User login
How high a priority is bariatric surgery during COVID-19?
The ASMBS statement, “Safer Through Surgery,” was published online in Surgery for Obesity and Related Diseases by the ASMBS executive committee.
It is a reaction to the fact that some U.S. states have placed metabolic and bariatric surgery in the same low-priority category as cosmetic surgery as examples of “elective” procedures that should be among the last to be restarted when pandemic restrictions are eased.
Rather, ASMBS argues, although obesity surgery must be postponed along with other nonemergency procedures when surges in the novel coronavirus make them unsafe, such operations should be resumed as soon as possible along with other medically necessary procedures.
“Metabolic and bariatric surgery is NOT elective. Metabolic and bariatric surgery is medically necessary and the best treatment for those with the life-threatening and life-limiting disease of severe obesity,” the statement says.
And obesity itself is a major risk factor for worse COVID-19 outcomes, ASMBS President Matt Hutter, MD, told Medscape Medical News, noting that individuals with obesity are “more likely to be in [intensive care units].”
“Mortality rates are higher, even in young patients. And [obesity] ... is associated with other comorbidities including diabetes and heart disease...We know the clock is ticking for some folks. For those with early diabetes, the sooner the [bariatric] surgery the more likely it is [for diabetes] to go into remission.”
Because the pandemic may be around for a while, “If we can make people [with obesity] safer ... because they’ve had surgery ... they may be better off,” should they get COVID-19 later, he pointed out.
Hutter noted that the ASMBS recorded a series of webinars, archived on the society’s website, with panels discussing in-depth issues to consider in prioritizing patients when restarting metabolic and bariatric surgery.
There are some differences of opinion, such as whether the sickest patients should be the first to have the surgeries upon reopening, or whether those individuals might be worse off if they contract COVID-19 in the perioperative setting.
“I don’t think there’s a right or wrong answer, but I think we have to figure out what’s right for the individual patient, considering their specific risks of having versus not having surgery, of waiting 1 month, 2 months, or 6 months. One thing we do know is that obesity is a significant disease.”
‘Before, during, and after COVID, obesity itself remains an epidemic’
Asked to comment on the ASMBS stance, Obesity Society president Lee M. Kaplan, MD, PhD, sent Medscape Medical News a statement.
“We do not fully understand which aspects of obesity pathophysiology ... are most responsible for the adverse COVID-19 outcomes, nor do we know the degree to which reduced access to care, social isolation, and other social and environmental determinants of health disproportionately affect COVID-19 patients with obesity,” he noted.
“At this early stage, we have not yet determined the impact of weight loss and various types of antiobesity therapies on these risks.”
Nonetheless, Kaplan said, “the extended COVID-19 pandemic underscores the importance of increasing, not diminishing, our commitment to understanding and treating obesity, using all available, evidence-based therapies, including lifestyle modification, antiobesity medications, bariatric surgery, and combinations thereof.”
As all health care delivery is being reorganized around the pandemic, Kaplan added: “Rethinking and changing our approach to obesity needs to be a central feature of this process.
“Before, during, and after COVID, obesity itself remains an epidemic. Its high global prevalence, increasing severity, and profound impact on all aspects of health and disease require that it be addressed more universally within the health care system, with the same commitment afforded to other chronic diseases.”
Obesity treatment isn’t generally considered an emergency, he noted, “because obesity is a chronic disease, whose adverse health effects often accumulate slowly and insidiously. Its generally slow progression allows for careful and coordinated care planning, and advanced scheduling of therapeutic interventions, including surgery. These characteristics, however, should not lead us to infer that treating obesity itself is optional.”
Hutter has reported receiving honoraria from Ethicon and Medtronic, and is a consultant for Vicarious Surgical and Sigilon Therapeutics. Kaplan has reported consulting for Boehringer Ingelheim, Fractyl, Gelesis, GI Dynamics, Johnson & Johnson, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, the National Institutes of Health, and the Department of State.
This article first appeared on Medscape.com.
The ASMBS statement, “Safer Through Surgery,” was published online in Surgery for Obesity and Related Diseases by the ASMBS executive committee.
It is a reaction to the fact that some U.S. states have placed metabolic and bariatric surgery in the same low-priority category as cosmetic surgery as examples of “elective” procedures that should be among the last to be restarted when pandemic restrictions are eased.
Rather, ASMBS argues, although obesity surgery must be postponed along with other nonemergency procedures when surges in the novel coronavirus make them unsafe, such operations should be resumed as soon as possible along with other medically necessary procedures.
“Metabolic and bariatric surgery is NOT elective. Metabolic and bariatric surgery is medically necessary and the best treatment for those with the life-threatening and life-limiting disease of severe obesity,” the statement says.
And obesity itself is a major risk factor for worse COVID-19 outcomes, ASMBS President Matt Hutter, MD, told Medscape Medical News, noting that individuals with obesity are “more likely to be in [intensive care units].”
“Mortality rates are higher, even in young patients. And [obesity] ... is associated with other comorbidities including diabetes and heart disease...We know the clock is ticking for some folks. For those with early diabetes, the sooner the [bariatric] surgery the more likely it is [for diabetes] to go into remission.”
Because the pandemic may be around for a while, “If we can make people [with obesity] safer ... because they’ve had surgery ... they may be better off,” should they get COVID-19 later, he pointed out.
Hutter noted that the ASMBS recorded a series of webinars, archived on the society’s website, with panels discussing in-depth issues to consider in prioritizing patients when restarting metabolic and bariatric surgery.
There are some differences of opinion, such as whether the sickest patients should be the first to have the surgeries upon reopening, or whether those individuals might be worse off if they contract COVID-19 in the perioperative setting.
“I don’t think there’s a right or wrong answer, but I think we have to figure out what’s right for the individual patient, considering their specific risks of having versus not having surgery, of waiting 1 month, 2 months, or 6 months. One thing we do know is that obesity is a significant disease.”
‘Before, during, and after COVID, obesity itself remains an epidemic’
Asked to comment on the ASMBS stance, Obesity Society president Lee M. Kaplan, MD, PhD, sent Medscape Medical News a statement.
“We do not fully understand which aspects of obesity pathophysiology ... are most responsible for the adverse COVID-19 outcomes, nor do we know the degree to which reduced access to care, social isolation, and other social and environmental determinants of health disproportionately affect COVID-19 patients with obesity,” he noted.
“At this early stage, we have not yet determined the impact of weight loss and various types of antiobesity therapies on these risks.”
Nonetheless, Kaplan said, “the extended COVID-19 pandemic underscores the importance of increasing, not diminishing, our commitment to understanding and treating obesity, using all available, evidence-based therapies, including lifestyle modification, antiobesity medications, bariatric surgery, and combinations thereof.”
As all health care delivery is being reorganized around the pandemic, Kaplan added: “Rethinking and changing our approach to obesity needs to be a central feature of this process.
“Before, during, and after COVID, obesity itself remains an epidemic. Its high global prevalence, increasing severity, and profound impact on all aspects of health and disease require that it be addressed more universally within the health care system, with the same commitment afforded to other chronic diseases.”
Obesity treatment isn’t generally considered an emergency, he noted, “because obesity is a chronic disease, whose adverse health effects often accumulate slowly and insidiously. Its generally slow progression allows for careful and coordinated care planning, and advanced scheduling of therapeutic interventions, including surgery. These characteristics, however, should not lead us to infer that treating obesity itself is optional.”
Hutter has reported receiving honoraria from Ethicon and Medtronic, and is a consultant for Vicarious Surgical and Sigilon Therapeutics. Kaplan has reported consulting for Boehringer Ingelheim, Fractyl, Gelesis, GI Dynamics, Johnson & Johnson, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, the National Institutes of Health, and the Department of State.
This article first appeared on Medscape.com.
The ASMBS statement, “Safer Through Surgery,” was published online in Surgery for Obesity and Related Diseases by the ASMBS executive committee.
It is a reaction to the fact that some U.S. states have placed metabolic and bariatric surgery in the same low-priority category as cosmetic surgery as examples of “elective” procedures that should be among the last to be restarted when pandemic restrictions are eased.
Rather, ASMBS argues, although obesity surgery must be postponed along with other nonemergency procedures when surges in the novel coronavirus make them unsafe, such operations should be resumed as soon as possible along with other medically necessary procedures.
“Metabolic and bariatric surgery is NOT elective. Metabolic and bariatric surgery is medically necessary and the best treatment for those with the life-threatening and life-limiting disease of severe obesity,” the statement says.
And obesity itself is a major risk factor for worse COVID-19 outcomes, ASMBS President Matt Hutter, MD, told Medscape Medical News, noting that individuals with obesity are “more likely to be in [intensive care units].”
“Mortality rates are higher, even in young patients. And [obesity] ... is associated with other comorbidities including diabetes and heart disease...We know the clock is ticking for some folks. For those with early diabetes, the sooner the [bariatric] surgery the more likely it is [for diabetes] to go into remission.”
Because the pandemic may be around for a while, “If we can make people [with obesity] safer ... because they’ve had surgery ... they may be better off,” should they get COVID-19 later, he pointed out.
Hutter noted that the ASMBS recorded a series of webinars, archived on the society’s website, with panels discussing in-depth issues to consider in prioritizing patients when restarting metabolic and bariatric surgery.
There are some differences of opinion, such as whether the sickest patients should be the first to have the surgeries upon reopening, or whether those individuals might be worse off if they contract COVID-19 in the perioperative setting.
“I don’t think there’s a right or wrong answer, but I think we have to figure out what’s right for the individual patient, considering their specific risks of having versus not having surgery, of waiting 1 month, 2 months, or 6 months. One thing we do know is that obesity is a significant disease.”
‘Before, during, and after COVID, obesity itself remains an epidemic’
Asked to comment on the ASMBS stance, Obesity Society president Lee M. Kaplan, MD, PhD, sent Medscape Medical News a statement.
“We do not fully understand which aspects of obesity pathophysiology ... are most responsible for the adverse COVID-19 outcomes, nor do we know the degree to which reduced access to care, social isolation, and other social and environmental determinants of health disproportionately affect COVID-19 patients with obesity,” he noted.
“At this early stage, we have not yet determined the impact of weight loss and various types of antiobesity therapies on these risks.”
Nonetheless, Kaplan said, “the extended COVID-19 pandemic underscores the importance of increasing, not diminishing, our commitment to understanding and treating obesity, using all available, evidence-based therapies, including lifestyle modification, antiobesity medications, bariatric surgery, and combinations thereof.”
As all health care delivery is being reorganized around the pandemic, Kaplan added: “Rethinking and changing our approach to obesity needs to be a central feature of this process.
“Before, during, and after COVID, obesity itself remains an epidemic. Its high global prevalence, increasing severity, and profound impact on all aspects of health and disease require that it be addressed more universally within the health care system, with the same commitment afforded to other chronic diseases.”
Obesity treatment isn’t generally considered an emergency, he noted, “because obesity is a chronic disease, whose adverse health effects often accumulate slowly and insidiously. Its generally slow progression allows for careful and coordinated care planning, and advanced scheduling of therapeutic interventions, including surgery. These characteristics, however, should not lead us to infer that treating obesity itself is optional.”
Hutter has reported receiving honoraria from Ethicon and Medtronic, and is a consultant for Vicarious Surgical and Sigilon Therapeutics. Kaplan has reported consulting for Boehringer Ingelheim, Fractyl, Gelesis, GI Dynamics, Johnson & Johnson, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, the National Institutes of Health, and the Department of State.
This article first appeared on Medscape.com.
Painful, swollen elbow
A 32-year-old woman presented to our clinic with left elbow swelling and pain of 6 days’ duration. She’d had a posterior interosseous nerve (PIN) injection (hydrodissection) at another facility 12 days earlier for refractory intersection syndrome.
During nerve hydrodissection, fluid is injected into the area surrounding the nerve in an effort to displace the muscles, tendons, and fascia and thus reduce friction on the nerve. This treatment, often completed with ultrasound guidance, is utilized by patients who want to obtain pain relief without undergoing surgery for nerve entrapment syndromes.
In this case, a combination of 1 mL (40 mg) of methylprednisolone acetate, 1 mL of lidocaine 2%, and 3 mL of normal saline was injected into the supinator muscle belly (proximal dorsal aspect of the forearm) under ultrasound guidance. Six days later, the patient began to experience elbow pain, redness, and swelling. The symptoms progressed within several hours and became so notable that she sought care at an urgent care facility the next morning. At this facility, she was told she had an infection and was prescribed oral levofloxacin 500 mg/d.
The patient presented to our clinic after 4 days of oral levofloxacin with no improvement of symptoms. She denied chills or fever and described her pain as moderate and radiating to her fingers. There was no history of trauma. The patient reported riding her bike more frequently, which had caused the original forearm pain that warranted the PIN injection. There were no other recent changes to activity. Her medical, social, and surgical histories were otherwise unremarkable.
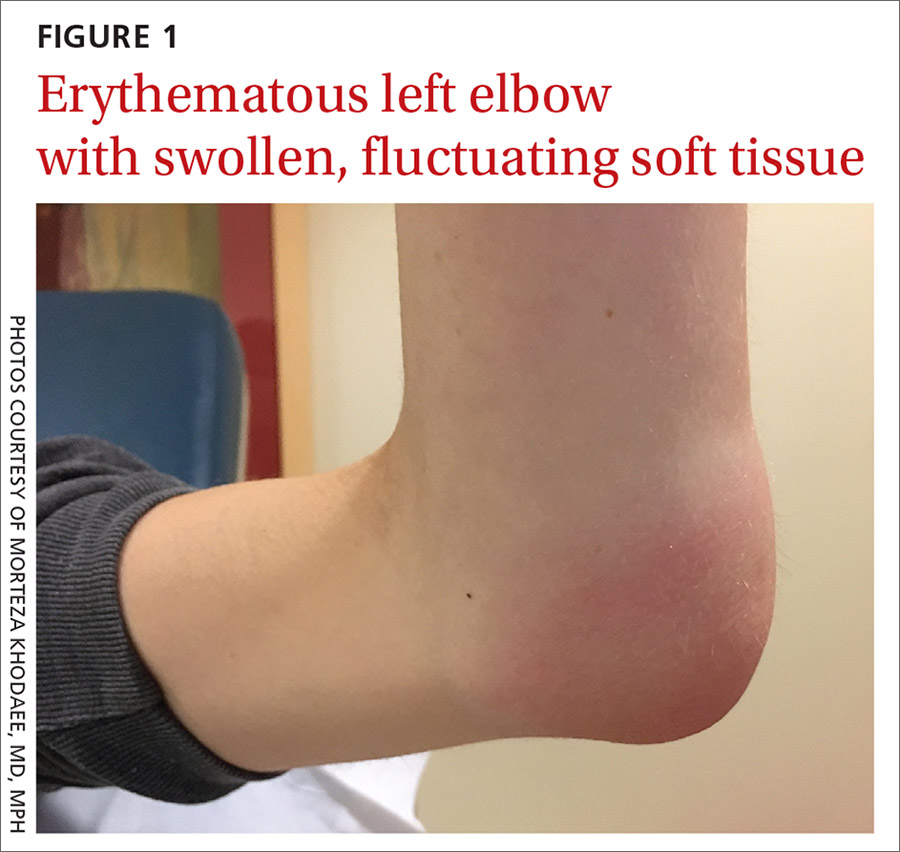
Her vital signs were normal. Physical exam revealed an erythematous and warm left elbow (FIGURE 1). Her left elbow range of motion (extension and flexion) was mildly decreased due to the pain and swelling.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Iatrogenic septic olecranon bursitis
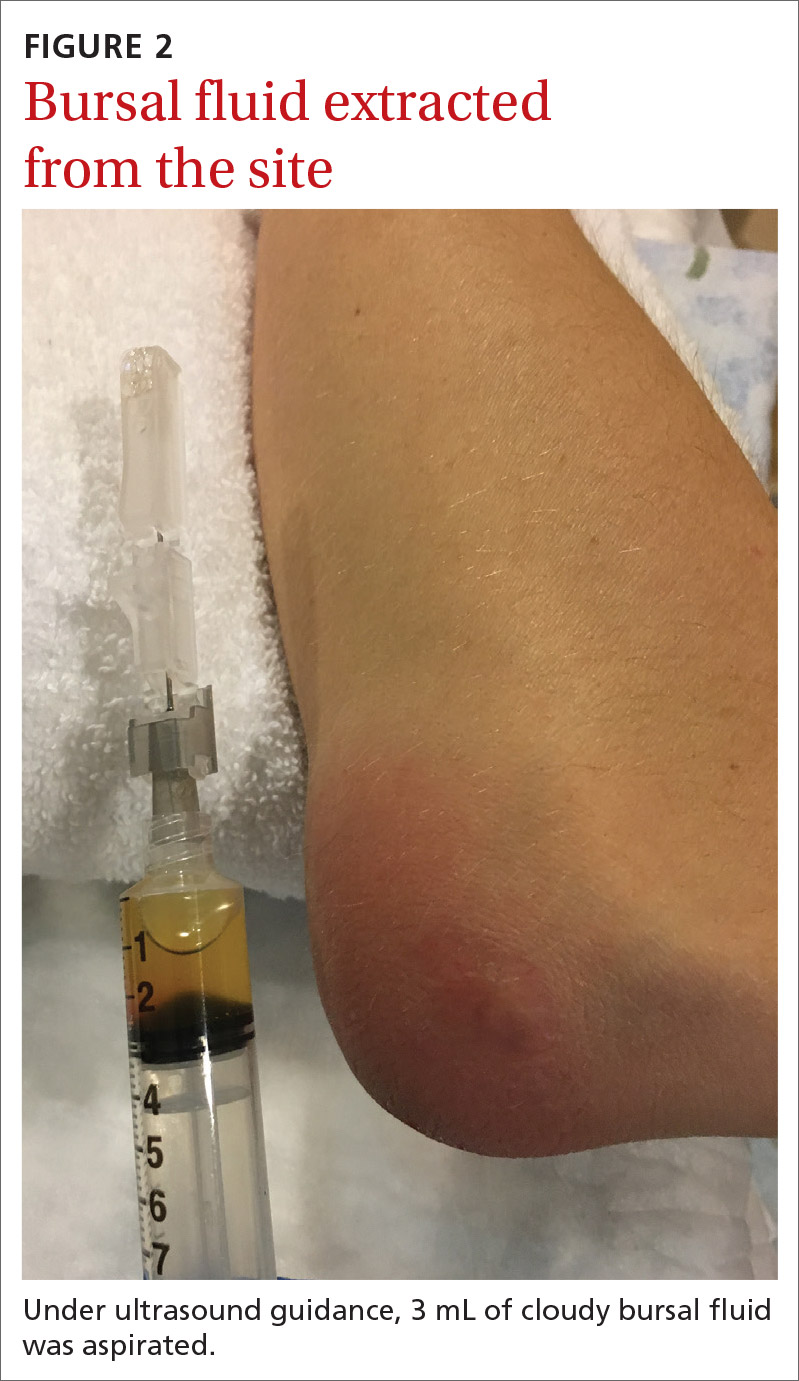
Aspiration of the patient’s olecranon bursa produced 3 mL of cloudy fluid (FIGURE 2). The patient’s painful, swollen, erythematous, warm elbow, cloudy aspirate, and history of preceding PIN hydrodissection were consistent with a diagnosis of septic olecranon bursitis.
Septic bursitis usually is caused by bacteria.1,2 Bursal infection can result from the spread of infection from nearby tissues or direct inoculation from skin trauma. It can also be iatrogenic and occur among healthy individuals.2,3 Injection anywhere close to the bursa can inoculate enough bacteria to progress to cellulitis first and then septic bursitis. Inflammatory conditions such as gout and rheumatoid arthritis also can cause acute and/or chronic superficial bursitis.1,2,4
Differentiating between septic and nonseptic bursitis can be challenging on history and physical exam alone, but specific signs and symptoms should warrant concern for infection.1,2,4,5 Fever is present in up to 75% of septic cases5; however, lack of fever does not rule out septic bursitis. Pain, erythema, warmth, and an overlying skin lesion also can indicate infection.4 Diagnostic imaging modalities may help distinguish different types of olecranon bursitis, but in most cases, they are not necessary.2
Other joint disorders factor into the differential
The differential diagnosis is broad and includes a variety of joint disorders in addition to septic (and nonseptic) bursitis.2,3
Septic arthritis is a deeper infection that involves the elbow joint and is considered an orthopedic emergency due to potential joint destruction.
Continue to: A simple joint effusion
A simple joint effusion also arises from the elbow joint, but this diagnosis becomes less likely when the joint aspirate appears cloudy. A simple joint effusion would not produce bacteria on gram stain and culture.
Crystalline inflammatory arthritis (gout, pseudogout) is due to intra-articular precipitation of crystals (uric acid crystals in gout, calcium pyrophosphate crystals in pseudogout).
Hematomas would produce gross blood or clot on joint aspiration.
Cellulitis is an infection of the superficial soft tissue (only) and thus, aspiration is not likely to yield fluid.
Diagnosis can be made with culture of fluid
Confirmation of septic olecranon bursitis is best attained by bursal needle aspiration and culture. Aspiration also can evaluate for other causes of elbow swelling. (If septic olecranon bursitis is suspected clinically, empiric antibiotics should be started while awaiting culture results.6) White blood cell counts from the aspirate also may be utilized but have a lower sensitivity and specificity for diagnosis.7
Continue to: In addition to aiding in diagnosis
In addition to aiding in diagnosis, bursal aspiration for a patient with septic bursitis can improve symptoms and reduce bacterial load.1-3,8 The use of a compressive bandage after aspiration may help reduce re-accumulation of the bursal fluid.1-3,8Staphylococcus aureus is responsible for the majority of septic olecranon bursitis cases.9-11
Tailoring the antibiotic regimen
There is wide variation in the treatment of septic olecranon bursitis due to the lack of strong evidence-based guidelines.1,2,8,11-13 When septic bursitis is strongly suspected (or confirmed) the patient should be started on an antibiotic regimen that covers S aureus.1,2 Once culture results and sensitivities return, the antibiotic regimen can be tailored appropriately.
In cases of mild-to-moderate septic olecranon bursitis in an immunocompetent host, the patient can be started on oral antibiotics and monitored closely as an outpatient.1-3,8 Patients with septic olecranon bursitis who meet the criteria for systemic inflammatory response syndrome or who are immunocompromised should be hospitalized and started on intravenous antibiotics.1-3 Recommended duration of antibiotic therapy varies but is usually about 10 to 14 days.1-3,8 In rare cases, surgical intervention with bursectomy may be necessary.1,2,14
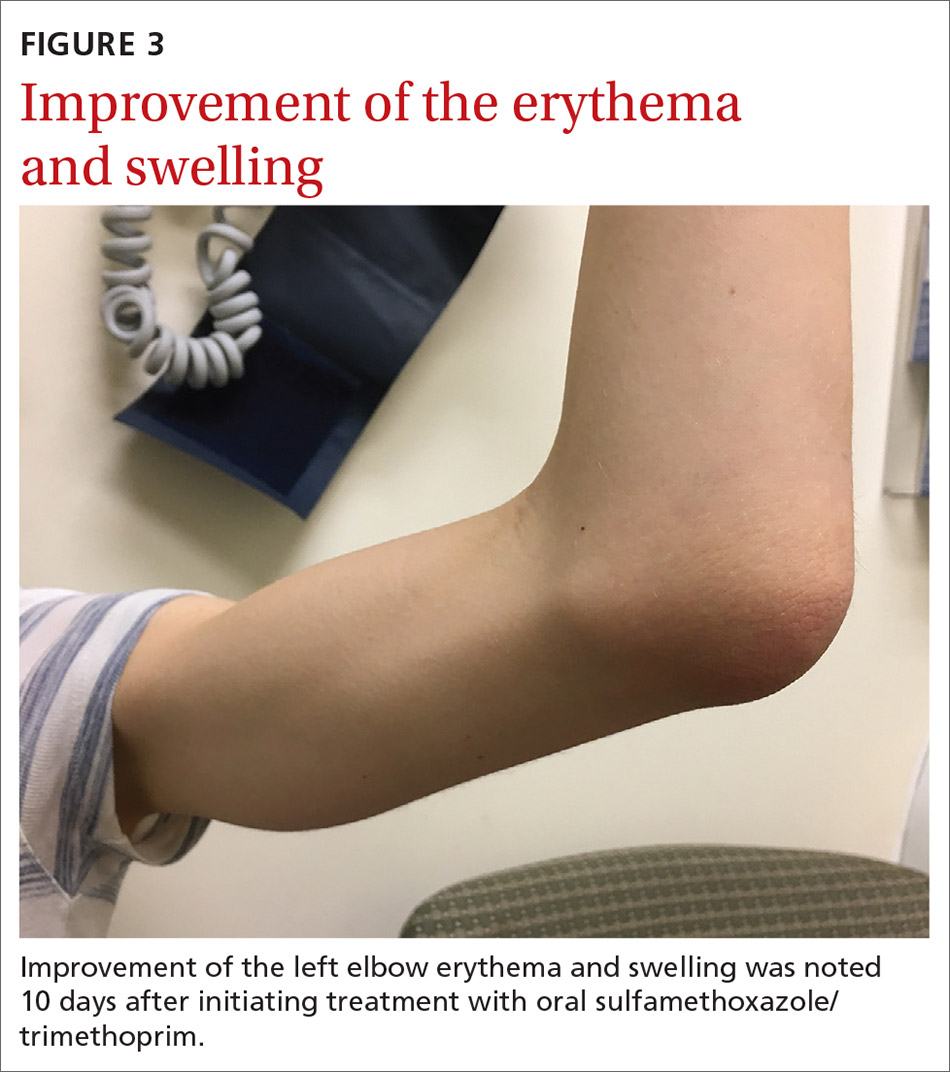
Our patient was given a dose of ceftriaxone 250 mg intramuscularly and was started on oral sulfamethoxazole/trimethoprim 800 mg/160 mg twice daily after aspiration of the bursa. Culture of the bursal fluid grew oxacillin-sensitive S aureus which was sensitive to a variety of antibiotics including levofloxacin and sulfamethoxazole/trimethoprim. Her symptoms gradually improved (FIGURE 3) and resolved after a 14-day course of oral sulfamethoxazole/trimethoprim.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine & Orthopedics, AFW Clinic, 3055 Roslyn St, Denver, CO 80238; morteza. khodaee@cuanschutz.edu
1. Baumbach SF, Lobo CM, Badyine I, et al. Prepatellar and olecranon bursitis: literature review and development of a treatment algorithm. Arch Orthop Trauma Surg. 2014;134:359-370.
2. Khodaee M. Common superficial bursitis. Am Fam Physician. 2017;95:224-231.
3. Harris-Spinks C, Nabhan D, Khodaee M. Noniatrogenic septic olecranon bursitis: report of two cases and review of the literature. Curr Sports Med Rep. 2016;15:33-37.
4. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25:158-167.
5. Blackwell JR, Hay BA, Bolt AM, et al. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6:182-190.
6. Del Buono A, Franceschi F, Palumbo A, et al. Diagnosis and management of olecranon bursitis. Surgeon. 2012;10:297-300.
7. Stell IM, Gransden WR. Simple tests for septic bursitis: comparative study. BMJ. 1998;316:1877.
8. Abzug JM, Chen NC, Jacoby SM. Septic olecranon bursitis. J Hand Surg Am. 2012;37:1252-1253.
9. Cea-Pereiro JC, Garcia-Meijide J, Mera-Varela A, et al. A comparison between septic bursitis caused by Staphylococcus aureus and those caused by other organisms. Clin Rheumatol. 2001;20:10-14.
10. Morrey BE. Bursitis. In: Morrey BE, Sanchez-Sotelo J, eds. The Elbow and its Disorders. 4th ed. Philadelphia, PA: Saunders Elsevier 2009:1164-1173.
11. Wingert NC, DeMaio M, Shenenberger DW. Septic olecranon bursitis, contact dermatitis, and pneumonitis in a gas turbine engine mechanic. J Shoulder Elbow Surg. 2012;21:E16-E20.
12. Baumbach SF, Michel M, Wyen H, et al. Current treatment concepts for olecranon and prepatellar bursitis in Austria. Z Orthop Unfall. 2013;151:149-155.
13. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134:1517-1536.
14. Ogilvie-Harris DJ, Gilbart M. Endoscopic bursal resection: the olecranon bursa and prepatellar bursa. Arthroscopy. 2000;16:249-253.
A 32-year-old woman presented to our clinic with left elbow swelling and pain of 6 days’ duration. She’d had a posterior interosseous nerve (PIN) injection (hydrodissection) at another facility 12 days earlier for refractory intersection syndrome.
During nerve hydrodissection, fluid is injected into the area surrounding the nerve in an effort to displace the muscles, tendons, and fascia and thus reduce friction on the nerve. This treatment, often completed with ultrasound guidance, is utilized by patients who want to obtain pain relief without undergoing surgery for nerve entrapment syndromes.
In this case, a combination of 1 mL (40 mg) of methylprednisolone acetate, 1 mL of lidocaine 2%, and 3 mL of normal saline was injected into the supinator muscle belly (proximal dorsal aspect of the forearm) under ultrasound guidance. Six days later, the patient began to experience elbow pain, redness, and swelling. The symptoms progressed within several hours and became so notable that she sought care at an urgent care facility the next morning. At this facility, she was told she had an infection and was prescribed oral levofloxacin 500 mg/d.
The patient presented to our clinic after 4 days of oral levofloxacin with no improvement of symptoms. She denied chills or fever and described her pain as moderate and radiating to her fingers. There was no history of trauma. The patient reported riding her bike more frequently, which had caused the original forearm pain that warranted the PIN injection. There were no other recent changes to activity. Her medical, social, and surgical histories were otherwise unremarkable.
Her vital signs were normal. Physical exam revealed an erythematous and warm left elbow (FIGURE 1). Her left elbow range of motion (extension and flexion) was mildly decreased due to the pain and swelling.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Iatrogenic septic olecranon bursitis
Aspiration of the patient’s olecranon bursa produced 3 mL of cloudy fluid (FIGURE 2). The patient’s painful, swollen, erythematous, warm elbow, cloudy aspirate, and history of preceding PIN hydrodissection were consistent with a diagnosis of septic olecranon bursitis.
Septic bursitis usually is caused by bacteria.1,2 Bursal infection can result from the spread of infection from nearby tissues or direct inoculation from skin trauma. It can also be iatrogenic and occur among healthy individuals.2,3 Injection anywhere close to the bursa can inoculate enough bacteria to progress to cellulitis first and then septic bursitis. Inflammatory conditions such as gout and rheumatoid arthritis also can cause acute and/or chronic superficial bursitis.1,2,4
Differentiating between septic and nonseptic bursitis can be challenging on history and physical exam alone, but specific signs and symptoms should warrant concern for infection.1,2,4,5 Fever is present in up to 75% of septic cases5; however, lack of fever does not rule out septic bursitis. Pain, erythema, warmth, and an overlying skin lesion also can indicate infection.4 Diagnostic imaging modalities may help distinguish different types of olecranon bursitis, but in most cases, they are not necessary.2
Other joint disorders factor into the differential
The differential diagnosis is broad and includes a variety of joint disorders in addition to septic (and nonseptic) bursitis.2,3
Septic arthritis is a deeper infection that involves the elbow joint and is considered an orthopedic emergency due to potential joint destruction.
Continue to: A simple joint effusion
A simple joint effusion also arises from the elbow joint, but this diagnosis becomes less likely when the joint aspirate appears cloudy. A simple joint effusion would not produce bacteria on gram stain and culture.
Crystalline inflammatory arthritis (gout, pseudogout) is due to intra-articular precipitation of crystals (uric acid crystals in gout, calcium pyrophosphate crystals in pseudogout).
Hematomas would produce gross blood or clot on joint aspiration.
Cellulitis is an infection of the superficial soft tissue (only) and thus, aspiration is not likely to yield fluid.
Diagnosis can be made with culture of fluid
Confirmation of septic olecranon bursitis is best attained by bursal needle aspiration and culture. Aspiration also can evaluate for other causes of elbow swelling. (If septic olecranon bursitis is suspected clinically, empiric antibiotics should be started while awaiting culture results.6) White blood cell counts from the aspirate also may be utilized but have a lower sensitivity and specificity for diagnosis.7
Continue to: In addition to aiding in diagnosis
In addition to aiding in diagnosis, bursal aspiration for a patient with septic bursitis can improve symptoms and reduce bacterial load.1-3,8 The use of a compressive bandage after aspiration may help reduce re-accumulation of the bursal fluid.1-3,8Staphylococcus aureus is responsible for the majority of septic olecranon bursitis cases.9-11
Tailoring the antibiotic regimen
There is wide variation in the treatment of septic olecranon bursitis due to the lack of strong evidence-based guidelines.1,2,8,11-13 When septic bursitis is strongly suspected (or confirmed) the patient should be started on an antibiotic regimen that covers S aureus.1,2 Once culture results and sensitivities return, the antibiotic regimen can be tailored appropriately.
In cases of mild-to-moderate septic olecranon bursitis in an immunocompetent host, the patient can be started on oral antibiotics and monitored closely as an outpatient.1-3,8 Patients with septic olecranon bursitis who meet the criteria for systemic inflammatory response syndrome or who are immunocompromised should be hospitalized and started on intravenous antibiotics.1-3 Recommended duration of antibiotic therapy varies but is usually about 10 to 14 days.1-3,8 In rare cases, surgical intervention with bursectomy may be necessary.1,2,14
Our patient was given a dose of ceftriaxone 250 mg intramuscularly and was started on oral sulfamethoxazole/trimethoprim 800 mg/160 mg twice daily after aspiration of the bursa. Culture of the bursal fluid grew oxacillin-sensitive S aureus which was sensitive to a variety of antibiotics including levofloxacin and sulfamethoxazole/trimethoprim. Her symptoms gradually improved (FIGURE 3) and resolved after a 14-day course of oral sulfamethoxazole/trimethoprim.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine & Orthopedics, AFW Clinic, 3055 Roslyn St, Denver, CO 80238; morteza. khodaee@cuanschutz.edu
A 32-year-old woman presented to our clinic with left elbow swelling and pain of 6 days’ duration. She’d had a posterior interosseous nerve (PIN) injection (hydrodissection) at another facility 12 days earlier for refractory intersection syndrome.
During nerve hydrodissection, fluid is injected into the area surrounding the nerve in an effort to displace the muscles, tendons, and fascia and thus reduce friction on the nerve. This treatment, often completed with ultrasound guidance, is utilized by patients who want to obtain pain relief without undergoing surgery for nerve entrapment syndromes.
In this case, a combination of 1 mL (40 mg) of methylprednisolone acetate, 1 mL of lidocaine 2%, and 3 mL of normal saline was injected into the supinator muscle belly (proximal dorsal aspect of the forearm) under ultrasound guidance. Six days later, the patient began to experience elbow pain, redness, and swelling. The symptoms progressed within several hours and became so notable that she sought care at an urgent care facility the next morning. At this facility, she was told she had an infection and was prescribed oral levofloxacin 500 mg/d.
The patient presented to our clinic after 4 days of oral levofloxacin with no improvement of symptoms. She denied chills or fever and described her pain as moderate and radiating to her fingers. There was no history of trauma. The patient reported riding her bike more frequently, which had caused the original forearm pain that warranted the PIN injection. There were no other recent changes to activity. Her medical, social, and surgical histories were otherwise unremarkable.
Her vital signs were normal. Physical exam revealed an erythematous and warm left elbow (FIGURE 1). Her left elbow range of motion (extension and flexion) was mildly decreased due to the pain and swelling.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Iatrogenic septic olecranon bursitis
Aspiration of the patient’s olecranon bursa produced 3 mL of cloudy fluid (FIGURE 2). The patient’s painful, swollen, erythematous, warm elbow, cloudy aspirate, and history of preceding PIN hydrodissection were consistent with a diagnosis of septic olecranon bursitis.
Septic bursitis usually is caused by bacteria.1,2 Bursal infection can result from the spread of infection from nearby tissues or direct inoculation from skin trauma. It can also be iatrogenic and occur among healthy individuals.2,3 Injection anywhere close to the bursa can inoculate enough bacteria to progress to cellulitis first and then septic bursitis. Inflammatory conditions such as gout and rheumatoid arthritis also can cause acute and/or chronic superficial bursitis.1,2,4
Differentiating between septic and nonseptic bursitis can be challenging on history and physical exam alone, but specific signs and symptoms should warrant concern for infection.1,2,4,5 Fever is present in up to 75% of septic cases5; however, lack of fever does not rule out septic bursitis. Pain, erythema, warmth, and an overlying skin lesion also can indicate infection.4 Diagnostic imaging modalities may help distinguish different types of olecranon bursitis, but in most cases, they are not necessary.2
Other joint disorders factor into the differential
The differential diagnosis is broad and includes a variety of joint disorders in addition to septic (and nonseptic) bursitis.2,3
Septic arthritis is a deeper infection that involves the elbow joint and is considered an orthopedic emergency due to potential joint destruction.
Continue to: A simple joint effusion
A simple joint effusion also arises from the elbow joint, but this diagnosis becomes less likely when the joint aspirate appears cloudy. A simple joint effusion would not produce bacteria on gram stain and culture.
Crystalline inflammatory arthritis (gout, pseudogout) is due to intra-articular precipitation of crystals (uric acid crystals in gout, calcium pyrophosphate crystals in pseudogout).
Hematomas would produce gross blood or clot on joint aspiration.
Cellulitis is an infection of the superficial soft tissue (only) and thus, aspiration is not likely to yield fluid.
Diagnosis can be made with culture of fluid
Confirmation of septic olecranon bursitis is best attained by bursal needle aspiration and culture. Aspiration also can evaluate for other causes of elbow swelling. (If septic olecranon bursitis is suspected clinically, empiric antibiotics should be started while awaiting culture results.6) White blood cell counts from the aspirate also may be utilized but have a lower sensitivity and specificity for diagnosis.7
Continue to: In addition to aiding in diagnosis
In addition to aiding in diagnosis, bursal aspiration for a patient with septic bursitis can improve symptoms and reduce bacterial load.1-3,8 The use of a compressive bandage after aspiration may help reduce re-accumulation of the bursal fluid.1-3,8Staphylococcus aureus is responsible for the majority of septic olecranon bursitis cases.9-11
Tailoring the antibiotic regimen
There is wide variation in the treatment of septic olecranon bursitis due to the lack of strong evidence-based guidelines.1,2,8,11-13 When septic bursitis is strongly suspected (or confirmed) the patient should be started on an antibiotic regimen that covers S aureus.1,2 Once culture results and sensitivities return, the antibiotic regimen can be tailored appropriately.
In cases of mild-to-moderate septic olecranon bursitis in an immunocompetent host, the patient can be started on oral antibiotics and monitored closely as an outpatient.1-3,8 Patients with septic olecranon bursitis who meet the criteria for systemic inflammatory response syndrome or who are immunocompromised should be hospitalized and started on intravenous antibiotics.1-3 Recommended duration of antibiotic therapy varies but is usually about 10 to 14 days.1-3,8 In rare cases, surgical intervention with bursectomy may be necessary.1,2,14
Our patient was given a dose of ceftriaxone 250 mg intramuscularly and was started on oral sulfamethoxazole/trimethoprim 800 mg/160 mg twice daily after aspiration of the bursa. Culture of the bursal fluid grew oxacillin-sensitive S aureus which was sensitive to a variety of antibiotics including levofloxacin and sulfamethoxazole/trimethoprim. Her symptoms gradually improved (FIGURE 3) and resolved after a 14-day course of oral sulfamethoxazole/trimethoprim.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine & Orthopedics, AFW Clinic, 3055 Roslyn St, Denver, CO 80238; morteza. khodaee@cuanschutz.edu
1. Baumbach SF, Lobo CM, Badyine I, et al. Prepatellar and olecranon bursitis: literature review and development of a treatment algorithm. Arch Orthop Trauma Surg. 2014;134:359-370.
2. Khodaee M. Common superficial bursitis. Am Fam Physician. 2017;95:224-231.
3. Harris-Spinks C, Nabhan D, Khodaee M. Noniatrogenic septic olecranon bursitis: report of two cases and review of the literature. Curr Sports Med Rep. 2016;15:33-37.
4. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25:158-167.
5. Blackwell JR, Hay BA, Bolt AM, et al. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6:182-190.
6. Del Buono A, Franceschi F, Palumbo A, et al. Diagnosis and management of olecranon bursitis. Surgeon. 2012;10:297-300.
7. Stell IM, Gransden WR. Simple tests for septic bursitis: comparative study. BMJ. 1998;316:1877.
8. Abzug JM, Chen NC, Jacoby SM. Septic olecranon bursitis. J Hand Surg Am. 2012;37:1252-1253.
9. Cea-Pereiro JC, Garcia-Meijide J, Mera-Varela A, et al. A comparison between septic bursitis caused by Staphylococcus aureus and those caused by other organisms. Clin Rheumatol. 2001;20:10-14.
10. Morrey BE. Bursitis. In: Morrey BE, Sanchez-Sotelo J, eds. The Elbow and its Disorders. 4th ed. Philadelphia, PA: Saunders Elsevier 2009:1164-1173.
11. Wingert NC, DeMaio M, Shenenberger DW. Septic olecranon bursitis, contact dermatitis, and pneumonitis in a gas turbine engine mechanic. J Shoulder Elbow Surg. 2012;21:E16-E20.
12. Baumbach SF, Michel M, Wyen H, et al. Current treatment concepts for olecranon and prepatellar bursitis in Austria. Z Orthop Unfall. 2013;151:149-155.
13. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134:1517-1536.
14. Ogilvie-Harris DJ, Gilbart M. Endoscopic bursal resection: the olecranon bursa and prepatellar bursa. Arthroscopy. 2000;16:249-253.
1. Baumbach SF, Lobo CM, Badyine I, et al. Prepatellar and olecranon bursitis: literature review and development of a treatment algorithm. Arch Orthop Trauma Surg. 2014;134:359-370.
2. Khodaee M. Common superficial bursitis. Am Fam Physician. 2017;95:224-231.
3. Harris-Spinks C, Nabhan D, Khodaee M. Noniatrogenic septic olecranon bursitis: report of two cases and review of the literature. Curr Sports Med Rep. 2016;15:33-37.
4. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25:158-167.
5. Blackwell JR, Hay BA, Bolt AM, et al. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6:182-190.
6. Del Buono A, Franceschi F, Palumbo A, et al. Diagnosis and management of olecranon bursitis. Surgeon. 2012;10:297-300.
7. Stell IM, Gransden WR. Simple tests for septic bursitis: comparative study. BMJ. 1998;316:1877.
8. Abzug JM, Chen NC, Jacoby SM. Septic olecranon bursitis. J Hand Surg Am. 2012;37:1252-1253.
9. Cea-Pereiro JC, Garcia-Meijide J, Mera-Varela A, et al. A comparison between septic bursitis caused by Staphylococcus aureus and those caused by other organisms. Clin Rheumatol. 2001;20:10-14.
10. Morrey BE. Bursitis. In: Morrey BE, Sanchez-Sotelo J, eds. The Elbow and its Disorders. 4th ed. Philadelphia, PA: Saunders Elsevier 2009:1164-1173.
11. Wingert NC, DeMaio M, Shenenberger DW. Septic olecranon bursitis, contact dermatitis, and pneumonitis in a gas turbine engine mechanic. J Shoulder Elbow Surg. 2012;21:E16-E20.
12. Baumbach SF, Michel M, Wyen H, et al. Current treatment concepts for olecranon and prepatellar bursitis in Austria. Z Orthop Unfall. 2013;151:149-155.
13. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134:1517-1536.
14. Ogilvie-Harris DJ, Gilbart M. Endoscopic bursal resection: the olecranon bursa and prepatellar bursa. Arthroscopy. 2000;16:249-253.
AAP releases new policy statement on barrier protection for teens
For adolescent patients, routinely take a sexual history, discuss the use of barrier methods, and perform relevant examinations, screenings, and vaccinations, according to a new policy statement on barrier protection use from the American Academy of Pediatrics’ Committee on Adolescence.
The policy statement has been expanded to cover multiple types of sexual activity and methods of barrier protection. These include not only traditional condoms, but also internal condoms (available in the United States only by prescription) and dental dams (for use during oral sex) or a latex sheet. “Pediatricians and other clinicians are encouraged to provide barrier methods within their offices and support availability within their communities,” said Laura K. Grubb, MD, MPH, of Tufts Medical Center in Boston, who authored both the policy statement and the technical report.
Counsel adolescents that abstaining from sexual intercourse is the best way to prevent genital sexually transmitted infections (STIs), HIV infection, and unplanned pregnancy. Also encourage and support consistent, correct barrier method use – in addition to other reliable contraception, if patients are sexually active or are thinking about becoming sexually active – the policy statement notes. Emphasize that all partners share responsibility to prevent STIs and unplanned pregnancies. “Adolescents with intellectual and physical disabilities are an overlooked group when it comes to sexual behavior, but they have similar rates of sexual behaviors when compared with their peers without disabilities,” Dr. Grubb and colleagues emphasized in the policy statement.
This is key because Centers for Disease Control and Prevention 2017 data showed that in the United States, “456,000 adolescent and young women younger than 20 years became pregnant; 448,000 of those pregnancies were among 15- to 19-year-olds, and 7,400 were among those 14 years of age and younger,” according to the technical report accompanying the policy statement. Also, “new cases of STIs increased 31% in the United States from 2013 to 2017, with half of the 2.3 million new STIs reported each year among young people 15 to 24 years of age.”
Parents may need support and encouragement to talk with their children about sex, sexuality, and the use of barrier methods to prevent STIs. Dr. Grubb and colleagues recommend via the policy statement: “Actively communicate to parents and communities that making barrier methods available to adolescents does not increase the onset or frequency of adolescent sexual activity, and that use of barrier methods can help decrease rates of unintended pregnancy and acquisition of STIs.”
Use Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents, Fourth Edition, for guidance on supporting parents and adolescents in promoting healthy sexual development and sexuality, including discussions of barrier methods.
Some groups of adolescents may use barrier methods less consistently because they perceive themselves to be lower risk. These include adolescents who use preexposure prophylaxis or nonbarrier contraception, who identify as bisexual or lesbian, or who are in established relationships. Monitor these patients to assess their risk and need for additional counseling. In the technical report, studies are cited finding that barrier methods are used less consistently during oral sex and that condom use is lower among cisgender and transgender females, and among adolescents who self-identify as gay, lesbian, or bisexual, compared with other groups.
In the policy statement, Dr. Grubb and colleagues call on pediatricians to advocate for more research and better access to barrier methods, especially for higher-risk adolescents and those living in underserved areas. In particular, school education programs on barrier methods can reach large adolescent groups and provide a “comprehensive array of educational and health care resources.”
Katie Brigham, MD, a pediatrician at MassGeneral Hospital for Children in Boston, affirmed the recommendations in the new policy statement (which she did not help write or research). “Even though the pregnancy rate is dropping in the United States, STI rates are increasing, so it is vital that pediatricians and other providers of adolescents and young adults counsel all their patients, regardless of gender and sexual orientation, of the importance of barrier methods when having oral, vaginal, or anal sex,” she said in an interview.
Dr. Brigham praised the technical report, adding that she found no major weaknesses in its methodology. “For future research, it would be interesting to see if there are different rates of pregnancy and STIs in pediatric practices that provide condoms and other barrier methods free to their patients, compared to those that do not.”
No external funding sources were reported. Dr. Grubb and Dr. Brigham reported having no relevant financial disclosures.
SOURCE: Grubb LK et al. Pediatrics. 2020 Jul 20. doi: 10.1542/peds.2020-007237.
For adolescent patients, routinely take a sexual history, discuss the use of barrier methods, and perform relevant examinations, screenings, and vaccinations, according to a new policy statement on barrier protection use from the American Academy of Pediatrics’ Committee on Adolescence.
The policy statement has been expanded to cover multiple types of sexual activity and methods of barrier protection. These include not only traditional condoms, but also internal condoms (available in the United States only by prescription) and dental dams (for use during oral sex) or a latex sheet. “Pediatricians and other clinicians are encouraged to provide barrier methods within their offices and support availability within their communities,” said Laura K. Grubb, MD, MPH, of Tufts Medical Center in Boston, who authored both the policy statement and the technical report.
Counsel adolescents that abstaining from sexual intercourse is the best way to prevent genital sexually transmitted infections (STIs), HIV infection, and unplanned pregnancy. Also encourage and support consistent, correct barrier method use – in addition to other reliable contraception, if patients are sexually active or are thinking about becoming sexually active – the policy statement notes. Emphasize that all partners share responsibility to prevent STIs and unplanned pregnancies. “Adolescents with intellectual and physical disabilities are an overlooked group when it comes to sexual behavior, but they have similar rates of sexual behaviors when compared with their peers without disabilities,” Dr. Grubb and colleagues emphasized in the policy statement.
This is key because Centers for Disease Control and Prevention 2017 data showed that in the United States, “456,000 adolescent and young women younger than 20 years became pregnant; 448,000 of those pregnancies were among 15- to 19-year-olds, and 7,400 were among those 14 years of age and younger,” according to the technical report accompanying the policy statement. Also, “new cases of STIs increased 31% in the United States from 2013 to 2017, with half of the 2.3 million new STIs reported each year among young people 15 to 24 years of age.”
Parents may need support and encouragement to talk with their children about sex, sexuality, and the use of barrier methods to prevent STIs. Dr. Grubb and colleagues recommend via the policy statement: “Actively communicate to parents and communities that making barrier methods available to adolescents does not increase the onset or frequency of adolescent sexual activity, and that use of barrier methods can help decrease rates of unintended pregnancy and acquisition of STIs.”
Use Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents, Fourth Edition, for guidance on supporting parents and adolescents in promoting healthy sexual development and sexuality, including discussions of barrier methods.
Some groups of adolescents may use barrier methods less consistently because they perceive themselves to be lower risk. These include adolescents who use preexposure prophylaxis or nonbarrier contraception, who identify as bisexual or lesbian, or who are in established relationships. Monitor these patients to assess their risk and need for additional counseling. In the technical report, studies are cited finding that barrier methods are used less consistently during oral sex and that condom use is lower among cisgender and transgender females, and among adolescents who self-identify as gay, lesbian, or bisexual, compared with other groups.
In the policy statement, Dr. Grubb and colleagues call on pediatricians to advocate for more research and better access to barrier methods, especially for higher-risk adolescents and those living in underserved areas. In particular, school education programs on barrier methods can reach large adolescent groups and provide a “comprehensive array of educational and health care resources.”
Katie Brigham, MD, a pediatrician at MassGeneral Hospital for Children in Boston, affirmed the recommendations in the new policy statement (which she did not help write or research). “Even though the pregnancy rate is dropping in the United States, STI rates are increasing, so it is vital that pediatricians and other providers of adolescents and young adults counsel all their patients, regardless of gender and sexual orientation, of the importance of barrier methods when having oral, vaginal, or anal sex,” she said in an interview.
Dr. Brigham praised the technical report, adding that she found no major weaknesses in its methodology. “For future research, it would be interesting to see if there are different rates of pregnancy and STIs in pediatric practices that provide condoms and other barrier methods free to their patients, compared to those that do not.”
No external funding sources were reported. Dr. Grubb and Dr. Brigham reported having no relevant financial disclosures.
SOURCE: Grubb LK et al. Pediatrics. 2020 Jul 20. doi: 10.1542/peds.2020-007237.
For adolescent patients, routinely take a sexual history, discuss the use of barrier methods, and perform relevant examinations, screenings, and vaccinations, according to a new policy statement on barrier protection use from the American Academy of Pediatrics’ Committee on Adolescence.
The policy statement has been expanded to cover multiple types of sexual activity and methods of barrier protection. These include not only traditional condoms, but also internal condoms (available in the United States only by prescription) and dental dams (for use during oral sex) or a latex sheet. “Pediatricians and other clinicians are encouraged to provide barrier methods within their offices and support availability within their communities,” said Laura K. Grubb, MD, MPH, of Tufts Medical Center in Boston, who authored both the policy statement and the technical report.
Counsel adolescents that abstaining from sexual intercourse is the best way to prevent genital sexually transmitted infections (STIs), HIV infection, and unplanned pregnancy. Also encourage and support consistent, correct barrier method use – in addition to other reliable contraception, if patients are sexually active or are thinking about becoming sexually active – the policy statement notes. Emphasize that all partners share responsibility to prevent STIs and unplanned pregnancies. “Adolescents with intellectual and physical disabilities are an overlooked group when it comes to sexual behavior, but they have similar rates of sexual behaviors when compared with their peers without disabilities,” Dr. Grubb and colleagues emphasized in the policy statement.
This is key because Centers for Disease Control and Prevention 2017 data showed that in the United States, “456,000 adolescent and young women younger than 20 years became pregnant; 448,000 of those pregnancies were among 15- to 19-year-olds, and 7,400 were among those 14 years of age and younger,” according to the technical report accompanying the policy statement. Also, “new cases of STIs increased 31% in the United States from 2013 to 2017, with half of the 2.3 million new STIs reported each year among young people 15 to 24 years of age.”
Parents may need support and encouragement to talk with their children about sex, sexuality, and the use of barrier methods to prevent STIs. Dr. Grubb and colleagues recommend via the policy statement: “Actively communicate to parents and communities that making barrier methods available to adolescents does not increase the onset or frequency of adolescent sexual activity, and that use of barrier methods can help decrease rates of unintended pregnancy and acquisition of STIs.”
Use Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents, Fourth Edition, for guidance on supporting parents and adolescents in promoting healthy sexual development and sexuality, including discussions of barrier methods.
Some groups of adolescents may use barrier methods less consistently because they perceive themselves to be lower risk. These include adolescents who use preexposure prophylaxis or nonbarrier contraception, who identify as bisexual or lesbian, or who are in established relationships. Monitor these patients to assess their risk and need for additional counseling. In the technical report, studies are cited finding that barrier methods are used less consistently during oral sex and that condom use is lower among cisgender and transgender females, and among adolescents who self-identify as gay, lesbian, or bisexual, compared with other groups.
In the policy statement, Dr. Grubb and colleagues call on pediatricians to advocate for more research and better access to barrier methods, especially for higher-risk adolescents and those living in underserved areas. In particular, school education programs on barrier methods can reach large adolescent groups and provide a “comprehensive array of educational and health care resources.”
Katie Brigham, MD, a pediatrician at MassGeneral Hospital for Children in Boston, affirmed the recommendations in the new policy statement (which she did not help write or research). “Even though the pregnancy rate is dropping in the United States, STI rates are increasing, so it is vital that pediatricians and other providers of adolescents and young adults counsel all their patients, regardless of gender and sexual orientation, of the importance of barrier methods when having oral, vaginal, or anal sex,” she said in an interview.
Dr. Brigham praised the technical report, adding that she found no major weaknesses in its methodology. “For future research, it would be interesting to see if there are different rates of pregnancy and STIs in pediatric practices that provide condoms and other barrier methods free to their patients, compared to those that do not.”
No external funding sources were reported. Dr. Grubb and Dr. Brigham reported having no relevant financial disclosures.
SOURCE: Grubb LK et al. Pediatrics. 2020 Jul 20. doi: 10.1542/peds.2020-007237.
FROM PEDIATRICS
Do-it-yourself cervical cancer screening?
ILLUSTRATIVE CASE
A 40-year-old woman presents to your office to establish care. During your interview you realize that she has never been screened for cervical cancer. In fact, she has not had a pelvic exam because she is fearful of the procedure. She would like to know if alternatives exist for cervical cancer screening. What can you suggest?
Although deaths from cervical cancer decreased in the United States from 1975 to 2017, demographic and social disparities in the burden of the disease remain.2,3 Data from 2016 reveal that cervical cancer incidence per 100,000 women is lowest among white (7.5), Asian-Pacific Islander (5.8), and American Indian/Alaska native (5.6) women, and highest among Hispanic (9.8) and black (8.7) women, which could be explained by lower screening rates in these populations.4,5 The National Cancer Institute’s publication on reducing cancer health disparities states that the most effective way to reduce cervical cancer incidence and mortality is by increasing screening rates among women who have not been screened or who have not been screened regularly.6
The US Food and Drug Administration (FDA) approved the first human papillomavirus (HPV) screening test in 2003.7 Evidence now suggests that high-risk HPV screening provides greater protection against cervical cancer than screening with cytology alone.8 The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) have changed their recommendations to include primary HPV testing as an alternative method to Pap smears for cervical cancer screening.9
An advantage of primary HPV screening is that it can be performed on a specimen collected by the patient, which could potentially increase rates of screening and help to decrease demographic and social disparities. A randomized trial of almost 2000 women ages 21 to 65 years that evaluated the acceptability of this method to patients revealed that more than half of women prefer the idea of a self-collected specimen to one that is collected by a clinician because it is more convenient and obviates the need for a pelvic exam.10
A meta-analysis of 36 studies and more than 150,000 women concluded that when self-collected samples were used with signal-based assays, the tests were not as sensitive or specific as when clinician-collected samples were used.11 However, the meta-analysis also found that some polymerase chain reaction (PCR)-based HPV tests were similarly sensitive for both self- and clinician-collected samples.
STUDY SUMMARY
PCR vs signal amplification HPV tests with collection by patients vs clinicians
This meta-analysis compared the accuracy of high-risk HPV self-screening with clinician collection of samples (56 diagnostic accuracy trials; total N not provided) in identifying cervical intraepithelial neoplasia grade 2 or worse (CIN 2+) with signal amplification and PCR tests evaluated separately.1 In addition, this review evaluated strategies to screen women who are underscreened or not screened, which was defined as women who were irregularly or never screened, or did not respond to reminder letters about cervical cancer screening (25 randomized controlled trials [RCTs]; total N not provided).
In the diagnostic accuracy studies, patients collected a vaginal sample themselves and then had a sample taken by a clinician. CIN 2+ or 3+ was confirmed by either colposcopy and biopsy performed on all patients or by a positive high-risk HPV test result. Studies were further divided into those using assays based on signal amplification or PCR.
Continue to: In signal amplification assays...
In signal amplification assays, the pooled sensitivity for CIN 2+ was lower in the group with the self-collected samples than in the clinician-collected sample group (77%; 95% confidence interval [CI], 69%-82% vs 93%; 95% CI, 89%-96%). The pooled specificity to exclude CIN 2+ was also lower in the group with the self-collected samples (84%; 95% CI, 77%-88% vs 86%; 95% CI, 81%-90%). In high-risk HPV assays based on PCR, there was no difference in sensitivity (96%) or specificity (79%) between the specimen groups.
With regard to the pooled relative sensitivity and specificity of signal amplification assays, those using self-swab samples were less sensitive and less specific for CIN 2+ (sensitivity ratio = 0.85; 95% CI, 0.80-0.89; specificity ratio = 0.96; 95% CI, 0.93-0.98) and CIN 3+ (sensitivity ratio = 0.86; 95% CI, 0.76-0.98; specificity ratio = 0.97; 95% CI, 0.95-0.99). Using PCR assays, there was no difference between groups in relative sensitivity for the diagnosis of CIN 2+ (sensitivity ratio = 0.99; 95% CI, 0.97-1.02) and CIN 3+ (sensitivity ratio = 0.99; 95% CI, 0.96-1.02). Relative specificity was slightly lower in the self-swab group for CIN 2+ (specificity ratio = 0.98; 95% CI, 0.97-0.99) and CIN 3+ (specificity ratio = 0.98; 95% CI, 0.97-0.99).
The second analysis to evaluate which outreach strategies are effective methods for screening underscreened/unscreened women found that delivering self-sample kits to patients was more effective than the control method, which was sending reminders to women to undergo conventional screening (95% vs 53%; mean difference [MD], 41%; 95% CI, 3%-78%). Similarly, mailing kits to patients compared favorably to the control method (25% vs 12%; MD, 13%; 95% CI, 10%-15%).
WHAT’S NEW
Self-collected specimens can beas reliable as clinician-collected ones
This is the first study to provide robust evidence that high-risk HPV PCR-based assays using patient self-collected specimens are as sensitive at diagnosing CIN 2+ or 3+ as using clinician-collected samples.
CAVEATS
Balancing lower specificity with reaching underscreened populations
Patients with a positive HPV test result require additional testing. The success rates for this follow-up are not known and could be a barrier to accurate diagnoses because of accessibility and patient willingness to follow up with a pelvic exam. In addition, self-collection may be less specific than cytology and could increase colposcopy referrals that lead to negative findings and overtreatment.12 However, the increased acceptance of this screening method could make it an effective strategy to reach underscreened or reluctant patients.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Availability of PCR-based HPV assays may be an issue
HPV PCR assays may not be available at all laboratories, but signal amplification HPV tests have been shown to be inferior to PCR assays. Physicians will have to confirm with their laboratories whether PCR-based HPV assays are available.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Arbyn M, Smith SB, Temin S, et al; Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self-samples: updated meta-analyses. BMJ. 2018;363:k4823.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: cervical cancer. www.seer.cancer.gov/statfacts/html/cervix.html. Accessed June 29, 2020.
3. Singh GK, Azuine RE, Siahpush M. Global inequalities in cervical cancer incidence and mortality are linked to deprivation, low socioeconomic status, and human development. Int J MCH AIDS. 2012;1:17‐30.
4. US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999-2016): US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. June 2019. www.cdc.gov/cancer/dataviz. Accessed June 29, 2020.
5. MacLaughlin KL, Jacobson RM, Breitkopf CR, et al. Trends over time in Pap and Pap-HPV cotesting for cervical cancer screening. J Womens Health. 2019;28:244-249.
6. Freeman HP, Wingrove BK. Excess Cervical Cancer Mortality: A Marker for Low Access to Health Care in Poor Communities. NIH Pub. No. 05–5282. Rockville, MD: National Cancer Institute, Center to Reduce Cancer Health Disparities, May 2005. www.cancer.gov/about-nci/organization/crchd/about-health-disparities/resources/excess-cervical-cancer-mortality.pdf. Accessed June 29, 2020.
7. FDA approves expanded use of HPV test. Infection Control Today. March 31, 2003. https://www.infectioncontroltoday.com/view/fda-approves-expanded-use-hpv-test. Accessed June 29, 2020.
8. Ronco G, Dillner J, Elfström K, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
9. CDC. Cervical cancer screening guidelines for average-risk women. www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. Accessed June 29, 2020.
10. Mao C, Kulasingam S, Whitham H, et al. Clinician and patient acceptability of self-collected human papillomavirus testing for cervical cancer screening. J Womens Health. 2017;26:609-615.
11. Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15:172-183.
12. Lazcano-Ponce E, Lorincz A, Cruz-Valdez A, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011;378:1868-1873.
ILLUSTRATIVE CASE
A 40-year-old woman presents to your office to establish care. During your interview you realize that she has never been screened for cervical cancer. In fact, she has not had a pelvic exam because she is fearful of the procedure. She would like to know if alternatives exist for cervical cancer screening. What can you suggest?
Although deaths from cervical cancer decreased in the United States from 1975 to 2017, demographic and social disparities in the burden of the disease remain.2,3 Data from 2016 reveal that cervical cancer incidence per 100,000 women is lowest among white (7.5), Asian-Pacific Islander (5.8), and American Indian/Alaska native (5.6) women, and highest among Hispanic (9.8) and black (8.7) women, which could be explained by lower screening rates in these populations.4,5 The National Cancer Institute’s publication on reducing cancer health disparities states that the most effective way to reduce cervical cancer incidence and mortality is by increasing screening rates among women who have not been screened or who have not been screened regularly.6
The US Food and Drug Administration (FDA) approved the first human papillomavirus (HPV) screening test in 2003.7 Evidence now suggests that high-risk HPV screening provides greater protection against cervical cancer than screening with cytology alone.8 The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) have changed their recommendations to include primary HPV testing as an alternative method to Pap smears for cervical cancer screening.9
An advantage of primary HPV screening is that it can be performed on a specimen collected by the patient, which could potentially increase rates of screening and help to decrease demographic and social disparities. A randomized trial of almost 2000 women ages 21 to 65 years that evaluated the acceptability of this method to patients revealed that more than half of women prefer the idea of a self-collected specimen to one that is collected by a clinician because it is more convenient and obviates the need for a pelvic exam.10
A meta-analysis of 36 studies and more than 150,000 women concluded that when self-collected samples were used with signal-based assays, the tests were not as sensitive or specific as when clinician-collected samples were used.11 However, the meta-analysis also found that some polymerase chain reaction (PCR)-based HPV tests were similarly sensitive for both self- and clinician-collected samples.
STUDY SUMMARY
PCR vs signal amplification HPV tests with collection by patients vs clinicians
This meta-analysis compared the accuracy of high-risk HPV self-screening with clinician collection of samples (56 diagnostic accuracy trials; total N not provided) in identifying cervical intraepithelial neoplasia grade 2 or worse (CIN 2+) with signal amplification and PCR tests evaluated separately.1 In addition, this review evaluated strategies to screen women who are underscreened or not screened, which was defined as women who were irregularly or never screened, or did not respond to reminder letters about cervical cancer screening (25 randomized controlled trials [RCTs]; total N not provided).
In the diagnostic accuracy studies, patients collected a vaginal sample themselves and then had a sample taken by a clinician. CIN 2+ or 3+ was confirmed by either colposcopy and biopsy performed on all patients or by a positive high-risk HPV test result. Studies were further divided into those using assays based on signal amplification or PCR.
Continue to: In signal amplification assays...
In signal amplification assays, the pooled sensitivity for CIN 2+ was lower in the group with the self-collected samples than in the clinician-collected sample group (77%; 95% confidence interval [CI], 69%-82% vs 93%; 95% CI, 89%-96%). The pooled specificity to exclude CIN 2+ was also lower in the group with the self-collected samples (84%; 95% CI, 77%-88% vs 86%; 95% CI, 81%-90%). In high-risk HPV assays based on PCR, there was no difference in sensitivity (96%) or specificity (79%) between the specimen groups.
With regard to the pooled relative sensitivity and specificity of signal amplification assays, those using self-swab samples were less sensitive and less specific for CIN 2+ (sensitivity ratio = 0.85; 95% CI, 0.80-0.89; specificity ratio = 0.96; 95% CI, 0.93-0.98) and CIN 3+ (sensitivity ratio = 0.86; 95% CI, 0.76-0.98; specificity ratio = 0.97; 95% CI, 0.95-0.99). Using PCR assays, there was no difference between groups in relative sensitivity for the diagnosis of CIN 2+ (sensitivity ratio = 0.99; 95% CI, 0.97-1.02) and CIN 3+ (sensitivity ratio = 0.99; 95% CI, 0.96-1.02). Relative specificity was slightly lower in the self-swab group for CIN 2+ (specificity ratio = 0.98; 95% CI, 0.97-0.99) and CIN 3+ (specificity ratio = 0.98; 95% CI, 0.97-0.99).
The second analysis to evaluate which outreach strategies are effective methods for screening underscreened/unscreened women found that delivering self-sample kits to patients was more effective than the control method, which was sending reminders to women to undergo conventional screening (95% vs 53%; mean difference [MD], 41%; 95% CI, 3%-78%). Similarly, mailing kits to patients compared favorably to the control method (25% vs 12%; MD, 13%; 95% CI, 10%-15%).
WHAT’S NEW
Self-collected specimens can beas reliable as clinician-collected ones
This is the first study to provide robust evidence that high-risk HPV PCR-based assays using patient self-collected specimens are as sensitive at diagnosing CIN 2+ or 3+ as using clinician-collected samples.
CAVEATS
Balancing lower specificity with reaching underscreened populations
Patients with a positive HPV test result require additional testing. The success rates for this follow-up are not known and could be a barrier to accurate diagnoses because of accessibility and patient willingness to follow up with a pelvic exam. In addition, self-collection may be less specific than cytology and could increase colposcopy referrals that lead to negative findings and overtreatment.12 However, the increased acceptance of this screening method could make it an effective strategy to reach underscreened or reluctant patients.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Availability of PCR-based HPV assays may be an issue
HPV PCR assays may not be available at all laboratories, but signal amplification HPV tests have been shown to be inferior to PCR assays. Physicians will have to confirm with their laboratories whether PCR-based HPV assays are available.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 40-year-old woman presents to your office to establish care. During your interview you realize that she has never been screened for cervical cancer. In fact, she has not had a pelvic exam because she is fearful of the procedure. She would like to know if alternatives exist for cervical cancer screening. What can you suggest?
Although deaths from cervical cancer decreased in the United States from 1975 to 2017, demographic and social disparities in the burden of the disease remain.2,3 Data from 2016 reveal that cervical cancer incidence per 100,000 women is lowest among white (7.5), Asian-Pacific Islander (5.8), and American Indian/Alaska native (5.6) women, and highest among Hispanic (9.8) and black (8.7) women, which could be explained by lower screening rates in these populations.4,5 The National Cancer Institute’s publication on reducing cancer health disparities states that the most effective way to reduce cervical cancer incidence and mortality is by increasing screening rates among women who have not been screened or who have not been screened regularly.6
The US Food and Drug Administration (FDA) approved the first human papillomavirus (HPV) screening test in 2003.7 Evidence now suggests that high-risk HPV screening provides greater protection against cervical cancer than screening with cytology alone.8 The American College of Obstetricians and Gynecologists (ACOG) and the US Preventive Services Task Force (USPSTF) have changed their recommendations to include primary HPV testing as an alternative method to Pap smears for cervical cancer screening.9
An advantage of primary HPV screening is that it can be performed on a specimen collected by the patient, which could potentially increase rates of screening and help to decrease demographic and social disparities. A randomized trial of almost 2000 women ages 21 to 65 years that evaluated the acceptability of this method to patients revealed that more than half of women prefer the idea of a self-collected specimen to one that is collected by a clinician because it is more convenient and obviates the need for a pelvic exam.10
A meta-analysis of 36 studies and more than 150,000 women concluded that when self-collected samples were used with signal-based assays, the tests were not as sensitive or specific as when clinician-collected samples were used.11 However, the meta-analysis also found that some polymerase chain reaction (PCR)-based HPV tests were similarly sensitive for both self- and clinician-collected samples.
STUDY SUMMARY
PCR vs signal amplification HPV tests with collection by patients vs clinicians
This meta-analysis compared the accuracy of high-risk HPV self-screening with clinician collection of samples (56 diagnostic accuracy trials; total N not provided) in identifying cervical intraepithelial neoplasia grade 2 or worse (CIN 2+) with signal amplification and PCR tests evaluated separately.1 In addition, this review evaluated strategies to screen women who are underscreened or not screened, which was defined as women who were irregularly or never screened, or did not respond to reminder letters about cervical cancer screening (25 randomized controlled trials [RCTs]; total N not provided).
In the diagnostic accuracy studies, patients collected a vaginal sample themselves and then had a sample taken by a clinician. CIN 2+ or 3+ was confirmed by either colposcopy and biopsy performed on all patients or by a positive high-risk HPV test result. Studies were further divided into those using assays based on signal amplification or PCR.
Continue to: In signal amplification assays...
In signal amplification assays, the pooled sensitivity for CIN 2+ was lower in the group with the self-collected samples than in the clinician-collected sample group (77%; 95% confidence interval [CI], 69%-82% vs 93%; 95% CI, 89%-96%). The pooled specificity to exclude CIN 2+ was also lower in the group with the self-collected samples (84%; 95% CI, 77%-88% vs 86%; 95% CI, 81%-90%). In high-risk HPV assays based on PCR, there was no difference in sensitivity (96%) or specificity (79%) between the specimen groups.
With regard to the pooled relative sensitivity and specificity of signal amplification assays, those using self-swab samples were less sensitive and less specific for CIN 2+ (sensitivity ratio = 0.85; 95% CI, 0.80-0.89; specificity ratio = 0.96; 95% CI, 0.93-0.98) and CIN 3+ (sensitivity ratio = 0.86; 95% CI, 0.76-0.98; specificity ratio = 0.97; 95% CI, 0.95-0.99). Using PCR assays, there was no difference between groups in relative sensitivity for the diagnosis of CIN 2+ (sensitivity ratio = 0.99; 95% CI, 0.97-1.02) and CIN 3+ (sensitivity ratio = 0.99; 95% CI, 0.96-1.02). Relative specificity was slightly lower in the self-swab group for CIN 2+ (specificity ratio = 0.98; 95% CI, 0.97-0.99) and CIN 3+ (specificity ratio = 0.98; 95% CI, 0.97-0.99).
The second analysis to evaluate which outreach strategies are effective methods for screening underscreened/unscreened women found that delivering self-sample kits to patients was more effective than the control method, which was sending reminders to women to undergo conventional screening (95% vs 53%; mean difference [MD], 41%; 95% CI, 3%-78%). Similarly, mailing kits to patients compared favorably to the control method (25% vs 12%; MD, 13%; 95% CI, 10%-15%).
WHAT’S NEW
Self-collected specimens can beas reliable as clinician-collected ones
This is the first study to provide robust evidence that high-risk HPV PCR-based assays using patient self-collected specimens are as sensitive at diagnosing CIN 2+ or 3+ as using clinician-collected samples.
CAVEATS
Balancing lower specificity with reaching underscreened populations
Patients with a positive HPV test result require additional testing. The success rates for this follow-up are not known and could be a barrier to accurate diagnoses because of accessibility and patient willingness to follow up with a pelvic exam. In addition, self-collection may be less specific than cytology and could increase colposcopy referrals that lead to negative findings and overtreatment.12 However, the increased acceptance of this screening method could make it an effective strategy to reach underscreened or reluctant patients.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Availability of PCR-based HPV assays may be an issue
HPV PCR assays may not be available at all laboratories, but signal amplification HPV tests have been shown to be inferior to PCR assays. Physicians will have to confirm with their laboratories whether PCR-based HPV assays are available.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Arbyn M, Smith SB, Temin S, et al; Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self-samples: updated meta-analyses. BMJ. 2018;363:k4823.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: cervical cancer. www.seer.cancer.gov/statfacts/html/cervix.html. Accessed June 29, 2020.
3. Singh GK, Azuine RE, Siahpush M. Global inequalities in cervical cancer incidence and mortality are linked to deprivation, low socioeconomic status, and human development. Int J MCH AIDS. 2012;1:17‐30.
4. US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999-2016): US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. June 2019. www.cdc.gov/cancer/dataviz. Accessed June 29, 2020.
5. MacLaughlin KL, Jacobson RM, Breitkopf CR, et al. Trends over time in Pap and Pap-HPV cotesting for cervical cancer screening. J Womens Health. 2019;28:244-249.
6. Freeman HP, Wingrove BK. Excess Cervical Cancer Mortality: A Marker for Low Access to Health Care in Poor Communities. NIH Pub. No. 05–5282. Rockville, MD: National Cancer Institute, Center to Reduce Cancer Health Disparities, May 2005. www.cancer.gov/about-nci/organization/crchd/about-health-disparities/resources/excess-cervical-cancer-mortality.pdf. Accessed June 29, 2020.
7. FDA approves expanded use of HPV test. Infection Control Today. March 31, 2003. https://www.infectioncontroltoday.com/view/fda-approves-expanded-use-hpv-test. Accessed June 29, 2020.
8. Ronco G, Dillner J, Elfström K, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
9. CDC. Cervical cancer screening guidelines for average-risk women. www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. Accessed June 29, 2020.
10. Mao C, Kulasingam S, Whitham H, et al. Clinician and patient acceptability of self-collected human papillomavirus testing for cervical cancer screening. J Womens Health. 2017;26:609-615.
11. Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15:172-183.
12. Lazcano-Ponce E, Lorincz A, Cruz-Valdez A, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011;378:1868-1873.
1. Arbyn M, Smith SB, Temin S, et al; Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self-samples: updated meta-analyses. BMJ. 2018;363:k4823.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: cervical cancer. www.seer.cancer.gov/statfacts/html/cervix.html. Accessed June 29, 2020.
3. Singh GK, Azuine RE, Siahpush M. Global inequalities in cervical cancer incidence and mortality are linked to deprivation, low socioeconomic status, and human development. Int J MCH AIDS. 2012;1:17‐30.
4. US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999-2016): US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. June 2019. www.cdc.gov/cancer/dataviz. Accessed June 29, 2020.
5. MacLaughlin KL, Jacobson RM, Breitkopf CR, et al. Trends over time in Pap and Pap-HPV cotesting for cervical cancer screening. J Womens Health. 2019;28:244-249.
6. Freeman HP, Wingrove BK. Excess Cervical Cancer Mortality: A Marker for Low Access to Health Care in Poor Communities. NIH Pub. No. 05–5282. Rockville, MD: National Cancer Institute, Center to Reduce Cancer Health Disparities, May 2005. www.cancer.gov/about-nci/organization/crchd/about-health-disparities/resources/excess-cervical-cancer-mortality.pdf. Accessed June 29, 2020.
7. FDA approves expanded use of HPV test. Infection Control Today. March 31, 2003. https://www.infectioncontroltoday.com/view/fda-approves-expanded-use-hpv-test. Accessed June 29, 2020.
8. Ronco G, Dillner J, Elfström K, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
9. CDC. Cervical cancer screening guidelines for average-risk women. www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. Accessed June 29, 2020.
10. Mao C, Kulasingam S, Whitham H, et al. Clinician and patient acceptability of self-collected human papillomavirus testing for cervical cancer screening. J Womens Health. 2017;26:609-615.
11. Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15:172-183.
12. Lazcano-Ponce E, Lorincz A, Cruz-Valdez A, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011;378:1868-1873.
PRACTICE CHANGER
Have patients who decline a pelvic examination self-collect a specimen for human papillomavirus polymerase chain reaction testing as an alternative to a clinician-collected one.
STRENGTH OF RECOMMENDATION
B: Meta-analysis of observational trials.1
Arbyn M, Smith SB, Temin S, et al; Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching under-screened women by using HPV testing on self-samples: updated meta-analyses. BMJ. 2018;363:k4823.
67-year-old woman • excessive flatulence • persistent heartburn • chronic cough • Dx?
THE CASE
A 67-year-old woman with type 2 diabetes mellitus and hypertension presented to our family medicine office for evaluation of excessive flatulence, belching, and bloating that had worsened over the previous 6 months. The patient said the symptoms occurred throughout the day but were most noticeable after eating meals. She had a 5-year history of heartburn and chronic cough. We initially suspected gastroesophageal reflux disease (GERD). However, trials with several different proton pump inhibitors (PPIs) over a 3-year period did not provide any relief. Lifestyle modifications such as losing weight; remaining upright for at least 3 hours after eating; and eliminating gluten, dairy, soy, and alcohol from her diet did not alleviate her symptoms.
At the current presentation, the physical examination was normal, and an upper endoscopy was unremarkable except for some mild gastric irritation. A urea breath test was negative for Helicobacter pylori, and a chest radiograph to investigate the cause of the chronic cough was normal. The patient’s increased symptoms after eating indicated that a sensitivity to food antibodies might be at work. The absence of urticaria and anaphylaxis correlated with an IgG-mediated rather than an IgE-mediated reaction.
Due to the high cost of IgG testing, we recommended that the patient start a 6-week elimination diet that excluded the most common culprits for food allergies: dairy, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, and soy.1 We also recommended that she eliminate alcohol (because of its role in exacerbating GERD); however, excluding these foods from her diet did not provide sufficient relief of her symptoms. We subsequently recommended a serum IgG food antibody test.
THE DIAGNOSIS
The results of the test were positive for IgG-mediated allergy to vegetables in the onion family, as indicated by a high (3+) antibody presence. The patient told us she consumed onions up to 3 times daily in her meals. We recommended that she eliminate onions from her diet. At a follow-up appointment 3 months later, the patient reported that the flatulence, belching, and bloating after eating had resolved and her heartburn had decreased. When we asked about her chronic cough, the patient mentioned she had not experienced it for a few months and had forgotten about it.
DISCUSSION
The most common food sensitivity test is the scratch test, which only measures IgE antibodies. However, past studies have suggested that IgE is not the only mediator in certain symptoms related to food allergy. It is thought that these symptoms may instead be IgG mediated.2 Normally, IgG antibodies do not form in the digestive tract because the epithelium creates a barrier that is impermeable to antigens. However, antigens can bypass the epithelium and reach immune cells in states of inflammation where the epithelium is damaged. This contact with immune cells provides an opportunity for development of IgG antibodies.3 Successive interactions with these antigens leads to defensive and inflammatory processes that manifest as food allergies.
Rather than the typical IgE-mediated presentations (eg, urticaria, anaphylaxis), patients with IgG-mediated allergies experience more subtle symptoms, such as nausea, abdominal pain, diarrhea, flatulence, cramping, bloating, heartburn, cough, bronchoconstriction, eczema, stiff joints, headache, and/or increased risk of infection.4 One study showed that eliminating IgG-sensitive foods (eg, dairy, eggs) improved symptoms in migraine patients.5 Likewise, a separate study showed that patients with irritable bowel syndrome experienced improved symptoms after eliminating foods for which they had high IgG sensitivity.6
Casting a wider net. Whereas scratch testing only looks at IgE-mediated allergies, serum IgG food antibody testing looks for both IgE- and IgG-mediated reactions. IgE-mediated food allergies are monitored via the scratch test as a visual expression of a histamine reaction on the skin. However, serum IgG food antibody testing identifies culprit foods via enzyme-linked immunosorbent assay.
Continue to: Furthermore, the serum antibody test...
Furthermore, the serum antibody test also identifies allergenic foods whose symptoms have a delayed onset of 4 to 72 hours.7 Without this test, those symptoms may be wrongfully attributed to other conditions, and prescribed treatments will not treat the root cause of the reaction.8 The information provided in the serum antibody test allows the patient to develop a tailored elimination diet and eliminate causative food(s) faster. Without this test, we may not have identified onions as the allergenic food in our patient.
THE TAKEAWAY
Recent guidelines emphasize that IgG testing plays no role in the diagnosis of food allergies or intolerance.1 This may indeed be true for the general population, but other studies have shown IgG testing to be of value for specific diagnoses such as migraines or irritable bowel syndrome.5,6 Given our patient’s unique presentation and lack of response to traditional treatments, IgG testing was warranted. This case demonstrates the importance of IgG food antibody testing as part of a second-tier diagnostic workup when a patient’s gastrointestinal symptoms are not alleviated by traditional interventions.
CORRESPONDENCE
Elizabeth A. Khan, MD, Personalized Longevity Medical Center, 1146 South Cedar Crest Boulevard, Allentown, PA 18103; info@plmc.life.
1. Boyce JA, Assa’ad A, Burks AW, et al; NIAID-sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored Expert Panel report. J Allergy Clin Immunol. 2010;126:1105-1118.
2. Kemeny DM, Urbanek R, Amlot PL, et al. Sub-class of IgG in an allergic disease. I. IgG sub-class antibodies in immediate and non-immediate food allergies. Clin Allergy. 1986;16:571-581.
3. Gocki J, Zbigniew B. Role of immunoglobulin G antibodies in diagnosis of food allergy. Postepy Dermatol Alergol. 2016;33:253-256.
4. Shaw W. Clinical usefulness of IgG food allergy testing. Integrative Medicine for Mental Health Web site. www.immh.org/article-source/2016/6/29/clinical-usefulness-of-igg-food-allergy-testing. Published November 16, 2015. Accessed June 29, 2020.
5. Arroyave Hernández CM, Echavarría Pinto M, Hernández Montiel HL. Food allergy mediated by IgG antibodies associated with migraine in adults. Rev Alerg Mex. 2007;54:162-168.
6. Guo H, Jiang T, Wang J, et al. The value of eliminating foods according to food-specific immunoglobulin G antibodies in irritable bowel syndrome with diarrhoea. J Int Med Res. 2012;40:204-210.
7. IgG food antibodies. Genova Diagnostics Web site. www.gdx.net/product/igg-food-antibodies-food-sensitivity-test-blood. Accessed June 29, 2020.
8. Atkinson W, Sheldon TA, Shaath N, et al. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459-1464.
THE CASE
A 67-year-old woman with type 2 diabetes mellitus and hypertension presented to our family medicine office for evaluation of excessive flatulence, belching, and bloating that had worsened over the previous 6 months. The patient said the symptoms occurred throughout the day but were most noticeable after eating meals. She had a 5-year history of heartburn and chronic cough. We initially suspected gastroesophageal reflux disease (GERD). However, trials with several different proton pump inhibitors (PPIs) over a 3-year period did not provide any relief. Lifestyle modifications such as losing weight; remaining upright for at least 3 hours after eating; and eliminating gluten, dairy, soy, and alcohol from her diet did not alleviate her symptoms.
At the current presentation, the physical examination was normal, and an upper endoscopy was unremarkable except for some mild gastric irritation. A urea breath test was negative for Helicobacter pylori, and a chest radiograph to investigate the cause of the chronic cough was normal. The patient’s increased symptoms after eating indicated that a sensitivity to food antibodies might be at work. The absence of urticaria and anaphylaxis correlated with an IgG-mediated rather than an IgE-mediated reaction.
Due to the high cost of IgG testing, we recommended that the patient start a 6-week elimination diet that excluded the most common culprits for food allergies: dairy, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, and soy.1 We also recommended that she eliminate alcohol (because of its role in exacerbating GERD); however, excluding these foods from her diet did not provide sufficient relief of her symptoms. We subsequently recommended a serum IgG food antibody test.
THE DIAGNOSIS
The results of the test were positive for IgG-mediated allergy to vegetables in the onion family, as indicated by a high (3+) antibody presence. The patient told us she consumed onions up to 3 times daily in her meals. We recommended that she eliminate onions from her diet. At a follow-up appointment 3 months later, the patient reported that the flatulence, belching, and bloating after eating had resolved and her heartburn had decreased. When we asked about her chronic cough, the patient mentioned she had not experienced it for a few months and had forgotten about it.
DISCUSSION
The most common food sensitivity test is the scratch test, which only measures IgE antibodies. However, past studies have suggested that IgE is not the only mediator in certain symptoms related to food allergy. It is thought that these symptoms may instead be IgG mediated.2 Normally, IgG antibodies do not form in the digestive tract because the epithelium creates a barrier that is impermeable to antigens. However, antigens can bypass the epithelium and reach immune cells in states of inflammation where the epithelium is damaged. This contact with immune cells provides an opportunity for development of IgG antibodies.3 Successive interactions with these antigens leads to defensive and inflammatory processes that manifest as food allergies.
Rather than the typical IgE-mediated presentations (eg, urticaria, anaphylaxis), patients with IgG-mediated allergies experience more subtle symptoms, such as nausea, abdominal pain, diarrhea, flatulence, cramping, bloating, heartburn, cough, bronchoconstriction, eczema, stiff joints, headache, and/or increased risk of infection.4 One study showed that eliminating IgG-sensitive foods (eg, dairy, eggs) improved symptoms in migraine patients.5 Likewise, a separate study showed that patients with irritable bowel syndrome experienced improved symptoms after eliminating foods for which they had high IgG sensitivity.6
Casting a wider net. Whereas scratch testing only looks at IgE-mediated allergies, serum IgG food antibody testing looks for both IgE- and IgG-mediated reactions. IgE-mediated food allergies are monitored via the scratch test as a visual expression of a histamine reaction on the skin. However, serum IgG food antibody testing identifies culprit foods via enzyme-linked immunosorbent assay.
Continue to: Furthermore, the serum antibody test...
Furthermore, the serum antibody test also identifies allergenic foods whose symptoms have a delayed onset of 4 to 72 hours.7 Without this test, those symptoms may be wrongfully attributed to other conditions, and prescribed treatments will not treat the root cause of the reaction.8 The information provided in the serum antibody test allows the patient to develop a tailored elimination diet and eliminate causative food(s) faster. Without this test, we may not have identified onions as the allergenic food in our patient.
THE TAKEAWAY
Recent guidelines emphasize that IgG testing plays no role in the diagnosis of food allergies or intolerance.1 This may indeed be true for the general population, but other studies have shown IgG testing to be of value for specific diagnoses such as migraines or irritable bowel syndrome.5,6 Given our patient’s unique presentation and lack of response to traditional treatments, IgG testing was warranted. This case demonstrates the importance of IgG food antibody testing as part of a second-tier diagnostic workup when a patient’s gastrointestinal symptoms are not alleviated by traditional interventions.
CORRESPONDENCE
Elizabeth A. Khan, MD, Personalized Longevity Medical Center, 1146 South Cedar Crest Boulevard, Allentown, PA 18103; info@plmc.life.
THE CASE
A 67-year-old woman with type 2 diabetes mellitus and hypertension presented to our family medicine office for evaluation of excessive flatulence, belching, and bloating that had worsened over the previous 6 months. The patient said the symptoms occurred throughout the day but were most noticeable after eating meals. She had a 5-year history of heartburn and chronic cough. We initially suspected gastroesophageal reflux disease (GERD). However, trials with several different proton pump inhibitors (PPIs) over a 3-year period did not provide any relief. Lifestyle modifications such as losing weight; remaining upright for at least 3 hours after eating; and eliminating gluten, dairy, soy, and alcohol from her diet did not alleviate her symptoms.
At the current presentation, the physical examination was normal, and an upper endoscopy was unremarkable except for some mild gastric irritation. A urea breath test was negative for Helicobacter pylori, and a chest radiograph to investigate the cause of the chronic cough was normal. The patient’s increased symptoms after eating indicated that a sensitivity to food antibodies might be at work. The absence of urticaria and anaphylaxis correlated with an IgG-mediated rather than an IgE-mediated reaction.
Due to the high cost of IgG testing, we recommended that the patient start a 6-week elimination diet that excluded the most common culprits for food allergies: dairy, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, and soy.1 We also recommended that she eliminate alcohol (because of its role in exacerbating GERD); however, excluding these foods from her diet did not provide sufficient relief of her symptoms. We subsequently recommended a serum IgG food antibody test.
THE DIAGNOSIS
The results of the test were positive for IgG-mediated allergy to vegetables in the onion family, as indicated by a high (3+) antibody presence. The patient told us she consumed onions up to 3 times daily in her meals. We recommended that she eliminate onions from her diet. At a follow-up appointment 3 months later, the patient reported that the flatulence, belching, and bloating after eating had resolved and her heartburn had decreased. When we asked about her chronic cough, the patient mentioned she had not experienced it for a few months and had forgotten about it.
DISCUSSION
The most common food sensitivity test is the scratch test, which only measures IgE antibodies. However, past studies have suggested that IgE is not the only mediator in certain symptoms related to food allergy. It is thought that these symptoms may instead be IgG mediated.2 Normally, IgG antibodies do not form in the digestive tract because the epithelium creates a barrier that is impermeable to antigens. However, antigens can bypass the epithelium and reach immune cells in states of inflammation where the epithelium is damaged. This contact with immune cells provides an opportunity for development of IgG antibodies.3 Successive interactions with these antigens leads to defensive and inflammatory processes that manifest as food allergies.
Rather than the typical IgE-mediated presentations (eg, urticaria, anaphylaxis), patients with IgG-mediated allergies experience more subtle symptoms, such as nausea, abdominal pain, diarrhea, flatulence, cramping, bloating, heartburn, cough, bronchoconstriction, eczema, stiff joints, headache, and/or increased risk of infection.4 One study showed that eliminating IgG-sensitive foods (eg, dairy, eggs) improved symptoms in migraine patients.5 Likewise, a separate study showed that patients with irritable bowel syndrome experienced improved symptoms after eliminating foods for which they had high IgG sensitivity.6
Casting a wider net. Whereas scratch testing only looks at IgE-mediated allergies, serum IgG food antibody testing looks for both IgE- and IgG-mediated reactions. IgE-mediated food allergies are monitored via the scratch test as a visual expression of a histamine reaction on the skin. However, serum IgG food antibody testing identifies culprit foods via enzyme-linked immunosorbent assay.
Continue to: Furthermore, the serum antibody test...
Furthermore, the serum antibody test also identifies allergenic foods whose symptoms have a delayed onset of 4 to 72 hours.7 Without this test, those symptoms may be wrongfully attributed to other conditions, and prescribed treatments will not treat the root cause of the reaction.8 The information provided in the serum antibody test allows the patient to develop a tailored elimination diet and eliminate causative food(s) faster. Without this test, we may not have identified onions as the allergenic food in our patient.
THE TAKEAWAY
Recent guidelines emphasize that IgG testing plays no role in the diagnosis of food allergies or intolerance.1 This may indeed be true for the general population, but other studies have shown IgG testing to be of value for specific diagnoses such as migraines or irritable bowel syndrome.5,6 Given our patient’s unique presentation and lack of response to traditional treatments, IgG testing was warranted. This case demonstrates the importance of IgG food antibody testing as part of a second-tier diagnostic workup when a patient’s gastrointestinal symptoms are not alleviated by traditional interventions.
CORRESPONDENCE
Elizabeth A. Khan, MD, Personalized Longevity Medical Center, 1146 South Cedar Crest Boulevard, Allentown, PA 18103; info@plmc.life.
1. Boyce JA, Assa’ad A, Burks AW, et al; NIAID-sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored Expert Panel report. J Allergy Clin Immunol. 2010;126:1105-1118.
2. Kemeny DM, Urbanek R, Amlot PL, et al. Sub-class of IgG in an allergic disease. I. IgG sub-class antibodies in immediate and non-immediate food allergies. Clin Allergy. 1986;16:571-581.
3. Gocki J, Zbigniew B. Role of immunoglobulin G antibodies in diagnosis of food allergy. Postepy Dermatol Alergol. 2016;33:253-256.
4. Shaw W. Clinical usefulness of IgG food allergy testing. Integrative Medicine for Mental Health Web site. www.immh.org/article-source/2016/6/29/clinical-usefulness-of-igg-food-allergy-testing. Published November 16, 2015. Accessed June 29, 2020.
5. Arroyave Hernández CM, Echavarría Pinto M, Hernández Montiel HL. Food allergy mediated by IgG antibodies associated with migraine in adults. Rev Alerg Mex. 2007;54:162-168.
6. Guo H, Jiang T, Wang J, et al. The value of eliminating foods according to food-specific immunoglobulin G antibodies in irritable bowel syndrome with diarrhoea. J Int Med Res. 2012;40:204-210.
7. IgG food antibodies. Genova Diagnostics Web site. www.gdx.net/product/igg-food-antibodies-food-sensitivity-test-blood. Accessed June 29, 2020.
8. Atkinson W, Sheldon TA, Shaath N, et al. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459-1464.
1. Boyce JA, Assa’ad A, Burks AW, et al; NIAID-sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored Expert Panel report. J Allergy Clin Immunol. 2010;126:1105-1118.
2. Kemeny DM, Urbanek R, Amlot PL, et al. Sub-class of IgG in an allergic disease. I. IgG sub-class antibodies in immediate and non-immediate food allergies. Clin Allergy. 1986;16:571-581.
3. Gocki J, Zbigniew B. Role of immunoglobulin G antibodies in diagnosis of food allergy. Postepy Dermatol Alergol. 2016;33:253-256.
4. Shaw W. Clinical usefulness of IgG food allergy testing. Integrative Medicine for Mental Health Web site. www.immh.org/article-source/2016/6/29/clinical-usefulness-of-igg-food-allergy-testing. Published November 16, 2015. Accessed June 29, 2020.
5. Arroyave Hernández CM, Echavarría Pinto M, Hernández Montiel HL. Food allergy mediated by IgG antibodies associated with migraine in adults. Rev Alerg Mex. 2007;54:162-168.
6. Guo H, Jiang T, Wang J, et al. The value of eliminating foods according to food-specific immunoglobulin G antibodies in irritable bowel syndrome with diarrhoea. J Int Med Res. 2012;40:204-210.
7. IgG food antibodies. Genova Diagnostics Web site. www.gdx.net/product/igg-food-antibodies-food-sensitivity-test-blood. Accessed June 29, 2020.
8. Atkinson W, Sheldon TA, Shaath N, et al. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459-1464.
COVID vaccine tested in people shows early promise
the company says in a news release.
Researchers also reported some side effects in the 45 people in the phase I study, but no significant safety issues, the news release says.
The vaccine is among hundreds being tested worldwide in an effort to halt the pandemic that has killed nearly 600,000 worldwide.
A researcher testing the vaccine called the results encouraging but cautioned more study is needed. “Importantly, the vaccine resulted in a robust immune response,” Evan Anderson, MD, principal investigator for the trial at Emory University, says in a news release. Emory and Kaiser Permanente Washington Health Research Institute were the two sites for the study.
The company is already testing the vaccine in a larger group of people, known as a phase II trial. It plans to begin phase III trials in late July. Phase III trials involve testing the vaccine on an even larger group and are the final step before FDA approval.
The study results are published in The New England Journal of Medicine. The study was led by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Moderna’s vaccine uses messenger RNA, also called mRNA. It carries the instruction for making the spike protein, a key protein on the surface of the virus that allows it to enter cells when a person is infected. After it’s injected, it goes to the immune cells and instructs them to make copies of the spike protein, acting as if the cells have been infected with the actual coronavirus. This allows other immune cells to develop immunity.
In the study, participants were divided into three groups of 15 people each. All groups received two vaccinations 28 days apart. Each group received a different strength of the vaccine – either 25, 100, or 250 micrograms.
Every person in the study developed antibodies that can block the infection. Most commonly reported side effects after the second vaccination in the 100-microgram group were fatigue, chills, headache, and muscle pains, ranging from mild to moderately severe.
The phase II study has 300 heathy adults ages 18-55, along with another 300 ages 55 and older
Moderna says it hopes to include about 30,000 participants at the 100-microgram dose level in the U.S. for the phase III trial. The estimated start date is July 27.
This article first appeared on WebMD.com.
the company says in a news release.
Researchers also reported some side effects in the 45 people in the phase I study, but no significant safety issues, the news release says.
The vaccine is among hundreds being tested worldwide in an effort to halt the pandemic that has killed nearly 600,000 worldwide.
A researcher testing the vaccine called the results encouraging but cautioned more study is needed. “Importantly, the vaccine resulted in a robust immune response,” Evan Anderson, MD, principal investigator for the trial at Emory University, says in a news release. Emory and Kaiser Permanente Washington Health Research Institute were the two sites for the study.
The company is already testing the vaccine in a larger group of people, known as a phase II trial. It plans to begin phase III trials in late July. Phase III trials involve testing the vaccine on an even larger group and are the final step before FDA approval.
The study results are published in The New England Journal of Medicine. The study was led by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Moderna’s vaccine uses messenger RNA, also called mRNA. It carries the instruction for making the spike protein, a key protein on the surface of the virus that allows it to enter cells when a person is infected. After it’s injected, it goes to the immune cells and instructs them to make copies of the spike protein, acting as if the cells have been infected with the actual coronavirus. This allows other immune cells to develop immunity.
In the study, participants were divided into three groups of 15 people each. All groups received two vaccinations 28 days apart. Each group received a different strength of the vaccine – either 25, 100, or 250 micrograms.
Every person in the study developed antibodies that can block the infection. Most commonly reported side effects after the second vaccination in the 100-microgram group were fatigue, chills, headache, and muscle pains, ranging from mild to moderately severe.
The phase II study has 300 heathy adults ages 18-55, along with another 300 ages 55 and older
Moderna says it hopes to include about 30,000 participants at the 100-microgram dose level in the U.S. for the phase III trial. The estimated start date is July 27.
This article first appeared on WebMD.com.
the company says in a news release.
Researchers also reported some side effects in the 45 people in the phase I study, but no significant safety issues, the news release says.
The vaccine is among hundreds being tested worldwide in an effort to halt the pandemic that has killed nearly 600,000 worldwide.
A researcher testing the vaccine called the results encouraging but cautioned more study is needed. “Importantly, the vaccine resulted in a robust immune response,” Evan Anderson, MD, principal investigator for the trial at Emory University, says in a news release. Emory and Kaiser Permanente Washington Health Research Institute were the two sites for the study.
The company is already testing the vaccine in a larger group of people, known as a phase II trial. It plans to begin phase III trials in late July. Phase III trials involve testing the vaccine on an even larger group and are the final step before FDA approval.
The study results are published in The New England Journal of Medicine. The study was led by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Moderna’s vaccine uses messenger RNA, also called mRNA. It carries the instruction for making the spike protein, a key protein on the surface of the virus that allows it to enter cells when a person is infected. After it’s injected, it goes to the immune cells and instructs them to make copies of the spike protein, acting as if the cells have been infected with the actual coronavirus. This allows other immune cells to develop immunity.
In the study, participants were divided into three groups of 15 people each. All groups received two vaccinations 28 days apart. Each group received a different strength of the vaccine – either 25, 100, or 250 micrograms.
Every person in the study developed antibodies that can block the infection. Most commonly reported side effects after the second vaccination in the 100-microgram group were fatigue, chills, headache, and muscle pains, ranging from mild to moderately severe.
The phase II study has 300 heathy adults ages 18-55, along with another 300 ages 55 and older
Moderna says it hopes to include about 30,000 participants at the 100-microgram dose level in the U.S. for the phase III trial. The estimated start date is July 27.
This article first appeared on WebMD.com.
Taking steps to slow the upswing in oral and pharyngeal cancers
A recent report by the Centers for Disease Control and Prevention (CDC) documents the trends in oral and pharyngeal cancers (OPC) in the United States over a 10-year period, 2007-2016.1 The rate of OPC began to increase in 1999 and has been increasing ever since. The age-adjusted rate in 2007 was 10.89/100,000 compared with 11.7/100,000 in 2016 (TABLE 11). This is an annual relative increase of about 6% per year. In absolute numbers, there were 35,076 cases in 2007 and 44,419 in 2016.1 The trends in incidence of OPC vary by anatomical site, with some increasing and others declining.
There are 3 known causal factors related to OPC: tobacco use, alcohol use, and human papillomavirus (HPV) infection. The CDC estimates that, overall, 70% of OPCs are caused by HPV.2 However, while cancers at some oropharyngeal sites are likely related to HPV infection, cancers at other sites are not. The rising overall incidence of OPC is being driven by increases in HPV-related cancers at an average rate of 2.1% per year, while the rates at non-HPV-associated sites have been declining by 0.4% per year.1 It is also important to appreciate that HPV causes cancer at other anatomical sites (TABLE 22) and is responsible for an estimated 35,000 cancers per year.2
Other trends of note in all OPCs combined are increasing rates among non-Hispanic whites and Asian-Pacific Islanders; decreasing rates among Hispanics and African Americans; increasing rates among males with no real change in rates among females; increasing rates in those 50 to 79 years of age; decreasing rates among those 40 to 49 years of age; and unchanged rates in other age groups.1
The role of the family physician
Preventing OPC and all HPV-related cancers begins by encouraging patients to reduce alcohol and tobacco use and by emphasizing the importance of HPV vaccination. Educate teens and parents/guardians about HPV vaccine and its safety. Screen for tobacco and alcohol use, and offer brief clinical interventions as needed to decrease usage.
Recommendations by the US Preventive Services Task Force regarding screening for, and reducing use of, tobacco and alcohol, as well as screening for cervical cancer, are listed in TABLE 3.3-6 Remember that cervical cancer screening is both a primary and secondary intervention: It can reduce mortality by preventing cervical cancer (via treatment of precancerous lesions) and by detecting cervical cancer early at more treatable stages.
HPV vaccination essentials. CDC recommendations for the use of HPV vaccine and the vaccine dosing schedule appear in TABLE 4.7 While it is true that the best evidence for HPV vaccine’s prevention of cancer comes from the study of cervical and anal cancers, it is reasonable to expect that it will also be proven over time to prevent other HPV-caused cancers as the rate of HPV infections declines.
HPV vaccine is underused. In a 2018 survey, only 68.1% of adolescents had received 1 or more doses of HPV vaccine, and only 51.1% were up to date.8 In contrast, 86.6% had received 1 or more doses of quadrivalent meningococcal vaccine; 88.9% had received 1 or more doses of tetanus, diphtheria & acellular pertussis vaccine; 91.9% were up to date with 2 or more doses of measles, mumps & rubella vaccine; and 92.1% were up to date with hepatitis B vaccine, with 3 or more doses.8
Continue to: Address parental concerns, including these 5 false beliefs
Address parental concerns, including these 5 false beliefs
One study found 5 major false beliefs parents hold about HPV vaccine9:
- Vaccination is not effective at preventing cancer.
- Pap smears are sufficient to prevent cervical cancer.
- HPV vaccination is not safe.
- HPV vaccination is not needed since most infections are naturally cleared by the immune system.
- Eleven to 12 years of age is too young to vaccinate.
There is some evidence that if clinicians actively engage with parents about these concerns and address them head on, same-day vaccination rates can improve.10
We can expect to see HPV-associated OPC decline in the coming years due to the delayed effects on cancer incidence by the HPV vaccine. These anticipated declines will be more dramatic if we can increase the uptake of the HPV vaccine.
1. Ellington TD, Henley SJ, Senkomago V, et al. Trends in the incidence of cancers of the oral cavity and pharynx—United States 2007-2016. MMWR Morb Mortal Wkly Rep. 2020;69:433-438.
2. CDC. HPV and cancer. 2019. https://www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed June 29, 2020.
3. USPSTF. Unhealthy alcohol use in adolescents and adults: screening and behavioral counseling interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/unhealthy-alcohol-use-in-adolescents-and-adults-screening-and-behavioral-counseling-interventions. Accessed June 29, 2020.
4. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed June 29, 2020.
5. USPSTF. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed June 29, 2020.
6. USPSTF. Cervical cancer: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening. Accessed June 29, 2020.
7. CDC. Vaccines and preventable diseases. HPV vaccine recommendations. 2020. www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed June 29, 2020.
8. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years-United States, 2018. MMWR Morb Mortal Wkly Rep. 2019:68:718-723.
9. Bednarczyk RA. Addressing HPV vaccine myths: practical information for healthcare providers. Hum Vaccin Immunother. 2019;15:1628-1638.
10. Shay LA, Baldwin AS, Betts AC, et al. Parent-provider communication of HPV vaccine hesitancy. Pediatrics 2018;141:e20172312.
A recent report by the Centers for Disease Control and Prevention (CDC) documents the trends in oral and pharyngeal cancers (OPC) in the United States over a 10-year period, 2007-2016.1 The rate of OPC began to increase in 1999 and has been increasing ever since. The age-adjusted rate in 2007 was 10.89/100,000 compared with 11.7/100,000 in 2016 (TABLE 11). This is an annual relative increase of about 6% per year. In absolute numbers, there were 35,076 cases in 2007 and 44,419 in 2016.1 The trends in incidence of OPC vary by anatomical site, with some increasing and others declining.
There are 3 known causal factors related to OPC: tobacco use, alcohol use, and human papillomavirus (HPV) infection. The CDC estimates that, overall, 70% of OPCs are caused by HPV.2 However, while cancers at some oropharyngeal sites are likely related to HPV infection, cancers at other sites are not. The rising overall incidence of OPC is being driven by increases in HPV-related cancers at an average rate of 2.1% per year, while the rates at non-HPV-associated sites have been declining by 0.4% per year.1 It is also important to appreciate that HPV causes cancer at other anatomical sites (TABLE 22) and is responsible for an estimated 35,000 cancers per year.2
Other trends of note in all OPCs combined are increasing rates among non-Hispanic whites and Asian-Pacific Islanders; decreasing rates among Hispanics and African Americans; increasing rates among males with no real change in rates among females; increasing rates in those 50 to 79 years of age; decreasing rates among those 40 to 49 years of age; and unchanged rates in other age groups.1
The role of the family physician
Preventing OPC and all HPV-related cancers begins by encouraging patients to reduce alcohol and tobacco use and by emphasizing the importance of HPV vaccination. Educate teens and parents/guardians about HPV vaccine and its safety. Screen for tobacco and alcohol use, and offer brief clinical interventions as needed to decrease usage.
Recommendations by the US Preventive Services Task Force regarding screening for, and reducing use of, tobacco and alcohol, as well as screening for cervical cancer, are listed in TABLE 3.3-6 Remember that cervical cancer screening is both a primary and secondary intervention: It can reduce mortality by preventing cervical cancer (via treatment of precancerous lesions) and by detecting cervical cancer early at more treatable stages.
HPV vaccination essentials. CDC recommendations for the use of HPV vaccine and the vaccine dosing schedule appear in TABLE 4.7 While it is true that the best evidence for HPV vaccine’s prevention of cancer comes from the study of cervical and anal cancers, it is reasonable to expect that it will also be proven over time to prevent other HPV-caused cancers as the rate of HPV infections declines.
HPV vaccine is underused. In a 2018 survey, only 68.1% of adolescents had received 1 or more doses of HPV vaccine, and only 51.1% were up to date.8 In contrast, 86.6% had received 1 or more doses of quadrivalent meningococcal vaccine; 88.9% had received 1 or more doses of tetanus, diphtheria & acellular pertussis vaccine; 91.9% were up to date with 2 or more doses of measles, mumps & rubella vaccine; and 92.1% were up to date with hepatitis B vaccine, with 3 or more doses.8
Continue to: Address parental concerns, including these 5 false beliefs
Address parental concerns, including these 5 false beliefs
One study found 5 major false beliefs parents hold about HPV vaccine9:
- Vaccination is not effective at preventing cancer.
- Pap smears are sufficient to prevent cervical cancer.
- HPV vaccination is not safe.
- HPV vaccination is not needed since most infections are naturally cleared by the immune system.
- Eleven to 12 years of age is too young to vaccinate.
There is some evidence that if clinicians actively engage with parents about these concerns and address them head on, same-day vaccination rates can improve.10
We can expect to see HPV-associated OPC decline in the coming years due to the delayed effects on cancer incidence by the HPV vaccine. These anticipated declines will be more dramatic if we can increase the uptake of the HPV vaccine.
A recent report by the Centers for Disease Control and Prevention (CDC) documents the trends in oral and pharyngeal cancers (OPC) in the United States over a 10-year period, 2007-2016.1 The rate of OPC began to increase in 1999 and has been increasing ever since. The age-adjusted rate in 2007 was 10.89/100,000 compared with 11.7/100,000 in 2016 (TABLE 11). This is an annual relative increase of about 6% per year. In absolute numbers, there were 35,076 cases in 2007 and 44,419 in 2016.1 The trends in incidence of OPC vary by anatomical site, with some increasing and others declining.
There are 3 known causal factors related to OPC: tobacco use, alcohol use, and human papillomavirus (HPV) infection. The CDC estimates that, overall, 70% of OPCs are caused by HPV.2 However, while cancers at some oropharyngeal sites are likely related to HPV infection, cancers at other sites are not. The rising overall incidence of OPC is being driven by increases in HPV-related cancers at an average rate of 2.1% per year, while the rates at non-HPV-associated sites have been declining by 0.4% per year.1 It is also important to appreciate that HPV causes cancer at other anatomical sites (TABLE 22) and is responsible for an estimated 35,000 cancers per year.2
Other trends of note in all OPCs combined are increasing rates among non-Hispanic whites and Asian-Pacific Islanders; decreasing rates among Hispanics and African Americans; increasing rates among males with no real change in rates among females; increasing rates in those 50 to 79 years of age; decreasing rates among those 40 to 49 years of age; and unchanged rates in other age groups.1
The role of the family physician
Preventing OPC and all HPV-related cancers begins by encouraging patients to reduce alcohol and tobacco use and by emphasizing the importance of HPV vaccination. Educate teens and parents/guardians about HPV vaccine and its safety. Screen for tobacco and alcohol use, and offer brief clinical interventions as needed to decrease usage.
Recommendations by the US Preventive Services Task Force regarding screening for, and reducing use of, tobacco and alcohol, as well as screening for cervical cancer, are listed in TABLE 3.3-6 Remember that cervical cancer screening is both a primary and secondary intervention: It can reduce mortality by preventing cervical cancer (via treatment of precancerous lesions) and by detecting cervical cancer early at more treatable stages.
HPV vaccination essentials. CDC recommendations for the use of HPV vaccine and the vaccine dosing schedule appear in TABLE 4.7 While it is true that the best evidence for HPV vaccine’s prevention of cancer comes from the study of cervical and anal cancers, it is reasonable to expect that it will also be proven over time to prevent other HPV-caused cancers as the rate of HPV infections declines.
HPV vaccine is underused. In a 2018 survey, only 68.1% of adolescents had received 1 or more doses of HPV vaccine, and only 51.1% were up to date.8 In contrast, 86.6% had received 1 or more doses of quadrivalent meningococcal vaccine; 88.9% had received 1 or more doses of tetanus, diphtheria & acellular pertussis vaccine; 91.9% were up to date with 2 or more doses of measles, mumps & rubella vaccine; and 92.1% were up to date with hepatitis B vaccine, with 3 or more doses.8
Continue to: Address parental concerns, including these 5 false beliefs
Address parental concerns, including these 5 false beliefs
One study found 5 major false beliefs parents hold about HPV vaccine9:
- Vaccination is not effective at preventing cancer.
- Pap smears are sufficient to prevent cervical cancer.
- HPV vaccination is not safe.
- HPV vaccination is not needed since most infections are naturally cleared by the immune system.
- Eleven to 12 years of age is too young to vaccinate.
There is some evidence that if clinicians actively engage with parents about these concerns and address them head on, same-day vaccination rates can improve.10
We can expect to see HPV-associated OPC decline in the coming years due to the delayed effects on cancer incidence by the HPV vaccine. These anticipated declines will be more dramatic if we can increase the uptake of the HPV vaccine.
1. Ellington TD, Henley SJ, Senkomago V, et al. Trends in the incidence of cancers of the oral cavity and pharynx—United States 2007-2016. MMWR Morb Mortal Wkly Rep. 2020;69:433-438.
2. CDC. HPV and cancer. 2019. https://www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed June 29, 2020.
3. USPSTF. Unhealthy alcohol use in adolescents and adults: screening and behavioral counseling interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/unhealthy-alcohol-use-in-adolescents-and-adults-screening-and-behavioral-counseling-interventions. Accessed June 29, 2020.
4. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed June 29, 2020.
5. USPSTF. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed June 29, 2020.
6. USPSTF. Cervical cancer: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening. Accessed June 29, 2020.
7. CDC. Vaccines and preventable diseases. HPV vaccine recommendations. 2020. www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed June 29, 2020.
8. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years-United States, 2018. MMWR Morb Mortal Wkly Rep. 2019:68:718-723.
9. Bednarczyk RA. Addressing HPV vaccine myths: practical information for healthcare providers. Hum Vaccin Immunother. 2019;15:1628-1638.
10. Shay LA, Baldwin AS, Betts AC, et al. Parent-provider communication of HPV vaccine hesitancy. Pediatrics 2018;141:e20172312.
1. Ellington TD, Henley SJ, Senkomago V, et al. Trends in the incidence of cancers of the oral cavity and pharynx—United States 2007-2016. MMWR Morb Mortal Wkly Rep. 2020;69:433-438.
2. CDC. HPV and cancer. 2019. https://www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed June 29, 2020.
3. USPSTF. Unhealthy alcohol use in adolescents and adults: screening and behavioral counseling interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/unhealthy-alcohol-use-in-adolescents-and-adults-screening-and-behavioral-counseling-interventions. Accessed June 29, 2020.
4. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed June 29, 2020.
5. USPSTF. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed June 29, 2020.
6. USPSTF. Cervical cancer: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening. Accessed June 29, 2020.
7. CDC. Vaccines and preventable diseases. HPV vaccine recommendations. 2020. www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed June 29, 2020.
8. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years-United States, 2018. MMWR Morb Mortal Wkly Rep. 2019:68:718-723.
9. Bednarczyk RA. Addressing HPV vaccine myths: practical information for healthcare providers. Hum Vaccin Immunother. 2019;15:1628-1638.
10. Shay LA, Baldwin AS, Betts AC, et al. Parent-provider communication of HPV vaccine hesitancy. Pediatrics 2018;141:e20172312.
Even mild obesity raises severe COVID-19 risks
People with a body mass index of 30 kg/m2 or above are at significantly increased risk for severe COVID-19, while a BMI of 35 and higher dramatically increases the risk for death, new research suggests.
The data, from nearly 500 patients hospitalized with COVID-19 in March and April 2020, were published in the European Journal of Endocrinology by Matteo Rottoli, MD, of the Alma Mater Studiorum, University of Bologna (Italy), and colleagues.
The data support the recent change by the Centers for Disease Control and Prevention to lower the cutoff for categorizing a person at increased risk from COVID-19 from a BMI of 40 down to 30. However, in the United Kingdom, the National Health Service still lists only a BMI of 40 or above as placing a person at “moderate risk (clinically vulnerable).”
“This finding calls for prevention and treatment strategies to reduce the risk of infection and hospitalization in patients with relevant degrees of obesity, supporting a revision of the BMI cutoff of 40 kg/m2, which was proposed as an independent risk factor for an adverse outcome of COVID-19 in the ... guidelines for social distancing in the United Kingdom: It may be appropriate to include patients with BMI >30 among those at higher risk for COVID-19 severe progression,” the authors wrote.
The study included 482 adults admitted with confirmed COVID-19 to a single Italian hospital between March 1 and April 20, 2020. Of those, 41.9% had a BMI of less than 25 (normal weight), 36.5% had a BMI of 25-29.9 (overweight), and 21.6% had BMI of at least 30 (obese). Of the obese group, 20 (4.1%) had BMIs of at least 35, while 18 patients (3.7%) had BMIs of less than 20 (underweight).
Among those with obesity, 51.9% experienced respiratory failure, 36.4% were admitted to the ICU, 25% required mechanical ventilation, and 29.8% died within 30 days of symptom onset.
Patients with BMIs of at least 30 had significantly increased risks for respiratory failure (odds ratio, 2.48; P = .001), ICU admission (OR, 5.28; P < .001), and death (2.35, P = .017), compared with those with lower BMIs. Within the group classified as obese, the risks of respiratory failure and ICU admission were higher, with BMIs of 30-34.9 (OR, 2.32; P = .004 and OR, 4.96; P < .001, respectively) and for BMIs of at least 35 (OR, 3.24; P = .019 and OR, 6.58; P < .001, respectively).
The risk of death was significantly higher among patients with a BMI of at least 35 (OR, 12.1; P < .001).
Every 1-unit increase in BMI was significantly associated with all outcomes, but there was no significant difference in any outcome between the 25-29.9 BMI category and normal weight. In all models, the BMI cutoff for increased risk was 30.
The authors reported no disclosures.
SOURCE: Rottoli M et al. Eur J Endocrinol. 2020 Jul 1. doi: 10.1530/EJE-20-054.
People with a body mass index of 30 kg/m2 or above are at significantly increased risk for severe COVID-19, while a BMI of 35 and higher dramatically increases the risk for death, new research suggests.
The data, from nearly 500 patients hospitalized with COVID-19 in March and April 2020, were published in the European Journal of Endocrinology by Matteo Rottoli, MD, of the Alma Mater Studiorum, University of Bologna (Italy), and colleagues.
The data support the recent change by the Centers for Disease Control and Prevention to lower the cutoff for categorizing a person at increased risk from COVID-19 from a BMI of 40 down to 30. However, in the United Kingdom, the National Health Service still lists only a BMI of 40 or above as placing a person at “moderate risk (clinically vulnerable).”
“This finding calls for prevention and treatment strategies to reduce the risk of infection and hospitalization in patients with relevant degrees of obesity, supporting a revision of the BMI cutoff of 40 kg/m2, which was proposed as an independent risk factor for an adverse outcome of COVID-19 in the ... guidelines for social distancing in the United Kingdom: It may be appropriate to include patients with BMI >30 among those at higher risk for COVID-19 severe progression,” the authors wrote.
The study included 482 adults admitted with confirmed COVID-19 to a single Italian hospital between March 1 and April 20, 2020. Of those, 41.9% had a BMI of less than 25 (normal weight), 36.5% had a BMI of 25-29.9 (overweight), and 21.6% had BMI of at least 30 (obese). Of the obese group, 20 (4.1%) had BMIs of at least 35, while 18 patients (3.7%) had BMIs of less than 20 (underweight).
Among those with obesity, 51.9% experienced respiratory failure, 36.4% were admitted to the ICU, 25% required mechanical ventilation, and 29.8% died within 30 days of symptom onset.
Patients with BMIs of at least 30 had significantly increased risks for respiratory failure (odds ratio, 2.48; P = .001), ICU admission (OR, 5.28; P < .001), and death (2.35, P = .017), compared with those with lower BMIs. Within the group classified as obese, the risks of respiratory failure and ICU admission were higher, with BMIs of 30-34.9 (OR, 2.32; P = .004 and OR, 4.96; P < .001, respectively) and for BMIs of at least 35 (OR, 3.24; P = .019 and OR, 6.58; P < .001, respectively).
The risk of death was significantly higher among patients with a BMI of at least 35 (OR, 12.1; P < .001).
Every 1-unit increase in BMI was significantly associated with all outcomes, but there was no significant difference in any outcome between the 25-29.9 BMI category and normal weight. In all models, the BMI cutoff for increased risk was 30.
The authors reported no disclosures.
SOURCE: Rottoli M et al. Eur J Endocrinol. 2020 Jul 1. doi: 10.1530/EJE-20-054.
People with a body mass index of 30 kg/m2 or above are at significantly increased risk for severe COVID-19, while a BMI of 35 and higher dramatically increases the risk for death, new research suggests.
The data, from nearly 500 patients hospitalized with COVID-19 in March and April 2020, were published in the European Journal of Endocrinology by Matteo Rottoli, MD, of the Alma Mater Studiorum, University of Bologna (Italy), and colleagues.
The data support the recent change by the Centers for Disease Control and Prevention to lower the cutoff for categorizing a person at increased risk from COVID-19 from a BMI of 40 down to 30. However, in the United Kingdom, the National Health Service still lists only a BMI of 40 or above as placing a person at “moderate risk (clinically vulnerable).”
“This finding calls for prevention and treatment strategies to reduce the risk of infection and hospitalization in patients with relevant degrees of obesity, supporting a revision of the BMI cutoff of 40 kg/m2, which was proposed as an independent risk factor for an adverse outcome of COVID-19 in the ... guidelines for social distancing in the United Kingdom: It may be appropriate to include patients with BMI >30 among those at higher risk for COVID-19 severe progression,” the authors wrote.
The study included 482 adults admitted with confirmed COVID-19 to a single Italian hospital between March 1 and April 20, 2020. Of those, 41.9% had a BMI of less than 25 (normal weight), 36.5% had a BMI of 25-29.9 (overweight), and 21.6% had BMI of at least 30 (obese). Of the obese group, 20 (4.1%) had BMIs of at least 35, while 18 patients (3.7%) had BMIs of less than 20 (underweight).
Among those with obesity, 51.9% experienced respiratory failure, 36.4% were admitted to the ICU, 25% required mechanical ventilation, and 29.8% died within 30 days of symptom onset.
Patients with BMIs of at least 30 had significantly increased risks for respiratory failure (odds ratio, 2.48; P = .001), ICU admission (OR, 5.28; P < .001), and death (2.35, P = .017), compared with those with lower BMIs. Within the group classified as obese, the risks of respiratory failure and ICU admission were higher, with BMIs of 30-34.9 (OR, 2.32; P = .004 and OR, 4.96; P < .001, respectively) and for BMIs of at least 35 (OR, 3.24; P = .019 and OR, 6.58; P < .001, respectively).
The risk of death was significantly higher among patients with a BMI of at least 35 (OR, 12.1; P < .001).
Every 1-unit increase in BMI was significantly associated with all outcomes, but there was no significant difference in any outcome between the 25-29.9 BMI category and normal weight. In all models, the BMI cutoff for increased risk was 30.
The authors reported no disclosures.
SOURCE: Rottoli M et al. Eur J Endocrinol. 2020 Jul 1. doi: 10.1530/EJE-20-054.
FROM THE EUROPEAN JOURNAL OF ENDOCRINOLOGY
Consider adverse childhood experiences during the pandemic
We live in historic times. A worldwide pandemic is surging in the United States, with millions infected and the world’s highest death rate. Many of our hospitals are overwhelmed. Schools have been closed for months. Businesses are struggling, and unemployment is at record levels. The murder of George Floyd unleashed an outpouring of grief and rage over police brutality and structural racism.
It is ironic that this age of adversity emerged at the same time that efforts to assess and address childhood adversity are gaining momentum. The effects of adverse childhood experiences (ACEs) have been well known for decades, but only recently have efforts at universal screening been initiated in primary care offices around the country. The multiple crises we face have made this work more pressing than ever. And
While there has long been awareness, especially among pediatricians, of the social determinants of health, it was only 1995 when Robert F. Anda, MD, and Vincent J. Felitti, MD, set about studying over 13,000 adult patients at Kaiser Permanente to understand the relationship between childhood trauma and chronic health problems in adulthood. In 1998 they published the results of this landmark study, establishing that childhood trauma was common and that it predicted chronic diseases and psychosocial problems in adulthood1.
They detailed 10 specific ACEs, and a patient’s ACE score was determined by how many of these experiences they had before they turned 18 years: neglect (emotional or physical), abuse (emotional, physical or sexual), and household dysfunction (parental divorce, incarceration of a parent, domestic violence, parental mental illness, or parental substance abuse). They found that more than half of adults studied had a score of at least 1, and 6% had scores of 4 or more. Those adults with an ACE score of 4 or more are twice as likely to be obese, twice as likely to smoke, and seven times as likely to abuse alcohol as the rest of the population. They are 4 times as likely to have emphysema, 5 times as likely to have depression, and 12 times as likely to attempt suicide. They have higher rates of heart disease, autoimmune disorders, and cancer. Those with ACE scores of 6 or more have their life expectancy shortened by an average of 20 years.
The value of knowing about these risk factors would seem self-evident; it would inform a patient’s health care from screening for cancer or heart disease, referral for mild depressive symptoms, and counseling about alcohol consumption. But this research did not lead to the establishment of routine screening for childhood adversity in primary care practices. There are multiple reasons for this, including growing pressure on physician time and discomfort with starting conversations about potentially traumatic material. But perhaps the greatest obstacle has been uncertainty about what to offer patients who screened in. What is the treatment for a high ACE score?
Even without treatments, we have learned much about childhood adversity since Dr. Anda and Dr. Felitti published their landmark study. Other more chronic adverse childhood experiences also contribute to adult health risk, such as poverty, homelessness, discrimination, community violence, parental chronic illness, or disability or placement in foster care. Having a high ACE score does not only affect health in adulthood. Children with an ACE score of 4 are 2 times as likely to have asthma2,3 and allergies3, 2 times as likely to be obese4, 3 times as likely to have headaches3 and dental problems5,6, 4 times as likely to have depression7,8, 5 times as likely to have ADHD8,9, 7 times as likely to have high rates of school absenteeism3 and aggression10, and over 30 times as likely to have learning or behavioral problems at school4. There is a growing body of knowledge about how chronic, severe stress in childhood affects can lead to pathological alterations in neuroendocrine and immune function. But this has not led to any concrete treatments that may be preventive or reparative.
Movement toward expanding screening nonetheless has accelerated. In California, Nadine Burke-Harris, MD, a pediatrician who studied ACEs and children’s health was named the state’s first Surgeon General in 2019 and spearheaded an effort to make screening for ACEs easier. Starting in 2020, MediCal will pay for annual screenings, and the state is offering training and resources on how to screen and what to do with the information to help patients and families.
The coronavirus pandemic has only highlighted the risks of childhood adversity. The burden of infection and mortality has been borne disproportionately by people of color and those with multiple chronic medical conditions (obesity, cardiovascular disease, diabetes, etc.). While viruses do not discriminate, they are more likely to infect those with higher risk of exposure and to kill those who are physiologically vulnerable.
And the pandemic increases the risk for adversity for today’s children and families. When children cannot attend school, financially vulnerable parents may have to choose between supervising them or feeding them. Families who suddenly are all in a small apartment together without school or other outside supports may be at higher risk for domestic violence and child abuse. Unemployment and financial uncertainty will increase the rates of substance abuse and depression amongst parents. And the serious illness or death of a parent will be a more common event for children in the year ahead. One of these risk factors may increase the likelihood of others.
Beyond the obvious need for substantial policy changes focused on housing, education, and health care, And resilience can build on itself, as children face subsequent challenges with the support of caring connected adults.
The critical first step is asking. Then listen calmly and supportively, normalizing for parents and children how common these experiences are. Explain how they affect health and well-being. Explain that adversity and its consequences are not their fault. Then educate them about what is in their control: the skills they can practice to buffer against the consequences of adversity and build resilience. They sound simple, but still require effort and work. And the pandemic has created some difficulty (social distancing) and opportunity (more family time, fewer school demands).
Sleep
Help parents establish and protect consistent, restful sleep for their children. They can set a consistent bedtime and a calm routine, with screens all off at least 30 minutes before sleep and reading before sleep. Restful sleep is physiologically and psychologically protective to everyone in a family.
Movement
Beyond directly improving physical health, establishing habits of exercise – especially outside – every day can effectively manage ongoing stress, build skills of self-regulation, and help with sleep.
Find out what parents and their children like to do together (walking the dog, shooting hoops, even dancing) and help them devise ways to create family routines around exercise.
Nutrition
Food should be a source of pleasure, but stress can make food into a source of comfort or escape. Help parents to create realistic ways to consistently offer healthy family meals and discourage unhealthy habits.
Even small changes like water instead of soda can help, and there are nutritional and emotional benefits to eating a healthy breakfast or dinner together as a family.
Connections
Nourishing social connections are protective. Help parents think about protecting time to spend with their children for talking, playing games, or even singing.
They should support their children’s connections to other caring adults, through community organizations (church, community centers, or sports), and they should know who their children’s reliable friends are. Parents will benefit from these supports for themselves, which in turn will benefit the full family.
Self-awareness
Activities that cultivate mindfulness are protective. Parents can simply ask how their children are feeling, physically or emotionally, and be able to bear it when it is uncomfortable. Work towards nonjudgmental awareness of how they are feeling. Learning what is relaxing or recharging for them (exercise, music, a hot bath, a good book, time with a friend) will protect against defaulting into maladaptive coping such as escape, numbing, or avoidance.
Of course, if you learn about symptoms that suggest PTSD, depression, or addiction, you should help your patient connect with effective treatment. The difficulty of referring to a mental health provider does not mean you should not try and bring as many people onto the team and into the orbit of the child and family at risk. It may be easier to access some therapy given the new availability of telemedicine visits across many more systems of care. Although the heaviest burdens of adversity are not being borne equally, the fact that adversity is currently a shared experience makes this a moment of promise.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Dr. Swick and Dr. Jellinek had no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Ann Allergy Asthma Immunol. 2015;114: 379-84.
3. BMC Public Health. 2018. doi: 10.1186/s12889-018-5699-8.
4. Child Abuse Negl. 2011 Jun;35(6):408-13.
5. Community Dent Oral Epidemiol. 2015;43:193-9.
6. Community Dent Oral Epidemiol. 2018 Oct;46(5): 442-8.
7. Pediatrics 2016 Apr. doi: 10.1542/peds.2015-4016.
8. Matern Child Health J. 2016 Apr. doi: 10.1007/s10995-015-1915-7.
9. Acad Pediatr. 2017 May-Jun. doi: 10.1016/j.acap.2016.08.013.
10. Pediatrics. 2010 Apr. doi: 10.1542/peds.2009-0597.
This article was updated 7/27/2020.
We live in historic times. A worldwide pandemic is surging in the United States, with millions infected and the world’s highest death rate. Many of our hospitals are overwhelmed. Schools have been closed for months. Businesses are struggling, and unemployment is at record levels. The murder of George Floyd unleashed an outpouring of grief and rage over police brutality and structural racism.
It is ironic that this age of adversity emerged at the same time that efforts to assess and address childhood adversity are gaining momentum. The effects of adverse childhood experiences (ACEs) have been well known for decades, but only recently have efforts at universal screening been initiated in primary care offices around the country. The multiple crises we face have made this work more pressing than ever. And
While there has long been awareness, especially among pediatricians, of the social determinants of health, it was only 1995 when Robert F. Anda, MD, and Vincent J. Felitti, MD, set about studying over 13,000 adult patients at Kaiser Permanente to understand the relationship between childhood trauma and chronic health problems in adulthood. In 1998 they published the results of this landmark study, establishing that childhood trauma was common and that it predicted chronic diseases and psychosocial problems in adulthood1.
They detailed 10 specific ACEs, and a patient’s ACE score was determined by how many of these experiences they had before they turned 18 years: neglect (emotional or physical), abuse (emotional, physical or sexual), and household dysfunction (parental divorce, incarceration of a parent, domestic violence, parental mental illness, or parental substance abuse). They found that more than half of adults studied had a score of at least 1, and 6% had scores of 4 or more. Those adults with an ACE score of 4 or more are twice as likely to be obese, twice as likely to smoke, and seven times as likely to abuse alcohol as the rest of the population. They are 4 times as likely to have emphysema, 5 times as likely to have depression, and 12 times as likely to attempt suicide. They have higher rates of heart disease, autoimmune disorders, and cancer. Those with ACE scores of 6 or more have their life expectancy shortened by an average of 20 years.
The value of knowing about these risk factors would seem self-evident; it would inform a patient’s health care from screening for cancer or heart disease, referral for mild depressive symptoms, and counseling about alcohol consumption. But this research did not lead to the establishment of routine screening for childhood adversity in primary care practices. There are multiple reasons for this, including growing pressure on physician time and discomfort with starting conversations about potentially traumatic material. But perhaps the greatest obstacle has been uncertainty about what to offer patients who screened in. What is the treatment for a high ACE score?
Even without treatments, we have learned much about childhood adversity since Dr. Anda and Dr. Felitti published their landmark study. Other more chronic adverse childhood experiences also contribute to adult health risk, such as poverty, homelessness, discrimination, community violence, parental chronic illness, or disability or placement in foster care. Having a high ACE score does not only affect health in adulthood. Children with an ACE score of 4 are 2 times as likely to have asthma2,3 and allergies3, 2 times as likely to be obese4, 3 times as likely to have headaches3 and dental problems5,6, 4 times as likely to have depression7,8, 5 times as likely to have ADHD8,9, 7 times as likely to have high rates of school absenteeism3 and aggression10, and over 30 times as likely to have learning or behavioral problems at school4. There is a growing body of knowledge about how chronic, severe stress in childhood affects can lead to pathological alterations in neuroendocrine and immune function. But this has not led to any concrete treatments that may be preventive or reparative.
Movement toward expanding screening nonetheless has accelerated. In California, Nadine Burke-Harris, MD, a pediatrician who studied ACEs and children’s health was named the state’s first Surgeon General in 2019 and spearheaded an effort to make screening for ACEs easier. Starting in 2020, MediCal will pay for annual screenings, and the state is offering training and resources on how to screen and what to do with the information to help patients and families.
The coronavirus pandemic has only highlighted the risks of childhood adversity. The burden of infection and mortality has been borne disproportionately by people of color and those with multiple chronic medical conditions (obesity, cardiovascular disease, diabetes, etc.). While viruses do not discriminate, they are more likely to infect those with higher risk of exposure and to kill those who are physiologically vulnerable.
And the pandemic increases the risk for adversity for today’s children and families. When children cannot attend school, financially vulnerable parents may have to choose between supervising them or feeding them. Families who suddenly are all in a small apartment together without school or other outside supports may be at higher risk for domestic violence and child abuse. Unemployment and financial uncertainty will increase the rates of substance abuse and depression amongst parents. And the serious illness or death of a parent will be a more common event for children in the year ahead. One of these risk factors may increase the likelihood of others.
Beyond the obvious need for substantial policy changes focused on housing, education, and health care, And resilience can build on itself, as children face subsequent challenges with the support of caring connected adults.
The critical first step is asking. Then listen calmly and supportively, normalizing for parents and children how common these experiences are. Explain how they affect health and well-being. Explain that adversity and its consequences are not their fault. Then educate them about what is in their control: the skills they can practice to buffer against the consequences of adversity and build resilience. They sound simple, but still require effort and work. And the pandemic has created some difficulty (social distancing) and opportunity (more family time, fewer school demands).
Sleep
Help parents establish and protect consistent, restful sleep for their children. They can set a consistent bedtime and a calm routine, with screens all off at least 30 minutes before sleep and reading before sleep. Restful sleep is physiologically and psychologically protective to everyone in a family.
Movement
Beyond directly improving physical health, establishing habits of exercise – especially outside – every day can effectively manage ongoing stress, build skills of self-regulation, and help with sleep.
Find out what parents and their children like to do together (walking the dog, shooting hoops, even dancing) and help them devise ways to create family routines around exercise.
Nutrition
Food should be a source of pleasure, but stress can make food into a source of comfort or escape. Help parents to create realistic ways to consistently offer healthy family meals and discourage unhealthy habits.
Even small changes like water instead of soda can help, and there are nutritional and emotional benefits to eating a healthy breakfast or dinner together as a family.
Connections
Nourishing social connections are protective. Help parents think about protecting time to spend with their children for talking, playing games, or even singing.
They should support their children’s connections to other caring adults, through community organizations (church, community centers, or sports), and they should know who their children’s reliable friends are. Parents will benefit from these supports for themselves, which in turn will benefit the full family.
Self-awareness
Activities that cultivate mindfulness are protective. Parents can simply ask how their children are feeling, physically or emotionally, and be able to bear it when it is uncomfortable. Work towards nonjudgmental awareness of how they are feeling. Learning what is relaxing or recharging for them (exercise, music, a hot bath, a good book, time with a friend) will protect against defaulting into maladaptive coping such as escape, numbing, or avoidance.
Of course, if you learn about symptoms that suggest PTSD, depression, or addiction, you should help your patient connect with effective treatment. The difficulty of referring to a mental health provider does not mean you should not try and bring as many people onto the team and into the orbit of the child and family at risk. It may be easier to access some therapy given the new availability of telemedicine visits across many more systems of care. Although the heaviest burdens of adversity are not being borne equally, the fact that adversity is currently a shared experience makes this a moment of promise.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Dr. Swick and Dr. Jellinek had no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Ann Allergy Asthma Immunol. 2015;114: 379-84.
3. BMC Public Health. 2018. doi: 10.1186/s12889-018-5699-8.
4. Child Abuse Negl. 2011 Jun;35(6):408-13.
5. Community Dent Oral Epidemiol. 2015;43:193-9.
6. Community Dent Oral Epidemiol. 2018 Oct;46(5): 442-8.
7. Pediatrics 2016 Apr. doi: 10.1542/peds.2015-4016.
8. Matern Child Health J. 2016 Apr. doi: 10.1007/s10995-015-1915-7.
9. Acad Pediatr. 2017 May-Jun. doi: 10.1016/j.acap.2016.08.013.
10. Pediatrics. 2010 Apr. doi: 10.1542/peds.2009-0597.
This article was updated 7/27/2020.
We live in historic times. A worldwide pandemic is surging in the United States, with millions infected and the world’s highest death rate. Many of our hospitals are overwhelmed. Schools have been closed for months. Businesses are struggling, and unemployment is at record levels. The murder of George Floyd unleashed an outpouring of grief and rage over police brutality and structural racism.
It is ironic that this age of adversity emerged at the same time that efforts to assess and address childhood adversity are gaining momentum. The effects of adverse childhood experiences (ACEs) have been well known for decades, but only recently have efforts at universal screening been initiated in primary care offices around the country. The multiple crises we face have made this work more pressing than ever. And
While there has long been awareness, especially among pediatricians, of the social determinants of health, it was only 1995 when Robert F. Anda, MD, and Vincent J. Felitti, MD, set about studying over 13,000 adult patients at Kaiser Permanente to understand the relationship between childhood trauma and chronic health problems in adulthood. In 1998 they published the results of this landmark study, establishing that childhood trauma was common and that it predicted chronic diseases and psychosocial problems in adulthood1.
They detailed 10 specific ACEs, and a patient’s ACE score was determined by how many of these experiences they had before they turned 18 years: neglect (emotional or physical), abuse (emotional, physical or sexual), and household dysfunction (parental divorce, incarceration of a parent, domestic violence, parental mental illness, or parental substance abuse). They found that more than half of adults studied had a score of at least 1, and 6% had scores of 4 or more. Those adults with an ACE score of 4 or more are twice as likely to be obese, twice as likely to smoke, and seven times as likely to abuse alcohol as the rest of the population. They are 4 times as likely to have emphysema, 5 times as likely to have depression, and 12 times as likely to attempt suicide. They have higher rates of heart disease, autoimmune disorders, and cancer. Those with ACE scores of 6 or more have their life expectancy shortened by an average of 20 years.
The value of knowing about these risk factors would seem self-evident; it would inform a patient’s health care from screening for cancer or heart disease, referral for mild depressive symptoms, and counseling about alcohol consumption. But this research did not lead to the establishment of routine screening for childhood adversity in primary care practices. There are multiple reasons for this, including growing pressure on physician time and discomfort with starting conversations about potentially traumatic material. But perhaps the greatest obstacle has been uncertainty about what to offer patients who screened in. What is the treatment for a high ACE score?
Even without treatments, we have learned much about childhood adversity since Dr. Anda and Dr. Felitti published their landmark study. Other more chronic adverse childhood experiences also contribute to adult health risk, such as poverty, homelessness, discrimination, community violence, parental chronic illness, or disability or placement in foster care. Having a high ACE score does not only affect health in adulthood. Children with an ACE score of 4 are 2 times as likely to have asthma2,3 and allergies3, 2 times as likely to be obese4, 3 times as likely to have headaches3 and dental problems5,6, 4 times as likely to have depression7,8, 5 times as likely to have ADHD8,9, 7 times as likely to have high rates of school absenteeism3 and aggression10, and over 30 times as likely to have learning or behavioral problems at school4. There is a growing body of knowledge about how chronic, severe stress in childhood affects can lead to pathological alterations in neuroendocrine and immune function. But this has not led to any concrete treatments that may be preventive or reparative.
Movement toward expanding screening nonetheless has accelerated. In California, Nadine Burke-Harris, MD, a pediatrician who studied ACEs and children’s health was named the state’s first Surgeon General in 2019 and spearheaded an effort to make screening for ACEs easier. Starting in 2020, MediCal will pay for annual screenings, and the state is offering training and resources on how to screen and what to do with the information to help patients and families.
The coronavirus pandemic has only highlighted the risks of childhood adversity. The burden of infection and mortality has been borne disproportionately by people of color and those with multiple chronic medical conditions (obesity, cardiovascular disease, diabetes, etc.). While viruses do not discriminate, they are more likely to infect those with higher risk of exposure and to kill those who are physiologically vulnerable.
And the pandemic increases the risk for adversity for today’s children and families. When children cannot attend school, financially vulnerable parents may have to choose between supervising them or feeding them. Families who suddenly are all in a small apartment together without school or other outside supports may be at higher risk for domestic violence and child abuse. Unemployment and financial uncertainty will increase the rates of substance abuse and depression amongst parents. And the serious illness or death of a parent will be a more common event for children in the year ahead. One of these risk factors may increase the likelihood of others.
Beyond the obvious need for substantial policy changes focused on housing, education, and health care, And resilience can build on itself, as children face subsequent challenges with the support of caring connected adults.
The critical first step is asking. Then listen calmly and supportively, normalizing for parents and children how common these experiences are. Explain how they affect health and well-being. Explain that adversity and its consequences are not their fault. Then educate them about what is in their control: the skills they can practice to buffer against the consequences of adversity and build resilience. They sound simple, but still require effort and work. And the pandemic has created some difficulty (social distancing) and opportunity (more family time, fewer school demands).
Sleep
Help parents establish and protect consistent, restful sleep for their children. They can set a consistent bedtime and a calm routine, with screens all off at least 30 minutes before sleep and reading before sleep. Restful sleep is physiologically and psychologically protective to everyone in a family.
Movement
Beyond directly improving physical health, establishing habits of exercise – especially outside – every day can effectively manage ongoing stress, build skills of self-regulation, and help with sleep.
Find out what parents and their children like to do together (walking the dog, shooting hoops, even dancing) and help them devise ways to create family routines around exercise.
Nutrition
Food should be a source of pleasure, but stress can make food into a source of comfort or escape. Help parents to create realistic ways to consistently offer healthy family meals and discourage unhealthy habits.
Even small changes like water instead of soda can help, and there are nutritional and emotional benefits to eating a healthy breakfast or dinner together as a family.
Connections
Nourishing social connections are protective. Help parents think about protecting time to spend with their children for talking, playing games, or even singing.
They should support their children’s connections to other caring adults, through community organizations (church, community centers, or sports), and they should know who their children’s reliable friends are. Parents will benefit from these supports for themselves, which in turn will benefit the full family.
Self-awareness
Activities that cultivate mindfulness are protective. Parents can simply ask how their children are feeling, physically or emotionally, and be able to bear it when it is uncomfortable. Work towards nonjudgmental awareness of how they are feeling. Learning what is relaxing or recharging for them (exercise, music, a hot bath, a good book, time with a friend) will protect against defaulting into maladaptive coping such as escape, numbing, or avoidance.
Of course, if you learn about symptoms that suggest PTSD, depression, or addiction, you should help your patient connect with effective treatment. The difficulty of referring to a mental health provider does not mean you should not try and bring as many people onto the team and into the orbit of the child and family at risk. It may be easier to access some therapy given the new availability of telemedicine visits across many more systems of care. Although the heaviest burdens of adversity are not being borne equally, the fact that adversity is currently a shared experience makes this a moment of promise.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Dr. Swick and Dr. Jellinek had no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Am J Prev Med. 1998 May;14(4):245-58.
2. Ann Allergy Asthma Immunol. 2015;114: 379-84.
3. BMC Public Health. 2018. doi: 10.1186/s12889-018-5699-8.
4. Child Abuse Negl. 2011 Jun;35(6):408-13.
5. Community Dent Oral Epidemiol. 2015;43:193-9.
6. Community Dent Oral Epidemiol. 2018 Oct;46(5): 442-8.
7. Pediatrics 2016 Apr. doi: 10.1542/peds.2015-4016.
8. Matern Child Health J. 2016 Apr. doi: 10.1007/s10995-015-1915-7.
9. Acad Pediatr. 2017 May-Jun. doi: 10.1016/j.acap.2016.08.013.
10. Pediatrics. 2010 Apr. doi: 10.1542/peds.2009-0597.
This article was updated 7/27/2020.
ECT more effective for psychotic vs. nonpsychotic depression?
For patients with psychotic depression, response to treatment, remission rates, and cognitive improvement are better following electroconvulsive therapy (ECT) than for patients with nonpsychotic depression, results from a new study suggest.
However, findings from another study suggest that at least some of these differences may be because psychotic patients are referred for ECT earlier in the disease course.
Both studies were presented at the European Psychiatric Association 2020 Congress, which was held online this year because of the COVID-19 pandemic.
Limited, old evidence
The first study was led by Christopher Yi Wen Chan, MD, Institute of Mental Health, Singapore. The investigators stated that they have “often observed” superior remission rates with ECT in psychotic versus nonpsychotic depression. However, the evidence base is “limited and mostly more than 10 years old.”
They conducted a retrospective case-control study that included 160 patients – 50 with psychotic depression, and 110 with nonpsychotic depression. All patients had a primary diagnosis of unipolar major depressive disorder and underwent ECT at a tertiary psychiatric institute between January 2016 and January 2018.
Baseline characteristics of the two groups were similar, although patients with psychosis were more likely to have had an involuntary hospital admission and to have had higher baseline scores on the Montreal Cognitive Assessment (MoCA) and Clinical Global Impression–Severity scale (CGI-S) than nonpsychotic patients.
Response rates to ECT were significantly higher for the patients with psychotic depression than for those with nonpsychotic depression (79% vs. 51%; P = .009), as were remission rates (71% vs. 36%; P = .001).
Both groups showed significant improvement following ECT in Montgomery-Åsberg Depression Rating Scale, CGI, and quality-of-life scores.
However, only the participants with psychotic depression showed a significant improvement in MoCA total score (P = .038), as well as on attention (P = .024), language (P = .008), and orientation (P = .021) subdomains.
Psychotic depression markers?
For the second study, a team led by Aida De Arriba Arnau, MD, Centro de Investigación Biomédica en Red de Salud Mental, Barcelona, Spain, retrospectively analyzed 66 patients with depression who had received ECT. Of these, 26 had psychotic depression, and 40 had nonpsychotic depression.
Response rates were again higher in patients with psychotic vs nonpsychotic depression (92.3% vs. 85.0%). A similar number of sessions was needed to achieve a response.
Improvements in Hamilton Depression Rating Scale scores were significant between the two groups from the start of treatment, although the difference became nonsignificant at week 6.
Arriba Arnau said that there were some notable differences between patients with psychotic depression and those with nonpsychotic depression. For example, the former had “poor functionality, shorter episode duration, and less pharmacological resistance before receiving ECT,” she said.
“So we hypothesized that they might be referred more promptly to ECT treatment,” she added.
The psychotic depression group was significantly older than the group with nonpsychotic depression, at an average of 67.81 years vs 58.96 years.
They also “showed more illness severity and cognitive disturbances at baseline and ... required less anesthetic doses and higher initial stimulus intensity,» Arriba Arnau noted.
“All these features could be the markers of psychotic depression as an entity,” she said. However, the potential impact of age on these differences should be “further studied.”
She added that other aspects, such as age at onset and number of previous episodes, were similar between the groups.
Confirmatory data
Commenting on the findings for Medscape Medical News, Georgios Petrides, MD, associate professor of psychiatry, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, East Garden City, New York, noted that differences in response to ECT between patients with psychotic depression and those with nonpsychotic depression are “well known.”
However, “it’s actually good to present more data that confirm what people are doing in clinical practice,” said Petrides, who was not involved with the research.
Petrides noted that some guidelines recommend ECT as first-line treatment for psychotic depression.
“For nonpsychotic depression, we’d try medications, psychotherapy, and everything else first,” he said. He noted that the current results are “a good replication of what is known so far.”
As to why ECT should be more effective for patients with psychotic depression, he said, “A lot of people think that the biology of psychotic depression is different from the biology of nonpsychotic depression.”
Petrides added.
That ECT is more effective in psychotic depression is an “indirect point of evidence” to support that theory.
One aspect that has traditionally dogged the use of ECT has been the stigma that surrounds the procedure, Petrides noted. That’s “always an issue, but it’s getting less and less over time,” he said.
He added that ECT is extremely safe and that it is associated with the “lowest mortality for any procedure performed under general anesthesia,” which helps to reduce the stigma around it, he noted.
The study authors and Petrides have reported no relevant financial relationships.
This article first appeared on Medscape.com.
For patients with psychotic depression, response to treatment, remission rates, and cognitive improvement are better following electroconvulsive therapy (ECT) than for patients with nonpsychotic depression, results from a new study suggest.
However, findings from another study suggest that at least some of these differences may be because psychotic patients are referred for ECT earlier in the disease course.
Both studies were presented at the European Psychiatric Association 2020 Congress, which was held online this year because of the COVID-19 pandemic.
Limited, old evidence
The first study was led by Christopher Yi Wen Chan, MD, Institute of Mental Health, Singapore. The investigators stated that they have “often observed” superior remission rates with ECT in psychotic versus nonpsychotic depression. However, the evidence base is “limited and mostly more than 10 years old.”
They conducted a retrospective case-control study that included 160 patients – 50 with psychotic depression, and 110 with nonpsychotic depression. All patients had a primary diagnosis of unipolar major depressive disorder and underwent ECT at a tertiary psychiatric institute between January 2016 and January 2018.
Baseline characteristics of the two groups were similar, although patients with psychosis were more likely to have had an involuntary hospital admission and to have had higher baseline scores on the Montreal Cognitive Assessment (MoCA) and Clinical Global Impression–Severity scale (CGI-S) than nonpsychotic patients.
Response rates to ECT were significantly higher for the patients with psychotic depression than for those with nonpsychotic depression (79% vs. 51%; P = .009), as were remission rates (71% vs. 36%; P = .001).
Both groups showed significant improvement following ECT in Montgomery-Åsberg Depression Rating Scale, CGI, and quality-of-life scores.
However, only the participants with psychotic depression showed a significant improvement in MoCA total score (P = .038), as well as on attention (P = .024), language (P = .008), and orientation (P = .021) subdomains.
Psychotic depression markers?
For the second study, a team led by Aida De Arriba Arnau, MD, Centro de Investigación Biomédica en Red de Salud Mental, Barcelona, Spain, retrospectively analyzed 66 patients with depression who had received ECT. Of these, 26 had psychotic depression, and 40 had nonpsychotic depression.
Response rates were again higher in patients with psychotic vs nonpsychotic depression (92.3% vs. 85.0%). A similar number of sessions was needed to achieve a response.
Improvements in Hamilton Depression Rating Scale scores were significant between the two groups from the start of treatment, although the difference became nonsignificant at week 6.
Arriba Arnau said that there were some notable differences between patients with psychotic depression and those with nonpsychotic depression. For example, the former had “poor functionality, shorter episode duration, and less pharmacological resistance before receiving ECT,” she said.
“So we hypothesized that they might be referred more promptly to ECT treatment,” she added.
The psychotic depression group was significantly older than the group with nonpsychotic depression, at an average of 67.81 years vs 58.96 years.
They also “showed more illness severity and cognitive disturbances at baseline and ... required less anesthetic doses and higher initial stimulus intensity,» Arriba Arnau noted.
“All these features could be the markers of psychotic depression as an entity,” she said. However, the potential impact of age on these differences should be “further studied.”
She added that other aspects, such as age at onset and number of previous episodes, were similar between the groups.
Confirmatory data
Commenting on the findings for Medscape Medical News, Georgios Petrides, MD, associate professor of psychiatry, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, East Garden City, New York, noted that differences in response to ECT between patients with psychotic depression and those with nonpsychotic depression are “well known.”
However, “it’s actually good to present more data that confirm what people are doing in clinical practice,” said Petrides, who was not involved with the research.
Petrides noted that some guidelines recommend ECT as first-line treatment for psychotic depression.
“For nonpsychotic depression, we’d try medications, psychotherapy, and everything else first,” he said. He noted that the current results are “a good replication of what is known so far.”
As to why ECT should be more effective for patients with psychotic depression, he said, “A lot of people think that the biology of psychotic depression is different from the biology of nonpsychotic depression.”
Petrides added.
That ECT is more effective in psychotic depression is an “indirect point of evidence” to support that theory.
One aspect that has traditionally dogged the use of ECT has been the stigma that surrounds the procedure, Petrides noted. That’s “always an issue, but it’s getting less and less over time,” he said.
He added that ECT is extremely safe and that it is associated with the “lowest mortality for any procedure performed under general anesthesia,” which helps to reduce the stigma around it, he noted.
The study authors and Petrides have reported no relevant financial relationships.
This article first appeared on Medscape.com.
For patients with psychotic depression, response to treatment, remission rates, and cognitive improvement are better following electroconvulsive therapy (ECT) than for patients with nonpsychotic depression, results from a new study suggest.
However, findings from another study suggest that at least some of these differences may be because psychotic patients are referred for ECT earlier in the disease course.
Both studies were presented at the European Psychiatric Association 2020 Congress, which was held online this year because of the COVID-19 pandemic.
Limited, old evidence
The first study was led by Christopher Yi Wen Chan, MD, Institute of Mental Health, Singapore. The investigators stated that they have “often observed” superior remission rates with ECT in psychotic versus nonpsychotic depression. However, the evidence base is “limited and mostly more than 10 years old.”
They conducted a retrospective case-control study that included 160 patients – 50 with psychotic depression, and 110 with nonpsychotic depression. All patients had a primary diagnosis of unipolar major depressive disorder and underwent ECT at a tertiary psychiatric institute between January 2016 and January 2018.
Baseline characteristics of the two groups were similar, although patients with psychosis were more likely to have had an involuntary hospital admission and to have had higher baseline scores on the Montreal Cognitive Assessment (MoCA) and Clinical Global Impression–Severity scale (CGI-S) than nonpsychotic patients.
Response rates to ECT were significantly higher for the patients with psychotic depression than for those with nonpsychotic depression (79% vs. 51%; P = .009), as were remission rates (71% vs. 36%; P = .001).
Both groups showed significant improvement following ECT in Montgomery-Åsberg Depression Rating Scale, CGI, and quality-of-life scores.
However, only the participants with psychotic depression showed a significant improvement in MoCA total score (P = .038), as well as on attention (P = .024), language (P = .008), and orientation (P = .021) subdomains.
Psychotic depression markers?
For the second study, a team led by Aida De Arriba Arnau, MD, Centro de Investigación Biomédica en Red de Salud Mental, Barcelona, Spain, retrospectively analyzed 66 patients with depression who had received ECT. Of these, 26 had psychotic depression, and 40 had nonpsychotic depression.
Response rates were again higher in patients with psychotic vs nonpsychotic depression (92.3% vs. 85.0%). A similar number of sessions was needed to achieve a response.
Improvements in Hamilton Depression Rating Scale scores were significant between the two groups from the start of treatment, although the difference became nonsignificant at week 6.
Arriba Arnau said that there were some notable differences between patients with psychotic depression and those with nonpsychotic depression. For example, the former had “poor functionality, shorter episode duration, and less pharmacological resistance before receiving ECT,” she said.
“So we hypothesized that they might be referred more promptly to ECT treatment,” she added.
The psychotic depression group was significantly older than the group with nonpsychotic depression, at an average of 67.81 years vs 58.96 years.
They also “showed more illness severity and cognitive disturbances at baseline and ... required less anesthetic doses and higher initial stimulus intensity,» Arriba Arnau noted.
“All these features could be the markers of psychotic depression as an entity,” she said. However, the potential impact of age on these differences should be “further studied.”
She added that other aspects, such as age at onset and number of previous episodes, were similar between the groups.
Confirmatory data
Commenting on the findings for Medscape Medical News, Georgios Petrides, MD, associate professor of psychiatry, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, East Garden City, New York, noted that differences in response to ECT between patients with psychotic depression and those with nonpsychotic depression are “well known.”
However, “it’s actually good to present more data that confirm what people are doing in clinical practice,” said Petrides, who was not involved with the research.
Petrides noted that some guidelines recommend ECT as first-line treatment for psychotic depression.
“For nonpsychotic depression, we’d try medications, psychotherapy, and everything else first,” he said. He noted that the current results are “a good replication of what is known so far.”
As to why ECT should be more effective for patients with psychotic depression, he said, “A lot of people think that the biology of psychotic depression is different from the biology of nonpsychotic depression.”
Petrides added.
That ECT is more effective in psychotic depression is an “indirect point of evidence” to support that theory.
One aspect that has traditionally dogged the use of ECT has been the stigma that surrounds the procedure, Petrides noted. That’s “always an issue, but it’s getting less and less over time,” he said.
He added that ECT is extremely safe and that it is associated with the “lowest mortality for any procedure performed under general anesthesia,” which helps to reduce the stigma around it, he noted.
The study authors and Petrides have reported no relevant financial relationships.
This article first appeared on Medscape.com.