User login
Intraventricular methotrexate may improve survival for medulloblastoma subtypes
according to research published in the Journal of Clinical Oncology.
Investigators conducted a prospective trial of 87 children diagnosed with nonmetastatic medulloblastoma before the age of 4 years who were treated from 2001-2011.
At 5 years after diagnosis, the 42 DMB/MBEN patients had a 93% progression-free survival (PFS) rate, a 100% overall survival (OS) rate, and a 93% craniospinal irradiation (CSI)–free survival rate.
“Our results suggest that ... poor outcomes of patients treated with systemic chemotherapy alone can be improved by the addition of intraventricular [methotrexate],” wrote Martin Mynarek, MD, of the University Medical Center Hamburg-Eppendorf (Germany), and colleagues.
However, it was a different story for the 45 patients with classic medulloblastoma (CMB) or large-cell/anaplastic medulloblastoma (CLA), in which outcomes are historically worse. At 5 years, the PFS was 37%, the OS was 62%, and the CSI-free survival was 39% in these patients.
In 2006, the CMB and CLA patients started receiving local radiotherapy in addition to chemotherapy and intraventricular methotrexate, but the radiotherapy did not seem to help with their disease.
“Because data suggest no effects, or even adverse effects, of local radiotherapy, additional development of this approach does not seem justified,” the investigators wrote.
“Interestingly, almost all patients with CMB/LCA had distant or combined relapses after local radiotherapy,” they added. “One might speculate that local radiotherapy reduced disease burden of the primary tumor and subclinical metastasis in the posterior fossa, leading to a survival advantage of distant subclinical metastasis over local residues.”
Treatment details
The 87 patients were enrolled in the BIS4 arm of the HIT 2000 trial (NCT00303810), which was designed to test six protocols and identify the optimal approach for treating young patients with medulloblastoma, supratentorial primitive neuroectodermal tumor, or ependymoma.
Patients started “HIT-SKK” chemotherapy within 2-4 weeks of surgery. They received three cycles of intravenous cyclophosphamide, vincristine, methotrexate (followed by leucovorin rescue after 42 hours), carboplatin, and etoposide, with concomitant intraventricular methotrexate, for a duration of 6 months (Neuro Oncol. 2011 Jun;13[6]:669-79).
Among patients who achieved a complete remission, treatment was ended after two additional cycles of chemotherapy. For other patients, secondary surgery, radiotherapy, and consolidation chemotherapy were recommended.
Starting in 2006, DMB and MBEN patients without complete remissions, as well as those with CMB or LCA, received 54 Gy of focal radiotherapy to the tumor bed after the first three treatment cycles.
SHH-I vs. SHH-II DMB/MBEN
DNA methylation profiling was available for 50 of the 87 patients in this analysis, 28 of whom had infantile sonic hedgehog (SHH)–activated DMB/MBEN. Data from these 28 patients – and 71 patients in a validation cohort – revealed no significant difference in 5-year PFS or OS based on methylation subtype.
The 5-year PFS was 73% in SHH-I patients and 83% in SHH-II patients (P = .25). The 5-year OS was 88% and 97%, respectively (P = .099).
“This suggests that the higher risk of relapse in the less favorable [SHH-I subtype] can be abrogated by the addition of intraventricular [methotrexate] to systemic chemotherapy,” the investigators wrote.
The results suggest SHH-I patients “markedly benefit” from the addition of intraventricular chemotherapy, according to the authors of a related editorial, Giles Robinson, MD, and Amar Gajjar, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
“However, cross-trial comparison of PFS among the SHH-II subtype does not suggest that SHH-II patients derive the same benefit,” the editorialists wrote.
These data divide SHH medulloblastoma into SHH-I, which benefits from chemotherapy with intraventricular methotrexate, and SHH-II, which can be cured without intraventricular methotrexate, high-dose chemotherapy, or focal radiotherapy, according to the editorialists.
This new information might prompt investigation of a risk-adapted approach. SHH-II patients would receive a reduced-intensity regimen with systemic chemotherapy only, and SHH-I patients would receive systemic chemotherapy combined with intraventricular methotrexate. This could avoid exposing young children to “more intensive therapy than necessary,” according to the editorialists.
Non-WNT/non-SHH disease
Patients with non-WNT/non-SHH medulloblastoma (CMB or LCA) were divided into molecularly defined subtypes group 3 (n = 14) and group 4 (n = 6). The patients in group 3 had lower survival rates than patients in group 4. The 5-year PFS rate was 36% and 83%, respectively (P < .001 ). The 5-year OS rate was 49% and 100%, respectively (P < .001).
“This represents the third recent publication to describe a poor PFS for group 3, and it signals a desperate need for better therapy,” the editorialists wrote. As for the “encouraging” survival rate for group 4 patients, there were only six subjects, which makes this finding “worthy of follow-up but not actionable.”
Even so, the investigators noted that “although poor survival has been reported for non-SHH CMB/LCA in almost every series of patients treated with CSI-sparing approaches, again, use of intraventricular [methotrexate] might be associated with slightly higher PFS, compared with conventional chemotherapy alone.”
As expected, IQ was significantly lower in patients who received CSI salvage. The mean IQ was 74 in patients who received CSI and 90 in patients who did not (P = .012). Neurocognitive outcomes were poor in general among CMB/LCA survivors, “which was closely related to use of radiotherapy,” according to the investigators.
“It is important to recognize that these studies were not designed to define outcome on the basis of molecular subgroup or subtype and that the sample size in all these studies is small,” Dr. Robinson and Dr. Gajjar wrote. “Thus, caution should be used when basing any treatment recommendation on these results, and it is our strong-held opinion that any treatment change be done on a well-planned and well-monitored clinical trial.”
This research was supported by the German Childhood Cancer Foundation, Styrian Childhood Cancer Foundation, and other organizations. Dr. Mynarek and colleagues disclosed relationships with Medac, Novartis, Eli Lilly, Bayer, Roche, and numerous other companies. Dr. Robinson disclosed relationships with Eli Lilly, Genentech, and Novartis. Dr. Gajjar reported relationships with Genentech and Kazia Therapeutics.
SOURCE: Mynarek M et al. J Clin Oncol. 2020 Jun 20;38(18):2028-40.
according to research published in the Journal of Clinical Oncology.
Investigators conducted a prospective trial of 87 children diagnosed with nonmetastatic medulloblastoma before the age of 4 years who were treated from 2001-2011.
At 5 years after diagnosis, the 42 DMB/MBEN patients had a 93% progression-free survival (PFS) rate, a 100% overall survival (OS) rate, and a 93% craniospinal irradiation (CSI)–free survival rate.
“Our results suggest that ... poor outcomes of patients treated with systemic chemotherapy alone can be improved by the addition of intraventricular [methotrexate],” wrote Martin Mynarek, MD, of the University Medical Center Hamburg-Eppendorf (Germany), and colleagues.
However, it was a different story for the 45 patients with classic medulloblastoma (CMB) or large-cell/anaplastic medulloblastoma (CLA), in which outcomes are historically worse. At 5 years, the PFS was 37%, the OS was 62%, and the CSI-free survival was 39% in these patients.
In 2006, the CMB and CLA patients started receiving local radiotherapy in addition to chemotherapy and intraventricular methotrexate, but the radiotherapy did not seem to help with their disease.
“Because data suggest no effects, or even adverse effects, of local radiotherapy, additional development of this approach does not seem justified,” the investigators wrote.
“Interestingly, almost all patients with CMB/LCA had distant or combined relapses after local radiotherapy,” they added. “One might speculate that local radiotherapy reduced disease burden of the primary tumor and subclinical metastasis in the posterior fossa, leading to a survival advantage of distant subclinical metastasis over local residues.”
Treatment details
The 87 patients were enrolled in the BIS4 arm of the HIT 2000 trial (NCT00303810), which was designed to test six protocols and identify the optimal approach for treating young patients with medulloblastoma, supratentorial primitive neuroectodermal tumor, or ependymoma.
Patients started “HIT-SKK” chemotherapy within 2-4 weeks of surgery. They received three cycles of intravenous cyclophosphamide, vincristine, methotrexate (followed by leucovorin rescue after 42 hours), carboplatin, and etoposide, with concomitant intraventricular methotrexate, for a duration of 6 months (Neuro Oncol. 2011 Jun;13[6]:669-79).
Among patients who achieved a complete remission, treatment was ended after two additional cycles of chemotherapy. For other patients, secondary surgery, radiotherapy, and consolidation chemotherapy were recommended.
Starting in 2006, DMB and MBEN patients without complete remissions, as well as those with CMB or LCA, received 54 Gy of focal radiotherapy to the tumor bed after the first three treatment cycles.
SHH-I vs. SHH-II DMB/MBEN
DNA methylation profiling was available for 50 of the 87 patients in this analysis, 28 of whom had infantile sonic hedgehog (SHH)–activated DMB/MBEN. Data from these 28 patients – and 71 patients in a validation cohort – revealed no significant difference in 5-year PFS or OS based on methylation subtype.
The 5-year PFS was 73% in SHH-I patients and 83% in SHH-II patients (P = .25). The 5-year OS was 88% and 97%, respectively (P = .099).
“This suggests that the higher risk of relapse in the less favorable [SHH-I subtype] can be abrogated by the addition of intraventricular [methotrexate] to systemic chemotherapy,” the investigators wrote.
The results suggest SHH-I patients “markedly benefit” from the addition of intraventricular chemotherapy, according to the authors of a related editorial, Giles Robinson, MD, and Amar Gajjar, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
“However, cross-trial comparison of PFS among the SHH-II subtype does not suggest that SHH-II patients derive the same benefit,” the editorialists wrote.
These data divide SHH medulloblastoma into SHH-I, which benefits from chemotherapy with intraventricular methotrexate, and SHH-II, which can be cured without intraventricular methotrexate, high-dose chemotherapy, or focal radiotherapy, according to the editorialists.
This new information might prompt investigation of a risk-adapted approach. SHH-II patients would receive a reduced-intensity regimen with systemic chemotherapy only, and SHH-I patients would receive systemic chemotherapy combined with intraventricular methotrexate. This could avoid exposing young children to “more intensive therapy than necessary,” according to the editorialists.
Non-WNT/non-SHH disease
Patients with non-WNT/non-SHH medulloblastoma (CMB or LCA) were divided into molecularly defined subtypes group 3 (n = 14) and group 4 (n = 6). The patients in group 3 had lower survival rates than patients in group 4. The 5-year PFS rate was 36% and 83%, respectively (P < .001 ). The 5-year OS rate was 49% and 100%, respectively (P < .001).
“This represents the third recent publication to describe a poor PFS for group 3, and it signals a desperate need for better therapy,” the editorialists wrote. As for the “encouraging” survival rate for group 4 patients, there were only six subjects, which makes this finding “worthy of follow-up but not actionable.”
Even so, the investigators noted that “although poor survival has been reported for non-SHH CMB/LCA in almost every series of patients treated with CSI-sparing approaches, again, use of intraventricular [methotrexate] might be associated with slightly higher PFS, compared with conventional chemotherapy alone.”
As expected, IQ was significantly lower in patients who received CSI salvage. The mean IQ was 74 in patients who received CSI and 90 in patients who did not (P = .012). Neurocognitive outcomes were poor in general among CMB/LCA survivors, “which was closely related to use of radiotherapy,” according to the investigators.
“It is important to recognize that these studies were not designed to define outcome on the basis of molecular subgroup or subtype and that the sample size in all these studies is small,” Dr. Robinson and Dr. Gajjar wrote. “Thus, caution should be used when basing any treatment recommendation on these results, and it is our strong-held opinion that any treatment change be done on a well-planned and well-monitored clinical trial.”
This research was supported by the German Childhood Cancer Foundation, Styrian Childhood Cancer Foundation, and other organizations. Dr. Mynarek and colleagues disclosed relationships with Medac, Novartis, Eli Lilly, Bayer, Roche, and numerous other companies. Dr. Robinson disclosed relationships with Eli Lilly, Genentech, and Novartis. Dr. Gajjar reported relationships with Genentech and Kazia Therapeutics.
SOURCE: Mynarek M et al. J Clin Oncol. 2020 Jun 20;38(18):2028-40.
according to research published in the Journal of Clinical Oncology.
Investigators conducted a prospective trial of 87 children diagnosed with nonmetastatic medulloblastoma before the age of 4 years who were treated from 2001-2011.
At 5 years after diagnosis, the 42 DMB/MBEN patients had a 93% progression-free survival (PFS) rate, a 100% overall survival (OS) rate, and a 93% craniospinal irradiation (CSI)–free survival rate.
“Our results suggest that ... poor outcomes of patients treated with systemic chemotherapy alone can be improved by the addition of intraventricular [methotrexate],” wrote Martin Mynarek, MD, of the University Medical Center Hamburg-Eppendorf (Germany), and colleagues.
However, it was a different story for the 45 patients with classic medulloblastoma (CMB) or large-cell/anaplastic medulloblastoma (CLA), in which outcomes are historically worse. At 5 years, the PFS was 37%, the OS was 62%, and the CSI-free survival was 39% in these patients.
In 2006, the CMB and CLA patients started receiving local radiotherapy in addition to chemotherapy and intraventricular methotrexate, but the radiotherapy did not seem to help with their disease.
“Because data suggest no effects, or even adverse effects, of local radiotherapy, additional development of this approach does not seem justified,” the investigators wrote.
“Interestingly, almost all patients with CMB/LCA had distant or combined relapses after local radiotherapy,” they added. “One might speculate that local radiotherapy reduced disease burden of the primary tumor and subclinical metastasis in the posterior fossa, leading to a survival advantage of distant subclinical metastasis over local residues.”
Treatment details
The 87 patients were enrolled in the BIS4 arm of the HIT 2000 trial (NCT00303810), which was designed to test six protocols and identify the optimal approach for treating young patients with medulloblastoma, supratentorial primitive neuroectodermal tumor, or ependymoma.
Patients started “HIT-SKK” chemotherapy within 2-4 weeks of surgery. They received three cycles of intravenous cyclophosphamide, vincristine, methotrexate (followed by leucovorin rescue after 42 hours), carboplatin, and etoposide, with concomitant intraventricular methotrexate, for a duration of 6 months (Neuro Oncol. 2011 Jun;13[6]:669-79).
Among patients who achieved a complete remission, treatment was ended after two additional cycles of chemotherapy. For other patients, secondary surgery, radiotherapy, and consolidation chemotherapy were recommended.
Starting in 2006, DMB and MBEN patients without complete remissions, as well as those with CMB or LCA, received 54 Gy of focal radiotherapy to the tumor bed after the first three treatment cycles.
SHH-I vs. SHH-II DMB/MBEN
DNA methylation profiling was available for 50 of the 87 patients in this analysis, 28 of whom had infantile sonic hedgehog (SHH)–activated DMB/MBEN. Data from these 28 patients – and 71 patients in a validation cohort – revealed no significant difference in 5-year PFS or OS based on methylation subtype.
The 5-year PFS was 73% in SHH-I patients and 83% in SHH-II patients (P = .25). The 5-year OS was 88% and 97%, respectively (P = .099).
“This suggests that the higher risk of relapse in the less favorable [SHH-I subtype] can be abrogated by the addition of intraventricular [methotrexate] to systemic chemotherapy,” the investigators wrote.
The results suggest SHH-I patients “markedly benefit” from the addition of intraventricular chemotherapy, according to the authors of a related editorial, Giles Robinson, MD, and Amar Gajjar, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
“However, cross-trial comparison of PFS among the SHH-II subtype does not suggest that SHH-II patients derive the same benefit,” the editorialists wrote.
These data divide SHH medulloblastoma into SHH-I, which benefits from chemotherapy with intraventricular methotrexate, and SHH-II, which can be cured without intraventricular methotrexate, high-dose chemotherapy, or focal radiotherapy, according to the editorialists.
This new information might prompt investigation of a risk-adapted approach. SHH-II patients would receive a reduced-intensity regimen with systemic chemotherapy only, and SHH-I patients would receive systemic chemotherapy combined with intraventricular methotrexate. This could avoid exposing young children to “more intensive therapy than necessary,” according to the editorialists.
Non-WNT/non-SHH disease
Patients with non-WNT/non-SHH medulloblastoma (CMB or LCA) were divided into molecularly defined subtypes group 3 (n = 14) and group 4 (n = 6). The patients in group 3 had lower survival rates than patients in group 4. The 5-year PFS rate was 36% and 83%, respectively (P < .001 ). The 5-year OS rate was 49% and 100%, respectively (P < .001).
“This represents the third recent publication to describe a poor PFS for group 3, and it signals a desperate need for better therapy,” the editorialists wrote. As for the “encouraging” survival rate for group 4 patients, there were only six subjects, which makes this finding “worthy of follow-up but not actionable.”
Even so, the investigators noted that “although poor survival has been reported for non-SHH CMB/LCA in almost every series of patients treated with CSI-sparing approaches, again, use of intraventricular [methotrexate] might be associated with slightly higher PFS, compared with conventional chemotherapy alone.”
As expected, IQ was significantly lower in patients who received CSI salvage. The mean IQ was 74 in patients who received CSI and 90 in patients who did not (P = .012). Neurocognitive outcomes were poor in general among CMB/LCA survivors, “which was closely related to use of radiotherapy,” according to the investigators.
“It is important to recognize that these studies were not designed to define outcome on the basis of molecular subgroup or subtype and that the sample size in all these studies is small,” Dr. Robinson and Dr. Gajjar wrote. “Thus, caution should be used when basing any treatment recommendation on these results, and it is our strong-held opinion that any treatment change be done on a well-planned and well-monitored clinical trial.”
This research was supported by the German Childhood Cancer Foundation, Styrian Childhood Cancer Foundation, and other organizations. Dr. Mynarek and colleagues disclosed relationships with Medac, Novartis, Eli Lilly, Bayer, Roche, and numerous other companies. Dr. Robinson disclosed relationships with Eli Lilly, Genentech, and Novartis. Dr. Gajjar reported relationships with Genentech and Kazia Therapeutics.
SOURCE: Mynarek M et al. J Clin Oncol. 2020 Jun 20;38(18):2028-40.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Rilzabrutinib shows positive results in phase 2b for pemphigus
accompanied by markedly reduced need for systemic corticosteroids in the phase 2b BELIEVE-PV trial, Dedee F. Murrell, MD, said at the virtual annual meeting of the American Academy of Dermatology.
Moreover, in sharp contrast to the standard first-line treatments for pemphigus – rituximab (Rituxan) and high-dose corticosteroids – the treatment-emergent adverse events that arose with 6 months of rilzabrutinib in BELIEVE-PV were uniformly mild to moderate and transient, added Dr. Murrell, professor of dermatology at the University of New South Wales and head of the department of dermatology at St. George University Hospital, Sydney.
The phase 2b BELIEVE-PV trial was a small, 24-week, open-label study that included six patients with newly diagnosed pemphigus and nine others with relapsing pemphigus. The primary endpoint was control of disease activity, defined as no new lesions appearing and established lesions beginning to heal. This was achieved in 9 of 15 patients (60%) at 4 weeks and in 13 patients by week 12. Meanwhile, the mean daily dose of prednisone fell from 21 mg at baseline to 6 mg at 24 weeks.
The mean score on the Pemphigus Disease Area Index (PDAI) dropped by 79% from a baseline of 15.5. Ten of 15 subjects improved to a PDAI of 0 or 1 – clear or almost clear skin – by week 24. The complete remission rate, defined as an absence of both new and established lesions while on no or a very low dose of prednisone, was 40% at week 24.
Treatment-emergent adverse events consisted of nausea in four patients, dizziness in two, and abdominal distension in two, all of which were grade 1 or 2.
Based upon these favorable results, the pivotal phase 3, double-blind, international PEGASUS trial is underway, led by Dr. Murrell. The trial will enroll 120 pemphigus patients, randomized to rilzabrutinib at 400 mg twice daily or placebo on top of background steroid tapering.
Rilzabrutinib is also in earlier-stage clinical trials for the treatment of immune thrombocytopenia.
Dr. Murrell reported serving as a consultant to Principia Biopharma, sponsor of the BELIEVE-PV and PEGASUS trials, and has received institutional research grants from numerous pharmaceutical companies.
SOURCE: Murrell DF. AAD 2020 LBCT.
accompanied by markedly reduced need for systemic corticosteroids in the phase 2b BELIEVE-PV trial, Dedee F. Murrell, MD, said at the virtual annual meeting of the American Academy of Dermatology.
Moreover, in sharp contrast to the standard first-line treatments for pemphigus – rituximab (Rituxan) and high-dose corticosteroids – the treatment-emergent adverse events that arose with 6 months of rilzabrutinib in BELIEVE-PV were uniformly mild to moderate and transient, added Dr. Murrell, professor of dermatology at the University of New South Wales and head of the department of dermatology at St. George University Hospital, Sydney.
The phase 2b BELIEVE-PV trial was a small, 24-week, open-label study that included six patients with newly diagnosed pemphigus and nine others with relapsing pemphigus. The primary endpoint was control of disease activity, defined as no new lesions appearing and established lesions beginning to heal. This was achieved in 9 of 15 patients (60%) at 4 weeks and in 13 patients by week 12. Meanwhile, the mean daily dose of prednisone fell from 21 mg at baseline to 6 mg at 24 weeks.
The mean score on the Pemphigus Disease Area Index (PDAI) dropped by 79% from a baseline of 15.5. Ten of 15 subjects improved to a PDAI of 0 or 1 – clear or almost clear skin – by week 24. The complete remission rate, defined as an absence of both new and established lesions while on no or a very low dose of prednisone, was 40% at week 24.
Treatment-emergent adverse events consisted of nausea in four patients, dizziness in two, and abdominal distension in two, all of which were grade 1 or 2.
Based upon these favorable results, the pivotal phase 3, double-blind, international PEGASUS trial is underway, led by Dr. Murrell. The trial will enroll 120 pemphigus patients, randomized to rilzabrutinib at 400 mg twice daily or placebo on top of background steroid tapering.
Rilzabrutinib is also in earlier-stage clinical trials for the treatment of immune thrombocytopenia.
Dr. Murrell reported serving as a consultant to Principia Biopharma, sponsor of the BELIEVE-PV and PEGASUS trials, and has received institutional research grants from numerous pharmaceutical companies.
SOURCE: Murrell DF. AAD 2020 LBCT.
accompanied by markedly reduced need for systemic corticosteroids in the phase 2b BELIEVE-PV trial, Dedee F. Murrell, MD, said at the virtual annual meeting of the American Academy of Dermatology.
Moreover, in sharp contrast to the standard first-line treatments for pemphigus – rituximab (Rituxan) and high-dose corticosteroids – the treatment-emergent adverse events that arose with 6 months of rilzabrutinib in BELIEVE-PV were uniformly mild to moderate and transient, added Dr. Murrell, professor of dermatology at the University of New South Wales and head of the department of dermatology at St. George University Hospital, Sydney.
The phase 2b BELIEVE-PV trial was a small, 24-week, open-label study that included six patients with newly diagnosed pemphigus and nine others with relapsing pemphigus. The primary endpoint was control of disease activity, defined as no new lesions appearing and established lesions beginning to heal. This was achieved in 9 of 15 patients (60%) at 4 weeks and in 13 patients by week 12. Meanwhile, the mean daily dose of prednisone fell from 21 mg at baseline to 6 mg at 24 weeks.
The mean score on the Pemphigus Disease Area Index (PDAI) dropped by 79% from a baseline of 15.5. Ten of 15 subjects improved to a PDAI of 0 or 1 – clear or almost clear skin – by week 24. The complete remission rate, defined as an absence of both new and established lesions while on no or a very low dose of prednisone, was 40% at week 24.
Treatment-emergent adverse events consisted of nausea in four patients, dizziness in two, and abdominal distension in two, all of which were grade 1 or 2.
Based upon these favorable results, the pivotal phase 3, double-blind, international PEGASUS trial is underway, led by Dr. Murrell. The trial will enroll 120 pemphigus patients, randomized to rilzabrutinib at 400 mg twice daily or placebo on top of background steroid tapering.
Rilzabrutinib is also in earlier-stage clinical trials for the treatment of immune thrombocytopenia.
Dr. Murrell reported serving as a consultant to Principia Biopharma, sponsor of the BELIEVE-PV and PEGASUS trials, and has received institutional research grants from numerous pharmaceutical companies.
SOURCE: Murrell DF. AAD 2020 LBCT.
FROM AAD 20
Early data support further study of ivosidenib in mIDH1 glioma
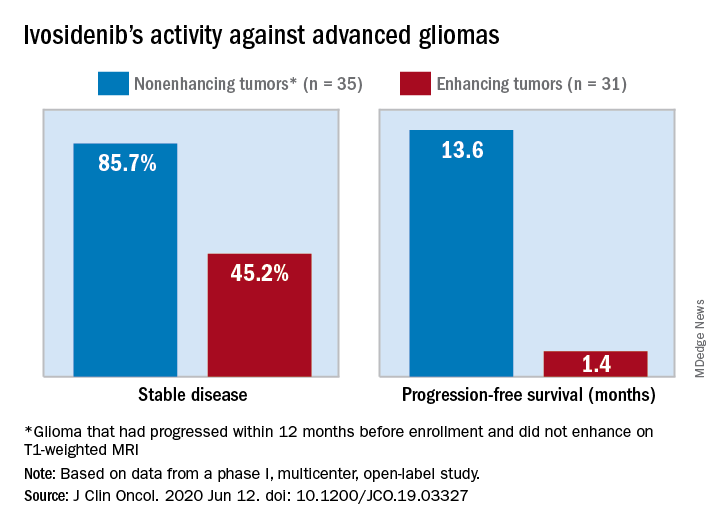
The median progression-free survival was 13.6 months for patients with nonenhancing tumors and 1.4 months for patients with enhancing tumors in a study of 66 adults with mIDH1 advanced glioma.
“On the basis of these data, additional clinical development of mIDH inhibitors for mIDH low-grade gliomas is warranted,” Ingo Mellinghoff, MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues wrote in the Journal of Clinical Oncology.
“This is not a home run but is of interest to the community,” said Lawrence Recht, MD, of Stanford (Calif.) University, who was not involved in this study. “Other companies are also developing agents like this.”
Considering that the ivosidenib study “is uncontrolled, one cannot say for sure that this wasn’t just the natural history of the disease,” Dr. Recht continued. “This type of tumor can behave very indolently, and patients can survive years without treatment, so this is rather a short interval to make a long-time statement. I think the authors are a bit overenthusiastic.”
The authors tested ivosidenib in 66 adults with mIDH1 glioma – 35 with nonenhancing glioma and 31 with enhancing glioma. Tumors had recurred after, or did not respond to, initial surgery, radiation, or chemotherapy.
The patients’ median age was 41 years (range, 21-71 years), and 25 patients (37.9%) were women. The most common tumor type at screening was oligodendroglioma in 23 patients (34.8%).
Patients received ivosidenib at doses ranging from 100 mg twice a day to 900 mg once a day. A total of 50 patients received the phase 2 recommended dose – 500 mg once a day. There were no dose-limiting toxicities, and there was no maximum-tolerated dose.
Adverse events of grade 3 or higher occurred in 19.7% of patients and included headache, seizure, hyperglycemia, neutropenia, and hypophosphatemia. Grade 3 or higher treatment-related adverse events occurred in two patients.
A total of 30 patients with nonenhancing tumors (85.7%) and 14 with enhancing tumors (45.2%) had a best response of stable disease. There was one partial response in a nonenhancing patient on 500 mg/day. The rest of the subjects had a best response of progressive disease.
The median treatment duration was 18.4 months among patients with nonenhancing tumors and 1.9 months among those with enhancing tumors. Discontinuation was caused byo progression in all but one case.
Among patients with measurable disease, tumor measurements decreased from baseline in 22 nonenhancing tumors (66.7%) and in 9 enhancing tumors (33.3%).
“Despite the heterogeneous patient population in our trial, the nonrandomized design, and the lack of central pathology review, the data from our trial suggest that ivosidenib has greater activity against nonenhancing gliomas than against enhancing gliomas,” the investigators wrote. “This finding may seem surprising because the absence of contrast enhancement is typically associated with impaired drug delivery.
“We hypothesize that ivosidenib may be more effective in nonenhancing gliomas because these tumors represent an earlier disease stage with fewer genetic alterations, reminiscent of the greater antitumor activity of the BCR-ABL inhibitor imatinib in earlier stages of chronic myeloid leukemia,” the investigators wrote.
The team also noted that the median progression-free survival for patients with nonenhancing gliomas in the current study “compares favorably to that reported for temozolomide” in advanced mIDH1 low-grade glioma, which was approximately 7 months.
This research was funded by Agios Pharmaceuticals, the company developing ivosidenib. Dr. Mellinghoff receives travel compensation from and is an adviser to the company. Several other investigators are employees. Dr. Recht disclosed no conflicts of interest.
SOURCE: Mellinghoff I et al. J Clin Oncol. 2020 Jun 12. doi: 10.1200/JCO.19.03327

The median progression-free survival was 13.6 months for patients with nonenhancing tumors and 1.4 months for patients with enhancing tumors in a study of 66 adults with mIDH1 advanced glioma.
“On the basis of these data, additional clinical development of mIDH inhibitors for mIDH low-grade gliomas is warranted,” Ingo Mellinghoff, MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues wrote in the Journal of Clinical Oncology.
“This is not a home run but is of interest to the community,” said Lawrence Recht, MD, of Stanford (Calif.) University, who was not involved in this study. “Other companies are also developing agents like this.”
Considering that the ivosidenib study “is uncontrolled, one cannot say for sure that this wasn’t just the natural history of the disease,” Dr. Recht continued. “This type of tumor can behave very indolently, and patients can survive years without treatment, so this is rather a short interval to make a long-time statement. I think the authors are a bit overenthusiastic.”
The authors tested ivosidenib in 66 adults with mIDH1 glioma – 35 with nonenhancing glioma and 31 with enhancing glioma. Tumors had recurred after, or did not respond to, initial surgery, radiation, or chemotherapy.
The patients’ median age was 41 years (range, 21-71 years), and 25 patients (37.9%) were women. The most common tumor type at screening was oligodendroglioma in 23 patients (34.8%).
Patients received ivosidenib at doses ranging from 100 mg twice a day to 900 mg once a day. A total of 50 patients received the phase 2 recommended dose – 500 mg once a day. There were no dose-limiting toxicities, and there was no maximum-tolerated dose.
Adverse events of grade 3 or higher occurred in 19.7% of patients and included headache, seizure, hyperglycemia, neutropenia, and hypophosphatemia. Grade 3 or higher treatment-related adverse events occurred in two patients.
A total of 30 patients with nonenhancing tumors (85.7%) and 14 with enhancing tumors (45.2%) had a best response of stable disease. There was one partial response in a nonenhancing patient on 500 mg/day. The rest of the subjects had a best response of progressive disease.
The median treatment duration was 18.4 months among patients with nonenhancing tumors and 1.9 months among those with enhancing tumors. Discontinuation was caused byo progression in all but one case.
Among patients with measurable disease, tumor measurements decreased from baseline in 22 nonenhancing tumors (66.7%) and in 9 enhancing tumors (33.3%).
“Despite the heterogeneous patient population in our trial, the nonrandomized design, and the lack of central pathology review, the data from our trial suggest that ivosidenib has greater activity against nonenhancing gliomas than against enhancing gliomas,” the investigators wrote. “This finding may seem surprising because the absence of contrast enhancement is typically associated with impaired drug delivery.
“We hypothesize that ivosidenib may be more effective in nonenhancing gliomas because these tumors represent an earlier disease stage with fewer genetic alterations, reminiscent of the greater antitumor activity of the BCR-ABL inhibitor imatinib in earlier stages of chronic myeloid leukemia,” the investigators wrote.
The team also noted that the median progression-free survival for patients with nonenhancing gliomas in the current study “compares favorably to that reported for temozolomide” in advanced mIDH1 low-grade glioma, which was approximately 7 months.
This research was funded by Agios Pharmaceuticals, the company developing ivosidenib. Dr. Mellinghoff receives travel compensation from and is an adviser to the company. Several other investigators are employees. Dr. Recht disclosed no conflicts of interest.
SOURCE: Mellinghoff I et al. J Clin Oncol. 2020 Jun 12. doi: 10.1200/JCO.19.03327

The median progression-free survival was 13.6 months for patients with nonenhancing tumors and 1.4 months for patients with enhancing tumors in a study of 66 adults with mIDH1 advanced glioma.
“On the basis of these data, additional clinical development of mIDH inhibitors for mIDH low-grade gliomas is warranted,” Ingo Mellinghoff, MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues wrote in the Journal of Clinical Oncology.
“This is not a home run but is of interest to the community,” said Lawrence Recht, MD, of Stanford (Calif.) University, who was not involved in this study. “Other companies are also developing agents like this.”
Considering that the ivosidenib study “is uncontrolled, one cannot say for sure that this wasn’t just the natural history of the disease,” Dr. Recht continued. “This type of tumor can behave very indolently, and patients can survive years without treatment, so this is rather a short interval to make a long-time statement. I think the authors are a bit overenthusiastic.”
The authors tested ivosidenib in 66 adults with mIDH1 glioma – 35 with nonenhancing glioma and 31 with enhancing glioma. Tumors had recurred after, or did not respond to, initial surgery, radiation, or chemotherapy.
The patients’ median age was 41 years (range, 21-71 years), and 25 patients (37.9%) were women. The most common tumor type at screening was oligodendroglioma in 23 patients (34.8%).
Patients received ivosidenib at doses ranging from 100 mg twice a day to 900 mg once a day. A total of 50 patients received the phase 2 recommended dose – 500 mg once a day. There were no dose-limiting toxicities, and there was no maximum-tolerated dose.
Adverse events of grade 3 or higher occurred in 19.7% of patients and included headache, seizure, hyperglycemia, neutropenia, and hypophosphatemia. Grade 3 or higher treatment-related adverse events occurred in two patients.
A total of 30 patients with nonenhancing tumors (85.7%) and 14 with enhancing tumors (45.2%) had a best response of stable disease. There was one partial response in a nonenhancing patient on 500 mg/day. The rest of the subjects had a best response of progressive disease.
The median treatment duration was 18.4 months among patients with nonenhancing tumors and 1.9 months among those with enhancing tumors. Discontinuation was caused byo progression in all but one case.
Among patients with measurable disease, tumor measurements decreased from baseline in 22 nonenhancing tumors (66.7%) and in 9 enhancing tumors (33.3%).
“Despite the heterogeneous patient population in our trial, the nonrandomized design, and the lack of central pathology review, the data from our trial suggest that ivosidenib has greater activity against nonenhancing gliomas than against enhancing gliomas,” the investigators wrote. “This finding may seem surprising because the absence of contrast enhancement is typically associated with impaired drug delivery.
“We hypothesize that ivosidenib may be more effective in nonenhancing gliomas because these tumors represent an earlier disease stage with fewer genetic alterations, reminiscent of the greater antitumor activity of the BCR-ABL inhibitor imatinib in earlier stages of chronic myeloid leukemia,” the investigators wrote.
The team also noted that the median progression-free survival for patients with nonenhancing gliomas in the current study “compares favorably to that reported for temozolomide” in advanced mIDH1 low-grade glioma, which was approximately 7 months.
This research was funded by Agios Pharmaceuticals, the company developing ivosidenib. Dr. Mellinghoff receives travel compensation from and is an adviser to the company. Several other investigators are employees. Dr. Recht disclosed no conflicts of interest.
SOURCE: Mellinghoff I et al. J Clin Oncol. 2020 Jun 12. doi: 10.1200/JCO.19.03327
FROM THE JOURNAL OF CLINICAL ONCOLOGY
One-week postsurgical interval for voiding trial increases pass rate
Women who underwent vaginal prolapse surgery and did not immediately have a successful voiding trial were seven times more likely to pass their second voiding trial if their follow-up was 7 days after surgery instead of 4 days, according to a study in the American Journal of Obstetrics and Gynecology.
“This information is useful for setting expectations and for counseling patients on when it might be best to repeat a voiding trial in those with transient incomplete bladder emptying on the day of surgery, especially for those who may not live close to their surgeon, or for those who have difficulty traveling to the office,” said Jeffrey S. Schachar, MD, of Wake Forest Baptist Health in Winston-Salem, N.C., and colleagues. “Despite a higher rate of initial unsuccessful office voiding trials, however, the early group did have significantly fewer days with an indwelling transurethral catheter, as well as total catheterization days,” including self-catheterization.
The researchers note that rates of temporary use of catheters after surgery vary widely, from 12% to 83%, likely because no consensus exists on how long to wait for voiding trials and what constitutes a successful trial.
“It is critical to identify patients with incomplete bladder emptying in order to prevent pain, myogenic and neurogenic damage, ureteral reflux and bladder overdistension that may further impair voiding function,” the authors wrote. “However, extending bladder drainage beyond the necessary recovery period may be associated with higher rates of urinary tract infection (UTI) and patient bother.”
To learn more about the best duration for postoperative catheter use, the researchers enrolled 102 patients before they underwent vaginal prolapse surgery at Wake Forest Baptist Health and Cleveland Clinic Florida from February 2017 to November 2019. The 29 patients with a successful voiding trial within 6 hours after surgery left the study, and 5 others were excluded for needing longer vaginal packing.
The voiding trial involved helping the patient stand to drain the bladder via the catheter, backfilling the bladder with 300 mL of saline solution through the catheter, removing the catheter to give women 1 hour to urinate, and then measuring the postvoid residual with a catheter or ultrasound. At least 100 mL postvoid residual was considered persistent incomplete bladder emptying.
The 60 remaining patients who did not pass the initial voiding trial and opted to remain in the study received a transurethral indwelling catheter and were randomly assigned to return for a second voiding trial either 2-4 days after surgery (depending on day of the week) or 7 days after surgery. The groups were demographically and clinically similar, with predominantly white postmenopausal, non-smoking women with stage II or III multicompartment pelvic organ prolapse.
Women without successful trials could continue with the transurethral catheter or give themselves intermittent catheterizations with a follow-up schedule determined by their surgeon. The researchers then tracked the women for 6 weeks to determine the rate of unsuccessful repeat voiding trials.
Among the women who returned 2-4 days post surgery, 23% had unsuccessful follow-up voiding trials, compared with 3% in the group returning 7 days after surgery (relative risk = 7; P = .02). The researchers calculated that one case of persistent postoperative incomplete bladder emptying was prevented for every five patients who used a catheter for 7 days after surgery.
Kevin A. Ault, MD, professor of obstetrics and gynecology at the University of Kansas Medical Center in Kansas City, said the study was well done, although the findings were unsurprising. He said the clinical implication is straightforward – to wait a week before doing a second voiding trial.
“I suspect these findings match the clinical experience of many surgeons. It is always good to see a well-done clinical trial on a topic,” Dr Ault said in an interview. “The most notable finding is how this impacts patient counseling. Gynecologists should tell their patients that it will take a week with a catheter when this problem arises.”
“The main limitation is whether this finding can be extrapolated to other gynecological surgeries, such as hysterectomy,” said Dr. Ault, who was not involved in the study. “Urinary retention is likely less common after that surgery, but it is still bothersome to patients.”
Dr. Schachar and associates also reported that patients in the earlier group “used significantly more morphine dose equivalents within 24 hours of the office voiding trial than the late-voiding trial group, which was expected given the proximity to surgery” (3 vs. 0.38; P = .005). However, new postoperative pain medication prescriptions and refills were similar in both groups.
Secondary endpoints included UTI rates, total days with a catheter, and patient experience of discomfort with the catheter. The two groups of women reported similar levels of catheter bother, but there was a nonsignificant difference in UTI rates: 23% in the earlier group, compared with 7% in the later group (P = .07).
The early-voiding trial group had an average 5 days with an indwelling transurethral catheter, compared with a significantly different 7 days in the later group (P = .0007). The early group also had fewer total days with an indwelling transurethral catheter and self-catheterization (6 days), compared with the late group (7 days; P = .0013). No patients had persistent incomplete bladder emptying after 17 days post surgery.
“Being able to adequately predict which patients are more likely to have unsuccessful postoperative voiding trials allows surgeons to better counsel their patients and may guide clinical decisions,” Dr. Schachar and associates said. They acknowledged, however, that their study’s biggest weakness is the small enrollment, which led to larger confidence intervals related to relative risk differences between the groups.
The study did not use external funding. Four of the investigators received grant, research funding, or honoraria from one or many medical device or pharmaceutical companies. The remaining researchers had no disclosures. Dr. Ault said he had no relevant financial disclosures.
SOURCE: Schachar JS et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.001.
Women who underwent vaginal prolapse surgery and did not immediately have a successful voiding trial were seven times more likely to pass their second voiding trial if their follow-up was 7 days after surgery instead of 4 days, according to a study in the American Journal of Obstetrics and Gynecology.
“This information is useful for setting expectations and for counseling patients on when it might be best to repeat a voiding trial in those with transient incomplete bladder emptying on the day of surgery, especially for those who may not live close to their surgeon, or for those who have difficulty traveling to the office,” said Jeffrey S. Schachar, MD, of Wake Forest Baptist Health in Winston-Salem, N.C., and colleagues. “Despite a higher rate of initial unsuccessful office voiding trials, however, the early group did have significantly fewer days with an indwelling transurethral catheter, as well as total catheterization days,” including self-catheterization.
The researchers note that rates of temporary use of catheters after surgery vary widely, from 12% to 83%, likely because no consensus exists on how long to wait for voiding trials and what constitutes a successful trial.
“It is critical to identify patients with incomplete bladder emptying in order to prevent pain, myogenic and neurogenic damage, ureteral reflux and bladder overdistension that may further impair voiding function,” the authors wrote. “However, extending bladder drainage beyond the necessary recovery period may be associated with higher rates of urinary tract infection (UTI) and patient bother.”
To learn more about the best duration for postoperative catheter use, the researchers enrolled 102 patients before they underwent vaginal prolapse surgery at Wake Forest Baptist Health and Cleveland Clinic Florida from February 2017 to November 2019. The 29 patients with a successful voiding trial within 6 hours after surgery left the study, and 5 others were excluded for needing longer vaginal packing.
The voiding trial involved helping the patient stand to drain the bladder via the catheter, backfilling the bladder with 300 mL of saline solution through the catheter, removing the catheter to give women 1 hour to urinate, and then measuring the postvoid residual with a catheter or ultrasound. At least 100 mL postvoid residual was considered persistent incomplete bladder emptying.
The 60 remaining patients who did not pass the initial voiding trial and opted to remain in the study received a transurethral indwelling catheter and were randomly assigned to return for a second voiding trial either 2-4 days after surgery (depending on day of the week) or 7 days after surgery. The groups were demographically and clinically similar, with predominantly white postmenopausal, non-smoking women with stage II or III multicompartment pelvic organ prolapse.
Women without successful trials could continue with the transurethral catheter or give themselves intermittent catheterizations with a follow-up schedule determined by their surgeon. The researchers then tracked the women for 6 weeks to determine the rate of unsuccessful repeat voiding trials.
Among the women who returned 2-4 days post surgery, 23% had unsuccessful follow-up voiding trials, compared with 3% in the group returning 7 days after surgery (relative risk = 7; P = .02). The researchers calculated that one case of persistent postoperative incomplete bladder emptying was prevented for every five patients who used a catheter for 7 days after surgery.
Kevin A. Ault, MD, professor of obstetrics and gynecology at the University of Kansas Medical Center in Kansas City, said the study was well done, although the findings were unsurprising. He said the clinical implication is straightforward – to wait a week before doing a second voiding trial.
“I suspect these findings match the clinical experience of many surgeons. It is always good to see a well-done clinical trial on a topic,” Dr Ault said in an interview. “The most notable finding is how this impacts patient counseling. Gynecologists should tell their patients that it will take a week with a catheter when this problem arises.”
“The main limitation is whether this finding can be extrapolated to other gynecological surgeries, such as hysterectomy,” said Dr. Ault, who was not involved in the study. “Urinary retention is likely less common after that surgery, but it is still bothersome to patients.”
Dr. Schachar and associates also reported that patients in the earlier group “used significantly more morphine dose equivalents within 24 hours of the office voiding trial than the late-voiding trial group, which was expected given the proximity to surgery” (3 vs. 0.38; P = .005). However, new postoperative pain medication prescriptions and refills were similar in both groups.
Secondary endpoints included UTI rates, total days with a catheter, and patient experience of discomfort with the catheter. The two groups of women reported similar levels of catheter bother, but there was a nonsignificant difference in UTI rates: 23% in the earlier group, compared with 7% in the later group (P = .07).
The early-voiding trial group had an average 5 days with an indwelling transurethral catheter, compared with a significantly different 7 days in the later group (P = .0007). The early group also had fewer total days with an indwelling transurethral catheter and self-catheterization (6 days), compared with the late group (7 days; P = .0013). No patients had persistent incomplete bladder emptying after 17 days post surgery.
“Being able to adequately predict which patients are more likely to have unsuccessful postoperative voiding trials allows surgeons to better counsel their patients and may guide clinical decisions,” Dr. Schachar and associates said. They acknowledged, however, that their study’s biggest weakness is the small enrollment, which led to larger confidence intervals related to relative risk differences between the groups.
The study did not use external funding. Four of the investigators received grant, research funding, or honoraria from one or many medical device or pharmaceutical companies. The remaining researchers had no disclosures. Dr. Ault said he had no relevant financial disclosures.
SOURCE: Schachar JS et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.001.
Women who underwent vaginal prolapse surgery and did not immediately have a successful voiding trial were seven times more likely to pass their second voiding trial if their follow-up was 7 days after surgery instead of 4 days, according to a study in the American Journal of Obstetrics and Gynecology.
“This information is useful for setting expectations and for counseling patients on when it might be best to repeat a voiding trial in those with transient incomplete bladder emptying on the day of surgery, especially for those who may not live close to their surgeon, or for those who have difficulty traveling to the office,” said Jeffrey S. Schachar, MD, of Wake Forest Baptist Health in Winston-Salem, N.C., and colleagues. “Despite a higher rate of initial unsuccessful office voiding trials, however, the early group did have significantly fewer days with an indwelling transurethral catheter, as well as total catheterization days,” including self-catheterization.
The researchers note that rates of temporary use of catheters after surgery vary widely, from 12% to 83%, likely because no consensus exists on how long to wait for voiding trials and what constitutes a successful trial.
“It is critical to identify patients with incomplete bladder emptying in order to prevent pain, myogenic and neurogenic damage, ureteral reflux and bladder overdistension that may further impair voiding function,” the authors wrote. “However, extending bladder drainage beyond the necessary recovery period may be associated with higher rates of urinary tract infection (UTI) and patient bother.”
To learn more about the best duration for postoperative catheter use, the researchers enrolled 102 patients before they underwent vaginal prolapse surgery at Wake Forest Baptist Health and Cleveland Clinic Florida from February 2017 to November 2019. The 29 patients with a successful voiding trial within 6 hours after surgery left the study, and 5 others were excluded for needing longer vaginal packing.
The voiding trial involved helping the patient stand to drain the bladder via the catheter, backfilling the bladder with 300 mL of saline solution through the catheter, removing the catheter to give women 1 hour to urinate, and then measuring the postvoid residual with a catheter or ultrasound. At least 100 mL postvoid residual was considered persistent incomplete bladder emptying.
The 60 remaining patients who did not pass the initial voiding trial and opted to remain in the study received a transurethral indwelling catheter and were randomly assigned to return for a second voiding trial either 2-4 days after surgery (depending on day of the week) or 7 days after surgery. The groups were demographically and clinically similar, with predominantly white postmenopausal, non-smoking women with stage II or III multicompartment pelvic organ prolapse.
Women without successful trials could continue with the transurethral catheter or give themselves intermittent catheterizations with a follow-up schedule determined by their surgeon. The researchers then tracked the women for 6 weeks to determine the rate of unsuccessful repeat voiding trials.
Among the women who returned 2-4 days post surgery, 23% had unsuccessful follow-up voiding trials, compared with 3% in the group returning 7 days after surgery (relative risk = 7; P = .02). The researchers calculated that one case of persistent postoperative incomplete bladder emptying was prevented for every five patients who used a catheter for 7 days after surgery.
Kevin A. Ault, MD, professor of obstetrics and gynecology at the University of Kansas Medical Center in Kansas City, said the study was well done, although the findings were unsurprising. He said the clinical implication is straightforward – to wait a week before doing a second voiding trial.
“I suspect these findings match the clinical experience of many surgeons. It is always good to see a well-done clinical trial on a topic,” Dr Ault said in an interview. “The most notable finding is how this impacts patient counseling. Gynecologists should tell their patients that it will take a week with a catheter when this problem arises.”
“The main limitation is whether this finding can be extrapolated to other gynecological surgeries, such as hysterectomy,” said Dr. Ault, who was not involved in the study. “Urinary retention is likely less common after that surgery, but it is still bothersome to patients.”
Dr. Schachar and associates also reported that patients in the earlier group “used significantly more morphine dose equivalents within 24 hours of the office voiding trial than the late-voiding trial group, which was expected given the proximity to surgery” (3 vs. 0.38; P = .005). However, new postoperative pain medication prescriptions and refills were similar in both groups.
Secondary endpoints included UTI rates, total days with a catheter, and patient experience of discomfort with the catheter. The two groups of women reported similar levels of catheter bother, but there was a nonsignificant difference in UTI rates: 23% in the earlier group, compared with 7% in the later group (P = .07).
The early-voiding trial group had an average 5 days with an indwelling transurethral catheter, compared with a significantly different 7 days in the later group (P = .0007). The early group also had fewer total days with an indwelling transurethral catheter and self-catheterization (6 days), compared with the late group (7 days; P = .0013). No patients had persistent incomplete bladder emptying after 17 days post surgery.
“Being able to adequately predict which patients are more likely to have unsuccessful postoperative voiding trials allows surgeons to better counsel their patients and may guide clinical decisions,” Dr. Schachar and associates said. They acknowledged, however, that their study’s biggest weakness is the small enrollment, which led to larger confidence intervals related to relative risk differences between the groups.
The study did not use external funding. Four of the investigators received grant, research funding, or honoraria from one or many medical device or pharmaceutical companies. The remaining researchers had no disclosures. Dr. Ault said he had no relevant financial disclosures.
SOURCE: Schachar JS et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.001.
FROM AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Bigotry and medical injustice
“We cannot teach people to withhold judgment; judgments are embedded in the way we view objects. I do not see a “tree”; I see a pleasant or an ugly tree. It is not possible without great, paralyzing effort to strip these small values we attach to matters. Likewise, it is not possible to hold a situation in one’s head without some element of bias” – Nassim Nicholas Taleb, MBA, PhD, “The Black Swan.”
Each morning I see the hungry ghosts congregate at the end of the alley behind my office waiting for their addiction clinic appointments (Maté G. “In the Realm of Hungry Ghosts, Close Encounters with Addiction” Berkeley, Calif.: North Atlantic Books, 2008). The fast food restaurant and the convenience store won’t let them linger, so there they sit on the curb in the saddest magpie’s row in the world. They have lip, nose, and eyebrow piercings, and lightning bolts tattooed up their cheeks. They all have backpacks, a few even rolling suitcases. They are opioid addicts, and almost all young, White adults. There they sit, once-innocent young girls, now worn and hardened, and vicious-looking young men, all with downcast empty eyes and miserable expressions. They are a frightening group marginalized by their addiction.
Opioid addiction became a national focus of attention with clarion calls for treatment, which resulted in legislative funding for treatment, restrictions on prescribing, and readily available Narcan. Physicians have greatly reduced their prescribing of narcotics and overdose death rates have dropped, but the drug crisis has not gone away, it has only been recently overshadowed by COVID-19.
The most ironic part of the current opioid epidemic and overdose deaths, and the other three bloodborne horsemen of death – endocarditis; hepatitis B, C, and D; and HIV – was that these scourges were affecting the Black community 40 years ago when, in my view, no one seemed to care. There was no addiction counseling, no treatment centers, and law enforcement would visit only with hopes of making a dealer’s arrest. Not until it became a White suburban issue, did this public health problem become recognized as something to act on. This is of course a result of racism, but there is a broader lesson here.
Humans may be naturally bigoted toward any marginalized or minority group. I recall working in the HIV clinic (before it was called HIV) in Dallas in the mid-1980s. The county refused to pay for zidovudine, which was very expensive at the time, and was sued to supply medication for a group marginalized by their sexual orientation. The AIDS epidemic was initially ignored, with the virus spreading to intravenous drug users and eventually to the broader population, which is when effective treatments became a priority.
Physicians and society should pay close attention to the ills of our marginalized communities. Because of isolation from health care, they are the medical canaries in the coal mine for all of us. Medical issues and infectious diseases identified there should be a priority and solutions sought and applied. This not only would benefit the marginalized group and ease their suffering, but would be salutary to society as a whole, because they surely will be coming everyone’s way.
COVID-19 highlights this. The working poor live in close quarters and most rely on crowded public transportation, and so a respiratory illness spreads rapidly in a population that cannot practically physically distance and probably cannot afford face masks, or alcohol hand gel.
As noted above, we have a persistent illegal drug epidemic. We also have a resurgence in venereal disease and tuberculosis, much of it drug resistant, which again is concentrated in our marginalized populations. Meanwhile, we have been cutting spending on public health, while we obviously need more resources devoted to public and community health.
When we step back and look, there are public health issues everywhere. We could eliminate 90% of cervical cancer and most of the oropharyngeal cancer with use of a very effective vaccine, but we struggle to get it paid for and to convince the public of its ultimate good.
Another example is in Ohio, where we raised the age to purchase tobacco to 21, which is laudable. But children of any age can still access tanning beds, which dramatically raises their lifetime risk of melanoma, often using a note from their “parents” that they write for each other on the car hood in the strip mall parking lot. This group of mostly young white women could also be considered a marginalized group despite their disposable income because of their belief in personal invincibility and false impressions of a tan conferring beauty and vitality repeated endlessly in their echo chamber of social media impressions.
Perhaps we should gauge the state of our public health by the health status of the most oppressed group of all, the incarcerated. Is it really possible that we don’t routinely test for and treat hepatitis C in many of our prisons? Is this indifference because the incarcerated are again a largely minority group and hepatitis C is spread by intravenous drug use?
Solutions and interventions for these problems range widely in cost, but all would eventually save the greater society money and alleviate great misery for those affected.
Perhaps we should be talking about the decriminalization of drug use. The drugs are already here and the consequences apparent, including overflowing prisons and out of control gun violence. This is a much thornier discussion, but seems at the root of many of our problems.
Bigotry is insidious and will take a long and continuing active effort to combat. As Dr. Taleb notes in the introductory quote, it requires a constant, tiring, deliberate mental effort to be mindful of one’s biases. As physicians, we have always been careful to try and treat all patients without bias, but this is not enough. We must become more insistent about the funding and application of public health measures.
Recognizing and treating the medical problems of our marginalized populations seems a doable first step while our greater society struggles with mental bias toward marginalized groups. Reducing the health burdens of these groups can only help them in their life struggles and will benefit all.
Someone once told me that the cold wind in the ghetto eventually blows out into the suburbs, and they were right. As physicians and a society, we should be insistent about correcting medical injustices beforehand. Let’s get started.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com
“We cannot teach people to withhold judgment; judgments are embedded in the way we view objects. I do not see a “tree”; I see a pleasant or an ugly tree. It is not possible without great, paralyzing effort to strip these small values we attach to matters. Likewise, it is not possible to hold a situation in one’s head without some element of bias” – Nassim Nicholas Taleb, MBA, PhD, “The Black Swan.”
Each morning I see the hungry ghosts congregate at the end of the alley behind my office waiting for their addiction clinic appointments (Maté G. “In the Realm of Hungry Ghosts, Close Encounters with Addiction” Berkeley, Calif.: North Atlantic Books, 2008). The fast food restaurant and the convenience store won’t let them linger, so there they sit on the curb in the saddest magpie’s row in the world. They have lip, nose, and eyebrow piercings, and lightning bolts tattooed up their cheeks. They all have backpacks, a few even rolling suitcases. They are opioid addicts, and almost all young, White adults. There they sit, once-innocent young girls, now worn and hardened, and vicious-looking young men, all with downcast empty eyes and miserable expressions. They are a frightening group marginalized by their addiction.
Opioid addiction became a national focus of attention with clarion calls for treatment, which resulted in legislative funding for treatment, restrictions on prescribing, and readily available Narcan. Physicians have greatly reduced their prescribing of narcotics and overdose death rates have dropped, but the drug crisis has not gone away, it has only been recently overshadowed by COVID-19.
The most ironic part of the current opioid epidemic and overdose deaths, and the other three bloodborne horsemen of death – endocarditis; hepatitis B, C, and D; and HIV – was that these scourges were affecting the Black community 40 years ago when, in my view, no one seemed to care. There was no addiction counseling, no treatment centers, and law enforcement would visit only with hopes of making a dealer’s arrest. Not until it became a White suburban issue, did this public health problem become recognized as something to act on. This is of course a result of racism, but there is a broader lesson here.
Humans may be naturally bigoted toward any marginalized or minority group. I recall working in the HIV clinic (before it was called HIV) in Dallas in the mid-1980s. The county refused to pay for zidovudine, which was very expensive at the time, and was sued to supply medication for a group marginalized by their sexual orientation. The AIDS epidemic was initially ignored, with the virus spreading to intravenous drug users and eventually to the broader population, which is when effective treatments became a priority.
Physicians and society should pay close attention to the ills of our marginalized communities. Because of isolation from health care, they are the medical canaries in the coal mine for all of us. Medical issues and infectious diseases identified there should be a priority and solutions sought and applied. This not only would benefit the marginalized group and ease their suffering, but would be salutary to society as a whole, because they surely will be coming everyone’s way.
COVID-19 highlights this. The working poor live in close quarters and most rely on crowded public transportation, and so a respiratory illness spreads rapidly in a population that cannot practically physically distance and probably cannot afford face masks, or alcohol hand gel.
As noted above, we have a persistent illegal drug epidemic. We also have a resurgence in venereal disease and tuberculosis, much of it drug resistant, which again is concentrated in our marginalized populations. Meanwhile, we have been cutting spending on public health, while we obviously need more resources devoted to public and community health.
When we step back and look, there are public health issues everywhere. We could eliminate 90% of cervical cancer and most of the oropharyngeal cancer with use of a very effective vaccine, but we struggle to get it paid for and to convince the public of its ultimate good.
Another example is in Ohio, where we raised the age to purchase tobacco to 21, which is laudable. But children of any age can still access tanning beds, which dramatically raises their lifetime risk of melanoma, often using a note from their “parents” that they write for each other on the car hood in the strip mall parking lot. This group of mostly young white women could also be considered a marginalized group despite their disposable income because of their belief in personal invincibility and false impressions of a tan conferring beauty and vitality repeated endlessly in their echo chamber of social media impressions.
Perhaps we should gauge the state of our public health by the health status of the most oppressed group of all, the incarcerated. Is it really possible that we don’t routinely test for and treat hepatitis C in many of our prisons? Is this indifference because the incarcerated are again a largely minority group and hepatitis C is spread by intravenous drug use?
Solutions and interventions for these problems range widely in cost, but all would eventually save the greater society money and alleviate great misery for those affected.
Perhaps we should be talking about the decriminalization of drug use. The drugs are already here and the consequences apparent, including overflowing prisons and out of control gun violence. This is a much thornier discussion, but seems at the root of many of our problems.
Bigotry is insidious and will take a long and continuing active effort to combat. As Dr. Taleb notes in the introductory quote, it requires a constant, tiring, deliberate mental effort to be mindful of one’s biases. As physicians, we have always been careful to try and treat all patients without bias, but this is not enough. We must become more insistent about the funding and application of public health measures.
Recognizing and treating the medical problems of our marginalized populations seems a doable first step while our greater society struggles with mental bias toward marginalized groups. Reducing the health burdens of these groups can only help them in their life struggles and will benefit all.
Someone once told me that the cold wind in the ghetto eventually blows out into the suburbs, and they were right. As physicians and a society, we should be insistent about correcting medical injustices beforehand. Let’s get started.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com
“We cannot teach people to withhold judgment; judgments are embedded in the way we view objects. I do not see a “tree”; I see a pleasant or an ugly tree. It is not possible without great, paralyzing effort to strip these small values we attach to matters. Likewise, it is not possible to hold a situation in one’s head without some element of bias” – Nassim Nicholas Taleb, MBA, PhD, “The Black Swan.”
Each morning I see the hungry ghosts congregate at the end of the alley behind my office waiting for their addiction clinic appointments (Maté G. “In the Realm of Hungry Ghosts, Close Encounters with Addiction” Berkeley, Calif.: North Atlantic Books, 2008). The fast food restaurant and the convenience store won’t let them linger, so there they sit on the curb in the saddest magpie’s row in the world. They have lip, nose, and eyebrow piercings, and lightning bolts tattooed up their cheeks. They all have backpacks, a few even rolling suitcases. They are opioid addicts, and almost all young, White adults. There they sit, once-innocent young girls, now worn and hardened, and vicious-looking young men, all with downcast empty eyes and miserable expressions. They are a frightening group marginalized by their addiction.
Opioid addiction became a national focus of attention with clarion calls for treatment, which resulted in legislative funding for treatment, restrictions on prescribing, and readily available Narcan. Physicians have greatly reduced their prescribing of narcotics and overdose death rates have dropped, but the drug crisis has not gone away, it has only been recently overshadowed by COVID-19.
The most ironic part of the current opioid epidemic and overdose deaths, and the other three bloodborne horsemen of death – endocarditis; hepatitis B, C, and D; and HIV – was that these scourges were affecting the Black community 40 years ago when, in my view, no one seemed to care. There was no addiction counseling, no treatment centers, and law enforcement would visit only with hopes of making a dealer’s arrest. Not until it became a White suburban issue, did this public health problem become recognized as something to act on. This is of course a result of racism, but there is a broader lesson here.
Humans may be naturally bigoted toward any marginalized or minority group. I recall working in the HIV clinic (before it was called HIV) in Dallas in the mid-1980s. The county refused to pay for zidovudine, which was very expensive at the time, and was sued to supply medication for a group marginalized by their sexual orientation. The AIDS epidemic was initially ignored, with the virus spreading to intravenous drug users and eventually to the broader population, which is when effective treatments became a priority.
Physicians and society should pay close attention to the ills of our marginalized communities. Because of isolation from health care, they are the medical canaries in the coal mine for all of us. Medical issues and infectious diseases identified there should be a priority and solutions sought and applied. This not only would benefit the marginalized group and ease their suffering, but would be salutary to society as a whole, because they surely will be coming everyone’s way.
COVID-19 highlights this. The working poor live in close quarters and most rely on crowded public transportation, and so a respiratory illness spreads rapidly in a population that cannot practically physically distance and probably cannot afford face masks, or alcohol hand gel.
As noted above, we have a persistent illegal drug epidemic. We also have a resurgence in venereal disease and tuberculosis, much of it drug resistant, which again is concentrated in our marginalized populations. Meanwhile, we have been cutting spending on public health, while we obviously need more resources devoted to public and community health.
When we step back and look, there are public health issues everywhere. We could eliminate 90% of cervical cancer and most of the oropharyngeal cancer with use of a very effective vaccine, but we struggle to get it paid for and to convince the public of its ultimate good.
Another example is in Ohio, where we raised the age to purchase tobacco to 21, which is laudable. But children of any age can still access tanning beds, which dramatically raises their lifetime risk of melanoma, often using a note from their “parents” that they write for each other on the car hood in the strip mall parking lot. This group of mostly young white women could also be considered a marginalized group despite their disposable income because of their belief in personal invincibility and false impressions of a tan conferring beauty and vitality repeated endlessly in their echo chamber of social media impressions.
Perhaps we should gauge the state of our public health by the health status of the most oppressed group of all, the incarcerated. Is it really possible that we don’t routinely test for and treat hepatitis C in many of our prisons? Is this indifference because the incarcerated are again a largely minority group and hepatitis C is spread by intravenous drug use?
Solutions and interventions for these problems range widely in cost, but all would eventually save the greater society money and alleviate great misery for those affected.
Perhaps we should be talking about the decriminalization of drug use. The drugs are already here and the consequences apparent, including overflowing prisons and out of control gun violence. This is a much thornier discussion, but seems at the root of many of our problems.
Bigotry is insidious and will take a long and continuing active effort to combat. As Dr. Taleb notes in the introductory quote, it requires a constant, tiring, deliberate mental effort to be mindful of one’s biases. As physicians, we have always been careful to try and treat all patients without bias, but this is not enough. We must become more insistent about the funding and application of public health measures.
Recognizing and treating the medical problems of our marginalized populations seems a doable first step while our greater society struggles with mental bias toward marginalized groups. Reducing the health burdens of these groups can only help them in their life struggles and will benefit all.
Someone once told me that the cold wind in the ghetto eventually blows out into the suburbs, and they were right. As physicians and a society, we should be insistent about correcting medical injustices beforehand. Let’s get started.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com
More U.S. psychiatrists restricting practices to self-pay patients
A growing number of psychiatrists in the United States no longer accept insurance and will only see patients who can pay upfront, out-of-pocket for office visits, a new trends analysis shows.
Ivy Benjenk, BSN, MPH, and Jie Chen, PhD, from the University of Maryland, College Park, wrote that psychiatrists may be more likely than other specialties to adopt a self-pay-only model because of low insurance reimbursement rates, particularly for psychotherapy, as well as a demand for psychiatric services that outstrips supply.
There is a “limited supply” of psychiatrists in the United States and a “great deal of administrative hoops that go into accepting insurance for pretty minimal payment. So it makes some sense for psychiatrists to move entirely into the self-pay market,” Ms. Benjenk said in an interview.
The study was published online July 15 in JAMA Psychiatry.
Barrier to care
To explore patterns in self-payment for office-based psychiatric services and changes over time, the researchers analyzed data from 2007 to 2016 from the National Ambulatory Medical Care Survey, a nationally representative survey of physicians who were not federally employed, were office based, and were primarily engaged in direct patient care.
Of 15,790 psychiatrist visits, 3445 (21.8%) were self-paid by patients, compared with 4336 of 119,749 primary care clinician visits (3.6%).
Of the 750 psychiatrists in the sample, 146 (19.5%) were reimbursed predominantly by self-payment, compared with 69 of 4,294 primary care clinicians (1.6%).
The percentage of self-paid psychiatrist office visits has trended upward (from 18.5% in 2007-2009 to 26.7% in 2014-2016), whereas the percentage of self-paid primary care visits has trended downward (from 4.1% in 2007-2009 to 2.8% in 2014-2016).
The percentage of psychiatrists who work in predominantly self-pay practices has also trended upward (from 16.4% in 2007-2009 to 26.4% in 2014-2016), whereas the percentage of primary care clinicians who work in predominantly self-pay practices has not changed significantly (from 1.5% to 1.7%).
Psychiatrists who are reimbursed predominantly by self-payment were more likely to work in solo practices than group practices. Most self-pay psychiatry patients were White men.
Self-pay visits lasted longer than visits paid for by a third party (average duration, 38.3 min vs. 28.8 min; P < .001). Self-pay patients also made more visits to their psychiatrist than patients with third-party payers (mean, 18.3 visits vs. 9.4 visits in preceding 12 months; P < .001).
Ms. Benjenk and Dr. Chen wrote that self-pay psychiatry is a “hurdle many patients cannot surmount,” even if a portion of that payment is eventually paid by insurance.
“Either they have to pay out-of-pocket entirely or try to bill their insurance after the fact. It’s a lot of work to try to get an insurance company to pay for something after the fact. Ms. Benjenk said.
Ms. Benjenk believes that, with COVID-19 and a large shift to telepsychiatry, more psychiatrists might be interested in accepting insurance because they may be able to see more patients in a day.
“With telehealth, psychiatrists also can practice anywhere in their state, so that opens up a whole new pool of patients. And it’s cost saving; they don’t have to travel to an office, worry about office space or about no-shows, and there can be less lag time between appointments,” Ms. Benjenk said.
The study had no specific funding. Ms. Benjenk and Dr. Chen disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A growing number of psychiatrists in the United States no longer accept insurance and will only see patients who can pay upfront, out-of-pocket for office visits, a new trends analysis shows.
Ivy Benjenk, BSN, MPH, and Jie Chen, PhD, from the University of Maryland, College Park, wrote that psychiatrists may be more likely than other specialties to adopt a self-pay-only model because of low insurance reimbursement rates, particularly for psychotherapy, as well as a demand for psychiatric services that outstrips supply.
There is a “limited supply” of psychiatrists in the United States and a “great deal of administrative hoops that go into accepting insurance for pretty minimal payment. So it makes some sense for psychiatrists to move entirely into the self-pay market,” Ms. Benjenk said in an interview.
The study was published online July 15 in JAMA Psychiatry.
Barrier to care
To explore patterns in self-payment for office-based psychiatric services and changes over time, the researchers analyzed data from 2007 to 2016 from the National Ambulatory Medical Care Survey, a nationally representative survey of physicians who were not federally employed, were office based, and were primarily engaged in direct patient care.
Of 15,790 psychiatrist visits, 3445 (21.8%) were self-paid by patients, compared with 4336 of 119,749 primary care clinician visits (3.6%).
Of the 750 psychiatrists in the sample, 146 (19.5%) were reimbursed predominantly by self-payment, compared with 69 of 4,294 primary care clinicians (1.6%).
The percentage of self-paid psychiatrist office visits has trended upward (from 18.5% in 2007-2009 to 26.7% in 2014-2016), whereas the percentage of self-paid primary care visits has trended downward (from 4.1% in 2007-2009 to 2.8% in 2014-2016).
The percentage of psychiatrists who work in predominantly self-pay practices has also trended upward (from 16.4% in 2007-2009 to 26.4% in 2014-2016), whereas the percentage of primary care clinicians who work in predominantly self-pay practices has not changed significantly (from 1.5% to 1.7%).
Psychiatrists who are reimbursed predominantly by self-payment were more likely to work in solo practices than group practices. Most self-pay psychiatry patients were White men.
Self-pay visits lasted longer than visits paid for by a third party (average duration, 38.3 min vs. 28.8 min; P < .001). Self-pay patients also made more visits to their psychiatrist than patients with third-party payers (mean, 18.3 visits vs. 9.4 visits in preceding 12 months; P < .001).
Ms. Benjenk and Dr. Chen wrote that self-pay psychiatry is a “hurdle many patients cannot surmount,” even if a portion of that payment is eventually paid by insurance.
“Either they have to pay out-of-pocket entirely or try to bill their insurance after the fact. It’s a lot of work to try to get an insurance company to pay for something after the fact. Ms. Benjenk said.
Ms. Benjenk believes that, with COVID-19 and a large shift to telepsychiatry, more psychiatrists might be interested in accepting insurance because they may be able to see more patients in a day.
“With telehealth, psychiatrists also can practice anywhere in their state, so that opens up a whole new pool of patients. And it’s cost saving; they don’t have to travel to an office, worry about office space or about no-shows, and there can be less lag time between appointments,” Ms. Benjenk said.
The study had no specific funding. Ms. Benjenk and Dr. Chen disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A growing number of psychiatrists in the United States no longer accept insurance and will only see patients who can pay upfront, out-of-pocket for office visits, a new trends analysis shows.
Ivy Benjenk, BSN, MPH, and Jie Chen, PhD, from the University of Maryland, College Park, wrote that psychiatrists may be more likely than other specialties to adopt a self-pay-only model because of low insurance reimbursement rates, particularly for psychotherapy, as well as a demand for psychiatric services that outstrips supply.
There is a “limited supply” of psychiatrists in the United States and a “great deal of administrative hoops that go into accepting insurance for pretty minimal payment. So it makes some sense for psychiatrists to move entirely into the self-pay market,” Ms. Benjenk said in an interview.
The study was published online July 15 in JAMA Psychiatry.
Barrier to care
To explore patterns in self-payment for office-based psychiatric services and changes over time, the researchers analyzed data from 2007 to 2016 from the National Ambulatory Medical Care Survey, a nationally representative survey of physicians who were not federally employed, were office based, and were primarily engaged in direct patient care.
Of 15,790 psychiatrist visits, 3445 (21.8%) were self-paid by patients, compared with 4336 of 119,749 primary care clinician visits (3.6%).
Of the 750 psychiatrists in the sample, 146 (19.5%) were reimbursed predominantly by self-payment, compared with 69 of 4,294 primary care clinicians (1.6%).
The percentage of self-paid psychiatrist office visits has trended upward (from 18.5% in 2007-2009 to 26.7% in 2014-2016), whereas the percentage of self-paid primary care visits has trended downward (from 4.1% in 2007-2009 to 2.8% in 2014-2016).
The percentage of psychiatrists who work in predominantly self-pay practices has also trended upward (from 16.4% in 2007-2009 to 26.4% in 2014-2016), whereas the percentage of primary care clinicians who work in predominantly self-pay practices has not changed significantly (from 1.5% to 1.7%).
Psychiatrists who are reimbursed predominantly by self-payment were more likely to work in solo practices than group practices. Most self-pay psychiatry patients were White men.
Self-pay visits lasted longer than visits paid for by a third party (average duration, 38.3 min vs. 28.8 min; P < .001). Self-pay patients also made more visits to their psychiatrist than patients with third-party payers (mean, 18.3 visits vs. 9.4 visits in preceding 12 months; P < .001).
Ms. Benjenk and Dr. Chen wrote that self-pay psychiatry is a “hurdle many patients cannot surmount,” even if a portion of that payment is eventually paid by insurance.
“Either they have to pay out-of-pocket entirely or try to bill their insurance after the fact. It’s a lot of work to try to get an insurance company to pay for something after the fact. Ms. Benjenk said.
Ms. Benjenk believes that, with COVID-19 and a large shift to telepsychiatry, more psychiatrists might be interested in accepting insurance because they may be able to see more patients in a day.
“With telehealth, psychiatrists also can practice anywhere in their state, so that opens up a whole new pool of patients. And it’s cost saving; they don’t have to travel to an office, worry about office space or about no-shows, and there can be less lag time between appointments,” Ms. Benjenk said.
The study had no specific funding. Ms. Benjenk and Dr. Chen disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Doctors say their COVID-19 protocol saves lives; others want proof
As COVID-19 cases mounted in Texas in late June, a local Houston news station shadowed Joseph Varon, MD, making rounds in the intensive care unit at United Memorial Medical Center in Houston. An unseen newscaster tells viewers that Varon credits his success against COVID-19 so far to an experimental and “controversial” drug protocol consisting of vitamins, steroids, and blood thinners.
“This is war. There’s no time to double-blind anything,” Varon tells the camera. “This is working. And if it’s working, I’m going to keep on doing it.”
But response to the protocol among other critical care physicians is mixed, with several physicians, in interviews with Medscape Medical News, urging caution because the benefits and relative risks of the combined medications have not been tested in randomized control trials.
From the earliest days of the pandemic, there’s been tension between the need for rigorous scientific study to understand a novel disease, which takes time, and the need to treat seriously ill patients immediately. Some treatments, like hydroxychloroquine, were promoted without randomized clinical trial data and then later were shown to be ineffective or even potentially harmful when tested.
“This pandemic has shown us there’s lots of ideas out there and they need to be tested and a theoretical basis is insufficient,” says Daniel Kaul, MD, a professor of infectious disease at the University of Michigan in Ann Arbor. The ups and downs with hydroxychloroquine offer a sobering example, he says. “I would argue we have an ethical obligation to do randomized controlled trials to see if our treatments work.”
Creating MATH+
MATH+ stands for methylprednisolone, ascorbic acid, thiamine, and heparin. The “+” holds a place for additional therapies like vitamin D, zinc, and melatonin. The protocol originated as a variation of the “HAT therapy,” a combination of hydrocortisone, ascorbic acid, and thiamine, which critical care specialist Paul Marik, MD, created for treating critically ill patients with sepsis.
Over a few weeks, the protocol evolved as Marik, chief of the division of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk, emailed with a small group of colleagues about treatments and their observations of SARS-CoV-2 in action, swapping in methylprednisolone and adding the anticoagulant heparin.
When Marik and colleagues created the protocol in early March, many healthcare organizations like the World Health Organization (WHO) were advising against steroids for COVID-19 patients. The MATH+ physicians decided they needed to spread a different message, and began publicizing the protocol with a website and a small communications team.
Marik says they tried to get their protocol in front of healthcare organizations – including the WHO, the Centers for Disease Control and Prevention, and the National Institutes of Health – but received no response. Marik went on Newt Gingrich’s podcast to discuss the protocol in the hopes it would make its way to the White House.
Senator Ron Johnson of Wisconsin saw the protocol and invited Pierre Kory, MD, MPA, who practices in Johnson’s home state, to testify remotely in front of the Senate Homeland Security Committee. Kory is a pulmonary critical care specialist about to start a new job at Aurora St. Luke’s Medical Center in Milwaukee.
In his testimony, Kory shared his positive experience using the protocol to treat patients and expressed his dismay that national healthcare organizations came out against the use of corticosteroids for COVID-19 from the early days of the pandemic based on what he called a “tragic error in analysis of medical data.” Although an analysis by national organizations suggested corticosteroids might be dangerous in COVID-19 patients, one of his colleagues came to the opposite conclusion, he said. But these organizations advised supportive care only, and against steroids. “We think that is a fatal and tragic flaw,” Kory said.
“The problem with the protocol early on was that it was heresy,” says Kory, referring to the protocol’s inclusion of corticosteroids before official treatment guidelines. During the height of the pandemic in New York this spring, Kory spent 5 weeks working in the ICU at Mount Sinai Beth Israel in Manhattan. Seeing patients flounder on supportive care, Kory says he used MATH+ successfully during his time in New York, using escalating and pulse doses of corticosteroids to stabilize rapidly deteriorating patients.
The website’s home page initially included an invitation for visitors to donate money to support “getting word of this effective treatment protocol out to physicians and hospitals around the world.” After Medscape Medical News brought up the donation prompt in questions, the physicians decided to remove all calls for donations from the website and social media, communications representative Betsy Ashton said. “Critics are misinterpreting this as some kind of fund-raising operation, when that could hardly be the case,” Ashton said in an email. “They are horrified that anyone would impugn their motives.”
Donations paid for the website designer, webmaster, and her work, Ashton said, and the physicians now have donors who will support publicizing the protocol without online calls for donations. “We have no commercial or vested interest,” Marik said. “I’m not going to make a single cent out of this and it’s obviously very time-consuming.”
The basis for the protocol
The protocol is based on common sense, an understanding of scientific literature, and an understanding of COVID-19, Marik says. The website includes links to past research trials and observational studies examining ascorbic acid and thiamine in critically ill patients and early looks at anticoagulants in COVID-19 patients.
They chose methylprednisolone as their corticosteroid based on the expertise of group member G. Umberto Meduri, MD, professor of medicine at the University of Tennessee Health Science Center in Memphis, Tennessee, who had found the steroid effective in treating acute respiratory distress syndrome. On the MATH+ website, the physicians link to multiple observational studies posted on preprint servers in April and May that suggest methylprednisolone helped COVID-19 patients.
“What’s happened with time is all the elements have been validated by scientific studies, which makes this so cool,” says Marik. The RECOVERY Trial results in particular validated the push to use corticosteroids in COVID-19 patients, he says. But that study used a different steroid, dexamethasone, in much smaller doses than what MATH+ recommends. Revised guidance from the Infectious Diseases Society of America recommends dexamethasone for severely ill patients, but says methylprednisolone and prednisone can be used as substitutes at equivalent doses.
Marik and Kory say that mortality rates for COVID-19 patients at their respective hospitals decreased after they began using the protocol. The physicians have been collecting observational data on their patients, but have not yet published any, and do not plan to conduct a randomized trial.
Several physicians who were not involved in the creation of the protocol say the evidence the physicians cite is not robust enough to warrant the promotion of MATH+ and call for randomized controlled trials. Coming up with a protocol is fine, says Kaul, but “you have to do the hard work of doing a randomized control trial to determine if those drugs given in those combinations work or not.”
“When I looked at it, I thought it was actually not very evidence based,” says Michelle Gong, MD, chief of the Division of Critical Care Medicine at Montefiore Health System in New York City. “It is not something I would recommend for my doctors to do outside of a clinical trial.”
The protocol authors push back against the necessity and feasibility of randomized control trials.
There is no time for a randomized control trial right now, says Jose Iglesias, DO, associate professor at Hackensack Meridian School of Medicine at Seton Hall and critical care specialist at Community Medical Center and Jersey Shore University Medical Center in New Jersey. “Time is limited. We’re busy bedside clinicians taking care of patients, and patients who are dying.”
Marik argues there is not equipoise: It wouldn’t be ethical to randomize patients in a placebo group when the physicians are confident the steroids will help. And the protocol is personalized for each patient, making the standardization required for a randomized control trial incredibly difficult, he says. He also cites “the people who are unwilling to accept our results and just think it’s too good to be true.”
Hugh Cassiere, MD, director of critical care medicine at Northwell Health’s North Shore University Hospital in Manhasset, New York, said he finds it “very disturbing that this is being propagated.” In the context of a pandemic in which physicians from other specialties are helping out colleagues in ICUs and might follow the protocol uncritically, he worries, “this could potentially lead to harm.”
“I understand the intention; everybody wants to do something, these patients are so sick and the crisis so sharp that we all want to do something to make patients better,” Gong said. “But as physicians taking care of patients we need to make sure we separate the noise from the evidence.”
Peer review
The physicians who reviewed MATH+ for Medscape Medical News differed on which parts of the protocol they support and which parts they would change.
Dexamethasone should be the corticosteroid of choice over methylprednisolone, says Cassiere, because it has now been proven effective in the randomized RECOVERY Trial, which also tested dosing and a timetable for treatment.
But Sam Parnia, MD, PhD, associate professor of medicine and director of critical care and resuscitation research at NYU Langone, thinks methylprednisolone may be effective, and that even higher doses over longer periods of time may stave off recurring pneumonia, based on his experience using the steroid to treat COVID-19 patients in New York.
“What I really like about this protocol is, these guys are very smart, they recommend the need to treat multiple different things at the same time,” says Parnia. COVID-19 is a complex condition, he notes: If physicians are only focused on solving one problem, like hypoxia, patients could still be dying from blood clots.
Despite general concerns about the protocol, Cassiere says he was excited about the inclusion of heparin. Given the extreme levels of clotting seen in COVID-19 patients, he would have included specific D-dimer levels to guide treatment and explored antiplatelet therapies like aspirin. Gong, however, cautioned that she had seen her patients on anticoagulants develop gastrointestinal bleeding, and reiterated the need for clinical evidence. (At least one clinical trial is currently testing the risks and benefits of heparin as an antithrombotic therapy for COVID-19 patients.)
Perhaps the most divisive part of the protocol is the inclusion of ascorbic acid. “That’s the civil war,” says Kory. “It’s the most polarizing medicine.” The authors of the MATH+ protocol were close colleagues before COVID-19 in part because of a mutual research interest in ascorbic acid, he says. Other physicians, including Cassiere, are extremely skeptical that ascorbic acid has any effect, citing recently published studies in the Journal of the American Medical Association that found ascorbic acid ineffective for treating sepsis.
The MATH+ creators say they are working on a literature review of the research behind the protocol, and they plan to write up the observational impacts of the protocol. Marik says he’s not optimistic about getting the findings published in a high-impact journal given the observational nature of the research; the relatively small number of patients treated at hospitals using the protocol (140 patients at Marik’s hospital in Virginia and 180 at Varon’s in Houston, according to Marik); and the vast number of COVID-19 papers being submitted to scientific journals right now.
“This is not a remedy with expensive designer drugs,” Marik said. “No one has any interest in treating patients with cheap, safe, readily available drugs.”
“I hope they’re right if they’re saying this combination of medicines dramatically decreases mortality,” says Taison Bell, MD, director of the medical intensive care unit and assistant professor of medicine at UVA Health in Charlottesville, Virginia.
But physicians have hurt patients in the past with medications they hoped would work, he says. “We have to make sure we’re balancing the risk and the harm with that benefit, and the only way to protect patients from those biases is by doing a randomized controlled trial.”
This article first appeared on Medscape.com.
As COVID-19 cases mounted in Texas in late June, a local Houston news station shadowed Joseph Varon, MD, making rounds in the intensive care unit at United Memorial Medical Center in Houston. An unseen newscaster tells viewers that Varon credits his success against COVID-19 so far to an experimental and “controversial” drug protocol consisting of vitamins, steroids, and blood thinners.
“This is war. There’s no time to double-blind anything,” Varon tells the camera. “This is working. And if it’s working, I’m going to keep on doing it.”
But response to the protocol among other critical care physicians is mixed, with several physicians, in interviews with Medscape Medical News, urging caution because the benefits and relative risks of the combined medications have not been tested in randomized control trials.
From the earliest days of the pandemic, there’s been tension between the need for rigorous scientific study to understand a novel disease, which takes time, and the need to treat seriously ill patients immediately. Some treatments, like hydroxychloroquine, were promoted without randomized clinical trial data and then later were shown to be ineffective or even potentially harmful when tested.
“This pandemic has shown us there’s lots of ideas out there and they need to be tested and a theoretical basis is insufficient,” says Daniel Kaul, MD, a professor of infectious disease at the University of Michigan in Ann Arbor. The ups and downs with hydroxychloroquine offer a sobering example, he says. “I would argue we have an ethical obligation to do randomized controlled trials to see if our treatments work.”
Creating MATH+
MATH+ stands for methylprednisolone, ascorbic acid, thiamine, and heparin. The “+” holds a place for additional therapies like vitamin D, zinc, and melatonin. The protocol originated as a variation of the “HAT therapy,” a combination of hydrocortisone, ascorbic acid, and thiamine, which critical care specialist Paul Marik, MD, created for treating critically ill patients with sepsis.
Over a few weeks, the protocol evolved as Marik, chief of the division of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk, emailed with a small group of colleagues about treatments and their observations of SARS-CoV-2 in action, swapping in methylprednisolone and adding the anticoagulant heparin.
When Marik and colleagues created the protocol in early March, many healthcare organizations like the World Health Organization (WHO) were advising against steroids for COVID-19 patients. The MATH+ physicians decided they needed to spread a different message, and began publicizing the protocol with a website and a small communications team.
Marik says they tried to get their protocol in front of healthcare organizations – including the WHO, the Centers for Disease Control and Prevention, and the National Institutes of Health – but received no response. Marik went on Newt Gingrich’s podcast to discuss the protocol in the hopes it would make its way to the White House.
Senator Ron Johnson of Wisconsin saw the protocol and invited Pierre Kory, MD, MPA, who practices in Johnson’s home state, to testify remotely in front of the Senate Homeland Security Committee. Kory is a pulmonary critical care specialist about to start a new job at Aurora St. Luke’s Medical Center in Milwaukee.
In his testimony, Kory shared his positive experience using the protocol to treat patients and expressed his dismay that national healthcare organizations came out against the use of corticosteroids for COVID-19 from the early days of the pandemic based on what he called a “tragic error in analysis of medical data.” Although an analysis by national organizations suggested corticosteroids might be dangerous in COVID-19 patients, one of his colleagues came to the opposite conclusion, he said. But these organizations advised supportive care only, and against steroids. “We think that is a fatal and tragic flaw,” Kory said.
“The problem with the protocol early on was that it was heresy,” says Kory, referring to the protocol’s inclusion of corticosteroids before official treatment guidelines. During the height of the pandemic in New York this spring, Kory spent 5 weeks working in the ICU at Mount Sinai Beth Israel in Manhattan. Seeing patients flounder on supportive care, Kory says he used MATH+ successfully during his time in New York, using escalating and pulse doses of corticosteroids to stabilize rapidly deteriorating patients.
The website’s home page initially included an invitation for visitors to donate money to support “getting word of this effective treatment protocol out to physicians and hospitals around the world.” After Medscape Medical News brought up the donation prompt in questions, the physicians decided to remove all calls for donations from the website and social media, communications representative Betsy Ashton said. “Critics are misinterpreting this as some kind of fund-raising operation, when that could hardly be the case,” Ashton said in an email. “They are horrified that anyone would impugn their motives.”
Donations paid for the website designer, webmaster, and her work, Ashton said, and the physicians now have donors who will support publicizing the protocol without online calls for donations. “We have no commercial or vested interest,” Marik said. “I’m not going to make a single cent out of this and it’s obviously very time-consuming.”
The basis for the protocol
The protocol is based on common sense, an understanding of scientific literature, and an understanding of COVID-19, Marik says. The website includes links to past research trials and observational studies examining ascorbic acid and thiamine in critically ill patients and early looks at anticoagulants in COVID-19 patients.
They chose methylprednisolone as their corticosteroid based on the expertise of group member G. Umberto Meduri, MD, professor of medicine at the University of Tennessee Health Science Center in Memphis, Tennessee, who had found the steroid effective in treating acute respiratory distress syndrome. On the MATH+ website, the physicians link to multiple observational studies posted on preprint servers in April and May that suggest methylprednisolone helped COVID-19 patients.
“What’s happened with time is all the elements have been validated by scientific studies, which makes this so cool,” says Marik. The RECOVERY Trial results in particular validated the push to use corticosteroids in COVID-19 patients, he says. But that study used a different steroid, dexamethasone, in much smaller doses than what MATH+ recommends. Revised guidance from the Infectious Diseases Society of America recommends dexamethasone for severely ill patients, but says methylprednisolone and prednisone can be used as substitutes at equivalent doses.
Marik and Kory say that mortality rates for COVID-19 patients at their respective hospitals decreased after they began using the protocol. The physicians have been collecting observational data on their patients, but have not yet published any, and do not plan to conduct a randomized trial.
Several physicians who were not involved in the creation of the protocol say the evidence the physicians cite is not robust enough to warrant the promotion of MATH+ and call for randomized controlled trials. Coming up with a protocol is fine, says Kaul, but “you have to do the hard work of doing a randomized control trial to determine if those drugs given in those combinations work or not.”
“When I looked at it, I thought it was actually not very evidence based,” says Michelle Gong, MD, chief of the Division of Critical Care Medicine at Montefiore Health System in New York City. “It is not something I would recommend for my doctors to do outside of a clinical trial.”
The protocol authors push back against the necessity and feasibility of randomized control trials.
There is no time for a randomized control trial right now, says Jose Iglesias, DO, associate professor at Hackensack Meridian School of Medicine at Seton Hall and critical care specialist at Community Medical Center and Jersey Shore University Medical Center in New Jersey. “Time is limited. We’re busy bedside clinicians taking care of patients, and patients who are dying.”
Marik argues there is not equipoise: It wouldn’t be ethical to randomize patients in a placebo group when the physicians are confident the steroids will help. And the protocol is personalized for each patient, making the standardization required for a randomized control trial incredibly difficult, he says. He also cites “the people who are unwilling to accept our results and just think it’s too good to be true.”
Hugh Cassiere, MD, director of critical care medicine at Northwell Health’s North Shore University Hospital in Manhasset, New York, said he finds it “very disturbing that this is being propagated.” In the context of a pandemic in which physicians from other specialties are helping out colleagues in ICUs and might follow the protocol uncritically, he worries, “this could potentially lead to harm.”
“I understand the intention; everybody wants to do something, these patients are so sick and the crisis so sharp that we all want to do something to make patients better,” Gong said. “But as physicians taking care of patients we need to make sure we separate the noise from the evidence.”
Peer review
The physicians who reviewed MATH+ for Medscape Medical News differed on which parts of the protocol they support and which parts they would change.
Dexamethasone should be the corticosteroid of choice over methylprednisolone, says Cassiere, because it has now been proven effective in the randomized RECOVERY Trial, which also tested dosing and a timetable for treatment.
But Sam Parnia, MD, PhD, associate professor of medicine and director of critical care and resuscitation research at NYU Langone, thinks methylprednisolone may be effective, and that even higher doses over longer periods of time may stave off recurring pneumonia, based on his experience using the steroid to treat COVID-19 patients in New York.
“What I really like about this protocol is, these guys are very smart, they recommend the need to treat multiple different things at the same time,” says Parnia. COVID-19 is a complex condition, he notes: If physicians are only focused on solving one problem, like hypoxia, patients could still be dying from blood clots.
Despite general concerns about the protocol, Cassiere says he was excited about the inclusion of heparin. Given the extreme levels of clotting seen in COVID-19 patients, he would have included specific D-dimer levels to guide treatment and explored antiplatelet therapies like aspirin. Gong, however, cautioned that she had seen her patients on anticoagulants develop gastrointestinal bleeding, and reiterated the need for clinical evidence. (At least one clinical trial is currently testing the risks and benefits of heparin as an antithrombotic therapy for COVID-19 patients.)
Perhaps the most divisive part of the protocol is the inclusion of ascorbic acid. “That’s the civil war,” says Kory. “It’s the most polarizing medicine.” The authors of the MATH+ protocol were close colleagues before COVID-19 in part because of a mutual research interest in ascorbic acid, he says. Other physicians, including Cassiere, are extremely skeptical that ascorbic acid has any effect, citing recently published studies in the Journal of the American Medical Association that found ascorbic acid ineffective for treating sepsis.
The MATH+ creators say they are working on a literature review of the research behind the protocol, and they plan to write up the observational impacts of the protocol. Marik says he’s not optimistic about getting the findings published in a high-impact journal given the observational nature of the research; the relatively small number of patients treated at hospitals using the protocol (140 patients at Marik’s hospital in Virginia and 180 at Varon’s in Houston, according to Marik); and the vast number of COVID-19 papers being submitted to scientific journals right now.
“This is not a remedy with expensive designer drugs,” Marik said. “No one has any interest in treating patients with cheap, safe, readily available drugs.”
“I hope they’re right if they’re saying this combination of medicines dramatically decreases mortality,” says Taison Bell, MD, director of the medical intensive care unit and assistant professor of medicine at UVA Health in Charlottesville, Virginia.
But physicians have hurt patients in the past with medications they hoped would work, he says. “We have to make sure we’re balancing the risk and the harm with that benefit, and the only way to protect patients from those biases is by doing a randomized controlled trial.”
This article first appeared on Medscape.com.
As COVID-19 cases mounted in Texas in late June, a local Houston news station shadowed Joseph Varon, MD, making rounds in the intensive care unit at United Memorial Medical Center in Houston. An unseen newscaster tells viewers that Varon credits his success against COVID-19 so far to an experimental and “controversial” drug protocol consisting of vitamins, steroids, and blood thinners.
“This is war. There’s no time to double-blind anything,” Varon tells the camera. “This is working. And if it’s working, I’m going to keep on doing it.”
But response to the protocol among other critical care physicians is mixed, with several physicians, in interviews with Medscape Medical News, urging caution because the benefits and relative risks of the combined medications have not been tested in randomized control trials.
From the earliest days of the pandemic, there’s been tension between the need for rigorous scientific study to understand a novel disease, which takes time, and the need to treat seriously ill patients immediately. Some treatments, like hydroxychloroquine, were promoted without randomized clinical trial data and then later were shown to be ineffective or even potentially harmful when tested.
“This pandemic has shown us there’s lots of ideas out there and they need to be tested and a theoretical basis is insufficient,” says Daniel Kaul, MD, a professor of infectious disease at the University of Michigan in Ann Arbor. The ups and downs with hydroxychloroquine offer a sobering example, he says. “I would argue we have an ethical obligation to do randomized controlled trials to see if our treatments work.”
Creating MATH+
MATH+ stands for methylprednisolone, ascorbic acid, thiamine, and heparin. The “+” holds a place for additional therapies like vitamin D, zinc, and melatonin. The protocol originated as a variation of the “HAT therapy,” a combination of hydrocortisone, ascorbic acid, and thiamine, which critical care specialist Paul Marik, MD, created for treating critically ill patients with sepsis.
Over a few weeks, the protocol evolved as Marik, chief of the division of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk, emailed with a small group of colleagues about treatments and their observations of SARS-CoV-2 in action, swapping in methylprednisolone and adding the anticoagulant heparin.
When Marik and colleagues created the protocol in early March, many healthcare organizations like the World Health Organization (WHO) were advising against steroids for COVID-19 patients. The MATH+ physicians decided they needed to spread a different message, and began publicizing the protocol with a website and a small communications team.
Marik says they tried to get their protocol in front of healthcare organizations – including the WHO, the Centers for Disease Control and Prevention, and the National Institutes of Health – but received no response. Marik went on Newt Gingrich’s podcast to discuss the protocol in the hopes it would make its way to the White House.
Senator Ron Johnson of Wisconsin saw the protocol and invited Pierre Kory, MD, MPA, who practices in Johnson’s home state, to testify remotely in front of the Senate Homeland Security Committee. Kory is a pulmonary critical care specialist about to start a new job at Aurora St. Luke’s Medical Center in Milwaukee.
In his testimony, Kory shared his positive experience using the protocol to treat patients and expressed his dismay that national healthcare organizations came out against the use of corticosteroids for COVID-19 from the early days of the pandemic based on what he called a “tragic error in analysis of medical data.” Although an analysis by national organizations suggested corticosteroids might be dangerous in COVID-19 patients, one of his colleagues came to the opposite conclusion, he said. But these organizations advised supportive care only, and against steroids. “We think that is a fatal and tragic flaw,” Kory said.
“The problem with the protocol early on was that it was heresy,” says Kory, referring to the protocol’s inclusion of corticosteroids before official treatment guidelines. During the height of the pandemic in New York this spring, Kory spent 5 weeks working in the ICU at Mount Sinai Beth Israel in Manhattan. Seeing patients flounder on supportive care, Kory says he used MATH+ successfully during his time in New York, using escalating and pulse doses of corticosteroids to stabilize rapidly deteriorating patients.
The website’s home page initially included an invitation for visitors to donate money to support “getting word of this effective treatment protocol out to physicians and hospitals around the world.” After Medscape Medical News brought up the donation prompt in questions, the physicians decided to remove all calls for donations from the website and social media, communications representative Betsy Ashton said. “Critics are misinterpreting this as some kind of fund-raising operation, when that could hardly be the case,” Ashton said in an email. “They are horrified that anyone would impugn their motives.”
Donations paid for the website designer, webmaster, and her work, Ashton said, and the physicians now have donors who will support publicizing the protocol without online calls for donations. “We have no commercial or vested interest,” Marik said. “I’m not going to make a single cent out of this and it’s obviously very time-consuming.”
The basis for the protocol
The protocol is based on common sense, an understanding of scientific literature, and an understanding of COVID-19, Marik says. The website includes links to past research trials and observational studies examining ascorbic acid and thiamine in critically ill patients and early looks at anticoagulants in COVID-19 patients.
They chose methylprednisolone as their corticosteroid based on the expertise of group member G. Umberto Meduri, MD, professor of medicine at the University of Tennessee Health Science Center in Memphis, Tennessee, who had found the steroid effective in treating acute respiratory distress syndrome. On the MATH+ website, the physicians link to multiple observational studies posted on preprint servers in April and May that suggest methylprednisolone helped COVID-19 patients.
“What’s happened with time is all the elements have been validated by scientific studies, which makes this so cool,” says Marik. The RECOVERY Trial results in particular validated the push to use corticosteroids in COVID-19 patients, he says. But that study used a different steroid, dexamethasone, in much smaller doses than what MATH+ recommends. Revised guidance from the Infectious Diseases Society of America recommends dexamethasone for severely ill patients, but says methylprednisolone and prednisone can be used as substitutes at equivalent doses.
Marik and Kory say that mortality rates for COVID-19 patients at their respective hospitals decreased after they began using the protocol. The physicians have been collecting observational data on their patients, but have not yet published any, and do not plan to conduct a randomized trial.
Several physicians who were not involved in the creation of the protocol say the evidence the physicians cite is not robust enough to warrant the promotion of MATH+ and call for randomized controlled trials. Coming up with a protocol is fine, says Kaul, but “you have to do the hard work of doing a randomized control trial to determine if those drugs given in those combinations work or not.”
“When I looked at it, I thought it was actually not very evidence based,” says Michelle Gong, MD, chief of the Division of Critical Care Medicine at Montefiore Health System in New York City. “It is not something I would recommend for my doctors to do outside of a clinical trial.”
The protocol authors push back against the necessity and feasibility of randomized control trials.
There is no time for a randomized control trial right now, says Jose Iglesias, DO, associate professor at Hackensack Meridian School of Medicine at Seton Hall and critical care specialist at Community Medical Center and Jersey Shore University Medical Center in New Jersey. “Time is limited. We’re busy bedside clinicians taking care of patients, and patients who are dying.”
Marik argues there is not equipoise: It wouldn’t be ethical to randomize patients in a placebo group when the physicians are confident the steroids will help. And the protocol is personalized for each patient, making the standardization required for a randomized control trial incredibly difficult, he says. He also cites “the people who are unwilling to accept our results and just think it’s too good to be true.”
Hugh Cassiere, MD, director of critical care medicine at Northwell Health’s North Shore University Hospital in Manhasset, New York, said he finds it “very disturbing that this is being propagated.” In the context of a pandemic in which physicians from other specialties are helping out colleagues in ICUs and might follow the protocol uncritically, he worries, “this could potentially lead to harm.”
“I understand the intention; everybody wants to do something, these patients are so sick and the crisis so sharp that we all want to do something to make patients better,” Gong said. “But as physicians taking care of patients we need to make sure we separate the noise from the evidence.”
Peer review
The physicians who reviewed MATH+ for Medscape Medical News differed on which parts of the protocol they support and which parts they would change.
Dexamethasone should be the corticosteroid of choice over methylprednisolone, says Cassiere, because it has now been proven effective in the randomized RECOVERY Trial, which also tested dosing and a timetable for treatment.
But Sam Parnia, MD, PhD, associate professor of medicine and director of critical care and resuscitation research at NYU Langone, thinks methylprednisolone may be effective, and that even higher doses over longer periods of time may stave off recurring pneumonia, based on his experience using the steroid to treat COVID-19 patients in New York.
“What I really like about this protocol is, these guys are very smart, they recommend the need to treat multiple different things at the same time,” says Parnia. COVID-19 is a complex condition, he notes: If physicians are only focused on solving one problem, like hypoxia, patients could still be dying from blood clots.
Despite general concerns about the protocol, Cassiere says he was excited about the inclusion of heparin. Given the extreme levels of clotting seen in COVID-19 patients, he would have included specific D-dimer levels to guide treatment and explored antiplatelet therapies like aspirin. Gong, however, cautioned that she had seen her patients on anticoagulants develop gastrointestinal bleeding, and reiterated the need for clinical evidence. (At least one clinical trial is currently testing the risks and benefits of heparin as an antithrombotic therapy for COVID-19 patients.)
Perhaps the most divisive part of the protocol is the inclusion of ascorbic acid. “That’s the civil war,” says Kory. “It’s the most polarizing medicine.” The authors of the MATH+ protocol were close colleagues before COVID-19 in part because of a mutual research interest in ascorbic acid, he says. Other physicians, including Cassiere, are extremely skeptical that ascorbic acid has any effect, citing recently published studies in the Journal of the American Medical Association that found ascorbic acid ineffective for treating sepsis.
The MATH+ creators say they are working on a literature review of the research behind the protocol, and they plan to write up the observational impacts of the protocol. Marik says he’s not optimistic about getting the findings published in a high-impact journal given the observational nature of the research; the relatively small number of patients treated at hospitals using the protocol (140 patients at Marik’s hospital in Virginia and 180 at Varon’s in Houston, according to Marik); and the vast number of COVID-19 papers being submitted to scientific journals right now.
“This is not a remedy with expensive designer drugs,” Marik said. “No one has any interest in treating patients with cheap, safe, readily available drugs.”
“I hope they’re right if they’re saying this combination of medicines dramatically decreases mortality,” says Taison Bell, MD, director of the medical intensive care unit and assistant professor of medicine at UVA Health in Charlottesville, Virginia.
But physicians have hurt patients in the past with medications they hoped would work, he says. “We have to make sure we’re balancing the risk and the harm with that benefit, and the only way to protect patients from those biases is by doing a randomized controlled trial.”
This article first appeared on Medscape.com.
Proton pump inhibitors tied to COVID-19 risk
In light of this finding, physicians should consider which patients truly need these powerful acid-lowering drugs, said Brennan Spiegel, MD, MSHS, professor of medicine and public health at Cedars Sinai Medical Center in Los Angeles, Calif.
“All it means is that we’re going to have a conversation with our patients,” he said in an interview. “We don’t normally have that conversation because we don’t live in an environment with a high risk of enteric infection. But now we’re in a pandemic.”
The study by Dr. Spiegel and his colleagues was published online on July 7 in the American Journal of Gastroenterology.
Use of PPIs has skyrocketed over the past 2 decades. For ambulatory care visits, their use increased from 1.6% in 1998 to 7.6% in 2015. The increase raised questions about overprescription.
Although studies have not borne out many of the other concerns raised about adverse reactions, they have shown that the drugs increase the risk for enteric infections, including infections by SARS-CoV-1, a virus that is related to the COVID-19 virus, SARS-CoV-2, Dr. Spiegel said.
SARS-CoV-2 uses the angiotensin-converting enzyme–2 receptor to invade enterocytes. Dr. Spiegel theorized that an increase in stomach pH above 3 as a result of use of PPIs might allow the virus to enter the GI tract more easily, leading to enteritis, colitis, and systemic spread to other organs, including the lungs. “There is a reason we have acid in our stomachs,” Dr. Spiegel said.
To see how PPI use relates to COVID-19 infections, Dr. Spiegel and his colleagues surveyed online a nationally representative sample of Americans between May 3 and June 24, 2020, as part of a larger survey on gastroenterologic health.
Participants answered questions about gastrointestinal symptoms, current use of PPIs, and COVID-19 test results. They also answered questions about histamine-2 receptor agonists (H2RAs), also known as H2 blockers, which are used to treat some of the same conditions as PPIs but that do not reduce stomach acid as much.
The surveying firm, Cint, contacted 264,058 people. Of the 86,602 eligible participants who completed the survey, 53,130 said they had experienced abdominal discomfort, acid reflux, heartburn, or regurgitation. These survey participants were subsequently asked about PPI and H2RA use.
Of these, 6.4% reported testing positive for SARS-CoV-2. The researchers adjusted for age, sex, race/ethnicity, education, marital status, household income, body mass index, smoking, alcohol consumption, U.S. region, insurance status, and the presence of irritable bowel syndrome, celiac disease, gastroesophageal reflux disease, liver cirrhosis, Crohn’s disease, ulcerative colitis, diabetes, and HIV/AIDS.
After adjusting for these factors, the researchers found that those who took PPIs up to once a day were twice as likely to have had a positive COVID-19 test result than those who did not take the drugs (odds ratio, 2.15; 95% confidence interval, 1.90-2.44).
Those who took PPIs twice a day were almost four times as likely to have tested positive for the disease (OR, 3.67; 95% CI, 2.93-4.60).
By contrast, those taking H2RA drugs once daily were 15% less likely to report a positive COVID-19 test result (OR, 0.85; 95% CI, 0.74-0.99). Research is currently underway to determine whether H2RAs might protect against the disease for reasons unrelated to pH balance.
Dr. Spiegel cautioned that the current data show only an association between PPI use and COVID-19 positivity; it cannot prove cause and effect.
Nevertheless, Dr. Spiegel said the findings should encourage physicians to prescribe PPIs only when clearly indicated. “If somebody is not yet on a PPI and you’re considering whether to start them on a PPI, it’s a good idea to consider H2 blockers,” he said.
People who need a daily dose of a PPI to control a severe condition can safely continue doing so, but such patients should take care to follow standard public health recommendations for avoiding exposure to the virus. These recommendations include wearing a mask, maintaining social distance, and washing hands frequently.
“People who are older, comorbid, or smokers – if they get infected, it could be severe,” he said. “[For] someone like that, it’s reasonable to ask, do we really need to be on twice-daily PPIs? There is good evidence that they are no better off than if they are taking once-daily doses.”
Brian Lacy, MD, PhD, a professor of medicine at the Mayo Clinic in Jacksonville, Fla., agreed that the study should prompt physicians to take a second look at their patients’ PPI prescriptions. “My view is that PPIs are frequently overused, and maybe this is one more piece of data that, if someone is on PPIs, maybe they don’t need to be on this medication.”
On the other hand, the drugs are important for treating conditions such as erosive esophagitis and healing ulcers, he said. The overall risk of contracting COVID-19 is low, so even this finding of a 3.7-fold increased risk should not lead patients or providers to stop taking or prescribing PPIs.
The study also lends support to the idea that the gastrointestinal tract could be involved in SARS-CoV-2 transmission, and it supports warnings about aerosols emitted from flushing toilets and through exhalation, Dr. Spiegel said. There is less evidence of the virus being transmitted through food. “It may not be fecal-oral; it may be fecal-respiratory,” he said.
The study was part of a larger project funded by Ironwood Pharmaceuticals. Dr. Spiegel reported relationships with Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Shire Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals. Dr. Lacy has disclosed no relevant financial relationships.
Help your patients better understand GERD and available treatment options by sharing the AGA patient education at http://ow.ly/ol2r30qYPpk.
A version of this article originally appeared on Medscape.com.
In light of this finding, physicians should consider which patients truly need these powerful acid-lowering drugs, said Brennan Spiegel, MD, MSHS, professor of medicine and public health at Cedars Sinai Medical Center in Los Angeles, Calif.
“All it means is that we’re going to have a conversation with our patients,” he said in an interview. “We don’t normally have that conversation because we don’t live in an environment with a high risk of enteric infection. But now we’re in a pandemic.”
The study by Dr. Spiegel and his colleagues was published online on July 7 in the American Journal of Gastroenterology.
Use of PPIs has skyrocketed over the past 2 decades. For ambulatory care visits, their use increased from 1.6% in 1998 to 7.6% in 2015. The increase raised questions about overprescription.
Although studies have not borne out many of the other concerns raised about adverse reactions, they have shown that the drugs increase the risk for enteric infections, including infections by SARS-CoV-1, a virus that is related to the COVID-19 virus, SARS-CoV-2, Dr. Spiegel said.
SARS-CoV-2 uses the angiotensin-converting enzyme–2 receptor to invade enterocytes. Dr. Spiegel theorized that an increase in stomach pH above 3 as a result of use of PPIs might allow the virus to enter the GI tract more easily, leading to enteritis, colitis, and systemic spread to other organs, including the lungs. “There is a reason we have acid in our stomachs,” Dr. Spiegel said.
To see how PPI use relates to COVID-19 infections, Dr. Spiegel and his colleagues surveyed online a nationally representative sample of Americans between May 3 and June 24, 2020, as part of a larger survey on gastroenterologic health.
Participants answered questions about gastrointestinal symptoms, current use of PPIs, and COVID-19 test results. They also answered questions about histamine-2 receptor agonists (H2RAs), also known as H2 blockers, which are used to treat some of the same conditions as PPIs but that do not reduce stomach acid as much.
The surveying firm, Cint, contacted 264,058 people. Of the 86,602 eligible participants who completed the survey, 53,130 said they had experienced abdominal discomfort, acid reflux, heartburn, or regurgitation. These survey participants were subsequently asked about PPI and H2RA use.
Of these, 6.4% reported testing positive for SARS-CoV-2. The researchers adjusted for age, sex, race/ethnicity, education, marital status, household income, body mass index, smoking, alcohol consumption, U.S. region, insurance status, and the presence of irritable bowel syndrome, celiac disease, gastroesophageal reflux disease, liver cirrhosis, Crohn’s disease, ulcerative colitis, diabetes, and HIV/AIDS.
After adjusting for these factors, the researchers found that those who took PPIs up to once a day were twice as likely to have had a positive COVID-19 test result than those who did not take the drugs (odds ratio, 2.15; 95% confidence interval, 1.90-2.44).
Those who took PPIs twice a day were almost four times as likely to have tested positive for the disease (OR, 3.67; 95% CI, 2.93-4.60).
By contrast, those taking H2RA drugs once daily were 15% less likely to report a positive COVID-19 test result (OR, 0.85; 95% CI, 0.74-0.99). Research is currently underway to determine whether H2RAs might protect against the disease for reasons unrelated to pH balance.
Dr. Spiegel cautioned that the current data show only an association between PPI use and COVID-19 positivity; it cannot prove cause and effect.
Nevertheless, Dr. Spiegel said the findings should encourage physicians to prescribe PPIs only when clearly indicated. “If somebody is not yet on a PPI and you’re considering whether to start them on a PPI, it’s a good idea to consider H2 blockers,” he said.
People who need a daily dose of a PPI to control a severe condition can safely continue doing so, but such patients should take care to follow standard public health recommendations for avoiding exposure to the virus. These recommendations include wearing a mask, maintaining social distance, and washing hands frequently.
“People who are older, comorbid, or smokers – if they get infected, it could be severe,” he said. “[For] someone like that, it’s reasonable to ask, do we really need to be on twice-daily PPIs? There is good evidence that they are no better off than if they are taking once-daily doses.”
Brian Lacy, MD, PhD, a professor of medicine at the Mayo Clinic in Jacksonville, Fla., agreed that the study should prompt physicians to take a second look at their patients’ PPI prescriptions. “My view is that PPIs are frequently overused, and maybe this is one more piece of data that, if someone is on PPIs, maybe they don’t need to be on this medication.”
On the other hand, the drugs are important for treating conditions such as erosive esophagitis and healing ulcers, he said. The overall risk of contracting COVID-19 is low, so even this finding of a 3.7-fold increased risk should not lead patients or providers to stop taking or prescribing PPIs.
The study also lends support to the idea that the gastrointestinal tract could be involved in SARS-CoV-2 transmission, and it supports warnings about aerosols emitted from flushing toilets and through exhalation, Dr. Spiegel said. There is less evidence of the virus being transmitted through food. “It may not be fecal-oral; it may be fecal-respiratory,” he said.
The study was part of a larger project funded by Ironwood Pharmaceuticals. Dr. Spiegel reported relationships with Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Shire Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals. Dr. Lacy has disclosed no relevant financial relationships.
Help your patients better understand GERD and available treatment options by sharing the AGA patient education at http://ow.ly/ol2r30qYPpk.
A version of this article originally appeared on Medscape.com.
In light of this finding, physicians should consider which patients truly need these powerful acid-lowering drugs, said Brennan Spiegel, MD, MSHS, professor of medicine and public health at Cedars Sinai Medical Center in Los Angeles, Calif.
“All it means is that we’re going to have a conversation with our patients,” he said in an interview. “We don’t normally have that conversation because we don’t live in an environment with a high risk of enteric infection. But now we’re in a pandemic.”
The study by Dr. Spiegel and his colleagues was published online on July 7 in the American Journal of Gastroenterology.
Use of PPIs has skyrocketed over the past 2 decades. For ambulatory care visits, their use increased from 1.6% in 1998 to 7.6% in 2015. The increase raised questions about overprescription.
Although studies have not borne out many of the other concerns raised about adverse reactions, they have shown that the drugs increase the risk for enteric infections, including infections by SARS-CoV-1, a virus that is related to the COVID-19 virus, SARS-CoV-2, Dr. Spiegel said.
SARS-CoV-2 uses the angiotensin-converting enzyme–2 receptor to invade enterocytes. Dr. Spiegel theorized that an increase in stomach pH above 3 as a result of use of PPIs might allow the virus to enter the GI tract more easily, leading to enteritis, colitis, and systemic spread to other organs, including the lungs. “There is a reason we have acid in our stomachs,” Dr. Spiegel said.
To see how PPI use relates to COVID-19 infections, Dr. Spiegel and his colleagues surveyed online a nationally representative sample of Americans between May 3 and June 24, 2020, as part of a larger survey on gastroenterologic health.
Participants answered questions about gastrointestinal symptoms, current use of PPIs, and COVID-19 test results. They also answered questions about histamine-2 receptor agonists (H2RAs), also known as H2 blockers, which are used to treat some of the same conditions as PPIs but that do not reduce stomach acid as much.
The surveying firm, Cint, contacted 264,058 people. Of the 86,602 eligible participants who completed the survey, 53,130 said they had experienced abdominal discomfort, acid reflux, heartburn, or regurgitation. These survey participants were subsequently asked about PPI and H2RA use.
Of these, 6.4% reported testing positive for SARS-CoV-2. The researchers adjusted for age, sex, race/ethnicity, education, marital status, household income, body mass index, smoking, alcohol consumption, U.S. region, insurance status, and the presence of irritable bowel syndrome, celiac disease, gastroesophageal reflux disease, liver cirrhosis, Crohn’s disease, ulcerative colitis, diabetes, and HIV/AIDS.
After adjusting for these factors, the researchers found that those who took PPIs up to once a day were twice as likely to have had a positive COVID-19 test result than those who did not take the drugs (odds ratio, 2.15; 95% confidence interval, 1.90-2.44).
Those who took PPIs twice a day were almost four times as likely to have tested positive for the disease (OR, 3.67; 95% CI, 2.93-4.60).
By contrast, those taking H2RA drugs once daily were 15% less likely to report a positive COVID-19 test result (OR, 0.85; 95% CI, 0.74-0.99). Research is currently underway to determine whether H2RAs might protect against the disease for reasons unrelated to pH balance.
Dr. Spiegel cautioned that the current data show only an association between PPI use and COVID-19 positivity; it cannot prove cause and effect.
Nevertheless, Dr. Spiegel said the findings should encourage physicians to prescribe PPIs only when clearly indicated. “If somebody is not yet on a PPI and you’re considering whether to start them on a PPI, it’s a good idea to consider H2 blockers,” he said.
People who need a daily dose of a PPI to control a severe condition can safely continue doing so, but such patients should take care to follow standard public health recommendations for avoiding exposure to the virus. These recommendations include wearing a mask, maintaining social distance, and washing hands frequently.
“People who are older, comorbid, or smokers – if they get infected, it could be severe,” he said. “[For] someone like that, it’s reasonable to ask, do we really need to be on twice-daily PPIs? There is good evidence that they are no better off than if they are taking once-daily doses.”
Brian Lacy, MD, PhD, a professor of medicine at the Mayo Clinic in Jacksonville, Fla., agreed that the study should prompt physicians to take a second look at their patients’ PPI prescriptions. “My view is that PPIs are frequently overused, and maybe this is one more piece of data that, if someone is on PPIs, maybe they don’t need to be on this medication.”
On the other hand, the drugs are important for treating conditions such as erosive esophagitis and healing ulcers, he said. The overall risk of contracting COVID-19 is low, so even this finding of a 3.7-fold increased risk should not lead patients or providers to stop taking or prescribing PPIs.
The study also lends support to the idea that the gastrointestinal tract could be involved in SARS-CoV-2 transmission, and it supports warnings about aerosols emitted from flushing toilets and through exhalation, Dr. Spiegel said. There is less evidence of the virus being transmitted through food. “It may not be fecal-oral; it may be fecal-respiratory,” he said.
The study was part of a larger project funded by Ironwood Pharmaceuticals. Dr. Spiegel reported relationships with Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Shire Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals. Dr. Lacy has disclosed no relevant financial relationships.
Help your patients better understand GERD and available treatment options by sharing the AGA patient education at http://ow.ly/ol2r30qYPpk.
A version of this article originally appeared on Medscape.com.
Liver biopsies show persistent FVIII after gene therapy for hemophilia A
Factor VIII expression was detected on liver biopsies at more than 2 years after a single infusion of adeno-associated virus (AAV) gene therapy for hemophilia A in two patients who were part of a recent phase 1/2 study and who participated in an optional liver biopsy substudy.
The persistent factor VIII (FVIII) expression seen in the two patients was consistent with the presence of circularized, full-length human FVIII-SQ DNA in the biopsy samples and was observed in one patients at week 201 after infusion with 6 x 1012 vg/kg of the AAV serotype 5 human FVIII-SQ gene therapy(valoctocogene roxaparvovec) and in another at week 140 after infusion with 4×1013 vg/kg, Sylvia Fong, PhD, reported during the International Society of Thrombosis and Haemostasis virtual congress.
The first patient had no FVIII detected in the plasma at the time of biopsy. The second had 28.4% of normal FVIII activity detected at the time of biopsy, said Dr. Fong of BioMarin Pharmaceutical, noting that alanine aminotransferase levels were normal at the time of biopsy for both subjects.
“Valoctocogene roxaparvovec is currently being evaluated in a phase 3 clinical study,” Dr. Fong said. “Data from the phase 1/2 trial have demonstrated preliminary proof of concept that valoctocogene roxaparvovec treatment, in many cases, eliminated spontaneous bleeds and the need for prophylactic factor VIII replacement.
“In addition, an acceptable safety profile was observed.”
Data from that trial were presented at the World Federation of Hemophilia virtual summit in June.
Liver biopsy for factor VIII expression
The current exploratory liver biopsy substudy – the first-in-human liver biopsy study after gene therapy for hemophilia A – was open to all phase 1/2 study participants and was initiated in September 2019 with multiple aims, including improved understanding of the durability and variability of AAV gene therapy, she explained.
Histopathological examination revealed normal liver architecture with mild steatosis and no evidence of steatohepatitis or significant inflammation in either participant. In the 6×1012 vg/kg– and 4×1013 vg/kg–treated participants, a dose-dependent increase was seen in the percentage of hepatocytes that stained positive for vector genomes (1.3% and 32%, respectively), she said.
“This was very exciting to see,” she said, referring to the 32% stain-positive rate in the patient who received the 4x1013 vg/kg dose. “Not only were we able to detect vector genomes more than 2 years post-dose, there seems to be quite a bit of signals in this patient sample.”
The findings were similar to those seen in preclinical nonhuman primate models, she noted.
liver hFVIII-SQ RNA levels were 7.67×102 copies/mcg and 6.77×104 copies/µg in the 6×1012 vg/kg–treated participant the 4×1013 vg/kg–treated participant, respectively.
The circularized genomes were present as monomers and concatemers in both participants, and “were presumably associated with long-term expression,” Dr. Fong said.
Both participants are clinically stable with no long-term hepatic issues, she said, noting that analyses of results from additional participants in the substudy will be shared as they become available.
To date, because of “precious small amounts” of tissue available from the biopsy samples, Dr. Fong said she and her colleagues had not looked for degradation of the vectors.
Session moderator Sebastien Lacroix-Desmazes, MD, of Centre de recherche des Cordeliers, Paris, asked: “Is there a plan [to do so] or not, because I guess it’s a very important point,” to which Dr. Fong said that it is a possibility if adequate samples become available.
Dr. Fong is an employee of BioMarin Pharmaceuticals.
SOURCE: Fong S. 2020 ISTH Congress, Abstract OC 03.4.
Factor VIII expression was detected on liver biopsies at more than 2 years after a single infusion of adeno-associated virus (AAV) gene therapy for hemophilia A in two patients who were part of a recent phase 1/2 study and who participated in an optional liver biopsy substudy.
The persistent factor VIII (FVIII) expression seen in the two patients was consistent with the presence of circularized, full-length human FVIII-SQ DNA in the biopsy samples and was observed in one patients at week 201 after infusion with 6 x 1012 vg/kg of the AAV serotype 5 human FVIII-SQ gene therapy(valoctocogene roxaparvovec) and in another at week 140 after infusion with 4×1013 vg/kg, Sylvia Fong, PhD, reported during the International Society of Thrombosis and Haemostasis virtual congress.
The first patient had no FVIII detected in the plasma at the time of biopsy. The second had 28.4% of normal FVIII activity detected at the time of biopsy, said Dr. Fong of BioMarin Pharmaceutical, noting that alanine aminotransferase levels were normal at the time of biopsy for both subjects.
“Valoctocogene roxaparvovec is currently being evaluated in a phase 3 clinical study,” Dr. Fong said. “Data from the phase 1/2 trial have demonstrated preliminary proof of concept that valoctocogene roxaparvovec treatment, in many cases, eliminated spontaneous bleeds and the need for prophylactic factor VIII replacement.
“In addition, an acceptable safety profile was observed.”
Data from that trial were presented at the World Federation of Hemophilia virtual summit in June.
Liver biopsy for factor VIII expression
The current exploratory liver biopsy substudy – the first-in-human liver biopsy study after gene therapy for hemophilia A – was open to all phase 1/2 study participants and was initiated in September 2019 with multiple aims, including improved understanding of the durability and variability of AAV gene therapy, she explained.
Histopathological examination revealed normal liver architecture with mild steatosis and no evidence of steatohepatitis or significant inflammation in either participant. In the 6×1012 vg/kg– and 4×1013 vg/kg–treated participants, a dose-dependent increase was seen in the percentage of hepatocytes that stained positive for vector genomes (1.3% and 32%, respectively), she said.
“This was very exciting to see,” she said, referring to the 32% stain-positive rate in the patient who received the 4x1013 vg/kg dose. “Not only were we able to detect vector genomes more than 2 years post-dose, there seems to be quite a bit of signals in this patient sample.”
The findings were similar to those seen in preclinical nonhuman primate models, she noted.
liver hFVIII-SQ RNA levels were 7.67×102 copies/mcg and 6.77×104 copies/µg in the 6×1012 vg/kg–treated participant the 4×1013 vg/kg–treated participant, respectively.
The circularized genomes were present as monomers and concatemers in both participants, and “were presumably associated with long-term expression,” Dr. Fong said.
Both participants are clinically stable with no long-term hepatic issues, she said, noting that analyses of results from additional participants in the substudy will be shared as they become available.
To date, because of “precious small amounts” of tissue available from the biopsy samples, Dr. Fong said she and her colleagues had not looked for degradation of the vectors.
Session moderator Sebastien Lacroix-Desmazes, MD, of Centre de recherche des Cordeliers, Paris, asked: “Is there a plan [to do so] or not, because I guess it’s a very important point,” to which Dr. Fong said that it is a possibility if adequate samples become available.
Dr. Fong is an employee of BioMarin Pharmaceuticals.
SOURCE: Fong S. 2020 ISTH Congress, Abstract OC 03.4.
Factor VIII expression was detected on liver biopsies at more than 2 years after a single infusion of adeno-associated virus (AAV) gene therapy for hemophilia A in two patients who were part of a recent phase 1/2 study and who participated in an optional liver biopsy substudy.
The persistent factor VIII (FVIII) expression seen in the two patients was consistent with the presence of circularized, full-length human FVIII-SQ DNA in the biopsy samples and was observed in one patients at week 201 after infusion with 6 x 1012 vg/kg of the AAV serotype 5 human FVIII-SQ gene therapy(valoctocogene roxaparvovec) and in another at week 140 after infusion with 4×1013 vg/kg, Sylvia Fong, PhD, reported during the International Society of Thrombosis and Haemostasis virtual congress.
The first patient had no FVIII detected in the plasma at the time of biopsy. The second had 28.4% of normal FVIII activity detected at the time of biopsy, said Dr. Fong of BioMarin Pharmaceutical, noting that alanine aminotransferase levels were normal at the time of biopsy for both subjects.
“Valoctocogene roxaparvovec is currently being evaluated in a phase 3 clinical study,” Dr. Fong said. “Data from the phase 1/2 trial have demonstrated preliminary proof of concept that valoctocogene roxaparvovec treatment, in many cases, eliminated spontaneous bleeds and the need for prophylactic factor VIII replacement.
“In addition, an acceptable safety profile was observed.”
Data from that trial were presented at the World Federation of Hemophilia virtual summit in June.
Liver biopsy for factor VIII expression
The current exploratory liver biopsy substudy – the first-in-human liver biopsy study after gene therapy for hemophilia A – was open to all phase 1/2 study participants and was initiated in September 2019 with multiple aims, including improved understanding of the durability and variability of AAV gene therapy, she explained.
Histopathological examination revealed normal liver architecture with mild steatosis and no evidence of steatohepatitis or significant inflammation in either participant. In the 6×1012 vg/kg– and 4×1013 vg/kg–treated participants, a dose-dependent increase was seen in the percentage of hepatocytes that stained positive for vector genomes (1.3% and 32%, respectively), she said.
“This was very exciting to see,” she said, referring to the 32% stain-positive rate in the patient who received the 4x1013 vg/kg dose. “Not only were we able to detect vector genomes more than 2 years post-dose, there seems to be quite a bit of signals in this patient sample.”
The findings were similar to those seen in preclinical nonhuman primate models, she noted.
liver hFVIII-SQ RNA levels were 7.67×102 copies/mcg and 6.77×104 copies/µg in the 6×1012 vg/kg–treated participant the 4×1013 vg/kg–treated participant, respectively.
The circularized genomes were present as monomers and concatemers in both participants, and “were presumably associated with long-term expression,” Dr. Fong said.
Both participants are clinically stable with no long-term hepatic issues, she said, noting that analyses of results from additional participants in the substudy will be shared as they become available.
To date, because of “precious small amounts” of tissue available from the biopsy samples, Dr. Fong said she and her colleagues had not looked for degradation of the vectors.
Session moderator Sebastien Lacroix-Desmazes, MD, of Centre de recherche des Cordeliers, Paris, asked: “Is there a plan [to do so] or not, because I guess it’s a very important point,” to which Dr. Fong said that it is a possibility if adequate samples become available.
Dr. Fong is an employee of BioMarin Pharmaceuticals.
SOURCE: Fong S. 2020 ISTH Congress, Abstract OC 03.4.
REPORTING FROM THE 2020 ISTH CONGRESS
Still no clear answer on intranasal insulin for MCI and Alzheimer’s disease
The randomized trial of nearly 300 patients showed that, although one insulin administration device produced marked benefit in terms of change in mean score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12) over 12 months, reliability was inconsistent. A second device, used on the majority of patients in the study’s intention-to-treat population, showed no difference in these measures between patients who did and those who did not receive intranasal insulin.
“The primary analysis of the study showed no benefit of intranasal insulin on any measures of cognition or cerebrospinal fluid Alzheimer’s disease biomarkers when using the new device,” said principal investigator Suzanne Craft, PhD.
“But when we looked at our planned secondary analysis with the original device – which has been successful in previous studies – we saw quite a different picture,” added Dr. Craft, director of the Alzheimer’s Disease Research Center at Wake Forest University, Winston-Salem, N.C.
“We found a pronounced benefit with that device, such that after 18 months of administration, participants who had been receiving insulin from the beginning of the study had a large and clinically significant advantage in the primary outcome measure.”
Dr. Craft described the findings as complex. “The primary results were negative,” she added. “But the secondary results replicated those of several earlier studies when we used the same device that was used in those.”
The study was published online June 22 in JAMA Neurology.
Important for brain function
Insulin has been shown to play several important roles in brain function. The hormone is associated with a variety of cognitive functions, including memory. Through its association with vasoreactivity, lipid metabolism, and inflammation, insulin also plays an important role in vascular function.
“In the normal brain in healthy individuals, insulin is very important for synaptic function and viability. Insulin also promotes dendritic growth and facilitates synaptic health. Through this role, it plays an important part in memory,” said Dr. Craft. Given these connections, it is not surprising that reduced insulin levels or activity in brain and cerebrospinal fluid have been documented in some, but not all, studies of Alzheimer’s disease. Markers of insulin resistance also have been detected in both neuronally derived exosomes and brain tissue from adults with Alzheimer’s disease.
In light of the several important roles that insulin plays in the brain – coupled with the evidence connecting dysregulation of brain insulin and AD pathology – restoring brain insulin function may offer therapeutic benefit for adults suffering either Alzheimer’s disease or MCI. “There are a number of ways to do this,” said Dr. Craft. “But one of the approaches that we’ve focused on is providing insulin directly to the brain through intranasal administration. “By doing this, you circumvent potential issues if you administered insulin systemically.”
Previous research has shown that through this mode of administration, insulin can bypass the blood-brain barrier and reach the brain through olfactory and trigeminal perivascular channels, with little effect on peripheral insulin or blood glucose levels.
As previously reported, an earlier pilot study, also conducted by Dr. Craft and her team, showed that 4 months of daily intranasal administration of 20 IU or 40 IU of insulin preserved cognitive performance in individuals with Alzheimer’s disease or MCI.
Deeper dive
In the current investigation, the researchers wanted to broaden these findings in a larger, longer, randomized double-blinded clinical trial. The investigators assessed the efficacy of intranasal insulin on cognition, function, and biomarkers of Alzheimer’s disease, as well as the safety and feasibility of the delivery method. The multicenter trial was conducted from 2014 to 2018 and included 27 sites.
Study participants were between the ages of 55 and 85 years and had been diagnosed with amnestic MCI or Alzheimer’s disease on the basis of National Institute on Aging–Alzheimer Association criteria, a score of 20 or higher on the Mini–Mental State Examination, a clinical dementia rating of 0.5 or 1.0, or a delayed logical memory score within a specified range.
In total, 289 participants were randomly assigned to receive 40 IU of insulin or placebo for 12 months, followed by a 6-month open-label extension phase. The first 49 participants (32 men; mean age, 71.9 years) underwent insulin administration with the same device the investigators used in previous trials.
Of these, 45 completed the blinded phase, and 42 completed the open-label extension. When this device, which uses an electronic nebulizer-like delivery system, proved unreliable, the researchers switched to a second device, which uses a liquid hydrofluoroalkane propellant to deliver a metered dose of insulin through a nose tip without electronic assistance. Device 2 was used for the remaining 240 participants (123 men; mean age, 70.8 years). These patients became the study’s primary intention-to-treat population.
The study’s primary outcome was the mean change in score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12), which was evaluated at 3-month intervals.
Secondary clinical outcomes were assessed at 6-month intervals. These included the mean change in scores for the Alzheimer Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment and the Clinical Dementia Rating Scale Sum of Boxes.
Safety and adherence were also assessed during each study visit. Physical and neurologic examinations were performed at baseline and at months 6, 12, and 18.
Of the primary intention-to-treat population of 240 patients, 121 were randomly assigned to receive intranasal insulin. The remaining 119 received placebo and served as controls. The two groups were demographically comparable.
Better cognitive performance
A total of 215 participants completed the blinded phase; 198 participants completed the open-label extension. Discontinuation rates were comparable in both arms. The researchers found no differences between groups with respect to mean change in ADAS-cog-12 score from baseline to month 12 (0.0258 points; 95% confidence interval, –1.771 to 1.822 points; P = .98). The two groups also proved comparable in terms of performance on all other cognitive tests.
The open-label portion yielded similar results. Participants originally assigned to the insulin arm and their counterparts in the placebo arm did not differ with respect to mean score change on the ADAS-cog-12 test (or any other outcome) at either month 15 or 18.
Cerebrospinal fluid insulin levels were unchanged between groups, as were blood glucose and hemoglobin A1c values. Indeed, levels of A-beta42, A-beta40, total tau protein, and tau p-181 were comparable for the patients who received intranasal insulin and those who received placebo.
The most common adverse events were infections, injuries, respiratory disorders, and nervous system disorders, though these did not differ between groups. In addition, there were no differences between groups with respect to severity of adverse events; most were rated as mild.
In contrast with the intention-to-treat population, the study’s secondary analysis – using data from the original administration device – yielded markedly different results. In the blinded phase, patients who received insulin had better ADAS-cog-12 performance at 12 months (−2.81 points; 95% CI, −6.09 to 0.45 points; P = .09) and nominally significant effects at 6 months (−3.78 points; 95% CI, −6.79 to −0.78 points; P = .01).
Device type critical
These effects persisted in the open-label analyses. Patients who received intranasal insulin had superior ADAS-cog-12 scores at month 15 (−5.70 points; 95% CI, −9.62 to −1.79 points; P = .004) and month 18 (−5.78 points; 95% CI, −10.55 to −1.01 points; P = .02), compared with their counterparts who received insulin via the second device. This part of the study also showed that, although individual biomarkers did not differ significantly between the two arms, the ratios of A-beta42 to A-beta40 (P = .01) and A-beta42 to total tau (P = .03) increased with use of the first device. The number, type, and severity of adverse events were comparable between the insulin and placebo groups in this arm of the study.
The mixed results revealed by the trial demonstrate that the device used for intranasal insulin administration is paramount in determining the therapy’s potential efficacy. “Our take-home message is that the device is a very important factor for these studies and that one needs to validate their ability to effectively deliver insulin to the CNS,” said Dr. Craft.
“We were quite confident that the first device was able to do that. On the other hand, the second device has never been tested in that way, and we still don’t know whether or not that device was able to successfully deliver insulin,” she said.
The investigators recognize the need for more research in the field. Such studies, Dr. Craft noted, will utilize administration devices that have been previously verified to have the ability to deliver insulin to the central nervous system. “We’re currently testing several devices,” she noted. “We’re using a protocol where we administer insulin with the devices and then conduct a lumbar puncture about 30 minutes later to verify that it is actually raising insulin levels in the cerebrospinal fluid.”
Not a failure
Commenting on the findings, Samuel E. Gandy, MD, PhD, who was not involved in the study, said the research illustrates the challenge when a new therapy, a new delivery device, and a cohort of cognitively impaired patients collide. “The result is not quite a slam dunk but is also by no means a failure,” commented Dr. Gandy, Mount Sinai Chair in Alzheimer’s Research at Mount Sinai Medical Center, New York.
“One looks forward to future iterations of the Craft et al. approach, wherein the trialists tweak the ligand and/or the delivery schedule and/or the device and/or the disease and/or the disease stage,” Dr. Gandy added. “Another ligand, VGF, also holds promise for intranasal delivery, based on work from Steve Salton, Michelle Ehrlich, and Eric Schadt, all from Mount Sinai. Perhaps the nose knows!”
For Dr. Craft, the potential upside of intranasal insulin for these patients is significant and warrants further investigation. “I understand why people who are not familiar with prior research in this area might be skeptical of our enthusiasm, given the results in the intention-to-treat population,” she said. “But those of us who have been working along with this for a while now, we feel like we’ve got to do the next study. But we need to have a device that we know works,” Dr. Craft added.
“If this is real, then there may be a very large clinical benefit in symptomatic patients, and there’s nothing so far that has really improved symptomatic disease.”
The study was supported by the National Institute on Aging. Eli Lilly provided diluent placebo for the blinded phase and insulin for the open-label phase of the clinical trial at no cost. Dr. Craft received grants from the National Institute on Aging and nonfinancial support from Eli Lilly during the conduct of the study and personal fees from T3D Therapeutics and vTv Therapeutics outside the submitted work.
A version of this article originally appeared on Medscape.com.
The randomized trial of nearly 300 patients showed that, although one insulin administration device produced marked benefit in terms of change in mean score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12) over 12 months, reliability was inconsistent. A second device, used on the majority of patients in the study’s intention-to-treat population, showed no difference in these measures between patients who did and those who did not receive intranasal insulin.
“The primary analysis of the study showed no benefit of intranasal insulin on any measures of cognition or cerebrospinal fluid Alzheimer’s disease biomarkers when using the new device,” said principal investigator Suzanne Craft, PhD.
“But when we looked at our planned secondary analysis with the original device – which has been successful in previous studies – we saw quite a different picture,” added Dr. Craft, director of the Alzheimer’s Disease Research Center at Wake Forest University, Winston-Salem, N.C.
“We found a pronounced benefit with that device, such that after 18 months of administration, participants who had been receiving insulin from the beginning of the study had a large and clinically significant advantage in the primary outcome measure.”
Dr. Craft described the findings as complex. “The primary results were negative,” she added. “But the secondary results replicated those of several earlier studies when we used the same device that was used in those.”
The study was published online June 22 in JAMA Neurology.
Important for brain function
Insulin has been shown to play several important roles in brain function. The hormone is associated with a variety of cognitive functions, including memory. Through its association with vasoreactivity, lipid metabolism, and inflammation, insulin also plays an important role in vascular function.
“In the normal brain in healthy individuals, insulin is very important for synaptic function and viability. Insulin also promotes dendritic growth and facilitates synaptic health. Through this role, it plays an important part in memory,” said Dr. Craft. Given these connections, it is not surprising that reduced insulin levels or activity in brain and cerebrospinal fluid have been documented in some, but not all, studies of Alzheimer’s disease. Markers of insulin resistance also have been detected in both neuronally derived exosomes and brain tissue from adults with Alzheimer’s disease.
In light of the several important roles that insulin plays in the brain – coupled with the evidence connecting dysregulation of brain insulin and AD pathology – restoring brain insulin function may offer therapeutic benefit for adults suffering either Alzheimer’s disease or MCI. “There are a number of ways to do this,” said Dr. Craft. “But one of the approaches that we’ve focused on is providing insulin directly to the brain through intranasal administration. “By doing this, you circumvent potential issues if you administered insulin systemically.”
Previous research has shown that through this mode of administration, insulin can bypass the blood-brain barrier and reach the brain through olfactory and trigeminal perivascular channels, with little effect on peripheral insulin or blood glucose levels.
As previously reported, an earlier pilot study, also conducted by Dr. Craft and her team, showed that 4 months of daily intranasal administration of 20 IU or 40 IU of insulin preserved cognitive performance in individuals with Alzheimer’s disease or MCI.
Deeper dive
In the current investigation, the researchers wanted to broaden these findings in a larger, longer, randomized double-blinded clinical trial. The investigators assessed the efficacy of intranasal insulin on cognition, function, and biomarkers of Alzheimer’s disease, as well as the safety and feasibility of the delivery method. The multicenter trial was conducted from 2014 to 2018 and included 27 sites.
Study participants were between the ages of 55 and 85 years and had been diagnosed with amnestic MCI or Alzheimer’s disease on the basis of National Institute on Aging–Alzheimer Association criteria, a score of 20 or higher on the Mini–Mental State Examination, a clinical dementia rating of 0.5 or 1.0, or a delayed logical memory score within a specified range.
In total, 289 participants were randomly assigned to receive 40 IU of insulin or placebo for 12 months, followed by a 6-month open-label extension phase. The first 49 participants (32 men; mean age, 71.9 years) underwent insulin administration with the same device the investigators used in previous trials.
Of these, 45 completed the blinded phase, and 42 completed the open-label extension. When this device, which uses an electronic nebulizer-like delivery system, proved unreliable, the researchers switched to a second device, which uses a liquid hydrofluoroalkane propellant to deliver a metered dose of insulin through a nose tip without electronic assistance. Device 2 was used for the remaining 240 participants (123 men; mean age, 70.8 years). These patients became the study’s primary intention-to-treat population.
The study’s primary outcome was the mean change in score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12), which was evaluated at 3-month intervals.
Secondary clinical outcomes were assessed at 6-month intervals. These included the mean change in scores for the Alzheimer Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment and the Clinical Dementia Rating Scale Sum of Boxes.
Safety and adherence were also assessed during each study visit. Physical and neurologic examinations were performed at baseline and at months 6, 12, and 18.
Of the primary intention-to-treat population of 240 patients, 121 were randomly assigned to receive intranasal insulin. The remaining 119 received placebo and served as controls. The two groups were demographically comparable.
Better cognitive performance
A total of 215 participants completed the blinded phase; 198 participants completed the open-label extension. Discontinuation rates were comparable in both arms. The researchers found no differences between groups with respect to mean change in ADAS-cog-12 score from baseline to month 12 (0.0258 points; 95% confidence interval, –1.771 to 1.822 points; P = .98). The two groups also proved comparable in terms of performance on all other cognitive tests.
The open-label portion yielded similar results. Participants originally assigned to the insulin arm and their counterparts in the placebo arm did not differ with respect to mean score change on the ADAS-cog-12 test (or any other outcome) at either month 15 or 18.
Cerebrospinal fluid insulin levels were unchanged between groups, as were blood glucose and hemoglobin A1c values. Indeed, levels of A-beta42, A-beta40, total tau protein, and tau p-181 were comparable for the patients who received intranasal insulin and those who received placebo.
The most common adverse events were infections, injuries, respiratory disorders, and nervous system disorders, though these did not differ between groups. In addition, there were no differences between groups with respect to severity of adverse events; most were rated as mild.
In contrast with the intention-to-treat population, the study’s secondary analysis – using data from the original administration device – yielded markedly different results. In the blinded phase, patients who received insulin had better ADAS-cog-12 performance at 12 months (−2.81 points; 95% CI, −6.09 to 0.45 points; P = .09) and nominally significant effects at 6 months (−3.78 points; 95% CI, −6.79 to −0.78 points; P = .01).
Device type critical
These effects persisted in the open-label analyses. Patients who received intranasal insulin had superior ADAS-cog-12 scores at month 15 (−5.70 points; 95% CI, −9.62 to −1.79 points; P = .004) and month 18 (−5.78 points; 95% CI, −10.55 to −1.01 points; P = .02), compared with their counterparts who received insulin via the second device. This part of the study also showed that, although individual biomarkers did not differ significantly between the two arms, the ratios of A-beta42 to A-beta40 (P = .01) and A-beta42 to total tau (P = .03) increased with use of the first device. The number, type, and severity of adverse events were comparable between the insulin and placebo groups in this arm of the study.
The mixed results revealed by the trial demonstrate that the device used for intranasal insulin administration is paramount in determining the therapy’s potential efficacy. “Our take-home message is that the device is a very important factor for these studies and that one needs to validate their ability to effectively deliver insulin to the CNS,” said Dr. Craft.
“We were quite confident that the first device was able to do that. On the other hand, the second device has never been tested in that way, and we still don’t know whether or not that device was able to successfully deliver insulin,” she said.
The investigators recognize the need for more research in the field. Such studies, Dr. Craft noted, will utilize administration devices that have been previously verified to have the ability to deliver insulin to the central nervous system. “We’re currently testing several devices,” she noted. “We’re using a protocol where we administer insulin with the devices and then conduct a lumbar puncture about 30 minutes later to verify that it is actually raising insulin levels in the cerebrospinal fluid.”
Not a failure
Commenting on the findings, Samuel E. Gandy, MD, PhD, who was not involved in the study, said the research illustrates the challenge when a new therapy, a new delivery device, and a cohort of cognitively impaired patients collide. “The result is not quite a slam dunk but is also by no means a failure,” commented Dr. Gandy, Mount Sinai Chair in Alzheimer’s Research at Mount Sinai Medical Center, New York.
“One looks forward to future iterations of the Craft et al. approach, wherein the trialists tweak the ligand and/or the delivery schedule and/or the device and/or the disease and/or the disease stage,” Dr. Gandy added. “Another ligand, VGF, also holds promise for intranasal delivery, based on work from Steve Salton, Michelle Ehrlich, and Eric Schadt, all from Mount Sinai. Perhaps the nose knows!”
For Dr. Craft, the potential upside of intranasal insulin for these patients is significant and warrants further investigation. “I understand why people who are not familiar with prior research in this area might be skeptical of our enthusiasm, given the results in the intention-to-treat population,” she said. “But those of us who have been working along with this for a while now, we feel like we’ve got to do the next study. But we need to have a device that we know works,” Dr. Craft added.
“If this is real, then there may be a very large clinical benefit in symptomatic patients, and there’s nothing so far that has really improved symptomatic disease.”
The study was supported by the National Institute on Aging. Eli Lilly provided diluent placebo for the blinded phase and insulin for the open-label phase of the clinical trial at no cost. Dr. Craft received grants from the National Institute on Aging and nonfinancial support from Eli Lilly during the conduct of the study and personal fees from T3D Therapeutics and vTv Therapeutics outside the submitted work.
A version of this article originally appeared on Medscape.com.
The randomized trial of nearly 300 patients showed that, although one insulin administration device produced marked benefit in terms of change in mean score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12) over 12 months, reliability was inconsistent. A second device, used on the majority of patients in the study’s intention-to-treat population, showed no difference in these measures between patients who did and those who did not receive intranasal insulin.
“The primary analysis of the study showed no benefit of intranasal insulin on any measures of cognition or cerebrospinal fluid Alzheimer’s disease biomarkers when using the new device,” said principal investigator Suzanne Craft, PhD.
“But when we looked at our planned secondary analysis with the original device – which has been successful in previous studies – we saw quite a different picture,” added Dr. Craft, director of the Alzheimer’s Disease Research Center at Wake Forest University, Winston-Salem, N.C.
“We found a pronounced benefit with that device, such that after 18 months of administration, participants who had been receiving insulin from the beginning of the study had a large and clinically significant advantage in the primary outcome measure.”
Dr. Craft described the findings as complex. “The primary results were negative,” she added. “But the secondary results replicated those of several earlier studies when we used the same device that was used in those.”
The study was published online June 22 in JAMA Neurology.
Important for brain function
Insulin has been shown to play several important roles in brain function. The hormone is associated with a variety of cognitive functions, including memory. Through its association with vasoreactivity, lipid metabolism, and inflammation, insulin also plays an important role in vascular function.
“In the normal brain in healthy individuals, insulin is very important for synaptic function and viability. Insulin also promotes dendritic growth and facilitates synaptic health. Through this role, it plays an important part in memory,” said Dr. Craft. Given these connections, it is not surprising that reduced insulin levels or activity in brain and cerebrospinal fluid have been documented in some, but not all, studies of Alzheimer’s disease. Markers of insulin resistance also have been detected in both neuronally derived exosomes and brain tissue from adults with Alzheimer’s disease.
In light of the several important roles that insulin plays in the brain – coupled with the evidence connecting dysregulation of brain insulin and AD pathology – restoring brain insulin function may offer therapeutic benefit for adults suffering either Alzheimer’s disease or MCI. “There are a number of ways to do this,” said Dr. Craft. “But one of the approaches that we’ve focused on is providing insulin directly to the brain through intranasal administration. “By doing this, you circumvent potential issues if you administered insulin systemically.”
Previous research has shown that through this mode of administration, insulin can bypass the blood-brain barrier and reach the brain through olfactory and trigeminal perivascular channels, with little effect on peripheral insulin or blood glucose levels.
As previously reported, an earlier pilot study, also conducted by Dr. Craft and her team, showed that 4 months of daily intranasal administration of 20 IU or 40 IU of insulin preserved cognitive performance in individuals with Alzheimer’s disease or MCI.
Deeper dive
In the current investigation, the researchers wanted to broaden these findings in a larger, longer, randomized double-blinded clinical trial. The investigators assessed the efficacy of intranasal insulin on cognition, function, and biomarkers of Alzheimer’s disease, as well as the safety and feasibility of the delivery method. The multicenter trial was conducted from 2014 to 2018 and included 27 sites.
Study participants were between the ages of 55 and 85 years and had been diagnosed with amnestic MCI or Alzheimer’s disease on the basis of National Institute on Aging–Alzheimer Association criteria, a score of 20 or higher on the Mini–Mental State Examination, a clinical dementia rating of 0.5 or 1.0, or a delayed logical memory score within a specified range.
In total, 289 participants were randomly assigned to receive 40 IU of insulin or placebo for 12 months, followed by a 6-month open-label extension phase. The first 49 participants (32 men; mean age, 71.9 years) underwent insulin administration with the same device the investigators used in previous trials.
Of these, 45 completed the blinded phase, and 42 completed the open-label extension. When this device, which uses an electronic nebulizer-like delivery system, proved unreliable, the researchers switched to a second device, which uses a liquid hydrofluoroalkane propellant to deliver a metered dose of insulin through a nose tip without electronic assistance. Device 2 was used for the remaining 240 participants (123 men; mean age, 70.8 years). These patients became the study’s primary intention-to-treat population.
The study’s primary outcome was the mean change in score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12), which was evaluated at 3-month intervals.
Secondary clinical outcomes were assessed at 6-month intervals. These included the mean change in scores for the Alzheimer Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment and the Clinical Dementia Rating Scale Sum of Boxes.
Safety and adherence were also assessed during each study visit. Physical and neurologic examinations were performed at baseline and at months 6, 12, and 18.
Of the primary intention-to-treat population of 240 patients, 121 were randomly assigned to receive intranasal insulin. The remaining 119 received placebo and served as controls. The two groups were demographically comparable.
Better cognitive performance
A total of 215 participants completed the blinded phase; 198 participants completed the open-label extension. Discontinuation rates were comparable in both arms. The researchers found no differences between groups with respect to mean change in ADAS-cog-12 score from baseline to month 12 (0.0258 points; 95% confidence interval, –1.771 to 1.822 points; P = .98). The two groups also proved comparable in terms of performance on all other cognitive tests.
The open-label portion yielded similar results. Participants originally assigned to the insulin arm and their counterparts in the placebo arm did not differ with respect to mean score change on the ADAS-cog-12 test (or any other outcome) at either month 15 or 18.
Cerebrospinal fluid insulin levels were unchanged between groups, as were blood glucose and hemoglobin A1c values. Indeed, levels of A-beta42, A-beta40, total tau protein, and tau p-181 were comparable for the patients who received intranasal insulin and those who received placebo.
The most common adverse events were infections, injuries, respiratory disorders, and nervous system disorders, though these did not differ between groups. In addition, there were no differences between groups with respect to severity of adverse events; most were rated as mild.
In contrast with the intention-to-treat population, the study’s secondary analysis – using data from the original administration device – yielded markedly different results. In the blinded phase, patients who received insulin had better ADAS-cog-12 performance at 12 months (−2.81 points; 95% CI, −6.09 to 0.45 points; P = .09) and nominally significant effects at 6 months (−3.78 points; 95% CI, −6.79 to −0.78 points; P = .01).
Device type critical
These effects persisted in the open-label analyses. Patients who received intranasal insulin had superior ADAS-cog-12 scores at month 15 (−5.70 points; 95% CI, −9.62 to −1.79 points; P = .004) and month 18 (−5.78 points; 95% CI, −10.55 to −1.01 points; P = .02), compared with their counterparts who received insulin via the second device. This part of the study also showed that, although individual biomarkers did not differ significantly between the two arms, the ratios of A-beta42 to A-beta40 (P = .01) and A-beta42 to total tau (P = .03) increased with use of the first device. The number, type, and severity of adverse events were comparable between the insulin and placebo groups in this arm of the study.
The mixed results revealed by the trial demonstrate that the device used for intranasal insulin administration is paramount in determining the therapy’s potential efficacy. “Our take-home message is that the device is a very important factor for these studies and that one needs to validate their ability to effectively deliver insulin to the CNS,” said Dr. Craft.
“We were quite confident that the first device was able to do that. On the other hand, the second device has never been tested in that way, and we still don’t know whether or not that device was able to successfully deliver insulin,” she said.
The investigators recognize the need for more research in the field. Such studies, Dr. Craft noted, will utilize administration devices that have been previously verified to have the ability to deliver insulin to the central nervous system. “We’re currently testing several devices,” she noted. “We’re using a protocol where we administer insulin with the devices and then conduct a lumbar puncture about 30 minutes later to verify that it is actually raising insulin levels in the cerebrospinal fluid.”
Not a failure
Commenting on the findings, Samuel E. Gandy, MD, PhD, who was not involved in the study, said the research illustrates the challenge when a new therapy, a new delivery device, and a cohort of cognitively impaired patients collide. “The result is not quite a slam dunk but is also by no means a failure,” commented Dr. Gandy, Mount Sinai Chair in Alzheimer’s Research at Mount Sinai Medical Center, New York.
“One looks forward to future iterations of the Craft et al. approach, wherein the trialists tweak the ligand and/or the delivery schedule and/or the device and/or the disease and/or the disease stage,” Dr. Gandy added. “Another ligand, VGF, also holds promise for intranasal delivery, based on work from Steve Salton, Michelle Ehrlich, and Eric Schadt, all from Mount Sinai. Perhaps the nose knows!”
For Dr. Craft, the potential upside of intranasal insulin for these patients is significant and warrants further investigation. “I understand why people who are not familiar with prior research in this area might be skeptical of our enthusiasm, given the results in the intention-to-treat population,” she said. “But those of us who have been working along with this for a while now, we feel like we’ve got to do the next study. But we need to have a device that we know works,” Dr. Craft added.
“If this is real, then there may be a very large clinical benefit in symptomatic patients, and there’s nothing so far that has really improved symptomatic disease.”
The study was supported by the National Institute on Aging. Eli Lilly provided diluent placebo for the blinded phase and insulin for the open-label phase of the clinical trial at no cost. Dr. Craft received grants from the National Institute on Aging and nonfinancial support from Eli Lilly during the conduct of the study and personal fees from T3D Therapeutics and vTv Therapeutics outside the submitted work.
A version of this article originally appeared on Medscape.com.
FROM JAMA NEUROLOGY



