User login
Patients who refuse to wear masks: Responses that won’t get you sued
What do you do now?
Your waiting room is filled with mask-wearing individuals, except for one person. Your staff offers a mask to this person, citing your office policy of requiring masks for all persons in order to prevent asymptomatic COVID-19 spread, and the patient refuses to put it on.
What can you/should you/must you do? Are you required to see a patient who refuses to wear a mask? If you ask the patient to leave without being seen, can you be accused of patient abandonment? If you allow the patient to stay, could you be liable for negligence for exposing others to a deadly illness?
The rules on mask-wearing, while initially downright confusing, have inexorably come to a rough consensus. By governors’ orders, masks are now mandatory in most states, though when and where they are required varies. For example, effective July 7, the governor of Washington has ordered that a business not allow a customer to enter without a face covering.
Nor do we have case law to help us determine whether patient abandonment would apply if a patient is sent home without being seen.
We can apply the legal principles and cases from other situations to this one, however, to tell us what constitutes negligence or patient abandonment. The practical questions, legally, are who might sue and on what basis?
Who might sue?
Someone who is injured in a public place may sue the owner for negligence if the owner knew or should have known of a danger and didn’t do anything about it. For example, individuals have sued grocery stores successfully after they slipped on a banana peel and fell. If, say, the banana peel was black, that indicates that it had been there for a while, and judges have found that the store management should have known about it and removed it.
Compare the banana peel scenario with the scenario where most news outlets and health departments are telling people, every day, to wear masks while in indoor public spaces, yet owners of a medical practice or facility allow individuals who are not wearing masks to sit in their waiting room. If an individual who was also in the waiting room with the unmasked individual develops COVID-19 2 days later, the ill individual may sue the medical practice for negligence for not removing the unmasked individual.
What about the individual’s responsibility to move away from the person not wearing a mask? That is the aspect of this scenario that attorneys and experts could argue about, for days, in a court case. But to go back to the banana peel case, one could argue that a customer in a grocery store should be looking out for banana peels on the floor and avoid them, yet courts have assigned liability to grocery stores when customers slip and fall.
Let’s review the four elements of negligence which a plaintiff would need to prove:
- Duty: Obligation of one person to another
- Breach: Improper act or omission, in the context of proper behavior to avoid imposing undue risks of harm to other persons and their property
- Damage
- Causation: That the act or omission caused the harm
Those who run medical offices and facilities have a duty to provide reasonably safe public spaces. Unmasked individuals are a risk to others nearby, so the “breach” element is satisfied if a practice fails to impose safety measures. Causation could be proven, or at least inferred, if contact tracing of an individual with COVID-19 showed that the only contact likely to have exposed the ill individual to the virus was an unmasked individual in a medical practice’s waiting room, especially if the unmasked individual was COVID-19 positive before, during, or shortly after the visit to the practice.
What about patient abandonment?
“Patient abandonment” is the legal term for terminating the physician-patient relationship in such a manner that the patient is denied necessary medical care. It is a form of negligence.
Refusing to see a patient unless the patient wears a mask is not denying care, in this attorney’s view, but rather establishing reasonable conditions for getting care. The patient simply needs to put on a mask.
What about the patient who refuses to wear a mask for medical reasons? There are exceptions in most of the governors’ orders for individuals with medical conditions that preclude covering nose and mouth with a mask. A medical office is the perfect place to test an individual’s ability or inability to breathe well while wearing a mask. “Put the mask on and we’ll see how you do” is a reasonable response. Monitor the patient visually and apply a pulse oximeter with mask off and mask on.
One physician recently wrote about measuring her own oxygen levels while wearing four different masks for 5 minutes each, with no change in breathing.
Editor’s note: Read more about mask exemptions in a Medscape interview with pulmonologist Albert Rizzo, MD, chief medical officer of the American Lung Association.
What are some practical tips?
Assuming that a patient is not in acute distress, options in this scenario include:
- Send the patient home and offer a return visit if masked or when the pandemic is over.
- Offer a telehealth visit, with the patient at home.
What if the unmasked person is not a patient but the companion of a patient? What if the individual refusing to wear a mask is an employee? In neither of these two hypotheticals is there a basis for legal action against a practice whose policy requires that everyone wear masks on the premises.
A companion who arrives without a mask should leave the office. An employee who refuses to mask up could be sent home. If the employee has a disability covered by the Americans with Disabilities Act, then the practice may need to make reasonable accommodations so that the employee works in a room alone if unable to work from home.
Those who manage medical practices should check the websites of the state health department and medical societies at least weekly, to see whether the agencies have issued guidance. For example, the Texas Medical Association has issued limited guidance.
A version of this article originally appeared on Medscape.com.
What do you do now?
Your waiting room is filled with mask-wearing individuals, except for one person. Your staff offers a mask to this person, citing your office policy of requiring masks for all persons in order to prevent asymptomatic COVID-19 spread, and the patient refuses to put it on.
What can you/should you/must you do? Are you required to see a patient who refuses to wear a mask? If you ask the patient to leave without being seen, can you be accused of patient abandonment? If you allow the patient to stay, could you be liable for negligence for exposing others to a deadly illness?
The rules on mask-wearing, while initially downright confusing, have inexorably come to a rough consensus. By governors’ orders, masks are now mandatory in most states, though when and where they are required varies. For example, effective July 7, the governor of Washington has ordered that a business not allow a customer to enter without a face covering.
Nor do we have case law to help us determine whether patient abandonment would apply if a patient is sent home without being seen.
We can apply the legal principles and cases from other situations to this one, however, to tell us what constitutes negligence or patient abandonment. The practical questions, legally, are who might sue and on what basis?
Who might sue?
Someone who is injured in a public place may sue the owner for negligence if the owner knew or should have known of a danger and didn’t do anything about it. For example, individuals have sued grocery stores successfully after they slipped on a banana peel and fell. If, say, the banana peel was black, that indicates that it had been there for a while, and judges have found that the store management should have known about it and removed it.
Compare the banana peel scenario with the scenario where most news outlets and health departments are telling people, every day, to wear masks while in indoor public spaces, yet owners of a medical practice or facility allow individuals who are not wearing masks to sit in their waiting room. If an individual who was also in the waiting room with the unmasked individual develops COVID-19 2 days later, the ill individual may sue the medical practice for negligence for not removing the unmasked individual.
What about the individual’s responsibility to move away from the person not wearing a mask? That is the aspect of this scenario that attorneys and experts could argue about, for days, in a court case. But to go back to the banana peel case, one could argue that a customer in a grocery store should be looking out for banana peels on the floor and avoid them, yet courts have assigned liability to grocery stores when customers slip and fall.
Let’s review the four elements of negligence which a plaintiff would need to prove:
- Duty: Obligation of one person to another
- Breach: Improper act or omission, in the context of proper behavior to avoid imposing undue risks of harm to other persons and their property
- Damage
- Causation: That the act or omission caused the harm
Those who run medical offices and facilities have a duty to provide reasonably safe public spaces. Unmasked individuals are a risk to others nearby, so the “breach” element is satisfied if a practice fails to impose safety measures. Causation could be proven, or at least inferred, if contact tracing of an individual with COVID-19 showed that the only contact likely to have exposed the ill individual to the virus was an unmasked individual in a medical practice’s waiting room, especially if the unmasked individual was COVID-19 positive before, during, or shortly after the visit to the practice.
What about patient abandonment?
“Patient abandonment” is the legal term for terminating the physician-patient relationship in such a manner that the patient is denied necessary medical care. It is a form of negligence.
Refusing to see a patient unless the patient wears a mask is not denying care, in this attorney’s view, but rather establishing reasonable conditions for getting care. The patient simply needs to put on a mask.
What about the patient who refuses to wear a mask for medical reasons? There are exceptions in most of the governors’ orders for individuals with medical conditions that preclude covering nose and mouth with a mask. A medical office is the perfect place to test an individual’s ability or inability to breathe well while wearing a mask. “Put the mask on and we’ll see how you do” is a reasonable response. Monitor the patient visually and apply a pulse oximeter with mask off and mask on.
One physician recently wrote about measuring her own oxygen levels while wearing four different masks for 5 minutes each, with no change in breathing.
Editor’s note: Read more about mask exemptions in a Medscape interview with pulmonologist Albert Rizzo, MD, chief medical officer of the American Lung Association.
What are some practical tips?
Assuming that a patient is not in acute distress, options in this scenario include:
- Send the patient home and offer a return visit if masked or when the pandemic is over.
- Offer a telehealth visit, with the patient at home.
What if the unmasked person is not a patient but the companion of a patient? What if the individual refusing to wear a mask is an employee? In neither of these two hypotheticals is there a basis for legal action against a practice whose policy requires that everyone wear masks on the premises.
A companion who arrives without a mask should leave the office. An employee who refuses to mask up could be sent home. If the employee has a disability covered by the Americans with Disabilities Act, then the practice may need to make reasonable accommodations so that the employee works in a room alone if unable to work from home.
Those who manage medical practices should check the websites of the state health department and medical societies at least weekly, to see whether the agencies have issued guidance. For example, the Texas Medical Association has issued limited guidance.
A version of this article originally appeared on Medscape.com.
What do you do now?
Your waiting room is filled with mask-wearing individuals, except for one person. Your staff offers a mask to this person, citing your office policy of requiring masks for all persons in order to prevent asymptomatic COVID-19 spread, and the patient refuses to put it on.
What can you/should you/must you do? Are you required to see a patient who refuses to wear a mask? If you ask the patient to leave without being seen, can you be accused of patient abandonment? If you allow the patient to stay, could you be liable for negligence for exposing others to a deadly illness?
The rules on mask-wearing, while initially downright confusing, have inexorably come to a rough consensus. By governors’ orders, masks are now mandatory in most states, though when and where they are required varies. For example, effective July 7, the governor of Washington has ordered that a business not allow a customer to enter without a face covering.
Nor do we have case law to help us determine whether patient abandonment would apply if a patient is sent home without being seen.
We can apply the legal principles and cases from other situations to this one, however, to tell us what constitutes negligence or patient abandonment. The practical questions, legally, are who might sue and on what basis?
Who might sue?
Someone who is injured in a public place may sue the owner for negligence if the owner knew or should have known of a danger and didn’t do anything about it. For example, individuals have sued grocery stores successfully after they slipped on a banana peel and fell. If, say, the banana peel was black, that indicates that it had been there for a while, and judges have found that the store management should have known about it and removed it.
Compare the banana peel scenario with the scenario where most news outlets and health departments are telling people, every day, to wear masks while in indoor public spaces, yet owners of a medical practice or facility allow individuals who are not wearing masks to sit in their waiting room. If an individual who was also in the waiting room with the unmasked individual develops COVID-19 2 days later, the ill individual may sue the medical practice for negligence for not removing the unmasked individual.
What about the individual’s responsibility to move away from the person not wearing a mask? That is the aspect of this scenario that attorneys and experts could argue about, for days, in a court case. But to go back to the banana peel case, one could argue that a customer in a grocery store should be looking out for banana peels on the floor and avoid them, yet courts have assigned liability to grocery stores when customers slip and fall.
Let’s review the four elements of negligence which a plaintiff would need to prove:
- Duty: Obligation of one person to another
- Breach: Improper act or omission, in the context of proper behavior to avoid imposing undue risks of harm to other persons and their property
- Damage
- Causation: That the act or omission caused the harm
Those who run medical offices and facilities have a duty to provide reasonably safe public spaces. Unmasked individuals are a risk to others nearby, so the “breach” element is satisfied if a practice fails to impose safety measures. Causation could be proven, or at least inferred, if contact tracing of an individual with COVID-19 showed that the only contact likely to have exposed the ill individual to the virus was an unmasked individual in a medical practice’s waiting room, especially if the unmasked individual was COVID-19 positive before, during, or shortly after the visit to the practice.
What about patient abandonment?
“Patient abandonment” is the legal term for terminating the physician-patient relationship in such a manner that the patient is denied necessary medical care. It is a form of negligence.
Refusing to see a patient unless the patient wears a mask is not denying care, in this attorney’s view, but rather establishing reasonable conditions for getting care. The patient simply needs to put on a mask.
What about the patient who refuses to wear a mask for medical reasons? There are exceptions in most of the governors’ orders for individuals with medical conditions that preclude covering nose and mouth with a mask. A medical office is the perfect place to test an individual’s ability or inability to breathe well while wearing a mask. “Put the mask on and we’ll see how you do” is a reasonable response. Monitor the patient visually and apply a pulse oximeter with mask off and mask on.
One physician recently wrote about measuring her own oxygen levels while wearing four different masks for 5 minutes each, with no change in breathing.
Editor’s note: Read more about mask exemptions in a Medscape interview with pulmonologist Albert Rizzo, MD, chief medical officer of the American Lung Association.
What are some practical tips?
Assuming that a patient is not in acute distress, options in this scenario include:
- Send the patient home and offer a return visit if masked or when the pandemic is over.
- Offer a telehealth visit, with the patient at home.
What if the unmasked person is not a patient but the companion of a patient? What if the individual refusing to wear a mask is an employee? In neither of these two hypotheticals is there a basis for legal action against a practice whose policy requires that everyone wear masks on the premises.
A companion who arrives without a mask should leave the office. An employee who refuses to mask up could be sent home. If the employee has a disability covered by the Americans with Disabilities Act, then the practice may need to make reasonable accommodations so that the employee works in a room alone if unable to work from home.
Those who manage medical practices should check the websites of the state health department and medical societies at least weekly, to see whether the agencies have issued guidance. For example, the Texas Medical Association has issued limited guidance.
A version of this article originally appeared on Medscape.com.
Early childhood overweight, obesity tied to high cardiometabolic syndrome risk
Children who were overweight or obese at ages 2-3 years and at 6-7 years were significantly more likely than were healthy-weight children to show cardiometabolic risk factors at 11-12 years in a population-based study of more than 5,000 children.
Previous studies of the impact of childhood body mass index on cardiovascular disease have used a single BMI measurement, wrote Kate Lycett, PhD, of Deakin University, Victoria, Australia, and colleagues. “This overlooks the considerable physiologic changes in BMI throughout childhood as part of typical growth.”
In a study published in Pediatrics, the researchers examined overweight and obesity at five time points in a cohort of 5,107 infants by measuring BMI every 2 years between the ages of 2-3 years and 10-11 years.
Overall, children with consistently high BMI trajectories from age 3 years had the highest risk of metabolic syndrome. At age 6-7 years, overweight and obese children had, respectively, higher metabolic syndrome risk scores by 0.23 and 0.76 mean standard deviation (SD) units, compared with healthy-weight children; these associations approximately doubled by age 11-12 years.
In addition, obese children had higher pulse wave velocity (PWV) from age 6-7 years (0.64-0.73 standard deviation units) and slightly higher carotid artery intima-media thickness (cIMT) at all measured ages, compared with healthy-weight children (0.20-0.30 SD units).
The findings were limited by several factors, including the inability to evaluate the effects of BMI on actual cardiovascular disease because of the young age of the study population, the researchers noted.
However, the “results are in keeping with previous studies but provide additional important insights that suggest BMI from as early as 2 to 3 years of age is predictive of preclinical cardiometabolic phenotypes by ages 11 to 12 years,” Dr. Lycett and associates said. The results have implications for public health by highlighting the subclinical effects of obesity in childhood and the importance of early intervention, they concluded.
“This important and comprehensive study has two important implications: first, high BMI by age 2 to 3 tends to stay high, and second, normal BMI occasionally increases to high BMI, but the reverse is rarely true,” Sarah Armstrong, MD, Jennifer S. Li, MD, and Asheley C. Skinner, PhD, wrote in an accompanying editorial (Pediatrics. 2020 Jul 6. doi: 10.1542/peds.2020-1353).
noted the editorialists, who are affiliated with Duke University, Durham, N.C.
“An important caveat is that although the relationships were significant, the amount of variance attributable directly to child BMI was small,” which highlights the complex relationship between obesity and health, they noted.
“Early-onset obesity is unlikely to change and, if it persists, will lead to detectable precursors of atherosclerosis by the time a child enters middle school,” and parents and primary care providers have an opportunity to “flatten the curve” by addressing BMI increases early in life to delay or prevent obesity, the editorialists concluded.
The study was supported by Australia’s National Health and Medical Research Council, The Royal Children’s Hospital Foundation, Murdoch Children’s Research Institute, The University of Melbourne, National Heart Foundation of Australia, Financial Markets Foundation for Children, and Victorian Deaf Education Institute. A number of the researchers were supported by grants from these and other universities and organizations. The researchers had no relevant financial disclosures. The editorialists had no financial conflicts to disclose.
SOURCE: Lycett K et al. Pediatrics. 2020 Jul 6. doi: 10.1542/peds.2019-3666.
Children who were overweight or obese at ages 2-3 years and at 6-7 years were significantly more likely than were healthy-weight children to show cardiometabolic risk factors at 11-12 years in a population-based study of more than 5,000 children.
Previous studies of the impact of childhood body mass index on cardiovascular disease have used a single BMI measurement, wrote Kate Lycett, PhD, of Deakin University, Victoria, Australia, and colleagues. “This overlooks the considerable physiologic changes in BMI throughout childhood as part of typical growth.”
In a study published in Pediatrics, the researchers examined overweight and obesity at five time points in a cohort of 5,107 infants by measuring BMI every 2 years between the ages of 2-3 years and 10-11 years.
Overall, children with consistently high BMI trajectories from age 3 years had the highest risk of metabolic syndrome. At age 6-7 years, overweight and obese children had, respectively, higher metabolic syndrome risk scores by 0.23 and 0.76 mean standard deviation (SD) units, compared with healthy-weight children; these associations approximately doubled by age 11-12 years.
In addition, obese children had higher pulse wave velocity (PWV) from age 6-7 years (0.64-0.73 standard deviation units) and slightly higher carotid artery intima-media thickness (cIMT) at all measured ages, compared with healthy-weight children (0.20-0.30 SD units).
The findings were limited by several factors, including the inability to evaluate the effects of BMI on actual cardiovascular disease because of the young age of the study population, the researchers noted.
However, the “results are in keeping with previous studies but provide additional important insights that suggest BMI from as early as 2 to 3 years of age is predictive of preclinical cardiometabolic phenotypes by ages 11 to 12 years,” Dr. Lycett and associates said. The results have implications for public health by highlighting the subclinical effects of obesity in childhood and the importance of early intervention, they concluded.
“This important and comprehensive study has two important implications: first, high BMI by age 2 to 3 tends to stay high, and second, normal BMI occasionally increases to high BMI, but the reverse is rarely true,” Sarah Armstrong, MD, Jennifer S. Li, MD, and Asheley C. Skinner, PhD, wrote in an accompanying editorial (Pediatrics. 2020 Jul 6. doi: 10.1542/peds.2020-1353).
noted the editorialists, who are affiliated with Duke University, Durham, N.C.
“An important caveat is that although the relationships were significant, the amount of variance attributable directly to child BMI was small,” which highlights the complex relationship between obesity and health, they noted.
“Early-onset obesity is unlikely to change and, if it persists, will lead to detectable precursors of atherosclerosis by the time a child enters middle school,” and parents and primary care providers have an opportunity to “flatten the curve” by addressing BMI increases early in life to delay or prevent obesity, the editorialists concluded.
The study was supported by Australia’s National Health and Medical Research Council, The Royal Children’s Hospital Foundation, Murdoch Children’s Research Institute, The University of Melbourne, National Heart Foundation of Australia, Financial Markets Foundation for Children, and Victorian Deaf Education Institute. A number of the researchers were supported by grants from these and other universities and organizations. The researchers had no relevant financial disclosures. The editorialists had no financial conflicts to disclose.
SOURCE: Lycett K et al. Pediatrics. 2020 Jul 6. doi: 10.1542/peds.2019-3666.
Children who were overweight or obese at ages 2-3 years and at 6-7 years were significantly more likely than were healthy-weight children to show cardiometabolic risk factors at 11-12 years in a population-based study of more than 5,000 children.
Previous studies of the impact of childhood body mass index on cardiovascular disease have used a single BMI measurement, wrote Kate Lycett, PhD, of Deakin University, Victoria, Australia, and colleagues. “This overlooks the considerable physiologic changes in BMI throughout childhood as part of typical growth.”
In a study published in Pediatrics, the researchers examined overweight and obesity at five time points in a cohort of 5,107 infants by measuring BMI every 2 years between the ages of 2-3 years and 10-11 years.
Overall, children with consistently high BMI trajectories from age 3 years had the highest risk of metabolic syndrome. At age 6-7 years, overweight and obese children had, respectively, higher metabolic syndrome risk scores by 0.23 and 0.76 mean standard deviation (SD) units, compared with healthy-weight children; these associations approximately doubled by age 11-12 years.
In addition, obese children had higher pulse wave velocity (PWV) from age 6-7 years (0.64-0.73 standard deviation units) and slightly higher carotid artery intima-media thickness (cIMT) at all measured ages, compared with healthy-weight children (0.20-0.30 SD units).
The findings were limited by several factors, including the inability to evaluate the effects of BMI on actual cardiovascular disease because of the young age of the study population, the researchers noted.
However, the “results are in keeping with previous studies but provide additional important insights that suggest BMI from as early as 2 to 3 years of age is predictive of preclinical cardiometabolic phenotypes by ages 11 to 12 years,” Dr. Lycett and associates said. The results have implications for public health by highlighting the subclinical effects of obesity in childhood and the importance of early intervention, they concluded.
“This important and comprehensive study has two important implications: first, high BMI by age 2 to 3 tends to stay high, and second, normal BMI occasionally increases to high BMI, but the reverse is rarely true,” Sarah Armstrong, MD, Jennifer S. Li, MD, and Asheley C. Skinner, PhD, wrote in an accompanying editorial (Pediatrics. 2020 Jul 6. doi: 10.1542/peds.2020-1353).
noted the editorialists, who are affiliated with Duke University, Durham, N.C.
“An important caveat is that although the relationships were significant, the amount of variance attributable directly to child BMI was small,” which highlights the complex relationship between obesity and health, they noted.
“Early-onset obesity is unlikely to change and, if it persists, will lead to detectable precursors of atherosclerosis by the time a child enters middle school,” and parents and primary care providers have an opportunity to “flatten the curve” by addressing BMI increases early in life to delay or prevent obesity, the editorialists concluded.
The study was supported by Australia’s National Health and Medical Research Council, The Royal Children’s Hospital Foundation, Murdoch Children’s Research Institute, The University of Melbourne, National Heart Foundation of Australia, Financial Markets Foundation for Children, and Victorian Deaf Education Institute. A number of the researchers were supported by grants from these and other universities and organizations. The researchers had no relevant financial disclosures. The editorialists had no financial conflicts to disclose.
SOURCE: Lycett K et al. Pediatrics. 2020 Jul 6. doi: 10.1542/peds.2019-3666.
FROM PEDIATRICS
Woody Erythematous Induration on the Posterior Neck
The Diagnosis: Scleredema Diabeticorum
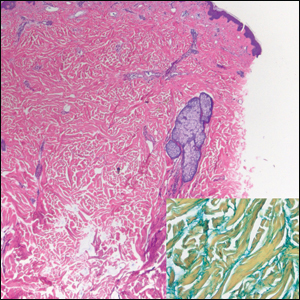
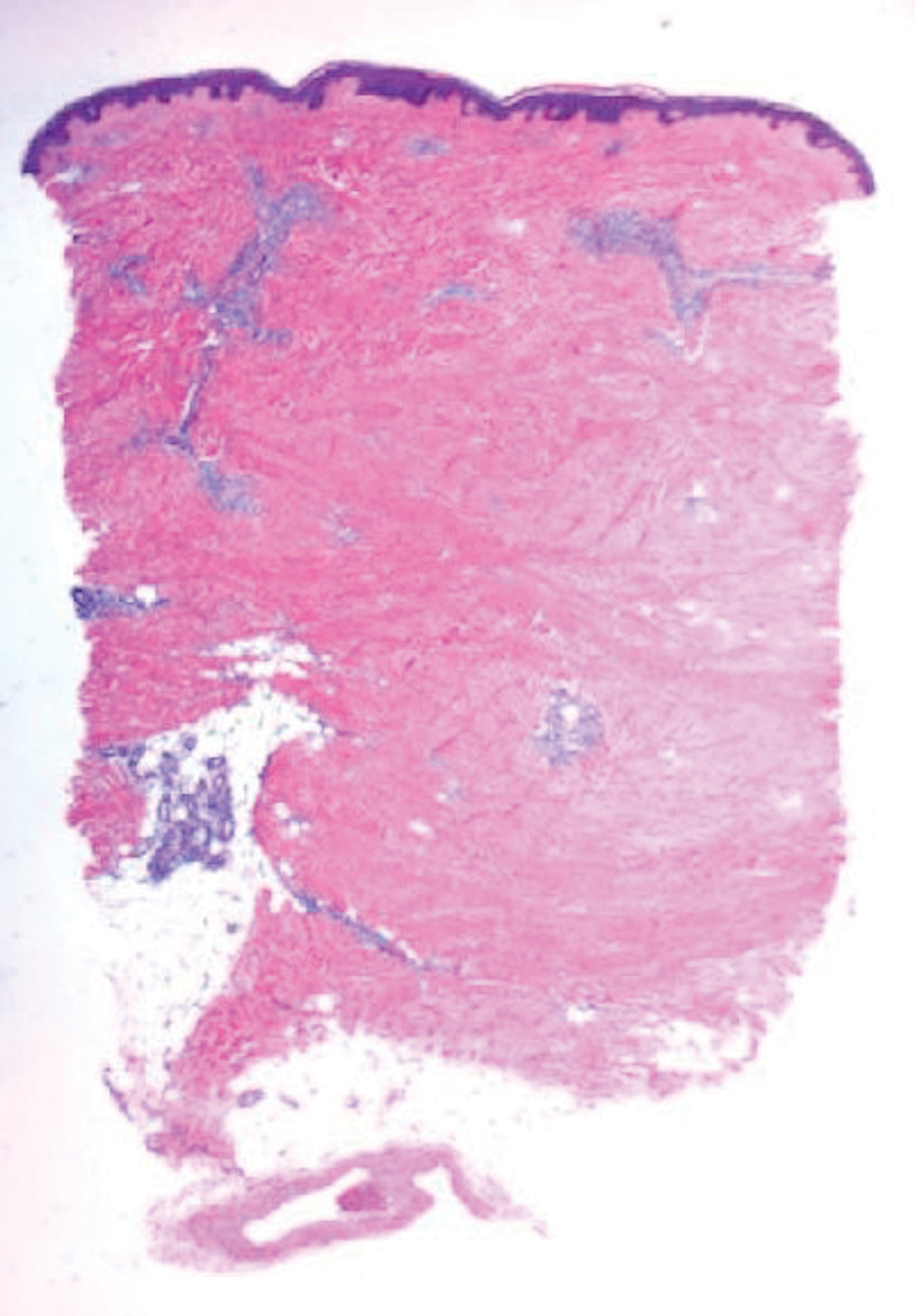
Histologically, scleredema is characterized by mucin deposition between collagen bundles in the deep dermis. Clinically, it is characterized by a progressive indurated plaque with associated stiffness of the involved area. It most commonly presents on the posterior aspect of the neck, though it can extend to involve the shoulders and upper torso.1 Scleredema is divided into 3 subtypes based on clinical associations. Type 1 often is preceded by an infection, most commonly group A Streptococcus. This type occurs acutely and often resolves completely over a few months.2 Type 2, which has progressive onset, is associated with monoclonal gammopathy.3 Type 3 is the most common type and is associated with diabetes mellitus. A study of 484 patients with type 2 diabetes mellitus demonstrated a prevalence of 2.5%.4 Although the exact pathogenesis has not been defined, it is hypothesized that irreversible glycosylation of collagen and alterations in collagenase activity may lead to accumulation of collagen and mucin in the dermis.5 Similar to type 2, type 3 scleredema appears subtly, progresses slowly, and tends to be chronic.1,6 Scleredema is characterized by marked dermal thickening and enlarged collagen bundles separated by mucin deposition (Figure 1). Fibroblast proliferation is characteristically absent.1
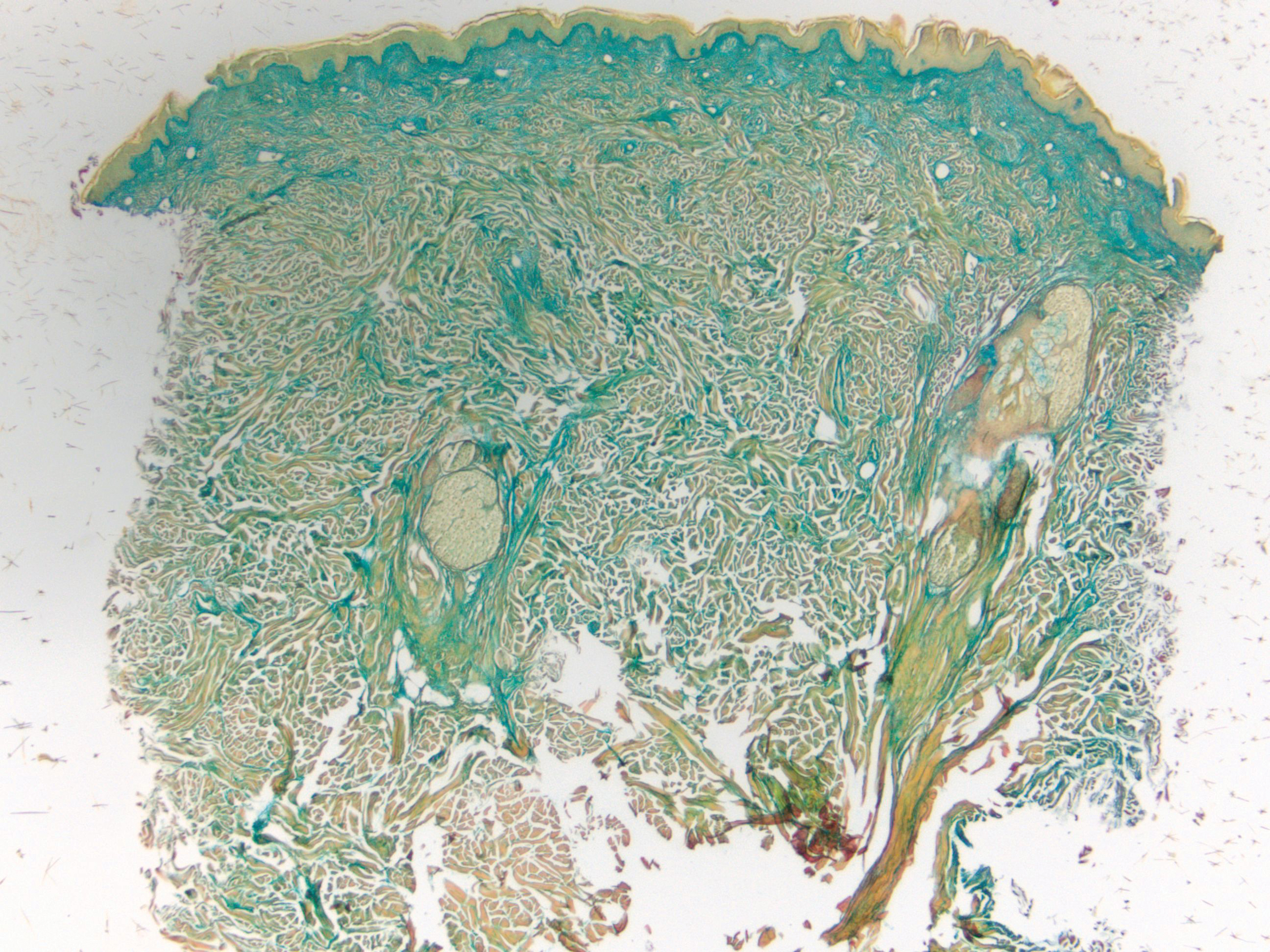
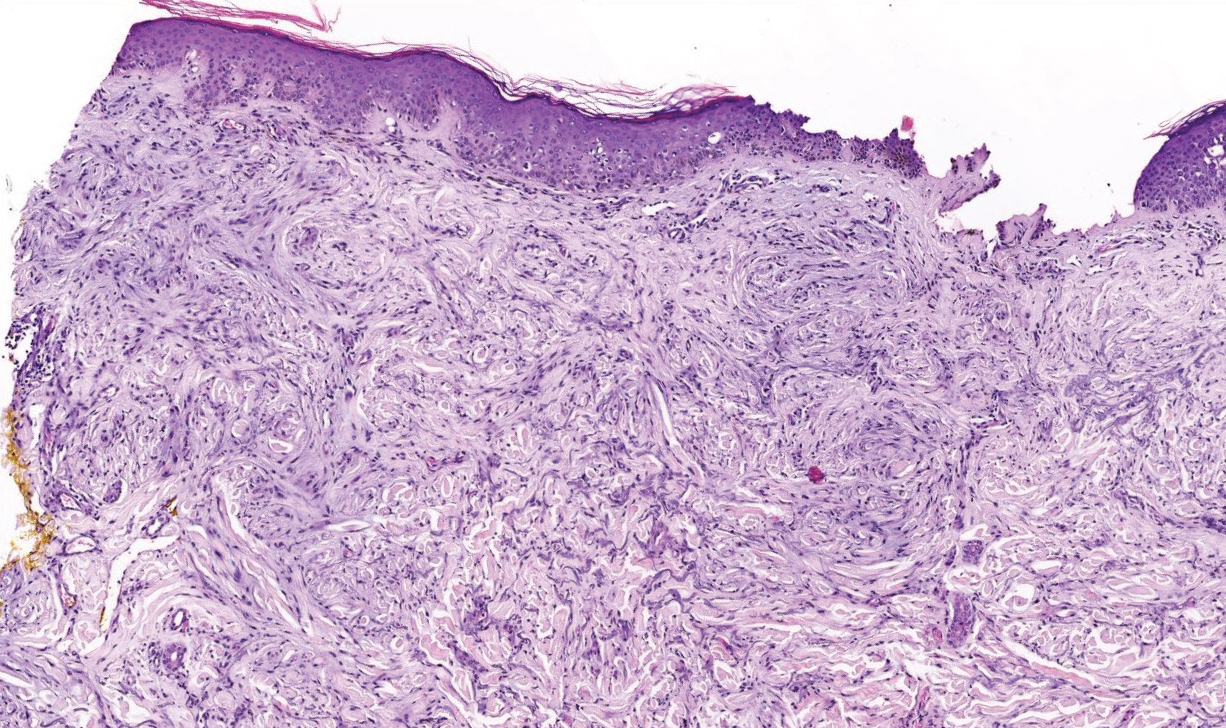
Clinically, tumid lupus erythematosus presents with erythematous edematous plaques on sun-exposed areas.7 Pretibial myxedema (PM) classically is associated with Graves disease; however, it can present in association with other types of thyroid dysfunction. Classically, PM presents on the pretibial regions as well-demarcated erythematous or hyperpigmented plaques.8 Similar to scleredema, histologic examination of tumid lupus erythematosus and PM reveals mucin deposition. Tumid lupus erythematosus also may demonstrate periadnexal and perivascular lymphocytic inflammation (Figure 2).7 The collagen bundles present in PM often are thin in comparison to scleredema (Figure 3).8

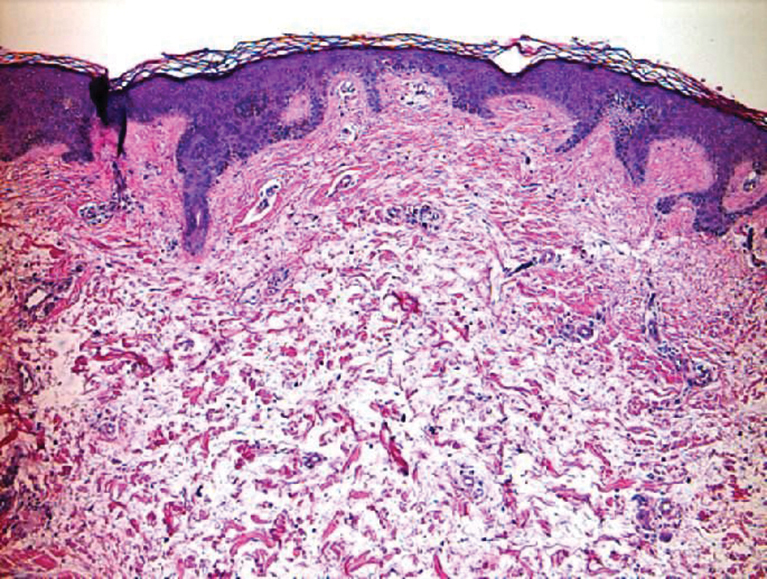
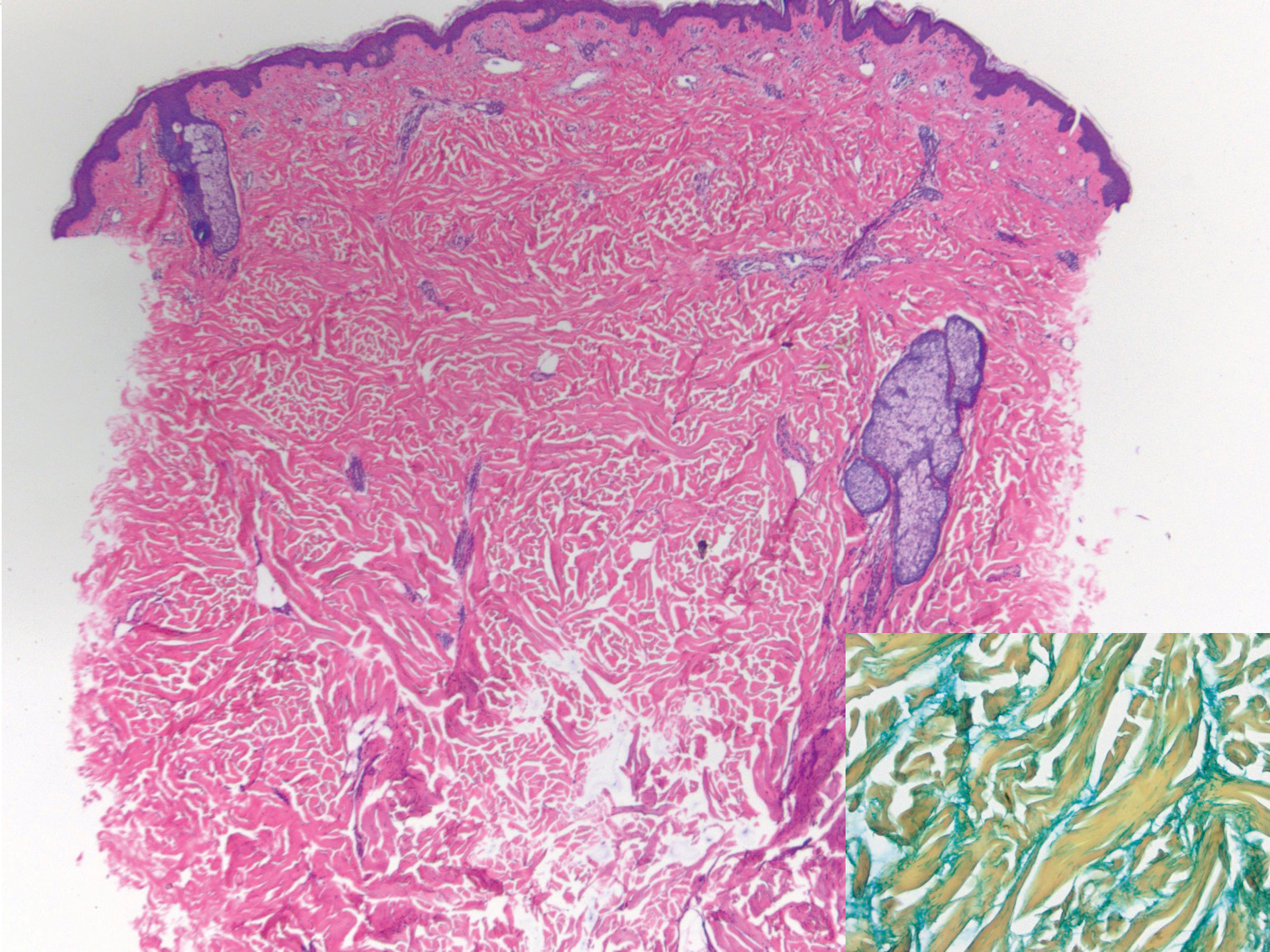
Scleroderma also presents with skin induration, erythema, and stiffening. However, unlike scleredema, scleroderma commonly involves the fingers, toes, and face. It presents with symptoms of Raynaud phenomenon, painful digital nonpitting edema, perioral skin tightening, mucocutaneous telangiectasia, and calcinosis cutis. Scleroderma also can involve organs such as the lungs, heart, kidneys, and gastrointestinal tract.9 Histologically, scleroderma is characterized by a compact dermis with closely packed collagen bundles. Other features of scleroderma can include perivascular mononuclear inflammatory cell infiltration, progressive atrophy of intradermal and perieccrine fat, and fibrosis (Figure 4).10
Scleromyxedema, also called papular mucinosis, is primary dermal mucinosis that often presents with waxy, dome-shaped papules that may coalesce into plaques. Similar to scleredema, scleromyxedema shows increased mucin deposition. However, scleromyxedema commonly is associated with fibroblast proliferation, which is characteristically absent in scleredema (Figure 5).11
- Beers WH, Ince A, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Cron RQ, Swetter SM. Scleredema revisited. a poststreptococcal complication. Clin Pediatr (Phila). 1994;33:606-610.
- Kövary PM, Vakilzadeh F, Macher E, et al. Monoclonal gammopathy in scleredema. observations in three cases. Arch Dermatol. 1981;117:536-539.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care. 1983;6:189-192.
- Namas R, Ashraf A. Scleredema of Buschke. Eur J Rheumatol. 2016;3:191-192.
- Knobler R, Moinzadeh P, Hunzelmann N, et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, part 2: scleromyxedema, scleredema and nephrogenic systemic fibrosis. J Eur Acad Dermatol Venereol. 2017;31:1581-1594.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus--a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. 2013;65:2737-2747.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336.
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72.
The Diagnosis: Scleredema Diabeticorum
Histologically, scleredema is characterized by mucin deposition between collagen bundles in the deep dermis. Clinically, it is characterized by a progressive indurated plaque with associated stiffness of the involved area. It most commonly presents on the posterior aspect of the neck, though it can extend to involve the shoulders and upper torso.1 Scleredema is divided into 3 subtypes based on clinical associations. Type 1 often is preceded by an infection, most commonly group A Streptococcus. This type occurs acutely and often resolves completely over a few months.2 Type 2, which has progressive onset, is associated with monoclonal gammopathy.3 Type 3 is the most common type and is associated with diabetes mellitus. A study of 484 patients with type 2 diabetes mellitus demonstrated a prevalence of 2.5%.4 Although the exact pathogenesis has not been defined, it is hypothesized that irreversible glycosylation of collagen and alterations in collagenase activity may lead to accumulation of collagen and mucin in the dermis.5 Similar to type 2, type 3 scleredema appears subtly, progresses slowly, and tends to be chronic.1,6 Scleredema is characterized by marked dermal thickening and enlarged collagen bundles separated by mucin deposition (Figure 1). Fibroblast proliferation is characteristically absent.1

Clinically, tumid lupus erythematosus presents with erythematous edematous plaques on sun-exposed areas.7 Pretibial myxedema (PM) classically is associated with Graves disease; however, it can present in association with other types of thyroid dysfunction. Classically, PM presents on the pretibial regions as well-demarcated erythematous or hyperpigmented plaques.8 Similar to scleredema, histologic examination of tumid lupus erythematosus and PM reveals mucin deposition. Tumid lupus erythematosus also may demonstrate periadnexal and perivascular lymphocytic inflammation (Figure 2).7 The collagen bundles present in PM often are thin in comparison to scleredema (Figure 3).8


Scleroderma also presents with skin induration, erythema, and stiffening. However, unlike scleredema, scleroderma commonly involves the fingers, toes, and face. It presents with symptoms of Raynaud phenomenon, painful digital nonpitting edema, perioral skin tightening, mucocutaneous telangiectasia, and calcinosis cutis. Scleroderma also can involve organs such as the lungs, heart, kidneys, and gastrointestinal tract.9 Histologically, scleroderma is characterized by a compact dermis with closely packed collagen bundles. Other features of scleroderma can include perivascular mononuclear inflammatory cell infiltration, progressive atrophy of intradermal and perieccrine fat, and fibrosis (Figure 4).10

Scleromyxedema, also called papular mucinosis, is primary dermal mucinosis that often presents with waxy, dome-shaped papules that may coalesce into plaques. Similar to scleredema, scleromyxedema shows increased mucin deposition. However, scleromyxedema commonly is associated with fibroblast proliferation, which is characteristically absent in scleredema (Figure 5).11

The Diagnosis: Scleredema Diabeticorum
Histologically, scleredema is characterized by mucin deposition between collagen bundles in the deep dermis. Clinically, it is characterized by a progressive indurated plaque with associated stiffness of the involved area. It most commonly presents on the posterior aspect of the neck, though it can extend to involve the shoulders and upper torso.1 Scleredema is divided into 3 subtypes based on clinical associations. Type 1 often is preceded by an infection, most commonly group A Streptococcus. This type occurs acutely and often resolves completely over a few months.2 Type 2, which has progressive onset, is associated with monoclonal gammopathy.3 Type 3 is the most common type and is associated with diabetes mellitus. A study of 484 patients with type 2 diabetes mellitus demonstrated a prevalence of 2.5%.4 Although the exact pathogenesis has not been defined, it is hypothesized that irreversible glycosylation of collagen and alterations in collagenase activity may lead to accumulation of collagen and mucin in the dermis.5 Similar to type 2, type 3 scleredema appears subtly, progresses slowly, and tends to be chronic.1,6 Scleredema is characterized by marked dermal thickening and enlarged collagen bundles separated by mucin deposition (Figure 1). Fibroblast proliferation is characteristically absent.1

Clinically, tumid lupus erythematosus presents with erythematous edematous plaques on sun-exposed areas.7 Pretibial myxedema (PM) classically is associated with Graves disease; however, it can present in association with other types of thyroid dysfunction. Classically, PM presents on the pretibial regions as well-demarcated erythematous or hyperpigmented plaques.8 Similar to scleredema, histologic examination of tumid lupus erythematosus and PM reveals mucin deposition. Tumid lupus erythematosus also may demonstrate periadnexal and perivascular lymphocytic inflammation (Figure 2).7 The collagen bundles present in PM often are thin in comparison to scleredema (Figure 3).8


Scleroderma also presents with skin induration, erythema, and stiffening. However, unlike scleredema, scleroderma commonly involves the fingers, toes, and face. It presents with symptoms of Raynaud phenomenon, painful digital nonpitting edema, perioral skin tightening, mucocutaneous telangiectasia, and calcinosis cutis. Scleroderma also can involve organs such as the lungs, heart, kidneys, and gastrointestinal tract.9 Histologically, scleroderma is characterized by a compact dermis with closely packed collagen bundles. Other features of scleroderma can include perivascular mononuclear inflammatory cell infiltration, progressive atrophy of intradermal and perieccrine fat, and fibrosis (Figure 4).10

Scleromyxedema, also called papular mucinosis, is primary dermal mucinosis that often presents with waxy, dome-shaped papules that may coalesce into plaques. Similar to scleredema, scleromyxedema shows increased mucin deposition. However, scleromyxedema commonly is associated with fibroblast proliferation, which is characteristically absent in scleredema (Figure 5).11

- Beers WH, Ince A, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Cron RQ, Swetter SM. Scleredema revisited. a poststreptococcal complication. Clin Pediatr (Phila). 1994;33:606-610.
- Kövary PM, Vakilzadeh F, Macher E, et al. Monoclonal gammopathy in scleredema. observations in three cases. Arch Dermatol. 1981;117:536-539.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care. 1983;6:189-192.
- Namas R, Ashraf A. Scleredema of Buschke. Eur J Rheumatol. 2016;3:191-192.
- Knobler R, Moinzadeh P, Hunzelmann N, et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, part 2: scleromyxedema, scleredema and nephrogenic systemic fibrosis. J Eur Acad Dermatol Venereol. 2017;31:1581-1594.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus--a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. 2013;65:2737-2747.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336.
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72.
- Beers WH, Ince A, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Cron RQ, Swetter SM. Scleredema revisited. a poststreptococcal complication. Clin Pediatr (Phila). 1994;33:606-610.
- Kövary PM, Vakilzadeh F, Macher E, et al. Monoclonal gammopathy in scleredema. observations in three cases. Arch Dermatol. 1981;117:536-539.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care. 1983;6:189-192.
- Namas R, Ashraf A. Scleredema of Buschke. Eur J Rheumatol. 2016;3:191-192.
- Knobler R, Moinzadeh P, Hunzelmann N, et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, part 2: scleromyxedema, scleredema and nephrogenic systemic fibrosis. J Eur Acad Dermatol Venereol. 2017;31:1581-1594.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus--a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. 2013;65:2737-2747.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336.
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72.
A 39-year-old white woman with a medical history of type 1 diabetes mellitus and rheumatoid arthritis presented to the dermatology clinic with pain and thickened skin on the posterior neck of 4 weeks’ duration. The patient noted stiffness in the neck and shoulders but denied any pain, pruritus, fever, chills, night sweats, fatigue, cough, dyspnea, dysphagia, weight loss, or change in appetite. Physical examination revealed a woody indurated plaque with slight erythema that was present diffusely on the posterior neck and upper back. The patient reported that a recent complete blood cell count and complete metabolic panel performed by her primary care physician were within reference range. Hemoglobin A1C was 8.6% of total hemoglobin (reference range, 4%–7%). A punch biopsy was performed.
Heavy menstrual bleeding difficult to control in young patients with inherited platelet disorders
Physician consensus and a broadly effective treatment for heavy menstrual bleeding was not found among young patients with inherited platelet function disorders, according to the results of a retrospective chart review reported in the Journal of Pediatric and Adolescent Gynecology.
Heavy menstrual bleeding (HMB) in girls with inherited platelet function disorders (IPFD) can be difficult to control despite ongoing follow-up and treatment changes, reported Christine M. Pennesi, MD, of the University of Michigan, Ann Arbor, and colleagues.
They assessed 34 young women and girls (ages 9-25 years) diagnosed with IPFDs referred to gynecology and/or hematology at a tertiary care hospital between 2006 and 2018.
Billing codes were used to determine hormonal or nonhormonal treatments, and outcomes over a 1- to 2-year period were collected. The initial treatment was defined as the first treatment prescribed after referral. The primary outcome was treatment failure, defined as a change in treatment method because of continued bleeding.
The majority (56%) of patients failed initial treatment (n = 19); among all 34 individuals followed in the study, an average of 2.7 total treatments were required.
Six patients (18%) remained uncontrolled despite numerous treatment changes (mean treatment changes, four; range, two to seven), and two patients (6%) remained uncontrolled because of noncompliance with treatment.
Overall, the researchers identified a 18% failure rate of successfully treatment of HMB in young women and girls with IPFDs over a 2-year follow-up period.
Of the 26 women who achieved control of HMB within 2-year follow-up, 54% (n = 14) were on hormonal treatments, 27% (n = 7) on nonhormonal treatments, 12% (n = 3) on combined treatments, and 8% (n = 2) on no treatment at time of control, the authors stated.
“The heterogeneity in treatments that were described in this study, clearly demonstrate that, in selecting treatment methods for HMB in young women, other considerations are often in play. This includes patient preference and need for contraception. Some patients or parents may have personal or religious objections to hormonal methods or worry about hormones in this young age group,” the researchers speculated.
“Appropriate counseling in these patients should include that it would not be unexpected for a patient to need more than one treatment before control of bleeding is achieved. This may help to alleviate the fear of teenagers when continued bleeding occurs after starting their initial treatment,” Dr. Pennesi and colleagues concluded.
One of the authors participated in funded trials and received funding from several pharmaceutical companies. The others reported having no disclosures.
SOURCE: Pennesi CM et al. J Pediatr Adolesc Gynecol. 2020 Jun 22. doi: 10.1016/j.jpag.2020.06.019.
Physician consensus and a broadly effective treatment for heavy menstrual bleeding was not found among young patients with inherited platelet function disorders, according to the results of a retrospective chart review reported in the Journal of Pediatric and Adolescent Gynecology.
Heavy menstrual bleeding (HMB) in girls with inherited platelet function disorders (IPFD) can be difficult to control despite ongoing follow-up and treatment changes, reported Christine M. Pennesi, MD, of the University of Michigan, Ann Arbor, and colleagues.
They assessed 34 young women and girls (ages 9-25 years) diagnosed with IPFDs referred to gynecology and/or hematology at a tertiary care hospital between 2006 and 2018.
Billing codes were used to determine hormonal or nonhormonal treatments, and outcomes over a 1- to 2-year period were collected. The initial treatment was defined as the first treatment prescribed after referral. The primary outcome was treatment failure, defined as a change in treatment method because of continued bleeding.
The majority (56%) of patients failed initial treatment (n = 19); among all 34 individuals followed in the study, an average of 2.7 total treatments were required.
Six patients (18%) remained uncontrolled despite numerous treatment changes (mean treatment changes, four; range, two to seven), and two patients (6%) remained uncontrolled because of noncompliance with treatment.
Overall, the researchers identified a 18% failure rate of successfully treatment of HMB in young women and girls with IPFDs over a 2-year follow-up period.
Of the 26 women who achieved control of HMB within 2-year follow-up, 54% (n = 14) were on hormonal treatments, 27% (n = 7) on nonhormonal treatments, 12% (n = 3) on combined treatments, and 8% (n = 2) on no treatment at time of control, the authors stated.
“The heterogeneity in treatments that were described in this study, clearly demonstrate that, in selecting treatment methods for HMB in young women, other considerations are often in play. This includes patient preference and need for contraception. Some patients or parents may have personal or religious objections to hormonal methods or worry about hormones in this young age group,” the researchers speculated.
“Appropriate counseling in these patients should include that it would not be unexpected for a patient to need more than one treatment before control of bleeding is achieved. This may help to alleviate the fear of teenagers when continued bleeding occurs after starting their initial treatment,” Dr. Pennesi and colleagues concluded.
One of the authors participated in funded trials and received funding from several pharmaceutical companies. The others reported having no disclosures.
SOURCE: Pennesi CM et al. J Pediatr Adolesc Gynecol. 2020 Jun 22. doi: 10.1016/j.jpag.2020.06.019.
Physician consensus and a broadly effective treatment for heavy menstrual bleeding was not found among young patients with inherited platelet function disorders, according to the results of a retrospective chart review reported in the Journal of Pediatric and Adolescent Gynecology.
Heavy menstrual bleeding (HMB) in girls with inherited platelet function disorders (IPFD) can be difficult to control despite ongoing follow-up and treatment changes, reported Christine M. Pennesi, MD, of the University of Michigan, Ann Arbor, and colleagues.
They assessed 34 young women and girls (ages 9-25 years) diagnosed with IPFDs referred to gynecology and/or hematology at a tertiary care hospital between 2006 and 2018.
Billing codes were used to determine hormonal or nonhormonal treatments, and outcomes over a 1- to 2-year period were collected. The initial treatment was defined as the first treatment prescribed after referral. The primary outcome was treatment failure, defined as a change in treatment method because of continued bleeding.
The majority (56%) of patients failed initial treatment (n = 19); among all 34 individuals followed in the study, an average of 2.7 total treatments were required.
Six patients (18%) remained uncontrolled despite numerous treatment changes (mean treatment changes, four; range, two to seven), and two patients (6%) remained uncontrolled because of noncompliance with treatment.
Overall, the researchers identified a 18% failure rate of successfully treatment of HMB in young women and girls with IPFDs over a 2-year follow-up period.
Of the 26 women who achieved control of HMB within 2-year follow-up, 54% (n = 14) were on hormonal treatments, 27% (n = 7) on nonhormonal treatments, 12% (n = 3) on combined treatments, and 8% (n = 2) on no treatment at time of control, the authors stated.
“The heterogeneity in treatments that were described in this study, clearly demonstrate that, in selecting treatment methods for HMB in young women, other considerations are often in play. This includes patient preference and need for contraception. Some patients or parents may have personal or religious objections to hormonal methods or worry about hormones in this young age group,” the researchers speculated.
“Appropriate counseling in these patients should include that it would not be unexpected for a patient to need more than one treatment before control of bleeding is achieved. This may help to alleviate the fear of teenagers when continued bleeding occurs after starting their initial treatment,” Dr. Pennesi and colleagues concluded.
One of the authors participated in funded trials and received funding from several pharmaceutical companies. The others reported having no disclosures.
SOURCE: Pennesi CM et al. J Pediatr Adolesc Gynecol. 2020 Jun 22. doi: 10.1016/j.jpag.2020.06.019.
FROM THE JOURNAL OF PEDIATRIC AND ADOLESCENT GYNECOLOGY
Oral difelikefalin quells severe chronic kidney disease–associated itch
, in a first-of-its-kind randomized clinical trial, Gil Yosipovitch, MD, said at the virtual annual meeting of the American Academy of Dermatology.
“Difelikefalin at 1.0 mg was associated with clinically meaningful improvements in pruritus. The improvement in itch was significant by week 2. And nearly 40% of patients achieved a complete response, which was more than two-and-one-half times more than with placebo,” noted Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami.
Pruritus associated with chronic kidney disease (CKD) is a common, underrecognized, and distressing condition that causes markedly impaired quality of life. It occurs in patients across all stages of CKD, not just in those on hemodialysis, as is widely but mistakenly believed. And at present there is no approved drug in any country for treatment of CKD-associated itch.
Difelikefalin, a novel selective agonist of peripheral kappa opioid receptors, is designed to have very limited CNS penetration. The drug, which is renally excreted, doesn’t bind to mu or delta opioid receptors. Its antipruritic effect arises from activation of kappa opioid receptors on peripheral sensory neurons and immune cells, the dermatologist explained.
Dr. Yosipovitch presented the results of a phase 2, randomized, double-blind, placebo-controlled, 12-week trial in which 240 patients with severe chronic pruritus and stage 3-5 CKD were assigned to once-daily oral difelikefalin at 0.25 mg, 0.5 mg, or 1.0 mg, or placebo. More than 80% of participants were not on dialysis. Indeed, this was the first-ever clinical trial targeting itch in patients across such a broad spectrum of CKD stages.
The primary study endpoint was change from baseline to week 12 in the weekly mean score on the 24-hour Worst Itching Intensity Numerical Rating Scale. The average baseline score was 7, considered severe pruritus on the 0-10 scale. Patients randomized to difelikefalin at 1.0 mg/day had a mean 4.4-point decrease, a significantly greater improvement than the 3.3-point reduction in placebo-treated controls.
“More than a 4-point decrease is considered a very meaningful itch reduction,” Dr. Yosipovitch noted.
The mean reductions in itch score in patients on 0.25 mg and 0.5 mg/day of difelikefalin were 4.0 and 3.8 points, respectively, which fell short of statistical significance versus placebo.
A key prespecified secondary endpoint was the proportion of subjects with at least a 3-point improvement in itch score over 12 weeks. This was achieved in 72% of patients on the top dose of difelikefalin, compared with 58% of controls, a significant difference. A 4-point or larger decrease in itch score occurred in 65% of patients on 1.0 mg/day of the kappa opioid recent agonist, versus 50% of controls, also a significant difference.
A complete response, defined as an itch score of 0 or 1 at least 80% of the time, was significantly more common in all three active treatment groups than in controls, with rates of 33%, 31.6%, and 38.6% at difelikefalin 0.25, 0.5, and 1.0 mg, compared with 4.4% among those on placebo.
Falls occurred in 1.5% of patients on difelikefalin. “The therapy does seem to increase the risk of dizziness, falls, fatigue, and GI complaints,” according to the investigator.
Still, most of these adverse events were mild or moderate in severity. Only about 1% of participants discontinued treatment for such reasons.
Earlier this year, a positive phase 3 trial of an intravenous formulation of difelikefalin for pruritus was reported in CKD patients on hemodialysis (N Engl J Med. 2020 Jan 16;382[3]:222-32).
In an interview, Dr. Yosipovitch said that this new phase 2 oral dose-finding study wasn’t powered to detect differences in treatment efficacy between the dialysis and nondialysis groups. However, the proportion of patients with at least a 3-point improvement in itch at week 12 was similar in the two groups.
“The oral formulation would of course be more convenient and would be preferred for patients not undergoing hemodialysis,” he said. “I would expect that the IV formulation would be the preferred route of administration for a patient undergoing hemodialysis. An IV formulation would be very convenient for such patients because it’s administered at the dialysis clinic at the end of the hemodialysis session.”
The oral difelikefalin phase 3 program is scheduled to start later in 2020.
CKD-associated itch poses a therapeutic challenge because it has so many contributory factors. These include CKD-induced peripheral neuropathy, functional and structural neuropathic changes in the brain, cutaneous mast cell activation, an imbalance between mu opioid receptor overexpression and kappa opioid receptor downregulation, secondary parathyroidism, and systemic accumulation of aluminum, beta 2 microglobulin, and other dialysis-related substances, the dermatologist observed.
Dr. Yosipovitch reported receiving research grants from a half-dozen pharmaceutical companies. He also serves as a consultant to numerous companies, including Cara Therapeutics, which sponsored the phase 2 trial.
, in a first-of-its-kind randomized clinical trial, Gil Yosipovitch, MD, said at the virtual annual meeting of the American Academy of Dermatology.
“Difelikefalin at 1.0 mg was associated with clinically meaningful improvements in pruritus. The improvement in itch was significant by week 2. And nearly 40% of patients achieved a complete response, which was more than two-and-one-half times more than with placebo,” noted Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami.
Pruritus associated with chronic kidney disease (CKD) is a common, underrecognized, and distressing condition that causes markedly impaired quality of life. It occurs in patients across all stages of CKD, not just in those on hemodialysis, as is widely but mistakenly believed. And at present there is no approved drug in any country for treatment of CKD-associated itch.
Difelikefalin, a novel selective agonist of peripheral kappa opioid receptors, is designed to have very limited CNS penetration. The drug, which is renally excreted, doesn’t bind to mu or delta opioid receptors. Its antipruritic effect arises from activation of kappa opioid receptors on peripheral sensory neurons and immune cells, the dermatologist explained.
Dr. Yosipovitch presented the results of a phase 2, randomized, double-blind, placebo-controlled, 12-week trial in which 240 patients with severe chronic pruritus and stage 3-5 CKD were assigned to once-daily oral difelikefalin at 0.25 mg, 0.5 mg, or 1.0 mg, or placebo. More than 80% of participants were not on dialysis. Indeed, this was the first-ever clinical trial targeting itch in patients across such a broad spectrum of CKD stages.
The primary study endpoint was change from baseline to week 12 in the weekly mean score on the 24-hour Worst Itching Intensity Numerical Rating Scale. The average baseline score was 7, considered severe pruritus on the 0-10 scale. Patients randomized to difelikefalin at 1.0 mg/day had a mean 4.4-point decrease, a significantly greater improvement than the 3.3-point reduction in placebo-treated controls.
“More than a 4-point decrease is considered a very meaningful itch reduction,” Dr. Yosipovitch noted.
The mean reductions in itch score in patients on 0.25 mg and 0.5 mg/day of difelikefalin were 4.0 and 3.8 points, respectively, which fell short of statistical significance versus placebo.
A key prespecified secondary endpoint was the proportion of subjects with at least a 3-point improvement in itch score over 12 weeks. This was achieved in 72% of patients on the top dose of difelikefalin, compared with 58% of controls, a significant difference. A 4-point or larger decrease in itch score occurred in 65% of patients on 1.0 mg/day of the kappa opioid recent agonist, versus 50% of controls, also a significant difference.
A complete response, defined as an itch score of 0 or 1 at least 80% of the time, was significantly more common in all three active treatment groups than in controls, with rates of 33%, 31.6%, and 38.6% at difelikefalin 0.25, 0.5, and 1.0 mg, compared with 4.4% among those on placebo.
Falls occurred in 1.5% of patients on difelikefalin. “The therapy does seem to increase the risk of dizziness, falls, fatigue, and GI complaints,” according to the investigator.
Still, most of these adverse events were mild or moderate in severity. Only about 1% of participants discontinued treatment for such reasons.
Earlier this year, a positive phase 3 trial of an intravenous formulation of difelikefalin for pruritus was reported in CKD patients on hemodialysis (N Engl J Med. 2020 Jan 16;382[3]:222-32).
In an interview, Dr. Yosipovitch said that this new phase 2 oral dose-finding study wasn’t powered to detect differences in treatment efficacy between the dialysis and nondialysis groups. However, the proportion of patients with at least a 3-point improvement in itch at week 12 was similar in the two groups.
“The oral formulation would of course be more convenient and would be preferred for patients not undergoing hemodialysis,” he said. “I would expect that the IV formulation would be the preferred route of administration for a patient undergoing hemodialysis. An IV formulation would be very convenient for such patients because it’s administered at the dialysis clinic at the end of the hemodialysis session.”
The oral difelikefalin phase 3 program is scheduled to start later in 2020.
CKD-associated itch poses a therapeutic challenge because it has so many contributory factors. These include CKD-induced peripheral neuropathy, functional and structural neuropathic changes in the brain, cutaneous mast cell activation, an imbalance between mu opioid receptor overexpression and kappa opioid receptor downregulation, secondary parathyroidism, and systemic accumulation of aluminum, beta 2 microglobulin, and other dialysis-related substances, the dermatologist observed.
Dr. Yosipovitch reported receiving research grants from a half-dozen pharmaceutical companies. He also serves as a consultant to numerous companies, including Cara Therapeutics, which sponsored the phase 2 trial.
, in a first-of-its-kind randomized clinical trial, Gil Yosipovitch, MD, said at the virtual annual meeting of the American Academy of Dermatology.
“Difelikefalin at 1.0 mg was associated with clinically meaningful improvements in pruritus. The improvement in itch was significant by week 2. And nearly 40% of patients achieved a complete response, which was more than two-and-one-half times more than with placebo,” noted Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami.
Pruritus associated with chronic kidney disease (CKD) is a common, underrecognized, and distressing condition that causes markedly impaired quality of life. It occurs in patients across all stages of CKD, not just in those on hemodialysis, as is widely but mistakenly believed. And at present there is no approved drug in any country for treatment of CKD-associated itch.
Difelikefalin, a novel selective agonist of peripheral kappa opioid receptors, is designed to have very limited CNS penetration. The drug, which is renally excreted, doesn’t bind to mu or delta opioid receptors. Its antipruritic effect arises from activation of kappa opioid receptors on peripheral sensory neurons and immune cells, the dermatologist explained.
Dr. Yosipovitch presented the results of a phase 2, randomized, double-blind, placebo-controlled, 12-week trial in which 240 patients with severe chronic pruritus and stage 3-5 CKD were assigned to once-daily oral difelikefalin at 0.25 mg, 0.5 mg, or 1.0 mg, or placebo. More than 80% of participants were not on dialysis. Indeed, this was the first-ever clinical trial targeting itch in patients across such a broad spectrum of CKD stages.
The primary study endpoint was change from baseline to week 12 in the weekly mean score on the 24-hour Worst Itching Intensity Numerical Rating Scale. The average baseline score was 7, considered severe pruritus on the 0-10 scale. Patients randomized to difelikefalin at 1.0 mg/day had a mean 4.4-point decrease, a significantly greater improvement than the 3.3-point reduction in placebo-treated controls.
“More than a 4-point decrease is considered a very meaningful itch reduction,” Dr. Yosipovitch noted.
The mean reductions in itch score in patients on 0.25 mg and 0.5 mg/day of difelikefalin were 4.0 and 3.8 points, respectively, which fell short of statistical significance versus placebo.
A key prespecified secondary endpoint was the proportion of subjects with at least a 3-point improvement in itch score over 12 weeks. This was achieved in 72% of patients on the top dose of difelikefalin, compared with 58% of controls, a significant difference. A 4-point or larger decrease in itch score occurred in 65% of patients on 1.0 mg/day of the kappa opioid recent agonist, versus 50% of controls, also a significant difference.
A complete response, defined as an itch score of 0 or 1 at least 80% of the time, was significantly more common in all three active treatment groups than in controls, with rates of 33%, 31.6%, and 38.6% at difelikefalin 0.25, 0.5, and 1.0 mg, compared with 4.4% among those on placebo.
Falls occurred in 1.5% of patients on difelikefalin. “The therapy does seem to increase the risk of dizziness, falls, fatigue, and GI complaints,” according to the investigator.
Still, most of these adverse events were mild or moderate in severity. Only about 1% of participants discontinued treatment for such reasons.
Earlier this year, a positive phase 3 trial of an intravenous formulation of difelikefalin for pruritus was reported in CKD patients on hemodialysis (N Engl J Med. 2020 Jan 16;382[3]:222-32).
In an interview, Dr. Yosipovitch said that this new phase 2 oral dose-finding study wasn’t powered to detect differences in treatment efficacy between the dialysis and nondialysis groups. However, the proportion of patients with at least a 3-point improvement in itch at week 12 was similar in the two groups.
“The oral formulation would of course be more convenient and would be preferred for patients not undergoing hemodialysis,” he said. “I would expect that the IV formulation would be the preferred route of administration for a patient undergoing hemodialysis. An IV formulation would be very convenient for such patients because it’s administered at the dialysis clinic at the end of the hemodialysis session.”
The oral difelikefalin phase 3 program is scheduled to start later in 2020.
CKD-associated itch poses a therapeutic challenge because it has so many contributory factors. These include CKD-induced peripheral neuropathy, functional and structural neuropathic changes in the brain, cutaneous mast cell activation, an imbalance between mu opioid receptor overexpression and kappa opioid receptor downregulation, secondary parathyroidism, and systemic accumulation of aluminum, beta 2 microglobulin, and other dialysis-related substances, the dermatologist observed.
Dr. Yosipovitch reported receiving research grants from a half-dozen pharmaceutical companies. He also serves as a consultant to numerous companies, including Cara Therapeutics, which sponsored the phase 2 trial.
FROM AAD 2020
As a black psychiatrist, she is ‘exhausted’ and ‘furious’
I didn’t have any doctors in my family. The only doctor I knew was my pediatrician. At 6 years old – and this gives you a glimpse into my personality – I told my parents I did not think he was a good doctor. I said, “When I grow up to be a doctor, I’m going to be a better doctor than him.” Fast forward to 7th grade, when I saw an orthopedic surgeon for my scoliosis. He was phenomenal. He listened. He explained to me all of the science and medicine and his rationale for decisions. I thought, “That is the kind of doctor I want to be.”
I went to medical school at Penn and didn’t think psychiatry was a medical specialty. I thought it was just Freud and laying on couches. I thought, “Where’s the science, where’s the physiology, where’s the genetics?” I was headed toward surgery.
Then, I rotated with an incredible psychiatrist. I saw behavior was biological, chemical, electrical, and physiological. I realize, looking back, that I had an interest because there is mental illness in my family. And there is so much stigma against psychiatric illnesses and addiction. It’s shocking how badly our patients get treated in the general medicine construct. So, I thought, “This field has science, the human body, activism, and marginalized patients? This is for me!”
I went to Howard University, which was the most freeing time of my life. There was no code-switching, no working hard to be a “presentable” Black person. When I started interviewing for medical schools, I was told by someone I interviewed with at one school that I should straighten my hair if I wanted to get accepted. I marked that school off my list. I decided right then that I would rather not go to medical school than straighten my hair to get into medical school. I went to Penn; they accepted me without my hair straight.
Penn Med was majorly White. There were six of us who were Black in a class of about 150 people. There was this feeling like “we let you in” even though every single one of us who was there was clearly at the top of the game to have been able to get there. I loved Penn Med. My class was amazing. I became the first Black president of medical student government there and I won a lot of awards.
When I was finishing up, my dean at the time, who was a White woman, said, “I’m so proud of you. You came in a piece of coal and look how we shined you up. “What do you say? I have a smart mouth, so I said, “I was already shiny when I got here.” She said, “See, that’s part of your problem, you don’t know how to take a compliment.” That was 2002, and I still remember every word of that conversation.
I was on the psychiatry unit rounding as a medical student and introduced myself to a patient. He said, “What’s your name?” And I thought, here it comes. I said, “Nzinga Ajabu,” my name at the time. He said “Nzinga? You probably have a spear in your closet.” When I tell these stories to White people, they’re always shocked. When I tell these stories to Black people, they say, “Yeah, that sounds about right.”
You can talk to Black medical students, Black interns, Black residents. When patients say something racist to you, nobody speaks up for you, nobody. It should be the attending that professionally approaches the patient and says something, anything. But they just laugh uncomfortably, they let it pass, they pretend they didn’t hear it. Meanwhile, you are fuming, and injured, and have to maintain your professionalism. It happens all the time. When people say, “Oh, you don’t look like a doctor,” I know what that means, but someone else may not even notice it’s an insult. When they do notice an insult, they don’t have the language or the courage to address it. And it’s not always a patient leveling racial insults. It very often is the attending, the fellow, the resident, or another medical student.
These things happen to me less now because I’m in a position of power. I’d say most insults that come my way now are overwhelmingly unintentional. I call people out on it 95% of the time. The other 5% of the time, I’m either exhausted, or I’m in some power structure where I decide it’s too risky. And those are the days – when I decide it’s too risky for me to speak up – when I come home exhausted. Because there will always be a power dynamic, as long as I’m alive, where you can’t speak up because you’re a Black woman, and that just wears me out.
Ultimately, I opted out of academic medicine because I thought it was too constraining, that I wouldn’t be able to raise my voice and do the activism I needed to do. – I’m able to advocate for people who are marginalized by medicine and, in treating addiction, advocate for people who are marginalized by psychiatry, which is marginalized by medicine.
A bias people have is that when you talk about Black people, they think you are talking about poor people. When we talk about police brutality, or being pulled over by the police, or dying in childbirth, our colleagues don’t think that’s happening to us. They think that’s happening to “those” Black people. Regardless of my socioeconomic status, I still have a higher chance of dying in childbirth or dying from COVID.
COVID had already turned my work up to 100 – we had staff losing loved ones and coming down with fevers themselves. And I had just launched my podcast. Then they killed Breonna Taylor, Ahmaud Arbery, Amy Cooper called the cops on Christian Cooper, and they killed George Floyd. This is how it happens. Bam. Bam. Bam.
The series of killings turned up my work at Physicians for Criminal Justice Reform, but it also turned up my work as a mother. My boys are 13 and 14. I personally can’t watch some of the videos because I see my own sons. I was already tired. Now I’m exhausted, I’m furious and I’m desperate to protect my kids. They have this on their backs already. Both of them have already had to deal with overt racism – they’ve had this burden since they were 5 years old, if not younger. I have to teach them to fight this war. Should that be how it is?
Nzinga Harrison, MD, 43, is a psychiatrist and the cofounder and chief medical officer of Eleanor Health, a network of physician clinics that treats people affected by addiction in North Carolina and New Jersey. She is also a cofounder of Physicians for Criminal Justice Reform. and host of the new podcast In Recovery. Harrison was raised in Indianapolis, went to college at Howard University and received her MD from the Perelman School of Medicine at the University of Pennsylvania in 2002. Her mother was an elementary school teacher. Her father, an electrical engineer, was commander of the local Black Panther Militia. Both supported her love of math and science and brought her with them to picket lines and marches.
This article first appeared on Medscape.com.
I didn’t have any doctors in my family. The only doctor I knew was my pediatrician. At 6 years old – and this gives you a glimpse into my personality – I told my parents I did not think he was a good doctor. I said, “When I grow up to be a doctor, I’m going to be a better doctor than him.” Fast forward to 7th grade, when I saw an orthopedic surgeon for my scoliosis. He was phenomenal. He listened. He explained to me all of the science and medicine and his rationale for decisions. I thought, “That is the kind of doctor I want to be.”
I went to medical school at Penn and didn’t think psychiatry was a medical specialty. I thought it was just Freud and laying on couches. I thought, “Where’s the science, where’s the physiology, where’s the genetics?” I was headed toward surgery.
Then, I rotated with an incredible psychiatrist. I saw behavior was biological, chemical, electrical, and physiological. I realize, looking back, that I had an interest because there is mental illness in my family. And there is so much stigma against psychiatric illnesses and addiction. It’s shocking how badly our patients get treated in the general medicine construct. So, I thought, “This field has science, the human body, activism, and marginalized patients? This is for me!”
I went to Howard University, which was the most freeing time of my life. There was no code-switching, no working hard to be a “presentable” Black person. When I started interviewing for medical schools, I was told by someone I interviewed with at one school that I should straighten my hair if I wanted to get accepted. I marked that school off my list. I decided right then that I would rather not go to medical school than straighten my hair to get into medical school. I went to Penn; they accepted me without my hair straight.
Penn Med was majorly White. There were six of us who were Black in a class of about 150 people. There was this feeling like “we let you in” even though every single one of us who was there was clearly at the top of the game to have been able to get there. I loved Penn Med. My class was amazing. I became the first Black president of medical student government there and I won a lot of awards.
When I was finishing up, my dean at the time, who was a White woman, said, “I’m so proud of you. You came in a piece of coal and look how we shined you up. “What do you say? I have a smart mouth, so I said, “I was already shiny when I got here.” She said, “See, that’s part of your problem, you don’t know how to take a compliment.” That was 2002, and I still remember every word of that conversation.
I was on the psychiatry unit rounding as a medical student and introduced myself to a patient. He said, “What’s your name?” And I thought, here it comes. I said, “Nzinga Ajabu,” my name at the time. He said “Nzinga? You probably have a spear in your closet.” When I tell these stories to White people, they’re always shocked. When I tell these stories to Black people, they say, “Yeah, that sounds about right.”
You can talk to Black medical students, Black interns, Black residents. When patients say something racist to you, nobody speaks up for you, nobody. It should be the attending that professionally approaches the patient and says something, anything. But they just laugh uncomfortably, they let it pass, they pretend they didn’t hear it. Meanwhile, you are fuming, and injured, and have to maintain your professionalism. It happens all the time. When people say, “Oh, you don’t look like a doctor,” I know what that means, but someone else may not even notice it’s an insult. When they do notice an insult, they don’t have the language or the courage to address it. And it’s not always a patient leveling racial insults. It very often is the attending, the fellow, the resident, or another medical student.
These things happen to me less now because I’m in a position of power. I’d say most insults that come my way now are overwhelmingly unintentional. I call people out on it 95% of the time. The other 5% of the time, I’m either exhausted, or I’m in some power structure where I decide it’s too risky. And those are the days – when I decide it’s too risky for me to speak up – when I come home exhausted. Because there will always be a power dynamic, as long as I’m alive, where you can’t speak up because you’re a Black woman, and that just wears me out.
Ultimately, I opted out of academic medicine because I thought it was too constraining, that I wouldn’t be able to raise my voice and do the activism I needed to do. – I’m able to advocate for people who are marginalized by medicine and, in treating addiction, advocate for people who are marginalized by psychiatry, which is marginalized by medicine.
A bias people have is that when you talk about Black people, they think you are talking about poor people. When we talk about police brutality, or being pulled over by the police, or dying in childbirth, our colleagues don’t think that’s happening to us. They think that’s happening to “those” Black people. Regardless of my socioeconomic status, I still have a higher chance of dying in childbirth or dying from COVID.
COVID had already turned my work up to 100 – we had staff losing loved ones and coming down with fevers themselves. And I had just launched my podcast. Then they killed Breonna Taylor, Ahmaud Arbery, Amy Cooper called the cops on Christian Cooper, and they killed George Floyd. This is how it happens. Bam. Bam. Bam.
The series of killings turned up my work at Physicians for Criminal Justice Reform, but it also turned up my work as a mother. My boys are 13 and 14. I personally can’t watch some of the videos because I see my own sons. I was already tired. Now I’m exhausted, I’m furious and I’m desperate to protect my kids. They have this on their backs already. Both of them have already had to deal with overt racism – they’ve had this burden since they were 5 years old, if not younger. I have to teach them to fight this war. Should that be how it is?
Nzinga Harrison, MD, 43, is a psychiatrist and the cofounder and chief medical officer of Eleanor Health, a network of physician clinics that treats people affected by addiction in North Carolina and New Jersey. She is also a cofounder of Physicians for Criminal Justice Reform. and host of the new podcast In Recovery. Harrison was raised in Indianapolis, went to college at Howard University and received her MD from the Perelman School of Medicine at the University of Pennsylvania in 2002. Her mother was an elementary school teacher. Her father, an electrical engineer, was commander of the local Black Panther Militia. Both supported her love of math and science and brought her with them to picket lines and marches.
This article first appeared on Medscape.com.
I didn’t have any doctors in my family. The only doctor I knew was my pediatrician. At 6 years old – and this gives you a glimpse into my personality – I told my parents I did not think he was a good doctor. I said, “When I grow up to be a doctor, I’m going to be a better doctor than him.” Fast forward to 7th grade, when I saw an orthopedic surgeon for my scoliosis. He was phenomenal. He listened. He explained to me all of the science and medicine and his rationale for decisions. I thought, “That is the kind of doctor I want to be.”
I went to medical school at Penn and didn’t think psychiatry was a medical specialty. I thought it was just Freud and laying on couches. I thought, “Where’s the science, where’s the physiology, where’s the genetics?” I was headed toward surgery.
Then, I rotated with an incredible psychiatrist. I saw behavior was biological, chemical, electrical, and physiological. I realize, looking back, that I had an interest because there is mental illness in my family. And there is so much stigma against psychiatric illnesses and addiction. It’s shocking how badly our patients get treated in the general medicine construct. So, I thought, “This field has science, the human body, activism, and marginalized patients? This is for me!”
I went to Howard University, which was the most freeing time of my life. There was no code-switching, no working hard to be a “presentable” Black person. When I started interviewing for medical schools, I was told by someone I interviewed with at one school that I should straighten my hair if I wanted to get accepted. I marked that school off my list. I decided right then that I would rather not go to medical school than straighten my hair to get into medical school. I went to Penn; they accepted me without my hair straight.
Penn Med was majorly White. There were six of us who were Black in a class of about 150 people. There was this feeling like “we let you in” even though every single one of us who was there was clearly at the top of the game to have been able to get there. I loved Penn Med. My class was amazing. I became the first Black president of medical student government there and I won a lot of awards.
When I was finishing up, my dean at the time, who was a White woman, said, “I’m so proud of you. You came in a piece of coal and look how we shined you up. “What do you say? I have a smart mouth, so I said, “I was already shiny when I got here.” She said, “See, that’s part of your problem, you don’t know how to take a compliment.” That was 2002, and I still remember every word of that conversation.
I was on the psychiatry unit rounding as a medical student and introduced myself to a patient. He said, “What’s your name?” And I thought, here it comes. I said, “Nzinga Ajabu,” my name at the time. He said “Nzinga? You probably have a spear in your closet.” When I tell these stories to White people, they’re always shocked. When I tell these stories to Black people, they say, “Yeah, that sounds about right.”
You can talk to Black medical students, Black interns, Black residents. When patients say something racist to you, nobody speaks up for you, nobody. It should be the attending that professionally approaches the patient and says something, anything. But they just laugh uncomfortably, they let it pass, they pretend they didn’t hear it. Meanwhile, you are fuming, and injured, and have to maintain your professionalism. It happens all the time. When people say, “Oh, you don’t look like a doctor,” I know what that means, but someone else may not even notice it’s an insult. When they do notice an insult, they don’t have the language or the courage to address it. And it’s not always a patient leveling racial insults. It very often is the attending, the fellow, the resident, or another medical student.
These things happen to me less now because I’m in a position of power. I’d say most insults that come my way now are overwhelmingly unintentional. I call people out on it 95% of the time. The other 5% of the time, I’m either exhausted, or I’m in some power structure where I decide it’s too risky. And those are the days – when I decide it’s too risky for me to speak up – when I come home exhausted. Because there will always be a power dynamic, as long as I’m alive, where you can’t speak up because you’re a Black woman, and that just wears me out.
Ultimately, I opted out of academic medicine because I thought it was too constraining, that I wouldn’t be able to raise my voice and do the activism I needed to do. – I’m able to advocate for people who are marginalized by medicine and, in treating addiction, advocate for people who are marginalized by psychiatry, which is marginalized by medicine.
A bias people have is that when you talk about Black people, they think you are talking about poor people. When we talk about police brutality, or being pulled over by the police, or dying in childbirth, our colleagues don’t think that’s happening to us. They think that’s happening to “those” Black people. Regardless of my socioeconomic status, I still have a higher chance of dying in childbirth or dying from COVID.
COVID had already turned my work up to 100 – we had staff losing loved ones and coming down with fevers themselves. And I had just launched my podcast. Then they killed Breonna Taylor, Ahmaud Arbery, Amy Cooper called the cops on Christian Cooper, and they killed George Floyd. This is how it happens. Bam. Bam. Bam.
The series of killings turned up my work at Physicians for Criminal Justice Reform, but it also turned up my work as a mother. My boys are 13 and 14. I personally can’t watch some of the videos because I see my own sons. I was already tired. Now I’m exhausted, I’m furious and I’m desperate to protect my kids. They have this on their backs already. Both of them have already had to deal with overt racism – they’ve had this burden since they were 5 years old, if not younger. I have to teach them to fight this war. Should that be how it is?
Nzinga Harrison, MD, 43, is a psychiatrist and the cofounder and chief medical officer of Eleanor Health, a network of physician clinics that treats people affected by addiction in North Carolina and New Jersey. She is also a cofounder of Physicians for Criminal Justice Reform. and host of the new podcast In Recovery. Harrison was raised in Indianapolis, went to college at Howard University and received her MD from the Perelman School of Medicine at the University of Pennsylvania in 2002. Her mother was an elementary school teacher. Her father, an electrical engineer, was commander of the local Black Panther Militia. Both supported her love of math and science and brought her with them to picket lines and marches.
This article first appeared on Medscape.com.
Injection beats pill for long-lasting HIV prevention
Injections of cabotegravir (ViiV Healthcare) given every other month are more effective in blocking HIV transmission than is the once-a-day combination of tenofovir disoproxil fumarate and emtricitabine (Truvada, Gilead Science), new data from the HPTN 083 trial show.
The findings “could transform the HIV prevention landscape for so many people,” said Megan Coleman, DNP, from Whitman-Walker Health in Washington, DC, who regularly prescribes Truvada as pre-exposure prophylaxis (PrEP).
At Whitman-Walker alone, about 3000 people were taking the pill in early 2020, but “for some people, taking a pill every day just isn’t a viable option,” said Coleman. “To have something that can support a patient’s choice and a patient’s ability to reduce their own risk of HIV is amazing.”
Final results from the trial — which looked at the drug in cisgender men and transgender women who have sex with men — were presented at the International AIDS Conference 2020.
Early Study Termination
Half of the 4566 study participants — from 43 sites in Africa, Asia, Latin America, and the United States — were younger than 30 years, 12.4% were transgender women, 29.7% were black, and 46.1% were Hispanic.
By design, ViiV Healthcare, the study sponsor, required that 50% of American participants be black to reflect the population at risk for HIV in the United States, said Raphael Landovitz, MD, from the UCLA David Geffen School of Medicine in Los Angeles, who is protocol chair for HPTN 083. In fact, 49.7% of the American cohort was black and 17.8% was Hispanic.
Patients randomized to the cabotegravir group received daily oral cabotegravir plus daily oral placebo for 5 weeks, to assess safety, followed by a cabotegravir injection at weeks 5 and 9 and every 2 months thereafter out to week 153 plus daily oral placebo. Patients randomized to the Truvada group received daily oral Truvada plus daily oral placebo for 5 weeks, followed by daily oral Truvada plus placebo injection, on the same schedule, out to week 153.
After the final injection, all participants continued on daily oral Truvada for 48 weeks.
The researchers expected to wait until 172 participants acquired HIV; they decided at the outset that this number would be sufficient to power a decision on whether or not cabotegravir injections are better than daily oral Truvada. But by May 2020, when 52 of the study participants had acquired HIV, the results were so lopsided in favor of cabotegravir that the trial was stopped. At that point, all participants were offered cabotegravir injections every 2 months.
Thirty-nine of the 52 (75%) new HIV infections occurred in the Truvada group. In fact, (hazard ratio, 0.34).
“This definitively establishes the superiority of cabotegravir,” said Landovitz.
He and his colleagues had been legitimately concerned that HIV acquisition would be so low in the trial that they wouldn’t be able to show how effective the injectable was. The success of Truvada PrEP has made it difficult to design prevention trials.
“We know that Truvada works extremely well, so the fact that we were able to show that cabotegravir in this population works better” is a powerful observation, said Landovitz. This is especially true because the rates of sexually transmitted infections — which are thought to increase risk for HIV transmission — were so high. Overall, 16.5% of the participants tested positive for syphilis during the trial, 13.3% tested positive for gonorrhea, and 21.1% tested positive for Chlamydia.
Five Surprising Seroconversions
Eleven of the 15 HIV infections in the cabotegravir group occurred in people who had received at least one injection. Three of these infections actually occurred during the first 5 weeks of the study when participants were taking oral cabotegravir, two occurred when participants chose to discontinue the injection and return to daily oral Truvada, and one occurred after a participant missed the injection for a prolonged period of time.
But five of the transmissions occurred in participants who appeared to be perfectly adherent.
Landovitz offered a number of possible reasons for this surprising finding.
“Number one could be that there’s something about these five particular individuals such that they grind up and eliminate the cabotegravir faster than other people, so an 8-week interval is too long for them,” he explained. “Another possibility, although pretty rare, is that there is a rare circulating virus that is intrinsically resistant to cabotegravir.”
Breakthrough HIV transmissions have been rare in people taking oral PrEP.
Disruptions caused by the COVID-19 pandemic have meant that the researchers don’t yet have the data on drug-resistant mutations or drug levels for these five participants, but they will.
“I suspect the truth is that there will never be a 100% failsafe HIV prevention mechanism,” said Landovitz.
“Impressive” Findings
The findings were greeted with excitement, although questions remain.
They are “impressive,” especially the data on black and Hispanic participants, said Paul Sax, MD, medical director of the Division of Infectious Diseases at Brigham and Women’s Hospital in Boston.
However, he said he is interested in the data showing that although participants in both groups gained weight during the study, there was early weight loss in the Truvada group, meaning that those in the cabotegravir group weighed more at the end of the study than those in the Truvada group.
“I’ve been watching the data on weight with integrase inhibitors,” he explained, including weight data specific to Truvada and to the combination of emtricitabine and tenofovir alafenamide (Descovy, Gilead). It looks like Truvada “has some sort of weight-suppressive effects. That’s going to be a thing we’re going to have to watch.”
Coleman said she is already thinking about patients at Whitman-Walker who might do well on cabotegravir and those who can start PrEP for the first time with this option.
“Not only would people probably switch to this option, but maybe people would be interested in starting a biomedical prevention approach that isn’t a pill every day,” she said. “It’s just exciting to have another option. Hopefully, in a few years, we’ll have implantable devices and rings; I can’t even imagine what all those brilliant minds are coming up with.”
But that’s still a ways off. First, cabotegravir has yet to be approved for HIV prevention, and ideally, eventually, there will be a way to determine if cabotegravir is safe for each patient that doesn’t involve a month of daily pills.
“We need to solve that problem because it’s so complicated to do an oral lead-in for a month or so,” said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases, National Institutes of Health. “Otherwise it’s not going to be feasible.”
We need to make sure this gets licensed for men and women and transgender individuals.
Even with these positive data, Dieffenbach and other officials are not keen to have ViiV apply for licensing right away. Last October, Descovy was the second oral PrEP pill approved for HIV prevention, but only for use by gay men and transgender women — it hadn’t been well studied in cisgender women — causing an outcry. Now, officials are suggesting that ViiV not make the same mistake.
They are urging the company to hold off until data from the sister study of the medication in women — HPTN 084 — is completed in 2022.
“We need to make sure this gets licensed for men and women and transgender individuals,” Dieffenbach told Medscape Medical News. “We just need to give this a little more time and then build a plan with contingencies, so that if something happens, we still have collected all the safety data in women so we can say it’s safe.”
ViiV seems to be making such a plan.
“Our goal is to seek approval across all genders and we will work with the FDA and other regulatory agencies to map out a plan to achieve this goal,” said Kimberly Smith, MD, head of research and development at ViiV Healthcare.
The World Health Organization (WHO), meanwhile, doesn’t expect to change its guidelines on HIV prevention medications until data from HPTN 084 are reported.
“What’s important when we look at guidelines is that we also look across populations,” said Meg Doherty, coordinator of treatment and care in the Department of HIV/AIDS at WHO. “We’re waiting to know more about how cabotegravir works in women, because we certainly want to have prevention drugs that can be used in men and women at different age ranges and, ideally, during pregnancy.”
International AIDS Conference 2020: Abstracts OAXLB01. Presented July 8, 2020.
This article first appeared on Medscape.com.
Injections of cabotegravir (ViiV Healthcare) given every other month are more effective in blocking HIV transmission than is the once-a-day combination of tenofovir disoproxil fumarate and emtricitabine (Truvada, Gilead Science), new data from the HPTN 083 trial show.
The findings “could transform the HIV prevention landscape for so many people,” said Megan Coleman, DNP, from Whitman-Walker Health in Washington, DC, who regularly prescribes Truvada as pre-exposure prophylaxis (PrEP).
At Whitman-Walker alone, about 3000 people were taking the pill in early 2020, but “for some people, taking a pill every day just isn’t a viable option,” said Coleman. “To have something that can support a patient’s choice and a patient’s ability to reduce their own risk of HIV is amazing.”
Final results from the trial — which looked at the drug in cisgender men and transgender women who have sex with men — were presented at the International AIDS Conference 2020.
Early Study Termination
Half of the 4566 study participants — from 43 sites in Africa, Asia, Latin America, and the United States — were younger than 30 years, 12.4% were transgender women, 29.7% were black, and 46.1% were Hispanic.
By design, ViiV Healthcare, the study sponsor, required that 50% of American participants be black to reflect the population at risk for HIV in the United States, said Raphael Landovitz, MD, from the UCLA David Geffen School of Medicine in Los Angeles, who is protocol chair for HPTN 083. In fact, 49.7% of the American cohort was black and 17.8% was Hispanic.
Patients randomized to the cabotegravir group received daily oral cabotegravir plus daily oral placebo for 5 weeks, to assess safety, followed by a cabotegravir injection at weeks 5 and 9 and every 2 months thereafter out to week 153 plus daily oral placebo. Patients randomized to the Truvada group received daily oral Truvada plus daily oral placebo for 5 weeks, followed by daily oral Truvada plus placebo injection, on the same schedule, out to week 153.
After the final injection, all participants continued on daily oral Truvada for 48 weeks.
The researchers expected to wait until 172 participants acquired HIV; they decided at the outset that this number would be sufficient to power a decision on whether or not cabotegravir injections are better than daily oral Truvada. But by May 2020, when 52 of the study participants had acquired HIV, the results were so lopsided in favor of cabotegravir that the trial was stopped. At that point, all participants were offered cabotegravir injections every 2 months.
Thirty-nine of the 52 (75%) new HIV infections occurred in the Truvada group. In fact, (hazard ratio, 0.34).
“This definitively establishes the superiority of cabotegravir,” said Landovitz.
He and his colleagues had been legitimately concerned that HIV acquisition would be so low in the trial that they wouldn’t be able to show how effective the injectable was. The success of Truvada PrEP has made it difficult to design prevention trials.
“We know that Truvada works extremely well, so the fact that we were able to show that cabotegravir in this population works better” is a powerful observation, said Landovitz. This is especially true because the rates of sexually transmitted infections — which are thought to increase risk for HIV transmission — were so high. Overall, 16.5% of the participants tested positive for syphilis during the trial, 13.3% tested positive for gonorrhea, and 21.1% tested positive for Chlamydia.
Five Surprising Seroconversions
Eleven of the 15 HIV infections in the cabotegravir group occurred in people who had received at least one injection. Three of these infections actually occurred during the first 5 weeks of the study when participants were taking oral cabotegravir, two occurred when participants chose to discontinue the injection and return to daily oral Truvada, and one occurred after a participant missed the injection for a prolonged period of time.
But five of the transmissions occurred in participants who appeared to be perfectly adherent.
Landovitz offered a number of possible reasons for this surprising finding.
“Number one could be that there’s something about these five particular individuals such that they grind up and eliminate the cabotegravir faster than other people, so an 8-week interval is too long for them,” he explained. “Another possibility, although pretty rare, is that there is a rare circulating virus that is intrinsically resistant to cabotegravir.”
Breakthrough HIV transmissions have been rare in people taking oral PrEP.
Disruptions caused by the COVID-19 pandemic have meant that the researchers don’t yet have the data on drug-resistant mutations or drug levels for these five participants, but they will.
“I suspect the truth is that there will never be a 100% failsafe HIV prevention mechanism,” said Landovitz.
“Impressive” Findings
The findings were greeted with excitement, although questions remain.
They are “impressive,” especially the data on black and Hispanic participants, said Paul Sax, MD, medical director of the Division of Infectious Diseases at Brigham and Women’s Hospital in Boston.
However, he said he is interested in the data showing that although participants in both groups gained weight during the study, there was early weight loss in the Truvada group, meaning that those in the cabotegravir group weighed more at the end of the study than those in the Truvada group.
“I’ve been watching the data on weight with integrase inhibitors,” he explained, including weight data specific to Truvada and to the combination of emtricitabine and tenofovir alafenamide (Descovy, Gilead). It looks like Truvada “has some sort of weight-suppressive effects. That’s going to be a thing we’re going to have to watch.”
Coleman said she is already thinking about patients at Whitman-Walker who might do well on cabotegravir and those who can start PrEP for the first time with this option.
“Not only would people probably switch to this option, but maybe people would be interested in starting a biomedical prevention approach that isn’t a pill every day,” she said. “It’s just exciting to have another option. Hopefully, in a few years, we’ll have implantable devices and rings; I can’t even imagine what all those brilliant minds are coming up with.”
But that’s still a ways off. First, cabotegravir has yet to be approved for HIV prevention, and ideally, eventually, there will be a way to determine if cabotegravir is safe for each patient that doesn’t involve a month of daily pills.
“We need to solve that problem because it’s so complicated to do an oral lead-in for a month or so,” said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases, National Institutes of Health. “Otherwise it’s not going to be feasible.”
We need to make sure this gets licensed for men and women and transgender individuals.
Even with these positive data, Dieffenbach and other officials are not keen to have ViiV apply for licensing right away. Last October, Descovy was the second oral PrEP pill approved for HIV prevention, but only for use by gay men and transgender women — it hadn’t been well studied in cisgender women — causing an outcry. Now, officials are suggesting that ViiV not make the same mistake.
They are urging the company to hold off until data from the sister study of the medication in women — HPTN 084 — is completed in 2022.
“We need to make sure this gets licensed for men and women and transgender individuals,” Dieffenbach told Medscape Medical News. “We just need to give this a little more time and then build a plan with contingencies, so that if something happens, we still have collected all the safety data in women so we can say it’s safe.”
ViiV seems to be making such a plan.
“Our goal is to seek approval across all genders and we will work with the FDA and other regulatory agencies to map out a plan to achieve this goal,” said Kimberly Smith, MD, head of research and development at ViiV Healthcare.
The World Health Organization (WHO), meanwhile, doesn’t expect to change its guidelines on HIV prevention medications until data from HPTN 084 are reported.
“What’s important when we look at guidelines is that we also look across populations,” said Meg Doherty, coordinator of treatment and care in the Department of HIV/AIDS at WHO. “We’re waiting to know more about how cabotegravir works in women, because we certainly want to have prevention drugs that can be used in men and women at different age ranges and, ideally, during pregnancy.”
International AIDS Conference 2020: Abstracts OAXLB01. Presented July 8, 2020.
This article first appeared on Medscape.com.
Injections of cabotegravir (ViiV Healthcare) given every other month are more effective in blocking HIV transmission than is the once-a-day combination of tenofovir disoproxil fumarate and emtricitabine (Truvada, Gilead Science), new data from the HPTN 083 trial show.
The findings “could transform the HIV prevention landscape for so many people,” said Megan Coleman, DNP, from Whitman-Walker Health in Washington, DC, who regularly prescribes Truvada as pre-exposure prophylaxis (PrEP).
At Whitman-Walker alone, about 3000 people were taking the pill in early 2020, but “for some people, taking a pill every day just isn’t a viable option,” said Coleman. “To have something that can support a patient’s choice and a patient’s ability to reduce their own risk of HIV is amazing.”
Final results from the trial — which looked at the drug in cisgender men and transgender women who have sex with men — were presented at the International AIDS Conference 2020.
Early Study Termination
Half of the 4566 study participants — from 43 sites in Africa, Asia, Latin America, and the United States — were younger than 30 years, 12.4% were transgender women, 29.7% were black, and 46.1% were Hispanic.
By design, ViiV Healthcare, the study sponsor, required that 50% of American participants be black to reflect the population at risk for HIV in the United States, said Raphael Landovitz, MD, from the UCLA David Geffen School of Medicine in Los Angeles, who is protocol chair for HPTN 083. In fact, 49.7% of the American cohort was black and 17.8% was Hispanic.
Patients randomized to the cabotegravir group received daily oral cabotegravir plus daily oral placebo for 5 weeks, to assess safety, followed by a cabotegravir injection at weeks 5 and 9 and every 2 months thereafter out to week 153 plus daily oral placebo. Patients randomized to the Truvada group received daily oral Truvada plus daily oral placebo for 5 weeks, followed by daily oral Truvada plus placebo injection, on the same schedule, out to week 153.
After the final injection, all participants continued on daily oral Truvada for 48 weeks.
The researchers expected to wait until 172 participants acquired HIV; they decided at the outset that this number would be sufficient to power a decision on whether or not cabotegravir injections are better than daily oral Truvada. But by May 2020, when 52 of the study participants had acquired HIV, the results were so lopsided in favor of cabotegravir that the trial was stopped. At that point, all participants were offered cabotegravir injections every 2 months.
Thirty-nine of the 52 (75%) new HIV infections occurred in the Truvada group. In fact, (hazard ratio, 0.34).
“This definitively establishes the superiority of cabotegravir,” said Landovitz.
He and his colleagues had been legitimately concerned that HIV acquisition would be so low in the trial that they wouldn’t be able to show how effective the injectable was. The success of Truvada PrEP has made it difficult to design prevention trials.
“We know that Truvada works extremely well, so the fact that we were able to show that cabotegravir in this population works better” is a powerful observation, said Landovitz. This is especially true because the rates of sexually transmitted infections — which are thought to increase risk for HIV transmission — were so high. Overall, 16.5% of the participants tested positive for syphilis during the trial, 13.3% tested positive for gonorrhea, and 21.1% tested positive for Chlamydia.
Five Surprising Seroconversions
Eleven of the 15 HIV infections in the cabotegravir group occurred in people who had received at least one injection. Three of these infections actually occurred during the first 5 weeks of the study when participants were taking oral cabotegravir, two occurred when participants chose to discontinue the injection and return to daily oral Truvada, and one occurred after a participant missed the injection for a prolonged period of time.
But five of the transmissions occurred in participants who appeared to be perfectly adherent.
Landovitz offered a number of possible reasons for this surprising finding.
“Number one could be that there’s something about these five particular individuals such that they grind up and eliminate the cabotegravir faster than other people, so an 8-week interval is too long for them,” he explained. “Another possibility, although pretty rare, is that there is a rare circulating virus that is intrinsically resistant to cabotegravir.”
Breakthrough HIV transmissions have been rare in people taking oral PrEP.
Disruptions caused by the COVID-19 pandemic have meant that the researchers don’t yet have the data on drug-resistant mutations or drug levels for these five participants, but they will.
“I suspect the truth is that there will never be a 100% failsafe HIV prevention mechanism,” said Landovitz.
“Impressive” Findings
The findings were greeted with excitement, although questions remain.
They are “impressive,” especially the data on black and Hispanic participants, said Paul Sax, MD, medical director of the Division of Infectious Diseases at Brigham and Women’s Hospital in Boston.
However, he said he is interested in the data showing that although participants in both groups gained weight during the study, there was early weight loss in the Truvada group, meaning that those in the cabotegravir group weighed more at the end of the study than those in the Truvada group.
“I’ve been watching the data on weight with integrase inhibitors,” he explained, including weight data specific to Truvada and to the combination of emtricitabine and tenofovir alafenamide (Descovy, Gilead). It looks like Truvada “has some sort of weight-suppressive effects. That’s going to be a thing we’re going to have to watch.”
Coleman said she is already thinking about patients at Whitman-Walker who might do well on cabotegravir and those who can start PrEP for the first time with this option.
“Not only would people probably switch to this option, but maybe people would be interested in starting a biomedical prevention approach that isn’t a pill every day,” she said. “It’s just exciting to have another option. Hopefully, in a few years, we’ll have implantable devices and rings; I can’t even imagine what all those brilliant minds are coming up with.”
But that’s still a ways off. First, cabotegravir has yet to be approved for HIV prevention, and ideally, eventually, there will be a way to determine if cabotegravir is safe for each patient that doesn’t involve a month of daily pills.
“We need to solve that problem because it’s so complicated to do an oral lead-in for a month or so,” said Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases, National Institutes of Health. “Otherwise it’s not going to be feasible.”
We need to make sure this gets licensed for men and women and transgender individuals.
Even with these positive data, Dieffenbach and other officials are not keen to have ViiV apply for licensing right away. Last October, Descovy was the second oral PrEP pill approved for HIV prevention, but only for use by gay men and transgender women — it hadn’t been well studied in cisgender women — causing an outcry. Now, officials are suggesting that ViiV not make the same mistake.
They are urging the company to hold off until data from the sister study of the medication in women — HPTN 084 — is completed in 2022.
“We need to make sure this gets licensed for men and women and transgender individuals,” Dieffenbach told Medscape Medical News. “We just need to give this a little more time and then build a plan with contingencies, so that if something happens, we still have collected all the safety data in women so we can say it’s safe.”
ViiV seems to be making such a plan.
“Our goal is to seek approval across all genders and we will work with the FDA and other regulatory agencies to map out a plan to achieve this goal,” said Kimberly Smith, MD, head of research and development at ViiV Healthcare.
The World Health Organization (WHO), meanwhile, doesn’t expect to change its guidelines on HIV prevention medications until data from HPTN 084 are reported.
“What’s important when we look at guidelines is that we also look across populations,” said Meg Doherty, coordinator of treatment and care in the Department of HIV/AIDS at WHO. “We’re waiting to know more about how cabotegravir works in women, because we certainly want to have prevention drugs that can be used in men and women at different age ranges and, ideally, during pregnancy.”
International AIDS Conference 2020: Abstracts OAXLB01. Presented July 8, 2020.
This article first appeared on Medscape.com.
Children rarely transmit SARS-CoV-2 within households
“Unlike with other viral respiratory infections, children do not seem to be a major vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, with most pediatric cases described inside familial clusters and no documentation of child-to-child or child-to-adult transmission,” said Klara M. Posfay-Barbe, MD, of the University of Geneva, Switzerland, and colleagues.
In a study published in Pediatrics, the researchers analyzed data from all COVID-19 patients younger than 16 years who were identified between March 10, 2020, and April 10, 2020, through a hospital surveillance network. Parents and household contacts were called for contact tracing.
In 31 of 39 (79%) households, at least one adult family member had a suspected or confirmed SARS-CoV-2 infection before onset of symptoms in the child. These findings support data from previous studies suggesting that children mainly become infected from adult family members rather than transmitting the virus to them, the researchers said
In only 3 of 39 (8%) households was the study child the first to develop symptoms. “Surprisingly, in 33% of households, symptomatic HHCs [household contacts] tested negative despite belonging to a familial cluster with confirmed SARS-CoV-2 cases, suggesting an underreporting of cases,” Dr. Posfay-Barbe and associates noted.
The findings were limited by several factors including potential underreporting of cases because those with mild or atypical presentations may not have sought medical care, and the inability to confirm child-to-adult transmission. The results were strengthened by the extensive contact tracing and very few individuals lost to follow-up, they said; however, more diagnostic screening and contact tracing are needed to improve understanding of household transmission of SARS-CoV-2, they concluded.
Resolving the issue of how much children contribute to transmission of SARS-CoV-2 is essential to making informed decisions about public health, including how to structure schools and child-care facility reopening, Benjamin Lee, MD, and William V. Raszka Jr., MD, both of the University of Vermont, Burlington, said in an accompanying editorial (Pediatrics. 2020 Jul 10. doi: 10.1542/peds/2020-004879).
The data in the current study support other studies of transmission among household contacts in China suggesting that, in most cases of childhood infections, “the child was not the source of infection and that children most frequently acquire COVID-19 from adults, rather than transmitting it to them,” they wrote.
In addition, the limited data on transmission of SARS-CoV-2 by children outside of the household show few cases of secondary infection from children identified with SARS-CoV-2 in school settings in studies from France and Australia, Dr. Lee and Dr. Raszka noted.
the editorialists wrote. “This would be another manner by which SARS-CoV2 differs drastically from influenza, for which school-based transmission is well recognized as a significant driver of epidemic disease and forms the basis for most evidence regarding school closures as public health strategy.”
“Therefore, serious consideration should be paid toward strategies that allow schools to remain open, even during periods of COVID-19 spread,” the editorialists concluded. “In doing so, we could minimize the potentially profound adverse social, developmental, and health costs that our children will continue to suffer until an effective treatment or vaccine can be developed and distributed or, failing that, until we reach herd immunity,” Dr. Lee and Dr. Raszka emphasized.
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
SOURCE: Posfay-Barbe KM et al. Pediatrics. 2020 Jul 10. doi: 10.1542/peds.2020-1576.
“Unlike with other viral respiratory infections, children do not seem to be a major vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, with most pediatric cases described inside familial clusters and no documentation of child-to-child or child-to-adult transmission,” said Klara M. Posfay-Barbe, MD, of the University of Geneva, Switzerland, and colleagues.
In a study published in Pediatrics, the researchers analyzed data from all COVID-19 patients younger than 16 years who were identified between March 10, 2020, and April 10, 2020, through a hospital surveillance network. Parents and household contacts were called for contact tracing.
In 31 of 39 (79%) households, at least one adult family member had a suspected or confirmed SARS-CoV-2 infection before onset of symptoms in the child. These findings support data from previous studies suggesting that children mainly become infected from adult family members rather than transmitting the virus to them, the researchers said
In only 3 of 39 (8%) households was the study child the first to develop symptoms. “Surprisingly, in 33% of households, symptomatic HHCs [household contacts] tested negative despite belonging to a familial cluster with confirmed SARS-CoV-2 cases, suggesting an underreporting of cases,” Dr. Posfay-Barbe and associates noted.
The findings were limited by several factors including potential underreporting of cases because those with mild or atypical presentations may not have sought medical care, and the inability to confirm child-to-adult transmission. The results were strengthened by the extensive contact tracing and very few individuals lost to follow-up, they said; however, more diagnostic screening and contact tracing are needed to improve understanding of household transmission of SARS-CoV-2, they concluded.
Resolving the issue of how much children contribute to transmission of SARS-CoV-2 is essential to making informed decisions about public health, including how to structure schools and child-care facility reopening, Benjamin Lee, MD, and William V. Raszka Jr., MD, both of the University of Vermont, Burlington, said in an accompanying editorial (Pediatrics. 2020 Jul 10. doi: 10.1542/peds/2020-004879).
The data in the current study support other studies of transmission among household contacts in China suggesting that, in most cases of childhood infections, “the child was not the source of infection and that children most frequently acquire COVID-19 from adults, rather than transmitting it to them,” they wrote.
In addition, the limited data on transmission of SARS-CoV-2 by children outside of the household show few cases of secondary infection from children identified with SARS-CoV-2 in school settings in studies from France and Australia, Dr. Lee and Dr. Raszka noted.
the editorialists wrote. “This would be another manner by which SARS-CoV2 differs drastically from influenza, for which school-based transmission is well recognized as a significant driver of epidemic disease and forms the basis for most evidence regarding school closures as public health strategy.”
“Therefore, serious consideration should be paid toward strategies that allow schools to remain open, even during periods of COVID-19 spread,” the editorialists concluded. “In doing so, we could minimize the potentially profound adverse social, developmental, and health costs that our children will continue to suffer until an effective treatment or vaccine can be developed and distributed or, failing that, until we reach herd immunity,” Dr. Lee and Dr. Raszka emphasized.
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
SOURCE: Posfay-Barbe KM et al. Pediatrics. 2020 Jul 10. doi: 10.1542/peds.2020-1576.
“Unlike with other viral respiratory infections, children do not seem to be a major vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, with most pediatric cases described inside familial clusters and no documentation of child-to-child or child-to-adult transmission,” said Klara M. Posfay-Barbe, MD, of the University of Geneva, Switzerland, and colleagues.
In a study published in Pediatrics, the researchers analyzed data from all COVID-19 patients younger than 16 years who were identified between March 10, 2020, and April 10, 2020, through a hospital surveillance network. Parents and household contacts were called for contact tracing.
In 31 of 39 (79%) households, at least one adult family member had a suspected or confirmed SARS-CoV-2 infection before onset of symptoms in the child. These findings support data from previous studies suggesting that children mainly become infected from adult family members rather than transmitting the virus to them, the researchers said
In only 3 of 39 (8%) households was the study child the first to develop symptoms. “Surprisingly, in 33% of households, symptomatic HHCs [household contacts] tested negative despite belonging to a familial cluster with confirmed SARS-CoV-2 cases, suggesting an underreporting of cases,” Dr. Posfay-Barbe and associates noted.
The findings were limited by several factors including potential underreporting of cases because those with mild or atypical presentations may not have sought medical care, and the inability to confirm child-to-adult transmission. The results were strengthened by the extensive contact tracing and very few individuals lost to follow-up, they said; however, more diagnostic screening and contact tracing are needed to improve understanding of household transmission of SARS-CoV-2, they concluded.
Resolving the issue of how much children contribute to transmission of SARS-CoV-2 is essential to making informed decisions about public health, including how to structure schools and child-care facility reopening, Benjamin Lee, MD, and William V. Raszka Jr., MD, both of the University of Vermont, Burlington, said in an accompanying editorial (Pediatrics. 2020 Jul 10. doi: 10.1542/peds/2020-004879).
The data in the current study support other studies of transmission among household contacts in China suggesting that, in most cases of childhood infections, “the child was not the source of infection and that children most frequently acquire COVID-19 from adults, rather than transmitting it to them,” they wrote.
In addition, the limited data on transmission of SARS-CoV-2 by children outside of the household show few cases of secondary infection from children identified with SARS-CoV-2 in school settings in studies from France and Australia, Dr. Lee and Dr. Raszka noted.
the editorialists wrote. “This would be another manner by which SARS-CoV2 differs drastically from influenza, for which school-based transmission is well recognized as a significant driver of epidemic disease and forms the basis for most evidence regarding school closures as public health strategy.”
“Therefore, serious consideration should be paid toward strategies that allow schools to remain open, even during periods of COVID-19 spread,” the editorialists concluded. “In doing so, we could minimize the potentially profound adverse social, developmental, and health costs that our children will continue to suffer until an effective treatment or vaccine can be developed and distributed or, failing that, until we reach herd immunity,” Dr. Lee and Dr. Raszka emphasized.
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
SOURCE: Posfay-Barbe KM et al. Pediatrics. 2020 Jul 10. doi: 10.1542/peds.2020-1576.
FROM PEDIATRICS
Perioperative sleep medicine: The Society of Anesthesia and Sleep Medicine
Obstructive sleep apnea (OSA) has been recognized to increase the risk of adverse cardiopulmonary perioperative outcomes for some time now.1 An ever growing body of literature supports this finding,2 including a large prospective study published in 2019 highlighting the significant risk of poor cardiac-related postoperative outcomes in patients with unrecognized OSA.3 As the majority of patients presenting for elective surgery with OSA will not be diagnosed at the time of presentation,3,4 many centers have developed preoperative screening programs to identify these patients, though the practice is not universal and a desire for better guidance is needed.5 In addition, best practices for patients with suspected or known OSA undergoing surgery have been a matter of debate. Out of these concerns, the Society of Anesthesia and Sleep Medicine (SASM) was formed over 10 years ago to promote interdisciplinary communication, education, and research into matters common to anesthesia and sleep.
Pulmonary and sleep medicine providers are often asked to provide preoperative clearance and recommendations for patients with suspected or known OSA. Recognizing the need for guidance in this area, a task force assembled by SASM obtained input from experts in anesthesiology, sleep medicine, and perioperative medicine to develop and publish an evidence-based / expert consensus guideline on the preoperative assessment and best practices for patients with suspected or known OSA.6 While specifics regarding logistics of preoperative screening and optimization of patients will vary based on each medical center’s infrastructure and organization, the recommendations presented should be able to be adapted by most, if not all, institutions. Preoperative evaluation and management is only part of the overall perioperative journey however, and SASM thus followed this document with guidelines for the intraoperative management of patients with OSA.7 To complete this set of recommendations, guidelines for the postoperative care of these patients are being planned. Guidelines for pediatric and obstetric perioperative OSA management are also currently being developed by SASM task forces to address these unique areas.
OSA is not the only sleep disorder where the perioperative environment may pose problems for our patients. Sleep disorders such as the hypersomnias and sleep-related movement disorders (including restless legs syndrome) may both impact and be impacted by the perioperative environment and may create safety concerns for some patients.8,9 These issues are also under active investigation by SASM. In addition, understanding the basic mechanisms determining unconsciousness in both anesthesia and sleep, as well as examination of the interrelationships between sleep disturbance, sedation and their effects on clinical outcomes, are areas of interest that have implications beyond the perioperative arena.
SASM is currently planning to host its 10th anniversary conference in Washington DC on October 1-2, public health issues permitting. The meeting has consistently enlisted expert speakers from anesthesia, sleep medicine, and other relevant fields, and this year will be no different. Given the host city, discussions on important healthcare policy issues will be included, as well. Registration for the meeting, as well as meeting updates, are on the SASM website (sasmhq.org).
Dr. Auckley is with the Division of Pulmonary, Critical Care and Sleep Medicine, MetroHealth Medical Center, Professor of Medicine, Case Western Reserve University, Cleveland, OH. He is the current president of the Society of Anesthesia and Sleep Medicine.
References
1. Gupta RM, et al. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: A case-control study. Mayo Clin Proc. 2001;76(9):897.
2. Opperer M, et al. Does obstructive sleep apnea influence perioperative outcome? A qualitative systematic review for the Society of Anesthesia and Sleep Medicine Task Force on Preoperative Preparation of Patients with Sleep-Disordered Breathing. Anesth Analg. 2016;122(5):1321.
3. Chan MTV, et al. Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery. JAMA. 2019;321(18):1788.
4. Finkel KJ, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic center. Sleep Med. 2009;10(7):753.
5. Auckley D, et al. Attitudes regarding perioperative care of patients with OSA: a survey study of four specialties in the United States. Sleep Breath. 2015;19(1):315.
6. Chung F, et al. Society of Anesthesia and Sleep Medicine Guidelines (SASM) on Preoperative Screening and Assessment of Adult Patients with Obstructive Sleep Apnea. Anesth Analg. 2016;123(2):452.
7. Memtsoudis SG, et al. Society of Anesthesia and Sleep Medicine Guideline (SASM) on Intraoperative Management of Adult Patients with Obstructive Sleep Apnea. Anesth Analg. 2018;127(4):967.
8. Hershner S, et al. Knowledge gaps in the perioperative management of adults with narcolepsy: A call for further research. Anesth Analg. 2019 Jul;129(1):204.
9. Goldstein C. Management of restless legs syndrome / Willis-Ekbom disease in hospitalized and perioperative patients. Sleep Med Clin. 2015;10(3):303.
Obstructive sleep apnea (OSA) has been recognized to increase the risk of adverse cardiopulmonary perioperative outcomes for some time now.1 An ever growing body of literature supports this finding,2 including a large prospective study published in 2019 highlighting the significant risk of poor cardiac-related postoperative outcomes in patients with unrecognized OSA.3 As the majority of patients presenting for elective surgery with OSA will not be diagnosed at the time of presentation,3,4 many centers have developed preoperative screening programs to identify these patients, though the practice is not universal and a desire for better guidance is needed.5 In addition, best practices for patients with suspected or known OSA undergoing surgery have been a matter of debate. Out of these concerns, the Society of Anesthesia and Sleep Medicine (SASM) was formed over 10 years ago to promote interdisciplinary communication, education, and research into matters common to anesthesia and sleep.
Pulmonary and sleep medicine providers are often asked to provide preoperative clearance and recommendations for patients with suspected or known OSA. Recognizing the need for guidance in this area, a task force assembled by SASM obtained input from experts in anesthesiology, sleep medicine, and perioperative medicine to develop and publish an evidence-based / expert consensus guideline on the preoperative assessment and best practices for patients with suspected or known OSA.6 While specifics regarding logistics of preoperative screening and optimization of patients will vary based on each medical center’s infrastructure and organization, the recommendations presented should be able to be adapted by most, if not all, institutions. Preoperative evaluation and management is only part of the overall perioperative journey however, and SASM thus followed this document with guidelines for the intraoperative management of patients with OSA.7 To complete this set of recommendations, guidelines for the postoperative care of these patients are being planned. Guidelines for pediatric and obstetric perioperative OSA management are also currently being developed by SASM task forces to address these unique areas.
OSA is not the only sleep disorder where the perioperative environment may pose problems for our patients. Sleep disorders such as the hypersomnias and sleep-related movement disorders (including restless legs syndrome) may both impact and be impacted by the perioperative environment and may create safety concerns for some patients.8,9 These issues are also under active investigation by SASM. In addition, understanding the basic mechanisms determining unconsciousness in both anesthesia and sleep, as well as examination of the interrelationships between sleep disturbance, sedation and their effects on clinical outcomes, are areas of interest that have implications beyond the perioperative arena.
SASM is currently planning to host its 10th anniversary conference in Washington DC on October 1-2, public health issues permitting. The meeting has consistently enlisted expert speakers from anesthesia, sleep medicine, and other relevant fields, and this year will be no different. Given the host city, discussions on important healthcare policy issues will be included, as well. Registration for the meeting, as well as meeting updates, are on the SASM website (sasmhq.org).
Dr. Auckley is with the Division of Pulmonary, Critical Care and Sleep Medicine, MetroHealth Medical Center, Professor of Medicine, Case Western Reserve University, Cleveland, OH. He is the current president of the Society of Anesthesia and Sleep Medicine.
References
1. Gupta RM, et al. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: A case-control study. Mayo Clin Proc. 2001;76(9):897.
2. Opperer M, et al. Does obstructive sleep apnea influence perioperative outcome? A qualitative systematic review for the Society of Anesthesia and Sleep Medicine Task Force on Preoperative Preparation of Patients with Sleep-Disordered Breathing. Anesth Analg. 2016;122(5):1321.
3. Chan MTV, et al. Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery. JAMA. 2019;321(18):1788.
4. Finkel KJ, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic center. Sleep Med. 2009;10(7):753.
5. Auckley D, et al. Attitudes regarding perioperative care of patients with OSA: a survey study of four specialties in the United States. Sleep Breath. 2015;19(1):315.
6. Chung F, et al. Society of Anesthesia and Sleep Medicine Guidelines (SASM) on Preoperative Screening and Assessment of Adult Patients with Obstructive Sleep Apnea. Anesth Analg. 2016;123(2):452.
7. Memtsoudis SG, et al. Society of Anesthesia and Sleep Medicine Guideline (SASM) on Intraoperative Management of Adult Patients with Obstructive Sleep Apnea. Anesth Analg. 2018;127(4):967.
8. Hershner S, et al. Knowledge gaps in the perioperative management of adults with narcolepsy: A call for further research. Anesth Analg. 2019 Jul;129(1):204.
9. Goldstein C. Management of restless legs syndrome / Willis-Ekbom disease in hospitalized and perioperative patients. Sleep Med Clin. 2015;10(3):303.
Obstructive sleep apnea (OSA) has been recognized to increase the risk of adverse cardiopulmonary perioperative outcomes for some time now.1 An ever growing body of literature supports this finding,2 including a large prospective study published in 2019 highlighting the significant risk of poor cardiac-related postoperative outcomes in patients with unrecognized OSA.3 As the majority of patients presenting for elective surgery with OSA will not be diagnosed at the time of presentation,3,4 many centers have developed preoperative screening programs to identify these patients, though the practice is not universal and a desire for better guidance is needed.5 In addition, best practices for patients with suspected or known OSA undergoing surgery have been a matter of debate. Out of these concerns, the Society of Anesthesia and Sleep Medicine (SASM) was formed over 10 years ago to promote interdisciplinary communication, education, and research into matters common to anesthesia and sleep.
Pulmonary and sleep medicine providers are often asked to provide preoperative clearance and recommendations for patients with suspected or known OSA. Recognizing the need for guidance in this area, a task force assembled by SASM obtained input from experts in anesthesiology, sleep medicine, and perioperative medicine to develop and publish an evidence-based / expert consensus guideline on the preoperative assessment and best practices for patients with suspected or known OSA.6 While specifics regarding logistics of preoperative screening and optimization of patients will vary based on each medical center’s infrastructure and organization, the recommendations presented should be able to be adapted by most, if not all, institutions. Preoperative evaluation and management is only part of the overall perioperative journey however, and SASM thus followed this document with guidelines for the intraoperative management of patients with OSA.7 To complete this set of recommendations, guidelines for the postoperative care of these patients are being planned. Guidelines for pediatric and obstetric perioperative OSA management are also currently being developed by SASM task forces to address these unique areas.
OSA is not the only sleep disorder where the perioperative environment may pose problems for our patients. Sleep disorders such as the hypersomnias and sleep-related movement disorders (including restless legs syndrome) may both impact and be impacted by the perioperative environment and may create safety concerns for some patients.8,9 These issues are also under active investigation by SASM. In addition, understanding the basic mechanisms determining unconsciousness in both anesthesia and sleep, as well as examination of the interrelationships between sleep disturbance, sedation and their effects on clinical outcomes, are areas of interest that have implications beyond the perioperative arena.
SASM is currently planning to host its 10th anniversary conference in Washington DC on October 1-2, public health issues permitting. The meeting has consistently enlisted expert speakers from anesthesia, sleep medicine, and other relevant fields, and this year will be no different. Given the host city, discussions on important healthcare policy issues will be included, as well. Registration for the meeting, as well as meeting updates, are on the SASM website (sasmhq.org).
Dr. Auckley is with the Division of Pulmonary, Critical Care and Sleep Medicine, MetroHealth Medical Center, Professor of Medicine, Case Western Reserve University, Cleveland, OH. He is the current president of the Society of Anesthesia and Sleep Medicine.
References
1. Gupta RM, et al. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: A case-control study. Mayo Clin Proc. 2001;76(9):897.
2. Opperer M, et al. Does obstructive sleep apnea influence perioperative outcome? A qualitative systematic review for the Society of Anesthesia and Sleep Medicine Task Force on Preoperative Preparation of Patients with Sleep-Disordered Breathing. Anesth Analg. 2016;122(5):1321.
3. Chan MTV, et al. Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery. JAMA. 2019;321(18):1788.
4. Finkel KJ, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic center. Sleep Med. 2009;10(7):753.
5. Auckley D, et al. Attitudes regarding perioperative care of patients with OSA: a survey study of four specialties in the United States. Sleep Breath. 2015;19(1):315.
6. Chung F, et al. Society of Anesthesia and Sleep Medicine Guidelines (SASM) on Preoperative Screening and Assessment of Adult Patients with Obstructive Sleep Apnea. Anesth Analg. 2016;123(2):452.
7. Memtsoudis SG, et al. Society of Anesthesia and Sleep Medicine Guideline (SASM) on Intraoperative Management of Adult Patients with Obstructive Sleep Apnea. Anesth Analg. 2018;127(4):967.
8. Hershner S, et al. Knowledge gaps in the perioperative management of adults with narcolepsy: A call for further research. Anesth Analg. 2019 Jul;129(1):204.
9. Goldstein C. Management of restless legs syndrome / Willis-Ekbom disease in hospitalized and perioperative patients. Sleep Med Clin. 2015;10(3):303.
COVID-19 and the future of telehealth for sleep medicine
On March 18, 2020, the doors to our sleep center were physically closed. Two potential exposures to COVID-19 within a few hours, the palpable anxiety of our team, and a poor grasp of the virus and the growing pandemic moved us to make this decision. Up to that point, we could not help but feel we were playing “catch up” with our evolving set of safety measures to the escalating risk. Like so many other sleep centers around the country, a complete transition to virtual care was needed to ensure the safety of our patients and our team. It was perhaps that moment that we felt the emotional impact that our world had changed, altering both our personal lives and sleep medicine practice as we knew it. This event, while unfortunate, also provided a transformative opportunity to reimagine our identity, accelerating the efforts to bring the future of sleep medicine into the present.
Our team’s clinical evolution and innovation efforts have been guided by efforts to reconsider sleep medicine paradigms. Innovation progress was deliberate with incremental implementations that typically required repeat business cases with multiple approving parties and budgetary access. Those barriers largely dissolved once COVID-19 intensified, and a large portion of the strategies on our roadmap were put into production. In a matter of a couple weeks, our services completely transitioned to remote and virtual care, while most of the team of 55 persons were moved to “work-from-home.” A suite of technologies (automated questionnaires, automated and two-way text messaging templates, consumer wearable technologies, and population management dashboards) were put on the table (Somnoware, Inc.), and each of our longitudinal care teams (eg, adult obstructive sleep apnea, pediatrics, chronic respiratory failure, commercial driver, insomnia programs, etc) worked to embed them into new care pathways. This effort further consolidated technology as the backbone of our work and the enabler of remote virtual collaboration between sleep center personnel (respiratory case managers, medical assistants and nursing team, and physician and leadership personnel) to enhance our team-based approach. Moreover, we felt this point in time was ripe to swallow the proverbial “red pill” and approach patient care with shifted paradigms. We discuss three areas of active effort to leverage technology in this COVID-19 environment to accelerate a transition toward how we envision the future of sleep medicine.
Reimagined sleep diagnostics
Our virtual obstructive sleep apnea (OSA) diagnostic process includes utilizing a disposable home sleep apnea test (HSAT) device with wireless data transfer (WatchPAT ONE, Itamar Medical) while HSAT and PAP (positive airway pressure) setups are supported by information sheets, online videos (YouTube), automated interactive platforms (Emmi Solutions; Hwang D. Am J Respir Crit Care Med. 2018 Jan 1;197[1]:117), and synchronous provider video visits. Our more radical shift, however, is in approaching OSA diagnosis based principally on symptoms and secondarily supported by physiologic measurements and response to therapy. This “clinical diagnosis” approach reduces our reliance on traditional sleep testing and allows patient wearables to provide supportive physiologic data (eg, oximetry) to help determine OSA severity and phenotype. Its immediate impact is in limiting the need to send and retrieve potentially contaminated equipment. Broader clinical advantages include overcoming the imprecise nature of the apnea-hypopnea index (which often has dramatic night-to-night variability) through data collection over extended durations, improving disease assessment due to availability of complementary sleep/activity data in the person’s usual setting, and tracking changes after therapy initiation.
Our post-COVID-19 re-opening of polysomnography (PSG) services, after a temporary shutdown, introduces home PSG (Type II) for approximately half our patients without suspected complex breathing conditions while reserving attended PSG (Type I) for those who may require noninvasive ventilation. The immediate incentive is in reducing viral exposure by limiting patient traffic and risk of PAP trial aerosolization while also improving access to accommodate the backlog of patients requiring PSG. This approach furthers the paradigm shift to emphasizing care in the home setting. Testing in the patient’s usual environment and enabling multiple night/day testing may be clinically advantageous.
Shift in emphasis to care management
The emphasis of sleep medicine has traditionally focused on diagnostics through performing PSG and HSAT. Our field has invested tremendous effort in developing guidelines for processing sleep studies, but the scoring and interpretation of those studies is extremely labor intensive. Reimagining the diagnostic approach reduces the need to manually process studies—wearable data are produced automatically, HSAT can be auto-scored, and artificial intelligence platforms can score PSGs (Goldstein CA. J Clin Sleep Med. 2020 Apr 15;16[4]:609), which allows a shift in resources and emphasis to follow-up care. A comprehensive discussion of technology-based tools to enhance care management is beyond the purview of this editorial. However, an overview of our current efforts includes: (1) utilizing population management dashboards to automatically risk stratify different cohorts of patients (eg, adult OSA, pediatrics, commercial drivers, chronic respiratory failure, etc) to identify patients “at-risk” (eg, based on OSA severity, symptoms, co-morbidities, and PAP adherence); (2) applying enhanced patient-provider interchange tools that include automated and “intelligent” electronic questionnaires, automated personalized text messaging/emails, and two-way messaging to deliver care; (3) utilizing remote patient monitoring to enhance holistic, personalized management, such as with remote activity/sleep trackers, blood pressure monitors, glucometers, and weight scales. We are engaged with efforts to validate the impact of these data to provide more personalized feedback, directly impact clinical outcomes, facilitate interdisciplinary collaboration, and identify acutely ill patients. Furthermore, a holistic approach beyond a narrow focus on PAP may create a positive collateral effect on adherence by targeting engagement with broader areas of health; and (4) implementing machine learning tools to directly support providers and patients (examples discussed in the next section.) Each of our teams has created workflows embedding these strategies throughout new care pathways. 
Generally, our emphasis during the first 3 months after PAP initiation focuses on achieving therapy adherence, and the post-3-month period broadens the efforts to target clinical outcomes. Recent trials with low PAP usage that failed to confirm the benefit of PAP on cardiovascular outcomes (McEvoy DR, et al. N Engl J Med. 2016;375:919) strongly suggest greater investment in cost-effective long-term strategies is imperative to increase our field’s relevance.
Application of artificial intelligence
We describe current efforts to apply artificial intelligence (AI) into clinical care: (1) We are implementing machine learning (ML) PSG scoring, which can potentially improve both the consistency and efficiency of scoring, further enabling greater investment in follow-up care. The future of sleep study processing, however, will likely depend on computer vision to “view” details inaccessible to the human eye and produce novel metrics that better inform clinical phenotypes (eg, cardiovascular risk, response to alternative therapies, etc). For example, “brain age” has been derived from EEG tracings that could reflect the degree of impact of sleep disorders on neurocognitive function (Fernandez C, unpublished data); (2) Machine learning clinical decision tools are in development to predict PAP adherence and timing of discontinuation, predict timing of cardiovascular disease onset and hospitalization, personalizing adherence targets, automating triaging of patients to home or PSG testing, and innumerable other predictions at clinical decision inflection points. Prediction outputs may be presented as risk profiles embedded in each patient’s “chart,” as personalized alerts, and in gamification strategies. For example, machine learning personalized cardiovascular risk scores can be regularly updated based on degree of PAP use to incentivize adherence; (3) Artificial providers may provide consistent, personalized, and holistic supplementary care. Many people rely on AI-bots for social support and cognitive-behavioral therapy (CBT) for depression. A sleep wellness bot, currently in planning stages, is intended to be the primary interface for many of the strategies described above that enhance engagement with PAP and therapies for comorbid conditions, provide CBT and lifestyle accountability, and collect patient reported data. This artificial provider would be a constant companion providing interactive, personalized, and continuous management to complement traditional intermittent live-person care.
The current health-care environment embodies the principle to “never let a serious crisis go to waste.” COVID-19 has accelerated the progression into the future by fostering an opening to embrace novel application of technologies to support changes in paradigms. Furthermore, health-care infrastructures that typically progress deliberately changed seemingly in a single moment. The Center for Medicare Services issued broad authorization to reimburse for telemedicine in response to COVID-19. Continued evolution in infrastructures will dictate progress with innovation, and a greater transition to outcomes-based incentives may be necessary to accommodate many of the strategies described above that rely on nonsynchronous care. But, we may be experiencing the moment when health care starts to catch up with the world in its embrace of technology. Sleep and pulmonary medicine can be a leader by providing a successful template for other specialties in optimizing chronic disease management.
Dr. Hwang is Medical Director, Kaiser Permanente SBC Sleep Center, and co-chair, Sleep Medicine, Kaiser Permanente Southern California.
On March 18, 2020, the doors to our sleep center were physically closed. Two potential exposures to COVID-19 within a few hours, the palpable anxiety of our team, and a poor grasp of the virus and the growing pandemic moved us to make this decision. Up to that point, we could not help but feel we were playing “catch up” with our evolving set of safety measures to the escalating risk. Like so many other sleep centers around the country, a complete transition to virtual care was needed to ensure the safety of our patients and our team. It was perhaps that moment that we felt the emotional impact that our world had changed, altering both our personal lives and sleep medicine practice as we knew it. This event, while unfortunate, also provided a transformative opportunity to reimagine our identity, accelerating the efforts to bring the future of sleep medicine into the present.
Our team’s clinical evolution and innovation efforts have been guided by efforts to reconsider sleep medicine paradigms. Innovation progress was deliberate with incremental implementations that typically required repeat business cases with multiple approving parties and budgetary access. Those barriers largely dissolved once COVID-19 intensified, and a large portion of the strategies on our roadmap were put into production. In a matter of a couple weeks, our services completely transitioned to remote and virtual care, while most of the team of 55 persons were moved to “work-from-home.” A suite of technologies (automated questionnaires, automated and two-way text messaging templates, consumer wearable technologies, and population management dashboards) were put on the table (Somnoware, Inc.), and each of our longitudinal care teams (eg, adult obstructive sleep apnea, pediatrics, chronic respiratory failure, commercial driver, insomnia programs, etc) worked to embed them into new care pathways. This effort further consolidated technology as the backbone of our work and the enabler of remote virtual collaboration between sleep center personnel (respiratory case managers, medical assistants and nursing team, and physician and leadership personnel) to enhance our team-based approach. Moreover, we felt this point in time was ripe to swallow the proverbial “red pill” and approach patient care with shifted paradigms. We discuss three areas of active effort to leverage technology in this COVID-19 environment to accelerate a transition toward how we envision the future of sleep medicine.
Reimagined sleep diagnostics
Our virtual obstructive sleep apnea (OSA) diagnostic process includes utilizing a disposable home sleep apnea test (HSAT) device with wireless data transfer (WatchPAT ONE, Itamar Medical) while HSAT and PAP (positive airway pressure) setups are supported by information sheets, online videos (YouTube), automated interactive platforms (Emmi Solutions; Hwang D. Am J Respir Crit Care Med. 2018 Jan 1;197[1]:117), and synchronous provider video visits. Our more radical shift, however, is in approaching OSA diagnosis based principally on symptoms and secondarily supported by physiologic measurements and response to therapy. This “clinical diagnosis” approach reduces our reliance on traditional sleep testing and allows patient wearables to provide supportive physiologic data (eg, oximetry) to help determine OSA severity and phenotype. Its immediate impact is in limiting the need to send and retrieve potentially contaminated equipment. Broader clinical advantages include overcoming the imprecise nature of the apnea-hypopnea index (which often has dramatic night-to-night variability) through data collection over extended durations, improving disease assessment due to availability of complementary sleep/activity data in the person’s usual setting, and tracking changes after therapy initiation.
Our post-COVID-19 re-opening of polysomnography (PSG) services, after a temporary shutdown, introduces home PSG (Type II) for approximately half our patients without suspected complex breathing conditions while reserving attended PSG (Type I) for those who may require noninvasive ventilation. The immediate incentive is in reducing viral exposure by limiting patient traffic and risk of PAP trial aerosolization while also improving access to accommodate the backlog of patients requiring PSG. This approach furthers the paradigm shift to emphasizing care in the home setting. Testing in the patient’s usual environment and enabling multiple night/day testing may be clinically advantageous.
Shift in emphasis to care management
The emphasis of sleep medicine has traditionally focused on diagnostics through performing PSG and HSAT. Our field has invested tremendous effort in developing guidelines for processing sleep studies, but the scoring and interpretation of those studies is extremely labor intensive. Reimagining the diagnostic approach reduces the need to manually process studies—wearable data are produced automatically, HSAT can be auto-scored, and artificial intelligence platforms can score PSGs (Goldstein CA. J Clin Sleep Med. 2020 Apr 15;16[4]:609), which allows a shift in resources and emphasis to follow-up care. A comprehensive discussion of technology-based tools to enhance care management is beyond the purview of this editorial. However, an overview of our current efforts includes: (1) utilizing population management dashboards to automatically risk stratify different cohorts of patients (eg, adult OSA, pediatrics, commercial drivers, chronic respiratory failure, etc) to identify patients “at-risk” (eg, based on OSA severity, symptoms, co-morbidities, and PAP adherence); (2) applying enhanced patient-provider interchange tools that include automated and “intelligent” electronic questionnaires, automated personalized text messaging/emails, and two-way messaging to deliver care; (3) utilizing remote patient monitoring to enhance holistic, personalized management, such as with remote activity/sleep trackers, blood pressure monitors, glucometers, and weight scales. We are engaged with efforts to validate the impact of these data to provide more personalized feedback, directly impact clinical outcomes, facilitate interdisciplinary collaboration, and identify acutely ill patients. Furthermore, a holistic approach beyond a narrow focus on PAP may create a positive collateral effect on adherence by targeting engagement with broader areas of health; and (4) implementing machine learning tools to directly support providers and patients (examples discussed in the next section.) Each of our teams has created workflows embedding these strategies throughout new care pathways. 
Generally, our emphasis during the first 3 months after PAP initiation focuses on achieving therapy adherence, and the post-3-month period broadens the efforts to target clinical outcomes. Recent trials with low PAP usage that failed to confirm the benefit of PAP on cardiovascular outcomes (McEvoy DR, et al. N Engl J Med. 2016;375:919) strongly suggest greater investment in cost-effective long-term strategies is imperative to increase our field’s relevance.
Application of artificial intelligence
We describe current efforts to apply artificial intelligence (AI) into clinical care: (1) We are implementing machine learning (ML) PSG scoring, which can potentially improve both the consistency and efficiency of scoring, further enabling greater investment in follow-up care. The future of sleep study processing, however, will likely depend on computer vision to “view” details inaccessible to the human eye and produce novel metrics that better inform clinical phenotypes (eg, cardiovascular risk, response to alternative therapies, etc). For example, “brain age” has been derived from EEG tracings that could reflect the degree of impact of sleep disorders on neurocognitive function (Fernandez C, unpublished data); (2) Machine learning clinical decision tools are in development to predict PAP adherence and timing of discontinuation, predict timing of cardiovascular disease onset and hospitalization, personalizing adherence targets, automating triaging of patients to home or PSG testing, and innumerable other predictions at clinical decision inflection points. Prediction outputs may be presented as risk profiles embedded in each patient’s “chart,” as personalized alerts, and in gamification strategies. For example, machine learning personalized cardiovascular risk scores can be regularly updated based on degree of PAP use to incentivize adherence; (3) Artificial providers may provide consistent, personalized, and holistic supplementary care. Many people rely on AI-bots for social support and cognitive-behavioral therapy (CBT) for depression. A sleep wellness bot, currently in planning stages, is intended to be the primary interface for many of the strategies described above that enhance engagement with PAP and therapies for comorbid conditions, provide CBT and lifestyle accountability, and collect patient reported data. This artificial provider would be a constant companion providing interactive, personalized, and continuous management to complement traditional intermittent live-person care.
The current health-care environment embodies the principle to “never let a serious crisis go to waste.” COVID-19 has accelerated the progression into the future by fostering an opening to embrace novel application of technologies to support changes in paradigms. Furthermore, health-care infrastructures that typically progress deliberately changed seemingly in a single moment. The Center for Medicare Services issued broad authorization to reimburse for telemedicine in response to COVID-19. Continued evolution in infrastructures will dictate progress with innovation, and a greater transition to outcomes-based incentives may be necessary to accommodate many of the strategies described above that rely on nonsynchronous care. But, we may be experiencing the moment when health care starts to catch up with the world in its embrace of technology. Sleep and pulmonary medicine can be a leader by providing a successful template for other specialties in optimizing chronic disease management.
Dr. Hwang is Medical Director, Kaiser Permanente SBC Sleep Center, and co-chair, Sleep Medicine, Kaiser Permanente Southern California.
On March 18, 2020, the doors to our sleep center were physically closed. Two potential exposures to COVID-19 within a few hours, the palpable anxiety of our team, and a poor grasp of the virus and the growing pandemic moved us to make this decision. Up to that point, we could not help but feel we were playing “catch up” with our evolving set of safety measures to the escalating risk. Like so many other sleep centers around the country, a complete transition to virtual care was needed to ensure the safety of our patients and our team. It was perhaps that moment that we felt the emotional impact that our world had changed, altering both our personal lives and sleep medicine practice as we knew it. This event, while unfortunate, also provided a transformative opportunity to reimagine our identity, accelerating the efforts to bring the future of sleep medicine into the present.
Our team’s clinical evolution and innovation efforts have been guided by efforts to reconsider sleep medicine paradigms. Innovation progress was deliberate with incremental implementations that typically required repeat business cases with multiple approving parties and budgetary access. Those barriers largely dissolved once COVID-19 intensified, and a large portion of the strategies on our roadmap were put into production. In a matter of a couple weeks, our services completely transitioned to remote and virtual care, while most of the team of 55 persons were moved to “work-from-home.” A suite of technologies (automated questionnaires, automated and two-way text messaging templates, consumer wearable technologies, and population management dashboards) were put on the table (Somnoware, Inc.), and each of our longitudinal care teams (eg, adult obstructive sleep apnea, pediatrics, chronic respiratory failure, commercial driver, insomnia programs, etc) worked to embed them into new care pathways. This effort further consolidated technology as the backbone of our work and the enabler of remote virtual collaboration between sleep center personnel (respiratory case managers, medical assistants and nursing team, and physician and leadership personnel) to enhance our team-based approach. Moreover, we felt this point in time was ripe to swallow the proverbial “red pill” and approach patient care with shifted paradigms. We discuss three areas of active effort to leverage technology in this COVID-19 environment to accelerate a transition toward how we envision the future of sleep medicine.
Reimagined sleep diagnostics
Our virtual obstructive sleep apnea (OSA) diagnostic process includes utilizing a disposable home sleep apnea test (HSAT) device with wireless data transfer (WatchPAT ONE, Itamar Medical) while HSAT and PAP (positive airway pressure) setups are supported by information sheets, online videos (YouTube), automated interactive platforms (Emmi Solutions; Hwang D. Am J Respir Crit Care Med. 2018 Jan 1;197[1]:117), and synchronous provider video visits. Our more radical shift, however, is in approaching OSA diagnosis based principally on symptoms and secondarily supported by physiologic measurements and response to therapy. This “clinical diagnosis” approach reduces our reliance on traditional sleep testing and allows patient wearables to provide supportive physiologic data (eg, oximetry) to help determine OSA severity and phenotype. Its immediate impact is in limiting the need to send and retrieve potentially contaminated equipment. Broader clinical advantages include overcoming the imprecise nature of the apnea-hypopnea index (which often has dramatic night-to-night variability) through data collection over extended durations, improving disease assessment due to availability of complementary sleep/activity data in the person’s usual setting, and tracking changes after therapy initiation.
Our post-COVID-19 re-opening of polysomnography (PSG) services, after a temporary shutdown, introduces home PSG (Type II) for approximately half our patients without suspected complex breathing conditions while reserving attended PSG (Type I) for those who may require noninvasive ventilation. The immediate incentive is in reducing viral exposure by limiting patient traffic and risk of PAP trial aerosolization while also improving access to accommodate the backlog of patients requiring PSG. This approach furthers the paradigm shift to emphasizing care in the home setting. Testing in the patient’s usual environment and enabling multiple night/day testing may be clinically advantageous.
Shift in emphasis to care management
The emphasis of sleep medicine has traditionally focused on diagnostics through performing PSG and HSAT. Our field has invested tremendous effort in developing guidelines for processing sleep studies, but the scoring and interpretation of those studies is extremely labor intensive. Reimagining the diagnostic approach reduces the need to manually process studies—wearable data are produced automatically, HSAT can be auto-scored, and artificial intelligence platforms can score PSGs (Goldstein CA. J Clin Sleep Med. 2020 Apr 15;16[4]:609), which allows a shift in resources and emphasis to follow-up care. A comprehensive discussion of technology-based tools to enhance care management is beyond the purview of this editorial. However, an overview of our current efforts includes: (1) utilizing population management dashboards to automatically risk stratify different cohorts of patients (eg, adult OSA, pediatrics, commercial drivers, chronic respiratory failure, etc) to identify patients “at-risk” (eg, based on OSA severity, symptoms, co-morbidities, and PAP adherence); (2) applying enhanced patient-provider interchange tools that include automated and “intelligent” electronic questionnaires, automated personalized text messaging/emails, and two-way messaging to deliver care; (3) utilizing remote patient monitoring to enhance holistic, personalized management, such as with remote activity/sleep trackers, blood pressure monitors, glucometers, and weight scales. We are engaged with efforts to validate the impact of these data to provide more personalized feedback, directly impact clinical outcomes, facilitate interdisciplinary collaboration, and identify acutely ill patients. Furthermore, a holistic approach beyond a narrow focus on PAP may create a positive collateral effect on adherence by targeting engagement with broader areas of health; and (4) implementing machine learning tools to directly support providers and patients (examples discussed in the next section.) Each of our teams has created workflows embedding these strategies throughout new care pathways. 
Generally, our emphasis during the first 3 months after PAP initiation focuses on achieving therapy adherence, and the post-3-month period broadens the efforts to target clinical outcomes. Recent trials with low PAP usage that failed to confirm the benefit of PAP on cardiovascular outcomes (McEvoy DR, et al. N Engl J Med. 2016;375:919) strongly suggest greater investment in cost-effective long-term strategies is imperative to increase our field’s relevance.
Application of artificial intelligence
We describe current efforts to apply artificial intelligence (AI) into clinical care: (1) We are implementing machine learning (ML) PSG scoring, which can potentially improve both the consistency and efficiency of scoring, further enabling greater investment in follow-up care. The future of sleep study processing, however, will likely depend on computer vision to “view” details inaccessible to the human eye and produce novel metrics that better inform clinical phenotypes (eg, cardiovascular risk, response to alternative therapies, etc). For example, “brain age” has been derived from EEG tracings that could reflect the degree of impact of sleep disorders on neurocognitive function (Fernandez C, unpublished data); (2) Machine learning clinical decision tools are in development to predict PAP adherence and timing of discontinuation, predict timing of cardiovascular disease onset and hospitalization, personalizing adherence targets, automating triaging of patients to home or PSG testing, and innumerable other predictions at clinical decision inflection points. Prediction outputs may be presented as risk profiles embedded in each patient’s “chart,” as personalized alerts, and in gamification strategies. For example, machine learning personalized cardiovascular risk scores can be regularly updated based on degree of PAP use to incentivize adherence; (3) Artificial providers may provide consistent, personalized, and holistic supplementary care. Many people rely on AI-bots for social support and cognitive-behavioral therapy (CBT) for depression. A sleep wellness bot, currently in planning stages, is intended to be the primary interface for many of the strategies described above that enhance engagement with PAP and therapies for comorbid conditions, provide CBT and lifestyle accountability, and collect patient reported data. This artificial provider would be a constant companion providing interactive, personalized, and continuous management to complement traditional intermittent live-person care.
The current health-care environment embodies the principle to “never let a serious crisis go to waste.” COVID-19 has accelerated the progression into the future by fostering an opening to embrace novel application of technologies to support changes in paradigms. Furthermore, health-care infrastructures that typically progress deliberately changed seemingly in a single moment. The Center for Medicare Services issued broad authorization to reimburse for telemedicine in response to COVID-19. Continued evolution in infrastructures will dictate progress with innovation, and a greater transition to outcomes-based incentives may be necessary to accommodate many of the strategies described above that rely on nonsynchronous care. But, we may be experiencing the moment when health care starts to catch up with the world in its embrace of technology. Sleep and pulmonary medicine can be a leader by providing a successful template for other specialties in optimizing chronic disease management.
Dr. Hwang is Medical Director, Kaiser Permanente SBC Sleep Center, and co-chair, Sleep Medicine, Kaiser Permanente Southern California.