User login
Command hallucinations, but is it really psychosis?
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.
Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.
TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
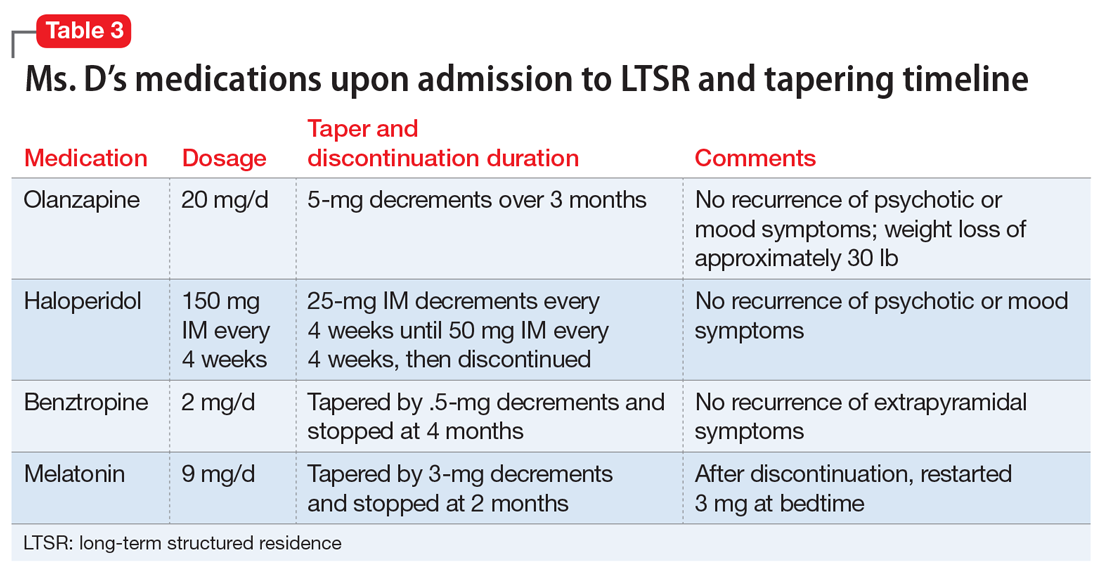
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.
Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.
Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.
TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.
Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.
Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.
TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.
Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
Polypharmacy in older adults
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15
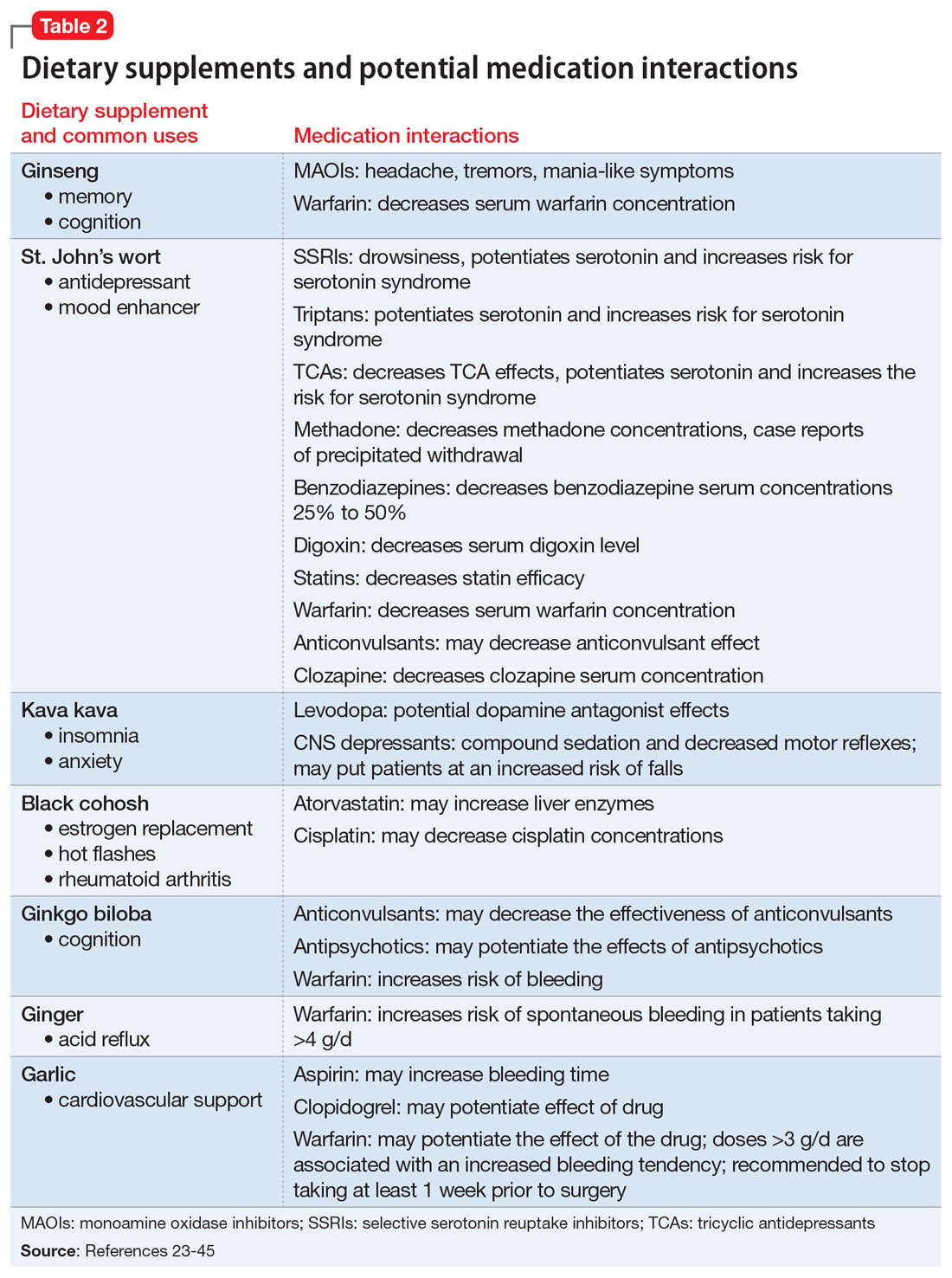
What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.
Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15
What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.
Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15
What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.
Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
More on ‘We are physicians, not providers’
I enjoyed Dr. Nasrallah’s recent editorial “We are physicians, not providers, and we treat patients, not clients!” (From the Editor,
A stethoscope, pressure cuff, and ophthalmoscope sit on the side table next to my psychotherapy chair, and I use them often. For years, I have administered electroconvulsive therapy and managed vagus nerve stimulation. I inject long-acting depot antipsychotics as well as depot buprenorphine and naltrexone, and I provide esketamine treatment. I spend significant parts of my patients’ visits explaining the treatments their other physicians have prescribed, in words they can understand. I still spend hours each week providing psychotherapy.
My psychiatric training afforded me the insight that my brother’s remark caused a lifelong change in my own behavior. Our capacity to blend psychology and medicine distinguishes us from our medical brethren and psychology colleagues.
I agree wholeheartedly that the medical bureaucracy/insurance industry degrades and demeans our profession, and we should do all in our power to resist this.
Norton Shores, Michigan
I congratulate Dr. Nasrallah on his forceful and clear editorial aimed at demystifying labels that only blur our identity and the nature of the patient-physician covenant.
Washington, DC
Continue to: I absolutely...
I absolutely agree with Dr. Nasrallah’s position that we are physicians, not “providers,” and that the people we care for are patients, not “clients.” Given the enthusiasm with which insurance companies have embraced the term provider (under the notion that we are indistinguishable from other types of trained professionals providing care), perhaps we should stop referring to them as “payors” because this does not seem to be their primary function. Instead, we can refer to them as “withholders” to better indicate the difference between what clinicians do (provide care) and what insurance companies do (withhold payment and thus delay care, sometimes with disastrous consequences).
Orefield, Pennsylvania
Regarding being called “providers,” I believe we have brought this on ourselves by allowing “management experts” to decide how we treat our patients.
Shyamala Vatsa, MBBS, DPM
Bengaluru, India
Being called a “provider” has been one of the most annoying and insulting things that we have had to endure in the last few years. Until Dr. Nasrallah’s editorial, I had never heard anyone raise this issue in a medical journal. The American Psychiatric Association (APA) and the American Medical Association (AMA) must help us with this. I have been saying for years that we are all going to be replaced by nurse practitioners (NPs)!
Chicago, Illinois
Continue to: I thank...
I thank Dr. Nasrallah very much for his editorial about being called “providers,” which has always irritated me. We worked hard to get our MDs. I’ve told my residents for a long time that the term “provider” sounds more like a wholesale grocery company. A similar term also confounds me: behavioral medicine. What is that, an old-fashioned reform school? We don’t change patients’ behavior to conform; we treat their illnesses and symptoms so that they can do better for themselves.
Bronx, New York
I commend Dr. Nasrallah’s recent editorial. Society must reconsider the concept that health care is a “product.” Patient education is key!
This editorial was translated into Portuguese by a colleague, and it has started a very interesting and fruitful debate.
Rio de Janeiro, Brasil
I was extremely impressed with the wisdom Dr. Nasrallah expressed in his editorial about how we should be referred to as physicians, not as “providers.” For many years, I have referred to my primary care colleagues as “PCPs,” which stands for primary care providers. I will do this no more and instead use the preferred and more accurate term primary care physicians. And yes, I do support his suggestion that the APA’s name be changed to the American Psychiatric Physicians Association, and hope that the plan to implement these improvements succeeds throughout the United States.
Rochester, New York
Continue to: I absolutely...
I absolutely loved Dr. Nasrallah's editorial and completely agree with him. Perhaps we could begin a letter-writing campaign urging Congress to amend Public Law 93-641 by replacing “provider” with “physicians and/or other health specialists” or something to that effect. With enough persuasion, the AMA might even take the lead on this.
Deborah Young, MD
Encinitas, California
I found Dr. Nasrallah's editorial extremely interesting and on target. I agree with his concern about the trend towards “bottom line” medicine.
I understand that, justifiably, Dr. Nasrallah's basic interest is in the realm of psychiatry (both clinicians and patients); however, shouldn’t that concern be broadened by recognizing that in all medical relationships, the individual (ie, physician assistant [PA], NP, registered nurse, respiratory therapist, etc.) is a “clinician” who cares for a patient
Also, while more and more people are willing to admit to being a patient undergoing mental health care, there still are many people who prefer not to called a patient when—or if—they describe the experience of receiving mental health care.
I teach a class called “Legal & Ethical Issues In Medicine” in a PA program, and one of the major objectives is to get the students to recognize and feel that they are becoming professionals. They are becoming clinicians who will help manage the health care of a patient.
Again, I appreciate Dr. Nasrallah's points, and wish that they could have a broader exposure.
Robert C. Grosz, EdD
Ft. Lauderdale, Florida
Continue to: I love...
I love Dr. Nasrallah's editorial. It was so refreshing to read, and we really need more of this all around. The language we use is important, and as a teaching faculty member, I see medical students and residents tossing around the term "provider" flippantly; it seems the term has been drilled into them. It is completely and totally demoralizing to our profession and has everything to do with the corporate takeover of health care. I lecture my students on physician advocacy, specifically regarding midlevel encroachment into medicine, but there is so much misuse of health care terminology. Now, everyone is a doctor, clinician, provider, or prescriber. Why not just identify everyone by their actual credentials? I also completely agree about the term “client,” which is also ridiculous.
I found Dr. Nasrallah's editorial to be a breath of fresh air. I thank him for it!
Laura K. Kendall, MD
Los Angeles, California
I want to congratulate Dr. Nasrallah for addressing the "provider" issue in his editorial. It is right on the mark and has been a thorn in my side for many years (as is the “client” vs “patient” issue). He worded it very well! I thank Dr. Nasrallah for discussing this.
René S. Kahn, MD, PhD
New York, New York
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
I thank my colleagues, whose letters confirm that being called “providers” instead of “physicians” is something that all psychiatrists resent and oppose. I hope each psychiatric physician reader of
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
I enjoyed Dr. Nasrallah’s recent editorial “We are physicians, not providers, and we treat patients, not clients!” (From the Editor,
A stethoscope, pressure cuff, and ophthalmoscope sit on the side table next to my psychotherapy chair, and I use them often. For years, I have administered electroconvulsive therapy and managed vagus nerve stimulation. I inject long-acting depot antipsychotics as well as depot buprenorphine and naltrexone, and I provide esketamine treatment. I spend significant parts of my patients’ visits explaining the treatments their other physicians have prescribed, in words they can understand. I still spend hours each week providing psychotherapy.
My psychiatric training afforded me the insight that my brother’s remark caused a lifelong change in my own behavior. Our capacity to blend psychology and medicine distinguishes us from our medical brethren and psychology colleagues.
I agree wholeheartedly that the medical bureaucracy/insurance industry degrades and demeans our profession, and we should do all in our power to resist this.
Norton Shores, Michigan
I congratulate Dr. Nasrallah on his forceful and clear editorial aimed at demystifying labels that only blur our identity and the nature of the patient-physician covenant.
Washington, DC
Continue to: I absolutely...
I absolutely agree with Dr. Nasrallah’s position that we are physicians, not “providers,” and that the people we care for are patients, not “clients.” Given the enthusiasm with which insurance companies have embraced the term provider (under the notion that we are indistinguishable from other types of trained professionals providing care), perhaps we should stop referring to them as “payors” because this does not seem to be their primary function. Instead, we can refer to them as “withholders” to better indicate the difference between what clinicians do (provide care) and what insurance companies do (withhold payment and thus delay care, sometimes with disastrous consequences).
Orefield, Pennsylvania
Regarding being called “providers,” I believe we have brought this on ourselves by allowing “management experts” to decide how we treat our patients.
Shyamala Vatsa, MBBS, DPM
Bengaluru, India
Being called a “provider” has been one of the most annoying and insulting things that we have had to endure in the last few years. Until Dr. Nasrallah’s editorial, I had never heard anyone raise this issue in a medical journal. The American Psychiatric Association (APA) and the American Medical Association (AMA) must help us with this. I have been saying for years that we are all going to be replaced by nurse practitioners (NPs)!
Chicago, Illinois
Continue to: I thank...
I thank Dr. Nasrallah very much for his editorial about being called “providers,” which has always irritated me. We worked hard to get our MDs. I’ve told my residents for a long time that the term “provider” sounds more like a wholesale grocery company. A similar term also confounds me: behavioral medicine. What is that, an old-fashioned reform school? We don’t change patients’ behavior to conform; we treat their illnesses and symptoms so that they can do better for themselves.
Bronx, New York
I commend Dr. Nasrallah’s recent editorial. Society must reconsider the concept that health care is a “product.” Patient education is key!
This editorial was translated into Portuguese by a colleague, and it has started a very interesting and fruitful debate.
Rio de Janeiro, Brasil
I was extremely impressed with the wisdom Dr. Nasrallah expressed in his editorial about how we should be referred to as physicians, not as “providers.” For many years, I have referred to my primary care colleagues as “PCPs,” which stands for primary care providers. I will do this no more and instead use the preferred and more accurate term primary care physicians. And yes, I do support his suggestion that the APA’s name be changed to the American Psychiatric Physicians Association, and hope that the plan to implement these improvements succeeds throughout the United States.
Rochester, New York
Continue to: I absolutely...
I absolutely loved Dr. Nasrallah's editorial and completely agree with him. Perhaps we could begin a letter-writing campaign urging Congress to amend Public Law 93-641 by replacing “provider” with “physicians and/or other health specialists” or something to that effect. With enough persuasion, the AMA might even take the lead on this.
Deborah Young, MD
Encinitas, California
I found Dr. Nasrallah's editorial extremely interesting and on target. I agree with his concern about the trend towards “bottom line” medicine.
I understand that, justifiably, Dr. Nasrallah's basic interest is in the realm of psychiatry (both clinicians and patients); however, shouldn’t that concern be broadened by recognizing that in all medical relationships, the individual (ie, physician assistant [PA], NP, registered nurse, respiratory therapist, etc.) is a “clinician” who cares for a patient
Also, while more and more people are willing to admit to being a patient undergoing mental health care, there still are many people who prefer not to called a patient when—or if—they describe the experience of receiving mental health care.
I teach a class called “Legal & Ethical Issues In Medicine” in a PA program, and one of the major objectives is to get the students to recognize and feel that they are becoming professionals. They are becoming clinicians who will help manage the health care of a patient.
Again, I appreciate Dr. Nasrallah's points, and wish that they could have a broader exposure.
Robert C. Grosz, EdD
Ft. Lauderdale, Florida
Continue to: I love...
I love Dr. Nasrallah's editorial. It was so refreshing to read, and we really need more of this all around. The language we use is important, and as a teaching faculty member, I see medical students and residents tossing around the term "provider" flippantly; it seems the term has been drilled into them. It is completely and totally demoralizing to our profession and has everything to do with the corporate takeover of health care. I lecture my students on physician advocacy, specifically regarding midlevel encroachment into medicine, but there is so much misuse of health care terminology. Now, everyone is a doctor, clinician, provider, or prescriber. Why not just identify everyone by their actual credentials? I also completely agree about the term “client,” which is also ridiculous.
I found Dr. Nasrallah's editorial to be a breath of fresh air. I thank him for it!
Laura K. Kendall, MD
Los Angeles, California
I want to congratulate Dr. Nasrallah for addressing the "provider" issue in his editorial. It is right on the mark and has been a thorn in my side for many years (as is the “client” vs “patient” issue). He worded it very well! I thank Dr. Nasrallah for discussing this.
René S. Kahn, MD, PhD
New York, New York
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
I thank my colleagues, whose letters confirm that being called “providers” instead of “physicians” is something that all psychiatrists resent and oppose. I hope each psychiatric physician reader of
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
I enjoyed Dr. Nasrallah’s recent editorial “We are physicians, not providers, and we treat patients, not clients!” (From the Editor,
A stethoscope, pressure cuff, and ophthalmoscope sit on the side table next to my psychotherapy chair, and I use them often. For years, I have administered electroconvulsive therapy and managed vagus nerve stimulation. I inject long-acting depot antipsychotics as well as depot buprenorphine and naltrexone, and I provide esketamine treatment. I spend significant parts of my patients’ visits explaining the treatments their other physicians have prescribed, in words they can understand. I still spend hours each week providing psychotherapy.
My psychiatric training afforded me the insight that my brother’s remark caused a lifelong change in my own behavior. Our capacity to blend psychology and medicine distinguishes us from our medical brethren and psychology colleagues.
I agree wholeheartedly that the medical bureaucracy/insurance industry degrades and demeans our profession, and we should do all in our power to resist this.
Norton Shores, Michigan
I congratulate Dr. Nasrallah on his forceful and clear editorial aimed at demystifying labels that only blur our identity and the nature of the patient-physician covenant.
Washington, DC
Continue to: I absolutely...
I absolutely agree with Dr. Nasrallah’s position that we are physicians, not “providers,” and that the people we care for are patients, not “clients.” Given the enthusiasm with which insurance companies have embraced the term provider (under the notion that we are indistinguishable from other types of trained professionals providing care), perhaps we should stop referring to them as “payors” because this does not seem to be their primary function. Instead, we can refer to them as “withholders” to better indicate the difference between what clinicians do (provide care) and what insurance companies do (withhold payment and thus delay care, sometimes with disastrous consequences).
Orefield, Pennsylvania
Regarding being called “providers,” I believe we have brought this on ourselves by allowing “management experts” to decide how we treat our patients.
Shyamala Vatsa, MBBS, DPM
Bengaluru, India
Being called a “provider” has been one of the most annoying and insulting things that we have had to endure in the last few years. Until Dr. Nasrallah’s editorial, I had never heard anyone raise this issue in a medical journal. The American Psychiatric Association (APA) and the American Medical Association (AMA) must help us with this. I have been saying for years that we are all going to be replaced by nurse practitioners (NPs)!
Chicago, Illinois
Continue to: I thank...
I thank Dr. Nasrallah very much for his editorial about being called “providers,” which has always irritated me. We worked hard to get our MDs. I’ve told my residents for a long time that the term “provider” sounds more like a wholesale grocery company. A similar term also confounds me: behavioral medicine. What is that, an old-fashioned reform school? We don’t change patients’ behavior to conform; we treat their illnesses and symptoms so that they can do better for themselves.
Bronx, New York
I commend Dr. Nasrallah’s recent editorial. Society must reconsider the concept that health care is a “product.” Patient education is key!
This editorial was translated into Portuguese by a colleague, and it has started a very interesting and fruitful debate.
Rio de Janeiro, Brasil
I was extremely impressed with the wisdom Dr. Nasrallah expressed in his editorial about how we should be referred to as physicians, not as “providers.” For many years, I have referred to my primary care colleagues as “PCPs,” which stands for primary care providers. I will do this no more and instead use the preferred and more accurate term primary care physicians. And yes, I do support his suggestion that the APA’s name be changed to the American Psychiatric Physicians Association, and hope that the plan to implement these improvements succeeds throughout the United States.
Rochester, New York
Continue to: I absolutely...
I absolutely loved Dr. Nasrallah's editorial and completely agree with him. Perhaps we could begin a letter-writing campaign urging Congress to amend Public Law 93-641 by replacing “provider” with “physicians and/or other health specialists” or something to that effect. With enough persuasion, the AMA might even take the lead on this.
Deborah Young, MD
Encinitas, California
I found Dr. Nasrallah's editorial extremely interesting and on target. I agree with his concern about the trend towards “bottom line” medicine.
I understand that, justifiably, Dr. Nasrallah's basic interest is in the realm of psychiatry (both clinicians and patients); however, shouldn’t that concern be broadened by recognizing that in all medical relationships, the individual (ie, physician assistant [PA], NP, registered nurse, respiratory therapist, etc.) is a “clinician” who cares for a patient
Also, while more and more people are willing to admit to being a patient undergoing mental health care, there still are many people who prefer not to called a patient when—or if—they describe the experience of receiving mental health care.
I teach a class called “Legal & Ethical Issues In Medicine” in a PA program, and one of the major objectives is to get the students to recognize and feel that they are becoming professionals. They are becoming clinicians who will help manage the health care of a patient.
Again, I appreciate Dr. Nasrallah's points, and wish that they could have a broader exposure.
Robert C. Grosz, EdD
Ft. Lauderdale, Florida
Continue to: I love...
I love Dr. Nasrallah's editorial. It was so refreshing to read, and we really need more of this all around. The language we use is important, and as a teaching faculty member, I see medical students and residents tossing around the term "provider" flippantly; it seems the term has been drilled into them. It is completely and totally demoralizing to our profession and has everything to do with the corporate takeover of health care. I lecture my students on physician advocacy, specifically regarding midlevel encroachment into medicine, but there is so much misuse of health care terminology. Now, everyone is a doctor, clinician, provider, or prescriber. Why not just identify everyone by their actual credentials? I also completely agree about the term “client,” which is also ridiculous.
I found Dr. Nasrallah's editorial to be a breath of fresh air. I thank him for it!
Laura K. Kendall, MD
Los Angeles, California
I want to congratulate Dr. Nasrallah for addressing the "provider" issue in his editorial. It is right on the mark and has been a thorn in my side for many years (as is the “client” vs “patient” issue). He worded it very well! I thank Dr. Nasrallah for discussing this.
René S. Kahn, MD, PhD
New York, New York
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
I thank my colleagues, whose letters confirm that being called “providers” instead of “physicians” is something that all psychiatrists resent and oppose. I hope each psychiatric physician reader of
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
Pre-authorization is illegal, unethical, and adversely disrupts patient care
Pre-authorization is a despicable scam. It’s a national racket by avaricious insurance companies, and it must be stopped. Since it first reared its ugly head 2 decades ago, it has inflicted great harm to countless patients, demoralized their physicians, and needlessly imposed higher costs in clinical practice while simultaneously depriving patients of the treatment their physicians prescribed for them.
Pre-authorization has become the nemesis of medical care. It recklessly and arbitrarily vetoes the clinical decision-making of competent physicians doing their best to address their patients’ medical needs. Yet, despite its outrageous disruption of the clinical practice of hundreds of thousands of practitioners, it continues unabated, without a forceful pushback. It has become the “new normal,” but in fact, it is the “new abnormal.” This harassment of clinicians must be outlawed.
Think about it: Pre-authorization is essentially practicing medicine without a license, which is a felony. When a remote and invisible insurance company staff member either prevents a patient from receiving a medication prescribed by that patient’s personal physician following a full diagnostic evaluation or pressures the physician to prescribe a different medication, he/she is basically deciding what the treatment should be for a patient who that insurance company employee has never seen, let alone examined. How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company? That is patently unethical, in addition to being a felonious practice of medicine by an absentee person unqualified to decide what a patient needs without a direct examination.
Consider the multiple malignant consequences of such brazen and egregious restriction or distortion of medical care:
1. The physician’s clinical judgment is abrogated, even when it is clearly in the patient’s best interest.
2. Patients are deprived of receiving the medication that their personal physician deemed optimal.
3. The physician in private practice has to spend an inordinate amount of time going to web sites, such as CoverMyMeds.com, to fill out extensive forms containing numerous questions about the patient’s illness and diagnosis, and then selecting from a list of medications that the insurance company ironically labels as “smart choices.” These medications often are not necessarily what the physician considers a smart choice, but are the cheapest (regardless of whether their efficacy, safety, or tolerability are the best fit for the patient). After the physician completes the forms, there is a waiting period, followed by additional questions that consume more valuable time and take the physician away from seeing more patients. Some busy colleagues told me they often take the pre-authorization “homework” with them to do at home, consuming part of what should be their family time. For physicians who see patients in an institutional “clinic,” medical assistants or nurses must be hired at significant expense to work full-time on pre-authorizations, adding to the overhead of the clinic while increasing the profits of the third-party insurer.
4. Patients who have been stable on a medication for months, even years, are forced to switch to another medication if they change jobs and become covered by a different insurance company that does not have the patient’s current medication on their infamous list of “approved drugs,” an evil euphemism for “cheapest drugs.” Switching medications is known to be a possibly hazardous process with lower efficacy and/or tolerability, but that appears to be irrelevant to the insurance company. The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs. We should all urge policymakers to pass legislation that goes beyond requiring insurance companies to cover “pre-existing conditions” and expands it to cover “pre-existing medications.”
Continue to: Often, frustrated physicians...
5. Often, frustrated physicians who do not want to see their patients receive a medication they do not believe is appropriate may spend valuable time writing letters of appeal, making phone calls, or printing and faxing scientific articles to the insurance company to convince them to authorize a medication that is not on the “approved list.” Based on my own clinical experience, that justification sometimes works and sometimes doesn’t.
6. Physicians are inevitably and understandably demoralized because their expertise and sound clinical judgment are arbitrarily dismissed and overruled by an invisible insurance employee whose knowledge about and compassion for the patient is miniscule at best.
7. New medication development has collided with the biased despotism of pre-authorization, which generally rejects any new medication (always costlier than generics) irrespective of whether the new medication was demonstrated in controlled clinical trials to have a measurably better profile than older generics. This has ominous implications for numerous medical disorders that do not have any approved medications (for psychiatry, a published study1 found that 82% of DSM disorders do not have a FDA-approved medication).
The lack of utilizing newly introduced medications has discouraged the pharmaceutical industry from investing to develop innovative new mechanisms of action for a variety of complex neuropsychiatric medical conditions. Some companies have already abandoned psychiatric drug development, which is dire for clinical care because pharmaceutical companies are the only entities that develop new treatments for our patients (some health care professionals wish the government had a pharmaceutical agency that develops medications for various illness, but no such agency has ever existed).
8. Hospitalization for a seriously ill patient is either denied, delayed, or eventually approved for an absurdly short period (a few days), which is woefully inadequate, culminating in discharging patients with unresolved symptoms. This can lead to disastrous consequences, including suicide, homicide, or incarceration.
Continue to: I have been personally infuriated...
I have been personally infuriated many times because of the adverse impact pre-authorization had on my patients. One example that still haunts me is a 23-year-old college graduate with severe treatment-resistant depression who failed multiple antidepressant trials, including IV ketamine. She harbored daily thoughts of suicide (throwing herself in front of a train, which she saw daily as she drove to work). She admitted to frequently contemplating which dress she should wear in her coffin. Based on several published double-blind studies showing that modafinil improved bipolar depression,2 I prescribed modafinil, 200 mg/d, as adjunctive treatment to venlafaxine, 300 mg/d, and she improved significantly for 10 months. Suddenly, the insurance company refused to renew her refill of modafinil, and it took 4 weeks of incessant communication (phone calls, faxes, letters, sending published articles) before it was finally approved. In the meantime, the patient deteriorated and began to have active suicidal urges. When she was restarted on modafinil, she never achieved the same level of improvement she had prior to discontinuing modafinil. The insurance company damaged this patient’s recovery with its refusal to authorize a medication that was “not approved” for depression despite the clear benefit it had provided this treatment-resistant patient for almost 1 year. Their motive was clearly to avoid covering the high cost of modafinil, regardless of this patient’s high risk of suicide.
Every physician can recite a litany of complaints about the evil of pre-authorizations. We must therefore unite and vigorously lobby legislators to pass laws that protect patients and uphold physicians’ authority to determine the right treatment for their patients. We must terminate the plague of pre-authorization that takes our patients hostage to the greed of insurance companies, who have no regard to the agony of patients who are prevented from receiving the medication that their personal physician prescribes. Physicians’ well-being would be greatly enhanced if they were not enslaved to the avarice of insurance companies.
The travesty of pre-authorization and its pervasive and deleterious effects on medical care, society, and citizens must be stopped. It’s a plague that sacrifices the practice of medicine on the altar of financial greed. Just because it has gone on for many years does not mean it should be accepted as the “new normal.” It must be condemned as the “new abnormal,” a cancerous lesion on health care delivery that must be excised and discarded.
1. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
2. Nunez NA, Singh B, Romo-Nava F, et al. Efficacy and tolerability of adjunctive modafinil/armodafinil in bipolar depression: a meta-analysis of randomized controlled trials. Bipolar Dipsord. 2019;10.1111/bdi.12859. doi: 10.1111/bdi.12859
Pre-authorization is a despicable scam. It’s a national racket by avaricious insurance companies, and it must be stopped. Since it first reared its ugly head 2 decades ago, it has inflicted great harm to countless patients, demoralized their physicians, and needlessly imposed higher costs in clinical practice while simultaneously depriving patients of the treatment their physicians prescribed for them.
Pre-authorization has become the nemesis of medical care. It recklessly and arbitrarily vetoes the clinical decision-making of competent physicians doing their best to address their patients’ medical needs. Yet, despite its outrageous disruption of the clinical practice of hundreds of thousands of practitioners, it continues unabated, without a forceful pushback. It has become the “new normal,” but in fact, it is the “new abnormal.” This harassment of clinicians must be outlawed.
Think about it: Pre-authorization is essentially practicing medicine without a license, which is a felony. When a remote and invisible insurance company staff member either prevents a patient from receiving a medication prescribed by that patient’s personal physician following a full diagnostic evaluation or pressures the physician to prescribe a different medication, he/she is basically deciding what the treatment should be for a patient who that insurance company employee has never seen, let alone examined. How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company? That is patently unethical, in addition to being a felonious practice of medicine by an absentee person unqualified to decide what a patient needs without a direct examination.
Consider the multiple malignant consequences of such brazen and egregious restriction or distortion of medical care:
1. The physician’s clinical judgment is abrogated, even when it is clearly in the patient’s best interest.
2. Patients are deprived of receiving the medication that their personal physician deemed optimal.
3. The physician in private practice has to spend an inordinate amount of time going to web sites, such as CoverMyMeds.com, to fill out extensive forms containing numerous questions about the patient’s illness and diagnosis, and then selecting from a list of medications that the insurance company ironically labels as “smart choices.” These medications often are not necessarily what the physician considers a smart choice, but are the cheapest (regardless of whether their efficacy, safety, or tolerability are the best fit for the patient). After the physician completes the forms, there is a waiting period, followed by additional questions that consume more valuable time and take the physician away from seeing more patients. Some busy colleagues told me they often take the pre-authorization “homework” with them to do at home, consuming part of what should be their family time. For physicians who see patients in an institutional “clinic,” medical assistants or nurses must be hired at significant expense to work full-time on pre-authorizations, adding to the overhead of the clinic while increasing the profits of the third-party insurer.
4. Patients who have been stable on a medication for months, even years, are forced to switch to another medication if they change jobs and become covered by a different insurance company that does not have the patient’s current medication on their infamous list of “approved drugs,” an evil euphemism for “cheapest drugs.” Switching medications is known to be a possibly hazardous process with lower efficacy and/or tolerability, but that appears to be irrelevant to the insurance company. The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs. We should all urge policymakers to pass legislation that goes beyond requiring insurance companies to cover “pre-existing conditions” and expands it to cover “pre-existing medications.”
Continue to: Often, frustrated physicians...
5. Often, frustrated physicians who do not want to see their patients receive a medication they do not believe is appropriate may spend valuable time writing letters of appeal, making phone calls, or printing and faxing scientific articles to the insurance company to convince them to authorize a medication that is not on the “approved list.” Based on my own clinical experience, that justification sometimes works and sometimes doesn’t.
6. Physicians are inevitably and understandably demoralized because their expertise and sound clinical judgment are arbitrarily dismissed and overruled by an invisible insurance employee whose knowledge about and compassion for the patient is miniscule at best.
7. New medication development has collided with the biased despotism of pre-authorization, which generally rejects any new medication (always costlier than generics) irrespective of whether the new medication was demonstrated in controlled clinical trials to have a measurably better profile than older generics. This has ominous implications for numerous medical disorders that do not have any approved medications (for psychiatry, a published study1 found that 82% of DSM disorders do not have a FDA-approved medication).
The lack of utilizing newly introduced medications has discouraged the pharmaceutical industry from investing to develop innovative new mechanisms of action for a variety of complex neuropsychiatric medical conditions. Some companies have already abandoned psychiatric drug development, which is dire for clinical care because pharmaceutical companies are the only entities that develop new treatments for our patients (some health care professionals wish the government had a pharmaceutical agency that develops medications for various illness, but no such agency has ever existed).
8. Hospitalization for a seriously ill patient is either denied, delayed, or eventually approved for an absurdly short period (a few days), which is woefully inadequate, culminating in discharging patients with unresolved symptoms. This can lead to disastrous consequences, including suicide, homicide, or incarceration.
Continue to: I have been personally infuriated...
I have been personally infuriated many times because of the adverse impact pre-authorization had on my patients. One example that still haunts me is a 23-year-old college graduate with severe treatment-resistant depression who failed multiple antidepressant trials, including IV ketamine. She harbored daily thoughts of suicide (throwing herself in front of a train, which she saw daily as she drove to work). She admitted to frequently contemplating which dress she should wear in her coffin. Based on several published double-blind studies showing that modafinil improved bipolar depression,2 I prescribed modafinil, 200 mg/d, as adjunctive treatment to venlafaxine, 300 mg/d, and she improved significantly for 10 months. Suddenly, the insurance company refused to renew her refill of modafinil, and it took 4 weeks of incessant communication (phone calls, faxes, letters, sending published articles) before it was finally approved. In the meantime, the patient deteriorated and began to have active suicidal urges. When she was restarted on modafinil, she never achieved the same level of improvement she had prior to discontinuing modafinil. The insurance company damaged this patient’s recovery with its refusal to authorize a medication that was “not approved” for depression despite the clear benefit it had provided this treatment-resistant patient for almost 1 year. Their motive was clearly to avoid covering the high cost of modafinil, regardless of this patient’s high risk of suicide.
Every physician can recite a litany of complaints about the evil of pre-authorizations. We must therefore unite and vigorously lobby legislators to pass laws that protect patients and uphold physicians’ authority to determine the right treatment for their patients. We must terminate the plague of pre-authorization that takes our patients hostage to the greed of insurance companies, who have no regard to the agony of patients who are prevented from receiving the medication that their personal physician prescribes. Physicians’ well-being would be greatly enhanced if they were not enslaved to the avarice of insurance companies.
The travesty of pre-authorization and its pervasive and deleterious effects on medical care, society, and citizens must be stopped. It’s a plague that sacrifices the practice of medicine on the altar of financial greed. Just because it has gone on for many years does not mean it should be accepted as the “new normal.” It must be condemned as the “new abnormal,” a cancerous lesion on health care delivery that must be excised and discarded.
Pre-authorization is a despicable scam. It’s a national racket by avaricious insurance companies, and it must be stopped. Since it first reared its ugly head 2 decades ago, it has inflicted great harm to countless patients, demoralized their physicians, and needlessly imposed higher costs in clinical practice while simultaneously depriving patients of the treatment their physicians prescribed for them.
Pre-authorization has become the nemesis of medical care. It recklessly and arbitrarily vetoes the clinical decision-making of competent physicians doing their best to address their patients’ medical needs. Yet, despite its outrageous disruption of the clinical practice of hundreds of thousands of practitioners, it continues unabated, without a forceful pushback. It has become the “new normal,” but in fact, it is the “new abnormal.” This harassment of clinicians must be outlawed.
Think about it: Pre-authorization is essentially practicing medicine without a license, which is a felony. When a remote and invisible insurance company staff member either prevents a patient from receiving a medication prescribed by that patient’s personal physician following a full diagnostic evaluation or pressures the physician to prescribe a different medication, he/she is basically deciding what the treatment should be for a patient who that insurance company employee has never seen, let alone examined. How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company? That is patently unethical, in addition to being a felonious practice of medicine by an absentee person unqualified to decide what a patient needs without a direct examination.
Consider the multiple malignant consequences of such brazen and egregious restriction or distortion of medical care:
1. The physician’s clinical judgment is abrogated, even when it is clearly in the patient’s best interest.
2. Patients are deprived of receiving the medication that their personal physician deemed optimal.
3. The physician in private practice has to spend an inordinate amount of time going to web sites, such as CoverMyMeds.com, to fill out extensive forms containing numerous questions about the patient’s illness and diagnosis, and then selecting from a list of medications that the insurance company ironically labels as “smart choices.” These medications often are not necessarily what the physician considers a smart choice, but are the cheapest (regardless of whether their efficacy, safety, or tolerability are the best fit for the patient). After the physician completes the forms, there is a waiting period, followed by additional questions that consume more valuable time and take the physician away from seeing more patients. Some busy colleagues told me they often take the pre-authorization “homework” with them to do at home, consuming part of what should be their family time. For physicians who see patients in an institutional “clinic,” medical assistants or nurses must be hired at significant expense to work full-time on pre-authorizations, adding to the overhead of the clinic while increasing the profits of the third-party insurer.
4. Patients who have been stable on a medication for months, even years, are forced to switch to another medication if they change jobs and become covered by a different insurance company that does not have the patient’s current medication on their infamous list of “approved drugs,” an evil euphemism for “cheapest drugs.” Switching medications is known to be a possibly hazardous process with lower efficacy and/or tolerability, but that appears to be irrelevant to the insurance company. The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs. We should all urge policymakers to pass legislation that goes beyond requiring insurance companies to cover “pre-existing conditions” and expands it to cover “pre-existing medications.”
Continue to: Often, frustrated physicians...
5. Often, frustrated physicians who do not want to see their patients receive a medication they do not believe is appropriate may spend valuable time writing letters of appeal, making phone calls, or printing and faxing scientific articles to the insurance company to convince them to authorize a medication that is not on the “approved list.” Based on my own clinical experience, that justification sometimes works and sometimes doesn’t.
6. Physicians are inevitably and understandably demoralized because their expertise and sound clinical judgment are arbitrarily dismissed and overruled by an invisible insurance employee whose knowledge about and compassion for the patient is miniscule at best.
7. New medication development has collided with the biased despotism of pre-authorization, which generally rejects any new medication (always costlier than generics) irrespective of whether the new medication was demonstrated in controlled clinical trials to have a measurably better profile than older generics. This has ominous implications for numerous medical disorders that do not have any approved medications (for psychiatry, a published study1 found that 82% of DSM disorders do not have a FDA-approved medication).
The lack of utilizing newly introduced medications has discouraged the pharmaceutical industry from investing to develop innovative new mechanisms of action for a variety of complex neuropsychiatric medical conditions. Some companies have already abandoned psychiatric drug development, which is dire for clinical care because pharmaceutical companies are the only entities that develop new treatments for our patients (some health care professionals wish the government had a pharmaceutical agency that develops medications for various illness, but no such agency has ever existed).
8. Hospitalization for a seriously ill patient is either denied, delayed, or eventually approved for an absurdly short period (a few days), which is woefully inadequate, culminating in discharging patients with unresolved symptoms. This can lead to disastrous consequences, including suicide, homicide, or incarceration.
Continue to: I have been personally infuriated...
I have been personally infuriated many times because of the adverse impact pre-authorization had on my patients. One example that still haunts me is a 23-year-old college graduate with severe treatment-resistant depression who failed multiple antidepressant trials, including IV ketamine. She harbored daily thoughts of suicide (throwing herself in front of a train, which she saw daily as she drove to work). She admitted to frequently contemplating which dress she should wear in her coffin. Based on several published double-blind studies showing that modafinil improved bipolar depression,2 I prescribed modafinil, 200 mg/d, as adjunctive treatment to venlafaxine, 300 mg/d, and she improved significantly for 10 months. Suddenly, the insurance company refused to renew her refill of modafinil, and it took 4 weeks of incessant communication (phone calls, faxes, letters, sending published articles) before it was finally approved. In the meantime, the patient deteriorated and began to have active suicidal urges. When she was restarted on modafinil, she never achieved the same level of improvement she had prior to discontinuing modafinil. The insurance company damaged this patient’s recovery with its refusal to authorize a medication that was “not approved” for depression despite the clear benefit it had provided this treatment-resistant patient for almost 1 year. Their motive was clearly to avoid covering the high cost of modafinil, regardless of this patient’s high risk of suicide.
Every physician can recite a litany of complaints about the evil of pre-authorizations. We must therefore unite and vigorously lobby legislators to pass laws that protect patients and uphold physicians’ authority to determine the right treatment for their patients. We must terminate the plague of pre-authorization that takes our patients hostage to the greed of insurance companies, who have no regard to the agony of patients who are prevented from receiving the medication that their personal physician prescribes. Physicians’ well-being would be greatly enhanced if they were not enslaved to the avarice of insurance companies.
The travesty of pre-authorization and its pervasive and deleterious effects on medical care, society, and citizens must be stopped. It’s a plague that sacrifices the practice of medicine on the altar of financial greed. Just because it has gone on for many years does not mean it should be accepted as the “new normal.” It must be condemned as the “new abnormal,” a cancerous lesion on health care delivery that must be excised and discarded.
1. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
2. Nunez NA, Singh B, Romo-Nava F, et al. Efficacy and tolerability of adjunctive modafinil/armodafinil in bipolar depression: a meta-analysis of randomized controlled trials. Bipolar Dipsord. 2019;10.1111/bdi.12859. doi: 10.1111/bdi.12859
1. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
2. Nunez NA, Singh B, Romo-Nava F, et al. Efficacy and tolerability of adjunctive modafinil/armodafinil in bipolar depression: a meta-analysis of randomized controlled trials. Bipolar Dipsord. 2019;10.1111/bdi.12859. doi: 10.1111/bdi.12859
Missing pieces
On the first day of my third postgraduate year, I sat at a table with my entire PGY-3 class and our attending physician. This was my first case discussion of the new academic year, and the attending was someone I hadn’t worked with previously. He was an older gentleman who primarily worked in private practice, but enjoyed teaching and maintained his academic affiliations. He started the discussion with a simple question: “Does anyone have a case they would like to discuss?”
The silence we were accustomed to as new interns on the first day of service fell over the group. Everyone seemed a bit apprehensive, as this attending was somewhat intimidating. He was educated at Hahnemann University Hospital, and classically trained in psychoanalysis. He had a wealth of research knowledge, and continued to publish in academic journals on a regular basis.
Finally, someone volunteered to present a case. The case involved a 45-year-old woman with a long history of depression. She had received multiple medication trials that did not result in remission. In fact, she had never experienced significant relief of any of her depressive symptoms. The case was clearly shaping up to look like treatment-resistant depression. The resident continued with the case and discussed the differential diagnosis and treatment plan. The treatment plan involved a combination of pharmacotherapy and psychotherapy—not much different from the previous treatments the patient had tried. I anxiously ant
After listening attentively and taking a moment to gather his thoughts, the attending responded with one word: “Egregious.” He was blunt, and clearly viewed the case formulation and management of this patient as “basic.” It was clear to me that I, and the rest of my class, were missing something. It was something that was not going to come from a textbook or treatment algorithm. He was the first attending in some time who was challenging us to truly think.
A profound point
I ruminated on his surprising response for a moment, as the treatment plan presented was commonly seen on the inpatient unit. It was not an unreasonable approach, but it lacked depth and sophistication. However, no attending I worked with in the past ever called it “egregious.” Now I was intrigued, and honestly, it had been some time since I felt excited about a case discussion. The attending’s point was simple: our patients are suffering, and they are coming to us in their most vulnerable state seeking answers. When we make decisions based on FDA approvals and blindly follow treatment algorithms, we fail to see the vast untapped potential to help patients that resides outside of these strict guidelines. This is not to say there is no place for algorithm-based psychiatry and FDA-approved medications; in fact, many times these will be the cornerstones of treatment.
During the discussion, this attending proceeded to make another profound statement that I continue to remind myself of each day. He said, “What would be the point of these patients coming to see you if you are going to practice psychiatry like a primary care provider?” I had to agree with him on many levels, because these patients are suffering, and they are looking for hope. If we simply offer them the same standard treatments, they are likely to get the same poor results. Our patients are coming to us because we are experts in the field of psychiatry; we owe them the respect to think outside the box. As specialists, the most complicated and difficult-to-treat cases will be referred to us. We need to possess a deep understanding of all treatment options, and know where to go when your first, second, and third options fail to produce the desired result.
The attending offered his thoughts on the case, and discussed his approach to treating this patient. He explained the importance of not being afraid to try medications in doses above the FDA-approved maximums in select cases. He explained the robust research behind monoamine oxidase inhibitors (MAOIs), and how to safely prescribe them. He explained why tricyclic antidepressants may be a more effective choice for some patients.
Continue to: These were discussions...
These were discussions I never had the opportunity to have in the past. In many instances, the possibility of using an MAOI would be quickly dismissed by my attendings as “too dangerous” or “better options are available.” In this attending’s view, it wasn’t the danger of an adverse outcome we are facing, but the danger of missing potentially life-changing treatments for our patients. The attending concluded with, “It’s sad that many of you will graduate without starting a patient on an MAOI, without titrating a tricyclic antidepressant and monitoring blood levels, and without ever really thinking for yourself.” These were powerful words, and he was speaking a truth that deep down I already knew.
When I reflect on this discussion and my first 2 years of training, I realize the value in learning structured methods of treating patients. I am aware of the need to practice in a safe manner that does not put the patient at unnecessary risk. However, I also realize I am going to face difficult cases where many smart and capable clinicians have attempted treatment and failed to get the desired outcome. It’s essential that as specialists we learn to use all the tools available to us to treat patients. If we limit ourselves out of fear, or blindly follow algorithms, we miss important opportunities to act boldly to help patients in their darkest moments.
On the first day of my third postgraduate year, I sat at a table with my entire PGY-3 class and our attending physician. This was my first case discussion of the new academic year, and the attending was someone I hadn’t worked with previously. He was an older gentleman who primarily worked in private practice, but enjoyed teaching and maintained his academic affiliations. He started the discussion with a simple question: “Does anyone have a case they would like to discuss?”
The silence we were accustomed to as new interns on the first day of service fell over the group. Everyone seemed a bit apprehensive, as this attending was somewhat intimidating. He was educated at Hahnemann University Hospital, and classically trained in psychoanalysis. He had a wealth of research knowledge, and continued to publish in academic journals on a regular basis.
Finally, someone volunteered to present a case. The case involved a 45-year-old woman with a long history of depression. She had received multiple medication trials that did not result in remission. In fact, she had never experienced significant relief of any of her depressive symptoms. The case was clearly shaping up to look like treatment-resistant depression. The resident continued with the case and discussed the differential diagnosis and treatment plan. The treatment plan involved a combination of pharmacotherapy and psychotherapy—not much different from the previous treatments the patient had tried. I anxiously ant
After listening attentively and taking a moment to gather his thoughts, the attending responded with one word: “Egregious.” He was blunt, and clearly viewed the case formulation and management of this patient as “basic.” It was clear to me that I, and the rest of my class, were missing something. It was something that was not going to come from a textbook or treatment algorithm. He was the first attending in some time who was challenging us to truly think.
A profound point
I ruminated on his surprising response for a moment, as the treatment plan presented was commonly seen on the inpatient unit. It was not an unreasonable approach, but it lacked depth and sophistication. However, no attending I worked with in the past ever called it “egregious.” Now I was intrigued, and honestly, it had been some time since I felt excited about a case discussion. The attending’s point was simple: our patients are suffering, and they are coming to us in their most vulnerable state seeking answers. When we make decisions based on FDA approvals and blindly follow treatment algorithms, we fail to see the vast untapped potential to help patients that resides outside of these strict guidelines. This is not to say there is no place for algorithm-based psychiatry and FDA-approved medications; in fact, many times these will be the cornerstones of treatment.
During the discussion, this attending proceeded to make another profound statement that I continue to remind myself of each day. He said, “What would be the point of these patients coming to see you if you are going to practice psychiatry like a primary care provider?” I had to agree with him on many levels, because these patients are suffering, and they are looking for hope. If we simply offer them the same standard treatments, they are likely to get the same poor results. Our patients are coming to us because we are experts in the field of psychiatry; we owe them the respect to think outside the box. As specialists, the most complicated and difficult-to-treat cases will be referred to us. We need to possess a deep understanding of all treatment options, and know where to go when your first, second, and third options fail to produce the desired result.
The attending offered his thoughts on the case, and discussed his approach to treating this patient. He explained the importance of not being afraid to try medications in doses above the FDA-approved maximums in select cases. He explained the robust research behind monoamine oxidase inhibitors (MAOIs), and how to safely prescribe them. He explained why tricyclic antidepressants may be a more effective choice for some patients.
Continue to: These were discussions...
These were discussions I never had the opportunity to have in the past. In many instances, the possibility of using an MAOI would be quickly dismissed by my attendings as “too dangerous” or “better options are available.” In this attending’s view, it wasn’t the danger of an adverse outcome we are facing, but the danger of missing potentially life-changing treatments for our patients. The attending concluded with, “It’s sad that many of you will graduate without starting a patient on an MAOI, without titrating a tricyclic antidepressant and monitoring blood levels, and without ever really thinking for yourself.” These were powerful words, and he was speaking a truth that deep down I already knew.
When I reflect on this discussion and my first 2 years of training, I realize the value in learning structured methods of treating patients. I am aware of the need to practice in a safe manner that does not put the patient at unnecessary risk. However, I also realize I am going to face difficult cases where many smart and capable clinicians have attempted treatment and failed to get the desired outcome. It’s essential that as specialists we learn to use all the tools available to us to treat patients. If we limit ourselves out of fear, or blindly follow algorithms, we miss important opportunities to act boldly to help patients in their darkest moments.
On the first day of my third postgraduate year, I sat at a table with my entire PGY-3 class and our attending physician. This was my first case discussion of the new academic year, and the attending was someone I hadn’t worked with previously. He was an older gentleman who primarily worked in private practice, but enjoyed teaching and maintained his academic affiliations. He started the discussion with a simple question: “Does anyone have a case they would like to discuss?”
The silence we were accustomed to as new interns on the first day of service fell over the group. Everyone seemed a bit apprehensive, as this attending was somewhat intimidating. He was educated at Hahnemann University Hospital, and classically trained in psychoanalysis. He had a wealth of research knowledge, and continued to publish in academic journals on a regular basis.
Finally, someone volunteered to present a case. The case involved a 45-year-old woman with a long history of depression. She had received multiple medication trials that did not result in remission. In fact, she had never experienced significant relief of any of her depressive symptoms. The case was clearly shaping up to look like treatment-resistant depression. The resident continued with the case and discussed the differential diagnosis and treatment plan. The treatment plan involved a combination of pharmacotherapy and psychotherapy—not much different from the previous treatments the patient had tried. I anxiously ant
After listening attentively and taking a moment to gather his thoughts, the attending responded with one word: “Egregious.” He was blunt, and clearly viewed the case formulation and management of this patient as “basic.” It was clear to me that I, and the rest of my class, were missing something. It was something that was not going to come from a textbook or treatment algorithm. He was the first attending in some time who was challenging us to truly think.
A profound point
I ruminated on his surprising response for a moment, as the treatment plan presented was commonly seen on the inpatient unit. It was not an unreasonable approach, but it lacked depth and sophistication. However, no attending I worked with in the past ever called it “egregious.” Now I was intrigued, and honestly, it had been some time since I felt excited about a case discussion. The attending’s point was simple: our patients are suffering, and they are coming to us in their most vulnerable state seeking answers. When we make decisions based on FDA approvals and blindly follow treatment algorithms, we fail to see the vast untapped potential to help patients that resides outside of these strict guidelines. This is not to say there is no place for algorithm-based psychiatry and FDA-approved medications; in fact, many times these will be the cornerstones of treatment.
During the discussion, this attending proceeded to make another profound statement that I continue to remind myself of each day. He said, “What would be the point of these patients coming to see you if you are going to practice psychiatry like a primary care provider?” I had to agree with him on many levels, because these patients are suffering, and they are looking for hope. If we simply offer them the same standard treatments, they are likely to get the same poor results. Our patients are coming to us because we are experts in the field of psychiatry; we owe them the respect to think outside the box. As specialists, the most complicated and difficult-to-treat cases will be referred to us. We need to possess a deep understanding of all treatment options, and know where to go when your first, second, and third options fail to produce the desired result.
The attending offered his thoughts on the case, and discussed his approach to treating this patient. He explained the importance of not being afraid to try medications in doses above the FDA-approved maximums in select cases. He explained the robust research behind monoamine oxidase inhibitors (MAOIs), and how to safely prescribe them. He explained why tricyclic antidepressants may be a more effective choice for some patients.
Continue to: These were discussions...
These were discussions I never had the opportunity to have in the past. In many instances, the possibility of using an MAOI would be quickly dismissed by my attendings as “too dangerous” or “better options are available.” In this attending’s view, it wasn’t the danger of an adverse outcome we are facing, but the danger of missing potentially life-changing treatments for our patients. The attending concluded with, “It’s sad that many of you will graduate without starting a patient on an MAOI, without titrating a tricyclic antidepressant and monitoring blood levels, and without ever really thinking for yourself.” These were powerful words, and he was speaking a truth that deep down I already knew.
When I reflect on this discussion and my first 2 years of training, I realize the value in learning structured methods of treating patients. I am aware of the need to practice in a safe manner that does not put the patient at unnecessary risk. However, I also realize I am going to face difficult cases where many smart and capable clinicians have attempted treatment and failed to get the desired outcome. It’s essential that as specialists we learn to use all the tools available to us to treat patients. If we limit ourselves out of fear, or blindly follow algorithms, we miss important opportunities to act boldly to help patients in their darkest moments.
The psychiatric clinic of the future
Despite the tremendous advances in psychiatry in recent years, the current clinical practice of psychiatry continues to rely on data from intermittent assessments along with subjective and unquantifiable accounts from patients and caregivers. Furthermore, there continues to be significant diagnostic variations among practitioners. Fortunately, technology to address these issues appears to be on the horizon.
How might the psychiatric clinic of the future look? What changes could we envision? These 4 critical factors may soon bring about dynamic changes in the way we practice psychiatry:
- precision psychiatry
- digital psychiatry
- technology-enhanced psychotherapy
- electronic health record (EHR) reforms.
In this article, we review how advances in each of these areas might lead to improved care for our patients.
Precision psychiatry
Precision psychiatry takes into account each patient’s variability in genes, environment, and lifestyle to determine individualized treatment and prevention strategies. It relies on pharmacogenomic testing as the primary tool. Pharmacogenomics is the study of variability in drug response due to heredity.
Emerging data on the clinical utility and cost-effectiveness of pharmacogenomic testing are encouraging, but its routine use is not well supported by current evidence.2 One limit to using pharmacogenomic testing is that many genes simultaneously exert an effect on the structure and function of neurons and associated pathophysiology. According to the International Society of Psychiatric Genetics, no single genetic variant is sufficient to cause psychiatric disorders such as depression, bipolar disorder, substance dependence, or schizophrenia. This limits the possibility of using genetic tests to establish a diagnosis.3
In the future, better algorithms could promote more accurate pharmacogenomics profiles for individual patients, which could influence treatment.
Precision psychiatry could lead to:
- identification of novel targets for new medications
- pharmacogenetic profiling of the patient to predict disease susceptibility and medication response
- personalized therapy: the right drug at the right dose for the right patient.
- improved efficacy and fewer adverse medication reactions.
Continue to: Digital psychiatry
Digital psychiatry
Integrating computer-based technology into psychiatric practice has given birth to a new frontier that could be called digital psychiatry. This might encompass the following:
- telepsychiatry
- social media with a mental health focus
- web-based applications/devices
- artificial intelligence (AI).
Telepsychiatry. Videoconferencing is the most widely used form of telepsychiatry. It provides patients with easier access to mental health treatment.4 Telepsychiatry has the potential to match patients and clinicians with similar cultural backgrounds, thus minimizing cultural gaps and misunderstandings. Most importantly, it is comparable to face-to-face interviews in terms of the reliability of assessment and treatment outcomes.5
Telepsychiatry might be particularly helpful for patients with restricted mobility, such as those who live in remote areas, nursing homes, or correctional facilities. In correctional settings, transferring prisoners is expensive and carries the risk of escape. In a small study (N = 86) conducted in Hong Kong, Chen et al6 found that using videoconferencing to conduct clinical interviews of inmates was cost-efficient and scored high in terms of patient acceptability.
Social media. Social media could be a powerful platform for early detection of mental illness. Staying connected with patients on social media could allow psychiatrists to be more aware of their patient’s mood fluctuations, which might lead to more timely assessments. Physicians could be automatically notified about changes in their patients’ social media activity that indicate changes in mental state, which could solicit immediate intervention and treatment. On the other hand, such use of social media could blur professional boundaries. Psychiatrists also could use social media to promote awareness of mental health and educate the public on ways to improve or maintain their mental well-being.7
Web-based applications/devices. Real-time monitoring through applications or internet-based smart devices creates a new avenue for patients to receive personalized assessments, treatment, and intervention.8 Smartwatches with internet connectivity may offer a glimpse of the wearer’s sleep architecture and duration, thus providing real-time data on patients who have insomnia. We can now passively collect objective data from devices, such as smartphones and laptops, to phenotype an individual’s mood and mental state, a process called digital phenotyping. The Table9 lists examples of the types of mental health–related metrics that can be captured by smartphones, smartwatches, and similar technology. Information from these devices can be accumulated to create a database that can be used to predict symptoms.10 For example, the way people use a smartphone’s keyboard, including latency time between space and character types, can be used to generate variables for data. This type of information is being studied for use in screening depression and passively assessing mood in real time.11
Continue to: Artificial intelligence
Artificial intelligence—the development of computer systems able to perform tasks that normally require human intelligence—is being increasingly used in psychiatry. Some studies have suggested AI can be used to identify patients’ risk of suicide12-15 or psychosis.16,17Kalanderian and Nasrallah18 reviewed several of these studies in
Other researchers have found clinical uses for machine learning, a subset of AI that uses methods to automatically detect patterns and make predictions based on those patterns. In one study, a machine learning analysis of functional MRI scans was able to identify 4 distinct subtypes of depression.19 In another study, a machine learning model was able to predict with 60% accuracy which patients with depression would respond to antidepressants.20
In the future, AI might be used to change mental health classification systems. Because many mental health disorders share similar symptom clusters, machine learning can help to identify associations between symptoms, behavior, brain function, and real-world function across different diagnoses, potentially affecting how we will classify mental disorders.21
Technology-enhanced psychotherapy
In the future, it might be common for psychotherapy to be provided by a computer, or “virtual therapist.” Several studies have evaluated the use of technology-enhanced psychotherapy.
Lucas et al22 investigated patients’ interactions with a virtual therapist. Participants were interviewed by an avatar named Ellie, who they saw on a TV screen. Half of the participants were told Ellie was not human, and half were told Ellie was being controlled remotely by a human. Three psychologists who were blinded to group allocations analyzed transcripts of the interviews and video recordings of participants’ facial expressions to quantify the participants’ fear, sadness, and other emotional responses during the interviews, as well as their openness to the questions. Participants who believed Ellie was fully automated reported significantly lower fear of self-disclosure and impression management (attempts to control how others perceive them) than participants who were told that Ellie was operated by a human. Additionally, participants who believed they were interacting with a computer were more open during the interview.22
Continue to: Researchers at the University of Southern California...
Researchers at the University of Southern California developed software that assessed 74 acoustic features, including pitch, volume, quality, shimmer, jitter, and prosody, to predict outcomes among patients receiving couples therapy. This software was able to predict marital discord at least as well as human therapists.23
Many mental health apps purport to implement specific components of psychotherapy. Many of these apps focus on cognitive-behavioral therapy worksheets, mindfulness exercises, and/or mood tracking. The features provided by such apps emulate the tasks and intended outcomes of traditional psychotherapy, but in an entirely decentralized venue.24
Some have expressed concern that an increased use of virtual therapists powered by AI might lead to a dehumanization of psychiatry (Box25,26).
Box
Whether there are aspects of the psychiatric patient encounter that cannot be managed by a “virtual clinician” created by artificial intelligence (AI) remains to be determined. Some of the benefits of using AI in this manner may be difficult to anticipate, or may be specific to an individual’s relationship with his/her clinician.25
On the other hand, AI systems blur previously assumed boundaries between reality and fiction, and this could have complex effects on patients. Similar to therapeutic relationships with a human clinician, there is the risk of transference of emotions, thoughts, and feelings to a virtual therapist powered by AI. Unlike with a psychiatrist or therapist, however, there is no person on the other side of this transference. Whether virtual clinicians will be able to manage such transference remains to be seen.
In Deep Medicine,26 cardiologist Eric Topol, MD, emphasizes a crucial component of a patient encounter that AI will be unlikely able to provide: empathy. Virtual therapists powered by AI will inherit the tasks best done by machines, leaving humans more time to do what they do best—providing empathy and being “present” for patients.
Electronic health record reforms
Although many clinicians find EHRs to be onerous and time-consuming, EHR technology is constantly improving, and EHRs have revolutionized documentation and order implementation. Several potential advances could improve clinical practice. For example, EHRs could incorporate a clinical decision support system that uses AI-based algorithms to assist psychiatrists with diagnosis, monitoring, and treatment.27 In the future, EHRs might have the ability to monitor and learn from errors and adverse events, and automatically design an algorithm to avoid them.28 They should be designed to better manage analysis of pharmacogenetic test results, which is challenging due to the amount and complexity of the data.29 Future EHRs should eliminate the non-intuitive and multi-click interfaces and cumbersome data searches of today’s EHRs.30
Technology brings new ethical considerations
Mental health interventions based on AI typically work with algorithms, and algorithms bring ethical issues. Mental health devices or systems that use AI could contain biases that have the potential to harm in unintended ways, such as a data-driven sexist or racist bias.31 This may require investing additional time to explain to patients (and their families) what an algorithm is and how it works in relation to the therapy provided.
Continue to: Another concern is patient...
Another concern is patient autonomy.32 For example, it would be ethically problematic if a patient were to assume that there was a human physician “at the other end” of a virtual therapist or other technology who is communicating or reviewing his/her messages. Similarly, an older adult or a patient with intellectual disabilities may not be able to understand advanced technology or what it does when it is installed in their home to monitor the patient’s activities. This would increase the risk of privacy violations, manipulation, or even coercion if the requirements for informed consent are not satisfied.
A flowchart for the future
Although current research and innovations typically target specific areas of psychiatry, these advances can be integrated by devising algorithms and protocols that will change the current practice of psychiatry. The Figure provides a glimpse of how the psychiatry clinic of the future might work. A maxim of management is that “the best way to predict the future is to create it.” However, the mere conception of a vision is not enough—working towards it is essential.
Bottom Line
With advances in technology, psychiatric practice will soon be radically different from what it is today. The expanded use of telepsychiatry, social media, artificial intelligence, and web-based applications/devices holds great promise for psychiatric assessment, diagnosis, and treatment, although certain ethical and privacy concerns need to be adequately addressed.
Related Resources
- National Institute of Mental Health. Technology and the future of mental health treatment. www.nimh.nih.gov/health/topics/technology-and-the-future-of-mental-health-treatment/index.shtml. Revised September 2019.
- Hays R, Farrell HM, Touros J. Mobile apps and mental health: using technology to quantify real-time clinical risk. Current Psychiatry. 2019;18(6):37-41.
- Torous J, Luo J, Chan SR. Mental health apps: what to tell patients. Current Psychiatry. 2018;17(3):21-25.
1. Pirmohamed M. Pharmacogenetics and pharmacogenomics. Br J Clin Pharmacol. 2001;52(4):345-347.
2. Benitez J, Cool CL, Scotti DJ. Use of combinatorial pharmacogenomic guidance in treating psychiatric disorders. Per Med. 2018;15(6):481-494.
3. Cannon TD. Candidate gene studies in the GWAS era: the MET proto-oncogene, neurocognition, and schizophrenia. Am J Psychiatry. 2010;167(4):4,369-372.
4. Greenwood J, Chamberlain C, Parker G. Evaluation of a rural telepsychiatry service. Australas Psychiatry. 2004;12(3):268-272.
5. Hubley S, Lynch SB, Schneck C, et al. Review of key telepsychiatry outcomes. World J Psychiatry. 2016;6(2):269-282.
6. Cheng KM, Siu BW, Yeung CC, et al. Telepsychiatry for stable Chinese psychiatric out-patients in custody in Hong Kong: a case-control pilot study. Hong Kong Med J. 2018;24(4):378-383.
7. Frankish K, Ryan C, Harris A. Psychiatry and online social media: potential, pitfalls and ethical guidelines for psychiatrists and trainees. Australasian Psychiatry. 2012;20(3):181-187.
8. de la Torre Díez I, Alonso SG, Hamrioui S, et al. IoT-based services and applications for mental health in the literature. J Med Syst. 2019;43(1):4-9.
9. Topol E. Deep Medicine. New York, NY: Basic Books; 2019:168.
10. Adams RA, Huys QJM, Roiser JP. Computational Psychiatry: towards a mathematically informed understanding of mental illness. J Neurol Neurosurg Psychiatry. 2016;87(1):53-63.
11. Insel TR. Bending the curve for mental health: technology for a public health approach. Am J Public Health. 2019;109(suppl 3):S168-S170.
12. Just MA, Pan L, Cherkassky VL, et al. Machine learning of neural representations of suicide and emotion concepts identifies suicidal youth. Nat Hum Behav. 2017;1:911-919.
13. Pestian J, Nasrallah H, Matykiewicz P, et al. Suicide note classification using natural language processing: a content analysis. Biomed Inform Insights. 2010;2010(3):19-28.
14. Walsh CG, Ribeiro JD, Franklin JC. Predicting risk of suicide attempts over time through machine learning. Clinical Psychological Science. 2017;5(3):457-469.
15. Pestian JP, Sorter M, Connolly B, et al; STM Research Group. A machine learning approach to identifying the thought markers of suicidal subjects: a prospective multicenter trial. Suicide Life Threat Behav. 2017;47(1):112-121.
16. Corcoran CM, Carrillo F, Fernández-Slezak D, et al. Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry. 2018;17(1):67-75.
17. Bedi G, Carrillo F, Cecchi GA, et al. Automated analysis of free speech predicts psychosis onset in high-risk youths. NPJ Schizophr. 2015;1:15030. doi: 10.1038/npjschz.2015.30.
18. Kalanderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019;18(8):33-38.
19. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
20. Chekroud AM, Zotti RJ, Shehzad Z, et al. Cross-trial prediction of treatment outcome in depression: a machine learning approach. Lancet Psychiatry. 2016;3(3):243-250.
21. Grisanzio KA, Goldstein-Piekarski AN, Wang MY, et al. Transdiagnostic symptom clusters and associations with brain, behavior, and daily function in mood, anxiety, and trauma disorders. JAMA Psychiatry. 2018;75(2):201-209.
22. Lucas G, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Computers in Human Behavior. 2014;37:94-100.
23. Nasir M, Baucom BR, Georgiou P, et al. Predicting couple therapy outcomes based on speech acoustic features. PLoS One. 2017;12(9):e0185123. doi: 10.1371/journal.pone.0185123.
24. Huguet A, Rao S, McGrath PJ, et al. A systematic review of cognitive behavioral therapy and behavioral activation apps for depression. PLoS One. 2016;11(5):e0154248. doi: 10.1371/journal.pone.0154248.
25. Scholten MR, Kelders SM, Van Gemert-Pijnen JE. Self-guided web-based interventions: scoping review on user needs and the potential of embodied conversational agents to address them. J Med Internet Res. 2017;19(11):e383.
26. Topol E. Deep Medicine. New York, NY: Basic Books; 2019:283-310.
27. Abramson EL, McGinnis S, Edwards A, et al. Electronic health record adoption and health information exchange among hospitals in New York State. J Eval Clin Pract. 2012;18(6):1156-1162.
28. Meeks DW, Smith MW, Taylor L, et al. An analysis of electronic health record-related patient safety concerns. J Am Med Inform Assoc. 2014;21(6):1053-1059.
29. Kho AN, Rasmussen LV, Connolly JJ, et al. Practical challenges in integrating genomic data into the electronic health record. Genet Med. 2013;15(10):772-778.
30. Ornstein SM, Oates RB, Fox GN. The computer-based medical record: current status. J Fam Pract. 1992;35(5):556-565.
31. Corea F. Machine ethics and artificial moral agents. In: Applied artificial intelligence: where AI can be used in business. Basel, Switzerland: Springer; 2019:33-41.
32. Beauchamp T, Childress J. Principles of biomedical ethics. 7th ed. New York, NY: Oxford University Press; 2012:44.
Despite the tremendous advances in psychiatry in recent years, the current clinical practice of psychiatry continues to rely on data from intermittent assessments along with subjective and unquantifiable accounts from patients and caregivers. Furthermore, there continues to be significant diagnostic variations among practitioners. Fortunately, technology to address these issues appears to be on the horizon.
How might the psychiatric clinic of the future look? What changes could we envision? These 4 critical factors may soon bring about dynamic changes in the way we practice psychiatry:
- precision psychiatry
- digital psychiatry
- technology-enhanced psychotherapy
- electronic health record (EHR) reforms.
In this article, we review how advances in each of these areas might lead to improved care for our patients.
Precision psychiatry
Precision psychiatry takes into account each patient’s variability in genes, environment, and lifestyle to determine individualized treatment and prevention strategies. It relies on pharmacogenomic testing as the primary tool. Pharmacogenomics is the study of variability in drug response due to heredity.
Emerging data on the clinical utility and cost-effectiveness of pharmacogenomic testing are encouraging, but its routine use is not well supported by current evidence.2 One limit to using pharmacogenomic testing is that many genes simultaneously exert an effect on the structure and function of neurons and associated pathophysiology. According to the International Society of Psychiatric Genetics, no single genetic variant is sufficient to cause psychiatric disorders such as depression, bipolar disorder, substance dependence, or schizophrenia. This limits the possibility of using genetic tests to establish a diagnosis.3
In the future, better algorithms could promote more accurate pharmacogenomics profiles for individual patients, which could influence treatment.
Precision psychiatry could lead to:
- identification of novel targets for new medications
- pharmacogenetic profiling of the patient to predict disease susceptibility and medication response
- personalized therapy: the right drug at the right dose for the right patient.
- improved efficacy and fewer adverse medication reactions.
Continue to: Digital psychiatry
Digital psychiatry
Integrating computer-based technology into psychiatric practice has given birth to a new frontier that could be called digital psychiatry. This might encompass the following:
- telepsychiatry
- social media with a mental health focus
- web-based applications/devices
- artificial intelligence (AI).
Telepsychiatry. Videoconferencing is the most widely used form of telepsychiatry. It provides patients with easier access to mental health treatment.4 Telepsychiatry has the potential to match patients and clinicians with similar cultural backgrounds, thus minimizing cultural gaps and misunderstandings. Most importantly, it is comparable to face-to-face interviews in terms of the reliability of assessment and treatment outcomes.5
Telepsychiatry might be particularly helpful for patients with restricted mobility, such as those who live in remote areas, nursing homes, or correctional facilities. In correctional settings, transferring prisoners is expensive and carries the risk of escape. In a small study (N = 86) conducted in Hong Kong, Chen et al6 found that using videoconferencing to conduct clinical interviews of inmates was cost-efficient and scored high in terms of patient acceptability.
Social media. Social media could be a powerful platform for early detection of mental illness. Staying connected with patients on social media could allow psychiatrists to be more aware of their patient’s mood fluctuations, which might lead to more timely assessments. Physicians could be automatically notified about changes in their patients’ social media activity that indicate changes in mental state, which could solicit immediate intervention and treatment. On the other hand, such use of social media could blur professional boundaries. Psychiatrists also could use social media to promote awareness of mental health and educate the public on ways to improve or maintain their mental well-being.7
Web-based applications/devices. Real-time monitoring through applications or internet-based smart devices creates a new avenue for patients to receive personalized assessments, treatment, and intervention.8 Smartwatches with internet connectivity may offer a glimpse of the wearer’s sleep architecture and duration, thus providing real-time data on patients who have insomnia. We can now passively collect objective data from devices, such as smartphones and laptops, to phenotype an individual’s mood and mental state, a process called digital phenotyping. The Table9 lists examples of the types of mental health–related metrics that can be captured by smartphones, smartwatches, and similar technology. Information from these devices can be accumulated to create a database that can be used to predict symptoms.10 For example, the way people use a smartphone’s keyboard, including latency time between space and character types, can be used to generate variables for data. This type of information is being studied for use in screening depression and passively assessing mood in real time.11
Continue to: Artificial intelligence
Artificial intelligence—the development of computer systems able to perform tasks that normally require human intelligence—is being increasingly used in psychiatry. Some studies have suggested AI can be used to identify patients’ risk of suicide12-15 or psychosis.16,17Kalanderian and Nasrallah18 reviewed several of these studies in
Other researchers have found clinical uses for machine learning, a subset of AI that uses methods to automatically detect patterns and make predictions based on those patterns. In one study, a machine learning analysis of functional MRI scans was able to identify 4 distinct subtypes of depression.19 In another study, a machine learning model was able to predict with 60% accuracy which patients with depression would respond to antidepressants.20
In the future, AI might be used to change mental health classification systems. Because many mental health disorders share similar symptom clusters, machine learning can help to identify associations between symptoms, behavior, brain function, and real-world function across different diagnoses, potentially affecting how we will classify mental disorders.21
Technology-enhanced psychotherapy
In the future, it might be common for psychotherapy to be provided by a computer, or “virtual therapist.” Several studies have evaluated the use of technology-enhanced psychotherapy.
Lucas et al22 investigated patients’ interactions with a virtual therapist. Participants were interviewed by an avatar named Ellie, who they saw on a TV screen. Half of the participants were told Ellie was not human, and half were told Ellie was being controlled remotely by a human. Three psychologists who were blinded to group allocations analyzed transcripts of the interviews and video recordings of participants’ facial expressions to quantify the participants’ fear, sadness, and other emotional responses during the interviews, as well as their openness to the questions. Participants who believed Ellie was fully automated reported significantly lower fear of self-disclosure and impression management (attempts to control how others perceive them) than participants who were told that Ellie was operated by a human. Additionally, participants who believed they were interacting with a computer were more open during the interview.22
Continue to: Researchers at the University of Southern California...
Researchers at the University of Southern California developed software that assessed 74 acoustic features, including pitch, volume, quality, shimmer, jitter, and prosody, to predict outcomes among patients receiving couples therapy. This software was able to predict marital discord at least as well as human therapists.23
Many mental health apps purport to implement specific components of psychotherapy. Many of these apps focus on cognitive-behavioral therapy worksheets, mindfulness exercises, and/or mood tracking. The features provided by such apps emulate the tasks and intended outcomes of traditional psychotherapy, but in an entirely decentralized venue.24
Some have expressed concern that an increased use of virtual therapists powered by AI might lead to a dehumanization of psychiatry (Box25,26).
Box
Whether there are aspects of the psychiatric patient encounter that cannot be managed by a “virtual clinician” created by artificial intelligence (AI) remains to be determined. Some of the benefits of using AI in this manner may be difficult to anticipate, or may be specific to an individual’s relationship with his/her clinician.25
On the other hand, AI systems blur previously assumed boundaries between reality and fiction, and this could have complex effects on patients. Similar to therapeutic relationships with a human clinician, there is the risk of transference of emotions, thoughts, and feelings to a virtual therapist powered by AI. Unlike with a psychiatrist or therapist, however, there is no person on the other side of this transference. Whether virtual clinicians will be able to manage such transference remains to be seen.
In Deep Medicine,26 cardiologist Eric Topol, MD, emphasizes a crucial component of a patient encounter that AI will be unlikely able to provide: empathy. Virtual therapists powered by AI will inherit the tasks best done by machines, leaving humans more time to do what they do best—providing empathy and being “present” for patients.
Electronic health record reforms
Although many clinicians find EHRs to be onerous and time-consuming, EHR technology is constantly improving, and EHRs have revolutionized documentation and order implementation. Several potential advances could improve clinical practice. For example, EHRs could incorporate a clinical decision support system that uses AI-based algorithms to assist psychiatrists with diagnosis, monitoring, and treatment.27 In the future, EHRs might have the ability to monitor and learn from errors and adverse events, and automatically design an algorithm to avoid them.28 They should be designed to better manage analysis of pharmacogenetic test results, which is challenging due to the amount and complexity of the data.29 Future EHRs should eliminate the non-intuitive and multi-click interfaces and cumbersome data searches of today’s EHRs.30
Technology brings new ethical considerations
Mental health interventions based on AI typically work with algorithms, and algorithms bring ethical issues. Mental health devices or systems that use AI could contain biases that have the potential to harm in unintended ways, such as a data-driven sexist or racist bias.31 This may require investing additional time to explain to patients (and their families) what an algorithm is and how it works in relation to the therapy provided.
Continue to: Another concern is patient...
Another concern is patient autonomy.32 For example, it would be ethically problematic if a patient were to assume that there was a human physician “at the other end” of a virtual therapist or other technology who is communicating or reviewing his/her messages. Similarly, an older adult or a patient with intellectual disabilities may not be able to understand advanced technology or what it does when it is installed in their home to monitor the patient’s activities. This would increase the risk of privacy violations, manipulation, or even coercion if the requirements for informed consent are not satisfied.
A flowchart for the future
Although current research and innovations typically target specific areas of psychiatry, these advances can be integrated by devising algorithms and protocols that will change the current practice of psychiatry. The Figure provides a glimpse of how the psychiatry clinic of the future might work. A maxim of management is that “the best way to predict the future is to create it.” However, the mere conception of a vision is not enough—working towards it is essential.
Bottom Line
With advances in technology, psychiatric practice will soon be radically different from what it is today. The expanded use of telepsychiatry, social media, artificial intelligence, and web-based applications/devices holds great promise for psychiatric assessment, diagnosis, and treatment, although certain ethical and privacy concerns need to be adequately addressed.
Related Resources
- National Institute of Mental Health. Technology and the future of mental health treatment. www.nimh.nih.gov/health/topics/technology-and-the-future-of-mental-health-treatment/index.shtml. Revised September 2019.
- Hays R, Farrell HM, Touros J. Mobile apps and mental health: using technology to quantify real-time clinical risk. Current Psychiatry. 2019;18(6):37-41.
- Torous J, Luo J, Chan SR. Mental health apps: what to tell patients. Current Psychiatry. 2018;17(3):21-25.
Despite the tremendous advances in psychiatry in recent years, the current clinical practice of psychiatry continues to rely on data from intermittent assessments along with subjective and unquantifiable accounts from patients and caregivers. Furthermore, there continues to be significant diagnostic variations among practitioners. Fortunately, technology to address these issues appears to be on the horizon.
How might the psychiatric clinic of the future look? What changes could we envision? These 4 critical factors may soon bring about dynamic changes in the way we practice psychiatry:
- precision psychiatry
- digital psychiatry
- technology-enhanced psychotherapy
- electronic health record (EHR) reforms.
In this article, we review how advances in each of these areas might lead to improved care for our patients.
Precision psychiatry
Precision psychiatry takes into account each patient’s variability in genes, environment, and lifestyle to determine individualized treatment and prevention strategies. It relies on pharmacogenomic testing as the primary tool. Pharmacogenomics is the study of variability in drug response due to heredity.
Emerging data on the clinical utility and cost-effectiveness of pharmacogenomic testing are encouraging, but its routine use is not well supported by current evidence.2 One limit to using pharmacogenomic testing is that many genes simultaneously exert an effect on the structure and function of neurons and associated pathophysiology. According to the International Society of Psychiatric Genetics, no single genetic variant is sufficient to cause psychiatric disorders such as depression, bipolar disorder, substance dependence, or schizophrenia. This limits the possibility of using genetic tests to establish a diagnosis.3
In the future, better algorithms could promote more accurate pharmacogenomics profiles for individual patients, which could influence treatment.
Precision psychiatry could lead to:
- identification of novel targets for new medications
- pharmacogenetic profiling of the patient to predict disease susceptibility and medication response
- personalized therapy: the right drug at the right dose for the right patient.
- improved efficacy and fewer adverse medication reactions.
Continue to: Digital psychiatry
Digital psychiatry
Integrating computer-based technology into psychiatric practice has given birth to a new frontier that could be called digital psychiatry. This might encompass the following:
- telepsychiatry
- social media with a mental health focus
- web-based applications/devices
- artificial intelligence (AI).
Telepsychiatry. Videoconferencing is the most widely used form of telepsychiatry. It provides patients with easier access to mental health treatment.4 Telepsychiatry has the potential to match patients and clinicians with similar cultural backgrounds, thus minimizing cultural gaps and misunderstandings. Most importantly, it is comparable to face-to-face interviews in terms of the reliability of assessment and treatment outcomes.5
Telepsychiatry might be particularly helpful for patients with restricted mobility, such as those who live in remote areas, nursing homes, or correctional facilities. In correctional settings, transferring prisoners is expensive and carries the risk of escape. In a small study (N = 86) conducted in Hong Kong, Chen et al6 found that using videoconferencing to conduct clinical interviews of inmates was cost-efficient and scored high in terms of patient acceptability.
Social media. Social media could be a powerful platform for early detection of mental illness. Staying connected with patients on social media could allow psychiatrists to be more aware of their patient’s mood fluctuations, which might lead to more timely assessments. Physicians could be automatically notified about changes in their patients’ social media activity that indicate changes in mental state, which could solicit immediate intervention and treatment. On the other hand, such use of social media could blur professional boundaries. Psychiatrists also could use social media to promote awareness of mental health and educate the public on ways to improve or maintain their mental well-being.7
Web-based applications/devices. Real-time monitoring through applications or internet-based smart devices creates a new avenue for patients to receive personalized assessments, treatment, and intervention.8 Smartwatches with internet connectivity may offer a glimpse of the wearer’s sleep architecture and duration, thus providing real-time data on patients who have insomnia. We can now passively collect objective data from devices, such as smartphones and laptops, to phenotype an individual’s mood and mental state, a process called digital phenotyping. The Table9 lists examples of the types of mental health–related metrics that can be captured by smartphones, smartwatches, and similar technology. Information from these devices can be accumulated to create a database that can be used to predict symptoms.10 For example, the way people use a smartphone’s keyboard, including latency time between space and character types, can be used to generate variables for data. This type of information is being studied for use in screening depression and passively assessing mood in real time.11
Continue to: Artificial intelligence
Artificial intelligence—the development of computer systems able to perform tasks that normally require human intelligence—is being increasingly used in psychiatry. Some studies have suggested AI can be used to identify patients’ risk of suicide12-15 or psychosis.16,17Kalanderian and Nasrallah18 reviewed several of these studies in
Other researchers have found clinical uses for machine learning, a subset of AI that uses methods to automatically detect patterns and make predictions based on those patterns. In one study, a machine learning analysis of functional MRI scans was able to identify 4 distinct subtypes of depression.19 In another study, a machine learning model was able to predict with 60% accuracy which patients with depression would respond to antidepressants.20
In the future, AI might be used to change mental health classification systems. Because many mental health disorders share similar symptom clusters, machine learning can help to identify associations between symptoms, behavior, brain function, and real-world function across different diagnoses, potentially affecting how we will classify mental disorders.21
Technology-enhanced psychotherapy
In the future, it might be common for psychotherapy to be provided by a computer, or “virtual therapist.” Several studies have evaluated the use of technology-enhanced psychotherapy.
Lucas et al22 investigated patients’ interactions with a virtual therapist. Participants were interviewed by an avatar named Ellie, who they saw on a TV screen. Half of the participants were told Ellie was not human, and half were told Ellie was being controlled remotely by a human. Three psychologists who were blinded to group allocations analyzed transcripts of the interviews and video recordings of participants’ facial expressions to quantify the participants’ fear, sadness, and other emotional responses during the interviews, as well as their openness to the questions. Participants who believed Ellie was fully automated reported significantly lower fear of self-disclosure and impression management (attempts to control how others perceive them) than participants who were told that Ellie was operated by a human. Additionally, participants who believed they were interacting with a computer were more open during the interview.22
Continue to: Researchers at the University of Southern California...
Researchers at the University of Southern California developed software that assessed 74 acoustic features, including pitch, volume, quality, shimmer, jitter, and prosody, to predict outcomes among patients receiving couples therapy. This software was able to predict marital discord at least as well as human therapists.23
Many mental health apps purport to implement specific components of psychotherapy. Many of these apps focus on cognitive-behavioral therapy worksheets, mindfulness exercises, and/or mood tracking. The features provided by such apps emulate the tasks and intended outcomes of traditional psychotherapy, but in an entirely decentralized venue.24
Some have expressed concern that an increased use of virtual therapists powered by AI might lead to a dehumanization of psychiatry (Box25,26).
Box
Whether there are aspects of the psychiatric patient encounter that cannot be managed by a “virtual clinician” created by artificial intelligence (AI) remains to be determined. Some of the benefits of using AI in this manner may be difficult to anticipate, or may be specific to an individual’s relationship with his/her clinician.25
On the other hand, AI systems blur previously assumed boundaries between reality and fiction, and this could have complex effects on patients. Similar to therapeutic relationships with a human clinician, there is the risk of transference of emotions, thoughts, and feelings to a virtual therapist powered by AI. Unlike with a psychiatrist or therapist, however, there is no person on the other side of this transference. Whether virtual clinicians will be able to manage such transference remains to be seen.
In Deep Medicine,26 cardiologist Eric Topol, MD, emphasizes a crucial component of a patient encounter that AI will be unlikely able to provide: empathy. Virtual therapists powered by AI will inherit the tasks best done by machines, leaving humans more time to do what they do best—providing empathy and being “present” for patients.
Electronic health record reforms
Although many clinicians find EHRs to be onerous and time-consuming, EHR technology is constantly improving, and EHRs have revolutionized documentation and order implementation. Several potential advances could improve clinical practice. For example, EHRs could incorporate a clinical decision support system that uses AI-based algorithms to assist psychiatrists with diagnosis, monitoring, and treatment.27 In the future, EHRs might have the ability to monitor and learn from errors and adverse events, and automatically design an algorithm to avoid them.28 They should be designed to better manage analysis of pharmacogenetic test results, which is challenging due to the amount and complexity of the data.29 Future EHRs should eliminate the non-intuitive and multi-click interfaces and cumbersome data searches of today’s EHRs.30
Technology brings new ethical considerations
Mental health interventions based on AI typically work with algorithms, and algorithms bring ethical issues. Mental health devices or systems that use AI could contain biases that have the potential to harm in unintended ways, such as a data-driven sexist or racist bias.31 This may require investing additional time to explain to patients (and their families) what an algorithm is and how it works in relation to the therapy provided.
Continue to: Another concern is patient...
Another concern is patient autonomy.32 For example, it would be ethically problematic if a patient were to assume that there was a human physician “at the other end” of a virtual therapist or other technology who is communicating or reviewing his/her messages. Similarly, an older adult or a patient with intellectual disabilities may not be able to understand advanced technology or what it does when it is installed in their home to monitor the patient’s activities. This would increase the risk of privacy violations, manipulation, or even coercion if the requirements for informed consent are not satisfied.
A flowchart for the future
Although current research and innovations typically target specific areas of psychiatry, these advances can be integrated by devising algorithms and protocols that will change the current practice of psychiatry. The Figure provides a glimpse of how the psychiatry clinic of the future might work. A maxim of management is that “the best way to predict the future is to create it.” However, the mere conception of a vision is not enough—working towards it is essential.
Bottom Line
With advances in technology, psychiatric practice will soon be radically different from what it is today. The expanded use of telepsychiatry, social media, artificial intelligence, and web-based applications/devices holds great promise for psychiatric assessment, diagnosis, and treatment, although certain ethical and privacy concerns need to be adequately addressed.
Related Resources
- National Institute of Mental Health. Technology and the future of mental health treatment. www.nimh.nih.gov/health/topics/technology-and-the-future-of-mental-health-treatment/index.shtml. Revised September 2019.
- Hays R, Farrell HM, Touros J. Mobile apps and mental health: using technology to quantify real-time clinical risk. Current Psychiatry. 2019;18(6):37-41.
- Torous J, Luo J, Chan SR. Mental health apps: what to tell patients. Current Psychiatry. 2018;17(3):21-25.
1. Pirmohamed M. Pharmacogenetics and pharmacogenomics. Br J Clin Pharmacol. 2001;52(4):345-347.
2. Benitez J, Cool CL, Scotti DJ. Use of combinatorial pharmacogenomic guidance in treating psychiatric disorders. Per Med. 2018;15(6):481-494.
3. Cannon TD. Candidate gene studies in the GWAS era: the MET proto-oncogene, neurocognition, and schizophrenia. Am J Psychiatry. 2010;167(4):4,369-372.
4. Greenwood J, Chamberlain C, Parker G. Evaluation of a rural telepsychiatry service. Australas Psychiatry. 2004;12(3):268-272.
5. Hubley S, Lynch SB, Schneck C, et al. Review of key telepsychiatry outcomes. World J Psychiatry. 2016;6(2):269-282.
6. Cheng KM, Siu BW, Yeung CC, et al. Telepsychiatry for stable Chinese psychiatric out-patients in custody in Hong Kong: a case-control pilot study. Hong Kong Med J. 2018;24(4):378-383.
7. Frankish K, Ryan C, Harris A. Psychiatry and online social media: potential, pitfalls and ethical guidelines for psychiatrists and trainees. Australasian Psychiatry. 2012;20(3):181-187.
8. de la Torre Díez I, Alonso SG, Hamrioui S, et al. IoT-based services and applications for mental health in the literature. J Med Syst. 2019;43(1):4-9.
9. Topol E. Deep Medicine. New York, NY: Basic Books; 2019:168.
10. Adams RA, Huys QJM, Roiser JP. Computational Psychiatry: towards a mathematically informed understanding of mental illness. J Neurol Neurosurg Psychiatry. 2016;87(1):53-63.
11. Insel TR. Bending the curve for mental health: technology for a public health approach. Am J Public Health. 2019;109(suppl 3):S168-S170.
12. Just MA, Pan L, Cherkassky VL, et al. Machine learning of neural representations of suicide and emotion concepts identifies suicidal youth. Nat Hum Behav. 2017;1:911-919.
13. Pestian J, Nasrallah H, Matykiewicz P, et al. Suicide note classification using natural language processing: a content analysis. Biomed Inform Insights. 2010;2010(3):19-28.
14. Walsh CG, Ribeiro JD, Franklin JC. Predicting risk of suicide attempts over time through machine learning. Clinical Psychological Science. 2017;5(3):457-469.
15. Pestian JP, Sorter M, Connolly B, et al; STM Research Group. A machine learning approach to identifying the thought markers of suicidal subjects: a prospective multicenter trial. Suicide Life Threat Behav. 2017;47(1):112-121.
16. Corcoran CM, Carrillo F, Fernández-Slezak D, et al. Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry. 2018;17(1):67-75.
17. Bedi G, Carrillo F, Cecchi GA, et al. Automated analysis of free speech predicts psychosis onset in high-risk youths. NPJ Schizophr. 2015;1:15030. doi: 10.1038/npjschz.2015.30.
18. Kalanderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019;18(8):33-38.
19. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
20. Chekroud AM, Zotti RJ, Shehzad Z, et al. Cross-trial prediction of treatment outcome in depression: a machine learning approach. Lancet Psychiatry. 2016;3(3):243-250.
21. Grisanzio KA, Goldstein-Piekarski AN, Wang MY, et al. Transdiagnostic symptom clusters and associations with brain, behavior, and daily function in mood, anxiety, and trauma disorders. JAMA Psychiatry. 2018;75(2):201-209.
22. Lucas G, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Computers in Human Behavior. 2014;37:94-100.
23. Nasir M, Baucom BR, Georgiou P, et al. Predicting couple therapy outcomes based on speech acoustic features. PLoS One. 2017;12(9):e0185123. doi: 10.1371/journal.pone.0185123.
24. Huguet A, Rao S, McGrath PJ, et al. A systematic review of cognitive behavioral therapy and behavioral activation apps for depression. PLoS One. 2016;11(5):e0154248. doi: 10.1371/journal.pone.0154248.
25. Scholten MR, Kelders SM, Van Gemert-Pijnen JE. Self-guided web-based interventions: scoping review on user needs and the potential of embodied conversational agents to address them. J Med Internet Res. 2017;19(11):e383.
26. Topol E. Deep Medicine. New York, NY: Basic Books; 2019:283-310.
27. Abramson EL, McGinnis S, Edwards A, et al. Electronic health record adoption and health information exchange among hospitals in New York State. J Eval Clin Pract. 2012;18(6):1156-1162.
28. Meeks DW, Smith MW, Taylor L, et al. An analysis of electronic health record-related patient safety concerns. J Am Med Inform Assoc. 2014;21(6):1053-1059.
29. Kho AN, Rasmussen LV, Connolly JJ, et al. Practical challenges in integrating genomic data into the electronic health record. Genet Med. 2013;15(10):772-778.
30. Ornstein SM, Oates RB, Fox GN. The computer-based medical record: current status. J Fam Pract. 1992;35(5):556-565.
31. Corea F. Machine ethics and artificial moral agents. In: Applied artificial intelligence: where AI can be used in business. Basel, Switzerland: Springer; 2019:33-41.
32. Beauchamp T, Childress J. Principles of biomedical ethics. 7th ed. New York, NY: Oxford University Press; 2012:44.
1. Pirmohamed M. Pharmacogenetics and pharmacogenomics. Br J Clin Pharmacol. 2001;52(4):345-347.
2. Benitez J, Cool CL, Scotti DJ. Use of combinatorial pharmacogenomic guidance in treating psychiatric disorders. Per Med. 2018;15(6):481-494.
3. Cannon TD. Candidate gene studies in the GWAS era: the MET proto-oncogene, neurocognition, and schizophrenia. Am J Psychiatry. 2010;167(4):4,369-372.
4. Greenwood J, Chamberlain C, Parker G. Evaluation of a rural telepsychiatry service. Australas Psychiatry. 2004;12(3):268-272.
5. Hubley S, Lynch SB, Schneck C, et al. Review of key telepsychiatry outcomes. World J Psychiatry. 2016;6(2):269-282.
6. Cheng KM, Siu BW, Yeung CC, et al. Telepsychiatry for stable Chinese psychiatric out-patients in custody in Hong Kong: a case-control pilot study. Hong Kong Med J. 2018;24(4):378-383.
7. Frankish K, Ryan C, Harris A. Psychiatry and online social media: potential, pitfalls and ethical guidelines for psychiatrists and trainees. Australasian Psychiatry. 2012;20(3):181-187.
8. de la Torre Díez I, Alonso SG, Hamrioui S, et al. IoT-based services and applications for mental health in the literature. J Med Syst. 2019;43(1):4-9.
9. Topol E. Deep Medicine. New York, NY: Basic Books; 2019:168.
10. Adams RA, Huys QJM, Roiser JP. Computational Psychiatry: towards a mathematically informed understanding of mental illness. J Neurol Neurosurg Psychiatry. 2016;87(1):53-63.
11. Insel TR. Bending the curve for mental health: technology for a public health approach. Am J Public Health. 2019;109(suppl 3):S168-S170.
12. Just MA, Pan L, Cherkassky VL, et al. Machine learning of neural representations of suicide and emotion concepts identifies suicidal youth. Nat Hum Behav. 2017;1:911-919.
13. Pestian J, Nasrallah H, Matykiewicz P, et al. Suicide note classification using natural language processing: a content analysis. Biomed Inform Insights. 2010;2010(3):19-28.
14. Walsh CG, Ribeiro JD, Franklin JC. Predicting risk of suicide attempts over time through machine learning. Clinical Psychological Science. 2017;5(3):457-469.
15. Pestian JP, Sorter M, Connolly B, et al; STM Research Group. A machine learning approach to identifying the thought markers of suicidal subjects: a prospective multicenter trial. Suicide Life Threat Behav. 2017;47(1):112-121.
16. Corcoran CM, Carrillo F, Fernández-Slezak D, et al. Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry. 2018;17(1):67-75.
17. Bedi G, Carrillo F, Cecchi GA, et al. Automated analysis of free speech predicts psychosis onset in high-risk youths. NPJ Schizophr. 2015;1:15030. doi: 10.1038/npjschz.2015.30.
18. Kalanderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019;18(8):33-38.
19. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
20. Chekroud AM, Zotti RJ, Shehzad Z, et al. Cross-trial prediction of treatment outcome in depression: a machine learning approach. Lancet Psychiatry. 2016;3(3):243-250.
21. Grisanzio KA, Goldstein-Piekarski AN, Wang MY, et al. Transdiagnostic symptom clusters and associations with brain, behavior, and daily function in mood, anxiety, and trauma disorders. JAMA Psychiatry. 2018;75(2):201-209.
22. Lucas G, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Computers in Human Behavior. 2014;37:94-100.
23. Nasir M, Baucom BR, Georgiou P, et al. Predicting couple therapy outcomes based on speech acoustic features. PLoS One. 2017;12(9):e0185123. doi: 10.1371/journal.pone.0185123.
24. Huguet A, Rao S, McGrath PJ, et al. A systematic review of cognitive behavioral therapy and behavioral activation apps for depression. PLoS One. 2016;11(5):e0154248. doi: 10.1371/journal.pone.0154248.
25. Scholten MR, Kelders SM, Van Gemert-Pijnen JE. Self-guided web-based interventions: scoping review on user needs and the potential of embodied conversational agents to address them. J Med Internet Res. 2017;19(11):e383.
26. Topol E. Deep Medicine. New York, NY: Basic Books; 2019:283-310.
27. Abramson EL, McGinnis S, Edwards A, et al. Electronic health record adoption and health information exchange among hospitals in New York State. J Eval Clin Pract. 2012;18(6):1156-1162.
28. Meeks DW, Smith MW, Taylor L, et al. An analysis of electronic health record-related patient safety concerns. J Am Med Inform Assoc. 2014;21(6):1053-1059.
29. Kho AN, Rasmussen LV, Connolly JJ, et al. Practical challenges in integrating genomic data into the electronic health record. Genet Med. 2013;15(10):772-778.
30. Ornstein SM, Oates RB, Fox GN. The computer-based medical record: current status. J Fam Pract. 1992;35(5):556-565.
31. Corea F. Machine ethics and artificial moral agents. In: Applied artificial intelligence: where AI can be used in business. Basel, Switzerland: Springer; 2019:33-41.
32. Beauchamp T, Childress J. Principles of biomedical ethics. 7th ed. New York, NY: Oxford University Press; 2012:44.
Does your patient have the right to refuse medications?
Ms. T, age 48, is brought to the psychiatric emergency department after the police find her walking along the highway at 3:00
Once involuntarily committed, does Ms. T have the right to refuse treatment?
Every psychiatrist has faced the predicament of a patient who refuses treatment. This creates an ethical dilemma between respecting the patient’s autonomy vs forcing treatment to ameliorate symptoms and reduce suffering. This article addresses case law related to the models for administering psychiatric medications over objection. We also discuss case law regarding court-appointed guardianship, and treating medical issues without consent. While this article provides valuable information on these scenarios, it is crucial to remember that the legal processes required to administer medications over patient objection are state-specific. In order to ensure the best practice and patient care, you must research the legal procedures specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
History of involuntary treatment
Prior to the 1960s, Ms. T would likely have been unable to refuse treatment. All patients were considered involuntary, and the course of treatment was decided solely by the psychiatric institution. Well into the 20th century, patients with psychiatric illness remained feared and stigmatized, which led to potent and potentially harsh methods of treatment. Some patients experienced extreme isolation, whipping, bloodletting, experimental use of chemicals, and starvation (Table 11-3).
With the advent of psychotropic medications and a focus on civil liberties, the psychiatric mindset began to change from hospital-based treatment to a community-based approach. The value of psychotherapy was recognized, and by the 1960s, the establishment of community mental health centers was gaining momentum.
In the context of these changes, the civil rights movement pressed for stronger legislation regarding autonomy and the quality of treatment available to patients with psychiatric illness. In the 1960s and 1970s, Rouse v Cameron4 and Wyatt v Stickney5 dealt with a patient’s right to receive treatment while involuntarily committed. However, it was not until the 1980s that the courts addressed the issue of a patient’s right to refuse treatment.
The judicial system: A primer
When reviewing case law and its applicability to your patients, it is important to understand the various court systems. The judicial system is divided into state and federal courts, which are subdivided into trial, appellate, and supreme courts. When decisions at either the state or federal level require an ultimate decision maker, the US Supreme Court can choose to hear the case, or grant certiorari, and make a ruling, which is then binding law.6 Decisions made by any court are based on various degrees of stringency, called standards of proof (Table 27).
Continue to: For Ms. T's case...
For Ms. T’s case, civil commitment and involuntary medication hearings are held in probate court, which is a civil (not criminal) court. In addition to overseeing civil commitment and involuntary medications, probate courts adjudicate will and estate contests, conservatorship, and guardianship. Conservatorship hearings deal with financial issues, and guardianship cases encompass personal and health-related needs. Regardless of the court, an individual is guaranteed due process under the 5th Amendment (federal) and 14th Amendment (state).
Individuals are presumed competent to make their own decisions, but a court may call this into question. Competencies are specific to a variety of areas, such as criminal proceedings, medical decision making, writing a will (testimonial capacity), etc. Because each field applies its own standard of competence, an individual may be competent in one area but incompetent in another. Competence in medical decision making varies by state but generally consists of being able to communicate a choice, understand relevant information, appreciate one’s illness and its likely consequences, and rationally manipulate information.8
Box
Administering medications despite a patient’s objection differs from situations in which medications are provided during a psychiatric emergency. In an emergency, courts do not have time to weigh in. Instead, emergency medications (most often given as IM injections) are administered based on the physician’s clinical judgment. The criteria for psychiatric emergencies are delineated at the state level, but typically are defined as when a person with a mental illness creates an imminent risk of harm to self or others. Alternative approaches to resolving the emergency may include verbal de-escalation, quiet time in a room devoid of stimuli, locked seclusion, or physical restraints. These measures are often exhausted before emergency medications are administered.
Source: Reference 9
It is important to note that the legal process required before administering involuntary medications is distinct from situations in which medication needs to be provided during a psychiatric emergency. The Box9 outlines the difference between these 2 scenarios.
4 Legal models
There are several legal models used to determine when a patient can be administered psychiatric medications over objection. Table 310,11 summarizes these models.
Rights-driven (Rogers) model. If Ms. T was involuntarily hospitalized in Massachusetts or another state that adopted the rights-driven model, she would retain the right to refuse treatment. These states require an external judicial review, and court approval is necessary before imposing any therapy. This model was established in Rogers v Commissioner,12 where 7 patients at the Boston State Hospital filed a lawsuit regarding their right to refuse medications. The Massachusetts Supreme Judicial Court ruled that, despite being involuntarily committed, a patient is considered competent to refuse treatment until found specifically incompetent to do so by the court. If a patient is found incompetent, the judge, using a full adversarial hearing, decides what the incompetent patient would have wanted if he/she were competent. The judge reaches a conclusion based on the substituted judgment model (Table 410). In Rogers v Commissioner,12 the court ruled that the right to decision making is not lost after becoming a patient at a mental health facility. The right is lost only if the patient is found incompetent by the judge. Thus, every individual has the right to “manage his own person” and “take care of himself.”
Continue to: An update to the rights-driven (Rogers) model
An update to the rights-driven (Rogers) model. Other states, such as Ohio, have adopted the Rogers model and addressed issues that arose subsequent to the aforementioned case. In Steele v Hamilton County,13 Jeffrey Steele was admitted and later civilly committed to the hospital. After 2 months, an involuntary medication hearing was completed in which 3 psychiatrists concluded that, although Mr. Steele was not a danger to himself or others while in the hospital, he would ultimately benefit from medications.
The probate court acknowledged that Mr. Steele lacked capacity and required hospitalization. However, because he was not imminently dangerous, medication should not be used involuntarily. After a series of appeals, the Ohio Supreme Court ruled that a court may authorize the administration of an antipsychotic medication against a patient’s wishes without a finding of dangerousness when clear and convincing evidence exists that:
- the patient lacks the capacity to give or withhold informed consent regarding treatment
- the proposed medication is in the patient’s best interest
- no less intrusive treatment will be as effective in treating the mental illness.
This ruling set a precedent that dangerousness is not a requirement for involuntary medications.
Treatment-driven (Rennie) model. As in the rights-driven model, in the treatment-driven model, Ms. T would retain the constitutional right to refuse treatment. However, the models differ in the amount of procedural due process required. The treatment-driven model derives from Rennie v Klein,14 in which John Rennie, a patient at Ancora State Psychiatric Hospital in New Jersey, filed a suit regarding the right of involuntarily committed patients to refuse antipsychotic medications. The Third Circuit Court of Appeals ruled that, if professional judgment deems a patient to be a danger to himself or others, then antipsychotics may be administered over individual objection. This professional judgment is typically based on the opinion of the treating physician, along with a second physician or panel.
Utah model. This model is based on A.E. and R.R. v Mitchell,15 in which the Utah District Court ruled that a civilly committed patient has no right to refuse treatment. This Utah model was created after state legislature determined that, in order to civilly commit a patient, hospitalization must be the least restrictive alternative and the patient is incompetent to consent to treatment. Unlike the 2 previous models, competency to refuse medications is not separated from a previous finding of civil commitment, but rather, they occur simultaneously.
Continue to: Rights in unique situations
Rights in unique situations
Correctional settings. If Ms. T was an inmate, would her right to refuse psychiatric medication change? This was addressed in the case of Washington v Harper.16 Walter Harper, serving time for a robbery conviction, filed a claim that his civil rights were being violated when he received involuntary medications based on the decision of a 3-person panel consisting of a psychiatrist, psychologist, and prison official. The US Supreme Court ruled that this process provided sufficient due process to mandate providing psychotropic medications against a patient’s will. This reduction in required procedures is related to the unique nature of the correctional environment and an increased need to maintain safety. This need was felt to outweigh an individual’s right to refuse medication.
Incompetent to stand trial. In Sell v U.S.,17 Charles Sell, a dentist, was charged with fraud and attempted murder. He underwent a competency evaluation and was found incompetent to stand trial because of delusional thinking. Mr. Sell was hospitalized for restorability but refused medications. The hospital held an administrative hearing to proceed with involuntary antipsychotic medications; however, Mr. Sell filed an order with the court to prevent this. Eventually, the US Supreme Court ruled that non-dangerous, incompetent defendants may be involuntarily medicated even if they do not pose a risk to self or others on the basis that it furthers the state’s interest in bringing to trial those charged with serious crimes. However, the following conditions must be met before involuntary medication can be administered:
- an important government issue must be at stake (determined case-by-case)
- a substantial probability must exist that the medication will enable the defendant to become competent without significant adverse effects
- the medication must be medically appropriate and necessary to restore competency, with no less restrictive alternative available.
This case suggests that, before one attempts to forcibly medicate a defendant for the purpose of competency restoration, one should exhaust the same judicial remedies one uses for civil patients first.
Court-appointed guardianship
In the case of Ms. T, what if her father requested to become her guardian? This question was explored in the matter of Guardianship of Richard Roe III.18 Mr. Roe was admitted to the Northampton State Hospital in Massachusetts, where he refused antipsychotic medications. Prior to his release, his father asked to be his guardian. The probate court obliged the request. However, Mr. Roe’s lawyer and guardian ad litem (a neutral temporary guardian often appointed when legal issues are pending) challenged the ruling, arguing the probate court cannot empower the guardian to consent to involuntary medication administration. On appeal, the court ruled:
- the guardianship was justified
- the standard of proof for establishment of a guardianship is preponderance of the evidence (Table 27)
- the guardian must seek from a court a “substituted judgment” to authorize forcible administration of antipsychotic medication.
The decision to establish the court as the final decision maker is based on the view that a patient’s relatives may be biased. Courts should take an objective approach that considers
- patient preference stated during periods of competency
- medication adverse effects
- consequences if treatment is refused
- prognosis with treatment
- religious beliefs
- impact on the patient’s family.
Continue to: This case set the stage for...
This case set the stage for later decisions that placed antipsychotic medications in the same category as electroconvulsive therapy and psychosurgery. This could mean a guardian would need specialized authorization to request antipsychotic treatment but could consent to an appendectomy without legal issue.
Fortunately, now most jurisdictions have remedied this cumbersome solution by requiring a higher standard of proof, clear and convincing evidence (Table 27), to establish guardianship but allowing the guardian more latitude to make decisions for their wards (such as those involving hospital admission or medications) without further court involvement.
Involuntary medical treatment
In order for a patient to consent for medical treatment, he/she must have the capacity to do so (Table 59). How do the courts handle the patient’s right to refuse medical treatment? This was addressed in the case of Georgetown College v Jones.19 Mrs. Jones, a 25-year-old Jehovah’s Witness and mother of a 7-month-old baby, suffered a ruptured ulcer and lost a life-threatening amount of blood. Due to her religious beliefs, Mrs. Jones refused a blood transfusion. The hospital quickly appealed to the court, who ruled the woman was help-seeking by going to the hospital, did not want to die, was in distress, and lacked capacity to make medical decisions. Acting in a parens patriae manner (when the government steps in to make decisions for its citizens who cannot), the court ordered the hospital to administer blood transfusions.
Proxy decision maker. When the situation is less emergent, a proxy decision maker can be appointed by the court. This was addressed in the case of Superintendent of Belchertown v Saikewicz.20 Mr. Saikewicz, a 67-year-old man with intellectual disability, was diagnosed with cancer and given weeks to months to live without treatment. However, treatment was only 50% effective and could potentially cause severe adverse effects. A guardian ad litem was appointed and recommended nontreatment, which the court upheld. The court ruled that the right to accept or reject medical treatment applies to both incompetent and competent persons. With incompetent persons, a “substituted judgment” analysis is used over the “best interest of the patient” doctrine.20 This falls in line with the Guardianship of Richard Roe III ruling,18 in which the court’s substituted judgment standard is enacted in an effort to respect patient autonomy.
Right to die. When does a patient have the right to die and what is the standard of proof? The US Supreme Court case Cruzan v Director21 addressed this. Nancy Cruzan was involved in a car crash, which left her in a persistent vegetative state with no significant cognitive function. She remained this way for 6 years before her parents sought to terminate life support. The hospital refused. The Missouri Supreme Court ruled that a standard of clear and convincing evidence (Table 27) is required to withdraw treatment, and in a 5-to-4 decision, the US Supreme Court upheld Missouri’s decision. This set the national standard for withdrawal of life-sustaining treatment. The moderate standard of proof is based on the court’s ruling that the decision to terminate life is a particularly important one.
Continue to: CASE
CASE CONTINUED
After having been civilly committed to your inpatient psychiatric facility, Ms. T’s paranoia and disorganized behavior persist. She continues to refuse medications.
There are 3 options: respect her decision, negotiate with her, or attempt to force medications through due process.11 In negotiating a compromise, it is best to understand the barriers to treatment. A patient may refuse medications due to poor insight into his/her illness, medication adverse effects, a preference for an alternative treatment, delusional concerns over contamination and/or poisoning, interpersonal conflicts with the treatment staff, a preference for symptoms (eg, mania) over wellness, medication ineffectiveness, length of treatment course, or stigma.22,23 However, a patient’s unwillingness to compromise creates the dilemma of autonomy vs treatment.
For Ms. T, the treatment team felt initiating involuntary medication was the best option for her quality of life and safety. Because she resides in Ohio, a Rogers-like model was applied. The probate court was petitioned and found her incompetent to make medical decisions. The court accepted the physician’s recommendation of treatment with antipsychotic medications. If this scenario took place in New Jersey, a Rennie model would apply, requiring due process through the second opinion of another physician. Lastly, if Ms. T lived in Utah, she would have been unable to refuse medications once civilly committed.
Pros and cons of each model
Over the years, various concerns about each of these models have been raised. Given the slow-moving wheels of justice, one concern was that perhaps patients would be left “rotting with their rights on,” or lingering in a psychotic state out of respect for their civil liberties.19 While court hearings do not always happen quickly, more often than not, a judge will agree with the psychiatrist seeking treatment because the judge likely has little experience with mental illness and will defer to the physician’s expertise. This means the Rogers model may be more likely to produce the desired outcome, just more slowly. With respect to the Rennie model, although it is often more expeditious, the second opinion of an independent psychiatrist may contradict that of the original physician because the consultant will rely on his/her own expertise. Finally, some were concerned that psychiatrists would view the Utah model as carte blanche to start whatever medications they wanted with no respect for patient preference. Based on our clinical experience, none of these concerns have come to fruition over time, and patients safely receive medications over objection in hospitals every day.
Consider why the patient refuses medication
Regardless of which involuntary medication model is employed, it is important to consider the underlying cause for medication refusal, because it may affect future compliance. If the refusal is the result of a religious belief, history of adverse effects, or other rational motive, then it may be reasonable to respect the patient’s autonomy.24 However, if the refusal is secondary to symptoms of mental illness, it is appropriate to move forward with an involuntary medication hearing and treat the underlying condition.
Continue to: In the case of Ms. T...
In the case of Ms. T, she appeared to be refusing medications because of her psychotic symptoms, which could be effectively treated with antipsychotic medications. Therefore, Ms. T’s current lack of capacity is hopefully a transient phenomenon that can be ameliorated by initiating medication. Typically, antipsychotic medications begin to reduce psychotic symptoms within the first week, with further improvement over time.25 The value of the inpatient psychiatric setting is that it allows for daily monitoring of a patient’s response to treatment. As capacity is regained, patient autonomy over medical decisions is reinstated.
Bottom Line
The legal processes required to administer medications over a patient’s objection are state-specific, and multiple models are used. In general, a patient’s right to refuse treatment can be overruled by obtaining adjudication through the courts (Rogers model) or the opinion of a second physician (Rennie model). In order to ensure the best practice and patient care, research the legal procedure specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
Related Resources
- Miller D. Is forced treatment in our outpatients’ best interests? Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/80277/forced-treatment-our-outpatients-best-interests.
- Miller D, Hanson A. Committed: The battle over involuntary psychiatric care. Baltimore, MD: Johns Hopkins University Press; 2016.
1. Laffey P. Psychiatric therapy in Georgian Britain. Psychol Med. 2003;33(7):1285-1297.
2. Porter R. Madness: a brief history. New York, NY: Oxford Press; 2002.
3. Stetka B, Watson J. Odd and outlandish psychiatric treatments throughout history. Medscape Psychiatry. https://www.medscape.com/features/slideshow/odd-psychiatric-treatments. Published April 13, 2016. Accessed February 26, 2020.
4. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
5. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
6. Administrative Office of the US Courts. Comparing federal and state Courts. United States Courts. https://www.uscourts.gov/about-federal-courts/court-role-and-structure/comparing-federal-state-courts. Accessed February 26, 2020.
7. Drogin E, Williams C. Introduction to the Legal System. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:80-83.
8. Appelbaum P, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
9. Kambam P. Informed consent and competence. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:115-121.
10. Wall B, Anfang S. Legal regulation of psychiatric treatment. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:306-333.
11. Pinals D, Nesbit A, Hoge S. Treatment refusal in psychiatric practice. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:155-163.
12. Rogers v Commissioner, 390 489 (Mass 1983).
13. Steele v Hamilton County, 90 Ohio St3d 176 (Ohio 2000).
14. Rennie v Klein, 462 F Supp 1131 (D NJ 1978).
15. AE and RR v Mitchell, 724 F.2d 864 (10th Cir 1983).
16. Washington v Harper, 494 US 210 (1990).
17. Sell v US, 539 US 166 (2003).
18. Guardianship of Richard Roe III, 383 415, 435 (Mass 1981).
19. Georgetown College v Jones, 331 F2d 1010 (DC Cir 1964).
20. Superintendent of Belchertown v Saikewicz, 370 NE 2d 417 (1977).
21. Cruzan v Director, 497 US 261 (1990).
22. Owiti J, Bowers L. A literature review: refusal of psychotropic medication in acute inpatient psychiatric care. J Psychiatr Ment Health Nurs. 2011;18(7):637-647.
23. Appelbaum P, Gutheil T. “Rotting with their rights on”: constitutional theory and clinical reality in drug refusal by psychiatric patients. Bull Am Acad Psychiatry Law. 1979;7(3):306-315.
24. Adelugba OO, Mela M, Haq IU. Psychotropic medication refusal: reasons and patients’ perception at a secure forensic psychiatric treatment centre. J Forensic Sci Med. 2016;2(1):12-17.
25. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228.
Ms. T, age 48, is brought to the psychiatric emergency department after the police find her walking along the highway at 3:00
Once involuntarily committed, does Ms. T have the right to refuse treatment?
Every psychiatrist has faced the predicament of a patient who refuses treatment. This creates an ethical dilemma between respecting the patient’s autonomy vs forcing treatment to ameliorate symptoms and reduce suffering. This article addresses case law related to the models for administering psychiatric medications over objection. We also discuss case law regarding court-appointed guardianship, and treating medical issues without consent. While this article provides valuable information on these scenarios, it is crucial to remember that the legal processes required to administer medications over patient objection are state-specific. In order to ensure the best practice and patient care, you must research the legal procedures specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
History of involuntary treatment
Prior to the 1960s, Ms. T would likely have been unable to refuse treatment. All patients were considered involuntary, and the course of treatment was decided solely by the psychiatric institution. Well into the 20th century, patients with psychiatric illness remained feared and stigmatized, which led to potent and potentially harsh methods of treatment. Some patients experienced extreme isolation, whipping, bloodletting, experimental use of chemicals, and starvation (Table 11-3).
With the advent of psychotropic medications and a focus on civil liberties, the psychiatric mindset began to change from hospital-based treatment to a community-based approach. The value of psychotherapy was recognized, and by the 1960s, the establishment of community mental health centers was gaining momentum.
In the context of these changes, the civil rights movement pressed for stronger legislation regarding autonomy and the quality of treatment available to patients with psychiatric illness. In the 1960s and 1970s, Rouse v Cameron4 and Wyatt v Stickney5 dealt with a patient’s right to receive treatment while involuntarily committed. However, it was not until the 1980s that the courts addressed the issue of a patient’s right to refuse treatment.
The judicial system: A primer
When reviewing case law and its applicability to your patients, it is important to understand the various court systems. The judicial system is divided into state and federal courts, which are subdivided into trial, appellate, and supreme courts. When decisions at either the state or federal level require an ultimate decision maker, the US Supreme Court can choose to hear the case, or grant certiorari, and make a ruling, which is then binding law.6 Decisions made by any court are based on various degrees of stringency, called standards of proof (Table 27).
Continue to: For Ms. T's case...
For Ms. T’s case, civil commitment and involuntary medication hearings are held in probate court, which is a civil (not criminal) court. In addition to overseeing civil commitment and involuntary medications, probate courts adjudicate will and estate contests, conservatorship, and guardianship. Conservatorship hearings deal with financial issues, and guardianship cases encompass personal and health-related needs. Regardless of the court, an individual is guaranteed due process under the 5th Amendment (federal) and 14th Amendment (state).
Individuals are presumed competent to make their own decisions, but a court may call this into question. Competencies are specific to a variety of areas, such as criminal proceedings, medical decision making, writing a will (testimonial capacity), etc. Because each field applies its own standard of competence, an individual may be competent in one area but incompetent in another. Competence in medical decision making varies by state but generally consists of being able to communicate a choice, understand relevant information, appreciate one’s illness and its likely consequences, and rationally manipulate information.8
Box
Administering medications despite a patient’s objection differs from situations in which medications are provided during a psychiatric emergency. In an emergency, courts do not have time to weigh in. Instead, emergency medications (most often given as IM injections) are administered based on the physician’s clinical judgment. The criteria for psychiatric emergencies are delineated at the state level, but typically are defined as when a person with a mental illness creates an imminent risk of harm to self or others. Alternative approaches to resolving the emergency may include verbal de-escalation, quiet time in a room devoid of stimuli, locked seclusion, or physical restraints. These measures are often exhausted before emergency medications are administered.
Source: Reference 9
It is important to note that the legal process required before administering involuntary medications is distinct from situations in which medication needs to be provided during a psychiatric emergency. The Box9 outlines the difference between these 2 scenarios.
4 Legal models
There are several legal models used to determine when a patient can be administered psychiatric medications over objection. Table 310,11 summarizes these models.
Rights-driven (Rogers) model. If Ms. T was involuntarily hospitalized in Massachusetts or another state that adopted the rights-driven model, she would retain the right to refuse treatment. These states require an external judicial review, and court approval is necessary before imposing any therapy. This model was established in Rogers v Commissioner,12 where 7 patients at the Boston State Hospital filed a lawsuit regarding their right to refuse medications. The Massachusetts Supreme Judicial Court ruled that, despite being involuntarily committed, a patient is considered competent to refuse treatment until found specifically incompetent to do so by the court. If a patient is found incompetent, the judge, using a full adversarial hearing, decides what the incompetent patient would have wanted if he/she were competent. The judge reaches a conclusion based on the substituted judgment model (Table 410). In Rogers v Commissioner,12 the court ruled that the right to decision making is not lost after becoming a patient at a mental health facility. The right is lost only if the patient is found incompetent by the judge. Thus, every individual has the right to “manage his own person” and “take care of himself.”
Continue to: An update to the rights-driven (Rogers) model
An update to the rights-driven (Rogers) model. Other states, such as Ohio, have adopted the Rogers model and addressed issues that arose subsequent to the aforementioned case. In Steele v Hamilton County,13 Jeffrey Steele was admitted and later civilly committed to the hospital. After 2 months, an involuntary medication hearing was completed in which 3 psychiatrists concluded that, although Mr. Steele was not a danger to himself or others while in the hospital, he would ultimately benefit from medications.
The probate court acknowledged that Mr. Steele lacked capacity and required hospitalization. However, because he was not imminently dangerous, medication should not be used involuntarily. After a series of appeals, the Ohio Supreme Court ruled that a court may authorize the administration of an antipsychotic medication against a patient’s wishes without a finding of dangerousness when clear and convincing evidence exists that:
- the patient lacks the capacity to give or withhold informed consent regarding treatment
- the proposed medication is in the patient’s best interest
- no less intrusive treatment will be as effective in treating the mental illness.
This ruling set a precedent that dangerousness is not a requirement for involuntary medications.
Treatment-driven (Rennie) model. As in the rights-driven model, in the treatment-driven model, Ms. T would retain the constitutional right to refuse treatment. However, the models differ in the amount of procedural due process required. The treatment-driven model derives from Rennie v Klein,14 in which John Rennie, a patient at Ancora State Psychiatric Hospital in New Jersey, filed a suit regarding the right of involuntarily committed patients to refuse antipsychotic medications. The Third Circuit Court of Appeals ruled that, if professional judgment deems a patient to be a danger to himself or others, then antipsychotics may be administered over individual objection. This professional judgment is typically based on the opinion of the treating physician, along with a second physician or panel.
Utah model. This model is based on A.E. and R.R. v Mitchell,15 in which the Utah District Court ruled that a civilly committed patient has no right to refuse treatment. This Utah model was created after state legislature determined that, in order to civilly commit a patient, hospitalization must be the least restrictive alternative and the patient is incompetent to consent to treatment. Unlike the 2 previous models, competency to refuse medications is not separated from a previous finding of civil commitment, but rather, they occur simultaneously.
Continue to: Rights in unique situations
Rights in unique situations
Correctional settings. If Ms. T was an inmate, would her right to refuse psychiatric medication change? This was addressed in the case of Washington v Harper.16 Walter Harper, serving time for a robbery conviction, filed a claim that his civil rights were being violated when he received involuntary medications based on the decision of a 3-person panel consisting of a psychiatrist, psychologist, and prison official. The US Supreme Court ruled that this process provided sufficient due process to mandate providing psychotropic medications against a patient’s will. This reduction in required procedures is related to the unique nature of the correctional environment and an increased need to maintain safety. This need was felt to outweigh an individual’s right to refuse medication.
Incompetent to stand trial. In Sell v U.S.,17 Charles Sell, a dentist, was charged with fraud and attempted murder. He underwent a competency evaluation and was found incompetent to stand trial because of delusional thinking. Mr. Sell was hospitalized for restorability but refused medications. The hospital held an administrative hearing to proceed with involuntary antipsychotic medications; however, Mr. Sell filed an order with the court to prevent this. Eventually, the US Supreme Court ruled that non-dangerous, incompetent defendants may be involuntarily medicated even if they do not pose a risk to self or others on the basis that it furthers the state’s interest in bringing to trial those charged with serious crimes. However, the following conditions must be met before involuntary medication can be administered:
- an important government issue must be at stake (determined case-by-case)
- a substantial probability must exist that the medication will enable the defendant to become competent without significant adverse effects
- the medication must be medically appropriate and necessary to restore competency, with no less restrictive alternative available.
This case suggests that, before one attempts to forcibly medicate a defendant for the purpose of competency restoration, one should exhaust the same judicial remedies one uses for civil patients first.
Court-appointed guardianship
In the case of Ms. T, what if her father requested to become her guardian? This question was explored in the matter of Guardianship of Richard Roe III.18 Mr. Roe was admitted to the Northampton State Hospital in Massachusetts, where he refused antipsychotic medications. Prior to his release, his father asked to be his guardian. The probate court obliged the request. However, Mr. Roe’s lawyer and guardian ad litem (a neutral temporary guardian often appointed when legal issues are pending) challenged the ruling, arguing the probate court cannot empower the guardian to consent to involuntary medication administration. On appeal, the court ruled:
- the guardianship was justified
- the standard of proof for establishment of a guardianship is preponderance of the evidence (Table 27)
- the guardian must seek from a court a “substituted judgment” to authorize forcible administration of antipsychotic medication.
The decision to establish the court as the final decision maker is based on the view that a patient’s relatives may be biased. Courts should take an objective approach that considers
- patient preference stated during periods of competency
- medication adverse effects
- consequences if treatment is refused
- prognosis with treatment
- religious beliefs
- impact on the patient’s family.
Continue to: This case set the stage for...
This case set the stage for later decisions that placed antipsychotic medications in the same category as electroconvulsive therapy and psychosurgery. This could mean a guardian would need specialized authorization to request antipsychotic treatment but could consent to an appendectomy without legal issue.
Fortunately, now most jurisdictions have remedied this cumbersome solution by requiring a higher standard of proof, clear and convincing evidence (Table 27), to establish guardianship but allowing the guardian more latitude to make decisions for their wards (such as those involving hospital admission or medications) without further court involvement.
Involuntary medical treatment
In order for a patient to consent for medical treatment, he/she must have the capacity to do so (Table 59). How do the courts handle the patient’s right to refuse medical treatment? This was addressed in the case of Georgetown College v Jones.19 Mrs. Jones, a 25-year-old Jehovah’s Witness and mother of a 7-month-old baby, suffered a ruptured ulcer and lost a life-threatening amount of blood. Due to her religious beliefs, Mrs. Jones refused a blood transfusion. The hospital quickly appealed to the court, who ruled the woman was help-seeking by going to the hospital, did not want to die, was in distress, and lacked capacity to make medical decisions. Acting in a parens patriae manner (when the government steps in to make decisions for its citizens who cannot), the court ordered the hospital to administer blood transfusions.
Proxy decision maker. When the situation is less emergent, a proxy decision maker can be appointed by the court. This was addressed in the case of Superintendent of Belchertown v Saikewicz.20 Mr. Saikewicz, a 67-year-old man with intellectual disability, was diagnosed with cancer and given weeks to months to live without treatment. However, treatment was only 50% effective and could potentially cause severe adverse effects. A guardian ad litem was appointed and recommended nontreatment, which the court upheld. The court ruled that the right to accept or reject medical treatment applies to both incompetent and competent persons. With incompetent persons, a “substituted judgment” analysis is used over the “best interest of the patient” doctrine.20 This falls in line with the Guardianship of Richard Roe III ruling,18 in which the court’s substituted judgment standard is enacted in an effort to respect patient autonomy.
Right to die. When does a patient have the right to die and what is the standard of proof? The US Supreme Court case Cruzan v Director21 addressed this. Nancy Cruzan was involved in a car crash, which left her in a persistent vegetative state with no significant cognitive function. She remained this way for 6 years before her parents sought to terminate life support. The hospital refused. The Missouri Supreme Court ruled that a standard of clear and convincing evidence (Table 27) is required to withdraw treatment, and in a 5-to-4 decision, the US Supreme Court upheld Missouri’s decision. This set the national standard for withdrawal of life-sustaining treatment. The moderate standard of proof is based on the court’s ruling that the decision to terminate life is a particularly important one.
Continue to: CASE
CASE CONTINUED
After having been civilly committed to your inpatient psychiatric facility, Ms. T’s paranoia and disorganized behavior persist. She continues to refuse medications.
There are 3 options: respect her decision, negotiate with her, or attempt to force medications through due process.11 In negotiating a compromise, it is best to understand the barriers to treatment. A patient may refuse medications due to poor insight into his/her illness, medication adverse effects, a preference for an alternative treatment, delusional concerns over contamination and/or poisoning, interpersonal conflicts with the treatment staff, a preference for symptoms (eg, mania) over wellness, medication ineffectiveness, length of treatment course, or stigma.22,23 However, a patient’s unwillingness to compromise creates the dilemma of autonomy vs treatment.
For Ms. T, the treatment team felt initiating involuntary medication was the best option for her quality of life and safety. Because she resides in Ohio, a Rogers-like model was applied. The probate court was petitioned and found her incompetent to make medical decisions. The court accepted the physician’s recommendation of treatment with antipsychotic medications. If this scenario took place in New Jersey, a Rennie model would apply, requiring due process through the second opinion of another physician. Lastly, if Ms. T lived in Utah, she would have been unable to refuse medications once civilly committed.
Pros and cons of each model
Over the years, various concerns about each of these models have been raised. Given the slow-moving wheels of justice, one concern was that perhaps patients would be left “rotting with their rights on,” or lingering in a psychotic state out of respect for their civil liberties.19 While court hearings do not always happen quickly, more often than not, a judge will agree with the psychiatrist seeking treatment because the judge likely has little experience with mental illness and will defer to the physician’s expertise. This means the Rogers model may be more likely to produce the desired outcome, just more slowly. With respect to the Rennie model, although it is often more expeditious, the second opinion of an independent psychiatrist may contradict that of the original physician because the consultant will rely on his/her own expertise. Finally, some were concerned that psychiatrists would view the Utah model as carte blanche to start whatever medications they wanted with no respect for patient preference. Based on our clinical experience, none of these concerns have come to fruition over time, and patients safely receive medications over objection in hospitals every day.
Consider why the patient refuses medication
Regardless of which involuntary medication model is employed, it is important to consider the underlying cause for medication refusal, because it may affect future compliance. If the refusal is the result of a religious belief, history of adverse effects, or other rational motive, then it may be reasonable to respect the patient’s autonomy.24 However, if the refusal is secondary to symptoms of mental illness, it is appropriate to move forward with an involuntary medication hearing and treat the underlying condition.
Continue to: In the case of Ms. T...
In the case of Ms. T, she appeared to be refusing medications because of her psychotic symptoms, which could be effectively treated with antipsychotic medications. Therefore, Ms. T’s current lack of capacity is hopefully a transient phenomenon that can be ameliorated by initiating medication. Typically, antipsychotic medications begin to reduce psychotic symptoms within the first week, with further improvement over time.25 The value of the inpatient psychiatric setting is that it allows for daily monitoring of a patient’s response to treatment. As capacity is regained, patient autonomy over medical decisions is reinstated.
Bottom Line
The legal processes required to administer medications over a patient’s objection are state-specific, and multiple models are used. In general, a patient’s right to refuse treatment can be overruled by obtaining adjudication through the courts (Rogers model) or the opinion of a second physician (Rennie model). In order to ensure the best practice and patient care, research the legal procedure specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
Related Resources
- Miller D. Is forced treatment in our outpatients’ best interests? Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/80277/forced-treatment-our-outpatients-best-interests.
- Miller D, Hanson A. Committed: The battle over involuntary psychiatric care. Baltimore, MD: Johns Hopkins University Press; 2016.
Ms. T, age 48, is brought to the psychiatric emergency department after the police find her walking along the highway at 3:00
Once involuntarily committed, does Ms. T have the right to refuse treatment?
Every psychiatrist has faced the predicament of a patient who refuses treatment. This creates an ethical dilemma between respecting the patient’s autonomy vs forcing treatment to ameliorate symptoms and reduce suffering. This article addresses case law related to the models for administering psychiatric medications over objection. We also discuss case law regarding court-appointed guardianship, and treating medical issues without consent. While this article provides valuable information on these scenarios, it is crucial to remember that the legal processes required to administer medications over patient objection are state-specific. In order to ensure the best practice and patient care, you must research the legal procedures specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
History of involuntary treatment
Prior to the 1960s, Ms. T would likely have been unable to refuse treatment. All patients were considered involuntary, and the course of treatment was decided solely by the psychiatric institution. Well into the 20th century, patients with psychiatric illness remained feared and stigmatized, which led to potent and potentially harsh methods of treatment. Some patients experienced extreme isolation, whipping, bloodletting, experimental use of chemicals, and starvation (Table 11-3).
With the advent of psychotropic medications and a focus on civil liberties, the psychiatric mindset began to change from hospital-based treatment to a community-based approach. The value of psychotherapy was recognized, and by the 1960s, the establishment of community mental health centers was gaining momentum.
In the context of these changes, the civil rights movement pressed for stronger legislation regarding autonomy and the quality of treatment available to patients with psychiatric illness. In the 1960s and 1970s, Rouse v Cameron4 and Wyatt v Stickney5 dealt with a patient’s right to receive treatment while involuntarily committed. However, it was not until the 1980s that the courts addressed the issue of a patient’s right to refuse treatment.
The judicial system: A primer
When reviewing case law and its applicability to your patients, it is important to understand the various court systems. The judicial system is divided into state and federal courts, which are subdivided into trial, appellate, and supreme courts. When decisions at either the state or federal level require an ultimate decision maker, the US Supreme Court can choose to hear the case, or grant certiorari, and make a ruling, which is then binding law.6 Decisions made by any court are based on various degrees of stringency, called standards of proof (Table 27).
Continue to: For Ms. T's case...
For Ms. T’s case, civil commitment and involuntary medication hearings are held in probate court, which is a civil (not criminal) court. In addition to overseeing civil commitment and involuntary medications, probate courts adjudicate will and estate contests, conservatorship, and guardianship. Conservatorship hearings deal with financial issues, and guardianship cases encompass personal and health-related needs. Regardless of the court, an individual is guaranteed due process under the 5th Amendment (federal) and 14th Amendment (state).
Individuals are presumed competent to make their own decisions, but a court may call this into question. Competencies are specific to a variety of areas, such as criminal proceedings, medical decision making, writing a will (testimonial capacity), etc. Because each field applies its own standard of competence, an individual may be competent in one area but incompetent in another. Competence in medical decision making varies by state but generally consists of being able to communicate a choice, understand relevant information, appreciate one’s illness and its likely consequences, and rationally manipulate information.8
Box
Administering medications despite a patient’s objection differs from situations in which medications are provided during a psychiatric emergency. In an emergency, courts do not have time to weigh in. Instead, emergency medications (most often given as IM injections) are administered based on the physician’s clinical judgment. The criteria for psychiatric emergencies are delineated at the state level, but typically are defined as when a person with a mental illness creates an imminent risk of harm to self or others. Alternative approaches to resolving the emergency may include verbal de-escalation, quiet time in a room devoid of stimuli, locked seclusion, or physical restraints. These measures are often exhausted before emergency medications are administered.
Source: Reference 9
It is important to note that the legal process required before administering involuntary medications is distinct from situations in which medication needs to be provided during a psychiatric emergency. The Box9 outlines the difference between these 2 scenarios.
4 Legal models
There are several legal models used to determine when a patient can be administered psychiatric medications over objection. Table 310,11 summarizes these models.
Rights-driven (Rogers) model. If Ms. T was involuntarily hospitalized in Massachusetts or another state that adopted the rights-driven model, she would retain the right to refuse treatment. These states require an external judicial review, and court approval is necessary before imposing any therapy. This model was established in Rogers v Commissioner,12 where 7 patients at the Boston State Hospital filed a lawsuit regarding their right to refuse medications. The Massachusetts Supreme Judicial Court ruled that, despite being involuntarily committed, a patient is considered competent to refuse treatment until found specifically incompetent to do so by the court. If a patient is found incompetent, the judge, using a full adversarial hearing, decides what the incompetent patient would have wanted if he/she were competent. The judge reaches a conclusion based on the substituted judgment model (Table 410). In Rogers v Commissioner,12 the court ruled that the right to decision making is not lost after becoming a patient at a mental health facility. The right is lost only if the patient is found incompetent by the judge. Thus, every individual has the right to “manage his own person” and “take care of himself.”
Continue to: An update to the rights-driven (Rogers) model
An update to the rights-driven (Rogers) model. Other states, such as Ohio, have adopted the Rogers model and addressed issues that arose subsequent to the aforementioned case. In Steele v Hamilton County,13 Jeffrey Steele was admitted and later civilly committed to the hospital. After 2 months, an involuntary medication hearing was completed in which 3 psychiatrists concluded that, although Mr. Steele was not a danger to himself or others while in the hospital, he would ultimately benefit from medications.
The probate court acknowledged that Mr. Steele lacked capacity and required hospitalization. However, because he was not imminently dangerous, medication should not be used involuntarily. After a series of appeals, the Ohio Supreme Court ruled that a court may authorize the administration of an antipsychotic medication against a patient’s wishes without a finding of dangerousness when clear and convincing evidence exists that:
- the patient lacks the capacity to give or withhold informed consent regarding treatment
- the proposed medication is in the patient’s best interest
- no less intrusive treatment will be as effective in treating the mental illness.
This ruling set a precedent that dangerousness is not a requirement for involuntary medications.
Treatment-driven (Rennie) model. As in the rights-driven model, in the treatment-driven model, Ms. T would retain the constitutional right to refuse treatment. However, the models differ in the amount of procedural due process required. The treatment-driven model derives from Rennie v Klein,14 in which John Rennie, a patient at Ancora State Psychiatric Hospital in New Jersey, filed a suit regarding the right of involuntarily committed patients to refuse antipsychotic medications. The Third Circuit Court of Appeals ruled that, if professional judgment deems a patient to be a danger to himself or others, then antipsychotics may be administered over individual objection. This professional judgment is typically based on the opinion of the treating physician, along with a second physician or panel.
Utah model. This model is based on A.E. and R.R. v Mitchell,15 in which the Utah District Court ruled that a civilly committed patient has no right to refuse treatment. This Utah model was created after state legislature determined that, in order to civilly commit a patient, hospitalization must be the least restrictive alternative and the patient is incompetent to consent to treatment. Unlike the 2 previous models, competency to refuse medications is not separated from a previous finding of civil commitment, but rather, they occur simultaneously.
Continue to: Rights in unique situations
Rights in unique situations
Correctional settings. If Ms. T was an inmate, would her right to refuse psychiatric medication change? This was addressed in the case of Washington v Harper.16 Walter Harper, serving time for a robbery conviction, filed a claim that his civil rights were being violated when he received involuntary medications based on the decision of a 3-person panel consisting of a psychiatrist, psychologist, and prison official. The US Supreme Court ruled that this process provided sufficient due process to mandate providing psychotropic medications against a patient’s will. This reduction in required procedures is related to the unique nature of the correctional environment and an increased need to maintain safety. This need was felt to outweigh an individual’s right to refuse medication.
Incompetent to stand trial. In Sell v U.S.,17 Charles Sell, a dentist, was charged with fraud and attempted murder. He underwent a competency evaluation and was found incompetent to stand trial because of delusional thinking. Mr. Sell was hospitalized for restorability but refused medications. The hospital held an administrative hearing to proceed with involuntary antipsychotic medications; however, Mr. Sell filed an order with the court to prevent this. Eventually, the US Supreme Court ruled that non-dangerous, incompetent defendants may be involuntarily medicated even if they do not pose a risk to self or others on the basis that it furthers the state’s interest in bringing to trial those charged with serious crimes. However, the following conditions must be met before involuntary medication can be administered:
- an important government issue must be at stake (determined case-by-case)
- a substantial probability must exist that the medication will enable the defendant to become competent without significant adverse effects
- the medication must be medically appropriate and necessary to restore competency, with no less restrictive alternative available.
This case suggests that, before one attempts to forcibly medicate a defendant for the purpose of competency restoration, one should exhaust the same judicial remedies one uses for civil patients first.
Court-appointed guardianship
In the case of Ms. T, what if her father requested to become her guardian? This question was explored in the matter of Guardianship of Richard Roe III.18 Mr. Roe was admitted to the Northampton State Hospital in Massachusetts, where he refused antipsychotic medications. Prior to his release, his father asked to be his guardian. The probate court obliged the request. However, Mr. Roe’s lawyer and guardian ad litem (a neutral temporary guardian often appointed when legal issues are pending) challenged the ruling, arguing the probate court cannot empower the guardian to consent to involuntary medication administration. On appeal, the court ruled:
- the guardianship was justified
- the standard of proof for establishment of a guardianship is preponderance of the evidence (Table 27)
- the guardian must seek from a court a “substituted judgment” to authorize forcible administration of antipsychotic medication.
The decision to establish the court as the final decision maker is based on the view that a patient’s relatives may be biased. Courts should take an objective approach that considers
- patient preference stated during periods of competency
- medication adverse effects
- consequences if treatment is refused
- prognosis with treatment
- religious beliefs
- impact on the patient’s family.
Continue to: This case set the stage for...
This case set the stage for later decisions that placed antipsychotic medications in the same category as electroconvulsive therapy and psychosurgery. This could mean a guardian would need specialized authorization to request antipsychotic treatment but could consent to an appendectomy without legal issue.
Fortunately, now most jurisdictions have remedied this cumbersome solution by requiring a higher standard of proof, clear and convincing evidence (Table 27), to establish guardianship but allowing the guardian more latitude to make decisions for their wards (such as those involving hospital admission or medications) without further court involvement.
Involuntary medical treatment
In order for a patient to consent for medical treatment, he/she must have the capacity to do so (Table 59). How do the courts handle the patient’s right to refuse medical treatment? This was addressed in the case of Georgetown College v Jones.19 Mrs. Jones, a 25-year-old Jehovah’s Witness and mother of a 7-month-old baby, suffered a ruptured ulcer and lost a life-threatening amount of blood. Due to her religious beliefs, Mrs. Jones refused a blood transfusion. The hospital quickly appealed to the court, who ruled the woman was help-seeking by going to the hospital, did not want to die, was in distress, and lacked capacity to make medical decisions. Acting in a parens patriae manner (when the government steps in to make decisions for its citizens who cannot), the court ordered the hospital to administer blood transfusions.
Proxy decision maker. When the situation is less emergent, a proxy decision maker can be appointed by the court. This was addressed in the case of Superintendent of Belchertown v Saikewicz.20 Mr. Saikewicz, a 67-year-old man with intellectual disability, was diagnosed with cancer and given weeks to months to live without treatment. However, treatment was only 50% effective and could potentially cause severe adverse effects. A guardian ad litem was appointed and recommended nontreatment, which the court upheld. The court ruled that the right to accept or reject medical treatment applies to both incompetent and competent persons. With incompetent persons, a “substituted judgment” analysis is used over the “best interest of the patient” doctrine.20 This falls in line with the Guardianship of Richard Roe III ruling,18 in which the court’s substituted judgment standard is enacted in an effort to respect patient autonomy.
Right to die. When does a patient have the right to die and what is the standard of proof? The US Supreme Court case Cruzan v Director21 addressed this. Nancy Cruzan was involved in a car crash, which left her in a persistent vegetative state with no significant cognitive function. She remained this way for 6 years before her parents sought to terminate life support. The hospital refused. The Missouri Supreme Court ruled that a standard of clear and convincing evidence (Table 27) is required to withdraw treatment, and in a 5-to-4 decision, the US Supreme Court upheld Missouri’s decision. This set the national standard for withdrawal of life-sustaining treatment. The moderate standard of proof is based on the court’s ruling that the decision to terminate life is a particularly important one.
Continue to: CASE
CASE CONTINUED
After having been civilly committed to your inpatient psychiatric facility, Ms. T’s paranoia and disorganized behavior persist. She continues to refuse medications.
There are 3 options: respect her decision, negotiate with her, or attempt to force medications through due process.11 In negotiating a compromise, it is best to understand the barriers to treatment. A patient may refuse medications due to poor insight into his/her illness, medication adverse effects, a preference for an alternative treatment, delusional concerns over contamination and/or poisoning, interpersonal conflicts with the treatment staff, a preference for symptoms (eg, mania) over wellness, medication ineffectiveness, length of treatment course, or stigma.22,23 However, a patient’s unwillingness to compromise creates the dilemma of autonomy vs treatment.
For Ms. T, the treatment team felt initiating involuntary medication was the best option for her quality of life and safety. Because she resides in Ohio, a Rogers-like model was applied. The probate court was petitioned and found her incompetent to make medical decisions. The court accepted the physician’s recommendation of treatment with antipsychotic medications. If this scenario took place in New Jersey, a Rennie model would apply, requiring due process through the second opinion of another physician. Lastly, if Ms. T lived in Utah, she would have been unable to refuse medications once civilly committed.
Pros and cons of each model
Over the years, various concerns about each of these models have been raised. Given the slow-moving wheels of justice, one concern was that perhaps patients would be left “rotting with their rights on,” or lingering in a psychotic state out of respect for their civil liberties.19 While court hearings do not always happen quickly, more often than not, a judge will agree with the psychiatrist seeking treatment because the judge likely has little experience with mental illness and will defer to the physician’s expertise. This means the Rogers model may be more likely to produce the desired outcome, just more slowly. With respect to the Rennie model, although it is often more expeditious, the second opinion of an independent psychiatrist may contradict that of the original physician because the consultant will rely on his/her own expertise. Finally, some were concerned that psychiatrists would view the Utah model as carte blanche to start whatever medications they wanted with no respect for patient preference. Based on our clinical experience, none of these concerns have come to fruition over time, and patients safely receive medications over objection in hospitals every day.
Consider why the patient refuses medication
Regardless of which involuntary medication model is employed, it is important to consider the underlying cause for medication refusal, because it may affect future compliance. If the refusal is the result of a religious belief, history of adverse effects, or other rational motive, then it may be reasonable to respect the patient’s autonomy.24 However, if the refusal is secondary to symptoms of mental illness, it is appropriate to move forward with an involuntary medication hearing and treat the underlying condition.
Continue to: In the case of Ms. T...
In the case of Ms. T, she appeared to be refusing medications because of her psychotic symptoms, which could be effectively treated with antipsychotic medications. Therefore, Ms. T’s current lack of capacity is hopefully a transient phenomenon that can be ameliorated by initiating medication. Typically, antipsychotic medications begin to reduce psychotic symptoms within the first week, with further improvement over time.25 The value of the inpatient psychiatric setting is that it allows for daily monitoring of a patient’s response to treatment. As capacity is regained, patient autonomy over medical decisions is reinstated.
Bottom Line
The legal processes required to administer medications over a patient’s objection are state-specific, and multiple models are used. In general, a patient’s right to refuse treatment can be overruled by obtaining adjudication through the courts (Rogers model) or the opinion of a second physician (Rennie model). In order to ensure the best practice and patient care, research the legal procedure specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
Related Resources
- Miller D. Is forced treatment in our outpatients’ best interests? Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/80277/forced-treatment-our-outpatients-best-interests.
- Miller D, Hanson A. Committed: The battle over involuntary psychiatric care. Baltimore, MD: Johns Hopkins University Press; 2016.
1. Laffey P. Psychiatric therapy in Georgian Britain. Psychol Med. 2003;33(7):1285-1297.
2. Porter R. Madness: a brief history. New York, NY: Oxford Press; 2002.
3. Stetka B, Watson J. Odd and outlandish psychiatric treatments throughout history. Medscape Psychiatry. https://www.medscape.com/features/slideshow/odd-psychiatric-treatments. Published April 13, 2016. Accessed February 26, 2020.
4. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
5. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
6. Administrative Office of the US Courts. Comparing federal and state Courts. United States Courts. https://www.uscourts.gov/about-federal-courts/court-role-and-structure/comparing-federal-state-courts. Accessed February 26, 2020.
7. Drogin E, Williams C. Introduction to the Legal System. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:80-83.
8. Appelbaum P, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
9. Kambam P. Informed consent and competence. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:115-121.
10. Wall B, Anfang S. Legal regulation of psychiatric treatment. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:306-333.
11. Pinals D, Nesbit A, Hoge S. Treatment refusal in psychiatric practice. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:155-163.
12. Rogers v Commissioner, 390 489 (Mass 1983).
13. Steele v Hamilton County, 90 Ohio St3d 176 (Ohio 2000).
14. Rennie v Klein, 462 F Supp 1131 (D NJ 1978).
15. AE and RR v Mitchell, 724 F.2d 864 (10th Cir 1983).
16. Washington v Harper, 494 US 210 (1990).
17. Sell v US, 539 US 166 (2003).
18. Guardianship of Richard Roe III, 383 415, 435 (Mass 1981).
19. Georgetown College v Jones, 331 F2d 1010 (DC Cir 1964).
20. Superintendent of Belchertown v Saikewicz, 370 NE 2d 417 (1977).
21. Cruzan v Director, 497 US 261 (1990).
22. Owiti J, Bowers L. A literature review: refusal of psychotropic medication in acute inpatient psychiatric care. J Psychiatr Ment Health Nurs. 2011;18(7):637-647.
23. Appelbaum P, Gutheil T. “Rotting with their rights on”: constitutional theory and clinical reality in drug refusal by psychiatric patients. Bull Am Acad Psychiatry Law. 1979;7(3):306-315.
24. Adelugba OO, Mela M, Haq IU. Psychotropic medication refusal: reasons and patients’ perception at a secure forensic psychiatric treatment centre. J Forensic Sci Med. 2016;2(1):12-17.
25. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228.
1. Laffey P. Psychiatric therapy in Georgian Britain. Psychol Med. 2003;33(7):1285-1297.
2. Porter R. Madness: a brief history. New York, NY: Oxford Press; 2002.
3. Stetka B, Watson J. Odd and outlandish psychiatric treatments throughout history. Medscape Psychiatry. https://www.medscape.com/features/slideshow/odd-psychiatric-treatments. Published April 13, 2016. Accessed February 26, 2020.
4. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
5. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
6. Administrative Office of the US Courts. Comparing federal and state Courts. United States Courts. https://www.uscourts.gov/about-federal-courts/court-role-and-structure/comparing-federal-state-courts. Accessed February 26, 2020.
7. Drogin E, Williams C. Introduction to the Legal System. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:80-83.
8. Appelbaum P, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
9. Kambam P. Informed consent and competence. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:115-121.
10. Wall B, Anfang S. Legal regulation of psychiatric treatment. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:306-333.
11. Pinals D, Nesbit A, Hoge S. Treatment refusal in psychiatric practice. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:155-163.
12. Rogers v Commissioner, 390 489 (Mass 1983).
13. Steele v Hamilton County, 90 Ohio St3d 176 (Ohio 2000).
14. Rennie v Klein, 462 F Supp 1131 (D NJ 1978).
15. AE and RR v Mitchell, 724 F.2d 864 (10th Cir 1983).
16. Washington v Harper, 494 US 210 (1990).
17. Sell v US, 539 US 166 (2003).
18. Guardianship of Richard Roe III, 383 415, 435 (Mass 1981).
19. Georgetown College v Jones, 331 F2d 1010 (DC Cir 1964).
20. Superintendent of Belchertown v Saikewicz, 370 NE 2d 417 (1977).
21. Cruzan v Director, 497 US 261 (1990).
22. Owiti J, Bowers L. A literature review: refusal of psychotropic medication in acute inpatient psychiatric care. J Psychiatr Ment Health Nurs. 2011;18(7):637-647.
23. Appelbaum P, Gutheil T. “Rotting with their rights on”: constitutional theory and clinical reality in drug refusal by psychiatric patients. Bull Am Acad Psychiatry Law. 1979;7(3):306-315.
24. Adelugba OO, Mela M, Haq IU. Psychotropic medication refusal: reasons and patients’ perception at a secure forensic psychiatric treatment centre. J Forensic Sci Med. 2016;2(1):12-17.
25. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228.
New lipid-lowering drug class slashes LDL in HoFH patients
Evinacumab, the first agent from a new class of lipid-lowering drugs, showed a “remarkable” and unprecedented level of LDL-cholesterol lowering in a pivotal trial with 65 patients with homozygous familial hypercholesterolemia.
Monthly intravenous infusions of evinacumab cut LDL cholesterol levels by an average of 135 mg/dL from baseline, a 47% mean reduction, after 24 weeks of treatment in 43 homozygous familial hypercholesterolemia (HoFH) patients, Frederick Raal, MBChB, said on March 30 in a video presentation of his research at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
Evinacumab is a human monoclonal antibody inhibitor of angiopoietin-like 3, a liver protein that boosts levels of LDL cholesterol and triglycerides (TG).
Another notable effect of the novel agent was that it was equally effective in the roughly one-third of patients with a minimal residual level of LDL receptor activity, patients know as having “null/null” mutations. “For the first time, we see HoFH patients getting to [lipid] targets that we never thought would be possible,” said Dr. Raal, professor and head of endocrinology and metabolism at the University of Witwatersrand in Johannesburg, South Africa. “This works in patients without residual LDL receptor function.” The drug was also generally very well tolerated, he said, causing no treatment-related serious adverse events during the brief treatment period of 24 weeks.
“One of the major, remarkable findings in this study was the effect on null/null patients,” which contrasts with the effects of other, more established drugs for treating dyslipidemia like statins and PCSK9 inhibitors, which work by increasing the number of LDL receptors on cells. The demonstrated efficacy and safety of evinacumab in null/null patients “is a definite advance,” commented Anne C. Goldberg, MD, a lipidologist and professor of medicine at Washington University in St. Louis.
The placebo-controlled trial randomized patients at 30 sites in 11 countries who were at least 12 years old and had documented mutations in both of their LDL receptor genes and a serum level of LDL cholesterol that was at least 500 mg/dL on no treatment. Patients averaged about 40 years of age; about 30% had null/null mutations, more than 90% were on statin treatment, and about three-quarters were receiving regular treatment with a PCSK9 inhibitor. At baseline, LDL cholesterol levels averaged about 250 mg/dL.
The study’s primary endpoint was the between-group percentage change in LDL cholesterol level after 24 weeks, which fell by 47% from baseline with evinacumab treatment and increased by an average of 2% among 22 patients who received placebo injections; so evinacumab cut this measure by 49%, compared with placebo after 24 weeks, a statistically significant difference. A cut of baseline LDL cholesterol by at least 50% occurred in 56% of the evinacumab-treated patients and in 5% of controls.
In addition to its LDL reduction, another notable effect of evinacumab was that it trimmed baseline triglyceride levels by half, consistent with prior reports of the drug’s effect on this measure, although average triglyceride levels in the enrolled patients fell within the normal range prior to treatment.
Evinacumab “will probably be very effective in treating patients with hypertriglyceridemia; those studies are ongoing,” noted Dr. Raal. But, he added, “this drug will probably be reserved for severe” dyslipidemia cases, not for “the garden variety of moderate hypertriglyceridemia or hypercholesterolemia.”
Evinacumab “may be a fairly broad-spectrum lipid-lowering drug, but it should be reserved for severe cases,” agreed Dirk Blom, MBChB, head of lipidology at the University of Capetown, South Africa. “This will likely remain a fairly expensive drug, and we wouldn’t want to use it across the board, but for difficult to treat patients with either severe hypercholesterolemia or hypertriglyceridemia, I think this will have very significant advantages,” he commented.
“Drugs that reduce triglycerides by large amounts may prove to have cardiovascular disease benefits, but that remains to be proven in large, long-term outcome trials,” commented Deepak Bhatt, MD, professor of medicine at Harvard Medical School and executive director of interventional cardiology programs at Brigham and Women’s Hospital, both in Boston. “But for right now, for most patients with more common forms of elevated LDL cholesterol, the treatment options include statins, ezetimibe [Zetia], and PCSK9 inhibitors, and for more common levels of elevated triglycerides, it’s icosapent ethyl [Vascepa],” Dr. Bhatt said.
The study was sponsored by Regeneron, the company developing evinacumab and which is partially owned by Sanofi. Dr. Raal has received personal fees and/or research funding from Regeneron, Sanofi Aventis, Amgen, and The Medicines Company. Dr. Goldberg has received research funding and/or consulting fees from Regeneron and Sanofi, Akcea, Amarin, Amgen, Esperion, Ionis, Merck, Novartis, and Pfizer. Dr. Blom has been a consultant to and/or received research funding from Regeneron, Sanofi, Aegerium, Akcea, Amgen, Amryt, AstraZeneca, Eli Lilly, Esperion, Gemphire, MSD, and Novo Nordisk. Dr. Bhatt has received research funding from many companies including Regeneron and Sanofi.
SOURCE: Raal F. ACC 20. Abstract 411-12.
Evinacumab, the first agent from a new class of lipid-lowering drugs, showed a “remarkable” and unprecedented level of LDL-cholesterol lowering in a pivotal trial with 65 patients with homozygous familial hypercholesterolemia.
Monthly intravenous infusions of evinacumab cut LDL cholesterol levels by an average of 135 mg/dL from baseline, a 47% mean reduction, after 24 weeks of treatment in 43 homozygous familial hypercholesterolemia (HoFH) patients, Frederick Raal, MBChB, said on March 30 in a video presentation of his research at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
Evinacumab is a human monoclonal antibody inhibitor of angiopoietin-like 3, a liver protein that boosts levels of LDL cholesterol and triglycerides (TG).
Another notable effect of the novel agent was that it was equally effective in the roughly one-third of patients with a minimal residual level of LDL receptor activity, patients know as having “null/null” mutations. “For the first time, we see HoFH patients getting to [lipid] targets that we never thought would be possible,” said Dr. Raal, professor and head of endocrinology and metabolism at the University of Witwatersrand in Johannesburg, South Africa. “This works in patients without residual LDL receptor function.” The drug was also generally very well tolerated, he said, causing no treatment-related serious adverse events during the brief treatment period of 24 weeks.
“One of the major, remarkable findings in this study was the effect on null/null patients,” which contrasts with the effects of other, more established drugs for treating dyslipidemia like statins and PCSK9 inhibitors, which work by increasing the number of LDL receptors on cells. The demonstrated efficacy and safety of evinacumab in null/null patients “is a definite advance,” commented Anne C. Goldberg, MD, a lipidologist and professor of medicine at Washington University in St. Louis.
The placebo-controlled trial randomized patients at 30 sites in 11 countries who were at least 12 years old and had documented mutations in both of their LDL receptor genes and a serum level of LDL cholesterol that was at least 500 mg/dL on no treatment. Patients averaged about 40 years of age; about 30% had null/null mutations, more than 90% were on statin treatment, and about three-quarters were receiving regular treatment with a PCSK9 inhibitor. At baseline, LDL cholesterol levels averaged about 250 mg/dL.
The study’s primary endpoint was the between-group percentage change in LDL cholesterol level after 24 weeks, which fell by 47% from baseline with evinacumab treatment and increased by an average of 2% among 22 patients who received placebo injections; so evinacumab cut this measure by 49%, compared with placebo after 24 weeks, a statistically significant difference. A cut of baseline LDL cholesterol by at least 50% occurred in 56% of the evinacumab-treated patients and in 5% of controls.
In addition to its LDL reduction, another notable effect of evinacumab was that it trimmed baseline triglyceride levels by half, consistent with prior reports of the drug’s effect on this measure, although average triglyceride levels in the enrolled patients fell within the normal range prior to treatment.
Evinacumab “will probably be very effective in treating patients with hypertriglyceridemia; those studies are ongoing,” noted Dr. Raal. But, he added, “this drug will probably be reserved for severe” dyslipidemia cases, not for “the garden variety of moderate hypertriglyceridemia or hypercholesterolemia.”
Evinacumab “may be a fairly broad-spectrum lipid-lowering drug, but it should be reserved for severe cases,” agreed Dirk Blom, MBChB, head of lipidology at the University of Capetown, South Africa. “This will likely remain a fairly expensive drug, and we wouldn’t want to use it across the board, but for difficult to treat patients with either severe hypercholesterolemia or hypertriglyceridemia, I think this will have very significant advantages,” he commented.
“Drugs that reduce triglycerides by large amounts may prove to have cardiovascular disease benefits, but that remains to be proven in large, long-term outcome trials,” commented Deepak Bhatt, MD, professor of medicine at Harvard Medical School and executive director of interventional cardiology programs at Brigham and Women’s Hospital, both in Boston. “But for right now, for most patients with more common forms of elevated LDL cholesterol, the treatment options include statins, ezetimibe [Zetia], and PCSK9 inhibitors, and for more common levels of elevated triglycerides, it’s icosapent ethyl [Vascepa],” Dr. Bhatt said.
The study was sponsored by Regeneron, the company developing evinacumab and which is partially owned by Sanofi. Dr. Raal has received personal fees and/or research funding from Regeneron, Sanofi Aventis, Amgen, and The Medicines Company. Dr. Goldberg has received research funding and/or consulting fees from Regeneron and Sanofi, Akcea, Amarin, Amgen, Esperion, Ionis, Merck, Novartis, and Pfizer. Dr. Blom has been a consultant to and/or received research funding from Regeneron, Sanofi, Aegerium, Akcea, Amgen, Amryt, AstraZeneca, Eli Lilly, Esperion, Gemphire, MSD, and Novo Nordisk. Dr. Bhatt has received research funding from many companies including Regeneron and Sanofi.
SOURCE: Raal F. ACC 20. Abstract 411-12.
Evinacumab, the first agent from a new class of lipid-lowering drugs, showed a “remarkable” and unprecedented level of LDL-cholesterol lowering in a pivotal trial with 65 patients with homozygous familial hypercholesterolemia.
Monthly intravenous infusions of evinacumab cut LDL cholesterol levels by an average of 135 mg/dL from baseline, a 47% mean reduction, after 24 weeks of treatment in 43 homozygous familial hypercholesterolemia (HoFH) patients, Frederick Raal, MBChB, said on March 30 in a video presentation of his research at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
Evinacumab is a human monoclonal antibody inhibitor of angiopoietin-like 3, a liver protein that boosts levels of LDL cholesterol and triglycerides (TG).
Another notable effect of the novel agent was that it was equally effective in the roughly one-third of patients with a minimal residual level of LDL receptor activity, patients know as having “null/null” mutations. “For the first time, we see HoFH patients getting to [lipid] targets that we never thought would be possible,” said Dr. Raal, professor and head of endocrinology and metabolism at the University of Witwatersrand in Johannesburg, South Africa. “This works in patients without residual LDL receptor function.” The drug was also generally very well tolerated, he said, causing no treatment-related serious adverse events during the brief treatment period of 24 weeks.
“One of the major, remarkable findings in this study was the effect on null/null patients,” which contrasts with the effects of other, more established drugs for treating dyslipidemia like statins and PCSK9 inhibitors, which work by increasing the number of LDL receptors on cells. The demonstrated efficacy and safety of evinacumab in null/null patients “is a definite advance,” commented Anne C. Goldberg, MD, a lipidologist and professor of medicine at Washington University in St. Louis.
The placebo-controlled trial randomized patients at 30 sites in 11 countries who were at least 12 years old and had documented mutations in both of their LDL receptor genes and a serum level of LDL cholesterol that was at least 500 mg/dL on no treatment. Patients averaged about 40 years of age; about 30% had null/null mutations, more than 90% were on statin treatment, and about three-quarters were receiving regular treatment with a PCSK9 inhibitor. At baseline, LDL cholesterol levels averaged about 250 mg/dL.
The study’s primary endpoint was the between-group percentage change in LDL cholesterol level after 24 weeks, which fell by 47% from baseline with evinacumab treatment and increased by an average of 2% among 22 patients who received placebo injections; so evinacumab cut this measure by 49%, compared with placebo after 24 weeks, a statistically significant difference. A cut of baseline LDL cholesterol by at least 50% occurred in 56% of the evinacumab-treated patients and in 5% of controls.
In addition to its LDL reduction, another notable effect of evinacumab was that it trimmed baseline triglyceride levels by half, consistent with prior reports of the drug’s effect on this measure, although average triglyceride levels in the enrolled patients fell within the normal range prior to treatment.
Evinacumab “will probably be very effective in treating patients with hypertriglyceridemia; those studies are ongoing,” noted Dr. Raal. But, he added, “this drug will probably be reserved for severe” dyslipidemia cases, not for “the garden variety of moderate hypertriglyceridemia or hypercholesterolemia.”
Evinacumab “may be a fairly broad-spectrum lipid-lowering drug, but it should be reserved for severe cases,” agreed Dirk Blom, MBChB, head of lipidology at the University of Capetown, South Africa. “This will likely remain a fairly expensive drug, and we wouldn’t want to use it across the board, but for difficult to treat patients with either severe hypercholesterolemia or hypertriglyceridemia, I think this will have very significant advantages,” he commented.
“Drugs that reduce triglycerides by large amounts may prove to have cardiovascular disease benefits, but that remains to be proven in large, long-term outcome trials,” commented Deepak Bhatt, MD, professor of medicine at Harvard Medical School and executive director of interventional cardiology programs at Brigham and Women’s Hospital, both in Boston. “But for right now, for most patients with more common forms of elevated LDL cholesterol, the treatment options include statins, ezetimibe [Zetia], and PCSK9 inhibitors, and for more common levels of elevated triglycerides, it’s icosapent ethyl [Vascepa],” Dr. Bhatt said.
The study was sponsored by Regeneron, the company developing evinacumab and which is partially owned by Sanofi. Dr. Raal has received personal fees and/or research funding from Regeneron, Sanofi Aventis, Amgen, and The Medicines Company. Dr. Goldberg has received research funding and/or consulting fees from Regeneron and Sanofi, Akcea, Amarin, Amgen, Esperion, Ionis, Merck, Novartis, and Pfizer. Dr. Blom has been a consultant to and/or received research funding from Regeneron, Sanofi, Aegerium, Akcea, Amgen, Amryt, AstraZeneca, Eli Lilly, Esperion, Gemphire, MSD, and Novo Nordisk. Dr. Bhatt has received research funding from many companies including Regeneron and Sanofi.
SOURCE: Raal F. ACC 20. Abstract 411-12.
REPORTING FROM ACC 2020
Alirocumab effective in homozygous FH
Alirocumab achieved a mean 63-mg/dL reduction in LDL cholesterol in the ODYSSEY HoFH study, the largest-ever randomized, placebo-controlled clinical trial of lipid-lowering in adults with homozygous familial hypercholesterolemia (HoFH), Dirk Blom, MD, said in a video presentation of his research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“We’re not getting most patients to goal, but we’re certainly getting them closer to goal. A lot of patients will still need further therapies that don’t rely on up-regulation of the LDL receptor, such as lipoprotein apheresis,” observed Dr. Blom, head of the division of lipidology at the University of Cape Town (South Africa).
Of the patients on alirocumab, 57%had at least a 30% reduction in LDL at 12 weeks, and 27% had a 50% reduction or more.
Alirocumab had salutary effects on other atherogenic lipids: roughly a 20% reduction from baseline in lipoprotein (a), a 23% decrease in apolipoprotein B, and a 25% reduction in non-HDL cholesterol.
Dr. Blom noted that, as is also the case for statins in HoFH, the LDL response to alirocumab in patients with this genetically complex disorder is more variable and generally weaker than in other hypercholesterolemic populations.
“We saw some patients getting up to 60%-70% LDL reduction in alirocumab, but a lot of patients getting much less,” he said.
Alirocumab was well tolerated in adults with HoFH, with the same favorable safety profile that’s been established in other patient populations.
Discussant Raul Santos, MD, commented that the ODYSSEY HoFH results are quite similar to those reported in patients with HoFH in an earlier study of evolocumab (Repatha), another PCSK9 inhibitor. The magnitude of LDL-lowering achieved with these biologic agents is such that, were treatment to start early in life, patients with HoFH might expect to experience an extra 10-15 years of life free of cardiovascular events.
“Certainly PCSK9 inhibitors should be the next step after statins and ezetimibe. They’re much less expensive and more available than apheresis,” said Dr. Santos, director of the lipid clinic at the Heart Institute of the University of São Paulo.
Since many patients with this rare disorder experience their first cardiovascular event in adolescence or young adulthood, Dr. Santos said, it’s very important to expand PCSK9 inhibitor therapy to the pediatric HoFH population. Two studies are ongoing in childlren.
The ODYSSEY HoFH trial was funded by Regeneron and Sanofi. Dr. Blom reported serving as a paid consultant to Sanofi, Akcea, Amgen, and Gemphire.
Alirocumab achieved a mean 63-mg/dL reduction in LDL cholesterol in the ODYSSEY HoFH study, the largest-ever randomized, placebo-controlled clinical trial of lipid-lowering in adults with homozygous familial hypercholesterolemia (HoFH), Dirk Blom, MD, said in a video presentation of his research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“We’re not getting most patients to goal, but we’re certainly getting them closer to goal. A lot of patients will still need further therapies that don’t rely on up-regulation of the LDL receptor, such as lipoprotein apheresis,” observed Dr. Blom, head of the division of lipidology at the University of Cape Town (South Africa).
Of the patients on alirocumab, 57%had at least a 30% reduction in LDL at 12 weeks, and 27% had a 50% reduction or more.
Alirocumab had salutary effects on other atherogenic lipids: roughly a 20% reduction from baseline in lipoprotein (a), a 23% decrease in apolipoprotein B, and a 25% reduction in non-HDL cholesterol.
Dr. Blom noted that, as is also the case for statins in HoFH, the LDL response to alirocumab in patients with this genetically complex disorder is more variable and generally weaker than in other hypercholesterolemic populations.
“We saw some patients getting up to 60%-70% LDL reduction in alirocumab, but a lot of patients getting much less,” he said.
Alirocumab was well tolerated in adults with HoFH, with the same favorable safety profile that’s been established in other patient populations.
Discussant Raul Santos, MD, commented that the ODYSSEY HoFH results are quite similar to those reported in patients with HoFH in an earlier study of evolocumab (Repatha), another PCSK9 inhibitor. The magnitude of LDL-lowering achieved with these biologic agents is such that, were treatment to start early in life, patients with HoFH might expect to experience an extra 10-15 years of life free of cardiovascular events.
“Certainly PCSK9 inhibitors should be the next step after statins and ezetimibe. They’re much less expensive and more available than apheresis,” said Dr. Santos, director of the lipid clinic at the Heart Institute of the University of São Paulo.
Since many patients with this rare disorder experience their first cardiovascular event in adolescence or young adulthood, Dr. Santos said, it’s very important to expand PCSK9 inhibitor therapy to the pediatric HoFH population. Two studies are ongoing in childlren.
The ODYSSEY HoFH trial was funded by Regeneron and Sanofi. Dr. Blom reported serving as a paid consultant to Sanofi, Akcea, Amgen, and Gemphire.
Alirocumab achieved a mean 63-mg/dL reduction in LDL cholesterol in the ODYSSEY HoFH study, the largest-ever randomized, placebo-controlled clinical trial of lipid-lowering in adults with homozygous familial hypercholesterolemia (HoFH), Dirk Blom, MD, said in a video presentation of his research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“We’re not getting most patients to goal, but we’re certainly getting them closer to goal. A lot of patients will still need further therapies that don’t rely on up-regulation of the LDL receptor, such as lipoprotein apheresis,” observed Dr. Blom, head of the division of lipidology at the University of Cape Town (South Africa).
Of the patients on alirocumab, 57%had at least a 30% reduction in LDL at 12 weeks, and 27% had a 50% reduction or more.
Alirocumab had salutary effects on other atherogenic lipids: roughly a 20% reduction from baseline in lipoprotein (a), a 23% decrease in apolipoprotein B, and a 25% reduction in non-HDL cholesterol.
Dr. Blom noted that, as is also the case for statins in HoFH, the LDL response to alirocumab in patients with this genetically complex disorder is more variable and generally weaker than in other hypercholesterolemic populations.
“We saw some patients getting up to 60%-70% LDL reduction in alirocumab, but a lot of patients getting much less,” he said.
Alirocumab was well tolerated in adults with HoFH, with the same favorable safety profile that’s been established in other patient populations.
Discussant Raul Santos, MD, commented that the ODYSSEY HoFH results are quite similar to those reported in patients with HoFH in an earlier study of evolocumab (Repatha), another PCSK9 inhibitor. The magnitude of LDL-lowering achieved with these biologic agents is such that, were treatment to start early in life, patients with HoFH might expect to experience an extra 10-15 years of life free of cardiovascular events.
“Certainly PCSK9 inhibitors should be the next step after statins and ezetimibe. They’re much less expensive and more available than apheresis,” said Dr. Santos, director of the lipid clinic at the Heart Institute of the University of São Paulo.
Since many patients with this rare disorder experience their first cardiovascular event in adolescence or young adulthood, Dr. Santos said, it’s very important to expand PCSK9 inhibitor therapy to the pediatric HoFH population. Two studies are ongoing in childlren.
The ODYSSEY HoFH trial was funded by Regeneron and Sanofi. Dr. Blom reported serving as a paid consultant to Sanofi, Akcea, Amgen, and Gemphire.
REPORTING FROM ACC 2020
Acid-suppressant medications in infants with bronchiolitis raises later allergy risk
Infants who are hospitalized for severe bronchiolitis and receive acid-suppressant medications may be at risk of developing allergic disease by age 3 years, according to recent research released as an abstract for the American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage
“Among children with a history of severe bronchiolitis during infancy, exposure to acid-suppressant medications during infancy further increases the risk of developing recurrent wheeze by age 3 years,” Lacey B. Robinson, MD, of the division of rheumatology, allergy, and immunology in the department of medicine at Massachusetts General Hospital in Boston, said in an interview.
Bronchiolitis is a risk factor in infants for developing conditions such as recurrent wheeze and childhood asthma in early childhood. Acid-suppressant medications like proton pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2RAs) may further increase the risk of allergic disease. One study by Mitre et al. published in JAMA Pediatrics showed use of acid-suppressant medications in infants up to 6 months raised the risk of allergic disease (JAMA Pediatr. 2018;172[6]:e180315). Some studies suggest between 30% and 50% of infants diagnosed with bronchiolitis requiring hospitalization will develop asthma by age 5 years (J Allergy Clin Immunol Pract. 2017 Jan - Feb;5[1]:92-6).
“Children with severe bronchiolitis during infancy are at a high risk of developing recurrent wheeze and subsequent asthma. There is limited evidence to suggest that exposure to acid suppressant medications [such as proton pump inhibitors and histamine-2 receptor antagonists] prenatally and during early childhood increases the risk of childhood asthma,” Dr. Robinson said. “It is not known if exposure to acid suppressant medications during infancy further increases the risk of developing recurrent wheeze among high-risk children, such as in those with a history of severe bronchiolitis during infancy.”
Dr. Robinson and colleagues performed a multicenter, prospective cohort study of 921 infants who were hospitalized for severe bronchiolitis between 2011 and 2014. The investigators reviewed the medical records of the infants for acid suppressant medication use, as well as parent report of acid suppressant medication use, during an infant’s first 12 months. Overall, 879 children were analyzed after excluding for patients who developed recurrent wheeze prior to receiving acid suppressant medications, as well as patients with incomplete data. The investigators used the National Institutes of Health Guidelines for the Diagnosis and Management of Asthma (EPR-3) to define recurrent wheeze. A Cox-proportional hazard model was used to analyze the time to event, which was stratified by age and adjusted for confounders.
Infants with a history of severe bronchiolitis were at greater risk of developing recurrent wheeze by age 3 years after being exposed to acid-suppressant medications, compared with infants who were not exposed, Dr. Robinson said. Of the 879 infants in the final analysis, 159 (18%) received acid-suppressant medications, and 68 of 159 patients (43%) went on to develop recurrent wheeze, compared with 206 of 720 infants (29%) who were not exposed (unadjusted hazard ratio, 1.63; 95% confidence interval, 1.24-2.14).
After adjustment for confounders such as gender, race and ethnicity; gestational age; delivery type; severity of bronchiolitis; respiratory syncytial virus (RSV) infection status; maternal atopy; use of acid-suppressant medications during pregnancy; median household income; and insurance status, the association between recurrent wheeze and acid-suppressant medication use during infancy remained (adjusted HR, 1.54; 95% CI, 1.15-2.07).
“More research is needed on this important topic including studies in other populations,” such as in healthy children, Dr. Robinson said. “We encourage future research on this important and understudied topic, including further research on the potential underlying mechanisms of this association.”
Dr. Robinson reported no relevant financial disclosures.
SOURCE: Robinson L. AAAAI 2020, Abstract L1
Infants who are hospitalized for severe bronchiolitis and receive acid-suppressant medications may be at risk of developing allergic disease by age 3 years, according to recent research released as an abstract for the American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage
“Among children with a history of severe bronchiolitis during infancy, exposure to acid-suppressant medications during infancy further increases the risk of developing recurrent wheeze by age 3 years,” Lacey B. Robinson, MD, of the division of rheumatology, allergy, and immunology in the department of medicine at Massachusetts General Hospital in Boston, said in an interview.
Bronchiolitis is a risk factor in infants for developing conditions such as recurrent wheeze and childhood asthma in early childhood. Acid-suppressant medications like proton pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2RAs) may further increase the risk of allergic disease. One study by Mitre et al. published in JAMA Pediatrics showed use of acid-suppressant medications in infants up to 6 months raised the risk of allergic disease (JAMA Pediatr. 2018;172[6]:e180315). Some studies suggest between 30% and 50% of infants diagnosed with bronchiolitis requiring hospitalization will develop asthma by age 5 years (J Allergy Clin Immunol Pract. 2017 Jan - Feb;5[1]:92-6).
“Children with severe bronchiolitis during infancy are at a high risk of developing recurrent wheeze and subsequent asthma. There is limited evidence to suggest that exposure to acid suppressant medications [such as proton pump inhibitors and histamine-2 receptor antagonists] prenatally and during early childhood increases the risk of childhood asthma,” Dr. Robinson said. “It is not known if exposure to acid suppressant medications during infancy further increases the risk of developing recurrent wheeze among high-risk children, such as in those with a history of severe bronchiolitis during infancy.”
Dr. Robinson and colleagues performed a multicenter, prospective cohort study of 921 infants who were hospitalized for severe bronchiolitis between 2011 and 2014. The investigators reviewed the medical records of the infants for acid suppressant medication use, as well as parent report of acid suppressant medication use, during an infant’s first 12 months. Overall, 879 children were analyzed after excluding for patients who developed recurrent wheeze prior to receiving acid suppressant medications, as well as patients with incomplete data. The investigators used the National Institutes of Health Guidelines for the Diagnosis and Management of Asthma (EPR-3) to define recurrent wheeze. A Cox-proportional hazard model was used to analyze the time to event, which was stratified by age and adjusted for confounders.
Infants with a history of severe bronchiolitis were at greater risk of developing recurrent wheeze by age 3 years after being exposed to acid-suppressant medications, compared with infants who were not exposed, Dr. Robinson said. Of the 879 infants in the final analysis, 159 (18%) received acid-suppressant medications, and 68 of 159 patients (43%) went on to develop recurrent wheeze, compared with 206 of 720 infants (29%) who were not exposed (unadjusted hazard ratio, 1.63; 95% confidence interval, 1.24-2.14).
After adjustment for confounders such as gender, race and ethnicity; gestational age; delivery type; severity of bronchiolitis; respiratory syncytial virus (RSV) infection status; maternal atopy; use of acid-suppressant medications during pregnancy; median household income; and insurance status, the association between recurrent wheeze and acid-suppressant medication use during infancy remained (adjusted HR, 1.54; 95% CI, 1.15-2.07).
“More research is needed on this important topic including studies in other populations,” such as in healthy children, Dr. Robinson said. “We encourage future research on this important and understudied topic, including further research on the potential underlying mechanisms of this association.”
Dr. Robinson reported no relevant financial disclosures.
SOURCE: Robinson L. AAAAI 2020, Abstract L1
Infants who are hospitalized for severe bronchiolitis and receive acid-suppressant medications may be at risk of developing allergic disease by age 3 years, according to recent research released as an abstract for the American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage
“Among children with a history of severe bronchiolitis during infancy, exposure to acid-suppressant medications during infancy further increases the risk of developing recurrent wheeze by age 3 years,” Lacey B. Robinson, MD, of the division of rheumatology, allergy, and immunology in the department of medicine at Massachusetts General Hospital in Boston, said in an interview.
Bronchiolitis is a risk factor in infants for developing conditions such as recurrent wheeze and childhood asthma in early childhood. Acid-suppressant medications like proton pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2RAs) may further increase the risk of allergic disease. One study by Mitre et al. published in JAMA Pediatrics showed use of acid-suppressant medications in infants up to 6 months raised the risk of allergic disease (JAMA Pediatr. 2018;172[6]:e180315). Some studies suggest between 30% and 50% of infants diagnosed with bronchiolitis requiring hospitalization will develop asthma by age 5 years (J Allergy Clin Immunol Pract. 2017 Jan - Feb;5[1]:92-6).
“Children with severe bronchiolitis during infancy are at a high risk of developing recurrent wheeze and subsequent asthma. There is limited evidence to suggest that exposure to acid suppressant medications [such as proton pump inhibitors and histamine-2 receptor antagonists] prenatally and during early childhood increases the risk of childhood asthma,” Dr. Robinson said. “It is not known if exposure to acid suppressant medications during infancy further increases the risk of developing recurrent wheeze among high-risk children, such as in those with a history of severe bronchiolitis during infancy.”
Dr. Robinson and colleagues performed a multicenter, prospective cohort study of 921 infants who were hospitalized for severe bronchiolitis between 2011 and 2014. The investigators reviewed the medical records of the infants for acid suppressant medication use, as well as parent report of acid suppressant medication use, during an infant’s first 12 months. Overall, 879 children were analyzed after excluding for patients who developed recurrent wheeze prior to receiving acid suppressant medications, as well as patients with incomplete data. The investigators used the National Institutes of Health Guidelines for the Diagnosis and Management of Asthma (EPR-3) to define recurrent wheeze. A Cox-proportional hazard model was used to analyze the time to event, which was stratified by age and adjusted for confounders.
Infants with a history of severe bronchiolitis were at greater risk of developing recurrent wheeze by age 3 years after being exposed to acid-suppressant medications, compared with infants who were not exposed, Dr. Robinson said. Of the 879 infants in the final analysis, 159 (18%) received acid-suppressant medications, and 68 of 159 patients (43%) went on to develop recurrent wheeze, compared with 206 of 720 infants (29%) who were not exposed (unadjusted hazard ratio, 1.63; 95% confidence interval, 1.24-2.14).
After adjustment for confounders such as gender, race and ethnicity; gestational age; delivery type; severity of bronchiolitis; respiratory syncytial virus (RSV) infection status; maternal atopy; use of acid-suppressant medications during pregnancy; median household income; and insurance status, the association between recurrent wheeze and acid-suppressant medication use during infancy remained (adjusted HR, 1.54; 95% CI, 1.15-2.07).
“More research is needed on this important topic including studies in other populations,” such as in healthy children, Dr. Robinson said. “We encourage future research on this important and understudied topic, including further research on the potential underlying mechanisms of this association.”
Dr. Robinson reported no relevant financial disclosures.
SOURCE: Robinson L. AAAAI 2020, Abstract L1
REPORTING FROM AAAAI 2020