User login
Hospital vs. outpatient management comparable for elderly syncope patients
Background: In the United States, there are over 1 million visits to EDs for syncope with a greater than 50% hospitalization rate for older adult patients. There remains uncertainty around which patients without an identified cause for the syncope could be discharged from the ED and managed as an outpatient.
Study design: Propensity score analysis.
Setting: EDs from 11 nonprofit academic hospitals.
Synopsis: Prospective data for 2,492 patients aged 60 years and older who did not have an identified cause in the ED for their presenting complaint of syncope were included in the propensity score analysis resulting in a sample size of 1,064 with 532 patients in each of the discharged and hospitalized groups. There was no significant difference in risk of 30-day post-ED serious adverse events between the hospitalized patients (4.89%; 95% confidence interval, 3.06%-6.72%) and discharged patients (2.82%; 95% CI, 1.41%-4.23%; risk difference 2.07%; 95% CI, –0.24% to 4.38%). There was also no statistically significant difference in 30-day mortality post–ED visit.
These results show no clinical benefit in hospitalization for older adults with unexplained syncope after ED evaluation suggesting that it would be reasonable to proceed with outpatient management and evaluation of these patients.
Bottom line: Consider discharging older patients home from the ED who do not have high risk factors and no identified cause of their syncope.
Citation: Probst MA et al. Clinical benefit of hospitalization for older adults with unexplained syncope: A propensity-matched analysis. Ann Emerg Med. 2019 Aug;74(2):260-9.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
Background: In the United States, there are over 1 million visits to EDs for syncope with a greater than 50% hospitalization rate for older adult patients. There remains uncertainty around which patients without an identified cause for the syncope could be discharged from the ED and managed as an outpatient.
Study design: Propensity score analysis.
Setting: EDs from 11 nonprofit academic hospitals.
Synopsis: Prospective data for 2,492 patients aged 60 years and older who did not have an identified cause in the ED for their presenting complaint of syncope were included in the propensity score analysis resulting in a sample size of 1,064 with 532 patients in each of the discharged and hospitalized groups. There was no significant difference in risk of 30-day post-ED serious adverse events between the hospitalized patients (4.89%; 95% confidence interval, 3.06%-6.72%) and discharged patients (2.82%; 95% CI, 1.41%-4.23%; risk difference 2.07%; 95% CI, –0.24% to 4.38%). There was also no statistically significant difference in 30-day mortality post–ED visit.
These results show no clinical benefit in hospitalization for older adults with unexplained syncope after ED evaluation suggesting that it would be reasonable to proceed with outpatient management and evaluation of these patients.
Bottom line: Consider discharging older patients home from the ED who do not have high risk factors and no identified cause of their syncope.
Citation: Probst MA et al. Clinical benefit of hospitalization for older adults with unexplained syncope: A propensity-matched analysis. Ann Emerg Med. 2019 Aug;74(2):260-9.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
Background: In the United States, there are over 1 million visits to EDs for syncope with a greater than 50% hospitalization rate for older adult patients. There remains uncertainty around which patients without an identified cause for the syncope could be discharged from the ED and managed as an outpatient.
Study design: Propensity score analysis.
Setting: EDs from 11 nonprofit academic hospitals.
Synopsis: Prospective data for 2,492 patients aged 60 years and older who did not have an identified cause in the ED for their presenting complaint of syncope were included in the propensity score analysis resulting in a sample size of 1,064 with 532 patients in each of the discharged and hospitalized groups. There was no significant difference in risk of 30-day post-ED serious adverse events between the hospitalized patients (4.89%; 95% confidence interval, 3.06%-6.72%) and discharged patients (2.82%; 95% CI, 1.41%-4.23%; risk difference 2.07%; 95% CI, –0.24% to 4.38%). There was also no statistically significant difference in 30-day mortality post–ED visit.
These results show no clinical benefit in hospitalization for older adults with unexplained syncope after ED evaluation suggesting that it would be reasonable to proceed with outpatient management and evaluation of these patients.
Bottom line: Consider discharging older patients home from the ED who do not have high risk factors and no identified cause of their syncope.
Citation: Probst MA et al. Clinical benefit of hospitalization for older adults with unexplained syncope: A propensity-matched analysis. Ann Emerg Med. 2019 Aug;74(2):260-9.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
New psoriasis guidelines focus on topical and alternative treatments, and severity measures
and the National Psoriasis Foundation.
The guidelines, published in the Journal of the American Academy of Dermatology, focus on treatment for adults, and follow the release of other AAD-NPF guidelines on biologics for psoriasis, psoriasis-related comorbidities, pediatric psoriasis, and phototherapy in 2019, and earlier this year, guidelines for systemic nonbiologic treatments. The latest guidelines’ section on topical treatment outlines evidence for the efficacy, effectiveness, and adverse events related to topical steroids, topical tacrolimus and pimecrolimus, vitamin D analogues, tazarotene, moisturizers, salicylic acid, anthralin, coal tar, combinations with biologic agents, and combinations with nonbiologic treatments (methotrexate, cyclosporine, acitretin, and apremilast).
The guidelines noted the “key role” of topical corticosteroids in treating psoriasis “especially for localized disease,” and include a review of the data on low-, moderate-, high-, and ultrahigh-potency topical steroids for psoriasis.
In general, all topical steroids can be used in combination with biologics, according to the guidelines, but the strongest recommendations based on the latest evidence include the addition of an ultra-high potency topical corticosteroid to standard dose etanercept for 12 weeks. Currently, 11 biologics are approved by the Food and Drug Administration for the treatment of psoriasis.
In addition, “while not FDA approved for psoriasis, the topical calcineurin inhibitors tacrolimus and pimecrolimus are often employed in the treatment of psoriasis,” can be helpful for “thinner skin such as facial and intertriginous areas,” and can be steroid sparing when used for more than 4 weeks, according to the guidelines.
Don’t discount the role of patient preferences when choosing topical treatments, the authors noted. “The optimal vehicle choice is the one the patient is mostly likely to use.”
The guidelines also address the evidence for effectiveness, and adverse events in the use of several alternative medicines for psoriasis including traditional Chinese medicine, and the herbal therapies aloe vera and St. John’s wort, as well as the potential role of dietary supplements including fish oil, vitamin D, turmeric, and zinc in managing psoriasis, and the potential role of a gluten-free diet.
In general, research on the efficacy, effectiveness, and potential adverse effects of these strategies are limited, according to the guidelines, although many patients express interest in supplements and herbal products. For example, “Many patients ask about the overall role of vitamin D in skin health. Rather than adding oral vitamin D supplementation, topical therapy with vitamin D agents is effective for the treatment of psoriasis,” the authors noted.
In addition, they noted that mind/body strategies, namely hypnosis and stress reduction or meditation techniques, have been shown to improve symptoms and can be helpful for some patients, but clinical evidence is limited.
The guidelines also addressed methods for assessing disease severity in psoriasis. They recommended using body surface area (BSA) to assess psoriasis severity and patient response to treatment in the clinical setting. However, BSA is a provider assessment tool that “does not take into account location on the body, clinical characteristics of the plaques, symptoms, or quality of life issues,” the authors noted. The Psoriasis Area and Severity Index (PASI) measures erythema, induration, and scaling and is more suited to assessing psoriasis severity and response to treatment in clinical trials rather than in practice, they said.
Prior AAD guidelines on psoriasis were published more than 10 years ago, and major developments including the availability of new biologic drugs and new data on comorbidities have been recognized in the past decade, working group cochair and author of the guidelines Alan Menter, MD, said in an interview.
The key game-changers from previous guidelines include the full section published on comorbidities plus the development of two new important cytokine classes: three IL-17 drugs and three new IL-23 drugs now available for moderate to severe psoriasis, said Dr. Menter, chairman of the division of dermatology at Baylor University Medical Center, Dallas.
Barriers to implementing the guidelines in practice may occur when “third party payers make the decision on which of the 11 biologic drugs now approved for moderate to severe psoriasis should be used,” he noted.
As for next steps in psoriasis studies, “new biomarker research is currently underway,” Dr. Menter said. With 11 biologic agents new formally approved by the FDA for moderate to severe psoriasis, the next steps are to determine which drug is likely to be the most appropriate for each individual patient.
Dr. Menter disclosed relationships with multiple companies that develop and manufacture psoriasis therapies, including Abbott Labs, AbbVie, Amgen, Eli Lilly and Company, Galderma USA, Janssen Pharmaceuticals, LEO Pharma US, Menlo Therapeutics, and Novartis. The updated guidelines were designed by a multidisciplinary work group of psoriasis experts including dermatologists, a rheumatologist, a cardiologist, and representatives from a patient advocacy organization.
SOURCE: Elmets CA et al. J Am Acad Dermatol. 2020 Jul 29. doi: 10.1016/j.jaad.2020.07.087.
and the National Psoriasis Foundation.
The guidelines, published in the Journal of the American Academy of Dermatology, focus on treatment for adults, and follow the release of other AAD-NPF guidelines on biologics for psoriasis, psoriasis-related comorbidities, pediatric psoriasis, and phototherapy in 2019, and earlier this year, guidelines for systemic nonbiologic treatments. The latest guidelines’ section on topical treatment outlines evidence for the efficacy, effectiveness, and adverse events related to topical steroids, topical tacrolimus and pimecrolimus, vitamin D analogues, tazarotene, moisturizers, salicylic acid, anthralin, coal tar, combinations with biologic agents, and combinations with nonbiologic treatments (methotrexate, cyclosporine, acitretin, and apremilast).
The guidelines noted the “key role” of topical corticosteroids in treating psoriasis “especially for localized disease,” and include a review of the data on low-, moderate-, high-, and ultrahigh-potency topical steroids for psoriasis.
In general, all topical steroids can be used in combination with biologics, according to the guidelines, but the strongest recommendations based on the latest evidence include the addition of an ultra-high potency topical corticosteroid to standard dose etanercept for 12 weeks. Currently, 11 biologics are approved by the Food and Drug Administration for the treatment of psoriasis.
In addition, “while not FDA approved for psoriasis, the topical calcineurin inhibitors tacrolimus and pimecrolimus are often employed in the treatment of psoriasis,” can be helpful for “thinner skin such as facial and intertriginous areas,” and can be steroid sparing when used for more than 4 weeks, according to the guidelines.
Don’t discount the role of patient preferences when choosing topical treatments, the authors noted. “The optimal vehicle choice is the one the patient is mostly likely to use.”
The guidelines also address the evidence for effectiveness, and adverse events in the use of several alternative medicines for psoriasis including traditional Chinese medicine, and the herbal therapies aloe vera and St. John’s wort, as well as the potential role of dietary supplements including fish oil, vitamin D, turmeric, and zinc in managing psoriasis, and the potential role of a gluten-free diet.
In general, research on the efficacy, effectiveness, and potential adverse effects of these strategies are limited, according to the guidelines, although many patients express interest in supplements and herbal products. For example, “Many patients ask about the overall role of vitamin D in skin health. Rather than adding oral vitamin D supplementation, topical therapy with vitamin D agents is effective for the treatment of psoriasis,” the authors noted.
In addition, they noted that mind/body strategies, namely hypnosis and stress reduction or meditation techniques, have been shown to improve symptoms and can be helpful for some patients, but clinical evidence is limited.
The guidelines also addressed methods for assessing disease severity in psoriasis. They recommended using body surface area (BSA) to assess psoriasis severity and patient response to treatment in the clinical setting. However, BSA is a provider assessment tool that “does not take into account location on the body, clinical characteristics of the plaques, symptoms, or quality of life issues,” the authors noted. The Psoriasis Area and Severity Index (PASI) measures erythema, induration, and scaling and is more suited to assessing psoriasis severity and response to treatment in clinical trials rather than in practice, they said.
Prior AAD guidelines on psoriasis were published more than 10 years ago, and major developments including the availability of new biologic drugs and new data on comorbidities have been recognized in the past decade, working group cochair and author of the guidelines Alan Menter, MD, said in an interview.
The key game-changers from previous guidelines include the full section published on comorbidities plus the development of two new important cytokine classes: three IL-17 drugs and three new IL-23 drugs now available for moderate to severe psoriasis, said Dr. Menter, chairman of the division of dermatology at Baylor University Medical Center, Dallas.
Barriers to implementing the guidelines in practice may occur when “third party payers make the decision on which of the 11 biologic drugs now approved for moderate to severe psoriasis should be used,” he noted.
As for next steps in psoriasis studies, “new biomarker research is currently underway,” Dr. Menter said. With 11 biologic agents new formally approved by the FDA for moderate to severe psoriasis, the next steps are to determine which drug is likely to be the most appropriate for each individual patient.
Dr. Menter disclosed relationships with multiple companies that develop and manufacture psoriasis therapies, including Abbott Labs, AbbVie, Amgen, Eli Lilly and Company, Galderma USA, Janssen Pharmaceuticals, LEO Pharma US, Menlo Therapeutics, and Novartis. The updated guidelines were designed by a multidisciplinary work group of psoriasis experts including dermatologists, a rheumatologist, a cardiologist, and representatives from a patient advocacy organization.
SOURCE: Elmets CA et al. J Am Acad Dermatol. 2020 Jul 29. doi: 10.1016/j.jaad.2020.07.087.
and the National Psoriasis Foundation.
The guidelines, published in the Journal of the American Academy of Dermatology, focus on treatment for adults, and follow the release of other AAD-NPF guidelines on biologics for psoriasis, psoriasis-related comorbidities, pediatric psoriasis, and phototherapy in 2019, and earlier this year, guidelines for systemic nonbiologic treatments. The latest guidelines’ section on topical treatment outlines evidence for the efficacy, effectiveness, and adverse events related to topical steroids, topical tacrolimus and pimecrolimus, vitamin D analogues, tazarotene, moisturizers, salicylic acid, anthralin, coal tar, combinations with biologic agents, and combinations with nonbiologic treatments (methotrexate, cyclosporine, acitretin, and apremilast).
The guidelines noted the “key role” of topical corticosteroids in treating psoriasis “especially for localized disease,” and include a review of the data on low-, moderate-, high-, and ultrahigh-potency topical steroids for psoriasis.
In general, all topical steroids can be used in combination with biologics, according to the guidelines, but the strongest recommendations based on the latest evidence include the addition of an ultra-high potency topical corticosteroid to standard dose etanercept for 12 weeks. Currently, 11 biologics are approved by the Food and Drug Administration for the treatment of psoriasis.
In addition, “while not FDA approved for psoriasis, the topical calcineurin inhibitors tacrolimus and pimecrolimus are often employed in the treatment of psoriasis,” can be helpful for “thinner skin such as facial and intertriginous areas,” and can be steroid sparing when used for more than 4 weeks, according to the guidelines.
Don’t discount the role of patient preferences when choosing topical treatments, the authors noted. “The optimal vehicle choice is the one the patient is mostly likely to use.”
The guidelines also address the evidence for effectiveness, and adverse events in the use of several alternative medicines for psoriasis including traditional Chinese medicine, and the herbal therapies aloe vera and St. John’s wort, as well as the potential role of dietary supplements including fish oil, vitamin D, turmeric, and zinc in managing psoriasis, and the potential role of a gluten-free diet.
In general, research on the efficacy, effectiveness, and potential adverse effects of these strategies are limited, according to the guidelines, although many patients express interest in supplements and herbal products. For example, “Many patients ask about the overall role of vitamin D in skin health. Rather than adding oral vitamin D supplementation, topical therapy with vitamin D agents is effective for the treatment of psoriasis,” the authors noted.
In addition, they noted that mind/body strategies, namely hypnosis and stress reduction or meditation techniques, have been shown to improve symptoms and can be helpful for some patients, but clinical evidence is limited.
The guidelines also addressed methods for assessing disease severity in psoriasis. They recommended using body surface area (BSA) to assess psoriasis severity and patient response to treatment in the clinical setting. However, BSA is a provider assessment tool that “does not take into account location on the body, clinical characteristics of the plaques, symptoms, or quality of life issues,” the authors noted. The Psoriasis Area and Severity Index (PASI) measures erythema, induration, and scaling and is more suited to assessing psoriasis severity and response to treatment in clinical trials rather than in practice, they said.
Prior AAD guidelines on psoriasis were published more than 10 years ago, and major developments including the availability of new biologic drugs and new data on comorbidities have been recognized in the past decade, working group cochair and author of the guidelines Alan Menter, MD, said in an interview.
The key game-changers from previous guidelines include the full section published on comorbidities plus the development of two new important cytokine classes: three IL-17 drugs and three new IL-23 drugs now available for moderate to severe psoriasis, said Dr. Menter, chairman of the division of dermatology at Baylor University Medical Center, Dallas.
Barriers to implementing the guidelines in practice may occur when “third party payers make the decision on which of the 11 biologic drugs now approved for moderate to severe psoriasis should be used,” he noted.
As for next steps in psoriasis studies, “new biomarker research is currently underway,” Dr. Menter said. With 11 biologic agents new formally approved by the FDA for moderate to severe psoriasis, the next steps are to determine which drug is likely to be the most appropriate for each individual patient.
Dr. Menter disclosed relationships with multiple companies that develop and manufacture psoriasis therapies, including Abbott Labs, AbbVie, Amgen, Eli Lilly and Company, Galderma USA, Janssen Pharmaceuticals, LEO Pharma US, Menlo Therapeutics, and Novartis. The updated guidelines were designed by a multidisciplinary work group of psoriasis experts including dermatologists, a rheumatologist, a cardiologist, and representatives from a patient advocacy organization.
SOURCE: Elmets CA et al. J Am Acad Dermatol. 2020 Jul 29. doi: 10.1016/j.jaad.2020.07.087.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Postpartum tubal ligation safe in obese women
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
FROM OBSTETRICS & GYNECOLOGY
FDA approves cannabidiol for tuberous sclerosis complex
The cannabidiol (CBD) oral solution Epidiolex has been approved by the Food and Drug Administration for the new indication of treatment of seizures associated with tuberous sclerosis complex in patients 1 year of age and older.
The drug was approved by the FDA in 2018 for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, as reported by Medscape Medical News.
This is the only FDA-approved drug that contains a purified drug substance derived from cannabis. It is also the second FDA approval of a drug for the treatment of seizures associated with tuberous sclerosis complex.
CBD is a chemical component of the cannabis sativa plant, but it does not cause intoxication or euphoria (the “high”) that comes from tetrahydrocannabinol (THC), which is the primary psychoactive component of cannabis.
“The FDA continues to believe the drug approval process represents the best way to make new medicines, including any drugs derived from cannabis, available to patients in need of appropriate medical therapy such as the treatment of seizures associated with these rare conditions,” Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said in an agency press release.
“This paradigm ensures new therapies are safe, effective, and manufactured to a high quality that provides uniform and reliable dosing for patients,” Dr. Throckmorton said.
He added that the FDA is committed to supporting research on the potential medical uses of cannabis-derived products.
Rare genetic disease
Tuberous sclerosis complex is a rare genetic disease that causes benign tumors to grow in the brain and other parts of the body, such as the eyes, heart, kidneys, lungs, and skin.
It usually affects the central nervous system and can result in a combination of symptoms, including seizures, developmental delay, and behavioral problems. The signs and symptoms of the condition, as well as the severity of symptoms, vary widely. The disease affects about 1 in 6,000 individuals.
The effectiveness of Epidiolex in the treatment of seizures associated with tuberous sclerosis complex was established in a randomized, double-blind, placebo-controlled trial in which 148 patients of a total of 224 in the study received the active drug, the FDA noted.
Results showed that for patients treated with CBD, there was a significantly greater reduction in seizure frequency during the treatment period than for patients who received placebo.
This effect was seen within 8 weeks and remained consistent throughout the 16-week treatment period.
The most common side effects that occurred in CBD-treated participants were diarrhea, elevated liver enzyme levels, decreased appetite, sleepiness, fever, and vomiting. Additional side effects that have been reported with the product include liver injury, decreased weight, anemia, and increased creatinine level.
As is true for all drugs that currently treat epilepsy, including Epidiolex, the most serious risks may include an increase in suicidal thoughts and behavior or thoughts of self-harm, the FDA reports.
Patients, their caregivers, and their families should be advised to monitor for any unusual changes in mood or behavior, such as worsening depression or suicidal thoughts or behavior. They should report behaviors of concern immediately to health care providers, the agency notes.
It also points out that Epidiolex can cause liver injury, of which most cases are generally mild. However, there is a risk for rare but more severe liver injury. More severe liver injury can cause nausea, vomiting, abdominal pain, fatigue, anorexia, jaundice, and/or dark urine.
A version of this story originally appeared on Medscape.com.
The cannabidiol (CBD) oral solution Epidiolex has been approved by the Food and Drug Administration for the new indication of treatment of seizures associated with tuberous sclerosis complex in patients 1 year of age and older.
The drug was approved by the FDA in 2018 for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, as reported by Medscape Medical News.
This is the only FDA-approved drug that contains a purified drug substance derived from cannabis. It is also the second FDA approval of a drug for the treatment of seizures associated with tuberous sclerosis complex.
CBD is a chemical component of the cannabis sativa plant, but it does not cause intoxication or euphoria (the “high”) that comes from tetrahydrocannabinol (THC), which is the primary psychoactive component of cannabis.
“The FDA continues to believe the drug approval process represents the best way to make new medicines, including any drugs derived from cannabis, available to patients in need of appropriate medical therapy such as the treatment of seizures associated with these rare conditions,” Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said in an agency press release.
“This paradigm ensures new therapies are safe, effective, and manufactured to a high quality that provides uniform and reliable dosing for patients,” Dr. Throckmorton said.
He added that the FDA is committed to supporting research on the potential medical uses of cannabis-derived products.
Rare genetic disease
Tuberous sclerosis complex is a rare genetic disease that causes benign tumors to grow in the brain and other parts of the body, such as the eyes, heart, kidneys, lungs, and skin.
It usually affects the central nervous system and can result in a combination of symptoms, including seizures, developmental delay, and behavioral problems. The signs and symptoms of the condition, as well as the severity of symptoms, vary widely. The disease affects about 1 in 6,000 individuals.
The effectiveness of Epidiolex in the treatment of seizures associated with tuberous sclerosis complex was established in a randomized, double-blind, placebo-controlled trial in which 148 patients of a total of 224 in the study received the active drug, the FDA noted.
Results showed that for patients treated with CBD, there was a significantly greater reduction in seizure frequency during the treatment period than for patients who received placebo.
This effect was seen within 8 weeks and remained consistent throughout the 16-week treatment period.
The most common side effects that occurred in CBD-treated participants were diarrhea, elevated liver enzyme levels, decreased appetite, sleepiness, fever, and vomiting. Additional side effects that have been reported with the product include liver injury, decreased weight, anemia, and increased creatinine level.
As is true for all drugs that currently treat epilepsy, including Epidiolex, the most serious risks may include an increase in suicidal thoughts and behavior or thoughts of self-harm, the FDA reports.
Patients, their caregivers, and their families should be advised to monitor for any unusual changes in mood or behavior, such as worsening depression or suicidal thoughts or behavior. They should report behaviors of concern immediately to health care providers, the agency notes.
It also points out that Epidiolex can cause liver injury, of which most cases are generally mild. However, there is a risk for rare but more severe liver injury. More severe liver injury can cause nausea, vomiting, abdominal pain, fatigue, anorexia, jaundice, and/or dark urine.
A version of this story originally appeared on Medscape.com.
The cannabidiol (CBD) oral solution Epidiolex has been approved by the Food and Drug Administration for the new indication of treatment of seizures associated with tuberous sclerosis complex in patients 1 year of age and older.
The drug was approved by the FDA in 2018 for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, as reported by Medscape Medical News.
This is the only FDA-approved drug that contains a purified drug substance derived from cannabis. It is also the second FDA approval of a drug for the treatment of seizures associated with tuberous sclerosis complex.
CBD is a chemical component of the cannabis sativa plant, but it does not cause intoxication or euphoria (the “high”) that comes from tetrahydrocannabinol (THC), which is the primary psychoactive component of cannabis.
“The FDA continues to believe the drug approval process represents the best way to make new medicines, including any drugs derived from cannabis, available to patients in need of appropriate medical therapy such as the treatment of seizures associated with these rare conditions,” Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said in an agency press release.
“This paradigm ensures new therapies are safe, effective, and manufactured to a high quality that provides uniform and reliable dosing for patients,” Dr. Throckmorton said.
He added that the FDA is committed to supporting research on the potential medical uses of cannabis-derived products.
Rare genetic disease
Tuberous sclerosis complex is a rare genetic disease that causes benign tumors to grow in the brain and other parts of the body, such as the eyes, heart, kidneys, lungs, and skin.
It usually affects the central nervous system and can result in a combination of symptoms, including seizures, developmental delay, and behavioral problems. The signs and symptoms of the condition, as well as the severity of symptoms, vary widely. The disease affects about 1 in 6,000 individuals.
The effectiveness of Epidiolex in the treatment of seizures associated with tuberous sclerosis complex was established in a randomized, double-blind, placebo-controlled trial in which 148 patients of a total of 224 in the study received the active drug, the FDA noted.
Results showed that for patients treated with CBD, there was a significantly greater reduction in seizure frequency during the treatment period than for patients who received placebo.
This effect was seen within 8 weeks and remained consistent throughout the 16-week treatment period.
The most common side effects that occurred in CBD-treated participants were diarrhea, elevated liver enzyme levels, decreased appetite, sleepiness, fever, and vomiting. Additional side effects that have been reported with the product include liver injury, decreased weight, anemia, and increased creatinine level.
As is true for all drugs that currently treat epilepsy, including Epidiolex, the most serious risks may include an increase in suicidal thoughts and behavior or thoughts of self-harm, the FDA reports.
Patients, their caregivers, and their families should be advised to monitor for any unusual changes in mood or behavior, such as worsening depression or suicidal thoughts or behavior. They should report behaviors of concern immediately to health care providers, the agency notes.
It also points out that Epidiolex can cause liver injury, of which most cases are generally mild. However, there is a risk for rare but more severe liver injury. More severe liver injury can cause nausea, vomiting, abdominal pain, fatigue, anorexia, jaundice, and/or dark urine.
A version of this story originally appeared on Medscape.com.
Biologics may delay psoriatic arthritis, study finds
(DMARDs), in a single center retrospective analysis in Argentina that followed patients for almost 2 decades.
About 30%-40% of patients with psoriasis go on to develop psoriatic arthritis (PsA), usually on average about 10 years after the onset of psoriasis. One potential mechanism of PsA onset is through enthesitis, which has been described at subclinical levels in psoriasis.
“It could be speculated that treatment with biologics in patients with psoriasis could prevent the development of psoriatic arthritis, perhaps by inhibiting the subclinical development of enthesitis,” Luciano Lo Giudice, MD, a rheumatology fellow at Hospital Italiano de Buenos Aires, said during his presentation at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Although these results do not prove that treatment of the underlying disease delays progression to PsA, it is suggestive, and highlights an emerging field of research, according to Diamant Thaçi, MD, PhD, professor of medicine at University Hospital Schleswig-Holstein, Germany, who led a live discussion following a prerecorded presentation of the results. “We’re going in this direction – how can we prevent psoriatic arthritis, how can we delay it. We are just starting to think about this,” Dr. Thaçi said in an interview.
The researchers examined medical records of 1,626 patients with psoriasis treated at their center between 2000 and 2019, with a total of 15,152 years of follow-up. Of these patients, 1,293 were treated with topical medication, 229 with conventional DMARDs (methotrexate in 77%, cyclosporine in 13%, and both in 10%), and 104 with biologics, including etanercept (34%), secukinumab (20%), adalimumab (20%), ustekinumab (12%), ixekizumab (9%), and infliximab (5%).
They found that 11% in the topical treatment group developed PsA, as did 3.5% in the conventional DMARD group, 1.9% in the biologics group, and 9.1% overall. Treatment with biologics was associated with a significantly lower odds of developing PsA compared with treatment with conventional DMARDs (3 versus 17.2 per 1,000 patient-years; incidence rate ratio [IRR], 0.17; P = .0177). There was a trend toward reduced odds of developing PsA among those on biologic therapy compared with those on topicals (3 versus 9.8 per 1,000 patient-years; IRR, 0.3; P = .0588).
The researchers confirmed all medical encounters using electronic medical records and the study had a long follow-up time, but was limited by the single center and its retrospective nature. It also could not associate reduced risk with specific biologics.
The findings probably reflect the presence of subclinical PsA that many clinicians don’t see, according to Dr. Thaçi. While a dermatology practice might find PsA in 2% or 3%, or at most, 10% of patients with psoriasis, “in our department it’s about 50 to 60 percent of patients who have psoriatic arthritis, because we diagnose it early,” he said.
He found the results of the study encouraging. “It looks like some of the biologics, for example IL [interleukin]-17 or even IL-23 [blockers] may have an influence on occurrence or delay the occurrence of psoriatic arthritis.”
Dr. Thaçi noted that early treatment of skin lesions can increase the probability of longer remissions, especially with IL-23 blockers. Still, that’s no guarantee the same would hold true for PsA risk. “Skin is skin and joints are joints,” Dr. Thaçi said.
Dr. Thaçi and Dr. Lo Giudice had no relevant financial disclosures.
(DMARDs), in a single center retrospective analysis in Argentina that followed patients for almost 2 decades.
About 30%-40% of patients with psoriasis go on to develop psoriatic arthritis (PsA), usually on average about 10 years after the onset of psoriasis. One potential mechanism of PsA onset is through enthesitis, which has been described at subclinical levels in psoriasis.
“It could be speculated that treatment with biologics in patients with psoriasis could prevent the development of psoriatic arthritis, perhaps by inhibiting the subclinical development of enthesitis,” Luciano Lo Giudice, MD, a rheumatology fellow at Hospital Italiano de Buenos Aires, said during his presentation at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Although these results do not prove that treatment of the underlying disease delays progression to PsA, it is suggestive, and highlights an emerging field of research, according to Diamant Thaçi, MD, PhD, professor of medicine at University Hospital Schleswig-Holstein, Germany, who led a live discussion following a prerecorded presentation of the results. “We’re going in this direction – how can we prevent psoriatic arthritis, how can we delay it. We are just starting to think about this,” Dr. Thaçi said in an interview.
The researchers examined medical records of 1,626 patients with psoriasis treated at their center between 2000 and 2019, with a total of 15,152 years of follow-up. Of these patients, 1,293 were treated with topical medication, 229 with conventional DMARDs (methotrexate in 77%, cyclosporine in 13%, and both in 10%), and 104 with biologics, including etanercept (34%), secukinumab (20%), adalimumab (20%), ustekinumab (12%), ixekizumab (9%), and infliximab (5%).
They found that 11% in the topical treatment group developed PsA, as did 3.5% in the conventional DMARD group, 1.9% in the biologics group, and 9.1% overall. Treatment with biologics was associated with a significantly lower odds of developing PsA compared with treatment with conventional DMARDs (3 versus 17.2 per 1,000 patient-years; incidence rate ratio [IRR], 0.17; P = .0177). There was a trend toward reduced odds of developing PsA among those on biologic therapy compared with those on topicals (3 versus 9.8 per 1,000 patient-years; IRR, 0.3; P = .0588).
The researchers confirmed all medical encounters using electronic medical records and the study had a long follow-up time, but was limited by the single center and its retrospective nature. It also could not associate reduced risk with specific biologics.
The findings probably reflect the presence of subclinical PsA that many clinicians don’t see, according to Dr. Thaçi. While a dermatology practice might find PsA in 2% or 3%, or at most, 10% of patients with psoriasis, “in our department it’s about 50 to 60 percent of patients who have psoriatic arthritis, because we diagnose it early,” he said.
He found the results of the study encouraging. “It looks like some of the biologics, for example IL [interleukin]-17 or even IL-23 [blockers] may have an influence on occurrence or delay the occurrence of psoriatic arthritis.”
Dr. Thaçi noted that early treatment of skin lesions can increase the probability of longer remissions, especially with IL-23 blockers. Still, that’s no guarantee the same would hold true for PsA risk. “Skin is skin and joints are joints,” Dr. Thaçi said.
Dr. Thaçi and Dr. Lo Giudice had no relevant financial disclosures.
(DMARDs), in a single center retrospective analysis in Argentina that followed patients for almost 2 decades.
About 30%-40% of patients with psoriasis go on to develop psoriatic arthritis (PsA), usually on average about 10 years after the onset of psoriasis. One potential mechanism of PsA onset is through enthesitis, which has been described at subclinical levels in psoriasis.
“It could be speculated that treatment with biologics in patients with psoriasis could prevent the development of psoriatic arthritis, perhaps by inhibiting the subclinical development of enthesitis,” Luciano Lo Giudice, MD, a rheumatology fellow at Hospital Italiano de Buenos Aires, said during his presentation at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Although these results do not prove that treatment of the underlying disease delays progression to PsA, it is suggestive, and highlights an emerging field of research, according to Diamant Thaçi, MD, PhD, professor of medicine at University Hospital Schleswig-Holstein, Germany, who led a live discussion following a prerecorded presentation of the results. “We’re going in this direction – how can we prevent psoriatic arthritis, how can we delay it. We are just starting to think about this,” Dr. Thaçi said in an interview.
The researchers examined medical records of 1,626 patients with psoriasis treated at their center between 2000 and 2019, with a total of 15,152 years of follow-up. Of these patients, 1,293 were treated with topical medication, 229 with conventional DMARDs (methotrexate in 77%, cyclosporine in 13%, and both in 10%), and 104 with biologics, including etanercept (34%), secukinumab (20%), adalimumab (20%), ustekinumab (12%), ixekizumab (9%), and infliximab (5%).
They found that 11% in the topical treatment group developed PsA, as did 3.5% in the conventional DMARD group, 1.9% in the biologics group, and 9.1% overall. Treatment with biologics was associated with a significantly lower odds of developing PsA compared with treatment with conventional DMARDs (3 versus 17.2 per 1,000 patient-years; incidence rate ratio [IRR], 0.17; P = .0177). There was a trend toward reduced odds of developing PsA among those on biologic therapy compared with those on topicals (3 versus 9.8 per 1,000 patient-years; IRR, 0.3; P = .0588).
The researchers confirmed all medical encounters using electronic medical records and the study had a long follow-up time, but was limited by the single center and its retrospective nature. It also could not associate reduced risk with specific biologics.
The findings probably reflect the presence of subclinical PsA that many clinicians don’t see, according to Dr. Thaçi. While a dermatology practice might find PsA in 2% or 3%, or at most, 10% of patients with psoriasis, “in our department it’s about 50 to 60 percent of patients who have psoriatic arthritis, because we diagnose it early,” he said.
He found the results of the study encouraging. “It looks like some of the biologics, for example IL [interleukin]-17 or even IL-23 [blockers] may have an influence on occurrence or delay the occurrence of psoriatic arthritis.”
Dr. Thaçi noted that early treatment of skin lesions can increase the probability of longer remissions, especially with IL-23 blockers. Still, that’s no guarantee the same would hold true for PsA risk. “Skin is skin and joints are joints,” Dr. Thaçi said.
Dr. Thaçi and Dr. Lo Giudice had no relevant financial disclosures.
FROM GRAPPA 2020 VIRTUAL ANNUAL MEETING
Addiction specialist charged in $681 million treatment fraud case
The federal government has charged a Florida addiction medicine specialist in what it says was a scheme to defraud Medicare and private insurers, charging them roughly $681 million over about a decade for lab tests, office visits, therapy sessions, and other services that were either unnecessary or never delivered.
The Department of Justice and the Attorney for the Southern District of Florida are prosecuting Michael Ligotti, DO, 46, saying that he preyed on individuals seeking substance abuse treatment. They have yet to issue a formal indictment.
“The substance abuse treatment fraud allegedly perpetrated by the defendant sacrificed the genuine care of vulnerable patients at a time when they urgently needed a trusted health care provider,” U.S. Attorney Ariana Fajardo Orshan, Southern District of Florida, said in a statement.
“Health care providers who allow greed to take precedence over their Hippocratic Oath and participate in these schemes are criminals and will be held accountable for their unscrupulous conduct,” she added.
Dr. Ligotti was charged July 31. The prosecutors were seeking to detain him until trial. However, a judge approved his bond today and he is free on bond, according to Ligotti’s attorney, Ben Curtis.
“As is always the case with any criminal matter, the burden of proof rests entirely with the government,” Mr. Curtis said in an interview.
“In this instance, we do not believe the US Department of Justice’s claims – and that is exactly what they are at this point, just one-sided claims – will reconcile with actual evidence at a future trial,” he said.
Mr. Curtis added that Dr. Ligotti “looks forward to establishing his innocence.”
Unnecessary urine tests
The government alleges that Dr. Ligotti played a central role in a scheme in which Medicare and private insurers paid about $121 million to cover some $680 million in charges from 2011 to 2020.
According to the prosecutors, Dr. Ligotti received a fee for becoming a “purported” medical director of about 50 addiction treatment facilities and sober homes – and that he issued 136 separate standing orders for medically unnecessary urinalysis (UA) tests.
The labs allegedly paid occasional kickbacks to the facilities and homes, and those facilities in turn were required to have their patients treated by Dr. Ligotti’s clinic, Whole Health, which is based in Delray Beach, Fla.
This allowed Dr. Ligotti to “bill hundreds of millions of dollars in additional fraudulent treatments, including unnecessary and expensive UAs, costly blood tests, nonexistent therapy sessions, office visits, and other unnecessary services, regardless of whether such treatment and testing were medically necessary and/or actually provided,” alleges the government.
Urine tests have been exploited before by addiction treatment clinics as a revenue generator. Kaiser Health News reported in 2017 that a single nurse practitioner at one pain clinic in Tennessee generated $1 million in billings to Medicare for drug-related urine tests in a single year.
Also in 2017, The New York Times reported that a single patient had been billed $260,000 for urine tests by his treatment center.
The federal government alleges that Dr. Ligotti also “billed for psychiatric services and therapy sessions that never happened, and that he and his staff were not qualified to conduct.”
In addition, they assert that Dr. Ligotti improperly prescribed controlled substances, including large quantities of buprenorphine/Suboxone, often exceeding the number of patients he was legally authorized to treat or giving it to patients who did not require the medications.
Dr. Ligotti may not be practicing any longer. His clinic’s website features a message that the practice will be closed as of Aug. 7.
This article first appeared on Medscape.com.
The federal government has charged a Florida addiction medicine specialist in what it says was a scheme to defraud Medicare and private insurers, charging them roughly $681 million over about a decade for lab tests, office visits, therapy sessions, and other services that were either unnecessary or never delivered.
The Department of Justice and the Attorney for the Southern District of Florida are prosecuting Michael Ligotti, DO, 46, saying that he preyed on individuals seeking substance abuse treatment. They have yet to issue a formal indictment.
“The substance abuse treatment fraud allegedly perpetrated by the defendant sacrificed the genuine care of vulnerable patients at a time when they urgently needed a trusted health care provider,” U.S. Attorney Ariana Fajardo Orshan, Southern District of Florida, said in a statement.
“Health care providers who allow greed to take precedence over their Hippocratic Oath and participate in these schemes are criminals and will be held accountable for their unscrupulous conduct,” she added.
Dr. Ligotti was charged July 31. The prosecutors were seeking to detain him until trial. However, a judge approved his bond today and he is free on bond, according to Ligotti’s attorney, Ben Curtis.
“As is always the case with any criminal matter, the burden of proof rests entirely with the government,” Mr. Curtis said in an interview.
“In this instance, we do not believe the US Department of Justice’s claims – and that is exactly what they are at this point, just one-sided claims – will reconcile with actual evidence at a future trial,” he said.
Mr. Curtis added that Dr. Ligotti “looks forward to establishing his innocence.”
Unnecessary urine tests
The government alleges that Dr. Ligotti played a central role in a scheme in which Medicare and private insurers paid about $121 million to cover some $680 million in charges from 2011 to 2020.
According to the prosecutors, Dr. Ligotti received a fee for becoming a “purported” medical director of about 50 addiction treatment facilities and sober homes – and that he issued 136 separate standing orders for medically unnecessary urinalysis (UA) tests.
The labs allegedly paid occasional kickbacks to the facilities and homes, and those facilities in turn were required to have their patients treated by Dr. Ligotti’s clinic, Whole Health, which is based in Delray Beach, Fla.
This allowed Dr. Ligotti to “bill hundreds of millions of dollars in additional fraudulent treatments, including unnecessary and expensive UAs, costly blood tests, nonexistent therapy sessions, office visits, and other unnecessary services, regardless of whether such treatment and testing were medically necessary and/or actually provided,” alleges the government.
Urine tests have been exploited before by addiction treatment clinics as a revenue generator. Kaiser Health News reported in 2017 that a single nurse practitioner at one pain clinic in Tennessee generated $1 million in billings to Medicare for drug-related urine tests in a single year.
Also in 2017, The New York Times reported that a single patient had been billed $260,000 for urine tests by his treatment center.
The federal government alleges that Dr. Ligotti also “billed for psychiatric services and therapy sessions that never happened, and that he and his staff were not qualified to conduct.”
In addition, they assert that Dr. Ligotti improperly prescribed controlled substances, including large quantities of buprenorphine/Suboxone, often exceeding the number of patients he was legally authorized to treat or giving it to patients who did not require the medications.
Dr. Ligotti may not be practicing any longer. His clinic’s website features a message that the practice will be closed as of Aug. 7.
This article first appeared on Medscape.com.
The federal government has charged a Florida addiction medicine specialist in what it says was a scheme to defraud Medicare and private insurers, charging them roughly $681 million over about a decade for lab tests, office visits, therapy sessions, and other services that were either unnecessary or never delivered.
The Department of Justice and the Attorney for the Southern District of Florida are prosecuting Michael Ligotti, DO, 46, saying that he preyed on individuals seeking substance abuse treatment. They have yet to issue a formal indictment.
“The substance abuse treatment fraud allegedly perpetrated by the defendant sacrificed the genuine care of vulnerable patients at a time when they urgently needed a trusted health care provider,” U.S. Attorney Ariana Fajardo Orshan, Southern District of Florida, said in a statement.
“Health care providers who allow greed to take precedence over their Hippocratic Oath and participate in these schemes are criminals and will be held accountable for their unscrupulous conduct,” she added.
Dr. Ligotti was charged July 31. The prosecutors were seeking to detain him until trial. However, a judge approved his bond today and he is free on bond, according to Ligotti’s attorney, Ben Curtis.
“As is always the case with any criminal matter, the burden of proof rests entirely with the government,” Mr. Curtis said in an interview.
“In this instance, we do not believe the US Department of Justice’s claims – and that is exactly what they are at this point, just one-sided claims – will reconcile with actual evidence at a future trial,” he said.
Mr. Curtis added that Dr. Ligotti “looks forward to establishing his innocence.”
Unnecessary urine tests
The government alleges that Dr. Ligotti played a central role in a scheme in which Medicare and private insurers paid about $121 million to cover some $680 million in charges from 2011 to 2020.
According to the prosecutors, Dr. Ligotti received a fee for becoming a “purported” medical director of about 50 addiction treatment facilities and sober homes – and that he issued 136 separate standing orders for medically unnecessary urinalysis (UA) tests.
The labs allegedly paid occasional kickbacks to the facilities and homes, and those facilities in turn were required to have their patients treated by Dr. Ligotti’s clinic, Whole Health, which is based in Delray Beach, Fla.
This allowed Dr. Ligotti to “bill hundreds of millions of dollars in additional fraudulent treatments, including unnecessary and expensive UAs, costly blood tests, nonexistent therapy sessions, office visits, and other unnecessary services, regardless of whether such treatment and testing were medically necessary and/or actually provided,” alleges the government.
Urine tests have been exploited before by addiction treatment clinics as a revenue generator. Kaiser Health News reported in 2017 that a single nurse practitioner at one pain clinic in Tennessee generated $1 million in billings to Medicare for drug-related urine tests in a single year.
Also in 2017, The New York Times reported that a single patient had been billed $260,000 for urine tests by his treatment center.
The federal government alleges that Dr. Ligotti also “billed for psychiatric services and therapy sessions that never happened, and that he and his staff were not qualified to conduct.”
In addition, they assert that Dr. Ligotti improperly prescribed controlled substances, including large quantities of buprenorphine/Suboxone, often exceeding the number of patients he was legally authorized to treat or giving it to patients who did not require the medications.
Dr. Ligotti may not be practicing any longer. His clinic’s website features a message that the practice will be closed as of Aug. 7.
This article first appeared on Medscape.com.
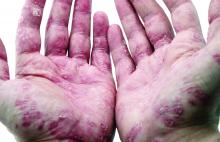
Painful Hemorrhagic Erosions
The Diagnosis: Kaposi Varicelliform Eruption (Eczema Herpeticum)
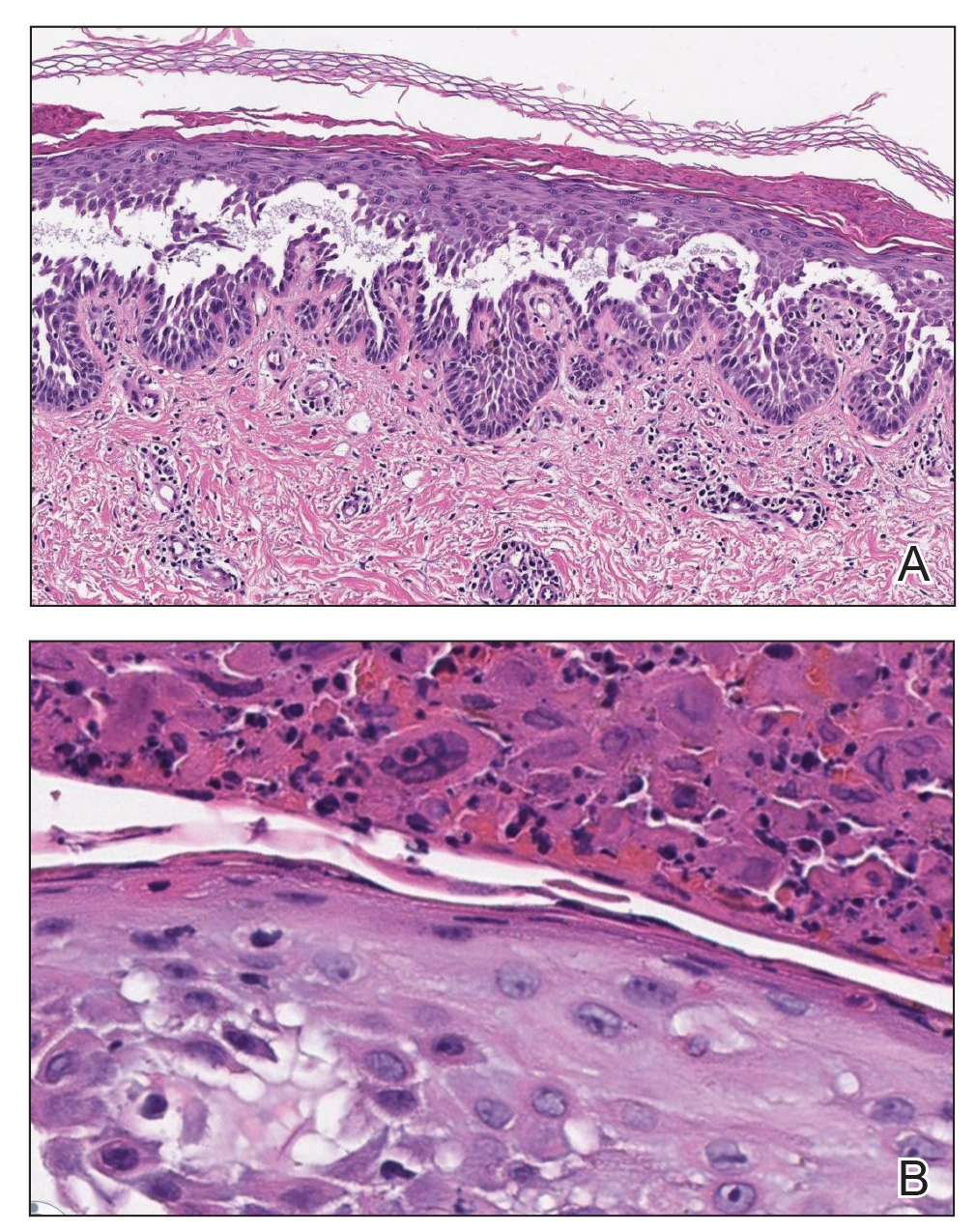
Polymerase chain reaction confirmed presence of herpes simplex virus (HSV) type 1, and the patient was started on intravenous acyclovir (10 mg/kg every 8 hours). Diagnosis was further supported by histopathologic examination with confirmatory immunohistochemistry (Figure 1). The patient's anemia and thrombocytopenia also were attributed to widespread HSV infection.
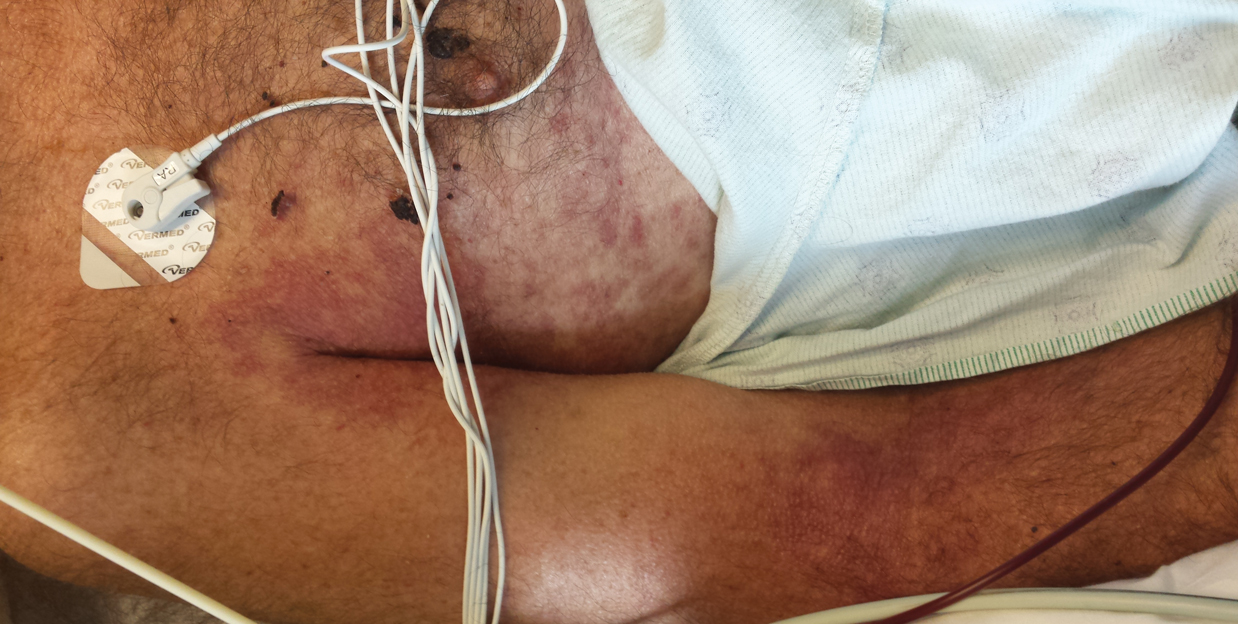
Approximately 8 hours after the patient was started on acyclovir, he developed increasing tremors, confusion, and impaired speech. Lumbar puncture confirmed the presence of HSV-1 in the cerebrospinal fluid. Despite ongoing intravenous antiviral therapy, he required intubation 6 days after hospitalization due to impaired mental status and myoclonic jerking. He remained intubated, unresponsive, and in critical condition for 9 days before he gradually began to demonstrate cognitive recovery. He subsequently was weaned off the ventilator, his mental status returned to normal, and his skin rash slowly resolved (Figure 2).
Hailey-Hailey disease (HHD), also known as familial benign chronic pemphigus, is a rare autosomal-dominant condition first described by Howard and Hugh Hailey in 1939.1 It is a chronic blistering process characterized by epidermal fragility, often manifesting as macerated fissured erosions in areas exposed to heat and friction (eg, axillae, groin). Hailey-Hailey disease results from a defective calcium transporter (ATP2C1 gene), leading to impaired keratinocyte adhesion.2
Eczema herpeticum refers to the dissemination of herpes infection to areas of compromised skin barrier. Although originally used to describe HSV infection in patients with atopic dermatitis, eczema herpeticum has been described in various conditions that affect the skin barrier function, including Darier disease, ichthyosis vulgaris, pemphigus foliaceus, pemphigus vulgaris, and mycosis fungoides, among others.3 When applied to skin conditions other than atopic dermatitis, it sometimes is referred to as Kaposi varicelliform eruption.2
Hailey-Hailey disease commonly is complicated by a bacterial or fungal infection, including impetigo, tinea, or candidiasis. The first case of HHD complicated by HSV infection was reported in 1973.4 A PubMed search of articles indexed for MEDLINE using the terms benign familial pemphigus AND herpes, Hailey-Hailey AND herpes, Hailey-Hailey AND eczema herpeticum, Hailey-Hailey AND Kaposi varicelliform eruption, and Hailey-Hailey herpeticum revealed 15 cases of HHD complicated by eczema herpeticum.4-6 Herpes simplex virus encephalitis is a rare and life-threatening complication of eczema herpeticum.7,8 We report a case of HSV encephalitis resulting from eczema herpeticum in a patient with HHD.
The clinical differential includes a flare of the patient's known HHD, secondary bacterial or fungal infection, or a superimposed viral infection (eg, HSV, zoster). Histologic evidence of herpetic infection would be absent in an uncomplicated flare of HHD. Impetigo is a superficial bacterial infection that can present in 2 clinical forms: a vesiculopustular type and less commonly a bullous type. It is caused by Staphylococcus aureus in most cases. In multiple myeloma with cutaneous dissemination, a monoclonal proliferation of plasma cells would be evident. Lastly, tinea corporis is caused by dermatophytes that can be seen on hematoxylin and eosin or periodic acid-Schiff staining.
The diagnosis of eczema herpeticum in a patient with HHD should be considered in patients who present with grouped vesicles or hemorrhagic or punched-out erosions in areas of pre-existing HHD. The diagnosis can be confirmed by Tzanck smear, viral culture, polymerase chain reaction, or histopathology (with or without immunohistochemistry).1,2,6 When eczema herpeticum is suspected, prompt antiviral administration is imperative to limit life-threatening systemic spread.
- Hailey J, Hailey H. Familial benign chronic pemphigus. Arch Dermatol. 1939;39:679-685.
- de Aquino Paulo Filho T, deFreitas YK, da Nóbrega MT, et al. Hailey-Hailey disease associated with herpetic eczema-the value of the Tzanck smear test. Dermatol Pract Concept. 2014;4:29-31.
- Flint ID, Spencer DM, Wilkin JK. Eczema herpeticum in association with familial benign chronic pemphigus. J Am Acad Dermatol. 1993;28(2, pt 1):257-259.
- Leppard B, Delaney TJ, Sanderson KV. Chronic benign familial pemphigus. induction of lesions by Herpesvirus hominis. Br J Dermatol. 1973;88:609-613.
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314.
- Zamperetti M, Pichler M, Perino F, et al. Ein fall von morbus Hailey-Hailey in verbindung mit einem eczema herpeticatum. J Dtsch Dermatol Ges. 2016;14:1035-1038.
- Ingrand D, Briquet I, Babinet JM, et al. Eczema herpeticum of the child. an unusual manifestation of herpes simplex virus infection. Clin Pediatr (Phila). 1985;24:660-663.
- Finlow C, Thomas J. Disseminated herpes simplex virus: a case of eczema herpeticum causing viral encephalitis. J R Coll Physicians Edinb. 2018;48:36-39.
The Diagnosis: Kaposi Varicelliform Eruption (Eczema Herpeticum)
Polymerase chain reaction confirmed presence of herpes simplex virus (HSV) type 1, and the patient was started on intravenous acyclovir (10 mg/kg every 8 hours). Diagnosis was further supported by histopathologic examination with confirmatory immunohistochemistry (Figure 1). The patient's anemia and thrombocytopenia also were attributed to widespread HSV infection.

Approximately 8 hours after the patient was started on acyclovir, he developed increasing tremors, confusion, and impaired speech. Lumbar puncture confirmed the presence of HSV-1 in the cerebrospinal fluid. Despite ongoing intravenous antiviral therapy, he required intubation 6 days after hospitalization due to impaired mental status and myoclonic jerking. He remained intubated, unresponsive, and in critical condition for 9 days before he gradually began to demonstrate cognitive recovery. He subsequently was weaned off the ventilator, his mental status returned to normal, and his skin rash slowly resolved (Figure 2).

Hailey-Hailey disease (HHD), also known as familial benign chronic pemphigus, is a rare autosomal-dominant condition first described by Howard and Hugh Hailey in 1939.1 It is a chronic blistering process characterized by epidermal fragility, often manifesting as macerated fissured erosions in areas exposed to heat and friction (eg, axillae, groin). Hailey-Hailey disease results from a defective calcium transporter (ATP2C1 gene), leading to impaired keratinocyte adhesion.2
Eczema herpeticum refers to the dissemination of herpes infection to areas of compromised skin barrier. Although originally used to describe HSV infection in patients with atopic dermatitis, eczema herpeticum has been described in various conditions that affect the skin barrier function, including Darier disease, ichthyosis vulgaris, pemphigus foliaceus, pemphigus vulgaris, and mycosis fungoides, among others.3 When applied to skin conditions other than atopic dermatitis, it sometimes is referred to as Kaposi varicelliform eruption.2
Hailey-Hailey disease commonly is complicated by a bacterial or fungal infection, including impetigo, tinea, or candidiasis. The first case of HHD complicated by HSV infection was reported in 1973.4 A PubMed search of articles indexed for MEDLINE using the terms benign familial pemphigus AND herpes, Hailey-Hailey AND herpes, Hailey-Hailey AND eczema herpeticum, Hailey-Hailey AND Kaposi varicelliform eruption, and Hailey-Hailey herpeticum revealed 15 cases of HHD complicated by eczema herpeticum.4-6 Herpes simplex virus encephalitis is a rare and life-threatening complication of eczema herpeticum.7,8 We report a case of HSV encephalitis resulting from eczema herpeticum in a patient with HHD.
The clinical differential includes a flare of the patient's known HHD, secondary bacterial or fungal infection, or a superimposed viral infection (eg, HSV, zoster). Histologic evidence of herpetic infection would be absent in an uncomplicated flare of HHD. Impetigo is a superficial bacterial infection that can present in 2 clinical forms: a vesiculopustular type and less commonly a bullous type. It is caused by Staphylococcus aureus in most cases. In multiple myeloma with cutaneous dissemination, a monoclonal proliferation of plasma cells would be evident. Lastly, tinea corporis is caused by dermatophytes that can be seen on hematoxylin and eosin or periodic acid-Schiff staining.
The diagnosis of eczema herpeticum in a patient with HHD should be considered in patients who present with grouped vesicles or hemorrhagic or punched-out erosions in areas of pre-existing HHD. The diagnosis can be confirmed by Tzanck smear, viral culture, polymerase chain reaction, or histopathology (with or without immunohistochemistry).1,2,6 When eczema herpeticum is suspected, prompt antiviral administration is imperative to limit life-threatening systemic spread.
The Diagnosis: Kaposi Varicelliform Eruption (Eczema Herpeticum)
Polymerase chain reaction confirmed presence of herpes simplex virus (HSV) type 1, and the patient was started on intravenous acyclovir (10 mg/kg every 8 hours). Diagnosis was further supported by histopathologic examination with confirmatory immunohistochemistry (Figure 1). The patient's anemia and thrombocytopenia also were attributed to widespread HSV infection.

Approximately 8 hours after the patient was started on acyclovir, he developed increasing tremors, confusion, and impaired speech. Lumbar puncture confirmed the presence of HSV-1 in the cerebrospinal fluid. Despite ongoing intravenous antiviral therapy, he required intubation 6 days after hospitalization due to impaired mental status and myoclonic jerking. He remained intubated, unresponsive, and in critical condition for 9 days before he gradually began to demonstrate cognitive recovery. He subsequently was weaned off the ventilator, his mental status returned to normal, and his skin rash slowly resolved (Figure 2).

Hailey-Hailey disease (HHD), also known as familial benign chronic pemphigus, is a rare autosomal-dominant condition first described by Howard and Hugh Hailey in 1939.1 It is a chronic blistering process characterized by epidermal fragility, often manifesting as macerated fissured erosions in areas exposed to heat and friction (eg, axillae, groin). Hailey-Hailey disease results from a defective calcium transporter (ATP2C1 gene), leading to impaired keratinocyte adhesion.2
Eczema herpeticum refers to the dissemination of herpes infection to areas of compromised skin barrier. Although originally used to describe HSV infection in patients with atopic dermatitis, eczema herpeticum has been described in various conditions that affect the skin barrier function, including Darier disease, ichthyosis vulgaris, pemphigus foliaceus, pemphigus vulgaris, and mycosis fungoides, among others.3 When applied to skin conditions other than atopic dermatitis, it sometimes is referred to as Kaposi varicelliform eruption.2
Hailey-Hailey disease commonly is complicated by a bacterial or fungal infection, including impetigo, tinea, or candidiasis. The first case of HHD complicated by HSV infection was reported in 1973.4 A PubMed search of articles indexed for MEDLINE using the terms benign familial pemphigus AND herpes, Hailey-Hailey AND herpes, Hailey-Hailey AND eczema herpeticum, Hailey-Hailey AND Kaposi varicelliform eruption, and Hailey-Hailey herpeticum revealed 15 cases of HHD complicated by eczema herpeticum.4-6 Herpes simplex virus encephalitis is a rare and life-threatening complication of eczema herpeticum.7,8 We report a case of HSV encephalitis resulting from eczema herpeticum in a patient with HHD.
The clinical differential includes a flare of the patient's known HHD, secondary bacterial or fungal infection, or a superimposed viral infection (eg, HSV, zoster). Histologic evidence of herpetic infection would be absent in an uncomplicated flare of HHD. Impetigo is a superficial bacterial infection that can present in 2 clinical forms: a vesiculopustular type and less commonly a bullous type. It is caused by Staphylococcus aureus in most cases. In multiple myeloma with cutaneous dissemination, a monoclonal proliferation of plasma cells would be evident. Lastly, tinea corporis is caused by dermatophytes that can be seen on hematoxylin and eosin or periodic acid-Schiff staining.
The diagnosis of eczema herpeticum in a patient with HHD should be considered in patients who present with grouped vesicles or hemorrhagic or punched-out erosions in areas of pre-existing HHD. The diagnosis can be confirmed by Tzanck smear, viral culture, polymerase chain reaction, or histopathology (with or without immunohistochemistry).1,2,6 When eczema herpeticum is suspected, prompt antiviral administration is imperative to limit life-threatening systemic spread.
- Hailey J, Hailey H. Familial benign chronic pemphigus. Arch Dermatol. 1939;39:679-685.
- de Aquino Paulo Filho T, deFreitas YK, da Nóbrega MT, et al. Hailey-Hailey disease associated with herpetic eczema-the value of the Tzanck smear test. Dermatol Pract Concept. 2014;4:29-31.
- Flint ID, Spencer DM, Wilkin JK. Eczema herpeticum in association with familial benign chronic pemphigus. J Am Acad Dermatol. 1993;28(2, pt 1):257-259.
- Leppard B, Delaney TJ, Sanderson KV. Chronic benign familial pemphigus. induction of lesions by Herpesvirus hominis. Br J Dermatol. 1973;88:609-613.
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314.
- Zamperetti M, Pichler M, Perino F, et al. Ein fall von morbus Hailey-Hailey in verbindung mit einem eczema herpeticatum. J Dtsch Dermatol Ges. 2016;14:1035-1038.
- Ingrand D, Briquet I, Babinet JM, et al. Eczema herpeticum of the child. an unusual manifestation of herpes simplex virus infection. Clin Pediatr (Phila). 1985;24:660-663.
- Finlow C, Thomas J. Disseminated herpes simplex virus: a case of eczema herpeticum causing viral encephalitis. J R Coll Physicians Edinb. 2018;48:36-39.
- Hailey J, Hailey H. Familial benign chronic pemphigus. Arch Dermatol. 1939;39:679-685.
- de Aquino Paulo Filho T, deFreitas YK, da Nóbrega MT, et al. Hailey-Hailey disease associated with herpetic eczema-the value of the Tzanck smear test. Dermatol Pract Concept. 2014;4:29-31.
- Flint ID, Spencer DM, Wilkin JK. Eczema herpeticum in association with familial benign chronic pemphigus. J Am Acad Dermatol. 1993;28(2, pt 1):257-259.
- Leppard B, Delaney TJ, Sanderson KV. Chronic benign familial pemphigus. induction of lesions by Herpesvirus hominis. Br J Dermatol. 1973;88:609-613.
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314.
- Zamperetti M, Pichler M, Perino F, et al. Ein fall von morbus Hailey-Hailey in verbindung mit einem eczema herpeticatum. J Dtsch Dermatol Ges. 2016;14:1035-1038.
- Ingrand D, Briquet I, Babinet JM, et al. Eczema herpeticum of the child. an unusual manifestation of herpes simplex virus infection. Clin Pediatr (Phila). 1985;24:660-663.
- Finlow C, Thomas J. Disseminated herpes simplex virus: a case of eczema herpeticum causing viral encephalitis. J R Coll Physicians Edinb. 2018;48:36-39.
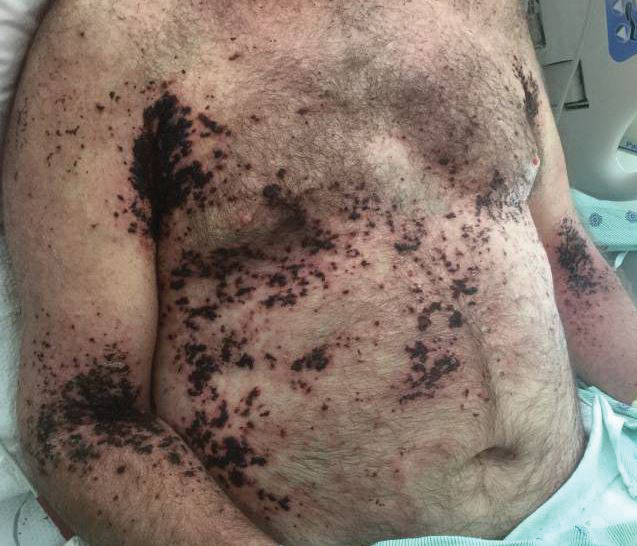
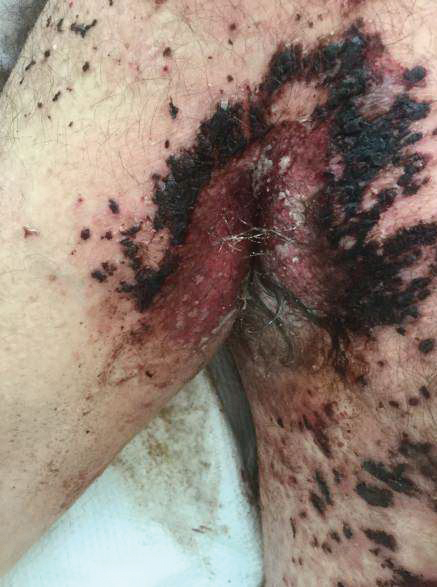
A 62-year-old man with a long-standing history (>40 years) of Hailey-Hailey disease was admitted from an outside hospital due to anemia (hemoglobin, 8.6 g/dL [reference range, 14.0–17.5 g/dL]), thrombocytopenia (platelets, 7×103 /µL [reference range, 150–350×103 /µL]), and worsening skin rash. The patient reported that his Hailey-Hailey disease worsened abruptly 1 month prior to admission and had progressed steadily since then. He described the rash as painful, especially with movement. Over the preceding month, he had been treated with topical triamcinolone, topical diphenhydramine, oral prednisone, fluconazole, and oral clindamycin, all without improvement. The skin lesions continued to worsen and persistently bled; he then presented to our institution for further care.
Physical examination demonstrated widespread shallow erosions with hemorrhagic drainage and crusting located on the lower back, chest, abdomen (top), axillae (bottom), groin, arms, and legs. No vesicles or pustules were noted. The patient had no cognitive dysfunction or focal neurologic deficits. A punch biopsy was performed.
Web-based fellowship interviews in the era of COVID 19: Tips and tricks
Fellowship interviews are an essential step – arguably the most important step – in the process of matching candidates to training programs. Until recently, most programs relied exclusively on on-site face-to face interviews. Since the appearance of the COVID-19 pandemic, the medical field has utilized web-based platforms. Despite inherent limitations, virtual meetings appear to be effective in providing patient care and in conducting administrative meetings.1,2
Because of uncertainty related to the pandemic, including changing guidelines regarding social distancing and travel restrictions, fellowship programs are expected to comply with CDC,3 state, and federal recommendations to avoid nonessential travel. Therefore, conducting web-based interviews exclusively will likely become a necessity.
While there may be some disadvantages to web-based interviews, many candidates find the overall experience satisfactory, thereby allowing them to understand the programs, express themselves, and comfortably rank the programs, as two studies have shown.4,5 Programs and candidates are encouraged to adapt to this new reality in order to achieve a successful match. After all, there are many potential advantages of web-based interviews. In addition to eliminating the risk of COVID-19 acquisition, web-based interviews have been described as helping to improve scheduling flexibility, reduce the financial burden, and allow conducting more interviews for candidates and programs (Table 1).6-8
There are different styles to the web-based interview.9 Some programs choose to offer a single group interview (or the so-called panel interview) in which all the interviewing faculties invite each candidate at a time. Alternatively, programs might choose to conduct separate interviews by each faculty in which the candidates would alternate. For the latter option, the program could use a single invitation link or multiple invitation links for each session.
General tips for a successful interview:9
1. Be pleasant and professional: Your communication with the program should reflect excellent manners and a professional attitude with everyone (i.e., faculties, coordinators, and fellows).
2. Know yourself and what you want: Review your CV and personal statement and reflect on your achievements, strengths and weaknesses. Identify examples from your experience that would speak well of you as a person and as a physician.
3. Communication is key:
- Respond to the interview invitations promptly.
- Send a brief thank you email to the interviewers and the coordinator. Avoid being generic; mention specific points of discussion and show your interest in the program.
- Proofread your emails carefully. Well-written emails that are devoid of grammatical or spelling errors send a positive message about the candidate.
4. Do your homework:
- Read the information posted on the website carefully and take notes. This should provide you with useful information to use when you rank the programs and could lead to questions that you might want to ask your interviewers. Besides, asking questions that are answered on the website reflects poorly on the candidate.
- Pay attention to various clues that could reflect how organized and how academically oriented a program is. For example, a program that provides details about their didactic lectures sends a message that quality teaching is a priority. On the other hand, a program that has a website that hasn’t been updated for years could dissuade rather than recruit applicants.
- Read about the faculty, their areas of interests, and publications. Learn how their names are pronounced and use them during the interview.
- Read about the city where the program is. It shows interest in the area where you might be living and will help you to stand out among candidates.
5. Be prepared for the classics; be honest, genuine, and authentic. Think about these common prompts:
- Tell me about yourself.
- Why did you choose gastroenterology?
- Where do you see yourself in 5 years?
- Why would you like to come to the city where the program is?
- Are there any certain areas in gastroenterology that you’re interested in more (e.g., hepatology, motility, IBD, advanced endoscopy)?
6. It is likely your interviewer will ask if you have questions. Ask questions that further allow you to assess the program and your fit into the program.
- What aspects of the program are you most proud of?
- Where would you like to see this program in 5 years?
- What keeps you at this program?
Tips for a successful web-based interview9,10 (Table 2):
1. Pay attention to the time zone of the city of the program. Be ready at least 10 minutes before the interview.
2. Ensure a fast and stable Internet service for an uninterrupted interview experience. Consider using an ethernet cable. Have a back-up plan such as using a phone as a hotspot.
3. Use a quiet and private room, preferably at home. Be aware of the background. A simple decoration is acceptable.
4. Consider recording yourself using the same device you’ll use for the interview to make sure audio and video are functioning properly.
5. When scheduling more than one interview in 1 day, allow at least 2 hours between interviews to avoid scheduling conflicts caused by unanticipated delays related to technical issues.
6. Have immediate access to the invitation link(s) that you received. Add the interviews to your device’s calendar. Note that sometimes a new invitation link is generated last minute because of technical issues.
7. While the advice for physical interviews is to turn off your phone (and smart watch), you’ll have to keep your phone on but on silent for the virtual interviews. Sometimes, you’ll receive a phone call from the program to update you about any last-minute changes.
7. It is recommended that a laptop or a tablet with a camera with good resolution and a microphone be used rather than using a phone. A wide screen allows better communication. Disable notification on that device to avoid interruptions.
8. Sit comfortably with the device being at or just below eye level. Avoid distractions and maintain eye contact.
9. Familiarize yourself with the platform used and its functions. Double check the audio and video before each interview.
10. Put your device on a desk or table to improve stability; don’t hold it in your hand.
11. Find a place where the view is best and your face appears in the middle of the screen; not too far or too close. Use a well-lit room but don’t have a source of light behind you. Many platforms allow you to select a background or blur the background. A background that is monochromatic and not distracting is recommended.
12. Use a pen and a paper to take notes during the interview. You would use these notes to generate “thank you” or “interest in program” emails. Additionally, they will be a helpful reference when ranking programs.
13. Do not type. Typing is much louder to the interviewer and can be distracting.
14. Dress professionally, just as you dress for an on-site interview. You never know when you might have to stand up during the interview for unplanned reasons.
15. Do a practice interview. Have a colleague set up a virtual web session using any available platform. This will allow you to get feedback on your dress, background, acoustics, and general ability to answer questions.
References
1. Dig Dis Sci. 2019;64:1150-7.
2. BMJ Open. 2017;7:e016242.
3. Centers for Disease Control and Prevention. Coronavirus and Travel in the United States, 2020.
4. J Bone Joint Surg Am. 2017;99:e114.
5. Am J Gastroenterol. 2014;109:155-9.
6. West J Emerg Med. 2018;19:80-6.
7. Int J Med Educ. 2016;7:102-8.
8. Aparajit Naram M. How COVID-19 changed our fellowship interview process for the better. KevinMD.com. April 17, 2020.
9. Association of American Medical Colleges. Virtual interviews: Tips for medical school applicants, 2020. Updated May 14, 2020.
10. Top 5 video interviewing tips for residency and fellowship programs. Thalamus: Connecting the Docs. April 2, 2020, 2020.
Dr. Kiwan is chief fellow in gastroenterology, division of gastroenterology, at Wayne State University, Detroit Medical Center, John D. Dingell VA Medical Center, all in Detroit. Dr. Judd is an assistant professor and associate program director in the division of gastroenterology, Wayne State University, Detroit Medical Center, John D. Dingell VA Medical Center. Dr. Al Masalmeh is in the department of internal medicine, Wayne State University, Detroit Medical Center. Dr. Levine is professor and the vice chair for education, Wayne State University, Detroit Medical Center.
Fellowship interviews are an essential step – arguably the most important step – in the process of matching candidates to training programs. Until recently, most programs relied exclusively on on-site face-to face interviews. Since the appearance of the COVID-19 pandemic, the medical field has utilized web-based platforms. Despite inherent limitations, virtual meetings appear to be effective in providing patient care and in conducting administrative meetings.1,2
Because of uncertainty related to the pandemic, including changing guidelines regarding social distancing and travel restrictions, fellowship programs are expected to comply with CDC,3 state, and federal recommendations to avoid nonessential travel. Therefore, conducting web-based interviews exclusively will likely become a necessity.
While there may be some disadvantages to web-based interviews, many candidates find the overall experience satisfactory, thereby allowing them to understand the programs, express themselves, and comfortably rank the programs, as two studies have shown.4,5 Programs and candidates are encouraged to adapt to this new reality in order to achieve a successful match. After all, there are many potential advantages of web-based interviews. In addition to eliminating the risk of COVID-19 acquisition, web-based interviews have been described as helping to improve scheduling flexibility, reduce the financial burden, and allow conducting more interviews for candidates and programs (Table 1).6-8
There are different styles to the web-based interview.9 Some programs choose to offer a single group interview (or the so-called panel interview) in which all the interviewing faculties invite each candidate at a time. Alternatively, programs might choose to conduct separate interviews by each faculty in which the candidates would alternate. For the latter option, the program could use a single invitation link or multiple invitation links for each session.
General tips for a successful interview:9
1. Be pleasant and professional: Your communication with the program should reflect excellent manners and a professional attitude with everyone (i.e., faculties, coordinators, and fellows).
2. Know yourself and what you want: Review your CV and personal statement and reflect on your achievements, strengths and weaknesses. Identify examples from your experience that would speak well of you as a person and as a physician.
3. Communication is key:
- Respond to the interview invitations promptly.
- Send a brief thank you email to the interviewers and the coordinator. Avoid being generic; mention specific points of discussion and show your interest in the program.
- Proofread your emails carefully. Well-written emails that are devoid of grammatical or spelling errors send a positive message about the candidate.
4. Do your homework:
- Read the information posted on the website carefully and take notes. This should provide you with useful information to use when you rank the programs and could lead to questions that you might want to ask your interviewers. Besides, asking questions that are answered on the website reflects poorly on the candidate.
- Pay attention to various clues that could reflect how organized and how academically oriented a program is. For example, a program that provides details about their didactic lectures sends a message that quality teaching is a priority. On the other hand, a program that has a website that hasn’t been updated for years could dissuade rather than recruit applicants.
- Read about the faculty, their areas of interests, and publications. Learn how their names are pronounced and use them during the interview.
- Read about the city where the program is. It shows interest in the area where you might be living and will help you to stand out among candidates.
5. Be prepared for the classics; be honest, genuine, and authentic. Think about these common prompts:
- Tell me about yourself.
- Why did you choose gastroenterology?
- Where do you see yourself in 5 years?
- Why would you like to come to the city where the program is?
- Are there any certain areas in gastroenterology that you’re interested in more (e.g., hepatology, motility, IBD, advanced endoscopy)?
6. It is likely your interviewer will ask if you have questions. Ask questions that further allow you to assess the program and your fit into the program.
- What aspects of the program are you most proud of?
- Where would you like to see this program in 5 years?
- What keeps you at this program?
Tips for a successful web-based interview9,10 (Table 2):
1. Pay attention to the time zone of the city of the program. Be ready at least 10 minutes before the interview.
2. Ensure a fast and stable Internet service for an uninterrupted interview experience. Consider using an ethernet cable. Have a back-up plan such as using a phone as a hotspot.
3. Use a quiet and private room, preferably at home. Be aware of the background. A simple decoration is acceptable.
4. Consider recording yourself using the same device you’ll use for the interview to make sure audio and video are functioning properly.
5. When scheduling more than one interview in 1 day, allow at least 2 hours between interviews to avoid scheduling conflicts caused by unanticipated delays related to technical issues.
6. Have immediate access to the invitation link(s) that you received. Add the interviews to your device’s calendar. Note that sometimes a new invitation link is generated last minute because of technical issues.
7. While the advice for physical interviews is to turn off your phone (and smart watch), you’ll have to keep your phone on but on silent for the virtual interviews. Sometimes, you’ll receive a phone call from the program to update you about any last-minute changes.
7. It is recommended that a laptop or a tablet with a camera with good resolution and a microphone be used rather than using a phone. A wide screen allows better communication. Disable notification on that device to avoid interruptions.
8. Sit comfortably with the device being at or just below eye level. Avoid distractions and maintain eye contact.
9. Familiarize yourself with the platform used and its functions. Double check the audio and video before each interview.
10. Put your device on a desk or table to improve stability; don’t hold it in your hand.
11. Find a place where the view is best and your face appears in the middle of the screen; not too far or too close. Use a well-lit room but don’t have a source of light behind you. Many platforms allow you to select a background or blur the background. A background that is monochromatic and not distracting is recommended.
12. Use a pen and a paper to take notes during the interview. You would use these notes to generate “thank you” or “interest in program” emails. Additionally, they will be a helpful reference when ranking programs.
13. Do not type. Typing is much louder to the interviewer and can be distracting.
14. Dress professionally, just as you dress for an on-site interview. You never know when you might have to stand up during the interview for unplanned reasons.
15. Do a practice interview. Have a colleague set up a virtual web session using any available platform. This will allow you to get feedback on your dress, background, acoustics, and general ability to answer questions.
References
1. Dig Dis Sci. 2019;64:1150-7.
2. BMJ Open. 2017;7:e016242.
3. Centers for Disease Control and Prevention. Coronavirus and Travel in the United States, 2020.
4. J Bone Joint Surg Am. 2017;99:e114.
5. Am J Gastroenterol. 2014;109:155-9.
6. West J Emerg Med. 2018;19:80-6.
7. Int J Med Educ. 2016;7:102-8.
8. Aparajit Naram M. How COVID-19 changed our fellowship interview process for the better. KevinMD.com. April 17, 2020.
9. Association of American Medical Colleges. Virtual interviews: Tips for medical school applicants, 2020. Updated May 14, 2020.
10. Top 5 video interviewing tips for residency and fellowship programs. Thalamus: Connecting the Docs. April 2, 2020, 2020.
Dr. Kiwan is chief fellow in gastroenterology, division of gastroenterology, at Wayne State University, Detroit Medical Center, John D. Dingell VA Medical Center, all in Detroit. Dr. Judd is an assistant professor and associate program director in the division of gastroenterology, Wayne State University, Detroit Medical Center, John D. Dingell VA Medical Center. Dr. Al Masalmeh is in the department of internal medicine, Wayne State University, Detroit Medical Center. Dr. Levine is professor and the vice chair for education, Wayne State University, Detroit Medical Center.
Fellowship interviews are an essential step – arguably the most important step – in the process of matching candidates to training programs. Until recently, most programs relied exclusively on on-site face-to face interviews. Since the appearance of the COVID-19 pandemic, the medical field has utilized web-based platforms. Despite inherent limitations, virtual meetings appear to be effective in providing patient care and in conducting administrative meetings.1,2
Because of uncertainty related to the pandemic, including changing guidelines regarding social distancing and travel restrictions, fellowship programs are expected to comply with CDC,3 state, and federal recommendations to avoid nonessential travel. Therefore, conducting web-based interviews exclusively will likely become a necessity.
While there may be some disadvantages to web-based interviews, many candidates find the overall experience satisfactory, thereby allowing them to understand the programs, express themselves, and comfortably rank the programs, as two studies have shown.4,5 Programs and candidates are encouraged to adapt to this new reality in order to achieve a successful match. After all, there are many potential advantages of web-based interviews. In addition to eliminating the risk of COVID-19 acquisition, web-based interviews have been described as helping to improve scheduling flexibility, reduce the financial burden, and allow conducting more interviews for candidates and programs (Table 1).6-8
There are different styles to the web-based interview.9 Some programs choose to offer a single group interview (or the so-called panel interview) in which all the interviewing faculties invite each candidate at a time. Alternatively, programs might choose to conduct separate interviews by each faculty in which the candidates would alternate. For the latter option, the program could use a single invitation link or multiple invitation links for each session.
General tips for a successful interview:9
1. Be pleasant and professional: Your communication with the program should reflect excellent manners and a professional attitude with everyone (i.e., faculties, coordinators, and fellows).
2. Know yourself and what you want: Review your CV and personal statement and reflect on your achievements, strengths and weaknesses. Identify examples from your experience that would speak well of you as a person and as a physician.
3. Communication is key:
- Respond to the interview invitations promptly.
- Send a brief thank you email to the interviewers and the coordinator. Avoid being generic; mention specific points of discussion and show your interest in the program.
- Proofread your emails carefully. Well-written emails that are devoid of grammatical or spelling errors send a positive message about the candidate.
4. Do your homework:
- Read the information posted on the website carefully and take notes. This should provide you with useful information to use when you rank the programs and could lead to questions that you might want to ask your interviewers. Besides, asking questions that are answered on the website reflects poorly on the candidate.
- Pay attention to various clues that could reflect how organized and how academically oriented a program is. For example, a program that provides details about their didactic lectures sends a message that quality teaching is a priority. On the other hand, a program that has a website that hasn’t been updated for years could dissuade rather than recruit applicants.
- Read about the faculty, their areas of interests, and publications. Learn how their names are pronounced and use them during the interview.
- Read about the city where the program is. It shows interest in the area where you might be living and will help you to stand out among candidates.
5. Be prepared for the classics; be honest, genuine, and authentic. Think about these common prompts:
- Tell me about yourself.
- Why did you choose gastroenterology?
- Where do you see yourself in 5 years?
- Why would you like to come to the city where the program is?
- Are there any certain areas in gastroenterology that you’re interested in more (e.g., hepatology, motility, IBD, advanced endoscopy)?
6. It is likely your interviewer will ask if you have questions. Ask questions that further allow you to assess the program and your fit into the program.
- What aspects of the program are you most proud of?
- Where would you like to see this program in 5 years?
- What keeps you at this program?
Tips for a successful web-based interview9,10 (Table 2):
1. Pay attention to the time zone of the city of the program. Be ready at least 10 minutes before the interview.
2. Ensure a fast and stable Internet service for an uninterrupted interview experience. Consider using an ethernet cable. Have a back-up plan such as using a phone as a hotspot.
3. Use a quiet and private room, preferably at home. Be aware of the background. A simple decoration is acceptable.
4. Consider recording yourself using the same device you’ll use for the interview to make sure audio and video are functioning properly.
5. When scheduling more than one interview in 1 day, allow at least 2 hours between interviews to avoid scheduling conflicts caused by unanticipated delays related to technical issues.
6. Have immediate access to the invitation link(s) that you received. Add the interviews to your device’s calendar. Note that sometimes a new invitation link is generated last minute because of technical issues.
7. While the advice for physical interviews is to turn off your phone (and smart watch), you’ll have to keep your phone on but on silent for the virtual interviews. Sometimes, you’ll receive a phone call from the program to update you about any last-minute changes.
7. It is recommended that a laptop or a tablet with a camera with good resolution and a microphone be used rather than using a phone. A wide screen allows better communication. Disable notification on that device to avoid interruptions.
8. Sit comfortably with the device being at or just below eye level. Avoid distractions and maintain eye contact.
9. Familiarize yourself with the platform used and its functions. Double check the audio and video before each interview.
10. Put your device on a desk or table to improve stability; don’t hold it in your hand.
11. Find a place where the view is best and your face appears in the middle of the screen; not too far or too close. Use a well-lit room but don’t have a source of light behind you. Many platforms allow you to select a background or blur the background. A background that is monochromatic and not distracting is recommended.
12. Use a pen and a paper to take notes during the interview. You would use these notes to generate “thank you” or “interest in program” emails. Additionally, they will be a helpful reference when ranking programs.
13. Do not type. Typing is much louder to the interviewer and can be distracting.
14. Dress professionally, just as you dress for an on-site interview. You never know when you might have to stand up during the interview for unplanned reasons.
15. Do a practice interview. Have a colleague set up a virtual web session using any available platform. This will allow you to get feedback on your dress, background, acoustics, and general ability to answer questions.
References
1. Dig Dis Sci. 2019;64:1150-7.
2. BMJ Open. 2017;7:e016242.
3. Centers for Disease Control and Prevention. Coronavirus and Travel in the United States, 2020.
4. J Bone Joint Surg Am. 2017;99:e114.
5. Am J Gastroenterol. 2014;109:155-9.
6. West J Emerg Med. 2018;19:80-6.
7. Int J Med Educ. 2016;7:102-8.
8. Aparajit Naram M. How COVID-19 changed our fellowship interview process for the better. KevinMD.com. April 17, 2020.
9. Association of American Medical Colleges. Virtual interviews: Tips for medical school applicants, 2020. Updated May 14, 2020.
10. Top 5 video interviewing tips for residency and fellowship programs. Thalamus: Connecting the Docs. April 2, 2020, 2020.
Dr. Kiwan is chief fellow in gastroenterology, division of gastroenterology, at Wayne State University, Detroit Medical Center, John D. Dingell VA Medical Center, all in Detroit. Dr. Judd is an assistant professor and associate program director in the division of gastroenterology, Wayne State University, Detroit Medical Center, John D. Dingell VA Medical Center. Dr. Al Masalmeh is in the department of internal medicine, Wayne State University, Detroit Medical Center. Dr. Levine is professor and the vice chair for education, Wayne State University, Detroit Medical Center.
The best and worst states for health care in 2020
according to the personal finance website WalletHub.
The Bay State finds itself at the top of the company’s annual ranking of state health care systems this year after finishing second in 2019 to Minnesota, which is now ranked second. Rhode Island is third this year, followed by Washington, D.C., and North Dakota, WalletHub reported Aug. 3.
The inclusion of Washington, D.C., allowed Georgia to finish 51st out of 50 states, just below the quartet of Louisiana (50th), Alabama (49th), North Carolina (48th), and Mississippi (47th). Alaska, which occupied the bottom spot in 2019, moved up to 42nd this year, the analysis showed.
The rankings are based on 44 (up from 43 last year) metrics that are grouped into three broad categories: cost (6 metrics), access (24 metrics), and outcomes (14 metrics). The one new measure added for 2020? That would be health infrastructure for coronavirus, which is itself based on a different WalletHub ranking.
Massachusetts’ top finish this year was driven by strong showings in such metrics as average monthly insurance premium (first), physicians per capita (second), insured children (first) and adults (first), and infant mortality rate (fourth). The state was 1st overall in outcomes and 4th in access but only 20th in cost, the company said.
Positive signs among the lowest-ranked states include Louisiana’s 18th-place finish in access, ahead of such top 10 states as Iowa and Hawaii, and Mississippi’s 17th in cost, which is higher than four of the states in the top 10, including Massachusetts, WalletHub said in the report.
Data for the analysis came from 22 different sources, including the Institute for Health Metrics and Evaluation, Centers for Medicare & Medicaid Services, Association of American Medical Colleges, and the American Telemedicine Association.
according to the personal finance website WalletHub.

The Bay State finds itself at the top of the company’s annual ranking of state health care systems this year after finishing second in 2019 to Minnesota, which is now ranked second. Rhode Island is third this year, followed by Washington, D.C., and North Dakota, WalletHub reported Aug. 3.
The inclusion of Washington, D.C., allowed Georgia to finish 51st out of 50 states, just below the quartet of Louisiana (50th), Alabama (49th), North Carolina (48th), and Mississippi (47th). Alaska, which occupied the bottom spot in 2019, moved up to 42nd this year, the analysis showed.
The rankings are based on 44 (up from 43 last year) metrics that are grouped into three broad categories: cost (6 metrics), access (24 metrics), and outcomes (14 metrics). The one new measure added for 2020? That would be health infrastructure for coronavirus, which is itself based on a different WalletHub ranking.
Massachusetts’ top finish this year was driven by strong showings in such metrics as average monthly insurance premium (first), physicians per capita (second), insured children (first) and adults (first), and infant mortality rate (fourth). The state was 1st overall in outcomes and 4th in access but only 20th in cost, the company said.
Positive signs among the lowest-ranked states include Louisiana’s 18th-place finish in access, ahead of such top 10 states as Iowa and Hawaii, and Mississippi’s 17th in cost, which is higher than four of the states in the top 10, including Massachusetts, WalletHub said in the report.
Data for the analysis came from 22 different sources, including the Institute for Health Metrics and Evaluation, Centers for Medicare & Medicaid Services, Association of American Medical Colleges, and the American Telemedicine Association.
according to the personal finance website WalletHub.

The Bay State finds itself at the top of the company’s annual ranking of state health care systems this year after finishing second in 2019 to Minnesota, which is now ranked second. Rhode Island is third this year, followed by Washington, D.C., and North Dakota, WalletHub reported Aug. 3.
The inclusion of Washington, D.C., allowed Georgia to finish 51st out of 50 states, just below the quartet of Louisiana (50th), Alabama (49th), North Carolina (48th), and Mississippi (47th). Alaska, which occupied the bottom spot in 2019, moved up to 42nd this year, the analysis showed.
The rankings are based on 44 (up from 43 last year) metrics that are grouped into three broad categories: cost (6 metrics), access (24 metrics), and outcomes (14 metrics). The one new measure added for 2020? That would be health infrastructure for coronavirus, which is itself based on a different WalletHub ranking.
Massachusetts’ top finish this year was driven by strong showings in such metrics as average monthly insurance premium (first), physicians per capita (second), insured children (first) and adults (first), and infant mortality rate (fourth). The state was 1st overall in outcomes and 4th in access but only 20th in cost, the company said.
Positive signs among the lowest-ranked states include Louisiana’s 18th-place finish in access, ahead of such top 10 states as Iowa and Hawaii, and Mississippi’s 17th in cost, which is higher than four of the states in the top 10, including Massachusetts, WalletHub said in the report.
Data for the analysis came from 22 different sources, including the Institute for Health Metrics and Evaluation, Centers for Medicare & Medicaid Services, Association of American Medical Colleges, and the American Telemedicine Association.
Unexpected rosuvastatin-canagliflozin adverse effect reported
A 76-year-old woman presented recently to a Toronto-area hospital with acute onset muscle pain, limb weakness, difficulty walking, and rhabdomyolysis associated with a sharp spike in her plasma level of rosuvastatin – a drug she had been on uneventfully for more than 5 years, within days of starting for the first time treatment with the SGLT2 inhibitor canagliflozin (Invokana).
The patient’s Canadian clinicians stopped her treatment with both rosuvastatin and canagliflozin, administered intravenous crystalloid fluids, and within days her pain subsided and her limb weakness gradually improved, allowing her discharge 10 days later while she was ambulating with a walker.
“To our knowledge this is the first published report of a drug interaction between rosuvastatin and canagliflozin,” wrote the authors of the case report (Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549). They cited the importance of the observation given the widespread use today of rosuvastatin for lowering low density lipoprotein cholesterol and exerting pleiotropic effects; and canagliflozin for its modest effects for reducing hyperglycemia, as well as its important role in reducing adverse cardiovascular outcomes, slowing progression of chronic kidney disease, and having a mild but important diuretic effect. “We encourage clinicians to remain vigilant for features of myotoxicity when canagliflozin and rosuvastatin are coprescribed,” they wrote, avoiding discussion of whether this may represent class or drug-specific effects.
“It’s reasonable to be mindful of this risk, but this is not a reason to not use rosuvastatin and canagliflozin in a patient,” nor for the time being to avoid any other combination of a statin and SGLT2 (sodium-glucose cotransporter 2) inhibitor, said David Juurlink, MD, head of the division of clinical pharmacology and toxicology at Sunnybrook Health Sciences Centre in Toronto and lead author of the report. “Few drug interactions have absolute contraindications. The admonition is just to be careful. It’s premature to say they shouldn’t be used together,” he said in an interview.
“We don’t know how much of an outlier this patient is. But it would be important to tell patients” on this or a similar combination to alert their clinicians if they start to have muscle aches, which should be a “red flag” to stop the statin, the SGLT2 inhibitor, or both until the situation can be fully assessed, Dr. Juurlink advised.
Sky high rosuvastatin levels
The linchpin of the observed adverse effects appeared to be a startlingly high elevation of the patient’s plasma rosuvastatin level when she was hospitalized 15 days after starting canagliflozin and 12 days after the onset of her thigh pain and weakness. Testing showed a plasma rosuvastatin concentration of 176 ng/mL, “more than 15-fold higher than the mean value expected” in patients taking 40 mg rosuvastatin daily, the maximum labeled dosage for the drug and what the affected patient had been taking without prior incident for more than 5 years. The patient’s canagliflozin dosage was 100 mg/day, the standard starting dosage according to the drug’s label.
The report’s authors noted that genetic assessment of the patient, a woman originally from the Philippines who was “high functioning,” and diagnosed with type 2 diabetes, showed she was heterozygous for a polymorphism, c.421C>A, which is linked with increased rosuvastatin plasma levels in the plasma. They also cited a report that canagliflozin can interact with proteins involved in hepatic drug uptake.
“We speculate that, in our patient, the addition of canagliflozin enhanced intestinal rosuvastatin absorption, inhibited its hepatocellular uptake, and impaired its excretion into bile canaliculi and the proximal tubule, resulting in rosuvastatin accumulation and leading to hepatotoxicity and myotoxicity,” the clinicians wrote in their report.
“There is little doubt this was a drug interaction, but it does not apply uniformly to everyone.” The severity of the interaction would depend on the dosages, the comorbidities a patient has, and their genetic profile, Dr. Juurlink said.
Concern and skepticism
Other clinicians who regularly prescribe these drugs expressed concern about the observation as well as skepticism about the prevalence of patients who could potentially experience similar effects.
“We don’t know how common are these genetic abnormalities. If this is extremely rare, then it doesn’t have many clinical implications, but if a large portion of the population has this [genetic] abnormality, it’s something we’d need to pay attention to,” Steven E. Nissen, MD, chair of cardiovascular medicine at the Cleveland Clinic Foundation, said in an interview. “It will be important to know the prevalence” of the genetic polymorphism carried by the reported patient, said Dr. Nissen, who has done research on lipid-lowering medications and drug safety.
“This could be important, or a very rare one-off. I can’t say which,” based on what’s currently known, he said. “There are many unanswered questions that make it hard to know how important this will be. It requires further investigation. There is a lot of uncertainty.”
Dr. Nissen particularly endorsed studies that approach this issue by looking at the prevalence rates of the implicated genetic polymorphism rather than pharmacovigilance studies that make epidemiologic assessments of adverse-effect prevalence. Studies that look for adverse-effect associations in large patient populations are “sloppy, and unless the interaction is incredibly intense they are not very sensitive,” he said.
But Dr. Juurlink, a pharmacoepidemiologist whose specialty includes studies of this sort, said that they could be useful if carefully designed. He suggested, for example, comparing in large patient databases the observed incidence of rhabdomyolysis among patients on an SGLT2 inhibitor and also on rosuvastatin with those on pravastatin, a statin with a different metabolic profile. Another approach to further examining the observation would be dosage studies with rosuvastatin and canagliflozin in healthy volunteers, he said.
Dr. Nissen noted that rosuvastatin is a key agent from the statin class because it’s the “most effective” for lowering low density lipoprotein cholesterol. “Rosuvastatin is a go-to drug,” he declared. On the other hand, canagliflozin is “a little less used” than other drugs in the SGLT2 inhibitor class, specifically dapagliflozin (Farxiga) and empagliflozin (Jardiance), he said.
One in a million?
“This was a freak accident. I don’t find it at all concerning. It was definitely one in a million,” Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif., said in an interview. “None of us have seen it” in either the several cardiovascular outcome trials now run on multiple drugs in the SGLT2 inhibitor class that included many patients also taking a statin, or in routine practice, he said. Dr. Handelsman noted that in his practice he had never seen a similar case despite treating “hundreds if not thousands of patients” with type 2 diabetes, virtually all of whom were on a statin and were also treated with an SGLT2 inhibitor, including many with canagliflozin.
Dr. Handelsman cited the notably low estimated glomerular filtration rate in the reported patient, who was described as having a serum creatinine level of 150 mcmol/L (1.7 mg/dL) prior to canagliflozin treatment that then rose to 194 mcmol/L (2.19 mg/dL) at the time of hospitalization, which corresponds to estimated glomerular filtration rates of 29-31 and 21-23 mL/min per 1.73 m2, respectively, depending on the calculator used, rates that were possibly below the labeled minimum rate of 30 mL/min per 1.73 m2 for patients starting canagliflozin treatment. The case report cited the patient as having stage 3B chronic kidney disease, which corresponds to a eGFR of 30-44* mL/min per 1.73 m2.
“I think the patient had acute kidney injury” on starting canagliflozin “that may have affected the [rosuvastatin] metabolism,” Dr. Handelsman suggested. “She had severe kidney dysfunction to start with that fell further with SGLT2 inhibitor treatment,” a well described and usually transient effect of starting drugs in this class because of changes the SGLT2 inhibitors cause in renal blood flow. He noted that the patient had not been receiving an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, which may have contributed to her acute problems with fluid balance. Most similar patients with type 2 diabetes, cardiovascular disease risk, and chronic kidney disease would be on stable treatment with a drug that inhibits the renin-angiotensin system before starting an SGLT2 inhibitor, and not already having a RAS inhibitor on board before starting canagliflozin may have somehow contributed to the observed adverse effects, Dr. Handelsman said.
Dr. Juurlink was skeptical that the kidneys played a major role. “An abrupt change in renal function can influence statin clearance, but this was a 15-fold increase. You can’t explain such a dramatic increase by a transient reduction in renal function,” he said.
Dr. Juurlink and coauthors had no disclosures. Dr. Nissen had no relevant disclosures. Dr. Handelsman has been a consultant to companies that market drugs in the SGLT2 inhibitor class.
SOURCE: Brailovski E et al. Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549.
*Correction: This value was missing from the original article.
A 76-year-old woman presented recently to a Toronto-area hospital with acute onset muscle pain, limb weakness, difficulty walking, and rhabdomyolysis associated with a sharp spike in her plasma level of rosuvastatin – a drug she had been on uneventfully for more than 5 years, within days of starting for the first time treatment with the SGLT2 inhibitor canagliflozin (Invokana).
The patient’s Canadian clinicians stopped her treatment with both rosuvastatin and canagliflozin, administered intravenous crystalloid fluids, and within days her pain subsided and her limb weakness gradually improved, allowing her discharge 10 days later while she was ambulating with a walker.
“To our knowledge this is the first published report of a drug interaction between rosuvastatin and canagliflozin,” wrote the authors of the case report (Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549). They cited the importance of the observation given the widespread use today of rosuvastatin for lowering low density lipoprotein cholesterol and exerting pleiotropic effects; and canagliflozin for its modest effects for reducing hyperglycemia, as well as its important role in reducing adverse cardiovascular outcomes, slowing progression of chronic kidney disease, and having a mild but important diuretic effect. “We encourage clinicians to remain vigilant for features of myotoxicity when canagliflozin and rosuvastatin are coprescribed,” they wrote, avoiding discussion of whether this may represent class or drug-specific effects.
“It’s reasonable to be mindful of this risk, but this is not a reason to not use rosuvastatin and canagliflozin in a patient,” nor for the time being to avoid any other combination of a statin and SGLT2 (sodium-glucose cotransporter 2) inhibitor, said David Juurlink, MD, head of the division of clinical pharmacology and toxicology at Sunnybrook Health Sciences Centre in Toronto and lead author of the report. “Few drug interactions have absolute contraindications. The admonition is just to be careful. It’s premature to say they shouldn’t be used together,” he said in an interview.
“We don’t know how much of an outlier this patient is. But it would be important to tell patients” on this or a similar combination to alert their clinicians if they start to have muscle aches, which should be a “red flag” to stop the statin, the SGLT2 inhibitor, or both until the situation can be fully assessed, Dr. Juurlink advised.
Sky high rosuvastatin levels
The linchpin of the observed adverse effects appeared to be a startlingly high elevation of the patient’s plasma rosuvastatin level when she was hospitalized 15 days after starting canagliflozin and 12 days after the onset of her thigh pain and weakness. Testing showed a plasma rosuvastatin concentration of 176 ng/mL, “more than 15-fold higher than the mean value expected” in patients taking 40 mg rosuvastatin daily, the maximum labeled dosage for the drug and what the affected patient had been taking without prior incident for more than 5 years. The patient’s canagliflozin dosage was 100 mg/day, the standard starting dosage according to the drug’s label.
The report’s authors noted that genetic assessment of the patient, a woman originally from the Philippines who was “high functioning,” and diagnosed with type 2 diabetes, showed she was heterozygous for a polymorphism, c.421C>A, which is linked with increased rosuvastatin plasma levels in the plasma. They also cited a report that canagliflozin can interact with proteins involved in hepatic drug uptake.
“We speculate that, in our patient, the addition of canagliflozin enhanced intestinal rosuvastatin absorption, inhibited its hepatocellular uptake, and impaired its excretion into bile canaliculi and the proximal tubule, resulting in rosuvastatin accumulation and leading to hepatotoxicity and myotoxicity,” the clinicians wrote in their report.
“There is little doubt this was a drug interaction, but it does not apply uniformly to everyone.” The severity of the interaction would depend on the dosages, the comorbidities a patient has, and their genetic profile, Dr. Juurlink said.
Concern and skepticism
Other clinicians who regularly prescribe these drugs expressed concern about the observation as well as skepticism about the prevalence of patients who could potentially experience similar effects.
“We don’t know how common are these genetic abnormalities. If this is extremely rare, then it doesn’t have many clinical implications, but if a large portion of the population has this [genetic] abnormality, it’s something we’d need to pay attention to,” Steven E. Nissen, MD, chair of cardiovascular medicine at the Cleveland Clinic Foundation, said in an interview. “It will be important to know the prevalence” of the genetic polymorphism carried by the reported patient, said Dr. Nissen, who has done research on lipid-lowering medications and drug safety.
“This could be important, or a very rare one-off. I can’t say which,” based on what’s currently known, he said. “There are many unanswered questions that make it hard to know how important this will be. It requires further investigation. There is a lot of uncertainty.”
Dr. Nissen particularly endorsed studies that approach this issue by looking at the prevalence rates of the implicated genetic polymorphism rather than pharmacovigilance studies that make epidemiologic assessments of adverse-effect prevalence. Studies that look for adverse-effect associations in large patient populations are “sloppy, and unless the interaction is incredibly intense they are not very sensitive,” he said.
But Dr. Juurlink, a pharmacoepidemiologist whose specialty includes studies of this sort, said that they could be useful if carefully designed. He suggested, for example, comparing in large patient databases the observed incidence of rhabdomyolysis among patients on an SGLT2 inhibitor and also on rosuvastatin with those on pravastatin, a statin with a different metabolic profile. Another approach to further examining the observation would be dosage studies with rosuvastatin and canagliflozin in healthy volunteers, he said.
Dr. Nissen noted that rosuvastatin is a key agent from the statin class because it’s the “most effective” for lowering low density lipoprotein cholesterol. “Rosuvastatin is a go-to drug,” he declared. On the other hand, canagliflozin is “a little less used” than other drugs in the SGLT2 inhibitor class, specifically dapagliflozin (Farxiga) and empagliflozin (Jardiance), he said.
One in a million?
“This was a freak accident. I don’t find it at all concerning. It was definitely one in a million,” Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif., said in an interview. “None of us have seen it” in either the several cardiovascular outcome trials now run on multiple drugs in the SGLT2 inhibitor class that included many patients also taking a statin, or in routine practice, he said. Dr. Handelsman noted that in his practice he had never seen a similar case despite treating “hundreds if not thousands of patients” with type 2 diabetes, virtually all of whom were on a statin and were also treated with an SGLT2 inhibitor, including many with canagliflozin.
Dr. Handelsman cited the notably low estimated glomerular filtration rate in the reported patient, who was described as having a serum creatinine level of 150 mcmol/L (1.7 mg/dL) prior to canagliflozin treatment that then rose to 194 mcmol/L (2.19 mg/dL) at the time of hospitalization, which corresponds to estimated glomerular filtration rates of 29-31 and 21-23 mL/min per 1.73 m2, respectively, depending on the calculator used, rates that were possibly below the labeled minimum rate of 30 mL/min per 1.73 m2 for patients starting canagliflozin treatment. The case report cited the patient as having stage 3B chronic kidney disease, which corresponds to a eGFR of 30-44* mL/min per 1.73 m2.
“I think the patient had acute kidney injury” on starting canagliflozin “that may have affected the [rosuvastatin] metabolism,” Dr. Handelsman suggested. “She had severe kidney dysfunction to start with that fell further with SGLT2 inhibitor treatment,” a well described and usually transient effect of starting drugs in this class because of changes the SGLT2 inhibitors cause in renal blood flow. He noted that the patient had not been receiving an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, which may have contributed to her acute problems with fluid balance. Most similar patients with type 2 diabetes, cardiovascular disease risk, and chronic kidney disease would be on stable treatment with a drug that inhibits the renin-angiotensin system before starting an SGLT2 inhibitor, and not already having a RAS inhibitor on board before starting canagliflozin may have somehow contributed to the observed adverse effects, Dr. Handelsman said.
Dr. Juurlink was skeptical that the kidneys played a major role. “An abrupt change in renal function can influence statin clearance, but this was a 15-fold increase. You can’t explain such a dramatic increase by a transient reduction in renal function,” he said.
Dr. Juurlink and coauthors had no disclosures. Dr. Nissen had no relevant disclosures. Dr. Handelsman has been a consultant to companies that market drugs in the SGLT2 inhibitor class.
SOURCE: Brailovski E et al. Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549.
*Correction: This value was missing from the original article.
A 76-year-old woman presented recently to a Toronto-area hospital with acute onset muscle pain, limb weakness, difficulty walking, and rhabdomyolysis associated with a sharp spike in her plasma level of rosuvastatin – a drug she had been on uneventfully for more than 5 years, within days of starting for the first time treatment with the SGLT2 inhibitor canagliflozin (Invokana).
The patient’s Canadian clinicians stopped her treatment with both rosuvastatin and canagliflozin, administered intravenous crystalloid fluids, and within days her pain subsided and her limb weakness gradually improved, allowing her discharge 10 days later while she was ambulating with a walker.
“To our knowledge this is the first published report of a drug interaction between rosuvastatin and canagliflozin,” wrote the authors of the case report (Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549). They cited the importance of the observation given the widespread use today of rosuvastatin for lowering low density lipoprotein cholesterol and exerting pleiotropic effects; and canagliflozin for its modest effects for reducing hyperglycemia, as well as its important role in reducing adverse cardiovascular outcomes, slowing progression of chronic kidney disease, and having a mild but important diuretic effect. “We encourage clinicians to remain vigilant for features of myotoxicity when canagliflozin and rosuvastatin are coprescribed,” they wrote, avoiding discussion of whether this may represent class or drug-specific effects.
“It’s reasonable to be mindful of this risk, but this is not a reason to not use rosuvastatin and canagliflozin in a patient,” nor for the time being to avoid any other combination of a statin and SGLT2 (sodium-glucose cotransporter 2) inhibitor, said David Juurlink, MD, head of the division of clinical pharmacology and toxicology at Sunnybrook Health Sciences Centre in Toronto and lead author of the report. “Few drug interactions have absolute contraindications. The admonition is just to be careful. It’s premature to say they shouldn’t be used together,” he said in an interview.
“We don’t know how much of an outlier this patient is. But it would be important to tell patients” on this or a similar combination to alert their clinicians if they start to have muscle aches, which should be a “red flag” to stop the statin, the SGLT2 inhibitor, or both until the situation can be fully assessed, Dr. Juurlink advised.
Sky high rosuvastatin levels
The linchpin of the observed adverse effects appeared to be a startlingly high elevation of the patient’s plasma rosuvastatin level when she was hospitalized 15 days after starting canagliflozin and 12 days after the onset of her thigh pain and weakness. Testing showed a plasma rosuvastatin concentration of 176 ng/mL, “more than 15-fold higher than the mean value expected” in patients taking 40 mg rosuvastatin daily, the maximum labeled dosage for the drug and what the affected patient had been taking without prior incident for more than 5 years. The patient’s canagliflozin dosage was 100 mg/day, the standard starting dosage according to the drug’s label.
The report’s authors noted that genetic assessment of the patient, a woman originally from the Philippines who was “high functioning,” and diagnosed with type 2 diabetes, showed she was heterozygous for a polymorphism, c.421C>A, which is linked with increased rosuvastatin plasma levels in the plasma. They also cited a report that canagliflozin can interact with proteins involved in hepatic drug uptake.
“We speculate that, in our patient, the addition of canagliflozin enhanced intestinal rosuvastatin absorption, inhibited its hepatocellular uptake, and impaired its excretion into bile canaliculi and the proximal tubule, resulting in rosuvastatin accumulation and leading to hepatotoxicity and myotoxicity,” the clinicians wrote in their report.
“There is little doubt this was a drug interaction, but it does not apply uniformly to everyone.” The severity of the interaction would depend on the dosages, the comorbidities a patient has, and their genetic profile, Dr. Juurlink said.
Concern and skepticism
Other clinicians who regularly prescribe these drugs expressed concern about the observation as well as skepticism about the prevalence of patients who could potentially experience similar effects.
“We don’t know how common are these genetic abnormalities. If this is extremely rare, then it doesn’t have many clinical implications, but if a large portion of the population has this [genetic] abnormality, it’s something we’d need to pay attention to,” Steven E. Nissen, MD, chair of cardiovascular medicine at the Cleveland Clinic Foundation, said in an interview. “It will be important to know the prevalence” of the genetic polymorphism carried by the reported patient, said Dr. Nissen, who has done research on lipid-lowering medications and drug safety.
“This could be important, or a very rare one-off. I can’t say which,” based on what’s currently known, he said. “There are many unanswered questions that make it hard to know how important this will be. It requires further investigation. There is a lot of uncertainty.”
Dr. Nissen particularly endorsed studies that approach this issue by looking at the prevalence rates of the implicated genetic polymorphism rather than pharmacovigilance studies that make epidemiologic assessments of adverse-effect prevalence. Studies that look for adverse-effect associations in large patient populations are “sloppy, and unless the interaction is incredibly intense they are not very sensitive,” he said.
But Dr. Juurlink, a pharmacoepidemiologist whose specialty includes studies of this sort, said that they could be useful if carefully designed. He suggested, for example, comparing in large patient databases the observed incidence of rhabdomyolysis among patients on an SGLT2 inhibitor and also on rosuvastatin with those on pravastatin, a statin with a different metabolic profile. Another approach to further examining the observation would be dosage studies with rosuvastatin and canagliflozin in healthy volunteers, he said.
Dr. Nissen noted that rosuvastatin is a key agent from the statin class because it’s the “most effective” for lowering low density lipoprotein cholesterol. “Rosuvastatin is a go-to drug,” he declared. On the other hand, canagliflozin is “a little less used” than other drugs in the SGLT2 inhibitor class, specifically dapagliflozin (Farxiga) and empagliflozin (Jardiance), he said.
One in a million?
“This was a freak accident. I don’t find it at all concerning. It was definitely one in a million,” Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif., said in an interview. “None of us have seen it” in either the several cardiovascular outcome trials now run on multiple drugs in the SGLT2 inhibitor class that included many patients also taking a statin, or in routine practice, he said. Dr. Handelsman noted that in his practice he had never seen a similar case despite treating “hundreds if not thousands of patients” with type 2 diabetes, virtually all of whom were on a statin and were also treated with an SGLT2 inhibitor, including many with canagliflozin.
Dr. Handelsman cited the notably low estimated glomerular filtration rate in the reported patient, who was described as having a serum creatinine level of 150 mcmol/L (1.7 mg/dL) prior to canagliflozin treatment that then rose to 194 mcmol/L (2.19 mg/dL) at the time of hospitalization, which corresponds to estimated glomerular filtration rates of 29-31 and 21-23 mL/min per 1.73 m2, respectively, depending on the calculator used, rates that were possibly below the labeled minimum rate of 30 mL/min per 1.73 m2 for patients starting canagliflozin treatment. The case report cited the patient as having stage 3B chronic kidney disease, which corresponds to a eGFR of 30-44* mL/min per 1.73 m2.
“I think the patient had acute kidney injury” on starting canagliflozin “that may have affected the [rosuvastatin] metabolism,” Dr. Handelsman suggested. “She had severe kidney dysfunction to start with that fell further with SGLT2 inhibitor treatment,” a well described and usually transient effect of starting drugs in this class because of changes the SGLT2 inhibitors cause in renal blood flow. He noted that the patient had not been receiving an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, which may have contributed to her acute problems with fluid balance. Most similar patients with type 2 diabetes, cardiovascular disease risk, and chronic kidney disease would be on stable treatment with a drug that inhibits the renin-angiotensin system before starting an SGLT2 inhibitor, and not already having a RAS inhibitor on board before starting canagliflozin may have somehow contributed to the observed adverse effects, Dr. Handelsman said.
Dr. Juurlink was skeptical that the kidneys played a major role. “An abrupt change in renal function can influence statin clearance, but this was a 15-fold increase. You can’t explain such a dramatic increase by a transient reduction in renal function,” he said.
Dr. Juurlink and coauthors had no disclosures. Dr. Nissen had no relevant disclosures. Dr. Handelsman has been a consultant to companies that market drugs in the SGLT2 inhibitor class.
SOURCE: Brailovski E et al. Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549.
*Correction: This value was missing from the original article.
FROM ANNALS OF INTERNAL MEDICINE