User login
Some women use prescription opioids during pregnancy
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.
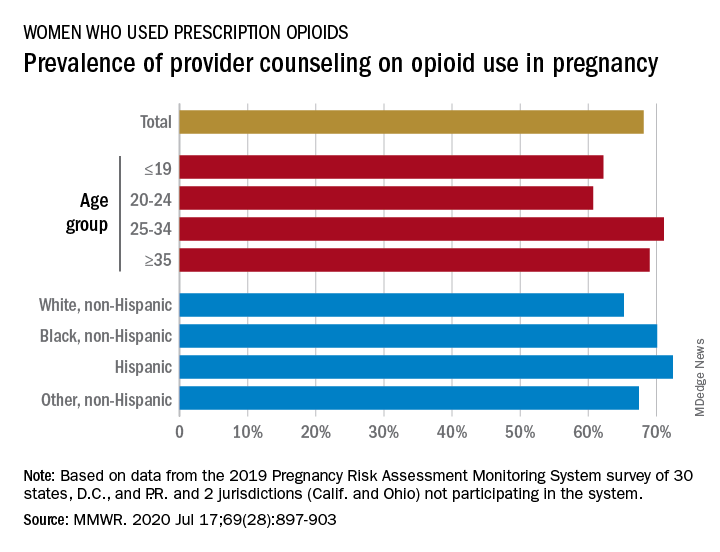
Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.

Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.

Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
FROM MMWR
Limit customized compounded hormones to special circumstances
The use of compounded bioidentical hormone therapies should be limited to patients who are not able to use a hormone therapy product approved by the Food and Drug Administration for reasons of allergy or dosage, according to a new report from the National Academies of Sciences, Engineering, and Medicine.
In recent years, compounded bioidentical hormone therapies (cBHTs) have been “marketed as a personalized and natural approach to enhanced wellness using tailored preparations that address a myriad of symptoms, including those associated with menopause and aging,” wrote Donald R. Mattison, MD, of the University of Ottawa, and chair of the committee charged with producing the report, and colleagues.
Although both cBHTs and bioidentical hormone therapies (BHTs) contain hormones that are structurally and chemically identical to those in the human body, cBHTs have not undergone the safety, efficacy, and quality control tests of approved FDA products, according to the report.
In addition, cBHTs have no standardization when it comes to medication doses, and the products often are available in topicals such as creams or ointments, as well as pills or pellets. The lack of standards in dosing or form can contribute to the risk of overdose, the report emphasized.
Various cBTH products continue to be marketed to the public for age-related hormone symptoms including hot flashes associated with menopause and decreased muscle mass associated with decreased testosterone. However, cBHTs are not approved by the FDA in part because the individually mixed products are not tested to verify the amount of hormone that may be absorbed.
In response to the increased use of cBHTs, the National Academies convened a Committee on the Clinical Utility of Treating Patients with Compounded Bioidentical Hormone Replacement Therapy and commissioned a report.
The two typical reasons to prescribe cBHT are either to provide a medication in an alternate dose not available in approved products or to omit components of a medication to which a patient is allergic, according to the report.
The report includes an algorithm to help guide clinicians in prescribing FDA-approved products, including off-label use of approved products, before cBHT products. “There is a dearth of high-quality evidence ... available to establish whether cBHT preparations are safe or efficacious for their prescribed uses,” the report states.
Of note, the committee also found no guidelines to recommend the use of cBHT products as a substitute for off-label use of FDA-approved BHT products for patients with female sexual dysfunction or gender dysphoria, two conditions for which no FDA-approved BHT products exist.
“The North American Menopause Society applauds the efforts of the National Academies of Sciences, Engineering, and Medicine (NASEM) and endorses their recommendations on compounded bioidentical hormone therapy,” Stephanie S. Faubion, MD, medical director of The North American Menopause Society, wrote in a statement. “As a society, we remain committed to improving the care of midlife women through the promotion of evidence-based research, education, and clinical care.”
A report on the use of cBHTs was important at this time because of the widespread and largely unregulated use of these products with little data to support their safety and efficacy, Dr. Faubion said in an interview.
“There are no indications for use of custom compounded hormone therapy aside from an allergy to a component in the FDA-approved products or lack of availability of the needed dose, which would be exceedingly rare given the variety of forms and doses available with FDA-approved products,” she said.
Main concerns regarding the use of cBHTs are the lack of safety and efficacy data, Dr. Faubion emphasized. “Women believe these products are safer than FDA-approved products because they do not receive a package insert outlining potential risks as they do with FDA-approved products.” A lack of data and safety monitoring of cBHTs means that adverse effects are not monitored and reported, she said. Also, safety concerns persist regarding some forms of cBHTs such as pellets, which were specifically highlighted in the report.
Dr. Faubion said that she “absolutely” agrees with the report’s limited circumstances in which the used of cBHTs would be appropriate. “There are very few reasons why women would need to use compounded hormones instead of the FDA-approved versions, which are regulated for quality, efficacy and safety, readily available in the local pharmacy, and often covered by insurance.”
In terms of the future, “we need more education for women as consumers and for medical providers on this topic,” Dr. Faubion noted. Also, “clearly, there is a dearth of research on the true efficacy and safety of these compounded hormone therapy products.”
The statement from the National Academies crystallizes what experts have been saying for decades, according to Lubna Pal, MBBS, director of the menopause program at Yale University, New Haven, Conn.
The formal recommendations to limit the use of cBHTs “are not novel, but certainly needed,” and the statement “offers guidance regardless of your specialty,” Dr. Pal said in an interview.
There is often a disconnect between consumers’ understanding of compounding and the reality of safety concerns, she said. “We are in a tabloid era,” and education is key to guiding patients toward the FDA-approved treatments with safety data and demonstrated effectiveness, she said. “Safety should be the driving factor.” In compounded products, “there is no consistency that what you get today is the same as what you get tomorrow,” and the lack of standardization of cBHTs increases the risk for adverse events, she emphasized.
For patients with special needs such as allergies or other specialized dosing requirements, as noted in the National Academies statement, clinicians should discuss the options with patients and monitor them regularly to head off potential adverse events such as the development of uterine cancer, said Dr. Pal, who is a member of the Ob.Gyn. News editorial advisory board.
The research involved in creating the report was supported by the Food and Drug Administration.
Dr. Faubion had no financial conflicts to disclose. Dr. Pal had no relevant financial disclosures.
obnews@mdedge.com
SOURCE: Mattison DR et al.; National Academies of Sciences, Engineering, and Medicine. The clinical utility of compounded bioidentical hormone therapy: A review of safety, effectiveness, and use. (Washington, DC: The National Academies Press. 2020.)
The use of compounded bioidentical hormone therapies should be limited to patients who are not able to use a hormone therapy product approved by the Food and Drug Administration for reasons of allergy or dosage, according to a new report from the National Academies of Sciences, Engineering, and Medicine.
In recent years, compounded bioidentical hormone therapies (cBHTs) have been “marketed as a personalized and natural approach to enhanced wellness using tailored preparations that address a myriad of symptoms, including those associated with menopause and aging,” wrote Donald R. Mattison, MD, of the University of Ottawa, and chair of the committee charged with producing the report, and colleagues.
Although both cBHTs and bioidentical hormone therapies (BHTs) contain hormones that are structurally and chemically identical to those in the human body, cBHTs have not undergone the safety, efficacy, and quality control tests of approved FDA products, according to the report.
In addition, cBHTs have no standardization when it comes to medication doses, and the products often are available in topicals such as creams or ointments, as well as pills or pellets. The lack of standards in dosing or form can contribute to the risk of overdose, the report emphasized.
Various cBTH products continue to be marketed to the public for age-related hormone symptoms including hot flashes associated with menopause and decreased muscle mass associated with decreased testosterone. However, cBHTs are not approved by the FDA in part because the individually mixed products are not tested to verify the amount of hormone that may be absorbed.
In response to the increased use of cBHTs, the National Academies convened a Committee on the Clinical Utility of Treating Patients with Compounded Bioidentical Hormone Replacement Therapy and commissioned a report.
The two typical reasons to prescribe cBHT are either to provide a medication in an alternate dose not available in approved products or to omit components of a medication to which a patient is allergic, according to the report.
The report includes an algorithm to help guide clinicians in prescribing FDA-approved products, including off-label use of approved products, before cBHT products. “There is a dearth of high-quality evidence ... available to establish whether cBHT preparations are safe or efficacious for their prescribed uses,” the report states.
Of note, the committee also found no guidelines to recommend the use of cBHT products as a substitute for off-label use of FDA-approved BHT products for patients with female sexual dysfunction or gender dysphoria, two conditions for which no FDA-approved BHT products exist.
“The North American Menopause Society applauds the efforts of the National Academies of Sciences, Engineering, and Medicine (NASEM) and endorses their recommendations on compounded bioidentical hormone therapy,” Stephanie S. Faubion, MD, medical director of The North American Menopause Society, wrote in a statement. “As a society, we remain committed to improving the care of midlife women through the promotion of evidence-based research, education, and clinical care.”
A report on the use of cBHTs was important at this time because of the widespread and largely unregulated use of these products with little data to support their safety and efficacy, Dr. Faubion said in an interview.
“There are no indications for use of custom compounded hormone therapy aside from an allergy to a component in the FDA-approved products or lack of availability of the needed dose, which would be exceedingly rare given the variety of forms and doses available with FDA-approved products,” she said.
Main concerns regarding the use of cBHTs are the lack of safety and efficacy data, Dr. Faubion emphasized. “Women believe these products are safer than FDA-approved products because they do not receive a package insert outlining potential risks as they do with FDA-approved products.” A lack of data and safety monitoring of cBHTs means that adverse effects are not monitored and reported, she said. Also, safety concerns persist regarding some forms of cBHTs such as pellets, which were specifically highlighted in the report.
Dr. Faubion said that she “absolutely” agrees with the report’s limited circumstances in which the used of cBHTs would be appropriate. “There are very few reasons why women would need to use compounded hormones instead of the FDA-approved versions, which are regulated for quality, efficacy and safety, readily available in the local pharmacy, and often covered by insurance.”
In terms of the future, “we need more education for women as consumers and for medical providers on this topic,” Dr. Faubion noted. Also, “clearly, there is a dearth of research on the true efficacy and safety of these compounded hormone therapy products.”
The statement from the National Academies crystallizes what experts have been saying for decades, according to Lubna Pal, MBBS, director of the menopause program at Yale University, New Haven, Conn.
The formal recommendations to limit the use of cBHTs “are not novel, but certainly needed,” and the statement “offers guidance regardless of your specialty,” Dr. Pal said in an interview.
There is often a disconnect between consumers’ understanding of compounding and the reality of safety concerns, she said. “We are in a tabloid era,” and education is key to guiding patients toward the FDA-approved treatments with safety data and demonstrated effectiveness, she said. “Safety should be the driving factor.” In compounded products, “there is no consistency that what you get today is the same as what you get tomorrow,” and the lack of standardization of cBHTs increases the risk for adverse events, she emphasized.
For patients with special needs such as allergies or other specialized dosing requirements, as noted in the National Academies statement, clinicians should discuss the options with patients and monitor them regularly to head off potential adverse events such as the development of uterine cancer, said Dr. Pal, who is a member of the Ob.Gyn. News editorial advisory board.
The research involved in creating the report was supported by the Food and Drug Administration.
Dr. Faubion had no financial conflicts to disclose. Dr. Pal had no relevant financial disclosures.
obnews@mdedge.com
SOURCE: Mattison DR et al.; National Academies of Sciences, Engineering, and Medicine. The clinical utility of compounded bioidentical hormone therapy: A review of safety, effectiveness, and use. (Washington, DC: The National Academies Press. 2020.)
The use of compounded bioidentical hormone therapies should be limited to patients who are not able to use a hormone therapy product approved by the Food and Drug Administration for reasons of allergy or dosage, according to a new report from the National Academies of Sciences, Engineering, and Medicine.
In recent years, compounded bioidentical hormone therapies (cBHTs) have been “marketed as a personalized and natural approach to enhanced wellness using tailored preparations that address a myriad of symptoms, including those associated with menopause and aging,” wrote Donald R. Mattison, MD, of the University of Ottawa, and chair of the committee charged with producing the report, and colleagues.
Although both cBHTs and bioidentical hormone therapies (BHTs) contain hormones that are structurally and chemically identical to those in the human body, cBHTs have not undergone the safety, efficacy, and quality control tests of approved FDA products, according to the report.
In addition, cBHTs have no standardization when it comes to medication doses, and the products often are available in topicals such as creams or ointments, as well as pills or pellets. The lack of standards in dosing or form can contribute to the risk of overdose, the report emphasized.
Various cBTH products continue to be marketed to the public for age-related hormone symptoms including hot flashes associated with menopause and decreased muscle mass associated with decreased testosterone. However, cBHTs are not approved by the FDA in part because the individually mixed products are not tested to verify the amount of hormone that may be absorbed.
In response to the increased use of cBHTs, the National Academies convened a Committee on the Clinical Utility of Treating Patients with Compounded Bioidentical Hormone Replacement Therapy and commissioned a report.
The two typical reasons to prescribe cBHT are either to provide a medication in an alternate dose not available in approved products or to omit components of a medication to which a patient is allergic, according to the report.
The report includes an algorithm to help guide clinicians in prescribing FDA-approved products, including off-label use of approved products, before cBHT products. “There is a dearth of high-quality evidence ... available to establish whether cBHT preparations are safe or efficacious for their prescribed uses,” the report states.
Of note, the committee also found no guidelines to recommend the use of cBHT products as a substitute for off-label use of FDA-approved BHT products for patients with female sexual dysfunction or gender dysphoria, two conditions for which no FDA-approved BHT products exist.
“The North American Menopause Society applauds the efforts of the National Academies of Sciences, Engineering, and Medicine (NASEM) and endorses their recommendations on compounded bioidentical hormone therapy,” Stephanie S. Faubion, MD, medical director of The North American Menopause Society, wrote in a statement. “As a society, we remain committed to improving the care of midlife women through the promotion of evidence-based research, education, and clinical care.”
A report on the use of cBHTs was important at this time because of the widespread and largely unregulated use of these products with little data to support their safety and efficacy, Dr. Faubion said in an interview.
“There are no indications for use of custom compounded hormone therapy aside from an allergy to a component in the FDA-approved products or lack of availability of the needed dose, which would be exceedingly rare given the variety of forms and doses available with FDA-approved products,” she said.
Main concerns regarding the use of cBHTs are the lack of safety and efficacy data, Dr. Faubion emphasized. “Women believe these products are safer than FDA-approved products because they do not receive a package insert outlining potential risks as they do with FDA-approved products.” A lack of data and safety monitoring of cBHTs means that adverse effects are not monitored and reported, she said. Also, safety concerns persist regarding some forms of cBHTs such as pellets, which were specifically highlighted in the report.
Dr. Faubion said that she “absolutely” agrees with the report’s limited circumstances in which the used of cBHTs would be appropriate. “There are very few reasons why women would need to use compounded hormones instead of the FDA-approved versions, which are regulated for quality, efficacy and safety, readily available in the local pharmacy, and often covered by insurance.”
In terms of the future, “we need more education for women as consumers and for medical providers on this topic,” Dr. Faubion noted. Also, “clearly, there is a dearth of research on the true efficacy and safety of these compounded hormone therapy products.”
The statement from the National Academies crystallizes what experts have been saying for decades, according to Lubna Pal, MBBS, director of the menopause program at Yale University, New Haven, Conn.
The formal recommendations to limit the use of cBHTs “are not novel, but certainly needed,” and the statement “offers guidance regardless of your specialty,” Dr. Pal said in an interview.
There is often a disconnect between consumers’ understanding of compounding and the reality of safety concerns, she said. “We are in a tabloid era,” and education is key to guiding patients toward the FDA-approved treatments with safety data and demonstrated effectiveness, she said. “Safety should be the driving factor.” In compounded products, “there is no consistency that what you get today is the same as what you get tomorrow,” and the lack of standardization of cBHTs increases the risk for adverse events, she emphasized.
For patients with special needs such as allergies or other specialized dosing requirements, as noted in the National Academies statement, clinicians should discuss the options with patients and monitor them regularly to head off potential adverse events such as the development of uterine cancer, said Dr. Pal, who is a member of the Ob.Gyn. News editorial advisory board.
The research involved in creating the report was supported by the Food and Drug Administration.
Dr. Faubion had no financial conflicts to disclose. Dr. Pal had no relevant financial disclosures.
obnews@mdedge.com
SOURCE: Mattison DR et al.; National Academies of Sciences, Engineering, and Medicine. The clinical utility of compounded bioidentical hormone therapy: A review of safety, effectiveness, and use. (Washington, DC: The National Academies Press. 2020.)
PSMA PET/CT may be new ‘gold standard’ for prostate cancer staging
The accuracy was 92% for PSMA PET/CT and 65% for CT and bone scintigraphy (P < .001), according to data reported at the virtual annual congress of the European Association of Urology and published in The Lancet.
In addition, PSMA PET/CT had greater effects on treatment. First-line imaging led to treatment changes in 28% of the PSMA PET/CT group and 15% of the CT/bone scan group. Second-line imaging led to treatment changes in 27% and 5% of patients, respectively.
“My strong view is that this is practice-changing data,” said study investigator Michael Hofman, MBBS, of the Peter MacCallum Cancer Centre in Melbourne.
Highly relevant secondary outcomes were included in the study, Dr. Hofman said, and results were all in favor of PSMA PET/CT over conventional imaging.
PSMA PET/CT was associated with a lower rate of equivocal or uncertain findings (7% vs. 23%), and half the radiation dose was needed with PSMA PET/CT (8 mSv vs. 19 mSv). Furthermore, PSMA PET/CT was more accurate when used after CT/bone scan than when CT/bone scan was used after PSMA PET/CT (19% vs. 2%).
“PSMA PET/CT has emerged as a potential new gold standard for imaging prostate cancer,” Dr. Hofman said. The images it can produce were “striking” compared to conventional CT, he added. Pelvic and abdominal metastases that are barely visible on CT were “lighting up very brightly” on PSMA PET/CT, he said.
The study also showed that PSMA PET/CT was superior to CT/bone scans for picking up metastases throughout the body. The detection rate was 91% and 59%, respectively, for pelvic nodal metastases and 95% and 74%, respectively, for distant metastases.
Study details
ProPSMA is a multicenter, phase 3 trial directly comparing PSMA PET/CT and the standard of imaging. Of 339 men assessed for inclusion across 10 centers in Australia, 302 were randomized. They had a median age of 69 years. All patients had high-risk prostate cancer, which was defined as a prostate-specific antigen level of 20 ng/mL, Gleason Grade Group 3-5, or clinical stage T3 or higher. They were all about to undergo either surgery or radiotherapy with the intention of curing their prostate cancer.
PSMA PET/CT was performed using the gallium-68-labelled PSMA-11 tracer, but the results would likely be no different if another tracer were used, Dr. Hofman said in the discussion following his talk.
Of the three available tracers, there were minor differences, mostly in how they were excreted. However, “they’re all extremely good. I’m not sure anyone’s ever going to undertake a head-to-head study comparing them,” Dr. Hofman said.
“Whichever one you can access, at the cheapest cost, I think, is going to be the best one in your center,” he added. “That really does vary geographically, but I really don’t think one is better or worse than the other.”
Praise and criticism
The latest European guidelines acknowledge that PSMA PET/CT is more sensitive for detecting lymph node and bone metastases than the classical workup of abdominopelvic CT and bone scintigraphy, according to invited discussant Matthias Heck, PD Dr. med, of the Technical University of Munich in Germany.
“Molecular imaging using PSMA PET/CT facilitates the detection of small lymph node metastasis, with the size of a few millimeters,” Dr. Heck said.
Although he commended the ProPSMA investigators, Dr. Heck had one criticism of the study design that may have resulted in over-sensitivity of PSMA PET/CT.
“As a urologist, I want to address as a discussion point the low number of histopathologic validation in the ProPSMA study,” he said. “Pelvic lymph node sampling was performed only in 66% of patients treated with radical prostatectomy for high-risk prostate cancer. Hard criteria to define the presence of metastasis were only used in 23% of patients with metastases. Therefore, it is possible that the sensitivity was overestimated by using mainly soft criteria.”
The sensitivity of PSMA PET/CT was 85%, while that of CT/bone scan was 38%. The respective specificities were 98% and 91%.
“What I like most about this study is that, when we perform a PSMA PET/CT, you see the whole body; you don’t see only pelvic lymph nodes,” Dr. Heck said. Since it was not possible to validate distant metastasis by histopathology, he added, this imaging method could clearly help determine the best treatment.
“If we have distant metastasis in the bones or in the lymph nodes outside of the pelvis, it’s clearly unnecessary to direct this patient to undergo local treatment, and we need to think about other treatments,” Dr. Heck said. “Therefore, I think it’s a very important question that is being raised by this study, and we all need to look at the whole body of the patient and not focus only on the pelvic lymph nodes.”
The study was funded by the Prostate Cancer Foundation of Australia. Dr. Hofman said he has no relevant conflicts of interest. Dr. Heck disclosed relationships with Astellas, Janssen, Ipsen, Amgen, Bayer, Heise, Merck, Sanofi, and Takeda.
SOURCES: Hofman M et al. Lancet. March 22, doi: https://doi.org/10.1016/S0140-6736(20)30314-7.
The accuracy was 92% for PSMA PET/CT and 65% for CT and bone scintigraphy (P < .001), according to data reported at the virtual annual congress of the European Association of Urology and published in The Lancet.
In addition, PSMA PET/CT had greater effects on treatment. First-line imaging led to treatment changes in 28% of the PSMA PET/CT group and 15% of the CT/bone scan group. Second-line imaging led to treatment changes in 27% and 5% of patients, respectively.
“My strong view is that this is practice-changing data,” said study investigator Michael Hofman, MBBS, of the Peter MacCallum Cancer Centre in Melbourne.
Highly relevant secondary outcomes were included in the study, Dr. Hofman said, and results were all in favor of PSMA PET/CT over conventional imaging.
PSMA PET/CT was associated with a lower rate of equivocal or uncertain findings (7% vs. 23%), and half the radiation dose was needed with PSMA PET/CT (8 mSv vs. 19 mSv). Furthermore, PSMA PET/CT was more accurate when used after CT/bone scan than when CT/bone scan was used after PSMA PET/CT (19% vs. 2%).
“PSMA PET/CT has emerged as a potential new gold standard for imaging prostate cancer,” Dr. Hofman said. The images it can produce were “striking” compared to conventional CT, he added. Pelvic and abdominal metastases that are barely visible on CT were “lighting up very brightly” on PSMA PET/CT, he said.
The study also showed that PSMA PET/CT was superior to CT/bone scans for picking up metastases throughout the body. The detection rate was 91% and 59%, respectively, for pelvic nodal metastases and 95% and 74%, respectively, for distant metastases.
Study details
ProPSMA is a multicenter, phase 3 trial directly comparing PSMA PET/CT and the standard of imaging. Of 339 men assessed for inclusion across 10 centers in Australia, 302 were randomized. They had a median age of 69 years. All patients had high-risk prostate cancer, which was defined as a prostate-specific antigen level of 20 ng/mL, Gleason Grade Group 3-5, or clinical stage T3 or higher. They were all about to undergo either surgery or radiotherapy with the intention of curing their prostate cancer.
PSMA PET/CT was performed using the gallium-68-labelled PSMA-11 tracer, but the results would likely be no different if another tracer were used, Dr. Hofman said in the discussion following his talk.
Of the three available tracers, there were minor differences, mostly in how they were excreted. However, “they’re all extremely good. I’m not sure anyone’s ever going to undertake a head-to-head study comparing them,” Dr. Hofman said.
“Whichever one you can access, at the cheapest cost, I think, is going to be the best one in your center,” he added. “That really does vary geographically, but I really don’t think one is better or worse than the other.”
Praise and criticism
The latest European guidelines acknowledge that PSMA PET/CT is more sensitive for detecting lymph node and bone metastases than the classical workup of abdominopelvic CT and bone scintigraphy, according to invited discussant Matthias Heck, PD Dr. med, of the Technical University of Munich in Germany.
“Molecular imaging using PSMA PET/CT facilitates the detection of small lymph node metastasis, with the size of a few millimeters,” Dr. Heck said.
Although he commended the ProPSMA investigators, Dr. Heck had one criticism of the study design that may have resulted in over-sensitivity of PSMA PET/CT.
“As a urologist, I want to address as a discussion point the low number of histopathologic validation in the ProPSMA study,” he said. “Pelvic lymph node sampling was performed only in 66% of patients treated with radical prostatectomy for high-risk prostate cancer. Hard criteria to define the presence of metastasis were only used in 23% of patients with metastases. Therefore, it is possible that the sensitivity was overestimated by using mainly soft criteria.”
The sensitivity of PSMA PET/CT was 85%, while that of CT/bone scan was 38%. The respective specificities were 98% and 91%.
“What I like most about this study is that, when we perform a PSMA PET/CT, you see the whole body; you don’t see only pelvic lymph nodes,” Dr. Heck said. Since it was not possible to validate distant metastasis by histopathology, he added, this imaging method could clearly help determine the best treatment.
“If we have distant metastasis in the bones or in the lymph nodes outside of the pelvis, it’s clearly unnecessary to direct this patient to undergo local treatment, and we need to think about other treatments,” Dr. Heck said. “Therefore, I think it’s a very important question that is being raised by this study, and we all need to look at the whole body of the patient and not focus only on the pelvic lymph nodes.”
The study was funded by the Prostate Cancer Foundation of Australia. Dr. Hofman said he has no relevant conflicts of interest. Dr. Heck disclosed relationships with Astellas, Janssen, Ipsen, Amgen, Bayer, Heise, Merck, Sanofi, and Takeda.
SOURCES: Hofman M et al. Lancet. March 22, doi: https://doi.org/10.1016/S0140-6736(20)30314-7.
The accuracy was 92% for PSMA PET/CT and 65% for CT and bone scintigraphy (P < .001), according to data reported at the virtual annual congress of the European Association of Urology and published in The Lancet.
In addition, PSMA PET/CT had greater effects on treatment. First-line imaging led to treatment changes in 28% of the PSMA PET/CT group and 15% of the CT/bone scan group. Second-line imaging led to treatment changes in 27% and 5% of patients, respectively.
“My strong view is that this is practice-changing data,” said study investigator Michael Hofman, MBBS, of the Peter MacCallum Cancer Centre in Melbourne.
Highly relevant secondary outcomes were included in the study, Dr. Hofman said, and results were all in favor of PSMA PET/CT over conventional imaging.
PSMA PET/CT was associated with a lower rate of equivocal or uncertain findings (7% vs. 23%), and half the radiation dose was needed with PSMA PET/CT (8 mSv vs. 19 mSv). Furthermore, PSMA PET/CT was more accurate when used after CT/bone scan than when CT/bone scan was used after PSMA PET/CT (19% vs. 2%).
“PSMA PET/CT has emerged as a potential new gold standard for imaging prostate cancer,” Dr. Hofman said. The images it can produce were “striking” compared to conventional CT, he added. Pelvic and abdominal metastases that are barely visible on CT were “lighting up very brightly” on PSMA PET/CT, he said.
The study also showed that PSMA PET/CT was superior to CT/bone scans for picking up metastases throughout the body. The detection rate was 91% and 59%, respectively, for pelvic nodal metastases and 95% and 74%, respectively, for distant metastases.
Study details
ProPSMA is a multicenter, phase 3 trial directly comparing PSMA PET/CT and the standard of imaging. Of 339 men assessed for inclusion across 10 centers in Australia, 302 were randomized. They had a median age of 69 years. All patients had high-risk prostate cancer, which was defined as a prostate-specific antigen level of 20 ng/mL, Gleason Grade Group 3-5, or clinical stage T3 or higher. They were all about to undergo either surgery or radiotherapy with the intention of curing their prostate cancer.
PSMA PET/CT was performed using the gallium-68-labelled PSMA-11 tracer, but the results would likely be no different if another tracer were used, Dr. Hofman said in the discussion following his talk.
Of the three available tracers, there were minor differences, mostly in how they were excreted. However, “they’re all extremely good. I’m not sure anyone’s ever going to undertake a head-to-head study comparing them,” Dr. Hofman said.
“Whichever one you can access, at the cheapest cost, I think, is going to be the best one in your center,” he added. “That really does vary geographically, but I really don’t think one is better or worse than the other.”
Praise and criticism
The latest European guidelines acknowledge that PSMA PET/CT is more sensitive for detecting lymph node and bone metastases than the classical workup of abdominopelvic CT and bone scintigraphy, according to invited discussant Matthias Heck, PD Dr. med, of the Technical University of Munich in Germany.
“Molecular imaging using PSMA PET/CT facilitates the detection of small lymph node metastasis, with the size of a few millimeters,” Dr. Heck said.
Although he commended the ProPSMA investigators, Dr. Heck had one criticism of the study design that may have resulted in over-sensitivity of PSMA PET/CT.
“As a urologist, I want to address as a discussion point the low number of histopathologic validation in the ProPSMA study,” he said. “Pelvic lymph node sampling was performed only in 66% of patients treated with radical prostatectomy for high-risk prostate cancer. Hard criteria to define the presence of metastasis were only used in 23% of patients with metastases. Therefore, it is possible that the sensitivity was overestimated by using mainly soft criteria.”
The sensitivity of PSMA PET/CT was 85%, while that of CT/bone scan was 38%. The respective specificities were 98% and 91%.
“What I like most about this study is that, when we perform a PSMA PET/CT, you see the whole body; you don’t see only pelvic lymph nodes,” Dr. Heck said. Since it was not possible to validate distant metastasis by histopathology, he added, this imaging method could clearly help determine the best treatment.
“If we have distant metastasis in the bones or in the lymph nodes outside of the pelvis, it’s clearly unnecessary to direct this patient to undergo local treatment, and we need to think about other treatments,” Dr. Heck said. “Therefore, I think it’s a very important question that is being raised by this study, and we all need to look at the whole body of the patient and not focus only on the pelvic lymph nodes.”
The study was funded by the Prostate Cancer Foundation of Australia. Dr. Hofman said he has no relevant conflicts of interest. Dr. Heck disclosed relationships with Astellas, Janssen, Ipsen, Amgen, Bayer, Heise, Merck, Sanofi, and Takeda.
SOURCES: Hofman M et al. Lancet. March 22, doi: https://doi.org/10.1016/S0140-6736(20)30314-7.
FROM EAU20
It’s time to rethink your approach to C diff infection
CASE 1
Beth O, a 63-year-old woman, presents to the emergency department (ED) with a 2-week history of diarrhea (6 very loose, watery stools per day) and lower abdominal pain. The patient denies any vomiting, sick contacts, or recent travel. Past medical history includes varicose veins. Her only active medication is loperamide, as needed, for the past 2 weeks. Ms. O also recently completed a 10-day course of clindamycin for an infected laceration on her finger.
Ms. O’s laboratory values are unremarkable, with a normal white blood cell (WBC) count and serum creatinine (SCr) level. Abdominal computed tomography (CT) reveals some abnormal bowel dilatation and a slight increase in colon wall thickness. There is a high suspicion for Clostridioides difficile (formerly Clostridium difficile) infection (CDI), and stool sent for polymerase chain reaction (PCR) testing comes back positive for C difficile toxin B. It is revealed to be a strain other than the BI/NAP1/027 epidemic strain (which has a higher mortality rate).
How should this patient be treated?
CASE 2
Sixty-eight-year-old Barbara Z presents to the ED from her skilled nursing facility with persistent diarrhea and abdominal cramping. She was diagnosed with CDI about 2 months ago and reports that her symptoms resolved within 4 to 5 days after starting a 14-day course of oral metronidazole.
Her past medical history is notable for multiple myeloma with bone metastasis, for which she is actively undergoing chemotherapy treatment. She also has chronic kidney disease (baseline SCr, 2.2 mg/dL), hypertension, and anemia of chronic disease. The patient’s medications include amlodipine and cholecalciferol. Her chemotherapy regimen consists of bortezomib, lenalidomide, and dexamethasone. CT of the abdomen shows diffuse colon wall thickening with surrounding inflammatory stranding—concerning for pancolitis. There is no evidence of toxic megacolon or ileus.
Ms. Z’s laboratory values are notable for a WBC count of 15,900 cells/mL and an SCr of 4.1 mg/dL. She is started on oral levofloxacin and metronidazole due to concern for an intra-abdominal infection. PCR testing is positive for C difficile, and an enzyme immunoassay (EIA) for C difficile toxin is positive.
What factors put Ms. Z at risk for C difficile, and how should she be treated?
Continue to: C difficile is one of the most...
C difficile is one of the most commonly reported pathogens in health care–associated infections and affects almost 1% of all hospitalized patients in the United States each year.1 From 2001 to 2010, the incidence of CDI doubled in patients discharged from hospitals,2 with an estimated cost of more than $5 billion annually.3 Furthermore, rates of community-associated CDI continue to increase and account for about 40% of cases.4
After colonization in the intestine, C difficile releases 2 toxins (TcdA and TcdB) that cause colitis.5 Patients may present with mild diarrhea that can progress to abdominal pain, cramping, fever, and leukocytosis. Fulminant CDI can lead to the formation of pseudomembranes in the colon, toxic megacolon, bowel perforation, shock, and death.2
Beginning in the early 2000s, hospitals reported increases in severe cases of CDI.6 A specific strain known as BI/NAP1/027 was identified and characterized by fluoroquinolone resistance, increased spore formation, and a higher mortality rate.6
Further complicating matters … Recurrent CDI occurs in up to 10% to 30% of patients,7 typically within 14 to 45 days of completion of antibiotic pharmacotherapy for CDI.8 Recurrence is characterized by new-onset diarrhea or abdominal symptoms after completion of treatment for CDI.5
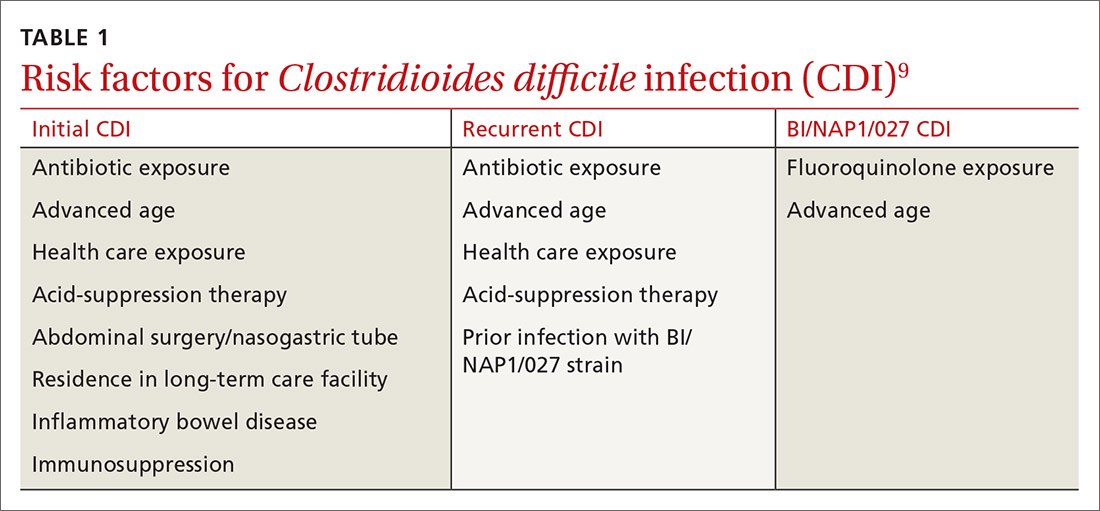
It typically begins with an antibiotic
Risk factors for CDI are listed in TABLE 1.9 The most important modifiable risk factor for initial and recurrent CDI is recent use of antibiotics.10 Most antibiotics can disrupt normal intestinal flora, causing colonization of C difficile, but the strongest association seems to be with third- and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and clindamycin.11 The risk for CDI occurs during antibiotic treatment, as well as up to 3 months after completion of antibiotic therapy.7 Exposure to multiple antibiotics and extended duration of antibacterial therapy can greatly increase the risk for CDI, so antimicrobial stewardship is key.11
Continue to: Continuing antibiotics while attempting...
Continuing antibiotics while attempting to treat CDI reduces the patient’s clinical response to CDI treatment, which can lead to recurrence.12 The Infectious Diseases Society of America (IDSA) guidelines include a strong recommendation to discontinue concurrent antibiotics as soon as possible in these scenarios.11
Acid-suppression therapy has also been associated with CDI. The mechanism is thought to be an interruption in the protection provided by stomach acid, and use over time may reduce the diversity of flora within the gut microbiome.13 The data demonstrating an association between acid-suppression therapy and CDI is conflicting, which may be a result of confounding factors such as the severity of CDI illness and diarrhea induced by use of proton pump inhibitors (PPIs).4 IDSA guidelines do not provide a recommendation regarding discontinuation of PPI therapy for the prevention of CDI, although inappropriate PPI therapy should always be discontinued.11
Advanced age is an important nonmodifiable risk factor for CDI. Older adults who live in long-term care facilities are at a higher risk for CDI, and these facilities have colonization rates as high as 50%.12
Community-associated risk. In an analysis of community-associated cases of CDI, 82% of patients reported some sort of health care exposure (ranging from physician office visit to surgery admission), 64% reported the receipt of antimicrobial therapy, and 31% reported the use of PPIs.14 Inflammatory bowel disease (IBD) may also put community dwellers at higher risk for CDI and its complications.15
CASES 1 & 2
Both CASE patients have risk factors for CDI. Ms. O (CASE 1) is likely at risk for CDI after completion of her recent course of clindamycin. Ms. Z (CASE 2) has several risk factors for recurrent CDI, including advanced age (≥ 65 years), residence in a long-term care facility, prior antibiotic exposure, and immunodeficiency because of chemotherapy/steroid use.
Continue to: Diagnosis
Diagnosis: Who and how to test
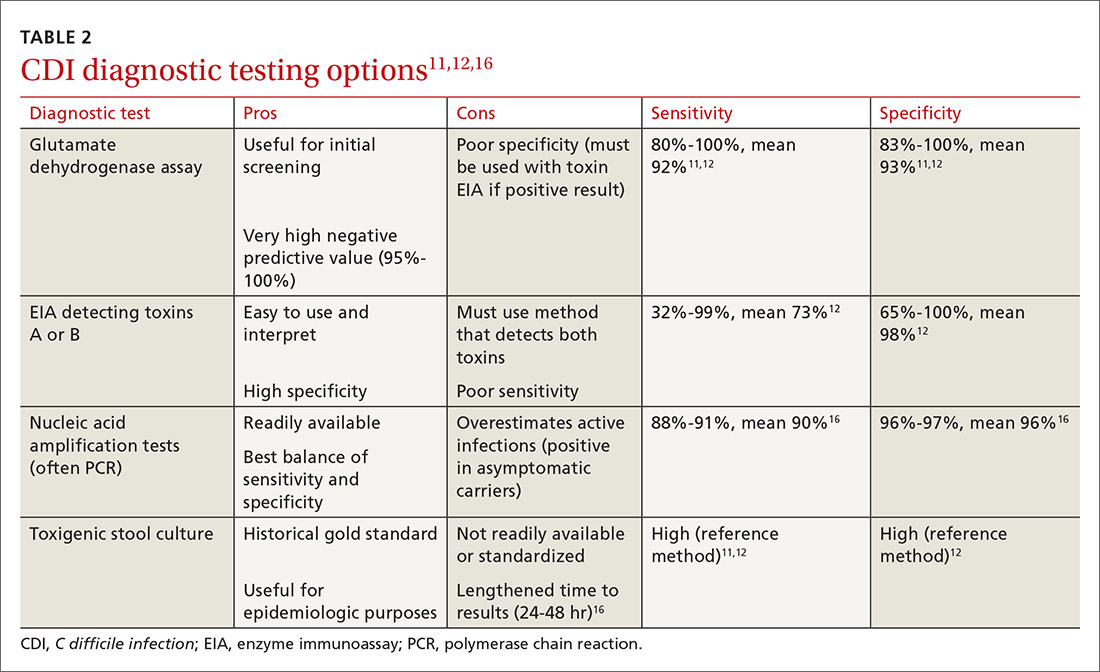
CDI should be both a clinical and laboratory-confirmed diagnosis. Patients should be tested for CDI if they have 3 or more episodes of unexplainable, new-onset unformed stools in 24 hours.11 Asymptomatic patients should not be tested to avoid unnecessary testing and treatment of those who are colonized but not infected.11 It is not recommended to routinely test patients who have taken laxatives within the previous 48 hours.11
There are several stool-based laboratory test options for the diagnosis of CDI (TABLE 211,12,16) but no definitive recommendation for all institutions.11 Many institutions have now implemented PCR testing for the diagnosis of CDI. However, while the benefits of this test include reduced need for repeat testing and possible identification of carriers, it’s estimated that reports of CDI increase more than 50% when an institution switches to PCR testing.1 Nonetheless, a one-step, highly sensitive test such as PCR may be used if strict criteria are implemented and followed.
The increase in positive PCR tests has prompted evaluation of using another test in addition to or in place of PCR. Multistep testing options include a glutamate dehydrogenase assay (GDH) with a toxin EIA, GDH with a toxin EIA and final decision via PCR, or PCR with toxin EIA.11 Use of a multistep diagnostic algorithm may increase overall specificity up to 100%, which may improve determination of asymptomatic colonization vs active infection.16 (Patients who have negative toxin results with positive PCR likely have colonization but not infection and often do not require treatment.) IDSA guidelines recommend that the stool toxin test should be part of a multistep algorithm for diagnosis, rather than PCR alone, if strict criteria are not implemented for stool test submission.11
There is no need to perform a test of cure after a patient has been treated for CDI, and no repeat testing should be performed within 7 days of the previous test.11 After successful treatment, patients will continue to shed spores and test positively via PCR for weeks to months.11 When patients have a positive PCR test, there are several important infection control efforts that institutions should consider; see “IDSA weighs in on measures to combat C difficile.”
SIDEBAR
IDSA weighs in on measures to combat C difficile
The spores produced by Clostridioides difficile can survive for 5 months or longer on dry surfaces because of resistance to heat, acid, antibiotics, and many cleaning products.38 Unfortunately, spores transmitted from health care workers and the environment are the most likely cause of infection spreading in health care institutions. To prevent transmission of C difficile infection (CDI) throughout institutions, appropriate infection control measures are necessary.
Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that patients with CDI be isolated to a private room with a dedicated toilet. Health care staff should wear gloves and gowns when entering the room of, or taking care of, a patient with CDI. For patients who are suspected of having CDI, contact precautions should be implemented while awaiting test results. When the diagnosis is confirmed, contact precautions should remain in place for at least 48 hours after resolution of diarrhea but may be continued until discharge.11
Practicing good hand hygiene is essential, especially in institutions with high rates of CDI or if fecal contamination is likely.11 Hand hygiene with soap and water is preferred, due to evidence of a higher spore removal rate, but alcohol-based alternatives may be used if necessary.11 In institutions with high rates of CDI, terminal (post-discharge) cleaning of rooms with a sporicidal agent should be considered.11
Asymptomatic carriers are also a concern for transmission of CDI in institutional settings. Screening and isolating patients who are carriers may prevent transmission, and some institutions have implemented this process to reduce the risk for CDI that originates in a health care facility.39 The IDSA guidelines do not make a recommendation regarding screening or isolation of asymptomatic carriers, so the decision is institution specific.11 These guidelines also recommend that patients presenting with similar infectious organisms be housed in the same room, if needed, to avoid cross-contamination to others or additional surfaces.11
For pediatric patients, testing recommendations vary by age. Testing is not generally recommended for neonates or infants ≤ 2 years of age with diarrhea because of the prevalence of colonization with C difficile.11 For children older than 2 years, testing for CDI is only recommended in the setting of prolonged or worsening diarrhea and if the patient has risk factors such as IBD, immunocompromised state, health care exposure, or recent antibiotic use.11 In addition, testing in this population should only be considered once other infectious and noninfectious causes of diarrhea have been excluded.11
Continue to: First-line treatment? Drug of choice has changed
First-line treatment? Drug of choice has changed
In 2018, the IDSA published new treatment guidelines that provide important updates from the 2010 guidelines.11 Chief among these was the elimination of metronidazole as a first-line therapy. Vancomycin or fidaxomicin are now recommended as first-line treatment options because of superior eradication of C difficile when compared with metronidazole.11 In the opinion of the authors, vancomycin should be considered the drug of choice because of cost. (See “The case for vancomycin.”)
SIDEBAR
The case for vancomycin
The majority of studies conducted prior to publication of the 2010 Infectious Diseases Society of America guidelines described numerically worse eradication rates of Clostridioides difficile infection (CDI) with metronidazole compared with vancomycin for all severities of infection, but statistical significance was not achieved. These studies also showed a nonsignificant increase in CDI recurrence with metronidazole.17,40,41
A 2005 systematic review demonstrated increased treatment failure rates with metronidazole.42 The rates of metronidazole discontinuation and transition to alternative options more than doubled in 2003-2004, to 25.7% of patients compared with 9.6% in earlier years.42 Metronidazole efficacy was further questioned in a prospective observational study conducted in 2005, in which only 50% of patients were cured after an initial course of treatment, while 28% had recurrence within 90 days.43
Vancomycin was found to be the superior treatment option to metronidazole and tolevamer in a 2014 randomized controlled trial.18 This study also demonstrated that vancomycin was the superior therapy when comparing treatment-naïve vs experienced patients and severity of CDI.18 A 2017 retrospective cohort study demonstrated decreased 30-day all-cause mortality for patients taking vancomycin vs metronidazole (adjusted relative risk = 0.86; 95% confidence interval, 0.74-0.98), although it should be noted that this difference was driven by those with severe CDI, and there was no statistically significant difference in mortality for patients with mild-to-moderate CDI.44
The results of these studies led to the recommendation of vancomycin over metronidazole as first-line pharmacotherapy for CDI in practice, despite the historical perspective that overutilization of oral vancomycin could potentially increase rates of vancomycinresistant Enterococcus.11
Metronidazole should only be used in the treatment of CDI as a lastresort medication because of cost or insurance coverage. Although the price of oral vancomycin is higher, favorable patient outcomes are substantially greater, and recent analyses have shown that vancomycin is actually more cost-effective than metronidazole as a result.24 Adverse effects for metronidazole include neurotoxicity, gastrointestinal discomfort, and disulfiram-like reaction.
Vancomycin does not harbor as many adverse effects because of extremely low systemic absorption when taken orally, but patients may experience gastrointestinal discomfort.45 While systemic exposure with oral administration of vancomycin is very low (< 1%), there have been case reports of nephrotoxicity and “red man syndrome” that are more typically seen with intravenous vancomycin.44
Given the low rate of systemic exposure, routine monitoring of renal function and serum drug levels is not usually necessary during oral vancomycin therapy. However, it may be appropriate to monitor renal function and serum levels of vancomycin in patients who have renal failure, have altered intestinal integrity, are age ≥ 65 years, or are receiving high doses of vancomycin.46
10-day vs 14-day treatment of CDI. Most studies for the treatment of CDI have used a 10-day regimen rather than increasing the duration to a 14-day regimen, and nearly all studies conducted have displayed high rates of symptom resolution at the end of 10 days of treatment.17,18 Thus, treatment duration beyond 10 days should only be considered for patients who continue to have symptoms or complications with CDI on Day 10 of treatment.
First recurrence. Metronidazole is no longer the recommended treatment for first recurrence of CDI treated initially with metronidazole; instead, a 10-day course of vancomycin should be used.11 For recurrent cases in patients initially treated with vancomycin, a tapered and pulsed regimen of vancomycin is recommended11:
- vancomycin PO 125 mg four times daily for 10 to 14 days followed by
- vancomycin PO 125 mg twice daily for 7 days, then
- vancomycin PO 125 mg once daily for 7 days, then
- vancomycin PO 125 mg every 2 to 3 days for 2 to 8 weeks.
Pediatric patients. The IDSA guidelines recommend use of metronidazole or vancomycin to treat an initial case or first recurrence of mild-to-moderate CDI in this population.11 Due to a lack of quality evidence, the drug of choice for initial treatment is inconclusive, so patient-specific factors and cost should be considered when choosing an agent.11 If not cost prohibitive, vancomycin should be the drug of choice for most cases of pediatric CDI, and for severe cases or multiple recurrences of CDI, vancomycin is clearly the drug of choice.
Recommended agents: A closer look
Oral vancomycin products. Vancocin, a capsule, and Firvanq, an oral solution, are 2 vancomycin products currently on the market for CDI. Although the capsules are a readily available treatment option, the cost of the full course of treatment can be a barrier for patients without insurance, or with high copays or deductibles (brand name, $4000; generic, $1252).19
Continue to: Historically, in an effort to keep costs down...
Historically, in an effort to keep costs down, an oral solution was often inexpensively compounded at hospitals or pharmacies.20
Fidaxomicin, an oral macrocyclic antibiotic with minimal systemic absorption, was first approved by the US Food and Drug Administration (FDA) for CDI in 2011.21 The IDSA guidelines recommend fidaxomicin for initial, and recurrent, cases of CDI as an alternative to vancomycin.11 This recommendation is based on 2 randomized double-blind trials comparing fidaxomicin to standard-dose oral vancomycin for initial or recurrent CDI.21,22
Pooled data from these 2 similar studies found that fidaxomicin was noninferior (10% noninferiority margin) to vancomycin for the primary outcome of clinical cure.23 Fidaxomicin was shown to be superior to vancomycin regarding rate of CDI recurrence (relative risk [RR] = 0.61; 95% confidence interval [CI], 0.43-0.87). These results were similar regardless of whether the CDI was an initial or recurrent case.23
Given the lack of systemic absorption, fidaxomicin is generally very well tolerated. The largest downside to fidaxomicin is its cost, which can be nearly $5000 for a standard 10-day course (vs as little as $165 for oral vancomycin).19 As a result, oral vancomycin solution is likely the most cost-effective therapy for initial cases of CDI.24 In patients with poor medication adherence, fidaxomicin offers the advantage of less-frequent dosing (twice daily vs 4 times daily with vancomycin).
For cases of recurrent CDI, when treatment failure occurred with vancomycin, fidaxomicin should be considered as an efficacious alternative. If fidaxomicin is used, it is advisable to verify coverage with the patient’s insurance plan, since prior authorization is frequently required.
Continue to: When meds fail, consider a fecal microbiota transplant
When meds fail, consider a fecal microbiota transplant
Another important change in the IDSA guidelines for CDI management is the strong recommendation for fecal microbiota transplantation (FMT) in patients with multiple recurrences of CDI for whom appropriate antibiotic treatment courses have failed.11,25 The goal of FMT is to “normalize” an abnormal gut microbiome by transplanting donor stool into a recipient.26
FMT has been shown to be highly effective in 5 randomized clinical trials conducted since 2013, with CDI cure rates between 85% and 94%.11 This rate of cure is particularly impressive given that the studies only included patients with refractory CDI.
Patients with recurrent CDI who may be candidates for FMT should be referred to a center or specialist with experience in FMT. These transplants can be expensive because of the screening process involved in obtaining donor samples. (Historically, a single FMT has cost $3000-$5000, and it is seldom covered by insurance.27) The emergence of universal stool banks offers a streamlined solution to this process.26
Fresh or frozen stool is considered equally effective in treating refractory CDI.26 Oral capsule and freeze-dried stool formulations have been studied, but their use is considered investigational at this time.26
Delivery via colonoscopy to the right colon is the preferred route of infusion; however, delivery via enema or nasogastric, nasojejunal, or nasoduodenal infusion can be considered as well.26
Continue to: In preparing for stool transplantation...
In preparing for stool transplantation, patients should be treated with standard doses of oral vancomycin or fidaxomicin for 3 days before the procedure to suppress intestinal C difficile, and the last dose of antibiotics should be given 12 to 48 hours before the procedure.26 Bowel lavage with polyethylene glycol is recommended, regardless of whether stool is delivered via colonoscopy or upper GI route.
Short-term adverse events associated with FMT appear to be minimal; data is lacking for long-term safety outcomes.28 While only recommended currently for cases of recurrent CDI, there is promising data emerging for use of FMT for severe cases, even without recurrence.29
The role of probiotics remains unclear
Probiotics have been explored in numerous trials to determine if they are effective in preventing CDI in patients who have been prescribed antibiotics.11 While no randomized trials have conclusively shown benefit, several meta-analyses have shown that the use of probiotics may result in a 60% to 65% relative risk reduction in CDI incidence.30,31
One proviso to these meta-analyses is that the incorporated studies have typically included patients at very high risk for CDI, and subanalyses have only found a reduction in CDI incidence when patients are at a very high baseline risk. In addition, there are many differences in probiotic types, formulations, treatment durations, and follow-up. As a result, the IDSA guidelines state that there is “insufficient data at this time” to recommend routine administration of probiotics for either primary or secondary CDI prophylaxis.11
Due to insufficient high-quality data, the IDSA guidelines do not provide a recommendation regarding use as an adjunct treatment option for acute CDI.11 Probiotics should not be routinely used to prevent CDI; however, they may provide benefit if reserved for patients at the highest risk for CDI (eg, history of CDI, prolonged use of broad-spectrum antibiotics, high local incidence).
Continue to: What about surgical intervention?
What about surgical intervention?
In severe cases of CDI, surgery may be necessary and can reduce mortality.32 The surgical procedure with the strongest recommendation in the IDSA guidelines is the subtotal colectomy, though the diverting loop ileostomy is an alternative option.11 Patients who may benefit from surgery include those with a WBC count ≥ 25,000; lactate > 5 mmol/L11; altered mental status; megacolon; perforation of the colon; acute abdomen on physical examination; or septic shock due to CDI.33 Although surgery can be beneficial, the mortality rate remains high for those with CDI who undergo colectomy.33
Reserve bezlotoxumab for prevention of recurrence
Bezlotoxumab, a human monoclonal immunoglobulin GI/kappa antibody, was approved by the FDA in 2016 for the prevention of recurrent CDI. Its mechanism of action is to bind and neutralize C difficile toxin B. It was approved as a single infusion for adults who are receiving active antibiotic therapy for CDI and are considered to be at high risk for recurrence.34
This approval was based on 2 trials of more than 2500 patients, in which participants received bezlotoxumab or placebo while receiving treatment for primary or recurrent CDI. The primary outcome of these studies was recurrent infection within 12 weeks after infusion, which was significantly lower for bezlotoxumab in both studies: 17% vs 28% (P < 0.001) in one trial and 16% vs 26% (P < 0.001) in the other trial.35
Bezlotoxumab should only be used as an adjunct to prevent recurrence.32 There is no recommendation for or against bezlotoxumab in the IDSA guidelines because of the recent date of the drug’s approval. Its frequency of use will likely depend on the number of patients who meet criteria as high risk for recurrence and its estimated cost of $4560 per dose.34,36
CASES
CASE 1: In light of Ms. O’s recent completion of a course of clindamycin and unremarkable lab work, she should be treated for mild-to-moderate CDI. She has no comorbid conditions to warrant fidaxomicin, and thus vancomycin (capsules or oral solution) would be the best treatment option. Ms. O is started on vancomycin PO 125 mg qid for 10 days. She is also advised to discontinue loperamide as soon as possible, based on poor outcomes data seen with the use of antimotility agents in CDI.37
Continue to: CASE 2
CASE 2: Ms. Z has several risk factors for recurrent CDI and has an elevated WBC count and SCr level (WBC ≥ 15,000 and SCr > 1.5 mg/dL). Thus, she is classified as having severe, recurrent CDI. Oral levofloxacin and metronidazole should be discontinued, because they increase the risk for treatment failure and development of more virulent CDI strains, such as BI/NAP1/027. Since Ms. Z used metronidazole for treatment of her initial CDI, vancomycin or fidaxomicin should be used at this time. Either vancomycin PO 125 mg qid for 10 days or fidaxomicin 200 mg bid for 10 days would be an appropriate regimen; however, because of cost and unknown insurance coverage, vancomycin is the most appropriate regimen.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, Saint Joseph Family Medicine Residency, 1000 E. University Avenue, Dept 3375, Laramie, WY 82071; jvandive@uwyo.edu
1. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792-1801.
2. Reveles KR, Lee GC, Boyd NK, et al. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028-1032.
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88-S92.
4. Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:784-791.
5. Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37:178-187.
6. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924.
7. Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C difficile infection. JAMA. 2009;301:954-962.
8. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
9. Napolitano LM, Edmiston CE Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery 2017;162:325-348.
10. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452-460.
11. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48.
12. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498; quiz 499.
13. Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
14. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359-1367.
15. Negrón ME, Rezaie A, Barkema HW, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study. Am J Gastroenterol. 2016;111:691-704.
16. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408.
17. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
18. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354.
19. Vancomycin: product details. Redbook Online. www.micromedexsolutions.com. Published 2018. Accessed June 13, 2020.
20. Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics. 2014;32:639-650.
21. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
22. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281-289.
23. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55 suppl 2:S93-103.
24. Ford DC, Schroeder MC, Ince D, et al. Cost-effectiveness analysis of initial treatment strategies for mild-to-moderate Clostridium difficile infection in hospitalized patients. Am J Health Syst Pharm. 2018;75:1110-1121.
25. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
26. Panchal P, Budree S, Scheeler A, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. 2018;20:14.
27. Arbel LT, Hsu E, McNally K. Cost-effectiveness of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection: a literature review. Cureus. 2017;9:e1599.
28. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580.
29. Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66:645-650.
30. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
31. Johnston BC, Lytvyn L, Lo CK, et al. Microbial preparations (probiotics) for the prevention of Clostridium difficile infection in adults and children: an individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018:1-11.
32. Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013;15:798-804.
33. Julien M, Wild JL, Blansfield J, et al. Severe complicated Clostridium difficile infection: can the UPMC proposed scoring system predict the need for surgery? J Trauma Acute Care Surg. 2016;81:221-228.
34. Merck & Co, Inc. Sharp M. ZinplavaTM (bezlotoxumab [package insert] US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Revised October 2016. Accessed May 29, 2020.
35. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-317.
36. Chahine EB, Cho JC, Worley MV. Bezlotoxumab for the Prevention of Clostridium difficile recurrence. Consult Pharm. 2018;33:89-97.
37. Koo HL, Koo DC, Musher DM, et al. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598-605.
38. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526-536.
39. Longtin Y, Paquet-Bolduc B, Gilca R, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176:796-804.
40. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-1046.
41. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
42. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
43. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
44. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177:546-553.
45. CutisPharma. FirvanqTM (vancomycin hydrochloride) for oral solution [package insert]. US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208910s000lbl.pdf. Revised January 2018. Accessed May 29, 2020.
46.
CASE 1
Beth O, a 63-year-old woman, presents to the emergency department (ED) with a 2-week history of diarrhea (6 very loose, watery stools per day) and lower abdominal pain. The patient denies any vomiting, sick contacts, or recent travel. Past medical history includes varicose veins. Her only active medication is loperamide, as needed, for the past 2 weeks. Ms. O also recently completed a 10-day course of clindamycin for an infected laceration on her finger.
Ms. O’s laboratory values are unremarkable, with a normal white blood cell (WBC) count and serum creatinine (SCr) level. Abdominal computed tomography (CT) reveals some abnormal bowel dilatation and a slight increase in colon wall thickness. There is a high suspicion for Clostridioides difficile (formerly Clostridium difficile) infection (CDI), and stool sent for polymerase chain reaction (PCR) testing comes back positive for C difficile toxin B. It is revealed to be a strain other than the BI/NAP1/027 epidemic strain (which has a higher mortality rate).
How should this patient be treated?
CASE 2
Sixty-eight-year-old Barbara Z presents to the ED from her skilled nursing facility with persistent diarrhea and abdominal cramping. She was diagnosed with CDI about 2 months ago and reports that her symptoms resolved within 4 to 5 days after starting a 14-day course of oral metronidazole.
Her past medical history is notable for multiple myeloma with bone metastasis, for which she is actively undergoing chemotherapy treatment. She also has chronic kidney disease (baseline SCr, 2.2 mg/dL), hypertension, and anemia of chronic disease. The patient’s medications include amlodipine and cholecalciferol. Her chemotherapy regimen consists of bortezomib, lenalidomide, and dexamethasone. CT of the abdomen shows diffuse colon wall thickening with surrounding inflammatory stranding—concerning for pancolitis. There is no evidence of toxic megacolon or ileus.
Ms. Z’s laboratory values are notable for a WBC count of 15,900 cells/mL and an SCr of 4.1 mg/dL. She is started on oral levofloxacin and metronidazole due to concern for an intra-abdominal infection. PCR testing is positive for C difficile, and an enzyme immunoassay (EIA) for C difficile toxin is positive.
What factors put Ms. Z at risk for C difficile, and how should she be treated?
Continue to: C difficile is one of the most...
C difficile is one of the most commonly reported pathogens in health care–associated infections and affects almost 1% of all hospitalized patients in the United States each year.1 From 2001 to 2010, the incidence of CDI doubled in patients discharged from hospitals,2 with an estimated cost of more than $5 billion annually.3 Furthermore, rates of community-associated CDI continue to increase and account for about 40% of cases.4
After colonization in the intestine, C difficile releases 2 toxins (TcdA and TcdB) that cause colitis.5 Patients may present with mild diarrhea that can progress to abdominal pain, cramping, fever, and leukocytosis. Fulminant CDI can lead to the formation of pseudomembranes in the colon, toxic megacolon, bowel perforation, shock, and death.2
Beginning in the early 2000s, hospitals reported increases in severe cases of CDI.6 A specific strain known as BI/NAP1/027 was identified and characterized by fluoroquinolone resistance, increased spore formation, and a higher mortality rate.6
Further complicating matters … Recurrent CDI occurs in up to 10% to 30% of patients,7 typically within 14 to 45 days of completion of antibiotic pharmacotherapy for CDI.8 Recurrence is characterized by new-onset diarrhea or abdominal symptoms after completion of treatment for CDI.5
It typically begins with an antibiotic
Risk factors for CDI are listed in TABLE 1.9 The most important modifiable risk factor for initial and recurrent CDI is recent use of antibiotics.10 Most antibiotics can disrupt normal intestinal flora, causing colonization of C difficile, but the strongest association seems to be with third- and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and clindamycin.11 The risk for CDI occurs during antibiotic treatment, as well as up to 3 months after completion of antibiotic therapy.7 Exposure to multiple antibiotics and extended duration of antibacterial therapy can greatly increase the risk for CDI, so antimicrobial stewardship is key.11
Continue to: Continuing antibiotics while attempting...
Continuing antibiotics while attempting to treat CDI reduces the patient’s clinical response to CDI treatment, which can lead to recurrence.12 The Infectious Diseases Society of America (IDSA) guidelines include a strong recommendation to discontinue concurrent antibiotics as soon as possible in these scenarios.11
Acid-suppression therapy has also been associated with CDI. The mechanism is thought to be an interruption in the protection provided by stomach acid, and use over time may reduce the diversity of flora within the gut microbiome.13 The data demonstrating an association between acid-suppression therapy and CDI is conflicting, which may be a result of confounding factors such as the severity of CDI illness and diarrhea induced by use of proton pump inhibitors (PPIs).4 IDSA guidelines do not provide a recommendation regarding discontinuation of PPI therapy for the prevention of CDI, although inappropriate PPI therapy should always be discontinued.11
Advanced age is an important nonmodifiable risk factor for CDI. Older adults who live in long-term care facilities are at a higher risk for CDI, and these facilities have colonization rates as high as 50%.12
Community-associated risk. In an analysis of community-associated cases of CDI, 82% of patients reported some sort of health care exposure (ranging from physician office visit to surgery admission), 64% reported the receipt of antimicrobial therapy, and 31% reported the use of PPIs.14 Inflammatory bowel disease (IBD) may also put community dwellers at higher risk for CDI and its complications.15
CASES 1 & 2
Both CASE patients have risk factors for CDI. Ms. O (CASE 1) is likely at risk for CDI after completion of her recent course of clindamycin. Ms. Z (CASE 2) has several risk factors for recurrent CDI, including advanced age (≥ 65 years), residence in a long-term care facility, prior antibiotic exposure, and immunodeficiency because of chemotherapy/steroid use.
Continue to: Diagnosis
Diagnosis: Who and how to test
CDI should be both a clinical and laboratory-confirmed diagnosis. Patients should be tested for CDI if they have 3 or more episodes of unexplainable, new-onset unformed stools in 24 hours.11 Asymptomatic patients should not be tested to avoid unnecessary testing and treatment of those who are colonized but not infected.11 It is not recommended to routinely test patients who have taken laxatives within the previous 48 hours.11
There are several stool-based laboratory test options for the diagnosis of CDI (TABLE 211,12,16) but no definitive recommendation for all institutions.11 Many institutions have now implemented PCR testing for the diagnosis of CDI. However, while the benefits of this test include reduced need for repeat testing and possible identification of carriers, it’s estimated that reports of CDI increase more than 50% when an institution switches to PCR testing.1 Nonetheless, a one-step, highly sensitive test such as PCR may be used if strict criteria are implemented and followed.
The increase in positive PCR tests has prompted evaluation of using another test in addition to or in place of PCR. Multistep testing options include a glutamate dehydrogenase assay (GDH) with a toxin EIA, GDH with a toxin EIA and final decision via PCR, or PCR with toxin EIA.11 Use of a multistep diagnostic algorithm may increase overall specificity up to 100%, which may improve determination of asymptomatic colonization vs active infection.16 (Patients who have negative toxin results with positive PCR likely have colonization but not infection and often do not require treatment.) IDSA guidelines recommend that the stool toxin test should be part of a multistep algorithm for diagnosis, rather than PCR alone, if strict criteria are not implemented for stool test submission.11
There is no need to perform a test of cure after a patient has been treated for CDI, and no repeat testing should be performed within 7 days of the previous test.11 After successful treatment, patients will continue to shed spores and test positively via PCR for weeks to months.11 When patients have a positive PCR test, there are several important infection control efforts that institutions should consider; see “IDSA weighs in on measures to combat C difficile.”
SIDEBAR
IDSA weighs in on measures to combat C difficile
The spores produced by Clostridioides difficile can survive for 5 months or longer on dry surfaces because of resistance to heat, acid, antibiotics, and many cleaning products.38 Unfortunately, spores transmitted from health care workers and the environment are the most likely cause of infection spreading in health care institutions. To prevent transmission of C difficile infection (CDI) throughout institutions, appropriate infection control measures are necessary.
Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that patients with CDI be isolated to a private room with a dedicated toilet. Health care staff should wear gloves and gowns when entering the room of, or taking care of, a patient with CDI. For patients who are suspected of having CDI, contact precautions should be implemented while awaiting test results. When the diagnosis is confirmed, contact precautions should remain in place for at least 48 hours after resolution of diarrhea but may be continued until discharge.11
Practicing good hand hygiene is essential, especially in institutions with high rates of CDI or if fecal contamination is likely.11 Hand hygiene with soap and water is preferred, due to evidence of a higher spore removal rate, but alcohol-based alternatives may be used if necessary.11 In institutions with high rates of CDI, terminal (post-discharge) cleaning of rooms with a sporicidal agent should be considered.11
Asymptomatic carriers are also a concern for transmission of CDI in institutional settings. Screening and isolating patients who are carriers may prevent transmission, and some institutions have implemented this process to reduce the risk for CDI that originates in a health care facility.39 The IDSA guidelines do not make a recommendation regarding screening or isolation of asymptomatic carriers, so the decision is institution specific.11 These guidelines also recommend that patients presenting with similar infectious organisms be housed in the same room, if needed, to avoid cross-contamination to others or additional surfaces.11
For pediatric patients, testing recommendations vary by age. Testing is not generally recommended for neonates or infants ≤ 2 years of age with diarrhea because of the prevalence of colonization with C difficile.11 For children older than 2 years, testing for CDI is only recommended in the setting of prolonged or worsening diarrhea and if the patient has risk factors such as IBD, immunocompromised state, health care exposure, or recent antibiotic use.11 In addition, testing in this population should only be considered once other infectious and noninfectious causes of diarrhea have been excluded.11
Continue to: First-line treatment? Drug of choice has changed
First-line treatment? Drug of choice has changed
In 2018, the IDSA published new treatment guidelines that provide important updates from the 2010 guidelines.11 Chief among these was the elimination of metronidazole as a first-line therapy. Vancomycin or fidaxomicin are now recommended as first-line treatment options because of superior eradication of C difficile when compared with metronidazole.11 In the opinion of the authors, vancomycin should be considered the drug of choice because of cost. (See “The case for vancomycin.”)
SIDEBAR
The case for vancomycin
The majority of studies conducted prior to publication of the 2010 Infectious Diseases Society of America guidelines described numerically worse eradication rates of Clostridioides difficile infection (CDI) with metronidazole compared with vancomycin for all severities of infection, but statistical significance was not achieved. These studies also showed a nonsignificant increase in CDI recurrence with metronidazole.17,40,41
A 2005 systematic review demonstrated increased treatment failure rates with metronidazole.42 The rates of metronidazole discontinuation and transition to alternative options more than doubled in 2003-2004, to 25.7% of patients compared with 9.6% in earlier years.42 Metronidazole efficacy was further questioned in a prospective observational study conducted in 2005, in which only 50% of patients were cured after an initial course of treatment, while 28% had recurrence within 90 days.43
Vancomycin was found to be the superior treatment option to metronidazole and tolevamer in a 2014 randomized controlled trial.18 This study also demonstrated that vancomycin was the superior therapy when comparing treatment-naïve vs experienced patients and severity of CDI.18 A 2017 retrospective cohort study demonstrated decreased 30-day all-cause mortality for patients taking vancomycin vs metronidazole (adjusted relative risk = 0.86; 95% confidence interval, 0.74-0.98), although it should be noted that this difference was driven by those with severe CDI, and there was no statistically significant difference in mortality for patients with mild-to-moderate CDI.44
The results of these studies led to the recommendation of vancomycin over metronidazole as first-line pharmacotherapy for CDI in practice, despite the historical perspective that overutilization of oral vancomycin could potentially increase rates of vancomycinresistant Enterococcus.11
Metronidazole should only be used in the treatment of CDI as a lastresort medication because of cost or insurance coverage. Although the price of oral vancomycin is higher, favorable patient outcomes are substantially greater, and recent analyses have shown that vancomycin is actually more cost-effective than metronidazole as a result.24 Adverse effects for metronidazole include neurotoxicity, gastrointestinal discomfort, and disulfiram-like reaction.
Vancomycin does not harbor as many adverse effects because of extremely low systemic absorption when taken orally, but patients may experience gastrointestinal discomfort.45 While systemic exposure with oral administration of vancomycin is very low (< 1%), there have been case reports of nephrotoxicity and “red man syndrome” that are more typically seen with intravenous vancomycin.44
Given the low rate of systemic exposure, routine monitoring of renal function and serum drug levels is not usually necessary during oral vancomycin therapy. However, it may be appropriate to monitor renal function and serum levels of vancomycin in patients who have renal failure, have altered intestinal integrity, are age ≥ 65 years, or are receiving high doses of vancomycin.46
10-day vs 14-day treatment of CDI. Most studies for the treatment of CDI have used a 10-day regimen rather than increasing the duration to a 14-day regimen, and nearly all studies conducted have displayed high rates of symptom resolution at the end of 10 days of treatment.17,18 Thus, treatment duration beyond 10 days should only be considered for patients who continue to have symptoms or complications with CDI on Day 10 of treatment.
First recurrence. Metronidazole is no longer the recommended treatment for first recurrence of CDI treated initially with metronidazole; instead, a 10-day course of vancomycin should be used.11 For recurrent cases in patients initially treated with vancomycin, a tapered and pulsed regimen of vancomycin is recommended11:
- vancomycin PO 125 mg four times daily for 10 to 14 days followed by
- vancomycin PO 125 mg twice daily for 7 days, then
- vancomycin PO 125 mg once daily for 7 days, then
- vancomycin PO 125 mg every 2 to 3 days for 2 to 8 weeks.
Pediatric patients. The IDSA guidelines recommend use of metronidazole or vancomycin to treat an initial case or first recurrence of mild-to-moderate CDI in this population.11 Due to a lack of quality evidence, the drug of choice for initial treatment is inconclusive, so patient-specific factors and cost should be considered when choosing an agent.11 If not cost prohibitive, vancomycin should be the drug of choice for most cases of pediatric CDI, and for severe cases or multiple recurrences of CDI, vancomycin is clearly the drug of choice.
Recommended agents: A closer look
Oral vancomycin products. Vancocin, a capsule, and Firvanq, an oral solution, are 2 vancomycin products currently on the market for CDI. Although the capsules are a readily available treatment option, the cost of the full course of treatment can be a barrier for patients without insurance, or with high copays or deductibles (brand name, $4000; generic, $1252).19
Continue to: Historically, in an effort to keep costs down...
Historically, in an effort to keep costs down, an oral solution was often inexpensively compounded at hospitals or pharmacies.20
Fidaxomicin, an oral macrocyclic antibiotic with minimal systemic absorption, was first approved by the US Food and Drug Administration (FDA) for CDI in 2011.21 The IDSA guidelines recommend fidaxomicin for initial, and recurrent, cases of CDI as an alternative to vancomycin.11 This recommendation is based on 2 randomized double-blind trials comparing fidaxomicin to standard-dose oral vancomycin for initial or recurrent CDI.21,22
Pooled data from these 2 similar studies found that fidaxomicin was noninferior (10% noninferiority margin) to vancomycin for the primary outcome of clinical cure.23 Fidaxomicin was shown to be superior to vancomycin regarding rate of CDI recurrence (relative risk [RR] = 0.61; 95% confidence interval [CI], 0.43-0.87). These results were similar regardless of whether the CDI was an initial or recurrent case.23
Given the lack of systemic absorption, fidaxomicin is generally very well tolerated. The largest downside to fidaxomicin is its cost, which can be nearly $5000 for a standard 10-day course (vs as little as $165 for oral vancomycin).19 As a result, oral vancomycin solution is likely the most cost-effective therapy for initial cases of CDI.24 In patients with poor medication adherence, fidaxomicin offers the advantage of less-frequent dosing (twice daily vs 4 times daily with vancomycin).
For cases of recurrent CDI, when treatment failure occurred with vancomycin, fidaxomicin should be considered as an efficacious alternative. If fidaxomicin is used, it is advisable to verify coverage with the patient’s insurance plan, since prior authorization is frequently required.
Continue to: When meds fail, consider a fecal microbiota transplant
When meds fail, consider a fecal microbiota transplant
Another important change in the IDSA guidelines for CDI management is the strong recommendation for fecal microbiota transplantation (FMT) in patients with multiple recurrences of CDI for whom appropriate antibiotic treatment courses have failed.11,25 The goal of FMT is to “normalize” an abnormal gut microbiome by transplanting donor stool into a recipient.26
FMT has been shown to be highly effective in 5 randomized clinical trials conducted since 2013, with CDI cure rates between 85% and 94%.11 This rate of cure is particularly impressive given that the studies only included patients with refractory CDI.
Patients with recurrent CDI who may be candidates for FMT should be referred to a center or specialist with experience in FMT. These transplants can be expensive because of the screening process involved in obtaining donor samples. (Historically, a single FMT has cost $3000-$5000, and it is seldom covered by insurance.27) The emergence of universal stool banks offers a streamlined solution to this process.26
Fresh or frozen stool is considered equally effective in treating refractory CDI.26 Oral capsule and freeze-dried stool formulations have been studied, but their use is considered investigational at this time.26
Delivery via colonoscopy to the right colon is the preferred route of infusion; however, delivery via enema or nasogastric, nasojejunal, or nasoduodenal infusion can be considered as well.26
Continue to: In preparing for stool transplantation...
In preparing for stool transplantation, patients should be treated with standard doses of oral vancomycin or fidaxomicin for 3 days before the procedure to suppress intestinal C difficile, and the last dose of antibiotics should be given 12 to 48 hours before the procedure.26 Bowel lavage with polyethylene glycol is recommended, regardless of whether stool is delivered via colonoscopy or upper GI route.
Short-term adverse events associated with FMT appear to be minimal; data is lacking for long-term safety outcomes.28 While only recommended currently for cases of recurrent CDI, there is promising data emerging for use of FMT for severe cases, even without recurrence.29
The role of probiotics remains unclear
Probiotics have been explored in numerous trials to determine if they are effective in preventing CDI in patients who have been prescribed antibiotics.11 While no randomized trials have conclusively shown benefit, several meta-analyses have shown that the use of probiotics may result in a 60% to 65% relative risk reduction in CDI incidence.30,31
One proviso to these meta-analyses is that the incorporated studies have typically included patients at very high risk for CDI, and subanalyses have only found a reduction in CDI incidence when patients are at a very high baseline risk. In addition, there are many differences in probiotic types, formulations, treatment durations, and follow-up. As a result, the IDSA guidelines state that there is “insufficient data at this time” to recommend routine administration of probiotics for either primary or secondary CDI prophylaxis.11
Due to insufficient high-quality data, the IDSA guidelines do not provide a recommendation regarding use as an adjunct treatment option for acute CDI.11 Probiotics should not be routinely used to prevent CDI; however, they may provide benefit if reserved for patients at the highest risk for CDI (eg, history of CDI, prolonged use of broad-spectrum antibiotics, high local incidence).
Continue to: What about surgical intervention?
What about surgical intervention?
In severe cases of CDI, surgery may be necessary and can reduce mortality.32 The surgical procedure with the strongest recommendation in the IDSA guidelines is the subtotal colectomy, though the diverting loop ileostomy is an alternative option.11 Patients who may benefit from surgery include those with a WBC count ≥ 25,000; lactate > 5 mmol/L11; altered mental status; megacolon; perforation of the colon; acute abdomen on physical examination; or septic shock due to CDI.33 Although surgery can be beneficial, the mortality rate remains high for those with CDI who undergo colectomy.33
Reserve bezlotoxumab for prevention of recurrence
Bezlotoxumab, a human monoclonal immunoglobulin GI/kappa antibody, was approved by the FDA in 2016 for the prevention of recurrent CDI. Its mechanism of action is to bind and neutralize C difficile toxin B. It was approved as a single infusion for adults who are receiving active antibiotic therapy for CDI and are considered to be at high risk for recurrence.34
This approval was based on 2 trials of more than 2500 patients, in which participants received bezlotoxumab or placebo while receiving treatment for primary or recurrent CDI. The primary outcome of these studies was recurrent infection within 12 weeks after infusion, which was significantly lower for bezlotoxumab in both studies: 17% vs 28% (P < 0.001) in one trial and 16% vs 26% (P < 0.001) in the other trial.35
Bezlotoxumab should only be used as an adjunct to prevent recurrence.32 There is no recommendation for or against bezlotoxumab in the IDSA guidelines because of the recent date of the drug’s approval. Its frequency of use will likely depend on the number of patients who meet criteria as high risk for recurrence and its estimated cost of $4560 per dose.34,36
CASES
CASE 1: In light of Ms. O’s recent completion of a course of clindamycin and unremarkable lab work, she should be treated for mild-to-moderate CDI. She has no comorbid conditions to warrant fidaxomicin, and thus vancomycin (capsules or oral solution) would be the best treatment option. Ms. O is started on vancomycin PO 125 mg qid for 10 days. She is also advised to discontinue loperamide as soon as possible, based on poor outcomes data seen with the use of antimotility agents in CDI.37
Continue to: CASE 2
CASE 2: Ms. Z has several risk factors for recurrent CDI and has an elevated WBC count and SCr level (WBC ≥ 15,000 and SCr > 1.5 mg/dL). Thus, she is classified as having severe, recurrent CDI. Oral levofloxacin and metronidazole should be discontinued, because they increase the risk for treatment failure and development of more virulent CDI strains, such as BI/NAP1/027. Since Ms. Z used metronidazole for treatment of her initial CDI, vancomycin or fidaxomicin should be used at this time. Either vancomycin PO 125 mg qid for 10 days or fidaxomicin 200 mg bid for 10 days would be an appropriate regimen; however, because of cost and unknown insurance coverage, vancomycin is the most appropriate regimen.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, Saint Joseph Family Medicine Residency, 1000 E. University Avenue, Dept 3375, Laramie, WY 82071; jvandive@uwyo.edu
CASE 1
Beth O, a 63-year-old woman, presents to the emergency department (ED) with a 2-week history of diarrhea (6 very loose, watery stools per day) and lower abdominal pain. The patient denies any vomiting, sick contacts, or recent travel. Past medical history includes varicose veins. Her only active medication is loperamide, as needed, for the past 2 weeks. Ms. O also recently completed a 10-day course of clindamycin for an infected laceration on her finger.
Ms. O’s laboratory values are unremarkable, with a normal white blood cell (WBC) count and serum creatinine (SCr) level. Abdominal computed tomography (CT) reveals some abnormal bowel dilatation and a slight increase in colon wall thickness. There is a high suspicion for Clostridioides difficile (formerly Clostridium difficile) infection (CDI), and stool sent for polymerase chain reaction (PCR) testing comes back positive for C difficile toxin B. It is revealed to be a strain other than the BI/NAP1/027 epidemic strain (which has a higher mortality rate).
How should this patient be treated?
CASE 2
Sixty-eight-year-old Barbara Z presents to the ED from her skilled nursing facility with persistent diarrhea and abdominal cramping. She was diagnosed with CDI about 2 months ago and reports that her symptoms resolved within 4 to 5 days after starting a 14-day course of oral metronidazole.
Her past medical history is notable for multiple myeloma with bone metastasis, for which she is actively undergoing chemotherapy treatment. She also has chronic kidney disease (baseline SCr, 2.2 mg/dL), hypertension, and anemia of chronic disease. The patient’s medications include amlodipine and cholecalciferol. Her chemotherapy regimen consists of bortezomib, lenalidomide, and dexamethasone. CT of the abdomen shows diffuse colon wall thickening with surrounding inflammatory stranding—concerning for pancolitis. There is no evidence of toxic megacolon or ileus.
Ms. Z’s laboratory values are notable for a WBC count of 15,900 cells/mL and an SCr of 4.1 mg/dL. She is started on oral levofloxacin and metronidazole due to concern for an intra-abdominal infection. PCR testing is positive for C difficile, and an enzyme immunoassay (EIA) for C difficile toxin is positive.
What factors put Ms. Z at risk for C difficile, and how should she be treated?
Continue to: C difficile is one of the most...
C difficile is one of the most commonly reported pathogens in health care–associated infections and affects almost 1% of all hospitalized patients in the United States each year.1 From 2001 to 2010, the incidence of CDI doubled in patients discharged from hospitals,2 with an estimated cost of more than $5 billion annually.3 Furthermore, rates of community-associated CDI continue to increase and account for about 40% of cases.4
After colonization in the intestine, C difficile releases 2 toxins (TcdA and TcdB) that cause colitis.5 Patients may present with mild diarrhea that can progress to abdominal pain, cramping, fever, and leukocytosis. Fulminant CDI can lead to the formation of pseudomembranes in the colon, toxic megacolon, bowel perforation, shock, and death.2
Beginning in the early 2000s, hospitals reported increases in severe cases of CDI.6 A specific strain known as BI/NAP1/027 was identified and characterized by fluoroquinolone resistance, increased spore formation, and a higher mortality rate.6
Further complicating matters … Recurrent CDI occurs in up to 10% to 30% of patients,7 typically within 14 to 45 days of completion of antibiotic pharmacotherapy for CDI.8 Recurrence is characterized by new-onset diarrhea or abdominal symptoms after completion of treatment for CDI.5
It typically begins with an antibiotic
Risk factors for CDI are listed in TABLE 1.9 The most important modifiable risk factor for initial and recurrent CDI is recent use of antibiotics.10 Most antibiotics can disrupt normal intestinal flora, causing colonization of C difficile, but the strongest association seems to be with third- and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and clindamycin.11 The risk for CDI occurs during antibiotic treatment, as well as up to 3 months after completion of antibiotic therapy.7 Exposure to multiple antibiotics and extended duration of antibacterial therapy can greatly increase the risk for CDI, so antimicrobial stewardship is key.11
Continue to: Continuing antibiotics while attempting...
Continuing antibiotics while attempting to treat CDI reduces the patient’s clinical response to CDI treatment, which can lead to recurrence.12 The Infectious Diseases Society of America (IDSA) guidelines include a strong recommendation to discontinue concurrent antibiotics as soon as possible in these scenarios.11
Acid-suppression therapy has also been associated with CDI. The mechanism is thought to be an interruption in the protection provided by stomach acid, and use over time may reduce the diversity of flora within the gut microbiome.13 The data demonstrating an association between acid-suppression therapy and CDI is conflicting, which may be a result of confounding factors such as the severity of CDI illness and diarrhea induced by use of proton pump inhibitors (PPIs).4 IDSA guidelines do not provide a recommendation regarding discontinuation of PPI therapy for the prevention of CDI, although inappropriate PPI therapy should always be discontinued.11
Advanced age is an important nonmodifiable risk factor for CDI. Older adults who live in long-term care facilities are at a higher risk for CDI, and these facilities have colonization rates as high as 50%.12
Community-associated risk. In an analysis of community-associated cases of CDI, 82% of patients reported some sort of health care exposure (ranging from physician office visit to surgery admission), 64% reported the receipt of antimicrobial therapy, and 31% reported the use of PPIs.14 Inflammatory bowel disease (IBD) may also put community dwellers at higher risk for CDI and its complications.15
CASES 1 & 2
Both CASE patients have risk factors for CDI. Ms. O (CASE 1) is likely at risk for CDI after completion of her recent course of clindamycin. Ms. Z (CASE 2) has several risk factors for recurrent CDI, including advanced age (≥ 65 years), residence in a long-term care facility, prior antibiotic exposure, and immunodeficiency because of chemotherapy/steroid use.
Continue to: Diagnosis
Diagnosis: Who and how to test
CDI should be both a clinical and laboratory-confirmed diagnosis. Patients should be tested for CDI if they have 3 or more episodes of unexplainable, new-onset unformed stools in 24 hours.11 Asymptomatic patients should not be tested to avoid unnecessary testing and treatment of those who are colonized but not infected.11 It is not recommended to routinely test patients who have taken laxatives within the previous 48 hours.11
There are several stool-based laboratory test options for the diagnosis of CDI (TABLE 211,12,16) but no definitive recommendation for all institutions.11 Many institutions have now implemented PCR testing for the diagnosis of CDI. However, while the benefits of this test include reduced need for repeat testing and possible identification of carriers, it’s estimated that reports of CDI increase more than 50% when an institution switches to PCR testing.1 Nonetheless, a one-step, highly sensitive test such as PCR may be used if strict criteria are implemented and followed.
The increase in positive PCR tests has prompted evaluation of using another test in addition to or in place of PCR. Multistep testing options include a glutamate dehydrogenase assay (GDH) with a toxin EIA, GDH with a toxin EIA and final decision via PCR, or PCR with toxin EIA.11 Use of a multistep diagnostic algorithm may increase overall specificity up to 100%, which may improve determination of asymptomatic colonization vs active infection.16 (Patients who have negative toxin results with positive PCR likely have colonization but not infection and often do not require treatment.) IDSA guidelines recommend that the stool toxin test should be part of a multistep algorithm for diagnosis, rather than PCR alone, if strict criteria are not implemented for stool test submission.11
There is no need to perform a test of cure after a patient has been treated for CDI, and no repeat testing should be performed within 7 days of the previous test.11 After successful treatment, patients will continue to shed spores and test positively via PCR for weeks to months.11 When patients have a positive PCR test, there are several important infection control efforts that institutions should consider; see “IDSA weighs in on measures to combat C difficile.”
SIDEBAR
IDSA weighs in on measures to combat C difficile
The spores produced by Clostridioides difficile can survive for 5 months or longer on dry surfaces because of resistance to heat, acid, antibiotics, and many cleaning products.38 Unfortunately, spores transmitted from health care workers and the environment are the most likely cause of infection spreading in health care institutions. To prevent transmission of C difficile infection (CDI) throughout institutions, appropriate infection control measures are necessary.
Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that patients with CDI be isolated to a private room with a dedicated toilet. Health care staff should wear gloves and gowns when entering the room of, or taking care of, a patient with CDI. For patients who are suspected of having CDI, contact precautions should be implemented while awaiting test results. When the diagnosis is confirmed, contact precautions should remain in place for at least 48 hours after resolution of diarrhea but may be continued until discharge.11
Practicing good hand hygiene is essential, especially in institutions with high rates of CDI or if fecal contamination is likely.11 Hand hygiene with soap and water is preferred, due to evidence of a higher spore removal rate, but alcohol-based alternatives may be used if necessary.11 In institutions with high rates of CDI, terminal (post-discharge) cleaning of rooms with a sporicidal agent should be considered.11
Asymptomatic carriers are also a concern for transmission of CDI in institutional settings. Screening and isolating patients who are carriers may prevent transmission, and some institutions have implemented this process to reduce the risk for CDI that originates in a health care facility.39 The IDSA guidelines do not make a recommendation regarding screening or isolation of asymptomatic carriers, so the decision is institution specific.11 These guidelines also recommend that patients presenting with similar infectious organisms be housed in the same room, if needed, to avoid cross-contamination to others or additional surfaces.11
For pediatric patients, testing recommendations vary by age. Testing is not generally recommended for neonates or infants ≤ 2 years of age with diarrhea because of the prevalence of colonization with C difficile.11 For children older than 2 years, testing for CDI is only recommended in the setting of prolonged or worsening diarrhea and if the patient has risk factors such as IBD, immunocompromised state, health care exposure, or recent antibiotic use.11 In addition, testing in this population should only be considered once other infectious and noninfectious causes of diarrhea have been excluded.11
Continue to: First-line treatment? Drug of choice has changed
First-line treatment? Drug of choice has changed
In 2018, the IDSA published new treatment guidelines that provide important updates from the 2010 guidelines.11 Chief among these was the elimination of metronidazole as a first-line therapy. Vancomycin or fidaxomicin are now recommended as first-line treatment options because of superior eradication of C difficile when compared with metronidazole.11 In the opinion of the authors, vancomycin should be considered the drug of choice because of cost. (See “The case for vancomycin.”)
SIDEBAR
The case for vancomycin
The majority of studies conducted prior to publication of the 2010 Infectious Diseases Society of America guidelines described numerically worse eradication rates of Clostridioides difficile infection (CDI) with metronidazole compared with vancomycin for all severities of infection, but statistical significance was not achieved. These studies also showed a nonsignificant increase in CDI recurrence with metronidazole.17,40,41
A 2005 systematic review demonstrated increased treatment failure rates with metronidazole.42 The rates of metronidazole discontinuation and transition to alternative options more than doubled in 2003-2004, to 25.7% of patients compared with 9.6% in earlier years.42 Metronidazole efficacy was further questioned in a prospective observational study conducted in 2005, in which only 50% of patients were cured after an initial course of treatment, while 28% had recurrence within 90 days.43
Vancomycin was found to be the superior treatment option to metronidazole and tolevamer in a 2014 randomized controlled trial.18 This study also demonstrated that vancomycin was the superior therapy when comparing treatment-naïve vs experienced patients and severity of CDI.18 A 2017 retrospective cohort study demonstrated decreased 30-day all-cause mortality for patients taking vancomycin vs metronidazole (adjusted relative risk = 0.86; 95% confidence interval, 0.74-0.98), although it should be noted that this difference was driven by those with severe CDI, and there was no statistically significant difference in mortality for patients with mild-to-moderate CDI.44
The results of these studies led to the recommendation of vancomycin over metronidazole as first-line pharmacotherapy for CDI in practice, despite the historical perspective that overutilization of oral vancomycin could potentially increase rates of vancomycinresistant Enterococcus.11
Metronidazole should only be used in the treatment of CDI as a lastresort medication because of cost or insurance coverage. Although the price of oral vancomycin is higher, favorable patient outcomes are substantially greater, and recent analyses have shown that vancomycin is actually more cost-effective than metronidazole as a result.24 Adverse effects for metronidazole include neurotoxicity, gastrointestinal discomfort, and disulfiram-like reaction.
Vancomycin does not harbor as many adverse effects because of extremely low systemic absorption when taken orally, but patients may experience gastrointestinal discomfort.45 While systemic exposure with oral administration of vancomycin is very low (< 1%), there have been case reports of nephrotoxicity and “red man syndrome” that are more typically seen with intravenous vancomycin.44
Given the low rate of systemic exposure, routine monitoring of renal function and serum drug levels is not usually necessary during oral vancomycin therapy. However, it may be appropriate to monitor renal function and serum levels of vancomycin in patients who have renal failure, have altered intestinal integrity, are age ≥ 65 years, or are receiving high doses of vancomycin.46
10-day vs 14-day treatment of CDI. Most studies for the treatment of CDI have used a 10-day regimen rather than increasing the duration to a 14-day regimen, and nearly all studies conducted have displayed high rates of symptom resolution at the end of 10 days of treatment.17,18 Thus, treatment duration beyond 10 days should only be considered for patients who continue to have symptoms or complications with CDI on Day 10 of treatment.
First recurrence. Metronidazole is no longer the recommended treatment for first recurrence of CDI treated initially with metronidazole; instead, a 10-day course of vancomycin should be used.11 For recurrent cases in patients initially treated with vancomycin, a tapered and pulsed regimen of vancomycin is recommended11:
- vancomycin PO 125 mg four times daily for 10 to 14 days followed by
- vancomycin PO 125 mg twice daily for 7 days, then
- vancomycin PO 125 mg once daily for 7 days, then
- vancomycin PO 125 mg every 2 to 3 days for 2 to 8 weeks.
Pediatric patients. The IDSA guidelines recommend use of metronidazole or vancomycin to treat an initial case or first recurrence of mild-to-moderate CDI in this population.11 Due to a lack of quality evidence, the drug of choice for initial treatment is inconclusive, so patient-specific factors and cost should be considered when choosing an agent.11 If not cost prohibitive, vancomycin should be the drug of choice for most cases of pediatric CDI, and for severe cases or multiple recurrences of CDI, vancomycin is clearly the drug of choice.
Recommended agents: A closer look
Oral vancomycin products. Vancocin, a capsule, and Firvanq, an oral solution, are 2 vancomycin products currently on the market for CDI. Although the capsules are a readily available treatment option, the cost of the full course of treatment can be a barrier for patients without insurance, or with high copays or deductibles (brand name, $4000; generic, $1252).19
Continue to: Historically, in an effort to keep costs down...
Historically, in an effort to keep costs down, an oral solution was often inexpensively compounded at hospitals or pharmacies.20
Fidaxomicin, an oral macrocyclic antibiotic with minimal systemic absorption, was first approved by the US Food and Drug Administration (FDA) for CDI in 2011.21 The IDSA guidelines recommend fidaxomicin for initial, and recurrent, cases of CDI as an alternative to vancomycin.11 This recommendation is based on 2 randomized double-blind trials comparing fidaxomicin to standard-dose oral vancomycin for initial or recurrent CDI.21,22
Pooled data from these 2 similar studies found that fidaxomicin was noninferior (10% noninferiority margin) to vancomycin for the primary outcome of clinical cure.23 Fidaxomicin was shown to be superior to vancomycin regarding rate of CDI recurrence (relative risk [RR] = 0.61; 95% confidence interval [CI], 0.43-0.87). These results were similar regardless of whether the CDI was an initial or recurrent case.23
Given the lack of systemic absorption, fidaxomicin is generally very well tolerated. The largest downside to fidaxomicin is its cost, which can be nearly $5000 for a standard 10-day course (vs as little as $165 for oral vancomycin).19 As a result, oral vancomycin solution is likely the most cost-effective therapy for initial cases of CDI.24 In patients with poor medication adherence, fidaxomicin offers the advantage of less-frequent dosing (twice daily vs 4 times daily with vancomycin).
For cases of recurrent CDI, when treatment failure occurred with vancomycin, fidaxomicin should be considered as an efficacious alternative. If fidaxomicin is used, it is advisable to verify coverage with the patient’s insurance plan, since prior authorization is frequently required.
Continue to: When meds fail, consider a fecal microbiota transplant
When meds fail, consider a fecal microbiota transplant
Another important change in the IDSA guidelines for CDI management is the strong recommendation for fecal microbiota transplantation (FMT) in patients with multiple recurrences of CDI for whom appropriate antibiotic treatment courses have failed.11,25 The goal of FMT is to “normalize” an abnormal gut microbiome by transplanting donor stool into a recipient.26
FMT has been shown to be highly effective in 5 randomized clinical trials conducted since 2013, with CDI cure rates between 85% and 94%.11 This rate of cure is particularly impressive given that the studies only included patients with refractory CDI.
Patients with recurrent CDI who may be candidates for FMT should be referred to a center or specialist with experience in FMT. These transplants can be expensive because of the screening process involved in obtaining donor samples. (Historically, a single FMT has cost $3000-$5000, and it is seldom covered by insurance.27) The emergence of universal stool banks offers a streamlined solution to this process.26
Fresh or frozen stool is considered equally effective in treating refractory CDI.26 Oral capsule and freeze-dried stool formulations have been studied, but their use is considered investigational at this time.26
Delivery via colonoscopy to the right colon is the preferred route of infusion; however, delivery via enema or nasogastric, nasojejunal, or nasoduodenal infusion can be considered as well.26
Continue to: In preparing for stool transplantation...
In preparing for stool transplantation, patients should be treated with standard doses of oral vancomycin or fidaxomicin for 3 days before the procedure to suppress intestinal C difficile, and the last dose of antibiotics should be given 12 to 48 hours before the procedure.26 Bowel lavage with polyethylene glycol is recommended, regardless of whether stool is delivered via colonoscopy or upper GI route.
Short-term adverse events associated with FMT appear to be minimal; data is lacking for long-term safety outcomes.28 While only recommended currently for cases of recurrent CDI, there is promising data emerging for use of FMT for severe cases, even without recurrence.29
The role of probiotics remains unclear
Probiotics have been explored in numerous trials to determine if they are effective in preventing CDI in patients who have been prescribed antibiotics.11 While no randomized trials have conclusively shown benefit, several meta-analyses have shown that the use of probiotics may result in a 60% to 65% relative risk reduction in CDI incidence.30,31
One proviso to these meta-analyses is that the incorporated studies have typically included patients at very high risk for CDI, and subanalyses have only found a reduction in CDI incidence when patients are at a very high baseline risk. In addition, there are many differences in probiotic types, formulations, treatment durations, and follow-up. As a result, the IDSA guidelines state that there is “insufficient data at this time” to recommend routine administration of probiotics for either primary or secondary CDI prophylaxis.11
Due to insufficient high-quality data, the IDSA guidelines do not provide a recommendation regarding use as an adjunct treatment option for acute CDI.11 Probiotics should not be routinely used to prevent CDI; however, they may provide benefit if reserved for patients at the highest risk for CDI (eg, history of CDI, prolonged use of broad-spectrum antibiotics, high local incidence).
Continue to: What about surgical intervention?
What about surgical intervention?
In severe cases of CDI, surgery may be necessary and can reduce mortality.32 The surgical procedure with the strongest recommendation in the IDSA guidelines is the subtotal colectomy, though the diverting loop ileostomy is an alternative option.11 Patients who may benefit from surgery include those with a WBC count ≥ 25,000; lactate > 5 mmol/L11; altered mental status; megacolon; perforation of the colon; acute abdomen on physical examination; or septic shock due to CDI.33 Although surgery can be beneficial, the mortality rate remains high for those with CDI who undergo colectomy.33
Reserve bezlotoxumab for prevention of recurrence
Bezlotoxumab, a human monoclonal immunoglobulin GI/kappa antibody, was approved by the FDA in 2016 for the prevention of recurrent CDI. Its mechanism of action is to bind and neutralize C difficile toxin B. It was approved as a single infusion for adults who are receiving active antibiotic therapy for CDI and are considered to be at high risk for recurrence.34
This approval was based on 2 trials of more than 2500 patients, in which participants received bezlotoxumab or placebo while receiving treatment for primary or recurrent CDI. The primary outcome of these studies was recurrent infection within 12 weeks after infusion, which was significantly lower for bezlotoxumab in both studies: 17% vs 28% (P < 0.001) in one trial and 16% vs 26% (P < 0.001) in the other trial.35
Bezlotoxumab should only be used as an adjunct to prevent recurrence.32 There is no recommendation for or against bezlotoxumab in the IDSA guidelines because of the recent date of the drug’s approval. Its frequency of use will likely depend on the number of patients who meet criteria as high risk for recurrence and its estimated cost of $4560 per dose.34,36
CASES
CASE 1: In light of Ms. O’s recent completion of a course of clindamycin and unremarkable lab work, she should be treated for mild-to-moderate CDI. She has no comorbid conditions to warrant fidaxomicin, and thus vancomycin (capsules or oral solution) would be the best treatment option. Ms. O is started on vancomycin PO 125 mg qid for 10 days. She is also advised to discontinue loperamide as soon as possible, based on poor outcomes data seen with the use of antimotility agents in CDI.37
Continue to: CASE 2
CASE 2: Ms. Z has several risk factors for recurrent CDI and has an elevated WBC count and SCr level (WBC ≥ 15,000 and SCr > 1.5 mg/dL). Thus, she is classified as having severe, recurrent CDI. Oral levofloxacin and metronidazole should be discontinued, because they increase the risk for treatment failure and development of more virulent CDI strains, such as BI/NAP1/027. Since Ms. Z used metronidazole for treatment of her initial CDI, vancomycin or fidaxomicin should be used at this time. Either vancomycin PO 125 mg qid for 10 days or fidaxomicin 200 mg bid for 10 days would be an appropriate regimen; however, because of cost and unknown insurance coverage, vancomycin is the most appropriate regimen.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, Saint Joseph Family Medicine Residency, 1000 E. University Avenue, Dept 3375, Laramie, WY 82071; jvandive@uwyo.edu
1. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792-1801.
2. Reveles KR, Lee GC, Boyd NK, et al. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028-1032.
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88-S92.
4. Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:784-791.
5. Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37:178-187.
6. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924.
7. Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C difficile infection. JAMA. 2009;301:954-962.
8. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
9. Napolitano LM, Edmiston CE Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery 2017;162:325-348.
10. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452-460.
11. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48.
12. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498; quiz 499.
13. Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
14. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359-1367.
15. Negrón ME, Rezaie A, Barkema HW, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study. Am J Gastroenterol. 2016;111:691-704.
16. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408.
17. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
18. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354.
19. Vancomycin: product details. Redbook Online. www.micromedexsolutions.com. Published 2018. Accessed June 13, 2020.
20. Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics. 2014;32:639-650.
21. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
22. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281-289.
23. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55 suppl 2:S93-103.
24. Ford DC, Schroeder MC, Ince D, et al. Cost-effectiveness analysis of initial treatment strategies for mild-to-moderate Clostridium difficile infection in hospitalized patients. Am J Health Syst Pharm. 2018;75:1110-1121.
25. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
26. Panchal P, Budree S, Scheeler A, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. 2018;20:14.
27. Arbel LT, Hsu E, McNally K. Cost-effectiveness of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection: a literature review. Cureus. 2017;9:e1599.
28. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580.
29. Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66:645-650.
30. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
31. Johnston BC, Lytvyn L, Lo CK, et al. Microbial preparations (probiotics) for the prevention of Clostridium difficile infection in adults and children: an individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018:1-11.
32. Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013;15:798-804.
33. Julien M, Wild JL, Blansfield J, et al. Severe complicated Clostridium difficile infection: can the UPMC proposed scoring system predict the need for surgery? J Trauma Acute Care Surg. 2016;81:221-228.
34. Merck & Co, Inc. Sharp M. ZinplavaTM (bezlotoxumab [package insert] US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Revised October 2016. Accessed May 29, 2020.
35. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-317.
36. Chahine EB, Cho JC, Worley MV. Bezlotoxumab for the Prevention of Clostridium difficile recurrence. Consult Pharm. 2018;33:89-97.
37. Koo HL, Koo DC, Musher DM, et al. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598-605.
38. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526-536.
39. Longtin Y, Paquet-Bolduc B, Gilca R, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176:796-804.
40. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-1046.
41. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
42. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
43. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
44. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177:546-553.
45. CutisPharma. FirvanqTM (vancomycin hydrochloride) for oral solution [package insert]. US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208910s000lbl.pdf. Revised January 2018. Accessed May 29, 2020.
46.
1. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792-1801.
2. Reveles KR, Lee GC, Boyd NK, et al. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028-1032.
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88-S92.
4. Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:784-791.
5. Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37:178-187.
6. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924.
7. Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C difficile infection. JAMA. 2009;301:954-962.
8. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
9. Napolitano LM, Edmiston CE Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery 2017;162:325-348.
10. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452-460.
11. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48.
12. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498; quiz 499.
13. Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
14. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359-1367.
15. Negrón ME, Rezaie A, Barkema HW, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study. Am J Gastroenterol. 2016;111:691-704.
16. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408.
17. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
18. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354.
19. Vancomycin: product details. Redbook Online. www.micromedexsolutions.com. Published 2018. Accessed June 13, 2020.
20. Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics. 2014;32:639-650.
21. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
22. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281-289.
23. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55 suppl 2:S93-103.
24. Ford DC, Schroeder MC, Ince D, et al. Cost-effectiveness analysis of initial treatment strategies for mild-to-moderate Clostridium difficile infection in hospitalized patients. Am J Health Syst Pharm. 2018;75:1110-1121.
25. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
26. Panchal P, Budree S, Scheeler A, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. 2018;20:14.
27. Arbel LT, Hsu E, McNally K. Cost-effectiveness of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection: a literature review. Cureus. 2017;9:e1599.
28. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580.
29. Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66:645-650.
30. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
31. Johnston BC, Lytvyn L, Lo CK, et al. Microbial preparations (probiotics) for the prevention of Clostridium difficile infection in adults and children: an individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018:1-11.
32. Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013;15:798-804.
33. Julien M, Wild JL, Blansfield J, et al. Severe complicated Clostridium difficile infection: can the UPMC proposed scoring system predict the need for surgery? J Trauma Acute Care Surg. 2016;81:221-228.
34. Merck & Co, Inc. Sharp M. ZinplavaTM (bezlotoxumab [package insert] US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Revised October 2016. Accessed May 29, 2020.
35. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-317.
36. Chahine EB, Cho JC, Worley MV. Bezlotoxumab for the Prevention of Clostridium difficile recurrence. Consult Pharm. 2018;33:89-97.
37. Koo HL, Koo DC, Musher DM, et al. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598-605.
38. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526-536.
39. Longtin Y, Paquet-Bolduc B, Gilca R, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176:796-804.
40. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-1046.
41. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
42. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
43. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
44. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177:546-553.
45. CutisPharma. FirvanqTM (vancomycin hydrochloride) for oral solution [package insert]. US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208910s000lbl.pdf. Revised January 2018. Accessed May 29, 2020.
46.
PRACTICE RECOMMENDATIONS
› Keep in mind that previous exposure to antibiotics is the most important risk factor for initial and recurrent Clostridioides difficile infection (CDI). Thus, appropriate antimicrobial stewardship is key to prevention. C
› Begin with vancomycin or fidaxomicin (over metronidazole) for first-line treatment of CDI in adults. A
› Consider fecal microbiota transplantation in high-risk patients with recurrent CDI for whom antimicrobial therapy has failed. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Dual antiplatelet Tx for stroke prevention: Worth the risk?
The incidence of ischemic stroke in the United States is estimated to be more than 795,000 events each year.1 After an initial stroke, the rate of recurrence is 5% to 20% within the first year, with the greatest prevalence in the first 90 days following an event.2-5 Although dual antiplatelet therapy, often with aspirin and a P2Y12 inhibitor such as clopidogrel, reduces the risk for recurrent cardiovascular events, cerebrovascular events, and death following acute coronary syndromes and percutaneous intervention, the role of combination antiplatelet therapy for secondary prevention of ischemic stroke continues to be debated.6 Reconciling currently available data can be challenging, as many studies vary considerably in both the time to antiplatelet initiation and duration of therapy.
For many years, aspirin alone was the drug of choice for secondary prevention of noncardioembolic ischemic stroke.7 Efficacy is similar at dosages anywhere between 50 and 1500 mg/d; higher doses incur a greater risk for gastrointestinal hemorrhage.7 Current secondary prevention guidelines recommend a dosage of aspirin somewhere between 50 and 325 mg/d.7
Alternative agents have also been evaluated for secondary stroke prevention, but only clopidogrel is currently considered an acceptable alternative for monotherapy based on a subgroup analysis of the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial.7,8 Other alternatives, including cilostazol, ticlopidine, and ticagrelor, are limited by a lack of data, adverse drug reactions, or unproven efficacy and are not recommended in current guidelines.7,9 The ongoing THALES (Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death) trial, assessing combination ticagrelor and aspirin, may identify an additional option for antiplatelet therapy following acute stroke.10
The current guidelines from the American Heart Association/American Stroke Association (AHA/ASA) support the combination of aspirin and extended-release dipyridamole (ASA-ERDP) as a long-term alternative to aspirin monotherapy.7,11 Additionally, the combination of clopidogrel and aspirin (CLO-ASA) is now recommended for limited duration in the early management of ischemic stroke.11
This review will explore the role of dual antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke or transient ischemic attack (TIA), with particular focus on acute use of CLO-ASA.
Clopidogrel and aspirin: When to initiate, when to stop
The combined use of clopidogrel and aspirin has been well-studied for secondary prevention of ischemic stroke and TIA. However, interpreting and applying the results of these trials can be challenging given key differences in both time to treatment initiation and the duration of combination therapy. Highlights of the major randomized controlled trials (RCTs) evaluating the safety and efficacy of CLO-ASA are detailed in TABLE 1.4,5,12-15
Initial trials evaluating CLO-ASA for secondary stroke prevention, including the MATCH (Management of ATherothrombosis with Clopidogrel in High-risk patients),12 SPS3 (Secondary Prevention of Small Subcortical Strokes),13 and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance)14 trials assessed the long-term benefits of combination therapy, with most patients initiating treatment a month or more following an initial stroke and continuing therapy for at least 18 months.12-14 Results from these trials indicate that long-term use (> 18 months) of CLO-ASA does not reduce recurrent events but increases rates of clinically significant bleeding.12-14
Continue to: A look at Tx timing
A look at Tx timing. Since these initial attempts failed to show a long-term benefit with CLO-ASA, subsequent trials attempted to establish an appropriate balance between the optimal time to initiate CLO-ASA and the optimal duration of therapy. The FASTER (Fast Assessment of Stroke and Transient ischaemic attack to prevent Early Recurrence) trial was a small pilot study of 392 patients randomized to CLO-ASA or aspirin within 24 hours of stroke or TIA onset and continued for only 3 months.15 While this trial did not find a significant reduction in ischemic or hemorrhagic stroke with combination therapy, there was a large numerical difference in event rates between the 2 groups (7.1% CLO-ASA vs 10.8% aspirin).15 An underpowered sample size (due to difficulty recruiting participants) is likely responsible for the lack of statistical significance.15 Despite the trial’s failure to show a benefit with acute use of CLO-ASA, it suggested a possible benefit that led to further investigation in the CHANCE (Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events)5 and POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) 4 trials.
The CHANCE trial conducted in China included more than 5000 patients with acute minor ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 3) or high-risk TIA (ABCD2 [a scale that assesses the risk of stroke on the basis of age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes] score ≥ 4).5 Similar to FASTER, patients were randomized within 24 hours of symptom onset to CLO-ASA or aspirin. However, CHANCE utilized combination therapy for only 21 days, after which the patients were continued on clopidogrel monotherapy for up to 90 days; the aspirin monotherapy group continued aspirin for 90 days.
After 90 days, patients initially using combination therapy had significantly lower rates of ischemic or hemorrhagic stroke vs those assigned to aspirin monotherapy. This result was driven heavily by the reduction in ischemic stroke (7.9% CLO-ASA vs 11.4% aspirin; P < .001). Additionally, there was no significant difference in moderate or severe bleeding events between the 2 groups.5 Efficacy and safety results were similar among a subgroup of patients who were randomized to treatment within 12 hours rather than 24 hours from symptom onset.16 The CHANCE trial was the first major study to demonstrate a clinical benefit of CLO-ASA to prevent recurrent stroke. Accordingly, the 2018 AHA/ASA guidelines included a new recommendation regarding secondary prevention for the use of CLO-ASA initiated within 24 hours and continued for 21 days following a minor stroke or TIA.11
A drawback of the CHANCE trial was its narrow patient population of only Chinese patients, which may limit applicability in clinical practice. There are known genetic variations in cytochrome P450 2C19 (CYP2C19) that may affect clopidogrel metabolism. CYP2C19 is responsible for the conversion of clopidogrel into its activated form in vivo. Carriers of a CYP2C19 loss-of-function allele may have reduced clopidogrel activation and subsequent reduced antiplatelet activity. Such loss-of-function alleles are more common in Asian populations vs non-Asian populations.17
A substudy of CHANCE found that CLO-ASA’s efficacy benefit was preserved in noncarrier patients; however, patients with the CYP2C19 loss-of-function allele did not benefit from combination therapy.18 Interestingly, these genetic differences did not affect bleeding outcomes. Given that approximately 60% of patients in the CHANCE substudy were loss-of-function allele carriers and that the overall study results still showed benefit with combination therapy, application of CHANCE’s findings to broader populations may not be a concern after all.18
Continue to: In efforts to gain insight...
In efforts to gain insight on CLO-ASA’s use in a more diverse patient population, the POINT trial included almost 5000 patients, with 82% from the United States, who were randomized within 12 hours of symptom onset to CLO-ASA or aspirin monotherapy for 90 days.4 Similar to the CHANCE study, the POINT study included patients with mild ischemic strokes (NIHSS ≤ 3) or high-risk TIA (ABCD2 ≥ 4). Combination therapy significantly reduced the primary endpoint of ischemic stroke, myocardial infarction (MI), or death from an ischemic event. Contrary to CHANCE, there was a significant increase in major bleeding in those assigned to combination therapy, which resulted in the trial being stopped early.4
A closer look at safety differences. CHANCE and POINT were the first major trials to show a benefit of CLO-ASA for secondary prevention of stroke, yet their differences in safety outcomes, specifically major hemorrhage, argued for a deeper reconciliation of their results.4,5 While both trials initiated secondary prevention within 24 hours of symptom onset, the difference in duration of combination therapy (21 days in CHANCE vs 90 days in POINT) likely impacted the rates of hemorrhage. When results from POINT were stratified by time period, particularly within the first 30 days of therapy (similar to the 21-day treatment duration of CHANCE), combination therapy significantly reduced the primary endpoint of ischemic stroke, MI, or death from an ischemic event (3.9% CLO-ASA vs 5.8% aspirin; P = .02) without an increased risk for major hemorrhage. Between 30 and 90 days, this efficacy benefit disappeared. However, bleeding rates between groups continued to separate throughout the 90-day course. In this light, the 30-day outcomes of POINT are largely similar to CHANCE and support the short-term use of CLO-ASA for secondary prevention without an associated increase in major bleeding.4,5
Antiplatelet dosing in POINT and CHANCE may also play a role in the contrasting safety results between the trials.4,5 While both studies utilized clopidogrel loading doses, POINT used 600 mg while CHANCE used 300 mg. Clopidogrel maintenance dosing was the same at 75 mg/d. In CHANCE, aspirin dosing was protocolized to 75 mg/d; however, in POINT, 31% of patients used > 100 mg/d aspirin.4,5 It is possible that the higher doses of both aspirin and clopidogrel in the POINT trial contributed to the difference in the occurrence of major hemorrhage between the treatment groups in these trials.
The takeaway. Based on currently available data, patients who are best suited to benefit from CLO-ASA are those who have had minor noncardioembolic ischemic strokes or high-risk TIAs.4,5,11 Clopidogrel should be given as a 300-mg loading dose followed by 75 mg/d given concomitantly with aspirin at a dose no higher than 100 mg/d. CLO-ASA therapy should be initiated within 24 hours of symptom onset and be continued for no longer than 1 month, after which chronic preventive therapy with either aspirin or clopidogrel monotherapy should be started.4,5,11
Dipyridamole and aspirin: A controversial option
Since the approval of the combination product ASA-ERDP, there has been considerable controversy about using this combination over other therapies, such as aspirin or clopidogrel, for recurrent ischemic stroke prevention. Much of this controversy arises from limitations in the trial designs.
Continue to: The first trial to show benefit...
The first trial to show benefit with ASA-ERDP was ESPS2 (European Stroke Prevention Study 2), which demonstrated superiority of the combination over placebo in reducing recurrent stroke when treatment was added within 3 months of an index stroke.19 A few studies have evaluated ASA-ERDP compared to aspirin monotherapy; however, most of these studies were small and did not show any difference in outcomes.20 Only ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)21 carried significant weight in a 2013 meta-analysis, which showed a significant reduction in recurrent events with the combination product compared to aspirin monotherapy.20
Both the ESPS2 and ESPRIT trials had significant limitations.19,21 Patients in both studies had vascular comorbidities including atherosclerotic cardiovascular disease (ASCVD); however, pharmacotherapies designated to treat these diseases were not mentioned in the demographic data, nor were these medications taken into consideration to limit potential bias.19,21 Retrospectively, a significant proportion of aspirin doses utilized as a control in ESPRIT were inferior to the guideline-recommended dosing with 42% to 46% of patients receiving 30 mg/d.21 Despite these controversies, ASA-ERDP is still considered an alternative to aspirin monotherapy in the guidelines.7
The timing of ASA-ERDP initiation appears to be inversely related to the efficacy of the combination over therapeutic alternatives. Studies in which the therapy was initiated 3 to 6 months from the index stroke indicated favorable outcomes for the combination when compared to ASA or ERDP monotherapy.19,21 Studies utilizing early initiation (ie, within 24 or 48 hours of the index event) or even within 3 weeks showed no difference in outcomes; however, this may be due in part to the use of clopidogrel or other combination antiplatelet therapy as active comparators.22-24
Early initiation of ASA-ERDP also demonstrated a higher risk of major and intracranial bleeding compared to clopidogrel.22 Additionally, use of triple therapy with ASA-ERDP plus clopidogrel increased bleeding events without improving efficacy.24 More recent studies of ASA-ERDP are focusing on earlier initiation of therapy; it is unknown whether the benefits of late initiation will be confirmed in future studies. Highlights of the major RCTs evaluating the safety and efficacy of ASA-ERDP are detailed in TABLE 219,21-24.
The takeaway. Methodological issues and potential confounding factors in many of the key trials for ASA-ERDP make it challenging to fully discern the role that ASA-ERDP may play in the secondary prevention of stroke. Further evidence utilizing appropriate controls, timing, and assessment of confounders is needed. Additionally, ASA-ERDP is plagued by tolerability issues such as headache, nausea, and vomiting, leading to higher rates of discontinuation than its comparators in clinical trials. Accordingly, the maintenance use of ASA-ERDP for secondary stroke prevention may be considered less preferred than other recommended alternatives such as aspirin or clopidogrel monotherapies.
CORRESPONDENCE
Robert S. Helmer, PharmD, BCPS, Department of Pharmacy Practice, Auburn University Harrison School of Pharmacy, 650 Clinic Drive, Suite 2100, Mobile, AL 36688; Rsh0011@auburn.edu.
1. CDC. Stroke Facts. Last updated January 31, 2020. www.cdc.gov/stroke/facts.htm. Accessed June 29, 2020.
2. Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
3. Amarenco P, Lavallee PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182-2190.
4. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
5. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
6. Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-966.
7. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
8. Gent M, Beaumont D, Blanchard J, et al. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
9. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
10. Johnston SC, Amarenco P, Denison H, et al. The acute stroke or transient ischemic attack treated with ticagrelor and aspirin for prevention of stroke and death (THALES) trial: rationale and design. Int J Stroke. 2019;14:745‐751.
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110.
12. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
13. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
14. Hankey GJ, Johnston SC, Easton JD, et al. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. Int J Stroke. 2011;6:3-9.
15. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
16. Li Z, Wang Y, Zhao X, et al. Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. J Am Heart Assoc. 2016;5:e003038.
17. Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317-323.
18. Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70-78.
19. Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
20. Li X, Zhou G, Zhou X, et al. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following TIA or stroke: a meta-analysis of randomized controlled trials. J Neurol Sci. 2013;332:92-96.
21. Halkes PH, van Gijn J, Kapelle IJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
22. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
23. Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
24. Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391:850-859.
The incidence of ischemic stroke in the United States is estimated to be more than 795,000 events each year.1 After an initial stroke, the rate of recurrence is 5% to 20% within the first year, with the greatest prevalence in the first 90 days following an event.2-5 Although dual antiplatelet therapy, often with aspirin and a P2Y12 inhibitor such as clopidogrel, reduces the risk for recurrent cardiovascular events, cerebrovascular events, and death following acute coronary syndromes and percutaneous intervention, the role of combination antiplatelet therapy for secondary prevention of ischemic stroke continues to be debated.6 Reconciling currently available data can be challenging, as many studies vary considerably in both the time to antiplatelet initiation and duration of therapy.
For many years, aspirin alone was the drug of choice for secondary prevention of noncardioembolic ischemic stroke.7 Efficacy is similar at dosages anywhere between 50 and 1500 mg/d; higher doses incur a greater risk for gastrointestinal hemorrhage.7 Current secondary prevention guidelines recommend a dosage of aspirin somewhere between 50 and 325 mg/d.7
Alternative agents have also been evaluated for secondary stroke prevention, but only clopidogrel is currently considered an acceptable alternative for monotherapy based on a subgroup analysis of the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial.7,8 Other alternatives, including cilostazol, ticlopidine, and ticagrelor, are limited by a lack of data, adverse drug reactions, or unproven efficacy and are not recommended in current guidelines.7,9 The ongoing THALES (Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death) trial, assessing combination ticagrelor and aspirin, may identify an additional option for antiplatelet therapy following acute stroke.10
The current guidelines from the American Heart Association/American Stroke Association (AHA/ASA) support the combination of aspirin and extended-release dipyridamole (ASA-ERDP) as a long-term alternative to aspirin monotherapy.7,11 Additionally, the combination of clopidogrel and aspirin (CLO-ASA) is now recommended for limited duration in the early management of ischemic stroke.11
This review will explore the role of dual antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke or transient ischemic attack (TIA), with particular focus on acute use of CLO-ASA.
Clopidogrel and aspirin: When to initiate, when to stop
The combined use of clopidogrel and aspirin has been well-studied for secondary prevention of ischemic stroke and TIA. However, interpreting and applying the results of these trials can be challenging given key differences in both time to treatment initiation and the duration of combination therapy. Highlights of the major randomized controlled trials (RCTs) evaluating the safety and efficacy of CLO-ASA are detailed in TABLE 1.4,5,12-15
Initial trials evaluating CLO-ASA for secondary stroke prevention, including the MATCH (Management of ATherothrombosis with Clopidogrel in High-risk patients),12 SPS3 (Secondary Prevention of Small Subcortical Strokes),13 and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance)14 trials assessed the long-term benefits of combination therapy, with most patients initiating treatment a month or more following an initial stroke and continuing therapy for at least 18 months.12-14 Results from these trials indicate that long-term use (> 18 months) of CLO-ASA does not reduce recurrent events but increases rates of clinically significant bleeding.12-14
Continue to: A look at Tx timing
A look at Tx timing. Since these initial attempts failed to show a long-term benefit with CLO-ASA, subsequent trials attempted to establish an appropriate balance between the optimal time to initiate CLO-ASA and the optimal duration of therapy. The FASTER (Fast Assessment of Stroke and Transient ischaemic attack to prevent Early Recurrence) trial was a small pilot study of 392 patients randomized to CLO-ASA or aspirin within 24 hours of stroke or TIA onset and continued for only 3 months.15 While this trial did not find a significant reduction in ischemic or hemorrhagic stroke with combination therapy, there was a large numerical difference in event rates between the 2 groups (7.1% CLO-ASA vs 10.8% aspirin).15 An underpowered sample size (due to difficulty recruiting participants) is likely responsible for the lack of statistical significance.15 Despite the trial’s failure to show a benefit with acute use of CLO-ASA, it suggested a possible benefit that led to further investigation in the CHANCE (Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events)5 and POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) 4 trials.
The CHANCE trial conducted in China included more than 5000 patients with acute minor ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 3) or high-risk TIA (ABCD2 [a scale that assesses the risk of stroke on the basis of age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes] score ≥ 4).5 Similar to FASTER, patients were randomized within 24 hours of symptom onset to CLO-ASA or aspirin. However, CHANCE utilized combination therapy for only 21 days, after which the patients were continued on clopidogrel monotherapy for up to 90 days; the aspirin monotherapy group continued aspirin for 90 days.
After 90 days, patients initially using combination therapy had significantly lower rates of ischemic or hemorrhagic stroke vs those assigned to aspirin monotherapy. This result was driven heavily by the reduction in ischemic stroke (7.9% CLO-ASA vs 11.4% aspirin; P < .001). Additionally, there was no significant difference in moderate or severe bleeding events between the 2 groups.5 Efficacy and safety results were similar among a subgroup of patients who were randomized to treatment within 12 hours rather than 24 hours from symptom onset.16 The CHANCE trial was the first major study to demonstrate a clinical benefit of CLO-ASA to prevent recurrent stroke. Accordingly, the 2018 AHA/ASA guidelines included a new recommendation regarding secondary prevention for the use of CLO-ASA initiated within 24 hours and continued for 21 days following a minor stroke or TIA.11
A drawback of the CHANCE trial was its narrow patient population of only Chinese patients, which may limit applicability in clinical practice. There are known genetic variations in cytochrome P450 2C19 (CYP2C19) that may affect clopidogrel metabolism. CYP2C19 is responsible for the conversion of clopidogrel into its activated form in vivo. Carriers of a CYP2C19 loss-of-function allele may have reduced clopidogrel activation and subsequent reduced antiplatelet activity. Such loss-of-function alleles are more common in Asian populations vs non-Asian populations.17
A substudy of CHANCE found that CLO-ASA’s efficacy benefit was preserved in noncarrier patients; however, patients with the CYP2C19 loss-of-function allele did not benefit from combination therapy.18 Interestingly, these genetic differences did not affect bleeding outcomes. Given that approximately 60% of patients in the CHANCE substudy were loss-of-function allele carriers and that the overall study results still showed benefit with combination therapy, application of CHANCE’s findings to broader populations may not be a concern after all.18
Continue to: In efforts to gain insight...
In efforts to gain insight on CLO-ASA’s use in a more diverse patient population, the POINT trial included almost 5000 patients, with 82% from the United States, who were randomized within 12 hours of symptom onset to CLO-ASA or aspirin monotherapy for 90 days.4 Similar to the CHANCE study, the POINT study included patients with mild ischemic strokes (NIHSS ≤ 3) or high-risk TIA (ABCD2 ≥ 4). Combination therapy significantly reduced the primary endpoint of ischemic stroke, myocardial infarction (MI), or death from an ischemic event. Contrary to CHANCE, there was a significant increase in major bleeding in those assigned to combination therapy, which resulted in the trial being stopped early.4
A closer look at safety differences. CHANCE and POINT were the first major trials to show a benefit of CLO-ASA for secondary prevention of stroke, yet their differences in safety outcomes, specifically major hemorrhage, argued for a deeper reconciliation of their results.4,5 While both trials initiated secondary prevention within 24 hours of symptom onset, the difference in duration of combination therapy (21 days in CHANCE vs 90 days in POINT) likely impacted the rates of hemorrhage. When results from POINT were stratified by time period, particularly within the first 30 days of therapy (similar to the 21-day treatment duration of CHANCE), combination therapy significantly reduced the primary endpoint of ischemic stroke, MI, or death from an ischemic event (3.9% CLO-ASA vs 5.8% aspirin; P = .02) without an increased risk for major hemorrhage. Between 30 and 90 days, this efficacy benefit disappeared. However, bleeding rates between groups continued to separate throughout the 90-day course. In this light, the 30-day outcomes of POINT are largely similar to CHANCE and support the short-term use of CLO-ASA for secondary prevention without an associated increase in major bleeding.4,5
Antiplatelet dosing in POINT and CHANCE may also play a role in the contrasting safety results between the trials.4,5 While both studies utilized clopidogrel loading doses, POINT used 600 mg while CHANCE used 300 mg. Clopidogrel maintenance dosing was the same at 75 mg/d. In CHANCE, aspirin dosing was protocolized to 75 mg/d; however, in POINT, 31% of patients used > 100 mg/d aspirin.4,5 It is possible that the higher doses of both aspirin and clopidogrel in the POINT trial contributed to the difference in the occurrence of major hemorrhage between the treatment groups in these trials.
The takeaway. Based on currently available data, patients who are best suited to benefit from CLO-ASA are those who have had minor noncardioembolic ischemic strokes or high-risk TIAs.4,5,11 Clopidogrel should be given as a 300-mg loading dose followed by 75 mg/d given concomitantly with aspirin at a dose no higher than 100 mg/d. CLO-ASA therapy should be initiated within 24 hours of symptom onset and be continued for no longer than 1 month, after which chronic preventive therapy with either aspirin or clopidogrel monotherapy should be started.4,5,11
Dipyridamole and aspirin: A controversial option
Since the approval of the combination product ASA-ERDP, there has been considerable controversy about using this combination over other therapies, such as aspirin or clopidogrel, for recurrent ischemic stroke prevention. Much of this controversy arises from limitations in the trial designs.
Continue to: The first trial to show benefit...
The first trial to show benefit with ASA-ERDP was ESPS2 (European Stroke Prevention Study 2), which demonstrated superiority of the combination over placebo in reducing recurrent stroke when treatment was added within 3 months of an index stroke.19 A few studies have evaluated ASA-ERDP compared to aspirin monotherapy; however, most of these studies were small and did not show any difference in outcomes.20 Only ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)21 carried significant weight in a 2013 meta-analysis, which showed a significant reduction in recurrent events with the combination product compared to aspirin monotherapy.20
Both the ESPS2 and ESPRIT trials had significant limitations.19,21 Patients in both studies had vascular comorbidities including atherosclerotic cardiovascular disease (ASCVD); however, pharmacotherapies designated to treat these diseases were not mentioned in the demographic data, nor were these medications taken into consideration to limit potential bias.19,21 Retrospectively, a significant proportion of aspirin doses utilized as a control in ESPRIT were inferior to the guideline-recommended dosing with 42% to 46% of patients receiving 30 mg/d.21 Despite these controversies, ASA-ERDP is still considered an alternative to aspirin monotherapy in the guidelines.7
The timing of ASA-ERDP initiation appears to be inversely related to the efficacy of the combination over therapeutic alternatives. Studies in which the therapy was initiated 3 to 6 months from the index stroke indicated favorable outcomes for the combination when compared to ASA or ERDP monotherapy.19,21 Studies utilizing early initiation (ie, within 24 or 48 hours of the index event) or even within 3 weeks showed no difference in outcomes; however, this may be due in part to the use of clopidogrel or other combination antiplatelet therapy as active comparators.22-24
Early initiation of ASA-ERDP also demonstrated a higher risk of major and intracranial bleeding compared to clopidogrel.22 Additionally, use of triple therapy with ASA-ERDP plus clopidogrel increased bleeding events without improving efficacy.24 More recent studies of ASA-ERDP are focusing on earlier initiation of therapy; it is unknown whether the benefits of late initiation will be confirmed in future studies. Highlights of the major RCTs evaluating the safety and efficacy of ASA-ERDP are detailed in TABLE 219,21-24.
The takeaway. Methodological issues and potential confounding factors in many of the key trials for ASA-ERDP make it challenging to fully discern the role that ASA-ERDP may play in the secondary prevention of stroke. Further evidence utilizing appropriate controls, timing, and assessment of confounders is needed. Additionally, ASA-ERDP is plagued by tolerability issues such as headache, nausea, and vomiting, leading to higher rates of discontinuation than its comparators in clinical trials. Accordingly, the maintenance use of ASA-ERDP for secondary stroke prevention may be considered less preferred than other recommended alternatives such as aspirin or clopidogrel monotherapies.
CORRESPONDENCE
Robert S. Helmer, PharmD, BCPS, Department of Pharmacy Practice, Auburn University Harrison School of Pharmacy, 650 Clinic Drive, Suite 2100, Mobile, AL 36688; Rsh0011@auburn.edu.
The incidence of ischemic stroke in the United States is estimated to be more than 795,000 events each year.1 After an initial stroke, the rate of recurrence is 5% to 20% within the first year, with the greatest prevalence in the first 90 days following an event.2-5 Although dual antiplatelet therapy, often with aspirin and a P2Y12 inhibitor such as clopidogrel, reduces the risk for recurrent cardiovascular events, cerebrovascular events, and death following acute coronary syndromes and percutaneous intervention, the role of combination antiplatelet therapy for secondary prevention of ischemic stroke continues to be debated.6 Reconciling currently available data can be challenging, as many studies vary considerably in both the time to antiplatelet initiation and duration of therapy.
For many years, aspirin alone was the drug of choice for secondary prevention of noncardioembolic ischemic stroke.7 Efficacy is similar at dosages anywhere between 50 and 1500 mg/d; higher doses incur a greater risk for gastrointestinal hemorrhage.7 Current secondary prevention guidelines recommend a dosage of aspirin somewhere between 50 and 325 mg/d.7
Alternative agents have also been evaluated for secondary stroke prevention, but only clopidogrel is currently considered an acceptable alternative for monotherapy based on a subgroup analysis of the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial.7,8 Other alternatives, including cilostazol, ticlopidine, and ticagrelor, are limited by a lack of data, adverse drug reactions, or unproven efficacy and are not recommended in current guidelines.7,9 The ongoing THALES (Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death) trial, assessing combination ticagrelor and aspirin, may identify an additional option for antiplatelet therapy following acute stroke.10
The current guidelines from the American Heart Association/American Stroke Association (AHA/ASA) support the combination of aspirin and extended-release dipyridamole (ASA-ERDP) as a long-term alternative to aspirin monotherapy.7,11 Additionally, the combination of clopidogrel and aspirin (CLO-ASA) is now recommended for limited duration in the early management of ischemic stroke.11
This review will explore the role of dual antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke or transient ischemic attack (TIA), with particular focus on acute use of CLO-ASA.
Clopidogrel and aspirin: When to initiate, when to stop
The combined use of clopidogrel and aspirin has been well-studied for secondary prevention of ischemic stroke and TIA. However, interpreting and applying the results of these trials can be challenging given key differences in both time to treatment initiation and the duration of combination therapy. Highlights of the major randomized controlled trials (RCTs) evaluating the safety and efficacy of CLO-ASA are detailed in TABLE 1.4,5,12-15
Initial trials evaluating CLO-ASA for secondary stroke prevention, including the MATCH (Management of ATherothrombosis with Clopidogrel in High-risk patients),12 SPS3 (Secondary Prevention of Small Subcortical Strokes),13 and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance)14 trials assessed the long-term benefits of combination therapy, with most patients initiating treatment a month or more following an initial stroke and continuing therapy for at least 18 months.12-14 Results from these trials indicate that long-term use (> 18 months) of CLO-ASA does not reduce recurrent events but increases rates of clinically significant bleeding.12-14
Continue to: A look at Tx timing
A look at Tx timing. Since these initial attempts failed to show a long-term benefit with CLO-ASA, subsequent trials attempted to establish an appropriate balance between the optimal time to initiate CLO-ASA and the optimal duration of therapy. The FASTER (Fast Assessment of Stroke and Transient ischaemic attack to prevent Early Recurrence) trial was a small pilot study of 392 patients randomized to CLO-ASA or aspirin within 24 hours of stroke or TIA onset and continued for only 3 months.15 While this trial did not find a significant reduction in ischemic or hemorrhagic stroke with combination therapy, there was a large numerical difference in event rates between the 2 groups (7.1% CLO-ASA vs 10.8% aspirin).15 An underpowered sample size (due to difficulty recruiting participants) is likely responsible for the lack of statistical significance.15 Despite the trial’s failure to show a benefit with acute use of CLO-ASA, it suggested a possible benefit that led to further investigation in the CHANCE (Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events)5 and POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) 4 trials.
The CHANCE trial conducted in China included more than 5000 patients with acute minor ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 3) or high-risk TIA (ABCD2 [a scale that assesses the risk of stroke on the basis of age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes] score ≥ 4).5 Similar to FASTER, patients were randomized within 24 hours of symptom onset to CLO-ASA or aspirin. However, CHANCE utilized combination therapy for only 21 days, after which the patients were continued on clopidogrel monotherapy for up to 90 days; the aspirin monotherapy group continued aspirin for 90 days.
After 90 days, patients initially using combination therapy had significantly lower rates of ischemic or hemorrhagic stroke vs those assigned to aspirin monotherapy. This result was driven heavily by the reduction in ischemic stroke (7.9% CLO-ASA vs 11.4% aspirin; P < .001). Additionally, there was no significant difference in moderate or severe bleeding events between the 2 groups.5 Efficacy and safety results were similar among a subgroup of patients who were randomized to treatment within 12 hours rather than 24 hours from symptom onset.16 The CHANCE trial was the first major study to demonstrate a clinical benefit of CLO-ASA to prevent recurrent stroke. Accordingly, the 2018 AHA/ASA guidelines included a new recommendation regarding secondary prevention for the use of CLO-ASA initiated within 24 hours and continued for 21 days following a minor stroke or TIA.11
A drawback of the CHANCE trial was its narrow patient population of only Chinese patients, which may limit applicability in clinical practice. There are known genetic variations in cytochrome P450 2C19 (CYP2C19) that may affect clopidogrel metabolism. CYP2C19 is responsible for the conversion of clopidogrel into its activated form in vivo. Carriers of a CYP2C19 loss-of-function allele may have reduced clopidogrel activation and subsequent reduced antiplatelet activity. Such loss-of-function alleles are more common in Asian populations vs non-Asian populations.17
A substudy of CHANCE found that CLO-ASA’s efficacy benefit was preserved in noncarrier patients; however, patients with the CYP2C19 loss-of-function allele did not benefit from combination therapy.18 Interestingly, these genetic differences did not affect bleeding outcomes. Given that approximately 60% of patients in the CHANCE substudy were loss-of-function allele carriers and that the overall study results still showed benefit with combination therapy, application of CHANCE’s findings to broader populations may not be a concern after all.18
Continue to: In efforts to gain insight...
In efforts to gain insight on CLO-ASA’s use in a more diverse patient population, the POINT trial included almost 5000 patients, with 82% from the United States, who were randomized within 12 hours of symptom onset to CLO-ASA or aspirin monotherapy for 90 days.4 Similar to the CHANCE study, the POINT study included patients with mild ischemic strokes (NIHSS ≤ 3) or high-risk TIA (ABCD2 ≥ 4). Combination therapy significantly reduced the primary endpoint of ischemic stroke, myocardial infarction (MI), or death from an ischemic event. Contrary to CHANCE, there was a significant increase in major bleeding in those assigned to combination therapy, which resulted in the trial being stopped early.4
A closer look at safety differences. CHANCE and POINT were the first major trials to show a benefit of CLO-ASA for secondary prevention of stroke, yet their differences in safety outcomes, specifically major hemorrhage, argued for a deeper reconciliation of their results.4,5 While both trials initiated secondary prevention within 24 hours of symptom onset, the difference in duration of combination therapy (21 days in CHANCE vs 90 days in POINT) likely impacted the rates of hemorrhage. When results from POINT were stratified by time period, particularly within the first 30 days of therapy (similar to the 21-day treatment duration of CHANCE), combination therapy significantly reduced the primary endpoint of ischemic stroke, MI, or death from an ischemic event (3.9% CLO-ASA vs 5.8% aspirin; P = .02) without an increased risk for major hemorrhage. Between 30 and 90 days, this efficacy benefit disappeared. However, bleeding rates between groups continued to separate throughout the 90-day course. In this light, the 30-day outcomes of POINT are largely similar to CHANCE and support the short-term use of CLO-ASA for secondary prevention without an associated increase in major bleeding.4,5
Antiplatelet dosing in POINT and CHANCE may also play a role in the contrasting safety results between the trials.4,5 While both studies utilized clopidogrel loading doses, POINT used 600 mg while CHANCE used 300 mg. Clopidogrel maintenance dosing was the same at 75 mg/d. In CHANCE, aspirin dosing was protocolized to 75 mg/d; however, in POINT, 31% of patients used > 100 mg/d aspirin.4,5 It is possible that the higher doses of both aspirin and clopidogrel in the POINT trial contributed to the difference in the occurrence of major hemorrhage between the treatment groups in these trials.
The takeaway. Based on currently available data, patients who are best suited to benefit from CLO-ASA are those who have had minor noncardioembolic ischemic strokes or high-risk TIAs.4,5,11 Clopidogrel should be given as a 300-mg loading dose followed by 75 mg/d given concomitantly with aspirin at a dose no higher than 100 mg/d. CLO-ASA therapy should be initiated within 24 hours of symptom onset and be continued for no longer than 1 month, after which chronic preventive therapy with either aspirin or clopidogrel monotherapy should be started.4,5,11
Dipyridamole and aspirin: A controversial option
Since the approval of the combination product ASA-ERDP, there has been considerable controversy about using this combination over other therapies, such as aspirin or clopidogrel, for recurrent ischemic stroke prevention. Much of this controversy arises from limitations in the trial designs.
Continue to: The first trial to show benefit...
The first trial to show benefit with ASA-ERDP was ESPS2 (European Stroke Prevention Study 2), which demonstrated superiority of the combination over placebo in reducing recurrent stroke when treatment was added within 3 months of an index stroke.19 A few studies have evaluated ASA-ERDP compared to aspirin monotherapy; however, most of these studies were small and did not show any difference in outcomes.20 Only ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)21 carried significant weight in a 2013 meta-analysis, which showed a significant reduction in recurrent events with the combination product compared to aspirin monotherapy.20
Both the ESPS2 and ESPRIT trials had significant limitations.19,21 Patients in both studies had vascular comorbidities including atherosclerotic cardiovascular disease (ASCVD); however, pharmacotherapies designated to treat these diseases were not mentioned in the demographic data, nor were these medications taken into consideration to limit potential bias.19,21 Retrospectively, a significant proportion of aspirin doses utilized as a control in ESPRIT were inferior to the guideline-recommended dosing with 42% to 46% of patients receiving 30 mg/d.21 Despite these controversies, ASA-ERDP is still considered an alternative to aspirin monotherapy in the guidelines.7
The timing of ASA-ERDP initiation appears to be inversely related to the efficacy of the combination over therapeutic alternatives. Studies in which the therapy was initiated 3 to 6 months from the index stroke indicated favorable outcomes for the combination when compared to ASA or ERDP monotherapy.19,21 Studies utilizing early initiation (ie, within 24 or 48 hours of the index event) or even within 3 weeks showed no difference in outcomes; however, this may be due in part to the use of clopidogrel or other combination antiplatelet therapy as active comparators.22-24
Early initiation of ASA-ERDP also demonstrated a higher risk of major and intracranial bleeding compared to clopidogrel.22 Additionally, use of triple therapy with ASA-ERDP plus clopidogrel increased bleeding events without improving efficacy.24 More recent studies of ASA-ERDP are focusing on earlier initiation of therapy; it is unknown whether the benefits of late initiation will be confirmed in future studies. Highlights of the major RCTs evaluating the safety and efficacy of ASA-ERDP are detailed in TABLE 219,21-24.
The takeaway. Methodological issues and potential confounding factors in many of the key trials for ASA-ERDP make it challenging to fully discern the role that ASA-ERDP may play in the secondary prevention of stroke. Further evidence utilizing appropriate controls, timing, and assessment of confounders is needed. Additionally, ASA-ERDP is plagued by tolerability issues such as headache, nausea, and vomiting, leading to higher rates of discontinuation than its comparators in clinical trials. Accordingly, the maintenance use of ASA-ERDP for secondary stroke prevention may be considered less preferred than other recommended alternatives such as aspirin or clopidogrel monotherapies.
CORRESPONDENCE
Robert S. Helmer, PharmD, BCPS, Department of Pharmacy Practice, Auburn University Harrison School of Pharmacy, 650 Clinic Drive, Suite 2100, Mobile, AL 36688; Rsh0011@auburn.edu.
1. CDC. Stroke Facts. Last updated January 31, 2020. www.cdc.gov/stroke/facts.htm. Accessed June 29, 2020.
2. Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
3. Amarenco P, Lavallee PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182-2190.
4. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
5. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
6. Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-966.
7. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
8. Gent M, Beaumont D, Blanchard J, et al. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
9. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
10. Johnston SC, Amarenco P, Denison H, et al. The acute stroke or transient ischemic attack treated with ticagrelor and aspirin for prevention of stroke and death (THALES) trial: rationale and design. Int J Stroke. 2019;14:745‐751.
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110.
12. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
13. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
14. Hankey GJ, Johnston SC, Easton JD, et al. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. Int J Stroke. 2011;6:3-9.
15. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
16. Li Z, Wang Y, Zhao X, et al. Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. J Am Heart Assoc. 2016;5:e003038.
17. Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317-323.
18. Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70-78.
19. Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
20. Li X, Zhou G, Zhou X, et al. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following TIA or stroke: a meta-analysis of randomized controlled trials. J Neurol Sci. 2013;332:92-96.
21. Halkes PH, van Gijn J, Kapelle IJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
22. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
23. Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
24. Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391:850-859.
1. CDC. Stroke Facts. Last updated January 31, 2020. www.cdc.gov/stroke/facts.htm. Accessed June 29, 2020.
2. Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
3. Amarenco P, Lavallee PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182-2190.
4. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
5. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
6. Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-966.
7. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
8. Gent M, Beaumont D, Blanchard J, et al. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
9. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
10. Johnston SC, Amarenco P, Denison H, et al. The acute stroke or transient ischemic attack treated with ticagrelor and aspirin for prevention of stroke and death (THALES) trial: rationale and design. Int J Stroke. 2019;14:745‐751.
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110.
12. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
13. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
14. Hankey GJ, Johnston SC, Easton JD, et al. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. Int J Stroke. 2011;6:3-9.
15. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
16. Li Z, Wang Y, Zhao X, et al. Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. J Am Heart Assoc. 2016;5:e003038.
17. Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317-323.
18. Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70-78.
19. Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
20. Li X, Zhou G, Zhou X, et al. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following TIA or stroke: a meta-analysis of randomized controlled trials. J Neurol Sci. 2013;332:92-96.
21. Halkes PH, van Gijn J, Kapelle IJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
22. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
23. Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
24. Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391:850-859.
PRACTICE RECOMMENDATIONS
› Initiate combined clopidogrel plus aspirin within 24 hours of a minor stroke or TIA and continue for no longer than 1 month; then switch patients to aspirin or clopidogrel monotherapy. A
› Do not use combined clopidogrel plus aspirin for long-term secondary stroke prevention. A
› Limit use of aspirin plus extended-release dipyridamole as a first choice for secondary stroke prevention because of limitations in efficacy and poor tolerability. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Part 4: Monitoring for CKD in Diabetes Patients
Previously, we discussed assessment and treatment for dyslipidemia in patients with diabetes. Now we’ll explore how to monitor for kidney disease in this population.
CASE CONTINUED
Mr. W’s basic metabolic panel includes an estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73 m2 (reference range, > 60 ml/min/1.73 m2). In the absence of any other markers of kidney disease, you obtain a spot urinary albumin-to-creatinine ratio (UACR). The UACR results show a ratio of 64 mg/g, confirming stage 3 chronic kidney disease (CKD).
Monitoring for Chronic Kidney Disease
CKD is characterized by persistent albuminuria, low eGFR, and manifestations of kidney damage, and it increases cardiovascular risk.2 According to the ADA, clinicians should obtain a UACR and eGFR at least annually in patients who have had type 1 diabetes for at least 5 years and in all patients with type 2 diabetes.2 Monitoring is needed twice a year for those who begin to show signs of albuminuria or a reduced eGFR. This helps define the presence or stage of CKD and allows for further treatment planning.
Notably, patients with an eGFR < 30 ml/min/1.73m2, an unclear cause of kidney disease, or signs of rapidly progressive disease (eg, decline in GFR category plus ≥ 25% decline in eGFR from baseline) should be seen by nephrology for further evaluation and treatment recommendations.2,36
Diabetes medications for kidney health. Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists may be good candidates to promote kidney health in patients such as Mr. W. Recent trials show that SGLT2 inhibitors reduce the risk for progressive diabetic kidney disease, and the ADA recommends these medications for patients with CKD.2,16,36 GLP-1 receptor agonists also may be associated with a lower rate of development and progression of diabetic kidney disease, but this effect appears to be less robust.7,15,16 ADA guidelines recommend SGLT2 inhibitors for patients whose eGFR is adequate.37
ADA and AACE guidelines offer specific treatment recommendations on the use of SGLT2 inhibitors and GLP-1 receptor agonists in the management of diabetes.10,37 Note that neither SGLT2 inhibitors nor GLP-1 agonists are strictly under the purview of endocrinologists. Rather, multiple guidelines state that they can be utilized safely by a variety of practitioners.6,38,39
In the concluding part of this series, we will explore how to screen for peripheral neuropathy and diabetic retinopathy—identification of which can improve the patient’s quality of life.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Previously, we discussed assessment and treatment for dyslipidemia in patients with diabetes. Now we’ll explore how to monitor for kidney disease in this population.
CASE CONTINUED
Mr. W’s basic metabolic panel includes an estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73 m2 (reference range, > 60 ml/min/1.73 m2). In the absence of any other markers of kidney disease, you obtain a spot urinary albumin-to-creatinine ratio (UACR). The UACR results show a ratio of 64 mg/g, confirming stage 3 chronic kidney disease (CKD).
Monitoring for Chronic Kidney Disease
CKD is characterized by persistent albuminuria, low eGFR, and manifestations of kidney damage, and it increases cardiovascular risk.2 According to the ADA, clinicians should obtain a UACR and eGFR at least annually in patients who have had type 1 diabetes for at least 5 years and in all patients with type 2 diabetes.2 Monitoring is needed twice a year for those who begin to show signs of albuminuria or a reduced eGFR. This helps define the presence or stage of CKD and allows for further treatment planning.
Notably, patients with an eGFR < 30 ml/min/1.73m2, an unclear cause of kidney disease, or signs of rapidly progressive disease (eg, decline in GFR category plus ≥ 25% decline in eGFR from baseline) should be seen by nephrology for further evaluation and treatment recommendations.2,36
Diabetes medications for kidney health. Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists may be good candidates to promote kidney health in patients such as Mr. W. Recent trials show that SGLT2 inhibitors reduce the risk for progressive diabetic kidney disease, and the ADA recommends these medications for patients with CKD.2,16,36 GLP-1 receptor agonists also may be associated with a lower rate of development and progression of diabetic kidney disease, but this effect appears to be less robust.7,15,16 ADA guidelines recommend SGLT2 inhibitors for patients whose eGFR is adequate.37
ADA and AACE guidelines offer specific treatment recommendations on the use of SGLT2 inhibitors and GLP-1 receptor agonists in the management of diabetes.10,37 Note that neither SGLT2 inhibitors nor GLP-1 agonists are strictly under the purview of endocrinologists. Rather, multiple guidelines state that they can be utilized safely by a variety of practitioners.6,38,39
In the concluding part of this series, we will explore how to screen for peripheral neuropathy and diabetic retinopathy—identification of which can improve the patient’s quality of life.
Previously, we discussed assessment and treatment for dyslipidemia in patients with diabetes. Now we’ll explore how to monitor for kidney disease in this population.
CASE CONTINUED
Mr. W’s basic metabolic panel includes an estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73 m2 (reference range, > 60 ml/min/1.73 m2). In the absence of any other markers of kidney disease, you obtain a spot urinary albumin-to-creatinine ratio (UACR). The UACR results show a ratio of 64 mg/g, confirming stage 3 chronic kidney disease (CKD).
Monitoring for Chronic Kidney Disease
CKD is characterized by persistent albuminuria, low eGFR, and manifestations of kidney damage, and it increases cardiovascular risk.2 According to the ADA, clinicians should obtain a UACR and eGFR at least annually in patients who have had type 1 diabetes for at least 5 years and in all patients with type 2 diabetes.2 Monitoring is needed twice a year for those who begin to show signs of albuminuria or a reduced eGFR. This helps define the presence or stage of CKD and allows for further treatment planning.
Notably, patients with an eGFR < 30 ml/min/1.73m2, an unclear cause of kidney disease, or signs of rapidly progressive disease (eg, decline in GFR category plus ≥ 25% decline in eGFR from baseline) should be seen by nephrology for further evaluation and treatment recommendations.2,36
Diabetes medications for kidney health. Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists may be good candidates to promote kidney health in patients such as Mr. W. Recent trials show that SGLT2 inhibitors reduce the risk for progressive diabetic kidney disease, and the ADA recommends these medications for patients with CKD.2,16,36 GLP-1 receptor agonists also may be associated with a lower rate of development and progression of diabetic kidney disease, but this effect appears to be less robust.7,15,16 ADA guidelines recommend SGLT2 inhibitors for patients whose eGFR is adequate.37
ADA and AACE guidelines offer specific treatment recommendations on the use of SGLT2 inhibitors and GLP-1 receptor agonists in the management of diabetes.10,37 Note that neither SGLT2 inhibitors nor GLP-1 agonists are strictly under the purview of endocrinologists. Rather, multiple guidelines state that they can be utilized safely by a variety of practitioners.6,38,39
In the concluding part of this series, we will explore how to screen for peripheral neuropathy and diabetic retinopathy—identification of which can improve the patient’s quality of life.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Confronting the epidemic of racism in ObGyn practice
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
Used together, troponin and coronary calcium improve CV risk assessment
If either high sensitivity cardiac troponin (hs-cTnT) or coronary artery calcium (CAC) are elevated, the 10-year risk of atherosclerotic cardiovascular disease (ASCVD) climbs substantially, which suggests these biomarkers yield more prognostic information when they are used together, according to a cohort study with a median 15 years of follow-up.
Among those with a double negative result, meaning hs-cTnT was less than the limit of detection (<3 ng/L) and the CAC score was zero, only 2.8% developed ASCVD within 10 years, but the rates climbed to 4.6% if hs-cTnT was detectable and to 9.8% if the CAC score exceeded zero even when the other biomarker was negative.
“The increased risk for ASCVD among those with discordant results indicate that their prognostic information is complementary, favoring their conjoined use for risk prediction,” reported a multicenter team of investigators led by Allan S. Jaffe, MD, professor of laboratory medicine and pathology, Mayo Clinic, Rochester, Minn.
The study was performed with data from 6,749 participants in the Multi-Ethnic Study of Atherosclerosis (MESA), which is a longitudinal, community-based study funded by the National Heart, Lung, and Blood Institute. Over the course of long-term follow-up in a patient population that was about half female, 39% non-Hispanic white, 28% Black, 22% Hispanic American, and 12% Asian, ASCVD events were evaluated in relation to both biomarkers measured at baseline.
At baseline, both biomarkers were negative in 22%, both positive in 40%, and discordant in 38%.
After a median follow-up of 15 years, when 1,002 ASCVD events had occurred, the crude rate of ASCVD was 2.8 per 1,000 person-years in the double-negative group. When compared with this, the adjusted hazard ratio for ASCVD among those with double positive biomarkers was 3.5 (P < .00001). Increased risk was also highly significant if just hs-cTnT was positive (HR, 1.59; P = .003) or if just CAC was positive (HR, 2.74; P < .00001).
The added value of using both biomarkers to identify individuals at very low risk of ASCVD makes sense, according to the authors of an accompanying editorial. Written by a team led by John W. McEvoy, MB, BCh, National University of Ireland, Galway, the editorial explained why the information is complementary.
“CAC indicates subclinical atherosclerosis, whereas hs-cTnT indicates myocardial ischemia or damage, not just from coronary stenosis but also due to other conditions like hypertensive heart and left ventricular hypertrophy,” the authors stated.
Although they maintained that adding N-terminal pro-brain natriuretic peptide, which could be drawn from the same blood sample as hs-cTnT, might prove to be an even better but still simple strategy to identify low-risk patients, they praised the concept of combining biomarkers.
“If one’s wish is to identify truly low-risk individuals, then it appears that it takes two negative ASCVD biomarkers to make that wish come true,” the authors of the editorial concluded.
Relative to alternative methods of ASCVD risk assessment, measurement of these biomarkers might be useful for sparing patients from interventions, such as lipid lowering with statin therapy, being considered on the basis of conventional risk factors alone.
Dr. Jaffe said in an interview that he considers the two-biomarker assessment to be a useful tool in the low-risk population that he studied, but he does not consider this strategy as a substitute for other methods, such as those outline in the 2019 ACC/AHA guidelines that address the entire spectrum of risk, although work is planned to see if this approach can be extended to this broader group.*
“The data we have presented now is a good start and suggests that these two objective measures can identify those who are at very low risk and avoid adding individuals who may not be at as low risk if only one of the two tests is used,” Dr. Jaffe explained.
“Given there are now techniques to measure coronary calcium from any chest CT study, and that high sensitivity cardiac troponin is a relatively inexpensive test, putting them together should really help risk stratify patients,” he added.
When asked whether this approach will eventually replace conventional methods of ASCVD risk assessment, such as those proposed in the 2019 American College of Cardiology/American Heart Association guidelines for the primary prevention of cardiovascular disease (Circulation. 2019;140:e596-e646), he said maybe.
“The answer is that we will probe that question in our ongoing studies using continuous data in an attempt to evaluate how to use this approach to risk stratify larger numbers of individuals,” Dr. Jaffe replied.
The senior investigator, Dr. Jaffe, has consulting relationships with many pharmaceutical companies. The editorial authors had no relevant disclosures.
SOURCE: Sandoval Y et al. J Am Coll Cardiol. 2020;76:357-370.
*Correction, 7/27/20: An earlier version of this article mischaracterized Dr. Jaffe's statement.
If either high sensitivity cardiac troponin (hs-cTnT) or coronary artery calcium (CAC) are elevated, the 10-year risk of atherosclerotic cardiovascular disease (ASCVD) climbs substantially, which suggests these biomarkers yield more prognostic information when they are used together, according to a cohort study with a median 15 years of follow-up.
Among those with a double negative result, meaning hs-cTnT was less than the limit of detection (<3 ng/L) and the CAC score was zero, only 2.8% developed ASCVD within 10 years, but the rates climbed to 4.6% if hs-cTnT was detectable and to 9.8% if the CAC score exceeded zero even when the other biomarker was negative.
“The increased risk for ASCVD among those with discordant results indicate that their prognostic information is complementary, favoring their conjoined use for risk prediction,” reported a multicenter team of investigators led by Allan S. Jaffe, MD, professor of laboratory medicine and pathology, Mayo Clinic, Rochester, Minn.
The study was performed with data from 6,749 participants in the Multi-Ethnic Study of Atherosclerosis (MESA), which is a longitudinal, community-based study funded by the National Heart, Lung, and Blood Institute. Over the course of long-term follow-up in a patient population that was about half female, 39% non-Hispanic white, 28% Black, 22% Hispanic American, and 12% Asian, ASCVD events were evaluated in relation to both biomarkers measured at baseline.
At baseline, both biomarkers were negative in 22%, both positive in 40%, and discordant in 38%.
After a median follow-up of 15 years, when 1,002 ASCVD events had occurred, the crude rate of ASCVD was 2.8 per 1,000 person-years in the double-negative group. When compared with this, the adjusted hazard ratio for ASCVD among those with double positive biomarkers was 3.5 (P < .00001). Increased risk was also highly significant if just hs-cTnT was positive (HR, 1.59; P = .003) or if just CAC was positive (HR, 2.74; P < .00001).
The added value of using both biomarkers to identify individuals at very low risk of ASCVD makes sense, according to the authors of an accompanying editorial. Written by a team led by John W. McEvoy, MB, BCh, National University of Ireland, Galway, the editorial explained why the information is complementary.
“CAC indicates subclinical atherosclerosis, whereas hs-cTnT indicates myocardial ischemia or damage, not just from coronary stenosis but also due to other conditions like hypertensive heart and left ventricular hypertrophy,” the authors stated.
Although they maintained that adding N-terminal pro-brain natriuretic peptide, which could be drawn from the same blood sample as hs-cTnT, might prove to be an even better but still simple strategy to identify low-risk patients, they praised the concept of combining biomarkers.
“If one’s wish is to identify truly low-risk individuals, then it appears that it takes two negative ASCVD biomarkers to make that wish come true,” the authors of the editorial concluded.
Relative to alternative methods of ASCVD risk assessment, measurement of these biomarkers might be useful for sparing patients from interventions, such as lipid lowering with statin therapy, being considered on the basis of conventional risk factors alone.
Dr. Jaffe said in an interview that he considers the two-biomarker assessment to be a useful tool in the low-risk population that he studied, but he does not consider this strategy as a substitute for other methods, such as those outline in the 2019 ACC/AHA guidelines that address the entire spectrum of risk, although work is planned to see if this approach can be extended to this broader group.*
“The data we have presented now is a good start and suggests that these two objective measures can identify those who are at very low risk and avoid adding individuals who may not be at as low risk if only one of the two tests is used,” Dr. Jaffe explained.
“Given there are now techniques to measure coronary calcium from any chest CT study, and that high sensitivity cardiac troponin is a relatively inexpensive test, putting them together should really help risk stratify patients,” he added.
When asked whether this approach will eventually replace conventional methods of ASCVD risk assessment, such as those proposed in the 2019 American College of Cardiology/American Heart Association guidelines for the primary prevention of cardiovascular disease (Circulation. 2019;140:e596-e646), he said maybe.
“The answer is that we will probe that question in our ongoing studies using continuous data in an attempt to evaluate how to use this approach to risk stratify larger numbers of individuals,” Dr. Jaffe replied.
The senior investigator, Dr. Jaffe, has consulting relationships with many pharmaceutical companies. The editorial authors had no relevant disclosures.
SOURCE: Sandoval Y et al. J Am Coll Cardiol. 2020;76:357-370.
*Correction, 7/27/20: An earlier version of this article mischaracterized Dr. Jaffe's statement.
If either high sensitivity cardiac troponin (hs-cTnT) or coronary artery calcium (CAC) are elevated, the 10-year risk of atherosclerotic cardiovascular disease (ASCVD) climbs substantially, which suggests these biomarkers yield more prognostic information when they are used together, according to a cohort study with a median 15 years of follow-up.
Among those with a double negative result, meaning hs-cTnT was less than the limit of detection (<3 ng/L) and the CAC score was zero, only 2.8% developed ASCVD within 10 years, but the rates climbed to 4.6% if hs-cTnT was detectable and to 9.8% if the CAC score exceeded zero even when the other biomarker was negative.
“The increased risk for ASCVD among those with discordant results indicate that their prognostic information is complementary, favoring their conjoined use for risk prediction,” reported a multicenter team of investigators led by Allan S. Jaffe, MD, professor of laboratory medicine and pathology, Mayo Clinic, Rochester, Minn.
The study was performed with data from 6,749 participants in the Multi-Ethnic Study of Atherosclerosis (MESA), which is a longitudinal, community-based study funded by the National Heart, Lung, and Blood Institute. Over the course of long-term follow-up in a patient population that was about half female, 39% non-Hispanic white, 28% Black, 22% Hispanic American, and 12% Asian, ASCVD events were evaluated in relation to both biomarkers measured at baseline.
At baseline, both biomarkers were negative in 22%, both positive in 40%, and discordant in 38%.
After a median follow-up of 15 years, when 1,002 ASCVD events had occurred, the crude rate of ASCVD was 2.8 per 1,000 person-years in the double-negative group. When compared with this, the adjusted hazard ratio for ASCVD among those with double positive biomarkers was 3.5 (P < .00001). Increased risk was also highly significant if just hs-cTnT was positive (HR, 1.59; P = .003) or if just CAC was positive (HR, 2.74; P < .00001).
The added value of using both biomarkers to identify individuals at very low risk of ASCVD makes sense, according to the authors of an accompanying editorial. Written by a team led by John W. McEvoy, MB, BCh, National University of Ireland, Galway, the editorial explained why the information is complementary.
“CAC indicates subclinical atherosclerosis, whereas hs-cTnT indicates myocardial ischemia or damage, not just from coronary stenosis but also due to other conditions like hypertensive heart and left ventricular hypertrophy,” the authors stated.
Although they maintained that adding N-terminal pro-brain natriuretic peptide, which could be drawn from the same blood sample as hs-cTnT, might prove to be an even better but still simple strategy to identify low-risk patients, they praised the concept of combining biomarkers.
“If one’s wish is to identify truly low-risk individuals, then it appears that it takes two negative ASCVD biomarkers to make that wish come true,” the authors of the editorial concluded.
Relative to alternative methods of ASCVD risk assessment, measurement of these biomarkers might be useful for sparing patients from interventions, such as lipid lowering with statin therapy, being considered on the basis of conventional risk factors alone.
Dr. Jaffe said in an interview that he considers the two-biomarker assessment to be a useful tool in the low-risk population that he studied, but he does not consider this strategy as a substitute for other methods, such as those outline in the 2019 ACC/AHA guidelines that address the entire spectrum of risk, although work is planned to see if this approach can be extended to this broader group.*
“The data we have presented now is a good start and suggests that these two objective measures can identify those who are at very low risk and avoid adding individuals who may not be at as low risk if only one of the two tests is used,” Dr. Jaffe explained.
“Given there are now techniques to measure coronary calcium from any chest CT study, and that high sensitivity cardiac troponin is a relatively inexpensive test, putting them together should really help risk stratify patients,” he added.
When asked whether this approach will eventually replace conventional methods of ASCVD risk assessment, such as those proposed in the 2019 American College of Cardiology/American Heart Association guidelines for the primary prevention of cardiovascular disease (Circulation. 2019;140:e596-e646), he said maybe.
“The answer is that we will probe that question in our ongoing studies using continuous data in an attempt to evaluate how to use this approach to risk stratify larger numbers of individuals,” Dr. Jaffe replied.
The senior investigator, Dr. Jaffe, has consulting relationships with many pharmaceutical companies. The editorial authors had no relevant disclosures.
SOURCE: Sandoval Y et al. J Am Coll Cardiol. 2020;76:357-370.
*Correction, 7/27/20: An earlier version of this article mischaracterized Dr. Jaffe's statement.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Hot-off-the-press insights on heart failure
Hospitalists frequently encounter patients with heart failure – a complex, clinical syndrome, which has high prevalence, mortality, hospitalization rates, and health care costs.
The HM20 Virtual session “Updates in Heart Failure” will provide literature updates for all types of heart failure patient scenarios – patients with acute and chronic heart failure, those who are hospitalized with heart failure, and patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). The popular session with questions and answers will be held on Aug. 25.
Presenter Dustin Smith, MD, SFHM, associate professor of medicine in the department of medicine at Emory University, Atlanta, and section chief for education in medical specialty at the Atlanta Veterans Affairs Medical Center, will discuss recent trends, diagnostics, therapeutics, and prognostics for heart failure. He’ll also provide a summary of recent changes to clinical practice guidelines.
“The significance of staying knowledgeable and updated regarding this common admission diagnosis cannot be overstated,” Dr. Smith said. Attendees of this clinical update should learn important practices from new evidence in literature, including an unearthed risk grade predictor of acute heart failure mortality, a diagnostic tool for HFpEF in euvolemic patients with unexplained dyspnea, an examination of the potassium “repletion reflex” in patients hospitalized with heart failure, dietary patterns associated with incident heart failure, and therapies efficacious for HFrEF and/or HFpEF.
“The goal of this session is for attendees to incorporate this new information into their clinical practice so they can optimally manage patients with heart failure,” Dr. Smith said.
The session is specifically curated to impact the clinical practice of hospitalists who provide care for patients with heart failure in the acute care setting and beyond. Key impact areas of clinical practice that will be tackled include:
- Augmenting one’s clinical acumen to diagnose HFpEF.
- Calculating mortality risk for patients with acute heart failure.
- Recognizing other predictors of risk for patients hospitalized with heart failure.
- Recommending dietary, medication, and interventional therapies to prevent future heart failure morbidity and mortality.
Dr. Smith will conclude each literature review with a summary of take-home learning points carefully selected to either change, modify, or confirm the current practice and teaching for providers who care for heart failure patients.
Although Dr. Smith has presented the “Updates in Heart Failure” session in various educational arenas in the past, this is a new update. He has gained vast experience and expertise in this area from conducting extensive and in-depth literature reviews on managing heart failure while preparing for presentations on this topic.
In addition, Dr. Smith has contributed to original research manuscripts, book chapters, and board review–style exam questions in cardiology – including heart failure – and evidence-based medicine topics as an author and editor. He has also sought out additional training and completed faculty development programs targeted at improving his knowledge and skill set to teach evidence-based clinical practice.
Dr. Smith had no relevant financial conflicts to disclose.
Updates in Heart Failure
Live Q&A – Tuesday, Aug. 25 1:00 p.m. to 2:00 p.m.
Hospitalists frequently encounter patients with heart failure – a complex, clinical syndrome, which has high prevalence, mortality, hospitalization rates, and health care costs.
The HM20 Virtual session “Updates in Heart Failure” will provide literature updates for all types of heart failure patient scenarios – patients with acute and chronic heart failure, those who are hospitalized with heart failure, and patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). The popular session with questions and answers will be held on Aug. 25.
Presenter Dustin Smith, MD, SFHM, associate professor of medicine in the department of medicine at Emory University, Atlanta, and section chief for education in medical specialty at the Atlanta Veterans Affairs Medical Center, will discuss recent trends, diagnostics, therapeutics, and prognostics for heart failure. He’ll also provide a summary of recent changes to clinical practice guidelines.
“The significance of staying knowledgeable and updated regarding this common admission diagnosis cannot be overstated,” Dr. Smith said. Attendees of this clinical update should learn important practices from new evidence in literature, including an unearthed risk grade predictor of acute heart failure mortality, a diagnostic tool for HFpEF in euvolemic patients with unexplained dyspnea, an examination of the potassium “repletion reflex” in patients hospitalized with heart failure, dietary patterns associated with incident heart failure, and therapies efficacious for HFrEF and/or HFpEF.
“The goal of this session is for attendees to incorporate this new information into their clinical practice so they can optimally manage patients with heart failure,” Dr. Smith said.
The session is specifically curated to impact the clinical practice of hospitalists who provide care for patients with heart failure in the acute care setting and beyond. Key impact areas of clinical practice that will be tackled include:
- Augmenting one’s clinical acumen to diagnose HFpEF.
- Calculating mortality risk for patients with acute heart failure.
- Recognizing other predictors of risk for patients hospitalized with heart failure.
- Recommending dietary, medication, and interventional therapies to prevent future heart failure morbidity and mortality.
Dr. Smith will conclude each literature review with a summary of take-home learning points carefully selected to either change, modify, or confirm the current practice and teaching for providers who care for heart failure patients.
Although Dr. Smith has presented the “Updates in Heart Failure” session in various educational arenas in the past, this is a new update. He has gained vast experience and expertise in this area from conducting extensive and in-depth literature reviews on managing heart failure while preparing for presentations on this topic.
In addition, Dr. Smith has contributed to original research manuscripts, book chapters, and board review–style exam questions in cardiology – including heart failure – and evidence-based medicine topics as an author and editor. He has also sought out additional training and completed faculty development programs targeted at improving his knowledge and skill set to teach evidence-based clinical practice.
Dr. Smith had no relevant financial conflicts to disclose.
Updates in Heart Failure
Live Q&A – Tuesday, Aug. 25 1:00 p.m. to 2:00 p.m.
Hospitalists frequently encounter patients with heart failure – a complex, clinical syndrome, which has high prevalence, mortality, hospitalization rates, and health care costs.
The HM20 Virtual session “Updates in Heart Failure” will provide literature updates for all types of heart failure patient scenarios – patients with acute and chronic heart failure, those who are hospitalized with heart failure, and patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). The popular session with questions and answers will be held on Aug. 25.
Presenter Dustin Smith, MD, SFHM, associate professor of medicine in the department of medicine at Emory University, Atlanta, and section chief for education in medical specialty at the Atlanta Veterans Affairs Medical Center, will discuss recent trends, diagnostics, therapeutics, and prognostics for heart failure. He’ll also provide a summary of recent changes to clinical practice guidelines.
“The significance of staying knowledgeable and updated regarding this common admission diagnosis cannot be overstated,” Dr. Smith said. Attendees of this clinical update should learn important practices from new evidence in literature, including an unearthed risk grade predictor of acute heart failure mortality, a diagnostic tool for HFpEF in euvolemic patients with unexplained dyspnea, an examination of the potassium “repletion reflex” in patients hospitalized with heart failure, dietary patterns associated with incident heart failure, and therapies efficacious for HFrEF and/or HFpEF.
“The goal of this session is for attendees to incorporate this new information into their clinical practice so they can optimally manage patients with heart failure,” Dr. Smith said.
The session is specifically curated to impact the clinical practice of hospitalists who provide care for patients with heart failure in the acute care setting and beyond. Key impact areas of clinical practice that will be tackled include:
- Augmenting one’s clinical acumen to diagnose HFpEF.
- Calculating mortality risk for patients with acute heart failure.
- Recognizing other predictors of risk for patients hospitalized with heart failure.
- Recommending dietary, medication, and interventional therapies to prevent future heart failure morbidity and mortality.
Dr. Smith will conclude each literature review with a summary of take-home learning points carefully selected to either change, modify, or confirm the current practice and teaching for providers who care for heart failure patients.
Although Dr. Smith has presented the “Updates in Heart Failure” session in various educational arenas in the past, this is a new update. He has gained vast experience and expertise in this area from conducting extensive and in-depth literature reviews on managing heart failure while preparing for presentations on this topic.
In addition, Dr. Smith has contributed to original research manuscripts, book chapters, and board review–style exam questions in cardiology – including heart failure – and evidence-based medicine topics as an author and editor. He has also sought out additional training and completed faculty development programs targeted at improving his knowledge and skill set to teach evidence-based clinical practice.
Dr. Smith had no relevant financial conflicts to disclose.
Updates in Heart Failure
Live Q&A – Tuesday, Aug. 25 1:00 p.m. to 2:00 p.m.
No link between topical steroids and fracture risk found in children with atopic dermatitis
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
FROM SPD 2020