User login
Does concurrent use of clopidogrel and PPIs increase CV risk in patients with ACS?
EVIDENCE SUMMARY
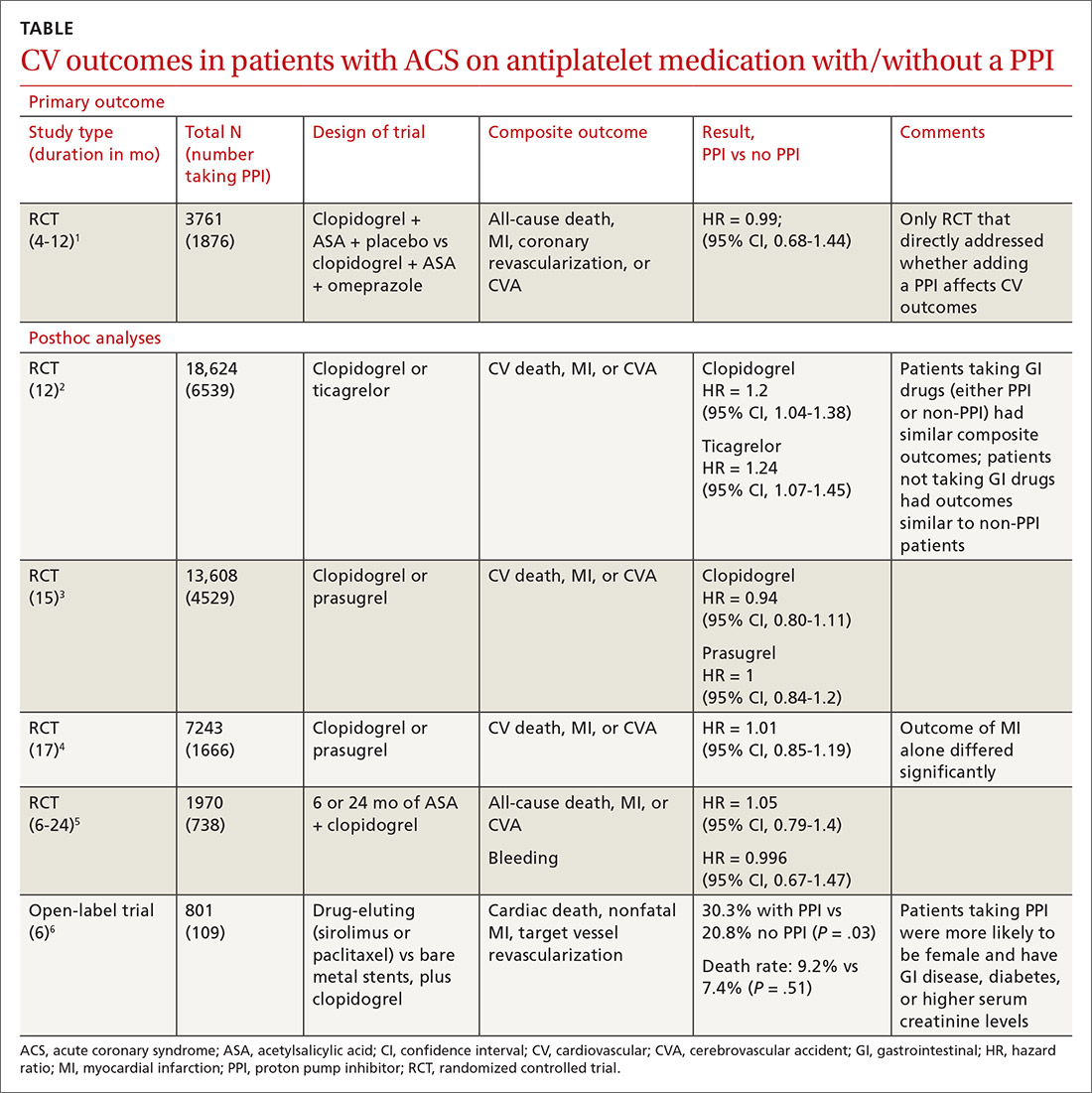
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.
Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
EVIDENCE SUMMARY
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.
Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
EVIDENCE SUMMARY
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.
Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
EVIDENCE-BASED ANSWER:
No. Adding a proton pump inhibitor (PPI) in patients taking antiplatelet medications such as clopidogrel for acute coronary syndrome (ACS) doesn’t increase the composite risk of cardiovascular (CV) events: CV death, myocardial infarction (MI), and cerebrovascular accident (CVA) (strength of recommendation: B, randomized, controlled trial [RCT] and prepon-derance of posthoc analyses of large RCTs).
Candidiasis: The essentials of diagnosis and treatment
CASE Woman with vulvar itch and white vaginal discharge
A 26-year-old sexually active nulligravid woman requests evaluation for moderately intense “itching in the vagina and on the vulva.” She uses combination oral contraceptives and has 2 current sexual partners. On physical examination, you note a thick, white, curd-like discharge that is adherent to the vaginal epithelium. The vulva is erythematous, and small “satellite lesions” are evident in the intertriginous folds.
- What is the most likely diagnosis?
- How should you treat this patient?
Approximately 75% of all women will have at least 1 episode of vulvovaginal candidiasis (VVC) in their lifetime.1 Candida albicans, the most commonly identified organism in these infections, colonizes the vagina of many individuals commensally; higher rates of colonization occur in women with diabetes, obesity, recent use of broad-spectrum antibiotics, steroid use and immunosuppression, and in women who are pregnant. Of special interest, pregnant women have an increased risk of symptomatic infection, and they respond less favorably to conventional treatment regimens.1
Deconstructing C albicans and other species
Historically, in more than 90% of cases, C albicans is the principal cause of VVC. While it remains the most prevalent Candida species in the United States, over the last 15 years studies have demonstrated that in some countries, such as India and Nigeria, C albicans constitutes less than half of the cultured species in women with VVC. This observation may be due to the widespread availability and use of common antifungal medications, which leads to resistance and selection for resistant species.1,2
In asymptomatic women, vaginal colonies of C albicans grow in the yeast form. This condition is usually well tolerated by the host and does not cause a major immune response. In periods of stress for the host micro- and mycobiomes, however (dysbiosis, immune suppression, trauma), C albicans is induced into morphogenesis, proliferating and forming hyphae that are thought to activate the host immune response. The vaginal epithelium becomes sensitized to the presence of C albicans and recruits large numbers of neutrophils that, in turn, drive the pathophysiology of VVC.3
There is a theory that the separation of the urethra and anus by the vagina has exerted evolutionary pressure to maintain the presence of commensal C albicans yeast colonies in the vagina. C albicans exerts an antagonistic effect on many bacteria and, therefore, may act as a “microbiologic barrier” between the anus and the urethra to prevent urinary tract infections that, before the modern antibiotic era, may have caused serious morbidity and even mortality.3
Other organisms that cause VVC include C glabrata, C parapsilosis, and C tropicalis. Ex vivo experiments have shown that co-infection of C albicans with C glabrata enhances the ability of C glabrata to invade tissue.2 C glabrata is more frequently resistant to commonly used antifungal compounds than C albicans,2,4 which suggests that identifying the specific fungal pathogen is becoming increasingly important in planning targeted therapy.
Continue to: A common infection...
A common infection
While three-quarters of women will experience VVC at least once in their lifetime, between 40% and 45% will experience it more than once, and 5% to 8% will develop recurrent VVC. Among pregnant women, 15% will develop symptomatic VVC.1,2
However, because VVC is not a reportable disease and antifungal medication is available over the counter without physician consultation, these numbers likely underestimate the true incidence of the infection.4
Complications in pregnancy
Vaginal infections, including VVC, bacterial vaginosis (BV), and trichomoniasis, may be associated with 40% of preterm deliveries.5 The high concentrations of estrogen and progesterone during pregnancy create a uniquely glycogen-rich vaginal environment in which Candida species can flourish.2,4 Even asymptomatic colonization of the vagina with Candida species has been associated with preterm labor, preterm birth, and low birth weight.1,6 This association appears to have more severe consequences if VVC occurs in the second trimester compared with the first trimester.6
Additionally, congenital candidiasis of the newborn may result from intrauterine Candida infection or heavy maternal vaginal colonization at delivery, and the infection is evident within 24 hours of birth. It presents typically as oropharyngeal candidiasis (thrush) of the newborn.1
Clinical manifestations of infection
The classic manifestations of Candida infection are similar in both the pregnant and nonpregnant patient: acute vaginal and vulvar pruritus and thick, white, malodorous “cottage cheese” vaginal discharge.1,4 Exercise caution, however, in treating presumptively based on these symptoms alone, especially in pregnancy, because they are not specific to candidiasis.4 Vaginal discharge is not always present, and it may vary in appearance and odor. Pruritus is the most specific symptom of Candida infection, but studies show that it is an accurate predictor in only 38% of cases.7
Other common signs and symptoms include the sensation of burning, dysuria, dyspareunia, fissures, excoriations, and pruritus ani. Physical examination demonstrates erythema and swelling of labial, vulvar, and vaginal structures, with a normal cervix and an adherent white or off-white discharge. When the discharge is removed from the vaginal wall, small bleeding points may appear.1,4
Making the diagnosis
As mentioned, history alone is not sufficient to make a definitive diagnosis of candidiasis. The diagnosis should be made by examining vaginal secretions under a microscope or by culture.4 A wet mount and KOH (potassium hydroxide) prep help differentiate VVC, BV, and trichomoniasis. Culture is particularly valuable in identifying less common fungal organisms, such as C glabrata and C tropicalis.
Vaginal pH testing is not conclusive for Candida because vaginal pH is normal in VVC. However, pH assessment can rule in other causative organisms if the value is abnormal (that is, elevated pH of 4.5 or greater with BV and trichomoniasis).1
Treatment options
Acute infection. A pregnant woman who tests positive for VVC may safely be treated in any trimester with a 7-day course of a topical azole.8 If the patient prefers the convenience of oral therapy, after the first trimester, oral fluconazole, 150 mg on day 1 and day 3, may be used for treatment. Note that fluconazole has been associated with an increased risk of spontaneous abortion and cardiac septal defects when used in the first trimester.1
The Centers for Disease Control and Prevention recommends a number of topical treatments for VVC (TABLE).8 Several of these drugs are available over the counter without a prescription. Topical azoles are more effective than nystatin in treating VVC, and posttreatment cultures are negative in up to 90% of treated patients.8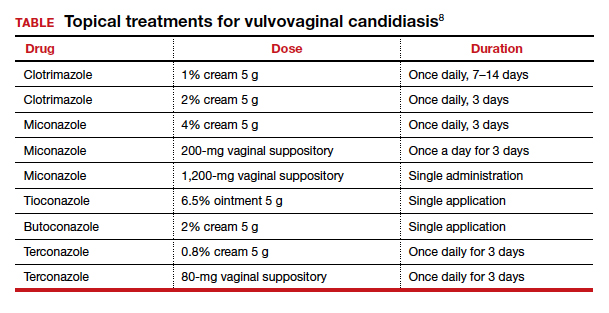
Recurrent infections. Recurrent VVC is defined as 4 or more episodes of symptomatic VVC within 12 months.8 Typical first-line treatment of recurrent infections in nonpregnant patients is a 6-month course of fluconazole, 150 mg weekly.9,10 As noted, however, fluconazole should not be used in the first trimester of pregnancy. It is acceptable therapy thereafter for patients who have troublesome recurrent or persistent infections.
Continue to: Strategies for preventing recurrence...
Strategies for preventing recurrence
While it is logical to consider antimycotic prophylaxis in women with a history of recurring VVC and/or a significant number of known risk factors, data suggest that extended prophylaxis with an azole does not consistently achieve long-term elimination of vaginal Candida organisms after cessation of the azole.9
At-risk women should be counseled to make lifestyle adjustments, such as wearing breathable cotton clothing, particularly undergarments; promptly changing out of damp clothing; and forgoing the use of commercial intravaginal feminine hygiene products.
Recent research has shown that the use of Saccharomyces cerevisiae–based probiotics has promise for controlling the burden of C albicans in women receiving antifungal drugs for VVC and also for preventing recurrence; however, this approach has undergone limited testing in humans, and its efficacy and safety in pregnancy is unknown.11 ●
- Duff P. Maternal and fetal infection. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:862.
- Goncalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927.
- Hall RA, Noverr MC. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr Opin Microbiol. 2017;40:58-64.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971.
- Holzer I, Farr A, Kiss H; et al. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. 2017;295:891-895.
- Farr A, Kiss H, Holzer I, et al. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94:989-996.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379.
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876-883.
- US Food and Drug Administration. FDA Drug Safety Communication: Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be#. Updated August 4, 2017. Accessed July 6, 2020.
- Gaziano R, Sabbatini S, Roselletti E, et al. Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front Microbiol. 2020;11:718.
CASE Woman with vulvar itch and white vaginal discharge
A 26-year-old sexually active nulligravid woman requests evaluation for moderately intense “itching in the vagina and on the vulva.” She uses combination oral contraceptives and has 2 current sexual partners. On physical examination, you note a thick, white, curd-like discharge that is adherent to the vaginal epithelium. The vulva is erythematous, and small “satellite lesions” are evident in the intertriginous folds.
- What is the most likely diagnosis?
- How should you treat this patient?
Approximately 75% of all women will have at least 1 episode of vulvovaginal candidiasis (VVC) in their lifetime.1 Candida albicans, the most commonly identified organism in these infections, colonizes the vagina of many individuals commensally; higher rates of colonization occur in women with diabetes, obesity, recent use of broad-spectrum antibiotics, steroid use and immunosuppression, and in women who are pregnant. Of special interest, pregnant women have an increased risk of symptomatic infection, and they respond less favorably to conventional treatment regimens.1
Deconstructing C albicans and other species
Historically, in more than 90% of cases, C albicans is the principal cause of VVC. While it remains the most prevalent Candida species in the United States, over the last 15 years studies have demonstrated that in some countries, such as India and Nigeria, C albicans constitutes less than half of the cultured species in women with VVC. This observation may be due to the widespread availability and use of common antifungal medications, which leads to resistance and selection for resistant species.1,2
In asymptomatic women, vaginal colonies of C albicans grow in the yeast form. This condition is usually well tolerated by the host and does not cause a major immune response. In periods of stress for the host micro- and mycobiomes, however (dysbiosis, immune suppression, trauma), C albicans is induced into morphogenesis, proliferating and forming hyphae that are thought to activate the host immune response. The vaginal epithelium becomes sensitized to the presence of C albicans and recruits large numbers of neutrophils that, in turn, drive the pathophysiology of VVC.3
There is a theory that the separation of the urethra and anus by the vagina has exerted evolutionary pressure to maintain the presence of commensal C albicans yeast colonies in the vagina. C albicans exerts an antagonistic effect on many bacteria and, therefore, may act as a “microbiologic barrier” between the anus and the urethra to prevent urinary tract infections that, before the modern antibiotic era, may have caused serious morbidity and even mortality.3
Other organisms that cause VVC include C glabrata, C parapsilosis, and C tropicalis. Ex vivo experiments have shown that co-infection of C albicans with C glabrata enhances the ability of C glabrata to invade tissue.2 C glabrata is more frequently resistant to commonly used antifungal compounds than C albicans,2,4 which suggests that identifying the specific fungal pathogen is becoming increasingly important in planning targeted therapy.
Continue to: A common infection...
A common infection
While three-quarters of women will experience VVC at least once in their lifetime, between 40% and 45% will experience it more than once, and 5% to 8% will develop recurrent VVC. Among pregnant women, 15% will develop symptomatic VVC.1,2
However, because VVC is not a reportable disease and antifungal medication is available over the counter without physician consultation, these numbers likely underestimate the true incidence of the infection.4
Complications in pregnancy
Vaginal infections, including VVC, bacterial vaginosis (BV), and trichomoniasis, may be associated with 40% of preterm deliveries.5 The high concentrations of estrogen and progesterone during pregnancy create a uniquely glycogen-rich vaginal environment in which Candida species can flourish.2,4 Even asymptomatic colonization of the vagina with Candida species has been associated with preterm labor, preterm birth, and low birth weight.1,6 This association appears to have more severe consequences if VVC occurs in the second trimester compared with the first trimester.6
Additionally, congenital candidiasis of the newborn may result from intrauterine Candida infection or heavy maternal vaginal colonization at delivery, and the infection is evident within 24 hours of birth. It presents typically as oropharyngeal candidiasis (thrush) of the newborn.1
Clinical manifestations of infection
The classic manifestations of Candida infection are similar in both the pregnant and nonpregnant patient: acute vaginal and vulvar pruritus and thick, white, malodorous “cottage cheese” vaginal discharge.1,4 Exercise caution, however, in treating presumptively based on these symptoms alone, especially in pregnancy, because they are not specific to candidiasis.4 Vaginal discharge is not always present, and it may vary in appearance and odor. Pruritus is the most specific symptom of Candida infection, but studies show that it is an accurate predictor in only 38% of cases.7
Other common signs and symptoms include the sensation of burning, dysuria, dyspareunia, fissures, excoriations, and pruritus ani. Physical examination demonstrates erythema and swelling of labial, vulvar, and vaginal structures, with a normal cervix and an adherent white or off-white discharge. When the discharge is removed from the vaginal wall, small bleeding points may appear.1,4
Making the diagnosis
As mentioned, history alone is not sufficient to make a definitive diagnosis of candidiasis. The diagnosis should be made by examining vaginal secretions under a microscope or by culture.4 A wet mount and KOH (potassium hydroxide) prep help differentiate VVC, BV, and trichomoniasis. Culture is particularly valuable in identifying less common fungal organisms, such as C glabrata and C tropicalis.
Vaginal pH testing is not conclusive for Candida because vaginal pH is normal in VVC. However, pH assessment can rule in other causative organisms if the value is abnormal (that is, elevated pH of 4.5 or greater with BV and trichomoniasis).1
Treatment options
Acute infection. A pregnant woman who tests positive for VVC may safely be treated in any trimester with a 7-day course of a topical azole.8 If the patient prefers the convenience of oral therapy, after the first trimester, oral fluconazole, 150 mg on day 1 and day 3, may be used for treatment. Note that fluconazole has been associated with an increased risk of spontaneous abortion and cardiac septal defects when used in the first trimester.1
The Centers for Disease Control and Prevention recommends a number of topical treatments for VVC (TABLE).8 Several of these drugs are available over the counter without a prescription. Topical azoles are more effective than nystatin in treating VVC, and posttreatment cultures are negative in up to 90% of treated patients.8
Recurrent infections. Recurrent VVC is defined as 4 or more episodes of symptomatic VVC within 12 months.8 Typical first-line treatment of recurrent infections in nonpregnant patients is a 6-month course of fluconazole, 150 mg weekly.9,10 As noted, however, fluconazole should not be used in the first trimester of pregnancy. It is acceptable therapy thereafter for patients who have troublesome recurrent or persistent infections.
Continue to: Strategies for preventing recurrence...
Strategies for preventing recurrence
While it is logical to consider antimycotic prophylaxis in women with a history of recurring VVC and/or a significant number of known risk factors, data suggest that extended prophylaxis with an azole does not consistently achieve long-term elimination of vaginal Candida organisms after cessation of the azole.9
At-risk women should be counseled to make lifestyle adjustments, such as wearing breathable cotton clothing, particularly undergarments; promptly changing out of damp clothing; and forgoing the use of commercial intravaginal feminine hygiene products.
Recent research has shown that the use of Saccharomyces cerevisiae–based probiotics has promise for controlling the burden of C albicans in women receiving antifungal drugs for VVC and also for preventing recurrence; however, this approach has undergone limited testing in humans, and its efficacy and safety in pregnancy is unknown.11 ●
CASE Woman with vulvar itch and white vaginal discharge
A 26-year-old sexually active nulligravid woman requests evaluation for moderately intense “itching in the vagina and on the vulva.” She uses combination oral contraceptives and has 2 current sexual partners. On physical examination, you note a thick, white, curd-like discharge that is adherent to the vaginal epithelium. The vulva is erythematous, and small “satellite lesions” are evident in the intertriginous folds.
- What is the most likely diagnosis?
- How should you treat this patient?
Approximately 75% of all women will have at least 1 episode of vulvovaginal candidiasis (VVC) in their lifetime.1 Candida albicans, the most commonly identified organism in these infections, colonizes the vagina of many individuals commensally; higher rates of colonization occur in women with diabetes, obesity, recent use of broad-spectrum antibiotics, steroid use and immunosuppression, and in women who are pregnant. Of special interest, pregnant women have an increased risk of symptomatic infection, and they respond less favorably to conventional treatment regimens.1
Deconstructing C albicans and other species
Historically, in more than 90% of cases, C albicans is the principal cause of VVC. While it remains the most prevalent Candida species in the United States, over the last 15 years studies have demonstrated that in some countries, such as India and Nigeria, C albicans constitutes less than half of the cultured species in women with VVC. This observation may be due to the widespread availability and use of common antifungal medications, which leads to resistance and selection for resistant species.1,2
In asymptomatic women, vaginal colonies of C albicans grow in the yeast form. This condition is usually well tolerated by the host and does not cause a major immune response. In periods of stress for the host micro- and mycobiomes, however (dysbiosis, immune suppression, trauma), C albicans is induced into morphogenesis, proliferating and forming hyphae that are thought to activate the host immune response. The vaginal epithelium becomes sensitized to the presence of C albicans and recruits large numbers of neutrophils that, in turn, drive the pathophysiology of VVC.3
There is a theory that the separation of the urethra and anus by the vagina has exerted evolutionary pressure to maintain the presence of commensal C albicans yeast colonies in the vagina. C albicans exerts an antagonistic effect on many bacteria and, therefore, may act as a “microbiologic barrier” between the anus and the urethra to prevent urinary tract infections that, before the modern antibiotic era, may have caused serious morbidity and even mortality.3
Other organisms that cause VVC include C glabrata, C parapsilosis, and C tropicalis. Ex vivo experiments have shown that co-infection of C albicans with C glabrata enhances the ability of C glabrata to invade tissue.2 C glabrata is more frequently resistant to commonly used antifungal compounds than C albicans,2,4 which suggests that identifying the specific fungal pathogen is becoming increasingly important in planning targeted therapy.
Continue to: A common infection...
A common infection
While three-quarters of women will experience VVC at least once in their lifetime, between 40% and 45% will experience it more than once, and 5% to 8% will develop recurrent VVC. Among pregnant women, 15% will develop symptomatic VVC.1,2
However, because VVC is not a reportable disease and antifungal medication is available over the counter without physician consultation, these numbers likely underestimate the true incidence of the infection.4
Complications in pregnancy
Vaginal infections, including VVC, bacterial vaginosis (BV), and trichomoniasis, may be associated with 40% of preterm deliveries.5 The high concentrations of estrogen and progesterone during pregnancy create a uniquely glycogen-rich vaginal environment in which Candida species can flourish.2,4 Even asymptomatic colonization of the vagina with Candida species has been associated with preterm labor, preterm birth, and low birth weight.1,6 This association appears to have more severe consequences if VVC occurs in the second trimester compared with the first trimester.6
Additionally, congenital candidiasis of the newborn may result from intrauterine Candida infection or heavy maternal vaginal colonization at delivery, and the infection is evident within 24 hours of birth. It presents typically as oropharyngeal candidiasis (thrush) of the newborn.1
Clinical manifestations of infection
The classic manifestations of Candida infection are similar in both the pregnant and nonpregnant patient: acute vaginal and vulvar pruritus and thick, white, malodorous “cottage cheese” vaginal discharge.1,4 Exercise caution, however, in treating presumptively based on these symptoms alone, especially in pregnancy, because they are not specific to candidiasis.4 Vaginal discharge is not always present, and it may vary in appearance and odor. Pruritus is the most specific symptom of Candida infection, but studies show that it is an accurate predictor in only 38% of cases.7
Other common signs and symptoms include the sensation of burning, dysuria, dyspareunia, fissures, excoriations, and pruritus ani. Physical examination demonstrates erythema and swelling of labial, vulvar, and vaginal structures, with a normal cervix and an adherent white or off-white discharge. When the discharge is removed from the vaginal wall, small bleeding points may appear.1,4
Making the diagnosis
As mentioned, history alone is not sufficient to make a definitive diagnosis of candidiasis. The diagnosis should be made by examining vaginal secretions under a microscope or by culture.4 A wet mount and KOH (potassium hydroxide) prep help differentiate VVC, BV, and trichomoniasis. Culture is particularly valuable in identifying less common fungal organisms, such as C glabrata and C tropicalis.
Vaginal pH testing is not conclusive for Candida because vaginal pH is normal in VVC. However, pH assessment can rule in other causative organisms if the value is abnormal (that is, elevated pH of 4.5 or greater with BV and trichomoniasis).1
Treatment options
Acute infection. A pregnant woman who tests positive for VVC may safely be treated in any trimester with a 7-day course of a topical azole.8 If the patient prefers the convenience of oral therapy, after the first trimester, oral fluconazole, 150 mg on day 1 and day 3, may be used for treatment. Note that fluconazole has been associated with an increased risk of spontaneous abortion and cardiac septal defects when used in the first trimester.1
The Centers for Disease Control and Prevention recommends a number of topical treatments for VVC (TABLE).8 Several of these drugs are available over the counter without a prescription. Topical azoles are more effective than nystatin in treating VVC, and posttreatment cultures are negative in up to 90% of treated patients.8
Recurrent infections. Recurrent VVC is defined as 4 or more episodes of symptomatic VVC within 12 months.8 Typical first-line treatment of recurrent infections in nonpregnant patients is a 6-month course of fluconazole, 150 mg weekly.9,10 As noted, however, fluconazole should not be used in the first trimester of pregnancy. It is acceptable therapy thereafter for patients who have troublesome recurrent or persistent infections.
Continue to: Strategies for preventing recurrence...
Strategies for preventing recurrence
While it is logical to consider antimycotic prophylaxis in women with a history of recurring VVC and/or a significant number of known risk factors, data suggest that extended prophylaxis with an azole does not consistently achieve long-term elimination of vaginal Candida organisms after cessation of the azole.9
At-risk women should be counseled to make lifestyle adjustments, such as wearing breathable cotton clothing, particularly undergarments; promptly changing out of damp clothing; and forgoing the use of commercial intravaginal feminine hygiene products.
Recent research has shown that the use of Saccharomyces cerevisiae–based probiotics has promise for controlling the burden of C albicans in women receiving antifungal drugs for VVC and also for preventing recurrence; however, this approach has undergone limited testing in humans, and its efficacy and safety in pregnancy is unknown.11 ●
- Duff P. Maternal and fetal infection. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:862.
- Goncalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927.
- Hall RA, Noverr MC. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr Opin Microbiol. 2017;40:58-64.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971.
- Holzer I, Farr A, Kiss H; et al. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. 2017;295:891-895.
- Farr A, Kiss H, Holzer I, et al. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94:989-996.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379.
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876-883.
- US Food and Drug Administration. FDA Drug Safety Communication: Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be#. Updated August 4, 2017. Accessed July 6, 2020.
- Gaziano R, Sabbatini S, Roselletti E, et al. Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front Microbiol. 2020;11:718.
- Duff P. Maternal and fetal infection. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:862.
- Goncalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927.
- Hall RA, Noverr MC. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr Opin Microbiol. 2017;40:58-64.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971.
- Holzer I, Farr A, Kiss H; et al. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. 2017;295:891-895.
- Farr A, Kiss H, Holzer I, et al. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94:989-996.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379.
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876-883.
- US Food and Drug Administration. FDA Drug Safety Communication: Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be#. Updated August 4, 2017. Accessed July 6, 2020.
- Gaziano R, Sabbatini S, Roselletti E, et al. Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front Microbiol. 2020;11:718.
Psoriatic disease inflammation linked to heart failure
Patients with psoriatic disease are known to be at increased risk of heart failure. A new cohort study suggests that part of the risk may be attributable to the disease itself, not just traditional cardiovascular risk factors like obesity and metabolic abnormalities that are common comorbidities in psoriatic disease. There may also be differences in the risk profiles of patients with ischemic and nonischemic heart failure.
Previous studies have shown that heart failure risk in patients with psoriatic arthritis is 32% higher than in the general population, and with psoriasis, it is 22%-53% higher. However, those studies were based on administrative databases with no clinical information to back up the accuracy of diagnoses, Sahil Koppikar, MD, from the University of Toronto, said during a presentation of the research at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
The finding that psoriatic disease inflammation may be a direct risk factor for heart failure might be good news for patients. “By controlling inflammation, we may be able to reduce the risk of heart failure in these patients,” Dr. Koppikar said.
During a question and answer session, discussant Deepak Jadon, MBChB, PhD, director of the rheumatology research unit and lead for psoriatic arthritis at Addenbrooke’s Hospital, Cambridge (England), noted that patients with conditions like lupus and systemic sclerosis may undergo regular echocardiograms, chest CTs, or other surveillance, and asked if Dr. Koppikar could recommend a framework for similar surveillance in psoriatic arthritis.
“With the current data we have, I don’t know if we can make recommendations. What we learned from our study is that patients that have elevated inflammatory disease, with elevated inflammatory markers for a prolonged period of time, were at higher risk than [if they had elevated markers only] just before the event. So poorly controlled patients might be something you should be more aware of, and maybe get cardiology involved. But I don’t think it’s something we should be doing right now for all patients,” Dr. Koppikar said.
The researchers analyzed data from a psoriasis cohort at the University of Toronto that began in 2006. Every 6-12 months, they were assessed by a rheumatologist and underwent imaging assessment and laboratory tests. The primary outcome of the study was the first heart failure event, which the researchers identified by linking the cohort database with provincial hospitalization and mortality databases. They verified all events by examining medical records. They also assessed the association between heart failure and disease activity over time rather than just before the event.
The analysis included 1,994 patients. A total of 64 new heart failure events occurred during a mean follow-up of 11.3 years (2.85 per 1,000 person-years), including 38 ischemic and 26 nonischemic events. A multivariate analysis found that heart failure was associated with adjusted mean (AM) tender joint count (hazard ratio, 1.51; P = .02), AM swollen joint count (HR, 1.82; P = .04), AM erythrocyte sedimentation rate (HR, 1.26; P = .009), AM C-reactive protein (HR, 1.27; P = .001), Health Assessment Questionnaire (HR, 1.95; P = .001), and minimum disease activity state (HR, 0.40; P = .04). The multivariate analysis was adjusted for sex, hypertension, diabetes mellitus, body mass index, ischemic heart disease, lipids, and smoking status.
When the researchers separated the analysis into ischemic and nonischemic heart failure, some interesting associations popped out. Nonischemic heart failure was associated with AM tender joint count (HR, 1.83; P = .004), but ischemic heart failure was not. Other factors associated with nonischemic but not ischemic heart failure included AM swollen joint count (HR, 3.56; P = .0003), damaged joint count (HR, 1.29; P = .04), and pain score (HR, 1.22; P = .047). Minimum disease activity had the opposite result: It was associated with only ischemic heart failure (HR, 0.40; P = .04).
The study cohort more closely resembles a rheumatology cohort than a dermatology cohort, and it suggests that patients with psoriatic arthritis have different cardiovascular comorbidities than those with pure psoriasis, according to Diamant Thaçi, MD, PhD, professor and chairman of the department of dermatology at the University of Lübeck (Germany). “It shows how it important it is to look for comorbidity in the rheumatologic setting,” Dr. Thaçi said in an interview.
The study was supported by the Arthritis Society. Dr. Koppikar and Dr. Thaçi have no relevant financial disclosures.
SOURCE: Koppikar S et al. GRAPPA 2020 Virtual Annual Meeting.
Patients with psoriatic disease are known to be at increased risk of heart failure. A new cohort study suggests that part of the risk may be attributable to the disease itself, not just traditional cardiovascular risk factors like obesity and metabolic abnormalities that are common comorbidities in psoriatic disease. There may also be differences in the risk profiles of patients with ischemic and nonischemic heart failure.
Previous studies have shown that heart failure risk in patients with psoriatic arthritis is 32% higher than in the general population, and with psoriasis, it is 22%-53% higher. However, those studies were based on administrative databases with no clinical information to back up the accuracy of diagnoses, Sahil Koppikar, MD, from the University of Toronto, said during a presentation of the research at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
The finding that psoriatic disease inflammation may be a direct risk factor for heart failure might be good news for patients. “By controlling inflammation, we may be able to reduce the risk of heart failure in these patients,” Dr. Koppikar said.
During a question and answer session, discussant Deepak Jadon, MBChB, PhD, director of the rheumatology research unit and lead for psoriatic arthritis at Addenbrooke’s Hospital, Cambridge (England), noted that patients with conditions like lupus and systemic sclerosis may undergo regular echocardiograms, chest CTs, or other surveillance, and asked if Dr. Koppikar could recommend a framework for similar surveillance in psoriatic arthritis.
“With the current data we have, I don’t know if we can make recommendations. What we learned from our study is that patients that have elevated inflammatory disease, with elevated inflammatory markers for a prolonged period of time, were at higher risk than [if they had elevated markers only] just before the event. So poorly controlled patients might be something you should be more aware of, and maybe get cardiology involved. But I don’t think it’s something we should be doing right now for all patients,” Dr. Koppikar said.
The researchers analyzed data from a psoriasis cohort at the University of Toronto that began in 2006. Every 6-12 months, they were assessed by a rheumatologist and underwent imaging assessment and laboratory tests. The primary outcome of the study was the first heart failure event, which the researchers identified by linking the cohort database with provincial hospitalization and mortality databases. They verified all events by examining medical records. They also assessed the association between heart failure and disease activity over time rather than just before the event.
The analysis included 1,994 patients. A total of 64 new heart failure events occurred during a mean follow-up of 11.3 years (2.85 per 1,000 person-years), including 38 ischemic and 26 nonischemic events. A multivariate analysis found that heart failure was associated with adjusted mean (AM) tender joint count (hazard ratio, 1.51; P = .02), AM swollen joint count (HR, 1.82; P = .04), AM erythrocyte sedimentation rate (HR, 1.26; P = .009), AM C-reactive protein (HR, 1.27; P = .001), Health Assessment Questionnaire (HR, 1.95; P = .001), and minimum disease activity state (HR, 0.40; P = .04). The multivariate analysis was adjusted for sex, hypertension, diabetes mellitus, body mass index, ischemic heart disease, lipids, and smoking status.
When the researchers separated the analysis into ischemic and nonischemic heart failure, some interesting associations popped out. Nonischemic heart failure was associated with AM tender joint count (HR, 1.83; P = .004), but ischemic heart failure was not. Other factors associated with nonischemic but not ischemic heart failure included AM swollen joint count (HR, 3.56; P = .0003), damaged joint count (HR, 1.29; P = .04), and pain score (HR, 1.22; P = .047). Minimum disease activity had the opposite result: It was associated with only ischemic heart failure (HR, 0.40; P = .04).
The study cohort more closely resembles a rheumatology cohort than a dermatology cohort, and it suggests that patients with psoriatic arthritis have different cardiovascular comorbidities than those with pure psoriasis, according to Diamant Thaçi, MD, PhD, professor and chairman of the department of dermatology at the University of Lübeck (Germany). “It shows how it important it is to look for comorbidity in the rheumatologic setting,” Dr. Thaçi said in an interview.
The study was supported by the Arthritis Society. Dr. Koppikar and Dr. Thaçi have no relevant financial disclosures.
SOURCE: Koppikar S et al. GRAPPA 2020 Virtual Annual Meeting.
Patients with psoriatic disease are known to be at increased risk of heart failure. A new cohort study suggests that part of the risk may be attributable to the disease itself, not just traditional cardiovascular risk factors like obesity and metabolic abnormalities that are common comorbidities in psoriatic disease. There may also be differences in the risk profiles of patients with ischemic and nonischemic heart failure.
Previous studies have shown that heart failure risk in patients with psoriatic arthritis is 32% higher than in the general population, and with psoriasis, it is 22%-53% higher. However, those studies were based on administrative databases with no clinical information to back up the accuracy of diagnoses, Sahil Koppikar, MD, from the University of Toronto, said during a presentation of the research at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
The finding that psoriatic disease inflammation may be a direct risk factor for heart failure might be good news for patients. “By controlling inflammation, we may be able to reduce the risk of heart failure in these patients,” Dr. Koppikar said.
During a question and answer session, discussant Deepak Jadon, MBChB, PhD, director of the rheumatology research unit and lead for psoriatic arthritis at Addenbrooke’s Hospital, Cambridge (England), noted that patients with conditions like lupus and systemic sclerosis may undergo regular echocardiograms, chest CTs, or other surveillance, and asked if Dr. Koppikar could recommend a framework for similar surveillance in psoriatic arthritis.
“With the current data we have, I don’t know if we can make recommendations. What we learned from our study is that patients that have elevated inflammatory disease, with elevated inflammatory markers for a prolonged period of time, were at higher risk than [if they had elevated markers only] just before the event. So poorly controlled patients might be something you should be more aware of, and maybe get cardiology involved. But I don’t think it’s something we should be doing right now for all patients,” Dr. Koppikar said.
The researchers analyzed data from a psoriasis cohort at the University of Toronto that began in 2006. Every 6-12 months, they were assessed by a rheumatologist and underwent imaging assessment and laboratory tests. The primary outcome of the study was the first heart failure event, which the researchers identified by linking the cohort database with provincial hospitalization and mortality databases. They verified all events by examining medical records. They also assessed the association between heart failure and disease activity over time rather than just before the event.
The analysis included 1,994 patients. A total of 64 new heart failure events occurred during a mean follow-up of 11.3 years (2.85 per 1,000 person-years), including 38 ischemic and 26 nonischemic events. A multivariate analysis found that heart failure was associated with adjusted mean (AM) tender joint count (hazard ratio, 1.51; P = .02), AM swollen joint count (HR, 1.82; P = .04), AM erythrocyte sedimentation rate (HR, 1.26; P = .009), AM C-reactive protein (HR, 1.27; P = .001), Health Assessment Questionnaire (HR, 1.95; P = .001), and minimum disease activity state (HR, 0.40; P = .04). The multivariate analysis was adjusted for sex, hypertension, diabetes mellitus, body mass index, ischemic heart disease, lipids, and smoking status.
When the researchers separated the analysis into ischemic and nonischemic heart failure, some interesting associations popped out. Nonischemic heart failure was associated with AM tender joint count (HR, 1.83; P = .004), but ischemic heart failure was not. Other factors associated with nonischemic but not ischemic heart failure included AM swollen joint count (HR, 3.56; P = .0003), damaged joint count (HR, 1.29; P = .04), and pain score (HR, 1.22; P = .047). Minimum disease activity had the opposite result: It was associated with only ischemic heart failure (HR, 0.40; P = .04).
The study cohort more closely resembles a rheumatology cohort than a dermatology cohort, and it suggests that patients with psoriatic arthritis have different cardiovascular comorbidities than those with pure psoriasis, according to Diamant Thaçi, MD, PhD, professor and chairman of the department of dermatology at the University of Lübeck (Germany). “It shows how it important it is to look for comorbidity in the rheumatologic setting,” Dr. Thaçi said in an interview.
The study was supported by the Arthritis Society. Dr. Koppikar and Dr. Thaçi have no relevant financial disclosures.
SOURCE: Koppikar S et al. GRAPPA 2020 Virtual Annual Meeting.
FROM GRAPPA 2020 VIRTUAL ANNUAL MEETING
Empagliflozin failed to improve exercise capacity in heart failure
Empagliflozin showed favorable effects on diuretic use and congestion symptoms in patients with heart failure with reduced ejection fraction (HFrEF), but the oral sodium glucose cotransporter 2 (SGLT2) inhibitor did not improve the primary endpoint of improved exercise capacity in the EMPERIAL-Reduced trial, investigators reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
In the matching EMPERIAL-Preserved trial, conducted in patients with heart failure with preserved ejection fraction (HFpEF), empagliflozin (Jardiance) produced modest improvements in diuretic use, as well as a reduction in unscheduled outpatient visits, compared with placebo-treated controls, although these trends failed to achieve statistical significance. And as in the EMPERIAL-Reduced trial, the SGLT2 inhibitor didn’t move the needle at all on the primary endpoint of improved exercise capacity as measured by 6-minute hall walk distance.
EMPERIAL-Reduced and -Preserved were identically designed, concurrent, phase 3, double-blind, 12-week randomized trials of empagliflozin versus placebo in 312 patients with HFrEF and 315 with HFpEF, defined in EMPERIAL-preserved as a left ventricular ejection fraction above 40%. The majority of participants had type 2 diabetes.
From a baseline median 6-minute walk distance of about 300 meters, the 6-minute walk distance at week 12 was actually 4.0 meters worse in the empagliflozin-treated HFrEF patients than it was in controls and a mere 4.0 meters better than with placebo in empagliflozin-treated patients with HFpEF, reported William T. Abraham, MD, professor of medicine, director of the division of cardiovascular medicine, and associate dean at Ohio State University, Columbus.
He indicated that the audience shouldn’t make too much of the failure to achieve the primary endpoint in the two trials in light of the studies’ major limitations: namely, their relatively small size for purposes of evaluating clinical outcomes and the relatively short 12-week duration.
“In many ways, I would say it’s remarkable that we can observe a positive signal, a favorable signal, in outcomes around congestion. In the case of HFrEF it’s statistically significant, and in HFpEF it’s a trend towards improvement. Of course, there are larger trials ongoing that may confirm these observations. Hopefully the EMPERIAL trials predict a good outcome for those ongoing trials,” Dr. Abraham said.
Piotr Ponikowski, MD, presented the study results for the secondary outcomes of congestion symptoms, diuretic use, and utilization of health care resources. In EMPERIAL-Reduced, intensification of diuretic therapy – often a prelude to acute decompensation and a trip to the hospital – occurred at a rate of 4.5% with empagliflozin and 16.1% with placebo, for a highly significant 73% relative risk reduction. Intensification of loop diuretics occurred in 2.6% of the empagliflozin group and 14.2% of controls, for a 82% risk reduction.
“That’s a pretty significant effect,” observed Dr. Ponikowski, professor of cardiology and head of the department of heart diseases at the Medical University of Wroclaw (Poland).
Moreover, a congestion symptoms score comprising a summary of orthopnea, jugular veinous distention, and edema improved by 47% after 12 weeks on empagliflozin, a statistically significant and clinically meaningful improvement that grew in magnitude over time and at 12 weeks was twice as large, compared with the reduction in placebo group, he added.
There was a trend for fewer unscheduled outpatient visits in the empagliflozin arm of EMPERIAL-Reduced with a rate of 10.4%, compared with 25.8% in controls; however, this 26% reduction in relative risk did not achieve statistical significance.
Intensification of loop diuretics occurred in 9% of EMPERIAL-Preserved participants on empagliflozin and 13.5% on placebo, but this 34% reduction in risk didn’t reach significance.
Adverse events in the EMPERIAL trials were similar across the active treatment and placebo arms. The benign safety profile was similar to what was seen in the earlier major clinical trials of empagliflozin for treatment of type 2 diabetes.
Session chair Stephane Heymans, MD, PhD, of the University of Maastricht (the Netherlands) noted that a substantial minority of patients in EMPERIAL-Reduced were on the combined neprilysin inhibitor sacubitril and the angiotensin receptor blocker valsartan (Entresto), whereas far fewer were in EMPERIAL-Preserved. He wondered if this greater use of background sacubitril/valsartan could explain empagliflozin’s greater efficacy in EMPERIAL-Reduced.
Highly unlikely, according to the investigators.
“It looks like, as is the case with most of our heart failure therapies, that we do see incremental value here. If you met the criteria for these trials, it appears you derived benefit from empagliflozin regardless of whether you were on an angiotensin receptor neprilysin inhibitor or not. I think that speaks to the incremental benefit of SGLT2 inhibitors on top of current guideline-directed medical therapy,” Dr. Abraham said.
Dr. Ponikowski observed that the same point was underscored in the DAPA-HF trial of the SGLT2 inhibitor dapagliflozin (Farxiga) in patients with heart failure (DAPA-HF: N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
“You’ll see that the mortality and morbidity and quality-of-life benefit is in those treated with dapagliflozin with or without angiotensin receptor neprilysin inhibition; so, regardless of background therapy. And the effect is especially clear in patients on both therapies,” the cardiologist said.
The EMPERIAL trials were sponsored by Boehringer Ingelheim. Dr. Abraham and Dr. Ponikowksi reported receiving consultant fees from the company for serving on the trials’ executive committee.
Empagliflozin showed favorable effects on diuretic use and congestion symptoms in patients with heart failure with reduced ejection fraction (HFrEF), but the oral sodium glucose cotransporter 2 (SGLT2) inhibitor did not improve the primary endpoint of improved exercise capacity in the EMPERIAL-Reduced trial, investigators reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
In the matching EMPERIAL-Preserved trial, conducted in patients with heart failure with preserved ejection fraction (HFpEF), empagliflozin (Jardiance) produced modest improvements in diuretic use, as well as a reduction in unscheduled outpatient visits, compared with placebo-treated controls, although these trends failed to achieve statistical significance. And as in the EMPERIAL-Reduced trial, the SGLT2 inhibitor didn’t move the needle at all on the primary endpoint of improved exercise capacity as measured by 6-minute hall walk distance.
EMPERIAL-Reduced and -Preserved were identically designed, concurrent, phase 3, double-blind, 12-week randomized trials of empagliflozin versus placebo in 312 patients with HFrEF and 315 with HFpEF, defined in EMPERIAL-preserved as a left ventricular ejection fraction above 40%. The majority of participants had type 2 diabetes.
From a baseline median 6-minute walk distance of about 300 meters, the 6-minute walk distance at week 12 was actually 4.0 meters worse in the empagliflozin-treated HFrEF patients than it was in controls and a mere 4.0 meters better than with placebo in empagliflozin-treated patients with HFpEF, reported William T. Abraham, MD, professor of medicine, director of the division of cardiovascular medicine, and associate dean at Ohio State University, Columbus.
He indicated that the audience shouldn’t make too much of the failure to achieve the primary endpoint in the two trials in light of the studies’ major limitations: namely, their relatively small size for purposes of evaluating clinical outcomes and the relatively short 12-week duration.
“In many ways, I would say it’s remarkable that we can observe a positive signal, a favorable signal, in outcomes around congestion. In the case of HFrEF it’s statistically significant, and in HFpEF it’s a trend towards improvement. Of course, there are larger trials ongoing that may confirm these observations. Hopefully the EMPERIAL trials predict a good outcome for those ongoing trials,” Dr. Abraham said.
Piotr Ponikowski, MD, presented the study results for the secondary outcomes of congestion symptoms, diuretic use, and utilization of health care resources. In EMPERIAL-Reduced, intensification of diuretic therapy – often a prelude to acute decompensation and a trip to the hospital – occurred at a rate of 4.5% with empagliflozin and 16.1% with placebo, for a highly significant 73% relative risk reduction. Intensification of loop diuretics occurred in 2.6% of the empagliflozin group and 14.2% of controls, for a 82% risk reduction.
“That’s a pretty significant effect,” observed Dr. Ponikowski, professor of cardiology and head of the department of heart diseases at the Medical University of Wroclaw (Poland).
Moreover, a congestion symptoms score comprising a summary of orthopnea, jugular veinous distention, and edema improved by 47% after 12 weeks on empagliflozin, a statistically significant and clinically meaningful improvement that grew in magnitude over time and at 12 weeks was twice as large, compared with the reduction in placebo group, he added.
There was a trend for fewer unscheduled outpatient visits in the empagliflozin arm of EMPERIAL-Reduced with a rate of 10.4%, compared with 25.8% in controls; however, this 26% reduction in relative risk did not achieve statistical significance.
Intensification of loop diuretics occurred in 9% of EMPERIAL-Preserved participants on empagliflozin and 13.5% on placebo, but this 34% reduction in risk didn’t reach significance.
Adverse events in the EMPERIAL trials were similar across the active treatment and placebo arms. The benign safety profile was similar to what was seen in the earlier major clinical trials of empagliflozin for treatment of type 2 diabetes.
Session chair Stephane Heymans, MD, PhD, of the University of Maastricht (the Netherlands) noted that a substantial minority of patients in EMPERIAL-Reduced were on the combined neprilysin inhibitor sacubitril and the angiotensin receptor blocker valsartan (Entresto), whereas far fewer were in EMPERIAL-Preserved. He wondered if this greater use of background sacubitril/valsartan could explain empagliflozin’s greater efficacy in EMPERIAL-Reduced.
Highly unlikely, according to the investigators.
“It looks like, as is the case with most of our heart failure therapies, that we do see incremental value here. If you met the criteria for these trials, it appears you derived benefit from empagliflozin regardless of whether you were on an angiotensin receptor neprilysin inhibitor or not. I think that speaks to the incremental benefit of SGLT2 inhibitors on top of current guideline-directed medical therapy,” Dr. Abraham said.
Dr. Ponikowski observed that the same point was underscored in the DAPA-HF trial of the SGLT2 inhibitor dapagliflozin (Farxiga) in patients with heart failure (DAPA-HF: N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
“You’ll see that the mortality and morbidity and quality-of-life benefit is in those treated with dapagliflozin with or without angiotensin receptor neprilysin inhibition; so, regardless of background therapy. And the effect is especially clear in patients on both therapies,” the cardiologist said.
The EMPERIAL trials were sponsored by Boehringer Ingelheim. Dr. Abraham and Dr. Ponikowksi reported receiving consultant fees from the company for serving on the trials’ executive committee.
Empagliflozin showed favorable effects on diuretic use and congestion symptoms in patients with heart failure with reduced ejection fraction (HFrEF), but the oral sodium glucose cotransporter 2 (SGLT2) inhibitor did not improve the primary endpoint of improved exercise capacity in the EMPERIAL-Reduced trial, investigators reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
In the matching EMPERIAL-Preserved trial, conducted in patients with heart failure with preserved ejection fraction (HFpEF), empagliflozin (Jardiance) produced modest improvements in diuretic use, as well as a reduction in unscheduled outpatient visits, compared with placebo-treated controls, although these trends failed to achieve statistical significance. And as in the EMPERIAL-Reduced trial, the SGLT2 inhibitor didn’t move the needle at all on the primary endpoint of improved exercise capacity as measured by 6-minute hall walk distance.
EMPERIAL-Reduced and -Preserved were identically designed, concurrent, phase 3, double-blind, 12-week randomized trials of empagliflozin versus placebo in 312 patients with HFrEF and 315 with HFpEF, defined in EMPERIAL-preserved as a left ventricular ejection fraction above 40%. The majority of participants had type 2 diabetes.
From a baseline median 6-minute walk distance of about 300 meters, the 6-minute walk distance at week 12 was actually 4.0 meters worse in the empagliflozin-treated HFrEF patients than it was in controls and a mere 4.0 meters better than with placebo in empagliflozin-treated patients with HFpEF, reported William T. Abraham, MD, professor of medicine, director of the division of cardiovascular medicine, and associate dean at Ohio State University, Columbus.
He indicated that the audience shouldn’t make too much of the failure to achieve the primary endpoint in the two trials in light of the studies’ major limitations: namely, their relatively small size for purposes of evaluating clinical outcomes and the relatively short 12-week duration.
“In many ways, I would say it’s remarkable that we can observe a positive signal, a favorable signal, in outcomes around congestion. In the case of HFrEF it’s statistically significant, and in HFpEF it’s a trend towards improvement. Of course, there are larger trials ongoing that may confirm these observations. Hopefully the EMPERIAL trials predict a good outcome for those ongoing trials,” Dr. Abraham said.
Piotr Ponikowski, MD, presented the study results for the secondary outcomes of congestion symptoms, diuretic use, and utilization of health care resources. In EMPERIAL-Reduced, intensification of diuretic therapy – often a prelude to acute decompensation and a trip to the hospital – occurred at a rate of 4.5% with empagliflozin and 16.1% with placebo, for a highly significant 73% relative risk reduction. Intensification of loop diuretics occurred in 2.6% of the empagliflozin group and 14.2% of controls, for a 82% risk reduction.
“That’s a pretty significant effect,” observed Dr. Ponikowski, professor of cardiology and head of the department of heart diseases at the Medical University of Wroclaw (Poland).
Moreover, a congestion symptoms score comprising a summary of orthopnea, jugular veinous distention, and edema improved by 47% after 12 weeks on empagliflozin, a statistically significant and clinically meaningful improvement that grew in magnitude over time and at 12 weeks was twice as large, compared with the reduction in placebo group, he added.
There was a trend for fewer unscheduled outpatient visits in the empagliflozin arm of EMPERIAL-Reduced with a rate of 10.4%, compared with 25.8% in controls; however, this 26% reduction in relative risk did not achieve statistical significance.
Intensification of loop diuretics occurred in 9% of EMPERIAL-Preserved participants on empagliflozin and 13.5% on placebo, but this 34% reduction in risk didn’t reach significance.
Adverse events in the EMPERIAL trials were similar across the active treatment and placebo arms. The benign safety profile was similar to what was seen in the earlier major clinical trials of empagliflozin for treatment of type 2 diabetes.
Session chair Stephane Heymans, MD, PhD, of the University of Maastricht (the Netherlands) noted that a substantial minority of patients in EMPERIAL-Reduced were on the combined neprilysin inhibitor sacubitril and the angiotensin receptor blocker valsartan (Entresto), whereas far fewer were in EMPERIAL-Preserved. He wondered if this greater use of background sacubitril/valsartan could explain empagliflozin’s greater efficacy in EMPERIAL-Reduced.
Highly unlikely, according to the investigators.
“It looks like, as is the case with most of our heart failure therapies, that we do see incremental value here. If you met the criteria for these trials, it appears you derived benefit from empagliflozin regardless of whether you were on an angiotensin receptor neprilysin inhibitor or not. I think that speaks to the incremental benefit of SGLT2 inhibitors on top of current guideline-directed medical therapy,” Dr. Abraham said.
Dr. Ponikowski observed that the same point was underscored in the DAPA-HF trial of the SGLT2 inhibitor dapagliflozin (Farxiga) in patients with heart failure (DAPA-HF: N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
“You’ll see that the mortality and morbidity and quality-of-life benefit is in those treated with dapagliflozin with or without angiotensin receptor neprilysin inhibition; so, regardless of background therapy. And the effect is especially clear in patients on both therapies,” the cardiologist said.
The EMPERIAL trials were sponsored by Boehringer Ingelheim. Dr. Abraham and Dr. Ponikowksi reported receiving consultant fees from the company for serving on the trials’ executive committee.
FROM ESC HEART FAILURE 2020
Ex-nursing assistant pleads guilty in West Virginia insulin deaths
A former nursing assistant and Army veteran pleaded guilty to federal murder charges this week in connection with the 2017-2018 deaths of seven patients in a West Virginia veteran’s hospital, according to news reports.
Prosecutors said in court documents filed on July 13 that Reta Mays, 46, injected lethal doses of insulin into seven veterans at the Louis A. Johnson VA Medical Center (VAMC) in rural Clarksburg, W.Va.
Their blood glucose levels plummeted, and each died shortly after their injections, according to the Tennessean.
An eighth patient, a 92-year-old man whom Mays is accused of assaulting with an insulin injection, initially survived after staff were able to stabilize him but died 2 weeks later at a nursing home, NPR reports.
According to NPR, US Attorney Jarod Douglas told the court Tuesday that the medical investigator could not determine whether the insulin contributed to the man’s death but that it was Mays’ intention to kill him.
“No one watched while she injected them with lethal doses of insulin during an 11-month killing rampage,” the Washington Post reported.
No motive offered
The Post article said no motive has been established, but after a 2-year investigation into a pattern of suspicious deaths that took the hospital almost a year to detect, Mays, who had denied any wrongdoing in multiple interviews with investigators, told a federal judge she preyed on some of the country›s most vulnerable service members.
An attorney for Mays, Brian Kornbrath, contacted by Medscape Medical News, said: “The defense team decided that we would have no public comment at this time.”
According to court documents from the Northern District of West Virginia, Mays was charged with seven counts of second-degree murder and one count of assault with intent to commit murder in connection with the patient who died later.
Mays was hired at the VAMC in Clarksburg in June 2015. She worked from 7:30 PM to 8:00 AM in the medical surgical unit, court documents say.
According to the documents, “VAMC Clarksburg did not require a nursing assistant to have a certification or licensure for initial appointment or as a condition of continuing employment.”
The documents indicate that in June 2018, a hospitalist employed by VAMC Clarksburg reported concern about several deaths from unexplained hypoglycemic events in the same ward and noted that many of the affected patients did not have diabetes.
By that time, according to the Tennessean, “at least eight patients had died under suspicious circumstances. Several had been embalmed and buried, destroying potential evidence. One veteran had been cremated.”
An internal investigation began, followed by a criminal investigation, and in July 2018, Mays was removed from patient care.
Mays fired in 2019 because of lies on resume; claims suffers from PTSD
The Post reports that Mays was fired from the hospital in 2019, 7 months after she was banned from patient care, «after it was discovered she had lied about her qualifications on her resume.»
Court documents indicate that her duties included acting as a sitter for patients, checking vital signs, intake and output, and testing blood glucose levels, but she was not qualified to administer medications, including insulin.
Similarities in the deaths were evident, the Post reported. Citing sources familiar with the case, the report said, “elderly patients in private rooms were injected in their abdomen and limbs with insulin the hospital had not ordered.”
The Post reported that Mays sobbed by the end of the hearing on Tuesday.
The article notes that Mays has three sons and served in the Army National Guard from November 2000 to April 2001 and again from February 2003 to May 2004, when she was deployed to Iraq and Kuwait. She told the judge she was taking medication for posttraumatic stress disorder.
By pleading guilty, she waived her right to have the case presented to a grand jury. A sentencing hearing has not been scheduled, the Post reports.
NPR notes that prosecutors have requested that Mays serve seven consecutive life sentences and an additional 20 years in prison.
“Our hearts go out to those affected by these tragic deaths”
A spokesman for VAMC Clarksburg said in a statement to Medscape Medical News: “Our hearts go out to those affected by these tragic deaths. Clarksburg VA Medical Center discovered these allegations and reported them to VA›s independent inspector general more than 2 years ago. Clarksburg VA Medical Center also fired the individual at the center of the allegations.
“We’re glad the Department of Justice stepped in to push this investigation across the finish line and hopeful our court system will deliver the justice Clarksburg-area Veterans and families deserve.”
According to the Tennessean, Michael Missal, inspector general for the Department of Veteran Affairs, said the agency is investigating the hospital’s practices, “including medication management and communications among staffers.”
This article first appeared on Medscape.com.
A former nursing assistant and Army veteran pleaded guilty to federal murder charges this week in connection with the 2017-2018 deaths of seven patients in a West Virginia veteran’s hospital, according to news reports.
Prosecutors said in court documents filed on July 13 that Reta Mays, 46, injected lethal doses of insulin into seven veterans at the Louis A. Johnson VA Medical Center (VAMC) in rural Clarksburg, W.Va.
Their blood glucose levels plummeted, and each died shortly after their injections, according to the Tennessean.
An eighth patient, a 92-year-old man whom Mays is accused of assaulting with an insulin injection, initially survived after staff were able to stabilize him but died 2 weeks later at a nursing home, NPR reports.
According to NPR, US Attorney Jarod Douglas told the court Tuesday that the medical investigator could not determine whether the insulin contributed to the man’s death but that it was Mays’ intention to kill him.
“No one watched while she injected them with lethal doses of insulin during an 11-month killing rampage,” the Washington Post reported.
No motive offered
The Post article said no motive has been established, but after a 2-year investigation into a pattern of suspicious deaths that took the hospital almost a year to detect, Mays, who had denied any wrongdoing in multiple interviews with investigators, told a federal judge she preyed on some of the country›s most vulnerable service members.
An attorney for Mays, Brian Kornbrath, contacted by Medscape Medical News, said: “The defense team decided that we would have no public comment at this time.”
According to court documents from the Northern District of West Virginia, Mays was charged with seven counts of second-degree murder and one count of assault with intent to commit murder in connection with the patient who died later.
Mays was hired at the VAMC in Clarksburg in June 2015. She worked from 7:30 PM to 8:00 AM in the medical surgical unit, court documents say.
According to the documents, “VAMC Clarksburg did not require a nursing assistant to have a certification or licensure for initial appointment or as a condition of continuing employment.”
The documents indicate that in June 2018, a hospitalist employed by VAMC Clarksburg reported concern about several deaths from unexplained hypoglycemic events in the same ward and noted that many of the affected patients did not have diabetes.
By that time, according to the Tennessean, “at least eight patients had died under suspicious circumstances. Several had been embalmed and buried, destroying potential evidence. One veteran had been cremated.”
An internal investigation began, followed by a criminal investigation, and in July 2018, Mays was removed from patient care.
Mays fired in 2019 because of lies on resume; claims suffers from PTSD
The Post reports that Mays was fired from the hospital in 2019, 7 months after she was banned from patient care, «after it was discovered she had lied about her qualifications on her resume.»
Court documents indicate that her duties included acting as a sitter for patients, checking vital signs, intake and output, and testing blood glucose levels, but she was not qualified to administer medications, including insulin.
Similarities in the deaths were evident, the Post reported. Citing sources familiar with the case, the report said, “elderly patients in private rooms were injected in their abdomen and limbs with insulin the hospital had not ordered.”
The Post reported that Mays sobbed by the end of the hearing on Tuesday.
The article notes that Mays has three sons and served in the Army National Guard from November 2000 to April 2001 and again from February 2003 to May 2004, when she was deployed to Iraq and Kuwait. She told the judge she was taking medication for posttraumatic stress disorder.
By pleading guilty, she waived her right to have the case presented to a grand jury. A sentencing hearing has not been scheduled, the Post reports.
NPR notes that prosecutors have requested that Mays serve seven consecutive life sentences and an additional 20 years in prison.
“Our hearts go out to those affected by these tragic deaths”
A spokesman for VAMC Clarksburg said in a statement to Medscape Medical News: “Our hearts go out to those affected by these tragic deaths. Clarksburg VA Medical Center discovered these allegations and reported them to VA›s independent inspector general more than 2 years ago. Clarksburg VA Medical Center also fired the individual at the center of the allegations.
“We’re glad the Department of Justice stepped in to push this investigation across the finish line and hopeful our court system will deliver the justice Clarksburg-area Veterans and families deserve.”
According to the Tennessean, Michael Missal, inspector general for the Department of Veteran Affairs, said the agency is investigating the hospital’s practices, “including medication management and communications among staffers.”
This article first appeared on Medscape.com.
A former nursing assistant and Army veteran pleaded guilty to federal murder charges this week in connection with the 2017-2018 deaths of seven patients in a West Virginia veteran’s hospital, according to news reports.
Prosecutors said in court documents filed on July 13 that Reta Mays, 46, injected lethal doses of insulin into seven veterans at the Louis A. Johnson VA Medical Center (VAMC) in rural Clarksburg, W.Va.
Their blood glucose levels plummeted, and each died shortly after their injections, according to the Tennessean.
An eighth patient, a 92-year-old man whom Mays is accused of assaulting with an insulin injection, initially survived after staff were able to stabilize him but died 2 weeks later at a nursing home, NPR reports.
According to NPR, US Attorney Jarod Douglas told the court Tuesday that the medical investigator could not determine whether the insulin contributed to the man’s death but that it was Mays’ intention to kill him.
“No one watched while she injected them with lethal doses of insulin during an 11-month killing rampage,” the Washington Post reported.
No motive offered
The Post article said no motive has been established, but after a 2-year investigation into a pattern of suspicious deaths that took the hospital almost a year to detect, Mays, who had denied any wrongdoing in multiple interviews with investigators, told a federal judge she preyed on some of the country›s most vulnerable service members.
An attorney for Mays, Brian Kornbrath, contacted by Medscape Medical News, said: “The defense team decided that we would have no public comment at this time.”
According to court documents from the Northern District of West Virginia, Mays was charged with seven counts of second-degree murder and one count of assault with intent to commit murder in connection with the patient who died later.
Mays was hired at the VAMC in Clarksburg in June 2015. She worked from 7:30 PM to 8:00 AM in the medical surgical unit, court documents say.
According to the documents, “VAMC Clarksburg did not require a nursing assistant to have a certification or licensure for initial appointment or as a condition of continuing employment.”
The documents indicate that in June 2018, a hospitalist employed by VAMC Clarksburg reported concern about several deaths from unexplained hypoglycemic events in the same ward and noted that many of the affected patients did not have diabetes.
By that time, according to the Tennessean, “at least eight patients had died under suspicious circumstances. Several had been embalmed and buried, destroying potential evidence. One veteran had been cremated.”
An internal investigation began, followed by a criminal investigation, and in July 2018, Mays was removed from patient care.
Mays fired in 2019 because of lies on resume; claims suffers from PTSD
The Post reports that Mays was fired from the hospital in 2019, 7 months after she was banned from patient care, «after it was discovered she had lied about her qualifications on her resume.»
Court documents indicate that her duties included acting as a sitter for patients, checking vital signs, intake and output, and testing blood glucose levels, but she was not qualified to administer medications, including insulin.
Similarities in the deaths were evident, the Post reported. Citing sources familiar with the case, the report said, “elderly patients in private rooms were injected in their abdomen and limbs with insulin the hospital had not ordered.”
The Post reported that Mays sobbed by the end of the hearing on Tuesday.
The article notes that Mays has three sons and served in the Army National Guard from November 2000 to April 2001 and again from February 2003 to May 2004, when she was deployed to Iraq and Kuwait. She told the judge she was taking medication for posttraumatic stress disorder.
By pleading guilty, she waived her right to have the case presented to a grand jury. A sentencing hearing has not been scheduled, the Post reports.
NPR notes that prosecutors have requested that Mays serve seven consecutive life sentences and an additional 20 years in prison.
“Our hearts go out to those affected by these tragic deaths”
A spokesman for VAMC Clarksburg said in a statement to Medscape Medical News: “Our hearts go out to those affected by these tragic deaths. Clarksburg VA Medical Center discovered these allegations and reported them to VA›s independent inspector general more than 2 years ago. Clarksburg VA Medical Center also fired the individual at the center of the allegations.
“We’re glad the Department of Justice stepped in to push this investigation across the finish line and hopeful our court system will deliver the justice Clarksburg-area Veterans and families deserve.”
According to the Tennessean, Michael Missal, inspector general for the Department of Veteran Affairs, said the agency is investigating the hospital’s practices, “including medication management and communications among staffers.”
This article first appeared on Medscape.com.
Chester Good, MD, MPH, on Value-Based Contracting in Multiple Sclerosis
Chester Good, MD, MPH, is professor of medicine and pharmacy, University of Pittsburgh School of Medicine and School of Pharmacy. He is also the senior medical director, UPMC Health Plan Insurance Division, as well as the director for the Center for Value-Based Pharmacy Initiatives.
What are the potential benefits of value-based contracting for patients with multiple sclerosis, as well as for health systems and payers?
CHESTER GOOD, MD, MPH: I think for all 3, value-based contracting really is about moving from volume to value. And in doing so, what we're trying to do is recognize the value of pharmaceuticals perhaps in ways that we previously have not.
Patients with conditions such as multiple sclerosis (MS) want to be able to live their lives where they can continue to work, maintain their activities of daily living, and maintain their quality of life. Value-based contracting ideally recognizes those patient values and leverages contracting of pharmaceuticals that have been shown to help maintain those qualities in patients. For health systems, our greatest duty is to our patients in terms of maintaining their quality of life, maintaining their health, and preventing disease progression. By developing value-based contracts in which we recognize these measurable outcomes, we're showing a commitment to our patients and trying to hopefully establish the value of our pharmacy benefit.
For most health plans and payers, MS especially has become an increasingly expensive disease, with MS-related medications representing a significant cost burden. It's no secret that MS drugs have gone up significantly in recent years.
In a paper published in JAMA Neurology,1 we looked at the costs of these self-administered disease-modifying therapies for MS. From 2006 to 2016, the costs more than quadrupled, from a mean of $18,660 a year to more than $75,000, which means they increased at an annual rate of almost 13%. For payers, pharmaceutical spending per 1000 beneficiaries increased tenfold; and out-of-pocket expenses to our patients also increased significantly.
So, that's the reality. But the other reality is that there have been significant advancements in the treatment of MS using pharmaceuticals. And some of these newer treatments are more effective. Moreover, some are easier to take than some of the older therapies, and some are better tolerated from a patient perspective.
As a result of dramatic increase in costs of MS drugs, payers are asking about what they are getting in return for all these dramatic increases in costs. One of the ways to demonstrate that value is to link paying for these drugs with a value-based contract.
Why is a disease such as MS a good fit for value-based contracting?
CHESTER GOOD, MD, MPH: MS is a fairly prevalent disease. Fortunately, pharmacotherapy has gotten better over the years, to the point where it's clear that the pharmacotherapy of MS has really changed the course of the disease for our patients, allowing them to maintain their quality of life and functional abilities.
On a personal level, I had a family member growing up who had MS. And she did not have the benefits of the newer drugs. And through my childhood and early adulthood, I watched as she went from a very productive working person to someone who could no longer work, was unable to take care of her children, and was institutionalized and eventually died from complications of MS. That would be unusual today.
In a value-based contract, what MS outcomes are linked to reimbursement, and how are they measured?
CHESTER GOOD, MD, MPH: Traditionally, outcomes-based agreements focused on things that would be easily measured through administrative data. These are things that either a drug manufacturer or insurer, or both, have identified as being what they think are important outcomes for that disease state, but also things that they think they would be able to measure. Oftentimes, these were intermediate outcomes. Did the patient remain on a drug? Were they adherent? What was the impact on hemoglobin A1c in patients with diabetes? Although these outcomes may be important, they may not be as important to our patients.
So for MS, I would argue that it's really important to try to expand on this idea of which MS-specific outcomes are meaningful to our patients, and then try to link them to reimbursement. One of the things that we do at the University of Pittsburgh Medical Center (UPMC) is survey our stakeholders, and we ask what outcomes are important, using the Delphi method. Our most important stakeholder, of course, is our patient. But we also survey the physicians who care for these patients, other clinicians who care for these patients, our pharmacists, pharmacy benefit managers, our industry partners, and sometimes other stakeholders such as employers.
Sometimes, the things that are important to the patients are easily measured. It may be that patients with heart disease don't want to have another heart attack, and typically with a heart attack you end up going into the hospital. In the case of other disease states, however, it may be that what's most important to our patients differs from what we think is most important. We don't make any assumptions. We measure what our patients tell us.
In the case of MS, before we entered into a value-based contract, we did a Delphi survey including patients, physicians, industry partners, payers, and our pharmacy benefits manager.2 We did several rounds of surveys, and in the second round we had our stakeholders rank outcomes. What we found was that the most important outcome was “worsening physical disability.” One hundred percent of our stakeholders—not just our patients, but everyone—ranked worsening physical disability as important.
I would've thought that patients would've said, “Well, I don't want an MS flare.” And we could argue that MS flares may result in worsening physical disability, but not always. When we asked what would be the easiest thing to measure, of course MS flares are the easiest thing, because they require an emergency department visit or use of corticosteroids or hospitalization.
Based on our survey, we picked disability progression as our outcome of interest and the basis for our value-based contract. We entered into a contract with Biogen for a couple of their drugs, dimethyl fumarate (Tecfidera) and interferon beta-1a (Avonex), 2 very important MS drugs. This is a classic patient-reported outcome, and of course that poses a lot of interesting challenges about how you actually measure it. So, we had to work with our subject matter experts in the clinics to identify and externally validate a patient-reported outcome that measured disability progression. We worked very hard so that we’re able to collect that in a way that doesn’t inconvenience our physicians or our patients.
There are other tools that can be used, one of which is the PROMIS tool developed through the National Institutes of Health (NIH). That's a way to quantify changes in patient-reported outcomes. The interesting thing is NIH specifically developed this tool with certain disease states in mind, one of which was MS. So, some of the PROMIS domains include pain, fatigue, physical functioning, emotional distress, cognitive functioning, and social functioning. And all these things could be very important to our MS patients.
We actually do use PROMIS measures and PROMIS questionnaires routinely with our patients who come to our neurology clinic. It's not part of our value-based contracts at this point, but it's something that is a potentially interesting way to measure patient-reported outcomes.
What are the key challenges to implementing a value-based contract?
CHESTER GOOD, MD, MPH: Value-based contracting has been far more laborious than I ever imagined. These are legal documents that are very long and complex, and quite daunting. We've tried our best to incorporate figures and diagrams to simplify issues and ensure that we all are understanding things similarly. But at the end of the day, despite our best efforts, these contracts remain very complex, and questions and unexpected situations develop as we implement them. So, that's been one of the challenges.
The pandemic has also posed some challenges. It has affected everything. That includes fewer patient clinic encounters, which is a challenge when some of our value-based contracts require vital signs, lab work, or other things dependent on patient visits. There are also fewer patients presenting with disease states. It’s been well documented that hospitalizations and patient visits to the emergency departments for various conditions have really plummeted in the face of the coronavirus. So, the pandemic is a clear confounder as we try to identify the value of these pharmaceuticals and how they've impacted care. That's been a huge challenge for us.
Another thing to mention is that we’ve tried to be very innovative in our value-based outcomes. But it's been very challenging to do the analyses. What seems to be clear in a contract may not be clear once you start to analyze things, as there are all sorts of scenarios that one cannot anticipate. That has required going back and forth with our industry partners. Fortunately, we have good working relationships with them, and we've been able to work out those wrinkles; but that's been a challenge.
I mentioned the importance of patient-reported outcomes, but they are a challenge to measure. How do you collect patient-reported outcomes in a way that does not pose a burden on our patients or clinicians?
Finally, a significant challenge for health care organizations and insurers that hope to do value-based contracting is that drug companies do not typically place a lot of risk on the table. While we've been very pleased with our value-based contracts thus far, in the future we hope to see greater risk in these contracts, especially for areas such as gene therapy.
What are the next steps in value-based contracting for MS?
CHESTER GOOD, MD, MPH: Multiple sclerosis can be a progressive disease, and it's over a lifetime. It can affect people's quality of life. It can affect their ability to work. It can affect the quality of their work and what sort of things that they can do.
Because MS can affect every aspect of life, it's not enough to simply measure things like disease flares or MRI lesions. Rather, we need to figure out ways to understand how these drugs impact our patients’ day-to-day lives, the quality of their lives, and their ability to function as normally as possible. I think these other patient-reported outcomes would represent important next steps in MS outcomes measurement. Our current value-based contract has 1 simple, albeit very important, outcome that we're looking at, but are there other ways to measure patients’ quality of life? Perhaps this will involve using PROMIS measures or other innovative ways to measure patient outcomes. We’ve discussed with some patient interest groups how to incorporate other measures that are very important to our patients.
From a payer perspective, it may not show up in terms of impacting total cost of care. Depending on the disease states, oftentimes if things progress slowly over years, it is difficult to demonstrate an impact. But it's not all only about decreasing our costs because we decrease hospitalizations; it’s also about improving the quality of our patients’ lives.
The next step for us at UPMC is to continue to gather our data and to analyze our outcomes. And based on that, we're hoping to expand our portfolio of drugs for the MS disease state and expand outcomes that we're measuring.
1. San-Juan-Rodriguez A, Good CB, Heyman RA, et al. Trends in prices, market share, and spending on self-administered disease-modifying therapies for multiple sclerosis in Medicare Part D. JAMA Neurol. 2019;76:1386-1390.
2. Swart ECS, Neilson LM, Good CB, et al. Determination of multiple sclerosis indicators for value-based contracting using the Delphi method. J Manag Care Spec Pharm. 2019;25:753-760.
Chester Good, MD, MPH, is professor of medicine and pharmacy, University of Pittsburgh School of Medicine and School of Pharmacy. He is also the senior medical director, UPMC Health Plan Insurance Division, as well as the director for the Center for Value-Based Pharmacy Initiatives.
What are the potential benefits of value-based contracting for patients with multiple sclerosis, as well as for health systems and payers?
CHESTER GOOD, MD, MPH: I think for all 3, value-based contracting really is about moving from volume to value. And in doing so, what we're trying to do is recognize the value of pharmaceuticals perhaps in ways that we previously have not.
Patients with conditions such as multiple sclerosis (MS) want to be able to live their lives where they can continue to work, maintain their activities of daily living, and maintain their quality of life. Value-based contracting ideally recognizes those patient values and leverages contracting of pharmaceuticals that have been shown to help maintain those qualities in patients. For health systems, our greatest duty is to our patients in terms of maintaining their quality of life, maintaining their health, and preventing disease progression. By developing value-based contracts in which we recognize these measurable outcomes, we're showing a commitment to our patients and trying to hopefully establish the value of our pharmacy benefit.
For most health plans and payers, MS especially has become an increasingly expensive disease, with MS-related medications representing a significant cost burden. It's no secret that MS drugs have gone up significantly in recent years.
In a paper published in JAMA Neurology,1 we looked at the costs of these self-administered disease-modifying therapies for MS. From 2006 to 2016, the costs more than quadrupled, from a mean of $18,660 a year to more than $75,000, which means they increased at an annual rate of almost 13%. For payers, pharmaceutical spending per 1000 beneficiaries increased tenfold; and out-of-pocket expenses to our patients also increased significantly.
So, that's the reality. But the other reality is that there have been significant advancements in the treatment of MS using pharmaceuticals. And some of these newer treatments are more effective. Moreover, some are easier to take than some of the older therapies, and some are better tolerated from a patient perspective.
As a result of dramatic increase in costs of MS drugs, payers are asking about what they are getting in return for all these dramatic increases in costs. One of the ways to demonstrate that value is to link paying for these drugs with a value-based contract.
Why is a disease such as MS a good fit for value-based contracting?
CHESTER GOOD, MD, MPH: MS is a fairly prevalent disease. Fortunately, pharmacotherapy has gotten better over the years, to the point where it's clear that the pharmacotherapy of MS has really changed the course of the disease for our patients, allowing them to maintain their quality of life and functional abilities.
On a personal level, I had a family member growing up who had MS. And she did not have the benefits of the newer drugs. And through my childhood and early adulthood, I watched as she went from a very productive working person to someone who could no longer work, was unable to take care of her children, and was institutionalized and eventually died from complications of MS. That would be unusual today.
In a value-based contract, what MS outcomes are linked to reimbursement, and how are they measured?
CHESTER GOOD, MD, MPH: Traditionally, outcomes-based agreements focused on things that would be easily measured through administrative data. These are things that either a drug manufacturer or insurer, or both, have identified as being what they think are important outcomes for that disease state, but also things that they think they would be able to measure. Oftentimes, these were intermediate outcomes. Did the patient remain on a drug? Were they adherent? What was the impact on hemoglobin A1c in patients with diabetes? Although these outcomes may be important, they may not be as important to our patients.
So for MS, I would argue that it's really important to try to expand on this idea of which MS-specific outcomes are meaningful to our patients, and then try to link them to reimbursement. One of the things that we do at the University of Pittsburgh Medical Center (UPMC) is survey our stakeholders, and we ask what outcomes are important, using the Delphi method. Our most important stakeholder, of course, is our patient. But we also survey the physicians who care for these patients, other clinicians who care for these patients, our pharmacists, pharmacy benefit managers, our industry partners, and sometimes other stakeholders such as employers.
Sometimes, the things that are important to the patients are easily measured. It may be that patients with heart disease don't want to have another heart attack, and typically with a heart attack you end up going into the hospital. In the case of other disease states, however, it may be that what's most important to our patients differs from what we think is most important. We don't make any assumptions. We measure what our patients tell us.
In the case of MS, before we entered into a value-based contract, we did a Delphi survey including patients, physicians, industry partners, payers, and our pharmacy benefits manager.2 We did several rounds of surveys, and in the second round we had our stakeholders rank outcomes. What we found was that the most important outcome was “worsening physical disability.” One hundred percent of our stakeholders—not just our patients, but everyone—ranked worsening physical disability as important.
I would've thought that patients would've said, “Well, I don't want an MS flare.” And we could argue that MS flares may result in worsening physical disability, but not always. When we asked what would be the easiest thing to measure, of course MS flares are the easiest thing, because they require an emergency department visit or use of corticosteroids or hospitalization.
Based on our survey, we picked disability progression as our outcome of interest and the basis for our value-based contract. We entered into a contract with Biogen for a couple of their drugs, dimethyl fumarate (Tecfidera) and interferon beta-1a (Avonex), 2 very important MS drugs. This is a classic patient-reported outcome, and of course that poses a lot of interesting challenges about how you actually measure it. So, we had to work with our subject matter experts in the clinics to identify and externally validate a patient-reported outcome that measured disability progression. We worked very hard so that we’re able to collect that in a way that doesn’t inconvenience our physicians or our patients.
There are other tools that can be used, one of which is the PROMIS tool developed through the National Institutes of Health (NIH). That's a way to quantify changes in patient-reported outcomes. The interesting thing is NIH specifically developed this tool with certain disease states in mind, one of which was MS. So, some of the PROMIS domains include pain, fatigue, physical functioning, emotional distress, cognitive functioning, and social functioning. And all these things could be very important to our MS patients.
We actually do use PROMIS measures and PROMIS questionnaires routinely with our patients who come to our neurology clinic. It's not part of our value-based contracts at this point, but it's something that is a potentially interesting way to measure patient-reported outcomes.
What are the key challenges to implementing a value-based contract?
CHESTER GOOD, MD, MPH: Value-based contracting has been far more laborious than I ever imagined. These are legal documents that are very long and complex, and quite daunting. We've tried our best to incorporate figures and diagrams to simplify issues and ensure that we all are understanding things similarly. But at the end of the day, despite our best efforts, these contracts remain very complex, and questions and unexpected situations develop as we implement them. So, that's been one of the challenges.
The pandemic has also posed some challenges. It has affected everything. That includes fewer patient clinic encounters, which is a challenge when some of our value-based contracts require vital signs, lab work, or other things dependent on patient visits. There are also fewer patients presenting with disease states. It’s been well documented that hospitalizations and patient visits to the emergency departments for various conditions have really plummeted in the face of the coronavirus. So, the pandemic is a clear confounder as we try to identify the value of these pharmaceuticals and how they've impacted care. That's been a huge challenge for us.
Another thing to mention is that we’ve tried to be very innovative in our value-based outcomes. But it's been very challenging to do the analyses. What seems to be clear in a contract may not be clear once you start to analyze things, as there are all sorts of scenarios that one cannot anticipate. That has required going back and forth with our industry partners. Fortunately, we have good working relationships with them, and we've been able to work out those wrinkles; but that's been a challenge.
I mentioned the importance of patient-reported outcomes, but they are a challenge to measure. How do you collect patient-reported outcomes in a way that does not pose a burden on our patients or clinicians?
Finally, a significant challenge for health care organizations and insurers that hope to do value-based contracting is that drug companies do not typically place a lot of risk on the table. While we've been very pleased with our value-based contracts thus far, in the future we hope to see greater risk in these contracts, especially for areas such as gene therapy.
What are the next steps in value-based contracting for MS?
CHESTER GOOD, MD, MPH: Multiple sclerosis can be a progressive disease, and it's over a lifetime. It can affect people's quality of life. It can affect their ability to work. It can affect the quality of their work and what sort of things that they can do.
Because MS can affect every aspect of life, it's not enough to simply measure things like disease flares or MRI lesions. Rather, we need to figure out ways to understand how these drugs impact our patients’ day-to-day lives, the quality of their lives, and their ability to function as normally as possible. I think these other patient-reported outcomes would represent important next steps in MS outcomes measurement. Our current value-based contract has 1 simple, albeit very important, outcome that we're looking at, but are there other ways to measure patients’ quality of life? Perhaps this will involve using PROMIS measures or other innovative ways to measure patient outcomes. We’ve discussed with some patient interest groups how to incorporate other measures that are very important to our patients.
From a payer perspective, it may not show up in terms of impacting total cost of care. Depending on the disease states, oftentimes if things progress slowly over years, it is difficult to demonstrate an impact. But it's not all only about decreasing our costs because we decrease hospitalizations; it’s also about improving the quality of our patients’ lives.
The next step for us at UPMC is to continue to gather our data and to analyze our outcomes. And based on that, we're hoping to expand our portfolio of drugs for the MS disease state and expand outcomes that we're measuring.
Chester Good, MD, MPH, is professor of medicine and pharmacy, University of Pittsburgh School of Medicine and School of Pharmacy. He is also the senior medical director, UPMC Health Plan Insurance Division, as well as the director for the Center for Value-Based Pharmacy Initiatives.
What are the potential benefits of value-based contracting for patients with multiple sclerosis, as well as for health systems and payers?
CHESTER GOOD, MD, MPH: I think for all 3, value-based contracting really is about moving from volume to value. And in doing so, what we're trying to do is recognize the value of pharmaceuticals perhaps in ways that we previously have not.
Patients with conditions such as multiple sclerosis (MS) want to be able to live their lives where they can continue to work, maintain their activities of daily living, and maintain their quality of life. Value-based contracting ideally recognizes those patient values and leverages contracting of pharmaceuticals that have been shown to help maintain those qualities in patients. For health systems, our greatest duty is to our patients in terms of maintaining their quality of life, maintaining their health, and preventing disease progression. By developing value-based contracts in which we recognize these measurable outcomes, we're showing a commitment to our patients and trying to hopefully establish the value of our pharmacy benefit.
For most health plans and payers, MS especially has become an increasingly expensive disease, with MS-related medications representing a significant cost burden. It's no secret that MS drugs have gone up significantly in recent years.
In a paper published in JAMA Neurology,1 we looked at the costs of these self-administered disease-modifying therapies for MS. From 2006 to 2016, the costs more than quadrupled, from a mean of $18,660 a year to more than $75,000, which means they increased at an annual rate of almost 13%. For payers, pharmaceutical spending per 1000 beneficiaries increased tenfold; and out-of-pocket expenses to our patients also increased significantly.
So, that's the reality. But the other reality is that there have been significant advancements in the treatment of MS using pharmaceuticals. And some of these newer treatments are more effective. Moreover, some are easier to take than some of the older therapies, and some are better tolerated from a patient perspective.
As a result of dramatic increase in costs of MS drugs, payers are asking about what they are getting in return for all these dramatic increases in costs. One of the ways to demonstrate that value is to link paying for these drugs with a value-based contract.
Why is a disease such as MS a good fit for value-based contracting?
CHESTER GOOD, MD, MPH: MS is a fairly prevalent disease. Fortunately, pharmacotherapy has gotten better over the years, to the point where it's clear that the pharmacotherapy of MS has really changed the course of the disease for our patients, allowing them to maintain their quality of life and functional abilities.
On a personal level, I had a family member growing up who had MS. And she did not have the benefits of the newer drugs. And through my childhood and early adulthood, I watched as she went from a very productive working person to someone who could no longer work, was unable to take care of her children, and was institutionalized and eventually died from complications of MS. That would be unusual today.
In a value-based contract, what MS outcomes are linked to reimbursement, and how are they measured?
CHESTER GOOD, MD, MPH: Traditionally, outcomes-based agreements focused on things that would be easily measured through administrative data. These are things that either a drug manufacturer or insurer, or both, have identified as being what they think are important outcomes for that disease state, but also things that they think they would be able to measure. Oftentimes, these were intermediate outcomes. Did the patient remain on a drug? Were they adherent? What was the impact on hemoglobin A1c in patients with diabetes? Although these outcomes may be important, they may not be as important to our patients.
So for MS, I would argue that it's really important to try to expand on this idea of which MS-specific outcomes are meaningful to our patients, and then try to link them to reimbursement. One of the things that we do at the University of Pittsburgh Medical Center (UPMC) is survey our stakeholders, and we ask what outcomes are important, using the Delphi method. Our most important stakeholder, of course, is our patient. But we also survey the physicians who care for these patients, other clinicians who care for these patients, our pharmacists, pharmacy benefit managers, our industry partners, and sometimes other stakeholders such as employers.
Sometimes, the things that are important to the patients are easily measured. It may be that patients with heart disease don't want to have another heart attack, and typically with a heart attack you end up going into the hospital. In the case of other disease states, however, it may be that what's most important to our patients differs from what we think is most important. We don't make any assumptions. We measure what our patients tell us.
In the case of MS, before we entered into a value-based contract, we did a Delphi survey including patients, physicians, industry partners, payers, and our pharmacy benefits manager.2 We did several rounds of surveys, and in the second round we had our stakeholders rank outcomes. What we found was that the most important outcome was “worsening physical disability.” One hundred percent of our stakeholders—not just our patients, but everyone—ranked worsening physical disability as important.
I would've thought that patients would've said, “Well, I don't want an MS flare.” And we could argue that MS flares may result in worsening physical disability, but not always. When we asked what would be the easiest thing to measure, of course MS flares are the easiest thing, because they require an emergency department visit or use of corticosteroids or hospitalization.
Based on our survey, we picked disability progression as our outcome of interest and the basis for our value-based contract. We entered into a contract with Biogen for a couple of their drugs, dimethyl fumarate (Tecfidera) and interferon beta-1a (Avonex), 2 very important MS drugs. This is a classic patient-reported outcome, and of course that poses a lot of interesting challenges about how you actually measure it. So, we had to work with our subject matter experts in the clinics to identify and externally validate a patient-reported outcome that measured disability progression. We worked very hard so that we’re able to collect that in a way that doesn’t inconvenience our physicians or our patients.
There are other tools that can be used, one of which is the PROMIS tool developed through the National Institutes of Health (NIH). That's a way to quantify changes in patient-reported outcomes. The interesting thing is NIH specifically developed this tool with certain disease states in mind, one of which was MS. So, some of the PROMIS domains include pain, fatigue, physical functioning, emotional distress, cognitive functioning, and social functioning. And all these things could be very important to our MS patients.
We actually do use PROMIS measures and PROMIS questionnaires routinely with our patients who come to our neurology clinic. It's not part of our value-based contracts at this point, but it's something that is a potentially interesting way to measure patient-reported outcomes.
What are the key challenges to implementing a value-based contract?
CHESTER GOOD, MD, MPH: Value-based contracting has been far more laborious than I ever imagined. These are legal documents that are very long and complex, and quite daunting. We've tried our best to incorporate figures and diagrams to simplify issues and ensure that we all are understanding things similarly. But at the end of the day, despite our best efforts, these contracts remain very complex, and questions and unexpected situations develop as we implement them. So, that's been one of the challenges.
The pandemic has also posed some challenges. It has affected everything. That includes fewer patient clinic encounters, which is a challenge when some of our value-based contracts require vital signs, lab work, or other things dependent on patient visits. There are also fewer patients presenting with disease states. It’s been well documented that hospitalizations and patient visits to the emergency departments for various conditions have really plummeted in the face of the coronavirus. So, the pandemic is a clear confounder as we try to identify the value of these pharmaceuticals and how they've impacted care. That's been a huge challenge for us.
Another thing to mention is that we’ve tried to be very innovative in our value-based outcomes. But it's been very challenging to do the analyses. What seems to be clear in a contract may not be clear once you start to analyze things, as there are all sorts of scenarios that one cannot anticipate. That has required going back and forth with our industry partners. Fortunately, we have good working relationships with them, and we've been able to work out those wrinkles; but that's been a challenge.
I mentioned the importance of patient-reported outcomes, but they are a challenge to measure. How do you collect patient-reported outcomes in a way that does not pose a burden on our patients or clinicians?
Finally, a significant challenge for health care organizations and insurers that hope to do value-based contracting is that drug companies do not typically place a lot of risk on the table. While we've been very pleased with our value-based contracts thus far, in the future we hope to see greater risk in these contracts, especially for areas such as gene therapy.
What are the next steps in value-based contracting for MS?
CHESTER GOOD, MD, MPH: Multiple sclerosis can be a progressive disease, and it's over a lifetime. It can affect people's quality of life. It can affect their ability to work. It can affect the quality of their work and what sort of things that they can do.
Because MS can affect every aspect of life, it's not enough to simply measure things like disease flares or MRI lesions. Rather, we need to figure out ways to understand how these drugs impact our patients’ day-to-day lives, the quality of their lives, and their ability to function as normally as possible. I think these other patient-reported outcomes would represent important next steps in MS outcomes measurement. Our current value-based contract has 1 simple, albeit very important, outcome that we're looking at, but are there other ways to measure patients’ quality of life? Perhaps this will involve using PROMIS measures or other innovative ways to measure patient outcomes. We’ve discussed with some patient interest groups how to incorporate other measures that are very important to our patients.
From a payer perspective, it may not show up in terms of impacting total cost of care. Depending on the disease states, oftentimes if things progress slowly over years, it is difficult to demonstrate an impact. But it's not all only about decreasing our costs because we decrease hospitalizations; it’s also about improving the quality of our patients’ lives.
The next step for us at UPMC is to continue to gather our data and to analyze our outcomes. And based on that, we're hoping to expand our portfolio of drugs for the MS disease state and expand outcomes that we're measuring.
1. San-Juan-Rodriguez A, Good CB, Heyman RA, et al. Trends in prices, market share, and spending on self-administered disease-modifying therapies for multiple sclerosis in Medicare Part D. JAMA Neurol. 2019;76:1386-1390.
2. Swart ECS, Neilson LM, Good CB, et al. Determination of multiple sclerosis indicators for value-based contracting using the Delphi method. J Manag Care Spec Pharm. 2019;25:753-760.
1. San-Juan-Rodriguez A, Good CB, Heyman RA, et al. Trends in prices, market share, and spending on self-administered disease-modifying therapies for multiple sclerosis in Medicare Part D. JAMA Neurol. 2019;76:1386-1390.
2. Swart ECS, Neilson LM, Good CB, et al. Determination of multiple sclerosis indicators for value-based contracting using the Delphi method. J Manag Care Spec Pharm. 2019;25:753-760.
‘Defund the police’: An important moment for society and psychiatry
Over the past months, society has reflected on the role of law enforcement. The shocking murder of George Floyd has forced Americans to reconsider the place of police officers in maintaining order.
The death of Mr. Floyd is certainly not a lone incident; in 2019, 1,098 people were killed by those tasked with protecting us.1 The United States holds 25% of the world’s incarcerated, though it makes up only 5% of the world’s population.2 Society is demanding a newer and better system.
The phrase “defund the police” can easily be dismissed because, to many, it implies an appeal to lawlessness. While we certainly cannot speak for any one protester, we think that many of the necessary changes are painfully obvious.3 Society wants law enforcement where force is not the default position but the last option. Society wants law enforcement where verbal conflict resolution is the primary focus of training and intervention. Society wants a correctional system that is more rehabilitative than it is punitive.4
Major U.S. cities spend up to 40% of their funds on police budgeting, much more than what is dedicated to community resources and infrastructure. This trend continues to increase between 1986 and 2013, state spending for correctional facilities increased by 141%.5 Yet, as psychiatrists, we are well aware that social determinants are a strong factor in future criminality.6 Increasing police budgets without addressing structural root causes and risk factors for future asocial behavior is not a wise approach to reducing unlawful behavior. Investing more into programs and policies that reduce these risks is essential.
Using the adverse childhood experiences (ACE) questionnaires, researchers have supported the idea that social programs are a key player in an improved criminal system. The ACE study identified 10 forms of childhood trauma in 17,000 patients, including abuse, neglect, abandonment, household dysfunction, and exposure to violence, that were strongly associated with negative psychological outcomes, engagement in high-risk behaviors, significant medical consequences, and even early death.7 More recent research has shown that those ACEs were four times more prevalent in a criminal offender group than in the general population.8 Psychiatry is in a unique position to address and provide education about ACEs as a tool to identify and help at-risk youths.
Many protesters have asked for mental health providers to have a primary role in this societal reflection and in providing a solution.9 This makes particular sense when considering that almost 20% of calls to law enforcement are for persons with impaired judgment from mental illness or intoxication, and one in four patients with mental illness has been arrested.10,11 We are humbled by this public trust and request. We believe psychiatry can provide many answers to this societal angst. After all, psychiatry is a specialty dedicated to addressing behavioral problems in an evidence-based way.
Yet, we should not forget psychiatry’s imperfect past and our own role in the creation of this system. While this article does not attempt to catalog psychiatry’s faults, one can start by recognizing that mass incarceration is partly a response to how poorly human beings were treated in asylums. Psychiatry was at one time a main enforcer of societal disenfranchisement. After most asylums were closed in 1963 with the Community Mental Health Act, correctional facilities became the largest purveyors of mental health care, often with damaging results.12 If psychiatry were to advocate for the reestablishment of asylums as a solution, we fear that psychiatry would have missed the point. We wonder whether the psychiatrists who have railed against deinstitutionalization since the 1970s do not realize that violence, unethical experimentation, and even racism were at times attributes of asylums.13
Psychiatry can and should be much more than what it once was. Instead of indirectly and inaccurately suggesting that our patients commit mass murders, we should improve research in the field of violence risk assessment and management. As many have already pointed out, violence risk assessment is permeated with overestimation of its potential and, more concerningly, tainted by evidence of implicit racism.14 Implicit racism extends to rights-limiting treatments as well. As previously studied, involuntary outpatient programs often referred to as assisted outpatient treatment are disproportionately levied on Black Americans.15 Instead of routinely seeking to expand abilities to involuntary treat and limit the rights of our patients, we should strive to be a violence-free alternative to law enforcement, not the medical version of police.
Psychiatrists should start actively training, practicing, and researching how to address nonviolent emergency calls. Training should include more robust deescalation training, techniques on the evaluation of patients outside of health care facilities (for example, the street), and a broadening of interventions to include proficiency in the treatment of subclinical populations seeking emergency care without the need to be formally labeled with a psychiatric disorder. Ride-alongs with police officers, volunteering at crisis hotlines, and home calls should not be volunteer or elective experiences for psychiatrists but a required part of training.
Thankfully, some local jurisdictions already have started promising practices that merit replication or at least academic review. Austin, Tex., recently implemented the capability of requesting mental health emergency calls when contacting 911.17 Eugene, Ore., has had the CAHOOTS (Crisis Assistance Helping Out On The Streets) program since 1989, where a medical provider and a mental health provider respond to calls without any law enforcement officers.18 Our own San Diego County has an innovative PERT (Psychiatric Emergency Response Team) program, which partners a mental health provider to a police patrol, allowing an ability to quickly provide different types of services.19 Programs like these show us what is possible. At this time, there is little research to evaluate many programs’ effectiveness.20 Psychiatry should seize this moment to be at the forefront of studying, then educating the public on what works and how to reproduce it.
Police officers have a difficult profession. They are tasked with preventing and predicting crime, often to the point of risking their own lives. Historically, police have been the first call to handle issues for which they are not equipped, ranging from fixing homelessness to arresting violent people using nonviolent means. The idea that police should be able to protect us in all situations has been mistakenly ingrained in our minds. Officers themselves do not feel adequately trained to handle mental health crises.21 “Defund the police” also means a recognition by governments, the public, and police themselves that officers should not be on the front lines for every emergency situation. We must diversify our first responders. Psychiatry should hear this call and be ready.
Since the death of Mr. Floyd, mental health professionals have attempted to voice empathy and warmth to those feeling left out and disenfranchised. Mental health professionals have voiced a desire to educate themselves on systemic biases and antiracism. However, we argue that psychiatry is not and has never been a bystander to the societal debate on the management of different and criminal behavior. While it may be enough for many fields to express sympathy from the sidelines, psychiatry has been and continues to be an active player in the disenfranchisement of minority populations in the criminal justice system. Society appears to be offering us a chance at repairing our past and helping the future. Let’s take it with honor and humility.
References
1. Collins S. Police killings can be captured in data. The terror police create cannot. Vox.com. 2020 Jun 19.
2. Lee MYH. Yes, U.S. locks people up at a higher rate than any other country. The Washington Post. 2015 Jul 7.
3. McDowell MG, Fernandez LA. Critical Criminology. 2018;26(3):373-91.
4. Thielo AJ et al. Criminology & Public Policy. 2016;15(1):137-70.
5. The Center for Popular Democracy. Freedom to Thrive.
6. Hipp JR. Criminology. 2007;45(3):665-97.
7. Felitti VJ et al. Am J Prev Med. 1998;14(4):245-58.
8. Reavis JA. Perm J. 2013 Spring;17(2):44-8.
9. McHarris PV, McHarris T. No more money for the police. The New York Times. 2020 May 20.
10. Kaminski RJ et al. Police Quarterly. 2004;7(3):311-38.
11. Livington JD. Psychiatr Serv. 2016 Aug 1;67(8):850-7.
12. Galanek JD. Cult Med Psychiatry. 2013 Mar;37(1):195-225.
13. Raz M. Nature. Book Review. 2020 Apr 21.
14. Dressel J, Farid H. Sci Adv. 2018 J 17;4(1):eaao5580.
15. Swartz MS et al. New York State assisted outpatient treatment program evaluation. 2009 Jun 30.
16. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7):784-6.
17. Fox A. Austin budget adds millions for mental health response in 911 services. efficientgov.com. 2019 Sep 13.
18. Elinson Z. When mental health experts, not police, are the first responders. The Wall Street Journal. 2018 Nov 14.
19. Improved responses in psychiatric crises: The Psychiatric Emergency Response Team.
20. Kane E et al. Crim Behav Ment Health. 2018 Apr;28(2):108-19.
21. Wells W, Schafer JA. Officer perceptions of police responses to persons with a mental illness, in “Policing: An International Journal of Police Strategies & Management,” 2006 Oct;29(4):578-61.
Dr. Malik is a first-year psychiatry resident at the University of California, San Diego. She has a background in policy and grassroots organizing through her time working at the National Coalition for the Homeless and the Women’s Law Project. Dr. Malik has no disclosures.
Dr. Amendolara is a first-year psychiatry resident at University of California, San Diego. He spent years advocating for survivors of rape and domestic violence at the Crime Victims Treatment Center in New York and conducted public health research at Lourdes Center for Public Health in Camden, N.J. Dr. Amendolara has no disclosures.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Over the past months, society has reflected on the role of law enforcement. The shocking murder of George Floyd has forced Americans to reconsider the place of police officers in maintaining order.
The death of Mr. Floyd is certainly not a lone incident; in 2019, 1,098 people were killed by those tasked with protecting us.1 The United States holds 25% of the world’s incarcerated, though it makes up only 5% of the world’s population.2 Society is demanding a newer and better system.
The phrase “defund the police” can easily be dismissed because, to many, it implies an appeal to lawlessness. While we certainly cannot speak for any one protester, we think that many of the necessary changes are painfully obvious.3 Society wants law enforcement where force is not the default position but the last option. Society wants law enforcement where verbal conflict resolution is the primary focus of training and intervention. Society wants a correctional system that is more rehabilitative than it is punitive.4
Major U.S. cities spend up to 40% of their funds on police budgeting, much more than what is dedicated to community resources and infrastructure. This trend continues to increase between 1986 and 2013, state spending for correctional facilities increased by 141%.5 Yet, as psychiatrists, we are well aware that social determinants are a strong factor in future criminality.6 Increasing police budgets without addressing structural root causes and risk factors for future asocial behavior is not a wise approach to reducing unlawful behavior. Investing more into programs and policies that reduce these risks is essential.
Using the adverse childhood experiences (ACE) questionnaires, researchers have supported the idea that social programs are a key player in an improved criminal system. The ACE study identified 10 forms of childhood trauma in 17,000 patients, including abuse, neglect, abandonment, household dysfunction, and exposure to violence, that were strongly associated with negative psychological outcomes, engagement in high-risk behaviors, significant medical consequences, and even early death.7 More recent research has shown that those ACEs were four times more prevalent in a criminal offender group than in the general population.8 Psychiatry is in a unique position to address and provide education about ACEs as a tool to identify and help at-risk youths.
Many protesters have asked for mental health providers to have a primary role in this societal reflection and in providing a solution.9 This makes particular sense when considering that almost 20% of calls to law enforcement are for persons with impaired judgment from mental illness or intoxication, and one in four patients with mental illness has been arrested.10,11 We are humbled by this public trust and request. We believe psychiatry can provide many answers to this societal angst. After all, psychiatry is a specialty dedicated to addressing behavioral problems in an evidence-based way.
Yet, we should not forget psychiatry’s imperfect past and our own role in the creation of this system. While this article does not attempt to catalog psychiatry’s faults, one can start by recognizing that mass incarceration is partly a response to how poorly human beings were treated in asylums. Psychiatry was at one time a main enforcer of societal disenfranchisement. After most asylums were closed in 1963 with the Community Mental Health Act, correctional facilities became the largest purveyors of mental health care, often with damaging results.12 If psychiatry were to advocate for the reestablishment of asylums as a solution, we fear that psychiatry would have missed the point. We wonder whether the psychiatrists who have railed against deinstitutionalization since the 1970s do not realize that violence, unethical experimentation, and even racism were at times attributes of asylums.13
Psychiatry can and should be much more than what it once was. Instead of indirectly and inaccurately suggesting that our patients commit mass murders, we should improve research in the field of violence risk assessment and management. As many have already pointed out, violence risk assessment is permeated with overestimation of its potential and, more concerningly, tainted by evidence of implicit racism.14 Implicit racism extends to rights-limiting treatments as well. As previously studied, involuntary outpatient programs often referred to as assisted outpatient treatment are disproportionately levied on Black Americans.15 Instead of routinely seeking to expand abilities to involuntary treat and limit the rights of our patients, we should strive to be a violence-free alternative to law enforcement, not the medical version of police.
Psychiatrists should start actively training, practicing, and researching how to address nonviolent emergency calls. Training should include more robust deescalation training, techniques on the evaluation of patients outside of health care facilities (for example, the street), and a broadening of interventions to include proficiency in the treatment of subclinical populations seeking emergency care without the need to be formally labeled with a psychiatric disorder. Ride-alongs with police officers, volunteering at crisis hotlines, and home calls should not be volunteer or elective experiences for psychiatrists but a required part of training.
Thankfully, some local jurisdictions already have started promising practices that merit replication or at least academic review. Austin, Tex., recently implemented the capability of requesting mental health emergency calls when contacting 911.17 Eugene, Ore., has had the CAHOOTS (Crisis Assistance Helping Out On The Streets) program since 1989, where a medical provider and a mental health provider respond to calls without any law enforcement officers.18 Our own San Diego County has an innovative PERT (Psychiatric Emergency Response Team) program, which partners a mental health provider to a police patrol, allowing an ability to quickly provide different types of services.19 Programs like these show us what is possible. At this time, there is little research to evaluate many programs’ effectiveness.20 Psychiatry should seize this moment to be at the forefront of studying, then educating the public on what works and how to reproduce it.
Police officers have a difficult profession. They are tasked with preventing and predicting crime, often to the point of risking their own lives. Historically, police have been the first call to handle issues for which they are not equipped, ranging from fixing homelessness to arresting violent people using nonviolent means. The idea that police should be able to protect us in all situations has been mistakenly ingrained in our minds. Officers themselves do not feel adequately trained to handle mental health crises.21 “Defund the police” also means a recognition by governments, the public, and police themselves that officers should not be on the front lines for every emergency situation. We must diversify our first responders. Psychiatry should hear this call and be ready.
Since the death of Mr. Floyd, mental health professionals have attempted to voice empathy and warmth to those feeling left out and disenfranchised. Mental health professionals have voiced a desire to educate themselves on systemic biases and antiracism. However, we argue that psychiatry is not and has never been a bystander to the societal debate on the management of different and criminal behavior. While it may be enough for many fields to express sympathy from the sidelines, psychiatry has been and continues to be an active player in the disenfranchisement of minority populations in the criminal justice system. Society appears to be offering us a chance at repairing our past and helping the future. Let’s take it with honor and humility.
References
1. Collins S. Police killings can be captured in data. The terror police create cannot. Vox.com. 2020 Jun 19.
2. Lee MYH. Yes, U.S. locks people up at a higher rate than any other country. The Washington Post. 2015 Jul 7.
3. McDowell MG, Fernandez LA. Critical Criminology. 2018;26(3):373-91.
4. Thielo AJ et al. Criminology & Public Policy. 2016;15(1):137-70.
5. The Center for Popular Democracy. Freedom to Thrive.
6. Hipp JR. Criminology. 2007;45(3):665-97.
7. Felitti VJ et al. Am J Prev Med. 1998;14(4):245-58.
8. Reavis JA. Perm J. 2013 Spring;17(2):44-8.
9. McHarris PV, McHarris T. No more money for the police. The New York Times. 2020 May 20.
10. Kaminski RJ et al. Police Quarterly. 2004;7(3):311-38.
11. Livington JD. Psychiatr Serv. 2016 Aug 1;67(8):850-7.
12. Galanek JD. Cult Med Psychiatry. 2013 Mar;37(1):195-225.
13. Raz M. Nature. Book Review. 2020 Apr 21.
14. Dressel J, Farid H. Sci Adv. 2018 J 17;4(1):eaao5580.
15. Swartz MS et al. New York State assisted outpatient treatment program evaluation. 2009 Jun 30.
16. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7):784-6.
17. Fox A. Austin budget adds millions for mental health response in 911 services. efficientgov.com. 2019 Sep 13.
18. Elinson Z. When mental health experts, not police, are the first responders. The Wall Street Journal. 2018 Nov 14.
19. Improved responses in psychiatric crises: The Psychiatric Emergency Response Team.
20. Kane E et al. Crim Behav Ment Health. 2018 Apr;28(2):108-19.
21. Wells W, Schafer JA. Officer perceptions of police responses to persons with a mental illness, in “Policing: An International Journal of Police Strategies & Management,” 2006 Oct;29(4):578-61.
Dr. Malik is a first-year psychiatry resident at the University of California, San Diego. She has a background in policy and grassroots organizing through her time working at the National Coalition for the Homeless and the Women’s Law Project. Dr. Malik has no disclosures.
Dr. Amendolara is a first-year psychiatry resident at University of California, San Diego. He spent years advocating for survivors of rape and domestic violence at the Crime Victims Treatment Center in New York and conducted public health research at Lourdes Center for Public Health in Camden, N.J. Dr. Amendolara has no disclosures.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Over the past months, society has reflected on the role of law enforcement. The shocking murder of George Floyd has forced Americans to reconsider the place of police officers in maintaining order.
The death of Mr. Floyd is certainly not a lone incident; in 2019, 1,098 people were killed by those tasked with protecting us.1 The United States holds 25% of the world’s incarcerated, though it makes up only 5% of the world’s population.2 Society is demanding a newer and better system.
The phrase “defund the police” can easily be dismissed because, to many, it implies an appeal to lawlessness. While we certainly cannot speak for any one protester, we think that many of the necessary changes are painfully obvious.3 Society wants law enforcement where force is not the default position but the last option. Society wants law enforcement where verbal conflict resolution is the primary focus of training and intervention. Society wants a correctional system that is more rehabilitative than it is punitive.4
Major U.S. cities spend up to 40% of their funds on police budgeting, much more than what is dedicated to community resources and infrastructure. This trend continues to increase between 1986 and 2013, state spending for correctional facilities increased by 141%.5 Yet, as psychiatrists, we are well aware that social determinants are a strong factor in future criminality.6 Increasing police budgets without addressing structural root causes and risk factors for future asocial behavior is not a wise approach to reducing unlawful behavior. Investing more into programs and policies that reduce these risks is essential.
Using the adverse childhood experiences (ACE) questionnaires, researchers have supported the idea that social programs are a key player in an improved criminal system. The ACE study identified 10 forms of childhood trauma in 17,000 patients, including abuse, neglect, abandonment, household dysfunction, and exposure to violence, that were strongly associated with negative psychological outcomes, engagement in high-risk behaviors, significant medical consequences, and even early death.7 More recent research has shown that those ACEs were four times more prevalent in a criminal offender group than in the general population.8 Psychiatry is in a unique position to address and provide education about ACEs as a tool to identify and help at-risk youths.
Many protesters have asked for mental health providers to have a primary role in this societal reflection and in providing a solution.9 This makes particular sense when considering that almost 20% of calls to law enforcement are for persons with impaired judgment from mental illness or intoxication, and one in four patients with mental illness has been arrested.10,11 We are humbled by this public trust and request. We believe psychiatry can provide many answers to this societal angst. After all, psychiatry is a specialty dedicated to addressing behavioral problems in an evidence-based way.
Yet, we should not forget psychiatry’s imperfect past and our own role in the creation of this system. While this article does not attempt to catalog psychiatry’s faults, one can start by recognizing that mass incarceration is partly a response to how poorly human beings were treated in asylums. Psychiatry was at one time a main enforcer of societal disenfranchisement. After most asylums were closed in 1963 with the Community Mental Health Act, correctional facilities became the largest purveyors of mental health care, often with damaging results.12 If psychiatry were to advocate for the reestablishment of asylums as a solution, we fear that psychiatry would have missed the point. We wonder whether the psychiatrists who have railed against deinstitutionalization since the 1970s do not realize that violence, unethical experimentation, and even racism were at times attributes of asylums.13
Psychiatry can and should be much more than what it once was. Instead of indirectly and inaccurately suggesting that our patients commit mass murders, we should improve research in the field of violence risk assessment and management. As many have already pointed out, violence risk assessment is permeated with overestimation of its potential and, more concerningly, tainted by evidence of implicit racism.14 Implicit racism extends to rights-limiting treatments as well. As previously studied, involuntary outpatient programs often referred to as assisted outpatient treatment are disproportionately levied on Black Americans.15 Instead of routinely seeking to expand abilities to involuntary treat and limit the rights of our patients, we should strive to be a violence-free alternative to law enforcement, not the medical version of police.
Psychiatrists should start actively training, practicing, and researching how to address nonviolent emergency calls. Training should include more robust deescalation training, techniques on the evaluation of patients outside of health care facilities (for example, the street), and a broadening of interventions to include proficiency in the treatment of subclinical populations seeking emergency care without the need to be formally labeled with a psychiatric disorder. Ride-alongs with police officers, volunteering at crisis hotlines, and home calls should not be volunteer or elective experiences for psychiatrists but a required part of training.
Thankfully, some local jurisdictions already have started promising practices that merit replication or at least academic review. Austin, Tex., recently implemented the capability of requesting mental health emergency calls when contacting 911.17 Eugene, Ore., has had the CAHOOTS (Crisis Assistance Helping Out On The Streets) program since 1989, where a medical provider and a mental health provider respond to calls without any law enforcement officers.18 Our own San Diego County has an innovative PERT (Psychiatric Emergency Response Team) program, which partners a mental health provider to a police patrol, allowing an ability to quickly provide different types of services.19 Programs like these show us what is possible. At this time, there is little research to evaluate many programs’ effectiveness.20 Psychiatry should seize this moment to be at the forefront of studying, then educating the public on what works and how to reproduce it.
Police officers have a difficult profession. They are tasked with preventing and predicting crime, often to the point of risking their own lives. Historically, police have been the first call to handle issues for which they are not equipped, ranging from fixing homelessness to arresting violent people using nonviolent means. The idea that police should be able to protect us in all situations has been mistakenly ingrained in our minds. Officers themselves do not feel adequately trained to handle mental health crises.21 “Defund the police” also means a recognition by governments, the public, and police themselves that officers should not be on the front lines for every emergency situation. We must diversify our first responders. Psychiatry should hear this call and be ready.
Since the death of Mr. Floyd, mental health professionals have attempted to voice empathy and warmth to those feeling left out and disenfranchised. Mental health professionals have voiced a desire to educate themselves on systemic biases and antiracism. However, we argue that psychiatry is not and has never been a bystander to the societal debate on the management of different and criminal behavior. While it may be enough for many fields to express sympathy from the sidelines, psychiatry has been and continues to be an active player in the disenfranchisement of minority populations in the criminal justice system. Society appears to be offering us a chance at repairing our past and helping the future. Let’s take it with honor and humility.
References
1. Collins S. Police killings can be captured in data. The terror police create cannot. Vox.com. 2020 Jun 19.
2. Lee MYH. Yes, U.S. locks people up at a higher rate than any other country. The Washington Post. 2015 Jul 7.
3. McDowell MG, Fernandez LA. Critical Criminology. 2018;26(3):373-91.
4. Thielo AJ et al. Criminology & Public Policy. 2016;15(1):137-70.
5. The Center for Popular Democracy. Freedom to Thrive.
6. Hipp JR. Criminology. 2007;45(3):665-97.
7. Felitti VJ et al. Am J Prev Med. 1998;14(4):245-58.
8. Reavis JA. Perm J. 2013 Spring;17(2):44-8.
9. McHarris PV, McHarris T. No more money for the police. The New York Times. 2020 May 20.
10. Kaminski RJ et al. Police Quarterly. 2004;7(3):311-38.
11. Livington JD. Psychiatr Serv. 2016 Aug 1;67(8):850-7.
12. Galanek JD. Cult Med Psychiatry. 2013 Mar;37(1):195-225.
13. Raz M. Nature. Book Review. 2020 Apr 21.
14. Dressel J, Farid H. Sci Adv. 2018 J 17;4(1):eaao5580.
15. Swartz MS et al. New York State assisted outpatient treatment program evaluation. 2009 Jun 30.
16. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7):784-6.
17. Fox A. Austin budget adds millions for mental health response in 911 services. efficientgov.com. 2019 Sep 13.
18. Elinson Z. When mental health experts, not police, are the first responders. The Wall Street Journal. 2018 Nov 14.
19. Improved responses in psychiatric crises: The Psychiatric Emergency Response Team.
20. Kane E et al. Crim Behav Ment Health. 2018 Apr;28(2):108-19.
21. Wells W, Schafer JA. Officer perceptions of police responses to persons with a mental illness, in “Policing: An International Journal of Police Strategies & Management,” 2006 Oct;29(4):578-61.
Dr. Malik is a first-year psychiatry resident at the University of California, San Diego. She has a background in policy and grassroots organizing through her time working at the National Coalition for the Homeless and the Women’s Law Project. Dr. Malik has no disclosures.
Dr. Amendolara is a first-year psychiatry resident at University of California, San Diego. He spent years advocating for survivors of rape and domestic violence at the Crime Victims Treatment Center in New York and conducted public health research at Lourdes Center for Public Health in Camden, N.J. Dr. Amendolara has no disclosures.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Cham, Switzerland: Springer, 2019). He has no disclosures.
Doctors hesitated to embrace biosimilar infliximab in first 2 years
Physicians have been slow to embrace biosimilar versions of infliximab, but are more likely to prescribe it to new patients, based on data from a review of nearly 50,000 infliximab claims through Medicare in the first 2 years that biosimilars were available in the United States.
“Although biosimilar versions are as safe and effective as the biologic, patients and physicians may be more reluctant to switch from a working biologic regimen in a chronic setting than an acute one,” wrote Alice J. Chen, PhD, of the University of Southern California, Los Angeles, and colleagues.
In a research letter published in JAMA Internal Medicine, the investigators examined prescribing patterns of physicians switching between the originator infliximab (Remicade) and two of its biosimilars (Inflectra and Renflexis).
They reviewed infliximab use and reimbursement in the 100% Medicare Part B quarterly claims database from Jan. 1, 2017, to Dec. 31, 2018. The study population included Medicare patients classified as new if they had no infliximab claims in the prior 6 months; those with claims were considered returning patients.
In a comparison of claims reflecting 49,771 patients and 4,289 physicians in 2018, a total of 1,418 new patients (17.4%) and 4,495 (10.8%) returning patients used a biosimilar. “Of returning patients, half used the biosimilar version exclusively, whereas the other half switched between biologic and biosimilar versions,” the researchers noted.
Of the 4,289 physicians who prescribed infliximab, 3,124 prescribed no biosimilars, 1,015 prescribed both biologics and biosimilars, and 150 prescribed biosimilars only. Of the physicians who prescribed both, approximately 61% switched some patients from the biologic to the biosimilar; “the remainder kept individual patients on only 1 version of the drug but treated patients with both versions,” the researchers wrote.
The adoption of biosimilars may be slower for chronic vs. acute conditions, the researchers noted. “Prescribers may hesitate to switch clinically stable chronic patients from biologic regimens if they are unfamiliar with the biosimilar or face financial disincentives from prescribing it.”
The study findings were limited by several factors including the use of only 2 years of data and a focus only on Medicare Part B. Switching medications may have been influenced by factors such as lower copays for patients and rebates or discounts for physicians; however, “further research is needed to better understand biosimilar pricing dynamics and the barriers to adopting biosimilars for chronic conditions,” they concluded.
The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
SOURCE: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
Help your patients understand biologics and biosimilars by using AGA resources for providers and patients available at www.gastro.org/biosimilars.
Physicians have been slow to embrace biosimilar versions of infliximab, but are more likely to prescribe it to new patients, based on data from a review of nearly 50,000 infliximab claims through Medicare in the first 2 years that biosimilars were available in the United States.
“Although biosimilar versions are as safe and effective as the biologic, patients and physicians may be more reluctant to switch from a working biologic regimen in a chronic setting than an acute one,” wrote Alice J. Chen, PhD, of the University of Southern California, Los Angeles, and colleagues.
In a research letter published in JAMA Internal Medicine, the investigators examined prescribing patterns of physicians switching between the originator infliximab (Remicade) and two of its biosimilars (Inflectra and Renflexis).
They reviewed infliximab use and reimbursement in the 100% Medicare Part B quarterly claims database from Jan. 1, 2017, to Dec. 31, 2018. The study population included Medicare patients classified as new if they had no infliximab claims in the prior 6 months; those with claims were considered returning patients.
In a comparison of claims reflecting 49,771 patients and 4,289 physicians in 2018, a total of 1,418 new patients (17.4%) and 4,495 (10.8%) returning patients used a biosimilar. “Of returning patients, half used the biosimilar version exclusively, whereas the other half switched between biologic and biosimilar versions,” the researchers noted.
Of the 4,289 physicians who prescribed infliximab, 3,124 prescribed no biosimilars, 1,015 prescribed both biologics and biosimilars, and 150 prescribed biosimilars only. Of the physicians who prescribed both, approximately 61% switched some patients from the biologic to the biosimilar; “the remainder kept individual patients on only 1 version of the drug but treated patients with both versions,” the researchers wrote.
The adoption of biosimilars may be slower for chronic vs. acute conditions, the researchers noted. “Prescribers may hesitate to switch clinically stable chronic patients from biologic regimens if they are unfamiliar with the biosimilar or face financial disincentives from prescribing it.”
The study findings were limited by several factors including the use of only 2 years of data and a focus only on Medicare Part B. Switching medications may have been influenced by factors such as lower copays for patients and rebates or discounts for physicians; however, “further research is needed to better understand biosimilar pricing dynamics and the barriers to adopting biosimilars for chronic conditions,” they concluded.
The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
SOURCE: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
Help your patients understand biologics and biosimilars by using AGA resources for providers and patients available at www.gastro.org/biosimilars.
Physicians have been slow to embrace biosimilar versions of infliximab, but are more likely to prescribe it to new patients, based on data from a review of nearly 50,000 infliximab claims through Medicare in the first 2 years that biosimilars were available in the United States.
“Although biosimilar versions are as safe and effective as the biologic, patients and physicians may be more reluctant to switch from a working biologic regimen in a chronic setting than an acute one,” wrote Alice J. Chen, PhD, of the University of Southern California, Los Angeles, and colleagues.
In a research letter published in JAMA Internal Medicine, the investigators examined prescribing patterns of physicians switching between the originator infliximab (Remicade) and two of its biosimilars (Inflectra and Renflexis).
They reviewed infliximab use and reimbursement in the 100% Medicare Part B quarterly claims database from Jan. 1, 2017, to Dec. 31, 2018. The study population included Medicare patients classified as new if they had no infliximab claims in the prior 6 months; those with claims were considered returning patients.
In a comparison of claims reflecting 49,771 patients and 4,289 physicians in 2018, a total of 1,418 new patients (17.4%) and 4,495 (10.8%) returning patients used a biosimilar. “Of returning patients, half used the biosimilar version exclusively, whereas the other half switched between biologic and biosimilar versions,” the researchers noted.
Of the 4,289 physicians who prescribed infliximab, 3,124 prescribed no biosimilars, 1,015 prescribed both biologics and biosimilars, and 150 prescribed biosimilars only. Of the physicians who prescribed both, approximately 61% switched some patients from the biologic to the biosimilar; “the remainder kept individual patients on only 1 version of the drug but treated patients with both versions,” the researchers wrote.
The adoption of biosimilars may be slower for chronic vs. acute conditions, the researchers noted. “Prescribers may hesitate to switch clinically stable chronic patients from biologic regimens if they are unfamiliar with the biosimilar or face financial disincentives from prescribing it.”
The study findings were limited by several factors including the use of only 2 years of data and a focus only on Medicare Part B. Switching medications may have been influenced by factors such as lower copays for patients and rebates or discounts for physicians; however, “further research is needed to better understand biosimilar pricing dynamics and the barriers to adopting biosimilars for chronic conditions,” they concluded.
The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
SOURCE: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
Help your patients understand biologics and biosimilars by using AGA resources for providers and patients available at www.gastro.org/biosimilars.
FROM JAMA INTERNAL MEDICINE
Doctors hesitated to embrace biosimilar infliximab in first 2 years
Physicians have been slow to embrace biosimilar versions of infliximab, but are more likely to prescribe it to new patients, based on data from a review of nearly 50,000 infliximab claims through Medicare in the first 2 years that biosimilars were available in the United States.
“Although biosimilar versions are as safe and effective as the biologic, patients and physicians may be more reluctant to switch from a working biologic regimen in a chronic setting than an acute one,” wrote Alice J. Chen, PhD, of the University of Southern California, Los Angeles, and colleagues.
In a research letter published in JAMA Internal Medicine, the investigators examined prescribing patterns of physicians switching between the originator infliximab (Remicade) and two of its biosimilars (Inflectra and Renflexis).
They reviewed infliximab use and reimbursement in the 100% Medicare Part B quarterly claims database from Jan. 1, 2017, to Dec. 31, 2018. The study population included Medicare patients classified as new if they had no infliximab claims in the prior 6 months; those with claims were considered returning patients.
In a comparison of claims reflecting 49,771 patients and 4,289 physicians in 2018, a total of 1,418 new patients (17.4%) and 4,495 (10.8%) returning patients used a biosimilar. “Of returning patients, half used the biosimilar version exclusively, whereas the other half switched between biologic and biosimilar versions,” the researchers noted.
Of the 4,289 physicians who prescribed infliximab, 3,124 prescribed no biosimilars, 1,015 prescribed both biologics and biosimilars, and 150 prescribed biosimilars only. Of the physicians who prescribed both, approximately 61% switched some patients from the biologic to the biosimilar; “the remainder kept individual patients on only 1 version of the drug but treated patients with both versions,” the researchers wrote.
The adoption of biosimilars may be slower for chronic vs. acute conditions, the researchers noted. “Prescribers may hesitate to switch clinically stable chronic patients from biologic regimens if they are unfamiliar with the biosimilar or face financial disincentives from prescribing it.”
The study findings were limited by several factors including the use of only 2 years of data and a focus only on Medicare Part B. Switching medications may have been influenced by factors such as lower copays for patients and rebates or discounts for physicians; however, “further research is needed to better understand biosimilar pricing dynamics and the barriers to adopting biosimilars for chronic conditions,” they concluded.
The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
SOURCE: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
Physicians have been slow to embrace biosimilar versions of infliximab, but are more likely to prescribe it to new patients, based on data from a review of nearly 50,000 infliximab claims through Medicare in the first 2 years that biosimilars were available in the United States.
“Although biosimilar versions are as safe and effective as the biologic, patients and physicians may be more reluctant to switch from a working biologic regimen in a chronic setting than an acute one,” wrote Alice J. Chen, PhD, of the University of Southern California, Los Angeles, and colleagues.
In a research letter published in JAMA Internal Medicine, the investigators examined prescribing patterns of physicians switching between the originator infliximab (Remicade) and two of its biosimilars (Inflectra and Renflexis).
They reviewed infliximab use and reimbursement in the 100% Medicare Part B quarterly claims database from Jan. 1, 2017, to Dec. 31, 2018. The study population included Medicare patients classified as new if they had no infliximab claims in the prior 6 months; those with claims were considered returning patients.
In a comparison of claims reflecting 49,771 patients and 4,289 physicians in 2018, a total of 1,418 new patients (17.4%) and 4,495 (10.8%) returning patients used a biosimilar. “Of returning patients, half used the biosimilar version exclusively, whereas the other half switched between biologic and biosimilar versions,” the researchers noted.
Of the 4,289 physicians who prescribed infliximab, 3,124 prescribed no biosimilars, 1,015 prescribed both biologics and biosimilars, and 150 prescribed biosimilars only. Of the physicians who prescribed both, approximately 61% switched some patients from the biologic to the biosimilar; “the remainder kept individual patients on only 1 version of the drug but treated patients with both versions,” the researchers wrote.
The adoption of biosimilars may be slower for chronic vs. acute conditions, the researchers noted. “Prescribers may hesitate to switch clinically stable chronic patients from biologic regimens if they are unfamiliar with the biosimilar or face financial disincentives from prescribing it.”
The study findings were limited by several factors including the use of only 2 years of data and a focus only on Medicare Part B. Switching medications may have been influenced by factors such as lower copays for patients and rebates or discounts for physicians; however, “further research is needed to better understand biosimilar pricing dynamics and the barriers to adopting biosimilars for chronic conditions,” they concluded.
The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
SOURCE: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
Physicians have been slow to embrace biosimilar versions of infliximab, but are more likely to prescribe it to new patients, based on data from a review of nearly 50,000 infliximab claims through Medicare in the first 2 years that biosimilars were available in the United States.
“Although biosimilar versions are as safe and effective as the biologic, patients and physicians may be more reluctant to switch from a working biologic regimen in a chronic setting than an acute one,” wrote Alice J. Chen, PhD, of the University of Southern California, Los Angeles, and colleagues.
In a research letter published in JAMA Internal Medicine, the investigators examined prescribing patterns of physicians switching between the originator infliximab (Remicade) and two of its biosimilars (Inflectra and Renflexis).
They reviewed infliximab use and reimbursement in the 100% Medicare Part B quarterly claims database from Jan. 1, 2017, to Dec. 31, 2018. The study population included Medicare patients classified as new if they had no infliximab claims in the prior 6 months; those with claims were considered returning patients.
In a comparison of claims reflecting 49,771 patients and 4,289 physicians in 2018, a total of 1,418 new patients (17.4%) and 4,495 (10.8%) returning patients used a biosimilar. “Of returning patients, half used the biosimilar version exclusively, whereas the other half switched between biologic and biosimilar versions,” the researchers noted.
Of the 4,289 physicians who prescribed infliximab, 3,124 prescribed no biosimilars, 1,015 prescribed both biologics and biosimilars, and 150 prescribed biosimilars only. Of the physicians who prescribed both, approximately 61% switched some patients from the biologic to the biosimilar; “the remainder kept individual patients on only 1 version of the drug but treated patients with both versions,” the researchers wrote.
The adoption of biosimilars may be slower for chronic vs. acute conditions, the researchers noted. “Prescribers may hesitate to switch clinically stable chronic patients from biologic regimens if they are unfamiliar with the biosimilar or face financial disincentives from prescribing it.”
The study findings were limited by several factors including the use of only 2 years of data and a focus only on Medicare Part B. Switching medications may have been influenced by factors such as lower copays for patients and rebates or discounts for physicians; however, “further research is needed to better understand biosimilar pricing dynamics and the barriers to adopting biosimilars for chronic conditions,” they concluded.
The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
SOURCE: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
FROM JAMA INTERNAL MEDICINE
Key clinical point: A total of 17% of patients new to infliximab received a biosimilar in 2018, compared with 11% of returning patients.
Major finding: Biosimilar infliximab accounted for 10% of the market share 2 years after the product was introduced.
Study details: The data come from a review of infliximab claims across 49,771 patients and 4,289 physicians who prescribed infliximab in 2018.
Disclosures: The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
Source: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
Colorism and dermatology
With the world currently really listening and engaged (hopefully) on making positive changes with regards to racism and systemic racial injustices, skin color has come to the forefront. Racism because of skin color has been an unfortunate part of our history and foundation of the United States with a capitalist society built and thriving on the profits of slavery, and a democracy founded on equality – unless you had black skin. These issues are at the forefront in the United States, but have also significantly impacted other parts of the world, including the Caribbean and South America having a significant African slave trade history and impacts, with Brazil currently facing the same systemic racial injustices and police brutality among black men, and King Leopold II of Belgium slaughtering an estimated 10-15 million Congolese people in the name of colonialism, slavery, and robbing resources (natural resources as well as servitude) in the Congo as late as the early 1900s.
These are just a few of the many historical examples of racial injustice, which remains ingrained in many parts of our society today. With this worldwide history, it has been advantageous for people to have lighter skin with regards to money, politics, jobs, education, the justice system, modeling/acting opportunities and contracts, home ownership, and opportunities for generational wealth for years to come. It has ingrained some unfortunate beliefs among some that having lighter skin is better, advantageous, and will make them more desirable or more beautiful.
and across the African continent. It is estimated that 77% of women in Nigeria and 55% of women in China use bleaching creams to achieve overall skin lightening. Unilever’s Fair & Lovely skin-whitening cream has long been a popular over-the-counter product in India, with an estimated market worth of 270 billion rupees ($4 billion USD). On June 25, 2020, Unilever vowed to rename and rebrand Fair & Lovely. With such an offensive name for a product that further promotes colorism, this is an effort in the right direction and has been a long time coming since its debut in 1975. Unilever’s Fair and Lovely Foundation for women’s causes still exists, and has not been renamed at the time of this writing.
Controversy remains on whether this product and other products such as these should exist for the purposes they are used for. Johnson & Johnson has decided that it will no longer produce and sell the Neutrogena Fine Fairness line, sold only in Asia and the Middle East, and the Clean & Clear Fairness line, sold in India. There are arguments to the contrary that halting production of skin-lightening products altogether may result in an influx of unsafe alternatives.
As dermatologists, we use skin-lightening products appropriately for the purposes of treating skin conditions such as postinflammatory hyperpigmentation, melasma, and photoaging. This is where the use of such products should largely end. While it is up to individuals about what they do with their skin and their bodies, we, as health care skin professionals, should be furthering the notion that all skin colors and types are beautiful. Moreover, we should not be encouraging the use of these products for overall skin whitening. Part of the issue is that these products are available often at high concentrations over the counter or in the illegal market, especially in parts of Asia and Africa where colorism is more common and skin whitening is more commonly practiced. The dangers are not only the risk of ochronosis with high concentrations or long term use of hydroquinone, but also what the Centre for Science and Environment found in a 2014 study, that 44% of the skin “fairness” creams in India contained mercury, which is illegal and a health concern.
In my practice, I have also had patients (several originally from Nigeria) who have admitted to long term use of skin-bleaching products for the purposes of all over face- and body-skin lightening who now suffer from very sensitive skin and experience bouts of eczematous dermatitis from time to time, despite having stopped using lightening cream. While there are adverse physical effects resulting from the use of these topicals for this purpose, the effects on the psyche are what concern me the most.
The beauty industry has also been an unfortunate part of furthering thoughts and attitudes concerning colorism over the years with lighter skin and Caucasian ideals being set as standards of beauty. One of many examples is a deodorant ad in the Middle East with the tagline “White is Purity” on a woman, which was pulled by Nivea in 2017 after it was slammed as racist. Another is the 2017 Dove ad for body wash that showed a smiling black woman peel off her brown shirt to reveal a white woman in a lighter-color shirt.
A shift has occurred in recent years with more ethnic images of beauty appearing in magazines and film. However, such opportunities are still less plentiful, pay discrepancies still occur, and sexual objectification of women of color as opposed to beautification is still rampant. As such, it is also up to us to do our part in studying and utilizing ethnic and racial differences in skin and beauty to maximize our efforts in promoting what is inherently beautiful as opposed to one standard of beauty. The education begins with the images we see, what we teach our children, loving ourselves, and as doctors, being knowledgeable about the right aesthetic choices for patients with different skin colors and types.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
With the world currently really listening and engaged (hopefully) on making positive changes with regards to racism and systemic racial injustices, skin color has come to the forefront. Racism because of skin color has been an unfortunate part of our history and foundation of the United States with a capitalist society built and thriving on the profits of slavery, and a democracy founded on equality – unless you had black skin. These issues are at the forefront in the United States, but have also significantly impacted other parts of the world, including the Caribbean and South America having a significant African slave trade history and impacts, with Brazil currently facing the same systemic racial injustices and police brutality among black men, and King Leopold II of Belgium slaughtering an estimated 10-15 million Congolese people in the name of colonialism, slavery, and robbing resources (natural resources as well as servitude) in the Congo as late as the early 1900s.
These are just a few of the many historical examples of racial injustice, which remains ingrained in many parts of our society today. With this worldwide history, it has been advantageous for people to have lighter skin with regards to money, politics, jobs, education, the justice system, modeling/acting opportunities and contracts, home ownership, and opportunities for generational wealth for years to come. It has ingrained some unfortunate beliefs among some that having lighter skin is better, advantageous, and will make them more desirable or more beautiful.
and across the African continent. It is estimated that 77% of women in Nigeria and 55% of women in China use bleaching creams to achieve overall skin lightening. Unilever’s Fair & Lovely skin-whitening cream has long been a popular over-the-counter product in India, with an estimated market worth of 270 billion rupees ($4 billion USD). On June 25, 2020, Unilever vowed to rename and rebrand Fair & Lovely. With such an offensive name for a product that further promotes colorism, this is an effort in the right direction and has been a long time coming since its debut in 1975. Unilever’s Fair and Lovely Foundation for women’s causes still exists, and has not been renamed at the time of this writing.
Controversy remains on whether this product and other products such as these should exist for the purposes they are used for. Johnson & Johnson has decided that it will no longer produce and sell the Neutrogena Fine Fairness line, sold only in Asia and the Middle East, and the Clean & Clear Fairness line, sold in India. There are arguments to the contrary that halting production of skin-lightening products altogether may result in an influx of unsafe alternatives.
As dermatologists, we use skin-lightening products appropriately for the purposes of treating skin conditions such as postinflammatory hyperpigmentation, melasma, and photoaging. This is where the use of such products should largely end. While it is up to individuals about what they do with their skin and their bodies, we, as health care skin professionals, should be furthering the notion that all skin colors and types are beautiful. Moreover, we should not be encouraging the use of these products for overall skin whitening. Part of the issue is that these products are available often at high concentrations over the counter or in the illegal market, especially in parts of Asia and Africa where colorism is more common and skin whitening is more commonly practiced. The dangers are not only the risk of ochronosis with high concentrations or long term use of hydroquinone, but also what the Centre for Science and Environment found in a 2014 study, that 44% of the skin “fairness” creams in India contained mercury, which is illegal and a health concern.
In my practice, I have also had patients (several originally from Nigeria) who have admitted to long term use of skin-bleaching products for the purposes of all over face- and body-skin lightening who now suffer from very sensitive skin and experience bouts of eczematous dermatitis from time to time, despite having stopped using lightening cream. While there are adverse physical effects resulting from the use of these topicals for this purpose, the effects on the psyche are what concern me the most.
The beauty industry has also been an unfortunate part of furthering thoughts and attitudes concerning colorism over the years with lighter skin and Caucasian ideals being set as standards of beauty. One of many examples is a deodorant ad in the Middle East with the tagline “White is Purity” on a woman, which was pulled by Nivea in 2017 after it was slammed as racist. Another is the 2017 Dove ad for body wash that showed a smiling black woman peel off her brown shirt to reveal a white woman in a lighter-color shirt.
A shift has occurred in recent years with more ethnic images of beauty appearing in magazines and film. However, such opportunities are still less plentiful, pay discrepancies still occur, and sexual objectification of women of color as opposed to beautification is still rampant. As such, it is also up to us to do our part in studying and utilizing ethnic and racial differences in skin and beauty to maximize our efforts in promoting what is inherently beautiful as opposed to one standard of beauty. The education begins with the images we see, what we teach our children, loving ourselves, and as doctors, being knowledgeable about the right aesthetic choices for patients with different skin colors and types.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
With the world currently really listening and engaged (hopefully) on making positive changes with regards to racism and systemic racial injustices, skin color has come to the forefront. Racism because of skin color has been an unfortunate part of our history and foundation of the United States with a capitalist society built and thriving on the profits of slavery, and a democracy founded on equality – unless you had black skin. These issues are at the forefront in the United States, but have also significantly impacted other parts of the world, including the Caribbean and South America having a significant African slave trade history and impacts, with Brazil currently facing the same systemic racial injustices and police brutality among black men, and King Leopold II of Belgium slaughtering an estimated 10-15 million Congolese people in the name of colonialism, slavery, and robbing resources (natural resources as well as servitude) in the Congo as late as the early 1900s.
These are just a few of the many historical examples of racial injustice, which remains ingrained in many parts of our society today. With this worldwide history, it has been advantageous for people to have lighter skin with regards to money, politics, jobs, education, the justice system, modeling/acting opportunities and contracts, home ownership, and opportunities for generational wealth for years to come. It has ingrained some unfortunate beliefs among some that having lighter skin is better, advantageous, and will make them more desirable or more beautiful.
and across the African continent. It is estimated that 77% of women in Nigeria and 55% of women in China use bleaching creams to achieve overall skin lightening. Unilever’s Fair & Lovely skin-whitening cream has long been a popular over-the-counter product in India, with an estimated market worth of 270 billion rupees ($4 billion USD). On June 25, 2020, Unilever vowed to rename and rebrand Fair & Lovely. With such an offensive name for a product that further promotes colorism, this is an effort in the right direction and has been a long time coming since its debut in 1975. Unilever’s Fair and Lovely Foundation for women’s causes still exists, and has not been renamed at the time of this writing.
Controversy remains on whether this product and other products such as these should exist for the purposes they are used for. Johnson & Johnson has decided that it will no longer produce and sell the Neutrogena Fine Fairness line, sold only in Asia and the Middle East, and the Clean & Clear Fairness line, sold in India. There are arguments to the contrary that halting production of skin-lightening products altogether may result in an influx of unsafe alternatives.
As dermatologists, we use skin-lightening products appropriately for the purposes of treating skin conditions such as postinflammatory hyperpigmentation, melasma, and photoaging. This is where the use of such products should largely end. While it is up to individuals about what they do with their skin and their bodies, we, as health care skin professionals, should be furthering the notion that all skin colors and types are beautiful. Moreover, we should not be encouraging the use of these products for overall skin whitening. Part of the issue is that these products are available often at high concentrations over the counter or in the illegal market, especially in parts of Asia and Africa where colorism is more common and skin whitening is more commonly practiced. The dangers are not only the risk of ochronosis with high concentrations or long term use of hydroquinone, but also what the Centre for Science and Environment found in a 2014 study, that 44% of the skin “fairness” creams in India contained mercury, which is illegal and a health concern.
In my practice, I have also had patients (several originally from Nigeria) who have admitted to long term use of skin-bleaching products for the purposes of all over face- and body-skin lightening who now suffer from very sensitive skin and experience bouts of eczematous dermatitis from time to time, despite having stopped using lightening cream. While there are adverse physical effects resulting from the use of these topicals for this purpose, the effects on the psyche are what concern me the most.
The beauty industry has also been an unfortunate part of furthering thoughts and attitudes concerning colorism over the years with lighter skin and Caucasian ideals being set as standards of beauty. One of many examples is a deodorant ad in the Middle East with the tagline “White is Purity” on a woman, which was pulled by Nivea in 2017 after it was slammed as racist. Another is the 2017 Dove ad for body wash that showed a smiling black woman peel off her brown shirt to reveal a white woman in a lighter-color shirt.
A shift has occurred in recent years with more ethnic images of beauty appearing in magazines and film. However, such opportunities are still less plentiful, pay discrepancies still occur, and sexual objectification of women of color as opposed to beautification is still rampant. As such, it is also up to us to do our part in studying and utilizing ethnic and racial differences in skin and beauty to maximize our efforts in promoting what is inherently beautiful as opposed to one standard of beauty. The education begins with the images we see, what we teach our children, loving ourselves, and as doctors, being knowledgeable about the right aesthetic choices for patients with different skin colors and types.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.