User login
This month in the journal CHEST®: Editor’s picks
Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China.By Dr. L. Shiyue, et al.
Effect of intermittent or continuous feed on muscle wasting in critical illness a phase II clinical trial. By Dr. A. McNelly, et al.
Triage of scarce critical care resources in COVID-19: An implementation guide for regional allocation: A CHEST and Task Force for Mass Critical Care Expert Panel Report.By Dr. J. Dichter, et al.
Managing Chronic Cough as a Symptom in Children and Management Algorithms: CHEST Guideline and Expert Panel Report. By Dr. A. Chang, et al.
Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China.By Dr. L. Shiyue, et al.
Effect of intermittent or continuous feed on muscle wasting in critical illness a phase II clinical trial. By Dr. A. McNelly, et al.
Triage of scarce critical care resources in COVID-19: An implementation guide for regional allocation: A CHEST and Task Force for Mass Critical Care Expert Panel Report.By Dr. J. Dichter, et al.
Managing Chronic Cough as a Symptom in Children and Management Algorithms: CHEST Guideline and Expert Panel Report. By Dr. A. Chang, et al.
Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China.By Dr. L. Shiyue, et al.
Effect of intermittent or continuous feed on muscle wasting in critical illness a phase II clinical trial. By Dr. A. McNelly, et al.
Triage of scarce critical care resources in COVID-19: An implementation guide for regional allocation: A CHEST and Task Force for Mass Critical Care Expert Panel Report.By Dr. J. Dichter, et al.
Managing Chronic Cough as a Symptom in Children and Management Algorithms: CHEST Guideline and Expert Panel Report. By Dr. A. Chang, et al.
Reflections on a virtual happy hour
On a Wednesday night in April, CHEST Women and Pulmonary Advisory Board hosted a virtual happy hour that was not just a webinar but also on Facebook Live, entitled Wellness Wednesday. During the 2-hour event, the hosts of the happy hour exchanged experiences during the pandemic, thoughts, hopes, and some very practical ideas on how to stay well in the midst of the pandemic. I was thrilled to co-host this event with Drs. Aneesa Das, Doreen Addrizzo-Harris, Margaret Pisani, Michele Cao, and Rachel Quaney.
We started off toasting with whatever drink people chose to have and each member shared what she was doing during the pandemic. There were many amazing stories of how these women adapted to the changing environment. Dr. Addrizzo-Harris told us how she and her husband literally split their apartment in half since they work in different hospitals and did not want to risk infecting not just one another but also their respective patients. Both she and her husband were working long shifts and most days of the week in the hospital and had not really seen each other since the lockdown started in New York. She also gave us an update on the pandemic and response in New York and reiterated her appreciation for health-care providers who came from elsewhere to help. Drs. Das and Quaney made a point to say that Ohio had done a great job planning for and preventing an onslaught of infected patients and that they were quite thankful to be able to do virtual visits and keep up with their patients.
With regards to work, a few panelists described not only the change in the hospital census and environment but also the impact on education for everyone. We shared ideas for keeping up with pulmonary and critical care that were not related to COVID-19 and ways to not feel overwhelmed by it. I mentioned that we kept our weekly clinical case conference for non-COVID cases and that our fellows and faculty found it refreshing and reinvigorating. Dr. Quaney, who is still in training, mentioned the impact the pandemic had on her education but was also thankful for all that was being done to mitigate that.
While several of us were going into the hospitals and working with COVID-19 patients, others were working from home. It may seem like that would be low stress but think about the challenges of doing virtual visits from home while young children are running around! Dr. Cao gave us a few stories about this and made us all laugh.
So much has changed in our lives and what we must do to care for ourselves, our families, and our patients. On this topic, many of the panelists mentioned that self-care is imperative, as well as all the other things we do. Many shared what they do to remain calm and to relieve stress, such as yoga, hiking, calls with friends and family, etc. Dr. Pisani in particular mentioned the importance of self-care while also lamenting that we have gone backwards with regard to delirium prevention in the ICU due to the isolation needed for COVID 19 patients.
The laughter and camaraderie amongst the panelist extended to the online participants. We had over 2,400 viewers either on Facebook live or via the webinar link! Many people who joined us asked questions or shared stories of how they were coping and what they miss about the pre-pandemic life. Most agreed that the lack of interpersonal interaction, especially with friends and family, has been difficult and that something as simple as this virtual happy hour was a welcome addition to all the other online meetings and patient visits. After the event, many online participants reached out personally and via social media to express how much they enjoyed it and hopes that we continue something like this going forward. I believe we all agreed at least a quarterly Wednesday Wellness event would be great, so I hope you will join us next time!
On a Wednesday night in April, CHEST Women and Pulmonary Advisory Board hosted a virtual happy hour that was not just a webinar but also on Facebook Live, entitled Wellness Wednesday. During the 2-hour event, the hosts of the happy hour exchanged experiences during the pandemic, thoughts, hopes, and some very practical ideas on how to stay well in the midst of the pandemic. I was thrilled to co-host this event with Drs. Aneesa Das, Doreen Addrizzo-Harris, Margaret Pisani, Michele Cao, and Rachel Quaney.
We started off toasting with whatever drink people chose to have and each member shared what she was doing during the pandemic. There were many amazing stories of how these women adapted to the changing environment. Dr. Addrizzo-Harris told us how she and her husband literally split their apartment in half since they work in different hospitals and did not want to risk infecting not just one another but also their respective patients. Both she and her husband were working long shifts and most days of the week in the hospital and had not really seen each other since the lockdown started in New York. She also gave us an update on the pandemic and response in New York and reiterated her appreciation for health-care providers who came from elsewhere to help. Drs. Das and Quaney made a point to say that Ohio had done a great job planning for and preventing an onslaught of infected patients and that they were quite thankful to be able to do virtual visits and keep up with their patients.
With regards to work, a few panelists described not only the change in the hospital census and environment but also the impact on education for everyone. We shared ideas for keeping up with pulmonary and critical care that were not related to COVID-19 and ways to not feel overwhelmed by it. I mentioned that we kept our weekly clinical case conference for non-COVID cases and that our fellows and faculty found it refreshing and reinvigorating. Dr. Quaney, who is still in training, mentioned the impact the pandemic had on her education but was also thankful for all that was being done to mitigate that.
While several of us were going into the hospitals and working with COVID-19 patients, others were working from home. It may seem like that would be low stress but think about the challenges of doing virtual visits from home while young children are running around! Dr. Cao gave us a few stories about this and made us all laugh.
So much has changed in our lives and what we must do to care for ourselves, our families, and our patients. On this topic, many of the panelists mentioned that self-care is imperative, as well as all the other things we do. Many shared what they do to remain calm and to relieve stress, such as yoga, hiking, calls with friends and family, etc. Dr. Pisani in particular mentioned the importance of self-care while also lamenting that we have gone backwards with regard to delirium prevention in the ICU due to the isolation needed for COVID 19 patients.
The laughter and camaraderie amongst the panelist extended to the online participants. We had over 2,400 viewers either on Facebook live or via the webinar link! Many people who joined us asked questions or shared stories of how they were coping and what they miss about the pre-pandemic life. Most agreed that the lack of interpersonal interaction, especially with friends and family, has been difficult and that something as simple as this virtual happy hour was a welcome addition to all the other online meetings and patient visits. After the event, many online participants reached out personally and via social media to express how much they enjoyed it and hopes that we continue something like this going forward. I believe we all agreed at least a quarterly Wednesday Wellness event would be great, so I hope you will join us next time!
On a Wednesday night in April, CHEST Women and Pulmonary Advisory Board hosted a virtual happy hour that was not just a webinar but also on Facebook Live, entitled Wellness Wednesday. During the 2-hour event, the hosts of the happy hour exchanged experiences during the pandemic, thoughts, hopes, and some very practical ideas on how to stay well in the midst of the pandemic. I was thrilled to co-host this event with Drs. Aneesa Das, Doreen Addrizzo-Harris, Margaret Pisani, Michele Cao, and Rachel Quaney.
We started off toasting with whatever drink people chose to have and each member shared what she was doing during the pandemic. There were many amazing stories of how these women adapted to the changing environment. Dr. Addrizzo-Harris told us how she and her husband literally split their apartment in half since they work in different hospitals and did not want to risk infecting not just one another but also their respective patients. Both she and her husband were working long shifts and most days of the week in the hospital and had not really seen each other since the lockdown started in New York. She also gave us an update on the pandemic and response in New York and reiterated her appreciation for health-care providers who came from elsewhere to help. Drs. Das and Quaney made a point to say that Ohio had done a great job planning for and preventing an onslaught of infected patients and that they were quite thankful to be able to do virtual visits and keep up with their patients.
With regards to work, a few panelists described not only the change in the hospital census and environment but also the impact on education for everyone. We shared ideas for keeping up with pulmonary and critical care that were not related to COVID-19 and ways to not feel overwhelmed by it. I mentioned that we kept our weekly clinical case conference for non-COVID cases and that our fellows and faculty found it refreshing and reinvigorating. Dr. Quaney, who is still in training, mentioned the impact the pandemic had on her education but was also thankful for all that was being done to mitigate that.
While several of us were going into the hospitals and working with COVID-19 patients, others were working from home. It may seem like that would be low stress but think about the challenges of doing virtual visits from home while young children are running around! Dr. Cao gave us a few stories about this and made us all laugh.
So much has changed in our lives and what we must do to care for ourselves, our families, and our patients. On this topic, many of the panelists mentioned that self-care is imperative, as well as all the other things we do. Many shared what they do to remain calm and to relieve stress, such as yoga, hiking, calls with friends and family, etc. Dr. Pisani in particular mentioned the importance of self-care while also lamenting that we have gone backwards with regard to delirium prevention in the ICU due to the isolation needed for COVID 19 patients.
The laughter and camaraderie amongst the panelist extended to the online participants. We had over 2,400 viewers either on Facebook live or via the webinar link! Many people who joined us asked questions or shared stories of how they were coping and what they miss about the pre-pandemic life. Most agreed that the lack of interpersonal interaction, especially with friends and family, has been difficult and that something as simple as this virtual happy hour was a welcome addition to all the other online meetings and patient visits. After the event, many online participants reached out personally and via social media to express how much they enjoyed it and hopes that we continue something like this going forward. I believe we all agreed at least a quarterly Wednesday Wellness event would be great, so I hope you will join us next time!
COVID-19 and asthma. Remdesivir for COVID-19. Burnout in unprecedented times. Advances in molecular imaging in pulmonary fibrosis.
Airways
COVID-19 and asthma: Much remains unknown
Viral-induced asthma exacerbations are common, but there has yet to be a published data set showing worse outcomes among asthmatics with COVID-19.
It is possible that inhaled corticosteroids (ICS) may provide some protection from viral infection. A 2014 study showed that ICS may reduce exacerbations by modulating inflammation and reducing airway viral receptors (Yamaya, et al. Respir Investig. 2014;52[4]:251). Analysis from the SARP-3 database showed ICS use associated with reduced expression of both ACE2 and transmembrane protease serine 2 (TMPRSS2), two receptors used by SARS-CoV-2 (Peters, et al. Am J Respir Crit Care Med. 2020. Online ahead of print). Another study showed a similar effect of ICS on the seasonal coronavirus strain HCoV-229E (Yamaya M, et al. Respir Investig. 2020;58[3]:155), and one study reported decreased ACE2 expression in allergic asthma (Jackson, et al. J Allergy Clin Immunol. Article in press, 2020). While these findings could support a hypothesis of reduced risk for COVID-19 infection among asthmatics using ICS, one would generally expect those with underlying lung disease, such as asthma, to be at higher risk for more severe infection.
Despite physiologic hypotheses of protective mechanisms, clinical outcomes may suffer as clinical operations and the American economy are impacted by this pandemic. Reduced access to or utilization of outpatient care, loss of employment, loss of health insurance, or a new difficulty in affording or accessing medications may all result in worsening asthma control for patients. Poorly controlled asthmatics are at higher risk for a more severe exacerbation of disease triggered by viral infection. Current recommendations are for patients to continue all controller medications; the use of systemic corticosteroids in treatment of COVID pneumonia is controversial, but their use in treating a COVID-associated asthma exacerbation should be based on individual assessment. As we care for asthma patients through this pandemic, much remains unknown but may be elucidated by further study.
Megan Conroy, MD
Fellow-in-Training Member
Steering Committee Member
Clinical Research and Quality Improvement
Remdesivir for COVID-19: A ray of hope?
The year 2020 witnessed a pandemic of unprecedented proportions, caused by a novel corona virus strain (SARS-CoV2). Across the globe, there have been more than 6.5 million positive cases of COVID-19 and more than 380,000 deaths. (WHO COVID-19 Dashboard [https://covid19.who.int]). Multiple therapeutic agents are currently being studied as potential treatment options for this novel disease. With negative trials so far on lopinavir-ritonavir and hydroxychloroquine, the only candidate drug showing benefit is remdesivir.
Results of the randomized double-blind placebo controlled Adaptive COVID-19 Treatment Trial (ACTT-1) trial (Beigel, J et al. N Engl J Med. 2020; e-pub ahead of print) shows remdesivir improved recovery time in COVID -19 patients as compared with control subjects. Remdesivir is an inhibitor of viral RNA polymerase that has been shown to inhibit coronaviruses in animal models and SARS-CoV2 in-vitro. The ACTT-1 trial enrolled 1,063 patients with 541 assigned to the remdesivir arm and 522 to the placebo group. Primary outcome measure was time to recovery. Mortality at 14 and 28 days and incidence of adverse events were also evaluated.
As interim analysis showed positive results, the data safety and monitoring board recommended early termination of the trial. Patients in the remdesivir group had a shorter time to recovery, with median recovery time of 11 days as compared with 15 days in placebo group (95% CI:1.12-1.55; P < .001). Hospitalized patients requiring supplemental oxygen (but not high-flow, mechanical ventilation or ECMO) derived the maximum benefit with a rate ratio of recovery being 1.47(95% CI:1.17-1.84). Thus, early drug administration may be beneficial. The difference in mortality at 14 days was not statistically significant and data on mortality difference at 28 days were not available at the time of publication.
In summary, this trial along with previous publications shows that remdesivir is a potential therapeutic option for COVID -19. The Food and Drug Administration (FDA) approved remdesivir under Emergency Use Authorization (EUA) for COVID-19 and larger trials are currently underway to study the full effect of this agent.
Aravind Menon, MD
Fellow-in-Training Member
Critical Care
Burnout in unprecedented times
Even in typical times, intensivists have a significantly higher rate of burnout compared with other medical specialties. We fight for lives, dealing with death, dying, and tragedy on a daily basis. Regrettably, we are no longer in ‘typical’ times. This is a prodigious and uncharted era.
The COVID-19 pandemic has created all new hardships. Added to the complex world of critical care, we undertake lack of appropriate medical equipment and PPE, the possibility of becoming ill or infecting our families, potential financial struggles, and the unpredictability of the future. Additionally, in our efforts to care for patients, we face increasing moral distress when placed in situations in which we cannot do what we feel is right. And we carry the burdens and guilt of patients’ families who cannot be with loved ones during this process, even during death.
What does burnout look like in this new era? Burnout is a continuum and can manifest differently depending on the individual. Even a typical day in the ICU may be cause for the symptoms of burnout including frustration, anger, anxiety, or sadness which can progress to feelings of powerlessness, self-doubt or depersonalization.
This crisis is a test of endurance. But we don’t have to face it alone. The ICU is a team environment, and we can help each other make it to the end. Consider beginning the shift with a group morale boosting activity. Perhaps debrief after the end of each shift to discuss ways of combatting these stressful times. Have a virtual happy hour with colleagues after work. Call on leadership for support. Watch each other’s back. Together we will get through these unprecedented times.
John P. Gaillard, MD
Steering Committee Member
Resources for confronting burnout:
http://ccsconline.org/optimizing-the-workforce/burnout
https://www.ama-assn.org/topics/physician-burnout
https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
Home-Based Mechanical Ventilation and Neuromuscular Disease
Use of modified RADs
Investigators have begun exploring ways to convert devices typically used to treat sleep-disordered breathing (respiratory assist device, RAD), with modifications to minimize risk of aerosolization of pathogen in the COVID-19 pandemic. These devices are presently not considered an effective means of treating acute respiratory distress syndrome (ARDS). In an emergency, however, it is reasonable to consider all the options available with a healthy respect for inherent device limitations.
A RAD could be converted from an open ventilation single-limb respiratory circuit to a closed ventilation circuit with a passive exhalation valve. This circuit could provide adequate minute ventilation and allow for adequate exhalation of CO2 to prevent rebreathing. Strategic placement of the passive exhalation valve proximal to a viricidal filter would allow the device to be used with either an endotracheal tube or a nonvented oronasal mask (Figure). These devices by design are pressure-regulated, and a backup rate would be necessary to control minute ventilation. Close monitoring would be necessary given lack of alarm capability for a critically ill patient and the need to ensure adequate oxygen bleed-in.
The primary limitation to these devices is the inability to achieve adequate mean airway pressure for ARDS. While such a converted device is not ready for prime time, it could be considered for patients who are close to weaning from conventional mechanical ventilation (i.e., freeing up a ventilator for a sicker patient) or temporizing a patient early in disease to stave off invasive ventilation.
MAJ Brian E. Foster, DO, USA
Fellow Member
Steering Committee Member
Interstitial and Diffuse Lung Disease NetWork
Advances in molecular imaging in pulmonary fibrosis
Fibrotic interstitial lung diseases (ILD), including idiopathic pulmonary fibrosis (IPF), have poor prognosis with marked heterogeneity in the clinical course. Treatment options, including antfibrotic drugs and immunosuppressants, are fairly limited for either conditions, and there is wide variability in drug responsiveness. Biomarkers that predict disease course and enable patient stratification to assess responsiveness to specific therapies play a crucial role in management of this fatal disease.
Molecular imaging has the ability to noninvasively provide both structural details, as well as functional/molecular information at the cellular level; it has thus developed into a powerful tool for several inflammatory and malignant disease processes. Probes that specifically target fibrosis-specific pathways utilizing positron emission tomography (PET) or magnetic resonance (MR) imaging have gained traction recently.
The most commonly used radiopharmaceutical for PET, 18F-FDG, is significantly increased in areas of established fibrosis in patients with IPF and autoimmune ILDs (Win, et al. Eur J Nucl Med Mol Imaging. 2018 May;45[5]:806; Uehara, et al. Mod Rheumatol. 2016;26[1]:121-7), as well as areas with seemingly normal morphologic appearance on HRCT scan (Win, et al. Eur J Nucl Med Mol Imaging. 2014 Feb;41[2]:337). While this probe was shown to have some potential for prognostication, there has been concern regarding the specificity of FDG uptake in fibrotic lung diseases. Hence, other probes that target specific fibrosis-related cellular mechanisms such as macrophages (Withana, et al. Nature Scientific Reports. 2016;6 [Jan 22):19755], and John, et al. J Nucl Med. 2013;54[12]:2146) and matrix proteins (Montesi, et al. Am J Respir Crit Care Med. 2019 Jul 15;200[2]:258) have been developed in preclinical fibrosis/lung injury models and are being translated to human subjects.
With the ability to capture early fibrogenesis and target engagement, molecular imaging has the potential to prognosticate patients, provide earlier evaluation of treatment responsiveness and have a promising application in clinical trial design for fibrotic lung diseases.
Tejaswini Kulkarni, MD
Steering Committee Member
Airways
COVID-19 and asthma: Much remains unknown
Viral-induced asthma exacerbations are common, but there has yet to be a published data set showing worse outcomes among asthmatics with COVID-19.
It is possible that inhaled corticosteroids (ICS) may provide some protection from viral infection. A 2014 study showed that ICS may reduce exacerbations by modulating inflammation and reducing airway viral receptors (Yamaya, et al. Respir Investig. 2014;52[4]:251). Analysis from the SARP-3 database showed ICS use associated with reduced expression of both ACE2 and transmembrane protease serine 2 (TMPRSS2), two receptors used by SARS-CoV-2 (Peters, et al. Am J Respir Crit Care Med. 2020. Online ahead of print). Another study showed a similar effect of ICS on the seasonal coronavirus strain HCoV-229E (Yamaya M, et al. Respir Investig. 2020;58[3]:155), and one study reported decreased ACE2 expression in allergic asthma (Jackson, et al. J Allergy Clin Immunol. Article in press, 2020). While these findings could support a hypothesis of reduced risk for COVID-19 infection among asthmatics using ICS, one would generally expect those with underlying lung disease, such as asthma, to be at higher risk for more severe infection.
Despite physiologic hypotheses of protective mechanisms, clinical outcomes may suffer as clinical operations and the American economy are impacted by this pandemic. Reduced access to or utilization of outpatient care, loss of employment, loss of health insurance, or a new difficulty in affording or accessing medications may all result in worsening asthma control for patients. Poorly controlled asthmatics are at higher risk for a more severe exacerbation of disease triggered by viral infection. Current recommendations are for patients to continue all controller medications; the use of systemic corticosteroids in treatment of COVID pneumonia is controversial, but their use in treating a COVID-associated asthma exacerbation should be based on individual assessment. As we care for asthma patients through this pandemic, much remains unknown but may be elucidated by further study.
Megan Conroy, MD
Fellow-in-Training Member
Steering Committee Member
Clinical Research and Quality Improvement
Remdesivir for COVID-19: A ray of hope?
The year 2020 witnessed a pandemic of unprecedented proportions, caused by a novel corona virus strain (SARS-CoV2). Across the globe, there have been more than 6.5 million positive cases of COVID-19 and more than 380,000 deaths. (WHO COVID-19 Dashboard [https://covid19.who.int]). Multiple therapeutic agents are currently being studied as potential treatment options for this novel disease. With negative trials so far on lopinavir-ritonavir and hydroxychloroquine, the only candidate drug showing benefit is remdesivir.
Results of the randomized double-blind placebo controlled Adaptive COVID-19 Treatment Trial (ACTT-1) trial (Beigel, J et al. N Engl J Med. 2020; e-pub ahead of print) shows remdesivir improved recovery time in COVID -19 patients as compared with control subjects. Remdesivir is an inhibitor of viral RNA polymerase that has been shown to inhibit coronaviruses in animal models and SARS-CoV2 in-vitro. The ACTT-1 trial enrolled 1,063 patients with 541 assigned to the remdesivir arm and 522 to the placebo group. Primary outcome measure was time to recovery. Mortality at 14 and 28 days and incidence of adverse events were also evaluated.
As interim analysis showed positive results, the data safety and monitoring board recommended early termination of the trial. Patients in the remdesivir group had a shorter time to recovery, with median recovery time of 11 days as compared with 15 days in placebo group (95% CI:1.12-1.55; P < .001). Hospitalized patients requiring supplemental oxygen (but not high-flow, mechanical ventilation or ECMO) derived the maximum benefit with a rate ratio of recovery being 1.47(95% CI:1.17-1.84). Thus, early drug administration may be beneficial. The difference in mortality at 14 days was not statistically significant and data on mortality difference at 28 days were not available at the time of publication.
In summary, this trial along with previous publications shows that remdesivir is a potential therapeutic option for COVID -19. The Food and Drug Administration (FDA) approved remdesivir under Emergency Use Authorization (EUA) for COVID-19 and larger trials are currently underway to study the full effect of this agent.
Aravind Menon, MD
Fellow-in-Training Member
Critical Care
Burnout in unprecedented times
Even in typical times, intensivists have a significantly higher rate of burnout compared with other medical specialties. We fight for lives, dealing with death, dying, and tragedy on a daily basis. Regrettably, we are no longer in ‘typical’ times. This is a prodigious and uncharted era.
The COVID-19 pandemic has created all new hardships. Added to the complex world of critical care, we undertake lack of appropriate medical equipment and PPE, the possibility of becoming ill or infecting our families, potential financial struggles, and the unpredictability of the future. Additionally, in our efforts to care for patients, we face increasing moral distress when placed in situations in which we cannot do what we feel is right. And we carry the burdens and guilt of patients’ families who cannot be with loved ones during this process, even during death.
What does burnout look like in this new era? Burnout is a continuum and can manifest differently depending on the individual. Even a typical day in the ICU may be cause for the symptoms of burnout including frustration, anger, anxiety, or sadness which can progress to feelings of powerlessness, self-doubt or depersonalization.
This crisis is a test of endurance. But we don’t have to face it alone. The ICU is a team environment, and we can help each other make it to the end. Consider beginning the shift with a group morale boosting activity. Perhaps debrief after the end of each shift to discuss ways of combatting these stressful times. Have a virtual happy hour with colleagues after work. Call on leadership for support. Watch each other’s back. Together we will get through these unprecedented times.
John P. Gaillard, MD
Steering Committee Member
Resources for confronting burnout:
http://ccsconline.org/optimizing-the-workforce/burnout
https://www.ama-assn.org/topics/physician-burnout
https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
Home-Based Mechanical Ventilation and Neuromuscular Disease
Use of modified RADs
Investigators have begun exploring ways to convert devices typically used to treat sleep-disordered breathing (respiratory assist device, RAD), with modifications to minimize risk of aerosolization of pathogen in the COVID-19 pandemic. These devices are presently not considered an effective means of treating acute respiratory distress syndrome (ARDS). In an emergency, however, it is reasonable to consider all the options available with a healthy respect for inherent device limitations.
A RAD could be converted from an open ventilation single-limb respiratory circuit to a closed ventilation circuit with a passive exhalation valve. This circuit could provide adequate minute ventilation and allow for adequate exhalation of CO2 to prevent rebreathing. Strategic placement of the passive exhalation valve proximal to a viricidal filter would allow the device to be used with either an endotracheal tube or a nonvented oronasal mask (Figure). These devices by design are pressure-regulated, and a backup rate would be necessary to control minute ventilation. Close monitoring would be necessary given lack of alarm capability for a critically ill patient and the need to ensure adequate oxygen bleed-in.
The primary limitation to these devices is the inability to achieve adequate mean airway pressure for ARDS. While such a converted device is not ready for prime time, it could be considered for patients who are close to weaning from conventional mechanical ventilation (i.e., freeing up a ventilator for a sicker patient) or temporizing a patient early in disease to stave off invasive ventilation.
MAJ Brian E. Foster, DO, USA
Fellow Member
Steering Committee Member
Interstitial and Diffuse Lung Disease NetWork
Advances in molecular imaging in pulmonary fibrosis
Fibrotic interstitial lung diseases (ILD), including idiopathic pulmonary fibrosis (IPF), have poor prognosis with marked heterogeneity in the clinical course. Treatment options, including antfibrotic drugs and immunosuppressants, are fairly limited for either conditions, and there is wide variability in drug responsiveness. Biomarkers that predict disease course and enable patient stratification to assess responsiveness to specific therapies play a crucial role in management of this fatal disease.
Molecular imaging has the ability to noninvasively provide both structural details, as well as functional/molecular information at the cellular level; it has thus developed into a powerful tool for several inflammatory and malignant disease processes. Probes that specifically target fibrosis-specific pathways utilizing positron emission tomography (PET) or magnetic resonance (MR) imaging have gained traction recently.
The most commonly used radiopharmaceutical for PET, 18F-FDG, is significantly increased in areas of established fibrosis in patients with IPF and autoimmune ILDs (Win, et al. Eur J Nucl Med Mol Imaging. 2018 May;45[5]:806; Uehara, et al. Mod Rheumatol. 2016;26[1]:121-7), as well as areas with seemingly normal morphologic appearance on HRCT scan (Win, et al. Eur J Nucl Med Mol Imaging. 2014 Feb;41[2]:337). While this probe was shown to have some potential for prognostication, there has been concern regarding the specificity of FDG uptake in fibrotic lung diseases. Hence, other probes that target specific fibrosis-related cellular mechanisms such as macrophages (Withana, et al. Nature Scientific Reports. 2016;6 [Jan 22):19755], and John, et al. J Nucl Med. 2013;54[12]:2146) and matrix proteins (Montesi, et al. Am J Respir Crit Care Med. 2019 Jul 15;200[2]:258) have been developed in preclinical fibrosis/lung injury models and are being translated to human subjects.
With the ability to capture early fibrogenesis and target engagement, molecular imaging has the potential to prognosticate patients, provide earlier evaluation of treatment responsiveness and have a promising application in clinical trial design for fibrotic lung diseases.
Tejaswini Kulkarni, MD
Steering Committee Member
Airways
COVID-19 and asthma: Much remains unknown
Viral-induced asthma exacerbations are common, but there has yet to be a published data set showing worse outcomes among asthmatics with COVID-19.
It is possible that inhaled corticosteroids (ICS) may provide some protection from viral infection. A 2014 study showed that ICS may reduce exacerbations by modulating inflammation and reducing airway viral receptors (Yamaya, et al. Respir Investig. 2014;52[4]:251). Analysis from the SARP-3 database showed ICS use associated with reduced expression of both ACE2 and transmembrane protease serine 2 (TMPRSS2), two receptors used by SARS-CoV-2 (Peters, et al. Am J Respir Crit Care Med. 2020. Online ahead of print). Another study showed a similar effect of ICS on the seasonal coronavirus strain HCoV-229E (Yamaya M, et al. Respir Investig. 2020;58[3]:155), and one study reported decreased ACE2 expression in allergic asthma (Jackson, et al. J Allergy Clin Immunol. Article in press, 2020). While these findings could support a hypothesis of reduced risk for COVID-19 infection among asthmatics using ICS, one would generally expect those with underlying lung disease, such as asthma, to be at higher risk for more severe infection.
Despite physiologic hypotheses of protective mechanisms, clinical outcomes may suffer as clinical operations and the American economy are impacted by this pandemic. Reduced access to or utilization of outpatient care, loss of employment, loss of health insurance, or a new difficulty in affording or accessing medications may all result in worsening asthma control for patients. Poorly controlled asthmatics are at higher risk for a more severe exacerbation of disease triggered by viral infection. Current recommendations are for patients to continue all controller medications; the use of systemic corticosteroids in treatment of COVID pneumonia is controversial, but their use in treating a COVID-associated asthma exacerbation should be based on individual assessment. As we care for asthma patients through this pandemic, much remains unknown but may be elucidated by further study.
Megan Conroy, MD
Fellow-in-Training Member
Steering Committee Member
Clinical Research and Quality Improvement
Remdesivir for COVID-19: A ray of hope?
The year 2020 witnessed a pandemic of unprecedented proportions, caused by a novel corona virus strain (SARS-CoV2). Across the globe, there have been more than 6.5 million positive cases of COVID-19 and more than 380,000 deaths. (WHO COVID-19 Dashboard [https://covid19.who.int]). Multiple therapeutic agents are currently being studied as potential treatment options for this novel disease. With negative trials so far on lopinavir-ritonavir and hydroxychloroquine, the only candidate drug showing benefit is remdesivir.
Results of the randomized double-blind placebo controlled Adaptive COVID-19 Treatment Trial (ACTT-1) trial (Beigel, J et al. N Engl J Med. 2020; e-pub ahead of print) shows remdesivir improved recovery time in COVID -19 patients as compared with control subjects. Remdesivir is an inhibitor of viral RNA polymerase that has been shown to inhibit coronaviruses in animal models and SARS-CoV2 in-vitro. The ACTT-1 trial enrolled 1,063 patients with 541 assigned to the remdesivir arm and 522 to the placebo group. Primary outcome measure was time to recovery. Mortality at 14 and 28 days and incidence of adverse events were also evaluated.
As interim analysis showed positive results, the data safety and monitoring board recommended early termination of the trial. Patients in the remdesivir group had a shorter time to recovery, with median recovery time of 11 days as compared with 15 days in placebo group (95% CI:1.12-1.55; P < .001). Hospitalized patients requiring supplemental oxygen (but not high-flow, mechanical ventilation or ECMO) derived the maximum benefit with a rate ratio of recovery being 1.47(95% CI:1.17-1.84). Thus, early drug administration may be beneficial. The difference in mortality at 14 days was not statistically significant and data on mortality difference at 28 days were not available at the time of publication.
In summary, this trial along with previous publications shows that remdesivir is a potential therapeutic option for COVID -19. The Food and Drug Administration (FDA) approved remdesivir under Emergency Use Authorization (EUA) for COVID-19 and larger trials are currently underway to study the full effect of this agent.
Aravind Menon, MD
Fellow-in-Training Member
Critical Care
Burnout in unprecedented times
Even in typical times, intensivists have a significantly higher rate of burnout compared with other medical specialties. We fight for lives, dealing with death, dying, and tragedy on a daily basis. Regrettably, we are no longer in ‘typical’ times. This is a prodigious and uncharted era.
The COVID-19 pandemic has created all new hardships. Added to the complex world of critical care, we undertake lack of appropriate medical equipment and PPE, the possibility of becoming ill or infecting our families, potential financial struggles, and the unpredictability of the future. Additionally, in our efforts to care for patients, we face increasing moral distress when placed in situations in which we cannot do what we feel is right. And we carry the burdens and guilt of patients’ families who cannot be with loved ones during this process, even during death.
What does burnout look like in this new era? Burnout is a continuum and can manifest differently depending on the individual. Even a typical day in the ICU may be cause for the symptoms of burnout including frustration, anger, anxiety, or sadness which can progress to feelings of powerlessness, self-doubt or depersonalization.
This crisis is a test of endurance. But we don’t have to face it alone. The ICU is a team environment, and we can help each other make it to the end. Consider beginning the shift with a group morale boosting activity. Perhaps debrief after the end of each shift to discuss ways of combatting these stressful times. Have a virtual happy hour with colleagues after work. Call on leadership for support. Watch each other’s back. Together we will get through these unprecedented times.
John P. Gaillard, MD
Steering Committee Member
Resources for confronting burnout:
http://ccsconline.org/optimizing-the-workforce/burnout
https://www.ama-assn.org/topics/physician-burnout
https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
Home-Based Mechanical Ventilation and Neuromuscular Disease
Use of modified RADs
Investigators have begun exploring ways to convert devices typically used to treat sleep-disordered breathing (respiratory assist device, RAD), with modifications to minimize risk of aerosolization of pathogen in the COVID-19 pandemic. These devices are presently not considered an effective means of treating acute respiratory distress syndrome (ARDS). In an emergency, however, it is reasonable to consider all the options available with a healthy respect for inherent device limitations.
A RAD could be converted from an open ventilation single-limb respiratory circuit to a closed ventilation circuit with a passive exhalation valve. This circuit could provide adequate minute ventilation and allow for adequate exhalation of CO2 to prevent rebreathing. Strategic placement of the passive exhalation valve proximal to a viricidal filter would allow the device to be used with either an endotracheal tube or a nonvented oronasal mask (Figure). These devices by design are pressure-regulated, and a backup rate would be necessary to control minute ventilation. Close monitoring would be necessary given lack of alarm capability for a critically ill patient and the need to ensure adequate oxygen bleed-in.
The primary limitation to these devices is the inability to achieve adequate mean airway pressure for ARDS. While such a converted device is not ready for prime time, it could be considered for patients who are close to weaning from conventional mechanical ventilation (i.e., freeing up a ventilator for a sicker patient) or temporizing a patient early in disease to stave off invasive ventilation.
MAJ Brian E. Foster, DO, USA
Fellow Member
Steering Committee Member
Interstitial and Diffuse Lung Disease NetWork
Advances in molecular imaging in pulmonary fibrosis
Fibrotic interstitial lung diseases (ILD), including idiopathic pulmonary fibrosis (IPF), have poor prognosis with marked heterogeneity in the clinical course. Treatment options, including antfibrotic drugs and immunosuppressants, are fairly limited for either conditions, and there is wide variability in drug responsiveness. Biomarkers that predict disease course and enable patient stratification to assess responsiveness to specific therapies play a crucial role in management of this fatal disease.
Molecular imaging has the ability to noninvasively provide both structural details, as well as functional/molecular information at the cellular level; it has thus developed into a powerful tool for several inflammatory and malignant disease processes. Probes that specifically target fibrosis-specific pathways utilizing positron emission tomography (PET) or magnetic resonance (MR) imaging have gained traction recently.
The most commonly used radiopharmaceutical for PET, 18F-FDG, is significantly increased in areas of established fibrosis in patients with IPF and autoimmune ILDs (Win, et al. Eur J Nucl Med Mol Imaging. 2018 May;45[5]:806; Uehara, et al. Mod Rheumatol. 2016;26[1]:121-7), as well as areas with seemingly normal morphologic appearance on HRCT scan (Win, et al. Eur J Nucl Med Mol Imaging. 2014 Feb;41[2]:337). While this probe was shown to have some potential for prognostication, there has been concern regarding the specificity of FDG uptake in fibrotic lung diseases. Hence, other probes that target specific fibrosis-related cellular mechanisms such as macrophages (Withana, et al. Nature Scientific Reports. 2016;6 [Jan 22):19755], and John, et al. J Nucl Med. 2013;54[12]:2146) and matrix proteins (Montesi, et al. Am J Respir Crit Care Med. 2019 Jul 15;200[2]:258) have been developed in preclinical fibrosis/lung injury models and are being translated to human subjects.
With the ability to capture early fibrogenesis and target engagement, molecular imaging has the potential to prognosticate patients, provide earlier evaluation of treatment responsiveness and have a promising application in clinical trial design for fibrotic lung diseases.
Tejaswini Kulkarni, MD
Steering Committee Member
CHEST Foundation
These last few months have been something that none of us has ever experienced. As many of you have witnessed firsthand, life is full of uncertainty and, as many of us try to get back to the “new normal,” we know that much of this uncertainty will persist. We are now not only dealing with a pandemic and caring for our patients but also addressing civil unrest and taking the time to grow and understand the importance of human life, no matter what race, ethnicity, or gender. In response, the CHEST Foundation has made efforts to further research in COVID-19 and increase our efforts in diversity and inclusion.
While we all race for solutions, we cannot overlook the immediate need in our local communities. The CHEST Foundation, along with partners across the nation, is taking a stand to deliver new resources and support now.
I proudly support the CHEST Foundation and am asking for your support, as well. Give a gift today, and together we can effect change for the better in our communities.
Warmest regards,
Doreen J. Addrizzo-Harris, MD, FCCP
Immediate Past President & Trustee
These last few months have been something that none of us has ever experienced. As many of you have witnessed firsthand, life is full of uncertainty and, as many of us try to get back to the “new normal,” we know that much of this uncertainty will persist. We are now not only dealing with a pandemic and caring for our patients but also addressing civil unrest and taking the time to grow and understand the importance of human life, no matter what race, ethnicity, or gender. In response, the CHEST Foundation has made efforts to further research in COVID-19 and increase our efforts in diversity and inclusion.
While we all race for solutions, we cannot overlook the immediate need in our local communities. The CHEST Foundation, along with partners across the nation, is taking a stand to deliver new resources and support now.
I proudly support the CHEST Foundation and am asking for your support, as well. Give a gift today, and together we can effect change for the better in our communities.
Warmest regards,
Doreen J. Addrizzo-Harris, MD, FCCP
Immediate Past President & Trustee
These last few months have been something that none of us has ever experienced. As many of you have witnessed firsthand, life is full of uncertainty and, as many of us try to get back to the “new normal,” we know that much of this uncertainty will persist. We are now not only dealing with a pandemic and caring for our patients but also addressing civil unrest and taking the time to grow and understand the importance of human life, no matter what race, ethnicity, or gender. In response, the CHEST Foundation has made efforts to further research in COVID-19 and increase our efforts in diversity and inclusion.
While we all race for solutions, we cannot overlook the immediate need in our local communities. The CHEST Foundation, along with partners across the nation, is taking a stand to deliver new resources and support now.
I proudly support the CHEST Foundation and am asking for your support, as well. Give a gift today, and together we can effect change for the better in our communities.
Warmest regards,
Doreen J. Addrizzo-Harris, MD, FCCP
Immediate Past President & Trustee
Calendar
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Aug. 13-14, Sept. 16-17, and Oct. 7-8, 2020
2-Day, In-Depth Coding and Billing Seminar
Become a certified GI coder with a 2-day, in-depth training course provided by McVey Associates.
Baltimore, Md. (Aug. 13-14); Atlanta, Ga. (Sept. 16-17); Las Vegas, Nev. (Oct. 7-8)
Aug. 15-16, 2020
2020 Principles of GI for the NP and PA
Because of COVID-19, the American Gastroenterological Association has transitioned the 2020 Principles of GI for the NP and PA course from a live meeting to a virtual course. The virtual course will provide you with team-based expert guidance on managing GI patients through case-based learning from faculty who are seasoned physicians and advanced practice providers. Register at https://bit.ly/38oeK4C.
AWARD DEADLINES
AGA-Pilot Research Award
This award provides $30,000 for 1 year to recipients at any career stage researching new directions in gastroenterology- or hepatology-related areas.
Application deadline: Sept. 2, 2020
AGA-Medtronic Pilot Research Award in Technology Innovation
This award provides $30,000 for 1 year to independent investigators at any career stage to support the research and development of novel devices or technologies that will potentially impact the diagnosis or treatment of digestive disease.
Application deadline: Sept. 2, 2020
AGA–Takeda Pharmaceuticals Research Scholar Award in Celiac Disease
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in celiac disease research.
Application deadline: Nov. 9, 2020
AGA Research Scholar Award (RSA)
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in digestive disease research.
Application deadline: Nov. 9, 2020
AGA–Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in inflammatory bowel disease (IBD) research.
Application deadline: Nov. 9, 2020
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early career (i.e., 35 years or younger at the time of Digestive Disease Week® [DDW]) basic, translational, or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb 26, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Aug. 13-14, Sept. 16-17, and Oct. 7-8, 2020
2-Day, In-Depth Coding and Billing Seminar
Become a certified GI coder with a 2-day, in-depth training course provided by McVey Associates.
Baltimore, Md. (Aug. 13-14); Atlanta, Ga. (Sept. 16-17); Las Vegas, Nev. (Oct. 7-8)
Aug. 15-16, 2020
2020 Principles of GI for the NP and PA
Because of COVID-19, the American Gastroenterological Association has transitioned the 2020 Principles of GI for the NP and PA course from a live meeting to a virtual course. The virtual course will provide you with team-based expert guidance on managing GI patients through case-based learning from faculty who are seasoned physicians and advanced practice providers. Register at https://bit.ly/38oeK4C.
AWARD DEADLINES
AGA-Pilot Research Award
This award provides $30,000 for 1 year to recipients at any career stage researching new directions in gastroenterology- or hepatology-related areas.
Application deadline: Sept. 2, 2020
AGA-Medtronic Pilot Research Award in Technology Innovation
This award provides $30,000 for 1 year to independent investigators at any career stage to support the research and development of novel devices or technologies that will potentially impact the diagnosis or treatment of digestive disease.
Application deadline: Sept. 2, 2020
AGA–Takeda Pharmaceuticals Research Scholar Award in Celiac Disease
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in celiac disease research.
Application deadline: Nov. 9, 2020
AGA Research Scholar Award (RSA)
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in digestive disease research.
Application deadline: Nov. 9, 2020
AGA–Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in inflammatory bowel disease (IBD) research.
Application deadline: Nov. 9, 2020
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early career (i.e., 35 years or younger at the time of Digestive Disease Week® [DDW]) basic, translational, or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb 26, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Aug. 13-14, Sept. 16-17, and Oct. 7-8, 2020
2-Day, In-Depth Coding and Billing Seminar
Become a certified GI coder with a 2-day, in-depth training course provided by McVey Associates.
Baltimore, Md. (Aug. 13-14); Atlanta, Ga. (Sept. 16-17); Las Vegas, Nev. (Oct. 7-8)
Aug. 15-16, 2020
2020 Principles of GI for the NP and PA
Because of COVID-19, the American Gastroenterological Association has transitioned the 2020 Principles of GI for the NP and PA course from a live meeting to a virtual course. The virtual course will provide you with team-based expert guidance on managing GI patients through case-based learning from faculty who are seasoned physicians and advanced practice providers. Register at https://bit.ly/38oeK4C.
AWARD DEADLINES
AGA-Pilot Research Award
This award provides $30,000 for 1 year to recipients at any career stage researching new directions in gastroenterology- or hepatology-related areas.
Application deadline: Sept. 2, 2020
AGA-Medtronic Pilot Research Award in Technology Innovation
This award provides $30,000 for 1 year to independent investigators at any career stage to support the research and development of novel devices or technologies that will potentially impact the diagnosis or treatment of digestive disease.
Application deadline: Sept. 2, 2020
AGA–Takeda Pharmaceuticals Research Scholar Award in Celiac Disease
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in celiac disease research.
Application deadline: Nov. 9, 2020
AGA Research Scholar Award (RSA)
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in digestive disease research.
Application deadline: Nov. 9, 2020
AGA–Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in inflammatory bowel disease (IBD) research.
Application deadline: Nov. 9, 2020
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early career (i.e., 35 years or younger at the time of Digestive Disease Week® [DDW]) basic, translational, or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb 26, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
Hypercalcemia Is of Uncertain Significance in Patients With Advanced Adenocarcinoma of the Prostate
Hypercalcemia is found when the corrected serum calcium level is > 10.5 mg/dL.1 Its symptoms are not specific and may include polyuria, dehydration, polydipsia, anorexia, nausea and/or vomiting, constipation, and other central nervous system manifestations, including confusion, delirium, cognitive impairment, muscle weakness, psychotic symptoms, and even coma.1,2
Hypercalcemia has varied etiologies; however, malignancy-induced hypercalcemia is one of the most common causes. In the US, the most common causes of malignancy-induced hypercalcemia are primary tumors of the lung or breast, multiple myeloma (MM), squamous cell carcinoma of the head or neck, renal cancer, and ovarian cancer.1
Men with prostate cancer and bone metastasis have relatively worse prognosis than do patient with no metastasis.3 In a recent meta-analysis of patients with bone-involved castration-resistant prostate cancer, the median survival was 21 months.3
Hypercalcemia is a rare manifestation of prostate cancer. In a retrospective study conducted between 2009 and 2013 using the Oncology Services Comprehensive Electronic Records (OSCER) warehouse of electronic health records (EHR), the rates of malignancy-induced hypercalcemia were the lowest among patients with prostate cancer, ranging from 1.4 to 2.1%.1
We present this case to discuss different pathophysiologic mechanisms leading to hypercalcemia in a patient with prostate cancer with bone metastasis and to study the role of humoral and growth factors in the pathogenesis of the disease.
Case Presentation
An African American man aged 69 years presented to the emergency department (ED) with generalized weakness, fatigue, and lower extremities muscle weakness. He reported a 40-lb weight loss over the past 3 months, intermittent lower back pain, and a 50 pack-year smoking history. A physical examination suggested clinical signs of dehydration.
Laboratory test results indicated hypercalcemia, macrocytic anemia, and thrombocytopenia: calcium 15.8 mg/dL, serum albumin 4.1 mg/dL, alkaline phosphatase 139 μ/L, blood urea nitrogen 55 mg/dL, creatinine 3.4 mg/dL (baseline 1.4-1.5 mg/dL), hemoglobin 8 g/dL, mean corpuscular volume 99.6 fL, and platelets 100,000/μL. The patient was admitted for hypercalcemia. His intact parathyroid hormone (iPTH) was suppressed at 16 pg/mL, phosphorous was 3.8 mg/dL, parathyroid hormone-related peptide (PTHrP) was < 0.74 pmol/L, vitamin D (25 hydroxy cholecalciferol) was mildly decreased at 17.2 ng/mL, and 1,25 dihydroxy cholecalciferol (calcitriol) was < 5.0 (normal range 20-79.3 pg/mL).
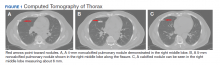
A computed tomography (CT) scan of the chest and abdomen was taken due to the patient’s heavy smoking history, an incidentally detected right lung base nodule on chest X-ray, and hypercalcemia. The CT scan showed multiple right middle lobe lung nodules with and without calcifications and calcified right hilar lymph nodes (Figure 1).
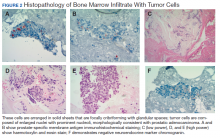
To evaluate the pancytopenia, a bone marrow biopsy was done, which showed that 80 to 90% of the marrow space was replaced by fibrosis and metastatic malignancy. Trilinear hematopoiesis was not seen (Figure 2). The tumor cells were positive for prostate- specific membrane antigen (PSMA) and negative for cytokeratin 7 and 20 (CK7 and CK20).4 The former is a membrane protein expressed on prostate tissues, including cancer; the latter is a form of protein used to identify adenocarcinoma of unknown primary origin (CK7 usually found in primary/ metastatic lung adenocarcinoma and CK20 usually in primary and some metastatic diseases of colon adenocarcinoma).5 A prostatic specific antigen (PSA) test was markedly elevated: 335.94 ng/mL (1.46 ng/mL on a previous 2011 test).
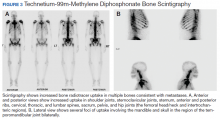
Metastatic adenocarcinoma of the prostate was diagnosed without a prostate biopsy. To determine the extent of bone metastases, a technetium-99m-methylene diphosphonate (MDP) bone scintigraphy demonstrated a superscan with intense foci of increased radiotracer uptake involving the bilateral shoulders, sternoclavicular joints, and sternum with heterogeneous uptake involving bilateral anterior and posterior ribs; cervical, thoracic, and lumbar spines; sacrum, pelvis, and bilateral hips, including the femoral head/neck and intertrochanteric regions. Also noted were several foci of radiotracer uptake involving the mandible and bilateral skull in the region of the temporomandibular joints (Figure 3).
The patient was initially treated with IV isotonic saline, followed by calcitonin and then pamidronate after kidney function improved. His calcium level responded to the therapy, and a plan was made by medical oncology to start androgen deprivation therapy (ADT) prior to discharge.
He was initially treated with bicalutamide, while a luteinizing hormone-releasing hormone agonist (leuprolide) was added 1 week later. Bicalutamide was then discontinued and a combined androgen blockade consisting of leuprolide, ketoconazole, and hydrocortisone was started. This therapy resulted in remission, and PSA declined to 1.73 ng/ mL 3 months later. At that time the patient enrolled in a clinical trial with leuprolide and bicalutamide combined therapy. About 6 months after his diagnosis, patient’s cancer progressed and became hormone refractory disease. At that time, bicalutamide was discontinued, and his therapy was switched to combined leuprolide and enzalutamide. After 6 months of therapy with enzalutamide, the patient’s cancer progressed again. He was later treated with docetaxel chemotherapy but died 16 months after diagnosis.
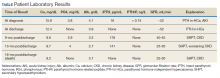
showed improvement of hypercalcemia at the time of discharge, but 9 months later and toward the time of expiration, our patient developed secondary hyperparathyroidism, with calcium maintained in the normal range, while iPTH was significantly elevated, a finding likely explained by a decline in kidney function and a fall in glomerular filtration rate (Table).
Discussion
Hypercalcemia in the setting of prostate cancer is a rare complication with an uncertain pathophysiology.6 Several mechanisms have been proposed for hypercalcemia of malignancy, these comprise humoral hypercalcemia of malignancy mediated by increased PTHrP; local osteolytic hypercalcemia with secretion of other humoral factors; excess extrarenal activation of vitamin D (1,25[OH]2D); PTH secretion, ectopic or primary; and multiple concurrent etiologies.7
PTHrP is the predominant mediator for hypercalcemia of malignancy and is estimated to account for 80% of hypercalcemia in patients with cancer. This protein shares a substantial sequence homology with PTH; in fact, 8 of the first 13 amino acids at the N-terminal portion of PTH were identical.8 PTHrP has multiple isoforms (PTHrP 141, PTHrP 139, and PTHrP 173). Like PTH, it enhances renal tubular reabsorption of calcium while increasing urinary phosphorus excretion.7 The result is both hypercalcemia and hypophosphatemia. However, unlike PTH, PTHrP does not increase 1,25(OH)2D and thus does not increase intestinal absorption of calcium and phosphorus. PTHrP acts on osteoblasts, leading to enhanced synthesis of receptor activator of nuclear factor-κB ligand (RANKL).7
In one study, PTHrP was detected immunohistochemically in prostate cancer cells. Iwamura and colleagues used 33 radical prostatectomy specimens from patients with clinically localized carcinoma of the prostate.9 None of these patients demonstrated hypercalcemia prior to the surgery. Using a mouse monoclonal antibody to an amino acid fragment, all cases demonstrated some degree of immunoreactivity throughout the cytoplasm of the tumor cells, but immunostaining was absent from inflammatory and stromal cells.9Furthermore, the intensity of the staining appeared to directly correlate with increasing tumor grade.9
Another study by Iwamura and colleagues suggested that PTHrP may play a significant role in the growth of prostate cancer by acting locally in an autocrine fashion.10 In this study, all prostate cancer cell lines from different sources expressed PTHrP immunoreactivity as well as evidence of DNA synthesis, the latter being measured by thymidine incorporation assay. Moreover, when these cells were incubated with various concentrations of mouse monoclonal antibody directed to PTHrP fragment, PTHrP-induced DNA synthesis was inhibited in a dose-dependent manner and almost completely neutralized at a specific concentration. Interestingly, the study demonstrated that cancer cell line derived from bone metastatic lesions secreted significantly greater amounts of PTHrP than did the cell line derived from the metastasis in the brain or in the lymph node. These findings suggest that PTHrP production may confer some advantage on the ability of prostate cancer cells to grow in bone.10
Ando and colleagues reported that neuroendocrine dedifferentiated prostate cancer can develop as a result of long-term ADT even after several years of therapy and has the potential to worsen and develop severe hypercalcemia.8 Neuron-specific enolase was used as the specific marker for the neuroendocrine cell, which suggested that the prostate cancer cell derived from the neuroendocrine cell might synthesize PTHrP and be responsible for the observed hypercalcemia.8
Other mechanisms cited for hypercalcemia of malignancy include other humoral factors associated with increased remodeling and comprise interleukin 1, 3, 6 (IL-1, IL-3, IL-6); tumor necrosis factor α; transforming growth factor A and B observed in metastatic bone lesions in breast cancer; lymphotoxin; E series prostaglandins; and macrophage inflammatory protein 1α seen in MM.
Local osteolytic hypercalcemia accounts for about 20% of cases and is usually associated with extensive bone metastases. It is most commonly seen in MM and metastatic breast cancer and less commonly in leukemia. The proposed mechanism is thought to be because of the release of local cytokines from the tumor, resulting in excess osteoclast activation and enhanced bone resorption often through RANK/RANKL interaction.
Extrarenal production of 1,25(OH)2D by the tumor accounts for about 1% of cases of hypercalcemia in malignancy. 1,25(OH)2D causes increased intestinal absorption of calcium and enhances osteolytic bone resorption, resulting in increased serum calcium. This mechanism is most commonly seen with Hodgkin and non-Hodgkin lymphoma and had been reported in ovarian dysgerminoma.7
In our patient, bone imaging showed osteoblastic lesions, a finding that likely contrasts the local osteolytic bone destruction theory. PTHrP was not significantly elevated in the serum, and PTH levels ruled out any form of primary hyperparathyroidism. In addition, histopathology showed no evidence of mosaicism or neuroendocrine dedifferentiation.
Findings in aggregate tell us that an exact pathophysiologic mechanism leading to hypercalcemia in prostate cancer is still unclear and may involve an interplay between growth factors and possible osteolytic materials, yet it must be studied thoroughly.
Conclusions
Hypercalcemia in pure metastatic adenocarcinoma of prostate is a rare finding and is of uncertain significance. Some studies suggested a search for unusual histopathologies, including neuroendocrine cancer and neuroendocrine dedifferentiation.8,11 However, in adenocarcinoma alone, it has an uncertain pathophysiology that needs to be further studied. Studies needed to investigate the role of PTHrP as a growth factor for both prostate cancer cells and development of hypercalcemia and possibly target-directed monoclonal antibody therapies may need to be extensively researched.
1. Gastanaga VM, Schwartzberg LS, Jain RK, et al. Prevalence of hypercalcemia among cancer patients in the United States. Cancer Med. 2016;5(8):2091‐2100. doi:10.1002/cam4.749
2. Grill V, Martin TJ. Hypercalcemia of malignancy. Rev Endocr Metab Disord. 2000;1(4):253‐263. doi:10.1023/a:1026597816193
3. Halabi S, Kelly WK, Ma H, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34(14):1652‐1659. doi:10.1200/JCO.2015.65.7270
4. Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(suppl 10):S13‐S18.
5. Kummar S, Fogarasi M, Canova A, Mota A, Ciesielski T. Cytokeratin 7 and 20 staining for the diagnosis of lung and colorectal adenocarcinoma. Br J Cancer. 2002;86(12):1884‐1887. doi:10.1038/sj.bjc.6600326
6. Avashia JH, Walsh TD, Thomas AJ Jr, Kaye M, Licata A. Metastatic carcinoma of the prostate with hypercalcemia [published correction appears in Cleve Clin J Med. 1991;58(3):284]. Cleve Clin J Med. 1990;57(7):636‐638. doi:10.3949/ccjm.57.7.636.
7. Goldner W. Cancer-related hypercalcemia. J Oncol Pract. 2016;12(5):426‐432. doi:10.1200/JOP.2016.011155.
8. Ando T, Watanabe K, Mizusawa T, Katagiri A. Hypercalcemia due to parathyroid hormone-related peptide secreted by neuroendocrine dedifferentiated prostate cancer. Urol Case Rep. 2018;22:67‐69. doi:10.1016/j.eucr.2018.11.001
9. Iwamura M, di Sant’Agnese PA, Wu G, et al. Immunohistochemical localization of parathyroid hormonerelated protein in human prostate cancer. Cancer Res. 1993;53(8):1724‐1726.
10. Iwamura M, Abrahamsson PA, Foss KA, Wu G, Cockett AT, Deftos LJ. Parathyroid hormone-related protein: a potential autocrine growth regulator in human prostate cancer cell lines. Urology. 1994;43(5):675‐679. doi:10.1016/0090-4295(94)90183-x
11. Smith DC, Tucker JA, Trump DL. Hypercalcemia and neuroendocrine carcinoma of the prostate: a report of three cases and a review of the literature. J Clin Oncol. 1992;10(3):499‐505. doi:10.1200/JCO.1992.10.3.499.
Hypercalcemia is found when the corrected serum calcium level is > 10.5 mg/dL.1 Its symptoms are not specific and may include polyuria, dehydration, polydipsia, anorexia, nausea and/or vomiting, constipation, and other central nervous system manifestations, including confusion, delirium, cognitive impairment, muscle weakness, psychotic symptoms, and even coma.1,2
Hypercalcemia has varied etiologies; however, malignancy-induced hypercalcemia is one of the most common causes. In the US, the most common causes of malignancy-induced hypercalcemia are primary tumors of the lung or breast, multiple myeloma (MM), squamous cell carcinoma of the head or neck, renal cancer, and ovarian cancer.1
Men with prostate cancer and bone metastasis have relatively worse prognosis than do patient with no metastasis.3 In a recent meta-analysis of patients with bone-involved castration-resistant prostate cancer, the median survival was 21 months.3
Hypercalcemia is a rare manifestation of prostate cancer. In a retrospective study conducted between 2009 and 2013 using the Oncology Services Comprehensive Electronic Records (OSCER) warehouse of electronic health records (EHR), the rates of malignancy-induced hypercalcemia were the lowest among patients with prostate cancer, ranging from 1.4 to 2.1%.1
We present this case to discuss different pathophysiologic mechanisms leading to hypercalcemia in a patient with prostate cancer with bone metastasis and to study the role of humoral and growth factors in the pathogenesis of the disease.
Case Presentation
An African American man aged 69 years presented to the emergency department (ED) with generalized weakness, fatigue, and lower extremities muscle weakness. He reported a 40-lb weight loss over the past 3 months, intermittent lower back pain, and a 50 pack-year smoking history. A physical examination suggested clinical signs of dehydration.
Laboratory test results indicated hypercalcemia, macrocytic anemia, and thrombocytopenia: calcium 15.8 mg/dL, serum albumin 4.1 mg/dL, alkaline phosphatase 139 μ/L, blood urea nitrogen 55 mg/dL, creatinine 3.4 mg/dL (baseline 1.4-1.5 mg/dL), hemoglobin 8 g/dL, mean corpuscular volume 99.6 fL, and platelets 100,000/μL. The patient was admitted for hypercalcemia. His intact parathyroid hormone (iPTH) was suppressed at 16 pg/mL, phosphorous was 3.8 mg/dL, parathyroid hormone-related peptide (PTHrP) was < 0.74 pmol/L, vitamin D (25 hydroxy cholecalciferol) was mildly decreased at 17.2 ng/mL, and 1,25 dihydroxy cholecalciferol (calcitriol) was < 5.0 (normal range 20-79.3 pg/mL).
A computed tomography (CT) scan of the chest and abdomen was taken due to the patient’s heavy smoking history, an incidentally detected right lung base nodule on chest X-ray, and hypercalcemia. The CT scan showed multiple right middle lobe lung nodules with and without calcifications and calcified right hilar lymph nodes (Figure 1).
To evaluate the pancytopenia, a bone marrow biopsy was done, which showed that 80 to 90% of the marrow space was replaced by fibrosis and metastatic malignancy. Trilinear hematopoiesis was not seen (Figure 2). The tumor cells were positive for prostate- specific membrane antigen (PSMA) and negative for cytokeratin 7 and 20 (CK7 and CK20).4 The former is a membrane protein expressed on prostate tissues, including cancer; the latter is a form of protein used to identify adenocarcinoma of unknown primary origin (CK7 usually found in primary/ metastatic lung adenocarcinoma and CK20 usually in primary and some metastatic diseases of colon adenocarcinoma).5 A prostatic specific antigen (PSA) test was markedly elevated: 335.94 ng/mL (1.46 ng/mL on a previous 2011 test).
Metastatic adenocarcinoma of the prostate was diagnosed without a prostate biopsy. To determine the extent of bone metastases, a technetium-99m-methylene diphosphonate (MDP) bone scintigraphy demonstrated a superscan with intense foci of increased radiotracer uptake involving the bilateral shoulders, sternoclavicular joints, and sternum with heterogeneous uptake involving bilateral anterior and posterior ribs; cervical, thoracic, and lumbar spines; sacrum, pelvis, and bilateral hips, including the femoral head/neck and intertrochanteric regions. Also noted were several foci of radiotracer uptake involving the mandible and bilateral skull in the region of the temporomandibular joints (Figure 3).
The patient was initially treated with IV isotonic saline, followed by calcitonin and then pamidronate after kidney function improved. His calcium level responded to the therapy, and a plan was made by medical oncology to start androgen deprivation therapy (ADT) prior to discharge.
He was initially treated with bicalutamide, while a luteinizing hormone-releasing hormone agonist (leuprolide) was added 1 week later. Bicalutamide was then discontinued and a combined androgen blockade consisting of leuprolide, ketoconazole, and hydrocortisone was started. This therapy resulted in remission, and PSA declined to 1.73 ng/ mL 3 months later. At that time the patient enrolled in a clinical trial with leuprolide and bicalutamide combined therapy. About 6 months after his diagnosis, patient’s cancer progressed and became hormone refractory disease. At that time, bicalutamide was discontinued, and his therapy was switched to combined leuprolide and enzalutamide. After 6 months of therapy with enzalutamide, the patient’s cancer progressed again. He was later treated with docetaxel chemotherapy but died 16 months after diagnosis.
showed improvement of hypercalcemia at the time of discharge, but 9 months later and toward the time of expiration, our patient developed secondary hyperparathyroidism, with calcium maintained in the normal range, while iPTH was significantly elevated, a finding likely explained by a decline in kidney function and a fall in glomerular filtration rate (Table).
Discussion
Hypercalcemia in the setting of prostate cancer is a rare complication with an uncertain pathophysiology.6 Several mechanisms have been proposed for hypercalcemia of malignancy, these comprise humoral hypercalcemia of malignancy mediated by increased PTHrP; local osteolytic hypercalcemia with secretion of other humoral factors; excess extrarenal activation of vitamin D (1,25[OH]2D); PTH secretion, ectopic or primary; and multiple concurrent etiologies.7
PTHrP is the predominant mediator for hypercalcemia of malignancy and is estimated to account for 80% of hypercalcemia in patients with cancer. This protein shares a substantial sequence homology with PTH; in fact, 8 of the first 13 amino acids at the N-terminal portion of PTH were identical.8 PTHrP has multiple isoforms (PTHrP 141, PTHrP 139, and PTHrP 173). Like PTH, it enhances renal tubular reabsorption of calcium while increasing urinary phosphorus excretion.7 The result is both hypercalcemia and hypophosphatemia. However, unlike PTH, PTHrP does not increase 1,25(OH)2D and thus does not increase intestinal absorption of calcium and phosphorus. PTHrP acts on osteoblasts, leading to enhanced synthesis of receptor activator of nuclear factor-κB ligand (RANKL).7
In one study, PTHrP was detected immunohistochemically in prostate cancer cells. Iwamura and colleagues used 33 radical prostatectomy specimens from patients with clinically localized carcinoma of the prostate.9 None of these patients demonstrated hypercalcemia prior to the surgery. Using a mouse monoclonal antibody to an amino acid fragment, all cases demonstrated some degree of immunoreactivity throughout the cytoplasm of the tumor cells, but immunostaining was absent from inflammatory and stromal cells.9Furthermore, the intensity of the staining appeared to directly correlate with increasing tumor grade.9
Another study by Iwamura and colleagues suggested that PTHrP may play a significant role in the growth of prostate cancer by acting locally in an autocrine fashion.10 In this study, all prostate cancer cell lines from different sources expressed PTHrP immunoreactivity as well as evidence of DNA synthesis, the latter being measured by thymidine incorporation assay. Moreover, when these cells were incubated with various concentrations of mouse monoclonal antibody directed to PTHrP fragment, PTHrP-induced DNA synthesis was inhibited in a dose-dependent manner and almost completely neutralized at a specific concentration. Interestingly, the study demonstrated that cancer cell line derived from bone metastatic lesions secreted significantly greater amounts of PTHrP than did the cell line derived from the metastasis in the brain or in the lymph node. These findings suggest that PTHrP production may confer some advantage on the ability of prostate cancer cells to grow in bone.10
Ando and colleagues reported that neuroendocrine dedifferentiated prostate cancer can develop as a result of long-term ADT even after several years of therapy and has the potential to worsen and develop severe hypercalcemia.8 Neuron-specific enolase was used as the specific marker for the neuroendocrine cell, which suggested that the prostate cancer cell derived from the neuroendocrine cell might synthesize PTHrP and be responsible for the observed hypercalcemia.8
Other mechanisms cited for hypercalcemia of malignancy include other humoral factors associated with increased remodeling and comprise interleukin 1, 3, 6 (IL-1, IL-3, IL-6); tumor necrosis factor α; transforming growth factor A and B observed in metastatic bone lesions in breast cancer; lymphotoxin; E series prostaglandins; and macrophage inflammatory protein 1α seen in MM.
Local osteolytic hypercalcemia accounts for about 20% of cases and is usually associated with extensive bone metastases. It is most commonly seen in MM and metastatic breast cancer and less commonly in leukemia. The proposed mechanism is thought to be because of the release of local cytokines from the tumor, resulting in excess osteoclast activation and enhanced bone resorption often through RANK/RANKL interaction.
Extrarenal production of 1,25(OH)2D by the tumor accounts for about 1% of cases of hypercalcemia in malignancy. 1,25(OH)2D causes increased intestinal absorption of calcium and enhances osteolytic bone resorption, resulting in increased serum calcium. This mechanism is most commonly seen with Hodgkin and non-Hodgkin lymphoma and had been reported in ovarian dysgerminoma.7
In our patient, bone imaging showed osteoblastic lesions, a finding that likely contrasts the local osteolytic bone destruction theory. PTHrP was not significantly elevated in the serum, and PTH levels ruled out any form of primary hyperparathyroidism. In addition, histopathology showed no evidence of mosaicism or neuroendocrine dedifferentiation.
Findings in aggregate tell us that an exact pathophysiologic mechanism leading to hypercalcemia in prostate cancer is still unclear and may involve an interplay between growth factors and possible osteolytic materials, yet it must be studied thoroughly.
Conclusions
Hypercalcemia in pure metastatic adenocarcinoma of prostate is a rare finding and is of uncertain significance. Some studies suggested a search for unusual histopathologies, including neuroendocrine cancer and neuroendocrine dedifferentiation.8,11 However, in adenocarcinoma alone, it has an uncertain pathophysiology that needs to be further studied. Studies needed to investigate the role of PTHrP as a growth factor for both prostate cancer cells and development of hypercalcemia and possibly target-directed monoclonal antibody therapies may need to be extensively researched.
Hypercalcemia is found when the corrected serum calcium level is > 10.5 mg/dL.1 Its symptoms are not specific and may include polyuria, dehydration, polydipsia, anorexia, nausea and/or vomiting, constipation, and other central nervous system manifestations, including confusion, delirium, cognitive impairment, muscle weakness, psychotic symptoms, and even coma.1,2
Hypercalcemia has varied etiologies; however, malignancy-induced hypercalcemia is one of the most common causes. In the US, the most common causes of malignancy-induced hypercalcemia are primary tumors of the lung or breast, multiple myeloma (MM), squamous cell carcinoma of the head or neck, renal cancer, and ovarian cancer.1
Men with prostate cancer and bone metastasis have relatively worse prognosis than do patient with no metastasis.3 In a recent meta-analysis of patients with bone-involved castration-resistant prostate cancer, the median survival was 21 months.3
Hypercalcemia is a rare manifestation of prostate cancer. In a retrospective study conducted between 2009 and 2013 using the Oncology Services Comprehensive Electronic Records (OSCER) warehouse of electronic health records (EHR), the rates of malignancy-induced hypercalcemia were the lowest among patients with prostate cancer, ranging from 1.4 to 2.1%.1
We present this case to discuss different pathophysiologic mechanisms leading to hypercalcemia in a patient with prostate cancer with bone metastasis and to study the role of humoral and growth factors in the pathogenesis of the disease.
Case Presentation
An African American man aged 69 years presented to the emergency department (ED) with generalized weakness, fatigue, and lower extremities muscle weakness. He reported a 40-lb weight loss over the past 3 months, intermittent lower back pain, and a 50 pack-year smoking history. A physical examination suggested clinical signs of dehydration.
Laboratory test results indicated hypercalcemia, macrocytic anemia, and thrombocytopenia: calcium 15.8 mg/dL, serum albumin 4.1 mg/dL, alkaline phosphatase 139 μ/L, blood urea nitrogen 55 mg/dL, creatinine 3.4 mg/dL (baseline 1.4-1.5 mg/dL), hemoglobin 8 g/dL, mean corpuscular volume 99.6 fL, and platelets 100,000/μL. The patient was admitted for hypercalcemia. His intact parathyroid hormone (iPTH) was suppressed at 16 pg/mL, phosphorous was 3.8 mg/dL, parathyroid hormone-related peptide (PTHrP) was < 0.74 pmol/L, vitamin D (25 hydroxy cholecalciferol) was mildly decreased at 17.2 ng/mL, and 1,25 dihydroxy cholecalciferol (calcitriol) was < 5.0 (normal range 20-79.3 pg/mL).
A computed tomography (CT) scan of the chest and abdomen was taken due to the patient’s heavy smoking history, an incidentally detected right lung base nodule on chest X-ray, and hypercalcemia. The CT scan showed multiple right middle lobe lung nodules with and without calcifications and calcified right hilar lymph nodes (Figure 1).
To evaluate the pancytopenia, a bone marrow biopsy was done, which showed that 80 to 90% of the marrow space was replaced by fibrosis and metastatic malignancy. Trilinear hematopoiesis was not seen (Figure 2). The tumor cells were positive for prostate- specific membrane antigen (PSMA) and negative for cytokeratin 7 and 20 (CK7 and CK20).4 The former is a membrane protein expressed on prostate tissues, including cancer; the latter is a form of protein used to identify adenocarcinoma of unknown primary origin (CK7 usually found in primary/ metastatic lung adenocarcinoma and CK20 usually in primary and some metastatic diseases of colon adenocarcinoma).5 A prostatic specific antigen (PSA) test was markedly elevated: 335.94 ng/mL (1.46 ng/mL on a previous 2011 test).
Metastatic adenocarcinoma of the prostate was diagnosed without a prostate biopsy. To determine the extent of bone metastases, a technetium-99m-methylene diphosphonate (MDP) bone scintigraphy demonstrated a superscan with intense foci of increased radiotracer uptake involving the bilateral shoulders, sternoclavicular joints, and sternum with heterogeneous uptake involving bilateral anterior and posterior ribs; cervical, thoracic, and lumbar spines; sacrum, pelvis, and bilateral hips, including the femoral head/neck and intertrochanteric regions. Also noted were several foci of radiotracer uptake involving the mandible and bilateral skull in the region of the temporomandibular joints (Figure 3).
The patient was initially treated with IV isotonic saline, followed by calcitonin and then pamidronate after kidney function improved. His calcium level responded to the therapy, and a plan was made by medical oncology to start androgen deprivation therapy (ADT) prior to discharge.
He was initially treated with bicalutamide, while a luteinizing hormone-releasing hormone agonist (leuprolide) was added 1 week later. Bicalutamide was then discontinued and a combined androgen blockade consisting of leuprolide, ketoconazole, and hydrocortisone was started. This therapy resulted in remission, and PSA declined to 1.73 ng/ mL 3 months later. At that time the patient enrolled in a clinical trial with leuprolide and bicalutamide combined therapy. About 6 months after his diagnosis, patient’s cancer progressed and became hormone refractory disease. At that time, bicalutamide was discontinued, and his therapy was switched to combined leuprolide and enzalutamide. After 6 months of therapy with enzalutamide, the patient’s cancer progressed again. He was later treated with docetaxel chemotherapy but died 16 months after diagnosis.
showed improvement of hypercalcemia at the time of discharge, but 9 months later and toward the time of expiration, our patient developed secondary hyperparathyroidism, with calcium maintained in the normal range, while iPTH was significantly elevated, a finding likely explained by a decline in kidney function and a fall in glomerular filtration rate (Table).
Discussion
Hypercalcemia in the setting of prostate cancer is a rare complication with an uncertain pathophysiology.6 Several mechanisms have been proposed for hypercalcemia of malignancy, these comprise humoral hypercalcemia of malignancy mediated by increased PTHrP; local osteolytic hypercalcemia with secretion of other humoral factors; excess extrarenal activation of vitamin D (1,25[OH]2D); PTH secretion, ectopic or primary; and multiple concurrent etiologies.7
PTHrP is the predominant mediator for hypercalcemia of malignancy and is estimated to account for 80% of hypercalcemia in patients with cancer. This protein shares a substantial sequence homology with PTH; in fact, 8 of the first 13 amino acids at the N-terminal portion of PTH were identical.8 PTHrP has multiple isoforms (PTHrP 141, PTHrP 139, and PTHrP 173). Like PTH, it enhances renal tubular reabsorption of calcium while increasing urinary phosphorus excretion.7 The result is both hypercalcemia and hypophosphatemia. However, unlike PTH, PTHrP does not increase 1,25(OH)2D and thus does not increase intestinal absorption of calcium and phosphorus. PTHrP acts on osteoblasts, leading to enhanced synthesis of receptor activator of nuclear factor-κB ligand (RANKL).7
In one study, PTHrP was detected immunohistochemically in prostate cancer cells. Iwamura and colleagues used 33 radical prostatectomy specimens from patients with clinically localized carcinoma of the prostate.9 None of these patients demonstrated hypercalcemia prior to the surgery. Using a mouse monoclonal antibody to an amino acid fragment, all cases demonstrated some degree of immunoreactivity throughout the cytoplasm of the tumor cells, but immunostaining was absent from inflammatory and stromal cells.9Furthermore, the intensity of the staining appeared to directly correlate with increasing tumor grade.9
Another study by Iwamura and colleagues suggested that PTHrP may play a significant role in the growth of prostate cancer by acting locally in an autocrine fashion.10 In this study, all prostate cancer cell lines from different sources expressed PTHrP immunoreactivity as well as evidence of DNA synthesis, the latter being measured by thymidine incorporation assay. Moreover, when these cells were incubated with various concentrations of mouse monoclonal antibody directed to PTHrP fragment, PTHrP-induced DNA synthesis was inhibited in a dose-dependent manner and almost completely neutralized at a specific concentration. Interestingly, the study demonstrated that cancer cell line derived from bone metastatic lesions secreted significantly greater amounts of PTHrP than did the cell line derived from the metastasis in the brain or in the lymph node. These findings suggest that PTHrP production may confer some advantage on the ability of prostate cancer cells to grow in bone.10
Ando and colleagues reported that neuroendocrine dedifferentiated prostate cancer can develop as a result of long-term ADT even after several years of therapy and has the potential to worsen and develop severe hypercalcemia.8 Neuron-specific enolase was used as the specific marker for the neuroendocrine cell, which suggested that the prostate cancer cell derived from the neuroendocrine cell might synthesize PTHrP and be responsible for the observed hypercalcemia.8
Other mechanisms cited for hypercalcemia of malignancy include other humoral factors associated with increased remodeling and comprise interleukin 1, 3, 6 (IL-1, IL-3, IL-6); tumor necrosis factor α; transforming growth factor A and B observed in metastatic bone lesions in breast cancer; lymphotoxin; E series prostaglandins; and macrophage inflammatory protein 1α seen in MM.
Local osteolytic hypercalcemia accounts for about 20% of cases and is usually associated with extensive bone metastases. It is most commonly seen in MM and metastatic breast cancer and less commonly in leukemia. The proposed mechanism is thought to be because of the release of local cytokines from the tumor, resulting in excess osteoclast activation and enhanced bone resorption often through RANK/RANKL interaction.
Extrarenal production of 1,25(OH)2D by the tumor accounts for about 1% of cases of hypercalcemia in malignancy. 1,25(OH)2D causes increased intestinal absorption of calcium and enhances osteolytic bone resorption, resulting in increased serum calcium. This mechanism is most commonly seen with Hodgkin and non-Hodgkin lymphoma and had been reported in ovarian dysgerminoma.7
In our patient, bone imaging showed osteoblastic lesions, a finding that likely contrasts the local osteolytic bone destruction theory. PTHrP was not significantly elevated in the serum, and PTH levels ruled out any form of primary hyperparathyroidism. In addition, histopathology showed no evidence of mosaicism or neuroendocrine dedifferentiation.
Findings in aggregate tell us that an exact pathophysiologic mechanism leading to hypercalcemia in prostate cancer is still unclear and may involve an interplay between growth factors and possible osteolytic materials, yet it must be studied thoroughly.
Conclusions
Hypercalcemia in pure metastatic adenocarcinoma of prostate is a rare finding and is of uncertain significance. Some studies suggested a search for unusual histopathologies, including neuroendocrine cancer and neuroendocrine dedifferentiation.8,11 However, in adenocarcinoma alone, it has an uncertain pathophysiology that needs to be further studied. Studies needed to investigate the role of PTHrP as a growth factor for both prostate cancer cells and development of hypercalcemia and possibly target-directed monoclonal antibody therapies may need to be extensively researched.
1. Gastanaga VM, Schwartzberg LS, Jain RK, et al. Prevalence of hypercalcemia among cancer patients in the United States. Cancer Med. 2016;5(8):2091‐2100. doi:10.1002/cam4.749
2. Grill V, Martin TJ. Hypercalcemia of malignancy. Rev Endocr Metab Disord. 2000;1(4):253‐263. doi:10.1023/a:1026597816193
3. Halabi S, Kelly WK, Ma H, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34(14):1652‐1659. doi:10.1200/JCO.2015.65.7270
4. Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(suppl 10):S13‐S18.
5. Kummar S, Fogarasi M, Canova A, Mota A, Ciesielski T. Cytokeratin 7 and 20 staining for the diagnosis of lung and colorectal adenocarcinoma. Br J Cancer. 2002;86(12):1884‐1887. doi:10.1038/sj.bjc.6600326
6. Avashia JH, Walsh TD, Thomas AJ Jr, Kaye M, Licata A. Metastatic carcinoma of the prostate with hypercalcemia [published correction appears in Cleve Clin J Med. 1991;58(3):284]. Cleve Clin J Med. 1990;57(7):636‐638. doi:10.3949/ccjm.57.7.636.
7. Goldner W. Cancer-related hypercalcemia. J Oncol Pract. 2016;12(5):426‐432. doi:10.1200/JOP.2016.011155.
8. Ando T, Watanabe K, Mizusawa T, Katagiri A. Hypercalcemia due to parathyroid hormone-related peptide secreted by neuroendocrine dedifferentiated prostate cancer. Urol Case Rep. 2018;22:67‐69. doi:10.1016/j.eucr.2018.11.001
9. Iwamura M, di Sant’Agnese PA, Wu G, et al. Immunohistochemical localization of parathyroid hormonerelated protein in human prostate cancer. Cancer Res. 1993;53(8):1724‐1726.
10. Iwamura M, Abrahamsson PA, Foss KA, Wu G, Cockett AT, Deftos LJ. Parathyroid hormone-related protein: a potential autocrine growth regulator in human prostate cancer cell lines. Urology. 1994;43(5):675‐679. doi:10.1016/0090-4295(94)90183-x
11. Smith DC, Tucker JA, Trump DL. Hypercalcemia and neuroendocrine carcinoma of the prostate: a report of three cases and a review of the literature. J Clin Oncol. 1992;10(3):499‐505. doi:10.1200/JCO.1992.10.3.499.
1. Gastanaga VM, Schwartzberg LS, Jain RK, et al. Prevalence of hypercalcemia among cancer patients in the United States. Cancer Med. 2016;5(8):2091‐2100. doi:10.1002/cam4.749
2. Grill V, Martin TJ. Hypercalcemia of malignancy. Rev Endocr Metab Disord. 2000;1(4):253‐263. doi:10.1023/a:1026597816193
3. Halabi S, Kelly WK, Ma H, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34(14):1652‐1659. doi:10.1200/JCO.2015.65.7270
4. Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(suppl 10):S13‐S18.
5. Kummar S, Fogarasi M, Canova A, Mota A, Ciesielski T. Cytokeratin 7 and 20 staining for the diagnosis of lung and colorectal adenocarcinoma. Br J Cancer. 2002;86(12):1884‐1887. doi:10.1038/sj.bjc.6600326
6. Avashia JH, Walsh TD, Thomas AJ Jr, Kaye M, Licata A. Metastatic carcinoma of the prostate with hypercalcemia [published correction appears in Cleve Clin J Med. 1991;58(3):284]. Cleve Clin J Med. 1990;57(7):636‐638. doi:10.3949/ccjm.57.7.636.
7. Goldner W. Cancer-related hypercalcemia. J Oncol Pract. 2016;12(5):426‐432. doi:10.1200/JOP.2016.011155.
8. Ando T, Watanabe K, Mizusawa T, Katagiri A. Hypercalcemia due to parathyroid hormone-related peptide secreted by neuroendocrine dedifferentiated prostate cancer. Urol Case Rep. 2018;22:67‐69. doi:10.1016/j.eucr.2018.11.001
9. Iwamura M, di Sant’Agnese PA, Wu G, et al. Immunohistochemical localization of parathyroid hormonerelated protein in human prostate cancer. Cancer Res. 1993;53(8):1724‐1726.
10. Iwamura M, Abrahamsson PA, Foss KA, Wu G, Cockett AT, Deftos LJ. Parathyroid hormone-related protein: a potential autocrine growth regulator in human prostate cancer cell lines. Urology. 1994;43(5):675‐679. doi:10.1016/0090-4295(94)90183-x
11. Smith DC, Tucker JA, Trump DL. Hypercalcemia and neuroendocrine carcinoma of the prostate: a report of three cases and a review of the literature. J Clin Oncol. 1992;10(3):499‐505. doi:10.1200/JCO.1992.10.3.499.
Combination probiotic formulations might improve outcomes in preterm infants
For preterm, low-birth-weight infants, probiotic formulations containing Lactobacillus and Bifidobacterium strains appear to be superior to single-strain probiotics and to other multiple-strain formulations for reducing the risk of all-cause mortality, according to the findings of a network meta-analysis of randomized clinical trials.
The results of a prior Cochrane review indicated that probiotics can help prevent severe necrotizing enterocolitis and all-cause mortality in preterm infants, but the most effective formulations remained unclear. Therefore, Rebecca L. Morgan, PhD, MPH, and her associates searched MEDLINE, EMBASE, Science Citation Index Expanded, CINAHL, Scopus, Cochrane CENTRAL, BIOSIS Previews, and Google Scholar through Jan. 1, 2019, to identify studies of single-strain and multistrain probiotic formulations in preterm, low-birth-weight neonates. A total of 63 studies involving 15,712 infants met inclusion criteria. “We used a frequentist approach for network meta-analysis and [a] GRADE approach to assess certainty of evidence,” they noted.
“High-certainty” evidence indicated that combination therapy with one or more Lactobacillus species and one or more Bifidobacterium species significantly reduced all-cause mortality, compared with placebo (odds ratio, 0.56; 95% confidence interval, 0.39-0.80), wrote Dr. Morgan, of McMaster University, Hamilton, Canada, and her coinvestigators. This was the only intervention to have moderate- or high-quality evidence for a reduction in mortality, the researchers wrote in Gastroenterology.
They added that, among the probiotic formulations with moderate- or high-quality evidence for efficacy, compared with placebo, those containing at least one species of Lactobacillus and at least one species of Bifidobacterium, and the single-strain probiotics containing Bifidobacterium animalis subspecies lactis, Lactobacillus reuteri, or Lactobacillus rhamnosus significantly reduced the risk of severe necrotizing enterocolitis (Bell stage II or higher), with statistically significant odds ratios of 0.35, 0.31, 0.55, and 0.44, respectively.
Three formulations were associated with “low-” or “very low-certainty” evidence for a reduction in risk for severe necrotizing enterocolitis, compared with placebo: Bacillus plus Enterococcus species, Lactobacillus plus Bifidobacterium plus Enterococcus species and Bifidobacterium plus Streptococcus salivarius subspecies thermophilus. Estimated odds ratios were 0.23 (risk difference, –4.9%), 0.28 (RD, –4.9%), and 0.38 (RD, –3.9%), respectively.
“The combinations of Bacillus species and Enterococcus species, and one or more Bifidobacterium species and S. salivarius subspecies thermophilus, might produce the largest reduction in necrotizing enterocolitis development,” the investigators wrote. “Further trials are needed.”
Compared with placebo, no probiotic formulation significantly improved the third primary outcome in the meta-analysis, culture-confirmed sepsis. However, several formulations were associated with moderate- or high-quality evidence for efficacy on secondary outcome measures. Compared with placebo, combinations of Lactobacillus and Bifidobacterium and Saccharomyces boulardii were associated with a significant decrease in the number of days to reach full feeding (mean reduction, 3.3 days; 95% CI, 5.9-0.7 days). Compared with placebo, single-strain therapy with B. animalis subspecies lactis or Lactobacillus reuteri was associated with a shorter duration of hospitalization, with mean reductions of 13.0 days (95% CI, 22.7-3.3 days) and 7.9 days (95% CI, 11.6-4.2 days), respectively.
“Multicenter and large randomized controlled trials should be prioritized to distinguish between the efficacy of single- and multiple-strain probiotics among preterm infants,” Dr. Morgan and her associates concluded. Such studies would further clarify the safety of probiotic formulations in this “fragile population,” they wrote. “Although the primary concern of live microbe administration, intestinal barrier translocation leading to sepsis, is decreased by several probiotic formulations, sound clinical judgement should be exercised.”
Partial support was provided by Mitacs Canada, in partnership with Nestlé Canada. The funder was not involved in designing or conducting the study or writing the manuscript. Dr. Morgan reported having no relevant conflicts of interest. One coinvestigator disclosed ties to AbbVie, Ferring, Janssen, and Takeda.
SOURCE: Morgan RL et al. Gastroenterology. 2020 Jun 24. doi: 10.1053/j.gastro.2020.05.096.
The demonstration of decreased risks of both death and necrotizing enterocolitis (NEC) in randomized placebo-controlled trials of probiotic microbes in very preterm babies is the most compelling case for administration of probiotics to date. Questions remain, including the optimal probiotic microbe(s) and dose for this population. The ideal studies would compare commercially available probiotic products and doses to each other (rather than to placebo). In the absence of these ideal studies, a network meta-analysis is a valuable tool to compare and rank multiple treatments. One of the drawbacks of a network meta-analysis is the assumption that all interventions have similar effects in all populations (an assumption that is challenging given the marked differences in the incidence of NEC between hospitals and populations).
The study conclusion that the combination of at least one Lactobacillus strain and at least one Bifidobacterium strain is most effective in preventing both death and NEC in very preterm infants is consistent with a previous network meta-analysis and with recent recommendations of the European Society for Paediatric Gastroenterology Hepatology and Nutrition and the American Gastroenterological Association.
Mark A. Underwood, MD, MAS, is a professor of pediatrics and chief of the division of neonatology in the department of pediatrics at the University of California, Davis. He has received honoraria from Abbott and conducted a clinical trial of probiotics that was funded by Evolve Biosystems.
The demonstration of decreased risks of both death and necrotizing enterocolitis (NEC) in randomized placebo-controlled trials of probiotic microbes in very preterm babies is the most compelling case for administration of probiotics to date. Questions remain, including the optimal probiotic microbe(s) and dose for this population. The ideal studies would compare commercially available probiotic products and doses to each other (rather than to placebo). In the absence of these ideal studies, a network meta-analysis is a valuable tool to compare and rank multiple treatments. One of the drawbacks of a network meta-analysis is the assumption that all interventions have similar effects in all populations (an assumption that is challenging given the marked differences in the incidence of NEC between hospitals and populations).
The study conclusion that the combination of at least one Lactobacillus strain and at least one Bifidobacterium strain is most effective in preventing both death and NEC in very preterm infants is consistent with a previous network meta-analysis and with recent recommendations of the European Society for Paediatric Gastroenterology Hepatology and Nutrition and the American Gastroenterological Association.
Mark A. Underwood, MD, MAS, is a professor of pediatrics and chief of the division of neonatology in the department of pediatrics at the University of California, Davis. He has received honoraria from Abbott and conducted a clinical trial of probiotics that was funded by Evolve Biosystems.
The demonstration of decreased risks of both death and necrotizing enterocolitis (NEC) in randomized placebo-controlled trials of probiotic microbes in very preterm babies is the most compelling case for administration of probiotics to date. Questions remain, including the optimal probiotic microbe(s) and dose for this population. The ideal studies would compare commercially available probiotic products and doses to each other (rather than to placebo). In the absence of these ideal studies, a network meta-analysis is a valuable tool to compare and rank multiple treatments. One of the drawbacks of a network meta-analysis is the assumption that all interventions have similar effects in all populations (an assumption that is challenging given the marked differences in the incidence of NEC between hospitals and populations).
The study conclusion that the combination of at least one Lactobacillus strain and at least one Bifidobacterium strain is most effective in preventing both death and NEC in very preterm infants is consistent with a previous network meta-analysis and with recent recommendations of the European Society for Paediatric Gastroenterology Hepatology and Nutrition and the American Gastroenterological Association.
Mark A. Underwood, MD, MAS, is a professor of pediatrics and chief of the division of neonatology in the department of pediatrics at the University of California, Davis. He has received honoraria from Abbott and conducted a clinical trial of probiotics that was funded by Evolve Biosystems.
For preterm, low-birth-weight infants, probiotic formulations containing Lactobacillus and Bifidobacterium strains appear to be superior to single-strain probiotics and to other multiple-strain formulations for reducing the risk of all-cause mortality, according to the findings of a network meta-analysis of randomized clinical trials.
The results of a prior Cochrane review indicated that probiotics can help prevent severe necrotizing enterocolitis and all-cause mortality in preterm infants, but the most effective formulations remained unclear. Therefore, Rebecca L. Morgan, PhD, MPH, and her associates searched MEDLINE, EMBASE, Science Citation Index Expanded, CINAHL, Scopus, Cochrane CENTRAL, BIOSIS Previews, and Google Scholar through Jan. 1, 2019, to identify studies of single-strain and multistrain probiotic formulations in preterm, low-birth-weight neonates. A total of 63 studies involving 15,712 infants met inclusion criteria. “We used a frequentist approach for network meta-analysis and [a] GRADE approach to assess certainty of evidence,” they noted.
“High-certainty” evidence indicated that combination therapy with one or more Lactobacillus species and one or more Bifidobacterium species significantly reduced all-cause mortality, compared with placebo (odds ratio, 0.56; 95% confidence interval, 0.39-0.80), wrote Dr. Morgan, of McMaster University, Hamilton, Canada, and her coinvestigators. This was the only intervention to have moderate- or high-quality evidence for a reduction in mortality, the researchers wrote in Gastroenterology.
They added that, among the probiotic formulations with moderate- or high-quality evidence for efficacy, compared with placebo, those containing at least one species of Lactobacillus and at least one species of Bifidobacterium, and the single-strain probiotics containing Bifidobacterium animalis subspecies lactis, Lactobacillus reuteri, or Lactobacillus rhamnosus significantly reduced the risk of severe necrotizing enterocolitis (Bell stage II or higher), with statistically significant odds ratios of 0.35, 0.31, 0.55, and 0.44, respectively.
Three formulations were associated with “low-” or “very low-certainty” evidence for a reduction in risk for severe necrotizing enterocolitis, compared with placebo: Bacillus plus Enterococcus species, Lactobacillus plus Bifidobacterium plus Enterococcus species and Bifidobacterium plus Streptococcus salivarius subspecies thermophilus. Estimated odds ratios were 0.23 (risk difference, –4.9%), 0.28 (RD, –4.9%), and 0.38 (RD, –3.9%), respectively.
“The combinations of Bacillus species and Enterococcus species, and one or more Bifidobacterium species and S. salivarius subspecies thermophilus, might produce the largest reduction in necrotizing enterocolitis development,” the investigators wrote. “Further trials are needed.”
Compared with placebo, no probiotic formulation significantly improved the third primary outcome in the meta-analysis, culture-confirmed sepsis. However, several formulations were associated with moderate- or high-quality evidence for efficacy on secondary outcome measures. Compared with placebo, combinations of Lactobacillus and Bifidobacterium and Saccharomyces boulardii were associated with a significant decrease in the number of days to reach full feeding (mean reduction, 3.3 days; 95% CI, 5.9-0.7 days). Compared with placebo, single-strain therapy with B. animalis subspecies lactis or Lactobacillus reuteri was associated with a shorter duration of hospitalization, with mean reductions of 13.0 days (95% CI, 22.7-3.3 days) and 7.9 days (95% CI, 11.6-4.2 days), respectively.
“Multicenter and large randomized controlled trials should be prioritized to distinguish between the efficacy of single- and multiple-strain probiotics among preterm infants,” Dr. Morgan and her associates concluded. Such studies would further clarify the safety of probiotic formulations in this “fragile population,” they wrote. “Although the primary concern of live microbe administration, intestinal barrier translocation leading to sepsis, is decreased by several probiotic formulations, sound clinical judgement should be exercised.”
Partial support was provided by Mitacs Canada, in partnership with Nestlé Canada. The funder was not involved in designing or conducting the study or writing the manuscript. Dr. Morgan reported having no relevant conflicts of interest. One coinvestigator disclosed ties to AbbVie, Ferring, Janssen, and Takeda.
SOURCE: Morgan RL et al. Gastroenterology. 2020 Jun 24. doi: 10.1053/j.gastro.2020.05.096.
For preterm, low-birth-weight infants, probiotic formulations containing Lactobacillus and Bifidobacterium strains appear to be superior to single-strain probiotics and to other multiple-strain formulations for reducing the risk of all-cause mortality, according to the findings of a network meta-analysis of randomized clinical trials.
The results of a prior Cochrane review indicated that probiotics can help prevent severe necrotizing enterocolitis and all-cause mortality in preterm infants, but the most effective formulations remained unclear. Therefore, Rebecca L. Morgan, PhD, MPH, and her associates searched MEDLINE, EMBASE, Science Citation Index Expanded, CINAHL, Scopus, Cochrane CENTRAL, BIOSIS Previews, and Google Scholar through Jan. 1, 2019, to identify studies of single-strain and multistrain probiotic formulations in preterm, low-birth-weight neonates. A total of 63 studies involving 15,712 infants met inclusion criteria. “We used a frequentist approach for network meta-analysis and [a] GRADE approach to assess certainty of evidence,” they noted.
“High-certainty” evidence indicated that combination therapy with one or more Lactobacillus species and one or more Bifidobacterium species significantly reduced all-cause mortality, compared with placebo (odds ratio, 0.56; 95% confidence interval, 0.39-0.80), wrote Dr. Morgan, of McMaster University, Hamilton, Canada, and her coinvestigators. This was the only intervention to have moderate- or high-quality evidence for a reduction in mortality, the researchers wrote in Gastroenterology.
They added that, among the probiotic formulations with moderate- or high-quality evidence for efficacy, compared with placebo, those containing at least one species of Lactobacillus and at least one species of Bifidobacterium, and the single-strain probiotics containing Bifidobacterium animalis subspecies lactis, Lactobacillus reuteri, or Lactobacillus rhamnosus significantly reduced the risk of severe necrotizing enterocolitis (Bell stage II or higher), with statistically significant odds ratios of 0.35, 0.31, 0.55, and 0.44, respectively.
Three formulations were associated with “low-” or “very low-certainty” evidence for a reduction in risk for severe necrotizing enterocolitis, compared with placebo: Bacillus plus Enterococcus species, Lactobacillus plus Bifidobacterium plus Enterococcus species and Bifidobacterium plus Streptococcus salivarius subspecies thermophilus. Estimated odds ratios were 0.23 (risk difference, –4.9%), 0.28 (RD, –4.9%), and 0.38 (RD, –3.9%), respectively.
“The combinations of Bacillus species and Enterococcus species, and one or more Bifidobacterium species and S. salivarius subspecies thermophilus, might produce the largest reduction in necrotizing enterocolitis development,” the investigators wrote. “Further trials are needed.”
Compared with placebo, no probiotic formulation significantly improved the third primary outcome in the meta-analysis, culture-confirmed sepsis. However, several formulations were associated with moderate- or high-quality evidence for efficacy on secondary outcome measures. Compared with placebo, combinations of Lactobacillus and Bifidobacterium and Saccharomyces boulardii were associated with a significant decrease in the number of days to reach full feeding (mean reduction, 3.3 days; 95% CI, 5.9-0.7 days). Compared with placebo, single-strain therapy with B. animalis subspecies lactis or Lactobacillus reuteri was associated with a shorter duration of hospitalization, with mean reductions of 13.0 days (95% CI, 22.7-3.3 days) and 7.9 days (95% CI, 11.6-4.2 days), respectively.
“Multicenter and large randomized controlled trials should be prioritized to distinguish between the efficacy of single- and multiple-strain probiotics among preterm infants,” Dr. Morgan and her associates concluded. Such studies would further clarify the safety of probiotic formulations in this “fragile population,” they wrote. “Although the primary concern of live microbe administration, intestinal barrier translocation leading to sepsis, is decreased by several probiotic formulations, sound clinical judgement should be exercised.”
Partial support was provided by Mitacs Canada, in partnership with Nestlé Canada. The funder was not involved in designing or conducting the study or writing the manuscript. Dr. Morgan reported having no relevant conflicts of interest. One coinvestigator disclosed ties to AbbVie, Ferring, Janssen, and Takeda.
SOURCE: Morgan RL et al. Gastroenterology. 2020 Jun 24. doi: 10.1053/j.gastro.2020.05.096.
FROM GASTROENTEROLOGY
Delayed diagnoses seen in children during COVID-19
There were also nine deaths where delayed presentation was considered a contributing factor, resulting mainly from sepsis and malignancy.
By comparison, over the same 2-week period of the survey there were three child deaths from COVID-19 directly, according to senior study author Shamez Ladhani, MRCPCH, PhD, chair of the British Paediatric Surveillance Unit (BPSU), Royal College of Paediatrics and Child Health, London.
“The unintended consequences of COVID are far greater, in children, than the disease itself. The way we are trying to prevent this is causing more harm than the disease,” he lamented.
One-third of senior U.K. pediatric specialists who responded to the survey reported dealing with so-called emergency delayed presentations in children who they would normally have expected to present much earlier.
After diabetes, the most commonly reported delayed diagnoses were sepsis and child protection issues. Cancer also featured prominently.
“We’ve found that there is great concern that children are not accessing healthcare as they should during lockdown and after,” Dr. Ladhani stressed. “Our emergency departments saw a 50% reduction during the peak, and now it is still 40% less than expected. The problem is improving but it remains.”
The survey findings were recently published online in Archives of Disease in Childhood, by first author Richard M. Lynn, MSc, of the Institute of Child Health, department of epidemiology and public health, University College London Research, and colleagues.
New diabetes cases presented very late during lockdown
Over the 2-week reporting period in mid-April 2020, type 1 diabetes was the most frequently reported delayed diagnosis, with 44 cases overall, 23 of which involved diabetic ketoacidosis.
“If you talk to the diabetes specialists, they tell us that generally, most cases of new diabetes arrive late because it has very nonspecific symptoms,” Dr. Ladhani explained.
However, he added, “pediatricians on the frontline know what to expect with diabetes. Those children who would have come in late prior to the pandemic are now arriving very late. Those consultants surveyed were not junior doctors but consultant pediatricians with many years of experience.”
In a recent article looking at pediatric delayed presentations, one patient with diabetes entered intensive care, and the BPSU report recorded one death possibly associated with diabetes, Dr. Ladhani pointed out.
“Pediatricians are worried that children are coming in late. We need to raise awareness that parents need to access healthcare and this message needs to go out now,” he said. “We can’t wait until a second wave. It has to be now because A&E [accident and emergency] attendance is still 40% [lower than] ... expected.”
BPSU survey covers over 90% of pediatricians in U.K. and Ireland
After numerous anecdotal reports of delayed presentations in the United Kingdom and abroad, the snapshot survey was conducted as part of routine monthly reports where pediatricians are asked to document any cases of rare conditions seen.
“We had heard stories of delayed presentations, but we wanted to know was this a real problem or just anecdotal?” Dr. Ladhani said.
The regular BPSU survey covers over 90% of U.K.- and Ireland-based pediatric consultants (numbering 4,075). On the back of this established communication, the BPSU decided to gauge the extent of delayed presentations during the peak weeks of the COVID-19 pandemic.
Over the next 7 days, 2,433 pediatricians, representing 60% of BPSU participants, responded.
“This response rate in 7 days highlights the importance given to the survey by pediatricians ... and the widespread professional concern about delayed presentations,” the authors wrote.
Participants were asked whether they had seen any children during the previous 14 days who, in their opinion, presented later than they would have expected prior to the COVID-19 pandemic.
“There’s no one definition for this but these senior clinicians know when something is unusual,” said Dr. Ladhani.
ED attendances were compared with figures for the same period last year. Overall, a total of 32% of 752 pediatricians working in EDs and pediatric assessment units reported witnessing delayed presentations, with 57 (8%) reporting at least three patients with delayed presentation.
“It was clear that those doctors on the frontline were seeing a lot of delayed presentations. Also, neonatologists reported women arriving late for labor, and community physicians said they just weren’t witnessing child protection cases anymore,” added Dr. Ladhani.
Other issues included early discharges following births because of COVID-19 concerns, before feeding had been established, prompting return visits because of feeding problems and dehydration.
The top five delayed diagnoses were diabetes (n = 44), sepsis (n = 21), child protection (n = 14), malignancy (n = 8), and appendicitis (n = 6). There were 10 delayed perinatal presentations.
Of the nine deaths, for which delayed presentation was considered to play a role, three were caused by sepsis, three were caused by new malignancy diagnoses, one was caused by new diagnosis of metabolic disease, and two did not have the cause reported.
The delays in presentation are likely to have been influenced by the U.K. government’s message to “stay at home” during the strict lockdown period, which perhaps was sometimes interpreted too literally, Dr. Ladhani suggested. “It was the right message socially, but not medically.”
Russell Viner, MB, PhD, president of the Royal College of Paediatrics and Child Health, said in a statement: “The impact for children is what we call ‘collateral damage’, including long absences from school and delays or interruptions to vital services. We know that parents adhered very strongly to the ‘stay at home’ [message] and we need to say clearly that this doesn’t apply if your child is very sick. Should we experience a second wave or regional outbreaks, it is vital that we get the message out to parents that we want to see unwell children at the earliest possible stage.”
Dr. Ladhani reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
There were also nine deaths where delayed presentation was considered a contributing factor, resulting mainly from sepsis and malignancy.
By comparison, over the same 2-week period of the survey there were three child deaths from COVID-19 directly, according to senior study author Shamez Ladhani, MRCPCH, PhD, chair of the British Paediatric Surveillance Unit (BPSU), Royal College of Paediatrics and Child Health, London.
“The unintended consequences of COVID are far greater, in children, than the disease itself. The way we are trying to prevent this is causing more harm than the disease,” he lamented.
One-third of senior U.K. pediatric specialists who responded to the survey reported dealing with so-called emergency delayed presentations in children who they would normally have expected to present much earlier.
After diabetes, the most commonly reported delayed diagnoses were sepsis and child protection issues. Cancer also featured prominently.
“We’ve found that there is great concern that children are not accessing healthcare as they should during lockdown and after,” Dr. Ladhani stressed. “Our emergency departments saw a 50% reduction during the peak, and now it is still 40% less than expected. The problem is improving but it remains.”
The survey findings were recently published online in Archives of Disease in Childhood, by first author Richard M. Lynn, MSc, of the Institute of Child Health, department of epidemiology and public health, University College London Research, and colleagues.
New diabetes cases presented very late during lockdown
Over the 2-week reporting period in mid-April 2020, type 1 diabetes was the most frequently reported delayed diagnosis, with 44 cases overall, 23 of which involved diabetic ketoacidosis.
“If you talk to the diabetes specialists, they tell us that generally, most cases of new diabetes arrive late because it has very nonspecific symptoms,” Dr. Ladhani explained.
However, he added, “pediatricians on the frontline know what to expect with diabetes. Those children who would have come in late prior to the pandemic are now arriving very late. Those consultants surveyed were not junior doctors but consultant pediatricians with many years of experience.”
In a recent article looking at pediatric delayed presentations, one patient with diabetes entered intensive care, and the BPSU report recorded one death possibly associated with diabetes, Dr. Ladhani pointed out.
“Pediatricians are worried that children are coming in late. We need to raise awareness that parents need to access healthcare and this message needs to go out now,” he said. “We can’t wait until a second wave. It has to be now because A&E [accident and emergency] attendance is still 40% [lower than] ... expected.”
BPSU survey covers over 90% of pediatricians in U.K. and Ireland
After numerous anecdotal reports of delayed presentations in the United Kingdom and abroad, the snapshot survey was conducted as part of routine monthly reports where pediatricians are asked to document any cases of rare conditions seen.
“We had heard stories of delayed presentations, but we wanted to know was this a real problem or just anecdotal?” Dr. Ladhani said.
The regular BPSU survey covers over 90% of U.K.- and Ireland-based pediatric consultants (numbering 4,075). On the back of this established communication, the BPSU decided to gauge the extent of delayed presentations during the peak weeks of the COVID-19 pandemic.
Over the next 7 days, 2,433 pediatricians, representing 60% of BPSU participants, responded.
“This response rate in 7 days highlights the importance given to the survey by pediatricians ... and the widespread professional concern about delayed presentations,” the authors wrote.
Participants were asked whether they had seen any children during the previous 14 days who, in their opinion, presented later than they would have expected prior to the COVID-19 pandemic.
“There’s no one definition for this but these senior clinicians know when something is unusual,” said Dr. Ladhani.
ED attendances were compared with figures for the same period last year. Overall, a total of 32% of 752 pediatricians working in EDs and pediatric assessment units reported witnessing delayed presentations, with 57 (8%) reporting at least three patients with delayed presentation.
“It was clear that those doctors on the frontline were seeing a lot of delayed presentations. Also, neonatologists reported women arriving late for labor, and community physicians said they just weren’t witnessing child protection cases anymore,” added Dr. Ladhani.
Other issues included early discharges following births because of COVID-19 concerns, before feeding had been established, prompting return visits because of feeding problems and dehydration.
The top five delayed diagnoses were diabetes (n = 44), sepsis (n = 21), child protection (n = 14), malignancy (n = 8), and appendicitis (n = 6). There were 10 delayed perinatal presentations.
Of the nine deaths, for which delayed presentation was considered to play a role, three were caused by sepsis, three were caused by new malignancy diagnoses, one was caused by new diagnosis of metabolic disease, and two did not have the cause reported.
The delays in presentation are likely to have been influenced by the U.K. government’s message to “stay at home” during the strict lockdown period, which perhaps was sometimes interpreted too literally, Dr. Ladhani suggested. “It was the right message socially, but not medically.”
Russell Viner, MB, PhD, president of the Royal College of Paediatrics and Child Health, said in a statement: “The impact for children is what we call ‘collateral damage’, including long absences from school and delays or interruptions to vital services. We know that parents adhered very strongly to the ‘stay at home’ [message] and we need to say clearly that this doesn’t apply if your child is very sick. Should we experience a second wave or regional outbreaks, it is vital that we get the message out to parents that we want to see unwell children at the earliest possible stage.”
Dr. Ladhani reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
There were also nine deaths where delayed presentation was considered a contributing factor, resulting mainly from sepsis and malignancy.
By comparison, over the same 2-week period of the survey there were three child deaths from COVID-19 directly, according to senior study author Shamez Ladhani, MRCPCH, PhD, chair of the British Paediatric Surveillance Unit (BPSU), Royal College of Paediatrics and Child Health, London.
“The unintended consequences of COVID are far greater, in children, than the disease itself. The way we are trying to prevent this is causing more harm than the disease,” he lamented.
One-third of senior U.K. pediatric specialists who responded to the survey reported dealing with so-called emergency delayed presentations in children who they would normally have expected to present much earlier.
After diabetes, the most commonly reported delayed diagnoses were sepsis and child protection issues. Cancer also featured prominently.
“We’ve found that there is great concern that children are not accessing healthcare as they should during lockdown and after,” Dr. Ladhani stressed. “Our emergency departments saw a 50% reduction during the peak, and now it is still 40% less than expected. The problem is improving but it remains.”
The survey findings were recently published online in Archives of Disease in Childhood, by first author Richard M. Lynn, MSc, of the Institute of Child Health, department of epidemiology and public health, University College London Research, and colleagues.
New diabetes cases presented very late during lockdown
Over the 2-week reporting period in mid-April 2020, type 1 diabetes was the most frequently reported delayed diagnosis, with 44 cases overall, 23 of which involved diabetic ketoacidosis.
“If you talk to the diabetes specialists, they tell us that generally, most cases of new diabetes arrive late because it has very nonspecific symptoms,” Dr. Ladhani explained.
However, he added, “pediatricians on the frontline know what to expect with diabetes. Those children who would have come in late prior to the pandemic are now arriving very late. Those consultants surveyed were not junior doctors but consultant pediatricians with many years of experience.”
In a recent article looking at pediatric delayed presentations, one patient with diabetes entered intensive care, and the BPSU report recorded one death possibly associated with diabetes, Dr. Ladhani pointed out.
“Pediatricians are worried that children are coming in late. We need to raise awareness that parents need to access healthcare and this message needs to go out now,” he said. “We can’t wait until a second wave. It has to be now because A&E [accident and emergency] attendance is still 40% [lower than] ... expected.”
BPSU survey covers over 90% of pediatricians in U.K. and Ireland
After numerous anecdotal reports of delayed presentations in the United Kingdom and abroad, the snapshot survey was conducted as part of routine monthly reports where pediatricians are asked to document any cases of rare conditions seen.
“We had heard stories of delayed presentations, but we wanted to know was this a real problem or just anecdotal?” Dr. Ladhani said.
The regular BPSU survey covers over 90% of U.K.- and Ireland-based pediatric consultants (numbering 4,075). On the back of this established communication, the BPSU decided to gauge the extent of delayed presentations during the peak weeks of the COVID-19 pandemic.
Over the next 7 days, 2,433 pediatricians, representing 60% of BPSU participants, responded.
“This response rate in 7 days highlights the importance given to the survey by pediatricians ... and the widespread professional concern about delayed presentations,” the authors wrote.
Participants were asked whether they had seen any children during the previous 14 days who, in their opinion, presented later than they would have expected prior to the COVID-19 pandemic.
“There’s no one definition for this but these senior clinicians know when something is unusual,” said Dr. Ladhani.
ED attendances were compared with figures for the same period last year. Overall, a total of 32% of 752 pediatricians working in EDs and pediatric assessment units reported witnessing delayed presentations, with 57 (8%) reporting at least three patients with delayed presentation.
“It was clear that those doctors on the frontline were seeing a lot of delayed presentations. Also, neonatologists reported women arriving late for labor, and community physicians said they just weren’t witnessing child protection cases anymore,” added Dr. Ladhani.
Other issues included early discharges following births because of COVID-19 concerns, before feeding had been established, prompting return visits because of feeding problems and dehydration.
The top five delayed diagnoses were diabetes (n = 44), sepsis (n = 21), child protection (n = 14), malignancy (n = 8), and appendicitis (n = 6). There were 10 delayed perinatal presentations.
Of the nine deaths, for which delayed presentation was considered to play a role, three were caused by sepsis, three were caused by new malignancy diagnoses, one was caused by new diagnosis of metabolic disease, and two did not have the cause reported.
The delays in presentation are likely to have been influenced by the U.K. government’s message to “stay at home” during the strict lockdown period, which perhaps was sometimes interpreted too literally, Dr. Ladhani suggested. “It was the right message socially, but not medically.”
Russell Viner, MB, PhD, president of the Royal College of Paediatrics and Child Health, said in a statement: “The impact for children is what we call ‘collateral damage’, including long absences from school and delays or interruptions to vital services. We know that parents adhered very strongly to the ‘stay at home’ [message] and we need to say clearly that this doesn’t apply if your child is very sick. Should we experience a second wave or regional outbreaks, it is vital that we get the message out to parents that we want to see unwell children at the earliest possible stage.”
Dr. Ladhani reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Primary prevention statins cut mortality even in the very elderly: VHA study
Patients in the Veterans Health Administration (VHA) system 75 years or older, free of cardiovascular (CV) disease and prescribed statins for the first time, had a one-fourth lower risk for death and a 20% lower risk for CV death over an average 7 years than that of comparable patients not prescribed the drugs in an observational study.
Ariela R. Orkaby, MD, MPH, lead author on the study, published in the July 7 issue of JAMA, said in an interview.
The very elderly are frequently undertreated, particularly in primary prevention, as many physicians consider it unnecessary for them to initiate or continue preventive measures, said Dr. Orkaby, of VA Boston Healthcare System and Harvard Medical School, Boston.
“From available data, we don’t really expect statins to start providing benefit in primary prevention until they’ve been taken for about 2 to 5 years. So for people who have very limited life expectancy, it may not be a great idea to add to their pill burden or increase the possibility that they might decline functionally,” Dr. Orkaby said.
“But what we saw in this study is that there is benefit to prescribing statins even in elderly patients, even within 2 years” of follow-up.
Despite being among the most studied drugs in the world, statins are understudied in older people. Fewer than 2% of the 186,854 participants in 28 statin trials were aged 75 years or older, wrote Dr. Orkaby and associates.
Most of what is known about initiating statin therapy in the 75-and-older age group comes from underpowered subgroup analyses and a few observational studies, Steven J. Nicholls, MBBS, PhD, Monash University, Melbourne, and Adam J. Nelson, MBBS, PhD, Duke Clinical Research Institute, Durham, N.C., wrote in an accompanying editorial. As a result, the evidence is conflicting, with some reports suggesting marked benefit and others possible harm.
The current findings, they wrote, “provide additional support for treatment guidelines that have increasingly advocated for more widespread use of statin therapy for ASCVD prevention in older individuals.”
Of the 326,981 people in the analysis, 57,178 (17.5%) were new statin users or initiated a statin during the study period, usually simvastatin. Their mean age was about 81 years, and 97.3% of the patients were men, 90% were white, and 72% were former smokers.
Using propensity scoring, the authors compared statin users with the other remaining patients who had the same likelihood of being prescribed a statin based on clinical characteristics but did not receive a prescription for a statin.
Michael W. Rich, MD, Washington University, St. Louis, who was not involved in the study but has previously worked with Dr. Orkaby, praised the analysis.
“It’s one of the best studies I’ve seen addressing this particular issue. It’s a large sample size, the analysis was very well done, and I think that it comes to a pretty unequivocal conclusion that, at least in this population, those individuals who were started on statins for the first time, and having no known prior ASCVD, clearly had a lower all-cause mortality and cardiovascular mortality, as well as a lower risk of composite cardiovascular events,” he said in an interview.
But the data have limitations, he added. The findings are still observational and could be confounded by unknown variables, and the select population – mostly white, male veterans – is known to be at somewhat higher risk for events than the general population.
Perhaps even more impressive than the risk reductions seen at a mean 6.8 years of follow-up, Dr. Rich said, are the sensitivity analyses at 2, 4, and 6 years that showed the benefit manifesting early.
The researchers saw a 32% reduction in all-cause mortality risk (P < .05) at 2 years, 21% at 4 years, and 13% at 6 years (P < .05 for all). Risk reductions for CV death followed a similar pattern, they wrote.
Dr. Rich said that the trial, although not a “slam dunk,” has persuaded him to shift from being very conservative about prescribing statins to elderly patients to being much more willing to consider it.
“This doesn’t mean that I will be running to routinely prescribe my 90-plus patients a statin, nor should we should be starting statins in everyone over 75, not even in all male former smokers over 75 – the type of people in this study – but I do think that it provides a stronger basis for talking to these patients about the possibility of starting a statin.”
There are two ongoing trials that may provide greater clarity, the authors observed. The STAREE trial has enrolled adults 70 years and older in Australia and includes serial evaluation of cognitive scores. Also, PREVENTABLE will examine the role of statins for prevention of dementia and disability-free survival in adults 75 years and older.
However, neither trial may fully resolve the question of primary prevention statin use in the elderly, they wrote. “While these trials are necessary to broaden the evidence base for older adults, it is unlikely that any trial will enroll large numbers of individuals at very advanced ages, black individuals, and those with dementia, as were included in this study.”
Dr. Orkaby had no disclosures; potential conflicts for the other authors are in the report. Dr. Rich reported having no conflicts of interest. Dr. Nicholls disclosed receiving research support from AstraZeneca, Amgen, Anthera, Eli Lilly, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi-Regeneron, and LipoScience; and receiving consulting fees or honoraria from AstraZeneca, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, and Boehringer Ingelheim. Dr. Nelson had no disclosures.
A version of this article originally appeared on Medscape.com.
Patients in the Veterans Health Administration (VHA) system 75 years or older, free of cardiovascular (CV) disease and prescribed statins for the first time, had a one-fourth lower risk for death and a 20% lower risk for CV death over an average 7 years than that of comparable patients not prescribed the drugs in an observational study.
Ariela R. Orkaby, MD, MPH, lead author on the study, published in the July 7 issue of JAMA, said in an interview.
The very elderly are frequently undertreated, particularly in primary prevention, as many physicians consider it unnecessary for them to initiate or continue preventive measures, said Dr. Orkaby, of VA Boston Healthcare System and Harvard Medical School, Boston.
“From available data, we don’t really expect statins to start providing benefit in primary prevention until they’ve been taken for about 2 to 5 years. So for people who have very limited life expectancy, it may not be a great idea to add to their pill burden or increase the possibility that they might decline functionally,” Dr. Orkaby said.
“But what we saw in this study is that there is benefit to prescribing statins even in elderly patients, even within 2 years” of follow-up.
Despite being among the most studied drugs in the world, statins are understudied in older people. Fewer than 2% of the 186,854 participants in 28 statin trials were aged 75 years or older, wrote Dr. Orkaby and associates.
Most of what is known about initiating statin therapy in the 75-and-older age group comes from underpowered subgroup analyses and a few observational studies, Steven J. Nicholls, MBBS, PhD, Monash University, Melbourne, and Adam J. Nelson, MBBS, PhD, Duke Clinical Research Institute, Durham, N.C., wrote in an accompanying editorial. As a result, the evidence is conflicting, with some reports suggesting marked benefit and others possible harm.
The current findings, they wrote, “provide additional support for treatment guidelines that have increasingly advocated for more widespread use of statin therapy for ASCVD prevention in older individuals.”
Of the 326,981 people in the analysis, 57,178 (17.5%) were new statin users or initiated a statin during the study period, usually simvastatin. Their mean age was about 81 years, and 97.3% of the patients were men, 90% were white, and 72% were former smokers.
Using propensity scoring, the authors compared statin users with the other remaining patients who had the same likelihood of being prescribed a statin based on clinical characteristics but did not receive a prescription for a statin.
Michael W. Rich, MD, Washington University, St. Louis, who was not involved in the study but has previously worked with Dr. Orkaby, praised the analysis.
“It’s one of the best studies I’ve seen addressing this particular issue. It’s a large sample size, the analysis was very well done, and I think that it comes to a pretty unequivocal conclusion that, at least in this population, those individuals who were started on statins for the first time, and having no known prior ASCVD, clearly had a lower all-cause mortality and cardiovascular mortality, as well as a lower risk of composite cardiovascular events,” he said in an interview.
But the data have limitations, he added. The findings are still observational and could be confounded by unknown variables, and the select population – mostly white, male veterans – is known to be at somewhat higher risk for events than the general population.
Perhaps even more impressive than the risk reductions seen at a mean 6.8 years of follow-up, Dr. Rich said, are the sensitivity analyses at 2, 4, and 6 years that showed the benefit manifesting early.
The researchers saw a 32% reduction in all-cause mortality risk (P < .05) at 2 years, 21% at 4 years, and 13% at 6 years (P < .05 for all). Risk reductions for CV death followed a similar pattern, they wrote.
Dr. Rich said that the trial, although not a “slam dunk,” has persuaded him to shift from being very conservative about prescribing statins to elderly patients to being much more willing to consider it.
“This doesn’t mean that I will be running to routinely prescribe my 90-plus patients a statin, nor should we should be starting statins in everyone over 75, not even in all male former smokers over 75 – the type of people in this study – but I do think that it provides a stronger basis for talking to these patients about the possibility of starting a statin.”
There are two ongoing trials that may provide greater clarity, the authors observed. The STAREE trial has enrolled adults 70 years and older in Australia and includes serial evaluation of cognitive scores. Also, PREVENTABLE will examine the role of statins for prevention of dementia and disability-free survival in adults 75 years and older.
However, neither trial may fully resolve the question of primary prevention statin use in the elderly, they wrote. “While these trials are necessary to broaden the evidence base for older adults, it is unlikely that any trial will enroll large numbers of individuals at very advanced ages, black individuals, and those with dementia, as were included in this study.”
Dr. Orkaby had no disclosures; potential conflicts for the other authors are in the report. Dr. Rich reported having no conflicts of interest. Dr. Nicholls disclosed receiving research support from AstraZeneca, Amgen, Anthera, Eli Lilly, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi-Regeneron, and LipoScience; and receiving consulting fees or honoraria from AstraZeneca, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, and Boehringer Ingelheim. Dr. Nelson had no disclosures.
A version of this article originally appeared on Medscape.com.
Patients in the Veterans Health Administration (VHA) system 75 years or older, free of cardiovascular (CV) disease and prescribed statins for the first time, had a one-fourth lower risk for death and a 20% lower risk for CV death over an average 7 years than that of comparable patients not prescribed the drugs in an observational study.
Ariela R. Orkaby, MD, MPH, lead author on the study, published in the July 7 issue of JAMA, said in an interview.
The very elderly are frequently undertreated, particularly in primary prevention, as many physicians consider it unnecessary for them to initiate or continue preventive measures, said Dr. Orkaby, of VA Boston Healthcare System and Harvard Medical School, Boston.
“From available data, we don’t really expect statins to start providing benefit in primary prevention until they’ve been taken for about 2 to 5 years. So for people who have very limited life expectancy, it may not be a great idea to add to their pill burden or increase the possibility that they might decline functionally,” Dr. Orkaby said.
“But what we saw in this study is that there is benefit to prescribing statins even in elderly patients, even within 2 years” of follow-up.
Despite being among the most studied drugs in the world, statins are understudied in older people. Fewer than 2% of the 186,854 participants in 28 statin trials were aged 75 years or older, wrote Dr. Orkaby and associates.
Most of what is known about initiating statin therapy in the 75-and-older age group comes from underpowered subgroup analyses and a few observational studies, Steven J. Nicholls, MBBS, PhD, Monash University, Melbourne, and Adam J. Nelson, MBBS, PhD, Duke Clinical Research Institute, Durham, N.C., wrote in an accompanying editorial. As a result, the evidence is conflicting, with some reports suggesting marked benefit and others possible harm.
The current findings, they wrote, “provide additional support for treatment guidelines that have increasingly advocated for more widespread use of statin therapy for ASCVD prevention in older individuals.”
Of the 326,981 people in the analysis, 57,178 (17.5%) were new statin users or initiated a statin during the study period, usually simvastatin. Their mean age was about 81 years, and 97.3% of the patients were men, 90% were white, and 72% were former smokers.
Using propensity scoring, the authors compared statin users with the other remaining patients who had the same likelihood of being prescribed a statin based on clinical characteristics but did not receive a prescription for a statin.
Michael W. Rich, MD, Washington University, St. Louis, who was not involved in the study but has previously worked with Dr. Orkaby, praised the analysis.
“It’s one of the best studies I’ve seen addressing this particular issue. It’s a large sample size, the analysis was very well done, and I think that it comes to a pretty unequivocal conclusion that, at least in this population, those individuals who were started on statins for the first time, and having no known prior ASCVD, clearly had a lower all-cause mortality and cardiovascular mortality, as well as a lower risk of composite cardiovascular events,” he said in an interview.
But the data have limitations, he added. The findings are still observational and could be confounded by unknown variables, and the select population – mostly white, male veterans – is known to be at somewhat higher risk for events than the general population.
Perhaps even more impressive than the risk reductions seen at a mean 6.8 years of follow-up, Dr. Rich said, are the sensitivity analyses at 2, 4, and 6 years that showed the benefit manifesting early.
The researchers saw a 32% reduction in all-cause mortality risk (P < .05) at 2 years, 21% at 4 years, and 13% at 6 years (P < .05 for all). Risk reductions for CV death followed a similar pattern, they wrote.
Dr. Rich said that the trial, although not a “slam dunk,” has persuaded him to shift from being very conservative about prescribing statins to elderly patients to being much more willing to consider it.
“This doesn’t mean that I will be running to routinely prescribe my 90-plus patients a statin, nor should we should be starting statins in everyone over 75, not even in all male former smokers over 75 – the type of people in this study – but I do think that it provides a stronger basis for talking to these patients about the possibility of starting a statin.”
There are two ongoing trials that may provide greater clarity, the authors observed. The STAREE trial has enrolled adults 70 years and older in Australia and includes serial evaluation of cognitive scores. Also, PREVENTABLE will examine the role of statins for prevention of dementia and disability-free survival in adults 75 years and older.
However, neither trial may fully resolve the question of primary prevention statin use in the elderly, they wrote. “While these trials are necessary to broaden the evidence base for older adults, it is unlikely that any trial will enroll large numbers of individuals at very advanced ages, black individuals, and those with dementia, as were included in this study.”
Dr. Orkaby had no disclosures; potential conflicts for the other authors are in the report. Dr. Rich reported having no conflicts of interest. Dr. Nicholls disclosed receiving research support from AstraZeneca, Amgen, Anthera, Eli Lilly, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi-Regeneron, and LipoScience; and receiving consulting fees or honoraria from AstraZeneca, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, and Boehringer Ingelheim. Dr. Nelson had no disclosures.
A version of this article originally appeared on Medscape.com.
Risky business: Longer-course prophylactic perioperative antimicrobials
Background: National guidelines recommend that surgical prophylactic antimicrobials be initiated within 1 hour prior to incision and discontinued 24 hours postoperatively. However, the risks and benefits of longer duration of antimicrobials are uncertain.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: After stratification by type of surgery and adjustment for covariates, antibiotic prophylaxis greater than 24 hours was not associated with lower SSI risk.
However, the odds of postoperative AKI increased with each additional day of prophylaxis (adjusted odds ratios, 1.82; 95% confidence interval,1.54-2.16 and aOR, 1.79; 95% CI, 1.27-2.53) with longer than 72 hours prophylaxis for cardiac and noncardiac surgery, respectively). Similarly, C. difficile infections increased with each additional day beyond 24 hours (aOR, 3.65; 95% CI, 2.40-5.55 with more than 72 hours of use).
Bottom line: Each day of perioperative antimicrobial prophylaxis beyond 24 hours increases the risk for postoperative AKI or C. difficile infection without reducing the risk of surgical site infection.
Citation: Branch-Elliman W et al. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 2019 Apr 24. doi: 10.1001/jamasurg.2019.0569.
Dr. Miller is a hospitalist at the University of Colorado at Denver, Aurora.
Background: National guidelines recommend that surgical prophylactic antimicrobials be initiated within 1 hour prior to incision and discontinued 24 hours postoperatively. However, the risks and benefits of longer duration of antimicrobials are uncertain.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: After stratification by type of surgery and adjustment for covariates, antibiotic prophylaxis greater than 24 hours was not associated with lower SSI risk.
However, the odds of postoperative AKI increased with each additional day of prophylaxis (adjusted odds ratios, 1.82; 95% confidence interval,1.54-2.16 and aOR, 1.79; 95% CI, 1.27-2.53) with longer than 72 hours prophylaxis for cardiac and noncardiac surgery, respectively). Similarly, C. difficile infections increased with each additional day beyond 24 hours (aOR, 3.65; 95% CI, 2.40-5.55 with more than 72 hours of use).
Bottom line: Each day of perioperative antimicrobial prophylaxis beyond 24 hours increases the risk for postoperative AKI or C. difficile infection without reducing the risk of surgical site infection.
Citation: Branch-Elliman W et al. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 2019 Apr 24. doi: 10.1001/jamasurg.2019.0569.
Dr. Miller is a hospitalist at the University of Colorado at Denver, Aurora.
Background: National guidelines recommend that surgical prophylactic antimicrobials be initiated within 1 hour prior to incision and discontinued 24 hours postoperatively. However, the risks and benefits of longer duration of antimicrobials are uncertain.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: After stratification by type of surgery and adjustment for covariates, antibiotic prophylaxis greater than 24 hours was not associated with lower SSI risk.
However, the odds of postoperative AKI increased with each additional day of prophylaxis (adjusted odds ratios, 1.82; 95% confidence interval,1.54-2.16 and aOR, 1.79; 95% CI, 1.27-2.53) with longer than 72 hours prophylaxis for cardiac and noncardiac surgery, respectively). Similarly, C. difficile infections increased with each additional day beyond 24 hours (aOR, 3.65; 95% CI, 2.40-5.55 with more than 72 hours of use).
Bottom line: Each day of perioperative antimicrobial prophylaxis beyond 24 hours increases the risk for postoperative AKI or C. difficile infection without reducing the risk of surgical site infection.
Citation: Branch-Elliman W et al. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 2019 Apr 24. doi: 10.1001/jamasurg.2019.0569.
Dr. Miller is a hospitalist at the University of Colorado at Denver, Aurora.