective of risk for statin-associated muscle symptoms, type 2 diabetes, or impaired cognition. These associations are often weak, Dr. Forman added.
Focusing on person-centered decisions
Ariel Green, MD, MPH, PhD, associate professor of medicine at Johns Hopkins University, Baltimore, said statin therapy “should be individualized” to weigh benefits, noncardiac risks, and other considerations.
Clinicians can incorporate life expectancy into prevention decisions using tools such as ePrognosis, from the University of California, San Francisco, Dr. Green said.
If life expectancy is greater than the time to benefit, statin therapy may help. Dr. Green cited research that showed that 2.5 years of statin therapy was needed to prevent one major adverse cardiovascular event (MACE) per 100 patients in a population aged 50-75. Other data show reductions in MACE for those older than 75, but overall, the data are limited in this population.
The proposed recommendation is to use tools such as life tables that include comorbid conditions and functional status to guide clinical decisions.
“Another aspect of assessing net benefits of statin therapy is to consider competing health risks,” Dr. Green said.
The group recommends considering using competing risk-adjusted CVD models, though these are not widely used.
The group also recommends integrating screenings for frailty (Clinical Frailty Scale), dementia (Mini-Cog), and functional status (Vulnerable Elders Scale–13) into assessments.
“The presence of these syndromes should prompt elicitation of patient values and preferences related to prevention and medication use,” Dr. Green said.
Clinicians can use decision aids, but these are not always practical, owing to obstacles such as patients’ cognitive problems, Dr. Green said.
“Another approach is asking people to prioritize a set of universal health outcomes that apply across health conditions, such as maintaining independence, staying alive, reducing, or eliminating symptoms and focusing on comfort,” Dr. Green said.
She addressed the evidence about deprescribing statins, with a focus on those with a life expectancy of less than a year. Researchers have found an increase in quality of life and no increases in cardiovascular events or death when statins were deprescribed.
A welcome framework
Cory Krueger, MD, an internal medicine and geriatric physician in Cornville, Ariz., who attended the talk, said he welcomed the presentation, in which preliminary recommendations were explained.
“This has been a controversial area in geriatrics,” Dr. Krueger said. “At least this gave me a framework for discussing this with my patients in a reasonable way.”
Dr. Forman and Dr. Krueger disclosed no relevant financial relationships. Dr. Green receives funding from the National Institute of Aging and Impact Collaboratory.
A version of this article first appeared on Medscape.com.
AT AGS 2023
Can this tool forecast peanut allergies?
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PAS 2023
Two phase 3 trials show benefits of dupilumab for prurigo nodularis
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
FROM NATURE MEDICINE
Bundled strategy increased preteen lipid screening
WASHINGTON – A bundled intervention combining point-of-care testing, electronic medical record support, and provider education significantly improved lipid screening rates in children aged 9-11 years, according to data from approximately 100 monthly visits over a 3-year period.
Guidelines from the National Heart, Lung, and Blood Institute currently recommend universal lipid screening for children aged 9-11 years, but screening rates in clinical practice remain low, according to Ruth E. Gardner, MD, of Penn State University, Hershey, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, Dr. Gardner and colleagues shared results of the implementation of a bundled testing protocol designed to improve screening.
The researchers reviewed data on lipid testing within 30 days for all 9- to 11-year-old well child visits at a single center between May 2019 and February 2022. The bundled intervention was introduced in May 2021.
The bundled protocol included in-office capillary testing and provider education. In addition, electronic medical record templates were modified to include prompts for lipid screening at relevant ages, and EMR orders were adjusted to include lipid testing. The researchers also collected targeted provider feedback on individualized screening rates in February 2022.
Screening rates were plotted monthly. For the period from May 2019 through May 2021, the rates averaged 6.5%. However, after the introduction of the bundled intervention, the rate increased to 29.9%. Following targeted provider feedback in February 2022, the researchers found an additional shift to 52.1% through March and April 2022.
The findings were limited by the use of data from a single center, and the researchers used an extended study period to account for disruptions to well-child care in the spring of 2020 related to the COVID-19 pandemic.
However, the results support the effectiveness of a bundled intervention for improving lipid screening rates in children aged 9-11 years, the researchers said, and targeted provider feedback and education could yield additional improvements, they concluded.
Preteen years are an optimal time for screening
“The current study is important because atherosclerosis begins in childhood, and screening at ages 9-11 is an optimal time to begin lifestyle changes to improve overall health and reduce risks of heart disease,” said Margaret Thew, DNP, FNP-BC, of the Medical College of Wisconsin, Milwaukee, in an interview.
Ms. Thew, who was not involved in the study, said, “The number of recommended and required screening items needed in pediatrics is vast, so many providers have to select which items to focus on for their health screenings with these ages.”
Overall, “I was impressed with the improvements that were made in this quality improvement study,” said Ms. Thew.
Barriers to lipid screening in this population include the reduced number of health screenings and immunizations recommended for this age group; the consequence is that access is limited to discuss preventive care opportunities, said Ms. Thew in an interview. Steps to overcome these barriers could include the use of many of the screening tools introduced in the current study, such as point-of-care testing in the office, use of the EMR to remind providers of testing, which can be done during well visits or school physicals, and educating providers about the current guidelines, she noted.
Other strategies to increase screening include moving the immunization series to provide more frequent appointments to children aged 9-11 years to offer education and preventive care, Ms. Thew added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
WASHINGTON – A bundled intervention combining point-of-care testing, electronic medical record support, and provider education significantly improved lipid screening rates in children aged 9-11 years, according to data from approximately 100 monthly visits over a 3-year period.
Guidelines from the National Heart, Lung, and Blood Institute currently recommend universal lipid screening for children aged 9-11 years, but screening rates in clinical practice remain low, according to Ruth E. Gardner, MD, of Penn State University, Hershey, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, Dr. Gardner and colleagues shared results of the implementation of a bundled testing protocol designed to improve screening.
The researchers reviewed data on lipid testing within 30 days for all 9- to 11-year-old well child visits at a single center between May 2019 and February 2022. The bundled intervention was introduced in May 2021.
The bundled protocol included in-office capillary testing and provider education. In addition, electronic medical record templates were modified to include prompts for lipid screening at relevant ages, and EMR orders were adjusted to include lipid testing. The researchers also collected targeted provider feedback on individualized screening rates in February 2022.
Screening rates were plotted monthly. For the period from May 2019 through May 2021, the rates averaged 6.5%. However, after the introduction of the bundled intervention, the rate increased to 29.9%. Following targeted provider feedback in February 2022, the researchers found an additional shift to 52.1% through March and April 2022.
The findings were limited by the use of data from a single center, and the researchers used an extended study period to account for disruptions to well-child care in the spring of 2020 related to the COVID-19 pandemic.
However, the results support the effectiveness of a bundled intervention for improving lipid screening rates in children aged 9-11 years, the researchers said, and targeted provider feedback and education could yield additional improvements, they concluded.
Preteen years are an optimal time for screening
“The current study is important because atherosclerosis begins in childhood, and screening at ages 9-11 is an optimal time to begin lifestyle changes to improve overall health and reduce risks of heart disease,” said Margaret Thew, DNP, FNP-BC, of the Medical College of Wisconsin, Milwaukee, in an interview.
Ms. Thew, who was not involved in the study, said, “The number of recommended and required screening items needed in pediatrics is vast, so many providers have to select which items to focus on for their health screenings with these ages.”
Overall, “I was impressed with the improvements that were made in this quality improvement study,” said Ms. Thew.
Barriers to lipid screening in this population include the reduced number of health screenings and immunizations recommended for this age group; the consequence is that access is limited to discuss preventive care opportunities, said Ms. Thew in an interview. Steps to overcome these barriers could include the use of many of the screening tools introduced in the current study, such as point-of-care testing in the office, use of the EMR to remind providers of testing, which can be done during well visits or school physicals, and educating providers about the current guidelines, she noted.
Other strategies to increase screening include moving the immunization series to provide more frequent appointments to children aged 9-11 years to offer education and preventive care, Ms. Thew added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
WASHINGTON – A bundled intervention combining point-of-care testing, electronic medical record support, and provider education significantly improved lipid screening rates in children aged 9-11 years, according to data from approximately 100 monthly visits over a 3-year period.
Guidelines from the National Heart, Lung, and Blood Institute currently recommend universal lipid screening for children aged 9-11 years, but screening rates in clinical practice remain low, according to Ruth E. Gardner, MD, of Penn State University, Hershey, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, Dr. Gardner and colleagues shared results of the implementation of a bundled testing protocol designed to improve screening.
The researchers reviewed data on lipid testing within 30 days for all 9- to 11-year-old well child visits at a single center between May 2019 and February 2022. The bundled intervention was introduced in May 2021.
The bundled protocol included in-office capillary testing and provider education. In addition, electronic medical record templates were modified to include prompts for lipid screening at relevant ages, and EMR orders were adjusted to include lipid testing. The researchers also collected targeted provider feedback on individualized screening rates in February 2022.
Screening rates were plotted monthly. For the period from May 2019 through May 2021, the rates averaged 6.5%. However, after the introduction of the bundled intervention, the rate increased to 29.9%. Following targeted provider feedback in February 2022, the researchers found an additional shift to 52.1% through March and April 2022.
The findings were limited by the use of data from a single center, and the researchers used an extended study period to account for disruptions to well-child care in the spring of 2020 related to the COVID-19 pandemic.
However, the results support the effectiveness of a bundled intervention for improving lipid screening rates in children aged 9-11 years, the researchers said, and targeted provider feedback and education could yield additional improvements, they concluded.
Preteen years are an optimal time for screening
“The current study is important because atherosclerosis begins in childhood, and screening at ages 9-11 is an optimal time to begin lifestyle changes to improve overall health and reduce risks of heart disease,” said Margaret Thew, DNP, FNP-BC, of the Medical College of Wisconsin, Milwaukee, in an interview.
Ms. Thew, who was not involved in the study, said, “The number of recommended and required screening items needed in pediatrics is vast, so many providers have to select which items to focus on for their health screenings with these ages.”
Overall, “I was impressed with the improvements that were made in this quality improvement study,” said Ms. Thew.
Barriers to lipid screening in this population include the reduced number of health screenings and immunizations recommended for this age group; the consequence is that access is limited to discuss preventive care opportunities, said Ms. Thew in an interview. Steps to overcome these barriers could include the use of many of the screening tools introduced in the current study, such as point-of-care testing in the office, use of the EMR to remind providers of testing, which can be done during well visits or school physicals, and educating providers about the current guidelines, she noted.
Other strategies to increase screening include moving the immunization series to provide more frequent appointments to children aged 9-11 years to offer education and preventive care, Ms. Thew added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
FROM PAS 2023
Boys may carry the weight, or overweight, of adults’ infertility
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Itchy pustules over hair follicles
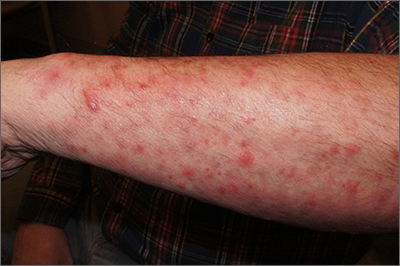
A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503

A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503