User login
Hepatitis screening now for all patients with cancer on therapy
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
Novel probiotic shows promise in treating type 2 diabetes
A novel probiotic product (Pendulum Glucose Control) containing gut bacteria strains that are deficient in people with type 2 diabetes modestly improves blood glucose levels, new research suggests.
The findings were published in BMJ Open Diabetes Research & Care by Fanny Perraudeau, PhD, and colleagues, all employees of Pendulum Therapeutics.
The product, classified as a medical food, is currently available for purchase on the company’s website without a prescription.
It contains the oligosaccharide-consuming Akkermansia muciniphila and Bifidobacterium infantis, the butyrate producers Anaerobutyricum hallii, Clostridium beijerinckii, and Clostridium butyricum, along with the “prebiotic” dietary fiber inulin.
In the 12-week trial of people with type 2 diabetes who were already taking metformin, with or without a sulfonylurea, 23 were randomized to the product and 26 received placebo capsules.
Participants in the active-treatment arm had significantly reduced glucose levels after a 3-hour standard meal-tolerance test, by 36.1 mg/dL (P = .05), and average A1c reduction of 0.6 percentage points (P = .054), compared with those taking placebo.
There were no major safety or tolerability issues, only transient gastrointestinal symptoms (nausea, diarrhea) lasting 3-5 days. No changes were seen in body weight, insulin sensitivity, or fasting blood glucose.
Asked to comment on the findings, Nanette I. Steinle, MD, an endocrinologist with expertise in nutrition who was not involved in the research, said that “to me it looks like the research was designed well and they didn’t overstate the results. ... I would say, for folks with mild to modest blood glucose elevations, it could be helpful to augment a healthy lifestyle.”
However, the product is not cheap, so cost could be a limiting factor for some patients, said Dr. Steinle, who is associate professor of medicine at the University of Maryland, Baltimore, and chief of the endocrine section, Maryland Veterans Affairs Health Care System.
Lead author Orville Kolterman, MD, chief medical officer at Pendulum, said in an interview that the formulation’s specificity distinguishes it from most commercially available probiotics.
“The ones sold in stores are reconfigurations of food probiotics, which are primarily aerobic organisms, whereas the abnormalities in the microbiome associated with type 2 diabetes reside in anaerobic organisms, which are more difficult to manufacture,” he explained.
The fiber component, inulin, is important as well, he said. “This product may make the dietary management of type 2 diabetes more effective, in that you need both the fiber and the microbes to ferment the fiber and produce short-chain fatty acids that appear to be very important for many reasons.”
The blood glucose-lowering effect is related in part to the three organisms’ production of butyrate, which binds to epithelial cells in the gut to secrete glucagonlike peptide–1, leading to inhibition of glucagon secretion among other actions.
And Akkermansia muciniphila protects the gut epithelium and has shown some evidence of improving insulin sensitivity and other beneficial metabolic effects in humans.
Dr. Kolterman, who was with Amylin Pharmaceuticals prior to moving to Pendulum, commented: “After doing this for 30 years or so, I’ve come to the strong appreciation that whenever you can do something to move back toward what Mother Nature set up, you’re doing a good thing.”
Clinically, Dr. Kolterman said, “I think perhaps the ideal place to try this would be shortly after diagnosis of type 2 diabetes, before patients go on to pharmacologic therapy.”
However, for practical reasons the study was done in patients who were already taking metformin, he said. “The results we have are that it’s beneficial above and beyond metformin, since [these] patients weren’t controlled with metformin.”
He also noted that it might benefit patients who can’t tolerate metformin or who have prediabetes; there’s an ongoing investigator-initiated study of the latter.
Dr. Steinle also endorsed the possibility that the product may benefit people with prediabetes. “I would suspect this could be very helpful to augment attempts to prevent diabetes. ... The group with prediabetes is huge.”
However, she cautioned, “if the blood glucose is over 200 [mg/dL], I wouldn’t think a probiotic would get them where they need to go.”
Overall, she pointed out that targeting the microbiome is a very active and potentially important field of medical research, and that it has received support from the National Institutes of Health. “I think we’re in the early stages of understanding how what grows in us, and on us, impacts our health and how we may be able to use these organisms to our benefit. I would expect we’ll see more of these probiotics being marketed in various forms.”
Dr. Kolterman is an employee of Pendulum. Dr. Steinle has reported receiving funding from the NIH, and she is conducting a study funded by Kowa through the VA.
A version of this article originally appeared on Medscape.com.
A novel probiotic product (Pendulum Glucose Control) containing gut bacteria strains that are deficient in people with type 2 diabetes modestly improves blood glucose levels, new research suggests.
The findings were published in BMJ Open Diabetes Research & Care by Fanny Perraudeau, PhD, and colleagues, all employees of Pendulum Therapeutics.
The product, classified as a medical food, is currently available for purchase on the company’s website without a prescription.
It contains the oligosaccharide-consuming Akkermansia muciniphila and Bifidobacterium infantis, the butyrate producers Anaerobutyricum hallii, Clostridium beijerinckii, and Clostridium butyricum, along with the “prebiotic” dietary fiber inulin.
In the 12-week trial of people with type 2 diabetes who were already taking metformin, with or without a sulfonylurea, 23 were randomized to the product and 26 received placebo capsules.
Participants in the active-treatment arm had significantly reduced glucose levels after a 3-hour standard meal-tolerance test, by 36.1 mg/dL (P = .05), and average A1c reduction of 0.6 percentage points (P = .054), compared with those taking placebo.
There were no major safety or tolerability issues, only transient gastrointestinal symptoms (nausea, diarrhea) lasting 3-5 days. No changes were seen in body weight, insulin sensitivity, or fasting blood glucose.
Asked to comment on the findings, Nanette I. Steinle, MD, an endocrinologist with expertise in nutrition who was not involved in the research, said that “to me it looks like the research was designed well and they didn’t overstate the results. ... I would say, for folks with mild to modest blood glucose elevations, it could be helpful to augment a healthy lifestyle.”
However, the product is not cheap, so cost could be a limiting factor for some patients, said Dr. Steinle, who is associate professor of medicine at the University of Maryland, Baltimore, and chief of the endocrine section, Maryland Veterans Affairs Health Care System.
Lead author Orville Kolterman, MD, chief medical officer at Pendulum, said in an interview that the formulation’s specificity distinguishes it from most commercially available probiotics.
“The ones sold in stores are reconfigurations of food probiotics, which are primarily aerobic organisms, whereas the abnormalities in the microbiome associated with type 2 diabetes reside in anaerobic organisms, which are more difficult to manufacture,” he explained.
The fiber component, inulin, is important as well, he said. “This product may make the dietary management of type 2 diabetes more effective, in that you need both the fiber and the microbes to ferment the fiber and produce short-chain fatty acids that appear to be very important for many reasons.”
The blood glucose-lowering effect is related in part to the three organisms’ production of butyrate, which binds to epithelial cells in the gut to secrete glucagonlike peptide–1, leading to inhibition of glucagon secretion among other actions.
And Akkermansia muciniphila protects the gut epithelium and has shown some evidence of improving insulin sensitivity and other beneficial metabolic effects in humans.
Dr. Kolterman, who was with Amylin Pharmaceuticals prior to moving to Pendulum, commented: “After doing this for 30 years or so, I’ve come to the strong appreciation that whenever you can do something to move back toward what Mother Nature set up, you’re doing a good thing.”
Clinically, Dr. Kolterman said, “I think perhaps the ideal place to try this would be shortly after diagnosis of type 2 diabetes, before patients go on to pharmacologic therapy.”
However, for practical reasons the study was done in patients who were already taking metformin, he said. “The results we have are that it’s beneficial above and beyond metformin, since [these] patients weren’t controlled with metformin.”
He also noted that it might benefit patients who can’t tolerate metformin or who have prediabetes; there’s an ongoing investigator-initiated study of the latter.
Dr. Steinle also endorsed the possibility that the product may benefit people with prediabetes. “I would suspect this could be very helpful to augment attempts to prevent diabetes. ... The group with prediabetes is huge.”
However, she cautioned, “if the blood glucose is over 200 [mg/dL], I wouldn’t think a probiotic would get them where they need to go.”
Overall, she pointed out that targeting the microbiome is a very active and potentially important field of medical research, and that it has received support from the National Institutes of Health. “I think we’re in the early stages of understanding how what grows in us, and on us, impacts our health and how we may be able to use these organisms to our benefit. I would expect we’ll see more of these probiotics being marketed in various forms.”
Dr. Kolterman is an employee of Pendulum. Dr. Steinle has reported receiving funding from the NIH, and she is conducting a study funded by Kowa through the VA.
A version of this article originally appeared on Medscape.com.
A novel probiotic product (Pendulum Glucose Control) containing gut bacteria strains that are deficient in people with type 2 diabetes modestly improves blood glucose levels, new research suggests.
The findings were published in BMJ Open Diabetes Research & Care by Fanny Perraudeau, PhD, and colleagues, all employees of Pendulum Therapeutics.
The product, classified as a medical food, is currently available for purchase on the company’s website without a prescription.
It contains the oligosaccharide-consuming Akkermansia muciniphila and Bifidobacterium infantis, the butyrate producers Anaerobutyricum hallii, Clostridium beijerinckii, and Clostridium butyricum, along with the “prebiotic” dietary fiber inulin.
In the 12-week trial of people with type 2 diabetes who were already taking metformin, with or without a sulfonylurea, 23 were randomized to the product and 26 received placebo capsules.
Participants in the active-treatment arm had significantly reduced glucose levels after a 3-hour standard meal-tolerance test, by 36.1 mg/dL (P = .05), and average A1c reduction of 0.6 percentage points (P = .054), compared with those taking placebo.
There were no major safety or tolerability issues, only transient gastrointestinal symptoms (nausea, diarrhea) lasting 3-5 days. No changes were seen in body weight, insulin sensitivity, or fasting blood glucose.
Asked to comment on the findings, Nanette I. Steinle, MD, an endocrinologist with expertise in nutrition who was not involved in the research, said that “to me it looks like the research was designed well and they didn’t overstate the results. ... I would say, for folks with mild to modest blood glucose elevations, it could be helpful to augment a healthy lifestyle.”
However, the product is not cheap, so cost could be a limiting factor for some patients, said Dr. Steinle, who is associate professor of medicine at the University of Maryland, Baltimore, and chief of the endocrine section, Maryland Veterans Affairs Health Care System.
Lead author Orville Kolterman, MD, chief medical officer at Pendulum, said in an interview that the formulation’s specificity distinguishes it from most commercially available probiotics.
“The ones sold in stores are reconfigurations of food probiotics, which are primarily aerobic organisms, whereas the abnormalities in the microbiome associated with type 2 diabetes reside in anaerobic organisms, which are more difficult to manufacture,” he explained.
The fiber component, inulin, is important as well, he said. “This product may make the dietary management of type 2 diabetes more effective, in that you need both the fiber and the microbes to ferment the fiber and produce short-chain fatty acids that appear to be very important for many reasons.”
The blood glucose-lowering effect is related in part to the three organisms’ production of butyrate, which binds to epithelial cells in the gut to secrete glucagonlike peptide–1, leading to inhibition of glucagon secretion among other actions.
And Akkermansia muciniphila protects the gut epithelium and has shown some evidence of improving insulin sensitivity and other beneficial metabolic effects in humans.
Dr. Kolterman, who was with Amylin Pharmaceuticals prior to moving to Pendulum, commented: “After doing this for 30 years or so, I’ve come to the strong appreciation that whenever you can do something to move back toward what Mother Nature set up, you’re doing a good thing.”
Clinically, Dr. Kolterman said, “I think perhaps the ideal place to try this would be shortly after diagnosis of type 2 diabetes, before patients go on to pharmacologic therapy.”
However, for practical reasons the study was done in patients who were already taking metformin, he said. “The results we have are that it’s beneficial above and beyond metformin, since [these] patients weren’t controlled with metformin.”
He also noted that it might benefit patients who can’t tolerate metformin or who have prediabetes; there’s an ongoing investigator-initiated study of the latter.
Dr. Steinle also endorsed the possibility that the product may benefit people with prediabetes. “I would suspect this could be very helpful to augment attempts to prevent diabetes. ... The group with prediabetes is huge.”
However, she cautioned, “if the blood glucose is over 200 [mg/dL], I wouldn’t think a probiotic would get them where they need to go.”
Overall, she pointed out that targeting the microbiome is a very active and potentially important field of medical research, and that it has received support from the National Institutes of Health. “I think we’re in the early stages of understanding how what grows in us, and on us, impacts our health and how we may be able to use these organisms to our benefit. I would expect we’ll see more of these probiotics being marketed in various forms.”
Dr. Kolterman is an employee of Pendulum. Dr. Steinle has reported receiving funding from the NIH, and she is conducting a study funded by Kowa through the VA.
A version of this article originally appeared on Medscape.com.
Cardiorespiratory fitness may alter AFib ablation outcomes
Higher baseline cardiorespiratory fitness (CRF) is associated with better outcomes after atrial fibrillation (AFib) ablation, according to new research.
In a single-center, retrospective cohort study, patients with the highest level of baseline CRF had significantly lower rates of arrhythmia recurrence and death than did patients with lower levels of CRF.
“It is stunning how just a simple measure, in this case walking on a treadmill, can predict whether atrial fibrillation ablation will be a successful endeavor or if it will fail,” senior author Wael A. Jaber, MD, professor of medicine, Cleveland Clinic, said in an interview.
“We found that ablation was not successful in most patients who had poor functional class and, conversely, that it was successful in most patients who were in tip-top shape when they walked on the treadmill. Our results can help clinicians inform patients about what they can expect after the procedure, depending on the baseline fitness level,” Dr. Jaber said.
The study was published online Aug. 2 in Heart Rhythm.
Several studies have shown a reduction in AFib incidence among individuals who report a physically active lifestyle, but the extent to which baseline CRF influences arrhythmia rates after AFib ablation is unknown, the authors note.
For the study, Dr. Jaber and colleagues analyzed results in 591 consecutive patients (mean age, 66.5 years; 75% male) with symptomatic paroxysmal or persistent AFib who underwent de novo AFib ablation at their institution. Only patients who had undergone an exercise stress test in the 12 months before AFib ablation (average, 4.5 months) were included.
Age- and sex-specific predicted metabolic equivalents (METs) were calculated using the St. James model for women and the Veterans Affairs referral model for men. The number of METs achieved was then divided by the predicted METs, and the patients were categorized into low (<85% predicted; n = 152), adequate (85%-100% predicted; n = 115), and high (>100% predicted; n = 324) CRF groups. Functional capacity was characterized as poor in 56 patients (9.5%), fair in 94 (16.0%), average in 225 (38.1%), good in 169 (28.6%), and high in 47 (8.0%).
During a mean follow-up of 32 months, arrhythmia recurrence was observed in 79% of patients in the low-CRF group, 54% of patients in the adequate-CRF group, and 27.5% of patients in the high-CRF group (P < .0001). Rates of repeat arrhythmia-related hospitalization, repeat rhythm-control procedures, and the need for ongoing antiarrhythmic therapy (ATT) were significantly lower in the high-CRF group. Specifically, ATT was stopped in 56% of patients in the high-CRF group, compared with 24% in the adequate-CRF group and 11% in the low-CRF group (P < .0001). Rehospitalization for arrhythmia was required in 18.5%, 38.0%, and 60.5% of cases, respectively, and repeat direct-current cardioversion or ablation was performed in 26.0%, 49.0%, and 65.0%, respectively (P < .0001 for both).
Death occurred in 11% of the low-CRF group, compared with 4% in the adequate-CRF group and 2.5% in the high-CRF group. Most (70%) of the deaths were caused by cardiovascular events, including heart failure, cardiac arrest, and coronary artery disease. The most common cause of noncardiac death was respiratory failure (13%), followed by sepsis (10%), malignancy (3%), and complications of Parkinson’s disease (3%).
“Although there was a statistically significant association between higher CRF and lower mortality in this cohort, the findings are to be viewed through the prism of a small sample size and relatively low death rate,” the authors wrote.
Don’t “overpromise” results
“The important message for clinicians is that when, you are discussing what to expect after atrial fibrillation ablation with your patients, do not overpromise the results. You can inform them that the success of the procedure depends more on how they perform on the baseline exercise test, and less on the ablation itself,” Dr. Jaber said.
Clinicians might want to consider advising their patients to become more active and increase their fitness level before undergoing the procedure, but whether doing so will improve outcomes is still unknown.
“This is what we don’t know. It makes sense. Hopefully, our results will encourage people to be more active before they arrive here for the procedure,” he said. “Our study is retrospective and is hypothesis generating, but we are planning a prospective study where patients will be referred to cardiac rehab prior to having ablation to try to improve their functional class to see if this will improve outcomes.”
Survival of the fittest
In an accompanying editorial commentary, Eric Black-Maier, MD, and Jonathan P. Piccini Sr, MD, from Duke University Medical Center, Durham, N.C., wrote that the findings have “important implications for clinical practice and raise important additional questions.”
They note that catheter ablation as a first-line rhythm-control strategy, per current recommendations, “seems reasonable” in individuals with high baseline cardiorespiratory fitness, but that the benefit is less clear for patients with poor baseline CRF and uncontrolled risk factors.
“Significant limitations in functional status may be at least partially attributable to uncontrolled [AFib], and patients with limited exercise capacity may stand to gain most from successful catheter ablation,” the editorialists wrote.
“Furthermore, because shorter time from [AFib] diagnosis to catheter ablation has been associated with improved outcomes, the decision to postpone ablation in favor of lifestyle modification is not without potential adverse consequences,” they added.
Dr. Black-Maier and Dr. Piccini agree with the need for additional prospective randomized clinical trials to confirm that exercise training to improve cardiorespiratory fitness before AFib ablation is practical and effective for reducing arrhythmia recurrence.
“Over the past 50-plus years, our understanding of cardiorespiratory fitness, exercise capacity, and arrhythmia occurrence in patients with [AFib] continues to evolve,” the editorialists concluded. Data from the study “clearly demonstrate that arrhythmia-free survival is indeed survival of the fittest. Time will tell if exercise training and improvements in cardiorespiratory fitness can change outcomes after ablation.”
The study was sponsored by the Cleveland Clinic. Dr. Jaber and Dr. Black-Maier report no relevant financial relationships. Dr. Piccini receives grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, MyoKardia, Sanofi, Philips, and UpToDate.
A version of this story originally appeared on Medscape.com.
Higher baseline cardiorespiratory fitness (CRF) is associated with better outcomes after atrial fibrillation (AFib) ablation, according to new research.
In a single-center, retrospective cohort study, patients with the highest level of baseline CRF had significantly lower rates of arrhythmia recurrence and death than did patients with lower levels of CRF.
“It is stunning how just a simple measure, in this case walking on a treadmill, can predict whether atrial fibrillation ablation will be a successful endeavor or if it will fail,” senior author Wael A. Jaber, MD, professor of medicine, Cleveland Clinic, said in an interview.
“We found that ablation was not successful in most patients who had poor functional class and, conversely, that it was successful in most patients who were in tip-top shape when they walked on the treadmill. Our results can help clinicians inform patients about what they can expect after the procedure, depending on the baseline fitness level,” Dr. Jaber said.
The study was published online Aug. 2 in Heart Rhythm.
Several studies have shown a reduction in AFib incidence among individuals who report a physically active lifestyle, but the extent to which baseline CRF influences arrhythmia rates after AFib ablation is unknown, the authors note.
For the study, Dr. Jaber and colleagues analyzed results in 591 consecutive patients (mean age, 66.5 years; 75% male) with symptomatic paroxysmal or persistent AFib who underwent de novo AFib ablation at their institution. Only patients who had undergone an exercise stress test in the 12 months before AFib ablation (average, 4.5 months) were included.
Age- and sex-specific predicted metabolic equivalents (METs) were calculated using the St. James model for women and the Veterans Affairs referral model for men. The number of METs achieved was then divided by the predicted METs, and the patients were categorized into low (<85% predicted; n = 152), adequate (85%-100% predicted; n = 115), and high (>100% predicted; n = 324) CRF groups. Functional capacity was characterized as poor in 56 patients (9.5%), fair in 94 (16.0%), average in 225 (38.1%), good in 169 (28.6%), and high in 47 (8.0%).
During a mean follow-up of 32 months, arrhythmia recurrence was observed in 79% of patients in the low-CRF group, 54% of patients in the adequate-CRF group, and 27.5% of patients in the high-CRF group (P < .0001). Rates of repeat arrhythmia-related hospitalization, repeat rhythm-control procedures, and the need for ongoing antiarrhythmic therapy (ATT) were significantly lower in the high-CRF group. Specifically, ATT was stopped in 56% of patients in the high-CRF group, compared with 24% in the adequate-CRF group and 11% in the low-CRF group (P < .0001). Rehospitalization for arrhythmia was required in 18.5%, 38.0%, and 60.5% of cases, respectively, and repeat direct-current cardioversion or ablation was performed in 26.0%, 49.0%, and 65.0%, respectively (P < .0001 for both).
Death occurred in 11% of the low-CRF group, compared with 4% in the adequate-CRF group and 2.5% in the high-CRF group. Most (70%) of the deaths were caused by cardiovascular events, including heart failure, cardiac arrest, and coronary artery disease. The most common cause of noncardiac death was respiratory failure (13%), followed by sepsis (10%), malignancy (3%), and complications of Parkinson’s disease (3%).
“Although there was a statistically significant association between higher CRF and lower mortality in this cohort, the findings are to be viewed through the prism of a small sample size and relatively low death rate,” the authors wrote.
Don’t “overpromise” results
“The important message for clinicians is that when, you are discussing what to expect after atrial fibrillation ablation with your patients, do not overpromise the results. You can inform them that the success of the procedure depends more on how they perform on the baseline exercise test, and less on the ablation itself,” Dr. Jaber said.
Clinicians might want to consider advising their patients to become more active and increase their fitness level before undergoing the procedure, but whether doing so will improve outcomes is still unknown.
“This is what we don’t know. It makes sense. Hopefully, our results will encourage people to be more active before they arrive here for the procedure,” he said. “Our study is retrospective and is hypothesis generating, but we are planning a prospective study where patients will be referred to cardiac rehab prior to having ablation to try to improve their functional class to see if this will improve outcomes.”
Survival of the fittest
In an accompanying editorial commentary, Eric Black-Maier, MD, and Jonathan P. Piccini Sr, MD, from Duke University Medical Center, Durham, N.C., wrote that the findings have “important implications for clinical practice and raise important additional questions.”
They note that catheter ablation as a first-line rhythm-control strategy, per current recommendations, “seems reasonable” in individuals with high baseline cardiorespiratory fitness, but that the benefit is less clear for patients with poor baseline CRF and uncontrolled risk factors.
“Significant limitations in functional status may be at least partially attributable to uncontrolled [AFib], and patients with limited exercise capacity may stand to gain most from successful catheter ablation,” the editorialists wrote.
“Furthermore, because shorter time from [AFib] diagnosis to catheter ablation has been associated with improved outcomes, the decision to postpone ablation in favor of lifestyle modification is not without potential adverse consequences,” they added.
Dr. Black-Maier and Dr. Piccini agree with the need for additional prospective randomized clinical trials to confirm that exercise training to improve cardiorespiratory fitness before AFib ablation is practical and effective for reducing arrhythmia recurrence.
“Over the past 50-plus years, our understanding of cardiorespiratory fitness, exercise capacity, and arrhythmia occurrence in patients with [AFib] continues to evolve,” the editorialists concluded. Data from the study “clearly demonstrate that arrhythmia-free survival is indeed survival of the fittest. Time will tell if exercise training and improvements in cardiorespiratory fitness can change outcomes after ablation.”
The study was sponsored by the Cleveland Clinic. Dr. Jaber and Dr. Black-Maier report no relevant financial relationships. Dr. Piccini receives grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, MyoKardia, Sanofi, Philips, and UpToDate.
A version of this story originally appeared on Medscape.com.
Higher baseline cardiorespiratory fitness (CRF) is associated with better outcomes after atrial fibrillation (AFib) ablation, according to new research.
In a single-center, retrospective cohort study, patients with the highest level of baseline CRF had significantly lower rates of arrhythmia recurrence and death than did patients with lower levels of CRF.
“It is stunning how just a simple measure, in this case walking on a treadmill, can predict whether atrial fibrillation ablation will be a successful endeavor or if it will fail,” senior author Wael A. Jaber, MD, professor of medicine, Cleveland Clinic, said in an interview.
“We found that ablation was not successful in most patients who had poor functional class and, conversely, that it was successful in most patients who were in tip-top shape when they walked on the treadmill. Our results can help clinicians inform patients about what they can expect after the procedure, depending on the baseline fitness level,” Dr. Jaber said.
The study was published online Aug. 2 in Heart Rhythm.
Several studies have shown a reduction in AFib incidence among individuals who report a physically active lifestyle, but the extent to which baseline CRF influences arrhythmia rates after AFib ablation is unknown, the authors note.
For the study, Dr. Jaber and colleagues analyzed results in 591 consecutive patients (mean age, 66.5 years; 75% male) with symptomatic paroxysmal or persistent AFib who underwent de novo AFib ablation at their institution. Only patients who had undergone an exercise stress test in the 12 months before AFib ablation (average, 4.5 months) were included.
Age- and sex-specific predicted metabolic equivalents (METs) were calculated using the St. James model for women and the Veterans Affairs referral model for men. The number of METs achieved was then divided by the predicted METs, and the patients were categorized into low (<85% predicted; n = 152), adequate (85%-100% predicted; n = 115), and high (>100% predicted; n = 324) CRF groups. Functional capacity was characterized as poor in 56 patients (9.5%), fair in 94 (16.0%), average in 225 (38.1%), good in 169 (28.6%), and high in 47 (8.0%).
During a mean follow-up of 32 months, arrhythmia recurrence was observed in 79% of patients in the low-CRF group, 54% of patients in the adequate-CRF group, and 27.5% of patients in the high-CRF group (P < .0001). Rates of repeat arrhythmia-related hospitalization, repeat rhythm-control procedures, and the need for ongoing antiarrhythmic therapy (ATT) were significantly lower in the high-CRF group. Specifically, ATT was stopped in 56% of patients in the high-CRF group, compared with 24% in the adequate-CRF group and 11% in the low-CRF group (P < .0001). Rehospitalization for arrhythmia was required in 18.5%, 38.0%, and 60.5% of cases, respectively, and repeat direct-current cardioversion or ablation was performed in 26.0%, 49.0%, and 65.0%, respectively (P < .0001 for both).
Death occurred in 11% of the low-CRF group, compared with 4% in the adequate-CRF group and 2.5% in the high-CRF group. Most (70%) of the deaths were caused by cardiovascular events, including heart failure, cardiac arrest, and coronary artery disease. The most common cause of noncardiac death was respiratory failure (13%), followed by sepsis (10%), malignancy (3%), and complications of Parkinson’s disease (3%).
“Although there was a statistically significant association between higher CRF and lower mortality in this cohort, the findings are to be viewed through the prism of a small sample size and relatively low death rate,” the authors wrote.
Don’t “overpromise” results
“The important message for clinicians is that when, you are discussing what to expect after atrial fibrillation ablation with your patients, do not overpromise the results. You can inform them that the success of the procedure depends more on how they perform on the baseline exercise test, and less on the ablation itself,” Dr. Jaber said.
Clinicians might want to consider advising their patients to become more active and increase their fitness level before undergoing the procedure, but whether doing so will improve outcomes is still unknown.
“This is what we don’t know. It makes sense. Hopefully, our results will encourage people to be more active before they arrive here for the procedure,” he said. “Our study is retrospective and is hypothesis generating, but we are planning a prospective study where patients will be referred to cardiac rehab prior to having ablation to try to improve their functional class to see if this will improve outcomes.”
Survival of the fittest
In an accompanying editorial commentary, Eric Black-Maier, MD, and Jonathan P. Piccini Sr, MD, from Duke University Medical Center, Durham, N.C., wrote that the findings have “important implications for clinical practice and raise important additional questions.”
They note that catheter ablation as a first-line rhythm-control strategy, per current recommendations, “seems reasonable” in individuals with high baseline cardiorespiratory fitness, but that the benefit is less clear for patients with poor baseline CRF and uncontrolled risk factors.
“Significant limitations in functional status may be at least partially attributable to uncontrolled [AFib], and patients with limited exercise capacity may stand to gain most from successful catheter ablation,” the editorialists wrote.
“Furthermore, because shorter time from [AFib] diagnosis to catheter ablation has been associated with improved outcomes, the decision to postpone ablation in favor of lifestyle modification is not without potential adverse consequences,” they added.
Dr. Black-Maier and Dr. Piccini agree with the need for additional prospective randomized clinical trials to confirm that exercise training to improve cardiorespiratory fitness before AFib ablation is practical and effective for reducing arrhythmia recurrence.
“Over the past 50-plus years, our understanding of cardiorespiratory fitness, exercise capacity, and arrhythmia occurrence in patients with [AFib] continues to evolve,” the editorialists concluded. Data from the study “clearly demonstrate that arrhythmia-free survival is indeed survival of the fittest. Time will tell if exercise training and improvements in cardiorespiratory fitness can change outcomes after ablation.”
The study was sponsored by the Cleveland Clinic. Dr. Jaber and Dr. Black-Maier report no relevant financial relationships. Dr. Piccini receives grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, MyoKardia, Sanofi, Philips, and UpToDate.
A version of this story originally appeared on Medscape.com.
Weight gain persists as HIV-treatment issue
People living with HIV who put on extra pounds and develop metabolic syndrome or related disorders linked in part to certain antiretroviral agents remain a concern today, even as the drugs used to suppress HIV infection have evolved over the decades.
Linkage of HIV treatment with lipodystrophy and insulin resistance or diabetes began in the 1990s with protease inhibitors (Clin Infect Dis. 2000 Jun;30[suppl 2]:s135-42). Several reports over the years also tied any form of effective antiretroviral therapy to weight gain in HIV patients (Antivir Ther. 2012;17[7]:1281-9). More recently, reports have rattled the HIV-treatment community by associating alarmingly high levels of weight gain with a useful and relatively new drug, tenofovir alafenamide fumarate (TAF) – a nucleoside reverse transcriptase inhibitor (NRTI) approved for use in the United States in late 2016, as well as certain agents from an entirely different antiretroviral therapy (ART) class, the integrase strand transfer inhibitors (INSTIs). Both TAF and the INSTIs have come to play major roles in the HIV-treatment landscape, despite relevant and concerning recent weight gain observations with these drugs, such as in a 2019 meta-analysis of eight trials with 5,680 treatment-naive patients who started ART during 2003-2015 (Clin Infect Dis. 2019 Oct 14;doi: 10.1093/cid/ciz999).
“Weight gain is clearly seen in studies of dolutegravir [DTG] or bictegravir [BTG] with TAF,” wrote W.D. Francois Venter, PhD and Andrew Hill, PhD in a recent published commentary on the topic (Lancet HIV. 2020 Jun 1;7[6]:e389-400). Both DTG and BTG are INSTI class members.
“Excessive weight gain, defined as more than 10% over baseline, has recently been observed among people with HIV initiating or switching to regimens incorporating TAF, an INSTI, or both, particularly DTG,” wrote Jordan E. Lake, MD, an HIV specialist at the University of Texas Health Science Center at Houston, in a recent commentary posted online. Women and Black patients “are at even greater risk for excessive weight gain,” Dr. Lake added.
“In recent times, it has emerged that weight gain is more pronounced with the integrase inhibitor class of agents, especially dolutegravir and bictegravir, the so-called second-generation” INSTIs, said Anna Maria Geretti, MD, a professor of clinical infection, microbiology, and immunology at the University of Liverpool, England. ”The effect is more pronounced in women and people of non-White ethnicity, and is of concern because of the associated potential risk of metabolic syndrome, cardiovascular disease, etc.,” Dr. Geretti said in an interview.
The unprecedented susceptibility to weight gain seen recently in non-White women may in part have resulted from the tendency of many earlier treatment trials to have cohorts comprised predominantly of White men, Dr. Venter noted in an interview.
Alarming weight gains reported
Perhaps the most eye-popping example of the potential for weight gain with the combination of TAF with an INSTI came in a recent report from the ADVANCE trial, a randomized, head-to-head comparison of three regimens in 1,053 HIV patients in South Africa. After 144 weeks on a regimen of TAF (Vemlidy), DTG (Tivicay), and FTC (emtricitabine, Emtriva), another NRTI, women gained an averaged of more than 12 kg, compared with their baseline weight, significantly more than in two comparator groups, Simiso Sokhela, MB, reported at the virtual meeting of the International AIDS conference. The women in ADVANCE on the TAF-DTG-FTC regimen also had an 11% rate of incident metabolic syndrome during their first 96 weeks on treatment, compared with rates of 8% among patients on a different form of tenofovir, tenofovir disoproxil fumarate (TDF), along with DTG-FTC, and 5% among those on TDF–EFV (efavirenz, Sustiva)–FTC said Dr. Sokhela, an HIV researcher at Ezintsha, a division of the University of the Witwatersrand in Johannesburg, South Africa.
“We believe that these results support the World Health Organization guidelines that reserve TAF for only patients with osteoporosis or impaired renal function,” Dr. Sokhela said during a press briefing at the conference. The WHO guidelines list the first-line regimen as TDF-DTG-3TC (lamivudine; Epivir) or FTC. “The risk for becoming obese continued to increase after 96 weeks” of chronic use of these drugs, she added.
“All regimens are now brilliant at viral control. Finding the ones that don’t make patients obese or have other long-term side effects is now the priority,” noted Dr. Venter, a professor and HIV researcher at University of the Witwatersrand, head of Ezintsha, and lead investigator of ADVANCE. Clinicians and researchers have recently thought that combining TAF and an INSTI plus FTC or a similar NRTI “would be the ultimate regimen to replace the nonnucleoside reverse transcriptase inhibitors (NNRTIs)” such as EFV, “but now we have a major headache” with unexpectedly high weight gains in some patients, Dr. Venter said.
Weight gains “over 10 kg are unlikely to be acceptable in any circumstances, especially when starting body mass index is already borderline overweight,” wrote Dr. Venter along with Dr. Hill in their commentary. Until recently, many clinicians chalked up weight gain on newly begun ART as a manifestation of the patient’s “return-to-health,” but this interpretation “gives a positive spin to a potentially serious and common side effect,” they added.
More from ADVANCE
The primary efficacy endpoint of ADVANCE was suppression of viral load to less than 50 RNA copies/mL after 48 weeks on treatment, and the result showed that the TAF-DTG-FTC regimen and the TDF-DTG-FTC regimen were each noninferior to the control regimen of TDF-EFV-FTC (New Engl J Med. 2019 Aug 29;381[9]:803-15). Virtually all of the enrolled patients were Black, and 59% were women. Planned follow-up of all patients ran for 96 weeks. After 48 weeks, weight gain among the women averaged 6.4 kg, 3.2 kg, and 1.7 kg in the TAF-DTG, TDF-DTG, and TDF-EFV arms respectively. After 96 weeks, the average weight gains among women were 8.2 kg, 4.6 kg, and 3.2 kg, respectively, in new results reported by Dr. Sokhela at the IAC. Follow-up to 144 weeks was partial and included about a quarter of the enrolled women, with gains averaging 12.3 kg, 7.4 kg, and 5.5 kg respectively. The pattern of weight gain among men tracked the pattern in women, but the magnitude of gain was less. Among men followed for 144 weeks, average gain among those on TAF-DTG-FTC was 7.2 kg, the largest gain seen among men on any regimen and at any follow-up time in the study.
Dr. Sokhela also reported data on body composition analyses, which showed that the weight gains were largely in fat rather than lean tissue, fat accumulation was significantly greater in women than men, and that in both sexes fat accumulated roughly equally in the trunk and on limbs.
An additional analysis looked at the incidence of new-onset obesity among the women who had a normal body mass index at baseline. After 96 weeks, incident obesity occurred in 14% of women on the TAG-DTG-FTC regimen, 8% on TDF-DTG-FTC, and in 2% of women maintained on TDF-EFV-FTC, said Dr. Hill in a separate report at the conference.
Weight starts to weigh in
“I am very mindful of weight gain potential, and I talk to patients about it. It doesn’t determine what regimen I choose for a patient” right now, “but it’s only a matter of time before it starts influencing what we do, particularly if we can achieve efficacy with fewer drugs,” commented Babafemi O. Taiwo, MD, professor of medicine and chief of infectious diseases at Northwestern University in Chicago. “I’ve had some patients show up with a weight gain of 20 kg, and that shouldn’t happen,” he said during a recent online educational session. Dr. Taiwo said his recent practice has been to warn patients about possible weight gain and to urge them to get back in touch with him quickly if it happens.
“Virologic suppression is the most important goal with ART, and the U.S. Department of Health and Human Services currently recommends INSTI-based ART for most PWH [people with HIV],” wrote Dr. Lake in April 2020. “I counsel all PWH initiating ART about the potential for weight gain, and I discuss their current diet and healthy lifestyle habits. I explain to patients that we will monitor their weight, and if weight gain seems more than either of us are comfortable with then we will reassess. Only a small percentage of patients experience excessive weight gain after starting ART.” Dr. Lake also stressed that she had not yet begun to change the regimen a patient is on solely because of weight gain. “We do not know whether this weight gain is reversible,” she noted.
“I do not anticipate that a risk of weight gain at present will dictate a change in guidelines,” said Dr. Geretti. “Drugs such as dolutegravir and bictegravir are very effective, and they are unlikely to cause drug resistance. Further data on the mechanism of weight gain and the reversibility after a change of treatment will help refine drug selection in the near future,” she predicted.
“I consider weight gain when prescribing because my patients hear about this. It’s a side effect that my patients really care about, and I don’t blame them,” said Lisa Hightow-Weidman, MD, a professor and HIV specialist at the University of North Carolina at Chapel Hill, during an on-line educational session. “If you don’t discuss it with a patient and then weight gain happens and the patient finds out [the known risk from their treatment] they may have an issue,” she noted. But weight gain is not a reason to avoid these drugs. “They are great medications in many ways, with once-daily regimens and few side effects.”
Weight gain during pregnancy a special concern
An additional analysis of data from ADVANCE presented at the conference highlighted what the observed weight gain on ART could mean for women who become pregnant while on treatment. Based on a systematic literature review, the ADVANCE investigators calculated the relative risk for six obesity-related pregnancy complications, compared with nonobese women: preterm delivery, gestational diabetes, gestational hypertension, preeclampsia, postpartum hemorrhage, and caesarean delivery. Based on the obesity changes among women on their assigned ART in ADVANCE, the researchers calculated the predicted incidence of these six complications. The analysis showed that for every 1,000 women, those on TAG-DTG-FTC would have an excess of 53 obesity-related pregnancy complications, those on TDF-DTG-FTC would develop 28 excess pregnancy complications, and those on TDG-EFV-FTC would have four excess complications, reported Dr. Hill at the International AIDS conference.
The researchers also ran a similar simulation for the incidence of neonatal complications that could result when mothers are obese because of their ART. The six neonatal complications included in this analysis were small for gestational age, large for gestational age, macrosomia, neonatal death, stillbirth, and neural tube defects. Based on the excess rate of incident obesity, they calculated that for every 1,000 pregnancies women on TAD-DTG-FTC would have 24 additional infants born with one of these complications, women on TDF-DTG-FTC would have an excess of 13 of these events, and women on TDG-EFV-FTC would have an excess of three such obesity-related neonatal complications, Dr. Hill said.
Sorting out the drugs
Results from several additional studies reported at the conference have started trying to discern exactly which ART drugs and regimens pose the greatest weight gain risk and which have the least risk while retaining high efficacy and resistance barriers.
Further evidence implicating any type of ART as a driver of increased weight came from a review of 8,256 adults infected with HIV and members of the Kaiser Permanente health system in three U.S. regions during 2000-2016. Researchers matched these cases using several demographic factors with just under 130,000 members without HIV. Those infected by HIV had half the prevalence of obesity as the matched controls at baseline. During 12 years of follow-up, those infected with HIV had a threefold higher rate of weight gain than those who were uninfected. Annual weight gain averaged 0.06 kg/year among the uninfected people and 0.22 kg/year among those infected with HIV, a statistically significant difference that was consistent regardless of whether people started the study at a normal body mass index, overweight, or obese, reported Michael J. Silverberg, PhD, an epidemiologist with Kaiser Permanente in Oakland, Calif.
Another study tried to focus on the weight gain impact when patients on three-drug ART regimens changed from taking TDF to TAF. This analysis used data collected in the OPERA (Observational Pharmaco-Epidemiology Research & Analysis) longitudinal cohort of about 115,000 U.S. PWH. The observational cohort included nearly 7,000 patients who made a TDF-to-TAF switch, including 3,288 patients who maintained treatment during this switch with an INSTI, 1,454 who maintained a background regimen based on a NNRTI, 1,430 patients who also switched from an INSTI to a different drug, and 747 patients maintained on a boosted dose of a protease inhibitor. All patients were well controlled on their baseline regimen, with at least two consecutive measures showing undetectable viral load.
Patients who maintained their background regimens while changing from TDF to TAF had a 2.0-2.6 kg increase in weight during the 9 months immediately following their switch to TAF, reported Patrick Mallon, MB, a professor of microbial diseases at University College Dublin. Among the patients who both switched to TAF and also switched to treatment with an INSTI, weight gain during the 9 months after the switch averaged 2.6-4.5 kg, depending on which INSTI was started. Patients who switched to treatment with elvitegravir/cobicistat (an INSTI plus a boosting agent) averaged a gain of 2.6 kg during 9 months, those who switched to DTG averaged a 3.1-kg gain, and those who switched to BTG averaged a 4.6-kg increase, Dr. Mallon reported at the conference.
These findings “give us a good sense that the weight gain is real. This is not just overeating or not exercising, but weight changes coincidental with a change in HIV treatment,” commented David Wohl, MD, professor of medicine and site leader of the HIV Prevention and Treatment Clinical Trials Unit at the University of North Carolina at Chapel Hill, during an online educational session.
Contrary to this evidence suggesting a consistent uptick in weight when patients start TAF treatment was a recent report on 629 HIV patients randomized to treatment with TAF-BTG-FTC or abacavir (an NRTI, Ziagen)–DTG-3TC, which found similar weight gains between these two regimens after 144 weeks on treatment (Lancet HIV. 2020 Jun;7[6]:e389-400). This finding had the effect of “strengthening the argument that TAF is simply an innocent bystander” and does not play a central role in weight gain, and supporting the notion that the alternative tenofovir formulation, TDF, differs from TAF by promoting weight loss, Dr. Venter and Dr. Hill suggested in their commentary that accompanied this report.
The new findings from Dr. Mallon raise “serious questions about the way we have moved to TAF as a replacement for TDF, especially because the benefits [from TAF] are for a small subgroup – patients with renal disease or osteoporosis,” Dr. Venter said in an interview. “The question is, will we see weight gain like this” if TAF was combined with a non-INSTI drug? he wondered.
While some study results have suggested a mitigating effect from TDF on weight gain, that wasn’t the case in the AFRICOS (African Cohort Study) study of 1,954 PWH who started treatment with TDF-DTG-FTC (742 patients) or a different three-drug regimen. After a median of 225 days on treatment, those who started on TDF-DTG-FTC had an adjusted, 85% higher rate of developing a high body mass index, compared with patients on a different ART regimen, Julie Ake, MD, reported in a talk at the conference. Her conclusion focused on the possible involvement of DTG: “Consistent with previous reports, dolutegravir was significantly associated with an increased risk of developing high body mass index,” said Dr. Ake, director of the U.S. Military HIV Research Program in Bethesda, Md. and leader of AFRICOS.
A potential workaround to some drugs that cause excessive the weight gain is to just not use them. That was part of the rationale for the TANGO study, which took 741 HIV-infected patients with successful viral suppression on a regimen of TAF-FTC plus one or two additional agents and switched half of them to a TAF-less, two-drug regimen of DTG-FTC. This open-label study’s primary endpoint was noninferiority for viral suppression of the DTG-FTC regimen, compared with patients who stayed on their starting regimen, and the results proved that DTG-FTC was just as effective over 48 weeks for this outcome (Clin Infect Dis. 2020 Jan 6. doi: 10.1093/cid/ciz1243).
At the conference, TANGO’s lead investigator, Jean van Wyk, MD, reported the weight and metabolic effects of the switch. The results showed a similar and small weight gain (on average less than 1 kg) during 48 week follow-up regardless of whether patients remained on their baseline, TAF-containing regimen or switched to DTG-FTC, said Dr. van Wyk, global medical lead for HIV treatment at Viiv Healthcare, the company that markets DTG. About three-quarters of patients in both arms received “boosted” dosages of their drugs, and in this subgroup, patients on DTG-FTC showed statistically significant benefits in several lipid levels, fasting glucose level, and in their degree of insulin resistance. Dr. van Wyk said. These between-group differences were not statistically significant among the “unboosted” patients, and the results failed to show a significant between-group difference in the incidence of metabolic syndrome.
Dr. Venter called these results “exciting,” and noted that he already uses the DTG-FTC two-drug combination “a lot” to treat PWH and renal disease.
A second alternative regimen showcased in a talk at the conference used the three-drug regimen of TDF-FTC plus the NNRTI, DOR (doravirine, Pifeltro). The DRIVE-SHIFT trial enrolled 670 HIV patients with successfully suppressed viral load on conventional regimens who were either switched to TDF-DOR-FTC or maintained on their baseline treatment. After 48 weeks, results confirmed the primary efficacy endpoint of noninferiority for maintenance of suppression with the investigational regimen (J Acquir Immune Defic Syndr. 2019 Aug;81[4]:463-72).
A post-hoc analysis looked at weight changes among these patients after as much as 144 weeks of follow-up. The results showed that patients switched to TDF-DOR-FTC had an average weight increase of 1.2-1.4 kg after more than 2 years on the new regimen, with fewer than 10% of patients having a 10% or greater weight gain with DOR, a “next-generation” NNRTI, reported Princy N. Kumar, MD, professor at Georgetown University and chief of infectious diseases at MedStar Georgetown University Hospital in Washington. “Weight gain was minimal, even over the long term,” she noted.
The tested DOR-based regimen also looks “very exciting,” but the populations it’s been tested have also been largely limited to White men, and limited data exist about the regimen’s performance in pregnant women, commented Dr. Venter. The DRIVE-SHIRT patient cohort was about 85% men, and about three-quarters White.
More weight data needed
HIV-treatment researchers and clinicians seem agreed that weight gain and other metabolic effects from HIV treatment need more assessment and evidence because current data, while suggestive, is also inconclusive.
“Clinical trials are desperately needed to understand the mechanisms of and potential therapeutic options for excessive weight gain on ART,” wrote Dr. Lake in her commentary in April. “While more research is needed,” the new data reported at the virtual International AIDS conference “get us closer to understanding the effects of integrase inhibitors and TAF on weight and the potential metabolic consequences,” she commented as chair of the conference session where these reports occurred.
“Further data on the mechanism of weight gain and its reversibility after a change of treatment will help refine drug selection in the near future,” predicted Dr. Geretti.
“It’s hard to understand physiologically how drugs from such different classes all seem to have weight effects; it’s maddening,” said Dr. Venter. “We need decent studies in all patient populations. That will now be the priority,” he declared. “Patients shouldn’t have to choose” between drugs that most effectively control their HIV infection and drugs that don’t pose a risk for weight gain or metabolic derangements. PWH “should not have to face obesity as their new epidemic,” he wrote with Dr. Hill.
ADVANCE was funded in part by Viiv, the company that markets dolutegravir (Tivicay), and received drugs supplied by Gilead and Viiv. TANGO was sponsored by Viiv. DRIVE-SHIFT was funded by Merck, the company that markets doravirine (Pifeltro). Dr. Lake, Dr. Sokhela, Dr. Ake, and Dr. Kumar had no disclosures, Dr. Venter has received personal fees from Adcock Ingraham, Aspen Healthcare, Johnson and Johnson, Merck, Mylan, Roche, and Viiv. Dr. Hill has received payments from Merck. Dr. Geretti has received honoraria and research funding from Gilead, Jansse, Roche, and Viiv. Dr. Taiwo has had financial relationships with Gilead, Janssen, and Viiv. Dr. Hightow-Weidman has received honoraria from Gilead and Jansse. Dr. Wohl has been a consultant to Gilead, Johnson and Johnson, and Merck. Dr. Silverberg received research funding from Gilead. Dr. Mallon has been an advisor to and speaker on behalf of Bristol-Myers Squibb, Cilag, Gilead, Jansse, Merck Sharp & Dohme, and Viiv. Dr. van Wyk is a Viiv employee.
People living with HIV who put on extra pounds and develop metabolic syndrome or related disorders linked in part to certain antiretroviral agents remain a concern today, even as the drugs used to suppress HIV infection have evolved over the decades.
Linkage of HIV treatment with lipodystrophy and insulin resistance or diabetes began in the 1990s with protease inhibitors (Clin Infect Dis. 2000 Jun;30[suppl 2]:s135-42). Several reports over the years also tied any form of effective antiretroviral therapy to weight gain in HIV patients (Antivir Ther. 2012;17[7]:1281-9). More recently, reports have rattled the HIV-treatment community by associating alarmingly high levels of weight gain with a useful and relatively new drug, tenofovir alafenamide fumarate (TAF) – a nucleoside reverse transcriptase inhibitor (NRTI) approved for use in the United States in late 2016, as well as certain agents from an entirely different antiretroviral therapy (ART) class, the integrase strand transfer inhibitors (INSTIs). Both TAF and the INSTIs have come to play major roles in the HIV-treatment landscape, despite relevant and concerning recent weight gain observations with these drugs, such as in a 2019 meta-analysis of eight trials with 5,680 treatment-naive patients who started ART during 2003-2015 (Clin Infect Dis. 2019 Oct 14;doi: 10.1093/cid/ciz999).
“Weight gain is clearly seen in studies of dolutegravir [DTG] or bictegravir [BTG] with TAF,” wrote W.D. Francois Venter, PhD and Andrew Hill, PhD in a recent published commentary on the topic (Lancet HIV. 2020 Jun 1;7[6]:e389-400). Both DTG and BTG are INSTI class members.
“Excessive weight gain, defined as more than 10% over baseline, has recently been observed among people with HIV initiating or switching to regimens incorporating TAF, an INSTI, or both, particularly DTG,” wrote Jordan E. Lake, MD, an HIV specialist at the University of Texas Health Science Center at Houston, in a recent commentary posted online. Women and Black patients “are at even greater risk for excessive weight gain,” Dr. Lake added.
“In recent times, it has emerged that weight gain is more pronounced with the integrase inhibitor class of agents, especially dolutegravir and bictegravir, the so-called second-generation” INSTIs, said Anna Maria Geretti, MD, a professor of clinical infection, microbiology, and immunology at the University of Liverpool, England. ”The effect is more pronounced in women and people of non-White ethnicity, and is of concern because of the associated potential risk of metabolic syndrome, cardiovascular disease, etc.,” Dr. Geretti said in an interview.
The unprecedented susceptibility to weight gain seen recently in non-White women may in part have resulted from the tendency of many earlier treatment trials to have cohorts comprised predominantly of White men, Dr. Venter noted in an interview.
Alarming weight gains reported
Perhaps the most eye-popping example of the potential for weight gain with the combination of TAF with an INSTI came in a recent report from the ADVANCE trial, a randomized, head-to-head comparison of three regimens in 1,053 HIV patients in South Africa. After 144 weeks on a regimen of TAF (Vemlidy), DTG (Tivicay), and FTC (emtricitabine, Emtriva), another NRTI, women gained an averaged of more than 12 kg, compared with their baseline weight, significantly more than in two comparator groups, Simiso Sokhela, MB, reported at the virtual meeting of the International AIDS conference. The women in ADVANCE on the TAF-DTG-FTC regimen also had an 11% rate of incident metabolic syndrome during their first 96 weeks on treatment, compared with rates of 8% among patients on a different form of tenofovir, tenofovir disoproxil fumarate (TDF), along with DTG-FTC, and 5% among those on TDF–EFV (efavirenz, Sustiva)–FTC said Dr. Sokhela, an HIV researcher at Ezintsha, a division of the University of the Witwatersrand in Johannesburg, South Africa.
“We believe that these results support the World Health Organization guidelines that reserve TAF for only patients with osteoporosis or impaired renal function,” Dr. Sokhela said during a press briefing at the conference. The WHO guidelines list the first-line regimen as TDF-DTG-3TC (lamivudine; Epivir) or FTC. “The risk for becoming obese continued to increase after 96 weeks” of chronic use of these drugs, she added.
“All regimens are now brilliant at viral control. Finding the ones that don’t make patients obese or have other long-term side effects is now the priority,” noted Dr. Venter, a professor and HIV researcher at University of the Witwatersrand, head of Ezintsha, and lead investigator of ADVANCE. Clinicians and researchers have recently thought that combining TAF and an INSTI plus FTC or a similar NRTI “would be the ultimate regimen to replace the nonnucleoside reverse transcriptase inhibitors (NNRTIs)” such as EFV, “but now we have a major headache” with unexpectedly high weight gains in some patients, Dr. Venter said.
Weight gains “over 10 kg are unlikely to be acceptable in any circumstances, especially when starting body mass index is already borderline overweight,” wrote Dr. Venter along with Dr. Hill in their commentary. Until recently, many clinicians chalked up weight gain on newly begun ART as a manifestation of the patient’s “return-to-health,” but this interpretation “gives a positive spin to a potentially serious and common side effect,” they added.
More from ADVANCE
The primary efficacy endpoint of ADVANCE was suppression of viral load to less than 50 RNA copies/mL after 48 weeks on treatment, and the result showed that the TAF-DTG-FTC regimen and the TDF-DTG-FTC regimen were each noninferior to the control regimen of TDF-EFV-FTC (New Engl J Med. 2019 Aug 29;381[9]:803-15). Virtually all of the enrolled patients were Black, and 59% were women. Planned follow-up of all patients ran for 96 weeks. After 48 weeks, weight gain among the women averaged 6.4 kg, 3.2 kg, and 1.7 kg in the TAF-DTG, TDF-DTG, and TDF-EFV arms respectively. After 96 weeks, the average weight gains among women were 8.2 kg, 4.6 kg, and 3.2 kg, respectively, in new results reported by Dr. Sokhela at the IAC. Follow-up to 144 weeks was partial and included about a quarter of the enrolled women, with gains averaging 12.3 kg, 7.4 kg, and 5.5 kg respectively. The pattern of weight gain among men tracked the pattern in women, but the magnitude of gain was less. Among men followed for 144 weeks, average gain among those on TAF-DTG-FTC was 7.2 kg, the largest gain seen among men on any regimen and at any follow-up time in the study.
Dr. Sokhela also reported data on body composition analyses, which showed that the weight gains were largely in fat rather than lean tissue, fat accumulation was significantly greater in women than men, and that in both sexes fat accumulated roughly equally in the trunk and on limbs.
An additional analysis looked at the incidence of new-onset obesity among the women who had a normal body mass index at baseline. After 96 weeks, incident obesity occurred in 14% of women on the TAG-DTG-FTC regimen, 8% on TDF-DTG-FTC, and in 2% of women maintained on TDF-EFV-FTC, said Dr. Hill in a separate report at the conference.
Weight starts to weigh in
“I am very mindful of weight gain potential, and I talk to patients about it. It doesn’t determine what regimen I choose for a patient” right now, “but it’s only a matter of time before it starts influencing what we do, particularly if we can achieve efficacy with fewer drugs,” commented Babafemi O. Taiwo, MD, professor of medicine and chief of infectious diseases at Northwestern University in Chicago. “I’ve had some patients show up with a weight gain of 20 kg, and that shouldn’t happen,” he said during a recent online educational session. Dr. Taiwo said his recent practice has been to warn patients about possible weight gain and to urge them to get back in touch with him quickly if it happens.
“Virologic suppression is the most important goal with ART, and the U.S. Department of Health and Human Services currently recommends INSTI-based ART for most PWH [people with HIV],” wrote Dr. Lake in April 2020. “I counsel all PWH initiating ART about the potential for weight gain, and I discuss their current diet and healthy lifestyle habits. I explain to patients that we will monitor their weight, and if weight gain seems more than either of us are comfortable with then we will reassess. Only a small percentage of patients experience excessive weight gain after starting ART.” Dr. Lake also stressed that she had not yet begun to change the regimen a patient is on solely because of weight gain. “We do not know whether this weight gain is reversible,” she noted.
“I do not anticipate that a risk of weight gain at present will dictate a change in guidelines,” said Dr. Geretti. “Drugs such as dolutegravir and bictegravir are very effective, and they are unlikely to cause drug resistance. Further data on the mechanism of weight gain and the reversibility after a change of treatment will help refine drug selection in the near future,” she predicted.
“I consider weight gain when prescribing because my patients hear about this. It’s a side effect that my patients really care about, and I don’t blame them,” said Lisa Hightow-Weidman, MD, a professor and HIV specialist at the University of North Carolina at Chapel Hill, during an on-line educational session. “If you don’t discuss it with a patient and then weight gain happens and the patient finds out [the known risk from their treatment] they may have an issue,” she noted. But weight gain is not a reason to avoid these drugs. “They are great medications in many ways, with once-daily regimens and few side effects.”
Weight gain during pregnancy a special concern
An additional analysis of data from ADVANCE presented at the conference highlighted what the observed weight gain on ART could mean for women who become pregnant while on treatment. Based on a systematic literature review, the ADVANCE investigators calculated the relative risk for six obesity-related pregnancy complications, compared with nonobese women: preterm delivery, gestational diabetes, gestational hypertension, preeclampsia, postpartum hemorrhage, and caesarean delivery. Based on the obesity changes among women on their assigned ART in ADVANCE, the researchers calculated the predicted incidence of these six complications. The analysis showed that for every 1,000 women, those on TAG-DTG-FTC would have an excess of 53 obesity-related pregnancy complications, those on TDF-DTG-FTC would develop 28 excess pregnancy complications, and those on TDG-EFV-FTC would have four excess complications, reported Dr. Hill at the International AIDS conference.
The researchers also ran a similar simulation for the incidence of neonatal complications that could result when mothers are obese because of their ART. The six neonatal complications included in this analysis were small for gestational age, large for gestational age, macrosomia, neonatal death, stillbirth, and neural tube defects. Based on the excess rate of incident obesity, they calculated that for every 1,000 pregnancies women on TAD-DTG-FTC would have 24 additional infants born with one of these complications, women on TDF-DTG-FTC would have an excess of 13 of these events, and women on TDG-EFV-FTC would have an excess of three such obesity-related neonatal complications, Dr. Hill said.
Sorting out the drugs
Results from several additional studies reported at the conference have started trying to discern exactly which ART drugs and regimens pose the greatest weight gain risk and which have the least risk while retaining high efficacy and resistance barriers.
Further evidence implicating any type of ART as a driver of increased weight came from a review of 8,256 adults infected with HIV and members of the Kaiser Permanente health system in three U.S. regions during 2000-2016. Researchers matched these cases using several demographic factors with just under 130,000 members without HIV. Those infected by HIV had half the prevalence of obesity as the matched controls at baseline. During 12 years of follow-up, those infected with HIV had a threefold higher rate of weight gain than those who were uninfected. Annual weight gain averaged 0.06 kg/year among the uninfected people and 0.22 kg/year among those infected with HIV, a statistically significant difference that was consistent regardless of whether people started the study at a normal body mass index, overweight, or obese, reported Michael J. Silverberg, PhD, an epidemiologist with Kaiser Permanente in Oakland, Calif.
Another study tried to focus on the weight gain impact when patients on three-drug ART regimens changed from taking TDF to TAF. This analysis used data collected in the OPERA (Observational Pharmaco-Epidemiology Research & Analysis) longitudinal cohort of about 115,000 U.S. PWH. The observational cohort included nearly 7,000 patients who made a TDF-to-TAF switch, including 3,288 patients who maintained treatment during this switch with an INSTI, 1,454 who maintained a background regimen based on a NNRTI, 1,430 patients who also switched from an INSTI to a different drug, and 747 patients maintained on a boosted dose of a protease inhibitor. All patients were well controlled on their baseline regimen, with at least two consecutive measures showing undetectable viral load.
Patients who maintained their background regimens while changing from TDF to TAF had a 2.0-2.6 kg increase in weight during the 9 months immediately following their switch to TAF, reported Patrick Mallon, MB, a professor of microbial diseases at University College Dublin. Among the patients who both switched to TAF and also switched to treatment with an INSTI, weight gain during the 9 months after the switch averaged 2.6-4.5 kg, depending on which INSTI was started. Patients who switched to treatment with elvitegravir/cobicistat (an INSTI plus a boosting agent) averaged a gain of 2.6 kg during 9 months, those who switched to DTG averaged a 3.1-kg gain, and those who switched to BTG averaged a 4.6-kg increase, Dr. Mallon reported at the conference.
These findings “give us a good sense that the weight gain is real. This is not just overeating or not exercising, but weight changes coincidental with a change in HIV treatment,” commented David Wohl, MD, professor of medicine and site leader of the HIV Prevention and Treatment Clinical Trials Unit at the University of North Carolina at Chapel Hill, during an online educational session.
Contrary to this evidence suggesting a consistent uptick in weight when patients start TAF treatment was a recent report on 629 HIV patients randomized to treatment with TAF-BTG-FTC or abacavir (an NRTI, Ziagen)–DTG-3TC, which found similar weight gains between these two regimens after 144 weeks on treatment (Lancet HIV. 2020 Jun;7[6]:e389-400). This finding had the effect of “strengthening the argument that TAF is simply an innocent bystander” and does not play a central role in weight gain, and supporting the notion that the alternative tenofovir formulation, TDF, differs from TAF by promoting weight loss, Dr. Venter and Dr. Hill suggested in their commentary that accompanied this report.
The new findings from Dr. Mallon raise “serious questions about the way we have moved to TAF as a replacement for TDF, especially because the benefits [from TAF] are for a small subgroup – patients with renal disease or osteoporosis,” Dr. Venter said in an interview. “The question is, will we see weight gain like this” if TAF was combined with a non-INSTI drug? he wondered.
While some study results have suggested a mitigating effect from TDF on weight gain, that wasn’t the case in the AFRICOS (African Cohort Study) study of 1,954 PWH who started treatment with TDF-DTG-FTC (742 patients) or a different three-drug regimen. After a median of 225 days on treatment, those who started on TDF-DTG-FTC had an adjusted, 85% higher rate of developing a high body mass index, compared with patients on a different ART regimen, Julie Ake, MD, reported in a talk at the conference. Her conclusion focused on the possible involvement of DTG: “Consistent with previous reports, dolutegravir was significantly associated with an increased risk of developing high body mass index,” said Dr. Ake, director of the U.S. Military HIV Research Program in Bethesda, Md. and leader of AFRICOS.
A potential workaround to some drugs that cause excessive the weight gain is to just not use them. That was part of the rationale for the TANGO study, which took 741 HIV-infected patients with successful viral suppression on a regimen of TAF-FTC plus one or two additional agents and switched half of them to a TAF-less, two-drug regimen of DTG-FTC. This open-label study’s primary endpoint was noninferiority for viral suppression of the DTG-FTC regimen, compared with patients who stayed on their starting regimen, and the results proved that DTG-FTC was just as effective over 48 weeks for this outcome (Clin Infect Dis. 2020 Jan 6. doi: 10.1093/cid/ciz1243).
At the conference, TANGO’s lead investigator, Jean van Wyk, MD, reported the weight and metabolic effects of the switch. The results showed a similar and small weight gain (on average less than 1 kg) during 48 week follow-up regardless of whether patients remained on their baseline, TAF-containing regimen or switched to DTG-FTC, said Dr. van Wyk, global medical lead for HIV treatment at Viiv Healthcare, the company that markets DTG. About three-quarters of patients in both arms received “boosted” dosages of their drugs, and in this subgroup, patients on DTG-FTC showed statistically significant benefits in several lipid levels, fasting glucose level, and in their degree of insulin resistance. Dr. van Wyk said. These between-group differences were not statistically significant among the “unboosted” patients, and the results failed to show a significant between-group difference in the incidence of metabolic syndrome.
Dr. Venter called these results “exciting,” and noted that he already uses the DTG-FTC two-drug combination “a lot” to treat PWH and renal disease.
A second alternative regimen showcased in a talk at the conference used the three-drug regimen of TDF-FTC plus the NNRTI, DOR (doravirine, Pifeltro). The DRIVE-SHIFT trial enrolled 670 HIV patients with successfully suppressed viral load on conventional regimens who were either switched to TDF-DOR-FTC or maintained on their baseline treatment. After 48 weeks, results confirmed the primary efficacy endpoint of noninferiority for maintenance of suppression with the investigational regimen (J Acquir Immune Defic Syndr. 2019 Aug;81[4]:463-72).
A post-hoc analysis looked at weight changes among these patients after as much as 144 weeks of follow-up. The results showed that patients switched to TDF-DOR-FTC had an average weight increase of 1.2-1.4 kg after more than 2 years on the new regimen, with fewer than 10% of patients having a 10% or greater weight gain with DOR, a “next-generation” NNRTI, reported Princy N. Kumar, MD, professor at Georgetown University and chief of infectious diseases at MedStar Georgetown University Hospital in Washington. “Weight gain was minimal, even over the long term,” she noted.
The tested DOR-based regimen also looks “very exciting,” but the populations it’s been tested have also been largely limited to White men, and limited data exist about the regimen’s performance in pregnant women, commented Dr. Venter. The DRIVE-SHIRT patient cohort was about 85% men, and about three-quarters White.
More weight data needed
HIV-treatment researchers and clinicians seem agreed that weight gain and other metabolic effects from HIV treatment need more assessment and evidence because current data, while suggestive, is also inconclusive.
“Clinical trials are desperately needed to understand the mechanisms of and potential therapeutic options for excessive weight gain on ART,” wrote Dr. Lake in her commentary in April. “While more research is needed,” the new data reported at the virtual International AIDS conference “get us closer to understanding the effects of integrase inhibitors and TAF on weight and the potential metabolic consequences,” she commented as chair of the conference session where these reports occurred.
“Further data on the mechanism of weight gain and its reversibility after a change of treatment will help refine drug selection in the near future,” predicted Dr. Geretti.
“It’s hard to understand physiologically how drugs from such different classes all seem to have weight effects; it’s maddening,” said Dr. Venter. “We need decent studies in all patient populations. That will now be the priority,” he declared. “Patients shouldn’t have to choose” between drugs that most effectively control their HIV infection and drugs that don’t pose a risk for weight gain or metabolic derangements. PWH “should not have to face obesity as their new epidemic,” he wrote with Dr. Hill.
ADVANCE was funded in part by Viiv, the company that markets dolutegravir (Tivicay), and received drugs supplied by Gilead and Viiv. TANGO was sponsored by Viiv. DRIVE-SHIFT was funded by Merck, the company that markets doravirine (Pifeltro). Dr. Lake, Dr. Sokhela, Dr. Ake, and Dr. Kumar had no disclosures, Dr. Venter has received personal fees from Adcock Ingraham, Aspen Healthcare, Johnson and Johnson, Merck, Mylan, Roche, and Viiv. Dr. Hill has received payments from Merck. Dr. Geretti has received honoraria and research funding from Gilead, Jansse, Roche, and Viiv. Dr. Taiwo has had financial relationships with Gilead, Janssen, and Viiv. Dr. Hightow-Weidman has received honoraria from Gilead and Jansse. Dr. Wohl has been a consultant to Gilead, Johnson and Johnson, and Merck. Dr. Silverberg received research funding from Gilead. Dr. Mallon has been an advisor to and speaker on behalf of Bristol-Myers Squibb, Cilag, Gilead, Jansse, Merck Sharp & Dohme, and Viiv. Dr. van Wyk is a Viiv employee.
People living with HIV who put on extra pounds and develop metabolic syndrome or related disorders linked in part to certain antiretroviral agents remain a concern today, even as the drugs used to suppress HIV infection have evolved over the decades.
Linkage of HIV treatment with lipodystrophy and insulin resistance or diabetes began in the 1990s with protease inhibitors (Clin Infect Dis. 2000 Jun;30[suppl 2]:s135-42). Several reports over the years also tied any form of effective antiretroviral therapy to weight gain in HIV patients (Antivir Ther. 2012;17[7]:1281-9). More recently, reports have rattled the HIV-treatment community by associating alarmingly high levels of weight gain with a useful and relatively new drug, tenofovir alafenamide fumarate (TAF) – a nucleoside reverse transcriptase inhibitor (NRTI) approved for use in the United States in late 2016, as well as certain agents from an entirely different antiretroviral therapy (ART) class, the integrase strand transfer inhibitors (INSTIs). Both TAF and the INSTIs have come to play major roles in the HIV-treatment landscape, despite relevant and concerning recent weight gain observations with these drugs, such as in a 2019 meta-analysis of eight trials with 5,680 treatment-naive patients who started ART during 2003-2015 (Clin Infect Dis. 2019 Oct 14;doi: 10.1093/cid/ciz999).
“Weight gain is clearly seen in studies of dolutegravir [DTG] or bictegravir [BTG] with TAF,” wrote W.D. Francois Venter, PhD and Andrew Hill, PhD in a recent published commentary on the topic (Lancet HIV. 2020 Jun 1;7[6]:e389-400). Both DTG and BTG are INSTI class members.
“Excessive weight gain, defined as more than 10% over baseline, has recently been observed among people with HIV initiating or switching to regimens incorporating TAF, an INSTI, or both, particularly DTG,” wrote Jordan E. Lake, MD, an HIV specialist at the University of Texas Health Science Center at Houston, in a recent commentary posted online. Women and Black patients “are at even greater risk for excessive weight gain,” Dr. Lake added.
“In recent times, it has emerged that weight gain is more pronounced with the integrase inhibitor class of agents, especially dolutegravir and bictegravir, the so-called second-generation” INSTIs, said Anna Maria Geretti, MD, a professor of clinical infection, microbiology, and immunology at the University of Liverpool, England. ”The effect is more pronounced in women and people of non-White ethnicity, and is of concern because of the associated potential risk of metabolic syndrome, cardiovascular disease, etc.,” Dr. Geretti said in an interview.
The unprecedented susceptibility to weight gain seen recently in non-White women may in part have resulted from the tendency of many earlier treatment trials to have cohorts comprised predominantly of White men, Dr. Venter noted in an interview.
Alarming weight gains reported
Perhaps the most eye-popping example of the potential for weight gain with the combination of TAF with an INSTI came in a recent report from the ADVANCE trial, a randomized, head-to-head comparison of three regimens in 1,053 HIV patients in South Africa. After 144 weeks on a regimen of TAF (Vemlidy), DTG (Tivicay), and FTC (emtricitabine, Emtriva), another NRTI, women gained an averaged of more than 12 kg, compared with their baseline weight, significantly more than in two comparator groups, Simiso Sokhela, MB, reported at the virtual meeting of the International AIDS conference. The women in ADVANCE on the TAF-DTG-FTC regimen also had an 11% rate of incident metabolic syndrome during their first 96 weeks on treatment, compared with rates of 8% among patients on a different form of tenofovir, tenofovir disoproxil fumarate (TDF), along with DTG-FTC, and 5% among those on TDF–EFV (efavirenz, Sustiva)–FTC said Dr. Sokhela, an HIV researcher at Ezintsha, a division of the University of the Witwatersrand in Johannesburg, South Africa.
“We believe that these results support the World Health Organization guidelines that reserve TAF for only patients with osteoporosis or impaired renal function,” Dr. Sokhela said during a press briefing at the conference. The WHO guidelines list the first-line regimen as TDF-DTG-3TC (lamivudine; Epivir) or FTC. “The risk for becoming obese continued to increase after 96 weeks” of chronic use of these drugs, she added.
“All regimens are now brilliant at viral control. Finding the ones that don’t make patients obese or have other long-term side effects is now the priority,” noted Dr. Venter, a professor and HIV researcher at University of the Witwatersrand, head of Ezintsha, and lead investigator of ADVANCE. Clinicians and researchers have recently thought that combining TAF and an INSTI plus FTC or a similar NRTI “would be the ultimate regimen to replace the nonnucleoside reverse transcriptase inhibitors (NNRTIs)” such as EFV, “but now we have a major headache” with unexpectedly high weight gains in some patients, Dr. Venter said.
Weight gains “over 10 kg are unlikely to be acceptable in any circumstances, especially when starting body mass index is already borderline overweight,” wrote Dr. Venter along with Dr. Hill in their commentary. Until recently, many clinicians chalked up weight gain on newly begun ART as a manifestation of the patient’s “return-to-health,” but this interpretation “gives a positive spin to a potentially serious and common side effect,” they added.
More from ADVANCE
The primary efficacy endpoint of ADVANCE was suppression of viral load to less than 50 RNA copies/mL after 48 weeks on treatment, and the result showed that the TAF-DTG-FTC regimen and the TDF-DTG-FTC regimen were each noninferior to the control regimen of TDF-EFV-FTC (New Engl J Med. 2019 Aug 29;381[9]:803-15). Virtually all of the enrolled patients were Black, and 59% were women. Planned follow-up of all patients ran for 96 weeks. After 48 weeks, weight gain among the women averaged 6.4 kg, 3.2 kg, and 1.7 kg in the TAF-DTG, TDF-DTG, and TDF-EFV arms respectively. After 96 weeks, the average weight gains among women were 8.2 kg, 4.6 kg, and 3.2 kg, respectively, in new results reported by Dr. Sokhela at the IAC. Follow-up to 144 weeks was partial and included about a quarter of the enrolled women, with gains averaging 12.3 kg, 7.4 kg, and 5.5 kg respectively. The pattern of weight gain among men tracked the pattern in women, but the magnitude of gain was less. Among men followed for 144 weeks, average gain among those on TAF-DTG-FTC was 7.2 kg, the largest gain seen among men on any regimen and at any follow-up time in the study.
Dr. Sokhela also reported data on body composition analyses, which showed that the weight gains were largely in fat rather than lean tissue, fat accumulation was significantly greater in women than men, and that in both sexes fat accumulated roughly equally in the trunk and on limbs.
An additional analysis looked at the incidence of new-onset obesity among the women who had a normal body mass index at baseline. After 96 weeks, incident obesity occurred in 14% of women on the TAG-DTG-FTC regimen, 8% on TDF-DTG-FTC, and in 2% of women maintained on TDF-EFV-FTC, said Dr. Hill in a separate report at the conference.
Weight starts to weigh in
“I am very mindful of weight gain potential, and I talk to patients about it. It doesn’t determine what regimen I choose for a patient” right now, “but it’s only a matter of time before it starts influencing what we do, particularly if we can achieve efficacy with fewer drugs,” commented Babafemi O. Taiwo, MD, professor of medicine and chief of infectious diseases at Northwestern University in Chicago. “I’ve had some patients show up with a weight gain of 20 kg, and that shouldn’t happen,” he said during a recent online educational session. Dr. Taiwo said his recent practice has been to warn patients about possible weight gain and to urge them to get back in touch with him quickly if it happens.
“Virologic suppression is the most important goal with ART, and the U.S. Department of Health and Human Services currently recommends INSTI-based ART for most PWH [people with HIV],” wrote Dr. Lake in April 2020. “I counsel all PWH initiating ART about the potential for weight gain, and I discuss their current diet and healthy lifestyle habits. I explain to patients that we will monitor their weight, and if weight gain seems more than either of us are comfortable with then we will reassess. Only a small percentage of patients experience excessive weight gain after starting ART.” Dr. Lake also stressed that she had not yet begun to change the regimen a patient is on solely because of weight gain. “We do not know whether this weight gain is reversible,” she noted.
“I do not anticipate that a risk of weight gain at present will dictate a change in guidelines,” said Dr. Geretti. “Drugs such as dolutegravir and bictegravir are very effective, and they are unlikely to cause drug resistance. Further data on the mechanism of weight gain and the reversibility after a change of treatment will help refine drug selection in the near future,” she predicted.
“I consider weight gain when prescribing because my patients hear about this. It’s a side effect that my patients really care about, and I don’t blame them,” said Lisa Hightow-Weidman, MD, a professor and HIV specialist at the University of North Carolina at Chapel Hill, during an on-line educational session. “If you don’t discuss it with a patient and then weight gain happens and the patient finds out [the known risk from their treatment] they may have an issue,” she noted. But weight gain is not a reason to avoid these drugs. “They are great medications in many ways, with once-daily regimens and few side effects.”
Weight gain during pregnancy a special concern
An additional analysis of data from ADVANCE presented at the conference highlighted what the observed weight gain on ART could mean for women who become pregnant while on treatment. Based on a systematic literature review, the ADVANCE investigators calculated the relative risk for six obesity-related pregnancy complications, compared with nonobese women: preterm delivery, gestational diabetes, gestational hypertension, preeclampsia, postpartum hemorrhage, and caesarean delivery. Based on the obesity changes among women on their assigned ART in ADVANCE, the researchers calculated the predicted incidence of these six complications. The analysis showed that for every 1,000 women, those on TAG-DTG-FTC would have an excess of 53 obesity-related pregnancy complications, those on TDF-DTG-FTC would develop 28 excess pregnancy complications, and those on TDG-EFV-FTC would have four excess complications, reported Dr. Hill at the International AIDS conference.
The researchers also ran a similar simulation for the incidence of neonatal complications that could result when mothers are obese because of their ART. The six neonatal complications included in this analysis were small for gestational age, large for gestational age, macrosomia, neonatal death, stillbirth, and neural tube defects. Based on the excess rate of incident obesity, they calculated that for every 1,000 pregnancies women on TAD-DTG-FTC would have 24 additional infants born with one of these complications, women on TDF-DTG-FTC would have an excess of 13 of these events, and women on TDG-EFV-FTC would have an excess of three such obesity-related neonatal complications, Dr. Hill said.
Sorting out the drugs
Results from several additional studies reported at the conference have started trying to discern exactly which ART drugs and regimens pose the greatest weight gain risk and which have the least risk while retaining high efficacy and resistance barriers.
Further evidence implicating any type of ART as a driver of increased weight came from a review of 8,256 adults infected with HIV and members of the Kaiser Permanente health system in three U.S. regions during 2000-2016. Researchers matched these cases using several demographic factors with just under 130,000 members without HIV. Those infected by HIV had half the prevalence of obesity as the matched controls at baseline. During 12 years of follow-up, those infected with HIV had a threefold higher rate of weight gain than those who were uninfected. Annual weight gain averaged 0.06 kg/year among the uninfected people and 0.22 kg/year among those infected with HIV, a statistically significant difference that was consistent regardless of whether people started the study at a normal body mass index, overweight, or obese, reported Michael J. Silverberg, PhD, an epidemiologist with Kaiser Permanente in Oakland, Calif.
Another study tried to focus on the weight gain impact when patients on three-drug ART regimens changed from taking TDF to TAF. This analysis used data collected in the OPERA (Observational Pharmaco-Epidemiology Research & Analysis) longitudinal cohort of about 115,000 U.S. PWH. The observational cohort included nearly 7,000 patients who made a TDF-to-TAF switch, including 3,288 patients who maintained treatment during this switch with an INSTI, 1,454 who maintained a background regimen based on a NNRTI, 1,430 patients who also switched from an INSTI to a different drug, and 747 patients maintained on a boosted dose of a protease inhibitor. All patients were well controlled on their baseline regimen, with at least two consecutive measures showing undetectable viral load.
Patients who maintained their background regimens while changing from TDF to TAF had a 2.0-2.6 kg increase in weight during the 9 months immediately following their switch to TAF, reported Patrick Mallon, MB, a professor of microbial diseases at University College Dublin. Among the patients who both switched to TAF and also switched to treatment with an INSTI, weight gain during the 9 months after the switch averaged 2.6-4.5 kg, depending on which INSTI was started. Patients who switched to treatment with elvitegravir/cobicistat (an INSTI plus a boosting agent) averaged a gain of 2.6 kg during 9 months, those who switched to DTG averaged a 3.1-kg gain, and those who switched to BTG averaged a 4.6-kg increase, Dr. Mallon reported at the conference.
These findings “give us a good sense that the weight gain is real. This is not just overeating or not exercising, but weight changes coincidental with a change in HIV treatment,” commented David Wohl, MD, professor of medicine and site leader of the HIV Prevention and Treatment Clinical Trials Unit at the University of North Carolina at Chapel Hill, during an online educational session.
Contrary to this evidence suggesting a consistent uptick in weight when patients start TAF treatment was a recent report on 629 HIV patients randomized to treatment with TAF-BTG-FTC or abacavir (an NRTI, Ziagen)–DTG-3TC, which found similar weight gains between these two regimens after 144 weeks on treatment (Lancet HIV. 2020 Jun;7[6]:e389-400). This finding had the effect of “strengthening the argument that TAF is simply an innocent bystander” and does not play a central role in weight gain, and supporting the notion that the alternative tenofovir formulation, TDF, differs from TAF by promoting weight loss, Dr. Venter and Dr. Hill suggested in their commentary that accompanied this report.
The new findings from Dr. Mallon raise “serious questions about the way we have moved to TAF as a replacement for TDF, especially because the benefits [from TAF] are for a small subgroup – patients with renal disease or osteoporosis,” Dr. Venter said in an interview. “The question is, will we see weight gain like this” if TAF was combined with a non-INSTI drug? he wondered.
While some study results have suggested a mitigating effect from TDF on weight gain, that wasn’t the case in the AFRICOS (African Cohort Study) study of 1,954 PWH who started treatment with TDF-DTG-FTC (742 patients) or a different three-drug regimen. After a median of 225 days on treatment, those who started on TDF-DTG-FTC had an adjusted, 85% higher rate of developing a high body mass index, compared with patients on a different ART regimen, Julie Ake, MD, reported in a talk at the conference. Her conclusion focused on the possible involvement of DTG: “Consistent with previous reports, dolutegravir was significantly associated with an increased risk of developing high body mass index,” said Dr. Ake, director of the U.S. Military HIV Research Program in Bethesda, Md. and leader of AFRICOS.
A potential workaround to some drugs that cause excessive the weight gain is to just not use them. That was part of the rationale for the TANGO study, which took 741 HIV-infected patients with successful viral suppression on a regimen of TAF-FTC plus one or two additional agents and switched half of them to a TAF-less, two-drug regimen of DTG-FTC. This open-label study’s primary endpoint was noninferiority for viral suppression of the DTG-FTC regimen, compared with patients who stayed on their starting regimen, and the results proved that DTG-FTC was just as effective over 48 weeks for this outcome (Clin Infect Dis. 2020 Jan 6. doi: 10.1093/cid/ciz1243).
At the conference, TANGO’s lead investigator, Jean van Wyk, MD, reported the weight and metabolic effects of the switch. The results showed a similar and small weight gain (on average less than 1 kg) during 48 week follow-up regardless of whether patients remained on their baseline, TAF-containing regimen or switched to DTG-FTC, said Dr. van Wyk, global medical lead for HIV treatment at Viiv Healthcare, the company that markets DTG. About three-quarters of patients in both arms received “boosted” dosages of their drugs, and in this subgroup, patients on DTG-FTC showed statistically significant benefits in several lipid levels, fasting glucose level, and in their degree of insulin resistance. Dr. van Wyk said. These between-group differences were not statistically significant among the “unboosted” patients, and the results failed to show a significant between-group difference in the incidence of metabolic syndrome.
Dr. Venter called these results “exciting,” and noted that he already uses the DTG-FTC two-drug combination “a lot” to treat PWH and renal disease.
A second alternative regimen showcased in a talk at the conference used the three-drug regimen of TDF-FTC plus the NNRTI, DOR (doravirine, Pifeltro). The DRIVE-SHIFT trial enrolled 670 HIV patients with successfully suppressed viral load on conventional regimens who were either switched to TDF-DOR-FTC or maintained on their baseline treatment. After 48 weeks, results confirmed the primary efficacy endpoint of noninferiority for maintenance of suppression with the investigational regimen (J Acquir Immune Defic Syndr. 2019 Aug;81[4]:463-72).
A post-hoc analysis looked at weight changes among these patients after as much as 144 weeks of follow-up. The results showed that patients switched to TDF-DOR-FTC had an average weight increase of 1.2-1.4 kg after more than 2 years on the new regimen, with fewer than 10% of patients having a 10% or greater weight gain with DOR, a “next-generation” NNRTI, reported Princy N. Kumar, MD, professor at Georgetown University and chief of infectious diseases at MedStar Georgetown University Hospital in Washington. “Weight gain was minimal, even over the long term,” she noted.
The tested DOR-based regimen also looks “very exciting,” but the populations it’s been tested have also been largely limited to White men, and limited data exist about the regimen’s performance in pregnant women, commented Dr. Venter. The DRIVE-SHIRT patient cohort was about 85% men, and about three-quarters White.
More weight data needed
HIV-treatment researchers and clinicians seem agreed that weight gain and other metabolic effects from HIV treatment need more assessment and evidence because current data, while suggestive, is also inconclusive.
“Clinical trials are desperately needed to understand the mechanisms of and potential therapeutic options for excessive weight gain on ART,” wrote Dr. Lake in her commentary in April. “While more research is needed,” the new data reported at the virtual International AIDS conference “get us closer to understanding the effects of integrase inhibitors and TAF on weight and the potential metabolic consequences,” she commented as chair of the conference session where these reports occurred.
“Further data on the mechanism of weight gain and its reversibility after a change of treatment will help refine drug selection in the near future,” predicted Dr. Geretti.
“It’s hard to understand physiologically how drugs from such different classes all seem to have weight effects; it’s maddening,” said Dr. Venter. “We need decent studies in all patient populations. That will now be the priority,” he declared. “Patients shouldn’t have to choose” between drugs that most effectively control their HIV infection and drugs that don’t pose a risk for weight gain or metabolic derangements. PWH “should not have to face obesity as their new epidemic,” he wrote with Dr. Hill.
ADVANCE was funded in part by Viiv, the company that markets dolutegravir (Tivicay), and received drugs supplied by Gilead and Viiv. TANGO was sponsored by Viiv. DRIVE-SHIFT was funded by Merck, the company that markets doravirine (Pifeltro). Dr. Lake, Dr. Sokhela, Dr. Ake, and Dr. Kumar had no disclosures, Dr. Venter has received personal fees from Adcock Ingraham, Aspen Healthcare, Johnson and Johnson, Merck, Mylan, Roche, and Viiv. Dr. Hill has received payments from Merck. Dr. Geretti has received honoraria and research funding from Gilead, Jansse, Roche, and Viiv. Dr. Taiwo has had financial relationships with Gilead, Janssen, and Viiv. Dr. Hightow-Weidman has received honoraria from Gilead and Jansse. Dr. Wohl has been a consultant to Gilead, Johnson and Johnson, and Merck. Dr. Silverberg received research funding from Gilead. Dr. Mallon has been an advisor to and speaker on behalf of Bristol-Myers Squibb, Cilag, Gilead, Jansse, Merck Sharp & Dohme, and Viiv. Dr. van Wyk is a Viiv employee.
FROM AIDS 2020
Large, painful facial cysts
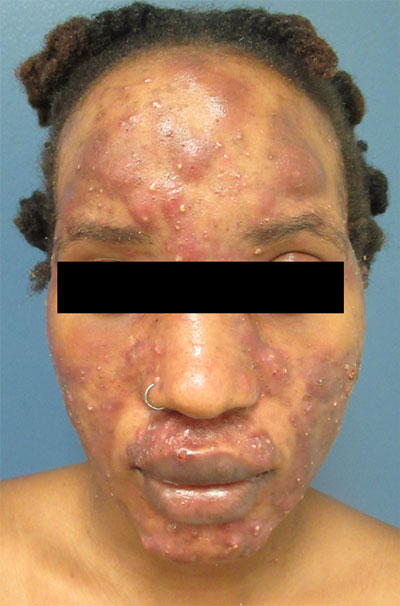
The abrupt onset of painful, violaceous coalescing papules, pustules, cysts, and nodules exclusively involving the centrofacial area with an overlying red-cyanotic erythema are the hallmarks of pyoderma faciale.
Pyoderma faciale is a rare disease that affects females in the second and third decades of life; 50% of these patients have a history of acne. The etiology of the condition remains unclear. Hormonal imbalance, inflammatory bowel disease, liver disease, and thyroid disease have been associated with the disorder. Ribavirin and interferon therapies for the treatment of hepatitis C along with high levels of vitamins (B6 and B12) have been identified as triggers. Culture of purulent drainage typically is sterile or may reveal commensal organisms.
The differential diagnosis includes acne fulminans and acne conglobata. Acne fulminans is not restricted to the face, as is pyoderma faciale, and it involves constitutional symptoms. Acne conglobata is a chronic process that affects males and females. It also involves purulent sinus tracts.
Prompt treatment of pyoderma faciale is essential to prevent widespread eruption, minimize the distress associated with the disfiguring nature of the disorder, and ultimately reduce scarring. Standard therapy consists of oral steroids (prednisone 1 mg/kg/d) for 1 week followed by a slow taper in combination with oral isotretinoin at a low dosage (0.2–0.5 mg/kg). The systemic retinoid is continued until all inflammatory lesions are healed.
In this case, a culture swab taken from the patient’s left cheek did not reveal any unexpected pathogens. The patient was started on oral doxycycline 100 mg bid and prednisone 50 mg/d tapered to 10 mg/d. She was counseled about the risks and benefits of isotretinoin and registered in the iPLEDGE system in anticipation of starting oral isotretinoin at 20 mg/d after negative pregnancy tests, 2 forms of contraception, and the 1-month qualification period.
Photo courtesy of Catherine N. Tchanque-Fossuo, MD, MS, and text courtesy of Catherine N. Tchanque-Fossuo, MD, MS, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Sharma RK, Pulimood S, Peter D, et al. A case report with review of literature on pyoderma faciale in pregnancy–a therapeutic dilemma. JDA Indian J Clin Dermatol. 2018;1:96-99.

The abrupt onset of painful, violaceous coalescing papules, pustules, cysts, and nodules exclusively involving the centrofacial area with an overlying red-cyanotic erythema are the hallmarks of pyoderma faciale.
Pyoderma faciale is a rare disease that affects females in the second and third decades of life; 50% of these patients have a history of acne. The etiology of the condition remains unclear. Hormonal imbalance, inflammatory bowel disease, liver disease, and thyroid disease have been associated with the disorder. Ribavirin and interferon therapies for the treatment of hepatitis C along with high levels of vitamins (B6 and B12) have been identified as triggers. Culture of purulent drainage typically is sterile or may reveal commensal organisms.
The differential diagnosis includes acne fulminans and acne conglobata. Acne fulminans is not restricted to the face, as is pyoderma faciale, and it involves constitutional symptoms. Acne conglobata is a chronic process that affects males and females. It also involves purulent sinus tracts.
Prompt treatment of pyoderma faciale is essential to prevent widespread eruption, minimize the distress associated with the disfiguring nature of the disorder, and ultimately reduce scarring. Standard therapy consists of oral steroids (prednisone 1 mg/kg/d) for 1 week followed by a slow taper in combination with oral isotretinoin at a low dosage (0.2–0.5 mg/kg). The systemic retinoid is continued until all inflammatory lesions are healed.
In this case, a culture swab taken from the patient’s left cheek did not reveal any unexpected pathogens. The patient was started on oral doxycycline 100 mg bid and prednisone 50 mg/d tapered to 10 mg/d. She was counseled about the risks and benefits of isotretinoin and registered in the iPLEDGE system in anticipation of starting oral isotretinoin at 20 mg/d after negative pregnancy tests, 2 forms of contraception, and the 1-month qualification period.
Photo courtesy of Catherine N. Tchanque-Fossuo, MD, MS, and text courtesy of Catherine N. Tchanque-Fossuo, MD, MS, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The abrupt onset of painful, violaceous coalescing papules, pustules, cysts, and nodules exclusively involving the centrofacial area with an overlying red-cyanotic erythema are the hallmarks of pyoderma faciale.
Pyoderma faciale is a rare disease that affects females in the second and third decades of life; 50% of these patients have a history of acne. The etiology of the condition remains unclear. Hormonal imbalance, inflammatory bowel disease, liver disease, and thyroid disease have been associated with the disorder. Ribavirin and interferon therapies for the treatment of hepatitis C along with high levels of vitamins (B6 and B12) have been identified as triggers. Culture of purulent drainage typically is sterile or may reveal commensal organisms.
The differential diagnosis includes acne fulminans and acne conglobata. Acne fulminans is not restricted to the face, as is pyoderma faciale, and it involves constitutional symptoms. Acne conglobata is a chronic process that affects males and females. It also involves purulent sinus tracts.
Prompt treatment of pyoderma faciale is essential to prevent widespread eruption, minimize the distress associated with the disfiguring nature of the disorder, and ultimately reduce scarring. Standard therapy consists of oral steroids (prednisone 1 mg/kg/d) for 1 week followed by a slow taper in combination with oral isotretinoin at a low dosage (0.2–0.5 mg/kg). The systemic retinoid is continued until all inflammatory lesions are healed.
In this case, a culture swab taken from the patient’s left cheek did not reveal any unexpected pathogens. The patient was started on oral doxycycline 100 mg bid and prednisone 50 mg/d tapered to 10 mg/d. She was counseled about the risks and benefits of isotretinoin and registered in the iPLEDGE system in anticipation of starting oral isotretinoin at 20 mg/d after negative pregnancy tests, 2 forms of contraception, and the 1-month qualification period.
Photo courtesy of Catherine N. Tchanque-Fossuo, MD, MS, and text courtesy of Catherine N. Tchanque-Fossuo, MD, MS, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Sharma RK, Pulimood S, Peter D, et al. A case report with review of literature on pyoderma faciale in pregnancy–a therapeutic dilemma. JDA Indian J Clin Dermatol. 2018;1:96-99.
Sharma RK, Pulimood S, Peter D, et al. A case report with review of literature on pyoderma faciale in pregnancy–a therapeutic dilemma. JDA Indian J Clin Dermatol. 2018;1:96-99.
SGLT2 inhibitors have a breakout year
The benefits from sodium-glucose cotransporter 2 inhibitor drugs proven during the past year for cutting heart failure hospitalization rates substantially in patients with heart failure with reduced ejection fraction and slowing progression of chronic kidney disease, all regardless of diabetes status, have thrust this drug class into the top tier of agents for potentially treating millions of patients with cardiac or renal disease.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors, first licensed for U.S. marketing in 2013 purely for glycemic control, have, during the 5 years since the first cardiovascular outcome trial results for the class came out, shown benefits in a range of patients reminiscent of what’s been established for ACE inhibitors and angiotensin receptor blockers (ARBs).
The wide-reaching benefits of SGLT2 inhibitors have recently become even more relevant by showing clinically meaningful effects in patients without type 2 diabetes (T2D). And in an uncanny coincidence, the SGLT2 inhibitors appear to act in complementary harmony with the ACE inhibitors and ARBs for preserving heart and renal function. These properties have made the SGLT2 inhibitors especially attractive as a new weapon for controlling the ascendant disorder of cardiorenal syndrome.
“SGLT2 inhibitors have a relatively greater impact on cardiovascular outcomes, compared with ACE inhibitors and ARBs, but the effects [of the two classes] are synergistic and ideally patients receive both,” said Peter McCullough, MD, a specialist in treating cardiorenal syndrome and other cardiovascular and renal disorders at Baylor, Scott, and White Heart and Vascular Hospital in Dallas. The SGLT2 inhibitors are among the drugs best suited to both treating and preventing cardiorenal syndrome by targeting both ends of the disorder, said Dr. McCullough, who chaired an American Heart Association panel that last year issued a scientific statement on cardiorenal syndrome (Circulation. 2019 Apr 16;139[16]:e840-78).
Although data on the SGLT2 inhibitors “are evolving,” the drug class is “going in the direction” of being “reasonably compared” with the ACE inhibitors and ARBs, said Javed Butler, MD, professor and chair of medicine at the University of Mississippi Medical Center, Jackson. “There are certainly complementary benefits that we see for both cardiovascular and renal outcomes.”
“We’ll think more and more about the SGLT2 inhibitors like renin-angiotensin system [RAS] inhibitors,” said David Z. Cherney, MD, referring to the drug class that includes ACE inhibitors and ARBs. “We should start to approach SGLT2 inhibitors like RAS inhibitors, with pleiotropic effects that go beyond glucose,” said Dr. Cherney, a nephrologist and professor of medicine at the University of Toronto, during the virtual annual scientific sessions of the American Diabetes Association in June 2020.
Working together in the nephron
One of the clearest complementary interactions between the SGLT2 inhibitors and the RAS inhibitors is their ability to reduce intraglomerular pressure, a key mechanism that slows nephron loss and progression of chronic kidney disease. SGLT2 inhibitors reduce sodium absorption in the proximal tubule that causes, through tubuloglomerular feedback, afferent arteriole constriction that lowers intraglomerular pressure, while the RAS inhibitors inhibit efferent arteriole constriction mediated by angiotensin II, also cutting intraglomerular pressure. Together, “they almost work in tandem,” explained Janani Rangaswami, MD, a nephrologist at Einstein Medical Center in Philadelphia, vice chair of the Kidney Council of the AHA, and first author of the 2019 cardiorenal syndrome AHA statement.
“Many had worried that if we target both the afferent and efferent arterioles simultaneously, it might increase the risk for acute kidney injury. What has been reassuring in both the recent data from the DAPA-HF trial and in recent meta-analysis was no evidence of increased risk for acute kidney injury with use of the SGLT2 inhibitor,” Dr. Rangaswami said in an interview. For example, a recent report on more than 39,000 Canadian patients with T2D who were at least 66 years old and newly begun on either an SGLT2 inhibitor or a different oral diabetes drug (a dipeptidyl peptidase–4 inhibitor), found a statistically significant 21% lower rate of acute kidney injury during the first 90 days on treatment with an SGLT2 inhibitor in a propensity score–matched analysis (CMAJ. 2020 Apr 6;192: e351-60).
Much of the concern about possible acute kidney injury stemmed from a property that the SGLT2 inhibitors share with RAS inhibitors: They cause an initial, reversible decline in glomerular filtration rate (GFR), followed by longer-term nephron preservation, a pattern attributable to reduced intraglomerular pressure. The question early on was: “ ‘Does this harm the kidney?’ But what we’ve seen is that patients do better over time, even with this initial hit. Whenever you offload the glomerulus you cut barotrauma and protect renal function,” explained Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center.
Dr. Inzucchi cautioned, however, that a small number of patients starting treatment with an SGLT2 inhibitor may have their GFR drop too sharply, especially if their GFR was low to start with. “You need to be careful, especially at the lower end of the GFR range. I recheck renal function after 1 month” after a patient starts an SGLT2. Patients whose level falls too low may need to discontinue. He added that it’s hard to set a uniform threshold for alarm, and instead assess patients on a case-by-case basis, but “you need some threshold in mind, where you will stop” treatment.
A smarter diuretic
One of the most intriguing renal effects of SGLT2 inhibitors is their diuretic action. During a talk at the virtual ADA scientific sessions, cardiologist Jeffrey Testani, MD, called them “smart” diuretics, because their effect on diuresis is relatively modest but comes without the neurohormonal price paid when patients take conventional loop diuretics.
”Loop diuretics are particularly bad,” causing neurohormonal activation that includes norepinephrine, renin, and vasopressin, said Dr. Testani, director of heart failure research at Yale. They also fail to produce a meaningful drop in blood volume despite causing substantial natriuresis.
In contrast, SGLT2 inhibitors cause “moderate” natriuresis while producing a significant cut in blood volume. “The body seems content with this lower plasma volume without activating catecholamines or renin, and that’s how the SGLT2 inhibitors differ from other diuretics,” said Dr. Inzucchi.
The class also maintains serum levels of potassium and magnesium, produces significant improvements in serum uric acid levels, and avoids the electrolyte abnormalities, volume depletion, and acute kidney injury that can occur with conventional distal diuretics, Dr. Testani said.
In short, the SGLT2 inhibitors “are safe and easy-to-use diuretics,” which allows them to fill a “huge unmet need for patients with heart failure.” As evidence accumulates for the benefits of the drug class in patients with heart failure and renal disease, “uptake will be extensive,” Dr. Testani predicted, driven in part by how easy it is to add the class to existing cardiorenal drug regimens.
Other standard therapies for patients with heart failure with reduced ejection fraction (HFrEF) risk electrolyte abnormalities, renal dysfunction, significantly lower blood pressure, often make patients feel worse, and involve a slow and laborious titration process, Dr. Testani noted. The SGLT2 inhibitor agents avoid these issues, a property that has played out in quality of life assessments of patients with HFrEF who received a drug from this class.
Outcomes met in trial after trial
In the DAPA-HF trial, with 4,443 patients with HFrEF and divided roughly equally between those with or without T2D, treatment with dapagliflozin (Farxiga) linked with significant improvements in health status and quality of life measured by the Kansas City Cardiomyopathy Questionnaire (Circulation. 2020 Jan 14;141[2]:90-9). “Not all treatments for HFrEF improve symptoms,” but in this study the SGTL2 inhibitor dapagliflozin did, boosting the Kansas City Cardiomyopathy Questionnaire score by about the same magnitude as treatment with a cardiac resynchronization device in patients with HFrEF, said Mikhail N. Kosiborod, MD, director of Cardiometabolic Research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., speaking at the virtual ADA scientific sessions.
Two more recent renal observations have further solidified the growing role of these drugs for kidney protection. Results from the CREDENCE trial that looked at canagliflozin (Invokana) treatment in 4,401 patients with T2D and albuminuria and chronic kidney disease showed canagliflozin treatment cut the primary, composite renal endpoint by a statistically significant 30%, compared with placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). The study stopped earlier than planned because of how effective canagliflozin appeared.
“Never before has a renal protection clinical trial stopped for overwhelming efficacy,” noted nephrologist Katherine R. Tuttle, MD, executive director for research at Providence Health Care in Spokane, Wash. “It’s very exciting to have a treatment that works on both the heart and kidney, given their interrelationship,” she said during the ADA sessions. Dr. Tuttle called the cardiorenal effects from the SGLT2 inhibitors “amazing.”
Just as the DAPA-HF trial’s primary outcome showed the ability of at least one drug from the class, dapagliflozin, to improve outcomes in HFrEF patients without T2D, topline results recently reported from the DAPA-CDK trial showed for the first time renal protection by an SGLT2 inhibitor in patients with chronic kidney disease but no T2D, in a study with about 4,300 patients.
Although detailed results from DAPA-CKD are not yet available, so far the outcomes seem consistent with the CREDENCE findings, and the cumulative renal findings for the class show the SGLT2 inhibitors have “potential for a profound impact on the patients we see in every nephrology clinic, and with dual cardiorenal disease,” said Dr. Rangaswami. The class is now established as “standard of care for patients with chronic kidney disease. The CREDENCE results made that clear.”
The DAPA-CKD findings in patients with chronic kidney disease regardless of their diabetes status “are very important. We really have not had any advances in this space for some time, and chronic kidney disease patients have very poor outcomes, both cardiovascular and renal,” commented Dr. Butler. The advantage from using this drug class in these patients “is huge.”
The DAPA-CKD findings are a “major advance,” agreed Dr. McCullough.
SGLT2 inhibitor use needs to grow
Experts lament that although the evidence favoring the class has been very bullish, prescribing uptake has been slow, perhaps partly explained by the retail U.S. cost for most of these agents, generally about $17/day.
Cost is, unfortunately, an issue right now for these drugs, said Dr. Butler. Generic formulations are imminent, “but we cannot accept waiting. Providing this therapy when insurance coverage is available,” is essential.
The FDA has already granted tentative approval to some generic formulations, although resolution of patent issues can delay generics actually reaching the market. “Generic dapagliflozin will have a major impact; the marketplace for these drugs will shift very quickly,” predicted Dr. McCullough.
But price may not be the sole barrier, cautioned Dr. Rangaswami. “I don’t think it’s just a cost issue. Several factors explain the slow uptake,” of the SGLT2 inhibitors. “The biggest barrier is that this is a new drug class, and understanding how to use the class is not yet where it needs to be in the physician community.” One of the biggest problems is that the SGLT2 inhibitors are still primarily regarded as drugs to treat hyperglycemia.
Physicians who treat patients with heart or renal disease “need to wrap their head around the idea that a drug with antihyperglycemic effects is now in their practice territory, and something they need to prescribe,” she noted. Currently “there is a reluctance to prescribe these drugs given the perception that they are antihyperglycemic agents, and usually get deferred to primary care physicians or endocrinologists. This results in huge missed opportunities by cardiologists and nephrologists in initiating these agents that have major cardiorenal risk reduction effects.”
The key role that cardiologists need to play in prescribing the SGLT2 inhibitors was brought home in a recent study of two representative U.S. health systems that showed patients with T2D were far more likely to see a cardiologist than an endocrinologist (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
“The SGLT2 inhibitors are definitely a game-changing drug class,” summed up Dr. Rangaswami. “We’re going to see a lot of use in patients with heart and kidney disease.”
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Butler has had financial relationships with numerous pharmaceutical companies. Dr. McCullough and Dr. Rangaswami had no disclosures. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Testani has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, cardionomic, FIRE1 Magenta Med, Novartis, Reprieve, Sanofi, and W.L. Gore. Dr. Kosiborod has been a consultant to or led trials for Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Glytec, Janssen, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi, and Vifor. Dr. Tuttle has been a consultant to AstraZeneca, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
mzoler@mdedge.com
The benefits from sodium-glucose cotransporter 2 inhibitor drugs proven during the past year for cutting heart failure hospitalization rates substantially in patients with heart failure with reduced ejection fraction and slowing progression of chronic kidney disease, all regardless of diabetes status, have thrust this drug class into the top tier of agents for potentially treating millions of patients with cardiac or renal disease.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors, first licensed for U.S. marketing in 2013 purely for glycemic control, have, during the 5 years since the first cardiovascular outcome trial results for the class came out, shown benefits in a range of patients reminiscent of what’s been established for ACE inhibitors and angiotensin receptor blockers (ARBs).
The wide-reaching benefits of SGLT2 inhibitors have recently become even more relevant by showing clinically meaningful effects in patients without type 2 diabetes (T2D). And in an uncanny coincidence, the SGLT2 inhibitors appear to act in complementary harmony with the ACE inhibitors and ARBs for preserving heart and renal function. These properties have made the SGLT2 inhibitors especially attractive as a new weapon for controlling the ascendant disorder of cardiorenal syndrome.
“SGLT2 inhibitors have a relatively greater impact on cardiovascular outcomes, compared with ACE inhibitors and ARBs, but the effects [of the two classes] are synergistic and ideally patients receive both,” said Peter McCullough, MD, a specialist in treating cardiorenal syndrome and other cardiovascular and renal disorders at Baylor, Scott, and White Heart and Vascular Hospital in Dallas. The SGLT2 inhibitors are among the drugs best suited to both treating and preventing cardiorenal syndrome by targeting both ends of the disorder, said Dr. McCullough, who chaired an American Heart Association panel that last year issued a scientific statement on cardiorenal syndrome (Circulation. 2019 Apr 16;139[16]:e840-78).
Although data on the SGLT2 inhibitors “are evolving,” the drug class is “going in the direction” of being “reasonably compared” with the ACE inhibitors and ARBs, said Javed Butler, MD, professor and chair of medicine at the University of Mississippi Medical Center, Jackson. “There are certainly complementary benefits that we see for both cardiovascular and renal outcomes.”
“We’ll think more and more about the SGLT2 inhibitors like renin-angiotensin system [RAS] inhibitors,” said David Z. Cherney, MD, referring to the drug class that includes ACE inhibitors and ARBs. “We should start to approach SGLT2 inhibitors like RAS inhibitors, with pleiotropic effects that go beyond glucose,” said Dr. Cherney, a nephrologist and professor of medicine at the University of Toronto, during the virtual annual scientific sessions of the American Diabetes Association in June 2020.
Working together in the nephron
One of the clearest complementary interactions between the SGLT2 inhibitors and the RAS inhibitors is their ability to reduce intraglomerular pressure, a key mechanism that slows nephron loss and progression of chronic kidney disease. SGLT2 inhibitors reduce sodium absorption in the proximal tubule that causes, through tubuloglomerular feedback, afferent arteriole constriction that lowers intraglomerular pressure, while the RAS inhibitors inhibit efferent arteriole constriction mediated by angiotensin II, also cutting intraglomerular pressure. Together, “they almost work in tandem,” explained Janani Rangaswami, MD, a nephrologist at Einstein Medical Center in Philadelphia, vice chair of the Kidney Council of the AHA, and first author of the 2019 cardiorenal syndrome AHA statement.
“Many had worried that if we target both the afferent and efferent arterioles simultaneously, it might increase the risk for acute kidney injury. What has been reassuring in both the recent data from the DAPA-HF trial and in recent meta-analysis was no evidence of increased risk for acute kidney injury with use of the SGLT2 inhibitor,” Dr. Rangaswami said in an interview. For example, a recent report on more than 39,000 Canadian patients with T2D who were at least 66 years old and newly begun on either an SGLT2 inhibitor or a different oral diabetes drug (a dipeptidyl peptidase–4 inhibitor), found a statistically significant 21% lower rate of acute kidney injury during the first 90 days on treatment with an SGLT2 inhibitor in a propensity score–matched analysis (CMAJ. 2020 Apr 6;192: e351-60).
Much of the concern about possible acute kidney injury stemmed from a property that the SGLT2 inhibitors share with RAS inhibitors: They cause an initial, reversible decline in glomerular filtration rate (GFR), followed by longer-term nephron preservation, a pattern attributable to reduced intraglomerular pressure. The question early on was: “ ‘Does this harm the kidney?’ But what we’ve seen is that patients do better over time, even with this initial hit. Whenever you offload the glomerulus you cut barotrauma and protect renal function,” explained Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center.
Dr. Inzucchi cautioned, however, that a small number of patients starting treatment with an SGLT2 inhibitor may have their GFR drop too sharply, especially if their GFR was low to start with. “You need to be careful, especially at the lower end of the GFR range. I recheck renal function after 1 month” after a patient starts an SGLT2. Patients whose level falls too low may need to discontinue. He added that it’s hard to set a uniform threshold for alarm, and instead assess patients on a case-by-case basis, but “you need some threshold in mind, where you will stop” treatment.
A smarter diuretic
One of the most intriguing renal effects of SGLT2 inhibitors is their diuretic action. During a talk at the virtual ADA scientific sessions, cardiologist Jeffrey Testani, MD, called them “smart” diuretics, because their effect on diuresis is relatively modest but comes without the neurohormonal price paid when patients take conventional loop diuretics.
”Loop diuretics are particularly bad,” causing neurohormonal activation that includes norepinephrine, renin, and vasopressin, said Dr. Testani, director of heart failure research at Yale. They also fail to produce a meaningful drop in blood volume despite causing substantial natriuresis.
In contrast, SGLT2 inhibitors cause “moderate” natriuresis while producing a significant cut in blood volume. “The body seems content with this lower plasma volume without activating catecholamines or renin, and that’s how the SGLT2 inhibitors differ from other diuretics,” said Dr. Inzucchi.
The class also maintains serum levels of potassium and magnesium, produces significant improvements in serum uric acid levels, and avoids the electrolyte abnormalities, volume depletion, and acute kidney injury that can occur with conventional distal diuretics, Dr. Testani said.
In short, the SGLT2 inhibitors “are safe and easy-to-use diuretics,” which allows them to fill a “huge unmet need for patients with heart failure.” As evidence accumulates for the benefits of the drug class in patients with heart failure and renal disease, “uptake will be extensive,” Dr. Testani predicted, driven in part by how easy it is to add the class to existing cardiorenal drug regimens.
Other standard therapies for patients with heart failure with reduced ejection fraction (HFrEF) risk electrolyte abnormalities, renal dysfunction, significantly lower blood pressure, often make patients feel worse, and involve a slow and laborious titration process, Dr. Testani noted. The SGLT2 inhibitor agents avoid these issues, a property that has played out in quality of life assessments of patients with HFrEF who received a drug from this class.
Outcomes met in trial after trial
In the DAPA-HF trial, with 4,443 patients with HFrEF and divided roughly equally between those with or without T2D, treatment with dapagliflozin (Farxiga) linked with significant improvements in health status and quality of life measured by the Kansas City Cardiomyopathy Questionnaire (Circulation. 2020 Jan 14;141[2]:90-9). “Not all treatments for HFrEF improve symptoms,” but in this study the SGTL2 inhibitor dapagliflozin did, boosting the Kansas City Cardiomyopathy Questionnaire score by about the same magnitude as treatment with a cardiac resynchronization device in patients with HFrEF, said Mikhail N. Kosiborod, MD, director of Cardiometabolic Research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., speaking at the virtual ADA scientific sessions.
Two more recent renal observations have further solidified the growing role of these drugs for kidney protection. Results from the CREDENCE trial that looked at canagliflozin (Invokana) treatment in 4,401 patients with T2D and albuminuria and chronic kidney disease showed canagliflozin treatment cut the primary, composite renal endpoint by a statistically significant 30%, compared with placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). The study stopped earlier than planned because of how effective canagliflozin appeared.
“Never before has a renal protection clinical trial stopped for overwhelming efficacy,” noted nephrologist Katherine R. Tuttle, MD, executive director for research at Providence Health Care in Spokane, Wash. “It’s very exciting to have a treatment that works on both the heart and kidney, given their interrelationship,” she said during the ADA sessions. Dr. Tuttle called the cardiorenal effects from the SGLT2 inhibitors “amazing.”
Just as the DAPA-HF trial’s primary outcome showed the ability of at least one drug from the class, dapagliflozin, to improve outcomes in HFrEF patients without T2D, topline results recently reported from the DAPA-CDK trial showed for the first time renal protection by an SGLT2 inhibitor in patients with chronic kidney disease but no T2D, in a study with about 4,300 patients.
Although detailed results from DAPA-CKD are not yet available, so far the outcomes seem consistent with the CREDENCE findings, and the cumulative renal findings for the class show the SGLT2 inhibitors have “potential for a profound impact on the patients we see in every nephrology clinic, and with dual cardiorenal disease,” said Dr. Rangaswami. The class is now established as “standard of care for patients with chronic kidney disease. The CREDENCE results made that clear.”
The DAPA-CKD findings in patients with chronic kidney disease regardless of their diabetes status “are very important. We really have not had any advances in this space for some time, and chronic kidney disease patients have very poor outcomes, both cardiovascular and renal,” commented Dr. Butler. The advantage from using this drug class in these patients “is huge.”
The DAPA-CKD findings are a “major advance,” agreed Dr. McCullough.
SGLT2 inhibitor use needs to grow
Experts lament that although the evidence favoring the class has been very bullish, prescribing uptake has been slow, perhaps partly explained by the retail U.S. cost for most of these agents, generally about $17/day.
Cost is, unfortunately, an issue right now for these drugs, said Dr. Butler. Generic formulations are imminent, “but we cannot accept waiting. Providing this therapy when insurance coverage is available,” is essential.
The FDA has already granted tentative approval to some generic formulations, although resolution of patent issues can delay generics actually reaching the market. “Generic dapagliflozin will have a major impact; the marketplace for these drugs will shift very quickly,” predicted Dr. McCullough.
But price may not be the sole barrier, cautioned Dr. Rangaswami. “I don’t think it’s just a cost issue. Several factors explain the slow uptake,” of the SGLT2 inhibitors. “The biggest barrier is that this is a new drug class, and understanding how to use the class is not yet where it needs to be in the physician community.” One of the biggest problems is that the SGLT2 inhibitors are still primarily regarded as drugs to treat hyperglycemia.
Physicians who treat patients with heart or renal disease “need to wrap their head around the idea that a drug with antihyperglycemic effects is now in their practice territory, and something they need to prescribe,” she noted. Currently “there is a reluctance to prescribe these drugs given the perception that they are antihyperglycemic agents, and usually get deferred to primary care physicians or endocrinologists. This results in huge missed opportunities by cardiologists and nephrologists in initiating these agents that have major cardiorenal risk reduction effects.”
The key role that cardiologists need to play in prescribing the SGLT2 inhibitors was brought home in a recent study of two representative U.S. health systems that showed patients with T2D were far more likely to see a cardiologist than an endocrinologist (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
“The SGLT2 inhibitors are definitely a game-changing drug class,” summed up Dr. Rangaswami. “We’re going to see a lot of use in patients with heart and kidney disease.”
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Butler has had financial relationships with numerous pharmaceutical companies. Dr. McCullough and Dr. Rangaswami had no disclosures. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Testani has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, cardionomic, FIRE1 Magenta Med, Novartis, Reprieve, Sanofi, and W.L. Gore. Dr. Kosiborod has been a consultant to or led trials for Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Glytec, Janssen, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi, and Vifor. Dr. Tuttle has been a consultant to AstraZeneca, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
mzoler@mdedge.com
The benefits from sodium-glucose cotransporter 2 inhibitor drugs proven during the past year for cutting heart failure hospitalization rates substantially in patients with heart failure with reduced ejection fraction and slowing progression of chronic kidney disease, all regardless of diabetes status, have thrust this drug class into the top tier of agents for potentially treating millions of patients with cardiac or renal disease.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors, first licensed for U.S. marketing in 2013 purely for glycemic control, have, during the 5 years since the first cardiovascular outcome trial results for the class came out, shown benefits in a range of patients reminiscent of what’s been established for ACE inhibitors and angiotensin receptor blockers (ARBs).
The wide-reaching benefits of SGLT2 inhibitors have recently become even more relevant by showing clinically meaningful effects in patients without type 2 diabetes (T2D). And in an uncanny coincidence, the SGLT2 inhibitors appear to act in complementary harmony with the ACE inhibitors and ARBs for preserving heart and renal function. These properties have made the SGLT2 inhibitors especially attractive as a new weapon for controlling the ascendant disorder of cardiorenal syndrome.
“SGLT2 inhibitors have a relatively greater impact on cardiovascular outcomes, compared with ACE inhibitors and ARBs, but the effects [of the two classes] are synergistic and ideally patients receive both,” said Peter McCullough, MD, a specialist in treating cardiorenal syndrome and other cardiovascular and renal disorders at Baylor, Scott, and White Heart and Vascular Hospital in Dallas. The SGLT2 inhibitors are among the drugs best suited to both treating and preventing cardiorenal syndrome by targeting both ends of the disorder, said Dr. McCullough, who chaired an American Heart Association panel that last year issued a scientific statement on cardiorenal syndrome (Circulation. 2019 Apr 16;139[16]:e840-78).
Although data on the SGLT2 inhibitors “are evolving,” the drug class is “going in the direction” of being “reasonably compared” with the ACE inhibitors and ARBs, said Javed Butler, MD, professor and chair of medicine at the University of Mississippi Medical Center, Jackson. “There are certainly complementary benefits that we see for both cardiovascular and renal outcomes.”
“We’ll think more and more about the SGLT2 inhibitors like renin-angiotensin system [RAS] inhibitors,” said David Z. Cherney, MD, referring to the drug class that includes ACE inhibitors and ARBs. “We should start to approach SGLT2 inhibitors like RAS inhibitors, with pleiotropic effects that go beyond glucose,” said Dr. Cherney, a nephrologist and professor of medicine at the University of Toronto, during the virtual annual scientific sessions of the American Diabetes Association in June 2020.
Working together in the nephron
One of the clearest complementary interactions between the SGLT2 inhibitors and the RAS inhibitors is their ability to reduce intraglomerular pressure, a key mechanism that slows nephron loss and progression of chronic kidney disease. SGLT2 inhibitors reduce sodium absorption in the proximal tubule that causes, through tubuloglomerular feedback, afferent arteriole constriction that lowers intraglomerular pressure, while the RAS inhibitors inhibit efferent arteriole constriction mediated by angiotensin II, also cutting intraglomerular pressure. Together, “they almost work in tandem,” explained Janani Rangaswami, MD, a nephrologist at Einstein Medical Center in Philadelphia, vice chair of the Kidney Council of the AHA, and first author of the 2019 cardiorenal syndrome AHA statement.
“Many had worried that if we target both the afferent and efferent arterioles simultaneously, it might increase the risk for acute kidney injury. What has been reassuring in both the recent data from the DAPA-HF trial and in recent meta-analysis was no evidence of increased risk for acute kidney injury with use of the SGLT2 inhibitor,” Dr. Rangaswami said in an interview. For example, a recent report on more than 39,000 Canadian patients with T2D who were at least 66 years old and newly begun on either an SGLT2 inhibitor or a different oral diabetes drug (a dipeptidyl peptidase–4 inhibitor), found a statistically significant 21% lower rate of acute kidney injury during the first 90 days on treatment with an SGLT2 inhibitor in a propensity score–matched analysis (CMAJ. 2020 Apr 6;192: e351-60).
Much of the concern about possible acute kidney injury stemmed from a property that the SGLT2 inhibitors share with RAS inhibitors: They cause an initial, reversible decline in glomerular filtration rate (GFR), followed by longer-term nephron preservation, a pattern attributable to reduced intraglomerular pressure. The question early on was: “ ‘Does this harm the kidney?’ But what we’ve seen is that patients do better over time, even with this initial hit. Whenever you offload the glomerulus you cut barotrauma and protect renal function,” explained Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center.
Dr. Inzucchi cautioned, however, that a small number of patients starting treatment with an SGLT2 inhibitor may have their GFR drop too sharply, especially if their GFR was low to start with. “You need to be careful, especially at the lower end of the GFR range. I recheck renal function after 1 month” after a patient starts an SGLT2. Patients whose level falls too low may need to discontinue. He added that it’s hard to set a uniform threshold for alarm, and instead assess patients on a case-by-case basis, but “you need some threshold in mind, where you will stop” treatment.
A smarter diuretic
One of the most intriguing renal effects of SGLT2 inhibitors is their diuretic action. During a talk at the virtual ADA scientific sessions, cardiologist Jeffrey Testani, MD, called them “smart” diuretics, because their effect on diuresis is relatively modest but comes without the neurohormonal price paid when patients take conventional loop diuretics.
”Loop diuretics are particularly bad,” causing neurohormonal activation that includes norepinephrine, renin, and vasopressin, said Dr. Testani, director of heart failure research at Yale. They also fail to produce a meaningful drop in blood volume despite causing substantial natriuresis.
In contrast, SGLT2 inhibitors cause “moderate” natriuresis while producing a significant cut in blood volume. “The body seems content with this lower plasma volume without activating catecholamines or renin, and that’s how the SGLT2 inhibitors differ from other diuretics,” said Dr. Inzucchi.
The class also maintains serum levels of potassium and magnesium, produces significant improvements in serum uric acid levels, and avoids the electrolyte abnormalities, volume depletion, and acute kidney injury that can occur with conventional distal diuretics, Dr. Testani said.
In short, the SGLT2 inhibitors “are safe and easy-to-use diuretics,” which allows them to fill a “huge unmet need for patients with heart failure.” As evidence accumulates for the benefits of the drug class in patients with heart failure and renal disease, “uptake will be extensive,” Dr. Testani predicted, driven in part by how easy it is to add the class to existing cardiorenal drug regimens.
Other standard therapies for patients with heart failure with reduced ejection fraction (HFrEF) risk electrolyte abnormalities, renal dysfunction, significantly lower blood pressure, often make patients feel worse, and involve a slow and laborious titration process, Dr. Testani noted. The SGLT2 inhibitor agents avoid these issues, a property that has played out in quality of life assessments of patients with HFrEF who received a drug from this class.
Outcomes met in trial after trial
In the DAPA-HF trial, with 4,443 patients with HFrEF and divided roughly equally between those with or without T2D, treatment with dapagliflozin (Farxiga) linked with significant improvements in health status and quality of life measured by the Kansas City Cardiomyopathy Questionnaire (Circulation. 2020 Jan 14;141[2]:90-9). “Not all treatments for HFrEF improve symptoms,” but in this study the SGTL2 inhibitor dapagliflozin did, boosting the Kansas City Cardiomyopathy Questionnaire score by about the same magnitude as treatment with a cardiac resynchronization device in patients with HFrEF, said Mikhail N. Kosiborod, MD, director of Cardiometabolic Research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., speaking at the virtual ADA scientific sessions.
Two more recent renal observations have further solidified the growing role of these drugs for kidney protection. Results from the CREDENCE trial that looked at canagliflozin (Invokana) treatment in 4,401 patients with T2D and albuminuria and chronic kidney disease showed canagliflozin treatment cut the primary, composite renal endpoint by a statistically significant 30%, compared with placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). The study stopped earlier than planned because of how effective canagliflozin appeared.
“Never before has a renal protection clinical trial stopped for overwhelming efficacy,” noted nephrologist Katherine R. Tuttle, MD, executive director for research at Providence Health Care in Spokane, Wash. “It’s very exciting to have a treatment that works on both the heart and kidney, given their interrelationship,” she said during the ADA sessions. Dr. Tuttle called the cardiorenal effects from the SGLT2 inhibitors “amazing.”
Just as the DAPA-HF trial’s primary outcome showed the ability of at least one drug from the class, dapagliflozin, to improve outcomes in HFrEF patients without T2D, topline results recently reported from the DAPA-CDK trial showed for the first time renal protection by an SGLT2 inhibitor in patients with chronic kidney disease but no T2D, in a study with about 4,300 patients.
Although detailed results from DAPA-CKD are not yet available, so far the outcomes seem consistent with the CREDENCE findings, and the cumulative renal findings for the class show the SGLT2 inhibitors have “potential for a profound impact on the patients we see in every nephrology clinic, and with dual cardiorenal disease,” said Dr. Rangaswami. The class is now established as “standard of care for patients with chronic kidney disease. The CREDENCE results made that clear.”
The DAPA-CKD findings in patients with chronic kidney disease regardless of their diabetes status “are very important. We really have not had any advances in this space for some time, and chronic kidney disease patients have very poor outcomes, both cardiovascular and renal,” commented Dr. Butler. The advantage from using this drug class in these patients “is huge.”
The DAPA-CKD findings are a “major advance,” agreed Dr. McCullough.
SGLT2 inhibitor use needs to grow
Experts lament that although the evidence favoring the class has been very bullish, prescribing uptake has been slow, perhaps partly explained by the retail U.S. cost for most of these agents, generally about $17/day.
Cost is, unfortunately, an issue right now for these drugs, said Dr. Butler. Generic formulations are imminent, “but we cannot accept waiting. Providing this therapy when insurance coverage is available,” is essential.
The FDA has already granted tentative approval to some generic formulations, although resolution of patent issues can delay generics actually reaching the market. “Generic dapagliflozin will have a major impact; the marketplace for these drugs will shift very quickly,” predicted Dr. McCullough.
But price may not be the sole barrier, cautioned Dr. Rangaswami. “I don’t think it’s just a cost issue. Several factors explain the slow uptake,” of the SGLT2 inhibitors. “The biggest barrier is that this is a new drug class, and understanding how to use the class is not yet where it needs to be in the physician community.” One of the biggest problems is that the SGLT2 inhibitors are still primarily regarded as drugs to treat hyperglycemia.
Physicians who treat patients with heart or renal disease “need to wrap their head around the idea that a drug with antihyperglycemic effects is now in their practice territory, and something they need to prescribe,” she noted. Currently “there is a reluctance to prescribe these drugs given the perception that they are antihyperglycemic agents, and usually get deferred to primary care physicians or endocrinologists. This results in huge missed opportunities by cardiologists and nephrologists in initiating these agents that have major cardiorenal risk reduction effects.”
The key role that cardiologists need to play in prescribing the SGLT2 inhibitors was brought home in a recent study of two representative U.S. health systems that showed patients with T2D were far more likely to see a cardiologist than an endocrinologist (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
“The SGLT2 inhibitors are definitely a game-changing drug class,” summed up Dr. Rangaswami. “We’re going to see a lot of use in patients with heart and kidney disease.”
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Butler has had financial relationships with numerous pharmaceutical companies. Dr. McCullough and Dr. Rangaswami had no disclosures. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Testani has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, cardionomic, FIRE1 Magenta Med, Novartis, Reprieve, Sanofi, and W.L. Gore. Dr. Kosiborod has been a consultant to or led trials for Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Glytec, Janssen, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi, and Vifor. Dr. Tuttle has been a consultant to AstraZeneca, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
mzoler@mdedge.com
AHA on cannabis: No evidence of heart benefits, but potential harms
Evidence for a link between cannabis use and cardiovascular health remains unsupported, and the potential risks outweigh any potential benefits, according to a scientific statement from the American Heart Association.
The increased legalization of cannabis and cannabis products in the United States has driven medical professionals to evaluate the safety and efficacy of cannabis in relation to health conditions, wrote Robert L. Page II, PharmD, of the University of Colorado, Aurora, and colleagues.
In a statement published in Circulation, the researchers noted that although cannabis has been shown to relieve pain and other symptoms in certain conditions, clinicians in the United States have been limited from studying its health effects because of federal law restrictions. “Cannabis remains a schedule I controlled substance, deeming no accepted medical use, a high potential for abuse, and an unacceptable safety profile,” the researchers wrote.
The statement addresses issues with the use of cannabis by individuals with cardiovascular disease or those at increased risk. Observational studies have shown no cardiovascular benefits associated with cannabis, the writers noted. The most common chemicals in cannabis include THC (tetrahydrocannabinolic acid) and CBD (cannabidiol).
Some research has shown associations between CBD cardiovascular features including lower blood pressure and reduced inflammation, the writers noted. However, THC, the component of cannabis associated with a “high” or intoxication, has been associated with heart rhythm abnormalities. The writers cited data suggesting an increased risk of heart attacks, atrial fibrillation and heart failure, although more research is needed.
The statement outlines common cannabis formulations including plant-based, extracts, crystalline forms, edible products, and tinctures. In addition, the statement notes that synthetic cannabis products are marketed and used in the United States without subject to regulation.
“Over the past 5 years, we have seen a surge in cannabis use, particularly during the COVID-19 pandemic here in Colorado, especially among adolescents and young adults,” Dr. Page said in an interview. Because of the surge, health care practitioners need to familiarize themselves with not only the benefits, but risks associated with cannabis use regardless of the formulation,” he said. As heart disease remains a leading cause of death in the United States, understanding the cardiovascular risks associated with cannabis is crucial at this time.
Dr. Page noted that popular attitudes about cannabis could pose risks to users’ cardiovascular health. “One leading misconception about cannabis is because it is ‘natural’ it must be safe,” Dr. Page said. “As with all medications, cannabis has side effects, some of which can be cardiovascular in nature,” he said. “Significant drug-drug interactions can occur as CBD and THC, both found in cannabis, inhibit CYP3A4, which metabolizes a large number of medications used to treat many cardiovascular conditions,” he noted.
“Unfortunately, much of the published data is observational in nature due to the federal restrictions on cannabis as a schedule I drug,” said Dr. Page. “Nonetheless, safety signals have emerged regarding cannabis use and adverse cardiovascular outcomes, including myocardial infarction, heart failure, and atrial fibrillation. Carefully designed prospective short- and long-term studies regarding cannabis use and cardiovascular safety are needed,” he emphasized.
Areas in particular need of additional research include the cardiovascular effects of cannabis in several vulnerable populations such as adolescents, older adults, pregnant women, transplant recipients, and those with underlying cardiovascular disease, said Dr. Page.
“Nonetheless, based on the safety signals described within this Clinical Science Statement, an open discussion regarding the risks of using cannabis needs to occur between patient and health care providers,” he said. “Furthermore, patients must be transparent regarding their cannabis use with their cardiologist and primary care provider. The cannabis story will continue to evolve and is a rapidly moving/changing target,” he said.
“Whether cannabis use is a definitive risk factor for cardiovascular disease as with tobacco use is still unknown, and both acute and long-term studies are desperately needed to address this issue,” he said.
Dr. Page had no relevant financial conflicts to disclose.
SOURCE: Page et al. Circulation. 2020 Aug 5. doi: 10.1161/CIR.0000000000000883.
Evidence for a link between cannabis use and cardiovascular health remains unsupported, and the potential risks outweigh any potential benefits, according to a scientific statement from the American Heart Association.
The increased legalization of cannabis and cannabis products in the United States has driven medical professionals to evaluate the safety and efficacy of cannabis in relation to health conditions, wrote Robert L. Page II, PharmD, of the University of Colorado, Aurora, and colleagues.
In a statement published in Circulation, the researchers noted that although cannabis has been shown to relieve pain and other symptoms in certain conditions, clinicians in the United States have been limited from studying its health effects because of federal law restrictions. “Cannabis remains a schedule I controlled substance, deeming no accepted medical use, a high potential for abuse, and an unacceptable safety profile,” the researchers wrote.
The statement addresses issues with the use of cannabis by individuals with cardiovascular disease or those at increased risk. Observational studies have shown no cardiovascular benefits associated with cannabis, the writers noted. The most common chemicals in cannabis include THC (tetrahydrocannabinolic acid) and CBD (cannabidiol).
Some research has shown associations between CBD cardiovascular features including lower blood pressure and reduced inflammation, the writers noted. However, THC, the component of cannabis associated with a “high” or intoxication, has been associated with heart rhythm abnormalities. The writers cited data suggesting an increased risk of heart attacks, atrial fibrillation and heart failure, although more research is needed.
The statement outlines common cannabis formulations including plant-based, extracts, crystalline forms, edible products, and tinctures. In addition, the statement notes that synthetic cannabis products are marketed and used in the United States without subject to regulation.
“Over the past 5 years, we have seen a surge in cannabis use, particularly during the COVID-19 pandemic here in Colorado, especially among adolescents and young adults,” Dr. Page said in an interview. Because of the surge, health care practitioners need to familiarize themselves with not only the benefits, but risks associated with cannabis use regardless of the formulation,” he said. As heart disease remains a leading cause of death in the United States, understanding the cardiovascular risks associated with cannabis is crucial at this time.
Dr. Page noted that popular attitudes about cannabis could pose risks to users’ cardiovascular health. “One leading misconception about cannabis is because it is ‘natural’ it must be safe,” Dr. Page said. “As with all medications, cannabis has side effects, some of which can be cardiovascular in nature,” he said. “Significant drug-drug interactions can occur as CBD and THC, both found in cannabis, inhibit CYP3A4, which metabolizes a large number of medications used to treat many cardiovascular conditions,” he noted.
“Unfortunately, much of the published data is observational in nature due to the federal restrictions on cannabis as a schedule I drug,” said Dr. Page. “Nonetheless, safety signals have emerged regarding cannabis use and adverse cardiovascular outcomes, including myocardial infarction, heart failure, and atrial fibrillation. Carefully designed prospective short- and long-term studies regarding cannabis use and cardiovascular safety are needed,” he emphasized.
Areas in particular need of additional research include the cardiovascular effects of cannabis in several vulnerable populations such as adolescents, older adults, pregnant women, transplant recipients, and those with underlying cardiovascular disease, said Dr. Page.
“Nonetheless, based on the safety signals described within this Clinical Science Statement, an open discussion regarding the risks of using cannabis needs to occur between patient and health care providers,” he said. “Furthermore, patients must be transparent regarding their cannabis use with their cardiologist and primary care provider. The cannabis story will continue to evolve and is a rapidly moving/changing target,” he said.
“Whether cannabis use is a definitive risk factor for cardiovascular disease as with tobacco use is still unknown, and both acute and long-term studies are desperately needed to address this issue,” he said.
Dr. Page had no relevant financial conflicts to disclose.
SOURCE: Page et al. Circulation. 2020 Aug 5. doi: 10.1161/CIR.0000000000000883.
Evidence for a link between cannabis use and cardiovascular health remains unsupported, and the potential risks outweigh any potential benefits, according to a scientific statement from the American Heart Association.
The increased legalization of cannabis and cannabis products in the United States has driven medical professionals to evaluate the safety and efficacy of cannabis in relation to health conditions, wrote Robert L. Page II, PharmD, of the University of Colorado, Aurora, and colleagues.
In a statement published in Circulation, the researchers noted that although cannabis has been shown to relieve pain and other symptoms in certain conditions, clinicians in the United States have been limited from studying its health effects because of federal law restrictions. “Cannabis remains a schedule I controlled substance, deeming no accepted medical use, a high potential for abuse, and an unacceptable safety profile,” the researchers wrote.
The statement addresses issues with the use of cannabis by individuals with cardiovascular disease or those at increased risk. Observational studies have shown no cardiovascular benefits associated with cannabis, the writers noted. The most common chemicals in cannabis include THC (tetrahydrocannabinolic acid) and CBD (cannabidiol).
Some research has shown associations between CBD cardiovascular features including lower blood pressure and reduced inflammation, the writers noted. However, THC, the component of cannabis associated with a “high” or intoxication, has been associated with heart rhythm abnormalities. The writers cited data suggesting an increased risk of heart attacks, atrial fibrillation and heart failure, although more research is needed.
The statement outlines common cannabis formulations including plant-based, extracts, crystalline forms, edible products, and tinctures. In addition, the statement notes that synthetic cannabis products are marketed and used in the United States without subject to regulation.
“Over the past 5 years, we have seen a surge in cannabis use, particularly during the COVID-19 pandemic here in Colorado, especially among adolescents and young adults,” Dr. Page said in an interview. Because of the surge, health care practitioners need to familiarize themselves with not only the benefits, but risks associated with cannabis use regardless of the formulation,” he said. As heart disease remains a leading cause of death in the United States, understanding the cardiovascular risks associated with cannabis is crucial at this time.
Dr. Page noted that popular attitudes about cannabis could pose risks to users’ cardiovascular health. “One leading misconception about cannabis is because it is ‘natural’ it must be safe,” Dr. Page said. “As with all medications, cannabis has side effects, some of which can be cardiovascular in nature,” he said. “Significant drug-drug interactions can occur as CBD and THC, both found in cannabis, inhibit CYP3A4, which metabolizes a large number of medications used to treat many cardiovascular conditions,” he noted.
“Unfortunately, much of the published data is observational in nature due to the federal restrictions on cannabis as a schedule I drug,” said Dr. Page. “Nonetheless, safety signals have emerged regarding cannabis use and adverse cardiovascular outcomes, including myocardial infarction, heart failure, and atrial fibrillation. Carefully designed prospective short- and long-term studies regarding cannabis use and cardiovascular safety are needed,” he emphasized.
Areas in particular need of additional research include the cardiovascular effects of cannabis in several vulnerable populations such as adolescents, older adults, pregnant women, transplant recipients, and those with underlying cardiovascular disease, said Dr. Page.
“Nonetheless, based on the safety signals described within this Clinical Science Statement, an open discussion regarding the risks of using cannabis needs to occur between patient and health care providers,” he said. “Furthermore, patients must be transparent regarding their cannabis use with their cardiologist and primary care provider. The cannabis story will continue to evolve and is a rapidly moving/changing target,” he said.
“Whether cannabis use is a definitive risk factor for cardiovascular disease as with tobacco use is still unknown, and both acute and long-term studies are desperately needed to address this issue,” he said.
Dr. Page had no relevant financial conflicts to disclose.
SOURCE: Page et al. Circulation. 2020 Aug 5. doi: 10.1161/CIR.0000000000000883.
FROM CIRCULATION
Does stirrup choice influence vaginal surgery outcome?
according to the first randomized controlled trial comparing both types of lithotomy stirrups.
“Participants positioned in candy cane stirrups had greater hip abduction than those positioned in boot stirrups, which could provide a rationale for our findings,” suggested Ankita Gupta, MD, MPH, of the University of Louisville (Ky.), and colleagues. Their report is in Obstetrics & Gynecology.
But one expert questions this interpretation, calling it a major limitation of the study.
“The only difference between the two arms of the study is associated with the angles between the femurs,” said Rosanne M. Kho, MD, a gynecologic surgeon at Cleveland Clinic, who was not involved in the study. “The difference of the angles at the femur is not inherent to the type of stirrup but in the method in which the patients were positioned using the two different types of stirrups,” she said. “The same wide angle between the femurs can be attained with the boot stirrups if the patient is not positioned properly. To determine if the same benefit in physical function is achieved with a lesser angle between the femur, the investigators should use only one type of stirrup (whether the candy cane or the boot stirrups) and change only the angles of the femur.”
The study was a single-masked, randomized controlled trial of women undergoing vaginal surgery at the University of Louisville’s division of urogynecology between March 2018 and Oct. 2019. Surgeries included any combination of vaginal hysterectomy, vaginal vault suspension (uterosacral or sacrospinous ligament fixation), vaginectomy (partial or total), mid-urethral slings, or other surgeries such as urethral diverticulectomy, fistula repair, or mesh excision.
Among the 138 women included in the intention-to-treat analysis, 72 were randomized to candy cane, and 66 to boot (Yellofin) stirrups. They were positioned in the assigned stirrup by the attending surgeon, with assistance from the surgical team, after administration of anesthesia and were not informed of their allocation until the end of the study at 6 weeks post surgery.
On day 1 post surgery, a 100-point visual analog scale (VAS) questionnaire was administered for pain in the lower back, hips, buttocks, thighs, knees, calves, and feet, followed by a series of questionnaires at 6 weeks post surgery, including the PROMIS (Patient-Reported Outcomes Measurement Information System) forms on physical function, pain intensity, and pain interference, as well as the Pelvic Floor Disability Index (PFDI-20) and the Patient Global Impression of Improvement forms.
While the authors acknowledged that neurologic injuries following vaginal surgery are rare, and therefore difficult to measure, physical function is a “prudent” alternative measurement.
Although the study was designed to compare lithotomy stirrups, patient positioning also was measured. Once the patient was anesthetized, the surgeon used a goniometer to measure flexion at the hip and knee joints, the angle of abduction and external rotation at the hip. The “angle between the femurs” was measured by placing the fulcrum of the goniometer at the anal opening.
While the angles of flexion at the hips and knees were similar between groups, the study found a significant difference between groups in the angle between the femurs (mean ± standard deviation, 88.7 ± 13.4 candy cane vs. 77.2 ± 13.3 boot, P < .01).
In addition, the primary outcome, change in physical function based on the PROMIS physical function shortform-20a, was significantly different between the two groups: While subjects in the candy cane group demonstrated a decline of 1.9 in mean physical function score at 6 weeks compared to baseline, those in the boot stirrup group showed an increase of 1.9 from baseline. The mean 6-week postoperative scores were 45.8 versus 49.8 for the candy cane and boot stirrup groups respectively (P < .01).
Although it was “well executed by a well-respected group of vaginal surgeons at a major academic institution,” the study has other limitations, noted Dr. Kho.
“Though the measurements were obtained with the goniometer at the beginning of the surgery, it does not appear that a repeat measurement was performed at the end of the case. Is it possible that positioning could have shifted and resulted in further change in the angle of the femur/hip/knees compared to the beginning of the surgery?” she asked.
In addition, “compared to the candy canes, the boot stirrup has bulky boots that could limit opportunities for bedside assistants who were standing next to the primary surgeon to lean against the patient’s thighs during the surgery. Were there measures done to ensure that assistants were not leaning against the [candy cane] patients?”
In terms of the 6-week outcome measure, Dr. Kho suggested PROMIS outcomes measured at 2 weeks and at 4 or 6 weeks “would have provided greater insight to the study question.
“The authors acknowledge that neuropathies due to patient positioning manifest soon after surgery and tend to be transient. Incidence of neuropathy is extremely low in both groups and is equivalent. Factors that could impair quick return to normal activity as a result of the neuromuscular effects due to patient positioning should have been measured earlier,” she suggested.
Finally, Dr. Kho noted that the authors “fail to provide any likely rationale for the impaired physical function measured at 6 weeks that can be attributed to the difference in the angles at the femur. The findings of decreased physical function at 6 weeks in the candy cane group may be incidental, and may be different if measured at an earlier time (which would be more pertinent for this study) or at a later time such as 3 months.”
Individual authors acknowledged personal funds from Society of Gynecologic Surgeons, Elsevier publishing, RBI Medical, and AMAG Pharmaceuticals. Dr. Kho had no relevant financial disclosures.
SOURCE: Gupta A et al. Obstet Gynecol. 2020 July 8. doi: 10.1097/AOG.0000000000003954.
according to the first randomized controlled trial comparing both types of lithotomy stirrups.
“Participants positioned in candy cane stirrups had greater hip abduction than those positioned in boot stirrups, which could provide a rationale for our findings,” suggested Ankita Gupta, MD, MPH, of the University of Louisville (Ky.), and colleagues. Their report is in Obstetrics & Gynecology.
But one expert questions this interpretation, calling it a major limitation of the study.
“The only difference between the two arms of the study is associated with the angles between the femurs,” said Rosanne M. Kho, MD, a gynecologic surgeon at Cleveland Clinic, who was not involved in the study. “The difference of the angles at the femur is not inherent to the type of stirrup but in the method in which the patients were positioned using the two different types of stirrups,” she said. “The same wide angle between the femurs can be attained with the boot stirrups if the patient is not positioned properly. To determine if the same benefit in physical function is achieved with a lesser angle between the femur, the investigators should use only one type of stirrup (whether the candy cane or the boot stirrups) and change only the angles of the femur.”
The study was a single-masked, randomized controlled trial of women undergoing vaginal surgery at the University of Louisville’s division of urogynecology between March 2018 and Oct. 2019. Surgeries included any combination of vaginal hysterectomy, vaginal vault suspension (uterosacral or sacrospinous ligament fixation), vaginectomy (partial or total), mid-urethral slings, or other surgeries such as urethral diverticulectomy, fistula repair, or mesh excision.
Among the 138 women included in the intention-to-treat analysis, 72 were randomized to candy cane, and 66 to boot (Yellofin) stirrups. They were positioned in the assigned stirrup by the attending surgeon, with assistance from the surgical team, after administration of anesthesia and were not informed of their allocation until the end of the study at 6 weeks post surgery.
On day 1 post surgery, a 100-point visual analog scale (VAS) questionnaire was administered for pain in the lower back, hips, buttocks, thighs, knees, calves, and feet, followed by a series of questionnaires at 6 weeks post surgery, including the PROMIS (Patient-Reported Outcomes Measurement Information System) forms on physical function, pain intensity, and pain interference, as well as the Pelvic Floor Disability Index (PFDI-20) and the Patient Global Impression of Improvement forms.
While the authors acknowledged that neurologic injuries following vaginal surgery are rare, and therefore difficult to measure, physical function is a “prudent” alternative measurement.
Although the study was designed to compare lithotomy stirrups, patient positioning also was measured. Once the patient was anesthetized, the surgeon used a goniometer to measure flexion at the hip and knee joints, the angle of abduction and external rotation at the hip. The “angle between the femurs” was measured by placing the fulcrum of the goniometer at the anal opening.
While the angles of flexion at the hips and knees were similar between groups, the study found a significant difference between groups in the angle between the femurs (mean ± standard deviation, 88.7 ± 13.4 candy cane vs. 77.2 ± 13.3 boot, P < .01).
In addition, the primary outcome, change in physical function based on the PROMIS physical function shortform-20a, was significantly different between the two groups: While subjects in the candy cane group demonstrated a decline of 1.9 in mean physical function score at 6 weeks compared to baseline, those in the boot stirrup group showed an increase of 1.9 from baseline. The mean 6-week postoperative scores were 45.8 versus 49.8 for the candy cane and boot stirrup groups respectively (P < .01).
Although it was “well executed by a well-respected group of vaginal surgeons at a major academic institution,” the study has other limitations, noted Dr. Kho.
“Though the measurements were obtained with the goniometer at the beginning of the surgery, it does not appear that a repeat measurement was performed at the end of the case. Is it possible that positioning could have shifted and resulted in further change in the angle of the femur/hip/knees compared to the beginning of the surgery?” she asked.
In addition, “compared to the candy canes, the boot stirrup has bulky boots that could limit opportunities for bedside assistants who were standing next to the primary surgeon to lean against the patient’s thighs during the surgery. Were there measures done to ensure that assistants were not leaning against the [candy cane] patients?”
In terms of the 6-week outcome measure, Dr. Kho suggested PROMIS outcomes measured at 2 weeks and at 4 or 6 weeks “would have provided greater insight to the study question.
“The authors acknowledge that neuropathies due to patient positioning manifest soon after surgery and tend to be transient. Incidence of neuropathy is extremely low in both groups and is equivalent. Factors that could impair quick return to normal activity as a result of the neuromuscular effects due to patient positioning should have been measured earlier,” she suggested.
Finally, Dr. Kho noted that the authors “fail to provide any likely rationale for the impaired physical function measured at 6 weeks that can be attributed to the difference in the angles at the femur. The findings of decreased physical function at 6 weeks in the candy cane group may be incidental, and may be different if measured at an earlier time (which would be more pertinent for this study) or at a later time such as 3 months.”
Individual authors acknowledged personal funds from Society of Gynecologic Surgeons, Elsevier publishing, RBI Medical, and AMAG Pharmaceuticals. Dr. Kho had no relevant financial disclosures.
SOURCE: Gupta A et al. Obstet Gynecol. 2020 July 8. doi: 10.1097/AOG.0000000000003954.
according to the first randomized controlled trial comparing both types of lithotomy stirrups.
“Participants positioned in candy cane stirrups had greater hip abduction than those positioned in boot stirrups, which could provide a rationale for our findings,” suggested Ankita Gupta, MD, MPH, of the University of Louisville (Ky.), and colleagues. Their report is in Obstetrics & Gynecology.
But one expert questions this interpretation, calling it a major limitation of the study.
“The only difference between the two arms of the study is associated with the angles between the femurs,” said Rosanne M. Kho, MD, a gynecologic surgeon at Cleveland Clinic, who was not involved in the study. “The difference of the angles at the femur is not inherent to the type of stirrup but in the method in which the patients were positioned using the two different types of stirrups,” she said. “The same wide angle between the femurs can be attained with the boot stirrups if the patient is not positioned properly. To determine if the same benefit in physical function is achieved with a lesser angle between the femur, the investigators should use only one type of stirrup (whether the candy cane or the boot stirrups) and change only the angles of the femur.”
The study was a single-masked, randomized controlled trial of women undergoing vaginal surgery at the University of Louisville’s division of urogynecology between March 2018 and Oct. 2019. Surgeries included any combination of vaginal hysterectomy, vaginal vault suspension (uterosacral or sacrospinous ligament fixation), vaginectomy (partial or total), mid-urethral slings, or other surgeries such as urethral diverticulectomy, fistula repair, or mesh excision.
Among the 138 women included in the intention-to-treat analysis, 72 were randomized to candy cane, and 66 to boot (Yellofin) stirrups. They were positioned in the assigned stirrup by the attending surgeon, with assistance from the surgical team, after administration of anesthesia and were not informed of their allocation until the end of the study at 6 weeks post surgery.
On day 1 post surgery, a 100-point visual analog scale (VAS) questionnaire was administered for pain in the lower back, hips, buttocks, thighs, knees, calves, and feet, followed by a series of questionnaires at 6 weeks post surgery, including the PROMIS (Patient-Reported Outcomes Measurement Information System) forms on physical function, pain intensity, and pain interference, as well as the Pelvic Floor Disability Index (PFDI-20) and the Patient Global Impression of Improvement forms.
While the authors acknowledged that neurologic injuries following vaginal surgery are rare, and therefore difficult to measure, physical function is a “prudent” alternative measurement.
Although the study was designed to compare lithotomy stirrups, patient positioning also was measured. Once the patient was anesthetized, the surgeon used a goniometer to measure flexion at the hip and knee joints, the angle of abduction and external rotation at the hip. The “angle between the femurs” was measured by placing the fulcrum of the goniometer at the anal opening.
While the angles of flexion at the hips and knees were similar between groups, the study found a significant difference between groups in the angle between the femurs (mean ± standard deviation, 88.7 ± 13.4 candy cane vs. 77.2 ± 13.3 boot, P < .01).
In addition, the primary outcome, change in physical function based on the PROMIS physical function shortform-20a, was significantly different between the two groups: While subjects in the candy cane group demonstrated a decline of 1.9 in mean physical function score at 6 weeks compared to baseline, those in the boot stirrup group showed an increase of 1.9 from baseline. The mean 6-week postoperative scores were 45.8 versus 49.8 for the candy cane and boot stirrup groups respectively (P < .01).
Although it was “well executed by a well-respected group of vaginal surgeons at a major academic institution,” the study has other limitations, noted Dr. Kho.
“Though the measurements were obtained with the goniometer at the beginning of the surgery, it does not appear that a repeat measurement was performed at the end of the case. Is it possible that positioning could have shifted and resulted in further change in the angle of the femur/hip/knees compared to the beginning of the surgery?” she asked.
In addition, “compared to the candy canes, the boot stirrup has bulky boots that could limit opportunities for bedside assistants who were standing next to the primary surgeon to lean against the patient’s thighs during the surgery. Were there measures done to ensure that assistants were not leaning against the [candy cane] patients?”
In terms of the 6-week outcome measure, Dr. Kho suggested PROMIS outcomes measured at 2 weeks and at 4 or 6 weeks “would have provided greater insight to the study question.
“The authors acknowledge that neuropathies due to patient positioning manifest soon after surgery and tend to be transient. Incidence of neuropathy is extremely low in both groups and is equivalent. Factors that could impair quick return to normal activity as a result of the neuromuscular effects due to patient positioning should have been measured earlier,” she suggested.
Finally, Dr. Kho noted that the authors “fail to provide any likely rationale for the impaired physical function measured at 6 weeks that can be attributed to the difference in the angles at the femur. The findings of decreased physical function at 6 weeks in the candy cane group may be incidental, and may be different if measured at an earlier time (which would be more pertinent for this study) or at a later time such as 3 months.”
Individual authors acknowledged personal funds from Society of Gynecologic Surgeons, Elsevier publishing, RBI Medical, and AMAG Pharmaceuticals. Dr. Kho had no relevant financial disclosures.
SOURCE: Gupta A et al. Obstet Gynecol. 2020 July 8. doi: 10.1097/AOG.0000000000003954.
FROM OBSTETRICS & GYNECOLOGY
How prostate cancer treatments affect quality of life
according to a presentation at the virtual annual congress of the European Association of Urology (EAU).
Results of EUPROMS – the first patient-driven, international, prostate cancer quality of life study – showed that fatigue, insomnia, urinary incontinence, and sexual function were worse with certain types of treatments.
“Quality of life is negatively impacted by any treatment for prostate cancer other than active surveillance,” said André Deschamps, the chairman of the patient advocacy movement Europa Uomo, which conducted the study with support from Erasmus University Medical Center in Rotterdam, the Netherlands.
Active surveillance “should be promoted as the first option for treatment for those men where it can be offered safely,” Mr. Deschamps said when presenting the study at the EAU congress.
The study showed that quality of life related to urinary incontinence was lowest in patients who had undergone radical prostatectomy, and sexual function was greatly affected by radiotherapy. Radiotherapy and chemotherapy had the greatest impact on patients’ levels of fatigue, and chemotherapy was associated with “the worst possible outcomes in quality of life,” Mr. Deschamps said.
Conversely, “reported quality of life scores are the best in patients where the cancer is discovered in an early, curable stage. Hence, efforts toward early detection and awareness are essential to avoid unnecessary deterioration in quality of life,” Mr. Deschamps said.
About the survey and respondents
Between August and November 2019, 2,943 prostate cancer patients from 24 European countries completed a web-based survey made available via the Europa Uomo website. The survey took around 20 minutes to complete and used three validated quality of life questionnaires, the EORTC-QLQ-C30, the EQ-5D-5L, and EPIC-26.
“The questionnaires were available in 19 languages, so every patient could answer in their mother tongue,” Mr. Deschamps pointed out, highlighting that this was a Europe-wide survey and was estimated to account for 0.1% of the patient population in Europe.
Countries with the highest number of respondents were Norway (n = 506), Sweden (n = 386), Belgium (n = 339), Germany (n = 253), Netherlands (n = 244), France (n = 234), Denmark (n = 188), the United Kingdom (n = 187), and Poland (n = 109).
The average age of respondents was 70 years at the time of the survey and 64 years at the time of diagnosis. Most patients (82%) were living with a partner.
Two-thirds of patients had received only one treatment for prostate cancer. This was most often radical prostatectomy, external beam radiotherapy, or active surveillance. Among the 22% of patients who had received two treatments, the therapies were most often a combination of surgery and radiotherapy, androgen deprivation therapy (ADT) and radiotherapy or chemotherapy, and active surveillance and surgery.
Fatigue and insomnia
According to the EORTC-QLQ-C30 symptoms questionnaire, fatigue and insomnia were particular problems for men with prostate cancer, as denoted by scores of 25 and 24, respectively, out of a possible 100. Low scores are associated with worse fatigue and insomnia.
The researchers focused their attention on how specific cancer treatments might influence fatigue. They found that radiotherapy doubled and chemotherapy tripled the number of patients reporting fatigue, when compared with active surveillance. The incidence of fatigue was 22% (n = 304), 33% (n = 246), and 11% (n = 179), respectively.
As for insomnia, “it’s bit of a mixed view,” Mr. Deschamps said. “We believe that the progression of disease is more important for insomnia. The only thing you can say is that chemotherapy leads to an increase in reported insomnia.”
Urinary continence and sexual function
The EPIC-26 questionnaire was used to look at the health-related quality of life domains of urinary and sexual function. Sexual function was the most impacted area.
“We often hear that decline in sexual functioning is a relatively small problem for prostate cancer patients, and the effect on their quality of life should not be exaggerated,” Mr. Deschamps said in a press statement.
“We also hear that prostate cancer is typically a disease of ‘old men,’ implying that the loss of sexual function is less relevant. This survey paints a different picture,” he added.
Higher EPIC-26 scores signify better function. For urinary incontinence, the score was 100/100 for active surveillance but 65/100 when active surveillance was combined with surgery and 71/100 for surgery alone. The combination of surgery and radiotherapy carried a score of 73/100 for urinary incontinence. Radiotherapy on its own had a score of 92/100, suggesting it was the addition of the surgery that was having a significant effect. The score for radiotherapy plus ADT was 100/100, and the score for chemotherapy was 86/100.
Chemotherapy appeared to have the worst effect on sexual function, with a score of just 12/100. Radiotherapy was not far behind at 17/100, and surgery alone was 21/100. When radiotherapy and surgery were combined, the score was 15/100.
Sexual function scores were also low for all the other treatments considered – 18/100 for radiotherapy and ADT, 26/100 for active surveillance and surgery, and 57/100 for active surveillance alone.
Implications for practice
“The data collected and the analysis done provide patients and healthcare professionals with a ‘snapshot’ on the impact of treatments based on the experience of fellow patients,” Mr. Deschamps said. “We hope these results will be used to establish and disseminate realistic expectations on the effects of different treatments for prostate cancer on [quality of life].”
“This study is important because it was initiated by patients and meant for patients,” noted Monique Roobol, PhD, professor of decision-making in urology at the Erasmus University Medical Center in Rotterdam, the Netherlands, where the survey data were analyzed.
“The questionnaires were completed unrelated to a hospital visit, which means respondents had more freedom to answer and provide insight into the effect of treatment on quality of life over a longer period,” she added.
“For me, the key point is that, as health care professionals, we have underestimated the impact on the quality of life for patients treated for prostate cancer,” said Hein van Poppel, MD, PhD, of University Hospitals Leuven (Belgium), who chaired the session in which the data were presented.
Arnulf Stenzl, MD, of Tübingen (Germany) University said in a statement that the survey provided valuable information. “It uses the same questionnaires used in standard clinical settings, but it is both qualitatively and quantitatively different to the kind of study usually undertaken, so it needs to be read alongside these previous studies,” Dr. Stenzl said.
There were several strong points, he said, such as the fact that EUPROMS was the largest study of its kind and thus would “reflect the impact of treatment on a wide range of patients, with different health systems.”
As an official EAU spokesperson, Dr. Stenzl added, “We completely agree that early detection and treatment is essential if we are to avoid problems with quality of life later on. It shows that, for many men, quality of life can be poor after most prostate cancer treatment, especially in advanced disease. This message is clear, and we need to listen to the voices of these patients.”
EUPROMS was conducted by Europa Uomo in conjunction with the Erasmus University Medical Centre in Rotterdam, the Netherlands. Funding was received from Bayer, Ipsen, and Janssen. The companies had no influence over any aspect of the study. The commentators did not have conflicts of interest to disclose.
according to a presentation at the virtual annual congress of the European Association of Urology (EAU).
Results of EUPROMS – the first patient-driven, international, prostate cancer quality of life study – showed that fatigue, insomnia, urinary incontinence, and sexual function were worse with certain types of treatments.
“Quality of life is negatively impacted by any treatment for prostate cancer other than active surveillance,” said André Deschamps, the chairman of the patient advocacy movement Europa Uomo, which conducted the study with support from Erasmus University Medical Center in Rotterdam, the Netherlands.
Active surveillance “should be promoted as the first option for treatment for those men where it can be offered safely,” Mr. Deschamps said when presenting the study at the EAU congress.
The study showed that quality of life related to urinary incontinence was lowest in patients who had undergone radical prostatectomy, and sexual function was greatly affected by radiotherapy. Radiotherapy and chemotherapy had the greatest impact on patients’ levels of fatigue, and chemotherapy was associated with “the worst possible outcomes in quality of life,” Mr. Deschamps said.
Conversely, “reported quality of life scores are the best in patients where the cancer is discovered in an early, curable stage. Hence, efforts toward early detection and awareness are essential to avoid unnecessary deterioration in quality of life,” Mr. Deschamps said.
About the survey and respondents
Between August and November 2019, 2,943 prostate cancer patients from 24 European countries completed a web-based survey made available via the Europa Uomo website. The survey took around 20 minutes to complete and used three validated quality of life questionnaires, the EORTC-QLQ-C30, the EQ-5D-5L, and EPIC-26.
“The questionnaires were available in 19 languages, so every patient could answer in their mother tongue,” Mr. Deschamps pointed out, highlighting that this was a Europe-wide survey and was estimated to account for 0.1% of the patient population in Europe.
Countries with the highest number of respondents were Norway (n = 506), Sweden (n = 386), Belgium (n = 339), Germany (n = 253), Netherlands (n = 244), France (n = 234), Denmark (n = 188), the United Kingdom (n = 187), and Poland (n = 109).
The average age of respondents was 70 years at the time of the survey and 64 years at the time of diagnosis. Most patients (82%) were living with a partner.
Two-thirds of patients had received only one treatment for prostate cancer. This was most often radical prostatectomy, external beam radiotherapy, or active surveillance. Among the 22% of patients who had received two treatments, the therapies were most often a combination of surgery and radiotherapy, androgen deprivation therapy (ADT) and radiotherapy or chemotherapy, and active surveillance and surgery.
Fatigue and insomnia
According to the EORTC-QLQ-C30 symptoms questionnaire, fatigue and insomnia were particular problems for men with prostate cancer, as denoted by scores of 25 and 24, respectively, out of a possible 100. Low scores are associated with worse fatigue and insomnia.
The researchers focused their attention on how specific cancer treatments might influence fatigue. They found that radiotherapy doubled and chemotherapy tripled the number of patients reporting fatigue, when compared with active surveillance. The incidence of fatigue was 22% (n = 304), 33% (n = 246), and 11% (n = 179), respectively.
As for insomnia, “it’s bit of a mixed view,” Mr. Deschamps said. “We believe that the progression of disease is more important for insomnia. The only thing you can say is that chemotherapy leads to an increase in reported insomnia.”
Urinary continence and sexual function
The EPIC-26 questionnaire was used to look at the health-related quality of life domains of urinary and sexual function. Sexual function was the most impacted area.
“We often hear that decline in sexual functioning is a relatively small problem for prostate cancer patients, and the effect on their quality of life should not be exaggerated,” Mr. Deschamps said in a press statement.
“We also hear that prostate cancer is typically a disease of ‘old men,’ implying that the loss of sexual function is less relevant. This survey paints a different picture,” he added.
Higher EPIC-26 scores signify better function. For urinary incontinence, the score was 100/100 for active surveillance but 65/100 when active surveillance was combined with surgery and 71/100 for surgery alone. The combination of surgery and radiotherapy carried a score of 73/100 for urinary incontinence. Radiotherapy on its own had a score of 92/100, suggesting it was the addition of the surgery that was having a significant effect. The score for radiotherapy plus ADT was 100/100, and the score for chemotherapy was 86/100.
Chemotherapy appeared to have the worst effect on sexual function, with a score of just 12/100. Radiotherapy was not far behind at 17/100, and surgery alone was 21/100. When radiotherapy and surgery were combined, the score was 15/100.
Sexual function scores were also low for all the other treatments considered – 18/100 for radiotherapy and ADT, 26/100 for active surveillance and surgery, and 57/100 for active surveillance alone.
Implications for practice
“The data collected and the analysis done provide patients and healthcare professionals with a ‘snapshot’ on the impact of treatments based on the experience of fellow patients,” Mr. Deschamps said. “We hope these results will be used to establish and disseminate realistic expectations on the effects of different treatments for prostate cancer on [quality of life].”
“This study is important because it was initiated by patients and meant for patients,” noted Monique Roobol, PhD, professor of decision-making in urology at the Erasmus University Medical Center in Rotterdam, the Netherlands, where the survey data were analyzed.
“The questionnaires were completed unrelated to a hospital visit, which means respondents had more freedom to answer and provide insight into the effect of treatment on quality of life over a longer period,” she added.
“For me, the key point is that, as health care professionals, we have underestimated the impact on the quality of life for patients treated for prostate cancer,” said Hein van Poppel, MD, PhD, of University Hospitals Leuven (Belgium), who chaired the session in which the data were presented.
Arnulf Stenzl, MD, of Tübingen (Germany) University said in a statement that the survey provided valuable information. “It uses the same questionnaires used in standard clinical settings, but it is both qualitatively and quantitatively different to the kind of study usually undertaken, so it needs to be read alongside these previous studies,” Dr. Stenzl said.
There were several strong points, he said, such as the fact that EUPROMS was the largest study of its kind and thus would “reflect the impact of treatment on a wide range of patients, with different health systems.”
As an official EAU spokesperson, Dr. Stenzl added, “We completely agree that early detection and treatment is essential if we are to avoid problems with quality of life later on. It shows that, for many men, quality of life can be poor after most prostate cancer treatment, especially in advanced disease. This message is clear, and we need to listen to the voices of these patients.”
EUPROMS was conducted by Europa Uomo in conjunction with the Erasmus University Medical Centre in Rotterdam, the Netherlands. Funding was received from Bayer, Ipsen, and Janssen. The companies had no influence over any aspect of the study. The commentators did not have conflicts of interest to disclose.
according to a presentation at the virtual annual congress of the European Association of Urology (EAU).
Results of EUPROMS – the first patient-driven, international, prostate cancer quality of life study – showed that fatigue, insomnia, urinary incontinence, and sexual function were worse with certain types of treatments.
“Quality of life is negatively impacted by any treatment for prostate cancer other than active surveillance,” said André Deschamps, the chairman of the patient advocacy movement Europa Uomo, which conducted the study with support from Erasmus University Medical Center in Rotterdam, the Netherlands.
Active surveillance “should be promoted as the first option for treatment for those men where it can be offered safely,” Mr. Deschamps said when presenting the study at the EAU congress.
The study showed that quality of life related to urinary incontinence was lowest in patients who had undergone radical prostatectomy, and sexual function was greatly affected by radiotherapy. Radiotherapy and chemotherapy had the greatest impact on patients’ levels of fatigue, and chemotherapy was associated with “the worst possible outcomes in quality of life,” Mr. Deschamps said.
Conversely, “reported quality of life scores are the best in patients where the cancer is discovered in an early, curable stage. Hence, efforts toward early detection and awareness are essential to avoid unnecessary deterioration in quality of life,” Mr. Deschamps said.
About the survey and respondents
Between August and November 2019, 2,943 prostate cancer patients from 24 European countries completed a web-based survey made available via the Europa Uomo website. The survey took around 20 minutes to complete and used three validated quality of life questionnaires, the EORTC-QLQ-C30, the EQ-5D-5L, and EPIC-26.
“The questionnaires were available in 19 languages, so every patient could answer in their mother tongue,” Mr. Deschamps pointed out, highlighting that this was a Europe-wide survey and was estimated to account for 0.1% of the patient population in Europe.
Countries with the highest number of respondents were Norway (n = 506), Sweden (n = 386), Belgium (n = 339), Germany (n = 253), Netherlands (n = 244), France (n = 234), Denmark (n = 188), the United Kingdom (n = 187), and Poland (n = 109).
The average age of respondents was 70 years at the time of the survey and 64 years at the time of diagnosis. Most patients (82%) were living with a partner.
Two-thirds of patients had received only one treatment for prostate cancer. This was most often radical prostatectomy, external beam radiotherapy, or active surveillance. Among the 22% of patients who had received two treatments, the therapies were most often a combination of surgery and radiotherapy, androgen deprivation therapy (ADT) and radiotherapy or chemotherapy, and active surveillance and surgery.
Fatigue and insomnia
According to the EORTC-QLQ-C30 symptoms questionnaire, fatigue and insomnia were particular problems for men with prostate cancer, as denoted by scores of 25 and 24, respectively, out of a possible 100. Low scores are associated with worse fatigue and insomnia.
The researchers focused their attention on how specific cancer treatments might influence fatigue. They found that radiotherapy doubled and chemotherapy tripled the number of patients reporting fatigue, when compared with active surveillance. The incidence of fatigue was 22% (n = 304), 33% (n = 246), and 11% (n = 179), respectively.
As for insomnia, “it’s bit of a mixed view,” Mr. Deschamps said. “We believe that the progression of disease is more important for insomnia. The only thing you can say is that chemotherapy leads to an increase in reported insomnia.”
Urinary continence and sexual function
The EPIC-26 questionnaire was used to look at the health-related quality of life domains of urinary and sexual function. Sexual function was the most impacted area.
“We often hear that decline in sexual functioning is a relatively small problem for prostate cancer patients, and the effect on their quality of life should not be exaggerated,” Mr. Deschamps said in a press statement.
“We also hear that prostate cancer is typically a disease of ‘old men,’ implying that the loss of sexual function is less relevant. This survey paints a different picture,” he added.
Higher EPIC-26 scores signify better function. For urinary incontinence, the score was 100/100 for active surveillance but 65/100 when active surveillance was combined with surgery and 71/100 for surgery alone. The combination of surgery and radiotherapy carried a score of 73/100 for urinary incontinence. Radiotherapy on its own had a score of 92/100, suggesting it was the addition of the surgery that was having a significant effect. The score for radiotherapy plus ADT was 100/100, and the score for chemotherapy was 86/100.
Chemotherapy appeared to have the worst effect on sexual function, with a score of just 12/100. Radiotherapy was not far behind at 17/100, and surgery alone was 21/100. When radiotherapy and surgery were combined, the score was 15/100.
Sexual function scores were also low for all the other treatments considered – 18/100 for radiotherapy and ADT, 26/100 for active surveillance and surgery, and 57/100 for active surveillance alone.
Implications for practice
“The data collected and the analysis done provide patients and healthcare professionals with a ‘snapshot’ on the impact of treatments based on the experience of fellow patients,” Mr. Deschamps said. “We hope these results will be used to establish and disseminate realistic expectations on the effects of different treatments for prostate cancer on [quality of life].”
“This study is important because it was initiated by patients and meant for patients,” noted Monique Roobol, PhD, professor of decision-making in urology at the Erasmus University Medical Center in Rotterdam, the Netherlands, where the survey data were analyzed.
“The questionnaires were completed unrelated to a hospital visit, which means respondents had more freedom to answer and provide insight into the effect of treatment on quality of life over a longer period,” she added.
“For me, the key point is that, as health care professionals, we have underestimated the impact on the quality of life for patients treated for prostate cancer,” said Hein van Poppel, MD, PhD, of University Hospitals Leuven (Belgium), who chaired the session in which the data were presented.
Arnulf Stenzl, MD, of Tübingen (Germany) University said in a statement that the survey provided valuable information. “It uses the same questionnaires used in standard clinical settings, but it is both qualitatively and quantitatively different to the kind of study usually undertaken, so it needs to be read alongside these previous studies,” Dr. Stenzl said.
There were several strong points, he said, such as the fact that EUPROMS was the largest study of its kind and thus would “reflect the impact of treatment on a wide range of patients, with different health systems.”
As an official EAU spokesperson, Dr. Stenzl added, “We completely agree that early detection and treatment is essential if we are to avoid problems with quality of life later on. It shows that, for many men, quality of life can be poor after most prostate cancer treatment, especially in advanced disease. This message is clear, and we need to listen to the voices of these patients.”
EUPROMS was conducted by Europa Uomo in conjunction with the Erasmus University Medical Centre in Rotterdam, the Netherlands. Funding was received from Bayer, Ipsen, and Janssen. The companies had no influence over any aspect of the study. The commentators did not have conflicts of interest to disclose.
FROM EAU20
Order errors not reduced with limiting number of open records
Background: An estimated 600,000 patients in U.S. hospitals had an order placed in their record that was meant for another patient in 2016. The Office of the National Coordinator for Health Information Technology and the Joint Commission recommend that EHRs limit the number of open records to one at a time based on expert opinion only. There is wide variation in the number of open records allowed among EHRs across the United States currently.
Study design: Randomized clinical trial.
Setting: Large health system in New York.
Synopsis: There were 3,356 clinicians (inpatient, outpatient, ED) randomized in a 1:1 ratio into either a restricted group (one open record at a time) or an unrestricted group (up to four open records at a time). In this study, 12,140,298 orders, in 4,486,631 order sessions, were analyzed with the Wrong-Patient Retract-and-Reorder (RAR) measure to identify wrong-patient orders. The proportion of wrong-patient order sessions were 90.7 vs. 88.0 per 100,000 order sessions for the restricted versus unrestricted groups (odds ratio, 1.03; 95% confidence interval, 0.90-1.20). There were no statistically significant differences in wrong-patient order sessions between the restricted and unrestricted groups in any clinical setting examined (inpatient, outpatient, ED).
Despite the ability to have up to four open records at one time in the unrestricted group, 66% of the order sessions were completed with only one record open in that group. This limited the power of the study to detect a difference in risk of order errors between the restricted and unrestricted groups.
Bottom line: Limiting clinicians to only one open record did not reduce the proportion of wrong-patient orders, compared with allowing up to four open records concurrently.
Citation: Adelman JS et al. Effect of restriction of the number of concurrently open records in an electronic health record on wrong-patient order errors: A randomized clinical trial. JAMA. 2019;32(18):1780-7.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
Background: An estimated 600,000 patients in U.S. hospitals had an order placed in their record that was meant for another patient in 2016. The Office of the National Coordinator for Health Information Technology and the Joint Commission recommend that EHRs limit the number of open records to one at a time based on expert opinion only. There is wide variation in the number of open records allowed among EHRs across the United States currently.
Study design: Randomized clinical trial.
Setting: Large health system in New York.
Synopsis: There were 3,356 clinicians (inpatient, outpatient, ED) randomized in a 1:1 ratio into either a restricted group (one open record at a time) or an unrestricted group (up to four open records at a time). In this study, 12,140,298 orders, in 4,486,631 order sessions, were analyzed with the Wrong-Patient Retract-and-Reorder (RAR) measure to identify wrong-patient orders. The proportion of wrong-patient order sessions were 90.7 vs. 88.0 per 100,000 order sessions for the restricted versus unrestricted groups (odds ratio, 1.03; 95% confidence interval, 0.90-1.20). There were no statistically significant differences in wrong-patient order sessions between the restricted and unrestricted groups in any clinical setting examined (inpatient, outpatient, ED).
Despite the ability to have up to four open records at one time in the unrestricted group, 66% of the order sessions were completed with only one record open in that group. This limited the power of the study to detect a difference in risk of order errors between the restricted and unrestricted groups.
Bottom line: Limiting clinicians to only one open record did not reduce the proportion of wrong-patient orders, compared with allowing up to four open records concurrently.
Citation: Adelman JS et al. Effect of restriction of the number of concurrently open records in an electronic health record on wrong-patient order errors: A randomized clinical trial. JAMA. 2019;32(18):1780-7.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
Background: An estimated 600,000 patients in U.S. hospitals had an order placed in their record that was meant for another patient in 2016. The Office of the National Coordinator for Health Information Technology and the Joint Commission recommend that EHRs limit the number of open records to one at a time based on expert opinion only. There is wide variation in the number of open records allowed among EHRs across the United States currently.
Study design: Randomized clinical trial.
Setting: Large health system in New York.
Synopsis: There were 3,356 clinicians (inpatient, outpatient, ED) randomized in a 1:1 ratio into either a restricted group (one open record at a time) or an unrestricted group (up to four open records at a time). In this study, 12,140,298 orders, in 4,486,631 order sessions, were analyzed with the Wrong-Patient Retract-and-Reorder (RAR) measure to identify wrong-patient orders. The proportion of wrong-patient order sessions were 90.7 vs. 88.0 per 100,000 order sessions for the restricted versus unrestricted groups (odds ratio, 1.03; 95% confidence interval, 0.90-1.20). There were no statistically significant differences in wrong-patient order sessions between the restricted and unrestricted groups in any clinical setting examined (inpatient, outpatient, ED).
Despite the ability to have up to four open records at one time in the unrestricted group, 66% of the order sessions were completed with only one record open in that group. This limited the power of the study to detect a difference in risk of order errors between the restricted and unrestricted groups.
Bottom line: Limiting clinicians to only one open record did not reduce the proportion of wrong-patient orders, compared with allowing up to four open records concurrently.
Citation: Adelman JS et al. Effect of restriction of the number of concurrently open records in an electronic health record on wrong-patient order errors: A randomized clinical trial. JAMA. 2019;32(18):1780-7.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.