User login
Characterization of norovirus immunity in nonsecretor adults might provide vaccine model for children
Among nonsecretors – individuals who express a less diverse array of fucosylated histoblood group antigen carbohydrates (HBGAs) and consequently are less susceptible to some norovirus strains – natural infection with norovirus strain GII.2 induced cellular and antibody immunity that lasted for at least 30 days for T cells, monocytes, and dendritic cells and for at least 180 days for blocking antibodies, researchers reported.
“Multiple cellular lineages expressing interferon-gamma and tumor necrosis factor [TNF]–alpha dominated the response. Both T-cell and B-cell responses were cross-reactive with other GII strains, but not GI strains,” Lisa C. Lindesmith of the University of North Carolina, Chapel Hill, and her associates wrote in Cellular and Molecular Gastroenterology and Hepatology. The researchers also found that bile salts enable GII.2 to bind HBGAs produced by nonsecretors. “[I]n addition to HBGAs, one or more specific components of bile also is likely to be an essential co-factor for human norovirus attachment and infection,” the researchers wrote.
Susceptibility to norovirus depends on whether individuals express secretor enzyme, which is encoded by the FUT2 gene. Nonsecretors (who are FUT2–/–) express less varied HBGA, are susceptible to fewer norovirus strains, and are resistant to the predominant norovirus strain, GII.4. “Because future human norovirus vaccines will comprise GII.4 antigen, and because secretor phenotype impacts GII.4 infection and immunity, nonsecretors may mimic young children immunologically in response to GII.4 vaccination,” the researchers explained. But until now, most vaccines have focused on adult secretors, they said.
Their study focused on a familial norovirus outbreak in Chapel Hill that was the first to be characterized among nonsecretors who were naturally infected with norovirus GII.2. Four adults provided blood samples, and one provided a stool sample from which the researchers isolated and cloned the G11.2 capsid gene sequence. They used neutralization assays to study serologic immunity and flow cytometry to assess cellular activation and cytokine production in blood samples from the four cases and from seven healthy donors.
Norovirus GII.2 infection activated both innate and adaptive immunity and typical production of antiviral helper T cell (Th)1 and Th2 cytokines. The cellular immune response lasted at least 30 days, “long after symptom resolution,” the investigators wrote.
Compared with healthy donors, blood specimens from infected nonsecretors showed increases in non-class-switched memory, transitional B cells, and plasmablast B cells, and both naive and memory B cells also were positive for activation markers for at least 30 days after infection. Activated interferon-gamma+ T cells, natural killer cells, TNF-alpha+ monocytes, IL-10+, TNF-alpha+ myeloid dendritic cells, and TNF plasmacytoid dendritic cells also persisted for at least 30 days. Cross-reactive GII immunity was evident for at least 180 days. “GII.2 infection boosted cross-reactive blocking antibodies to GII.3, GII.14, and GII.17, as well as T-cell responses to GII.4, despite the lack of clear serologic evidence of previous GII.4 exposure,” the investigators wrote.
Based on prior reports that bile enhances norovirus growth or ligand binding, they inoculated specimens with chenodeoxycholic acid (CDCA) and glycochenodeoxycholic acid (GCDCA), pig bile, ox bile, or human bile. “Strikingly, the addition of bile enabled GII.2 Chapel Hill outbreak virus-like particle to bind to saliva from the four nonsecretor donors,” the researchers wrote. Bile acids “may override the genetic advantage of less-diverse HBGA expression in nonsecretors by improving the avidity of GII.2 binding to nonsecretor HBGAs, potentially paving the way for infection.” However, bile salts did not enable the GII.2 strain to replicate in human intestinal enteroid cells, which suggests that additional factors play into how norovirus enters human cells, according to the researchers.
The findings, they wrote, “support development of within-genogroup, cross-reactive antibody and T-cell immunity, key outcomes that may provide the foundation for eliciting broad immune responses after GII.4 vaccination in individuals with limited GII.4 immunity, including young children.”
The National Institutes of Health, the Wellcome Trust, the Centers for Disease Control and Prevention, and a Cancer Center Core support provided funding. Ms. Lindesmith and her associates reported having no relevant conflicts of interest.
SOURCE: Lindesmith LC et al. Cell Molec Gastroenterol Hepatol. 2020;10:245-67.
Noroviruses belonging to genogroup II.4 are the leading cause of acute gastroenteritis, but our understanding of norovirus immunity remains incomplete. Most studies have focused on humoral responses and have shown that antibodies may be short lived, strain specific, and not always protective against rechallenge. On the other hand, human innate and T-cell immunity have received little attention despite evidence from the mouse norovirus model that they are critical for limiting viral spread and clearing antigen.
In this study, Lindesmith et al. conducted broad phenotypic and functional analysis of innate and adaptive immune responses following infection with a GII.2 strain of norovirus. Their cohort consists of “nonsecretors,” subjects who express a limited repertoire of histoblood group antigens and are therefore naturally resistant to GII.4 infection. Since nonsecretors have no pre-existing immunity against GII.4 viruses, this system enables the authors to test cross-reactivity of GII.2-specific T cells against GII.4 virus-like particles (VLPs).
The authors showed broad immune activation against natural norovirus infection. Following GII.2 infection, T-cell responses persist for at least a month and, importantly, are cross-reactive against GII.4 VLPs. These findings suggest that T cells may target conserved viral epitopes and play an important role in long-term protection against reinfection.
Developing an effective norovirus vaccine will require a detailed understanding of immune correlates of protection, and this study is a step in the right direction. In future work, tracking epitope-specific T cells must further define the phenotype, functionality, and localization of the norovirus T-cell repertoire.
Vesselin Tomov, MD, PhD, is assistant professor of medicine at the Hospital of the University of Pennsylvania, Philadelphia. He has no conflicts of interest.
Noroviruses belonging to genogroup II.4 are the leading cause of acute gastroenteritis, but our understanding of norovirus immunity remains incomplete. Most studies have focused on humoral responses and have shown that antibodies may be short lived, strain specific, and not always protective against rechallenge. On the other hand, human innate and T-cell immunity have received little attention despite evidence from the mouse norovirus model that they are critical for limiting viral spread and clearing antigen.
In this study, Lindesmith et al. conducted broad phenotypic and functional analysis of innate and adaptive immune responses following infection with a GII.2 strain of norovirus. Their cohort consists of “nonsecretors,” subjects who express a limited repertoire of histoblood group antigens and are therefore naturally resistant to GII.4 infection. Since nonsecretors have no pre-existing immunity against GII.4 viruses, this system enables the authors to test cross-reactivity of GII.2-specific T cells against GII.4 virus-like particles (VLPs).
The authors showed broad immune activation against natural norovirus infection. Following GII.2 infection, T-cell responses persist for at least a month and, importantly, are cross-reactive against GII.4 VLPs. These findings suggest that T cells may target conserved viral epitopes and play an important role in long-term protection against reinfection.
Developing an effective norovirus vaccine will require a detailed understanding of immune correlates of protection, and this study is a step in the right direction. In future work, tracking epitope-specific T cells must further define the phenotype, functionality, and localization of the norovirus T-cell repertoire.
Vesselin Tomov, MD, PhD, is assistant professor of medicine at the Hospital of the University of Pennsylvania, Philadelphia. He has no conflicts of interest.
Noroviruses belonging to genogroup II.4 are the leading cause of acute gastroenteritis, but our understanding of norovirus immunity remains incomplete. Most studies have focused on humoral responses and have shown that antibodies may be short lived, strain specific, and not always protective against rechallenge. On the other hand, human innate and T-cell immunity have received little attention despite evidence from the mouse norovirus model that they are critical for limiting viral spread and clearing antigen.
In this study, Lindesmith et al. conducted broad phenotypic and functional analysis of innate and adaptive immune responses following infection with a GII.2 strain of norovirus. Their cohort consists of “nonsecretors,” subjects who express a limited repertoire of histoblood group antigens and are therefore naturally resistant to GII.4 infection. Since nonsecretors have no pre-existing immunity against GII.4 viruses, this system enables the authors to test cross-reactivity of GII.2-specific T cells against GII.4 virus-like particles (VLPs).
The authors showed broad immune activation against natural norovirus infection. Following GII.2 infection, T-cell responses persist for at least a month and, importantly, are cross-reactive against GII.4 VLPs. These findings suggest that T cells may target conserved viral epitopes and play an important role in long-term protection against reinfection.
Developing an effective norovirus vaccine will require a detailed understanding of immune correlates of protection, and this study is a step in the right direction. In future work, tracking epitope-specific T cells must further define the phenotype, functionality, and localization of the norovirus T-cell repertoire.
Vesselin Tomov, MD, PhD, is assistant professor of medicine at the Hospital of the University of Pennsylvania, Philadelphia. He has no conflicts of interest.
Among nonsecretors – individuals who express a less diverse array of fucosylated histoblood group antigen carbohydrates (HBGAs) and consequently are less susceptible to some norovirus strains – natural infection with norovirus strain GII.2 induced cellular and antibody immunity that lasted for at least 30 days for T cells, monocytes, and dendritic cells and for at least 180 days for blocking antibodies, researchers reported.
“Multiple cellular lineages expressing interferon-gamma and tumor necrosis factor [TNF]–alpha dominated the response. Both T-cell and B-cell responses were cross-reactive with other GII strains, but not GI strains,” Lisa C. Lindesmith of the University of North Carolina, Chapel Hill, and her associates wrote in Cellular and Molecular Gastroenterology and Hepatology. The researchers also found that bile salts enable GII.2 to bind HBGAs produced by nonsecretors. “[I]n addition to HBGAs, one or more specific components of bile also is likely to be an essential co-factor for human norovirus attachment and infection,” the researchers wrote.
Susceptibility to norovirus depends on whether individuals express secretor enzyme, which is encoded by the FUT2 gene. Nonsecretors (who are FUT2–/–) express less varied HBGA, are susceptible to fewer norovirus strains, and are resistant to the predominant norovirus strain, GII.4. “Because future human norovirus vaccines will comprise GII.4 antigen, and because secretor phenotype impacts GII.4 infection and immunity, nonsecretors may mimic young children immunologically in response to GII.4 vaccination,” the researchers explained. But until now, most vaccines have focused on adult secretors, they said.
Their study focused on a familial norovirus outbreak in Chapel Hill that was the first to be characterized among nonsecretors who were naturally infected with norovirus GII.2. Four adults provided blood samples, and one provided a stool sample from which the researchers isolated and cloned the G11.2 capsid gene sequence. They used neutralization assays to study serologic immunity and flow cytometry to assess cellular activation and cytokine production in blood samples from the four cases and from seven healthy donors.
Norovirus GII.2 infection activated both innate and adaptive immunity and typical production of antiviral helper T cell (Th)1 and Th2 cytokines. The cellular immune response lasted at least 30 days, “long after symptom resolution,” the investigators wrote.
Compared with healthy donors, blood specimens from infected nonsecretors showed increases in non-class-switched memory, transitional B cells, and plasmablast B cells, and both naive and memory B cells also were positive for activation markers for at least 30 days after infection. Activated interferon-gamma+ T cells, natural killer cells, TNF-alpha+ monocytes, IL-10+, TNF-alpha+ myeloid dendritic cells, and TNF plasmacytoid dendritic cells also persisted for at least 30 days. Cross-reactive GII immunity was evident for at least 180 days. “GII.2 infection boosted cross-reactive blocking antibodies to GII.3, GII.14, and GII.17, as well as T-cell responses to GII.4, despite the lack of clear serologic evidence of previous GII.4 exposure,” the investigators wrote.
Based on prior reports that bile enhances norovirus growth or ligand binding, they inoculated specimens with chenodeoxycholic acid (CDCA) and glycochenodeoxycholic acid (GCDCA), pig bile, ox bile, or human bile. “Strikingly, the addition of bile enabled GII.2 Chapel Hill outbreak virus-like particle to bind to saliva from the four nonsecretor donors,” the researchers wrote. Bile acids “may override the genetic advantage of less-diverse HBGA expression in nonsecretors by improving the avidity of GII.2 binding to nonsecretor HBGAs, potentially paving the way for infection.” However, bile salts did not enable the GII.2 strain to replicate in human intestinal enteroid cells, which suggests that additional factors play into how norovirus enters human cells, according to the researchers.
The findings, they wrote, “support development of within-genogroup, cross-reactive antibody and T-cell immunity, key outcomes that may provide the foundation for eliciting broad immune responses after GII.4 vaccination in individuals with limited GII.4 immunity, including young children.”
The National Institutes of Health, the Wellcome Trust, the Centers for Disease Control and Prevention, and a Cancer Center Core support provided funding. Ms. Lindesmith and her associates reported having no relevant conflicts of interest.
SOURCE: Lindesmith LC et al. Cell Molec Gastroenterol Hepatol. 2020;10:245-67.
Among nonsecretors – individuals who express a less diverse array of fucosylated histoblood group antigen carbohydrates (HBGAs) and consequently are less susceptible to some norovirus strains – natural infection with norovirus strain GII.2 induced cellular and antibody immunity that lasted for at least 30 days for T cells, monocytes, and dendritic cells and for at least 180 days for blocking antibodies, researchers reported.
“Multiple cellular lineages expressing interferon-gamma and tumor necrosis factor [TNF]–alpha dominated the response. Both T-cell and B-cell responses were cross-reactive with other GII strains, but not GI strains,” Lisa C. Lindesmith of the University of North Carolina, Chapel Hill, and her associates wrote in Cellular and Molecular Gastroenterology and Hepatology. The researchers also found that bile salts enable GII.2 to bind HBGAs produced by nonsecretors. “[I]n addition to HBGAs, one or more specific components of bile also is likely to be an essential co-factor for human norovirus attachment and infection,” the researchers wrote.
Susceptibility to norovirus depends on whether individuals express secretor enzyme, which is encoded by the FUT2 gene. Nonsecretors (who are FUT2–/–) express less varied HBGA, are susceptible to fewer norovirus strains, and are resistant to the predominant norovirus strain, GII.4. “Because future human norovirus vaccines will comprise GII.4 antigen, and because secretor phenotype impacts GII.4 infection and immunity, nonsecretors may mimic young children immunologically in response to GII.4 vaccination,” the researchers explained. But until now, most vaccines have focused on adult secretors, they said.
Their study focused on a familial norovirus outbreak in Chapel Hill that was the first to be characterized among nonsecretors who were naturally infected with norovirus GII.2. Four adults provided blood samples, and one provided a stool sample from which the researchers isolated and cloned the G11.2 capsid gene sequence. They used neutralization assays to study serologic immunity and flow cytometry to assess cellular activation and cytokine production in blood samples from the four cases and from seven healthy donors.
Norovirus GII.2 infection activated both innate and adaptive immunity and typical production of antiviral helper T cell (Th)1 and Th2 cytokines. The cellular immune response lasted at least 30 days, “long after symptom resolution,” the investigators wrote.
Compared with healthy donors, blood specimens from infected nonsecretors showed increases in non-class-switched memory, transitional B cells, and plasmablast B cells, and both naive and memory B cells also were positive for activation markers for at least 30 days after infection. Activated interferon-gamma+ T cells, natural killer cells, TNF-alpha+ monocytes, IL-10+, TNF-alpha+ myeloid dendritic cells, and TNF plasmacytoid dendritic cells also persisted for at least 30 days. Cross-reactive GII immunity was evident for at least 180 days. “GII.2 infection boosted cross-reactive blocking antibodies to GII.3, GII.14, and GII.17, as well as T-cell responses to GII.4, despite the lack of clear serologic evidence of previous GII.4 exposure,” the investigators wrote.
Based on prior reports that bile enhances norovirus growth or ligand binding, they inoculated specimens with chenodeoxycholic acid (CDCA) and glycochenodeoxycholic acid (GCDCA), pig bile, ox bile, or human bile. “Strikingly, the addition of bile enabled GII.2 Chapel Hill outbreak virus-like particle to bind to saliva from the four nonsecretor donors,” the researchers wrote. Bile acids “may override the genetic advantage of less-diverse HBGA expression in nonsecretors by improving the avidity of GII.2 binding to nonsecretor HBGAs, potentially paving the way for infection.” However, bile salts did not enable the GII.2 strain to replicate in human intestinal enteroid cells, which suggests that additional factors play into how norovirus enters human cells, according to the researchers.
The findings, they wrote, “support development of within-genogroup, cross-reactive antibody and T-cell immunity, key outcomes that may provide the foundation for eliciting broad immune responses after GII.4 vaccination in individuals with limited GII.4 immunity, including young children.”
The National Institutes of Health, the Wellcome Trust, the Centers for Disease Control and Prevention, and a Cancer Center Core support provided funding. Ms. Lindesmith and her associates reported having no relevant conflicts of interest.
SOURCE: Lindesmith LC et al. Cell Molec Gastroenterol Hepatol. 2020;10:245-67.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Creating a student-staffed family call line to alleviate clinical burden
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
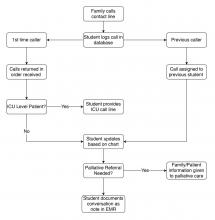
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
The wheels on the bus take lung cancer screening to rural areas
Results from a pilot study, published online July 13 in The Annals of Thoracic Surgery, show that the scheme is both practical and financially sustainable.
During a 10-month test run, the mobile unit screened 548 individuals at 104 sites. Five lung cancers (four of which were early stage) and a type B thymoma were discovered, and all of these individuals went on to have treatment.
Significant pulmonary findings were also discovered in 52 individuals, who were advised to undergo further testing, as well as significant nonpulmonary findings in 152 individuals (of whom 13 required further testing, but none went on to have treatment). These findings included severe coronary disease and thyroid abnormalities.
The bus reached the estimated financial break-even point of 428 scans, but future economic viability of such a program will likely rely on additional revenue from the treatment of patients with incidental findings from low-dose CT screens, acknowledged the authors, led by James R. Headrick Jr, MD, MBA, from the University of Tennessee College of Medicine in Chattanooga.
The real value of the Breathe Easy program, however, comes from bringing both patient education and lung cancer screening services to a high-risk population who might otherwise be overlooked, Headrick said in an interview with Medscape Medical News.
“We were all excited when lung screening was approved, and we got the recommendation from the United States Preventive Services Task Force [USPSTF], and the Centers for Medicare & Medicaid Services signed off on it, and we sat in our offices and clinics and hospitals — and nobody showed up. We were thinking, ‘Wow, we have this simple test, the easiest screening tool in the world, and nobody’s coming,’ “ he said.
“There was certainly an educational issue that needed to be solved,” he continued, “but we were also dealing with a population that had been told that if they smoked and didn’t live life well, there was a 100% chance they were going to get lung cancer and die,” he said.
The individuals screened in the program were very heavy smokers.
The mean pack-years of smoking was 41 — 11 pack-years higher than the minimum recommended under current lung cancer screening guidelines, and 21 pack-years higher than that recently recommended under proposed low-dose CT screening guidelines by the USPSTF.
Albert Rizzo, MD, chief medical officer for the American Lung Association, who was not involved in the study, told Medscape Medical News any initiative that can expand lung cancer screening is welcome, particularly when a program may be self-sustaining.
“The interesting part of this article included the downstream revenue to help make something like this viable,” Rizzo said. “Just doing the scans is probably not going to cover the cost of the mobile unit itself, but if you take into account that other things are being found in addition to lung cancer, such as coronary abnormalities, then it becomes more cost-effective, especially if those patients are then treated at the site where the mobile unit is coming from,” he said.
Starting at square one
The first mobile CT scanner was launched in Nagano Prefecture, a rural area in Japan, in 1996. Since then, mobile screening units, primarily mounted on tractor trailers, have brought screening to centralized areas, such as shopping mall parking lots. The Levine Cancer Institute in Charlotte, North Carolina, also has a mobile CT-screening unit mounted in a modified box truck.
For Headrick and colleagues the goal was not to reinvent the wheel, but to see if a mobile lung cancer screening program could improve access and also pay for itself in a time of parsimonious support for preventive medicine.
Their first challenge was the mobile unit itself.
“CT scanners are sensitive, complex electrical machines that require climate control and a level environment to operate. Historically, they have been placed in tractor trailers and parked on level concrete slabs connected to external power supplies. We needed mobility, self-leveling, independent power, climate control, patient comfort, and drivability,” they wrote.
They assembled a team of engineers from CT and vehicle makers, and input was also provided by a thoracic surgeon, pulmonologist, radiologist, CT technician, and driver with a commercial driver’s license. Together, they designed and built the bus over 8 months. Funds for the total cost of the prototype vehicle ($650,000) came from two local nonprofit foundations. The estimated cost for a commercial version of the same vehicle was $850,000.
The Breathe Easy pilot began operation in early 2018, with the initial plan to drive the bus within a 2-hour radius of CHI Memorial Hospital, Chattanooga, Tennessee, to avoid overnight trips. The radius was later shortened to 1.5 hours when operators realized it was a burden for patients with significant screening findings to travel to as much as 4 hours (round trip) to Chattanooga for further testing.
Each screening visit takes about 15 minutes.
Cancer and other significant findings
As noted before, the bus traveled to 104 sites over 10 months, and 548 patients with a mean age of 62 were screened. Five lung cancers were identified, including two stage 1A, one stage 1A2, one stage 1B, and one stage 3A.
Two patients with early stage disease underwent stereotactic body radiation therapy, and two underwent minimally invasive surgery (a segmentectomy and a lobectomy). The patient with stage 3A disease underwent curative chemotherapy and radiation therapy. One patient with a type B1 thymoma underwent robotic-assisted thoracoscopic resection with en bloc pericardial resection and reconstruction.
A total of 51 patients had a significant pulmonary finding of Lung CT Screening Reporting and Data System (Lung-RADS) 3 or 4 and were advised to follow up with further testing, but 17 patients in this group did not pursue further testing. Of these 17 patients, 15 had been screened in a health clinic for the homeless at a rural site.
Significant nonpulmonary findings included moderate to severe coronary artery disease in 101 patients, abdominal findings in 15, thyroid abnormalities in 14, other thoracic findings in 10, and ascending aortic dilatation in 9. Of the 152 patients with nonpulmonary findings, only 13 required further testing and none required treatment.
Revisions, improvements, and priorities
The Breathe Easy bus has been in operation for more than 2 years, performing an average of approximately 100 screenings per month, with a goal of 200. The bus continued to operate throughout the COVID-19 pandemic because many patients viewed it as a safer alternative to a hospital visit, Headrick said.
Design changes planned to improve performance of the bus include a stronger chassis and structural components, as well as swapping out the 16-slice CT unit for a specially designed 64-slice mobile unit that can be operated with an iPad and provide gated coronary calcium scores.
When challenged about whether the cost of lung cancer screening is the best use of limited resources, Headrick said, “if it’s not, then when we need to go back to the drawing board and jump-start lung cancer screening.”
“When I spent a year in 2014-2015 trying to talk to radio stations, news stations, media, nobody really cared about lung cancer screening,” he said. “But as soon as I had this shiny object, which is the bus, which we labeled as the easiest and most valuable doctor visit, people had an interest.”
The pilot study was supported by local nonprofit foundations through the CHI Memorial Foundation.
This article first appeared on Medscape.com.
Results from a pilot study, published online July 13 in The Annals of Thoracic Surgery, show that the scheme is both practical and financially sustainable.
During a 10-month test run, the mobile unit screened 548 individuals at 104 sites. Five lung cancers (four of which were early stage) and a type B thymoma were discovered, and all of these individuals went on to have treatment.
Significant pulmonary findings were also discovered in 52 individuals, who were advised to undergo further testing, as well as significant nonpulmonary findings in 152 individuals (of whom 13 required further testing, but none went on to have treatment). These findings included severe coronary disease and thyroid abnormalities.
The bus reached the estimated financial break-even point of 428 scans, but future economic viability of such a program will likely rely on additional revenue from the treatment of patients with incidental findings from low-dose CT screens, acknowledged the authors, led by James R. Headrick Jr, MD, MBA, from the University of Tennessee College of Medicine in Chattanooga.
The real value of the Breathe Easy program, however, comes from bringing both patient education and lung cancer screening services to a high-risk population who might otherwise be overlooked, Headrick said in an interview with Medscape Medical News.
“We were all excited when lung screening was approved, and we got the recommendation from the United States Preventive Services Task Force [USPSTF], and the Centers for Medicare & Medicaid Services signed off on it, and we sat in our offices and clinics and hospitals — and nobody showed up. We were thinking, ‘Wow, we have this simple test, the easiest screening tool in the world, and nobody’s coming,’ “ he said.
“There was certainly an educational issue that needed to be solved,” he continued, “but we were also dealing with a population that had been told that if they smoked and didn’t live life well, there was a 100% chance they were going to get lung cancer and die,” he said.
The individuals screened in the program were very heavy smokers.
The mean pack-years of smoking was 41 — 11 pack-years higher than the minimum recommended under current lung cancer screening guidelines, and 21 pack-years higher than that recently recommended under proposed low-dose CT screening guidelines by the USPSTF.
Albert Rizzo, MD, chief medical officer for the American Lung Association, who was not involved in the study, told Medscape Medical News any initiative that can expand lung cancer screening is welcome, particularly when a program may be self-sustaining.
“The interesting part of this article included the downstream revenue to help make something like this viable,” Rizzo said. “Just doing the scans is probably not going to cover the cost of the mobile unit itself, but if you take into account that other things are being found in addition to lung cancer, such as coronary abnormalities, then it becomes more cost-effective, especially if those patients are then treated at the site where the mobile unit is coming from,” he said.
Starting at square one
The first mobile CT scanner was launched in Nagano Prefecture, a rural area in Japan, in 1996. Since then, mobile screening units, primarily mounted on tractor trailers, have brought screening to centralized areas, such as shopping mall parking lots. The Levine Cancer Institute in Charlotte, North Carolina, also has a mobile CT-screening unit mounted in a modified box truck.
For Headrick and colleagues the goal was not to reinvent the wheel, but to see if a mobile lung cancer screening program could improve access and also pay for itself in a time of parsimonious support for preventive medicine.
Their first challenge was the mobile unit itself.
“CT scanners are sensitive, complex electrical machines that require climate control and a level environment to operate. Historically, they have been placed in tractor trailers and parked on level concrete slabs connected to external power supplies. We needed mobility, self-leveling, independent power, climate control, patient comfort, and drivability,” they wrote.
They assembled a team of engineers from CT and vehicle makers, and input was also provided by a thoracic surgeon, pulmonologist, radiologist, CT technician, and driver with a commercial driver’s license. Together, they designed and built the bus over 8 months. Funds for the total cost of the prototype vehicle ($650,000) came from two local nonprofit foundations. The estimated cost for a commercial version of the same vehicle was $850,000.
The Breathe Easy pilot began operation in early 2018, with the initial plan to drive the bus within a 2-hour radius of CHI Memorial Hospital, Chattanooga, Tennessee, to avoid overnight trips. The radius was later shortened to 1.5 hours when operators realized it was a burden for patients with significant screening findings to travel to as much as 4 hours (round trip) to Chattanooga for further testing.
Each screening visit takes about 15 minutes.
Cancer and other significant findings
As noted before, the bus traveled to 104 sites over 10 months, and 548 patients with a mean age of 62 were screened. Five lung cancers were identified, including two stage 1A, one stage 1A2, one stage 1B, and one stage 3A.
Two patients with early stage disease underwent stereotactic body radiation therapy, and two underwent minimally invasive surgery (a segmentectomy and a lobectomy). The patient with stage 3A disease underwent curative chemotherapy and radiation therapy. One patient with a type B1 thymoma underwent robotic-assisted thoracoscopic resection with en bloc pericardial resection and reconstruction.
A total of 51 patients had a significant pulmonary finding of Lung CT Screening Reporting and Data System (Lung-RADS) 3 or 4 and were advised to follow up with further testing, but 17 patients in this group did not pursue further testing. Of these 17 patients, 15 had been screened in a health clinic for the homeless at a rural site.
Significant nonpulmonary findings included moderate to severe coronary artery disease in 101 patients, abdominal findings in 15, thyroid abnormalities in 14, other thoracic findings in 10, and ascending aortic dilatation in 9. Of the 152 patients with nonpulmonary findings, only 13 required further testing and none required treatment.
Revisions, improvements, and priorities
The Breathe Easy bus has been in operation for more than 2 years, performing an average of approximately 100 screenings per month, with a goal of 200. The bus continued to operate throughout the COVID-19 pandemic because many patients viewed it as a safer alternative to a hospital visit, Headrick said.
Design changes planned to improve performance of the bus include a stronger chassis and structural components, as well as swapping out the 16-slice CT unit for a specially designed 64-slice mobile unit that can be operated with an iPad and provide gated coronary calcium scores.
When challenged about whether the cost of lung cancer screening is the best use of limited resources, Headrick said, “if it’s not, then when we need to go back to the drawing board and jump-start lung cancer screening.”
“When I spent a year in 2014-2015 trying to talk to radio stations, news stations, media, nobody really cared about lung cancer screening,” he said. “But as soon as I had this shiny object, which is the bus, which we labeled as the easiest and most valuable doctor visit, people had an interest.”
The pilot study was supported by local nonprofit foundations through the CHI Memorial Foundation.
This article first appeared on Medscape.com.
Results from a pilot study, published online July 13 in The Annals of Thoracic Surgery, show that the scheme is both practical and financially sustainable.
During a 10-month test run, the mobile unit screened 548 individuals at 104 sites. Five lung cancers (four of which were early stage) and a type B thymoma were discovered, and all of these individuals went on to have treatment.
Significant pulmonary findings were also discovered in 52 individuals, who were advised to undergo further testing, as well as significant nonpulmonary findings in 152 individuals (of whom 13 required further testing, but none went on to have treatment). These findings included severe coronary disease and thyroid abnormalities.
The bus reached the estimated financial break-even point of 428 scans, but future economic viability of such a program will likely rely on additional revenue from the treatment of patients with incidental findings from low-dose CT screens, acknowledged the authors, led by James R. Headrick Jr, MD, MBA, from the University of Tennessee College of Medicine in Chattanooga.
The real value of the Breathe Easy program, however, comes from bringing both patient education and lung cancer screening services to a high-risk population who might otherwise be overlooked, Headrick said in an interview with Medscape Medical News.
“We were all excited when lung screening was approved, and we got the recommendation from the United States Preventive Services Task Force [USPSTF], and the Centers for Medicare & Medicaid Services signed off on it, and we sat in our offices and clinics and hospitals — and nobody showed up. We were thinking, ‘Wow, we have this simple test, the easiest screening tool in the world, and nobody’s coming,’ “ he said.
“There was certainly an educational issue that needed to be solved,” he continued, “but we were also dealing with a population that had been told that if they smoked and didn’t live life well, there was a 100% chance they were going to get lung cancer and die,” he said.
The individuals screened in the program were very heavy smokers.
The mean pack-years of smoking was 41 — 11 pack-years higher than the minimum recommended under current lung cancer screening guidelines, and 21 pack-years higher than that recently recommended under proposed low-dose CT screening guidelines by the USPSTF.
Albert Rizzo, MD, chief medical officer for the American Lung Association, who was not involved in the study, told Medscape Medical News any initiative that can expand lung cancer screening is welcome, particularly when a program may be self-sustaining.
“The interesting part of this article included the downstream revenue to help make something like this viable,” Rizzo said. “Just doing the scans is probably not going to cover the cost of the mobile unit itself, but if you take into account that other things are being found in addition to lung cancer, such as coronary abnormalities, then it becomes more cost-effective, especially if those patients are then treated at the site where the mobile unit is coming from,” he said.
Starting at square one
The first mobile CT scanner was launched in Nagano Prefecture, a rural area in Japan, in 1996. Since then, mobile screening units, primarily mounted on tractor trailers, have brought screening to centralized areas, such as shopping mall parking lots. The Levine Cancer Institute in Charlotte, North Carolina, also has a mobile CT-screening unit mounted in a modified box truck.
For Headrick and colleagues the goal was not to reinvent the wheel, but to see if a mobile lung cancer screening program could improve access and also pay for itself in a time of parsimonious support for preventive medicine.
Their first challenge was the mobile unit itself.
“CT scanners are sensitive, complex electrical machines that require climate control and a level environment to operate. Historically, they have been placed in tractor trailers and parked on level concrete slabs connected to external power supplies. We needed mobility, self-leveling, independent power, climate control, patient comfort, and drivability,” they wrote.
They assembled a team of engineers from CT and vehicle makers, and input was also provided by a thoracic surgeon, pulmonologist, radiologist, CT technician, and driver with a commercial driver’s license. Together, they designed and built the bus over 8 months. Funds for the total cost of the prototype vehicle ($650,000) came from two local nonprofit foundations. The estimated cost for a commercial version of the same vehicle was $850,000.
The Breathe Easy pilot began operation in early 2018, with the initial plan to drive the bus within a 2-hour radius of CHI Memorial Hospital, Chattanooga, Tennessee, to avoid overnight trips. The radius was later shortened to 1.5 hours when operators realized it was a burden for patients with significant screening findings to travel to as much as 4 hours (round trip) to Chattanooga for further testing.
Each screening visit takes about 15 minutes.
Cancer and other significant findings
As noted before, the bus traveled to 104 sites over 10 months, and 548 patients with a mean age of 62 were screened. Five lung cancers were identified, including two stage 1A, one stage 1A2, one stage 1B, and one stage 3A.
Two patients with early stage disease underwent stereotactic body radiation therapy, and two underwent minimally invasive surgery (a segmentectomy and a lobectomy). The patient with stage 3A disease underwent curative chemotherapy and radiation therapy. One patient with a type B1 thymoma underwent robotic-assisted thoracoscopic resection with en bloc pericardial resection and reconstruction.
A total of 51 patients had a significant pulmonary finding of Lung CT Screening Reporting and Data System (Lung-RADS) 3 or 4 and were advised to follow up with further testing, but 17 patients in this group did not pursue further testing. Of these 17 patients, 15 had been screened in a health clinic for the homeless at a rural site.
Significant nonpulmonary findings included moderate to severe coronary artery disease in 101 patients, abdominal findings in 15, thyroid abnormalities in 14, other thoracic findings in 10, and ascending aortic dilatation in 9. Of the 152 patients with nonpulmonary findings, only 13 required further testing and none required treatment.
Revisions, improvements, and priorities
The Breathe Easy bus has been in operation for more than 2 years, performing an average of approximately 100 screenings per month, with a goal of 200. The bus continued to operate throughout the COVID-19 pandemic because many patients viewed it as a safer alternative to a hospital visit, Headrick said.
Design changes planned to improve performance of the bus include a stronger chassis and structural components, as well as swapping out the 16-slice CT unit for a specially designed 64-slice mobile unit that can be operated with an iPad and provide gated coronary calcium scores.
When challenged about whether the cost of lung cancer screening is the best use of limited resources, Headrick said, “if it’s not, then when we need to go back to the drawing board and jump-start lung cancer screening.”
“When I spent a year in 2014-2015 trying to talk to radio stations, news stations, media, nobody really cared about lung cancer screening,” he said. “But as soon as I had this shiny object, which is the bus, which we labeled as the easiest and most valuable doctor visit, people had an interest.”
The pilot study was supported by local nonprofit foundations through the CHI Memorial Foundation.
This article first appeared on Medscape.com.
Revisiting Xanax amid the coronavirus crisis
One of the more alarming trends that has emerged during the coronavirus crisis is the concomitant rise in the use of benzodiazepines, such as Xanax. It has been reported that at-risk individuals began seeking prescription anxiolytics as early as mid-February with a consequent peak of 34% the following month, coinciding with the World Health Organization’s declaration of a global pandemic.1
Consistent with the available literature indicating that women are twice as likely to be affected by anxiety disorders, the prescription spikes were almost double when compared with those of their male counterparts.2 The pandemic has instilled a sense of fear in people, leading to social repercussions, such as estrangement, insomnia, and paranoia for at-risk populations.3,4
“Benzos” are commonly prescribed to help people sleep or to assist them in overcoming a host of anxiety disorders. The rapid onset of effects make Xanax a desirable and efficacious benzodiazepine.5 The use of these medications might not be an immediate cause for concern because patients might be taking it as intended. Nevertheless, clinicians are shying away from medical management in favor of counseling or therapy.
Dangerous trends
Numerous factors might contribute to this grim scenario, including patient dependence on benzodiazepines, paranoia about engaging with health care professionals because of fear tied to potential COVID-19 exposure, and/or increased access to illicit counterfeit pills from drug dealers or the dark web markets.
Lessons can be gleaned from the most extensive dark web drug busts in Britain’s history, in which a deluge of “pharmaceutical grade” Xanax pills made it to the hands of drug dealers and consumers between 2015 and 2017.6 A similar phenomenon emerged stateside.7 Virtually indistinguishable from recognized 2-mg Xanax pills, these fake pills posed a serious challenge to forensic scientists.8 The threat of overdose is very real for users targeted by the counterfeit Xanax trade, especially since those at risk often bypass professional health care guidelines.
In broad daylight, the drug dealers ran their operations revolving around two fake Xanax products: a primary knockoff and a limited edition – and vastly more potent “Red Devil” variant that was intentionally dyed for branding purposes. Because the “Red Devil” formulations contained 2.5 times the dose of the 2-mg pill, it had even more pronounced tolerance, dependence, and withdrawal effects (for example, panic attacks, anxiety, and/or hallucinations) – fatal consequences for users involved in consuming other drugs, such as alcohol or opioids. Preexisting drug users tend to gravitate toward benzodiazepines, such as alprazolam (Xanax), perhaps in part, because of its relatively rapid onset of action. Xanax also is known for inducing proeuphoric states at higher doses, hence the appeal of the “Red Devil” pills.
Benzodiazepines, as a class of drugs, facilitate the neurotransmitter gamma-aminobutryric acid’s (GABA) effect on the brain, producing anxiolytic, hypnotic, and/or anticonvulsant states within the user.9 Unbeknownst to numerous users is the fact that drugs such as alcohol and opioids, like Xanax, also serve as respiratory depressants, overriding the brain’s governance of the breathing mechanism. This, in turn, leads to unintended overdose deaths, even among seasoned drug seekers.
Overdose deaths have been steadily climbing over the years because it is common for some users to consume alcohol while being on Xanax therapy – without realizing that both substances are depressants and that taking them together can lead to side effects such as respiratory depression.
Forensic cases also have revealed that preexisting opioid consumers were drawn to Xanax; the drug’s potent mechanism of action would likely appeal to habituated users. A typical behavioral pattern has emerged among users and must be addressed. According to Australian Professor Shane Darke: “So they take their Xanax, they take their painkiller, then they get drunk, that could be enough to kill them.”
Fatalities are more likely when benzodiazepines are combined with other drug classes or if the existing supply is contaminated or laced (for example, with fentanyl).8
As far as deaths by accidental benzodiazepine overdose are concerned, a similar epidemic has been recorded in the United States. In 2013, almost one-third of all prescription overdose deaths can be attributed to the use of benzodiazepines (for example, Xanax, Valium, and Ativan). However, media attention has been considerably muted, especially when compared with that of narcotic abuse. This is even more puzzling when taking into account that three-quarters of benzodiazepine mortalities co-occur within the context of narcotic consumption. Substance Abuse and Mental Health Services Administration data confirm the ubiquitous nature of benzodiazepine (such as alprazolam) coprescriptions, accounting for roughly half of the 176,000 emergency department cases for 2011. The Centers for Disease Control and Prevention noted that there was a 67% increase in benzodiazepine prescriptions between 1996 and 2013, which warranted more stringent regulations for this particular class of drugs.
In 2016, the CDC issued new guidelines for opioid use acknowledging the danger of benzodiazepine coprescriptions. Food and Drug Administration “black box” warnings now grace the prescriptions of both of these drug classes.10 This trend remains on an upward trajectory, even more so during the pandemic, as there are 9.7 million prescriptions of anxiolytics/hypnotics such as Xanax, Ativan, and Klonopin in the United States as of March 2020, which represents a 10% increase over the previous year. , as well as the implementation of urine drug screening monitoring for drug adherence/compliance and diversion in those with suspected benzodiazepine addiction or a history of polysubstance abuse.11,12
Clinical correlates
For patients who present acutely with Xanax toxicity in the emergency room setting, we will need to initially stabilize the vital signs and address the ongoing symptoms. It is advisable to arrange health care accommodations for patients with physical dependence to monitor and treat their withdrawal symptoms. The patient should be enrolled in a comprehensive addiction facility after undergoing formal detoxification; a tapered treatment protocol will need to be implemented because quitting “cold turkey” can lead to convulsions and, in some cases, death. Patient education, talk therapy, and alternatives to benzodiazepines should be discussed with the clinician.13,145
However, to truly address the elephant in the room, we will need to consider institutional reforms to prevent a similar situation from arising in the future. Primary care physician shortages are compounded by changes in insurance policies. Nurses and physician assistants will need to be trained to manage benzodiazepine prescriptions. If there are community shortages in physicians, patients might turn to illegal means to secure their benzodiazepine supply, and it is imperative that we have the necessary fellowship and education programs to educate nonphysician health care clinicians with benzodiazepine management. Because physicians were prescribing benzodiazepines liberally, the Prescription Drug Monitoring Programs (PDMP) was enacted to monitor physician practices. Unfortunately, this ultimately intimidated physicians and effectively curbed reasonable physician prescribing patterns. It might be necessary to revisit existing prescription monitoring programs, encourage drug evaluations and guidelines based on evidence-based medicine and embrace telemedicine in order to facilitate patient-physician communication.
As of now, it is too early to prescribe Xanax routinely for ongoing anxiety experienced during the coronavirus crisis, and several physicians are cautious about prescribing antianxiety medications for more than a few months.17 Surprisingly, researchers in Barcelona have even explored the role of Xanax as potentially inhibiting Mpro, the primary protease of coronavirus, thereby forestalling the virus’s ability to replicate.16 However, it is worth noting that, given the preliminary nature of the results, any attempts at conclusively integrating Xanax within the context of coronavirus therapy would be premature.
References
1. Luhby T. Anti-anxiety medication prescriptions up 34% since coronavirus. CNN. 2020 Apr 16.
2. Women and Anxiety. Anxiety and Depression Association of America.
3. Shigemura J et al. Psychiatry Clin Neurosci. 2012 Apr 7;74(4):281-2.
4. Petersen A. More people are taking drugs for anxiety and insomnia, and doctors are worried. The Wall Street Journal. 2020 May 25.
5. Downey M. Xanax overdose and related deaths. National Drug & Alcohol Research Centre. UNSW Sydney.
6. Bryant B. Fake Xanax: The UK’s biggest ever dark net drugs bust. BBC. 2018 Mar 10.
7. Reinberg S. Fatal overdoses rising from sedatives like Valium, Xanax. HealthDay. 2016 Feb.
8. Is counterfeit Xanax dangerous? American Addiction Centers. Updated 2018 Nov 14.
9. McLaren E. Xanax history and statistics. Drugabuse.com.
10. Benzodiazepines and opioids. National Institute on Drug Abuse. 2018 Mar 15.
11. Choudhry Z et al. J Psychiatry. 2015;18(5). doi: 10.4172/2378-5756.1000319.
12. Islam FA et al. Current Psychiatry. 2018 Dec 17(12):43-4.
13. Adams M. Xanax death rate on the rise. White Sands Treatment. 2017 Sept.NEED LINK
14. Storrs C. Benzodiazepine overdose deaths soared in recent years, study finds. CNN. 2016 Feb. 18.
15. Hanscom DA. Plan A – Thrive and survive COVID-19. Back in Control. 2020.
16. Smith C. Xanax, a common anxiety medication, might actually block coronavirus. BGR. 2020 May 29.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Mr. Choudhry is a research assistant at the IMCHF. He has no disclosures.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF and is Mr. Choudhry’s father. He has no disclosures.
One of the more alarming trends that has emerged during the coronavirus crisis is the concomitant rise in the use of benzodiazepines, such as Xanax. It has been reported that at-risk individuals began seeking prescription anxiolytics as early as mid-February with a consequent peak of 34% the following month, coinciding with the World Health Organization’s declaration of a global pandemic.1
Consistent with the available literature indicating that women are twice as likely to be affected by anxiety disorders, the prescription spikes were almost double when compared with those of their male counterparts.2 The pandemic has instilled a sense of fear in people, leading to social repercussions, such as estrangement, insomnia, and paranoia for at-risk populations.3,4
“Benzos” are commonly prescribed to help people sleep or to assist them in overcoming a host of anxiety disorders. The rapid onset of effects make Xanax a desirable and efficacious benzodiazepine.5 The use of these medications might not be an immediate cause for concern because patients might be taking it as intended. Nevertheless, clinicians are shying away from medical management in favor of counseling or therapy.
Dangerous trends
Numerous factors might contribute to this grim scenario, including patient dependence on benzodiazepines, paranoia about engaging with health care professionals because of fear tied to potential COVID-19 exposure, and/or increased access to illicit counterfeit pills from drug dealers or the dark web markets.
Lessons can be gleaned from the most extensive dark web drug busts in Britain’s history, in which a deluge of “pharmaceutical grade” Xanax pills made it to the hands of drug dealers and consumers between 2015 and 2017.6 A similar phenomenon emerged stateside.7 Virtually indistinguishable from recognized 2-mg Xanax pills, these fake pills posed a serious challenge to forensic scientists.8 The threat of overdose is very real for users targeted by the counterfeit Xanax trade, especially since those at risk often bypass professional health care guidelines.
In broad daylight, the drug dealers ran their operations revolving around two fake Xanax products: a primary knockoff and a limited edition – and vastly more potent “Red Devil” variant that was intentionally dyed for branding purposes. Because the “Red Devil” formulations contained 2.5 times the dose of the 2-mg pill, it had even more pronounced tolerance, dependence, and withdrawal effects (for example, panic attacks, anxiety, and/or hallucinations) – fatal consequences for users involved in consuming other drugs, such as alcohol or opioids. Preexisting drug users tend to gravitate toward benzodiazepines, such as alprazolam (Xanax), perhaps in part, because of its relatively rapid onset of action. Xanax also is known for inducing proeuphoric states at higher doses, hence the appeal of the “Red Devil” pills.
Benzodiazepines, as a class of drugs, facilitate the neurotransmitter gamma-aminobutryric acid’s (GABA) effect on the brain, producing anxiolytic, hypnotic, and/or anticonvulsant states within the user.9 Unbeknownst to numerous users is the fact that drugs such as alcohol and opioids, like Xanax, also serve as respiratory depressants, overriding the brain’s governance of the breathing mechanism. This, in turn, leads to unintended overdose deaths, even among seasoned drug seekers.
Overdose deaths have been steadily climbing over the years because it is common for some users to consume alcohol while being on Xanax therapy – without realizing that both substances are depressants and that taking them together can lead to side effects such as respiratory depression.
Forensic cases also have revealed that preexisting opioid consumers were drawn to Xanax; the drug’s potent mechanism of action would likely appeal to habituated users. A typical behavioral pattern has emerged among users and must be addressed. According to Australian Professor Shane Darke: “So they take their Xanax, they take their painkiller, then they get drunk, that could be enough to kill them.”
Fatalities are more likely when benzodiazepines are combined with other drug classes or if the existing supply is contaminated or laced (for example, with fentanyl).8
As far as deaths by accidental benzodiazepine overdose are concerned, a similar epidemic has been recorded in the United States. In 2013, almost one-third of all prescription overdose deaths can be attributed to the use of benzodiazepines (for example, Xanax, Valium, and Ativan). However, media attention has been considerably muted, especially when compared with that of narcotic abuse. This is even more puzzling when taking into account that three-quarters of benzodiazepine mortalities co-occur within the context of narcotic consumption. Substance Abuse and Mental Health Services Administration data confirm the ubiquitous nature of benzodiazepine (such as alprazolam) coprescriptions, accounting for roughly half of the 176,000 emergency department cases for 2011. The Centers for Disease Control and Prevention noted that there was a 67% increase in benzodiazepine prescriptions between 1996 and 2013, which warranted more stringent regulations for this particular class of drugs.
In 2016, the CDC issued new guidelines for opioid use acknowledging the danger of benzodiazepine coprescriptions. Food and Drug Administration “black box” warnings now grace the prescriptions of both of these drug classes.10 This trend remains on an upward trajectory, even more so during the pandemic, as there are 9.7 million prescriptions of anxiolytics/hypnotics such as Xanax, Ativan, and Klonopin in the United States as of March 2020, which represents a 10% increase over the previous year. , as well as the implementation of urine drug screening monitoring for drug adherence/compliance and diversion in those with suspected benzodiazepine addiction or a history of polysubstance abuse.11,12
Clinical correlates
For patients who present acutely with Xanax toxicity in the emergency room setting, we will need to initially stabilize the vital signs and address the ongoing symptoms. It is advisable to arrange health care accommodations for patients with physical dependence to monitor and treat their withdrawal symptoms. The patient should be enrolled in a comprehensive addiction facility after undergoing formal detoxification; a tapered treatment protocol will need to be implemented because quitting “cold turkey” can lead to convulsions and, in some cases, death. Patient education, talk therapy, and alternatives to benzodiazepines should be discussed with the clinician.13,145
However, to truly address the elephant in the room, we will need to consider institutional reforms to prevent a similar situation from arising in the future. Primary care physician shortages are compounded by changes in insurance policies. Nurses and physician assistants will need to be trained to manage benzodiazepine prescriptions. If there are community shortages in physicians, patients might turn to illegal means to secure their benzodiazepine supply, and it is imperative that we have the necessary fellowship and education programs to educate nonphysician health care clinicians with benzodiazepine management. Because physicians were prescribing benzodiazepines liberally, the Prescription Drug Monitoring Programs (PDMP) was enacted to monitor physician practices. Unfortunately, this ultimately intimidated physicians and effectively curbed reasonable physician prescribing patterns. It might be necessary to revisit existing prescription monitoring programs, encourage drug evaluations and guidelines based on evidence-based medicine and embrace telemedicine in order to facilitate patient-physician communication.
As of now, it is too early to prescribe Xanax routinely for ongoing anxiety experienced during the coronavirus crisis, and several physicians are cautious about prescribing antianxiety medications for more than a few months.17 Surprisingly, researchers in Barcelona have even explored the role of Xanax as potentially inhibiting Mpro, the primary protease of coronavirus, thereby forestalling the virus’s ability to replicate.16 However, it is worth noting that, given the preliminary nature of the results, any attempts at conclusively integrating Xanax within the context of coronavirus therapy would be premature.
References
1. Luhby T. Anti-anxiety medication prescriptions up 34% since coronavirus. CNN. 2020 Apr 16.
2. Women and Anxiety. Anxiety and Depression Association of America.
3. Shigemura J et al. Psychiatry Clin Neurosci. 2012 Apr 7;74(4):281-2.
4. Petersen A. More people are taking drugs for anxiety and insomnia, and doctors are worried. The Wall Street Journal. 2020 May 25.
5. Downey M. Xanax overdose and related deaths. National Drug & Alcohol Research Centre. UNSW Sydney.
6. Bryant B. Fake Xanax: The UK’s biggest ever dark net drugs bust. BBC. 2018 Mar 10.
7. Reinberg S. Fatal overdoses rising from sedatives like Valium, Xanax. HealthDay. 2016 Feb.
8. Is counterfeit Xanax dangerous? American Addiction Centers. Updated 2018 Nov 14.
9. McLaren E. Xanax history and statistics. Drugabuse.com.
10. Benzodiazepines and opioids. National Institute on Drug Abuse. 2018 Mar 15.
11. Choudhry Z et al. J Psychiatry. 2015;18(5). doi: 10.4172/2378-5756.1000319.
12. Islam FA et al. Current Psychiatry. 2018 Dec 17(12):43-4.
13. Adams M. Xanax death rate on the rise. White Sands Treatment. 2017 Sept.NEED LINK
14. Storrs C. Benzodiazepine overdose deaths soared in recent years, study finds. CNN. 2016 Feb. 18.
15. Hanscom DA. Plan A – Thrive and survive COVID-19. Back in Control. 2020.
16. Smith C. Xanax, a common anxiety medication, might actually block coronavirus. BGR. 2020 May 29.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Mr. Choudhry is a research assistant at the IMCHF. He has no disclosures.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF and is Mr. Choudhry’s father. He has no disclosures.
One of the more alarming trends that has emerged during the coronavirus crisis is the concomitant rise in the use of benzodiazepines, such as Xanax. It has been reported that at-risk individuals began seeking prescription anxiolytics as early as mid-February with a consequent peak of 34% the following month, coinciding with the World Health Organization’s declaration of a global pandemic.1
Consistent with the available literature indicating that women are twice as likely to be affected by anxiety disorders, the prescription spikes were almost double when compared with those of their male counterparts.2 The pandemic has instilled a sense of fear in people, leading to social repercussions, such as estrangement, insomnia, and paranoia for at-risk populations.3,4
“Benzos” are commonly prescribed to help people sleep or to assist them in overcoming a host of anxiety disorders. The rapid onset of effects make Xanax a desirable and efficacious benzodiazepine.5 The use of these medications might not be an immediate cause for concern because patients might be taking it as intended. Nevertheless, clinicians are shying away from medical management in favor of counseling or therapy.
Dangerous trends
Numerous factors might contribute to this grim scenario, including patient dependence on benzodiazepines, paranoia about engaging with health care professionals because of fear tied to potential COVID-19 exposure, and/or increased access to illicit counterfeit pills from drug dealers or the dark web markets.
Lessons can be gleaned from the most extensive dark web drug busts in Britain’s history, in which a deluge of “pharmaceutical grade” Xanax pills made it to the hands of drug dealers and consumers between 2015 and 2017.6 A similar phenomenon emerged stateside.7 Virtually indistinguishable from recognized 2-mg Xanax pills, these fake pills posed a serious challenge to forensic scientists.8 The threat of overdose is very real for users targeted by the counterfeit Xanax trade, especially since those at risk often bypass professional health care guidelines.
In broad daylight, the drug dealers ran their operations revolving around two fake Xanax products: a primary knockoff and a limited edition – and vastly more potent “Red Devil” variant that was intentionally dyed for branding purposes. Because the “Red Devil” formulations contained 2.5 times the dose of the 2-mg pill, it had even more pronounced tolerance, dependence, and withdrawal effects (for example, panic attacks, anxiety, and/or hallucinations) – fatal consequences for users involved in consuming other drugs, such as alcohol or opioids. Preexisting drug users tend to gravitate toward benzodiazepines, such as alprazolam (Xanax), perhaps in part, because of its relatively rapid onset of action. Xanax also is known for inducing proeuphoric states at higher doses, hence the appeal of the “Red Devil” pills.
Benzodiazepines, as a class of drugs, facilitate the neurotransmitter gamma-aminobutryric acid’s (GABA) effect on the brain, producing anxiolytic, hypnotic, and/or anticonvulsant states within the user.9 Unbeknownst to numerous users is the fact that drugs such as alcohol and opioids, like Xanax, also serve as respiratory depressants, overriding the brain’s governance of the breathing mechanism. This, in turn, leads to unintended overdose deaths, even among seasoned drug seekers.
Overdose deaths have been steadily climbing over the years because it is common for some users to consume alcohol while being on Xanax therapy – without realizing that both substances are depressants and that taking them together can lead to side effects such as respiratory depression.
Forensic cases also have revealed that preexisting opioid consumers were drawn to Xanax; the drug’s potent mechanism of action would likely appeal to habituated users. A typical behavioral pattern has emerged among users and must be addressed. According to Australian Professor Shane Darke: “So they take their Xanax, they take their painkiller, then they get drunk, that could be enough to kill them.”
Fatalities are more likely when benzodiazepines are combined with other drug classes or if the existing supply is contaminated or laced (for example, with fentanyl).8
As far as deaths by accidental benzodiazepine overdose are concerned, a similar epidemic has been recorded in the United States. In 2013, almost one-third of all prescription overdose deaths can be attributed to the use of benzodiazepines (for example, Xanax, Valium, and Ativan). However, media attention has been considerably muted, especially when compared with that of narcotic abuse. This is even more puzzling when taking into account that three-quarters of benzodiazepine mortalities co-occur within the context of narcotic consumption. Substance Abuse and Mental Health Services Administration data confirm the ubiquitous nature of benzodiazepine (such as alprazolam) coprescriptions, accounting for roughly half of the 176,000 emergency department cases for 2011. The Centers for Disease Control and Prevention noted that there was a 67% increase in benzodiazepine prescriptions between 1996 and 2013, which warranted more stringent regulations for this particular class of drugs.
In 2016, the CDC issued new guidelines for opioid use acknowledging the danger of benzodiazepine coprescriptions. Food and Drug Administration “black box” warnings now grace the prescriptions of both of these drug classes.10 This trend remains on an upward trajectory, even more so during the pandemic, as there are 9.7 million prescriptions of anxiolytics/hypnotics such as Xanax, Ativan, and Klonopin in the United States as of March 2020, which represents a 10% increase over the previous year. , as well as the implementation of urine drug screening monitoring for drug adherence/compliance and diversion in those with suspected benzodiazepine addiction or a history of polysubstance abuse.11,12
Clinical correlates
For patients who present acutely with Xanax toxicity in the emergency room setting, we will need to initially stabilize the vital signs and address the ongoing symptoms. It is advisable to arrange health care accommodations for patients with physical dependence to monitor and treat their withdrawal symptoms. The patient should be enrolled in a comprehensive addiction facility after undergoing formal detoxification; a tapered treatment protocol will need to be implemented because quitting “cold turkey” can lead to convulsions and, in some cases, death. Patient education, talk therapy, and alternatives to benzodiazepines should be discussed with the clinician.13,145
However, to truly address the elephant in the room, we will need to consider institutional reforms to prevent a similar situation from arising in the future. Primary care physician shortages are compounded by changes in insurance policies. Nurses and physician assistants will need to be trained to manage benzodiazepine prescriptions. If there are community shortages in physicians, patients might turn to illegal means to secure their benzodiazepine supply, and it is imperative that we have the necessary fellowship and education programs to educate nonphysician health care clinicians with benzodiazepine management. Because physicians were prescribing benzodiazepines liberally, the Prescription Drug Monitoring Programs (PDMP) was enacted to monitor physician practices. Unfortunately, this ultimately intimidated physicians and effectively curbed reasonable physician prescribing patterns. It might be necessary to revisit existing prescription monitoring programs, encourage drug evaluations and guidelines based on evidence-based medicine and embrace telemedicine in order to facilitate patient-physician communication.
As of now, it is too early to prescribe Xanax routinely for ongoing anxiety experienced during the coronavirus crisis, and several physicians are cautious about prescribing antianxiety medications for more than a few months.17 Surprisingly, researchers in Barcelona have even explored the role of Xanax as potentially inhibiting Mpro, the primary protease of coronavirus, thereby forestalling the virus’s ability to replicate.16 However, it is worth noting that, given the preliminary nature of the results, any attempts at conclusively integrating Xanax within the context of coronavirus therapy would be premature.
References
1. Luhby T. Anti-anxiety medication prescriptions up 34% since coronavirus. CNN. 2020 Apr 16.
2. Women and Anxiety. Anxiety and Depression Association of America.
3. Shigemura J et al. Psychiatry Clin Neurosci. 2012 Apr 7;74(4):281-2.
4. Petersen A. More people are taking drugs for anxiety and insomnia, and doctors are worried. The Wall Street Journal. 2020 May 25.
5. Downey M. Xanax overdose and related deaths. National Drug & Alcohol Research Centre. UNSW Sydney.
6. Bryant B. Fake Xanax: The UK’s biggest ever dark net drugs bust. BBC. 2018 Mar 10.
7. Reinberg S. Fatal overdoses rising from sedatives like Valium, Xanax. HealthDay. 2016 Feb.
8. Is counterfeit Xanax dangerous? American Addiction Centers. Updated 2018 Nov 14.
9. McLaren E. Xanax history and statistics. Drugabuse.com.
10. Benzodiazepines and opioids. National Institute on Drug Abuse. 2018 Mar 15.
11. Choudhry Z et al. J Psychiatry. 2015;18(5). doi: 10.4172/2378-5756.1000319.
12. Islam FA et al. Current Psychiatry. 2018 Dec 17(12):43-4.
13. Adams M. Xanax death rate on the rise. White Sands Treatment. 2017 Sept.NEED LINK
14. Storrs C. Benzodiazepine overdose deaths soared in recent years, study finds. CNN. 2016 Feb. 18.
15. Hanscom DA. Plan A – Thrive and survive COVID-19. Back in Control. 2020.
16. Smith C. Xanax, a common anxiety medication, might actually block coronavirus. BGR. 2020 May 29.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Mr. Choudhry is a research assistant at the IMCHF. He has no disclosures.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF and is Mr. Choudhry’s father. He has no disclosures.
Be wary of ‘for eczema’ claims on labels of popular moisturizers
Be wary of “for eczema” advertising claims contained on the labels of popular skin moisturizers.
Results from a study presented during the virtual annual meeting of the Society for Pediatric Dermatology found that .
“Prescription medications are important for managing eczema flares, but a lot of the work in treating eczema is preventative, done by consistently moisturizing the skin at home with drug store products,” co-first study author Catherine L. Ludwig, said in an interview. “Allergic contact dermatitis occurs more commonly in people with eczema. A previous study was done in characterizing the allergenic potential of drug store moisturizers and found that 88% of moisturizers contain at least one common allergen. Many moisturizers are marketed specifically to eczema, but the allergen content of these products are unknown.”
For the current study, Ms. Ludwig, a medical student at the University of Illinois at Chicago and co-first author Alyssa M. Thompson, a medical student at the University of Arizona, Tucson, and their colleagues compiled a list of the top 30 moisturizers “for eczema” sold by Amazon, Target, and Walmart. For each moisturizer they recorded common ingredients and marketing claims related to benefits for atopic dermatitis, including eczema relief, sensitive/gentle skin, hypoallergenic, anti-itch, anti-inflammatory, clinically proven, oatmeal, dermatologist recommended/approved, organic, fragrance-free, for baby, or National Eczema Association approved. To establish allergenic potential, the researchers used MATLAB to compare ingredient lists to compounds listed as common allergens in the American Contact Dermatitis Society’s Contact Allergen Management Program database (ACDS CAMP). Next, they used the Mann-Whitney U test to evaluate differences in allergen count between products with and without specific marketing claims.
Ms. Ludwig and her associates found that 28 of 30 products analyzed (93%) contained at least one allergen, with an overall average allergen count of 3.60. The three most prevalent allergens were cetyl alcohol (70%), phenoxyethanol (50%), and aloe (33%). “Anti-inflammatory” moisturizers had the greatest average number of allergens (4.00), followed by “anti-itch” (3.71) and “oatmeal” (3.71). Only products claiming to be “hypoallergenic” had significantly lower allergenic ingredient count (an average of 2.45) than those without the claim (P = .011).
“It was validating to see that eczema moisturizer products marketed as ‘hypoallergenic’ truly do have fewer allergenic ingredients than moisturizers without the claim,” Ms. Ludwig said. “However, it was surprising to see that even products marketed to eczema patients, who have a higher prevalence of allergic contact dermatitis, contain an average of 3.6 common allergens. As dermatology providers, we can relay to patients and parents that relying solely on ‘for eczema’ claims is not advisable. Clinicians should acquaint themselves with the top allergens (cetyl alcohol, phenoxyethanol, and aloe) and keep these ingredients, as well as affordability and patient preferences, in mind when making product recommendations.”
The study’s senior author, Vivian Y. Shi, MD, is a stock shareholder of Learn Health and has served as an advisory board member and/or investigator, and/or received research funding from AbbVie, Burt’s Bees, GpSkin, LEO Pharma, Eli Lilly, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Skin Actives Scientific, and SUN Pharma, and the Foundation for Atopic Dermatitis, Global Parents for Eczema Research, and the National Eczema Association. The other study authors reported having no financial disclosures.
Be wary of “for eczema” advertising claims contained on the labels of popular skin moisturizers.
Results from a study presented during the virtual annual meeting of the Society for Pediatric Dermatology found that .
“Prescription medications are important for managing eczema flares, but a lot of the work in treating eczema is preventative, done by consistently moisturizing the skin at home with drug store products,” co-first study author Catherine L. Ludwig, said in an interview. “Allergic contact dermatitis occurs more commonly in people with eczema. A previous study was done in characterizing the allergenic potential of drug store moisturizers and found that 88% of moisturizers contain at least one common allergen. Many moisturizers are marketed specifically to eczema, but the allergen content of these products are unknown.”
For the current study, Ms. Ludwig, a medical student at the University of Illinois at Chicago and co-first author Alyssa M. Thompson, a medical student at the University of Arizona, Tucson, and their colleagues compiled a list of the top 30 moisturizers “for eczema” sold by Amazon, Target, and Walmart. For each moisturizer they recorded common ingredients and marketing claims related to benefits for atopic dermatitis, including eczema relief, sensitive/gentle skin, hypoallergenic, anti-itch, anti-inflammatory, clinically proven, oatmeal, dermatologist recommended/approved, organic, fragrance-free, for baby, or National Eczema Association approved. To establish allergenic potential, the researchers used MATLAB to compare ingredient lists to compounds listed as common allergens in the American Contact Dermatitis Society’s Contact Allergen Management Program database (ACDS CAMP). Next, they used the Mann-Whitney U test to evaluate differences in allergen count between products with and without specific marketing claims.
Ms. Ludwig and her associates found that 28 of 30 products analyzed (93%) contained at least one allergen, with an overall average allergen count of 3.60. The three most prevalent allergens were cetyl alcohol (70%), phenoxyethanol (50%), and aloe (33%). “Anti-inflammatory” moisturizers had the greatest average number of allergens (4.00), followed by “anti-itch” (3.71) and “oatmeal” (3.71). Only products claiming to be “hypoallergenic” had significantly lower allergenic ingredient count (an average of 2.45) than those without the claim (P = .011).
“It was validating to see that eczema moisturizer products marketed as ‘hypoallergenic’ truly do have fewer allergenic ingredients than moisturizers without the claim,” Ms. Ludwig said. “However, it was surprising to see that even products marketed to eczema patients, who have a higher prevalence of allergic contact dermatitis, contain an average of 3.6 common allergens. As dermatology providers, we can relay to patients and parents that relying solely on ‘for eczema’ claims is not advisable. Clinicians should acquaint themselves with the top allergens (cetyl alcohol, phenoxyethanol, and aloe) and keep these ingredients, as well as affordability and patient preferences, in mind when making product recommendations.”
The study’s senior author, Vivian Y. Shi, MD, is a stock shareholder of Learn Health and has served as an advisory board member and/or investigator, and/or received research funding from AbbVie, Burt’s Bees, GpSkin, LEO Pharma, Eli Lilly, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Skin Actives Scientific, and SUN Pharma, and the Foundation for Atopic Dermatitis, Global Parents for Eczema Research, and the National Eczema Association. The other study authors reported having no financial disclosures.
Be wary of “for eczema” advertising claims contained on the labels of popular skin moisturizers.
Results from a study presented during the virtual annual meeting of the Society for Pediatric Dermatology found that .
“Prescription medications are important for managing eczema flares, but a lot of the work in treating eczema is preventative, done by consistently moisturizing the skin at home with drug store products,” co-first study author Catherine L. Ludwig, said in an interview. “Allergic contact dermatitis occurs more commonly in people with eczema. A previous study was done in characterizing the allergenic potential of drug store moisturizers and found that 88% of moisturizers contain at least one common allergen. Many moisturizers are marketed specifically to eczema, but the allergen content of these products are unknown.”
For the current study, Ms. Ludwig, a medical student at the University of Illinois at Chicago and co-first author Alyssa M. Thompson, a medical student at the University of Arizona, Tucson, and their colleagues compiled a list of the top 30 moisturizers “for eczema” sold by Amazon, Target, and Walmart. For each moisturizer they recorded common ingredients and marketing claims related to benefits for atopic dermatitis, including eczema relief, sensitive/gentle skin, hypoallergenic, anti-itch, anti-inflammatory, clinically proven, oatmeal, dermatologist recommended/approved, organic, fragrance-free, for baby, or National Eczema Association approved. To establish allergenic potential, the researchers used MATLAB to compare ingredient lists to compounds listed as common allergens in the American Contact Dermatitis Society’s Contact Allergen Management Program database (ACDS CAMP). Next, they used the Mann-Whitney U test to evaluate differences in allergen count between products with and without specific marketing claims.
Ms. Ludwig and her associates found that 28 of 30 products analyzed (93%) contained at least one allergen, with an overall average allergen count of 3.60. The three most prevalent allergens were cetyl alcohol (70%), phenoxyethanol (50%), and aloe (33%). “Anti-inflammatory” moisturizers had the greatest average number of allergens (4.00), followed by “anti-itch” (3.71) and “oatmeal” (3.71). Only products claiming to be “hypoallergenic” had significantly lower allergenic ingredient count (an average of 2.45) than those without the claim (P = .011).
“It was validating to see that eczema moisturizer products marketed as ‘hypoallergenic’ truly do have fewer allergenic ingredients than moisturizers without the claim,” Ms. Ludwig said. “However, it was surprising to see that even products marketed to eczema patients, who have a higher prevalence of allergic contact dermatitis, contain an average of 3.6 common allergens. As dermatology providers, we can relay to patients and parents that relying solely on ‘for eczema’ claims is not advisable. Clinicians should acquaint themselves with the top allergens (cetyl alcohol, phenoxyethanol, and aloe) and keep these ingredients, as well as affordability and patient preferences, in mind when making product recommendations.”
The study’s senior author, Vivian Y. Shi, MD, is a stock shareholder of Learn Health and has served as an advisory board member and/or investigator, and/or received research funding from AbbVie, Burt’s Bees, GpSkin, LEO Pharma, Eli Lilly, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Skin Actives Scientific, and SUN Pharma, and the Foundation for Atopic Dermatitis, Global Parents for Eczema Research, and the National Eczema Association. The other study authors reported having no financial disclosures.
FROM SPD 2020
Less REM sleep tied to higher mortality
Less rapid eye movement (REM) sleep is associated with an increased risk for death in middle-aged and older adults, new research suggests.
Investigators at the University of California, San Diego, found that, over a 12-year period, each 5% reduction in REM sleep was associated with a 13% increase in mortality rate. However, the investigators noted that this is only an association and does not indicate cause and effect.
“Determining causality can be difficult,” study investigator Sonia Ancoli-Israel, PhD, professor emeritus of psychiatry at the University of California, San Diego, said in an interview.
“It is therefore important that physicians and the public understand that our findings suggest an increased risk, but that does not mean that reduced REM will always result in shorter survival. With all the self-monitoring sleep gadgets available to the public, I would caution against any panic if one notices reduced REM. But mentioning it to a physician may be a clue to examine what else might be going on with that patient that could more easily be targeted,” Dr. Ancoli-Israel added.
The research was published online July 6 in JAMA Neurology.
Negative consequences
Approximately 50-70 million Americans have problems with sleep. Such problems have a multitude of consequences for health, including cardiovascular disease; metabolic, psychiatric, and cognitive disorders; lower quality of life; and increased mortality.
The investigators noted that the aspects of sleep that may be driving this association remain unclear. Because decreased REM sleep has been associated with poor mental and physical health outcomes, the researchers hypothesized that decreased REM sleep may be associated with an increased risk for death.
To test this hypothesis, they conducted a multicenter, population-based, cross-sectional investigation using data from independent cohorts – the Outcomes of Sleep Disorders in Older Men (MrOS) Sleep Study and the Wisconsin Sleep Cohort (WSC). The MrOS cohort included 2,675 men (mean age, 76.3 years) who were recruited from December 2003 to March 2005 at six U.S. centers and were followed for a median of 12.1 years. The WSC cohort included 1,386 individuals (54.3% men; mean age, 51.5 years) and had a median follow-up of 20.8 years. Data from this study were used to replicate the findings from the MrOS study.
Primary outcome measures included all-cause and cause-specific mortality, which were confirmed using death certificates.
Participants in both cohorts underwent polysomnography and evaluation with the Epworth Sleepiness Scale. For MrOS participants, investigators calculated the total number of minutes per night spent in REM sleep and the corresponding percentage of total sleep time.
Less sleep, more death
Self-report sleep measures in MrOS participants were collected using the Pittsburgh Sleep Quality Index and the Functional Outcomes of Sleep Questionnaire
The investigators contacted participants in MrOS every 4 months to determine vital status. Cause of death was categorized by the ICD-9 as cardiovascular, cancer, and other. In WSC, the researchers identified deaths by matching participants’ social security numbers with national and state registries. The cause of death was categorized in the same manner as in the MrOS cohort.
Approximately half (53%) of the MrOS cohort died during follow-up. For each mortality category, the highest percentage of deaths occurred among those in the lowest quartile percentage of REM sleep. Adjusted analyses revealed that the MrOS participants had a 13% higher mortality rate for every 5% reduction in REM sleep (hazard ratio, 1.13; 95% confidence interval, 1.08-1.19). These findings were similar for cardiovascular and other causes of death but were not significant for cancer-related mortality. For all mortality categories, the mortality rate was higher for participants who had less than 15% REM sleep per night in comparison with individuals who had 15% or more.
The findings were similar in the WSC cohort despite its younger age, the inclusion of women, and longer follow-up (HR, 1.13; 95% CI, 1.08-1.19). Compared with MrOS participants, WSC participants were more likely to be obese and to use more antidepressants or sedatives. Overall, the mean percentage of REM sleep was 19.2%. Participants in the lowest quartile of REM sleep generally were older, had higher rates of antidepressant use, hypertension, heart attack, and transient ischemic attack, as well as engaging in less physical activity.
Ask about sleep
When the data were stratified by sex, the association between decreased REM sleep and mortality was significant for women but not for men.
“Obtaining a sleep study, representative of the patient’s usual sleep, that shows reduced REM time should alert the neurologist to look for reasons for low REM,” the study’s coinvestigator, Susan Redline, MD, MPH, Peter C. Farrell Professor of Sleep Medicine at Harvard Medical School in Boston, said in an interview.
Dr. Redline added that measures to promote sleep health, such as encouraging regular, sufficient nightly sleep; offering guidance on avoiding alcohol before bedtime and on other healthy sleep practices; and treating sleep disorders may be beneficial.
Low REM time, especially interpreted with other relevant clinical information, may alert the neurologist that a patient may have risk factors for poorer health, she added.
Sleep studies are expensive and are in high demand, so “the most realistic approach is for the neurologist to be asking each and every patient about their sleep,” said Ancoli-Israel.
“By asking a few more questions in every intake, the neurologist is more likely to determine if there are any occult sleep disorders that need to be addressed. By improving sleep in general, one is more likely to also improve any REM abnormalities,” she said.
Disease indicator?
In an accompanying editorial, Michael S. Jaffee, MD, vice chair of neurology at the University of Florida in Gainesville, and colleagues noted that the study raises the question of whether REM sleep “could serve as a biomarker for general health.”
“Since the known roles of REM sleep do not easily suggest a causal link with mortality ... it seems more likely that REM sleep reduction is either a crude marker of health or specific disease states that decrease REM sleep may play an important role in contributing to mortality,” they wrote.
Neurologists should remember that certain medications affect sleep architecture, the editorialists advised. They note that serotonin reuptake inhibitors, selective serotonin and norepinephrine reuptake inhibitors, and tricyclic antidepressants reduce REM sleep, and that gabapentin, prazosin, and bupropion, on the other hand, increase REM sleep. However, data regarding whether these medications have an effect on mortality are insufficient.
The editorialists wrote that the study findings are a “welcome addition to the literature and demonstrate definitively that the association between sleep and mortality extends beyond the simple measure of total sleep time.”
Funding for the MrOS and WSC studies was provided by the National Institutes of Health and the National Institute on Aging. Dr. Ancoli-Israel consults for Eisai and Merck on matters unrelated to the study. Dr. Redline has received grants and personal fees from Jazz Pharmaceuticals, consulting fees from Respicardia, and personal fees from Eisai unrelated to the study. Dr. Jaffee served on a data and safety monitoring board for Helius Medical Technologies and consulted for the National Collegiate Athletic Association and the Department of Defense.
A version of this article originally appeared on Medscape.com.
Less rapid eye movement (REM) sleep is associated with an increased risk for death in middle-aged and older adults, new research suggests.
Investigators at the University of California, San Diego, found that, over a 12-year period, each 5% reduction in REM sleep was associated with a 13% increase in mortality rate. However, the investigators noted that this is only an association and does not indicate cause and effect.
“Determining causality can be difficult,” study investigator Sonia Ancoli-Israel, PhD, professor emeritus of psychiatry at the University of California, San Diego, said in an interview.
“It is therefore important that physicians and the public understand that our findings suggest an increased risk, but that does not mean that reduced REM will always result in shorter survival. With all the self-monitoring sleep gadgets available to the public, I would caution against any panic if one notices reduced REM. But mentioning it to a physician may be a clue to examine what else might be going on with that patient that could more easily be targeted,” Dr. Ancoli-Israel added.
The research was published online July 6 in JAMA Neurology.
Negative consequences
Approximately 50-70 million Americans have problems with sleep. Such problems have a multitude of consequences for health, including cardiovascular disease; metabolic, psychiatric, and cognitive disorders; lower quality of life; and increased mortality.
The investigators noted that the aspects of sleep that may be driving this association remain unclear. Because decreased REM sleep has been associated with poor mental and physical health outcomes, the researchers hypothesized that decreased REM sleep may be associated with an increased risk for death.
To test this hypothesis, they conducted a multicenter, population-based, cross-sectional investigation using data from independent cohorts – the Outcomes of Sleep Disorders in Older Men (MrOS) Sleep Study and the Wisconsin Sleep Cohort (WSC). The MrOS cohort included 2,675 men (mean age, 76.3 years) who were recruited from December 2003 to March 2005 at six U.S. centers and were followed for a median of 12.1 years. The WSC cohort included 1,386 individuals (54.3% men; mean age, 51.5 years) and had a median follow-up of 20.8 years. Data from this study were used to replicate the findings from the MrOS study.
Primary outcome measures included all-cause and cause-specific mortality, which were confirmed using death certificates.
Participants in both cohorts underwent polysomnography and evaluation with the Epworth Sleepiness Scale. For MrOS participants, investigators calculated the total number of minutes per night spent in REM sleep and the corresponding percentage of total sleep time.
Less sleep, more death
Self-report sleep measures in MrOS participants were collected using the Pittsburgh Sleep Quality Index and the Functional Outcomes of Sleep Questionnaire
The investigators contacted participants in MrOS every 4 months to determine vital status. Cause of death was categorized by the ICD-9 as cardiovascular, cancer, and other. In WSC, the researchers identified deaths by matching participants’ social security numbers with national and state registries. The cause of death was categorized in the same manner as in the MrOS cohort.
Approximately half (53%) of the MrOS cohort died during follow-up. For each mortality category, the highest percentage of deaths occurred among those in the lowest quartile percentage of REM sleep. Adjusted analyses revealed that the MrOS participants had a 13% higher mortality rate for every 5% reduction in REM sleep (hazard ratio, 1.13; 95% confidence interval, 1.08-1.19). These findings were similar for cardiovascular and other causes of death but were not significant for cancer-related mortality. For all mortality categories, the mortality rate was higher for participants who had less than 15% REM sleep per night in comparison with individuals who had 15% or more.
The findings were similar in the WSC cohort despite its younger age, the inclusion of women, and longer follow-up (HR, 1.13; 95% CI, 1.08-1.19). Compared with MrOS participants, WSC participants were more likely to be obese and to use more antidepressants or sedatives. Overall, the mean percentage of REM sleep was 19.2%. Participants in the lowest quartile of REM sleep generally were older, had higher rates of antidepressant use, hypertension, heart attack, and transient ischemic attack, as well as engaging in less physical activity.
Ask about sleep
When the data were stratified by sex, the association between decreased REM sleep and mortality was significant for women but not for men.
“Obtaining a sleep study, representative of the patient’s usual sleep, that shows reduced REM time should alert the neurologist to look for reasons for low REM,” the study’s coinvestigator, Susan Redline, MD, MPH, Peter C. Farrell Professor of Sleep Medicine at Harvard Medical School in Boston, said in an interview.
Dr. Redline added that measures to promote sleep health, such as encouraging regular, sufficient nightly sleep; offering guidance on avoiding alcohol before bedtime and on other healthy sleep practices; and treating sleep disorders may be beneficial.
Low REM time, especially interpreted with other relevant clinical information, may alert the neurologist that a patient may have risk factors for poorer health, she added.
Sleep studies are expensive and are in high demand, so “the most realistic approach is for the neurologist to be asking each and every patient about their sleep,” said Ancoli-Israel.
“By asking a few more questions in every intake, the neurologist is more likely to determine if there are any occult sleep disorders that need to be addressed. By improving sleep in general, one is more likely to also improve any REM abnormalities,” she said.
Disease indicator?
In an accompanying editorial, Michael S. Jaffee, MD, vice chair of neurology at the University of Florida in Gainesville, and colleagues noted that the study raises the question of whether REM sleep “could serve as a biomarker for general health.”
“Since the known roles of REM sleep do not easily suggest a causal link with mortality ... it seems more likely that REM sleep reduction is either a crude marker of health or specific disease states that decrease REM sleep may play an important role in contributing to mortality,” they wrote.
Neurologists should remember that certain medications affect sleep architecture, the editorialists advised. They note that serotonin reuptake inhibitors, selective serotonin and norepinephrine reuptake inhibitors, and tricyclic antidepressants reduce REM sleep, and that gabapentin, prazosin, and bupropion, on the other hand, increase REM sleep. However, data regarding whether these medications have an effect on mortality are insufficient.
The editorialists wrote that the study findings are a “welcome addition to the literature and demonstrate definitively that the association between sleep and mortality extends beyond the simple measure of total sleep time.”
Funding for the MrOS and WSC studies was provided by the National Institutes of Health and the National Institute on Aging. Dr. Ancoli-Israel consults for Eisai and Merck on matters unrelated to the study. Dr. Redline has received grants and personal fees from Jazz Pharmaceuticals, consulting fees from Respicardia, and personal fees from Eisai unrelated to the study. Dr. Jaffee served on a data and safety monitoring board for Helius Medical Technologies and consulted for the National Collegiate Athletic Association and the Department of Defense.
A version of this article originally appeared on Medscape.com.
Less rapid eye movement (REM) sleep is associated with an increased risk for death in middle-aged and older adults, new research suggests.
Investigators at the University of California, San Diego, found that, over a 12-year period, each 5% reduction in REM sleep was associated with a 13% increase in mortality rate. However, the investigators noted that this is only an association and does not indicate cause and effect.
“Determining causality can be difficult,” study investigator Sonia Ancoli-Israel, PhD, professor emeritus of psychiatry at the University of California, San Diego, said in an interview.
“It is therefore important that physicians and the public understand that our findings suggest an increased risk, but that does not mean that reduced REM will always result in shorter survival. With all the self-monitoring sleep gadgets available to the public, I would caution against any panic if one notices reduced REM. But mentioning it to a physician may be a clue to examine what else might be going on with that patient that could more easily be targeted,” Dr. Ancoli-Israel added.
The research was published online July 6 in JAMA Neurology.
Negative consequences
Approximately 50-70 million Americans have problems with sleep. Such problems have a multitude of consequences for health, including cardiovascular disease; metabolic, psychiatric, and cognitive disorders; lower quality of life; and increased mortality.
The investigators noted that the aspects of sleep that may be driving this association remain unclear. Because decreased REM sleep has been associated with poor mental and physical health outcomes, the researchers hypothesized that decreased REM sleep may be associated with an increased risk for death.
To test this hypothesis, they conducted a multicenter, population-based, cross-sectional investigation using data from independent cohorts – the Outcomes of Sleep Disorders in Older Men (MrOS) Sleep Study and the Wisconsin Sleep Cohort (WSC). The MrOS cohort included 2,675 men (mean age, 76.3 years) who were recruited from December 2003 to March 2005 at six U.S. centers and were followed for a median of 12.1 years. The WSC cohort included 1,386 individuals (54.3% men; mean age, 51.5 years) and had a median follow-up of 20.8 years. Data from this study were used to replicate the findings from the MrOS study.
Primary outcome measures included all-cause and cause-specific mortality, which were confirmed using death certificates.
Participants in both cohorts underwent polysomnography and evaluation with the Epworth Sleepiness Scale. For MrOS participants, investigators calculated the total number of minutes per night spent in REM sleep and the corresponding percentage of total sleep time.
Less sleep, more death
Self-report sleep measures in MrOS participants were collected using the Pittsburgh Sleep Quality Index and the Functional Outcomes of Sleep Questionnaire
The investigators contacted participants in MrOS every 4 months to determine vital status. Cause of death was categorized by the ICD-9 as cardiovascular, cancer, and other. In WSC, the researchers identified deaths by matching participants’ social security numbers with national and state registries. The cause of death was categorized in the same manner as in the MrOS cohort.
Approximately half (53%) of the MrOS cohort died during follow-up. For each mortality category, the highest percentage of deaths occurred among those in the lowest quartile percentage of REM sleep. Adjusted analyses revealed that the MrOS participants had a 13% higher mortality rate for every 5% reduction in REM sleep (hazard ratio, 1.13; 95% confidence interval, 1.08-1.19). These findings were similar for cardiovascular and other causes of death but were not significant for cancer-related mortality. For all mortality categories, the mortality rate was higher for participants who had less than 15% REM sleep per night in comparison with individuals who had 15% or more.
The findings were similar in the WSC cohort despite its younger age, the inclusion of women, and longer follow-up (HR, 1.13; 95% CI, 1.08-1.19). Compared with MrOS participants, WSC participants were more likely to be obese and to use more antidepressants or sedatives. Overall, the mean percentage of REM sleep was 19.2%. Participants in the lowest quartile of REM sleep generally were older, had higher rates of antidepressant use, hypertension, heart attack, and transient ischemic attack, as well as engaging in less physical activity.
Ask about sleep
When the data were stratified by sex, the association between decreased REM sleep and mortality was significant for women but not for men.
“Obtaining a sleep study, representative of the patient’s usual sleep, that shows reduced REM time should alert the neurologist to look for reasons for low REM,” the study’s coinvestigator, Susan Redline, MD, MPH, Peter C. Farrell Professor of Sleep Medicine at Harvard Medical School in Boston, said in an interview.
Dr. Redline added that measures to promote sleep health, such as encouraging regular, sufficient nightly sleep; offering guidance on avoiding alcohol before bedtime and on other healthy sleep practices; and treating sleep disorders may be beneficial.
Low REM time, especially interpreted with other relevant clinical information, may alert the neurologist that a patient may have risk factors for poorer health, she added.
Sleep studies are expensive and are in high demand, so “the most realistic approach is for the neurologist to be asking each and every patient about their sleep,” said Ancoli-Israel.
“By asking a few more questions in every intake, the neurologist is more likely to determine if there are any occult sleep disorders that need to be addressed. By improving sleep in general, one is more likely to also improve any REM abnormalities,” she said.
Disease indicator?
In an accompanying editorial, Michael S. Jaffee, MD, vice chair of neurology at the University of Florida in Gainesville, and colleagues noted that the study raises the question of whether REM sleep “could serve as a biomarker for general health.”
“Since the known roles of REM sleep do not easily suggest a causal link with mortality ... it seems more likely that REM sleep reduction is either a crude marker of health or specific disease states that decrease REM sleep may play an important role in contributing to mortality,” they wrote.
Neurologists should remember that certain medications affect sleep architecture, the editorialists advised. They note that serotonin reuptake inhibitors, selective serotonin and norepinephrine reuptake inhibitors, and tricyclic antidepressants reduce REM sleep, and that gabapentin, prazosin, and bupropion, on the other hand, increase REM sleep. However, data regarding whether these medications have an effect on mortality are insufficient.
The editorialists wrote that the study findings are a “welcome addition to the literature and demonstrate definitively that the association between sleep and mortality extends beyond the simple measure of total sleep time.”
Funding for the MrOS and WSC studies was provided by the National Institutes of Health and the National Institute on Aging. Dr. Ancoli-Israel consults for Eisai and Merck on matters unrelated to the study. Dr. Redline has received grants and personal fees from Jazz Pharmaceuticals, consulting fees from Respicardia, and personal fees from Eisai unrelated to the study. Dr. Jaffee served on a data and safety monitoring board for Helius Medical Technologies and consulted for the National Collegiate Athletic Association and the Department of Defense.
A version of this article originally appeared on Medscape.com.
Is bufexamac worth the risk?
Bufexamac, a nonsteroidal anti-inflammatory drug agent used cutaneously and rectally, is well known globally as an initiator of allergic contact dermatitis. In fact, it has been removed from the European market (except Switzerland) for inducing allergic reactions, and is also banned in Japan, New Zealand, and the United States (where it was never approved).1 This column will primarily discuss recent findings in human trials and weigh in on the issue.
Antioxidant activity
. In 2003, Trommer and Neubert demonstrated that bufexamac displayed antioxidant activity in lipid models and HaCaT keratinocytes, as measured through mass spectrometry.2 In a 2005 in vitro study of the impact of 47 drugs, plant extracts and ingredients, and polysaccharides on lipid peroxidation engendered by UV irradiation, Trommer and Neubert found that bufexamac was among the drugs shown to exhibit antioxidant activity.3
Minor allergen? Worth using?
In a 2009 study on the prevalence and risk factors for allergic contact dermatitis to topical atopic dermatitis (AD) treatments, Mailhol et al. patch tested 641 children with AD using seven then-common ingredients (chlorhexidine, hexamidine, budesonide, tixocortol pivalate, bufexamac, sodium fusidate and with the current emollient used by the child). Bufexamac was identified as an allergen in only 2.5% of the 41 positive patch tests.4
To ban or not to ban
In 2012, the European Medicines Agency’s Committee for Medicinal Products for Human Use recommended that the marketing of formulations containing bufexamac be disallowed throughout the European Union because of a tendency toward inducing severe allergic contact dermatitis.5
Given its continuing use in Australia for the local treatment of several dermatoses, Pan and Nixon, in 2012, retrospectively reviewed patch-test data at the Skin and Cancer Foundation Inc. and found 19 cases of positive reactions to bufexamac (5% petrolatum) from 451 people patch tested. In 13 of 19 patients (68%), the reaction to bufexamac was considered to be associated with the identified dermatitis. The authors concluded that allergic contact dermatitis from bufexamac exposure is underreported in the English-language literature and cautioned that physicians should consider bufexamac allergy in patients who have a history of exposure.5
Bufexamac remained available over the counter in topical formulations in Australia as of early 2019. In response, Harris et al. presented several cases of patients who experienced severe skin eruptions after using such preparations in support of their advocacy to the Therapeutic Goods Administration in Australia to ban its use.6
In the middle of that year, Wong et al. reported on the hospitalization of a 41-year-old administrative worker who applied a first aid cream containing bufexamac (5%), lignocaine (1%), and chlorhexidine (0.1%) to a superficial right foot abrasion and who developed facial edema and widespread polymorphic eruptions 2 hours later. The authors suggested that this case reinforced the need to remove bufexamac from the markets where it remains because of the tendency to provoke severe allergic contact dermatoses and lack of efficacy.1
Conclusion
Bufexamac offers the somewhat rare opportunity for advocacy. That is to say, I think there is sufficient evidence to justify the removal of this potent allergen from the market in Australia, Switzerland, and other countries where it may be available.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. She had no relevant disclosures. Write to her at dermnews@mdedge.com.
References
1. Wong GN et al. Contact Dermatitis. 2019 Jun;80(6):395-7.
2. Trommer H et al. J Pharm Pharmacol. 2003 Oct;55(10):1379-88.
3. Trommer H, Neubert RH. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
4. Mailhol C et al. Allergy. 2009 May;64(5):801-6.
5. Pan Y, Nixon R. Australas J Dermatol. 2012 Aug;53(3):207-10.
6. Harris AG et al. Australas J Dermatol. 2019 Feb;60(1):53-6.
Bufexamac, a nonsteroidal anti-inflammatory drug agent used cutaneously and rectally, is well known globally as an initiator of allergic contact dermatitis. In fact, it has been removed from the European market (except Switzerland) for inducing allergic reactions, and is also banned in Japan, New Zealand, and the United States (where it was never approved).1 This column will primarily discuss recent findings in human trials and weigh in on the issue.
Antioxidant activity
. In 2003, Trommer and Neubert demonstrated that bufexamac displayed antioxidant activity in lipid models and HaCaT keratinocytes, as measured through mass spectrometry.2 In a 2005 in vitro study of the impact of 47 drugs, plant extracts and ingredients, and polysaccharides on lipid peroxidation engendered by UV irradiation, Trommer and Neubert found that bufexamac was among the drugs shown to exhibit antioxidant activity.3
Minor allergen? Worth using?
In a 2009 study on the prevalence and risk factors for allergic contact dermatitis to topical atopic dermatitis (AD) treatments, Mailhol et al. patch tested 641 children with AD using seven then-common ingredients (chlorhexidine, hexamidine, budesonide, tixocortol pivalate, bufexamac, sodium fusidate and with the current emollient used by the child). Bufexamac was identified as an allergen in only 2.5% of the 41 positive patch tests.4
To ban or not to ban
In 2012, the European Medicines Agency’s Committee for Medicinal Products for Human Use recommended that the marketing of formulations containing bufexamac be disallowed throughout the European Union because of a tendency toward inducing severe allergic contact dermatitis.5
Given its continuing use in Australia for the local treatment of several dermatoses, Pan and Nixon, in 2012, retrospectively reviewed patch-test data at the Skin and Cancer Foundation Inc. and found 19 cases of positive reactions to bufexamac (5% petrolatum) from 451 people patch tested. In 13 of 19 patients (68%), the reaction to bufexamac was considered to be associated with the identified dermatitis. The authors concluded that allergic contact dermatitis from bufexamac exposure is underreported in the English-language literature and cautioned that physicians should consider bufexamac allergy in patients who have a history of exposure.5
Bufexamac remained available over the counter in topical formulations in Australia as of early 2019. In response, Harris et al. presented several cases of patients who experienced severe skin eruptions after using such preparations in support of their advocacy to the Therapeutic Goods Administration in Australia to ban its use.6
In the middle of that year, Wong et al. reported on the hospitalization of a 41-year-old administrative worker who applied a first aid cream containing bufexamac (5%), lignocaine (1%), and chlorhexidine (0.1%) to a superficial right foot abrasion and who developed facial edema and widespread polymorphic eruptions 2 hours later. The authors suggested that this case reinforced the need to remove bufexamac from the markets where it remains because of the tendency to provoke severe allergic contact dermatoses and lack of efficacy.1
Conclusion
Bufexamac offers the somewhat rare opportunity for advocacy. That is to say, I think there is sufficient evidence to justify the removal of this potent allergen from the market in Australia, Switzerland, and other countries where it may be available.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. She had no relevant disclosures. Write to her at dermnews@mdedge.com.
References
1. Wong GN et al. Contact Dermatitis. 2019 Jun;80(6):395-7.
2. Trommer H et al. J Pharm Pharmacol. 2003 Oct;55(10):1379-88.
3. Trommer H, Neubert RH. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
4. Mailhol C et al. Allergy. 2009 May;64(5):801-6.
5. Pan Y, Nixon R. Australas J Dermatol. 2012 Aug;53(3):207-10.
6. Harris AG et al. Australas J Dermatol. 2019 Feb;60(1):53-6.
Bufexamac, a nonsteroidal anti-inflammatory drug agent used cutaneously and rectally, is well known globally as an initiator of allergic contact dermatitis. In fact, it has been removed from the European market (except Switzerland) for inducing allergic reactions, and is also banned in Japan, New Zealand, and the United States (where it was never approved).1 This column will primarily discuss recent findings in human trials and weigh in on the issue.
Antioxidant activity
. In 2003, Trommer and Neubert demonstrated that bufexamac displayed antioxidant activity in lipid models and HaCaT keratinocytes, as measured through mass spectrometry.2 In a 2005 in vitro study of the impact of 47 drugs, plant extracts and ingredients, and polysaccharides on lipid peroxidation engendered by UV irradiation, Trommer and Neubert found that bufexamac was among the drugs shown to exhibit antioxidant activity.3
Minor allergen? Worth using?
In a 2009 study on the prevalence and risk factors for allergic contact dermatitis to topical atopic dermatitis (AD) treatments, Mailhol et al. patch tested 641 children with AD using seven then-common ingredients (chlorhexidine, hexamidine, budesonide, tixocortol pivalate, bufexamac, sodium fusidate and with the current emollient used by the child). Bufexamac was identified as an allergen in only 2.5% of the 41 positive patch tests.4
To ban or not to ban
In 2012, the European Medicines Agency’s Committee for Medicinal Products for Human Use recommended that the marketing of formulations containing bufexamac be disallowed throughout the European Union because of a tendency toward inducing severe allergic contact dermatitis.5
Given its continuing use in Australia for the local treatment of several dermatoses, Pan and Nixon, in 2012, retrospectively reviewed patch-test data at the Skin and Cancer Foundation Inc. and found 19 cases of positive reactions to bufexamac (5% petrolatum) from 451 people patch tested. In 13 of 19 patients (68%), the reaction to bufexamac was considered to be associated with the identified dermatitis. The authors concluded that allergic contact dermatitis from bufexamac exposure is underreported in the English-language literature and cautioned that physicians should consider bufexamac allergy in patients who have a history of exposure.5
Bufexamac remained available over the counter in topical formulations in Australia as of early 2019. In response, Harris et al. presented several cases of patients who experienced severe skin eruptions after using such preparations in support of their advocacy to the Therapeutic Goods Administration in Australia to ban its use.6
In the middle of that year, Wong et al. reported on the hospitalization of a 41-year-old administrative worker who applied a first aid cream containing bufexamac (5%), lignocaine (1%), and chlorhexidine (0.1%) to a superficial right foot abrasion and who developed facial edema and widespread polymorphic eruptions 2 hours later. The authors suggested that this case reinforced the need to remove bufexamac from the markets where it remains because of the tendency to provoke severe allergic contact dermatoses and lack of efficacy.1
Conclusion
Bufexamac offers the somewhat rare opportunity for advocacy. That is to say, I think there is sufficient evidence to justify the removal of this potent allergen from the market in Australia, Switzerland, and other countries where it may be available.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. She had no relevant disclosures. Write to her at dermnews@mdedge.com.
References
1. Wong GN et al. Contact Dermatitis. 2019 Jun;80(6):395-7.
2. Trommer H et al. J Pharm Pharmacol. 2003 Oct;55(10):1379-88.
3. Trommer H, Neubert RH. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
4. Mailhol C et al. Allergy. 2009 May;64(5):801-6.
5. Pan Y, Nixon R. Australas J Dermatol. 2012 Aug;53(3):207-10.
6. Harris AG et al. Australas J Dermatol. 2019 Feb;60(1):53-6.
AGA News
Rep. Suzan DelBene (D-Wash.) leads prior authorization reform
As a member of the powerful Ways and Means Committee, which has jurisdiction over the Medicare program, Rep. DelBene has worked closely with the American Gastroenterological Association.
When Rep. DelBene was first elected to Congress in 2012, we met with her to share AGA’s policy priorities. We knew instantly that we had a voice for many of our issues. Rep. DelBene started her career as a young investigator before continuing her education and launching a career in the biotechnology industry. From her firsthand experience, she understands the need for investments in National Institutes of Health research and for access to and coverage of colorectal cancer screenings since a member of her family had the disease.
Since Rep. DelBene has been in office, she has taken the lead on several policy priorities affecting our profession, including patient access and protections and regulatory relief. Rep. DelBene is the lead Democratic sponsor of H.R. 3107, the Improving Seniors’ Timely Access to Care Act, legislation that would streamline prior authorization in Medicare Advantage plans. The legislation hit a milestone of securing 218 cosponsors in the House, which is a majority of the members. We look forward to continuing to work with Rep. DelBene on advancing AGA’s policy priorities.
Featured microbiome investigator: Josephine Ni, MD
We’re checking in with a rising star in microbiome research: Dr. Josephine Ni from the University of Pennsylvania, Philadelphia.
Dr. Ni is an instructor of medicine at the University of Pennsylvania, and 2017 recipient of the AGA–Takeda Pharmaceuticals Research Scholar Award in IBD from the AGA Research Foundation.
Congrats to Dr. Ni! While Dr. Ni’s AGA Research Scholar Award concludes at the end of June 2020, we’re proud to share that she has secured two significant grants to continue her work: an NIH KO8 grant and a Burroughs Welcome Fund Award. We catch up with Dr. Ni in the Q&A below.
How would you sum up your research in one sentence?
I am interested in better understanding bacterial colonization of the healthy and inflamed intestinal tract; specifically, my current research focuses on characterizing the role of biofilm formation on intestinal colonization.
What effect do you hope your research will have on patients?
I hope that my work on understanding intestinal colonization will allow us to engineer the microbiota in predictable ways, which will pave the way to exclude enteropathogens, deliver specific compounds, and prevent dysbiosis.
What inspired you to focus your research career on the gut microbiome?
Being able to use data and observations from patient cohorts to generate research hypotheses and then translate those hypotheses into mouse models to explore mechanisms has been a very gratifying experience that I learned from my mentor, Gary Wu, MD. There is still so much to learn about the effects of the microbiome on intestinal health and I’m excited to be a part of this process.
What recent publication from your lab best represents your work if anyone wants to learn more?
Ni J et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med. 2017 Nov 15;9(416):eaah6888.
Gastroenterology invites submissions for an issue focused on colorectal cancer
Share your innovative basic and clinical research for consideration.
The past decade has seen significant milestones in our understanding of the epidemiology, clinical and genetic risk factors, and underlying biological mechanisms of colorectal cancer. This progress has also emphasized the need for further advances. To this end, Gastroenterology will publish a thematic issue in honor of Colorectal Cancer (CRC) Awareness Month in March 2021. The aim is to cover research highlighting novel pathways with human correlates, discoveries related to clinical interventions, clinical trials, and high-profile epidemiologic studies.
Help drive progress of CRC understanding and care by contributing your work. Enhanced promotion of the full issue and automatic indexing of your article to PubMed will increase the visibility of your research in the scientific community and beyond.
Submit your research through Gastroenterology‘s streamlined submission system: www.editorialmanager.com/gastro by Sept. 30, 2020. Original articles and brief communications are welcome.
For more information, please contact Gastroenterology’s Managing Editor, Christopher Lowe, at clowe@gastro.org.
AGA journals select editorial fellows for 2020-2021 academic year
The AGA journals Gastroenterology, Clinical Gastroenterology and Hepatology (CGH), and Cellular and Molecular Gastroenterology and Hepatology (CMGH) recently selected the recipients of their editorial fellowships, which runs from July 2020 through June 2021. The editorial fellowship program is in its fourth year.
The editorial fellows for each journal are:
Gastroenterology
Ruben Colman, MD
Cincinnati Children’s Hospital Medical Center
John Gubatan, MD
Stanford (Calif.) University Medical Center
CGH
Blake Jones, MD
University of Colorado at Denver, Aurora
Nikhil Thiruvengadam, MD
University of California, San Francisco
CMGH
Samuel Hinman, PhD
University of Washington, Seattle
The editorial fellows will be mentored on the journals’ editorial processes, including peer review and the publication process from manuscript submission to acceptance. They will participate in discussions and conferences with the boards of editors and work closely with the AGA editorial staff. Additionally, the fellows will participate in AGA’s new reviewer education program and will also be offered the opportunity to contribute content to their respective journals.
The journals’ board of editors and editorial staff congratulate the fellows and are excited to work with them over the next year.
AGA welcomes new president, M. Bishr Omary, MD, PhD, AGAF
M. Bishr Omary, MD, PhD, AGAF, will begin his term as the 115th president of the AGA Institute on June 1, 2020.
Dr. Omary, an international leader in GI biology and physiology, currently serves as senior vice chancellor for academic affairs and research for Rutgers Biomedical and Health Sciences schools, centers, and institutes at Rutgers University, Newark, N.J.
Eldest of three siblings, Dr. Omary was born and raised to Syrian parents in New York. After his father obtained his MS degree in political science from Columbia University in New York, the family returned to Damascus, Syria, where his father worked in the Ministry of Urban Planning. The family emigrated to the United States in 1968.
“I am eternally grateful to my parents from whom I learned the meaning of hard work and unconditional love. The opportunities in the U.S. open so many doors, compared with many other countries, including Syria then and especially now given the ongoing 9-year civil war that has ravaged the country,” shared Dr. Omary.
When asked about how he will approach his presidency during a global COVID-19 pandemic, Dr. Omary expressed his commitment to urgently working with and for patients, as well as our community of gastroenterologists, researchers, trainees, and other AGA members, to overcome the disruptions created by the pandemic and ultimately be in a better place than we were before. Dr. Omary holds steadfast to AGA’s vision, a world free from digestive diseases.
Dr. Omary’s primary focus, as an internationally recognized biomedical investigator, is understanding the mechanism and developing therapies for several diseases including lipodystrophies, acute liver failure, and porphyrias. He served as chief of gastroenterology and hepatology at Stanford University, then chair of physiology and chief scientific officer while at the University of Michigan, Ann Arbor, before moving to Rutgers.
Dr. Omary has been a long-time AGA leader, most notably chairing the AGA Institute Research Awards Panel and serving as senior associate editor (2006-2011) then editor in chief (2011-2016) of Gastroenterology, AGA’s premier journal.
Dr. Omary has been on the AGA Governing Board for 2 years as vice president then president-elect; his term as AGA president concludes May 2021.
Rep. Suzan DelBene (D-Wash.) leads prior authorization reform
As a member of the powerful Ways and Means Committee, which has jurisdiction over the Medicare program, Rep. DelBene has worked closely with the American Gastroenterological Association.
When Rep. DelBene was first elected to Congress in 2012, we met with her to share AGA’s policy priorities. We knew instantly that we had a voice for many of our issues. Rep. DelBene started her career as a young investigator before continuing her education and launching a career in the biotechnology industry. From her firsthand experience, she understands the need for investments in National Institutes of Health research and for access to and coverage of colorectal cancer screenings since a member of her family had the disease.
Since Rep. DelBene has been in office, she has taken the lead on several policy priorities affecting our profession, including patient access and protections and regulatory relief. Rep. DelBene is the lead Democratic sponsor of H.R. 3107, the Improving Seniors’ Timely Access to Care Act, legislation that would streamline prior authorization in Medicare Advantage plans. The legislation hit a milestone of securing 218 cosponsors in the House, which is a majority of the members. We look forward to continuing to work with Rep. DelBene on advancing AGA’s policy priorities.
Featured microbiome investigator: Josephine Ni, MD
We’re checking in with a rising star in microbiome research: Dr. Josephine Ni from the University of Pennsylvania, Philadelphia.
Dr. Ni is an instructor of medicine at the University of Pennsylvania, and 2017 recipient of the AGA–Takeda Pharmaceuticals Research Scholar Award in IBD from the AGA Research Foundation.
Congrats to Dr. Ni! While Dr. Ni’s AGA Research Scholar Award concludes at the end of June 2020, we’re proud to share that she has secured two significant grants to continue her work: an NIH KO8 grant and a Burroughs Welcome Fund Award. We catch up with Dr. Ni in the Q&A below.
How would you sum up your research in one sentence?
I am interested in better understanding bacterial colonization of the healthy and inflamed intestinal tract; specifically, my current research focuses on characterizing the role of biofilm formation on intestinal colonization.
What effect do you hope your research will have on patients?
I hope that my work on understanding intestinal colonization will allow us to engineer the microbiota in predictable ways, which will pave the way to exclude enteropathogens, deliver specific compounds, and prevent dysbiosis.
What inspired you to focus your research career on the gut microbiome?
Being able to use data and observations from patient cohorts to generate research hypotheses and then translate those hypotheses into mouse models to explore mechanisms has been a very gratifying experience that I learned from my mentor, Gary Wu, MD. There is still so much to learn about the effects of the microbiome on intestinal health and I’m excited to be a part of this process.
What recent publication from your lab best represents your work if anyone wants to learn more?
Ni J et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med. 2017 Nov 15;9(416):eaah6888.
Gastroenterology invites submissions for an issue focused on colorectal cancer
Share your innovative basic and clinical research for consideration.
The past decade has seen significant milestones in our understanding of the epidemiology, clinical and genetic risk factors, and underlying biological mechanisms of colorectal cancer. This progress has also emphasized the need for further advances. To this end, Gastroenterology will publish a thematic issue in honor of Colorectal Cancer (CRC) Awareness Month in March 2021. The aim is to cover research highlighting novel pathways with human correlates, discoveries related to clinical interventions, clinical trials, and high-profile epidemiologic studies.
Help drive progress of CRC understanding and care by contributing your work. Enhanced promotion of the full issue and automatic indexing of your article to PubMed will increase the visibility of your research in the scientific community and beyond.
Submit your research through Gastroenterology‘s streamlined submission system: www.editorialmanager.com/gastro by Sept. 30, 2020. Original articles and brief communications are welcome.
For more information, please contact Gastroenterology’s Managing Editor, Christopher Lowe, at clowe@gastro.org.
AGA journals select editorial fellows for 2020-2021 academic year
The AGA journals Gastroenterology, Clinical Gastroenterology and Hepatology (CGH), and Cellular and Molecular Gastroenterology and Hepatology (CMGH) recently selected the recipients of their editorial fellowships, which runs from July 2020 through June 2021. The editorial fellowship program is in its fourth year.
The editorial fellows for each journal are:
Gastroenterology
Ruben Colman, MD
Cincinnati Children’s Hospital Medical Center
John Gubatan, MD
Stanford (Calif.) University Medical Center
CGH
Blake Jones, MD
University of Colorado at Denver, Aurora
Nikhil Thiruvengadam, MD
University of California, San Francisco
CMGH
Samuel Hinman, PhD
University of Washington, Seattle
The editorial fellows will be mentored on the journals’ editorial processes, including peer review and the publication process from manuscript submission to acceptance. They will participate in discussions and conferences with the boards of editors and work closely with the AGA editorial staff. Additionally, the fellows will participate in AGA’s new reviewer education program and will also be offered the opportunity to contribute content to their respective journals.
The journals’ board of editors and editorial staff congratulate the fellows and are excited to work with them over the next year.
AGA welcomes new president, M. Bishr Omary, MD, PhD, AGAF
M. Bishr Omary, MD, PhD, AGAF, will begin his term as the 115th president of the AGA Institute on June 1, 2020.
Dr. Omary, an international leader in GI biology and physiology, currently serves as senior vice chancellor for academic affairs and research for Rutgers Biomedical and Health Sciences schools, centers, and institutes at Rutgers University, Newark, N.J.
Eldest of three siblings, Dr. Omary was born and raised to Syrian parents in New York. After his father obtained his MS degree in political science from Columbia University in New York, the family returned to Damascus, Syria, where his father worked in the Ministry of Urban Planning. The family emigrated to the United States in 1968.
“I am eternally grateful to my parents from whom I learned the meaning of hard work and unconditional love. The opportunities in the U.S. open so many doors, compared with many other countries, including Syria then and especially now given the ongoing 9-year civil war that has ravaged the country,” shared Dr. Omary.
When asked about how he will approach his presidency during a global COVID-19 pandemic, Dr. Omary expressed his commitment to urgently working with and for patients, as well as our community of gastroenterologists, researchers, trainees, and other AGA members, to overcome the disruptions created by the pandemic and ultimately be in a better place than we were before. Dr. Omary holds steadfast to AGA’s vision, a world free from digestive diseases.
Dr. Omary’s primary focus, as an internationally recognized biomedical investigator, is understanding the mechanism and developing therapies for several diseases including lipodystrophies, acute liver failure, and porphyrias. He served as chief of gastroenterology and hepatology at Stanford University, then chair of physiology and chief scientific officer while at the University of Michigan, Ann Arbor, before moving to Rutgers.
Dr. Omary has been a long-time AGA leader, most notably chairing the AGA Institute Research Awards Panel and serving as senior associate editor (2006-2011) then editor in chief (2011-2016) of Gastroenterology, AGA’s premier journal.
Dr. Omary has been on the AGA Governing Board for 2 years as vice president then president-elect; his term as AGA president concludes May 2021.
Rep. Suzan DelBene (D-Wash.) leads prior authorization reform
As a member of the powerful Ways and Means Committee, which has jurisdiction over the Medicare program, Rep. DelBene has worked closely with the American Gastroenterological Association.
When Rep. DelBene was first elected to Congress in 2012, we met with her to share AGA’s policy priorities. We knew instantly that we had a voice for many of our issues. Rep. DelBene started her career as a young investigator before continuing her education and launching a career in the biotechnology industry. From her firsthand experience, she understands the need for investments in National Institutes of Health research and for access to and coverage of colorectal cancer screenings since a member of her family had the disease.
Since Rep. DelBene has been in office, she has taken the lead on several policy priorities affecting our profession, including patient access and protections and regulatory relief. Rep. DelBene is the lead Democratic sponsor of H.R. 3107, the Improving Seniors’ Timely Access to Care Act, legislation that would streamline prior authorization in Medicare Advantage plans. The legislation hit a milestone of securing 218 cosponsors in the House, which is a majority of the members. We look forward to continuing to work with Rep. DelBene on advancing AGA’s policy priorities.
Featured microbiome investigator: Josephine Ni, MD
We’re checking in with a rising star in microbiome research: Dr. Josephine Ni from the University of Pennsylvania, Philadelphia.
Dr. Ni is an instructor of medicine at the University of Pennsylvania, and 2017 recipient of the AGA–Takeda Pharmaceuticals Research Scholar Award in IBD from the AGA Research Foundation.
Congrats to Dr. Ni! While Dr. Ni’s AGA Research Scholar Award concludes at the end of June 2020, we’re proud to share that she has secured two significant grants to continue her work: an NIH KO8 grant and a Burroughs Welcome Fund Award. We catch up with Dr. Ni in the Q&A below.
How would you sum up your research in one sentence?
I am interested in better understanding bacterial colonization of the healthy and inflamed intestinal tract; specifically, my current research focuses on characterizing the role of biofilm formation on intestinal colonization.
What effect do you hope your research will have on patients?
I hope that my work on understanding intestinal colonization will allow us to engineer the microbiota in predictable ways, which will pave the way to exclude enteropathogens, deliver specific compounds, and prevent dysbiosis.
What inspired you to focus your research career on the gut microbiome?
Being able to use data and observations from patient cohorts to generate research hypotheses and then translate those hypotheses into mouse models to explore mechanisms has been a very gratifying experience that I learned from my mentor, Gary Wu, MD. There is still so much to learn about the effects of the microbiome on intestinal health and I’m excited to be a part of this process.
What recent publication from your lab best represents your work if anyone wants to learn more?
Ni J et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med. 2017 Nov 15;9(416):eaah6888.
Gastroenterology invites submissions for an issue focused on colorectal cancer
Share your innovative basic and clinical research for consideration.
The past decade has seen significant milestones in our understanding of the epidemiology, clinical and genetic risk factors, and underlying biological mechanisms of colorectal cancer. This progress has also emphasized the need for further advances. To this end, Gastroenterology will publish a thematic issue in honor of Colorectal Cancer (CRC) Awareness Month in March 2021. The aim is to cover research highlighting novel pathways with human correlates, discoveries related to clinical interventions, clinical trials, and high-profile epidemiologic studies.
Help drive progress of CRC understanding and care by contributing your work. Enhanced promotion of the full issue and automatic indexing of your article to PubMed will increase the visibility of your research in the scientific community and beyond.
Submit your research through Gastroenterology‘s streamlined submission system: www.editorialmanager.com/gastro by Sept. 30, 2020. Original articles and brief communications are welcome.
For more information, please contact Gastroenterology’s Managing Editor, Christopher Lowe, at clowe@gastro.org.
AGA journals select editorial fellows for 2020-2021 academic year
The AGA journals Gastroenterology, Clinical Gastroenterology and Hepatology (CGH), and Cellular and Molecular Gastroenterology and Hepatology (CMGH) recently selected the recipients of their editorial fellowships, which runs from July 2020 through June 2021. The editorial fellowship program is in its fourth year.
The editorial fellows for each journal are:
Gastroenterology
Ruben Colman, MD
Cincinnati Children’s Hospital Medical Center
John Gubatan, MD
Stanford (Calif.) University Medical Center
CGH
Blake Jones, MD
University of Colorado at Denver, Aurora
Nikhil Thiruvengadam, MD
University of California, San Francisco
CMGH
Samuel Hinman, PhD
University of Washington, Seattle
The editorial fellows will be mentored on the journals’ editorial processes, including peer review and the publication process from manuscript submission to acceptance. They will participate in discussions and conferences with the boards of editors and work closely with the AGA editorial staff. Additionally, the fellows will participate in AGA’s new reviewer education program and will also be offered the opportunity to contribute content to their respective journals.
The journals’ board of editors and editorial staff congratulate the fellows and are excited to work with them over the next year.
AGA welcomes new president, M. Bishr Omary, MD, PhD, AGAF
M. Bishr Omary, MD, PhD, AGAF, will begin his term as the 115th president of the AGA Institute on June 1, 2020.
Dr. Omary, an international leader in GI biology and physiology, currently serves as senior vice chancellor for academic affairs and research for Rutgers Biomedical and Health Sciences schools, centers, and institutes at Rutgers University, Newark, N.J.
Eldest of three siblings, Dr. Omary was born and raised to Syrian parents in New York. After his father obtained his MS degree in political science from Columbia University in New York, the family returned to Damascus, Syria, where his father worked in the Ministry of Urban Planning. The family emigrated to the United States in 1968.
“I am eternally grateful to my parents from whom I learned the meaning of hard work and unconditional love. The opportunities in the U.S. open so many doors, compared with many other countries, including Syria then and especially now given the ongoing 9-year civil war that has ravaged the country,” shared Dr. Omary.
When asked about how he will approach his presidency during a global COVID-19 pandemic, Dr. Omary expressed his commitment to urgently working with and for patients, as well as our community of gastroenterologists, researchers, trainees, and other AGA members, to overcome the disruptions created by the pandemic and ultimately be in a better place than we were before. Dr. Omary holds steadfast to AGA’s vision, a world free from digestive diseases.
Dr. Omary’s primary focus, as an internationally recognized biomedical investigator, is understanding the mechanism and developing therapies for several diseases including lipodystrophies, acute liver failure, and porphyrias. He served as chief of gastroenterology and hepatology at Stanford University, then chair of physiology and chief scientific officer while at the University of Michigan, Ann Arbor, before moving to Rutgers.
Dr. Omary has been a long-time AGA leader, most notably chairing the AGA Institute Research Awards Panel and serving as senior associate editor (2006-2011) then editor in chief (2011-2016) of Gastroenterology, AGA’s premier journal.
Dr. Omary has been on the AGA Governing Board for 2 years as vice president then president-elect; his term as AGA president concludes May 2021.
FDA approves Tremfya (guselkumab) for psoriatic arthritis
, according to a July 14 announcement from its manufacturer, Janssen.
The FDA’s approval marks the second indication for guselkumab, which was first approved for adults with plaque psoriasis in 2017.
The agency based its approval on two pivotal phase 3 clinical trials, DISCOVER-1 and DISCOVER-2, which tested the biologic in 1,120 adults with active PsA who were naive to biologics (both trials) or had an inadequate response or intolerance to one or two tumor necrosis factor inhibitors (in about 30% of patients in DISCOVER-1). Part of this pretrial standard treatment could include at least 4 months of Otezla (apremilast), at least 3 months of nonbiologic disease-modifying antirheumatic drugs (DMARDs), or at least 4 weeks of NSAIDs. In both trials, about 58% of patients took methotrexate.
Participants who took guselkumab achieved 20% improvement in American College of Rheumatology response criteria at week 24 at rates of 52% in DISCOVER-1 and 64% in DISCOVER-2, whereas placebo-treated patients had rates of 22% and 33%, respectively.
Guselkumab improved patients’ other symptoms, including skin manifestations of psoriasis, physical functioning, enthesitis, dactylitis, and fatigue, according to the Janssen release.
Guselkumab, a fully human monoclonal antibody that selectively binds to the p19 subunit of IL-23, is administered as a 100-mg subcutaneous injection every 8 weeks, following two starter doses at weeks 0 and 4, and can be used alone or in combination with a conventional DMARD.
In guselkumab clinical trials of patients with PsA, a minority had bronchitis or a decreased neutrophil count, but the safety profile was otherwise generally consistent with what has been seen in patients with plaque psoriasis, according to the company release. Other common side effects described in 1% or more of patients have included upper respiratory infections, headache, injection-site reactions, arthralgia, diarrhea, gastroenteritis, tinea infections, and herpes simplex infections.
, according to a July 14 announcement from its manufacturer, Janssen.
The FDA’s approval marks the second indication for guselkumab, which was first approved for adults with plaque psoriasis in 2017.
The agency based its approval on two pivotal phase 3 clinical trials, DISCOVER-1 and DISCOVER-2, which tested the biologic in 1,120 adults with active PsA who were naive to biologics (both trials) or had an inadequate response or intolerance to one or two tumor necrosis factor inhibitors (in about 30% of patients in DISCOVER-1). Part of this pretrial standard treatment could include at least 4 months of Otezla (apremilast), at least 3 months of nonbiologic disease-modifying antirheumatic drugs (DMARDs), or at least 4 weeks of NSAIDs. In both trials, about 58% of patients took methotrexate.
Participants who took guselkumab achieved 20% improvement in American College of Rheumatology response criteria at week 24 at rates of 52% in DISCOVER-1 and 64% in DISCOVER-2, whereas placebo-treated patients had rates of 22% and 33%, respectively.
Guselkumab improved patients’ other symptoms, including skin manifestations of psoriasis, physical functioning, enthesitis, dactylitis, and fatigue, according to the Janssen release.
Guselkumab, a fully human monoclonal antibody that selectively binds to the p19 subunit of IL-23, is administered as a 100-mg subcutaneous injection every 8 weeks, following two starter doses at weeks 0 and 4, and can be used alone or in combination with a conventional DMARD.
In guselkumab clinical trials of patients with PsA, a minority had bronchitis or a decreased neutrophil count, but the safety profile was otherwise generally consistent with what has been seen in patients with plaque psoriasis, according to the company release. Other common side effects described in 1% or more of patients have included upper respiratory infections, headache, injection-site reactions, arthralgia, diarrhea, gastroenteritis, tinea infections, and herpes simplex infections.
, according to a July 14 announcement from its manufacturer, Janssen.
The FDA’s approval marks the second indication for guselkumab, which was first approved for adults with plaque psoriasis in 2017.
The agency based its approval on two pivotal phase 3 clinical trials, DISCOVER-1 and DISCOVER-2, which tested the biologic in 1,120 adults with active PsA who were naive to biologics (both trials) or had an inadequate response or intolerance to one or two tumor necrosis factor inhibitors (in about 30% of patients in DISCOVER-1). Part of this pretrial standard treatment could include at least 4 months of Otezla (apremilast), at least 3 months of nonbiologic disease-modifying antirheumatic drugs (DMARDs), or at least 4 weeks of NSAIDs. In both trials, about 58% of patients took methotrexate.
Participants who took guselkumab achieved 20% improvement in American College of Rheumatology response criteria at week 24 at rates of 52% in DISCOVER-1 and 64% in DISCOVER-2, whereas placebo-treated patients had rates of 22% and 33%, respectively.
Guselkumab improved patients’ other symptoms, including skin manifestations of psoriasis, physical functioning, enthesitis, dactylitis, and fatigue, according to the Janssen release.
Guselkumab, a fully human monoclonal antibody that selectively binds to the p19 subunit of IL-23, is administered as a 100-mg subcutaneous injection every 8 weeks, following two starter doses at weeks 0 and 4, and can be used alone or in combination with a conventional DMARD.
In guselkumab clinical trials of patients with PsA, a minority had bronchitis or a decreased neutrophil count, but the safety profile was otherwise generally consistent with what has been seen in patients with plaque psoriasis, according to the company release. Other common side effects described in 1% or more of patients have included upper respiratory infections, headache, injection-site reactions, arthralgia, diarrhea, gastroenteritis, tinea infections, and herpes simplex infections.
The public’s trust in science
Having been a bench research scientist 30 years ago, I am flabbergasted at what is and is not currently possible. In a few weeks, scientists sequenced a novel coronavirus and used the genetic sequence to select candidate molecules for a vaccine. But we still can’t reliably say how much protection a cloth mask provides. Worse yet, even if/when we could reliably quantify contagion, it isn’t clear that the public will believe us anyhow.
The good news is that the public worldwide did believe scientists about the threat of a pandemic and the need to flatten the curve. Saving lives has not been about the strength of an antibiotic or the skill in managing a ventilator, but the credibility of medical scientists. The degree of acceptance was variable and subject to a variety of delays caused by regional politicians, but The bad news is that the public’s trust in that scientific advice has waned, the willingness to accept onerous restrictions has fatigued, and the cooperation for maintaining these social changes is evaporating.
I will leave pontificating about the spread of COVID-19 to other experts in other forums. My focus is on the public’s trust in the professionalism of physicians, nurses, medical scientists, and the health care industry as a whole. That trust has been our most valuable tool in fighting the pandemic so far. There have been situations in which weaknesses in modern science have let society down during the pandemic of the century. In my February 2020 column, at the beginning of the outbreak, a month before it was declared a pandemic, when its magnitude was still unclear, I emphasized the importance of having a trusted scientific spokesperson providing timely, accurate information to the public. That, obviously, did not happen in the United States and the degree of the ensuing disaster is still to be revealed.
Scientists have made some wrong decisions about this novel threat. The advice on masks is an illustrative example. For many years, infection control nurses have insisted that medical students wear a mask to protect themselves, even if they were observing rounds from just inside the doorway of a room of a baby with bronchiolitis. The landfills are full of briefly worn surgical masks. Now the story goes: Surgical masks don’t protect staff; they protect others. Changes like that contribute to a credibility gap.
For 3 months, there was conflicting advice about the appropriateness of masks. In early March 2020, some health care workers were disciplined for wearing personal masks. Now, most scientists recommend the public use masks to reduce contagion. Significant subgroups in the U.S. population have refused, mostly to signal their contrarian politics. In June there was an anecdote of a success story from the Show Me state of Missouri, where a mask is credited for preventing an outbreak from a sick hair stylist.
It is hard to find something more reliable than an anecdote. On June 1, a meta-analysis funded by the World Health Organization was published online by Lancet. It supports the idea that masks are beneficial. It is mostly forest plots, so you can try to interpret it yourself. There were 172 observational studies in the systematic review, and the meta-analysis contains 44 relevant comparative studies and 0 randomized controlled trials. Most of those forest plots have an I2 of 75% or worse, which to me indicates that they are not much more reliable than a good anecdote. My primary conclusion was that modern academic science, in an era with a shortage of toilet paper, should convert to printing on soft tissue paper.
It is important to note that the guesstimated overall benefit of cloth masks was a relative risk of 0.30. That benefit is easily nullified if the false security of a mask causes people to congregate together in groups three times larger or for three times more minutes. N95 masks were more effective.
A different article was published in PNAS on June 11. Its senior author was awarded the Nobel Prize in Chemistry in 1995. That article touted the benefits of masks. The article is facing heavy criticism for flaws in methodology and flaws in the peer review process. A long list of signatories have joined a letter asking for the article’s retraction.
This article, when combined with the two instances of prominent articles being retracted in the prior month by the New England Journal of Medicine and The Lancet, is accumulating evidence the peer review system is not working as intended.
There are many heroes in this pandemic, from the frontline health care workers in hotspots to the grocery workers and cleaning staff. There is hope, indeed some faith, that medical scientists in the foreseeable future will provide treatments and a vaccine for this viral plague. This month, the credibility of scientists again plays a major role as communities respond to outbreaks related to reopening the economy. Let’s celebrate the victories, resolve to fix the impure system, and restore a high level of public trust in science. Lives depend on it.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
Having been a bench research scientist 30 years ago, I am flabbergasted at what is and is not currently possible. In a few weeks, scientists sequenced a novel coronavirus and used the genetic sequence to select candidate molecules for a vaccine. But we still can’t reliably say how much protection a cloth mask provides. Worse yet, even if/when we could reliably quantify contagion, it isn’t clear that the public will believe us anyhow.
The good news is that the public worldwide did believe scientists about the threat of a pandemic and the need to flatten the curve. Saving lives has not been about the strength of an antibiotic or the skill in managing a ventilator, but the credibility of medical scientists. The degree of acceptance was variable and subject to a variety of delays caused by regional politicians, but The bad news is that the public’s trust in that scientific advice has waned, the willingness to accept onerous restrictions has fatigued, and the cooperation for maintaining these social changes is evaporating.
I will leave pontificating about the spread of COVID-19 to other experts in other forums. My focus is on the public’s trust in the professionalism of physicians, nurses, medical scientists, and the health care industry as a whole. That trust has been our most valuable tool in fighting the pandemic so far. There have been situations in which weaknesses in modern science have let society down during the pandemic of the century. In my February 2020 column, at the beginning of the outbreak, a month before it was declared a pandemic, when its magnitude was still unclear, I emphasized the importance of having a trusted scientific spokesperson providing timely, accurate information to the public. That, obviously, did not happen in the United States and the degree of the ensuing disaster is still to be revealed.
Scientists have made some wrong decisions about this novel threat. The advice on masks is an illustrative example. For many years, infection control nurses have insisted that medical students wear a mask to protect themselves, even if they were observing rounds from just inside the doorway of a room of a baby with bronchiolitis. The landfills are full of briefly worn surgical masks. Now the story goes: Surgical masks don’t protect staff; they protect others. Changes like that contribute to a credibility gap.
For 3 months, there was conflicting advice about the appropriateness of masks. In early March 2020, some health care workers were disciplined for wearing personal masks. Now, most scientists recommend the public use masks to reduce contagion. Significant subgroups in the U.S. population have refused, mostly to signal their contrarian politics. In June there was an anecdote of a success story from the Show Me state of Missouri, where a mask is credited for preventing an outbreak from a sick hair stylist.
It is hard to find something more reliable than an anecdote. On June 1, a meta-analysis funded by the World Health Organization was published online by Lancet. It supports the idea that masks are beneficial. It is mostly forest plots, so you can try to interpret it yourself. There were 172 observational studies in the systematic review, and the meta-analysis contains 44 relevant comparative studies and 0 randomized controlled trials. Most of those forest plots have an I2 of 75% or worse, which to me indicates that they are not much more reliable than a good anecdote. My primary conclusion was that modern academic science, in an era with a shortage of toilet paper, should convert to printing on soft tissue paper.
It is important to note that the guesstimated overall benefit of cloth masks was a relative risk of 0.30. That benefit is easily nullified if the false security of a mask causes people to congregate together in groups three times larger or for three times more minutes. N95 masks were more effective.
A different article was published in PNAS on June 11. Its senior author was awarded the Nobel Prize in Chemistry in 1995. That article touted the benefits of masks. The article is facing heavy criticism for flaws in methodology and flaws in the peer review process. A long list of signatories have joined a letter asking for the article’s retraction.
This article, when combined with the two instances of prominent articles being retracted in the prior month by the New England Journal of Medicine and The Lancet, is accumulating evidence the peer review system is not working as intended.
There are many heroes in this pandemic, from the frontline health care workers in hotspots to the grocery workers and cleaning staff. There is hope, indeed some faith, that medical scientists in the foreseeable future will provide treatments and a vaccine for this viral plague. This month, the credibility of scientists again plays a major role as communities respond to outbreaks related to reopening the economy. Let’s celebrate the victories, resolve to fix the impure system, and restore a high level of public trust in science. Lives depend on it.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
Having been a bench research scientist 30 years ago, I am flabbergasted at what is and is not currently possible. In a few weeks, scientists sequenced a novel coronavirus and used the genetic sequence to select candidate molecules for a vaccine. But we still can’t reliably say how much protection a cloth mask provides. Worse yet, even if/when we could reliably quantify contagion, it isn’t clear that the public will believe us anyhow.
The good news is that the public worldwide did believe scientists about the threat of a pandemic and the need to flatten the curve. Saving lives has not been about the strength of an antibiotic or the skill in managing a ventilator, but the credibility of medical scientists. The degree of acceptance was variable and subject to a variety of delays caused by regional politicians, but The bad news is that the public’s trust in that scientific advice has waned, the willingness to accept onerous restrictions has fatigued, and the cooperation for maintaining these social changes is evaporating.
I will leave pontificating about the spread of COVID-19 to other experts in other forums. My focus is on the public’s trust in the professionalism of physicians, nurses, medical scientists, and the health care industry as a whole. That trust has been our most valuable tool in fighting the pandemic so far. There have been situations in which weaknesses in modern science have let society down during the pandemic of the century. In my February 2020 column, at the beginning of the outbreak, a month before it was declared a pandemic, when its magnitude was still unclear, I emphasized the importance of having a trusted scientific spokesperson providing timely, accurate information to the public. That, obviously, did not happen in the United States and the degree of the ensuing disaster is still to be revealed.
Scientists have made some wrong decisions about this novel threat. The advice on masks is an illustrative example. For many years, infection control nurses have insisted that medical students wear a mask to protect themselves, even if they were observing rounds from just inside the doorway of a room of a baby with bronchiolitis. The landfills are full of briefly worn surgical masks. Now the story goes: Surgical masks don’t protect staff; they protect others. Changes like that contribute to a credibility gap.
For 3 months, there was conflicting advice about the appropriateness of masks. In early March 2020, some health care workers were disciplined for wearing personal masks. Now, most scientists recommend the public use masks to reduce contagion. Significant subgroups in the U.S. population have refused, mostly to signal their contrarian politics. In June there was an anecdote of a success story from the Show Me state of Missouri, where a mask is credited for preventing an outbreak from a sick hair stylist.
It is hard to find something more reliable than an anecdote. On June 1, a meta-analysis funded by the World Health Organization was published online by Lancet. It supports the idea that masks are beneficial. It is mostly forest plots, so you can try to interpret it yourself. There were 172 observational studies in the systematic review, and the meta-analysis contains 44 relevant comparative studies and 0 randomized controlled trials. Most of those forest plots have an I2 of 75% or worse, which to me indicates that they are not much more reliable than a good anecdote. My primary conclusion was that modern academic science, in an era with a shortage of toilet paper, should convert to printing on soft tissue paper.
It is important to note that the guesstimated overall benefit of cloth masks was a relative risk of 0.30. That benefit is easily nullified if the false security of a mask causes people to congregate together in groups three times larger or for three times more minutes. N95 masks were more effective.
A different article was published in PNAS on June 11. Its senior author was awarded the Nobel Prize in Chemistry in 1995. That article touted the benefits of masks. The article is facing heavy criticism for flaws in methodology and flaws in the peer review process. A long list of signatories have joined a letter asking for the article’s retraction.
This article, when combined with the two instances of prominent articles being retracted in the prior month by the New England Journal of Medicine and The Lancet, is accumulating evidence the peer review system is not working as intended.
There are many heroes in this pandemic, from the frontline health care workers in hotspots to the grocery workers and cleaning staff. There is hope, indeed some faith, that medical scientists in the foreseeable future will provide treatments and a vaccine for this viral plague. This month, the credibility of scientists again plays a major role as communities respond to outbreaks related to reopening the economy. Let’s celebrate the victories, resolve to fix the impure system, and restore a high level of public trust in science. Lives depend on it.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.