User login
CYP450 interactions between illicit substances and prescription medications
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.
Pharmacokinetic interactions
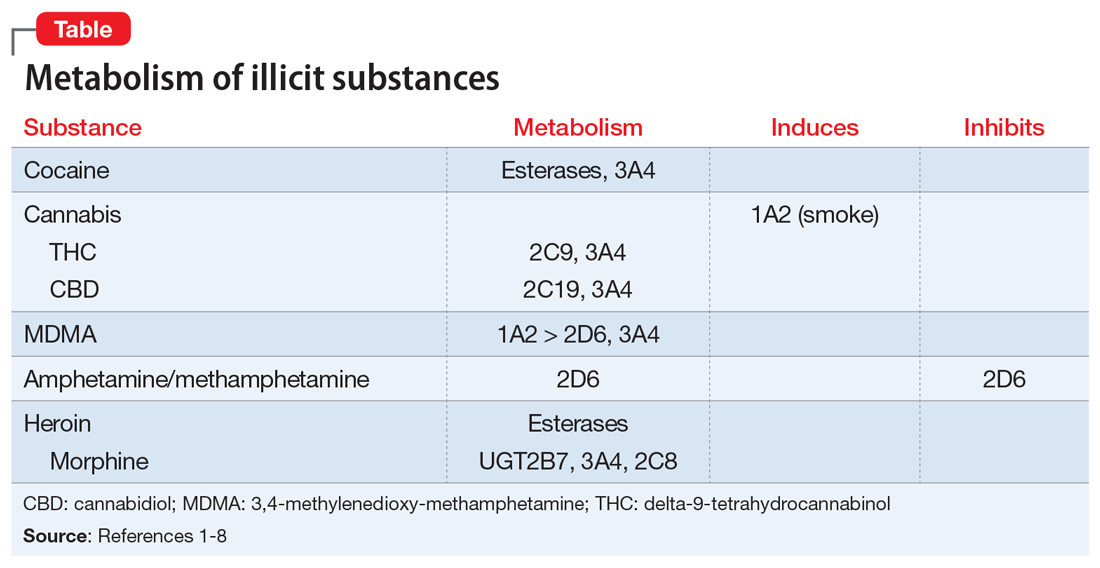
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.
Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.
Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.
Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.
Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.
Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
When the worry is worse than the actual illness
CASE Distraught over a medical illness
Ms. S, age 16, presents to the emergency department (ED) accompanied by her mother with superficial lacerations on her arm. Ms. S states, “I cut my arm because I was afraid I was going to do something serious if I didn’t get to go to the ED.” She says that 6 months earlier, she was diagnosed with superior mesenteric artery syndrome (SMAS), a rare, potentially life-threatening condition that occurs when the duodenum is compressed between the aorta and the superior mesenteric artery, causing a partial or complete blockage of the duodenum. Since receiving this diagnosis, Ms. S reports feeling anxious, depressed, and overwhelmed by both the pain she is experiencing from her illness and uncertainty about her prognosis.
HISTORY In pain and isolated
Since being diagnosed with SMAS, Ms. S has had approximately 30 medical and 7 ED visits for SMAS-related pain. Ms. S was referred to the outpatient clinic for ongoing support and treatment for SMAS.
Because of her pain and anxiety, Ms. S, a junior in high school, no longer attends school but has been working with a tutor. Ms. S says that some of her loneliness and hopelessness are due to the social isolation of being tutored at home. She states that she has been “out of sight and out of mind” from her friends. She also reports feeling different from them due to the pain brought on by SMAS.
Ms. S and her mother live in public housing. Ms. S says that overall, she has a good relationship with her mother, but that in certain situations, her mother’s anxiety causes her significant frustration and anxiety.
EVALUATION Transient suicidal thoughts
A physical examination reveals superficial lacerations to Ms. S’s left arm. Although she appears thin, her current body mass index (BMI) is 20.4 kg/m2, which is within normal range. She says she sees herself as “underweight” and “not fat at all.” Ms. S reports that she likes food and enjoyed eating until it became too painful following her SMAS diagnosis. Ms. S denies a history of binging or purging. Results from her laboratory workup and all values are within normal limits.
During the initial interview, Ms. S’s mother says they came to the ED because Ms. S urgently needs a psychiatric evaluation so she can be cleared for gastrointestinal (GI) surgery and placement of a nasogastric tube. Her mother says a surgeon from a different hospital told them that her insurance company required a psychiatric evaluation to rule out anorexia nervosa before they would authorize the GI surgery. When asked why psychiatry at this hospital was not consulted, Ms. S’s mother does not answer.
When asked about the symptoms she has been experiencing, Ms. S says that her sleep has been poor because of increased pain and excessive worrying about her health. She has limited her food intake. Ms. S reports that after eating, she lays on her left side to alleviate pain and help the food move through her body.
Continue to: Ms. S says...
Ms. S says she feels anxious and depressed due to her SMAS diagnosis, her mother’s online research and oversharing of poor prognoses, and being isolated from her friends. Most of her time outside the home is spent attending medical appointments with specialists. Several months ago, Ms. S had seen a psychotherapist, but her mother was unhappy with the treatment recommendations, which included seeking care from a nutritionist and joining group therapy. Ms. S’s mother says she ended her daughter’s psychotherapy because she was unable to obtain a signature ruling out anorexia nervosa within the first few appointments.
Ms. S also says she has had passive suicidal thoughts during the past month, usually twice a week. She reports that these thoughts lasted as long as several hours and were difficult to control, but she has no specific plan or intent. Ms. S denies current suicidal thoughts or ideation, and works with the treatment team to complete a safety plan, which she signs. Other than her recent visit to the ED, Ms. S denies any other thoughts or behaviors of self-injury or suicide.
[polldaddy:10586905]
The authors’ observations
The treatment team considered the following conditions as part of Ms. S’s differential diagnosis:
Major depressive disorder. The team was able to rule out MDD because Ms. S’s depression was attributed to SMAS. Ms. S reported that all depressive symptoms were manageable or nonexistent before the onset of pain from SMAS. There was no direct pathophysiological consequence of another medical condition. Ms. S was clear that her symptoms of anxiety and depression began after she was isolated from her friends and began having difficulty understanding her diagnosis and prognosis.
Anorexia nervosa also was ruled out. According to the DSM-5, a diagnosis of anorexia nervosa requires the following 3 criteria1:
- restriction of food intake resulting in significantly low body weight (defined as weight that is less than “minimally normal”) relative to age, gender, or development
- intense fear of gaining weight, or persistent behaviors that interfere with weight gain
- disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or lack of insight with regard to seriousness of current low body weight.
Continue to: Although Ms. S appeared...
Although Ms. S appeared thin, her BMI was within normal range. She added that she likes food and enjoyed eating, but that her medical condition made it too painful. Lastly, Ms. S denied a history of binging or purging.
Somatic symptom disorder.
Factitious disorder imposed on self. An individual with FDIS chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient.
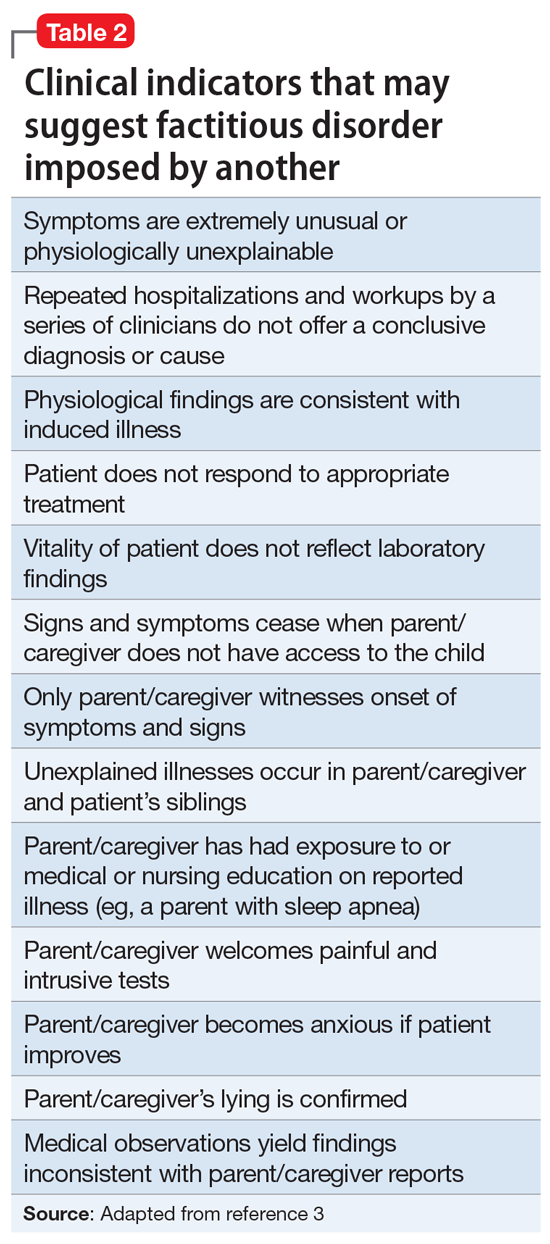
Factitious disorder imposed on another is the deliberate feigning or production of symptoms in another individual who is under the perpetrator’s supervision.1Table 23 lists clinical indicators that raise suspicion for FDIA.
Before a diagnosis of somatic symptom disorder, FDIS, or FDIA could be established or ruled out, it was imperative to gather collateral information from other clinicians involved in Ms. S’s care. Ms. S and her mother had sought out help from a pediatric surgeon, a pediatric gastroenterologist, a pediatrician, and a psychotherapist.
Continue to: EVALUATION Collateral information
EVALUATION Collateral information
After Ms. S’s mother signs consent forms for exchange of information, the treatment team reaches out to the other clinicians. The therapist confirms that Ms. S’s mother had ended her daughter’s treatment after she was unable to quickly obtain documentation to rule out anorexia nervosa.
Both the pediatric surgeon and gastroenterologist report concerns of FDIA, which is why both clinicians had referred Ms. S and her mother to psychiatry. The pediatric surgeon states that on one occasion when he interviewed Ms. S separately from her mother, she seemed to be going down a checklist of symptoms. The surgeon reports that there was a partial occlusion of the superior mesenteric artery, confirming the diagnosis of SMAS, but he believed it was not severe enough to explain the symptoms Ms. S reported. The surgeon had scheduled another imaging appointment for 1 month later.
The pediatric gastroenterologist reports that Ms. S’s mother had demanded surgery and nasogastric tube placement for her daughter, which raised suspicion of FDIA. The gastroenterologist had convinced Ms. S and her mother to start low-dose doxepin, 20 mg twice a day, for anxiety, sleep, and abdominal pain.
Lastly, the pediatrician reports that she had not seen Ms. S for several months but stated that Ms. S always has been in the low normal BMI range. The pediatrician also reports that 6 months ago, the patient and her mother were frantically visiting EDs and scheduling doctor’s appointments.
[polldaddy:10586906]
The authors’ observations
The treatment team decided that Ms. S was not in imminent danger, and felt it was important to keep her in treatment without raising her mother’s suspicion. The team agreed to raise these concerns to the police, child protective services, and risk management if Ms. S’s health suddenly deteriorated or if her mother decided to remove Ms. S from our care.
Continue to: The treatment team...
The treatment team at the outpatient psychiatry clinic agreed that Ms. S did not currently meet criteria for anorexia nervosa, MDD, FDIS, or FDIA. However, Ms. S reported worries particular to persistent abdominal pain that was exacerbated by either eating or going to bed at night, which indicated that somatic symptom disorder was her likely diagnosis. Further, she endorsed a high level of anxiety and depression with regard to this somatic complaint that interfered with her daily activities and consumed an excessive amount of time, which also pointed to somatic symptom disorder. As a result of this diagnosis, the treatment team helped Ms. S manage her somatic symptoms and monitored for any other changes in her symptoms
Generally, cognitive-behavioral therapy (CBT) and mindfulness-based therapy may help relieve symptoms associated with somatic symptom disorder.4
TREATMENT Therapy sessions and medication management
At the psychiatric clinic, Ms. S is scheduled for biweekly therapy sessions with a social worker and biweekly appointments with a senior psychiatry resident for medication management. At each visit, Ms. S’s vital signs, height, and weight are measured. In the therapy sessions, she is taught mindfulness skills as well as CBT. The senior psychiatry resident maintains regular communication with the other clinicians involved in Ms. S’s care.
After the first month of treatment, Ms. S undergoes repeat imaging at the gastroenterologist’s office that indicates her SMAS is no longer occluded. Ms. S continues to report somatic symptoms, but with mild improvement.
Over the course of approximately 4 months, Ms. S begins to show signs of improvement in her pain, anxiety, and depression. Ms. S begins to feel well enough to get a summer job at a nursing home and expresses enthusiasm when her weight begins to increase. Her mother also became enthused and verbalized her appreciation that her daughter appeared to be improving.
Continue to: In the fall...
In the fall, Ms. S returns to high school for her senior year but has difficulty getting back into the routine and relating to her old friends. Ms. S continues to perseverate on thoughts of getting sick and her physical symptoms become overwhelming once again. She continues to be focused on any new symptoms she experiences, and to limit the types of foods she eats due to fear of the abdominal pain returning.
After several more months of psychiatric treatment, Ms. S reports significant relief from her abdominal pain, and no longer seeks corrective surgery for her SMAS. Although she occasionally struggles with perseverating thoughts and anxiety about her somatic symptoms such as abdominal pain and worrying about the types of foods she eats and becoming ill, she continues to work through symptoms of her somatic symptom disorder.
The authors’ observations
The main challenge of somatic symptom disorder is the patient’s “abnormal illness behavior.”2,5,6 For pediatric patients, there may an association between a parent’s psychological status and the patient’s somatic symptoms. Abdominal symptoms in a pediatric patient have a strong association with a parent who presents with depression, anxiety, or somatization. The effects of the parent’s psychological status could also manifest in the form of modeling catastrophic thinking or through reinforcement. Parents with certain traits, such as disproportionate worry about pain, may pay more attention to their child’s symptoms, and hence, reward the child when he/she reports somatic symptoms.7,8 In the case of Ms. S, her mother did not participate in therapy and the mother’s psychiatric history was never obtained.
OUTCOMES Making personal strides
Ms. S continues to use mindfulness skills as well as CBT to manage her symptoms of somatic symptom disorder. She continues to celebrate her weight gains, denies any thoughts of suicide or self-harm behaviors, and prepares for college by scheduling campus visits and completing admissions applications.
Bottom Line
Patients with somatic symptom disorder tend to have very high levels of worry about illness. Somatic symptoms in such patients may or may not have a medical explanation. Accurate diagnosis and careful management are necessary to reduce patient distress. Cognitive-behavioral therapy and mindfulness-based therapy may help relieve symptoms associated with this disorder.
Related Resources
- Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-91.
- Rosic T, Kalra S, Samaan Z. Somatic symptom disorder, a new DSM-5 diagnosis of an old clinical challenge. BMJ Case Rep. 2016: bcr2015212553. doi: 10.1136/bcr-2015-212553.
Drug Brand Name
Doxepin • Silenor
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern T, Freudenreich O, Smith F, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 7th ed. New York, NY: Elsevier; 2017.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Association; 1997.
4. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry, 11th ed. Philadelphia, PA: Wolters Kluwer; 2014:470.
5. Pilowsky I. The concept of abnormal illness behavior. Psychosomatics. 1990;31(2):207-213.
6. Kirmayer LJ, Looper KJ. Abnormal illness behavior: physiological, psychological and social dimensions of coping with stress. Curr Opin Psychiatry. 2006;19(1):54-60.
7. Walker LS, Garber J, Greene JW. Somatic complaints in pediatric patients: a prospective study of the role of negative life events, child social and academic competence, and parental somatic symptoms. J Consult Clin Psychology. 1994;62(6):1213-1221.
8. Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355-1367.
CASE Distraught over a medical illness
Ms. S, age 16, presents to the emergency department (ED) accompanied by her mother with superficial lacerations on her arm. Ms. S states, “I cut my arm because I was afraid I was going to do something serious if I didn’t get to go to the ED.” She says that 6 months earlier, she was diagnosed with superior mesenteric artery syndrome (SMAS), a rare, potentially life-threatening condition that occurs when the duodenum is compressed between the aorta and the superior mesenteric artery, causing a partial or complete blockage of the duodenum. Since receiving this diagnosis, Ms. S reports feeling anxious, depressed, and overwhelmed by both the pain she is experiencing from her illness and uncertainty about her prognosis.
HISTORY In pain and isolated
Since being diagnosed with SMAS, Ms. S has had approximately 30 medical and 7 ED visits for SMAS-related pain. Ms. S was referred to the outpatient clinic for ongoing support and treatment for SMAS.
Because of her pain and anxiety, Ms. S, a junior in high school, no longer attends school but has been working with a tutor. Ms. S says that some of her loneliness and hopelessness are due to the social isolation of being tutored at home. She states that she has been “out of sight and out of mind” from her friends. She also reports feeling different from them due to the pain brought on by SMAS.
Ms. S and her mother live in public housing. Ms. S says that overall, she has a good relationship with her mother, but that in certain situations, her mother’s anxiety causes her significant frustration and anxiety.
EVALUATION Transient suicidal thoughts
A physical examination reveals superficial lacerations to Ms. S’s left arm. Although she appears thin, her current body mass index (BMI) is 20.4 kg/m2, which is within normal range. She says she sees herself as “underweight” and “not fat at all.” Ms. S reports that she likes food and enjoyed eating until it became too painful following her SMAS diagnosis. Ms. S denies a history of binging or purging. Results from her laboratory workup and all values are within normal limits.
During the initial interview, Ms. S’s mother says they came to the ED because Ms. S urgently needs a psychiatric evaluation so she can be cleared for gastrointestinal (GI) surgery and placement of a nasogastric tube. Her mother says a surgeon from a different hospital told them that her insurance company required a psychiatric evaluation to rule out anorexia nervosa before they would authorize the GI surgery. When asked why psychiatry at this hospital was not consulted, Ms. S’s mother does not answer.
When asked about the symptoms she has been experiencing, Ms. S says that her sleep has been poor because of increased pain and excessive worrying about her health. She has limited her food intake. Ms. S reports that after eating, she lays on her left side to alleviate pain and help the food move through her body.
Continue to: Ms. S says...
Ms. S says she feels anxious and depressed due to her SMAS diagnosis, her mother’s online research and oversharing of poor prognoses, and being isolated from her friends. Most of her time outside the home is spent attending medical appointments with specialists. Several months ago, Ms. S had seen a psychotherapist, but her mother was unhappy with the treatment recommendations, which included seeking care from a nutritionist and joining group therapy. Ms. S’s mother says she ended her daughter’s psychotherapy because she was unable to obtain a signature ruling out anorexia nervosa within the first few appointments.
Ms. S also says she has had passive suicidal thoughts during the past month, usually twice a week. She reports that these thoughts lasted as long as several hours and were difficult to control, but she has no specific plan or intent. Ms. S denies current suicidal thoughts or ideation, and works with the treatment team to complete a safety plan, which she signs. Other than her recent visit to the ED, Ms. S denies any other thoughts or behaviors of self-injury or suicide.
[polldaddy:10586905]
The authors’ observations
The treatment team considered the following conditions as part of Ms. S’s differential diagnosis:
Major depressive disorder. The team was able to rule out MDD because Ms. S’s depression was attributed to SMAS. Ms. S reported that all depressive symptoms were manageable or nonexistent before the onset of pain from SMAS. There was no direct pathophysiological consequence of another medical condition. Ms. S was clear that her symptoms of anxiety and depression began after she was isolated from her friends and began having difficulty understanding her diagnosis and prognosis.
Anorexia nervosa also was ruled out. According to the DSM-5, a diagnosis of anorexia nervosa requires the following 3 criteria1:
- restriction of food intake resulting in significantly low body weight (defined as weight that is less than “minimally normal”) relative to age, gender, or development
- intense fear of gaining weight, or persistent behaviors that interfere with weight gain
- disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or lack of insight with regard to seriousness of current low body weight.
Continue to: Although Ms. S appeared...
Although Ms. S appeared thin, her BMI was within normal range. She added that she likes food and enjoyed eating, but that her medical condition made it too painful. Lastly, Ms. S denied a history of binging or purging.
Somatic symptom disorder.
Factitious disorder imposed on self. An individual with FDIS chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient.
Factitious disorder imposed on another is the deliberate feigning or production of symptoms in another individual who is under the perpetrator’s supervision.1Table 23 lists clinical indicators that raise suspicion for FDIA.
Before a diagnosis of somatic symptom disorder, FDIS, or FDIA could be established or ruled out, it was imperative to gather collateral information from other clinicians involved in Ms. S’s care. Ms. S and her mother had sought out help from a pediatric surgeon, a pediatric gastroenterologist, a pediatrician, and a psychotherapist.
Continue to: EVALUATION Collateral information
EVALUATION Collateral information
After Ms. S’s mother signs consent forms for exchange of information, the treatment team reaches out to the other clinicians. The therapist confirms that Ms. S’s mother had ended her daughter’s treatment after she was unable to quickly obtain documentation to rule out anorexia nervosa.
Both the pediatric surgeon and gastroenterologist report concerns of FDIA, which is why both clinicians had referred Ms. S and her mother to psychiatry. The pediatric surgeon states that on one occasion when he interviewed Ms. S separately from her mother, she seemed to be going down a checklist of symptoms. The surgeon reports that there was a partial occlusion of the superior mesenteric artery, confirming the diagnosis of SMAS, but he believed it was not severe enough to explain the symptoms Ms. S reported. The surgeon had scheduled another imaging appointment for 1 month later.
The pediatric gastroenterologist reports that Ms. S’s mother had demanded surgery and nasogastric tube placement for her daughter, which raised suspicion of FDIA. The gastroenterologist had convinced Ms. S and her mother to start low-dose doxepin, 20 mg twice a day, for anxiety, sleep, and abdominal pain.
Lastly, the pediatrician reports that she had not seen Ms. S for several months but stated that Ms. S always has been in the low normal BMI range. The pediatrician also reports that 6 months ago, the patient and her mother were frantically visiting EDs and scheduling doctor’s appointments.
[polldaddy:10586906]
The authors’ observations
The treatment team decided that Ms. S was not in imminent danger, and felt it was important to keep her in treatment without raising her mother’s suspicion. The team agreed to raise these concerns to the police, child protective services, and risk management if Ms. S’s health suddenly deteriorated or if her mother decided to remove Ms. S from our care.
Continue to: The treatment team...
The treatment team at the outpatient psychiatry clinic agreed that Ms. S did not currently meet criteria for anorexia nervosa, MDD, FDIS, or FDIA. However, Ms. S reported worries particular to persistent abdominal pain that was exacerbated by either eating or going to bed at night, which indicated that somatic symptom disorder was her likely diagnosis. Further, she endorsed a high level of anxiety and depression with regard to this somatic complaint that interfered with her daily activities and consumed an excessive amount of time, which also pointed to somatic symptom disorder. As a result of this diagnosis, the treatment team helped Ms. S manage her somatic symptoms and monitored for any other changes in her symptoms
Generally, cognitive-behavioral therapy (CBT) and mindfulness-based therapy may help relieve symptoms associated with somatic symptom disorder.4
TREATMENT Therapy sessions and medication management
At the psychiatric clinic, Ms. S is scheduled for biweekly therapy sessions with a social worker and biweekly appointments with a senior psychiatry resident for medication management. At each visit, Ms. S’s vital signs, height, and weight are measured. In the therapy sessions, she is taught mindfulness skills as well as CBT. The senior psychiatry resident maintains regular communication with the other clinicians involved in Ms. S’s care.
After the first month of treatment, Ms. S undergoes repeat imaging at the gastroenterologist’s office that indicates her SMAS is no longer occluded. Ms. S continues to report somatic symptoms, but with mild improvement.
Over the course of approximately 4 months, Ms. S begins to show signs of improvement in her pain, anxiety, and depression. Ms. S begins to feel well enough to get a summer job at a nursing home and expresses enthusiasm when her weight begins to increase. Her mother also became enthused and verbalized her appreciation that her daughter appeared to be improving.
Continue to: In the fall...
In the fall, Ms. S returns to high school for her senior year but has difficulty getting back into the routine and relating to her old friends. Ms. S continues to perseverate on thoughts of getting sick and her physical symptoms become overwhelming once again. She continues to be focused on any new symptoms she experiences, and to limit the types of foods she eats due to fear of the abdominal pain returning.
After several more months of psychiatric treatment, Ms. S reports significant relief from her abdominal pain, and no longer seeks corrective surgery for her SMAS. Although she occasionally struggles with perseverating thoughts and anxiety about her somatic symptoms such as abdominal pain and worrying about the types of foods she eats and becoming ill, she continues to work through symptoms of her somatic symptom disorder.
The authors’ observations
The main challenge of somatic symptom disorder is the patient’s “abnormal illness behavior.”2,5,6 For pediatric patients, there may an association between a parent’s psychological status and the patient’s somatic symptoms. Abdominal symptoms in a pediatric patient have a strong association with a parent who presents with depression, anxiety, or somatization. The effects of the parent’s psychological status could also manifest in the form of modeling catastrophic thinking or through reinforcement. Parents with certain traits, such as disproportionate worry about pain, may pay more attention to their child’s symptoms, and hence, reward the child when he/she reports somatic symptoms.7,8 In the case of Ms. S, her mother did not participate in therapy and the mother’s psychiatric history was never obtained.
OUTCOMES Making personal strides
Ms. S continues to use mindfulness skills as well as CBT to manage her symptoms of somatic symptom disorder. She continues to celebrate her weight gains, denies any thoughts of suicide or self-harm behaviors, and prepares for college by scheduling campus visits and completing admissions applications.
Bottom Line
Patients with somatic symptom disorder tend to have very high levels of worry about illness. Somatic symptoms in such patients may or may not have a medical explanation. Accurate diagnosis and careful management are necessary to reduce patient distress. Cognitive-behavioral therapy and mindfulness-based therapy may help relieve symptoms associated with this disorder.
Related Resources
- Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-91.
- Rosic T, Kalra S, Samaan Z. Somatic symptom disorder, a new DSM-5 diagnosis of an old clinical challenge. BMJ Case Rep. 2016: bcr2015212553. doi: 10.1136/bcr-2015-212553.
Drug Brand Name
Doxepin • Silenor
CASE Distraught over a medical illness
Ms. S, age 16, presents to the emergency department (ED) accompanied by her mother with superficial lacerations on her arm. Ms. S states, “I cut my arm because I was afraid I was going to do something serious if I didn’t get to go to the ED.” She says that 6 months earlier, she was diagnosed with superior mesenteric artery syndrome (SMAS), a rare, potentially life-threatening condition that occurs when the duodenum is compressed between the aorta and the superior mesenteric artery, causing a partial or complete blockage of the duodenum. Since receiving this diagnosis, Ms. S reports feeling anxious, depressed, and overwhelmed by both the pain she is experiencing from her illness and uncertainty about her prognosis.
HISTORY In pain and isolated
Since being diagnosed with SMAS, Ms. S has had approximately 30 medical and 7 ED visits for SMAS-related pain. Ms. S was referred to the outpatient clinic for ongoing support and treatment for SMAS.
Because of her pain and anxiety, Ms. S, a junior in high school, no longer attends school but has been working with a tutor. Ms. S says that some of her loneliness and hopelessness are due to the social isolation of being tutored at home. She states that she has been “out of sight and out of mind” from her friends. She also reports feeling different from them due to the pain brought on by SMAS.
Ms. S and her mother live in public housing. Ms. S says that overall, she has a good relationship with her mother, but that in certain situations, her mother’s anxiety causes her significant frustration and anxiety.
EVALUATION Transient suicidal thoughts
A physical examination reveals superficial lacerations to Ms. S’s left arm. Although she appears thin, her current body mass index (BMI) is 20.4 kg/m2, which is within normal range. She says she sees herself as “underweight” and “not fat at all.” Ms. S reports that she likes food and enjoyed eating until it became too painful following her SMAS diagnosis. Ms. S denies a history of binging or purging. Results from her laboratory workup and all values are within normal limits.
During the initial interview, Ms. S’s mother says they came to the ED because Ms. S urgently needs a psychiatric evaluation so she can be cleared for gastrointestinal (GI) surgery and placement of a nasogastric tube. Her mother says a surgeon from a different hospital told them that her insurance company required a psychiatric evaluation to rule out anorexia nervosa before they would authorize the GI surgery. When asked why psychiatry at this hospital was not consulted, Ms. S’s mother does not answer.
When asked about the symptoms she has been experiencing, Ms. S says that her sleep has been poor because of increased pain and excessive worrying about her health. She has limited her food intake. Ms. S reports that after eating, she lays on her left side to alleviate pain and help the food move through her body.
Continue to: Ms. S says...
Ms. S says she feels anxious and depressed due to her SMAS diagnosis, her mother’s online research and oversharing of poor prognoses, and being isolated from her friends. Most of her time outside the home is spent attending medical appointments with specialists. Several months ago, Ms. S had seen a psychotherapist, but her mother was unhappy with the treatment recommendations, which included seeking care from a nutritionist and joining group therapy. Ms. S’s mother says she ended her daughter’s psychotherapy because she was unable to obtain a signature ruling out anorexia nervosa within the first few appointments.
Ms. S also says she has had passive suicidal thoughts during the past month, usually twice a week. She reports that these thoughts lasted as long as several hours and were difficult to control, but she has no specific plan or intent. Ms. S denies current suicidal thoughts or ideation, and works with the treatment team to complete a safety plan, which she signs. Other than her recent visit to the ED, Ms. S denies any other thoughts or behaviors of self-injury or suicide.
[polldaddy:10586905]
The authors’ observations
The treatment team considered the following conditions as part of Ms. S’s differential diagnosis:
Major depressive disorder. The team was able to rule out MDD because Ms. S’s depression was attributed to SMAS. Ms. S reported that all depressive symptoms were manageable or nonexistent before the onset of pain from SMAS. There was no direct pathophysiological consequence of another medical condition. Ms. S was clear that her symptoms of anxiety and depression began after she was isolated from her friends and began having difficulty understanding her diagnosis and prognosis.
Anorexia nervosa also was ruled out. According to the DSM-5, a diagnosis of anorexia nervosa requires the following 3 criteria1:
- restriction of food intake resulting in significantly low body weight (defined as weight that is less than “minimally normal”) relative to age, gender, or development
- intense fear of gaining weight, or persistent behaviors that interfere with weight gain
- disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or lack of insight with regard to seriousness of current low body weight.
Continue to: Although Ms. S appeared...
Although Ms. S appeared thin, her BMI was within normal range. She added that she likes food and enjoyed eating, but that her medical condition made it too painful. Lastly, Ms. S denied a history of binging or purging.
Somatic symptom disorder.
Factitious disorder imposed on self. An individual with FDIS chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient.
Factitious disorder imposed on another is the deliberate feigning or production of symptoms in another individual who is under the perpetrator’s supervision.1Table 23 lists clinical indicators that raise suspicion for FDIA.
Before a diagnosis of somatic symptom disorder, FDIS, or FDIA could be established or ruled out, it was imperative to gather collateral information from other clinicians involved in Ms. S’s care. Ms. S and her mother had sought out help from a pediatric surgeon, a pediatric gastroenterologist, a pediatrician, and a psychotherapist.
Continue to: EVALUATION Collateral information
EVALUATION Collateral information
After Ms. S’s mother signs consent forms for exchange of information, the treatment team reaches out to the other clinicians. The therapist confirms that Ms. S’s mother had ended her daughter’s treatment after she was unable to quickly obtain documentation to rule out anorexia nervosa.
Both the pediatric surgeon and gastroenterologist report concerns of FDIA, which is why both clinicians had referred Ms. S and her mother to psychiatry. The pediatric surgeon states that on one occasion when he interviewed Ms. S separately from her mother, she seemed to be going down a checklist of symptoms. The surgeon reports that there was a partial occlusion of the superior mesenteric artery, confirming the diagnosis of SMAS, but he believed it was not severe enough to explain the symptoms Ms. S reported. The surgeon had scheduled another imaging appointment for 1 month later.
The pediatric gastroenterologist reports that Ms. S’s mother had demanded surgery and nasogastric tube placement for her daughter, which raised suspicion of FDIA. The gastroenterologist had convinced Ms. S and her mother to start low-dose doxepin, 20 mg twice a day, for anxiety, sleep, and abdominal pain.
Lastly, the pediatrician reports that she had not seen Ms. S for several months but stated that Ms. S always has been in the low normal BMI range. The pediatrician also reports that 6 months ago, the patient and her mother were frantically visiting EDs and scheduling doctor’s appointments.
[polldaddy:10586906]
The authors’ observations
The treatment team decided that Ms. S was not in imminent danger, and felt it was important to keep her in treatment without raising her mother’s suspicion. The team agreed to raise these concerns to the police, child protective services, and risk management if Ms. S’s health suddenly deteriorated or if her mother decided to remove Ms. S from our care.
Continue to: The treatment team...
The treatment team at the outpatient psychiatry clinic agreed that Ms. S did not currently meet criteria for anorexia nervosa, MDD, FDIS, or FDIA. However, Ms. S reported worries particular to persistent abdominal pain that was exacerbated by either eating or going to bed at night, which indicated that somatic symptom disorder was her likely diagnosis. Further, she endorsed a high level of anxiety and depression with regard to this somatic complaint that interfered with her daily activities and consumed an excessive amount of time, which also pointed to somatic symptom disorder. As a result of this diagnosis, the treatment team helped Ms. S manage her somatic symptoms and monitored for any other changes in her symptoms
Generally, cognitive-behavioral therapy (CBT) and mindfulness-based therapy may help relieve symptoms associated with somatic symptom disorder.4
TREATMENT Therapy sessions and medication management
At the psychiatric clinic, Ms. S is scheduled for biweekly therapy sessions with a social worker and biweekly appointments with a senior psychiatry resident for medication management. At each visit, Ms. S’s vital signs, height, and weight are measured. In the therapy sessions, she is taught mindfulness skills as well as CBT. The senior psychiatry resident maintains regular communication with the other clinicians involved in Ms. S’s care.
After the first month of treatment, Ms. S undergoes repeat imaging at the gastroenterologist’s office that indicates her SMAS is no longer occluded. Ms. S continues to report somatic symptoms, but with mild improvement.
Over the course of approximately 4 months, Ms. S begins to show signs of improvement in her pain, anxiety, and depression. Ms. S begins to feel well enough to get a summer job at a nursing home and expresses enthusiasm when her weight begins to increase. Her mother also became enthused and verbalized her appreciation that her daughter appeared to be improving.
Continue to: In the fall...
In the fall, Ms. S returns to high school for her senior year but has difficulty getting back into the routine and relating to her old friends. Ms. S continues to perseverate on thoughts of getting sick and her physical symptoms become overwhelming once again. She continues to be focused on any new symptoms she experiences, and to limit the types of foods she eats due to fear of the abdominal pain returning.
After several more months of psychiatric treatment, Ms. S reports significant relief from her abdominal pain, and no longer seeks corrective surgery for her SMAS. Although she occasionally struggles with perseverating thoughts and anxiety about her somatic symptoms such as abdominal pain and worrying about the types of foods she eats and becoming ill, she continues to work through symptoms of her somatic symptom disorder.
The authors’ observations
The main challenge of somatic symptom disorder is the patient’s “abnormal illness behavior.”2,5,6 For pediatric patients, there may an association between a parent’s psychological status and the patient’s somatic symptoms. Abdominal symptoms in a pediatric patient have a strong association with a parent who presents with depression, anxiety, or somatization. The effects of the parent’s psychological status could also manifest in the form of modeling catastrophic thinking or through reinforcement. Parents with certain traits, such as disproportionate worry about pain, may pay more attention to their child’s symptoms, and hence, reward the child when he/she reports somatic symptoms.7,8 In the case of Ms. S, her mother did not participate in therapy and the mother’s psychiatric history was never obtained.
OUTCOMES Making personal strides
Ms. S continues to use mindfulness skills as well as CBT to manage her symptoms of somatic symptom disorder. She continues to celebrate her weight gains, denies any thoughts of suicide or self-harm behaviors, and prepares for college by scheduling campus visits and completing admissions applications.
Bottom Line
Patients with somatic symptom disorder tend to have very high levels of worry about illness. Somatic symptoms in such patients may or may not have a medical explanation. Accurate diagnosis and careful management are necessary to reduce patient distress. Cognitive-behavioral therapy and mindfulness-based therapy may help relieve symptoms associated with this disorder.
Related Resources
- Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-91.
- Rosic T, Kalra S, Samaan Z. Somatic symptom disorder, a new DSM-5 diagnosis of an old clinical challenge. BMJ Case Rep. 2016: bcr2015212553. doi: 10.1136/bcr-2015-212553.
Drug Brand Name
Doxepin • Silenor
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern T, Freudenreich O, Smith F, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 7th ed. New York, NY: Elsevier; 2017.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Association; 1997.
4. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry, 11th ed. Philadelphia, PA: Wolters Kluwer; 2014:470.
5. Pilowsky I. The concept of abnormal illness behavior. Psychosomatics. 1990;31(2):207-213.
6. Kirmayer LJ, Looper KJ. Abnormal illness behavior: physiological, psychological and social dimensions of coping with stress. Curr Opin Psychiatry. 2006;19(1):54-60.
7. Walker LS, Garber J, Greene JW. Somatic complaints in pediatric patients: a prospective study of the role of negative life events, child social and academic competence, and parental somatic symptoms. J Consult Clin Psychology. 1994;62(6):1213-1221.
8. Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355-1367.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern T, Freudenreich O, Smith F, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 7th ed. New York, NY: Elsevier; 2017.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Association; 1997.
4. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry, 11th ed. Philadelphia, PA: Wolters Kluwer; 2014:470.
5. Pilowsky I. The concept of abnormal illness behavior. Psychosomatics. 1990;31(2):207-213.
6. Kirmayer LJ, Looper KJ. Abnormal illness behavior: physiological, psychological and social dimensions of coping with stress. Curr Opin Psychiatry. 2006;19(1):54-60.
7. Walker LS, Garber J, Greene JW. Somatic complaints in pediatric patients: a prospective study of the role of negative life events, child social and academic competence, and parental somatic symptoms. J Consult Clin Psychology. 1994;62(6):1213-1221.
8. Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355-1367.
What to tell parents whose child saw them having sex
Many parents find themselves in a difficult situation when their child accidentally sees them having sex. These patients may ask us, as their clinicians, if they should discuss the incident with their child, and if so, what to say. If parents do not address the subject appropriately, their child might be confused and uncomfortable with his/her interpretation of the encounter.1 Some older research suggests that after witnessing their parents in a sexual encounter, children may have difficulty with affectional love, fears of being alone, or feelings of vulnerability.2,3 Clinicians may find themselves at a loss when parents ask them how to handle these situations. Although there are no evidence-based guidelines to consult, consider the following suggestions:
Relax. For patients who have not yet experienced this situation, tell them it is important not to panic if their child witnesses them having sex. They should cover their bodies and calmly respond to their child’s presence. Calm responsiveness is a key to diffusing this awkward situation. Otherwise, children may sense their parents’ embarrassment and conclude that sex is shameful. Parents should gently explain to their child that they are having a private, adult moment. They should ask their child if something is needed immediately, or if it could wait.
Accept that it happened. Parents should not avoid discussing the incident, but should promptly follow up with their child at an appropriate time and place. Waiting for a child to raise the topic puts the responsibility on him/her, instead of on the parent. Although some forthright children may ask questions, others may feel too ashamed or nervous to broach the topic and will prefer their parents to take the lead.
Discuss what happened. Tell parents to explore their child’s impression of what he/she saw. Tailoring the discussion to the child’s age is important. For example, a 3-year-old might wonder if anyone was harmed, and might need reassurance, whereas a 12-year-old is likely to have a better understanding of sex but still feel uncomfortable. Educational conversations about sexuality might be appropriate for children age 8 to 12. The parents’ goal should be to answer questions honestly without oversharing, and to leave the door open—so to speak—for future conversations.
Recommend that parents use plain, factual language to answer any questions their child asks. Statements such as “We were having a private, adult moment” can be helpful. Parents can categorize sex as a universal activity that is not harmful or scary by telling their child something such as, “This is what all parents do.” Parents should avoid providing unnecessary information or answering questions their child is not asking. The Table4 offers guidance on how parents might handle such conversations.
Consider potentially positive outcomes. Although parents may feel guilty or describe this as a terrible situation, remind them that there are some potentially healthy outcomes. For example, such incidents may help reassure the child that their parents love each other, which might give him/her a sense of happiness and security.
Take steps to prevent this from happening again. Advise parents to lock the door when having sex. Remind them to consider the proximity of rooms because their child might hear noises and become curious.
1. Blum HP. On the concept and consequences of the primal scene. Psychoanal Q. 1979;48(1):27-47.
2. Hoyt MF. On the psychology and psychopathology of primal-scene experience. J Am Acad Psychoanal. 1980;8(3):311-335.
3. Ikonen P, Rechardt E. On the universal nature of primal scene fantasies. Int J Psychoanal. 1984;65(pt 1):63-72.
4. Pelly J. Four things my four-year-old already knows about sex. Today’s Parent. https://www.todaysparent.com/family/parenting/4-things-my-4-year-old-already-knows-about-sex/. Published February 1, 2020. Accessed June 20, 2020.
Many parents find themselves in a difficult situation when their child accidentally sees them having sex. These patients may ask us, as their clinicians, if they should discuss the incident with their child, and if so, what to say. If parents do not address the subject appropriately, their child might be confused and uncomfortable with his/her interpretation of the encounter.1 Some older research suggests that after witnessing their parents in a sexual encounter, children may have difficulty with affectional love, fears of being alone, or feelings of vulnerability.2,3 Clinicians may find themselves at a loss when parents ask them how to handle these situations. Although there are no evidence-based guidelines to consult, consider the following suggestions:
Relax. For patients who have not yet experienced this situation, tell them it is important not to panic if their child witnesses them having sex. They should cover their bodies and calmly respond to their child’s presence. Calm responsiveness is a key to diffusing this awkward situation. Otherwise, children may sense their parents’ embarrassment and conclude that sex is shameful. Parents should gently explain to their child that they are having a private, adult moment. They should ask their child if something is needed immediately, or if it could wait.
Accept that it happened. Parents should not avoid discussing the incident, but should promptly follow up with their child at an appropriate time and place. Waiting for a child to raise the topic puts the responsibility on him/her, instead of on the parent. Although some forthright children may ask questions, others may feel too ashamed or nervous to broach the topic and will prefer their parents to take the lead.
Discuss what happened. Tell parents to explore their child’s impression of what he/she saw. Tailoring the discussion to the child’s age is important. For example, a 3-year-old might wonder if anyone was harmed, and might need reassurance, whereas a 12-year-old is likely to have a better understanding of sex but still feel uncomfortable. Educational conversations about sexuality might be appropriate for children age 8 to 12. The parents’ goal should be to answer questions honestly without oversharing, and to leave the door open—so to speak—for future conversations.
Recommend that parents use plain, factual language to answer any questions their child asks. Statements such as “We were having a private, adult moment” can be helpful. Parents can categorize sex as a universal activity that is not harmful or scary by telling their child something such as, “This is what all parents do.” Parents should avoid providing unnecessary information or answering questions their child is not asking. The Table4 offers guidance on how parents might handle such conversations.
Consider potentially positive outcomes. Although parents may feel guilty or describe this as a terrible situation, remind them that there are some potentially healthy outcomes. For example, such incidents may help reassure the child that their parents love each other, which might give him/her a sense of happiness and security.
Take steps to prevent this from happening again. Advise parents to lock the door when having sex. Remind them to consider the proximity of rooms because their child might hear noises and become curious.
Many parents find themselves in a difficult situation when their child accidentally sees them having sex. These patients may ask us, as their clinicians, if they should discuss the incident with their child, and if so, what to say. If parents do not address the subject appropriately, their child might be confused and uncomfortable with his/her interpretation of the encounter.1 Some older research suggests that after witnessing their parents in a sexual encounter, children may have difficulty with affectional love, fears of being alone, or feelings of vulnerability.2,3 Clinicians may find themselves at a loss when parents ask them how to handle these situations. Although there are no evidence-based guidelines to consult, consider the following suggestions:
Relax. For patients who have not yet experienced this situation, tell them it is important not to panic if their child witnesses them having sex. They should cover their bodies and calmly respond to their child’s presence. Calm responsiveness is a key to diffusing this awkward situation. Otherwise, children may sense their parents’ embarrassment and conclude that sex is shameful. Parents should gently explain to their child that they are having a private, adult moment. They should ask their child if something is needed immediately, or if it could wait.
Accept that it happened. Parents should not avoid discussing the incident, but should promptly follow up with their child at an appropriate time and place. Waiting for a child to raise the topic puts the responsibility on him/her, instead of on the parent. Although some forthright children may ask questions, others may feel too ashamed or nervous to broach the topic and will prefer their parents to take the lead.
Discuss what happened. Tell parents to explore their child’s impression of what he/she saw. Tailoring the discussion to the child’s age is important. For example, a 3-year-old might wonder if anyone was harmed, and might need reassurance, whereas a 12-year-old is likely to have a better understanding of sex but still feel uncomfortable. Educational conversations about sexuality might be appropriate for children age 8 to 12. The parents’ goal should be to answer questions honestly without oversharing, and to leave the door open—so to speak—for future conversations.
Recommend that parents use plain, factual language to answer any questions their child asks. Statements such as “We were having a private, adult moment” can be helpful. Parents can categorize sex as a universal activity that is not harmful or scary by telling their child something such as, “This is what all parents do.” Parents should avoid providing unnecessary information or answering questions their child is not asking. The Table4 offers guidance on how parents might handle such conversations.
Consider potentially positive outcomes. Although parents may feel guilty or describe this as a terrible situation, remind them that there are some potentially healthy outcomes. For example, such incidents may help reassure the child that their parents love each other, which might give him/her a sense of happiness and security.
Take steps to prevent this from happening again. Advise parents to lock the door when having sex. Remind them to consider the proximity of rooms because their child might hear noises and become curious.
1. Blum HP. On the concept and consequences of the primal scene. Psychoanal Q. 1979;48(1):27-47.
2. Hoyt MF. On the psychology and psychopathology of primal-scene experience. J Am Acad Psychoanal. 1980;8(3):311-335.
3. Ikonen P, Rechardt E. On the universal nature of primal scene fantasies. Int J Psychoanal. 1984;65(pt 1):63-72.
4. Pelly J. Four things my four-year-old already knows about sex. Today’s Parent. https://www.todaysparent.com/family/parenting/4-things-my-4-year-old-already-knows-about-sex/. Published February 1, 2020. Accessed June 20, 2020.
1. Blum HP. On the concept and consequences of the primal scene. Psychoanal Q. 1979;48(1):27-47.
2. Hoyt MF. On the psychology and psychopathology of primal-scene experience. J Am Acad Psychoanal. 1980;8(3):311-335.
3. Ikonen P, Rechardt E. On the universal nature of primal scene fantasies. Int J Psychoanal. 1984;65(pt 1):63-72.
4. Pelly J. Four things my four-year-old already knows about sex. Today’s Parent. https://www.todaysparent.com/family/parenting/4-things-my-4-year-old-already-knows-about-sex/. Published February 1, 2020. Accessed June 20, 2020.
Kleptomania: 4 Tips for better diagnosis and treatment
Kleptomania is characterized by a recurrent failure to resist impulses to steal objects that are not needed for personal use or their monetary value.1 It is a rare disorder; an estimated 0.3% to 0.6% of the general population meet DSM-5 criteria for kleptomania (Table 1).1 Kleptomania usually begins in early adolescence and is more common among females than males (3:1).1 Although DSM-5 does not outline how long symptoms need to be present for patients to meet the diagnostic criteria, the disorder may persist for years, even when patients face legal consequences.1
Due to the clinical ambiguities surrounding kleptomania, it remains one of psychiatry’s most poorly understood diagnoses2 and regularly goes undiagnosed and untreated. Here we provide 4 tips for better diagnosis and treatment of this condition.
1. Screen for kleptomania in patients with other psychiatric disorders because kleptomania often is comorbid with other mental illnesses. Patients who present for evaluation of a mood disorder, substance use, anxiety disorders, eating disorders, impulse control disorders, conduct disorder, and obsessive-compulsive disorder should be screened for kleptomania.1,3,4 Patients with kleptomania often are reluctant to discuss their stealing because they may experience humiliation and guilt related to theft.1,4 Undiagnosed kleptomania can be fatal; a study of suicide attempts in 107 individuals with kleptomania found that 92% of the patients attributed their attempt specifically to kleptomania.5 Table 21 offers screening questions based on the DSM-5 criteria for kleptomania.
2. Distinguish kleptomania from other diagnoses that can include stealing. Because stealing can be a symptom of several other psychiatric disorders, misdiagnosis is fairly common.1 The differential can include bipolar disorder, borderline personality disorder, antisocial personality disorder, and eating disorder. Table 31,3 describes how to differentiate these diagnoses from kleptomania.
3. Select an appropriate treatment. There are no FDA-approved medications for kleptomania, but some agents may help. In an 8-week, double-blind, placebo-controlled trial, 25 patients with kleptomania who received naltrexone (50 to 150 mg/d) demonstrated significant reductions in stealing urges and behavior.6 Some evidence suggests a combination of pharmacologic and behavioral therapy (cognitive-behavioral therapy, covert sensitization, and systemic desensitization) may be the optimal treatment strategy for kleptomania.4
4. Monitor progress. After initiating treatment, use the Kleptomania Symptom Assessment Scale7 (K-SAS) to determine treatment efficacy. The K-SAS is an 11-item self-report questionnaire that assesses the severity of kleptomania symptoms during the past week.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry. 1991;148:986-996.
3. Yao S, Kuja‐Halkola R, Thornton LM, et al. Risk of being convicted of theft and other crimes in anorexia nervosa and bulimia nervosa: a prospective cohort study in a Swedish female population. Int J Eat Disord. 2017;50(9):1095-1103.
4. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients of kleptomania. Compr Psychiatry. 2002;43(5):378-384.
5. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
6. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7):600-606.
7. Grant JE, Odlaug BL, Kim SW. Kleptomania: clinical characteristics and relationship to substance use disorders. Am J Drug Alcohol Abuse. 2010;36(5):291-295.
Kleptomania is characterized by a recurrent failure to resist impulses to steal objects that are not needed for personal use or their monetary value.1 It is a rare disorder; an estimated 0.3% to 0.6% of the general population meet DSM-5 criteria for kleptomania (Table 1).1 Kleptomania usually begins in early adolescence and is more common among females than males (3:1).1 Although DSM-5 does not outline how long symptoms need to be present for patients to meet the diagnostic criteria, the disorder may persist for years, even when patients face legal consequences.1
Due to the clinical ambiguities surrounding kleptomania, it remains one of psychiatry’s most poorly understood diagnoses2 and regularly goes undiagnosed and untreated. Here we provide 4 tips for better diagnosis and treatment of this condition.
1. Screen for kleptomania in patients with other psychiatric disorders because kleptomania often is comorbid with other mental illnesses. Patients who present for evaluation of a mood disorder, substance use, anxiety disorders, eating disorders, impulse control disorders, conduct disorder, and obsessive-compulsive disorder should be screened for kleptomania.1,3,4 Patients with kleptomania often are reluctant to discuss their stealing because they may experience humiliation and guilt related to theft.1,4 Undiagnosed kleptomania can be fatal; a study of suicide attempts in 107 individuals with kleptomania found that 92% of the patients attributed their attempt specifically to kleptomania.5 Table 21 offers screening questions based on the DSM-5 criteria for kleptomania.
2. Distinguish kleptomania from other diagnoses that can include stealing. Because stealing can be a symptom of several other psychiatric disorders, misdiagnosis is fairly common.1 The differential can include bipolar disorder, borderline personality disorder, antisocial personality disorder, and eating disorder. Table 31,3 describes how to differentiate these diagnoses from kleptomania.
3. Select an appropriate treatment. There are no FDA-approved medications for kleptomania, but some agents may help. In an 8-week, double-blind, placebo-controlled trial, 25 patients with kleptomania who received naltrexone (50 to 150 mg/d) demonstrated significant reductions in stealing urges and behavior.6 Some evidence suggests a combination of pharmacologic and behavioral therapy (cognitive-behavioral therapy, covert sensitization, and systemic desensitization) may be the optimal treatment strategy for kleptomania.4
4. Monitor progress. After initiating treatment, use the Kleptomania Symptom Assessment Scale7 (K-SAS) to determine treatment efficacy. The K-SAS is an 11-item self-report questionnaire that assesses the severity of kleptomania symptoms during the past week.
Kleptomania is characterized by a recurrent failure to resist impulses to steal objects that are not needed for personal use or their monetary value.1 It is a rare disorder; an estimated 0.3% to 0.6% of the general population meet DSM-5 criteria for kleptomania (Table 1).1 Kleptomania usually begins in early adolescence and is more common among females than males (3:1).1 Although DSM-5 does not outline how long symptoms need to be present for patients to meet the diagnostic criteria, the disorder may persist for years, even when patients face legal consequences.1
Due to the clinical ambiguities surrounding kleptomania, it remains one of psychiatry’s most poorly understood diagnoses2 and regularly goes undiagnosed and untreated. Here we provide 4 tips for better diagnosis and treatment of this condition.
1. Screen for kleptomania in patients with other psychiatric disorders because kleptomania often is comorbid with other mental illnesses. Patients who present for evaluation of a mood disorder, substance use, anxiety disorders, eating disorders, impulse control disorders, conduct disorder, and obsessive-compulsive disorder should be screened for kleptomania.1,3,4 Patients with kleptomania often are reluctant to discuss their stealing because they may experience humiliation and guilt related to theft.1,4 Undiagnosed kleptomania can be fatal; a study of suicide attempts in 107 individuals with kleptomania found that 92% of the patients attributed their attempt specifically to kleptomania.5 Table 21 offers screening questions based on the DSM-5 criteria for kleptomania.
2. Distinguish kleptomania from other diagnoses that can include stealing. Because stealing can be a symptom of several other psychiatric disorders, misdiagnosis is fairly common.1 The differential can include bipolar disorder, borderline personality disorder, antisocial personality disorder, and eating disorder. Table 31,3 describes how to differentiate these diagnoses from kleptomania.
3. Select an appropriate treatment. There are no FDA-approved medications for kleptomania, but some agents may help. In an 8-week, double-blind, placebo-controlled trial, 25 patients with kleptomania who received naltrexone (50 to 150 mg/d) demonstrated significant reductions in stealing urges and behavior.6 Some evidence suggests a combination of pharmacologic and behavioral therapy (cognitive-behavioral therapy, covert sensitization, and systemic desensitization) may be the optimal treatment strategy for kleptomania.4
4. Monitor progress. After initiating treatment, use the Kleptomania Symptom Assessment Scale7 (K-SAS) to determine treatment efficacy. The K-SAS is an 11-item self-report questionnaire that assesses the severity of kleptomania symptoms during the past week.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry. 1991;148:986-996.
3. Yao S, Kuja‐Halkola R, Thornton LM, et al. Risk of being convicted of theft and other crimes in anorexia nervosa and bulimia nervosa: a prospective cohort study in a Swedish female population. Int J Eat Disord. 2017;50(9):1095-1103.
4. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients of kleptomania. Compr Psychiatry. 2002;43(5):378-384.
5. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
6. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7):600-606.
7. Grant JE, Odlaug BL, Kim SW. Kleptomania: clinical characteristics and relationship to substance use disorders. Am J Drug Alcohol Abuse. 2010;36(5):291-295.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry. 1991;148:986-996.
3. Yao S, Kuja‐Halkola R, Thornton LM, et al. Risk of being convicted of theft and other crimes in anorexia nervosa and bulimia nervosa: a prospective cohort study in a Swedish female population. Int J Eat Disord. 2017;50(9):1095-1103.
4. Grant JE, Kim SW. Clinical characteristics and associated psychopathology of 22 patients of kleptomania. Compr Psychiatry. 2002;43(5):378-384.
5. Odlaug BL, Grant JE, Kim SW. Suicide attempts in 107 adolescents and adults with kleptomania. Arch Suicide Res. 2012;16(4):348-359.
6. Grant JE, Kim SW, Odlaug BL. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol Psychiatry. 2009;65(7):600-606.
7. Grant JE, Odlaug BL, Kim SW. Kleptomania: clinical characteristics and relationship to substance use disorders. Am J Drug Alcohol Abuse. 2010;36(5):291-295.
Improving your experience with electronic health records
The electronic health record (EHR) was introduced to improve how clinicians document patient information, contribute to medical research, and allow for medical records to be universally transferable.1 However, many clinicians find EHRs to be burdensome, time-consuming, and inefficient. Clinicians often spend multiple hours each day navigating their EHR system, which reduces the amount of time they spend interacting with patients and contributes to physician burnout.1-3 For example, in a study of 142 family medicine physicians, clinicians reported that they spent approximately 6 hours per work day interacting with their EHR.3
Clearly, the EHR needs a fundamental revision. In the meantime, how can we adapt to improve the situation? Here I suggest practical steps clinicians can take to improve their experience with their EHR system.4-8
Steps to take during patient visits
Because entering information into the EHR can be distracting, be prepared to multitask during each clinical encounter.1-7 Be ready to address pertinent inquiries and issues your patient raises, and provide instructions on therapies and interventions. Because interpersonal relations are important during clinical encounters, establish interaction with your patient by acknowledging them and maintaining frequent eye contact.7 Consider allowing your patient to view the EHR screen because doing so might increase his/her involvement in the visit.
So that you can pay closer attention to your patient, consider taking notes during the visit and entering the information into the EHR later. Consider improving your typing skills to increase the speed of your note-taking. Alternatively, using a voice-recognition recording tool to transcribe your notes via speech might help you spend less time on note-taking.3 Whenever possible, finish charting for one patient before meeting with the next because doing so will save time and help you to better remember details.7
In addition, lowering your overall stress might help reduce the burden of using the EHR.3-5 Adopt healthy behaviors, including good sleep, nutrition, exercise, and hobbies, and strive for balance in your routines. Attend to any personal medical or psychiatric conditions, and avoid misusing alcohol, medications, or other substances.
Optimize how your practice functions
With your clinical group and colleagues, create a comfortable environment, good patient-to-doctor interactions, and a smooth flow within the practice. Simplify registration. Ask patients to complete screening forms before an appointment; this information could be entered directly into their EHR.3 Consider using physician-extender staff and other personnel, such as scribes, to complete documentation into the EHR.3,8 This may help reduce burnout, create more time for clinical care, and improve face-to-face patient interactions.8 Employing scribes can allow doctors to be better able to directly attend to their patients while complying with record-keeping needs. Although scribes make charting easier, they are an additional expense, and must be trained.
Consider EHR training
EHR training sessions can teach you how to use your EHR system more efficiently.6 Such education may help boost confidence, aid documentation, and reduce the amount of time spent correcting coding errors. In a study of 3,500 physicians who underwent a 3-day intensive EHR training course, 85% to 98% reported having improved the quality, readability, and clinical accuracy of their documentation.6
Help shape future EHRs
Individual doctors and professional groups can promote EHR improvements through their state, regional, and/or national organizations and medical societies. These bodies should deliver EHR revision recommendations to government officials, who can craft laws and regulations, and can influence regulators and/or insurance companies. Clinicians also can communicate with EHR developers on ways to simplify the usability of these tools, such as reducing the amount of steps the EHR’s interface requires.5 With a more efficient EHR, we can better concentrate on patient care, which will reduce expenses and should yield better outcomes.
1. Ehrenfeld JM, Wonderer JP. Technology as friend or foe? Do electronic health records increase burnout? Curr Opin Anesthesiol. 2018;31(3):357-360.
2. Meigs SL, Solomon M. Electronic health record use a bitter pill for many physicians. Perspect Health Inf Manag. 2016;13:1d.
3. Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15(5):419-426.
4. Fogarty CT, Winters P, Farah S. Improving patient-centered communication while using an electronic health record: report from a curriculum evaluation. Int J Psych Med. 2016;51(4):379-389.
5. Guo U, Chen L, Mehta PH. Electronic health record innovations: helping physicians - one less click at a time. Health Inf Manag. 2017;46(3):140-144.
6. Robinson KE, Kersey JA. Novel electronic health record (EHR) education intervention in large healthcare organization improves quality, efficiency, time, and impact on burnout. Medicine. 2018;91(38):e123419. doi: 10.1097/MD.0000000000012319.
7. Fogarty CT. Getting your notes done on time. Fam Pract Manag. 2016;23(2):40.
8. DeChant PF, Acs A, Rhee KB, et al. Effect of organization-directed workplace interventions on physician burnout: a systematic review. Mayo Clin Proc Innov Qual Outcomes. 2019;3(4):384-408.
The electronic health record (EHR) was introduced to improve how clinicians document patient information, contribute to medical research, and allow for medical records to be universally transferable.1 However, many clinicians find EHRs to be burdensome, time-consuming, and inefficient. Clinicians often spend multiple hours each day navigating their EHR system, which reduces the amount of time they spend interacting with patients and contributes to physician burnout.1-3 For example, in a study of 142 family medicine physicians, clinicians reported that they spent approximately 6 hours per work day interacting with their EHR.3
Clearly, the EHR needs a fundamental revision. In the meantime, how can we adapt to improve the situation? Here I suggest practical steps clinicians can take to improve their experience with their EHR system.4-8
Steps to take during patient visits
Because entering information into the EHR can be distracting, be prepared to multitask during each clinical encounter.1-7 Be ready to address pertinent inquiries and issues your patient raises, and provide instructions on therapies and interventions. Because interpersonal relations are important during clinical encounters, establish interaction with your patient by acknowledging them and maintaining frequent eye contact.7 Consider allowing your patient to view the EHR screen because doing so might increase his/her involvement in the visit.
So that you can pay closer attention to your patient, consider taking notes during the visit and entering the information into the EHR later. Consider improving your typing skills to increase the speed of your note-taking. Alternatively, using a voice-recognition recording tool to transcribe your notes via speech might help you spend less time on note-taking.3 Whenever possible, finish charting for one patient before meeting with the next because doing so will save time and help you to better remember details.7
In addition, lowering your overall stress might help reduce the burden of using the EHR.3-5 Adopt healthy behaviors, including good sleep, nutrition, exercise, and hobbies, and strive for balance in your routines. Attend to any personal medical or psychiatric conditions, and avoid misusing alcohol, medications, or other substances.
Optimize how your practice functions
With your clinical group and colleagues, create a comfortable environment, good patient-to-doctor interactions, and a smooth flow within the practice. Simplify registration. Ask patients to complete screening forms before an appointment; this information could be entered directly into their EHR.3 Consider using physician-extender staff and other personnel, such as scribes, to complete documentation into the EHR.3,8 This may help reduce burnout, create more time for clinical care, and improve face-to-face patient interactions.8 Employing scribes can allow doctors to be better able to directly attend to their patients while complying with record-keeping needs. Although scribes make charting easier, they are an additional expense, and must be trained.
Consider EHR training
EHR training sessions can teach you how to use your EHR system more efficiently.6 Such education may help boost confidence, aid documentation, and reduce the amount of time spent correcting coding errors. In a study of 3,500 physicians who underwent a 3-day intensive EHR training course, 85% to 98% reported having improved the quality, readability, and clinical accuracy of their documentation.6
Help shape future EHRs
Individual doctors and professional groups can promote EHR improvements through their state, regional, and/or national organizations and medical societies. These bodies should deliver EHR revision recommendations to government officials, who can craft laws and regulations, and can influence regulators and/or insurance companies. Clinicians also can communicate with EHR developers on ways to simplify the usability of these tools, such as reducing the amount of steps the EHR’s interface requires.5 With a more efficient EHR, we can better concentrate on patient care, which will reduce expenses and should yield better outcomes.
The electronic health record (EHR) was introduced to improve how clinicians document patient information, contribute to medical research, and allow for medical records to be universally transferable.1 However, many clinicians find EHRs to be burdensome, time-consuming, and inefficient. Clinicians often spend multiple hours each day navigating their EHR system, which reduces the amount of time they spend interacting with patients and contributes to physician burnout.1-3 For example, in a study of 142 family medicine physicians, clinicians reported that they spent approximately 6 hours per work day interacting with their EHR.3
Clearly, the EHR needs a fundamental revision. In the meantime, how can we adapt to improve the situation? Here I suggest practical steps clinicians can take to improve their experience with their EHR system.4-8
Steps to take during patient visits
Because entering information into the EHR can be distracting, be prepared to multitask during each clinical encounter.1-7 Be ready to address pertinent inquiries and issues your patient raises, and provide instructions on therapies and interventions. Because interpersonal relations are important during clinical encounters, establish interaction with your patient by acknowledging them and maintaining frequent eye contact.7 Consider allowing your patient to view the EHR screen because doing so might increase his/her involvement in the visit.
So that you can pay closer attention to your patient, consider taking notes during the visit and entering the information into the EHR later. Consider improving your typing skills to increase the speed of your note-taking. Alternatively, using a voice-recognition recording tool to transcribe your notes via speech might help you spend less time on note-taking.3 Whenever possible, finish charting for one patient before meeting with the next because doing so will save time and help you to better remember details.7
In addition, lowering your overall stress might help reduce the burden of using the EHR.3-5 Adopt healthy behaviors, including good sleep, nutrition, exercise, and hobbies, and strive for balance in your routines. Attend to any personal medical or psychiatric conditions, and avoid misusing alcohol, medications, or other substances.
Optimize how your practice functions
With your clinical group and colleagues, create a comfortable environment, good patient-to-doctor interactions, and a smooth flow within the practice. Simplify registration. Ask patients to complete screening forms before an appointment; this information could be entered directly into their EHR.3 Consider using physician-extender staff and other personnel, such as scribes, to complete documentation into the EHR.3,8 This may help reduce burnout, create more time for clinical care, and improve face-to-face patient interactions.8 Employing scribes can allow doctors to be better able to directly attend to their patients while complying with record-keeping needs. Although scribes make charting easier, they are an additional expense, and must be trained.
Consider EHR training
EHR training sessions can teach you how to use your EHR system more efficiently.6 Such education may help boost confidence, aid documentation, and reduce the amount of time spent correcting coding errors. In a study of 3,500 physicians who underwent a 3-day intensive EHR training course, 85% to 98% reported having improved the quality, readability, and clinical accuracy of their documentation.6
Help shape future EHRs
Individual doctors and professional groups can promote EHR improvements through their state, regional, and/or national organizations and medical societies. These bodies should deliver EHR revision recommendations to government officials, who can craft laws and regulations, and can influence regulators and/or insurance companies. Clinicians also can communicate with EHR developers on ways to simplify the usability of these tools, such as reducing the amount of steps the EHR’s interface requires.5 With a more efficient EHR, we can better concentrate on patient care, which will reduce expenses and should yield better outcomes.
1. Ehrenfeld JM, Wonderer JP. Technology as friend or foe? Do electronic health records increase burnout? Curr Opin Anesthesiol. 2018;31(3):357-360.
2. Meigs SL, Solomon M. Electronic health record use a bitter pill for many physicians. Perspect Health Inf Manag. 2016;13:1d.
3. Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15(5):419-426.
4. Fogarty CT, Winters P, Farah S. Improving patient-centered communication while using an electronic health record: report from a curriculum evaluation. Int J Psych Med. 2016;51(4):379-389.
5. Guo U, Chen L, Mehta PH. Electronic health record innovations: helping physicians - one less click at a time. Health Inf Manag. 2017;46(3):140-144.
6. Robinson KE, Kersey JA. Novel electronic health record (EHR) education intervention in large healthcare organization improves quality, efficiency, time, and impact on burnout. Medicine. 2018;91(38):e123419. doi: 10.1097/MD.0000000000012319.
7. Fogarty CT. Getting your notes done on time. Fam Pract Manag. 2016;23(2):40.
8. DeChant PF, Acs A, Rhee KB, et al. Effect of organization-directed workplace interventions on physician burnout: a systematic review. Mayo Clin Proc Innov Qual Outcomes. 2019;3(4):384-408.
1. Ehrenfeld JM, Wonderer JP. Technology as friend or foe? Do electronic health records increase burnout? Curr Opin Anesthesiol. 2018;31(3):357-360.
2. Meigs SL, Solomon M. Electronic health record use a bitter pill for many physicians. Perspect Health Inf Manag. 2016;13:1d.
3. Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15(5):419-426.
4. Fogarty CT, Winters P, Farah S. Improving patient-centered communication while using an electronic health record: report from a curriculum evaluation. Int J Psych Med. 2016;51(4):379-389.
5. Guo U, Chen L, Mehta PH. Electronic health record innovations: helping physicians - one less click at a time. Health Inf Manag. 2017;46(3):140-144.
6. Robinson KE, Kersey JA. Novel electronic health record (EHR) education intervention in large healthcare organization improves quality, efficiency, time, and impact on burnout. Medicine. 2018;91(38):e123419. doi: 10.1097/MD.0000000000012319.
7. Fogarty CT. Getting your notes done on time. Fam Pract Manag. 2016;23(2):40.
8. DeChant PF, Acs A, Rhee KB, et al. Effect of organization-directed workplace interventions on physician burnout: a systematic review. Mayo Clin Proc Innov Qual Outcomes. 2019;3(4):384-408.
Eosinophilic esophagitis: Frequently asked questions (and answers) for the early-career gastroenterologist
Introduction
Eosinophilic esophagitis (EoE) has transformed over the past 3 decades from a rarely encountered entity to one of the most common causes of dysphagia in adults.1 Given the marked rise in prevalence, the early-career gastroenterologist will undoubtedly be involved with managing this disease.2 The typical presentation includes a young, atopic male presenting with dysphagia in the outpatient setting or, more acutely, with a food impaction when on call. As every fellow is keenly aware, the calls often come late at night as patients commonly have meat impactions while consuming dinner. Current management focuses on symptomatic, histologic, and endoscopic improvement with medication, dietary, and mechanical (i.e., dilation) modalities.
EoE is defined by the presence of esophageal dysfunction and esophageal eosinophilic inflammation with ≥15 eosinophils/high-powered field (eos/hpf) required for the diagnosis. With better understanding of the pathogenesis of EoE involving the complex interaction of environmental, host, and genetic factors, advancements have been made as it relates to the diagnostic criteria, endoscopic evaluation, and therapeutic options. In this article, we review the current management of adult patients with EoE and offer practical guidance to key questions for the young gastroenterologist as well as insights into future areas of interest.
What should I consider when diagnosing EoE?
Symptoms are central to the diagnosis and clinical presentation of EoE. In assessing symptoms, clinicians should be aware of adaptive “IMPACT” strategies patients often subconsciously develop in response to their chronic and progressive condition: Imbibing fluids with meals, modifying foods by cutting or pureeing, prolonging meal times, avoiding harder texture foods, chewing excessively, and turning away tablets/pills.3 Failure to query such adaptive behaviors may lead to an underestimation of disease activity and severity.
An important aspect to confirming the diagnosis of EoE is to exclude other causes of esophageal eosinophilia. Gastroesophageal reflux disease (GERD) is known to cause esophageal eosinophilia and historically has been viewed as a distinct disease process. In fact, initial guidelines included lack of response to a proton pump inhibitor (PPI) trial or normal esophageal pH monitoring as diagnostic criteria.4 However, as experience was garnered, it became clear that PPI therapy was effective at improving inflammation in 30%-50% of patients with clinical presentations and histologic features consistent with EoE. As such, the concept of PPI–responsive esophageal eosinophilia (PPI-REE) was introduced in 2011.5 Further investigation then highlighted that PPI-REE and EoE had nearly identical clinical, endoscopic, and histologic features as well as eosinophil biomarker and gene expression profiles. Hence, recent international guidelines no longer necessitate a PPI trial to establish a diagnosis of EoE.6
The young gastroenterologist should also be mindful of other issues related to the initial diagnosis of EoE. EoE may present concomitantly with other disease entities including GERD, “extra-esophageal” eosinophilic gastrointestinal diseases, concomitant IgE-mediated food allergy, hypereosinophilic syndromes, connective tissue disorders, autoimmune diseases, celiac disease, and inflammatory bowel disease.3 It has been speculated that some of these disorders share common aspects of genetic and environmental predisposing factors as well as shared pathogenesis. Careful history taking should include a full review of atopic conditions and GI-related symptoms and endoscopy should carefully inspect not only the esophagus, but also gastric and duodenal mucosa. The endoscopic features almost always reveal edema, rings, exudates, furrows, and strictures and can be assessed using the EoE Endoscopic Reference Scoring system (EREFS).7 EREFS allows for systematic identification of abnormalities that can inform decisions regarding treatment efficacy and decisions on the need for esophageal dilation. When the esophageal mucosa is evaluated for biopsies, furrows and exudates should be targeted, if present, and multiple biopsies (minimum of five to six) should be taken throughout the esophagus given the patchy nature of the disease.
How do I choose an initial therapy?
The choice of initial therapy considers patient preferences, medication availability, disease severity, impact on quality of life, and need for repeated endoscopies. While there are many novel agents currently being investigated in phase 2 and 3 clinical trials, the current mainstays of treatment include PPI therapy, topical steroids, dietary therapy, and dilation. Of note, there have been no head-to-head trials comparing these different modalities. A recent systematic review reported that PPIs can induce histologic remission in 42% of patients.8 The ease of use and availability of PPI therapy make this an attractive first choice for patients. Pooled estimates show that topical steroids can induce remission in 66% of patients.8 It is important to note that there is currently no Food and Drug Administration–approved formulation of steroids for the treatment of EoE. As such, there are several practical aspects to consider when instructing patients to use agents not designed for esophageal delivery (Figure 1).
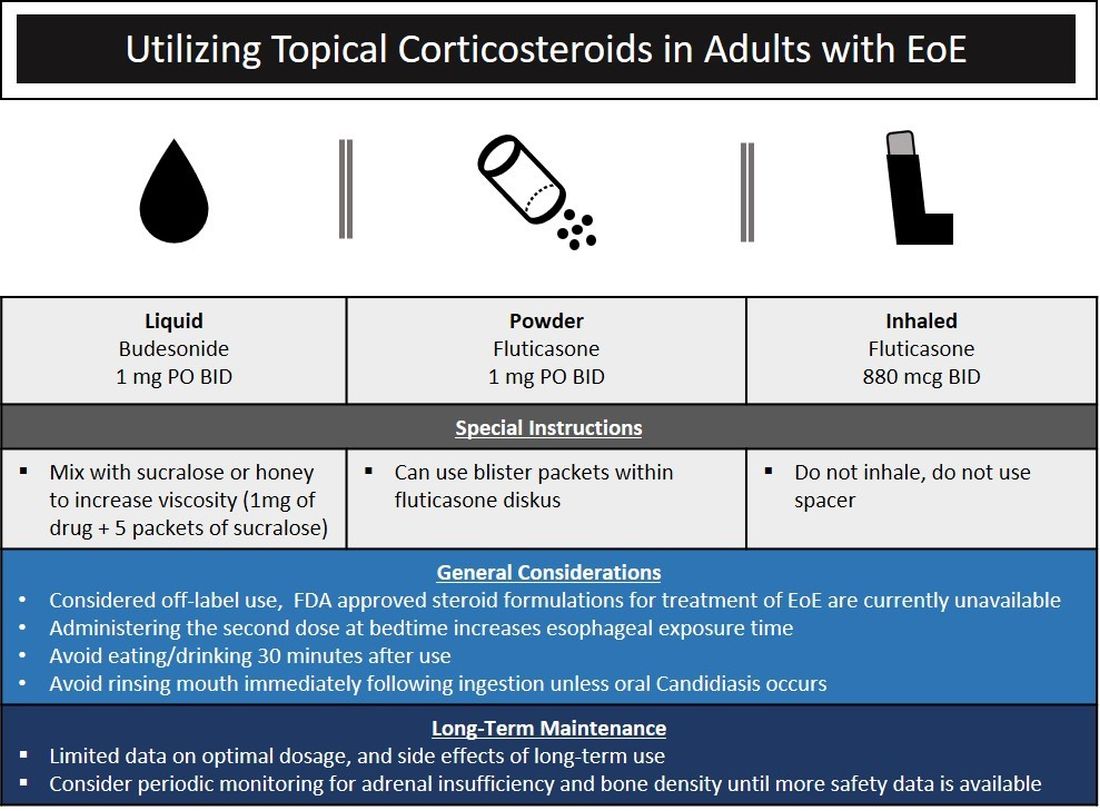
Source: Dr. Patel, Dr. Hirano
Lack of insurance coverage for topical steroids can make cost of a prescription a deterrent to use. While topical steroids are well tolerated, concerns for candidiasis and adrenal insufficiency are being monitored in prospective, long-term clinical trials. Concomitant use of steroids with PPI would be appropriate for EoE patients with coexisting GERD (severe heartburn, erosive esophagitis, Barrett’s esophagus). In addition, we often combine steroids with PPI therapy for EoE patients who demonstrate a convincing but incomplete response to PPI monotherapy (i.e., reduction of baseline inflammation from 75 eos/hpf to 20 eos/hpf).
Diet therapy is a popular choice for management of EoE by patients, given the ability to remove food triggers that initiate the immune dysregulation and to avoid chronic medication use. Three dietary options have been described including an elemental, amino acid–based diet which eliminates all common food allergens, allergy testing–directed elimination diet, and an empiric elimination diet. Though elemental diets have shown the most efficacy, practical aspects of implementing, maintaining, and identifying triggers restrict their adoption by most patients and clinicians.9 Allergy-directed elimination diets, where allergens are eliminated based on office-based allergy testing, initially seemed promising, though studies have shown limited histologic remission, compared with other diet therapies as well as the inability to identify true food triggers. Advancement of office-based testing to identify food triggers is needed to streamline this dietary approach. In the adult patient, the empiric elimination diet remains an attractive choice of the available dietary therapies. In this dietary approach, which has shown efficacy in both children and adults, the most common food allergens (milk, wheat, soy, egg, nuts, and seafood) are eliminated.9
How do I make dietary therapy work in clinical practice?
Before dietary therapy is initiated, it is important that your practice is situated to support this approach and that patients fully understand the process. A multidisciplinary approach optimizes dietary therapy. Dietitians provide expert guidance on eliminating trigger foods, maintaining nutrition, and avoiding inadvertent cross-contamination. Patient questions may include the safety of consumption of non–cow-based cheese/milk, alcoholic beverages, wheat alternatives, and restaurant food. Allergists address concerns for a concomitant IgE food allergy based on a clinical history or previous testing. Patients should be informed that identifying a food trigger often takes several months and multiple endoscopies. Clinicians should be aware of potential food cost and accessibility issues as well as the reported, albeit uncommon, development of de novo IgE-mediated food allergy during reintroduction. Timing of diet therapy is also a factor in success. Patients should avoid starting diets during major holidays, family celebrations, college years, and busy travel months.
Particularly empiric elimination diets, frequently used in adults, several approaches have been described (Figure 2).
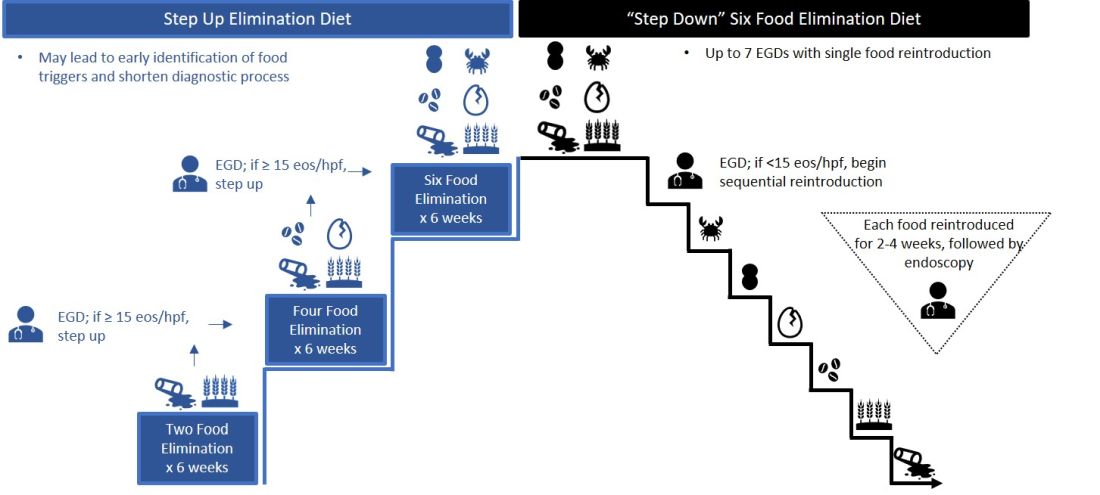
Source: Dr. Patel, Dr. Hirano
Initially, a step-down approach was described, with patients pursuing a six-food elimination diet (SFED), which eliminates the six most common triggers: milk, wheat, soy/legumes, egg, nuts, and seafood. Once in histologic remission, patients then systematically reintroduce foods in order to identify a causative trigger. Given that many patients have only one or two identified food triggers, other approaches were created including a single-food elimination diet eliminating milk, the two-food elimination diet (TFED) eliminating milk and wheat, and the four-food elimination diet (FFED) eliminating milk, wheat, soy/legumes, and eggs. A novel step-up approach has also now been described where patients start with the TFED and progress to the FFED and then potentially SFED based on histologic response.10 This approach has the potential to more readily identify triggers, decrease diagnostic time, and reduce endoscopic interventions. There are pros and cons to each elimination diet approach that should be discussed with patients. Many patients may find a one- or two-food elimination diet more feasible than a full SFED.
What should I consider when performing dilation?
Esophageal dilation is frequently used to address the fibrostenotic complications of EoE that do not as readily respond to PPI, steroid, or diet therapy. The majority of patients note symptomatic improvement following dilation, though dilation alone does not address the inflammatory component of disease.8 With a conservative approach, the complication rates of esophageal dilation in EoE are similar to that of benign, esophageal strictures. Endoscopists should be aware that endoscopy alone can miss strictures and consider both practical and technical aspects when performing dilations (Table 1).11,12
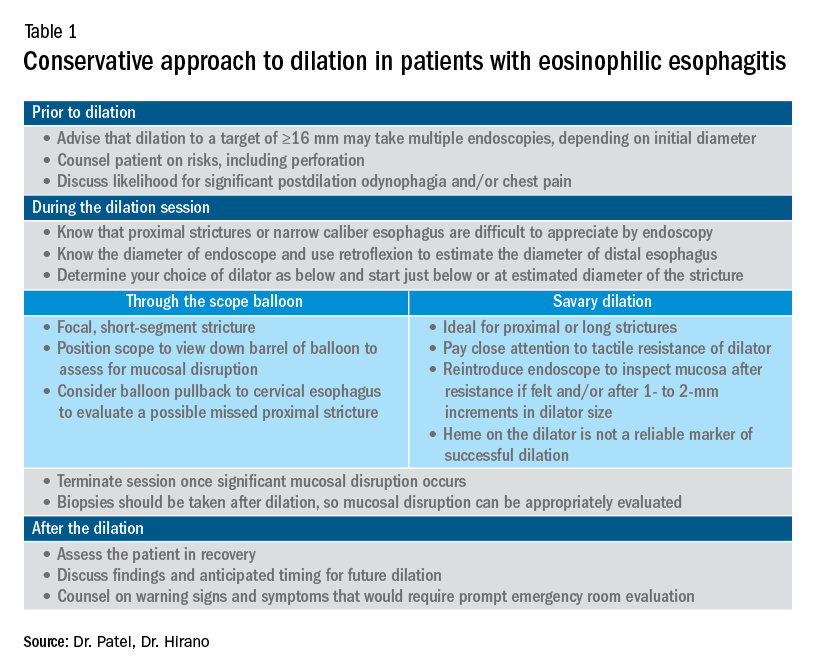
When should an allergist be consulted?
The role of the allergist in the management of patients with EoE varies by patient and practice. IgE serologic or skin testing have limited accuracy in identifying food triggers for EoE. Nevertheless, the majority of patients with EoE have an atopic condition which may include asthma, allergic rhinitis, atopic dermatitis, or IgE-mediated food allergy. Although EoE is thought to primarily occur from an immune response to ingested oral allergens, aeroallergens may exacerbate disease as evidenced by the seasonal variation in EoE symptoms in some patients. The allergist provides treatment for these “extraesophageal” atopic conditions which may, in turn, have synergistic effects on the treatment of EoE. Furthermore, allergists may prescribe biologic therapies that are FDA approved for the treatment of atopic dermatitis, asthma, and allergic rhinitis. While not approved for EoE, several of these agents have shown efficacy in phase 2 clinical trials in EoE. In some practice settings, allergists primarily manage EoE patients with the assistance of gastroenterologists for periodic endoscopic activity assessment.
What are the key aspects of maintenance therapy?
The goals of treatment focus on symptomatic, histologic, and endoscopic improvement, and the prevention of future or ongoing fibrostenotic complications.2 Because of the adaptive eating behaviors discussed above, symptom response may not reliably correlate with histologic and/or endoscopic improvement. Moreover, dysphagia is related to strictures that often do not resolve in spite of resolution of mucosal inflammation. As such, histology and endoscopy are more objective and reliable targets of a successful response to therapy. Though studies have used variable esophageal density levels for response, using a cutoff of <15 eos/hpf as a therapeutic endpoint is reasonable for both initial response to therapy and long-term monitoring.13 We advocate for standardization of reporting endoscopic findings to better track change over time using the EREFS scoring system.7 While inflammatory features improve, the fibrostenotic features may persist despite improvement in histology. Dilation is often performed in these situations, especially for symptomatic individuals.
During clinical follow-up, the frequency of monitoring as it relates to symptom and endoscopic assessment is not well defined. It is reasonable to repeat endoscopic intervention following changes in therapy (i.e., reduction in steroid dosing or reintroduction of putative food triggers) or in symptoms.13 It is unclear if patients benefit from repeated endoscopies at set intervals without symptom change and after histologic response has been confirmed. In our practice, endoscopies are often considered on an annual basis. This interval is increased for patients with demonstrated stability of disease.
For patients who opt for dietary therapy and have one or two food triggers identified, long-term maintenance therapy can be straightforward with ongoing food avoidance. Limited data exist regarding long-term effectiveness of dietary therapy but loss of initial response has been reported that is often attributed to problems with adherence. Use of “diet holidays” or “planned cheats” to allow for intermittent consumption of trigger foods, often under the cover of short-term use of steroids, may improve the long-term feasibility of diet approaches.
In the recent American Gastroenterological Association guidelines, continuation of swallowed, topical steroids is recommended following remission with short-term treatment. The recurrence of both symptoms and inflammation following medication withdrawal supports this practice. Furthermore, natural history studies demonstrate progression of esophageal strictures with untreated disease.
There are no clear guidelines for long-term dosage and use of PPI or topical steroid therapy. Our practice is to down-titrate the dose of PPI or steroid following remission with short-term therapy, often starting with a reduction from twice a day to daily dosing. Although topical steroid therapy has fewer side effects, compared with systemic steroids, patients should be aware of the potential for adrenal suppression especially in an atopic population who may be exposed to multiple forms of topical steroids. Shared decision-making between patients and providers is recommended to determine comfort level with long-term use of prescription medications and dosage.
What’s on the horizon?
Several areas of development are underway to better assess and manage EoE. Novel histologic scoring tools now assess characteristics on pathology beyond eosinophil density, office-based testing modalities have been developed to assess inflammatory activity and thereby obviate the need for endoscopy, new technology can provide measures of esophageal remodeling and provide assessment of disease severity, and several biologic agents are being studied that target specific allergic mediators of the immune response in EoE.3,14-18 These novel tools, technologies, and therapies will undoubtedly change the management approach to EoE. Referral of patients into ongoing clinical trials will help inform advances in the field.
Conclusion
As an increasingly prevalent disease with a high degree of upper GI morbidity, EoE has transitioned from a rare entity to a commonly encountered disease. The new gastroenterologist will confront both straightforward as well as complex patients with EoE, and we offer several practical aspects on management. In the years ahead, the care of patients with EoE will continue to evolve to a more streamlined, effective, and personalized approach.
References
1. Kidambi T et al. World J Gastroenterol. 2012;18:4335-41.
2. Dellon ES et al. Gastroenterology. 2018;154:319-32 e3.
3. Hirano I et al. Gastroenterology. 2020;158:840-51.
4. Furuta GT et al. Gastroenterology. 2007;133:1342-63.
5. Liacouras CA et al. J Allergy Clin Immunol. 2011;128:3-20 e6; quiz 1-2.
6. Dellon ES et al. Gastroenterology. 2018;155:1022-33 e10.
7. Hirano I et al. Gut. 2013;62:489-95.
8. Rank MA et al. Gastroenterology. 2020;158:1789-810 e15.
9. Arias A et al. Gastroenterology. 2014;146:1639-48.
10. Molina-Infante J et al. J Allergy Clin Immunol. 2018;141:1365-72.
11. Gentile N et al. Aliment Pharmacol Ther. 2014;40:1333-40.
12. Hirano I. Gastroenterology. 2018;155:601-6.
13. Hirano I et al. Gastroenterology. 2020;158:1776-86.
14. Collins MH et al. Dis Esophagus. 2017;30:1-8.
15. Furuta GT et al. Gut. 2013;62:1395-405.
16. Katzka DA et al. Clin Gastroenterol Hepatol. 2015;13:77-83 e2.
17. Kwiatek MA et al. Gastroenterology. 2011;140:82-90.
18. Nicodeme F et al. Clin Gastroenterol Hepatol. 2013;11:1101-7 e1.
Introduction
Eosinophilic esophagitis (EoE) has transformed over the past 3 decades from a rarely encountered entity to one of the most common causes of dysphagia in adults.1 Given the marked rise in prevalence, the early-career gastroenterologist will undoubtedly be involved with managing this disease.2 The typical presentation includes a young, atopic male presenting with dysphagia in the outpatient setting or, more acutely, with a food impaction when on call. As every fellow is keenly aware, the calls often come late at night as patients commonly have meat impactions while consuming dinner. Current management focuses on symptomatic, histologic, and endoscopic improvement with medication, dietary, and mechanical (i.e., dilation) modalities.
EoE is defined by the presence of esophageal dysfunction and esophageal eosinophilic inflammation with ≥15 eosinophils/high-powered field (eos/hpf) required for the diagnosis. With better understanding of the pathogenesis of EoE involving the complex interaction of environmental, host, and genetic factors, advancements have been made as it relates to the diagnostic criteria, endoscopic evaluation, and therapeutic options. In this article, we review the current management of adult patients with EoE and offer practical guidance to key questions for the young gastroenterologist as well as insights into future areas of interest.
What should I consider when diagnosing EoE?
Symptoms are central to the diagnosis and clinical presentation of EoE. In assessing symptoms, clinicians should be aware of adaptive “IMPACT” strategies patients often subconsciously develop in response to their chronic and progressive condition: Imbibing fluids with meals, modifying foods by cutting or pureeing, prolonging meal times, avoiding harder texture foods, chewing excessively, and turning away tablets/pills.3 Failure to query such adaptive behaviors may lead to an underestimation of disease activity and severity.
An important aspect to confirming the diagnosis of EoE is to exclude other causes of esophageal eosinophilia. Gastroesophageal reflux disease (GERD) is known to cause esophageal eosinophilia and historically has been viewed as a distinct disease process. In fact, initial guidelines included lack of response to a proton pump inhibitor (PPI) trial or normal esophageal pH monitoring as diagnostic criteria.4 However, as experience was garnered, it became clear that PPI therapy was effective at improving inflammation in 30%-50% of patients with clinical presentations and histologic features consistent with EoE. As such, the concept of PPI–responsive esophageal eosinophilia (PPI-REE) was introduced in 2011.5 Further investigation then highlighted that PPI-REE and EoE had nearly identical clinical, endoscopic, and histologic features as well as eosinophil biomarker and gene expression profiles. Hence, recent international guidelines no longer necessitate a PPI trial to establish a diagnosis of EoE.6
The young gastroenterologist should also be mindful of other issues related to the initial diagnosis of EoE. EoE may present concomitantly with other disease entities including GERD, “extra-esophageal” eosinophilic gastrointestinal diseases, concomitant IgE-mediated food allergy, hypereosinophilic syndromes, connective tissue disorders, autoimmune diseases, celiac disease, and inflammatory bowel disease.3 It has been speculated that some of these disorders share common aspects of genetic and environmental predisposing factors as well as shared pathogenesis. Careful history taking should include a full review of atopic conditions and GI-related symptoms and endoscopy should carefully inspect not only the esophagus, but also gastric and duodenal mucosa. The endoscopic features almost always reveal edema, rings, exudates, furrows, and strictures and can be assessed using the EoE Endoscopic Reference Scoring system (EREFS).7 EREFS allows for systematic identification of abnormalities that can inform decisions regarding treatment efficacy and decisions on the need for esophageal dilation. When the esophageal mucosa is evaluated for biopsies, furrows and exudates should be targeted, if present, and multiple biopsies (minimum of five to six) should be taken throughout the esophagus given the patchy nature of the disease.
How do I choose an initial therapy?
The choice of initial therapy considers patient preferences, medication availability, disease severity, impact on quality of life, and need for repeated endoscopies. While there are many novel agents currently being investigated in phase 2 and 3 clinical trials, the current mainstays of treatment include PPI therapy, topical steroids, dietary therapy, and dilation. Of note, there have been no head-to-head trials comparing these different modalities. A recent systematic review reported that PPIs can induce histologic remission in 42% of patients.8 The ease of use and availability of PPI therapy make this an attractive first choice for patients. Pooled estimates show that topical steroids can induce remission in 66% of patients.8 It is important to note that there is currently no Food and Drug Administration–approved formulation of steroids for the treatment of EoE. As such, there are several practical aspects to consider when instructing patients to use agents not designed for esophageal delivery (Figure 1).

Source: Dr. Patel, Dr. Hirano
Lack of insurance coverage for topical steroids can make cost of a prescription a deterrent to use. While topical steroids are well tolerated, concerns for candidiasis and adrenal insufficiency are being monitored in prospective, long-term clinical trials. Concomitant use of steroids with PPI would be appropriate for EoE patients with coexisting GERD (severe heartburn, erosive esophagitis, Barrett’s esophagus). In addition, we often combine steroids with PPI therapy for EoE patients who demonstrate a convincing but incomplete response to PPI monotherapy (i.e., reduction of baseline inflammation from 75 eos/hpf to 20 eos/hpf).
Diet therapy is a popular choice for management of EoE by patients, given the ability to remove food triggers that initiate the immune dysregulation and to avoid chronic medication use. Three dietary options have been described including an elemental, amino acid–based diet which eliminates all common food allergens, allergy testing–directed elimination diet, and an empiric elimination diet. Though elemental diets have shown the most efficacy, practical aspects of implementing, maintaining, and identifying triggers restrict their adoption by most patients and clinicians.9 Allergy-directed elimination diets, where allergens are eliminated based on office-based allergy testing, initially seemed promising, though studies have shown limited histologic remission, compared with other diet therapies as well as the inability to identify true food triggers. Advancement of office-based testing to identify food triggers is needed to streamline this dietary approach. In the adult patient, the empiric elimination diet remains an attractive choice of the available dietary therapies. In this dietary approach, which has shown efficacy in both children and adults, the most common food allergens (milk, wheat, soy, egg, nuts, and seafood) are eliminated.9
How do I make dietary therapy work in clinical practice?
Before dietary therapy is initiated, it is important that your practice is situated to support this approach and that patients fully understand the process. A multidisciplinary approach optimizes dietary therapy. Dietitians provide expert guidance on eliminating trigger foods, maintaining nutrition, and avoiding inadvertent cross-contamination. Patient questions may include the safety of consumption of non–cow-based cheese/milk, alcoholic beverages, wheat alternatives, and restaurant food. Allergists address concerns for a concomitant IgE food allergy based on a clinical history or previous testing. Patients should be informed that identifying a food trigger often takes several months and multiple endoscopies. Clinicians should be aware of potential food cost and accessibility issues as well as the reported, albeit uncommon, development of de novo IgE-mediated food allergy during reintroduction. Timing of diet therapy is also a factor in success. Patients should avoid starting diets during major holidays, family celebrations, college years, and busy travel months.
Particularly empiric elimination diets, frequently used in adults, several approaches have been described (Figure 2).

Source: Dr. Patel, Dr. Hirano
Initially, a step-down approach was described, with patients pursuing a six-food elimination diet (SFED), which eliminates the six most common triggers: milk, wheat, soy/legumes, egg, nuts, and seafood. Once in histologic remission, patients then systematically reintroduce foods in order to identify a causative trigger. Given that many patients have only one or two identified food triggers, other approaches were created including a single-food elimination diet eliminating milk, the two-food elimination diet (TFED) eliminating milk and wheat, and the four-food elimination diet (FFED) eliminating milk, wheat, soy/legumes, and eggs. A novel step-up approach has also now been described where patients start with the TFED and progress to the FFED and then potentially SFED based on histologic response.10 This approach has the potential to more readily identify triggers, decrease diagnostic time, and reduce endoscopic interventions. There are pros and cons to each elimination diet approach that should be discussed with patients. Many patients may find a one- or two-food elimination diet more feasible than a full SFED.
What should I consider when performing dilation?
Esophageal dilation is frequently used to address the fibrostenotic complications of EoE that do not as readily respond to PPI, steroid, or diet therapy. The majority of patients note symptomatic improvement following dilation, though dilation alone does not address the inflammatory component of disease.8 With a conservative approach, the complication rates of esophageal dilation in EoE are similar to that of benign, esophageal strictures. Endoscopists should be aware that endoscopy alone can miss strictures and consider both practical and technical aspects when performing dilations (Table 1).11,12

When should an allergist be consulted?
The role of the allergist in the management of patients with EoE varies by patient and practice. IgE serologic or skin testing have limited accuracy in identifying food triggers for EoE. Nevertheless, the majority of patients with EoE have an atopic condition which may include asthma, allergic rhinitis, atopic dermatitis, or IgE-mediated food allergy. Although EoE is thought to primarily occur from an immune response to ingested oral allergens, aeroallergens may exacerbate disease as evidenced by the seasonal variation in EoE symptoms in some patients. The allergist provides treatment for these “extraesophageal” atopic conditions which may, in turn, have synergistic effects on the treatment of EoE. Furthermore, allergists may prescribe biologic therapies that are FDA approved for the treatment of atopic dermatitis, asthma, and allergic rhinitis. While not approved for EoE, several of these agents have shown efficacy in phase 2 clinical trials in EoE. In some practice settings, allergists primarily manage EoE patients with the assistance of gastroenterologists for periodic endoscopic activity assessment.
What are the key aspects of maintenance therapy?
The goals of treatment focus on symptomatic, histologic, and endoscopic improvement, and the prevention of future or ongoing fibrostenotic complications.2 Because of the adaptive eating behaviors discussed above, symptom response may not reliably correlate with histologic and/or endoscopic improvement. Moreover, dysphagia is related to strictures that often do not resolve in spite of resolution of mucosal inflammation. As such, histology and endoscopy are more objective and reliable targets of a successful response to therapy. Though studies have used variable esophageal density levels for response, using a cutoff of <15 eos/hpf as a therapeutic endpoint is reasonable for both initial response to therapy and long-term monitoring.13 We advocate for standardization of reporting endoscopic findings to better track change over time using the EREFS scoring system.7 While inflammatory features improve, the fibrostenotic features may persist despite improvement in histology. Dilation is often performed in these situations, especially for symptomatic individuals.
During clinical follow-up, the frequency of monitoring as it relates to symptom and endoscopic assessment is not well defined. It is reasonable to repeat endoscopic intervention following changes in therapy (i.e., reduction in steroid dosing or reintroduction of putative food triggers) or in symptoms.13 It is unclear if patients benefit from repeated endoscopies at set intervals without symptom change and after histologic response has been confirmed. In our practice, endoscopies are often considered on an annual basis. This interval is increased for patients with demonstrated stability of disease.
For patients who opt for dietary therapy and have one or two food triggers identified, long-term maintenance therapy can be straightforward with ongoing food avoidance. Limited data exist regarding long-term effectiveness of dietary therapy but loss of initial response has been reported that is often attributed to problems with adherence. Use of “diet holidays” or “planned cheats” to allow for intermittent consumption of trigger foods, often under the cover of short-term use of steroids, may improve the long-term feasibility of diet approaches.
In the recent American Gastroenterological Association guidelines, continuation of swallowed, topical steroids is recommended following remission with short-term treatment. The recurrence of both symptoms and inflammation following medication withdrawal supports this practice. Furthermore, natural history studies demonstrate progression of esophageal strictures with untreated disease.
There are no clear guidelines for long-term dosage and use of PPI or topical steroid therapy. Our practice is to down-titrate the dose of PPI or steroid following remission with short-term therapy, often starting with a reduction from twice a day to daily dosing. Although topical steroid therapy has fewer side effects, compared with systemic steroids, patients should be aware of the potential for adrenal suppression especially in an atopic population who may be exposed to multiple forms of topical steroids. Shared decision-making between patients and providers is recommended to determine comfort level with long-term use of prescription medications and dosage.
What’s on the horizon?
Several areas of development are underway to better assess and manage EoE. Novel histologic scoring tools now assess characteristics on pathology beyond eosinophil density, office-based testing modalities have been developed to assess inflammatory activity and thereby obviate the need for endoscopy, new technology can provide measures of esophageal remodeling and provide assessment of disease severity, and several biologic agents are being studied that target specific allergic mediators of the immune response in EoE.3,14-18 These novel tools, technologies, and therapies will undoubtedly change the management approach to EoE. Referral of patients into ongoing clinical trials will help inform advances in the field.
Conclusion
As an increasingly prevalent disease with a high degree of upper GI morbidity, EoE has transitioned from a rare entity to a commonly encountered disease. The new gastroenterologist will confront both straightforward as well as complex patients with EoE, and we offer several practical aspects on management. In the years ahead, the care of patients with EoE will continue to evolve to a more streamlined, effective, and personalized approach.
References
1. Kidambi T et al. World J Gastroenterol. 2012;18:4335-41.
2. Dellon ES et al. Gastroenterology. 2018;154:319-32 e3.
3. Hirano I et al. Gastroenterology. 2020;158:840-51.
4. Furuta GT et al. Gastroenterology. 2007;133:1342-63.
5. Liacouras CA et al. J Allergy Clin Immunol. 2011;128:3-20 e6; quiz 1-2.
6. Dellon ES et al. Gastroenterology. 2018;155:1022-33 e10.
7. Hirano I et al. Gut. 2013;62:489-95.
8. Rank MA et al. Gastroenterology. 2020;158:1789-810 e15.
9. Arias A et al. Gastroenterology. 2014;146:1639-48.
10. Molina-Infante J et al. J Allergy Clin Immunol. 2018;141:1365-72.
11. Gentile N et al. Aliment Pharmacol Ther. 2014;40:1333-40.
12. Hirano I. Gastroenterology. 2018;155:601-6.
13. Hirano I et al. Gastroenterology. 2020;158:1776-86.
14. Collins MH et al. Dis Esophagus. 2017;30:1-8.
15. Furuta GT et al. Gut. 2013;62:1395-405.
16. Katzka DA et al. Clin Gastroenterol Hepatol. 2015;13:77-83 e2.
17. Kwiatek MA et al. Gastroenterology. 2011;140:82-90.
18. Nicodeme F et al. Clin Gastroenterol Hepatol. 2013;11:1101-7 e1.
Introduction
Eosinophilic esophagitis (EoE) has transformed over the past 3 decades from a rarely encountered entity to one of the most common causes of dysphagia in adults.1 Given the marked rise in prevalence, the early-career gastroenterologist will undoubtedly be involved with managing this disease.2 The typical presentation includes a young, atopic male presenting with dysphagia in the outpatient setting or, more acutely, with a food impaction when on call. As every fellow is keenly aware, the calls often come late at night as patients commonly have meat impactions while consuming dinner. Current management focuses on symptomatic, histologic, and endoscopic improvement with medication, dietary, and mechanical (i.e., dilation) modalities.
EoE is defined by the presence of esophageal dysfunction and esophageal eosinophilic inflammation with ≥15 eosinophils/high-powered field (eos/hpf) required for the diagnosis. With better understanding of the pathogenesis of EoE involving the complex interaction of environmental, host, and genetic factors, advancements have been made as it relates to the diagnostic criteria, endoscopic evaluation, and therapeutic options. In this article, we review the current management of adult patients with EoE and offer practical guidance to key questions for the young gastroenterologist as well as insights into future areas of interest.
What should I consider when diagnosing EoE?
Symptoms are central to the diagnosis and clinical presentation of EoE. In assessing symptoms, clinicians should be aware of adaptive “IMPACT” strategies patients often subconsciously develop in response to their chronic and progressive condition: Imbibing fluids with meals, modifying foods by cutting or pureeing, prolonging meal times, avoiding harder texture foods, chewing excessively, and turning away tablets/pills.3 Failure to query such adaptive behaviors may lead to an underestimation of disease activity and severity.
An important aspect to confirming the diagnosis of EoE is to exclude other causes of esophageal eosinophilia. Gastroesophageal reflux disease (GERD) is known to cause esophageal eosinophilia and historically has been viewed as a distinct disease process. In fact, initial guidelines included lack of response to a proton pump inhibitor (PPI) trial or normal esophageal pH monitoring as diagnostic criteria.4 However, as experience was garnered, it became clear that PPI therapy was effective at improving inflammation in 30%-50% of patients with clinical presentations and histologic features consistent with EoE. As such, the concept of PPI–responsive esophageal eosinophilia (PPI-REE) was introduced in 2011.5 Further investigation then highlighted that PPI-REE and EoE had nearly identical clinical, endoscopic, and histologic features as well as eosinophil biomarker and gene expression profiles. Hence, recent international guidelines no longer necessitate a PPI trial to establish a diagnosis of EoE.6
The young gastroenterologist should also be mindful of other issues related to the initial diagnosis of EoE. EoE may present concomitantly with other disease entities including GERD, “extra-esophageal” eosinophilic gastrointestinal diseases, concomitant IgE-mediated food allergy, hypereosinophilic syndromes, connective tissue disorders, autoimmune diseases, celiac disease, and inflammatory bowel disease.3 It has been speculated that some of these disorders share common aspects of genetic and environmental predisposing factors as well as shared pathogenesis. Careful history taking should include a full review of atopic conditions and GI-related symptoms and endoscopy should carefully inspect not only the esophagus, but also gastric and duodenal mucosa. The endoscopic features almost always reveal edema, rings, exudates, furrows, and strictures and can be assessed using the EoE Endoscopic Reference Scoring system (EREFS).7 EREFS allows for systematic identification of abnormalities that can inform decisions regarding treatment efficacy and decisions on the need for esophageal dilation. When the esophageal mucosa is evaluated for biopsies, furrows and exudates should be targeted, if present, and multiple biopsies (minimum of five to six) should be taken throughout the esophagus given the patchy nature of the disease.
How do I choose an initial therapy?
The choice of initial therapy considers patient preferences, medication availability, disease severity, impact on quality of life, and need for repeated endoscopies. While there are many novel agents currently being investigated in phase 2 and 3 clinical trials, the current mainstays of treatment include PPI therapy, topical steroids, dietary therapy, and dilation. Of note, there have been no head-to-head trials comparing these different modalities. A recent systematic review reported that PPIs can induce histologic remission in 42% of patients.8 The ease of use and availability of PPI therapy make this an attractive first choice for patients. Pooled estimates show that topical steroids can induce remission in 66% of patients.8 It is important to note that there is currently no Food and Drug Administration–approved formulation of steroids for the treatment of EoE. As such, there are several practical aspects to consider when instructing patients to use agents not designed for esophageal delivery (Figure 1).

Source: Dr. Patel, Dr. Hirano
Lack of insurance coverage for topical steroids can make cost of a prescription a deterrent to use. While topical steroids are well tolerated, concerns for candidiasis and adrenal insufficiency are being monitored in prospective, long-term clinical trials. Concomitant use of steroids with PPI would be appropriate for EoE patients with coexisting GERD (severe heartburn, erosive esophagitis, Barrett’s esophagus). In addition, we often combine steroids with PPI therapy for EoE patients who demonstrate a convincing but incomplete response to PPI monotherapy (i.e., reduction of baseline inflammation from 75 eos/hpf to 20 eos/hpf).
Diet therapy is a popular choice for management of EoE by patients, given the ability to remove food triggers that initiate the immune dysregulation and to avoid chronic medication use. Three dietary options have been described including an elemental, amino acid–based diet which eliminates all common food allergens, allergy testing–directed elimination diet, and an empiric elimination diet. Though elemental diets have shown the most efficacy, practical aspects of implementing, maintaining, and identifying triggers restrict their adoption by most patients and clinicians.9 Allergy-directed elimination diets, where allergens are eliminated based on office-based allergy testing, initially seemed promising, though studies have shown limited histologic remission, compared with other diet therapies as well as the inability to identify true food triggers. Advancement of office-based testing to identify food triggers is needed to streamline this dietary approach. In the adult patient, the empiric elimination diet remains an attractive choice of the available dietary therapies. In this dietary approach, which has shown efficacy in both children and adults, the most common food allergens (milk, wheat, soy, egg, nuts, and seafood) are eliminated.9
How do I make dietary therapy work in clinical practice?
Before dietary therapy is initiated, it is important that your practice is situated to support this approach and that patients fully understand the process. A multidisciplinary approach optimizes dietary therapy. Dietitians provide expert guidance on eliminating trigger foods, maintaining nutrition, and avoiding inadvertent cross-contamination. Patient questions may include the safety of consumption of non–cow-based cheese/milk, alcoholic beverages, wheat alternatives, and restaurant food. Allergists address concerns for a concomitant IgE food allergy based on a clinical history or previous testing. Patients should be informed that identifying a food trigger often takes several months and multiple endoscopies. Clinicians should be aware of potential food cost and accessibility issues as well as the reported, albeit uncommon, development of de novo IgE-mediated food allergy during reintroduction. Timing of diet therapy is also a factor in success. Patients should avoid starting diets during major holidays, family celebrations, college years, and busy travel months.
Particularly empiric elimination diets, frequently used in adults, several approaches have been described (Figure 2).

Source: Dr. Patel, Dr. Hirano
Initially, a step-down approach was described, with patients pursuing a six-food elimination diet (SFED), which eliminates the six most common triggers: milk, wheat, soy/legumes, egg, nuts, and seafood. Once in histologic remission, patients then systematically reintroduce foods in order to identify a causative trigger. Given that many patients have only one or two identified food triggers, other approaches were created including a single-food elimination diet eliminating milk, the two-food elimination diet (TFED) eliminating milk and wheat, and the four-food elimination diet (FFED) eliminating milk, wheat, soy/legumes, and eggs. A novel step-up approach has also now been described where patients start with the TFED and progress to the FFED and then potentially SFED based on histologic response.10 This approach has the potential to more readily identify triggers, decrease diagnostic time, and reduce endoscopic interventions. There are pros and cons to each elimination diet approach that should be discussed with patients. Many patients may find a one- or two-food elimination diet more feasible than a full SFED.
What should I consider when performing dilation?
Esophageal dilation is frequently used to address the fibrostenotic complications of EoE that do not as readily respond to PPI, steroid, or diet therapy. The majority of patients note symptomatic improvement following dilation, though dilation alone does not address the inflammatory component of disease.8 With a conservative approach, the complication rates of esophageal dilation in EoE are similar to that of benign, esophageal strictures. Endoscopists should be aware that endoscopy alone can miss strictures and consider both practical and technical aspects when performing dilations (Table 1).11,12

When should an allergist be consulted?
The role of the allergist in the management of patients with EoE varies by patient and practice. IgE serologic or skin testing have limited accuracy in identifying food triggers for EoE. Nevertheless, the majority of patients with EoE have an atopic condition which may include asthma, allergic rhinitis, atopic dermatitis, or IgE-mediated food allergy. Although EoE is thought to primarily occur from an immune response to ingested oral allergens, aeroallergens may exacerbate disease as evidenced by the seasonal variation in EoE symptoms in some patients. The allergist provides treatment for these “extraesophageal” atopic conditions which may, in turn, have synergistic effects on the treatment of EoE. Furthermore, allergists may prescribe biologic therapies that are FDA approved for the treatment of atopic dermatitis, asthma, and allergic rhinitis. While not approved for EoE, several of these agents have shown efficacy in phase 2 clinical trials in EoE. In some practice settings, allergists primarily manage EoE patients with the assistance of gastroenterologists for periodic endoscopic activity assessment.
What are the key aspects of maintenance therapy?
The goals of treatment focus on symptomatic, histologic, and endoscopic improvement, and the prevention of future or ongoing fibrostenotic complications.2 Because of the adaptive eating behaviors discussed above, symptom response may not reliably correlate with histologic and/or endoscopic improvement. Moreover, dysphagia is related to strictures that often do not resolve in spite of resolution of mucosal inflammation. As such, histology and endoscopy are more objective and reliable targets of a successful response to therapy. Though studies have used variable esophageal density levels for response, using a cutoff of <15 eos/hpf as a therapeutic endpoint is reasonable for both initial response to therapy and long-term monitoring.13 We advocate for standardization of reporting endoscopic findings to better track change over time using the EREFS scoring system.7 While inflammatory features improve, the fibrostenotic features may persist despite improvement in histology. Dilation is often performed in these situations, especially for symptomatic individuals.
During clinical follow-up, the frequency of monitoring as it relates to symptom and endoscopic assessment is not well defined. It is reasonable to repeat endoscopic intervention following changes in therapy (i.e., reduction in steroid dosing or reintroduction of putative food triggers) or in symptoms.13 It is unclear if patients benefit from repeated endoscopies at set intervals without symptom change and after histologic response has been confirmed. In our practice, endoscopies are often considered on an annual basis. This interval is increased for patients with demonstrated stability of disease.
For patients who opt for dietary therapy and have one or two food triggers identified, long-term maintenance therapy can be straightforward with ongoing food avoidance. Limited data exist regarding long-term effectiveness of dietary therapy but loss of initial response has been reported that is often attributed to problems with adherence. Use of “diet holidays” or “planned cheats” to allow for intermittent consumption of trigger foods, often under the cover of short-term use of steroids, may improve the long-term feasibility of diet approaches.
In the recent American Gastroenterological Association guidelines, continuation of swallowed, topical steroids is recommended following remission with short-term treatment. The recurrence of both symptoms and inflammation following medication withdrawal supports this practice. Furthermore, natural history studies demonstrate progression of esophageal strictures with untreated disease.
There are no clear guidelines for long-term dosage and use of PPI or topical steroid therapy. Our practice is to down-titrate the dose of PPI or steroid following remission with short-term therapy, often starting with a reduction from twice a day to daily dosing. Although topical steroid therapy has fewer side effects, compared with systemic steroids, patients should be aware of the potential for adrenal suppression especially in an atopic population who may be exposed to multiple forms of topical steroids. Shared decision-making between patients and providers is recommended to determine comfort level with long-term use of prescription medications and dosage.
What’s on the horizon?
Several areas of development are underway to better assess and manage EoE. Novel histologic scoring tools now assess characteristics on pathology beyond eosinophil density, office-based testing modalities have been developed to assess inflammatory activity and thereby obviate the need for endoscopy, new technology can provide measures of esophageal remodeling and provide assessment of disease severity, and several biologic agents are being studied that target specific allergic mediators of the immune response in EoE.3,14-18 These novel tools, technologies, and therapies will undoubtedly change the management approach to EoE. Referral of patients into ongoing clinical trials will help inform advances in the field.
Conclusion
As an increasingly prevalent disease with a high degree of upper GI morbidity, EoE has transitioned from a rare entity to a commonly encountered disease. The new gastroenterologist will confront both straightforward as well as complex patients with EoE, and we offer several practical aspects on management. In the years ahead, the care of patients with EoE will continue to evolve to a more streamlined, effective, and personalized approach.
References
1. Kidambi T et al. World J Gastroenterol. 2012;18:4335-41.
2. Dellon ES et al. Gastroenterology. 2018;154:319-32 e3.
3. Hirano I et al. Gastroenterology. 2020;158:840-51.
4. Furuta GT et al. Gastroenterology. 2007;133:1342-63.
5. Liacouras CA et al. J Allergy Clin Immunol. 2011;128:3-20 e6; quiz 1-2.
6. Dellon ES et al. Gastroenterology. 2018;155:1022-33 e10.
7. Hirano I et al. Gut. 2013;62:489-95.
8. Rank MA et al. Gastroenterology. 2020;158:1789-810 e15.
9. Arias A et al. Gastroenterology. 2014;146:1639-48.
10. Molina-Infante J et al. J Allergy Clin Immunol. 2018;141:1365-72.
11. Gentile N et al. Aliment Pharmacol Ther. 2014;40:1333-40.
12. Hirano I. Gastroenterology. 2018;155:601-6.
13. Hirano I et al. Gastroenterology. 2020;158:1776-86.
14. Collins MH et al. Dis Esophagus. 2017;30:1-8.
15. Furuta GT et al. Gut. 2013;62:1395-405.
16. Katzka DA et al. Clin Gastroenterol Hepatol. 2015;13:77-83 e2.
17. Kwiatek MA et al. Gastroenterology. 2011;140:82-90.
18. Nicodeme F et al. Clin Gastroenterol Hepatol. 2013;11:1101-7 e1.
Letter from the Editor: Stay safe and, please, wear a mask
“Beginning immediately, the University of Michigan will require all students, staff, faculty, and visitors to wear a face covering that covers the mouth and nose while anywhere on campus grounds.” (Mark S. Schlissel MD, PhD – President, University of Michigan – July 15).
Executive Order 2020-147 (Michigan’s Governor Whitmer) mandated appropriate facial covering for all indoor spaces and crowded outdoor spaces. Additionally, businesses will be held responsible if they allow entry to anyone not wearing a mask.
While enforcement is proving to be a nightmare, masking combined with social distancing, hand washing, and staying home are the only effective levers we have to slow the spread of COVID-19. As of today, 138,000 Americans have died, and we anticipate 240,000 deaths by November 1. By now, most of us (myself included) have had a friend or relative die of this virus. America is not winning this battle and we have yet to see an effective, coordinated national response. Four forces are killing our citizens: COVID-19, structural racism, economic/health inequities, and divisive politics. We should do better.
Although Michigan and Minnesota (my home states) have slowed the virus enough to maintain resource capacity, just last weekend a single house party in a suburb near Ann Arbor resulted in 40 new infections. Thirty-nine states have rising case numbers, hospitalizations, and deaths. We are still in the early innings of this game. Michigan Medicine is actively planning our response to a second surge, which will be combined with increases of influenza and RSV infections.
This month we continue to cover the rapidly emerging information about COVID-19 and digestive implications. There are other interesting articles, including guidance around BRCA risk for colorectal cancer, eosinophilic esophagitis, probiotics, and the emerging impact of AI on endoscopy. Enjoy – stay safe, wash hands, socially distance, and please, wear a mask.
“Respect science, respect nature, respect each other” (Thomas Friedman).
John I. Allen, MD, MBA, AGAF
Editor in Chief
“Beginning immediately, the University of Michigan will require all students, staff, faculty, and visitors to wear a face covering that covers the mouth and nose while anywhere on campus grounds.” (Mark S. Schlissel MD, PhD – President, University of Michigan – July 15).
Executive Order 2020-147 (Michigan’s Governor Whitmer) mandated appropriate facial covering for all indoor spaces and crowded outdoor spaces. Additionally, businesses will be held responsible if they allow entry to anyone not wearing a mask.
While enforcement is proving to be a nightmare, masking combined with social distancing, hand washing, and staying home are the only effective levers we have to slow the spread of COVID-19. As of today, 138,000 Americans have died, and we anticipate 240,000 deaths by November 1. By now, most of us (myself included) have had a friend or relative die of this virus. America is not winning this battle and we have yet to see an effective, coordinated national response. Four forces are killing our citizens: COVID-19, structural racism, economic/health inequities, and divisive politics. We should do better.
Although Michigan and Minnesota (my home states) have slowed the virus enough to maintain resource capacity, just last weekend a single house party in a suburb near Ann Arbor resulted in 40 new infections. Thirty-nine states have rising case numbers, hospitalizations, and deaths. We are still in the early innings of this game. Michigan Medicine is actively planning our response to a second surge, which will be combined with increases of influenza and RSV infections.
This month we continue to cover the rapidly emerging information about COVID-19 and digestive implications. There are other interesting articles, including guidance around BRCA risk for colorectal cancer, eosinophilic esophagitis, probiotics, and the emerging impact of AI on endoscopy. Enjoy – stay safe, wash hands, socially distance, and please, wear a mask.
“Respect science, respect nature, respect each other” (Thomas Friedman).
John I. Allen, MD, MBA, AGAF
Editor in Chief
“Beginning immediately, the University of Michigan will require all students, staff, faculty, and visitors to wear a face covering that covers the mouth and nose while anywhere on campus grounds.” (Mark S. Schlissel MD, PhD – President, University of Michigan – July 15).
Executive Order 2020-147 (Michigan’s Governor Whitmer) mandated appropriate facial covering for all indoor spaces and crowded outdoor spaces. Additionally, businesses will be held responsible if they allow entry to anyone not wearing a mask.
While enforcement is proving to be a nightmare, masking combined with social distancing, hand washing, and staying home are the only effective levers we have to slow the spread of COVID-19. As of today, 138,000 Americans have died, and we anticipate 240,000 deaths by November 1. By now, most of us (myself included) have had a friend or relative die of this virus. America is not winning this battle and we have yet to see an effective, coordinated national response. Four forces are killing our citizens: COVID-19, structural racism, economic/health inequities, and divisive politics. We should do better.
Although Michigan and Minnesota (my home states) have slowed the virus enough to maintain resource capacity, just last weekend a single house party in a suburb near Ann Arbor resulted in 40 new infections. Thirty-nine states have rising case numbers, hospitalizations, and deaths. We are still in the early innings of this game. Michigan Medicine is actively planning our response to a second surge, which will be combined with increases of influenza and RSV infections.
This month we continue to cover the rapidly emerging information about COVID-19 and digestive implications. There are other interesting articles, including guidance around BRCA risk for colorectal cancer, eosinophilic esophagitis, probiotics, and the emerging impact of AI on endoscopy. Enjoy – stay safe, wash hands, socially distance, and please, wear a mask.
“Respect science, respect nature, respect each other” (Thomas Friedman).
John I. Allen, MD, MBA, AGAF
Editor in Chief