User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
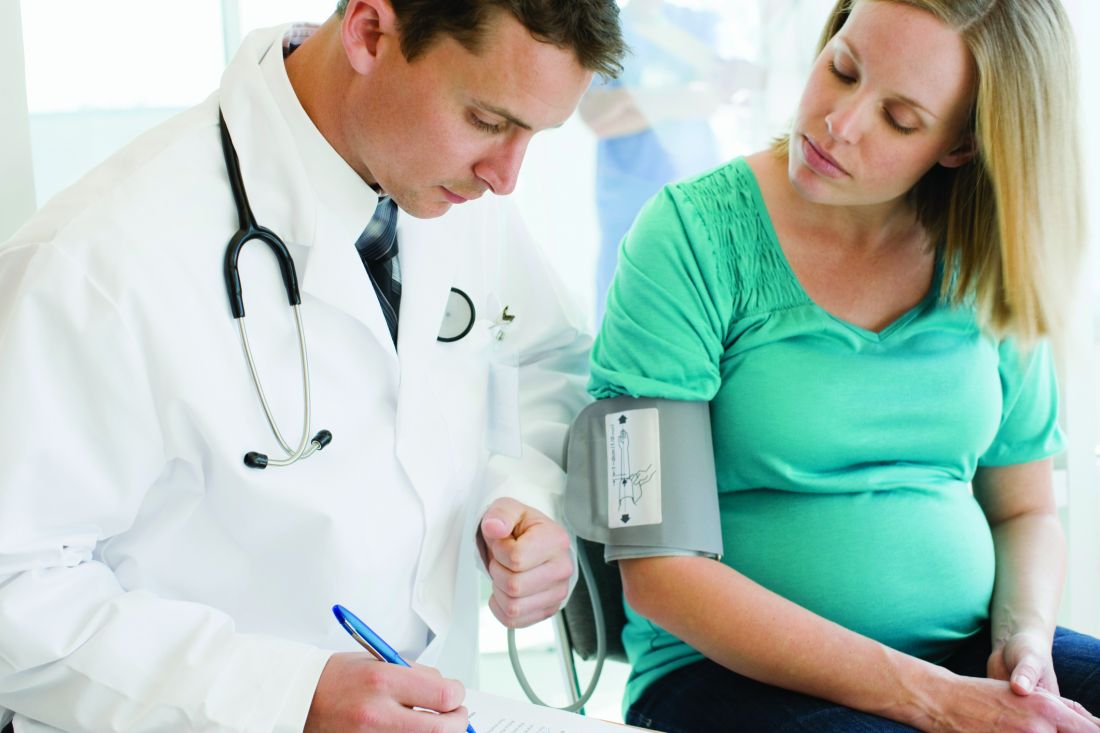
Gestational HTN, preeclampsia worsen long-term risk for ischemic, nonischemic heart failure
, an observational study suggests.
The risks were most pronounced, jumping more than sixfold in the case of ischemic HF, during the first 6 years after the pregnancy. They then receded to plateau at a lower, still significantly elevated level of risk that persisted even years later, in the analysis of women in a Swedish medical birth registry.
The case-matching study compared women with no history of cardiovascular (CV) disease and a first successful pregnancy during which they either developed or did not experience gestational hypertension or preeclampsia.
It’s among the first studies to explore the impact of pregnancy-induced hypertensive disease on subsequent HF risk separately for both ischemic and nonischemic HF and to find that the severity of such risk differs for the two HF etiologies, according to a report published in JACC: Heart Failure.
The adjusted risk for any HF during a median of 13 years after the pregnancy rose 70% for those who had developed gestational hypertension or preeclampsia. Their risk of nonischemic HF went up 60%, and their risk of ischemic HF more than doubled.
Hypertensive disorders of pregnancy “are so much more than short-term disorders confined to the pregnancy period. They have long-term implications throughout a lifetime,” lead author Ängla Mantel, MD, PhD, said in an interview.
Obstetric history doesn’t figure into any formal HF risk scoring systems, observed Dr. Mantel of Karolinska Institutet, Stockholm. Still, women who develop gestational hypertension, preeclampsia, or other pregnancy complications “should be considered a high-risk population even after the pregnancy and monitored for cardiovascular risk factors regularly throughout life.”
In many studies, she said, “knowledge of women-specific risk factors for cardiovascular disease is poor among both clinicians and patients.” The current findings should help raise awareness about such obstetric risk factors for HF, “especially” in patients with HF with preserved ejection fraction (HFpEF), which isn’t closely related to a number of traditional CV risk factors.
Even though pregnancy complications such as gestational hypertension and preeclampsia don’t feature in risk calculators, “they are actually risk enhancers per the 2019 primary prevention guidelines,” Natalie A. Bello, MD, MPH, who was not involved in the current study, said in an interview.
“We’re working to educate physicians and cardiovascular team members to take a pregnancy history” for risk stratification of women in primary prevention,” said Dr. Bello, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
The current study, she said, “is an important step” for its finding that hypertensive disorders of pregnancy are associated separately with both ischemic and nonischemic HF.
She pointed out, however, that because the study excluded women with peripartum cardiomyopathy, a form of nonischemic HF, it may “underestimate the impact of hypertensive disorders on the short-term risk of nonischemic heart failure.” Women who had peripartum cardiomyopathy were excluded to avoid misclassification of other HF outcomes, the authors stated.
Also, Dr. Bello said, the study’s inclusion of patients with either gestational hypertension or preeclampsia may complicate its interpretation. Compared with the former condition, she said, preeclampsia “involves more inflammation and more endothelial dysfunction. It may cause a different impact on the heart and the vasculature.”
In the analysis, about 79,000 women with gestational hypertension or preeclampsia were identified among more than 1.4 million primiparous women who entered the Swedish Medical Birth Register over a period of about 30 years. They were matched with about 396,000 women in the registry who had normotensive pregnancies.
Excluded, besides women with peripartum cardiomyopathy, were women with a prepregnancy history of HF, hypertension, ischemic heart disease, atrial fibrillation, or valvular heart disease.
Hazard ratios (HRs) for HF, ischemic HF, and nonischemic HF were significantly elevated over among the women with gestational hypertension or preeclampsia compared to those with normotensive pregnancies:
- Any HF: HR, 1.70 (95% confidence interval [CI], 1.51-1.91)
- Nonischemic HF: HR, 1.60 (95% CI, 1.40-1.83)
- Ischemic HF: HR, 2.28 (95% CI, 1.74-2.98)
The analyses were adjusted for maternal age at delivery, year of delivery, prepregnancy comorbidities, maternal education level, smoking status, and body mass index.
Sharper risk increases were seen among women with gestational hypertension or preeclampsia who delivered prior to gestational week 34:
- Any HF: HR, 2.46 (95% CI, 1.82-3.32)
- Nonischemic HF: HR, 2.33 (95% CI, 1.65-3.31)
- Ischemic HF: HR, 3.64 (95% CI, 1.97-6.74)
Risks for HF developing within 6 years of pregnancy characterized by gestational hypertension or preeclampsia were far more pronounced for ischemic HF than for nonischemic HF:
- Any HF: HR, 2.09 (95% CI, 1.52-2.89)
- Nonischemic HF: HR, 1.86 (95% CI, 1.32-2.61)
- Ischemic HF: HR, 6.52 (95% CI, 2.00-12.34).
The study couldn’t directly explore potential mechanisms for the associations between pregnancy-induced hypertensive disorders and different forms of HF, but it may have provided clues, Dr. Mantel said.
The hypertensive disorders and ischemic HF appear to share risk factors that could lead to both conditions, she noted. Also, hypertension itself is a risk factor for ischemic heart disease.
In contrast, “the risk of nonischemic heart failure might be driven by other factors, such as the inflammatory profile, endothelial dysfunction, and cardiac remodeling induced by preeclampsia or gestational hypertension.”
Those disorders, moreover, are associated with cardiac structural changes that are also seen in HFpEF, Dr. Mantel said. And both HFpEF and preeclampsia are characterized by systemic inflammation and endothelial dysfunction.
“These pathophysiological similarities,” she proposed, “might explain the link between pregnancy-induced hypertensive disorder and HFpEF.”
The authors have disclosed no relevant financial relationships. Dr. Bello has received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, an observational study suggests.
The risks were most pronounced, jumping more than sixfold in the case of ischemic HF, during the first 6 years after the pregnancy. They then receded to plateau at a lower, still significantly elevated level of risk that persisted even years later, in the analysis of women in a Swedish medical birth registry.
The case-matching study compared women with no history of cardiovascular (CV) disease and a first successful pregnancy during which they either developed or did not experience gestational hypertension or preeclampsia.
It’s among the first studies to explore the impact of pregnancy-induced hypertensive disease on subsequent HF risk separately for both ischemic and nonischemic HF and to find that the severity of such risk differs for the two HF etiologies, according to a report published in JACC: Heart Failure.
The adjusted risk for any HF during a median of 13 years after the pregnancy rose 70% for those who had developed gestational hypertension or preeclampsia. Their risk of nonischemic HF went up 60%, and their risk of ischemic HF more than doubled.
Hypertensive disorders of pregnancy “are so much more than short-term disorders confined to the pregnancy period. They have long-term implications throughout a lifetime,” lead author Ängla Mantel, MD, PhD, said in an interview.
Obstetric history doesn’t figure into any formal HF risk scoring systems, observed Dr. Mantel of Karolinska Institutet, Stockholm. Still, women who develop gestational hypertension, preeclampsia, or other pregnancy complications “should be considered a high-risk population even after the pregnancy and monitored for cardiovascular risk factors regularly throughout life.”
In many studies, she said, “knowledge of women-specific risk factors for cardiovascular disease is poor among both clinicians and patients.” The current findings should help raise awareness about such obstetric risk factors for HF, “especially” in patients with HF with preserved ejection fraction (HFpEF), which isn’t closely related to a number of traditional CV risk factors.
Even though pregnancy complications such as gestational hypertension and preeclampsia don’t feature in risk calculators, “they are actually risk enhancers per the 2019 primary prevention guidelines,” Natalie A. Bello, MD, MPH, who was not involved in the current study, said in an interview.
“We’re working to educate physicians and cardiovascular team members to take a pregnancy history” for risk stratification of women in primary prevention,” said Dr. Bello, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
The current study, she said, “is an important step” for its finding that hypertensive disorders of pregnancy are associated separately with both ischemic and nonischemic HF.
She pointed out, however, that because the study excluded women with peripartum cardiomyopathy, a form of nonischemic HF, it may “underestimate the impact of hypertensive disorders on the short-term risk of nonischemic heart failure.” Women who had peripartum cardiomyopathy were excluded to avoid misclassification of other HF outcomes, the authors stated.
Also, Dr. Bello said, the study’s inclusion of patients with either gestational hypertension or preeclampsia may complicate its interpretation. Compared with the former condition, she said, preeclampsia “involves more inflammation and more endothelial dysfunction. It may cause a different impact on the heart and the vasculature.”
In the analysis, about 79,000 women with gestational hypertension or preeclampsia were identified among more than 1.4 million primiparous women who entered the Swedish Medical Birth Register over a period of about 30 years. They were matched with about 396,000 women in the registry who had normotensive pregnancies.
Excluded, besides women with peripartum cardiomyopathy, were women with a prepregnancy history of HF, hypertension, ischemic heart disease, atrial fibrillation, or valvular heart disease.
Hazard ratios (HRs) for HF, ischemic HF, and nonischemic HF were significantly elevated over among the women with gestational hypertension or preeclampsia compared to those with normotensive pregnancies:
- Any HF: HR, 1.70 (95% confidence interval [CI], 1.51-1.91)
- Nonischemic HF: HR, 1.60 (95% CI, 1.40-1.83)
- Ischemic HF: HR, 2.28 (95% CI, 1.74-2.98)
The analyses were adjusted for maternal age at delivery, year of delivery, prepregnancy comorbidities, maternal education level, smoking status, and body mass index.
Sharper risk increases were seen among women with gestational hypertension or preeclampsia who delivered prior to gestational week 34:
- Any HF: HR, 2.46 (95% CI, 1.82-3.32)
- Nonischemic HF: HR, 2.33 (95% CI, 1.65-3.31)
- Ischemic HF: HR, 3.64 (95% CI, 1.97-6.74)
Risks for HF developing within 6 years of pregnancy characterized by gestational hypertension or preeclampsia were far more pronounced for ischemic HF than for nonischemic HF:
- Any HF: HR, 2.09 (95% CI, 1.52-2.89)
- Nonischemic HF: HR, 1.86 (95% CI, 1.32-2.61)
- Ischemic HF: HR, 6.52 (95% CI, 2.00-12.34).
The study couldn’t directly explore potential mechanisms for the associations between pregnancy-induced hypertensive disorders and different forms of HF, but it may have provided clues, Dr. Mantel said.
The hypertensive disorders and ischemic HF appear to share risk factors that could lead to both conditions, she noted. Also, hypertension itself is a risk factor for ischemic heart disease.
In contrast, “the risk of nonischemic heart failure might be driven by other factors, such as the inflammatory profile, endothelial dysfunction, and cardiac remodeling induced by preeclampsia or gestational hypertension.”
Those disorders, moreover, are associated with cardiac structural changes that are also seen in HFpEF, Dr. Mantel said. And both HFpEF and preeclampsia are characterized by systemic inflammation and endothelial dysfunction.
“These pathophysiological similarities,” she proposed, “might explain the link between pregnancy-induced hypertensive disorder and HFpEF.”
The authors have disclosed no relevant financial relationships. Dr. Bello has received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, an observational study suggests.
The risks were most pronounced, jumping more than sixfold in the case of ischemic HF, during the first 6 years after the pregnancy. They then receded to plateau at a lower, still significantly elevated level of risk that persisted even years later, in the analysis of women in a Swedish medical birth registry.
The case-matching study compared women with no history of cardiovascular (CV) disease and a first successful pregnancy during which they either developed or did not experience gestational hypertension or preeclampsia.
It’s among the first studies to explore the impact of pregnancy-induced hypertensive disease on subsequent HF risk separately for both ischemic and nonischemic HF and to find that the severity of such risk differs for the two HF etiologies, according to a report published in JACC: Heart Failure.
The adjusted risk for any HF during a median of 13 years after the pregnancy rose 70% for those who had developed gestational hypertension or preeclampsia. Their risk of nonischemic HF went up 60%, and their risk of ischemic HF more than doubled.
Hypertensive disorders of pregnancy “are so much more than short-term disorders confined to the pregnancy period. They have long-term implications throughout a lifetime,” lead author Ängla Mantel, MD, PhD, said in an interview.
Obstetric history doesn’t figure into any formal HF risk scoring systems, observed Dr. Mantel of Karolinska Institutet, Stockholm. Still, women who develop gestational hypertension, preeclampsia, or other pregnancy complications “should be considered a high-risk population even after the pregnancy and monitored for cardiovascular risk factors regularly throughout life.”
In many studies, she said, “knowledge of women-specific risk factors for cardiovascular disease is poor among both clinicians and patients.” The current findings should help raise awareness about such obstetric risk factors for HF, “especially” in patients with HF with preserved ejection fraction (HFpEF), which isn’t closely related to a number of traditional CV risk factors.
Even though pregnancy complications such as gestational hypertension and preeclampsia don’t feature in risk calculators, “they are actually risk enhancers per the 2019 primary prevention guidelines,” Natalie A. Bello, MD, MPH, who was not involved in the current study, said in an interview.
“We’re working to educate physicians and cardiovascular team members to take a pregnancy history” for risk stratification of women in primary prevention,” said Dr. Bello, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
The current study, she said, “is an important step” for its finding that hypertensive disorders of pregnancy are associated separately with both ischemic and nonischemic HF.
She pointed out, however, that because the study excluded women with peripartum cardiomyopathy, a form of nonischemic HF, it may “underestimate the impact of hypertensive disorders on the short-term risk of nonischemic heart failure.” Women who had peripartum cardiomyopathy were excluded to avoid misclassification of other HF outcomes, the authors stated.
Also, Dr. Bello said, the study’s inclusion of patients with either gestational hypertension or preeclampsia may complicate its interpretation. Compared with the former condition, she said, preeclampsia “involves more inflammation and more endothelial dysfunction. It may cause a different impact on the heart and the vasculature.”
In the analysis, about 79,000 women with gestational hypertension or preeclampsia were identified among more than 1.4 million primiparous women who entered the Swedish Medical Birth Register over a period of about 30 years. They were matched with about 396,000 women in the registry who had normotensive pregnancies.
Excluded, besides women with peripartum cardiomyopathy, were women with a prepregnancy history of HF, hypertension, ischemic heart disease, atrial fibrillation, or valvular heart disease.
Hazard ratios (HRs) for HF, ischemic HF, and nonischemic HF were significantly elevated over among the women with gestational hypertension or preeclampsia compared to those with normotensive pregnancies:
- Any HF: HR, 1.70 (95% confidence interval [CI], 1.51-1.91)
- Nonischemic HF: HR, 1.60 (95% CI, 1.40-1.83)
- Ischemic HF: HR, 2.28 (95% CI, 1.74-2.98)
The analyses were adjusted for maternal age at delivery, year of delivery, prepregnancy comorbidities, maternal education level, smoking status, and body mass index.
Sharper risk increases were seen among women with gestational hypertension or preeclampsia who delivered prior to gestational week 34:
- Any HF: HR, 2.46 (95% CI, 1.82-3.32)
- Nonischemic HF: HR, 2.33 (95% CI, 1.65-3.31)
- Ischemic HF: HR, 3.64 (95% CI, 1.97-6.74)
Risks for HF developing within 6 years of pregnancy characterized by gestational hypertension or preeclampsia were far more pronounced for ischemic HF than for nonischemic HF:
- Any HF: HR, 2.09 (95% CI, 1.52-2.89)
- Nonischemic HF: HR, 1.86 (95% CI, 1.32-2.61)
- Ischemic HF: HR, 6.52 (95% CI, 2.00-12.34).
The study couldn’t directly explore potential mechanisms for the associations between pregnancy-induced hypertensive disorders and different forms of HF, but it may have provided clues, Dr. Mantel said.
The hypertensive disorders and ischemic HF appear to share risk factors that could lead to both conditions, she noted. Also, hypertension itself is a risk factor for ischemic heart disease.
In contrast, “the risk of nonischemic heart failure might be driven by other factors, such as the inflammatory profile, endothelial dysfunction, and cardiac remodeling induced by preeclampsia or gestational hypertension.”
Those disorders, moreover, are associated with cardiac structural changes that are also seen in HFpEF, Dr. Mantel said. And both HFpEF and preeclampsia are characterized by systemic inflammation and endothelial dysfunction.
“These pathophysiological similarities,” she proposed, “might explain the link between pregnancy-induced hypertensive disorder and HFpEF.”
The authors have disclosed no relevant financial relationships. Dr. Bello has received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM JACC: HEART FAILURE
Could vitamin D supplementation help in long COVID?
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECE 2023
Nonpharmacologic therapies for T2D: Five things to know
According to the Centers for Disease Control and Prevention National Diabetes Statistic Report, there are more than 37 million adults aged 18 years or older with diabetes in the United States, representing 14.7% of the adult population. Approximately 90%-95% of people diagnosed with diabetes have type 2 diabetes (T2D). An increasing aging population with T2D and a disparate incidence and burden of disease in African American and Hispanic populations raise important care considerations in effective disease assessment and management, especially in primary care, where the majority of diabetes management occurs.
This extends to the need for quality patient education in an effort to give persons with diabetes a better understanding of what it’s like to live with the disease.
Here are five things to know about nonpharmacologic therapies for effective T2D management.
1. Understand and treat the person before the disease.
Diabetes is a complex and unrelenting disease of self-management, requiring an individualized care approach to achieve optimal health outcomes and quality of life for persons living with this condition. Over 90% of care is provided by the person with diabetes, therefore understanding the lived world of the person with diabetes and its connected impact on self-care is critical to establishing effective treatment recommendations, especially for people from racial and ethnic minority groups and lower socioeconomic status where diabetes disparities are highest. Disease prevalence, cost of care, and disease burden are driven by social determinants of health (SDOH) factors that need to be assessed, and strategies addressing causative factors need to be implemented. SDOH factors, including the built environment, safety, financial status, education, food access, health care access, and social support, directly affect the ability of a person with diabetes to effectively implement treatment recommendations, including access to new medications. The adoption of a shared decision-making approach is key to person-centered care. Shared decision-making promotes a positive communication feedback loop, therapeutic patient-care team relationship, and collaborative plan of care between the person with diabetes and the care team. It also supports the establishment of mutual respect between the person with diabetes and the care team members. This cultivates the strong, open, and authentic partnership needed for effective chronic disease management.
2. Quality diabetes education is the foundation for effective self-care.
Diabetes self-management education and support (DSMES) is a fundamental component of diabetes care and ensures patients have the knowledge, skills, motivation, and resources necessary for effectively managing this condition. Despite treatment advances and the evidence base for DSMES, less than 5% of Medicare beneficiaries and 6.8% of privately insured beneficiaries have utilized its services, and this is a likely contributor to the lack of improvement for achieving national diabetes clinical targets. The Association of Diabetes Care and Education Specialists (ADCES7) Self-Care Behaviors provides an evidence-based framework for an optimal DSMES curriculum, incorporating the self-care behaviors of healthy coping (e.g., having a positive attitude toward diabetes self-management), nutritious eating, being active, taking medication, monitoring, reducing risk, and problem-solving.
There are four core times to implement and adapt referral for DSMES: (1) at diagnosis, (2) annually or when not meeting targets, (3) when complications arise, and (4) with transitions in life and care. DSMES referrals should be made for programs accredited by the ADCES or American Diabetes Association (ADA) and led by expert Certified Diabetes Care and Education Specialists (CDCES). The multidisciplinary composition and clinical skill level of CDCES make them a highly valued member of the diabetes care team. CDCES have demonstrated not only diabetes education expertise but are involved in broader health care roles to include population health management, technology integration, mitigation of therapeutic inertia, quality improvement activity, and delivery of cost-effective care.
3. Establish a strong foundation in lifestyle medicine.
Lifestyle medicine encompasses healthy eating, physical activity, restorative sleep, stress management, avoidance of risky behaviors, and positive social connections. It has also been strongly connected as a primary modality to prevent and treat chronic conditions like T2D. Lifestyle modifications have been noted in reducing the incidence of developing diabetes, reversing disease, improving clinical markers such as A1c and lipids, weight reduction, reducing use of medications, and improving quality of life. The multidisciplinary care team and CDCES can support the empowerment of individuals with T2D to develop the life skills and knowledge needed to establish positive self-care behaviors and successfully achieve health goals. Lifestyle medicine is not a replacement for pharmacologic interventions but rather serves as an adjunct when medication management is required.
4. Harness technology in diabetes treatment and care delivery.
Diabetes technology is advancing swiftly and includes glucose monitors, medication delivery devices, data-sharing platforms, and disease self-management applications. Combined with education and support, diabetes technology has been shown to have a positive clinical and personal impact on disease outcomes and quality of life. Regardless of its benefits, at times technology can seem overwhelming for the person with diabetes and the care team. Diabetes Care and Education Specialists (DCES) can support the care team and people living with diabetes to effectively identify, implement, and evaluate patient-centered diabetes technologies, as well as implement processes to drive clinical efficiencies and sustainability. Patient-generated health data reports can provide the care team with effective and proficient evaluation of diabetes care and needed treatment changes.
The expansion of telehealth during the COVID-19 pandemic, including real-time and asynchronous approaches, coupled with in-person care team visits, has resulted in improved access to diabetes care and education. Moreover, there continues to be an expanding health system focus on improving access to care beyond traditional brick and mortar solutions. Telehealth poses one possible access solution for people living with diabetes for whom factors such as transportation, remote geographies, and physical limitations affect their ability to attend in-person care visits.
5. Assess and address diabetes-related distress.
The persistent nature of diabetes self-care expectations and the impact on lifestyle behaviors, medication adherence, and glycemic control demands the need for assessment and treatment of diabetes-related distress (DRD). DRD can be expressed as shame, guilt, anger, fear, and frustration in combination with the everyday context of life priorities and stressors. An assessment of diabetes distress, utilizing a simple scale, should be included as part of an annual therapeutic diabetes care plan. The ADA Standards of Care in Diabetes recommends assessing patients’ psychological and social situations as an ongoing part of medical management, including an annual screening for depression and other psychological problems. The prevalence of depression is nearly twice as high in people with T2D than in the general population and can significantly influence patients’ ability to self-manage their diabetes and achieve healthy outcomes. Assessment and treatment of psychosocial components of care can result in significant improvements in A1c and other positive outcomes, including quality of life.
Kellie M. Rodriguez, director of the global diabetes program at Parkland Health, Dallas, Tex., disclosed ties with the Association of Diabetes Care and Education Specialists.
A version of this article originally appeared on Medscape.com.
According to the Centers for Disease Control and Prevention National Diabetes Statistic Report, there are more than 37 million adults aged 18 years or older with diabetes in the United States, representing 14.7% of the adult population. Approximately 90%-95% of people diagnosed with diabetes have type 2 diabetes (T2D). An increasing aging population with T2D and a disparate incidence and burden of disease in African American and Hispanic populations raise important care considerations in effective disease assessment and management, especially in primary care, where the majority of diabetes management occurs.
This extends to the need for quality patient education in an effort to give persons with diabetes a better understanding of what it’s like to live with the disease.
Here are five things to know about nonpharmacologic therapies for effective T2D management.
1. Understand and treat the person before the disease.
Diabetes is a complex and unrelenting disease of self-management, requiring an individualized care approach to achieve optimal health outcomes and quality of life for persons living with this condition. Over 90% of care is provided by the person with diabetes, therefore understanding the lived world of the person with diabetes and its connected impact on self-care is critical to establishing effective treatment recommendations, especially for people from racial and ethnic minority groups and lower socioeconomic status where diabetes disparities are highest. Disease prevalence, cost of care, and disease burden are driven by social determinants of health (SDOH) factors that need to be assessed, and strategies addressing causative factors need to be implemented. SDOH factors, including the built environment, safety, financial status, education, food access, health care access, and social support, directly affect the ability of a person with diabetes to effectively implement treatment recommendations, including access to new medications. The adoption of a shared decision-making approach is key to person-centered care. Shared decision-making promotes a positive communication feedback loop, therapeutic patient-care team relationship, and collaborative plan of care between the person with diabetes and the care team. It also supports the establishment of mutual respect between the person with diabetes and the care team members. This cultivates the strong, open, and authentic partnership needed for effective chronic disease management.
2. Quality diabetes education is the foundation for effective self-care.
Diabetes self-management education and support (DSMES) is a fundamental component of diabetes care and ensures patients have the knowledge, skills, motivation, and resources necessary for effectively managing this condition. Despite treatment advances and the evidence base for DSMES, less than 5% of Medicare beneficiaries and 6.8% of privately insured beneficiaries have utilized its services, and this is a likely contributor to the lack of improvement for achieving national diabetes clinical targets. The Association of Diabetes Care and Education Specialists (ADCES7) Self-Care Behaviors provides an evidence-based framework for an optimal DSMES curriculum, incorporating the self-care behaviors of healthy coping (e.g., having a positive attitude toward diabetes self-management), nutritious eating, being active, taking medication, monitoring, reducing risk, and problem-solving.
There are four core times to implement and adapt referral for DSMES: (1) at diagnosis, (2) annually or when not meeting targets, (3) when complications arise, and (4) with transitions in life and care. DSMES referrals should be made for programs accredited by the ADCES or American Diabetes Association (ADA) and led by expert Certified Diabetes Care and Education Specialists (CDCES). The multidisciplinary composition and clinical skill level of CDCES make them a highly valued member of the diabetes care team. CDCES have demonstrated not only diabetes education expertise but are involved in broader health care roles to include population health management, technology integration, mitigation of therapeutic inertia, quality improvement activity, and delivery of cost-effective care.
3. Establish a strong foundation in lifestyle medicine.
Lifestyle medicine encompasses healthy eating, physical activity, restorative sleep, stress management, avoidance of risky behaviors, and positive social connections. It has also been strongly connected as a primary modality to prevent and treat chronic conditions like T2D. Lifestyle modifications have been noted in reducing the incidence of developing diabetes, reversing disease, improving clinical markers such as A1c and lipids, weight reduction, reducing use of medications, and improving quality of life. The multidisciplinary care team and CDCES can support the empowerment of individuals with T2D to develop the life skills and knowledge needed to establish positive self-care behaviors and successfully achieve health goals. Lifestyle medicine is not a replacement for pharmacologic interventions but rather serves as an adjunct when medication management is required.
4. Harness technology in diabetes treatment and care delivery.
Diabetes technology is advancing swiftly and includes glucose monitors, medication delivery devices, data-sharing platforms, and disease self-management applications. Combined with education and support, diabetes technology has been shown to have a positive clinical and personal impact on disease outcomes and quality of life. Regardless of its benefits, at times technology can seem overwhelming for the person with diabetes and the care team. Diabetes Care and Education Specialists (DCES) can support the care team and people living with diabetes to effectively identify, implement, and evaluate patient-centered diabetes technologies, as well as implement processes to drive clinical efficiencies and sustainability. Patient-generated health data reports can provide the care team with effective and proficient evaluation of diabetes care and needed treatment changes.
The expansion of telehealth during the COVID-19 pandemic, including real-time and asynchronous approaches, coupled with in-person care team visits, has resulted in improved access to diabetes care and education. Moreover, there continues to be an expanding health system focus on improving access to care beyond traditional brick and mortar solutions. Telehealth poses one possible access solution for people living with diabetes for whom factors such as transportation, remote geographies, and physical limitations affect their ability to attend in-person care visits.
5. Assess and address diabetes-related distress.
The persistent nature of diabetes self-care expectations and the impact on lifestyle behaviors, medication adherence, and glycemic control demands the need for assessment and treatment of diabetes-related distress (DRD). DRD can be expressed as shame, guilt, anger, fear, and frustration in combination with the everyday context of life priorities and stressors. An assessment of diabetes distress, utilizing a simple scale, should be included as part of an annual therapeutic diabetes care plan. The ADA Standards of Care in Diabetes recommends assessing patients’ psychological and social situations as an ongoing part of medical management, including an annual screening for depression and other psychological problems. The prevalence of depression is nearly twice as high in people with T2D than in the general population and can significantly influence patients’ ability to self-manage their diabetes and achieve healthy outcomes. Assessment and treatment of psychosocial components of care can result in significant improvements in A1c and other positive outcomes, including quality of life.
Kellie M. Rodriguez, director of the global diabetes program at Parkland Health, Dallas, Tex., disclosed ties with the Association of Diabetes Care and Education Specialists.
A version of this article originally appeared on Medscape.com.
According to the Centers for Disease Control and Prevention National Diabetes Statistic Report, there are more than 37 million adults aged 18 years or older with diabetes in the United States, representing 14.7% of the adult population. Approximately 90%-95% of people diagnosed with diabetes have type 2 diabetes (T2D). An increasing aging population with T2D and a disparate incidence and burden of disease in African American and Hispanic populations raise important care considerations in effective disease assessment and management, especially in primary care, where the majority of diabetes management occurs.
This extends to the need for quality patient education in an effort to give persons with diabetes a better understanding of what it’s like to live with the disease.
Here are five things to know about nonpharmacologic therapies for effective T2D management.
1. Understand and treat the person before the disease.
Diabetes is a complex and unrelenting disease of self-management, requiring an individualized care approach to achieve optimal health outcomes and quality of life for persons living with this condition. Over 90% of care is provided by the person with diabetes, therefore understanding the lived world of the person with diabetes and its connected impact on self-care is critical to establishing effective treatment recommendations, especially for people from racial and ethnic minority groups and lower socioeconomic status where diabetes disparities are highest. Disease prevalence, cost of care, and disease burden are driven by social determinants of health (SDOH) factors that need to be assessed, and strategies addressing causative factors need to be implemented. SDOH factors, including the built environment, safety, financial status, education, food access, health care access, and social support, directly affect the ability of a person with diabetes to effectively implement treatment recommendations, including access to new medications. The adoption of a shared decision-making approach is key to person-centered care. Shared decision-making promotes a positive communication feedback loop, therapeutic patient-care team relationship, and collaborative plan of care between the person with diabetes and the care team. It also supports the establishment of mutual respect between the person with diabetes and the care team members. This cultivates the strong, open, and authentic partnership needed for effective chronic disease management.
2. Quality diabetes education is the foundation for effective self-care.
Diabetes self-management education and support (DSMES) is a fundamental component of diabetes care and ensures patients have the knowledge, skills, motivation, and resources necessary for effectively managing this condition. Despite treatment advances and the evidence base for DSMES, less than 5% of Medicare beneficiaries and 6.8% of privately insured beneficiaries have utilized its services, and this is a likely contributor to the lack of improvement for achieving national diabetes clinical targets. The Association of Diabetes Care and Education Specialists (ADCES7) Self-Care Behaviors provides an evidence-based framework for an optimal DSMES curriculum, incorporating the self-care behaviors of healthy coping (e.g., having a positive attitude toward diabetes self-management), nutritious eating, being active, taking medication, monitoring, reducing risk, and problem-solving.
There are four core times to implement and adapt referral for DSMES: (1) at diagnosis, (2) annually or when not meeting targets, (3) when complications arise, and (4) with transitions in life and care. DSMES referrals should be made for programs accredited by the ADCES or American Diabetes Association (ADA) and led by expert Certified Diabetes Care and Education Specialists (CDCES). The multidisciplinary composition and clinical skill level of CDCES make them a highly valued member of the diabetes care team. CDCES have demonstrated not only diabetes education expertise but are involved in broader health care roles to include population health management, technology integration, mitigation of therapeutic inertia, quality improvement activity, and delivery of cost-effective care.
3. Establish a strong foundation in lifestyle medicine.
Lifestyle medicine encompasses healthy eating, physical activity, restorative sleep, stress management, avoidance of risky behaviors, and positive social connections. It has also been strongly connected as a primary modality to prevent and treat chronic conditions like T2D. Lifestyle modifications have been noted in reducing the incidence of developing diabetes, reversing disease, improving clinical markers such as A1c and lipids, weight reduction, reducing use of medications, and improving quality of life. The multidisciplinary care team and CDCES can support the empowerment of individuals with T2D to develop the life skills and knowledge needed to establish positive self-care behaviors and successfully achieve health goals. Lifestyle medicine is not a replacement for pharmacologic interventions but rather serves as an adjunct when medication management is required.
4. Harness technology in diabetes treatment and care delivery.
Diabetes technology is advancing swiftly and includes glucose monitors, medication delivery devices, data-sharing platforms, and disease self-management applications. Combined with education and support, diabetes technology has been shown to have a positive clinical and personal impact on disease outcomes and quality of life. Regardless of its benefits, at times technology can seem overwhelming for the person with diabetes and the care team. Diabetes Care and Education Specialists (DCES) can support the care team and people living with diabetes to effectively identify, implement, and evaluate patient-centered diabetes technologies, as well as implement processes to drive clinical efficiencies and sustainability. Patient-generated health data reports can provide the care team with effective and proficient evaluation of diabetes care and needed treatment changes.
The expansion of telehealth during the COVID-19 pandemic, including real-time and asynchronous approaches, coupled with in-person care team visits, has resulted in improved access to diabetes care and education. Moreover, there continues to be an expanding health system focus on improving access to care beyond traditional brick and mortar solutions. Telehealth poses one possible access solution for people living with diabetes for whom factors such as transportation, remote geographies, and physical limitations affect their ability to attend in-person care visits.
5. Assess and address diabetes-related distress.
The persistent nature of diabetes self-care expectations and the impact on lifestyle behaviors, medication adherence, and glycemic control demands the need for assessment and treatment of diabetes-related distress (DRD). DRD can be expressed as shame, guilt, anger, fear, and frustration in combination with the everyday context of life priorities and stressors. An assessment of diabetes distress, utilizing a simple scale, should be included as part of an annual therapeutic diabetes care plan. The ADA Standards of Care in Diabetes recommends assessing patients’ psychological and social situations as an ongoing part of medical management, including an annual screening for depression and other psychological problems. The prevalence of depression is nearly twice as high in people with T2D than in the general population and can significantly influence patients’ ability to self-manage their diabetes and achieve healthy outcomes. Assessment and treatment of psychosocial components of care can result in significant improvements in A1c and other positive outcomes, including quality of life.
Kellie M. Rodriguez, director of the global diabetes program at Parkland Health, Dallas, Tex., disclosed ties with the Association of Diabetes Care and Education Specialists.
A version of this article originally appeared on Medscape.com.
Foot ulcers red flag for eye disease in diabetes
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARVO 2023
Overcoming death anxiety: Understanding our lives and legacies
Disappointment – “I failed this exam, my life is ruined” or regret – “I am getting a divorce, I wasted so much of my life.” Patients present with a wide variety of complaints that can be understood as a form of death anxiety.
Fundamentally, patients come to see us to understand and explain their lives. One can reinterpret this as a patient asking, “If I died today, would my life have been good enough?” or “When I die, how will I look back at this moment in time and judge the choices I made?”
Other patients come to us attempting to use the same maladaptive defenses that did not serve them well in the past in the hopes of achieving a new outcome that will validate their lives. While it may be understandable that a child dissociates when facing abuse, hoping that this defense mechanism – as an adult – will work, it is unlikely to be fruitful and will certainly not validate or repair the past. This hope to repair one’s past can be interpreted as a fear of death – “I cannot die without correcting this.” This psychic conflict can intensify if one does not adopt a more adaptive understanding of his or her life.
Death anxiety is the feeling associated with the finality of life. Not only is life final, but a constant reminder of that fact is the idea that any one moment is final. Other than in science fiction, one cannot return to a prior moment and repair the past in the hope of a better future. Time goes only in one direction and death is the natural outcome of all life.
Death may have some evolutionary purpose that encourages the promotion of newer and more fitter genes, but one doesn’t have to consider its origin and reason to admit death’s constancy throughout humanity. People die and that is an anxiety-provoking fact of life. Death anxiety can feel especially tangible in our connected world. In a world of constant news, it can feel – for many people – that if your house wasn’t displaced because of global warming or that you are not a war refugee, you don’t deserve to be seen and heard.
This can be a particularly strong feeling for and among physicians, who don’t think that the mental health challenges generated by their own tough circumstances deserve to be labeled a mental disorder, so they designate themselves as having “burnout”1 – as they don’t deserve the sympathy of having the clinically significant impairments of “depression.” Our traumas don’t seem important enough to deserve notice, and thus we may feel like we could die without ever having truly mattered.
This can also be applied in the reverse fashion. Certain individuals, like celebrities, live such extravagant lives that our simpler achievements can feel futile in comparison. While the neighbor’s grass has always felt greener, we are now constantly exposed to perfectly manicured lawns on social media. When compounded, the idea that our successes and our pains are both simultaneously irrelevant can lead one to have very palpable death anxiety – my life will never matter if none of the things I do matter, or my life will never matter because I will never achieve the requisite number of “likes” or “views” on social media required to believe that one’s life was worth living.
A way of alleviating death anxiety can be through the concept of legacy, or what we leave behind. How will people remember me? Will people remember me, or will I disappear like a shadow into the distant memory of my near and dear ones? The idea of being forgotten or lost to memory is intolerable to some and can be a strong driving force to “make a name” for oneself. For those who crave fame, whether a celebrity or a generous alumnus, part of this is likely related to remaining well known after death. After all, one can argue that you are not truly dead as long as you continue to live in the memory and/or genes of others.
Legacy thus serves as a form of posthumous transitional object; a way of calming our fears about how we will be remembered. For many, reconciling their feelings towards their legacy is an avenue to tame death anxiety.
A case study
The case of Mr. B illustrates this. As a 72-year-old male with a long history of generalized anxiety, he once had a nightmare as a child, similar to the plot of Sleeping Beauty. In his dream, he walks up a spiral staircase in a castle and touches the spindle on a spinning wheel, thus ending his life. The dream was vivid and marked him.
His fear of death has subsequently reared its head throughout his life. In more recent years, he has suffered from cardiovascular disease. Although he is now quite stable on his current cardiac medications, he is constantly fearful that he will experience a cardiac event while asleep and suddenly die. He is so anxious about not waking up in the morning that falling asleep is nearly impossible.
Mr. B is single, with no close family besides a sister who lives in another state. He has a dog and few friends. He worries about what will happen to his dog if he doesn’t wake up in the morning, but perhaps most distressing to him is “there’s so much left for me to do, I have so much to write!” As an accomplished author, he continues to write, and hopes to publish many more novels in his lifetime. It is unsurprising that someone without a strong social network may fear death and feel pressured to somehow make a mark on the world before the curtain falls. It is scary to think that even without us, life goes on.
By bringing to Mr. B’s attention that his ever-present anxiety is rooted in fear of death, he was able to gain more insight into his own defensive behaviors. By confronting his death anxiety and processing his definition of a life well lived together in therapy, he’s acknowledged his lack of social connection as demoralizing, and has made significant strides to remedy this. He’s been able to focus on a more fulfilling life day to day, with less emphasis on his to-do list and aspirations. Instead, he’s connected more with his faith and members of his church. He’s gotten close to several neighbors and enjoys long dinners with them on his back patio.
At a recent meeting, he confessed that he feels “lighter” and not as fearful about sudden cardiac death, and thus has noticed that his overall anxiety has diminished greatly. He concluded that experiencing meaningful relationships in the present moment would give him greater joy than spending his remaining time engaged in preserving a future identity for himself. It seems elementary, but if we look within, we may find that we all suffer similarly: How much of our daily actions, thoughts, and fears are tied to the looming threat of death?
Conclusion
While modern psychiatry continues to advance with better understandings of our neurobiology, improved knowledge of pathophysiological processes of mental illness, and expanding discovery of novel pharmacotherapeutics, the modern psychiatrist should not forget fundamental truths of behavior and humanity that were once the staple of psychiatry.
Death anxiety is one of those truths; it is the ultimate stressor that we will all face and should be regular study and practice for psychiatrists. In this article, we explored some of those facets most meaningful to us but recommend you expand your study to the many more available.
Patients often come to physicians seeking validation of their lives or trying to use the same maladaptive defense mechanisms that did not serve them well in the past to achieve a better outcome.
In today’s world, death anxiety can feel palpable due to the constant exposure to global news and social media that can make us feel irrelevant. However, legacy, or what we leave behind, can serve as a way to alleviate death anxiety. For many, reconciling their feelings toward their legacy is an avenue to tame death anxiety. Therapy can help individuals gain insight into their defensive behaviors and process their definition of a life well lived. By focusing on a life worth living, individuals can alleviate their death anxiety and gain a sense of fulfillment.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
Reference
1. Badre N. Burnout: A concept that rebrands mental illness for professionals. Clinical Psychiatry News. 2020 Mar 5.
Disappointment – “I failed this exam, my life is ruined” or regret – “I am getting a divorce, I wasted so much of my life.” Patients present with a wide variety of complaints that can be understood as a form of death anxiety.
Fundamentally, patients come to see us to understand and explain their lives. One can reinterpret this as a patient asking, “If I died today, would my life have been good enough?” or “When I die, how will I look back at this moment in time and judge the choices I made?”
Other patients come to us attempting to use the same maladaptive defenses that did not serve them well in the past in the hopes of achieving a new outcome that will validate their lives. While it may be understandable that a child dissociates when facing abuse, hoping that this defense mechanism – as an adult – will work, it is unlikely to be fruitful and will certainly not validate or repair the past. This hope to repair one’s past can be interpreted as a fear of death – “I cannot die without correcting this.” This psychic conflict can intensify if one does not adopt a more adaptive understanding of his or her life.
Death anxiety is the feeling associated with the finality of life. Not only is life final, but a constant reminder of that fact is the idea that any one moment is final. Other than in science fiction, one cannot return to a prior moment and repair the past in the hope of a better future. Time goes only in one direction and death is the natural outcome of all life.
Death may have some evolutionary purpose that encourages the promotion of newer and more fitter genes, but one doesn’t have to consider its origin and reason to admit death’s constancy throughout humanity. People die and that is an anxiety-provoking fact of life. Death anxiety can feel especially tangible in our connected world. In a world of constant news, it can feel – for many people – that if your house wasn’t displaced because of global warming or that you are not a war refugee, you don’t deserve to be seen and heard.
This can be a particularly strong feeling for and among physicians, who don’t think that the mental health challenges generated by their own tough circumstances deserve to be labeled a mental disorder, so they designate themselves as having “burnout”1 – as they don’t deserve the sympathy of having the clinically significant impairments of “depression.” Our traumas don’t seem important enough to deserve notice, and thus we may feel like we could die without ever having truly mattered.
This can also be applied in the reverse fashion. Certain individuals, like celebrities, live such extravagant lives that our simpler achievements can feel futile in comparison. While the neighbor’s grass has always felt greener, we are now constantly exposed to perfectly manicured lawns on social media. When compounded, the idea that our successes and our pains are both simultaneously irrelevant can lead one to have very palpable death anxiety – my life will never matter if none of the things I do matter, or my life will never matter because I will never achieve the requisite number of “likes” or “views” on social media required to believe that one’s life was worth living.
A way of alleviating death anxiety can be through the concept of legacy, or what we leave behind. How will people remember me? Will people remember me, or will I disappear like a shadow into the distant memory of my near and dear ones? The idea of being forgotten or lost to memory is intolerable to some and can be a strong driving force to “make a name” for oneself. For those who crave fame, whether a celebrity or a generous alumnus, part of this is likely related to remaining well known after death. After all, one can argue that you are not truly dead as long as you continue to live in the memory and/or genes of others.
Legacy thus serves as a form of posthumous transitional object; a way of calming our fears about how we will be remembered. For many, reconciling their feelings towards their legacy is an avenue to tame death anxiety.
A case study
The case of Mr. B illustrates this. As a 72-year-old male with a long history of generalized anxiety, he once had a nightmare as a child, similar to the plot of Sleeping Beauty. In his dream, he walks up a spiral staircase in a castle and touches the spindle on a spinning wheel, thus ending his life. The dream was vivid and marked him.
His fear of death has subsequently reared its head throughout his life. In more recent years, he has suffered from cardiovascular disease. Although he is now quite stable on his current cardiac medications, he is constantly fearful that he will experience a cardiac event while asleep and suddenly die. He is so anxious about not waking up in the morning that falling asleep is nearly impossible.
Mr. B is single, with no close family besides a sister who lives in another state. He has a dog and few friends. He worries about what will happen to his dog if he doesn’t wake up in the morning, but perhaps most distressing to him is “there’s so much left for me to do, I have so much to write!” As an accomplished author, he continues to write, and hopes to publish many more novels in his lifetime. It is unsurprising that someone without a strong social network may fear death and feel pressured to somehow make a mark on the world before the curtain falls. It is scary to think that even without us, life goes on.
By bringing to Mr. B’s attention that his ever-present anxiety is rooted in fear of death, he was able to gain more insight into his own defensive behaviors. By confronting his death anxiety and processing his definition of a life well lived together in therapy, he’s acknowledged his lack of social connection as demoralizing, and has made significant strides to remedy this. He’s been able to focus on a more fulfilling life day to day, with less emphasis on his to-do list and aspirations. Instead, he’s connected more with his faith and members of his church. He’s gotten close to several neighbors and enjoys long dinners with them on his back patio.
At a recent meeting, he confessed that he feels “lighter” and not as fearful about sudden cardiac death, and thus has noticed that his overall anxiety has diminished greatly. He concluded that experiencing meaningful relationships in the present moment would give him greater joy than spending his remaining time engaged in preserving a future identity for himself. It seems elementary, but if we look within, we may find that we all suffer similarly: How much of our daily actions, thoughts, and fears are tied to the looming threat of death?
Conclusion
While modern psychiatry continues to advance with better understandings of our neurobiology, improved knowledge of pathophysiological processes of mental illness, and expanding discovery of novel pharmacotherapeutics, the modern psychiatrist should not forget fundamental truths of behavior and humanity that were once the staple of psychiatry.
Death anxiety is one of those truths; it is the ultimate stressor that we will all face and should be regular study and practice for psychiatrists. In this article, we explored some of those facets most meaningful to us but recommend you expand your study to the many more available.
Patients often come to physicians seeking validation of their lives or trying to use the same maladaptive defense mechanisms that did not serve them well in the past to achieve a better outcome.
In today’s world, death anxiety can feel palpable due to the constant exposure to global news and social media that can make us feel irrelevant. However, legacy, or what we leave behind, can serve as a way to alleviate death anxiety. For many, reconciling their feelings toward their legacy is an avenue to tame death anxiety. Therapy can help individuals gain insight into their defensive behaviors and process their definition of a life well lived. By focusing on a life worth living, individuals can alleviate their death anxiety and gain a sense of fulfillment.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
Reference
1. Badre N. Burnout: A concept that rebrands mental illness for professionals. Clinical Psychiatry News. 2020 Mar 5.
Disappointment – “I failed this exam, my life is ruined” or regret – “I am getting a divorce, I wasted so much of my life.” Patients present with a wide variety of complaints that can be understood as a form of death anxiety.
Fundamentally, patients come to see us to understand and explain their lives. One can reinterpret this as a patient asking, “If I died today, would my life have been good enough?” or “When I die, how will I look back at this moment in time and judge the choices I made?”
Other patients come to us attempting to use the same maladaptive defenses that did not serve them well in the past in the hopes of achieving a new outcome that will validate their lives. While it may be understandable that a child dissociates when facing abuse, hoping that this defense mechanism – as an adult – will work, it is unlikely to be fruitful and will certainly not validate or repair the past. This hope to repair one’s past can be interpreted as a fear of death – “I cannot die without correcting this.” This psychic conflict can intensify if one does not adopt a more adaptive understanding of his or her life.
Death anxiety is the feeling associated with the finality of life. Not only is life final, but a constant reminder of that fact is the idea that any one moment is final. Other than in science fiction, one cannot return to a prior moment and repair the past in the hope of a better future. Time goes only in one direction and death is the natural outcome of all life.
Death may have some evolutionary purpose that encourages the promotion of newer and more fitter genes, but one doesn’t have to consider its origin and reason to admit death’s constancy throughout humanity. People die and that is an anxiety-provoking fact of life. Death anxiety can feel especially tangible in our connected world. In a world of constant news, it can feel – for many people – that if your house wasn’t displaced because of global warming or that you are not a war refugee, you don’t deserve to be seen and heard.
This can be a particularly strong feeling for and among physicians, who don’t think that the mental health challenges generated by their own tough circumstances deserve to be labeled a mental disorder, so they designate themselves as having “burnout”1 – as they don’t deserve the sympathy of having the clinically significant impairments of “depression.” Our traumas don’t seem important enough to deserve notice, and thus we may feel like we could die without ever having truly mattered.
This can also be applied in the reverse fashion. Certain individuals, like celebrities, live such extravagant lives that our simpler achievements can feel futile in comparison. While the neighbor’s grass has always felt greener, we are now constantly exposed to perfectly manicured lawns on social media. When compounded, the idea that our successes and our pains are both simultaneously irrelevant can lead one to have very palpable death anxiety – my life will never matter if none of the things I do matter, or my life will never matter because I will never achieve the requisite number of “likes” or “views” on social media required to believe that one’s life was worth living.
A way of alleviating death anxiety can be through the concept of legacy, or what we leave behind. How will people remember me? Will people remember me, or will I disappear like a shadow into the distant memory of my near and dear ones? The idea of being forgotten or lost to memory is intolerable to some and can be a strong driving force to “make a name” for oneself. For those who crave fame, whether a celebrity or a generous alumnus, part of this is likely related to remaining well known after death. After all, one can argue that you are not truly dead as long as you continue to live in the memory and/or genes of others.
Legacy thus serves as a form of posthumous transitional object; a way of calming our fears about how we will be remembered. For many, reconciling their feelings towards their legacy is an avenue to tame death anxiety.
A case study
The case of Mr. B illustrates this. As a 72-year-old male with a long history of generalized anxiety, he once had a nightmare as a child, similar to the plot of Sleeping Beauty. In his dream, he walks up a spiral staircase in a castle and touches the spindle on a spinning wheel, thus ending his life. The dream was vivid and marked him.
His fear of death has subsequently reared its head throughout his life. In more recent years, he has suffered from cardiovascular disease. Although he is now quite stable on his current cardiac medications, he is constantly fearful that he will experience a cardiac event while asleep and suddenly die. He is so anxious about not waking up in the morning that falling asleep is nearly impossible.
Mr. B is single, with no close family besides a sister who lives in another state. He has a dog and few friends. He worries about what will happen to his dog if he doesn’t wake up in the morning, but perhaps most distressing to him is “there’s so much left for me to do, I have so much to write!” As an accomplished author, he continues to write, and hopes to publish many more novels in his lifetime. It is unsurprising that someone without a strong social network may fear death and feel pressured to somehow make a mark on the world before the curtain falls. It is scary to think that even without us, life goes on.
By bringing to Mr. B’s attention that his ever-present anxiety is rooted in fear of death, he was able to gain more insight into his own defensive behaviors. By confronting his death anxiety and processing his definition of a life well lived together in therapy, he’s acknowledged his lack of social connection as demoralizing, and has made significant strides to remedy this. He’s been able to focus on a more fulfilling life day to day, with less emphasis on his to-do list and aspirations. Instead, he’s connected more with his faith and members of his church. He’s gotten close to several neighbors and enjoys long dinners with them on his back patio.
At a recent meeting, he confessed that he feels “lighter” and not as fearful about sudden cardiac death, and thus has noticed that his overall anxiety has diminished greatly. He concluded that experiencing meaningful relationships in the present moment would give him greater joy than spending his remaining time engaged in preserving a future identity for himself. It seems elementary, but if we look within, we may find that we all suffer similarly: How much of our daily actions, thoughts, and fears are tied to the looming threat of death?
Conclusion
While modern psychiatry continues to advance with better understandings of our neurobiology, improved knowledge of pathophysiological processes of mental illness, and expanding discovery of novel pharmacotherapeutics, the modern psychiatrist should not forget fundamental truths of behavior and humanity that were once the staple of psychiatry.
Death anxiety is one of those truths; it is the ultimate stressor that we will all face and should be regular study and practice for psychiatrists. In this article, we explored some of those facets most meaningful to us but recommend you expand your study to the many more available.
Patients often come to physicians seeking validation of their lives or trying to use the same maladaptive defense mechanisms that did not serve them well in the past to achieve a better outcome.
In today’s world, death anxiety can feel palpable due to the constant exposure to global news and social media that can make us feel irrelevant. However, legacy, or what we leave behind, can serve as a way to alleviate death anxiety. For many, reconciling their feelings toward their legacy is an avenue to tame death anxiety. Therapy can help individuals gain insight into their defensive behaviors and process their definition of a life well lived. By focusing on a life worth living, individuals can alleviate their death anxiety and gain a sense of fulfillment.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
Reference
1. Badre N. Burnout: A concept that rebrands mental illness for professionals. Clinical Psychiatry News. 2020 Mar 5.
CDC: Drug-resistant ringworm reported in New York
BY ALICIA AULT
The in New York.
Tinea, or ringworm, one of the most common fungal infections, is responsible for almost 5 million outpatient visits and 690 hospitalizations annually, according to the CDC.
Over the past 10 years, severe, antifungal-resistant tinea has spread in South Asia, in part because of the rise of a new dermatophyte species known as Trichophyton indotineae, wrote the authors of a report on the two patients with the drug-resistant strain. This epidemic “has likely been driven by misuse and overuse of topical antifungals and corticosteroids,” added the authors, in Morbidity and Mortality Weekly Report.
The cases were detected by a New York City dermatologist. In the first case, a 28-year-old woman developed a widespread pruritic eruption in the summer of 2021. She did not consult a dermatologist until December, when she was in the third trimester of pregnancy. She had large, annular, scaly, pruritic plaques on her neck, abdomen, pubic region, and buttocks, but had no underlying medical conditions, no known exposures to someone with a similar rash, and no recent international travel history.
After she gave birth in January, she started oral terbinafine therapy but had no improvement after 2 weeks. Clinicians administered a 4-week course of itraconazole, which resolved the infection.
The second patient, a 47-year-old woman with no medical conditions, developed a rash while in Bangladesh in the summer of 2022. Other family members had a similar rash. She was treated with topical antifungal and steroid combination creams but had no resolution. Back in the United States, she was prescribed hydrocortisone 2.5% ointment and diphenhydramine, clotrimazole cream, and terbinafine cream in three successive emergency department visits. In December 2022, dermatologists, observing widespread, discrete, scaly, annular, pruritic plaques on the thighs and buttocks, prescribed a 4-week course of oral terbinafine. When the rash did not resolve, she was given 4 weeks of griseofulvin. The rash persisted, although there was 80% improvement. Clinicians are now considering itraconazole. The woman’s son and husband are also being evaluated, as they have similar rashes.
In both cases, skin culture isolates were initially identified as Trichophyton mentagrophytes. Further analysis at the New York State Department of Health’s lab, using Sanger sequencing of the internal transcribed spacer region of the ribosomal gene, followed by phylogenetic analysis, identified the isolates as T. indotineae.
The authors note that culture-based techniques used by most clinical laboratories typically misidentify T. indotineae as T. mentagrophytes or T. interdigitale. Genomic sequencing must be used to properly identify T. indotineae, they wrote.
Clinicians should consider T. indotineae in patients with widespread ringworm, especially if they do not improve with topical antifungals or oral terbinafine, said the authors. If T. indotineae is suspected, state or local public health departments can direct clinicians to testing.
The authors report no relevant financial relationships.
BY ALICIA AULT
The in New York.
Tinea, or ringworm, one of the most common fungal infections, is responsible for almost 5 million outpatient visits and 690 hospitalizations annually, according to the CDC.
Over the past 10 years, severe, antifungal-resistant tinea has spread in South Asia, in part because of the rise of a new dermatophyte species known as Trichophyton indotineae, wrote the authors of a report on the two patients with the drug-resistant strain. This epidemic “has likely been driven by misuse and overuse of topical antifungals and corticosteroids,” added the authors, in Morbidity and Mortality Weekly Report.
The cases were detected by a New York City dermatologist. In the first case, a 28-year-old woman developed a widespread pruritic eruption in the summer of 2021. She did not consult a dermatologist until December, when she was in the third trimester of pregnancy. She had large, annular, scaly, pruritic plaques on her neck, abdomen, pubic region, and buttocks, but had no underlying medical conditions, no known exposures to someone with a similar rash, and no recent international travel history.
After she gave birth in January, she started oral terbinafine therapy but had no improvement after 2 weeks. Clinicians administered a 4-week course of itraconazole, which resolved the infection.
The second patient, a 47-year-old woman with no medical conditions, developed a rash while in Bangladesh in the summer of 2022. Other family members had a similar rash. She was treated with topical antifungal and steroid combination creams but had no resolution. Back in the United States, she was prescribed hydrocortisone 2.5% ointment and diphenhydramine, clotrimazole cream, and terbinafine cream in three successive emergency department visits. In December 2022, dermatologists, observing widespread, discrete, scaly, annular, pruritic plaques on the thighs and buttocks, prescribed a 4-week course of oral terbinafine. When the rash did not resolve, she was given 4 weeks of griseofulvin. The rash persisted, although there was 80% improvement. Clinicians are now considering itraconazole. The woman’s son and husband are also being evaluated, as they have similar rashes.
In both cases, skin culture isolates were initially identified as Trichophyton mentagrophytes. Further analysis at the New York State Department of Health’s lab, using Sanger sequencing of the internal transcribed spacer region of the ribosomal gene, followed by phylogenetic analysis, identified the isolates as T. indotineae.
The authors note that culture-based techniques used by most clinical laboratories typically misidentify T. indotineae as T. mentagrophytes or T. interdigitale. Genomic sequencing must be used to properly identify T. indotineae, they wrote.
Clinicians should consider T. indotineae in patients with widespread ringworm, especially if they do not improve with topical antifungals or oral terbinafine, said the authors. If T. indotineae is suspected, state or local public health departments can direct clinicians to testing.
The authors report no relevant financial relationships.
BY ALICIA AULT
The in New York.
Tinea, or ringworm, one of the most common fungal infections, is responsible for almost 5 million outpatient visits and 690 hospitalizations annually, according to the CDC.
Over the past 10 years, severe, antifungal-resistant tinea has spread in South Asia, in part because of the rise of a new dermatophyte species known as Trichophyton indotineae, wrote the authors of a report on the two patients with the drug-resistant strain. This epidemic “has likely been driven by misuse and overuse of topical antifungals and corticosteroids,” added the authors, in Morbidity and Mortality Weekly Report.
The cases were detected by a New York City dermatologist. In the first case, a 28-year-old woman developed a widespread pruritic eruption in the summer of 2021. She did not consult a dermatologist until December, when she was in the third trimester of pregnancy. She had large, annular, scaly, pruritic plaques on her neck, abdomen, pubic region, and buttocks, but had no underlying medical conditions, no known exposures to someone with a similar rash, and no recent international travel history.
After she gave birth in January, she started oral terbinafine therapy but had no improvement after 2 weeks. Clinicians administered a 4-week course of itraconazole, which resolved the infection.
The second patient, a 47-year-old woman with no medical conditions, developed a rash while in Bangladesh in the summer of 2022. Other family members had a similar rash. She was treated with topical antifungal and steroid combination creams but had no resolution. Back in the United States, she was prescribed hydrocortisone 2.5% ointment and diphenhydramine, clotrimazole cream, and terbinafine cream in three successive emergency department visits. In December 2022, dermatologists, observing widespread, discrete, scaly, annular, pruritic plaques on the thighs and buttocks, prescribed a 4-week course of oral terbinafine. When the rash did not resolve, she was given 4 weeks of griseofulvin. The rash persisted, although there was 80% improvement. Clinicians are now considering itraconazole. The woman’s son and husband are also being evaluated, as they have similar rashes.
In both cases, skin culture isolates were initially identified as Trichophyton mentagrophytes. Further analysis at the New York State Department of Health’s lab, using Sanger sequencing of the internal transcribed spacer region of the ribosomal gene, followed by phylogenetic analysis, identified the isolates as T. indotineae.
The authors note that culture-based techniques used by most clinical laboratories typically misidentify T. indotineae as T. mentagrophytes or T. interdigitale. Genomic sequencing must be used to properly identify T. indotineae, they wrote.
Clinicians should consider T. indotineae in patients with widespread ringworm, especially if they do not improve with topical antifungals or oral terbinafine, said the authors. If T. indotineae is suspected, state or local public health departments can direct clinicians to testing.
The authors report no relevant financial relationships.
Statins appear to guard against liver disease progression
The Swedish population-based study found that adults with noncirrhotic CLD who were on statin therapy had a statistically significant 40% lower risk of developing severe liver disease, compared with matched patients who were not on statin therapy.
The statin users were also less apt to progress to cirrhosis or hepatocellular carcinoma (HCC) and to die of liver disease, Rajani Sharma, MD, MSc, division of digestive and liver diseases, Columbia University Irving Medical Center, New York, and colleagues reported.
Their study was published online in Clinical Gastroenterology and Hepatology.
More than just cholesterol lowering
The study “continues the theme that cholesterol-lowering statins are good for a lot more things than just lowering cholesterol,” William Carey, MD, who wasn’t involved with the study, said in an interview.
The results are “very consistent with other trials that show that people with liver disease on statins do better in many respects than those who are not on statins,” said Dr. Carey, acting head of the hepatology section, department of gastroenterology, hepatology, and nutrition, Cleveland Clinic.
“The effects are not trivial,” Dr. Carey added. “It’s a very significant advantage in terms of fibrosis progression and survival.”
Statins have been shown to inhibit inflammatory pathways, promote endothelial cell function, and reduce hepatic stellate cell activity, suggesting that statins could lessen the progression of liver fibrosis, Dr. Sharma and coauthors wrote.
A few prior studies have looked at the effects of statins in noncirrhotic CLD specifically, but most only included patients with viral hepatitis, and the identification of precirrhotic liver disease was largely based on fibrosis scores or ICD coding, leading to a risk for misclassification and heterogeneity in results, they wrote.
Using histopathology data in a nationwide Swedish cohort, Dr. Sharma and colleagues identified 3862 adults with noncirrhotic CLD who were statin users and a like number of propensity score–matched nonstatin users with noncirrhotic CLD. The adults with CLD included in the study were required to have a liver biopsy showing fibrosis or inflammation between the years 1969 and 2017 and at least one ICD code for CLD.
In both groups, 45% of patients had nonalcoholic fatty liver disease (NAFLD), 22% had alcohol-related liver disease (ALD), 18% had viral hepatitis, and 15% had autoimmune hepatitis (AIH).
The analysis found 234 (6.1%) statin users developed severe liver disease versus 276 (7.1%) nonusers, with incidence rates of 10.5 versus 18.1 per 1,000 person-years, respectively.
Statin use was associated with a statistically significant 40% lower rate of severe liver disease (hazard ratio, 0.60; 95% confidence interval, 0.48-0.74).
This was the case in ALD (HR, 0.30; 95% CI, 0.19-0.49) and NAFLD (HR, 0.68; 95% CI 0.45-1.00), but the results were not statistically significant for individuals with viral hepatitis (HR, 0.76; 95% CI, 0.51-1.14) or AIH (HR, 0.88; 0.48-1.58).
Statin use had a protective association in both prefibrosis and fibrosis stages at diagnosis, the researchers reported.
Statin use was also associated with lower rates of progression to cirrhosis (HR, 0.62; 95% CI, 0.49-0.78), HCC (HR, 0.44; 95% CI, 0.27-0.71) and liver-related death or liver transplant (HR, 0.55; 95% CI, 0.36-0.82).
The authors noted that their “study provides the most robust estimates available thus far.” However, they cautioned that “prospective randomized controlled trials are necessary in order to recommend statin use in clinical practice.”
‘Reassuring and pleasantly surprising’
The study is “very interesting, reassuring, and pleasantly surprising,” Scott L. Friedman, MD, chief of the division of liver diseases and dean for therapeutic discovery at the Icahn School of Medicine at Mount Sinai. New York, said in an interview.
“Statins have been around for a long time, and in earlier days, there was fear of using them because they might induce liver injury. But ample and consistent data exclude the possibility that they are more toxic in patients with liver disease,” said Dr. Friedman, who was not associated with this research.
“What’s interesting and new about this paper is that those studies that have looked at the effects of statins on liver disease have primarily focused on patients who have cirrhosis because there’s some scientific evidence [that] statins can lead to vasodilation and reduce the elevated liver blood flow that occurs in cirrhosis,” he explained.
“Instead, this study, which is quite sizable, includes patients who do not have evidence of cirrhosis based on biopsies. The results suggest that statins have a significant protective effect in these patients,” Dr. Friedman said.
The study was supported by the Karolinska Institute in Stockholm, the Columbia University Irving Medical Center, the Swedish Research Council, the Swedish Cancer Society, and the U.S. National Institutes of Health. Dr. Sharma is a consultant for Takeda and Volv. Other coauthors reported current or past relationships with Bristol-Myers Squibb, Gilead, Salix, and GlaxoSmithKline. Dr. Carey and Dr. Friedman reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Swedish population-based study found that adults with noncirrhotic CLD who were on statin therapy had a statistically significant 40% lower risk of developing severe liver disease, compared with matched patients who were not on statin therapy.
The statin users were also less apt to progress to cirrhosis or hepatocellular carcinoma (HCC) and to die of liver disease, Rajani Sharma, MD, MSc, division of digestive and liver diseases, Columbia University Irving Medical Center, New York, and colleagues reported.
Their study was published online in Clinical Gastroenterology and Hepatology.
More than just cholesterol lowering
The study “continues the theme that cholesterol-lowering statins are good for a lot more things than just lowering cholesterol,” William Carey, MD, who wasn’t involved with the study, said in an interview.
The results are “very consistent with other trials that show that people with liver disease on statins do better in many respects than those who are not on statins,” said Dr. Carey, acting head of the hepatology section, department of gastroenterology, hepatology, and nutrition, Cleveland Clinic.
“The effects are not trivial,” Dr. Carey added. “It’s a very significant advantage in terms of fibrosis progression and survival.”
Statins have been shown to inhibit inflammatory pathways, promote endothelial cell function, and reduce hepatic stellate cell activity, suggesting that statins could lessen the progression of liver fibrosis, Dr. Sharma and coauthors wrote.
A few prior studies have looked at the effects of statins in noncirrhotic CLD specifically, but most only included patients with viral hepatitis, and the identification of precirrhotic liver disease was largely based on fibrosis scores or ICD coding, leading to a risk for misclassification and heterogeneity in results, they wrote.
Using histopathology data in a nationwide Swedish cohort, Dr. Sharma and colleagues identified 3862 adults with noncirrhotic CLD who were statin users and a like number of propensity score–matched nonstatin users with noncirrhotic CLD. The adults with CLD included in the study were required to have a liver biopsy showing fibrosis or inflammation between the years 1969 and 2017 and at least one ICD code for CLD.
In both groups, 45% of patients had nonalcoholic fatty liver disease (NAFLD), 22% had alcohol-related liver disease (ALD), 18% had viral hepatitis, and 15% had autoimmune hepatitis (AIH).
The analysis found 234 (6.1%) statin users developed severe liver disease versus 276 (7.1%) nonusers, with incidence rates of 10.5 versus 18.1 per 1,000 person-years, respectively.
Statin use was associated with a statistically significant 40% lower rate of severe liver disease (hazard ratio, 0.60; 95% confidence interval, 0.48-0.74).
This was the case in ALD (HR, 0.30; 95% CI, 0.19-0.49) and NAFLD (HR, 0.68; 95% CI 0.45-1.00), but the results were not statistically significant for individuals with viral hepatitis (HR, 0.76; 95% CI, 0.51-1.14) or AIH (HR, 0.88; 0.48-1.58).
Statin use had a protective association in both prefibrosis and fibrosis stages at diagnosis, the researchers reported.
Statin use was also associated with lower rates of progression to cirrhosis (HR, 0.62; 95% CI, 0.49-0.78), HCC (HR, 0.44; 95% CI, 0.27-0.71) and liver-related death or liver transplant (HR, 0.55; 95% CI, 0.36-0.82).
The authors noted that their “study provides the most robust estimates available thus far.” However, they cautioned that “prospective randomized controlled trials are necessary in order to recommend statin use in clinical practice.”
‘Reassuring and pleasantly surprising’
The study is “very interesting, reassuring, and pleasantly surprising,” Scott L. Friedman, MD, chief of the division of liver diseases and dean for therapeutic discovery at the Icahn School of Medicine at Mount Sinai. New York, said in an interview.
“Statins have been around for a long time, and in earlier days, there was fear of using them because they might induce liver injury. But ample and consistent data exclude the possibility that they are more toxic in patients with liver disease,” said Dr. Friedman, who was not associated with this research.
“What’s interesting and new about this paper is that those studies that have looked at the effects of statins on liver disease have primarily focused on patients who have cirrhosis because there’s some scientific evidence [that] statins can lead to vasodilation and reduce the elevated liver blood flow that occurs in cirrhosis,” he explained.
“Instead, this study, which is quite sizable, includes patients who do not have evidence of cirrhosis based on biopsies. The results suggest that statins have a significant protective effect in these patients,” Dr. Friedman said.
The study was supported by the Karolinska Institute in Stockholm, the Columbia University Irving Medical Center, the Swedish Research Council, the Swedish Cancer Society, and the U.S. National Institutes of Health. Dr. Sharma is a consultant for Takeda and Volv. Other coauthors reported current or past relationships with Bristol-Myers Squibb, Gilead, Salix, and GlaxoSmithKline. Dr. Carey and Dr. Friedman reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Swedish population-based study found that adults with noncirrhotic CLD who were on statin therapy had a statistically significant 40% lower risk of developing severe liver disease, compared with matched patients who were not on statin therapy.
The statin users were also less apt to progress to cirrhosis or hepatocellular carcinoma (HCC) and to die of liver disease, Rajani Sharma, MD, MSc, division of digestive and liver diseases, Columbia University Irving Medical Center, New York, and colleagues reported.
Their study was published online in Clinical Gastroenterology and Hepatology.
More than just cholesterol lowering
The study “continues the theme that cholesterol-lowering statins are good for a lot more things than just lowering cholesterol,” William Carey, MD, who wasn’t involved with the study, said in an interview.
The results are “very consistent with other trials that show that people with liver disease on statins do better in many respects than those who are not on statins,” said Dr. Carey, acting head of the hepatology section, department of gastroenterology, hepatology, and nutrition, Cleveland Clinic.
“The effects are not trivial,” Dr. Carey added. “It’s a very significant advantage in terms of fibrosis progression and survival.”
Statins have been shown to inhibit inflammatory pathways, promote endothelial cell function, and reduce hepatic stellate cell activity, suggesting that statins could lessen the progression of liver fibrosis, Dr. Sharma and coauthors wrote.
A few prior studies have looked at the effects of statins in noncirrhotic CLD specifically, but most only included patients with viral hepatitis, and the identification of precirrhotic liver disease was largely based on fibrosis scores or ICD coding, leading to a risk for misclassification and heterogeneity in results, they wrote.
Using histopathology data in a nationwide Swedish cohort, Dr. Sharma and colleagues identified 3862 adults with noncirrhotic CLD who were statin users and a like number of propensity score–matched nonstatin users with noncirrhotic CLD. The adults with CLD included in the study were required to have a liver biopsy showing fibrosis or inflammation between the years 1969 and 2017 and at least one ICD code for CLD.
In both groups, 45% of patients had nonalcoholic fatty liver disease (NAFLD), 22% had alcohol-related liver disease (ALD), 18% had viral hepatitis, and 15% had autoimmune hepatitis (AIH).
The analysis found 234 (6.1%) statin users developed severe liver disease versus 276 (7.1%) nonusers, with incidence rates of 10.5 versus 18.1 per 1,000 person-years, respectively.
Statin use was associated with a statistically significant 40% lower rate of severe liver disease (hazard ratio, 0.60; 95% confidence interval, 0.48-0.74).
This was the case in ALD (HR, 0.30; 95% CI, 0.19-0.49) and NAFLD (HR, 0.68; 95% CI 0.45-1.00), but the results were not statistically significant for individuals with viral hepatitis (HR, 0.76; 95% CI, 0.51-1.14) or AIH (HR, 0.88; 0.48-1.58).
Statin use had a protective association in both prefibrosis and fibrosis stages at diagnosis, the researchers reported.
Statin use was also associated with lower rates of progression to cirrhosis (HR, 0.62; 95% CI, 0.49-0.78), HCC (HR, 0.44; 95% CI, 0.27-0.71) and liver-related death or liver transplant (HR, 0.55; 95% CI, 0.36-0.82).
The authors noted that their “study provides the most robust estimates available thus far.” However, they cautioned that “prospective randomized controlled trials are necessary in order to recommend statin use in clinical practice.”
‘Reassuring and pleasantly surprising’
The study is “very interesting, reassuring, and pleasantly surprising,” Scott L. Friedman, MD, chief of the division of liver diseases and dean for therapeutic discovery at the Icahn School of Medicine at Mount Sinai. New York, said in an interview.
“Statins have been around for a long time, and in earlier days, there was fear of using them because they might induce liver injury. But ample and consistent data exclude the possibility that they are more toxic in patients with liver disease,” said Dr. Friedman, who was not associated with this research.
“What’s interesting and new about this paper is that those studies that have looked at the effects of statins on liver disease have primarily focused on patients who have cirrhosis because there’s some scientific evidence [that] statins can lead to vasodilation and reduce the elevated liver blood flow that occurs in cirrhosis,” he explained.
“Instead, this study, which is quite sizable, includes patients who do not have evidence of cirrhosis based on biopsies. The results suggest that statins have a significant protective effect in these patients,” Dr. Friedman said.
The study was supported by the Karolinska Institute in Stockholm, the Columbia University Irving Medical Center, the Swedish Research Council, the Swedish Cancer Society, and the U.S. National Institutes of Health. Dr. Sharma is a consultant for Takeda and Volv. Other coauthors reported current or past relationships with Bristol-Myers Squibb, Gilead, Salix, and GlaxoSmithKline. Dr. Carey and Dr. Friedman reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Facial, hand, and foot dermatitis: Lebrikizumab and dupilumab show efficacy in new studies
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
AT RAD 2023
CGM completes picture of A1c in type 2 diabetes
in a post hoc analysis of the SWITCH PRO clinical trial.
TIR was inversely related to A1c, with the strongest correlation following treatment intensification.
However, “there was a wide scatter of data, indicating that TIR (and other metrics) provides information about glycemic control that cannot be discerned from A1c alone, and which at least complements it,” Ronald M. Goldenberg, MD, from LMC Diabetes & Endocrinology in Thornhill, Ont., and colleagues write in their article published in Diabetes Therapy.
Other work has shown that more than a third of people with type 2 diabetes are not achieving the internationally recommended A1c target of < 7% to 8.5%, they note.
When used with A1c, CGM data – such as TIR, time below range (TBR), and time above range (TAR) – “provide a more complete picture of glucose levels throughout the day and night,” they write.
“This may help empower people with diabetes to better manage their condition, giving them practical insights into the factors driving daily fluctuations in glucose levels, such as diet, exercise, insulin dosage, and insulin timing,” they add. “These metrics may also be used to inform treatment decisions by health care professionals.”
“Ultimately,” the researchers conclude, “it is hoped that the use of these new metrics should lead to an improved quality of glycemic control and, in turn, to a reduction in the number of diabetes-related complications.”
‘Important study’
Invited to comment, Celeste C. Thomas, MD, who was not involved with the research, said: “This study is important because it is consistent with previous analyses that found a correlation between TIR and A1c.”
But, “I was surprised by the scatter plots which identified participants with TIR of 70% that also had A1c > 9%,” she added. “This highlights the importance of using multiple glycemic metrics to understand an individual’s risk for diabetes complications and to be aware of the limitations of the metrics.”
Dr. Thomas, from the University of Chicago, also noted that CGM is used in endocrinology clinics and increasingly in primary care clinics, “often to determine glycemic patterns to optimize therapeutic management but also to review TIR and, importantly, time below range to reduce the incidence of hypoglycemia.”
And people with type 2 diabetes are using CGM, Dr. Thomas noted, to understand their individual responses to medications, food choices, sleep quality and duration, exercise, and other day-to-day variables that affect glucose levels. “In my clinical practice, the information provided by personal CGM is empowering,” she said.
Effective April 4, 2023, Medicare “allows for the coverage of CGM in patients [with type 2 diabetes] treated with one injection of insulin daily and those not taking insulin but with a history of hypoglycemia,” Dr. Thomas noted, whereas “previously, patients needed to be prescribed at least three injections of insulin daily. Other insurers will hopefully soon follow.”
“I foresee CGM and TIR being widely used in clinical practice for people living with type 2 diabetes,” she said, “especially those who have ever had an A1c over 8%, those with a history of hypoglycemia, and those treated with medications that are known to cause hypoglycemia.”
How does TIR compare with A1c?
Dr. Goldenberg and colleagues set out to better understand how the emerging TIR metric compares with the traditional A1c value.
They performed a post-hoc analysis of data from the phase 4 SWITCH PRO study of basal insulin–treated patients with type 2 diabetes with at least one risk factor for hypoglycemia.
The patients were treated with insulin degludec or glargine 100 during a 16-week titration and 2-week maintenance phase, and then crossed over to the other treatment for the same time periods.
Glycemic control was evaluated using a blinded professional CGM (Abbott Freestyle Libro Pro). The primary outcome was TIR, which was defined as the percentage of time spent in the blood glucose range of 70-180 mg/dL.
There were 419 participants in the full analysis. Patients were a mean age of 63 and 48% were men. They had a mean body mass index of 32 kg/m2 and had diabetes for a mean of 15 years.
There was a moderate inverse linear correlation between TIR and A1c at baseline, which became stronger following treatment intensification during the maintenance periods in the full cohort, and in a subgroup of patients with median A1c ≥ 7.5% (212 patients).
This correlation between TIR and A1c was poorer in the subgroup of patients with baseline median A1c < 7.5% (307 patients).
The data were widely scattered, “supporting the premise that A1c and TIR can be relatively crude surrogates of each other when it comes to individual patients,” Dr. Goldenberg and colleagues note.
Where individual patients have both low A1c and low TIR values, this might indicate frequent episodes of hypoglycemia.
A few individual patients had TIR > 70% but A1c approaching 9%. These patients may have different red blood cell physiology whereby A1c does not reflect average glycemic values, the researchers suggest.
The study was sponsored by Novo Nordisk and several authors are Novo Nordisk employees. The complete author disclosures are listed with the article. Dr. Thomas has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a post hoc analysis of the SWITCH PRO clinical trial.
TIR was inversely related to A1c, with the strongest correlation following treatment intensification.
However, “there was a wide scatter of data, indicating that TIR (and other metrics) provides information about glycemic control that cannot be discerned from A1c alone, and which at least complements it,” Ronald M. Goldenberg, MD, from LMC Diabetes & Endocrinology in Thornhill, Ont., and colleagues write in their article published in Diabetes Therapy.
Other work has shown that more than a third of people with type 2 diabetes are not achieving the internationally recommended A1c target of < 7% to 8.5%, they note.
When used with A1c, CGM data – such as TIR, time below range (TBR), and time above range (TAR) – “provide a more complete picture of glucose levels throughout the day and night,” they write.
“This may help empower people with diabetes to better manage their condition, giving them practical insights into the factors driving daily fluctuations in glucose levels, such as diet, exercise, insulin dosage, and insulin timing,” they add. “These metrics may also be used to inform treatment decisions by health care professionals.”
“Ultimately,” the researchers conclude, “it is hoped that the use of these new metrics should lead to an improved quality of glycemic control and, in turn, to a reduction in the number of diabetes-related complications.”
‘Important study’
Invited to comment, Celeste C. Thomas, MD, who was not involved with the research, said: “This study is important because it is consistent with previous analyses that found a correlation between TIR and A1c.”
But, “I was surprised by the scatter plots which identified participants with TIR of 70% that also had A1c > 9%,” she added. “This highlights the importance of using multiple glycemic metrics to understand an individual’s risk for diabetes complications and to be aware of the limitations of the metrics.”
Dr. Thomas, from the University of Chicago, also noted that CGM is used in endocrinology clinics and increasingly in primary care clinics, “often to determine glycemic patterns to optimize therapeutic management but also to review TIR and, importantly, time below range to reduce the incidence of hypoglycemia.”
And people with type 2 diabetes are using CGM, Dr. Thomas noted, to understand their individual responses to medications, food choices, sleep quality and duration, exercise, and other day-to-day variables that affect glucose levels. “In my clinical practice, the information provided by personal CGM is empowering,” she said.
Effective April 4, 2023, Medicare “allows for the coverage of CGM in patients [with type 2 diabetes] treated with one injection of insulin daily and those not taking insulin but with a history of hypoglycemia,” Dr. Thomas noted, whereas “previously, patients needed to be prescribed at least three injections of insulin daily. Other insurers will hopefully soon follow.”
“I foresee CGM and TIR being widely used in clinical practice for people living with type 2 diabetes,” she said, “especially those who have ever had an A1c over 8%, those with a history of hypoglycemia, and those treated with medications that are known to cause hypoglycemia.”
How does TIR compare with A1c?
Dr. Goldenberg and colleagues set out to better understand how the emerging TIR metric compares with the traditional A1c value.
They performed a post-hoc analysis of data from the phase 4 SWITCH PRO study of basal insulin–treated patients with type 2 diabetes with at least one risk factor for hypoglycemia.
The patients were treated with insulin degludec or glargine 100 during a 16-week titration and 2-week maintenance phase, and then crossed over to the other treatment for the same time periods.
Glycemic control was evaluated using a blinded professional CGM (Abbott Freestyle Libro Pro). The primary outcome was TIR, which was defined as the percentage of time spent in the blood glucose range of 70-180 mg/dL.
There were 419 participants in the full analysis. Patients were a mean age of 63 and 48% were men. They had a mean body mass index of 32 kg/m2 and had diabetes for a mean of 15 years.
There was a moderate inverse linear correlation between TIR and A1c at baseline, which became stronger following treatment intensification during the maintenance periods in the full cohort, and in a subgroup of patients with median A1c ≥ 7.5% (212 patients).
This correlation between TIR and A1c was poorer in the subgroup of patients with baseline median A1c < 7.5% (307 patients).
The data were widely scattered, “supporting the premise that A1c and TIR can be relatively crude surrogates of each other when it comes to individual patients,” Dr. Goldenberg and colleagues note.
Where individual patients have both low A1c and low TIR values, this might indicate frequent episodes of hypoglycemia.
A few individual patients had TIR > 70% but A1c approaching 9%. These patients may have different red blood cell physiology whereby A1c does not reflect average glycemic values, the researchers suggest.
The study was sponsored by Novo Nordisk and several authors are Novo Nordisk employees. The complete author disclosures are listed with the article. Dr. Thomas has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a post hoc analysis of the SWITCH PRO clinical trial.
TIR was inversely related to A1c, with the strongest correlation following treatment intensification.
However, “there was a wide scatter of data, indicating that TIR (and other metrics) provides information about glycemic control that cannot be discerned from A1c alone, and which at least complements it,” Ronald M. Goldenberg, MD, from LMC Diabetes & Endocrinology in Thornhill, Ont., and colleagues write in their article published in Diabetes Therapy.
Other work has shown that more than a third of people with type 2 diabetes are not achieving the internationally recommended A1c target of < 7% to 8.5%, they note.
When used with A1c, CGM data – such as TIR, time below range (TBR), and time above range (TAR) – “provide a more complete picture of glucose levels throughout the day and night,” they write.
“This may help empower people with diabetes to better manage their condition, giving them practical insights into the factors driving daily fluctuations in glucose levels, such as diet, exercise, insulin dosage, and insulin timing,” they add. “These metrics may also be used to inform treatment decisions by health care professionals.”
“Ultimately,” the researchers conclude, “it is hoped that the use of these new metrics should lead to an improved quality of glycemic control and, in turn, to a reduction in the number of diabetes-related complications.”
‘Important study’
Invited to comment, Celeste C. Thomas, MD, who was not involved with the research, said: “This study is important because it is consistent with previous analyses that found a correlation between TIR and A1c.”
But, “I was surprised by the scatter plots which identified participants with TIR of 70% that also had A1c > 9%,” she added. “This highlights the importance of using multiple glycemic metrics to understand an individual’s risk for diabetes complications and to be aware of the limitations of the metrics.”
Dr. Thomas, from the University of Chicago, also noted that CGM is used in endocrinology clinics and increasingly in primary care clinics, “often to determine glycemic patterns to optimize therapeutic management but also to review TIR and, importantly, time below range to reduce the incidence of hypoglycemia.”
And people with type 2 diabetes are using CGM, Dr. Thomas noted, to understand their individual responses to medications, food choices, sleep quality and duration, exercise, and other day-to-day variables that affect glucose levels. “In my clinical practice, the information provided by personal CGM is empowering,” she said.
Effective April 4, 2023, Medicare “allows for the coverage of CGM in patients [with type 2 diabetes] treated with one injection of insulin daily and those not taking insulin but with a history of hypoglycemia,” Dr. Thomas noted, whereas “previously, patients needed to be prescribed at least three injections of insulin daily. Other insurers will hopefully soon follow.”
“I foresee CGM and TIR being widely used in clinical practice for people living with type 2 diabetes,” she said, “especially those who have ever had an A1c over 8%, those with a history of hypoglycemia, and those treated with medications that are known to cause hypoglycemia.”
How does TIR compare with A1c?
Dr. Goldenberg and colleagues set out to better understand how the emerging TIR metric compares with the traditional A1c value.
They performed a post-hoc analysis of data from the phase 4 SWITCH PRO study of basal insulin–treated patients with type 2 diabetes with at least one risk factor for hypoglycemia.
The patients were treated with insulin degludec or glargine 100 during a 16-week titration and 2-week maintenance phase, and then crossed over to the other treatment for the same time periods.
Glycemic control was evaluated using a blinded professional CGM (Abbott Freestyle Libro Pro). The primary outcome was TIR, which was defined as the percentage of time spent in the blood glucose range of 70-180 mg/dL.
There were 419 participants in the full analysis. Patients were a mean age of 63 and 48% were men. They had a mean body mass index of 32 kg/m2 and had diabetes for a mean of 15 years.
There was a moderate inverse linear correlation between TIR and A1c at baseline, which became stronger following treatment intensification during the maintenance periods in the full cohort, and in a subgroup of patients with median A1c ≥ 7.5% (212 patients).
This correlation between TIR and A1c was poorer in the subgroup of patients with baseline median A1c < 7.5% (307 patients).
The data were widely scattered, “supporting the premise that A1c and TIR can be relatively crude surrogates of each other when it comes to individual patients,” Dr. Goldenberg and colleagues note.
Where individual patients have both low A1c and low TIR values, this might indicate frequent episodes of hypoglycemia.
A few individual patients had TIR > 70% but A1c approaching 9%. These patients may have different red blood cell physiology whereby A1c does not reflect average glycemic values, the researchers suggest.
The study was sponsored by Novo Nordisk and several authors are Novo Nordisk employees. The complete author disclosures are listed with the article. Dr. Thomas has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETES THERAPY
Key red flags for early-onset colorectal cancer
As the number of cases of early-onset colorectal cancer (CRC) diagnosed before age 50 continues to rise, early detection has become increasingly important.
The signs and symptoms are abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia.
Two symptoms in particular – rectal bleeding and iron-deficiency anemia – point to the need for timely endoscopy and follow-up, the researchers say.
“Colorectal cancer is not simply a disease affecting older people; we want younger adults to be aware of and act on these potentially very telling signs and symptoms – particularly because people under 50 are considered to be at low risk, and they don’t receive routine colorectal cancer screening,” senior investigator Yin Cao, ScD, with Washington University School of Medicine, St. Louis, said in a news release.
“It’s also crucial to spread awareness among primary care doctors, gastroenterologists, and emergency medicine doctors,” Dr. Cao added. “To date, many early-onset colorectal cancers are detected in emergency rooms, and there often are significant diagnostic delays with this cancer.”
The study was published online in the Journal of the National Cancer Institute.
Although previous research has identified rectal bleeding, iron-deficiency anemia, and rectal/abdominal pain as symptoms of early-onset CRC, most studies “have aggregated symptoms till the time of diagnosis,” which limits their use for early detection, the authors explain.
In the current study, the researchers analyzed data from more than 5,000 cases of early-onset CRC and from more than 22,000 control patients using the IBM MarketScan commercial database.
Dr. Cao and colleagues found that between 3 months and 2 years before diagnosis, abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia each indicated an increased risk for early-onset CRC.
Among patients with early-onset CRC, 19.3% presented with one or more of the four red flags between 3 months and 2 years prior to the index date; 15.6% had one symptom, and 3.7% had two or more.
After multivariable adjustment, having one symptom almost doubled the risk for early-onset CRC (odds ratio, 1.94); having two symptoms increased risk by more than threefold (OR, 3.59); and having three or more boosted the risk by more than 6.5-fold (OR, 6.52).
Abdominal pain was associated with a 34% higher risk of early-onset CRC (11.6% among case patients vs. 7.7% among controls; OR, 1.34).
Although not as common, rectal bleeding was associated with the highest odds for early-onset CRC (7.2% case patients vs. 1.3% controls; OR, 5.13).
The other predictive signs and symptoms included diarrhea (2.8% case patients vs. 1.4% controls; OR, 1.43) and iron-deficiency anemia (2.3% case patients vs. 0.9% controls; OR, 2.07).
No differences were observed by gender for each sign or symptom.
Among patients with a red-flag symptom who presented between 3 months and 2 years before diagnosis, for those with early-onset CRC, the median diagnostic interval was 8.7 months.
The researchers suggest that clinicians prioritize prompt diagnostic workups for patients younger than 50 who present with rectal bleeding and/or iron-deficiency anemia and that they also keep abdominal pain and diarrhea in mind as early symptoms.
Dr. Cao noted that since most early-onset CRC cases “have been and will continue to be diagnosed after symptom presentation, it is crucial to recognize these red-flag signs and symptoms promptly and conduct a diagnostic workup as soon as possible.
“By doing so, we can diagnose the disease earlier, which in turn can reduce the need for more aggressive treatment and improve patients’ quality of life and survival rates,” said Dr. Cao.
The study was supported by grants from the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article originally appeared on Medscape.com.
As the number of cases of early-onset colorectal cancer (CRC) diagnosed before age 50 continues to rise, early detection has become increasingly important.
The signs and symptoms are abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia.
Two symptoms in particular – rectal bleeding and iron-deficiency anemia – point to the need for timely endoscopy and follow-up, the researchers say.
“Colorectal cancer is not simply a disease affecting older people; we want younger adults to be aware of and act on these potentially very telling signs and symptoms – particularly because people under 50 are considered to be at low risk, and they don’t receive routine colorectal cancer screening,” senior investigator Yin Cao, ScD, with Washington University School of Medicine, St. Louis, said in a news release.
“It’s also crucial to spread awareness among primary care doctors, gastroenterologists, and emergency medicine doctors,” Dr. Cao added. “To date, many early-onset colorectal cancers are detected in emergency rooms, and there often are significant diagnostic delays with this cancer.”
The study was published online in the Journal of the National Cancer Institute.
Although previous research has identified rectal bleeding, iron-deficiency anemia, and rectal/abdominal pain as symptoms of early-onset CRC, most studies “have aggregated symptoms till the time of diagnosis,” which limits their use for early detection, the authors explain.
In the current study, the researchers analyzed data from more than 5,000 cases of early-onset CRC and from more than 22,000 control patients using the IBM MarketScan commercial database.
Dr. Cao and colleagues found that between 3 months and 2 years before diagnosis, abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia each indicated an increased risk for early-onset CRC.
Among patients with early-onset CRC, 19.3% presented with one or more of the four red flags between 3 months and 2 years prior to the index date; 15.6% had one symptom, and 3.7% had two or more.
After multivariable adjustment, having one symptom almost doubled the risk for early-onset CRC (odds ratio, 1.94); having two symptoms increased risk by more than threefold (OR, 3.59); and having three or more boosted the risk by more than 6.5-fold (OR, 6.52).
Abdominal pain was associated with a 34% higher risk of early-onset CRC (11.6% among case patients vs. 7.7% among controls; OR, 1.34).
Although not as common, rectal bleeding was associated with the highest odds for early-onset CRC (7.2% case patients vs. 1.3% controls; OR, 5.13).
The other predictive signs and symptoms included diarrhea (2.8% case patients vs. 1.4% controls; OR, 1.43) and iron-deficiency anemia (2.3% case patients vs. 0.9% controls; OR, 2.07).
No differences were observed by gender for each sign or symptom.
Among patients with a red-flag symptom who presented between 3 months and 2 years before diagnosis, for those with early-onset CRC, the median diagnostic interval was 8.7 months.
The researchers suggest that clinicians prioritize prompt diagnostic workups for patients younger than 50 who present with rectal bleeding and/or iron-deficiency anemia and that they also keep abdominal pain and diarrhea in mind as early symptoms.
Dr. Cao noted that since most early-onset CRC cases “have been and will continue to be diagnosed after symptom presentation, it is crucial to recognize these red-flag signs and symptoms promptly and conduct a diagnostic workup as soon as possible.
“By doing so, we can diagnose the disease earlier, which in turn can reduce the need for more aggressive treatment and improve patients’ quality of life and survival rates,” said Dr. Cao.
The study was supported by grants from the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article originally appeared on Medscape.com.
As the number of cases of early-onset colorectal cancer (CRC) diagnosed before age 50 continues to rise, early detection has become increasingly important.
The signs and symptoms are abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia.
Two symptoms in particular – rectal bleeding and iron-deficiency anemia – point to the need for timely endoscopy and follow-up, the researchers say.
“Colorectal cancer is not simply a disease affecting older people; we want younger adults to be aware of and act on these potentially very telling signs and symptoms – particularly because people under 50 are considered to be at low risk, and they don’t receive routine colorectal cancer screening,” senior investigator Yin Cao, ScD, with Washington University School of Medicine, St. Louis, said in a news release.
“It’s also crucial to spread awareness among primary care doctors, gastroenterologists, and emergency medicine doctors,” Dr. Cao added. “To date, many early-onset colorectal cancers are detected in emergency rooms, and there often are significant diagnostic delays with this cancer.”
The study was published online in the Journal of the National Cancer Institute.
Although previous research has identified rectal bleeding, iron-deficiency anemia, and rectal/abdominal pain as symptoms of early-onset CRC, most studies “have aggregated symptoms till the time of diagnosis,” which limits their use for early detection, the authors explain.
In the current study, the researchers analyzed data from more than 5,000 cases of early-onset CRC and from more than 22,000 control patients using the IBM MarketScan commercial database.
Dr. Cao and colleagues found that between 3 months and 2 years before diagnosis, abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia each indicated an increased risk for early-onset CRC.
Among patients with early-onset CRC, 19.3% presented with one or more of the four red flags between 3 months and 2 years prior to the index date; 15.6% had one symptom, and 3.7% had two or more.
After multivariable adjustment, having one symptom almost doubled the risk for early-onset CRC (odds ratio, 1.94); having two symptoms increased risk by more than threefold (OR, 3.59); and having three or more boosted the risk by more than 6.5-fold (OR, 6.52).
Abdominal pain was associated with a 34% higher risk of early-onset CRC (11.6% among case patients vs. 7.7% among controls; OR, 1.34).
Although not as common, rectal bleeding was associated with the highest odds for early-onset CRC (7.2% case patients vs. 1.3% controls; OR, 5.13).
The other predictive signs and symptoms included diarrhea (2.8% case patients vs. 1.4% controls; OR, 1.43) and iron-deficiency anemia (2.3% case patients vs. 0.9% controls; OR, 2.07).
No differences were observed by gender for each sign or symptom.
Among patients with a red-flag symptom who presented between 3 months and 2 years before diagnosis, for those with early-onset CRC, the median diagnostic interval was 8.7 months.
The researchers suggest that clinicians prioritize prompt diagnostic workups for patients younger than 50 who present with rectal bleeding and/or iron-deficiency anemia and that they also keep abdominal pain and diarrhea in mind as early symptoms.
Dr. Cao noted that since most early-onset CRC cases “have been and will continue to be diagnosed after symptom presentation, it is crucial to recognize these red-flag signs and symptoms promptly and conduct a diagnostic workup as soon as possible.
“By doing so, we can diagnose the disease earlier, which in turn can reduce the need for more aggressive treatment and improve patients’ quality of life and survival rates,” said Dr. Cao.
The study was supported by grants from the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article originally appeared on Medscape.com.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE