User login
OSHA in the COVID-19 era
As more and more reopened medical practices ramp up toward normal activity, the safety of patients and health care workers alike remains paramount. As always,
Most of the modified guidelines are already familiar: wear masks (and other personal protective equipment as necessary); maintain social distancing; have hand cleaner, soap, and water readily available; and sanitize between patient examinations.
It is also important to remember that COVID-19 is now a reportable disease; check with your local health authorities as to where and how. Also remember that, if you decide to screen employees and/or patients for fevers and other symptoms of COVID-19, those data are subject to HIPAA rules and must be kept confidential.
Now might be a good time to confirm that you remain in compliance with both the new and old regulations. Even if you hold regular safety meetings – which often is not the case – it is always a good idea to occasionally conduct a comprehensive review, which could save you a lot in fines.
So get your OSHA logs out, and walk through your office. Start by making sure you have an official OSHA poster, which enumerates employee rights and explains how to file complaints. Every office must have one posted in plain site, and is what an OSHA inspector will look for first. They are available for free at OSHA’s website or you can order one by calling 800-321-OSHA.
How long have you had your written exposure control plan for blood-borne pathogens? This plan should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, as well as your implementation of universal precautions. It should be updated annually to reflect changes in technology – and new threats, such as COVID-19.
Review your list of hazardous substances, which all employees have a right to know about. OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are classified as “hazardous.” Also remember that you’re probably using new disinfectants, which may need to be added to your list. For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
It is not necessary to adopt every new safety device as it comes on the market, but you should document which ones you are using and which ones you decide not to use – and why. For example, if you and your employees decide against buying a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it is worth, you still should document how you made that decision and why you believe that your current protocol is as good or better.
All at-risk employees should be provided with hepatitis B vaccine at no cost to them. And after any exposure to dangerous pathogens – which now include COVID-19 – you also must provide and pay for appropriate medical treatment and follow-up.
Another important consideration in your review: Electrical devices and their power sources in the office. All electrically powered equipment – medical or clerical – must operate safely and should all be examined. It is particularly important to check how wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Medical and dental offices are not required to keep an injury and illness log under federal OSHA regulations, which other businesses must. However, your state may have a requirement that supersedes the federal law so you should check with your state, or with your local OSHA office, regarding any such requirements.
It is important to take OSHA regulations seriously because failure to comply with them can result in stiff penalties running into many thousands of dollars.
To be certain you are complying with all the rules, you can call your local OSHA office and request an inspection. This is the easiest and cheapest way because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they discover.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
As more and more reopened medical practices ramp up toward normal activity, the safety of patients and health care workers alike remains paramount. As always,
Most of the modified guidelines are already familiar: wear masks (and other personal protective equipment as necessary); maintain social distancing; have hand cleaner, soap, and water readily available; and sanitize between patient examinations.
It is also important to remember that COVID-19 is now a reportable disease; check with your local health authorities as to where and how. Also remember that, if you decide to screen employees and/or patients for fevers and other symptoms of COVID-19, those data are subject to HIPAA rules and must be kept confidential.
Now might be a good time to confirm that you remain in compliance with both the new and old regulations. Even if you hold regular safety meetings – which often is not the case – it is always a good idea to occasionally conduct a comprehensive review, which could save you a lot in fines.
So get your OSHA logs out, and walk through your office. Start by making sure you have an official OSHA poster, which enumerates employee rights and explains how to file complaints. Every office must have one posted in plain site, and is what an OSHA inspector will look for first. They are available for free at OSHA’s website or you can order one by calling 800-321-OSHA.
How long have you had your written exposure control plan for blood-borne pathogens? This plan should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, as well as your implementation of universal precautions. It should be updated annually to reflect changes in technology – and new threats, such as COVID-19.
Review your list of hazardous substances, which all employees have a right to know about. OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are classified as “hazardous.” Also remember that you’re probably using new disinfectants, which may need to be added to your list. For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
It is not necessary to adopt every new safety device as it comes on the market, but you should document which ones you are using and which ones you decide not to use – and why. For example, if you and your employees decide against buying a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it is worth, you still should document how you made that decision and why you believe that your current protocol is as good or better.
All at-risk employees should be provided with hepatitis B vaccine at no cost to them. And after any exposure to dangerous pathogens – which now include COVID-19 – you also must provide and pay for appropriate medical treatment and follow-up.
Another important consideration in your review: Electrical devices and their power sources in the office. All electrically powered equipment – medical or clerical – must operate safely and should all be examined. It is particularly important to check how wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Medical and dental offices are not required to keep an injury and illness log under federal OSHA regulations, which other businesses must. However, your state may have a requirement that supersedes the federal law so you should check with your state, or with your local OSHA office, regarding any such requirements.
It is important to take OSHA regulations seriously because failure to comply with them can result in stiff penalties running into many thousands of dollars.
To be certain you are complying with all the rules, you can call your local OSHA office and request an inspection. This is the easiest and cheapest way because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they discover.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
As more and more reopened medical practices ramp up toward normal activity, the safety of patients and health care workers alike remains paramount. As always,
Most of the modified guidelines are already familiar: wear masks (and other personal protective equipment as necessary); maintain social distancing; have hand cleaner, soap, and water readily available; and sanitize between patient examinations.
It is also important to remember that COVID-19 is now a reportable disease; check with your local health authorities as to where and how. Also remember that, if you decide to screen employees and/or patients for fevers and other symptoms of COVID-19, those data are subject to HIPAA rules and must be kept confidential.
Now might be a good time to confirm that you remain in compliance with both the new and old regulations. Even if you hold regular safety meetings – which often is not the case – it is always a good idea to occasionally conduct a comprehensive review, which could save you a lot in fines.
So get your OSHA logs out, and walk through your office. Start by making sure you have an official OSHA poster, which enumerates employee rights and explains how to file complaints. Every office must have one posted in plain site, and is what an OSHA inspector will look for first. They are available for free at OSHA’s website or you can order one by calling 800-321-OSHA.
How long have you had your written exposure control plan for blood-borne pathogens? This plan should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, as well as your implementation of universal precautions. It should be updated annually to reflect changes in technology – and new threats, such as COVID-19.
Review your list of hazardous substances, which all employees have a right to know about. OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are classified as “hazardous.” Also remember that you’re probably using new disinfectants, which may need to be added to your list. For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
It is not necessary to adopt every new safety device as it comes on the market, but you should document which ones you are using and which ones you decide not to use – and why. For example, if you and your employees decide against buying a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it is worth, you still should document how you made that decision and why you believe that your current protocol is as good or better.
All at-risk employees should be provided with hepatitis B vaccine at no cost to them. And after any exposure to dangerous pathogens – which now include COVID-19 – you also must provide and pay for appropriate medical treatment and follow-up.
Another important consideration in your review: Electrical devices and their power sources in the office. All electrically powered equipment – medical or clerical – must operate safely and should all be examined. It is particularly important to check how wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it and that circuit breakers are present and functioning.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Medical and dental offices are not required to keep an injury and illness log under federal OSHA regulations, which other businesses must. However, your state may have a requirement that supersedes the federal law so you should check with your state, or with your local OSHA office, regarding any such requirements.
It is important to take OSHA regulations seriously because failure to comply with them can result in stiff penalties running into many thousands of dollars.
To be certain you are complying with all the rules, you can call your local OSHA office and request an inspection. This is the easiest and cheapest way because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they discover.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Medications may drive postmenopausal weight gain
based on data from more than 76,000 individuals in the Women’s Health Initiative.
“Many of the medications prescribed to treat obesity-related comorbidities such as hypertension, type 2 diabetes, and depression have been linked to weight gain,” but the impact of such medications in relation to changes in body mass index (BMI) and waist circumference in postmenopausal women in particular has not been studied, wrote Fatima Cody Stanford, MD, of Harvard Medical School, Boston, and colleagues.
“Postmenopausal women are of significant interest as those who have obesity and normal weight central obesity are at increased risk for conditions such as invasive breast cancer, sleep disturbances, and type 2 diabetes, as well as mortality,” they wrote.
In a study published in the journal Menopause, the researchers identified 76,252 postmenopausal women aged 50-79 years and measured body mass index at baseline and after 3 years. Medication use was determined by a medication inventory of pill bottles brought to baseline and year-3 visits.
During a 3-year follow-up period, the average BMI increase was 0.37 kg/m2 in women taking at least one weight-promoting medication, compared with an average increase of 0.27 kg/m2 in women not taking such medications (P = .0045). Weight-promoting medications in the study included antidepressants, beta-blockers, insulin, and/or glucocorticosteroids. The researchers used generalized linear models to assess the impact of these medications on increased BMI and waist circumference.
In addition, the average increase in waist circumference was 1.10 cm in women taking at least one weight-promoting medication, compared with 0.89 cm (P = .0077) for women not on such medications.
“Type of medication, dosage, and race/ethnicity may have important interrelationships,” in postmenopausal weight gain, as do individual susceptibility and genetics, the researchers noted. “Options to mitigate the weight gain may include proactive lifestyle modifications, reduction in dose, change to another agent, or discontinuation of the medication altogether. If alternative medications are not an option, lifestyle factors such as diet quality, physical activity level, and sleep quality and duration warrant emphasis.”
The study findings were limited by several factors, including a lack of data on indications and underlying health conditions surrounding the prescription of various medications, notably psychotropics and antipsychotics, the researchers wrote.
However, the data “may help to inform clinical decision-making and support increased attention to lifestyle modifications and other strategies” to mitigate the potential for weight gain in a population already at risk for overweight and obesity over time, they concluded.
“Given the obesity epidemic, addressing factors contributing to weight gain in midlife [a time associated with weight gain] women is critical,” Stephanie S. Faubion, MD, of the Mayo Clinic in Jacksonville, Fla., said in an interview. Dr. Faubion said that the study findings were not surprising given the widespread use of known weight-promoting medications by midlife women for such as hypertension, diabetes, and depression.
“Clinicians need to ensure that they prescribe medications that are truly needed and utilize the lowest dose required to achieve treatment goals,” Dr. Faubion said. “When possible, alternative therapies that do not cause weight gain should be considered. In addition, patients should be warned of the potential for weight gain, and clinicians should advocate for lifestyle measures aimed at mitigating these effects.”
The findings do not encourage the use of alternative therapies for menopausal symptoms per se, added Dr. Faubion, who is also medical director of the North American Menopause Society. “Hormone therapy is not associated with weight gain, and if anything, it is weight favorable and associated with less weight around the midsection. It is the alternative strategies for management of hot flashes that are associated with weight gain, such as antidepressants and gabapentin.
“We need to focus efforts on strategies to prevent weight gain in midlife to avoid the development of conditions that necessitate initiation of many of these weight-promoting medications,” Dr. Faubion said.
The study was supported by the National Institutes of Health and Massachusetts General Hospital Executive Committee on Research, the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases. The researchers had no financial conflicts to disclose. Dr. Faubion had no financial conflicts to disclose.
SOURCE: Stanford FC et al. Menopause. 2020 Jul 13. doi: 10.1097/GME.0000000000001589.
based on data from more than 76,000 individuals in the Women’s Health Initiative.
“Many of the medications prescribed to treat obesity-related comorbidities such as hypertension, type 2 diabetes, and depression have been linked to weight gain,” but the impact of such medications in relation to changes in body mass index (BMI) and waist circumference in postmenopausal women in particular has not been studied, wrote Fatima Cody Stanford, MD, of Harvard Medical School, Boston, and colleagues.
“Postmenopausal women are of significant interest as those who have obesity and normal weight central obesity are at increased risk for conditions such as invasive breast cancer, sleep disturbances, and type 2 diabetes, as well as mortality,” they wrote.
In a study published in the journal Menopause, the researchers identified 76,252 postmenopausal women aged 50-79 years and measured body mass index at baseline and after 3 years. Medication use was determined by a medication inventory of pill bottles brought to baseline and year-3 visits.
During a 3-year follow-up period, the average BMI increase was 0.37 kg/m2 in women taking at least one weight-promoting medication, compared with an average increase of 0.27 kg/m2 in women not taking such medications (P = .0045). Weight-promoting medications in the study included antidepressants, beta-blockers, insulin, and/or glucocorticosteroids. The researchers used generalized linear models to assess the impact of these medications on increased BMI and waist circumference.
In addition, the average increase in waist circumference was 1.10 cm in women taking at least one weight-promoting medication, compared with 0.89 cm (P = .0077) for women not on such medications.
“Type of medication, dosage, and race/ethnicity may have important interrelationships,” in postmenopausal weight gain, as do individual susceptibility and genetics, the researchers noted. “Options to mitigate the weight gain may include proactive lifestyle modifications, reduction in dose, change to another agent, or discontinuation of the medication altogether. If alternative medications are not an option, lifestyle factors such as diet quality, physical activity level, and sleep quality and duration warrant emphasis.”
The study findings were limited by several factors, including a lack of data on indications and underlying health conditions surrounding the prescription of various medications, notably psychotropics and antipsychotics, the researchers wrote.
However, the data “may help to inform clinical decision-making and support increased attention to lifestyle modifications and other strategies” to mitigate the potential for weight gain in a population already at risk for overweight and obesity over time, they concluded.
“Given the obesity epidemic, addressing factors contributing to weight gain in midlife [a time associated with weight gain] women is critical,” Stephanie S. Faubion, MD, of the Mayo Clinic in Jacksonville, Fla., said in an interview. Dr. Faubion said that the study findings were not surprising given the widespread use of known weight-promoting medications by midlife women for such as hypertension, diabetes, and depression.
“Clinicians need to ensure that they prescribe medications that are truly needed and utilize the lowest dose required to achieve treatment goals,” Dr. Faubion said. “When possible, alternative therapies that do not cause weight gain should be considered. In addition, patients should be warned of the potential for weight gain, and clinicians should advocate for lifestyle measures aimed at mitigating these effects.”
The findings do not encourage the use of alternative therapies for menopausal symptoms per se, added Dr. Faubion, who is also medical director of the North American Menopause Society. “Hormone therapy is not associated with weight gain, and if anything, it is weight favorable and associated with less weight around the midsection. It is the alternative strategies for management of hot flashes that are associated with weight gain, such as antidepressants and gabapentin.
“We need to focus efforts on strategies to prevent weight gain in midlife to avoid the development of conditions that necessitate initiation of many of these weight-promoting medications,” Dr. Faubion said.
The study was supported by the National Institutes of Health and Massachusetts General Hospital Executive Committee on Research, the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases. The researchers had no financial conflicts to disclose. Dr. Faubion had no financial conflicts to disclose.
SOURCE: Stanford FC et al. Menopause. 2020 Jul 13. doi: 10.1097/GME.0000000000001589.
based on data from more than 76,000 individuals in the Women’s Health Initiative.
“Many of the medications prescribed to treat obesity-related comorbidities such as hypertension, type 2 diabetes, and depression have been linked to weight gain,” but the impact of such medications in relation to changes in body mass index (BMI) and waist circumference in postmenopausal women in particular has not been studied, wrote Fatima Cody Stanford, MD, of Harvard Medical School, Boston, and colleagues.
“Postmenopausal women are of significant interest as those who have obesity and normal weight central obesity are at increased risk for conditions such as invasive breast cancer, sleep disturbances, and type 2 diabetes, as well as mortality,” they wrote.
In a study published in the journal Menopause, the researchers identified 76,252 postmenopausal women aged 50-79 years and measured body mass index at baseline and after 3 years. Medication use was determined by a medication inventory of pill bottles brought to baseline and year-3 visits.
During a 3-year follow-up period, the average BMI increase was 0.37 kg/m2 in women taking at least one weight-promoting medication, compared with an average increase of 0.27 kg/m2 in women not taking such medications (P = .0045). Weight-promoting medications in the study included antidepressants, beta-blockers, insulin, and/or glucocorticosteroids. The researchers used generalized linear models to assess the impact of these medications on increased BMI and waist circumference.
In addition, the average increase in waist circumference was 1.10 cm in women taking at least one weight-promoting medication, compared with 0.89 cm (P = .0077) for women not on such medications.
“Type of medication, dosage, and race/ethnicity may have important interrelationships,” in postmenopausal weight gain, as do individual susceptibility and genetics, the researchers noted. “Options to mitigate the weight gain may include proactive lifestyle modifications, reduction in dose, change to another agent, or discontinuation of the medication altogether. If alternative medications are not an option, lifestyle factors such as diet quality, physical activity level, and sleep quality and duration warrant emphasis.”
The study findings were limited by several factors, including a lack of data on indications and underlying health conditions surrounding the prescription of various medications, notably psychotropics and antipsychotics, the researchers wrote.
However, the data “may help to inform clinical decision-making and support increased attention to lifestyle modifications and other strategies” to mitigate the potential for weight gain in a population already at risk for overweight and obesity over time, they concluded.
“Given the obesity epidemic, addressing factors contributing to weight gain in midlife [a time associated with weight gain] women is critical,” Stephanie S. Faubion, MD, of the Mayo Clinic in Jacksonville, Fla., said in an interview. Dr. Faubion said that the study findings were not surprising given the widespread use of known weight-promoting medications by midlife women for such as hypertension, diabetes, and depression.
“Clinicians need to ensure that they prescribe medications that are truly needed and utilize the lowest dose required to achieve treatment goals,” Dr. Faubion said. “When possible, alternative therapies that do not cause weight gain should be considered. In addition, patients should be warned of the potential for weight gain, and clinicians should advocate for lifestyle measures aimed at mitigating these effects.”
The findings do not encourage the use of alternative therapies for menopausal symptoms per se, added Dr. Faubion, who is also medical director of the North American Menopause Society. “Hormone therapy is not associated with weight gain, and if anything, it is weight favorable and associated with less weight around the midsection. It is the alternative strategies for management of hot flashes that are associated with weight gain, such as antidepressants and gabapentin.
“We need to focus efforts on strategies to prevent weight gain in midlife to avoid the development of conditions that necessitate initiation of many of these weight-promoting medications,” Dr. Faubion said.
The study was supported by the National Institutes of Health and Massachusetts General Hospital Executive Committee on Research, the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases. The researchers had no financial conflicts to disclose. Dr. Faubion had no financial conflicts to disclose.
SOURCE: Stanford FC et al. Menopause. 2020 Jul 13. doi: 10.1097/GME.0000000000001589.
FROM MENOPAUSE
Leadership & Professional Development: Dis-Missed: Cultural and Gender Barriers to Graceful Self-Promotion
“The world accommodates you for fitting in, but only rewards you for standing out.”
—Matshona Dhliwayo
Graceful self-promotion—a way of speaking diplomatically and strategically about yourself and your accomplishments—is a key behavior to achieve professional success in medicine. However, some of us are uncomfortable with promoting ourselves in the workplace because of concerns about receiving negative backlash for bragging. These concerns may have roots in our cultural and gender backgrounds, norms that strongly influence our social behaviors. Cultures that emphasize collectivism (eg, East Asia, Scandinavia, Latin America), which is associated with modesty and a focus on “we,” may not approve of self-promotion in contrast to cultures that emphasize individualism (eg, United States, Canada, and parts of Western Europe).1 Additionally, societal gender roles across different cultures focus on women conforming to a “modesty norm,” by which they are socialized to “be nice” and “not too demanding.” Female physicians practicing self-promotion for career advancement may experience a backlash with social penalties and career repercussions.2
One’s avoiding self-promotion may lead others to prematurely dismiss a physician’s capability, competence, ambition, and qualifications for leadership and other opportunities. These oversights may be a contributing factor in the existing inequities in physician compensation, faculty promotions, leadership roles, speaking engagements, journal editorial boards, and more. Women make up over 50% of all US medical students, yet only 18% are hospital CEOs, 16% are deans and department chairs, and 7% are editors-in-chief of high-impact medical journals.3
So how do you get started overcoming cultural and gender barriers and embrace graceful self-promotion? Start small!
First, write a reference or nominating letter for a colleague. The exercise of synthesizing someone else’s accomplishments, skills, and experiences for a specific audience and purpose will give you a template to apply to yourself.
Second, identify an accomplishment with an outcome that educates others about you, your ideas, and your impact. Practice with a trusted peer to frame your accomplishment and its context as a story; for example: “Dr. X, I am pleased to share that I will present a key workshop on Y at the upcoming national Z meeting, based largely on the outcomes from a QI initiative that I developed and oversaw with support from my hospitalist team. We overcame initial staff resistance by recruiting project champions among the interdisciplinary team and successfully reduced readmissions for Y from A% to B% over a 12-month period.”
Third, consider when and how to strategically promote the accomplishment with your medical director, clinical leadership, department leadership, etc. Start out gracefully self-promoting in person or via email with a leader with whom you already have a relationship. If you want to share your accomplishment with a leader who does not yet know you (but may be important to your career), nudge a mentor or sponsor for an introductory conversation.
Finally, ask yourself the next time you are doing a performance review or attending a hospital committee meeting: Am I contributing to a culture in which everyone is encouraged to share their accomplishments? Which qualified candidates who don’t speak out about themselves can I nominate, sponsor, mentor, or encourage for an upcoming opportunity to increase cultural and gender representation? After all, paying it forward helps foster the success of others.
Graceful self-promotion is an important tool for personal and professional development in healthcare. Cultural and gender-based barriers to self-promotion can be surmounted through self-awareness, practice with trusted peers, and recognition of the importance of storytelling gracefully. A medical workplace culture that encourages sharing achievements and celebrates individual and team accomplishments can go a long way toward helping people change their perception of self-promotion and overcome their hesitations.
1. Lalwani AK, Shavitt S. The “me” I claim to be: cultural self-construal elicits self-presentational goal pursuit. J Pers Soc Psychol. 2009;97(1):88-102. https://doi.org/10.1037/a0014100
2. Templeton K, Bernstein CA, Sukhera J, Nora LM, et al. Gender-based differences in burnout: issues faced by women physicians. NAM Perspectives. 2019. Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/201905a
3. Mangurian C, Linos E, Sarkar U, Rodriguez C, Jagsi R. What’s holding women in medicine back from leadership. Harvard Business Review. 2018. https://hbr.org/2018/06/whats-holding-women-in-medicine-back-from-leadership
“The world accommodates you for fitting in, but only rewards you for standing out.”
—Matshona Dhliwayo
Graceful self-promotion—a way of speaking diplomatically and strategically about yourself and your accomplishments—is a key behavior to achieve professional success in medicine. However, some of us are uncomfortable with promoting ourselves in the workplace because of concerns about receiving negative backlash for bragging. These concerns may have roots in our cultural and gender backgrounds, norms that strongly influence our social behaviors. Cultures that emphasize collectivism (eg, East Asia, Scandinavia, Latin America), which is associated with modesty and a focus on “we,” may not approve of self-promotion in contrast to cultures that emphasize individualism (eg, United States, Canada, and parts of Western Europe).1 Additionally, societal gender roles across different cultures focus on women conforming to a “modesty norm,” by which they are socialized to “be nice” and “not too demanding.” Female physicians practicing self-promotion for career advancement may experience a backlash with social penalties and career repercussions.2
One’s avoiding self-promotion may lead others to prematurely dismiss a physician’s capability, competence, ambition, and qualifications for leadership and other opportunities. These oversights may be a contributing factor in the existing inequities in physician compensation, faculty promotions, leadership roles, speaking engagements, journal editorial boards, and more. Women make up over 50% of all US medical students, yet only 18% are hospital CEOs, 16% are deans and department chairs, and 7% are editors-in-chief of high-impact medical journals.3
So how do you get started overcoming cultural and gender barriers and embrace graceful self-promotion? Start small!
First, write a reference or nominating letter for a colleague. The exercise of synthesizing someone else’s accomplishments, skills, and experiences for a specific audience and purpose will give you a template to apply to yourself.
Second, identify an accomplishment with an outcome that educates others about you, your ideas, and your impact. Practice with a trusted peer to frame your accomplishment and its context as a story; for example: “Dr. X, I am pleased to share that I will present a key workshop on Y at the upcoming national Z meeting, based largely on the outcomes from a QI initiative that I developed and oversaw with support from my hospitalist team. We overcame initial staff resistance by recruiting project champions among the interdisciplinary team and successfully reduced readmissions for Y from A% to B% over a 12-month period.”
Third, consider when and how to strategically promote the accomplishment with your medical director, clinical leadership, department leadership, etc. Start out gracefully self-promoting in person or via email with a leader with whom you already have a relationship. If you want to share your accomplishment with a leader who does not yet know you (but may be important to your career), nudge a mentor or sponsor for an introductory conversation.
Finally, ask yourself the next time you are doing a performance review or attending a hospital committee meeting: Am I contributing to a culture in which everyone is encouraged to share their accomplishments? Which qualified candidates who don’t speak out about themselves can I nominate, sponsor, mentor, or encourage for an upcoming opportunity to increase cultural and gender representation? After all, paying it forward helps foster the success of others.
Graceful self-promotion is an important tool for personal and professional development in healthcare. Cultural and gender-based barriers to self-promotion can be surmounted through self-awareness, practice with trusted peers, and recognition of the importance of storytelling gracefully. A medical workplace culture that encourages sharing achievements and celebrates individual and team accomplishments can go a long way toward helping people change their perception of self-promotion and overcome their hesitations.
“The world accommodates you for fitting in, but only rewards you for standing out.”
—Matshona Dhliwayo
Graceful self-promotion—a way of speaking diplomatically and strategically about yourself and your accomplishments—is a key behavior to achieve professional success in medicine. However, some of us are uncomfortable with promoting ourselves in the workplace because of concerns about receiving negative backlash for bragging. These concerns may have roots in our cultural and gender backgrounds, norms that strongly influence our social behaviors. Cultures that emphasize collectivism (eg, East Asia, Scandinavia, Latin America), which is associated with modesty and a focus on “we,” may not approve of self-promotion in contrast to cultures that emphasize individualism (eg, United States, Canada, and parts of Western Europe).1 Additionally, societal gender roles across different cultures focus on women conforming to a “modesty norm,” by which they are socialized to “be nice” and “not too demanding.” Female physicians practicing self-promotion for career advancement may experience a backlash with social penalties and career repercussions.2
One’s avoiding self-promotion may lead others to prematurely dismiss a physician’s capability, competence, ambition, and qualifications for leadership and other opportunities. These oversights may be a contributing factor in the existing inequities in physician compensation, faculty promotions, leadership roles, speaking engagements, journal editorial boards, and more. Women make up over 50% of all US medical students, yet only 18% are hospital CEOs, 16% are deans and department chairs, and 7% are editors-in-chief of high-impact medical journals.3
So how do you get started overcoming cultural and gender barriers and embrace graceful self-promotion? Start small!
First, write a reference or nominating letter for a colleague. The exercise of synthesizing someone else’s accomplishments, skills, and experiences for a specific audience and purpose will give you a template to apply to yourself.
Second, identify an accomplishment with an outcome that educates others about you, your ideas, and your impact. Practice with a trusted peer to frame your accomplishment and its context as a story; for example: “Dr. X, I am pleased to share that I will present a key workshop on Y at the upcoming national Z meeting, based largely on the outcomes from a QI initiative that I developed and oversaw with support from my hospitalist team. We overcame initial staff resistance by recruiting project champions among the interdisciplinary team and successfully reduced readmissions for Y from A% to B% over a 12-month period.”
Third, consider when and how to strategically promote the accomplishment with your medical director, clinical leadership, department leadership, etc. Start out gracefully self-promoting in person or via email with a leader with whom you already have a relationship. If you want to share your accomplishment with a leader who does not yet know you (but may be important to your career), nudge a mentor or sponsor for an introductory conversation.
Finally, ask yourself the next time you are doing a performance review or attending a hospital committee meeting: Am I contributing to a culture in which everyone is encouraged to share their accomplishments? Which qualified candidates who don’t speak out about themselves can I nominate, sponsor, mentor, or encourage for an upcoming opportunity to increase cultural and gender representation? After all, paying it forward helps foster the success of others.
Graceful self-promotion is an important tool for personal and professional development in healthcare. Cultural and gender-based barriers to self-promotion can be surmounted through self-awareness, practice with trusted peers, and recognition of the importance of storytelling gracefully. A medical workplace culture that encourages sharing achievements and celebrates individual and team accomplishments can go a long way toward helping people change their perception of self-promotion and overcome their hesitations.
1. Lalwani AK, Shavitt S. The “me” I claim to be: cultural self-construal elicits self-presentational goal pursuit. J Pers Soc Psychol. 2009;97(1):88-102. https://doi.org/10.1037/a0014100
2. Templeton K, Bernstein CA, Sukhera J, Nora LM, et al. Gender-based differences in burnout: issues faced by women physicians. NAM Perspectives. 2019. Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/201905a
3. Mangurian C, Linos E, Sarkar U, Rodriguez C, Jagsi R. What’s holding women in medicine back from leadership. Harvard Business Review. 2018. https://hbr.org/2018/06/whats-holding-women-in-medicine-back-from-leadership
1. Lalwani AK, Shavitt S. The “me” I claim to be: cultural self-construal elicits self-presentational goal pursuit. J Pers Soc Psychol. 2009;97(1):88-102. https://doi.org/10.1037/a0014100
2. Templeton K, Bernstein CA, Sukhera J, Nora LM, et al. Gender-based differences in burnout: issues faced by women physicians. NAM Perspectives. 2019. Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/201905a
3. Mangurian C, Linos E, Sarkar U, Rodriguez C, Jagsi R. What’s holding women in medicine back from leadership. Harvard Business Review. 2018. https://hbr.org/2018/06/whats-holding-women-in-medicine-back-from-leadership
© 2020 Society of Hospital Medicine
Comanagement of Hip Fracture Patients
We read with interest the article by Maxwell and Mirza.1 We appreciate using the large National Surgical Quality Improvement Project (NSQIP) database to assess comanagement outcomes, although we have concerns about the study design. Propensity score–matching (PSM) studies are limited; PSMs generate an average effect that neither establishes whether a treatment is optimal for a given patient nor control for unmeasured confounders.2 Some baseline characteristics suggest that the comanaged and noncomanaged populations are quite different and, therefore, likely had unmeasured confounders that contributed to not detecting true effects. Also, as suggested by the authors, the NSQIP definitions of comanagement and standardized hip fracture program are broad. Recent studies in hip fracture comanagement attribute best outcomes to an organized program, shared decision making, expert comanagers, and each service having full responsibility including writing their own orders.3-5 As no large database captures this distinction, it is not yet possible to perform a large, multicenter analysis. This type of comanagement cannot be studied in a randomized controlled trial. We recommend caution in overinterpreting the conclusions because there is substantial evidence in favor of optimized comanagement.
1. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project J Hosp Med. 2020;15:468-474. https://doi.org/10.12788/jhm.3343
2. Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53(6):1112-1117. https://doi.org/10.1093/ejcts/ezy167
3. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. https://doi.org/10.1111/j.1532-5415.2008.01770.x
4. Schnell S, Friedman SM, Mendelson DA, Bingham KW, Kates SL. The 1-year mortality of patients treated in a hip fracture program for elders. Geriatr Orthop Surg Rehabil. 2010;1(1):6-14. https://doi.org/10.1177/2151458510378105
5. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. https://doi.org/10.1016/j.cger.2014.01.016
We read with interest the article by Maxwell and Mirza.1 We appreciate using the large National Surgical Quality Improvement Project (NSQIP) database to assess comanagement outcomes, although we have concerns about the study design. Propensity score–matching (PSM) studies are limited; PSMs generate an average effect that neither establishes whether a treatment is optimal for a given patient nor control for unmeasured confounders.2 Some baseline characteristics suggest that the comanaged and noncomanaged populations are quite different and, therefore, likely had unmeasured confounders that contributed to not detecting true effects. Also, as suggested by the authors, the NSQIP definitions of comanagement and standardized hip fracture program are broad. Recent studies in hip fracture comanagement attribute best outcomes to an organized program, shared decision making, expert comanagers, and each service having full responsibility including writing their own orders.3-5 As no large database captures this distinction, it is not yet possible to perform a large, multicenter analysis. This type of comanagement cannot be studied in a randomized controlled trial. We recommend caution in overinterpreting the conclusions because there is substantial evidence in favor of optimized comanagement.
We read with interest the article by Maxwell and Mirza.1 We appreciate using the large National Surgical Quality Improvement Project (NSQIP) database to assess comanagement outcomes, although we have concerns about the study design. Propensity score–matching (PSM) studies are limited; PSMs generate an average effect that neither establishes whether a treatment is optimal for a given patient nor control for unmeasured confounders.2 Some baseline characteristics suggest that the comanaged and noncomanaged populations are quite different and, therefore, likely had unmeasured confounders that contributed to not detecting true effects. Also, as suggested by the authors, the NSQIP definitions of comanagement and standardized hip fracture program are broad. Recent studies in hip fracture comanagement attribute best outcomes to an organized program, shared decision making, expert comanagers, and each service having full responsibility including writing their own orders.3-5 As no large database captures this distinction, it is not yet possible to perform a large, multicenter analysis. This type of comanagement cannot be studied in a randomized controlled trial. We recommend caution in overinterpreting the conclusions because there is substantial evidence in favor of optimized comanagement.
1. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project J Hosp Med. 2020;15:468-474. https://doi.org/10.12788/jhm.3343
2. Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53(6):1112-1117. https://doi.org/10.1093/ejcts/ezy167
3. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. https://doi.org/10.1111/j.1532-5415.2008.01770.x
4. Schnell S, Friedman SM, Mendelson DA, Bingham KW, Kates SL. The 1-year mortality of patients treated in a hip fracture program for elders. Geriatr Orthop Surg Rehabil. 2010;1(1):6-14. https://doi.org/10.1177/2151458510378105
5. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. https://doi.org/10.1016/j.cger.2014.01.016
1. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project J Hosp Med. 2020;15:468-474. https://doi.org/10.12788/jhm.3343
2. Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53(6):1112-1117. https://doi.org/10.1093/ejcts/ezy167
3. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. https://doi.org/10.1111/j.1532-5415.2008.01770.x
4. Schnell S, Friedman SM, Mendelson DA, Bingham KW, Kates SL. The 1-year mortality of patients treated in a hip fracture program for elders. Geriatr Orthop Surg Rehabil. 2010;1(1):6-14. https://doi.org/10.1177/2151458510378105
5. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. https://doi.org/10.1016/j.cger.2014.01.016
© 2020 Society of Hospital Medicine
Surgical Comanagement for Hip Fracture: Time for a Randomized Trial
The growth in the hospitalist workforce has been one of the major trends shaping US (and international) inpatient medicine over the last 25 years.1 Hospitalists’ clinical work is typically split among serving as the primary attending for admitted patients (termed “most responsible physician,” or MRP, in Canada), outpatient clinics, medical consults, and comanagement.2,3 Comanagement typically involves the cooperative efforts of hospitalists and subspecialists ranging from general surgery to orthopedics to medical oncology. Comanagement differs from typical medical consultation because comanaging hospitalists are commonly given broad discretion to directly write orders, manage intercurrent medical illness (eg, hyperglycemia), and even discharge patients from the hospital when appropriate. There can be significant heterogeneity in how comanagement is implemented across institutions.4
With respect to hip fractures, literature suggests that subspecialists value comanagement and that comanagement is associated with reductions in hospital length of stay, timelier surgical repair, and potential cost savings for hospitals.5-7 Some studies have found reductions in in-hospital and 1-year mortality (including one meta-analysis on ortho-geriatric comanagement)8 and complications,9 but others have found no such benefits.10,11
In the current issue of the Journal of Hospital Medicine, Maxwell and Mirza used data from the National Surgical Quality Improvement Program (NSQIP) Participant Use Data File (PUF)—specifically, from the Hip Fracture PUF—to investigate the relationship between comanagement and mortality and major morbidity among more than 15,000 patients hospitalized with hip fracture.12 The investigators did not find that comanagement was associated with a reduction in either morbidity or mortality.
Several factors give gravitas to their analysis. First, the NSQIP PUF is an extremely rigorous data source for evaluating surgical outcomes. Originally developed in the US Veterans Health Administration in the 1980’s to standardize data elements needed for quality improvement and hospital benchmarking, today NSQIP involves more than 600 hospitals in 9 different countries submitting hundreds of thousands of cases annually.13 Second, the authors recognized that the comanagement and noncomanagement groups differed substantially and used propensity score matching in an effort to account for these differences. Surprisingly, they found that the comanagement had significantly higher mortality and morbidity than the noncomanagement group, even after propensity score matching.
These results are important in testing the assumption of the inherent “good” of comanagement. Does this study provide definitive evidence that surgical comanagement does not improve outcomes? We would suggest that this study be interpreted in light of certain considerations.
First, comanagement is a broad term including a variety of operationalizations, such as geriatrician vs hospitalist comanagement, involvement before vs after surgery, and varying divisions of responsibility between the surgical and medical services. Research indicates that successful comanagement models tend to incorporate multidisciplinary teams, embrace the “dual primary caregiver” nature of comanagement, and shared goals among primary caregivers, specifically anticipating prevention of complications.5 The NSQIP data do not provide sufficient granularity to allow for investigation of these crucial nuances that may ultimately determine whether comanagement programs are effective. Additionally, comanagement often (but not always) coexists with a care pathway, and so deficiencies in or absence of a care pathway add additional heterogeneity to the comanagement group which is not captured in the NSQIP PUF.
Second, it is important to consider the potential for unmeasured confounding. The propensity score matching did seem to achieve balance in the distribution of most baseline variables between the comanagement and noncomanagement groups, though differences remain for certain covariates. A key assumption in propensity score matching (and in observational research more broadly) is the principle of “no unmeasured confounders” (ie, the assumption that all variables that might influence treatment assignment and outcomes are measured).14 For the NSQIP PUF this absence of unmeasured confounders is clearly not the case because hospital and surgeon variables are omitted from the PUF for reasons of confidentiality. Inclusion of hospital and surgeon variables could well be important because outcomes may vary by hospital or by surgeon, and simultaneously, different hospitals and different surgeons will have different protocols and preferences regarding comanagement. Furthermore, confounding is virtually guaranteed to the extent that hospitals and surgeons do not randomly assign hip fracture patients to comanagement or usual care. The finding of higher mortality in the comanagement group, even after adjustment and matching, suggests the presence of residual confounding. Even if residual confounding is the explanation for the worse outcomes observed in the comanagement group, the finding of a lack of benefit of comanagement is noteworthy and should not be dismissed out of hand.
Limitations aside, these results suggest a need for humility among strong proponents of comanagement, at least in the hip fracture population. While it may still be reasonable to claim that comanagement improves efficiency and may enhance certain aspects of patient or physician satisfaction, the lack of an impact on mortality highlights a need to examine the benefits of these programs more carefully. From a clinical perspective, hospitalists and orthopedic surgeons should consider which hip fracture patients might be most likely to benefit from comanagement.4 From a research perspective, the current study highlights the pressing need for a randomized trial of comanagement to definitively address the effectiveness of these programs.
1. Wachter RM, Goldman L. Zero to 50,000 — the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
3. Soong C, Eddy Fan, Eric E Howell, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag . 2009;16(2):69
4. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
5. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: an economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4
6. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
7. Soong C, Cram P, Chezar K, et al. Impact of an integrated hip fracture inpatient program on length of stay and costs. J Orthop Trauma. 2016;30(12):647-652. https://doi.org/10.1097/BOT.0000000000000691
8. Grigoryan KV, Javedan H, Rudolph JL. Ortho-geriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49-e55. https://doi.org/10.1097/BOT.0b013e3182a5a045
9. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x
10. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
11. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. http://doi.org/10.1001/archinte.167.17.1869
12. Maxwell B, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score matched retrospective cohort analysis of the national surgical quality improvement project. J Hosp Med. 2020;15:468-474. http://doi.org/10.12788/jhm.3343
13. Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336–46.e1. https://doi.org/10.1016/j.jamcollsurg.2013.02.027
14. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786
The growth in the hospitalist workforce has been one of the major trends shaping US (and international) inpatient medicine over the last 25 years.1 Hospitalists’ clinical work is typically split among serving as the primary attending for admitted patients (termed “most responsible physician,” or MRP, in Canada), outpatient clinics, medical consults, and comanagement.2,3 Comanagement typically involves the cooperative efforts of hospitalists and subspecialists ranging from general surgery to orthopedics to medical oncology. Comanagement differs from typical medical consultation because comanaging hospitalists are commonly given broad discretion to directly write orders, manage intercurrent medical illness (eg, hyperglycemia), and even discharge patients from the hospital when appropriate. There can be significant heterogeneity in how comanagement is implemented across institutions.4
With respect to hip fractures, literature suggests that subspecialists value comanagement and that comanagement is associated with reductions in hospital length of stay, timelier surgical repair, and potential cost savings for hospitals.5-7 Some studies have found reductions in in-hospital and 1-year mortality (including one meta-analysis on ortho-geriatric comanagement)8 and complications,9 but others have found no such benefits.10,11
In the current issue of the Journal of Hospital Medicine, Maxwell and Mirza used data from the National Surgical Quality Improvement Program (NSQIP) Participant Use Data File (PUF)—specifically, from the Hip Fracture PUF—to investigate the relationship between comanagement and mortality and major morbidity among more than 15,000 patients hospitalized with hip fracture.12 The investigators did not find that comanagement was associated with a reduction in either morbidity or mortality.
Several factors give gravitas to their analysis. First, the NSQIP PUF is an extremely rigorous data source for evaluating surgical outcomes. Originally developed in the US Veterans Health Administration in the 1980’s to standardize data elements needed for quality improvement and hospital benchmarking, today NSQIP involves more than 600 hospitals in 9 different countries submitting hundreds of thousands of cases annually.13 Second, the authors recognized that the comanagement and noncomanagement groups differed substantially and used propensity score matching in an effort to account for these differences. Surprisingly, they found that the comanagement had significantly higher mortality and morbidity than the noncomanagement group, even after propensity score matching.
These results are important in testing the assumption of the inherent “good” of comanagement. Does this study provide definitive evidence that surgical comanagement does not improve outcomes? We would suggest that this study be interpreted in light of certain considerations.
First, comanagement is a broad term including a variety of operationalizations, such as geriatrician vs hospitalist comanagement, involvement before vs after surgery, and varying divisions of responsibility between the surgical and medical services. Research indicates that successful comanagement models tend to incorporate multidisciplinary teams, embrace the “dual primary caregiver” nature of comanagement, and shared goals among primary caregivers, specifically anticipating prevention of complications.5 The NSQIP data do not provide sufficient granularity to allow for investigation of these crucial nuances that may ultimately determine whether comanagement programs are effective. Additionally, comanagement often (but not always) coexists with a care pathway, and so deficiencies in or absence of a care pathway add additional heterogeneity to the comanagement group which is not captured in the NSQIP PUF.
Second, it is important to consider the potential for unmeasured confounding. The propensity score matching did seem to achieve balance in the distribution of most baseline variables between the comanagement and noncomanagement groups, though differences remain for certain covariates. A key assumption in propensity score matching (and in observational research more broadly) is the principle of “no unmeasured confounders” (ie, the assumption that all variables that might influence treatment assignment and outcomes are measured).14 For the NSQIP PUF this absence of unmeasured confounders is clearly not the case because hospital and surgeon variables are omitted from the PUF for reasons of confidentiality. Inclusion of hospital and surgeon variables could well be important because outcomes may vary by hospital or by surgeon, and simultaneously, different hospitals and different surgeons will have different protocols and preferences regarding comanagement. Furthermore, confounding is virtually guaranteed to the extent that hospitals and surgeons do not randomly assign hip fracture patients to comanagement or usual care. The finding of higher mortality in the comanagement group, even after adjustment and matching, suggests the presence of residual confounding. Even if residual confounding is the explanation for the worse outcomes observed in the comanagement group, the finding of a lack of benefit of comanagement is noteworthy and should not be dismissed out of hand.
Limitations aside, these results suggest a need for humility among strong proponents of comanagement, at least in the hip fracture population. While it may still be reasonable to claim that comanagement improves efficiency and may enhance certain aspects of patient or physician satisfaction, the lack of an impact on mortality highlights a need to examine the benefits of these programs more carefully. From a clinical perspective, hospitalists and orthopedic surgeons should consider which hip fracture patients might be most likely to benefit from comanagement.4 From a research perspective, the current study highlights the pressing need for a randomized trial of comanagement to definitively address the effectiveness of these programs.
The growth in the hospitalist workforce has been one of the major trends shaping US (and international) inpatient medicine over the last 25 years.1 Hospitalists’ clinical work is typically split among serving as the primary attending for admitted patients (termed “most responsible physician,” or MRP, in Canada), outpatient clinics, medical consults, and comanagement.2,3 Comanagement typically involves the cooperative efforts of hospitalists and subspecialists ranging from general surgery to orthopedics to medical oncology. Comanagement differs from typical medical consultation because comanaging hospitalists are commonly given broad discretion to directly write orders, manage intercurrent medical illness (eg, hyperglycemia), and even discharge patients from the hospital when appropriate. There can be significant heterogeneity in how comanagement is implemented across institutions.4
With respect to hip fractures, literature suggests that subspecialists value comanagement and that comanagement is associated with reductions in hospital length of stay, timelier surgical repair, and potential cost savings for hospitals.5-7 Some studies have found reductions in in-hospital and 1-year mortality (including one meta-analysis on ortho-geriatric comanagement)8 and complications,9 but others have found no such benefits.10,11
In the current issue of the Journal of Hospital Medicine, Maxwell and Mirza used data from the National Surgical Quality Improvement Program (NSQIP) Participant Use Data File (PUF)—specifically, from the Hip Fracture PUF—to investigate the relationship between comanagement and mortality and major morbidity among more than 15,000 patients hospitalized with hip fracture.12 The investigators did not find that comanagement was associated with a reduction in either morbidity or mortality.
Several factors give gravitas to their analysis. First, the NSQIP PUF is an extremely rigorous data source for evaluating surgical outcomes. Originally developed in the US Veterans Health Administration in the 1980’s to standardize data elements needed for quality improvement and hospital benchmarking, today NSQIP involves more than 600 hospitals in 9 different countries submitting hundreds of thousands of cases annually.13 Second, the authors recognized that the comanagement and noncomanagement groups differed substantially and used propensity score matching in an effort to account for these differences. Surprisingly, they found that the comanagement had significantly higher mortality and morbidity than the noncomanagement group, even after propensity score matching.
These results are important in testing the assumption of the inherent “good” of comanagement. Does this study provide definitive evidence that surgical comanagement does not improve outcomes? We would suggest that this study be interpreted in light of certain considerations.
First, comanagement is a broad term including a variety of operationalizations, such as geriatrician vs hospitalist comanagement, involvement before vs after surgery, and varying divisions of responsibility between the surgical and medical services. Research indicates that successful comanagement models tend to incorporate multidisciplinary teams, embrace the “dual primary caregiver” nature of comanagement, and shared goals among primary caregivers, specifically anticipating prevention of complications.5 The NSQIP data do not provide sufficient granularity to allow for investigation of these crucial nuances that may ultimately determine whether comanagement programs are effective. Additionally, comanagement often (but not always) coexists with a care pathway, and so deficiencies in or absence of a care pathway add additional heterogeneity to the comanagement group which is not captured in the NSQIP PUF.
Second, it is important to consider the potential for unmeasured confounding. The propensity score matching did seem to achieve balance in the distribution of most baseline variables between the comanagement and noncomanagement groups, though differences remain for certain covariates. A key assumption in propensity score matching (and in observational research more broadly) is the principle of “no unmeasured confounders” (ie, the assumption that all variables that might influence treatment assignment and outcomes are measured).14 For the NSQIP PUF this absence of unmeasured confounders is clearly not the case because hospital and surgeon variables are omitted from the PUF for reasons of confidentiality. Inclusion of hospital and surgeon variables could well be important because outcomes may vary by hospital or by surgeon, and simultaneously, different hospitals and different surgeons will have different protocols and preferences regarding comanagement. Furthermore, confounding is virtually guaranteed to the extent that hospitals and surgeons do not randomly assign hip fracture patients to comanagement or usual care. The finding of higher mortality in the comanagement group, even after adjustment and matching, suggests the presence of residual confounding. Even if residual confounding is the explanation for the worse outcomes observed in the comanagement group, the finding of a lack of benefit of comanagement is noteworthy and should not be dismissed out of hand.
Limitations aside, these results suggest a need for humility among strong proponents of comanagement, at least in the hip fracture population. While it may still be reasonable to claim that comanagement improves efficiency and may enhance certain aspects of patient or physician satisfaction, the lack of an impact on mortality highlights a need to examine the benefits of these programs more carefully. From a clinical perspective, hospitalists and orthopedic surgeons should consider which hip fracture patients might be most likely to benefit from comanagement.4 From a research perspective, the current study highlights the pressing need for a randomized trial of comanagement to definitively address the effectiveness of these programs.
1. Wachter RM, Goldman L. Zero to 50,000 — the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
3. Soong C, Eddy Fan, Eric E Howell, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag . 2009;16(2):69
4. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
5. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: an economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4
6. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
7. Soong C, Cram P, Chezar K, et al. Impact of an integrated hip fracture inpatient program on length of stay and costs. J Orthop Trauma. 2016;30(12):647-652. https://doi.org/10.1097/BOT.0000000000000691
8. Grigoryan KV, Javedan H, Rudolph JL. Ortho-geriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49-e55. https://doi.org/10.1097/BOT.0b013e3182a5a045
9. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x
10. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
11. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. http://doi.org/10.1001/archinte.167.17.1869
12. Maxwell B, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score matched retrospective cohort analysis of the national surgical quality improvement project. J Hosp Med. 2020;15:468-474. http://doi.org/10.12788/jhm.3343
13. Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336–46.e1. https://doi.org/10.1016/j.jamcollsurg.2013.02.027
14. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786
1. Wachter RM, Goldman L. Zero to 50,000 — the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
3. Soong C, Eddy Fan, Eric E Howell, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag . 2009;16(2):69
4. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
5. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: an economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4
6. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
7. Soong C, Cram P, Chezar K, et al. Impact of an integrated hip fracture inpatient program on length of stay and costs. J Orthop Trauma. 2016;30(12):647-652. https://doi.org/10.1097/BOT.0000000000000691
8. Grigoryan KV, Javedan H, Rudolph JL. Ortho-geriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49-e55. https://doi.org/10.1097/BOT.0b013e3182a5a045
9. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x
10. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
11. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. http://doi.org/10.1001/archinte.167.17.1869
12. Maxwell B, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score matched retrospective cohort analysis of the national surgical quality improvement project. J Hosp Med. 2020;15:468-474. http://doi.org/10.12788/jhm.3343
13. Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336–46.e1. https://doi.org/10.1016/j.jamcollsurg.2013.02.027
14. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786
© 2020 Society of Hospital Medicine
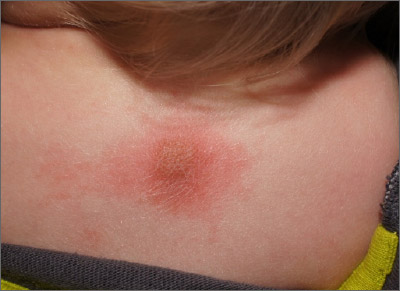
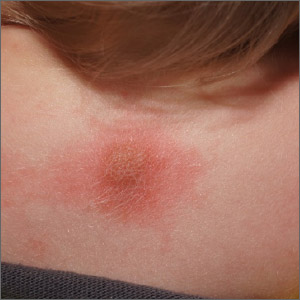
Fixed scaly lesion
The patient’s history of a recurrent wheal was consistent with a diagnosis of mastocytoma, the most common and least concerning form of cutaneous mastocytosis. Mastocytomas commonly appear in infants as 1 to 3 firm 1- to 5-cm papules (or a plaque) caused by a histamine release from a group of mast cells with abnormal growth receptors. When flaring, the surface may have a prominent orange peel texture because of tethered adnexal structures. When uninflamed, the skin surface may be slightly raised and flesh-colored to pink.
When first noticed, mastocytomas are easily mistaken for insect bites or congenital nevi. However, mastocytomas don’t resolve completely, as would an insect bite, and they become recurrently inflamed (spontaneously or with trauma). Inflammation that can be elicited with pressure or scratching is called Darrier sign and is helpful in making the diagnosis and distinguishing these lesions from congenital nevi.
Dermoscopy of a mastocytoma lacks signs of a melanocytic nevi, which further adds to the clinical diagnosis. Blood tests and biopsy are unnecessary, but if a biopsy is performed, it is important to mention the possibility of mast cell disease to the lab so that appropriate immunostaining for mast cells can be carried out.
Mastocytomas that appear in infancy usually resolve spontaneously in early childhood or by puberty, at the latest. If there is any notable itching or discomfort, oral antihistamines are helpful, as are topical steroids and topical tacrolimus. In this case, the diagnosis was made clinically and the patient’s parents were content to observe the area.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46.
The patient’s history of a recurrent wheal was consistent with a diagnosis of mastocytoma, the most common and least concerning form of cutaneous mastocytosis. Mastocytomas commonly appear in infants as 1 to 3 firm 1- to 5-cm papules (or a plaque) caused by a histamine release from a group of mast cells with abnormal growth receptors. When flaring, the surface may have a prominent orange peel texture because of tethered adnexal structures. When uninflamed, the skin surface may be slightly raised and flesh-colored to pink.
When first noticed, mastocytomas are easily mistaken for insect bites or congenital nevi. However, mastocytomas don’t resolve completely, as would an insect bite, and they become recurrently inflamed (spontaneously or with trauma). Inflammation that can be elicited with pressure or scratching is called Darrier sign and is helpful in making the diagnosis and distinguishing these lesions from congenital nevi.
Dermoscopy of a mastocytoma lacks signs of a melanocytic nevi, which further adds to the clinical diagnosis. Blood tests and biopsy are unnecessary, but if a biopsy is performed, it is important to mention the possibility of mast cell disease to the lab so that appropriate immunostaining for mast cells can be carried out.
Mastocytomas that appear in infancy usually resolve spontaneously in early childhood or by puberty, at the latest. If there is any notable itching or discomfort, oral antihistamines are helpful, as are topical steroids and topical tacrolimus. In this case, the diagnosis was made clinically and the patient’s parents were content to observe the area.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
The patient’s history of a recurrent wheal was consistent with a diagnosis of mastocytoma, the most common and least concerning form of cutaneous mastocytosis. Mastocytomas commonly appear in infants as 1 to 3 firm 1- to 5-cm papules (or a plaque) caused by a histamine release from a group of mast cells with abnormal growth receptors. When flaring, the surface may have a prominent orange peel texture because of tethered adnexal structures. When uninflamed, the skin surface may be slightly raised and flesh-colored to pink.
When first noticed, mastocytomas are easily mistaken for insect bites or congenital nevi. However, mastocytomas don’t resolve completely, as would an insect bite, and they become recurrently inflamed (spontaneously or with trauma). Inflammation that can be elicited with pressure or scratching is called Darrier sign and is helpful in making the diagnosis and distinguishing these lesions from congenital nevi.
Dermoscopy of a mastocytoma lacks signs of a melanocytic nevi, which further adds to the clinical diagnosis. Blood tests and biopsy are unnecessary, but if a biopsy is performed, it is important to mention the possibility of mast cell disease to the lab so that appropriate immunostaining for mast cells can be carried out.
Mastocytomas that appear in infancy usually resolve spontaneously in early childhood or by puberty, at the latest. If there is any notable itching or discomfort, oral antihistamines are helpful, as are topical steroids and topical tacrolimus. In this case, the diagnosis was made clinically and the patient’s parents were content to observe the area.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46.
Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46.
Applications for the CUTIS 2021 Resident Corner Column
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears (msears@mdedge.com) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears (msears@mdedge.com) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears (msears@mdedge.com) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
Survey: Most FPs live at or below their means
a Medscape survey has found.
According to the Medscape Family Physician Debt and Net Worth Report 2020, almost half of FPs (46%) reported having that amount as their net worth, compared with the 18% of gastroenterologists and 19% of urologists who fell into that category.
And whereas 19% of orthopedists reported at least $5 million in net worth, only 3% of FPs did.
A third are paying off student loans
FPs were also more likely, along with physical medicine and rehabilitation physicians, at 34%, to report that they are continuing to pay off student loans. Conversely, 14% of gastroenterologists and 15% of nephrologists and rheumatologists said they were still paying off the loans.
Student loan debt was third on the list for FPs. Two-thirds of FPs were paying off a mortgage, and 41% had car loan payments.
Overall, FPs appear to manage their finances well and are living within their means. Only 6% of FPs said they live above their means, whereas 51% said they live at their means, and 42% said they live below that threshold.
Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minn., said in an interview he recommends saving 20% of gross salary each year.
The survey was completed before Feb. 11 and before the financial effects of the COVID-19 pandemic could be known. The report is based on responses from more than 17,000 physicians across 30 specialties.
A lower level of net worth among FPs corresponds with their being close to the bottom among physicians in compensation. They made $234,000 on average, according to the report. By contrast, orthopedists made more than twice as much, at $511,000.
Smaller homes, less mortgage debt
FPs were among the least likely to indicate that they had a home of more than 5,000 square feet. That was true for only 6% of FPs; it was true for 22% of plastic surgeons and orthopedists. Most (61%) lived in dwellings of 3,000 square feet or less.
At the same time, FPs reported smaller mortgages than many of their colleagues.
Nearly half (49%) of FPs have mortgages of $300,000 or less; 26% have no mortgage at all. That figure was much higher than the 37% of physicians overall who had mortgages of $300,000 or less, although almost the same percentage had no mortgage at all.
Most had no financial loss in the past year
In further good news, most FPs (70%) said they did not experience a financial loss in the past year. Of those who did experience a loss, the top reasons were problems with their practice, such as reimbursement changes or changes in practice situations, or bad investments.
FPs socked away more into tax-deferred than taxable accounts, the survey showed.
More than half (54%) of FPs put at least $1,000 into tax-deferred accounts, such as college savings or retirement accounts, although 14% said they do not regularly contribute to such accounts.
Fewer (29%) contributed at least $1,000 to a taxable account.
As for who pays the day-to-day bills in households, 56% of FPs said they pool resources with a spouse or partner and pay bills from a common fund. Only 4% split the bills equally, no matter the income difference. One in four said they do not have joint finances with a spouse or partner.
FPs were divided as to whether they are currently working with a financial planner (38%) or had not worked with one (37%); the remainder said they had met with or used one in the past.
A version of this article originally appeared on Medscape.com.
a Medscape survey has found.
According to the Medscape Family Physician Debt and Net Worth Report 2020, almost half of FPs (46%) reported having that amount as their net worth, compared with the 18% of gastroenterologists and 19% of urologists who fell into that category.
And whereas 19% of orthopedists reported at least $5 million in net worth, only 3% of FPs did.
A third are paying off student loans
FPs were also more likely, along with physical medicine and rehabilitation physicians, at 34%, to report that they are continuing to pay off student loans. Conversely, 14% of gastroenterologists and 15% of nephrologists and rheumatologists said they were still paying off the loans.
Student loan debt was third on the list for FPs. Two-thirds of FPs were paying off a mortgage, and 41% had car loan payments.
Overall, FPs appear to manage their finances well and are living within their means. Only 6% of FPs said they live above their means, whereas 51% said they live at their means, and 42% said they live below that threshold.
Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minn., said in an interview he recommends saving 20% of gross salary each year.
The survey was completed before Feb. 11 and before the financial effects of the COVID-19 pandemic could be known. The report is based on responses from more than 17,000 physicians across 30 specialties.
A lower level of net worth among FPs corresponds with their being close to the bottom among physicians in compensation. They made $234,000 on average, according to the report. By contrast, orthopedists made more than twice as much, at $511,000.
Smaller homes, less mortgage debt
FPs were among the least likely to indicate that they had a home of more than 5,000 square feet. That was true for only 6% of FPs; it was true for 22% of plastic surgeons and orthopedists. Most (61%) lived in dwellings of 3,000 square feet or less.
At the same time, FPs reported smaller mortgages than many of their colleagues.
Nearly half (49%) of FPs have mortgages of $300,000 or less; 26% have no mortgage at all. That figure was much higher than the 37% of physicians overall who had mortgages of $300,000 or less, although almost the same percentage had no mortgage at all.
Most had no financial loss in the past year
In further good news, most FPs (70%) said they did not experience a financial loss in the past year. Of those who did experience a loss, the top reasons were problems with their practice, such as reimbursement changes or changes in practice situations, or bad investments.
FPs socked away more into tax-deferred than taxable accounts, the survey showed.
More than half (54%) of FPs put at least $1,000 into tax-deferred accounts, such as college savings or retirement accounts, although 14% said they do not regularly contribute to such accounts.
Fewer (29%) contributed at least $1,000 to a taxable account.
As for who pays the day-to-day bills in households, 56% of FPs said they pool resources with a spouse or partner and pay bills from a common fund. Only 4% split the bills equally, no matter the income difference. One in four said they do not have joint finances with a spouse or partner.
FPs were divided as to whether they are currently working with a financial planner (38%) or had not worked with one (37%); the remainder said they had met with or used one in the past.
A version of this article originally appeared on Medscape.com.
a Medscape survey has found.
According to the Medscape Family Physician Debt and Net Worth Report 2020, almost half of FPs (46%) reported having that amount as their net worth, compared with the 18% of gastroenterologists and 19% of urologists who fell into that category.
And whereas 19% of orthopedists reported at least $5 million in net worth, only 3% of FPs did.
A third are paying off student loans
FPs were also more likely, along with physical medicine and rehabilitation physicians, at 34%, to report that they are continuing to pay off student loans. Conversely, 14% of gastroenterologists and 15% of nephrologists and rheumatologists said they were still paying off the loans.
Student loan debt was third on the list for FPs. Two-thirds of FPs were paying off a mortgage, and 41% had car loan payments.
Overall, FPs appear to manage their finances well and are living within their means. Only 6% of FPs said they live above their means, whereas 51% said they live at their means, and 42% said they live below that threshold.
Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minn., said in an interview he recommends saving 20% of gross salary each year.
The survey was completed before Feb. 11 and before the financial effects of the COVID-19 pandemic could be known. The report is based on responses from more than 17,000 physicians across 30 specialties.
A lower level of net worth among FPs corresponds with their being close to the bottom among physicians in compensation. They made $234,000 on average, according to the report. By contrast, orthopedists made more than twice as much, at $511,000.
Smaller homes, less mortgage debt
FPs were among the least likely to indicate that they had a home of more than 5,000 square feet. That was true for only 6% of FPs; it was true for 22% of plastic surgeons and orthopedists. Most (61%) lived in dwellings of 3,000 square feet or less.
At the same time, FPs reported smaller mortgages than many of their colleagues.
Nearly half (49%) of FPs have mortgages of $300,000 or less; 26% have no mortgage at all. That figure was much higher than the 37% of physicians overall who had mortgages of $300,000 or less, although almost the same percentage had no mortgage at all.
Most had no financial loss in the past year
In further good news, most FPs (70%) said they did not experience a financial loss in the past year. Of those who did experience a loss, the top reasons were problems with their practice, such as reimbursement changes or changes in practice situations, or bad investments.
FPs socked away more into tax-deferred than taxable accounts, the survey showed.
More than half (54%) of FPs put at least $1,000 into tax-deferred accounts, such as college savings or retirement accounts, although 14% said they do not regularly contribute to such accounts.
Fewer (29%) contributed at least $1,000 to a taxable account.
As for who pays the day-to-day bills in households, 56% of FPs said they pool resources with a spouse or partner and pay bills from a common fund. Only 4% split the bills equally, no matter the income difference. One in four said they do not have joint finances with a spouse or partner.
FPs were divided as to whether they are currently working with a financial planner (38%) or had not worked with one (37%); the remainder said they had met with or used one in the past.
A version of this article originally appeared on Medscape.com.
FDA approves low-sodium treatment option for narcolepsy
Xywav is a novel oxybate product with a unique composition of cations, resulting in 92% less sodium than sodium oxybate (Xyrem, Jazz Pharmaceuticals) at the recommended dosage range of 6 to 9 grams, the company said in a news release.
The FDA approved the drug based on a phase 3 trial involving 201 patients who had narcolepsy with cataplexy.
As reported by Medscape Medical News from the World Sleep 2019 meeting, Xywav demonstrated highly statistically significant differences (P < .0001) in weekly number of cataplexy attacks (primary efficacy endpoint) and Epworth Sleepiness Scale scores (key secondary outcome) vs placebo.
“Based on the efficacy demonstrated in the clinical program, the approval of Xywav is important for people living with cataplexy or EDS associated with narcolepsy,” lead investigator Richard K. Bogan, MD, said in the company’s news release.
He noted that the average American consumes too much sodium. “Excess sodium intake has been linked with increases in blood pressure, hypertension, stroke, and other cardiovascular disease,” said Dr. Bogan, associate clinical professor at the University of South Carolina School of Medicine, Columbia.
“Xywav makes it possible for patients to have a lower-sodium oxybate treatment option. This may help patients taking sodium oxybate better align with daily sodium intake recommendations, including those by the American Heart Association,” he added.
The overall safety profile of Xywav is in line with sodium oxybate, the company said. The most common adverse reactions in adults, occurring in at least 5% of participants, were headache, nausea, dizziness, decreased appetite, parasomnia, diarrhea, hyperhidrosis (excessive sweating), anxiety, and vomiting.
Xywav has a boxed warning as a CNS depressant and for its potential for abuse and misuse. As a result, the drug is only available through a Risk Evaluation and Mitigation Strategy (REMS) program.
The US Drug Enforcement Agency has designated Xywav as a schedule III drug, meaning it has a moderate to low potential for physical and psychological dependence.
The company plans to launch Xywav by the end of the year. Full prescribing information and a medication guide are available online.
This article first appeared on Medscape.com.
Xywav is a novel oxybate product with a unique composition of cations, resulting in 92% less sodium than sodium oxybate (Xyrem, Jazz Pharmaceuticals) at the recommended dosage range of 6 to 9 grams, the company said in a news release.
The FDA approved the drug based on a phase 3 trial involving 201 patients who had narcolepsy with cataplexy.
As reported by Medscape Medical News from the World Sleep 2019 meeting, Xywav demonstrated highly statistically significant differences (P < .0001) in weekly number of cataplexy attacks (primary efficacy endpoint) and Epworth Sleepiness Scale scores (key secondary outcome) vs placebo.
“Based on the efficacy demonstrated in the clinical program, the approval of Xywav is important for people living with cataplexy or EDS associated with narcolepsy,” lead investigator Richard K. Bogan, MD, said in the company’s news release.
He noted that the average American consumes too much sodium. “Excess sodium intake has been linked with increases in blood pressure, hypertension, stroke, and other cardiovascular disease,” said Dr. Bogan, associate clinical professor at the University of South Carolina School of Medicine, Columbia.
“Xywav makes it possible for patients to have a lower-sodium oxybate treatment option. This may help patients taking sodium oxybate better align with daily sodium intake recommendations, including those by the American Heart Association,” he added.
The overall safety profile of Xywav is in line with sodium oxybate, the company said. The most common adverse reactions in adults, occurring in at least 5% of participants, were headache, nausea, dizziness, decreased appetite, parasomnia, diarrhea, hyperhidrosis (excessive sweating), anxiety, and vomiting.
Xywav has a boxed warning as a CNS depressant and for its potential for abuse and misuse. As a result, the drug is only available through a Risk Evaluation and Mitigation Strategy (REMS) program.
The US Drug Enforcement Agency has designated Xywav as a schedule III drug, meaning it has a moderate to low potential for physical and psychological dependence.
The company plans to launch Xywav by the end of the year. Full prescribing information and a medication guide are available online.
This article first appeared on Medscape.com.
Xywav is a novel oxybate product with a unique composition of cations, resulting in 92% less sodium than sodium oxybate (Xyrem, Jazz Pharmaceuticals) at the recommended dosage range of 6 to 9 grams, the company said in a news release.
The FDA approved the drug based on a phase 3 trial involving 201 patients who had narcolepsy with cataplexy.
As reported by Medscape Medical News from the World Sleep 2019 meeting, Xywav demonstrated highly statistically significant differences (P < .0001) in weekly number of cataplexy attacks (primary efficacy endpoint) and Epworth Sleepiness Scale scores (key secondary outcome) vs placebo.
“Based on the efficacy demonstrated in the clinical program, the approval of Xywav is important for people living with cataplexy or EDS associated with narcolepsy,” lead investigator Richard K. Bogan, MD, said in the company’s news release.
He noted that the average American consumes too much sodium. “Excess sodium intake has been linked with increases in blood pressure, hypertension, stroke, and other cardiovascular disease,” said Dr. Bogan, associate clinical professor at the University of South Carolina School of Medicine, Columbia.
“Xywav makes it possible for patients to have a lower-sodium oxybate treatment option. This may help patients taking sodium oxybate better align with daily sodium intake recommendations, including those by the American Heart Association,” he added.
The overall safety profile of Xywav is in line with sodium oxybate, the company said. The most common adverse reactions in adults, occurring in at least 5% of participants, were headache, nausea, dizziness, decreased appetite, parasomnia, diarrhea, hyperhidrosis (excessive sweating), anxiety, and vomiting.
Xywav has a boxed warning as a CNS depressant and for its potential for abuse and misuse. As a result, the drug is only available through a Risk Evaluation and Mitigation Strategy (REMS) program.
The US Drug Enforcement Agency has designated Xywav as a schedule III drug, meaning it has a moderate to low potential for physical and psychological dependence.
The company plans to launch Xywav by the end of the year. Full prescribing information and a medication guide are available online.
This article first appeared on Medscape.com.
Heavy toll from ongoing cancer referral delays
Delays in cancer referrals caused by the COVID-19 pandemic and the ensuing shutdown in cancer services will lead to thousands of additional deaths and tens of thousands of life-years lost, suggest two new modeling studies from the United Kingdom.
Clearing the backlog in cancer diagnoses will require a coordinated effort from the government and the National Health Service (NHS), say the authors, inasmuch as services were already running at “full capacity” before the pandemic.
Both studies were published in The Lancet Oncology on July 20.
When the UK-wide lockdown to combat the COVID-19 pandemic was implemented on March 23, cancer screening and routine outpatient referrals in the NHS were suspended, and treatment of cancer patients either halted or slowed down.
Moreover, because of physical distancing measures, which are expected to continue for up to a year, urgent 3-week referrals for suspected cancer cases have fallen by as much as 80%.
To estimate the potential impact on cancer deaths, Ajay Aggarwal, MD, from the London School of Hygiene and Tropical Medicine, United Kingdom, and colleagues conducted a population-based modeling study.
They collected data on 32,583 patients with breast cancer, 24,975 with colorectal cancer, 6744 with esophageal cancer, and 29,305 with lung cancer. Patients were diagnosed between 2010 and 2012 and were followed to 2015.
The investigators used that data to estimate the impact of diagnostic delays resulting from 12 months of physical distancing.
For breast cancer, this would lead to a 7.9%-9.6% increase in the number of cancer deaths within 5 years after diagnosis, or to 281-344 additional deaths.
For colorectal cancer, there would be a 15.3%-16.7% increase in mortality over 5 years, or an additional 1,445-1,563 deaths.
For lung cancer, there would a 4.8%-5.3% increase in mortality, or an additional 1235-1372 deaths.
For esophageal cancer, the mortality increase over 5 years would be 5.8%-6.0%, leading to 330-342 additional deaths.
Across the four tumor types, 59,204-63,229 life-years would be lost because of physical distancing compared to the prepandemic era.
Resources need to be increased
These additional deaths are not inevitable, the researchers suggest.
To prevent the increase in colorectal cancer deaths, for example, Aggarwal said, “It is vital that more resources are made urgently available for endoscopy and colonoscopy services, which are managing significant backlogs currently.
“Whilst currently attention is being focused on diagnostic pathways where cancer is suspected, the issue is that a significant number of cancers are diagnosed in patients awaiting investigation for symptoms not considered related to be cancer,” he added in a statement.
“Therefore we need a whole system approach to avoid the predicted excess deaths.”
Coauthor Bernard Rachet, PhD, also from the London School of Hygiene and Tropical Medicine, added that “to absorb the cancer patient backlog, the healthcare community also needs to establish clear criteria to prioritise patients on clinical grounds, in order to maintain equitability in care delivery.”
It will not be easy “to pin down the exact number of additional cancer deaths we expect to see over the coming years, but studies like this help us to understand the devastating long-term effect a pandemic like COVID-19 will have on the lives of thousands of cancer patients,” commented Michelle Mitchell, chief executive of Cancer Research UK.
Underlining the “enormous backlog” of cancer care that has built up during the pandemic, she said: “Diagnosing and treating people swiftly is vital to give people with cancer the greatest chances of survival.
“The government must work closely with the NHS to ensure it has sufficient staff and equipment to clear the backlog while giving patients the care that they need, quickly and safely,” Mitchell added.
Increasing resources will not be easy. In an accompanying editorial, William Hamilton, MD, PhD, University of Exeter, United Kingdom, warns that many NHS imaging departments, for example, were “working at full capacity before the COVID-19 pandemic.”
Consequently, they “might not be able to meet the increase in demand” resulting from the backlog in patients, especially as “the need to keep patients separate and to clean equipment has reduced their efficiency.
“The UK has had a long-term shortage of diagnostic capacity, although this shortage is not simply of equipment, but also of personnel, which is not so easily improved,” he cautions.
Another study, similar estimates
For the second study, Clare Turnbull, PhD, Institute of Cancer Research, London, and colleagues obtained age- and stage-stratified 10-year cancer survival estimates for patients in England diagnosed with 20 common tumor types between 2008 and 2017.
They also gathered data on cancer diagnoses made via urgent 2-week referrals between 2013 and 2016. They estimate that 6,281 patients were diagnosed with cancer of stages I-III per month.
Of those, 1,691 (27%) would die within 10 years of their diagnosis, they found.
They then calculated that delays in 2-week referrals during a 3-month lockdown would lead to an average delay in presentation of 2 months per patient.
A resulting 25% backlog in referrals would lead to 181 additional lives and 3,316 life-years lost. With a 75% backlog in referrals, an additional 276 lives and 5,075 life-years would be lost.
The team says that additional diagnostic delays spread over 3-8 months after the lockdown could increase the impact of a 25% backlog in referrals to 401 additional lives and 14,873 life-years lost.
For a 75% backlog in referrals, the additional lives lost would rise to 1,231, and the number of life-years lost would reach 22,635.
“Substantial additional deaths from diagnostic delays on top of those expected from delays in presentation – because many people are simply too afraid to visit their GP or hospital – are likely, especially if rapid provision of additional capacity, including technical provision and increased staffing, is not forthcoming,” Turnbull commented in a statement.
The study by Aggarwal and colleagues was funded by the U.K. Research and Innovation Economic and Social Research Council. Several of the researchers were supported by Cancer Research UK and Breast Cancer Now. Turnbull reports receiving support from the Movember Foundation.
This article first appeared on Medscape.com.
Delays in cancer referrals caused by the COVID-19 pandemic and the ensuing shutdown in cancer services will lead to thousands of additional deaths and tens of thousands of life-years lost, suggest two new modeling studies from the United Kingdom.
Clearing the backlog in cancer diagnoses will require a coordinated effort from the government and the National Health Service (NHS), say the authors, inasmuch as services were already running at “full capacity” before the pandemic.
Both studies were published in The Lancet Oncology on July 20.
When the UK-wide lockdown to combat the COVID-19 pandemic was implemented on March 23, cancer screening and routine outpatient referrals in the NHS were suspended, and treatment of cancer patients either halted or slowed down.
Moreover, because of physical distancing measures, which are expected to continue for up to a year, urgent 3-week referrals for suspected cancer cases have fallen by as much as 80%.
To estimate the potential impact on cancer deaths, Ajay Aggarwal, MD, from the London School of Hygiene and Tropical Medicine, United Kingdom, and colleagues conducted a population-based modeling study.
They collected data on 32,583 patients with breast cancer, 24,975 with colorectal cancer, 6744 with esophageal cancer, and 29,305 with lung cancer. Patients were diagnosed between 2010 and 2012 and were followed to 2015.
The investigators used that data to estimate the impact of diagnostic delays resulting from 12 months of physical distancing.
For breast cancer, this would lead to a 7.9%-9.6% increase in the number of cancer deaths within 5 years after diagnosis, or to 281-344 additional deaths.
For colorectal cancer, there would be a 15.3%-16.7% increase in mortality over 5 years, or an additional 1,445-1,563 deaths.
For lung cancer, there would a 4.8%-5.3% increase in mortality, or an additional 1235-1372 deaths.
For esophageal cancer, the mortality increase over 5 years would be 5.8%-6.0%, leading to 330-342 additional deaths.
Across the four tumor types, 59,204-63,229 life-years would be lost because of physical distancing compared to the prepandemic era.
Resources need to be increased
These additional deaths are not inevitable, the researchers suggest.
To prevent the increase in colorectal cancer deaths, for example, Aggarwal said, “It is vital that more resources are made urgently available for endoscopy and colonoscopy services, which are managing significant backlogs currently.
“Whilst currently attention is being focused on diagnostic pathways where cancer is suspected, the issue is that a significant number of cancers are diagnosed in patients awaiting investigation for symptoms not considered related to be cancer,” he added in a statement.
“Therefore we need a whole system approach to avoid the predicted excess deaths.”
Coauthor Bernard Rachet, PhD, also from the London School of Hygiene and Tropical Medicine, added that “to absorb the cancer patient backlog, the healthcare community also needs to establish clear criteria to prioritise patients on clinical grounds, in order to maintain equitability in care delivery.”
It will not be easy “to pin down the exact number of additional cancer deaths we expect to see over the coming years, but studies like this help us to understand the devastating long-term effect a pandemic like COVID-19 will have on the lives of thousands of cancer patients,” commented Michelle Mitchell, chief executive of Cancer Research UK.
Underlining the “enormous backlog” of cancer care that has built up during the pandemic, she said: “Diagnosing and treating people swiftly is vital to give people with cancer the greatest chances of survival.
“The government must work closely with the NHS to ensure it has sufficient staff and equipment to clear the backlog while giving patients the care that they need, quickly and safely,” Mitchell added.
Increasing resources will not be easy. In an accompanying editorial, William Hamilton, MD, PhD, University of Exeter, United Kingdom, warns that many NHS imaging departments, for example, were “working at full capacity before the COVID-19 pandemic.”
Consequently, they “might not be able to meet the increase in demand” resulting from the backlog in patients, especially as “the need to keep patients separate and to clean equipment has reduced their efficiency.
“The UK has had a long-term shortage of diagnostic capacity, although this shortage is not simply of equipment, but also of personnel, which is not so easily improved,” he cautions.
Another study, similar estimates
For the second study, Clare Turnbull, PhD, Institute of Cancer Research, London, and colleagues obtained age- and stage-stratified 10-year cancer survival estimates for patients in England diagnosed with 20 common tumor types between 2008 and 2017.
They also gathered data on cancer diagnoses made via urgent 2-week referrals between 2013 and 2016. They estimate that 6,281 patients were diagnosed with cancer of stages I-III per month.
Of those, 1,691 (27%) would die within 10 years of their diagnosis, they found.
They then calculated that delays in 2-week referrals during a 3-month lockdown would lead to an average delay in presentation of 2 months per patient.
A resulting 25% backlog in referrals would lead to 181 additional lives and 3,316 life-years lost. With a 75% backlog in referrals, an additional 276 lives and 5,075 life-years would be lost.
The team says that additional diagnostic delays spread over 3-8 months after the lockdown could increase the impact of a 25% backlog in referrals to 401 additional lives and 14,873 life-years lost.
For a 75% backlog in referrals, the additional lives lost would rise to 1,231, and the number of life-years lost would reach 22,635.
“Substantial additional deaths from diagnostic delays on top of those expected from delays in presentation – because many people are simply too afraid to visit their GP or hospital – are likely, especially if rapid provision of additional capacity, including technical provision and increased staffing, is not forthcoming,” Turnbull commented in a statement.
The study by Aggarwal and colleagues was funded by the U.K. Research and Innovation Economic and Social Research Council. Several of the researchers were supported by Cancer Research UK and Breast Cancer Now. Turnbull reports receiving support from the Movember Foundation.
This article first appeared on Medscape.com.
Delays in cancer referrals caused by the COVID-19 pandemic and the ensuing shutdown in cancer services will lead to thousands of additional deaths and tens of thousands of life-years lost, suggest two new modeling studies from the United Kingdom.
Clearing the backlog in cancer diagnoses will require a coordinated effort from the government and the National Health Service (NHS), say the authors, inasmuch as services were already running at “full capacity” before the pandemic.
Both studies were published in The Lancet Oncology on July 20.
When the UK-wide lockdown to combat the COVID-19 pandemic was implemented on March 23, cancer screening and routine outpatient referrals in the NHS were suspended, and treatment of cancer patients either halted or slowed down.
Moreover, because of physical distancing measures, which are expected to continue for up to a year, urgent 3-week referrals for suspected cancer cases have fallen by as much as 80%.
To estimate the potential impact on cancer deaths, Ajay Aggarwal, MD, from the London School of Hygiene and Tropical Medicine, United Kingdom, and colleagues conducted a population-based modeling study.
They collected data on 32,583 patients with breast cancer, 24,975 with colorectal cancer, 6744 with esophageal cancer, and 29,305 with lung cancer. Patients were diagnosed between 2010 and 2012 and were followed to 2015.
The investigators used that data to estimate the impact of diagnostic delays resulting from 12 months of physical distancing.
For breast cancer, this would lead to a 7.9%-9.6% increase in the number of cancer deaths within 5 years after diagnosis, or to 281-344 additional deaths.
For colorectal cancer, there would be a 15.3%-16.7% increase in mortality over 5 years, or an additional 1,445-1,563 deaths.
For lung cancer, there would a 4.8%-5.3% increase in mortality, or an additional 1235-1372 deaths.
For esophageal cancer, the mortality increase over 5 years would be 5.8%-6.0%, leading to 330-342 additional deaths.
Across the four tumor types, 59,204-63,229 life-years would be lost because of physical distancing compared to the prepandemic era.
Resources need to be increased
These additional deaths are not inevitable, the researchers suggest.
To prevent the increase in colorectal cancer deaths, for example, Aggarwal said, “It is vital that more resources are made urgently available for endoscopy and colonoscopy services, which are managing significant backlogs currently.
“Whilst currently attention is being focused on diagnostic pathways where cancer is suspected, the issue is that a significant number of cancers are diagnosed in patients awaiting investigation for symptoms not considered related to be cancer,” he added in a statement.
“Therefore we need a whole system approach to avoid the predicted excess deaths.”
Coauthor Bernard Rachet, PhD, also from the London School of Hygiene and Tropical Medicine, added that “to absorb the cancer patient backlog, the healthcare community also needs to establish clear criteria to prioritise patients on clinical grounds, in order to maintain equitability in care delivery.”
It will not be easy “to pin down the exact number of additional cancer deaths we expect to see over the coming years, but studies like this help us to understand the devastating long-term effect a pandemic like COVID-19 will have on the lives of thousands of cancer patients,” commented Michelle Mitchell, chief executive of Cancer Research UK.
Underlining the “enormous backlog” of cancer care that has built up during the pandemic, she said: “Diagnosing and treating people swiftly is vital to give people with cancer the greatest chances of survival.
“The government must work closely with the NHS to ensure it has sufficient staff and equipment to clear the backlog while giving patients the care that they need, quickly and safely,” Mitchell added.
Increasing resources will not be easy. In an accompanying editorial, William Hamilton, MD, PhD, University of Exeter, United Kingdom, warns that many NHS imaging departments, for example, were “working at full capacity before the COVID-19 pandemic.”
Consequently, they “might not be able to meet the increase in demand” resulting from the backlog in patients, especially as “the need to keep patients separate and to clean equipment has reduced their efficiency.
“The UK has had a long-term shortage of diagnostic capacity, although this shortage is not simply of equipment, but also of personnel, which is not so easily improved,” he cautions.
Another study, similar estimates
For the second study, Clare Turnbull, PhD, Institute of Cancer Research, London, and colleagues obtained age- and stage-stratified 10-year cancer survival estimates for patients in England diagnosed with 20 common tumor types between 2008 and 2017.
They also gathered data on cancer diagnoses made via urgent 2-week referrals between 2013 and 2016. They estimate that 6,281 patients were diagnosed with cancer of stages I-III per month.
Of those, 1,691 (27%) would die within 10 years of their diagnosis, they found.
They then calculated that delays in 2-week referrals during a 3-month lockdown would lead to an average delay in presentation of 2 months per patient.
A resulting 25% backlog in referrals would lead to 181 additional lives and 3,316 life-years lost. With a 75% backlog in referrals, an additional 276 lives and 5,075 life-years would be lost.
The team says that additional diagnostic delays spread over 3-8 months after the lockdown could increase the impact of a 25% backlog in referrals to 401 additional lives and 14,873 life-years lost.
For a 75% backlog in referrals, the additional lives lost would rise to 1,231, and the number of life-years lost would reach 22,635.
“Substantial additional deaths from diagnostic delays on top of those expected from delays in presentation – because many people are simply too afraid to visit their GP or hospital – are likely, especially if rapid provision of additional capacity, including technical provision and increased staffing, is not forthcoming,” Turnbull commented in a statement.
The study by Aggarwal and colleagues was funded by the U.K. Research and Innovation Economic and Social Research Council. Several of the researchers were supported by Cancer Research UK and Breast Cancer Now. Turnbull reports receiving support from the Movember Foundation.
This article first appeared on Medscape.com.