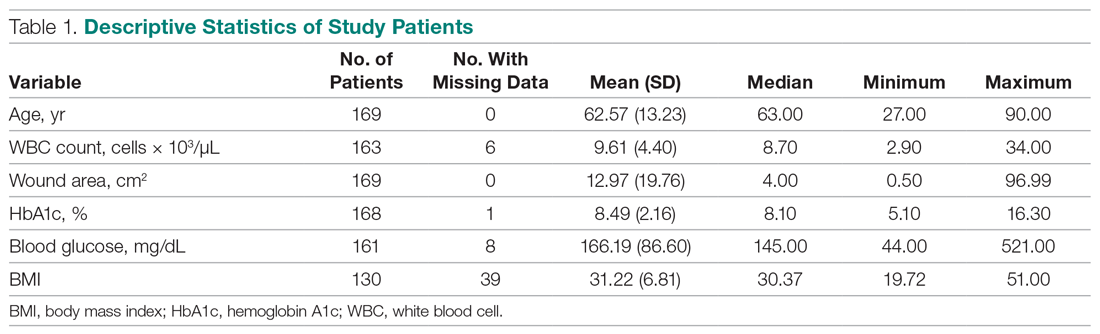
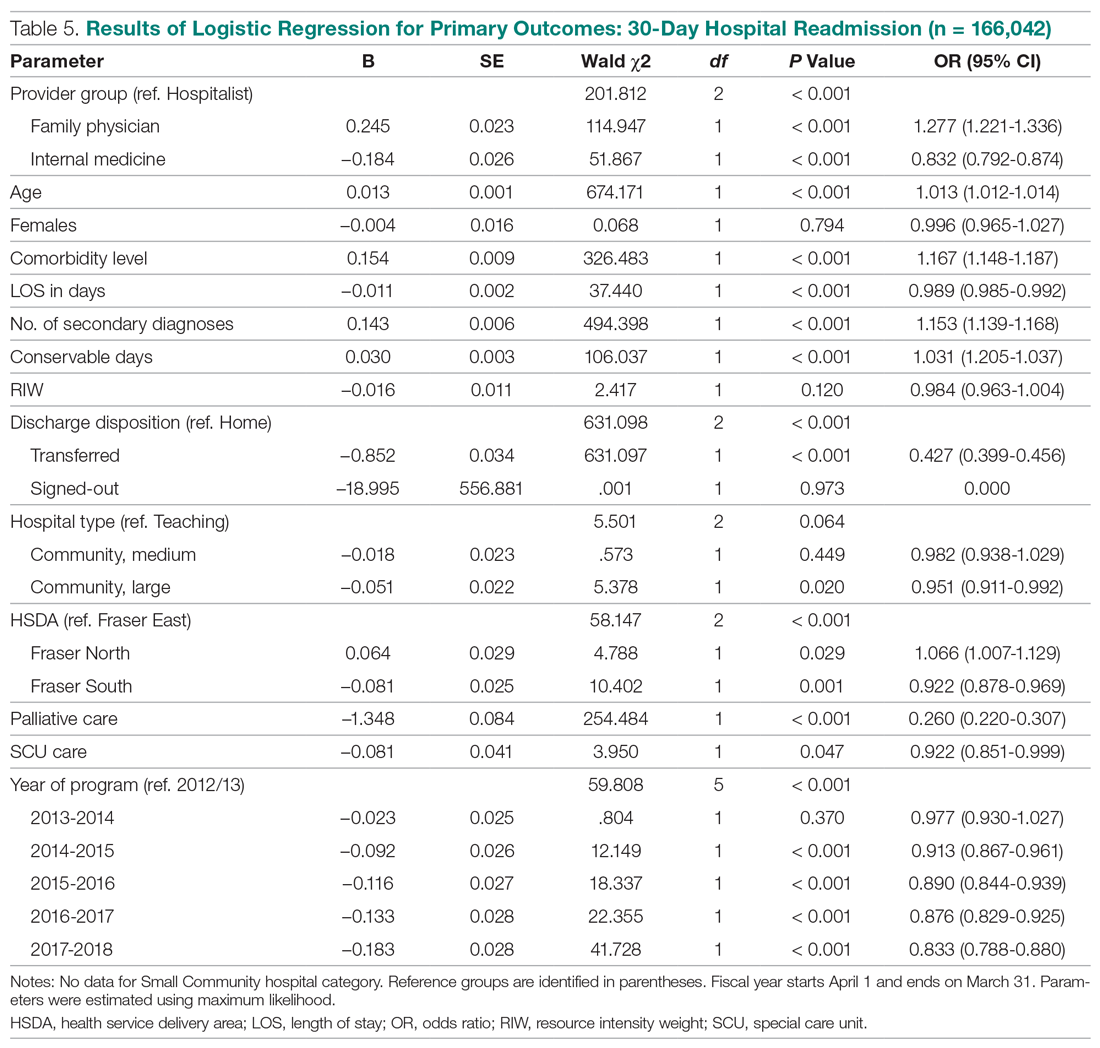
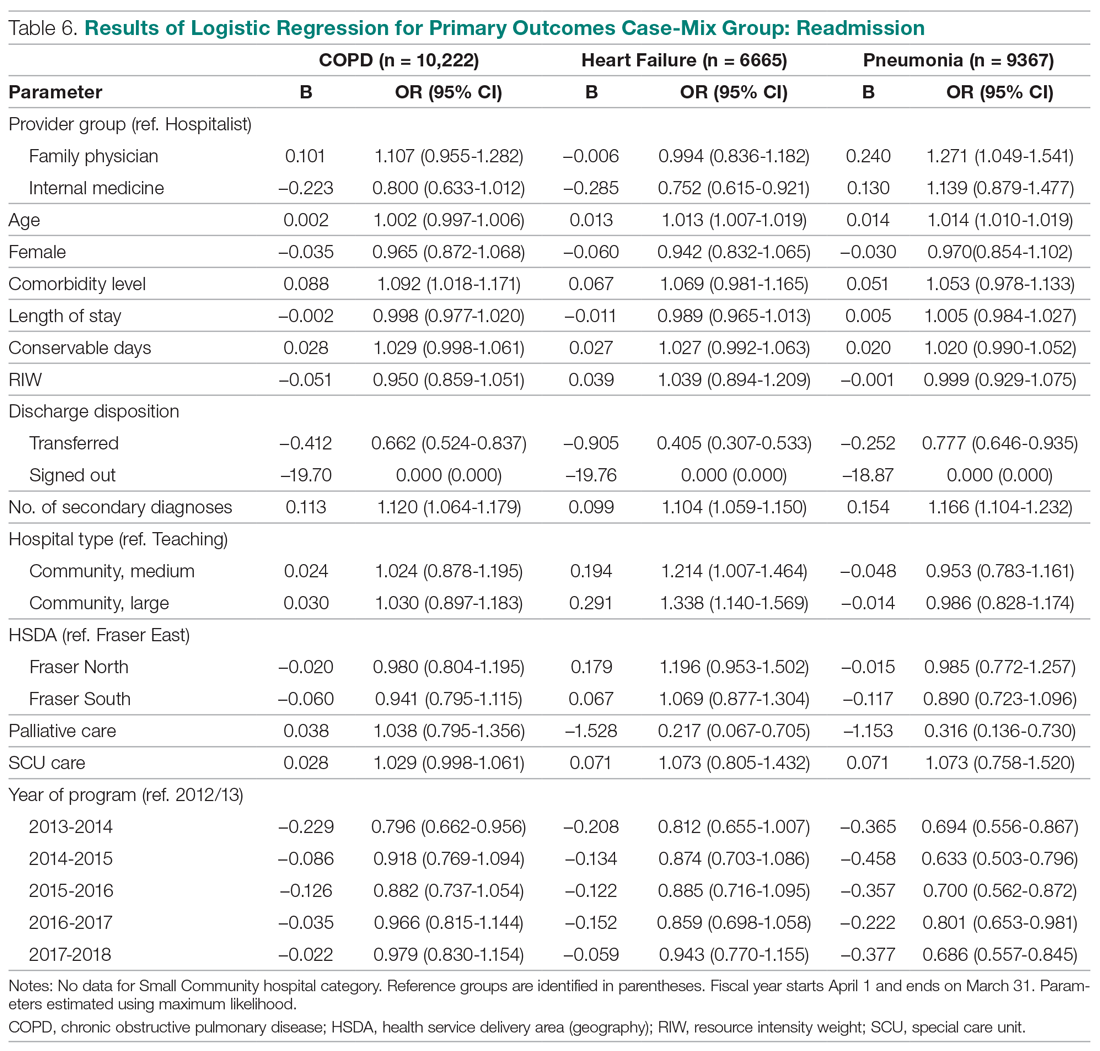
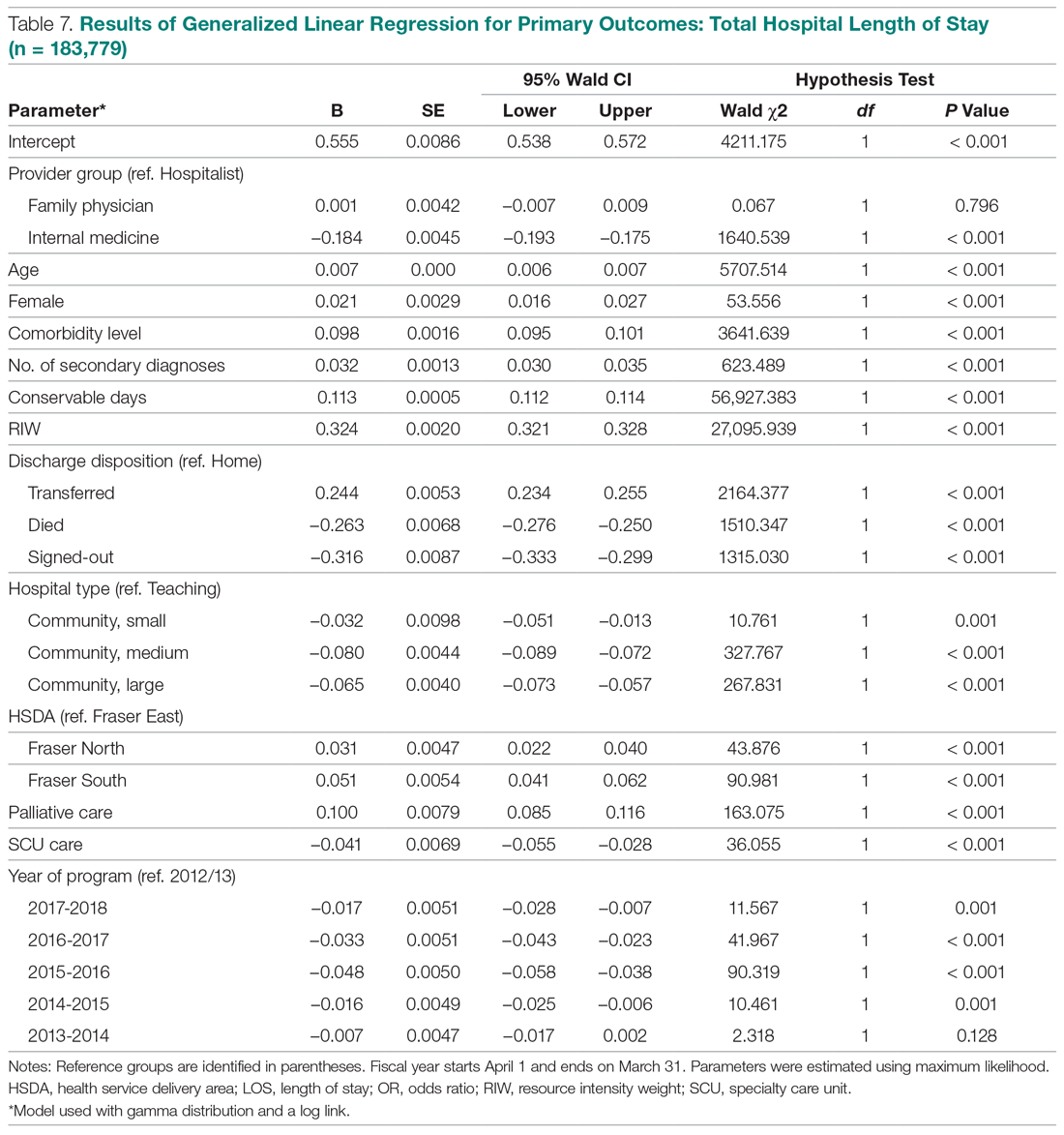
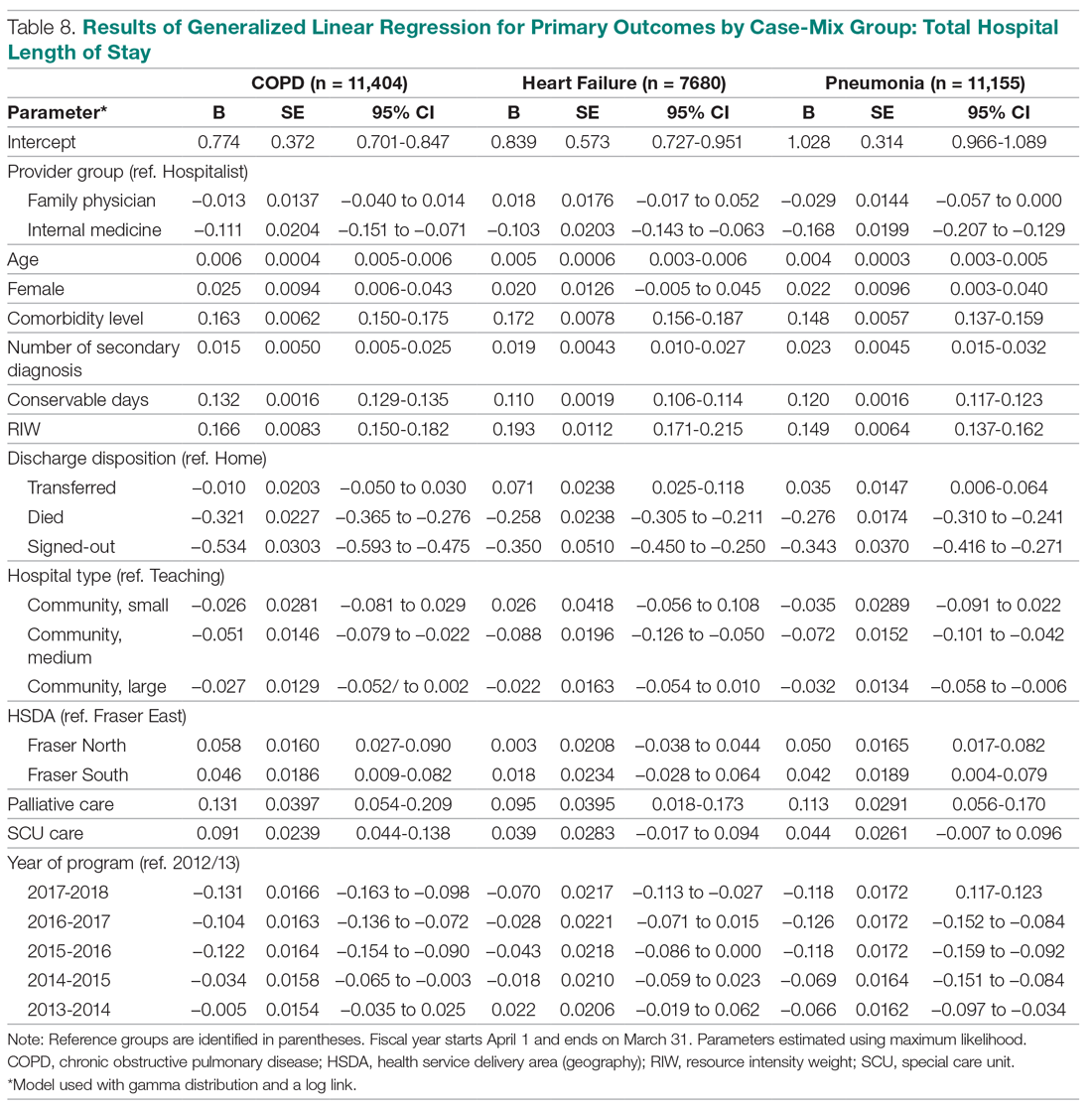
User login
Critical care and COVID-19: Dr. Matt Aldrich
Matt Aldrich, MD, is an anesthesiologist and medical director of critical care at UCSF Health in San Francisco. Robert Wachter, MD,MHM, spoke with him about critical care issues in COVID-19, including clinical presentation, PPE in the ICU, whether the health system has enough ventilators for a surge, and ethical dilemmas that ICUs may face during the pandemic.
Matt Aldrich, MD, is an anesthesiologist and medical director of critical care at UCSF Health in San Francisco. Robert Wachter, MD,MHM, spoke with him about critical care issues in COVID-19, including clinical presentation, PPE in the ICU, whether the health system has enough ventilators for a surge, and ethical dilemmas that ICUs may face during the pandemic.
Matt Aldrich, MD, is an anesthesiologist and medical director of critical care at UCSF Health in San Francisco. Robert Wachter, MD,MHM, spoke with him about critical care issues in COVID-19, including clinical presentation, PPE in the ICU, whether the health system has enough ventilators for a surge, and ethical dilemmas that ICUs may face during the pandemic.
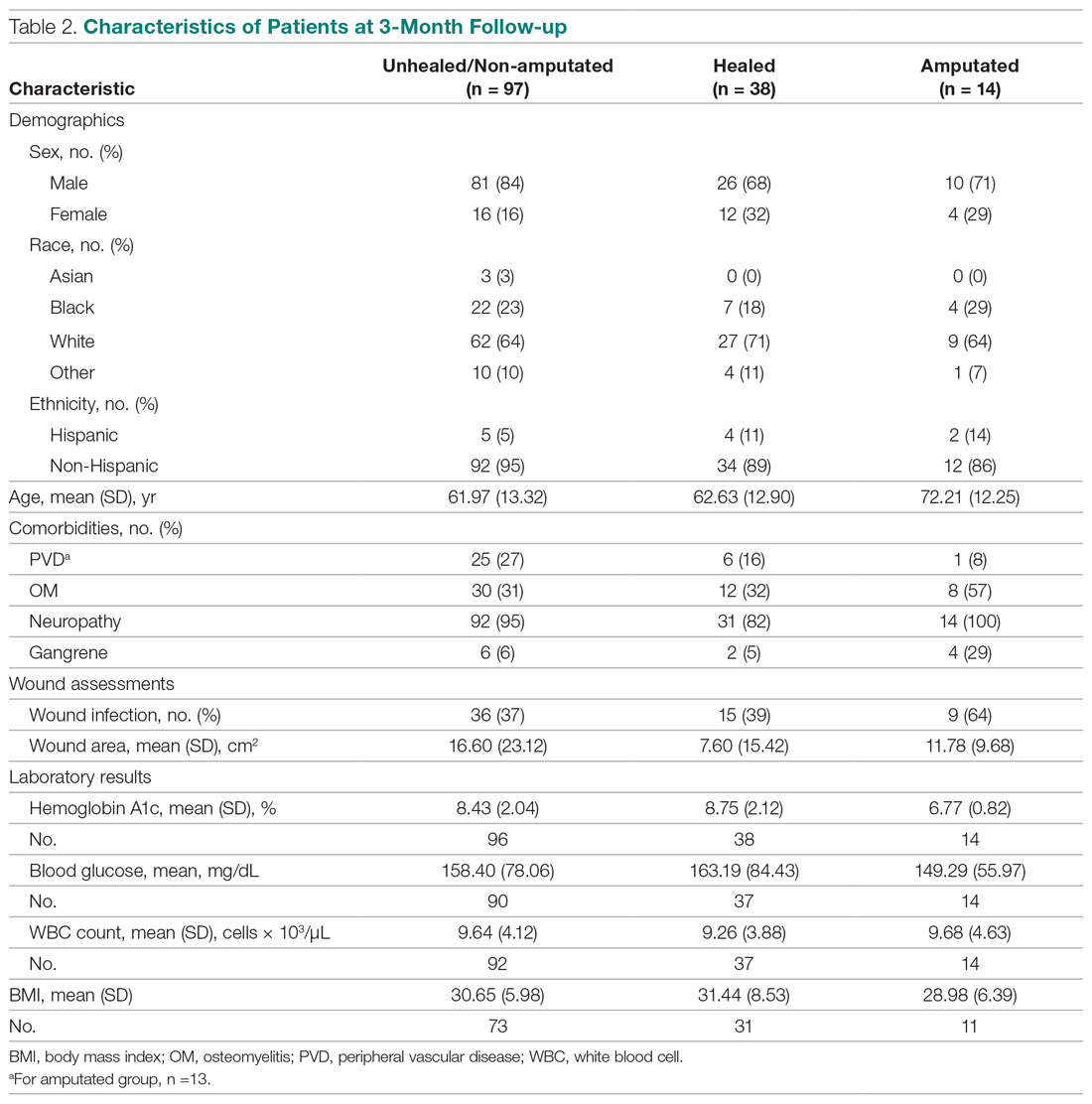
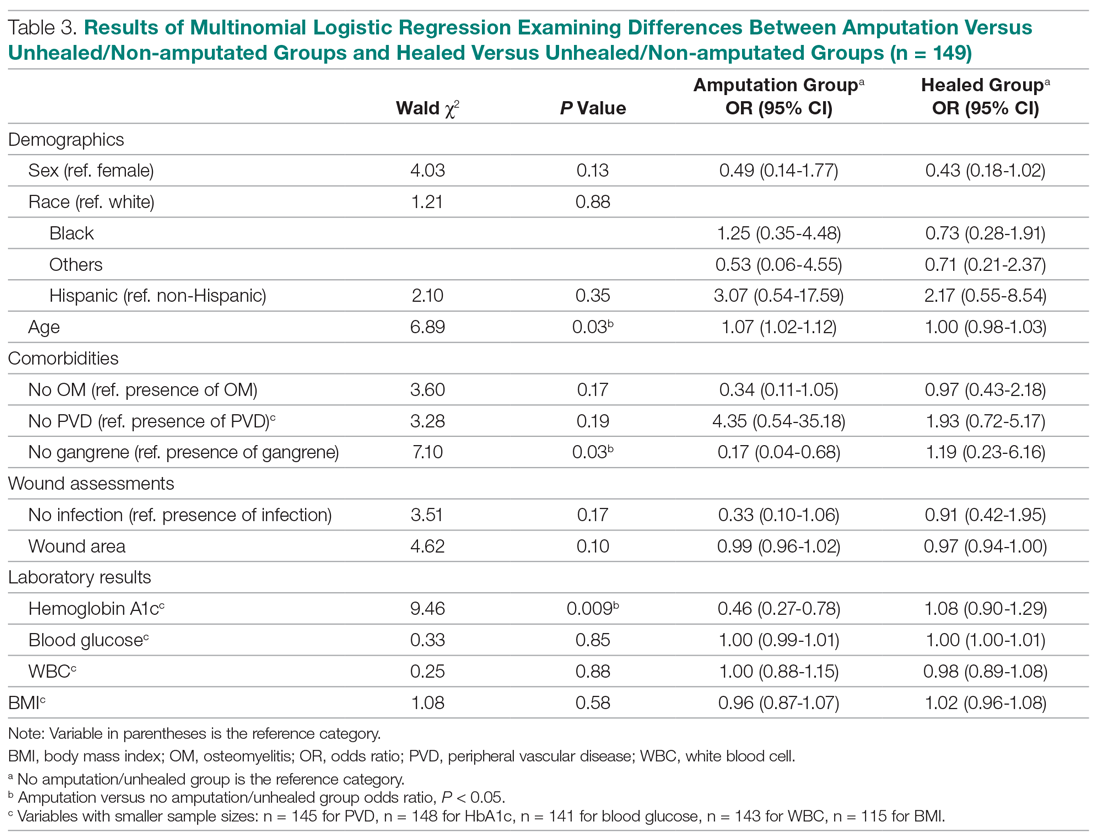
No benefit to trastuzumab after progression in HER2-positive gastric, GEJ cancers
While continuing trastuzumab after disease progression may provide clinical benefit in HER2-positive breast cancer, results of a phase 2 study failed to show the same in patients with HER2-positive gastric cancers.
Second-line treatment with paclitaxel plus trastuzumab didn’t improve progression-free survival, compared with paclitaxel alone, in the small, randomized study of patients with HER2-positive advanced gastric or gastroesophageal junction (GEJ) cancers who progressed on trastuzumab.
The failure of the paclitaxel/trastuzumab regimen in this study might be because of reduced HER2 positivity rather than acquired resistance to trastuzumab, according to the researchers, led by Akitaka Makiyama, MD, PhD, of Kyushu University Hospital in Fukuoka, Japan.
In any case, this approach of continuing trastuzumab beyond progression can’t be recommended as a standard of care, Dr. Makiyama and coauthors wrote in their report, which appears in the Journal of Clinical Oncology.
Drug development is meanwhile proceeding rapidly for several novel HER2-targeted treatments in this setting, the authors noted, including trastuzumab deruxtecan, an antibody-drug conjugate; ZW25, a bispecific antibody; and margetuximab, a chimeric monoclonal antibody with a modified Fc domain.
“We hope these new agents could confer survival benefit in HER2-positive gastric cancer by large-scale clinical trials comparing paclitaxel with ramucirumab as a standard arm,” the authors wrote, noting that paclitaxel with ramucirumab is considered a standard second-line regimen for advanced disease, regardless of HER2 status, based on analysis of the RAINBOW trial (Lancet Oncol. 2014 Oct;15[11]:1224-35).
The approval of novel therapies could be a significant advance for tumors in which HER2 is overexpressed or amplified, which represents about 10%-20% of gastric/GEJ cancers, according to Dr. Makiyama and coauthors.
In their phase 2 study, known as T-ACT (Trial to Assess the Concept of Using Trastuzumab Beyond Progression), Dr. Makiyama and colleagues included patients with HER2-positive advanced gastric or GEJ cancer that had progressed on a standard first-line chemotherapy regimen containing trastuzumab. Patients were enrolled at 52 centers in the West Japan Oncology Group.
A total of 91 patients were randomized to receive either paclitaxel at 80 mg/m2 on days 1, 8, and 15 every 4 weeks or the same paclitaxel dose and schedule plus trastuzumab, given at an initial dose of 8 mg/kg and subsequently at 6 mg/kg every 3 weeks.
The median progression-free survival was 3.2 months in the paclitaxel arm and 3.7 months in the paclitaxel/trastuzumab arm (P = .33). The median overall survival was 10.0 months and 10.2 months, respectively (P = .20). Responses were seen in about a third of patients in each group, and adverse events were similar between arms.
In biomarker analyses of tumor tissue samples obtained after first-line treatment, only 5 of 16 patients (31%) maintained HER2 positivity. Of those five patients, two received paclitaxel plus trastuzumab, and one had long-term stable disease (9.4 months).
“Reevaluation of HER2 expression status would be essential, particularly for developing new generations of HER2-targeted therapeutic agents in patients treated with prior trastuzumab,” Dr. Makiyama and colleagues concluded.
Partial support for this study came from a Japan Society of Clinical Oncology grant program. The authors disclosed relationships with Chugai Pharma, Lilly, Takeda, and numerous other companies.
SOURCE: Makiyama A et al. J Clin Oncol. 2020 Mar 24. doi: 10.1200/JCO.19.03077.
While continuing trastuzumab after disease progression may provide clinical benefit in HER2-positive breast cancer, results of a phase 2 study failed to show the same in patients with HER2-positive gastric cancers.
Second-line treatment with paclitaxel plus trastuzumab didn’t improve progression-free survival, compared with paclitaxel alone, in the small, randomized study of patients with HER2-positive advanced gastric or gastroesophageal junction (GEJ) cancers who progressed on trastuzumab.
The failure of the paclitaxel/trastuzumab regimen in this study might be because of reduced HER2 positivity rather than acquired resistance to trastuzumab, according to the researchers, led by Akitaka Makiyama, MD, PhD, of Kyushu University Hospital in Fukuoka, Japan.
In any case, this approach of continuing trastuzumab beyond progression can’t be recommended as a standard of care, Dr. Makiyama and coauthors wrote in their report, which appears in the Journal of Clinical Oncology.
Drug development is meanwhile proceeding rapidly for several novel HER2-targeted treatments in this setting, the authors noted, including trastuzumab deruxtecan, an antibody-drug conjugate; ZW25, a bispecific antibody; and margetuximab, a chimeric monoclonal antibody with a modified Fc domain.
“We hope these new agents could confer survival benefit in HER2-positive gastric cancer by large-scale clinical trials comparing paclitaxel with ramucirumab as a standard arm,” the authors wrote, noting that paclitaxel with ramucirumab is considered a standard second-line regimen for advanced disease, regardless of HER2 status, based on analysis of the RAINBOW trial (Lancet Oncol. 2014 Oct;15[11]:1224-35).
The approval of novel therapies could be a significant advance for tumors in which HER2 is overexpressed or amplified, which represents about 10%-20% of gastric/GEJ cancers, according to Dr. Makiyama and coauthors.
In their phase 2 study, known as T-ACT (Trial to Assess the Concept of Using Trastuzumab Beyond Progression), Dr. Makiyama and colleagues included patients with HER2-positive advanced gastric or GEJ cancer that had progressed on a standard first-line chemotherapy regimen containing trastuzumab. Patients were enrolled at 52 centers in the West Japan Oncology Group.
A total of 91 patients were randomized to receive either paclitaxel at 80 mg/m2 on days 1, 8, and 15 every 4 weeks or the same paclitaxel dose and schedule plus trastuzumab, given at an initial dose of 8 mg/kg and subsequently at 6 mg/kg every 3 weeks.
The median progression-free survival was 3.2 months in the paclitaxel arm and 3.7 months in the paclitaxel/trastuzumab arm (P = .33). The median overall survival was 10.0 months and 10.2 months, respectively (P = .20). Responses were seen in about a third of patients in each group, and adverse events were similar between arms.
In biomarker analyses of tumor tissue samples obtained after first-line treatment, only 5 of 16 patients (31%) maintained HER2 positivity. Of those five patients, two received paclitaxel plus trastuzumab, and one had long-term stable disease (9.4 months).
“Reevaluation of HER2 expression status would be essential, particularly for developing new generations of HER2-targeted therapeutic agents in patients treated with prior trastuzumab,” Dr. Makiyama and colleagues concluded.
Partial support for this study came from a Japan Society of Clinical Oncology grant program. The authors disclosed relationships with Chugai Pharma, Lilly, Takeda, and numerous other companies.
SOURCE: Makiyama A et al. J Clin Oncol. 2020 Mar 24. doi: 10.1200/JCO.19.03077.
While continuing trastuzumab after disease progression may provide clinical benefit in HER2-positive breast cancer, results of a phase 2 study failed to show the same in patients with HER2-positive gastric cancers.
Second-line treatment with paclitaxel plus trastuzumab didn’t improve progression-free survival, compared with paclitaxel alone, in the small, randomized study of patients with HER2-positive advanced gastric or gastroesophageal junction (GEJ) cancers who progressed on trastuzumab.
The failure of the paclitaxel/trastuzumab regimen in this study might be because of reduced HER2 positivity rather than acquired resistance to trastuzumab, according to the researchers, led by Akitaka Makiyama, MD, PhD, of Kyushu University Hospital in Fukuoka, Japan.
In any case, this approach of continuing trastuzumab beyond progression can’t be recommended as a standard of care, Dr. Makiyama and coauthors wrote in their report, which appears in the Journal of Clinical Oncology.
Drug development is meanwhile proceeding rapidly for several novel HER2-targeted treatments in this setting, the authors noted, including trastuzumab deruxtecan, an antibody-drug conjugate; ZW25, a bispecific antibody; and margetuximab, a chimeric monoclonal antibody with a modified Fc domain.
“We hope these new agents could confer survival benefit in HER2-positive gastric cancer by large-scale clinical trials comparing paclitaxel with ramucirumab as a standard arm,” the authors wrote, noting that paclitaxel with ramucirumab is considered a standard second-line regimen for advanced disease, regardless of HER2 status, based on analysis of the RAINBOW trial (Lancet Oncol. 2014 Oct;15[11]:1224-35).
The approval of novel therapies could be a significant advance for tumors in which HER2 is overexpressed or amplified, which represents about 10%-20% of gastric/GEJ cancers, according to Dr. Makiyama and coauthors.
In their phase 2 study, known as T-ACT (Trial to Assess the Concept of Using Trastuzumab Beyond Progression), Dr. Makiyama and colleagues included patients with HER2-positive advanced gastric or GEJ cancer that had progressed on a standard first-line chemotherapy regimen containing trastuzumab. Patients were enrolled at 52 centers in the West Japan Oncology Group.
A total of 91 patients were randomized to receive either paclitaxel at 80 mg/m2 on days 1, 8, and 15 every 4 weeks or the same paclitaxel dose and schedule plus trastuzumab, given at an initial dose of 8 mg/kg and subsequently at 6 mg/kg every 3 weeks.
The median progression-free survival was 3.2 months in the paclitaxel arm and 3.7 months in the paclitaxel/trastuzumab arm (P = .33). The median overall survival was 10.0 months and 10.2 months, respectively (P = .20). Responses were seen in about a third of patients in each group, and adverse events were similar between arms.
In biomarker analyses of tumor tissue samples obtained after first-line treatment, only 5 of 16 patients (31%) maintained HER2 positivity. Of those five patients, two received paclitaxel plus trastuzumab, and one had long-term stable disease (9.4 months).
“Reevaluation of HER2 expression status would be essential, particularly for developing new generations of HER2-targeted therapeutic agents in patients treated with prior trastuzumab,” Dr. Makiyama and colleagues concluded.
Partial support for this study came from a Japan Society of Clinical Oncology grant program. The authors disclosed relationships with Chugai Pharma, Lilly, Takeda, and numerous other companies.
SOURCE: Makiyama A et al. J Clin Oncol. 2020 Mar 24. doi: 10.1200/JCO.19.03077.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Systemic Treatment for Advanced Hepatocellular Carcinoma
From the University of Alabama at Birmingham, Division of Hematology Oncology, Birmingham, AL, and the University of South Alabama, Division of Hematology Oncology, Mobile, AL. Dr. Paluri and Dr. Hatic contributed equally to this article.
Abstract
- Objective: To review systemic treatment options for patients with locally advanced unresectable hepatocellular carcinoma (HCC).
- Methods: Review of the literature.
- Results: The paradigm of what constitutes first-line treatment for advanced HCC is shifting. Until recently, many patients with advanced HCC were treated with repeated locoregional therapies, such as transartertial embolization (TACE). However, retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
- Conclusion: Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
Keywords: liver cancer; molecular therapy; immunotherapy.
Hepatocellular carcinoma (HCC) represents 90% of primary liver malignancies. It is the fifth most common malignancy in males and the ninth most common in females worldwide.1 In contrast to other major cancers (colon, breast, prostate), the incidence of and mortality from HCC has increased over the past decade, following a brief decline between 1999 and 2004.2 The epidemiology and incidence of HCC is closely linked to chronic liver disease and conditions predisposing to liver cirrhosis. Worldwide, hepatitis B virus infection is the leading cause of liver cirrhosis and, hence, HCC. In the United States, 50% of HCC cases are linked to hepatitis C virus (HCV) infection. Diabetes mellitus and alcoholic and nonalcoholic steatohepatitis are the other major etiologies of HCC. Indeed, the metabolic syndrome, independent of other factors, is associated with a 2-fold increase in the risk of HCC.3
Although most cases of HCC are predated by liver cirrhosis, in about 20% of patients HCC occurs without liver cirrhosis.4 Similar to other malignancies, surgery in the form of resection (for isolated lesions in the context of good liver function) or liver transplant (for low-volume disease with mildly impaired liver function) provides the best chance of a cure. Locoregional therapies involving hepatic artery–directed therapy are offered for patients with more advanced disease that is limited to the liver, while systemic therapy is offered for advanced unresectable disease that involves portal vein invasion, lymph nodes, and distant metastasis. The
Molecular Pathogenesis
Similar to other malignancies, a multistep process of carcinogenesis, with accumulation of genomic alterations at the molecular and cellular levels, is recognized in HCC. In about 80% of cases, repeated and chronic injury, inflammation, and repair lead to a distortion of normal liver architecture and development of cirrhotic nodules. Exome sequencing of HCC tissues has identified risk factor–specific mutational signatures, including those related to the tumor microenvironment, and defined the extensive landscape of altered genes and pathways in HCC (eg, angiogenic and MET pathways).7 In the Schulze et al study, the frequency of alterations that could be targeted by available Food and Drug Administration (FDA)–approved drugs comprised either amplifications or mutations of FLTs (6%), FGF3 or 4 or 19 (4%), PDGFRs (3%), VEGFA (1%), HGF (3%), MTOR (2%), EGFR (1%), FGFRs (1%), and MET (1%).7 Epigenetic modification of growth-factor expression, particularly insulin-like growth factor 2 and transforming growth factor alpha, and structural alterations that lead to loss of heterozygosity are early events that cause hepatocyte proliferation and progression of dysplastic nodules.8,9 Advances in whole-exome sequencing have identified TERT promoter mutations, leading to activation of telomerase, as an early event in HCC pathogenesis. Other commonly altered genes include CTNNB1 (B-Catenin) and TP53. As a group, alterations in the MAP kinase pathway genes occur in about 40% of HCC cases.
Actionable oncogenic driver alterations are not as common as tumor suppressor pathway alterations in HCC, making targeted drug development challenging.10,11 The FGFR (fibroblast growth factor receptor) pathway, which plays a critical role in carcinogenesis-related cell growth, survival, neo-angiogenesis, and acquired resistance to other cancer treatments, is being explored as a treatment target.12 The molecular characterization of HCC may help with identifying new biomarkers and present opportunities for developing therapeutic targets.
CASE PRESENTATION
A 61-year-old man with a history of chronic hepatitis C and hypertension presents to his primary care physician due to right upper quadrant pain. Laboratory evaluation shows transaminases elevated 2 times the upper limit of normal. This leads to an ultrasound and follow-up magnetic resonance imaging. Imaging shows diffuse cirrhotic changes, with a 6-cm, well-circumscribed lesion within the left lobe of the liver that shows rapid arterial enhancement with venous washout. These vascular characteristics are consistent with HCC. In addition, 2 satellite lesions in the left lobe and sonographic evidence of invasion into the portal vein are present. Periportal lymph nodes are pathologically enlarged.
The physical examination is unremarkable, except for mild tenderness over the right upper quadrant of the abdomen. Serum bilirubin, albumin, platelets, and international normalized ratio are normal, and alpha fetoprotein (AFP) is elevated at 1769 ng/mL. The patient’s family history is unremarkable for any major illness or cancer. Computed tomography scan of the chest and pelvis shows no evidence of other lesions. His liver disease is classified as Child–Pugh A. Due to locally advanced presentation, the tumor is deemed to be nontransplantable and unresectable, and is staged as BCLC-C. The patient continues to work and his performance status is ECOG (
What systemic treatment would you recommend for this patient with locally advanced unresectable HCC with nodal metastasis?
First-Line Therapeutic Options
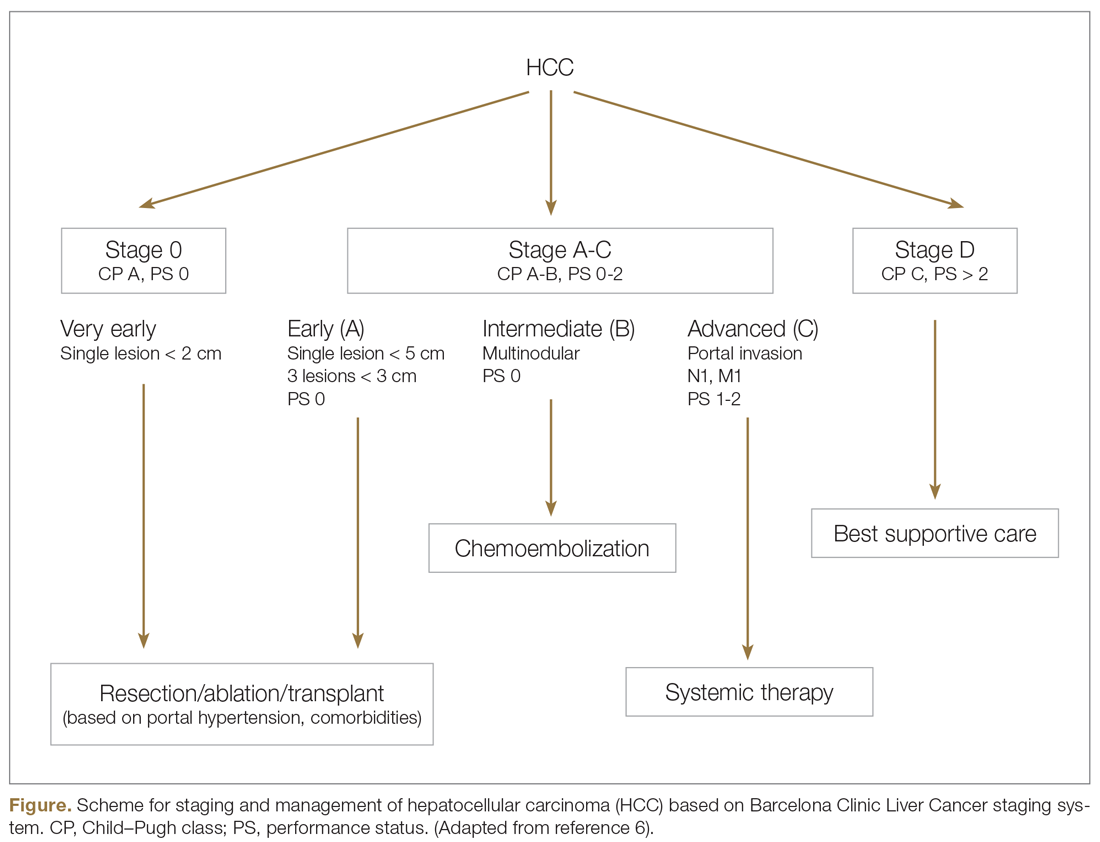
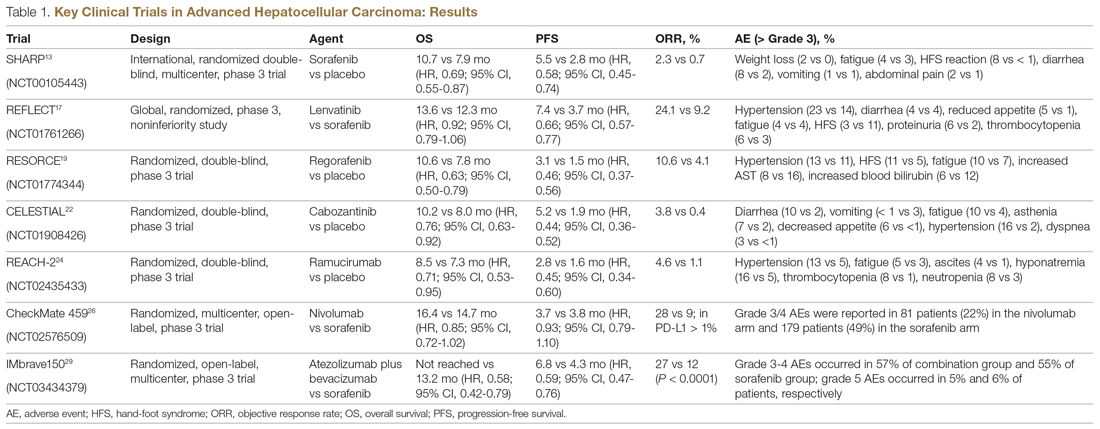
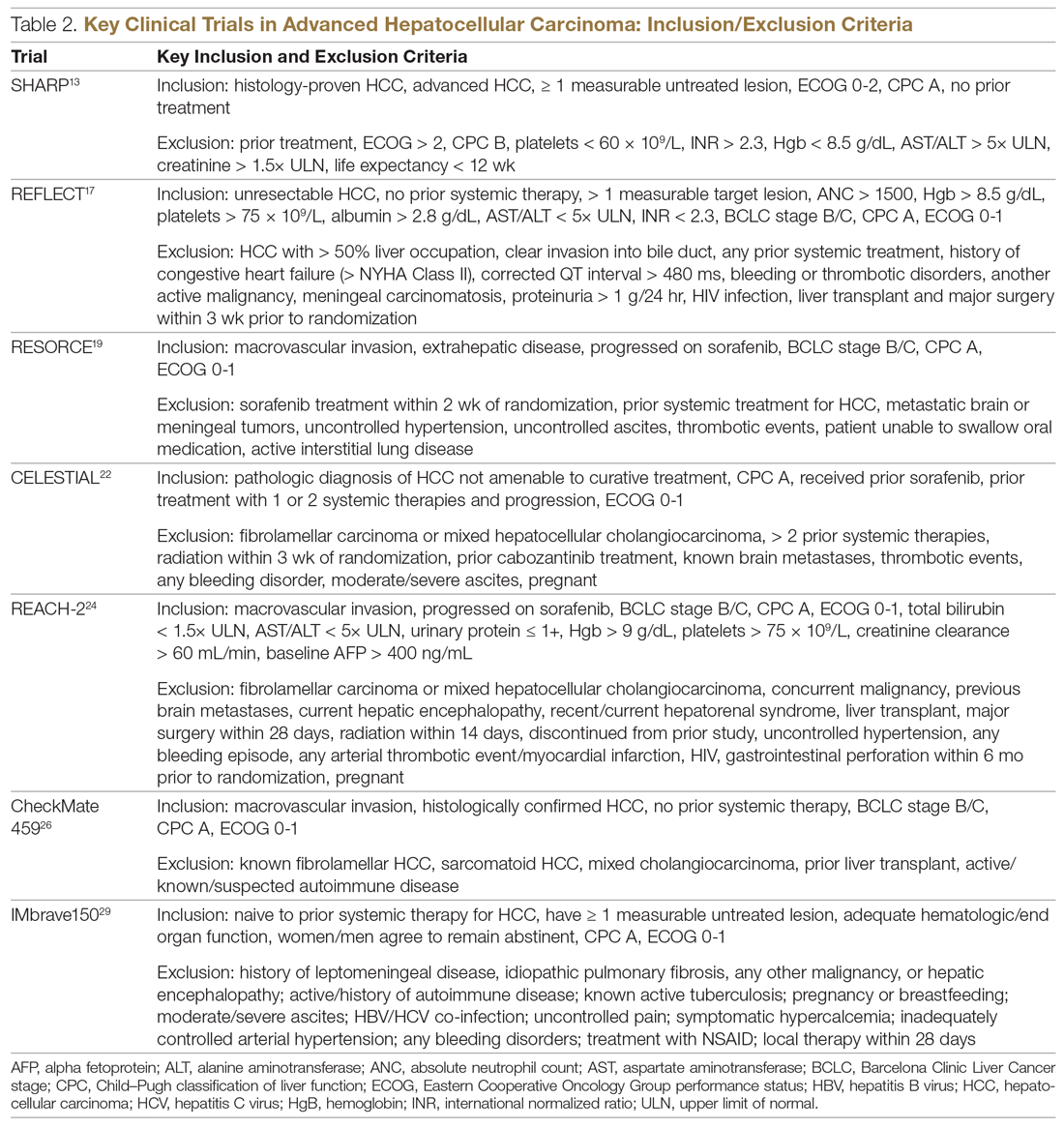
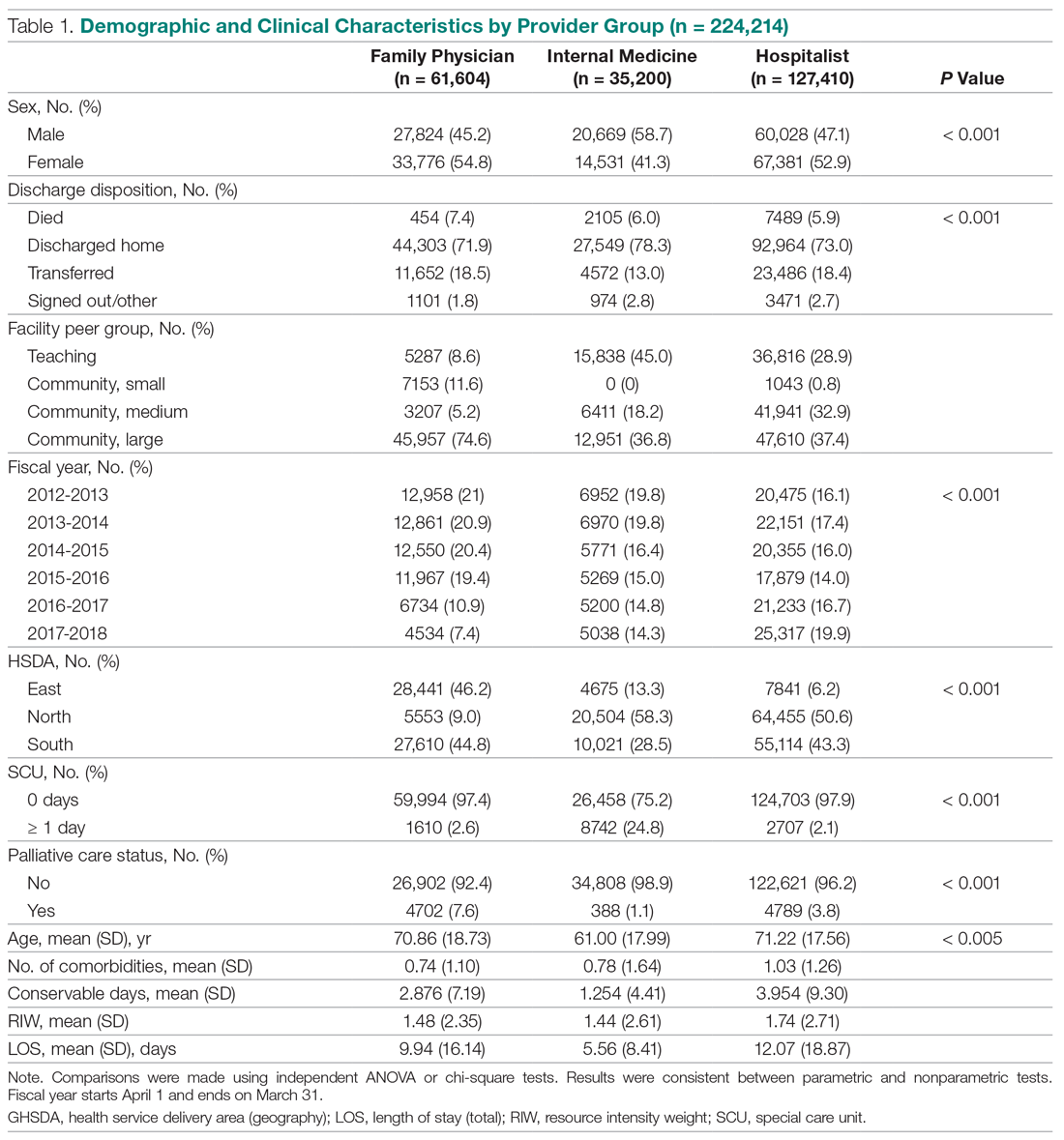
Systemic treatment of HCC is challenging because of the underlying liver cirrhosis and hepatic dysfunction present in most patients. Overall prognosis is therefore dependent on the disease biology and burden and on the degree of hepatic dysfunction. These factors must be considered in patients with advanced disease who present for systemic therapy. As such, patients with BCLC class D HCC with poor performance status and impaired liver function are better off with best supportive care and hospice services (Figure). Table 1 and Table 2 outline the landmark trials that led to the approval of agents for advanced HCC treatment.
Sorafenib
In the patient with BCLC class C HCC who has preserved liver function (traditionally based on a Child–Pugh score of ≤ 6 and a decent functional status [ECOG performance status 1-2]), sorafenib is the first FDA-approved first-line treatment. Sorafenib is a small-molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) kinase signaling, in addition to many other tyrosine kinase pathways (including the platelet-derived growth factor and Raf-Ras pathways). Evidence for the clinical benefit of sorafenib comes from the SHARP trial.13 This was a multinational, but primarily European, randomized phase 3 study that compared sorafenib to best supportive care for advanced HCC in patients with a Child–Pugh score ≤ 6A and a robust performance status (ECOG 0 and 1). Overall survival (OS) with placebo and sorafenib was 7.9 months and 10.7 months, respectively. There was no difference in time to radiologic progression, and the progression-free survival (PFS) at 4 months was 62% with sorafenib and 42% with placebo. Patients with HCV-associated HCC appeared to derive a more substantial benefit from sorafenib.14 In a smaller randomized study of sorafenib in Asian patients with predominantly hepatitis B–associated HCC, OS in the sorafenib and best supportive care arms was lower than that reported in the SHARP study (6.5 months vs 4.2 months), although OS still was longer in the sorafenib group.15
Significant adverse events reported with sorafenib include fatigue (30%), hand and foot syndrome (30%), diarrhea (15%), and mucositis (10%). Major proportions of patients in the clinical setting have not tolerated the standard dose of 400 mg twice daily. Dose-adjusted administration of sorafenib has been advocated in patients with more impaired liver function (Child–Pugh class 7B) and bilirubin of 1.5 to 3 times the upper limit of normal, although it is unclear whether these patients are deriving any benefit from sorafenib.16 At this time, in a patient with preserved liver function, starting with 400 mg twice daily, followed by dose reduction based on toxicity, remains standard.
Lenvatinib
After multiple attempts to develop newer first-line treatments for HCC,
Second-Line Therapeutic Options
Following the sorafenib approval, clinical studies of several other agents did not meet their primary endpoint and failed to show improvement in clinical outcomes compared to sorafenib. However, over the past years the treatment landscape for advanced HCC has been changed with the approval of several agents in the second line. The overall response rate (ORR) has become the new theme for management of advanced disease. With multiple therapeutic options available, optimal sequencing now plays a critical role, especially for transitioning from locoregional to systemic therapy. Five drugs are now indicated for second-line treatment of patients who progressed on or were intolerant to sorafenib: regorafenib, cabozantinib, ramucirumab, nivolumab, and pembrolizumab.
Regorafenib
Regorafenib was evaluated in the advanced HCC setting in a single-arm, phase 2 trial involving 36 patients with Child–Pugh class A liver disease who had progressed on prior sorafenib.18 Patients received regorafenib 160 mg orally once daily for 3 weeks on/1 week off cycles. Disease control was achieved in 72% of patients, with stable disease in 25 patients (69%). Based on these results, regorafenib was further evaluated in the multicenter, phase 3, 2:1 randomized, double-blind, placebo-controlled RESORCE study, which enrolled 573 patients.19 Due to the overlapping safety profiles of sorafenib and regorafenib, the inclusion criteria required patients to have tolerated a sorafenib dose of at least 400 mg daily for 20 of the past 28 days of treatment prior to enrollment. The primary endpoint of the study, OS, was met (median OS of 10.6 months in regorafenib arm versus 7.8 months in placebo arm; hazard ratio [HR], 0.63; P < 0.0001).
Cabozantinib
CELESTIAL was a phase 3, double-blind study that assessed the efficacy of cabozantinib versus placebo in patients with advanced HCC who had received prior sorafenib.22 In this study, 707 patients with Child–Pugh class A liver disease who progressed on at least 1 prior systemic therapy were randomized in a 2:1 ratio to treatment with cabozantinib at 60 mg daily or placebo. Patients treated with cabozantinib had a longer OS (10.2 months vs 8.0 months), resulting in a 24% reduction in the risk of death (HR, 0.76), and a longer median PFS (5.2 months versus 1.9 months). The disease control rate was higher with cabozantinib (64% vs 33%) as well. The incidence of high‐grade adverse events in the cabozantinib group was twice that of the placebo group. Common adverse events reported with cabozantinib included HFSR (17%), hypertension (16%), increased aspartate aminotransferase (12%), fatigue (10%), and diarrhea (10%).
Ramucirumab
REACH was a phase 3 study exploring the efficacy of ramucirumab that did not meet its primary endpoint; however, the subgroup analysis in AFP-high patients showed an OS improvement with ramucirumab.23 This led to the phase 3 REACH-2 trial, a multicenter, randomized, double-blind biomarker study in patients with advanced HCC who either progressed on or were intolerant to sorafenib and had an AFP level ≥ 400 ng/mL.24 Patients were randomized to ramucirumab 8 mg/kg every 2 weeks or placebo. The study met its primary endpoint, showing improved OS (8.5 months vs 7.3 months; P = 0.0059). The most common treatment-related adverse events in the ramucirumab group were ascites (5%), hypertension (12%), asthenia (5%), malignant neoplasm progression (6%), increased aspartate aminotransferase concentration (5%), and thrombocytopenia.
Immunotherapy
HCC is considered an inflammation-induced cancer, which renders immunotherapeutic strategies more appealing. The PD-L1/PD-1 pathway is the critical immune checkpoint mechanism and is an important target for treatment. HCC uses a complex, overlapping set of mechanisms to evade cancer-specific immunity and to suppress the immune system. Initial efforts to develop immunotherapies for HCC focused on anti-PD-1 and anti-PD-L1 antibodies. CheckMate 040 evaluated nivolumab in 262 sorafenib-naïve and -treated patients with advanced HCC (98% with Child–Pugh scores of 5 or 6), with a median follow-up of 12.9 months.25 In sorafenib-naïve patients (n = 80), the ORR was 23%, and the disease control rate was 63%. In sorafenib-treated patients (n = 182), the ORR was 18%. Response was not associated with PD-L1 expression. Durable objective responses, a manageable safety profile, and promising efficacy led the FDA to grant accelerated approval of nivolumab for the treatment of patients with HCC who have been previously treated with sorafenib. Based on this, the phase 3 randomized CheckMate-459 trial evaluated the efficacy of nivolumab versus sorafenib in the first-line. Median OS and ORR were better with nivolumab (16.4 months vs 14.7 months; HR 0.85; P = 0.752; and 15% [with 5 complete responses] vs 7%), as was the safety profile (22% vs 49% reporting grade 3 and 4 adverse events). 26
The KEYNOTE-224 study27 evaluated pembrolizumab in 104 patients with previously treated advanced HCC. This study showed an ORR of 17%, with 1 complete response and 17 partial responses. One-third of the patients had progressive disease, while 46 had stable disease. Among those who responded, 56% maintained a durable response for more than 1 year. Subsequently, in KEYNOTE 240, pembrolizumab showed an improvement in OS (13.9 months vs 10.6 months; HR, 0.78; P = 0.0238) and PFS (3.0 months versus 2.8 months; HR, 0.78; P = 0.0186) compared with placebo.28 The ORR for pembrolizumab was 16.9% (95% confidence interval [CI], 12.7%-21.8%) versus 2.2% (95% CI, 0.5%-6.4%; P = 0.00001) for placebo. Mean duration of response was 13.8 months.
In the IMbrave150 trial, atezolizumab/bevacizumab combination, compared to sorafenib, had better OS (not estimable vs 13.2 months; P = 0.0006), PFS (6.8 months vs 4.5 months, P < 0.0001), and ORR (33% vs 13%, P < 0.0001), but grade 3-4 events were similar.29 This combination has potential for first-line approval. The COSMIC–312 study is comparing the combination of cabozantinib and atezolizumab to sorafenib monotherapy and cabozantinib monotherapy in advanced HCC.
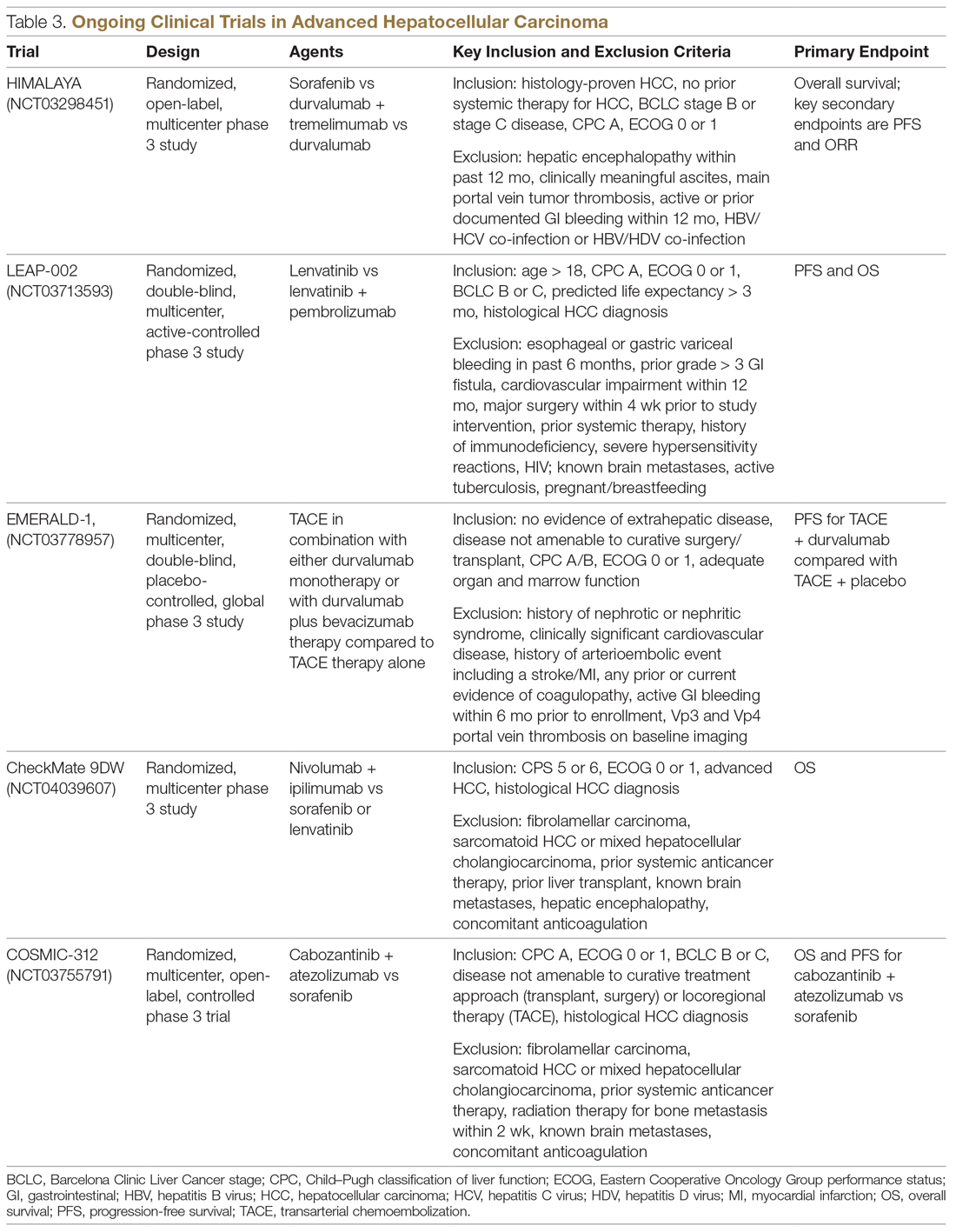
Resistance to immunotherapy can be extrinsic, associated with activation mechanisms of T-cells, or intrinsic, related to immune recognition, gene expression, and cell-signaling pathways.30 Tumor-immune heterogeneity and antigen presentation contribute to complex resistance mechanisms.31,32 Although clinical outcomes have improved with immune checkpoint inhibitors, the response rate is low and responses are inconsistent, likely due to an immunosuppressive tumor microenvironment.33 Therefore, several novel combinations of checkpoint inhibitors and targeted drugs are being evaluated to bypass some of the resistance mechanisms (Table 3).
Chemotherapy
Multiple combinations of cytotoxic regimens have been evaluated, but efficacy has been modest, suggesting the limited role for traditional chemotherapy in the systemic management of advanced HCC. Response rates to chemotherapy are low and responses are not durable. Gemcitabine- and doxorubicin-based treatment and FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) are some regimens that have been studied, with a median OS of less than 1 year for these regimens.34-36 FOLFOX had a higher response rate (8.15% vs 2.67%; P = 0.02) and longer median OS (6.40 months versus 4.97 months; HR, 0.80; 95% CI, 0.63-1.02; P = 0.07) than doxorubicin.34 With the gemcitabine/oxaliplatin combination, ORR was 18%, with stable disease in 58% of patients, and median PFS and OS were 6.3 months and 11.5 months, respectively.35 In a study that compared doxorubicin and PIAF (cisplatin/interferon a-2b/doxorubicin/5-fluorouracil), median OS was 6.83 months and 8.67 months, respectively (P = 0.83). The hazard ratio for death from any cause in the PIAF group compared with the doxorubicin group was 0.97 (95% CI, 0.71-1.32). PIAF had a higher ORR (20.9%; 95% CI, 12.5%-29.2%) than doxorubicin (10.5%; 95% CI, 3.9%-16.9%).
The phase 3 ALLIANCE study evaluated the combination of sorafenib and doxorubicin in treatment-naïve HCC patients with Child–Pugh class A liver disease, and did not demonstrate superiority with the addition of cytotoxic chemotherapy.37 Indeed, the combination of chemotherapy with sorafenib appears harmful in terms of lower OS (9.3 months vs 10.6 months; HR, 1.06; 95% CI, 0.8-1.4) and worse toxicity. Patients treated with the combination experienced more hematologic (37.8% vs 8.1%) and nonhematologic adverse events (63.6% vs 61.5%).
Locoregional Therapy
The role of locoregional therapy in advanced HCC remains the subject of intense debate. Patients with BCLC stage C HCC with metastatic disease and those with lymph node involvement are candidates for systemic therapy. The optimal candidate for locoregional therapy is the patient with localized intermediate-stage disease, particularly hepatic artery–delivered therapeutic interventions. However, the presence of a solitary large tumor or portal vein involvement constitutes gray areas regarding which therapy to deliver directly to the tumor via the hepatic artery, and increasingly stereotactic body radiation therapy is being offered.
Transarterial Chemoembolization
Transarterial chemoembolization (TACE), with or without chemotherapy, is the most widely adopted locoregional therapy in the management of HCC. TACE exploits the differential vascular supply to the HCC and normal liver parenchyma. Normal liver receives only one-fourth of its blood supply from the hepatic artery (three-fourths from the portal vein), whereas HCC is mainly supplied by the hepatic artery. A survival benefit for TACE compared to best supportive care is widely acknowledged for intermediate-stage HCC, and transarterial embolization (TAE) with gelatin sponge or microspheres is noninferior to TACE.38,39 Overall safety profile and efficacy inform therapy selection in advanced HCC, although the evidence for TACE in advanced HCC is less robust. Although single-institution experiences suggest survival numbers similar to and even superior to sorafenib,40,41 there is a scarcity of large randomized clinical trial data to back this up. Based on this, patients with advanced HCC should only be offered liver-directed therapy within a clinical trial or on a case-by-case basis under multidisciplinary tumor board consensus.
A serious adverse effect of TACE is post-embolization syndrome, which occurs in about 30% of patients and may be associated with poor prognosis.42 The syndrome consists of right upper quadrant abdominal pain, malaise, and worsening nausea/vomiting following the embolization procedure. Laboratory abnormalities and other complications may persist for up to 30 days after the procedure. This is a concern, because post-embolization syndrome may affect the ability to deliver systemic therapy.
Transarterial Radioembolization
In the past few years, there has been an uptick in the utilization of transarterial radioembolization (TARE), which instead of delivering glass beads, as done in TAE, or chemotherapy-infused beads, as done in TACE, delivers the radioisotope Y-90 to the tumor via the hepatic artery. TARE is able to administer larger doses of radiation to the tumor while avoiding normal liver tissue, as compared to external-beam radiation. There has been no head-to-head comparison of these different intra-arterial therapy approaches, but TARE with Y-90 has been shown to be safe in patients with portal vein thrombosis. A recent multicenter retrospective study of TARE demonstrated a median OS of 8.8 to 10.8 months in patients with BCLC C HCC,43 and in a large randomized study of Y-90 compared to sorafenib in advanced and previously treated intermediate HCC, there was no difference in median OS between the treatment modalities (8 months for selective internal radiotherapy, 9 months for sorafenib; P = 0.18). Treatment with Y-90 was better tolerated.44 A major impediment to the adoption of TARE is the time it takes to order, plan, and deliver Y-90 to patients. Radio-embolization-induced liver disease, similar to post-embolization syndrome, is characterized by jaundice and ascites, which may occur 4 to 8 weeks postprocedure and is more common in patients with HCC who do not have cirrhosis. Compared to TACE, TARE may offer a better adverse effect profile, with improvement in quality of life.
Combination of Systemic and Locoregional Therapy
Even in carefully selected patients with intermediate- and advanced-stage HCC, locoregional therapy is not curative. Tumor embolization may promote more angiogenesis, and hence tumor progression, by causing hypoxia and upregulation of hypoxia-inducible factor.45 This upregulation of angiogenesis as a resistance mechanism to tumor embolization provides a rationale for combining systemic therapy (typically based on abrogating angiogenesis) with TACE/TAE. Most of the experience has been with sorafenib in intermediate-stage disease, and the results have been disappointing. The administration of sorafenib after at least a partial response with TACE did not provide additional benefit in terms of time to progression.46 Similarly, in the SPACE trial, concurrent therapy with TACE-doxorubicin-eluting beads and sorafenib compared to TACE-doxorubicin-eluting beads and placebo yielded similar time to progression numbers for both treatment modalities.47 While the data have been disappointing in intermediate-stage disease, as described earlier, registry data suggest that patients with advanced-stage disease may benefit from this approach.48
In the phase 2 TACTICS trial, 156 patients with unresectable HCC were randomized to receive TACE alone or sorafenib plus TACE, with a novel endpoint, time to untreatable progression (TTUP) and/or progression to TACE refractoriness.49 Treatment with sorafenib following TACE was continued until TTUP, decline in liver function to Child–Pugh class C, or the development of vascular invasion or extrahepatic spread. Development of new lesions while on sorafenib was not considered as progressive disease as long as the lesions were amenable to TACE. In this study, PFS was longer with sorafenib-TACE compared to TACE alone (26.7 months vs 20.6 months; P = 0.02). However, the TTUP endpoint needs further validation, and we are still awaiting the survival outcomes of this study. At this time, there are insufficient data to recommend the combination of liver-directed locoregional therapy and sorafenib or other systemic therapy options outside of a clinical trial setting.
Current Treatment Approach for Advanced HCC (BCLC-C)
Although progress is being made in the development of effective therapies, advanced HCC is generally incurable. These patients experience significant symptom burden throughout the course of the disease. Therefore, the optimal treatment plan must focus on improving or maintaining quality of life, in addition to overall efficacy. It is important to actively involve patients in treatment decisions for an individualized treatment plan, and to discuss the best strategy for incorporating current advances in targeted and immunotherapies. The paradigm of what constitutes first-line treatment for advanced HCC is shifting due to the recent systemic therapy approvals. Prior to the availability of these therapies, many patients with advanced HCC were treated with repeated locoregional therapies. For instance, TACE was often used to treat unresectable HCC multiple times beyond progression. There was no consensus on the definition of TACE failure, and hence it was used in broader, unselected populations. Retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial, and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
CASE CONCLUSION
An important part of evaluating a new patient with HCC is to determine whether they are a candidate for curative therapies, such as transplant or surgical resection. These are no longer an option for patients with intermediate disease. For patients with advanced disease characteristics, such as vascular invasion or systemic metastasis, the evidence supports using systemic therapy with sorafenib or lenvatinib. Lenvatinib, with a better tolerance profile and response rate, is the treatment of choice for the patient described in the case scenario. Lenvatinib is also indicated for first-line treatment of advanced HCC, and is useful in very aggressive tumors, such as those with an AFP level exceeding 200 ng/mL.
Future Directions
The emerging role of novel systemic therapeutics, including immunotherapy, has drastically changed the treatment landscape for hepatocellular cancers, with 6 new drugs for treating advanced hepatocellular cancers approved recently. While these systemic drugs have improved survival in advanced HCC in the past decade, patient selection and treatment sequencing remain a challenge, due to a lack of biomarkers capable of predicting antitumor responses. In addition, there is an unmet need for treatment options for patients with Child–Pugh class B7 and C liver disease and poor performance status.
The goal of future management should be to achieve personalized care aimed at improved safety and efficacy by targeting multiple cancer pathways in the HCC cascade with combination treatments. Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
2. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491.
3. Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471.
4. Schutte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117.
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
6. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314.
7. Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
8. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346.
9. Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5.
10. Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
11. Cancer Genome Atlas Research Network. Electronic address: wheeler@bcm.edu; Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327-1134.
12. Chae YK, Ranganath K, Hammerman PS, et al: Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2016;8:16052-16074.
13. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
14. Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622-628.
15. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
16. Da Fonseca LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol. 2015;3:793-796.
17. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
18. Bruix J, Tak W-Y, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412-3419.
19. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
20. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36:412-412.
21. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358.
22. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36:207-207.
23. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncology. 2015;16:859-870.
24. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased αfetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology. 2019;20:282-296.
25. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502.
26. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2020;30:v874-v875.
27. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36:942-952.
28. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193-202.
29. Cheng A-L, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30 (suppl_9):ix183-ix202.
30. Jiang Y, Han Q-J, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167.
31. Xu F, Jin T, Zhu Y, et al. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110.
32. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
33. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700.
34. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508.
35. Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC). Cancer. 2007;109:1384-1390.
36. Tang A, Chan AT, Zee B, et al. A randomized phase iii study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538.
37. Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol. 2016;34:192.
38. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
39. Brown KT, Do RK, Gonen M, et al. randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34:2046-2053.
40. Kirstein MM, Voigtlander T, Schweitzer N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J. 2018;6:238-246.
41. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599.
42. Mason MC, Massarweh NN, Salami A, et al. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137-1144.
43. Sangro B, Maini CL, Ettorre GM, et al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721-1730.
44. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636.
45. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921.
46. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127.
47. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098.
48. Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14:585-587.
49. Kudo M, Ueshima K, Ikeda M, et al. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol. 2018;36:206.
From the University of Alabama at Birmingham, Division of Hematology Oncology, Birmingham, AL, and the University of South Alabama, Division of Hematology Oncology, Mobile, AL. Dr. Paluri and Dr. Hatic contributed equally to this article.
Abstract
- Objective: To review systemic treatment options for patients with locally advanced unresectable hepatocellular carcinoma (HCC).
- Methods: Review of the literature.
- Results: The paradigm of what constitutes first-line treatment for advanced HCC is shifting. Until recently, many patients with advanced HCC were treated with repeated locoregional therapies, such as transartertial embolization (TACE). However, retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
- Conclusion: Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
Keywords: liver cancer; molecular therapy; immunotherapy.
Hepatocellular carcinoma (HCC) represents 90% of primary liver malignancies. It is the fifth most common malignancy in males and the ninth most common in females worldwide.1 In contrast to other major cancers (colon, breast, prostate), the incidence of and mortality from HCC has increased over the past decade, following a brief decline between 1999 and 2004.2 The epidemiology and incidence of HCC is closely linked to chronic liver disease and conditions predisposing to liver cirrhosis. Worldwide, hepatitis B virus infection is the leading cause of liver cirrhosis and, hence, HCC. In the United States, 50% of HCC cases are linked to hepatitis C virus (HCV) infection. Diabetes mellitus and alcoholic and nonalcoholic steatohepatitis are the other major etiologies of HCC. Indeed, the metabolic syndrome, independent of other factors, is associated with a 2-fold increase in the risk of HCC.3
Although most cases of HCC are predated by liver cirrhosis, in about 20% of patients HCC occurs without liver cirrhosis.4 Similar to other malignancies, surgery in the form of resection (for isolated lesions in the context of good liver function) or liver transplant (for low-volume disease with mildly impaired liver function) provides the best chance of a cure. Locoregional therapies involving hepatic artery–directed therapy are offered for patients with more advanced disease that is limited to the liver, while systemic therapy is offered for advanced unresectable disease that involves portal vein invasion, lymph nodes, and distant metastasis. The
Molecular Pathogenesis
Similar to other malignancies, a multistep process of carcinogenesis, with accumulation of genomic alterations at the molecular and cellular levels, is recognized in HCC. In about 80% of cases, repeated and chronic injury, inflammation, and repair lead to a distortion of normal liver architecture and development of cirrhotic nodules. Exome sequencing of HCC tissues has identified risk factor–specific mutational signatures, including those related to the tumor microenvironment, and defined the extensive landscape of altered genes and pathways in HCC (eg, angiogenic and MET pathways).7 In the Schulze et al study, the frequency of alterations that could be targeted by available Food and Drug Administration (FDA)–approved drugs comprised either amplifications or mutations of FLTs (6%), FGF3 or 4 or 19 (4%), PDGFRs (3%), VEGFA (1%), HGF (3%), MTOR (2%), EGFR (1%), FGFRs (1%), and MET (1%).7 Epigenetic modification of growth-factor expression, particularly insulin-like growth factor 2 and transforming growth factor alpha, and structural alterations that lead to loss of heterozygosity are early events that cause hepatocyte proliferation and progression of dysplastic nodules.8,9 Advances in whole-exome sequencing have identified TERT promoter mutations, leading to activation of telomerase, as an early event in HCC pathogenesis. Other commonly altered genes include CTNNB1 (B-Catenin) and TP53. As a group, alterations in the MAP kinase pathway genes occur in about 40% of HCC cases.
Actionable oncogenic driver alterations are not as common as tumor suppressor pathway alterations in HCC, making targeted drug development challenging.10,11 The FGFR (fibroblast growth factor receptor) pathway, which plays a critical role in carcinogenesis-related cell growth, survival, neo-angiogenesis, and acquired resistance to other cancer treatments, is being explored as a treatment target.12 The molecular characterization of HCC may help with identifying new biomarkers and present opportunities for developing therapeutic targets.
CASE PRESENTATION
A 61-year-old man with a history of chronic hepatitis C and hypertension presents to his primary care physician due to right upper quadrant pain. Laboratory evaluation shows transaminases elevated 2 times the upper limit of normal. This leads to an ultrasound and follow-up magnetic resonance imaging. Imaging shows diffuse cirrhotic changes, with a 6-cm, well-circumscribed lesion within the left lobe of the liver that shows rapid arterial enhancement with venous washout. These vascular characteristics are consistent with HCC. In addition, 2 satellite lesions in the left lobe and sonographic evidence of invasion into the portal vein are present. Periportal lymph nodes are pathologically enlarged.
The physical examination is unremarkable, except for mild tenderness over the right upper quadrant of the abdomen. Serum bilirubin, albumin, platelets, and international normalized ratio are normal, and alpha fetoprotein (AFP) is elevated at 1769 ng/mL. The patient’s family history is unremarkable for any major illness or cancer. Computed tomography scan of the chest and pelvis shows no evidence of other lesions. His liver disease is classified as Child–Pugh A. Due to locally advanced presentation, the tumor is deemed to be nontransplantable and unresectable, and is staged as BCLC-C. The patient continues to work and his performance status is ECOG (
What systemic treatment would you recommend for this patient with locally advanced unresectable HCC with nodal metastasis?
First-Line Therapeutic Options
Systemic treatment of HCC is challenging because of the underlying liver cirrhosis and hepatic dysfunction present in most patients. Overall prognosis is therefore dependent on the disease biology and burden and on the degree of hepatic dysfunction. These factors must be considered in patients with advanced disease who present for systemic therapy. As such, patients with BCLC class D HCC with poor performance status and impaired liver function are better off with best supportive care and hospice services (Figure). Table 1 and Table 2 outline the landmark trials that led to the approval of agents for advanced HCC treatment.
Sorafenib
In the patient with BCLC class C HCC who has preserved liver function (traditionally based on a Child–Pugh score of ≤ 6 and a decent functional status [ECOG performance status 1-2]), sorafenib is the first FDA-approved first-line treatment. Sorafenib is a small-molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) kinase signaling, in addition to many other tyrosine kinase pathways (including the platelet-derived growth factor and Raf-Ras pathways). Evidence for the clinical benefit of sorafenib comes from the SHARP trial.13 This was a multinational, but primarily European, randomized phase 3 study that compared sorafenib to best supportive care for advanced HCC in patients with a Child–Pugh score ≤ 6A and a robust performance status (ECOG 0 and 1). Overall survival (OS) with placebo and sorafenib was 7.9 months and 10.7 months, respectively. There was no difference in time to radiologic progression, and the progression-free survival (PFS) at 4 months was 62% with sorafenib and 42% with placebo. Patients with HCV-associated HCC appeared to derive a more substantial benefit from sorafenib.14 In a smaller randomized study of sorafenib in Asian patients with predominantly hepatitis B–associated HCC, OS in the sorafenib and best supportive care arms was lower than that reported in the SHARP study (6.5 months vs 4.2 months), although OS still was longer in the sorafenib group.15
Significant adverse events reported with sorafenib include fatigue (30%), hand and foot syndrome (30%), diarrhea (15%), and mucositis (10%). Major proportions of patients in the clinical setting have not tolerated the standard dose of 400 mg twice daily. Dose-adjusted administration of sorafenib has been advocated in patients with more impaired liver function (Child–Pugh class 7B) and bilirubin of 1.5 to 3 times the upper limit of normal, although it is unclear whether these patients are deriving any benefit from sorafenib.16 At this time, in a patient with preserved liver function, starting with 400 mg twice daily, followed by dose reduction based on toxicity, remains standard.
Lenvatinib
After multiple attempts to develop newer first-line treatments for HCC,
Second-Line Therapeutic Options
Following the sorafenib approval, clinical studies of several other agents did not meet their primary endpoint and failed to show improvement in clinical outcomes compared to sorafenib. However, over the past years the treatment landscape for advanced HCC has been changed with the approval of several agents in the second line. The overall response rate (ORR) has become the new theme for management of advanced disease. With multiple therapeutic options available, optimal sequencing now plays a critical role, especially for transitioning from locoregional to systemic therapy. Five drugs are now indicated for second-line treatment of patients who progressed on or were intolerant to sorafenib: regorafenib, cabozantinib, ramucirumab, nivolumab, and pembrolizumab.
Regorafenib
Regorafenib was evaluated in the advanced HCC setting in a single-arm, phase 2 trial involving 36 patients with Child–Pugh class A liver disease who had progressed on prior sorafenib.18 Patients received regorafenib 160 mg orally once daily for 3 weeks on/1 week off cycles. Disease control was achieved in 72% of patients, with stable disease in 25 patients (69%). Based on these results, regorafenib was further evaluated in the multicenter, phase 3, 2:1 randomized, double-blind, placebo-controlled RESORCE study, which enrolled 573 patients.19 Due to the overlapping safety profiles of sorafenib and regorafenib, the inclusion criteria required patients to have tolerated a sorafenib dose of at least 400 mg daily for 20 of the past 28 days of treatment prior to enrollment. The primary endpoint of the study, OS, was met (median OS of 10.6 months in regorafenib arm versus 7.8 months in placebo arm; hazard ratio [HR], 0.63; P < 0.0001).
Cabozantinib
CELESTIAL was a phase 3, double-blind study that assessed the efficacy of cabozantinib versus placebo in patients with advanced HCC who had received prior sorafenib.22 In this study, 707 patients with Child–Pugh class A liver disease who progressed on at least 1 prior systemic therapy were randomized in a 2:1 ratio to treatment with cabozantinib at 60 mg daily or placebo. Patients treated with cabozantinib had a longer OS (10.2 months vs 8.0 months), resulting in a 24% reduction in the risk of death (HR, 0.76), and a longer median PFS (5.2 months versus 1.9 months). The disease control rate was higher with cabozantinib (64% vs 33%) as well. The incidence of high‐grade adverse events in the cabozantinib group was twice that of the placebo group. Common adverse events reported with cabozantinib included HFSR (17%), hypertension (16%), increased aspartate aminotransferase (12%), fatigue (10%), and diarrhea (10%).
Ramucirumab
REACH was a phase 3 study exploring the efficacy of ramucirumab that did not meet its primary endpoint; however, the subgroup analysis in AFP-high patients showed an OS improvement with ramucirumab.23 This led to the phase 3 REACH-2 trial, a multicenter, randomized, double-blind biomarker study in patients with advanced HCC who either progressed on or were intolerant to sorafenib and had an AFP level ≥ 400 ng/mL.24 Patients were randomized to ramucirumab 8 mg/kg every 2 weeks or placebo. The study met its primary endpoint, showing improved OS (8.5 months vs 7.3 months; P = 0.0059). The most common treatment-related adverse events in the ramucirumab group were ascites (5%), hypertension (12%), asthenia (5%), malignant neoplasm progression (6%), increased aspartate aminotransferase concentration (5%), and thrombocytopenia.
Immunotherapy
HCC is considered an inflammation-induced cancer, which renders immunotherapeutic strategies more appealing. The PD-L1/PD-1 pathway is the critical immune checkpoint mechanism and is an important target for treatment. HCC uses a complex, overlapping set of mechanisms to evade cancer-specific immunity and to suppress the immune system. Initial efforts to develop immunotherapies for HCC focused on anti-PD-1 and anti-PD-L1 antibodies. CheckMate 040 evaluated nivolumab in 262 sorafenib-naïve and -treated patients with advanced HCC (98% with Child–Pugh scores of 5 or 6), with a median follow-up of 12.9 months.25 In sorafenib-naïve patients (n = 80), the ORR was 23%, and the disease control rate was 63%. In sorafenib-treated patients (n = 182), the ORR was 18%. Response was not associated with PD-L1 expression. Durable objective responses, a manageable safety profile, and promising efficacy led the FDA to grant accelerated approval of nivolumab for the treatment of patients with HCC who have been previously treated with sorafenib. Based on this, the phase 3 randomized CheckMate-459 trial evaluated the efficacy of nivolumab versus sorafenib in the first-line. Median OS and ORR were better with nivolumab (16.4 months vs 14.7 months; HR 0.85; P = 0.752; and 15% [with 5 complete responses] vs 7%), as was the safety profile (22% vs 49% reporting grade 3 and 4 adverse events). 26
The KEYNOTE-224 study27 evaluated pembrolizumab in 104 patients with previously treated advanced HCC. This study showed an ORR of 17%, with 1 complete response and 17 partial responses. One-third of the patients had progressive disease, while 46 had stable disease. Among those who responded, 56% maintained a durable response for more than 1 year. Subsequently, in KEYNOTE 240, pembrolizumab showed an improvement in OS (13.9 months vs 10.6 months; HR, 0.78; P = 0.0238) and PFS (3.0 months versus 2.8 months; HR, 0.78; P = 0.0186) compared with placebo.28 The ORR for pembrolizumab was 16.9% (95% confidence interval [CI], 12.7%-21.8%) versus 2.2% (95% CI, 0.5%-6.4%; P = 0.00001) for placebo. Mean duration of response was 13.8 months.
In the IMbrave150 trial, atezolizumab/bevacizumab combination, compared to sorafenib, had better OS (not estimable vs 13.2 months; P = 0.0006), PFS (6.8 months vs 4.5 months, P < 0.0001), and ORR (33% vs 13%, P < 0.0001), but grade 3-4 events were similar.29 This combination has potential for first-line approval. The COSMIC–312 study is comparing the combination of cabozantinib and atezolizumab to sorafenib monotherapy and cabozantinib monotherapy in advanced HCC.
Resistance to immunotherapy can be extrinsic, associated with activation mechanisms of T-cells, or intrinsic, related to immune recognition, gene expression, and cell-signaling pathways.30 Tumor-immune heterogeneity and antigen presentation contribute to complex resistance mechanisms.31,32 Although clinical outcomes have improved with immune checkpoint inhibitors, the response rate is low and responses are inconsistent, likely due to an immunosuppressive tumor microenvironment.33 Therefore, several novel combinations of checkpoint inhibitors and targeted drugs are being evaluated to bypass some of the resistance mechanisms (Table 3).
Chemotherapy
Multiple combinations of cytotoxic regimens have been evaluated, but efficacy has been modest, suggesting the limited role for traditional chemotherapy in the systemic management of advanced HCC. Response rates to chemotherapy are low and responses are not durable. Gemcitabine- and doxorubicin-based treatment and FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) are some regimens that have been studied, with a median OS of less than 1 year for these regimens.34-36 FOLFOX had a higher response rate (8.15% vs 2.67%; P = 0.02) and longer median OS (6.40 months versus 4.97 months; HR, 0.80; 95% CI, 0.63-1.02; P = 0.07) than doxorubicin.34 With the gemcitabine/oxaliplatin combination, ORR was 18%, with stable disease in 58% of patients, and median PFS and OS were 6.3 months and 11.5 months, respectively.35 In a study that compared doxorubicin and PIAF (cisplatin/interferon a-2b/doxorubicin/5-fluorouracil), median OS was 6.83 months and 8.67 months, respectively (P = 0.83). The hazard ratio for death from any cause in the PIAF group compared with the doxorubicin group was 0.97 (95% CI, 0.71-1.32). PIAF had a higher ORR (20.9%; 95% CI, 12.5%-29.2%) than doxorubicin (10.5%; 95% CI, 3.9%-16.9%).
The phase 3 ALLIANCE study evaluated the combination of sorafenib and doxorubicin in treatment-naïve HCC patients with Child–Pugh class A liver disease, and did not demonstrate superiority with the addition of cytotoxic chemotherapy.37 Indeed, the combination of chemotherapy with sorafenib appears harmful in terms of lower OS (9.3 months vs 10.6 months; HR, 1.06; 95% CI, 0.8-1.4) and worse toxicity. Patients treated with the combination experienced more hematologic (37.8% vs 8.1%) and nonhematologic adverse events (63.6% vs 61.5%).
Locoregional Therapy
The role of locoregional therapy in advanced HCC remains the subject of intense debate. Patients with BCLC stage C HCC with metastatic disease and those with lymph node involvement are candidates for systemic therapy. The optimal candidate for locoregional therapy is the patient with localized intermediate-stage disease, particularly hepatic artery–delivered therapeutic interventions. However, the presence of a solitary large tumor or portal vein involvement constitutes gray areas regarding which therapy to deliver directly to the tumor via the hepatic artery, and increasingly stereotactic body radiation therapy is being offered.
Transarterial Chemoembolization
Transarterial chemoembolization (TACE), with or without chemotherapy, is the most widely adopted locoregional therapy in the management of HCC. TACE exploits the differential vascular supply to the HCC and normal liver parenchyma. Normal liver receives only one-fourth of its blood supply from the hepatic artery (three-fourths from the portal vein), whereas HCC is mainly supplied by the hepatic artery. A survival benefit for TACE compared to best supportive care is widely acknowledged for intermediate-stage HCC, and transarterial embolization (TAE) with gelatin sponge or microspheres is noninferior to TACE.38,39 Overall safety profile and efficacy inform therapy selection in advanced HCC, although the evidence for TACE in advanced HCC is less robust. Although single-institution experiences suggest survival numbers similar to and even superior to sorafenib,40,41 there is a scarcity of large randomized clinical trial data to back this up. Based on this, patients with advanced HCC should only be offered liver-directed therapy within a clinical trial or on a case-by-case basis under multidisciplinary tumor board consensus.
A serious adverse effect of TACE is post-embolization syndrome, which occurs in about 30% of patients and may be associated with poor prognosis.42 The syndrome consists of right upper quadrant abdominal pain, malaise, and worsening nausea/vomiting following the embolization procedure. Laboratory abnormalities and other complications may persist for up to 30 days after the procedure. This is a concern, because post-embolization syndrome may affect the ability to deliver systemic therapy.
Transarterial Radioembolization
In the past few years, there has been an uptick in the utilization of transarterial radioembolization (TARE), which instead of delivering glass beads, as done in TAE, or chemotherapy-infused beads, as done in TACE, delivers the radioisotope Y-90 to the tumor via the hepatic artery. TARE is able to administer larger doses of radiation to the tumor while avoiding normal liver tissue, as compared to external-beam radiation. There has been no head-to-head comparison of these different intra-arterial therapy approaches, but TARE with Y-90 has been shown to be safe in patients with portal vein thrombosis. A recent multicenter retrospective study of TARE demonstrated a median OS of 8.8 to 10.8 months in patients with BCLC C HCC,43 and in a large randomized study of Y-90 compared to sorafenib in advanced and previously treated intermediate HCC, there was no difference in median OS between the treatment modalities (8 months for selective internal radiotherapy, 9 months for sorafenib; P = 0.18). Treatment with Y-90 was better tolerated.44 A major impediment to the adoption of TARE is the time it takes to order, plan, and deliver Y-90 to patients. Radio-embolization-induced liver disease, similar to post-embolization syndrome, is characterized by jaundice and ascites, which may occur 4 to 8 weeks postprocedure and is more common in patients with HCC who do not have cirrhosis. Compared to TACE, TARE may offer a better adverse effect profile, with improvement in quality of life.
Combination of Systemic and Locoregional Therapy
Even in carefully selected patients with intermediate- and advanced-stage HCC, locoregional therapy is not curative. Tumor embolization may promote more angiogenesis, and hence tumor progression, by causing hypoxia and upregulation of hypoxia-inducible factor.45 This upregulation of angiogenesis as a resistance mechanism to tumor embolization provides a rationale for combining systemic therapy (typically based on abrogating angiogenesis) with TACE/TAE. Most of the experience has been with sorafenib in intermediate-stage disease, and the results have been disappointing. The administration of sorafenib after at least a partial response with TACE did not provide additional benefit in terms of time to progression.46 Similarly, in the SPACE trial, concurrent therapy with TACE-doxorubicin-eluting beads and sorafenib compared to TACE-doxorubicin-eluting beads and placebo yielded similar time to progression numbers for both treatment modalities.47 While the data have been disappointing in intermediate-stage disease, as described earlier, registry data suggest that patients with advanced-stage disease may benefit from this approach.48
In the phase 2 TACTICS trial, 156 patients with unresectable HCC were randomized to receive TACE alone or sorafenib plus TACE, with a novel endpoint, time to untreatable progression (TTUP) and/or progression to TACE refractoriness.49 Treatment with sorafenib following TACE was continued until TTUP, decline in liver function to Child–Pugh class C, or the development of vascular invasion or extrahepatic spread. Development of new lesions while on sorafenib was not considered as progressive disease as long as the lesions were amenable to TACE. In this study, PFS was longer with sorafenib-TACE compared to TACE alone (26.7 months vs 20.6 months; P = 0.02). However, the TTUP endpoint needs further validation, and we are still awaiting the survival outcomes of this study. At this time, there are insufficient data to recommend the combination of liver-directed locoregional therapy and sorafenib or other systemic therapy options outside of a clinical trial setting.
Current Treatment Approach for Advanced HCC (BCLC-C)
Although progress is being made in the development of effective therapies, advanced HCC is generally incurable. These patients experience significant symptom burden throughout the course of the disease. Therefore, the optimal treatment plan must focus on improving or maintaining quality of life, in addition to overall efficacy. It is important to actively involve patients in treatment decisions for an individualized treatment plan, and to discuss the best strategy for incorporating current advances in targeted and immunotherapies. The paradigm of what constitutes first-line treatment for advanced HCC is shifting due to the recent systemic therapy approvals. Prior to the availability of these therapies, many patients with advanced HCC were treated with repeated locoregional therapies. For instance, TACE was often used to treat unresectable HCC multiple times beyond progression. There was no consensus on the definition of TACE failure, and hence it was used in broader, unselected populations. Retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial, and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
CASE CONCLUSION
An important part of evaluating a new patient with HCC is to determine whether they are a candidate for curative therapies, such as transplant or surgical resection. These are no longer an option for patients with intermediate disease. For patients with advanced disease characteristics, such as vascular invasion or systemic metastasis, the evidence supports using systemic therapy with sorafenib or lenvatinib. Lenvatinib, with a better tolerance profile and response rate, is the treatment of choice for the patient described in the case scenario. Lenvatinib is also indicated for first-line treatment of advanced HCC, and is useful in very aggressive tumors, such as those with an AFP level exceeding 200 ng/mL.
Future Directions
The emerging role of novel systemic therapeutics, including immunotherapy, has drastically changed the treatment landscape for hepatocellular cancers, with 6 new drugs for treating advanced hepatocellular cancers approved recently. While these systemic drugs have improved survival in advanced HCC in the past decade, patient selection and treatment sequencing remain a challenge, due to a lack of biomarkers capable of predicting antitumor responses. In addition, there is an unmet need for treatment options for patients with Child–Pugh class B7 and C liver disease and poor performance status.
The goal of future management should be to achieve personalized care aimed at improved safety and efficacy by targeting multiple cancer pathways in the HCC cascade with combination treatments. Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
From the University of Alabama at Birmingham, Division of Hematology Oncology, Birmingham, AL, and the University of South Alabama, Division of Hematology Oncology, Mobile, AL. Dr. Paluri and Dr. Hatic contributed equally to this article.
Abstract
- Objective: To review systemic treatment options for patients with locally advanced unresectable hepatocellular carcinoma (HCC).
- Methods: Review of the literature.
- Results: The paradigm of what constitutes first-line treatment for advanced HCC is shifting. Until recently, many patients with advanced HCC were treated with repeated locoregional therapies, such as transartertial embolization (TACE). However, retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
- Conclusion: Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
Keywords: liver cancer; molecular therapy; immunotherapy.
Hepatocellular carcinoma (HCC) represents 90% of primary liver malignancies. It is the fifth most common malignancy in males and the ninth most common in females worldwide.1 In contrast to other major cancers (colon, breast, prostate), the incidence of and mortality from HCC has increased over the past decade, following a brief decline between 1999 and 2004.2 The epidemiology and incidence of HCC is closely linked to chronic liver disease and conditions predisposing to liver cirrhosis. Worldwide, hepatitis B virus infection is the leading cause of liver cirrhosis and, hence, HCC. In the United States, 50% of HCC cases are linked to hepatitis C virus (HCV) infection. Diabetes mellitus and alcoholic and nonalcoholic steatohepatitis are the other major etiologies of HCC. Indeed, the metabolic syndrome, independent of other factors, is associated with a 2-fold increase in the risk of HCC.3
Although most cases of HCC are predated by liver cirrhosis, in about 20% of patients HCC occurs without liver cirrhosis.4 Similar to other malignancies, surgery in the form of resection (for isolated lesions in the context of good liver function) or liver transplant (for low-volume disease with mildly impaired liver function) provides the best chance of a cure. Locoregional therapies involving hepatic artery–directed therapy are offered for patients with more advanced disease that is limited to the liver, while systemic therapy is offered for advanced unresectable disease that involves portal vein invasion, lymph nodes, and distant metastasis. The
Molecular Pathogenesis
Similar to other malignancies, a multistep process of carcinogenesis, with accumulation of genomic alterations at the molecular and cellular levels, is recognized in HCC. In about 80% of cases, repeated and chronic injury, inflammation, and repair lead to a distortion of normal liver architecture and development of cirrhotic nodules. Exome sequencing of HCC tissues has identified risk factor–specific mutational signatures, including those related to the tumor microenvironment, and defined the extensive landscape of altered genes and pathways in HCC (eg, angiogenic and MET pathways).7 In the Schulze et al study, the frequency of alterations that could be targeted by available Food and Drug Administration (FDA)–approved drugs comprised either amplifications or mutations of FLTs (6%), FGF3 or 4 or 19 (4%), PDGFRs (3%), VEGFA (1%), HGF (3%), MTOR (2%), EGFR (1%), FGFRs (1%), and MET (1%).7 Epigenetic modification of growth-factor expression, particularly insulin-like growth factor 2 and transforming growth factor alpha, and structural alterations that lead to loss of heterozygosity are early events that cause hepatocyte proliferation and progression of dysplastic nodules.8,9 Advances in whole-exome sequencing have identified TERT promoter mutations, leading to activation of telomerase, as an early event in HCC pathogenesis. Other commonly altered genes include CTNNB1 (B-Catenin) and TP53. As a group, alterations in the MAP kinase pathway genes occur in about 40% of HCC cases.
Actionable oncogenic driver alterations are not as common as tumor suppressor pathway alterations in HCC, making targeted drug development challenging.10,11 The FGFR (fibroblast growth factor receptor) pathway, which plays a critical role in carcinogenesis-related cell growth, survival, neo-angiogenesis, and acquired resistance to other cancer treatments, is being explored as a treatment target.12 The molecular characterization of HCC may help with identifying new biomarkers and present opportunities for developing therapeutic targets.
CASE PRESENTATION
A 61-year-old man with a history of chronic hepatitis C and hypertension presents to his primary care physician due to right upper quadrant pain. Laboratory evaluation shows transaminases elevated 2 times the upper limit of normal. This leads to an ultrasound and follow-up magnetic resonance imaging. Imaging shows diffuse cirrhotic changes, with a 6-cm, well-circumscribed lesion within the left lobe of the liver that shows rapid arterial enhancement with venous washout. These vascular characteristics are consistent with HCC. In addition, 2 satellite lesions in the left lobe and sonographic evidence of invasion into the portal vein are present. Periportal lymph nodes are pathologically enlarged.
The physical examination is unremarkable, except for mild tenderness over the right upper quadrant of the abdomen. Serum bilirubin, albumin, platelets, and international normalized ratio are normal, and alpha fetoprotein (AFP) is elevated at 1769 ng/mL. The patient’s family history is unremarkable for any major illness or cancer. Computed tomography scan of the chest and pelvis shows no evidence of other lesions. His liver disease is classified as Child–Pugh A. Due to locally advanced presentation, the tumor is deemed to be nontransplantable and unresectable, and is staged as BCLC-C. The patient continues to work and his performance status is ECOG (
What systemic treatment would you recommend for this patient with locally advanced unresectable HCC with nodal metastasis?
First-Line Therapeutic Options
Systemic treatment of HCC is challenging because of the underlying liver cirrhosis and hepatic dysfunction present in most patients. Overall prognosis is therefore dependent on the disease biology and burden and on the degree of hepatic dysfunction. These factors must be considered in patients with advanced disease who present for systemic therapy. As such, patients with BCLC class D HCC with poor performance status and impaired liver function are better off with best supportive care and hospice services (Figure). Table 1 and Table 2 outline the landmark trials that led to the approval of agents for advanced HCC treatment.
Sorafenib
In the patient with BCLC class C HCC who has preserved liver function (traditionally based on a Child–Pugh score of ≤ 6 and a decent functional status [ECOG performance status 1-2]), sorafenib is the first FDA-approved first-line treatment. Sorafenib is a small-molecule tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) kinase signaling, in addition to many other tyrosine kinase pathways (including the platelet-derived growth factor and Raf-Ras pathways). Evidence for the clinical benefit of sorafenib comes from the SHARP trial.13 This was a multinational, but primarily European, randomized phase 3 study that compared sorafenib to best supportive care for advanced HCC in patients with a Child–Pugh score ≤ 6A and a robust performance status (ECOG 0 and 1). Overall survival (OS) with placebo and sorafenib was 7.9 months and 10.7 months, respectively. There was no difference in time to radiologic progression, and the progression-free survival (PFS) at 4 months was 62% with sorafenib and 42% with placebo. Patients with HCV-associated HCC appeared to derive a more substantial benefit from sorafenib.14 In a smaller randomized study of sorafenib in Asian patients with predominantly hepatitis B–associated HCC, OS in the sorafenib and best supportive care arms was lower than that reported in the SHARP study (6.5 months vs 4.2 months), although OS still was longer in the sorafenib group.15
Significant adverse events reported with sorafenib include fatigue (30%), hand and foot syndrome (30%), diarrhea (15%), and mucositis (10%). Major proportions of patients in the clinical setting have not tolerated the standard dose of 400 mg twice daily. Dose-adjusted administration of sorafenib has been advocated in patients with more impaired liver function (Child–Pugh class 7B) and bilirubin of 1.5 to 3 times the upper limit of normal, although it is unclear whether these patients are deriving any benefit from sorafenib.16 At this time, in a patient with preserved liver function, starting with 400 mg twice daily, followed by dose reduction based on toxicity, remains standard.
Lenvatinib
After multiple attempts to develop newer first-line treatments for HCC,
Second-Line Therapeutic Options
Following the sorafenib approval, clinical studies of several other agents did not meet their primary endpoint and failed to show improvement in clinical outcomes compared to sorafenib. However, over the past years the treatment landscape for advanced HCC has been changed with the approval of several agents in the second line. The overall response rate (ORR) has become the new theme for management of advanced disease. With multiple therapeutic options available, optimal sequencing now plays a critical role, especially for transitioning from locoregional to systemic therapy. Five drugs are now indicated for second-line treatment of patients who progressed on or were intolerant to sorafenib: regorafenib, cabozantinib, ramucirumab, nivolumab, and pembrolizumab.
Regorafenib
Regorafenib was evaluated in the advanced HCC setting in a single-arm, phase 2 trial involving 36 patients with Child–Pugh class A liver disease who had progressed on prior sorafenib.18 Patients received regorafenib 160 mg orally once daily for 3 weeks on/1 week off cycles. Disease control was achieved in 72% of patients, with stable disease in 25 patients (69%). Based on these results, regorafenib was further evaluated in the multicenter, phase 3, 2:1 randomized, double-blind, placebo-controlled RESORCE study, which enrolled 573 patients.19 Due to the overlapping safety profiles of sorafenib and regorafenib, the inclusion criteria required patients to have tolerated a sorafenib dose of at least 400 mg daily for 20 of the past 28 days of treatment prior to enrollment. The primary endpoint of the study, OS, was met (median OS of 10.6 months in regorafenib arm versus 7.8 months in placebo arm; hazard ratio [HR], 0.63; P < 0.0001).
Cabozantinib
CELESTIAL was a phase 3, double-blind study that assessed the efficacy of cabozantinib versus placebo in patients with advanced HCC who had received prior sorafenib.22 In this study, 707 patients with Child–Pugh class A liver disease who progressed on at least 1 prior systemic therapy were randomized in a 2:1 ratio to treatment with cabozantinib at 60 mg daily or placebo. Patients treated with cabozantinib had a longer OS (10.2 months vs 8.0 months), resulting in a 24% reduction in the risk of death (HR, 0.76), and a longer median PFS (5.2 months versus 1.9 months). The disease control rate was higher with cabozantinib (64% vs 33%) as well. The incidence of high‐grade adverse events in the cabozantinib group was twice that of the placebo group. Common adverse events reported with cabozantinib included HFSR (17%), hypertension (16%), increased aspartate aminotransferase (12%), fatigue (10%), and diarrhea (10%).
Ramucirumab
REACH was a phase 3 study exploring the efficacy of ramucirumab that did not meet its primary endpoint; however, the subgroup analysis in AFP-high patients showed an OS improvement with ramucirumab.23 This led to the phase 3 REACH-2 trial, a multicenter, randomized, double-blind biomarker study in patients with advanced HCC who either progressed on or were intolerant to sorafenib and had an AFP level ≥ 400 ng/mL.24 Patients were randomized to ramucirumab 8 mg/kg every 2 weeks or placebo. The study met its primary endpoint, showing improved OS (8.5 months vs 7.3 months; P = 0.0059). The most common treatment-related adverse events in the ramucirumab group were ascites (5%), hypertension (12%), asthenia (5%), malignant neoplasm progression (6%), increased aspartate aminotransferase concentration (5%), and thrombocytopenia.
Immunotherapy
HCC is considered an inflammation-induced cancer, which renders immunotherapeutic strategies more appealing. The PD-L1/PD-1 pathway is the critical immune checkpoint mechanism and is an important target for treatment. HCC uses a complex, overlapping set of mechanisms to evade cancer-specific immunity and to suppress the immune system. Initial efforts to develop immunotherapies for HCC focused on anti-PD-1 and anti-PD-L1 antibodies. CheckMate 040 evaluated nivolumab in 262 sorafenib-naïve and -treated patients with advanced HCC (98% with Child–Pugh scores of 5 or 6), with a median follow-up of 12.9 months.25 In sorafenib-naïve patients (n = 80), the ORR was 23%, and the disease control rate was 63%. In sorafenib-treated patients (n = 182), the ORR was 18%. Response was not associated with PD-L1 expression. Durable objective responses, a manageable safety profile, and promising efficacy led the FDA to grant accelerated approval of nivolumab for the treatment of patients with HCC who have been previously treated with sorafenib. Based on this, the phase 3 randomized CheckMate-459 trial evaluated the efficacy of nivolumab versus sorafenib in the first-line. Median OS and ORR were better with nivolumab (16.4 months vs 14.7 months; HR 0.85; P = 0.752; and 15% [with 5 complete responses] vs 7%), as was the safety profile (22% vs 49% reporting grade 3 and 4 adverse events). 26
The KEYNOTE-224 study27 evaluated pembrolizumab in 104 patients with previously treated advanced HCC. This study showed an ORR of 17%, with 1 complete response and 17 partial responses. One-third of the patients had progressive disease, while 46 had stable disease. Among those who responded, 56% maintained a durable response for more than 1 year. Subsequently, in KEYNOTE 240, pembrolizumab showed an improvement in OS (13.9 months vs 10.6 months; HR, 0.78; P = 0.0238) and PFS (3.0 months versus 2.8 months; HR, 0.78; P = 0.0186) compared with placebo.28 The ORR for pembrolizumab was 16.9% (95% confidence interval [CI], 12.7%-21.8%) versus 2.2% (95% CI, 0.5%-6.4%; P = 0.00001) for placebo. Mean duration of response was 13.8 months.
In the IMbrave150 trial, atezolizumab/bevacizumab combination, compared to sorafenib, had better OS (not estimable vs 13.2 months; P = 0.0006), PFS (6.8 months vs 4.5 months, P < 0.0001), and ORR (33% vs 13%, P < 0.0001), but grade 3-4 events were similar.29 This combination has potential for first-line approval. The COSMIC–312 study is comparing the combination of cabozantinib and atezolizumab to sorafenib monotherapy and cabozantinib monotherapy in advanced HCC.
Resistance to immunotherapy can be extrinsic, associated with activation mechanisms of T-cells, or intrinsic, related to immune recognition, gene expression, and cell-signaling pathways.30 Tumor-immune heterogeneity and antigen presentation contribute to complex resistance mechanisms.31,32 Although clinical outcomes have improved with immune checkpoint inhibitors, the response rate is low and responses are inconsistent, likely due to an immunosuppressive tumor microenvironment.33 Therefore, several novel combinations of checkpoint inhibitors and targeted drugs are being evaluated to bypass some of the resistance mechanisms (Table 3).
Chemotherapy
Multiple combinations of cytotoxic regimens have been evaluated, but efficacy has been modest, suggesting the limited role for traditional chemotherapy in the systemic management of advanced HCC. Response rates to chemotherapy are low and responses are not durable. Gemcitabine- and doxorubicin-based treatment and FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) are some regimens that have been studied, with a median OS of less than 1 year for these regimens.34-36 FOLFOX had a higher response rate (8.15% vs 2.67%; P = 0.02) and longer median OS (6.40 months versus 4.97 months; HR, 0.80; 95% CI, 0.63-1.02; P = 0.07) than doxorubicin.34 With the gemcitabine/oxaliplatin combination, ORR was 18%, with stable disease in 58% of patients, and median PFS and OS were 6.3 months and 11.5 months, respectively.35 In a study that compared doxorubicin and PIAF (cisplatin/interferon a-2b/doxorubicin/5-fluorouracil), median OS was 6.83 months and 8.67 months, respectively (P = 0.83). The hazard ratio for death from any cause in the PIAF group compared with the doxorubicin group was 0.97 (95% CI, 0.71-1.32). PIAF had a higher ORR (20.9%; 95% CI, 12.5%-29.2%) than doxorubicin (10.5%; 95% CI, 3.9%-16.9%).
The phase 3 ALLIANCE study evaluated the combination of sorafenib and doxorubicin in treatment-naïve HCC patients with Child–Pugh class A liver disease, and did not demonstrate superiority with the addition of cytotoxic chemotherapy.37 Indeed, the combination of chemotherapy with sorafenib appears harmful in terms of lower OS (9.3 months vs 10.6 months; HR, 1.06; 95% CI, 0.8-1.4) and worse toxicity. Patients treated with the combination experienced more hematologic (37.8% vs 8.1%) and nonhematologic adverse events (63.6% vs 61.5%).
Locoregional Therapy
The role of locoregional therapy in advanced HCC remains the subject of intense debate. Patients with BCLC stage C HCC with metastatic disease and those with lymph node involvement are candidates for systemic therapy. The optimal candidate for locoregional therapy is the patient with localized intermediate-stage disease, particularly hepatic artery–delivered therapeutic interventions. However, the presence of a solitary large tumor or portal vein involvement constitutes gray areas regarding which therapy to deliver directly to the tumor via the hepatic artery, and increasingly stereotactic body radiation therapy is being offered.
Transarterial Chemoembolization
Transarterial chemoembolization (TACE), with or without chemotherapy, is the most widely adopted locoregional therapy in the management of HCC. TACE exploits the differential vascular supply to the HCC and normal liver parenchyma. Normal liver receives only one-fourth of its blood supply from the hepatic artery (three-fourths from the portal vein), whereas HCC is mainly supplied by the hepatic artery. A survival benefit for TACE compared to best supportive care is widely acknowledged for intermediate-stage HCC, and transarterial embolization (TAE) with gelatin sponge or microspheres is noninferior to TACE.38,39 Overall safety profile and efficacy inform therapy selection in advanced HCC, although the evidence for TACE in advanced HCC is less robust. Although single-institution experiences suggest survival numbers similar to and even superior to sorafenib,40,41 there is a scarcity of large randomized clinical trial data to back this up. Based on this, patients with advanced HCC should only be offered liver-directed therapy within a clinical trial or on a case-by-case basis under multidisciplinary tumor board consensus.
A serious adverse effect of TACE is post-embolization syndrome, which occurs in about 30% of patients and may be associated with poor prognosis.42 The syndrome consists of right upper quadrant abdominal pain, malaise, and worsening nausea/vomiting following the embolization procedure. Laboratory abnormalities and other complications may persist for up to 30 days after the procedure. This is a concern, because post-embolization syndrome may affect the ability to deliver systemic therapy.
Transarterial Radioembolization
In the past few years, there has been an uptick in the utilization of transarterial radioembolization (TARE), which instead of delivering glass beads, as done in TAE, or chemotherapy-infused beads, as done in TACE, delivers the radioisotope Y-90 to the tumor via the hepatic artery. TARE is able to administer larger doses of radiation to the tumor while avoiding normal liver tissue, as compared to external-beam radiation. There has been no head-to-head comparison of these different intra-arterial therapy approaches, but TARE with Y-90 has been shown to be safe in patients with portal vein thrombosis. A recent multicenter retrospective study of TARE demonstrated a median OS of 8.8 to 10.8 months in patients with BCLC C HCC,43 and in a large randomized study of Y-90 compared to sorafenib in advanced and previously treated intermediate HCC, there was no difference in median OS between the treatment modalities (8 months for selective internal radiotherapy, 9 months for sorafenib; P = 0.18). Treatment with Y-90 was better tolerated.44 A major impediment to the adoption of TARE is the time it takes to order, plan, and deliver Y-90 to patients. Radio-embolization-induced liver disease, similar to post-embolization syndrome, is characterized by jaundice and ascites, which may occur 4 to 8 weeks postprocedure and is more common in patients with HCC who do not have cirrhosis. Compared to TACE, TARE may offer a better adverse effect profile, with improvement in quality of life.
Combination of Systemic and Locoregional Therapy
Even in carefully selected patients with intermediate- and advanced-stage HCC, locoregional therapy is not curative. Tumor embolization may promote more angiogenesis, and hence tumor progression, by causing hypoxia and upregulation of hypoxia-inducible factor.45 This upregulation of angiogenesis as a resistance mechanism to tumor embolization provides a rationale for combining systemic therapy (typically based on abrogating angiogenesis) with TACE/TAE. Most of the experience has been with sorafenib in intermediate-stage disease, and the results have been disappointing. The administration of sorafenib after at least a partial response with TACE did not provide additional benefit in terms of time to progression.46 Similarly, in the SPACE trial, concurrent therapy with TACE-doxorubicin-eluting beads and sorafenib compared to TACE-doxorubicin-eluting beads and placebo yielded similar time to progression numbers for both treatment modalities.47 While the data have been disappointing in intermediate-stage disease, as described earlier, registry data suggest that patients with advanced-stage disease may benefit from this approach.48
In the phase 2 TACTICS trial, 156 patients with unresectable HCC were randomized to receive TACE alone or sorafenib plus TACE, with a novel endpoint, time to untreatable progression (TTUP) and/or progression to TACE refractoriness.49 Treatment with sorafenib following TACE was continued until TTUP, decline in liver function to Child–Pugh class C, or the development of vascular invasion or extrahepatic spread. Development of new lesions while on sorafenib was not considered as progressive disease as long as the lesions were amenable to TACE. In this study, PFS was longer with sorafenib-TACE compared to TACE alone (26.7 months vs 20.6 months; P = 0.02). However, the TTUP endpoint needs further validation, and we are still awaiting the survival outcomes of this study. At this time, there are insufficient data to recommend the combination of liver-directed locoregional therapy and sorafenib or other systemic therapy options outside of a clinical trial setting.
Current Treatment Approach for Advanced HCC (BCLC-C)
Although progress is being made in the development of effective therapies, advanced HCC is generally incurable. These patients experience significant symptom burden throughout the course of the disease. Therefore, the optimal treatment plan must focus on improving or maintaining quality of life, in addition to overall efficacy. It is important to actively involve patients in treatment decisions for an individualized treatment plan, and to discuss the best strategy for incorporating current advances in targeted and immunotherapies. The paradigm of what constitutes first-line treatment for advanced HCC is shifting due to the recent systemic therapy approvals. Prior to the availability of these therapies, many patients with advanced HCC were treated with repeated locoregional therapies. For instance, TACE was often used to treat unresectable HCC multiple times beyond progression. There was no consensus on the definition of TACE failure, and hence it was used in broader, unselected populations. Retrospective studies suggest that continuing TACE after refractoriness or failure may not be beneficial, and may delay subsequent treatments because of deterioration of liver function or declines in performance status. With recent approvals of several systemic therapy options, including immunotherapy, it is vital to conduct a risk-benefit assessment prior to repeating TACE after failure, so that patients are not denied the use of available systemic therapeutic options due to declined performance status or organ function from these procedures. The optimal timing and the sequence of systemic and locoregional therapy must be carefully evaluated by a multidisciplinary team.
CASE CONCLUSION
An important part of evaluating a new patient with HCC is to determine whether they are a candidate for curative therapies, such as transplant or surgical resection. These are no longer an option for patients with intermediate disease. For patients with advanced disease characteristics, such as vascular invasion or systemic metastasis, the evidence supports using systemic therapy with sorafenib or lenvatinib. Lenvatinib, with a better tolerance profile and response rate, is the treatment of choice for the patient described in the case scenario. Lenvatinib is also indicated for first-line treatment of advanced HCC, and is useful in very aggressive tumors, such as those with an AFP level exceeding 200 ng/mL.
Future Directions
The emerging role of novel systemic therapeutics, including immunotherapy, has drastically changed the treatment landscape for hepatocellular cancers, with 6 new drugs for treating advanced hepatocellular cancers approved recently. While these systemic drugs have improved survival in advanced HCC in the past decade, patient selection and treatment sequencing remain a challenge, due to a lack of biomarkers capable of predicting antitumor responses. In addition, there is an unmet need for treatment options for patients with Child–Pugh class B7 and C liver disease and poor performance status.
The goal of future management should be to achieve personalized care aimed at improved safety and efficacy by targeting multiple cancer pathways in the HCC cascade with combination treatments. Randomized clinical trials to improve patient selection and determine the proper sequence of treatments are needed. Given the heterogeneity of HCC, molecular profiling of the tumor to differentiate responders from nonresponders may elucidate potential biomarkers to effectively guide treatment recommendations.
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
2. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491.
3. Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471.
4. Schutte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117.
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
6. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314.
7. Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
8. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346.
9. Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5.
10. Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
11. Cancer Genome Atlas Research Network. Electronic address: wheeler@bcm.edu; Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327-1134.
12. Chae YK, Ranganath K, Hammerman PS, et al: Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2016;8:16052-16074.
13. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
14. Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622-628.
15. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
16. Da Fonseca LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol. 2015;3:793-796.
17. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
18. Bruix J, Tak W-Y, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412-3419.
19. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
20. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36:412-412.
21. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358.
22. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36:207-207.
23. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncology. 2015;16:859-870.
24. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased αfetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology. 2019;20:282-296.
25. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502.
26. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2020;30:v874-v875.
27. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36:942-952.
28. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193-202.
29. Cheng A-L, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30 (suppl_9):ix183-ix202.
30. Jiang Y, Han Q-J, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167.
31. Xu F, Jin T, Zhu Y, et al. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110.
32. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
33. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700.
34. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508.
35. Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC). Cancer. 2007;109:1384-1390.
36. Tang A, Chan AT, Zee B, et al. A randomized phase iii study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538.
37. Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol. 2016;34:192.
38. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
39. Brown KT, Do RK, Gonen M, et al. randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34:2046-2053.
40. Kirstein MM, Voigtlander T, Schweitzer N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J. 2018;6:238-246.
41. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599.
42. Mason MC, Massarweh NN, Salami A, et al. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137-1144.
43. Sangro B, Maini CL, Ettorre GM, et al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721-1730.
44. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636.
45. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921.
46. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127.
47. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098.
48. Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14:585-587.
49. Kudo M, Ueshima K, Ikeda M, et al. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol. 2018;36:206.
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
2. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491.
3. Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471.
4. Schutte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117.
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338.
6. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314.
7. Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
8. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346.
9. Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5.
10. Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511.
11. Cancer Genome Atlas Research Network. Electronic address: wheeler@bcm.edu; Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327-1134.
12. Chae YK, Ranganath K, Hammerman PS, et al: Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2016;8:16052-16074.
13. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
14. Jackson R, Psarelli EE, Berhane S, et al. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622-628.
15. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34.
16. Da Fonseca LG, Barroso-Sousa R, Bento AD, et al. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol. 2015;3:793-796.
17. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
18. Bruix J, Tak W-Y, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412-3419.
19. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
20. Bruix J, Merle P, Granito A, et al. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36:412-412.
21. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358.
22. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36:207-207.
23. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncology. 2015;16:859-870.
24. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased αfetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology. 2019;20:282-296.
25. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502.
26. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2020;30:v874-v875.
27. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36:942-952.
28. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193-202.
29. Cheng A-L, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30 (suppl_9):ix183-ix202.
30. Jiang Y, Han Q-J, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167.
31. Xu F, Jin T, Zhu Y, et al. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110.
32. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
33. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700.
34. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508.
35. Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC). Cancer. 2007;109:1384-1390.
36. Tang A, Chan AT, Zee B, et al. A randomized phase iii study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538.
37. Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol. 2016;34:192.
38. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
39. Brown KT, Do RK, Gonen M, et al. randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34:2046-2053.
40. Kirstein MM, Voigtlander T, Schweitzer N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J. 2018;6:238-246.
41. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590-599.
42. Mason MC, Massarweh NN, Salami A, et al. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137-1144.
43. Sangro B, Maini CL, Ettorre GM, et al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018;45:1721-1730.
44. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636.
45. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921.
46. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127.
47. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098.
48. Geschwind JF, Chapiro J. Sorafenib in combination with transarterial chemoembolization for the treatment of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2016;14:585-587.
49. Kudo M, Ueshima K, Ikeda M, et al. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol. 2018;36:206.
Use of an Electronic Alert Tool to Prevent Readmissions Following Coronary Artery Bypass Graft Surgery
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; smithsd@uncw.edu.
Funding disclosures: None.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; smithsd@uncw.edu.
Funding disclosures: None.
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; smithsd@uncw.edu.
Funding disclosures: None.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
Factors Associated With Lower-Extremity Amputation in Patients With Diabetic Foot Ulcers
From Northwell Health System, Lake Success, NY.
Abstract
- Objective: To explore factors associated with lower-extremity amputation (LEA) in patients with diabetic foot ulcers using data from the Online Wound Electronic Medical Record Database.
- Design: Retrospective analysis of medical records.
- Setting and participants: Data from 169 individuals with previously diagnosed diabetes mellitus who received wound care for a 6-month period within a span of 2 years was analyzed. A baseline evaluation was obtained and wound(s) were treated, managed, and monitored.
Treatment continued until the patient healed, required an LEA, or phased out of the study, neither healing nor undergoing an amputation. Of the 149 patients who completed the study, 38 had healed ulcers, 14 underwent amputation, and 97 neither healed nor underwent an amputation. All patients were treated under the care of vascular and/or podiatric surgeons. - Measurements: Variables included wound status (healed, amputated, and unhealed/non-amputated); size of wound area; age, gender, race, and ethnicity; white blood cell (WBC) count, hemoglobin A1c (HbA1c), blood glucose, and body mass index (BMI); and presence of osteomyelitis, gangrene, and peripheral vascular disease.
- Results: As compared to the healed and unhealed/non-amputated group, the group of patients who underwent LEA was older and had higher percentages of males, Hispanics, and African Americans; had a higher WBC count, larger wound area, and higher rates of wound infection, osteomyelitis, and neuropathy; and had lower average values of HbA1c, blood glucose, and BMI and a lower rate of peripheral vascular disease.
- Conclusion: The association between HbA1c and LEA highlights a window of relative safety among an at-risk population. By identifying and focusing on factors associated with LEA, health care professionals may be able to decrease the prevalence of LEA in patients with diabetes.
Keywords: diabetic foot ulcer; lower-extremity amputation; risk factors; HbA1c.
An estimated 30.3 million people, or 9.4% of the US population, has diabetes. In 2014, approximately 108,000 amputations were performed on adults with diagnosed diabetes.1 Furthermore, patients with diabetes have a 10-fold increased risk for lower-extremity amputation (LEA), as compared with patients without diabetes.2 The frequency of amputations in the diabetic population is a public health crisis.
Amputation has significant, life-altering consequences. Patients who undergo LEA often face debilitation in their daily activities and must undergo intense rehabilitation to learn basic tasks. Amputations can also impact individuals’ psychological well-being as they come to terms with their altered body and may face challenges in self-perception, confidence, self-esteem, work life, and relationships. In addition, the mortality rate for patients with diabetes 5 years after undergoing LEA is 30%.2 However, public health studies estimate that more than half of LEAs in patients with diabetes are preventable.3
Although studies have explored the relationship between diabetes and LEA, few have sought to identify factors directly correlated with wound care. In the United States, patients with diabetic ulcerations are typically treated in wound care facilities; however, previous studies have concentrated on the conditions that lead to the formation of an ulcer or amputation, viewing amputation and ulcer as 2 separate entities. Our study took into account systemic variables, patient demographics, and specific wound characteristics to explore factors associated with LEA in a high-risk group of patients with diabetes. This study was designed to assess ailments that are prevalent in patients who require a LEA.
Methods
Patients and Setting
A total of 169 patients who were treated at the Comprehensive Wound Healing and Hyperbaric Center (Lake Success, NY), a tertiary facility of the Northwell Health system, participated in this retrospective study. The data for this study were obtained in conjunction with the development of the New York University School of Medicine’s Online Wound Electronic Medical Record to Decrease Limb Amputations in Persons with Diabetes (OWEMR) database. The OWEMR collects individual patient data from satellite locations across the country. Using this database, researchers can analyze similarities and differences between patients who undergo LEA.
This study utilized patient data specific to the Northwell Health facility. All of the patients in our study were enrolled under the criteria of the OWEMR database. In order to be included in the OWEMR database, patients had to be diagnosed with type 1 or type 2 diabetes; have a break in the skin ≥ 0.5 cm2; be 18 years of age or older; and have a measured hemoglobin A1c (HbA1c) value within the past 120 days. Study patients signed an informed consent and committed to being available for follow-up visits to the wound care facility for 6 months after entering the study. Patients were enrolled between 2012 and 2014, and each patient was monitored for a period of 6 months within this time period. Participants were treated with current standards of care using diet, lifestyle, and pharmacologic interventions. This study was approved by the Northwell Health System Institutional Review Board Human Research Protection Program (Manhasset, NY).
Data Collection
On their first visit to the facility, patients were given a physical examination and initial interview regarding their medical history. Clinicians were required to select 1 ulcer that would be examined for the duration of the study. The selection of the ulcer was based on a point system that awarded points for pedal pulses, the ability to be probed to the bone, the location of the ulcer (ie, located on the foot rather than a toe), and the presence of multiple ulcerations. The ulcer with the highest score was selected for the study. If numerous ulcers were evaluated with the same score, the largest and deepest was selected. Wagner classification of the wound was recorded at baseline and taken at each subsequent patient visit. In addition, peripheral sensation was assessed for signs of neuropathy using Semmes-Weinstein monofilament testing.
Once selected, the wound was clinically evaluated, samples for culture were obtained, and blood tests were performed to detect the presence of wound infection. The patient’s blood was drawn for a full laboratory analysis, including white blood cell (WBC) count and measurement of blood glucose and HbA1c levels. Bone biopsy, magnetic resonance imaging, and bone scans were used to detect the presence of osteomyelitis at the discretion of the health care provider. Wounds suspected of infection, underlying osteomyelitis, or gangrene at baseline were excluded. Patients would then return for follow-up visits at least once every 6 weeks, plus or minus 2 weeks, for a maximum of 6 months.
Statistical Analysis
Utilizing SAS version 9.3 (Cary, NC), descriptive statistics (minimum, maximum, mean, median, and SD) were calculated for the following variables: age, WBC count, wound area, HbA1c, blood glucose, and body mass index (BMI). These variables were collected for each patient as per the OWEMR protocol and provided a basis for which to compare patients who underwent amputation and those who did not. Twenty patients were lost to follow-up, and therefore we altered the window of our statistics from 6 months to 3 months to provide the most accurate data, as 6-month follow-up data were limited. The patients were classified into the following categories: healed, amputated, and unhealed/non-amputated. Descriptive statistics were calculated for these 3 groups, analyzing the same variables (age, WBC count, wound area, HbA1c, blood glucose, and BMI). Additional statistical computations were utilized in order to show the prevalence and frequency of our categorical variables: gender, race, ethnicity, osteomyelitis, gangrene, and peripheral vascular disease. The baseline values of WBC count, HbA1c, wound area, and BMI of the 3 groups were analyzed with descriptive statistics for comparison. A multinomial logistic regression was then performed using a 3-level outcome variable: healed, amputated, or unhealed/non-amputated. Each predictor variable was analyzed independently due to the small sample size.
Results
Of the 169 registered patients treated at the Northwell Health facility, all qualified for the OWEMR study and met the study criteria. In the original 169 patients, there were 19 amputations: 6 toe, 6 trans-metatarsal, 6 below knee, and 1 above knee (Table 1).
The descriptive statistics of 149 patients grouped into 3 categories (healed, amputated, unhealed/non-amputated) are shown in Table 2.
The results of the logistic regression exploring the differences between the amputation and healed groups and the unhealed/non-amputated group are shown in Table 3. The amputation group had a higher mean age and WBC count and greater wound area. Increased age was determined to be a significant predictor of the odds of amputation (P = 0.0089). For each year increase in age, the odds of amputation increased by 6.5% (odds ratio, 1.07 [95% confidence interval {CI}, 1.02-1.12]). Patients in the amputation group were more likely to be male, Hispanic, and African American and to have wound infections and comorbidities (osteomyelitis, neuropathy, and gangrene).
The presence of gangrene was significantly associated with LEA (P = 0.03). Specifically, the odds of patients without gangrene undergoing a LEA were substantially lower compared with their counterparts with gangrene (odds ratio, 0.17; 95% CI, 0.04-0.68; P = 0.0131). However, the presence of gangrene was not associated with the odds of healing compared with the odds of neither healing nor undergoing amputation (P = 0.84; not shown in Table 3).
The amputation group had lower mean values for HbA1c, BMI, and blood glucose levels and a lower rate of peripheral vascular disease. Only the relationship between lower HbA1c and increased odds of amputation versus not healing/non-amputation was found to be statistically significant (95% CI, 0.27-0.78; P = 0.009).
Discussion
This retrospective study was undertaken to evaluate factors associated with LEA in patients with diabetic foot ulcers. Patients with diabetes being treated at a wound care facility often require continuous surgical and metabolic intervention to promote optimal healing: drainage, surgical debridement, irrigation, culturing for infection, and monitoring of blood glucose levels. This treatment requires strict compliance with medical directions and, oftentimes, additional care, such as home-care nursing visits, to maintain a curative environment for the wound. Frequently, wounds on the lower extremity further complicate the healing process by reducing the patient’s mobility and daily life. Due to these factors, many patients progress to LEA. The link between diabetic ulcers and amputation has already been well described in previous studies, with studies showing that history of diabetic foot ulcer significantly predisposes an individual to LEA.4 However, few studies have further investigated demographic factors associated with risk for an amputation. Our study analyzed several categories of patient data taken from a baseline visit. We found that those with highly elevated HbA1c values were less likely to have an amputation than persons with relatively lower levels, a finding that is contrary to previous studies.
Our study’s findings suggest a higher risk for LEA with increased age. The amputation group was, on average, 7 years older than the other 2 groups. A recent study showed that risk for amputation is directly correlated to patient age, as is the mortality rate after undergoing LEA (2.3%; P < 0.05).5 Our study found that with each increase in age of 1 year, the odds of amputation increased by 6.5%. However, recent evidence on LEA risk and aging suggests that age is of less consequence than the duration of diabetes. One study found that the propensity to develop diabetic foot ulcers increases with the duration of diabetes.6 The same study found that prevalence of ulceration was correlated with age, but the relationship between age and LEA was less significant. A follow-up study for LEA could be done to examine the role of disease duration versus age in LEA.
A consensus among previous studies is that men have a higher risk for LEA.5,7 Men comprised the majority in all 3 groups in our study. In addition, the amputation group in our study had the lowest BMI. Higher BMI generally is associated with an increased risk for health complications. However, a past study conducted in Taiwan reported that obese patients with diabetes were less likely to undergo LEA than those within the normal range for BMI.8 Neither study suggests that obesity is a deterrent for LEA, but both studies may suggest that risk of amputation may approach a maximum frequency at a specific BMI range, and then decrease. This unconfirmed “cyclic” relationship should be evaluated further in a larger sample size.
Most patients in our analysis were Caucasian, followed by African American and South Asian. African Americans were the only racial group with an increased frequency in the amputation group. This finding is supported by a previous study that found that the rate of LEA among patients with diabetes in low-income, predominantly African-American neighborhoods was nearly double that in wealthier, predominantly Caucasian areas.9 A potential problem in the comparison between our data with previous studies is that the studies did not analyze patients with our inclusion criteria. All patients with diabetes in previous investigations were grouped by race, but were not necessarily required to have 1 or more ulcers. Multiple ulcers may predispose an individual to a greater risk for amputation.
Multinomial logistic regression did not suggest an association between initial size of a patient’s wound and the risk of amputation. However, the descriptive data suggests a trend. Patients who did not heal or require an amputation had the largest average wound area. This finding is not surprising in that our study followed individuals for only 3 months. Many wounds require a long course of treatment, especially in patients with diabetes, who may have poor vascularization. However, in comparison to the healed patients, the patients who required an amputation had a larger average wound area. A larger wound requires a plentiful vascular supply for the delivery of clotting factors and nutrients to the damaged area. As wound size increases, an individual’s body must transmit an increased quantity of these factors and nutrients for the regeneration of tissue. In addition, wounds that possess a larger surface area require more debridement and present a greater opportunity for infection. This may also foreshadow a longer, more costly course of treatment. Additionally, individuals coping with large ulcerations are burdened by more elaborate and complex wound dressings.
Elevated levels of HbA1c are associated with increased adverse effects of diabetes, including end-stage renal disease, neuropathy, and infection.10 In a previous study, the risk for amputation was 1.2 times higher in patients with elevated HbA1c.11 In contrast, our study suggested the odds of LEA versus not healing/not undergoing amputation decreased as HbA1c increased. As a patient’s HbA1c level increased by a value of 1, their odds for LEA decreased by 54.3%. This finding contradicts prior studies that have found a positive association between HbA1c and LEA risk, including a study where each percentage increase in HbA1c correlated with a 13% to 15% increased risk of LEA.12 The finding that patients who underwent amputation in our study had lower levels of HbA1c and blood glucose cannot be fully explained. The maximum HbA1c value in the amputated group was 7.9%. The average values for healed patients and those who underwent LEA were 8.75% and 6.77%, respectively.
Blood glucose levels were also found to be the lowest in the amputated group in our study (mean, 149.29 mg/dL vs 163.19 mg/dL in the healed group). Similar results were found in a Brazilian study, in which patients who did not require amputation had higher HbA1c levels. This study also found an association between blood glucose levels above 200 mg/dL and amputations.3 These findings provide interesting opportunities for repeat studies, preferably with a larger number of participants.
Our study is limited by the small sample size. The sample population had to be reduced, as many patients were lost to follow-up. Although this paring down of the sample size can introduce bias, we are confident that our study is representative of the demographic of patients treated in our facility. The loss of patients to follow-up in turn caused the window of analysis to be narrowed, as long-term outcome data were not available. A multisite study observing various population samples can better explore the relationship between HbA1c and risk of amputation.
Conclusion
This retrospective study exploring factors associated with LEA was unique in that all our participants had 1 or more diabetic foot ulcerations, and thus already had an extremely high risk for amputation, in contrast to previous studies that followed persons at risk for developing diabetic foot ulcerations. In contrast to several previous studies, we found that the risk for amputation actually decreased as baseline measurements of HbA1c increased. The results of this study offer many opportunities for future investigations, preferably with a larger sample size. By further isolating and scrutinizing specific factors associated with LEA, researchers can help clinicians focus on providing wound care that promotes limb salvage.
Corresponding author: Alisha Oropallo, MD, MS, Northwell Health Comprehensive Wound Care Healing Center and Hyperbarics, 1999 Marcus Avenue, Suite M6, Lake Success, NY 11042; aoropallo@northwell.edu.
Financial disclosures: Funding for this research was provided by a multi-institutional AHRQ governmental grant.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
2. Uccioli L, Giurato L, Meloni M, et al. Comment on Hoffstad et al. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38:1852-1857.
3. Gamba MA, Gotlieb SLD, Bergamaschi DP, Vianna LAC. Lower extremity amputations in diabetic patients: a case-control study. Rev Saúde Pública. 2004;38:399-404.
4. Martins-Mendes D, Monteiro-Soares M, Boyko EJ, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632-638.
5. Lipsky BA, Weigelt JA, Sun X, et al. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695-1700.
6. Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446.
7. Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852-857.
8. Lin C, Hsu BR, Tsai J, et al. Effect of limb preservation status and body mass index on the survival of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications. 2017;31:180-185.
9. Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff. 2014;33:1383-1390.
10. Liao L, Li C, Liu C, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan diabetes study. PLoS One. 2015; 10:e0130828.
11. Miyajima S, Shirai A, Yamamoto S, et al. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006;71:272-279.
12. Zhao W, Katzmarzyk PT, Horswell R, et al. HbA1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care. 2013;36:3591-3598.
From Northwell Health System, Lake Success, NY.
Abstract
- Objective: To explore factors associated with lower-extremity amputation (LEA) in patients with diabetic foot ulcers using data from the Online Wound Electronic Medical Record Database.
- Design: Retrospective analysis of medical records.
- Setting and participants: Data from 169 individuals with previously diagnosed diabetes mellitus who received wound care for a 6-month period within a span of 2 years was analyzed. A baseline evaluation was obtained and wound(s) were treated, managed, and monitored.
Treatment continued until the patient healed, required an LEA, or phased out of the study, neither healing nor undergoing an amputation. Of the 149 patients who completed the study, 38 had healed ulcers, 14 underwent amputation, and 97 neither healed nor underwent an amputation. All patients were treated under the care of vascular and/or podiatric surgeons. - Measurements: Variables included wound status (healed, amputated, and unhealed/non-amputated); size of wound area; age, gender, race, and ethnicity; white blood cell (WBC) count, hemoglobin A1c (HbA1c), blood glucose, and body mass index (BMI); and presence of osteomyelitis, gangrene, and peripheral vascular disease.
- Results: As compared to the healed and unhealed/non-amputated group, the group of patients who underwent LEA was older and had higher percentages of males, Hispanics, and African Americans; had a higher WBC count, larger wound area, and higher rates of wound infection, osteomyelitis, and neuropathy; and had lower average values of HbA1c, blood glucose, and BMI and a lower rate of peripheral vascular disease.
- Conclusion: The association between HbA1c and LEA highlights a window of relative safety among an at-risk population. By identifying and focusing on factors associated with LEA, health care professionals may be able to decrease the prevalence of LEA in patients with diabetes.
Keywords: diabetic foot ulcer; lower-extremity amputation; risk factors; HbA1c.
An estimated 30.3 million people, or 9.4% of the US population, has diabetes. In 2014, approximately 108,000 amputations were performed on adults with diagnosed diabetes.1 Furthermore, patients with diabetes have a 10-fold increased risk for lower-extremity amputation (LEA), as compared with patients without diabetes.2 The frequency of amputations in the diabetic population is a public health crisis.
Amputation has significant, life-altering consequences. Patients who undergo LEA often face debilitation in their daily activities and must undergo intense rehabilitation to learn basic tasks. Amputations can also impact individuals’ psychological well-being as they come to terms with their altered body and may face challenges in self-perception, confidence, self-esteem, work life, and relationships. In addition, the mortality rate for patients with diabetes 5 years after undergoing LEA is 30%.2 However, public health studies estimate that more than half of LEAs in patients with diabetes are preventable.3
Although studies have explored the relationship between diabetes and LEA, few have sought to identify factors directly correlated with wound care. In the United States, patients with diabetic ulcerations are typically treated in wound care facilities; however, previous studies have concentrated on the conditions that lead to the formation of an ulcer or amputation, viewing amputation and ulcer as 2 separate entities. Our study took into account systemic variables, patient demographics, and specific wound characteristics to explore factors associated with LEA in a high-risk group of patients with diabetes. This study was designed to assess ailments that are prevalent in patients who require a LEA.
Methods
Patients and Setting
A total of 169 patients who were treated at the Comprehensive Wound Healing and Hyperbaric Center (Lake Success, NY), a tertiary facility of the Northwell Health system, participated in this retrospective study. The data for this study were obtained in conjunction with the development of the New York University School of Medicine’s Online Wound Electronic Medical Record to Decrease Limb Amputations in Persons with Diabetes (OWEMR) database. The OWEMR collects individual patient data from satellite locations across the country. Using this database, researchers can analyze similarities and differences between patients who undergo LEA.
This study utilized patient data specific to the Northwell Health facility. All of the patients in our study were enrolled under the criteria of the OWEMR database. In order to be included in the OWEMR database, patients had to be diagnosed with type 1 or type 2 diabetes; have a break in the skin ≥ 0.5 cm2; be 18 years of age or older; and have a measured hemoglobin A1c (HbA1c) value within the past 120 days. Study patients signed an informed consent and committed to being available for follow-up visits to the wound care facility for 6 months after entering the study. Patients were enrolled between 2012 and 2014, and each patient was monitored for a period of 6 months within this time period. Participants were treated with current standards of care using diet, lifestyle, and pharmacologic interventions. This study was approved by the Northwell Health System Institutional Review Board Human Research Protection Program (Manhasset, NY).
Data Collection
On their first visit to the facility, patients were given a physical examination and initial interview regarding their medical history. Clinicians were required to select 1 ulcer that would be examined for the duration of the study. The selection of the ulcer was based on a point system that awarded points for pedal pulses, the ability to be probed to the bone, the location of the ulcer (ie, located on the foot rather than a toe), and the presence of multiple ulcerations. The ulcer with the highest score was selected for the study. If numerous ulcers were evaluated with the same score, the largest and deepest was selected. Wagner classification of the wound was recorded at baseline and taken at each subsequent patient visit. In addition, peripheral sensation was assessed for signs of neuropathy using Semmes-Weinstein monofilament testing.
Once selected, the wound was clinically evaluated, samples for culture were obtained, and blood tests were performed to detect the presence of wound infection. The patient’s blood was drawn for a full laboratory analysis, including white blood cell (WBC) count and measurement of blood glucose and HbA1c levels. Bone biopsy, magnetic resonance imaging, and bone scans were used to detect the presence of osteomyelitis at the discretion of the health care provider. Wounds suspected of infection, underlying osteomyelitis, or gangrene at baseline were excluded. Patients would then return for follow-up visits at least once every 6 weeks, plus or minus 2 weeks, for a maximum of 6 months.
Statistical Analysis
Utilizing SAS version 9.3 (Cary, NC), descriptive statistics (minimum, maximum, mean, median, and SD) were calculated for the following variables: age, WBC count, wound area, HbA1c, blood glucose, and body mass index (BMI). These variables were collected for each patient as per the OWEMR protocol and provided a basis for which to compare patients who underwent amputation and those who did not. Twenty patients were lost to follow-up, and therefore we altered the window of our statistics from 6 months to 3 months to provide the most accurate data, as 6-month follow-up data were limited. The patients were classified into the following categories: healed, amputated, and unhealed/non-amputated. Descriptive statistics were calculated for these 3 groups, analyzing the same variables (age, WBC count, wound area, HbA1c, blood glucose, and BMI). Additional statistical computations were utilized in order to show the prevalence and frequency of our categorical variables: gender, race, ethnicity, osteomyelitis, gangrene, and peripheral vascular disease. The baseline values of WBC count, HbA1c, wound area, and BMI of the 3 groups were analyzed with descriptive statistics for comparison. A multinomial logistic regression was then performed using a 3-level outcome variable: healed, amputated, or unhealed/non-amputated. Each predictor variable was analyzed independently due to the small sample size.
Results
Of the 169 registered patients treated at the Northwell Health facility, all qualified for the OWEMR study and met the study criteria. In the original 169 patients, there were 19 amputations: 6 toe, 6 trans-metatarsal, 6 below knee, and 1 above knee (Table 1).
The descriptive statistics of 149 patients grouped into 3 categories (healed, amputated, unhealed/non-amputated) are shown in Table 2.
The results of the logistic regression exploring the differences between the amputation and healed groups and the unhealed/non-amputated group are shown in Table 3. The amputation group had a higher mean age and WBC count and greater wound area. Increased age was determined to be a significant predictor of the odds of amputation (P = 0.0089). For each year increase in age, the odds of amputation increased by 6.5% (odds ratio, 1.07 [95% confidence interval {CI}, 1.02-1.12]). Patients in the amputation group were more likely to be male, Hispanic, and African American and to have wound infections and comorbidities (osteomyelitis, neuropathy, and gangrene).
The presence of gangrene was significantly associated with LEA (P = 0.03). Specifically, the odds of patients without gangrene undergoing a LEA were substantially lower compared with their counterparts with gangrene (odds ratio, 0.17; 95% CI, 0.04-0.68; P = 0.0131). However, the presence of gangrene was not associated with the odds of healing compared with the odds of neither healing nor undergoing amputation (P = 0.84; not shown in Table 3).
The amputation group had lower mean values for HbA1c, BMI, and blood glucose levels and a lower rate of peripheral vascular disease. Only the relationship between lower HbA1c and increased odds of amputation versus not healing/non-amputation was found to be statistically significant (95% CI, 0.27-0.78; P = 0.009).
Discussion
This retrospective study was undertaken to evaluate factors associated with LEA in patients with diabetic foot ulcers. Patients with diabetes being treated at a wound care facility often require continuous surgical and metabolic intervention to promote optimal healing: drainage, surgical debridement, irrigation, culturing for infection, and monitoring of blood glucose levels. This treatment requires strict compliance with medical directions and, oftentimes, additional care, such as home-care nursing visits, to maintain a curative environment for the wound. Frequently, wounds on the lower extremity further complicate the healing process by reducing the patient’s mobility and daily life. Due to these factors, many patients progress to LEA. The link between diabetic ulcers and amputation has already been well described in previous studies, with studies showing that history of diabetic foot ulcer significantly predisposes an individual to LEA.4 However, few studies have further investigated demographic factors associated with risk for an amputation. Our study analyzed several categories of patient data taken from a baseline visit. We found that those with highly elevated HbA1c values were less likely to have an amputation than persons with relatively lower levels, a finding that is contrary to previous studies.
Our study’s findings suggest a higher risk for LEA with increased age. The amputation group was, on average, 7 years older than the other 2 groups. A recent study showed that risk for amputation is directly correlated to patient age, as is the mortality rate after undergoing LEA (2.3%; P < 0.05).5 Our study found that with each increase in age of 1 year, the odds of amputation increased by 6.5%. However, recent evidence on LEA risk and aging suggests that age is of less consequence than the duration of diabetes. One study found that the propensity to develop diabetic foot ulcers increases with the duration of diabetes.6 The same study found that prevalence of ulceration was correlated with age, but the relationship between age and LEA was less significant. A follow-up study for LEA could be done to examine the role of disease duration versus age in LEA.
A consensus among previous studies is that men have a higher risk for LEA.5,7 Men comprised the majority in all 3 groups in our study. In addition, the amputation group in our study had the lowest BMI. Higher BMI generally is associated with an increased risk for health complications. However, a past study conducted in Taiwan reported that obese patients with diabetes were less likely to undergo LEA than those within the normal range for BMI.8 Neither study suggests that obesity is a deterrent for LEA, but both studies may suggest that risk of amputation may approach a maximum frequency at a specific BMI range, and then decrease. This unconfirmed “cyclic” relationship should be evaluated further in a larger sample size.
Most patients in our analysis were Caucasian, followed by African American and South Asian. African Americans were the only racial group with an increased frequency in the amputation group. This finding is supported by a previous study that found that the rate of LEA among patients with diabetes in low-income, predominantly African-American neighborhoods was nearly double that in wealthier, predominantly Caucasian areas.9 A potential problem in the comparison between our data with previous studies is that the studies did not analyze patients with our inclusion criteria. All patients with diabetes in previous investigations were grouped by race, but were not necessarily required to have 1 or more ulcers. Multiple ulcers may predispose an individual to a greater risk for amputation.
Multinomial logistic regression did not suggest an association between initial size of a patient’s wound and the risk of amputation. However, the descriptive data suggests a trend. Patients who did not heal or require an amputation had the largest average wound area. This finding is not surprising in that our study followed individuals for only 3 months. Many wounds require a long course of treatment, especially in patients with diabetes, who may have poor vascularization. However, in comparison to the healed patients, the patients who required an amputation had a larger average wound area. A larger wound requires a plentiful vascular supply for the delivery of clotting factors and nutrients to the damaged area. As wound size increases, an individual’s body must transmit an increased quantity of these factors and nutrients for the regeneration of tissue. In addition, wounds that possess a larger surface area require more debridement and present a greater opportunity for infection. This may also foreshadow a longer, more costly course of treatment. Additionally, individuals coping with large ulcerations are burdened by more elaborate and complex wound dressings.
Elevated levels of HbA1c are associated with increased adverse effects of diabetes, including end-stage renal disease, neuropathy, and infection.10 In a previous study, the risk for amputation was 1.2 times higher in patients with elevated HbA1c.11 In contrast, our study suggested the odds of LEA versus not healing/not undergoing amputation decreased as HbA1c increased. As a patient’s HbA1c level increased by a value of 1, their odds for LEA decreased by 54.3%. This finding contradicts prior studies that have found a positive association between HbA1c and LEA risk, including a study where each percentage increase in HbA1c correlated with a 13% to 15% increased risk of LEA.12 The finding that patients who underwent amputation in our study had lower levels of HbA1c and blood glucose cannot be fully explained. The maximum HbA1c value in the amputated group was 7.9%. The average values for healed patients and those who underwent LEA were 8.75% and 6.77%, respectively.
Blood glucose levels were also found to be the lowest in the amputated group in our study (mean, 149.29 mg/dL vs 163.19 mg/dL in the healed group). Similar results were found in a Brazilian study, in which patients who did not require amputation had higher HbA1c levels. This study also found an association between blood glucose levels above 200 mg/dL and amputations.3 These findings provide interesting opportunities for repeat studies, preferably with a larger number of participants.
Our study is limited by the small sample size. The sample population had to be reduced, as many patients were lost to follow-up. Although this paring down of the sample size can introduce bias, we are confident that our study is representative of the demographic of patients treated in our facility. The loss of patients to follow-up in turn caused the window of analysis to be narrowed, as long-term outcome data were not available. A multisite study observing various population samples can better explore the relationship between HbA1c and risk of amputation.
Conclusion
This retrospective study exploring factors associated with LEA was unique in that all our participants had 1 or more diabetic foot ulcerations, and thus already had an extremely high risk for amputation, in contrast to previous studies that followed persons at risk for developing diabetic foot ulcerations. In contrast to several previous studies, we found that the risk for amputation actually decreased as baseline measurements of HbA1c increased. The results of this study offer many opportunities for future investigations, preferably with a larger sample size. By further isolating and scrutinizing specific factors associated with LEA, researchers can help clinicians focus on providing wound care that promotes limb salvage.
Corresponding author: Alisha Oropallo, MD, MS, Northwell Health Comprehensive Wound Care Healing Center and Hyperbarics, 1999 Marcus Avenue, Suite M6, Lake Success, NY 11042; aoropallo@northwell.edu.
Financial disclosures: Funding for this research was provided by a multi-institutional AHRQ governmental grant.
From Northwell Health System, Lake Success, NY.
Abstract
- Objective: To explore factors associated with lower-extremity amputation (LEA) in patients with diabetic foot ulcers using data from the Online Wound Electronic Medical Record Database.
- Design: Retrospective analysis of medical records.
- Setting and participants: Data from 169 individuals with previously diagnosed diabetes mellitus who received wound care for a 6-month period within a span of 2 years was analyzed. A baseline evaluation was obtained and wound(s) were treated, managed, and monitored.
Treatment continued until the patient healed, required an LEA, or phased out of the study, neither healing nor undergoing an amputation. Of the 149 patients who completed the study, 38 had healed ulcers, 14 underwent amputation, and 97 neither healed nor underwent an amputation. All patients were treated under the care of vascular and/or podiatric surgeons. - Measurements: Variables included wound status (healed, amputated, and unhealed/non-amputated); size of wound area; age, gender, race, and ethnicity; white blood cell (WBC) count, hemoglobin A1c (HbA1c), blood glucose, and body mass index (BMI); and presence of osteomyelitis, gangrene, and peripheral vascular disease.
- Results: As compared to the healed and unhealed/non-amputated group, the group of patients who underwent LEA was older and had higher percentages of males, Hispanics, and African Americans; had a higher WBC count, larger wound area, and higher rates of wound infection, osteomyelitis, and neuropathy; and had lower average values of HbA1c, blood glucose, and BMI and a lower rate of peripheral vascular disease.
- Conclusion: The association between HbA1c and LEA highlights a window of relative safety among an at-risk population. By identifying and focusing on factors associated with LEA, health care professionals may be able to decrease the prevalence of LEA in patients with diabetes.
Keywords: diabetic foot ulcer; lower-extremity amputation; risk factors; HbA1c.
An estimated 30.3 million people, or 9.4% of the US population, has diabetes. In 2014, approximately 108,000 amputations were performed on adults with diagnosed diabetes.1 Furthermore, patients with diabetes have a 10-fold increased risk for lower-extremity amputation (LEA), as compared with patients without diabetes.2 The frequency of amputations in the diabetic population is a public health crisis.
Amputation has significant, life-altering consequences. Patients who undergo LEA often face debilitation in their daily activities and must undergo intense rehabilitation to learn basic tasks. Amputations can also impact individuals’ psychological well-being as they come to terms with their altered body and may face challenges in self-perception, confidence, self-esteem, work life, and relationships. In addition, the mortality rate for patients with diabetes 5 years after undergoing LEA is 30%.2 However, public health studies estimate that more than half of LEAs in patients with diabetes are preventable.3
Although studies have explored the relationship between diabetes and LEA, few have sought to identify factors directly correlated with wound care. In the United States, patients with diabetic ulcerations are typically treated in wound care facilities; however, previous studies have concentrated on the conditions that lead to the formation of an ulcer or amputation, viewing amputation and ulcer as 2 separate entities. Our study took into account systemic variables, patient demographics, and specific wound characteristics to explore factors associated with LEA in a high-risk group of patients with diabetes. This study was designed to assess ailments that are prevalent in patients who require a LEA.
Methods
Patients and Setting
A total of 169 patients who were treated at the Comprehensive Wound Healing and Hyperbaric Center (Lake Success, NY), a tertiary facility of the Northwell Health system, participated in this retrospective study. The data for this study were obtained in conjunction with the development of the New York University School of Medicine’s Online Wound Electronic Medical Record to Decrease Limb Amputations in Persons with Diabetes (OWEMR) database. The OWEMR collects individual patient data from satellite locations across the country. Using this database, researchers can analyze similarities and differences between patients who undergo LEA.
This study utilized patient data specific to the Northwell Health facility. All of the patients in our study were enrolled under the criteria of the OWEMR database. In order to be included in the OWEMR database, patients had to be diagnosed with type 1 or type 2 diabetes; have a break in the skin ≥ 0.5 cm2; be 18 years of age or older; and have a measured hemoglobin A1c (HbA1c) value within the past 120 days. Study patients signed an informed consent and committed to being available for follow-up visits to the wound care facility for 6 months after entering the study. Patients were enrolled between 2012 and 2014, and each patient was monitored for a period of 6 months within this time period. Participants were treated with current standards of care using diet, lifestyle, and pharmacologic interventions. This study was approved by the Northwell Health System Institutional Review Board Human Research Protection Program (Manhasset, NY).
Data Collection
On their first visit to the facility, patients were given a physical examination and initial interview regarding their medical history. Clinicians were required to select 1 ulcer that would be examined for the duration of the study. The selection of the ulcer was based on a point system that awarded points for pedal pulses, the ability to be probed to the bone, the location of the ulcer (ie, located on the foot rather than a toe), and the presence of multiple ulcerations. The ulcer with the highest score was selected for the study. If numerous ulcers were evaluated with the same score, the largest and deepest was selected. Wagner classification of the wound was recorded at baseline and taken at each subsequent patient visit. In addition, peripheral sensation was assessed for signs of neuropathy using Semmes-Weinstein monofilament testing.
Once selected, the wound was clinically evaluated, samples for culture were obtained, and blood tests were performed to detect the presence of wound infection. The patient’s blood was drawn for a full laboratory analysis, including white blood cell (WBC) count and measurement of blood glucose and HbA1c levels. Bone biopsy, magnetic resonance imaging, and bone scans were used to detect the presence of osteomyelitis at the discretion of the health care provider. Wounds suspected of infection, underlying osteomyelitis, or gangrene at baseline were excluded. Patients would then return for follow-up visits at least once every 6 weeks, plus or minus 2 weeks, for a maximum of 6 months.
Statistical Analysis
Utilizing SAS version 9.3 (Cary, NC), descriptive statistics (minimum, maximum, mean, median, and SD) were calculated for the following variables: age, WBC count, wound area, HbA1c, blood glucose, and body mass index (BMI). These variables were collected for each patient as per the OWEMR protocol and provided a basis for which to compare patients who underwent amputation and those who did not. Twenty patients were lost to follow-up, and therefore we altered the window of our statistics from 6 months to 3 months to provide the most accurate data, as 6-month follow-up data were limited. The patients were classified into the following categories: healed, amputated, and unhealed/non-amputated. Descriptive statistics were calculated for these 3 groups, analyzing the same variables (age, WBC count, wound area, HbA1c, blood glucose, and BMI). Additional statistical computations were utilized in order to show the prevalence and frequency of our categorical variables: gender, race, ethnicity, osteomyelitis, gangrene, and peripheral vascular disease. The baseline values of WBC count, HbA1c, wound area, and BMI of the 3 groups were analyzed with descriptive statistics for comparison. A multinomial logistic regression was then performed using a 3-level outcome variable: healed, amputated, or unhealed/non-amputated. Each predictor variable was analyzed independently due to the small sample size.
Results
Of the 169 registered patients treated at the Northwell Health facility, all qualified for the OWEMR study and met the study criteria. In the original 169 patients, there were 19 amputations: 6 toe, 6 trans-metatarsal, 6 below knee, and 1 above knee (Table 1).
The descriptive statistics of 149 patients grouped into 3 categories (healed, amputated, unhealed/non-amputated) are shown in Table 2.
The results of the logistic regression exploring the differences between the amputation and healed groups and the unhealed/non-amputated group are shown in Table 3. The amputation group had a higher mean age and WBC count and greater wound area. Increased age was determined to be a significant predictor of the odds of amputation (P = 0.0089). For each year increase in age, the odds of amputation increased by 6.5% (odds ratio, 1.07 [95% confidence interval {CI}, 1.02-1.12]). Patients in the amputation group were more likely to be male, Hispanic, and African American and to have wound infections and comorbidities (osteomyelitis, neuropathy, and gangrene).
The presence of gangrene was significantly associated with LEA (P = 0.03). Specifically, the odds of patients without gangrene undergoing a LEA were substantially lower compared with their counterparts with gangrene (odds ratio, 0.17; 95% CI, 0.04-0.68; P = 0.0131). However, the presence of gangrene was not associated with the odds of healing compared with the odds of neither healing nor undergoing amputation (P = 0.84; not shown in Table 3).
The amputation group had lower mean values for HbA1c, BMI, and blood glucose levels and a lower rate of peripheral vascular disease. Only the relationship between lower HbA1c and increased odds of amputation versus not healing/non-amputation was found to be statistically significant (95% CI, 0.27-0.78; P = 0.009).
Discussion
This retrospective study was undertaken to evaluate factors associated with LEA in patients with diabetic foot ulcers. Patients with diabetes being treated at a wound care facility often require continuous surgical and metabolic intervention to promote optimal healing: drainage, surgical debridement, irrigation, culturing for infection, and monitoring of blood glucose levels. This treatment requires strict compliance with medical directions and, oftentimes, additional care, such as home-care nursing visits, to maintain a curative environment for the wound. Frequently, wounds on the lower extremity further complicate the healing process by reducing the patient’s mobility and daily life. Due to these factors, many patients progress to LEA. The link between diabetic ulcers and amputation has already been well described in previous studies, with studies showing that history of diabetic foot ulcer significantly predisposes an individual to LEA.4 However, few studies have further investigated demographic factors associated with risk for an amputation. Our study analyzed several categories of patient data taken from a baseline visit. We found that those with highly elevated HbA1c values were less likely to have an amputation than persons with relatively lower levels, a finding that is contrary to previous studies.
Our study’s findings suggest a higher risk for LEA with increased age. The amputation group was, on average, 7 years older than the other 2 groups. A recent study showed that risk for amputation is directly correlated to patient age, as is the mortality rate after undergoing LEA (2.3%; P < 0.05).5 Our study found that with each increase in age of 1 year, the odds of amputation increased by 6.5%. However, recent evidence on LEA risk and aging suggests that age is of less consequence than the duration of diabetes. One study found that the propensity to develop diabetic foot ulcers increases with the duration of diabetes.6 The same study found that prevalence of ulceration was correlated with age, but the relationship between age and LEA was less significant. A follow-up study for LEA could be done to examine the role of disease duration versus age in LEA.
A consensus among previous studies is that men have a higher risk for LEA.5,7 Men comprised the majority in all 3 groups in our study. In addition, the amputation group in our study had the lowest BMI. Higher BMI generally is associated with an increased risk for health complications. However, a past study conducted in Taiwan reported that obese patients with diabetes were less likely to undergo LEA than those within the normal range for BMI.8 Neither study suggests that obesity is a deterrent for LEA, but both studies may suggest that risk of amputation may approach a maximum frequency at a specific BMI range, and then decrease. This unconfirmed “cyclic” relationship should be evaluated further in a larger sample size.
Most patients in our analysis were Caucasian, followed by African American and South Asian. African Americans were the only racial group with an increased frequency in the amputation group. This finding is supported by a previous study that found that the rate of LEA among patients with diabetes in low-income, predominantly African-American neighborhoods was nearly double that in wealthier, predominantly Caucasian areas.9 A potential problem in the comparison between our data with previous studies is that the studies did not analyze patients with our inclusion criteria. All patients with diabetes in previous investigations were grouped by race, but were not necessarily required to have 1 or more ulcers. Multiple ulcers may predispose an individual to a greater risk for amputation.
Multinomial logistic regression did not suggest an association between initial size of a patient’s wound and the risk of amputation. However, the descriptive data suggests a trend. Patients who did not heal or require an amputation had the largest average wound area. This finding is not surprising in that our study followed individuals for only 3 months. Many wounds require a long course of treatment, especially in patients with diabetes, who may have poor vascularization. However, in comparison to the healed patients, the patients who required an amputation had a larger average wound area. A larger wound requires a plentiful vascular supply for the delivery of clotting factors and nutrients to the damaged area. As wound size increases, an individual’s body must transmit an increased quantity of these factors and nutrients for the regeneration of tissue. In addition, wounds that possess a larger surface area require more debridement and present a greater opportunity for infection. This may also foreshadow a longer, more costly course of treatment. Additionally, individuals coping with large ulcerations are burdened by more elaborate and complex wound dressings.
Elevated levels of HbA1c are associated with increased adverse effects of diabetes, including end-stage renal disease, neuropathy, and infection.10 In a previous study, the risk for amputation was 1.2 times higher in patients with elevated HbA1c.11 In contrast, our study suggested the odds of LEA versus not healing/not undergoing amputation decreased as HbA1c increased. As a patient’s HbA1c level increased by a value of 1, their odds for LEA decreased by 54.3%. This finding contradicts prior studies that have found a positive association between HbA1c and LEA risk, including a study where each percentage increase in HbA1c correlated with a 13% to 15% increased risk of LEA.12 The finding that patients who underwent amputation in our study had lower levels of HbA1c and blood glucose cannot be fully explained. The maximum HbA1c value in the amputated group was 7.9%. The average values for healed patients and those who underwent LEA were 8.75% and 6.77%, respectively.
Blood glucose levels were also found to be the lowest in the amputated group in our study (mean, 149.29 mg/dL vs 163.19 mg/dL in the healed group). Similar results were found in a Brazilian study, in which patients who did not require amputation had higher HbA1c levels. This study also found an association between blood glucose levels above 200 mg/dL and amputations.3 These findings provide interesting opportunities for repeat studies, preferably with a larger number of participants.
Our study is limited by the small sample size. The sample population had to be reduced, as many patients were lost to follow-up. Although this paring down of the sample size can introduce bias, we are confident that our study is representative of the demographic of patients treated in our facility. The loss of patients to follow-up in turn caused the window of analysis to be narrowed, as long-term outcome data were not available. A multisite study observing various population samples can better explore the relationship between HbA1c and risk of amputation.
Conclusion
This retrospective study exploring factors associated with LEA was unique in that all our participants had 1 or more diabetic foot ulcerations, and thus already had an extremely high risk for amputation, in contrast to previous studies that followed persons at risk for developing diabetic foot ulcerations. In contrast to several previous studies, we found that the risk for amputation actually decreased as baseline measurements of HbA1c increased. The results of this study offer many opportunities for future investigations, preferably with a larger sample size. By further isolating and scrutinizing specific factors associated with LEA, researchers can help clinicians focus on providing wound care that promotes limb salvage.
Corresponding author: Alisha Oropallo, MD, MS, Northwell Health Comprehensive Wound Care Healing Center and Hyperbarics, 1999 Marcus Avenue, Suite M6, Lake Success, NY 11042; aoropallo@northwell.edu.
Financial disclosures: Funding for this research was provided by a multi-institutional AHRQ governmental grant.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
2. Uccioli L, Giurato L, Meloni M, et al. Comment on Hoffstad et al. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38:1852-1857.
3. Gamba MA, Gotlieb SLD, Bergamaschi DP, Vianna LAC. Lower extremity amputations in diabetic patients: a case-control study. Rev Saúde Pública. 2004;38:399-404.
4. Martins-Mendes D, Monteiro-Soares M, Boyko EJ, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632-638.
5. Lipsky BA, Weigelt JA, Sun X, et al. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695-1700.
6. Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446.
7. Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852-857.
8. Lin C, Hsu BR, Tsai J, et al. Effect of limb preservation status and body mass index on the survival of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications. 2017;31:180-185.
9. Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff. 2014;33:1383-1390.
10. Liao L, Li C, Liu C, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan diabetes study. PLoS One. 2015; 10:e0130828.
11. Miyajima S, Shirai A, Yamamoto S, et al. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006;71:272-279.
12. Zhao W, Katzmarzyk PT, Horswell R, et al. HbA1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care. 2013;36:3591-3598.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
2. Uccioli L, Giurato L, Meloni M, et al. Comment on Hoffstad et al. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38:1852-1857.
3. Gamba MA, Gotlieb SLD, Bergamaschi DP, Vianna LAC. Lower extremity amputations in diabetic patients: a case-control study. Rev Saúde Pública. 2004;38:399-404.
4. Martins-Mendes D, Monteiro-Soares M, Boyko EJ, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632-638.
5. Lipsky BA, Weigelt JA, Sun X, et al. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695-1700.
6. Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446.
7. Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852-857.
8. Lin C, Hsu BR, Tsai J, et al. Effect of limb preservation status and body mass index on the survival of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications. 2017;31:180-185.
9. Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff. 2014;33:1383-1390.
10. Liao L, Li C, Liu C, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan diabetes study. PLoS One. 2015; 10:e0130828.
11. Miyajima S, Shirai A, Yamamoto S, et al. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006;71:272-279.
12. Zhao W, Katzmarzyk PT, Horswell R, et al. HbA1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care. 2013;36:3591-3598.
Impact of Hospitalists on Care Outcomes in a Large Integrated Health System in British Columbia
From the Fraser Health Authority, Surrey, British Columbia, Canada.
Abstract
- Objective: To study care outcomes associated with a network of hospitalist services compared to traditional providers.
- Design: Retrospective review of administrative data.
- Setting and participants: Patients from a large integrated health care system in British Columbia in western Canada admitted and cared for by 3 provider groups between April 1, 2012, and March 31, 2018: hospitalists, family physicians (FP), and internal medicine (IM) physicians:
- Measurements: Average total length of stay (LOS), 30-day readmission, in-hospital mortality, and hospital standardized mortality ratio (HSMR) were the study outcome measures. Multiple logistic regression or generalized regression were completed to determine the relationship between provider groups and outcomes.
- Results: A total of 248,412 hospitalizations were included. Compared to patients admitted to hospitalists, patients admitted to other providers had higher odds of mortality (odds ratio [OR] for FP, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Compared to hospitalist care, FP care was associated with higher readmission (OR, 1.27; 95% CI, 1.22-1.33), while IM care showed lower odds of readmission (OR, 0.83; 95% CI, 0.79-0.87). Patients admitted to the IM group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to patients admitted to hospitalists (mean, 7.37 days; CI, 7.26-7.49) and FPs (mean, 7.30 days; 95% CI, 7.19-7.41). In a subgroup analysis of patients presenting with congestive heart failure, chronic obstructive pulmonary disease, and pneumonia, these general tendencies broadly persisted for mortality and LOS comparisons between FPs and hospitalists, but results were mixed for hospital readmissions.
- Conclusion: Care provided by hospitalists was associated with lower mortality and readmission rates compared with care provided by FPs, despite similar LOS. These findings may reflect differences in volume of services delivered by individual physicians, on-site availability to address urgent medical issues, and evolving specialization of clinical and nonclinical care processes in the acute care setting.
Keywords: hospital medicine; length of stay; readmission; mortality.
The hospitalist model of care has undergone rapid growth globally in recent years.1 The first hospitalist programs in Canada began around the same time as those in the United States and share many similarities in design and operations with their counterparts.2-4 However, unlike in the United States, where the hospitalist model has successfully established itself as an emerging specialty, debates about the merits of the model and its value proposition continue among Canadian observers.5-9
Historically, the type of physicians who acted as the most responsible provider (MRP) in Canadian hospitals depended on setting and geography.10 In large urban areas, groups of general internists or specialists have historically looked after general medicine patients as part of university-affiliated teaching services.11,12 Patients admitted to community hospitals have traditionally been cared for by their own primary care providers, typically general practitioners or family physicians (FPs). In the mid-1990s, many primary care providers in urban centers began to withdraw from inpatient care and primarily focused their practices in the outpatient setting.13-15 Hospitalist programs emerged as health care administrators sought to fill the resulting gap in MRP coverage.2,10
To date, attempts to understand the impact of hospitalist programs in Canada have been limited. A number of early studies aimed to describe16 the role of hospitalists in Canada and suggested improvements in length of stay (LOS) and staff satisfaction.17 However, these studies relied on unadjusted before-after comparisons and lacked methodological rigor to draw robust conclusions. More recently, a few studies have evaluated care outcomes associated with hospitalists using administrative databases, which attempted to control for potential confounding factors.18-21
While these studies are beginning to shed some light on the impact of hospital medicine programs in Canada, there are a number of issues that limit their generalizability. For example, the majority of studies to date focus on hospital medicine programs in Canada’s largest province (Ontario), and most describe experiences from single institutions. Since each of the 13 provincial and territorial governments organizes its health care system differently,22 results from 1 province may not be generalizable to other parts of the country. Moreover, hospitalists in Ontario are more diverse in their training backgrounds, with a larger percentage having trained in general internal medicine (IM), as compared to other parts of Canada, where the majority of hospitalists are overwhelmingly trained as FPs.3
We aimed to study care outcomes associated with a network of hospitalist services compared to “traditional” providers (community-based FPs and IM specialists) in a large integrated health care system in the province of British Columbia in western Canada. The hospital medicine services in this network span a range of community and academic hospitals, and collectively constitute 1 of the largest regional programs in the country. This provides a unique opportunity to understand the impact of hospitalists on outcome measures across a range of acute care institutions.
Methods
Setting and Population
Fraser Health Authority is 1 of 5 regional health authorities in British Columbia that emerged in 2001.23,24 It operates a network of hospitalist programs in 10 of its 12 acute care hospitals. In addition to hospitalists, there are a variable number of “traditional” physician providers who continue to act as MRPs. These include community-based FPs who continue to see their own patients in the hospital, either as part of a solo-practice model or a clinic-based call group. There are also a number of general internists and other subspecialists who accept MRP roles for general medicine patients who may present with higher-acuity conditions. As a result, patients requiring hospitalization due to nonsurgical or noncritical care conditions at each Fraser Health hospital may be cared for by a physician belonging to 1 of 3 groups, depending on local circumstances: an FP, a hospitalist, or an internist.
Inclusion and Exclusion Criteria
In order to evaluate comparative outcomes associated with hospitalist care, we included all patients admitted to a physician in each of the 3 provider groups between April 1, 2012, and March 31, 2018. We chose this time period for 2 reasons: first, we wanted to ensure comparability over an extended period of time, given the methodological changes implemented in 2009 by the Canadian Institute for Health Information (CIHI), the federal organization in the country responsible for setting standards for health care measures.25 Second, previous internal reviews had suggested that data quality prior to this year was inconsistent. We only considered hospitalizations where patients were admitted to and discharged by the same service, and excluded 2 acute care facilities and 1 free-standing rehabilitation facility without a hospitalist service during this period. We also excluded patients who resided in a location beyond the geographic catchment area of Fraser Health. Further details about data collection are outlined in the Appendix.
Measures
We used the framework developed by White and Glazier26 to inform the selection of our outcome measures, as well as relevant variables that may impact them. This framework proposes that the design of the inpatient care model (structures and processes of care) directly affects care outcomes. The model also proposes that patient and provider attributes can modulate this relationship, and suggests that a comprehensive evaluation of hospitalist performance needs to take these factors into account. We identified average total LOS, 30-day readmission rate, in-hospital mortality, and hospital standardized mortality ratio (HSMR)27 as primary outcome measures. HSMR is defined as actual over expected mortality and is measured by CIHI through a formula that takes into account patient illness attributes (eg, the most responsible diagnosis, comorbidity levels) and baseline population mortality rates.27 We chose these measures because they are clinically relevant and easy to obtain and have been utilized in previous similar studies in Canada and the United States.18-21,26
Statistical Analysis
Baseline demographic and clinical differences in patient outcomes were examined using independent t-tests or chi-square tests. Furthermore, baseline differences based on provider groups were explored using analysis of variance or chi-square tests. Multiple logistic regression analyses were completed to determine the relationship between provider groups and readmission and mortality, while the relationship between provider groups and hospital LOS was determined with generalized linear regression (using gamma distribution and a log link). Gamma distribution with a log link analysis is appropriate with outcome measures that are positively skewed (eg, hospital LOS). It assumes that data are sampled from an exponential family of distributions, thus mimicking a log-normal distribution, and minimizes estimation bias and standard errors. These analyses were completed while controlling for the effects of age, gender, and other potential confounding factors.
We initially attempted to control for case mix by incorporating case-mix groups (CMGs) in our multivariate analysis. However, we identified 475 CMGs with at least 1 patient in our study population. We then explored the inclusion of major clinical categories (MCCs) that broadly group CMGs into various higher order/organ-system level categories (eg, diseases of the respiratory system); however, we could not aggregate them into sufficiently homogenous groups to be entered into regression models. Instead, we conducted subgroup analyses on patients in our study population who were hospitalized with 1 of the following 3 CMGs: chronic obstructive pulmonary disease (COPD, n = 11,404 patients), congestive heart failure without coronary angiography (CHF, n = 7680), and pneumonia (itself an aggregate of 3 separate CMGs: aspiration pneumonia, bacterial pneumonia, viral/unspecified pneumonia, n = 11,155). We chose these CMGs as they are among the top 8 presentations for all 3 provider groups.
For all outcome measures, we excluded atypical patients (defined by CIHI as those with atypically long stays) and patients who had been transferred between facilities. For the readmission analysis, we also excluded patients who died in the hospital (Appendix A). Data analyses were completed in IBM SPSS, version 21. For all analyses, significance was determined using 2-tailed test and alpha < 0.05.
Ethics
The Fraser Health Department of Research and Evaluation reviewed this project to determine need for formal Ethics Review Board review, and granted an exemption based on institutional guidelines for program evaluations.
Results
A total of 132,178 patients were admitted to and discharged by 1 of the 3 study provider groups during the study period, accounting for a total of 248,412 hospitalizations. After excluding patients cared for in Fraser Health facilities without a hospitalist service and those who resided in a geographic area beyond Fraser Health, a total of 224,214 admissions were included in the final analysis.
Patient Characteristics
The demographic and clinical characteristics of patients by provider group are summarized in Table 1. Patients admitted to IM providers were substantially younger than those admitted to either FPs or hospitalists (61.00 vs 70.86 and 71.22 years, respectively; P < 0.005). However, patients admitted to hospitalists had higher degrees of complexity (as measured by higher comorbidity levels, number of secondary diagnoses, and higher resource intensity weights [RIWs]; P < 000.1 for all comparisons). Overall, the most common CMGs seen by FPs and hospitalists were similar, while IM providers primarily saw patients with cardiac conditions (Table 2).
Trends Over Time
During the study period, the number of patients admitted to the hospitalist services increased by 24%, while admissions to FPs and IM providers declined steadily (Figure). During this time, LOS for hospitalists progressively declined, while LOS for FPs and IM providers increased. Similar trends were observed for measures of mortality, while readmission rates remained constant for FPs, despite a decline observed for other providers.
Mortality
Table 3 summarizes the relationship between provider groups and in-hospital mortality (n = 183,779). Controlling for other variables, patients admitted to FP and IM providers had higher odds of mortality when compared to hospitalists (odds ratio [OR] for FPs, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Older age, higher comorbidity level, higher number of secondary diagnoses, higher use of hospital resources (as measured by RIWs), longer than expected hospital stay (as measured by conservable days), and male gender were also associated with higher mortality. Similarly, patients receiving palliative care and those who spent at least 1 day in a special care unit (critical care, observation, and monitored care units) also had higher odds of mortality. On the other hand, admission to nonteaching medium facilities and longer hospital stay were associated with lower mortality. Compared to the first year of this analysis, lower mortality rates were observed in subsequent fiscal years. Finally, there appear to be geographic variations in mortality within Fraser Health.
Our analysis of patients with COPD, CHF, and pneumonia showed mixed results (Table 4). Patients admitted to the FP provider group with CHF and pneumonia had higher mortality compared to hospitalists (OR for CHF, 1.77; 95% CI, 1.38-2.27; OR for pneumonia, 1.53; 95% CI, 1.25-1.88), with a similar but nonstatistically significant trend observed for patients with COPD (OR, 1.29; 95% CI, 0.99-1.70). On the other hand, the higher observed mortality associated with the IM provider group in the overall study population only persisted for patients with COPD (OR, 2.71; 95% CI, 1.94-3.80), with no statistically significant differences for patients with CHF (OR, 1.18; 95% CI, 0.84-1.65) and pneumonia (OR, 0.93; 95% CI, 0.69-1.25).
We also studied adjusted mortality as measured by HSMRs. Currently, our Health Information Management system calculates an HSMR value for each patient admitted to our acute care facilities using the methodology developed by CIHI. Prior internal audits demonstrated that our internal calculations closely approximate those reported nationally. Our analysis suggests that over time, HSMR rates for the 3 provider groups have diverged, with patients admitted to IM providers having a higher mortality rate than what would be expected based on the presenting clinical conditions and comorbidity levels (Figure, part D).
Readmission
The results of our multiple logistic regression for readmission are summarized in Table 5 (n = 166,042). The impact of provider group on 30-day readmission is mixed, with higher odds associated with FPs compared to hospitalists (OR, 1.27; 95% CI, 1.22-1.34) and lower odds associated with IM physicians (OR, 0.83; 95% CI, 0.79-0.87). Gender and RIW did not show any significant associations, but increasing age, higher number of secondary diagnoses, higher comorbidity levels, and longer than expected LOS (as measure by conservable days) were associated with higher odds of readmission. Conversely, longer hospitalization, admission to a large community hospital, palliative status, admission to a special care unit, geography, and fiscal year were associated with lower odds of readmission.
The above differences between provider groups were no longer consistently present when we analyzed patients presenting with COPD, CHF, and pneumonias (Table 6). Only patients admitted to the FP provider group with pneumonia had higher odds of readmission compared to hospitalists (OR, 1.27; 95% CI, 1.05-1.54). Conversely, only patients admitted to the IM provider group with CHF showed lower readmission (OR, 0.75; 95% CI, 0.62-0.92).
Total LOS
Results using generalized linear regressions for total LOS are presented in Table 7 (n = 183,779). Patients admitted to the IM provider group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to the hospitalist (mean, 7.37 days; 95% CI, 7.26-7.49) and FP (mean, 7.30 days; 95% CI, 7.19-7.41) groups, with no significant differences between the latter 2 groups. Older patients, females, patients with higher comorbidity levels or number of secondary diagnoses, higher RIW, palliative patients, and discharge to a facility other than the patient’s home were associated with a significantly longer LOS. On the other hand, admission to nonteaching hospitals and admission to a special care unit was associated with lower LOS.
When we compared total LOS for patients admitted with COPD, CHF, and pneumonias, the same differences observed for the broader comparisons persisted: IM patients consistently showed shorter LOS compared to hospitalist patients, while LOS associated with FP patients was similar (Table 8).
Discussion
To our knowledge, our evaluation is the largest study to date designed to understand outcomes associated with hospitalist care in Canada. Our analyses suggest that patients admitted to our large network of hospitalist services present with clinical conditions that are very similar to those of general medicine patients in other Canadian provinces.28,29 They also show that patients cared for by hospitalists experience lower mortality rates compared to those cared for by FPs. Our findings are similar to previous studies, which have suggested a 12% to 75% reduction in odds of mortality associated with hospitalist care.18,19 These differences persisted even when we focused on patients presenting with specific clinical conditions (CHF, COPD, and pneumonias).
White and colleagues have previously demonstrated that generalist physicians who had higher volumes of inpatient care activity also had lower mortality rates compared to those who cared for hospitalized patients less frequently.19 An association between higher physician caseloads and better outcomes has been established for many surgical and medical conditions.30-32 Given that 85% of hospitalists in our program have post-graduate medical training in family medicine (internal department surveys, data not shown), it is less likely that training background can explain differences in outcomes. Instead, differences in patient volumes and the dedicated focus of hospitalists on acute care are likely more important contributors to lower mortality. In our program, a full-time hospitalist spends an average of 2000 hours annually providing services in the hospital setting. The continuous on-site presence of hospitalists enhances their clinical experience with regards to the management of common medical conditions, and increases their exposure to less common presentations of illnesses. The ability to respond to deteriorating patients in a timely manner may be another factor in explaining the differences in mortality rates between dedicated hospital-based generalist providers and similarly trained physicians with a primarily community-based focus.
In our study, hospitalist care was also broadly associated with lower mortality compared to the IM providers, although these differences were not consistently present when patients with specific diagnoses were compared. This may be partly explained by the relationship between caseload and outcomes, but other factors may also be important. For example, patients admitted by IM providers spend significantly more time in specialized units. They also predominantly present with cardiac conditions, and as such may have higher acuity levels and require more invasive interventions. While this may explain the higher observed mortality, a within-group comparison still suggests higher than expected mortality for IM patients. The HSMR methodology measures actual mortality rates compared to what would be expected based on clinical presentation and baseline population characteristics. Calculating HSMR is highly dependent on proper documentation and chart abstraction,33,34 and it is possible that some of the differences observed are due to incomplete physician documentation. However, a more in-depth analysis of care processes will be required to clarify the observed trends.
Compared to hospitalists, patients cared for by FPs also had higher odds of readmission within 30 days, which is consistent with prior studies.18,19 One of the criticisms of the hospitalist model has been the inherent discontinuity of care that is built into the model, which can contribute to suboptimal transitions of care between the acute and community settings.35 The expectation is that FPs who admit their own patients do not face this challenge, and as a result their patients should be readmitted less frequently after discharge. Our data and those from previous studies do not support this hypothesis. At the same time, when we studied patients with specific clinical diagnoses, only those hospitalized for pneumonias continued to demonstrate higher readmission odds. This suggests that hospital readmission rate is a complex measure that may be influenced by a multitude of hospital and community factors, and may be different for patients who present with different clinical diagnoses. Further research is required to better understand the relationship between provider type and experience with hospital readmission for patients with various clinical presentations.
Unlike the United States, where hospitalist care has been associated with reductions in LOS,26,36 studies in the Canadian health care setting have shown mixed results.17-21 In our evaluation, hospitalist care is not associated with reductions in total LOS compared to care provided by FPs or IM physicians. This could be due to a number of factors. First, unlike FPs, who know their patients, hospitalists may have a more conservative risk tolerance in discharging patients with whom they are not familiar. Similarly, physicians who have trained in IM may have a lower threshold for discharging patients than hospitalists, whose training background is mainly rooted in family medicine.3 Second, discontinuity of care has been associated with longer LOS for hospitalized patients.37,38 Hospitalists generally work for 7- to 10-day rotations. As a result, a patient may see a number of different hospitalists during the same hospital stay, which could nullify any gains in LOS that may be expected from better familiarity with hospital processes. Third, whereas a FP or an internist may only have a few inpatients under their care at any given time, each hospitalist typically cares for 17 to 22 patients every day. Increasing hospitalist workload has been shown to negatively impact LOS and may result in lower efficiency.39 Finally, many patients in our health system who require more time to recuperate or need complex discharge planning are usually transferred to the care of the hospitalist service from other services, or are preferentially admitted to hospitalists from the emergency department. As a result, hospitalists may look after a disproportionately higher number of long-stay patients. Despite all this, hospitalists in our population perform similarly to FPs, regardless of the clinical diagnoses of hospitalized patients.
Our study has a number of notable limitations. First, we used administrative data to conduct our evaluation and could only control for factors that are available in our data systems. As a result, some potential confounders may not have been taken into consideration. For example, our databases do not contain provider characteristics (eg, age, years of clinical experience) that have been deemed to be relevant by White and Glazier.26 Similarly, we did not have all the necessary information about the characteristics of the various MRP programs (eg, number of physicians involved in group practices, the schedule model of community FP call groups) and were not able to account for the potential impact of these on observed outcomes. Second, although our findings mirror prior studies from other parts of Canada, they may not be applicable to hospitalist programs in other jurisdictions or in health systems that are not regionalized or integrated. Third, our IM provider group is heterogeneous, with a number of different IM subspecialties (cardiologists, gastroenterologists, general internists) grouped under the IM category in our database. As a result, comparisons between the IM provider group and the other 2 provider groups, which are more homogenous, should be interpreted with caution.
Finally, we included only patients admitted to facilities in which a hospitalist service existed during the study period. As a result, a medium-size community hospital without a hospitalist service where patients are cared for exclusively by FPs and IM physicians was not included in the comparisons, and in 4 of the 10 facilities included, the number of FP patients was less than 10% of total hospitalized patients at the site (Appendix A). This may have resulted in an under-representation of FP patients.
Conclusion
Debates about the merits of the hospitalist model in Canada continue, and are in part fueled by a paucity of robust evidence about its impact on care outcomes compared to more traditional ways of providing inpatient care. In our evaluation, care provided by hospitalists is associated with lower mortality and readmission rates, despite similar LOS compared with FPs. Hospitalist care is also associated with lower mortality compared to IM providers. Hospitalists also demonstrated progressive improvement over time, with decreasing LOS and mortality rates and a stable readmission rate. Our results suggest that physicians with a focus on inpatient care can have positive contributions to quality and efficiency of care in Canada.
Corresponding author: Vandad Yousefi MD, CCFP, FHM, Fraser Health Authority, 400, 13450–102 Avenue, Surrey BC V3T 0H1, Canada.
Financial disclosures: None.
1. Kisuule F, Howell E. Hospital medicine beyond the United States. Int J Gen Med. 2018;11:65-71.
2. Yousefi V, Wilton D. Dedesigning hospital care: learning from the experience of hospital medicine in Canada. J Global Health Care Syst. 2011;1(3).
3. Soong C, Fan E, Howell E, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag. 2009;16:69-76.
4. Yousefi V. How Canadian hospitalists spend their time - A work-sampling study within a hospital medicine program in Ontario. J Clin Outcomes Manag. 2011;18:159-166.
5. Wilson G. Are inpatients’ needs better served by hospitalists than by their family doctors? No. Can Fam Physician. 2008;54:1101-1103.
6. Samoil D. Are inpatients’ needs better served by hospitalists than by their family doctors: Yes? Can Fam Physician. 2008;54:1100-1101.
7. Nicolson B. Where’s Marcus Welby when you need him? BC Medical J. 2016;58:63-64.
8. Lemire F. Enhanced skills in family medicine: Update. Can Fam Physician. 2018;64:160.
9. Lerner J. Wanting family medicine without primary care. Can Fam Physician. 2018; 64:155.
10. Canadian Society of Hospital Medicine. Core Competencies in Hospital Medicine - Care of the Medical Inpatient. 2015.
11. Redelmeier DA. A Canadian perspective on the American hospitalist movement. Arch Intern Med. 1999;159:1665-1668.
12. Ghali WA, Greenberg PB, Mejia R, et al. International perspectives on general internal medicine and the case for “globalization” of a discipline. J Gen Intern Med. 2006;21:197-200.
13. Day A, MacMillan L. Neglect of the inpatient: The hospitalist movement in Canada responds. Hosp Q. 2001;4:36.
14. Sullivan P. Enter the hospitalist: New type of patient creating a new type of specialist. CMAJ. 2000;162:1345-1346.
15. Chan BTB. The declining comprehensiveness of primary care. CMAJ. 2002;166:429-434.
16. Abenhaim HA, Kahn SR, Raffoul J, Becker MR. Program description: A hospitalist-run, medical short-stay unit in a teaching hospital. CMAJ. 2000;163:1477-1480.
17. McGowan B, Nightingale M. The hospitalist program a new specialty on the horizon in acute care medicine a hospital case study. BC Med J. 2003;45:391-394.
18. Yousefi V, Chong C. Does implementation of a hospitalist program in a Canadian community hospital improve measures of quality of care and utilization? An observational comparative analysis of hospitalists vs. traditional care providers. BMC Health Serv Res. 2013;13:204.
19. White HL. Assessing the prevalence, penetration and performance of hospital physicians in Ontario: Implications for the quality and efficiency of inpatient care. ProQuest Dissertations Publishing; 2016.
20. Gutierrez CA, Norris M, Chail M. Impact of a newly established hospitalist training program on patient LOS and RIW. Poster presented at the 9th Annual Canadian Society of Hospital Medicine Conference, September 23-25, 2011; Banff, Alberta.
21. Seth P, Nicholson K, Habbous S, Menard J. Implementation of a hospitalist medicine model in a full-service community hospital: Examining impact two years post-implementation on health resource use andpatient satisfaction. Poster presented at the 13th Annual Canadian Society of Hospital Medicine Conference. 2015; Niagara Falls, Ontario.
22. Lewis S. A system in name only--access, variation, and reform in Canada’s provinces. N Engl J Med. 2015;372:497-500.
23. Lewis S, Kouri D. Regionalization: Making sense of the Canadian experience. Healthcare Papers. 2004;5:12-31.
24. Fraser Health Authority. About Fraser health. www.fraserhealth.ca/about-us/about-fraser-health#.XFJrl9JKiUk. Updated 2018. Accessed January 30, 2019.
25. Canadian Institute for Health Information. CMG+. https://www.cihi.ca/en/cmg. Accessed January 30, 2019.
26. White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
27. Canadian Institute for Health Information. Hospital standardized mortality ratio technical notes. 2008. www.cihi.ca/sites/default/files/document/hsmr-tech-notes_en_0.pdf.
28. McAlister FA, Youngson E, Bakal JA, et al. Physician experience and outcomes among patients admitted to general internal medicine teaching wards. CMAJ. 2015;187:1041-1048.
29. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: The general medicine inpatient initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5:E849.
30. Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: A systematic review of systematic reviews. Syst Rev. 2016;5:204.
31. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511-520.
32. Chen CH, Chen YH, Lin HC, Lin HC. Association between physician caseload and patient outcome for sepsis treatment. Infect Control Hosp Epidemiol. 2009;30:556-562.
33. van Gestel YR, Lemmens VE, Lingsma HF, et al. The hospital standardized mortality ratio fallacy: A narrative review. Med Care. 2012;50:662-667.
34. Scott IA, Brand CA, Phelps GE, et al. Using hospital standardised mortality ratios to assess quality of care—proceed with extreme caution. Med J Aust. 2011; 194:645-648.
35. Wachter RM. Hospitalists in the United States -- mission accomplished or work in progress? N Engl J Med. 2004;350:1935-1936.
36. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84:248-254.
37. Chandra S, Wright SM, Howell EE. The creating incentives and continuity leading to efficiency staffing model: A quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87:364-371.
38. Epstein K, Juarez E, Epstein A, et al. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335-338.
39. Elliott DJ, Young RS, Brice J, et al. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174:786-793.
From the Fraser Health Authority, Surrey, British Columbia, Canada.
Abstract
- Objective: To study care outcomes associated with a network of hospitalist services compared to traditional providers.
- Design: Retrospective review of administrative data.
- Setting and participants: Patients from a large integrated health care system in British Columbia in western Canada admitted and cared for by 3 provider groups between April 1, 2012, and March 31, 2018: hospitalists, family physicians (FP), and internal medicine (IM) physicians:
- Measurements: Average total length of stay (LOS), 30-day readmission, in-hospital mortality, and hospital standardized mortality ratio (HSMR) were the study outcome measures. Multiple logistic regression or generalized regression were completed to determine the relationship between provider groups and outcomes.
- Results: A total of 248,412 hospitalizations were included. Compared to patients admitted to hospitalists, patients admitted to other providers had higher odds of mortality (odds ratio [OR] for FP, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Compared to hospitalist care, FP care was associated with higher readmission (OR, 1.27; 95% CI, 1.22-1.33), while IM care showed lower odds of readmission (OR, 0.83; 95% CI, 0.79-0.87). Patients admitted to the IM group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to patients admitted to hospitalists (mean, 7.37 days; CI, 7.26-7.49) and FPs (mean, 7.30 days; 95% CI, 7.19-7.41). In a subgroup analysis of patients presenting with congestive heart failure, chronic obstructive pulmonary disease, and pneumonia, these general tendencies broadly persisted for mortality and LOS comparisons between FPs and hospitalists, but results were mixed for hospital readmissions.
- Conclusion: Care provided by hospitalists was associated with lower mortality and readmission rates compared with care provided by FPs, despite similar LOS. These findings may reflect differences in volume of services delivered by individual physicians, on-site availability to address urgent medical issues, and evolving specialization of clinical and nonclinical care processes in the acute care setting.
Keywords: hospital medicine; length of stay; readmission; mortality.
The hospitalist model of care has undergone rapid growth globally in recent years.1 The first hospitalist programs in Canada began around the same time as those in the United States and share many similarities in design and operations with their counterparts.2-4 However, unlike in the United States, where the hospitalist model has successfully established itself as an emerging specialty, debates about the merits of the model and its value proposition continue among Canadian observers.5-9
Historically, the type of physicians who acted as the most responsible provider (MRP) in Canadian hospitals depended on setting and geography.10 In large urban areas, groups of general internists or specialists have historically looked after general medicine patients as part of university-affiliated teaching services.11,12 Patients admitted to community hospitals have traditionally been cared for by their own primary care providers, typically general practitioners or family physicians (FPs). In the mid-1990s, many primary care providers in urban centers began to withdraw from inpatient care and primarily focused their practices in the outpatient setting.13-15 Hospitalist programs emerged as health care administrators sought to fill the resulting gap in MRP coverage.2,10
To date, attempts to understand the impact of hospitalist programs in Canada have been limited. A number of early studies aimed to describe16 the role of hospitalists in Canada and suggested improvements in length of stay (LOS) and staff satisfaction.17 However, these studies relied on unadjusted before-after comparisons and lacked methodological rigor to draw robust conclusions. More recently, a few studies have evaluated care outcomes associated with hospitalists using administrative databases, which attempted to control for potential confounding factors.18-21
While these studies are beginning to shed some light on the impact of hospital medicine programs in Canada, there are a number of issues that limit their generalizability. For example, the majority of studies to date focus on hospital medicine programs in Canada’s largest province (Ontario), and most describe experiences from single institutions. Since each of the 13 provincial and territorial governments organizes its health care system differently,22 results from 1 province may not be generalizable to other parts of the country. Moreover, hospitalists in Ontario are more diverse in their training backgrounds, with a larger percentage having trained in general internal medicine (IM), as compared to other parts of Canada, where the majority of hospitalists are overwhelmingly trained as FPs.3
We aimed to study care outcomes associated with a network of hospitalist services compared to “traditional” providers (community-based FPs and IM specialists) in a large integrated health care system in the province of British Columbia in western Canada. The hospital medicine services in this network span a range of community and academic hospitals, and collectively constitute 1 of the largest regional programs in the country. This provides a unique opportunity to understand the impact of hospitalists on outcome measures across a range of acute care institutions.
Methods
Setting and Population
Fraser Health Authority is 1 of 5 regional health authorities in British Columbia that emerged in 2001.23,24 It operates a network of hospitalist programs in 10 of its 12 acute care hospitals. In addition to hospitalists, there are a variable number of “traditional” physician providers who continue to act as MRPs. These include community-based FPs who continue to see their own patients in the hospital, either as part of a solo-practice model or a clinic-based call group. There are also a number of general internists and other subspecialists who accept MRP roles for general medicine patients who may present with higher-acuity conditions. As a result, patients requiring hospitalization due to nonsurgical or noncritical care conditions at each Fraser Health hospital may be cared for by a physician belonging to 1 of 3 groups, depending on local circumstances: an FP, a hospitalist, or an internist.
Inclusion and Exclusion Criteria
In order to evaluate comparative outcomes associated with hospitalist care, we included all patients admitted to a physician in each of the 3 provider groups between April 1, 2012, and March 31, 2018. We chose this time period for 2 reasons: first, we wanted to ensure comparability over an extended period of time, given the methodological changes implemented in 2009 by the Canadian Institute for Health Information (CIHI), the federal organization in the country responsible for setting standards for health care measures.25 Second, previous internal reviews had suggested that data quality prior to this year was inconsistent. We only considered hospitalizations where patients were admitted to and discharged by the same service, and excluded 2 acute care facilities and 1 free-standing rehabilitation facility without a hospitalist service during this period. We also excluded patients who resided in a location beyond the geographic catchment area of Fraser Health. Further details about data collection are outlined in the Appendix.
Measures
We used the framework developed by White and Glazier26 to inform the selection of our outcome measures, as well as relevant variables that may impact them. This framework proposes that the design of the inpatient care model (structures and processes of care) directly affects care outcomes. The model also proposes that patient and provider attributes can modulate this relationship, and suggests that a comprehensive evaluation of hospitalist performance needs to take these factors into account. We identified average total LOS, 30-day readmission rate, in-hospital mortality, and hospital standardized mortality ratio (HSMR)27 as primary outcome measures. HSMR is defined as actual over expected mortality and is measured by CIHI through a formula that takes into account patient illness attributes (eg, the most responsible diagnosis, comorbidity levels) and baseline population mortality rates.27 We chose these measures because they are clinically relevant and easy to obtain and have been utilized in previous similar studies in Canada and the United States.18-21,26
Statistical Analysis
Baseline demographic and clinical differences in patient outcomes were examined using independent t-tests or chi-square tests. Furthermore, baseline differences based on provider groups were explored using analysis of variance or chi-square tests. Multiple logistic regression analyses were completed to determine the relationship between provider groups and readmission and mortality, while the relationship between provider groups and hospital LOS was determined with generalized linear regression (using gamma distribution and a log link). Gamma distribution with a log link analysis is appropriate with outcome measures that are positively skewed (eg, hospital LOS). It assumes that data are sampled from an exponential family of distributions, thus mimicking a log-normal distribution, and minimizes estimation bias and standard errors. These analyses were completed while controlling for the effects of age, gender, and other potential confounding factors.
We initially attempted to control for case mix by incorporating case-mix groups (CMGs) in our multivariate analysis. However, we identified 475 CMGs with at least 1 patient in our study population. We then explored the inclusion of major clinical categories (MCCs) that broadly group CMGs into various higher order/organ-system level categories (eg, diseases of the respiratory system); however, we could not aggregate them into sufficiently homogenous groups to be entered into regression models. Instead, we conducted subgroup analyses on patients in our study population who were hospitalized with 1 of the following 3 CMGs: chronic obstructive pulmonary disease (COPD, n = 11,404 patients), congestive heart failure without coronary angiography (CHF, n = 7680), and pneumonia (itself an aggregate of 3 separate CMGs: aspiration pneumonia, bacterial pneumonia, viral/unspecified pneumonia, n = 11,155). We chose these CMGs as they are among the top 8 presentations for all 3 provider groups.
For all outcome measures, we excluded atypical patients (defined by CIHI as those with atypically long stays) and patients who had been transferred between facilities. For the readmission analysis, we also excluded patients who died in the hospital (Appendix A). Data analyses were completed in IBM SPSS, version 21. For all analyses, significance was determined using 2-tailed test and alpha < 0.05.
Ethics
The Fraser Health Department of Research and Evaluation reviewed this project to determine need for formal Ethics Review Board review, and granted an exemption based on institutional guidelines for program evaluations.
Results
A total of 132,178 patients were admitted to and discharged by 1 of the 3 study provider groups during the study period, accounting for a total of 248,412 hospitalizations. After excluding patients cared for in Fraser Health facilities without a hospitalist service and those who resided in a geographic area beyond Fraser Health, a total of 224,214 admissions were included in the final analysis.
Patient Characteristics
The demographic and clinical characteristics of patients by provider group are summarized in Table 1. Patients admitted to IM providers were substantially younger than those admitted to either FPs or hospitalists (61.00 vs 70.86 and 71.22 years, respectively; P < 0.005). However, patients admitted to hospitalists had higher degrees of complexity (as measured by higher comorbidity levels, number of secondary diagnoses, and higher resource intensity weights [RIWs]; P < 000.1 for all comparisons). Overall, the most common CMGs seen by FPs and hospitalists were similar, while IM providers primarily saw patients with cardiac conditions (Table 2).
Trends Over Time
During the study period, the number of patients admitted to the hospitalist services increased by 24%, while admissions to FPs and IM providers declined steadily (Figure). During this time, LOS for hospitalists progressively declined, while LOS for FPs and IM providers increased. Similar trends were observed for measures of mortality, while readmission rates remained constant for FPs, despite a decline observed for other providers.
Mortality
Table 3 summarizes the relationship between provider groups and in-hospital mortality (n = 183,779). Controlling for other variables, patients admitted to FP and IM providers had higher odds of mortality when compared to hospitalists (odds ratio [OR] for FPs, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Older age, higher comorbidity level, higher number of secondary diagnoses, higher use of hospital resources (as measured by RIWs), longer than expected hospital stay (as measured by conservable days), and male gender were also associated with higher mortality. Similarly, patients receiving palliative care and those who spent at least 1 day in a special care unit (critical care, observation, and monitored care units) also had higher odds of mortality. On the other hand, admission to nonteaching medium facilities and longer hospital stay were associated with lower mortality. Compared to the first year of this analysis, lower mortality rates were observed in subsequent fiscal years. Finally, there appear to be geographic variations in mortality within Fraser Health.
Our analysis of patients with COPD, CHF, and pneumonia showed mixed results (Table 4). Patients admitted to the FP provider group with CHF and pneumonia had higher mortality compared to hospitalists (OR for CHF, 1.77; 95% CI, 1.38-2.27; OR for pneumonia, 1.53; 95% CI, 1.25-1.88), with a similar but nonstatistically significant trend observed for patients with COPD (OR, 1.29; 95% CI, 0.99-1.70). On the other hand, the higher observed mortality associated with the IM provider group in the overall study population only persisted for patients with COPD (OR, 2.71; 95% CI, 1.94-3.80), with no statistically significant differences for patients with CHF (OR, 1.18; 95% CI, 0.84-1.65) and pneumonia (OR, 0.93; 95% CI, 0.69-1.25).
We also studied adjusted mortality as measured by HSMRs. Currently, our Health Information Management system calculates an HSMR value for each patient admitted to our acute care facilities using the methodology developed by CIHI. Prior internal audits demonstrated that our internal calculations closely approximate those reported nationally. Our analysis suggests that over time, HSMR rates for the 3 provider groups have diverged, with patients admitted to IM providers having a higher mortality rate than what would be expected based on the presenting clinical conditions and comorbidity levels (Figure, part D).
Readmission
The results of our multiple logistic regression for readmission are summarized in Table 5 (n = 166,042). The impact of provider group on 30-day readmission is mixed, with higher odds associated with FPs compared to hospitalists (OR, 1.27; 95% CI, 1.22-1.34) and lower odds associated with IM physicians (OR, 0.83; 95% CI, 0.79-0.87). Gender and RIW did not show any significant associations, but increasing age, higher number of secondary diagnoses, higher comorbidity levels, and longer than expected LOS (as measure by conservable days) were associated with higher odds of readmission. Conversely, longer hospitalization, admission to a large community hospital, palliative status, admission to a special care unit, geography, and fiscal year were associated with lower odds of readmission.
The above differences between provider groups were no longer consistently present when we analyzed patients presenting with COPD, CHF, and pneumonias (Table 6). Only patients admitted to the FP provider group with pneumonia had higher odds of readmission compared to hospitalists (OR, 1.27; 95% CI, 1.05-1.54). Conversely, only patients admitted to the IM provider group with CHF showed lower readmission (OR, 0.75; 95% CI, 0.62-0.92).
Total LOS
Results using generalized linear regressions for total LOS are presented in Table 7 (n = 183,779). Patients admitted to the IM provider group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to the hospitalist (mean, 7.37 days; 95% CI, 7.26-7.49) and FP (mean, 7.30 days; 95% CI, 7.19-7.41) groups, with no significant differences between the latter 2 groups. Older patients, females, patients with higher comorbidity levels or number of secondary diagnoses, higher RIW, palliative patients, and discharge to a facility other than the patient’s home were associated with a significantly longer LOS. On the other hand, admission to nonteaching hospitals and admission to a special care unit was associated with lower LOS.
When we compared total LOS for patients admitted with COPD, CHF, and pneumonias, the same differences observed for the broader comparisons persisted: IM patients consistently showed shorter LOS compared to hospitalist patients, while LOS associated with FP patients was similar (Table 8).
Discussion
To our knowledge, our evaluation is the largest study to date designed to understand outcomes associated with hospitalist care in Canada. Our analyses suggest that patients admitted to our large network of hospitalist services present with clinical conditions that are very similar to those of general medicine patients in other Canadian provinces.28,29 They also show that patients cared for by hospitalists experience lower mortality rates compared to those cared for by FPs. Our findings are similar to previous studies, which have suggested a 12% to 75% reduction in odds of mortality associated with hospitalist care.18,19 These differences persisted even when we focused on patients presenting with specific clinical conditions (CHF, COPD, and pneumonias).
White and colleagues have previously demonstrated that generalist physicians who had higher volumes of inpatient care activity also had lower mortality rates compared to those who cared for hospitalized patients less frequently.19 An association between higher physician caseloads and better outcomes has been established for many surgical and medical conditions.30-32 Given that 85% of hospitalists in our program have post-graduate medical training in family medicine (internal department surveys, data not shown), it is less likely that training background can explain differences in outcomes. Instead, differences in patient volumes and the dedicated focus of hospitalists on acute care are likely more important contributors to lower mortality. In our program, a full-time hospitalist spends an average of 2000 hours annually providing services in the hospital setting. The continuous on-site presence of hospitalists enhances their clinical experience with regards to the management of common medical conditions, and increases their exposure to less common presentations of illnesses. The ability to respond to deteriorating patients in a timely manner may be another factor in explaining the differences in mortality rates between dedicated hospital-based generalist providers and similarly trained physicians with a primarily community-based focus.
In our study, hospitalist care was also broadly associated with lower mortality compared to the IM providers, although these differences were not consistently present when patients with specific diagnoses were compared. This may be partly explained by the relationship between caseload and outcomes, but other factors may also be important. For example, patients admitted by IM providers spend significantly more time in specialized units. They also predominantly present with cardiac conditions, and as such may have higher acuity levels and require more invasive interventions. While this may explain the higher observed mortality, a within-group comparison still suggests higher than expected mortality for IM patients. The HSMR methodology measures actual mortality rates compared to what would be expected based on clinical presentation and baseline population characteristics. Calculating HSMR is highly dependent on proper documentation and chart abstraction,33,34 and it is possible that some of the differences observed are due to incomplete physician documentation. However, a more in-depth analysis of care processes will be required to clarify the observed trends.
Compared to hospitalists, patients cared for by FPs also had higher odds of readmission within 30 days, which is consistent with prior studies.18,19 One of the criticisms of the hospitalist model has been the inherent discontinuity of care that is built into the model, which can contribute to suboptimal transitions of care between the acute and community settings.35 The expectation is that FPs who admit their own patients do not face this challenge, and as a result their patients should be readmitted less frequently after discharge. Our data and those from previous studies do not support this hypothesis. At the same time, when we studied patients with specific clinical diagnoses, only those hospitalized for pneumonias continued to demonstrate higher readmission odds. This suggests that hospital readmission rate is a complex measure that may be influenced by a multitude of hospital and community factors, and may be different for patients who present with different clinical diagnoses. Further research is required to better understand the relationship between provider type and experience with hospital readmission for patients with various clinical presentations.
Unlike the United States, where hospitalist care has been associated with reductions in LOS,26,36 studies in the Canadian health care setting have shown mixed results.17-21 In our evaluation, hospitalist care is not associated with reductions in total LOS compared to care provided by FPs or IM physicians. This could be due to a number of factors. First, unlike FPs, who know their patients, hospitalists may have a more conservative risk tolerance in discharging patients with whom they are not familiar. Similarly, physicians who have trained in IM may have a lower threshold for discharging patients than hospitalists, whose training background is mainly rooted in family medicine.3 Second, discontinuity of care has been associated with longer LOS for hospitalized patients.37,38 Hospitalists generally work for 7- to 10-day rotations. As a result, a patient may see a number of different hospitalists during the same hospital stay, which could nullify any gains in LOS that may be expected from better familiarity with hospital processes. Third, whereas a FP or an internist may only have a few inpatients under their care at any given time, each hospitalist typically cares for 17 to 22 patients every day. Increasing hospitalist workload has been shown to negatively impact LOS and may result in lower efficiency.39 Finally, many patients in our health system who require more time to recuperate or need complex discharge planning are usually transferred to the care of the hospitalist service from other services, or are preferentially admitted to hospitalists from the emergency department. As a result, hospitalists may look after a disproportionately higher number of long-stay patients. Despite all this, hospitalists in our population perform similarly to FPs, regardless of the clinical diagnoses of hospitalized patients.
Our study has a number of notable limitations. First, we used administrative data to conduct our evaluation and could only control for factors that are available in our data systems. As a result, some potential confounders may not have been taken into consideration. For example, our databases do not contain provider characteristics (eg, age, years of clinical experience) that have been deemed to be relevant by White and Glazier.26 Similarly, we did not have all the necessary information about the characteristics of the various MRP programs (eg, number of physicians involved in group practices, the schedule model of community FP call groups) and were not able to account for the potential impact of these on observed outcomes. Second, although our findings mirror prior studies from other parts of Canada, they may not be applicable to hospitalist programs in other jurisdictions or in health systems that are not regionalized or integrated. Third, our IM provider group is heterogeneous, with a number of different IM subspecialties (cardiologists, gastroenterologists, general internists) grouped under the IM category in our database. As a result, comparisons between the IM provider group and the other 2 provider groups, which are more homogenous, should be interpreted with caution.
Finally, we included only patients admitted to facilities in which a hospitalist service existed during the study period. As a result, a medium-size community hospital without a hospitalist service where patients are cared for exclusively by FPs and IM physicians was not included in the comparisons, and in 4 of the 10 facilities included, the number of FP patients was less than 10% of total hospitalized patients at the site (Appendix A). This may have resulted in an under-representation of FP patients.
Conclusion
Debates about the merits of the hospitalist model in Canada continue, and are in part fueled by a paucity of robust evidence about its impact on care outcomes compared to more traditional ways of providing inpatient care. In our evaluation, care provided by hospitalists is associated with lower mortality and readmission rates, despite similar LOS compared with FPs. Hospitalist care is also associated with lower mortality compared to IM providers. Hospitalists also demonstrated progressive improvement over time, with decreasing LOS and mortality rates and a stable readmission rate. Our results suggest that physicians with a focus on inpatient care can have positive contributions to quality and efficiency of care in Canada.
Corresponding author: Vandad Yousefi MD, CCFP, FHM, Fraser Health Authority, 400, 13450–102 Avenue, Surrey BC V3T 0H1, Canada.
Financial disclosures: None.
From the Fraser Health Authority, Surrey, British Columbia, Canada.
Abstract
- Objective: To study care outcomes associated with a network of hospitalist services compared to traditional providers.
- Design: Retrospective review of administrative data.
- Setting and participants: Patients from a large integrated health care system in British Columbia in western Canada admitted and cared for by 3 provider groups between April 1, 2012, and March 31, 2018: hospitalists, family physicians (FP), and internal medicine (IM) physicians:
- Measurements: Average total length of stay (LOS), 30-day readmission, in-hospital mortality, and hospital standardized mortality ratio (HSMR) were the study outcome measures. Multiple logistic regression or generalized regression were completed to determine the relationship between provider groups and outcomes.
- Results: A total of 248,412 hospitalizations were included. Compared to patients admitted to hospitalists, patients admitted to other providers had higher odds of mortality (odds ratio [OR] for FP, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Compared to hospitalist care, FP care was associated with higher readmission (OR, 1.27; 95% CI, 1.22-1.33), while IM care showed lower odds of readmission (OR, 0.83; 95% CI, 0.79-0.87). Patients admitted to the IM group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to patients admitted to hospitalists (mean, 7.37 days; CI, 7.26-7.49) and FPs (mean, 7.30 days; 95% CI, 7.19-7.41). In a subgroup analysis of patients presenting with congestive heart failure, chronic obstructive pulmonary disease, and pneumonia, these general tendencies broadly persisted for mortality and LOS comparisons between FPs and hospitalists, but results were mixed for hospital readmissions.
- Conclusion: Care provided by hospitalists was associated with lower mortality and readmission rates compared with care provided by FPs, despite similar LOS. These findings may reflect differences in volume of services delivered by individual physicians, on-site availability to address urgent medical issues, and evolving specialization of clinical and nonclinical care processes in the acute care setting.
Keywords: hospital medicine; length of stay; readmission; mortality.
The hospitalist model of care has undergone rapid growth globally in recent years.1 The first hospitalist programs in Canada began around the same time as those in the United States and share many similarities in design and operations with their counterparts.2-4 However, unlike in the United States, where the hospitalist model has successfully established itself as an emerging specialty, debates about the merits of the model and its value proposition continue among Canadian observers.5-9
Historically, the type of physicians who acted as the most responsible provider (MRP) in Canadian hospitals depended on setting and geography.10 In large urban areas, groups of general internists or specialists have historically looked after general medicine patients as part of university-affiliated teaching services.11,12 Patients admitted to community hospitals have traditionally been cared for by their own primary care providers, typically general practitioners or family physicians (FPs). In the mid-1990s, many primary care providers in urban centers began to withdraw from inpatient care and primarily focused their practices in the outpatient setting.13-15 Hospitalist programs emerged as health care administrators sought to fill the resulting gap in MRP coverage.2,10
To date, attempts to understand the impact of hospitalist programs in Canada have been limited. A number of early studies aimed to describe16 the role of hospitalists in Canada and suggested improvements in length of stay (LOS) and staff satisfaction.17 However, these studies relied on unadjusted before-after comparisons and lacked methodological rigor to draw robust conclusions. More recently, a few studies have evaluated care outcomes associated with hospitalists using administrative databases, which attempted to control for potential confounding factors.18-21
While these studies are beginning to shed some light on the impact of hospital medicine programs in Canada, there are a number of issues that limit their generalizability. For example, the majority of studies to date focus on hospital medicine programs in Canada’s largest province (Ontario), and most describe experiences from single institutions. Since each of the 13 provincial and territorial governments organizes its health care system differently,22 results from 1 province may not be generalizable to other parts of the country. Moreover, hospitalists in Ontario are more diverse in their training backgrounds, with a larger percentage having trained in general internal medicine (IM), as compared to other parts of Canada, where the majority of hospitalists are overwhelmingly trained as FPs.3
We aimed to study care outcomes associated with a network of hospitalist services compared to “traditional” providers (community-based FPs and IM specialists) in a large integrated health care system in the province of British Columbia in western Canada. The hospital medicine services in this network span a range of community and academic hospitals, and collectively constitute 1 of the largest regional programs in the country. This provides a unique opportunity to understand the impact of hospitalists on outcome measures across a range of acute care institutions.
Methods
Setting and Population
Fraser Health Authority is 1 of 5 regional health authorities in British Columbia that emerged in 2001.23,24 It operates a network of hospitalist programs in 10 of its 12 acute care hospitals. In addition to hospitalists, there are a variable number of “traditional” physician providers who continue to act as MRPs. These include community-based FPs who continue to see their own patients in the hospital, either as part of a solo-practice model or a clinic-based call group. There are also a number of general internists and other subspecialists who accept MRP roles for general medicine patients who may present with higher-acuity conditions. As a result, patients requiring hospitalization due to nonsurgical or noncritical care conditions at each Fraser Health hospital may be cared for by a physician belonging to 1 of 3 groups, depending on local circumstances: an FP, a hospitalist, or an internist.
Inclusion and Exclusion Criteria
In order to evaluate comparative outcomes associated with hospitalist care, we included all patients admitted to a physician in each of the 3 provider groups between April 1, 2012, and March 31, 2018. We chose this time period for 2 reasons: first, we wanted to ensure comparability over an extended period of time, given the methodological changes implemented in 2009 by the Canadian Institute for Health Information (CIHI), the federal organization in the country responsible for setting standards for health care measures.25 Second, previous internal reviews had suggested that data quality prior to this year was inconsistent. We only considered hospitalizations where patients were admitted to and discharged by the same service, and excluded 2 acute care facilities and 1 free-standing rehabilitation facility without a hospitalist service during this period. We also excluded patients who resided in a location beyond the geographic catchment area of Fraser Health. Further details about data collection are outlined in the Appendix.
Measures
We used the framework developed by White and Glazier26 to inform the selection of our outcome measures, as well as relevant variables that may impact them. This framework proposes that the design of the inpatient care model (structures and processes of care) directly affects care outcomes. The model also proposes that patient and provider attributes can modulate this relationship, and suggests that a comprehensive evaluation of hospitalist performance needs to take these factors into account. We identified average total LOS, 30-day readmission rate, in-hospital mortality, and hospital standardized mortality ratio (HSMR)27 as primary outcome measures. HSMR is defined as actual over expected mortality and is measured by CIHI through a formula that takes into account patient illness attributes (eg, the most responsible diagnosis, comorbidity levels) and baseline population mortality rates.27 We chose these measures because they are clinically relevant and easy to obtain and have been utilized in previous similar studies in Canada and the United States.18-21,26
Statistical Analysis
Baseline demographic and clinical differences in patient outcomes were examined using independent t-tests or chi-square tests. Furthermore, baseline differences based on provider groups were explored using analysis of variance or chi-square tests. Multiple logistic regression analyses were completed to determine the relationship between provider groups and readmission and mortality, while the relationship between provider groups and hospital LOS was determined with generalized linear regression (using gamma distribution and a log link). Gamma distribution with a log link analysis is appropriate with outcome measures that are positively skewed (eg, hospital LOS). It assumes that data are sampled from an exponential family of distributions, thus mimicking a log-normal distribution, and minimizes estimation bias and standard errors. These analyses were completed while controlling for the effects of age, gender, and other potential confounding factors.
We initially attempted to control for case mix by incorporating case-mix groups (CMGs) in our multivariate analysis. However, we identified 475 CMGs with at least 1 patient in our study population. We then explored the inclusion of major clinical categories (MCCs) that broadly group CMGs into various higher order/organ-system level categories (eg, diseases of the respiratory system); however, we could not aggregate them into sufficiently homogenous groups to be entered into regression models. Instead, we conducted subgroup analyses on patients in our study population who were hospitalized with 1 of the following 3 CMGs: chronic obstructive pulmonary disease (COPD, n = 11,404 patients), congestive heart failure without coronary angiography (CHF, n = 7680), and pneumonia (itself an aggregate of 3 separate CMGs: aspiration pneumonia, bacterial pneumonia, viral/unspecified pneumonia, n = 11,155). We chose these CMGs as they are among the top 8 presentations for all 3 provider groups.
For all outcome measures, we excluded atypical patients (defined by CIHI as those with atypically long stays) and patients who had been transferred between facilities. For the readmission analysis, we also excluded patients who died in the hospital (Appendix A). Data analyses were completed in IBM SPSS, version 21. For all analyses, significance was determined using 2-tailed test and alpha < 0.05.
Ethics
The Fraser Health Department of Research and Evaluation reviewed this project to determine need for formal Ethics Review Board review, and granted an exemption based on institutional guidelines for program evaluations.
Results
A total of 132,178 patients were admitted to and discharged by 1 of the 3 study provider groups during the study period, accounting for a total of 248,412 hospitalizations. After excluding patients cared for in Fraser Health facilities without a hospitalist service and those who resided in a geographic area beyond Fraser Health, a total of 224,214 admissions were included in the final analysis.
Patient Characteristics
The demographic and clinical characteristics of patients by provider group are summarized in Table 1. Patients admitted to IM providers were substantially younger than those admitted to either FPs or hospitalists (61.00 vs 70.86 and 71.22 years, respectively; P < 0.005). However, patients admitted to hospitalists had higher degrees of complexity (as measured by higher comorbidity levels, number of secondary diagnoses, and higher resource intensity weights [RIWs]; P < 000.1 for all comparisons). Overall, the most common CMGs seen by FPs and hospitalists were similar, while IM providers primarily saw patients with cardiac conditions (Table 2).
Trends Over Time
During the study period, the number of patients admitted to the hospitalist services increased by 24%, while admissions to FPs and IM providers declined steadily (Figure). During this time, LOS for hospitalists progressively declined, while LOS for FPs and IM providers increased. Similar trends were observed for measures of mortality, while readmission rates remained constant for FPs, despite a decline observed for other providers.
Mortality
Table 3 summarizes the relationship between provider groups and in-hospital mortality (n = 183,779). Controlling for other variables, patients admitted to FP and IM providers had higher odds of mortality when compared to hospitalists (odds ratio [OR] for FPs, 1.29; 95% confidence interval [CI], 1.21-1.37; OR for IM, 1.24; 95% CI, 1.15-1.33). Older age, higher comorbidity level, higher number of secondary diagnoses, higher use of hospital resources (as measured by RIWs), longer than expected hospital stay (as measured by conservable days), and male gender were also associated with higher mortality. Similarly, patients receiving palliative care and those who spent at least 1 day in a special care unit (critical care, observation, and monitored care units) also had higher odds of mortality. On the other hand, admission to nonteaching medium facilities and longer hospital stay were associated with lower mortality. Compared to the first year of this analysis, lower mortality rates were observed in subsequent fiscal years. Finally, there appear to be geographic variations in mortality within Fraser Health.
Our analysis of patients with COPD, CHF, and pneumonia showed mixed results (Table 4). Patients admitted to the FP provider group with CHF and pneumonia had higher mortality compared to hospitalists (OR for CHF, 1.77; 95% CI, 1.38-2.27; OR for pneumonia, 1.53; 95% CI, 1.25-1.88), with a similar but nonstatistically significant trend observed for patients with COPD (OR, 1.29; 95% CI, 0.99-1.70). On the other hand, the higher observed mortality associated with the IM provider group in the overall study population only persisted for patients with COPD (OR, 2.71; 95% CI, 1.94-3.80), with no statistically significant differences for patients with CHF (OR, 1.18; 95% CI, 0.84-1.65) and pneumonia (OR, 0.93; 95% CI, 0.69-1.25).
We also studied adjusted mortality as measured by HSMRs. Currently, our Health Information Management system calculates an HSMR value for each patient admitted to our acute care facilities using the methodology developed by CIHI. Prior internal audits demonstrated that our internal calculations closely approximate those reported nationally. Our analysis suggests that over time, HSMR rates for the 3 provider groups have diverged, with patients admitted to IM providers having a higher mortality rate than what would be expected based on the presenting clinical conditions and comorbidity levels (Figure, part D).
Readmission
The results of our multiple logistic regression for readmission are summarized in Table 5 (n = 166,042). The impact of provider group on 30-day readmission is mixed, with higher odds associated with FPs compared to hospitalists (OR, 1.27; 95% CI, 1.22-1.34) and lower odds associated with IM physicians (OR, 0.83; 95% CI, 0.79-0.87). Gender and RIW did not show any significant associations, but increasing age, higher number of secondary diagnoses, higher comorbidity levels, and longer than expected LOS (as measure by conservable days) were associated with higher odds of readmission. Conversely, longer hospitalization, admission to a large community hospital, palliative status, admission to a special care unit, geography, and fiscal year were associated with lower odds of readmission.
The above differences between provider groups were no longer consistently present when we analyzed patients presenting with COPD, CHF, and pneumonias (Table 6). Only patients admitted to the FP provider group with pneumonia had higher odds of readmission compared to hospitalists (OR, 1.27; 95% CI, 1.05-1.54). Conversely, only patients admitted to the IM provider group with CHF showed lower readmission (OR, 0.75; 95% CI, 0.62-0.92).
Total LOS
Results using generalized linear regressions for total LOS are presented in Table 7 (n = 183,779). Patients admitted to the IM provider group had significantly lower total LOS (mean, 5.13 days; 95% CI, 5.04-5.21) compared to the hospitalist (mean, 7.37 days; 95% CI, 7.26-7.49) and FP (mean, 7.30 days; 95% CI, 7.19-7.41) groups, with no significant differences between the latter 2 groups. Older patients, females, patients with higher comorbidity levels or number of secondary diagnoses, higher RIW, palliative patients, and discharge to a facility other than the patient’s home were associated with a significantly longer LOS. On the other hand, admission to nonteaching hospitals and admission to a special care unit was associated with lower LOS.
When we compared total LOS for patients admitted with COPD, CHF, and pneumonias, the same differences observed for the broader comparisons persisted: IM patients consistently showed shorter LOS compared to hospitalist patients, while LOS associated with FP patients was similar (Table 8).
Discussion
To our knowledge, our evaluation is the largest study to date designed to understand outcomes associated with hospitalist care in Canada. Our analyses suggest that patients admitted to our large network of hospitalist services present with clinical conditions that are very similar to those of general medicine patients in other Canadian provinces.28,29 They also show that patients cared for by hospitalists experience lower mortality rates compared to those cared for by FPs. Our findings are similar to previous studies, which have suggested a 12% to 75% reduction in odds of mortality associated with hospitalist care.18,19 These differences persisted even when we focused on patients presenting with specific clinical conditions (CHF, COPD, and pneumonias).
White and colleagues have previously demonstrated that generalist physicians who had higher volumes of inpatient care activity also had lower mortality rates compared to those who cared for hospitalized patients less frequently.19 An association between higher physician caseloads and better outcomes has been established for many surgical and medical conditions.30-32 Given that 85% of hospitalists in our program have post-graduate medical training in family medicine (internal department surveys, data not shown), it is less likely that training background can explain differences in outcomes. Instead, differences in patient volumes and the dedicated focus of hospitalists on acute care are likely more important contributors to lower mortality. In our program, a full-time hospitalist spends an average of 2000 hours annually providing services in the hospital setting. The continuous on-site presence of hospitalists enhances their clinical experience with regards to the management of common medical conditions, and increases their exposure to less common presentations of illnesses. The ability to respond to deteriorating patients in a timely manner may be another factor in explaining the differences in mortality rates between dedicated hospital-based generalist providers and similarly trained physicians with a primarily community-based focus.
In our study, hospitalist care was also broadly associated with lower mortality compared to the IM providers, although these differences were not consistently present when patients with specific diagnoses were compared. This may be partly explained by the relationship between caseload and outcomes, but other factors may also be important. For example, patients admitted by IM providers spend significantly more time in specialized units. They also predominantly present with cardiac conditions, and as such may have higher acuity levels and require more invasive interventions. While this may explain the higher observed mortality, a within-group comparison still suggests higher than expected mortality for IM patients. The HSMR methodology measures actual mortality rates compared to what would be expected based on clinical presentation and baseline population characteristics. Calculating HSMR is highly dependent on proper documentation and chart abstraction,33,34 and it is possible that some of the differences observed are due to incomplete physician documentation. However, a more in-depth analysis of care processes will be required to clarify the observed trends.
Compared to hospitalists, patients cared for by FPs also had higher odds of readmission within 30 days, which is consistent with prior studies.18,19 One of the criticisms of the hospitalist model has been the inherent discontinuity of care that is built into the model, which can contribute to suboptimal transitions of care between the acute and community settings.35 The expectation is that FPs who admit their own patients do not face this challenge, and as a result their patients should be readmitted less frequently after discharge. Our data and those from previous studies do not support this hypothesis. At the same time, when we studied patients with specific clinical diagnoses, only those hospitalized for pneumonias continued to demonstrate higher readmission odds. This suggests that hospital readmission rate is a complex measure that may be influenced by a multitude of hospital and community factors, and may be different for patients who present with different clinical diagnoses. Further research is required to better understand the relationship between provider type and experience with hospital readmission for patients with various clinical presentations.
Unlike the United States, where hospitalist care has been associated with reductions in LOS,26,36 studies in the Canadian health care setting have shown mixed results.17-21 In our evaluation, hospitalist care is not associated with reductions in total LOS compared to care provided by FPs or IM physicians. This could be due to a number of factors. First, unlike FPs, who know their patients, hospitalists may have a more conservative risk tolerance in discharging patients with whom they are not familiar. Similarly, physicians who have trained in IM may have a lower threshold for discharging patients than hospitalists, whose training background is mainly rooted in family medicine.3 Second, discontinuity of care has been associated with longer LOS for hospitalized patients.37,38 Hospitalists generally work for 7- to 10-day rotations. As a result, a patient may see a number of different hospitalists during the same hospital stay, which could nullify any gains in LOS that may be expected from better familiarity with hospital processes. Third, whereas a FP or an internist may only have a few inpatients under their care at any given time, each hospitalist typically cares for 17 to 22 patients every day. Increasing hospitalist workload has been shown to negatively impact LOS and may result in lower efficiency.39 Finally, many patients in our health system who require more time to recuperate or need complex discharge planning are usually transferred to the care of the hospitalist service from other services, or are preferentially admitted to hospitalists from the emergency department. As a result, hospitalists may look after a disproportionately higher number of long-stay patients. Despite all this, hospitalists in our population perform similarly to FPs, regardless of the clinical diagnoses of hospitalized patients.
Our study has a number of notable limitations. First, we used administrative data to conduct our evaluation and could only control for factors that are available in our data systems. As a result, some potential confounders may not have been taken into consideration. For example, our databases do not contain provider characteristics (eg, age, years of clinical experience) that have been deemed to be relevant by White and Glazier.26 Similarly, we did not have all the necessary information about the characteristics of the various MRP programs (eg, number of physicians involved in group practices, the schedule model of community FP call groups) and were not able to account for the potential impact of these on observed outcomes. Second, although our findings mirror prior studies from other parts of Canada, they may not be applicable to hospitalist programs in other jurisdictions or in health systems that are not regionalized or integrated. Third, our IM provider group is heterogeneous, with a number of different IM subspecialties (cardiologists, gastroenterologists, general internists) grouped under the IM category in our database. As a result, comparisons between the IM provider group and the other 2 provider groups, which are more homogenous, should be interpreted with caution.
Finally, we included only patients admitted to facilities in which a hospitalist service existed during the study period. As a result, a medium-size community hospital without a hospitalist service where patients are cared for exclusively by FPs and IM physicians was not included in the comparisons, and in 4 of the 10 facilities included, the number of FP patients was less than 10% of total hospitalized patients at the site (Appendix A). This may have resulted in an under-representation of FP patients.
Conclusion
Debates about the merits of the hospitalist model in Canada continue, and are in part fueled by a paucity of robust evidence about its impact on care outcomes compared to more traditional ways of providing inpatient care. In our evaluation, care provided by hospitalists is associated with lower mortality and readmission rates, despite similar LOS compared with FPs. Hospitalist care is also associated with lower mortality compared to IM providers. Hospitalists also demonstrated progressive improvement over time, with decreasing LOS and mortality rates and a stable readmission rate. Our results suggest that physicians with a focus on inpatient care can have positive contributions to quality and efficiency of care in Canada.
Corresponding author: Vandad Yousefi MD, CCFP, FHM, Fraser Health Authority, 400, 13450–102 Avenue, Surrey BC V3T 0H1, Canada.
Financial disclosures: None.
1. Kisuule F, Howell E. Hospital medicine beyond the United States. Int J Gen Med. 2018;11:65-71.
2. Yousefi V, Wilton D. Dedesigning hospital care: learning from the experience of hospital medicine in Canada. J Global Health Care Syst. 2011;1(3).
3. Soong C, Fan E, Howell E, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag. 2009;16:69-76.
4. Yousefi V. How Canadian hospitalists spend their time - A work-sampling study within a hospital medicine program in Ontario. J Clin Outcomes Manag. 2011;18:159-166.
5. Wilson G. Are inpatients’ needs better served by hospitalists than by their family doctors? No. Can Fam Physician. 2008;54:1101-1103.
6. Samoil D. Are inpatients’ needs better served by hospitalists than by their family doctors: Yes? Can Fam Physician. 2008;54:1100-1101.
7. Nicolson B. Where’s Marcus Welby when you need him? BC Medical J. 2016;58:63-64.
8. Lemire F. Enhanced skills in family medicine: Update. Can Fam Physician. 2018;64:160.
9. Lerner J. Wanting family medicine without primary care. Can Fam Physician. 2018; 64:155.
10. Canadian Society of Hospital Medicine. Core Competencies in Hospital Medicine - Care of the Medical Inpatient. 2015.
11. Redelmeier DA. A Canadian perspective on the American hospitalist movement. Arch Intern Med. 1999;159:1665-1668.
12. Ghali WA, Greenberg PB, Mejia R, et al. International perspectives on general internal medicine and the case for “globalization” of a discipline. J Gen Intern Med. 2006;21:197-200.
13. Day A, MacMillan L. Neglect of the inpatient: The hospitalist movement in Canada responds. Hosp Q. 2001;4:36.
14. Sullivan P. Enter the hospitalist: New type of patient creating a new type of specialist. CMAJ. 2000;162:1345-1346.
15. Chan BTB. The declining comprehensiveness of primary care. CMAJ. 2002;166:429-434.
16. Abenhaim HA, Kahn SR, Raffoul J, Becker MR. Program description: A hospitalist-run, medical short-stay unit in a teaching hospital. CMAJ. 2000;163:1477-1480.
17. McGowan B, Nightingale M. The hospitalist program a new specialty on the horizon in acute care medicine a hospital case study. BC Med J. 2003;45:391-394.
18. Yousefi V, Chong C. Does implementation of a hospitalist program in a Canadian community hospital improve measures of quality of care and utilization? An observational comparative analysis of hospitalists vs. traditional care providers. BMC Health Serv Res. 2013;13:204.
19. White HL. Assessing the prevalence, penetration and performance of hospital physicians in Ontario: Implications for the quality and efficiency of inpatient care. ProQuest Dissertations Publishing; 2016.
20. Gutierrez CA, Norris M, Chail M. Impact of a newly established hospitalist training program on patient LOS and RIW. Poster presented at the 9th Annual Canadian Society of Hospital Medicine Conference, September 23-25, 2011; Banff, Alberta.
21. Seth P, Nicholson K, Habbous S, Menard J. Implementation of a hospitalist medicine model in a full-service community hospital: Examining impact two years post-implementation on health resource use andpatient satisfaction. Poster presented at the 13th Annual Canadian Society of Hospital Medicine Conference. 2015; Niagara Falls, Ontario.
22. Lewis S. A system in name only--access, variation, and reform in Canada’s provinces. N Engl J Med. 2015;372:497-500.
23. Lewis S, Kouri D. Regionalization: Making sense of the Canadian experience. Healthcare Papers. 2004;5:12-31.
24. Fraser Health Authority. About Fraser health. www.fraserhealth.ca/about-us/about-fraser-health#.XFJrl9JKiUk. Updated 2018. Accessed January 30, 2019.
25. Canadian Institute for Health Information. CMG+. https://www.cihi.ca/en/cmg. Accessed January 30, 2019.
26. White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
27. Canadian Institute for Health Information. Hospital standardized mortality ratio technical notes. 2008. www.cihi.ca/sites/default/files/document/hsmr-tech-notes_en_0.pdf.
28. McAlister FA, Youngson E, Bakal JA, et al. Physician experience and outcomes among patients admitted to general internal medicine teaching wards. CMAJ. 2015;187:1041-1048.
29. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: The general medicine inpatient initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5:E849.
30. Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: A systematic review of systematic reviews. Syst Rev. 2016;5:204.
31. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511-520.
32. Chen CH, Chen YH, Lin HC, Lin HC. Association between physician caseload and patient outcome for sepsis treatment. Infect Control Hosp Epidemiol. 2009;30:556-562.
33. van Gestel YR, Lemmens VE, Lingsma HF, et al. The hospital standardized mortality ratio fallacy: A narrative review. Med Care. 2012;50:662-667.
34. Scott IA, Brand CA, Phelps GE, et al. Using hospital standardised mortality ratios to assess quality of care—proceed with extreme caution. Med J Aust. 2011; 194:645-648.
35. Wachter RM. Hospitalists in the United States -- mission accomplished or work in progress? N Engl J Med. 2004;350:1935-1936.
36. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84:248-254.
37. Chandra S, Wright SM, Howell EE. The creating incentives and continuity leading to efficiency staffing model: A quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87:364-371.
38. Epstein K, Juarez E, Epstein A, et al. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335-338.
39. Elliott DJ, Young RS, Brice J, et al. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174:786-793.
1. Kisuule F, Howell E. Hospital medicine beyond the United States. Int J Gen Med. 2018;11:65-71.
2. Yousefi V, Wilton D. Dedesigning hospital care: learning from the experience of hospital medicine in Canada. J Global Health Care Syst. 2011;1(3).
3. Soong C, Fan E, Howell E, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag. 2009;16:69-76.
4. Yousefi V. How Canadian hospitalists spend their time - A work-sampling study within a hospital medicine program in Ontario. J Clin Outcomes Manag. 2011;18:159-166.
5. Wilson G. Are inpatients’ needs better served by hospitalists than by their family doctors? No. Can Fam Physician. 2008;54:1101-1103.
6. Samoil D. Are inpatients’ needs better served by hospitalists than by their family doctors: Yes? Can Fam Physician. 2008;54:1100-1101.
7. Nicolson B. Where’s Marcus Welby when you need him? BC Medical J. 2016;58:63-64.
8. Lemire F. Enhanced skills in family medicine: Update. Can Fam Physician. 2018;64:160.
9. Lerner J. Wanting family medicine without primary care. Can Fam Physician. 2018; 64:155.
10. Canadian Society of Hospital Medicine. Core Competencies in Hospital Medicine - Care of the Medical Inpatient. 2015.
11. Redelmeier DA. A Canadian perspective on the American hospitalist movement. Arch Intern Med. 1999;159:1665-1668.
12. Ghali WA, Greenberg PB, Mejia R, et al. International perspectives on general internal medicine and the case for “globalization” of a discipline. J Gen Intern Med. 2006;21:197-200.
13. Day A, MacMillan L. Neglect of the inpatient: The hospitalist movement in Canada responds. Hosp Q. 2001;4:36.
14. Sullivan P. Enter the hospitalist: New type of patient creating a new type of specialist. CMAJ. 2000;162:1345-1346.
15. Chan BTB. The declining comprehensiveness of primary care. CMAJ. 2002;166:429-434.
16. Abenhaim HA, Kahn SR, Raffoul J, Becker MR. Program description: A hospitalist-run, medical short-stay unit in a teaching hospital. CMAJ. 2000;163:1477-1480.
17. McGowan B, Nightingale M. The hospitalist program a new specialty on the horizon in acute care medicine a hospital case study. BC Med J. 2003;45:391-394.
18. Yousefi V, Chong C. Does implementation of a hospitalist program in a Canadian community hospital improve measures of quality of care and utilization? An observational comparative analysis of hospitalists vs. traditional care providers. BMC Health Serv Res. 2013;13:204.
19. White HL. Assessing the prevalence, penetration and performance of hospital physicians in Ontario: Implications for the quality and efficiency of inpatient care. ProQuest Dissertations Publishing; 2016.
20. Gutierrez CA, Norris M, Chail M. Impact of a newly established hospitalist training program on patient LOS and RIW. Poster presented at the 9th Annual Canadian Society of Hospital Medicine Conference, September 23-25, 2011; Banff, Alberta.
21. Seth P, Nicholson K, Habbous S, Menard J. Implementation of a hospitalist medicine model in a full-service community hospital: Examining impact two years post-implementation on health resource use andpatient satisfaction. Poster presented at the 13th Annual Canadian Society of Hospital Medicine Conference. 2015; Niagara Falls, Ontario.
22. Lewis S. A system in name only--access, variation, and reform in Canada’s provinces. N Engl J Med. 2015;372:497-500.
23. Lewis S, Kouri D. Regionalization: Making sense of the Canadian experience. Healthcare Papers. 2004;5:12-31.
24. Fraser Health Authority. About Fraser health. www.fraserhealth.ca/about-us/about-fraser-health#.XFJrl9JKiUk. Updated 2018. Accessed January 30, 2019.
25. Canadian Institute for Health Information. CMG+. https://www.cihi.ca/en/cmg. Accessed January 30, 2019.
26. White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
27. Canadian Institute for Health Information. Hospital standardized mortality ratio technical notes. 2008. www.cihi.ca/sites/default/files/document/hsmr-tech-notes_en_0.pdf.
28. McAlister FA, Youngson E, Bakal JA, et al. Physician experience and outcomes among patients admitted to general internal medicine teaching wards. CMAJ. 2015;187:1041-1048.
29. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: The general medicine inpatient initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5:E849.
30. Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: A systematic review of systematic reviews. Syst Rev. 2016;5:204.
31. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511-520.
32. Chen CH, Chen YH, Lin HC, Lin HC. Association between physician caseload and patient outcome for sepsis treatment. Infect Control Hosp Epidemiol. 2009;30:556-562.
33. van Gestel YR, Lemmens VE, Lingsma HF, et al. The hospital standardized mortality ratio fallacy: A narrative review. Med Care. 2012;50:662-667.
34. Scott IA, Brand CA, Phelps GE, et al. Using hospital standardised mortality ratios to assess quality of care—proceed with extreme caution. Med J Aust. 2011; 194:645-648.
35. Wachter RM. Hospitalists in the United States -- mission accomplished or work in progress? N Engl J Med. 2004;350:1935-1936.
36. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84:248-254.
37. Chandra S, Wright SM, Howell EE. The creating incentives and continuity leading to efficiency staffing model: A quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87:364-371.
38. Epstein K, Juarez E, Epstein A, et al. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335-338.
39. Elliott DJ, Young RS, Brice J, et al. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174:786-793.
Geriatric Assessment and Collaborative Medication Review for Older Adults With Polypharmacy
Study Overview
Objective. To examine the effect of clinical geriatric assessments and collaborative medication review by geriatricians and family physicians on quality of life and other patient outcomes in home-dwelling older adults with polypharmacy.
Design. The study was a single-blind, cluster randomized clinical trial enrolling home-dwelling adults aged 70 years and older who were taking 7 or more medications. Family physicians in Norway were recruited to participate in the trial with their patients. Randomization was at the family physician level to avoid contamination between intervention and control groups.
Setting and participants. The study was conducted in Akershus and Oslo, Norway. Family physicians were recruited to participate in the trial with their patients. A total of 84 family physicians were recruited, of which 70 were included in the trial and randomized to intervention versus control; 14 were excluded because they had no eligible patients. The cluster size of each family physician was limited to 5 patients per physician to avoid large variation in cluster sizes. Patients were eligible for enrollment if they were home-dwelling, aged 70 years or older, and were taking 7 or more systemic medications regularly and had medications administered by the home nursing service. Patients were excluded if they were expected to die or be institutionalized within 6 months, or if they were discouraged from participation by their family physician. A total of 174 patients were recruited, with 87 patients in each group (34 family physicians were in the control group and 36 in the intervention group).
Intervention. The intervention included a geriatric assessment performed by a physician trained in geriatric medicine and supervised by a senior consultant. The geriatric assessment consisted of review of medical history; systematic screening for current problems; clinical examination; supplementary tests, if indicated; and review of each medication being used. The review of medication included the indication for each medication, dosage, adverse effects, and interactions. The geriatric assessment consultation took 1 hour to complete, on average. After the geriatric assessment, the family physician and the geriatrician met to discuss each medication and to establish a collaborative plan for adjustments and follow-up; this meeting was approximately 15 minutes in duration. Lastly, clinical follow-up with the older adult was conducted by the geriatrician or the family physician, as agreed upon in the plan, with most follow-up conducted by the family physician. Participants randomized to the control group received usual care without any intervention.
Main outcome measures. Outcomes were assessed at 16-week and 24-week follow-up. The main study outcome measure was health-related quality of life (HRQoL), as measured by the 15D instrument, at 16 weeks. The quality-of-life measure included the following aspects, each rated on an ordinal scale of 5 levels: mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort or symptoms, depression, distress, vitality, and sexual activity. The index scale including all aspects is in the range of 0 to 1, with a higher score indicating better quality of life. A predetermined change of 0.015 or more is considered clinically important, and a positive change of 0.035 indicates much better HRQoL. Other outcomes included: appropriateness of medications measured by the Medication Appropriateness Index and the Assessment of Underutilization; physical function (short Physical Performance battery); gait speed; grip strength; cognitive functioning; physical and cognitive disability (Functional Independence Measure); caregiver burden (Relative Stress Scale); physical measures, including orthostatic blood pressure, falls, and weight; hospital admissions; use of home nursing service; incidence of institutionalization; and mortality.
Main results. The study included 174 patients with an average age of 83.3 years (SD, 7.3); 67.8% were women. Of those who were randomized to the intervention and control groups, 158 (90.8%) completed the trial. The average number of regularly used medications was 10.1 (SD, 2.7) in the intervention group and 9.5 (SD, 2.6) in the control group. At week 16 of follow-up, patients in the intervention group had an improved HRQoL score measured by the 15D instrument; the difference between the intervention group and control groups was 0.045 (95% confidence interval [CI], 0.004 -0.086; P = 0.03). Medication appropriateness was better in the intervention group, as compared with the control group at both 16 weeks and 24 weeks. Nearly all (99%) patients in the intervention group experienced medication changes, which included withdrawal of medications, dosage adjustment, or new drug regimens. There was a trend towards a higher rate of hospitalization during follow-up in the intervention group (adjusted risk ratio, 2.03; 95% CI, 0.98-4.24; P = 0.06). Other secondary outcomes were not substantially different between the intervention and control groups.
Conclusion. The study demonstrated that a clinical geriatric assessment and collaborative medication review by geriatrician and family physician led to improved HRQoL and improved medication use.
Commentary
The use of multiple medications in older adults is common, with almost 20% of older adults over age 65 taking 10 or more medications.1 Polypharmacy in older adults is associated with lower adherence rates and increases the potential for interactions between medications.2 Age-related changes, such as changes in absorption, metabolism, and excretion, affect pharmacokinetics of medications and potentiate adverse drug reactions, requiring adjustments in use and dosing to optimize safety and outcomes. Recognizing the potential effects of medications in older adults, evidence-based guidelines, such as the Beers criteria3 and START/STOPP criteria,4 have been developed to identify potentially inappropriate medications in older adults and to improve prescribing. Randomized trials using the START/STOPP criteria have demonstrated improved medication appropriateness, reduced polypharmacy, and reduced adverse drug reactions.5 Although this study did not use a criteria-based approach for improving medication use, it demonstrated that in a population of older adults with polypharmacy, medication review with geriatricians can lead to improved HRQoL while improving medication appropriateness. The collaborative approach between the family physician and geriatrician, rather than a consultative approach with recommendations from a geriatrician, may have contributed to increased uptake of medication changes. Such an approach may be a reasonable strategy to improve medication use in older adults.
A limitation of the study is that the improvement in HRQoL could have been the result of medication changes, but could also have been due to other changes in the plan of care that resulted from the geriatric assessment. As noted by the authors, the increase in hospital admissions, though not statistically significant, could have resulted from the medication modifications; however, it was also noted that the geriatric assessments could have identified severe illnesses that required hospitalization, as the timeline from geriatric assessment to hospitalization suggested was the case. Thus, the increase in hospitalization resulting from timely identification of severe illness was more likely a benefit than an adverse effect; however, further studies should be done to elucidate this.
Applications for Clinical Practice
Older adults with multiple chronic conditions and complex medication regimens are at risk for poor health outcomes, and a purposeful medication review to improve medication use, leading to the removal of unnecessary and potentially harmful medications, adjustment of dosages, and initiation of appropriate medications, may yield health benefits, such as improved HRQoL. The present study utilized an approach that could be scalable, which is important given the limited number of clinicians with geriatrics expertise. For health systems with geriatrics clinical expertise, it may be reasonable to consider adopting a similar collaborative approach in order to improve care for older adults most at risk. Further reports on how patients and family physicians perceive this intervention will enhance our understanding of whether it could be implemented widely.
–William W. Hung, MD, MPH
1. Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium”. JAMA. 2010;304:1592-1601.
2. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
3. American Geriatrics Society 2015 Updated Beers criteria for potentially inappropriate medication use in older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
4. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38:360-372.
5. O’Mahony D. STOPP/START criteria for potentially inappropriate medications/ potential prescribing omissions in older people: origin and progress. Expert Rev Clin Pharmacol. 2020;13:15-22.
Study Overview
Objective. To examine the effect of clinical geriatric assessments and collaborative medication review by geriatricians and family physicians on quality of life and other patient outcomes in home-dwelling older adults with polypharmacy.
Design. The study was a single-blind, cluster randomized clinical trial enrolling home-dwelling adults aged 70 years and older who were taking 7 or more medications. Family physicians in Norway were recruited to participate in the trial with their patients. Randomization was at the family physician level to avoid contamination between intervention and control groups.
Setting and participants. The study was conducted in Akershus and Oslo, Norway. Family physicians were recruited to participate in the trial with their patients. A total of 84 family physicians were recruited, of which 70 were included in the trial and randomized to intervention versus control; 14 were excluded because they had no eligible patients. The cluster size of each family physician was limited to 5 patients per physician to avoid large variation in cluster sizes. Patients were eligible for enrollment if they were home-dwelling, aged 70 years or older, and were taking 7 or more systemic medications regularly and had medications administered by the home nursing service. Patients were excluded if they were expected to die or be institutionalized within 6 months, or if they were discouraged from participation by their family physician. A total of 174 patients were recruited, with 87 patients in each group (34 family physicians were in the control group and 36 in the intervention group).
Intervention. The intervention included a geriatric assessment performed by a physician trained in geriatric medicine and supervised by a senior consultant. The geriatric assessment consisted of review of medical history; systematic screening for current problems; clinical examination; supplementary tests, if indicated; and review of each medication being used. The review of medication included the indication for each medication, dosage, adverse effects, and interactions. The geriatric assessment consultation took 1 hour to complete, on average. After the geriatric assessment, the family physician and the geriatrician met to discuss each medication and to establish a collaborative plan for adjustments and follow-up; this meeting was approximately 15 minutes in duration. Lastly, clinical follow-up with the older adult was conducted by the geriatrician or the family physician, as agreed upon in the plan, with most follow-up conducted by the family physician. Participants randomized to the control group received usual care without any intervention.
Main outcome measures. Outcomes were assessed at 16-week and 24-week follow-up. The main study outcome measure was health-related quality of life (HRQoL), as measured by the 15D instrument, at 16 weeks. The quality-of-life measure included the following aspects, each rated on an ordinal scale of 5 levels: mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort or symptoms, depression, distress, vitality, and sexual activity. The index scale including all aspects is in the range of 0 to 1, with a higher score indicating better quality of life. A predetermined change of 0.015 or more is considered clinically important, and a positive change of 0.035 indicates much better HRQoL. Other outcomes included: appropriateness of medications measured by the Medication Appropriateness Index and the Assessment of Underutilization; physical function (short Physical Performance battery); gait speed; grip strength; cognitive functioning; physical and cognitive disability (Functional Independence Measure); caregiver burden (Relative Stress Scale); physical measures, including orthostatic blood pressure, falls, and weight; hospital admissions; use of home nursing service; incidence of institutionalization; and mortality.
Main results. The study included 174 patients with an average age of 83.3 years (SD, 7.3); 67.8% were women. Of those who were randomized to the intervention and control groups, 158 (90.8%) completed the trial. The average number of regularly used medications was 10.1 (SD, 2.7) in the intervention group and 9.5 (SD, 2.6) in the control group. At week 16 of follow-up, patients in the intervention group had an improved HRQoL score measured by the 15D instrument; the difference between the intervention group and control groups was 0.045 (95% confidence interval [CI], 0.004 -0.086; P = 0.03). Medication appropriateness was better in the intervention group, as compared with the control group at both 16 weeks and 24 weeks. Nearly all (99%) patients in the intervention group experienced medication changes, which included withdrawal of medications, dosage adjustment, or new drug regimens. There was a trend towards a higher rate of hospitalization during follow-up in the intervention group (adjusted risk ratio, 2.03; 95% CI, 0.98-4.24; P = 0.06). Other secondary outcomes were not substantially different between the intervention and control groups.
Conclusion. The study demonstrated that a clinical geriatric assessment and collaborative medication review by geriatrician and family physician led to improved HRQoL and improved medication use.
Commentary
The use of multiple medications in older adults is common, with almost 20% of older adults over age 65 taking 10 or more medications.1 Polypharmacy in older adults is associated with lower adherence rates and increases the potential for interactions between medications.2 Age-related changes, such as changes in absorption, metabolism, and excretion, affect pharmacokinetics of medications and potentiate adverse drug reactions, requiring adjustments in use and dosing to optimize safety and outcomes. Recognizing the potential effects of medications in older adults, evidence-based guidelines, such as the Beers criteria3 and START/STOPP criteria,4 have been developed to identify potentially inappropriate medications in older adults and to improve prescribing. Randomized trials using the START/STOPP criteria have demonstrated improved medication appropriateness, reduced polypharmacy, and reduced adverse drug reactions.5 Although this study did not use a criteria-based approach for improving medication use, it demonstrated that in a population of older adults with polypharmacy, medication review with geriatricians can lead to improved HRQoL while improving medication appropriateness. The collaborative approach between the family physician and geriatrician, rather than a consultative approach with recommendations from a geriatrician, may have contributed to increased uptake of medication changes. Such an approach may be a reasonable strategy to improve medication use in older adults.
A limitation of the study is that the improvement in HRQoL could have been the result of medication changes, but could also have been due to other changes in the plan of care that resulted from the geriatric assessment. As noted by the authors, the increase in hospital admissions, though not statistically significant, could have resulted from the medication modifications; however, it was also noted that the geriatric assessments could have identified severe illnesses that required hospitalization, as the timeline from geriatric assessment to hospitalization suggested was the case. Thus, the increase in hospitalization resulting from timely identification of severe illness was more likely a benefit than an adverse effect; however, further studies should be done to elucidate this.
Applications for Clinical Practice
Older adults with multiple chronic conditions and complex medication regimens are at risk for poor health outcomes, and a purposeful medication review to improve medication use, leading to the removal of unnecessary and potentially harmful medications, adjustment of dosages, and initiation of appropriate medications, may yield health benefits, such as improved HRQoL. The present study utilized an approach that could be scalable, which is important given the limited number of clinicians with geriatrics expertise. For health systems with geriatrics clinical expertise, it may be reasonable to consider adopting a similar collaborative approach in order to improve care for older adults most at risk. Further reports on how patients and family physicians perceive this intervention will enhance our understanding of whether it could be implemented widely.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine the effect of clinical geriatric assessments and collaborative medication review by geriatricians and family physicians on quality of life and other patient outcomes in home-dwelling older adults with polypharmacy.
Design. The study was a single-blind, cluster randomized clinical trial enrolling home-dwelling adults aged 70 years and older who were taking 7 or more medications. Family physicians in Norway were recruited to participate in the trial with their patients. Randomization was at the family physician level to avoid contamination between intervention and control groups.
Setting and participants. The study was conducted in Akershus and Oslo, Norway. Family physicians were recruited to participate in the trial with their patients. A total of 84 family physicians were recruited, of which 70 were included in the trial and randomized to intervention versus control; 14 were excluded because they had no eligible patients. The cluster size of each family physician was limited to 5 patients per physician to avoid large variation in cluster sizes. Patients were eligible for enrollment if they were home-dwelling, aged 70 years or older, and were taking 7 or more systemic medications regularly and had medications administered by the home nursing service. Patients were excluded if they were expected to die or be institutionalized within 6 months, or if they were discouraged from participation by their family physician. A total of 174 patients were recruited, with 87 patients in each group (34 family physicians were in the control group and 36 in the intervention group).
Intervention. The intervention included a geriatric assessment performed by a physician trained in geriatric medicine and supervised by a senior consultant. The geriatric assessment consisted of review of medical history; systematic screening for current problems; clinical examination; supplementary tests, if indicated; and review of each medication being used. The review of medication included the indication for each medication, dosage, adverse effects, and interactions. The geriatric assessment consultation took 1 hour to complete, on average. After the geriatric assessment, the family physician and the geriatrician met to discuss each medication and to establish a collaborative plan for adjustments and follow-up; this meeting was approximately 15 minutes in duration. Lastly, clinical follow-up with the older adult was conducted by the geriatrician or the family physician, as agreed upon in the plan, with most follow-up conducted by the family physician. Participants randomized to the control group received usual care without any intervention.
Main outcome measures. Outcomes were assessed at 16-week and 24-week follow-up. The main study outcome measure was health-related quality of life (HRQoL), as measured by the 15D instrument, at 16 weeks. The quality-of-life measure included the following aspects, each rated on an ordinal scale of 5 levels: mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort or symptoms, depression, distress, vitality, and sexual activity. The index scale including all aspects is in the range of 0 to 1, with a higher score indicating better quality of life. A predetermined change of 0.015 or more is considered clinically important, and a positive change of 0.035 indicates much better HRQoL. Other outcomes included: appropriateness of medications measured by the Medication Appropriateness Index and the Assessment of Underutilization; physical function (short Physical Performance battery); gait speed; grip strength; cognitive functioning; physical and cognitive disability (Functional Independence Measure); caregiver burden (Relative Stress Scale); physical measures, including orthostatic blood pressure, falls, and weight; hospital admissions; use of home nursing service; incidence of institutionalization; and mortality.
Main results. The study included 174 patients with an average age of 83.3 years (SD, 7.3); 67.8% were women. Of those who were randomized to the intervention and control groups, 158 (90.8%) completed the trial. The average number of regularly used medications was 10.1 (SD, 2.7) in the intervention group and 9.5 (SD, 2.6) in the control group. At week 16 of follow-up, patients in the intervention group had an improved HRQoL score measured by the 15D instrument; the difference between the intervention group and control groups was 0.045 (95% confidence interval [CI], 0.004 -0.086; P = 0.03). Medication appropriateness was better in the intervention group, as compared with the control group at both 16 weeks and 24 weeks. Nearly all (99%) patients in the intervention group experienced medication changes, which included withdrawal of medications, dosage adjustment, or new drug regimens. There was a trend towards a higher rate of hospitalization during follow-up in the intervention group (adjusted risk ratio, 2.03; 95% CI, 0.98-4.24; P = 0.06). Other secondary outcomes were not substantially different between the intervention and control groups.
Conclusion. The study demonstrated that a clinical geriatric assessment and collaborative medication review by geriatrician and family physician led to improved HRQoL and improved medication use.
Commentary
The use of multiple medications in older adults is common, with almost 20% of older adults over age 65 taking 10 or more medications.1 Polypharmacy in older adults is associated with lower adherence rates and increases the potential for interactions between medications.2 Age-related changes, such as changes in absorption, metabolism, and excretion, affect pharmacokinetics of medications and potentiate adverse drug reactions, requiring adjustments in use and dosing to optimize safety and outcomes. Recognizing the potential effects of medications in older adults, evidence-based guidelines, such as the Beers criteria3 and START/STOPP criteria,4 have been developed to identify potentially inappropriate medications in older adults and to improve prescribing. Randomized trials using the START/STOPP criteria have demonstrated improved medication appropriateness, reduced polypharmacy, and reduced adverse drug reactions.5 Although this study did not use a criteria-based approach for improving medication use, it demonstrated that in a population of older adults with polypharmacy, medication review with geriatricians can lead to improved HRQoL while improving medication appropriateness. The collaborative approach between the family physician and geriatrician, rather than a consultative approach with recommendations from a geriatrician, may have contributed to increased uptake of medication changes. Such an approach may be a reasonable strategy to improve medication use in older adults.
A limitation of the study is that the improvement in HRQoL could have been the result of medication changes, but could also have been due to other changes in the plan of care that resulted from the geriatric assessment. As noted by the authors, the increase in hospital admissions, though not statistically significant, could have resulted from the medication modifications; however, it was also noted that the geriatric assessments could have identified severe illnesses that required hospitalization, as the timeline from geriatric assessment to hospitalization suggested was the case. Thus, the increase in hospitalization resulting from timely identification of severe illness was more likely a benefit than an adverse effect; however, further studies should be done to elucidate this.
Applications for Clinical Practice
Older adults with multiple chronic conditions and complex medication regimens are at risk for poor health outcomes, and a purposeful medication review to improve medication use, leading to the removal of unnecessary and potentially harmful medications, adjustment of dosages, and initiation of appropriate medications, may yield health benefits, such as improved HRQoL. The present study utilized an approach that could be scalable, which is important given the limited number of clinicians with geriatrics expertise. For health systems with geriatrics clinical expertise, it may be reasonable to consider adopting a similar collaborative approach in order to improve care for older adults most at risk. Further reports on how patients and family physicians perceive this intervention will enhance our understanding of whether it could be implemented widely.
–William W. Hung, MD, MPH
1. Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium”. JAMA. 2010;304:1592-1601.
2. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
3. American Geriatrics Society 2015 Updated Beers criteria for potentially inappropriate medication use in older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
4. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38:360-372.
5. O’Mahony D. STOPP/START criteria for potentially inappropriate medications/ potential prescribing omissions in older people: origin and progress. Expert Rev Clin Pharmacol. 2020;13:15-22.
1. Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium”. JAMA. 2010;304:1592-1601.
2. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
3. American Geriatrics Society 2015 Updated Beers criteria for potentially inappropriate medication use in older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
4. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38:360-372.
5. O’Mahony D. STOPP/START criteria for potentially inappropriate medications/ potential prescribing omissions in older people: origin and progress. Expert Rev Clin Pharmacol. 2020;13:15-22.
Pembrolizumab Plus Neoadjuvant Chemotherapy Improves Pathologic Complete Response Rates in Triple-Negative Breast Cancer
Study Overview
Objective. To evaluate the efficacy and safety of pembrolizumab in combination with neoadjuvant chemotherapy followed by adjuvant pembrolizumab in early-stage triple-negative breast cancer.
Design. International, multicenter, randomized, double-blind, phase 3 trial.
Intervention. Patients were randomly assigned in a 2:1 fashion to receive either pembrolizumab or placebo. Patients received 4 cycles of neoadjuvant pembrolizumab or placebo once every 3 weeks, in addition to weekly paclitaxel 80 mg/m2 plus carboplatin AUC5 once every 3 weeks. This was followed by 4 cycles of pembrolizumab or placebo plus doxorubicin 60 mg/m2 or epirubicin 90 mg/m2 plus cyclophosphamide 600 mg/m2 once every 3 weeks. Patients then underwent definitive surgery 3 to 6 weeks after completion of neoadjuvant therapy. In the adjuvant setting, patients received pembrolizumab or placebo once every 3 weeks for up to 9 cycles. Adjuvant capecitabine was not allowed.
Setting and participants. A total of 1174 patients underwent randomization: 784 patients in the pembrolizumab/chemotherapy group and 390 patients in the placebo/chemotherapy group. Eligible patients had newly diagnosed, centrally confirmed triple-negative breast cancer (nonmetastatic: T1c, N1-2 or T2-4, N0-2). Patients were eligible regardless of PD-L1 status, and those with inflammatory breast cancer and multifocal primaries were eligible.
Main outcome measures. The primary endpoints of this study were pathologic complete response (pCR) rate (defined as ypT0/ypTis, ypN0) at the time of surgery and event-free survival (EFS) in the intention-to-treat population. Secondary endpoints included pCR in all patients, pCR among patients with PD-L1–positive tumors, EFS among patients with PD-L1–positive tumors, and overall survival among all patients and those with PD-L1–positive tumors. PD-L1 expression was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent, Santa Clara, CA). Expression was characterized according to the combined positive score, with a score of 1% or greater being considered positive.
Results. The baseline characteristics were well balanced between the treatment arms. At the time of the second interim analysis, the median duration of follow-up was 15.5 months. The pCR rate among the first 602 patients who were randomized was 64.8% in the pembrolizumab/chemotherapy group and 51.2% in the placebo group (P < 0.001; 95% confidence interval, 5.4-21.8). The pCR rate in the PD-L1–positive population was 68.9% in the pembrolizumab/chemotherapy group, as compared to 54.9% in the placebo group. In the PD-L1–negative population, the pCR rate was 45.3% in the pembrolizumab/chemotherapy group, as compared to 30.3% in the placebo group. At the time of analysis, 104 events had occurred, and the estimated percentage of patients at 18 months who were alive without disease progression was 91% in the pembrolizumab group and 85% in the placebo group. The median was not reached in either group.
Grade 3 or higher adverse events in the neoadjuvant phase were seen in 76.8% and 72.2% of patients in the pembrolizumab and placebo arms, respectively. Serious treatment-related adverse events occurred in 32% of patients in the pembrolizumab group compared to 19% in the placebo group. Febrile neutropenia and anemia were the most common. Discontinuation of the trial drug due to adverse events occurred in 23% of patients in the pembrolizumab arm and in 12% in the placebo arm. The majority of treatment-related adverse events occurred in the neoadjuvant phase. In the adjuvant phase, treatment-related adverse events occurred in 48% and 43% of patients in the pembrolizumab and placebo groups, respectively.
Conclusion. The combination of neoadjuvant chemotherapy and pembrolizumab in patients with newly diagnosed, early-stage, triple-negative breast cancer yielded a higher percentage of patients achieving a pCR as compared with chemotherapy plus placebo.
Commentary
The current study adds to the growing body of literature outlining the efficacy of immune checkpoint inhibition in triple-negative breast cancer. The previously published IMpassion130 trial showed that the addition of the PD-L1 antibody atezolizumab to nab-paclitaxel improved progression-free survival in patients with PD-L1–positive (1% or greater), metastatic triple-negative breast cancer.1 Similarly, in the phase 2 I-SPY2 trial, the addition of pembrolizumab to standard neoadjuvant chemotherapy led to a near tripling of the pCR rates in triple-negative breast cancer.2 While the current study demonstrated improved pCR rates with pembrolizumab, no difference in EFS has yet been demonstrated; however, longer-term follow-up will be required. There certainly are numerous studies documenting an association between pCR and improved disease-free survival and possibly overall survival. Cortazar and colleagues performed a pooled analysis of 12 international trials, which demonstrated an association between pCR and improved EFS (hazard ratio [HR], 0.24) and overall survival (HR, 0.16) in patients with triple-negative breast cancer.3 The results of the current study will require longer-term follow-up to confirm such an association.
The current study appears to have demonstrated a benefit with the addition of pembrolizumab across treatment subgroups, particularly in the PD-L1–positive and PD-L1–negative populations. While this differs from the findings of the IMpassion130 trial, it is quite difficult to draw definitive conclusions because the 2 trials studied different antibodies, and thus used a different assay to define PD-L1 positivity. Notable differences exist in determination of PD-L1 status across assays, and it is important for providers to use the appropriate assay for each antibody. These differences highlight the need for more informative biomarkers to predict a benefit from immune checkpoint inhibition.
It is also noteworthy that the control arm in the current trial was a platinum-based regimen. Platinum-based neoadjuvant regimens previously have been shown to induce higher pCR rates in triple-negative breast cancer; however, the incorporation of carboplatin as standard of care remains a topic of debate.4 Nevertheless, a similar trial evaluating the efficacy of atezolizumab combined with platinum-based neoadjuvant chemotherapy in triple-negative breast cancer, NSABP B-59 (NCT03281954), is underway, with the control arm also incorporating carboplatin. The results of this study will also help validate the role of checkpoint inhibitors in the neoadjuvant setting in triple-negative breast cancer. Of note, this trial did not allow for the use of adjuvant capecitabine, which has been previously shown in the CREATE-X trial to prolong survival in this population.5 How the use of adjuvant capecitabine would impact these results is completely unknown.6 The incidence of grade 3 or higher toxicities in the current trial appeared to be similar in both groups. There did appear to be a higher incidence of infusion reactions and skin reactions in the pembrolizumab groups. Immune-related adverse events were consistent with prior pembrolizumab data.
Applications for Clinical Practice
KEYNOTE-522 adds to the growing evidence suggesting that incorporation of immune checkpoint inhibitors into neoadjuvant therapy in patients with triple-negative breast cancer can improve pCR rates; however, its use as a standard of care will require longer-term follow-up to ensure the noted findings translate into improvement in EFS and, ultimately, overall survival.
– Daniel Isaac, DO, MS
1. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121.
2. Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J Clin Oncol. 2017;35: Suppl:506. Abstract 506.
3. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172.
4. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant one-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33:13-21.
5. Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147-2159.
6. von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747-756.
Study Overview
Objective. To evaluate the efficacy and safety of pembrolizumab in combination with neoadjuvant chemotherapy followed by adjuvant pembrolizumab in early-stage triple-negative breast cancer.
Design. International, multicenter, randomized, double-blind, phase 3 trial.
Intervention. Patients were randomly assigned in a 2:1 fashion to receive either pembrolizumab or placebo. Patients received 4 cycles of neoadjuvant pembrolizumab or placebo once every 3 weeks, in addition to weekly paclitaxel 80 mg/m2 plus carboplatin AUC5 once every 3 weeks. This was followed by 4 cycles of pembrolizumab or placebo plus doxorubicin 60 mg/m2 or epirubicin 90 mg/m2 plus cyclophosphamide 600 mg/m2 once every 3 weeks. Patients then underwent definitive surgery 3 to 6 weeks after completion of neoadjuvant therapy. In the adjuvant setting, patients received pembrolizumab or placebo once every 3 weeks for up to 9 cycles. Adjuvant capecitabine was not allowed.
Setting and participants. A total of 1174 patients underwent randomization: 784 patients in the pembrolizumab/chemotherapy group and 390 patients in the placebo/chemotherapy group. Eligible patients had newly diagnosed, centrally confirmed triple-negative breast cancer (nonmetastatic: T1c, N1-2 or T2-4, N0-2). Patients were eligible regardless of PD-L1 status, and those with inflammatory breast cancer and multifocal primaries were eligible.
Main outcome measures. The primary endpoints of this study were pathologic complete response (pCR) rate (defined as ypT0/ypTis, ypN0) at the time of surgery and event-free survival (EFS) in the intention-to-treat population. Secondary endpoints included pCR in all patients, pCR among patients with PD-L1–positive tumors, EFS among patients with PD-L1–positive tumors, and overall survival among all patients and those with PD-L1–positive tumors. PD-L1 expression was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent, Santa Clara, CA). Expression was characterized according to the combined positive score, with a score of 1% or greater being considered positive.
Results. The baseline characteristics were well balanced between the treatment arms. At the time of the second interim analysis, the median duration of follow-up was 15.5 months. The pCR rate among the first 602 patients who were randomized was 64.8% in the pembrolizumab/chemotherapy group and 51.2% in the placebo group (P < 0.001; 95% confidence interval, 5.4-21.8). The pCR rate in the PD-L1–positive population was 68.9% in the pembrolizumab/chemotherapy group, as compared to 54.9% in the placebo group. In the PD-L1–negative population, the pCR rate was 45.3% in the pembrolizumab/chemotherapy group, as compared to 30.3% in the placebo group. At the time of analysis, 104 events had occurred, and the estimated percentage of patients at 18 months who were alive without disease progression was 91% in the pembrolizumab group and 85% in the placebo group. The median was not reached in either group.
Grade 3 or higher adverse events in the neoadjuvant phase were seen in 76.8% and 72.2% of patients in the pembrolizumab and placebo arms, respectively. Serious treatment-related adverse events occurred in 32% of patients in the pembrolizumab group compared to 19% in the placebo group. Febrile neutropenia and anemia were the most common. Discontinuation of the trial drug due to adverse events occurred in 23% of patients in the pembrolizumab arm and in 12% in the placebo arm. The majority of treatment-related adverse events occurred in the neoadjuvant phase. In the adjuvant phase, treatment-related adverse events occurred in 48% and 43% of patients in the pembrolizumab and placebo groups, respectively.
Conclusion. The combination of neoadjuvant chemotherapy and pembrolizumab in patients with newly diagnosed, early-stage, triple-negative breast cancer yielded a higher percentage of patients achieving a pCR as compared with chemotherapy plus placebo.
Commentary
The current study adds to the growing body of literature outlining the efficacy of immune checkpoint inhibition in triple-negative breast cancer. The previously published IMpassion130 trial showed that the addition of the PD-L1 antibody atezolizumab to nab-paclitaxel improved progression-free survival in patients with PD-L1–positive (1% or greater), metastatic triple-negative breast cancer.1 Similarly, in the phase 2 I-SPY2 trial, the addition of pembrolizumab to standard neoadjuvant chemotherapy led to a near tripling of the pCR rates in triple-negative breast cancer.2 While the current study demonstrated improved pCR rates with pembrolizumab, no difference in EFS has yet been demonstrated; however, longer-term follow-up will be required. There certainly are numerous studies documenting an association between pCR and improved disease-free survival and possibly overall survival. Cortazar and colleagues performed a pooled analysis of 12 international trials, which demonstrated an association between pCR and improved EFS (hazard ratio [HR], 0.24) and overall survival (HR, 0.16) in patients with triple-negative breast cancer.3 The results of the current study will require longer-term follow-up to confirm such an association.
The current study appears to have demonstrated a benefit with the addition of pembrolizumab across treatment subgroups, particularly in the PD-L1–positive and PD-L1–negative populations. While this differs from the findings of the IMpassion130 trial, it is quite difficult to draw definitive conclusions because the 2 trials studied different antibodies, and thus used a different assay to define PD-L1 positivity. Notable differences exist in determination of PD-L1 status across assays, and it is important for providers to use the appropriate assay for each antibody. These differences highlight the need for more informative biomarkers to predict a benefit from immune checkpoint inhibition.
It is also noteworthy that the control arm in the current trial was a platinum-based regimen. Platinum-based neoadjuvant regimens previously have been shown to induce higher pCR rates in triple-negative breast cancer; however, the incorporation of carboplatin as standard of care remains a topic of debate.4 Nevertheless, a similar trial evaluating the efficacy of atezolizumab combined with platinum-based neoadjuvant chemotherapy in triple-negative breast cancer, NSABP B-59 (NCT03281954), is underway, with the control arm also incorporating carboplatin. The results of this study will also help validate the role of checkpoint inhibitors in the neoadjuvant setting in triple-negative breast cancer. Of note, this trial did not allow for the use of adjuvant capecitabine, which has been previously shown in the CREATE-X trial to prolong survival in this population.5 How the use of adjuvant capecitabine would impact these results is completely unknown.6 The incidence of grade 3 or higher toxicities in the current trial appeared to be similar in both groups. There did appear to be a higher incidence of infusion reactions and skin reactions in the pembrolizumab groups. Immune-related adverse events were consistent with prior pembrolizumab data.
Applications for Clinical Practice
KEYNOTE-522 adds to the growing evidence suggesting that incorporation of immune checkpoint inhibitors into neoadjuvant therapy in patients with triple-negative breast cancer can improve pCR rates; however, its use as a standard of care will require longer-term follow-up to ensure the noted findings translate into improvement in EFS and, ultimately, overall survival.
– Daniel Isaac, DO, MS
Study Overview
Objective. To evaluate the efficacy and safety of pembrolizumab in combination with neoadjuvant chemotherapy followed by adjuvant pembrolizumab in early-stage triple-negative breast cancer.
Design. International, multicenter, randomized, double-blind, phase 3 trial.
Intervention. Patients were randomly assigned in a 2:1 fashion to receive either pembrolizumab or placebo. Patients received 4 cycles of neoadjuvant pembrolizumab or placebo once every 3 weeks, in addition to weekly paclitaxel 80 mg/m2 plus carboplatin AUC5 once every 3 weeks. This was followed by 4 cycles of pembrolizumab or placebo plus doxorubicin 60 mg/m2 or epirubicin 90 mg/m2 plus cyclophosphamide 600 mg/m2 once every 3 weeks. Patients then underwent definitive surgery 3 to 6 weeks after completion of neoadjuvant therapy. In the adjuvant setting, patients received pembrolizumab or placebo once every 3 weeks for up to 9 cycles. Adjuvant capecitabine was not allowed.
Setting and participants. A total of 1174 patients underwent randomization: 784 patients in the pembrolizumab/chemotherapy group and 390 patients in the placebo/chemotherapy group. Eligible patients had newly diagnosed, centrally confirmed triple-negative breast cancer (nonmetastatic: T1c, N1-2 or T2-4, N0-2). Patients were eligible regardless of PD-L1 status, and those with inflammatory breast cancer and multifocal primaries were eligible.
Main outcome measures. The primary endpoints of this study were pathologic complete response (pCR) rate (defined as ypT0/ypTis, ypN0) at the time of surgery and event-free survival (EFS) in the intention-to-treat population. Secondary endpoints included pCR in all patients, pCR among patients with PD-L1–positive tumors, EFS among patients with PD-L1–positive tumors, and overall survival among all patients and those with PD-L1–positive tumors. PD-L1 expression was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent, Santa Clara, CA). Expression was characterized according to the combined positive score, with a score of 1% or greater being considered positive.
Results. The baseline characteristics were well balanced between the treatment arms. At the time of the second interim analysis, the median duration of follow-up was 15.5 months. The pCR rate among the first 602 patients who were randomized was 64.8% in the pembrolizumab/chemotherapy group and 51.2% in the placebo group (P < 0.001; 95% confidence interval, 5.4-21.8). The pCR rate in the PD-L1–positive population was 68.9% in the pembrolizumab/chemotherapy group, as compared to 54.9% in the placebo group. In the PD-L1–negative population, the pCR rate was 45.3% in the pembrolizumab/chemotherapy group, as compared to 30.3% in the placebo group. At the time of analysis, 104 events had occurred, and the estimated percentage of patients at 18 months who were alive without disease progression was 91% in the pembrolizumab group and 85% in the placebo group. The median was not reached in either group.
Grade 3 or higher adverse events in the neoadjuvant phase were seen in 76.8% and 72.2% of patients in the pembrolizumab and placebo arms, respectively. Serious treatment-related adverse events occurred in 32% of patients in the pembrolizumab group compared to 19% in the placebo group. Febrile neutropenia and anemia were the most common. Discontinuation of the trial drug due to adverse events occurred in 23% of patients in the pembrolizumab arm and in 12% in the placebo arm. The majority of treatment-related adverse events occurred in the neoadjuvant phase. In the adjuvant phase, treatment-related adverse events occurred in 48% and 43% of patients in the pembrolizumab and placebo groups, respectively.
Conclusion. The combination of neoadjuvant chemotherapy and pembrolizumab in patients with newly diagnosed, early-stage, triple-negative breast cancer yielded a higher percentage of patients achieving a pCR as compared with chemotherapy plus placebo.
Commentary
The current study adds to the growing body of literature outlining the efficacy of immune checkpoint inhibition in triple-negative breast cancer. The previously published IMpassion130 trial showed that the addition of the PD-L1 antibody atezolizumab to nab-paclitaxel improved progression-free survival in patients with PD-L1–positive (1% or greater), metastatic triple-negative breast cancer.1 Similarly, in the phase 2 I-SPY2 trial, the addition of pembrolizumab to standard neoadjuvant chemotherapy led to a near tripling of the pCR rates in triple-negative breast cancer.2 While the current study demonstrated improved pCR rates with pembrolizumab, no difference in EFS has yet been demonstrated; however, longer-term follow-up will be required. There certainly are numerous studies documenting an association between pCR and improved disease-free survival and possibly overall survival. Cortazar and colleagues performed a pooled analysis of 12 international trials, which demonstrated an association between pCR and improved EFS (hazard ratio [HR], 0.24) and overall survival (HR, 0.16) in patients with triple-negative breast cancer.3 The results of the current study will require longer-term follow-up to confirm such an association.
The current study appears to have demonstrated a benefit with the addition of pembrolizumab across treatment subgroups, particularly in the PD-L1–positive and PD-L1–negative populations. While this differs from the findings of the IMpassion130 trial, it is quite difficult to draw definitive conclusions because the 2 trials studied different antibodies, and thus used a different assay to define PD-L1 positivity. Notable differences exist in determination of PD-L1 status across assays, and it is important for providers to use the appropriate assay for each antibody. These differences highlight the need for more informative biomarkers to predict a benefit from immune checkpoint inhibition.
It is also noteworthy that the control arm in the current trial was a platinum-based regimen. Platinum-based neoadjuvant regimens previously have been shown to induce higher pCR rates in triple-negative breast cancer; however, the incorporation of carboplatin as standard of care remains a topic of debate.4 Nevertheless, a similar trial evaluating the efficacy of atezolizumab combined with platinum-based neoadjuvant chemotherapy in triple-negative breast cancer, NSABP B-59 (NCT03281954), is underway, with the control arm also incorporating carboplatin. The results of this study will also help validate the role of checkpoint inhibitors in the neoadjuvant setting in triple-negative breast cancer. Of note, this trial did not allow for the use of adjuvant capecitabine, which has been previously shown in the CREATE-X trial to prolong survival in this population.5 How the use of adjuvant capecitabine would impact these results is completely unknown.6 The incidence of grade 3 or higher toxicities in the current trial appeared to be similar in both groups. There did appear to be a higher incidence of infusion reactions and skin reactions in the pembrolizumab groups. Immune-related adverse events were consistent with prior pembrolizumab data.
Applications for Clinical Practice
KEYNOTE-522 adds to the growing evidence suggesting that incorporation of immune checkpoint inhibitors into neoadjuvant therapy in patients with triple-negative breast cancer can improve pCR rates; however, its use as a standard of care will require longer-term follow-up to ensure the noted findings translate into improvement in EFS and, ultimately, overall survival.
– Daniel Isaac, DO, MS
1. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121.
2. Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J Clin Oncol. 2017;35: Suppl:506. Abstract 506.
3. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172.
4. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant one-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33:13-21.
5. Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147-2159.
6. von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747-756.
1. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121.
2. Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J Clin Oncol. 2017;35: Suppl:506. Abstract 506.
3. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172.
4. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant one-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33:13-21.
5. Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147-2159.
6. von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747-756.
Renal denervation shown safe and effective in pivotal trial
Catheter-based renal denervation took a step closer to attaining legitimacy as a nonpharmacologic treatment for hypertension with presentation of the primary results of the SPYRAL HTN-OFF MED pivotal trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“We saw clinically meaningful blood pressure reductions at 3 months,” reported Michael Boehm, MD, chief of cardiology at Saarland University Hospital in Homburg, Germany.
That’s encouraging news, as renal denervation (RDN) was nearly abandoned as a potential treatment for hypertension in the wake of the unexpectedly negative results of the SYMPLICITY HTN-3 trial (N Engl J Med. 2014;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution, and a more rigorous development program for the percutaneous device-based therapy is well underway.
The SPYRAL HTN-OFF MED pivotal trial was designed under Food and Drug Administration guidance to show whether RDN reduces blood pressure in patients with untreated hypertension. The prospective study included 331 off-medication patients in nine countries who were randomized to RDN or a sham procedure, then followed in double-blind fashion for 3 months.
The primary outcome was change in 24-hour ambulatory systolic blood pressure from baseline to 3 months. From a mean baseline 24-hour ambulatory blood pressure of 151.4/98 mm Hg, patients in the RDN group averaged a 4.7 mm Hg decrease in 24-hour SBP, which was 4 mm Hg more than in sham-treated controls. Statistically, this translated to a greater than 99.9% probability that RDN was superior to sham therapy. The RDN group also experienced a mean 3.7–mm Hg reduction in 24-hour DBP, compared with a 0.8–mm Hg decrease in controls.
Office SBP – the secondary endpoint – decreased by a mean of 9.2 mm Hg with RDN, compared with 2.5 mm Hg in controls.
These results probably understate the true antihypertensive effect of RDN for two reasons, Dr. Boehm noted. For one, previous studies have shown that the magnitude of blood pressure lowering continues to increase for up to 1-2 years following the procedure, whereas the off-medication assessment in SPYRAL HTN-OFF MED ended at 3 months for ethical and safety reasons. Also, 17% of patients in the control arm were withdrawn from the study and placed on antihypertensive medication because their office SBP reached 180 mm Hg or more, as compared to 9.6% of the RDN group.
A key finding was that RDN lowered blood pressure around the clock, including nighttime and early morning, the hours of greatest cardiovascular risk and a time when some antihypertensive medications are less effective at blood pressure control, the cardiologist observed.
The RDN safety picture was reassuring, with no strokes, myocardial infarctions, major bleeding, or acute deterioration in kidney function.
A surprising finding was that, even though participants underwent blood and urine testing for the presence of antihypertensive drugs at baseline to ensure they were off medication, and were told they would be retested at 3 months, 5%-9% nonetheless tested positive at the second test.
That elicited a comment from session chair Richard A. Chazal, MD, of Fort Myers, Fla.: “I must say, as a clinician who sometimes has trouble getting his patients to take antihypertensives, it’s fascinating that some of the people that you asked not to take the medications were taking them.”
While the primary outcome in SPYRAL HTN-OFF MED was the 3-month reduction in blood pressure while off of antihypertensive medication, the ongoing second phase of the trial may have greater clinical relevance. At 3 months, participants are being placed on antihypertensive medication and uptitrated to target, with unblinding at 6 months. The purpose is to see how many RDN recipients don’t need antihypertensive drugs, as well as whether those that do require less medication than the patients who didn’t undergo RDN.
Dr. Boehm characterized RDN as a work in progress. Two major limitations that are the focus of intense research are the lack of a predictor as to which patients are most likely to respond to what is after all an invasive procedure, and the current inability intraprocedurally to tell if sufficient RDN has been achieved.
“Frankly speaking, there is no technology during the procedure to see how efficacious the procedure was,” he explained.
Discussant Dhanunaja Lakkireddy, MD, deemed the mean 4.7–mm Hg reduction in 24-hour SBP “reasonably impressive – that’s actually a pretty good number for an antihypertensive clinical trial.” He was also favorably impressed by RDN’s safety in a 44-site study.
“The drops in blood pressure are not enough to really make a case for renal denervation to be a standalone therapy. But adding it as an adjunct to standard medications may be a very reasonable strategy to adopt. This is a fantastic signal for something that can be brought along as a long-term add-on to antihypertensive medications,” commented Dr. Lakkireddy, chair of the ACC Electrophysiology Council and medical director of the Kansas City Heart Rhythm Institute.
Simultaneous with Dr. Boehm’s presentation, the SPYRAL HTN-OFF MED Pivotal Trial details were published online (Lancet 2020 Mar 29. doi: 10.1016/S0140-6736(20)30554-7).
The study was sponsored by Medtronic. Dr. Boehm reported serving as a consultant to that company and Abbott, Amgen, Astra, Boehringer-Ingelheim, Cytokinetics, Novartis, ReCor, Servier, and Vifor.
SOURCE: Boehm M. ACC 2020, Abstract 406-15.
Catheter-based renal denervation took a step closer to attaining legitimacy as a nonpharmacologic treatment for hypertension with presentation of the primary results of the SPYRAL HTN-OFF MED pivotal trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“We saw clinically meaningful blood pressure reductions at 3 months,” reported Michael Boehm, MD, chief of cardiology at Saarland University Hospital in Homburg, Germany.
That’s encouraging news, as renal denervation (RDN) was nearly abandoned as a potential treatment for hypertension in the wake of the unexpectedly negative results of the SYMPLICITY HTN-3 trial (N Engl J Med. 2014;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution, and a more rigorous development program for the percutaneous device-based therapy is well underway.
The SPYRAL HTN-OFF MED pivotal trial was designed under Food and Drug Administration guidance to show whether RDN reduces blood pressure in patients with untreated hypertension. The prospective study included 331 off-medication patients in nine countries who were randomized to RDN or a sham procedure, then followed in double-blind fashion for 3 months.
The primary outcome was change in 24-hour ambulatory systolic blood pressure from baseline to 3 months. From a mean baseline 24-hour ambulatory blood pressure of 151.4/98 mm Hg, patients in the RDN group averaged a 4.7 mm Hg decrease in 24-hour SBP, which was 4 mm Hg more than in sham-treated controls. Statistically, this translated to a greater than 99.9% probability that RDN was superior to sham therapy. The RDN group also experienced a mean 3.7–mm Hg reduction in 24-hour DBP, compared with a 0.8–mm Hg decrease in controls.
Office SBP – the secondary endpoint – decreased by a mean of 9.2 mm Hg with RDN, compared with 2.5 mm Hg in controls.
These results probably understate the true antihypertensive effect of RDN for two reasons, Dr. Boehm noted. For one, previous studies have shown that the magnitude of blood pressure lowering continues to increase for up to 1-2 years following the procedure, whereas the off-medication assessment in SPYRAL HTN-OFF MED ended at 3 months for ethical and safety reasons. Also, 17% of patients in the control arm were withdrawn from the study and placed on antihypertensive medication because their office SBP reached 180 mm Hg or more, as compared to 9.6% of the RDN group.
A key finding was that RDN lowered blood pressure around the clock, including nighttime and early morning, the hours of greatest cardiovascular risk and a time when some antihypertensive medications are less effective at blood pressure control, the cardiologist observed.
The RDN safety picture was reassuring, with no strokes, myocardial infarctions, major bleeding, or acute deterioration in kidney function.
A surprising finding was that, even though participants underwent blood and urine testing for the presence of antihypertensive drugs at baseline to ensure they were off medication, and were told they would be retested at 3 months, 5%-9% nonetheless tested positive at the second test.
That elicited a comment from session chair Richard A. Chazal, MD, of Fort Myers, Fla.: “I must say, as a clinician who sometimes has trouble getting his patients to take antihypertensives, it’s fascinating that some of the people that you asked not to take the medications were taking them.”
While the primary outcome in SPYRAL HTN-OFF MED was the 3-month reduction in blood pressure while off of antihypertensive medication, the ongoing second phase of the trial may have greater clinical relevance. At 3 months, participants are being placed on antihypertensive medication and uptitrated to target, with unblinding at 6 months. The purpose is to see how many RDN recipients don’t need antihypertensive drugs, as well as whether those that do require less medication than the patients who didn’t undergo RDN.
Dr. Boehm characterized RDN as a work in progress. Two major limitations that are the focus of intense research are the lack of a predictor as to which patients are most likely to respond to what is after all an invasive procedure, and the current inability intraprocedurally to tell if sufficient RDN has been achieved.
“Frankly speaking, there is no technology during the procedure to see how efficacious the procedure was,” he explained.
Discussant Dhanunaja Lakkireddy, MD, deemed the mean 4.7–mm Hg reduction in 24-hour SBP “reasonably impressive – that’s actually a pretty good number for an antihypertensive clinical trial.” He was also favorably impressed by RDN’s safety in a 44-site study.
“The drops in blood pressure are not enough to really make a case for renal denervation to be a standalone therapy. But adding it as an adjunct to standard medications may be a very reasonable strategy to adopt. This is a fantastic signal for something that can be brought along as a long-term add-on to antihypertensive medications,” commented Dr. Lakkireddy, chair of the ACC Electrophysiology Council and medical director of the Kansas City Heart Rhythm Institute.
Simultaneous with Dr. Boehm’s presentation, the SPYRAL HTN-OFF MED Pivotal Trial details were published online (Lancet 2020 Mar 29. doi: 10.1016/S0140-6736(20)30554-7).
The study was sponsored by Medtronic. Dr. Boehm reported serving as a consultant to that company and Abbott, Amgen, Astra, Boehringer-Ingelheim, Cytokinetics, Novartis, ReCor, Servier, and Vifor.
SOURCE: Boehm M. ACC 2020, Abstract 406-15.
Catheter-based renal denervation took a step closer to attaining legitimacy as a nonpharmacologic treatment for hypertension with presentation of the primary results of the SPYRAL HTN-OFF MED pivotal trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“We saw clinically meaningful blood pressure reductions at 3 months,” reported Michael Boehm, MD, chief of cardiology at Saarland University Hospital in Homburg, Germany.
That’s encouraging news, as renal denervation (RDN) was nearly abandoned as a potential treatment for hypertension in the wake of the unexpectedly negative results of the SYMPLICITY HTN-3 trial (N Engl J Med. 2014;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution, and a more rigorous development program for the percutaneous device-based therapy is well underway.
The SPYRAL HTN-OFF MED pivotal trial was designed under Food and Drug Administration guidance to show whether RDN reduces blood pressure in patients with untreated hypertension. The prospective study included 331 off-medication patients in nine countries who were randomized to RDN or a sham procedure, then followed in double-blind fashion for 3 months.
The primary outcome was change in 24-hour ambulatory systolic blood pressure from baseline to 3 months. From a mean baseline 24-hour ambulatory blood pressure of 151.4/98 mm Hg, patients in the RDN group averaged a 4.7 mm Hg decrease in 24-hour SBP, which was 4 mm Hg more than in sham-treated controls. Statistically, this translated to a greater than 99.9% probability that RDN was superior to sham therapy. The RDN group also experienced a mean 3.7–mm Hg reduction in 24-hour DBP, compared with a 0.8–mm Hg decrease in controls.
Office SBP – the secondary endpoint – decreased by a mean of 9.2 mm Hg with RDN, compared with 2.5 mm Hg in controls.
These results probably understate the true antihypertensive effect of RDN for two reasons, Dr. Boehm noted. For one, previous studies have shown that the magnitude of blood pressure lowering continues to increase for up to 1-2 years following the procedure, whereas the off-medication assessment in SPYRAL HTN-OFF MED ended at 3 months for ethical and safety reasons. Also, 17% of patients in the control arm were withdrawn from the study and placed on antihypertensive medication because their office SBP reached 180 mm Hg or more, as compared to 9.6% of the RDN group.
A key finding was that RDN lowered blood pressure around the clock, including nighttime and early morning, the hours of greatest cardiovascular risk and a time when some antihypertensive medications are less effective at blood pressure control, the cardiologist observed.
The RDN safety picture was reassuring, with no strokes, myocardial infarctions, major bleeding, or acute deterioration in kidney function.
A surprising finding was that, even though participants underwent blood and urine testing for the presence of antihypertensive drugs at baseline to ensure they were off medication, and were told they would be retested at 3 months, 5%-9% nonetheless tested positive at the second test.
That elicited a comment from session chair Richard A. Chazal, MD, of Fort Myers, Fla.: “I must say, as a clinician who sometimes has trouble getting his patients to take antihypertensives, it’s fascinating that some of the people that you asked not to take the medications were taking them.”
While the primary outcome in SPYRAL HTN-OFF MED was the 3-month reduction in blood pressure while off of antihypertensive medication, the ongoing second phase of the trial may have greater clinical relevance. At 3 months, participants are being placed on antihypertensive medication and uptitrated to target, with unblinding at 6 months. The purpose is to see how many RDN recipients don’t need antihypertensive drugs, as well as whether those that do require less medication than the patients who didn’t undergo RDN.
Dr. Boehm characterized RDN as a work in progress. Two major limitations that are the focus of intense research are the lack of a predictor as to which patients are most likely to respond to what is after all an invasive procedure, and the current inability intraprocedurally to tell if sufficient RDN has been achieved.
“Frankly speaking, there is no technology during the procedure to see how efficacious the procedure was,” he explained.
Discussant Dhanunaja Lakkireddy, MD, deemed the mean 4.7–mm Hg reduction in 24-hour SBP “reasonably impressive – that’s actually a pretty good number for an antihypertensive clinical trial.” He was also favorably impressed by RDN’s safety in a 44-site study.
“The drops in blood pressure are not enough to really make a case for renal denervation to be a standalone therapy. But adding it as an adjunct to standard medications may be a very reasonable strategy to adopt. This is a fantastic signal for something that can be brought along as a long-term add-on to antihypertensive medications,” commented Dr. Lakkireddy, chair of the ACC Electrophysiology Council and medical director of the Kansas City Heart Rhythm Institute.
Simultaneous with Dr. Boehm’s presentation, the SPYRAL HTN-OFF MED Pivotal Trial details were published online (Lancet 2020 Mar 29. doi: 10.1016/S0140-6736(20)30554-7).
The study was sponsored by Medtronic. Dr. Boehm reported serving as a consultant to that company and Abbott, Amgen, Astra, Boehringer-Ingelheim, Cytokinetics, Novartis, ReCor, Servier, and Vifor.
SOURCE: Boehm M. ACC 2020, Abstract 406-15.
REPORTING FROM ACC 20
Outcomes-based measurement of TAVR program quality goes live
The long-sought goal of measuring the quality of U.S. transcatheter aortic valve replacement (TAVR) programs by patient outcomes rather than by the surrogate measure of case volume is about to be realized.
Starting more or less immediately, the U.S. national register for all TAVR cases that’s mandated by Food and Drug Administration labeling of these devices and run through a collaboration of the American College of Cardiology and the Society of Thoracic Surgeons will start applying a newly developed and validated five-item metric for measuring 30-day patient outcomes and designed to gauge the quality of TAVR programs.
At first, the only recipients of the data will be the programs themselves, but starting in about a year, by sometime in 2021, the STS/ACC TVT (transcatheter valve therapy) Registry will start to make its star-based rating of TAVR programs available to the public, Nimesh D. Desai, MD, said on March 29 at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic. These societies already make star-based ratings of U.S. programs available to the public for several other types of cardiac interventions, including coronary artery bypass surgery and MI management.
The new, composite metric based on 30-day outcome data “is appropriate for high-stakes applications such as public reporting,” said Dr. Desai, a thoracic surgeon and director of thoracic aortic surgery research at the University of Pennsylvania in Philadelphia. “We’re confident this model can be used for public reporting. It’s undergone extensive testing of its validity.” The steering committee of the STS/ACC TVT Registry commissioned development of the metric, and it’s now “considered approved,” and ready for use, he explained.
To create the new metric, Dr. Desai and his associates used data from 52,561 patients who underwent transfemoral TAVR during 2015-2017 at any of 301 U.S. sites. These data came from a total pool of more than 114,000 patients at 556 sites, but data from many sites weren’t usable because they were not adequately complete. The researchers then identified the top four measures taken during the 30 days following intervention (hospitalization included) that best correlated with 1-year survival and patients’ quality-of-life scores on the Kansas City Cardiomyopathy Questionnaire: stroke; major, life-threatening, or disabling bleed; acute kidney injury (stage III); and moderate or severe paravalvular leak. These outcomes “matter most to patients,” Dr. Desai said.
They used these four outcomes plus 30-day mortality to calculate the programs’ ratings. Among the 52,561 patients, 14% had at least one of these adverse outcomes. The researchers then used a logistic regression model that adjusted for 46 measured variables to calculate how each program performed relative to the average performance of all the programs. Programs with outcomes that fell within the 95% confidence intervals of average performance were rated as performing as expected; those outside rated as performing either better or worse than expected. The results showed 34 centers (11%) had worse than expected outcomes and 25 (8%) had better than expected outcomes, Dr. Desai said.
“This is a major step forward in measuring TAVR quality,” commented Michael Mack, MD, a cardiac surgeon with Baylor Scott & White Health in Dallas who has been very active in studying TAVR. “Until now, we used volume as a surrogate for quality, but the precision was not great. This is an extremely welcome metric.” The next step is to eventually use 1-year follow-up data instead of 30-day outcomes, he added.
“With the rapid expansion of TAVR over the past 6-8 years, we’re now at the point to start to do this. It’s an ethical obligation This will be one of the most high-fidelity, valid models for public reporting” of clinical outcomes,” said Joseph Cleveland, MD, a professor of surgery at the University of Colorado at Denver in Aurora. “It’s reassuring that about 90% of the program performed as expected or better than expected,” he added.
“Transparency and outcomes should drive how TAVR is delivered,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medicine Heart Institute in Phoenix who estimated that he performs about 150 TAVR procedures annually. “This is a step forward. I’ve been waiting for this for a long time. Until now, volume has been used as a surrogate outcome, but we know it’s not accurate. I’m confident that this model is a good starting point.” But Dr. Pershad also had concern that this new approach “can lend itself to some degree of gaming,” like a bleeding event getting classified as minor when it was really major, or outlier patients getting dropped from reports.
The temptation to cut corners may be higher for TAVR than it’s been for the cardiac-disease metrics that already get publicly reported, like bypass surgery and myocardial infarction management, because of TAVR’s higher cost and higher profile, Dr. Pershad said. Existing measures “have not been as linked to financial disincentive as TAVR might be” because TAVR reimbursements can run as high as $50,000 per case. “The stakes with TAVR are higher,” he said.
Ultimately, the reliable examination of TAVR outcomes that this new metric allows may lead to a shake-up of TAVR programs, Dr. Pershad predicted. “This is clearly a step toward closing down some programs that [consistently] underperform.”
The STS/ACC TVT Registry receives no commercial funding. Dr. Desai has been a consultant to, speaker on behalf of, and received research funding from Gore, and he has also spoken on behalf of Cook, Medtronic, and Terumo Aortic. Dr. Cleveland, Dr. Mack, and Dr. Pershad had no disclosures.
The long-sought goal of measuring the quality of U.S. transcatheter aortic valve replacement (TAVR) programs by patient outcomes rather than by the surrogate measure of case volume is about to be realized.
Starting more or less immediately, the U.S. national register for all TAVR cases that’s mandated by Food and Drug Administration labeling of these devices and run through a collaboration of the American College of Cardiology and the Society of Thoracic Surgeons will start applying a newly developed and validated five-item metric for measuring 30-day patient outcomes and designed to gauge the quality of TAVR programs.
At first, the only recipients of the data will be the programs themselves, but starting in about a year, by sometime in 2021, the STS/ACC TVT (transcatheter valve therapy) Registry will start to make its star-based rating of TAVR programs available to the public, Nimesh D. Desai, MD, said on March 29 at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic. These societies already make star-based ratings of U.S. programs available to the public for several other types of cardiac interventions, including coronary artery bypass surgery and MI management.
The new, composite metric based on 30-day outcome data “is appropriate for high-stakes applications such as public reporting,” said Dr. Desai, a thoracic surgeon and director of thoracic aortic surgery research at the University of Pennsylvania in Philadelphia. “We’re confident this model can be used for public reporting. It’s undergone extensive testing of its validity.” The steering committee of the STS/ACC TVT Registry commissioned development of the metric, and it’s now “considered approved,” and ready for use, he explained.
To create the new metric, Dr. Desai and his associates used data from 52,561 patients who underwent transfemoral TAVR during 2015-2017 at any of 301 U.S. sites. These data came from a total pool of more than 114,000 patients at 556 sites, but data from many sites weren’t usable because they were not adequately complete. The researchers then identified the top four measures taken during the 30 days following intervention (hospitalization included) that best correlated with 1-year survival and patients’ quality-of-life scores on the Kansas City Cardiomyopathy Questionnaire: stroke; major, life-threatening, or disabling bleed; acute kidney injury (stage III); and moderate or severe paravalvular leak. These outcomes “matter most to patients,” Dr. Desai said.
They used these four outcomes plus 30-day mortality to calculate the programs’ ratings. Among the 52,561 patients, 14% had at least one of these adverse outcomes. The researchers then used a logistic regression model that adjusted for 46 measured variables to calculate how each program performed relative to the average performance of all the programs. Programs with outcomes that fell within the 95% confidence intervals of average performance were rated as performing as expected; those outside rated as performing either better or worse than expected. The results showed 34 centers (11%) had worse than expected outcomes and 25 (8%) had better than expected outcomes, Dr. Desai said.
“This is a major step forward in measuring TAVR quality,” commented Michael Mack, MD, a cardiac surgeon with Baylor Scott & White Health in Dallas who has been very active in studying TAVR. “Until now, we used volume as a surrogate for quality, but the precision was not great. This is an extremely welcome metric.” The next step is to eventually use 1-year follow-up data instead of 30-day outcomes, he added.
“With the rapid expansion of TAVR over the past 6-8 years, we’re now at the point to start to do this. It’s an ethical obligation This will be one of the most high-fidelity, valid models for public reporting” of clinical outcomes,” said Joseph Cleveland, MD, a professor of surgery at the University of Colorado at Denver in Aurora. “It’s reassuring that about 90% of the program performed as expected or better than expected,” he added.
“Transparency and outcomes should drive how TAVR is delivered,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medicine Heart Institute in Phoenix who estimated that he performs about 150 TAVR procedures annually. “This is a step forward. I’ve been waiting for this for a long time. Until now, volume has been used as a surrogate outcome, but we know it’s not accurate. I’m confident that this model is a good starting point.” But Dr. Pershad also had concern that this new approach “can lend itself to some degree of gaming,” like a bleeding event getting classified as minor when it was really major, or outlier patients getting dropped from reports.
The temptation to cut corners may be higher for TAVR than it’s been for the cardiac-disease metrics that already get publicly reported, like bypass surgery and myocardial infarction management, because of TAVR’s higher cost and higher profile, Dr. Pershad said. Existing measures “have not been as linked to financial disincentive as TAVR might be” because TAVR reimbursements can run as high as $50,000 per case. “The stakes with TAVR are higher,” he said.
Ultimately, the reliable examination of TAVR outcomes that this new metric allows may lead to a shake-up of TAVR programs, Dr. Pershad predicted. “This is clearly a step toward closing down some programs that [consistently] underperform.”
The STS/ACC TVT Registry receives no commercial funding. Dr. Desai has been a consultant to, speaker on behalf of, and received research funding from Gore, and he has also spoken on behalf of Cook, Medtronic, and Terumo Aortic. Dr. Cleveland, Dr. Mack, and Dr. Pershad had no disclosures.
The long-sought goal of measuring the quality of U.S. transcatheter aortic valve replacement (TAVR) programs by patient outcomes rather than by the surrogate measure of case volume is about to be realized.
Starting more or less immediately, the U.S. national register for all TAVR cases that’s mandated by Food and Drug Administration labeling of these devices and run through a collaboration of the American College of Cardiology and the Society of Thoracic Surgeons will start applying a newly developed and validated five-item metric for measuring 30-day patient outcomes and designed to gauge the quality of TAVR programs.
At first, the only recipients of the data will be the programs themselves, but starting in about a year, by sometime in 2021, the STS/ACC TVT (transcatheter valve therapy) Registry will start to make its star-based rating of TAVR programs available to the public, Nimesh D. Desai, MD, said on March 29 at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic. These societies already make star-based ratings of U.S. programs available to the public for several other types of cardiac interventions, including coronary artery bypass surgery and MI management.
The new, composite metric based on 30-day outcome data “is appropriate for high-stakes applications such as public reporting,” said Dr. Desai, a thoracic surgeon and director of thoracic aortic surgery research at the University of Pennsylvania in Philadelphia. “We’re confident this model can be used for public reporting. It’s undergone extensive testing of its validity.” The steering committee of the STS/ACC TVT Registry commissioned development of the metric, and it’s now “considered approved,” and ready for use, he explained.
To create the new metric, Dr. Desai and his associates used data from 52,561 patients who underwent transfemoral TAVR during 2015-2017 at any of 301 U.S. sites. These data came from a total pool of more than 114,000 patients at 556 sites, but data from many sites weren’t usable because they were not adequately complete. The researchers then identified the top four measures taken during the 30 days following intervention (hospitalization included) that best correlated with 1-year survival and patients’ quality-of-life scores on the Kansas City Cardiomyopathy Questionnaire: stroke; major, life-threatening, or disabling bleed; acute kidney injury (stage III); and moderate or severe paravalvular leak. These outcomes “matter most to patients,” Dr. Desai said.
They used these four outcomes plus 30-day mortality to calculate the programs’ ratings. Among the 52,561 patients, 14% had at least one of these adverse outcomes. The researchers then used a logistic regression model that adjusted for 46 measured variables to calculate how each program performed relative to the average performance of all the programs. Programs with outcomes that fell within the 95% confidence intervals of average performance were rated as performing as expected; those outside rated as performing either better or worse than expected. The results showed 34 centers (11%) had worse than expected outcomes and 25 (8%) had better than expected outcomes, Dr. Desai said.
“This is a major step forward in measuring TAVR quality,” commented Michael Mack, MD, a cardiac surgeon with Baylor Scott & White Health in Dallas who has been very active in studying TAVR. “Until now, we used volume as a surrogate for quality, but the precision was not great. This is an extremely welcome metric.” The next step is to eventually use 1-year follow-up data instead of 30-day outcomes, he added.
“With the rapid expansion of TAVR over the past 6-8 years, we’re now at the point to start to do this. It’s an ethical obligation This will be one of the most high-fidelity, valid models for public reporting” of clinical outcomes,” said Joseph Cleveland, MD, a professor of surgery at the University of Colorado at Denver in Aurora. “It’s reassuring that about 90% of the program performed as expected or better than expected,” he added.
“Transparency and outcomes should drive how TAVR is delivered,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medicine Heart Institute in Phoenix who estimated that he performs about 150 TAVR procedures annually. “This is a step forward. I’ve been waiting for this for a long time. Until now, volume has been used as a surrogate outcome, but we know it’s not accurate. I’m confident that this model is a good starting point.” But Dr. Pershad also had concern that this new approach “can lend itself to some degree of gaming,” like a bleeding event getting classified as minor when it was really major, or outlier patients getting dropped from reports.
The temptation to cut corners may be higher for TAVR than it’s been for the cardiac-disease metrics that already get publicly reported, like bypass surgery and myocardial infarction management, because of TAVR’s higher cost and higher profile, Dr. Pershad said. Existing measures “have not been as linked to financial disincentive as TAVR might be” because TAVR reimbursements can run as high as $50,000 per case. “The stakes with TAVR are higher,” he said.
Ultimately, the reliable examination of TAVR outcomes that this new metric allows may lead to a shake-up of TAVR programs, Dr. Pershad predicted. “This is clearly a step toward closing down some programs that [consistently] underperform.”
The STS/ACC TVT Registry receives no commercial funding. Dr. Desai has been a consultant to, speaker on behalf of, and received research funding from Gore, and he has also spoken on behalf of Cook, Medtronic, and Terumo Aortic. Dr. Cleveland, Dr. Mack, and Dr. Pershad had no disclosures.
REPORTING FROM ACC 20