User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
The Role of Dermatology in Identifying and Reporting a Primary Varicella Outbreak
To the Editor:
Cases of primary varicella-zoster virus (VZV) are relatively uncommon in the United States since the introduction of the varicella vaccine in 1995, with an overall decline in cases of more than 97%.1 Prior to the vaccine, 70% of hospitalizations occurred in children; subsequently, hospitalizations among the pediatric population (aged ≤20 years) declined by 97%. Compared to children, adults and immunocompromised patients with VZV infection may present with more severe disease and experience more complications.1
Most children in the United States are vaccinated against VZV, with 90.3% receiving at least 1 dose by 24 months of age.2 However, many countries do not implement universal varicella vaccination for infants.3 As a result, physicians should remember to include primary varicella in the differential when clinically correlated, especially when evaluating patients who have immigrated to the United States or who may be living in unvaccinated communities. We report 2 cases of primary VZV manifesting in adults to remind readers of the salient clinical features of this disease and how dermatologists play a critical role in early and accurate identification of diseases that can have wide-reaching public health implications.
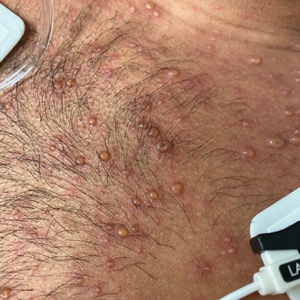
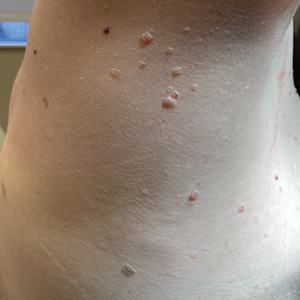
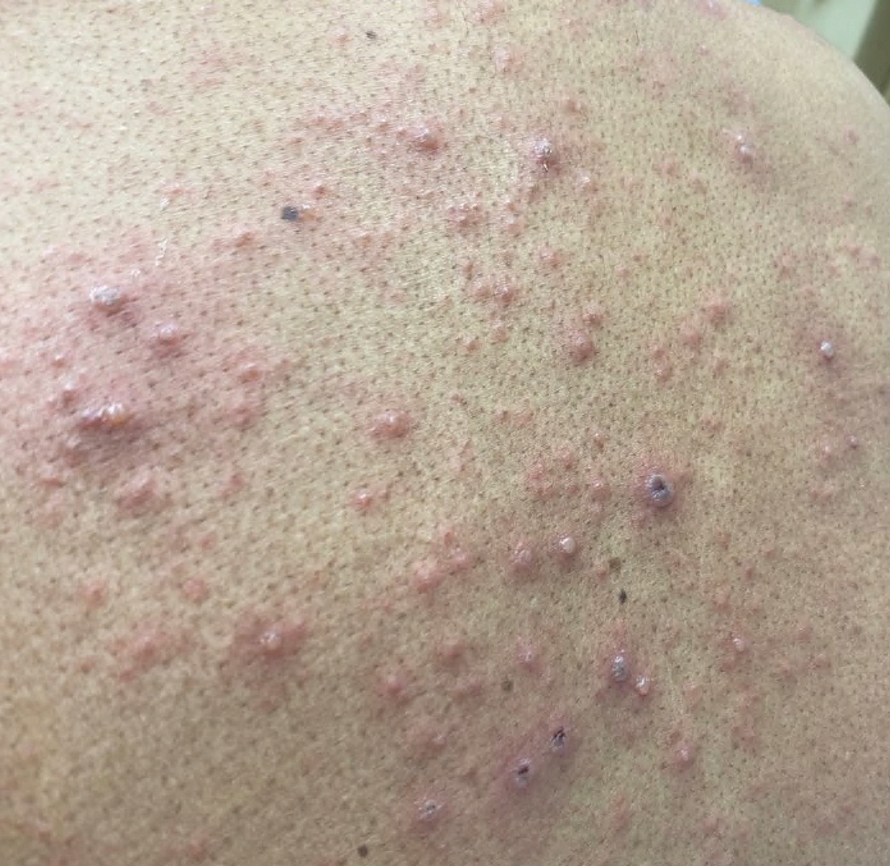
A 26-year-old man with no relevant medical history presented to the emergency department with an itchy and painful rash of 5 days’ duration that began on the trunk and spread to the face, lips, feet, hands, arms, and legs. He also reported shortness of breath, cough, and chills, and he had a temperature of 100.8 °F (38.2 °C). Physical examination revealed numerous erythematous papules and vesiculopustules, some with central umbilication and some with overlying gold crusts (Figure 1).
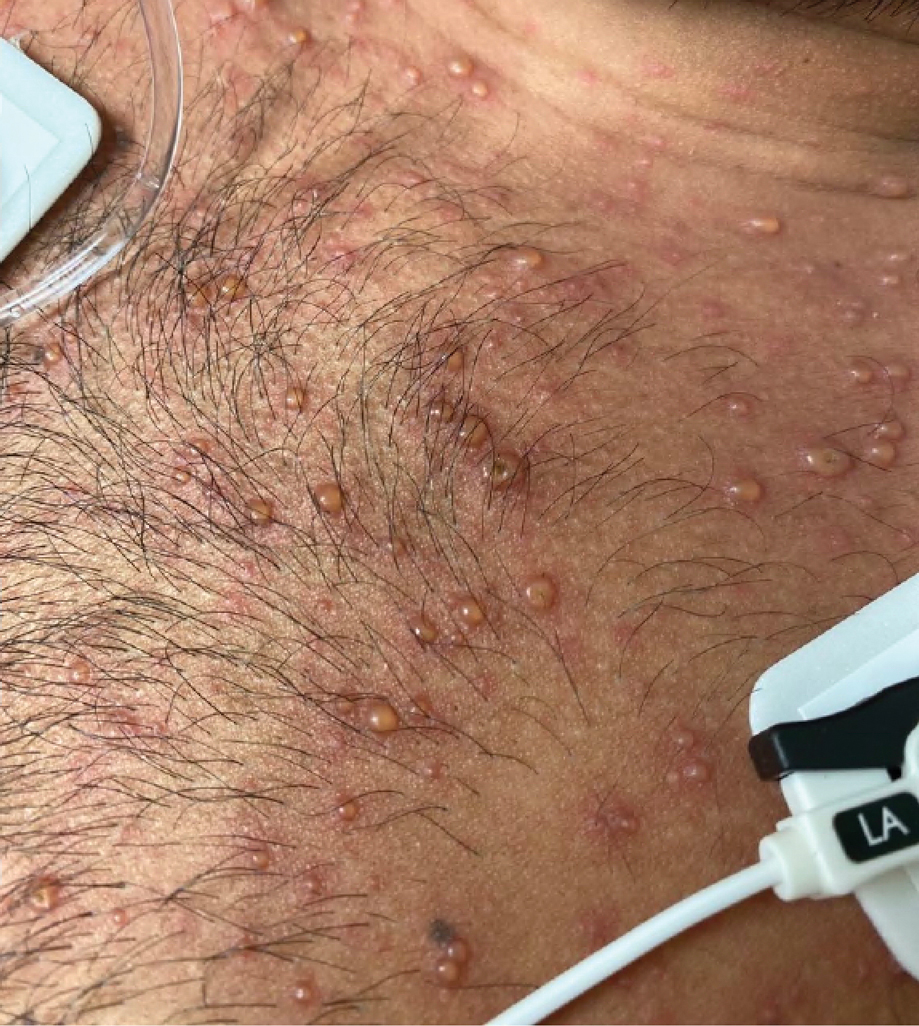
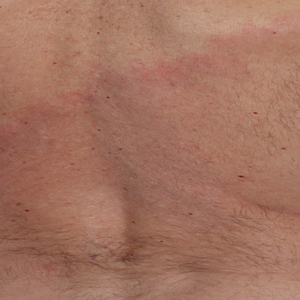
Later that day, a 47-year-old man with no relevant medical history presented to the same emergency department with a rash along with self-reported fever and sore throat of 3 days’ duration. Physical examination found innumerable erythematous vesicopustules scattered on the face, scalp, neck, trunk, arms, and legs, some with a “dew drop on a rose petal” appearance and some with overlying hemorrhagic crust (Figure 2).
Although infection was of primary concern for the first patient, the presentation of the second patient prompted specific concern for primary VZV infection in both patients, who were placed on airborne and contact isolation precautions.
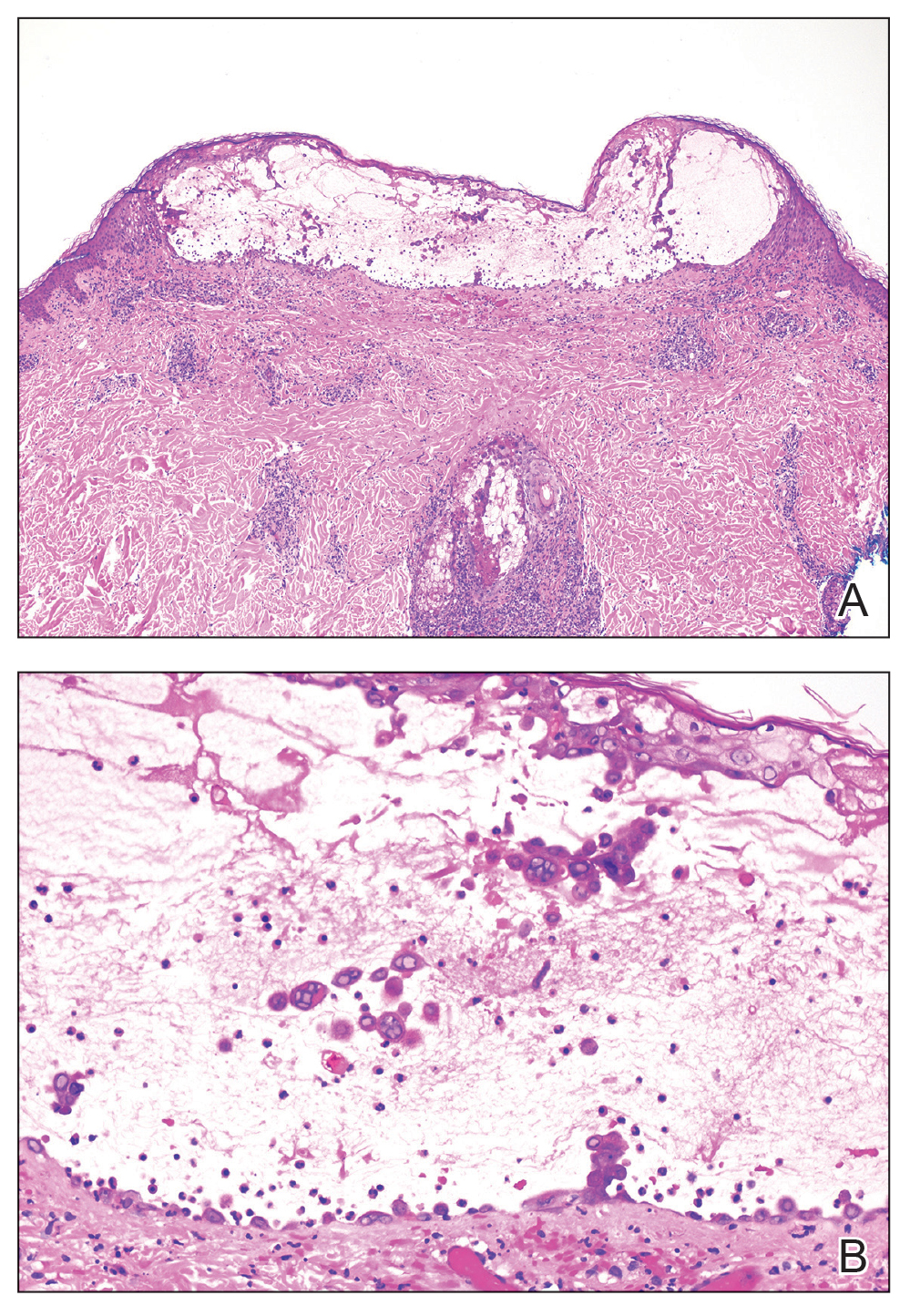
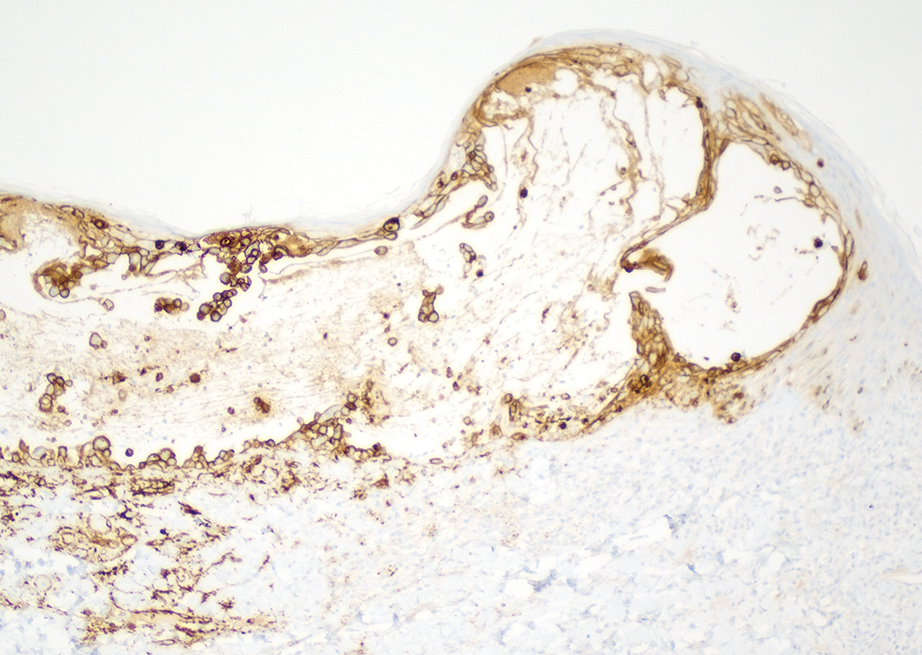
Skin biopsies from both patients showed acantholytic blisters, hair follicle necrosis, and marked dermal inflammation (Figure 3). Herpetic viral changes were seen in keratinocytes, with steel-grey nuclei, multinucleated keratinocytes, and chromatin margination. An immunostain for VZV was diffusely positive, and VZV antibody IgG was positive (Figure 4).
Upon additional questioning, both patients reported recent exposure to VZV-like illnesses in family members without a history of international travel. Neither of the patients was sure of their vaccination status or prior infection history. Both patients received intravenous acyclovir 10 mg/kg administered every 8 hours. Both patients experienced improvement and were discharged after 3 days on oral valacyclovir (1 g 3 times daily for a 7-day treatment course).
The similar presentation and timing of these 2 VZV cases caused concern for an unidentified community outbreak. The infection control team was notified; additionally, per hospital protocol the state health department was alerted as well as the clinicians and staff of the hospital with a request to be vigilant for further cases.
Despite high vaccination rates in the United States, outbreaks of varicella still occur, particularly among unvaccinated individuals, and a robust and efficient response is necessary to control the spread of such outbreaks.4 Many states, including Arkansas where our cases occurred, have laws mandating report of VZV cases to the department of health.5 Dermatologists play an important role in reporting cases, aiding in diagnosis through recognition of the physical examination findings, obtaining appropriate biopsy, and recommending additional laboratory testing.
Typical skin manifestations include a pruritic rash of macules, papules, vesicles, and crusted lesions distributed throughout the trunk, face, arms, and legs. Because new lesions appear over several days, they will be in different stages of healing, resulting in the simultaneous presence of papules, vesicles, and crusted lesions.6 This unique characteristic helps distinguish VZV from other skin diseases such as smallpox or mpox (monkeypox), which generally show lesions in similar stages of evolution.
Biopsy also can aid in identification. Viruses in the herpes family reveal similar histopathologic characteristics, including acantholysis and vesicle formation, intranuclear inclusions with margination of chromatin, multinucleation, and nuclear molding.7 Immunohistochemistry can be used to differentiate VZV from herpes simplex virus; however, neither microscopic examination nor immunohistochemistry distinguish primary VZV infection from herpes zoster (HZ).8
The mpox rash progresses more slowly than a VZV rash and has a centrifugal rather than central distribution that can involve the palms and soles. Lymphadenopathy is a characteristic finding in mpox.9 Rickettsialpox is distinguished from VZV primarily by the appearance of brown or black eschar after the original papulovesicular lesions dry out.10 Atypical hand, foot, and mouth disease can manifest in adults as widespread papulovesicular lesions. This form is associated with coxsackievirus A6 and may require direct fluorescent antibody assay or polymerase chain reaction of keratinocytes to rule out VZV.11
Herpes zoster occurs in older adults with a history of primary VZV.6 It manifests as vesicular lesions confined to 1 or 2 adjacent dermatomes vs the diffuse spread of VZV over the entire body. However, HZ can become disseminated in immunocompromised individuals, making it difficult to clinically distinguish from VZV.6 Serology can be helpful, as high IgM titers indicate an acute primary VZV infection. Subsequently increased IgG titers steadily wane over time and spike during reactivation.12
Dermatology and infectious disease consultations in our cases yielded a preliminary diagnosis through physical examination that was confirmed by biopsy and subsequent laboratory testing, which allowed for a swift response by the infection control team including isolation precautions to control a potential outbreak. Patients with VZV should remain in respiratory isolation until all lesions have crusted over.6
Individuals who had face-to-face indoor contact for at least 5 minutes or who shared a living space with an infected individual should be assessed for VZV immunity, which is defined as confirmed prior immunization or infection.5,13 Lack of VZV immunity requires postexposure prophylaxis—active immunization for the immunocompetent and passive immunization for the immunocompromised.13 Ultimately, no additional cases were reported in the community where our patients resided.
Immunocompetent children with primary VZV require supportive care only. Oral antiviral therapy is the treatment of choice for immunocompetent adults or anyone at increased risk for complications, while intravenous antivirals are recommended for the immunocompromised or those with VZV-related complications.14 A similar approach is used for HZ. Uncomplicated cases are treated with oral antivirals, and complicated cases (eg, HZ ophthalmicus) are treated with intravenous antivirals.15 Commonly used antivirals include acyclovir, valacyclovir, and famciclovir.14
Our cases highlight the ongoing risk for varicella outbreaks in unvaccinated or undervaccinated communities. Physician vigilance is necessary, and dermatology plays a particularly important role in swift and accurate detection of VZV, as demonstrated in our cases by the recognition of classic physical examination findings of erythematous and vesicular papules in each of the patients. Because primary VZV infection can result in life-threatening complications including hepatitis, encephalitis, and pancreatitis, prompt identification and initiation of therapy is important.6 Similarly, quick notification of public health officials about detected primary VZV cases is vital to containing potential community outbreaks.
- Centers for Disease Control and Prevention. Chickenpox (varicella) for healthcare professionals. Published October 21, 2022. Accessed March 6, 2024. https://www.cdc.gov/chickenpox/hcp/index.html#vaccination-impact
- National Center for Health Statistics. Immunization. Published June 13, 2023. Accessed March 6, 2024. https://www.cdc.gov/nchs/fastats/immunize.htm
- Lee YH, Choe YJ, Lee J, et al. Global varicella vaccination programs. Clin Exp Pediatr. 2022;65:555. doi:10.3345/CEP.2021.01564
- Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the Varicella Vaccination Program, 1995–2019. J Infect Dis. 2022;226(suppl 4):S400-S406.
- Arkansas Department of Health. Rules Pertaining to Reportable Diseases. Published September 11, 2023. Accessed March 6, 2024. https://www.healthy.arkansas.gov/images/uploads/rules/ReportableDiseaseList.pdf
- Pergam S, Limaye A; The AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV). Am J Transplant. 2009;9(suppl 4):S108-S115. doi:10.1111/J.1600-9143.2009.02901.X
- Hoyt B, Bhawan J. Histological spectrum of cutaneous herpes infections. Am J Dermatopathol. 2014;36:609-619. doi:10.1097/DAD.0000000000000148
- Oumarou Hama H, Aboudharam G, Barbieri R, et al. Immunohistochemical diagnosis of human infectious diseases: a review. Diagn Pathol. 2022;17. doi:10.1186/S13000-022-01197-5
- World Health Organization. Mpox (monkeypox). Published April 18, 2023. Accessed March 7, 2024. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Akram SM, Jamil RT, Gossman W. Rickettsia akari (Rickettsialpox). StatPearls [Internet]. Updated May 8, 2023. Accessed February 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448081/
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736. doi:10.1016/J.JAAD.2013.07.024
- Petrun B, Williams V, Brice S. Disseminated varicella-zoster virus in an immunocompetent adult. Dermatol Online J. 2015;21. doi:10.5070/D3213022343
- Kimberlin D, Barnett E, Lynfield R, et al. Exposure to specific pathogens. In: Red Book: 2021-2024 Report of the Committee of Infectious Disease. 32nd ed. American Academy of Pediatrics; 2021:1007-1009.
- Treatment of varicella (chickenpox) infection. UpToDate [Internet]. Updated February 7, 2024. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-varicella-chickenpox-infection
- Treatment of herpes zoster in the immunocompetent host. UpToDate [Internet]. Updated November 29, 2023. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-herpes-zoster
To the Editor:
Cases of primary varicella-zoster virus (VZV) are relatively uncommon in the United States since the introduction of the varicella vaccine in 1995, with an overall decline in cases of more than 97%.1 Prior to the vaccine, 70% of hospitalizations occurred in children; subsequently, hospitalizations among the pediatric population (aged ≤20 years) declined by 97%. Compared to children, adults and immunocompromised patients with VZV infection may present with more severe disease and experience more complications.1
Most children in the United States are vaccinated against VZV, with 90.3% receiving at least 1 dose by 24 months of age.2 However, many countries do not implement universal varicella vaccination for infants.3 As a result, physicians should remember to include primary varicella in the differential when clinically correlated, especially when evaluating patients who have immigrated to the United States or who may be living in unvaccinated communities. We report 2 cases of primary VZV manifesting in adults to remind readers of the salient clinical features of this disease and how dermatologists play a critical role in early and accurate identification of diseases that can have wide-reaching public health implications.
A 26-year-old man with no relevant medical history presented to the emergency department with an itchy and painful rash of 5 days’ duration that began on the trunk and spread to the face, lips, feet, hands, arms, and legs. He also reported shortness of breath, cough, and chills, and he had a temperature of 100.8 °F (38.2 °C). Physical examination revealed numerous erythematous papules and vesiculopustules, some with central umbilication and some with overlying gold crusts (Figure 1).

Later that day, a 47-year-old man with no relevant medical history presented to the same emergency department with a rash along with self-reported fever and sore throat of 3 days’ duration. Physical examination found innumerable erythematous vesicopustules scattered on the face, scalp, neck, trunk, arms, and legs, some with a “dew drop on a rose petal” appearance and some with overlying hemorrhagic crust (Figure 2).

Although infection was of primary concern for the first patient, the presentation of the second patient prompted specific concern for primary VZV infection in both patients, who were placed on airborne and contact isolation precautions.
Skin biopsies from both patients showed acantholytic blisters, hair follicle necrosis, and marked dermal inflammation (Figure 3). Herpetic viral changes were seen in keratinocytes, with steel-grey nuclei, multinucleated keratinocytes, and chromatin margination. An immunostain for VZV was diffusely positive, and VZV antibody IgG was positive (Figure 4).

Upon additional questioning, both patients reported recent exposure to VZV-like illnesses in family members without a history of international travel. Neither of the patients was sure of their vaccination status or prior infection history. Both patients received intravenous acyclovir 10 mg/kg administered every 8 hours. Both patients experienced improvement and were discharged after 3 days on oral valacyclovir (1 g 3 times daily for a 7-day treatment course).

The similar presentation and timing of these 2 VZV cases caused concern for an unidentified community outbreak. The infection control team was notified; additionally, per hospital protocol the state health department was alerted as well as the clinicians and staff of the hospital with a request to be vigilant for further cases.
Despite high vaccination rates in the United States, outbreaks of varicella still occur, particularly among unvaccinated individuals, and a robust and efficient response is necessary to control the spread of such outbreaks.4 Many states, including Arkansas where our cases occurred, have laws mandating report of VZV cases to the department of health.5 Dermatologists play an important role in reporting cases, aiding in diagnosis through recognition of the physical examination findings, obtaining appropriate biopsy, and recommending additional laboratory testing.
Typical skin manifestations include a pruritic rash of macules, papules, vesicles, and crusted lesions distributed throughout the trunk, face, arms, and legs. Because new lesions appear over several days, they will be in different stages of healing, resulting in the simultaneous presence of papules, vesicles, and crusted lesions.6 This unique characteristic helps distinguish VZV from other skin diseases such as smallpox or mpox (monkeypox), which generally show lesions in similar stages of evolution.
Biopsy also can aid in identification. Viruses in the herpes family reveal similar histopathologic characteristics, including acantholysis and vesicle formation, intranuclear inclusions with margination of chromatin, multinucleation, and nuclear molding.7 Immunohistochemistry can be used to differentiate VZV from herpes simplex virus; however, neither microscopic examination nor immunohistochemistry distinguish primary VZV infection from herpes zoster (HZ).8
The mpox rash progresses more slowly than a VZV rash and has a centrifugal rather than central distribution that can involve the palms and soles. Lymphadenopathy is a characteristic finding in mpox.9 Rickettsialpox is distinguished from VZV primarily by the appearance of brown or black eschar after the original papulovesicular lesions dry out.10 Atypical hand, foot, and mouth disease can manifest in adults as widespread papulovesicular lesions. This form is associated with coxsackievirus A6 and may require direct fluorescent antibody assay or polymerase chain reaction of keratinocytes to rule out VZV.11
Herpes zoster occurs in older adults with a history of primary VZV.6 It manifests as vesicular lesions confined to 1 or 2 adjacent dermatomes vs the diffuse spread of VZV over the entire body. However, HZ can become disseminated in immunocompromised individuals, making it difficult to clinically distinguish from VZV.6 Serology can be helpful, as high IgM titers indicate an acute primary VZV infection. Subsequently increased IgG titers steadily wane over time and spike during reactivation.12
Dermatology and infectious disease consultations in our cases yielded a preliminary diagnosis through physical examination that was confirmed by biopsy and subsequent laboratory testing, which allowed for a swift response by the infection control team including isolation precautions to control a potential outbreak. Patients with VZV should remain in respiratory isolation until all lesions have crusted over.6
Individuals who had face-to-face indoor contact for at least 5 minutes or who shared a living space with an infected individual should be assessed for VZV immunity, which is defined as confirmed prior immunization or infection.5,13 Lack of VZV immunity requires postexposure prophylaxis—active immunization for the immunocompetent and passive immunization for the immunocompromised.13 Ultimately, no additional cases were reported in the community where our patients resided.
Immunocompetent children with primary VZV require supportive care only. Oral antiviral therapy is the treatment of choice for immunocompetent adults or anyone at increased risk for complications, while intravenous antivirals are recommended for the immunocompromised or those with VZV-related complications.14 A similar approach is used for HZ. Uncomplicated cases are treated with oral antivirals, and complicated cases (eg, HZ ophthalmicus) are treated with intravenous antivirals.15 Commonly used antivirals include acyclovir, valacyclovir, and famciclovir.14
Our cases highlight the ongoing risk for varicella outbreaks in unvaccinated or undervaccinated communities. Physician vigilance is necessary, and dermatology plays a particularly important role in swift and accurate detection of VZV, as demonstrated in our cases by the recognition of classic physical examination findings of erythematous and vesicular papules in each of the patients. Because primary VZV infection can result in life-threatening complications including hepatitis, encephalitis, and pancreatitis, prompt identification and initiation of therapy is important.6 Similarly, quick notification of public health officials about detected primary VZV cases is vital to containing potential community outbreaks.
To the Editor:
Cases of primary varicella-zoster virus (VZV) are relatively uncommon in the United States since the introduction of the varicella vaccine in 1995, with an overall decline in cases of more than 97%.1 Prior to the vaccine, 70% of hospitalizations occurred in children; subsequently, hospitalizations among the pediatric population (aged ≤20 years) declined by 97%. Compared to children, adults and immunocompromised patients with VZV infection may present with more severe disease and experience more complications.1
Most children in the United States are vaccinated against VZV, with 90.3% receiving at least 1 dose by 24 months of age.2 However, many countries do not implement universal varicella vaccination for infants.3 As a result, physicians should remember to include primary varicella in the differential when clinically correlated, especially when evaluating patients who have immigrated to the United States or who may be living in unvaccinated communities. We report 2 cases of primary VZV manifesting in adults to remind readers of the salient clinical features of this disease and how dermatologists play a critical role in early and accurate identification of diseases that can have wide-reaching public health implications.
A 26-year-old man with no relevant medical history presented to the emergency department with an itchy and painful rash of 5 days’ duration that began on the trunk and spread to the face, lips, feet, hands, arms, and legs. He also reported shortness of breath, cough, and chills, and he had a temperature of 100.8 °F (38.2 °C). Physical examination revealed numerous erythematous papules and vesiculopustules, some with central umbilication and some with overlying gold crusts (Figure 1).

Later that day, a 47-year-old man with no relevant medical history presented to the same emergency department with a rash along with self-reported fever and sore throat of 3 days’ duration. Physical examination found innumerable erythematous vesicopustules scattered on the face, scalp, neck, trunk, arms, and legs, some with a “dew drop on a rose petal” appearance and some with overlying hemorrhagic crust (Figure 2).

Although infection was of primary concern for the first patient, the presentation of the second patient prompted specific concern for primary VZV infection in both patients, who were placed on airborne and contact isolation precautions.
Skin biopsies from both patients showed acantholytic blisters, hair follicle necrosis, and marked dermal inflammation (Figure 3). Herpetic viral changes were seen in keratinocytes, with steel-grey nuclei, multinucleated keratinocytes, and chromatin margination. An immunostain for VZV was diffusely positive, and VZV antibody IgG was positive (Figure 4).

Upon additional questioning, both patients reported recent exposure to VZV-like illnesses in family members without a history of international travel. Neither of the patients was sure of their vaccination status or prior infection history. Both patients received intravenous acyclovir 10 mg/kg administered every 8 hours. Both patients experienced improvement and were discharged after 3 days on oral valacyclovir (1 g 3 times daily for a 7-day treatment course).

The similar presentation and timing of these 2 VZV cases caused concern for an unidentified community outbreak. The infection control team was notified; additionally, per hospital protocol the state health department was alerted as well as the clinicians and staff of the hospital with a request to be vigilant for further cases.
Despite high vaccination rates in the United States, outbreaks of varicella still occur, particularly among unvaccinated individuals, and a robust and efficient response is necessary to control the spread of such outbreaks.4 Many states, including Arkansas where our cases occurred, have laws mandating report of VZV cases to the department of health.5 Dermatologists play an important role in reporting cases, aiding in diagnosis through recognition of the physical examination findings, obtaining appropriate biopsy, and recommending additional laboratory testing.
Typical skin manifestations include a pruritic rash of macules, papules, vesicles, and crusted lesions distributed throughout the trunk, face, arms, and legs. Because new lesions appear over several days, they will be in different stages of healing, resulting in the simultaneous presence of papules, vesicles, and crusted lesions.6 This unique characteristic helps distinguish VZV from other skin diseases such as smallpox or mpox (monkeypox), which generally show lesions in similar stages of evolution.
Biopsy also can aid in identification. Viruses in the herpes family reveal similar histopathologic characteristics, including acantholysis and vesicle formation, intranuclear inclusions with margination of chromatin, multinucleation, and nuclear molding.7 Immunohistochemistry can be used to differentiate VZV from herpes simplex virus; however, neither microscopic examination nor immunohistochemistry distinguish primary VZV infection from herpes zoster (HZ).8
The mpox rash progresses more slowly than a VZV rash and has a centrifugal rather than central distribution that can involve the palms and soles. Lymphadenopathy is a characteristic finding in mpox.9 Rickettsialpox is distinguished from VZV primarily by the appearance of brown or black eschar after the original papulovesicular lesions dry out.10 Atypical hand, foot, and mouth disease can manifest in adults as widespread papulovesicular lesions. This form is associated with coxsackievirus A6 and may require direct fluorescent antibody assay or polymerase chain reaction of keratinocytes to rule out VZV.11
Herpes zoster occurs in older adults with a history of primary VZV.6 It manifests as vesicular lesions confined to 1 or 2 adjacent dermatomes vs the diffuse spread of VZV over the entire body. However, HZ can become disseminated in immunocompromised individuals, making it difficult to clinically distinguish from VZV.6 Serology can be helpful, as high IgM titers indicate an acute primary VZV infection. Subsequently increased IgG titers steadily wane over time and spike during reactivation.12
Dermatology and infectious disease consultations in our cases yielded a preliminary diagnosis through physical examination that was confirmed by biopsy and subsequent laboratory testing, which allowed for a swift response by the infection control team including isolation precautions to control a potential outbreak. Patients with VZV should remain in respiratory isolation until all lesions have crusted over.6
Individuals who had face-to-face indoor contact for at least 5 minutes or who shared a living space with an infected individual should be assessed for VZV immunity, which is defined as confirmed prior immunization or infection.5,13 Lack of VZV immunity requires postexposure prophylaxis—active immunization for the immunocompetent and passive immunization for the immunocompromised.13 Ultimately, no additional cases were reported in the community where our patients resided.
Immunocompetent children with primary VZV require supportive care only. Oral antiviral therapy is the treatment of choice for immunocompetent adults or anyone at increased risk for complications, while intravenous antivirals are recommended for the immunocompromised or those with VZV-related complications.14 A similar approach is used for HZ. Uncomplicated cases are treated with oral antivirals, and complicated cases (eg, HZ ophthalmicus) are treated with intravenous antivirals.15 Commonly used antivirals include acyclovir, valacyclovir, and famciclovir.14
Our cases highlight the ongoing risk for varicella outbreaks in unvaccinated or undervaccinated communities. Physician vigilance is necessary, and dermatology plays a particularly important role in swift and accurate detection of VZV, as demonstrated in our cases by the recognition of classic physical examination findings of erythematous and vesicular papules in each of the patients. Because primary VZV infection can result in life-threatening complications including hepatitis, encephalitis, and pancreatitis, prompt identification and initiation of therapy is important.6 Similarly, quick notification of public health officials about detected primary VZV cases is vital to containing potential community outbreaks.
- Centers for Disease Control and Prevention. Chickenpox (varicella) for healthcare professionals. Published October 21, 2022. Accessed March 6, 2024. https://www.cdc.gov/chickenpox/hcp/index.html#vaccination-impact
- National Center for Health Statistics. Immunization. Published June 13, 2023. Accessed March 6, 2024. https://www.cdc.gov/nchs/fastats/immunize.htm
- Lee YH, Choe YJ, Lee J, et al. Global varicella vaccination programs. Clin Exp Pediatr. 2022;65:555. doi:10.3345/CEP.2021.01564
- Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the Varicella Vaccination Program, 1995–2019. J Infect Dis. 2022;226(suppl 4):S400-S406.
- Arkansas Department of Health. Rules Pertaining to Reportable Diseases. Published September 11, 2023. Accessed March 6, 2024. https://www.healthy.arkansas.gov/images/uploads/rules/ReportableDiseaseList.pdf
- Pergam S, Limaye A; The AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV). Am J Transplant. 2009;9(suppl 4):S108-S115. doi:10.1111/J.1600-9143.2009.02901.X
- Hoyt B, Bhawan J. Histological spectrum of cutaneous herpes infections. Am J Dermatopathol. 2014;36:609-619. doi:10.1097/DAD.0000000000000148
- Oumarou Hama H, Aboudharam G, Barbieri R, et al. Immunohistochemical diagnosis of human infectious diseases: a review. Diagn Pathol. 2022;17. doi:10.1186/S13000-022-01197-5
- World Health Organization. Mpox (monkeypox). Published April 18, 2023. Accessed March 7, 2024. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Akram SM, Jamil RT, Gossman W. Rickettsia akari (Rickettsialpox). StatPearls [Internet]. Updated May 8, 2023. Accessed February 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448081/
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736. doi:10.1016/J.JAAD.2013.07.024
- Petrun B, Williams V, Brice S. Disseminated varicella-zoster virus in an immunocompetent adult. Dermatol Online J. 2015;21. doi:10.5070/D3213022343
- Kimberlin D, Barnett E, Lynfield R, et al. Exposure to specific pathogens. In: Red Book: 2021-2024 Report of the Committee of Infectious Disease. 32nd ed. American Academy of Pediatrics; 2021:1007-1009.
- Treatment of varicella (chickenpox) infection. UpToDate [Internet]. Updated February 7, 2024. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-varicella-chickenpox-infection
- Treatment of herpes zoster in the immunocompetent host. UpToDate [Internet]. Updated November 29, 2023. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-herpes-zoster
- Centers for Disease Control and Prevention. Chickenpox (varicella) for healthcare professionals. Published October 21, 2022. Accessed March 6, 2024. https://www.cdc.gov/chickenpox/hcp/index.html#vaccination-impact
- National Center for Health Statistics. Immunization. Published June 13, 2023. Accessed March 6, 2024. https://www.cdc.gov/nchs/fastats/immunize.htm
- Lee YH, Choe YJ, Lee J, et al. Global varicella vaccination programs. Clin Exp Pediatr. 2022;65:555. doi:10.3345/CEP.2021.01564
- Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the Varicella Vaccination Program, 1995–2019. J Infect Dis. 2022;226(suppl 4):S400-S406.
- Arkansas Department of Health. Rules Pertaining to Reportable Diseases. Published September 11, 2023. Accessed March 6, 2024. https://www.healthy.arkansas.gov/images/uploads/rules/ReportableDiseaseList.pdf
- Pergam S, Limaye A; The AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV). Am J Transplant. 2009;9(suppl 4):S108-S115. doi:10.1111/J.1600-9143.2009.02901.X
- Hoyt B, Bhawan J. Histological spectrum of cutaneous herpes infections. Am J Dermatopathol. 2014;36:609-619. doi:10.1097/DAD.0000000000000148
- Oumarou Hama H, Aboudharam G, Barbieri R, et al. Immunohistochemical diagnosis of human infectious diseases: a review. Diagn Pathol. 2022;17. doi:10.1186/S13000-022-01197-5
- World Health Organization. Mpox (monkeypox). Published April 18, 2023. Accessed March 7, 2024. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Akram SM, Jamil RT, Gossman W. Rickettsia akari (Rickettsialpox). StatPearls [Internet]. Updated May 8, 2023. Accessed February 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448081/
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736. doi:10.1016/J.JAAD.2013.07.024
- Petrun B, Williams V, Brice S. Disseminated varicella-zoster virus in an immunocompetent adult. Dermatol Online J. 2015;21. doi:10.5070/D3213022343
- Kimberlin D, Barnett E, Lynfield R, et al. Exposure to specific pathogens. In: Red Book: 2021-2024 Report of the Committee of Infectious Disease. 32nd ed. American Academy of Pediatrics; 2021:1007-1009.
- Treatment of varicella (chickenpox) infection. UpToDate [Internet]. Updated February 7, 2024. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-varicella-chickenpox-infection
- Treatment of herpes zoster in the immunocompetent host. UpToDate [Internet]. Updated November 29, 2023. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-herpes-zoster
Practice Points
- Primary varicella is a relatively infrequent occurrence since the introduction of vaccination, creating the need for a reminder on the importance of including it in the differential when clinically appropriate.
- When outbreaks do happen, typically among unvaccinated communities, swift identification via physical examination and histology is imperative to allow infection control teams and public health officials to quickly take action.
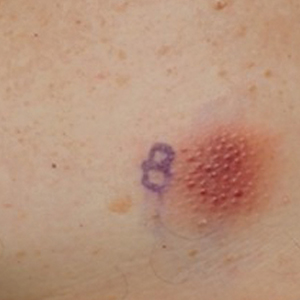
Asymptomatic Erythematous Plaque in an Outdoorsman
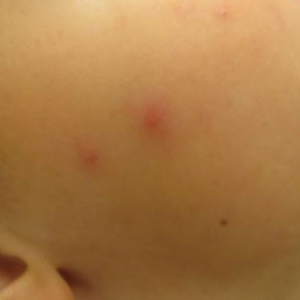
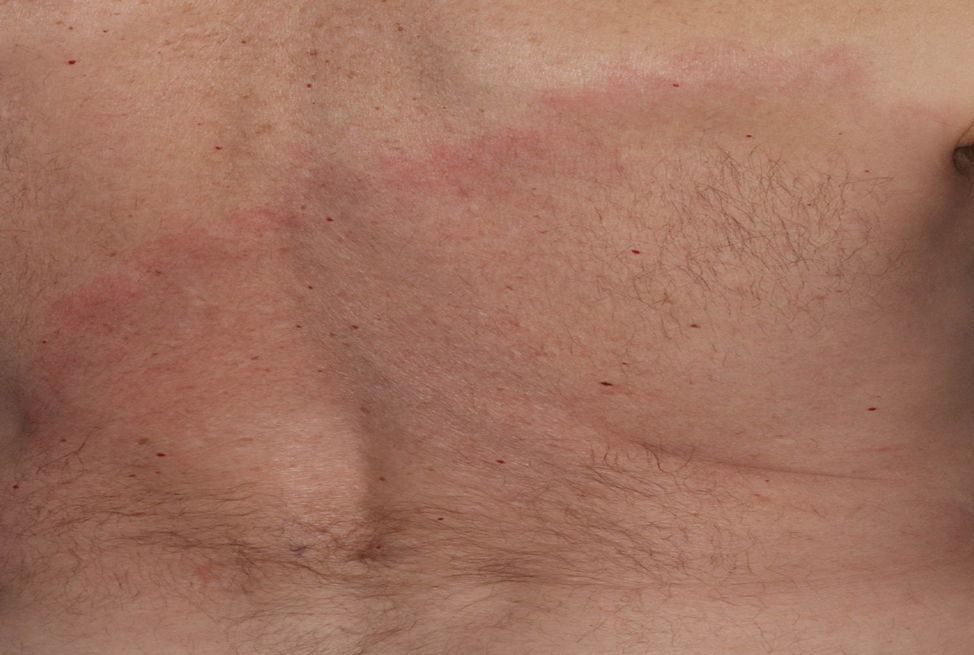
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
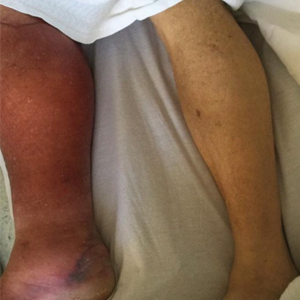
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
A middle-aged man presented with a well-demarcated, hyperpigmented, erythematous patch with an annular erythematous border that extended from the mid-back to the lower back. The patient was otherwise asymptomatic. He was an avid gardener who resided in South Carolina and had recently adopted 2 puppies.
DermGPT Can Help Improve Your Office Productivity
For anyone (physicians included) concerned about whether generative artificial intelligence (AI) tools will take your job, the likely answer is no—but those who use generative AI will have an advantage, according to Faranak (Fara) Kamangar, MD, Department Chair, Palo Alto Medical Foundation, who presented on AI at the 2024 Annual Meeting of the American Academy of Dermatology, San Diego, California.
Dr. Kamangar is a dermatologist and inventor of DermGPT, an AI tool created specifically to help health care providers with clinical tasks to increase productivity. According to Dr. Kamangar, “For every 8 hours of scheduled patient time, ambulatory physicians spend more than 5 hours on the [electronic health record].” Her advice is to use AI when you can to complete clinical tasks and move on.
DermGPT utilizes a learned language model that is based on dermatology knowledge acquired from more than 3000 peer-reviewed articles and texts (eg, systematic literature reviews, other published sources in the field of dermatology). Search output includes citations so that users can confirm that the answers and sources are accurate. “Still, with all of these safeguards, all AI models can create inaccuracies and it is important to proofread all content,” says Dr. Kamangar.
During her presentation, Dr. Kamangar gave the following examples of potentially useful DermGPT prompts for dermatologists:
- Can you help me write a response to a denial letter to an insurance company for upadacitinib in a patient with atopic dermatitis?
- I am seeing a patient with blisters. What is the differential diagnosis?
- I am prescribing bimekizumab. What labs do I need to check?
Other potential time-saving uses for a dermatologist include:
- prior authorization letters
- coding support
- responses to common patient messages
- letters of recommendation
- information on new treatments.
In Dr. Kamangar’s practice, they have been able to save at least 30 to 60 minutes at the end of the day that is usually spent updating the electronic health record. “This allows us to complete the clinic day much earlier and does not leave work that will spill over to the next day,” she shares. “During my workday, I have [DermGPT] open on my computer, and as clinical tasks arise, if they require more information or assistance, I turn to DermGPT to help de-escalate the task from a moderate to difficult level to an easy task that can be easily managed.”
The next stage of health technology—AI—is here, and physicians are understandably cautious. Board-certified dermatologists, dermatology residents, fellows, and medical students can try DermGPT for free at https://www.dermgpt.com/.
Dr. Kamangar is the founder of DermGPT.
Melissa Sears is the Director, Editorial, of Cutis.
For anyone (physicians included) concerned about whether generative artificial intelligence (AI) tools will take your job, the likely answer is no—but those who use generative AI will have an advantage, according to Faranak (Fara) Kamangar, MD, Department Chair, Palo Alto Medical Foundation, who presented on AI at the 2024 Annual Meeting of the American Academy of Dermatology, San Diego, California.
Dr. Kamangar is a dermatologist and inventor of DermGPT, an AI tool created specifically to help health care providers with clinical tasks to increase productivity. According to Dr. Kamangar, “For every 8 hours of scheduled patient time, ambulatory physicians spend more than 5 hours on the [electronic health record].” Her advice is to use AI when you can to complete clinical tasks and move on.
DermGPT utilizes a learned language model that is based on dermatology knowledge acquired from more than 3000 peer-reviewed articles and texts (eg, systematic literature reviews, other published sources in the field of dermatology). Search output includes citations so that users can confirm that the answers and sources are accurate. “Still, with all of these safeguards, all AI models can create inaccuracies and it is important to proofread all content,” says Dr. Kamangar.
During her presentation, Dr. Kamangar gave the following examples of potentially useful DermGPT prompts for dermatologists:
- Can you help me write a response to a denial letter to an insurance company for upadacitinib in a patient with atopic dermatitis?
- I am seeing a patient with blisters. What is the differential diagnosis?
- I am prescribing bimekizumab. What labs do I need to check?
Other potential time-saving uses for a dermatologist include:
- prior authorization letters
- coding support
- responses to common patient messages
- letters of recommendation
- information on new treatments.
In Dr. Kamangar’s practice, they have been able to save at least 30 to 60 minutes at the end of the day that is usually spent updating the electronic health record. “This allows us to complete the clinic day much earlier and does not leave work that will spill over to the next day,” she shares. “During my workday, I have [DermGPT] open on my computer, and as clinical tasks arise, if they require more information or assistance, I turn to DermGPT to help de-escalate the task from a moderate to difficult level to an easy task that can be easily managed.”
The next stage of health technology—AI—is here, and physicians are understandably cautious. Board-certified dermatologists, dermatology residents, fellows, and medical students can try DermGPT for free at https://www.dermgpt.com/.
Dr. Kamangar is the founder of DermGPT.
Melissa Sears is the Director, Editorial, of Cutis.
For anyone (physicians included) concerned about whether generative artificial intelligence (AI) tools will take your job, the likely answer is no—but those who use generative AI will have an advantage, according to Faranak (Fara) Kamangar, MD, Department Chair, Palo Alto Medical Foundation, who presented on AI at the 2024 Annual Meeting of the American Academy of Dermatology, San Diego, California.
Dr. Kamangar is a dermatologist and inventor of DermGPT, an AI tool created specifically to help health care providers with clinical tasks to increase productivity. According to Dr. Kamangar, “For every 8 hours of scheduled patient time, ambulatory physicians spend more than 5 hours on the [electronic health record].” Her advice is to use AI when you can to complete clinical tasks and move on.
DermGPT utilizes a learned language model that is based on dermatology knowledge acquired from more than 3000 peer-reviewed articles and texts (eg, systematic literature reviews, other published sources in the field of dermatology). Search output includes citations so that users can confirm that the answers and sources are accurate. “Still, with all of these safeguards, all AI models can create inaccuracies and it is important to proofread all content,” says Dr. Kamangar.
During her presentation, Dr. Kamangar gave the following examples of potentially useful DermGPT prompts for dermatologists:
- Can you help me write a response to a denial letter to an insurance company for upadacitinib in a patient with atopic dermatitis?
- I am seeing a patient with blisters. What is the differential diagnosis?
- I am prescribing bimekizumab. What labs do I need to check?
Other potential time-saving uses for a dermatologist include:
- prior authorization letters
- coding support
- responses to common patient messages
- letters of recommendation
- information on new treatments.
In Dr. Kamangar’s practice, they have been able to save at least 30 to 60 minutes at the end of the day that is usually spent updating the electronic health record. “This allows us to complete the clinic day much earlier and does not leave work that will spill over to the next day,” she shares. “During my workday, I have [DermGPT] open on my computer, and as clinical tasks arise, if they require more information or assistance, I turn to DermGPT to help de-escalate the task from a moderate to difficult level to an easy task that can be easily managed.”
The next stage of health technology—AI—is here, and physicians are understandably cautious. Board-certified dermatologists, dermatology residents, fellows, and medical students can try DermGPT for free at https://www.dermgpt.com/.
Dr. Kamangar is the founder of DermGPT.
Melissa Sears is the Director, Editorial, of Cutis.
Skin Lesions on the Face and Chest
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
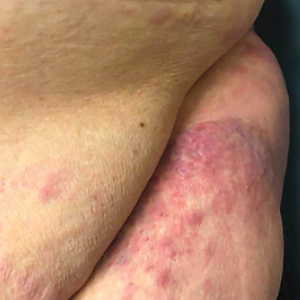
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.
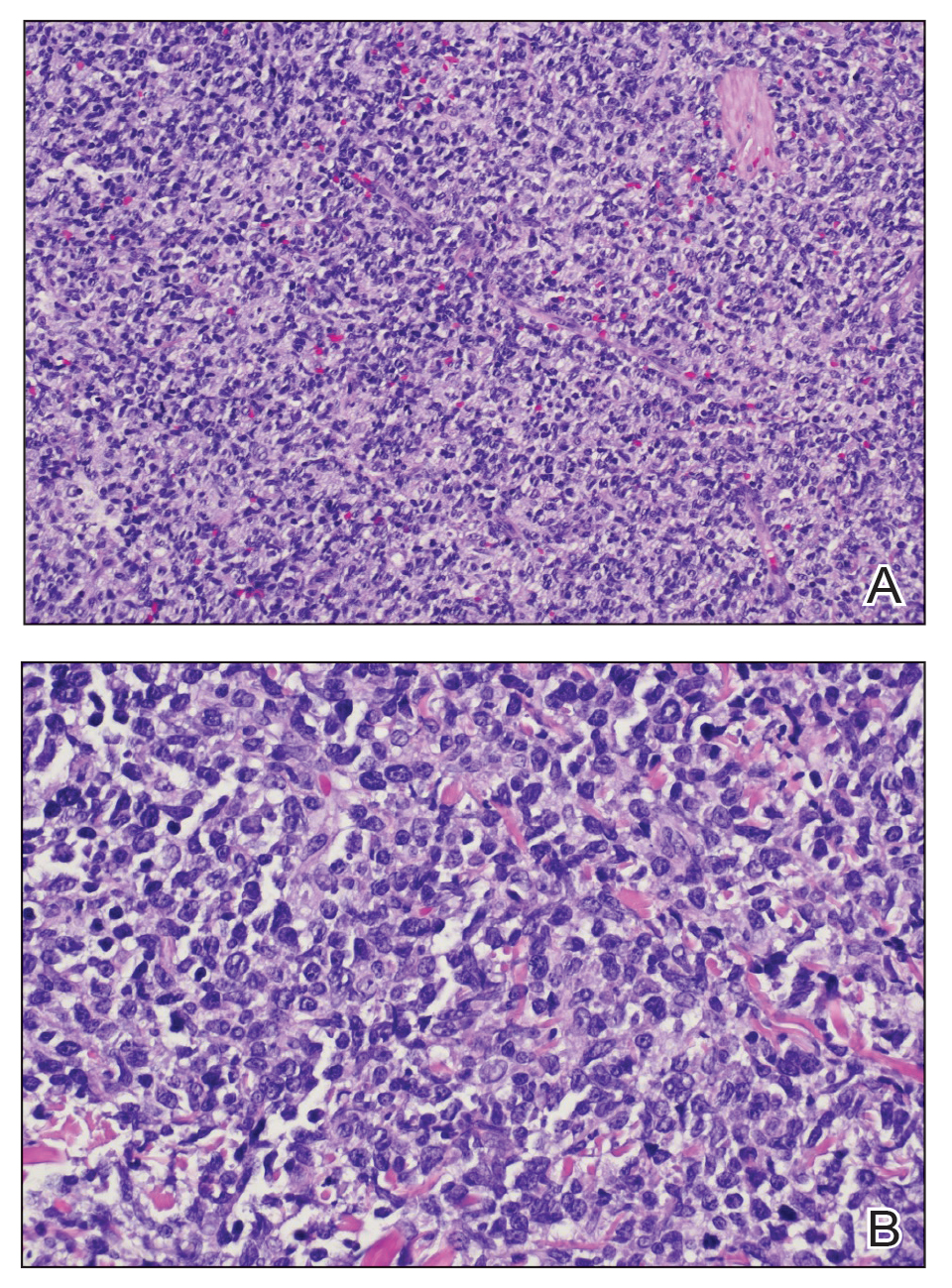
Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.

Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.

Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
A 79-year-old man presented to the dermatology clinic with multiple skin lesions of 4 months’ duration. The patient had a history of monoclonal gammopathy and reported no changes in medication, travel, or trauma. He reported tenderness only when trying to comb hair over the left occipital nodule. He denied fevers, night sweats, weight loss, or poor appetite. Physical examination revealed 4 concerning skin lesions: a 3×3-cm violaceous nodule with underlying ecchymosis on the right medial jaw (top), a 3×2.5-cm violaceous nodule on the posterior occiput, a pink plaque with 1-mm vascular papules on the right mid-chest (bottom), and a 4×2.5-cm oval pink patch on the left side of the lower back. Punch biopsies were performed on the right medial jaw nodule and right mid-chest plaque.
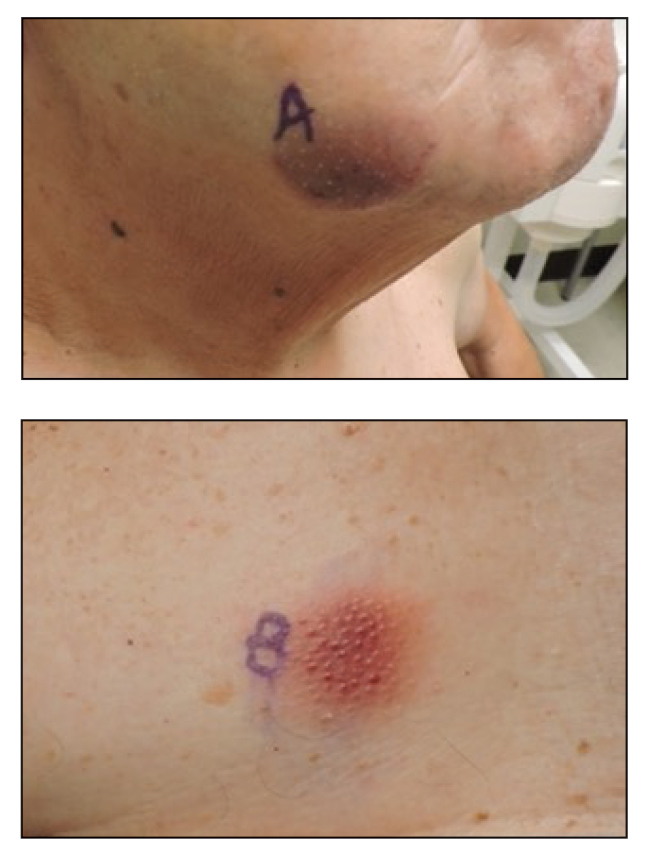
Rare Cutaneous Presentation of Burkitt Lymphoma
To the Editor:
A 73-year-old man was admitted to the hospital with progressive abdominal and hip pain of several weeks’ duration that was accompanied by unilateral swelling of the left leg. He had a medical history of hypertension, hyperlipidemia, and prediabetes. Computed tomography (CT) showed extensive intra-abdominal, retroperitoneal, and pelvic lymphadenopathy in addition to poorly defined hepatic lesions.
A CT-guided core biopsy of a left inguinal lymph node showed Burkitt lymphoma. Fluorescence in situ hybridization was positive for oncogene c-MYC rearrangement on chromosome 8q24 and negative for B-cell lymphoma 2 (BCL2) and B-cell lymphoma 6 (BCL6) gene rearrangements. Flow cytometry demonstrated an aberrant population of κ light chain-restricted CD5−CD10+ B lymphocytes.
The patient’s overall disease burden was consistent with stage IV Burkitt lymphoma. R-miniCHOP chemotherapy—rituximab plus a reduced dose of cyclophosphamide, doxorubicin, vincristine sulfate, and prednisone—was initiated. Approximately 2 weeks after chemotherapy was initiated, the patient developed a firm erythematous eruption on the left hip (Figure 1A). His regimen was then switched to R-EPOCH—rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin—at the time of discharge, and he was referred to dermatology due to an initial concern of an adverse reaction to R-EPOCH chemotherapy. The patient denied any pain, pruritus, or irritation. Physical examination showed multifocal, subcutaneous, indurated, erythematous and violaceous nodules without epidermal changes. Some nodules on the lateral aspect of the hip coalesced to form firm plaques.
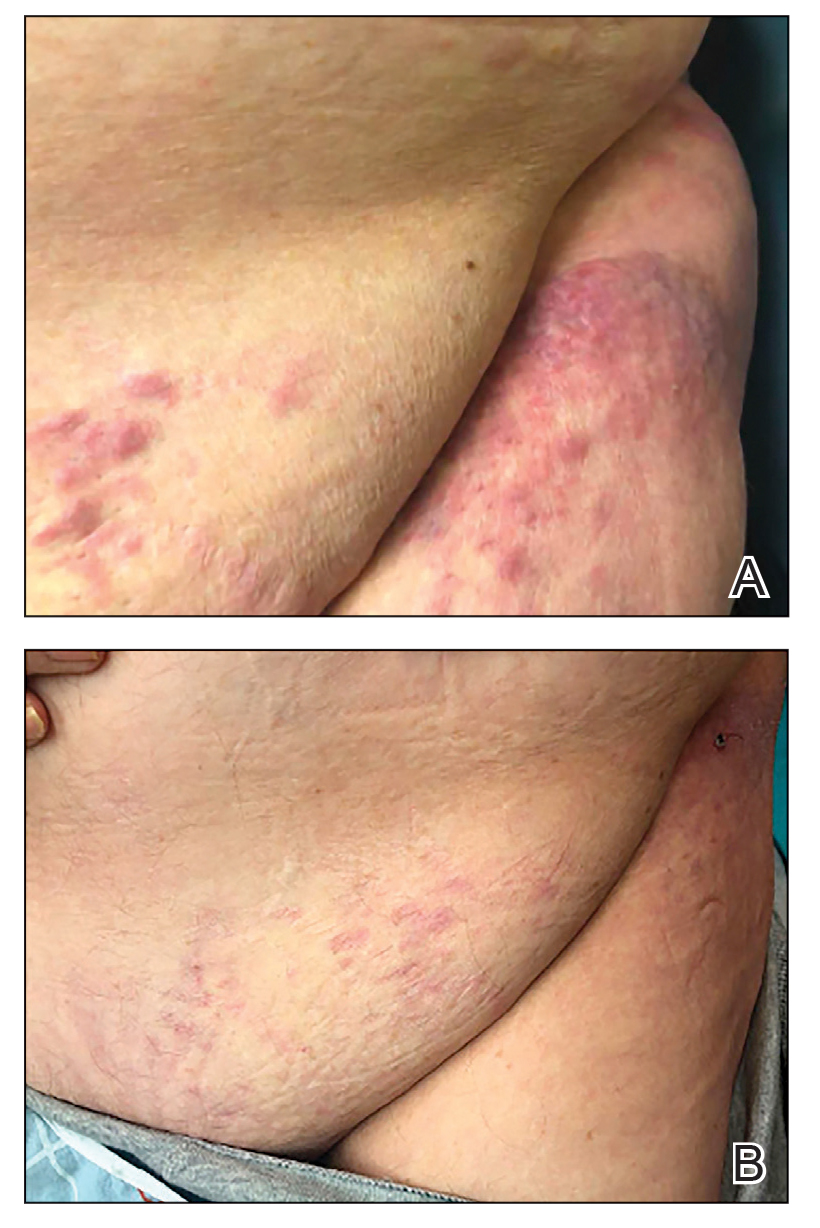
A punch biopsy specimen showed markedly atypical lymphocytes with enlarged nuclei and scant cytoplasm present throughout the dermis (Figures 2A and 2B). Numerous apoptotic cells and cellular debris were seen. Immunohistochemical staining demonstrated that the lymphocytic infiltrate comprised CD79a+ B cells that were positive for Bcl-6 and CD10 and negative for Bcl-2 (Figures 2C and 2D). There also was diminished focal expression of CD20. Ki-67 protein staining was intensely positive and demonstrated a very high proliferative index.
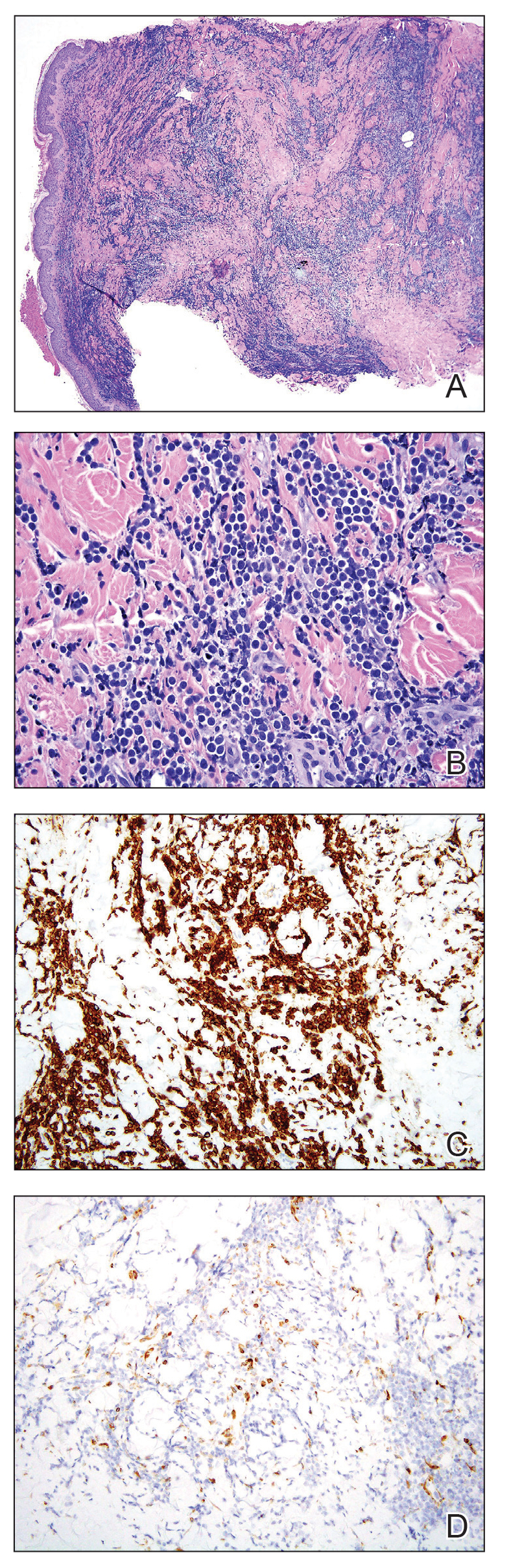
Taken together, these findings were consistent with a diagnosis of cutaneous metastasis of Burkitt lymphoma. The patient’s cutaneous lesions improved after continued aggressive chemotherapy. At follow-up 2 weeks after biopsy, he was receiving his second round of R-EPOCH chemotherapy with appreciable regression of skin lesions (Figure 1B). However, he then developed right-side double vision, ptosis, and right-side facial paresthesia. Although magnetic resonance imaging of the brain and lumbar puncture did not show evidence of central nervous system involvement, the chemotherapy regimen was switched to dose-adjusted CVAD-R—hypercyclophosphamide, vincristine, doxorubicin hydrochloride, and dexamethasone plus rituximab—for empiric treatment of central nervous system disease. Although treatment was complicated by sepsis with extended-spectrum β-lactamase-producing Enterobacter cloacae, Burkitt lymphoma was found to be in remission after 3 cycles of CVAD-R and 5 months of chemotherapy.
Burkitt lymphoma is a B-cell non-Hodgkin malignancy caused by translocation of chromosome 8 and chromosome 14, leading to overexpression of c-MYC and subsequent hyperproliferation of B lymphocytes.1,2 The disease is divided into 3 major categories: sporadic, endemic, and immunodeficiency related.3 The endemic variant is the most prevalent subtype in Africa and is associated with Plasmodium falciparum malaria; the sporadic variant is the most common subtype in the rest of the world.4
Burkitt lymphoma is highly aggressive and is characterized by unusually high rates of mitosis and apoptosis that result in abundant cellular debris and a distinctive starry-sky pattern on histopathology.5,6 Extranodal metastasis is common,7 but cutaneous involvement is exceedingly rare, with only a few cases having been reported.8-14 Cutaneous metastasis of Burkitt lymphoma often is associated with a high overall disease burden and poor prognosis.8,11
Immunodeficiency-related Burkitt lymphoma is particularly aggressive. Notably, 3 of 7 (42.9%) reported cases of cutaneous Burkitt lymphoma occurred in HIV-positive patients.11,13 In one case, cutaneous involvement was the first sign of relapsed disease that had been in remission.12
Although c-MYC rearrangement is required to make a diagnosis of Burkitt lymphoma, the disease also is present in a minority of cases of diffuse large B-cell lymphoma (DLBCL)(6%).15 Although DLBCL typically can be differentiated from Burkitt lymphoma by the large nuclear size and characteristic vesicular nuclei of B cells, few cases of DLBCL with c-MYC rearrangement histologically mimic Burkitt lymphoma. However, key features such as immunohistochemical staining for Bcl-2 and CD10 can be used to distinguish these 2 entities.16 Bcl-2 negativity and CD10 positivity, as seen in our patient, is considered more characteristic of Burkitt lymphoma. This staining pattern in combination with a high Ki-67 fraction (>95%) and the presence of monomorphic medium-sized cells is more consistent with a diagnosis of Burkitt lymphoma than of DLBCL.17
Earlier case reports have documented that cutaneous lesions of Burkitt lymphoma can occur in a variety of ways. Hematogenous spread is the likely route of metastasis for lesions distant to the primary site or those that have widespread distribution.18 Alternatively, other reports have suggested that cutaneous metastases can occur from local invasion and subcutaneous extension of malignant cells after a surgical procedure.10,19 For example, cutaneous Burkitt lymphoma has been reported in the setting of celioscopy, occurring directly at the surgical site.19 In our patient, we believe that the route of metastatic spread likely was through subcutaneous invasion secondary to CT-guided core biopsy, which was supported by the observation that the onset of cutaneous manifestations was temporally related to the procedure and that the lesions occurred on the skin directly overlying the biopsy site.
In conclusion, we describe an exceedingly rare presentation of cutaneous Burkitt lymphoma in which a surgical procedure likely served as an inciting event that triggered seeding of malignant cells to the skin. Cutaneous spread of Burkitt lymphoma is infrequently reported; all such reports that provide long-term follow-up data have described it in association with high disease burden and often a lethal outcome.8,11,12 Our patient had complete resolution of cutaneous lesions with chemotherapy. It is unclear if the presence of cutaneous lesions can serve as a prognostic indicator and requires further investigation. However, our case provides preliminary evidence to suggest that cutaneous metastases do not always represent aggressive disease and that cutaneous lesions may respond well to chemotherapy.
- Kalisz K, Alessandrino F, Beck R, et al. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019;10:56. doi:10.1186/s13244-019-0733-7
- Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375. doi:10.1182/blood-2018-02-778480
- Noy A. Burkitt lymphoma—subtypes, pathogenesis, and treatment strategies. Clin Lymphoma Myeloma Leuk. 2020;20(Suppl 1):S37-S38. doi:10.1016/S2152-2650(20)30455-9
- Lenze D, Leoncini L, Hummel M, et al. The different epidemiologic subtypes of Burkitt lymphoma share a homogenous micro RNA profile distinct from diffuse large B-cell lymphoma. Leukemia. 2011;25:1869-1876. doi:10.1038/leu.2011.156
- Bellan C, Lazzi S, De Falco G, et al. Burkitt’s lymphoma: new insights into molecular pathogenesis. J Clin Pathol. 2003;56:188-192. doi:10.1136/jcp.56.3.188
- Chuang S-S, Ye H, Du M-Q, et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol. 2007;128:558-564. doi:10.1309/EQJR3D3V0CCQGP04
- Baker PS, Gold KG, Lane KA, et al. Orbital burkitt lymphoma in immunocompetent patients: a report of 3 cases and a review of the literature. Ophthalmic Plast Reconstr Surg. 2009;25:464-468. doi:10.1097/IOP.0b013e3181b80fde
- Fuhrmann TL, Ignatovich YV, Pentland A. Cutaneous metastatic disease: Burkitt lymphoma. J Am Acad Dermatol. 2011;64:1196-1197. doi:10.1016/j.jaad.2009.08.033
- Burns CA, Scott GA, Miller CC. Leukemia cutis at the site of trauma in a patient with Burkitt leukemia. Cutis. 2005;75:54-56.
- Jacobson MA, Hutcheson ACS, Hurray DH, et al. Cutaneous involvement by Burkitt lymphoma. J Am Acad Dermatol. 2006;54:1111-1113. doi:10.1016/j.jaad.2006.02.030
- Berk DR, Cheng A, Lind AC, et al. Burkitt lymphoma with cutaneous involvement. Dermatol Online J. 2008;14:14.
- Bachmeyer C, Bazarbachi A, Rio B, et al. Specific cutaneous involvement indicating relapse of Burkitt’s lymphoma. Am J Hematol. 1997;54:176. doi:10.1002/(sici)1096-8652(199702)54:2<176::aid-ajh20>3.0.co;2-c
- Rogers A, Graves M, Toscano M, et al. A unique cutaneous presentation of Burkitt lymphoma. Am J Dermatopathol. 2014;36:997-1001. doi:10.1097/DAD.0000000000000004
- Thakkar D, Lipi L, Misra R, et al. Skin involvement in Burkitt’s lymphoma. Hematol Oncol Stem Cell Ther. 2018;11:251-252. doi:10.1016/j.hemonc.2018.01.002
- Akasaka T, Akasaka H, Ueda C, et al. Molecular and clinical features of non-Burkitt’s, diffuse large-cell lymphoma of B-cell type associated with the c-MYC/immunoglobulin heavy-chain fusion gene. J Clin Oncol. 2000;18:510-518. doi:10.1200/JCO.2000.18.3.510
- Nakamura N, Nakamine H, Tamaru J-I, et al. The distinction between Burkitt lymphoma and diffuse large B-cell lymphoma with c-myc rearrangement. Mod Pathol. 2002;15:771-776. doi:10.1097/01.MP.0000019577.73786.64
- Bellan C, Stefano L, Giulia de F, et al. Burkitt lymphoma versus diffuse large B-cell lymphoma: a practical approach. Hematol Oncol. 2010;28:53-56. doi:10.1002/hon.916
- Amonchaisakda N, Aiempanakit K, Apinantriyo B. Burkitt lymphoma initially mimicking varicella zoster infection. IDCases. 2020;21:E00818. doi:10.1016/j.idcr.2020.e00818
- Aractingi S, Marolleau JP, Daniel MT, et al. Subcutaneous localizations of Burkitt lymphoma after celioscopy. Am J Hematol. 1993;42:408. doi:10.1002/ajh.2830420421
To the Editor:
A 73-year-old man was admitted to the hospital with progressive abdominal and hip pain of several weeks’ duration that was accompanied by unilateral swelling of the left leg. He had a medical history of hypertension, hyperlipidemia, and prediabetes. Computed tomography (CT) showed extensive intra-abdominal, retroperitoneal, and pelvic lymphadenopathy in addition to poorly defined hepatic lesions.
A CT-guided core biopsy of a left inguinal lymph node showed Burkitt lymphoma. Fluorescence in situ hybridization was positive for oncogene c-MYC rearrangement on chromosome 8q24 and negative for B-cell lymphoma 2 (BCL2) and B-cell lymphoma 6 (BCL6) gene rearrangements. Flow cytometry demonstrated an aberrant population of κ light chain-restricted CD5−CD10+ B lymphocytes.
The patient’s overall disease burden was consistent with stage IV Burkitt lymphoma. R-miniCHOP chemotherapy—rituximab plus a reduced dose of cyclophosphamide, doxorubicin, vincristine sulfate, and prednisone—was initiated. Approximately 2 weeks after chemotherapy was initiated, the patient developed a firm erythematous eruption on the left hip (Figure 1A). His regimen was then switched to R-EPOCH—rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin—at the time of discharge, and he was referred to dermatology due to an initial concern of an adverse reaction to R-EPOCH chemotherapy. The patient denied any pain, pruritus, or irritation. Physical examination showed multifocal, subcutaneous, indurated, erythematous and violaceous nodules without epidermal changes. Some nodules on the lateral aspect of the hip coalesced to form firm plaques.

A punch biopsy specimen showed markedly atypical lymphocytes with enlarged nuclei and scant cytoplasm present throughout the dermis (Figures 2A and 2B). Numerous apoptotic cells and cellular debris were seen. Immunohistochemical staining demonstrated that the lymphocytic infiltrate comprised CD79a+ B cells that were positive for Bcl-6 and CD10 and negative for Bcl-2 (Figures 2C and 2D). There also was diminished focal expression of CD20. Ki-67 protein staining was intensely positive and demonstrated a very high proliferative index.

Taken together, these findings were consistent with a diagnosis of cutaneous metastasis of Burkitt lymphoma. The patient’s cutaneous lesions improved after continued aggressive chemotherapy. At follow-up 2 weeks after biopsy, he was receiving his second round of R-EPOCH chemotherapy with appreciable regression of skin lesions (Figure 1B). However, he then developed right-side double vision, ptosis, and right-side facial paresthesia. Although magnetic resonance imaging of the brain and lumbar puncture did not show evidence of central nervous system involvement, the chemotherapy regimen was switched to dose-adjusted CVAD-R—hypercyclophosphamide, vincristine, doxorubicin hydrochloride, and dexamethasone plus rituximab—for empiric treatment of central nervous system disease. Although treatment was complicated by sepsis with extended-spectrum β-lactamase-producing Enterobacter cloacae, Burkitt lymphoma was found to be in remission after 3 cycles of CVAD-R and 5 months of chemotherapy.
Burkitt lymphoma is a B-cell non-Hodgkin malignancy caused by translocation of chromosome 8 and chromosome 14, leading to overexpression of c-MYC and subsequent hyperproliferation of B lymphocytes.1,2 The disease is divided into 3 major categories: sporadic, endemic, and immunodeficiency related.3 The endemic variant is the most prevalent subtype in Africa and is associated with Plasmodium falciparum malaria; the sporadic variant is the most common subtype in the rest of the world.4
Burkitt lymphoma is highly aggressive and is characterized by unusually high rates of mitosis and apoptosis that result in abundant cellular debris and a distinctive starry-sky pattern on histopathology.5,6 Extranodal metastasis is common,7 but cutaneous involvement is exceedingly rare, with only a few cases having been reported.8-14 Cutaneous metastasis of Burkitt lymphoma often is associated with a high overall disease burden and poor prognosis.8,11
Immunodeficiency-related Burkitt lymphoma is particularly aggressive. Notably, 3 of 7 (42.9%) reported cases of cutaneous Burkitt lymphoma occurred in HIV-positive patients.11,13 In one case, cutaneous involvement was the first sign of relapsed disease that had been in remission.12
Although c-MYC rearrangement is required to make a diagnosis of Burkitt lymphoma, the disease also is present in a minority of cases of diffuse large B-cell lymphoma (DLBCL)(6%).15 Although DLBCL typically can be differentiated from Burkitt lymphoma by the large nuclear size and characteristic vesicular nuclei of B cells, few cases of DLBCL with c-MYC rearrangement histologically mimic Burkitt lymphoma. However, key features such as immunohistochemical staining for Bcl-2 and CD10 can be used to distinguish these 2 entities.16 Bcl-2 negativity and CD10 positivity, as seen in our patient, is considered more characteristic of Burkitt lymphoma. This staining pattern in combination with a high Ki-67 fraction (>95%) and the presence of monomorphic medium-sized cells is more consistent with a diagnosis of Burkitt lymphoma than of DLBCL.17
Earlier case reports have documented that cutaneous lesions of Burkitt lymphoma can occur in a variety of ways. Hematogenous spread is the likely route of metastasis for lesions distant to the primary site or those that have widespread distribution.18 Alternatively, other reports have suggested that cutaneous metastases can occur from local invasion and subcutaneous extension of malignant cells after a surgical procedure.10,19 For example, cutaneous Burkitt lymphoma has been reported in the setting of celioscopy, occurring directly at the surgical site.19 In our patient, we believe that the route of metastatic spread likely was through subcutaneous invasion secondary to CT-guided core biopsy, which was supported by the observation that the onset of cutaneous manifestations was temporally related to the procedure and that the lesions occurred on the skin directly overlying the biopsy site.
In conclusion, we describe an exceedingly rare presentation of cutaneous Burkitt lymphoma in which a surgical procedure likely served as an inciting event that triggered seeding of malignant cells to the skin. Cutaneous spread of Burkitt lymphoma is infrequently reported; all such reports that provide long-term follow-up data have described it in association with high disease burden and often a lethal outcome.8,11,12 Our patient had complete resolution of cutaneous lesions with chemotherapy. It is unclear if the presence of cutaneous lesions can serve as a prognostic indicator and requires further investigation. However, our case provides preliminary evidence to suggest that cutaneous metastases do not always represent aggressive disease and that cutaneous lesions may respond well to chemotherapy.
To the Editor:
A 73-year-old man was admitted to the hospital with progressive abdominal and hip pain of several weeks’ duration that was accompanied by unilateral swelling of the left leg. He had a medical history of hypertension, hyperlipidemia, and prediabetes. Computed tomography (CT) showed extensive intra-abdominal, retroperitoneal, and pelvic lymphadenopathy in addition to poorly defined hepatic lesions.
A CT-guided core biopsy of a left inguinal lymph node showed Burkitt lymphoma. Fluorescence in situ hybridization was positive for oncogene c-MYC rearrangement on chromosome 8q24 and negative for B-cell lymphoma 2 (BCL2) and B-cell lymphoma 6 (BCL6) gene rearrangements. Flow cytometry demonstrated an aberrant population of κ light chain-restricted CD5−CD10+ B lymphocytes.
The patient’s overall disease burden was consistent with stage IV Burkitt lymphoma. R-miniCHOP chemotherapy—rituximab plus a reduced dose of cyclophosphamide, doxorubicin, vincristine sulfate, and prednisone—was initiated. Approximately 2 weeks after chemotherapy was initiated, the patient developed a firm erythematous eruption on the left hip (Figure 1A). His regimen was then switched to R-EPOCH—rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin—at the time of discharge, and he was referred to dermatology due to an initial concern of an adverse reaction to R-EPOCH chemotherapy. The patient denied any pain, pruritus, or irritation. Physical examination showed multifocal, subcutaneous, indurated, erythematous and violaceous nodules without epidermal changes. Some nodules on the lateral aspect of the hip coalesced to form firm plaques.

A punch biopsy specimen showed markedly atypical lymphocytes with enlarged nuclei and scant cytoplasm present throughout the dermis (Figures 2A and 2B). Numerous apoptotic cells and cellular debris were seen. Immunohistochemical staining demonstrated that the lymphocytic infiltrate comprised CD79a+ B cells that were positive for Bcl-6 and CD10 and negative for Bcl-2 (Figures 2C and 2D). There also was diminished focal expression of CD20. Ki-67 protein staining was intensely positive and demonstrated a very high proliferative index.

Taken together, these findings were consistent with a diagnosis of cutaneous metastasis of Burkitt lymphoma. The patient’s cutaneous lesions improved after continued aggressive chemotherapy. At follow-up 2 weeks after biopsy, he was receiving his second round of R-EPOCH chemotherapy with appreciable regression of skin lesions (Figure 1B). However, he then developed right-side double vision, ptosis, and right-side facial paresthesia. Although magnetic resonance imaging of the brain and lumbar puncture did not show evidence of central nervous system involvement, the chemotherapy regimen was switched to dose-adjusted CVAD-R—hypercyclophosphamide, vincristine, doxorubicin hydrochloride, and dexamethasone plus rituximab—for empiric treatment of central nervous system disease. Although treatment was complicated by sepsis with extended-spectrum β-lactamase-producing Enterobacter cloacae, Burkitt lymphoma was found to be in remission after 3 cycles of CVAD-R and 5 months of chemotherapy.
Burkitt lymphoma is a B-cell non-Hodgkin malignancy caused by translocation of chromosome 8 and chromosome 14, leading to overexpression of c-MYC and subsequent hyperproliferation of B lymphocytes.1,2 The disease is divided into 3 major categories: sporadic, endemic, and immunodeficiency related.3 The endemic variant is the most prevalent subtype in Africa and is associated with Plasmodium falciparum malaria; the sporadic variant is the most common subtype in the rest of the world.4
Burkitt lymphoma is highly aggressive and is characterized by unusually high rates of mitosis and apoptosis that result in abundant cellular debris and a distinctive starry-sky pattern on histopathology.5,6 Extranodal metastasis is common,7 but cutaneous involvement is exceedingly rare, with only a few cases having been reported.8-14 Cutaneous metastasis of Burkitt lymphoma often is associated with a high overall disease burden and poor prognosis.8,11
Immunodeficiency-related Burkitt lymphoma is particularly aggressive. Notably, 3 of 7 (42.9%) reported cases of cutaneous Burkitt lymphoma occurred in HIV-positive patients.11,13 In one case, cutaneous involvement was the first sign of relapsed disease that had been in remission.12
Although c-MYC rearrangement is required to make a diagnosis of Burkitt lymphoma, the disease also is present in a minority of cases of diffuse large B-cell lymphoma (DLBCL)(6%).15 Although DLBCL typically can be differentiated from Burkitt lymphoma by the large nuclear size and characteristic vesicular nuclei of B cells, few cases of DLBCL with c-MYC rearrangement histologically mimic Burkitt lymphoma. However, key features such as immunohistochemical staining for Bcl-2 and CD10 can be used to distinguish these 2 entities.16 Bcl-2 negativity and CD10 positivity, as seen in our patient, is considered more characteristic of Burkitt lymphoma. This staining pattern in combination with a high Ki-67 fraction (>95%) and the presence of monomorphic medium-sized cells is more consistent with a diagnosis of Burkitt lymphoma than of DLBCL.17
Earlier case reports have documented that cutaneous lesions of Burkitt lymphoma can occur in a variety of ways. Hematogenous spread is the likely route of metastasis for lesions distant to the primary site or those that have widespread distribution.18 Alternatively, other reports have suggested that cutaneous metastases can occur from local invasion and subcutaneous extension of malignant cells after a surgical procedure.10,19 For example, cutaneous Burkitt lymphoma has been reported in the setting of celioscopy, occurring directly at the surgical site.19 In our patient, we believe that the route of metastatic spread likely was through subcutaneous invasion secondary to CT-guided core biopsy, which was supported by the observation that the onset of cutaneous manifestations was temporally related to the procedure and that the lesions occurred on the skin directly overlying the biopsy site.
In conclusion, we describe an exceedingly rare presentation of cutaneous Burkitt lymphoma in which a surgical procedure likely served as an inciting event that triggered seeding of malignant cells to the skin. Cutaneous spread of Burkitt lymphoma is infrequently reported; all such reports that provide long-term follow-up data have described it in association with high disease burden and often a lethal outcome.8,11,12 Our patient had complete resolution of cutaneous lesions with chemotherapy. It is unclear if the presence of cutaneous lesions can serve as a prognostic indicator and requires further investigation. However, our case provides preliminary evidence to suggest that cutaneous metastases do not always represent aggressive disease and that cutaneous lesions may respond well to chemotherapy.
- Kalisz K, Alessandrino F, Beck R, et al. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019;10:56. doi:10.1186/s13244-019-0733-7
- Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375. doi:10.1182/blood-2018-02-778480
- Noy A. Burkitt lymphoma—subtypes, pathogenesis, and treatment strategies. Clin Lymphoma Myeloma Leuk. 2020;20(Suppl 1):S37-S38. doi:10.1016/S2152-2650(20)30455-9
- Lenze D, Leoncini L, Hummel M, et al. The different epidemiologic subtypes of Burkitt lymphoma share a homogenous micro RNA profile distinct from diffuse large B-cell lymphoma. Leukemia. 2011;25:1869-1876. doi:10.1038/leu.2011.156
- Bellan C, Lazzi S, De Falco G, et al. Burkitt’s lymphoma: new insights into molecular pathogenesis. J Clin Pathol. 2003;56:188-192. doi:10.1136/jcp.56.3.188
- Chuang S-S, Ye H, Du M-Q, et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol. 2007;128:558-564. doi:10.1309/EQJR3D3V0CCQGP04
- Baker PS, Gold KG, Lane KA, et al. Orbital burkitt lymphoma in immunocompetent patients: a report of 3 cases and a review of the literature. Ophthalmic Plast Reconstr Surg. 2009;25:464-468. doi:10.1097/IOP.0b013e3181b80fde
- Fuhrmann TL, Ignatovich YV, Pentland A. Cutaneous metastatic disease: Burkitt lymphoma. J Am Acad Dermatol. 2011;64:1196-1197. doi:10.1016/j.jaad.2009.08.033
- Burns CA, Scott GA, Miller CC. Leukemia cutis at the site of trauma in a patient with Burkitt leukemia. Cutis. 2005;75:54-56.
- Jacobson MA, Hutcheson ACS, Hurray DH, et al. Cutaneous involvement by Burkitt lymphoma. J Am Acad Dermatol. 2006;54:1111-1113. doi:10.1016/j.jaad.2006.02.030
- Berk DR, Cheng A, Lind AC, et al. Burkitt lymphoma with cutaneous involvement. Dermatol Online J. 2008;14:14.
- Bachmeyer C, Bazarbachi A, Rio B, et al. Specific cutaneous involvement indicating relapse of Burkitt’s lymphoma. Am J Hematol. 1997;54:176. doi:10.1002/(sici)1096-8652(199702)54:2<176::aid-ajh20>3.0.co;2-c
- Rogers A, Graves M, Toscano M, et al. A unique cutaneous presentation of Burkitt lymphoma. Am J Dermatopathol. 2014;36:997-1001. doi:10.1097/DAD.0000000000000004
- Thakkar D, Lipi L, Misra R, et al. Skin involvement in Burkitt’s lymphoma. Hematol Oncol Stem Cell Ther. 2018;11:251-252. doi:10.1016/j.hemonc.2018.01.002
- Akasaka T, Akasaka H, Ueda C, et al. Molecular and clinical features of non-Burkitt’s, diffuse large-cell lymphoma of B-cell type associated with the c-MYC/immunoglobulin heavy-chain fusion gene. J Clin Oncol. 2000;18:510-518. doi:10.1200/JCO.2000.18.3.510
- Nakamura N, Nakamine H, Tamaru J-I, et al. The distinction between Burkitt lymphoma and diffuse large B-cell lymphoma with c-myc rearrangement. Mod Pathol. 2002;15:771-776. doi:10.1097/01.MP.0000019577.73786.64
- Bellan C, Stefano L, Giulia de F, et al. Burkitt lymphoma versus diffuse large B-cell lymphoma: a practical approach. Hematol Oncol. 2010;28:53-56. doi:10.1002/hon.916
- Amonchaisakda N, Aiempanakit K, Apinantriyo B. Burkitt lymphoma initially mimicking varicella zoster infection. IDCases. 2020;21:E00818. doi:10.1016/j.idcr.2020.e00818
- Aractingi S, Marolleau JP, Daniel MT, et al. Subcutaneous localizations of Burkitt lymphoma after celioscopy. Am J Hematol. 1993;42:408. doi:10.1002/ajh.2830420421
- Kalisz K, Alessandrino F, Beck R, et al. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019;10:56. doi:10.1186/s13244-019-0733-7
- Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375. doi:10.1182/blood-2018-02-778480
- Noy A. Burkitt lymphoma—subtypes, pathogenesis, and treatment strategies. Clin Lymphoma Myeloma Leuk. 2020;20(Suppl 1):S37-S38. doi:10.1016/S2152-2650(20)30455-9
- Lenze D, Leoncini L, Hummel M, et al. The different epidemiologic subtypes of Burkitt lymphoma share a homogenous micro RNA profile distinct from diffuse large B-cell lymphoma. Leukemia. 2011;25:1869-1876. doi:10.1038/leu.2011.156
- Bellan C, Lazzi S, De Falco G, et al. Burkitt’s lymphoma: new insights into molecular pathogenesis. J Clin Pathol. 2003;56:188-192. doi:10.1136/jcp.56.3.188
- Chuang S-S, Ye H, Du M-Q, et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol. 2007;128:558-564. doi:10.1309/EQJR3D3V0CCQGP04
- Baker PS, Gold KG, Lane KA, et al. Orbital burkitt lymphoma in immunocompetent patients: a report of 3 cases and a review of the literature. Ophthalmic Plast Reconstr Surg. 2009;25:464-468. doi:10.1097/IOP.0b013e3181b80fde
- Fuhrmann TL, Ignatovich YV, Pentland A. Cutaneous metastatic disease: Burkitt lymphoma. J Am Acad Dermatol. 2011;64:1196-1197. doi:10.1016/j.jaad.2009.08.033
- Burns CA, Scott GA, Miller CC. Leukemia cutis at the site of trauma in a patient with Burkitt leukemia. Cutis. 2005;75:54-56.
- Jacobson MA, Hutcheson ACS, Hurray DH, et al. Cutaneous involvement by Burkitt lymphoma. J Am Acad Dermatol. 2006;54:1111-1113. doi:10.1016/j.jaad.2006.02.030
- Berk DR, Cheng A, Lind AC, et al. Burkitt lymphoma with cutaneous involvement. Dermatol Online J. 2008;14:14.
- Bachmeyer C, Bazarbachi A, Rio B, et al. Specific cutaneous involvement indicating relapse of Burkitt’s lymphoma. Am J Hematol. 1997;54:176. doi:10.1002/(sici)1096-8652(199702)54:2<176::aid-ajh20>3.0.co;2-c
- Rogers A, Graves M, Toscano M, et al. A unique cutaneous presentation of Burkitt lymphoma. Am J Dermatopathol. 2014;36:997-1001. doi:10.1097/DAD.0000000000000004
- Thakkar D, Lipi L, Misra R, et al. Skin involvement in Burkitt’s lymphoma. Hematol Oncol Stem Cell Ther. 2018;11:251-252. doi:10.1016/j.hemonc.2018.01.002
- Akasaka T, Akasaka H, Ueda C, et al. Molecular and clinical features of non-Burkitt’s, diffuse large-cell lymphoma of B-cell type associated with the c-MYC/immunoglobulin heavy-chain fusion gene. J Clin Oncol. 2000;18:510-518. doi:10.1200/JCO.2000.18.3.510
- Nakamura N, Nakamine H, Tamaru J-I, et al. The distinction between Burkitt lymphoma and diffuse large B-cell lymphoma with c-myc rearrangement. Mod Pathol. 2002;15:771-776. doi:10.1097/01.MP.0000019577.73786.64
- Bellan C, Stefano L, Giulia de F, et al. Burkitt lymphoma versus diffuse large B-cell lymphoma: a practical approach. Hematol Oncol. 2010;28:53-56. doi:10.1002/hon.916
- Amonchaisakda N, Aiempanakit K, Apinantriyo B. Burkitt lymphoma initially mimicking varicella zoster infection. IDCases. 2020;21:E00818. doi:10.1016/j.idcr.2020.e00818
- Aractingi S, Marolleau JP, Daniel MT, et al. Subcutaneous localizations of Burkitt lymphoma after celioscopy. Am J Hematol. 1993;42:408. doi:10.1002/ajh.2830420421
Practice Points
- Cutaneous metastasis is exceedingly rare in Burkitt lymphoma. When cutaneous involvement does occur, it can represent an uncommon consequence of a surgical procedure, serving as the inciting event for hematogenous spread and local tumor extension into the skin.
- Although cutaneous metasis of Burkitt lymphoma typically is associated with high disease burden and mortality, our case demonstrated that cutaneous spread can be present even in a patient who has a positive outcome. Our patient was able to achieve disease remission and complete resolution of cutaneous lesions with continued chemotherapy, suggesting that cutaneous metastasis does not always portend a poor prognosis.
Papules on the Breast, Flank, and Arm Following Breast Cancer Treatment
The Diagnosis: Acquired Cutaneous Lymphangiectasia
Histopathology showed a cluster of widely ectatic, thin-walled lymphatic spaces immediately subjacent to the epidermis and flanked by an epidermal collarette (Figure, A). The vessels did not extend any further than the papillary dermis and were not accompanied by any notable inflammation (Figure, B). A single layer of bland endothelial cells lined each lymphatic space (Figure, C). A diagnosis of acquired cutaneous lymphangiectasia secondary to surgical and radiation treatment of breast cancer was made. Clinical monitoring was recommended, but no treatment was required unless symptoms arose. At 2-year follow-up, she continued to do well.
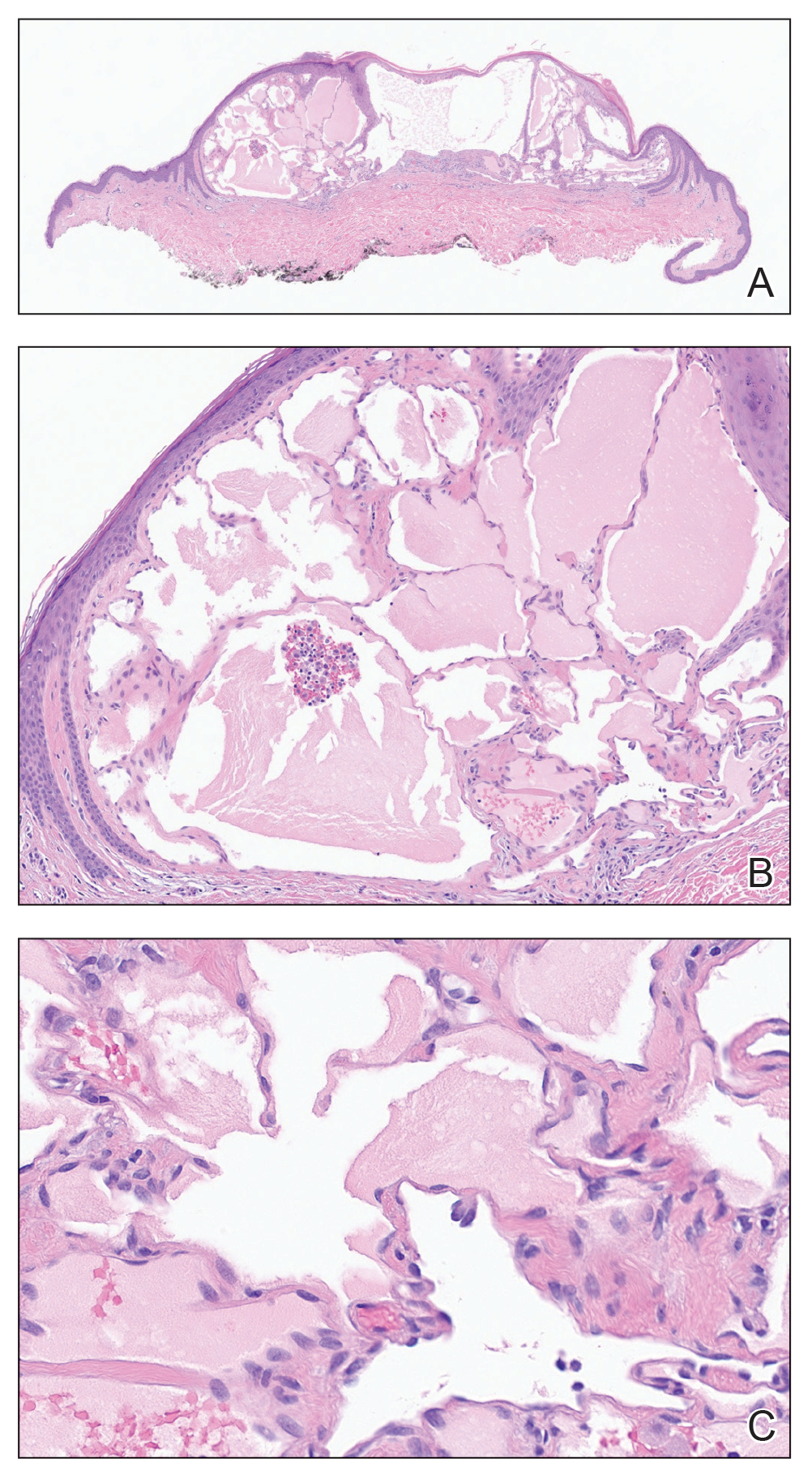
Acquired cutaneous lymphangiectasia is characterized by benign dilations of surface lymphatic vessels, likely resulting from disruption of the lymphatic system.1 This finding most commonly occurs on the external genitalia following combined surgical and radiation treatment of malignancy, though in a minority of cases it is seen with surgical or radiation treatment alone.2 Acquired cutaneous lymphangiectasia secondary to radical mastectomy for breast cancer was first reported in 1956 in a patient with persistent ipsilateral lymphadenopathy.3 The presentation in a patient with Cowden syndrome is rare. Cowden syndrome (also called PTEN hamartoma tumor syndrome) is a rare autosomal-dominant disorder caused by mutations in the tumor suppressor phosphatase and tensin homolog gene, PTEN. It is characterized by multiple hamartomas and substantially increased risk for breast, endometrial, and thyroid malignancy.4 In addition to breast cancer, our patient had a history of papillary thyroid carcinoma, cerebellar dysplastic gangliocytoma, and multiple cutaneous fibromas and angiolipomas.
A diagnosis of syringomas—benign tumors that arise from the intraepidermal aspect of eccrine sweat ducts— could be considered in the differential diagnosis. Cases of eruptive syringoma on the breast have been reported, but the biopsy would show a circumscribed proliferation of tadpole-shaped tubules comprised of secretory cells in a sclerotic stroma.5 Hidrocystomas are benign sweat gland cysts that present on the face, especially around the eyes, but rarely have been reported on the trunk, particularly the axillae.6 Although they clinically manifest as translucent papules, histopathology shows fluid-filled cysts lined by a layer of secretory columnar epithelium.7 Metastatic breast carcinoma was considered, given the patient’s history of breast cancer. Cutaneous metastases often are found on the chest wall but also can occur at distant sites. Histopathology can reveal various patterns, including islands of tumor cells with glandular formation or single files of cells infiltrating through dermal collagen.
Angiosarcoma also must be considered in the setting of any vasoformative proliferation arising on previously irradiated skin. Angiosarcomas can sometimes be well differentiated with paradoxically bland cytomorphology but characteristically have anastomosing vessels and infiltrative architecture, which were not identified in our patient. Other diagnostic features of angiosarcoma include endothelial nuclear atypia, multilayering, and mitoses. Radiation-associated angiosarcomas amplify MYC, a transcription factor that affects multiple aspects of the cell cycle and is an oncogene implicated in several different types of malignancy.8MYC immunohistochemistry testing should be performed whenever a vasoformative proliferation on irradiated skin is partially sampled or shows any features concerning for angiosarcoma. Lastly, the term postradiation atypical vascular lesion has been introduced to describe discrete papular proliferations that show close histopathologic overlap with lymphangioma/lymphatic malformations. In contrast, atypical vascular lesions show wedge-shaped intradermal growth that can cause diagnostic confusion with well-differentiated angiosarcoma. Unlike angiosarcomas, they do not express MYC. Postradiation atypical vascular lesions sometimes have an associated inflammatory infiltrate.9 Considerable histomorphologic overlap among lymphangiomas, atypical vascular lesions, and well-differentiated angiosarcomas exists; thus, lesions should be removed in their perceived totality whenever possible to help permit diagnostic distinction. In our patient, the abrupt discontinuation of vessels at the interface of the papillary and reticular dermis was reassuring of benignancy.
Our patient’s diagnosis of acquired cutaneous lymphangiectasia was a benign adverse effect of prior breast cancer treatments. This case demonstrates a rare dermatologic sequela that may arise in patients who receive surgical or radiation treatment of breast cancer. Given the heightened risk for angiosarcoma after radiation therapy as well as the increased risk for malignancy in patients with Cowden syndrome, biopsy can be an important diagnostic step in the management of these patients.
- Valdés F, Peteiro C, Toribio J. Acquired lymphangiectases and breast cancer. Actas Dermosifiliogr (Engl Ed). 2007;98:347-350.
- Chiyomaru K, Nishigori C. Acquired lymphangiectasia associated with treatment for preceding malignant neoplasm: a retrospective series of 73 Japanese patients. AMA Arch Derm. 2009;145:841-842.
- Plotnick H, Richfield D. Tuberous lymphangiectatic varices secondary to radical mastectomy. AMA Arch Derm. 1956;74:466-468.
- Pilarski R, Burt R, Kohlman W, et al. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607-1616.
- Müller CSL, Tilgen W, Pföhler C. Clinicopathological diversity of syringomas: a study on current clinical and histopathologic concepts. Dermatoendocrinol. 2009;1:282-288.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. MedGenMed. 2006;8:57.
- Ahmadi SE, Rahimi S, Zarandi B, et al. MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies. J Hematol Oncol. 2021;14:121. doi:10.1186/s13045-021-01111-4
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
The Diagnosis: Acquired Cutaneous Lymphangiectasia
Histopathology showed a cluster of widely ectatic, thin-walled lymphatic spaces immediately subjacent to the epidermis and flanked by an epidermal collarette (Figure, A). The vessels did not extend any further than the papillary dermis and were not accompanied by any notable inflammation (Figure, B). A single layer of bland endothelial cells lined each lymphatic space (Figure, C). A diagnosis of acquired cutaneous lymphangiectasia secondary to surgical and radiation treatment of breast cancer was made. Clinical monitoring was recommended, but no treatment was required unless symptoms arose. At 2-year follow-up, she continued to do well.

Acquired cutaneous lymphangiectasia is characterized by benign dilations of surface lymphatic vessels, likely resulting from disruption of the lymphatic system.1 This finding most commonly occurs on the external genitalia following combined surgical and radiation treatment of malignancy, though in a minority of cases it is seen with surgical or radiation treatment alone.2 Acquired cutaneous lymphangiectasia secondary to radical mastectomy for breast cancer was first reported in 1956 in a patient with persistent ipsilateral lymphadenopathy.3 The presentation in a patient with Cowden syndrome is rare. Cowden syndrome (also called PTEN hamartoma tumor syndrome) is a rare autosomal-dominant disorder caused by mutations in the tumor suppressor phosphatase and tensin homolog gene, PTEN. It is characterized by multiple hamartomas and substantially increased risk for breast, endometrial, and thyroid malignancy.4 In addition to breast cancer, our patient had a history of papillary thyroid carcinoma, cerebellar dysplastic gangliocytoma, and multiple cutaneous fibromas and angiolipomas.
A diagnosis of syringomas—benign tumors that arise from the intraepidermal aspect of eccrine sweat ducts— could be considered in the differential diagnosis. Cases of eruptive syringoma on the breast have been reported, but the biopsy would show a circumscribed proliferation of tadpole-shaped tubules comprised of secretory cells in a sclerotic stroma.5 Hidrocystomas are benign sweat gland cysts that present on the face, especially around the eyes, but rarely have been reported on the trunk, particularly the axillae.6 Although they clinically manifest as translucent papules, histopathology shows fluid-filled cysts lined by a layer of secretory columnar epithelium.7 Metastatic breast carcinoma was considered, given the patient’s history of breast cancer. Cutaneous metastases often are found on the chest wall but also can occur at distant sites. Histopathology can reveal various patterns, including islands of tumor cells with glandular formation or single files of cells infiltrating through dermal collagen.
Angiosarcoma also must be considered in the setting of any vasoformative proliferation arising on previously irradiated skin. Angiosarcomas can sometimes be well differentiated with paradoxically bland cytomorphology but characteristically have anastomosing vessels and infiltrative architecture, which were not identified in our patient. Other diagnostic features of angiosarcoma include endothelial nuclear atypia, multilayering, and mitoses. Radiation-associated angiosarcomas amplify MYC, a transcription factor that affects multiple aspects of the cell cycle and is an oncogene implicated in several different types of malignancy.8MYC immunohistochemistry testing should be performed whenever a vasoformative proliferation on irradiated skin is partially sampled or shows any features concerning for angiosarcoma. Lastly, the term postradiation atypical vascular lesion has been introduced to describe discrete papular proliferations that show close histopathologic overlap with lymphangioma/lymphatic malformations. In contrast, atypical vascular lesions show wedge-shaped intradermal growth that can cause diagnostic confusion with well-differentiated angiosarcoma. Unlike angiosarcomas, they do not express MYC. Postradiation atypical vascular lesions sometimes have an associated inflammatory infiltrate.9 Considerable histomorphologic overlap among lymphangiomas, atypical vascular lesions, and well-differentiated angiosarcomas exists; thus, lesions should be removed in their perceived totality whenever possible to help permit diagnostic distinction. In our patient, the abrupt discontinuation of vessels at the interface of the papillary and reticular dermis was reassuring of benignancy.
Our patient’s diagnosis of acquired cutaneous lymphangiectasia was a benign adverse effect of prior breast cancer treatments. This case demonstrates a rare dermatologic sequela that may arise in patients who receive surgical or radiation treatment of breast cancer. Given the heightened risk for angiosarcoma after radiation therapy as well as the increased risk for malignancy in patients with Cowden syndrome, biopsy can be an important diagnostic step in the management of these patients.
The Diagnosis: Acquired Cutaneous Lymphangiectasia
Histopathology showed a cluster of widely ectatic, thin-walled lymphatic spaces immediately subjacent to the epidermis and flanked by an epidermal collarette (Figure, A). The vessels did not extend any further than the papillary dermis and were not accompanied by any notable inflammation (Figure, B). A single layer of bland endothelial cells lined each lymphatic space (Figure, C). A diagnosis of acquired cutaneous lymphangiectasia secondary to surgical and radiation treatment of breast cancer was made. Clinical monitoring was recommended, but no treatment was required unless symptoms arose. At 2-year follow-up, she continued to do well.

Acquired cutaneous lymphangiectasia is characterized by benign dilations of surface lymphatic vessels, likely resulting from disruption of the lymphatic system.1 This finding most commonly occurs on the external genitalia following combined surgical and radiation treatment of malignancy, though in a minority of cases it is seen with surgical or radiation treatment alone.2 Acquired cutaneous lymphangiectasia secondary to radical mastectomy for breast cancer was first reported in 1956 in a patient with persistent ipsilateral lymphadenopathy.3 The presentation in a patient with Cowden syndrome is rare. Cowden syndrome (also called PTEN hamartoma tumor syndrome) is a rare autosomal-dominant disorder caused by mutations in the tumor suppressor phosphatase and tensin homolog gene, PTEN. It is characterized by multiple hamartomas and substantially increased risk for breast, endometrial, and thyroid malignancy.4 In addition to breast cancer, our patient had a history of papillary thyroid carcinoma, cerebellar dysplastic gangliocytoma, and multiple cutaneous fibromas and angiolipomas.
A diagnosis of syringomas—benign tumors that arise from the intraepidermal aspect of eccrine sweat ducts— could be considered in the differential diagnosis. Cases of eruptive syringoma on the breast have been reported, but the biopsy would show a circumscribed proliferation of tadpole-shaped tubules comprised of secretory cells in a sclerotic stroma.5 Hidrocystomas are benign sweat gland cysts that present on the face, especially around the eyes, but rarely have been reported on the trunk, particularly the axillae.6 Although they clinically manifest as translucent papules, histopathology shows fluid-filled cysts lined by a layer of secretory columnar epithelium.7 Metastatic breast carcinoma was considered, given the patient’s history of breast cancer. Cutaneous metastases often are found on the chest wall but also can occur at distant sites. Histopathology can reveal various patterns, including islands of tumor cells with glandular formation or single files of cells infiltrating through dermal collagen.
Angiosarcoma also must be considered in the setting of any vasoformative proliferation arising on previously irradiated skin. Angiosarcomas can sometimes be well differentiated with paradoxically bland cytomorphology but characteristically have anastomosing vessels and infiltrative architecture, which were not identified in our patient. Other diagnostic features of angiosarcoma include endothelial nuclear atypia, multilayering, and mitoses. Radiation-associated angiosarcomas amplify MYC, a transcription factor that affects multiple aspects of the cell cycle and is an oncogene implicated in several different types of malignancy.8MYC immunohistochemistry testing should be performed whenever a vasoformative proliferation on irradiated skin is partially sampled or shows any features concerning for angiosarcoma. Lastly, the term postradiation atypical vascular lesion has been introduced to describe discrete papular proliferations that show close histopathologic overlap with lymphangioma/lymphatic malformations. In contrast, atypical vascular lesions show wedge-shaped intradermal growth that can cause diagnostic confusion with well-differentiated angiosarcoma. Unlike angiosarcomas, they do not express MYC. Postradiation atypical vascular lesions sometimes have an associated inflammatory infiltrate.9 Considerable histomorphologic overlap among lymphangiomas, atypical vascular lesions, and well-differentiated angiosarcomas exists; thus, lesions should be removed in their perceived totality whenever possible to help permit diagnostic distinction. In our patient, the abrupt discontinuation of vessels at the interface of the papillary and reticular dermis was reassuring of benignancy.
Our patient’s diagnosis of acquired cutaneous lymphangiectasia was a benign adverse effect of prior breast cancer treatments. This case demonstrates a rare dermatologic sequela that may arise in patients who receive surgical or radiation treatment of breast cancer. Given the heightened risk for angiosarcoma after radiation therapy as well as the increased risk for malignancy in patients with Cowden syndrome, biopsy can be an important diagnostic step in the management of these patients.
- Valdés F, Peteiro C, Toribio J. Acquired lymphangiectases and breast cancer. Actas Dermosifiliogr (Engl Ed). 2007;98:347-350.
- Chiyomaru K, Nishigori C. Acquired lymphangiectasia associated with treatment for preceding malignant neoplasm: a retrospective series of 73 Japanese patients. AMA Arch Derm. 2009;145:841-842.
- Plotnick H, Richfield D. Tuberous lymphangiectatic varices secondary to radical mastectomy. AMA Arch Derm. 1956;74:466-468.
- Pilarski R, Burt R, Kohlman W, et al. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607-1616.
- Müller CSL, Tilgen W, Pföhler C. Clinicopathological diversity of syringomas: a study on current clinical and histopathologic concepts. Dermatoendocrinol. 2009;1:282-288.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. MedGenMed. 2006;8:57.
- Ahmadi SE, Rahimi S, Zarandi B, et al. MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies. J Hematol Oncol. 2021;14:121. doi:10.1186/s13045-021-01111-4
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Valdés F, Peteiro C, Toribio J. Acquired lymphangiectases and breast cancer. Actas Dermosifiliogr (Engl Ed). 2007;98:347-350.
- Chiyomaru K, Nishigori C. Acquired lymphangiectasia associated with treatment for preceding malignant neoplasm: a retrospective series of 73 Japanese patients. AMA Arch Derm. 2009;145:841-842.
- Plotnick H, Richfield D. Tuberous lymphangiectatic varices secondary to radical mastectomy. AMA Arch Derm. 1956;74:466-468.
- Pilarski R, Burt R, Kohlman W, et al. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607-1616.
- Müller CSL, Tilgen W, Pföhler C. Clinicopathological diversity of syringomas: a study on current clinical and histopathologic concepts. Dermatoendocrinol. 2009;1:282-288.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. MedGenMed. 2006;8:57.
- Ahmadi SE, Rahimi S, Zarandi B, et al. MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies. J Hematol Oncol. 2021;14:121. doi:10.1186/s13045-021-01111-4
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
A 47-year-old woman with Cowden syndrome presented to the dermatology clinic with asymptomatic papules on and near the right breast that had increased in number over the last year. She had a medical history of breast cancer treated with mastectomy, chemotherapy, and radiation; papillary thyroid carcinoma treated with thyroidectomy and subsequent thyroid hormone replacement; dysplastic cerebellar gangliocytoma treated with surgical excision; and multiple cutaneous fibromas and angiolipomas. Physical examination revealed multiple clustered, 1- to 5-mm, translucent to red papules on the right breast, flank, and upper arm. A shave biopsy of a papule from the right lateral breast was performed.
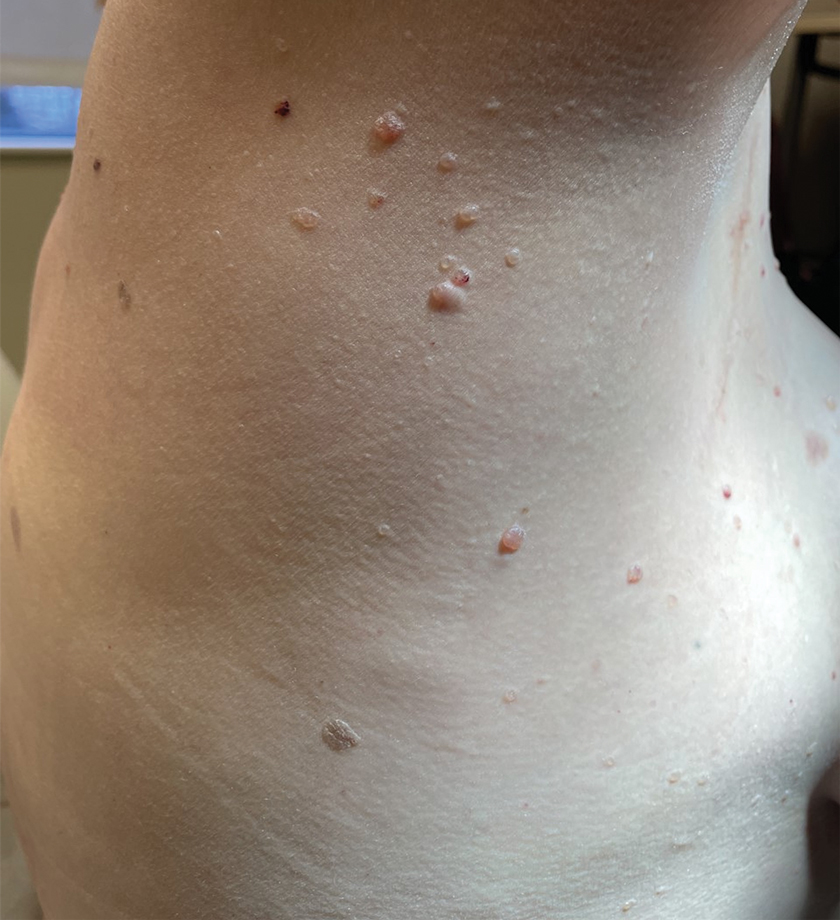
What’s Eating You? Carpet Beetles (Dermestidae)
Carpet beetle larvae of the family Dermestidae have been documented to cause both acute and delayed hypersensitivity reactions in susceptible individuals. These larvae have specialized horizontal rows of spear-shaped hairs called hastisetae, which detach easily into the surrounding environment and are small enough to travel by air. Exposure to hastisetae has been tied to adverse effects ranging from dermatitis to rhinoconjunctivitis and acute asthma, with treatment being mostly empiric and symptom based. Due to the pervasiveness of carpet beetles in homes, improved awareness of dermestid-induced manifestations is valuable for clinicians.
Beetles in the Dermestidae family do not bite humans but have been reported to cause skin reactions in addition to other symptoms typical of an allergic reaction. Skin contact with larval hairs (hastisetae) of these insects—known as carpet, larder, or hide beetles — may cause urticarial or edematous papules that are mistaken for papular urticaria or arthropod bites. 1 There are approximately 500 to 700 species of carpet beetles worldwide. Carpet beetles are a clinically underrecognized cause of allergic contact dermatitis given their frequent presence in homes across the world. 2 Carpet beetle larvae feed on shed skin, feathers, hair, wool, book bindings, felt, leather, wood, silk, and sometimes grains and thus can be found nearly anywhere. Most symptom-inducing exposures to Dermestidae beetles occur occupationally, such as in museum curators working hands-on with collection materials and workers handling infested materials such as wool. 3,4 In-home Dermestidae exposure may lead to symptoms, especially if regularly worn clothing and bedding materials are infested. The broad palate of dermestid members has resulted in substantial contamination of stored materials such as flour and fabric in addition to the destruction of museum collections. 5-7
The larvae of some dermestid species, most commonly of the genera Anthrenus and Dermestes, are 2 to 3 mm in length and have detachable hairlike hastisetae that shed into the surrounding environment throughout larval development (Figure 1).8 The hastisetae, located on the thoracic and abdominal segments (tergites), serve as a larval defense mechanism. When prodded, the round, hairy, wormlike larvae tense up and can raise their abdominal tergites while splaying the hastisetae out in a fanlike manner.9 Similar to porcupine quills, the hastisetae easily detach and can entrap the appendages of invertebrate predators. Hastisetae are not known to be sharp enough to puncture human skin, but friction and irritation from skin contact and superficial sticking of the hastisetae into mucous membranes and noncornified epithelium, such as in the bronchial airways, are thought to induce hypersensitivity reactions in susceptible individuals.

Additionally, hastisetae and the exoskeletons of both adult and larval dermestid beetles are composed mostly of chitin, which is highly allergenic. Chitin has been found to play a proinflammatory role in ocular inflammation, asthma, and bronchial reactivity via T helper cell (TH2)–mediated cellular interactions.10-12 Larvae shed their exoskeletons, including hastisetae, multiple times over the course of their development, which contributes to their potential allergen burden (Figure 2). Reports of positive prick and/or patch testing to larval components indicate some cases of both acute type 1 and delayed type 4 hypersensitivity reactions.4,8,13
Clinical Presentation and Diagnosis
Multiple erythematous urticarial papules, papulopustules, and papulovesicles are the typical manifestations of dermestid dermatitis.3,4,13-16 Figure 3 demonstrates several characteristic edematous papules with background erythema. Unlike the clusters seen with flea and bed bug bites, dermestid-induced lesions typically are single and scattered, with a propensity for exposed limbs and the face. Exposure to hastisetae commonly results in classic allergic symptoms including rhinitis, conjunctivitis, coughing, wheezing, sneezing, and intranasal and periocular pruritus, even in those with no personal history of atopy.17-19 Lymphadenopathy, vasculitis, and allergic alveolitis also have been reported.20 A large infestation in which many individual beetles as well as larvae can be found in 1 or more areas of the inhabited structure has been reported to cause more severe symptoms, including acute eczema, otitis externa, lymphocytic vasculitis, and allergic alveolitis, all of which resolved within 3 months of thorough deinfestation cleaning.21

Skin-prick and/or patch testing is not necessary for this clinical diagnosis of dermestid-induced allergic contact dermatitis. This diagnosis is bolstered by (but does not require a history of) repeated symptom induction upon performing certain activities (eg, handling taxidermy specimens) and/or in certain environments (eg, only at home). Because of individual differences in hypersensitivity to dermestid parts, it is not typical for all members of a household to be affected.
When there are multiple potential suspected allergens or an unknown cause for symptoms despite a detailed history, allergy testing can be useful in confirming a diagnosis and directing management. Immediate-onset type 1 hypersensitivity reactions are evaluated using skin-prick testing or serum IgE levels, whereas delayed type 4 hypersensitivity reactions can be evaluated using patch testing. Type 1 reactions tend to present with classic allergy symptoms, especially where there are abundant mast cells to degranulate in the skin and mucosa of the gastrointestinal and respiratory tracts; these symptoms range from mild wheezing, urticaria, periorbital pruritus, and sneezing to outright asthma, diarrhea, rhinoconjunctivitis, and even anaphylaxis. With these reactions, initial exposure to an antigen such as chitin in the hastisetae leads to an asymptomatic sensitization against the antigen in which its introduction leads to a TH2-skewed cellular response, which promotes B-cell production of IgE antibodies. Upon subsequent exposure to this antigen, IgE antibodies bound to mast cells will lead them to degranulate with release of histamine and other proinflammatory molecules, resulting in clinical manifestations. The skin-prick test relies on introduction of potential antigens through the epidermis into the dermis with a sharp lancet to induce IgE antibody activation and then degranulation of the patient’s mast cells, resulting in a pruritic erythematous wheal. This IgE-mediated process has been shown to occur in response to dermestid larval parts among household dust, resulting in chronic coughing, sneezing, nasal pruritus, and asthma.15,17,22
Type 4 hypersensitivity reactions are T-cell mediated and also include a sensitization phase followed by symptom manifestation upon repeat exposure; however, these reactions usually are not immediate and can take up to 72 hours after exposure to manifest.23 This is because T cells specific to the antigen do not lead a process resulting in antibodies but instead recruit numerous other TH1-polarized mediators upon re-exposure to activate cytotoxic CD8+ T cells and macrophages to attempt to neutralize the antigen. Many type 4 reactions result in mostly cutaneous manifestations, such as contact dermatitis. Patch testing involves adhering potential allergens to the skin for a time with assessments at regular intervals to evaluate the level of reaction from weakly positive to severe. At minimum, most reports of dermestid-related manifestations include a rash such as erythematous papules, and several published cases involving patch testing have yielded positive results to various preparations of larval parts.3,14,21
Management and Treatment
Prevention of dermestid exposure is difficult given the myriad materials eaten by the larvae. An insect exterminator should verify and treat a carpet beetle infestation, while a dermatologist can treat symptomatic individuals. Treatment is driven by the severity of the patient’s discomfort and is aimed at both symptomatic relief and reducing dermestid exposure moving forward. Although in certain environments it will be nearly impossible to eradicate Dermestidae, cleaning thoroughly and regularly may go far to reduce exposure and associated symptoms.
Clothing and other materials such as bedding that will have direct skin contact should be washed to remove hastisetae and be stored in airtight containers in addition to items made with animal fibers, such as wool sweaters and down blankets. Mattresses, flooring, rugs, curtains, and other amenable areas should be vacuumed thoroughly, and the vacuum bag should be placed in the trash afterward. Protective pillow and mattress covers should be used. Stuffed animals in infested areas should be thrown away if not able to be completely washed and dried. Air conditioning systems may spread larval hairs away from the site of infestation and should be cleaned as much as possible. Surfaces where beetles and larvae also are commonly seen, such as windowsills, and hidden among closet and pantry items should also be wiped clean to remove both insects and potential substrate. In one case, scraping the wood flooring and applying a thick coat of varnish in addition to removing all stuffed animals from an affected individual’s home allowed for resolution of symptoms.17
Treatment for symptoms includes topical anti-inflammatory agents and/or oral antihistamines, with improvement in symptoms typically occurring within days and resolution dependent on level of exposure moving forward.
Final Thoughts
- Gumina ME, Yan AC. Carpet beetle dermatitis mimicking bullous impetigo. Pediatr Dermatol. 2021;38:329-331. doi:10.1111/pde.14453
- Bertone MA, Leong M, Bayless KM, et al. Arthropods of the great indoors: characterizing diversity inside urban and suburban homes. PeerJ. 2016;4:E1582. doi:10.7717/peerj.1582
- Siegel S, Lee N, Rohr A, et. al. Evaluation of dermestid sensitivity in museum personnel. J Allergy Clin Immunol. 1991;87:190. doi:10.1016/0091-6749(91)91488-F
- Brito FF, Mur P, Barber D, et al. Occupational rhinoconjunctivitis and asthma in a wool worker caused by Dermestidae spp. Allergy. 2002;57:1191-1194.
- Stengaard HL, Akerlund M, Grontoft T, et al. Future pest status of an insect pest in museums, Attagenus smirnovi: distribution and food consumption in relation to climate change. J Cult Herit. 2012;13:22l-227.
- Veer V, Negi BK, Rao KM. Dermestid beetles and some other insect pests associated with stored silkworm cocoons in India, including a world list of dermestid species found attacking this commodity. J Stored Products Research. 1996;32:69-89.
- Veer V, Prasad R, Rao KM. Taxonomic and biological notes on Attagenus and Anthrenus spp. (Coleoptera: Dermestidae) found damaging stored woolen fabrics in India. J Stored Products Research. 1991;27:189-198.
- Háva J. World Catalogue of Insects. Volume 13. Dermestidae (Coleoptera). Brill; 2015.
- Ruzzier E, Kadej M, Di Giulio A, et al. Entangling the enemy: ecological, systematic, and medical implications of dermestid beetle Hastisetae. Insects. 2021;12:436. doi:10.3390/insects12050436
- Arae K, Morita H, Unno H, et al. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1β production by DCs. Sci Rep. 2018;8:11721.
- Brinchmann BC, Bayat M, Brøgger T, et. al. A possible role of chitin in the pathogenesis of asthma and allergy. Ann Agric Environ Med. 2011;18:7-12.
- Bucolo C, Musumeci M, Musumeci S, et al. Acidic mammalian chitinase and the eye: implications for ocular inflammatory diseases. Front Pharmacol. 2011;2:1-4.
- Hoverson K, Wohltmann WE, Pollack RJ, et al. Dermestid dermatitis in a 2-year-old girl: case report and review of the literature. Pediatr Dermatol. 2015;32:E228-E233. doi:10.1111/pde.12641
- Simon L, Boukari F, Oumarou H, et al. Anthrenus sp. and an uncommon cluster of dermatitis. Emerg Infect Dis. 2021;27:1940-1943. doi:10.3201/eid2707.203245
- Ahmed R, Moy R, Barr R, et al. Carpet beetle dermatitis. J Am Acad Dermatol. 1981;5:428-432.
- MacArthur K, Richardson V, Novoa R, et al. Carpet beetle dermatitis: a possibly under-recognized entity. Int J Dermatol. 2016;55:577-579.
- Cuesta-Herranz J, de las Heras M, Sastre J, et al. Asthma caused by Dermestidae (black carpet beetle): a new allergen in house dust. J Allergy Clin Immunol. 1997;99(1 Pt 1):147-149.
- Bernstein J, Morgan M, Ghosh D, et al. Respiratory sensitization of a worker to the warehouse beetle Trogoderma variabile: an index case report. J Allergy Clin Immunol. 2009;123:1413-1416.
- Gorgojo IE, De Las Heras M, Pastor C, et al. Allergy to Dermestidae: a new indoor allergen? [abstract] J Allergy Clin Immunol. 2015;135:AB105.
- Ruzzier E, Kadej M, Battisti A. Occurrence, ecological function and medical importance of dermestid beetle hastisetae. PeerJ. 2020;8:E8340. doi:10.7717/peerj.8340
- Ramachandran J, Hern J, Almeyda J, et al. Contact dermatitis with cervical lymphadenopathy following exposure to the hide beetle, Dermestes peruvianus. Br J Dermatol. 1997;136:943-945.
- Horster S, Prinz J, Holm N, et al. Anthrenus-dermatitis. Hautarzt. 2002;53:328-331.
- Justiz Vaillant AA, Vashisht R, Zito PM. Immediate hypersensitivity reactions. In: StatPearls. StatPearls Publishing; 2023.
Carpet beetle larvae of the family Dermestidae have been documented to cause both acute and delayed hypersensitivity reactions in susceptible individuals. These larvae have specialized horizontal rows of spear-shaped hairs called hastisetae, which detach easily into the surrounding environment and are small enough to travel by air. Exposure to hastisetae has been tied to adverse effects ranging from dermatitis to rhinoconjunctivitis and acute asthma, with treatment being mostly empiric and symptom based. Due to the pervasiveness of carpet beetles in homes, improved awareness of dermestid-induced manifestations is valuable for clinicians.
Beetles in the Dermestidae family do not bite humans but have been reported to cause skin reactions in addition to other symptoms typical of an allergic reaction. Skin contact with larval hairs (hastisetae) of these insects—known as carpet, larder, or hide beetles — may cause urticarial or edematous papules that are mistaken for papular urticaria or arthropod bites. 1 There are approximately 500 to 700 species of carpet beetles worldwide. Carpet beetles are a clinically underrecognized cause of allergic contact dermatitis given their frequent presence in homes across the world. 2 Carpet beetle larvae feed on shed skin, feathers, hair, wool, book bindings, felt, leather, wood, silk, and sometimes grains and thus can be found nearly anywhere. Most symptom-inducing exposures to Dermestidae beetles occur occupationally, such as in museum curators working hands-on with collection materials and workers handling infested materials such as wool. 3,4 In-home Dermestidae exposure may lead to symptoms, especially if regularly worn clothing and bedding materials are infested. The broad palate of dermestid members has resulted in substantial contamination of stored materials such as flour and fabric in addition to the destruction of museum collections. 5-7
The larvae of some dermestid species, most commonly of the genera Anthrenus and Dermestes, are 2 to 3 mm in length and have detachable hairlike hastisetae that shed into the surrounding environment throughout larval development (Figure 1).8 The hastisetae, located on the thoracic and abdominal segments (tergites), serve as a larval defense mechanism. When prodded, the round, hairy, wormlike larvae tense up and can raise their abdominal tergites while splaying the hastisetae out in a fanlike manner.9 Similar to porcupine quills, the hastisetae easily detach and can entrap the appendages of invertebrate predators. Hastisetae are not known to be sharp enough to puncture human skin, but friction and irritation from skin contact and superficial sticking of the hastisetae into mucous membranes and noncornified epithelium, such as in the bronchial airways, are thought to induce hypersensitivity reactions in susceptible individuals.

Additionally, hastisetae and the exoskeletons of both adult and larval dermestid beetles are composed mostly of chitin, which is highly allergenic. Chitin has been found to play a proinflammatory role in ocular inflammation, asthma, and bronchial reactivity via T helper cell (TH2)–mediated cellular interactions.10-12 Larvae shed their exoskeletons, including hastisetae, multiple times over the course of their development, which contributes to their potential allergen burden (Figure 2). Reports of positive prick and/or patch testing to larval components indicate some cases of both acute type 1 and delayed type 4 hypersensitivity reactions.4,8,13

Clinical Presentation and Diagnosis
Multiple erythematous urticarial papules, papulopustules, and papulovesicles are the typical manifestations of dermestid dermatitis.3,4,13-16 Figure 3 demonstrates several characteristic edematous papules with background erythema. Unlike the clusters seen with flea and bed bug bites, dermestid-induced lesions typically are single and scattered, with a propensity for exposed limbs and the face. Exposure to hastisetae commonly results in classic allergic symptoms including rhinitis, conjunctivitis, coughing, wheezing, sneezing, and intranasal and periocular pruritus, even in those with no personal history of atopy.17-19 Lymphadenopathy, vasculitis, and allergic alveolitis also have been reported.20 A large infestation in which many individual beetles as well as larvae can be found in 1 or more areas of the inhabited structure has been reported to cause more severe symptoms, including acute eczema, otitis externa, lymphocytic vasculitis, and allergic alveolitis, all of which resolved within 3 months of thorough deinfestation cleaning.21

Skin-prick and/or patch testing is not necessary for this clinical diagnosis of dermestid-induced allergic contact dermatitis. This diagnosis is bolstered by (but does not require a history of) repeated symptom induction upon performing certain activities (eg, handling taxidermy specimens) and/or in certain environments (eg, only at home). Because of individual differences in hypersensitivity to dermestid parts, it is not typical for all members of a household to be affected.
When there are multiple potential suspected allergens or an unknown cause for symptoms despite a detailed history, allergy testing can be useful in confirming a diagnosis and directing management. Immediate-onset type 1 hypersensitivity reactions are evaluated using skin-prick testing or serum IgE levels, whereas delayed type 4 hypersensitivity reactions can be evaluated using patch testing. Type 1 reactions tend to present with classic allergy symptoms, especially where there are abundant mast cells to degranulate in the skin and mucosa of the gastrointestinal and respiratory tracts; these symptoms range from mild wheezing, urticaria, periorbital pruritus, and sneezing to outright asthma, diarrhea, rhinoconjunctivitis, and even anaphylaxis. With these reactions, initial exposure to an antigen such as chitin in the hastisetae leads to an asymptomatic sensitization against the antigen in which its introduction leads to a TH2-skewed cellular response, which promotes B-cell production of IgE antibodies. Upon subsequent exposure to this antigen, IgE antibodies bound to mast cells will lead them to degranulate with release of histamine and other proinflammatory molecules, resulting in clinical manifestations. The skin-prick test relies on introduction of potential antigens through the epidermis into the dermis with a sharp lancet to induce IgE antibody activation and then degranulation of the patient’s mast cells, resulting in a pruritic erythematous wheal. This IgE-mediated process has been shown to occur in response to dermestid larval parts among household dust, resulting in chronic coughing, sneezing, nasal pruritus, and asthma.15,17,22
Type 4 hypersensitivity reactions are T-cell mediated and also include a sensitization phase followed by symptom manifestation upon repeat exposure; however, these reactions usually are not immediate and can take up to 72 hours after exposure to manifest.23 This is because T cells specific to the antigen do not lead a process resulting in antibodies but instead recruit numerous other TH1-polarized mediators upon re-exposure to activate cytotoxic CD8+ T cells and macrophages to attempt to neutralize the antigen. Many type 4 reactions result in mostly cutaneous manifestations, such as contact dermatitis. Patch testing involves adhering potential allergens to the skin for a time with assessments at regular intervals to evaluate the level of reaction from weakly positive to severe. At minimum, most reports of dermestid-related manifestations include a rash such as erythematous papules, and several published cases involving patch testing have yielded positive results to various preparations of larval parts.3,14,21
Management and Treatment
Prevention of dermestid exposure is difficult given the myriad materials eaten by the larvae. An insect exterminator should verify and treat a carpet beetle infestation, while a dermatologist can treat symptomatic individuals. Treatment is driven by the severity of the patient’s discomfort and is aimed at both symptomatic relief and reducing dermestid exposure moving forward. Although in certain environments it will be nearly impossible to eradicate Dermestidae, cleaning thoroughly and regularly may go far to reduce exposure and associated symptoms.
Clothing and other materials such as bedding that will have direct skin contact should be washed to remove hastisetae and be stored in airtight containers in addition to items made with animal fibers, such as wool sweaters and down blankets. Mattresses, flooring, rugs, curtains, and other amenable areas should be vacuumed thoroughly, and the vacuum bag should be placed in the trash afterward. Protective pillow and mattress covers should be used. Stuffed animals in infested areas should be thrown away if not able to be completely washed and dried. Air conditioning systems may spread larval hairs away from the site of infestation and should be cleaned as much as possible. Surfaces where beetles and larvae also are commonly seen, such as windowsills, and hidden among closet and pantry items should also be wiped clean to remove both insects and potential substrate. In one case, scraping the wood flooring and applying a thick coat of varnish in addition to removing all stuffed animals from an affected individual’s home allowed for resolution of symptoms.17
Treatment for symptoms includes topical anti-inflammatory agents and/or oral antihistamines, with improvement in symptoms typically occurring within days and resolution dependent on level of exposure moving forward.
Final Thoughts
Carpet beetle larvae of the family Dermestidae have been documented to cause both acute and delayed hypersensitivity reactions in susceptible individuals. These larvae have specialized horizontal rows of spear-shaped hairs called hastisetae, which detach easily into the surrounding environment and are small enough to travel by air. Exposure to hastisetae has been tied to adverse effects ranging from dermatitis to rhinoconjunctivitis and acute asthma, with treatment being mostly empiric and symptom based. Due to the pervasiveness of carpet beetles in homes, improved awareness of dermestid-induced manifestations is valuable for clinicians.
Beetles in the Dermestidae family do not bite humans but have been reported to cause skin reactions in addition to other symptoms typical of an allergic reaction. Skin contact with larval hairs (hastisetae) of these insects—known as carpet, larder, or hide beetles — may cause urticarial or edematous papules that are mistaken for papular urticaria or arthropod bites. 1 There are approximately 500 to 700 species of carpet beetles worldwide. Carpet beetles are a clinically underrecognized cause of allergic contact dermatitis given their frequent presence in homes across the world. 2 Carpet beetle larvae feed on shed skin, feathers, hair, wool, book bindings, felt, leather, wood, silk, and sometimes grains and thus can be found nearly anywhere. Most symptom-inducing exposures to Dermestidae beetles occur occupationally, such as in museum curators working hands-on with collection materials and workers handling infested materials such as wool. 3,4 In-home Dermestidae exposure may lead to symptoms, especially if regularly worn clothing and bedding materials are infested. The broad palate of dermestid members has resulted in substantial contamination of stored materials such as flour and fabric in addition to the destruction of museum collections. 5-7
The larvae of some dermestid species, most commonly of the genera Anthrenus and Dermestes, are 2 to 3 mm in length and have detachable hairlike hastisetae that shed into the surrounding environment throughout larval development (Figure 1).8 The hastisetae, located on the thoracic and abdominal segments (tergites), serve as a larval defense mechanism. When prodded, the round, hairy, wormlike larvae tense up and can raise their abdominal tergites while splaying the hastisetae out in a fanlike manner.9 Similar to porcupine quills, the hastisetae easily detach and can entrap the appendages of invertebrate predators. Hastisetae are not known to be sharp enough to puncture human skin, but friction and irritation from skin contact and superficial sticking of the hastisetae into mucous membranes and noncornified epithelium, such as in the bronchial airways, are thought to induce hypersensitivity reactions in susceptible individuals.

Additionally, hastisetae and the exoskeletons of both adult and larval dermestid beetles are composed mostly of chitin, which is highly allergenic. Chitin has been found to play a proinflammatory role in ocular inflammation, asthma, and bronchial reactivity via T helper cell (TH2)–mediated cellular interactions.10-12 Larvae shed their exoskeletons, including hastisetae, multiple times over the course of their development, which contributes to their potential allergen burden (Figure 2). Reports of positive prick and/or patch testing to larval components indicate some cases of both acute type 1 and delayed type 4 hypersensitivity reactions.4,8,13

Clinical Presentation and Diagnosis
Multiple erythematous urticarial papules, papulopustules, and papulovesicles are the typical manifestations of dermestid dermatitis.3,4,13-16 Figure 3 demonstrates several characteristic edematous papules with background erythema. Unlike the clusters seen with flea and bed bug bites, dermestid-induced lesions typically are single and scattered, with a propensity for exposed limbs and the face. Exposure to hastisetae commonly results in classic allergic symptoms including rhinitis, conjunctivitis, coughing, wheezing, sneezing, and intranasal and periocular pruritus, even in those with no personal history of atopy.17-19 Lymphadenopathy, vasculitis, and allergic alveolitis also have been reported.20 A large infestation in which many individual beetles as well as larvae can be found in 1 or more areas of the inhabited structure has been reported to cause more severe symptoms, including acute eczema, otitis externa, lymphocytic vasculitis, and allergic alveolitis, all of which resolved within 3 months of thorough deinfestation cleaning.21

Skin-prick and/or patch testing is not necessary for this clinical diagnosis of dermestid-induced allergic contact dermatitis. This diagnosis is bolstered by (but does not require a history of) repeated symptom induction upon performing certain activities (eg, handling taxidermy specimens) and/or in certain environments (eg, only at home). Because of individual differences in hypersensitivity to dermestid parts, it is not typical for all members of a household to be affected.
When there are multiple potential suspected allergens or an unknown cause for symptoms despite a detailed history, allergy testing can be useful in confirming a diagnosis and directing management. Immediate-onset type 1 hypersensitivity reactions are evaluated using skin-prick testing or serum IgE levels, whereas delayed type 4 hypersensitivity reactions can be evaluated using patch testing. Type 1 reactions tend to present with classic allergy symptoms, especially where there are abundant mast cells to degranulate in the skin and mucosa of the gastrointestinal and respiratory tracts; these symptoms range from mild wheezing, urticaria, periorbital pruritus, and sneezing to outright asthma, diarrhea, rhinoconjunctivitis, and even anaphylaxis. With these reactions, initial exposure to an antigen such as chitin in the hastisetae leads to an asymptomatic sensitization against the antigen in which its introduction leads to a TH2-skewed cellular response, which promotes B-cell production of IgE antibodies. Upon subsequent exposure to this antigen, IgE antibodies bound to mast cells will lead them to degranulate with release of histamine and other proinflammatory molecules, resulting in clinical manifestations. The skin-prick test relies on introduction of potential antigens through the epidermis into the dermis with a sharp lancet to induce IgE antibody activation and then degranulation of the patient’s mast cells, resulting in a pruritic erythematous wheal. This IgE-mediated process has been shown to occur in response to dermestid larval parts among household dust, resulting in chronic coughing, sneezing, nasal pruritus, and asthma.15,17,22
Type 4 hypersensitivity reactions are T-cell mediated and also include a sensitization phase followed by symptom manifestation upon repeat exposure; however, these reactions usually are not immediate and can take up to 72 hours after exposure to manifest.23 This is because T cells specific to the antigen do not lead a process resulting in antibodies but instead recruit numerous other TH1-polarized mediators upon re-exposure to activate cytotoxic CD8+ T cells and macrophages to attempt to neutralize the antigen. Many type 4 reactions result in mostly cutaneous manifestations, such as contact dermatitis. Patch testing involves adhering potential allergens to the skin for a time with assessments at regular intervals to evaluate the level of reaction from weakly positive to severe. At minimum, most reports of dermestid-related manifestations include a rash such as erythematous papules, and several published cases involving patch testing have yielded positive results to various preparations of larval parts.3,14,21
Management and Treatment
Prevention of dermestid exposure is difficult given the myriad materials eaten by the larvae. An insect exterminator should verify and treat a carpet beetle infestation, while a dermatologist can treat symptomatic individuals. Treatment is driven by the severity of the patient’s discomfort and is aimed at both symptomatic relief and reducing dermestid exposure moving forward. Although in certain environments it will be nearly impossible to eradicate Dermestidae, cleaning thoroughly and regularly may go far to reduce exposure and associated symptoms.
Clothing and other materials such as bedding that will have direct skin contact should be washed to remove hastisetae and be stored in airtight containers in addition to items made with animal fibers, such as wool sweaters and down blankets. Mattresses, flooring, rugs, curtains, and other amenable areas should be vacuumed thoroughly, and the vacuum bag should be placed in the trash afterward. Protective pillow and mattress covers should be used. Stuffed animals in infested areas should be thrown away if not able to be completely washed and dried. Air conditioning systems may spread larval hairs away from the site of infestation and should be cleaned as much as possible. Surfaces where beetles and larvae also are commonly seen, such as windowsills, and hidden among closet and pantry items should also be wiped clean to remove both insects and potential substrate. In one case, scraping the wood flooring and applying a thick coat of varnish in addition to removing all stuffed animals from an affected individual’s home allowed for resolution of symptoms.17
Treatment for symptoms includes topical anti-inflammatory agents and/or oral antihistamines, with improvement in symptoms typically occurring within days and resolution dependent on level of exposure moving forward.
Final Thoughts
- Gumina ME, Yan AC. Carpet beetle dermatitis mimicking bullous impetigo. Pediatr Dermatol. 2021;38:329-331. doi:10.1111/pde.14453
- Bertone MA, Leong M, Bayless KM, et al. Arthropods of the great indoors: characterizing diversity inside urban and suburban homes. PeerJ. 2016;4:E1582. doi:10.7717/peerj.1582
- Siegel S, Lee N, Rohr A, et. al. Evaluation of dermestid sensitivity in museum personnel. J Allergy Clin Immunol. 1991;87:190. doi:10.1016/0091-6749(91)91488-F
- Brito FF, Mur P, Barber D, et al. Occupational rhinoconjunctivitis and asthma in a wool worker caused by Dermestidae spp. Allergy. 2002;57:1191-1194.
- Stengaard HL, Akerlund M, Grontoft T, et al. Future pest status of an insect pest in museums, Attagenus smirnovi: distribution and food consumption in relation to climate change. J Cult Herit. 2012;13:22l-227.
- Veer V, Negi BK, Rao KM. Dermestid beetles and some other insect pests associated with stored silkworm cocoons in India, including a world list of dermestid species found attacking this commodity. J Stored Products Research. 1996;32:69-89.
- Veer V, Prasad R, Rao KM. Taxonomic and biological notes on Attagenus and Anthrenus spp. (Coleoptera: Dermestidae) found damaging stored woolen fabrics in India. J Stored Products Research. 1991;27:189-198.
- Háva J. World Catalogue of Insects. Volume 13. Dermestidae (Coleoptera). Brill; 2015.
- Ruzzier E, Kadej M, Di Giulio A, et al. Entangling the enemy: ecological, systematic, and medical implications of dermestid beetle Hastisetae. Insects. 2021;12:436. doi:10.3390/insects12050436
- Arae K, Morita H, Unno H, et al. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1β production by DCs. Sci Rep. 2018;8:11721.
- Brinchmann BC, Bayat M, Brøgger T, et. al. A possible role of chitin in the pathogenesis of asthma and allergy. Ann Agric Environ Med. 2011;18:7-12.
- Bucolo C, Musumeci M, Musumeci S, et al. Acidic mammalian chitinase and the eye: implications for ocular inflammatory diseases. Front Pharmacol. 2011;2:1-4.
- Hoverson K, Wohltmann WE, Pollack RJ, et al. Dermestid dermatitis in a 2-year-old girl: case report and review of the literature. Pediatr Dermatol. 2015;32:E228-E233. doi:10.1111/pde.12641
- Simon L, Boukari F, Oumarou H, et al. Anthrenus sp. and an uncommon cluster of dermatitis. Emerg Infect Dis. 2021;27:1940-1943. doi:10.3201/eid2707.203245
- Ahmed R, Moy R, Barr R, et al. Carpet beetle dermatitis. J Am Acad Dermatol. 1981;5:428-432.
- MacArthur K, Richardson V, Novoa R, et al. Carpet beetle dermatitis: a possibly under-recognized entity. Int J Dermatol. 2016;55:577-579.
- Cuesta-Herranz J, de las Heras M, Sastre J, et al. Asthma caused by Dermestidae (black carpet beetle): a new allergen in house dust. J Allergy Clin Immunol. 1997;99(1 Pt 1):147-149.
- Bernstein J, Morgan M, Ghosh D, et al. Respiratory sensitization of a worker to the warehouse beetle Trogoderma variabile: an index case report. J Allergy Clin Immunol. 2009;123:1413-1416.
- Gorgojo IE, De Las Heras M, Pastor C, et al. Allergy to Dermestidae: a new indoor allergen? [abstract] J Allergy Clin Immunol. 2015;135:AB105.
- Ruzzier E, Kadej M, Battisti A. Occurrence, ecological function and medical importance of dermestid beetle hastisetae. PeerJ. 2020;8:E8340. doi:10.7717/peerj.8340
- Ramachandran J, Hern J, Almeyda J, et al. Contact dermatitis with cervical lymphadenopathy following exposure to the hide beetle, Dermestes peruvianus. Br J Dermatol. 1997;136:943-945.
- Horster S, Prinz J, Holm N, et al. Anthrenus-dermatitis. Hautarzt. 2002;53:328-331.
- Justiz Vaillant AA, Vashisht R, Zito PM. Immediate hypersensitivity reactions. In: StatPearls. StatPearls Publishing; 2023.
- Gumina ME, Yan AC. Carpet beetle dermatitis mimicking bullous impetigo. Pediatr Dermatol. 2021;38:329-331. doi:10.1111/pde.14453
- Bertone MA, Leong M, Bayless KM, et al. Arthropods of the great indoors: characterizing diversity inside urban and suburban homes. PeerJ. 2016;4:E1582. doi:10.7717/peerj.1582
- Siegel S, Lee N, Rohr A, et. al. Evaluation of dermestid sensitivity in museum personnel. J Allergy Clin Immunol. 1991;87:190. doi:10.1016/0091-6749(91)91488-F
- Brito FF, Mur P, Barber D, et al. Occupational rhinoconjunctivitis and asthma in a wool worker caused by Dermestidae spp. Allergy. 2002;57:1191-1194.
- Stengaard HL, Akerlund M, Grontoft T, et al. Future pest status of an insect pest in museums, Attagenus smirnovi: distribution and food consumption in relation to climate change. J Cult Herit. 2012;13:22l-227.
- Veer V, Negi BK, Rao KM. Dermestid beetles and some other insect pests associated with stored silkworm cocoons in India, including a world list of dermestid species found attacking this commodity. J Stored Products Research. 1996;32:69-89.
- Veer V, Prasad R, Rao KM. Taxonomic and biological notes on Attagenus and Anthrenus spp. (Coleoptera: Dermestidae) found damaging stored woolen fabrics in India. J Stored Products Research. 1991;27:189-198.
- Háva J. World Catalogue of Insects. Volume 13. Dermestidae (Coleoptera). Brill; 2015.
- Ruzzier E, Kadej M, Di Giulio A, et al. Entangling the enemy: ecological, systematic, and medical implications of dermestid beetle Hastisetae. Insects. 2021;12:436. doi:10.3390/insects12050436
- Arae K, Morita H, Unno H, et al. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1β production by DCs. Sci Rep. 2018;8:11721.
- Brinchmann BC, Bayat M, Brøgger T, et. al. A possible role of chitin in the pathogenesis of asthma and allergy. Ann Agric Environ Med. 2011;18:7-12.
- Bucolo C, Musumeci M, Musumeci S, et al. Acidic mammalian chitinase and the eye: implications for ocular inflammatory diseases. Front Pharmacol. 2011;2:1-4.
- Hoverson K, Wohltmann WE, Pollack RJ, et al. Dermestid dermatitis in a 2-year-old girl: case report and review of the literature. Pediatr Dermatol. 2015;32:E228-E233. doi:10.1111/pde.12641
- Simon L, Boukari F, Oumarou H, et al. Anthrenus sp. and an uncommon cluster of dermatitis. Emerg Infect Dis. 2021;27:1940-1943. doi:10.3201/eid2707.203245
- Ahmed R, Moy R, Barr R, et al. Carpet beetle dermatitis. J Am Acad Dermatol. 1981;5:428-432.
- MacArthur K, Richardson V, Novoa R, et al. Carpet beetle dermatitis: a possibly under-recognized entity. Int J Dermatol. 2016;55:577-579.
- Cuesta-Herranz J, de las Heras M, Sastre J, et al. Asthma caused by Dermestidae (black carpet beetle): a new allergen in house dust. J Allergy Clin Immunol. 1997;99(1 Pt 1):147-149.
- Bernstein J, Morgan M, Ghosh D, et al. Respiratory sensitization of a worker to the warehouse beetle Trogoderma variabile: an index case report. J Allergy Clin Immunol. 2009;123:1413-1416.
- Gorgojo IE, De Las Heras M, Pastor C, et al. Allergy to Dermestidae: a new indoor allergen? [abstract] J Allergy Clin Immunol. 2015;135:AB105.
- Ruzzier E, Kadej M, Battisti A. Occurrence, ecological function and medical importance of dermestid beetle hastisetae. PeerJ. 2020;8:E8340. doi:10.7717/peerj.8340
- Ramachandran J, Hern J, Almeyda J, et al. Contact dermatitis with cervical lymphadenopathy following exposure to the hide beetle, Dermestes peruvianus. Br J Dermatol. 1997;136:943-945.
- Horster S, Prinz J, Holm N, et al. Anthrenus-dermatitis. Hautarzt. 2002;53:328-331.
- Justiz Vaillant AA, Vashisht R, Zito PM. Immediate hypersensitivity reactions. In: StatPearls. StatPearls Publishing; 2023.
Practice Points
- Given their ubiquity, dermatologists should be aware of the potential for hypersensitivity reactions to carpet beetles (Dermestidae).
- Pruritic erythematous papules, pustules, and vesicles are the most common manifestations of exposure to larval hairs.
- Treatment is symptom based, and future exposure can be greatly diminished with thorough cleaning of the patient’s environment.
Bartonella henselae Infection May Occasionally Distract Immune Control of Latent Human Herpesviruses
To the Editor:
We read with interest the September 2023 Cutis article by Swink et al,1 “Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl.” The authors documented the possibility of Bartonella henselae infection as another causative agent for pityriasis rosea (PR) even though the association of PR with human herpesvirus (HHV) 6 and HHV-7 infection is based on several consistent observations and not on occasional findings. The association of PR with endogenous systemic reactivation of HHV-6 and HHV-7 has been identified with different investigations and laboratory techniques. Using polymerase chain reaction, real-time calibrated quantitative polymerase chain reaction, in situ hybridization, immunohistochemistry, and electron microscopy, HHV-6 and HHV-7 have been detected in plasma (a marker of active viral replication), peripheral blood mononuclear cells, and skin lesions from patients with PR.2 In addition, HHV-6 and HHV-7 messenger RNA expression and their specific antigens have been detected in PR skin lesions and herpesvirus virions in various stages of morphogenesis as well as in the supernatant of co-cultured peripheral blood mononuclear cells of patients with PR.2,3 Lastly, the increased levels of several particular cytokines and chemokinesin the sera of patients with PR support a viral role in its pathogenesis.4
Bartonella henselae is a gram-negative intracellular facultative bacterium that is commonly implicated in causing zoonotic infections worldwide. The incidence of cat-scratch disease (CSD) was reported to be 6.4 cases per 100,000 population in adults and 9.4 cases per 100,000 population in children aged 5 to 9 years globally.5 Approximately 24,000 cases of CSD are reported in the United States every year.6 Therefore, considering these data, if B henselae was a causative agent for PR, the eruption would be observed frequently in many patients with CSD, which is not the case. On the contrary, it is possible that B henselae infection may have reactivated HHV-6 and/or HHV-7 infection. It is well established that B henselae causes a robust cell-mediated immune response by activating natural killer and helper T cells (TH1) and enhancement of cytotoxic T lymphocytes.7 It could be assumed that by strongly stimulating the immune response and polarizing it to a specific antigen cell response, B henselae infection may temporarily distract the T cell-mediated control of the latent infections, such as HHV-6 and HHV-7, which may reactivate and cause PR.
It is important to point out that a case of concomitant B henselae and Epstein-Barr virus infection has been described.8 Even in that case, the B henselae infection may have reactivated Epstein-Barr virus as well as HHV-6 and HHV-7 in the case described by Swink et al.1 Epstein-Barr virus reactivation has been detected in one case8 through serologic testing—IgM, IgG, Epstein-Barr virus nuclear antigen IgG, and heterophile antibodies—as there were no dermatologic manifestations that may be related to Epstein-Barr virus reactivation from latency.9
In conclusion, a viral or bacterial infection such as Epstein-Barr virus or B henselae may have a transactivating function allowing another (latent) virus such as HHV-6 or HHV-7 to reactivate. Indeed, it has been described that SARS-CoV-2 may act as a transactivator agent triggering HHV-6/HHV-7 reactivation, thereby indirectly causing PR clinical manifestation.10
- Swink SM, Rhodes LP, Levin J. Cat scratch disease presenting with concurrent pityriasis rosea in a 10-year-old girl. Cutis. 2023;112:E24-E26. doi:10.12788/cutis.0861
- Broccolo F, Drago F, Careddu AM, et al. Additional evidence that pityriasis rosea is associated with reactivation of human herpesvirus-6 and -7. J Invest Dermatol. 2005;124:1234-1240.
- Rebora A, Ciccarese G, Herzum A, et al. Pityriasis rosea and other infectious eruptions during pregnancy: possible life-threatening health conditions for the fetus. Clin Dermatol. 2020;38:105-112.
- Drago F, Ciccarese G, Broccolo F, et al. The role of cytokines, chemokines, and growth factors in the pathogenesis of pityriasis rosea. Mediators Inflamm. 2015;2015:438963. doi:10.1155/2015/438963
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge. Zoonoses Public Health. 2018;65:67-73.
- Ackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993;83:1707-1711.
- Resto-Ruiz S, Burgess A, Anderson BE. The role of the host immune response in pathogenesis of Bartonella henselae. DNA Cell Biol. 2003; 22:431-440.
- Aparicio-Casares H, Puente-Rico MH, Tomé-Nestal C, et al. A pediatric case of Bartonella henselae and Epstein Barr virus disease with bone and hepatosplenic involvement. Bol Med Hosp Infant Mex. 2021;78:467-473.
- Ciccarese G, Trave I, Herzum A, et al. Dermatological manifestations of Epstein-Barr virus systemic infection: a case report and literature review. Int J Dermatol. 2020;59:1202-1209.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;40:586-590.
To the Editor:
We read with interest the September 2023 Cutis article by Swink et al,1 “Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl.” The authors documented the possibility of Bartonella henselae infection as another causative agent for pityriasis rosea (PR) even though the association of PR with human herpesvirus (HHV) 6 and HHV-7 infection is based on several consistent observations and not on occasional findings. The association of PR with endogenous systemic reactivation of HHV-6 and HHV-7 has been identified with different investigations and laboratory techniques. Using polymerase chain reaction, real-time calibrated quantitative polymerase chain reaction, in situ hybridization, immunohistochemistry, and electron microscopy, HHV-6 and HHV-7 have been detected in plasma (a marker of active viral replication), peripheral blood mononuclear cells, and skin lesions from patients with PR.2 In addition, HHV-6 and HHV-7 messenger RNA expression and their specific antigens have been detected in PR skin lesions and herpesvirus virions in various stages of morphogenesis as well as in the supernatant of co-cultured peripheral blood mononuclear cells of patients with PR.2,3 Lastly, the increased levels of several particular cytokines and chemokinesin the sera of patients with PR support a viral role in its pathogenesis.4
Bartonella henselae is a gram-negative intracellular facultative bacterium that is commonly implicated in causing zoonotic infections worldwide. The incidence of cat-scratch disease (CSD) was reported to be 6.4 cases per 100,000 population in adults and 9.4 cases per 100,000 population in children aged 5 to 9 years globally.5 Approximately 24,000 cases of CSD are reported in the United States every year.6 Therefore, considering these data, if B henselae was a causative agent for PR, the eruption would be observed frequently in many patients with CSD, which is not the case. On the contrary, it is possible that B henselae infection may have reactivated HHV-6 and/or HHV-7 infection. It is well established that B henselae causes a robust cell-mediated immune response by activating natural killer and helper T cells (TH1) and enhancement of cytotoxic T lymphocytes.7 It could be assumed that by strongly stimulating the immune response and polarizing it to a specific antigen cell response, B henselae infection may temporarily distract the T cell-mediated control of the latent infections, such as HHV-6 and HHV-7, which may reactivate and cause PR.
It is important to point out that a case of concomitant B henselae and Epstein-Barr virus infection has been described.8 Even in that case, the B henselae infection may have reactivated Epstein-Barr virus as well as HHV-6 and HHV-7 in the case described by Swink et al.1 Epstein-Barr virus reactivation has been detected in one case8 through serologic testing—IgM, IgG, Epstein-Barr virus nuclear antigen IgG, and heterophile antibodies—as there were no dermatologic manifestations that may be related to Epstein-Barr virus reactivation from latency.9
In conclusion, a viral or bacterial infection such as Epstein-Barr virus or B henselae may have a transactivating function allowing another (latent) virus such as HHV-6 or HHV-7 to reactivate. Indeed, it has been described that SARS-CoV-2 may act as a transactivator agent triggering HHV-6/HHV-7 reactivation, thereby indirectly causing PR clinical manifestation.10
To the Editor:
We read with interest the September 2023 Cutis article by Swink et al,1 “Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl.” The authors documented the possibility of Bartonella henselae infection as another causative agent for pityriasis rosea (PR) even though the association of PR with human herpesvirus (HHV) 6 and HHV-7 infection is based on several consistent observations and not on occasional findings. The association of PR with endogenous systemic reactivation of HHV-6 and HHV-7 has been identified with different investigations and laboratory techniques. Using polymerase chain reaction, real-time calibrated quantitative polymerase chain reaction, in situ hybridization, immunohistochemistry, and electron microscopy, HHV-6 and HHV-7 have been detected in plasma (a marker of active viral replication), peripheral blood mononuclear cells, and skin lesions from patients with PR.2 In addition, HHV-6 and HHV-7 messenger RNA expression and their specific antigens have been detected in PR skin lesions and herpesvirus virions in various stages of morphogenesis as well as in the supernatant of co-cultured peripheral blood mononuclear cells of patients with PR.2,3 Lastly, the increased levels of several particular cytokines and chemokinesin the sera of patients with PR support a viral role in its pathogenesis.4
Bartonella henselae is a gram-negative intracellular facultative bacterium that is commonly implicated in causing zoonotic infections worldwide. The incidence of cat-scratch disease (CSD) was reported to be 6.4 cases per 100,000 population in adults and 9.4 cases per 100,000 population in children aged 5 to 9 years globally.5 Approximately 24,000 cases of CSD are reported in the United States every year.6 Therefore, considering these data, if B henselae was a causative agent for PR, the eruption would be observed frequently in many patients with CSD, which is not the case. On the contrary, it is possible that B henselae infection may have reactivated HHV-6 and/or HHV-7 infection. It is well established that B henselae causes a robust cell-mediated immune response by activating natural killer and helper T cells (TH1) and enhancement of cytotoxic T lymphocytes.7 It could be assumed that by strongly stimulating the immune response and polarizing it to a specific antigen cell response, B henselae infection may temporarily distract the T cell-mediated control of the latent infections, such as HHV-6 and HHV-7, which may reactivate and cause PR.
It is important to point out that a case of concomitant B henselae and Epstein-Barr virus infection has been described.8 Even in that case, the B henselae infection may have reactivated Epstein-Barr virus as well as HHV-6 and HHV-7 in the case described by Swink et al.1 Epstein-Barr virus reactivation has been detected in one case8 through serologic testing—IgM, IgG, Epstein-Barr virus nuclear antigen IgG, and heterophile antibodies—as there were no dermatologic manifestations that may be related to Epstein-Barr virus reactivation from latency.9
In conclusion, a viral or bacterial infection such as Epstein-Barr virus or B henselae may have a transactivating function allowing another (latent) virus such as HHV-6 or HHV-7 to reactivate. Indeed, it has been described that SARS-CoV-2 may act as a transactivator agent triggering HHV-6/HHV-7 reactivation, thereby indirectly causing PR clinical manifestation.10
- Swink SM, Rhodes LP, Levin J. Cat scratch disease presenting with concurrent pityriasis rosea in a 10-year-old girl. Cutis. 2023;112:E24-E26. doi:10.12788/cutis.0861
- Broccolo F, Drago F, Careddu AM, et al. Additional evidence that pityriasis rosea is associated with reactivation of human herpesvirus-6 and -7. J Invest Dermatol. 2005;124:1234-1240.
- Rebora A, Ciccarese G, Herzum A, et al. Pityriasis rosea and other infectious eruptions during pregnancy: possible life-threatening health conditions for the fetus. Clin Dermatol. 2020;38:105-112.
- Drago F, Ciccarese G, Broccolo F, et al. The role of cytokines, chemokines, and growth factors in the pathogenesis of pityriasis rosea. Mediators Inflamm. 2015;2015:438963. doi:10.1155/2015/438963
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge. Zoonoses Public Health. 2018;65:67-73.
- Ackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993;83:1707-1711.
- Resto-Ruiz S, Burgess A, Anderson BE. The role of the host immune response in pathogenesis of Bartonella henselae. DNA Cell Biol. 2003; 22:431-440.
- Aparicio-Casares H, Puente-Rico MH, Tomé-Nestal C, et al. A pediatric case of Bartonella henselae and Epstein Barr virus disease with bone and hepatosplenic involvement. Bol Med Hosp Infant Mex. 2021;78:467-473.
- Ciccarese G, Trave I, Herzum A, et al. Dermatological manifestations of Epstein-Barr virus systemic infection: a case report and literature review. Int J Dermatol. 2020;59:1202-1209.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;40:586-590.
- Swink SM, Rhodes LP, Levin J. Cat scratch disease presenting with concurrent pityriasis rosea in a 10-year-old girl. Cutis. 2023;112:E24-E26. doi:10.12788/cutis.0861
- Broccolo F, Drago F, Careddu AM, et al. Additional evidence that pityriasis rosea is associated with reactivation of human herpesvirus-6 and -7. J Invest Dermatol. 2005;124:1234-1240.
- Rebora A, Ciccarese G, Herzum A, et al. Pityriasis rosea and other infectious eruptions during pregnancy: possible life-threatening health conditions for the fetus. Clin Dermatol. 2020;38:105-112.
- Drago F, Ciccarese G, Broccolo F, et al. The role of cytokines, chemokines, and growth factors in the pathogenesis of pityriasis rosea. Mediators Inflamm. 2015;2015:438963. doi:10.1155/2015/438963
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge. Zoonoses Public Health. 2018;65:67-73.
- Ackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993;83:1707-1711.
- Resto-Ruiz S, Burgess A, Anderson BE. The role of the host immune response in pathogenesis of Bartonella henselae. DNA Cell Biol. 2003; 22:431-440.
- Aparicio-Casares H, Puente-Rico MH, Tomé-Nestal C, et al. A pediatric case of Bartonella henselae and Epstein Barr virus disease with bone and hepatosplenic involvement. Bol Med Hosp Infant Mex. 2021;78:467-473.
- Ciccarese G, Trave I, Herzum A, et al. Dermatological manifestations of Epstein-Barr virus systemic infection: a case report and literature review. Int J Dermatol. 2020;59:1202-1209.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;40:586-590.
Hemorrhagic Crescent Sign in Pseudocellulitis
To the Editor:
Cellulitis is the most common reason for skin-related hospital admissions.1 Despite its frequency, it is suspected that many cases of cellulitis are misdiagnosed as other etiologies presenting with similar symptoms such as a ruptured Baker cyst. These cysts are located behind the knee and can present with calf pain, peripheral edema, and erythema when ruptured. Symptoms of a ruptured Baker cyst can be indistinguishable from cellulitis as well as deep vein thrombosis (DVT), both manifesting with lower extremity pain, swelling, and erythema, making diagnosis challenging.2 The hemorrhagic crescent sign—a crescent of ecchymosis distal to the medial malleolus and on the foot that results from synovial injury or rupture—can be a useful diagnostic tool to differentiate between the causes of acute swelling and pain of the leg.2 When observed, the hemorrhagic crescent sign supports testing for synovial pathology at the knee.
A 63-year-old man presented to an outside hospital for evaluation of a fever (temperature, 101 °F [38.3 °C]), as well as pain, edema, and erythema of the right lower leg of 2 days’ duration. He had a history of leg cellulitis, gout, diabetes mellitus, lymphedema, and peripheral neuropathy. On admission, he was found to have elevated C-reactive protein (45 mg/L [reference range, <8 mg/L]) and mild leukocytosis (13,500 cells/μL [reference range, 4500–11,000 cells/μL]). A venous duplex scan did not demonstrate signs of thrombosis. Antibiotic therapy was started for suspected cellulitis including levofloxacin, piperacillin-tazobactam, and linezolid. Despite broad-spectrum antibiotic coverage, the patient continued to be febrile and experienced progressive erythema and swelling of the right lower leg, at which point he was transferred to our institution. A new antibiotic regimen of vancomycin, cefepime, and clindamycin was started and showed no improvement, after which dermatology was consulted.
Physical examination revealed unilateral edema and calor of the right lower leg with a demarcated erythematous rash extending to the level of the knee. Furthermore, a hemorrhagic crescent sign was present below the right medial malleolus (Figure). The popliteal fossa was supple, though the patient was adamant that he had a Baker cyst. Punch biopsies demonstrated epidermal spongiosis and extensive edema with perivascular inflammation. No organisms were found by stain and culture. Ultrasound records confirmed a Baker cyst present at least 4 months prior; however, a repeat ultrasound showed resolution. A diagnosis of pseudocellulitis secondary to Baker cyst rupture was made, and corticosteroids were started, resulting in marked reduction in erythema and edema of the lower leg and hospital discharge.
This case highlights the importance of early involvement of dermatology when cellulitis is suspected. A study of 635 patients in the United Kingdom referred to dermatology for lower limb cellulitis found that 210 (33%) patients did not have cellulitis and only 18 (3%) required hospital admission.3 Dermatology consultations have been shown to benefit patients with inflammatory skin disease by decreasing length of stay and reducing readmissions.4
Our patient had several risk factors for cellulitis, including obesity, lymphedema, and chronic kidney disease, in addition to having fevers and unilateral involvement. However, failure of symptoms to improve with broad-spectrum antibiotics made a diagnosis of cellulitis less likely. In this case, a severe immune response to the ruptured Baker cyst mimicked the presentation of cellulitis.
Ruptured Baker cysts have been reported to cause acute leg swelling, mimicking the symptoms of cellulitis or DVT.2,5 The presence of a hemorrhagic crescent sign can be a useful diagnostic tool, as in our patient, because it has been reported as an indication of synovial injury or rupture, supporting the exclusion of cellulitis or DVT when it is observed.6 Prior reports have observed ecchymosis on the foot in as little as 1 day after the onset of calf swelling and at the lateral malleolus 3 days after the onset of calf swelling.5
Following suspicion of a ruptured Baker cyst causing pseudocellulitis, an ultrasound can be used to confirm the diagnosis. Ultrasonography shows a large hypoechoic space behind the calf muscles, which is pathognomonic of a ruptured Baker cyst.7
In conclusion, when a hemorrhagic crescent sign is observed, one should be suspicious for a ruptured Baker cyst or other synovial pathology as an etiology of pseudocellulitis. Early recognition of the hemorrhagic crescent sign can help rule out cellulitis and DVT, thereby reducing the amount of intravenous antibiotic prescribed, decreasing the length of hospital stay, and reducing readmission.
- Feldman SR, Fleischer AB, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730. doi:10.1001/archinte.158.7.726
- Von Schroeder HP, Ameli FM, Piazza D, et al. Ruptured Baker’s cyst causes ecchymosis of the foot. J Bone Joint Surg Br. 1993;75:316-317.
- Levell NJ, Wingfield CG, Garioch JJ. Severe lower limb cellulitis is best diagnosed by dermatologists and managed with shared care between primary and secondary care. Br J Dermatol. 2011;164:1326-1328.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;53:523-528.
- Dunlop D, Parker PJ, Keating JF. Ruptured Baker’s cyst causing posterior compartment syndrome. Injury. 1997;28:561-562.
- Kraag G, Thevathasan EM, Gordon DA, et al. The hemorrhagic crescent sign of acute synovial rupture. Ann Intern Med. 1976;85:477-478.
- Sato O, Kondoh K, Iyori K, et al. Midcalf ultrasonography for the diagnosis of ruptured Baker’s cysts. Surg Today. 2001;31:410-413. doi:10.1007/s005950170131
To the Editor:
Cellulitis is the most common reason for skin-related hospital admissions.1 Despite its frequency, it is suspected that many cases of cellulitis are misdiagnosed as other etiologies presenting with similar symptoms such as a ruptured Baker cyst. These cysts are located behind the knee and can present with calf pain, peripheral edema, and erythema when ruptured. Symptoms of a ruptured Baker cyst can be indistinguishable from cellulitis as well as deep vein thrombosis (DVT), both manifesting with lower extremity pain, swelling, and erythema, making diagnosis challenging.2 The hemorrhagic crescent sign—a crescent of ecchymosis distal to the medial malleolus and on the foot that results from synovial injury or rupture—can be a useful diagnostic tool to differentiate between the causes of acute swelling and pain of the leg.2 When observed, the hemorrhagic crescent sign supports testing for synovial pathology at the knee.
A 63-year-old man presented to an outside hospital for evaluation of a fever (temperature, 101 °F [38.3 °C]), as well as pain, edema, and erythema of the right lower leg of 2 days’ duration. He had a history of leg cellulitis, gout, diabetes mellitus, lymphedema, and peripheral neuropathy. On admission, he was found to have elevated C-reactive protein (45 mg/L [reference range, <8 mg/L]) and mild leukocytosis (13,500 cells/μL [reference range, 4500–11,000 cells/μL]). A venous duplex scan did not demonstrate signs of thrombosis. Antibiotic therapy was started for suspected cellulitis including levofloxacin, piperacillin-tazobactam, and linezolid. Despite broad-spectrum antibiotic coverage, the patient continued to be febrile and experienced progressive erythema and swelling of the right lower leg, at which point he was transferred to our institution. A new antibiotic regimen of vancomycin, cefepime, and clindamycin was started and showed no improvement, after which dermatology was consulted.
Physical examination revealed unilateral edema and calor of the right lower leg with a demarcated erythematous rash extending to the level of the knee. Furthermore, a hemorrhagic crescent sign was present below the right medial malleolus (Figure). The popliteal fossa was supple, though the patient was adamant that he had a Baker cyst. Punch biopsies demonstrated epidermal spongiosis and extensive edema with perivascular inflammation. No organisms were found by stain and culture. Ultrasound records confirmed a Baker cyst present at least 4 months prior; however, a repeat ultrasound showed resolution. A diagnosis of pseudocellulitis secondary to Baker cyst rupture was made, and corticosteroids were started, resulting in marked reduction in erythema and edema of the lower leg and hospital discharge.

This case highlights the importance of early involvement of dermatology when cellulitis is suspected. A study of 635 patients in the United Kingdom referred to dermatology for lower limb cellulitis found that 210 (33%) patients did not have cellulitis and only 18 (3%) required hospital admission.3 Dermatology consultations have been shown to benefit patients with inflammatory skin disease by decreasing length of stay and reducing readmissions.4
Our patient had several risk factors for cellulitis, including obesity, lymphedema, and chronic kidney disease, in addition to having fevers and unilateral involvement. However, failure of symptoms to improve with broad-spectrum antibiotics made a diagnosis of cellulitis less likely. In this case, a severe immune response to the ruptured Baker cyst mimicked the presentation of cellulitis.
Ruptured Baker cysts have been reported to cause acute leg swelling, mimicking the symptoms of cellulitis or DVT.2,5 The presence of a hemorrhagic crescent sign can be a useful diagnostic tool, as in our patient, because it has been reported as an indication of synovial injury or rupture, supporting the exclusion of cellulitis or DVT when it is observed.6 Prior reports have observed ecchymosis on the foot in as little as 1 day after the onset of calf swelling and at the lateral malleolus 3 days after the onset of calf swelling.5
Following suspicion of a ruptured Baker cyst causing pseudocellulitis, an ultrasound can be used to confirm the diagnosis. Ultrasonography shows a large hypoechoic space behind the calf muscles, which is pathognomonic of a ruptured Baker cyst.7
In conclusion, when a hemorrhagic crescent sign is observed, one should be suspicious for a ruptured Baker cyst or other synovial pathology as an etiology of pseudocellulitis. Early recognition of the hemorrhagic crescent sign can help rule out cellulitis and DVT, thereby reducing the amount of intravenous antibiotic prescribed, decreasing the length of hospital stay, and reducing readmission.
To the Editor:
Cellulitis is the most common reason for skin-related hospital admissions.1 Despite its frequency, it is suspected that many cases of cellulitis are misdiagnosed as other etiologies presenting with similar symptoms such as a ruptured Baker cyst. These cysts are located behind the knee and can present with calf pain, peripheral edema, and erythema when ruptured. Symptoms of a ruptured Baker cyst can be indistinguishable from cellulitis as well as deep vein thrombosis (DVT), both manifesting with lower extremity pain, swelling, and erythema, making diagnosis challenging.2 The hemorrhagic crescent sign—a crescent of ecchymosis distal to the medial malleolus and on the foot that results from synovial injury or rupture—can be a useful diagnostic tool to differentiate between the causes of acute swelling and pain of the leg.2 When observed, the hemorrhagic crescent sign supports testing for synovial pathology at the knee.
A 63-year-old man presented to an outside hospital for evaluation of a fever (temperature, 101 °F [38.3 °C]), as well as pain, edema, and erythema of the right lower leg of 2 days’ duration. He had a history of leg cellulitis, gout, diabetes mellitus, lymphedema, and peripheral neuropathy. On admission, he was found to have elevated C-reactive protein (45 mg/L [reference range, <8 mg/L]) and mild leukocytosis (13,500 cells/μL [reference range, 4500–11,000 cells/μL]). A venous duplex scan did not demonstrate signs of thrombosis. Antibiotic therapy was started for suspected cellulitis including levofloxacin, piperacillin-tazobactam, and linezolid. Despite broad-spectrum antibiotic coverage, the patient continued to be febrile and experienced progressive erythema and swelling of the right lower leg, at which point he was transferred to our institution. A new antibiotic regimen of vancomycin, cefepime, and clindamycin was started and showed no improvement, after which dermatology was consulted.
Physical examination revealed unilateral edema and calor of the right lower leg with a demarcated erythematous rash extending to the level of the knee. Furthermore, a hemorrhagic crescent sign was present below the right medial malleolus (Figure). The popliteal fossa was supple, though the patient was adamant that he had a Baker cyst. Punch biopsies demonstrated epidermal spongiosis and extensive edema with perivascular inflammation. No organisms were found by stain and culture. Ultrasound records confirmed a Baker cyst present at least 4 months prior; however, a repeat ultrasound showed resolution. A diagnosis of pseudocellulitis secondary to Baker cyst rupture was made, and corticosteroids were started, resulting in marked reduction in erythema and edema of the lower leg and hospital discharge.

This case highlights the importance of early involvement of dermatology when cellulitis is suspected. A study of 635 patients in the United Kingdom referred to dermatology for lower limb cellulitis found that 210 (33%) patients did not have cellulitis and only 18 (3%) required hospital admission.3 Dermatology consultations have been shown to benefit patients with inflammatory skin disease by decreasing length of stay and reducing readmissions.4
Our patient had several risk factors for cellulitis, including obesity, lymphedema, and chronic kidney disease, in addition to having fevers and unilateral involvement. However, failure of symptoms to improve with broad-spectrum antibiotics made a diagnosis of cellulitis less likely. In this case, a severe immune response to the ruptured Baker cyst mimicked the presentation of cellulitis.
Ruptured Baker cysts have been reported to cause acute leg swelling, mimicking the symptoms of cellulitis or DVT.2,5 The presence of a hemorrhagic crescent sign can be a useful diagnostic tool, as in our patient, because it has been reported as an indication of synovial injury or rupture, supporting the exclusion of cellulitis or DVT when it is observed.6 Prior reports have observed ecchymosis on the foot in as little as 1 day after the onset of calf swelling and at the lateral malleolus 3 days after the onset of calf swelling.5
Following suspicion of a ruptured Baker cyst causing pseudocellulitis, an ultrasound can be used to confirm the diagnosis. Ultrasonography shows a large hypoechoic space behind the calf muscles, which is pathognomonic of a ruptured Baker cyst.7
In conclusion, when a hemorrhagic crescent sign is observed, one should be suspicious for a ruptured Baker cyst or other synovial pathology as an etiology of pseudocellulitis. Early recognition of the hemorrhagic crescent sign can help rule out cellulitis and DVT, thereby reducing the amount of intravenous antibiotic prescribed, decreasing the length of hospital stay, and reducing readmission.
- Feldman SR, Fleischer AB, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730. doi:10.1001/archinte.158.7.726
- Von Schroeder HP, Ameli FM, Piazza D, et al. Ruptured Baker’s cyst causes ecchymosis of the foot. J Bone Joint Surg Br. 1993;75:316-317.
- Levell NJ, Wingfield CG, Garioch JJ. Severe lower limb cellulitis is best diagnosed by dermatologists and managed with shared care between primary and secondary care. Br J Dermatol. 2011;164:1326-1328.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;53:523-528.
- Dunlop D, Parker PJ, Keating JF. Ruptured Baker’s cyst causing posterior compartment syndrome. Injury. 1997;28:561-562.
- Kraag G, Thevathasan EM, Gordon DA, et al. The hemorrhagic crescent sign of acute synovial rupture. Ann Intern Med. 1976;85:477-478.
- Sato O, Kondoh K, Iyori K, et al. Midcalf ultrasonography for the diagnosis of ruptured Baker’s cysts. Surg Today. 2001;31:410-413. doi:10.1007/s005950170131
- Feldman SR, Fleischer AB, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730. doi:10.1001/archinte.158.7.726
- Von Schroeder HP, Ameli FM, Piazza D, et al. Ruptured Baker’s cyst causes ecchymosis of the foot. J Bone Joint Surg Br. 1993;75:316-317.
- Levell NJ, Wingfield CG, Garioch JJ. Severe lower limb cellulitis is best diagnosed by dermatologists and managed with shared care between primary and secondary care. Br J Dermatol. 2011;164:1326-1328.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;53:523-528.
- Dunlop D, Parker PJ, Keating JF. Ruptured Baker’s cyst causing posterior compartment syndrome. Injury. 1997;28:561-562.
- Kraag G, Thevathasan EM, Gordon DA, et al. The hemorrhagic crescent sign of acute synovial rupture. Ann Intern Med. 1976;85:477-478.
- Sato O, Kondoh K, Iyori K, et al. Midcalf ultrasonography for the diagnosis of ruptured Baker’s cysts. Surg Today. 2001;31:410-413. doi:10.1007/s005950170131
Practice Points
- Pseudocellulitis is common in patients presenting with cellulitislike symptoms.
- A hemorrhagic crescent at the medial malleolus should lead to the suspicion on bursa or joint pathology as a cause of pseudocellulitis.