User login
Ready for PRIME time? Newly ID’d cells predict RA flares
A newly identified circulating cell type may be a reliable marker for impending RA flares. The discovery and description of the cells, which bear a “striking” similarity to synovial fibroblasts, provide important clues to the origins of RA and progressive joint inflammation, investigators say.
By studying longitudinally collected blood samples from four patients with RA over 4 years, Dana E. Orange, MD and colleagues at Rockefeller University, New York, identified a pattern of B-cell activation and expansion of circulating cells that are negative for CD45 and CD31 expression, and positive for PDPN, dubbed preinflammatory mesenchymal or “PRIME” cells.
Expansion of PRIME cells in circulation increased dramatically in the weeks leading up to a flare and decreased during a flare, suggesting the possibility of a serum assay for predicting flares and allowing for early intervention to ameliorate or prevent disabling consequences, the investigators wrote in a study published in the New England Journal of Medicine.
“Our hope is that this will be a diagnostic in the future, but we need to study it in more patients to see how it will perform,” Dr. Orange said in an interview, adding that the cells, if shown to be pathogenic, could also be targets for new therapeutic strategies.
RNA sequencing
Dr. Orange and colleagues discovered the PRIME cells through a novel clinical and technical protocol involving home collection of blood by patients and longitudinal RNA sequencing to study gene expression profiles during times of both disease quiescence and flares, and noticed a distinct pattern of PRIME cell expansion, depletion, and gene expression.
“Looking at their gene expression profiles, they overlapped with fibroblasts that reside in inflamed rheumatoid arthritis synovium, and in an animal model those types of fibroblasts were important for allowing entry of inflammatory infiltrates around the joint,” she said.
PRIME cells may be a precursor of synovial fibroblasts, which have been implicated by some researchers in the spread of RA between joints, Dr. Orange added.
Patients do homework
The investigators began by enrolling four patients, followed for 1-4 years, who met 2010 American College of Rheumatology–European League Against Rheumatism criteria and who were seropositive for anti–cyclic citrullinated peptide antibodies.
They assessed disease activity from patient homes weekly or during escalation of flares up to four times daily, with the Routine Assessment of Patient Index Data 3 (RAPID3) questionnaire, as well as monthly clinic visits. At clinic visits during flares, disease activity was assessed using both the RAPID3 and 28-joint Disease Activity Score.
The patients performed fingerstick blood collection and mailed the samples overnight each week to Rockefeller University, where RNA was extracted and sequenced. The investigators identified gene transcripts that were differentially expressed in blood prior to flares, and compared them with data profiles derived from synovial single-cell RNA sequencing.
To validate the findings, the researchers used flow cytometry and sorted blood-cell RNA sequencing of samples from an additional 19 patients with RA.
They found that a total of 2,613 genes were differentially expressed during a flare, compared with baseline, and that expression of 1,437 of these genes was increased during a flare, with the remaining 1,176 decreased during flares.
Before the storm
Focusing on two flare-antecedent clusters of genes, they identified one cluster of transcripts that increased 2 weeks before a flare, enriched with genes coding for developmental pathways for naive B cells (that is, not yet exposed to antigens) and leukocytes.
The second cluster included gene transcripts that increased during the week before a flare, then decreased over the duration of the flare. Genes in this cluster were enriched for pathways that were unexpected in typical blood specimens, including genes involved in cartilage morphogenesis, endochondral bone growth, and extracellular matrix organization. The gene activity suggested the presence of a mesenchymal cell, they wrote.
The RNA expression profiles of these newly identified PRIME cells were very similar to those of synovial fibroblasts, and the investigators speculated that PRIME cells may be synovial fibroblast precursors.
They proposed a model of RA exacerbation in which PRIME cells become activated by B cells in the weeks immediately preceding a flare, and then migrate out of blood into the synovium.
The investigators are currently investigating “how reproducible this signature is in different flares in patients on different types of background therapy, and then we’re very interested in looking at the upstream triggers of the B cell and the PRIME cell,” Dr. Orange said.
“One of the reasons this is very exciting is that there are these signatures that can be found when patients are clearly asymptomatic but about to flare, and if we can intervene at that time, then the patients won’t have to live through a flare, they won’t have to have that experience,” she said.
Pros and cons
In an editorial accompanying the study, Ellen M. Gravallese, MD, from Brigham and Women’s Hospital in Boston and William H. Robinson, MD, PhD, from Stanford (Calif.) University and the Veterans Affairs Palo Alto (Calif.) Health Care System wrote that the study demonstrates an important method for identifying genetic contributions to many different types of disease.
“Orange and colleagues show that intensively collected longitudinal data from a small sample of patients can be used to identify dysregulated transcriptional signatures that are not recognized by classical cross-sectional studies. This study illustrates the exciting potential of longitudinal genomics to identify key antecedents of disease flares in an approach that may be applicable to the investigation of pathogenic and protective immune responses in a wide range of human diseases,” they wrote.
Rheumatology researcher Christopher D. Buckley, MBBS, DPhil, from the University of Birmingham (England), who was not involved in the study, said that the use of blood samples is both a strength and a weakness of the study.
“Blood is much easier to get than synovial tissue, but synovial tissue is important. If I’m trying to look at the blood and trying to make an inference about what’s going on in the synovium, if I don’t look at the synovium I don’t know what the link between the blood and synovium is,” he said in an interview.
On the plus side, “the big advantage about looking at blood is that you do multiple time points, which is really cool,” he said.
Dr. Buckley is a coauthor of a recent paper in Nature Medicine – published after the study by Dr. Orange and colleagues was accepted by the New England Journal of Medicine – showing that a population of macrophages in synovium was highly predictive of remission in patients with RA, and that therapeutic modulation of these macrophages has the potential as a treatment strategy for RA.
“We are very keen to understand the cellular basis of disease. We’re very good at understanding genes, but genes have to work in cells, and cells make organs, so the cells are critical,” he said.
The paper adds fuel to a controversy that has been raging among rheumatology researchers for more than a decade: the “flying fibroblast” hypothesis, which suggests that fibroblasts can migrate from one joint to another, hence spreading the disease in a manner akin to cancer metastases.
“It’s been quite controversial whether these cells like fibroblasts can exist in the blood, or whether they’re found in sufficient number in the blood,” Dr. Buckley said. “The fact that they have identified these PRIME cells is fascinating, because that’s going to cause us to go back and reinvestigate the whole flying fibroblast story.”
His colleague John Isaacs, MBBS, PhD, from Newcastle (England) University, is principal investigator for the BIO-FLARE study, in which participants with RA stop taking their disease-modifying antirheumatic drugs under close supervision of researchers. The investigators then study the patients looking for flare signals as well as the biology of flares themselves.
“As it happens, our protocols would not pick up this particular cell, because we have not been focusing on the stroma, at least not in peripheral blood. We’ve all been looking at synovium as part of BIO-FLARE,” he said in an interview. “We will be looking for this cell now that we have seen this research, and certainly we would want to replicate.”
He agreed with Dr. Buckley’s observation that the PRIME cell data may revive the flying fibroblast hypothesis. “This is a great paper in a top clinical journal. What isn’t there is mechanism. That’s the thing we’re all going to want to understand now: Where do the cells come from, how do they actually trigger flares, and how do they go down as flare starts?”
Both Dr. Buckley and Dr. Isaacs agreed that the study findings point to important new avenues of research, but also noted that the study was small, involving a total of only 23 patients, and that replication of the findings and elucidation of the mechanism of PRIME cell generation and disposition will be required.
The study was supported by grants from the National Institutes of Health, Simons Foundation, Robertson Foundation, Rheumatology Research Foundation, Bernard and Irene Schwartz Foundation, the Iris and Junming Le Foundation, and Rockefeller University. Dr. Orange disclosed a provisional patent for the discovery of the PRIME cells. Dr. Buckley and Dr. Isaacs reported no relevant conflicts of interest.
SOURCE: Orange DE et al. N Engl J Med. 2020 Jul 15. doi: 10.1056/NEJMoa2004114.
A newly identified circulating cell type may be a reliable marker for impending RA flares. The discovery and description of the cells, which bear a “striking” similarity to synovial fibroblasts, provide important clues to the origins of RA and progressive joint inflammation, investigators say.
By studying longitudinally collected blood samples from four patients with RA over 4 years, Dana E. Orange, MD and colleagues at Rockefeller University, New York, identified a pattern of B-cell activation and expansion of circulating cells that are negative for CD45 and CD31 expression, and positive for PDPN, dubbed preinflammatory mesenchymal or “PRIME” cells.
Expansion of PRIME cells in circulation increased dramatically in the weeks leading up to a flare and decreased during a flare, suggesting the possibility of a serum assay for predicting flares and allowing for early intervention to ameliorate or prevent disabling consequences, the investigators wrote in a study published in the New England Journal of Medicine.
“Our hope is that this will be a diagnostic in the future, but we need to study it in more patients to see how it will perform,” Dr. Orange said in an interview, adding that the cells, if shown to be pathogenic, could also be targets for new therapeutic strategies.
RNA sequencing
Dr. Orange and colleagues discovered the PRIME cells through a novel clinical and technical protocol involving home collection of blood by patients and longitudinal RNA sequencing to study gene expression profiles during times of both disease quiescence and flares, and noticed a distinct pattern of PRIME cell expansion, depletion, and gene expression.
“Looking at their gene expression profiles, they overlapped with fibroblasts that reside in inflamed rheumatoid arthritis synovium, and in an animal model those types of fibroblasts were important for allowing entry of inflammatory infiltrates around the joint,” she said.
PRIME cells may be a precursor of synovial fibroblasts, which have been implicated by some researchers in the spread of RA between joints, Dr. Orange added.
Patients do homework
The investigators began by enrolling four patients, followed for 1-4 years, who met 2010 American College of Rheumatology–European League Against Rheumatism criteria and who were seropositive for anti–cyclic citrullinated peptide antibodies.
They assessed disease activity from patient homes weekly or during escalation of flares up to four times daily, with the Routine Assessment of Patient Index Data 3 (RAPID3) questionnaire, as well as monthly clinic visits. At clinic visits during flares, disease activity was assessed using both the RAPID3 and 28-joint Disease Activity Score.
The patients performed fingerstick blood collection and mailed the samples overnight each week to Rockefeller University, where RNA was extracted and sequenced. The investigators identified gene transcripts that were differentially expressed in blood prior to flares, and compared them with data profiles derived from synovial single-cell RNA sequencing.
To validate the findings, the researchers used flow cytometry and sorted blood-cell RNA sequencing of samples from an additional 19 patients with RA.
They found that a total of 2,613 genes were differentially expressed during a flare, compared with baseline, and that expression of 1,437 of these genes was increased during a flare, with the remaining 1,176 decreased during flares.
Before the storm
Focusing on two flare-antecedent clusters of genes, they identified one cluster of transcripts that increased 2 weeks before a flare, enriched with genes coding for developmental pathways for naive B cells (that is, not yet exposed to antigens) and leukocytes.
The second cluster included gene transcripts that increased during the week before a flare, then decreased over the duration of the flare. Genes in this cluster were enriched for pathways that were unexpected in typical blood specimens, including genes involved in cartilage morphogenesis, endochondral bone growth, and extracellular matrix organization. The gene activity suggested the presence of a mesenchymal cell, they wrote.
The RNA expression profiles of these newly identified PRIME cells were very similar to those of synovial fibroblasts, and the investigators speculated that PRIME cells may be synovial fibroblast precursors.
They proposed a model of RA exacerbation in which PRIME cells become activated by B cells in the weeks immediately preceding a flare, and then migrate out of blood into the synovium.
The investigators are currently investigating “how reproducible this signature is in different flares in patients on different types of background therapy, and then we’re very interested in looking at the upstream triggers of the B cell and the PRIME cell,” Dr. Orange said.
“One of the reasons this is very exciting is that there are these signatures that can be found when patients are clearly asymptomatic but about to flare, and if we can intervene at that time, then the patients won’t have to live through a flare, they won’t have to have that experience,” she said.
Pros and cons
In an editorial accompanying the study, Ellen M. Gravallese, MD, from Brigham and Women’s Hospital in Boston and William H. Robinson, MD, PhD, from Stanford (Calif.) University and the Veterans Affairs Palo Alto (Calif.) Health Care System wrote that the study demonstrates an important method for identifying genetic contributions to many different types of disease.
“Orange and colleagues show that intensively collected longitudinal data from a small sample of patients can be used to identify dysregulated transcriptional signatures that are not recognized by classical cross-sectional studies. This study illustrates the exciting potential of longitudinal genomics to identify key antecedents of disease flares in an approach that may be applicable to the investigation of pathogenic and protective immune responses in a wide range of human diseases,” they wrote.
Rheumatology researcher Christopher D. Buckley, MBBS, DPhil, from the University of Birmingham (England), who was not involved in the study, said that the use of blood samples is both a strength and a weakness of the study.
“Blood is much easier to get than synovial tissue, but synovial tissue is important. If I’m trying to look at the blood and trying to make an inference about what’s going on in the synovium, if I don’t look at the synovium I don’t know what the link between the blood and synovium is,” he said in an interview.
On the plus side, “the big advantage about looking at blood is that you do multiple time points, which is really cool,” he said.
Dr. Buckley is a coauthor of a recent paper in Nature Medicine – published after the study by Dr. Orange and colleagues was accepted by the New England Journal of Medicine – showing that a population of macrophages in synovium was highly predictive of remission in patients with RA, and that therapeutic modulation of these macrophages has the potential as a treatment strategy for RA.
“We are very keen to understand the cellular basis of disease. We’re very good at understanding genes, but genes have to work in cells, and cells make organs, so the cells are critical,” he said.
The paper adds fuel to a controversy that has been raging among rheumatology researchers for more than a decade: the “flying fibroblast” hypothesis, which suggests that fibroblasts can migrate from one joint to another, hence spreading the disease in a manner akin to cancer metastases.
“It’s been quite controversial whether these cells like fibroblasts can exist in the blood, or whether they’re found in sufficient number in the blood,” Dr. Buckley said. “The fact that they have identified these PRIME cells is fascinating, because that’s going to cause us to go back and reinvestigate the whole flying fibroblast story.”
His colleague John Isaacs, MBBS, PhD, from Newcastle (England) University, is principal investigator for the BIO-FLARE study, in which participants with RA stop taking their disease-modifying antirheumatic drugs under close supervision of researchers. The investigators then study the patients looking for flare signals as well as the biology of flares themselves.
“As it happens, our protocols would not pick up this particular cell, because we have not been focusing on the stroma, at least not in peripheral blood. We’ve all been looking at synovium as part of BIO-FLARE,” he said in an interview. “We will be looking for this cell now that we have seen this research, and certainly we would want to replicate.”
He agreed with Dr. Buckley’s observation that the PRIME cell data may revive the flying fibroblast hypothesis. “This is a great paper in a top clinical journal. What isn’t there is mechanism. That’s the thing we’re all going to want to understand now: Where do the cells come from, how do they actually trigger flares, and how do they go down as flare starts?”
Both Dr. Buckley and Dr. Isaacs agreed that the study findings point to important new avenues of research, but also noted that the study was small, involving a total of only 23 patients, and that replication of the findings and elucidation of the mechanism of PRIME cell generation and disposition will be required.
The study was supported by grants from the National Institutes of Health, Simons Foundation, Robertson Foundation, Rheumatology Research Foundation, Bernard and Irene Schwartz Foundation, the Iris and Junming Le Foundation, and Rockefeller University. Dr. Orange disclosed a provisional patent for the discovery of the PRIME cells. Dr. Buckley and Dr. Isaacs reported no relevant conflicts of interest.
SOURCE: Orange DE et al. N Engl J Med. 2020 Jul 15. doi: 10.1056/NEJMoa2004114.
A newly identified circulating cell type may be a reliable marker for impending RA flares. The discovery and description of the cells, which bear a “striking” similarity to synovial fibroblasts, provide important clues to the origins of RA and progressive joint inflammation, investigators say.
By studying longitudinally collected blood samples from four patients with RA over 4 years, Dana E. Orange, MD and colleagues at Rockefeller University, New York, identified a pattern of B-cell activation and expansion of circulating cells that are negative for CD45 and CD31 expression, and positive for PDPN, dubbed preinflammatory mesenchymal or “PRIME” cells.
Expansion of PRIME cells in circulation increased dramatically in the weeks leading up to a flare and decreased during a flare, suggesting the possibility of a serum assay for predicting flares and allowing for early intervention to ameliorate or prevent disabling consequences, the investigators wrote in a study published in the New England Journal of Medicine.
“Our hope is that this will be a diagnostic in the future, but we need to study it in more patients to see how it will perform,” Dr. Orange said in an interview, adding that the cells, if shown to be pathogenic, could also be targets for new therapeutic strategies.
RNA sequencing
Dr. Orange and colleagues discovered the PRIME cells through a novel clinical and technical protocol involving home collection of blood by patients and longitudinal RNA sequencing to study gene expression profiles during times of both disease quiescence and flares, and noticed a distinct pattern of PRIME cell expansion, depletion, and gene expression.
“Looking at their gene expression profiles, they overlapped with fibroblasts that reside in inflamed rheumatoid arthritis synovium, and in an animal model those types of fibroblasts were important for allowing entry of inflammatory infiltrates around the joint,” she said.
PRIME cells may be a precursor of synovial fibroblasts, which have been implicated by some researchers in the spread of RA between joints, Dr. Orange added.
Patients do homework
The investigators began by enrolling four patients, followed for 1-4 years, who met 2010 American College of Rheumatology–European League Against Rheumatism criteria and who were seropositive for anti–cyclic citrullinated peptide antibodies.
They assessed disease activity from patient homes weekly or during escalation of flares up to four times daily, with the Routine Assessment of Patient Index Data 3 (RAPID3) questionnaire, as well as monthly clinic visits. At clinic visits during flares, disease activity was assessed using both the RAPID3 and 28-joint Disease Activity Score.
The patients performed fingerstick blood collection and mailed the samples overnight each week to Rockefeller University, where RNA was extracted and sequenced. The investigators identified gene transcripts that were differentially expressed in blood prior to flares, and compared them with data profiles derived from synovial single-cell RNA sequencing.
To validate the findings, the researchers used flow cytometry and sorted blood-cell RNA sequencing of samples from an additional 19 patients with RA.
They found that a total of 2,613 genes were differentially expressed during a flare, compared with baseline, and that expression of 1,437 of these genes was increased during a flare, with the remaining 1,176 decreased during flares.
Before the storm
Focusing on two flare-antecedent clusters of genes, they identified one cluster of transcripts that increased 2 weeks before a flare, enriched with genes coding for developmental pathways for naive B cells (that is, not yet exposed to antigens) and leukocytes.
The second cluster included gene transcripts that increased during the week before a flare, then decreased over the duration of the flare. Genes in this cluster were enriched for pathways that were unexpected in typical blood specimens, including genes involved in cartilage morphogenesis, endochondral bone growth, and extracellular matrix organization. The gene activity suggested the presence of a mesenchymal cell, they wrote.
The RNA expression profiles of these newly identified PRIME cells were very similar to those of synovial fibroblasts, and the investigators speculated that PRIME cells may be synovial fibroblast precursors.
They proposed a model of RA exacerbation in which PRIME cells become activated by B cells in the weeks immediately preceding a flare, and then migrate out of blood into the synovium.
The investigators are currently investigating “how reproducible this signature is in different flares in patients on different types of background therapy, and then we’re very interested in looking at the upstream triggers of the B cell and the PRIME cell,” Dr. Orange said.
“One of the reasons this is very exciting is that there are these signatures that can be found when patients are clearly asymptomatic but about to flare, and if we can intervene at that time, then the patients won’t have to live through a flare, they won’t have to have that experience,” she said.
Pros and cons
In an editorial accompanying the study, Ellen M. Gravallese, MD, from Brigham and Women’s Hospital in Boston and William H. Robinson, MD, PhD, from Stanford (Calif.) University and the Veterans Affairs Palo Alto (Calif.) Health Care System wrote that the study demonstrates an important method for identifying genetic contributions to many different types of disease.
“Orange and colleagues show that intensively collected longitudinal data from a small sample of patients can be used to identify dysregulated transcriptional signatures that are not recognized by classical cross-sectional studies. This study illustrates the exciting potential of longitudinal genomics to identify key antecedents of disease flares in an approach that may be applicable to the investigation of pathogenic and protective immune responses in a wide range of human diseases,” they wrote.
Rheumatology researcher Christopher D. Buckley, MBBS, DPhil, from the University of Birmingham (England), who was not involved in the study, said that the use of blood samples is both a strength and a weakness of the study.
“Blood is much easier to get than synovial tissue, but synovial tissue is important. If I’m trying to look at the blood and trying to make an inference about what’s going on in the synovium, if I don’t look at the synovium I don’t know what the link between the blood and synovium is,” he said in an interview.
On the plus side, “the big advantage about looking at blood is that you do multiple time points, which is really cool,” he said.
Dr. Buckley is a coauthor of a recent paper in Nature Medicine – published after the study by Dr. Orange and colleagues was accepted by the New England Journal of Medicine – showing that a population of macrophages in synovium was highly predictive of remission in patients with RA, and that therapeutic modulation of these macrophages has the potential as a treatment strategy for RA.
“We are very keen to understand the cellular basis of disease. We’re very good at understanding genes, but genes have to work in cells, and cells make organs, so the cells are critical,” he said.
The paper adds fuel to a controversy that has been raging among rheumatology researchers for more than a decade: the “flying fibroblast” hypothesis, which suggests that fibroblasts can migrate from one joint to another, hence spreading the disease in a manner akin to cancer metastases.
“It’s been quite controversial whether these cells like fibroblasts can exist in the blood, or whether they’re found in sufficient number in the blood,” Dr. Buckley said. “The fact that they have identified these PRIME cells is fascinating, because that’s going to cause us to go back and reinvestigate the whole flying fibroblast story.”
His colleague John Isaacs, MBBS, PhD, from Newcastle (England) University, is principal investigator for the BIO-FLARE study, in which participants with RA stop taking their disease-modifying antirheumatic drugs under close supervision of researchers. The investigators then study the patients looking for flare signals as well as the biology of flares themselves.
“As it happens, our protocols would not pick up this particular cell, because we have not been focusing on the stroma, at least not in peripheral blood. We’ve all been looking at synovium as part of BIO-FLARE,” he said in an interview. “We will be looking for this cell now that we have seen this research, and certainly we would want to replicate.”
He agreed with Dr. Buckley’s observation that the PRIME cell data may revive the flying fibroblast hypothesis. “This is a great paper in a top clinical journal. What isn’t there is mechanism. That’s the thing we’re all going to want to understand now: Where do the cells come from, how do they actually trigger flares, and how do they go down as flare starts?”
Both Dr. Buckley and Dr. Isaacs agreed that the study findings point to important new avenues of research, but also noted that the study was small, involving a total of only 23 patients, and that replication of the findings and elucidation of the mechanism of PRIME cell generation and disposition will be required.
The study was supported by grants from the National Institutes of Health, Simons Foundation, Robertson Foundation, Rheumatology Research Foundation, Bernard and Irene Schwartz Foundation, the Iris and Junming Le Foundation, and Rockefeller University. Dr. Orange disclosed a provisional patent for the discovery of the PRIME cells. Dr. Buckley and Dr. Isaacs reported no relevant conflicts of interest.
SOURCE: Orange DE et al. N Engl J Med. 2020 Jul 15. doi: 10.1056/NEJMoa2004114.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: A newly identified cell type may be predictive of RA flares.
Major finding: Preinflammatory mesenchymal cells were expanded in the week preceding a flare and decreased during the flare.
Study details: A longitudinal observational study of 23 patients with RA.
Disclosures: The study was supported by grants from the National Institutes of Health, Simons Foundation, Robertson Foundation, Rheumatology Research Foundation, Bernard and Irene Schwartz Foundation, the Iris and Junming Le Foundation, and Rockefeller University. Dr. Orange disclosed a provisional patent for the discovery of the preinflammatory mesenchymal cells.
Source: Orange DE et al. N Engl J Med. 2020 Jul 15. doi: 10.1056/NEJMoa2004114.
Novel program cuts weight retention after gestational diabetes
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ADA 2020
COVID-19: A primary care perspective
With the COVID-19 pandemic, we are experiencing a once-in-a-100-year event. Dr. Steven A. Schulz, who is serving children on the front line in upstate New York, and I outline some of the challenges primary care pediatricians have been facing and solutions that have succeeded.
Reduction in direct patient care and its consequences
Because of the unknowns of COVID-19, many parents have not wanted to bring their children to a medical office because of fear of contracting SARS-CoV-2. At the same time, pediatricians have restricted in-person visits to prevent spread of SARS-CoV-2 and to help flatten the curve of infection. Use of pediatric medical professional services, compared with last year, dropped by 52% in March 2020 and by 58% in April, according to FAIR Health, a nonprofit organization that manages a database of 31 million claims. This is resulting in decreased immunization rates, which increases concern for secondary spikes of other preventable illnesses; for example, data from the Centers for Disease Control and Prevention showed that, from mid-March to mid-April 2020, physicians in the Vaccines for Children program ordered 2.5 million fewer doses of vaccines and 250,000 fewer doses of measles-containing vaccines, compared with the same period in 2019. Fewer children are being seen for well visits, which means opportunities are lost for adequate monitoring of growth, development, physical wellness, and social determinants of health.
This is occurring at a time when families have been experiencing increased stress in terms of finances, social isolation, finding adequate child care, and serving as parent, teacher, and breadwinner. An increase in injuries is occurring because of inadequate parental supervision because many parents have been distracted while working from home. An increase in cases of severe abuse is occurring because schools, child care providers, physicians, and other mandated reporters in the community have decreased interaction with children. Children’s Hospital Colorado in Colorado Springs saw a 118% increase in the number of trauma cases in its ED between January and April 2020. Some of these were accidental injuries caused by falls or bicycle accidents, but there was a 200% increase in nonaccidental trauma, which was associated with a steep fall in calls to the state’s child abuse hotline. Academic gains are being lost, and there has been worry for a prolonged “summer slide” risk, especially for children living in poverty and children with developmental disabilities.
The COVID-19 pandemic also is affecting physicians and staff. As frontline personnel, we are at risk to contract the virus, and news media reminds us of severe illness and deaths among health care workers. The pandemic is affecting financial viability; estimated revenue of pediatric offices fell by 45% in March 2020 and 48% in April, compared with the previous year, according to FAIR Health. Nurses and staff have been furloughed. Practices have had to apply for grants and Paycheck Protection Program funds while extending credit lines.
Limited testing capability for SARS-CoV-2
Testing for SARS-CoV-2 has been variably available. There have been problems with false positive and especially false negative results (BMJ. 2020 May 12. doi: 10.1136/bmj.m1808).The best specimen collection method has yet to be determined. Blood testing for antibody has been touted, but it remains unclear if there is clinical benefit because a positive result offers no guarantee of immunity, and immunity may quickly wane. Perhaps widespread primary care office–based testing will be in place by the fall, with hope for future reliable point of care results.
Evolving knowledge regarding SARS-CoV-2 and MIS-C
It initially was thought that children were relatively spared from serious illness caused by COVID-19. Then reports of cases of newly identified multisystem inflammatory syndrome of children occurred. It has been unclear how children contribute to the spread of COVID-19 illness, although emerging evidence indicates it is lower than adult transmission. What will happen when children return to school and daycare in the fall?
The challenges have led to creative solutions for how to deliver care.
Adapting to telehealth to provide care
At least for the short term, HIPAA regulations have been relaxed to allow for video visits using platforms such as FaceTime, Skype, Zoom, Doximity, and Doxy.me. Some of these platforms are HIPAA compliant and will be long-term solutions; however, electronic medical record portals allowing for video visits are the more secure option, according to HIPAA.
It has been a learning experience to see what can be accomplished with a video visit. Taking a history and visual examination of injuries and rashes has been possible. Addressing mental health concerns through the video exchange generally has been effective.
However, video visits change the provider-patient interpersonal dynamic and offer only visual exam capabilities, compared with an in-person visit. We cannot look in ears, palpate a liver and spleen, touch and examine a joint or bone, or feel a rash. Video visits also are dependent on the quality of patient Internet access, sufficient data plans, and mutual capabilities to address the inevitable technological glitches on the provider’s end as well. Expanding information technology infrastructure ability and added licensure costs have occurred. Practices and health systems have been working with insurance companies to ensure telephone and video visits are reimbursed on a comparable level to in-office visits.
A new type of office visit and developing appropriate safety plans
Patients must be universally screened prior to arrival during appointment scheduling for well and illness visits. Patients aged older than 2 years and caregivers must wear masks on entering the facility. In many practices, patients are scheduled during specific sick or well visit time slots throughout the day. Waiting rooms chairs need to be spaced for 6-foot social distancing, and cars in the parking lot often serve as waiting rooms until staff can meet patients at the door and take them to the exam room. Alternate entrances, car-side exams, and drive-by and/or tent testing facilities often have become part of the new normal everyday practice. Creating virtual visit time blocks in provider’s schedules has allowed for decreased office congestion. Patients often are checked out from their room, as opposed to waiting in a line at a check out desk. Nurse triage protocols also have been adapted and enhanced to meet needs and concerns.
With the need for summer physicals and many regions opening up, a gradual return toward baseline has been evolving, although some of the twists of a “new normal” will stay in place. The new normal has been for providers and staff to wear surgical masks and face shields; sometimes N95 masks, gloves, and gowns have been needed. Cleaning rooms and equipment between patient visits has become a major, new time-consuming task. Acquiring and maintaining adequate supplies has been a challenge.
Summary
The American Academy of Pediatrics, CDC, and state and local health departments have been providing informative and regular updates, webinars, and best practices guidelines. Pediatricians, community organizations, schools, and mental health professionals have been collaborating, overcoming hurdles, and working together to help mitigate the effects of the pandemic on children, their families, and our communities. Continued education, cooperation, and adaptation will be needed in the months ahead. If there is a silver lining to this pandemic experience, it may be that families have grown closer together as they sheltered in place (and we have grown closer to our own families as well). One day perhaps a child who lived through this pandemic might be asked what it was like, and their recollection might be that it was a wonderful time because their parents stayed home all the time, took care of them, taught them their school work, and took lots of long family walks.
Dr. Schulz is pediatric medical director, Rochester (N.Y.) Regional Health. Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz and Dr. Pichichero said they have no relevant financial disclosures. Email them at pdnews@mdedge.com.
This article was updated 7/16/2020.
With the COVID-19 pandemic, we are experiencing a once-in-a-100-year event. Dr. Steven A. Schulz, who is serving children on the front line in upstate New York, and I outline some of the challenges primary care pediatricians have been facing and solutions that have succeeded.
Reduction in direct patient care and its consequences
Because of the unknowns of COVID-19, many parents have not wanted to bring their children to a medical office because of fear of contracting SARS-CoV-2. At the same time, pediatricians have restricted in-person visits to prevent spread of SARS-CoV-2 and to help flatten the curve of infection. Use of pediatric medical professional services, compared with last year, dropped by 52% in March 2020 and by 58% in April, according to FAIR Health, a nonprofit organization that manages a database of 31 million claims. This is resulting in decreased immunization rates, which increases concern for secondary spikes of other preventable illnesses; for example, data from the Centers for Disease Control and Prevention showed that, from mid-March to mid-April 2020, physicians in the Vaccines for Children program ordered 2.5 million fewer doses of vaccines and 250,000 fewer doses of measles-containing vaccines, compared with the same period in 2019. Fewer children are being seen for well visits, which means opportunities are lost for adequate monitoring of growth, development, physical wellness, and social determinants of health.
This is occurring at a time when families have been experiencing increased stress in terms of finances, social isolation, finding adequate child care, and serving as parent, teacher, and breadwinner. An increase in injuries is occurring because of inadequate parental supervision because many parents have been distracted while working from home. An increase in cases of severe abuse is occurring because schools, child care providers, physicians, and other mandated reporters in the community have decreased interaction with children. Children’s Hospital Colorado in Colorado Springs saw a 118% increase in the number of trauma cases in its ED between January and April 2020. Some of these were accidental injuries caused by falls or bicycle accidents, but there was a 200% increase in nonaccidental trauma, which was associated with a steep fall in calls to the state’s child abuse hotline. Academic gains are being lost, and there has been worry for a prolonged “summer slide” risk, especially for children living in poverty and children with developmental disabilities.
The COVID-19 pandemic also is affecting physicians and staff. As frontline personnel, we are at risk to contract the virus, and news media reminds us of severe illness and deaths among health care workers. The pandemic is affecting financial viability; estimated revenue of pediatric offices fell by 45% in March 2020 and 48% in April, compared with the previous year, according to FAIR Health. Nurses and staff have been furloughed. Practices have had to apply for grants and Paycheck Protection Program funds while extending credit lines.
Limited testing capability for SARS-CoV-2
Testing for SARS-CoV-2 has been variably available. There have been problems with false positive and especially false negative results (BMJ. 2020 May 12. doi: 10.1136/bmj.m1808).The best specimen collection method has yet to be determined. Blood testing for antibody has been touted, but it remains unclear if there is clinical benefit because a positive result offers no guarantee of immunity, and immunity may quickly wane. Perhaps widespread primary care office–based testing will be in place by the fall, with hope for future reliable point of care results.
Evolving knowledge regarding SARS-CoV-2 and MIS-C
It initially was thought that children were relatively spared from serious illness caused by COVID-19. Then reports of cases of newly identified multisystem inflammatory syndrome of children occurred. It has been unclear how children contribute to the spread of COVID-19 illness, although emerging evidence indicates it is lower than adult transmission. What will happen when children return to school and daycare in the fall?
The challenges have led to creative solutions for how to deliver care.
Adapting to telehealth to provide care
At least for the short term, HIPAA regulations have been relaxed to allow for video visits using platforms such as FaceTime, Skype, Zoom, Doximity, and Doxy.me. Some of these platforms are HIPAA compliant and will be long-term solutions; however, electronic medical record portals allowing for video visits are the more secure option, according to HIPAA.
It has been a learning experience to see what can be accomplished with a video visit. Taking a history and visual examination of injuries and rashes has been possible. Addressing mental health concerns through the video exchange generally has been effective.
However, video visits change the provider-patient interpersonal dynamic and offer only visual exam capabilities, compared with an in-person visit. We cannot look in ears, palpate a liver and spleen, touch and examine a joint or bone, or feel a rash. Video visits also are dependent on the quality of patient Internet access, sufficient data plans, and mutual capabilities to address the inevitable technological glitches on the provider’s end as well. Expanding information technology infrastructure ability and added licensure costs have occurred. Practices and health systems have been working with insurance companies to ensure telephone and video visits are reimbursed on a comparable level to in-office visits.
A new type of office visit and developing appropriate safety plans
Patients must be universally screened prior to arrival during appointment scheduling for well and illness visits. Patients aged older than 2 years and caregivers must wear masks on entering the facility. In many practices, patients are scheduled during specific sick or well visit time slots throughout the day. Waiting rooms chairs need to be spaced for 6-foot social distancing, and cars in the parking lot often serve as waiting rooms until staff can meet patients at the door and take them to the exam room. Alternate entrances, car-side exams, and drive-by and/or tent testing facilities often have become part of the new normal everyday practice. Creating virtual visit time blocks in provider’s schedules has allowed for decreased office congestion. Patients often are checked out from their room, as opposed to waiting in a line at a check out desk. Nurse triage protocols also have been adapted and enhanced to meet needs and concerns.
With the need for summer physicals and many regions opening up, a gradual return toward baseline has been evolving, although some of the twists of a “new normal” will stay in place. The new normal has been for providers and staff to wear surgical masks and face shields; sometimes N95 masks, gloves, and gowns have been needed. Cleaning rooms and equipment between patient visits has become a major, new time-consuming task. Acquiring and maintaining adequate supplies has been a challenge.
Summary
The American Academy of Pediatrics, CDC, and state and local health departments have been providing informative and regular updates, webinars, and best practices guidelines. Pediatricians, community organizations, schools, and mental health professionals have been collaborating, overcoming hurdles, and working together to help mitigate the effects of the pandemic on children, their families, and our communities. Continued education, cooperation, and adaptation will be needed in the months ahead. If there is a silver lining to this pandemic experience, it may be that families have grown closer together as they sheltered in place (and we have grown closer to our own families as well). One day perhaps a child who lived through this pandemic might be asked what it was like, and their recollection might be that it was a wonderful time because their parents stayed home all the time, took care of them, taught them their school work, and took lots of long family walks.
Dr. Schulz is pediatric medical director, Rochester (N.Y.) Regional Health. Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz and Dr. Pichichero said they have no relevant financial disclosures. Email them at pdnews@mdedge.com.
This article was updated 7/16/2020.
With the COVID-19 pandemic, we are experiencing a once-in-a-100-year event. Dr. Steven A. Schulz, who is serving children on the front line in upstate New York, and I outline some of the challenges primary care pediatricians have been facing and solutions that have succeeded.
Reduction in direct patient care and its consequences
Because of the unknowns of COVID-19, many parents have not wanted to bring their children to a medical office because of fear of contracting SARS-CoV-2. At the same time, pediatricians have restricted in-person visits to prevent spread of SARS-CoV-2 and to help flatten the curve of infection. Use of pediatric medical professional services, compared with last year, dropped by 52% in March 2020 and by 58% in April, according to FAIR Health, a nonprofit organization that manages a database of 31 million claims. This is resulting in decreased immunization rates, which increases concern for secondary spikes of other preventable illnesses; for example, data from the Centers for Disease Control and Prevention showed that, from mid-March to mid-April 2020, physicians in the Vaccines for Children program ordered 2.5 million fewer doses of vaccines and 250,000 fewer doses of measles-containing vaccines, compared with the same period in 2019. Fewer children are being seen for well visits, which means opportunities are lost for adequate monitoring of growth, development, physical wellness, and social determinants of health.
This is occurring at a time when families have been experiencing increased stress in terms of finances, social isolation, finding adequate child care, and serving as parent, teacher, and breadwinner. An increase in injuries is occurring because of inadequate parental supervision because many parents have been distracted while working from home. An increase in cases of severe abuse is occurring because schools, child care providers, physicians, and other mandated reporters in the community have decreased interaction with children. Children’s Hospital Colorado in Colorado Springs saw a 118% increase in the number of trauma cases in its ED between January and April 2020. Some of these were accidental injuries caused by falls or bicycle accidents, but there was a 200% increase in nonaccidental trauma, which was associated with a steep fall in calls to the state’s child abuse hotline. Academic gains are being lost, and there has been worry for a prolonged “summer slide” risk, especially for children living in poverty and children with developmental disabilities.
The COVID-19 pandemic also is affecting physicians and staff. As frontline personnel, we are at risk to contract the virus, and news media reminds us of severe illness and deaths among health care workers. The pandemic is affecting financial viability; estimated revenue of pediatric offices fell by 45% in March 2020 and 48% in April, compared with the previous year, according to FAIR Health. Nurses and staff have been furloughed. Practices have had to apply for grants and Paycheck Protection Program funds while extending credit lines.
Limited testing capability for SARS-CoV-2
Testing for SARS-CoV-2 has been variably available. There have been problems with false positive and especially false negative results (BMJ. 2020 May 12. doi: 10.1136/bmj.m1808).The best specimen collection method has yet to be determined. Blood testing for antibody has been touted, but it remains unclear if there is clinical benefit because a positive result offers no guarantee of immunity, and immunity may quickly wane. Perhaps widespread primary care office–based testing will be in place by the fall, with hope for future reliable point of care results.
Evolving knowledge regarding SARS-CoV-2 and MIS-C
It initially was thought that children were relatively spared from serious illness caused by COVID-19. Then reports of cases of newly identified multisystem inflammatory syndrome of children occurred. It has been unclear how children contribute to the spread of COVID-19 illness, although emerging evidence indicates it is lower than adult transmission. What will happen when children return to school and daycare in the fall?
The challenges have led to creative solutions for how to deliver care.
Adapting to telehealth to provide care
At least for the short term, HIPAA regulations have been relaxed to allow for video visits using platforms such as FaceTime, Skype, Zoom, Doximity, and Doxy.me. Some of these platforms are HIPAA compliant and will be long-term solutions; however, electronic medical record portals allowing for video visits are the more secure option, according to HIPAA.
It has been a learning experience to see what can be accomplished with a video visit. Taking a history and visual examination of injuries and rashes has been possible. Addressing mental health concerns through the video exchange generally has been effective.
However, video visits change the provider-patient interpersonal dynamic and offer only visual exam capabilities, compared with an in-person visit. We cannot look in ears, palpate a liver and spleen, touch and examine a joint or bone, or feel a rash. Video visits also are dependent on the quality of patient Internet access, sufficient data plans, and mutual capabilities to address the inevitable technological glitches on the provider’s end as well. Expanding information technology infrastructure ability and added licensure costs have occurred. Practices and health systems have been working with insurance companies to ensure telephone and video visits are reimbursed on a comparable level to in-office visits.
A new type of office visit and developing appropriate safety plans
Patients must be universally screened prior to arrival during appointment scheduling for well and illness visits. Patients aged older than 2 years and caregivers must wear masks on entering the facility. In many practices, patients are scheduled during specific sick or well visit time slots throughout the day. Waiting rooms chairs need to be spaced for 6-foot social distancing, and cars in the parking lot often serve as waiting rooms until staff can meet patients at the door and take them to the exam room. Alternate entrances, car-side exams, and drive-by and/or tent testing facilities often have become part of the new normal everyday practice. Creating virtual visit time blocks in provider’s schedules has allowed for decreased office congestion. Patients often are checked out from their room, as opposed to waiting in a line at a check out desk. Nurse triage protocols also have been adapted and enhanced to meet needs and concerns.
With the need for summer physicals and many regions opening up, a gradual return toward baseline has been evolving, although some of the twists of a “new normal” will stay in place. The new normal has been for providers and staff to wear surgical masks and face shields; sometimes N95 masks, gloves, and gowns have been needed. Cleaning rooms and equipment between patient visits has become a major, new time-consuming task. Acquiring and maintaining adequate supplies has been a challenge.
Summary
The American Academy of Pediatrics, CDC, and state and local health departments have been providing informative and regular updates, webinars, and best practices guidelines. Pediatricians, community organizations, schools, and mental health professionals have been collaborating, overcoming hurdles, and working together to help mitigate the effects of the pandemic on children, their families, and our communities. Continued education, cooperation, and adaptation will be needed in the months ahead. If there is a silver lining to this pandemic experience, it may be that families have grown closer together as they sheltered in place (and we have grown closer to our own families as well). One day perhaps a child who lived through this pandemic might be asked what it was like, and their recollection might be that it was a wonderful time because their parents stayed home all the time, took care of them, taught them their school work, and took lots of long family walks.
Dr. Schulz is pediatric medical director, Rochester (N.Y.) Regional Health. Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz and Dr. Pichichero said they have no relevant financial disclosures. Email them at pdnews@mdedge.com.
This article was updated 7/16/2020.
PD-1 Signaling in Extramammary Paget Disease
Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts that presents as an erythematous patch on cutaneous sites rich with apocrine glands.1 Primary EMPD can be in situ or invasive with the potential to become metastatic.2 Treatment of primary EMPD is challenging due to the difficulty of achieving clear surgical margins, as the tumor has microscopic spread throughout the epidermis in a skipping fashion.3 Mohs micrographic surgery is the treatment of choice; however, there is a clinical need to identify additional treatment modalities, especially for patients with unresectable, invasive, or metastatic primary EMPD,4 which partly is due to lack of data to understand the pathogenesis of primary EMPD. Recently, there have been studies investigating the genetic characteristics of EMPD tumors. The interaction between the programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) is one of the pathways recently studied and has been reported to be a potential target in EMPD.5-7 Programmed cell death receptor 1 signaling constitutes an immune checkpoint pathway that regulates the activation of tumor-specific T cells.8 In several malignancies, cancer cells express PD-L1 on their surface to activate PD-1 signaling in T cells as a mechanism to dampen the tumor-specific immune response and evade antitumor immunity.9 Thus, blocking PD-1 signaling widely is used to activate tumor-specific T cells and decrease tumor burden.10 Given the advances of immunotherapy in many neoplasms and the paucity of effective agents to treat EMPD, this article serves to shed light on recent data studying PD-1 signaling in EMPD and highlights the potential clinical use of immunotherapy for EMPD.
EMPD and Its Subtypes
Extramammary Paget disease is a rare adenocarcinoma typically affecting older patients (age >60 years) in cutaneous sites with abundant apocrine glands such as the genital and perianal skin.3 Extramammary Paget disease presents as an erythematous patch and frequently is treated initially as a skin dermatosis, resulting in a delay in diagnosis. Histologically, EMPD is characterized by the presence of single cells or a nest of cells having abundant pale cytoplasm and large vesicular nuclei distributed in the epidermis in a pagetoid fashion.11
Extramammary Paget disease can be primary or secondary; the 2 subtypes behave differently both clinically and prognostically. Although primary EMPD is considered to be an adnexal carcinoma of the apocrine gland ducts, secondary EMPD is considered to be an intraepithelial extension of malignant cells from an underlying internal neoplasm.12 The underlying malignancies usually are located within dermal adnexal glands or organs in the vicinity of the cutaneous lesion, such as the colon in the case of perianal EMPD. Histologically, primary and secondary EMPD can be differentiated based on their immunophenotypic staining profiles. Although all cases of EMPD show positive immunohistochemistry staining for cytokeratin 7, carcinoembryonic antigen, and epithelial membrane antigen, only primary EMPD will additionally stain for GCDFP-15 (gross cystic disease fluid protein 15) and GATA.11 Regardless of the immunohistochemistry stains, every patient newly diagnosed with EMPD deserves a full workup for malignancy screening, including a colonoscopy, cystoscopy, mammography and Papanicolaou test in women, pelvic ultrasound, and computed tomography of the abdomen and pelvis.13
The first-line treatment of EMPD is surgery; however, obtaining clear surgical margins can be a challenge, with high recurrence rates due to the microscopic spread of the disease throughout the epidermis.4 In addition, anatomic location affects the surgical approach and patient survival. Recent studies on EMPD mortality outcomes in women show that mortality is higher in patients with vaginal EMPD than in those with vulvar/labial EMPD, partly due to the sensitive location that makes it difficult to perform wide local excisions.13,14 Assessing the entire margins with tissue preservation using Mohs micrographic surgery has been shown to be successful in decreasing the recurrence rate, especially when coupled with the use of cytokeratin 7 immunohistochemistry.4 Other treatment modalities include radiation, topical imiquimod, and photodynamic therapy.15,16 Regardless of treatment modality, EMPD requires long‐term follow-up to monitor for disease recurrence, regional lymphadenopathy, distant metastasis, or development of an internal malignancy.
The pathogenesis of primary EMPD remains unclear. The tumor is thought to be derived from Toker cells, which are pluripotent adnexal stem cells located in the epidermis that normally give rise to apocrine glands.17 There have been few studies investigating the genetic characteristics of EMPD lesions in an attempt to understand pathogenesis as well as to find druggable targets. Current data for targeted therapy have focused on HER2 (human epidermal growth factor receptor 2) hormone receptor expression,18 ERBB (erythroblastic oncogene B) amplification,19 CDK4 (cyclin-dependent kinase 4)–cyclin D1 signaling,20 and most recently PD-1/PD-L1 pathway.5-7
PD-1 Expression in EMPD: Implication for Immunotherapy
Most tumors display novel antigens that are recognized by the host immune system and thus stimulate cell-mediated and humoral pathways. The immune system naturally provides regulatory immune checkpoints to T cell–mediated immune responses. One of these checkpoints involves the interaction between PD-1 on T cells and its ligand PD-L1 on tumor cells.21 When PD-1 binds to PD-L1 on tumor cells, there is inhibition of T-cell proliferation, a decrease in cytokine production, and induction of T-cell cytolysis.22 The Figure summarizes the dynamics for T-cell regulation.

Naturally, tumor-infiltrating T cells trigger their own inhibition by binding to PD-L1. However, certain tumor cells constitutively upregulate the expression of PD-L1. With that, the tumor cells gain the ability to suppress T cells and avoid T cell–mediated cytotoxicity,23 which is known as the adoptive immune resistance mechanism. There have been several studies in the literature investigating the PD-1 signaling pathway in EMPD as a way to determine if EMPD would be susceptible to immune checkpoint blockade. The success of checkpoint inhibitor immunotherapy generally correlates with increased PD-L1 expression by tumor cells.
One study evaluated the expression of PD-L1 in tumor cells and tumor-infiltrating T cells in 18 cases of EMPD.6 The authors identified that even though tumor cell PD-L1 expression was detected in only 3 (17%) cases, tumor-infiltrating lymphocytes expressed PD-L1 in the majority of the cases analyzed and in all of the cases positive for tumor cell PD-L1.6
Another study evaluated PD-1 and PD-L1 expression in EMPD tumor cells and tumor-associated immune infiltrate.5 They found that PD-1 was expressed heavily by the tumor-associated immune infiltrate in all EMPD cases analyzed. Similar to the previously mentioned study,6 PD-L1 was expressed by tumor cells in a few cases only. Interestingly, they found that the density of CD3 in the tumor-associated immune infiltrate was significantly (P=.049) higher in patients who were alive than in those who died, suggesting the importance of an exuberant T-cell response for survival in EMPD.5
A third study investigated protein expression of the B7 family members as well as PD-1 and PD-L1/2 in 55 EMPD samples. In this study the authors also found that tumor cell PD-L1 was minimal. Interestingly, they also found that tumor cells expressed B7 proteins in the majority of the cases.7
Finally, another study examined activity levels of T cells in EMPD by measuring the number and expression levels of cytotoxic T-cell cytokines.24 The authors first found that EMPD tumors had a significantly higher number of CD8+ tumor-infiltrating lymphocytes compared to peripheral blood (P<.01). These CD8+ tumor-infiltrating lymphocytes also had a significantly higher expression of PD-1 (P<.01). They also found that tumor cells produced an immunosuppressive molecule called indoleamine 2,3-dyoxygenae that functions by suppressing T-cell activity levels. They concluded that in EMPD, tumor-specific T lymphocytes have an exhausted phenotype due to PD-1 activation as well as indoleamine 2,3-dyoxygenase release to the tumor microenvironment.24
These studies highlight that restoring the effector functions of tumor-specific T lymphocytes could be an effective treatment strategy for EMPD. In fact, immunotherapy has been used with success for EMPD in the form of topical immunomodulators such as imiquimod.16,25 More than 40 cases of EMPD treated with imiquimod 5% have been published; of these, only 6 were considered nonresponders,5 which suggests that EMPD may respond to other immunotherapies such as checkpoint inhibitors. It is an exciting time for immunotherapy as more checkpoint inhibitors are being developed. Among the newer agents is cemiplimab, which is a PD-1 inhibitor now US Food and Drug Administration approved for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in patients who are not candidates for curative surgery or curative radiation.26 Programmed cell death receptor 1 signaling can serve as a potential target in EMPD, and further studies need to be performed to test the clinical efficacy, especially in unresectable or invasive/metastatic EMPD. As the PD-1 pathway is more studied in EMPD, and as more PD-1 inhibitors get developed, it would be a clinical need to establish clinical studies for PD-1 inhibitors in EMPD.
- Ito T, Kaku-Ito Y, Furue M. The diagnosis and management of extramammary Paget’s disease. Expert Rev Anticancer Ther. 2018;18:543-553.
- van der Zwan JM, Siesling S, Blokx WAM, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214-221.
- Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58:871-879.
- Wollina U, Goldman A, Bieneck A, et al. Surgical treatment for extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:27.
- Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget disease: implications for immune-targeted therapy. Cancers (Basel). 2019;11:754.
- Fowler MR, Flanigan KL, Googe PB. PD-L1 expression in extramammary Paget disease [published online March 6, 2020]. Am J Dermatopathol. doi:10.1097/dad.0000000000001622.
- Pourmaleki M, Young JH, Socci ND, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3, B7-H4, PD-L1, PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. 2019;10:6152-6167.
- Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764-782.
- Dany M, Nganga R, Chidiac A, et al. Advances in immunotherapy for melanoma management. Hum Vaccines Immunother. 2016;12:2501-2511.
- Richter MD, Hughes GC, Chung SH, et al. Immunologic adverse events from immune checkpoint therapy [published online April 13, 2020]. Best Pract Res Clin Rheumatol. doi:10.1016/j.berh.2020.101511.
- Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol. 2015;8:13233-13240.
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83:234-239.
- Hatta N. Prognostic factors of extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:47.
- Yao H, Xie M, Fu S, et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18:403.
- Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget’s disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22:1625-1630.
- Sanderson P, Innamaa A, Palmer J, et al. Imiquimod therapy for extramammary Paget’s disease of the vulva: a viable non-surgical alternative. J Obstet Gynaecol. 2013;33:479-483.
- Smith AA. Pre-Paget cells: evidence of keratinocyte origin of extramammary Paget’s disease. Intractable Rare Dis Res. 2019;8:203-205.
- Garganese G, Inzani F, Mantovani G, et al. The vulvar immunohistochemical panel (VIP) project: molecular profiles of vulvar Paget’s disease. J Cancer Res Clin Oncol. 2019;145:2211-2225.
- Dias-Santagata D, Lam Q, Bergethon K, et al. A potential role for targeted therapy in a subset of metastasizing adnexal carcinomas. Mod Pathol. 2011;24:974-982.
- Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol. 2015;40:473-478.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069-1086.
- Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. 2018;67:1481-1489.
- Cui C, Yu B, Jiang Q, et al. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol. 2019;46:3-10.
- Iga N, Otsuka A, Yamamoto Y, et al. Accumulation of exhausted CD8+ T cells in extramammary Paget’s disease. PLoS One. 2019;14:E0211135.
- Frances L, Pascual JC, Leiva-Salinas M, et al. Extramammary Paget disease successfully treated with topical imiquimod 5% and tazarotene. Dermatol Ther. 2014;27:19-20.
- Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs. 2020;80:813-819.
Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts that presents as an erythematous patch on cutaneous sites rich with apocrine glands.1 Primary EMPD can be in situ or invasive with the potential to become metastatic.2 Treatment of primary EMPD is challenging due to the difficulty of achieving clear surgical margins, as the tumor has microscopic spread throughout the epidermis in a skipping fashion.3 Mohs micrographic surgery is the treatment of choice; however, there is a clinical need to identify additional treatment modalities, especially for patients with unresectable, invasive, or metastatic primary EMPD,4 which partly is due to lack of data to understand the pathogenesis of primary EMPD. Recently, there have been studies investigating the genetic characteristics of EMPD tumors. The interaction between the programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) is one of the pathways recently studied and has been reported to be a potential target in EMPD.5-7 Programmed cell death receptor 1 signaling constitutes an immune checkpoint pathway that regulates the activation of tumor-specific T cells.8 In several malignancies, cancer cells express PD-L1 on their surface to activate PD-1 signaling in T cells as a mechanism to dampen the tumor-specific immune response and evade antitumor immunity.9 Thus, blocking PD-1 signaling widely is used to activate tumor-specific T cells and decrease tumor burden.10 Given the advances of immunotherapy in many neoplasms and the paucity of effective agents to treat EMPD, this article serves to shed light on recent data studying PD-1 signaling in EMPD and highlights the potential clinical use of immunotherapy for EMPD.
EMPD and Its Subtypes
Extramammary Paget disease is a rare adenocarcinoma typically affecting older patients (age >60 years) in cutaneous sites with abundant apocrine glands such as the genital and perianal skin.3 Extramammary Paget disease presents as an erythematous patch and frequently is treated initially as a skin dermatosis, resulting in a delay in diagnosis. Histologically, EMPD is characterized by the presence of single cells or a nest of cells having abundant pale cytoplasm and large vesicular nuclei distributed in the epidermis in a pagetoid fashion.11
Extramammary Paget disease can be primary or secondary; the 2 subtypes behave differently both clinically and prognostically. Although primary EMPD is considered to be an adnexal carcinoma of the apocrine gland ducts, secondary EMPD is considered to be an intraepithelial extension of malignant cells from an underlying internal neoplasm.12 The underlying malignancies usually are located within dermal adnexal glands or organs in the vicinity of the cutaneous lesion, such as the colon in the case of perianal EMPD. Histologically, primary and secondary EMPD can be differentiated based on their immunophenotypic staining profiles. Although all cases of EMPD show positive immunohistochemistry staining for cytokeratin 7, carcinoembryonic antigen, and epithelial membrane antigen, only primary EMPD will additionally stain for GCDFP-15 (gross cystic disease fluid protein 15) and GATA.11 Regardless of the immunohistochemistry stains, every patient newly diagnosed with EMPD deserves a full workup for malignancy screening, including a colonoscopy, cystoscopy, mammography and Papanicolaou test in women, pelvic ultrasound, and computed tomography of the abdomen and pelvis.13
The first-line treatment of EMPD is surgery; however, obtaining clear surgical margins can be a challenge, with high recurrence rates due to the microscopic spread of the disease throughout the epidermis.4 In addition, anatomic location affects the surgical approach and patient survival. Recent studies on EMPD mortality outcomes in women show that mortality is higher in patients with vaginal EMPD than in those with vulvar/labial EMPD, partly due to the sensitive location that makes it difficult to perform wide local excisions.13,14 Assessing the entire margins with tissue preservation using Mohs micrographic surgery has been shown to be successful in decreasing the recurrence rate, especially when coupled with the use of cytokeratin 7 immunohistochemistry.4 Other treatment modalities include radiation, topical imiquimod, and photodynamic therapy.15,16 Regardless of treatment modality, EMPD requires long‐term follow-up to monitor for disease recurrence, regional lymphadenopathy, distant metastasis, or development of an internal malignancy.
The pathogenesis of primary EMPD remains unclear. The tumor is thought to be derived from Toker cells, which are pluripotent adnexal stem cells located in the epidermis that normally give rise to apocrine glands.17 There have been few studies investigating the genetic characteristics of EMPD lesions in an attempt to understand pathogenesis as well as to find druggable targets. Current data for targeted therapy have focused on HER2 (human epidermal growth factor receptor 2) hormone receptor expression,18 ERBB (erythroblastic oncogene B) amplification,19 CDK4 (cyclin-dependent kinase 4)–cyclin D1 signaling,20 and most recently PD-1/PD-L1 pathway.5-7
PD-1 Expression in EMPD: Implication for Immunotherapy
Most tumors display novel antigens that are recognized by the host immune system and thus stimulate cell-mediated and humoral pathways. The immune system naturally provides regulatory immune checkpoints to T cell–mediated immune responses. One of these checkpoints involves the interaction between PD-1 on T cells and its ligand PD-L1 on tumor cells.21 When PD-1 binds to PD-L1 on tumor cells, there is inhibition of T-cell proliferation, a decrease in cytokine production, and induction of T-cell cytolysis.22 The Figure summarizes the dynamics for T-cell regulation.

Naturally, tumor-infiltrating T cells trigger their own inhibition by binding to PD-L1. However, certain tumor cells constitutively upregulate the expression of PD-L1. With that, the tumor cells gain the ability to suppress T cells and avoid T cell–mediated cytotoxicity,23 which is known as the adoptive immune resistance mechanism. There have been several studies in the literature investigating the PD-1 signaling pathway in EMPD as a way to determine if EMPD would be susceptible to immune checkpoint blockade. The success of checkpoint inhibitor immunotherapy generally correlates with increased PD-L1 expression by tumor cells.
One study evaluated the expression of PD-L1 in tumor cells and tumor-infiltrating T cells in 18 cases of EMPD.6 The authors identified that even though tumor cell PD-L1 expression was detected in only 3 (17%) cases, tumor-infiltrating lymphocytes expressed PD-L1 in the majority of the cases analyzed and in all of the cases positive for tumor cell PD-L1.6
Another study evaluated PD-1 and PD-L1 expression in EMPD tumor cells and tumor-associated immune infiltrate.5 They found that PD-1 was expressed heavily by the tumor-associated immune infiltrate in all EMPD cases analyzed. Similar to the previously mentioned study,6 PD-L1 was expressed by tumor cells in a few cases only. Interestingly, they found that the density of CD3 in the tumor-associated immune infiltrate was significantly (P=.049) higher in patients who were alive than in those who died, suggesting the importance of an exuberant T-cell response for survival in EMPD.5
A third study investigated protein expression of the B7 family members as well as PD-1 and PD-L1/2 in 55 EMPD samples. In this study the authors also found that tumor cell PD-L1 was minimal. Interestingly, they also found that tumor cells expressed B7 proteins in the majority of the cases.7
Finally, another study examined activity levels of T cells in EMPD by measuring the number and expression levels of cytotoxic T-cell cytokines.24 The authors first found that EMPD tumors had a significantly higher number of CD8+ tumor-infiltrating lymphocytes compared to peripheral blood (P<.01). These CD8+ tumor-infiltrating lymphocytes also had a significantly higher expression of PD-1 (P<.01). They also found that tumor cells produced an immunosuppressive molecule called indoleamine 2,3-dyoxygenae that functions by suppressing T-cell activity levels. They concluded that in EMPD, tumor-specific T lymphocytes have an exhausted phenotype due to PD-1 activation as well as indoleamine 2,3-dyoxygenase release to the tumor microenvironment.24
These studies highlight that restoring the effector functions of tumor-specific T lymphocytes could be an effective treatment strategy for EMPD. In fact, immunotherapy has been used with success for EMPD in the form of topical immunomodulators such as imiquimod.16,25 More than 40 cases of EMPD treated with imiquimod 5% have been published; of these, only 6 were considered nonresponders,5 which suggests that EMPD may respond to other immunotherapies such as checkpoint inhibitors. It is an exciting time for immunotherapy as more checkpoint inhibitors are being developed. Among the newer agents is cemiplimab, which is a PD-1 inhibitor now US Food and Drug Administration approved for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in patients who are not candidates for curative surgery or curative radiation.26 Programmed cell death receptor 1 signaling can serve as a potential target in EMPD, and further studies need to be performed to test the clinical efficacy, especially in unresectable or invasive/metastatic EMPD. As the PD-1 pathway is more studied in EMPD, and as more PD-1 inhibitors get developed, it would be a clinical need to establish clinical studies for PD-1 inhibitors in EMPD.
Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts that presents as an erythematous patch on cutaneous sites rich with apocrine glands.1 Primary EMPD can be in situ or invasive with the potential to become metastatic.2 Treatment of primary EMPD is challenging due to the difficulty of achieving clear surgical margins, as the tumor has microscopic spread throughout the epidermis in a skipping fashion.3 Mohs micrographic surgery is the treatment of choice; however, there is a clinical need to identify additional treatment modalities, especially for patients with unresectable, invasive, or metastatic primary EMPD,4 which partly is due to lack of data to understand the pathogenesis of primary EMPD. Recently, there have been studies investigating the genetic characteristics of EMPD tumors. The interaction between the programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) is one of the pathways recently studied and has been reported to be a potential target in EMPD.5-7 Programmed cell death receptor 1 signaling constitutes an immune checkpoint pathway that regulates the activation of tumor-specific T cells.8 In several malignancies, cancer cells express PD-L1 on their surface to activate PD-1 signaling in T cells as a mechanism to dampen the tumor-specific immune response and evade antitumor immunity.9 Thus, blocking PD-1 signaling widely is used to activate tumor-specific T cells and decrease tumor burden.10 Given the advances of immunotherapy in many neoplasms and the paucity of effective agents to treat EMPD, this article serves to shed light on recent data studying PD-1 signaling in EMPD and highlights the potential clinical use of immunotherapy for EMPD.
EMPD and Its Subtypes
Extramammary Paget disease is a rare adenocarcinoma typically affecting older patients (age >60 years) in cutaneous sites with abundant apocrine glands such as the genital and perianal skin.3 Extramammary Paget disease presents as an erythematous patch and frequently is treated initially as a skin dermatosis, resulting in a delay in diagnosis. Histologically, EMPD is characterized by the presence of single cells or a nest of cells having abundant pale cytoplasm and large vesicular nuclei distributed in the epidermis in a pagetoid fashion.11
Extramammary Paget disease can be primary or secondary; the 2 subtypes behave differently both clinically and prognostically. Although primary EMPD is considered to be an adnexal carcinoma of the apocrine gland ducts, secondary EMPD is considered to be an intraepithelial extension of malignant cells from an underlying internal neoplasm.12 The underlying malignancies usually are located within dermal adnexal glands or organs in the vicinity of the cutaneous lesion, such as the colon in the case of perianal EMPD. Histologically, primary and secondary EMPD can be differentiated based on their immunophenotypic staining profiles. Although all cases of EMPD show positive immunohistochemistry staining for cytokeratin 7, carcinoembryonic antigen, and epithelial membrane antigen, only primary EMPD will additionally stain for GCDFP-15 (gross cystic disease fluid protein 15) and GATA.11 Regardless of the immunohistochemistry stains, every patient newly diagnosed with EMPD deserves a full workup for malignancy screening, including a colonoscopy, cystoscopy, mammography and Papanicolaou test in women, pelvic ultrasound, and computed tomography of the abdomen and pelvis.13
The first-line treatment of EMPD is surgery; however, obtaining clear surgical margins can be a challenge, with high recurrence rates due to the microscopic spread of the disease throughout the epidermis.4 In addition, anatomic location affects the surgical approach and patient survival. Recent studies on EMPD mortality outcomes in women show that mortality is higher in patients with vaginal EMPD than in those with vulvar/labial EMPD, partly due to the sensitive location that makes it difficult to perform wide local excisions.13,14 Assessing the entire margins with tissue preservation using Mohs micrographic surgery has been shown to be successful in decreasing the recurrence rate, especially when coupled with the use of cytokeratin 7 immunohistochemistry.4 Other treatment modalities include radiation, topical imiquimod, and photodynamic therapy.15,16 Regardless of treatment modality, EMPD requires long‐term follow-up to monitor for disease recurrence, regional lymphadenopathy, distant metastasis, or development of an internal malignancy.
The pathogenesis of primary EMPD remains unclear. The tumor is thought to be derived from Toker cells, which are pluripotent adnexal stem cells located in the epidermis that normally give rise to apocrine glands.17 There have been few studies investigating the genetic characteristics of EMPD lesions in an attempt to understand pathogenesis as well as to find druggable targets. Current data for targeted therapy have focused on HER2 (human epidermal growth factor receptor 2) hormone receptor expression,18 ERBB (erythroblastic oncogene B) amplification,19 CDK4 (cyclin-dependent kinase 4)–cyclin D1 signaling,20 and most recently PD-1/PD-L1 pathway.5-7
PD-1 Expression in EMPD: Implication for Immunotherapy
Most tumors display novel antigens that are recognized by the host immune system and thus stimulate cell-mediated and humoral pathways. The immune system naturally provides regulatory immune checkpoints to T cell–mediated immune responses. One of these checkpoints involves the interaction between PD-1 on T cells and its ligand PD-L1 on tumor cells.21 When PD-1 binds to PD-L1 on tumor cells, there is inhibition of T-cell proliferation, a decrease in cytokine production, and induction of T-cell cytolysis.22 The Figure summarizes the dynamics for T-cell regulation.

Naturally, tumor-infiltrating T cells trigger their own inhibition by binding to PD-L1. However, certain tumor cells constitutively upregulate the expression of PD-L1. With that, the tumor cells gain the ability to suppress T cells and avoid T cell–mediated cytotoxicity,23 which is known as the adoptive immune resistance mechanism. There have been several studies in the literature investigating the PD-1 signaling pathway in EMPD as a way to determine if EMPD would be susceptible to immune checkpoint blockade. The success of checkpoint inhibitor immunotherapy generally correlates with increased PD-L1 expression by tumor cells.
One study evaluated the expression of PD-L1 in tumor cells and tumor-infiltrating T cells in 18 cases of EMPD.6 The authors identified that even though tumor cell PD-L1 expression was detected in only 3 (17%) cases, tumor-infiltrating lymphocytes expressed PD-L1 in the majority of the cases analyzed and in all of the cases positive for tumor cell PD-L1.6
Another study evaluated PD-1 and PD-L1 expression in EMPD tumor cells and tumor-associated immune infiltrate.5 They found that PD-1 was expressed heavily by the tumor-associated immune infiltrate in all EMPD cases analyzed. Similar to the previously mentioned study,6 PD-L1 was expressed by tumor cells in a few cases only. Interestingly, they found that the density of CD3 in the tumor-associated immune infiltrate was significantly (P=.049) higher in patients who were alive than in those who died, suggesting the importance of an exuberant T-cell response for survival in EMPD.5
A third study investigated protein expression of the B7 family members as well as PD-1 and PD-L1/2 in 55 EMPD samples. In this study the authors also found that tumor cell PD-L1 was minimal. Interestingly, they also found that tumor cells expressed B7 proteins in the majority of the cases.7
Finally, another study examined activity levels of T cells in EMPD by measuring the number and expression levels of cytotoxic T-cell cytokines.24 The authors first found that EMPD tumors had a significantly higher number of CD8+ tumor-infiltrating lymphocytes compared to peripheral blood (P<.01). These CD8+ tumor-infiltrating lymphocytes also had a significantly higher expression of PD-1 (P<.01). They also found that tumor cells produced an immunosuppressive molecule called indoleamine 2,3-dyoxygenae that functions by suppressing T-cell activity levels. They concluded that in EMPD, tumor-specific T lymphocytes have an exhausted phenotype due to PD-1 activation as well as indoleamine 2,3-dyoxygenase release to the tumor microenvironment.24
These studies highlight that restoring the effector functions of tumor-specific T lymphocytes could be an effective treatment strategy for EMPD. In fact, immunotherapy has been used with success for EMPD in the form of topical immunomodulators such as imiquimod.16,25 More than 40 cases of EMPD treated with imiquimod 5% have been published; of these, only 6 were considered nonresponders,5 which suggests that EMPD may respond to other immunotherapies such as checkpoint inhibitors. It is an exciting time for immunotherapy as more checkpoint inhibitors are being developed. Among the newer agents is cemiplimab, which is a PD-1 inhibitor now US Food and Drug Administration approved for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in patients who are not candidates for curative surgery or curative radiation.26 Programmed cell death receptor 1 signaling can serve as a potential target in EMPD, and further studies need to be performed to test the clinical efficacy, especially in unresectable or invasive/metastatic EMPD. As the PD-1 pathway is more studied in EMPD, and as more PD-1 inhibitors get developed, it would be a clinical need to establish clinical studies for PD-1 inhibitors in EMPD.
- Ito T, Kaku-Ito Y, Furue M. The diagnosis and management of extramammary Paget’s disease. Expert Rev Anticancer Ther. 2018;18:543-553.
- van der Zwan JM, Siesling S, Blokx WAM, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214-221.
- Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58:871-879.
- Wollina U, Goldman A, Bieneck A, et al. Surgical treatment for extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:27.
- Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget disease: implications for immune-targeted therapy. Cancers (Basel). 2019;11:754.
- Fowler MR, Flanigan KL, Googe PB. PD-L1 expression in extramammary Paget disease [published online March 6, 2020]. Am J Dermatopathol. doi:10.1097/dad.0000000000001622.
- Pourmaleki M, Young JH, Socci ND, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3, B7-H4, PD-L1, PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. 2019;10:6152-6167.
- Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764-782.
- Dany M, Nganga R, Chidiac A, et al. Advances in immunotherapy for melanoma management. Hum Vaccines Immunother. 2016;12:2501-2511.
- Richter MD, Hughes GC, Chung SH, et al. Immunologic adverse events from immune checkpoint therapy [published online April 13, 2020]. Best Pract Res Clin Rheumatol. doi:10.1016/j.berh.2020.101511.
- Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol. 2015;8:13233-13240.
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83:234-239.
- Hatta N. Prognostic factors of extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:47.
- Yao H, Xie M, Fu S, et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18:403.
- Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget’s disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22:1625-1630.
- Sanderson P, Innamaa A, Palmer J, et al. Imiquimod therapy for extramammary Paget’s disease of the vulva: a viable non-surgical alternative. J Obstet Gynaecol. 2013;33:479-483.
- Smith AA. Pre-Paget cells: evidence of keratinocyte origin of extramammary Paget’s disease. Intractable Rare Dis Res. 2019;8:203-205.
- Garganese G, Inzani F, Mantovani G, et al. The vulvar immunohistochemical panel (VIP) project: molecular profiles of vulvar Paget’s disease. J Cancer Res Clin Oncol. 2019;145:2211-2225.
- Dias-Santagata D, Lam Q, Bergethon K, et al. A potential role for targeted therapy in a subset of metastasizing adnexal carcinomas. Mod Pathol. 2011;24:974-982.
- Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol. 2015;40:473-478.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069-1086.
- Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. 2018;67:1481-1489.
- Cui C, Yu B, Jiang Q, et al. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol. 2019;46:3-10.
- Iga N, Otsuka A, Yamamoto Y, et al. Accumulation of exhausted CD8+ T cells in extramammary Paget’s disease. PLoS One. 2019;14:E0211135.
- Frances L, Pascual JC, Leiva-Salinas M, et al. Extramammary Paget disease successfully treated with topical imiquimod 5% and tazarotene. Dermatol Ther. 2014;27:19-20.
- Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs. 2020;80:813-819.
- Ito T, Kaku-Ito Y, Furue M. The diagnosis and management of extramammary Paget’s disease. Expert Rev Anticancer Ther. 2018;18:543-553.
- van der Zwan JM, Siesling S, Blokx WAM, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214-221.
- Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58:871-879.
- Wollina U, Goldman A, Bieneck A, et al. Surgical treatment for extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:27.
- Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget disease: implications for immune-targeted therapy. Cancers (Basel). 2019;11:754.
- Fowler MR, Flanigan KL, Googe PB. PD-L1 expression in extramammary Paget disease [published online March 6, 2020]. Am J Dermatopathol. doi:10.1097/dad.0000000000001622.
- Pourmaleki M, Young JH, Socci ND, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3, B7-H4, PD-L1, PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. 2019;10:6152-6167.
- Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764-782.
- Dany M, Nganga R, Chidiac A, et al. Advances in immunotherapy for melanoma management. Hum Vaccines Immunother. 2016;12:2501-2511.
- Richter MD, Hughes GC, Chung SH, et al. Immunologic adverse events from immune checkpoint therapy [published online April 13, 2020]. Best Pract Res Clin Rheumatol. doi:10.1016/j.berh.2020.101511.
- Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol. 2015;8:13233-13240.
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83:234-239.
- Hatta N. Prognostic factors of extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:47.
- Yao H, Xie M, Fu S, et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18:403.
- Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget’s disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22:1625-1630.
- Sanderson P, Innamaa A, Palmer J, et al. Imiquimod therapy for extramammary Paget’s disease of the vulva: a viable non-surgical alternative. J Obstet Gynaecol. 2013;33:479-483.
- Smith AA. Pre-Paget cells: evidence of keratinocyte origin of extramammary Paget’s disease. Intractable Rare Dis Res. 2019;8:203-205.
- Garganese G, Inzani F, Mantovani G, et al. The vulvar immunohistochemical panel (VIP) project: molecular profiles of vulvar Paget’s disease. J Cancer Res Clin Oncol. 2019;145:2211-2225.
- Dias-Santagata D, Lam Q, Bergethon K, et al. A potential role for targeted therapy in a subset of metastasizing adnexal carcinomas. Mod Pathol. 2011;24:974-982.
- Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol. 2015;40:473-478.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069-1086.
- Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. 2018;67:1481-1489.
- Cui C, Yu B, Jiang Q, et al. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol. 2019;46:3-10.
- Iga N, Otsuka A, Yamamoto Y, et al. Accumulation of exhausted CD8+ T cells in extramammary Paget’s disease. PLoS One. 2019;14:E0211135.
- Frances L, Pascual JC, Leiva-Salinas M, et al. Extramammary Paget disease successfully treated with topical imiquimod 5% and tazarotene. Dermatol Ther. 2014;27:19-20.
- Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs. 2020;80:813-819.
Resident Pearls
- Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts, while secondary EMPD is an extension of malignant cells from an underlying internal neoplasm.
- Surgical margin clearance in EMPD often is problematic, with high recurrence rates indicating the need for additional treatment modalities.
- Programmed cell death receptor 1 (PD-1) signaling can serve as a potential target in EMPD. Further studies and clinical trials are needed to test the efficacy of PD-1 inhibitors in unresectable or invasive/metastatic EMPD.
MRI reliably identifies significant prostate cancer
Prostate cancers that are missed on multiparametric (mp) MRI are small and “not life-threatening,” according to an analysis of data from the Prostate MR Imaging Study (PROMIS).
“Our work suggests that MRI scans of the prostate appear to deliver crucial information about a man’s risk of dying from prostate cancer, even before he has a biopsy,” said Joseph Norris, BM BS, from University College London.
“This may mean that we can finally move prostate cancer to a position in which we can use imaging as the primary tool to direct further investigations, treatment, and prediction of risk,” he told Medscape Medical News.
This is “a position that all other solid organ cancers have reached,” said Norris, who will present the findings at the upcoming virtual European Association of Urology 2020 Congress.
All 576 PROMIS participants underwent an mpMRI scan, a transrectal ultrasonography (TRUS)–guided biopsy, and a template prostate mapping (TPM) biopsy taken at 5-mm intervals across the entire prostate.
PROMIS researchers previously showed that mpMRI had a 93% sensitivity for clinically significant cancer, whereas TRUS biopsy had only a 48% sensitivity, as reported by Medscape Medical News. And they concluded that the use of mpMRI as a first-line diagnostic tool could prevent 27% of all biopsies, which can have serious adverse effects, such as pain, urinary problems, infection, bleeding, and erectile dysfunction.
However, in their study looking at the accuracy of mpMRI and TRUS biopsy, the researchers did not investigate the severity of the 7% of cancers that mpMRI missed. “What if those missed cancers are, in fact, aggressive? That’s what we set out to examine,” Norris explained.
So he and his colleagues conducted a post ad hoc analysis of the PROMIS participants in whom clinically significant cancer had been detected with TPM biopsy to see which of those cancers had been detected with mpMRI. The findings were published online in European Urology.
Cancers met the strict definition of clinically significant if they had a Gleason score of at least 4+3 for a tumor of any length, or a maximum cancer core length (MCCL) greater than 6 mm for a cancer of any grade. They met the less-strict definition if they had a Gleason score of at least 3+4 for a tumor of any length, or a MCCL greater than 4 mm for a cancer of any grade.
In PROMIS, TPM biopsy detected 230 cancers that met the strict definition of clinically significant and 331 that met the less-strict definition.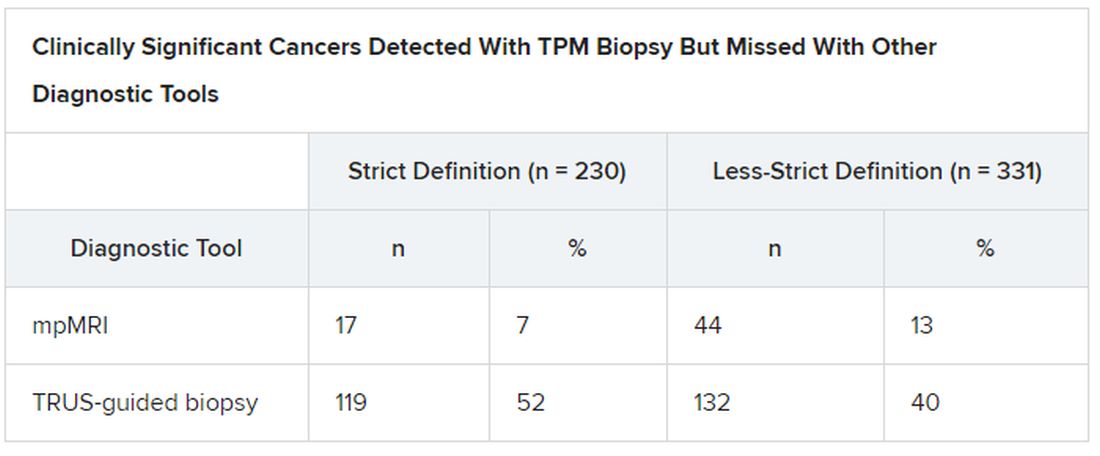
Overall Gleason scores were significantly lower for the 17 strict-definition cancers not detected with 1.5 T mpMRI than for those detected with mpMRI (P = .0007), as were maximum Gleason scores (P < .0001).
Median MCCL was 3 mm shorter for all 17 tumors missed with mpMRI than for those detected with mpMRI (5 vs 8 mm; P < .0001).
mpMRI detected all tumors identified on TPM biopsy that had an overall Gleason score greater than 3+4 (Gleason grades 3 to 5) or a maximum Gleason score greater than 4+3 (Gleason grades 4 and 5).
“This finding is important, given that in PROMIS, no men with an overall Gleason score of 4+3 had cancer missed by MRI, indicating that actually MRI may be able to identify all truly significant cancers,” said Norris.
Adding PSA Density Threshold
To further assess cancers missed on mpMRI, the researchers looked at prostate-specific antigen (PSA) density, calculated as total PSA level (ng/mL) divided by prostate volume (mL).
“We found that if we applied a threshold PSA density to men with normal-looking MRI scans, we could reduce the proportion of missed significant cancer to just 5%. This is exciting; it means we can make MRI an even more effective test for prostate cancer in a very simple way,” Norris reported.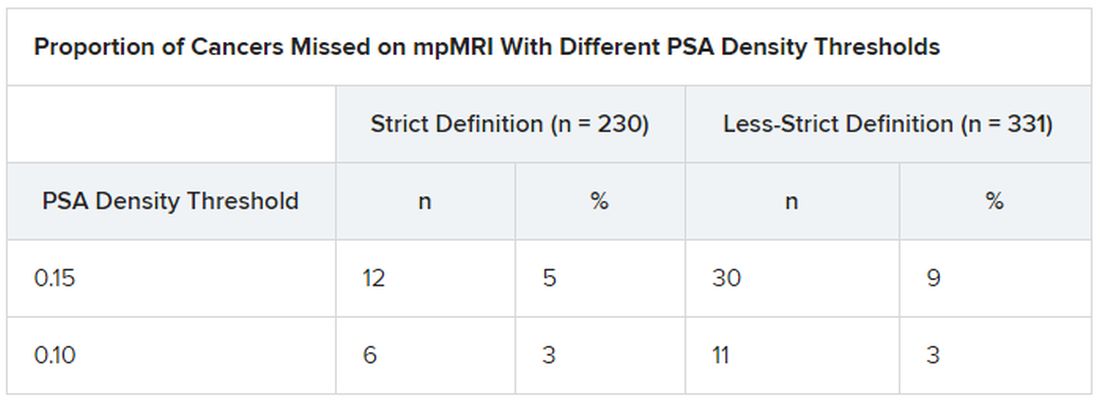
“These data show that no highly aggressive prostate cancers were missed by MRI, either at the level of the whole prostate or at the individual needle level,” said Norris. This should lead to positive outcomes in the long term.
And since the PROMIS data were gathered, MRI technology has improved, he said. The “MRI scanners in PROMIS were 1.5 Tesla,” whereas today’s machines are 3.0 T, which could increase the detection of significant prostate cancer.
In fact, “our analysis here potentially overestimates the amount of undetected disease,” he noted.
Prostate cancer that is not clinically significant is often monitored with active surveillance, so “invisible” cancers missed on mpMRI could actually be looked at in a positive light, he explained.
Variation in technique, interpretation
But the quality of care when it comes to the diagnosis of prostate cancer is not equal everywhere, said Gerald Andriole, MD, from the Washington School of Medicine in St. Louis, Missouri.
“When you get an MRI in a center that doesn’t do a lot of prostate cancer testing, you may not have the best software and you may have a radiologist who is not that experienced,” he told Medscape Medical News. Specialized cancer centers of excellence tend to do a great job finding prostate cancer, “but other centers have high significant-miss rates or high overcall rates.”
“The elephant in the room remains the considerable variation in technique and interobserver interpretation of prostate mpMRI,” write Steven Monda, MD, and Marc Dall’Era, MD, both from UC Davis Health in Sacramento, California, in an editorial that accompanies the new PROMIS analysis.
“These problems must be addressed and remedied in each institution before relying on results from PROMIS to drive changes in clinical practice,” they add.
Norris, Andriole, Monda, and Dall’Era have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Prostate cancers that are missed on multiparametric (mp) MRI are small and “not life-threatening,” according to an analysis of data from the Prostate MR Imaging Study (PROMIS).
“Our work suggests that MRI scans of the prostate appear to deliver crucial information about a man’s risk of dying from prostate cancer, even before he has a biopsy,” said Joseph Norris, BM BS, from University College London.
“This may mean that we can finally move prostate cancer to a position in which we can use imaging as the primary tool to direct further investigations, treatment, and prediction of risk,” he told Medscape Medical News.
This is “a position that all other solid organ cancers have reached,” said Norris, who will present the findings at the upcoming virtual European Association of Urology 2020 Congress.
All 576 PROMIS participants underwent an mpMRI scan, a transrectal ultrasonography (TRUS)–guided biopsy, and a template prostate mapping (TPM) biopsy taken at 5-mm intervals across the entire prostate.
PROMIS researchers previously showed that mpMRI had a 93% sensitivity for clinically significant cancer, whereas TRUS biopsy had only a 48% sensitivity, as reported by Medscape Medical News. And they concluded that the use of mpMRI as a first-line diagnostic tool could prevent 27% of all biopsies, which can have serious adverse effects, such as pain, urinary problems, infection, bleeding, and erectile dysfunction.
However, in their study looking at the accuracy of mpMRI and TRUS biopsy, the researchers did not investigate the severity of the 7% of cancers that mpMRI missed. “What if those missed cancers are, in fact, aggressive? That’s what we set out to examine,” Norris explained.
So he and his colleagues conducted a post ad hoc analysis of the PROMIS participants in whom clinically significant cancer had been detected with TPM biopsy to see which of those cancers had been detected with mpMRI. The findings were published online in European Urology.
Cancers met the strict definition of clinically significant if they had a Gleason score of at least 4+3 for a tumor of any length, or a maximum cancer core length (MCCL) greater than 6 mm for a cancer of any grade. They met the less-strict definition if they had a Gleason score of at least 3+4 for a tumor of any length, or a MCCL greater than 4 mm for a cancer of any grade.
In PROMIS, TPM biopsy detected 230 cancers that met the strict definition of clinically significant and 331 that met the less-strict definition.
Overall Gleason scores were significantly lower for the 17 strict-definition cancers not detected with 1.5 T mpMRI than for those detected with mpMRI (P = .0007), as were maximum Gleason scores (P < .0001).
Median MCCL was 3 mm shorter for all 17 tumors missed with mpMRI than for those detected with mpMRI (5 vs 8 mm; P < .0001).
mpMRI detected all tumors identified on TPM biopsy that had an overall Gleason score greater than 3+4 (Gleason grades 3 to 5) or a maximum Gleason score greater than 4+3 (Gleason grades 4 and 5).
“This finding is important, given that in PROMIS, no men with an overall Gleason score of 4+3 had cancer missed by MRI, indicating that actually MRI may be able to identify all truly significant cancers,” said Norris.
Adding PSA Density Threshold
To further assess cancers missed on mpMRI, the researchers looked at prostate-specific antigen (PSA) density, calculated as total PSA level (ng/mL) divided by prostate volume (mL).
“We found that if we applied a threshold PSA density to men with normal-looking MRI scans, we could reduce the proportion of missed significant cancer to just 5%. This is exciting; it means we can make MRI an even more effective test for prostate cancer in a very simple way,” Norris reported.
“These data show that no highly aggressive prostate cancers were missed by MRI, either at the level of the whole prostate or at the individual needle level,” said Norris. This should lead to positive outcomes in the long term.
And since the PROMIS data were gathered, MRI technology has improved, he said. The “MRI scanners in PROMIS were 1.5 Tesla,” whereas today’s machines are 3.0 T, which could increase the detection of significant prostate cancer.
In fact, “our analysis here potentially overestimates the amount of undetected disease,” he noted.
Prostate cancer that is not clinically significant is often monitored with active surveillance, so “invisible” cancers missed on mpMRI could actually be looked at in a positive light, he explained.
Variation in technique, interpretation
But the quality of care when it comes to the diagnosis of prostate cancer is not equal everywhere, said Gerald Andriole, MD, from the Washington School of Medicine in St. Louis, Missouri.
“When you get an MRI in a center that doesn’t do a lot of prostate cancer testing, you may not have the best software and you may have a radiologist who is not that experienced,” he told Medscape Medical News. Specialized cancer centers of excellence tend to do a great job finding prostate cancer, “but other centers have high significant-miss rates or high overcall rates.”
“The elephant in the room remains the considerable variation in technique and interobserver interpretation of prostate mpMRI,” write Steven Monda, MD, and Marc Dall’Era, MD, both from UC Davis Health in Sacramento, California, in an editorial that accompanies the new PROMIS analysis.
“These problems must be addressed and remedied in each institution before relying on results from PROMIS to drive changes in clinical practice,” they add.
Norris, Andriole, Monda, and Dall’Era have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Prostate cancers that are missed on multiparametric (mp) MRI are small and “not life-threatening,” according to an analysis of data from the Prostate MR Imaging Study (PROMIS).
“Our work suggests that MRI scans of the prostate appear to deliver crucial information about a man’s risk of dying from prostate cancer, even before he has a biopsy,” said Joseph Norris, BM BS, from University College London.
“This may mean that we can finally move prostate cancer to a position in which we can use imaging as the primary tool to direct further investigations, treatment, and prediction of risk,” he told Medscape Medical News.
This is “a position that all other solid organ cancers have reached,” said Norris, who will present the findings at the upcoming virtual European Association of Urology 2020 Congress.
All 576 PROMIS participants underwent an mpMRI scan, a transrectal ultrasonography (TRUS)–guided biopsy, and a template prostate mapping (TPM) biopsy taken at 5-mm intervals across the entire prostate.
PROMIS researchers previously showed that mpMRI had a 93% sensitivity for clinically significant cancer, whereas TRUS biopsy had only a 48% sensitivity, as reported by Medscape Medical News. And they concluded that the use of mpMRI as a first-line diagnostic tool could prevent 27% of all biopsies, which can have serious adverse effects, such as pain, urinary problems, infection, bleeding, and erectile dysfunction.
However, in their study looking at the accuracy of mpMRI and TRUS biopsy, the researchers did not investigate the severity of the 7% of cancers that mpMRI missed. “What if those missed cancers are, in fact, aggressive? That’s what we set out to examine,” Norris explained.
So he and his colleagues conducted a post ad hoc analysis of the PROMIS participants in whom clinically significant cancer had been detected with TPM biopsy to see which of those cancers had been detected with mpMRI. The findings were published online in European Urology.
Cancers met the strict definition of clinically significant if they had a Gleason score of at least 4+3 for a tumor of any length, or a maximum cancer core length (MCCL) greater than 6 mm for a cancer of any grade. They met the less-strict definition if they had a Gleason score of at least 3+4 for a tumor of any length, or a MCCL greater than 4 mm for a cancer of any grade.
In PROMIS, TPM biopsy detected 230 cancers that met the strict definition of clinically significant and 331 that met the less-strict definition.
Overall Gleason scores were significantly lower for the 17 strict-definition cancers not detected with 1.5 T mpMRI than for those detected with mpMRI (P = .0007), as were maximum Gleason scores (P < .0001).
Median MCCL was 3 mm shorter for all 17 tumors missed with mpMRI than for those detected with mpMRI (5 vs 8 mm; P < .0001).
mpMRI detected all tumors identified on TPM biopsy that had an overall Gleason score greater than 3+4 (Gleason grades 3 to 5) or a maximum Gleason score greater than 4+3 (Gleason grades 4 and 5).
“This finding is important, given that in PROMIS, no men with an overall Gleason score of 4+3 had cancer missed by MRI, indicating that actually MRI may be able to identify all truly significant cancers,” said Norris.
Adding PSA Density Threshold
To further assess cancers missed on mpMRI, the researchers looked at prostate-specific antigen (PSA) density, calculated as total PSA level (ng/mL) divided by prostate volume (mL).
“We found that if we applied a threshold PSA density to men with normal-looking MRI scans, we could reduce the proportion of missed significant cancer to just 5%. This is exciting; it means we can make MRI an even more effective test for prostate cancer in a very simple way,” Norris reported.
“These data show that no highly aggressive prostate cancers were missed by MRI, either at the level of the whole prostate or at the individual needle level,” said Norris. This should lead to positive outcomes in the long term.
And since the PROMIS data were gathered, MRI technology has improved, he said. The “MRI scanners in PROMIS were 1.5 Tesla,” whereas today’s machines are 3.0 T, which could increase the detection of significant prostate cancer.
In fact, “our analysis here potentially overestimates the amount of undetected disease,” he noted.
Prostate cancer that is not clinically significant is often monitored with active surveillance, so “invisible” cancers missed on mpMRI could actually be looked at in a positive light, he explained.
Variation in technique, interpretation
But the quality of care when it comes to the diagnosis of prostate cancer is not equal everywhere, said Gerald Andriole, MD, from the Washington School of Medicine in St. Louis, Missouri.
“When you get an MRI in a center that doesn’t do a lot of prostate cancer testing, you may not have the best software and you may have a radiologist who is not that experienced,” he told Medscape Medical News. Specialized cancer centers of excellence tend to do a great job finding prostate cancer, “but other centers have high significant-miss rates or high overcall rates.”
“The elephant in the room remains the considerable variation in technique and interobserver interpretation of prostate mpMRI,” write Steven Monda, MD, and Marc Dall’Era, MD, both from UC Davis Health in Sacramento, California, in an editorial that accompanies the new PROMIS analysis.
“These problems must be addressed and remedied in each institution before relying on results from PROMIS to drive changes in clinical practice,” they add.
Norris, Andriole, Monda, and Dall’Era have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Psychiatry trainees subjected to high levels of physical, sexual, verbal abuse from patients
More than 80% of psychiatric trainees have experienced some kind of verbal, physical, or sexual assault from patients, and approximately one-third have been physically attacked multiple times, new survey results show.
Such incidents, said study investigator Victor Pereira-Sanchez, MD, from the department of child and adolescent psychiatry at New York University take a toll on the trainees’ well-being and may ultimately affect the quality of patient care.
“The extent of violence against psychiatric trainees is alarming and calls for the implementation of effective training, prevention, and intervention measures,” Dr. Pereira-Sanchez said in an interview.
The findings were presented at the European Psychiatric Association (EPA) 2020 Congress, which was virtual this year because of the COVID-19 pandemic.
Widespread problem
Violence against health care professionals is widespread among clinicians in EDs with psychiatry trainees “more exposed and vulnerable,” Dr. Pereira-Sanchez said during his presentation.
In 2017, the European Federation of Psychiatric Trainees established a group of researchers to describe “the extent and consequences of violence against psychiatric trainees in Europe and beyond,” he said. The group developed a 15-item questionnaire asking young clinicians about experiences of physical, sexual, and verbal assault at work. The survey was posted online by partner institutions via social media.
A total of 827 psychiatric trainees, the majority of whom were from France and the United Kingdom, completed the survey. Respondents had an average age of 31 years, and 68% were women. On average, respondents had completed 51.3% of their psychiatric training.
with 92.0% reporting verbal assaults, 44.1% physical assaults, and 9.3% sexual assaults. In addition, 14.2% had been assaulted once, 51.9% had been assaulted two to five times, and 33.9% had been assaulted more than five times during their training. Results also showed that assaults were more likely to occur on an inpatient ward (63.4%) or the ED (56.9%), although 37.2% occurred in an outpatient setting and 4.2% in community settings. The majority of respondents (69.0%) did not report their assaults, and 67.3% did not call police or security personnel.
The most common emotions experienced by trainees following an assault were fear, rage, and anxiety. Guilt, sadness, feeling unsupported, and self-doubt were also reported.
Dr. Pereira-Sanchez noted the low rate of reported assaults is likely because trainees view it as “part of the job to get insulted, it’s part of the job to suffer minor physical violence.”
Individuals who did report assaults tended to be those who had been assaulted more than five times and those who felt more anxiety, rage, and fear.
“Basically, those who experience more emotional consequences and physical consequences tend to report more,” he said.
In addition, trainees tended to report assaults if they worked in an institution that provided protocols and training in prevention and management of patient aggression.
However, he added, most respondents reported they were not aware of their center’s protocols with respect to assaults and were not trained in the management or prevention of patient violence.
Management tools key
Commenting on the study in an interview, Renee Binder, MD, professor of psychiatry at University of California, San Francisco, said the findings show that, “when patients are out of control, they may act inappropriately, including verbal, physical, and sexual assaults.”
Consequently, “clinicians should be prepared and have management tools,” said Dr. Binder, who was not involved in the research.
She noted that derogatory statements and racial slurs were included among the verbal assaults, which is particularly common in inpatient units and EDs where “patients may be acutely psychotic or manic and out of control,” she said.
However, Dr. Binder pointed out that the investigators did not separate mild and more severe forms of physical and sexual assault.
“If the authors had more finely separated out the types of physical and sexual assaults, they probably would have found that mild types of assaults are much more common than more severe assaults,” she said.
Dr. Pereira-Sanchez’s fellowship program is funded by Fundacion Alicia Koplowitz. He and Dr. Binder have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
More than 80% of psychiatric trainees have experienced some kind of verbal, physical, or sexual assault from patients, and approximately one-third have been physically attacked multiple times, new survey results show.
Such incidents, said study investigator Victor Pereira-Sanchez, MD, from the department of child and adolescent psychiatry at New York University take a toll on the trainees’ well-being and may ultimately affect the quality of patient care.
“The extent of violence against psychiatric trainees is alarming and calls for the implementation of effective training, prevention, and intervention measures,” Dr. Pereira-Sanchez said in an interview.
The findings were presented at the European Psychiatric Association (EPA) 2020 Congress, which was virtual this year because of the COVID-19 pandemic.
Widespread problem
Violence against health care professionals is widespread among clinicians in EDs with psychiatry trainees “more exposed and vulnerable,” Dr. Pereira-Sanchez said during his presentation.
In 2017, the European Federation of Psychiatric Trainees established a group of researchers to describe “the extent and consequences of violence against psychiatric trainees in Europe and beyond,” he said. The group developed a 15-item questionnaire asking young clinicians about experiences of physical, sexual, and verbal assault at work. The survey was posted online by partner institutions via social media.
A total of 827 psychiatric trainees, the majority of whom were from France and the United Kingdom, completed the survey. Respondents had an average age of 31 years, and 68% were women. On average, respondents had completed 51.3% of their psychiatric training.
with 92.0% reporting verbal assaults, 44.1% physical assaults, and 9.3% sexual assaults. In addition, 14.2% had been assaulted once, 51.9% had been assaulted two to five times, and 33.9% had been assaulted more than five times during their training. Results also showed that assaults were more likely to occur on an inpatient ward (63.4%) or the ED (56.9%), although 37.2% occurred in an outpatient setting and 4.2% in community settings. The majority of respondents (69.0%) did not report their assaults, and 67.3% did not call police or security personnel.
The most common emotions experienced by trainees following an assault were fear, rage, and anxiety. Guilt, sadness, feeling unsupported, and self-doubt were also reported.
Dr. Pereira-Sanchez noted the low rate of reported assaults is likely because trainees view it as “part of the job to get insulted, it’s part of the job to suffer minor physical violence.”
Individuals who did report assaults tended to be those who had been assaulted more than five times and those who felt more anxiety, rage, and fear.
“Basically, those who experience more emotional consequences and physical consequences tend to report more,” he said.
In addition, trainees tended to report assaults if they worked in an institution that provided protocols and training in prevention and management of patient aggression.
However, he added, most respondents reported they were not aware of their center’s protocols with respect to assaults and were not trained in the management or prevention of patient violence.
Management tools key
Commenting on the study in an interview, Renee Binder, MD, professor of psychiatry at University of California, San Francisco, said the findings show that, “when patients are out of control, they may act inappropriately, including verbal, physical, and sexual assaults.”
Consequently, “clinicians should be prepared and have management tools,” said Dr. Binder, who was not involved in the research.
She noted that derogatory statements and racial slurs were included among the verbal assaults, which is particularly common in inpatient units and EDs where “patients may be acutely psychotic or manic and out of control,” she said.
However, Dr. Binder pointed out that the investigators did not separate mild and more severe forms of physical and sexual assault.
“If the authors had more finely separated out the types of physical and sexual assaults, they probably would have found that mild types of assaults are much more common than more severe assaults,” she said.
Dr. Pereira-Sanchez’s fellowship program is funded by Fundacion Alicia Koplowitz. He and Dr. Binder have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
More than 80% of psychiatric trainees have experienced some kind of verbal, physical, or sexual assault from patients, and approximately one-third have been physically attacked multiple times, new survey results show.
Such incidents, said study investigator Victor Pereira-Sanchez, MD, from the department of child and adolescent psychiatry at New York University take a toll on the trainees’ well-being and may ultimately affect the quality of patient care.
“The extent of violence against psychiatric trainees is alarming and calls for the implementation of effective training, prevention, and intervention measures,” Dr. Pereira-Sanchez said in an interview.
The findings were presented at the European Psychiatric Association (EPA) 2020 Congress, which was virtual this year because of the COVID-19 pandemic.
Widespread problem
Violence against health care professionals is widespread among clinicians in EDs with psychiatry trainees “more exposed and vulnerable,” Dr. Pereira-Sanchez said during his presentation.
In 2017, the European Federation of Psychiatric Trainees established a group of researchers to describe “the extent and consequences of violence against psychiatric trainees in Europe and beyond,” he said. The group developed a 15-item questionnaire asking young clinicians about experiences of physical, sexual, and verbal assault at work. The survey was posted online by partner institutions via social media.
A total of 827 psychiatric trainees, the majority of whom were from France and the United Kingdom, completed the survey. Respondents had an average age of 31 years, and 68% were women. On average, respondents had completed 51.3% of their psychiatric training.
with 92.0% reporting verbal assaults, 44.1% physical assaults, and 9.3% sexual assaults. In addition, 14.2% had been assaulted once, 51.9% had been assaulted two to five times, and 33.9% had been assaulted more than five times during their training. Results also showed that assaults were more likely to occur on an inpatient ward (63.4%) or the ED (56.9%), although 37.2% occurred in an outpatient setting and 4.2% in community settings. The majority of respondents (69.0%) did not report their assaults, and 67.3% did not call police or security personnel.
The most common emotions experienced by trainees following an assault were fear, rage, and anxiety. Guilt, sadness, feeling unsupported, and self-doubt were also reported.
Dr. Pereira-Sanchez noted the low rate of reported assaults is likely because trainees view it as “part of the job to get insulted, it’s part of the job to suffer minor physical violence.”
Individuals who did report assaults tended to be those who had been assaulted more than five times and those who felt more anxiety, rage, and fear.
“Basically, those who experience more emotional consequences and physical consequences tend to report more,” he said.
In addition, trainees tended to report assaults if they worked in an institution that provided protocols and training in prevention and management of patient aggression.
However, he added, most respondents reported they were not aware of their center’s protocols with respect to assaults and were not trained in the management or prevention of patient violence.
Management tools key
Commenting on the study in an interview, Renee Binder, MD, professor of psychiatry at University of California, San Francisco, said the findings show that, “when patients are out of control, they may act inappropriately, including verbal, physical, and sexual assaults.”
Consequently, “clinicians should be prepared and have management tools,” said Dr. Binder, who was not involved in the research.
She noted that derogatory statements and racial slurs were included among the verbal assaults, which is particularly common in inpatient units and EDs where “patients may be acutely psychotic or manic and out of control,” she said.
However, Dr. Binder pointed out that the investigators did not separate mild and more severe forms of physical and sexual assault.
“If the authors had more finely separated out the types of physical and sexual assaults, they probably would have found that mild types of assaults are much more common than more severe assaults,” she said.
Dr. Pereira-Sanchez’s fellowship program is funded by Fundacion Alicia Koplowitz. He and Dr. Binder have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Move over supplements, here come medical foods
As the Food and Drug Administration focuses on other issues, companies, both big and small, are looking to boost physician and consumer interest in their “medical foods” – products that fall somewhere between drugs and supplements and promise to mitigate symptoms, or even address underlying pathologies, of a range of diseases.
Manufacturers now market an array of medical foods, ranging from powders and capsules for Alzheimer disease to low-protein spaghetti for chronic kidney disease (CKD). The FDA has not been completely absent; it takes a narrow view of what medical conditions qualify for treatment with food products and has warned some manufacturers that their misbranded products are acting more like unapproved drugs.
By the FDA’s definition, medical food is limited to products that provide crucial therapy for patients with inborn errors of metabolism (IEM). An example is specialized baby formula for infants with phenylketonuria. Unlike supplements, medical foods are supposed to be used under the supervision of a physician. This has prompted some sales reps to turn up in the clinic, and most manufacturers have online approval forms for doctors to sign. Manufacturers, advisers, and regulators were interviewed for a closer look at this burgeoning industry.
The market
The global market for medical foods – about $18 billion in 2019 – is expected to grow steadily in the near future. It is drawing more interest, especially in Europe, where medical foods are more accepted by physicians and consumers, Meghan Donnelly, MS, RDN, said in an interview. She is a registered dietitian who conducts physician outreach in the United States for Flavis, a division of Dr. Schär. That company, based in northern Italy, started out targeting IEMs but now also sells gluten-free foods for celiac disease and low-protein foods for CKD.
It is still a niche market in the United States – and isn’t likely to ever approach the size of the supplement market, according to Marcus Charuvastra, the managing director of Targeted Medical Pharma, which markets Theramine capsules for pain management, among many other products. But it could still be a big win for a manufacturer if they get a small slice of a big market, such as for Alzheimer disease.
Defining medical food
According to an update of the Orphan Drug Act in 1988, a medical food is “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” The FDA issued regulations to accompany that law in 1993 but has since only issued a guidance document that is not legally binding.
Medical foods are not drugs and they are not supplements (the latter are intended only for healthy people). The FDA doesn’t require formal approval of a medical food, but, by law, the ingredients must be generally recognized as safe, and manufacturers must follow good manufacturing practices. However, the agency has taken a narrow view of what conditions require medical foods.
Policing medical foods hasn’t been a priority for the FDA, which is why there has been a proliferation of products that don’t meet the FDA’s view of the statutory definition of medical foods, according to Miriam Guggenheim, a food and drug law attorney in Washington, D.C. The FDA usually takes enforcement action when it sees a risk to the public’s health.
The agency’s stance has led to confusion – among manufacturers, physicians, consumers, and even regulators – making the market a kind of Wild West, according to Paul Hyman, a Washington, D.C.–based attorney who has represented medical food companies.
George A. Burdock, PhD, an Orlando-based regulatory consultant who has worked with medical food makers, believes the FDA will be forced to expand their narrow definition. He foresees a reconsideration of many medical food products in light of an October 2019 White House executive order prohibiting federal agencies from issuing guidance in lieu of rules.
Manufacturers and the FDA differ
One example of a product about which regulators and manufacturers differ is Theramine, which is described as “specially designed to supply the nervous system with the fuel it needs to meet the altered metabolic requirements of chronic pain and inflammatory disorders.”
It is not considered a medical food by the FDA, and the company has had numerous discussions with the agency about their diverging views, according to Mr. Charuvastra. “We’ve had our warning letters and we’ve had our sit downs, and we just had an inspection.”
Targeted Medical Pharma continues to market its products as medical foods but steers away from making any claims that they are like drugs, he said.
Confusion about medical foods has been exposed in the California Workers’ Compensation System by Leslie Wilson, PhD, and colleagues at the University of California, San Francisco. They found that physicians regularly wrote medical food prescriptions for non–FDA-approved uses and that the system reimbursed the majority of the products at a cost of $15.5 million from 2011 to 2013. More than half of these prescriptions were for Theramine.
Dr. Wilson reported that, for most products, no evidence supported effectiveness, and they were frequently mislabeled – for all 36 that were studied, submissions for reimbursement were made using a National Drug Code, an impossibility because medical foods are not drugs, and 14 were labeled “Rx only.”
Big-name companies joining in
The FDA does not keep a list of approved medical foods or manufacturers. Both small businesses and big food companies like Danone, Nestlé, and Abbott are players. Most products are sold online.

In the United States, Danone’s Nutricia division sells formulas and low-protein foods for IEMs. They also sell Ketocal, a powder or ready-to-drink liquid that is pitched as a balanced medical food to simplify and optimize the ketogenic diet for children with intractable epilepsy. Yet the FDA does not include epilepsy among the conditions that medical foods can treat.
Nestlé sells traditional medical foods for IEMs and also markets a range of what it calls nutritional therapies for such conditions as irritable bowel syndrome and dysphagia.
Nestlé is a minority shareholder in Axona, a product originally developed by Accera (Cerecin as of 2018). Jacquelyn Campo, senior director of global communications at Nestlé Health Sciences, said that the company is not actively involved in the operations management of Cerecin. However, on its website, Nestlé touts Axona, which is only available in the United States, as a “medical food” that “is intended for the clinical dietary management of mild to moderate Alzheimer disease.” The Axona site claims that the main ingredient, caprylic triglyceride, is broken down into ketones that provide fuel to treat cerebral hypometabolism, a precursor to Alzheimer disease. In a 2009 study, daily dosing of a preliminary formulation was associated with improved cognitive performance compared with placebo in patients with mild to moderate Alzheimer disease.
In 2013, the FDA warned Accera that it was misbranding Axona as a medical food and that the therapeutic claims the company was making would make the product an unapproved drug. Ms. Campo said Nestlé is aware of the agency’s warning, but added, “to our knowledge, Cerecin provided answers to the issues raised by the FDA.”
With the goal of getting drug approval, Accera went on to test a tweaked formulation in a 400-patient randomized, placebo-controlled trial called NOURISH AD that ultimately failed. Nevertheless, Axona is still marketed as a medical food. It costs about $100 for a month’s supply.
Repeated requests for comment from Cerecin were not answered. Danielle Schor, an FDA spokesperson, said the agency will not discuss the status of individual products.
More disputes and insurance coverage
Mary Ann DeMarco, executive director of sales and marketing for the Scottsdale, Ariz.–based medical food maker Primus Pharmaceuticals, said the company believes its products fit within the FDA’s medical foods rubric.
These include Fosteum Plus capsules, which it markets “for the clinical dietary management of the metabolic processes of osteopenia and osteoporosis.” The capsules contain a combination of genistein, zinc, calcium, phosphate, vitamin K2, and vitamin D. As proof of effectiveness, the company cites clinical data on some of the ingredients – not the product itself.
Primus has run afoul of the FDA before when it similarly positioned another product, called Limbrel, as a medical food for osteoarthritis. From 2007 to 2017, the FDA received 194 adverse event reports associated with Limbrel, including reports of drug-induced liver injury, pancreatitis, and hypersensitivity pneumonitis. In December 2017, the agency urged Primus to recall Limbrel, a move that it said was “necessary to protect the public health and welfare.” Primus withdrew the product but laid out a defense of Limbrel on a devoted website.
The FDA would not comment any further, said Ms. Schor. Ms. DeMarco said that Primus is working with the FDA to bring Limbrel back to market.
A lack of insurance coverage – even for approved medical foods for IEMs – has frustrated advocates, parents, and manufacturers. They are putting their weight behind the Medical Nutrition Equity Act, which would mandate public and private payer coverage of medical foods for IEMs and digestive conditions such as Crohn disease. That 2019 House bill has 56 cosponsors; there is no Senate companion bill.
“If you can get reimbursement, it really makes the market,” for Primus and the other manufacturers, Mr. Hyman said.
Primus Pharmaceuticals has launched its own campaign, Cover My Medical Foods, to enlist consumers and others to the cause.
Partnering with advocates
Although its low-protein breads, pastas, and baking products are not considered medical foods by the FDA, Dr. Schär is marketing them as such in the United States. They are trying to make a mark in CKD, according to Ms. Donnelly. She added that Dr. Schär has been successful in Europe, where nutrition therapy is more integrated in the health care system.
In 2019, Flavis and the National Kidney Foundation joined forces to raise awareness of nutritional interventions and to build enthusiasm for the Flavis products. The partnership has now ended, mostly because Flavis could no longer afford it, according to Ms. Donnelly.
“Information on diet and nutrition is the most requested subject matter from the NKF,” said Anthony Gucciardo, senior vice president of strategic partnerships at the foundation. The partnership “has never been necessarily about promoting their products per se; it’s promoting a healthy diet and really a diet specific for CKD.”
The NKF developed cobranded materials on low-protein foods for physicians and a teaching tool they could use with patients. Consumers could access nutrition information and a discount on Flavis products on a dedicated webpage. The foundation didn’t describe the low-protein products as medical foods, said Mr. Gucciardo, even if Flavis promoted them as such.
In patients with CKD, dietary management can help prevent the progression to end-stage renal disease. Although Medicare covers medical nutrition therapy – in which patients receive personalized assessments and dietary advice – uptake is abysmally low, according to a 2018 study.
Dr. Burdock thinks low-protein foods for CKD do meet the FDA’s criteria for a medical food but that the agency might not necessarily agree with him. The FDA would not comment.
Physician beware
When it comes to medical foods, the FDA has often looked the other way because the ingredients may already have been proven safe and the danger to an individual or to the public’s health is relatively low, according to Dr. Burdock and Mr. Hyman.
However, if the agency “feels that a medical food will prevent people from seeking medical care or there is potential to defraud the public, it is justified in taking action against the company,” said Dr. Burdock.
According to Dr. Wilson, the pharmacist who reported on the inappropriate medical food prescriptions in the California system, the FDA could help by creating a list of approved medical foods. Physicians should take time to learn about the difference between medical foods and supplements, she said, adding that they should also not hesitate to “question the veracity of the claims for them.”
Ms. Guggenheim believed doctors need to know that, for the most part, these are not FDA-approved products. She emphasized the importance of evaluating the products and looking at the data of their impact on a disease or condition.
“Many of these companies strongly believe that the products work and help people, so clinicians need to be very data driven,” she said.
A version of this article originally appeared on Medscape.com.
As the Food and Drug Administration focuses on other issues, companies, both big and small, are looking to boost physician and consumer interest in their “medical foods” – products that fall somewhere between drugs and supplements and promise to mitigate symptoms, or even address underlying pathologies, of a range of diseases.
Manufacturers now market an array of medical foods, ranging from powders and capsules for Alzheimer disease to low-protein spaghetti for chronic kidney disease (CKD). The FDA has not been completely absent; it takes a narrow view of what medical conditions qualify for treatment with food products and has warned some manufacturers that their misbranded products are acting more like unapproved drugs.
By the FDA’s definition, medical food is limited to products that provide crucial therapy for patients with inborn errors of metabolism (IEM). An example is specialized baby formula for infants with phenylketonuria. Unlike supplements, medical foods are supposed to be used under the supervision of a physician. This has prompted some sales reps to turn up in the clinic, and most manufacturers have online approval forms for doctors to sign. Manufacturers, advisers, and regulators were interviewed for a closer look at this burgeoning industry.
The market
The global market for medical foods – about $18 billion in 2019 – is expected to grow steadily in the near future. It is drawing more interest, especially in Europe, where medical foods are more accepted by physicians and consumers, Meghan Donnelly, MS, RDN, said in an interview. She is a registered dietitian who conducts physician outreach in the United States for Flavis, a division of Dr. Schär. That company, based in northern Italy, started out targeting IEMs but now also sells gluten-free foods for celiac disease and low-protein foods for CKD.
It is still a niche market in the United States – and isn’t likely to ever approach the size of the supplement market, according to Marcus Charuvastra, the managing director of Targeted Medical Pharma, which markets Theramine capsules for pain management, among many other products. But it could still be a big win for a manufacturer if they get a small slice of a big market, such as for Alzheimer disease.
Defining medical food
According to an update of the Orphan Drug Act in 1988, a medical food is “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” The FDA issued regulations to accompany that law in 1993 but has since only issued a guidance document that is not legally binding.
Medical foods are not drugs and they are not supplements (the latter are intended only for healthy people). The FDA doesn’t require formal approval of a medical food, but, by law, the ingredients must be generally recognized as safe, and manufacturers must follow good manufacturing practices. However, the agency has taken a narrow view of what conditions require medical foods.
Policing medical foods hasn’t been a priority for the FDA, which is why there has been a proliferation of products that don’t meet the FDA’s view of the statutory definition of medical foods, according to Miriam Guggenheim, a food and drug law attorney in Washington, D.C. The FDA usually takes enforcement action when it sees a risk to the public’s health.
The agency’s stance has led to confusion – among manufacturers, physicians, consumers, and even regulators – making the market a kind of Wild West, according to Paul Hyman, a Washington, D.C.–based attorney who has represented medical food companies.
George A. Burdock, PhD, an Orlando-based regulatory consultant who has worked with medical food makers, believes the FDA will be forced to expand their narrow definition. He foresees a reconsideration of many medical food products in light of an October 2019 White House executive order prohibiting federal agencies from issuing guidance in lieu of rules.
Manufacturers and the FDA differ
One example of a product about which regulators and manufacturers differ is Theramine, which is described as “specially designed to supply the nervous system with the fuel it needs to meet the altered metabolic requirements of chronic pain and inflammatory disorders.”
It is not considered a medical food by the FDA, and the company has had numerous discussions with the agency about their diverging views, according to Mr. Charuvastra. “We’ve had our warning letters and we’ve had our sit downs, and we just had an inspection.”
Targeted Medical Pharma continues to market its products as medical foods but steers away from making any claims that they are like drugs, he said.
Confusion about medical foods has been exposed in the California Workers’ Compensation System by Leslie Wilson, PhD, and colleagues at the University of California, San Francisco. They found that physicians regularly wrote medical food prescriptions for non–FDA-approved uses and that the system reimbursed the majority of the products at a cost of $15.5 million from 2011 to 2013. More than half of these prescriptions were for Theramine.
Dr. Wilson reported that, for most products, no evidence supported effectiveness, and they were frequently mislabeled – for all 36 that were studied, submissions for reimbursement were made using a National Drug Code, an impossibility because medical foods are not drugs, and 14 were labeled “Rx only.”
Big-name companies joining in
The FDA does not keep a list of approved medical foods or manufacturers. Both small businesses and big food companies like Danone, Nestlé, and Abbott are players. Most products are sold online.

In the United States, Danone’s Nutricia division sells formulas and low-protein foods for IEMs. They also sell Ketocal, a powder or ready-to-drink liquid that is pitched as a balanced medical food to simplify and optimize the ketogenic diet for children with intractable epilepsy. Yet the FDA does not include epilepsy among the conditions that medical foods can treat.
Nestlé sells traditional medical foods for IEMs and also markets a range of what it calls nutritional therapies for such conditions as irritable bowel syndrome and dysphagia.
Nestlé is a minority shareholder in Axona, a product originally developed by Accera (Cerecin as of 2018). Jacquelyn Campo, senior director of global communications at Nestlé Health Sciences, said that the company is not actively involved in the operations management of Cerecin. However, on its website, Nestlé touts Axona, which is only available in the United States, as a “medical food” that “is intended for the clinical dietary management of mild to moderate Alzheimer disease.” The Axona site claims that the main ingredient, caprylic triglyceride, is broken down into ketones that provide fuel to treat cerebral hypometabolism, a precursor to Alzheimer disease. In a 2009 study, daily dosing of a preliminary formulation was associated with improved cognitive performance compared with placebo in patients with mild to moderate Alzheimer disease.
In 2013, the FDA warned Accera that it was misbranding Axona as a medical food and that the therapeutic claims the company was making would make the product an unapproved drug. Ms. Campo said Nestlé is aware of the agency’s warning, but added, “to our knowledge, Cerecin provided answers to the issues raised by the FDA.”
With the goal of getting drug approval, Accera went on to test a tweaked formulation in a 400-patient randomized, placebo-controlled trial called NOURISH AD that ultimately failed. Nevertheless, Axona is still marketed as a medical food. It costs about $100 for a month’s supply.
Repeated requests for comment from Cerecin were not answered. Danielle Schor, an FDA spokesperson, said the agency will not discuss the status of individual products.
More disputes and insurance coverage
Mary Ann DeMarco, executive director of sales and marketing for the Scottsdale, Ariz.–based medical food maker Primus Pharmaceuticals, said the company believes its products fit within the FDA’s medical foods rubric.
These include Fosteum Plus capsules, which it markets “for the clinical dietary management of the metabolic processes of osteopenia and osteoporosis.” The capsules contain a combination of genistein, zinc, calcium, phosphate, vitamin K2, and vitamin D. As proof of effectiveness, the company cites clinical data on some of the ingredients – not the product itself.
Primus has run afoul of the FDA before when it similarly positioned another product, called Limbrel, as a medical food for osteoarthritis. From 2007 to 2017, the FDA received 194 adverse event reports associated with Limbrel, including reports of drug-induced liver injury, pancreatitis, and hypersensitivity pneumonitis. In December 2017, the agency urged Primus to recall Limbrel, a move that it said was “necessary to protect the public health and welfare.” Primus withdrew the product but laid out a defense of Limbrel on a devoted website.
The FDA would not comment any further, said Ms. Schor. Ms. DeMarco said that Primus is working with the FDA to bring Limbrel back to market.
A lack of insurance coverage – even for approved medical foods for IEMs – has frustrated advocates, parents, and manufacturers. They are putting their weight behind the Medical Nutrition Equity Act, which would mandate public and private payer coverage of medical foods for IEMs and digestive conditions such as Crohn disease. That 2019 House bill has 56 cosponsors; there is no Senate companion bill.
“If you can get reimbursement, it really makes the market,” for Primus and the other manufacturers, Mr. Hyman said.
Primus Pharmaceuticals has launched its own campaign, Cover My Medical Foods, to enlist consumers and others to the cause.
Partnering with advocates
Although its low-protein breads, pastas, and baking products are not considered medical foods by the FDA, Dr. Schär is marketing them as such in the United States. They are trying to make a mark in CKD, according to Ms. Donnelly. She added that Dr. Schär has been successful in Europe, where nutrition therapy is more integrated in the health care system.
In 2019, Flavis and the National Kidney Foundation joined forces to raise awareness of nutritional interventions and to build enthusiasm for the Flavis products. The partnership has now ended, mostly because Flavis could no longer afford it, according to Ms. Donnelly.
“Information on diet and nutrition is the most requested subject matter from the NKF,” said Anthony Gucciardo, senior vice president of strategic partnerships at the foundation. The partnership “has never been necessarily about promoting their products per se; it’s promoting a healthy diet and really a diet specific for CKD.”
The NKF developed cobranded materials on low-protein foods for physicians and a teaching tool they could use with patients. Consumers could access nutrition information and a discount on Flavis products on a dedicated webpage. The foundation didn’t describe the low-protein products as medical foods, said Mr. Gucciardo, even if Flavis promoted them as such.
In patients with CKD, dietary management can help prevent the progression to end-stage renal disease. Although Medicare covers medical nutrition therapy – in which patients receive personalized assessments and dietary advice – uptake is abysmally low, according to a 2018 study.
Dr. Burdock thinks low-protein foods for CKD do meet the FDA’s criteria for a medical food but that the agency might not necessarily agree with him. The FDA would not comment.
Physician beware
When it comes to medical foods, the FDA has often looked the other way because the ingredients may already have been proven safe and the danger to an individual or to the public’s health is relatively low, according to Dr. Burdock and Mr. Hyman.
However, if the agency “feels that a medical food will prevent people from seeking medical care or there is potential to defraud the public, it is justified in taking action against the company,” said Dr. Burdock.
According to Dr. Wilson, the pharmacist who reported on the inappropriate medical food prescriptions in the California system, the FDA could help by creating a list of approved medical foods. Physicians should take time to learn about the difference between medical foods and supplements, she said, adding that they should also not hesitate to “question the veracity of the claims for them.”
Ms. Guggenheim believed doctors need to know that, for the most part, these are not FDA-approved products. She emphasized the importance of evaluating the products and looking at the data of their impact on a disease or condition.
“Many of these companies strongly believe that the products work and help people, so clinicians need to be very data driven,” she said.
A version of this article originally appeared on Medscape.com.
As the Food and Drug Administration focuses on other issues, companies, both big and small, are looking to boost physician and consumer interest in their “medical foods” – products that fall somewhere between drugs and supplements and promise to mitigate symptoms, or even address underlying pathologies, of a range of diseases.
Manufacturers now market an array of medical foods, ranging from powders and capsules for Alzheimer disease to low-protein spaghetti for chronic kidney disease (CKD). The FDA has not been completely absent; it takes a narrow view of what medical conditions qualify for treatment with food products and has warned some manufacturers that their misbranded products are acting more like unapproved drugs.
By the FDA’s definition, medical food is limited to products that provide crucial therapy for patients with inborn errors of metabolism (IEM). An example is specialized baby formula for infants with phenylketonuria. Unlike supplements, medical foods are supposed to be used under the supervision of a physician. This has prompted some sales reps to turn up in the clinic, and most manufacturers have online approval forms for doctors to sign. Manufacturers, advisers, and regulators were interviewed for a closer look at this burgeoning industry.
The market
The global market for medical foods – about $18 billion in 2019 – is expected to grow steadily in the near future. It is drawing more interest, especially in Europe, where medical foods are more accepted by physicians and consumers, Meghan Donnelly, MS, RDN, said in an interview. She is a registered dietitian who conducts physician outreach in the United States for Flavis, a division of Dr. Schär. That company, based in northern Italy, started out targeting IEMs but now also sells gluten-free foods for celiac disease and low-protein foods for CKD.
It is still a niche market in the United States – and isn’t likely to ever approach the size of the supplement market, according to Marcus Charuvastra, the managing director of Targeted Medical Pharma, which markets Theramine capsules for pain management, among many other products. But it could still be a big win for a manufacturer if they get a small slice of a big market, such as for Alzheimer disease.
Defining medical food
According to an update of the Orphan Drug Act in 1988, a medical food is “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” The FDA issued regulations to accompany that law in 1993 but has since only issued a guidance document that is not legally binding.
Medical foods are not drugs and they are not supplements (the latter are intended only for healthy people). The FDA doesn’t require formal approval of a medical food, but, by law, the ingredients must be generally recognized as safe, and manufacturers must follow good manufacturing practices. However, the agency has taken a narrow view of what conditions require medical foods.
Policing medical foods hasn’t been a priority for the FDA, which is why there has been a proliferation of products that don’t meet the FDA’s view of the statutory definition of medical foods, according to Miriam Guggenheim, a food and drug law attorney in Washington, D.C. The FDA usually takes enforcement action when it sees a risk to the public’s health.
The agency’s stance has led to confusion – among manufacturers, physicians, consumers, and even regulators – making the market a kind of Wild West, according to Paul Hyman, a Washington, D.C.–based attorney who has represented medical food companies.
George A. Burdock, PhD, an Orlando-based regulatory consultant who has worked with medical food makers, believes the FDA will be forced to expand their narrow definition. He foresees a reconsideration of many medical food products in light of an October 2019 White House executive order prohibiting federal agencies from issuing guidance in lieu of rules.
Manufacturers and the FDA differ
One example of a product about which regulators and manufacturers differ is Theramine, which is described as “specially designed to supply the nervous system with the fuel it needs to meet the altered metabolic requirements of chronic pain and inflammatory disorders.”
It is not considered a medical food by the FDA, and the company has had numerous discussions with the agency about their diverging views, according to Mr. Charuvastra. “We’ve had our warning letters and we’ve had our sit downs, and we just had an inspection.”
Targeted Medical Pharma continues to market its products as medical foods but steers away from making any claims that they are like drugs, he said.
Confusion about medical foods has been exposed in the California Workers’ Compensation System by Leslie Wilson, PhD, and colleagues at the University of California, San Francisco. They found that physicians regularly wrote medical food prescriptions for non–FDA-approved uses and that the system reimbursed the majority of the products at a cost of $15.5 million from 2011 to 2013. More than half of these prescriptions were for Theramine.
Dr. Wilson reported that, for most products, no evidence supported effectiveness, and they were frequently mislabeled – for all 36 that were studied, submissions for reimbursement were made using a National Drug Code, an impossibility because medical foods are not drugs, and 14 were labeled “Rx only.”
Big-name companies joining in
The FDA does not keep a list of approved medical foods or manufacturers. Both small businesses and big food companies like Danone, Nestlé, and Abbott are players. Most products are sold online.

In the United States, Danone’s Nutricia division sells formulas and low-protein foods for IEMs. They also sell Ketocal, a powder or ready-to-drink liquid that is pitched as a balanced medical food to simplify and optimize the ketogenic diet for children with intractable epilepsy. Yet the FDA does not include epilepsy among the conditions that medical foods can treat.
Nestlé sells traditional medical foods for IEMs and also markets a range of what it calls nutritional therapies for such conditions as irritable bowel syndrome and dysphagia.
Nestlé is a minority shareholder in Axona, a product originally developed by Accera (Cerecin as of 2018). Jacquelyn Campo, senior director of global communications at Nestlé Health Sciences, said that the company is not actively involved in the operations management of Cerecin. However, on its website, Nestlé touts Axona, which is only available in the United States, as a “medical food” that “is intended for the clinical dietary management of mild to moderate Alzheimer disease.” The Axona site claims that the main ingredient, caprylic triglyceride, is broken down into ketones that provide fuel to treat cerebral hypometabolism, a precursor to Alzheimer disease. In a 2009 study, daily dosing of a preliminary formulation was associated with improved cognitive performance compared with placebo in patients with mild to moderate Alzheimer disease.
In 2013, the FDA warned Accera that it was misbranding Axona as a medical food and that the therapeutic claims the company was making would make the product an unapproved drug. Ms. Campo said Nestlé is aware of the agency’s warning, but added, “to our knowledge, Cerecin provided answers to the issues raised by the FDA.”
With the goal of getting drug approval, Accera went on to test a tweaked formulation in a 400-patient randomized, placebo-controlled trial called NOURISH AD that ultimately failed. Nevertheless, Axona is still marketed as a medical food. It costs about $100 for a month’s supply.
Repeated requests for comment from Cerecin were not answered. Danielle Schor, an FDA spokesperson, said the agency will not discuss the status of individual products.
More disputes and insurance coverage
Mary Ann DeMarco, executive director of sales and marketing for the Scottsdale, Ariz.–based medical food maker Primus Pharmaceuticals, said the company believes its products fit within the FDA’s medical foods rubric.
These include Fosteum Plus capsules, which it markets “for the clinical dietary management of the metabolic processes of osteopenia and osteoporosis.” The capsules contain a combination of genistein, zinc, calcium, phosphate, vitamin K2, and vitamin D. As proof of effectiveness, the company cites clinical data on some of the ingredients – not the product itself.
Primus has run afoul of the FDA before when it similarly positioned another product, called Limbrel, as a medical food for osteoarthritis. From 2007 to 2017, the FDA received 194 adverse event reports associated with Limbrel, including reports of drug-induced liver injury, pancreatitis, and hypersensitivity pneumonitis. In December 2017, the agency urged Primus to recall Limbrel, a move that it said was “necessary to protect the public health and welfare.” Primus withdrew the product but laid out a defense of Limbrel on a devoted website.
The FDA would not comment any further, said Ms. Schor. Ms. DeMarco said that Primus is working with the FDA to bring Limbrel back to market.
A lack of insurance coverage – even for approved medical foods for IEMs – has frustrated advocates, parents, and manufacturers. They are putting their weight behind the Medical Nutrition Equity Act, which would mandate public and private payer coverage of medical foods for IEMs and digestive conditions such as Crohn disease. That 2019 House bill has 56 cosponsors; there is no Senate companion bill.
“If you can get reimbursement, it really makes the market,” for Primus and the other manufacturers, Mr. Hyman said.
Primus Pharmaceuticals has launched its own campaign, Cover My Medical Foods, to enlist consumers and others to the cause.
Partnering with advocates
Although its low-protein breads, pastas, and baking products are not considered medical foods by the FDA, Dr. Schär is marketing them as such in the United States. They are trying to make a mark in CKD, according to Ms. Donnelly. She added that Dr. Schär has been successful in Europe, where nutrition therapy is more integrated in the health care system.
In 2019, Flavis and the National Kidney Foundation joined forces to raise awareness of nutritional interventions and to build enthusiasm for the Flavis products. The partnership has now ended, mostly because Flavis could no longer afford it, according to Ms. Donnelly.
“Information on diet and nutrition is the most requested subject matter from the NKF,” said Anthony Gucciardo, senior vice president of strategic partnerships at the foundation. The partnership “has never been necessarily about promoting their products per se; it’s promoting a healthy diet and really a diet specific for CKD.”
The NKF developed cobranded materials on low-protein foods for physicians and a teaching tool they could use with patients. Consumers could access nutrition information and a discount on Flavis products on a dedicated webpage. The foundation didn’t describe the low-protein products as medical foods, said Mr. Gucciardo, even if Flavis promoted them as such.
In patients with CKD, dietary management can help prevent the progression to end-stage renal disease. Although Medicare covers medical nutrition therapy – in which patients receive personalized assessments and dietary advice – uptake is abysmally low, according to a 2018 study.
Dr. Burdock thinks low-protein foods for CKD do meet the FDA’s criteria for a medical food but that the agency might not necessarily agree with him. The FDA would not comment.
Physician beware
When it comes to medical foods, the FDA has often looked the other way because the ingredients may already have been proven safe and the danger to an individual or to the public’s health is relatively low, according to Dr. Burdock and Mr. Hyman.
However, if the agency “feels that a medical food will prevent people from seeking medical care or there is potential to defraud the public, it is justified in taking action against the company,” said Dr. Burdock.
According to Dr. Wilson, the pharmacist who reported on the inappropriate medical food prescriptions in the California system, the FDA could help by creating a list of approved medical foods. Physicians should take time to learn about the difference between medical foods and supplements, she said, adding that they should also not hesitate to “question the veracity of the claims for them.”
Ms. Guggenheim believed doctors need to know that, for the most part, these are not FDA-approved products. She emphasized the importance of evaluating the products and looking at the data of their impact on a disease or condition.
“Many of these companies strongly believe that the products work and help people, so clinicians need to be very data driven,” she said.
A version of this article originally appeared on Medscape.com.
Pediatric News welcomes Margaret Thew to the editorial advisory board
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee, in addition to working casually among Children’s Wisconsin pediatric urgent cares within the city of Milwaukee and surrounding suburbs. She has published articles on Nexplanon complications and management of the malnourished state of the eating disorder patient. She currently is involved in a longitudinal study on the health and nutrition of high school endurance runners. Ms. Thew has presented her research both nationally and internationally. Her most recent podium presentation was given at the International Conference for Eating Disorders May 2020 on her research working with high school endurance athletes.
Ms. Thew serves on several committees within Children’s Wisconsin including chair of the domestic violence committee and adjunct on the electronic health record provider committee. She is leading a quality improvement project on adolescent confidential care and the judicious use of the adolescent sensitive note. In addition, she is active within her state nursing organizations; she sits on the Wisconsin Nursing Association board as the advanced practice registered nurse director at large.
Ms. Thew was selected and graduated from the exclusive Duke Johnson & Johnson Nurse Leadership Fellowship in 2016 and was selected as the keynote speaker at graduation by her peers. She was asked to speak to this year’s cohort on her accomplishments as a leader at their virtual graduation April 2020.
Ms. Thew received her Doctor of Nursing Practice, Executive Nurse Leadership May 2020 from Concordia University Wisconsin, and her master’s degree in nursing specializing in family practice from the University of Wisconsin–Milwaukee in December 1997. She is presently enrolled in the nurse educator certificate program at Concordia University Wisconsin. She has worked in the department of adolescent medicine specializing in eating disorders and adolescent gynecology for 6 years and was named the medical director in October 2019. In addition to her work in adolescent medicine, she has an extensive history working as a nurse practitioner in pediatric hematology, oncology, and primary care.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee, in addition to working casually among Children’s Wisconsin pediatric urgent cares within the city of Milwaukee and surrounding suburbs. She has published articles on Nexplanon complications and management of the malnourished state of the eating disorder patient. She currently is involved in a longitudinal study on the health and nutrition of high school endurance runners. Ms. Thew has presented her research both nationally and internationally. Her most recent podium presentation was given at the International Conference for Eating Disorders May 2020 on her research working with high school endurance athletes.
Ms. Thew serves on several committees within Children’s Wisconsin including chair of the domestic violence committee and adjunct on the electronic health record provider committee. She is leading a quality improvement project on adolescent confidential care and the judicious use of the adolescent sensitive note. In addition, she is active within her state nursing organizations; she sits on the Wisconsin Nursing Association board as the advanced practice registered nurse director at large.
Ms. Thew was selected and graduated from the exclusive Duke Johnson & Johnson Nurse Leadership Fellowship in 2016 and was selected as the keynote speaker at graduation by her peers. She was asked to speak to this year’s cohort on her accomplishments as a leader at their virtual graduation April 2020.
Ms. Thew received her Doctor of Nursing Practice, Executive Nurse Leadership May 2020 from Concordia University Wisconsin, and her master’s degree in nursing specializing in family practice from the University of Wisconsin–Milwaukee in December 1997. She is presently enrolled in the nurse educator certificate program at Concordia University Wisconsin. She has worked in the department of adolescent medicine specializing in eating disorders and adolescent gynecology for 6 years and was named the medical director in October 2019. In addition to her work in adolescent medicine, she has an extensive history working as a nurse practitioner in pediatric hematology, oncology, and primary care.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee, in addition to working casually among Children’s Wisconsin pediatric urgent cares within the city of Milwaukee and surrounding suburbs. She has published articles on Nexplanon complications and management of the malnourished state of the eating disorder patient. She currently is involved in a longitudinal study on the health and nutrition of high school endurance runners. Ms. Thew has presented her research both nationally and internationally. Her most recent podium presentation was given at the International Conference for Eating Disorders May 2020 on her research working with high school endurance athletes.
Ms. Thew serves on several committees within Children’s Wisconsin including chair of the domestic violence committee and adjunct on the electronic health record provider committee. She is leading a quality improvement project on adolescent confidential care and the judicious use of the adolescent sensitive note. In addition, she is active within her state nursing organizations; she sits on the Wisconsin Nursing Association board as the advanced practice registered nurse director at large.
Ms. Thew was selected and graduated from the exclusive Duke Johnson & Johnson Nurse Leadership Fellowship in 2016 and was selected as the keynote speaker at graduation by her peers. She was asked to speak to this year’s cohort on her accomplishments as a leader at their virtual graduation April 2020.
Ms. Thew received her Doctor of Nursing Practice, Executive Nurse Leadership May 2020 from Concordia University Wisconsin, and her master’s degree in nursing specializing in family practice from the University of Wisconsin–Milwaukee in December 1997. She is presently enrolled in the nurse educator certificate program at Concordia University Wisconsin. She has worked in the department of adolescent medicine specializing in eating disorders and adolescent gynecology for 6 years and was named the medical director in October 2019. In addition to her work in adolescent medicine, she has an extensive history working as a nurse practitioner in pediatric hematology, oncology, and primary care.
How to not miss something
It’s a mad, mad, mad world. In California, we seem bent on swelling our curve. We’d just begun bringing our patients back into the office. We felt safe, back to business. Then air raid sirens again. Retreat to the Underground. Minimize waiting room waiting, convert to telephone and video. Do what we can to protect our patients and people.
As doctors, we’ve gotten proficient at being triage nurses, examining each appointment request, and sorting who should be seen in person and who could be cared for virtually. We do it for every clinic now.
My 11 a.m. patient last Thursday was an 83-year-old Filipino man with at least a 13-year history of hand dermatitis (based on his long electronic medical record). He had plenty of betamethasone refills. There were even photos of his large, brown hands in his chart. Grandpa hands, calloused by tending his garden and scarred from fixing bikes, building sheds, and doing oil changes for any nephew or niece who asked. The most recent uploads showed a bit of fingertip fissuring, some lichenified plaques. Not much different than they looked after planting persimmon trees a decade ago. I called him early that morning to offer a phone appointment. Perhaps I could save him from venturing out.
“I see that you have an appointment with me in a few hours. If you’d like, I might be able to help you by phone instead.” “Oh, thank you, doc,” he replied. “It’s so kind of you to call. But doc, I think maybe it is better if I come in to see you.” “Are you sure?” “Oh, yes. I will be careful.”
He checked in at 10:45. When I walked into the room he was wearing a face mask and a face shield – good job! He also had a cane and U.S. Navy Destroyer hat. And on the bottom left of his plastic shield was a sticker decal of a U.S. Navy Chief Petty Officer, dress blue insignia. His hands looked just like the photos: no purpura, plenty of lentigines. Fissures, calluses, lichenified plaques. I touched them. In the unaffected areas, his skin was remarkably soft. What stories these hands told. “I was 20 years in the Navy, doc,” he said. “I would have stayed longer but my wife, who’s younger, wanted me back home.” He talked about his nine grandchildren, some of whom went on to join the navy too – but as officers, he noted with pride. Now he spends his days caring for his wife; she has dementia. He can’t stay long because she’s in the waiting room and is likely to get confused if alone for too long.
We quickly reviewed good hand care. I ordered clobetasol ointment. He was pleased; that seemed to work years ago and he was glad to have it again.
So, why did he need to come in? Clearly I could have done this remotely. “Thank you so much for seeing me, doc,” as he stood to walk out. “Proper inspections have to be done in person, right?” Yes, I thought. Otherwise, you might miss something.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
It’s a mad, mad, mad world. In California, we seem bent on swelling our curve. We’d just begun bringing our patients back into the office. We felt safe, back to business. Then air raid sirens again. Retreat to the Underground. Minimize waiting room waiting, convert to telephone and video. Do what we can to protect our patients and people.
As doctors, we’ve gotten proficient at being triage nurses, examining each appointment request, and sorting who should be seen in person and who could be cared for virtually. We do it for every clinic now.
My 11 a.m. patient last Thursday was an 83-year-old Filipino man with at least a 13-year history of hand dermatitis (based on his long electronic medical record). He had plenty of betamethasone refills. There were even photos of his large, brown hands in his chart. Grandpa hands, calloused by tending his garden and scarred from fixing bikes, building sheds, and doing oil changes for any nephew or niece who asked. The most recent uploads showed a bit of fingertip fissuring, some lichenified plaques. Not much different than they looked after planting persimmon trees a decade ago. I called him early that morning to offer a phone appointment. Perhaps I could save him from venturing out.
“I see that you have an appointment with me in a few hours. If you’d like, I might be able to help you by phone instead.” “Oh, thank you, doc,” he replied. “It’s so kind of you to call. But doc, I think maybe it is better if I come in to see you.” “Are you sure?” “Oh, yes. I will be careful.”
He checked in at 10:45. When I walked into the room he was wearing a face mask and a face shield – good job! He also had a cane and U.S. Navy Destroyer hat. And on the bottom left of his plastic shield was a sticker decal of a U.S. Navy Chief Petty Officer, dress blue insignia. His hands looked just like the photos: no purpura, plenty of lentigines. Fissures, calluses, lichenified plaques. I touched them. In the unaffected areas, his skin was remarkably soft. What stories these hands told. “I was 20 years in the Navy, doc,” he said. “I would have stayed longer but my wife, who’s younger, wanted me back home.” He talked about his nine grandchildren, some of whom went on to join the navy too – but as officers, he noted with pride. Now he spends his days caring for his wife; she has dementia. He can’t stay long because she’s in the waiting room and is likely to get confused if alone for too long.
We quickly reviewed good hand care. I ordered clobetasol ointment. He was pleased; that seemed to work years ago and he was glad to have it again.
So, why did he need to come in? Clearly I could have done this remotely. “Thank you so much for seeing me, doc,” as he stood to walk out. “Proper inspections have to be done in person, right?” Yes, I thought. Otherwise, you might miss something.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
It’s a mad, mad, mad world. In California, we seem bent on swelling our curve. We’d just begun bringing our patients back into the office. We felt safe, back to business. Then air raid sirens again. Retreat to the Underground. Minimize waiting room waiting, convert to telephone and video. Do what we can to protect our patients and people.
As doctors, we’ve gotten proficient at being triage nurses, examining each appointment request, and sorting who should be seen in person and who could be cared for virtually. We do it for every clinic now.
My 11 a.m. patient last Thursday was an 83-year-old Filipino man with at least a 13-year history of hand dermatitis (based on his long electronic medical record). He had plenty of betamethasone refills. There were even photos of his large, brown hands in his chart. Grandpa hands, calloused by tending his garden and scarred from fixing bikes, building sheds, and doing oil changes for any nephew or niece who asked. The most recent uploads showed a bit of fingertip fissuring, some lichenified plaques. Not much different than they looked after planting persimmon trees a decade ago. I called him early that morning to offer a phone appointment. Perhaps I could save him from venturing out.
“I see that you have an appointment with me in a few hours. If you’d like, I might be able to help you by phone instead.” “Oh, thank you, doc,” he replied. “It’s so kind of you to call. But doc, I think maybe it is better if I come in to see you.” “Are you sure?” “Oh, yes. I will be careful.”
He checked in at 10:45. When I walked into the room he was wearing a face mask and a face shield – good job! He also had a cane and U.S. Navy Destroyer hat. And on the bottom left of his plastic shield was a sticker decal of a U.S. Navy Chief Petty Officer, dress blue insignia. His hands looked just like the photos: no purpura, plenty of lentigines. Fissures, calluses, lichenified plaques. I touched them. In the unaffected areas, his skin was remarkably soft. What stories these hands told. “I was 20 years in the Navy, doc,” he said. “I would have stayed longer but my wife, who’s younger, wanted me back home.” He talked about his nine grandchildren, some of whom went on to join the navy too – but as officers, he noted with pride. Now he spends his days caring for his wife; she has dementia. He can’t stay long because she’s in the waiting room and is likely to get confused if alone for too long.
We quickly reviewed good hand care. I ordered clobetasol ointment. He was pleased; that seemed to work years ago and he was glad to have it again.
So, why did he need to come in? Clearly I could have done this remotely. “Thank you so much for seeing me, doc,” as he stood to walk out. “Proper inspections have to be done in person, right?” Yes, I thought. Otherwise, you might miss something.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Women suffer less NAFLD but more advanced fibrosis
Women have a lower risk of nonalcoholic fatty liver disease compared with men, but those who do develop the disease are significantly more likely than are men to develop advanced fibrosis, according to data from a meta-analysis of more than 62,000 individuals.
Sex disparity persists in most chronic liver diseases, with more cases and risk of progression reported in men, but the effect of sex on nonalcoholic fatty liver disease (NAFLD) remains unclear, wrote Maya Balakrishnan, MD, of Baylor College of Medicine, Houston, and colleagues. “Knowing whether and how [sex] influences the risk and severity of NAFLD is important for risk stratification, risk modification as well as prognostication,” they said.
In a study published in Clinical Gastroenterology and Hepatology, the researchers conducted a review and meta-analysis of 54 studies, including data from 62,239 patients with NAFLD, 5,428 with nonalcoholic steatohepatitis (NASH), and 6,444 with advanced NAFLD fibrosis.
Overall, women had a 19% lower risk of developing NAFLD compared with men (pooled risk ratio 0.81), a similar risk to men of developing NASH (RR, 1.00), and a 37% increased risk of advanced fibrosis (RR, 1.37) compared with men.
The risk of more severe disease in women increased with age. Among women aged 50 years and older, the risks of NASH and advanced fibrosis were significantly higher, at 17% and 56%, respectively (RR, 1.17 and RR, 1.56). The sex-specific prevalence of advanced fibrosis was not significantly different in patients younger than 50 years.
“Our findings of an increased prevalence of severe phenotypes of NAFLD – NASH and advanced fibrosis – among older women fits well into the current understanding of disease pathogenesis,” the researchers noted.
The findings were limited by several factors, including the cross-sectional nature and heterogeneity of the included studies and lack of data on possible contributions to NASH and NAFLD such as polycystic ovarian syndrome, cumulative use of hormone therapy, and pregnancy, the researchers noted.
However, the results were strengthened by the large patient population. “Given the higher risk of advanced fibrosis observed among women compared to men with NAFLD in our meta-analysis, it is plausible that cirrhosis and its complications may occur with greater frequency among women than in men,” the researchers said. Consequently, women older than 50 years with NAFLD should be evaluated frequently for advanced disease, they noted. In addition, “more focused and intensified efforts may be warranted to target lifestyle modifications and weight loss among young women with NAFLD, particularly in the presence of NASH and/or advanced fibrosis,” the researchers concluded.
Conducting the study at this time was important because of conjectures of sex-based differences in NAFLD prevalence and NAFLD progression, Dr. Balakrishnan said in an interview. “However, the findings from studies conducted across different study populations have been disparate. Therefore, it was important to perform a systematic review and meta-analysis to determine whether there are differences in NAFLD and NAFLD severity risk between the [sexes],” she said.
Dr. Balakrishnan said she was surprised by the higher risk of severe NASH fibrosis in women compared with men once NAFLD is established. “This was surprising and sets NAFLD apart from other highly prevalent chronic liver disease etiologies,” she said. “Other common liver diseases, for example hepatitis B and hepatitis C, tend to be more common among men and tend to progress more rapidly, and tend to be more severe among men compared to women,” she noted.
The take-home message for clinicians is that NAFLD is at least equally, if not more, aggressive in women compared with men, and should be evaluated with equal aggressiveness, Dr. Balakrishnan emphasized. “Moreover, in the future we may expect to see the burden of cirrhosis distributed more equally among women and men than we have to date. This has implications for liver disease screening and women’s health,” she said. The next steps for research are to determine the specific reasons for the higher risk of NAFLD fibrosis in women compared with men, she added.
The study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Balakrishnan M et al. Clin Gastroenterol Hepatol. 2020 Apr 30. doi: 10.1016/j.cgh.2020.04.067.
Women have a lower risk of nonalcoholic fatty liver disease compared with men, but those who do develop the disease are significantly more likely than are men to develop advanced fibrosis, according to data from a meta-analysis of more than 62,000 individuals.
Sex disparity persists in most chronic liver diseases, with more cases and risk of progression reported in men, but the effect of sex on nonalcoholic fatty liver disease (NAFLD) remains unclear, wrote Maya Balakrishnan, MD, of Baylor College of Medicine, Houston, and colleagues. “Knowing whether and how [sex] influences the risk and severity of NAFLD is important for risk stratification, risk modification as well as prognostication,” they said.
In a study published in Clinical Gastroenterology and Hepatology, the researchers conducted a review and meta-analysis of 54 studies, including data from 62,239 patients with NAFLD, 5,428 with nonalcoholic steatohepatitis (NASH), and 6,444 with advanced NAFLD fibrosis.
Overall, women had a 19% lower risk of developing NAFLD compared with men (pooled risk ratio 0.81), a similar risk to men of developing NASH (RR, 1.00), and a 37% increased risk of advanced fibrosis (RR, 1.37) compared with men.
The risk of more severe disease in women increased with age. Among women aged 50 years and older, the risks of NASH and advanced fibrosis were significantly higher, at 17% and 56%, respectively (RR, 1.17 and RR, 1.56). The sex-specific prevalence of advanced fibrosis was not significantly different in patients younger than 50 years.
“Our findings of an increased prevalence of severe phenotypes of NAFLD – NASH and advanced fibrosis – among older women fits well into the current understanding of disease pathogenesis,” the researchers noted.
The findings were limited by several factors, including the cross-sectional nature and heterogeneity of the included studies and lack of data on possible contributions to NASH and NAFLD such as polycystic ovarian syndrome, cumulative use of hormone therapy, and pregnancy, the researchers noted.
However, the results were strengthened by the large patient population. “Given the higher risk of advanced fibrosis observed among women compared to men with NAFLD in our meta-analysis, it is plausible that cirrhosis and its complications may occur with greater frequency among women than in men,” the researchers said. Consequently, women older than 50 years with NAFLD should be evaluated frequently for advanced disease, they noted. In addition, “more focused and intensified efforts may be warranted to target lifestyle modifications and weight loss among young women with NAFLD, particularly in the presence of NASH and/or advanced fibrosis,” the researchers concluded.
Conducting the study at this time was important because of conjectures of sex-based differences in NAFLD prevalence and NAFLD progression, Dr. Balakrishnan said in an interview. “However, the findings from studies conducted across different study populations have been disparate. Therefore, it was important to perform a systematic review and meta-analysis to determine whether there are differences in NAFLD and NAFLD severity risk between the [sexes],” she said.
Dr. Balakrishnan said she was surprised by the higher risk of severe NASH fibrosis in women compared with men once NAFLD is established. “This was surprising and sets NAFLD apart from other highly prevalent chronic liver disease etiologies,” she said. “Other common liver diseases, for example hepatitis B and hepatitis C, tend to be more common among men and tend to progress more rapidly, and tend to be more severe among men compared to women,” she noted.
The take-home message for clinicians is that NAFLD is at least equally, if not more, aggressive in women compared with men, and should be evaluated with equal aggressiveness, Dr. Balakrishnan emphasized. “Moreover, in the future we may expect to see the burden of cirrhosis distributed more equally among women and men than we have to date. This has implications for liver disease screening and women’s health,” she said. The next steps for research are to determine the specific reasons for the higher risk of NAFLD fibrosis in women compared with men, she added.
The study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Balakrishnan M et al. Clin Gastroenterol Hepatol. 2020 Apr 30. doi: 10.1016/j.cgh.2020.04.067.
Women have a lower risk of nonalcoholic fatty liver disease compared with men, but those who do develop the disease are significantly more likely than are men to develop advanced fibrosis, according to data from a meta-analysis of more than 62,000 individuals.
Sex disparity persists in most chronic liver diseases, with more cases and risk of progression reported in men, but the effect of sex on nonalcoholic fatty liver disease (NAFLD) remains unclear, wrote Maya Balakrishnan, MD, of Baylor College of Medicine, Houston, and colleagues. “Knowing whether and how [sex] influences the risk and severity of NAFLD is important for risk stratification, risk modification as well as prognostication,” they said.
In a study published in Clinical Gastroenterology and Hepatology, the researchers conducted a review and meta-analysis of 54 studies, including data from 62,239 patients with NAFLD, 5,428 with nonalcoholic steatohepatitis (NASH), and 6,444 with advanced NAFLD fibrosis.
Overall, women had a 19% lower risk of developing NAFLD compared with men (pooled risk ratio 0.81), a similar risk to men of developing NASH (RR, 1.00), and a 37% increased risk of advanced fibrosis (RR, 1.37) compared with men.
The risk of more severe disease in women increased with age. Among women aged 50 years and older, the risks of NASH and advanced fibrosis were significantly higher, at 17% and 56%, respectively (RR, 1.17 and RR, 1.56). The sex-specific prevalence of advanced fibrosis was not significantly different in patients younger than 50 years.
“Our findings of an increased prevalence of severe phenotypes of NAFLD – NASH and advanced fibrosis – among older women fits well into the current understanding of disease pathogenesis,” the researchers noted.
The findings were limited by several factors, including the cross-sectional nature and heterogeneity of the included studies and lack of data on possible contributions to NASH and NAFLD such as polycystic ovarian syndrome, cumulative use of hormone therapy, and pregnancy, the researchers noted.
However, the results were strengthened by the large patient population. “Given the higher risk of advanced fibrosis observed among women compared to men with NAFLD in our meta-analysis, it is plausible that cirrhosis and its complications may occur with greater frequency among women than in men,” the researchers said. Consequently, women older than 50 years with NAFLD should be evaluated frequently for advanced disease, they noted. In addition, “more focused and intensified efforts may be warranted to target lifestyle modifications and weight loss among young women with NAFLD, particularly in the presence of NASH and/or advanced fibrosis,” the researchers concluded.
Conducting the study at this time was important because of conjectures of sex-based differences in NAFLD prevalence and NAFLD progression, Dr. Balakrishnan said in an interview. “However, the findings from studies conducted across different study populations have been disparate. Therefore, it was important to perform a systematic review and meta-analysis to determine whether there are differences in NAFLD and NAFLD severity risk between the [sexes],” she said.
Dr. Balakrishnan said she was surprised by the higher risk of severe NASH fibrosis in women compared with men once NAFLD is established. “This was surprising and sets NAFLD apart from other highly prevalent chronic liver disease etiologies,” she said. “Other common liver diseases, for example hepatitis B and hepatitis C, tend to be more common among men and tend to progress more rapidly, and tend to be more severe among men compared to women,” she noted.
The take-home message for clinicians is that NAFLD is at least equally, if not more, aggressive in women compared with men, and should be evaluated with equal aggressiveness, Dr. Balakrishnan emphasized. “Moreover, in the future we may expect to see the burden of cirrhosis distributed more equally among women and men than we have to date. This has implications for liver disease screening and women’s health,” she said. The next steps for research are to determine the specific reasons for the higher risk of NAFLD fibrosis in women compared with men, she added.
The study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Balakrishnan M et al. Clin Gastroenterol Hepatol. 2020 Apr 30. doi: 10.1016/j.cgh.2020.04.067.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY














