User login
Strategies of Female Teaching Attending Physicians to Navigate Gender-Based Challenges: An Exploratory Qualitative Study
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
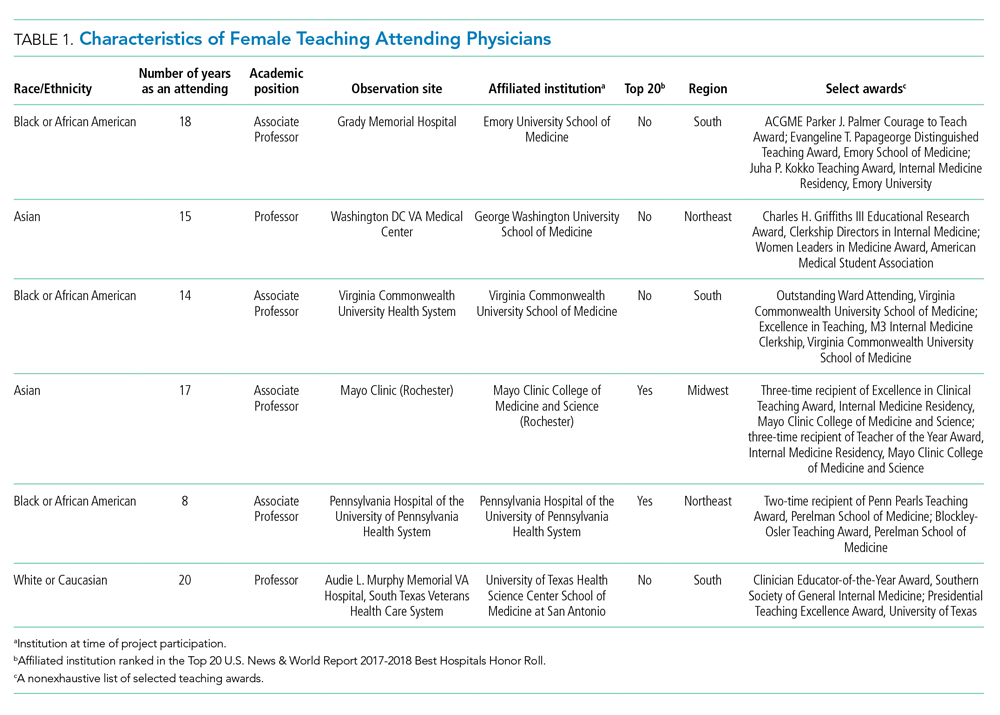
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).
The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
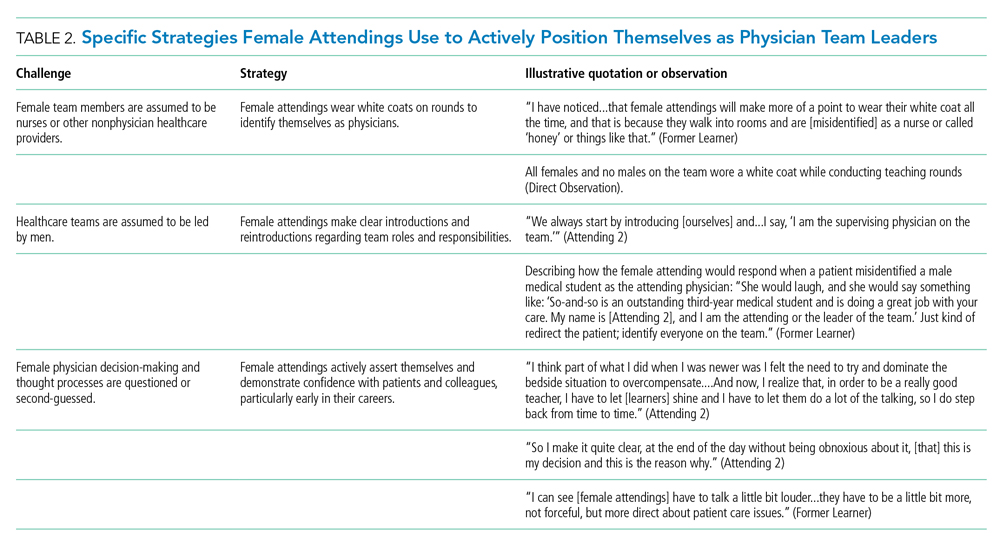
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.
We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.
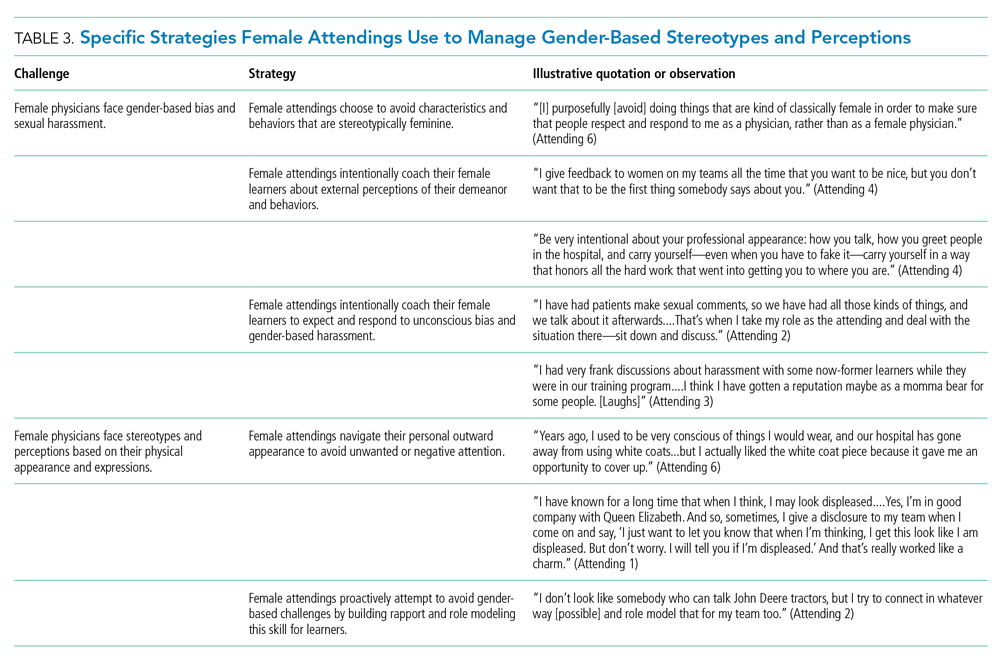
Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
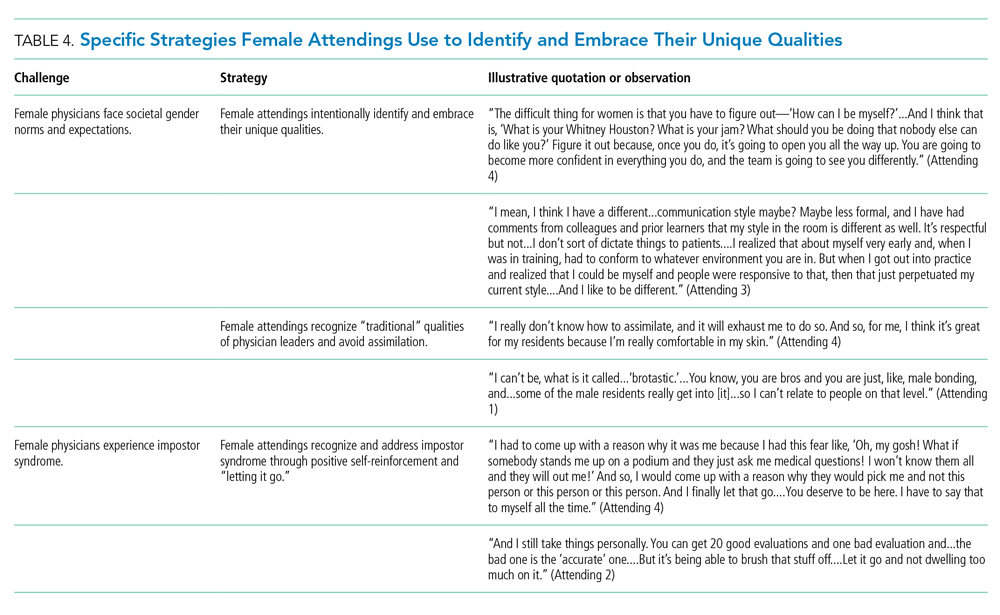
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”
Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).

The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.

We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.

Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”

Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).

The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.

We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.

Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”

Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
© 2020 Society of Hospital Medicine
‘Knowledge is power’: Knowing BRCA1/2 status tied to survival
The study, conducted among Ashkenazi Jewish women in Israel, showed that among women who knew their carrier status before they developed breast cancer, diagnoses were made at an earlier disease stage and 5-year survival was improved compared to women who learned their carrier status only after their disease had been diagnosed.
The study was published online on July 9 in JAMA Oncology.
“I don’t want to belittle the complexities of knowing that you’re a carrier. But I think these results really show that knowledge is power,” first author Ephrat Levy-Lahad, MD, director of the medical genetics unit at Shaare Zedek Medical Center in Jerusalem, Israel, told Medscape Medical News.
Carrying a BRCA1/2 pathogenic mutation is associated with a 70% to 80% lifetime risk for breast cancer and about a 10% to 50% lifetime risk for ovarian cancer, depending on the specific mutation. Only about 10% of carriers will not develop either cancer during their lifetime.
The study provides support for genetic screening for pathogenic BRCA1/2 mutations, especially in high-risk populations, according to Levy-Lahad.
“For me, the results are part of a bigger picture.... I think we should be moving towards general population screening, certainly in high-risk populations like Ashkenazi Jews,” she said.
In Israel, that decision has already been made: a new policy, introduced in January 2020, offers testing for common BRCA1/2 mutations for all Ashkenazi Jewish women.
However, women in other countries may also benefit from testing, she argues. About half of BRCA1/2 carriers in a general population like that of the United States do not have a family history that would indicate a need for testing. That means many women who carry these mutations may not be taking advantage of recommended surveillance and prevention measures, she said.
But screening for BRCA1/2 mutations becomes more complicated when applied to more general populations, she acknowledged.
About 2.5% of women of Ashkenazi Jewish descent carry pathogenic mutations for BRCA1/2, compared to 0.5% in the general White population.
Also, screening in the Ashkenazi Jewish population is probably simpler than in the general population. Just three mutations are definitely known to cause disease and need to be tested for among Ashkenazi Jews. Screening in a larger population would require full sequencing of the gene. That increases the likelihood of finding variants of unknown significance (VUSs), which muddies the water. Knowledge is incomplete about whether some of these VUSs increase cancer risk, and physicians do not always know how to manage them in women who test positive.
Moreover, Israel has a national health system. Screening in a country without universal health insurance such as the United States raises questions about whether follow-up would be covered by insurance carriers for women who test positive.
Mehmet Copur, MD, an oncologist at Morrison Cancer Center in Hastings, Nebraska, questions how general population screening could be done in “real life.”
“These findings should be taken into consideration in the context of the patient population who would agree to genetic testing, who would agree to comply with the recommended guidelines for risk reduction, and who would have insurance coverage or resources to comply with the recommendations,” Copur told Medscape Medical News.
“If BRCA-positive patients did not or could not follow these recommendations, the results would different,” he added.
The most crucial component of screening for these mutations is genetic counselors, who are in short supply in the United States, according to Copur.
Another issue is that of cost. Genetic counseling is not always covered by insurance, especially for individuals who do not have a family history of BRCA-related cancers. Genetic testing is not cheap, and the costs of monitoring women who test positive could be prohibitive, especially in a healthcare system burdened by COVID-19.
“Whether our current healthcare system could bear the cost of such a change is up for debate. The screening itself may be feasible, but offering lifelong surveillance to every woman identified with mutations could present huge capacity issues,” Copur said. “Maybe in the future, the healthcare system can be ready for such an undertaking, but I don’t think we are there yet.”
Although she acknowledges the differences in risk between Ashkenazi Jews and the general population, Levy-Lahad thinks not having screening is like “throwing the baby out with the bath water.”
“Maybe we’re not ready for total general population screening, but I think we have to start thinking along those lines,” she said. “We have this incredible tool for cancer prevention, and we should really be using it, certainly in populations like Ashkenazi Jews.”
Researchers conducted a retrospective analysis that included 105 women diagnosed with breast cancer at Shaare Zedek Medical Center in Jerusalem between 2005 and 2016. Forty-two women knew they were carriers before their breast cancer diagnosis, and 63 learned of their carrier status only after diagnosis. Of the participants, 83% were Ashkenazi Jews. For both prediagnosis and postdiagnosis groups, the age at diagnosis was the same (50.4 years). For both groups, distributions of pathogenic mutations were similar. There were no significant differences in hormone receptor or ERBB2 status.
Among women who knew they were carriers before diagnosis, 80.9% (34/42) were diagnosed either with ductal carcinoma in situ or stage 1 disease. Only 9.5% (4/42) of these women were diagnosed with disease of stage 2 or higher.
In comparison, among women who learned their carrier status after diagnosis, 30% (19/63) had early-stage disease at diagnosis, and 52.4% (33/63) were diagnosed at stage 2 or higher (P < .001).
Compared to women who knew their carrier status before diagnosis, women who found out after diagnosis had 12 times higher odds of being diagnosed with disease of advanced clinical stage (P = .001) and eight times higher odds of being diagnosed with disease of advanced pathologic stage (P = .002).
A sentinel node biopsy was sufficient in 85.7% (36/42) of women who knew their carrier status before diagnosis; 7.2% (3/42) of these women needed a full lymph node dissection. In contrast, 3.2% (2/63) of women who learned their carrier status after diagnosis underwent sentinel node biopsy, and 34.9% (25/105) needed a full lymph node dissection (P < .001).
Among women who knew their carrier status before diagnosis, 54.8% (23/42) did not need chemotherapy at all, and none needed neoadjuvant chemotherapy. Only 4.8% (3/63) of women who learned their mutation status after diagnosis were able to forgo chemotherapy (P < .001); 22.2% (14/63) needed neoadjuvant therapy (P = .001).
These findings appeared to translate into better outcomes. Overall 5-year survival was significantly higher among women who knew their carrier status before diagnosis compared to women who found out afterward (94% [SE 4%] vs 78% [SE 5%]; P = .03). Only two of 42 women (4.8%) in the prediagnosis group died, compared to 16 of 63 (25.4%) in the postdiagnosis group.
Analyses that controlled for year at diagnosis showed that women who learned their carrier status before diagnosis had significantly lower risk for overall mortality compared with those who found out after diagnosis (hazard ratio [HR], 0.20; 95% CI, 0.04 – 0.93; P = .04). However, these results lost significance when controlled for age, socioeconomic index, family history, and gene variant (HR, 0.16; 95% CI, 0.02 – 1.4; P = .10).
Higher socioeconomic status (HR, 0.76; 95% CI, 0.6 – 0.97; P = .03), gene variant (BRCA2 vs BRCA1: HR, 0.15; 95% CI, 0.03 – 0.75; P = .02), and age at diagnosis (HR, 1.047; 95% CI, 1.003 – 1.093; P = .04) were all associated with overall mortality.
“I can’t infer causation, but we suspect that the reason for these results is the difference in follow-up,” Levy-Lahad said.
Most of the women (95.2%, 40/42) who knew their carrier status before diagnosis received their follow-up at the medical center’s high-risk carrier clinic. Twenty-seven of 42 (64.3%) of these women were diagnosed with breast MRI. By contrast, only 1.6% (1/63) of women who found out their carrier status after diagnosis were diagnosed with breast MRI. Breast MRI is not routinely used for breast cancer screening but can be more sensitive than mammography for detecting breast cancer.
The study was funded by the Breast Cancer Research Foundation and by a gift from Ellie and David Werber to ShaareZedek Medical Center.
Levy-Lahad received grants from the Breast Cancer Research Foundation and from the Israel Cancer Association during the conduct of the study and personal fees from AstraZeneca outside the submitted work. Copur has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The study, conducted among Ashkenazi Jewish women in Israel, showed that among women who knew their carrier status before they developed breast cancer, diagnoses were made at an earlier disease stage and 5-year survival was improved compared to women who learned their carrier status only after their disease had been diagnosed.
The study was published online on July 9 in JAMA Oncology.
“I don’t want to belittle the complexities of knowing that you’re a carrier. But I think these results really show that knowledge is power,” first author Ephrat Levy-Lahad, MD, director of the medical genetics unit at Shaare Zedek Medical Center in Jerusalem, Israel, told Medscape Medical News.
Carrying a BRCA1/2 pathogenic mutation is associated with a 70% to 80% lifetime risk for breast cancer and about a 10% to 50% lifetime risk for ovarian cancer, depending on the specific mutation. Only about 10% of carriers will not develop either cancer during their lifetime.
The study provides support for genetic screening for pathogenic BRCA1/2 mutations, especially in high-risk populations, according to Levy-Lahad.
“For me, the results are part of a bigger picture.... I think we should be moving towards general population screening, certainly in high-risk populations like Ashkenazi Jews,” she said.
In Israel, that decision has already been made: a new policy, introduced in January 2020, offers testing for common BRCA1/2 mutations for all Ashkenazi Jewish women.
However, women in other countries may also benefit from testing, she argues. About half of BRCA1/2 carriers in a general population like that of the United States do not have a family history that would indicate a need for testing. That means many women who carry these mutations may not be taking advantage of recommended surveillance and prevention measures, she said.
But screening for BRCA1/2 mutations becomes more complicated when applied to more general populations, she acknowledged.
About 2.5% of women of Ashkenazi Jewish descent carry pathogenic mutations for BRCA1/2, compared to 0.5% in the general White population.
Also, screening in the Ashkenazi Jewish population is probably simpler than in the general population. Just three mutations are definitely known to cause disease and need to be tested for among Ashkenazi Jews. Screening in a larger population would require full sequencing of the gene. That increases the likelihood of finding variants of unknown significance (VUSs), which muddies the water. Knowledge is incomplete about whether some of these VUSs increase cancer risk, and physicians do not always know how to manage them in women who test positive.
Moreover, Israel has a national health system. Screening in a country without universal health insurance such as the United States raises questions about whether follow-up would be covered by insurance carriers for women who test positive.
Mehmet Copur, MD, an oncologist at Morrison Cancer Center in Hastings, Nebraska, questions how general population screening could be done in “real life.”
“These findings should be taken into consideration in the context of the patient population who would agree to genetic testing, who would agree to comply with the recommended guidelines for risk reduction, and who would have insurance coverage or resources to comply with the recommendations,” Copur told Medscape Medical News.
“If BRCA-positive patients did not or could not follow these recommendations, the results would different,” he added.
The most crucial component of screening for these mutations is genetic counselors, who are in short supply in the United States, according to Copur.
Another issue is that of cost. Genetic counseling is not always covered by insurance, especially for individuals who do not have a family history of BRCA-related cancers. Genetic testing is not cheap, and the costs of monitoring women who test positive could be prohibitive, especially in a healthcare system burdened by COVID-19.
“Whether our current healthcare system could bear the cost of such a change is up for debate. The screening itself may be feasible, but offering lifelong surveillance to every woman identified with mutations could present huge capacity issues,” Copur said. “Maybe in the future, the healthcare system can be ready for such an undertaking, but I don’t think we are there yet.”
Although she acknowledges the differences in risk between Ashkenazi Jews and the general population, Levy-Lahad thinks not having screening is like “throwing the baby out with the bath water.”
“Maybe we’re not ready for total general population screening, but I think we have to start thinking along those lines,” she said. “We have this incredible tool for cancer prevention, and we should really be using it, certainly in populations like Ashkenazi Jews.”
Researchers conducted a retrospective analysis that included 105 women diagnosed with breast cancer at Shaare Zedek Medical Center in Jerusalem between 2005 and 2016. Forty-two women knew they were carriers before their breast cancer diagnosis, and 63 learned of their carrier status only after diagnosis. Of the participants, 83% were Ashkenazi Jews. For both prediagnosis and postdiagnosis groups, the age at diagnosis was the same (50.4 years). For both groups, distributions of pathogenic mutations were similar. There were no significant differences in hormone receptor or ERBB2 status.
Among women who knew they were carriers before diagnosis, 80.9% (34/42) were diagnosed either with ductal carcinoma in situ or stage 1 disease. Only 9.5% (4/42) of these women were diagnosed with disease of stage 2 or higher.
In comparison, among women who learned their carrier status after diagnosis, 30% (19/63) had early-stage disease at diagnosis, and 52.4% (33/63) were diagnosed at stage 2 or higher (P < .001).
Compared to women who knew their carrier status before diagnosis, women who found out after diagnosis had 12 times higher odds of being diagnosed with disease of advanced clinical stage (P = .001) and eight times higher odds of being diagnosed with disease of advanced pathologic stage (P = .002).
A sentinel node biopsy was sufficient in 85.7% (36/42) of women who knew their carrier status before diagnosis; 7.2% (3/42) of these women needed a full lymph node dissection. In contrast, 3.2% (2/63) of women who learned their carrier status after diagnosis underwent sentinel node biopsy, and 34.9% (25/105) needed a full lymph node dissection (P < .001).
Among women who knew their carrier status before diagnosis, 54.8% (23/42) did not need chemotherapy at all, and none needed neoadjuvant chemotherapy. Only 4.8% (3/63) of women who learned their mutation status after diagnosis were able to forgo chemotherapy (P < .001); 22.2% (14/63) needed neoadjuvant therapy (P = .001).
These findings appeared to translate into better outcomes. Overall 5-year survival was significantly higher among women who knew their carrier status before diagnosis compared to women who found out afterward (94% [SE 4%] vs 78% [SE 5%]; P = .03). Only two of 42 women (4.8%) in the prediagnosis group died, compared to 16 of 63 (25.4%) in the postdiagnosis group.
Analyses that controlled for year at diagnosis showed that women who learned their carrier status before diagnosis had significantly lower risk for overall mortality compared with those who found out after diagnosis (hazard ratio [HR], 0.20; 95% CI, 0.04 – 0.93; P = .04). However, these results lost significance when controlled for age, socioeconomic index, family history, and gene variant (HR, 0.16; 95% CI, 0.02 – 1.4; P = .10).
Higher socioeconomic status (HR, 0.76; 95% CI, 0.6 – 0.97; P = .03), gene variant (BRCA2 vs BRCA1: HR, 0.15; 95% CI, 0.03 – 0.75; P = .02), and age at diagnosis (HR, 1.047; 95% CI, 1.003 – 1.093; P = .04) were all associated with overall mortality.
“I can’t infer causation, but we suspect that the reason for these results is the difference in follow-up,” Levy-Lahad said.
Most of the women (95.2%, 40/42) who knew their carrier status before diagnosis received their follow-up at the medical center’s high-risk carrier clinic. Twenty-seven of 42 (64.3%) of these women were diagnosed with breast MRI. By contrast, only 1.6% (1/63) of women who found out their carrier status after diagnosis were diagnosed with breast MRI. Breast MRI is not routinely used for breast cancer screening but can be more sensitive than mammography for detecting breast cancer.
The study was funded by the Breast Cancer Research Foundation and by a gift from Ellie and David Werber to ShaareZedek Medical Center.
Levy-Lahad received grants from the Breast Cancer Research Foundation and from the Israel Cancer Association during the conduct of the study and personal fees from AstraZeneca outside the submitted work. Copur has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The study, conducted among Ashkenazi Jewish women in Israel, showed that among women who knew their carrier status before they developed breast cancer, diagnoses were made at an earlier disease stage and 5-year survival was improved compared to women who learned their carrier status only after their disease had been diagnosed.
The study was published online on July 9 in JAMA Oncology.
“I don’t want to belittle the complexities of knowing that you’re a carrier. But I think these results really show that knowledge is power,” first author Ephrat Levy-Lahad, MD, director of the medical genetics unit at Shaare Zedek Medical Center in Jerusalem, Israel, told Medscape Medical News.
Carrying a BRCA1/2 pathogenic mutation is associated with a 70% to 80% lifetime risk for breast cancer and about a 10% to 50% lifetime risk for ovarian cancer, depending on the specific mutation. Only about 10% of carriers will not develop either cancer during their lifetime.
The study provides support for genetic screening for pathogenic BRCA1/2 mutations, especially in high-risk populations, according to Levy-Lahad.
“For me, the results are part of a bigger picture.... I think we should be moving towards general population screening, certainly in high-risk populations like Ashkenazi Jews,” she said.
In Israel, that decision has already been made: a new policy, introduced in January 2020, offers testing for common BRCA1/2 mutations for all Ashkenazi Jewish women.
However, women in other countries may also benefit from testing, she argues. About half of BRCA1/2 carriers in a general population like that of the United States do not have a family history that would indicate a need for testing. That means many women who carry these mutations may not be taking advantage of recommended surveillance and prevention measures, she said.
But screening for BRCA1/2 mutations becomes more complicated when applied to more general populations, she acknowledged.
About 2.5% of women of Ashkenazi Jewish descent carry pathogenic mutations for BRCA1/2, compared to 0.5% in the general White population.
Also, screening in the Ashkenazi Jewish population is probably simpler than in the general population. Just three mutations are definitely known to cause disease and need to be tested for among Ashkenazi Jews. Screening in a larger population would require full sequencing of the gene. That increases the likelihood of finding variants of unknown significance (VUSs), which muddies the water. Knowledge is incomplete about whether some of these VUSs increase cancer risk, and physicians do not always know how to manage them in women who test positive.
Moreover, Israel has a national health system. Screening in a country without universal health insurance such as the United States raises questions about whether follow-up would be covered by insurance carriers for women who test positive.
Mehmet Copur, MD, an oncologist at Morrison Cancer Center in Hastings, Nebraska, questions how general population screening could be done in “real life.”
“These findings should be taken into consideration in the context of the patient population who would agree to genetic testing, who would agree to comply with the recommended guidelines for risk reduction, and who would have insurance coverage or resources to comply with the recommendations,” Copur told Medscape Medical News.
“If BRCA-positive patients did not or could not follow these recommendations, the results would different,” he added.
The most crucial component of screening for these mutations is genetic counselors, who are in short supply in the United States, according to Copur.
Another issue is that of cost. Genetic counseling is not always covered by insurance, especially for individuals who do not have a family history of BRCA-related cancers. Genetic testing is not cheap, and the costs of monitoring women who test positive could be prohibitive, especially in a healthcare system burdened by COVID-19.
“Whether our current healthcare system could bear the cost of such a change is up for debate. The screening itself may be feasible, but offering lifelong surveillance to every woman identified with mutations could present huge capacity issues,” Copur said. “Maybe in the future, the healthcare system can be ready for such an undertaking, but I don’t think we are there yet.”
Although she acknowledges the differences in risk between Ashkenazi Jews and the general population, Levy-Lahad thinks not having screening is like “throwing the baby out with the bath water.”
“Maybe we’re not ready for total general population screening, but I think we have to start thinking along those lines,” she said. “We have this incredible tool for cancer prevention, and we should really be using it, certainly in populations like Ashkenazi Jews.”
Researchers conducted a retrospective analysis that included 105 women diagnosed with breast cancer at Shaare Zedek Medical Center in Jerusalem between 2005 and 2016. Forty-two women knew they were carriers before their breast cancer diagnosis, and 63 learned of their carrier status only after diagnosis. Of the participants, 83% were Ashkenazi Jews. For both prediagnosis and postdiagnosis groups, the age at diagnosis was the same (50.4 years). For both groups, distributions of pathogenic mutations were similar. There were no significant differences in hormone receptor or ERBB2 status.
Among women who knew they were carriers before diagnosis, 80.9% (34/42) were diagnosed either with ductal carcinoma in situ or stage 1 disease. Only 9.5% (4/42) of these women were diagnosed with disease of stage 2 or higher.
In comparison, among women who learned their carrier status after diagnosis, 30% (19/63) had early-stage disease at diagnosis, and 52.4% (33/63) were diagnosed at stage 2 or higher (P < .001).
Compared to women who knew their carrier status before diagnosis, women who found out after diagnosis had 12 times higher odds of being diagnosed with disease of advanced clinical stage (P = .001) and eight times higher odds of being diagnosed with disease of advanced pathologic stage (P = .002).
A sentinel node biopsy was sufficient in 85.7% (36/42) of women who knew their carrier status before diagnosis; 7.2% (3/42) of these women needed a full lymph node dissection. In contrast, 3.2% (2/63) of women who learned their carrier status after diagnosis underwent sentinel node biopsy, and 34.9% (25/105) needed a full lymph node dissection (P < .001).
Among women who knew their carrier status before diagnosis, 54.8% (23/42) did not need chemotherapy at all, and none needed neoadjuvant chemotherapy. Only 4.8% (3/63) of women who learned their mutation status after diagnosis were able to forgo chemotherapy (P < .001); 22.2% (14/63) needed neoadjuvant therapy (P = .001).
These findings appeared to translate into better outcomes. Overall 5-year survival was significantly higher among women who knew their carrier status before diagnosis compared to women who found out afterward (94% [SE 4%] vs 78% [SE 5%]; P = .03). Only two of 42 women (4.8%) in the prediagnosis group died, compared to 16 of 63 (25.4%) in the postdiagnosis group.
Analyses that controlled for year at diagnosis showed that women who learned their carrier status before diagnosis had significantly lower risk for overall mortality compared with those who found out after diagnosis (hazard ratio [HR], 0.20; 95% CI, 0.04 – 0.93; P = .04). However, these results lost significance when controlled for age, socioeconomic index, family history, and gene variant (HR, 0.16; 95% CI, 0.02 – 1.4; P = .10).
Higher socioeconomic status (HR, 0.76; 95% CI, 0.6 – 0.97; P = .03), gene variant (BRCA2 vs BRCA1: HR, 0.15; 95% CI, 0.03 – 0.75; P = .02), and age at diagnosis (HR, 1.047; 95% CI, 1.003 – 1.093; P = .04) were all associated with overall mortality.
“I can’t infer causation, but we suspect that the reason for these results is the difference in follow-up,” Levy-Lahad said.
Most of the women (95.2%, 40/42) who knew their carrier status before diagnosis received their follow-up at the medical center’s high-risk carrier clinic. Twenty-seven of 42 (64.3%) of these women were diagnosed with breast MRI. By contrast, only 1.6% (1/63) of women who found out their carrier status after diagnosis were diagnosed with breast MRI. Breast MRI is not routinely used for breast cancer screening but can be more sensitive than mammography for detecting breast cancer.
The study was funded by the Breast Cancer Research Foundation and by a gift from Ellie and David Werber to ShaareZedek Medical Center.
Levy-Lahad received grants from the Breast Cancer Research Foundation and from the Israel Cancer Association during the conduct of the study and personal fees from AstraZeneca outside the submitted work. Copur has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Behind the mask
Bicycling has always been part of who I am because it offered me the freedom to explore as a preteen. As an adult I have always been a bicycle commuter and a very visible part of the community as I pedal around town to do my errands. But, I didn’t always wear a helmet ... because well, I just didn’t. I saw the helmet as a nuisance with very little benefit to myself. Eventually, when bike races required helmets I bought one just for the competitions. Until one day about 30 years ago when the mother of a child I was seeing in the office said, “Dr. Wilkoff, you know as an influential member of this community, particularly its children, you should be wearing a helmet.” My wife had been badgering me for years but this woman’s courage to speak up embarrassed me into changing my ways.
For some, maybe many, people, wearing a mask during the COVID-19 pandemic is a nuisance and an assault on their independence just as I viewed a bicycle helmet. Initially there was some information being circulated that any mask less robust than a N-95 had very little if any effect, either as protection or as way to decrease spread. I certainly had my doubts about the value of mask other than as a statement of solidarity. However, we are now learning that masks can serve an important role along with social distancing in a comprehensive community effort to minimize contagion.
In light of this new information, why are there are still people who won’t wear a mask? It may be that they are receiving their news filtered through a lens that discredits science. But, it is more likely the result of the same mindset that permeates the anti-vaccine faction that the common good is less important than personal freedom to follow their beliefs.
Do we have any tools at our disposal to increase the number of folks wearing masks? Based on our experience with attempts to convince those who are anti-vaccine, education will be ineffective in shifting the focus from personal freedom to a commitment to the welfare of the community at large. Shaming might be effective, but it runs the risk of igniting conflicts and further widening the gaps in our society. Some establishments have been effective in simply saying “no mask, no entry,” but this runs the same risk of creating friction depending on the community and the situation.
The ship may have already sailed on our best opportunity to achieve community compliance when the leaders of our national government have chosen to ignore their obligation to set an example by refusing to wear masks. I fear that the wedge has already been set and the widening of the gap between those who see their responsibility to the community at large and those who do not will continue to grow.
I am fortunate to live in a town whose residents look out for each other and have relied on local leaders to set an example in the absence of leadership on a national level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Bicycling has always been part of who I am because it offered me the freedom to explore as a preteen. As an adult I have always been a bicycle commuter and a very visible part of the community as I pedal around town to do my errands. But, I didn’t always wear a helmet ... because well, I just didn’t. I saw the helmet as a nuisance with very little benefit to myself. Eventually, when bike races required helmets I bought one just for the competitions. Until one day about 30 years ago when the mother of a child I was seeing in the office said, “Dr. Wilkoff, you know as an influential member of this community, particularly its children, you should be wearing a helmet.” My wife had been badgering me for years but this woman’s courage to speak up embarrassed me into changing my ways.
For some, maybe many, people, wearing a mask during the COVID-19 pandemic is a nuisance and an assault on their independence just as I viewed a bicycle helmet. Initially there was some information being circulated that any mask less robust than a N-95 had very little if any effect, either as protection or as way to decrease spread. I certainly had my doubts about the value of mask other than as a statement of solidarity. However, we are now learning that masks can serve an important role along with social distancing in a comprehensive community effort to minimize contagion.
In light of this new information, why are there are still people who won’t wear a mask? It may be that they are receiving their news filtered through a lens that discredits science. But, it is more likely the result of the same mindset that permeates the anti-vaccine faction that the common good is less important than personal freedom to follow their beliefs.
Do we have any tools at our disposal to increase the number of folks wearing masks? Based on our experience with attempts to convince those who are anti-vaccine, education will be ineffective in shifting the focus from personal freedom to a commitment to the welfare of the community at large. Shaming might be effective, but it runs the risk of igniting conflicts and further widening the gaps in our society. Some establishments have been effective in simply saying “no mask, no entry,” but this runs the same risk of creating friction depending on the community and the situation.
The ship may have already sailed on our best opportunity to achieve community compliance when the leaders of our national government have chosen to ignore their obligation to set an example by refusing to wear masks. I fear that the wedge has already been set and the widening of the gap between those who see their responsibility to the community at large and those who do not will continue to grow.
I am fortunate to live in a town whose residents look out for each other and have relied on local leaders to set an example in the absence of leadership on a national level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Bicycling has always been part of who I am because it offered me the freedom to explore as a preteen. As an adult I have always been a bicycle commuter and a very visible part of the community as I pedal around town to do my errands. But, I didn’t always wear a helmet ... because well, I just didn’t. I saw the helmet as a nuisance with very little benefit to myself. Eventually, when bike races required helmets I bought one just for the competitions. Until one day about 30 years ago when the mother of a child I was seeing in the office said, “Dr. Wilkoff, you know as an influential member of this community, particularly its children, you should be wearing a helmet.” My wife had been badgering me for years but this woman’s courage to speak up embarrassed me into changing my ways.
For some, maybe many, people, wearing a mask during the COVID-19 pandemic is a nuisance and an assault on their independence just as I viewed a bicycle helmet. Initially there was some information being circulated that any mask less robust than a N-95 had very little if any effect, either as protection or as way to decrease spread. I certainly had my doubts about the value of mask other than as a statement of solidarity. However, we are now learning that masks can serve an important role along with social distancing in a comprehensive community effort to minimize contagion.
In light of this new information, why are there are still people who won’t wear a mask? It may be that they are receiving their news filtered through a lens that discredits science. But, it is more likely the result of the same mindset that permeates the anti-vaccine faction that the common good is less important than personal freedom to follow their beliefs.
Do we have any tools at our disposal to increase the number of folks wearing masks? Based on our experience with attempts to convince those who are anti-vaccine, education will be ineffective in shifting the focus from personal freedom to a commitment to the welfare of the community at large. Shaming might be effective, but it runs the risk of igniting conflicts and further widening the gaps in our society. Some establishments have been effective in simply saying “no mask, no entry,” but this runs the same risk of creating friction depending on the community and the situation.
The ship may have already sailed on our best opportunity to achieve community compliance when the leaders of our national government have chosen to ignore their obligation to set an example by refusing to wear masks. I fear that the wedge has already been set and the widening of the gap between those who see their responsibility to the community at large and those who do not will continue to grow.
I am fortunate to live in a town whose residents look out for each other and have relied on local leaders to set an example in the absence of leadership on a national level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
In remission for 10 years: Long-term toxicity data on CAR T cells
When a patient with cancer hears there isn’t much left that doctors can do, it always stays fresh in the mind.
Doug Olson was first diagnosed with chronic lymphocytic leukemia (CLL) over 20 years ago, in 1996. For several years, his doctors used the watch-and-wait approach. But then his cancer progressed and needed treatment. By 2010, it had mutated so much that it no longer responded to standard therapy.
He was rapidly running out of options. Back then, the only treatment left was a bone marrow transplant. Without one, his doctors said, he would have 1 or 2 years left to live.
“I was really trying to avoid a bone marrow transplant. You’re playing your last card if that doesn’t work. It’s a pretty rough procedure,” Olson told Medscape Medical News.
Looking back, Olson counts himself as lucky – for being in the right place, at the right time, with the right doctor. His oncologist was David Porter, MD, the principal investigator on a trial at the University of Pennsylvania that was investigating a brand new approach to treating cancer: chimeric antigen receptor (CAR) T-cell therapy.
CAR T-cell therapy uses a patient’s own T cells engineered to express a receptor that targets proteins on cancer cells. CAR T cells are considered “living drugs” because they expand inside the body and stick around for years – maybe for a lifetime – to fight the cancer if it tries to come back.
“I was certainly intrigued by the approach. It had worked in mice, and it was the sort of thing that looked like it would work,” Olson recalled.
Science is not a foreign language to Olson. He holds a PhD in medicinal chemistry, spent most of his career in the in vitro diagnostics industry, and currently acts as chief executive officer of Buhlmann Diagnostics Corp.
So he read the clinical protocol for the first in-human trial of CAR T cells and agreed to become patient number two.
Olson’s T cells were harvested, engineered to attack the CD19 antigen found on malignant and normal B lymphocytes, and then were expanded into millions in the lab. After undergoing preconditioning with chemotherapy to minimize rejection and boost the CAR T cells’ expansion inside the body, he received several infusions of the new therapy over the course of 3 days.
Nothing really happened for 2 weeks. Then he developed severe flu-like symptoms – so bad that he was hospitalized.
Ironically, getting sick was a sign that the CAR T cells were working. Olson was experiencing one of the main short-term effects of CAR T-cell therapy: cytokine release syndrome. Symptoms include extremely high fevers and dangerous drops in blood pressure that can potentially cause end-organ damage.
In the early trials of these products, some patients experienced such a severe reaction that they needed intensive care, and some died. With increasing clinical experience, doctors have learned to control the reaction with the use of steroids and interleukein-6 inhibitors such as tocilizumab (Actemra).
Fortunately for Olson, the reaction passed, and he was eventually discharged.
Then the “aha moment” happened. Four weeks after receiving the CAR T cells, Olson found out that he was cancer free.
“It still gives me shivers,” he said. “Dr Porter said, ‘Your bone marrow’s completely free. We just can’t find a cancer cell anywhere.’ “
The remission has lasted, and it is now 10 years later.
Balancing long-term risks vs benefits
Long-term data have been accumulating for these novel therapies since Olson’s treatment in 2010. This is particularly important for CAR T-cell therapy, because of its longevity. Because these are living cells and are expected to persist in the body for years, there is great interest in longer-term data, especially the risks for toxicity.
The FDA requires clinical follow-up for at least 15 years for patients treated with CAR T-cell therapy or any other genetically modified cells.
So far, most of the experience with CAR T cells comes from anti-CD19-directed therapy, which has shown “remarkable” remission rates in the 50% to 85% range, said Nirali Shah, MD, head of the hematologic malignancies section of the Pediatric Oncology Branch at the National Cancer Institute (NCI).
The most recent results presented at this year’s annual meeting of the American Society of Clinical Oncology support earlier efficacy data, she noted. In the longest follow-up to date, researchers reported remissions lasting over 9 years in patients with relapsed/refractory B-cell lymphoma or CLL treated with Kite›s axicaptagene cilleucel (Yescarta), one of two anti-CD19-directed CAR T-cell therapies approved by the FDA in 2017 (the other is Novartis’ tisagenlecleucel [Kymriah]).
This study included 43 patients and showed an overall remission rate of 76%. Complete remission was achieved in 54% of patients, and 22% had partial remission.
The other focus is long-term safety. Although some of the long-term adverse effects are known and are manageable, others fall into the theoretical realm. In early May 2020, the NCI held a multidisciplinary virtual conference on CAR T-cell therapy «to encourage collaborative research about the subacute and potentially long-term toxicity profile of these treatments.»
“We know just a little at this point about late- and long-term effects of CAR-T therapy, because we are relatively early in the era of CAR T cells,” said Merav Bar, MD, from the Fred Hutchinson Cancer Research Center in Seattle, Washington.
B-cell aplasia and risk for new infections
What is known is that B-cell aplasia represents the most common long-term adverse effect of CAR T-cell therapy. B-cell aplasia results when anti-CD19 CAR-T therapy wipes out healthy B cells as well as the malignant ones responsible for leukemia/lymphoma.
As major players in the immune system, B cells are a key defense against viruses. So B-cell aplasia represents a very specific type of immunosuppression. It is generally less severe than immunosuppression that occurs after organ transplant, which hits the immune system pretty much across the board and carries a much higher risk for infection.
The main concern is what happens when someone with B-cell aplasia encounters a new pathogen, such as SARS-CoV-2.
After infection, B cells generate memory cells, which are not killed off by anti-CD19 therapy and that stick around for life. So a patient such as Olson would still make antibodies that fight infections they experienced before receiving CAR-T therapy, such as childhood chickenpox. But now they are unable to make new memory cells, so these patients receive monthly immunoglobulin infusions to protect against pathogens they have not previously encountered.
Olson takes this in stride and says he isn’t overly worried about COVID-19. He follows the recommended precautions for a man his age. He wears a mask, washes his hands frequently, and tries to maintain social distancing. But he doesn’t stay locked up in his New Hampshire home.
“I took the attitude when I was diagnosed with cancer that I’m going to live my life,” he said. “Quality of life to me is more important than quantity.”
Neuropsychiatric toxicity
Another problem is the possibility of neuropsychiatric toxicity. Past studies have reported a wide range of such toxicities associated with CAR T-cell therapy, including seizures and hallucinations. Most have occurred early in the course of treatment and appear to be short-lived and reversible. However, there remain questions about long-term neuropsychiatric problems.
In a long-term study of 40 patients with relapsed/refractory CLL, non-Hodgkin lymphoma, and ALL, nearly half of patients (47.5%, 19/40) self-reported at least one clinically meaningful negative neuropsychiatric outcome (anxiety, depression, or cognitive difficulty) 1 to 5 years after anti-CD19 CAR T-cell therapy. In addition, 37.5% (15/40) self-reported cognitive difficulties.
“Patients with more severe neurotoxicity showed a trend for more cognitive difficulties afterwards,» said Bar, senior author of the study.
However, teasing out the role that CAR T-cell therapy plays in these problems poses a challenge. All of these patients had been heavily pretreated with previous cancer therapy, which has also been associated with neuropsychiatric problems.
“So far, we don’t know what caused it,” Bar said. “Nevertheless, people need to pay attention to neuropsychiatric symptoms in CAR T-cell therapy. It is important to continue to monitor these patients for these issues.”
Graft-vs-host disease
Another potential problem is graft-vs-host disease (GVHD). This is not uncommon after hematopoietic stem cell transplants. It develops when the donor T cells view antigens on healthy recipient cells as foreign and attack them.
For patients who are treated with CAR T cells, GVHD is mostly a concern among individuals who have previously had a transplant and who are already at increased risk for it.
In a study of late effects among 86 adults treated with anti-CD19 CAR T cells for relapsed/refractory non-Hodgkin lymphoma, Bar and colleagues found that GVHD occurred only among patients who had received a previous donor stem cell transplant. Of these, 20% (3/15) developed GVHD about 28 months after CAR-T therapy.
“The data for CAR T cells causing GVHD really hasn’t shown that it’s a huge problem, although we have seen it and are continuing to monitor for it,” the NCI’s Shah commented to Medscape Medical News.
Other Long-term Adverse Effects
A range of other long-term adverse effects have been reported with CAR-T therapy, including prolonged cytopenias (reduced mature blood cells), myelodysplasia (bone marrow failure), and second malignancies.
In the study with the longest follow-up to date, 16% (7/43) of patients developed second malignancies, which is comparable to data from Bar’s study in Seattle (15%, 13/86). The researchers in this study consider this rate to be no higher than expected: these patients had already received extensive chemotherapy, which increases the risk for other cancers, they point out.
However, this brings up theoretical concerns about the long-term effects of gene modification. CAR T cells are engineered using retroviruses (mainly lentiviruses), which randomly insert the CAR genes into the host genome. Doing so may cause mutations that could promote cancer. These lentiviruses also carry the theoretical risk of becoming capable of viral replication once inside the body.
To address these concerns, viruses used to engineer CAR T cells go through comprehensive safety testing. After therapy, patients are checked every few months during the first year and annually after that.
So far, there have been no reports of cancers associated with CAR T-cell therapy.
“Any type of cancer is a very theoretical risk,” Bar told Medscape Medical News. «Most likely the malignancies in our study were related to prior treatment that the patients received. None of them had any evidence of replication-competent lentivirus, or any other evidence that the malignancies were related to the CAR T cells.»
Another theoretical concern is the possibility of new-onset autoimmune disease, although, once again, no cases have been reported so far.
“We think of it as a theoretic possibility. Whenever you jack up the immune system, autoimmune disease is a potential risk,” said Carl June, MD, director of the Center for Cellular Immunotherapies at the University of Pennsylvania.
June was the co–principal investigator of the trial in which Olson participated. He is also the inventor on patents for CAR T cells licensed by the University of Pennsylvania to Novartis and Tmunity and is a scientific founder with equity in Tmunity.
Still, autoimmunity could occur, and scientists are looking out for it.
“We are continuing to be vigilant in our monitoring for autoimmune disease,” Shah added. “We’ve been doing CAR T-cell therapy since 2012, and I think we have yet to see true autoimmunity beyond GVHD.”
Future directions
In the 10 years since Olson received CAR T-cell therapy, an entire industry has sprung up. Over 100 companies worldwide are now developing CAR T-cell therapies targeting various antigens. These therapies are directed at about 60 different tumor types, including solid tumors. Nearly 200 clinical trials are underway, though most are still in early stages: as of September 2019, only 5% had reached phase 3.
Clinical data show promising results for CAR T-cell therapy directed against CD22 (overexpressed on ALL cells), and BCMA (found on almost all multiple myeloma cells). Yet questions remain as to whether CAR T cells will be as effective if they target antigens other than CD19 or cells other than B lymphocytes. One of the biggest research questions is whether they will be effective against solid tumors.
One research avenue being watched with great interest is the development of universal CAR T cells. So far, such products are at very early stages of development (phase 1 trials), but they are attractive because of the potential advantages they offer over bespoke CAR T cells. Automating the process holds the promise of immediate availability, standardizing production, expanding access, and lowering costs. And because the T cells for this universal product come from healthy donors, they may function better than T cells that have been battered and bruised by past cancer treatments, or even the cancer itself.
However, precisely because they are developed from healthy donor T cells, universal CAR T cells may pose increased risk for GVHD. Scientists are trying to get around this problem by engineering universal CAR T cells that lack the T-cell receptor involved in GVHD.
There are also other concerns. Nature has a penchant for mutation. Engineering CAR T cells without T-cell receptors means the body may no longer detect or reject a universal CAR T cell if it goes rogue. Also, gene insertion in universal CAR-T therapy is targeted rather than random (as in bespoke CAR T cells), which could create off-target effects. Both issues create a theoretical risk of such products inducing an untreatable CAR T-cell therapy–associated cancer.
“The theoretic risk with universal cells is that their safety profile may not be as good for long term,” June commented.
Hope for the future
From that first trial in which June and Porter used CAR T cells, two of three patients they treated are still alive 10 years later.
Olson is one of these two, and he still undergoes monitoring every 3 months to check for relapse. So far, none of his tests have shown signs of his cancer returning.
After going into remission, Doug spent the next 6 to 9 months regaining his health and strength.
“I figured if I had this amazing treatment that saved my life, I had an obligation to stay alive,” he said. “I’d better not die of something like a heart attack!”
He took up long distance running and has completed six half marathons. He became involved in the Leukemia and Lymphoma Society, participating in fund-raising and helping newly diagnosed patients. Over the years, he has also given talks for researchers, people with cancer, and healthcare providers.
Doug is now 73. Today, he marvels at how rapidly the CAR-T field has progressed.
“Twenty years ago, if you had cancer, your prospects weren’t nearly as good as these days. In 2010, people still didn’t believe in CAR T-cell therapy,” he said. “My goal always in telling my story is a message of hope.”
This article first appeared on Medscape.com.
When a patient with cancer hears there isn’t much left that doctors can do, it always stays fresh in the mind.
Doug Olson was first diagnosed with chronic lymphocytic leukemia (CLL) over 20 years ago, in 1996. For several years, his doctors used the watch-and-wait approach. But then his cancer progressed and needed treatment. By 2010, it had mutated so much that it no longer responded to standard therapy.
He was rapidly running out of options. Back then, the only treatment left was a bone marrow transplant. Without one, his doctors said, he would have 1 or 2 years left to live.
“I was really trying to avoid a bone marrow transplant. You’re playing your last card if that doesn’t work. It’s a pretty rough procedure,” Olson told Medscape Medical News.
Looking back, Olson counts himself as lucky – for being in the right place, at the right time, with the right doctor. His oncologist was David Porter, MD, the principal investigator on a trial at the University of Pennsylvania that was investigating a brand new approach to treating cancer: chimeric antigen receptor (CAR) T-cell therapy.
CAR T-cell therapy uses a patient’s own T cells engineered to express a receptor that targets proteins on cancer cells. CAR T cells are considered “living drugs” because they expand inside the body and stick around for years – maybe for a lifetime – to fight the cancer if it tries to come back.
“I was certainly intrigued by the approach. It had worked in mice, and it was the sort of thing that looked like it would work,” Olson recalled.
Science is not a foreign language to Olson. He holds a PhD in medicinal chemistry, spent most of his career in the in vitro diagnostics industry, and currently acts as chief executive officer of Buhlmann Diagnostics Corp.
So he read the clinical protocol for the first in-human trial of CAR T cells and agreed to become patient number two.
Olson’s T cells were harvested, engineered to attack the CD19 antigen found on malignant and normal B lymphocytes, and then were expanded into millions in the lab. After undergoing preconditioning with chemotherapy to minimize rejection and boost the CAR T cells’ expansion inside the body, he received several infusions of the new therapy over the course of 3 days.
Nothing really happened for 2 weeks. Then he developed severe flu-like symptoms – so bad that he was hospitalized.
Ironically, getting sick was a sign that the CAR T cells were working. Olson was experiencing one of the main short-term effects of CAR T-cell therapy: cytokine release syndrome. Symptoms include extremely high fevers and dangerous drops in blood pressure that can potentially cause end-organ damage.
In the early trials of these products, some patients experienced such a severe reaction that they needed intensive care, and some died. With increasing clinical experience, doctors have learned to control the reaction with the use of steroids and interleukein-6 inhibitors such as tocilizumab (Actemra).
Fortunately for Olson, the reaction passed, and he was eventually discharged.
Then the “aha moment” happened. Four weeks after receiving the CAR T cells, Olson found out that he was cancer free.
“It still gives me shivers,” he said. “Dr Porter said, ‘Your bone marrow’s completely free. We just can’t find a cancer cell anywhere.’ “
The remission has lasted, and it is now 10 years later.
Balancing long-term risks vs benefits
Long-term data have been accumulating for these novel therapies since Olson’s treatment in 2010. This is particularly important for CAR T-cell therapy, because of its longevity. Because these are living cells and are expected to persist in the body for years, there is great interest in longer-term data, especially the risks for toxicity.
The FDA requires clinical follow-up for at least 15 years for patients treated with CAR T-cell therapy or any other genetically modified cells.
So far, most of the experience with CAR T cells comes from anti-CD19-directed therapy, which has shown “remarkable” remission rates in the 50% to 85% range, said Nirali Shah, MD, head of the hematologic malignancies section of the Pediatric Oncology Branch at the National Cancer Institute (NCI).
The most recent results presented at this year’s annual meeting of the American Society of Clinical Oncology support earlier efficacy data, she noted. In the longest follow-up to date, researchers reported remissions lasting over 9 years in patients with relapsed/refractory B-cell lymphoma or CLL treated with Kite›s axicaptagene cilleucel (Yescarta), one of two anti-CD19-directed CAR T-cell therapies approved by the FDA in 2017 (the other is Novartis’ tisagenlecleucel [Kymriah]).
This study included 43 patients and showed an overall remission rate of 76%. Complete remission was achieved in 54% of patients, and 22% had partial remission.
The other focus is long-term safety. Although some of the long-term adverse effects are known and are manageable, others fall into the theoretical realm. In early May 2020, the NCI held a multidisciplinary virtual conference on CAR T-cell therapy «to encourage collaborative research about the subacute and potentially long-term toxicity profile of these treatments.»
“We know just a little at this point about late- and long-term effects of CAR-T therapy, because we are relatively early in the era of CAR T cells,” said Merav Bar, MD, from the Fred Hutchinson Cancer Research Center in Seattle, Washington.
B-cell aplasia and risk for new infections
What is known is that B-cell aplasia represents the most common long-term adverse effect of CAR T-cell therapy. B-cell aplasia results when anti-CD19 CAR-T therapy wipes out healthy B cells as well as the malignant ones responsible for leukemia/lymphoma.
As major players in the immune system, B cells are a key defense against viruses. So B-cell aplasia represents a very specific type of immunosuppression. It is generally less severe than immunosuppression that occurs after organ transplant, which hits the immune system pretty much across the board and carries a much higher risk for infection.
The main concern is what happens when someone with B-cell aplasia encounters a new pathogen, such as SARS-CoV-2.
After infection, B cells generate memory cells, which are not killed off by anti-CD19 therapy and that stick around for life. So a patient such as Olson would still make antibodies that fight infections they experienced before receiving CAR-T therapy, such as childhood chickenpox. But now they are unable to make new memory cells, so these patients receive monthly immunoglobulin infusions to protect against pathogens they have not previously encountered.
Olson takes this in stride and says he isn’t overly worried about COVID-19. He follows the recommended precautions for a man his age. He wears a mask, washes his hands frequently, and tries to maintain social distancing. But he doesn’t stay locked up in his New Hampshire home.
“I took the attitude when I was diagnosed with cancer that I’m going to live my life,” he said. “Quality of life to me is more important than quantity.”
Neuropsychiatric toxicity
Another problem is the possibility of neuropsychiatric toxicity. Past studies have reported a wide range of such toxicities associated with CAR T-cell therapy, including seizures and hallucinations. Most have occurred early in the course of treatment and appear to be short-lived and reversible. However, there remain questions about long-term neuropsychiatric problems.
In a long-term study of 40 patients with relapsed/refractory CLL, non-Hodgkin lymphoma, and ALL, nearly half of patients (47.5%, 19/40) self-reported at least one clinically meaningful negative neuropsychiatric outcome (anxiety, depression, or cognitive difficulty) 1 to 5 years after anti-CD19 CAR T-cell therapy. In addition, 37.5% (15/40) self-reported cognitive difficulties.
“Patients with more severe neurotoxicity showed a trend for more cognitive difficulties afterwards,» said Bar, senior author of the study.
However, teasing out the role that CAR T-cell therapy plays in these problems poses a challenge. All of these patients had been heavily pretreated with previous cancer therapy, which has also been associated with neuropsychiatric problems.
“So far, we don’t know what caused it,” Bar said. “Nevertheless, people need to pay attention to neuropsychiatric symptoms in CAR T-cell therapy. It is important to continue to monitor these patients for these issues.”
Graft-vs-host disease
Another potential problem is graft-vs-host disease (GVHD). This is not uncommon after hematopoietic stem cell transplants. It develops when the donor T cells view antigens on healthy recipient cells as foreign and attack them.
For patients who are treated with CAR T cells, GVHD is mostly a concern among individuals who have previously had a transplant and who are already at increased risk for it.
In a study of late effects among 86 adults treated with anti-CD19 CAR T cells for relapsed/refractory non-Hodgkin lymphoma, Bar and colleagues found that GVHD occurred only among patients who had received a previous donor stem cell transplant. Of these, 20% (3/15) developed GVHD about 28 months after CAR-T therapy.
“The data for CAR T cells causing GVHD really hasn’t shown that it’s a huge problem, although we have seen it and are continuing to monitor for it,” the NCI’s Shah commented to Medscape Medical News.
Other Long-term Adverse Effects
A range of other long-term adverse effects have been reported with CAR-T therapy, including prolonged cytopenias (reduced mature blood cells), myelodysplasia (bone marrow failure), and second malignancies.
In the study with the longest follow-up to date, 16% (7/43) of patients developed second malignancies, which is comparable to data from Bar’s study in Seattle (15%, 13/86). The researchers in this study consider this rate to be no higher than expected: these patients had already received extensive chemotherapy, which increases the risk for other cancers, they point out.
However, this brings up theoretical concerns about the long-term effects of gene modification. CAR T cells are engineered using retroviruses (mainly lentiviruses), which randomly insert the CAR genes into the host genome. Doing so may cause mutations that could promote cancer. These lentiviruses also carry the theoretical risk of becoming capable of viral replication once inside the body.
To address these concerns, viruses used to engineer CAR T cells go through comprehensive safety testing. After therapy, patients are checked every few months during the first year and annually after that.
So far, there have been no reports of cancers associated with CAR T-cell therapy.
“Any type of cancer is a very theoretical risk,” Bar told Medscape Medical News. «Most likely the malignancies in our study were related to prior treatment that the patients received. None of them had any evidence of replication-competent lentivirus, or any other evidence that the malignancies were related to the CAR T cells.»
Another theoretical concern is the possibility of new-onset autoimmune disease, although, once again, no cases have been reported so far.
“We think of it as a theoretic possibility. Whenever you jack up the immune system, autoimmune disease is a potential risk,” said Carl June, MD, director of the Center for Cellular Immunotherapies at the University of Pennsylvania.
June was the co–principal investigator of the trial in which Olson participated. He is also the inventor on patents for CAR T cells licensed by the University of Pennsylvania to Novartis and Tmunity and is a scientific founder with equity in Tmunity.
Still, autoimmunity could occur, and scientists are looking out for it.
“We are continuing to be vigilant in our monitoring for autoimmune disease,” Shah added. “We’ve been doing CAR T-cell therapy since 2012, and I think we have yet to see true autoimmunity beyond GVHD.”
Future directions
In the 10 years since Olson received CAR T-cell therapy, an entire industry has sprung up. Over 100 companies worldwide are now developing CAR T-cell therapies targeting various antigens. These therapies are directed at about 60 different tumor types, including solid tumors. Nearly 200 clinical trials are underway, though most are still in early stages: as of September 2019, only 5% had reached phase 3.
Clinical data show promising results for CAR T-cell therapy directed against CD22 (overexpressed on ALL cells), and BCMA (found on almost all multiple myeloma cells). Yet questions remain as to whether CAR T cells will be as effective if they target antigens other than CD19 or cells other than B lymphocytes. One of the biggest research questions is whether they will be effective against solid tumors.
One research avenue being watched with great interest is the development of universal CAR T cells. So far, such products are at very early stages of development (phase 1 trials), but they are attractive because of the potential advantages they offer over bespoke CAR T cells. Automating the process holds the promise of immediate availability, standardizing production, expanding access, and lowering costs. And because the T cells for this universal product come from healthy donors, they may function better than T cells that have been battered and bruised by past cancer treatments, or even the cancer itself.
However, precisely because they are developed from healthy donor T cells, universal CAR T cells may pose increased risk for GVHD. Scientists are trying to get around this problem by engineering universal CAR T cells that lack the T-cell receptor involved in GVHD.
There are also other concerns. Nature has a penchant for mutation. Engineering CAR T cells without T-cell receptors means the body may no longer detect or reject a universal CAR T cell if it goes rogue. Also, gene insertion in universal CAR-T therapy is targeted rather than random (as in bespoke CAR T cells), which could create off-target effects. Both issues create a theoretical risk of such products inducing an untreatable CAR T-cell therapy–associated cancer.
“The theoretic risk with universal cells is that their safety profile may not be as good for long term,” June commented.
Hope for the future
From that first trial in which June and Porter used CAR T cells, two of three patients they treated are still alive 10 years later.
Olson is one of these two, and he still undergoes monitoring every 3 months to check for relapse. So far, none of his tests have shown signs of his cancer returning.
After going into remission, Doug spent the next 6 to 9 months regaining his health and strength.
“I figured if I had this amazing treatment that saved my life, I had an obligation to stay alive,” he said. “I’d better not die of something like a heart attack!”
He took up long distance running and has completed six half marathons. He became involved in the Leukemia and Lymphoma Society, participating in fund-raising and helping newly diagnosed patients. Over the years, he has also given talks for researchers, people with cancer, and healthcare providers.
Doug is now 73. Today, he marvels at how rapidly the CAR-T field has progressed.
“Twenty years ago, if you had cancer, your prospects weren’t nearly as good as these days. In 2010, people still didn’t believe in CAR T-cell therapy,” he said. “My goal always in telling my story is a message of hope.”
This article first appeared on Medscape.com.
When a patient with cancer hears there isn’t much left that doctors can do, it always stays fresh in the mind.
Doug Olson was first diagnosed with chronic lymphocytic leukemia (CLL) over 20 years ago, in 1996. For several years, his doctors used the watch-and-wait approach. But then his cancer progressed and needed treatment. By 2010, it had mutated so much that it no longer responded to standard therapy.
He was rapidly running out of options. Back then, the only treatment left was a bone marrow transplant. Without one, his doctors said, he would have 1 or 2 years left to live.
“I was really trying to avoid a bone marrow transplant. You’re playing your last card if that doesn’t work. It’s a pretty rough procedure,” Olson told Medscape Medical News.
Looking back, Olson counts himself as lucky – for being in the right place, at the right time, with the right doctor. His oncologist was David Porter, MD, the principal investigator on a trial at the University of Pennsylvania that was investigating a brand new approach to treating cancer: chimeric antigen receptor (CAR) T-cell therapy.
CAR T-cell therapy uses a patient’s own T cells engineered to express a receptor that targets proteins on cancer cells. CAR T cells are considered “living drugs” because they expand inside the body and stick around for years – maybe for a lifetime – to fight the cancer if it tries to come back.
“I was certainly intrigued by the approach. It had worked in mice, and it was the sort of thing that looked like it would work,” Olson recalled.
Science is not a foreign language to Olson. He holds a PhD in medicinal chemistry, spent most of his career in the in vitro diagnostics industry, and currently acts as chief executive officer of Buhlmann Diagnostics Corp.
So he read the clinical protocol for the first in-human trial of CAR T cells and agreed to become patient number two.
Olson’s T cells were harvested, engineered to attack the CD19 antigen found on malignant and normal B lymphocytes, and then were expanded into millions in the lab. After undergoing preconditioning with chemotherapy to minimize rejection and boost the CAR T cells’ expansion inside the body, he received several infusions of the new therapy over the course of 3 days.
Nothing really happened for 2 weeks. Then he developed severe flu-like symptoms – so bad that he was hospitalized.
Ironically, getting sick was a sign that the CAR T cells were working. Olson was experiencing one of the main short-term effects of CAR T-cell therapy: cytokine release syndrome. Symptoms include extremely high fevers and dangerous drops in blood pressure that can potentially cause end-organ damage.
In the early trials of these products, some patients experienced such a severe reaction that they needed intensive care, and some died. With increasing clinical experience, doctors have learned to control the reaction with the use of steroids and interleukein-6 inhibitors such as tocilizumab (Actemra).
Fortunately for Olson, the reaction passed, and he was eventually discharged.
Then the “aha moment” happened. Four weeks after receiving the CAR T cells, Olson found out that he was cancer free.
“It still gives me shivers,” he said. “Dr Porter said, ‘Your bone marrow’s completely free. We just can’t find a cancer cell anywhere.’ “
The remission has lasted, and it is now 10 years later.
Balancing long-term risks vs benefits
Long-term data have been accumulating for these novel therapies since Olson’s treatment in 2010. This is particularly important for CAR T-cell therapy, because of its longevity. Because these are living cells and are expected to persist in the body for years, there is great interest in longer-term data, especially the risks for toxicity.
The FDA requires clinical follow-up for at least 15 years for patients treated with CAR T-cell therapy or any other genetically modified cells.
So far, most of the experience with CAR T cells comes from anti-CD19-directed therapy, which has shown “remarkable” remission rates in the 50% to 85% range, said Nirali Shah, MD, head of the hematologic malignancies section of the Pediatric Oncology Branch at the National Cancer Institute (NCI).
The most recent results presented at this year’s annual meeting of the American Society of Clinical Oncology support earlier efficacy data, she noted. In the longest follow-up to date, researchers reported remissions lasting over 9 years in patients with relapsed/refractory B-cell lymphoma or CLL treated with Kite›s axicaptagene cilleucel (Yescarta), one of two anti-CD19-directed CAR T-cell therapies approved by the FDA in 2017 (the other is Novartis’ tisagenlecleucel [Kymriah]).
This study included 43 patients and showed an overall remission rate of 76%. Complete remission was achieved in 54% of patients, and 22% had partial remission.
The other focus is long-term safety. Although some of the long-term adverse effects are known and are manageable, others fall into the theoretical realm. In early May 2020, the NCI held a multidisciplinary virtual conference on CAR T-cell therapy «to encourage collaborative research about the subacute and potentially long-term toxicity profile of these treatments.»
“We know just a little at this point about late- and long-term effects of CAR-T therapy, because we are relatively early in the era of CAR T cells,” said Merav Bar, MD, from the Fred Hutchinson Cancer Research Center in Seattle, Washington.
B-cell aplasia and risk for new infections
What is known is that B-cell aplasia represents the most common long-term adverse effect of CAR T-cell therapy. B-cell aplasia results when anti-CD19 CAR-T therapy wipes out healthy B cells as well as the malignant ones responsible for leukemia/lymphoma.
As major players in the immune system, B cells are a key defense against viruses. So B-cell aplasia represents a very specific type of immunosuppression. It is generally less severe than immunosuppression that occurs after organ transplant, which hits the immune system pretty much across the board and carries a much higher risk for infection.
The main concern is what happens when someone with B-cell aplasia encounters a new pathogen, such as SARS-CoV-2.
After infection, B cells generate memory cells, which are not killed off by anti-CD19 therapy and that stick around for life. So a patient such as Olson would still make antibodies that fight infections they experienced before receiving CAR-T therapy, such as childhood chickenpox. But now they are unable to make new memory cells, so these patients receive monthly immunoglobulin infusions to protect against pathogens they have not previously encountered.
Olson takes this in stride and says he isn’t overly worried about COVID-19. He follows the recommended precautions for a man his age. He wears a mask, washes his hands frequently, and tries to maintain social distancing. But he doesn’t stay locked up in his New Hampshire home.
“I took the attitude when I was diagnosed with cancer that I’m going to live my life,” he said. “Quality of life to me is more important than quantity.”
Neuropsychiatric toxicity
Another problem is the possibility of neuropsychiatric toxicity. Past studies have reported a wide range of such toxicities associated with CAR T-cell therapy, including seizures and hallucinations. Most have occurred early in the course of treatment and appear to be short-lived and reversible. However, there remain questions about long-term neuropsychiatric problems.
In a long-term study of 40 patients with relapsed/refractory CLL, non-Hodgkin lymphoma, and ALL, nearly half of patients (47.5%, 19/40) self-reported at least one clinically meaningful negative neuropsychiatric outcome (anxiety, depression, or cognitive difficulty) 1 to 5 years after anti-CD19 CAR T-cell therapy. In addition, 37.5% (15/40) self-reported cognitive difficulties.
“Patients with more severe neurotoxicity showed a trend for more cognitive difficulties afterwards,» said Bar, senior author of the study.
However, teasing out the role that CAR T-cell therapy plays in these problems poses a challenge. All of these patients had been heavily pretreated with previous cancer therapy, which has also been associated with neuropsychiatric problems.
“So far, we don’t know what caused it,” Bar said. “Nevertheless, people need to pay attention to neuropsychiatric symptoms in CAR T-cell therapy. It is important to continue to monitor these patients for these issues.”
Graft-vs-host disease
Another potential problem is graft-vs-host disease (GVHD). This is not uncommon after hematopoietic stem cell transplants. It develops when the donor T cells view antigens on healthy recipient cells as foreign and attack them.
For patients who are treated with CAR T cells, GVHD is mostly a concern among individuals who have previously had a transplant and who are already at increased risk for it.
In a study of late effects among 86 adults treated with anti-CD19 CAR T cells for relapsed/refractory non-Hodgkin lymphoma, Bar and colleagues found that GVHD occurred only among patients who had received a previous donor stem cell transplant. Of these, 20% (3/15) developed GVHD about 28 months after CAR-T therapy.
“The data for CAR T cells causing GVHD really hasn’t shown that it’s a huge problem, although we have seen it and are continuing to monitor for it,” the NCI’s Shah commented to Medscape Medical News.
Other Long-term Adverse Effects
A range of other long-term adverse effects have been reported with CAR-T therapy, including prolonged cytopenias (reduced mature blood cells), myelodysplasia (bone marrow failure), and second malignancies.
In the study with the longest follow-up to date, 16% (7/43) of patients developed second malignancies, which is comparable to data from Bar’s study in Seattle (15%, 13/86). The researchers in this study consider this rate to be no higher than expected: these patients had already received extensive chemotherapy, which increases the risk for other cancers, they point out.
However, this brings up theoretical concerns about the long-term effects of gene modification. CAR T cells are engineered using retroviruses (mainly lentiviruses), which randomly insert the CAR genes into the host genome. Doing so may cause mutations that could promote cancer. These lentiviruses also carry the theoretical risk of becoming capable of viral replication once inside the body.
To address these concerns, viruses used to engineer CAR T cells go through comprehensive safety testing. After therapy, patients are checked every few months during the first year and annually after that.
So far, there have been no reports of cancers associated with CAR T-cell therapy.
“Any type of cancer is a very theoretical risk,” Bar told Medscape Medical News. «Most likely the malignancies in our study were related to prior treatment that the patients received. None of them had any evidence of replication-competent lentivirus, or any other evidence that the malignancies were related to the CAR T cells.»
Another theoretical concern is the possibility of new-onset autoimmune disease, although, once again, no cases have been reported so far.
“We think of it as a theoretic possibility. Whenever you jack up the immune system, autoimmune disease is a potential risk,” said Carl June, MD, director of the Center for Cellular Immunotherapies at the University of Pennsylvania.
June was the co–principal investigator of the trial in which Olson participated. He is also the inventor on patents for CAR T cells licensed by the University of Pennsylvania to Novartis and Tmunity and is a scientific founder with equity in Tmunity.
Still, autoimmunity could occur, and scientists are looking out for it.
“We are continuing to be vigilant in our monitoring for autoimmune disease,” Shah added. “We’ve been doing CAR T-cell therapy since 2012, and I think we have yet to see true autoimmunity beyond GVHD.”
Future directions
In the 10 years since Olson received CAR T-cell therapy, an entire industry has sprung up. Over 100 companies worldwide are now developing CAR T-cell therapies targeting various antigens. These therapies are directed at about 60 different tumor types, including solid tumors. Nearly 200 clinical trials are underway, though most are still in early stages: as of September 2019, only 5% had reached phase 3.
Clinical data show promising results for CAR T-cell therapy directed against CD22 (overexpressed on ALL cells), and BCMA (found on almost all multiple myeloma cells). Yet questions remain as to whether CAR T cells will be as effective if they target antigens other than CD19 or cells other than B lymphocytes. One of the biggest research questions is whether they will be effective against solid tumors.
One research avenue being watched with great interest is the development of universal CAR T cells. So far, such products are at very early stages of development (phase 1 trials), but they are attractive because of the potential advantages they offer over bespoke CAR T cells. Automating the process holds the promise of immediate availability, standardizing production, expanding access, and lowering costs. And because the T cells for this universal product come from healthy donors, they may function better than T cells that have been battered and bruised by past cancer treatments, or even the cancer itself.
However, precisely because they are developed from healthy donor T cells, universal CAR T cells may pose increased risk for GVHD. Scientists are trying to get around this problem by engineering universal CAR T cells that lack the T-cell receptor involved in GVHD.
There are also other concerns. Nature has a penchant for mutation. Engineering CAR T cells without T-cell receptors means the body may no longer detect or reject a universal CAR T cell if it goes rogue. Also, gene insertion in universal CAR-T therapy is targeted rather than random (as in bespoke CAR T cells), which could create off-target effects. Both issues create a theoretical risk of such products inducing an untreatable CAR T-cell therapy–associated cancer.
“The theoretic risk with universal cells is that their safety profile may not be as good for long term,” June commented.
Hope for the future
From that first trial in which June and Porter used CAR T cells, two of three patients they treated are still alive 10 years later.
Olson is one of these two, and he still undergoes monitoring every 3 months to check for relapse. So far, none of his tests have shown signs of his cancer returning.
After going into remission, Doug spent the next 6 to 9 months regaining his health and strength.
“I figured if I had this amazing treatment that saved my life, I had an obligation to stay alive,” he said. “I’d better not die of something like a heart attack!”
He took up long distance running and has completed six half marathons. He became involved in the Leukemia and Lymphoma Society, participating in fund-raising and helping newly diagnosed patients. Over the years, he has also given talks for researchers, people with cancer, and healthcare providers.
Doug is now 73. Today, he marvels at how rapidly the CAR-T field has progressed.
“Twenty years ago, if you had cancer, your prospects weren’t nearly as good as these days. In 2010, people still didn’t believe in CAR T-cell therapy,” he said. “My goal always in telling my story is a message of hope.”
This article first appeared on Medscape.com.
Psychiatry trainees drive COVID-19 palliative care in New York
As SARS-CoV-2 cases surged in New York this past spring, one hospital system met the growing demand for palliative care in COVID-19 patients in acute care and emergency settings by training and redeploying psychiatry trainees, producing 100 consultations during a crisis period. Developers of this program wrote about their experience in the Journal of Pain and Symptom Management.
Research shows that psychiatrists can play an important, complementary role in palliative care, but not many models have explored this in practice. Over a 45-day period in March and April, New York Presbyterian/Columbia University Irving Medical Center saw an influx of 7,600 COVID-19 patients. Many were critically ill, and palliative care needs skyrocketed. Initial efforts to install a palliative care team at the emergency department and a proactive consultation model in the step-down units failed to meet demand for consults.
COVID-19 patients present unique challenges. Their clinical trajectory is less clear than those with cancer or other illnesses, Daniel Shalev, MD, a fellow in hospice and palliative medicine at Columbia University/New York State Psychiatric Institute, New York, and the study’s first author, said in an interview. “Ethical and systems issues around distribution of scarce resources may inflect patients’ and physicians’ responses,” Dr. Shalev said. “And families may not be able to be at the bedside with patients.”
To rapidly expand the palliative care workforce and meet patient needs, Dr. Shalev and colleagues recruited 16 psychiatry trainees from NYP, Columbia University Irving Medical Center, and Weill Cornell Medicine to work at NYP/Columbia University Irving Medical Center’s section of adult palliative medicine. Senior general psychiatry residents, child and adolescent psychiatry fellows, addiction psychiatry fellows, and postresidency T32 research fellows became part of a psychiatry-palliative care liaison team, offering psychosocial support and care goal strategies to patients and families.
Already well-versed in serious illness communication and psychosocial aspects of medical illness, the residents and fellows received additional training and education about SARS-CoV-2 and goals-of-care conversations. Child and adolescent psychiatry fellows participated in a communication workshop about the virus at Weill Cornell Medicine.
Working closely with the medical center’s palliative care service, the liaison team did consults around the clock at the ED under the supervision of a consultation-liaison (C-L) psychiatrist specializing in primary palliative care skills. The team managed 16 cases a day during the peak of New York’s COVID-19 outbreak, operating on a rotating schedule of one to three shifts weekly. Some shifts took place remotely to reduce exposure to the virus.
“We were fortunate that New York Presbyterian was early and aggressive in ensuring all clinical staff had personal protective equipment” in the treatment of COVID-19 patients, Dr. Shalev said.
The C-L psychiatry coordinator served as a traffic controller of sorts, overseeing daily staffing changes, maintaining a psychiatry–palliative care liaison team–shared patient list, and ensuring follow-up and continuity on patient care. The rotating schedule freed up time for trainees to meet other research and outpatient obligations.
The liaison team held a meeting each morning and accompanied the adult palliative care service on its daily virtual rounds to help streamline case management and care coordination among the various palliative care channels. Modifications in personnel took place as cases started to recede. Overall, the team participated in 100 consultations.
The findings show that there is significant overlap in psychiatry and palliative care skill sets, Dr. Shalev said. “Furthermore, many patients benefiting from palliative care services have mental health needs. But there are gaps between psychiatry and palliative care, including a lack of collaboration and cross-training. Our model showed how easily our disciplines can work together to improve the care available to all patients,” he added.
Some things could have gone more smoothly. Working under the duress of a pandemic, project leaders didn’t have enough time to train and supervise the team about advanced symptom management. Psychiatry staff members also weren’t as comfortable with nonpsychiatric symptom management as serious illness communication and psychiatric symptom management. Dr. Shalev expects these growth areas to improve over time.
The model could easily translate to other facilities, he believes. As of this writing, the liaison team was transitioning to a longer-term assignment involving patients on mechanical ventilation and their families.
The program increased access to care during a time of limited resources,and successfully combined psychiatric and palliative services – two specialties that, at times, can have conflicting recommendations, noted Maria I. Lapid, MD, a professor of psychiatry at the Mayo Clinic in Rochester, Minn., and a faculty member of the Mayo Clinic Center for Palliative Medicine, who was not part of the study. As urgent training for psychiatric trainees proved useful in the current crisis, long-term psychiatric programs will need to explore and consider how to integrate palliative care training into the psychiatric curriculum.
“Not only is this relevant in the current pandemic, but this will continue to be relevant in the context of the rapidly aging population” in the United States, said Dr. Lapid.
Dr. Shalev and colleagues declared no conflicts of interest in their study. Their research received no funds or grants from public, commercial, or nonprofit agencies.
SOURCE: Shalev D et al. J Pain Symptom Manage. 2020 Jun 13. doi.org/10.1016/j.jpainsymman.2020.06.009.
As SARS-CoV-2 cases surged in New York this past spring, one hospital system met the growing demand for palliative care in COVID-19 patients in acute care and emergency settings by training and redeploying psychiatry trainees, producing 100 consultations during a crisis period. Developers of this program wrote about their experience in the Journal of Pain and Symptom Management.
Research shows that psychiatrists can play an important, complementary role in palliative care, but not many models have explored this in practice. Over a 45-day period in March and April, New York Presbyterian/Columbia University Irving Medical Center saw an influx of 7,600 COVID-19 patients. Many were critically ill, and palliative care needs skyrocketed. Initial efforts to install a palliative care team at the emergency department and a proactive consultation model in the step-down units failed to meet demand for consults.
COVID-19 patients present unique challenges. Their clinical trajectory is less clear than those with cancer or other illnesses, Daniel Shalev, MD, a fellow in hospice and palliative medicine at Columbia University/New York State Psychiatric Institute, New York, and the study’s first author, said in an interview. “Ethical and systems issues around distribution of scarce resources may inflect patients’ and physicians’ responses,” Dr. Shalev said. “And families may not be able to be at the bedside with patients.”
To rapidly expand the palliative care workforce and meet patient needs, Dr. Shalev and colleagues recruited 16 psychiatry trainees from NYP, Columbia University Irving Medical Center, and Weill Cornell Medicine to work at NYP/Columbia University Irving Medical Center’s section of adult palliative medicine. Senior general psychiatry residents, child and adolescent psychiatry fellows, addiction psychiatry fellows, and postresidency T32 research fellows became part of a psychiatry-palliative care liaison team, offering psychosocial support and care goal strategies to patients and families.
Already well-versed in serious illness communication and psychosocial aspects of medical illness, the residents and fellows received additional training and education about SARS-CoV-2 and goals-of-care conversations. Child and adolescent psychiatry fellows participated in a communication workshop about the virus at Weill Cornell Medicine.
Working closely with the medical center’s palliative care service, the liaison team did consults around the clock at the ED under the supervision of a consultation-liaison (C-L) psychiatrist specializing in primary palliative care skills. The team managed 16 cases a day during the peak of New York’s COVID-19 outbreak, operating on a rotating schedule of one to three shifts weekly. Some shifts took place remotely to reduce exposure to the virus.
“We were fortunate that New York Presbyterian was early and aggressive in ensuring all clinical staff had personal protective equipment” in the treatment of COVID-19 patients, Dr. Shalev said.
The C-L psychiatry coordinator served as a traffic controller of sorts, overseeing daily staffing changes, maintaining a psychiatry–palliative care liaison team–shared patient list, and ensuring follow-up and continuity on patient care. The rotating schedule freed up time for trainees to meet other research and outpatient obligations.
The liaison team held a meeting each morning and accompanied the adult palliative care service on its daily virtual rounds to help streamline case management and care coordination among the various palliative care channels. Modifications in personnel took place as cases started to recede. Overall, the team participated in 100 consultations.
The findings show that there is significant overlap in psychiatry and palliative care skill sets, Dr. Shalev said. “Furthermore, many patients benefiting from palliative care services have mental health needs. But there are gaps between psychiatry and palliative care, including a lack of collaboration and cross-training. Our model showed how easily our disciplines can work together to improve the care available to all patients,” he added.
Some things could have gone more smoothly. Working under the duress of a pandemic, project leaders didn’t have enough time to train and supervise the team about advanced symptom management. Psychiatry staff members also weren’t as comfortable with nonpsychiatric symptom management as serious illness communication and psychiatric symptom management. Dr. Shalev expects these growth areas to improve over time.
The model could easily translate to other facilities, he believes. As of this writing, the liaison team was transitioning to a longer-term assignment involving patients on mechanical ventilation and their families.
The program increased access to care during a time of limited resources,and successfully combined psychiatric and palliative services – two specialties that, at times, can have conflicting recommendations, noted Maria I. Lapid, MD, a professor of psychiatry at the Mayo Clinic in Rochester, Minn., and a faculty member of the Mayo Clinic Center for Palliative Medicine, who was not part of the study. As urgent training for psychiatric trainees proved useful in the current crisis, long-term psychiatric programs will need to explore and consider how to integrate palliative care training into the psychiatric curriculum.
“Not only is this relevant in the current pandemic, but this will continue to be relevant in the context of the rapidly aging population” in the United States, said Dr. Lapid.
Dr. Shalev and colleagues declared no conflicts of interest in their study. Their research received no funds or grants from public, commercial, or nonprofit agencies.
SOURCE: Shalev D et al. J Pain Symptom Manage. 2020 Jun 13. doi.org/10.1016/j.jpainsymman.2020.06.009.
As SARS-CoV-2 cases surged in New York this past spring, one hospital system met the growing demand for palliative care in COVID-19 patients in acute care and emergency settings by training and redeploying psychiatry trainees, producing 100 consultations during a crisis period. Developers of this program wrote about their experience in the Journal of Pain and Symptom Management.
Research shows that psychiatrists can play an important, complementary role in palliative care, but not many models have explored this in practice. Over a 45-day period in March and April, New York Presbyterian/Columbia University Irving Medical Center saw an influx of 7,600 COVID-19 patients. Many were critically ill, and palliative care needs skyrocketed. Initial efforts to install a palliative care team at the emergency department and a proactive consultation model in the step-down units failed to meet demand for consults.
COVID-19 patients present unique challenges. Their clinical trajectory is less clear than those with cancer or other illnesses, Daniel Shalev, MD, a fellow in hospice and palliative medicine at Columbia University/New York State Psychiatric Institute, New York, and the study’s first author, said in an interview. “Ethical and systems issues around distribution of scarce resources may inflect patients’ and physicians’ responses,” Dr. Shalev said. “And families may not be able to be at the bedside with patients.”
To rapidly expand the palliative care workforce and meet patient needs, Dr. Shalev and colleagues recruited 16 psychiatry trainees from NYP, Columbia University Irving Medical Center, and Weill Cornell Medicine to work at NYP/Columbia University Irving Medical Center’s section of adult palliative medicine. Senior general psychiatry residents, child and adolescent psychiatry fellows, addiction psychiatry fellows, and postresidency T32 research fellows became part of a psychiatry-palliative care liaison team, offering psychosocial support and care goal strategies to patients and families.
Already well-versed in serious illness communication and psychosocial aspects of medical illness, the residents and fellows received additional training and education about SARS-CoV-2 and goals-of-care conversations. Child and adolescent psychiatry fellows participated in a communication workshop about the virus at Weill Cornell Medicine.
Working closely with the medical center’s palliative care service, the liaison team did consults around the clock at the ED under the supervision of a consultation-liaison (C-L) psychiatrist specializing in primary palliative care skills. The team managed 16 cases a day during the peak of New York’s COVID-19 outbreak, operating on a rotating schedule of one to three shifts weekly. Some shifts took place remotely to reduce exposure to the virus.
“We were fortunate that New York Presbyterian was early and aggressive in ensuring all clinical staff had personal protective equipment” in the treatment of COVID-19 patients, Dr. Shalev said.
The C-L psychiatry coordinator served as a traffic controller of sorts, overseeing daily staffing changes, maintaining a psychiatry–palliative care liaison team–shared patient list, and ensuring follow-up and continuity on patient care. The rotating schedule freed up time for trainees to meet other research and outpatient obligations.
The liaison team held a meeting each morning and accompanied the adult palliative care service on its daily virtual rounds to help streamline case management and care coordination among the various palliative care channels. Modifications in personnel took place as cases started to recede. Overall, the team participated in 100 consultations.
The findings show that there is significant overlap in psychiatry and palliative care skill sets, Dr. Shalev said. “Furthermore, many patients benefiting from palliative care services have mental health needs. But there are gaps between psychiatry and palliative care, including a lack of collaboration and cross-training. Our model showed how easily our disciplines can work together to improve the care available to all patients,” he added.
Some things could have gone more smoothly. Working under the duress of a pandemic, project leaders didn’t have enough time to train and supervise the team about advanced symptom management. Psychiatry staff members also weren’t as comfortable with nonpsychiatric symptom management as serious illness communication and psychiatric symptom management. Dr. Shalev expects these growth areas to improve over time.
The model could easily translate to other facilities, he believes. As of this writing, the liaison team was transitioning to a longer-term assignment involving patients on mechanical ventilation and their families.
The program increased access to care during a time of limited resources,and successfully combined psychiatric and palliative services – two specialties that, at times, can have conflicting recommendations, noted Maria I. Lapid, MD, a professor of psychiatry at the Mayo Clinic in Rochester, Minn., and a faculty member of the Mayo Clinic Center for Palliative Medicine, who was not part of the study. As urgent training for psychiatric trainees proved useful in the current crisis, long-term psychiatric programs will need to explore and consider how to integrate palliative care training into the psychiatric curriculum.
“Not only is this relevant in the current pandemic, but this will continue to be relevant in the context of the rapidly aging population” in the United States, said Dr. Lapid.
Dr. Shalev and colleagues declared no conflicts of interest in their study. Their research received no funds or grants from public, commercial, or nonprofit agencies.
SOURCE: Shalev D et al. J Pain Symptom Manage. 2020 Jun 13. doi.org/10.1016/j.jpainsymman.2020.06.009.
Hyperpigmentation of the legs
A 90-year-old man was admitted from the Emergency Department (ED) to our inpatient service for difficulty urinating and hematuria. In the ED, a complete blood count (CBC) with differential and a urinalysis were performed. CBC showed a mild normocytic anemia, consistent with the patient’s known chronic kidney disease. The urinalysis revealed moderate blood, trace ketones, proteinuria, small leukocyte esterases, positive nitrites, and more than 182 red blood cells—findings suspicious for a urinary tract infection. Computed tomography of the abdomen and pelvis was notable for a soft-tissue mass in the bladder.
He had a history of coronary artery disease (treated with stent placement), atrial fibrillation, congestive heart failure, hypothyroidism, gastroesophageal reflux disease, gastrointestinal bleeding, chronic obstructive pulmonary disease, a 60-pack-per-year history of tobacco dependence, chronic kidney disease, prostate cancer, benign prostatic hypertrophy, peripheral vascular disease, and gout. Medications included digoxin, metoprolol, torsemide, aspirin, levothyroxine, fluticasone, albuterol, omeprazole, diclofenac, escitalopram, and minocycline.
About 5 years earlier, doctors had discovered a popliteal thrombosis that required emergent thrombectomy of the infragenicular popliteal artery, thromboembolectomy of the right posterior tibial artery, graft angioplasty of the right posterior tibial artery, and right anterior fasciotomy for compartment syndrome.
Ten months later, an abscess formed at the incision site. His physician irrigated the popliteal wound and prescribed intravenous (IV) vancomycin. However, the patient developed an allergy and IV daptomycin was initiated and followed by chronic antibiotic suppression with oral minocycline 100 mg bid for about 3.5 years. Skin discoloration appeared within a year of starting the minocycline.
During his hospitalization on our service, we noted black pigmentation of both legs (FIGURE). He had intact strength and sensation in his legs, 1+ pitting edema, no pain upon palpation, and 2+ distal pulses. The patient was well appearing and in no acute distress.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Minocycline-induced hyperpigmentation
The patient’s clinical presentation of chronic blue-black hyperpigmentation on the anterior shins of both legs after a prolonged antibiotic course led us to conclude that this was an adverse effect of minocycline. Commonly, doctors use minocycline to treat acne, rosacea, and rheumatoid arthritis. In this case, it was used to provide chronic antimicrobial suppression.
Not an uncommon reaction for a patient like ours. One small study conducted in an orthopedic patient population found that 54% of patients receiving long-term minocycline suppression developed hyperpigmentation after a mean follow-up of nearly 5 years.1 The hyperpigmentation is solely cosmetic and without known clinical complications, but it can be distressing for patients.
There are 3 types of minocycline-induced hyperpigmentation:
- Type I is a circumscribed blue-black pigmentation that manifests in skin that previously was inflamed or scarred, such as facial acne scars.2 Histopathologic findings include black pigment granules in macrophages and throughout the dermis that stain with Perls Prussian blue iron.3
- Type II (which our patient had) is circumscribed blue-black pigmentation that appears in previously normal skin of the forearms or lower legs—especially the shins.3 On histopathology, black pigment granules are found in the dermis with macrophages that stain with Perls Prussian blue iron and Fontana-Masson.3
- Type III is a diffuse muddy brown hyperpigmentation in previously normal, sun-exposed skin.2 Histopathologic findings include increased melanin in basal keratinocytes and dermal melanophages that stain with Fontana-Masson.3
Types II and III may be related to cumulative dosing, whereas type I can occur at any point during treatment.2
Differential includes pigmentation disorders
The differential diagnosis includes Addison disease, argyria, hemochromatosis, and polycythemia vera, which all can cause diffuse blue-gray patches.4 Brown-violet pigmentation on sun-exposed areas, redness, and itching are more typical of Riehl melanosis.4
Continue to: Diltiazem
Diltiazem can produce slate-gray to blue-gray reticulated hyperpigmentation.5 Other drugs that can induce slate-gray macules or patches include amiodarone, chlorpromazine, imipramine, and desipramine.5
Treatment is simple, resolution takes time
The treatment for this condition is cessation of minocycline use. Pigmentation fades slowly and may persist for years. There has been successful treatment of type I and III minocycline-induced hyperpigmentation with the alexandrite 755 nm Q-switched laser combined with fractional photothermolysis.3,6 Unfortunately, insurance coverage is limited because these treatments are cosmetic in nature.
Given that hyperpigmentation is a known adverse effect of minocycline use, it’s important to counsel patients about the possibility prior to initiating treatment. It’s also important to monitor for signs of changing pigmentation to prevent psychological distress.
In this case, a biopsy was deemed unnecessary, as the antibiotic was the most likely cause of the pigmentation. The patient’s outpatient dermatologist recommended changing therapy if a medically appropriate alternative was available. Doxycycline would have been a reasonable alternative; however, the patient died shortly after his presentation to our hospital due to his multiple comorbidities.
CORRESPONDENCE
Bich-May Nguyen, MD, MPH, 14023 Southwest Freeway, Sugar Land, TX 77478; Bich-May.Nguyen@memorialhermann.org
1. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3:ofv107.
2. Mouton RW, Jordaan HF, Schneider JW. A new type of minocycline-induced cutaneous hyperpigmentation. Clin Exp Dermatol. 2004;29:8-14.
3. D’Agostino ML, Risser J, Robinson-Bostom L. Imipramine-induced hyperpigmentation: a case report and review of the literature. J Cutan Pathol. 2009;36:799-803.
4. Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162.
5. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation. Arch Dermatol. 2001;137:179-182.
6. Vangipuram RK, DeLozier WL, Geddes E, et al. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med. 2016;48:234-237.
A 90-year-old man was admitted from the Emergency Department (ED) to our inpatient service for difficulty urinating and hematuria. In the ED, a complete blood count (CBC) with differential and a urinalysis were performed. CBC showed a mild normocytic anemia, consistent with the patient’s known chronic kidney disease. The urinalysis revealed moderate blood, trace ketones, proteinuria, small leukocyte esterases, positive nitrites, and more than 182 red blood cells—findings suspicious for a urinary tract infection. Computed tomography of the abdomen and pelvis was notable for a soft-tissue mass in the bladder.
He had a history of coronary artery disease (treated with stent placement), atrial fibrillation, congestive heart failure, hypothyroidism, gastroesophageal reflux disease, gastrointestinal bleeding, chronic obstructive pulmonary disease, a 60-pack-per-year history of tobacco dependence, chronic kidney disease, prostate cancer, benign prostatic hypertrophy, peripheral vascular disease, and gout. Medications included digoxin, metoprolol, torsemide, aspirin, levothyroxine, fluticasone, albuterol, omeprazole, diclofenac, escitalopram, and minocycline.
About 5 years earlier, doctors had discovered a popliteal thrombosis that required emergent thrombectomy of the infragenicular popliteal artery, thromboembolectomy of the right posterior tibial artery, graft angioplasty of the right posterior tibial artery, and right anterior fasciotomy for compartment syndrome.
Ten months later, an abscess formed at the incision site. His physician irrigated the popliteal wound and prescribed intravenous (IV) vancomycin. However, the patient developed an allergy and IV daptomycin was initiated and followed by chronic antibiotic suppression with oral minocycline 100 mg bid for about 3.5 years. Skin discoloration appeared within a year of starting the minocycline.
During his hospitalization on our service, we noted black pigmentation of both legs (FIGURE). He had intact strength and sensation in his legs, 1+ pitting edema, no pain upon palpation, and 2+ distal pulses. The patient was well appearing and in no acute distress.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Minocycline-induced hyperpigmentation
The patient’s clinical presentation of chronic blue-black hyperpigmentation on the anterior shins of both legs after a prolonged antibiotic course led us to conclude that this was an adverse effect of minocycline. Commonly, doctors use minocycline to treat acne, rosacea, and rheumatoid arthritis. In this case, it was used to provide chronic antimicrobial suppression.
Not an uncommon reaction for a patient like ours. One small study conducted in an orthopedic patient population found that 54% of patients receiving long-term minocycline suppression developed hyperpigmentation after a mean follow-up of nearly 5 years.1 The hyperpigmentation is solely cosmetic and without known clinical complications, but it can be distressing for patients.
There are 3 types of minocycline-induced hyperpigmentation:
- Type I is a circumscribed blue-black pigmentation that manifests in skin that previously was inflamed or scarred, such as facial acne scars.2 Histopathologic findings include black pigment granules in macrophages and throughout the dermis that stain with Perls Prussian blue iron.3
- Type II (which our patient had) is circumscribed blue-black pigmentation that appears in previously normal skin of the forearms or lower legs—especially the shins.3 On histopathology, black pigment granules are found in the dermis with macrophages that stain with Perls Prussian blue iron and Fontana-Masson.3
- Type III is a diffuse muddy brown hyperpigmentation in previously normal, sun-exposed skin.2 Histopathologic findings include increased melanin in basal keratinocytes and dermal melanophages that stain with Fontana-Masson.3
Types II and III may be related to cumulative dosing, whereas type I can occur at any point during treatment.2
Differential includes pigmentation disorders
The differential diagnosis includes Addison disease, argyria, hemochromatosis, and polycythemia vera, which all can cause diffuse blue-gray patches.4 Brown-violet pigmentation on sun-exposed areas, redness, and itching are more typical of Riehl melanosis.4
Continue to: Diltiazem
Diltiazem can produce slate-gray to blue-gray reticulated hyperpigmentation.5 Other drugs that can induce slate-gray macules or patches include amiodarone, chlorpromazine, imipramine, and desipramine.5
Treatment is simple, resolution takes time
The treatment for this condition is cessation of minocycline use. Pigmentation fades slowly and may persist for years. There has been successful treatment of type I and III minocycline-induced hyperpigmentation with the alexandrite 755 nm Q-switched laser combined with fractional photothermolysis.3,6 Unfortunately, insurance coverage is limited because these treatments are cosmetic in nature.
Given that hyperpigmentation is a known adverse effect of minocycline use, it’s important to counsel patients about the possibility prior to initiating treatment. It’s also important to monitor for signs of changing pigmentation to prevent psychological distress.
In this case, a biopsy was deemed unnecessary, as the antibiotic was the most likely cause of the pigmentation. The patient’s outpatient dermatologist recommended changing therapy if a medically appropriate alternative was available. Doxycycline would have been a reasonable alternative; however, the patient died shortly after his presentation to our hospital due to his multiple comorbidities.
CORRESPONDENCE
Bich-May Nguyen, MD, MPH, 14023 Southwest Freeway, Sugar Land, TX 77478; Bich-May.Nguyen@memorialhermann.org
A 90-year-old man was admitted from the Emergency Department (ED) to our inpatient service for difficulty urinating and hematuria. In the ED, a complete blood count (CBC) with differential and a urinalysis were performed. CBC showed a mild normocytic anemia, consistent with the patient’s known chronic kidney disease. The urinalysis revealed moderate blood, trace ketones, proteinuria, small leukocyte esterases, positive nitrites, and more than 182 red blood cells—findings suspicious for a urinary tract infection. Computed tomography of the abdomen and pelvis was notable for a soft-tissue mass in the bladder.
He had a history of coronary artery disease (treated with stent placement), atrial fibrillation, congestive heart failure, hypothyroidism, gastroesophageal reflux disease, gastrointestinal bleeding, chronic obstructive pulmonary disease, a 60-pack-per-year history of tobacco dependence, chronic kidney disease, prostate cancer, benign prostatic hypertrophy, peripheral vascular disease, and gout. Medications included digoxin, metoprolol, torsemide, aspirin, levothyroxine, fluticasone, albuterol, omeprazole, diclofenac, escitalopram, and minocycline.
About 5 years earlier, doctors had discovered a popliteal thrombosis that required emergent thrombectomy of the infragenicular popliteal artery, thromboembolectomy of the right posterior tibial artery, graft angioplasty of the right posterior tibial artery, and right anterior fasciotomy for compartment syndrome.
Ten months later, an abscess formed at the incision site. His physician irrigated the popliteal wound and prescribed intravenous (IV) vancomycin. However, the patient developed an allergy and IV daptomycin was initiated and followed by chronic antibiotic suppression with oral minocycline 100 mg bid for about 3.5 years. Skin discoloration appeared within a year of starting the minocycline.
During his hospitalization on our service, we noted black pigmentation of both legs (FIGURE). He had intact strength and sensation in his legs, 1+ pitting edema, no pain upon palpation, and 2+ distal pulses. The patient was well appearing and in no acute distress.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Minocycline-induced hyperpigmentation
The patient’s clinical presentation of chronic blue-black hyperpigmentation on the anterior shins of both legs after a prolonged antibiotic course led us to conclude that this was an adverse effect of minocycline. Commonly, doctors use minocycline to treat acne, rosacea, and rheumatoid arthritis. In this case, it was used to provide chronic antimicrobial suppression.
Not an uncommon reaction for a patient like ours. One small study conducted in an orthopedic patient population found that 54% of patients receiving long-term minocycline suppression developed hyperpigmentation after a mean follow-up of nearly 5 years.1 The hyperpigmentation is solely cosmetic and without known clinical complications, but it can be distressing for patients.
There are 3 types of minocycline-induced hyperpigmentation:
- Type I is a circumscribed blue-black pigmentation that manifests in skin that previously was inflamed or scarred, such as facial acne scars.2 Histopathologic findings include black pigment granules in macrophages and throughout the dermis that stain with Perls Prussian blue iron.3
- Type II (which our patient had) is circumscribed blue-black pigmentation that appears in previously normal skin of the forearms or lower legs—especially the shins.3 On histopathology, black pigment granules are found in the dermis with macrophages that stain with Perls Prussian blue iron and Fontana-Masson.3
- Type III is a diffuse muddy brown hyperpigmentation in previously normal, sun-exposed skin.2 Histopathologic findings include increased melanin in basal keratinocytes and dermal melanophages that stain with Fontana-Masson.3
Types II and III may be related to cumulative dosing, whereas type I can occur at any point during treatment.2
Differential includes pigmentation disorders
The differential diagnosis includes Addison disease, argyria, hemochromatosis, and polycythemia vera, which all can cause diffuse blue-gray patches.4 Brown-violet pigmentation on sun-exposed areas, redness, and itching are more typical of Riehl melanosis.4
Continue to: Diltiazem
Diltiazem can produce slate-gray to blue-gray reticulated hyperpigmentation.5 Other drugs that can induce slate-gray macules or patches include amiodarone, chlorpromazine, imipramine, and desipramine.5
Treatment is simple, resolution takes time
The treatment for this condition is cessation of minocycline use. Pigmentation fades slowly and may persist for years. There has been successful treatment of type I and III minocycline-induced hyperpigmentation with the alexandrite 755 nm Q-switched laser combined with fractional photothermolysis.3,6 Unfortunately, insurance coverage is limited because these treatments are cosmetic in nature.
Given that hyperpigmentation is a known adverse effect of minocycline use, it’s important to counsel patients about the possibility prior to initiating treatment. It’s also important to monitor for signs of changing pigmentation to prevent psychological distress.
In this case, a biopsy was deemed unnecessary, as the antibiotic was the most likely cause of the pigmentation. The patient’s outpatient dermatologist recommended changing therapy if a medically appropriate alternative was available. Doxycycline would have been a reasonable alternative; however, the patient died shortly after his presentation to our hospital due to his multiple comorbidities.
CORRESPONDENCE
Bich-May Nguyen, MD, MPH, 14023 Southwest Freeway, Sugar Land, TX 77478; Bich-May.Nguyen@memorialhermann.org
1. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3:ofv107.
2. Mouton RW, Jordaan HF, Schneider JW. A new type of minocycline-induced cutaneous hyperpigmentation. Clin Exp Dermatol. 2004;29:8-14.
3. D’Agostino ML, Risser J, Robinson-Bostom L. Imipramine-induced hyperpigmentation: a case report and review of the literature. J Cutan Pathol. 2009;36:799-803.
4. Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162.
5. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation. Arch Dermatol. 2001;137:179-182.
6. Vangipuram RK, DeLozier WL, Geddes E, et al. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med. 2016;48:234-237.
1. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3:ofv107.
2. Mouton RW, Jordaan HF, Schneider JW. A new type of minocycline-induced cutaneous hyperpigmentation. Clin Exp Dermatol. 2004;29:8-14.
3. D’Agostino ML, Risser J, Robinson-Bostom L. Imipramine-induced hyperpigmentation: a case report and review of the literature. J Cutan Pathol. 2009;36:799-803.
4. Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162.
5. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation. Arch Dermatol. 2001;137:179-182.
6. Vangipuram RK, DeLozier WL, Geddes E, et al. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med. 2016;48:234-237.
CHEST 2020: Premier education from the convenience of your home
After careful consideration, CHEST has decided to cancel the live, in-person CHEST Annual Meeting in Chicago, Illinois, this October and replace it with a 100% virtual event. The COVID-19 pandemic has provided the opportunity to look at different approaches for delivering education, and over the past several months, CHEST has done just that.
Due to the pandemic, we moved the CHEST Congress 2020, originally scheduled to take place in Bologna, Italy, to June 2021. On June 30, in partnership with the Italian Delegation, the CHEST Virtual Congress event took place with over 3,200 people registered, spanning over 100 countries. This event featured a robust program that included an international COVID panel, additional educational sessions, over 300 recorded poster presentations, and live, interactive games that kept attendees engaged throughout the day. There was also a surprise welcome message delivered by Dr. Anthony Fauci, the Director of the National Institute of Allergy and Infectious Diseases. We are excited to use the success of this virtual event as an opportunity to expand our knowledge and expertise, and deliver a fun, memorable CHEST 2020.
This October, CHEST will bring you the premier virtual education event in pulmonary, critical care, and sleep medicine, all from the comfort and safety of your home or institution. This year’s virtual Annual Meeting will include live, interactive education, including panel and case-based discussions, virtual networking opportunities, CHEST GAMES, and the space for you to connect, learn, and recharge with your peers…virtually.
Top faculty from across the field will bring you the latest in clinical developments related to the diagnosis, treatment, and management of pulmonary diseases, critical care complications, and sleep disorders. Nonclinical topics, like cultural diversity and burnout, that feature more prominently than ever in day-to-day practice, will be given equal weight. Sessions like, Being Me: Understanding ‘Otherness’ and Issues of Diversity, will rely on audience interaction to address scenarios involving bias and racism faced by the panel of presenters and members of the audience.
Crucial and quickly evolving information on COVID-19 will be front and center, including complications with COVID-19 recovery, COVID-19 management in complex situations, and additional discussions on updated drug trials, treatment plans, and practice management changes. We will focus on other challenges the pandemic has highlighted, helping educators with sessions such as APCCMPD: Education Lessons During a Pandemic and sharing key reminders to all on the fundamentals of pandemic preparation with When the Theoretical Becomes Real: Lessons from a Pandemic.
It is more important than ever to stay up to date on developments in health and medicine, but CHEST is putting equal weight on ensuring the experience of CHEST 2020 is a respite from the mental and physical exhaustion our community is experiencing during these unprecedented times. As ever, we will ensure you meet your educational needs. But together, we will also focus on supporting you in building resilience and giving you the tools to continue to find joy in medicine, even amidst the chaos of a pandemic. Thank you for your continued trust in CHEST, and we look forward to “seeing” you at CHEST 2020 October 18-21!
After careful consideration, CHEST has decided to cancel the live, in-person CHEST Annual Meeting in Chicago, Illinois, this October and replace it with a 100% virtual event. The COVID-19 pandemic has provided the opportunity to look at different approaches for delivering education, and over the past several months, CHEST has done just that.
Due to the pandemic, we moved the CHEST Congress 2020, originally scheduled to take place in Bologna, Italy, to June 2021. On June 30, in partnership with the Italian Delegation, the CHEST Virtual Congress event took place with over 3,200 people registered, spanning over 100 countries. This event featured a robust program that included an international COVID panel, additional educational sessions, over 300 recorded poster presentations, and live, interactive games that kept attendees engaged throughout the day. There was also a surprise welcome message delivered by Dr. Anthony Fauci, the Director of the National Institute of Allergy and Infectious Diseases. We are excited to use the success of this virtual event as an opportunity to expand our knowledge and expertise, and deliver a fun, memorable CHEST 2020.
This October, CHEST will bring you the premier virtual education event in pulmonary, critical care, and sleep medicine, all from the comfort and safety of your home or institution. This year’s virtual Annual Meeting will include live, interactive education, including panel and case-based discussions, virtual networking opportunities, CHEST GAMES, and the space for you to connect, learn, and recharge with your peers…virtually.
Top faculty from across the field will bring you the latest in clinical developments related to the diagnosis, treatment, and management of pulmonary diseases, critical care complications, and sleep disorders. Nonclinical topics, like cultural diversity and burnout, that feature more prominently than ever in day-to-day practice, will be given equal weight. Sessions like, Being Me: Understanding ‘Otherness’ and Issues of Diversity, will rely on audience interaction to address scenarios involving bias and racism faced by the panel of presenters and members of the audience.
Crucial and quickly evolving information on COVID-19 will be front and center, including complications with COVID-19 recovery, COVID-19 management in complex situations, and additional discussions on updated drug trials, treatment plans, and practice management changes. We will focus on other challenges the pandemic has highlighted, helping educators with sessions such as APCCMPD: Education Lessons During a Pandemic and sharing key reminders to all on the fundamentals of pandemic preparation with When the Theoretical Becomes Real: Lessons from a Pandemic.
It is more important than ever to stay up to date on developments in health and medicine, but CHEST is putting equal weight on ensuring the experience of CHEST 2020 is a respite from the mental and physical exhaustion our community is experiencing during these unprecedented times. As ever, we will ensure you meet your educational needs. But together, we will also focus on supporting you in building resilience and giving you the tools to continue to find joy in medicine, even amidst the chaos of a pandemic. Thank you for your continued trust in CHEST, and we look forward to “seeing” you at CHEST 2020 October 18-21!
After careful consideration, CHEST has decided to cancel the live, in-person CHEST Annual Meeting in Chicago, Illinois, this October and replace it with a 100% virtual event. The COVID-19 pandemic has provided the opportunity to look at different approaches for delivering education, and over the past several months, CHEST has done just that.
Due to the pandemic, we moved the CHEST Congress 2020, originally scheduled to take place in Bologna, Italy, to June 2021. On June 30, in partnership with the Italian Delegation, the CHEST Virtual Congress event took place with over 3,200 people registered, spanning over 100 countries. This event featured a robust program that included an international COVID panel, additional educational sessions, over 300 recorded poster presentations, and live, interactive games that kept attendees engaged throughout the day. There was also a surprise welcome message delivered by Dr. Anthony Fauci, the Director of the National Institute of Allergy and Infectious Diseases. We are excited to use the success of this virtual event as an opportunity to expand our knowledge and expertise, and deliver a fun, memorable CHEST 2020.
This October, CHEST will bring you the premier virtual education event in pulmonary, critical care, and sleep medicine, all from the comfort and safety of your home or institution. This year’s virtual Annual Meeting will include live, interactive education, including panel and case-based discussions, virtual networking opportunities, CHEST GAMES, and the space for you to connect, learn, and recharge with your peers…virtually.
Top faculty from across the field will bring you the latest in clinical developments related to the diagnosis, treatment, and management of pulmonary diseases, critical care complications, and sleep disorders. Nonclinical topics, like cultural diversity and burnout, that feature more prominently than ever in day-to-day practice, will be given equal weight. Sessions like, Being Me: Understanding ‘Otherness’ and Issues of Diversity, will rely on audience interaction to address scenarios involving bias and racism faced by the panel of presenters and members of the audience.
Crucial and quickly evolving information on COVID-19 will be front and center, including complications with COVID-19 recovery, COVID-19 management in complex situations, and additional discussions on updated drug trials, treatment plans, and practice management changes. We will focus on other challenges the pandemic has highlighted, helping educators with sessions such as APCCMPD: Education Lessons During a Pandemic and sharing key reminders to all on the fundamentals of pandemic preparation with When the Theoretical Becomes Real: Lessons from a Pandemic.
It is more important than ever to stay up to date on developments in health and medicine, but CHEST is putting equal weight on ensuring the experience of CHEST 2020 is a respite from the mental and physical exhaustion our community is experiencing during these unprecedented times. As ever, we will ensure you meet your educational needs. But together, we will also focus on supporting you in building resilience and giving you the tools to continue to find joy in medicine, even amidst the chaos of a pandemic. Thank you for your continued trust in CHEST, and we look forward to “seeing” you at CHEST 2020 October 18-21!
USPSTF expands options for cervical cancer screening
ILLUSTRATIVE CASE
A 35-year-old healthy woman without a history of high-grade precancerous cervical lesions, immunodeficiency, or exposure to diethylstilbestrol presents to your office for her routine health visit. During your conversation with her, she shares, “I read on the Internet that I only need to be tested for human papillomavirus, but I’m wondering how I’ll be checked for cervical cancer.” She asks for your opinion about cervical cancer screening methods.
The National Cancer Institute predicts that there will be 13,800 new cases of cervical cancer this year, with an estimated 4290 deaths.3 This type of cancer is primarily caused by high-risk human papillomavirus (hrHPV) infections. Fortunately, high-grade precancerous cervical lesions and cervical cancer can be detected with routine Papanicolaou (Pap) smears, which have led to a substantial decrease in the number of deaths from cervical cancer in the United States—from 2.8 per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015.3 In addition to hrHPV infection, risk factors for cervical cancer include low socioeconomic status, cigarette smoking, marrying before 18 years of age, young age at first coitus, multiple sexual partners, multiple sexual partners of a partner, and multiple childbirths.4
Cervical cancer is associated with numerous negative outcomes, including a decrease in quality of life, decreased libido, poor mental health, infertility, negative body image, and death.5 This is particularly true among women of lower socioeconomic status or whose language differs from that of their primary health care provider.1,5
Given the enormous impact cervical cancer screening has made on the detection and mortality rate of this devastating disease,4,5 it is crucial to identify the types of screening tests and screening intervals that lead to the greatest benefit and least harm for all patient populations. The US Preventive Services Task Force (USPSTF) previously addressed this issue in 2012, concluding that cytology alone every 3 years for women ages 21 to 65 years and cytology alone every 3 years or co-testing with cytology and hrHPV every 5 years in women ages 30 to 65 years was of substantial benefit (strength of recommendation [SOR]: A).6
STUDY SUMMARY
Another option for some women: hrHPV testing alone every 5 years
In this 2018 systematic review and modeling study by the USPSTF, randomized controlled trials (RCTs) and cohort studies that compared cytology to hrHPV testing alone or co-testing (cytology with hrHPV) were used to determine the optimal frequency of, and age group for, cervical cancer screening that would yield the least harm and the most benefit from each of these screening methods.7-9
Similar to the previous recommendation, the USPSTF found that screening women < 21 years or > 65 years if previously adequately screened (defined as 3 consecutive negative screenings or 2 negative screenings within the past 10 years with the most recent being within the past 5 years) led to more harm than benefit. They therefore concluded that women in these age groups should not be screened routinely (SOR: D). The USPSTF also recommends against cervical cancer screening in women who have had a hysterectomy with removal of the cervix and who do not have a history of a high-grade precancerous lesion or cervical cancer (SOR: D).
However, for women ages 21 to 65 years, the USPSTF found that screening substantially reduces cervical cancer incidence and mortality, and that for women ages 21 to 29 years, screening every 3 years with cytology alone offers the best balance of benefits and harms (SOR: A). For women ages 30 to 65 years, the USPSTF recommends screening every 3 years with cytology alone or every 5 years with either primary hrHPV testing or co-testing (hrHPV with cytology) (SOR: A). The recommendations apply to all asymptomatic women with a cervix; exceptions include those with a history of a high-grade precancerous cervical lesion or cancer, in utero exposure to diethylstilbestrol, or a compromised immune system.
Continue to: The change
The change in this current set of recommendations by the USPSTF is the inclusion of screening with hrHPV alone every 5 years as an additional cervical cancer screening option for women ages 30 to 65 years. The decision to include this option was based largely on a decision analysis model commissioned by the USPSTF and reviewed along with clinical trials and cohort studies. The modeling studies found that both primary hrHPV testing alone and co-testing every 5 years prevented a similar number of cervical cancer cases and required a similar number of colposcopies.
Finally, the USPSTF emphasized that screening alone is not sufficient for the prevention of cervical cancer and that efforts should be made to create equitable access to follow-up of abnormal results and the provision of appropriate treatment.1,2
WHAT’S NEW
When it comes to cervical cancer screening, 3 solid options now exist
The previous USPSTF recommendation concluded that women ages 30 to 65 years should be screened with either cytology alone every 3 years or co-testing (cytology and hrHPV) every 5 years. This systematic review and modeling study concluded that any one of the stated screening methods would be adequately sensitive for detecting precancerous high-grade cervical lesions or cervical cancer: cytology every 3 years, primary hrHPV every 5 years, or co-testing every 5 years.7-9
CAVEATS
No studies comparing hrHPVto co-testing and no meta-analysis
No studies were found that directly compared primary hrHPV testing with co-testing.1 A meta-analysis could not be performed due to the methodological differences in RCTs and cohort studies reviewed. The new recommendation is unique in its reliance on modeling to simulate a direct comparison of these 2 screening methods.
CHALLENGES TO IMPLEMENTATION
Getting the word out and increasing comfort levels
The principal challenge to implementation lies in practitioners’ knowledge of this new recommendation and a possible low comfort level with ordering hrHPV testing alone. Patients will need to be engaged in shared decision-making to understand and make use of the 3 options.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686.
2. Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: a systematic evidence review for the US Preventive Services Task Force. Evidence Synthesis No. 158. Rockville, MD: Agency for Healthcare Research and Quality; 2018.
3. National Cancer Institute. Cancer Stat Facts. Cervix uteri. https://seer.cancer.gov/statfacts/. Accessed July 1, 2020.
4. Momenimovahed Z, Salehiniya H. Incidence, mortality and risk factors of cervical cancer in the world. Biomed Res Ther. 2017;4:1795-1811.
5. Ashing-Giwa KT, Kagawa-Singer M, Padilla GV, et al. The impact of cervical cancer and dysplasia: a qualitative, multiethnic study. Psychooncology. 2004;13:709-728.
6. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 156:880-891.
7. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol. 2010;11:249-257.
8. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492-501.
9. Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer. 2010;10:111.
ILLUSTRATIVE CASE
A 35-year-old healthy woman without a history of high-grade precancerous cervical lesions, immunodeficiency, or exposure to diethylstilbestrol presents to your office for her routine health visit. During your conversation with her, she shares, “I read on the Internet that I only need to be tested for human papillomavirus, but I’m wondering how I’ll be checked for cervical cancer.” She asks for your opinion about cervical cancer screening methods.
The National Cancer Institute predicts that there will be 13,800 new cases of cervical cancer this year, with an estimated 4290 deaths.3 This type of cancer is primarily caused by high-risk human papillomavirus (hrHPV) infections. Fortunately, high-grade precancerous cervical lesions and cervical cancer can be detected with routine Papanicolaou (Pap) smears, which have led to a substantial decrease in the number of deaths from cervical cancer in the United States—from 2.8 per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015.3 In addition to hrHPV infection, risk factors for cervical cancer include low socioeconomic status, cigarette smoking, marrying before 18 years of age, young age at first coitus, multiple sexual partners, multiple sexual partners of a partner, and multiple childbirths.4
Cervical cancer is associated with numerous negative outcomes, including a decrease in quality of life, decreased libido, poor mental health, infertility, negative body image, and death.5 This is particularly true among women of lower socioeconomic status or whose language differs from that of their primary health care provider.1,5
Given the enormous impact cervical cancer screening has made on the detection and mortality rate of this devastating disease,4,5 it is crucial to identify the types of screening tests and screening intervals that lead to the greatest benefit and least harm for all patient populations. The US Preventive Services Task Force (USPSTF) previously addressed this issue in 2012, concluding that cytology alone every 3 years for women ages 21 to 65 years and cytology alone every 3 years or co-testing with cytology and hrHPV every 5 years in women ages 30 to 65 years was of substantial benefit (strength of recommendation [SOR]: A).6
STUDY SUMMARY
Another option for some women: hrHPV testing alone every 5 years
In this 2018 systematic review and modeling study by the USPSTF, randomized controlled trials (RCTs) and cohort studies that compared cytology to hrHPV testing alone or co-testing (cytology with hrHPV) were used to determine the optimal frequency of, and age group for, cervical cancer screening that would yield the least harm and the most benefit from each of these screening methods.7-9
Similar to the previous recommendation, the USPSTF found that screening women < 21 years or > 65 years if previously adequately screened (defined as 3 consecutive negative screenings or 2 negative screenings within the past 10 years with the most recent being within the past 5 years) led to more harm than benefit. They therefore concluded that women in these age groups should not be screened routinely (SOR: D). The USPSTF also recommends against cervical cancer screening in women who have had a hysterectomy with removal of the cervix and who do not have a history of a high-grade precancerous lesion or cervical cancer (SOR: D).
However, for women ages 21 to 65 years, the USPSTF found that screening substantially reduces cervical cancer incidence and mortality, and that for women ages 21 to 29 years, screening every 3 years with cytology alone offers the best balance of benefits and harms (SOR: A). For women ages 30 to 65 years, the USPSTF recommends screening every 3 years with cytology alone or every 5 years with either primary hrHPV testing or co-testing (hrHPV with cytology) (SOR: A). The recommendations apply to all asymptomatic women with a cervix; exceptions include those with a history of a high-grade precancerous cervical lesion or cancer, in utero exposure to diethylstilbestrol, or a compromised immune system.
Continue to: The change
The change in this current set of recommendations by the USPSTF is the inclusion of screening with hrHPV alone every 5 years as an additional cervical cancer screening option for women ages 30 to 65 years. The decision to include this option was based largely on a decision analysis model commissioned by the USPSTF and reviewed along with clinical trials and cohort studies. The modeling studies found that both primary hrHPV testing alone and co-testing every 5 years prevented a similar number of cervical cancer cases and required a similar number of colposcopies.
Finally, the USPSTF emphasized that screening alone is not sufficient for the prevention of cervical cancer and that efforts should be made to create equitable access to follow-up of abnormal results and the provision of appropriate treatment.1,2
WHAT’S NEW
When it comes to cervical cancer screening, 3 solid options now exist
The previous USPSTF recommendation concluded that women ages 30 to 65 years should be screened with either cytology alone every 3 years or co-testing (cytology and hrHPV) every 5 years. This systematic review and modeling study concluded that any one of the stated screening methods would be adequately sensitive for detecting precancerous high-grade cervical lesions or cervical cancer: cytology every 3 years, primary hrHPV every 5 years, or co-testing every 5 years.7-9
CAVEATS
No studies comparing hrHPVto co-testing and no meta-analysis
No studies were found that directly compared primary hrHPV testing with co-testing.1 A meta-analysis could not be performed due to the methodological differences in RCTs and cohort studies reviewed. The new recommendation is unique in its reliance on modeling to simulate a direct comparison of these 2 screening methods.
CHALLENGES TO IMPLEMENTATION
Getting the word out and increasing comfort levels
The principal challenge to implementation lies in practitioners’ knowledge of this new recommendation and a possible low comfort level with ordering hrHPV testing alone. Patients will need to be engaged in shared decision-making to understand and make use of the 3 options.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 35-year-old healthy woman without a history of high-grade precancerous cervical lesions, immunodeficiency, or exposure to diethylstilbestrol presents to your office for her routine health visit. During your conversation with her, she shares, “I read on the Internet that I only need to be tested for human papillomavirus, but I’m wondering how I’ll be checked for cervical cancer.” She asks for your opinion about cervical cancer screening methods.
The National Cancer Institute predicts that there will be 13,800 new cases of cervical cancer this year, with an estimated 4290 deaths.3 This type of cancer is primarily caused by high-risk human papillomavirus (hrHPV) infections. Fortunately, high-grade precancerous cervical lesions and cervical cancer can be detected with routine Papanicolaou (Pap) smears, which have led to a substantial decrease in the number of deaths from cervical cancer in the United States—from 2.8 per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015.3 In addition to hrHPV infection, risk factors for cervical cancer include low socioeconomic status, cigarette smoking, marrying before 18 years of age, young age at first coitus, multiple sexual partners, multiple sexual partners of a partner, and multiple childbirths.4
Cervical cancer is associated with numerous negative outcomes, including a decrease in quality of life, decreased libido, poor mental health, infertility, negative body image, and death.5 This is particularly true among women of lower socioeconomic status or whose language differs from that of their primary health care provider.1,5
Given the enormous impact cervical cancer screening has made on the detection and mortality rate of this devastating disease,4,5 it is crucial to identify the types of screening tests and screening intervals that lead to the greatest benefit and least harm for all patient populations. The US Preventive Services Task Force (USPSTF) previously addressed this issue in 2012, concluding that cytology alone every 3 years for women ages 21 to 65 years and cytology alone every 3 years or co-testing with cytology and hrHPV every 5 years in women ages 30 to 65 years was of substantial benefit (strength of recommendation [SOR]: A).6
STUDY SUMMARY
Another option for some women: hrHPV testing alone every 5 years
In this 2018 systematic review and modeling study by the USPSTF, randomized controlled trials (RCTs) and cohort studies that compared cytology to hrHPV testing alone or co-testing (cytology with hrHPV) were used to determine the optimal frequency of, and age group for, cervical cancer screening that would yield the least harm and the most benefit from each of these screening methods.7-9
Similar to the previous recommendation, the USPSTF found that screening women < 21 years or > 65 years if previously adequately screened (defined as 3 consecutive negative screenings or 2 negative screenings within the past 10 years with the most recent being within the past 5 years) led to more harm than benefit. They therefore concluded that women in these age groups should not be screened routinely (SOR: D). The USPSTF also recommends against cervical cancer screening in women who have had a hysterectomy with removal of the cervix and who do not have a history of a high-grade precancerous lesion or cervical cancer (SOR: D).
However, for women ages 21 to 65 years, the USPSTF found that screening substantially reduces cervical cancer incidence and mortality, and that for women ages 21 to 29 years, screening every 3 years with cytology alone offers the best balance of benefits and harms (SOR: A). For women ages 30 to 65 years, the USPSTF recommends screening every 3 years with cytology alone or every 5 years with either primary hrHPV testing or co-testing (hrHPV with cytology) (SOR: A). The recommendations apply to all asymptomatic women with a cervix; exceptions include those with a history of a high-grade precancerous cervical lesion or cancer, in utero exposure to diethylstilbestrol, or a compromised immune system.
Continue to: The change
The change in this current set of recommendations by the USPSTF is the inclusion of screening with hrHPV alone every 5 years as an additional cervical cancer screening option for women ages 30 to 65 years. The decision to include this option was based largely on a decision analysis model commissioned by the USPSTF and reviewed along with clinical trials and cohort studies. The modeling studies found that both primary hrHPV testing alone and co-testing every 5 years prevented a similar number of cervical cancer cases and required a similar number of colposcopies.
Finally, the USPSTF emphasized that screening alone is not sufficient for the prevention of cervical cancer and that efforts should be made to create equitable access to follow-up of abnormal results and the provision of appropriate treatment.1,2
WHAT’S NEW
When it comes to cervical cancer screening, 3 solid options now exist
The previous USPSTF recommendation concluded that women ages 30 to 65 years should be screened with either cytology alone every 3 years or co-testing (cytology and hrHPV) every 5 years. This systematic review and modeling study concluded that any one of the stated screening methods would be adequately sensitive for detecting precancerous high-grade cervical lesions or cervical cancer: cytology every 3 years, primary hrHPV every 5 years, or co-testing every 5 years.7-9
CAVEATS
No studies comparing hrHPVto co-testing and no meta-analysis
No studies were found that directly compared primary hrHPV testing with co-testing.1 A meta-analysis could not be performed due to the methodological differences in RCTs and cohort studies reviewed. The new recommendation is unique in its reliance on modeling to simulate a direct comparison of these 2 screening methods.
CHALLENGES TO IMPLEMENTATION
Getting the word out and increasing comfort levels
The principal challenge to implementation lies in practitioners’ knowledge of this new recommendation and a possible low comfort level with ordering hrHPV testing alone. Patients will need to be engaged in shared decision-making to understand and make use of the 3 options.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686.
2. Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: a systematic evidence review for the US Preventive Services Task Force. Evidence Synthesis No. 158. Rockville, MD: Agency for Healthcare Research and Quality; 2018.
3. National Cancer Institute. Cancer Stat Facts. Cervix uteri. https://seer.cancer.gov/statfacts/. Accessed July 1, 2020.
4. Momenimovahed Z, Salehiniya H. Incidence, mortality and risk factors of cervical cancer in the world. Biomed Res Ther. 2017;4:1795-1811.
5. Ashing-Giwa KT, Kagawa-Singer M, Padilla GV, et al. The impact of cervical cancer and dysplasia: a qualitative, multiethnic study. Psychooncology. 2004;13:709-728.
6. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 156:880-891.
7. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol. 2010;11:249-257.
8. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492-501.
9. Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer. 2010;10:111.
1. Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686.
2. Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: a systematic evidence review for the US Preventive Services Task Force. Evidence Synthesis No. 158. Rockville, MD: Agency for Healthcare Research and Quality; 2018.
3. National Cancer Institute. Cancer Stat Facts. Cervix uteri. https://seer.cancer.gov/statfacts/. Accessed July 1, 2020.
4. Momenimovahed Z, Salehiniya H. Incidence, mortality and risk factors of cervical cancer in the world. Biomed Res Ther. 2017;4:1795-1811.
5. Ashing-Giwa KT, Kagawa-Singer M, Padilla GV, et al. The impact of cervical cancer and dysplasia: a qualitative, multiethnic study. Psychooncology. 2004;13:709-728.
6. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 156:880-891.
7. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol. 2010;11:249-257.
8. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492-501.
9. Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer. 2010;10:111.
PRACTICE CHANGER
Offer women ages 30 to 65 years the option of being screened for cervical cancer using a high-risk human papillomavirus assay every 5 years.1,2
STRENGTH OF RECOMMENDATION
A: Based on a US Preventive Services Task Force recommendation statement.
Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686.
Nine states have no board-certified pediatric dermatologist, analysis reveals
In fact, nine states do not have a single pediatric dermatologist.
The findings come from a cross-sectional analysis of national data presented by Sepideh Ashrafzadeh at the virtual annual meeting of the Society for Pediatric Dermatology.
“Nearly 82% of pediatricians report that their patients have difficulty accessing pediatric dermatologists [and] over 25% of pediatric dermatologists have a wait time of greater than 10 weeks for new patient appointments,” Ms. Ashrafzadeh, a student at Harvard Medical School, Boston, and associates wrote in their poster abstract. “While the shortage of pediatric dermatologists is well documented, little is known about the distribution of pediatric dermatologists across the U.S., which in turn affects families’ travel time and access to pediatric dermatologists. Defining the specific regions with greatest need for pediatric dermatology can help shape recruitment efforts and initiatives to increase access to pediatric dermatologists in areas with the greatest need.”
For the current study, the researchers drew from the SPD Directory in March 2020 to identify all U.S. board-certified pediatric dermatologists. They used the 2020 American Board of Pediatrics Directory and the 2020 Centers for Medicaid & Medicare Physician Compare Database to identify pediatric generalists, which were defined as pediatricians and family medicine physicians. They used the 2018 American Community Survey, published by the U.S. Census Bureau, to obtain the number of children ages 0-17 years in each county and state.
Next, Ms. Ashrafzadeh and colleagues tabulated the number of children, pediatric dermatologists, and pediatric generalists in each county and state, and calculated ratios of pediatric dermatologists and generalists to number of children. The Gini index, a standardized scale where 0 signifies equal distribution and 1 signifies complete maldistribution, was calculated for pediatric dermatologists and generalists relative to the population of children at the state level.
Of the 317 pediatric dermatologists included in the analysis, 243 (77%) were female, 194 (61%) worked in an academic center, and 311 (98%) worked in a metropolitan county. A pediatric dermatologist was present in 41 of 50 states (82%) and in 142 of 3,228 counties (4%). There was not a single pediatric dermatologist in 73 out of 158 counties (46%) with over 100,000 children, 19 out of 66 counties (29%) with over 200,000 children, and 4 out of 13 counties (31%) with over 500,000 children. Nine states had no pediatric dermatologists: Delaware, Idaho, Maine, Mississippi, Montana, Nevada, North Dakota, South Dakota, and Wyoming. States with the greatest density of pediatric dermatologists (range, 10.1-15.2 pediatric dermatologists per 1,000,000 children) were Wisconsin, Massachusetts, Rhode Island, and New Hampshire. The Gini index for the distribution of pediatric dermatologists relative to the population of children was 0.488, compared with 0.132 for that of pediatric generalists.
“To address the unmet pediatric dermatology need, educators and policymakers can create initiatives to recruit pediatric dermatologists and expand access to telehealth pediatric dermatology services in these high priority states and counties,” the researchers wrote in their abstract. “Future studies need to be done quantifying travel distances to pediatric dermatologists across the US as travel distances can further identify areas that are in great need of pediatric dermatologists.”
They acknowledged certain limitations of the study, including the fact that they may have missed board-certified pediatric dermatologists who are not listed in the SPD Directory. Ms. Ashrafzadeh and colleagues reported having no financial disclosures.
In fact, nine states do not have a single pediatric dermatologist.
The findings come from a cross-sectional analysis of national data presented by Sepideh Ashrafzadeh at the virtual annual meeting of the Society for Pediatric Dermatology.
“Nearly 82% of pediatricians report that their patients have difficulty accessing pediatric dermatologists [and] over 25% of pediatric dermatologists have a wait time of greater than 10 weeks for new patient appointments,” Ms. Ashrafzadeh, a student at Harvard Medical School, Boston, and associates wrote in their poster abstract. “While the shortage of pediatric dermatologists is well documented, little is known about the distribution of pediatric dermatologists across the U.S., which in turn affects families’ travel time and access to pediatric dermatologists. Defining the specific regions with greatest need for pediatric dermatology can help shape recruitment efforts and initiatives to increase access to pediatric dermatologists in areas with the greatest need.”
For the current study, the researchers drew from the SPD Directory in March 2020 to identify all U.S. board-certified pediatric dermatologists. They used the 2020 American Board of Pediatrics Directory and the 2020 Centers for Medicaid & Medicare Physician Compare Database to identify pediatric generalists, which were defined as pediatricians and family medicine physicians. They used the 2018 American Community Survey, published by the U.S. Census Bureau, to obtain the number of children ages 0-17 years in each county and state.
Next, Ms. Ashrafzadeh and colleagues tabulated the number of children, pediatric dermatologists, and pediatric generalists in each county and state, and calculated ratios of pediatric dermatologists and generalists to number of children. The Gini index, a standardized scale where 0 signifies equal distribution and 1 signifies complete maldistribution, was calculated for pediatric dermatologists and generalists relative to the population of children at the state level.
Of the 317 pediatric dermatologists included in the analysis, 243 (77%) were female, 194 (61%) worked in an academic center, and 311 (98%) worked in a metropolitan county. A pediatric dermatologist was present in 41 of 50 states (82%) and in 142 of 3,228 counties (4%). There was not a single pediatric dermatologist in 73 out of 158 counties (46%) with over 100,000 children, 19 out of 66 counties (29%) with over 200,000 children, and 4 out of 13 counties (31%) with over 500,000 children. Nine states had no pediatric dermatologists: Delaware, Idaho, Maine, Mississippi, Montana, Nevada, North Dakota, South Dakota, and Wyoming. States with the greatest density of pediatric dermatologists (range, 10.1-15.2 pediatric dermatologists per 1,000,000 children) were Wisconsin, Massachusetts, Rhode Island, and New Hampshire. The Gini index for the distribution of pediatric dermatologists relative to the population of children was 0.488, compared with 0.132 for that of pediatric generalists.
“To address the unmet pediatric dermatology need, educators and policymakers can create initiatives to recruit pediatric dermatologists and expand access to telehealth pediatric dermatology services in these high priority states and counties,” the researchers wrote in their abstract. “Future studies need to be done quantifying travel distances to pediatric dermatologists across the US as travel distances can further identify areas that are in great need of pediatric dermatologists.”
They acknowledged certain limitations of the study, including the fact that they may have missed board-certified pediatric dermatologists who are not listed in the SPD Directory. Ms. Ashrafzadeh and colleagues reported having no financial disclosures.
In fact, nine states do not have a single pediatric dermatologist.
The findings come from a cross-sectional analysis of national data presented by Sepideh Ashrafzadeh at the virtual annual meeting of the Society for Pediatric Dermatology.
“Nearly 82% of pediatricians report that their patients have difficulty accessing pediatric dermatologists [and] over 25% of pediatric dermatologists have a wait time of greater than 10 weeks for new patient appointments,” Ms. Ashrafzadeh, a student at Harvard Medical School, Boston, and associates wrote in their poster abstract. “While the shortage of pediatric dermatologists is well documented, little is known about the distribution of pediatric dermatologists across the U.S., which in turn affects families’ travel time and access to pediatric dermatologists. Defining the specific regions with greatest need for pediatric dermatology can help shape recruitment efforts and initiatives to increase access to pediatric dermatologists in areas with the greatest need.”
For the current study, the researchers drew from the SPD Directory in March 2020 to identify all U.S. board-certified pediatric dermatologists. They used the 2020 American Board of Pediatrics Directory and the 2020 Centers for Medicaid & Medicare Physician Compare Database to identify pediatric generalists, which were defined as pediatricians and family medicine physicians. They used the 2018 American Community Survey, published by the U.S. Census Bureau, to obtain the number of children ages 0-17 years in each county and state.
Next, Ms. Ashrafzadeh and colleagues tabulated the number of children, pediatric dermatologists, and pediatric generalists in each county and state, and calculated ratios of pediatric dermatologists and generalists to number of children. The Gini index, a standardized scale where 0 signifies equal distribution and 1 signifies complete maldistribution, was calculated for pediatric dermatologists and generalists relative to the population of children at the state level.
Of the 317 pediatric dermatologists included in the analysis, 243 (77%) were female, 194 (61%) worked in an academic center, and 311 (98%) worked in a metropolitan county. A pediatric dermatologist was present in 41 of 50 states (82%) and in 142 of 3,228 counties (4%). There was not a single pediatric dermatologist in 73 out of 158 counties (46%) with over 100,000 children, 19 out of 66 counties (29%) with over 200,000 children, and 4 out of 13 counties (31%) with over 500,000 children. Nine states had no pediatric dermatologists: Delaware, Idaho, Maine, Mississippi, Montana, Nevada, North Dakota, South Dakota, and Wyoming. States with the greatest density of pediatric dermatologists (range, 10.1-15.2 pediatric dermatologists per 1,000,000 children) were Wisconsin, Massachusetts, Rhode Island, and New Hampshire. The Gini index for the distribution of pediatric dermatologists relative to the population of children was 0.488, compared with 0.132 for that of pediatric generalists.
“To address the unmet pediatric dermatology need, educators and policymakers can create initiatives to recruit pediatric dermatologists and expand access to telehealth pediatric dermatology services in these high priority states and counties,” the researchers wrote in their abstract. “Future studies need to be done quantifying travel distances to pediatric dermatologists across the US as travel distances can further identify areas that are in great need of pediatric dermatologists.”
They acknowledged certain limitations of the study, including the fact that they may have missed board-certified pediatric dermatologists who are not listed in the SPD Directory. Ms. Ashrafzadeh and colleagues reported having no financial disclosures.
FROM SPD 2020
Quitting smoking after MI has huge benefits in young adults
Young adult smokers who stop smoking in the first year after an initial myocardial infarction are far less likely to die over the next 10 years than their peers who continue to smoke. Yet nearly two-thirds keep smoking after the event, according to new data from the Partners YOUNG-MI Registry.
“Smoking is one of the most common risk factors for developing an MI at a young age. ... This reinforces the need to have more young individuals avoid, or quit, the use of tobacco,” Ron Blankstein, MD, Brigham and Women’s Hospital and Harvard Medical School, Boston, said in an interview.
Yet, the finding that 62% of young adults continue to smoke 1 year after MI points to an “enormous need for better smoking cessation efforts following a heart attack,” he said.
“Powerful” message for clinicians
“This study joins an incredibly powerful body of evidence that says if you quit smoking, you’re going to live longer,” said Michael Fiore, MD, MPH, MBA, director of the University of Wisconsin Center for Tobacco Research and Intervention, Madison, who wasn’t involved in the study.
“As physicians, there is nothing we can do that will have a greater impact for our patients than quitting smoking. The study is a powerful call for clinicians to intervene with their patients that smoke – both if you have an MI or if you don’t,” Dr. Fiore told this news organization.
The study involved 2,072 individuals 50 years or younger (median age, 45 years; 81% male) who were hospitalized for an initial MI at two large academic medical centers in Boston. Of these, 33.9% were never-smokers, 13.6% were former smokers, and 52.5% were smokers at the time of their MI.
During a median follow-up of 10.2 years, those who quit smoking had a significantly lower rate of death from any cause (unadjusted hazard ratio, 0.35; 95% confidence interval, 0.19-0.63; P < .001) and a cardiovascular cause (HR, 0.29; 95% CI, 0.11-0.79; P = .02), relative to those who continued to smoke.
The results remained statistically significant in a propensity-matched analysis for both all-cause (HR, 0.30; 95% CI, 0.16-0.56; P < .001) and CV mortality (HR, 0.19; 95% CI, 0.06-0.56; P = .003).
“Although patients who quit smoking were similar to those who continued to smoke with respect to their baseline characteristics, smoking cessation was associated with an approximate 70%-80% reduction in all-cause and CV mortality,” the authors note in their article, published online July 8 in JAMA Network Open.
They say it’s also noteworthy that long-term death rates of never-smokers and former smokers who quit before the MI were nearly identical.
‘A failure of our health care system’
The bottom line, said Dr. Blankstein, is that it is “never too late to quit, and those who experience an MI should do so right away. Our health care system must help promote such efforts, as there is immense room for improvement.”
Dr. Fiore said: “When I see an article like this, it just reminds me that, if you’re really thinking about staying healthy, there is nothing better you can do to improve the quality and longevity of your life than quitting smoking.”
The observation that many patients continue to smoke after MI is a “failure of our health care system, and it’s an individual failure in that these individuals are not able to overcome their powerful nicotine dependence. It’s an unfortunate occurrence that’s resulting in unnecessary deaths,” said Dr. Fiore.
There is no “magic bullet” to overcome nicotine addiction, but there are approved treatments that can “substantially boost quit rates,” he noted.
The two most effective smoking-cessation treatments are varenicline (Chantix) and combination nicotine replacement therapy, a patch combined ideally with nicotine mini lozenges, particularly when combined with some brief counseling, said Fiore.
He encourages cardiologists to get their patients to commit to quitting and then link them to resources such as 1-800-QUIT-NOW or SmokeFree.gov.
Funding for the study was provided by grants from the National Heart, Lung, and Blood Institute. Dr. Blankstein reported receiving research support from Amgen and Astellas. Dr. Fiore had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Young adult smokers who stop smoking in the first year after an initial myocardial infarction are far less likely to die over the next 10 years than their peers who continue to smoke. Yet nearly two-thirds keep smoking after the event, according to new data from the Partners YOUNG-MI Registry.
“Smoking is one of the most common risk factors for developing an MI at a young age. ... This reinforces the need to have more young individuals avoid, or quit, the use of tobacco,” Ron Blankstein, MD, Brigham and Women’s Hospital and Harvard Medical School, Boston, said in an interview.
Yet, the finding that 62% of young adults continue to smoke 1 year after MI points to an “enormous need for better smoking cessation efforts following a heart attack,” he said.
“Powerful” message for clinicians
“This study joins an incredibly powerful body of evidence that says if you quit smoking, you’re going to live longer,” said Michael Fiore, MD, MPH, MBA, director of the University of Wisconsin Center for Tobacco Research and Intervention, Madison, who wasn’t involved in the study.
“As physicians, there is nothing we can do that will have a greater impact for our patients than quitting smoking. The study is a powerful call for clinicians to intervene with their patients that smoke – both if you have an MI or if you don’t,” Dr. Fiore told this news organization.
The study involved 2,072 individuals 50 years or younger (median age, 45 years; 81% male) who were hospitalized for an initial MI at two large academic medical centers in Boston. Of these, 33.9% were never-smokers, 13.6% were former smokers, and 52.5% were smokers at the time of their MI.
During a median follow-up of 10.2 years, those who quit smoking had a significantly lower rate of death from any cause (unadjusted hazard ratio, 0.35; 95% confidence interval, 0.19-0.63; P < .001) and a cardiovascular cause (HR, 0.29; 95% CI, 0.11-0.79; P = .02), relative to those who continued to smoke.
The results remained statistically significant in a propensity-matched analysis for both all-cause (HR, 0.30; 95% CI, 0.16-0.56; P < .001) and CV mortality (HR, 0.19; 95% CI, 0.06-0.56; P = .003).
“Although patients who quit smoking were similar to those who continued to smoke with respect to their baseline characteristics, smoking cessation was associated with an approximate 70%-80% reduction in all-cause and CV mortality,” the authors note in their article, published online July 8 in JAMA Network Open.
They say it’s also noteworthy that long-term death rates of never-smokers and former smokers who quit before the MI were nearly identical.
‘A failure of our health care system’
The bottom line, said Dr. Blankstein, is that it is “never too late to quit, and those who experience an MI should do so right away. Our health care system must help promote such efforts, as there is immense room for improvement.”
Dr. Fiore said: “When I see an article like this, it just reminds me that, if you’re really thinking about staying healthy, there is nothing better you can do to improve the quality and longevity of your life than quitting smoking.”
The observation that many patients continue to smoke after MI is a “failure of our health care system, and it’s an individual failure in that these individuals are not able to overcome their powerful nicotine dependence. It’s an unfortunate occurrence that’s resulting in unnecessary deaths,” said Dr. Fiore.
There is no “magic bullet” to overcome nicotine addiction, but there are approved treatments that can “substantially boost quit rates,” he noted.
The two most effective smoking-cessation treatments are varenicline (Chantix) and combination nicotine replacement therapy, a patch combined ideally with nicotine mini lozenges, particularly when combined with some brief counseling, said Fiore.
He encourages cardiologists to get their patients to commit to quitting and then link them to resources such as 1-800-QUIT-NOW or SmokeFree.gov.
Funding for the study was provided by grants from the National Heart, Lung, and Blood Institute. Dr. Blankstein reported receiving research support from Amgen and Astellas. Dr. Fiore had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Young adult smokers who stop smoking in the first year after an initial myocardial infarction are far less likely to die over the next 10 years than their peers who continue to smoke. Yet nearly two-thirds keep smoking after the event, according to new data from the Partners YOUNG-MI Registry.
“Smoking is one of the most common risk factors for developing an MI at a young age. ... This reinforces the need to have more young individuals avoid, or quit, the use of tobacco,” Ron Blankstein, MD, Brigham and Women’s Hospital and Harvard Medical School, Boston, said in an interview.
Yet, the finding that 62% of young adults continue to smoke 1 year after MI points to an “enormous need for better smoking cessation efforts following a heart attack,” he said.
“Powerful” message for clinicians
“This study joins an incredibly powerful body of evidence that says if you quit smoking, you’re going to live longer,” said Michael Fiore, MD, MPH, MBA, director of the University of Wisconsin Center for Tobacco Research and Intervention, Madison, who wasn’t involved in the study.
“As physicians, there is nothing we can do that will have a greater impact for our patients than quitting smoking. The study is a powerful call for clinicians to intervene with their patients that smoke – both if you have an MI or if you don’t,” Dr. Fiore told this news organization.
The study involved 2,072 individuals 50 years or younger (median age, 45 years; 81% male) who were hospitalized for an initial MI at two large academic medical centers in Boston. Of these, 33.9% were never-smokers, 13.6% were former smokers, and 52.5% were smokers at the time of their MI.
During a median follow-up of 10.2 years, those who quit smoking had a significantly lower rate of death from any cause (unadjusted hazard ratio, 0.35; 95% confidence interval, 0.19-0.63; P < .001) and a cardiovascular cause (HR, 0.29; 95% CI, 0.11-0.79; P = .02), relative to those who continued to smoke.
The results remained statistically significant in a propensity-matched analysis for both all-cause (HR, 0.30; 95% CI, 0.16-0.56; P < .001) and CV mortality (HR, 0.19; 95% CI, 0.06-0.56; P = .003).
“Although patients who quit smoking were similar to those who continued to smoke with respect to their baseline characteristics, smoking cessation was associated with an approximate 70%-80% reduction in all-cause and CV mortality,” the authors note in their article, published online July 8 in JAMA Network Open.
They say it’s also noteworthy that long-term death rates of never-smokers and former smokers who quit before the MI were nearly identical.
‘A failure of our health care system’
The bottom line, said Dr. Blankstein, is that it is “never too late to quit, and those who experience an MI should do so right away. Our health care system must help promote such efforts, as there is immense room for improvement.”
Dr. Fiore said: “When I see an article like this, it just reminds me that, if you’re really thinking about staying healthy, there is nothing better you can do to improve the quality and longevity of your life than quitting smoking.”
The observation that many patients continue to smoke after MI is a “failure of our health care system, and it’s an individual failure in that these individuals are not able to overcome their powerful nicotine dependence. It’s an unfortunate occurrence that’s resulting in unnecessary deaths,” said Dr. Fiore.
There is no “magic bullet” to overcome nicotine addiction, but there are approved treatments that can “substantially boost quit rates,” he noted.
The two most effective smoking-cessation treatments are varenicline (Chantix) and combination nicotine replacement therapy, a patch combined ideally with nicotine mini lozenges, particularly when combined with some brief counseling, said Fiore.
He encourages cardiologists to get their patients to commit to quitting and then link them to resources such as 1-800-QUIT-NOW or SmokeFree.gov.
Funding for the study was provided by grants from the National Heart, Lung, and Blood Institute. Dr. Blankstein reported receiving research support from Amgen and Astellas. Dr. Fiore had no relevant disclosures.
A version of this article originally appeared on Medscape.com.