User login
How can hospitalists address health disparities for LGBTQ+ patients?
It is well established that lesbian, gay, bisexual, transgender, and queer (LGBTQ) patients suffer worse health outcomes, relative to patients who are heterosexual and cisgender – that is, those whose sense of personal identity and gender corresponds with their birth sex. The reasons for these disparities are multifactorial but include discrimination and limited provider knowledge about LGBTQ-specific health concerns.
These disparities – and what hospitalists can do to try to ameliorate them on the job – will be explored in a session at HM20 Virtual, “When the Answers Aren’t Straight Forward: Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Health Updates for the Hospitalist.”
, according to Tyler Anstett, DO, copresenter and assistant professor in the division of hospital medicine at the University of Colorado. He and copresenter Keshav Khanijow, MD, an assistant professor in the division of hospital medicine, Northwestern University, Chicago, will share results from the Q-HEALTH (Quantifying Hospitalist Education and Awareness of LGBTQ Topics in Health) national survey of SHM members about their knowledge and attitudes regarding LGBTQ health. This survey, sponsored by SHM’s Education Committee, identified knowledge and comfort gaps in caring for LGBTQ+ patients. Most respondents say they are interested in receiving more didactic training on this topic, building on an introductory session on LGBTQ+ health presented at last year’s SHM Annual Conference. They also named the Annual Conference as one of their top venues for receiving such training.
The session at HM20 Virtual will cover the health disparities identified in LGBTQ+ populations, with case examples that highlight those disparities, Dr. Anstett said. “We will review results from Q-HEALTH, the SHM-wide survey on provider attitudes, knowledge, and comfort in caring for LGBTQ+ patients. Finally, the session will cover basic LGBTQ+ terminology and, through clinical scenarios, provide attendees with some basic skills for improving their practice for LGBTQ+ patients.”
With over 11 million Americans who identify as lesbian, gay, bisexual, transgender, and/or queer, hospitalists will certainly encounter patients of diverse sexual orientations and gender identities, Dr. Anstett said. Hospitalists should serve as allies for their patients, including for those who are LGBTQ+. Through this session, attendees can reflect on individual practice and learn how to educate others on LGBTQ+ health basics.
“We hope the cases we present will provide attendees with an introduction to the health issues the LGBTQ+ community faces with greater prevalence, and what hospitalists can be thinking about when they approach these issues,” Dr. Khanijow added.
Dr. Anstett and Dr. Khanijow had no relevant financial conflicts to disclose.
When the Answers Aren’t Straight Forward: Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Health Updates for the Hospitalist
It is well established that lesbian, gay, bisexual, transgender, and queer (LGBTQ) patients suffer worse health outcomes, relative to patients who are heterosexual and cisgender – that is, those whose sense of personal identity and gender corresponds with their birth sex. The reasons for these disparities are multifactorial but include discrimination and limited provider knowledge about LGBTQ-specific health concerns.
These disparities – and what hospitalists can do to try to ameliorate them on the job – will be explored in a session at HM20 Virtual, “When the Answers Aren’t Straight Forward: Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Health Updates for the Hospitalist.”
, according to Tyler Anstett, DO, copresenter and assistant professor in the division of hospital medicine at the University of Colorado. He and copresenter Keshav Khanijow, MD, an assistant professor in the division of hospital medicine, Northwestern University, Chicago, will share results from the Q-HEALTH (Quantifying Hospitalist Education and Awareness of LGBTQ Topics in Health) national survey of SHM members about their knowledge and attitudes regarding LGBTQ health. This survey, sponsored by SHM’s Education Committee, identified knowledge and comfort gaps in caring for LGBTQ+ patients. Most respondents say they are interested in receiving more didactic training on this topic, building on an introductory session on LGBTQ+ health presented at last year’s SHM Annual Conference. They also named the Annual Conference as one of their top venues for receiving such training.
The session at HM20 Virtual will cover the health disparities identified in LGBTQ+ populations, with case examples that highlight those disparities, Dr. Anstett said. “We will review results from Q-HEALTH, the SHM-wide survey on provider attitudes, knowledge, and comfort in caring for LGBTQ+ patients. Finally, the session will cover basic LGBTQ+ terminology and, through clinical scenarios, provide attendees with some basic skills for improving their practice for LGBTQ+ patients.”
With over 11 million Americans who identify as lesbian, gay, bisexual, transgender, and/or queer, hospitalists will certainly encounter patients of diverse sexual orientations and gender identities, Dr. Anstett said. Hospitalists should serve as allies for their patients, including for those who are LGBTQ+. Through this session, attendees can reflect on individual practice and learn how to educate others on LGBTQ+ health basics.
“We hope the cases we present will provide attendees with an introduction to the health issues the LGBTQ+ community faces with greater prevalence, and what hospitalists can be thinking about when they approach these issues,” Dr. Khanijow added.
Dr. Anstett and Dr. Khanijow had no relevant financial conflicts to disclose.
When the Answers Aren’t Straight Forward: Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Health Updates for the Hospitalist
It is well established that lesbian, gay, bisexual, transgender, and queer (LGBTQ) patients suffer worse health outcomes, relative to patients who are heterosexual and cisgender – that is, those whose sense of personal identity and gender corresponds with their birth sex. The reasons for these disparities are multifactorial but include discrimination and limited provider knowledge about LGBTQ-specific health concerns.
These disparities – and what hospitalists can do to try to ameliorate them on the job – will be explored in a session at HM20 Virtual, “When the Answers Aren’t Straight Forward: Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Health Updates for the Hospitalist.”
, according to Tyler Anstett, DO, copresenter and assistant professor in the division of hospital medicine at the University of Colorado. He and copresenter Keshav Khanijow, MD, an assistant professor in the division of hospital medicine, Northwestern University, Chicago, will share results from the Q-HEALTH (Quantifying Hospitalist Education and Awareness of LGBTQ Topics in Health) national survey of SHM members about their knowledge and attitudes regarding LGBTQ health. This survey, sponsored by SHM’s Education Committee, identified knowledge and comfort gaps in caring for LGBTQ+ patients. Most respondents say they are interested in receiving more didactic training on this topic, building on an introductory session on LGBTQ+ health presented at last year’s SHM Annual Conference. They also named the Annual Conference as one of their top venues for receiving such training.
The session at HM20 Virtual will cover the health disparities identified in LGBTQ+ populations, with case examples that highlight those disparities, Dr. Anstett said. “We will review results from Q-HEALTH, the SHM-wide survey on provider attitudes, knowledge, and comfort in caring for LGBTQ+ patients. Finally, the session will cover basic LGBTQ+ terminology and, through clinical scenarios, provide attendees with some basic skills for improving their practice for LGBTQ+ patients.”
With over 11 million Americans who identify as lesbian, gay, bisexual, transgender, and/or queer, hospitalists will certainly encounter patients of diverse sexual orientations and gender identities, Dr. Anstett said. Hospitalists should serve as allies for their patients, including for those who are LGBTQ+. Through this session, attendees can reflect on individual practice and learn how to educate others on LGBTQ+ health basics.
“We hope the cases we present will provide attendees with an introduction to the health issues the LGBTQ+ community faces with greater prevalence, and what hospitalists can be thinking about when they approach these issues,” Dr. Khanijow added.
Dr. Anstett and Dr. Khanijow had no relevant financial conflicts to disclose.
When the Answers Aren’t Straight Forward: Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Health Updates for the Hospitalist
Early recognition of oncologic emergencies deemed ‘crucial’
During an oncologic emergency, making a clinical decision during the early diagnostic period is one of the most critical things a hospitalist can do when caring for patients with cancer. Hospitalists may not always be well versed in the symptoms of oncologic emergencies, though, particularly with newer treatments like immunotherapy and targeted therapies. They also may be tempted to contact colleagues in oncology when they may be qualified to handle these emergencies on their own.
At the end of her question-and-answer session, “Getting to Know Oncology Emergencies: Recognition and Management” to be presented on Aug. 12 at HM20 Virtual, the virtual annual meeting of the Society of Hospital Medicine, Megan Kruse, MD, hopes hospitalists will be able to recognize the signs and symptoms of “classic” oncologic emergencies they are likely to see in routine practice, as well as side effects of newer therapies they may not have encountered. Attendees will know how to manage these situations and understand when they need to involve a cancer specialist.
“Early recognition of these emergencies is crucial, and there are simple initial interventions that can make a big difference in patient outcomes,” said Dr. Kruse, an oncologist at the Cleveland Clinic.
In her presentation, Dr. Kruse will review oncologic emergencies that can occur in patients with acute leukemia such as acute blast crisis, as well as spinal cord compression and neutropenic fever. These complications are common in patients with cancer: Many cancers, such as multiple myeloma, lung cancer, and breast cancer, can cause spinal metastases that lead to spinal cord compression, while studies have shown neutropenic fever can occur in up to 80% of patients who undergo chemotherapy.
The presentation also will outline how hospitalists can manage specific side effects of immunotherapy and targeted therapies during an emergency situation. Dr. Kruse noted the session also will focus on when to start steroids for immune-related adverse event concerns and when to think about adding alternate immunosuppression. Complications of these therapies can differ from those of traditional chemotherapy, and not all hospitalists may be expecting them. Side effects from cancer therapy also can present months after treatment, further complicating the nature of oncologic emergencies in a hospital setting.
Recognizing the signs of such emergencies can be crucial for patients, especially if clinical decisions are made before a hospitalist can reach an oncologist for consult. Some decisions can be made by hospitalists themselves, while others may require specialty knowledge from an oncologist, Dr. Kruse noted. Regardless, it is important to consider cancer treatment history in a patient’s differential diagnosis.
Dr. Kruse has given presentations on oncologic emergencies at SHM annual conferences in the past, but notes this year’s virtual presentation will include more cases and examples of complications to improve recognition of these conditions. in patients with oncologic emergencies.
“I hope that attendees will leave with a better idea of what symptoms should be, warning signs of impending oncologic emergencies/complications, and what measures can be taken to treat these conditions prior to oncology service involvement,” Dr. Kruse said.
Dr. Kruse reported advisory board involvement for Novartis Oncology and consulting for Puma Biotechnology.
Getting to Know Oncology Emergencies: Recognition and Management
Live Q&A: Wednesday, Aug. 12, 1:00 p.m. to 2:00 p.m.
During an oncologic emergency, making a clinical decision during the early diagnostic period is one of the most critical things a hospitalist can do when caring for patients with cancer. Hospitalists may not always be well versed in the symptoms of oncologic emergencies, though, particularly with newer treatments like immunotherapy and targeted therapies. They also may be tempted to contact colleagues in oncology when they may be qualified to handle these emergencies on their own.
At the end of her question-and-answer session, “Getting to Know Oncology Emergencies: Recognition and Management” to be presented on Aug. 12 at HM20 Virtual, the virtual annual meeting of the Society of Hospital Medicine, Megan Kruse, MD, hopes hospitalists will be able to recognize the signs and symptoms of “classic” oncologic emergencies they are likely to see in routine practice, as well as side effects of newer therapies they may not have encountered. Attendees will know how to manage these situations and understand when they need to involve a cancer specialist.
“Early recognition of these emergencies is crucial, and there are simple initial interventions that can make a big difference in patient outcomes,” said Dr. Kruse, an oncologist at the Cleveland Clinic.
In her presentation, Dr. Kruse will review oncologic emergencies that can occur in patients with acute leukemia such as acute blast crisis, as well as spinal cord compression and neutropenic fever. These complications are common in patients with cancer: Many cancers, such as multiple myeloma, lung cancer, and breast cancer, can cause spinal metastases that lead to spinal cord compression, while studies have shown neutropenic fever can occur in up to 80% of patients who undergo chemotherapy.
The presentation also will outline how hospitalists can manage specific side effects of immunotherapy and targeted therapies during an emergency situation. Dr. Kruse noted the session also will focus on when to start steroids for immune-related adverse event concerns and when to think about adding alternate immunosuppression. Complications of these therapies can differ from those of traditional chemotherapy, and not all hospitalists may be expecting them. Side effects from cancer therapy also can present months after treatment, further complicating the nature of oncologic emergencies in a hospital setting.
Recognizing the signs of such emergencies can be crucial for patients, especially if clinical decisions are made before a hospitalist can reach an oncologist for consult. Some decisions can be made by hospitalists themselves, while others may require specialty knowledge from an oncologist, Dr. Kruse noted. Regardless, it is important to consider cancer treatment history in a patient’s differential diagnosis.
Dr. Kruse has given presentations on oncologic emergencies at SHM annual conferences in the past, but notes this year’s virtual presentation will include more cases and examples of complications to improve recognition of these conditions. in patients with oncologic emergencies.
“I hope that attendees will leave with a better idea of what symptoms should be, warning signs of impending oncologic emergencies/complications, and what measures can be taken to treat these conditions prior to oncology service involvement,” Dr. Kruse said.
Dr. Kruse reported advisory board involvement for Novartis Oncology and consulting for Puma Biotechnology.
Getting to Know Oncology Emergencies: Recognition and Management
Live Q&A: Wednesday, Aug. 12, 1:00 p.m. to 2:00 p.m.
During an oncologic emergency, making a clinical decision during the early diagnostic period is one of the most critical things a hospitalist can do when caring for patients with cancer. Hospitalists may not always be well versed in the symptoms of oncologic emergencies, though, particularly with newer treatments like immunotherapy and targeted therapies. They also may be tempted to contact colleagues in oncology when they may be qualified to handle these emergencies on their own.
At the end of her question-and-answer session, “Getting to Know Oncology Emergencies: Recognition and Management” to be presented on Aug. 12 at HM20 Virtual, the virtual annual meeting of the Society of Hospital Medicine, Megan Kruse, MD, hopes hospitalists will be able to recognize the signs and symptoms of “classic” oncologic emergencies they are likely to see in routine practice, as well as side effects of newer therapies they may not have encountered. Attendees will know how to manage these situations and understand when they need to involve a cancer specialist.
“Early recognition of these emergencies is crucial, and there are simple initial interventions that can make a big difference in patient outcomes,” said Dr. Kruse, an oncologist at the Cleveland Clinic.
In her presentation, Dr. Kruse will review oncologic emergencies that can occur in patients with acute leukemia such as acute blast crisis, as well as spinal cord compression and neutropenic fever. These complications are common in patients with cancer: Many cancers, such as multiple myeloma, lung cancer, and breast cancer, can cause spinal metastases that lead to spinal cord compression, while studies have shown neutropenic fever can occur in up to 80% of patients who undergo chemotherapy.
The presentation also will outline how hospitalists can manage specific side effects of immunotherapy and targeted therapies during an emergency situation. Dr. Kruse noted the session also will focus on when to start steroids for immune-related adverse event concerns and when to think about adding alternate immunosuppression. Complications of these therapies can differ from those of traditional chemotherapy, and not all hospitalists may be expecting them. Side effects from cancer therapy also can present months after treatment, further complicating the nature of oncologic emergencies in a hospital setting.
Recognizing the signs of such emergencies can be crucial for patients, especially if clinical decisions are made before a hospitalist can reach an oncologist for consult. Some decisions can be made by hospitalists themselves, while others may require specialty knowledge from an oncologist, Dr. Kruse noted. Regardless, it is important to consider cancer treatment history in a patient’s differential diagnosis.
Dr. Kruse has given presentations on oncologic emergencies at SHM annual conferences in the past, but notes this year’s virtual presentation will include more cases and examples of complications to improve recognition of these conditions. in patients with oncologic emergencies.
“I hope that attendees will leave with a better idea of what symptoms should be, warning signs of impending oncologic emergencies/complications, and what measures can be taken to treat these conditions prior to oncology service involvement,” Dr. Kruse said.
Dr. Kruse reported advisory board involvement for Novartis Oncology and consulting for Puma Biotechnology.
Getting to Know Oncology Emergencies: Recognition and Management
Live Q&A: Wednesday, Aug. 12, 1:00 p.m. to 2:00 p.m.
Trio of antibodies may enable earlier diagnosis of axSpA
Three autoantibodies to newly discovered axial spondyloarthritis peptides may improve early diagnosis of the disease, according to a cross-sectional cohort study reported in Arthritis & Rheumatology.
The Assessment in SpondyloArthritis International Society (ASAS) classification criteria were not intended for diagnosis and do not differentiate well between patients with early axial spondyloarthritis (axSpA) and patients with nonspecific chronic low back pain, note the investigators, who conducted their research under senior investigator Veerle Somers, PhD, professor of molecular biology at Hasselt (Belgium) University and vice dean of the School of Life Sciences at Transnationale Universiteit Limburg, also in Hasselt.
“Therefore, for many patients, axSpA diagnosis may be challenging and is often delayed by several years after the occurrence of first clinical symptoms, posing a problem for early treatment initiation,” they wrote.
The investigators used plasma samples from patients with early disease and an axSpA complementary DNA phage display library developed with synovial tissue to screen for IgG antibodies that displayed significantly higher reactivity to plasma pools from the early axSpA patients than healthy controls.
They then assessed presence of the antibodies with enzyme-linked immunosorbent assays in a mixed cohort (76 patients with early axSpA having mean disease duration of 2.8 years, 75 control patients with nonspecific chronic low back pain, 60 patients with RA, and 94 healthy controls) and in an axSpA-only cohort (174 patients, 79 of whom had early disease with mean disease duration of 1.4 years).
Screening identified antibodies to nine novel peptides – eight peptides showing partial homology to human proteins and one novel axSpA autoantigen, double homeobox protein 4 (DUX4) – that were more commonly present in patients with early axSpA than in healthy controls, Dr. Somers and coinvestigators reported.
Subsequent analyses focused on the three antibodies having the highest positive likelihood ratios for differentiating axSpA from chronic low back pain.
Some 14.2% of the combined group of all patients with early axSpA had at least one antibody in this panel, compared with just 5.3% of the patients with chronic low back pain (P = .0484), corresponding to 95% specificity.
Prevalence did not differ significantly from that in patients with RA (10.0%; P = .5025) or healthy controls (8.4%; P = .2292).
The positive likelihood ratio for confirming early axSpA using the three antibodies was 2.7, on par with the historical ratio of 2.5 seen for C-reactive protein (CRP), the currently used laboratory marker, the investigators noted.
Among the patients with chronic low back pain, the posttest probability for axSpA increased from 79% with presence of inflammatory back pain and positive test results for HLA-B27 and CRP to 91% with addition of testing for the three antibodies.
The researchers proposed that, “in combination with other laboratory markers such as HLA-B27 and CRP, antibodies against our [three peptides] ... could provide a novel tool for the diagnosis of a subset of axSpA patients,” but the three-peptide panel needs to be studied more in larger cohorts of early axSpA patients and controls with low back pain.
Findings in context
“The authors did a number of steps laudably,” James T. Rosenbaum, MD, chair of the division of arthritis and rheumatic diseases and the Edward E. Rosenbaum Professor of Inflammation Research at Oregon Health & Science University, Portland, commented in an interview. Specifically, they used a variety of appropriate controls, had discovery and validation sets, achieved a fairly good sample size, and applied the phage library technique.
“Despite this technological tour de force and the need for a sensitive and specific blood test to diagnose nonradiographic axSpA, this study is preliminary,” he cautioned. “For example, the authors found antibodies to DUX4 in 8% of axSpA patients versus 3% of healthy controls, 4% of patients with chronic low back pain, and 7% with RA. It took a combination of antigens to enhance the diagnostic accuracy of the ASAS criteria to diagnose axSpA. For each antigen that was studied, more than 80% of the axSpA patients had no detectable antibodies.”
Importantly, rheumatic diseases are often immune mediated without being autoimmune, calling into question the role of the antibodies, according to Dr. Rosenbaum.
“Even if further studies validate these observations, additional research needs to be done to support the concept that these antibodies cause disease as opposed to being mere epiphenomena as is suggested by the low prevalence,” he concluded. “Current hypotheses as to the cause of ankylosing spondylitis now point to the microbiome and autoinflammatory rather than autoimmune pathways, but the jury is still out.”
Dr. Somers and three coauthors disclosed having a patent pending on the markers. The study was funded by a personal grant from the Agency for Innovation by Science and Technology Flanders. Dr. Rosenbaum disclosed that he consults for AbbVie, Gilead, Novartis, Pfizer, Roche, and UCB.
SOURCE: Quaden D et al. Arthritis Rheumatol. 2020 Jul 8. doi: 10.1002/art.41427.
Three autoantibodies to newly discovered axial spondyloarthritis peptides may improve early diagnosis of the disease, according to a cross-sectional cohort study reported in Arthritis & Rheumatology.
The Assessment in SpondyloArthritis International Society (ASAS) classification criteria were not intended for diagnosis and do not differentiate well between patients with early axial spondyloarthritis (axSpA) and patients with nonspecific chronic low back pain, note the investigators, who conducted their research under senior investigator Veerle Somers, PhD, professor of molecular biology at Hasselt (Belgium) University and vice dean of the School of Life Sciences at Transnationale Universiteit Limburg, also in Hasselt.
“Therefore, for many patients, axSpA diagnosis may be challenging and is often delayed by several years after the occurrence of first clinical symptoms, posing a problem for early treatment initiation,” they wrote.
The investigators used plasma samples from patients with early disease and an axSpA complementary DNA phage display library developed with synovial tissue to screen for IgG antibodies that displayed significantly higher reactivity to plasma pools from the early axSpA patients than healthy controls.
They then assessed presence of the antibodies with enzyme-linked immunosorbent assays in a mixed cohort (76 patients with early axSpA having mean disease duration of 2.8 years, 75 control patients with nonspecific chronic low back pain, 60 patients with RA, and 94 healthy controls) and in an axSpA-only cohort (174 patients, 79 of whom had early disease with mean disease duration of 1.4 years).
Screening identified antibodies to nine novel peptides – eight peptides showing partial homology to human proteins and one novel axSpA autoantigen, double homeobox protein 4 (DUX4) – that were more commonly present in patients with early axSpA than in healthy controls, Dr. Somers and coinvestigators reported.
Subsequent analyses focused on the three antibodies having the highest positive likelihood ratios for differentiating axSpA from chronic low back pain.
Some 14.2% of the combined group of all patients with early axSpA had at least one antibody in this panel, compared with just 5.3% of the patients with chronic low back pain (P = .0484), corresponding to 95% specificity.
Prevalence did not differ significantly from that in patients with RA (10.0%; P = .5025) or healthy controls (8.4%; P = .2292).
The positive likelihood ratio for confirming early axSpA using the three antibodies was 2.7, on par with the historical ratio of 2.5 seen for C-reactive protein (CRP), the currently used laboratory marker, the investigators noted.
Among the patients with chronic low back pain, the posttest probability for axSpA increased from 79% with presence of inflammatory back pain and positive test results for HLA-B27 and CRP to 91% with addition of testing for the three antibodies.
The researchers proposed that, “in combination with other laboratory markers such as HLA-B27 and CRP, antibodies against our [three peptides] ... could provide a novel tool for the diagnosis of a subset of axSpA patients,” but the three-peptide panel needs to be studied more in larger cohorts of early axSpA patients and controls with low back pain.
Findings in context
“The authors did a number of steps laudably,” James T. Rosenbaum, MD, chair of the division of arthritis and rheumatic diseases and the Edward E. Rosenbaum Professor of Inflammation Research at Oregon Health & Science University, Portland, commented in an interview. Specifically, they used a variety of appropriate controls, had discovery and validation sets, achieved a fairly good sample size, and applied the phage library technique.
“Despite this technological tour de force and the need for a sensitive and specific blood test to diagnose nonradiographic axSpA, this study is preliminary,” he cautioned. “For example, the authors found antibodies to DUX4 in 8% of axSpA patients versus 3% of healthy controls, 4% of patients with chronic low back pain, and 7% with RA. It took a combination of antigens to enhance the diagnostic accuracy of the ASAS criteria to diagnose axSpA. For each antigen that was studied, more than 80% of the axSpA patients had no detectable antibodies.”
Importantly, rheumatic diseases are often immune mediated without being autoimmune, calling into question the role of the antibodies, according to Dr. Rosenbaum.
“Even if further studies validate these observations, additional research needs to be done to support the concept that these antibodies cause disease as opposed to being mere epiphenomena as is suggested by the low prevalence,” he concluded. “Current hypotheses as to the cause of ankylosing spondylitis now point to the microbiome and autoinflammatory rather than autoimmune pathways, but the jury is still out.”
Dr. Somers and three coauthors disclosed having a patent pending on the markers. The study was funded by a personal grant from the Agency for Innovation by Science and Technology Flanders. Dr. Rosenbaum disclosed that he consults for AbbVie, Gilead, Novartis, Pfizer, Roche, and UCB.
SOURCE: Quaden D et al. Arthritis Rheumatol. 2020 Jul 8. doi: 10.1002/art.41427.
Three autoantibodies to newly discovered axial spondyloarthritis peptides may improve early diagnosis of the disease, according to a cross-sectional cohort study reported in Arthritis & Rheumatology.
The Assessment in SpondyloArthritis International Society (ASAS) classification criteria were not intended for diagnosis and do not differentiate well between patients with early axial spondyloarthritis (axSpA) and patients with nonspecific chronic low back pain, note the investigators, who conducted their research under senior investigator Veerle Somers, PhD, professor of molecular biology at Hasselt (Belgium) University and vice dean of the School of Life Sciences at Transnationale Universiteit Limburg, also in Hasselt.
“Therefore, for many patients, axSpA diagnosis may be challenging and is often delayed by several years after the occurrence of first clinical symptoms, posing a problem for early treatment initiation,” they wrote.
The investigators used plasma samples from patients with early disease and an axSpA complementary DNA phage display library developed with synovial tissue to screen for IgG antibodies that displayed significantly higher reactivity to plasma pools from the early axSpA patients than healthy controls.
They then assessed presence of the antibodies with enzyme-linked immunosorbent assays in a mixed cohort (76 patients with early axSpA having mean disease duration of 2.8 years, 75 control patients with nonspecific chronic low back pain, 60 patients with RA, and 94 healthy controls) and in an axSpA-only cohort (174 patients, 79 of whom had early disease with mean disease duration of 1.4 years).
Screening identified antibodies to nine novel peptides – eight peptides showing partial homology to human proteins and one novel axSpA autoantigen, double homeobox protein 4 (DUX4) – that were more commonly present in patients with early axSpA than in healthy controls, Dr. Somers and coinvestigators reported.
Subsequent analyses focused on the three antibodies having the highest positive likelihood ratios for differentiating axSpA from chronic low back pain.
Some 14.2% of the combined group of all patients with early axSpA had at least one antibody in this panel, compared with just 5.3% of the patients with chronic low back pain (P = .0484), corresponding to 95% specificity.
Prevalence did not differ significantly from that in patients with RA (10.0%; P = .5025) or healthy controls (8.4%; P = .2292).
The positive likelihood ratio for confirming early axSpA using the three antibodies was 2.7, on par with the historical ratio of 2.5 seen for C-reactive protein (CRP), the currently used laboratory marker, the investigators noted.
Among the patients with chronic low back pain, the posttest probability for axSpA increased from 79% with presence of inflammatory back pain and positive test results for HLA-B27 and CRP to 91% with addition of testing for the three antibodies.
The researchers proposed that, “in combination with other laboratory markers such as HLA-B27 and CRP, antibodies against our [three peptides] ... could provide a novel tool for the diagnosis of a subset of axSpA patients,” but the three-peptide panel needs to be studied more in larger cohorts of early axSpA patients and controls with low back pain.
Findings in context
“The authors did a number of steps laudably,” James T. Rosenbaum, MD, chair of the division of arthritis and rheumatic diseases and the Edward E. Rosenbaum Professor of Inflammation Research at Oregon Health & Science University, Portland, commented in an interview. Specifically, they used a variety of appropriate controls, had discovery and validation sets, achieved a fairly good sample size, and applied the phage library technique.
“Despite this technological tour de force and the need for a sensitive and specific blood test to diagnose nonradiographic axSpA, this study is preliminary,” he cautioned. “For example, the authors found antibodies to DUX4 in 8% of axSpA patients versus 3% of healthy controls, 4% of patients with chronic low back pain, and 7% with RA. It took a combination of antigens to enhance the diagnostic accuracy of the ASAS criteria to diagnose axSpA. For each antigen that was studied, more than 80% of the axSpA patients had no detectable antibodies.”
Importantly, rheumatic diseases are often immune mediated without being autoimmune, calling into question the role of the antibodies, according to Dr. Rosenbaum.
“Even if further studies validate these observations, additional research needs to be done to support the concept that these antibodies cause disease as opposed to being mere epiphenomena as is suggested by the low prevalence,” he concluded. “Current hypotheses as to the cause of ankylosing spondylitis now point to the microbiome and autoinflammatory rather than autoimmune pathways, but the jury is still out.”
Dr. Somers and three coauthors disclosed having a patent pending on the markers. The study was funded by a personal grant from the Agency for Innovation by Science and Technology Flanders. Dr. Rosenbaum disclosed that he consults for AbbVie, Gilead, Novartis, Pfizer, Roche, and UCB.
SOURCE: Quaden D et al. Arthritis Rheumatol. 2020 Jul 8. doi: 10.1002/art.41427.
FROM ARTHRITIS & RHEUMATOLOGY
Simplifying the antibiotic selection process
Hospitalists are constantly battling infection.
James Soo Kim, MD, a hospitalist and assistant professor at Emory Healthcare in Atlanta, a presenter of the session “Antibiotics Made Ridiculously Simple” during HM20 Virtual, said that while he has given this talk at previous Society of Hospital Medicine Annual Conferences, the presentation has undergone significant changes over the years as the landscape of infectious disease treatment has shifted.
He hopes attendees of HM20 Virtual will appreciate the changes and encourages those who have attended his presentation in previous years to come see what is new, but admitted newcomers may think the presentation’s title is a bit of a misnomer.
“Despite the title of the talk, there really isn’t any way to make antibiotics ridiculously simple,” he said.
Dr. Kim, who is also an editorial board member for The Hospitalist, said the origin of “Antibiotics Made Ridiculously Simple” took place during his residency, where he had an interest in infectious disease. This interest carried over to his time in fellowship at the Keck School of Medicine of the University of Southern California – and was enough to become board certified in infectious disease by the American Board of Internal Medicine. Infectious disease continues to interest him now as an attending, he said, and since he joined Emory Healthcare in 2012, he has given a version of this presentation every year.
HM20 Virtual attendees will come away from the presentation with an idea of how to choose an antibiotic regimen, Dr. Kim said, including how to select an antibiotic when you’re worried about Pseudomonas, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus or other likely organisms. “There are a variety of drugs out there that have activity against our ‘usual suspects,’ ” he said.
Attendees will also learn to select antibiotic options that have empiric coverage during a shortage of piperacillin/tazobactam (Zosyn), vancomycin, or your preferred drug of choice for treating common infections. He will also review the latest drugs that have been released over the past few years so attendees can add them to their armamentarium.
“I won’t necessarily expect attendees to use everything I talk about, but if you have a patient on service that infectious disease started Vabomere on, you’ll at least have a general idea of what they were worried about,” Dr. Kim said.
One practice pearl he hopes attendees take away from his presentation: Allergies to beta-lactam antibiotics like penicillin (PCN) derivatives are not as common as most providers and patients believe, and not giving these antibiotics to patients can actually decrease the chance that the patient gets appropriate therapy while also increasing the cost of care.
“I hope that my talk changes practice by making people aware of how infrequent true clinically significant PCN cross-reactions are so that patients can get more cost-effective and medically effective therapy,” he said.Dr. Kim reports no relevant financial disclosures.
Antibiotics Made Ridiculously Simple Live Q&A: Tuesday, August 18, 3:30-4:30 p.m.
Hospitalists are constantly battling infection.
James Soo Kim, MD, a hospitalist and assistant professor at Emory Healthcare in Atlanta, a presenter of the session “Antibiotics Made Ridiculously Simple” during HM20 Virtual, said that while he has given this talk at previous Society of Hospital Medicine Annual Conferences, the presentation has undergone significant changes over the years as the landscape of infectious disease treatment has shifted.
He hopes attendees of HM20 Virtual will appreciate the changes and encourages those who have attended his presentation in previous years to come see what is new, but admitted newcomers may think the presentation’s title is a bit of a misnomer.
“Despite the title of the talk, there really isn’t any way to make antibiotics ridiculously simple,” he said.
Dr. Kim, who is also an editorial board member for The Hospitalist, said the origin of “Antibiotics Made Ridiculously Simple” took place during his residency, where he had an interest in infectious disease. This interest carried over to his time in fellowship at the Keck School of Medicine of the University of Southern California – and was enough to become board certified in infectious disease by the American Board of Internal Medicine. Infectious disease continues to interest him now as an attending, he said, and since he joined Emory Healthcare in 2012, he has given a version of this presentation every year.
HM20 Virtual attendees will come away from the presentation with an idea of how to choose an antibiotic regimen, Dr. Kim said, including how to select an antibiotic when you’re worried about Pseudomonas, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus or other likely organisms. “There are a variety of drugs out there that have activity against our ‘usual suspects,’ ” he said.
Attendees will also learn to select antibiotic options that have empiric coverage during a shortage of piperacillin/tazobactam (Zosyn), vancomycin, or your preferred drug of choice for treating common infections. He will also review the latest drugs that have been released over the past few years so attendees can add them to their armamentarium.
“I won’t necessarily expect attendees to use everything I talk about, but if you have a patient on service that infectious disease started Vabomere on, you’ll at least have a general idea of what they were worried about,” Dr. Kim said.
One practice pearl he hopes attendees take away from his presentation: Allergies to beta-lactam antibiotics like penicillin (PCN) derivatives are not as common as most providers and patients believe, and not giving these antibiotics to patients can actually decrease the chance that the patient gets appropriate therapy while also increasing the cost of care.
“I hope that my talk changes practice by making people aware of how infrequent true clinically significant PCN cross-reactions are so that patients can get more cost-effective and medically effective therapy,” he said.Dr. Kim reports no relevant financial disclosures.
Antibiotics Made Ridiculously Simple Live Q&A: Tuesday, August 18, 3:30-4:30 p.m.
Hospitalists are constantly battling infection.
James Soo Kim, MD, a hospitalist and assistant professor at Emory Healthcare in Atlanta, a presenter of the session “Antibiotics Made Ridiculously Simple” during HM20 Virtual, said that while he has given this talk at previous Society of Hospital Medicine Annual Conferences, the presentation has undergone significant changes over the years as the landscape of infectious disease treatment has shifted.
He hopes attendees of HM20 Virtual will appreciate the changes and encourages those who have attended his presentation in previous years to come see what is new, but admitted newcomers may think the presentation’s title is a bit of a misnomer.
“Despite the title of the talk, there really isn’t any way to make antibiotics ridiculously simple,” he said.
Dr. Kim, who is also an editorial board member for The Hospitalist, said the origin of “Antibiotics Made Ridiculously Simple” took place during his residency, where he had an interest in infectious disease. This interest carried over to his time in fellowship at the Keck School of Medicine of the University of Southern California – and was enough to become board certified in infectious disease by the American Board of Internal Medicine. Infectious disease continues to interest him now as an attending, he said, and since he joined Emory Healthcare in 2012, he has given a version of this presentation every year.
HM20 Virtual attendees will come away from the presentation with an idea of how to choose an antibiotic regimen, Dr. Kim said, including how to select an antibiotic when you’re worried about Pseudomonas, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus or other likely organisms. “There are a variety of drugs out there that have activity against our ‘usual suspects,’ ” he said.
Attendees will also learn to select antibiotic options that have empiric coverage during a shortage of piperacillin/tazobactam (Zosyn), vancomycin, or your preferred drug of choice for treating common infections. He will also review the latest drugs that have been released over the past few years so attendees can add them to their armamentarium.
“I won’t necessarily expect attendees to use everything I talk about, but if you have a patient on service that infectious disease started Vabomere on, you’ll at least have a general idea of what they were worried about,” Dr. Kim said.
One practice pearl he hopes attendees take away from his presentation: Allergies to beta-lactam antibiotics like penicillin (PCN) derivatives are not as common as most providers and patients believe, and not giving these antibiotics to patients can actually decrease the chance that the patient gets appropriate therapy while also increasing the cost of care.
“I hope that my talk changes practice by making people aware of how infrequent true clinically significant PCN cross-reactions are so that patients can get more cost-effective and medically effective therapy,” he said.Dr. Kim reports no relevant financial disclosures.
Antibiotics Made Ridiculously Simple Live Q&A: Tuesday, August 18, 3:30-4:30 p.m.
Hospital medicine update highlights research from ‘extended family’
The annual “Update in Hospital Medicine” session will go a step further by highlighting the work and insights of what Dr. Pfeifer affectionately calls the “extended family.”
Scott Kaatz, DO, MSc, SFHM, a hospitalist at Henry Ford Hospital in Detroit, explained that “the Update has a long-standing tradition at the national meeting as an overview of the most impactful or insightful publications relevant to clinicians working in the hospital, which includes internists, pediatricians, obstetricians, family physicians, nurse practitioners, physician assistants, and other specialties.”
Why does the Update embrace such a wide focus? Because there’s a need for a broader perspective, according to Dr. Pfeifer, professor of medicine at the Medical College of Wisconsin, Milwaukee. “The Society of Hospital Medicine Annual Conference has many superb offerings with specific focuses that help attendees fill knowledge and practice gaps and network with individuals with similar interests,” he said. “All of those different offerings highlight something that is very cool about hospital medicine – its diversity. However, it’s also important for us to come together as one big family to support each other and advocate for the larger cause of hospital medicine. With the “Update in Hospital Medicine,” attendees can specifically hear about the clinical changes happening in their “extended family.”
“We will be giving an overview of key new literature across the spectrum of hospital medicine in areas such as sepsis, inclusion/diversity, co-management, and hospital staffing models,” Dr. Kaatz said. “We will also highlight the various different focuses/practices within hospital medicine and the wonderful diversity within the Society of Hospital Medicine. We have coordinated our selection of topics with the Special Interest Groups (SIGs) and the Chapters to make sure we include the voices of our wider membership. This will also allow us to celebrate our diversity by giving shout outs to our SIGs and chapters and showcase the wonderful things going on in hospital medicine, including advances being made by our very own members.”
Dr. Kaatz added that he and Dr. Pfeifer are grateful to the organizers for allowing them to try something new. “Presented papers will reflect the interests of SHM members via a ‘learner needs assessment’ survey,” he said. “Several of the special interest groups and local chapters surveyed their membership and voted on the most impactful papers in the past year. It has been very gratifying to see the level of engagement in our society and to be able to share this important research with a large audience.”
Dr. Pfeifer has no relevant disclosures. Dr. Kaatz discloses research funding to institution (BMS) and consultant/advisory board relationships (BMS, Pfizer and Janssen).
“Update in Hospital Medicine”
The annual “Update in Hospital Medicine” session will go a step further by highlighting the work and insights of what Dr. Pfeifer affectionately calls the “extended family.”
Scott Kaatz, DO, MSc, SFHM, a hospitalist at Henry Ford Hospital in Detroit, explained that “the Update has a long-standing tradition at the national meeting as an overview of the most impactful or insightful publications relevant to clinicians working in the hospital, which includes internists, pediatricians, obstetricians, family physicians, nurse practitioners, physician assistants, and other specialties.”
Why does the Update embrace such a wide focus? Because there’s a need for a broader perspective, according to Dr. Pfeifer, professor of medicine at the Medical College of Wisconsin, Milwaukee. “The Society of Hospital Medicine Annual Conference has many superb offerings with specific focuses that help attendees fill knowledge and practice gaps and network with individuals with similar interests,” he said. “All of those different offerings highlight something that is very cool about hospital medicine – its diversity. However, it’s also important for us to come together as one big family to support each other and advocate for the larger cause of hospital medicine. With the “Update in Hospital Medicine,” attendees can specifically hear about the clinical changes happening in their “extended family.”
“We will be giving an overview of key new literature across the spectrum of hospital medicine in areas such as sepsis, inclusion/diversity, co-management, and hospital staffing models,” Dr. Kaatz said. “We will also highlight the various different focuses/practices within hospital medicine and the wonderful diversity within the Society of Hospital Medicine. We have coordinated our selection of topics with the Special Interest Groups (SIGs) and the Chapters to make sure we include the voices of our wider membership. This will also allow us to celebrate our diversity by giving shout outs to our SIGs and chapters and showcase the wonderful things going on in hospital medicine, including advances being made by our very own members.”
Dr. Kaatz added that he and Dr. Pfeifer are grateful to the organizers for allowing them to try something new. “Presented papers will reflect the interests of SHM members via a ‘learner needs assessment’ survey,” he said. “Several of the special interest groups and local chapters surveyed their membership and voted on the most impactful papers in the past year. It has been very gratifying to see the level of engagement in our society and to be able to share this important research with a large audience.”
Dr. Pfeifer has no relevant disclosures. Dr. Kaatz discloses research funding to institution (BMS) and consultant/advisory board relationships (BMS, Pfizer and Janssen).
“Update in Hospital Medicine”
The annual “Update in Hospital Medicine” session will go a step further by highlighting the work and insights of what Dr. Pfeifer affectionately calls the “extended family.”
Scott Kaatz, DO, MSc, SFHM, a hospitalist at Henry Ford Hospital in Detroit, explained that “the Update has a long-standing tradition at the national meeting as an overview of the most impactful or insightful publications relevant to clinicians working in the hospital, which includes internists, pediatricians, obstetricians, family physicians, nurse practitioners, physician assistants, and other specialties.”
Why does the Update embrace such a wide focus? Because there’s a need for a broader perspective, according to Dr. Pfeifer, professor of medicine at the Medical College of Wisconsin, Milwaukee. “The Society of Hospital Medicine Annual Conference has many superb offerings with specific focuses that help attendees fill knowledge and practice gaps and network with individuals with similar interests,” he said. “All of those different offerings highlight something that is very cool about hospital medicine – its diversity. However, it’s also important for us to come together as one big family to support each other and advocate for the larger cause of hospital medicine. With the “Update in Hospital Medicine,” attendees can specifically hear about the clinical changes happening in their “extended family.”
“We will be giving an overview of key new literature across the spectrum of hospital medicine in areas such as sepsis, inclusion/diversity, co-management, and hospital staffing models,” Dr. Kaatz said. “We will also highlight the various different focuses/practices within hospital medicine and the wonderful diversity within the Society of Hospital Medicine. We have coordinated our selection of topics with the Special Interest Groups (SIGs) and the Chapters to make sure we include the voices of our wider membership. This will also allow us to celebrate our diversity by giving shout outs to our SIGs and chapters and showcase the wonderful things going on in hospital medicine, including advances being made by our very own members.”
Dr. Kaatz added that he and Dr. Pfeifer are grateful to the organizers for allowing them to try something new. “Presented papers will reflect the interests of SHM members via a ‘learner needs assessment’ survey,” he said. “Several of the special interest groups and local chapters surveyed their membership and voted on the most impactful papers in the past year. It has been very gratifying to see the level of engagement in our society and to be able to share this important research with a large audience.”
Dr. Pfeifer has no relevant disclosures. Dr. Kaatz discloses research funding to institution (BMS) and consultant/advisory board relationships (BMS, Pfizer and Janssen).
“Update in Hospital Medicine”
Get updated: Latest ATS/ISDA guidelines for pneumonia
according to Joanna M. Bonsall, MD, PhD, SFHM, chief of hospital medicine at Grady Memorial Hospital and associate professor of medicine at Emory University, both in Atlanta.
Last year, the American Thoracic Society and the Infectious Diseases Society of America updated their clinical guidelines on community-acquired pneumonia (CAP) for the first time since 2007. The guidelines were published in the Oct. 1, 2019 issue of the American Journal of Respiratory and Critical Care Medicine.
CAP is one of the most common reasons for hospitalization in the United States, and it is estimated that CAP comprises over 4.5 million outpatient and ED visits each year, according to the National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey in 2009-2010. It is also the most common cause of death from infection disease, according to the Centers for Disease Control and Prevention.
Dr. Bonsall will present “Updates in Pneumonia” at HM20 Virtual, the virtual annual meeting of the Society of Hospital Medicine; a live question-and-answer session will be held online Aug. 20. In her session, Dr. Bonsall said she plans to cover the new ATS/IDSA guidelines for CAP, which will include what initial testing to order, which empiric antibiotics to use, and how to manage patients at risk for resistant organisms, formerly known as health care–associated pneumonia (HCAP). Dr. Bonsall also will outline the evidence for use of steroids, especially in cases of severe pneumonia, and review the 2016 ATS/IDSA guidelines for hospital-acquired pneumonia with a focus on antibiotic selection.
One major change for 2019: The ATS/IDSA CAP guideline authors issued a strong recommendation to abandon use of the term HCAP as a “distinct clinical entity” when considering antibiotics for patients with CAP. In addition, methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa should only be empirically covered in patients with CAP if they present with locally validated risk factors for either pathogen, according to the guidelines.
“Order pretreatment testing based on severity of illness as well as risk factors for drug-resistant pathogens,” Dr. Bonsall said. Hospitalists also should avoid using procalcitonin levels as a benchmark for whether a patient should be started on antibiotics. Once the recommended antibiotic treatment has been initiated, attendees should use culture results to narrow down the possibilities, especially in cases of drug-resistant pathogens.
The ATS/IDSA guidelines also state that corticosteroids should not be routinely used for patients with nonsevere CAP, but attendees should also be aware of the limitations and interpretations of the evidence, Dr. Bonsall said. Avoiding routine corticosteroid use in patients with severe CAP or in patients with severe influenza pneumonia carries a conditional recommendation with a moderate and low quality of evidence, respectively. In general, cases of CAP should be treated for no more than 5 days, or 3 days of treatment after the patient becomes clinically stable.
Attendees at HM20 Virtual should walk away from the session knowing what testing is necessary and what testing is unnecessary, and how to reduce antibiotic exposure for both broad spectrum use and duration. “At the end of the session, you should feel comfortable using both the CAP and HAP guidelines,” Dr. Bonsall said.
Dr. Bonsall reported no relevant financial disclosures.
Updates in Pneumonia
Live Q&A: Thursday, Aug. 20, 2:15 p.m to 3:15 p.m.
according to Joanna M. Bonsall, MD, PhD, SFHM, chief of hospital medicine at Grady Memorial Hospital and associate professor of medicine at Emory University, both in Atlanta.
Last year, the American Thoracic Society and the Infectious Diseases Society of America updated their clinical guidelines on community-acquired pneumonia (CAP) for the first time since 2007. The guidelines were published in the Oct. 1, 2019 issue of the American Journal of Respiratory and Critical Care Medicine.
CAP is one of the most common reasons for hospitalization in the United States, and it is estimated that CAP comprises over 4.5 million outpatient and ED visits each year, according to the National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey in 2009-2010. It is also the most common cause of death from infection disease, according to the Centers for Disease Control and Prevention.
Dr. Bonsall will present “Updates in Pneumonia” at HM20 Virtual, the virtual annual meeting of the Society of Hospital Medicine; a live question-and-answer session will be held online Aug. 20. In her session, Dr. Bonsall said she plans to cover the new ATS/IDSA guidelines for CAP, which will include what initial testing to order, which empiric antibiotics to use, and how to manage patients at risk for resistant organisms, formerly known as health care–associated pneumonia (HCAP). Dr. Bonsall also will outline the evidence for use of steroids, especially in cases of severe pneumonia, and review the 2016 ATS/IDSA guidelines for hospital-acquired pneumonia with a focus on antibiotic selection.
One major change for 2019: The ATS/IDSA CAP guideline authors issued a strong recommendation to abandon use of the term HCAP as a “distinct clinical entity” when considering antibiotics for patients with CAP. In addition, methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa should only be empirically covered in patients with CAP if they present with locally validated risk factors for either pathogen, according to the guidelines.
“Order pretreatment testing based on severity of illness as well as risk factors for drug-resistant pathogens,” Dr. Bonsall said. Hospitalists also should avoid using procalcitonin levels as a benchmark for whether a patient should be started on antibiotics. Once the recommended antibiotic treatment has been initiated, attendees should use culture results to narrow down the possibilities, especially in cases of drug-resistant pathogens.
The ATS/IDSA guidelines also state that corticosteroids should not be routinely used for patients with nonsevere CAP, but attendees should also be aware of the limitations and interpretations of the evidence, Dr. Bonsall said. Avoiding routine corticosteroid use in patients with severe CAP or in patients with severe influenza pneumonia carries a conditional recommendation with a moderate and low quality of evidence, respectively. In general, cases of CAP should be treated for no more than 5 days, or 3 days of treatment after the patient becomes clinically stable.
Attendees at HM20 Virtual should walk away from the session knowing what testing is necessary and what testing is unnecessary, and how to reduce antibiotic exposure for both broad spectrum use and duration. “At the end of the session, you should feel comfortable using both the CAP and HAP guidelines,” Dr. Bonsall said.
Dr. Bonsall reported no relevant financial disclosures.
Updates in Pneumonia
Live Q&A: Thursday, Aug. 20, 2:15 p.m to 3:15 p.m.
according to Joanna M. Bonsall, MD, PhD, SFHM, chief of hospital medicine at Grady Memorial Hospital and associate professor of medicine at Emory University, both in Atlanta.
Last year, the American Thoracic Society and the Infectious Diseases Society of America updated their clinical guidelines on community-acquired pneumonia (CAP) for the first time since 2007. The guidelines were published in the Oct. 1, 2019 issue of the American Journal of Respiratory and Critical Care Medicine.
CAP is one of the most common reasons for hospitalization in the United States, and it is estimated that CAP comprises over 4.5 million outpatient and ED visits each year, according to the National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey in 2009-2010. It is also the most common cause of death from infection disease, according to the Centers for Disease Control and Prevention.
Dr. Bonsall will present “Updates in Pneumonia” at HM20 Virtual, the virtual annual meeting of the Society of Hospital Medicine; a live question-and-answer session will be held online Aug. 20. In her session, Dr. Bonsall said she plans to cover the new ATS/IDSA guidelines for CAP, which will include what initial testing to order, which empiric antibiotics to use, and how to manage patients at risk for resistant organisms, formerly known as health care–associated pneumonia (HCAP). Dr. Bonsall also will outline the evidence for use of steroids, especially in cases of severe pneumonia, and review the 2016 ATS/IDSA guidelines for hospital-acquired pneumonia with a focus on antibiotic selection.
One major change for 2019: The ATS/IDSA CAP guideline authors issued a strong recommendation to abandon use of the term HCAP as a “distinct clinical entity” when considering antibiotics for patients with CAP. In addition, methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa should only be empirically covered in patients with CAP if they present with locally validated risk factors for either pathogen, according to the guidelines.
“Order pretreatment testing based on severity of illness as well as risk factors for drug-resistant pathogens,” Dr. Bonsall said. Hospitalists also should avoid using procalcitonin levels as a benchmark for whether a patient should be started on antibiotics. Once the recommended antibiotic treatment has been initiated, attendees should use culture results to narrow down the possibilities, especially in cases of drug-resistant pathogens.
The ATS/IDSA guidelines also state that corticosteroids should not be routinely used for patients with nonsevere CAP, but attendees should also be aware of the limitations and interpretations of the evidence, Dr. Bonsall said. Avoiding routine corticosteroid use in patients with severe CAP or in patients with severe influenza pneumonia carries a conditional recommendation with a moderate and low quality of evidence, respectively. In general, cases of CAP should be treated for no more than 5 days, or 3 days of treatment after the patient becomes clinically stable.
Attendees at HM20 Virtual should walk away from the session knowing what testing is necessary and what testing is unnecessary, and how to reduce antibiotic exposure for both broad spectrum use and duration. “At the end of the session, you should feel comfortable using both the CAP and HAP guidelines,” Dr. Bonsall said.
Dr. Bonsall reported no relevant financial disclosures.
Updates in Pneumonia
Live Q&A: Thursday, Aug. 20, 2:15 p.m to 3:15 p.m.
Sepsis: Vitamin C, thiamine, glucocorticoids remain controversial
Sepsis is the number one killer in U.S. hospitals. About one in three patient deaths in a hospital are attributable to sepsis, according to the Centers for Disease Control and Prevention, and it is the leading cause of readmission for U.S. hospitals as well.
Patricia Kritek MD, EdM, of the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle, hopes to bring attendees up to speed on sepsis with her presentation, “Put SIRS on the SOFA and Let’s get Septic! Update in Sepsis” at HM20 Virtual.
Each year, approximately 1.7 million American adults develop sepsis, and nearly 270,000 Americans will die from sepsis annually. Although sepsis disproportionately affects young children, older adults, patients with chronic diseases, and those with a weak immune system, the disease can affect anyone.
With that reputation, sepsis is on the forefront of hospitalists’ minds. Hospitalists are traditionally well versed in current sepsis guidelines, but time to treatment is paramount, and it can be difficult to stay up to date on the latest studies in the field.
The title of Dr. Kritek’s presentation hints at the theme: Hospitalists may have learned the systemic inflammatory response syndrome (SIRS) criteria for diagnosing sepsis, but the Sequential Organ Failure Assessment (SOFA) Score developed by the Third International Consensus Definitions for Sepsis and Septic Shock – previously known as the sepsis-related organ failure assessment score – has been the new method since 2016 to assess the clinical outcomes of patients with sepsis.
The quick SOFA score (qSOFA), developed by the Society of Critical Care and European Society of Intensive Care Medicine in 2016 guidelines, further helps hospitalists and other hospital physicians identify those patients at highest risk of mortality from sepsis outside an intensive care unit setting.
Dr. Kritek, who is a board-certified critical care medicine physician, has previously presented this talk at the Society for Hospital Medicine Annual Conference in the past. This year the presentation will include a number of studies that examine what role vitamin C, thiamine, and glucocorticoids have in treating patients with sepsis, she said. For example, it is thought that parenteral administration of vitamin C could raise plasma levels and reduce multiorgan failure. Thiamine could be useful in sepsis treatment because of its role in glucose metabolism and lactate production, while glucocorticoids could help improve the mortality rate of patients with sepsis.
While Dr. Kritek said she is not going to be advocating for the benefit of vitamin C and thiamine during the session, “this is an area of ongoing debate, and we will walk through the most recent data to try to make sense of it,” she said.
Dr. Kritek noted that the role of balanced crystalloids in resuscitation will be discussed versus when to use saline, as well as the potential of new vasopressors for the treatment of septic shock.
“Our goal will be to integrate the most recent literature into day-to-day practice,” Dr. Kritek said.Dr. Kritek reports no conflicts of interest.
“Put SIRS on the SOFA and Let’s get Septic! Update in Sepsis”
Sepsis is the number one killer in U.S. hospitals. About one in three patient deaths in a hospital are attributable to sepsis, according to the Centers for Disease Control and Prevention, and it is the leading cause of readmission for U.S. hospitals as well.
Patricia Kritek MD, EdM, of the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle, hopes to bring attendees up to speed on sepsis with her presentation, “Put SIRS on the SOFA and Let’s get Septic! Update in Sepsis” at HM20 Virtual.
Each year, approximately 1.7 million American adults develop sepsis, and nearly 270,000 Americans will die from sepsis annually. Although sepsis disproportionately affects young children, older adults, patients with chronic diseases, and those with a weak immune system, the disease can affect anyone.
With that reputation, sepsis is on the forefront of hospitalists’ minds. Hospitalists are traditionally well versed in current sepsis guidelines, but time to treatment is paramount, and it can be difficult to stay up to date on the latest studies in the field.
The title of Dr. Kritek’s presentation hints at the theme: Hospitalists may have learned the systemic inflammatory response syndrome (SIRS) criteria for diagnosing sepsis, but the Sequential Organ Failure Assessment (SOFA) Score developed by the Third International Consensus Definitions for Sepsis and Septic Shock – previously known as the sepsis-related organ failure assessment score – has been the new method since 2016 to assess the clinical outcomes of patients with sepsis.
The quick SOFA score (qSOFA), developed by the Society of Critical Care and European Society of Intensive Care Medicine in 2016 guidelines, further helps hospitalists and other hospital physicians identify those patients at highest risk of mortality from sepsis outside an intensive care unit setting.
Dr. Kritek, who is a board-certified critical care medicine physician, has previously presented this talk at the Society for Hospital Medicine Annual Conference in the past. This year the presentation will include a number of studies that examine what role vitamin C, thiamine, and glucocorticoids have in treating patients with sepsis, she said. For example, it is thought that parenteral administration of vitamin C could raise plasma levels and reduce multiorgan failure. Thiamine could be useful in sepsis treatment because of its role in glucose metabolism and lactate production, while glucocorticoids could help improve the mortality rate of patients with sepsis.
While Dr. Kritek said she is not going to be advocating for the benefit of vitamin C and thiamine during the session, “this is an area of ongoing debate, and we will walk through the most recent data to try to make sense of it,” she said.
Dr. Kritek noted that the role of balanced crystalloids in resuscitation will be discussed versus when to use saline, as well as the potential of new vasopressors for the treatment of septic shock.
“Our goal will be to integrate the most recent literature into day-to-day practice,” Dr. Kritek said.Dr. Kritek reports no conflicts of interest.
“Put SIRS on the SOFA and Let’s get Septic! Update in Sepsis”
Sepsis is the number one killer in U.S. hospitals. About one in three patient deaths in a hospital are attributable to sepsis, according to the Centers for Disease Control and Prevention, and it is the leading cause of readmission for U.S. hospitals as well.
Patricia Kritek MD, EdM, of the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle, hopes to bring attendees up to speed on sepsis with her presentation, “Put SIRS on the SOFA and Let’s get Septic! Update in Sepsis” at HM20 Virtual.
Each year, approximately 1.7 million American adults develop sepsis, and nearly 270,000 Americans will die from sepsis annually. Although sepsis disproportionately affects young children, older adults, patients with chronic diseases, and those with a weak immune system, the disease can affect anyone.
With that reputation, sepsis is on the forefront of hospitalists’ minds. Hospitalists are traditionally well versed in current sepsis guidelines, but time to treatment is paramount, and it can be difficult to stay up to date on the latest studies in the field.
The title of Dr. Kritek’s presentation hints at the theme: Hospitalists may have learned the systemic inflammatory response syndrome (SIRS) criteria for diagnosing sepsis, but the Sequential Organ Failure Assessment (SOFA) Score developed by the Third International Consensus Definitions for Sepsis and Septic Shock – previously known as the sepsis-related organ failure assessment score – has been the new method since 2016 to assess the clinical outcomes of patients with sepsis.
The quick SOFA score (qSOFA), developed by the Society of Critical Care and European Society of Intensive Care Medicine in 2016 guidelines, further helps hospitalists and other hospital physicians identify those patients at highest risk of mortality from sepsis outside an intensive care unit setting.
Dr. Kritek, who is a board-certified critical care medicine physician, has previously presented this talk at the Society for Hospital Medicine Annual Conference in the past. This year the presentation will include a number of studies that examine what role vitamin C, thiamine, and glucocorticoids have in treating patients with sepsis, she said. For example, it is thought that parenteral administration of vitamin C could raise plasma levels and reduce multiorgan failure. Thiamine could be useful in sepsis treatment because of its role in glucose metabolism and lactate production, while glucocorticoids could help improve the mortality rate of patients with sepsis.
While Dr. Kritek said she is not going to be advocating for the benefit of vitamin C and thiamine during the session, “this is an area of ongoing debate, and we will walk through the most recent data to try to make sense of it,” she said.
Dr. Kritek noted that the role of balanced crystalloids in resuscitation will be discussed versus when to use saline, as well as the potential of new vasopressors for the treatment of septic shock.
“Our goal will be to integrate the most recent literature into day-to-day practice,” Dr. Kritek said.Dr. Kritek reports no conflicts of interest.
“Put SIRS on the SOFA and Let’s get Septic! Update in Sepsis”
Travel times to opioid addiction programs drive a lack of access to treatment
If US pharmacies were permitted to dispense methadone for opioid use disorder (OUD) it would improve national access to treatment and save costs, new research suggests.
Under current federal regulations, only opioid treatment programs (OTPs) are permitted to dispense methadone maintenance treatment. This stands in sharp contrast to how methadone is dispensed in Canada, Australia, and the United Kingdom, where patients can obtain daily doses of methadone maintenance from community pharmacies.
“It’s challenging for patients in many parts of the US to access methadone treatment,” Robert Kleinman, MD, of Stanford University School of Medicine, Stanford, California, said in a JAMA Psychiatry podcast.
“It’s important for policymakers to consider strategies that enhance access to methadone maintenance treatment, in that it’s associated with large reductions in mortality from opioid use disorder. One possibility is to use pharmacies as dispensing sites,” said Kleinman.
The study was published online July 15 in JAMA Psychiatry.
An Hour vs 10 Minutes
Kleinman examined how pharmacy-based dispensing would affect drive times to the nearest OTP for the general US population. The analysis included all 1682 OTP locations, 69,475 unique pharmacy locations, and 72,443 census tracts.
The average drive time to OTPs in the US is 20.4 minutes vs a drive time of 4.5 minutes to pharmacies.
Driving times to OTPs are particularly long in nonmetropolitan counties while pharmacies remain “relatively easily accessible” in nonmetropolitan counties, he said.
In “micropolitan” counties, for example, the drive time to OTPs was 48.4 minutes vs 7 minutes to pharmacies. In the most rural counties, the drive time to OTPs is 60.9 minutes vs 9.1 minutes to pharmacies.
“This suggests that pharmacy-based dispensing has the potential to reduce urban or rural inequities, and access to methadone treatment,” Kleinman said.
In a mileage cost analysis, Kleinman determined that the average cost of one-way trip to an OTP in the US is $3.12 compared with 45 cents to a pharmacy. In the most rural counties, the average cost one-way is $11.10 vs $1.27 to a pharmacy.
Kleinman says decreasing drive times, distance, and costs for patients seeking methadone treatment by allowing pharmacies to dispense the medication may help achieve several public health goals.
“Patients dissuaded from obtaining treatment because of extended travel, particularly patients with disabilities, unreliable access to transportation, or from rural regions, would have reduced barriers to care. Quality of life may be increased for the more than 380,000 individuals currently receiving methadone treatment if less time is spent commuting,” he writes.
Time for Change
The authors of an accompanying editorial, say the “regulatory burden” on methadone provision in the US “effectively prohibits the integration of methadone prescribing into primary care, even in rural communities where there may exist no specialty substance use treatment options.”
However, federal and state agencies are starting to take action to expand geographic access to methadone treatment, note Paul Joudrey, MD, MPH, and coauthors from Yale School of Medicine, New Haven, Connecticut.
, and Ohio and Kentucky have passed laws to allow greater use of federally qualified health centers and other facilities for methadone dispensing.
“While these policies are welcomed, the results here by Kleinman and others suggest they fall short of needed expansion if patients’ rights to evidence-based care for OUD are to be ensured. Importantly, even with broad adoption of mobile or pharmacy-based dispensing, patients would still face a long drive time to a central OTP before starting methadone,” Joudrey and colleagues write.
In their view, the only way to address this barrier is to modify federal law, and this “should be urgently pursued in the context of the ongoing overdose epidemic. It is time for policies that truly support methadone treatment for OUD as opposed to focusing on diversion.”
This article first appeared on Medscape.com.
If US pharmacies were permitted to dispense methadone for opioid use disorder (OUD) it would improve national access to treatment and save costs, new research suggests.
Under current federal regulations, only opioid treatment programs (OTPs) are permitted to dispense methadone maintenance treatment. This stands in sharp contrast to how methadone is dispensed in Canada, Australia, and the United Kingdom, where patients can obtain daily doses of methadone maintenance from community pharmacies.
“It’s challenging for patients in many parts of the US to access methadone treatment,” Robert Kleinman, MD, of Stanford University School of Medicine, Stanford, California, said in a JAMA Psychiatry podcast.
“It’s important for policymakers to consider strategies that enhance access to methadone maintenance treatment, in that it’s associated with large reductions in mortality from opioid use disorder. One possibility is to use pharmacies as dispensing sites,” said Kleinman.
The study was published online July 15 in JAMA Psychiatry.
An Hour vs 10 Minutes
Kleinman examined how pharmacy-based dispensing would affect drive times to the nearest OTP for the general US population. The analysis included all 1682 OTP locations, 69,475 unique pharmacy locations, and 72,443 census tracts.
The average drive time to OTPs in the US is 20.4 minutes vs a drive time of 4.5 minutes to pharmacies.
Driving times to OTPs are particularly long in nonmetropolitan counties while pharmacies remain “relatively easily accessible” in nonmetropolitan counties, he said.
In “micropolitan” counties, for example, the drive time to OTPs was 48.4 minutes vs 7 minutes to pharmacies. In the most rural counties, the drive time to OTPs is 60.9 minutes vs 9.1 minutes to pharmacies.
“This suggests that pharmacy-based dispensing has the potential to reduce urban or rural inequities, and access to methadone treatment,” Kleinman said.
In a mileage cost analysis, Kleinman determined that the average cost of one-way trip to an OTP in the US is $3.12 compared with 45 cents to a pharmacy. In the most rural counties, the average cost one-way is $11.10 vs $1.27 to a pharmacy.
Kleinman says decreasing drive times, distance, and costs for patients seeking methadone treatment by allowing pharmacies to dispense the medication may help achieve several public health goals.
“Patients dissuaded from obtaining treatment because of extended travel, particularly patients with disabilities, unreliable access to transportation, or from rural regions, would have reduced barriers to care. Quality of life may be increased for the more than 380,000 individuals currently receiving methadone treatment if less time is spent commuting,” he writes.
Time for Change
The authors of an accompanying editorial, say the “regulatory burden” on methadone provision in the US “effectively prohibits the integration of methadone prescribing into primary care, even in rural communities where there may exist no specialty substance use treatment options.”
However, federal and state agencies are starting to take action to expand geographic access to methadone treatment, note Paul Joudrey, MD, MPH, and coauthors from Yale School of Medicine, New Haven, Connecticut.
, and Ohio and Kentucky have passed laws to allow greater use of federally qualified health centers and other facilities for methadone dispensing.
“While these policies are welcomed, the results here by Kleinman and others suggest they fall short of needed expansion if patients’ rights to evidence-based care for OUD are to be ensured. Importantly, even with broad adoption of mobile or pharmacy-based dispensing, patients would still face a long drive time to a central OTP before starting methadone,” Joudrey and colleagues write.
In their view, the only way to address this barrier is to modify federal law, and this “should be urgently pursued in the context of the ongoing overdose epidemic. It is time for policies that truly support methadone treatment for OUD as opposed to focusing on diversion.”
This article first appeared on Medscape.com.
If US pharmacies were permitted to dispense methadone for opioid use disorder (OUD) it would improve national access to treatment and save costs, new research suggests.
Under current federal regulations, only opioid treatment programs (OTPs) are permitted to dispense methadone maintenance treatment. This stands in sharp contrast to how methadone is dispensed in Canada, Australia, and the United Kingdom, where patients can obtain daily doses of methadone maintenance from community pharmacies.
“It’s challenging for patients in many parts of the US to access methadone treatment,” Robert Kleinman, MD, of Stanford University School of Medicine, Stanford, California, said in a JAMA Psychiatry podcast.
“It’s important for policymakers to consider strategies that enhance access to methadone maintenance treatment, in that it’s associated with large reductions in mortality from opioid use disorder. One possibility is to use pharmacies as dispensing sites,” said Kleinman.
The study was published online July 15 in JAMA Psychiatry.
An Hour vs 10 Minutes
Kleinman examined how pharmacy-based dispensing would affect drive times to the nearest OTP for the general US population. The analysis included all 1682 OTP locations, 69,475 unique pharmacy locations, and 72,443 census tracts.
The average drive time to OTPs in the US is 20.4 minutes vs a drive time of 4.5 minutes to pharmacies.
Driving times to OTPs are particularly long in nonmetropolitan counties while pharmacies remain “relatively easily accessible” in nonmetropolitan counties, he said.
In “micropolitan” counties, for example, the drive time to OTPs was 48.4 minutes vs 7 minutes to pharmacies. In the most rural counties, the drive time to OTPs is 60.9 minutes vs 9.1 minutes to pharmacies.
“This suggests that pharmacy-based dispensing has the potential to reduce urban or rural inequities, and access to methadone treatment,” Kleinman said.
In a mileage cost analysis, Kleinman determined that the average cost of one-way trip to an OTP in the US is $3.12 compared with 45 cents to a pharmacy. In the most rural counties, the average cost one-way is $11.10 vs $1.27 to a pharmacy.
Kleinman says decreasing drive times, distance, and costs for patients seeking methadone treatment by allowing pharmacies to dispense the medication may help achieve several public health goals.
“Patients dissuaded from obtaining treatment because of extended travel, particularly patients with disabilities, unreliable access to transportation, or from rural regions, would have reduced barriers to care. Quality of life may be increased for the more than 380,000 individuals currently receiving methadone treatment if less time is spent commuting,” he writes.
Time for Change
The authors of an accompanying editorial, say the “regulatory burden” on methadone provision in the US “effectively prohibits the integration of methadone prescribing into primary care, even in rural communities where there may exist no specialty substance use treatment options.”
However, federal and state agencies are starting to take action to expand geographic access to methadone treatment, note Paul Joudrey, MD, MPH, and coauthors from Yale School of Medicine, New Haven, Connecticut.
, and Ohio and Kentucky have passed laws to allow greater use of federally qualified health centers and other facilities for methadone dispensing.
“While these policies are welcomed, the results here by Kleinman and others suggest they fall short of needed expansion if patients’ rights to evidence-based care for OUD are to be ensured. Importantly, even with broad adoption of mobile or pharmacy-based dispensing, patients would still face a long drive time to a central OTP before starting methadone,” Joudrey and colleagues write.
In their view, the only way to address this barrier is to modify federal law, and this “should be urgently pursued in the context of the ongoing overdose epidemic. It is time for policies that truly support methadone treatment for OUD as opposed to focusing on diversion.”
This article first appeared on Medscape.com.
Tips and tools for safe opioid prescribing
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1
The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16
Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30
Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,
At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).
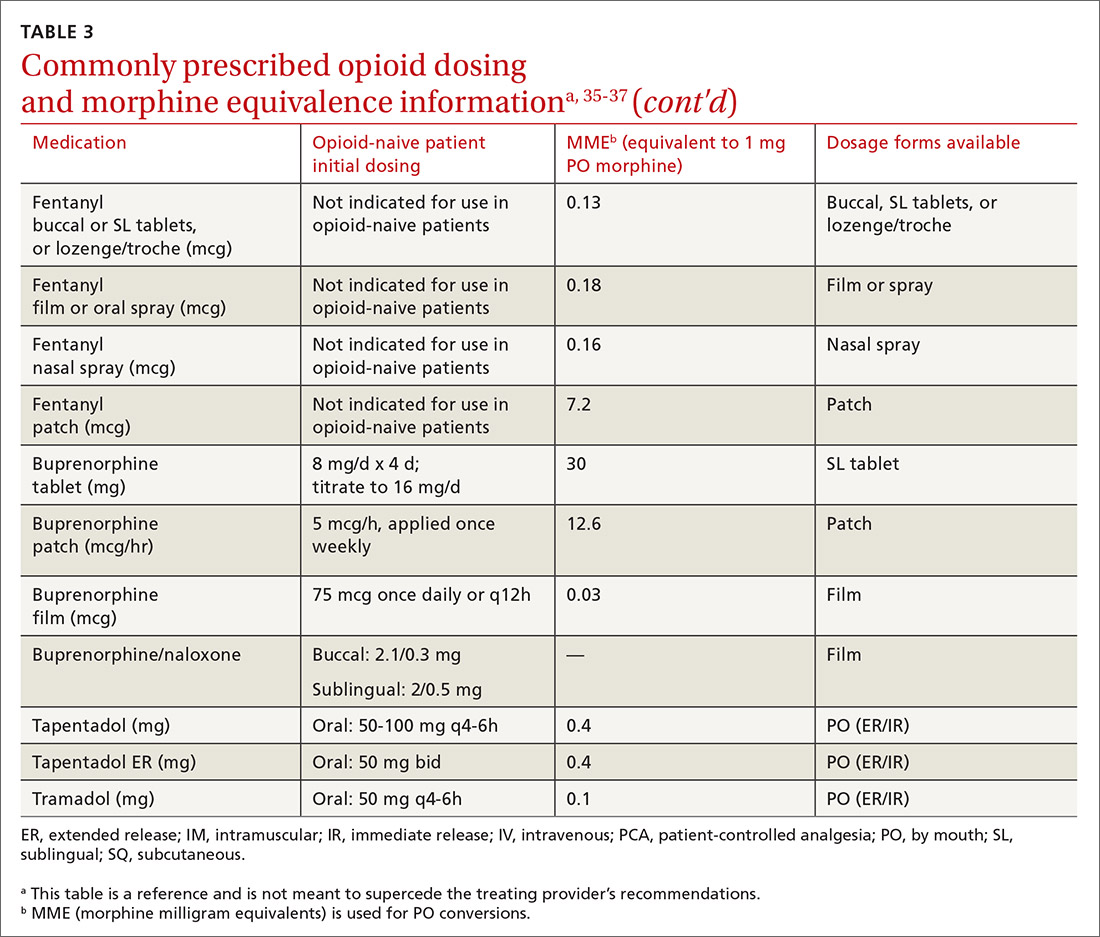
Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28
CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.
Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31
Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44
General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; tbaher@uwyo.edu.
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1
The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16
Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30
Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,
At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).

Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28
CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.
Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31
Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44
General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; tbaher@uwyo.edu.
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1
The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16
Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30
Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,
At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).

Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28
CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.
Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31
Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44
General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; tbaher@uwyo.edu.
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
PRACTICE RECOMMENDATIONS
› Use a screening instrument such as the Opioid Risk Tool or the DIRE assessment to gauge a patient’s risk of opioid misuse and determine the frequency of monitoring. C
› Give as much priority to improving functional activity and minimizing adverse opioid effects as you do to relieving pain. C
› Prescribe an immediate-release, short-acting agent at first instead of a long-acting formulation; start with the lowest effective dosage and calculate total daily dose in terms of morphine milligram equivalents (MME). C
› Reduce the original MME dose by 5% to 10% every week when discontinuing an opioid. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Patients usually understand and agree with physicians’ notes
Overall, 93% of respondents said the notes accurately described the visit; only 6% reported that something important was missing, write Suzanne G. Leveille, RN, PhD, of the University of Massachusetts, Boston, and colleagues in the Journal of General Internal Medicine.
“I think it’s wonderful news,” commented Howard Levy, MD, PhD, who spearheaded the implementation of open notes at Johns Hopkins University, Baltimore. “I’m thrilled with this report.”
Currently, 50 million Americans have access to their notes, the researchers report. Starting Nov. 2, 2020, the 21st Century Cures Act will require all US physicians to provide this access.
The regulation follows a movement to involve patients more actively in their care. Previous research has shown that access to visit notes improves patients’ feelings of control, helps them adhere to their medication regimens, and enables them to better understand their care plans.
Although physicians often feel that giving patients access to notes will lead to unnecessary conversations that will waste their time, previous studies have not borne that out. “Most clinical providers don’t notice a thing,” Levy told Medscape Medical News. “There was no change in the volume of work.”
Leveille and colleagues wanted to know how patients viewed the clarity, accuracy, and completeness of the notes they were reading and whether they had suggestions for improvements.
They surveyed all 136,815 adult outpatients affiliated with Beth Israel Deaconess Medical Center in Boston, Massachusetts; the University of Washington Medicine, in Seattle; and the Geisinger Health System, based in Danville, Pennsylvania. These systems all offer patients access to physicians’ notes.
The researchers asked the patients to recall one note written by a doctor, nurse practitioner, physician assistant, or mental health professional.
They received responses from 21,664 patients who had read at least one note. Of these, two thirds were women, three quarters were aged 45 years or older, and 85% were White.
Seventy-two percent had completed college. Although 85% reported being in good or excellent health, more of the respondents than nonrespondents had chronic health problems.
Ninety-seven percent of those with college educations understood their notes, compared with 92% of those who had not completed college, a finding that conflicted with the researchers’ expectations. “Good gracious, that’s wonderful,” Levy said. “In medicine we almost never get a 92% success rate in anything we do.”
Of the patients in fair or poor health, 88.6% said the note was accurate, compared with 94.4% of those in better health. Those in worse health were also more likely to say something important was missing.
When patients didn’t understand something, 35% searched the Internet, 27% asked a clinician, 7% asked a friend or family member, and 27% didn’t get help. (The researchers did not account for the other 4%.)
Of those patients whose note was written by a physician, 95% reported that the note accurately described the visit, compared with 92% of those whose note was written by a nurse practitioner and 90% of those whose note was written by a physician assistant.
Of patients reporting on a primary care note, 97% understood the note, compared with 94% of those reporting on a note from a visit to a specialist.
Ninety-three percent of those who understood their note were likely to recommend their clinician, compared with 77% of those who didn’t completely understand their note.
Asked how the notes could be improved, 3,812 people responded with comments of at least five words. These responses were included in the analysis.
Most commonly, patients wanted new information to be prominently featured at the top of the note, with clear instructions about next steps, referrals, and explanations of test results.
Often, they complained of old information or templates that felt impersonal. They stumbled over medical jargon and suggested links to glossaries. They bristled at such terms as “obese” and “patient denies.” Some wanted a way to comment on the notes.
Regarding the portals in which the notes were found, some patients said the notes were sometimes hard to find. Some said the notes were not posted quickly enough after the visits.
Levy said physicians should learn to write notes more succinctly, and he expects new regulations from the Centers for Medicare & Medicaid Services to encourage that. Previous regulations may have given physicians the impression that longer notes would allow them to bill at higher rates, he said. “The change in billing requirements will make it easier for healthcare providers to feel comfortable that they don’t have to restate information that had already been stated,” he said.
On the other hand, physicians should continue to use medical terminology, he said. “At times we use jargon, because it conveys rich, dense information in a few words,” he said. “That’s something that we should not have to give up.” Patients can research terms they don’t understand, he said.
Family physician Doug Iliff, MD, thinks it’s about time that his colleagues share their notes. He’s been doing it since he opened his solo practice in Topeka, Kansas, in 1984.
He still does it the way he always did, with carbonless copy paper. After each visit, he simply tears off the copy and hands it to the patient.
“It makes them know we’re on the same page,” he told Medscape Medical News. “It gives them confidence that I’m telling them what I really think.”
He has one comment on the work of Leveille and her colleagues. “Why are they studying this? Isn’t it obvious that it’s a good thing?”
The study was funded by the Robert Wood Johnson Foundation, the Gordon and Betty Moore Foundation, the Peterson Center on Healthcare, and the Cambia Health Foundation. The study authors, Iliff, and Levy have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Overall, 93% of respondents said the notes accurately described the visit; only 6% reported that something important was missing, write Suzanne G. Leveille, RN, PhD, of the University of Massachusetts, Boston, and colleagues in the Journal of General Internal Medicine.
“I think it’s wonderful news,” commented Howard Levy, MD, PhD, who spearheaded the implementation of open notes at Johns Hopkins University, Baltimore. “I’m thrilled with this report.”
Currently, 50 million Americans have access to their notes, the researchers report. Starting Nov. 2, 2020, the 21st Century Cures Act will require all US physicians to provide this access.
The regulation follows a movement to involve patients more actively in their care. Previous research has shown that access to visit notes improves patients’ feelings of control, helps them adhere to their medication regimens, and enables them to better understand their care plans.
Although physicians often feel that giving patients access to notes will lead to unnecessary conversations that will waste their time, previous studies have not borne that out. “Most clinical providers don’t notice a thing,” Levy told Medscape Medical News. “There was no change in the volume of work.”
Leveille and colleagues wanted to know how patients viewed the clarity, accuracy, and completeness of the notes they were reading and whether they had suggestions for improvements.
They surveyed all 136,815 adult outpatients affiliated with Beth Israel Deaconess Medical Center in Boston, Massachusetts; the University of Washington Medicine, in Seattle; and the Geisinger Health System, based in Danville, Pennsylvania. These systems all offer patients access to physicians’ notes.
The researchers asked the patients to recall one note written by a doctor, nurse practitioner, physician assistant, or mental health professional.
They received responses from 21,664 patients who had read at least one note. Of these, two thirds were women, three quarters were aged 45 years or older, and 85% were White.
Seventy-two percent had completed college. Although 85% reported being in good or excellent health, more of the respondents than nonrespondents had chronic health problems.
Ninety-seven percent of those with college educations understood their notes, compared with 92% of those who had not completed college, a finding that conflicted with the researchers’ expectations. “Good gracious, that’s wonderful,” Levy said. “In medicine we almost never get a 92% success rate in anything we do.”
Of the patients in fair or poor health, 88.6% said the note was accurate, compared with 94.4% of those in better health. Those in worse health were also more likely to say something important was missing.
When patients didn’t understand something, 35% searched the Internet, 27% asked a clinician, 7% asked a friend or family member, and 27% didn’t get help. (The researchers did not account for the other 4%.)
Of those patients whose note was written by a physician, 95% reported that the note accurately described the visit, compared with 92% of those whose note was written by a nurse practitioner and 90% of those whose note was written by a physician assistant.
Of patients reporting on a primary care note, 97% understood the note, compared with 94% of those reporting on a note from a visit to a specialist.
Ninety-three percent of those who understood their note were likely to recommend their clinician, compared with 77% of those who didn’t completely understand their note.
Asked how the notes could be improved, 3,812 people responded with comments of at least five words. These responses were included in the analysis.
Most commonly, patients wanted new information to be prominently featured at the top of the note, with clear instructions about next steps, referrals, and explanations of test results.
Often, they complained of old information or templates that felt impersonal. They stumbled over medical jargon and suggested links to glossaries. They bristled at such terms as “obese” and “patient denies.” Some wanted a way to comment on the notes.
Regarding the portals in which the notes were found, some patients said the notes were sometimes hard to find. Some said the notes were not posted quickly enough after the visits.
Levy said physicians should learn to write notes more succinctly, and he expects new regulations from the Centers for Medicare & Medicaid Services to encourage that. Previous regulations may have given physicians the impression that longer notes would allow them to bill at higher rates, he said. “The change in billing requirements will make it easier for healthcare providers to feel comfortable that they don’t have to restate information that had already been stated,” he said.
On the other hand, physicians should continue to use medical terminology, he said. “At times we use jargon, because it conveys rich, dense information in a few words,” he said. “That’s something that we should not have to give up.” Patients can research terms they don’t understand, he said.
Family physician Doug Iliff, MD, thinks it’s about time that his colleagues share their notes. He’s been doing it since he opened his solo practice in Topeka, Kansas, in 1984.
He still does it the way he always did, with carbonless copy paper. After each visit, he simply tears off the copy and hands it to the patient.
“It makes them know we’re on the same page,” he told Medscape Medical News. “It gives them confidence that I’m telling them what I really think.”
He has one comment on the work of Leveille and her colleagues. “Why are they studying this? Isn’t it obvious that it’s a good thing?”
The study was funded by the Robert Wood Johnson Foundation, the Gordon and Betty Moore Foundation, the Peterson Center on Healthcare, and the Cambia Health Foundation. The study authors, Iliff, and Levy have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Overall, 93% of respondents said the notes accurately described the visit; only 6% reported that something important was missing, write Suzanne G. Leveille, RN, PhD, of the University of Massachusetts, Boston, and colleagues in the Journal of General Internal Medicine.
“I think it’s wonderful news,” commented Howard Levy, MD, PhD, who spearheaded the implementation of open notes at Johns Hopkins University, Baltimore. “I’m thrilled with this report.”
Currently, 50 million Americans have access to their notes, the researchers report. Starting Nov. 2, 2020, the 21st Century Cures Act will require all US physicians to provide this access.
The regulation follows a movement to involve patients more actively in their care. Previous research has shown that access to visit notes improves patients’ feelings of control, helps them adhere to their medication regimens, and enables them to better understand their care plans.
Although physicians often feel that giving patients access to notes will lead to unnecessary conversations that will waste their time, previous studies have not borne that out. “Most clinical providers don’t notice a thing,” Levy told Medscape Medical News. “There was no change in the volume of work.”
Leveille and colleagues wanted to know how patients viewed the clarity, accuracy, and completeness of the notes they were reading and whether they had suggestions for improvements.
They surveyed all 136,815 adult outpatients affiliated with Beth Israel Deaconess Medical Center in Boston, Massachusetts; the University of Washington Medicine, in Seattle; and the Geisinger Health System, based in Danville, Pennsylvania. These systems all offer patients access to physicians’ notes.
The researchers asked the patients to recall one note written by a doctor, nurse practitioner, physician assistant, or mental health professional.
They received responses from 21,664 patients who had read at least one note. Of these, two thirds were women, three quarters were aged 45 years or older, and 85% were White.
Seventy-two percent had completed college. Although 85% reported being in good or excellent health, more of the respondents than nonrespondents had chronic health problems.
Ninety-seven percent of those with college educations understood their notes, compared with 92% of those who had not completed college, a finding that conflicted with the researchers’ expectations. “Good gracious, that’s wonderful,” Levy said. “In medicine we almost never get a 92% success rate in anything we do.”
Of the patients in fair or poor health, 88.6% said the note was accurate, compared with 94.4% of those in better health. Those in worse health were also more likely to say something important was missing.
When patients didn’t understand something, 35% searched the Internet, 27% asked a clinician, 7% asked a friend or family member, and 27% didn’t get help. (The researchers did not account for the other 4%.)
Of those patients whose note was written by a physician, 95% reported that the note accurately described the visit, compared with 92% of those whose note was written by a nurse practitioner and 90% of those whose note was written by a physician assistant.
Of patients reporting on a primary care note, 97% understood the note, compared with 94% of those reporting on a note from a visit to a specialist.
Ninety-three percent of those who understood their note were likely to recommend their clinician, compared with 77% of those who didn’t completely understand their note.
Asked how the notes could be improved, 3,812 people responded with comments of at least five words. These responses were included in the analysis.
Most commonly, patients wanted new information to be prominently featured at the top of the note, with clear instructions about next steps, referrals, and explanations of test results.
Often, they complained of old information or templates that felt impersonal. They stumbled over medical jargon and suggested links to glossaries. They bristled at such terms as “obese” and “patient denies.” Some wanted a way to comment on the notes.
Regarding the portals in which the notes were found, some patients said the notes were sometimes hard to find. Some said the notes were not posted quickly enough after the visits.
Levy said physicians should learn to write notes more succinctly, and he expects new regulations from the Centers for Medicare & Medicaid Services to encourage that. Previous regulations may have given physicians the impression that longer notes would allow them to bill at higher rates, he said. “The change in billing requirements will make it easier for healthcare providers to feel comfortable that they don’t have to restate information that had already been stated,” he said.
On the other hand, physicians should continue to use medical terminology, he said. “At times we use jargon, because it conveys rich, dense information in a few words,” he said. “That’s something that we should not have to give up.” Patients can research terms they don’t understand, he said.
Family physician Doug Iliff, MD, thinks it’s about time that his colleagues share their notes. He’s been doing it since he opened his solo practice in Topeka, Kansas, in 1984.
He still does it the way he always did, with carbonless copy paper. After each visit, he simply tears off the copy and hands it to the patient.
“It makes them know we’re on the same page,” he told Medscape Medical News. “It gives them confidence that I’m telling them what I really think.”
He has one comment on the work of Leveille and her colleagues. “Why are they studying this? Isn’t it obvious that it’s a good thing?”
The study was funded by the Robert Wood Johnson Foundation, the Gordon and Betty Moore Foundation, the Peterson Center on Healthcare, and the Cambia Health Foundation. The study authors, Iliff, and Levy have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.