User login
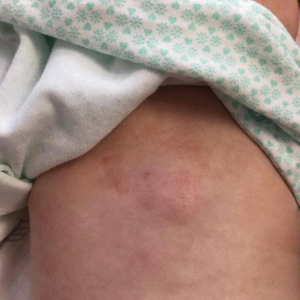
Erythematous Plaque on the Back of a Newborn
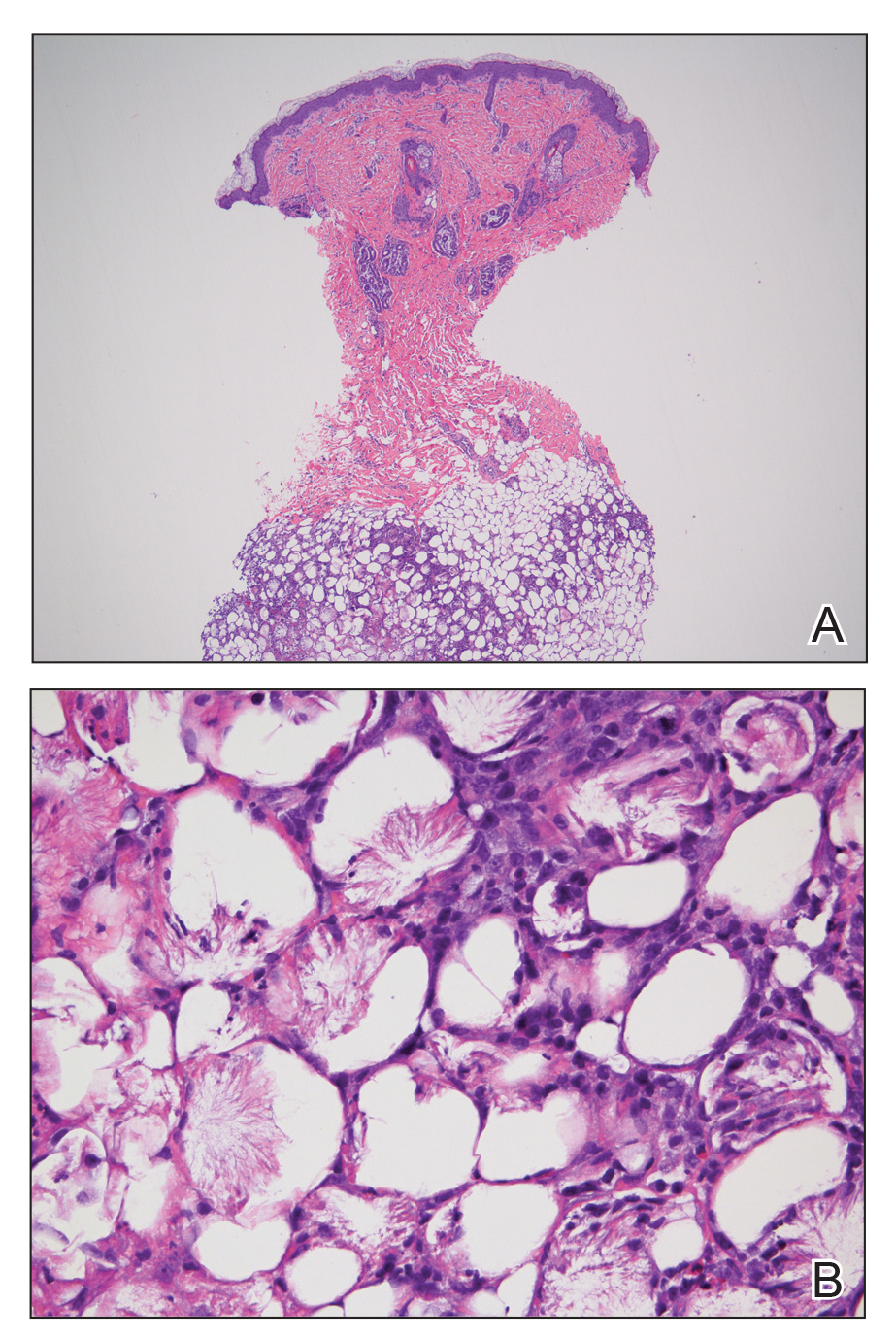
Subcutaneous fat necrosis of the newborn is a benign and self-limited condition that commonly occurs in term to postterm infants.1 However, it is an important diagnosis to recognize, as the potential exists for co-occurring metabolic derangements, most commonly hypercalcemia.1-4 Subcutaneous fat necrosis of the newborn is characterized by a panniculitis, most often on the back, shoulders, face, and buttocks. Lesions commonly present as erythematous nodules and plaques with overlying induration and can appear from birth to up to the first 6 weeks of life; calcification can be present in long-standing cases.2 Biopsy is diagnostic, showing a normal epidermis and dermis with a diffuse lobular panniculitis (Figure, A). Fat degeneration, radial crystal formation, and interstitial histiocytes also can be seen (Figure, B).
Patients with suspected subcutaneous fat necrosis should have their calcium levels checked, as up to 25% of patients may have coexisting hypercalcemia, which can contribute to morbidity and mortality.2 The hypercalcemia can occur with the onset of the lesions; however, it may be seen after they resolve completely.3 Thus, it is recommended that calcium levels be monitored for at least 1 month after lesions resolve. The exact etiology of subcutaneous fat necrosis is unknown, but it has been associated with perinatal stress and neonatal and maternal risk factors such as umbilical cord prolapse, meconium aspiration, neonatal sepsis, preeclampsia, and Rh incompatibility.1 The prognosis generally is excellent, with no treatment necessary for the skin lesions, as they resolve within a few months without subsequent sequelae or scarring.1,2 Patients with hypercalcemia should be treated appropriately with measures such as hydration and restriction of vitamin D; severe cases can be treated with bisphosphonates or loop diuretics.4
Cutis marmorata presents symmetrically on the trunk and may affect the upper and lower extremities as a reticulated erythema, often in response to cold temperature. Lesions are transient and resolve with warming. The isolated location of the skin lesions on the back, consistent course, and induration is unlikely to be seen in cutis marmorata. Infantile hemangiomas present several weeks to months after birth, and they undergo a rapid growth phase and subsequent slower involution phase. Furthermore, infantile hemangiomas have a rubbery feel and typically are not hard plaques, as seen in our patient.5 Patients with bacterial cellulitis often have systemic symptoms such as fever or chills, and the lesion generally is an ill-defined area of erythema and edema that can enlarge and become fluctuant.6 Sclerema neonatorum is a rare condition characterized by diffuse thickening of the skin that occurs in premature infants.7 These patients often are severely ill, as opposed to our asymptomatic full-term patient.
- Rubin G, Spagnut G, Morandi F, et al. Subcutaneous fat necrosis of the newborn. Clin Case Rep. 2015;3:1017-1020.
- de Campos Luciano Gomes MP, Porro AM, Simões da Silva Enokihara MM, et al. Subcutaneous fat necrosis of the newborn: clinical manifestations in two cases. An Bras Dermatol. 2013;88(6 suppl 1):154-157.
- Karochristou K, Siahanidou T, Kakourou-Tsivitanidou T, et al. Subcutaneous fat necrosis associated with severe hypocalcemia in a neonate. J Perinatol. 2005;26:64-66.
- Salas IV, Miralbell AR, Peinado CM, et al. Subcutaneous fat necrosis of the newborn and hypercalcemia: a case report. J Am Acad Dermatol. 2014;70:AB149.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Linder KA, Malani PN. Cellulitis. JAMA. 2017;317:2142.
- Jardine D, Atherton DJ, Trompeter RS. Sclerema neonaturm and subcutaneous fat necrosis of the newborn in the same infant. Eur J Pediatr. 1990;150:125-126.
Subcutaneous fat necrosis of the newborn is a benign and self-limited condition that commonly occurs in term to postterm infants.1 However, it is an important diagnosis to recognize, as the potential exists for co-occurring metabolic derangements, most commonly hypercalcemia.1-4 Subcutaneous fat necrosis of the newborn is characterized by a panniculitis, most often on the back, shoulders, face, and buttocks. Lesions commonly present as erythematous nodules and plaques with overlying induration and can appear from birth to up to the first 6 weeks of life; calcification can be present in long-standing cases.2 Biopsy is diagnostic, showing a normal epidermis and dermis with a diffuse lobular panniculitis (Figure, A). Fat degeneration, radial crystal formation, and interstitial histiocytes also can be seen (Figure, B).

Patients with suspected subcutaneous fat necrosis should have their calcium levels checked, as up to 25% of patients may have coexisting hypercalcemia, which can contribute to morbidity and mortality.2 The hypercalcemia can occur with the onset of the lesions; however, it may be seen after they resolve completely.3 Thus, it is recommended that calcium levels be monitored for at least 1 month after lesions resolve. The exact etiology of subcutaneous fat necrosis is unknown, but it has been associated with perinatal stress and neonatal and maternal risk factors such as umbilical cord prolapse, meconium aspiration, neonatal sepsis, preeclampsia, and Rh incompatibility.1 The prognosis generally is excellent, with no treatment necessary for the skin lesions, as they resolve within a few months without subsequent sequelae or scarring.1,2 Patients with hypercalcemia should be treated appropriately with measures such as hydration and restriction of vitamin D; severe cases can be treated with bisphosphonates or loop diuretics.4
Cutis marmorata presents symmetrically on the trunk and may affect the upper and lower extremities as a reticulated erythema, often in response to cold temperature. Lesions are transient and resolve with warming. The isolated location of the skin lesions on the back, consistent course, and induration is unlikely to be seen in cutis marmorata. Infantile hemangiomas present several weeks to months after birth, and they undergo a rapid growth phase and subsequent slower involution phase. Furthermore, infantile hemangiomas have a rubbery feel and typically are not hard plaques, as seen in our patient.5 Patients with bacterial cellulitis often have systemic symptoms such as fever or chills, and the lesion generally is an ill-defined area of erythema and edema that can enlarge and become fluctuant.6 Sclerema neonatorum is a rare condition characterized by diffuse thickening of the skin that occurs in premature infants.7 These patients often are severely ill, as opposed to our asymptomatic full-term patient.
Subcutaneous fat necrosis of the newborn is a benign and self-limited condition that commonly occurs in term to postterm infants.1 However, it is an important diagnosis to recognize, as the potential exists for co-occurring metabolic derangements, most commonly hypercalcemia.1-4 Subcutaneous fat necrosis of the newborn is characterized by a panniculitis, most often on the back, shoulders, face, and buttocks. Lesions commonly present as erythematous nodules and plaques with overlying induration and can appear from birth to up to the first 6 weeks of life; calcification can be present in long-standing cases.2 Biopsy is diagnostic, showing a normal epidermis and dermis with a diffuse lobular panniculitis (Figure, A). Fat degeneration, radial crystal formation, and interstitial histiocytes also can be seen (Figure, B).

Patients with suspected subcutaneous fat necrosis should have their calcium levels checked, as up to 25% of patients may have coexisting hypercalcemia, which can contribute to morbidity and mortality.2 The hypercalcemia can occur with the onset of the lesions; however, it may be seen after they resolve completely.3 Thus, it is recommended that calcium levels be monitored for at least 1 month after lesions resolve. The exact etiology of subcutaneous fat necrosis is unknown, but it has been associated with perinatal stress and neonatal and maternal risk factors such as umbilical cord prolapse, meconium aspiration, neonatal sepsis, preeclampsia, and Rh incompatibility.1 The prognosis generally is excellent, with no treatment necessary for the skin lesions, as they resolve within a few months without subsequent sequelae or scarring.1,2 Patients with hypercalcemia should be treated appropriately with measures such as hydration and restriction of vitamin D; severe cases can be treated with bisphosphonates or loop diuretics.4
Cutis marmorata presents symmetrically on the trunk and may affect the upper and lower extremities as a reticulated erythema, often in response to cold temperature. Lesions are transient and resolve with warming. The isolated location of the skin lesions on the back, consistent course, and induration is unlikely to be seen in cutis marmorata. Infantile hemangiomas present several weeks to months after birth, and they undergo a rapid growth phase and subsequent slower involution phase. Furthermore, infantile hemangiomas have a rubbery feel and typically are not hard plaques, as seen in our patient.5 Patients with bacterial cellulitis often have systemic symptoms such as fever or chills, and the lesion generally is an ill-defined area of erythema and edema that can enlarge and become fluctuant.6 Sclerema neonatorum is a rare condition characterized by diffuse thickening of the skin that occurs in premature infants.7 These patients often are severely ill, as opposed to our asymptomatic full-term patient.
- Rubin G, Spagnut G, Morandi F, et al. Subcutaneous fat necrosis of the newborn. Clin Case Rep. 2015;3:1017-1020.
- de Campos Luciano Gomes MP, Porro AM, Simões da Silva Enokihara MM, et al. Subcutaneous fat necrosis of the newborn: clinical manifestations in two cases. An Bras Dermatol. 2013;88(6 suppl 1):154-157.
- Karochristou K, Siahanidou T, Kakourou-Tsivitanidou T, et al. Subcutaneous fat necrosis associated with severe hypocalcemia in a neonate. J Perinatol. 2005;26:64-66.
- Salas IV, Miralbell AR, Peinado CM, et al. Subcutaneous fat necrosis of the newborn and hypercalcemia: a case report. J Am Acad Dermatol. 2014;70:AB149.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Linder KA, Malani PN. Cellulitis. JAMA. 2017;317:2142.
- Jardine D, Atherton DJ, Trompeter RS. Sclerema neonaturm and subcutaneous fat necrosis of the newborn in the same infant. Eur J Pediatr. 1990;150:125-126.
- Rubin G, Spagnut G, Morandi F, et al. Subcutaneous fat necrosis of the newborn. Clin Case Rep. 2015;3:1017-1020.
- de Campos Luciano Gomes MP, Porro AM, Simões da Silva Enokihara MM, et al. Subcutaneous fat necrosis of the newborn: clinical manifestations in two cases. An Bras Dermatol. 2013;88(6 suppl 1):154-157.
- Karochristou K, Siahanidou T, Kakourou-Tsivitanidou T, et al. Subcutaneous fat necrosis associated with severe hypocalcemia in a neonate. J Perinatol. 2005;26:64-66.
- Salas IV, Miralbell AR, Peinado CM, et al. Subcutaneous fat necrosis of the newborn and hypercalcemia: a case report. J Am Acad Dermatol. 2014;70:AB149.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Linder KA, Malani PN. Cellulitis. JAMA. 2017;317:2142.
- Jardine D, Atherton DJ, Trompeter RS. Sclerema neonaturm and subcutaneous fat necrosis of the newborn in the same infant. Eur J Pediatr. 1990;150:125-126.
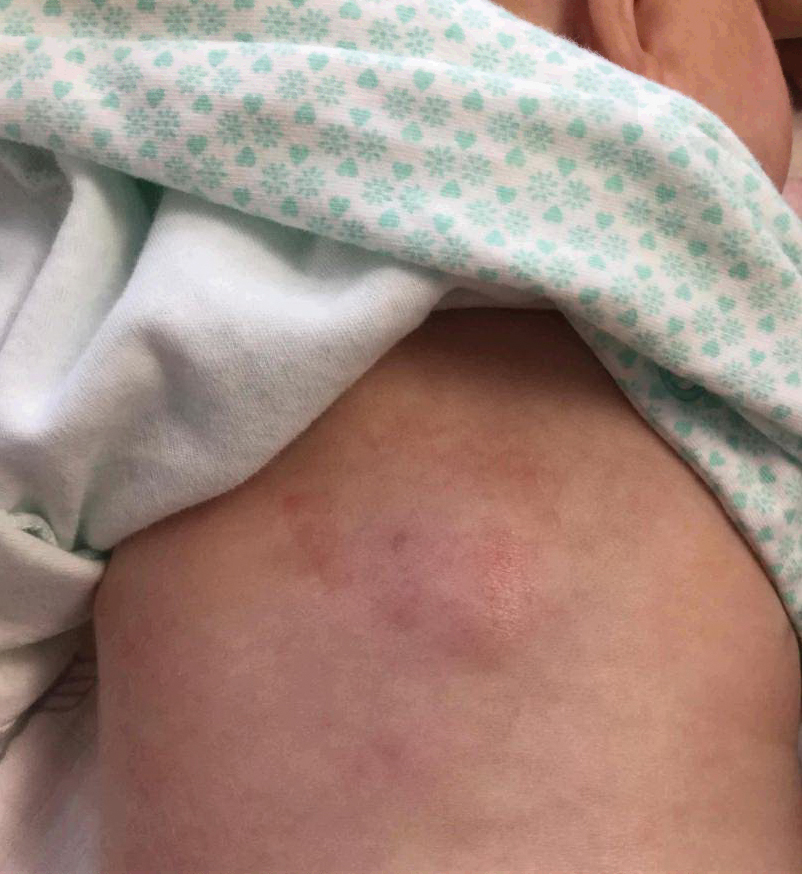
An 8-day-old female infant presented with a mass on the lower back that had been present since birth. The patient was well appearing, alert, and active. Physical examination revealed a 6×5-cm, erythematous, ill-defined, indurated plaque on the lower thoracic back. There was no associated family history of similar findings. According to the mother, the patient was feeding well with no recent fever, irritability, or lethargy. The patient was born via elective induction of labor at term due to maternal intrauterine infection from chorioamnionitis. The birth was complicated by shoulder dystocia with an Erb palsy, and she was hospitalized for 5 days after delivery for management of hypotension and ABO isoimmunization and to rule out sepsis; blood cultures were negative for neonatal infection.
Caution urged for antidepressant use in bipolar depression
Although patients with bipolar disorder commonly experience depressive symptoms, clinicians should be very cautious about treating them with antidepressants, especially as monotherapy, experts asserted in a recent debate on the topic as part of the European Psychiatric Association (EPA) 2020 Congress.
At the Congress, which was virtual this year because of the COVID-19 pandemic, psychiatric experts said that clinicians should also screen patients for mixed symptoms that are better treated with mood stabilizers. These same experts also raised concerns over long-term antidepressant use, recommending continued use only in patients who relapse after stopping antidepressants.
Isabella Pacchiarotti, MD, PhD, Centro de Investigación Biomédica en Red de Salud Mental, Barcelona, Spain, argued against the use of antidepressants in treating bipolar disorder; Guy Goodwin, PhD, however, took the “pro” stance.
Goodwin, a professor of psychiatry at the University of Oxford in the UK, admitted that there is a “paucity of data” on the role of antidepressants in bipolar disorder.
Nevertheless, there are “circumstances that one really has to treat with antidepressants simply because other things have been tried and have not worked,” he told conference attendees.
Challenging, Controversial Topic
The debate was chaired by Eduard Vieta, MD, PhD, chair of the Department of Psychiatry and Psychology at the University of Barcelona Hospital Clinic, Spain.
Vieta said the question over whether antidepressants should be used in the depressive phase of bipolar illness is “perhaps the most challenging ... especially in the area of bipolar disorder.”
At the beginning of the presentation, Vieta asked the audience for their opinion in order to have a “baseline” for the debate: among 164 respondents, 73% were in favor of using antidepressants in bipolar depression.
“Clearly there is a majority, so Isabella [Dr Pacchiarotti] is going to have a hard time improving these numbers,” Vieta noted.
Up first, Pacchiarotti began by noting that this topic remains “an area of big controversy.” However, the real question “should not be the pros and cons of antidepressants but more when and how to use them.»
Of the three phases of bipolar disorder, acute depression «poses the greatest difficulties,» she added.
This is because of the relative paucity of studies in the area, the often heated debates on the specific role of antidepressants, the discrepancy in conclusions between meta-analyses, and the currently approved therapeutic options being associated with “not very high response rates,” Pacchiarotti said.
The diagnostic criteria for unipolar and bipolar depression are “basically the same,” she noted. However, it’s important to be able to distinguish between the two conditions, as up to one fifth of patients with unipolar depression suffer from undiagnosed bipolar disorder, she explained.
Moreover, several studies have identified key symptoms in bipolar depression, such as hyperphagia and hypersomnia, increased anxiety, and psychotic and psychomotor symptoms.
As previously reported by Medscape Medical News, a task force report was released in 2013 by the International Society for Bipolar Disorder (ISBD) on antidepressant use in bipolar disorders. Pacchiarotti and Goodwin were among the report’s authors, which concluded that available evidence on this issue is methodologically weak.
This is largely because of a lack of placebo-controlled studies in this patient population (bipolar depression, alongside suicidal ideation, is often an exclusion criteria in clinical antidepressant trials).
Many guidelines consequently do not consider antidepressants to be a first-line option as monotherapy in bipolar depression, although some name the drugs as second- or third-line options.
In 2013, the ISBD recommended that antidepressant monotherapy should be “avoided” in bipolar I disorder; and in bipolar I and II depression, the treatment should be accompanied by at least two concomitant core manic symptoms.
“What Has Changed?”
Antidepressants should be used “only if there is a history of a positive response,” whereas maintenance therapy should be considered if a patient relapses into a depressive episode after stopping the drugs, the report notes.
Pacchiarotti noted that since the recommendations were published nothing has changed, noting that antidepressant efficacy in bipolar depression “remains unproven.”
The issue is not whether antidepressants are effective in bipolar depression but rather are there subpopulations where these medications are helpful or harmful, she added.
The key to understanding the heterogeneity of responses to antidepressants, she said, is the concept of a bipolar spectrum and a dimensional approach to distinguishing between bipolar disorder and unipolar depression.
In addition, the definition of a mixed episode in the DSM-IV-TR differs from that of an episode with mixed characteristics in the DSM-5, which Pacchiarotti said offers a better understanding of the phenomenon while seemingly disposing with the idea of mixed depression.
Based on previous research, there is some suggestion that a depressive state exists between major depressive disorder and bipolar I disorder with mixed features, and hypomania state between bipolar II and I disorder, also with mixed features.
Pacchiarotti said the role of antidepressants in the treatment of bipolar depression remains “controversial” and there is a need for both short- and long-term studies of their use in both bipolar I and bipolar II disorder with real-world inclusion criteria.
The concept of a bipolar spectrum needs to be considered a more “dimensional approach” to depression, with mixed features seen as a “transversal” contraindication for antidepressant use, she concluded.
In Favor — With Caveats
Taking the opposite position and arguing in favor of antidepressant use, albeit cautiously, Goodwin said previous work has shown that stable patients with bipolar disorder experience depression of variable severity about 50% of the time.
The truth is that patients do not have a depressive episode for extended periods but instead have depressive symptoms, he said. “So how we manage and treat depression really matters.”
In an analysis, Goodwin and his colleagues estimated that the cost of bipolar disorder is approximately £12,600 ($16,000) per patient per year, of which only 30.6% is attributable to healthcare costs and 68.1% to indirect costs. This means the impact on the patient is also felt by society.
He agreed with Pacchiarotti’s assertion of a bipolar spectrum and the need for a dimensional approach.
“All the patients along the spectrum have the symptoms of depression and they differ in the extent to which they show symptoms of mania, which will include irritability,” he added.
Goodwin argued that there is no evidence to suggest that the depression experienced at one end of the scale is any different from that at the other. However, safety issues around antidepressant use “really relate to the additional symptoms you see with increasing evidence of bipolarity.”
In addition, the whole discussion is confounded by comorbidity, “with symptoms that sometimes coalesce into our concept of borderline personality disorder” or attention deficit hyperactivity disorder, he said.
Goodwin said there is “very little doubt” that antidepressants have an effect vs placebo. “The argument is over whether the effect is large and whether we should regard it as clinically significant.”
He noted that previous studies have shown a range of effect sizes with antidepressants, but the “massive” confidence intervals mean that “one is free to believe pretty much what one likes.”
The only antidepressant medication that is statistically significantly different from placebo is fluoxetine combined with olanzapine. However, that conclusion is based on “little data,” said Goodwin.
In terms of long-term management, there is “extremely little” randomized data for maintenance treatment with antidepressants in bipolar disorder. “So this does not support” long-term use, he added.
Still, although choice of antidepressant remains a guess, there is “just about support” for using them, Goodwin noted.
He urged clinicians not to dismiss antidepressant use, but to use them only where there is a clinical need and for as little time as possible. Patients with bipolar disorder should continue to take antidepressants if they relapse after they come off these medications.
However, all of that sits “in contrast” to how they’re currently used in clinical practice, Goodwin said.
Caution Urged
After the debate, the audience was asked to vote again. This time, The remaining 12% voted against the practice.
Summarizing the discussion, Vieta said that “we should be cautious” when using antidepressants in bipolar depression. However, “we should be able to use them when necessary,” he added.
Although their use as monotherapy is not best practice, especially in bipolar I disorder, there may be a subset of bipolar II patients in whom monotherapy “might still be acceptable; but I don’t think it’s a good idea,” Vieta said.
He added that clinicians should very carefully screen for mixed symptoms, which call for the prescription of other drugs, such as olanzapine and fluoxetine.
“The other important message is that we have to be even more cautious in the long term with the use of antidepressants, and we should be able to use them when there is a comorbidity” that calls for their use, Vieta concluded.
Pacchiarotti reported having received speaker fees and educational grants from Adamed, AstraZeneca, Janssen-Cilag, and Lundbeck. Goodwin reported having received honoraria from Angellini, Medscape, Pfizer, Servier, Shire, and Sun; having shares in P1vital Products; past employment as medical director of P1vital Products; and advisory board membership for Compass Pathways, Minerva, MSD, Novartis, Lundbeck, Sage, Servier, and Shire. Vieta has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Although patients with bipolar disorder commonly experience depressive symptoms, clinicians should be very cautious about treating them with antidepressants, especially as monotherapy, experts asserted in a recent debate on the topic as part of the European Psychiatric Association (EPA) 2020 Congress.
At the Congress, which was virtual this year because of the COVID-19 pandemic, psychiatric experts said that clinicians should also screen patients for mixed symptoms that are better treated with mood stabilizers. These same experts also raised concerns over long-term antidepressant use, recommending continued use only in patients who relapse after stopping antidepressants.
Isabella Pacchiarotti, MD, PhD, Centro de Investigación Biomédica en Red de Salud Mental, Barcelona, Spain, argued against the use of antidepressants in treating bipolar disorder; Guy Goodwin, PhD, however, took the “pro” stance.
Goodwin, a professor of psychiatry at the University of Oxford in the UK, admitted that there is a “paucity of data” on the role of antidepressants in bipolar disorder.
Nevertheless, there are “circumstances that one really has to treat with antidepressants simply because other things have been tried and have not worked,” he told conference attendees.
Challenging, Controversial Topic
The debate was chaired by Eduard Vieta, MD, PhD, chair of the Department of Psychiatry and Psychology at the University of Barcelona Hospital Clinic, Spain.
Vieta said the question over whether antidepressants should be used in the depressive phase of bipolar illness is “perhaps the most challenging ... especially in the area of bipolar disorder.”
At the beginning of the presentation, Vieta asked the audience for their opinion in order to have a “baseline” for the debate: among 164 respondents, 73% were in favor of using antidepressants in bipolar depression.
“Clearly there is a majority, so Isabella [Dr Pacchiarotti] is going to have a hard time improving these numbers,” Vieta noted.
Up first, Pacchiarotti began by noting that this topic remains “an area of big controversy.” However, the real question “should not be the pros and cons of antidepressants but more when and how to use them.»
Of the three phases of bipolar disorder, acute depression «poses the greatest difficulties,» she added.
This is because of the relative paucity of studies in the area, the often heated debates on the specific role of antidepressants, the discrepancy in conclusions between meta-analyses, and the currently approved therapeutic options being associated with “not very high response rates,” Pacchiarotti said.
The diagnostic criteria for unipolar and bipolar depression are “basically the same,” she noted. However, it’s important to be able to distinguish between the two conditions, as up to one fifth of patients with unipolar depression suffer from undiagnosed bipolar disorder, she explained.
Moreover, several studies have identified key symptoms in bipolar depression, such as hyperphagia and hypersomnia, increased anxiety, and psychotic and psychomotor symptoms.
As previously reported by Medscape Medical News, a task force report was released in 2013 by the International Society for Bipolar Disorder (ISBD) on antidepressant use in bipolar disorders. Pacchiarotti and Goodwin were among the report’s authors, which concluded that available evidence on this issue is methodologically weak.
This is largely because of a lack of placebo-controlled studies in this patient population (bipolar depression, alongside suicidal ideation, is often an exclusion criteria in clinical antidepressant trials).
Many guidelines consequently do not consider antidepressants to be a first-line option as monotherapy in bipolar depression, although some name the drugs as second- or third-line options.
In 2013, the ISBD recommended that antidepressant monotherapy should be “avoided” in bipolar I disorder; and in bipolar I and II depression, the treatment should be accompanied by at least two concomitant core manic symptoms.
“What Has Changed?”
Antidepressants should be used “only if there is a history of a positive response,” whereas maintenance therapy should be considered if a patient relapses into a depressive episode after stopping the drugs, the report notes.
Pacchiarotti noted that since the recommendations were published nothing has changed, noting that antidepressant efficacy in bipolar depression “remains unproven.”
The issue is not whether antidepressants are effective in bipolar depression but rather are there subpopulations where these medications are helpful or harmful, she added.
The key to understanding the heterogeneity of responses to antidepressants, she said, is the concept of a bipolar spectrum and a dimensional approach to distinguishing between bipolar disorder and unipolar depression.
In addition, the definition of a mixed episode in the DSM-IV-TR differs from that of an episode with mixed characteristics in the DSM-5, which Pacchiarotti said offers a better understanding of the phenomenon while seemingly disposing with the idea of mixed depression.
Based on previous research, there is some suggestion that a depressive state exists between major depressive disorder and bipolar I disorder with mixed features, and hypomania state between bipolar II and I disorder, also with mixed features.
Pacchiarotti said the role of antidepressants in the treatment of bipolar depression remains “controversial” and there is a need for both short- and long-term studies of their use in both bipolar I and bipolar II disorder with real-world inclusion criteria.
The concept of a bipolar spectrum needs to be considered a more “dimensional approach” to depression, with mixed features seen as a “transversal” contraindication for antidepressant use, she concluded.
In Favor — With Caveats
Taking the opposite position and arguing in favor of antidepressant use, albeit cautiously, Goodwin said previous work has shown that stable patients with bipolar disorder experience depression of variable severity about 50% of the time.
The truth is that patients do not have a depressive episode for extended periods but instead have depressive symptoms, he said. “So how we manage and treat depression really matters.”
In an analysis, Goodwin and his colleagues estimated that the cost of bipolar disorder is approximately £12,600 ($16,000) per patient per year, of which only 30.6% is attributable to healthcare costs and 68.1% to indirect costs. This means the impact on the patient is also felt by society.
He agreed with Pacchiarotti’s assertion of a bipolar spectrum and the need for a dimensional approach.
“All the patients along the spectrum have the symptoms of depression and they differ in the extent to which they show symptoms of mania, which will include irritability,” he added.
Goodwin argued that there is no evidence to suggest that the depression experienced at one end of the scale is any different from that at the other. However, safety issues around antidepressant use “really relate to the additional symptoms you see with increasing evidence of bipolarity.”
In addition, the whole discussion is confounded by comorbidity, “with symptoms that sometimes coalesce into our concept of borderline personality disorder” or attention deficit hyperactivity disorder, he said.
Goodwin said there is “very little doubt” that antidepressants have an effect vs placebo. “The argument is over whether the effect is large and whether we should regard it as clinically significant.”
He noted that previous studies have shown a range of effect sizes with antidepressants, but the “massive” confidence intervals mean that “one is free to believe pretty much what one likes.”
The only antidepressant medication that is statistically significantly different from placebo is fluoxetine combined with olanzapine. However, that conclusion is based on “little data,” said Goodwin.
In terms of long-term management, there is “extremely little” randomized data for maintenance treatment with antidepressants in bipolar disorder. “So this does not support” long-term use, he added.
Still, although choice of antidepressant remains a guess, there is “just about support” for using them, Goodwin noted.
He urged clinicians not to dismiss antidepressant use, but to use them only where there is a clinical need and for as little time as possible. Patients with bipolar disorder should continue to take antidepressants if they relapse after they come off these medications.
However, all of that sits “in contrast” to how they’re currently used in clinical practice, Goodwin said.
Caution Urged
After the debate, the audience was asked to vote again. This time, The remaining 12% voted against the practice.
Summarizing the discussion, Vieta said that “we should be cautious” when using antidepressants in bipolar depression. However, “we should be able to use them when necessary,” he added.
Although their use as monotherapy is not best practice, especially in bipolar I disorder, there may be a subset of bipolar II patients in whom monotherapy “might still be acceptable; but I don’t think it’s a good idea,” Vieta said.
He added that clinicians should very carefully screen for mixed symptoms, which call for the prescription of other drugs, such as olanzapine and fluoxetine.
“The other important message is that we have to be even more cautious in the long term with the use of antidepressants, and we should be able to use them when there is a comorbidity” that calls for their use, Vieta concluded.
Pacchiarotti reported having received speaker fees and educational grants from Adamed, AstraZeneca, Janssen-Cilag, and Lundbeck. Goodwin reported having received honoraria from Angellini, Medscape, Pfizer, Servier, Shire, and Sun; having shares in P1vital Products; past employment as medical director of P1vital Products; and advisory board membership for Compass Pathways, Minerva, MSD, Novartis, Lundbeck, Sage, Servier, and Shire. Vieta has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Although patients with bipolar disorder commonly experience depressive symptoms, clinicians should be very cautious about treating them with antidepressants, especially as monotherapy, experts asserted in a recent debate on the topic as part of the European Psychiatric Association (EPA) 2020 Congress.
At the Congress, which was virtual this year because of the COVID-19 pandemic, psychiatric experts said that clinicians should also screen patients for mixed symptoms that are better treated with mood stabilizers. These same experts also raised concerns over long-term antidepressant use, recommending continued use only in patients who relapse after stopping antidepressants.
Isabella Pacchiarotti, MD, PhD, Centro de Investigación Biomédica en Red de Salud Mental, Barcelona, Spain, argued against the use of antidepressants in treating bipolar disorder; Guy Goodwin, PhD, however, took the “pro” stance.
Goodwin, a professor of psychiatry at the University of Oxford in the UK, admitted that there is a “paucity of data” on the role of antidepressants in bipolar disorder.
Nevertheless, there are “circumstances that one really has to treat with antidepressants simply because other things have been tried and have not worked,” he told conference attendees.
Challenging, Controversial Topic
The debate was chaired by Eduard Vieta, MD, PhD, chair of the Department of Psychiatry and Psychology at the University of Barcelona Hospital Clinic, Spain.
Vieta said the question over whether antidepressants should be used in the depressive phase of bipolar illness is “perhaps the most challenging ... especially in the area of bipolar disorder.”
At the beginning of the presentation, Vieta asked the audience for their opinion in order to have a “baseline” for the debate: among 164 respondents, 73% were in favor of using antidepressants in bipolar depression.
“Clearly there is a majority, so Isabella [Dr Pacchiarotti] is going to have a hard time improving these numbers,” Vieta noted.
Up first, Pacchiarotti began by noting that this topic remains “an area of big controversy.” However, the real question “should not be the pros and cons of antidepressants but more when and how to use them.»
Of the three phases of bipolar disorder, acute depression «poses the greatest difficulties,» she added.
This is because of the relative paucity of studies in the area, the often heated debates on the specific role of antidepressants, the discrepancy in conclusions between meta-analyses, and the currently approved therapeutic options being associated with “not very high response rates,” Pacchiarotti said.
The diagnostic criteria for unipolar and bipolar depression are “basically the same,” she noted. However, it’s important to be able to distinguish between the two conditions, as up to one fifth of patients with unipolar depression suffer from undiagnosed bipolar disorder, she explained.
Moreover, several studies have identified key symptoms in bipolar depression, such as hyperphagia and hypersomnia, increased anxiety, and psychotic and psychomotor symptoms.
As previously reported by Medscape Medical News, a task force report was released in 2013 by the International Society for Bipolar Disorder (ISBD) on antidepressant use in bipolar disorders. Pacchiarotti and Goodwin were among the report’s authors, which concluded that available evidence on this issue is methodologically weak.
This is largely because of a lack of placebo-controlled studies in this patient population (bipolar depression, alongside suicidal ideation, is often an exclusion criteria in clinical antidepressant trials).
Many guidelines consequently do not consider antidepressants to be a first-line option as monotherapy in bipolar depression, although some name the drugs as second- or third-line options.
In 2013, the ISBD recommended that antidepressant monotherapy should be “avoided” in bipolar I disorder; and in bipolar I and II depression, the treatment should be accompanied by at least two concomitant core manic symptoms.
“What Has Changed?”
Antidepressants should be used “only if there is a history of a positive response,” whereas maintenance therapy should be considered if a patient relapses into a depressive episode after stopping the drugs, the report notes.
Pacchiarotti noted that since the recommendations were published nothing has changed, noting that antidepressant efficacy in bipolar depression “remains unproven.”
The issue is not whether antidepressants are effective in bipolar depression but rather are there subpopulations where these medications are helpful or harmful, she added.
The key to understanding the heterogeneity of responses to antidepressants, she said, is the concept of a bipolar spectrum and a dimensional approach to distinguishing between bipolar disorder and unipolar depression.
In addition, the definition of a mixed episode in the DSM-IV-TR differs from that of an episode with mixed characteristics in the DSM-5, which Pacchiarotti said offers a better understanding of the phenomenon while seemingly disposing with the idea of mixed depression.
Based on previous research, there is some suggestion that a depressive state exists between major depressive disorder and bipolar I disorder with mixed features, and hypomania state between bipolar II and I disorder, also with mixed features.
Pacchiarotti said the role of antidepressants in the treatment of bipolar depression remains “controversial” and there is a need for both short- and long-term studies of their use in both bipolar I and bipolar II disorder with real-world inclusion criteria.
The concept of a bipolar spectrum needs to be considered a more “dimensional approach” to depression, with mixed features seen as a “transversal” contraindication for antidepressant use, she concluded.
In Favor — With Caveats
Taking the opposite position and arguing in favor of antidepressant use, albeit cautiously, Goodwin said previous work has shown that stable patients with bipolar disorder experience depression of variable severity about 50% of the time.
The truth is that patients do not have a depressive episode for extended periods but instead have depressive symptoms, he said. “So how we manage and treat depression really matters.”
In an analysis, Goodwin and his colleagues estimated that the cost of bipolar disorder is approximately £12,600 ($16,000) per patient per year, of which only 30.6% is attributable to healthcare costs and 68.1% to indirect costs. This means the impact on the patient is also felt by society.
He agreed with Pacchiarotti’s assertion of a bipolar spectrum and the need for a dimensional approach.
“All the patients along the spectrum have the symptoms of depression and they differ in the extent to which they show symptoms of mania, which will include irritability,” he added.
Goodwin argued that there is no evidence to suggest that the depression experienced at one end of the scale is any different from that at the other. However, safety issues around antidepressant use “really relate to the additional symptoms you see with increasing evidence of bipolarity.”
In addition, the whole discussion is confounded by comorbidity, “with symptoms that sometimes coalesce into our concept of borderline personality disorder” or attention deficit hyperactivity disorder, he said.
Goodwin said there is “very little doubt” that antidepressants have an effect vs placebo. “The argument is over whether the effect is large and whether we should regard it as clinically significant.”
He noted that previous studies have shown a range of effect sizes with antidepressants, but the “massive” confidence intervals mean that “one is free to believe pretty much what one likes.”
The only antidepressant medication that is statistically significantly different from placebo is fluoxetine combined with olanzapine. However, that conclusion is based on “little data,” said Goodwin.
In terms of long-term management, there is “extremely little” randomized data for maintenance treatment with antidepressants in bipolar disorder. “So this does not support” long-term use, he added.
Still, although choice of antidepressant remains a guess, there is “just about support” for using them, Goodwin noted.
He urged clinicians not to dismiss antidepressant use, but to use them only where there is a clinical need and for as little time as possible. Patients with bipolar disorder should continue to take antidepressants if they relapse after they come off these medications.
However, all of that sits “in contrast” to how they’re currently used in clinical practice, Goodwin said.
Caution Urged
After the debate, the audience was asked to vote again. This time, The remaining 12% voted against the practice.
Summarizing the discussion, Vieta said that “we should be cautious” when using antidepressants in bipolar depression. However, “we should be able to use them when necessary,” he added.
Although their use as monotherapy is not best practice, especially in bipolar I disorder, there may be a subset of bipolar II patients in whom monotherapy “might still be acceptable; but I don’t think it’s a good idea,” Vieta said.
He added that clinicians should very carefully screen for mixed symptoms, which call for the prescription of other drugs, such as olanzapine and fluoxetine.
“The other important message is that we have to be even more cautious in the long term with the use of antidepressants, and we should be able to use them when there is a comorbidity” that calls for their use, Vieta concluded.
Pacchiarotti reported having received speaker fees and educational grants from Adamed, AstraZeneca, Janssen-Cilag, and Lundbeck. Goodwin reported having received honoraria from Angellini, Medscape, Pfizer, Servier, Shire, and Sun; having shares in P1vital Products; past employment as medical director of P1vital Products; and advisory board membership for Compass Pathways, Minerva, MSD, Novartis, Lundbeck, Sage, Servier, and Shire. Vieta has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
It’s been surreal
Hopefully 2020 will be the strangest year in modern memory, but who knows?
Things continue to be surreal at my office. I haven’t seen my staff since mid-March, even though I’m in touch with them all day long. Fortunately we live in an age where many things can be handled from home.
At the office I’d started to see an increase in patients, but that has dropped off again as the infection rate in Arizona has soared out of control. I’m not complaining about patients staying home; many neurology patients are frail or on immune-suppressing agents, and should not be out and about.
Normally I’m a stickler for stable patients coming in once a year for refills, but in 2020 I’m letting that slide. Sumatriptan, levetiracetam, and nortriptyline are better filled for 90 days to minimize potential COVID-19 contacts on all parts – including mine.
Originally I thought that some degree of normalcy would be back by August, but clearly that won’t be the case. Arizona, and many other states, continue to get worse as political ambitions trounce sound science.
A year ago I routinely fielded calls asking whether various supplements would fend off Alzheimer’s disease as the manufacturers claimed (NO! THEY DON’T!). Today similar calls come in asking about stuff marketed to prevent and cure COVID-19 (same answer).
I have no idea when this will improve. My kids are scheduled to move back into their dorms in about a month, but realistically I don’t see that safely happening. Classrooms, with the reduced capacity needed and cost of frequent cleanings, seem impractical, compared with Zoom.
The college football season is almost certainly going to be canceled. The NFL maybe. Basketball and baseball are playing out reduced seasons in sterilized bubbles. Sports, next to holidays and school, are the cyclical touchstones our society is measured by. Their disruption reflects the strangeness of the year as a whole.
As always during the Phoenix summer, I’m hiding in an air-conditioned office, waiting for patients to come in. It’s quieter without my secretary and her energetic 4-year-old daughter. But I’m still here. It’s strange with the unfamiliar silence, but the routine of coming to work each day, even on a reduced schedule, brings a sense of normalcy. There may not be as many patients, but those who need me come in, and as long as I’m able to, I’ll be here to help them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Hopefully 2020 will be the strangest year in modern memory, but who knows?
Things continue to be surreal at my office. I haven’t seen my staff since mid-March, even though I’m in touch with them all day long. Fortunately we live in an age where many things can be handled from home.
At the office I’d started to see an increase in patients, but that has dropped off again as the infection rate in Arizona has soared out of control. I’m not complaining about patients staying home; many neurology patients are frail or on immune-suppressing agents, and should not be out and about.
Normally I’m a stickler for stable patients coming in once a year for refills, but in 2020 I’m letting that slide. Sumatriptan, levetiracetam, and nortriptyline are better filled for 90 days to minimize potential COVID-19 contacts on all parts – including mine.
Originally I thought that some degree of normalcy would be back by August, but clearly that won’t be the case. Arizona, and many other states, continue to get worse as political ambitions trounce sound science.
A year ago I routinely fielded calls asking whether various supplements would fend off Alzheimer’s disease as the manufacturers claimed (NO! THEY DON’T!). Today similar calls come in asking about stuff marketed to prevent and cure COVID-19 (same answer).
I have no idea when this will improve. My kids are scheduled to move back into their dorms in about a month, but realistically I don’t see that safely happening. Classrooms, with the reduced capacity needed and cost of frequent cleanings, seem impractical, compared with Zoom.
The college football season is almost certainly going to be canceled. The NFL maybe. Basketball and baseball are playing out reduced seasons in sterilized bubbles. Sports, next to holidays and school, are the cyclical touchstones our society is measured by. Their disruption reflects the strangeness of the year as a whole.
As always during the Phoenix summer, I’m hiding in an air-conditioned office, waiting for patients to come in. It’s quieter without my secretary and her energetic 4-year-old daughter. But I’m still here. It’s strange with the unfamiliar silence, but the routine of coming to work each day, even on a reduced schedule, brings a sense of normalcy. There may not be as many patients, but those who need me come in, and as long as I’m able to, I’ll be here to help them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Hopefully 2020 will be the strangest year in modern memory, but who knows?
Things continue to be surreal at my office. I haven’t seen my staff since mid-March, even though I’m in touch with them all day long. Fortunately we live in an age where many things can be handled from home.
At the office I’d started to see an increase in patients, but that has dropped off again as the infection rate in Arizona has soared out of control. I’m not complaining about patients staying home; many neurology patients are frail or on immune-suppressing agents, and should not be out and about.
Normally I’m a stickler for stable patients coming in once a year for refills, but in 2020 I’m letting that slide. Sumatriptan, levetiracetam, and nortriptyline are better filled for 90 days to minimize potential COVID-19 contacts on all parts – including mine.
Originally I thought that some degree of normalcy would be back by August, but clearly that won’t be the case. Arizona, and many other states, continue to get worse as political ambitions trounce sound science.
A year ago I routinely fielded calls asking whether various supplements would fend off Alzheimer’s disease as the manufacturers claimed (NO! THEY DON’T!). Today similar calls come in asking about stuff marketed to prevent and cure COVID-19 (same answer).
I have no idea when this will improve. My kids are scheduled to move back into their dorms in about a month, but realistically I don’t see that safely happening. Classrooms, with the reduced capacity needed and cost of frequent cleanings, seem impractical, compared with Zoom.
The college football season is almost certainly going to be canceled. The NFL maybe. Basketball and baseball are playing out reduced seasons in sterilized bubbles. Sports, next to holidays and school, are the cyclical touchstones our society is measured by. Their disruption reflects the strangeness of the year as a whole.
As always during the Phoenix summer, I’m hiding in an air-conditioned office, waiting for patients to come in. It’s quieter without my secretary and her energetic 4-year-old daughter. But I’m still here. It’s strange with the unfamiliar silence, but the routine of coming to work each day, even on a reduced schedule, brings a sense of normalcy. There may not be as many patients, but those who need me come in, and as long as I’m able to, I’ll be here to help them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
New hope for ALS
. Both studies investigated potential benefits of suppressing the toxic activity in cells of a mutant gene (SOD1) that encodes superoxide dismutase 1 (SOD1) in patients with ALS.
One study investigated the antisense oligonucleotide (ASO) tofersen (Biogen); the other study examined viral vector–mediated gene suppression.
The studies’ promising results signal “the beginning of a new precision medicine–based approach towards treating ALS,” said Orla Hardiman, BSc, MB, BCh, BAO, MD, a consultant neurologist and professor of neurology at Trinity College and Beaumont Hospital in Dublin, Ireland. Dr. Hardiman co-authored an editorial that accompanied the two studies, which were published July 9 in the New England Journal of Medicine.
Genetic culprits
ALS is a disorder of progressive degeneration of upper and lower motor neurons. It typically leads to death from ventilatory failure within 5 years of symptom onset.
Genetic factors are responsible for about half the risk variance of ALS. In populations of European origin, variants in SOD1 account for an estimated 13% to 20% of familial ALS, although this rate varies around the world. Although SOD1 is not the most common variant in ALS, it is the one that researchers are most familiar with and has been studied in an animal model.
In the first study, investigators evaluated the safety, pharmacokinetics, and pharmacodynamics of the ASO tofersen in adults with ALS.
An ASO is a small piece of nucleic acid that enters neurons in the spinal cord and brain, explained co-investigator Toby A. Ferguson, MD, PhD, vice president and head of the neuromuscular development unit at Biogen.
ASO binds to the SOD1 gene and knocks down the SOD1 protein, which is the “toxic engine [that] drives the disease, kills neurons, and causes patients to have loss of function and eventually to die,” said Dr. Ferguson. “The ASO turns off the motor that produces that toxic protein,” he added.
Animal studies have shown that ASOs that target SOD1 messenger RNA transcripts prolong survival, improve motor performance, and reduce SOD1 protein concentrations.
The new phase 1/2 double-blind study included 50 adults at 18 sites in the United States, Canada, and four European countries. All had muscle weakness attributed to ALS and a documented SOD1 mutation. Participants were randomly assigned to receive one of four doses of tofersen—20, 40, 60, or 100 mg—or placebo. Treatment was administered via a lumbar intrathecal bolus injection. The study included a screening period followed by a 12-week intervention period and a 12-week follow-up.
Adverse events
A primary outcome was the incidence of adverse events (AEs) and serious AEs. Results showed that all participants reported one or more AEs. The most common AEs were headache, pain at the injection site, post–lumbar puncture syndrome, and falls. Three deaths occurred, one in the placebo group, one in the 20-mg dose group, and one in the 60-mg dose group. There were no serious AEs in the 100-mg group.
Although the investigators found an increase in cerebrospinal fluid (CSF) protein and white cell counts, there was no clear association between these observations and higher doses of tofersen or longer duration of exposure.
“We don’t know the implications of this, and it’s something we need to keep an eye on as we move these studies forward,” Dr. Ferguson said.
None of the AEs or CSF abnormalities led to trial discontinuation.
A secondary outcome was change in SOD1 protein concentration in CSF at day 85. The study showed that SOD1 concentrations decreased by 36% among the participants who received tofersen 100 mg and by lesser amounts in the patients who received lower doses. Concentrations in the placebo group were reduced by 3%.
The 36% reduction in the highest dose group is likely meaningful and “foundational to the concept of what this molecule can do,” Dr. Ferguson said.
“If the number one cause of SOD1 ALS is accumulation of toxic SOD1 protein, then the demonstration that we can reduce SOD1 protein in the CSF ... is saying that’s the first step on the way to showing the molecule is doing what it should do,” he added.
Emerging tool
In patients with ALS, neurofilament concentrations typically increase as the disease progresses. However, this study documented a reduction in these CSF concentrations. “One interpretation of that could be that there is less neurodegeneration or neuro injury” in patients treated with tofersen, Dr. Ferguson said.
He noted that neurofilament is “an emerging tool” for understanding neurodegeneration. It could also “be another sort of biochemical signal that the molecule is doing something important,” he added.
However, he noted that neurofilament concentration is still an exploratory marker.
Exploratory analyses suggested a possible slowing of functional loss, as measured by the ALS Functional Rating Scale–Revised (ALSFRS-R) score and the handheld dynamometry megascore. The latter assesses strength in 16 muscle groups in the arms and legs. The investigators noted that no conclusions can be drawn from these outcomes.
A post hoc analysis showed that among patients with SOD1 mutations associated with a fast-progressing disease course, the slope of clinical decline might have been gentler, and there was a greater decrease in CSF neurofilament concentration compared among those whose disease followed a slower course.
This suggests that “if you pick the right target,” even patients with severe disease can be treated, Dr. Ferguson said.
He acknowledged that in a relatively short study such as this one, it may be easier to see benefits in patients whose disease is progressing rapidly. However, he’s convinced that the treatment “would work for all SOD1 ALS patients, not just fast patients.”
Dr. Ferguson said the study investigators are encouraged by the new data, which “really suggest that we may be developing a meaningful treatment for SOD1 ALS.” However, “it’s still early” in terms of rolling out this therapy for patients with ALS, he said.
The safety and efficacy of tofersen are currently being evaluated in a phase 3, randomized, double-blind, placebo-controlled trial.
Limitations of the current study were the small number of participants, the short duration of treatment and follow-up, the exploratory nature of efficacy outcomes, and the post hoc methods for defining the fast-progressing subgroup.
Although an advantage of tofersen is that it can enter the nucleus of the cell, perhaps boosting effectiveness, a drawback might be that patients need several treatments administered via lumbar puncture. Following three initial doses, the drug is given every month.
An alternative approach might be a viral vector approach.
“Stunning” finding
In the second study, investigators assessed the safety of a single intrathecal infusion of a viral vector therapy designed to target SOD1 in two patients with familial ALS. The two patients were a 22-year-old man whose mother had died of ALS at age 45 and a 56-year-old man who had a family history of ALS.
The aim of the viral vector therapy is to continually suppress mutant gene activity, said study co-investigator Robert H. Brown, Jr, MD, professor of neurology, University of Massachusetts Medical School, Worcester.
“The virus essentially drops off a piece of DNA, and that DNA keeps making the agent that suppresses the gene,” Dr. Brown said.
He noted that the first patient had a mutation that causes a rapidly developing, “horribly devastating” disease.
Initially, the patient’s right leg, in which movement had been worsening over several weeks, “seemed to get stronger and remain strong for quite a long time. I’ve never seen that in this kind of mutation,” said Dr. Brown.
The patient died of ALS. At autopsy, there was evidence of suppression of SOD1 in the spinal cord. There was some preservation of motor neurons on the right side of the spinal cord, which Dr. Brown called a “stunning” finding.
“We have never seen preservation of motor neurons in an autopsy of a patient with this kind of mutation before,” he said.
Prior to the patient’s death, there were some initial signs of a decrease of SOD1 in CSF. However, the patient developed an inflammatory response in the lining of the CSF known as meningoradiculitis.
“In that setting, the SOD1 level went back up, so we could not say that we produced a significant lasting decrease,” Dr. Brown said.
One and done
Because meningoradiculitis occurred in the first patient, immunosuppressive drugs were administered to the second patient.
The functional status and vital capacity of the second patient were relatively stable during a 60-week period, a course that could be typical of the slow disease progression in patients with this SOD1 genotype.
As with the first patient, this man did not experience a substantial change in SOD1 protein levels in CSF, and he did not show clinical improvement.
The main advantage of a viral gene therapy is that it could be a one-time treatment; ideally, it could be used to replace a single missing gene in conditions such as cystic fibrosis. “The hope is that the virus will drop off the gene modulator or the gene itself of interest, depending on the disease, and that the gene will be there more or less indefinitely,” said Dr. Brown. “So the cliché is, ‘one and done’—if all goes well.”
This small study illustrates that gene therapy safely “turns off genes and that the extent of suppression of genes can be significant,” said Dr. Brown.
Most SOD1 mutations could be treated with this microRNA viral vector, he added. More than 180 such mutations have been identified in ALS.
Additional studies are now needed to determine the results of this method in a larger number of patients who have ALS with SOD1 mutations, the investigators wrote.
Within reach
Both studies are encouraging in that they show that a precision-medicine approach to ALS associated with single mutated genes “may be within reach,” said Dr. Hardiman.
She noted that gene therapies have been used successfully in other motor neuron conditions. For example, an ASO and a viral vector have “very significant efficacy” in a form of spinal muscular atrophy that occurs in infants. “So the underlying proof of principle is already there.”
The reduction in SOD1 levels among the highest-dose tofersen group in the first study indicates “target engagement,” Dr. Hardiman said.
In that study, the documented decreased protein in the CSF appeared to be dose related, as was the effect for neurofilaments, which is biomarker evidence of neuronal damage, she noted.
In the second study, the pathologic evidence from the first patient also suggests “evidence of target engagement,” Dr. Hardiman said.
However, she added, “We don’t know very much about the outcome of the second case other than immunosuppression seemed to be beneficial.”
New hope
Both studies have caveats, said Dr. Hardiman. For example, it is unclear whether the treatments would be beneficial for every variant in SOD1.
“These are very expensive therapies, and we will need to have some level of certainty in order to be able to determine whether this should be a treatment for a patient or not,” said Dr. Hardiman.
She also noted that the studies were not powered to provide evidence of efficacy and that they raise questions about the accuracy of the ALSFRS-R.
One issue is that the respiratory part of that scale is “very insensitive”; another is that the scale doesn’t capture nonmotor elements, such as cognition and behavior, she said.
Utilizing a combination of the ALSFRS-R slope and survival would “probably be more beneficial,” Dr. Hardiman said.
Understanding how to alter the genetic influence in a disorder is important to be able to identify successful treatments, Dr. Hardiman added. For example, the discovery of the BRCA gene led oncologists to develop a precision medicine approach to the treatment of breast cancer.
In regard to ALS, by starting with subgroups that have specific genomic features, “investigators are providing new hope for patients at genetic risk for this devastating fatal disease,” said Dr. Hardiman.
The first study was funded by Biogen. The second study was funded by a fellowship grant from the Alzheimer’s Association, a Jack Satter Foundation Award, the ALS Association, the Angel Fund for ALS Research, ALS Finding a Cure, ALS-One, Project ALS, the Massachusetts General Hospital, the Max Rosenfeld and Cellucci Funds for ALS Research, and several senior members of Bain Capital. Dr. Ferguson is employed by and holds stock in Biogen. Dr. Brown receives grant support from the National Institute of Neurological Disorders and Stroke. He is also co-founder of Apic Bio. Dr. Hardiman is the editor-in-chief of the Journal of Amyotrophic Lateral Sclerosis and Frontotemporal Degenerations, has consulted for Cytokinetics, Mitsubishi, and Wave, and holds research grants from Novartis and Merck. During the past 2 years, she has also been a principal investigator on ALS clinical trials sponsored by Orion and Cytokinetics and is currently on the data and safety monitoring board of Accelsior.
This article first appeared on Medscape.com.
. Both studies investigated potential benefits of suppressing the toxic activity in cells of a mutant gene (SOD1) that encodes superoxide dismutase 1 (SOD1) in patients with ALS.
One study investigated the antisense oligonucleotide (ASO) tofersen (Biogen); the other study examined viral vector–mediated gene suppression.
The studies’ promising results signal “the beginning of a new precision medicine–based approach towards treating ALS,” said Orla Hardiman, BSc, MB, BCh, BAO, MD, a consultant neurologist and professor of neurology at Trinity College and Beaumont Hospital in Dublin, Ireland. Dr. Hardiman co-authored an editorial that accompanied the two studies, which were published July 9 in the New England Journal of Medicine.
Genetic culprits
ALS is a disorder of progressive degeneration of upper and lower motor neurons. It typically leads to death from ventilatory failure within 5 years of symptom onset.
Genetic factors are responsible for about half the risk variance of ALS. In populations of European origin, variants in SOD1 account for an estimated 13% to 20% of familial ALS, although this rate varies around the world. Although SOD1 is not the most common variant in ALS, it is the one that researchers are most familiar with and has been studied in an animal model.
In the first study, investigators evaluated the safety, pharmacokinetics, and pharmacodynamics of the ASO tofersen in adults with ALS.
An ASO is a small piece of nucleic acid that enters neurons in the spinal cord and brain, explained co-investigator Toby A. Ferguson, MD, PhD, vice president and head of the neuromuscular development unit at Biogen.
ASO binds to the SOD1 gene and knocks down the SOD1 protein, which is the “toxic engine [that] drives the disease, kills neurons, and causes patients to have loss of function and eventually to die,” said Dr. Ferguson. “The ASO turns off the motor that produces that toxic protein,” he added.
Animal studies have shown that ASOs that target SOD1 messenger RNA transcripts prolong survival, improve motor performance, and reduce SOD1 protein concentrations.
The new phase 1/2 double-blind study included 50 adults at 18 sites in the United States, Canada, and four European countries. All had muscle weakness attributed to ALS and a documented SOD1 mutation. Participants were randomly assigned to receive one of four doses of tofersen—20, 40, 60, or 100 mg—or placebo. Treatment was administered via a lumbar intrathecal bolus injection. The study included a screening period followed by a 12-week intervention period and a 12-week follow-up.
Adverse events
A primary outcome was the incidence of adverse events (AEs) and serious AEs. Results showed that all participants reported one or more AEs. The most common AEs were headache, pain at the injection site, post–lumbar puncture syndrome, and falls. Three deaths occurred, one in the placebo group, one in the 20-mg dose group, and one in the 60-mg dose group. There were no serious AEs in the 100-mg group.
Although the investigators found an increase in cerebrospinal fluid (CSF) protein and white cell counts, there was no clear association between these observations and higher doses of tofersen or longer duration of exposure.
“We don’t know the implications of this, and it’s something we need to keep an eye on as we move these studies forward,” Dr. Ferguson said.
None of the AEs or CSF abnormalities led to trial discontinuation.
A secondary outcome was change in SOD1 protein concentration in CSF at day 85. The study showed that SOD1 concentrations decreased by 36% among the participants who received tofersen 100 mg and by lesser amounts in the patients who received lower doses. Concentrations in the placebo group were reduced by 3%.
The 36% reduction in the highest dose group is likely meaningful and “foundational to the concept of what this molecule can do,” Dr. Ferguson said.
“If the number one cause of SOD1 ALS is accumulation of toxic SOD1 protein, then the demonstration that we can reduce SOD1 protein in the CSF ... is saying that’s the first step on the way to showing the molecule is doing what it should do,” he added.
Emerging tool
In patients with ALS, neurofilament concentrations typically increase as the disease progresses. However, this study documented a reduction in these CSF concentrations. “One interpretation of that could be that there is less neurodegeneration or neuro injury” in patients treated with tofersen, Dr. Ferguson said.
He noted that neurofilament is “an emerging tool” for understanding neurodegeneration. It could also “be another sort of biochemical signal that the molecule is doing something important,” he added.
However, he noted that neurofilament concentration is still an exploratory marker.
Exploratory analyses suggested a possible slowing of functional loss, as measured by the ALS Functional Rating Scale–Revised (ALSFRS-R) score and the handheld dynamometry megascore. The latter assesses strength in 16 muscle groups in the arms and legs. The investigators noted that no conclusions can be drawn from these outcomes.
A post hoc analysis showed that among patients with SOD1 mutations associated with a fast-progressing disease course, the slope of clinical decline might have been gentler, and there was a greater decrease in CSF neurofilament concentration compared among those whose disease followed a slower course.
This suggests that “if you pick the right target,” even patients with severe disease can be treated, Dr. Ferguson said.
He acknowledged that in a relatively short study such as this one, it may be easier to see benefits in patients whose disease is progressing rapidly. However, he’s convinced that the treatment “would work for all SOD1 ALS patients, not just fast patients.”
Dr. Ferguson said the study investigators are encouraged by the new data, which “really suggest that we may be developing a meaningful treatment for SOD1 ALS.” However, “it’s still early” in terms of rolling out this therapy for patients with ALS, he said.
The safety and efficacy of tofersen are currently being evaluated in a phase 3, randomized, double-blind, placebo-controlled trial.
Limitations of the current study were the small number of participants, the short duration of treatment and follow-up, the exploratory nature of efficacy outcomes, and the post hoc methods for defining the fast-progressing subgroup.
Although an advantage of tofersen is that it can enter the nucleus of the cell, perhaps boosting effectiveness, a drawback might be that patients need several treatments administered via lumbar puncture. Following three initial doses, the drug is given every month.
An alternative approach might be a viral vector approach.
“Stunning” finding
In the second study, investigators assessed the safety of a single intrathecal infusion of a viral vector therapy designed to target SOD1 in two patients with familial ALS. The two patients were a 22-year-old man whose mother had died of ALS at age 45 and a 56-year-old man who had a family history of ALS.
The aim of the viral vector therapy is to continually suppress mutant gene activity, said study co-investigator Robert H. Brown, Jr, MD, professor of neurology, University of Massachusetts Medical School, Worcester.
“The virus essentially drops off a piece of DNA, and that DNA keeps making the agent that suppresses the gene,” Dr. Brown said.
He noted that the first patient had a mutation that causes a rapidly developing, “horribly devastating” disease.
Initially, the patient’s right leg, in which movement had been worsening over several weeks, “seemed to get stronger and remain strong for quite a long time. I’ve never seen that in this kind of mutation,” said Dr. Brown.
The patient died of ALS. At autopsy, there was evidence of suppression of SOD1 in the spinal cord. There was some preservation of motor neurons on the right side of the spinal cord, which Dr. Brown called a “stunning” finding.
“We have never seen preservation of motor neurons in an autopsy of a patient with this kind of mutation before,” he said.
Prior to the patient’s death, there were some initial signs of a decrease of SOD1 in CSF. However, the patient developed an inflammatory response in the lining of the CSF known as meningoradiculitis.
“In that setting, the SOD1 level went back up, so we could not say that we produced a significant lasting decrease,” Dr. Brown said.
One and done
Because meningoradiculitis occurred in the first patient, immunosuppressive drugs were administered to the second patient.
The functional status and vital capacity of the second patient were relatively stable during a 60-week period, a course that could be typical of the slow disease progression in patients with this SOD1 genotype.
As with the first patient, this man did not experience a substantial change in SOD1 protein levels in CSF, and he did not show clinical improvement.
The main advantage of a viral gene therapy is that it could be a one-time treatment; ideally, it could be used to replace a single missing gene in conditions such as cystic fibrosis. “The hope is that the virus will drop off the gene modulator or the gene itself of interest, depending on the disease, and that the gene will be there more or less indefinitely,” said Dr. Brown. “So the cliché is, ‘one and done’—if all goes well.”
This small study illustrates that gene therapy safely “turns off genes and that the extent of suppression of genes can be significant,” said Dr. Brown.
Most SOD1 mutations could be treated with this microRNA viral vector, he added. More than 180 such mutations have been identified in ALS.
Additional studies are now needed to determine the results of this method in a larger number of patients who have ALS with SOD1 mutations, the investigators wrote.
Within reach
Both studies are encouraging in that they show that a precision-medicine approach to ALS associated with single mutated genes “may be within reach,” said Dr. Hardiman.
She noted that gene therapies have been used successfully in other motor neuron conditions. For example, an ASO and a viral vector have “very significant efficacy” in a form of spinal muscular atrophy that occurs in infants. “So the underlying proof of principle is already there.”
The reduction in SOD1 levels among the highest-dose tofersen group in the first study indicates “target engagement,” Dr. Hardiman said.
In that study, the documented decreased protein in the CSF appeared to be dose related, as was the effect for neurofilaments, which is biomarker evidence of neuronal damage, she noted.
In the second study, the pathologic evidence from the first patient also suggests “evidence of target engagement,” Dr. Hardiman said.
However, she added, “We don’t know very much about the outcome of the second case other than immunosuppression seemed to be beneficial.”
New hope
Both studies have caveats, said Dr. Hardiman. For example, it is unclear whether the treatments would be beneficial for every variant in SOD1.
“These are very expensive therapies, and we will need to have some level of certainty in order to be able to determine whether this should be a treatment for a patient or not,” said Dr. Hardiman.
She also noted that the studies were not powered to provide evidence of efficacy and that they raise questions about the accuracy of the ALSFRS-R.
One issue is that the respiratory part of that scale is “very insensitive”; another is that the scale doesn’t capture nonmotor elements, such as cognition and behavior, she said.
Utilizing a combination of the ALSFRS-R slope and survival would “probably be more beneficial,” Dr. Hardiman said.
Understanding how to alter the genetic influence in a disorder is important to be able to identify successful treatments, Dr. Hardiman added. For example, the discovery of the BRCA gene led oncologists to develop a precision medicine approach to the treatment of breast cancer.
In regard to ALS, by starting with subgroups that have specific genomic features, “investigators are providing new hope for patients at genetic risk for this devastating fatal disease,” said Dr. Hardiman.
The first study was funded by Biogen. The second study was funded by a fellowship grant from the Alzheimer’s Association, a Jack Satter Foundation Award, the ALS Association, the Angel Fund for ALS Research, ALS Finding a Cure, ALS-One, Project ALS, the Massachusetts General Hospital, the Max Rosenfeld and Cellucci Funds for ALS Research, and several senior members of Bain Capital. Dr. Ferguson is employed by and holds stock in Biogen. Dr. Brown receives grant support from the National Institute of Neurological Disorders and Stroke. He is also co-founder of Apic Bio. Dr. Hardiman is the editor-in-chief of the Journal of Amyotrophic Lateral Sclerosis and Frontotemporal Degenerations, has consulted for Cytokinetics, Mitsubishi, and Wave, and holds research grants from Novartis and Merck. During the past 2 years, she has also been a principal investigator on ALS clinical trials sponsored by Orion and Cytokinetics and is currently on the data and safety monitoring board of Accelsior.
This article first appeared on Medscape.com.
. Both studies investigated potential benefits of suppressing the toxic activity in cells of a mutant gene (SOD1) that encodes superoxide dismutase 1 (SOD1) in patients with ALS.
One study investigated the antisense oligonucleotide (ASO) tofersen (Biogen); the other study examined viral vector–mediated gene suppression.
The studies’ promising results signal “the beginning of a new precision medicine–based approach towards treating ALS,” said Orla Hardiman, BSc, MB, BCh, BAO, MD, a consultant neurologist and professor of neurology at Trinity College and Beaumont Hospital in Dublin, Ireland. Dr. Hardiman co-authored an editorial that accompanied the two studies, which were published July 9 in the New England Journal of Medicine.
Genetic culprits
ALS is a disorder of progressive degeneration of upper and lower motor neurons. It typically leads to death from ventilatory failure within 5 years of symptom onset.
Genetic factors are responsible for about half the risk variance of ALS. In populations of European origin, variants in SOD1 account for an estimated 13% to 20% of familial ALS, although this rate varies around the world. Although SOD1 is not the most common variant in ALS, it is the one that researchers are most familiar with and has been studied in an animal model.
In the first study, investigators evaluated the safety, pharmacokinetics, and pharmacodynamics of the ASO tofersen in adults with ALS.
An ASO is a small piece of nucleic acid that enters neurons in the spinal cord and brain, explained co-investigator Toby A. Ferguson, MD, PhD, vice president and head of the neuromuscular development unit at Biogen.
ASO binds to the SOD1 gene and knocks down the SOD1 protein, which is the “toxic engine [that] drives the disease, kills neurons, and causes patients to have loss of function and eventually to die,” said Dr. Ferguson. “The ASO turns off the motor that produces that toxic protein,” he added.
Animal studies have shown that ASOs that target SOD1 messenger RNA transcripts prolong survival, improve motor performance, and reduce SOD1 protein concentrations.
The new phase 1/2 double-blind study included 50 adults at 18 sites in the United States, Canada, and four European countries. All had muscle weakness attributed to ALS and a documented SOD1 mutation. Participants were randomly assigned to receive one of four doses of tofersen—20, 40, 60, or 100 mg—or placebo. Treatment was administered via a lumbar intrathecal bolus injection. The study included a screening period followed by a 12-week intervention period and a 12-week follow-up.
Adverse events
A primary outcome was the incidence of adverse events (AEs) and serious AEs. Results showed that all participants reported one or more AEs. The most common AEs were headache, pain at the injection site, post–lumbar puncture syndrome, and falls. Three deaths occurred, one in the placebo group, one in the 20-mg dose group, and one in the 60-mg dose group. There were no serious AEs in the 100-mg group.
Although the investigators found an increase in cerebrospinal fluid (CSF) protein and white cell counts, there was no clear association between these observations and higher doses of tofersen or longer duration of exposure.
“We don’t know the implications of this, and it’s something we need to keep an eye on as we move these studies forward,” Dr. Ferguson said.
None of the AEs or CSF abnormalities led to trial discontinuation.
A secondary outcome was change in SOD1 protein concentration in CSF at day 85. The study showed that SOD1 concentrations decreased by 36% among the participants who received tofersen 100 mg and by lesser amounts in the patients who received lower doses. Concentrations in the placebo group were reduced by 3%.
The 36% reduction in the highest dose group is likely meaningful and “foundational to the concept of what this molecule can do,” Dr. Ferguson said.
“If the number one cause of SOD1 ALS is accumulation of toxic SOD1 protein, then the demonstration that we can reduce SOD1 protein in the CSF ... is saying that’s the first step on the way to showing the molecule is doing what it should do,” he added.
Emerging tool
In patients with ALS, neurofilament concentrations typically increase as the disease progresses. However, this study documented a reduction in these CSF concentrations. “One interpretation of that could be that there is less neurodegeneration or neuro injury” in patients treated with tofersen, Dr. Ferguson said.
He noted that neurofilament is “an emerging tool” for understanding neurodegeneration. It could also “be another sort of biochemical signal that the molecule is doing something important,” he added.
However, he noted that neurofilament concentration is still an exploratory marker.
Exploratory analyses suggested a possible slowing of functional loss, as measured by the ALS Functional Rating Scale–Revised (ALSFRS-R) score and the handheld dynamometry megascore. The latter assesses strength in 16 muscle groups in the arms and legs. The investigators noted that no conclusions can be drawn from these outcomes.
A post hoc analysis showed that among patients with SOD1 mutations associated with a fast-progressing disease course, the slope of clinical decline might have been gentler, and there was a greater decrease in CSF neurofilament concentration compared among those whose disease followed a slower course.
This suggests that “if you pick the right target,” even patients with severe disease can be treated, Dr. Ferguson said.
He acknowledged that in a relatively short study such as this one, it may be easier to see benefits in patients whose disease is progressing rapidly. However, he’s convinced that the treatment “would work for all SOD1 ALS patients, not just fast patients.”
Dr. Ferguson said the study investigators are encouraged by the new data, which “really suggest that we may be developing a meaningful treatment for SOD1 ALS.” However, “it’s still early” in terms of rolling out this therapy for patients with ALS, he said.
The safety and efficacy of tofersen are currently being evaluated in a phase 3, randomized, double-blind, placebo-controlled trial.
Limitations of the current study were the small number of participants, the short duration of treatment and follow-up, the exploratory nature of efficacy outcomes, and the post hoc methods for defining the fast-progressing subgroup.
Although an advantage of tofersen is that it can enter the nucleus of the cell, perhaps boosting effectiveness, a drawback might be that patients need several treatments administered via lumbar puncture. Following three initial doses, the drug is given every month.
An alternative approach might be a viral vector approach.
“Stunning” finding
In the second study, investigators assessed the safety of a single intrathecal infusion of a viral vector therapy designed to target SOD1 in two patients with familial ALS. The two patients were a 22-year-old man whose mother had died of ALS at age 45 and a 56-year-old man who had a family history of ALS.
The aim of the viral vector therapy is to continually suppress mutant gene activity, said study co-investigator Robert H. Brown, Jr, MD, professor of neurology, University of Massachusetts Medical School, Worcester.
“The virus essentially drops off a piece of DNA, and that DNA keeps making the agent that suppresses the gene,” Dr. Brown said.
He noted that the first patient had a mutation that causes a rapidly developing, “horribly devastating” disease.
Initially, the patient’s right leg, in which movement had been worsening over several weeks, “seemed to get stronger and remain strong for quite a long time. I’ve never seen that in this kind of mutation,” said Dr. Brown.
The patient died of ALS. At autopsy, there was evidence of suppression of SOD1 in the spinal cord. There was some preservation of motor neurons on the right side of the spinal cord, which Dr. Brown called a “stunning” finding.
“We have never seen preservation of motor neurons in an autopsy of a patient with this kind of mutation before,” he said.
Prior to the patient’s death, there were some initial signs of a decrease of SOD1 in CSF. However, the patient developed an inflammatory response in the lining of the CSF known as meningoradiculitis.
“In that setting, the SOD1 level went back up, so we could not say that we produced a significant lasting decrease,” Dr. Brown said.
One and done
Because meningoradiculitis occurred in the first patient, immunosuppressive drugs were administered to the second patient.
The functional status and vital capacity of the second patient were relatively stable during a 60-week period, a course that could be typical of the slow disease progression in patients with this SOD1 genotype.
As with the first patient, this man did not experience a substantial change in SOD1 protein levels in CSF, and he did not show clinical improvement.
The main advantage of a viral gene therapy is that it could be a one-time treatment; ideally, it could be used to replace a single missing gene in conditions such as cystic fibrosis. “The hope is that the virus will drop off the gene modulator or the gene itself of interest, depending on the disease, and that the gene will be there more or less indefinitely,” said Dr. Brown. “So the cliché is, ‘one and done’—if all goes well.”
This small study illustrates that gene therapy safely “turns off genes and that the extent of suppression of genes can be significant,” said Dr. Brown.
Most SOD1 mutations could be treated with this microRNA viral vector, he added. More than 180 such mutations have been identified in ALS.
Additional studies are now needed to determine the results of this method in a larger number of patients who have ALS with SOD1 mutations, the investigators wrote.
Within reach
Both studies are encouraging in that they show that a precision-medicine approach to ALS associated with single mutated genes “may be within reach,” said Dr. Hardiman.
She noted that gene therapies have been used successfully in other motor neuron conditions. For example, an ASO and a viral vector have “very significant efficacy” in a form of spinal muscular atrophy that occurs in infants. “So the underlying proof of principle is already there.”
The reduction in SOD1 levels among the highest-dose tofersen group in the first study indicates “target engagement,” Dr. Hardiman said.
In that study, the documented decreased protein in the CSF appeared to be dose related, as was the effect for neurofilaments, which is biomarker evidence of neuronal damage, she noted.
In the second study, the pathologic evidence from the first patient also suggests “evidence of target engagement,” Dr. Hardiman said.
However, she added, “We don’t know very much about the outcome of the second case other than immunosuppression seemed to be beneficial.”
New hope
Both studies have caveats, said Dr. Hardiman. For example, it is unclear whether the treatments would be beneficial for every variant in SOD1.
“These are very expensive therapies, and we will need to have some level of certainty in order to be able to determine whether this should be a treatment for a patient or not,” said Dr. Hardiman.
She also noted that the studies were not powered to provide evidence of efficacy and that they raise questions about the accuracy of the ALSFRS-R.
One issue is that the respiratory part of that scale is “very insensitive”; another is that the scale doesn’t capture nonmotor elements, such as cognition and behavior, she said.
Utilizing a combination of the ALSFRS-R slope and survival would “probably be more beneficial,” Dr. Hardiman said.
Understanding how to alter the genetic influence in a disorder is important to be able to identify successful treatments, Dr. Hardiman added. For example, the discovery of the BRCA gene led oncologists to develop a precision medicine approach to the treatment of breast cancer.
In regard to ALS, by starting with subgroups that have specific genomic features, “investigators are providing new hope for patients at genetic risk for this devastating fatal disease,” said Dr. Hardiman.
The first study was funded by Biogen. The second study was funded by a fellowship grant from the Alzheimer’s Association, a Jack Satter Foundation Award, the ALS Association, the Angel Fund for ALS Research, ALS Finding a Cure, ALS-One, Project ALS, the Massachusetts General Hospital, the Max Rosenfeld and Cellucci Funds for ALS Research, and several senior members of Bain Capital. Dr. Ferguson is employed by and holds stock in Biogen. Dr. Brown receives grant support from the National Institute of Neurological Disorders and Stroke. He is also co-founder of Apic Bio. Dr. Hardiman is the editor-in-chief of the Journal of Amyotrophic Lateral Sclerosis and Frontotemporal Degenerations, has consulted for Cytokinetics, Mitsubishi, and Wave, and holds research grants from Novartis and Merck. During the past 2 years, she has also been a principal investigator on ALS clinical trials sponsored by Orion and Cytokinetics and is currently on the data and safety monitoring board of Accelsior.
This article first appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Intubation boxes may do more harm than good in COVID-19 risk
Clear aerosol boxes designed to keep COVID-19 patients’ airborne droplets from infecting health care workers during intubation may actually increase providers’ exposure to the virus, a small study suggests.
Joanna P. Simpson, MbChB, an intensivist in the department of anaesthesia and perioperative medicine at Eastern Health in Melbourne, and colleagues tested five models of barriers used for protection while intubating simulated “patients” with COVID-19 and compared the interventions with a control of having no protection. They published their findings online in Anaesthesia.
Coauthor Peter Chan, MBBS, also an intensivist at Eastern Health, said in an interview that the virus essentially concentrates inside the box and because the box has holes on the sides to allow providers’ arms in, the gaps “act as nozzles, so when a patient coughs, it creates a sudden wave of air that pushes all these particles out the path of least resistance” and into the face of the intubator.
Their institution stopped using any such aerosol-containment devices during intubation until safety can be proven.
Many forms for boxes
The boxes take different forms and are made by various designers and manufacturers around the world, including in the United States, but they generally cover the head and upper body of patients and allow providers to reach through holes to intubate.
The U.S. Food and Drug Administration on May 1 issued an emergency use authorization (EUA) for “protective barrier enclosures ... to prevent [health care provider] exposure to pathogenic biological airborne particulates by providing an extra layer of barrier protection in addition to personal protective equipment [PPE].”
Others refer to them as “intubation boxes.” A search of GoFundMe campaigns showed hundreds of campaigns for intubation boxes.
Dr. Simpson and colleagues used an in-situ simulation model to evaluate laryngoscopist exposure to airborne particles sized 0.3-5.0 mcm using five aerosol containment devices (aerosol box, sealed box with suction, sealed box without suction, vertical drapes, and horizontal drapes) compared with no aerosol containment device.
Nebulized saline was used in an aerosol-generating model for 300 seconds, at which point the devices were removed to gauge particle spread for another 60 seconds.
Compared with no device use, the sealed intubation box with suction resulted in a decreased exposure for particle sizes of 0.3, 0.5, 1.0, and 2.5 mcm – but not 5.0 mcm – over all time periods (P = .003 for all time periods, which ranged from 30 to 360 seconds).
Conversely, the aerosol box, compared with no device use, showed an increase in 1.0, 2.5, and 5.0 mcm airborne-particle exposure at 300 seconds (P = .002, 0.008, and .002, respectively). Compared with no device use, neither horizontal nor vertical drapes showed any difference in any particle size exposure at any time.
The researchers used seven volunteers who took turns acting as the patient or the intubator. As each of the seven volunteers did all six trials (the five interventions plus no intervention), the study generated 42 sets of results.
More evidence passive boxes are ineffective
Plastic surgeon Dave Turer, MD, MS, who is also an electrical and biomedical engineer, and some emergency physician colleagues had doubts about these boxes early on and wrote about the need for thorough testing.
He told this news organization, “I find it kind of infuriating that if you search for ‘intubation box’ there are all these companies making claims that are totally unsubstantiated.”
A desperate need to stop the virus is leading to unacceptable practices, he said.
His team at the University of Pittsburgh Medical Center in Pennsylvania tested commercially available boxes using white vapor to simulate patients› exhaled breath and found the vapor billowed into the surrounding environment.
He said Simpson and colleagues had similar findings: The boxes didn’t contain the patients’ breaths and may even increase the stream heading toward intubators.
Dr. Turer said his team has designed a different kind of box, without armholes for the intubators, and with active airflow and filtering and have submitted their design and research to the FDA for an EUA.
The FDA’s current EUA is for boxes “that are no different from a face shield or a splash shield,” Dr. Turer said, adding that “they specifically state that they are not designed or intended to contain aerosol.”
He said while this study is a good start, his team’s findings will help demonstrate why the common passive boxes should not be used.
One of the most prevalent designs, he pointed out, was one by Taiwanese anesthesiologist Hsien Yung Lai that was widely circulated in March.
David W. Kaczka, MD, PhD, associate professor of anesthesia, biomedical engineering, and radiology at University of Iowa in Iowa City, is one of the researchers who modified that design and made prototypes. He said in an interview he thinks the study conclusion by Simpson et al is “not as dismal as the authors are making it out to be.”
He pointed to the relative success of the sealed box with suction. His team’s adapted model added a suction port to generate a negative pressure field around the patient.
The biggest critique he had of the study, Dr. Kaczka said, was a lack of a true control group.
“They tested all their conditions with nebulized saline,” he pointed out. “I think a more appropriately designed study would have also looked at a group where no saline was being nebulized and see what the particle counts were afterwards. It’s not clear how the device would distinguish between a particle coming from a saline nebulizer vs. coming from a simulated patient vs. coming from the laryngoscopist.”
He also noted that what comes out of a patient is not going to be saline and will have different density and viscosity.
That said, the study by Dr. Simpson and colleagues highlights the need to take a hard look at these boxes with more research, he said, adding, “I think there’s some hope there.”
He noted that a letter to the editor by Boston researchers, published online April 3 in the New England Journal of Medicine, describes how they used fluorescent dye forced from a balloon to simulate a patient’s cough to see whether an aerosol box protected intubators.
That letter concludes, “We suggest that our ad hoc barrier enclosure provided a modicum of additional protection and could be considered to be an adjunct to standard PPE.”
The Anaesthesia findings come as a second global wave becomes more likely as does awareness of the potential of airborne droplets to spread the virus.
Scientists from 32 countries warned the World Health Organization that the spread of COVID-19 through airborne droplets may have been severely underestimated.
On Wednesday, the World Health Organization formally acknowledged evidence regarding potential spread of the virus through these droplets and on Thursday issued an updated brief.
Intellectual property surrounding the device invented by Dr. Turer’s team is owned by UPMC. Dr. Chan and Dr. Kaczka have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clear aerosol boxes designed to keep COVID-19 patients’ airborne droplets from infecting health care workers during intubation may actually increase providers’ exposure to the virus, a small study suggests.
Joanna P. Simpson, MbChB, an intensivist in the department of anaesthesia and perioperative medicine at Eastern Health in Melbourne, and colleagues tested five models of barriers used for protection while intubating simulated “patients” with COVID-19 and compared the interventions with a control of having no protection. They published their findings online in Anaesthesia.
Coauthor Peter Chan, MBBS, also an intensivist at Eastern Health, said in an interview that the virus essentially concentrates inside the box and because the box has holes on the sides to allow providers’ arms in, the gaps “act as nozzles, so when a patient coughs, it creates a sudden wave of air that pushes all these particles out the path of least resistance” and into the face of the intubator.
Their institution stopped using any such aerosol-containment devices during intubation until safety can be proven.
Many forms for boxes
The boxes take different forms and are made by various designers and manufacturers around the world, including in the United States, but they generally cover the head and upper body of patients and allow providers to reach through holes to intubate.
The U.S. Food and Drug Administration on May 1 issued an emergency use authorization (EUA) for “protective barrier enclosures ... to prevent [health care provider] exposure to pathogenic biological airborne particulates by providing an extra layer of barrier protection in addition to personal protective equipment [PPE].”
Others refer to them as “intubation boxes.” A search of GoFundMe campaigns showed hundreds of campaigns for intubation boxes.
Dr. Simpson and colleagues used an in-situ simulation model to evaluate laryngoscopist exposure to airborne particles sized 0.3-5.0 mcm using five aerosol containment devices (aerosol box, sealed box with suction, sealed box without suction, vertical drapes, and horizontal drapes) compared with no aerosol containment device.
Nebulized saline was used in an aerosol-generating model for 300 seconds, at which point the devices were removed to gauge particle spread for another 60 seconds.
Compared with no device use, the sealed intubation box with suction resulted in a decreased exposure for particle sizes of 0.3, 0.5, 1.0, and 2.5 mcm – but not 5.0 mcm – over all time periods (P = .003 for all time periods, which ranged from 30 to 360 seconds).
Conversely, the aerosol box, compared with no device use, showed an increase in 1.0, 2.5, and 5.0 mcm airborne-particle exposure at 300 seconds (P = .002, 0.008, and .002, respectively). Compared with no device use, neither horizontal nor vertical drapes showed any difference in any particle size exposure at any time.
The researchers used seven volunteers who took turns acting as the patient or the intubator. As each of the seven volunteers did all six trials (the five interventions plus no intervention), the study generated 42 sets of results.
More evidence passive boxes are ineffective
Plastic surgeon Dave Turer, MD, MS, who is also an electrical and biomedical engineer, and some emergency physician colleagues had doubts about these boxes early on and wrote about the need for thorough testing.
He told this news organization, “I find it kind of infuriating that if you search for ‘intubation box’ there are all these companies making claims that are totally unsubstantiated.”
A desperate need to stop the virus is leading to unacceptable practices, he said.
His team at the University of Pittsburgh Medical Center in Pennsylvania tested commercially available boxes using white vapor to simulate patients› exhaled breath and found the vapor billowed into the surrounding environment.
He said Simpson and colleagues had similar findings: The boxes didn’t contain the patients’ breaths and may even increase the stream heading toward intubators.
Dr. Turer said his team has designed a different kind of box, without armholes for the intubators, and with active airflow and filtering and have submitted their design and research to the FDA for an EUA.
The FDA’s current EUA is for boxes “that are no different from a face shield or a splash shield,” Dr. Turer said, adding that “they specifically state that they are not designed or intended to contain aerosol.”
He said while this study is a good start, his team’s findings will help demonstrate why the common passive boxes should not be used.
One of the most prevalent designs, he pointed out, was one by Taiwanese anesthesiologist Hsien Yung Lai that was widely circulated in March.
David W. Kaczka, MD, PhD, associate professor of anesthesia, biomedical engineering, and radiology at University of Iowa in Iowa City, is one of the researchers who modified that design and made prototypes. He said in an interview he thinks the study conclusion by Simpson et al is “not as dismal as the authors are making it out to be.”
He pointed to the relative success of the sealed box with suction. His team’s adapted model added a suction port to generate a negative pressure field around the patient.
The biggest critique he had of the study, Dr. Kaczka said, was a lack of a true control group.
“They tested all their conditions with nebulized saline,” he pointed out. “I think a more appropriately designed study would have also looked at a group where no saline was being nebulized and see what the particle counts were afterwards. It’s not clear how the device would distinguish between a particle coming from a saline nebulizer vs. coming from a simulated patient vs. coming from the laryngoscopist.”
He also noted that what comes out of a patient is not going to be saline and will have different density and viscosity.
That said, the study by Dr. Simpson and colleagues highlights the need to take a hard look at these boxes with more research, he said, adding, “I think there’s some hope there.”
He noted that a letter to the editor by Boston researchers, published online April 3 in the New England Journal of Medicine, describes how they used fluorescent dye forced from a balloon to simulate a patient’s cough to see whether an aerosol box protected intubators.
That letter concludes, “We suggest that our ad hoc barrier enclosure provided a modicum of additional protection and could be considered to be an adjunct to standard PPE.”
The Anaesthesia findings come as a second global wave becomes more likely as does awareness of the potential of airborne droplets to spread the virus.
Scientists from 32 countries warned the World Health Organization that the spread of COVID-19 through airborne droplets may have been severely underestimated.
On Wednesday, the World Health Organization formally acknowledged evidence regarding potential spread of the virus through these droplets and on Thursday issued an updated brief.
Intellectual property surrounding the device invented by Dr. Turer’s team is owned by UPMC. Dr. Chan and Dr. Kaczka have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clear aerosol boxes designed to keep COVID-19 patients’ airborne droplets from infecting health care workers during intubation may actually increase providers’ exposure to the virus, a small study suggests.
Joanna P. Simpson, MbChB, an intensivist in the department of anaesthesia and perioperative medicine at Eastern Health in Melbourne, and colleagues tested five models of barriers used for protection while intubating simulated “patients” with COVID-19 and compared the interventions with a control of having no protection. They published their findings online in Anaesthesia.
Coauthor Peter Chan, MBBS, also an intensivist at Eastern Health, said in an interview that the virus essentially concentrates inside the box and because the box has holes on the sides to allow providers’ arms in, the gaps “act as nozzles, so when a patient coughs, it creates a sudden wave of air that pushes all these particles out the path of least resistance” and into the face of the intubator.
Their institution stopped using any such aerosol-containment devices during intubation until safety can be proven.
Many forms for boxes
The boxes take different forms and are made by various designers and manufacturers around the world, including in the United States, but they generally cover the head and upper body of patients and allow providers to reach through holes to intubate.
The U.S. Food and Drug Administration on May 1 issued an emergency use authorization (EUA) for “protective barrier enclosures ... to prevent [health care provider] exposure to pathogenic biological airborne particulates by providing an extra layer of barrier protection in addition to personal protective equipment [PPE].”
Others refer to them as “intubation boxes.” A search of GoFundMe campaigns showed hundreds of campaigns for intubation boxes.
Dr. Simpson and colleagues used an in-situ simulation model to evaluate laryngoscopist exposure to airborne particles sized 0.3-5.0 mcm using five aerosol containment devices (aerosol box, sealed box with suction, sealed box without suction, vertical drapes, and horizontal drapes) compared with no aerosol containment device.
Nebulized saline was used in an aerosol-generating model for 300 seconds, at which point the devices were removed to gauge particle spread for another 60 seconds.
Compared with no device use, the sealed intubation box with suction resulted in a decreased exposure for particle sizes of 0.3, 0.5, 1.0, and 2.5 mcm – but not 5.0 mcm – over all time periods (P = .003 for all time periods, which ranged from 30 to 360 seconds).
Conversely, the aerosol box, compared with no device use, showed an increase in 1.0, 2.5, and 5.0 mcm airborne-particle exposure at 300 seconds (P = .002, 0.008, and .002, respectively). Compared with no device use, neither horizontal nor vertical drapes showed any difference in any particle size exposure at any time.
The researchers used seven volunteers who took turns acting as the patient or the intubator. As each of the seven volunteers did all six trials (the five interventions plus no intervention), the study generated 42 sets of results.
More evidence passive boxes are ineffective
Plastic surgeon Dave Turer, MD, MS, who is also an electrical and biomedical engineer, and some emergency physician colleagues had doubts about these boxes early on and wrote about the need for thorough testing.
He told this news organization, “I find it kind of infuriating that if you search for ‘intubation box’ there are all these companies making claims that are totally unsubstantiated.”
A desperate need to stop the virus is leading to unacceptable practices, he said.
His team at the University of Pittsburgh Medical Center in Pennsylvania tested commercially available boxes using white vapor to simulate patients› exhaled breath and found the vapor billowed into the surrounding environment.
He said Simpson and colleagues had similar findings: The boxes didn’t contain the patients’ breaths and may even increase the stream heading toward intubators.
Dr. Turer said his team has designed a different kind of box, without armholes for the intubators, and with active airflow and filtering and have submitted their design and research to the FDA for an EUA.
The FDA’s current EUA is for boxes “that are no different from a face shield or a splash shield,” Dr. Turer said, adding that “they specifically state that they are not designed or intended to contain aerosol.”
He said while this study is a good start, his team’s findings will help demonstrate why the common passive boxes should not be used.
One of the most prevalent designs, he pointed out, was one by Taiwanese anesthesiologist Hsien Yung Lai that was widely circulated in March.
David W. Kaczka, MD, PhD, associate professor of anesthesia, biomedical engineering, and radiology at University of Iowa in Iowa City, is one of the researchers who modified that design and made prototypes. He said in an interview he thinks the study conclusion by Simpson et al is “not as dismal as the authors are making it out to be.”
He pointed to the relative success of the sealed box with suction. His team’s adapted model added a suction port to generate a negative pressure field around the patient.
The biggest critique he had of the study, Dr. Kaczka said, was a lack of a true control group.
“They tested all their conditions with nebulized saline,” he pointed out. “I think a more appropriately designed study would have also looked at a group where no saline was being nebulized and see what the particle counts were afterwards. It’s not clear how the device would distinguish between a particle coming from a saline nebulizer vs. coming from a simulated patient vs. coming from the laryngoscopist.”
He also noted that what comes out of a patient is not going to be saline and will have different density and viscosity.
That said, the study by Dr. Simpson and colleagues highlights the need to take a hard look at these boxes with more research, he said, adding, “I think there’s some hope there.”
He noted that a letter to the editor by Boston researchers, published online April 3 in the New England Journal of Medicine, describes how they used fluorescent dye forced from a balloon to simulate a patient’s cough to see whether an aerosol box protected intubators.
That letter concludes, “We suggest that our ad hoc barrier enclosure provided a modicum of additional protection and could be considered to be an adjunct to standard PPE.”
The Anaesthesia findings come as a second global wave becomes more likely as does awareness of the potential of airborne droplets to spread the virus.
Scientists from 32 countries warned the World Health Organization that the spread of COVID-19 through airborne droplets may have been severely underestimated.
On Wednesday, the World Health Organization formally acknowledged evidence regarding potential spread of the virus through these droplets and on Thursday issued an updated brief.
Intellectual property surrounding the device invented by Dr. Turer’s team is owned by UPMC. Dr. Chan and Dr. Kaczka have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Part 3: Lipid Management in Diabetes Patients
Previously, we explored blood pressure control in a patient with diabetes. Now, we’ll discuss the value of a fasting lipid panel and treatment for dyslipidemia in this population.
CASE CONTINUED
Mr. W completed a fasting lipid panel, which revealed the following: triglycerides, 145 mg/dL; high-density lipoprotein (HDL) level, 32 mg/dL; and low-density lipoprotein (LDL) level, 108 mg/dL. He is currently receiving low-dose statin therapy. Based on these results, Mr. W fits the criteria for dyslipidemia.
Dyslipidemia
Dyslipidemia marked by elevated LDL levels—as observed in Mr. W—is a well-known contributing factor to development of cardiovascular disease in patients with diabetes. Elevated triglycerides and low HDL levels also are often noted in these patients. Patients with diabetes are particularly vulnerable to atherosclerosis due to a combination of pro-inflammatory factors and hyperglycemic effects. Both the ADA and the AACE agree that lipid management, including fasting lipid panels and appropriate treatment, is of paramount importance in patients with diabetes.7,8
Fasting Lipid Panels
The AACE recommends administering at least annual fasting lipid panels in all adults with diabetes, and LDL goal levels should be based on the cardiovascular risk of the patient.7 For patients with
- established ASCVD, the LDL goal is < 55 mg/dL
- risk factors for ASCVD (eg, hypertension, tobacco use, family history of ASCVD) in addition to diabetes, the LDL goal is < 70 mg/dL
- no risk factors, the LDL goal is < 100 mg/dL.7
Statin Therapy
Research indicates that statins reduce the risk for cardiovascular events and are recommended as first-line treatment for dyslipidemia.2,7 Statin therapy is recommended for patients with LDL levels above goal without contraindications.10 Higher-dose statins have been shown to help improve cardiovascular outcomes, and most—if not all—guidelines recommend up-titration of these medications as tolerated by the patient. 7,8,29 After initiation of statin therapy, clinicians should continue to monitor lipid levels every 4 to 12 weeks after a change in lipid therapy and then schedule monitoring annually.2
Unfortunately, a recent large-scale retrospective study of the medical records of 125,464 patients with type 2 diabetes showed that although 99% of the patients were at high risk for or already had ASCVD, only 63% were receiving the recommended statin therapy.30 Therefore, all patients with diabetes at risk for ASCVD require evaluation to determine the need for statins.
Additional treatments. If the patient’s levels remain above goal, strong consideration should be given to additional therapies. Ezetimibe has been shown to have some benefit in reducing LDL levels and cardiovascular risk.31 PCSK9 inhibitors are a newer treatment for cardiovascular disease and are particularly beneficial for patients with known ASCVD. The FOURIER and ODYSSEY trials demonstrated that PCSK9 inhibitors had relative risk reductions of 48% to 53% for major ASCVD events and showed that these medications help reduce LDL levels and, most importantly, cardiovascular risk.32,33
Continue to: Recommendations for other lipid components
Recommendations for other lipid components—non–HDL-C, apolipoprotein B, or LDL-P—are very specific and consideration may be given for referral to an endocrinologist or lipidologist for evaluation and treatment.7,8 Evidence on reducing cardiovascular risk with therapies for decreasing triglyceride levels is limited. Recently though, icosapent ethyl received FDA approval as an adjunct to maximally tolerated statin therapy to reduce the risk for cardiovascular events in patients with elevated triglyceride levels (≥ 150 mg/dL).34,35 ADA guidelines recommend icosapent ethyl for patients with diabetes, 1 additional cardiovascular risk factor, and triglyceride levels between 135 and 499 mg/dL.2
In Part 4, I’ll explore how clinicians can best monitor for chronic kidney disease in patients with diabetes. We’ll also discuss the medications used for improving kidney health in these patients.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Previously, we explored blood pressure control in a patient with diabetes. Now, we’ll discuss the value of a fasting lipid panel and treatment for dyslipidemia in this population.
CASE CONTINUED
Mr. W completed a fasting lipid panel, which revealed the following: triglycerides, 145 mg/dL; high-density lipoprotein (HDL) level, 32 mg/dL; and low-density lipoprotein (LDL) level, 108 mg/dL. He is currently receiving low-dose statin therapy. Based on these results, Mr. W fits the criteria for dyslipidemia.
Dyslipidemia
Dyslipidemia marked by elevated LDL levels—as observed in Mr. W—is a well-known contributing factor to development of cardiovascular disease in patients with diabetes. Elevated triglycerides and low HDL levels also are often noted in these patients. Patients with diabetes are particularly vulnerable to atherosclerosis due to a combination of pro-inflammatory factors and hyperglycemic effects. Both the ADA and the AACE agree that lipid management, including fasting lipid panels and appropriate treatment, is of paramount importance in patients with diabetes.7,8
Fasting Lipid Panels
The AACE recommends administering at least annual fasting lipid panels in all adults with diabetes, and LDL goal levels should be based on the cardiovascular risk of the patient.7 For patients with
- established ASCVD, the LDL goal is < 55 mg/dL
- risk factors for ASCVD (eg, hypertension, tobacco use, family history of ASCVD) in addition to diabetes, the LDL goal is < 70 mg/dL
- no risk factors, the LDL goal is < 100 mg/dL.7
Statin Therapy
Research indicates that statins reduce the risk for cardiovascular events and are recommended as first-line treatment for dyslipidemia.2,7 Statin therapy is recommended for patients with LDL levels above goal without contraindications.10 Higher-dose statins have been shown to help improve cardiovascular outcomes, and most—if not all—guidelines recommend up-titration of these medications as tolerated by the patient. 7,8,29 After initiation of statin therapy, clinicians should continue to monitor lipid levels every 4 to 12 weeks after a change in lipid therapy and then schedule monitoring annually.2
Unfortunately, a recent large-scale retrospective study of the medical records of 125,464 patients with type 2 diabetes showed that although 99% of the patients were at high risk for or already had ASCVD, only 63% were receiving the recommended statin therapy.30 Therefore, all patients with diabetes at risk for ASCVD require evaluation to determine the need for statins.
Additional treatments. If the patient’s levels remain above goal, strong consideration should be given to additional therapies. Ezetimibe has been shown to have some benefit in reducing LDL levels and cardiovascular risk.31 PCSK9 inhibitors are a newer treatment for cardiovascular disease and are particularly beneficial for patients with known ASCVD. The FOURIER and ODYSSEY trials demonstrated that PCSK9 inhibitors had relative risk reductions of 48% to 53% for major ASCVD events and showed that these medications help reduce LDL levels and, most importantly, cardiovascular risk.32,33
Continue to: Recommendations for other lipid components
Recommendations for other lipid components—non–HDL-C, apolipoprotein B, or LDL-P—are very specific and consideration may be given for referral to an endocrinologist or lipidologist for evaluation and treatment.7,8 Evidence on reducing cardiovascular risk with therapies for decreasing triglyceride levels is limited. Recently though, icosapent ethyl received FDA approval as an adjunct to maximally tolerated statin therapy to reduce the risk for cardiovascular events in patients with elevated triglyceride levels (≥ 150 mg/dL).34,35 ADA guidelines recommend icosapent ethyl for patients with diabetes, 1 additional cardiovascular risk factor, and triglyceride levels between 135 and 499 mg/dL.2
In Part 4, I’ll explore how clinicians can best monitor for chronic kidney disease in patients with diabetes. We’ll also discuss the medications used for improving kidney health in these patients.
Previously, we explored blood pressure control in a patient with diabetes. Now, we’ll discuss the value of a fasting lipid panel and treatment for dyslipidemia in this population.
CASE CONTINUED
Mr. W completed a fasting lipid panel, which revealed the following: triglycerides, 145 mg/dL; high-density lipoprotein (HDL) level, 32 mg/dL; and low-density lipoprotein (LDL) level, 108 mg/dL. He is currently receiving low-dose statin therapy. Based on these results, Mr. W fits the criteria for dyslipidemia.
Dyslipidemia
Dyslipidemia marked by elevated LDL levels—as observed in Mr. W—is a well-known contributing factor to development of cardiovascular disease in patients with diabetes. Elevated triglycerides and low HDL levels also are often noted in these patients. Patients with diabetes are particularly vulnerable to atherosclerosis due to a combination of pro-inflammatory factors and hyperglycemic effects. Both the ADA and the AACE agree that lipid management, including fasting lipid panels and appropriate treatment, is of paramount importance in patients with diabetes.7,8
Fasting Lipid Panels
The AACE recommends administering at least annual fasting lipid panels in all adults with diabetes, and LDL goal levels should be based on the cardiovascular risk of the patient.7 For patients with
- established ASCVD, the LDL goal is < 55 mg/dL
- risk factors for ASCVD (eg, hypertension, tobacco use, family history of ASCVD) in addition to diabetes, the LDL goal is < 70 mg/dL
- no risk factors, the LDL goal is < 100 mg/dL.7
Statin Therapy
Research indicates that statins reduce the risk for cardiovascular events and are recommended as first-line treatment for dyslipidemia.2,7 Statin therapy is recommended for patients with LDL levels above goal without contraindications.10 Higher-dose statins have been shown to help improve cardiovascular outcomes, and most—if not all—guidelines recommend up-titration of these medications as tolerated by the patient. 7,8,29 After initiation of statin therapy, clinicians should continue to monitor lipid levels every 4 to 12 weeks after a change in lipid therapy and then schedule monitoring annually.2
Unfortunately, a recent large-scale retrospective study of the medical records of 125,464 patients with type 2 diabetes showed that although 99% of the patients were at high risk for or already had ASCVD, only 63% were receiving the recommended statin therapy.30 Therefore, all patients with diabetes at risk for ASCVD require evaluation to determine the need for statins.
Additional treatments. If the patient’s levels remain above goal, strong consideration should be given to additional therapies. Ezetimibe has been shown to have some benefit in reducing LDL levels and cardiovascular risk.31 PCSK9 inhibitors are a newer treatment for cardiovascular disease and are particularly beneficial for patients with known ASCVD. The FOURIER and ODYSSEY trials demonstrated that PCSK9 inhibitors had relative risk reductions of 48% to 53% for major ASCVD events and showed that these medications help reduce LDL levels and, most importantly, cardiovascular risk.32,33
Continue to: Recommendations for other lipid components
Recommendations for other lipid components—non–HDL-C, apolipoprotein B, or LDL-P—are very specific and consideration may be given for referral to an endocrinologist or lipidologist for evaluation and treatment.7,8 Evidence on reducing cardiovascular risk with therapies for decreasing triglyceride levels is limited. Recently though, icosapent ethyl received FDA approval as an adjunct to maximally tolerated statin therapy to reduce the risk for cardiovascular events in patients with elevated triglyceride levels (≥ 150 mg/dL).34,35 ADA guidelines recommend icosapent ethyl for patients with diabetes, 1 additional cardiovascular risk factor, and triglyceride levels between 135 and 499 mg/dL.2
In Part 4, I’ll explore how clinicians can best monitor for chronic kidney disease in patients with diabetes. We’ll also discuss the medications used for improving kidney health in these patients.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Six snags docs hit when seeing patients again
Sachin Dave, MD, an internist in Greenwood, Ind., never thought he’d tell his patients to avoid coming into the office. But these days, he must balance the need for face-to-face visits with the risk for COVID-19 transmission. Although he connects with most patients by telehealth, some patients still demand in-office care.
“My older patients actually insist on coming to see me in person,” said Dr. Dave, who is part of Indiana Internal Medicine Consultants, a large group practice near Indianapolis. “I have to tell them it’s not safe.”
It’s a minor hitch as his practice ramps up again – but one of those things you can’t overlook, he said. “We need to educate our patients and communicate the risk to them.”
senior vice president of patient safety and risk management for the Doctors Company, a physician-owned malpractice insurer. “It’s about minimizing risk.”
As practices increase patient volume, physicians are juggling a desire for a return to patient care and increased revenue with a need to maximize patient and staff safety. Avoiding some of these common snags may help make the transition smoother.
1. Unclear or nonexistent polices and protocols
Some physicians know what general rules they want to follow, but they haven’t conveyed them in a readily available document. Although you and your staff may have a sense of what they are, patients may be less aware of how mandatory you consider them. It’s important to develop a formal framework that you will follow and to make sure patients and staff know it.
Dr. Dave and colleagues have stringent safety protocols in place for the small percentage of patients he does feel a need to be seen in person. Masks are mandatory for staff and patients. The waiting room is set up for social distancing. If it begins getting crowded, patients are asked to wait in their cars until an exam room is ready.
“I’m not going to see a patient who refuses to put a mask on, because when I put a mask on, I’m trying to protect my patients,” said Dr. Dave. He makes it clear that he expects the same from his patients; they must wear a mask to protect his staff and himself.
“I am going to let them in with the caveat that they don’t have qualms about wearing a mask. If they have qualms about wearing a mask, then I have qualms about seeing them in person,” he said.
Be sure that all patients understand and will adhere to your protocols before they come to the office. Patients should be triaged over the phone before arriving, according to Centers for Disease Control and Prevention recommendations. (Remember that refusing assessment or care could lead to issues of patient abandonment.)
When you don’t really have a framework to follow, you don’t really know what the structure is going to be and how your practice is going to provide care. The question is, how do you build a framework for right now? said Ron Holder, chief operations officer of the Medical Group Management Association. “The first step is do no harm.”
2. Trying to see too many patients too soon
On average, practices have reported a 55% decrease in revenue and a 60% decrease in patient volume since the beginning of the COVID-19 crisis, according to the MGMA. It’s natural that many want to ramp up immediately and go back to their prior patient volume. But they need to take it slow and ensure that the correct safety protocols are in place, Mr. Holder said.
For example, telehealth is still reimbursable at parity, so physicians should keep taking advantage of that. MGMA’s practice reopening checklist has links to additional resources and considerations.
Some doctors want to see an overload of patients and want to get back to how they practiced before the pandemic, says orthopedic surgeon Charles Ruotolo, MD, president of Total Orthopedics and Sports Medicine in Massapequa, N.Y., and chairman of the department of orthopedics, Nassau University Medical Center, East Meadow, N.Y., “but at the same time, you know we still have to limit how many people are coming into the office.”
It’s not fair if some doctors in your practice are seeing 45 patients daily as they did previously whereas others are seeing half that many, he explained. “We must remain cognizant and constantly review schedules and remember we have to still keep the numbers down.”
“COVID is not going to be completely over in our lifetime,” says Evan Levine, MD, a cardiologist in Ridgefield, Conn. Taking advantage of technologies is one way to reduce risk.
He predicts that the demand will continue to increase as patients become more comfortable with virtual visits. Using Bluetooth and WiFi devices to assess patients is no longer futuristic and can help reduce the number of people in the waiting room, according to Dr. Levine, a solo practitioner and author of “What Your Doctor Won’t (or Can’t) Tell You.” “That’s a very good thing, especially as we look to fall and to flu season.”
3. Undercommunicating with patients and staff
Don’t assume patients know that you’ve opened back up and are seeing people in the office, Mr. Holder said. Update your practice website, send letters or newsletters to patients’ homes, maintain telephone and email contact, and post signs at the facility explaining your reopening process. The CDC has an excellent phone script that practices can adapt. Everyone should know what to expect and what’s expected of them.
He advised overcommunicating – more than you think is necessary – to your staff and patients. Tell them about the extra steps you’re taking. Let them know that their safety and health are the most important thing and that you are taking all these extra measures to make sure that they feel comfortable.
Keep staff appraised of policy changes. Stress what you’re doing to ensure the safety of your team members. “Even though you could be doing all those things, if you’re not communicating, then no one knows it,” said Mr. Holder.
He predicted the practices that emerge stronger from this crisis will be those with great patient education that have built up a lot of goodwill. Patients should know they can go to this practice’s patient portal as a trusted resource about COVID-19 and safety-related measures. This approach will pay dividends over the long term.
4. Giving inadequate staff training and holding too-high expectations
Staff members are scared, really scared, Ms. Bashaw said. Some may not return because they’re unsure what to expect; others may have to stay home to care for children or older relatives. Clear guidance on what is being done to ensure everyone’s safety, what is expected from staff, and flexibility with scheduling can help address these issues.
Most practices’ staff are not used to donning and removing personal protective equipment, and they’re not used to wearing masks when working with patients. Expect some mistakes.
“We had a scenario where a provider was in a room with an older patient, and the provider pulled his mask down so the patient could hear him better. He then kept the mask down while giving the patient an injection. When the family found out, they were very upset,” Ms. Bashaw related. “It was done with good intentions, to improve communication, but it’s a slip-up that could have found him liable if she became ill.”
Dr. Ruotolo had to implement new policies throughout his practice’s multiple locations in the New York metro area. They encompassed everything from staggering appointments and staff to establishing designated employee eating areas so front desk staff weren’t taking their masks off to snack.
Having specific guidelines for staff helps reassure patients that safety protocols are being adhered to. “Patients want to see we’re all doing the right thing,” he said.
Have those policies clearly written so everyone’s on the same page, Dr. Ruotolo advised. Also make sure staff knows what the rules are for patients.
Dr. Ruotolo’s reception staff hand every patient a disinfectant wipe when they arrive. They are asked to wipe down the check-in kiosk before and after using it. Assistants know not to cut corners when disinfecting exam rooms, equipment, or tables. “It’s the little things you have to think about, and make sure it’s reiterated with your staff so they’re doing it.”
If your practice isn’t back up to full staffing volume, it’s a good idea to cross train staff members so some jobs overlap, suggests Mr. Holder. Although smaller practices may already do this, at larger practices, staff members’ roles may be more specific. “You may be able to pull employees from other positions in the practice, but it’s a good idea to have some redundancy.”
5. Neglecting to document everything – even more so than before
The standard of care is changing every day, and so are the regulations, says Ms. Bashaw. Many physicians who work in larger practices or for health systems don’t take advantage of internal risk management departments, which can help them keep tabs on all of these changes.
Writing down simple protocols and having a consistent work flow are extremely important right now. What have you told staff and patients? Are they comfortable with how you’re minimizing their risk? Physicians can find a seven-page checklist that helps practitioners organize and methodically go through reopening process at the Doctors Company website.
Implementing state and local statutes or public health requirements and keeping track of when things stop and start can be complex, says Ms. Bashaw. Take a look at your pre–COVID-19 policies and procedures, and make sure you’re on top of the current standards for your office, including staff education. The most important step is connecting with your local public health authority and taking direction from them.
Ms. Bashaw strongly encouraged physicians to conduct huddles with their staff; it’s an evidence-based leadership practice that’s important from a medical malpractice perspective. Review the day’s game plan, then conduct a debriefing at the end of the day.
Discuss what worked well, what didn’t, and what tomorrow looks like. And be sure to document it all. “A standard routine and debrief gets everyone on the same page and shows due diligence,” she said.
Keep an administrative file so 2 years down the road, you remember what you did and when. That way, if there’s a problem or a breach or the standard isn’t adhered to, it’s documented in the file. Note what happened and when and what was done to mitigate it or what corrective action was taken.
All practices need to stay on top of regulatory changes. Smaller practices don’t have full-time staff dedicated to monitoring what’s happening in Washington. Associations such as the MGMA can help target what’s important and actionable.
6. Forgetting about your own and your staff’s physical and mental health
Physicians need to be worried about burnout and mental health problems from their team members, their colleagues, their patients, and themselves, according to Mr. Holder.
“There’s a mental exhaustion that is just pervasive in the world and the United States right now about all this COVID stuff and stress, not to mention all the other things that are going on,” he said.
That’s going to carry over, so physicians must make sure there’s a positive culture at the practice, where everyone’s taking care of and watching out for each other.
A version of this article originally appeared on Medscape.com.
Sachin Dave, MD, an internist in Greenwood, Ind., never thought he’d tell his patients to avoid coming into the office. But these days, he must balance the need for face-to-face visits with the risk for COVID-19 transmission. Although he connects with most patients by telehealth, some patients still demand in-office care.
“My older patients actually insist on coming to see me in person,” said Dr. Dave, who is part of Indiana Internal Medicine Consultants, a large group practice near Indianapolis. “I have to tell them it’s not safe.”
It’s a minor hitch as his practice ramps up again – but one of those things you can’t overlook, he said. “We need to educate our patients and communicate the risk to them.”
senior vice president of patient safety and risk management for the Doctors Company, a physician-owned malpractice insurer. “It’s about minimizing risk.”
As practices increase patient volume, physicians are juggling a desire for a return to patient care and increased revenue with a need to maximize patient and staff safety. Avoiding some of these common snags may help make the transition smoother.
1. Unclear or nonexistent polices and protocols
Some physicians know what general rules they want to follow, but they haven’t conveyed them in a readily available document. Although you and your staff may have a sense of what they are, patients may be less aware of how mandatory you consider them. It’s important to develop a formal framework that you will follow and to make sure patients and staff know it.
Dr. Dave and colleagues have stringent safety protocols in place for the small percentage of patients he does feel a need to be seen in person. Masks are mandatory for staff and patients. The waiting room is set up for social distancing. If it begins getting crowded, patients are asked to wait in their cars until an exam room is ready.
“I’m not going to see a patient who refuses to put a mask on, because when I put a mask on, I’m trying to protect my patients,” said Dr. Dave. He makes it clear that he expects the same from his patients; they must wear a mask to protect his staff and himself.
“I am going to let them in with the caveat that they don’t have qualms about wearing a mask. If they have qualms about wearing a mask, then I have qualms about seeing them in person,” he said.
Be sure that all patients understand and will adhere to your protocols before they come to the office. Patients should be triaged over the phone before arriving, according to Centers for Disease Control and Prevention recommendations. (Remember that refusing assessment or care could lead to issues of patient abandonment.)
When you don’t really have a framework to follow, you don’t really know what the structure is going to be and how your practice is going to provide care. The question is, how do you build a framework for right now? said Ron Holder, chief operations officer of the Medical Group Management Association. “The first step is do no harm.”
2. Trying to see too many patients too soon
On average, practices have reported a 55% decrease in revenue and a 60% decrease in patient volume since the beginning of the COVID-19 crisis, according to the MGMA. It’s natural that many want to ramp up immediately and go back to their prior patient volume. But they need to take it slow and ensure that the correct safety protocols are in place, Mr. Holder said.
For example, telehealth is still reimbursable at parity, so physicians should keep taking advantage of that. MGMA’s practice reopening checklist has links to additional resources and considerations.
Some doctors want to see an overload of patients and want to get back to how they practiced before the pandemic, says orthopedic surgeon Charles Ruotolo, MD, president of Total Orthopedics and Sports Medicine in Massapequa, N.Y., and chairman of the department of orthopedics, Nassau University Medical Center, East Meadow, N.Y., “but at the same time, you know we still have to limit how many people are coming into the office.”
It’s not fair if some doctors in your practice are seeing 45 patients daily as they did previously whereas others are seeing half that many, he explained. “We must remain cognizant and constantly review schedules and remember we have to still keep the numbers down.”
“COVID is not going to be completely over in our lifetime,” says Evan Levine, MD, a cardiologist in Ridgefield, Conn. Taking advantage of technologies is one way to reduce risk.
He predicts that the demand will continue to increase as patients become more comfortable with virtual visits. Using Bluetooth and WiFi devices to assess patients is no longer futuristic and can help reduce the number of people in the waiting room, according to Dr. Levine, a solo practitioner and author of “What Your Doctor Won’t (or Can’t) Tell You.” “That’s a very good thing, especially as we look to fall and to flu season.”
3. Undercommunicating with patients and staff
Don’t assume patients know that you’ve opened back up and are seeing people in the office, Mr. Holder said. Update your practice website, send letters or newsletters to patients’ homes, maintain telephone and email contact, and post signs at the facility explaining your reopening process. The CDC has an excellent phone script that practices can adapt. Everyone should know what to expect and what’s expected of them.
He advised overcommunicating – more than you think is necessary – to your staff and patients. Tell them about the extra steps you’re taking. Let them know that their safety and health are the most important thing and that you are taking all these extra measures to make sure that they feel comfortable.
Keep staff appraised of policy changes. Stress what you’re doing to ensure the safety of your team members. “Even though you could be doing all those things, if you’re not communicating, then no one knows it,” said Mr. Holder.
He predicted the practices that emerge stronger from this crisis will be those with great patient education that have built up a lot of goodwill. Patients should know they can go to this practice’s patient portal as a trusted resource about COVID-19 and safety-related measures. This approach will pay dividends over the long term.
4. Giving inadequate staff training and holding too-high expectations
Staff members are scared, really scared, Ms. Bashaw said. Some may not return because they’re unsure what to expect; others may have to stay home to care for children or older relatives. Clear guidance on what is being done to ensure everyone’s safety, what is expected from staff, and flexibility with scheduling can help address these issues.
Most practices’ staff are not used to donning and removing personal protective equipment, and they’re not used to wearing masks when working with patients. Expect some mistakes.
“We had a scenario where a provider was in a room with an older patient, and the provider pulled his mask down so the patient could hear him better. He then kept the mask down while giving the patient an injection. When the family found out, they were very upset,” Ms. Bashaw related. “It was done with good intentions, to improve communication, but it’s a slip-up that could have found him liable if she became ill.”
Dr. Ruotolo had to implement new policies throughout his practice’s multiple locations in the New York metro area. They encompassed everything from staggering appointments and staff to establishing designated employee eating areas so front desk staff weren’t taking their masks off to snack.
Having specific guidelines for staff helps reassure patients that safety protocols are being adhered to. “Patients want to see we’re all doing the right thing,” he said.
Have those policies clearly written so everyone’s on the same page, Dr. Ruotolo advised. Also make sure staff knows what the rules are for patients.
Dr. Ruotolo’s reception staff hand every patient a disinfectant wipe when they arrive. They are asked to wipe down the check-in kiosk before and after using it. Assistants know not to cut corners when disinfecting exam rooms, equipment, or tables. “It’s the little things you have to think about, and make sure it’s reiterated with your staff so they’re doing it.”
If your practice isn’t back up to full staffing volume, it’s a good idea to cross train staff members so some jobs overlap, suggests Mr. Holder. Although smaller practices may already do this, at larger practices, staff members’ roles may be more specific. “You may be able to pull employees from other positions in the practice, but it’s a good idea to have some redundancy.”
5. Neglecting to document everything – even more so than before
The standard of care is changing every day, and so are the regulations, says Ms. Bashaw. Many physicians who work in larger practices or for health systems don’t take advantage of internal risk management departments, which can help them keep tabs on all of these changes.
Writing down simple protocols and having a consistent work flow are extremely important right now. What have you told staff and patients? Are they comfortable with how you’re minimizing their risk? Physicians can find a seven-page checklist that helps practitioners organize and methodically go through reopening process at the Doctors Company website.
Implementing state and local statutes or public health requirements and keeping track of when things stop and start can be complex, says Ms. Bashaw. Take a look at your pre–COVID-19 policies and procedures, and make sure you’re on top of the current standards for your office, including staff education. The most important step is connecting with your local public health authority and taking direction from them.
Ms. Bashaw strongly encouraged physicians to conduct huddles with their staff; it’s an evidence-based leadership practice that’s important from a medical malpractice perspective. Review the day’s game plan, then conduct a debriefing at the end of the day.
Discuss what worked well, what didn’t, and what tomorrow looks like. And be sure to document it all. “A standard routine and debrief gets everyone on the same page and shows due diligence,” she said.
Keep an administrative file so 2 years down the road, you remember what you did and when. That way, if there’s a problem or a breach or the standard isn’t adhered to, it’s documented in the file. Note what happened and when and what was done to mitigate it or what corrective action was taken.
All practices need to stay on top of regulatory changes. Smaller practices don’t have full-time staff dedicated to monitoring what’s happening in Washington. Associations such as the MGMA can help target what’s important and actionable.
6. Forgetting about your own and your staff’s physical and mental health
Physicians need to be worried about burnout and mental health problems from their team members, their colleagues, their patients, and themselves, according to Mr. Holder.
“There’s a mental exhaustion that is just pervasive in the world and the United States right now about all this COVID stuff and stress, not to mention all the other things that are going on,” he said.
That’s going to carry over, so physicians must make sure there’s a positive culture at the practice, where everyone’s taking care of and watching out for each other.
A version of this article originally appeared on Medscape.com.
Sachin Dave, MD, an internist in Greenwood, Ind., never thought he’d tell his patients to avoid coming into the office. But these days, he must balance the need for face-to-face visits with the risk for COVID-19 transmission. Although he connects with most patients by telehealth, some patients still demand in-office care.
“My older patients actually insist on coming to see me in person,” said Dr. Dave, who is part of Indiana Internal Medicine Consultants, a large group practice near Indianapolis. “I have to tell them it’s not safe.”
It’s a minor hitch as his practice ramps up again – but one of those things you can’t overlook, he said. “We need to educate our patients and communicate the risk to them.”
senior vice president of patient safety and risk management for the Doctors Company, a physician-owned malpractice insurer. “It’s about minimizing risk.”
As practices increase patient volume, physicians are juggling a desire for a return to patient care and increased revenue with a need to maximize patient and staff safety. Avoiding some of these common snags may help make the transition smoother.
1. Unclear or nonexistent polices and protocols
Some physicians know what general rules they want to follow, but they haven’t conveyed them in a readily available document. Although you and your staff may have a sense of what they are, patients may be less aware of how mandatory you consider them. It’s important to develop a formal framework that you will follow and to make sure patients and staff know it.
Dr. Dave and colleagues have stringent safety protocols in place for the small percentage of patients he does feel a need to be seen in person. Masks are mandatory for staff and patients. The waiting room is set up for social distancing. If it begins getting crowded, patients are asked to wait in their cars until an exam room is ready.
“I’m not going to see a patient who refuses to put a mask on, because when I put a mask on, I’m trying to protect my patients,” said Dr. Dave. He makes it clear that he expects the same from his patients; they must wear a mask to protect his staff and himself.
“I am going to let them in with the caveat that they don’t have qualms about wearing a mask. If they have qualms about wearing a mask, then I have qualms about seeing them in person,” he said.
Be sure that all patients understand and will adhere to your protocols before they come to the office. Patients should be triaged over the phone before arriving, according to Centers for Disease Control and Prevention recommendations. (Remember that refusing assessment or care could lead to issues of patient abandonment.)
When you don’t really have a framework to follow, you don’t really know what the structure is going to be and how your practice is going to provide care. The question is, how do you build a framework for right now? said Ron Holder, chief operations officer of the Medical Group Management Association. “The first step is do no harm.”
2. Trying to see too many patients too soon
On average, practices have reported a 55% decrease in revenue and a 60% decrease in patient volume since the beginning of the COVID-19 crisis, according to the MGMA. It’s natural that many want to ramp up immediately and go back to their prior patient volume. But they need to take it slow and ensure that the correct safety protocols are in place, Mr. Holder said.
For example, telehealth is still reimbursable at parity, so physicians should keep taking advantage of that. MGMA’s practice reopening checklist has links to additional resources and considerations.
Some doctors want to see an overload of patients and want to get back to how they practiced before the pandemic, says orthopedic surgeon Charles Ruotolo, MD, president of Total Orthopedics and Sports Medicine in Massapequa, N.Y., and chairman of the department of orthopedics, Nassau University Medical Center, East Meadow, N.Y., “but at the same time, you know we still have to limit how many people are coming into the office.”
It’s not fair if some doctors in your practice are seeing 45 patients daily as they did previously whereas others are seeing half that many, he explained. “We must remain cognizant and constantly review schedules and remember we have to still keep the numbers down.”
“COVID is not going to be completely over in our lifetime,” says Evan Levine, MD, a cardiologist in Ridgefield, Conn. Taking advantage of technologies is one way to reduce risk.
He predicts that the demand will continue to increase as patients become more comfortable with virtual visits. Using Bluetooth and WiFi devices to assess patients is no longer futuristic and can help reduce the number of people in the waiting room, according to Dr. Levine, a solo practitioner and author of “What Your Doctor Won’t (or Can’t) Tell You.” “That’s a very good thing, especially as we look to fall and to flu season.”
3. Undercommunicating with patients and staff
Don’t assume patients know that you’ve opened back up and are seeing people in the office, Mr. Holder said. Update your practice website, send letters or newsletters to patients’ homes, maintain telephone and email contact, and post signs at the facility explaining your reopening process. The CDC has an excellent phone script that practices can adapt. Everyone should know what to expect and what’s expected of them.
He advised overcommunicating – more than you think is necessary – to your staff and patients. Tell them about the extra steps you’re taking. Let them know that their safety and health are the most important thing and that you are taking all these extra measures to make sure that they feel comfortable.
Keep staff appraised of policy changes. Stress what you’re doing to ensure the safety of your team members. “Even though you could be doing all those things, if you’re not communicating, then no one knows it,” said Mr. Holder.
He predicted the practices that emerge stronger from this crisis will be those with great patient education that have built up a lot of goodwill. Patients should know they can go to this practice’s patient portal as a trusted resource about COVID-19 and safety-related measures. This approach will pay dividends over the long term.
4. Giving inadequate staff training and holding too-high expectations
Staff members are scared, really scared, Ms. Bashaw said. Some may not return because they’re unsure what to expect; others may have to stay home to care for children or older relatives. Clear guidance on what is being done to ensure everyone’s safety, what is expected from staff, and flexibility with scheduling can help address these issues.
Most practices’ staff are not used to donning and removing personal protective equipment, and they’re not used to wearing masks when working with patients. Expect some mistakes.
“We had a scenario where a provider was in a room with an older patient, and the provider pulled his mask down so the patient could hear him better. He then kept the mask down while giving the patient an injection. When the family found out, they were very upset,” Ms. Bashaw related. “It was done with good intentions, to improve communication, but it’s a slip-up that could have found him liable if she became ill.”
Dr. Ruotolo had to implement new policies throughout his practice’s multiple locations in the New York metro area. They encompassed everything from staggering appointments and staff to establishing designated employee eating areas so front desk staff weren’t taking their masks off to snack.
Having specific guidelines for staff helps reassure patients that safety protocols are being adhered to. “Patients want to see we’re all doing the right thing,” he said.
Have those policies clearly written so everyone’s on the same page, Dr. Ruotolo advised. Also make sure staff knows what the rules are for patients.
Dr. Ruotolo’s reception staff hand every patient a disinfectant wipe when they arrive. They are asked to wipe down the check-in kiosk before and after using it. Assistants know not to cut corners when disinfecting exam rooms, equipment, or tables. “It’s the little things you have to think about, and make sure it’s reiterated with your staff so they’re doing it.”
If your practice isn’t back up to full staffing volume, it’s a good idea to cross train staff members so some jobs overlap, suggests Mr. Holder. Although smaller practices may already do this, at larger practices, staff members’ roles may be more specific. “You may be able to pull employees from other positions in the practice, but it’s a good idea to have some redundancy.”
5. Neglecting to document everything – even more so than before
The standard of care is changing every day, and so are the regulations, says Ms. Bashaw. Many physicians who work in larger practices or for health systems don’t take advantage of internal risk management departments, which can help them keep tabs on all of these changes.
Writing down simple protocols and having a consistent work flow are extremely important right now. What have you told staff and patients? Are they comfortable with how you’re minimizing their risk? Physicians can find a seven-page checklist that helps practitioners organize and methodically go through reopening process at the Doctors Company website.
Implementing state and local statutes or public health requirements and keeping track of when things stop and start can be complex, says Ms. Bashaw. Take a look at your pre–COVID-19 policies and procedures, and make sure you’re on top of the current standards for your office, including staff education. The most important step is connecting with your local public health authority and taking direction from them.
Ms. Bashaw strongly encouraged physicians to conduct huddles with their staff; it’s an evidence-based leadership practice that’s important from a medical malpractice perspective. Review the day’s game plan, then conduct a debriefing at the end of the day.
Discuss what worked well, what didn’t, and what tomorrow looks like. And be sure to document it all. “A standard routine and debrief gets everyone on the same page and shows due diligence,” she said.
Keep an administrative file so 2 years down the road, you remember what you did and when. That way, if there’s a problem or a breach or the standard isn’t adhered to, it’s documented in the file. Note what happened and when and what was done to mitigate it or what corrective action was taken.
All practices need to stay on top of regulatory changes. Smaller practices don’t have full-time staff dedicated to monitoring what’s happening in Washington. Associations such as the MGMA can help target what’s important and actionable.
6. Forgetting about your own and your staff’s physical and mental health
Physicians need to be worried about burnout and mental health problems from their team members, their colleagues, their patients, and themselves, according to Mr. Holder.
“There’s a mental exhaustion that is just pervasive in the world and the United States right now about all this COVID stuff and stress, not to mention all the other things that are going on,” he said.
That’s going to carry over, so physicians must make sure there’s a positive culture at the practice, where everyone’s taking care of and watching out for each other.
A version of this article originally appeared on Medscape.com.
New insight into neurobehavioral effects of legalized cannabis
Researchers have published one of the first studies to characterize the association between the consumption of legal cannabis and subsequent pharmacologic and neurobehavioral outcomes, with somewhat surprising results.
The study showed that, although cannabis consumption did not affect most short-term neurobehavioral measures, it delayed recall memory and impaired balance.
The investigation also showed that users of much more potent cannabis concentrates actually demonstrated similar or lower levels of subjective drug intoxication and short-term impairment than did their counterparts who used lower-potency forms of the cannabis flower.
“It does not appear that the potency being used matters that much,” senior investigator Kent E. Hutchison, PhD, said in an interview. “People seem to be titrating to a certain level of intoxication or a certain level of feeling high. And for some people that requires a lot of drug, and for other people not as much.”
added Dr. Hutchison, a professor of psychology and neuroscience at the University of Colorado Boulder.
The study was published online June 10 in JAMA Psychiatry.
Widespread availability, little research
Recreational cannabis is now legal in 11 states and the District of Columbia, while medical cannabis is legal in 33. However, despite growing popularity of cannabis, there is little research on its potential health and biobehavioral risks, largely because of federal restrictions on cannabis research.
Cannabis users typically consume various forms of the cannabis flower, which can boast concentrations of the psychoactive cannabinoid delta-9-tetrahydrocannabinol (THC) of up to 30%. However, use of concentrated forms of cannabis – which are made by extracting plant cannabinoids into a different form – is increasing.
Such formulations can boast THC concentrations as high as 90%. Nevertheless, data regarding the relative risks of these higher-strength products are limited.
Previous research has shown a variety of negative short-term and long-term neurobehavioral effects associated with cannabis use, including harmful cognitive and motor effects. Extended exposure to THC may also negatively affect brain regions that are associated with the control of coordinated movement, and create brain-activation deficits in motor control regions that persist well beyond the effects of short-term intoxication.
Despite such findings, Dr. Hutchison said the existing literature on the subject does not yield a real-world view of current cannabis use because it tends to focus on low-THC products that are increasingly less common in today’s legal market.
Given such shortcomings, the investigators wanted to address persistent questions surrounding the neurobehavioral effects of legal cannabis flower products (16% or 24% THC) and cannabis concentrate products (70% or 90% THC). In doing so, they examined three primary topics:
- The association between short-term use of these products and THC plasma levels; subjective intoxication; and mood, cognitive performance, and balance
- Differences in such associations between users of cannabis flower and concentrate products
- Potential variations in these associations by THC potency
High- versus low-potency varieties
The study included 133 individuals (aged 21-70 years), who were designated as either cannabis flower users or cannabis concentrate users. Participants had all used cannabis at least four times in the previous month with no adverse reaction and were not receiving treatment for a psychotic disorder or bipolar disorder.
Participants were randomly assigned to consume either higher-potency or lower-potency products that had been purchased from a local dispensary. Flower users were randomized to purchase 3 g of either a 16% THC or 24% THC product, while concentrate users were randomized to purchase 1g of either 70% THC or 90% THC.
Participants completed a series of four assessments, one at baseline and three others at a mobile laboratory. The mobile laboratory assessments occurred before, immediately after, and one hour after participants had all consumed their cannabis ad libitum.
Of the original cohort of 133 participants, 55 flower cannabis users (mean age, 28.8 years; 46% women) and 66 concentrate cannabis users (mean age, 28.3 years; 45% women) complied with the study’s instructions and had complete data.
The study’s primary outcome measures included plasma cannabinoids, subjective drug intoxication and mood, and neurobehavioral outcomes such as attention, memory, inhibitory control, and balance.
Mixed results
With respect to cannabis concentrations, results showed that users of concentrate exhibited higher levels of both plasma THC and the active metabolite of THC (11-hydroxy-delta9-THC) across all points than did their counterparts who used cannabis flower products.
Specifically, mean plasma THC levels were 1,016 ± 1,380 mcg/ml in concentrate users and 455±503 mcg/mL in flower users after ad libitum cannabis consumption. Nevertheless, self-reported levels of intoxication were no different between users of cannabis flower or concentrate products.
Although results also showed that most neurobehavioral measures were not altered by short-term cannabis consumption, there were some notable exceptions. There was a negative linear effect with delayed verbal recall errors, suggesting poorer performance after cannabis use (F1, 203 = 32.31; P < .001).
On the other hand, investigators found a positive linear effect with inhibitory control and working memory, which actually suggests better performance after cannabis use. This finding, the researchers note, may be the result of a practice effect. Cannabis flower users performed better across all inhibitory control assessments.
The researchers also tested participants’ balance with their eyes open and closed. In the eyes-open condition, they found a trend toward impaired balance after cannabis use, though this normalized within an hour. When subjects closed their eyes, however, researchers observed a significant short-term increase in sway after cannabis use, which fell back to pre-use levels one hour after use (F1, 203 = 18.88; P < .001).
Of note, outcomes did not differ between groups according to the type of cannabis product consumed or its relative potency.
The study yielded several surprising findings, beginning with self-reported intoxication levels, which were not statistically significant between different cannabis flower and concentrate users, despite significantly different plasma THC levels between the two groups.
Dr. Hutchison explained that this may be the result of greater THC tolerance among concentrate users, THC saturation of cannabinoid receptors, or interindividual differences among users with respect to cannabis metabolism or sensitivity.
“I thought for sure that high-potency users would be much more compromised,” he said. “I guess it just goes to show we have a lot to learn about how these things work.”
Additionally, there were virtually no significant changes in acute performance after cannabis use, with the exception of delayed verbal recall. In fact, the most marked change observed in the study was the effect of cannabis on balance immediately after drug use, though these changes seemed to abate within an hour.
Nevertheless, the study highlights several potential public health implications of cannabis consumption, Dr. Hutchison added. “What happens when people with high blood concentrations decide to quit?” he asked. “Do they have trouble quitting? Do they have withdrawal symptoms?”
The long-term effects of cannabis use is another important question that still needs to be answered, he added.
Finally, Dr. Hutchison noted that, although the study showed little difference between users of cannabis flower and concentrates, study participants were all experienced users.
“There is certainly the potential for harm when a naive person uses cannabis concentrate,” he said. “Suddenly they have way more THC than they thought they were going to get, and that’s where a lot of people get into trouble with cannabis.”
Pitfalls and hurdles
In an accompanying editorial, Margaret Haney, PhD, of Columbia University Irving Medical Center, New York, explained that cannabis’ awkward position as simultaneously legal and illegal, medical and recreational, has hampered researchers’ ability to study its effects as comprehensively as they would otherwise like.
“With a federally illegal drug legalized in individual states, scientists constrained, and federal agencies somewhat silent, clinicians have none of the data that guide their decisions for other medications (eg, which indication, product, cannabinoid ratio, dose, or route of administration; what risks for individual patients [eg, pregnant, adolescent, psychiatric?]),” Dr. Haney wrote.
These pitfalls are compounded by the significant regulatory hurdles.
“The FDA is appropriately cautious about what it allows scientists to test in patients, and none of the products available in dispensaries or online have undergone the safety and manufacturing procedures needed for FDA approval,” she continued. “How then to conduct the studies so needed?”
Yet as Haney noted, giving cannabinoid researchers a Schedule I exemption may help address many of the barriers facing these scientists. Such a move, she said, would increase the number of randomized controlled trials being performed, “and thereby begin to breach the divide between the use of these products and empirical evidence.”
Dr. Hutchison has disclosed no relevant financial relationships. Dr. Haney disclosed funding from the US National Institute on Drug Abuse and from the Thompson Family Foundation Initiative. The study was funded by the NIH and Colorado Department of Public Health and Environment.
A version of this article originally appeared on Medscape.com.
Researchers have published one of the first studies to characterize the association between the consumption of legal cannabis and subsequent pharmacologic and neurobehavioral outcomes, with somewhat surprising results.
The study showed that, although cannabis consumption did not affect most short-term neurobehavioral measures, it delayed recall memory and impaired balance.
The investigation also showed that users of much more potent cannabis concentrates actually demonstrated similar or lower levels of subjective drug intoxication and short-term impairment than did their counterparts who used lower-potency forms of the cannabis flower.
“It does not appear that the potency being used matters that much,” senior investigator Kent E. Hutchison, PhD, said in an interview. “People seem to be titrating to a certain level of intoxication or a certain level of feeling high. And for some people that requires a lot of drug, and for other people not as much.”
added Dr. Hutchison, a professor of psychology and neuroscience at the University of Colorado Boulder.
The study was published online June 10 in JAMA Psychiatry.
Widespread availability, little research
Recreational cannabis is now legal in 11 states and the District of Columbia, while medical cannabis is legal in 33. However, despite growing popularity of cannabis, there is little research on its potential health and biobehavioral risks, largely because of federal restrictions on cannabis research.
Cannabis users typically consume various forms of the cannabis flower, which can boast concentrations of the psychoactive cannabinoid delta-9-tetrahydrocannabinol (THC) of up to 30%. However, use of concentrated forms of cannabis – which are made by extracting plant cannabinoids into a different form – is increasing.
Such formulations can boast THC concentrations as high as 90%. Nevertheless, data regarding the relative risks of these higher-strength products are limited.
Previous research has shown a variety of negative short-term and long-term neurobehavioral effects associated with cannabis use, including harmful cognitive and motor effects. Extended exposure to THC may also negatively affect brain regions that are associated with the control of coordinated movement, and create brain-activation deficits in motor control regions that persist well beyond the effects of short-term intoxication.
Despite such findings, Dr. Hutchison said the existing literature on the subject does not yield a real-world view of current cannabis use because it tends to focus on low-THC products that are increasingly less common in today’s legal market.
Given such shortcomings, the investigators wanted to address persistent questions surrounding the neurobehavioral effects of legal cannabis flower products (16% or 24% THC) and cannabis concentrate products (70% or 90% THC). In doing so, they examined three primary topics:
- The association between short-term use of these products and THC plasma levels; subjective intoxication; and mood, cognitive performance, and balance
- Differences in such associations between users of cannabis flower and concentrate products
- Potential variations in these associations by THC potency
High- versus low-potency varieties
The study included 133 individuals (aged 21-70 years), who were designated as either cannabis flower users or cannabis concentrate users. Participants had all used cannabis at least four times in the previous month with no adverse reaction and were not receiving treatment for a psychotic disorder or bipolar disorder.
Participants were randomly assigned to consume either higher-potency or lower-potency products that had been purchased from a local dispensary. Flower users were randomized to purchase 3 g of either a 16% THC or 24% THC product, while concentrate users were randomized to purchase 1g of either 70% THC or 90% THC.
Participants completed a series of four assessments, one at baseline and three others at a mobile laboratory. The mobile laboratory assessments occurred before, immediately after, and one hour after participants had all consumed their cannabis ad libitum.
Of the original cohort of 133 participants, 55 flower cannabis users (mean age, 28.8 years; 46% women) and 66 concentrate cannabis users (mean age, 28.3 years; 45% women) complied with the study’s instructions and had complete data.
The study’s primary outcome measures included plasma cannabinoids, subjective drug intoxication and mood, and neurobehavioral outcomes such as attention, memory, inhibitory control, and balance.
Mixed results
With respect to cannabis concentrations, results showed that users of concentrate exhibited higher levels of both plasma THC and the active metabolite of THC (11-hydroxy-delta9-THC) across all points than did their counterparts who used cannabis flower products.
Specifically, mean plasma THC levels were 1,016 ± 1,380 mcg/ml in concentrate users and 455±503 mcg/mL in flower users after ad libitum cannabis consumption. Nevertheless, self-reported levels of intoxication were no different between users of cannabis flower or concentrate products.
Although results also showed that most neurobehavioral measures were not altered by short-term cannabis consumption, there were some notable exceptions. There was a negative linear effect with delayed verbal recall errors, suggesting poorer performance after cannabis use (F1, 203 = 32.31; P < .001).
On the other hand, investigators found a positive linear effect with inhibitory control and working memory, which actually suggests better performance after cannabis use. This finding, the researchers note, may be the result of a practice effect. Cannabis flower users performed better across all inhibitory control assessments.
The researchers also tested participants’ balance with their eyes open and closed. In the eyes-open condition, they found a trend toward impaired balance after cannabis use, though this normalized within an hour. When subjects closed their eyes, however, researchers observed a significant short-term increase in sway after cannabis use, which fell back to pre-use levels one hour after use (F1, 203 = 18.88; P < .001).
Of note, outcomes did not differ between groups according to the type of cannabis product consumed or its relative potency.
The study yielded several surprising findings, beginning with self-reported intoxication levels, which were not statistically significant between different cannabis flower and concentrate users, despite significantly different plasma THC levels between the two groups.
Dr. Hutchison explained that this may be the result of greater THC tolerance among concentrate users, THC saturation of cannabinoid receptors, or interindividual differences among users with respect to cannabis metabolism or sensitivity.
“I thought for sure that high-potency users would be much more compromised,” he said. “I guess it just goes to show we have a lot to learn about how these things work.”
Additionally, there were virtually no significant changes in acute performance after cannabis use, with the exception of delayed verbal recall. In fact, the most marked change observed in the study was the effect of cannabis on balance immediately after drug use, though these changes seemed to abate within an hour.
Nevertheless, the study highlights several potential public health implications of cannabis consumption, Dr. Hutchison added. “What happens when people with high blood concentrations decide to quit?” he asked. “Do they have trouble quitting? Do they have withdrawal symptoms?”
The long-term effects of cannabis use is another important question that still needs to be answered, he added.
Finally, Dr. Hutchison noted that, although the study showed little difference between users of cannabis flower and concentrates, study participants were all experienced users.
“There is certainly the potential for harm when a naive person uses cannabis concentrate,” he said. “Suddenly they have way more THC than they thought they were going to get, and that’s where a lot of people get into trouble with cannabis.”
Pitfalls and hurdles
In an accompanying editorial, Margaret Haney, PhD, of Columbia University Irving Medical Center, New York, explained that cannabis’ awkward position as simultaneously legal and illegal, medical and recreational, has hampered researchers’ ability to study its effects as comprehensively as they would otherwise like.
“With a federally illegal drug legalized in individual states, scientists constrained, and federal agencies somewhat silent, clinicians have none of the data that guide their decisions for other medications (eg, which indication, product, cannabinoid ratio, dose, or route of administration; what risks for individual patients [eg, pregnant, adolescent, psychiatric?]),” Dr. Haney wrote.
These pitfalls are compounded by the significant regulatory hurdles.
“The FDA is appropriately cautious about what it allows scientists to test in patients, and none of the products available in dispensaries or online have undergone the safety and manufacturing procedures needed for FDA approval,” she continued. “How then to conduct the studies so needed?”
Yet as Haney noted, giving cannabinoid researchers a Schedule I exemption may help address many of the barriers facing these scientists. Such a move, she said, would increase the number of randomized controlled trials being performed, “and thereby begin to breach the divide between the use of these products and empirical evidence.”
Dr. Hutchison has disclosed no relevant financial relationships. Dr. Haney disclosed funding from the US National Institute on Drug Abuse and from the Thompson Family Foundation Initiative. The study was funded by the NIH and Colorado Department of Public Health and Environment.
A version of this article originally appeared on Medscape.com.
Researchers have published one of the first studies to characterize the association between the consumption of legal cannabis and subsequent pharmacologic and neurobehavioral outcomes, with somewhat surprising results.
The study showed that, although cannabis consumption did not affect most short-term neurobehavioral measures, it delayed recall memory and impaired balance.
The investigation also showed that users of much more potent cannabis concentrates actually demonstrated similar or lower levels of subjective drug intoxication and short-term impairment than did their counterparts who used lower-potency forms of the cannabis flower.
“It does not appear that the potency being used matters that much,” senior investigator Kent E. Hutchison, PhD, said in an interview. “People seem to be titrating to a certain level of intoxication or a certain level of feeling high. And for some people that requires a lot of drug, and for other people not as much.”
added Dr. Hutchison, a professor of psychology and neuroscience at the University of Colorado Boulder.
The study was published online June 10 in JAMA Psychiatry.
Widespread availability, little research
Recreational cannabis is now legal in 11 states and the District of Columbia, while medical cannabis is legal in 33. However, despite growing popularity of cannabis, there is little research on its potential health and biobehavioral risks, largely because of federal restrictions on cannabis research.
Cannabis users typically consume various forms of the cannabis flower, which can boast concentrations of the psychoactive cannabinoid delta-9-tetrahydrocannabinol (THC) of up to 30%. However, use of concentrated forms of cannabis – which are made by extracting plant cannabinoids into a different form – is increasing.
Such formulations can boast THC concentrations as high as 90%. Nevertheless, data regarding the relative risks of these higher-strength products are limited.
Previous research has shown a variety of negative short-term and long-term neurobehavioral effects associated with cannabis use, including harmful cognitive and motor effects. Extended exposure to THC may also negatively affect brain regions that are associated with the control of coordinated movement, and create brain-activation deficits in motor control regions that persist well beyond the effects of short-term intoxication.
Despite such findings, Dr. Hutchison said the existing literature on the subject does not yield a real-world view of current cannabis use because it tends to focus on low-THC products that are increasingly less common in today’s legal market.
Given such shortcomings, the investigators wanted to address persistent questions surrounding the neurobehavioral effects of legal cannabis flower products (16% or 24% THC) and cannabis concentrate products (70% or 90% THC). In doing so, they examined three primary topics:
- The association between short-term use of these products and THC plasma levels; subjective intoxication; and mood, cognitive performance, and balance
- Differences in such associations between users of cannabis flower and concentrate products
- Potential variations in these associations by THC potency
High- versus low-potency varieties
The study included 133 individuals (aged 21-70 years), who were designated as either cannabis flower users or cannabis concentrate users. Participants had all used cannabis at least four times in the previous month with no adverse reaction and were not receiving treatment for a psychotic disorder or bipolar disorder.
Participants were randomly assigned to consume either higher-potency or lower-potency products that had been purchased from a local dispensary. Flower users were randomized to purchase 3 g of either a 16% THC or 24% THC product, while concentrate users were randomized to purchase 1g of either 70% THC or 90% THC.
Participants completed a series of four assessments, one at baseline and three others at a mobile laboratory. The mobile laboratory assessments occurred before, immediately after, and one hour after participants had all consumed their cannabis ad libitum.
Of the original cohort of 133 participants, 55 flower cannabis users (mean age, 28.8 years; 46% women) and 66 concentrate cannabis users (mean age, 28.3 years; 45% women) complied with the study’s instructions and had complete data.
The study’s primary outcome measures included plasma cannabinoids, subjective drug intoxication and mood, and neurobehavioral outcomes such as attention, memory, inhibitory control, and balance.
Mixed results
With respect to cannabis concentrations, results showed that users of concentrate exhibited higher levels of both plasma THC and the active metabolite of THC (11-hydroxy-delta9-THC) across all points than did their counterparts who used cannabis flower products.
Specifically, mean plasma THC levels were 1,016 ± 1,380 mcg/ml in concentrate users and 455±503 mcg/mL in flower users after ad libitum cannabis consumption. Nevertheless, self-reported levels of intoxication were no different between users of cannabis flower or concentrate products.
Although results also showed that most neurobehavioral measures were not altered by short-term cannabis consumption, there were some notable exceptions. There was a negative linear effect with delayed verbal recall errors, suggesting poorer performance after cannabis use (F1, 203 = 32.31; P < .001).
On the other hand, investigators found a positive linear effect with inhibitory control and working memory, which actually suggests better performance after cannabis use. This finding, the researchers note, may be the result of a practice effect. Cannabis flower users performed better across all inhibitory control assessments.
The researchers also tested participants’ balance with their eyes open and closed. In the eyes-open condition, they found a trend toward impaired balance after cannabis use, though this normalized within an hour. When subjects closed their eyes, however, researchers observed a significant short-term increase in sway after cannabis use, which fell back to pre-use levels one hour after use (F1, 203 = 18.88; P < .001).
Of note, outcomes did not differ between groups according to the type of cannabis product consumed or its relative potency.
The study yielded several surprising findings, beginning with self-reported intoxication levels, which were not statistically significant between different cannabis flower and concentrate users, despite significantly different plasma THC levels between the two groups.
Dr. Hutchison explained that this may be the result of greater THC tolerance among concentrate users, THC saturation of cannabinoid receptors, or interindividual differences among users with respect to cannabis metabolism or sensitivity.
“I thought for sure that high-potency users would be much more compromised,” he said. “I guess it just goes to show we have a lot to learn about how these things work.”
Additionally, there were virtually no significant changes in acute performance after cannabis use, with the exception of delayed verbal recall. In fact, the most marked change observed in the study was the effect of cannabis on balance immediately after drug use, though these changes seemed to abate within an hour.
Nevertheless, the study highlights several potential public health implications of cannabis consumption, Dr. Hutchison added. “What happens when people with high blood concentrations decide to quit?” he asked. “Do they have trouble quitting? Do they have withdrawal symptoms?”
The long-term effects of cannabis use is another important question that still needs to be answered, he added.
Finally, Dr. Hutchison noted that, although the study showed little difference between users of cannabis flower and concentrates, study participants were all experienced users.
“There is certainly the potential for harm when a naive person uses cannabis concentrate,” he said. “Suddenly they have way more THC than they thought they were going to get, and that’s where a lot of people get into trouble with cannabis.”
Pitfalls and hurdles
In an accompanying editorial, Margaret Haney, PhD, of Columbia University Irving Medical Center, New York, explained that cannabis’ awkward position as simultaneously legal and illegal, medical and recreational, has hampered researchers’ ability to study its effects as comprehensively as they would otherwise like.
“With a federally illegal drug legalized in individual states, scientists constrained, and federal agencies somewhat silent, clinicians have none of the data that guide their decisions for other medications (eg, which indication, product, cannabinoid ratio, dose, or route of administration; what risks for individual patients [eg, pregnant, adolescent, psychiatric?]),” Dr. Haney wrote.
These pitfalls are compounded by the significant regulatory hurdles.
“The FDA is appropriately cautious about what it allows scientists to test in patients, and none of the products available in dispensaries or online have undergone the safety and manufacturing procedures needed for FDA approval,” she continued. “How then to conduct the studies so needed?”
Yet as Haney noted, giving cannabinoid researchers a Schedule I exemption may help address many of the barriers facing these scientists. Such a move, she said, would increase the number of randomized controlled trials being performed, “and thereby begin to breach the divide between the use of these products and empirical evidence.”
Dr. Hutchison has disclosed no relevant financial relationships. Dr. Haney disclosed funding from the US National Institute on Drug Abuse and from the Thompson Family Foundation Initiative. The study was funded by the NIH and Colorado Department of Public Health and Environment.
A version of this article originally appeared on Medscape.com.
Cardiac CT scans can be used for osteoporosis screening
A new study has determined a benefit of cardiac CT scans beyond assessing heart health: Evaluating fracture rate and potential osteoporosis through the bone mineral density (BMD) of thoracic vertebrae.
“Our results represent a step toward appraisal and recognition of the clinical utility of opportunistic BMD screening from cardiac CT,” wrote Josephine Therkildsen, MD, of Hospital Unit West in Herning, Denmark, and coauthors. The study was published July 14 in Radiology.
To determine if further analysis of cardiac CT could help determine BMD and its association with fracture rate, the investigators launched a prospective observational study of 1,487 Danish patients with potential coronary artery disease who underwent cardiac CT scans between September 2014 and March 2016. Their mean age was 57 years (standard deviation, 9; range, 40-80). Nearly all of the patients were white, and 52.5% (n = 781) were women.
All participants underwent a noncontrast-enhanced cardiac CT, from which volumetric BMD of three thoracic vertebrae was measured via commercially available semiautomatic software. Their mean BMD was 119 mg/cm3 (SD, 34) with no significant difference noted between male and female patients. Of the 1,487 participants, 695 were defined as having normal BMD (> 120 mg/cm3), 613 as having low BMD (80-120 mg/cm3), and 179 as having very low BMD (< 80 mg/cm3). Median follow-up was 3.1 years (interquartile range, 2.7-3.4).
Incident fracture occurred in 80 patients (5.4%), of whom 48 were women and 32 were men. Patients who suffered fractures were significantly older than patients with no fractures (mean 59 years vs. 57 years; P = .03). Of the 80 patients with fractures, 31 were osteoporosis related.
In an unadjusted analysis, participants with very low BMD had a greater rate of any fracture (hazard ratio [HR], 2.6; 95% confidence interval, 1.4-4.7; P = .002) and of osteoporosis-related fracture (HR, 8.1; 95% CI, 2.4-27.0; P = .001). After adjustment for age and sex, their rates remained significantly greater for any fracture (HR, 2.1; 95% CI, 1.1-4.2; P = .03) and for osteoporosis-related fracture (HR, 4.0; 95% CI, 1.1-15.0; P = .04).
“Opportunistic” use of scans benefits both physicians and patients
“The concept of using a CT scan that was done for a different purpose allows you to be opportunistic,” Ethel S. Siris, MD, the Madeline C. Stabile Professor of Clinical Medicine in the department of medicine at Columbia University and director of the Toni Stabile Osteoporosis Center of the Columbia University Medical Center, New York–Presbyterian Hospital, New York, said in an interview. “If you’re dealing with older patients, and if you have the software for your radiologist to use to reanalyze the CT scan and say something about the bone, it’s certainly a way of estimating who may be at risk of future fractures.
“From a practical point of view, it’s hard to imagine that it would ever replace conventional bone mineral density testing via DXA [dual-energy x-ray absorptiometry],” she added. “That said, osteoporosis is woefully underdiagnosed because people don’t get DXA tested. This study showed that, if you have access to the scan of the thoracic or even the lumbar spine and if you have the necessary software, you can make legitimate statements about the numbers being low or very low. What that would lead to, I would hope, is some internists to say, ‘This could be a predictor of fracture risk. We should put you on treatment.’ And then follow up with a conventional DXA test.
“Is that going to happen? I don’t know. But the bottom line of the study is: Anything that may enhance the physician’s drive to evaluate a patient for fracture risk is good.”
Whatever the reason for the scan, CT can help diagnose osteoporosis
This study reinforces that CT exams – of the chest, in particular – can serve a valuable dual purpose as osteoporosis screenings, Miriam A. Bredella, MD, professor of radiology at Harvard Medical School and vice chair of the department of radiology at Massachusetts General Hospital, Boston, wrote in an accompanying editorial.
“In the United States, more than 80 million CT examinations are performed each year, many of which could be used to screen for osteoporosis without additional costs or radiation exposure,” she wrote. And thanks to the findings of the study by Therkildsen et al., which relied on both established and new BMD thresholds, the link between thoracic spine BMD and fracture risk is clearer than ever.
“I hope this study will ignite interest in using chest CT examinations performed for other purposes, such as lung cancer screening, for opportunistic osteoporosis screening and prediction of fractures in vulnerable populations,” she added.
The authors acknowledged their study’s limitations, including a small number of fracture events overall and the inability to evaluate associations between BMD and fracture rate at specific locations. In addition, their cohort was largely made up of white participants with a certain coronary artery disease risk profile; because of ethnical differences in BMD measurements, their results “cannot be extrapolated to other ethnical groups.”
Several of the study’s authors reported potential conflicts of interest, including receiving grants and money for consultancies and board memberships from various councils, associations, and pharmaceutical companies. Dr. Bredella reported no conflicts of interest. Dr. Siris has no relevant disclosures.
SOURCE: Therkildsen J et al. Radiology. 2020 Jul 14. doi: 10.1148/radiol.2020192706.
A new study has determined a benefit of cardiac CT scans beyond assessing heart health: Evaluating fracture rate and potential osteoporosis through the bone mineral density (BMD) of thoracic vertebrae.
“Our results represent a step toward appraisal and recognition of the clinical utility of opportunistic BMD screening from cardiac CT,” wrote Josephine Therkildsen, MD, of Hospital Unit West in Herning, Denmark, and coauthors. The study was published July 14 in Radiology.
To determine if further analysis of cardiac CT could help determine BMD and its association with fracture rate, the investigators launched a prospective observational study of 1,487 Danish patients with potential coronary artery disease who underwent cardiac CT scans between September 2014 and March 2016. Their mean age was 57 years (standard deviation, 9; range, 40-80). Nearly all of the patients were white, and 52.5% (n = 781) were women.
All participants underwent a noncontrast-enhanced cardiac CT, from which volumetric BMD of three thoracic vertebrae was measured via commercially available semiautomatic software. Their mean BMD was 119 mg/cm3 (SD, 34) with no significant difference noted between male and female patients. Of the 1,487 participants, 695 were defined as having normal BMD (> 120 mg/cm3), 613 as having low BMD (80-120 mg/cm3), and 179 as having very low BMD (< 80 mg/cm3). Median follow-up was 3.1 years (interquartile range, 2.7-3.4).
Incident fracture occurred in 80 patients (5.4%), of whom 48 were women and 32 were men. Patients who suffered fractures were significantly older than patients with no fractures (mean 59 years vs. 57 years; P = .03). Of the 80 patients with fractures, 31 were osteoporosis related.
In an unadjusted analysis, participants with very low BMD had a greater rate of any fracture (hazard ratio [HR], 2.6; 95% confidence interval, 1.4-4.7; P = .002) and of osteoporosis-related fracture (HR, 8.1; 95% CI, 2.4-27.0; P = .001). After adjustment for age and sex, their rates remained significantly greater for any fracture (HR, 2.1; 95% CI, 1.1-4.2; P = .03) and for osteoporosis-related fracture (HR, 4.0; 95% CI, 1.1-15.0; P = .04).
“Opportunistic” use of scans benefits both physicians and patients
“The concept of using a CT scan that was done for a different purpose allows you to be opportunistic,” Ethel S. Siris, MD, the Madeline C. Stabile Professor of Clinical Medicine in the department of medicine at Columbia University and director of the Toni Stabile Osteoporosis Center of the Columbia University Medical Center, New York–Presbyterian Hospital, New York, said in an interview. “If you’re dealing with older patients, and if you have the software for your radiologist to use to reanalyze the CT scan and say something about the bone, it’s certainly a way of estimating who may be at risk of future fractures.
“From a practical point of view, it’s hard to imagine that it would ever replace conventional bone mineral density testing via DXA [dual-energy x-ray absorptiometry],” she added. “That said, osteoporosis is woefully underdiagnosed because people don’t get DXA tested. This study showed that, if you have access to the scan of the thoracic or even the lumbar spine and if you have the necessary software, you can make legitimate statements about the numbers being low or very low. What that would lead to, I would hope, is some internists to say, ‘This could be a predictor of fracture risk. We should put you on treatment.’ And then follow up with a conventional DXA test.
“Is that going to happen? I don’t know. But the bottom line of the study is: Anything that may enhance the physician’s drive to evaluate a patient for fracture risk is good.”
Whatever the reason for the scan, CT can help diagnose osteoporosis
This study reinforces that CT exams – of the chest, in particular – can serve a valuable dual purpose as osteoporosis screenings, Miriam A. Bredella, MD, professor of radiology at Harvard Medical School and vice chair of the department of radiology at Massachusetts General Hospital, Boston, wrote in an accompanying editorial.
“In the United States, more than 80 million CT examinations are performed each year, many of which could be used to screen for osteoporosis without additional costs or radiation exposure,” she wrote. And thanks to the findings of the study by Therkildsen et al., which relied on both established and new BMD thresholds, the link between thoracic spine BMD and fracture risk is clearer than ever.
“I hope this study will ignite interest in using chest CT examinations performed for other purposes, such as lung cancer screening, for opportunistic osteoporosis screening and prediction of fractures in vulnerable populations,” she added.
The authors acknowledged their study’s limitations, including a small number of fracture events overall and the inability to evaluate associations between BMD and fracture rate at specific locations. In addition, their cohort was largely made up of white participants with a certain coronary artery disease risk profile; because of ethnical differences in BMD measurements, their results “cannot be extrapolated to other ethnical groups.”
Several of the study’s authors reported potential conflicts of interest, including receiving grants and money for consultancies and board memberships from various councils, associations, and pharmaceutical companies. Dr. Bredella reported no conflicts of interest. Dr. Siris has no relevant disclosures.
SOURCE: Therkildsen J et al. Radiology. 2020 Jul 14. doi: 10.1148/radiol.2020192706.
A new study has determined a benefit of cardiac CT scans beyond assessing heart health: Evaluating fracture rate and potential osteoporosis through the bone mineral density (BMD) of thoracic vertebrae.
“Our results represent a step toward appraisal and recognition of the clinical utility of opportunistic BMD screening from cardiac CT,” wrote Josephine Therkildsen, MD, of Hospital Unit West in Herning, Denmark, and coauthors. The study was published July 14 in Radiology.
To determine if further analysis of cardiac CT could help determine BMD and its association with fracture rate, the investigators launched a prospective observational study of 1,487 Danish patients with potential coronary artery disease who underwent cardiac CT scans between September 2014 and March 2016. Their mean age was 57 years (standard deviation, 9; range, 40-80). Nearly all of the patients were white, and 52.5% (n = 781) were women.
All participants underwent a noncontrast-enhanced cardiac CT, from which volumetric BMD of three thoracic vertebrae was measured via commercially available semiautomatic software. Their mean BMD was 119 mg/cm3 (SD, 34) with no significant difference noted between male and female patients. Of the 1,487 participants, 695 were defined as having normal BMD (> 120 mg/cm3), 613 as having low BMD (80-120 mg/cm3), and 179 as having very low BMD (< 80 mg/cm3). Median follow-up was 3.1 years (interquartile range, 2.7-3.4).
Incident fracture occurred in 80 patients (5.4%), of whom 48 were women and 32 were men. Patients who suffered fractures were significantly older than patients with no fractures (mean 59 years vs. 57 years; P = .03). Of the 80 patients with fractures, 31 were osteoporosis related.
In an unadjusted analysis, participants with very low BMD had a greater rate of any fracture (hazard ratio [HR], 2.6; 95% confidence interval, 1.4-4.7; P = .002) and of osteoporosis-related fracture (HR, 8.1; 95% CI, 2.4-27.0; P = .001). After adjustment for age and sex, their rates remained significantly greater for any fracture (HR, 2.1; 95% CI, 1.1-4.2; P = .03) and for osteoporosis-related fracture (HR, 4.0; 95% CI, 1.1-15.0; P = .04).
“Opportunistic” use of scans benefits both physicians and patients
“The concept of using a CT scan that was done for a different purpose allows you to be opportunistic,” Ethel S. Siris, MD, the Madeline C. Stabile Professor of Clinical Medicine in the department of medicine at Columbia University and director of the Toni Stabile Osteoporosis Center of the Columbia University Medical Center, New York–Presbyterian Hospital, New York, said in an interview. “If you’re dealing with older patients, and if you have the software for your radiologist to use to reanalyze the CT scan and say something about the bone, it’s certainly a way of estimating who may be at risk of future fractures.
“From a practical point of view, it’s hard to imagine that it would ever replace conventional bone mineral density testing via DXA [dual-energy x-ray absorptiometry],” she added. “That said, osteoporosis is woefully underdiagnosed because people don’t get DXA tested. This study showed that, if you have access to the scan of the thoracic or even the lumbar spine and if you have the necessary software, you can make legitimate statements about the numbers being low or very low. What that would lead to, I would hope, is some internists to say, ‘This could be a predictor of fracture risk. We should put you on treatment.’ And then follow up with a conventional DXA test.
“Is that going to happen? I don’t know. But the bottom line of the study is: Anything that may enhance the physician’s drive to evaluate a patient for fracture risk is good.”
Whatever the reason for the scan, CT can help diagnose osteoporosis
This study reinforces that CT exams – of the chest, in particular – can serve a valuable dual purpose as osteoporosis screenings, Miriam A. Bredella, MD, professor of radiology at Harvard Medical School and vice chair of the department of radiology at Massachusetts General Hospital, Boston, wrote in an accompanying editorial.
“In the United States, more than 80 million CT examinations are performed each year, many of which could be used to screen for osteoporosis without additional costs or radiation exposure,” she wrote. And thanks to the findings of the study by Therkildsen et al., which relied on both established and new BMD thresholds, the link between thoracic spine BMD and fracture risk is clearer than ever.
“I hope this study will ignite interest in using chest CT examinations performed for other purposes, such as lung cancer screening, for opportunistic osteoporosis screening and prediction of fractures in vulnerable populations,” she added.
The authors acknowledged their study’s limitations, including a small number of fracture events overall and the inability to evaluate associations between BMD and fracture rate at specific locations. In addition, their cohort was largely made up of white participants with a certain coronary artery disease risk profile; because of ethnical differences in BMD measurements, their results “cannot be extrapolated to other ethnical groups.”
Several of the study’s authors reported potential conflicts of interest, including receiving grants and money for consultancies and board memberships from various councils, associations, and pharmaceutical companies. Dr. Bredella reported no conflicts of interest. Dr. Siris has no relevant disclosures.
SOURCE: Therkildsen J et al. Radiology. 2020 Jul 14. doi: 10.1148/radiol.2020192706.
FROM RADIOLOGY
Wave, surge, or tsunami
Different COVID-19 models and predicting inpatient bed capacity
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
Different COVID-19 models and predicting inpatient bed capacity
Different COVID-19 models and predicting inpatient bed capacity
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.